User login
Running in place: The uncertain future of primary care internal medicine
“My dear, here we must run as fast as we can, just to stay in place. And if you wish to go anywhere you must run twice as fast as that.”
—Lewis Carroll
Alice’s Adventures in Wonderland
The future of primary care internal medicine physicians is uncertain. According to a 2018 survey of internal medicine residents conducted by the American College of Physicians, only 11% were considering primary care as a career path.1 In 1998, that number was 54%.2
Possible reasons are many:
- Lower pay compared with subspecialists in a pay system that rewards procedural competency over mental effort
- Work schedules less flexible than in other specialties (eg, hospital medicine practitioners may have 1 week on and 1 week off)
- Perceived lack of respect
- Increasing regulatory and record-keeping burdens
- Tyranny of 15- to 20-minute appointments (irrespective of patient complexity)
- Scope-of-practice concerns as other providers seek primary care equivalency status (eg, pharmacists, walk-in clinics, advanced practice providers, telemedicine providers).
The result is a projected shortage of primary care physicians of 21,100 to 55,200 by 2030, according to a 2019 report by the Association of American Medical Colleges,3 despite an expected growth in advanced practice providers in primary care such as nurse practitioners and physician assistants.
A practical result of this shortage will be even less patient access to primary care physicians. A 2017 national survey found that the average wait time for a new patient-physician appointment has already increased by 30% since 2014.4 The wait time to see a primary care physician varied between 29 days in major metropolitan areas (up 50% from 2014) and 56 days in mid-sized markets. The longest waits by market size were 109 days for new patients in Boston, MA, and 122 days for those living in Albany, NY.
What are the implications?
In this issue, Pravia and Diaz5 make the case that primary care providers must adapt their practices to meet the needs of younger generations by increasing their use of technology. We agree that telemedicine, wearable medical devices, and enhanced patient communication through the electronic medical record (EMR) are here to stay and should be embraced.
However, we have seen the challenges of adopting technologic advances without first making an adjustment to the volume-driven patient schedule. For such advances to be successfully integrated into a clinical practice, it is vital to be cognizant of the current challenges encountered in primary care internal medicine.
UNIQUE BURDENS ON PRIMARY CARE
In addition to the stress of addressing multiple complex medical problems within a short time, evaluating multiple medical problems often leads to increases in results to review, forms to complete, and calls to patients. Even treatment plans initiated by specialists are often deferred to primary care providers for dosing adjustments, follow-up laboratory testing, and monitoring.
Moreover, patients often seek a second opinion from their primary care provider regarding care provided by subspecialists, as they consider their primary care provider to be the doctor who knows them best. And though it can be personally gratifying to be considered a trusted partner in the patient’s care, these requests often result in additional phone calls to the office or another thing to address within a complex visit.
A large in-box can be daunting in the setting of increased EMR demands. Whether you have difficulty putting in basic orders or are an advanced user, each upgrade can make you feel like you’re using the EMR for the first time. This is a problem for all specialties, but in primary care, one is addressing a large spectrum of concerns, so there is less opportunity to use standardized templates that can help buffer the problem.
A study of primary care providers found that nearly 75% of each patient visit was spent on activities other than face-to-face patient care, including working with the EMR.6 Similarly, a study using in-office observations and after-hours diaries found that physicians from various specialties spend 2 hours on administrative duties for each hour that they see patients in the office, followed by an additional 1 to 2 hours of work after clinic, mostly devoted to the EMR.7
Clinicians using scribes to help with record-keeping duties often need to see more patients to compensate for the cost. Adding 2 to 3 patients to a daily schedule usually means adding more medical conditions to manage, with an exponential increase in testing and in-box burden.
The additional burden this coverage creates in primary care is often not well understood by those in other specialties.
GUIDELINE CONFUSION AND THE DEATH OF THE ANNUAL PREVENTIVE VISIT
Another burden unique to primary care providers is the nearly continuous publication of guidelines that are often confusing and discrepant. Because many high-impact guidelines represent expert consensus or evidence from specialist perspectives, they may not fit the primary care model or values: eg, primary care guidelines tend to place more emphasis on harms associated with screening.
Screening for breast and prostate cancers is a prime example. Both require shared decision-making based on patient preferences and values.8,9 Detailed discussions about preventive screening can be difficult to achieve within the context of a medical visit owing to time limitations, especially if other medical conditions being addressed are equally controversial, such as blood pressure target goals. A decade ago, one could easily declare, “It’s time for your annual PSA test,” and move on to other concerns. Given the changing evidence, an informed patient is now likely to question whether this test should be done, how often it should be done, and whether a prostate examination should also be included.
The push toward population health has raised questions about the value of a preventive wellness visit, especially in healthy patients.10,11 Arguments against the annual physical do not account for the value of these visits, which provide the opportunity to have time-intensive shared decision-making conversations and build a trusting patient-physician relationship. The value of the annual physical is not simply to do examinations for which there is limited evidence; it is a time for us to get to know our patients, to update their preventive needs (and the medical record), and to discuss which screening tests they may safely forgo to avoid unnecessary false-positives, leading to excess cost and harm.
This trusting relationship, developed over years, is likely to save both the patient and the healthcare system significant money. For example, it enables us to reassure patients that an antibiotic is not needed for their upper respiratory infection, to encourage them to try a dietary change before proceeding with computed tomography for their abdominal pain, or to discourage them from inappropriately aggressive screening tests that may result in overtesting or overdiagnosis.
Unfortunately, it is nearly impossible to accurately quantify these substantial benefits to the healthcare system and patients. And there is a real potential that recommendations against the annual physical may eventually affect future reimbursement, which would add to the time pressures of an already overburdened primary care workforce.
DO PRIMARY CARE PHYSICIANS MAKE A DIFFERENCE?
As medicine and technology evolve, patients have more ways to access care. However, the Internet also provides patients with access to more conflicting information than ever before, making it even more important for clinicians, as trusted partners in their patients’ health, to help patients navigate the waters of information and misinformation.
Studies have shown that having a primary care physician is associated with a longer life span, higher likelihood of reporting good health, and similar clinical outcomes for common conditions such as diabetes and hypertension when compared with subspecialty care, but at a lower cost and with less resource utilization.12,13 In a study published in 2019, Basu et al12 found that for every 10 additional primary care physicians per 100,000 population, there was an associated 51.5-day increase in life expectancy, compared with a 19.2-day increase for specialists. Cost savings also occur. Similarly, a review by the American College of Physicians13 found that each additional primary care physician per 10,000 population in a US state increased the state’s health quality ranking by more than 10 spots and reduced their overall spending per Medicare beneficiary. In contrast, an increase of 1 specialist per 10,000 population was linked to a 9-spot decrease in health-quality ranking and an increase in spending.
