User login
FDA, FTC uniting to promote biosimilars
The Food and Drug Administration is collaborating with the Federal Trade Commission (FTC) to expand the biosimilars market.
The two agencies signed a joint statement on Feb. 3, 2020, outlining four sets of goals aimed at creating meaningful competition from biosimilars against their reference biologic products.
“Competition is key for helping American patients have access to affordable medicines,” FDA Commissioner Stephen Hahn, MD, said in a statement. “Strengthening efforts to curtail and discourage anticompetitive behavior is key for facilitating robust competition for patients in the biologics marketplace, including through biosimilars, bringing down the costs of these crucial products for patients.”
“We appreciate and applaud the FDA and FTC in recognizing that biosimilar development and approval has not been as robust as many stakeholders had hoped,” said Colin Edgerton, MD, chair of the American College of Rheumatology’s Committee on Rheumatologic Care. “We continue to see anticompetitive activities that prevent manufacturers from developing biosimilar products. We hope that a greater focus on these practices will pave the way for more biosimilars to be developed.”
The statement highlighted four goals. First is that the agencies will coordinate to promote greater competition in the biologic market, including the development of materials to educate the market about biosimilars. The FDA and FTC also will be sponsoring a public workshop on March 9 to discuss competition for biologics.
“This workshop is the first step,” Dr. Edgerton said. “ACR will continue to work with other organizations and patient groups to help educate providers and patients on the scientific rigor that is required in developing and approving biosimilars. Additionally, we look forward to working with the FDA and FTC to continue this conversation on ways to encourage more development of biosimilar products and greater education for the providers and patients.”
The second goal has the FDA and FTC working together “to deter behavior that impedes access to samples needed for the development of biologics, including biosimilars,” the joint statement notes.
Third, the agencies will crack down on “false or misleading communications about biologics, including biosimilars, within their respective authorities,” according to the joint statement.
“FDA and FTC, as authorized by their respective statutes, will work together to address false or misleading communications about biologics, including biosimilars,” the statement continues. “In particular, if a communication makes a false or misleading comparison between a reference product and a biosimilar in a manner that misrepresents the safety or efficacy of biosimilars, deceives consumers, or deters competition, FDA and FTC intend to take appropriate action within their respective authorities. FDA intends to take appropriate action to address such communications where those communications have the potential to impact public health.”
Finally, the FTC committed to review patent settlement agreements involving biologics, including biosimilars, for antitrust violations.
Dr. Edgerton highlighted why this agreement between the two agencies is so important.
“Biologics are life-changing treatments for many of our patients,” he said. “Due to the high cost of discovery and development, the cost of biologics has resulted in delayed access and financial hardships for so many. It has always been our hope that biosimilars would offer the same life-changing treatment for patients at a lower price point. A robust biosimilars market is imperative to allow greater access to these treatments that can help patients to have a better quality of life.”
Separately, the FDA issued a draft guidance document for comment on manufacturers seeking licensure of biosimilar products that do not cover all the approved uses of the reference product, as well as how to add uses over time that were not part of the initial license of the biosimilar product. The draft guidance covers licensure of products, labeling of biosimilars with fewer indications than the reference product, supplemental applications for indications not on the initial biosimilar application but covered by the reference product, and the timing of applications.
The FDA notes in the draft guidance that this is needed to cover situations such as when some indications on the reference product are covered by exclusivity, although it does encourage a biosimilar manufacturer to seek licensure for all indications that the reference product does have.
The Food and Drug Administration is collaborating with the Federal Trade Commission (FTC) to expand the biosimilars market.
The two agencies signed a joint statement on Feb. 3, 2020, outlining four sets of goals aimed at creating meaningful competition from biosimilars against their reference biologic products.
“Competition is key for helping American patients have access to affordable medicines,” FDA Commissioner Stephen Hahn, MD, said in a statement. “Strengthening efforts to curtail and discourage anticompetitive behavior is key for facilitating robust competition for patients in the biologics marketplace, including through biosimilars, bringing down the costs of these crucial products for patients.”
“We appreciate and applaud the FDA and FTC in recognizing that biosimilar development and approval has not been as robust as many stakeholders had hoped,” said Colin Edgerton, MD, chair of the American College of Rheumatology’s Committee on Rheumatologic Care. “We continue to see anticompetitive activities that prevent manufacturers from developing biosimilar products. We hope that a greater focus on these practices will pave the way for more biosimilars to be developed.”
The statement highlighted four goals. First is that the agencies will coordinate to promote greater competition in the biologic market, including the development of materials to educate the market about biosimilars. The FDA and FTC also will be sponsoring a public workshop on March 9 to discuss competition for biologics.
“This workshop is the first step,” Dr. Edgerton said. “ACR will continue to work with other organizations and patient groups to help educate providers and patients on the scientific rigor that is required in developing and approving biosimilars. Additionally, we look forward to working with the FDA and FTC to continue this conversation on ways to encourage more development of biosimilar products and greater education for the providers and patients.”
The second goal has the FDA and FTC working together “to deter behavior that impedes access to samples needed for the development of biologics, including biosimilars,” the joint statement notes.
Third, the agencies will crack down on “false or misleading communications about biologics, including biosimilars, within their respective authorities,” according to the joint statement.
“FDA and FTC, as authorized by their respective statutes, will work together to address false or misleading communications about biologics, including biosimilars,” the statement continues. “In particular, if a communication makes a false or misleading comparison between a reference product and a biosimilar in a manner that misrepresents the safety or efficacy of biosimilars, deceives consumers, or deters competition, FDA and FTC intend to take appropriate action within their respective authorities. FDA intends to take appropriate action to address such communications where those communications have the potential to impact public health.”
Finally, the FTC committed to review patent settlement agreements involving biologics, including biosimilars, for antitrust violations.
Dr. Edgerton highlighted why this agreement between the two agencies is so important.
“Biologics are life-changing treatments for many of our patients,” he said. “Due to the high cost of discovery and development, the cost of biologics has resulted in delayed access and financial hardships for so many. It has always been our hope that biosimilars would offer the same life-changing treatment for patients at a lower price point. A robust biosimilars market is imperative to allow greater access to these treatments that can help patients to have a better quality of life.”
Separately, the FDA issued a draft guidance document for comment on manufacturers seeking licensure of biosimilar products that do not cover all the approved uses of the reference product, as well as how to add uses over time that were not part of the initial license of the biosimilar product. The draft guidance covers licensure of products, labeling of biosimilars with fewer indications than the reference product, supplemental applications for indications not on the initial biosimilar application but covered by the reference product, and the timing of applications.
The FDA notes in the draft guidance that this is needed to cover situations such as when some indications on the reference product are covered by exclusivity, although it does encourage a biosimilar manufacturer to seek licensure for all indications that the reference product does have.
The Food and Drug Administration is collaborating with the Federal Trade Commission (FTC) to expand the biosimilars market.
The two agencies signed a joint statement on Feb. 3, 2020, outlining four sets of goals aimed at creating meaningful competition from biosimilars against their reference biologic products.
“Competition is key for helping American patients have access to affordable medicines,” FDA Commissioner Stephen Hahn, MD, said in a statement. “Strengthening efforts to curtail and discourage anticompetitive behavior is key for facilitating robust competition for patients in the biologics marketplace, including through biosimilars, bringing down the costs of these crucial products for patients.”
“We appreciate and applaud the FDA and FTC in recognizing that biosimilar development and approval has not been as robust as many stakeholders had hoped,” said Colin Edgerton, MD, chair of the American College of Rheumatology’s Committee on Rheumatologic Care. “We continue to see anticompetitive activities that prevent manufacturers from developing biosimilar products. We hope that a greater focus on these practices will pave the way for more biosimilars to be developed.”
The statement highlighted four goals. First is that the agencies will coordinate to promote greater competition in the biologic market, including the development of materials to educate the market about biosimilars. The FDA and FTC also will be sponsoring a public workshop on March 9 to discuss competition for biologics.
“This workshop is the first step,” Dr. Edgerton said. “ACR will continue to work with other organizations and patient groups to help educate providers and patients on the scientific rigor that is required in developing and approving biosimilars. Additionally, we look forward to working with the FDA and FTC to continue this conversation on ways to encourage more development of biosimilar products and greater education for the providers and patients.”
The second goal has the FDA and FTC working together “to deter behavior that impedes access to samples needed for the development of biologics, including biosimilars,” the joint statement notes.
Third, the agencies will crack down on “false or misleading communications about biologics, including biosimilars, within their respective authorities,” according to the joint statement.
“FDA and FTC, as authorized by their respective statutes, will work together to address false or misleading communications about biologics, including biosimilars,” the statement continues. “In particular, if a communication makes a false or misleading comparison between a reference product and a biosimilar in a manner that misrepresents the safety or efficacy of biosimilars, deceives consumers, or deters competition, FDA and FTC intend to take appropriate action within their respective authorities. FDA intends to take appropriate action to address such communications where those communications have the potential to impact public health.”
Finally, the FTC committed to review patent settlement agreements involving biologics, including biosimilars, for antitrust violations.
Dr. Edgerton highlighted why this agreement between the two agencies is so important.
“Biologics are life-changing treatments for many of our patients,” he said. “Due to the high cost of discovery and development, the cost of biologics has resulted in delayed access and financial hardships for so many. It has always been our hope that biosimilars would offer the same life-changing treatment for patients at a lower price point. A robust biosimilars market is imperative to allow greater access to these treatments that can help patients to have a better quality of life.”
Separately, the FDA issued a draft guidance document for comment on manufacturers seeking licensure of biosimilar products that do not cover all the approved uses of the reference product, as well as how to add uses over time that were not part of the initial license of the biosimilar product. The draft guidance covers licensure of products, labeling of biosimilars with fewer indications than the reference product, supplemental applications for indications not on the initial biosimilar application but covered by the reference product, and the timing of applications.
The FDA notes in the draft guidance that this is needed to cover situations such as when some indications on the reference product are covered by exclusivity, although it does encourage a biosimilar manufacturer to seek licensure for all indications that the reference product does have.
FDA opens the door to biosimilar insulin
The Food and Drug Administration published Feb. 21 in the Federal Register a final rule that transitions insulin and other products from regulation as a drug to a biologic. This will provide manufacturers access to the biosimilars approval pathway and is expected to bring more competition to the insulin market. The move comes as insulin manufacturers continue to get increased scrutiny over the significantly increased pricing of their products in recent years.
The transition was required under a provision of the Biologics Price Competition and Innovation Act of 2009.
