User login
Psoriasis is a common chronic inflammatory skin disease affecting 1% to 8% of the world population, depending on the country.1 Psoriasis can greatly impact quality of life in affected individuals, even in those with limited body surface involvement.2 Studies have demonstrated a high degree of psychological distress associated with psoriasis, leading to depression and poor self-esteem.3
Over the last decade, our improved understanding of the autoimmune inflammatory pathways and the associated changing concepts in psoriasis pathogenesis have led to the development of biological drugs targeting specific components of effector immune mechanisms, and these biological drugs have revolutionized the treatment of psoriasis.4 Although response rates of these biological agents are greater compared to those of conventional systemic drugs,5 current biological drugs fail to demonstrate efficacy in some patients or lose their efficacy over time. In addition to the high costs associated with these drugs, these limitations have driven a continued search for alternative therapies.
Helper T cells (TH17) and the proinflammatory cytokine IL-17 have been shown to play a key role in the pathophysiology of psoriasis, bridging innate and adaptive immune responses. IL-17 is involved in the modulation of proinflammatory cytokines, hematopoietic growth factors, antimicrobial peptides, and chemokines. Increased TH17 activity and high levels of IL-17 have been found in psoriatic plaques, and increased levels of TH17 are found in the plasma of psoriasis patients.6 Increased IL-17 induces neutrophilia, inflammation, and angiogenesis.7 Other cytokines that are highly upregulated in involved skin are tumor necrosis factor a (TNF-α), IL-23, IL-22, and IL-21.8 IL-23 is involved in regulating TH17 cells and is a potent activator of keratinocyte proliferation.9 Blockade of IL-12/23 causes downregulation of TH17 and TH22 cell responses.10 As IL-17 has a key role in protecting skin and mucous membranes from bacterial and fungal infections, IL-17 inhibition can potentially interfere with the inflammatory cascade. However, available data suggest that sufficient residual IL-17 activity remains to maintain immunity against infections.11
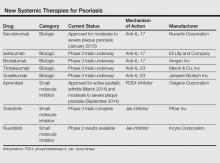
Currently approved biological agents for psoriasis target proinflammatory cytokines such as TNF-α, or the p40 subunit of IL-12 and IL-23. A number of novel targeted therapies including biologics as well as small molecule inhibitors targeting various cytokines and molecules involved in the pathogenesis of psoriasis are currently in different stages of development (Table). These drugs include 3 IL-17 inhibitors (secukinumab, ixekizumab, and brodalumab); 2 IL-23 blockers (tildrakizumab and guselkumab); and small molecule inhibitors that target the kinase pathway including apremilast (a phosphodiesterase 4 [PDE4] inhibitor), as well as tofacitinib, baricitinib, and ruxolitinib (Janus kinase [Jak] inhibitors). Small molecule inhibitors can be administered orally and are less expensive to produce than biological agents. This article reviews available data on these new systemic agents in the pipeline.
Novel Biologics
Secukinumab
Secukinumab is a fully human monoclonal IgG1k antibody that selectively binds and neutralizes IL-17A.12 It is the first of the IL-17 antibodies to receive approval for the treatment of moderate to severe psoriasis. In 2 phase 3, double-blind, 52-week trials—ERASURE (Efficacy of Response and Safety of Two Fixed Secukinumab Regimens in Psoriasis) and FIXTURE (Full Year Investigative Examination of Secukinumab vs Etanercept Using Two Dosing Regimens to Determine Efficacy in Psoriasis)—participants were randomly assigned to receive subcutaneous secukinumab at doses of 300 mg (n=245 and n=327, respectively) or 150 mg (n=245 and n=327, respectively) once weekly for 5 weeks then every 4 weeks, or placebo (n=248 and n=326, respectively); in the FIXTURE study only, an etanercept group (n=326) was given a 50-mg dose twice weekly for 12 weeks then once weekly.13
In the ERASURE study, the proportion of participants showing a reduction of 75% or more in psoriasis area and severity index (PASI) score from baseline to week 12 was 81.6% with secukinumab 300 mg, 71.6% with secukinumab 150 mg, and 4.5% with placebo.13 Secondary end point results demonstrated the proportion of participants showing a 90% reduction in PASI score was 59.2% with secukinumab 300 mg and 39.1% with secukinumab 150 mg, which were both superior to placebo (1.2%). The proportion of participants who met the criteria for 100% reduction in PASI score at week 12 also was greater with each secukinumab dose than with placebo.13
In the FIXTURE study, the proportion of participants showing a reduction of 75% or more from baseline in PASI score at week 12 was 77.1% with secukinumab 300 mg, 67.0% with secukinumab 150 mg, 44.0% with etanercept, and 4.9% with placebo.13 Secondary end point results demonstrated the proportion of participants showing a 90% reduction in PASI score was 54.2% with secukinumab 300 mg, 41.9% with secukinumab 150 mg, 20.7% with etanercept, and 1.5% with placebo. The speed of response, which was assessed as the median time to a 50% reduction in mean PASI score from baseline, was significantly shorter with both doses of secukinumab (3.0 weeks and 3.9 weeks, respectively) than with etanercept (7.0 weeks)(P<.001 for both).13
In the FIXTURE study, incidences of adverse events (AEs) were similar in the secukinumab and etanercept groups during both the induction period and the entire treatment period.13 The most common AEs in the secukinumab groups were nasopharyngitis, headache, and diarrhea. The rates of infections or infestations during the induction period were 26.7% with secukinumab 300 mg, 30.9% with secukinumab 150 mg, 24.5% with etanercept, and 19.3% with placebo. Candidal infections were more common with secukinumab than with etanercept during the entire treatment period (4.7% and 2.3% of participants in the secukinumab 300 mg and 150 mg groups, respectively, reported mild or moderate candidal infections). None of these infections resulted in chronic mucocutaneous candidiasis or discontinuation of the study drug and all resolved on their own or with standard therapy. Candidal infection was reported in 1.2% of participants in the etanercept group. Responses at week 12 were sustained in the majority of participants through week 52 with continued secukinumab therapy every 4 weeks. Grade 3 neutropenia occurred in 1.0% of secukinumab-treated participants and in none of the participants in the etanercept group. There were no apparent dose-related differences between the secukinumab groups with respect to AEs, with the exception of mild and moderate candidal infections.13
These efficacy data are impressive and no specific serious safety concerns have been identified to date. However, IL-17A plays a key role in host defense, particularly in mucocutaneous immunity against Candida albicans,14 as well as in hematopoiesis through stimulation of granulopoiesis and neutrophil trafficking,15 and thus we need to remain watchful with regards to Candida albicans infections and neutropenia.
Ixekizumab
Ixekizumab is a humanized IgG4 anti–IL-17A monoclonal antibody. In a phase 2, double-blind, placebo-controlled trial, 142 participants with chronic moderate to severe plaque psoriasis were randomly assigned to receive 150-mg (n=28), 75-mg (n=29), 25-mg (n=30), or 10-mg (n=28) subcutaneous injections of ixekizumab or placebo (n=27) at weeks 0, 2, 4, 8, 12, and 16.16 At 12 weeks, the percentage of participants who achieved a 75% reduction in PASI score from baseline was significantly greater with ixekizumab (82.1% with 150-mg dose, 82.8% with 75-mg dose, 76.7% with 25-mg dose) than with placebo (7.7%)(P<.001 for each comparison), except with the 10-mg dose. Similarly, a greater percentage of participants in the same ixekizumab groups achieved a 90% reduction (71.4% with 150-mg dose, 58.6% with 75-mg dose, 50.0% with 25-mg dose) and a 100% reduction (39.3% with 150-mg dose, 37.9% with 75-mg dose) in PASI score compared to placebo (0%)(P<.001 for each comparison). Significant reductions in PASI scores were evident as early as week 1 in the 150-mg and 75-mg groups, and these reductions were sustained for 20 weeks (P<.05).16 Phase 3 studies of ixekizumab currently are underway.