WHY CHOOSE PRIMARY CARE?
As medical students, we fell in love with internal medicine because of the complexity and intellectual challenges of working through a diagnostic dilemma. There is a certain excitement in not knowing what type of patients will show up that day.
Primary care’s focus on continuity and developing long-standing relationships with patients and their families is largely unmatched in the subspecialty field. It is satisfying to have a general knowledge of the human body, and the central vantage point with which to weigh different subspecialty recommendations. We feel such sentiments are common to those interested in primary care, but sadly, we believe these are not enough to sustain the future of primary care internal medicine.
IS THE FUTURE BRIGHT OR BLEAK?
Primary care internists must resist the call to “run twice as fast.” Instead, we need to look for ways where our unique skill sets can benefit the health of our nation while attracting students to internal medicine primary care. The following are potential areas for moving forward.
The aging of America
The US Census Bureau projects that by the year 2035, older adults will outnumber children for the first time in US history, and by the year 2060, nearly 25% of the US population will be 65 or older.14 The rise of the geriatric patient and the need for comprehensive care will create a continued demand for primary care internists. There certainly aren’t enough geriatricians to meet this need, and primary care internists are well trained to fill this gap.
The rise of the team approach
As we are learning, complex disease management benefits from a team approach. The rise of new models of care delivery such as accountable care organizations and patient-centered medical homes echo this reality. The day of a single provider “doing it all” is fading.
The focus on population health in these models has given rise to multidisciplinary teams—including physicians, nurses, advanced practice providers, social workers, and pharmacists—whose function is to help manage and improve the physical, mental, and social care of patients, often in a capitated payment system. The primary care internist can play a key role in leading these teams, and such partnerships may help lessen reliance on the current primary care hustle of 15- to 20-minute visits. In such models, it is possible that the internist will need to see each patient only once or twice a year, in a longer appointment slot, instead of 4 to 6 times per year in rushed visits. The hope is that this will encourage the relationship-building that is so important in primary care and reduce the time and volume scheduling burdens seen in the current fee-for-service system.
Technology and advanced diagnostics
The joy of digging into a diagnostic dilemma has been a hallmark of internal medicine. The rise of technology should enable primary care internists to increase their diagnostic capabilities in the office without an overreliance on subspecialists.
Examples of technology that may benefit primary care are artificial intelligence with real-time diagnostic support, precision medicine, and office-based point-of-care ultrasonography.15–17 By increasing the diagnostic power of an office-based visit, we hope that the prestige factor of primary care medicine will increase as internists incorporate such advances into their clinics—not to mention the joy of making an appropriate diagnosis in real time.
Reimbursement and the value of time
Time is a valuable commodity for primary care internists. Unfortunately, there seems to be less of it in today’s practice. Gone are the days when we could go to the doctors’ dining room to decompress, chat, and break bread with colleagues. Today, we are more likely to be found in front of our computers over lunch answering patients’ messages. Time is also a key reason that physicians express frustration with issues such as prior authorizations for medications. These tasks routinely take time away from what is valuable—the care of our patients.
The rise of innovative practice models such as direct primary care and concierge medicine can be seen as a market response to the frustrations of increasing regulatory complexity, billing hassles, and lack of time. However, some have cautioned that such models have the potential to worsen healthcare disparities because patients pay out of pocket for some or all of their care in these practices.18
Interestingly, the Centers for Medicare and Medicaid Services recently unveiled new voluntary payment models for primary care that go into effect in 2020. These models may allow for increased practice innovation. The 2 proposed options are Primary Care First (designed for small primary care practices) and Direct Contracting (aimed at larger practices). These models are designed to provide a predictable up-front payment stream (a set payment per beneficiary) to the primary care practice. Hopefully, these options will move primary care away from the current fee-for-service, multiple-patient-visit model.
The primary care model allows practices to “assume financial risk in exchange for reduced administrative burden and performance-based payments” and “introduces new, higher payments for practices that care for complex, chronically ill patients.”19 It is too soon to know the effectiveness of such models, but any reimbursement innovation should be met with cautious optimism.
In addition, the Centers for Medicare and Medicaid Services has recently moved to reduce requirements for documentation. For example, one can fully bill with a medical student note without needing to repeat visit notes.20,21 Such changes should decrease the time needed to document the EMR and free up more time to care for patients.
A CALL TO ACTION
The national shortage of primary care providers points to the fact that this is a difficult career, and one that remains undervalued. One step we need to take is to protect the time we have with patients. It is doubtful that seeing a greater number of sicker patients each day, in addition to the responsibilities of proactive population-based care (“panel management”), will attract younger generations of physicians to fill this void, no matter what technology we adopt.
Keys to facilitating this change include understanding the value of primary care physicians, decreasing the burden of documentation, facilitating team-care options to support them, and expanding diagnostic tools available to use within primary care. If we don’t demand change, who will be there to take care of us when we grow old?
- American College of Physicians. Internal Medicine In-Training Examination® 2018 Residents Survey: Report of Findings, unpublished data. [Summary and analysis of residents' answers to questions about training] Philadelphia: American College of Physicians; 2019.
- American College of Physicians. Internal Medicine In-Training Examination® 1998 Residents Survey: Report of Findings, unpublished data. [Summary and analysis of residents' answers to questions about training] Philadelphia: American College of Physicians; 1999.
- Association of American Medical Colleges. New findings confirm predictions on physician shortage. news.aamc.org/press-releases/article/2019-workforce-projections-update. Accessed July 3, 2019.
- Merritt Hawkins Associates. 2017 Survey of physician appointment wait times and Medicare and Medicaid acceptance rates. www.merritthawkins.com/news-and-insights/thought-leadership/survey/survey-of-physician-appointment-wait-times. Accessed July 3, 2019.
