User login
Screening for Depression in Rosacea Patients
Rosacea is a chronic skin condition that can be classified into 4 subtypes: erythematotelangiectatic, papulopustular, phymatous, and ocular. Erythematotelangiectatic rosacea is characterized by redness of the face and excessive blushing. Papulopustular rosacea is a more severe form of disease that is characterized by papules and pustules of the central face. If left untreated, these subtypes may progress to phymatous rosacea, which is characterized by skin thickening, fibrosis, and cosmetic disfigurement. Ocular rosacea is characterized by redness and irritation of the eyes.1 Rosacea patients often are burdened with embarrassment, social anxiety, and psychiatric comorbidities.
The Patient Health Questionnaire 9 (PHQ-9) is a validated and reliable self-administered tool for diagnosis of depression and designation of depression severity. This instrument could prove useful in screening for depression in rosacea patients given the high incidence of psychiatric comorbidities in this patient population.2 The PHQ-9 consists of 9 questions that assess for criteria used to define depressive disorders in the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition).3 The questionnaire is brief, easy to administer, and has 88% specificity and sensitivity.4
Other studies have evaluated the relationship between rosacea and psychiatric illness, but the PHQ-9 was not used as a screening tool.7,8 Rosacea patients are at increased risk for having psychiatrist-diagnosed depression.5 In one assessment, a positive correlation between rosacea and psychiatric illness was noted using the Dermatology Life Quality Index, the rejection scale of the Questionnaire on Experience with Skin Complaints, and the German version of the Hospital Anxiety and Depression Scale.6 Interpretation of Rosacea Quality of Life and Dermatology Life Quality Index scores indicated that rosacea has a negative impact on quality of life.7
The purpose of this study was to examine the relationship between self-assessed rosacea severity scores and level of depression using the validated rosacea self-assessment tool and the PHQ-9 questionnaire, respectively.
Methods
Study Population
Study participants were adult patients from the Wake Forest Baptist Medical Center (Winston-Salem, North Carolina) dermatology clinic from January 2011 to December 2014 who had received a diagnosis of rosacea (International Classification of Diseases, Ninth Revision [ICD-9] code 695.3) from a Wake Forest dermatologist. Institutional review board approval was obtained prior to initiation of the study. Data collection occurred from October 2014 through February 2015. A total of 478 patients met criteria for participation in the study and were identified from the Wake Forest Baptist Hospital Transitional Data Warehouse and the electronic medical record. Because rosacea typically is not diagnosed in children and the data measures are not validated in children, this demographic group was excluded from participation.
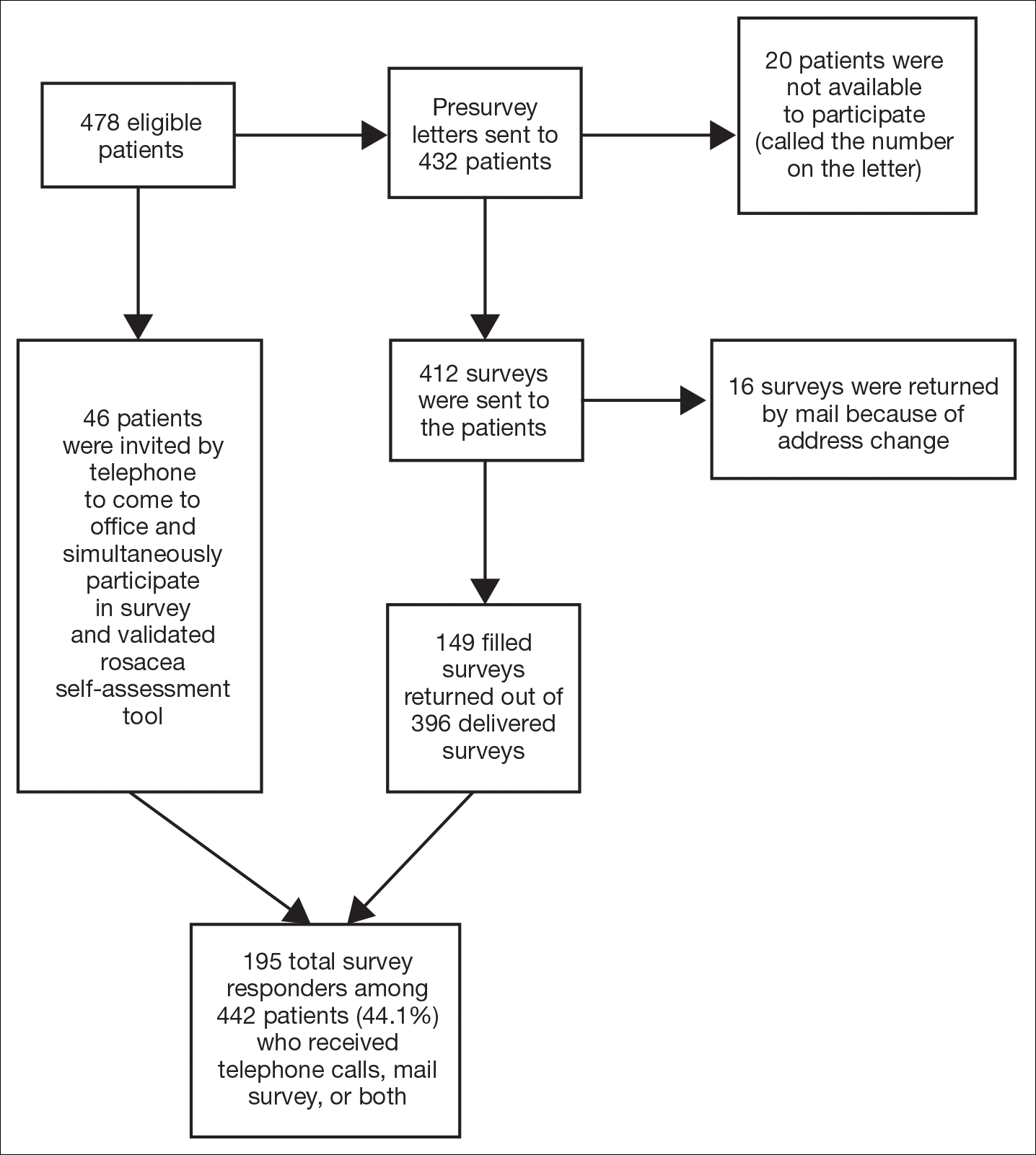
Of 478 eligible patients who were invited to participate via mail or telephone, 46 completed the rosacea self-assessment tool and PHQ-9 survey in person. A total of 432 patients were mailed a presurvey recruitment letter notifying them that they would be receiving a survey in the mail unless they contacted the study team to decline participation. An email address and telephone number for the study team was provided. Twenty patients declined to participate in the study; surveys were then mailed to the remaining 412 patients. Sixteen of the mailed surveys were returned by the post office due to an incorrect address.
Self-assessments
Patients selected images to self-identify the severity of their rosacea symptoms, including erythema, papulopustular lesions, ocular symptoms, and nasal involvement by looking at photographs on the self-assessment tool, which showed various rosacea severity levels. Scores ranged from 2 (least severe) to 8 (most severe). The PHQ-9 survey was completed by participants to assess mental health and mood.
Statistical Analysis
Results were reported using descriptive statistics. Regression analysis was performed to identify independent outcome predictors. To study the relationship between age and demographic variables, the population was divided into 2 groups: patients aged 60 years and older and patients younger than 60 years. Correlation of variables with duration of disease also was studied by creating 2 groups: patients with a disease duration of 11 years or longer and patients with a disease duration of less than 11 years. Comparisons were completed between groups using χ2 tests for proportions and t tests or analysis of variance for continuous variables. Analysis of variance was applied among all patients classified according to the following levels of depression: nondepressed, minimal depression symptoms, minor depression, major depression (moderate), and major depression (severe).
Results
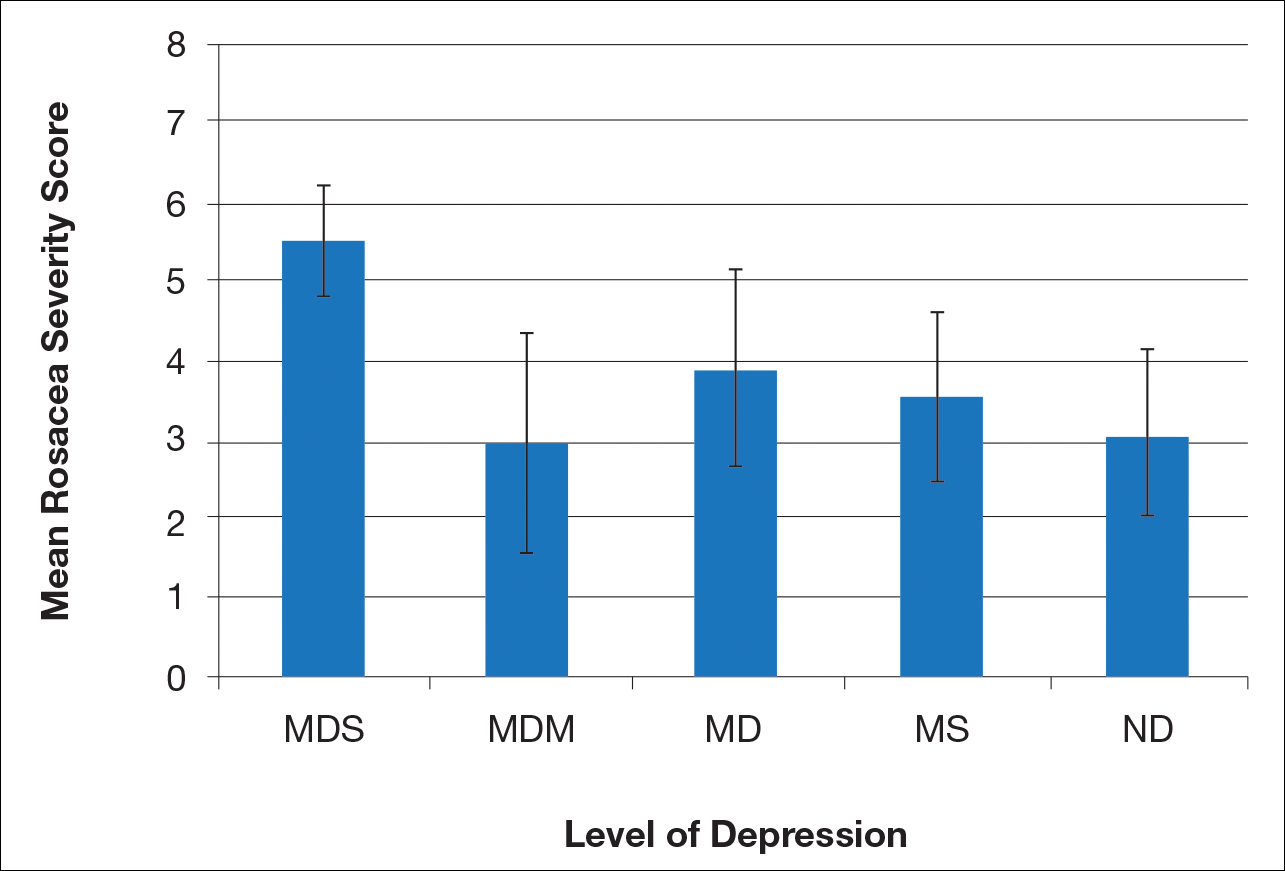
There is a direct relationship between rosacea severity and depression when comparing across the following levels of depression: nondepressed, minimal depression symptoms, minor depression, major depression (moderate), and major depression (severe)(P=.006; F=5.18; N=183)
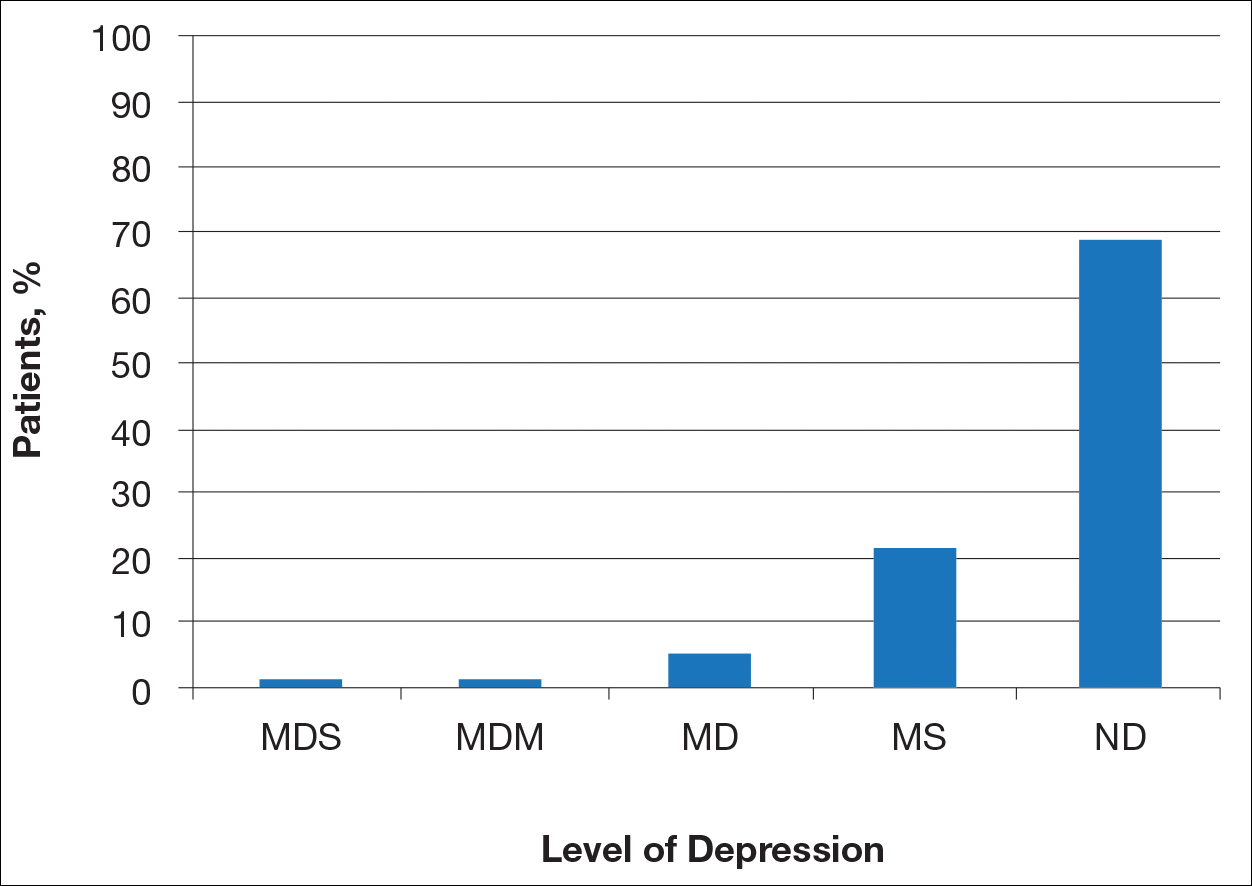
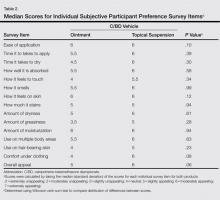
Most patients reported they were nondepressed (68.9%). As measured by the PHQ-9, 31.1% of patients experienced some level of depression: 21.9% reported minimal depression symptoms, 7.1% reported minor depression, 1.1% reported major depression (moderate), and 1.1% reported major depression (severe)(Table).
Comment
There is a direct relationship between rosacea severity and level of depression. In our study, nearly one-third of patients reported some degree of depression. The reason for this correlation may be due to disease stigmatization and decreased quality of life due to the somatic symptoms of rosacea. Our study reinforced the results of other studies evaluating the psychosocial impact of rosacea.8,9 Depression is associated with poor treatment adherence and poor outcomes in rosacea patients; therefore, depression may serve as an important outcome measure.10 The psychosocial impact of rosacea can be severe, but with disease improvement, there often is an improvement in the patient’s psychosocial status.7
There are several limitations to our study. The study population consisted of patients at a university dermatology clinic who may not be representative of patients in the general population; however, our hospital system does not require referral and provides care to a large percentage of the surrounding community.
Clinical implementation of the validated rosacea self-assessment tool and PHQ-9 may have several benefits. Patient-assessed rosacea severity and psychosocial impact obtained via use of these tools would provide physicians with information to fine-tune rosacea treatment regimens. Patients with the greatest social impact may require a more aggressive treatment approach. Early detection of depression in the rosacea population is important in informing treatment strategy and improving outcomes. Physicians should pay close attention to signs of depression in rosacea patients and determine if psychiatric treatment or referral for psychiatric evaluation is indicated. The correlation between rosacea and depression underscores the importance of treating this highly impactful disease; however, the low number of responders from the major depression (moderate) subgroup prevented us from making any strong conclusion about this specific subgroup.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6, suppl 1):S15-S26.
- Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychol Ann. 2002;32:509-515.
- America Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
- Gupta MA, Gupta AK, Chen SJ, et al. Comorbidity of rosacea and depression: an analysis of the National Ambulatory Medical Care Survey and National Hospital Ambulatory Care Survey—outpatient department data collected by the US National Center for Health Statistics from 1995 to 2002. Br J Dermatol. 2005;153:1176-1181.
- Böhm D, Schwanitz P, Stock Gissendanner S, et al. Symptom severity and psychological sequelae in rosacea: results of a survey. Psychol Health Med. 2014;19:586-591.
- Moustafa F, Lewallen RS, Feldman SR. The psychological impact of rosacea and the influence of current management options. J Am Acad Dermatol. 2014;71:973-980.
- Halioua B, Cribier B, Frey M, et al. Feelings of stigmatization in patients with rosacea [published online June 21, 2016]. J Eur Acad Dermatol Venereol. 2016;31:163-168.
- Bewley A, Fowler J, Schöfer H, et al. Erythema of rosacea impairs health-related quality of life: results of a meta-analysis [published online March 16, 2016]. Dermatol Ther (Heidelb). 2016;6:237-247.
- Korman AM, Hill D, Alikhan A, et al. Impact and management of depression in psoriasis patients [published online January 4, 2016]. Expert Opin Pharmacother. 2016;17:147-152.
Rosacea is a chronic skin condition that can be classified into 4 subtypes: erythematotelangiectatic, papulopustular, phymatous, and ocular. Erythematotelangiectatic rosacea is characterized by redness of the face and excessive blushing. Papulopustular rosacea is a more severe form of disease that is characterized by papules and pustules of the central face. If left untreated, these subtypes may progress to phymatous rosacea, which is characterized by skin thickening, fibrosis, and cosmetic disfigurement. Ocular rosacea is characterized by redness and irritation of the eyes.1 Rosacea patients often are burdened with embarrassment, social anxiety, and psychiatric comorbidities.
The Patient Health Questionnaire 9 (PHQ-9) is a validated and reliable self-administered tool for diagnosis of depression and designation of depression severity. This instrument could prove useful in screening for depression in rosacea patients given the high incidence of psychiatric comorbidities in this patient population.2 The PHQ-9 consists of 9 questions that assess for criteria used to define depressive disorders in the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition).3 The questionnaire is brief, easy to administer, and has 88% specificity and sensitivity.4
Other studies have evaluated the relationship between rosacea and psychiatric illness, but the PHQ-9 was not used as a screening tool.7,8 Rosacea patients are at increased risk for having psychiatrist-diagnosed depression.5 In one assessment, a positive correlation between rosacea and psychiatric illness was noted using the Dermatology Life Quality Index, the rejection scale of the Questionnaire on Experience with Skin Complaints, and the German version of the Hospital Anxiety and Depression Scale.6 Interpretation of Rosacea Quality of Life and Dermatology Life Quality Index scores indicated that rosacea has a negative impact on quality of life.7
The purpose of this study was to examine the relationship between self-assessed rosacea severity scores and level of depression using the validated rosacea self-assessment tool and the PHQ-9 questionnaire, respectively.
Methods
Study Population
Study participants were adult patients from the Wake Forest Baptist Medical Center (Winston-Salem, North Carolina) dermatology clinic from January 2011 to December 2014 who had received a diagnosis of rosacea (International Classification of Diseases, Ninth Revision [ICD-9] code 695.3) from a Wake Forest dermatologist. Institutional review board approval was obtained prior to initiation of the study. Data collection occurred from October 2014 through February 2015. A total of 478 patients met criteria for participation in the study and were identified from the Wake Forest Baptist Hospital Transitional Data Warehouse and the electronic medical record. Because rosacea typically is not diagnosed in children and the data measures are not validated in children, this demographic group was excluded from participation.
Of 478 eligible patients who were invited to participate via mail or telephone, 46 completed the rosacea self-assessment tool and PHQ-9 survey in person. A total of 432 patients were mailed a presurvey recruitment letter notifying them that they would be receiving a survey in the mail unless they contacted the study team to decline participation. An email address and telephone number for the study team was provided. Twenty patients declined to participate in the study; surveys were then mailed to the remaining 412 patients. Sixteen of the mailed surveys were returned by the post office due to an incorrect address.

Self-assessments
Patients selected images to self-identify the severity of their rosacea symptoms, including erythema, papulopustular lesions, ocular symptoms, and nasal involvement by looking at photographs on the self-assessment tool, which showed various rosacea severity levels. Scores ranged from 2 (least severe) to 8 (most severe). The PHQ-9 survey was completed by participants to assess mental health and mood.