The move is expected to have no impact on the distribution of insulin and other products affected by the transition.
“In general, prescribers should continue to prescribe and order insulin and other biological products the same way they did before the transition,” the FDA said in an FAQ on the transition for physicians and other health care workers. “In general, pharmacists should continue to dispense and counsel about insulin and other biological products the same way they did before the transition. Prescribers and pharmacists should ensure their patients understand there are no changes to the product and they should continue to use the product the same way as before the transition.”
Other products affected by the transition include human growth hormone (somatropin), pancrelipase, chorionic gonadotropin, follitropin alfa, and menotropins. Information on all the transitioning products will move from the Orange Book (which lists FDA-approved drug products with therapeutic equivalent evaluations) to the Purple Book (which lists FDA-licensed biological products with reference product exclusivity data and biosimilar/interchangeability evaluations).
The FDA in the FAQ reiterated its commitment to reviewing any applications for these transition products within 12 months of submission.
The Food and Drug Administration published Feb. 21 in the Federal Register a final rule that transitions insulin and other products from regulation as a drug to a biologic. This will provide manufacturers access to the biosimilars approval pathway and is expected to bring more competition to the insulin market. The move comes as insulin manufacturers continue to get increased scrutiny over the significantly increased pricing of their products in recent years.
The transition was required under a provision of the Biologics Price Competition and Innovation Act of 2009.
The move is expected to have no impact on the distribution of insulin and other products affected by the transition.
“In general, prescribers should continue to prescribe and order insulin and other biological products the same way they did before the transition,” the FDA said in an FAQ on the transition for physicians and other health care workers. “In general, pharmacists should continue to dispense and counsel about insulin and other biological products the same way they did before the transition. Prescribers and pharmacists should ensure their patients understand there are no changes to the product and they should continue to use the product the same way as before the transition.”
Other products affected by the transition include human growth hormone (somatropin), pancrelipase, chorionic gonadotropin, follitropin alfa, and menotropins. Information on all the transitioning products will move from the Orange Book (which lists FDA-approved drug products with therapeutic equivalent evaluations) to the Purple Book (which lists FDA-licensed biological products with reference product exclusivity data and biosimilar/interchangeability evaluations).
The FDA in the FAQ reiterated its commitment to reviewing any applications for these transition products within 12 months of submission.
The Food and Drug Administration published Feb. 21 in the Federal Register a final rule that transitions insulin and other products from regulation as a drug to a biologic. This will provide manufacturers access to the biosimilars approval pathway and is expected to bring more competition to the insulin market. The move comes as insulin manufacturers continue to get increased scrutiny over the significantly increased pricing of their products in recent years.
The transition was required under a provision of the Biologics Price Competition and Innovation Act of 2009.
The move is expected to have no impact on the distribution of insulin and other products affected by the transition.
“In general, prescribers should continue to prescribe and order insulin and other biological products the same way they did before the transition,” the FDA said in an FAQ on the transition for physicians and other health care workers. “In general, pharmacists should continue to dispense and counsel about insulin and other biological products the same way they did before the transition. Prescribers and pharmacists should ensure their patients understand there are no changes to the product and they should continue to use the product the same way as before the transition.”
Other products affected by the transition include human growth hormone (somatropin), pancrelipase, chorionic gonadotropin, follitropin alfa, and menotropins. Information on all the transitioning products will move from the Orange Book (which lists FDA-approved drug products with therapeutic equivalent evaluations) to the Purple Book (which lists FDA-licensed biological products with reference product exclusivity data and biosimilar/interchangeability evaluations).
The FDA in the FAQ reiterated its commitment to reviewing any applications for these transition products within 12 months of submission.
Two new Novel Coronavirus cases confirmed among quarantined U.S. patients
The Centers for Disease Control and Prevention announced two new patients now have the 2019 Novel Coronavirus (2019-nCoV), bringing the case total in the United States to 15.
The 14th case was discovered in California among a group of people under federal quarantine after returning from the Hubei Province in China. That patient was on a U.S. State Department–chartered flight that arrived in the United States on Feb. 7.
The 15th case was discovered in Texas among a group of people who also are under federal quarantine. That patient arrived on a State Department–chartered flight that arrived on Feb. 7. It is the first person in Texas that has tested positive for 2019-nCoV.
CDC said in a statement announcing the Texas case that there “will likely be additional cases in the coming days and weeks, including among other people recently returned from Wuhan.” Officials noted that more than 600 people who have returned as part of State Department–chartered flights are currently under that 14-day quarantine.
The agency is preparing for more widespread cases of 2019-nCoV.
Nancy Messonnier, MD, director of the CDC National Center for Immunization and Respiratory Diseases, said that containment has been the early focus for the agency.
“The goal of the measures we have taken to date are to slow the introduction and impact of this disease in the United States, but at some point, we are likely to see community spread in the U.S.,” Dr. Messonnier said during a Feb. 12 teleconference with reporters. She added that the federal response will change over time as the virus spreads.
Dr. Messonnier noted that public health officials are planning for the increased demands that a wider outbreak of 2019-nCov would place on the health care delivery system, including ensuring an adequate supply of medical equipment.
The Centers for Disease Control and Prevention announced two new patients now have the 2019 Novel Coronavirus (2019-nCoV), bringing the case total in the United States to 15.
The 14th case was discovered in California among a group of people under federal quarantine after returning from the Hubei Province in China. That patient was on a U.S. State Department–chartered flight that arrived in the United States on Feb. 7.
The 15th case was discovered in Texas among a group of people who also are under federal quarantine. That patient arrived on a State Department–chartered flight that arrived on Feb. 7. It is the first person in Texas that has tested positive for 2019-nCoV.
CDC said in a statement announcing the Texas case that there “will likely be additional cases in the coming days and weeks, including among other people recently returned from Wuhan.” Officials noted that more than 600 people who have returned as part of State Department–chartered flights are currently under that 14-day quarantine.
The agency is preparing for more widespread cases of 2019-nCoV.
Nancy Messonnier, MD, director of the CDC National Center for Immunization and Respiratory Diseases, said that containment has been the early focus for the agency.
“The goal of the measures we have taken to date are to slow the introduction and impact of this disease in the United States, but at some point, we are likely to see community spread in the U.S.,” Dr. Messonnier said during a Feb. 12 teleconference with reporters. She added that the federal response will change over time as the virus spreads.
Dr. Messonnier noted that public health officials are planning for the increased demands that a wider outbreak of 2019-nCov would place on the health care delivery system, including ensuring an adequate supply of medical equipment.
The Centers for Disease Control and Prevention announced two new patients now have the 2019 Novel Coronavirus (2019-nCoV), bringing the case total in the United States to 15.
The 14th case was discovered in California among a group of people under federal quarantine after returning from the Hubei Province in China. That patient was on a U.S. State Department–chartered flight that arrived in the United States on Feb. 7.
The 15th case was discovered in Texas among a group of people who also are under federal quarantine. That patient arrived on a State Department–chartered flight that arrived on Feb. 7. It is the first person in Texas that has tested positive for 2019-nCoV.
CDC said in a statement announcing the Texas case that there “will likely be additional cases in the coming days and weeks, including among other people recently returned from Wuhan.” Officials noted that more than 600 people who have returned as part of State Department–chartered flights are currently under that 14-day quarantine.
The agency is preparing for more widespread cases of 2019-nCoV.
Nancy Messonnier, MD, director of the CDC National Center for Immunization and Respiratory Diseases, said that containment has been the early focus for the agency.
“The goal of the measures we have taken to date are to slow the introduction and impact of this disease in the United States, but at some point, we are likely to see community spread in the U.S.,” Dr. Messonnier said during a Feb. 12 teleconference with reporters. She added that the federal response will change over time as the virus spreads.
Dr. Messonnier noted that public health officials are planning for the increased demands that a wider outbreak of 2019-nCov would place on the health care delivery system, including ensuring an adequate supply of medical equipment.
CDC confirms 13th case of coronavirus in U.S.
The Centers for Disease Control and Prevention announced the number of confirmed cases of the 2019 Novel Coronavirus (2019-nCoV) in the United States has reached 13.
The latest case, announced Feb. 11, 2020, by the CDC, was in a person in California who was previously under federal quarantine because the patient had traveled to Wuhan, China.
The CDC is currently looking into who the patient may have come in contact with to understand the potential for further spread of the coronavirus.
“The contact investigation is ongoing,” CDC principal deputy director Anne Schuchat, MD, said during a Feb. 11 press conference to provide an update on coronavirus containment activities being taken by the CDC.
Dr. Schuchat also addressed issues related to the laboratory test, as the patient in California was initially thought to be negative for the coronavirus.
“With other cases around the country that we are evaluating, we have been doing serial tests to understand whether they are still infectious” and to gather other information about how results change over time, Dr. Schuchat said.
She noted that the CDC does not “have as much information as we would like on the severity of the virus,” noting that there are many cases in China with severe reactions, while the 13 cases in the United States represent a much more mild reaction to the virus so far.
With the latest case in California, she noted that there was “probably a mix-up and the original test wasn’t negative,” although she did not elaborate on what the nature of the mix-up was, stating that was all the information that she had.
In general, Dr. Schuchat touted the actions taken by the CDC and the federal government focused primarily on containing the spread of the virus in the United States, including the implementation of travel advisories, quarantining passengers returning from China, as well as the new test kits that are being distributed by the agency across the nation and around the world. She also mentioned CDC staff are being deployed around the world to monitor the spreading of the disease and highlighted the outreach efforts to keep the public informed.
Dr. Schuchat highlighted the fact that, of the 13 cases in the United States, 11 were with patients that were in Wuhan, and only 2 were because of close contact with a patient, something that she attributed to the actions being taken.
She also noted that cases in the United States have not been as severe as they have been in China, where deaths have been attributed to the coronavirus outbreak. She added that there have been only two deaths outside of mainland China attributed to the coronavirus.
“Some of the steps the CDC has taken have really put us in better shape should widespread transmission occur in the United States,” she said.
Dr. Schuchat also highlighted that the first charter flight of people quarantined after returning from Wuhan have reached the 14-day milestone and should be on their way home beginning today.
The Centers for Disease Control and Prevention announced the number of confirmed cases of the 2019 Novel Coronavirus (2019-nCoV) in the United States has reached 13.
The latest case, announced Feb. 11, 2020, by the CDC, was in a person in California who was previously under federal quarantine because the patient had traveled to Wuhan, China.