- Pravia CI, Diaz YM. Primary care: practice meets technology. Cleve Clin J Med 2019; 86(8):525–528. doi:10.3949/ccjm.86a.18122
- Young RA, Burge SK, Kumar KA, Wilson JM, Ortiz DF. A time-motion study of primary care physicians’ work in the electronic health record era. Fam Med 2018; 50(2):91–99. doi:10.22454/FamMed.2018.184803
- Sinsky C, Colligan L, Li L, et al. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med 2016; 165(11):753–760. doi:10.7326/M16-0961
- O'Callaghan ME, Kichenadasse G, Vatandoust S, Moretti K. Informed decision making about prostate cancer screening. Ann Intern Med 2015; 162(6):457. doi:10.7326/L15-5063
- Batur P, Walsh J. Annual mammography starting at age 40: More talk, less action? Cleve Clin J Med 2015; 82(5):272–275. doi:10.3949/ccjm.82a.14156
- Mehrotra A, Prochazka A. Improving value in health care—against the annual physical. N Engl J Med 2015; 373(16):1485–1487. doi:10.1056/NEJMp1507485
- Krogsboll LT, Jorgensen KJ, Gotzsche PC. General health checks in adults for reducing morbidity and mortality from disease. Cochrane Database Syst Rev 2019; 1:CD009009. doi:10.1002/14651858.CD009009.pub3
- Basu S, Berkowitz SA, Phillips RL, Bitton A, Landon BE, Phillips RS. Association of primary care physician supply with population mortality in the United States, 2005–2015. JAMA Intern Med 2019; 179(4):506–514. doi:10.1001/jamainternmed.2018.7624
- American College of Physicians. How is a shortage of primary care physicians affecting the quality and cost of medical care? www.acponline.org/acp_policy/policies/primary_care_shortage_affecting_hc_2008.pdf. Accessed July 3, 2019.
- Vespa, J, Armstrong D, Medina L. Demographic Turning Points for the United States: Population Projections for 2020 to 2060. www.census.gov/content/dam/Census/library/publications/2018/demo/P25_1144.pdf. Accessed July 3, 2019.
- Lin S, Mahoney M, Sinsky C. Ten ways artificial intelligence will transform primary care. J Gen Intern Med 2019. doi:10.1007/s11606-019-05035-1. Epub ahead of print.
- Feero WG. Is “precision medicine” ready to use in primary care practice? Yes: It offers patients more individualized ways of managing their health. Am Fam Physician 2017; 96(12):767–768. pmid:29431374
- Bornemann P, Jayasekera N, Bergman K, Ramos M, Gerhart J. Point-of-care ultrasound: coming soon to primary care? J Fam Pract 2018; 67(2):70–80. pmid:29400896
- Doherty R; Medical Practice and Quality Committee of the American College of Physicians. Assessing the patient care implications of “concierge” and other direct patient contracting practices: a policy position paper from the American College of Physicians. Ann Intern Med 2015; 163(12):949–952. doi:10.7326/M15-0366
- Centers for Medicare and Medicaid Services. Primary care first model options. innovation.cms.gov/initiatives/primary-care-first-model-options. Accessed July 29, 2019.
- Centers for Medicare and Medicaid Services. Final Policy, Payment, and Quality Provisions Changes to the Medicare Physician Fee Schedule for Calendar Year 2019. www.cms.gov/newsroom/fact-sheets/final-policy-payment-and-quality-provisions-changes-medicare-physician-fee-schedule-calendar-year. Accessed July 3, 2019.
- Centers for Medicare and Medicaid Services. E/M Service Documentation Provided By Students. www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/MM10412.pdf. Accessed July 3, 2019.
“My dear, here we must run as fast as we can, just to stay in place. And if you wish to go anywhere you must run twice as fast as that.”
—Lewis Carroll
Alice’s Adventures in Wonderland
The future of primary care internal medicine physicians is uncertain. According to a 2018 survey of internal medicine residents conducted by the American College of Physicians, only 11% were considering primary care as a career path.1 In 1998, that number was 54%.2
Possible reasons are many:
- Lower pay compared with subspecialists in a pay system that rewards procedural competency over mental effort
- Work schedules less flexible than in other specialties (eg, hospital medicine practitioners may have 1 week on and 1 week off)
- Perceived lack of respect
- Increasing regulatory and record-keeping burdens
- Tyranny of 15- to 20-minute appointments (irrespective of patient complexity)
- Scope-of-practice concerns as other providers seek primary care equivalency status (eg, pharmacists, walk-in clinics, advanced practice providers, telemedicine providers).
The result is a projected shortage of primary care physicians of 21,100 to 55,200 by 2030, according to a 2019 report by the Association of American Medical Colleges,3 despite an expected growth in advanced practice providers in primary care such as nurse practitioners and physician assistants.
A practical result of this shortage will be even less patient access to primary care physicians. A 2017 national survey found that the average wait time for a new patient-physician appointment has already increased by 30% since 2014.4 The wait time to see a primary care physician varied between 29 days in major metropolitan areas (up 50% from 2014) and 56 days in mid-sized markets. The longest waits by market size were 109 days for new patients in Boston, MA, and 122 days for those living in Albany, NY.
What are the implications?
In this issue, Pravia and Diaz5 make the case that primary care providers must adapt their practices to meet the needs of younger generations by increasing their use of technology. We agree that telemedicine, wearable medical devices, and enhanced patient communication through the electronic medical record (EMR) are here to stay and should be embraced.
However, we have seen the challenges of adopting technologic advances without first making an adjustment to the volume-driven patient schedule. For such advances to be successfully integrated into a clinical practice, it is vital to be cognizant of the current challenges encountered in primary care internal medicine.
UNIQUE BURDENS ON PRIMARY CARE
In addition to the stress of addressing multiple complex medical problems within a short time, evaluating multiple medical problems often leads to increases in results to review, forms to complete, and calls to patients. Even treatment plans initiated by specialists are often deferred to primary care providers for dosing adjustments, follow-up laboratory testing, and monitoring.
Moreover, patients often seek a second opinion from their primary care provider regarding care provided by subspecialists, as they consider their primary care provider to be the doctor who knows them best. And though it can be personally gratifying to be considered a trusted partner in the patient’s care, these requests often result in additional phone calls to the office or another thing to address within a complex visit.
A large in-box can be daunting in the setting of increased EMR demands. Whether you have difficulty putting in basic orders or are an advanced user, each upgrade can make you feel like you’re using the EMR for the first time. This is a problem for all specialties, but in primary care, one is addressing a large spectrum of concerns, so there is less opportunity to use standardized templates that can help buffer the problem.