Statistical Analysis
Results were reported using descriptive statistics. Regression analysis was performed to identify independent outcome predictors. To study the relationship between age and demographic variables, the population was divided into 2 groups: patients aged 60 years and older and patients younger than 60 years. Correlation of variables with duration of disease also was studied by creating 2 groups: patients with a disease duration of 11 years or longer and patients with a disease duration of less than 11 years. Comparisons were completed between groups using χ2 tests for proportions and t tests or analysis of variance for continuous variables. Analysis of variance was applied among all patients classified according to the following levels of depression: nondepressed, minimal depression symptoms, minor depression, major depression (moderate), and major depression (severe).
Results
There is a direct relationship between rosacea severity and depression when comparing across the following levels of depression: nondepressed, minimal depression symptoms, minor depression, major depression (moderate), and major depression (severe)(P=.006; F=5.18; N=183)


Most patients reported they were nondepressed (68.9%). As measured by the PHQ-9, 31.1% of patients experienced some level of depression: 21.9% reported minimal depression symptoms, 7.1% reported minor depression, 1.1% reported major depression (moderate), and 1.1% reported major depression (severe)(Table).
Comment
There is a direct relationship between rosacea severity and level of depression. In our study, nearly one-third of patients reported some degree of depression. The reason for this correlation may be due to disease stigmatization and decreased quality of life due to the somatic symptoms of rosacea. Our study reinforced the results of other studies evaluating the psychosocial impact of rosacea.8,9 Depression is associated with poor treatment adherence and poor outcomes in rosacea patients; therefore, depression may serve as an important outcome measure.10 The psychosocial impact of rosacea can be severe, but with disease improvement, there often is an improvement in the patient’s psychosocial status.7
There are several limitations to our study. The study population consisted of patients at a university dermatology clinic who may not be representative of patients in the general population; however, our hospital system does not require referral and provides care to a large percentage of the surrounding community.
Clinical implementation of the validated rosacea self-assessment tool and PHQ-9 may have several benefits. Patient-assessed rosacea severity and psychosocial impact obtained via use of these tools would provide physicians with information to fine-tune rosacea treatment regimens. Patients with the greatest social impact may require a more aggressive treatment approach. Early detection of depression in the rosacea population is important in informing treatment strategy and improving outcomes. Physicians should pay close attention to signs of depression in rosacea patients and determine if psychiatric treatment or referral for psychiatric evaluation is indicated. The correlation between rosacea and depression underscores the importance of treating this highly impactful disease; however, the low number of responders from the major depression (moderate) subgroup prevented us from making any strong conclusion about this specific subgroup.
Rosacea is a chronic skin condition that can be classified into 4 subtypes: erythematotelangiectatic, papulopustular, phymatous, and ocular. Erythematotelangiectatic rosacea is characterized by redness of the face and excessive blushing. Papulopustular rosacea is a more severe form of disease that is characterized by papules and pustules of the central face. If left untreated, these subtypes may progress to phymatous rosacea, which is characterized by skin thickening, fibrosis, and cosmetic disfigurement. Ocular rosacea is characterized by redness and irritation of the eyes.1 Rosacea patients often are burdened with embarrassment, social anxiety, and psychiatric comorbidities.
The Patient Health Questionnaire 9 (PHQ-9) is a validated and reliable self-administered tool for diagnosis of depression and designation of depression severity. This instrument could prove useful in screening for depression in rosacea patients given the high incidence of psychiatric comorbidities in this patient population.2 The PHQ-9 consists of 9 questions that assess for criteria used to define depressive disorders in the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition).3 The questionnaire is brief, easy to administer, and has 88% specificity and sensitivity.4
Other studies have evaluated the relationship between rosacea and psychiatric illness, but the PHQ-9 was not used as a screening tool.7,8 Rosacea patients are at increased risk for having psychiatrist-diagnosed depression.5 In one assessment, a positive correlation between rosacea and psychiatric illness was noted using the Dermatology Life Quality Index, the rejection scale of the Questionnaire on Experience with Skin Complaints, and the German version of the Hospital Anxiety and Depression Scale.6 Interpretation of Rosacea Quality of Life and Dermatology Life Quality Index scores indicated that rosacea has a negative impact on quality of life.7
The purpose of this study was to examine the relationship between self-assessed rosacea severity scores and level of depression using the validated rosacea self-assessment tool and the PHQ-9 questionnaire, respectively.
Methods
Study Population
Study participants were adult patients from the Wake Forest Baptist Medical Center (Winston-Salem, North Carolina) dermatology clinic from January 2011 to December 2014 who had received a diagnosis of rosacea (International Classification of Diseases, Ninth Revision [ICD-9] code 695.3) from a Wake Forest dermatologist. Institutional review board approval was obtained prior to initiation of the study. Data collection occurred from October 2014 through February 2015. A total of 478 patients met criteria for participation in the study and were identified from the Wake Forest Baptist Hospital Transitional Data Warehouse and the electronic medical record. Because rosacea typically is not diagnosed in children and the data measures are not validated in children, this demographic group was excluded from participation.
Of 478 eligible patients who were invited to participate via mail or telephone, 46 completed the rosacea self-assessment tool and PHQ-9 survey in person. A total of 432 patients were mailed a presurvey recruitment letter notifying them that they would be receiving a survey in the mail unless they contacted the study team to decline participation. An email address and telephone number for the study team was provided. Twenty patients declined to participate in the study; surveys were then mailed to the remaining 412 patients. Sixteen of the mailed surveys were returned by the post office due to an incorrect address.

Self-assessments
Patients selected images to self-identify the severity of their rosacea symptoms, including erythema, papulopustular lesions, ocular symptoms, and nasal involvement by looking at photographs on the self-assessment tool, which showed various rosacea severity levels. Scores ranged from 2 (least severe) to 8 (most severe). The PHQ-9 survey was completed by participants to assess mental health and mood.
Statistical Analysis
Results were reported using descriptive statistics. Regression analysis was performed to identify independent outcome predictors. To study the relationship between age and demographic variables, the population was divided into 2 groups: patients aged 60 years and older and patients younger than 60 years. Correlation of variables with duration of disease also was studied by creating 2 groups: patients with a disease duration of 11 years or longer and patients with a disease duration of less than 11 years. Comparisons were completed between groups using χ2 tests for proportions and t tests or analysis of variance for continuous variables. Analysis of variance was applied among all patients classified according to the following levels of depression: nondepressed, minimal depression symptoms, minor depression, major depression (moderate), and major depression (severe).
Results
There is a direct relationship between rosacea severity and depression when comparing across the following levels of depression: nondepressed, minimal depression symptoms, minor depression, major depression (moderate), and major depression (severe)(P=.006; F=5.18; N=183)


Most patients reported they were nondepressed (68.9%). As measured by the PHQ-9, 31.1% of patients experienced some level of depression: 21.9% reported minimal depression symptoms, 7.1% reported minor depression, 1.1% reported major depression (moderate), and 1.1% reported major depression (severe)(Table).
Comment
There is a direct relationship between rosacea severity and level of depression. In our study, nearly one-third of patients reported some degree of depression. The reason for this correlation may be due to disease stigmatization and decreased quality of life due to the somatic symptoms of rosacea. Our study reinforced the results of other studies evaluating the psychosocial impact of rosacea.8,9 Depression is associated with poor treatment adherence and poor outcomes in rosacea patients; therefore, depression may serve as an important outcome measure.10 The psychosocial impact of rosacea can be severe, but with disease improvement, there often is an improvement in the patient’s psychosocial status.7
There are several limitations to our study. The study population consisted of patients at a university dermatology clinic who may not be representative of patients in the general population; however, our hospital system does not require referral and provides care to a large percentage of the surrounding community.
Clinical implementation of the validated rosacea self-assessment tool and PHQ-9 may have several benefits. Patient-assessed rosacea severity and psychosocial impact obtained via use of these tools would provide physicians with information to fine-tune rosacea treatment regimens. Patients with the greatest social impact may require a more aggressive treatment approach. Early detection of depression in the rosacea population is important in informing treatment strategy and improving outcomes. Physicians should pay close attention to signs of depression in rosacea patients and determine if psychiatric treatment or referral for psychiatric evaluation is indicated. The correlation between rosacea and depression underscores the importance of treating this highly impactful disease; however, the low number of responders from the major depression (moderate) subgroup prevented us from making any strong conclusion about this specific subgroup.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6, suppl 1):S15-S26.
- Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychol Ann. 2002;32:509-515.
- America Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
- Gupta MA, Gupta AK, Chen SJ, et al. Comorbidity of rosacea and depression: an analysis of the National Ambulatory Medical Care Survey and National Hospital Ambulatory Care Survey—outpatient department data collected by the US National Center for Health Statistics from 1995 to 2002. Br J Dermatol. 2005;153:1176-1181.
- Böhm D, Schwanitz P, Stock Gissendanner S, et al. Symptom severity and psychological sequelae in rosacea: results of a survey. Psychol Health Med. 2014;19:586-591.
- Moustafa F, Lewallen RS, Feldman SR. The psychological impact of rosacea and the influence of current management options. J Am Acad Dermatol. 2014;71:973-980.
- Halioua B, Cribier B, Frey M, et al. Feelings of stigmatization in patients with rosacea [published online June 21, 2016]. J Eur Acad Dermatol Venereol. 2016;31:163-168.
- Bewley A, Fowler J, Schöfer H, et al. Erythema of rosacea impairs health-related quality of life: results of a meta-analysis [published online March 16, 2016]. Dermatol Ther (Heidelb). 2016;6:237-247.
- Korman AM, Hill D, Alikhan A, et al. Impact and management of depression in psoriasis patients [published online January 4, 2016]. Expert Opin Pharmacother. 2016;17:147-152.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6, suppl 1):S15-S26.
- Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychol Ann. 2002;32:509-515.
- America Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
- Gupta MA, Gupta AK, Chen SJ, et al. Comorbidity of rosacea and depression: an analysis of the National Ambulatory Medical Care Survey and National Hospital Ambulatory Care Survey—outpatient department data collected by the US National Center for Health Statistics from 1995 to 2002. Br J Dermatol. 2005;153:1176-1181.
- Böhm D, Schwanitz P, Stock Gissendanner S, et al. Symptom severity and psychological sequelae in rosacea: results of a survey. Psychol Health Med. 2014;19:586-591.
- Moustafa F, Lewallen RS, Feldman SR. The psychological impact of rosacea and the influence of current management options. J Am Acad Dermatol. 2014;71:973-980.
- Halioua B, Cribier B, Frey M, et al. Feelings of stigmatization in patients with rosacea [published online June 21, 2016]. J Eur Acad Dermatol Venereol. 2016;31:163-168.
- Bewley A, Fowler J, Schöfer H, et al. Erythema of rosacea impairs health-related quality of life: results of a meta-analysis [published online March 16, 2016]. Dermatol Ther (Heidelb). 2016;6:237-247.
- Korman AM, Hill D, Alikhan A, et al. Impact and management of depression in psoriasis patients [published online January 4, 2016]. Expert Opin Pharmacother. 2016;17:147-152.
Practice Points
- Rosacea patients often are burdened with embarrassment, social anxiety, and psychiatric comorbidities.
- There is a direct relationship between rosacea severity and degree of depression.
- Physicians should pay close attention to signs of depression in rosacea patients and determine if psychiatric treatment or referral for psychiatric evaluation is indicated.
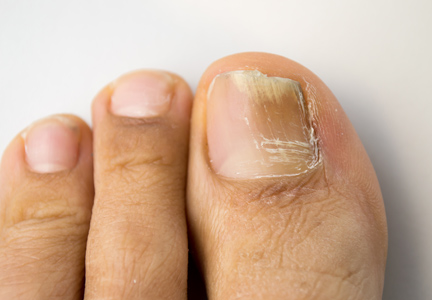
Onychomycosis Treatment in the United States
Onychomycosis is a common progressive infection of the nails caused by dermatophytes, nondermatophyte molds, and yeasts, with Trichophyton rubrum being the most common causative organism.1-3 Onychomycosis affects approximately 2% to 26% of different populations worldwide. It represents 20% to 50% of onychopathies and approximately 30% of fungal cutaneous infections.4-9 Less than 30% of infected persons seek medical advice or treatment even in developed areas of the world.10 Onychomycosis may be a source of more widespread fungal skin infections or give rise to complications such as cellulitis. Chronic, long-lasting infection may result in nail dystrophy and can lead to pain, absence from work, and decreased quality of life.1,11 Because the dermatophyte can contaminate communal bathing facilities and spread to others,12 it is important to effectively target and treat patients with onychomycosis, thus reducing the rate of related morbidities.1,9
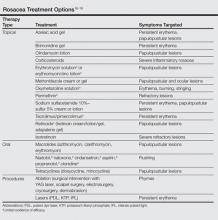
The primary aim of onychomycosis treatment is to cure the infection and prevent relapse. Both topical and oral agents are available for the treatment of fungal nail infections. Generally, systemic therapy for onychomycosis is more successful than topical treatment, likely due to poor penetration of topical medications into the nail plate.1,2,9 However, newer topical drugs have shown promising results in treating some types of onychomycosis.13 In its guidelines for treatment of onychomycosis, the British Association of Dermatologists recommends use of topical treatment under the following conditions: (1) when there is not extensive involvement of the nail plate (eg, candidal paronychia, superficial white onychomycosis, early stages of distal and lateral subungual onychomycosis), (2) when systemic therapy is contraindicated, or (3) in combination with systemic therapy.1 Although there are multiple treatments for fungal nail infections, there are limited reports on the ways in which physicians actually use these treatments or the frequency with which they prescribe them.
This study provides a representative portrayal of onychomycosis visits in the US outpatient setting using a large nationally sampled survey. In particular, we aimed to assess the number of visits related to onychomycosis, the demographics of patients, and the treatments being prescribed for onychomycosis.
Methods
Study Design
Data from January 1, 1993, to December 31, 2010, were collected from the National Ambulatory Medical Care Survey (NAMCS), an ongoing survey of nonfederal employed US office-based physicians who are primarily engaged in direct patient care. The NAMCS has been conducted by the National Center for Health Statistics every year since 1989 to estimate the utilization of ambulatory care services in the United States. Since 1989 including 1993 to 2010, the NAMCS sampled approximately 30,000 visits per year. For each visit sampled, a 1-page patient log including demographic data, physicians’ diagnoses, services provided, and medications was completed. In the NAMCS survey, visits were divided into 2 groups: (1) visits from established patients that have been seen in that office before for any reason, and (2) visits for new (ie, first-time) patients. The current study included all visits in which fungal nail infection (code 110.1 according to the International Classification of Diseases, Ninth Revision [ICD-9]) was listed as 1 of 3 possible diagnoses.
Statistical Analysis
Sampling weights were applied to data to produce estimates for the total US outpatient setting.14 Data were analyzed using SAS version 9.2, and SAS survey analysis procedures were used to account for the clustered sampling of the survey. The total numbers of visits for which onychomycosis was 1 of 3 possible diagnoses and for which it was the sole diagnosis were reported. Visit rates per population by demographic characteristics (ie, patient sex, age, race, and ethnicity) were calculated. Population estimates were based on the 2001 NAMCS Public Micro-Data File Documentation records of the US census estimates for noninstitutionalized civilian persons.15 Trends in proportion of visits linked with an onychomycosis diagnosis over time were evaluated using the SAS SURVEYREG procedure. Types of physicians who attended to these visits as well as leading comorbidities that had been diagnosed and documented in the medical record were characterized. Onychomycosis-related medications prescribed at these visits were reported and prescribing trends over time were evaluated. Differences in the treatment prescribed according to the type of visit (ie, first-time or return visit); physician specialty; and patients’ gender, race, and health conditions (eg, obesity, diabetes mellitus) were examined. To exclude the possibility that fluconazole and other broad-spectrum antifungals were being used for secondary diagnoses, we determined the number of visits that had an additional diagnosis of either candidiasis (ICD-9 codes 112.0–112.9) or “other specified erythematous conditions” (ICD-9 code 695.89).
Results
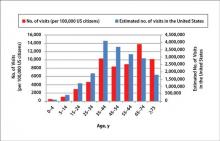
During the 18-year study period, 636 visits with a diagnosis of onychomycosis were recorded in the NAMCS database. This unweighted number of visits corresponded with approximately 19,350,000 visits (an average of 1,075,000 visits per year) to physicians’ offices with a diagnosis of onychomycosis in the United States during this period. Among these visits, there were an estimated 4,250,000 visits with fungal nail infection as the only diagnosis (no other comorbidities recorded). The recorded visits included more female (57.6%) than male (42.4%) patients, and 85% of patients were white (Table). Patients aged 35 to 44 years accounted for the largest number of visits; however, the estimated rate of onychomycosis visits per 100,000 US citizens was highest among those aged 65 to 74 years (Figure 1).
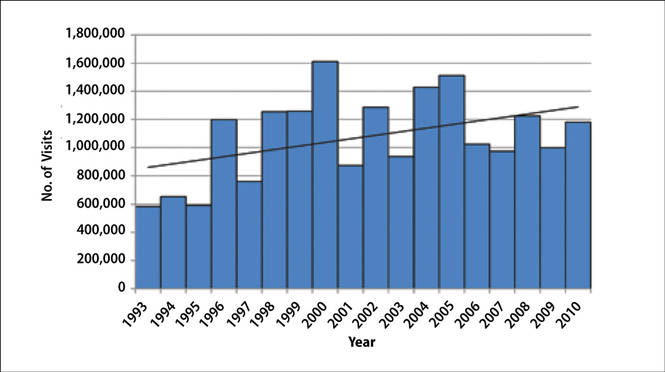
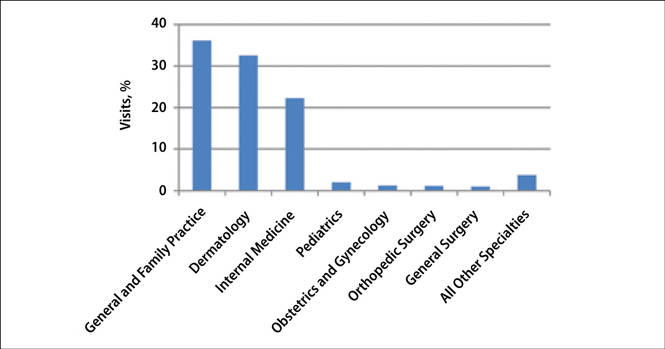
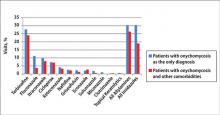
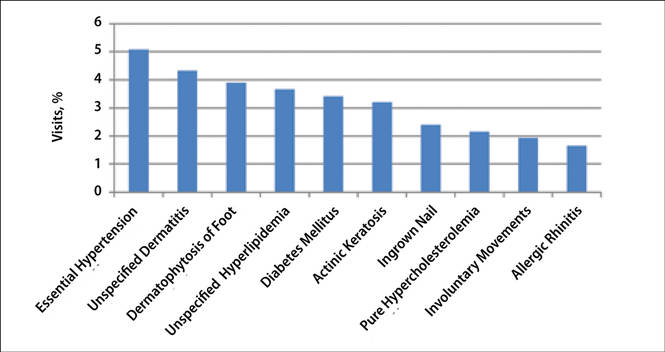
The number of US outpatient visits with a recorded diagnosis of onychomycosis increased from 1993 to 2010 (Figure 2); however, there was no change in the ratio of onychomycosis visits to the total number of recorded visits in NAMCS database during the study period (P=.9). A combined total of 91% of onychomycosis visits were to general and family practitioners, dermatologists, or internal medicine practitioners (Figure 3). Although cardiovascular diseases and diabetes mellitus accounted for a large proportion of comorbidities, conditions affecting the feet (eg, tinea pedis, ingrown nails) also were among the most common comorbidities (Figure 4).
 |
In both topical and systemic form, terbinafine was the most commonly prescribed antifungal agent, followed by systemic fluconazole, systemic itraconazole, and topical ciclopirox (Figure 5). Over the 18-year study period, there was an increasing trend in the frequency of terbinafine prescription (regression coefficient [r]=0.01319; P=.004); a decreasing trend for fluconazole (r=-0.0053851; P=.04), itraconazole (r=-0.0113988; P<.001), griseofulvin (r=-0.0073942; P<.001), and econazole prescription (r=-0.0032405; P=.01); and no significant trend for ketoconazole (r=-0.0034553; P=.1), naftifine (r=-0.0029067; P=.06), sulconazole (r=-0.0001619; P=.8), ciclopirox (r=0.0032684; P=.1), and miconazole prescription (r=0.0002074; P=.5).
Eighty-six percent of visits were for established patients who had been seen in the related office with any diagnosis before the recorded visit and 14% of visits were for new (first-time) patients. Fluconazole was the most frequently used antifungal drug for new patients, while terbinafine was the most frequently used in other visits. Terbinafine was the most frequently prescribed antifungal drug by general and family practitioners, dermatologists, internal medicine practitioners, and all other specialties not listed.
Terbinafine was the most frequently prescribed antifungal drug in both genders and in white and black patients. Itraconazole was the most frequently prescribed antifungal drug for Hispanic patients and those of other ethnicities not listed. Terbinafine was the most frequently prescribed antifungal drug for patients with diabetes and obesity (ie, body mass index ≥30). In 19,330,000 of 19,350,000 total estimated visits included in this study, onychomycosis was the only diagnosis with a potential indication for an antifungal drug therapy, ruling out the possibility that fluconazole or other drugs were used for patients who also had candidiasis or “other specified erythematous conditions.”