The CDC is currently looking into who the patient may have come in contact with to understand the potential for further spread of the coronavirus.
“The contact investigation is ongoing,” CDC principal deputy director Anne Schuchat, MD, said during a Feb. 11 press conference to provide an update on coronavirus containment activities being taken by the CDC.
Dr. Schuchat also addressed issues related to the laboratory test, as the patient in California was initially thought to be negative for the coronavirus.
“With other cases around the country that we are evaluating, we have been doing serial tests to understand whether they are still infectious” and to gather other information about how results change over time, Dr. Schuchat said.
She noted that the CDC does not “have as much information as we would like on the severity of the virus,” noting that there are many cases in China with severe reactions, while the 13 cases in the United States represent a much more mild reaction to the virus so far.
With the latest case in California, she noted that there was “probably a mix-up and the original test wasn’t negative,” although she did not elaborate on what the nature of the mix-up was, stating that was all the information that she had.
In general, Dr. Schuchat touted the actions taken by the CDC and the federal government focused primarily on containing the spread of the virus in the United States, including the implementation of travel advisories, quarantining passengers returning from China, as well as the new test kits that are being distributed by the agency across the nation and around the world. She also mentioned CDC staff are being deployed around the world to monitor the spreading of the disease and highlighted the outreach efforts to keep the public informed.
Dr. Schuchat highlighted the fact that, of the 13 cases in the United States, 11 were with patients that were in Wuhan, and only 2 were because of close contact with a patient, something that she attributed to the actions being taken.
She also noted that cases in the United States have not been as severe as they have been in China, where deaths have been attributed to the coronavirus outbreak. She added that there have been only two deaths outside of mainland China attributed to the coronavirus.
“Some of the steps the CDC has taken have really put us in better shape should widespread transmission occur in the United States,” she said.
Dr. Schuchat also highlighted that the first charter flight of people quarantined after returning from Wuhan have reached the 14-day milestone and should be on their way home beginning today.
The Centers for Disease Control and Prevention announced the number of confirmed cases of the 2019 Novel Coronavirus (2019-nCoV) in the United States has reached 13.
The latest case, announced Feb. 11, 2020, by the CDC, was in a person in California who was previously under federal quarantine because the patient had traveled to Wuhan, China.
The CDC is currently looking into who the patient may have come in contact with to understand the potential for further spread of the coronavirus.
“The contact investigation is ongoing,” CDC principal deputy director Anne Schuchat, MD, said during a Feb. 11 press conference to provide an update on coronavirus containment activities being taken by the CDC.
Dr. Schuchat also addressed issues related to the laboratory test, as the patient in California was initially thought to be negative for the coronavirus.
“With other cases around the country that we are evaluating, we have been doing serial tests to understand whether they are still infectious” and to gather other information about how results change over time, Dr. Schuchat said.
She noted that the CDC does not “have as much information as we would like on the severity of the virus,” noting that there are many cases in China with severe reactions, while the 13 cases in the United States represent a much more mild reaction to the virus so far.
With the latest case in California, she noted that there was “probably a mix-up and the original test wasn’t negative,” although she did not elaborate on what the nature of the mix-up was, stating that was all the information that she had.
In general, Dr. Schuchat touted the actions taken by the CDC and the federal government focused primarily on containing the spread of the virus in the United States, including the implementation of travel advisories, quarantining passengers returning from China, as well as the new test kits that are being distributed by the agency across the nation and around the world. She also mentioned CDC staff are being deployed around the world to monitor the spreading of the disease and highlighted the outreach efforts to keep the public informed.
Dr. Schuchat highlighted the fact that, of the 13 cases in the United States, 11 were with patients that were in Wuhan, and only 2 were because of close contact with a patient, something that she attributed to the actions being taken.
She also noted that cases in the United States have not been as severe as they have been in China, where deaths have been attributed to the coronavirus outbreak. She added that there have been only two deaths outside of mainland China attributed to the coronavirus.
“Some of the steps the CDC has taken have really put us in better shape should widespread transmission occur in the United States,” she said.
Dr. Schuchat also highlighted that the first charter flight of people quarantined after returning from Wuhan have reached the 14-day milestone and should be on their way home beginning today.
CMS proposes second specialty tier for Medicare drugs
The Centers for Medicare & Medicaid Services’ latest maneuver to combat rising drug prices is the proposed addition of a second specialty drug tier for the Medicare Part D prescription drug benefit.
The proposal is part of a broader proposed update to Medicare Parts C and D for contract years 2021 and 2022.
In a fact sheet highlighting various elements of the overall proposal, CMS noted that Part D plan sponsors and pharmacy benefit managers have been requesting the option to add a second “preferred” specialty tier that would “encourage the use of more preferred, less expensive agents, reduce enrollee cost sharing, and reduce costs to CMS.”
Currently, all pharmaceuticals with a cost greater than $670 are placed in a single specialty tier.
During a Feb. 5 press briefing, CMS Administrator Seema Verma described this change as “giving plans more negotiating power so they can lower prices for beneficiaries even further.”
Ms. Verma used a hypothetical example of two rheumatoid arthritis drugs to illustrate how the change will work. Currently, if both are over the $670 threshold, they would both be on the specialty tier with the same cost sharing. “Creating a second preferred specialty tier would allow for a different copay and fosters a more competitive environment that places Part D plans in a better position to negotiate the price of similar drugs and pass those savings onto the patient through lower cost sharing,” she said.
CMS is proposing to allow plans to implement a preferred specialty tier for the 2021 plan year.
The agency is also seeking to drive more generic drug use as a means of lowering costs.
Ms. Verma noted that, typically, even after a generic drug is launched, health plan sponsors prefer to drive patients to the brand name product, if they can secure a greater rebate from the manufacturer.
In a separate Feb. 5 blog post, Ms. Verma noted that when a brand was included on a formulary, the generic was also on the formulary 91.8% of the time. For the times in which the generic was not, it was typically because the wholesale cost of the generic was only 5%-15% lower than the brand wholesale cost.
In an effort to encourage use of generics, CMS is seeking comment on the development of measures of generic and biosimilar use in Medicare Part D that could be incorporated in health plan star ratings.
Some of the measures proposed in the blog post include the generic substitution rate, the generic therapeutic alternative opportunity rate (which measures the number of brand fills divided by the sum of the brand and generic fills when both are available), and the biosimilar utilization rate.
gtwachtman@mdedge.com
The Centers for Medicare & Medicaid Services’ latest maneuver to combat rising drug prices is the proposed addition of a second specialty drug tier for the Medicare Part D prescription drug benefit.
The proposal is part of a broader proposed update to Medicare Parts C and D for contract years 2021 and 2022.
In a fact sheet highlighting various elements of the overall proposal, CMS noted that Part D plan sponsors and pharmacy benefit managers have been requesting the option to add a second “preferred” specialty tier that would “encourage the use of more preferred, less expensive agents, reduce enrollee cost sharing, and reduce costs to CMS.”
Currently, all pharmaceuticals with a cost greater than $670 are placed in a single specialty tier.
During a Feb. 5 press briefing, CMS Administrator Seema Verma described this change as “giving plans more negotiating power so they can lower prices for beneficiaries even further.”
Ms. Verma used a hypothetical example of two rheumatoid arthritis drugs to illustrate how the change will work. Currently, if both are over the $670 threshold, they would both be on the specialty tier with the same cost sharing. “Creating a second preferred specialty tier would allow for a different copay and fosters a more competitive environment that places Part D plans in a better position to negotiate the price of similar drugs and pass those savings onto the patient through lower cost sharing,” she said.
CMS is proposing to allow plans to implement a preferred specialty tier for the 2021 plan year.
The agency is also seeking to drive more generic drug use as a means of lowering costs.
Ms. Verma noted that, typically, even after a generic drug is launched, health plan sponsors prefer to drive patients to the brand name product, if they can secure a greater rebate from the manufacturer.
In a separate Feb. 5 blog post, Ms. Verma noted that when a brand was included on a formulary, the generic was also on the formulary 91.8% of the time. For the times in which the generic was not, it was typically because the wholesale cost of the generic was only 5%-15% lower than the brand wholesale cost.
In an effort to encourage use of generics, CMS is seeking comment on the development of measures of generic and biosimilar use in Medicare Part D that could be incorporated in health plan star ratings.
Some of the measures proposed in the blog post include the generic substitution rate, the generic therapeutic alternative opportunity rate (which measures the number of brand fills divided by the sum of the brand and generic fills when both are available), and the biosimilar utilization rate.
gtwachtman@mdedge.com
The Centers for Medicare & Medicaid Services’ latest maneuver to combat rising drug prices is the proposed addition of a second specialty drug tier for the Medicare Part D prescription drug benefit.
The proposal is part of a broader proposed update to Medicare Parts C and D for contract years 2021 and 2022.
In a fact sheet highlighting various elements of the overall proposal, CMS noted that Part D plan sponsors and pharmacy benefit managers have been requesting the option to add a second “preferred” specialty tier that would “encourage the use of more preferred, less expensive agents, reduce enrollee cost sharing, and reduce costs to CMS.”
Currently, all pharmaceuticals with a cost greater than $670 are placed in a single specialty tier.
During a Feb. 5 press briefing, CMS Administrator Seema Verma described this change as “giving plans more negotiating power so they can lower prices for beneficiaries even further.”
Ms. Verma used a hypothetical example of two rheumatoid arthritis drugs to illustrate how the change will work. Currently, if both are over the $670 threshold, they would both be on the specialty tier with the same cost sharing. “Creating a second preferred specialty tier would allow for a different copay and fosters a more competitive environment that places Part D plans in a better position to negotiate the price of similar drugs and pass those savings onto the patient through lower cost sharing,” she said.
CMS is proposing to allow plans to implement a preferred specialty tier for the 2021 plan year.
The agency is also seeking to drive more generic drug use as a means of lowering costs.
Ms. Verma noted that, typically, even after a generic drug is launched, health plan sponsors prefer to drive patients to the brand name product, if they can secure a greater rebate from the manufacturer.
In a separate Feb. 5 blog post, Ms. Verma noted that when a brand was included on a formulary, the generic was also on the formulary 91.8% of the time. For the times in which the generic was not, it was typically because the wholesale cost of the generic was only 5%-15% lower than the brand wholesale cost.
In an effort to encourage use of generics, CMS is seeking comment on the development of measures of generic and biosimilar use in Medicare Part D that could be incorporated in health plan star ratings.