A study of primary care providers found that nearly 75% of each patient visit was spent on activities other than face-to-face patient care, including working with the EMR.6 Similarly, a study using in-office observations and after-hours diaries found that physicians from various specialties spend 2 hours on administrative duties for each hour that they see patients in the office, followed by an additional 1 to 2 hours of work after clinic, mostly devoted to the EMR.7
Clinicians using scribes to help with record-keeping duties often need to see more patients to compensate for the cost. Adding 2 to 3 patients to a daily schedule usually means adding more medical conditions to manage, with an exponential increase in testing and in-box burden.
The additional burden this coverage creates in primary care is often not well understood by those in other specialties.
GUIDELINE CONFUSION AND THE DEATH OF THE ANNUAL PREVENTIVE VISIT
Another burden unique to primary care providers is the nearly continuous publication of guidelines that are often confusing and discrepant. Because many high-impact guidelines represent expert consensus or evidence from specialist perspectives, they may not fit the primary care model or values: eg, primary care guidelines tend to place more emphasis on harms associated with screening.
Screening for breast and prostate cancers is a prime example. Both require shared decision-making based on patient preferences and values.8,9 Detailed discussions about preventive screening can be difficult to achieve within the context of a medical visit owing to time limitations, especially if other medical conditions being addressed are equally controversial, such as blood pressure target goals. A decade ago, one could easily declare, “It’s time for your annual PSA test,” and move on to other concerns. Given the changing evidence, an informed patient is now likely to question whether this test should be done, how often it should be done, and whether a prostate examination should also be included.
The push toward population health has raised questions about the value of a preventive wellness visit, especially in healthy patients.10,11 Arguments against the annual physical do not account for the value of these visits, which provide the opportunity to have time-intensive shared decision-making conversations and build a trusting patient-physician relationship. The value of the annual physical is not simply to do examinations for which there is limited evidence; it is a time for us to get to know our patients, to update their preventive needs (and the medical record), and to discuss which screening tests they may safely forgo to avoid unnecessary false-positives, leading to excess cost and harm.
This trusting relationship, developed over years, is likely to save both the patient and the healthcare system significant money. For example, it enables us to reassure patients that an antibiotic is not needed for their upper respiratory infection, to encourage them to try a dietary change before proceeding with computed tomography for their abdominal pain, or to discourage them from inappropriately aggressive screening tests that may result in overtesting or overdiagnosis.
Unfortunately, it is nearly impossible to accurately quantify these substantial benefits to the healthcare system and patients. And there is a real potential that recommendations against the annual physical may eventually affect future reimbursement, which would add to the time pressures of an already overburdened primary care workforce.
DO PRIMARY CARE PHYSICIANS MAKE A DIFFERENCE?
As medicine and technology evolve, patients have more ways to access care. However, the Internet also provides patients with access to more conflicting information than ever before, making it even more important for clinicians, as trusted partners in their patients’ health, to help patients navigate the waters of information and misinformation.
Studies have shown that having a primary care physician is associated with a longer life span, higher likelihood of reporting good health, and similar clinical outcomes for common conditions such as diabetes and hypertension when compared with subspecialty care, but at a lower cost and with less resource utilization.12,13 In a study published in 2019, Basu et al12 found that for every 10 additional primary care physicians per 100,000 population, there was an associated 51.5-day increase in life expectancy, compared with a 19.2-day increase for specialists. Cost savings also occur. Similarly, a review by the American College of Physicians13 found that each additional primary care physician per 10,000 population in a US state increased the state’s health quality ranking by more than 10 spots and reduced their overall spending per Medicare beneficiary. In contrast, an increase of 1 specialist per 10,000 population was linked to a 9-spot decrease in health-quality ranking and an increase in spending.
WHY CHOOSE PRIMARY CARE?
As medical students, we fell in love with internal medicine because of the complexity and intellectual challenges of working through a diagnostic dilemma. There is a certain excitement in not knowing what type of patients will show up that day.
Primary care’s focus on continuity and developing long-standing relationships with patients and their families is largely unmatched in the subspecialty field. It is satisfying to have a general knowledge of the human body, and the central vantage point with which to weigh different subspecialty recommendations. We feel such sentiments are common to those interested in primary care, but sadly, we believe these are not enough to sustain the future of primary care internal medicine.
IS THE FUTURE BRIGHT OR BLEAK?
Primary care internists must resist the call to “run twice as fast.” Instead, we need to look for ways where our unique skill sets can benefit the health of our nation while attracting students to internal medicine primary care. The following are potential areas for moving forward.
The aging of America
The US Census Bureau projects that by the year 2035, older adults will outnumber children for the first time in US history, and by the year 2060, nearly 25% of the US population will be 65 or older.14 The rise of the geriatric patient and the need for comprehensive care will create a continued demand for primary care internists. There certainly aren’t enough geriatricians to meet this need, and primary care internists are well trained to fill this gap.
The rise of the team approach
As we are learning, complex disease management benefits from a team approach. The rise of new models of care delivery such as accountable care organizations and patient-centered medical homes echo this reality. The day of a single provider “doing it all” is fading.
The focus on population health in these models has given rise to multidisciplinary teams—including physicians, nurses, advanced practice providers, social workers, and pharmacists—whose function is to help manage and improve the physical, mental, and social care of patients, often in a capitated payment system. The primary care internist can play a key role in leading these teams, and such partnerships may help lessen reliance on the current primary care hustle of 15- to 20-minute visits. In such models, it is possible that the internist will need to see each patient only once or twice a year, in a longer appointment slot, instead of 4 to 6 times per year in rushed visits. The hope is that this will encourage the relationship-building that is so important in primary care and reduce the time and volume scheduling burdens seen in the current fee-for-service system.
Technology and advanced diagnostics
The joy of digging into a diagnostic dilemma has been a hallmark of internal medicine. The rise of technology should enable primary care internists to increase their diagnostic capabilities in the office without an overreliance on subspecialists.
Examples of technology that may benefit primary care are artificial intelligence with real-time diagnostic support, precision medicine, and office-based point-of-care ultrasonography.15–17 By increasing the diagnostic power of an office-based visit, we hope that the prestige factor of primary care medicine will increase as internists incorporate such advances into their clinics—not to mention the joy of making an appropriate diagnosis in real time.
Reimbursement and the value of time
Time is a valuable commodity for primary care internists. Unfortunately, there seems to be less of it in today’s practice. Gone are the days when we could go to the doctors’ dining room to decompress, chat, and break bread with colleagues. Today, we are more likely to be found in front of our computers over lunch answering patients’ messages. Time is also a key reason that physicians express frustration with issues such as prior authorizations for medications. These tasks routinely take time away from what is valuable—the care of our patients.