Discussion
Onychomycosis is a common progressive infection of the nails that is more prevalent in older age groups, with equal prevalence in both genders and a higher prevalence in males. The NAMCS data showed higher rates of onychomycosis visits among older age groups, which is in agreement with results from prior studies.16,17 In the current study, we observed a higher prevalence of onychomycosis visits among females as well as white and Hispanic patients. These results may be due to a higher prevalence of onychomycosis in these populations or simply a result of difference in socioeconomic level or importance of aesthetics. Although there are limited data regarding the prevalence of onychomycosis among different races and ethnicities in the United States, a high incidence of onychomycosis has been reported in Mexico.18
Repeated trauma to the great toenail from ill-fitting shoes is a predisposing factor for onychomycosis.16 In the current study, ingrown nails were among the most common comorbidities found in onychomycosis patients. Although nail dystrophy caused by onychomycosis may lead to ingrown nails, it also is possible that both conditions may be caused by trauma.
Patients with immunodeficiencies (eg, diabetes) may be predisposed to onychomycosis as well as its associated complications and morbidities (eg, cellulitis).16,19 Diabetes affects 4% to 22% of patients with onychomycosis in different populations, including Denmark, Mexico, and India.18,20,21 In our study, diabetes was among the most common recorded comorbidities reported during onychomycosis visits, with a prevalence of 3.4%. It is likely that many more visits involved patients with diabetes that had not been diagnosed or reported. With the increased risk for complications with diabetes, it is important for physicians to treat these patients when they have a nail infection.
The available systemic therapies for treatment of onychomycosis include griseofulvin, allylamines, and imidazoles. Comparison of griseofulvin with newer systemic antifungal agents such as terbinafine and itraconazole suggests that griseofulvin has lower efficacy and therefore is not a first-line treatment of onychomycosis.1 Terbinafine is the most active of the currently available antidermatophyte drugs both in vitro and in vivo, with synergistic effects with imidazoles and ciclopirox.1,22-27 A combination of topical and systemic therapies may improve cure rates of onychomycosis or possibly shorten the duration of therapy with the systemic agent.1,2 Treatment strategies can vary according to the specialty of the treating physician, with general practitioners often preferring monotherapies and dermatologists preferring combination therapies.28 In Europe, the most commonly prescribed medication for onychomycosis was topical amorolfine followed by systemic terbinafine and itraconazole.28 In the current study, we could not separate data for topical versus systemic terbinafine because the NAMCS uses similar names for reporting the drug; however, the rates of prescription for allylamines and imidazoles were nearly equal (Figure 5), with terbinafine showing an increased use over time as opposed to a decreased use of imidazoles. Although fluconazole is not approved by the US Food and Drug Administration for treatment of onychomycosis, oral fluconazole was the second most common treatment prescribed in our study. Griseofulvin, which is not considered as a drug of choice in onychomycosis,1 was prescribed in a small fraction of the visits, with a decreasing trend of usage over time.
Conclusion
Our analysis of the NAMCS data revealed that the treatment of onychomycosis in the United States is in accordance with recommendations in current guidelines. An encouraging finding was the notable downward trend in use of griseofulvin, suggesting that health care providers are changing practice to meet standard of care. Increased efforts must be made to uniformly modify practices in compliance with evidence-based recommendations and to minimize unnecessary risk and cost associated with use of drugs with lower efficacy.
1. Roberts DT, Taylor WD, Boyle J; British Association of Dermatologists. Guidelines for treatment of onychomycosis. Br J Dermatol. 2003;148:402-410.
2. Seebacher C, Brasch J, Abeck D, et al. Onychomycosis. Mycoses. 2007;50:321-327.
3. Summerbell RC, Kane J, Krajden S. Onychomycosis, tinea pedis and tinea manuum caused by non-dermatophytic filamentous fungi. Mycoses. 1989;32:609-619.
4. Murray SC, Dawber RP. Onychomycosis of toenails: orthopaedic and podiatric considerations. Australas J Dermatol. 2002;43:105-112.
5. Achten G, Wanet-Rouard J. Onychomycoses in the laboratory. Mykosen Suppl. 1978;1:125-127.
6. Haneke E, Roseeuw D. The scope of onychomycosis: epidemiology and clinical features. Int J Dermatol. 1999;38(suppl 2):7-12.
7. Haneke E. Fungal infections of the nail. Semin Dermatol. 1991;10:41-53.
8. Karmakar S, Kalla G, Joshi KR, et al. Dermatophytoses in a desert district of Western Rajasthan. Indian J Dermatol Venereol Leprol. 1995;61:280-283.
9. Drake LA. Guidelines of care for superficial mycotic infections of the skin: onychomycosis. Guidelines/Outcomes Committee. American Academy of Dermatology. J Am Acad Dermatol. 1996;34:116-121.
10. Roberts DT. Prevalence of dermatophyte onychomycosis in the United Kingdom: results of an omnibus survey. Br J Dermatol. 1992;126(suppl 39):23-27.
11. Drake LA, Scher RK, Smith EB, et al. Effect of onychomycosis on quality of life. J Am Acad Dermatol. 1998;38(5 pt 1):702-704.
12. Detandt M, Nolard N. Fungal contamination of the floors of swimming pools, particularly subtropical swimming paradises. Mycoses. 1995;38:509-513.
13. Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
14. Fleischer AB Jr, Feldman SR, Bradham DD. Office-based physician services provided by dermatologists in the United States in 1990. J Invest Dermatol. 1994;102:93-97.
15. 2001 NAMCS Micro-Data File Documentation. http://www.nber.org/namcs/docs/namcs2001.pdf. National Bureau of Economic Research Web site. Accessed April 27, 2015.
16. Williams HC. The epidemiology of onychomycosis in Britain. Br J Dermatol. 1993;129:101-109.
17. Elewski BE, Charif MA. Prevalence of onychomycosis in patients attending a dermatology clinic in northeastern Ohio for other conditions. Arch Dermatol. 1997;133:1172-1173.
18. Arenas R, Bonifaz A, Padilla MC, et al. Onychomycosis. a Mexican survey. Eur J Dermatol. 2010;20:611-614.
19. Faergemann J, Baran R. Epidemiology, clinical presentation and diagnosis of onychomycosis. Br J Dermatol. 2003;149(suppl 65):1-4.
20. Sarma S, Capoor MR, Deb M, et al. Epidemiologic and clinicomycologic profile of onychomycosis from north India. Int J Dermatol. 2008;47:584-587.
21. Svejgaard EL, Nilsson J. Onychomycosis in Denmark: prevalence of fungal nail infection in general practice. Mycoses. 2004;47:131-135.
22. Santos DA, Hamdan JS. In vitro antifungal oral drug and drug-combination activity against onychomycosis causative dermatophytes. Med Mycol. 2006;44:357-362.
23. Gupta AK, Kohli Y. In vitro susceptibility testing of ciclopirox, terbinafine, ketoconazole and itraconazole against dermatophytes and nondermatophytes, and in vitro evaluation of combination antifungal activity. Br J Dermatol. 2003;149:296-305.
24. Gupta AK, Lynch LE. Management of onychomycosis: examining the role of monotherapy and dual, triple, or quadruple therapies. Cutis. 2004;74(suppl 1):5-9.
25. Harman S, Ashbee HR, Evans EG. Testing of antifungal combinations against yeasts and dermatophytes. J Dermatolog Treat. 2004;15:104-107.
26. Spader TB, Venturini TP, Rossato L, et al. Synergisms of voriconazole or itraconazole combined with other antifungal agents against Fusarium spp. Rev Iberoam Micol. 2013;30:200-204.
27. Biancalana FS, Lyra L, Moretti ML, et al. Susceptibility testing of terbinafine alone and in combination with amphotericin B, itraconazole, or voriconazole against conidia and hyphae of dematiaceous molds. Diagn Microbiol Infect Dis. 2011;71:378-385.
28. Effendy I, Lecha M, Feuilhade de CM, et al. Epidemiology and clinical classification of onychomycosis. J Eur Acad Dermatol Venereol. 2005;19(suppl 1):8-12.
Onychomycosis is a common progressive infection of the nails caused by dermatophytes, nondermatophyte molds, and yeasts, with Trichophyton rubrum being the most common causative organism.1-3 Onychomycosis affects approximately 2% to 26% of different populations worldwide. It represents 20% to 50% of onychopathies and approximately 30% of fungal cutaneous infections.4-9 Less than 30% of infected persons seek medical advice or treatment even in developed areas of the world.10 Onychomycosis may be a source of more widespread fungal skin infections or give rise to complications such as cellulitis. Chronic, long-lasting infection may result in nail dystrophy and can lead to pain, absence from work, and decreased quality of life.1,11 Because the dermatophyte can contaminate communal bathing facilities and spread to others,12 it is important to effectively target and treat patients with onychomycosis, thus reducing the rate of related morbidities.1,9
The primary aim of onychomycosis treatment is to cure the infection and prevent relapse. Both topical and oral agents are available for the treatment of fungal nail infections. Generally, systemic therapy for onychomycosis is more successful than topical treatment, likely due to poor penetration of topical medications into the nail plate.1,2,9 However, newer topical drugs have shown promising results in treating some types of onychomycosis.13 In its guidelines for treatment of onychomycosis, the British Association of Dermatologists recommends use of topical treatment under the following conditions: (1) when there is not extensive involvement of the nail plate (eg, candidal paronychia, superficial white onychomycosis, early stages of distal and lateral subungual onychomycosis), (2) when systemic therapy is contraindicated, or (3) in combination with systemic therapy.1 Although there are multiple treatments for fungal nail infections, there are limited reports on the ways in which physicians actually use these treatments or the frequency with which they prescribe them.
This study provides a representative portrayal of onychomycosis visits in the US outpatient setting using a large nationally sampled survey. In particular, we aimed to assess the number of visits related to onychomycosis, the demographics of patients, and the treatments being prescribed for onychomycosis.
Methods
Study Design
Data from January 1, 1993, to December 31, 2010, were collected from the National Ambulatory Medical Care Survey (NAMCS), an ongoing survey of nonfederal employed US office-based physicians who are primarily engaged in direct patient care. The NAMCS has been conducted by the National Center for Health Statistics every year since 1989 to estimate the utilization of ambulatory care services in the United States. Since 1989 including 1993 to 2010, the NAMCS sampled approximately 30,000 visits per year. For each visit sampled, a 1-page patient log including demographic data, physicians’ diagnoses, services provided, and medications was completed. In the NAMCS survey, visits were divided into 2 groups: (1) visits from established patients that have been seen in that office before for any reason, and (2) visits for new (ie, first-time) patients. The current study included all visits in which fungal nail infection (code 110.1 according to the International Classification of Diseases, Ninth Revision [ICD-9]) was listed as 1 of 3 possible diagnoses.
Statistical Analysis
Sampling weights were applied to data to produce estimates for the total US outpatient setting.14 Data were analyzed using SAS version 9.2, and SAS survey analysis procedures were used to account for the clustered sampling of the survey. The total numbers of visits for which onychomycosis was 1 of 3 possible diagnoses and for which it was the sole diagnosis were reported. Visit rates per population by demographic characteristics (ie, patient sex, age, race, and ethnicity) were calculated. Population estimates were based on the 2001 NAMCS Public Micro-Data File Documentation records of the US census estimates for noninstitutionalized civilian persons.15 Trends in proportion of visits linked with an onychomycosis diagnosis over time were evaluated using the SAS SURVEYREG procedure. Types of physicians who attended to these visits as well as leading comorbidities that had been diagnosed and documented in the medical record were characterized. Onychomycosis-related medications prescribed at these visits were reported and prescribing trends over time were evaluated. Differences in the treatment prescribed according to the type of visit (ie, first-time or return visit); physician specialty; and patients’ gender, race, and health conditions (eg, obesity, diabetes mellitus) were examined. To exclude the possibility that fluconazole and other broad-spectrum antifungals were being used for secondary diagnoses, we determined the number of visits that had an additional diagnosis of either candidiasis (ICD-9 codes 112.0–112.9) or “other specified erythematous conditions” (ICD-9 code 695.89).
Results
During the 18-year study period, 636 visits with a diagnosis of onychomycosis were recorded in the NAMCS database. This unweighted number of visits corresponded with approximately 19,350,000 visits (an average of 1,075,000 visits per year) to physicians’ offices with a diagnosis of onychomycosis in the United States during this period. Among these visits, there were an estimated 4,250,000 visits with fungal nail infection as the only diagnosis (no other comorbidities recorded). The recorded visits included more female (57.6%) than male (42.4%) patients, and 85% of patients were white (Table). Patients aged 35 to 44 years accounted for the largest number of visits; however, the estimated rate of onychomycosis visits per 100,000 US citizens was highest among those aged 65 to 74 years (Figure 1).
The number of US outpatient visits with a recorded diagnosis of onychomycosis increased from 1993 to 2010 (Figure 2); however, there was no change in the ratio of onychomycosis visits to the total number of recorded visits in NAMCS database during the study period (P=.9). A combined total of 91% of onychomycosis visits were to general and family practitioners, dermatologists, or internal medicine practitioners (Figure 3). Although cardiovascular diseases and diabetes mellitus accounted for a large proportion of comorbidities, conditions affecting the feet (eg, tinea pedis, ingrown nails) also were among the most common comorbidities (Figure 4).
 |
In both topical and systemic form, terbinafine was the most commonly prescribed antifungal agent, followed by systemic fluconazole, systemic itraconazole, and topical ciclopirox (Figure 5). Over the 18-year study period, there was an increasing trend in the frequency of terbinafine prescription (regression coefficient [r]=0.01319; P=.004); a decreasing trend for fluconazole (r=-0.0053851; P=.04), itraconazole (r=-0.0113988; P<.001), griseofulvin (r=-0.0073942; P<.001), and econazole prescription (r=-0.0032405; P=.01); and no significant trend for ketoconazole (r=-0.0034553; P=.1), naftifine (r=-0.0029067; P=.06), sulconazole (r=-0.0001619; P=.8), ciclopirox (r=0.0032684; P=.1), and miconazole prescription (r=0.0002074; P=.5).
Eighty-six percent of visits were for established patients who had been seen in the related office with any diagnosis before the recorded visit and 14% of visits were for new (first-time) patients. Fluconazole was the most frequently used antifungal drug for new patients, while terbinafine was the most frequently used in other visits. Terbinafine was the most frequently prescribed antifungal drug by general and family practitioners, dermatologists, internal medicine practitioners, and all other specialties not listed.
Terbinafine was the most frequently prescribed antifungal drug in both genders and in white and black patients. Itraconazole was the most frequently prescribed antifungal drug for Hispanic patients and those of other ethnicities not listed. Terbinafine was the most frequently prescribed antifungal drug for patients with diabetes and obesity (ie, body mass index ≥30). In 19,330,000 of 19,350,000 total estimated visits included in this study, onychomycosis was the only diagnosis with a potential indication for an antifungal drug therapy, ruling out the possibility that fluconazole or other drugs were used for patients who also had candidiasis or “other specified erythematous conditions.”
Discussion
Onychomycosis is a common progressive infection of the nails that is more prevalent in older age groups, with equal prevalence in both genders and a higher prevalence in males. The NAMCS data showed higher rates of onychomycosis visits among older age groups, which is in agreement with results from prior studies.16,17 In the current study, we observed a higher prevalence of onychomycosis visits among females as well as white and Hispanic patients. These results may be due to a higher prevalence of onychomycosis in these populations or simply a result of difference in socioeconomic level or importance of aesthetics. Although there are limited data regarding the prevalence of onychomycosis among different races and ethnicities in the United States, a high incidence of onychomycosis has been reported in Mexico.18
Repeated trauma to the great toenail from ill-fitting shoes is a predisposing factor for onychomycosis.16 In the current study, ingrown nails were among the most common comorbidities found in onychomycosis patients. Although nail dystrophy caused by onychomycosis may lead to ingrown nails, it also is possible that both conditions may be caused by trauma.
Patients with immunodeficiencies (eg, diabetes) may be predisposed to onychomycosis as well as its associated complications and morbidities (eg, cellulitis).16,19 Diabetes affects 4% to 22% of patients with onychomycosis in different populations, including Denmark, Mexico, and India.18,20,21 In our study, diabetes was among the most common recorded comorbidities reported during onychomycosis visits, with a prevalence of 3.4%. It is likely that many more visits involved patients with diabetes that had not been diagnosed or reported. With the increased risk for complications with diabetes, it is important for physicians to treat these patients when they have a nail infection.
The available systemic therapies for treatment of onychomycosis include griseofulvin, allylamines, and imidazoles. Comparison of griseofulvin with newer systemic antifungal agents such as terbinafine and itraconazole suggests that griseofulvin has lower efficacy and therefore is not a first-line treatment of onychomycosis.1 Terbinafine is the most active of the currently available antidermatophyte drugs both in vitro and in vivo, with synergistic effects with imidazoles and ciclopirox.1,22-27 A combination of topical and systemic therapies may improve cure rates of onychomycosis or possibly shorten the duration of therapy with the systemic agent.1,2 Treatment strategies can vary according to the specialty of the treating physician, with general practitioners often preferring monotherapies and dermatologists preferring combination therapies.28 In Europe, the most commonly prescribed medication for onychomycosis was topical amorolfine followed by systemic terbinafine and itraconazole.28 In the current study, we could not separate data for topical versus systemic terbinafine because the NAMCS uses similar names for reporting the drug; however, the rates of prescription for allylamines and imidazoles were nearly equal (Figure 5), with terbinafine showing an increased use over time as opposed to a decreased use of imidazoles. Although fluconazole is not approved by the US Food and Drug Administration for treatment of onychomycosis, oral fluconazole was the second most common treatment prescribed in our study. Griseofulvin, which is not considered as a drug of choice in onychomycosis,1 was prescribed in a small fraction of the visits, with a decreasing trend of usage over time.
Conclusion
Our analysis of the NAMCS data revealed that the treatment of onychomycosis in the United States is in accordance with recommendations in current guidelines. An encouraging finding was the notable downward trend in use of griseofulvin, suggesting that health care providers are changing practice to meet standard of care. Increased efforts must be made to uniformly modify practices in compliance with evidence-based recommendations and to minimize unnecessary risk and cost associated with use of drugs with lower efficacy.
Onychomycosis is a common progressive infection of the nails caused by dermatophytes, nondermatophyte molds, and yeasts, with Trichophyton rubrum being the most common causative organism.1-3 Onychomycosis affects approximately 2% to 26% of different populations worldwide. It represents 20% to 50% of onychopathies and approximately 30% of fungal cutaneous infections.4-9 Less than 30% of infected persons seek medical advice or treatment even in developed areas of the world.10 Onychomycosis may be a source of more widespread fungal skin infections or give rise to complications such as cellulitis. Chronic, long-lasting infection may result in nail dystrophy and can lead to pain, absence from work, and decreased quality of life.1,11 Because the dermatophyte can contaminate communal bathing facilities and spread to others,12 it is important to effectively target and treat patients with onychomycosis, thus reducing the rate of related morbidities.1,9
The primary aim of onychomycosis treatment is to cure the infection and prevent relapse. Both topical and oral agents are available for the treatment of fungal nail infections. Generally, systemic therapy for onychomycosis is more successful than topical treatment, likely due to poor penetration of topical medications into the nail plate.1,2,9 However, newer topical drugs have shown promising results in treating some types of onychomycosis.13 In its guidelines for treatment of onychomycosis, the British Association of Dermatologists recommends use of topical treatment under the following conditions: (1) when there is not extensive involvement of the nail plate (eg, candidal paronychia, superficial white onychomycosis, early stages of distal and lateral subungual onychomycosis), (2) when systemic therapy is contraindicated, or (3) in combination with systemic therapy.1 Although there are multiple treatments for fungal nail infections, there are limited reports on the ways in which physicians actually use these treatments or the frequency with which they prescribe them.
This study provides a representative portrayal of onychomycosis visits in the US outpatient setting using a large nationally sampled survey. In particular, we aimed to assess the number of visits related to onychomycosis, the demographics of patients, and the treatments being prescribed for onychomycosis.
Methods
Study Design
Data from January 1, 1993, to December 31, 2010, were collected from the National Ambulatory Medical Care Survey (NAMCS), an ongoing survey of nonfederal employed US office-based physicians who are primarily engaged in direct patient care. The NAMCS has been conducted by the National Center for Health Statistics every year since 1989 to estimate the utilization of ambulatory care services in the United States. Since 1989 including 1993 to 2010, the NAMCS sampled approximately 30,000 visits per year. For each visit sampled, a 1-page patient log including demographic data, physicians’ diagnoses, services provided, and medications was completed. In the NAMCS survey, visits were divided into 2 groups: (1) visits from established patients that have been seen in that office before for any reason, and (2) visits for new (ie, first-time) patients. The current study included all visits in which fungal nail infection (code 110.1 according to the International Classification of Diseases, Ninth Revision [ICD-9]) was listed as 1 of 3 possible diagnoses.