Some of the measures proposed in the blog post include the generic substitution rate, the generic therapeutic alternative opportunity rate (which measures the number of brand fills divided by the sum of the brand and generic fills when both are available), and the biosimilar utilization rate.
gtwachtman@mdedge.com
CDC begins coronavirus diagnostic test kit distribution; new case confirmed in Wisconsin
The Centers for Disease Control and Prevention and the Wisconsin Department of Health Services confirmed a new case of the 2019 Novel Coronavirus (2019-nCoV) on Feb. 5, 2020, bringing the total number of cases in the United States to 12.*
Earlier in the day, Nancy Messonnier, MD, director of the CDC National Center for Immunization and Respiratory Diseases, told reporters that 206 individuals under investigation had tested negative for infection with the novel virus and that tests were pending on another 76 individuals.
The agency also announced during a press briefing call that diagnostic test kits will begin shipping on Feb. 5, less than 24 hours after receiving an emergency use authorization from the Food and Drug Administration. Full information is available in an article published in the Morbidity and Mortality Weekly Report.
The emergency use authorization will allow for broader use of the CDC’s 2019-nCoV Real Time RT-PCR Diagnostic Panel, which to date has been limited for use at CDC laboratories. Under the emergency use authorization, the diagnostic kit is authorized for patients who meed the CDC criteria for 2019-nCoV testing. The diagnostic test is a reverse transcriptase polymerase chain reaction test that provides presumptive detection of 2019-nCoV from respiratory secretions, such as nasal or oral swabs. A positive test indicates likely infection, although a negative test does not preclude infection and should not be the sole determination for patient management decisions.
“Today, the test kits will start shipping to over 100 U.S. public health labs,” she said. “Each of these labs is required to perform international verification for [Clinical Laboratory Improvement Amendments] compliance prior to reporting out. This process is expected to take a few days.”
Dr. Messonnier said that 200 test kits will be distributed to domestic labs and another 200 test kits will go to select international labs. Each kit can perform diagnostics on 700-800 patient samples.
“What that means is that, by the start of next week, we expect there to be much enhanced capacity for laboratory testing closer to our patients,” she said, adding that additional test kits are being produced and will be available for ordering in the future. Each laboratory that places an order will receive one test kit.
“Distribution of these tests will improve the global capacity to detect and respond to this new virus,” Dr. Messonnier said. “Availability of this test is a starting place for greater commercial availability of diagnostic testing for nCoV.”
The CDC also said that the next batch of passengers arriving from Wuhan, China, will be arriving in one of four locations: Travis Air Force Base, Fairfield, Calif.; Marine Corps Air Station Miramar, San Diego; Lackland Air Force Base, San Antonio; and Eppley Airfield, Omaha, Neb. Passengers will be quarantined for up to 14 days from the day the flight left Wuhan and medical care will be provided if needed.
“We do not believe these people pose a threat to the communities where they are being housed as we are taking measures to minimize any contact,” she said, adding that confirmed infections are expected among these and other returning travelers.
Dr. Messonnier warned that the quarantine measures “may not catch every single returning traveler returning with novel coronavirus, given the nature of this virus and how it is spreading. But if we can catch the majority of them, that will slow the entry of this virus into the United States.”
*This story was updated on 02/05/2020.
The Centers for Disease Control and Prevention and the Wisconsin Department of Health Services confirmed a new case of the 2019 Novel Coronavirus (2019-nCoV) on Feb. 5, 2020, bringing the total number of cases in the United States to 12.*
Earlier in the day, Nancy Messonnier, MD, director of the CDC National Center for Immunization and Respiratory Diseases, told reporters that 206 individuals under investigation had tested negative for infection with the novel virus and that tests were pending on another 76 individuals.
The agency also announced during a press briefing call that diagnostic test kits will begin shipping on Feb. 5, less than 24 hours after receiving an emergency use authorization from the Food and Drug Administration. Full information is available in an article published in the Morbidity and Mortality Weekly Report.
The emergency use authorization will allow for broader use of the CDC’s 2019-nCoV Real Time RT-PCR Diagnostic Panel, which to date has been limited for use at CDC laboratories. Under the emergency use authorization, the diagnostic kit is authorized for patients who meed the CDC criteria for 2019-nCoV testing. The diagnostic test is a reverse transcriptase polymerase chain reaction test that provides presumptive detection of 2019-nCoV from respiratory secretions, such as nasal or oral swabs. A positive test indicates likely infection, although a negative test does not preclude infection and should not be the sole determination for patient management decisions.
“Today, the test kits will start shipping to over 100 U.S. public health labs,” she said. “Each of these labs is required to perform international verification for [Clinical Laboratory Improvement Amendments] compliance prior to reporting out. This process is expected to take a few days.”
Dr. Messonnier said that 200 test kits will be distributed to domestic labs and another 200 test kits will go to select international labs. Each kit can perform diagnostics on 700-800 patient samples.
“What that means is that, by the start of next week, we expect there to be much enhanced capacity for laboratory testing closer to our patients,” she said, adding that additional test kits are being produced and will be available for ordering in the future. Each laboratory that places an order will receive one test kit.
“Distribution of these tests will improve the global capacity to detect and respond to this new virus,” Dr. Messonnier said. “Availability of this test is a starting place for greater commercial availability of diagnostic testing for nCoV.”
The CDC also said that the next batch of passengers arriving from Wuhan, China, will be arriving in one of four locations: Travis Air Force Base, Fairfield, Calif.; Marine Corps Air Station Miramar, San Diego; Lackland Air Force Base, San Antonio; and Eppley Airfield, Omaha, Neb. Passengers will be quarantined for up to 14 days from the day the flight left Wuhan and medical care will be provided if needed.
“We do not believe these people pose a threat to the communities where they are being housed as we are taking measures to minimize any contact,” she said, adding that confirmed infections are expected among these and other returning travelers.
Dr. Messonnier warned that the quarantine measures “may not catch every single returning traveler returning with novel coronavirus, given the nature of this virus and how it is spreading. But if we can catch the majority of them, that will slow the entry of this virus into the United States.”
*This story was updated on 02/05/2020.
The Centers for Disease Control and Prevention and the Wisconsin Department of Health Services confirmed a new case of the 2019 Novel Coronavirus (2019-nCoV) on Feb. 5, 2020, bringing the total number of cases in the United States to 12.*
Earlier in the day, Nancy Messonnier, MD, director of the CDC National Center for Immunization and Respiratory Diseases, told reporters that 206 individuals under investigation had tested negative for infection with the novel virus and that tests were pending on another 76 individuals.
The agency also announced during a press briefing call that diagnostic test kits will begin shipping on Feb. 5, less than 24 hours after receiving an emergency use authorization from the Food and Drug Administration. Full information is available in an article published in the Morbidity and Mortality Weekly Report.
The emergency use authorization will allow for broader use of the CDC’s 2019-nCoV Real Time RT-PCR Diagnostic Panel, which to date has been limited for use at CDC laboratories. Under the emergency use authorization, the diagnostic kit is authorized for patients who meed the CDC criteria for 2019-nCoV testing. The diagnostic test is a reverse transcriptase polymerase chain reaction test that provides presumptive detection of 2019-nCoV from respiratory secretions, such as nasal or oral swabs. A positive test indicates likely infection, although a negative test does not preclude infection and should not be the sole determination for patient management decisions.
“Today, the test kits will start shipping to over 100 U.S. public health labs,” she said. “Each of these labs is required to perform international verification for [Clinical Laboratory Improvement Amendments] compliance prior to reporting out. This process is expected to take a few days.”
Dr. Messonnier said that 200 test kits will be distributed to domestic labs and another 200 test kits will go to select international labs. Each kit can perform diagnostics on 700-800 patient samples.
“What that means is that, by the start of next week, we expect there to be much enhanced capacity for laboratory testing closer to our patients,” she said, adding that additional test kits are being produced and will be available for ordering in the future. Each laboratory that places an order will receive one test kit.
“Distribution of these tests will improve the global capacity to detect and respond to this new virus,” Dr. Messonnier said. “Availability of this test is a starting place for greater commercial availability of diagnostic testing for nCoV.”
The CDC also said that the next batch of passengers arriving from Wuhan, China, will be arriving in one of four locations: Travis Air Force Base, Fairfield, Calif.; Marine Corps Air Station Miramar, San Diego; Lackland Air Force Base, San Antonio; and Eppley Airfield, Omaha, Neb. Passengers will be quarantined for up to 14 days from the day the flight left Wuhan and medical care will be provided if needed.
“We do not believe these people pose a threat to the communities where they are being housed as we are taking measures to minimize any contact,” she said, adding that confirmed infections are expected among these and other returning travelers.
Dr. Messonnier warned that the quarantine measures “may not catch every single returning traveler returning with novel coronavirus, given the nature of this virus and how it is spreading. But if we can catch the majority of them, that will slow the entry of this virus into the United States.”
*This story was updated on 02/05/2020.
Physician groups push back on Medicaid block grant plan
It took less than a day for physician groups to start pushing back at the Centers for Medicare & Medicaid Services over its new Medicaid block grant plan, which was introduced on Jan. 30.
Dubbed “Healthy Adult Opportunity,” the agency is offering all states the chance to participate in a block grant program through the 1115 waiver process.
According to a fact sheet issued by the agency, the program will focus on “adults under age 65 who are not eligible for Medicaid on the basis of disability or their need for long term care services and supports, and who are not eligible under a state plan. Other very low-income parents, children, pregnant women, elderly adults, and people eligible on the basis of a disability will not be directly affected – except from the improvement that results from states reinvesting savings into strengthening their overall programs.”
States will be operating within a defined budget when participating in the program and expenditures exceeding that defined budget will not be eligible for additional federal funding. Budgets will be based on a state’s historic costs, as well as national and regional trends, and will be tied to inflation with the potential to have adjustments made for extraordinary events. States can set their baseline using the prior year’s total spending or a per-enrollee spending model.
A Jan. 30 letter to state Medicaid directors notes that states participating in the program “will be granted extensive flexibility to test alternative approaches to implementing their Medicaid programs, including the ability to make many ongoing program adjustments without the need for demonstration or state plan amendments that require prior approval.”
Among the activities states can engage in under this plan are adjusting cost-sharing requirements, adopting a closed formulary, and applying additional conditions of eligibility. Requests, if approved, will be approved for a 5-year initial period, with a renewal option of up to 10 years.
But physician groups are not seeing a benefit with this new block grant program.