Brodalumab
The third IL-17 blocker in the pipeline is brodalumab, a human monoclonal antibody against IL-17RA, which blocks signaling of IL-17A and IL-17F as well as the IL-17A/F heterodimer, all of which are involved in the inflammatory process of psoriasis. Brodalumab was evaluated in a phase 2, double-blind, placebo-controlled, dose-ranging study of 198 participants who were randomized to receive 70 mg (n=39), 140 mg (n=39), 210 mg (n=40), or 280 mg (n=42) of brodalumab or placebo (n=38).17 At week 12, improvements of at least 75% and at least 90% in PASI score were achieved by 77% and 72%, respectively, in the 140-mg group, and 82% and 75%, respectively, in the 210-mg group compared to 0% of the placebo group (P<.001 for all comparisons). One hundred percent improvement in PASI was achieved by 38% of participants in the 140-mg group and 62% in the 210-mg group. No participants in the placebo group demonstrated improvement of 75% or higher. The most common AEs were nasopharyngitis, upper respiratory tract infection, arthralgia, and injection-site erythema. Serious AEs reported during the study included renal colic (1 participant), ec-topic pregnancy (1 participant), and grade 3 asymptomatic neutropenia (2 participants). Both cases of neutropenia were noted at the first assessment after brodalumab initiation (week 2) and resolved when the study drug was withheld.17
Results for this new IL-17 blocker are encouraging, but phase 3 data of brodalumab will need to be awaited.
Tildrakizumab
Tildrakizumab is a humanized IgG1 monoclonal antibody that blocks the p19 subunit of IL-23. In a randomized, double-blind, phase 2b trial, 355 adults with moderate to severe psoriasis were randomized to receive subcutaneous injections of tildrakizumab (5 mg, 25 mg, 100 mg, or 200 mg) or placebo.18 In part 1 of the study, injections were administered at weeks 0 and 4. Part 2 of the study started at week 16. In part 2, responders with a 75% improvement in PASI score in the 5- and 25-mg groups continued their dose, while responders in the 100- or 200-mg groups were randomized again to continue the same dose or a reduced dose (100 mg to 25 mg; 200 mg to 100 mg) every 12 weeks from weeks 16 to 52. Those in the placebo group received tildrakizumab 25 mg every 12 weeks in part 2. The primary end point was the mean change in PASI score from baseline to week 16, which was significantly greater in all tildrakizumab groups than in the placebo group (P<.001 for all comparisons). Improvements of 75% in PASI score were achieved by 74% in the 200-mg group, 66% in the 100-mg group, 64% in the 25-mg group, and 33% in the 5-mg group. In contrast, 4.9% in the placebo group achieved an improvement of 75%. At week 52, no loss of efficacy was seen in those participants who had achieved 75% improvement in PASI score at week 16 and had continued their prior doses. The rates of AEs seen in the tildrakizumab groups were 60% to 71% compared to 69% in the placebo group. The most common AE was nasopharyngitis, occurring in 12% to 20% of participants in each group. Serious AEs were uncommon.18 Phase 3 studies are currently underway.19
Guselkumab
Guselkumab is a human IgG1 monoclonal antibody in clinical development that specifically blocks the p19 subunit of IL-23. In a double-blind, placebo-controlled, phase 1 study, 24 participants with moderate to severe plaque psoriasis were randomized to receive a single 10-mg (n=5), 30-mg (n=5), 100-mg (n=5), or 300-mg (n=5) dose of guselkumab or placebo (n=4).20 At week 12, 50% in the 10-mg group, 60% in both the 30- and 100-mg groups, and 100% in the 300-mg group showed 75% improvement in PASI score versus 0% in the placebo group. Improvements in PASI scores were generally maintained through week 24. The rates of AEs were 65% (13/20) in the combined guselkumab group and 50% (2/4) in the placebo group.20
Small Molecule Inhibitors
In contrast to biologics, which mainly target soluble cytokine or cellular receptors, small molecule inhibitors target enzymes within signaling pathways. Small molecule inhibitors have some advantages over biologics in that they are relatively inexpensive to produce and can be administered orally; thus, they may be preferred by some patients over injectable drugs. There are several agents that are undergoing clinical trials in psoriasis, including PDE4 inhibitors and Jak inhibitors.
Apremilast
Apremilast is an oral small molecule PDE4 inhibitor that was approved by the US Food and Drug Administration in March 2014 for the treatment of adult patients with active psoriatic arthritis; an indication for moderate to severe plaque psoriasis was approved in September 2014.21 Phosphodiesterase 4 is a cyclic adenosine monophosphate–specific phosphodiesterase inhibitor, which is dominant in inflammatory cells. Inhibition of PDE4 increases intracellular cyclic adenosine monophosphate levels, thus downregulating proinflammatory cytokines such as TNF-α, IFN-γ, IL-2, IL-12, and IL-23, and increasing the production of anti-inflammatory cytokines such as IL-10.22
Phase 2 and phase 3 studies have demonstrated the clinical efficacy of apremilast in the treatment of patients with moderate to severe plaque psoriasis. In a 16-week randomized, placebo-controlled, phase 3 trial (ESTEEM 2), 408 participants were randomized to receive oral apremilast 30 mg twice daily (n=275) or placebo (n=138).23 Improvement of 75% in PASI score was achieved by 29% of participants in the apremilast group at week 16. The most common AEs were diarrhea (16%) and nausea (18%), which were predominantly mild, occurring most commonly in the first week and resolving within 1 month. No cases of severe diarrhea or severe nausea were reported. Apremilast had no apparent effect on the results of hematological or serum chemistry tests.23 Although the US Food and Drug Administration warns of a possible link between apremilast and depression,24 data are mostly related to roflumilast, another PDE4 inhibitor. Studies in patients with chronic obstructive pulmonary disease have noted increased cases of depression (1.21% vs 0.82%) and suicidal ideation/attempt (0.03% vs 0.02%) in patients treated with roflumilast versus placebo.25
Jak Inhibitors
Janus kinases are a family of intracellular tyrosine kinases that connect several cytokine receptors to the signal transducer and activator of transcription pathways.26 There are 4 Jak family members: Jak1, Jak2, Jak3, and tyrosine kinase 2. Janus kinases 1 and 2 have roles in interferon signaling, while Jak3 transduces signals from IL-2, IL-7, IL-15, and IL-21, which are T-cell growth and survival factors.
Tofacitinib is a novel oral signal transduction molecule that blocks the Jak3 pathway. A phase 2b, 12-week, dose-ranging study was conducted to assess the efficacy and safety of 3 twice-daily regimens of tofacitinib versus placebo in patients with moderate to severe chronic plaque psoriasis.27 One hundred and ninety-seven participants were randomized to receive oral tofacitinib (2 mg, 5 mg, or 15 mg; n=49 each) or placebo (n=50) twice daily for 12 weeks with a 4-week follow-up period. The primary end point was the proportion of participants achieving at least a 75% reduction in PASI score at week 12 (25.0% with 2 mg, 40.8% with 5 mg, 66.7% with 15 mg, 2.0% with placebo). Similarly, a higher proportion of participants achieving 90% reduction in PASI score was seen at weeks 8 and 12 in all tofacitinib-treated participants versus placebo. The most common AEs were upper respiratory tract infection, sinusitis, nasopharyngitis, and headache. A number of changes in laboratory parameters occurred in the tofacitinib groups. Mild dose-related decreases in hemoglobin were noted at week 12 for all tofacitinib groups, and a small increase (mean, 0.04 mg/dL) in serum creatinine levels was observed in the 15-mg group. Decreases in neutrophil counts were observed with higher doses of tofacitinib, with the maximum mean decrease of 0.9×103/mm3 from baseline observed in the 15-mg group at week 4. After weeks 4 through 8, mean neutrophil counts began to return to baseline levels. Dose-related increases in total cholesterol, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol were observed by week 2 and remained at this level through week 12; mean lipid levels decreased to baseline levels after cessation of active treatment. One participant in the 15-mg group developed an elevated alanine aminotransferase level that was more than 2.5 times the highest normal limit. Three participants experienced 5 serious AEs.27 These early results show that tofacitinib can be a safe and effective treatment in patients with psoriasis, but further data from phase 3 studies will need to be awaited.