Statistical Analysis
Sampling weights were applied to data to produce estimates for the total US outpatient setting.14 Data were analyzed using SAS version 9.2, and SAS survey analysis procedures were used to account for the clustered sampling of the survey. The total numbers of visits for which onychomycosis was 1 of 3 possible diagnoses and for which it was the sole diagnosis were reported. Visit rates per population by demographic characteristics (ie, patient sex, age, race, and ethnicity) were calculated. Population estimates were based on the 2001 NAMCS Public Micro-Data File Documentation records of the US census estimates for noninstitutionalized civilian persons.15 Trends in proportion of visits linked with an onychomycosis diagnosis over time were evaluated using the SAS SURVEYREG procedure. Types of physicians who attended to these visits as well as leading comorbidities that had been diagnosed and documented in the medical record were characterized. Onychomycosis-related medications prescribed at these visits were reported and prescribing trends over time were evaluated. Differences in the treatment prescribed according to the type of visit (ie, first-time or return visit); physician specialty; and patients’ gender, race, and health conditions (eg, obesity, diabetes mellitus) were examined. To exclude the possibility that fluconazole and other broad-spectrum antifungals were being used for secondary diagnoses, we determined the number of visits that had an additional diagnosis of either candidiasis (ICD-9 codes 112.0–112.9) or “other specified erythematous conditions” (ICD-9 code 695.89).
Results
During the 18-year study period, 636 visits with a diagnosis of onychomycosis were recorded in the NAMCS database. This unweighted number of visits corresponded with approximately 19,350,000 visits (an average of 1,075,000 visits per year) to physicians’ offices with a diagnosis of onychomycosis in the United States during this period. Among these visits, there were an estimated 4,250,000 visits with fungal nail infection as the only diagnosis (no other comorbidities recorded). The recorded visits included more female (57.6%) than male (42.4%) patients, and 85% of patients were white (Table). Patients aged 35 to 44 years accounted for the largest number of visits; however, the estimated rate of onychomycosis visits per 100,000 US citizens was highest among those aged 65 to 74 years (Figure 1).
The number of US outpatient visits with a recorded diagnosis of onychomycosis increased from 1993 to 2010 (Figure 2); however, there was no change in the ratio of onychomycosis visits to the total number of recorded visits in NAMCS database during the study period (P=.9). A combined total of 91% of onychomycosis visits were to general and family practitioners, dermatologists, or internal medicine practitioners (Figure 3). Although cardiovascular diseases and diabetes mellitus accounted for a large proportion of comorbidities, conditions affecting the feet (eg, tinea pedis, ingrown nails) also were among the most common comorbidities (Figure 4).
 |
In both topical and systemic form, terbinafine was the most commonly prescribed antifungal agent, followed by systemic fluconazole, systemic itraconazole, and topical ciclopirox (Figure 5). Over the 18-year study period, there was an increasing trend in the frequency of terbinafine prescription (regression coefficient [r]=0.01319; P=.004); a decreasing trend for fluconazole (r=-0.0053851; P=.04), itraconazole (r=-0.0113988; P<.001), griseofulvin (r=-0.0073942; P<.001), and econazole prescription (r=-0.0032405; P=.01); and no significant trend for ketoconazole (r=-0.0034553; P=.1), naftifine (r=-0.0029067; P=.06), sulconazole (r=-0.0001619; P=.8), ciclopirox (r=0.0032684; P=.1), and miconazole prescription (r=0.0002074; P=.5).
Eighty-six percent of visits were for established patients who had been seen in the related office with any diagnosis before the recorded visit and 14% of visits were for new (first-time) patients. Fluconazole was the most frequently used antifungal drug for new patients, while terbinafine was the most frequently used in other visits. Terbinafine was the most frequently prescribed antifungal drug by general and family practitioners, dermatologists, internal medicine practitioners, and all other specialties not listed.
Terbinafine was the most frequently prescribed antifungal drug in both genders and in white and black patients. Itraconazole was the most frequently prescribed antifungal drug for Hispanic patients and those of other ethnicities not listed. Terbinafine was the most frequently prescribed antifungal drug for patients with diabetes and obesity (ie, body mass index ≥30). In 19,330,000 of 19,350,000 total estimated visits included in this study, onychomycosis was the only diagnosis with a potential indication for an antifungal drug therapy, ruling out the possibility that fluconazole or other drugs were used for patients who also had candidiasis or “other specified erythematous conditions.”
Discussion
Onychomycosis is a common progressive infection of the nails that is more prevalent in older age groups, with equal prevalence in both genders and a higher prevalence in males. The NAMCS data showed higher rates of onychomycosis visits among older age groups, which is in agreement with results from prior studies.16,17 In the current study, we observed a higher prevalence of onychomycosis visits among females as well as white and Hispanic patients. These results may be due to a higher prevalence of onychomycosis in these populations or simply a result of difference in socioeconomic level or importance of aesthetics. Although there are limited data regarding the prevalence of onychomycosis among different races and ethnicities in the United States, a high incidence of onychomycosis has been reported in Mexico.18
Repeated trauma to the great toenail from ill-fitting shoes is a predisposing factor for onychomycosis.16 In the current study, ingrown nails were among the most common comorbidities found in onychomycosis patients. Although nail dystrophy caused by onychomycosis may lead to ingrown nails, it also is possible that both conditions may be caused by trauma.
Patients with immunodeficiencies (eg, diabetes) may be predisposed to onychomycosis as well as its associated complications and morbidities (eg, cellulitis).16,19 Diabetes affects 4% to 22% of patients with onychomycosis in different populations, including Denmark, Mexico, and India.18,20,21 In our study, diabetes was among the most common recorded comorbidities reported during onychomycosis visits, with a prevalence of 3.4%. It is likely that many more visits involved patients with diabetes that had not been diagnosed or reported. With the increased risk for complications with diabetes, it is important for physicians to treat these patients when they have a nail infection.
The available systemic therapies for treatment of onychomycosis include griseofulvin, allylamines, and imidazoles. Comparison of griseofulvin with newer systemic antifungal agents such as terbinafine and itraconazole suggests that griseofulvin has lower efficacy and therefore is not a first-line treatment of onychomycosis.1 Terbinafine is the most active of the currently available antidermatophyte drugs both in vitro and in vivo, with synergistic effects with imidazoles and ciclopirox.1,22-27 A combination of topical and systemic therapies may improve cure rates of onychomycosis or possibly shorten the duration of therapy with the systemic agent.1,2 Treatment strategies can vary according to the specialty of the treating physician, with general practitioners often preferring monotherapies and dermatologists preferring combination therapies.28 In Europe, the most commonly prescribed medication for onychomycosis was topical amorolfine followed by systemic terbinafine and itraconazole.28 In the current study, we could not separate data for topical versus systemic terbinafine because the NAMCS uses similar names for reporting the drug; however, the rates of prescription for allylamines and imidazoles were nearly equal (Figure 5), with terbinafine showing an increased use over time as opposed to a decreased use of imidazoles. Although fluconazole is not approved by the US Food and Drug Administration for treatment of onychomycosis, oral fluconazole was the second most common treatment prescribed in our study. Griseofulvin, which is not considered as a drug of choice in onychomycosis,1 was prescribed in a small fraction of the visits, with a decreasing trend of usage over time.
Conclusion
Our analysis of the NAMCS data revealed that the treatment of onychomycosis in the United States is in accordance with recommendations in current guidelines. An encouraging finding was the notable downward trend in use of griseofulvin, suggesting that health care providers are changing practice to meet standard of care. Increased efforts must be made to uniformly modify practices in compliance with evidence-based recommendations and to minimize unnecessary risk and cost associated with use of drugs with lower efficacy.
1. Roberts DT, Taylor WD, Boyle J; British Association of Dermatologists. Guidelines for treatment of onychomycosis. Br J Dermatol. 2003;148:402-410.
2. Seebacher C, Brasch J, Abeck D, et al. Onychomycosis. Mycoses. 2007;50:321-327.
3. Summerbell RC, Kane J, Krajden S. Onychomycosis, tinea pedis and tinea manuum caused by non-dermatophytic filamentous fungi. Mycoses. 1989;32:609-619.
4. Murray SC, Dawber RP. Onychomycosis of toenails: orthopaedic and podiatric considerations. Australas J Dermatol. 2002;43:105-112.
5. Achten G, Wanet-Rouard J. Onychomycoses in the laboratory. Mykosen Suppl. 1978;1:125-127.
6. Haneke E, Roseeuw D. The scope of onychomycosis: epidemiology and clinical features. Int J Dermatol. 1999;38(suppl 2):7-12.
7. Haneke E. Fungal infections of the nail. Semin Dermatol. 1991;10:41-53.
8. Karmakar S, Kalla G, Joshi KR, et al. Dermatophytoses in a desert district of Western Rajasthan. Indian J Dermatol Venereol Leprol. 1995;61:280-283.
9. Drake LA. Guidelines of care for superficial mycotic infections of the skin: onychomycosis. Guidelines/Outcomes Committee. American Academy of Dermatology. J Am Acad Dermatol. 1996;34:116-121.
10. Roberts DT. Prevalence of dermatophyte onychomycosis in the United Kingdom: results of an omnibus survey. Br J Dermatol. 1992;126(suppl 39):23-27.
11. Drake LA, Scher RK, Smith EB, et al. Effect of onychomycosis on quality of life. J Am Acad Dermatol. 1998;38(5 pt 1):702-704.
12. Detandt M, Nolard N. Fungal contamination of the floors of swimming pools, particularly subtropical swimming paradises. Mycoses. 1995;38:509-513.
13. Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
14. Fleischer AB Jr, Feldman SR, Bradham DD. Office-based physician services provided by dermatologists in the United States in 1990. J Invest Dermatol. 1994;102:93-97.
15. 2001 NAMCS Micro-Data File Documentation. http://www.nber.org/namcs/docs/namcs2001.pdf. National Bureau of Economic Research Web site. Accessed April 27, 2015.
16. Williams HC. The epidemiology of onychomycosis in Britain. Br J Dermatol. 1993;129:101-109.
17. Elewski BE, Charif MA. Prevalence of onychomycosis in patients attending a dermatology clinic in northeastern Ohio for other conditions. Arch Dermatol. 1997;133:1172-1173.
18. Arenas R, Bonifaz A, Padilla MC, et al. Onychomycosis. a Mexican survey. Eur J Dermatol. 2010;20:611-614.
19. Faergemann J, Baran R. Epidemiology, clinical presentation and diagnosis of onychomycosis. Br J Dermatol. 2003;149(suppl 65):1-4.
20. Sarma S, Capoor MR, Deb M, et al. Epidemiologic and clinicomycologic profile of onychomycosis from north India. Int J Dermatol. 2008;47:584-587.
21. Svejgaard EL, Nilsson J. Onychomycosis in Denmark: prevalence of fungal nail infection in general practice. Mycoses. 2004;47:131-135.
22. Santos DA, Hamdan JS. In vitro antifungal oral drug and drug-combination activity against onychomycosis causative dermatophytes. Med Mycol. 2006;44:357-362.
23. Gupta AK, Kohli Y. In vitro susceptibility testing of ciclopirox, terbinafine, ketoconazole and itraconazole against dermatophytes and nondermatophytes, and in vitro evaluation of combination antifungal activity. Br J Dermatol. 2003;149:296-305.
24. Gupta AK, Lynch LE. Management of onychomycosis: examining the role of monotherapy and dual, triple, or quadruple therapies. Cutis. 2004;74(suppl 1):5-9.
25. Harman S, Ashbee HR, Evans EG. Testing of antifungal combinations against yeasts and dermatophytes. J Dermatolog Treat. 2004;15:104-107.
26. Spader TB, Venturini TP, Rossato L, et al. Synergisms of voriconazole or itraconazole combined with other antifungal agents against Fusarium spp. Rev Iberoam Micol. 2013;30:200-204.
27. Biancalana FS, Lyra L, Moretti ML, et al. Susceptibility testing of terbinafine alone and in combination with amphotericin B, itraconazole, or voriconazole against conidia and hyphae of dematiaceous molds. Diagn Microbiol Infect Dis. 2011;71:378-385.
28. Effendy I, Lecha M, Feuilhade de CM, et al. Epidemiology and clinical classification of onychomycosis. J Eur Acad Dermatol Venereol. 2005;19(suppl 1):8-12.
1. Roberts DT, Taylor WD, Boyle J; British Association of Dermatologists. Guidelines for treatment of onychomycosis. Br J Dermatol. 2003;148:402-410.
2. Seebacher C, Brasch J, Abeck D, et al. Onychomycosis. Mycoses. 2007;50:321-327.
3. Summerbell RC, Kane J, Krajden S. Onychomycosis, tinea pedis and tinea manuum caused by non-dermatophytic filamentous fungi. Mycoses. 1989;32:609-619.
4. Murray SC, Dawber RP. Onychomycosis of toenails: orthopaedic and podiatric considerations. Australas J Dermatol. 2002;43:105-112.
5. Achten G, Wanet-Rouard J. Onychomycoses in the laboratory. Mykosen Suppl. 1978;1:125-127.
6. Haneke E, Roseeuw D. The scope of onychomycosis: epidemiology and clinical features. Int J Dermatol. 1999;38(suppl 2):7-12.
7. Haneke E. Fungal infections of the nail. Semin Dermatol. 1991;10:41-53.
8. Karmakar S, Kalla G, Joshi KR, et al. Dermatophytoses in a desert district of Western Rajasthan. Indian J Dermatol Venereol Leprol. 1995;61:280-283.
9. Drake LA. Guidelines of care for superficial mycotic infections of the skin: onychomycosis. Guidelines/Outcomes Committee. American Academy of Dermatology. J Am Acad Dermatol. 1996;34:116-121.
10. Roberts DT. Prevalence of dermatophyte onychomycosis in the United Kingdom: results of an omnibus survey. Br J Dermatol. 1992;126(suppl 39):23-27.
11. Drake LA, Scher RK, Smith EB, et al. Effect of onychomycosis on quality of life. J Am Acad Dermatol. 1998;38(5 pt 1):702-704.
12. Detandt M, Nolard N. Fungal contamination of the floors of swimming pools, particularly subtropical swimming paradises. Mycoses. 1995;38:509-513.
13. Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
14. Fleischer AB Jr, Feldman SR, Bradham DD. Office-based physician services provided by dermatologists in the United States in 1990. J Invest Dermatol. 1994;102:93-97.
15. 2001 NAMCS Micro-Data File Documentation. http://www.nber.org/namcs/docs/namcs2001.pdf. National Bureau of Economic Research Web site. Accessed April 27, 2015.
16. Williams HC. The epidemiology of onychomycosis in Britain. Br J Dermatol. 1993;129:101-109.
17. Elewski BE, Charif MA. Prevalence of onychomycosis in patients attending a dermatology clinic in northeastern Ohio for other conditions. Arch Dermatol. 1997;133:1172-1173.
18. Arenas R, Bonifaz A, Padilla MC, et al. Onychomycosis. a Mexican survey. Eur J Dermatol. 2010;20:611-614.
19. Faergemann J, Baran R. Epidemiology, clinical presentation and diagnosis of onychomycosis. Br J Dermatol. 2003;149(suppl 65):1-4.
20. Sarma S, Capoor MR, Deb M, et al. Epidemiologic and clinicomycologic profile of onychomycosis from north India. Int J Dermatol. 2008;47:584-587.
21. Svejgaard EL, Nilsson J. Onychomycosis in Denmark: prevalence of fungal nail infection in general practice. Mycoses. 2004;47:131-135.
22. Santos DA, Hamdan JS. In vitro antifungal oral drug and drug-combination activity against onychomycosis causative dermatophytes. Med Mycol. 2006;44:357-362.
23. Gupta AK, Kohli Y. In vitro susceptibility testing of ciclopirox, terbinafine, ketoconazole and itraconazole against dermatophytes and nondermatophytes, and in vitro evaluation of combination antifungal activity. Br J Dermatol. 2003;149:296-305.
24. Gupta AK, Lynch LE. Management of onychomycosis: examining the role of monotherapy and dual, triple, or quadruple therapies. Cutis. 2004;74(suppl 1):5-9.
25. Harman S, Ashbee HR, Evans EG. Testing of antifungal combinations against yeasts and dermatophytes. J Dermatolog Treat. 2004;15:104-107.
26. Spader TB, Venturini TP, Rossato L, et al. Synergisms of voriconazole or itraconazole combined with other antifungal agents against Fusarium spp. Rev Iberoam Micol. 2013;30:200-204.
27. Biancalana FS, Lyra L, Moretti ML, et al. Susceptibility testing of terbinafine alone and in combination with amphotericin B, itraconazole, or voriconazole against conidia and hyphae of dematiaceous molds. Diagn Microbiol Infect Dis. 2011;71:378-385.
28. Effendy I, Lecha M, Feuilhade de CM, et al. Epidemiology and clinical classification of onychomycosis. J Eur Acad Dermatol Venereol. 2005;19(suppl 1):8-12.
Practice Points
- Onychomycosis is a common progressive infection of the nails that may result in remarkable morbidity. Effective treatment may reduce the rate of transmission and related morbidities.
- Onychomycosis is most commonly found in patients older than 35 years.
- Terbinafine has been the most commonly prescribed antifungal agent for onychomycosis in the United States between 1993 and 2010, followed by systemic fluconazole, systemic itraconazole, and topical ciclopirox.
The Rosacea Patient Journey: A Novel Approach to Conceptualizing Patient Experiences
Rosacea patients experience symptoms ranging from flushing to persistent acnelike rashes that can cause low self-esteem and anxiety, leading to social and professional isolation.1 Although it is estimated that 16 million individuals in the United States have rosacea, only 10% seek treatment.2,3 The motivation for patients to seek and adhere to treatment is not well characterized.
A patient journey is a map of the steps a patient takes as he/she progresses through different segments of the disease from diagnosis to management, including all the influences that can push him/her toward or away from certain decisions. The patient journey model provides a structure for understanding key issues in rosacea management, including barriers to successful treatment outcomes.
The patient journey model progresses from development of disease and diagnosis to treatment and disease management (Figure). We sought to examine each step of the rosacea patient journey to better understand key patient care boundaries faced by rosacea patients. We assessed the current literature regarding each step of the patient experience and identified areas of the patient journey with limited research.
Click here to view the figure as a PDF to print for future reference.
Researching the Patient Experience
A PubMed search of articles indexed for MEDLINE as well as a search of the National Rosacea Society Web site (http://www.rosacea.org) were conducted to identify articles and materials that quantitatively or qualitatively described rosacea patient experiences. Search terms included rosacea, rosacea patient experience, rosacea treatment, rosacea adherence, and rosacea quality of life. A Google search also was conducted using the same terms to obtain current news articles online. Current literature pertaining to the patient journey was summarized.
To create a model for the rosacea patient journey, we refined a rheumatoid arthritis patient journey map4 and included the critical components of the journey for rosacea patients. We organized the journey into stages, including prediagnosis, diagnosis, treatment, adherence, and management. We first explored what occurs prior to diagnosis, which includes the patient’s symptoms before visiting a physician. We then examined the process of diagnosis and the implementation of a treatment plan. Treatment adherence was then explored, ending with the ways patients self-manage their disease beyond the physician’s office.
Prediagnosis: What Motivates Patients to Seek Treatment
Rosacea can present with many symptoms that may lead patients to see a physician, including facial erythema and telangiectases, papules and pustules, phymatous changes, and ocular manifestations.5 The most common concern is temporary facial flushing, followed by persistent redness, then bumps and pimples.6 Many patients seek treatment after persistent facial flushing and an intolerable burning sensation. Some middle-aged patients decide to see a dermatologist for the first time when they break out in acne lesions after a history of clear skin. Others seek treatment because they can no longer tolerate the pain and embarrassment associated with their symptoms. However, patients who seek treatment only account for a small proportion of patients with rosacea, as only 10% of patients seek conventional medical treatment.7 Furthermore, symptomatic patients on average wait 7 months to 5 years before receiving a diagnosis.8,9
Care often is delayed or not pursued because many rosacea symptoms are mild when they first appear and may not initially bother the patient. Patients may not think anything of their symptoms and dismiss them as either acne vulgaris or sunburn. Due to the relapsing and remitting nature of the disease course, patients may feel their symptoms will resolve. Of patients diagnosed with rosacea, only one-half have heard of the condition prior to diagnosis,8 which can largely be attributed to lack of patient education on the signs and symptoms of rosacea, a concern that prompted the National Rosacea Society to designate the month of April as rosacea awareness month.5
With sales of antiredness facial care products growing 35% from 2002 to 2007, accounting for an increase of $300 million in revenue, patients also may be turning to over-the-counter products first.10 Men with rosacea tend to present with more severe symptoms such as rhinophyma, which may be due to their desire to wait until their symptoms reached more advanced stages of disease before seeking medical help.5
Diagnosis of Rosacea
After the patient decides that his/her symptoms are unusual, severe, or intolerable enough to seek treatment, the issues of access to dermatologic care and receiving the correct diagnosis come into play. Accessing dermatologic care can be difficult, as appointments may be hard to obtain, and even if the patient is able to get an appointment, it could be many weeks later.11 For some rosacea patients, the anxiety of waiting for their appointment prompts them to seek support and advice from online message boards (eg, http://www.rosacea-support.org). The long wait for appointments may be attributed to the increased demand for dermatologists for cosmetic procedures.12 Additionally, disparities according to insurance type can contribute to difficulties procuring an appointment. In one study, privately insured dermatology patients demonstrated a 91% acceptance rate and shorter wait times for appointments compared to publicly insured patients who were limited to a 29.8% acceptance rate and longer wait times.11 Many patients then are left to wait for an appointment with a dermatologist or instead turn to a primary care physician. Of patients diagnosed with rosacea in one study (N=2847), the majority of patients were seen by a dermatologist (79%), while the other patients were diagnosed by a family physician (14%) or other types of physicians such as internists and ophthalmologists (7%).6
The diagnosis of rosacea usually is not a major hurdle for dermatologists, but misdiagnoses can sometimes occur. The Rosacea Research & Development Institute compiled multiple patient anecdotes describing the struggles of finally reaching the correct diagnosis of rosacea; however, no estimates as to the frequency of misdiagnoses was estimated.13 Even with an accurate diagnosis of rosacea, correct classification of the 4 types of rosacea (ie, erythematotelangiectatic, papulopustular, phymatous, ocular) is necessary to avoid incorrect treatment recommendations. For example, patients with flushing often cannot tolerate topical medications in contrast to patients with the papulopustular subtype who benefit from them.14 In the meantime, the patients who are misdiagnosed may be met with frustration, as treatment was either delayed or incorrectly prescribed.