“Moving to a block grant system will likely limit the ability of Medicaid patients to receive preventive and needed medical care from their family physicians, and it will only increase the health disparities that exist in these communities, worsen overall health outcomes, and ultimately increase costs,” Gary LeRoy, MD, president of the American Academy of Family Physicians, said in a statement.
The American Medical Association concurred.
“The AMA opposes caps on federal Medicaid funding, such as block grants, because they would increase the number of uninsured and undermine Medicaid’s role as an indispensable safety net,” Patrice Harris, MD, the AMA’s president, said in a statement. “The AMA supports flexibility in Medicaid and encourages CMS to work with states to develop and test new Medicaid models that best meet the needs and priorities of low-income patients. While encouraging flexibility, the AMA is mindful that expanding Medicaid has been a literal lifesaver for low-income patients. We need to find ways to build on this success. We look forward to reviewing the proposal in detail.”
Officials at the American College of Obstetricians and Gynecologists said the changes have the potential to harm women and children’s health, as well as negatively impact physician reimbursement and ultimately access to care.
“Limits on the federal contribution to the Medicaid program would negatively impact patients by forcing states to reduce the number of people who are eligible for Medicaid coverage, eliminate covered services, and increase beneficiary cost-sharing,” ACOG President Ted Anderson, MD, said in a statement. “ACOG is also concerned that this block grant opportunity could lower physician reimbursement for certain services, forcing providers out of the program and jeopardizing patients’ ability to access health care services. Given our nation’s stark rates of maternal mortality and severe maternal morbidity, we are alarmed by the Administration’s willingness to weaken physician payment in Medicaid.”
It took less than a day for physician groups to start pushing back at the Centers for Medicare & Medicaid Services over its new Medicaid block grant plan, which was introduced on Jan. 30.
Dubbed “Healthy Adult Opportunity,” the agency is offering all states the chance to participate in a block grant program through the 1115 waiver process.
According to a fact sheet issued by the agency, the program will focus on “adults under age 65 who are not eligible for Medicaid on the basis of disability or their need for long term care services and supports, and who are not eligible under a state plan. Other very low-income parents, children, pregnant women, elderly adults, and people eligible on the basis of a disability will not be directly affected – except from the improvement that results from states reinvesting savings into strengthening their overall programs.”
States will be operating within a defined budget when participating in the program and expenditures exceeding that defined budget will not be eligible for additional federal funding. Budgets will be based on a state’s historic costs, as well as national and regional trends, and will be tied to inflation with the potential to have adjustments made for extraordinary events. States can set their baseline using the prior year’s total spending or a per-enrollee spending model.
A Jan. 30 letter to state Medicaid directors notes that states participating in the program “will be granted extensive flexibility to test alternative approaches to implementing their Medicaid programs, including the ability to make many ongoing program adjustments without the need for demonstration or state plan amendments that require prior approval.”
Among the activities states can engage in under this plan are adjusting cost-sharing requirements, adopting a closed formulary, and applying additional conditions of eligibility. Requests, if approved, will be approved for a 5-year initial period, with a renewal option of up to 10 years.
But physician groups are not seeing a benefit with this new block grant program.
“Moving to a block grant system will likely limit the ability of Medicaid patients to receive preventive and needed medical care from their family physicians, and it will only increase the health disparities that exist in these communities, worsen overall health outcomes, and ultimately increase costs,” Gary LeRoy, MD, president of the American Academy of Family Physicians, said in a statement.
The American Medical Association concurred.
“The AMA opposes caps on federal Medicaid funding, such as block grants, because they would increase the number of uninsured and undermine Medicaid’s role as an indispensable safety net,” Patrice Harris, MD, the AMA’s president, said in a statement. “The AMA supports flexibility in Medicaid and encourages CMS to work with states to develop and test new Medicaid models that best meet the needs and priorities of low-income patients. While encouraging flexibility, the AMA is mindful that expanding Medicaid has been a literal lifesaver for low-income patients. We need to find ways to build on this success. We look forward to reviewing the proposal in detail.”
Officials at the American College of Obstetricians and Gynecologists said the changes have the potential to harm women and children’s health, as well as negatively impact physician reimbursement and ultimately access to care.
“Limits on the federal contribution to the Medicaid program would negatively impact patients by forcing states to reduce the number of people who are eligible for Medicaid coverage, eliminate covered services, and increase beneficiary cost-sharing,” ACOG President Ted Anderson, MD, said in a statement. “ACOG is also concerned that this block grant opportunity could lower physician reimbursement for certain services, forcing providers out of the program and jeopardizing patients’ ability to access health care services. Given our nation’s stark rates of maternal mortality and severe maternal morbidity, we are alarmed by the Administration’s willingness to weaken physician payment in Medicaid.”
It took less than a day for physician groups to start pushing back at the Centers for Medicare & Medicaid Services over its new Medicaid block grant plan, which was introduced on Jan. 30.
Dubbed “Healthy Adult Opportunity,” the agency is offering all states the chance to participate in a block grant program through the 1115 waiver process.
According to a fact sheet issued by the agency, the program will focus on “adults under age 65 who are not eligible for Medicaid on the basis of disability or their need for long term care services and supports, and who are not eligible under a state plan. Other very low-income parents, children, pregnant women, elderly adults, and people eligible on the basis of a disability will not be directly affected – except from the improvement that results from states reinvesting savings into strengthening their overall programs.”
States will be operating within a defined budget when participating in the program and expenditures exceeding that defined budget will not be eligible for additional federal funding. Budgets will be based on a state’s historic costs, as well as national and regional trends, and will be tied to inflation with the potential to have adjustments made for extraordinary events. States can set their baseline using the prior year’s total spending or a per-enrollee spending model.
A Jan. 30 letter to state Medicaid directors notes that states participating in the program “will be granted extensive flexibility to test alternative approaches to implementing their Medicaid programs, including the ability to make many ongoing program adjustments without the need for demonstration or state plan amendments that require prior approval.”
Among the activities states can engage in under this plan are adjusting cost-sharing requirements, adopting a closed formulary, and applying additional conditions of eligibility. Requests, if approved, will be approved for a 5-year initial period, with a renewal option of up to 10 years.
But physician groups are not seeing a benefit with this new block grant program.
“Moving to a block grant system will likely limit the ability of Medicaid patients to receive preventive and needed medical care from their family physicians, and it will only increase the health disparities that exist in these communities, worsen overall health outcomes, and ultimately increase costs,” Gary LeRoy, MD, president of the American Academy of Family Physicians, said in a statement.
The American Medical Association concurred.
“The AMA opposes caps on federal Medicaid funding, such as block grants, because they would increase the number of uninsured and undermine Medicaid’s role as an indispensable safety net,” Patrice Harris, MD, the AMA’s president, said in a statement. “The AMA supports flexibility in Medicaid and encourages CMS to work with states to develop and test new Medicaid models that best meet the needs and priorities of low-income patients. While encouraging flexibility, the AMA is mindful that expanding Medicaid has been a literal lifesaver for low-income patients. We need to find ways to build on this success. We look forward to reviewing the proposal in detail.”
Officials at the American College of Obstetricians and Gynecologists said the changes have the potential to harm women and children’s health, as well as negatively impact physician reimbursement and ultimately access to care.
“Limits on the federal contribution to the Medicaid program would negatively impact patients by forcing states to reduce the number of people who are eligible for Medicaid coverage, eliminate covered services, and increase beneficiary cost-sharing,” ACOG President Ted Anderson, MD, said in a statement. “ACOG is also concerned that this block grant opportunity could lower physician reimbursement for certain services, forcing providers out of the program and jeopardizing patients’ ability to access health care services. Given our nation’s stark rates of maternal mortality and severe maternal morbidity, we are alarmed by the Administration’s willingness to weaken physician payment in Medicaid.”
Costs are keeping Americans out of the doctor’s office
The cost of health care is keeping more Americans from seeing a doctor, even as the number of individuals with insurance coverage increases, according to a new study.
“Despite short-term gains owing to the [Affordable Care Act], over the past 20 years the portion of adults aged 18-64 years unable to see a physician owing to the cost increased, mostly because of an increase among persons with insurance,” Laura Hawks, MD, of Cambridge (Mass.) Health Alliance and Harvard Medical School in Boston and colleagues wrote in a new research report published in JAMA Internal Medicine.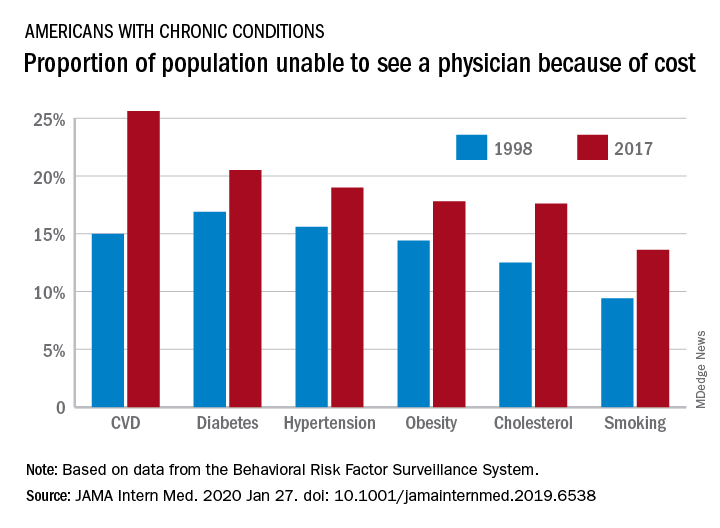
“In 2017, nearly one-fifth of individuals with any chronic condition (diabetes, obesity, or cardiovascular disease) said they were unable to see a physician owing to cost,” they continued.
Researchers examined 20 years of data (January 1998 through December 2017) from the Centers for Disease Control and Prevention’s Behavioral Risk Factor Surveillance System to identify trends in unmet need for physician and preventive services.
Among adults aged 18-64 years who responded to the survey in 1998 and 2017, uninsurance decreased by 2.1 percentage points, falling from 16.9% to 14.8%. But at the same time, the portion of adults who were unable to see a physician because of cost rose by 2.7 percentage points, from 11.4% to 15.7%. Looking specifically at adults who had insurance coverage, the researchers found that cost was a barrier for 11.5% of them in 2017, up from 7.1% in 1998.
These results come against a backdrop of growing medical costs, increasing deductibles and copayments, an increasing use of cost containment measures like prior authorization, and narrow provider networks in the wake of the transition to value-based payment structures, the authors noted.