Another Jak inhibitor under investigationfor the treatment of psoriasis is ruxolitinib, an inhibitor of Jak1 and Jak2, which has been primarily studied as a topical agent for milder cases of the disease.28
Conclusion
Many new drugs are currently on the horizon and will increase our armamentarium for treating psoriasis. Some of these agents promise greater levels of efficacy than currently used therapies. Although this review focuses on systemic agents, there also are a number of topical formulations in the pipeline. These new agents will certainly increase our options when choosing the most suitable treatment for a patient with psoriasis, but safety will remain a primary concern, and time and experience will tell whether efficacy outweighs any potential side effects.
1. Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
2. Rapp SR, Feldman SR, Exum ML, et al. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999;41(3 pt 1):401-407.
3. Shah R, Bewley A. Psoriasis: ‘the badge of shame.’ a case report of a psychological intervention to reduce and potentially clear chronic skin disease. Clin Exp Dermatol. 2014;39:600-603.
4. Vincent FB, Morand EF, Murphy K, et al. Antidrug antibodies (ADAb) to tumour necrosis factor (TNF)-specific neutralising agents in chronic inflammatory diseases: a real issue, a clinical perspective. Ann Rheum Dis. 2013;72:165-178.
5. Chi CC, Wang SH. Efficacy and cost-efficacy of biologic therapies for moderate to severe psoriasis: a meta-analysis and cost-efficacy analysis using the intention-to-treat principle. Biomed Res Int. 2014;2014:862851.
6. Kagami S, Rizzo HL, Lee JJ, et al. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010;130:1373-1383.
7. Leonardi CL, Gordon KB. New and emerging therapies in psoriasis. Semin Cutan Med Surg. 2014;33(2, suppl 2):S37-S41.
8. Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207-1211.
9. Nickoloff BJ, Qin JZ, Nestle FO. Immunopathogenesis of psoriasis. Clin Rev Allergy Immunol. 2007;33:45-56.
10. Fitch E, Harper E, Skorcheva I, et al. Pathophysiology of psoriasis: recent advances on IL-23 and Th17 cytokines. Curr Rheumatol Rep. 2007;9:461-467.
11. Adami S, Cavani A, Rossi F, et al. The role of interleukin-17A in psoriatic disease. BioDrugs. 2014;28:487-497.
12. Hueber W, Patel DD, Dryja T, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2:52ra72.
13. Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338.
14. Puel A, Cypowyj S, Bustamante J, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65-68.
15. Krstic A, Mojsilovic S, Jovcic G, et al. The potential of interleukin-17 to mediate hematopoietic response. Immunol Res. 2012;52:34-41.
16. Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
17. Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181-1189.
18. Langley RGB, Thaci D, Papp KA, et al. MK-3222, an anti–IL-23p19 humanized monoclonal antibody, provides significant improvement in psoriasis over 52 weeks of treatment that is maintained after discontinuation of dosing. Poster presented at: 73rd Annual Meeting of the American Academy of Dermatology; March 21-25, 2014; Denver, CO. Poster 8056.
19. Tausend W, Downing C, Tyring S. Systematic review of interleukin-12, interleukin-17, and interleukin-23 pathway inhibitors for the treatment of moderate-to-severe chronic plaque psoriasis: ustekinumab, briakinumab, tildrakizumab, guselkumab, secukinumab, ixekizumab, and brodalumab. J Cutan Med Surg. 2014;18:156-169.
20. Sofen H, Smith S, Matheson RT, et al. Guselkumab (an IL-23-specific mAb) demonstrates clinical and molecular response in patients with moderate-to-severe psoriasis. J Allergy Clin Immunol. 2014;133:1032-1040.
21. Schafer PH, Parton A, Capone L, et al. Apremilast is a selective PDE4 inhibitor with regulatory effects on innate immunity. Cell Signal. 2014;26:2016-2029.
22. van de Kerkhof PC. Apremilast: a step forward in the treatment of psoriasis? Lancet. 2012;380:708-709.
23. Paul C, Crowley J, Cather J, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe psoriasis: 16-week results of a phase 3, randomized, controlled trial (ESTEEM 2). Poster presented at: 73rd Annual Meeting of the American Academy of Dermatology; March 21-25, 2014; Denver, CO. Poster 8412.
24. Otezla [product insert]. Summit, NJ: Celgene Corporation; 2014.
25. Rabe KF. Update on roflumilast, a phosphodiesterase 4 inhibitor for the treatment of chronic obstructive pulmonary disease. Br J Pharmacol. 2011;163:53-67.
26. Palanivel JA, Macbeth AE, Chetty NC, et al. An insight into JAK-STAT signalling in dermatology. Clin Exp Dermatol. 2014;39:513-518.
27. Papp KA, Menter A, Strober B, et al. Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: a phase 2b randomized placebo-controlled dose-ranging study. Br J Dermatol. 2012;167:668-677.
28. Hsu L, Armstrong AW. JAK inhibitors: treatment efficacy and safety profile in patients with psoriasis. J Immunol Res. 2014;2014:283617.
Psoriasis is a common chronic inflammatory skin disease affecting 1% to 8% of the world population, depending on the country.1 Psoriasis can greatly impact quality of life in affected individuals, even in those with limited body surface involvement.2 Studies have demonstrated a high degree of psychological distress associated with psoriasis, leading to depression and poor self-esteem.3
Over the last decade, our improved understanding of the autoimmune inflammatory pathways and the associated changing concepts in psoriasis pathogenesis have led to the development of biological drugs targeting specific components of effector immune mechanisms, and these biological drugs have revolutionized the treatment of psoriasis.4 Although response rates of these biological agents are greater compared to those of conventional systemic drugs,5 current biological drugs fail to demonstrate efficacy in some patients or lose their efficacy over time. In addition to the high costs associated with these drugs, these limitations have driven a continued search for alternative therapies.
Helper T cells (TH17) and the proinflammatory cytokine IL-17 have been shown to play a key role in the pathophysiology of psoriasis, bridging innate and adaptive immune responses. IL-17 is involved in the modulation of proinflammatory cytokines, hematopoietic growth factors, antimicrobial peptides, and chemokines. Increased TH17 activity and high levels of IL-17 have been found in psoriatic plaques, and increased levels of TH17 are found in the plasma of psoriasis patients.6 Increased IL-17 induces neutrophilia, inflammation, and angiogenesis.7 Other cytokines that are highly upregulated in involved skin are tumor necrosis factor a (TNF-α), IL-23, IL-22, and IL-21.8 IL-23 is involved in regulating TH17 cells and is a potent activator of keratinocyte proliferation.9 Blockade of IL-12/23 causes downregulation of TH17 and TH22 cell responses.10 As IL-17 has a key role in protecting skin and mucous membranes from bacterial and fungal infections, IL-17 inhibition can potentially interfere with the inflammatory cascade. However, available data suggest that sufficient residual IL-17 activity remains to maintain immunity against infections.11
Currently approved biological agents for psoriasis target proinflammatory cytokines such as TNF-α, or the p40 subunit of IL-12 and IL-23. A number of novel targeted therapies including biologics as well as small molecule inhibitors targeting various cytokines and molecules involved in the pathogenesis of psoriasis are currently in different stages of development (Table). These drugs include 3 IL-17 inhibitors (secukinumab, ixekizumab, and brodalumab); 2 IL-23 blockers (tildrakizumab and guselkumab); and small molecule inhibitors that target the kinase pathway including apremilast (a phosphodiesterase 4 [PDE4] inhibitor), as well as tofacitinib, baricitinib, and ruxolitinib (Janus kinase [Jak] inhibitors). Small molecule inhibitors can be administered orally and are less expensive to produce than biological agents. This article reviews available data on these new systemic agents in the pipeline.