Although there are limited data regarding patient reactions after receiving a diagnosis of rosacea, it can be assumed that patients would be hopeful that diagnosis would lead to correct treatment. In a 2008 article in The New York Times, a rosacea patient was described as feeling relieved to be diagnosed with rosacea because it was an explanation for the development of pimples on the cheeks in her late 40s.10
Implementation of a Treatment Plan
After recognizing the symptoms and receiving a correct diagnosis, the next step in the patient journey is treatment. Long-term management of incurable conditions such as rosacea is difficult. The main goals of treatment are to relieve symptoms, improve appearance, delay progression to advanced stages, and maintain remission.15 There are only a few reliable clinical trials regarding therapies for rosacea, so treatment has mostly relied on clinical experience (Table). The efficacy and safety of many older treatments has not been assessed.15 Mainstays of treatment include both topical agents and oral medications. The use of topical metronidazole, oral tetracycline, and oral isotretinoin have been found to improve both skin lesions and quality of life.18 Initially, a combination of a topical and an oral medication may be used for at least the first 12 weeks, and improvement is usually gradual, taking many weeks to become evident.15 Long-term treatment with topical medications often is required for maintenance, which can last another 6 months or more.19,20
Besides using pharmacologic therapies, some patients also may choose to undergo various procedures. The most common procedure is laser therapy, followed by dermabrasion, chemical peels, hot loop electrocoagulation, and surgical sculpting or plastic surgery.6 The use of these adjunct therapies may suggest impatience from the patient for improvement; it also indicates the lengths patients will go to and willingness to pay for improvement of symptoms.
Along with medication, patients are recommended to make changes to their skin care regimen and lifestyle. Rosacea patients typically have sensitive skin that may include symptoms such as dryness, scaling, stinging, burning, and pruritus.16 Skin care recommendations for rosacea patients include using a gentle cleanser and regularly applying sunscreen.5 Issues with physical appearance can be addressed with the use of cosmetic products such as green-tinted makeup to conceal skin lesions.21 Remission can be maintained by identifying certain triggers (eg, red wine, spicy foods, extreme temperatures, prolonged sun exposure, vigorous exercise) that can cause flare-ups.15 The most common trigger is sun exposure, making photoprotection an important component of the rosacea patient’s skin care regimen.6
Adherence
With a diagnosis and treatment plan in effect, the patient journey reaches the stage of treatment adherence, which should include ongoing education about the condition. Self-reported statistics from rosacea patients indicated that 28% of patients took time off from their treatment regimen,6 but actual nonadherence rates likely are higher. The most commonly reported reason for poor treatment adherence among rosacea patients was the impression that the symptoms had resolved or were adequately controlled.6 Treatment also must be affordable. In a national survey of rosacea patients, 24% of 427 patients receiving pharmacologic therapy planned on switching medications because of cost, and 17% of 769 patients discontinued medications due to co-pay/insurance issues.6 Other reasons cited for discontinuation of treatment included patient perception that symptoms were not that serious, co-pay/insurance issues, ineffectiveness of the medication, and side effects.6 Adherence to topical medications is lower than oral medications due to the time and inconvenience required for application.22 For some patients, topical medications may be too messy, have a strange odor, or stain clothing.
It is promising that most rosacea patients have reported the intent to continue using pharmacologic agents because the medication prevented worsening of their symptoms.6 However, there are still patients who switch or discontinue therapies without physician direction. These patients often cite that they desire more information at the time of diagnosis, particularly related to causes of flare-ups, physical symptoms to expect, drug treatment options, makeup to cover up visible symptoms, surgical or laser treatment options, psychological symptoms, patient support groups, and counseling options.6
Management
The last part of the journey is disease management, which occurs when the patient learns how to control his/her symptoms long-term. Important factors contributing to long-term control of rosacea flares are medication adherence and avoiding lifestyle triggers.23,24 Through the other stages of the journey, the patient has learned which treatments work and which factors may lead to exacerbation of symptoms.
Educating Patients on the Journey
The patient journey is a concept that can be applied to any disease state and brings to light roadblocks that patients may face from the initial diagnosis to successful disease management. Rosacea patients are faced with confusing and aggravating symptoms that can cause anxiety and may lead them to seek treatment from a physician. Facial flushing and phymatous changes of the nose can be mistaken for alcohol abuse, leading rosacea to be a socially stigmatizing disease.15 Because rosacea involves mostly the facial skin, it can disrupt social and professional interactions, leading to quality-of-life effects such as difficulty functioning on a day-to-day basis, which can be detrimental because patients usually are aged 30 to 50 years and may be perceived based on their appearance in the workforce.3 A lack of confidence, low self-esteem, embarrassment, and anxiety can even lead to serious psychiatric conditions such as depression and body dysmorphic disorder.25 Because the severity of rosacea increases over time, it is important to educate patients about seeking early treatment; therefore, understanding and awareness of rosacea symptoms are necessary to prompt patients to see a medical professional to either confirm or refute the diagnosis.
Rosacea is a clinical diagnosis that relies on patterns of primary and secondary features, as outlined in a 2002 report by the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea.5 Even with this consensus grading system, it appears that additional fine-tuning of the criteria is needed in the disease definition. Importantly, because much of the pathogenesis and progression of rosacea is still not completely understood, there is no laboratory benchmark test that can be utilized for correct diagnosis.14 Moreover, many of the clinical manifestations of rosacea are shared with other conditions, and patients may present with different symptoms or varying combinations.26
Treatment of rosacea is multifactorial and behavioral, as patients must not only be able to obtain and adhere to oral and topical regimens and possible procedures but also avoid various lifestyle and environmental triggers and learn to cope with emotional distress caused by their symptoms. Although patients who discontinue use of medications appear to be in the minority, education is still needed to stress the chronic nature of rosacea and the importance of the continuation of treatment. Collaboration between the physician and patient is needed to determine why a certain medication may not be effective and explore other treatment options. Treatment ineffectiveness could be due to incorrect use of the product, failure to use an adjunct skin care regimen, or inability to control rosacea triggers. Adequate early follow-up also is needed to maximize patient adherence to treatment.27 Working together with the patient to develop a treatment plan that can be followed is necessary for long-term control of rosacea symptoms.
There is little information on how to address the psychological needs of patients, but patients can find support from various avenues. For instance, the National Rosacea Society, a large advocacy group, produces newsletters and educational materials for both physicians and patients.28,29 There also are online support groups for rosacea patients that have thousands of members who exchange stories and provide words of encouragement. Although there are not many face-to-face support groups, physicians may consider developing live support groups for their rosacea patients. As patients achieve the later stages of the rosacea patient journey, they hopefully will have controlled their symptoms by following a treatment regimen and learning to adapt to a new life of successful disease management.
Many aspects of the rosacea patient journey have yet to be explored. It is uncertain how long patients with symptoms of rosacea wait before seeking treatment, what methods they use to control their rosacea before they receive a prescribed treatment or physician recommendations, and how they react to their diagnosis. It also is unknown how many rosacea patients receive an initial misdiagnosis of another condition and which physicians typically make the misdiagnosis. We also need to know more about the role of psychological issues in addressing patient adherence to treatment. Similarly, what role do support groups such as online forums play on adherence? There is a need for more patient education and awareness of rosacea.
Conclusion
Patients may be relieved that rosacea is not a life-threatening condition, but they may be disappointed that there is no cure for rosacea. As the patient and dermatologist work together to find an appropriate treatment plan, identify certain triggers, and modify the skin care routine, the patient can become disciplined in controlling rosacea symptoms. Ultimately, with the alleviation of visible symptoms, the patient’s quality of life also can improve. Better understanding of the rosacea patient perspective can lead to a more efficient health care system, improved patient care, and better patient satisfaction.
1. Baldwin HE. Systemic therapy for rosacea. Skin Therapy Lett. 2007;12:1-5, 9.
2. Drake L. Rosacea now estimated to affect at least 16 million Americans. Rosacea Review. Winter 2010. http://www.rosacea.org/rr/2010/winter/article_1.php. Accessed December 11, 2014.
3. Rosacea as an inflammatory disease: an expert interview with Brian Berman, MD, PhD. Medscape Web site. http://www.medscape.org/viewarticle/722156. Published May 27, 2010. Accessed December 11, 2014.
4. HealthEd Group, Inc. Rheumatoid arthritis patient journey map. http://visual.ly/rheumatoid-arthritis-patient-journey-map. Accessed December 19, 2014.
5. Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
6. Elewski BE. Results of a national rosacea patient survey: common issues that concern rosacea sufferers. J Drugs Dermatol. 2009;8:120-123.
7. Del Rosso J. Management of rosacea in the United States: analysis based on recent prescribing patterns and insurance claims. J Am Acad Dermatol. 2008;58:AB13.
8. New survey reveals first impressions may not always be rosy for people with the widespread skin condition rosacea. Medical News Today Web site. http://www.medicalnew today.com/releases/185491.php. Updated April 15, 2010. Accessed December 12, 2014.
9. Shear NH, Levine C. Needs survey of Canadian rosacea patients. J Cutan Med Surg. 1999;3:178-181.
10. Sweeney C. In a perfect world, rosacea remains a problem. New York Times. April 24, 2008. http://www.nytimes.com/2008/04/24/fashion/24SKIN.html?pagewanted=all. Accessed December 12, 2014.
11. Alghothani L, Jacks SK, Vander HA, et al. Disparities in access to dermatologic care according to insurance type. Arch Dermatol. 2012;148:956-957.
12. Resneck J Jr. Too few or too many dermatologists? difficulties in assessing optimal workforce size. Arch Dermatol. 2001;137:1295-1301.
13. Rosacea Research & Development Institute Web site. http://irosacea.org/misdiagnosed_rosacea.html. Accessed December 19, 2014.
14. Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341.
15. Elewski BE, Draelos Z, Dreno B, et al. Rosacea—global diversity and optimized outcome: proposed international consensus from the Rosacea International Expert Group. J Eur Acad Dermatol Venereol. 2011;25:188-200.
16. Del Rosso JQ, Baldwin H, Webster G. American Acne & Rosacea Society rosacea medical management guidelines. J Drugs Dermatol. 2008;7:531-533.
17. Fowler J Jr, Jackson M, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12:650-656.
18. Aksoy B, Altaykan-Hapa A, Egemen D, et al. The impact of rosacea on quality of life: effects of demographic and clinical characteristics and various treatment modalities. Br J Dermatol. 2010;163:719-725.
19. Dahl MV, Katz HI, Krueger GG, et al. Topical metronidazole maintains remissions of rosacea. Arch Dermatol. 1998;134:679-683.
20. Thiboutot DM, Fleischer AB, Del Rosso JQ, et al. A multicenter study of topical azelaic acid 15% gel in combination with oral doxycycline as initial therapy and azelaic acid 15% gel as maintenance monotherapy. J Drugs Dermatol. 2009;8:639-648.
21. Boehncke WH, Ochsendorf F, Paeslack I, et al. Decorative cosmetics improve the quality of life in patients with disfiguring skin diseases. Eur J Dermatol. 2002;12:577-580.
22. Jackson JM, Pelle M. Topical rosacea therapy: the importance of vehicles for efficacy, tolerability and compliance. J Drugs Dermatol. 2011;10:627-633.
23. Wolf JE Jr. Medication adherence: a key factor in effective management of rosacea. Adv Ther. 2001;18:272-281.
24. Managing rosacea. National Rosacea Society Web site. http://www.rosacea.org/patients/materials/managing/lifestyle.php. Accessed December 19, 2014.
25. van Zuuren EJ, Fedorowicz Z. Lack of ‘appropriately assessed’ patient-reported outcomes in randomized controlled trials assessing the effectiveness of interventions for rosacea. Br J Dermatol. 2013;168:442-444.
26. Del Rosso JQ. Advances in understanding and managing rosacea: part 2: the central role, evaluation, and medical management of diffuse and persistent facial erythema of rosacea. J Clin Aesthet Dermatol. 2012;5:26-36.
27. Davis SA, Lin HC, Yu CH, et al. Underuse of early follow-up visits: a missed opportunity to improve patients’ adherence. 2014;13:833-836.
28. If you have rosacea, you’re not alone. National Rosacea Society Web site. http://www.rosacea.org/patients/index.php. Accessed December 19, 2014.
29. Tools for the professional. National Rosacea Society Web site. http://www.rosacea.org/physicians/index.php. Accessed December 19, 2014.
Rosacea patients experience symptoms ranging from flushing to persistent acnelike rashes that can cause low self-esteem and anxiety, leading to social and professional isolation.1 Although it is estimated that 16 million individuals in the United States have rosacea, only 10% seek treatment.2,3 The motivation for patients to seek and adhere to treatment is not well characterized.
A patient journey is a map of the steps a patient takes as he/she progresses through different segments of the disease from diagnosis to management, including all the influences that can push him/her toward or away from certain decisions. The patient journey model provides a structure for understanding key issues in rosacea management, including barriers to successful treatment outcomes.
The patient journey model progresses from development of disease and diagnosis to treatment and disease management (Figure). We sought to examine each step of the rosacea patient journey to better understand key patient care boundaries faced by rosacea patients. We assessed the current literature regarding each step of the patient experience and identified areas of the patient journey with limited research.
Click here to view the figure as a PDF to print for future reference.
Researching the Patient Experience
A PubMed search of articles indexed for MEDLINE as well as a search of the National Rosacea Society Web site (http://www.rosacea.org) were conducted to identify articles and materials that quantitatively or qualitatively described rosacea patient experiences. Search terms included rosacea, rosacea patient experience, rosacea treatment, rosacea adherence, and rosacea quality of life. A Google search also was conducted using the same terms to obtain current news articles online. Current literature pertaining to the patient journey was summarized.
To create a model for the rosacea patient journey, we refined a rheumatoid arthritis patient journey map4 and included the critical components of the journey for rosacea patients. We organized the journey into stages, including prediagnosis, diagnosis, treatment, adherence, and management. We first explored what occurs prior to diagnosis, which includes the patient’s symptoms before visiting a physician. We then examined the process of diagnosis and the implementation of a treatment plan. Treatment adherence was then explored, ending with the ways patients self-manage their disease beyond the physician’s office.
Prediagnosis: What Motivates Patients to Seek Treatment
Rosacea can present with many symptoms that may lead patients to see a physician, including facial erythema and telangiectases, papules and pustules, phymatous changes, and ocular manifestations.5 The most common concern is temporary facial flushing, followed by persistent redness, then bumps and pimples.6 Many patients seek treatment after persistent facial flushing and an intolerable burning sensation. Some middle-aged patients decide to see a dermatologist for the first time when they break out in acne lesions after a history of clear skin. Others seek treatment because they can no longer tolerate the pain and embarrassment associated with their symptoms. However, patients who seek treatment only account for a small proportion of patients with rosacea, as only 10% of patients seek conventional medical treatment.7 Furthermore, symptomatic patients on average wait 7 months to 5 years before receiving a diagnosis.8,9
Care often is delayed or not pursued because many rosacea symptoms are mild when they first appear and may not initially bother the patient. Patients may not think anything of their symptoms and dismiss them as either acne vulgaris or sunburn. Due to the relapsing and remitting nature of the disease course, patients may feel their symptoms will resolve. Of patients diagnosed with rosacea, only one-half have heard of the condition prior to diagnosis,8 which can largely be attributed to lack of patient education on the signs and symptoms of rosacea, a concern that prompted the National Rosacea Society to designate the month of April as rosacea awareness month.5
With sales of antiredness facial care products growing 35% from 2002 to 2007, accounting for an increase of $300 million in revenue, patients also may be turning to over-the-counter products first.10 Men with rosacea tend to present with more severe symptoms such as rhinophyma, which may be due to their desire to wait until their symptoms reached more advanced stages of disease before seeking medical help.5
Diagnosis of Rosacea
After the patient decides that his/her symptoms are unusual, severe, or intolerable enough to seek treatment, the issues of access to dermatologic care and receiving the correct diagnosis come into play. Accessing dermatologic care can be difficult, as appointments may be hard to obtain, and even if the patient is able to get an appointment, it could be many weeks later.11 For some rosacea patients, the anxiety of waiting for their appointment prompts them to seek support and advice from online message boards (eg, http://www.rosacea-support.org). The long wait for appointments may be attributed to the increased demand for dermatologists for cosmetic procedures.12 Additionally, disparities according to insurance type can contribute to difficulties procuring an appointment. In one study, privately insured dermatology patients demonstrated a 91% acceptance rate and shorter wait times for appointments compared to publicly insured patients who were limited to a 29.8% acceptance rate and longer wait times.11 Many patients then are left to wait for an appointment with a dermatologist or instead turn to a primary care physician. Of patients diagnosed with rosacea in one study (N=2847), the majority of patients were seen by a dermatologist (79%), while the other patients were diagnosed by a family physician (14%) or other types of physicians such as internists and ophthalmologists (7%).6
The diagnosis of rosacea usually is not a major hurdle for dermatologists, but misdiagnoses can sometimes occur. The Rosacea Research & Development Institute compiled multiple patient anecdotes describing the struggles of finally reaching the correct diagnosis of rosacea; however, no estimates as to the frequency of misdiagnoses was estimated.13 Even with an accurate diagnosis of rosacea, correct classification of the 4 types of rosacea (ie, erythematotelangiectatic, papulopustular, phymatous, ocular) is necessary to avoid incorrect treatment recommendations. For example, patients with flushing often cannot tolerate topical medications in contrast to patients with the papulopustular subtype who benefit from them.14 In the meantime, the patients who are misdiagnosed may be met with frustration, as treatment was either delayed or incorrectly prescribed.
Although there are limited data regarding patient reactions after receiving a diagnosis of rosacea, it can be assumed that patients would be hopeful that diagnosis would lead to correct treatment. In a 2008 article in The New York Times, a rosacea patient was described as feeling relieved to be diagnosed with rosacea because it was an explanation for the development of pimples on the cheeks in her late 40s.10
Implementation of a Treatment Plan
After recognizing the symptoms and receiving a correct diagnosis, the next step in the patient journey is treatment. Long-term management of incurable conditions such as rosacea is difficult. The main goals of treatment are to relieve symptoms, improve appearance, delay progression to advanced stages, and maintain remission.15 There are only a few reliable clinical trials regarding therapies for rosacea, so treatment has mostly relied on clinical experience (Table). The efficacy and safety of many older treatments has not been assessed.15 Mainstays of treatment include both topical agents and oral medications. The use of topical metronidazole, oral tetracycline, and oral isotretinoin have been found to improve both skin lesions and quality of life.18 Initially, a combination of a topical and an oral medication may be used for at least the first 12 weeks, and improvement is usually gradual, taking many weeks to become evident.15 Long-term treatment with topical medications often is required for maintenance, which can last another 6 months or more.19,20
Besides using pharmacologic therapies, some patients also may choose to undergo various procedures. The most common procedure is laser therapy, followed by dermabrasion, chemical peels, hot loop electrocoagulation, and surgical sculpting or plastic surgery.6 The use of these adjunct therapies may suggest impatience from the patient for improvement; it also indicates the lengths patients will go to and willingness to pay for improvement of symptoms.
Along with medication, patients are recommended to make changes to their skin care regimen and lifestyle. Rosacea patients typically have sensitive skin that may include symptoms such as dryness, scaling, stinging, burning, and pruritus.16 Skin care recommendations for rosacea patients include using a gentle cleanser and regularly applying sunscreen.5 Issues with physical appearance can be addressed with the use of cosmetic products such as green-tinted makeup to conceal skin lesions.21 Remission can be maintained by identifying certain triggers (eg, red wine, spicy foods, extreme temperatures, prolonged sun exposure, vigorous exercise) that can cause flare-ups.15 The most common trigger is sun exposure, making photoprotection an important component of the rosacea patient’s skin care regimen.6
Adherence
With a diagnosis and treatment plan in effect, the patient journey reaches the stage of treatment adherence, which should include ongoing education about the condition. Self-reported statistics from rosacea patients indicated that 28% of patients took time off from their treatment regimen,6 but actual nonadherence rates likely are higher. The most commonly reported reason for poor treatment adherence among rosacea patients was the impression that the symptoms had resolved or were adequately controlled.6 Treatment also must be affordable. In a national survey of rosacea patients, 24% of 427 patients receiving pharmacologic therapy planned on switching medications because of cost, and 17% of 769 patients discontinued medications due to co-pay/insurance issues.6 Other reasons cited for discontinuation of treatment included patient perception that symptoms were not that serious, co-pay/insurance issues, ineffectiveness of the medication, and side effects.6 Adherence to topical medications is lower than oral medications due to the time and inconvenience required for application.22 For some patients, topical medications may be too messy, have a strange odor, or stain clothing.