“Our finding that financial access to physician care worsened is concerning,” Dr. Hawks and her colleagues wrote. “Persons with conditions such as diabetes, hypertension, cardiovascular disease, and poor health status risk substantial harms if they forgo physician care. Financial barriers to care have been associated with increased hospitalizations and worse health outcomes in patients with cardiovascular disease and hypertension and increased morbidity among patients with diabetes.”
One of the trends highlighted by the study authors is the growing number of employers offering plans with a high deductible.
“Enrollment in a high-deductible health plan, which has become increasingly common in the last decade, a trend uninterrupted by the ACA, is associated with forgoing needed care, especially among those of lower socioeconomic status,” the authors wrote. “Other changes in insurance benefit design, such as imposing tiered copayments and coinsurance obligations, eliminating coverage for some services (e.g., eyeglasses) and narrowing provider networks (which can force some patients to go out-of-network for care) may also have undermined the affordability of care.”
There was some positive news among the findings, however.
“The main encouraging finding from our analysis is the increase in the proportion of persons – both insured and uninsured – receiving cholesterol checks and flu shots,” Dr. Hawk and her colleagues wrote, adding that this increase “may be attributable to the increasing implementation of quality metrics, financial incentives, and improved systems for the delivery of these services.”
However, not all preventive services that had cost barriers eliminated under the ACA saw improvement, such as cancer screening. They note that the proportion of women who did not receive mammography increased during the study period and then plateaued, but did not improve following the implementation of the ACA. The authors described the reasons for this as “unclear.”
Dr. Hawks received funding support from an Institutional National Research Service award and from Cambridge Health Alliance, her employer. Other authors reported membership in Physicians for a National Health Program.
SOURCE: Hawks L et al. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6538.
The cost of health care is keeping more Americans from seeing a doctor, even as the number of individuals with insurance coverage increases, according to a new study.
“Despite short-term gains owing to the [Affordable Care Act], over the past 20 years the portion of adults aged 18-64 years unable to see a physician owing to the cost increased, mostly because of an increase among persons with insurance,” Laura Hawks, MD, of Cambridge (Mass.) Health Alliance and Harvard Medical School in Boston and colleagues wrote in a new research report published in JAMA Internal Medicine.
“In 2017, nearly one-fifth of individuals with any chronic condition (diabetes, obesity, or cardiovascular disease) said they were unable to see a physician owing to cost,” they continued.
Researchers examined 20 years of data (January 1998 through December 2017) from the Centers for Disease Control and Prevention’s Behavioral Risk Factor Surveillance System to identify trends in unmet need for physician and preventive services.
Among adults aged 18-64 years who responded to the survey in 1998 and 2017, uninsurance decreased by 2.1 percentage points, falling from 16.9% to 14.8%. But at the same time, the portion of adults who were unable to see a physician because of cost rose by 2.7 percentage points, from 11.4% to 15.7%. Looking specifically at adults who had insurance coverage, the researchers found that cost was a barrier for 11.5% of them in 2017, up from 7.1% in 1998.
These results come against a backdrop of growing medical costs, increasing deductibles and copayments, an increasing use of cost containment measures like prior authorization, and narrow provider networks in the wake of the transition to value-based payment structures, the authors noted.
“Our finding that financial access to physician care worsened is concerning,” Dr. Hawks and her colleagues wrote. “Persons with conditions such as diabetes, hypertension, cardiovascular disease, and poor health status risk substantial harms if they forgo physician care. Financial barriers to care have been associated with increased hospitalizations and worse health outcomes in patients with cardiovascular disease and hypertension and increased morbidity among patients with diabetes.”
One of the trends highlighted by the study authors is the growing number of employers offering plans with a high deductible.
“Enrollment in a high-deductible health plan, which has become increasingly common in the last decade, a trend uninterrupted by the ACA, is associated with forgoing needed care, especially among those of lower socioeconomic status,” the authors wrote. “Other changes in insurance benefit design, such as imposing tiered copayments and coinsurance obligations, eliminating coverage for some services (e.g., eyeglasses) and narrowing provider networks (which can force some patients to go out-of-network for care) may also have undermined the affordability of care.”
There was some positive news among the findings, however.
“The main encouraging finding from our analysis is the increase in the proportion of persons – both insured and uninsured – receiving cholesterol checks and flu shots,” Dr. Hawk and her colleagues wrote, adding that this increase “may be attributable to the increasing implementation of quality metrics, financial incentives, and improved systems for the delivery of these services.”
However, not all preventive services that had cost barriers eliminated under the ACA saw improvement, such as cancer screening. They note that the proportion of women who did not receive mammography increased during the study period and then plateaued, but did not improve following the implementation of the ACA. The authors described the reasons for this as “unclear.”
Dr. Hawks received funding support from an Institutional National Research Service award and from Cambridge Health Alliance, her employer. Other authors reported membership in Physicians for a National Health Program.
SOURCE: Hawks L et al. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6538.
The cost of health care is keeping more Americans from seeing a doctor, even as the number of individuals with insurance coverage increases, according to a new study.
“Despite short-term gains owing to the [Affordable Care Act], over the past 20 years the portion of adults aged 18-64 years unable to see a physician owing to the cost increased, mostly because of an increase among persons with insurance,” Laura Hawks, MD, of Cambridge (Mass.) Health Alliance and Harvard Medical School in Boston and colleagues wrote in a new research report published in JAMA Internal Medicine.
“In 2017, nearly one-fifth of individuals with any chronic condition (diabetes, obesity, or cardiovascular disease) said they were unable to see a physician owing to cost,” they continued.
Researchers examined 20 years of data (January 1998 through December 2017) from the Centers for Disease Control and Prevention’s Behavioral Risk Factor Surveillance System to identify trends in unmet need for physician and preventive services.
Among adults aged 18-64 years who responded to the survey in 1998 and 2017, uninsurance decreased by 2.1 percentage points, falling from 16.9% to 14.8%. But at the same time, the portion of adults who were unable to see a physician because of cost rose by 2.7 percentage points, from 11.4% to 15.7%. Looking specifically at adults who had insurance coverage, the researchers found that cost was a barrier for 11.5% of them in 2017, up from 7.1% in 1998.
These results come against a backdrop of growing medical costs, increasing deductibles and copayments, an increasing use of cost containment measures like prior authorization, and narrow provider networks in the wake of the transition to value-based payment structures, the authors noted.
“Our finding that financial access to physician care worsened is concerning,” Dr. Hawks and her colleagues wrote. “Persons with conditions such as diabetes, hypertension, cardiovascular disease, and poor health status risk substantial harms if they forgo physician care. Financial barriers to care have been associated with increased hospitalizations and worse health outcomes in patients with cardiovascular disease and hypertension and increased morbidity among patients with diabetes.”
One of the trends highlighted by the study authors is the growing number of employers offering plans with a high deductible.
“Enrollment in a high-deductible health plan, which has become increasingly common in the last decade, a trend uninterrupted by the ACA, is associated with forgoing needed care, especially among those of lower socioeconomic status,” the authors wrote. “Other changes in insurance benefit design, such as imposing tiered copayments and coinsurance obligations, eliminating coverage for some services (e.g., eyeglasses) and narrowing provider networks (which can force some patients to go out-of-network for care) may also have undermined the affordability of care.”
There was some positive news among the findings, however.
“The main encouraging finding from our analysis is the increase in the proportion of persons – both insured and uninsured – receiving cholesterol checks and flu shots,” Dr. Hawk and her colleagues wrote, adding that this increase “may be attributable to the increasing implementation of quality metrics, financial incentives, and improved systems for the delivery of these services.”
However, not all preventive services that had cost barriers eliminated under the ACA saw improvement, such as cancer screening. They note that the proportion of women who did not receive mammography increased during the study period and then plateaued, but did not improve following the implementation of the ACA. The authors described the reasons for this as “unclear.”
Dr. Hawks received funding support from an Institutional National Research Service award and from Cambridge Health Alliance, her employer. Other authors reported membership in Physicians for a National Health Program.
SOURCE: Hawks L et al. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6538.
FROM JAMA INTERNAL MEDICINE
ACP maps two potential paths to universal health care
The American College of Physicians is recommending either a single-payer system or a public option within a regulated private insurance system to help deliver universal and affordable access to health care for all Americans.
“We came to the conclusion that two directions or approaches could get us to where we need to be,” ACP President Robert McLean, MD, said in an interview. “We need ... a system that provides universal, affordable access to care.”
After examining the evidence, ACP discarded one option: a direct market-based approach.
“Direct market-based approaches won’t work,” Dr. McLean explained. “If you look at where direct marketplace approaches ... have been implemented, they just will not get you to a place where you are going to get universal coverage, portability, essential benefits, and preexisting condition protection and administrative simplification.”
Dr. McLean highlighted two paths that could achieve universal coverage and better access to health care: a single-payer–financed system, or a publicly financed coverage option within a system of regulated private insurance.
It’s the first time ACP has endorsed a single-payer approach. The college supported the public option that wasn’t included as part of the Affordable Care Act. But ACP’s latest publicly financed proposal offers a deeper level of detail on how to make that option work in the context of a private insurance system.
While the health reform conversation may be a political, ACP doesn’t want to make it a partisan one. ACP’s policy recommendations represent a carefully researched series of ideas backed by evidence-based research, Dr. McLean said.
“There is a lot of nuance behind” the two recommendations, he noted, and those nuances are explored in a series of articles and editorials published Jan. 21 in Annals of Internal Medicine.
Sizing up single payer
The ACP acknowledges that for its single-payer system, the transition could be “politically difficult and strain the federal budget,” according to Ryan A. Crowley, senior analyst at ACP, and colleagues in an article outlining the organization’s vision. “Taxes would probably replace premiums, and private insurance would have a reduced role or be eliminated altogether.”
However, the authors note that a single-payer system could be designed to address concerns from a generally skeptical public, such as providing bulk funding or setting minimum standards to guide state operations. It also could include private insurance to provide supplemental coverage.
Even so, “adopting a single-payer system would be highly disruptive and could lead to price controls that would perpetuate flaws in the current Medicare payment system, including the undervaluation of primary care,” Mr. Crowley and colleagues wrote. “If prices are set too low, it could lead to shortages and longer wait times for services. Without sufficient cost controls, however, the cost of a single-payer system could be too high to be feasible.”
Pondering the public option
Given a single-payer plan’s potential challenges, ACP also is endorsing a public option model, which provides the choice of a government-sponsored health insurance plan to compete with existing private insurance options.