Novel Biologics
Secukinumab
Secukinumab is a fully human monoclonal IgG1k antibody that selectively binds and neutralizes IL-17A.12 It is the first of the IL-17 antibodies to receive approval for the treatment of moderate to severe psoriasis. In 2 phase 3, double-blind, 52-week trials—ERASURE (Efficacy of Response and Safety of Two Fixed Secukinumab Regimens in Psoriasis) and FIXTURE (Full Year Investigative Examination of Secukinumab vs Etanercept Using Two Dosing Regimens to Determine Efficacy in Psoriasis)—participants were randomly assigned to receive subcutaneous secukinumab at doses of 300 mg (n=245 and n=327, respectively) or 150 mg (n=245 and n=327, respectively) once weekly for 5 weeks then every 4 weeks, or placebo (n=248 and n=326, respectively); in the FIXTURE study only, an etanercept group (n=326) was given a 50-mg dose twice weekly for 12 weeks then once weekly.13
In the ERASURE study, the proportion of participants showing a reduction of 75% or more in psoriasis area and severity index (PASI) score from baseline to week 12 was 81.6% with secukinumab 300 mg, 71.6% with secukinumab 150 mg, and 4.5% with placebo.13 Secondary end point results demonstrated the proportion of participants showing a 90% reduction in PASI score was 59.2% with secukinumab 300 mg and 39.1% with secukinumab 150 mg, which were both superior to placebo (1.2%). The proportion of participants who met the criteria for 100% reduction in PASI score at week 12 also was greater with each secukinumab dose than with placebo.13
In the FIXTURE study, the proportion of participants showing a reduction of 75% or more from baseline in PASI score at week 12 was 77.1% with secukinumab 300 mg, 67.0% with secukinumab 150 mg, 44.0% with etanercept, and 4.9% with placebo.13 Secondary end point results demonstrated the proportion of participants showing a 90% reduction in PASI score was 54.2% with secukinumab 300 mg, 41.9% with secukinumab 150 mg, 20.7% with etanercept, and 1.5% with placebo. The speed of response, which was assessed as the median time to a 50% reduction in mean PASI score from baseline, was significantly shorter with both doses of secukinumab (3.0 weeks and 3.9 weeks, respectively) than with etanercept (7.0 weeks)(P<.001 for both).13
In the FIXTURE study, incidences of adverse events (AEs) were similar in the secukinumab and etanercept groups during both the induction period and the entire treatment period.13 The most common AEs in the secukinumab groups were nasopharyngitis, headache, and diarrhea. The rates of infections or infestations during the induction period were 26.7% with secukinumab 300 mg, 30.9% with secukinumab 150 mg, 24.5% with etanercept, and 19.3% with placebo. Candidal infections were more common with secukinumab than with etanercept during the entire treatment period (4.7% and 2.3% of participants in the secukinumab 300 mg and 150 mg groups, respectively, reported mild or moderate candidal infections). None of these infections resulted in chronic mucocutaneous candidiasis or discontinuation of the study drug and all resolved on their own or with standard therapy. Candidal infection was reported in 1.2% of participants in the etanercept group. Responses at week 12 were sustained in the majority of participants through week 52 with continued secukinumab therapy every 4 weeks. Grade 3 neutropenia occurred in 1.0% of secukinumab-treated participants and in none of the participants in the etanercept group. There were no apparent dose-related differences between the secukinumab groups with respect to AEs, with the exception of mild and moderate candidal infections.13
These efficacy data are impressive and no specific serious safety concerns have been identified to date. However, IL-17A plays a key role in host defense, particularly in mucocutaneous immunity against Candida albicans,14 as well as in hematopoiesis through stimulation of granulopoiesis and neutrophil trafficking,15 and thus we need to remain watchful with regards to Candida albicans infections and neutropenia.
Ixekizumab
Ixekizumab is a humanized IgG4 anti–IL-17A monoclonal antibody. In a phase 2, double-blind, placebo-controlled trial, 142 participants with chronic moderate to severe plaque psoriasis were randomly assigned to receive 150-mg (n=28), 75-mg (n=29), 25-mg (n=30), or 10-mg (n=28) subcutaneous injections of ixekizumab or placebo (n=27) at weeks 0, 2, 4, 8, 12, and 16.16 At 12 weeks, the percentage of participants who achieved a 75% reduction in PASI score from baseline was significantly greater with ixekizumab (82.1% with 150-mg dose, 82.8% with 75-mg dose, 76.7% with 25-mg dose) than with placebo (7.7%)(P<.001 for each comparison), except with the 10-mg dose. Similarly, a greater percentage of participants in the same ixekizumab groups achieved a 90% reduction (71.4% with 150-mg dose, 58.6% with 75-mg dose, 50.0% with 25-mg dose) and a 100% reduction (39.3% with 150-mg dose, 37.9% with 75-mg dose) in PASI score compared to placebo (0%)(P<.001 for each comparison). Significant reductions in PASI scores were evident as early as week 1 in the 150-mg and 75-mg groups, and these reductions were sustained for 20 weeks (P<.05).16 Phase 3 studies of ixekizumab currently are underway.
Brodalumab
The third IL-17 blocker in the pipeline is brodalumab, a human monoclonal antibody against IL-17RA, which blocks signaling of IL-17A and IL-17F as well as the IL-17A/F heterodimer, all of which are involved in the inflammatory process of psoriasis. Brodalumab was evaluated in a phase 2, double-blind, placebo-controlled, dose-ranging study of 198 participants who were randomized to receive 70 mg (n=39), 140 mg (n=39), 210 mg (n=40), or 280 mg (n=42) of brodalumab or placebo (n=38).17 At week 12, improvements of at least 75% and at least 90% in PASI score were achieved by 77% and 72%, respectively, in the 140-mg group, and 82% and 75%, respectively, in the 210-mg group compared to 0% of the placebo group (P<.001 for all comparisons). One hundred percent improvement in PASI was achieved by 38% of participants in the 140-mg group and 62% in the 210-mg group. No participants in the placebo group demonstrated improvement of 75% or higher. The most common AEs were nasopharyngitis, upper respiratory tract infection, arthralgia, and injection-site erythema. Serious AEs reported during the study included renal colic (1 participant), ec-topic pregnancy (1 participant), and grade 3 asymptomatic neutropenia (2 participants). Both cases of neutropenia were noted at the first assessment after brodalumab initiation (week 2) and resolved when the study drug was withheld.17
Results for this new IL-17 blocker are encouraging, but phase 3 data of brodalumab will need to be awaited.
Tildrakizumab
Tildrakizumab is a humanized IgG1 monoclonal antibody that blocks the p19 subunit of IL-23. In a randomized, double-blind, phase 2b trial, 355 adults with moderate to severe psoriasis were randomized to receive subcutaneous injections of tildrakizumab (5 mg, 25 mg, 100 mg, or 200 mg) or placebo.18 In part 1 of the study, injections were administered at weeks 0 and 4. Part 2 of the study started at week 16. In part 2, responders with a 75% improvement in PASI score in the 5- and 25-mg groups continued their dose, while responders in the 100- or 200-mg groups were randomized again to continue the same dose or a reduced dose (100 mg to 25 mg; 200 mg to 100 mg) every 12 weeks from weeks 16 to 52. Those in the placebo group received tildrakizumab 25 mg every 12 weeks in part 2. The primary end point was the mean change in PASI score from baseline to week 16, which was significantly greater in all tildrakizumab groups than in the placebo group (P<.001 for all comparisons). Improvements of 75% in PASI score were achieved by 74% in the 200-mg group, 66% in the 100-mg group, 64% in the 25-mg group, and 33% in the 5-mg group. In contrast, 4.9% in the placebo group achieved an improvement of 75%. At week 52, no loss of efficacy was seen in those participants who had achieved 75% improvement in PASI score at week 16 and had continued their prior doses. The rates of AEs seen in the tildrakizumab groups were 60% to 71% compared to 69% in the placebo group. The most common AE was nasopharyngitis, occurring in 12% to 20% of participants in each group. Serious AEs were uncommon.18 Phase 3 studies are currently underway.19
Guselkumab
Guselkumab is a human IgG1 monoclonal antibody in clinical development that specifically blocks the p19 subunit of IL-23. In a double-blind, placebo-controlled, phase 1 study, 24 participants with moderate to severe plaque psoriasis were randomized to receive a single 10-mg (n=5), 30-mg (n=5), 100-mg (n=5), or 300-mg (n=5) dose of guselkumab or placebo (n=4).20 At week 12, 50% in the 10-mg group, 60% in both the 30- and 100-mg groups, and 100% in the 300-mg group showed 75% improvement in PASI score versus 0% in the placebo group. Improvements in PASI scores were generally maintained through week 24. The rates of AEs were 65% (13/20) in the combined guselkumab group and 50% (2/4) in the placebo group.20
Small Molecule Inhibitors
In contrast to biologics, which mainly target soluble cytokine or cellular receptors, small molecule inhibitors target enzymes within signaling pathways. Small molecule inhibitors have some advantages over biologics in that they are relatively inexpensive to produce and can be administered orally; thus, they may be preferred by some patients over injectable drugs. There are several agents that are undergoing clinical trials in psoriasis, including PDE4 inhibitors and Jak inhibitors.