It is promising that most rosacea patients have reported the intent to continue using pharmacologic agents because the medication prevented worsening of their symptoms.6 However, there are still patients who switch or discontinue therapies without physician direction. These patients often cite that they desire more information at the time of diagnosis, particularly related to causes of flare-ups, physical symptoms to expect, drug treatment options, makeup to cover up visible symptoms, surgical or laser treatment options, psychological symptoms, patient support groups, and counseling options.6
Management
The last part of the journey is disease management, which occurs when the patient learns how to control his/her symptoms long-term. Important factors contributing to long-term control of rosacea flares are medication adherence and avoiding lifestyle triggers.23,24 Through the other stages of the journey, the patient has learned which treatments work and which factors may lead to exacerbation of symptoms.
Educating Patients on the Journey
The patient journey is a concept that can be applied to any disease state and brings to light roadblocks that patients may face from the initial diagnosis to successful disease management. Rosacea patients are faced with confusing and aggravating symptoms that can cause anxiety and may lead them to seek treatment from a physician. Facial flushing and phymatous changes of the nose can be mistaken for alcohol abuse, leading rosacea to be a socially stigmatizing disease.15 Because rosacea involves mostly the facial skin, it can disrupt social and professional interactions, leading to quality-of-life effects such as difficulty functioning on a day-to-day basis, which can be detrimental because patients usually are aged 30 to 50 years and may be perceived based on their appearance in the workforce.3 A lack of confidence, low self-esteem, embarrassment, and anxiety can even lead to serious psychiatric conditions such as depression and body dysmorphic disorder.25 Because the severity of rosacea increases over time, it is important to educate patients about seeking early treatment; therefore, understanding and awareness of rosacea symptoms are necessary to prompt patients to see a medical professional to either confirm or refute the diagnosis.
Rosacea is a clinical diagnosis that relies on patterns of primary and secondary features, as outlined in a 2002 report by the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea.5 Even with this consensus grading system, it appears that additional fine-tuning of the criteria is needed in the disease definition. Importantly, because much of the pathogenesis and progression of rosacea is still not completely understood, there is no laboratory benchmark test that can be utilized for correct diagnosis.14 Moreover, many of the clinical manifestations of rosacea are shared with other conditions, and patients may present with different symptoms or varying combinations.26
Treatment of rosacea is multifactorial and behavioral, as patients must not only be able to obtain and adhere to oral and topical regimens and possible procedures but also avoid various lifestyle and environmental triggers and learn to cope with emotional distress caused by their symptoms. Although patients who discontinue use of medications appear to be in the minority, education is still needed to stress the chronic nature of rosacea and the importance of the continuation of treatment. Collaboration between the physician and patient is needed to determine why a certain medication may not be effective and explore other treatment options. Treatment ineffectiveness could be due to incorrect use of the product, failure to use an adjunct skin care regimen, or inability to control rosacea triggers. Adequate early follow-up also is needed to maximize patient adherence to treatment.27 Working together with the patient to develop a treatment plan that can be followed is necessary for long-term control of rosacea symptoms.
There is little information on how to address the psychological needs of patients, but patients can find support from various avenues. For instance, the National Rosacea Society, a large advocacy group, produces newsletters and educational materials for both physicians and patients.28,29 There also are online support groups for rosacea patients that have thousands of members who exchange stories and provide words of encouragement. Although there are not many face-to-face support groups, physicians may consider developing live support groups for their rosacea patients. As patients achieve the later stages of the rosacea patient journey, they hopefully will have controlled their symptoms by following a treatment regimen and learning to adapt to a new life of successful disease management.
Many aspects of the rosacea patient journey have yet to be explored. It is uncertain how long patients with symptoms of rosacea wait before seeking treatment, what methods they use to control their rosacea before they receive a prescribed treatment or physician recommendations, and how they react to their diagnosis. It also is unknown how many rosacea patients receive an initial misdiagnosis of another condition and which physicians typically make the misdiagnosis. We also need to know more about the role of psychological issues in addressing patient adherence to treatment. Similarly, what role do support groups such as online forums play on adherence? There is a need for more patient education and awareness of rosacea.
Conclusion
Patients may be relieved that rosacea is not a life-threatening condition, but they may be disappointed that there is no cure for rosacea. As the patient and dermatologist work together to find an appropriate treatment plan, identify certain triggers, and modify the skin care routine, the patient can become disciplined in controlling rosacea symptoms. Ultimately, with the alleviation of visible symptoms, the patient’s quality of life also can improve. Better understanding of the rosacea patient perspective can lead to a more efficient health care system, improved patient care, and better patient satisfaction.
Rosacea patients experience symptoms ranging from flushing to persistent acnelike rashes that can cause low self-esteem and anxiety, leading to social and professional isolation.1 Although it is estimated that 16 million individuals in the United States have rosacea, only 10% seek treatment.2,3 The motivation for patients to seek and adhere to treatment is not well characterized.
A patient journey is a map of the steps a patient takes as he/she progresses through different segments of the disease from diagnosis to management, including all the influences that can push him/her toward or away from certain decisions. The patient journey model provides a structure for understanding key issues in rosacea management, including barriers to successful treatment outcomes.
The patient journey model progresses from development of disease and diagnosis to treatment and disease management (Figure). We sought to examine each step of the rosacea patient journey to better understand key patient care boundaries faced by rosacea patients. We assessed the current literature regarding each step of the patient experience and identified areas of the patient journey with limited research.
Click here to view the figure as a PDF to print for future reference.
Researching the Patient Experience
A PubMed search of articles indexed for MEDLINE as well as a search of the National Rosacea Society Web site (http://www.rosacea.org) were conducted to identify articles and materials that quantitatively or qualitatively described rosacea patient experiences. Search terms included rosacea, rosacea patient experience, rosacea treatment, rosacea adherence, and rosacea quality of life. A Google search also was conducted using the same terms to obtain current news articles online. Current literature pertaining to the patient journey was summarized.
To create a model for the rosacea patient journey, we refined a rheumatoid arthritis patient journey map4 and included the critical components of the journey for rosacea patients. We organized the journey into stages, including prediagnosis, diagnosis, treatment, adherence, and management. We first explored what occurs prior to diagnosis, which includes the patient’s symptoms before visiting a physician. We then examined the process of diagnosis and the implementation of a treatment plan. Treatment adherence was then explored, ending with the ways patients self-manage their disease beyond the physician’s office.
Prediagnosis: What Motivates Patients to Seek Treatment
Rosacea can present with many symptoms that may lead patients to see a physician, including facial erythema and telangiectases, papules and pustules, phymatous changes, and ocular manifestations.5 The most common concern is temporary facial flushing, followed by persistent redness, then bumps and pimples.6 Many patients seek treatment after persistent facial flushing and an intolerable burning sensation. Some middle-aged patients decide to see a dermatologist for the first time when they break out in acne lesions after a history of clear skin. Others seek treatment because they can no longer tolerate the pain and embarrassment associated with their symptoms. However, patients who seek treatment only account for a small proportion of patients with rosacea, as only 10% of patients seek conventional medical treatment.7 Furthermore, symptomatic patients on average wait 7 months to 5 years before receiving a diagnosis.8,9
Care often is delayed or not pursued because many rosacea symptoms are mild when they first appear and may not initially bother the patient. Patients may not think anything of their symptoms and dismiss them as either acne vulgaris or sunburn. Due to the relapsing and remitting nature of the disease course, patients may feel their symptoms will resolve. Of patients diagnosed with rosacea, only one-half have heard of the condition prior to diagnosis,8 which can largely be attributed to lack of patient education on the signs and symptoms of rosacea, a concern that prompted the National Rosacea Society to designate the month of April as rosacea awareness month.5
With sales of antiredness facial care products growing 35% from 2002 to 2007, accounting for an increase of $300 million in revenue, patients also may be turning to over-the-counter products first.10 Men with rosacea tend to present with more severe symptoms such as rhinophyma, which may be due to their desire to wait until their symptoms reached more advanced stages of disease before seeking medical help.5
Diagnosis of Rosacea
After the patient decides that his/her symptoms are unusual, severe, or intolerable enough to seek treatment, the issues of access to dermatologic care and receiving the correct diagnosis come into play. Accessing dermatologic care can be difficult, as appointments may be hard to obtain, and even if the patient is able to get an appointment, it could be many weeks later.11 For some rosacea patients, the anxiety of waiting for their appointment prompts them to seek support and advice from online message boards (eg, http://www.rosacea-support.org). The long wait for appointments may be attributed to the increased demand for dermatologists for cosmetic procedures.12 Additionally, disparities according to insurance type can contribute to difficulties procuring an appointment. In one study, privately insured dermatology patients demonstrated a 91% acceptance rate and shorter wait times for appointments compared to publicly insured patients who were limited to a 29.8% acceptance rate and longer wait times.11 Many patients then are left to wait for an appointment with a dermatologist or instead turn to a primary care physician. Of patients diagnosed with rosacea in one study (N=2847), the majority of patients were seen by a dermatologist (79%), while the other patients were diagnosed by a family physician (14%) or other types of physicians such as internists and ophthalmologists (7%).6
The diagnosis of rosacea usually is not a major hurdle for dermatologists, but misdiagnoses can sometimes occur. The Rosacea Research & Development Institute compiled multiple patient anecdotes describing the struggles of finally reaching the correct diagnosis of rosacea; however, no estimates as to the frequency of misdiagnoses was estimated.13 Even with an accurate diagnosis of rosacea, correct classification of the 4 types of rosacea (ie, erythematotelangiectatic, papulopustular, phymatous, ocular) is necessary to avoid incorrect treatment recommendations. For example, patients with flushing often cannot tolerate topical medications in contrast to patients with the papulopustular subtype who benefit from them.14 In the meantime, the patients who are misdiagnosed may be met with frustration, as treatment was either delayed or incorrectly prescribed.
Although there are limited data regarding patient reactions after receiving a diagnosis of rosacea, it can be assumed that patients would be hopeful that diagnosis would lead to correct treatment. In a 2008 article in The New York Times, a rosacea patient was described as feeling relieved to be diagnosed with rosacea because it was an explanation for the development of pimples on the cheeks in her late 40s.10
Implementation of a Treatment Plan
After recognizing the symptoms and receiving a correct diagnosis, the next step in the patient journey is treatment. Long-term management of incurable conditions such as rosacea is difficult. The main goals of treatment are to relieve symptoms, improve appearance, delay progression to advanced stages, and maintain remission.15 There are only a few reliable clinical trials regarding therapies for rosacea, so treatment has mostly relied on clinical experience (Table). The efficacy and safety of many older treatments has not been assessed.15 Mainstays of treatment include both topical agents and oral medications. The use of topical metronidazole, oral tetracycline, and oral isotretinoin have been found to improve both skin lesions and quality of life.18 Initially, a combination of a topical and an oral medication may be used for at least the first 12 weeks, and improvement is usually gradual, taking many weeks to become evident.15 Long-term treatment with topical medications often is required for maintenance, which can last another 6 months or more.19,20
Besides using pharmacologic therapies, some patients also may choose to undergo various procedures. The most common procedure is laser therapy, followed by dermabrasion, chemical peels, hot loop electrocoagulation, and surgical sculpting or plastic surgery.6 The use of these adjunct therapies may suggest impatience from the patient for improvement; it also indicates the lengths patients will go to and willingness to pay for improvement of symptoms.
Along with medication, patients are recommended to make changes to their skin care regimen and lifestyle. Rosacea patients typically have sensitive skin that may include symptoms such as dryness, scaling, stinging, burning, and pruritus.16 Skin care recommendations for rosacea patients include using a gentle cleanser and regularly applying sunscreen.5 Issues with physical appearance can be addressed with the use of cosmetic products such as green-tinted makeup to conceal skin lesions.21 Remission can be maintained by identifying certain triggers (eg, red wine, spicy foods, extreme temperatures, prolonged sun exposure, vigorous exercise) that can cause flare-ups.15 The most common trigger is sun exposure, making photoprotection an important component of the rosacea patient’s skin care regimen.6
Adherence
With a diagnosis and treatment plan in effect, the patient journey reaches the stage of treatment adherence, which should include ongoing education about the condition. Self-reported statistics from rosacea patients indicated that 28% of patients took time off from their treatment regimen,6 but actual nonadherence rates likely are higher. The most commonly reported reason for poor treatment adherence among rosacea patients was the impression that the symptoms had resolved or were adequately controlled.6 Treatment also must be affordable. In a national survey of rosacea patients, 24% of 427 patients receiving pharmacologic therapy planned on switching medications because of cost, and 17% of 769 patients discontinued medications due to co-pay/insurance issues.6 Other reasons cited for discontinuation of treatment included patient perception that symptoms were not that serious, co-pay/insurance issues, ineffectiveness of the medication, and side effects.6 Adherence to topical medications is lower than oral medications due to the time and inconvenience required for application.22 For some patients, topical medications may be too messy, have a strange odor, or stain clothing.
It is promising that most rosacea patients have reported the intent to continue using pharmacologic agents because the medication prevented worsening of their symptoms.6 However, there are still patients who switch or discontinue therapies without physician direction. These patients often cite that they desire more information at the time of diagnosis, particularly related to causes of flare-ups, physical symptoms to expect, drug treatment options, makeup to cover up visible symptoms, surgical or laser treatment options, psychological symptoms, patient support groups, and counseling options.6
Management
The last part of the journey is disease management, which occurs when the patient learns how to control his/her symptoms long-term. Important factors contributing to long-term control of rosacea flares are medication adherence and avoiding lifestyle triggers.23,24 Through the other stages of the journey, the patient has learned which treatments work and which factors may lead to exacerbation of symptoms.
Educating Patients on the Journey
The patient journey is a concept that can be applied to any disease state and brings to light roadblocks that patients may face from the initial diagnosis to successful disease management. Rosacea patients are faced with confusing and aggravating symptoms that can cause anxiety and may lead them to seek treatment from a physician. Facial flushing and phymatous changes of the nose can be mistaken for alcohol abuse, leading rosacea to be a socially stigmatizing disease.15 Because rosacea involves mostly the facial skin, it can disrupt social and professional interactions, leading to quality-of-life effects such as difficulty functioning on a day-to-day basis, which can be detrimental because patients usually are aged 30 to 50 years and may be perceived based on their appearance in the workforce.3 A lack of confidence, low self-esteem, embarrassment, and anxiety can even lead to serious psychiatric conditions such as depression and body dysmorphic disorder.25 Because the severity of rosacea increases over time, it is important to educate patients about seeking early treatment; therefore, understanding and awareness of rosacea symptoms are necessary to prompt patients to see a medical professional to either confirm or refute the diagnosis.
Rosacea is a clinical diagnosis that relies on patterns of primary and secondary features, as outlined in a 2002 report by the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea.5 Even with this consensus grading system, it appears that additional fine-tuning of the criteria is needed in the disease definition. Importantly, because much of the pathogenesis and progression of rosacea is still not completely understood, there is no laboratory benchmark test that can be utilized for correct diagnosis.14 Moreover, many of the clinical manifestations of rosacea are shared with other conditions, and patients may present with different symptoms or varying combinations.26
Treatment of rosacea is multifactorial and behavioral, as patients must not only be able to obtain and adhere to oral and topical regimens and possible procedures but also avoid various lifestyle and environmental triggers and learn to cope with emotional distress caused by their symptoms. Although patients who discontinue use of medications appear to be in the minority, education is still needed to stress the chronic nature of rosacea and the importance of the continuation of treatment. Collaboration between the physician and patient is needed to determine why a certain medication may not be effective and explore other treatment options. Treatment ineffectiveness could be due to incorrect use of the product, failure to use an adjunct skin care regimen, or inability to control rosacea triggers. Adequate early follow-up also is needed to maximize patient adherence to treatment.27 Working together with the patient to develop a treatment plan that can be followed is necessary for long-term control of rosacea symptoms.
There is little information on how to address the psychological needs of patients, but patients can find support from various avenues. For instance, the National Rosacea Society, a large advocacy group, produces newsletters and educational materials for both physicians and patients.28,29 There also are online support groups for rosacea patients that have thousands of members who exchange stories and provide words of encouragement. Although there are not many face-to-face support groups, physicians may consider developing live support groups for their rosacea patients. As patients achieve the later stages of the rosacea patient journey, they hopefully will have controlled their symptoms by following a treatment regimen and learning to adapt to a new life of successful disease management.
Many aspects of the rosacea patient journey have yet to be explored. It is uncertain how long patients with symptoms of rosacea wait before seeking treatment, what methods they use to control their rosacea before they receive a prescribed treatment or physician recommendations, and how they react to their diagnosis. It also is unknown how many rosacea patients receive an initial misdiagnosis of another condition and which physicians typically make the misdiagnosis. We also need to know more about the role of psychological issues in addressing patient adherence to treatment. Similarly, what role do support groups such as online forums play on adherence? There is a need for more patient education and awareness of rosacea.
Conclusion
Patients may be relieved that rosacea is not a life-threatening condition, but they may be disappointed that there is no cure for rosacea. As the patient and dermatologist work together to find an appropriate treatment plan, identify certain triggers, and modify the skin care routine, the patient can become disciplined in controlling rosacea symptoms. Ultimately, with the alleviation of visible symptoms, the patient’s quality of life also can improve. Better understanding of the rosacea patient perspective can lead to a more efficient health care system, improved patient care, and better patient satisfaction.
1. Baldwin HE. Systemic therapy for rosacea. Skin Therapy Lett. 2007;12:1-5, 9.
2. Drake L. Rosacea now estimated to affect at least 16 million Americans. Rosacea Review. Winter 2010. http://www.rosacea.org/rr/2010/winter/article_1.php. Accessed December 11, 2014.
3. Rosacea as an inflammatory disease: an expert interview with Brian Berman, MD, PhD. Medscape Web site. http://www.medscape.org/viewarticle/722156. Published May 27, 2010. Accessed December 11, 2014.
4. HealthEd Group, Inc. Rheumatoid arthritis patient journey map. http://visual.ly/rheumatoid-arthritis-patient-journey-map. Accessed December 19, 2014.
5. Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
6. Elewski BE. Results of a national rosacea patient survey: common issues that concern rosacea sufferers. J Drugs Dermatol. 2009;8:120-123.
7. Del Rosso J. Management of rosacea in the United States: analysis based on recent prescribing patterns and insurance claims. J Am Acad Dermatol. 2008;58:AB13.
8. New survey reveals first impressions may not always be rosy for people with the widespread skin condition rosacea. Medical News Today Web site. http://www.medicalnew today.com/releases/185491.php. Updated April 15, 2010. Accessed December 12, 2014.
9. Shear NH, Levine C. Needs survey of Canadian rosacea patients. J Cutan Med Surg. 1999;3:178-181.
10. Sweeney C. In a perfect world, rosacea remains a problem. New York Times. April 24, 2008. http://www.nytimes.com/2008/04/24/fashion/24SKIN.html?pagewanted=all. Accessed December 12, 2014.
11. Alghothani L, Jacks SK, Vander HA, et al. Disparities in access to dermatologic care according to insurance type. Arch Dermatol. 2012;148:956-957.
12. Resneck J Jr. Too few or too many dermatologists? difficulties in assessing optimal workforce size. Arch Dermatol. 2001;137:1295-1301.
13. Rosacea Research & Development Institute Web site. http://irosacea.org/misdiagnosed_rosacea.html. Accessed December 19, 2014.
14. Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341.
15. Elewski BE, Draelos Z, Dreno B, et al. Rosacea—global diversity and optimized outcome: proposed international consensus from the Rosacea International Expert Group. J Eur Acad Dermatol Venereol. 2011;25:188-200.
16. Del Rosso JQ, Baldwin H, Webster G. American Acne & Rosacea Society rosacea medical management guidelines. J Drugs Dermatol. 2008;7:531-533.
17. Fowler J Jr, Jackson M, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12:650-656.
18. Aksoy B, Altaykan-Hapa A, Egemen D, et al. The impact of rosacea on quality of life: effects of demographic and clinical characteristics and various treatment modalities. Br J Dermatol. 2010;163:719-725.
19. Dahl MV, Katz HI, Krueger GG, et al. Topical metronidazole maintains remissions of rosacea. Arch Dermatol. 1998;134:679-683.
20. Thiboutot DM, Fleischer AB, Del Rosso JQ, et al. A multicenter study of topical azelaic acid 15% gel in combination with oral doxycycline as initial therapy and azelaic acid 15% gel as maintenance monotherapy. J Drugs Dermatol. 2009;8:639-648.
21. Boehncke WH, Ochsendorf F, Paeslack I, et al. Decorative cosmetics improve the quality of life in patients with disfiguring skin diseases. Eur J Dermatol. 2002;12:577-580.
22. Jackson JM, Pelle M. Topical rosacea therapy: the importance of vehicles for efficacy, tolerability and compliance. J Drugs Dermatol. 2011;10:627-633.
23. Wolf JE Jr. Medication adherence: a key factor in effective management of rosacea. Adv Ther. 2001;18:272-281.
24. Managing rosacea. National Rosacea Society Web site. http://www.rosacea.org/patients/materials/managing/lifestyle.php. Accessed December 19, 2014.