“Depending on its structure and implementation, a public choice (or public option) model available to all could help to achieve universal coverage, better access, and improved outcomes without the disruption of a single-payer approach,” the ACP authors noted.
The public option has its own drawbacks, they acknowledge. Those include an inability to achieve better savings on prescription drugs, compared with a single-payer system. The public option approach also doesn’t do away with the current administrative burden, and access issues related to narrow provider networks would persist.
Dr. McLean noted that a more highly regulated insurance market would be needed to help make the public option model work.
“Insurance companies don’t have regulation in a lot of things that they do,” Dr. McLean said. “We see that as quite problematic. They are kind of running amok at this point.”
Expanding the role of primary care
In either reform scenario, primary care would play a much greater role.
“We need to promote primary care,” Dr. McLean said. That includes better incentives to draw physicians to it. “We have to pay them enough,” he added.
The health care models will need to move away from higher pay to specialties for high-cost, high-volume procedural reimbursement. And they’ll need to recognize the need for placing a higher value on the cognitive services provided at the primary care level.
Also in need of change: physicians’ administrative burdens. Reforms need to address the burden created by value-based care and the poor application and misapplication of quality measures.
Migration to a single-payer environment could would make reducing the administrative burden a lot easier, Dr. McLean said. But it also could be done with a public option approach.
That’s where regulators can play a big role in working with insurers to help address administrative burden – streamlining prior authorization of procedures, the types of forms used, and other policies, Dr. McLean explained.
“The number of insurers and their ability to have their own rules and regulations [make it] incredibly complex for patients as well as physicians trying to figure out how to deliver the care that they need,” he noted.
Dr. McLean hopes that the ACP’s papers will spark conversation, particularly among legislators and regulators.
“The bottom line is we cannot afford to not do something bold,” he cautioned. “It is just not working. Our patients deserve better, and we can do better.”
The American College of Physicians is recommending either a single-payer system or a public option within a regulated private insurance system to help deliver universal and affordable access to health care for all Americans.
“We came to the conclusion that two directions or approaches could get us to where we need to be,” ACP President Robert McLean, MD, said in an interview. “We need ... a system that provides universal, affordable access to care.”
After examining the evidence, ACP discarded one option: a direct market-based approach.
“Direct market-based approaches won’t work,” Dr. McLean explained. “If you look at where direct marketplace approaches ... have been implemented, they just will not get you to a place where you are going to get universal coverage, portability, essential benefits, and preexisting condition protection and administrative simplification.”
Dr. McLean highlighted two paths that could achieve universal coverage and better access to health care: a single-payer–financed system, or a publicly financed coverage option within a system of regulated private insurance.
It’s the first time ACP has endorsed a single-payer approach. The college supported the public option that wasn’t included as part of the Affordable Care Act. But ACP’s latest publicly financed proposal offers a deeper level of detail on how to make that option work in the context of a private insurance system.
While the health reform conversation may be a political, ACP doesn’t want to make it a partisan one. ACP’s policy recommendations represent a carefully researched series of ideas backed by evidence-based research, Dr. McLean said.
“There is a lot of nuance behind” the two recommendations, he noted, and those nuances are explored in a series of articles and editorials published Jan. 21 in Annals of Internal Medicine.
Sizing up single payer
The ACP acknowledges that for its single-payer system, the transition could be “politically difficult and strain the federal budget,” according to Ryan A. Crowley, senior analyst at ACP, and colleagues in an article outlining the organization’s vision. “Taxes would probably replace premiums, and private insurance would have a reduced role or be eliminated altogether.”
However, the authors note that a single-payer system could be designed to address concerns from a generally skeptical public, such as providing bulk funding or setting minimum standards to guide state operations. It also could include private insurance to provide supplemental coverage.
Even so, “adopting a single-payer system would be highly disruptive and could lead to price controls that would perpetuate flaws in the current Medicare payment system, including the undervaluation of primary care,” Mr. Crowley and colleagues wrote. “If prices are set too low, it could lead to shortages and longer wait times for services. Without sufficient cost controls, however, the cost of a single-payer system could be too high to be feasible.”
Pondering the public option
Given a single-payer plan’s potential challenges, ACP also is endorsing a public option model, which provides the choice of a government-sponsored health insurance plan to compete with existing private insurance options.
“Depending on its structure and implementation, a public choice (or public option) model available to all could help to achieve universal coverage, better access, and improved outcomes without the disruption of a single-payer approach,” the ACP authors noted.
The public option has its own drawbacks, they acknowledge. Those include an inability to achieve better savings on prescription drugs, compared with a single-payer system. The public option approach also doesn’t do away with the current administrative burden, and access issues related to narrow provider networks would persist.
Dr. McLean noted that a more highly regulated insurance market would be needed to help make the public option model work.
“Insurance companies don’t have regulation in a lot of things that they do,” Dr. McLean said. “We see that as quite problematic. They are kind of running amok at this point.”
Expanding the role of primary care
In either reform scenario, primary care would play a much greater role.
“We need to promote primary care,” Dr. McLean said. That includes better incentives to draw physicians to it. “We have to pay them enough,” he added.
The health care models will need to move away from higher pay to specialties for high-cost, high-volume procedural reimbursement. And they’ll need to recognize the need for placing a higher value on the cognitive services provided at the primary care level.
Also in need of change: physicians’ administrative burdens. Reforms need to address the burden created by value-based care and the poor application and misapplication of quality measures.
Migration to a single-payer environment could would make reducing the administrative burden a lot easier, Dr. McLean said. But it also could be done with a public option approach.
That’s where regulators can play a big role in working with insurers to help address administrative burden – streamlining prior authorization of procedures, the types of forms used, and other policies, Dr. McLean explained.
“The number of insurers and their ability to have their own rules and regulations [make it] incredibly complex for patients as well as physicians trying to figure out how to deliver the care that they need,” he noted.
Dr. McLean hopes that the ACP’s papers will spark conversation, particularly among legislators and regulators.
“The bottom line is we cannot afford to not do something bold,” he cautioned. “It is just not working. Our patients deserve better, and we can do better.”
The American College of Physicians is recommending either a single-payer system or a public option within a regulated private insurance system to help deliver universal and affordable access to health care for all Americans.
“We came to the conclusion that two directions or approaches could get us to where we need to be,” ACP President Robert McLean, MD, said in an interview. “We need ... a system that provides universal, affordable access to care.”
After examining the evidence, ACP discarded one option: a direct market-based approach.
“Direct market-based approaches won’t work,” Dr. McLean explained. “If you look at where direct marketplace approaches ... have been implemented, they just will not get you to a place where you are going to get universal coverage, portability, essential benefits, and preexisting condition protection and administrative simplification.”
Dr. McLean highlighted two paths that could achieve universal coverage and better access to health care: a single-payer–financed system, or a publicly financed coverage option within a system of regulated private insurance.
It’s the first time ACP has endorsed a single-payer approach. The college supported the public option that wasn’t included as part of the Affordable Care Act. But ACP’s latest publicly financed proposal offers a deeper level of detail on how to make that option work in the context of a private insurance system.
While the health reform conversation may be a political, ACP doesn’t want to make it a partisan one. ACP’s policy recommendations represent a carefully researched series of ideas backed by evidence-based research, Dr. McLean said.
“There is a lot of nuance behind” the two recommendations, he noted, and those nuances are explored in a series of articles and editorials published Jan. 21 in Annals of Internal Medicine.
Sizing up single payer
The ACP acknowledges that for its single-payer system, the transition could be “politically difficult and strain the federal budget,” according to Ryan A. Crowley, senior analyst at ACP, and colleagues in an article outlining the organization’s vision. “Taxes would probably replace premiums, and private insurance would have a reduced role or be eliminated altogether.”
However, the authors note that a single-payer system could be designed to address concerns from a generally skeptical public, such as providing bulk funding or setting minimum standards to guide state operations. It also could include private insurance to provide supplemental coverage.
Even so, “adopting a single-payer system would be highly disruptive and could lead to price controls that would perpetuate flaws in the current Medicare payment system, including the undervaluation of primary care,” Mr. Crowley and colleagues wrote. “If prices are set too low, it could lead to shortages and longer wait times for services. Without sufficient cost controls, however, the cost of a single-payer system could be too high to be feasible.”
Pondering the public option
Given a single-payer plan’s potential challenges, ACP also is endorsing a public option model, which provides the choice of a government-sponsored health insurance plan to compete with existing private insurance options.
“Depending on its structure and implementation, a public choice (or public option) model available to all could help to achieve universal coverage, better access, and improved outcomes without the disruption of a single-payer approach,” the ACP authors noted.
The public option has its own drawbacks, they acknowledge. Those include an inability to achieve better savings on prescription drugs, compared with a single-payer system. The public option approach also doesn’t do away with the current administrative burden, and access issues related to narrow provider networks would persist.
Dr. McLean noted that a more highly regulated insurance market would be needed to help make the public option model work.
“Insurance companies don’t have regulation in a lot of things that they do,” Dr. McLean said. “We see that as quite problematic. They are kind of running amok at this point.”
Expanding the role of primary care
In either reform scenario, primary care would play a much greater role.
“We need to promote primary care,” Dr. McLean said. That includes better incentives to draw physicians to it. “We have to pay them enough,” he added.
The health care models will need to move away from higher pay to specialties for high-cost, high-volume procedural reimbursement. And they’ll need to recognize the need for placing a higher value on the cognitive services provided at the primary care level.
Also in need of change: physicians’ administrative burdens. Reforms need to address the burden created by value-based care and the poor application and misapplication of quality measures.
Migration to a single-payer environment could would make reducing the administrative burden a lot easier, Dr. McLean said. But it also could be done with a public option approach.
That’s where regulators can play a big role in working with insurers to help address administrative burden – streamlining prior authorization of procedures, the types of forms used, and other policies, Dr. McLean explained.
“The number of insurers and their ability to have their own rules and regulations [make it] incredibly complex for patients as well as physicians trying to figure out how to deliver the care that they need,” he noted.
Dr. McLean hopes that the ACP’s papers will spark conversation, particularly among legislators and regulators.
“The bottom line is we cannot afford to not do something bold,” he cautioned. “It is just not working. Our patients deserve better, and we can do better.”
FROM ANNALS OF INTERNAL MEDICINE
Medscape survey points to generational differences in physician burnout
Burnout among physicians appears to have decreased slightly in the past few years, but remains a significant problem for the medical profession, according to the Medscape National Physician Burnout & Suicide Report 2020: The Generational Divide.