Apremilast
Apremilast is an oral small molecule PDE4 inhibitor that was approved by the US Food and Drug Administration in March 2014 for the treatment of adult patients with active psoriatic arthritis; an indication for moderate to severe plaque psoriasis was approved in September 2014.21 Phosphodiesterase 4 is a cyclic adenosine monophosphate–specific phosphodiesterase inhibitor, which is dominant in inflammatory cells. Inhibition of PDE4 increases intracellular cyclic adenosine monophosphate levels, thus downregulating proinflammatory cytokines such as TNF-α, IFN-γ, IL-2, IL-12, and IL-23, and increasing the production of anti-inflammatory cytokines such as IL-10.22
Phase 2 and phase 3 studies have demonstrated the clinical efficacy of apremilast in the treatment of patients with moderate to severe plaque psoriasis. In a 16-week randomized, placebo-controlled, phase 3 trial (ESTEEM 2), 408 participants were randomized to receive oral apremilast 30 mg twice daily (n=275) or placebo (n=138).23 Improvement of 75% in PASI score was achieved by 29% of participants in the apremilast group at week 16. The most common AEs were diarrhea (16%) and nausea (18%), which were predominantly mild, occurring most commonly in the first week and resolving within 1 month. No cases of severe diarrhea or severe nausea were reported. Apremilast had no apparent effect on the results of hematological or serum chemistry tests.23 Although the US Food and Drug Administration warns of a possible link between apremilast and depression,24 data are mostly related to roflumilast, another PDE4 inhibitor. Studies in patients with chronic obstructive pulmonary disease have noted increased cases of depression (1.21% vs 0.82%) and suicidal ideation/attempt (0.03% vs 0.02%) in patients treated with roflumilast versus placebo.25
Jak Inhibitors
Janus kinases are a family of intracellular tyrosine kinases that connect several cytokine receptors to the signal transducer and activator of transcription pathways.26 There are 4 Jak family members: Jak1, Jak2, Jak3, and tyrosine kinase 2. Janus kinases 1 and 2 have roles in interferon signaling, while Jak3 transduces signals from IL-2, IL-7, IL-15, and IL-21, which are T-cell growth and survival factors.
Tofacitinib is a novel oral signal transduction molecule that blocks the Jak3 pathway. A phase 2b, 12-week, dose-ranging study was conducted to assess the efficacy and safety of 3 twice-daily regimens of tofacitinib versus placebo in patients with moderate to severe chronic plaque psoriasis.27 One hundred and ninety-seven participants were randomized to receive oral tofacitinib (2 mg, 5 mg, or 15 mg; n=49 each) or placebo (n=50) twice daily for 12 weeks with a 4-week follow-up period. The primary end point was the proportion of participants achieving at least a 75% reduction in PASI score at week 12 (25.0% with 2 mg, 40.8% with 5 mg, 66.7% with 15 mg, 2.0% with placebo). Similarly, a higher proportion of participants achieving 90% reduction in PASI score was seen at weeks 8 and 12 in all tofacitinib-treated participants versus placebo. The most common AEs were upper respiratory tract infection, sinusitis, nasopharyngitis, and headache. A number of changes in laboratory parameters occurred in the tofacitinib groups. Mild dose-related decreases in hemoglobin were noted at week 12 for all tofacitinib groups, and a small increase (mean, 0.04 mg/dL) in serum creatinine levels was observed in the 15-mg group. Decreases in neutrophil counts were observed with higher doses of tofacitinib, with the maximum mean decrease of 0.9×103/mm3 from baseline observed in the 15-mg group at week 4. After weeks 4 through 8, mean neutrophil counts began to return to baseline levels. Dose-related increases in total cholesterol, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol were observed by week 2 and remained at this level through week 12; mean lipid levels decreased to baseline levels after cessation of active treatment. One participant in the 15-mg group developed an elevated alanine aminotransferase level that was more than 2.5 times the highest normal limit. Three participants experienced 5 serious AEs.27 These early results show that tofacitinib can be a safe and effective treatment in patients with psoriasis, but further data from phase 3 studies will need to be awaited.
Another Jak inhibitor under investigationfor the treatment of psoriasis is ruxolitinib, an inhibitor of Jak1 and Jak2, which has been primarily studied as a topical agent for milder cases of the disease.28
Conclusion
Many new drugs are currently on the horizon and will increase our armamentarium for treating psoriasis. Some of these agents promise greater levels of efficacy than currently used therapies. Although this review focuses on systemic agents, there also are a number of topical formulations in the pipeline. These new agents will certainly increase our options when choosing the most suitable treatment for a patient with psoriasis, but safety will remain a primary concern, and time and experience will tell whether efficacy outweighs any potential side effects.
Psoriasis is a common chronic inflammatory skin disease affecting 1% to 8% of the world population, depending on the country.1 Psoriasis can greatly impact quality of life in affected individuals, even in those with limited body surface involvement.2 Studies have demonstrated a high degree of psychological distress associated with psoriasis, leading to depression and poor self-esteem.3
Over the last decade, our improved understanding of the autoimmune inflammatory pathways and the associated changing concepts in psoriasis pathogenesis have led to the development of biological drugs targeting specific components of effector immune mechanisms, and these biological drugs have revolutionized the treatment of psoriasis.4 Although response rates of these biological agents are greater compared to those of conventional systemic drugs,5 current biological drugs fail to demonstrate efficacy in some patients or lose their efficacy over time. In addition to the high costs associated with these drugs, these limitations have driven a continued search for alternative therapies.
Helper T cells (TH17) and the proinflammatory cytokine IL-17 have been shown to play a key role in the pathophysiology of psoriasis, bridging innate and adaptive immune responses. IL-17 is involved in the modulation of proinflammatory cytokines, hematopoietic growth factors, antimicrobial peptides, and chemokines. Increased TH17 activity and high levels of IL-17 have been found in psoriatic plaques, and increased levels of TH17 are found in the plasma of psoriasis patients.6 Increased IL-17 induces neutrophilia, inflammation, and angiogenesis.7 Other cytokines that are highly upregulated in involved skin are tumor necrosis factor a (TNF-α), IL-23, IL-22, and IL-21.8 IL-23 is involved in regulating TH17 cells and is a potent activator of keratinocyte proliferation.9 Blockade of IL-12/23 causes downregulation of TH17 and TH22 cell responses.10 As IL-17 has a key role in protecting skin and mucous membranes from bacterial and fungal infections, IL-17 inhibition can potentially interfere with the inflammatory cascade. However, available data suggest that sufficient residual IL-17 activity remains to maintain immunity against infections.11
Currently approved biological agents for psoriasis target proinflammatory cytokines such as TNF-α, or the p40 subunit of IL-12 and IL-23. A number of novel targeted therapies including biologics as well as small molecule inhibitors targeting various cytokines and molecules involved in the pathogenesis of psoriasis are currently in different stages of development (Table). These drugs include 3 IL-17 inhibitors (secukinumab, ixekizumab, and brodalumab); 2 IL-23 blockers (tildrakizumab and guselkumab); and small molecule inhibitors that target the kinase pathway including apremilast (a phosphodiesterase 4 [PDE4] inhibitor), as well as tofacitinib, baricitinib, and ruxolitinib (Janus kinase [Jak] inhibitors). Small molecule inhibitors can be administered orally and are less expensive to produce than biological agents. This article reviews available data on these new systemic agents in the pipeline.