The rise of innovative practice models such as direct primary care and concierge medicine can be seen as a market response to the frustrations of increasing regulatory complexity, billing hassles, and lack of time. However, some have cautioned that such models have the potential to worsen healthcare disparities because patients pay out of pocket for some or all of their care in these practices.18
Interestingly, the Centers for Medicare and Medicaid Services recently unveiled new voluntary payment models for primary care that go into effect in 2020. These models may allow for increased practice innovation. The 2 proposed options are Primary Care First (designed for small primary care practices) and Direct Contracting (aimed at larger practices). These models are designed to provide a predictable up-front payment stream (a set payment per beneficiary) to the primary care practice. Hopefully, these options will move primary care away from the current fee-for-service, multiple-patient-visit model.
The primary care model allows practices to “assume financial risk in exchange for reduced administrative burden and performance-based payments” and “introduces new, higher payments for practices that care for complex, chronically ill patients.”19 It is too soon to know the effectiveness of such models, but any reimbursement innovation should be met with cautious optimism.
In addition, the Centers for Medicare and Medicaid Services has recently moved to reduce requirements for documentation. For example, one can fully bill with a medical student note without needing to repeat visit notes.20,21 Such changes should decrease the time needed to document the EMR and free up more time to care for patients.
A CALL TO ACTION
The national shortage of primary care providers points to the fact that this is a difficult career, and one that remains undervalued. One step we need to take is to protect the time we have with patients. It is doubtful that seeing a greater number of sicker patients each day, in addition to the responsibilities of proactive population-based care (“panel management”), will attract younger generations of physicians to fill this void, no matter what technology we adopt.
Keys to facilitating this change include understanding the value of primary care physicians, decreasing the burden of documentation, facilitating team-care options to support them, and expanding diagnostic tools available to use within primary care. If we don’t demand change, who will be there to take care of us when we grow old?
“My dear, here we must run as fast as we can, just to stay in place. And if you wish to go anywhere you must run twice as fast as that.”
—Lewis Carroll
Alice’s Adventures in Wonderland
The future of primary care internal medicine physicians is uncertain. According to a 2018 survey of internal medicine residents conducted by the American College of Physicians, only 11% were considering primary care as a career path.1 In 1998, that number was 54%.2
Possible reasons are many:
- Lower pay compared with subspecialists in a pay system that rewards procedural competency over mental effort
- Work schedules less flexible than in other specialties (eg, hospital medicine practitioners may have 1 week on and 1 week off)
- Perceived lack of respect
- Increasing regulatory and record-keeping burdens
- Tyranny of 15- to 20-minute appointments (irrespective of patient complexity)
- Scope-of-practice concerns as other providers seek primary care equivalency status (eg, pharmacists, walk-in clinics, advanced practice providers, telemedicine providers).
The result is a projected shortage of primary care physicians of 21,100 to 55,200 by 2030, according to a 2019 report by the Association of American Medical Colleges,3 despite an expected growth in advanced practice providers in primary care such as nurse practitioners and physician assistants.
A practical result of this shortage will be even less patient access to primary care physicians. A 2017 national survey found that the average wait time for a new patient-physician appointment has already increased by 30% since 2014.4 The wait time to see a primary care physician varied between 29 days in major metropolitan areas (up 50% from 2014) and 56 days in mid-sized markets. The longest waits by market size were 109 days for new patients in Boston, MA, and 122 days for those living in Albany, NY.
What are the implications?
In this issue, Pravia and Diaz5 make the case that primary care providers must adapt their practices to meet the needs of younger generations by increasing their use of technology. We agree that telemedicine, wearable medical devices, and enhanced patient communication through the electronic medical record (EMR) are here to stay and should be embraced.
However, we have seen the challenges of adopting technologic advances without first making an adjustment to the volume-driven patient schedule. For such advances to be successfully integrated into a clinical practice, it is vital to be cognizant of the current challenges encountered in primary care internal medicine.
UNIQUE BURDENS ON PRIMARY CARE
In addition to the stress of addressing multiple complex medical problems within a short time, evaluating multiple medical problems often leads to increases in results to review, forms to complete, and calls to patients. Even treatment plans initiated by specialists are often deferred to primary care providers for dosing adjustments, follow-up laboratory testing, and monitoring.
Moreover, patients often seek a second opinion from their primary care provider regarding care provided by subspecialists, as they consider their primary care provider to be the doctor who knows them best. And though it can be personally gratifying to be considered a trusted partner in the patient’s care, these requests often result in additional phone calls to the office or another thing to address within a complex visit.
A large in-box can be daunting in the setting of increased EMR demands. Whether you have difficulty putting in basic orders or are an advanced user, each upgrade can make you feel like you’re using the EMR for the first time. This is a problem for all specialties, but in primary care, one is addressing a large spectrum of concerns, so there is less opportunity to use standardized templates that can help buffer the problem.
A study of primary care providers found that nearly 75% of each patient visit was spent on activities other than face-to-face patient care, including working with the EMR.6 Similarly, a study using in-office observations and after-hours diaries found that physicians from various specialties spend 2 hours on administrative duties for each hour that they see patients in the office, followed by an additional 1 to 2 hours of work after clinic, mostly devoted to the EMR.7
Clinicians using scribes to help with record-keeping duties often need to see more patients to compensate for the cost. Adding 2 to 3 patients to a daily schedule usually means adding more medical conditions to manage, with an exponential increase in testing and in-box burden.
The additional burden this coverage creates in primary care is often not well understood by those in other specialties.
GUIDELINE CONFUSION AND THE DEATH OF THE ANNUAL PREVENTIVE VISIT
Another burden unique to primary care providers is the nearly continuous publication of guidelines that are often confusing and discrepant. Because many high-impact guidelines represent expert consensus or evidence from specialist perspectives, they may not fit the primary care model or values: eg, primary care guidelines tend to place more emphasis on harms associated with screening.
Screening for breast and prostate cancers is a prime example. Both require shared decision-making based on patient preferences and values.8,9 Detailed discussions about preventive screening can be difficult to achieve within the context of a medical visit owing to time limitations, especially if other medical conditions being addressed are equally controversial, such as blood pressure target goals. A decade ago, one could easily declare, “It’s time for your annual PSA test,” and move on to other concerns. Given the changing evidence, an informed patient is now likely to question whether this test should be done, how often it should be done, and whether a prostate examination should also be included.