A survey of more than 15,000 physicians revealed that 42% reported being burned out, down from 46% who responded to the survey 5 years ago. However, there are variations in the rates based on certain demographic factors such as specialty, age, and gender.
Urology sits at the top of the list as the specialty that is experiencing the highest rate of burnout, with 54% of urologists responding to the survey reporting burnout. Neurology and nephrology followed with rates of burnout at 50% and 49%, respectively. The next five specialties on the list all reported burnout rates of 46%: diabetes and endocrinology, family medicine, radiology, ob.gyn., and rheumatology. Pulmonology specialists reported a burnout rate of 41%. Gastroenterologists reported burnout rates of 37%.
The survey divided participants into three age categories – Millennial (ages 25-39 years), Generation X (ages 40-54 years), and Baby Boomer (ages 55-73 years). Both Millennials and Baby Boomers reported similar rates of burnout (38% and 39%, respectively) and those in Generation X reported a higher rate of burnout (48%).
This higher rate is not unexpected. The survey results cite Carol Bernstein, MD, of the Albert Einstein College of Medicine, New York, as noting that midcareer “is typically the time of highest burnout, which is where Gen Xers are in their career trajectory, suggesting a number of factors outside of work such as caring for children and elderly parents, planning for retirement, can play a role in contributing to burnout.”
Women also reported a higher rate of burnout, although the rate has dropped from the survey conducted 5 years ago. The rate of burnout among women reported for the 2020 survey was 48%, down from 51% reported 5 years ago. By comparison, the rate of burnout for men was 37% in 2020, down from 43% in 2015.
In terms of what is causing burnout, the biggest contributor is the bureaucratic tasks (charting and paperwork, for example) that physicians must complete, which 55% of respondents to the survey said was the leading cause of burnout. Next was spending too many hours at work (33%); lack of respect from administrators, employers, colleagues, and staff (32%); and the increased computerization of the practice, including the use of electronic health records (30%).
When broken down by age category, the bureaucratic tasks was tops in all three groups (57% for Millennials, 56% for Generation X, and 54% for Baby Boomers), but what ranks next differs slightly by age group. For Millennials, the next two factors were too many hours at work (38%) and lack of respect (35%). Generation X respondents cited the same two factors, both at 33%. Baby Boomers cited computerization as their second-highest factor (41%) and spending too many hours at work as the third-highest factor (31%).
The generations had different approaches to coping with burnout. Millennials (56%) reported sleep as their top-ranked coping strategy, while Gen Xers and Baby Boomers ranked exercise and personal isolation as their top choice. For these two older groups, sleep was ranked last, after other activities such as talking with family and friends.
The survey also asked about depression, and respondents reported a similar rate across all age groups (15%, 18%, and 16%, respectively). Among those who said they were depressed, the three age groups had similar rates of suicidal thoughts (21%, 24%, and 22%).
Perhaps the most striking finding of the survey is the number of physicians who would take a pay cut to achieve a better work-life balance. Among Millennials, 52% would accept a pay cut, compared with 48% of Generation X and 49% of Baby Boomers. A surprising number (36%, 34%, and 31%, respectively, reported that they would accept a $10,000-$20,000 pay cut to have a 20% reduction in work hours. gtwachtman@mdedge.com
*This story was updated on 1/22/2020.
SOURCE: Kane L et al. Medscape National Physician Burnout & Suicide Report 2020: The Generational Divide. Medscape. 2020 Jan 15.
Burnout among physicians appears to have decreased slightly in the past few years, but remains a significant problem for the medical profession, according to the Medscape National Physician Burnout & Suicide Report 2020: The Generational Divide.
A survey of more than 15,000 physicians revealed that 42% reported being burned out, down from 46% who responded to the survey 5 years ago. However, there are variations in the rates based on certain demographic factors such as specialty, age, and gender.
Urology sits at the top of the list as the specialty that is experiencing the highest rate of burnout, with 54% of urologists responding to the survey reporting burnout. Neurology and nephrology followed with rates of burnout at 50% and 49%, respectively. The next five specialties on the list all reported burnout rates of 46%: diabetes and endocrinology, family medicine, radiology, ob.gyn., and rheumatology. Pulmonology specialists reported a burnout rate of 41%. Gastroenterologists reported burnout rates of 37%.
The survey divided participants into three age categories – Millennial (ages 25-39 years), Generation X (ages 40-54 years), and Baby Boomer (ages 55-73 years). Both Millennials and Baby Boomers reported similar rates of burnout (38% and 39%, respectively) and those in Generation X reported a higher rate of burnout (48%).
This higher rate is not unexpected. The survey results cite Carol Bernstein, MD, of the Albert Einstein College of Medicine, New York, as noting that midcareer “is typically the time of highest burnout, which is where Gen Xers are in their career trajectory, suggesting a number of factors outside of work such as caring for children and elderly parents, planning for retirement, can play a role in contributing to burnout.”
Women also reported a higher rate of burnout, although the rate has dropped from the survey conducted 5 years ago. The rate of burnout among women reported for the 2020 survey was 48%, down from 51% reported 5 years ago. By comparison, the rate of burnout for men was 37% in 2020, down from 43% in 2015.
In terms of what is causing burnout, the biggest contributor is the bureaucratic tasks (charting and paperwork, for example) that physicians must complete, which 55% of respondents to the survey said was the leading cause of burnout. Next was spending too many hours at work (33%); lack of respect from administrators, employers, colleagues, and staff (32%); and the increased computerization of the practice, including the use of electronic health records (30%).
When broken down by age category, the bureaucratic tasks was tops in all three groups (57% for Millennials, 56% for Generation X, and 54% for Baby Boomers), but what ranks next differs slightly by age group. For Millennials, the next two factors were too many hours at work (38%) and lack of respect (35%). Generation X respondents cited the same two factors, both at 33%. Baby Boomers cited computerization as their second-highest factor (41%) and spending too many hours at work as the third-highest factor (31%).
The generations had different approaches to coping with burnout. Millennials (56%) reported sleep as their top-ranked coping strategy, while Gen Xers and Baby Boomers ranked exercise and personal isolation as their top choice. For these two older groups, sleep was ranked last, after other activities such as talking with family and friends.
The survey also asked about depression, and respondents reported a similar rate across all age groups (15%, 18%, and 16%, respectively). Among those who said they were depressed, the three age groups had similar rates of suicidal thoughts (21%, 24%, and 22%).
Perhaps the most striking finding of the survey is the number of physicians who would take a pay cut to achieve a better work-life balance. Among Millennials, 52% would accept a pay cut, compared with 48% of Generation X and 49% of Baby Boomers. A surprising number (36%, 34%, and 31%, respectively, reported that they would accept a $10,000-$20,000 pay cut to have a 20% reduction in work hours. gtwachtman@mdedge.com
*This story was updated on 1/22/2020.
SOURCE: Kane L et al. Medscape National Physician Burnout & Suicide Report 2020: The Generational Divide. Medscape. 2020 Jan 15.
Burnout among physicians appears to have decreased slightly in the past few years, but remains a significant problem for the medical profession, according to the Medscape National Physician Burnout & Suicide Report 2020: The Generational Divide.
A survey of more than 15,000 physicians revealed that 42% reported being burned out, down from 46% who responded to the survey 5 years ago. However, there are variations in the rates based on certain demographic factors such as specialty, age, and gender.
Urology sits at the top of the list as the specialty that is experiencing the highest rate of burnout, with 54% of urologists responding to the survey reporting burnout. Neurology and nephrology followed with rates of burnout at 50% and 49%, respectively. The next five specialties on the list all reported burnout rates of 46%: diabetes and endocrinology, family medicine, radiology, ob.gyn., and rheumatology. Pulmonology specialists reported a burnout rate of 41%. Gastroenterologists reported burnout rates of 37%.
The survey divided participants into three age categories – Millennial (ages 25-39 years), Generation X (ages 40-54 years), and Baby Boomer (ages 55-73 years). Both Millennials and Baby Boomers reported similar rates of burnout (38% and 39%, respectively) and those in Generation X reported a higher rate of burnout (48%).
This higher rate is not unexpected. The survey results cite Carol Bernstein, MD, of the Albert Einstein College of Medicine, New York, as noting that midcareer “is typically the time of highest burnout, which is where Gen Xers are in their career trajectory, suggesting a number of factors outside of work such as caring for children and elderly parents, planning for retirement, can play a role in contributing to burnout.”
Women also reported a higher rate of burnout, although the rate has dropped from the survey conducted 5 years ago. The rate of burnout among women reported for the 2020 survey was 48%, down from 51% reported 5 years ago. By comparison, the rate of burnout for men was 37% in 2020, down from 43% in 2015.
In terms of what is causing burnout, the biggest contributor is the bureaucratic tasks (charting and paperwork, for example) that physicians must complete, which 55% of respondents to the survey said was the leading cause of burnout. Next was spending too many hours at work (33%); lack of respect from administrators, employers, colleagues, and staff (32%); and the increased computerization of the practice, including the use of electronic health records (30%).
When broken down by age category, the bureaucratic tasks was tops in all three groups (57% for Millennials, 56% for Generation X, and 54% for Baby Boomers), but what ranks next differs slightly by age group. For Millennials, the next two factors were too many hours at work (38%) and lack of respect (35%). Generation X respondents cited the same two factors, both at 33%. Baby Boomers cited computerization as their second-highest factor (41%) and spending too many hours at work as the third-highest factor (31%).
The generations had different approaches to coping with burnout. Millennials (56%) reported sleep as their top-ranked coping strategy, while Gen Xers and Baby Boomers ranked exercise and personal isolation as their top choice. For these two older groups, sleep was ranked last, after other activities such as talking with family and friends.
The survey also asked about depression, and respondents reported a similar rate across all age groups (15%, 18%, and 16%, respectively). Among those who said they were depressed, the three age groups had similar rates of suicidal thoughts (21%, 24%, and 22%).
Perhaps the most striking finding of the survey is the number of physicians who would take a pay cut to achieve a better work-life balance. Among Millennials, 52% would accept a pay cut, compared with 48% of Generation X and 49% of Baby Boomers. A surprising number (36%, 34%, and 31%, respectively, reported that they would accept a $10,000-$20,000 pay cut to have a 20% reduction in work hours. gtwachtman@mdedge.com
*This story was updated on 1/22/2020.
SOURCE: Kane L et al. Medscape National Physician Burnout & Suicide Report 2020: The Generational Divide. Medscape. 2020 Jan 15.