Novel Biologics
Secukinumab
Secukinumab is a fully human monoclonal IgG1k antibody that selectively binds and neutralizes IL-17A.12 It is the first of the IL-17 antibodies to receive approval for the treatment of moderate to severe psoriasis. In 2 phase 3, double-blind, 52-week trials—ERASURE (Efficacy of Response and Safety of Two Fixed Secukinumab Regimens in Psoriasis) and FIXTURE (Full Year Investigative Examination of Secukinumab vs Etanercept Using Two Dosing Regimens to Determine Efficacy in Psoriasis)—participants were randomly assigned to receive subcutaneous secukinumab at doses of 300 mg (n=245 and n=327, respectively) or 150 mg (n=245 and n=327, respectively) once weekly for 5 weeks then every 4 weeks, or placebo (n=248 and n=326, respectively); in the FIXTURE study only, an etanercept group (n=326) was given a 50-mg dose twice weekly for 12 weeks then once weekly.13
In the ERASURE study, the proportion of participants showing a reduction of 75% or more in psoriasis area and severity index (PASI) score from baseline to week 12 was 81.6% with secukinumab 300 mg, 71.6% with secukinumab 150 mg, and 4.5% with placebo.13 Secondary end point results demonstrated the proportion of participants showing a 90% reduction in PASI score was 59.2% with secukinumab 300 mg and 39.1% with secukinumab 150 mg, which were both superior to placebo (1.2%). The proportion of participants who met the criteria for 100% reduction in PASI score at week 12 also was greater with each secukinumab dose than with placebo.13
In the FIXTURE study, the proportion of participants showing a reduction of 75% or more from baseline in PASI score at week 12 was 77.1% with secukinumab 300 mg, 67.0% with secukinumab 150 mg, 44.0% with etanercept, and 4.9% with placebo.13 Secondary end point results demonstrated the proportion of participants showing a 90% reduction in PASI score was 54.2% with secukinumab 300 mg, 41.9% with secukinumab 150 mg, 20.7% with etanercept, and 1.5% with placebo. The speed of response, which was assessed as the median time to a 50% reduction in mean PASI score from baseline, was significantly shorter with both doses of secukinumab (3.0 weeks and 3.9 weeks, respectively) than with etanercept (7.0 weeks)(P<.001 for both).13
In the FIXTURE study, incidences of adverse events (AEs) were similar in the secukinumab and etanercept groups during both the induction period and the entire treatment period.13 The most common AEs in the secukinumab groups were nasopharyngitis, headache, and diarrhea. The rates of infections or infestations during the induction period were 26.7% with secukinumab 300 mg, 30.9% with secukinumab 150 mg, 24.5% with etanercept, and 19.3% with placebo. Candidal infections were more common with secukinumab than with etanercept during the entire treatment period (4.7% and 2.3% of participants in the secukinumab 300 mg and 150 mg groups, respectively, reported mild or moderate candidal infections). None of these infections resulted in chronic mucocutaneous candidiasis or discontinuation of the study drug and all resolved on their own or with standard therapy. Candidal infection was reported in 1.2% of participants in the etanercept group. Responses at week 12 were sustained in the majority of participants through week 52 with continued secukinumab therapy every 4 weeks. Grade 3 neutropenia occurred in 1.0% of secukinumab-treated participants and in none of the participants in the etanercept group. There were no apparent dose-related differences between the secukinumab groups with respect to AEs, with the exception of mild and moderate candidal infections.13
These efficacy data are impressive and no specific serious safety concerns have been identified to date. However, IL-17A plays a key role in host defense, particularly in mucocutaneous immunity against Candida albicans,14 as well as in hematopoiesis through stimulation of granulopoiesis and neutrophil trafficking,15 and thus we need to remain watchful with regards to Candida albicans infections and neutropenia.
Ixekizumab
Ixekizumab is a humanized IgG4 anti–IL-17A monoclonal antibody. In a phase 2, double-blind, placebo-controlled trial, 142 participants with chronic moderate to severe plaque psoriasis were randomly assigned to receive 150-mg (n=28), 75-mg (n=29), 25-mg (n=30), or 10-mg (n=28) subcutaneous injections of ixekizumab or placebo (n=27) at weeks 0, 2, 4, 8, 12, and 16.16 At 12 weeks, the percentage of participants who achieved a 75% reduction in PASI score from baseline was significantly greater with ixekizumab (82.1% with 150-mg dose, 82.8% with 75-mg dose, 76.7% with 25-mg dose) than with placebo (7.7%)(P<.001 for each comparison), except with the 10-mg dose. Similarly, a greater percentage of participants in the same ixekizumab groups achieved a 90% reduction (71.4% with 150-mg dose, 58.6% with 75-mg dose, 50.0% with 25-mg dose) and a 100% reduction (39.3% with 150-mg dose, 37.9% with 75-mg dose) in PASI score compared to placebo (0%)(P<.001 for each comparison). Significant reductions in PASI scores were evident as early as week 1 in the 150-mg and 75-mg groups, and these reductions were sustained for 20 weeks (P<.05).16 Phase 3 studies of ixekizumab currently are underway.
Brodalumab
The third IL-17 blocker in the pipeline is brodalumab, a human monoclonal antibody against IL-17RA, which blocks signaling of IL-17A and IL-17F as well as the IL-17A/F heterodimer, all of which are involved in the inflammatory process of psoriasis. Brodalumab was evaluated in a phase 2, double-blind, placebo-controlled, dose-ranging study of 198 participants who were randomized to receive 70 mg (n=39), 140 mg (n=39), 210 mg (n=40), or 280 mg (n=42) of brodalumab or placebo (n=38).17 At week 12, improvements of at least 75% and at least 90% in PASI score were achieved by 77% and 72%, respectively, in the 140-mg group, and 82% and 75%, respectively, in the 210-mg group compared to 0% of the placebo group (P<.001 for all comparisons). One hundred percent improvement in PASI was achieved by 38% of participants in the 140-mg group and 62% in the 210-mg group. No participants in the placebo group demonstrated improvement of 75% or higher. The most common AEs were nasopharyngitis, upper respiratory tract infection, arthralgia, and injection-site erythema. Serious AEs reported during the study included renal colic (1 participant), ec-topic pregnancy (1 participant), and grade 3 asymptomatic neutropenia (2 participants). Both cases of neutropenia were noted at the first assessment after brodalumab initiation (week 2) and resolved when the study drug was withheld.17
Results for this new IL-17 blocker are encouraging, but phase 3 data of brodalumab will need to be awaited.