25. van Zuuren EJ, Fedorowicz Z. Lack of ‘appropriately assessed’ patient-reported outcomes in randomized controlled trials assessing the effectiveness of interventions for rosacea. Br J Dermatol. 2013;168:442-444.
26. Del Rosso JQ. Advances in understanding and managing rosacea: part 2: the central role, evaluation, and medical management of diffuse and persistent facial erythema of rosacea. J Clin Aesthet Dermatol. 2012;5:26-36.
27. Davis SA, Lin HC, Yu CH, et al. Underuse of early follow-up visits: a missed opportunity to improve patients’ adherence. 2014;13:833-836.
28. If you have rosacea, you’re not alone. National Rosacea Society Web site. http://www.rosacea.org/patients/index.php. Accessed December 19, 2014.
29. Tools for the professional. National Rosacea Society Web site. http://www.rosacea.org/physicians/index.php. Accessed December 19, 2014.
1. Baldwin HE. Systemic therapy for rosacea. Skin Therapy Lett. 2007;12:1-5, 9.
2. Drake L. Rosacea now estimated to affect at least 16 million Americans. Rosacea Review. Winter 2010. http://www.rosacea.org/rr/2010/winter/article_1.php. Accessed December 11, 2014.
3. Rosacea as an inflammatory disease: an expert interview with Brian Berman, MD, PhD. Medscape Web site. http://www.medscape.org/viewarticle/722156. Published May 27, 2010. Accessed December 11, 2014.
4. HealthEd Group, Inc. Rheumatoid arthritis patient journey map. http://visual.ly/rheumatoid-arthritis-patient-journey-map. Accessed December 19, 2014.
5. Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
6. Elewski BE. Results of a national rosacea patient survey: common issues that concern rosacea sufferers. J Drugs Dermatol. 2009;8:120-123.
7. Del Rosso J. Management of rosacea in the United States: analysis based on recent prescribing patterns and insurance claims. J Am Acad Dermatol. 2008;58:AB13.
8. New survey reveals first impressions may not always be rosy for people with the widespread skin condition rosacea. Medical News Today Web site. http://www.medicalnew today.com/releases/185491.php. Updated April 15, 2010. Accessed December 12, 2014.
9. Shear NH, Levine C. Needs survey of Canadian rosacea patients. J Cutan Med Surg. 1999;3:178-181.
10. Sweeney C. In a perfect world, rosacea remains a problem. New York Times. April 24, 2008. http://www.nytimes.com/2008/04/24/fashion/24SKIN.html?pagewanted=all. Accessed December 12, 2014.
11. Alghothani L, Jacks SK, Vander HA, et al. Disparities in access to dermatologic care according to insurance type. Arch Dermatol. 2012;148:956-957.
12. Resneck J Jr. Too few or too many dermatologists? difficulties in assessing optimal workforce size. Arch Dermatol. 2001;137:1295-1301.
13. Rosacea Research & Development Institute Web site. http://irosacea.org/misdiagnosed_rosacea.html. Accessed December 19, 2014.
14. Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341.
15. Elewski BE, Draelos Z, Dreno B, et al. Rosacea—global diversity and optimized outcome: proposed international consensus from the Rosacea International Expert Group. J Eur Acad Dermatol Venereol. 2011;25:188-200.
16. Del Rosso JQ, Baldwin H, Webster G. American Acne & Rosacea Society rosacea medical management guidelines. J Drugs Dermatol. 2008;7:531-533.
17. Fowler J Jr, Jackson M, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12:650-656.
18. Aksoy B, Altaykan-Hapa A, Egemen D, et al. The impact of rosacea on quality of life: effects of demographic and clinical characteristics and various treatment modalities. Br J Dermatol. 2010;163:719-725.
19. Dahl MV, Katz HI, Krueger GG, et al. Topical metronidazole maintains remissions of rosacea. Arch Dermatol. 1998;134:679-683.
20. Thiboutot DM, Fleischer AB, Del Rosso JQ, et al. A multicenter study of topical azelaic acid 15% gel in combination with oral doxycycline as initial therapy and azelaic acid 15% gel as maintenance monotherapy. J Drugs Dermatol. 2009;8:639-648.
21. Boehncke WH, Ochsendorf F, Paeslack I, et al. Decorative cosmetics improve the quality of life in patients with disfiguring skin diseases. Eur J Dermatol. 2002;12:577-580.
22. Jackson JM, Pelle M. Topical rosacea therapy: the importance of vehicles for efficacy, tolerability and compliance. J Drugs Dermatol. 2011;10:627-633.
23. Wolf JE Jr. Medication adherence: a key factor in effective management of rosacea. Adv Ther. 2001;18:272-281.
24. Managing rosacea. National Rosacea Society Web site. http://www.rosacea.org/patients/materials/managing/lifestyle.php. Accessed December 19, 2014.
25. van Zuuren EJ, Fedorowicz Z. Lack of ‘appropriately assessed’ patient-reported outcomes in randomized controlled trials assessing the effectiveness of interventions for rosacea. Br J Dermatol. 2013;168:442-444.
26. Del Rosso JQ. Advances in understanding and managing rosacea: part 2: the central role, evaluation, and medical management of diffuse and persistent facial erythema of rosacea. J Clin Aesthet Dermatol. 2012;5:26-36.
27. Davis SA, Lin HC, Yu CH, et al. Underuse of early follow-up visits: a missed opportunity to improve patients’ adherence. 2014;13:833-836.
28. If you have rosacea, you’re not alone. National Rosacea Society Web site. http://www.rosacea.org/patients/index.php. Accessed December 19, 2014.
29. Tools for the professional. National Rosacea Society Web site. http://www.rosacea.org/physicians/index.php. Accessed December 19, 2014.
Practice Points
- For patients who are emotionally distressed by their rosacea and who lack a social support network, several rosacea-focused online support systems are available.
- An early follow-up visit to evaluate newly prescribed treatments can positively influence disease management.
Calcipotriene 0.005%–Betamethasone Dipropionate 0.064% Ointment Versus Topical Suspension in the Treatment of Plaque Psoriasis: A Randomized Pilot Study of Patient Preference
Psoriasis is a chronic relapsing inflammatory skin and joint disease that affects 1% to 3% of the US population.1 In cases of mild to moderate disease, topical agents including corticosteroids, vitamin D analogues, and retinoids are the mainstay of therapy. The need for long-term treatment can be frustrating for patients and treatment adherence often is problematic, resulting in poor outcomes. Reported adherence rates to topical psoriasis treatments range from 27% to 73%.2-6
Topical agents for treatment of psoriasis are available in various formulations, including creams, lotions, gels, ointments, solutions, and shampoos. For topical psoriasis therapies, vehicle formulation plays a major role in both delivery of the active drug and treatment adherence. Patients often cite poor cosmetic characteristics (eg, product feels too sticky or greasy, product feels unpleasant/has a bad texture, product is too messy, product application is too time consuming/takes too long to rub in) as reasons for poor treatment adherence.2,6-9 Psoriasis patients tend to prefer formulations that are not as messy such as solutions and foams versus creams, gels, and ointments.10
Ointments have been favored by physicians for the treatment of psoriasis because of the belief that their occlusive properties result in greater potency; however, a systematic review of clinical trials of different formulations of clobetasol propionate did not find that ointments were more effective than other vehicles.11 Furthermore, if a patient finds an ointment to be cosmetically unacceptable, he/she will be less inclined to use the medication as prescribed, regardless of its potency.
The objective of this study was to conduct a preliminary assessment of patient preference for ointment versus topical suspension formulations of calcipotriene 0.005%–betamethasone dipropionate 0.064% for treatment of plaque psoriasis. The specific attributes that were found to be appealing or unappealing by participants for each formulation also were evaluated.
Methods
Study Design and Participants
This open-label, investigator-blinded, crossover, prospective, single-center study evaluated patient preference for ointment versus topical suspension formulations of calcipo-triene 0.005%–betamethasone dipropionate 0.064% in the treatment of plaque psoriasis. The study protocol was approved by the Wake Forest School of Medicine (Winston-Salem, North Carolina) institutional review board.
Participants were recruited from the Wake Forest School of Medicine dermatology clinic. Inclusion criteria included men and women with mild to moderate plaque-type psoriasis who were 18 years of age or older; participants also were required to have an investigator global assessment (IGA) score of 2 (mild) or 3 (moderate) on a 5-point scale and 1% to 10% body surface involvement on the trunk or extremities.
Exclusion criteria included use of a topical or systemic psoriasis treatment within 2 or 4 weeks of baseline, respectively. Women who were pregnant, breastfeeding, planning to become pregnant, or could potentially become pregnant and were not using a medically accepted form of contraception also were excluded from the study. Patients with other serious skin conditions or any other chronic medical conditions that were not well controlled also were considered ineligible.
As a pilot study, a sample size of 20 participants was needed based on available funding.
Assessments
At baseline, the diagnosis of plaque-type psoriasis in each participant was confirmed by the investigator. Each participant’s medical history was obtained and all prior and current medications were reviewed to ensure eligibility criteria were met. Female participants of childbearing potential also underwent a urine pregnancy test. Consent was obtained from all enrolled participants. Investigators assessed the severity of psoriasis at baseline using the IGA.
All participants then were randomized (1:1 randomization) for treatment with either calcipotriene 0.005%–betamethasone dipropionate 0.064% ointment or calcipotriene 0.005%–betamethasone dipropionate 0.064% topical suspension. A simple random sampling chart was prepared and used by the study staff to determine treatment group allotment. Participants were instructed to apply the assigned study drug to affected areas of the body, excluding the scalp, face, and intertriginous areas, once daily for 3 days. Participants and study staff were aware of the study product being used, while investigators remained blinded. Participants also were asked to maintain a daily medication diary noting when the medication was applied.
Participants returned on day 3 for crossover treatment. Participants were asked to complete a subjective participant preference survey and study staff reviewed concomitant medications and adverse events. The packaging for the initial study drug was collected and weighed, and the crossover drug was dispensed to each participant to be applied once daily for 3 days.
Participants returned on day 6 or 7 for follow-up and were again asked to complete the subjective participant preference survey; study staff reviewed concomitant medications and adverse events. The packaging for the crossover study drug was collected and weighed, and the participant’s medication diary also was collected.
Subjective Participant Preference Survey
The subjective participant preference survey consisted of 15 questions relating to the participant’s experience with the study drug (eg, how the product felt to touch, amount of greasiness, time it took to apply). The final survey question asked participants to rate the overall appeal of the vehicle. Participants responded to the questions using a 7-point grading scale (1=extremely unappealing; 4=neutral; 7=extremely appealing). Total preference scores could range from 15 to 105.
End Points
The primary end point was the mean total preference score for each study drug obtained from the subjective participant preference surveys. Secondary end points included median values for individual survey questions and treatment adherence, which was measured from self-reported medication diary entries.
Statistical Analysis
Participant characteristics were reported with percentages for dichotomous data and median and ranges for other data. Subjective participant preference survey scores were calculated by taking the mean (standard deviation [SD]) sum of the scores for each individual survey item for both products. A generalized linear model that accounted for possible carryover and period effects was used to compare the difference of individual participant scores for the 2 products using SAS software. The mean (SD) amount of product used was reported and correlated to the preference score using a Spearman rank correlation. Total and individual survey scores were compared between sexes using Wilcoxon rank sum tests.
Results
Participants were enrolled in and completed the study from January 2013 to March 2013. The Figure presents a diagram of the Consolidated Standards of Reporting Trials. Thirty patients were screened; 10 patients did not meet eligibility criteria. Twenty patients were enrolled in the study with 10 patients randomized to each study arm. All 20 participants completed the study.
Participant demographics are described in Table 1. The median age was 48 years (range 29–64 years). The majority of participants were male (13/20) and white (18/20). The median IGA score was 3 (range, 2–3).
The mean (SD) total preference score for the calcipotriene 0.005%–betamethasone dipropionate 0.064% ointment formulation was 73.5 (19.4) and 80 (20.2) for the topical suspension formulation. The difference between means was -6.5 (95% confidence interval, -19.7-6.8; P=.32 after adjusting for possible carryover and period effects). Participants used a mean (SD) of 11 (11.4) g of product per study arm for the ointment formulation and 8.8 (6.6) g for the topical suspension formulation. There was no correlation between the amount of product used and preference for product (Spearman r=-0.01; P=.94). No statistically significant difference in product preference among men versus women was noted when considering total preference score or median scores of individual survey questions. Median overall appeal rating for the ointment formulation was 5 (slightly appealing) versus 6 (moderately appealing) for the topical suspension formulation, approaching statistical significance with P=.06 (Table 2). No significant carryover effects from one product to the other were noted (P=.64). The mean (SD) total preference scores were 81.1 (18.4) and 77.6 (21.2) in participants who used the topical suspension first followed by the ointment. In participants who used the ointment first followed by the topical suspension, the mean (SD) total preference scores were 69.4 (17.6) and 78.9 (22.8). Self-reported treatment adherence according to the participant’s daily medication diary was 100%.
Adverse effects during the study included 1 report of neck and back muscle pain and 1 report of sinusitis; neither was considered to be related to the study drugs.
Comment
Psoriasis is a chronic disease that can be difficult to treat, and treatment compliance often is poor. Multiple topical agents often are needed for adequate disease control, and adherence can be an even greater hurdle than with monotherapy. Combination products such as calcipotriene–betamethasone dipropionate offer the potential advantage of once-daily application. Adherence to once-daily application regimens for treatment of psoriasis has been shown to be greater than twice-daily application (82% vs 44%).4 However, the vehicle may be an adherence barrier for some patients.
Calcipotriene 0.005%–betamethasone dipropionate 0.064% topical suspension originally was indicated for the treatment of psoriasis of the scalp; however, it is now also indicated for treatment of psoriasis of the body.12 This topical suspension formulation is less messy, which could potentially be more cosmetically appealing and useful for improving treatment adherence.
Overall, the participants in our small pilot study showed a preference for the topical suspension versus the ointment formulation. The difference was substantial but was not statistically significant. This result is consistent with a previous study in which patients were found to prefer solutions that were less greasy compared to messy sticky ointments.10 Although the topical suspension received a higher average rating from participants for how it felt to touch and how it felt under clothing than the ointment and the ointment was rated on average as more greasy (none of these individual items achieved statistical significance), the calcipotriene–betamethasone dipropionate ointment was still rated as slightly appealing overall (Table 2). This result supports the need for physicians to discuss individual patient preferences when choosing the most appropriate vehicle for topical psoriasis treatment.
In our study, participants were found to use less product during treatment with the topical suspension versus the ointment, likely because the topical suspension formulation is thinner and spreads easier; however, participants rated the ease of application of the 2 products equally (Table 2). Ease of application was rated moderately appealing and time to apply was rated slightly to moderately appealing, which is important because patients often cite these factors as barriers to treatment adherence.2,6,13
The occlusive properties of ointment formulations provide moisturization by preventing water loss, a property that can be desirable when treating psoriatic plaques. Unlike the ointment, which was formulated with petrolatum and mineral oil, the calcipotriene–betamethasone dipropionate topical suspension was formulated with hydrogenated castor oil and mineral oil to provide moisturization. Nevertheless, participants found both the ointment and topical suspension to be moderately appealing (median score, 6; P=.94) for moisturization.
Limitations of this pilot study include the small sample size, which restricted the extent of subgroup analyses and the generalizability of our results. The small sample size also may or may not have contributed to the lack of statistical significance in the majority of the outcomes. This study provides pilot data that can be used to define a larger study; however, we do not think that a larger study is needed, as patients can be offered both vehicles in a practical clinical setting and can choose the product that is right for them. The short treatment duration of 3 days for each formulation also is a limitation, as a patient’s preference may change over time with longer use of the product. Treatment efficacy, which was not measured in this study, also could have an effect on patient preference, which could be assessed over a longer treatment period. Additionally, the study drugs may not be representative of all ointments or topical suspensions in their cosmetic appeal.
Conclusion
In this small cohort of plaque psoriasis patients, a calcipotriene–betamethasone dipropionate topical suspension was preferred over an ointment formulation, but in clinical practice it may be best to allow patients to choose the vehicle formulation that is most desirable on an individual basis. The topical suspension provides clinicians with an alternative that not only has the benefits of a combination product but also has been found to be appealing to patients.
1. Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
2. Brown KK, Rehmus WE, Kimball AB. Determining the relative importance of patient motivations for nonadherence to topical corticosteroid therapy in psoriasis. J Am Acad Dermatol. 2006;55:607-613.
3. Carroll CL, Feldman SR, Camacho FT, et al. Adherence to topical therapy decreases during the course of an 8-week psoriasis clinical trial: commonly used methods of measuring adherence to topical therapy overestimate actual use. J Am Acad Dermatol. 2004;51:212-216.
4. Zaghloul SS, Goodfield MJ. Objective assessment of compliance with psoriasis treatment. Arch Dermatol. 2004;140:408-414.
5. Richards HL, Fortune DG, O’Sullivan TM, et al. Patients with psoriasis and their compliance with medication. J Am Acad Dermatol. 1999;41:581-583.
6. Fouere S, Adjadj L, Pawin H. How patients experience psoriasis: results from a European survey. J Eur Acad Dermatol Venereol. 2005;19:S2-S6.
7. van de Kerkhof PC, Steegers-Theunissen RP, Kuipers MV. Evaluation of topical drug treatment in psoriasis. Dermatology. 1998;197:31-36.
8. Devaux S, Castela A, Archier E, et al. Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):S61-S67.
9. Chan SA, Hussain F, Lawson LG, et al. Factors affecting adherence to treatment of psoriasis: comparing biologic therapy to other modalities. J Dermatolog Treat. 2013;24:64-69.
10. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
11. Warino L, Balkrishnan R, Feldman SR. Clobetasol propionate for psoriasis: are ointments really more potent? J Drugs Dermatol. 2006;5:527-532.
12. Menter A, Gold LS, Bukhalo M, et al. Calcipotriene plus betamethasone dipropionate topical suspension for the treatment of mild to moderate psoriasis vulgaris on the body: a randomized, double-blind, vehicle-controlled trial. J Drugs Dermatol. 2013;12:92-98.
13. Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
Psoriasis is a chronic relapsing inflammatory skin and joint disease that affects 1% to 3% of the US population.1 In cases of mild to moderate disease, topical agents including corticosteroids, vitamin D analogues, and retinoids are the mainstay of therapy. The need for long-term treatment can be frustrating for patients and treatment adherence often is problematic, resulting in poor outcomes. Reported adherence rates to topical psoriasis treatments range from 27% to 73%.2-6
Topical agents for treatment of psoriasis are available in various formulations, including creams, lotions, gels, ointments, solutions, and shampoos. For topical psoriasis therapies, vehicle formulation plays a major role in both delivery of the active drug and treatment adherence. Patients often cite poor cosmetic characteristics (eg, product feels too sticky or greasy, product feels unpleasant/has a bad texture, product is too messy, product application is too time consuming/takes too long to rub in) as reasons for poor treatment adherence.2,6-9 Psoriasis patients tend to prefer formulations that are not as messy such as solutions and foams versus creams, gels, and ointments.10
Ointments have been favored by physicians for the treatment of psoriasis because of the belief that their occlusive properties result in greater potency; however, a systematic review of clinical trials of different formulations of clobetasol propionate did not find that ointments were more effective than other vehicles.11 Furthermore, if a patient finds an ointment to be cosmetically unacceptable, he/she will be less inclined to use the medication as prescribed, regardless of its potency.
The objective of this study was to conduct a preliminary assessment of patient preference for ointment versus topical suspension formulations of calcipotriene 0.005%–betamethasone dipropionate 0.064% for treatment of plaque psoriasis. The specific attributes that were found to be appealing or unappealing by participants for each formulation also were evaluated.
Methods
Study Design and Participants
This open-label, investigator-blinded, crossover, prospective, single-center study evaluated patient preference for ointment versus topical suspension formulations of calcipo-triene 0.005%–betamethasone dipropionate 0.064% in the treatment of plaque psoriasis. The study protocol was approved by the Wake Forest School of Medicine (Winston-Salem, North Carolina) institutional review board.
Participants were recruited from the Wake Forest School of Medicine dermatology clinic. Inclusion criteria included men and women with mild to moderate plaque-type psoriasis who were 18 years of age or older; participants also were required to have an investigator global assessment (IGA) score of 2 (mild) or 3 (moderate) on a 5-point scale and 1% to 10% body surface involvement on the trunk or extremities.
Exclusion criteria included use of a topical or systemic psoriasis treatment within 2 or 4 weeks of baseline, respectively. Women who were pregnant, breastfeeding, planning to become pregnant, or could potentially become pregnant and were not using a medically accepted form of contraception also were excluded from the study. Patients with other serious skin conditions or any other chronic medical conditions that were not well controlled also were considered ineligible.
As a pilot study, a sample size of 20 participants was needed based on available funding.
Assessments
At baseline, the diagnosis of plaque-type psoriasis in each participant was confirmed by the investigator. Each participant’s medical history was obtained and all prior and current medications were reviewed to ensure eligibility criteria were met. Female participants of childbearing potential also underwent a urine pregnancy test. Consent was obtained from all enrolled participants. Investigators assessed the severity of psoriasis at baseline using the IGA.