The push toward population health has raised questions about the value of a preventive wellness visit, especially in healthy patients.10,11 Arguments against the annual physical do not account for the value of these visits, which provide the opportunity to have time-intensive shared decision-making conversations and build a trusting patient-physician relationship. The value of the annual physical is not simply to do examinations for which there is limited evidence; it is a time for us to get to know our patients, to update their preventive needs (and the medical record), and to discuss which screening tests they may safely forgo to avoid unnecessary false-positives, leading to excess cost and harm.
This trusting relationship, developed over years, is likely to save both the patient and the healthcare system significant money. For example, it enables us to reassure patients that an antibiotic is not needed for their upper respiratory infection, to encourage them to try a dietary change before proceeding with computed tomography for their abdominal pain, or to discourage them from inappropriately aggressive screening tests that may result in overtesting or overdiagnosis.
Unfortunately, it is nearly impossible to accurately quantify these substantial benefits to the healthcare system and patients. And there is a real potential that recommendations against the annual physical may eventually affect future reimbursement, which would add to the time pressures of an already overburdened primary care workforce.
DO PRIMARY CARE PHYSICIANS MAKE A DIFFERENCE?
As medicine and technology evolve, patients have more ways to access care. However, the Internet also provides patients with access to more conflicting information than ever before, making it even more important for clinicians, as trusted partners in their patients’ health, to help patients navigate the waters of information and misinformation.
Studies have shown that having a primary care physician is associated with a longer life span, higher likelihood of reporting good health, and similar clinical outcomes for common conditions such as diabetes and hypertension when compared with subspecialty care, but at a lower cost and with less resource utilization.12,13 In a study published in 2019, Basu et al12 found that for every 10 additional primary care physicians per 100,000 population, there was an associated 51.5-day increase in life expectancy, compared with a 19.2-day increase for specialists. Cost savings also occur. Similarly, a review by the American College of Physicians13 found that each additional primary care physician per 10,000 population in a US state increased the state’s health quality ranking by more than 10 spots and reduced their overall spending per Medicare beneficiary. In contrast, an increase of 1 specialist per 10,000 population was linked to a 9-spot decrease in health-quality ranking and an increase in spending.
WHY CHOOSE PRIMARY CARE?
As medical students, we fell in love with internal medicine because of the complexity and intellectual challenges of working through a diagnostic dilemma. There is a certain excitement in not knowing what type of patients will show up that day.
Primary care’s focus on continuity and developing long-standing relationships with patients and their families is largely unmatched in the subspecialty field. It is satisfying to have a general knowledge of the human body, and the central vantage point with which to weigh different subspecialty recommendations. We feel such sentiments are common to those interested in primary care, but sadly, we believe these are not enough to sustain the future of primary care internal medicine.
IS THE FUTURE BRIGHT OR BLEAK?
Primary care internists must resist the call to “run twice as fast.” Instead, we need to look for ways where our unique skill sets can benefit the health of our nation while attracting students to internal medicine primary care. The following are potential areas for moving forward.
The aging of America
The US Census Bureau projects that by the year 2035, older adults will outnumber children for the first time in US history, and by the year 2060, nearly 25% of the US population will be 65 or older.14 The rise of the geriatric patient and the need for comprehensive care will create a continued demand for primary care internists. There certainly aren’t enough geriatricians to meet this need, and primary care internists are well trained to fill this gap.
The rise of the team approach
As we are learning, complex disease management benefits from a team approach. The rise of new models of care delivery such as accountable care organizations and patient-centered medical homes echo this reality. The day of a single provider “doing it all” is fading.
The focus on population health in these models has given rise to multidisciplinary teams—including physicians, nurses, advanced practice providers, social workers, and pharmacists—whose function is to help manage and improve the physical, mental, and social care of patients, often in a capitated payment system. The primary care internist can play a key role in leading these teams, and such partnerships may help lessen reliance on the current primary care hustle of 15- to 20-minute visits. In such models, it is possible that the internist will need to see each patient only once or twice a year, in a longer appointment slot, instead of 4 to 6 times per year in rushed visits. The hope is that this will encourage the relationship-building that is so important in primary care and reduce the time and volume scheduling burdens seen in the current fee-for-service system.
Technology and advanced diagnostics
The joy of digging into a diagnostic dilemma has been a hallmark of internal medicine. The rise of technology should enable primary care internists to increase their diagnostic capabilities in the office without an overreliance on subspecialists.
Examples of technology that may benefit primary care are artificial intelligence with real-time diagnostic support, precision medicine, and office-based point-of-care ultrasonography.15–17 By increasing the diagnostic power of an office-based visit, we hope that the prestige factor of primary care medicine will increase as internists incorporate such advances into their clinics—not to mention the joy of making an appropriate diagnosis in real time.
Reimbursement and the value of time
Time is a valuable commodity for primary care internists. Unfortunately, there seems to be less of it in today’s practice. Gone are the days when we could go to the doctors’ dining room to decompress, chat, and break bread with colleagues. Today, we are more likely to be found in front of our computers over lunch answering patients’ messages. Time is also a key reason that physicians express frustration with issues such as prior authorizations for medications. These tasks routinely take time away from what is valuable—the care of our patients.
The rise of innovative practice models such as direct primary care and concierge medicine can be seen as a market response to the frustrations of increasing regulatory complexity, billing hassles, and lack of time. However, some have cautioned that such models have the potential to worsen healthcare disparities because patients pay out of pocket for some or all of their care in these practices.18
Interestingly, the Centers for Medicare and Medicaid Services recently unveiled new voluntary payment models for primary care that go into effect in 2020. These models may allow for increased practice innovation. The 2 proposed options are Primary Care First (designed for small primary care practices) and Direct Contracting (aimed at larger practices). These models are designed to provide a predictable up-front payment stream (a set payment per beneficiary) to the primary care practice. Hopefully, these options will move primary care away from the current fee-for-service, multiple-patient-visit model.
The primary care model allows practices to “assume financial risk in exchange for reduced administrative burden and performance-based payments” and “introduces new, higher payments for practices that care for complex, chronically ill patients.”19 It is too soon to know the effectiveness of such models, but any reimbursement innovation should be met with cautious optimism.