Tildrakizumab
Tildrakizumab is a humanized IgG1 monoclonal antibody that blocks the p19 subunit of IL-23. In a randomized, double-blind, phase 2b trial, 355 adults with moderate to severe psoriasis were randomized to receive subcutaneous injections of tildrakizumab (5 mg, 25 mg, 100 mg, or 200 mg) or placebo.18 In part 1 of the study, injections were administered at weeks 0 and 4. Part 2 of the study started at week 16. In part 2, responders with a 75% improvement in PASI score in the 5- and 25-mg groups continued their dose, while responders in the 100- or 200-mg groups were randomized again to continue the same dose or a reduced dose (100 mg to 25 mg; 200 mg to 100 mg) every 12 weeks from weeks 16 to 52. Those in the placebo group received tildrakizumab 25 mg every 12 weeks in part 2. The primary end point was the mean change in PASI score from baseline to week 16, which was significantly greater in all tildrakizumab groups than in the placebo group (P<.001 for all comparisons). Improvements of 75% in PASI score were achieved by 74% in the 200-mg group, 66% in the 100-mg group, 64% in the 25-mg group, and 33% in the 5-mg group. In contrast, 4.9% in the placebo group achieved an improvement of 75%. At week 52, no loss of efficacy was seen in those participants who had achieved 75% improvement in PASI score at week 16 and had continued their prior doses. The rates of AEs seen in the tildrakizumab groups were 60% to 71% compared to 69% in the placebo group. The most common AE was nasopharyngitis, occurring in 12% to 20% of participants in each group. Serious AEs were uncommon.18 Phase 3 studies are currently underway.19
Guselkumab
Guselkumab is a human IgG1 monoclonal antibody in clinical development that specifically blocks the p19 subunit of IL-23. In a double-blind, placebo-controlled, phase 1 study, 24 participants with moderate to severe plaque psoriasis were randomized to receive a single 10-mg (n=5), 30-mg (n=5), 100-mg (n=5), or 300-mg (n=5) dose of guselkumab or placebo (n=4).20 At week 12, 50% in the 10-mg group, 60% in both the 30- and 100-mg groups, and 100% in the 300-mg group showed 75% improvement in PASI score versus 0% in the placebo group. Improvements in PASI scores were generally maintained through week 24. The rates of AEs were 65% (13/20) in the combined guselkumab group and 50% (2/4) in the placebo group.20
Small Molecule Inhibitors
In contrast to biologics, which mainly target soluble cytokine or cellular receptors, small molecule inhibitors target enzymes within signaling pathways. Small molecule inhibitors have some advantages over biologics in that they are relatively inexpensive to produce and can be administered orally; thus, they may be preferred by some patients over injectable drugs. There are several agents that are undergoing clinical trials in psoriasis, including PDE4 inhibitors and Jak inhibitors.
Apremilast
Apremilast is an oral small molecule PDE4 inhibitor that was approved by the US Food and Drug Administration in March 2014 for the treatment of adult patients with active psoriatic arthritis; an indication for moderate to severe plaque psoriasis was approved in September 2014.21 Phosphodiesterase 4 is a cyclic adenosine monophosphate–specific phosphodiesterase inhibitor, which is dominant in inflammatory cells. Inhibition of PDE4 increases intracellular cyclic adenosine monophosphate levels, thus downregulating proinflammatory cytokines such as TNF-α, IFN-γ, IL-2, IL-12, and IL-23, and increasing the production of anti-inflammatory cytokines such as IL-10.22
Phase 2 and phase 3 studies have demonstrated the clinical efficacy of apremilast in the treatment of patients with moderate to severe plaque psoriasis. In a 16-week randomized, placebo-controlled, phase 3 trial (ESTEEM 2), 408 participants were randomized to receive oral apremilast 30 mg twice daily (n=275) or placebo (n=138).23 Improvement of 75% in PASI score was achieved by 29% of participants in the apremilast group at week 16. The most common AEs were diarrhea (16%) and nausea (18%), which were predominantly mild, occurring most commonly in the first week and resolving within 1 month. No cases of severe diarrhea or severe nausea were reported. Apremilast had no apparent effect on the results of hematological or serum chemistry tests.23 Although the US Food and Drug Administration warns of a possible link between apremilast and depression,24 data are mostly related to roflumilast, another PDE4 inhibitor. Studies in patients with chronic obstructive pulmonary disease have noted increased cases of depression (1.21% vs 0.82%) and suicidal ideation/attempt (0.03% vs 0.02%) in patients treated with roflumilast versus placebo.25
Jak Inhibitors
Janus kinases are a family of intracellular tyrosine kinases that connect several cytokine receptors to the signal transducer and activator of transcription pathways.26 There are 4 Jak family members: Jak1, Jak2, Jak3, and tyrosine kinase 2. Janus kinases 1 and 2 have roles in interferon signaling, while Jak3 transduces signals from IL-2, IL-7, IL-15, and IL-21, which are T-cell growth and survival factors.
Tofacitinib is a novel oral signal transduction molecule that blocks the Jak3 pathway. A phase 2b, 12-week, dose-ranging study was conducted to assess the efficacy and safety of 3 twice-daily regimens of tofacitinib versus placebo in patients with moderate to severe chronic plaque psoriasis.27 One hundred and ninety-seven participants were randomized to receive oral tofacitinib (2 mg, 5 mg, or 15 mg; n=49 each) or placebo (n=50) twice daily for 12 weeks with a 4-week follow-up period. The primary end point was the proportion of participants achieving at least a 75% reduction in PASI score at week 12 (25.0% with 2 mg, 40.8% with 5 mg, 66.7% with 15 mg, 2.0% with placebo). Similarly, a higher proportion of participants achieving 90% reduction in PASI score was seen at weeks 8 and 12 in all tofacitinib-treated participants versus placebo. The most common AEs were upper respiratory tract infection, sinusitis, nasopharyngitis, and headache. A number of changes in laboratory parameters occurred in the tofacitinib groups. Mild dose-related decreases in hemoglobin were noted at week 12 for all tofacitinib groups, and a small increase (mean, 0.04 mg/dL) in serum creatinine levels was observed in the 15-mg group. Decreases in neutrophil counts were observed with higher doses of tofacitinib, with the maximum mean decrease of 0.9×103/mm3 from baseline observed in the 15-mg group at week 4. After weeks 4 through 8, mean neutrophil counts began to return to baseline levels. Dose-related increases in total cholesterol, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol were observed by week 2 and remained at this level through week 12; mean lipid levels decreased to baseline levels after cessation of active treatment. One participant in the 15-mg group developed an elevated alanine aminotransferase level that was more than 2.5 times the highest normal limit. Three participants experienced 5 serious AEs.27 These early results show that tofacitinib can be a safe and effective treatment in patients with psoriasis, but further data from phase 3 studies will need to be awaited.
Another Jak inhibitor under investigationfor the treatment of psoriasis is ruxolitinib, an inhibitor of Jak1 and Jak2, which has been primarily studied as a topical agent for milder cases of the disease.28
Conclusion
Many new drugs are currently on the horizon and will increase our armamentarium for treating psoriasis. Some of these agents promise greater levels of efficacy than currently used therapies. Although this review focuses on systemic agents, there also are a number of topical formulations in the pipeline. These new agents will certainly increase our options when choosing the most suitable treatment for a patient with psoriasis, but safety will remain a primary concern, and time and experience will tell whether efficacy outweighs any potential side effects.
1. Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
2. Rapp SR, Feldman SR, Exum ML, et al. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999;41(3 pt 1):401-407.
3. Shah R, Bewley A. Psoriasis: ‘the badge of shame.’ a case report of a psychological intervention to reduce and potentially clear chronic skin disease. Clin Exp Dermatol. 2014;39:600-603.
4. Vincent FB, Morand EF, Murphy K, et al. Antidrug antibodies (ADAb) to tumour necrosis factor (TNF)-specific neutralising agents in chronic inflammatory diseases: a real issue, a clinical perspective. Ann Rheum Dis. 2013;72:165-178.
5. Chi CC, Wang SH. Efficacy and cost-efficacy of biologic therapies for moderate to severe psoriasis: a meta-analysis and cost-efficacy analysis using the intention-to-treat principle. Biomed Res Int. 2014;2014:862851.
6. Kagami S, Rizzo HL, Lee JJ, et al. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010;130:1373-1383.
7. Leonardi CL, Gordon KB. New and emerging therapies in psoriasis. Semin Cutan Med Surg. 2014;33(2, suppl 2):S37-S41.
8. Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207-1211.