All participants then were randomized (1:1 randomization) for treatment with either calcipotriene 0.005%–betamethasone dipropionate 0.064% ointment or calcipotriene 0.005%–betamethasone dipropionate 0.064% topical suspension. A simple random sampling chart was prepared and used by the study staff to determine treatment group allotment. Participants were instructed to apply the assigned study drug to affected areas of the body, excluding the scalp, face, and intertriginous areas, once daily for 3 days. Participants and study staff were aware of the study product being used, while investigators remained blinded. Participants also were asked to maintain a daily medication diary noting when the medication was applied.
Participants returned on day 3 for crossover treatment. Participants were asked to complete a subjective participant preference survey and study staff reviewed concomitant medications and adverse events. The packaging for the initial study drug was collected and weighed, and the crossover drug was dispensed to each participant to be applied once daily for 3 days.
Participants returned on day 6 or 7 for follow-up and were again asked to complete the subjective participant preference survey; study staff reviewed concomitant medications and adverse events. The packaging for the crossover study drug was collected and weighed, and the participant’s medication diary also was collected.
Subjective Participant Preference Survey
The subjective participant preference survey consisted of 15 questions relating to the participant’s experience with the study drug (eg, how the product felt to touch, amount of greasiness, time it took to apply). The final survey question asked participants to rate the overall appeal of the vehicle. Participants responded to the questions using a 7-point grading scale (1=extremely unappealing; 4=neutral; 7=extremely appealing). Total preference scores could range from 15 to 105.
End Points
The primary end point was the mean total preference score for each study drug obtained from the subjective participant preference surveys. Secondary end points included median values for individual survey questions and treatment adherence, which was measured from self-reported medication diary entries.
Statistical Analysis
Participant characteristics were reported with percentages for dichotomous data and median and ranges for other data. Subjective participant preference survey scores were calculated by taking the mean (standard deviation [SD]) sum of the scores for each individual survey item for both products. A generalized linear model that accounted for possible carryover and period effects was used to compare the difference of individual participant scores for the 2 products using SAS software. The mean (SD) amount of product used was reported and correlated to the preference score using a Spearman rank correlation. Total and individual survey scores were compared between sexes using Wilcoxon rank sum tests.
Results
Participants were enrolled in and completed the study from January 2013 to March 2013. The Figure presents a diagram of the Consolidated Standards of Reporting Trials. Thirty patients were screened; 10 patients did not meet eligibility criteria. Twenty patients were enrolled in the study with 10 patients randomized to each study arm. All 20 participants completed the study.
Participant demographics are described in Table 1. The median age was 48 years (range 29–64 years). The majority of participants were male (13/20) and white (18/20). The median IGA score was 3 (range, 2–3).
The mean (SD) total preference score for the calcipotriene 0.005%–betamethasone dipropionate 0.064% ointment formulation was 73.5 (19.4) and 80 (20.2) for the topical suspension formulation. The difference between means was -6.5 (95% confidence interval, -19.7-6.8; P=.32 after adjusting for possible carryover and period effects). Participants used a mean (SD) of 11 (11.4) g of product per study arm for the ointment formulation and 8.8 (6.6) g for the topical suspension formulation. There was no correlation between the amount of product used and preference for product (Spearman r=-0.01; P=.94). No statistically significant difference in product preference among men versus women was noted when considering total preference score or median scores of individual survey questions. Median overall appeal rating for the ointment formulation was 5 (slightly appealing) versus 6 (moderately appealing) for the topical suspension formulation, approaching statistical significance with P=.06 (Table 2). No significant carryover effects from one product to the other were noted (P=.64). The mean (SD) total preference scores were 81.1 (18.4) and 77.6 (21.2) in participants who used the topical suspension first followed by the ointment. In participants who used the ointment first followed by the topical suspension, the mean (SD) total preference scores were 69.4 (17.6) and 78.9 (22.8). Self-reported treatment adherence according to the participant’s daily medication diary was 100%.
Adverse effects during the study included 1 report of neck and back muscle pain and 1 report of sinusitis; neither was considered to be related to the study drugs.
Comment
Psoriasis is a chronic disease that can be difficult to treat, and treatment compliance often is poor. Multiple topical agents often are needed for adequate disease control, and adherence can be an even greater hurdle than with monotherapy. Combination products such as calcipotriene–betamethasone dipropionate offer the potential advantage of once-daily application. Adherence to once-daily application regimens for treatment of psoriasis has been shown to be greater than twice-daily application (82% vs 44%).4 However, the vehicle may be an adherence barrier for some patients.
Calcipotriene 0.005%–betamethasone dipropionate 0.064% topical suspension originally was indicated for the treatment of psoriasis of the scalp; however, it is now also indicated for treatment of psoriasis of the body.12 This topical suspension formulation is less messy, which could potentially be more cosmetically appealing and useful for improving treatment adherence.
Overall, the participants in our small pilot study showed a preference for the topical suspension versus the ointment formulation. The difference was substantial but was not statistically significant. This result is consistent with a previous study in which patients were found to prefer solutions that were less greasy compared to messy sticky ointments.10 Although the topical suspension received a higher average rating from participants for how it felt to touch and how it felt under clothing than the ointment and the ointment was rated on average as more greasy (none of these individual items achieved statistical significance), the calcipotriene–betamethasone dipropionate ointment was still rated as slightly appealing overall (Table 2). This result supports the need for physicians to discuss individual patient preferences when choosing the most appropriate vehicle for topical psoriasis treatment.
In our study, participants were found to use less product during treatment with the topical suspension versus the ointment, likely because the topical suspension formulation is thinner and spreads easier; however, participants rated the ease of application of the 2 products equally (Table 2). Ease of application was rated moderately appealing and time to apply was rated slightly to moderately appealing, which is important because patients often cite these factors as barriers to treatment adherence.2,6,13
The occlusive properties of ointment formulations provide moisturization by preventing water loss, a property that can be desirable when treating psoriatic plaques. Unlike the ointment, which was formulated with petrolatum and mineral oil, the calcipotriene–betamethasone dipropionate topical suspension was formulated with hydrogenated castor oil and mineral oil to provide moisturization. Nevertheless, participants found both the ointment and topical suspension to be moderately appealing (median score, 6; P=.94) for moisturization.
Limitations of this pilot study include the small sample size, which restricted the extent of subgroup analyses and the generalizability of our results. The small sample size also may or may not have contributed to the lack of statistical significance in the majority of the outcomes. This study provides pilot data that can be used to define a larger study; however, we do not think that a larger study is needed, as patients can be offered both vehicles in a practical clinical setting and can choose the product that is right for them. The short treatment duration of 3 days for each formulation also is a limitation, as a patient’s preference may change over time with longer use of the product. Treatment efficacy, which was not measured in this study, also could have an effect on patient preference, which could be assessed over a longer treatment period. Additionally, the study drugs may not be representative of all ointments or topical suspensions in their cosmetic appeal.
Conclusion
In this small cohort of plaque psoriasis patients, a calcipotriene–betamethasone dipropionate topical suspension was preferred over an ointment formulation, but in clinical practice it may be best to allow patients to choose the vehicle formulation that is most desirable on an individual basis. The topical suspension provides clinicians with an alternative that not only has the benefits of a combination product but also has been found to be appealing to patients.
Psoriasis is a chronic relapsing inflammatory skin and joint disease that affects 1% to 3% of the US population.1 In cases of mild to moderate disease, topical agents including corticosteroids, vitamin D analogues, and retinoids are the mainstay of therapy. The need for long-term treatment can be frustrating for patients and treatment adherence often is problematic, resulting in poor outcomes. Reported adherence rates to topical psoriasis treatments range from 27% to 73%.2-6
Topical agents for treatment of psoriasis are available in various formulations, including creams, lotions, gels, ointments, solutions, and shampoos. For topical psoriasis therapies, vehicle formulation plays a major role in both delivery of the active drug and treatment adherence. Patients often cite poor cosmetic characteristics (eg, product feels too sticky or greasy, product feels unpleasant/has a bad texture, product is too messy, product application is too time consuming/takes too long to rub in) as reasons for poor treatment adherence.2,6-9 Psoriasis patients tend to prefer formulations that are not as messy such as solutions and foams versus creams, gels, and ointments.10
Ointments have been favored by physicians for the treatment of psoriasis because of the belief that their occlusive properties result in greater potency; however, a systematic review of clinical trials of different formulations of clobetasol propionate did not find that ointments were more effective than other vehicles.11 Furthermore, if a patient finds an ointment to be cosmetically unacceptable, he/she will be less inclined to use the medication as prescribed, regardless of its potency.
The objective of this study was to conduct a preliminary assessment of patient preference for ointment versus topical suspension formulations of calcipotriene 0.005%–betamethasone dipropionate 0.064% for treatment of plaque psoriasis. The specific attributes that were found to be appealing or unappealing by participants for each formulation also were evaluated.
Methods
Study Design and Participants
This open-label, investigator-blinded, crossover, prospective, single-center study evaluated patient preference for ointment versus topical suspension formulations of calcipo-triene 0.005%–betamethasone dipropionate 0.064% in the treatment of plaque psoriasis. The study protocol was approved by the Wake Forest School of Medicine (Winston-Salem, North Carolina) institutional review board.
Participants were recruited from the Wake Forest School of Medicine dermatology clinic. Inclusion criteria included men and women with mild to moderate plaque-type psoriasis who were 18 years of age or older; participants also were required to have an investigator global assessment (IGA) score of 2 (mild) or 3 (moderate) on a 5-point scale and 1% to 10% body surface involvement on the trunk or extremities.
Exclusion criteria included use of a topical or systemic psoriasis treatment within 2 or 4 weeks of baseline, respectively. Women who were pregnant, breastfeeding, planning to become pregnant, or could potentially become pregnant and were not using a medically accepted form of contraception also were excluded from the study. Patients with other serious skin conditions or any other chronic medical conditions that were not well controlled also were considered ineligible.
As a pilot study, a sample size of 20 participants was needed based on available funding.
Assessments
At baseline, the diagnosis of plaque-type psoriasis in each participant was confirmed by the investigator. Each participant’s medical history was obtained and all prior and current medications were reviewed to ensure eligibility criteria were met. Female participants of childbearing potential also underwent a urine pregnancy test. Consent was obtained from all enrolled participants. Investigators assessed the severity of psoriasis at baseline using the IGA.
All participants then were randomized (1:1 randomization) for treatment with either calcipotriene 0.005%–betamethasone dipropionate 0.064% ointment or calcipotriene 0.005%–betamethasone dipropionate 0.064% topical suspension. A simple random sampling chart was prepared and used by the study staff to determine treatment group allotment. Participants were instructed to apply the assigned study drug to affected areas of the body, excluding the scalp, face, and intertriginous areas, once daily for 3 days. Participants and study staff were aware of the study product being used, while investigators remained blinded. Participants also were asked to maintain a daily medication diary noting when the medication was applied.
Participants returned on day 3 for crossover treatment. Participants were asked to complete a subjective participant preference survey and study staff reviewed concomitant medications and adverse events. The packaging for the initial study drug was collected and weighed, and the crossover drug was dispensed to each participant to be applied once daily for 3 days.
Participants returned on day 6 or 7 for follow-up and were again asked to complete the subjective participant preference survey; study staff reviewed concomitant medications and adverse events. The packaging for the crossover study drug was collected and weighed, and the participant’s medication diary also was collected.
Subjective Participant Preference Survey
The subjective participant preference survey consisted of 15 questions relating to the participant’s experience with the study drug (eg, how the product felt to touch, amount of greasiness, time it took to apply). The final survey question asked participants to rate the overall appeal of the vehicle. Participants responded to the questions using a 7-point grading scale (1=extremely unappealing; 4=neutral; 7=extremely appealing). Total preference scores could range from 15 to 105.
End Points
The primary end point was the mean total preference score for each study drug obtained from the subjective participant preference surveys. Secondary end points included median values for individual survey questions and treatment adherence, which was measured from self-reported medication diary entries.
Statistical Analysis
Participant characteristics were reported with percentages for dichotomous data and median and ranges for other data. Subjective participant preference survey scores were calculated by taking the mean (standard deviation [SD]) sum of the scores for each individual survey item for both products. A generalized linear model that accounted for possible carryover and period effects was used to compare the difference of individual participant scores for the 2 products using SAS software. The mean (SD) amount of product used was reported and correlated to the preference score using a Spearman rank correlation. Total and individual survey scores were compared between sexes using Wilcoxon rank sum tests.
Results
Participants were enrolled in and completed the study from January 2013 to March 2013. The Figure presents a diagram of the Consolidated Standards of Reporting Trials. Thirty patients were screened; 10 patients did not meet eligibility criteria. Twenty patients were enrolled in the study with 10 patients randomized to each study arm. All 20 participants completed the study.
Participant demographics are described in Table 1. The median age was 48 years (range 29–64 years). The majority of participants were male (13/20) and white (18/20). The median IGA score was 3 (range, 2–3).
The mean (SD) total preference score for the calcipotriene 0.005%–betamethasone dipropionate 0.064% ointment formulation was 73.5 (19.4) and 80 (20.2) for the topical suspension formulation. The difference between means was -6.5 (95% confidence interval, -19.7-6.8; P=.32 after adjusting for possible carryover and period effects). Participants used a mean (SD) of 11 (11.4) g of product per study arm for the ointment formulation and 8.8 (6.6) g for the topical suspension formulation. There was no correlation between the amount of product used and preference for product (Spearman r=-0.01; P=.94). No statistically significant difference in product preference among men versus women was noted when considering total preference score or median scores of individual survey questions. Median overall appeal rating for the ointment formulation was 5 (slightly appealing) versus 6 (moderately appealing) for the topical suspension formulation, approaching statistical significance with P=.06 (Table 2). No significant carryover effects from one product to the other were noted (P=.64). The mean (SD) total preference scores were 81.1 (18.4) and 77.6 (21.2) in participants who used the topical suspension first followed by the ointment. In participants who used the ointment first followed by the topical suspension, the mean (SD) total preference scores were 69.4 (17.6) and 78.9 (22.8). Self-reported treatment adherence according to the participant’s daily medication diary was 100%.
Adverse effects during the study included 1 report of neck and back muscle pain and 1 report of sinusitis; neither was considered to be related to the study drugs.
Comment
Psoriasis is a chronic disease that can be difficult to treat, and treatment compliance often is poor. Multiple topical agents often are needed for adequate disease control, and adherence can be an even greater hurdle than with monotherapy. Combination products such as calcipotriene–betamethasone dipropionate offer the potential advantage of once-daily application. Adherence to once-daily application regimens for treatment of psoriasis has been shown to be greater than twice-daily application (82% vs 44%).4 However, the vehicle may be an adherence barrier for some patients.
Calcipotriene 0.005%–betamethasone dipropionate 0.064% topical suspension originally was indicated for the treatment of psoriasis of the scalp; however, it is now also indicated for treatment of psoriasis of the body.12 This topical suspension formulation is less messy, which could potentially be more cosmetically appealing and useful for improving treatment adherence.
Overall, the participants in our small pilot study showed a preference for the topical suspension versus the ointment formulation. The difference was substantial but was not statistically significant. This result is consistent with a previous study in which patients were found to prefer solutions that were less greasy compared to messy sticky ointments.10 Although the topical suspension received a higher average rating from participants for how it felt to touch and how it felt under clothing than the ointment and the ointment was rated on average as more greasy (none of these individual items achieved statistical significance), the calcipotriene–betamethasone dipropionate ointment was still rated as slightly appealing overall (Table 2). This result supports the need for physicians to discuss individual patient preferences when choosing the most appropriate vehicle for topical psoriasis treatment.
In our study, participants were found to use less product during treatment with the topical suspension versus the ointment, likely because the topical suspension formulation is thinner and spreads easier; however, participants rated the ease of application of the 2 products equally (Table 2). Ease of application was rated moderately appealing and time to apply was rated slightly to moderately appealing, which is important because patients often cite these factors as barriers to treatment adherence.2,6,13
The occlusive properties of ointment formulations provide moisturization by preventing water loss, a property that can be desirable when treating psoriatic plaques. Unlike the ointment, which was formulated with petrolatum and mineral oil, the calcipotriene–betamethasone dipropionate topical suspension was formulated with hydrogenated castor oil and mineral oil to provide moisturization. Nevertheless, participants found both the ointment and topical suspension to be moderately appealing (median score, 6; P=.94) for moisturization.
Limitations of this pilot study include the small sample size, which restricted the extent of subgroup analyses and the generalizability of our results. The small sample size also may or may not have contributed to the lack of statistical significance in the majority of the outcomes. This study provides pilot data that can be used to define a larger study; however, we do not think that a larger study is needed, as patients can be offered both vehicles in a practical clinical setting and can choose the product that is right for them. The short treatment duration of 3 days for each formulation also is a limitation, as a patient’s preference may change over time with longer use of the product. Treatment efficacy, which was not measured in this study, also could have an effect on patient preference, which could be assessed over a longer treatment period. Additionally, the study drugs may not be representative of all ointments or topical suspensions in their cosmetic appeal.
Conclusion
In this small cohort of plaque psoriasis patients, a calcipotriene–betamethasone dipropionate topical suspension was preferred over an ointment formulation, but in clinical practice it may be best to allow patients to choose the vehicle formulation that is most desirable on an individual basis. The topical suspension provides clinicians with an alternative that not only has the benefits of a combination product but also has been found to be appealing to patients.
1. Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
2. Brown KK, Rehmus WE, Kimball AB. Determining the relative importance of patient motivations for nonadherence to topical corticosteroid therapy in psoriasis. J Am Acad Dermatol. 2006;55:607-613.
3. Carroll CL, Feldman SR, Camacho FT, et al. Adherence to topical therapy decreases during the course of an 8-week psoriasis clinical trial: commonly used methods of measuring adherence to topical therapy overestimate actual use. J Am Acad Dermatol. 2004;51:212-216.
4. Zaghloul SS, Goodfield MJ. Objective assessment of compliance with psoriasis treatment. Arch Dermatol. 2004;140:408-414.
5. Richards HL, Fortune DG, O’Sullivan TM, et al. Patients with psoriasis and their compliance with medication. J Am Acad Dermatol. 1999;41:581-583.
6. Fouere S, Adjadj L, Pawin H. How patients experience psoriasis: results from a European survey. J Eur Acad Dermatol Venereol. 2005;19:S2-S6.
7. van de Kerkhof PC, Steegers-Theunissen RP, Kuipers MV. Evaluation of topical drug treatment in psoriasis. Dermatology. 1998;197:31-36.
8. Devaux S, Castela A, Archier E, et al. Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):S61-S67.
9. Chan SA, Hussain F, Lawson LG, et al. Factors affecting adherence to treatment of psoriasis: comparing biologic therapy to other modalities. J Dermatolog Treat. 2013;24:64-69.
10. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
11. Warino L, Balkrishnan R, Feldman SR. Clobetasol propionate for psoriasis: are ointments really more potent? J Drugs Dermatol. 2006;5:527-532.
12. Menter A, Gold LS, Bukhalo M, et al. Calcipotriene plus betamethasone dipropionate topical suspension for the treatment of mild to moderate psoriasis vulgaris on the body: a randomized, double-blind, vehicle-controlled trial. J Drugs Dermatol. 2013;12:92-98.
13. Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
1. Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
2. Brown KK, Rehmus WE, Kimball AB. Determining the relative importance of patient motivations for nonadherence to topical corticosteroid therapy in psoriasis. J Am Acad Dermatol. 2006;55:607-613.
3. Carroll CL, Feldman SR, Camacho FT, et al. Adherence to topical therapy decreases during the course of an 8-week psoriasis clinical trial: commonly used methods of measuring adherence to topical therapy overestimate actual use. J Am Acad Dermatol. 2004;51:212-216.
4. Zaghloul SS, Goodfield MJ. Objective assessment of compliance with psoriasis treatment. Arch Dermatol. 2004;140:408-414.
5. Richards HL, Fortune DG, O’Sullivan TM, et al. Patients with psoriasis and their compliance with medication. J Am Acad Dermatol. 1999;41:581-583.
6. Fouere S, Adjadj L, Pawin H. How patients experience psoriasis: results from a European survey. J Eur Acad Dermatol Venereol. 2005;19:S2-S6.
7. van de Kerkhof PC, Steegers-Theunissen RP, Kuipers MV. Evaluation of topical drug treatment in psoriasis. Dermatology. 1998;197:31-36.
8. Devaux S, Castela A, Archier E, et al. Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):S61-S67.
9. Chan SA, Hussain F, Lawson LG, et al. Factors affecting adherence to treatment of psoriasis: comparing biologic therapy to other modalities. J Dermatolog Treat. 2013;24:64-69.
10. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
11. Warino L, Balkrishnan R, Feldman SR. Clobetasol propionate for psoriasis: are ointments really more potent? J Drugs Dermatol. 2006;5:527-532.
12. Menter A, Gold LS, Bukhalo M, et al. Calcipotriene plus betamethasone dipropionate topical suspension for the treatment of mild to moderate psoriasis vulgaris on the body: a randomized, double-blind, vehicle-controlled trial. J Drugs Dermatol. 2013;12:92-98.
13. Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
Practice Points
- Patient preference plays an important role in adherence to treatment regimens in chronic skin diseases such as psoriasis.
- The topical suspension formulation of calcipotriene 0.005%–betamethasone dipropionate 0.064% was preferred by psoriasis patients over the ointment; however, the availability of the 2 formulations provides patients with options and allows them to choose which product they prefer.