In addition, the Centers for Medicare and Medicaid Services has recently moved to reduce requirements for documentation. For example, one can fully bill with a medical student note without needing to repeat visit notes.20,21 Such changes should decrease the time needed to document the EMR and free up more time to care for patients.
A CALL TO ACTION
The national shortage of primary care providers points to the fact that this is a difficult career, and one that remains undervalued. One step we need to take is to protect the time we have with patients. It is doubtful that seeing a greater number of sicker patients each day, in addition to the responsibilities of proactive population-based care (“panel management”), will attract younger generations of physicians to fill this void, no matter what technology we adopt.
Keys to facilitating this change include understanding the value of primary care physicians, decreasing the burden of documentation, facilitating team-care options to support them, and expanding diagnostic tools available to use within primary care. If we don’t demand change, who will be there to take care of us when we grow old?
- American College of Physicians. Internal Medicine In-Training Examination® 2018 Residents Survey: Report of Findings, unpublished data. [Summary and analysis of residents' answers to questions about training] Philadelphia: American College of Physicians; 2019.
- American College of Physicians. Internal Medicine In-Training Examination® 1998 Residents Survey: Report of Findings, unpublished data. [Summary and analysis of residents' answers to questions about training] Philadelphia: American College of Physicians; 1999.
- Association of American Medical Colleges. New findings confirm predictions on physician shortage. news.aamc.org/press-releases/article/2019-workforce-projections-update. Accessed July 3, 2019.
- Merritt Hawkins Associates. 2017 Survey of physician appointment wait times and Medicare and Medicaid acceptance rates. www.merritthawkins.com/news-and-insights/thought-leadership/survey/survey-of-physician-appointment-wait-times. Accessed July 3, 2019.
- Pravia CI, Diaz YM. Primary care: practice meets technology. Cleve Clin J Med 2019; 86(8):525–528. doi:10.3949/ccjm.86a.18122
- Young RA, Burge SK, Kumar KA, Wilson JM, Ortiz DF. A time-motion study of primary care physicians’ work in the electronic health record era. Fam Med 2018; 50(2):91–99. doi:10.22454/FamMed.2018.184803
- Sinsky C, Colligan L, Li L, et al. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med 2016; 165(11):753–760. doi:10.7326/M16-0961
- O'Callaghan ME, Kichenadasse G, Vatandoust S, Moretti K. Informed decision making about prostate cancer screening. Ann Intern Med 2015; 162(6):457. doi:10.7326/L15-5063
- Batur P, Walsh J. Annual mammography starting at age 40: More talk, less action? Cleve Clin J Med 2015; 82(5):272–275. doi:10.3949/ccjm.82a.14156
- Mehrotra A, Prochazka A. Improving value in health care—against the annual physical. N Engl J Med 2015; 373(16):1485–1487. doi:10.1056/NEJMp1507485
- Krogsboll LT, Jorgensen KJ, Gotzsche PC. General health checks in adults for reducing morbidity and mortality from disease. Cochrane Database Syst Rev 2019; 1:CD009009. doi:10.1002/14651858.CD009009.pub3
- Basu S, Berkowitz SA, Phillips RL, Bitton A, Landon BE, Phillips RS. Association of primary care physician supply with population mortality in the United States, 2005–2015. JAMA Intern Med 2019; 179(4):506–514. doi:10.1001/jamainternmed.2018.7624
- American College of Physicians. How is a shortage of primary care physicians affecting the quality and cost of medical care? www.acponline.org/acp_policy/policies/primary_care_shortage_affecting_hc_2008.pdf. Accessed July 3, 2019.
- Vespa, J, Armstrong D, Medina L. Demographic Turning Points for the United States: Population Projections for 2020 to 2060. www.census.gov/content/dam/Census/library/publications/2018/demo/P25_1144.pdf. Accessed July 3, 2019.
- Lin S, Mahoney M, Sinsky C. Ten ways artificial intelligence will transform primary care. J Gen Intern Med 2019. doi:10.1007/s11606-019-05035-1. Epub ahead of print.
- Feero WG. Is “precision medicine” ready to use in primary care practice? Yes: It offers patients more individualized ways of managing their health. Am Fam Physician 2017; 96(12):767–768. pmid:29431374
- Bornemann P, Jayasekera N, Bergman K, Ramos M, Gerhart J. Point-of-care ultrasound: coming soon to primary care? J Fam Pract 2018; 67(2):70–80. pmid:29400896
- Doherty R; Medical Practice and Quality Committee of the American College of Physicians. Assessing the patient care implications of “concierge” and other direct patient contracting practices: a policy position paper from the American College of Physicians. Ann Intern Med 2015; 163(12):949–952. doi:10.7326/M15-0366
- Centers for Medicare and Medicaid Services. Primary care first model options. innovation.cms.gov/initiatives/primary-care-first-model-options. Accessed July 29, 2019.
- Centers for Medicare and Medicaid Services. Final Policy, Payment, and Quality Provisions Changes to the Medicare Physician Fee Schedule for Calendar Year 2019. www.cms.gov/newsroom/fact-sheets/final-policy-payment-and-quality-provisions-changes-medicare-physician-fee-schedule-calendar-year. Accessed July 3, 2019.
- Centers for Medicare and Medicaid Services. E/M Service Documentation Provided By Students. www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/MM10412.pdf. Accessed July 3, 2019.
- American College of Physicians. Internal Medicine In-Training Examination® 2018 Residents Survey: Report of Findings, unpublished data. [Summary and analysis of residents' answers to questions about training] Philadelphia: American College of Physicians; 2019.
- American College of Physicians. Internal Medicine In-Training Examination® 1998 Residents Survey: Report of Findings, unpublished data. [Summary and analysis of residents' answers to questions about training] Philadelphia: American College of Physicians; 1999.
- Association of American Medical Colleges. New findings confirm predictions on physician shortage. news.aamc.org/press-releases/article/2019-workforce-projections-update. Accessed July 3, 2019.
- Merritt Hawkins Associates. 2017 Survey of physician appointment wait times and Medicare and Medicaid acceptance rates. www.merritthawkins.com/news-and-insights/thought-leadership/survey/survey-of-physician-appointment-wait-times. Accessed July 3, 2019.