9. Nickoloff BJ, Qin JZ, Nestle FO. Immunopathogenesis of psoriasis. Clin Rev Allergy Immunol. 2007;33:45-56.
10. Fitch E, Harper E, Skorcheva I, et al. Pathophysiology of psoriasis: recent advances on IL-23 and Th17 cytokines. Curr Rheumatol Rep. 2007;9:461-467.
11. Adami S, Cavani A, Rossi F, et al. The role of interleukin-17A in psoriatic disease. BioDrugs. 2014;28:487-497.
12. Hueber W, Patel DD, Dryja T, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2:52ra72.
13. Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338.
14. Puel A, Cypowyj S, Bustamante J, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65-68.
15. Krstic A, Mojsilovic S, Jovcic G, et al. The potential of interleukin-17 to mediate hematopoietic response. Immunol Res. 2012;52:34-41.
16. Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
17. Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181-1189.
18. Langley RGB, Thaci D, Papp KA, et al. MK-3222, an anti–IL-23p19 humanized monoclonal antibody, provides significant improvement in psoriasis over 52 weeks of treatment that is maintained after discontinuation of dosing. Poster presented at: 73rd Annual Meeting of the American Academy of Dermatology; March 21-25, 2014; Denver, CO. Poster 8056.
19. Tausend W, Downing C, Tyring S. Systematic review of interleukin-12, interleukin-17, and interleukin-23 pathway inhibitors for the treatment of moderate-to-severe chronic plaque psoriasis: ustekinumab, briakinumab, tildrakizumab, guselkumab, secukinumab, ixekizumab, and brodalumab. J Cutan Med Surg. 2014;18:156-169.
20. Sofen H, Smith S, Matheson RT, et al. Guselkumab (an IL-23-specific mAb) demonstrates clinical and molecular response in patients with moderate-to-severe psoriasis. J Allergy Clin Immunol. 2014;133:1032-1040.
21. Schafer PH, Parton A, Capone L, et al. Apremilast is a selective PDE4 inhibitor with regulatory effects on innate immunity. Cell Signal. 2014;26:2016-2029.
22. van de Kerkhof PC. Apremilast: a step forward in the treatment of psoriasis? Lancet. 2012;380:708-709.
23. Paul C, Crowley J, Cather J, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe psoriasis: 16-week results of a phase 3, randomized, controlled trial (ESTEEM 2). Poster presented at: 73rd Annual Meeting of the American Academy of Dermatology; March 21-25, 2014; Denver, CO. Poster 8412.
24. Otezla [product insert]. Summit, NJ: Celgene Corporation; 2014.
25. Rabe KF. Update on roflumilast, a phosphodiesterase 4 inhibitor for the treatment of chronic obstructive pulmonary disease. Br J Pharmacol. 2011;163:53-67.
26. Palanivel JA, Macbeth AE, Chetty NC, et al. An insight into JAK-STAT signalling in dermatology. Clin Exp Dermatol. 2014;39:513-518.
27. Papp KA, Menter A, Strober B, et al. Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: a phase 2b randomized placebo-controlled dose-ranging study. Br J Dermatol. 2012;167:668-677.
28. Hsu L, Armstrong AW. JAK inhibitors: treatment efficacy and safety profile in patients with psoriasis. J Immunol Res. 2014;2014:283617.
1. Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
2. Rapp SR, Feldman SR, Exum ML, et al. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999;41(3 pt 1):401-407.
3. Shah R, Bewley A. Psoriasis: ‘the badge of shame.’ a case report of a psychological intervention to reduce and potentially clear chronic skin disease. Clin Exp Dermatol. 2014;39:600-603.
4. Vincent FB, Morand EF, Murphy K, et al. Antidrug antibodies (ADAb) to tumour necrosis factor (TNF)-specific neutralising agents in chronic inflammatory diseases: a real issue, a clinical perspective. Ann Rheum Dis. 2013;72:165-178.
5. Chi CC, Wang SH. Efficacy and cost-efficacy of biologic therapies for moderate to severe psoriasis: a meta-analysis and cost-efficacy analysis using the intention-to-treat principle. Biomed Res Int. 2014;2014:862851.
6. Kagami S, Rizzo HL, Lee JJ, et al. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010;130:1373-1383.
7. Leonardi CL, Gordon KB. New and emerging therapies in psoriasis. Semin Cutan Med Surg. 2014;33(2, suppl 2):S37-S41.
8. Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207-1211.
9. Nickoloff BJ, Qin JZ, Nestle FO. Immunopathogenesis of psoriasis. Clin Rev Allergy Immunol. 2007;33:45-56.
10. Fitch E, Harper E, Skorcheva I, et al. Pathophysiology of psoriasis: recent advances on IL-23 and Th17 cytokines. Curr Rheumatol Rep. 2007;9:461-467.
11. Adami S, Cavani A, Rossi F, et al. The role of interleukin-17A in psoriatic disease. BioDrugs. 2014;28:487-497.
12. Hueber W, Patel DD, Dryja T, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2:52ra72.
13. Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338.
14. Puel A, Cypowyj S, Bustamante J, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65-68.
15. Krstic A, Mojsilovic S, Jovcic G, et al. The potential of interleukin-17 to mediate hematopoietic response. Immunol Res. 2012;52:34-41.
16. Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
17. Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181-1189.
18. Langley RGB, Thaci D, Papp KA, et al. MK-3222, an anti–IL-23p19 humanized monoclonal antibody, provides significant improvement in psoriasis over 52 weeks of treatment that is maintained after discontinuation of dosing. Poster presented at: 73rd Annual Meeting of the American Academy of Dermatology; March 21-25, 2014; Denver, CO. Poster 8056.
19. Tausend W, Downing C, Tyring S. Systematic review of interleukin-12, interleukin-17, and interleukin-23 pathway inhibitors for the treatment of moderate-to-severe chronic plaque psoriasis: ustekinumab, briakinumab, tildrakizumab, guselkumab, secukinumab, ixekizumab, and brodalumab. J Cutan Med Surg. 2014;18:156-169.
20. Sofen H, Smith S, Matheson RT, et al. Guselkumab (an IL-23-specific mAb) demonstrates clinical and molecular response in patients with moderate-to-severe psoriasis. J Allergy Clin Immunol. 2014;133:1032-1040.
21. Schafer PH, Parton A, Capone L, et al. Apremilast is a selective PDE4 inhibitor with regulatory effects on innate immunity. Cell Signal. 2014;26:2016-2029.
22. van de Kerkhof PC. Apremilast: a step forward in the treatment of psoriasis? Lancet. 2012;380:708-709.
23. Paul C, Crowley J, Cather J, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe psoriasis: 16-week results of a phase 3, randomized, controlled trial (ESTEEM 2). Poster presented at: 73rd Annual Meeting of the American Academy of Dermatology; March 21-25, 2014; Denver, CO. Poster 8412.
24. Otezla [product insert]. Summit, NJ: Celgene Corporation; 2014.
25. Rabe KF. Update on roflumilast, a phosphodiesterase 4 inhibitor for the treatment of chronic obstructive pulmonary disease. Br J Pharmacol. 2011;163:53-67.
26. Palanivel JA, Macbeth AE, Chetty NC, et al. An insight into JAK-STAT signalling in dermatology. Clin Exp Dermatol. 2014;39:513-518.
27. Papp KA, Menter A, Strober B, et al. Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: a phase 2b randomized placebo-controlled dose-ranging study. Br J Dermatol. 2012;167:668-677.
28. Hsu L, Armstrong AW. JAK inhibitors: treatment efficacy and safety profile in patients with psoriasis. J Immunol Res. 2014;2014:283617.
Practice Points
- Secukinumab is an anti–IL-17 antibody approved for the treatment of psoriasis. It is indicated for the treatment of moderate to severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy.
- The new biological agents have shown promising results with some patients achieving psoriasis area and severity index scores of 90 and 100.
- A number of small molecule inhibitors also are in the pipeline, with apremilast the first one to have reached approval for psoriasis.