- Pravia CI, Diaz YM. Primary care: practice meets technology. Cleve Clin J Med 2019; 86(8):525–528. doi:10.3949/ccjm.86a.18122
- Young RA, Burge SK, Kumar KA, Wilson JM, Ortiz DF. A time-motion study of primary care physicians’ work in the electronic health record era. Fam Med 2018; 50(2):91–99. doi:10.22454/FamMed.2018.184803
- Sinsky C, Colligan L, Li L, et al. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med 2016; 165(11):753–760. doi:10.7326/M16-0961
- O'Callaghan ME, Kichenadasse G, Vatandoust S, Moretti K. Informed decision making about prostate cancer screening. Ann Intern Med 2015; 162(6):457. doi:10.7326/L15-5063
- Batur P, Walsh J. Annual mammography starting at age 40: More talk, less action? Cleve Clin J Med 2015; 82(5):272–275. doi:10.3949/ccjm.82a.14156
- Mehrotra A, Prochazka A. Improving value in health care—against the annual physical. N Engl J Med 2015; 373(16):1485–1487. doi:10.1056/NEJMp1507485
- Krogsboll LT, Jorgensen KJ, Gotzsche PC. General health checks in adults for reducing morbidity and mortality from disease. Cochrane Database Syst Rev 2019; 1:CD009009. doi:10.1002/14651858.CD009009.pub3
- Basu S, Berkowitz SA, Phillips RL, Bitton A, Landon BE, Phillips RS. Association of primary care physician supply with population mortality in the United States, 2005–2015. JAMA Intern Med 2019; 179(4):506–514. doi:10.1001/jamainternmed.2018.7624
- American College of Physicians. How is a shortage of primary care physicians affecting the quality and cost of medical care? www.acponline.org/acp_policy/policies/primary_care_shortage_affecting_hc_2008.pdf. Accessed July 3, 2019.
- Vespa, J, Armstrong D, Medina L. Demographic Turning Points for the United States: Population Projections for 2020 to 2060. www.census.gov/content/dam/Census/library/publications/2018/demo/P25_1144.pdf. Accessed July 3, 2019.
- Lin S, Mahoney M, Sinsky C. Ten ways artificial intelligence will transform primary care. J Gen Intern Med 2019. doi:10.1007/s11606-019-05035-1. Epub ahead of print.
- Feero WG. Is “precision medicine” ready to use in primary care practice? Yes: It offers patients more individualized ways of managing their health. Am Fam Physician 2017; 96(12):767–768. pmid:29431374
- Bornemann P, Jayasekera N, Bergman K, Ramos M, Gerhart J. Point-of-care ultrasound: coming soon to primary care? J Fam Pract 2018; 67(2):70–80. pmid:29400896
- Doherty R; Medical Practice and Quality Committee of the American College of Physicians. Assessing the patient care implications of “concierge” and other direct patient contracting practices: a policy position paper from the American College of Physicians. Ann Intern Med 2015; 163(12):949–952. doi:10.7326/M15-0366
- Centers for Medicare and Medicaid Services. Primary care first model options. innovation.cms.gov/initiatives/primary-care-first-model-options. Accessed July 29, 2019.
- Centers for Medicare and Medicaid Services. Final Policy, Payment, and Quality Provisions Changes to the Medicare Physician Fee Schedule for Calendar Year 2019. www.cms.gov/newsroom/fact-sheets/final-policy-payment-and-quality-provisions-changes-medicare-physician-fee-schedule-calendar-year. Accessed July 3, 2019.
- Centers for Medicare and Medicaid Services. E/M Service Documentation Provided By Students. www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/MM10412.pdf. Accessed July 3, 2019.
Medication management in older adults
Medications started for appropriate indications in middle age may need to be monitored more closely as the patient ages. Some drugs may become unnecessary or even dangerous as the patient ages, functional status and renal function decline, and goals of care change.
Older adults tend to have multiple illnesses and therefore take more drugs, and polypharmacy increases the risk of poor outcomes. The number of medications a person uses is a risk factor for adverse drug reactions, nonadherence, financial burden, drug-drug interactions, and worse outcomes.1
The prevalence of polypharmacy increased from an estimated 8.2% to 15% from 1999 to 2011 based on the National Health and Nutrition Examination Survey.2 Guideline-based therapy for specific diseases may lead to the addition of more medications to reach disease targets.3 Most older adults in the United States compound the risk of prescribed medications by also taking over-the-counter medications and dietary supplements.4
In addition, medications are often used in older adults based on studies of younger persons without significant comorbidities. Applying clinical guidelines based on these studies to older adults with comorbidity and functional impairment is challenging.5 Age-related pharmacokinetic and pharmacodynamic changes increase the risk of adverse drug reactions.6
In this article, we review commonly used medications that are potentially inappropriate based on clinical practice. We also review tools to evaluate appropriate drug therapy in older adults.
DRUGS THAT ARE COMMONLY USED, BUT POTENTIALLY INAPPROPRIATE
Statins
Statins are effective when used as secondary prevention in older adults,7 but their efficacy when used as primary prevention of atherosclerotic cardiovascular disease in people age 75 and older is questionable.8 Nevertheless, they are widely used for this purpose. For example, before the 2013 joint guidelines of the American College of Cardiology and the American Heart Association (ACC/AHA) were released, 22% of patients age 80 and older in the Geisinger health system were taking a statin for primary prevention.9
The 2013 ACC/AHA guidelines included a limited recommendation for statins for primary prevention of atherosclerotic cardiovascular disease in adults age 75 and older.10 The guideline noted, however, that few data were available to support this recommendation.10
In a systematic review of 18 randomized clinical trials of statins for primary prevention of atherosclerotic cardiovascular disease, the mean age was 57, yet conclusions were extrapolated to an older patient population.11 The estimated 10-year risk of atherosclerotic cardiovascular disease based on pooled cohort risk equations of adults age 75 and older always exceeds the 7.5% treatment threshold recommended by the guidelines.8
Myopathy is a common adverse effect of statins. In addition, statins interact with other drugs that inhibit the cytochrome P450 3A4 isoenzyme, such as amlodipine, amiodarone, and diltiazem.8,12 If statin therapy caused no functional limitation due to muscle pain or weakness, statins for primary prevention would be cost-effective, but even a small increase in adverse effects in an elderly patient can offset the cardiovascular benefit.13 A recent post hoc secondary analysis found no benefit of pravastatin for primary prevention in adults age 75 and older.14
Thus, statin treatment for primary prevention in older patients should be individualized, based on life expectancy, function, and cardiovascular risk. Statin therapy does not replace modification of other risk factors.
Anticholinergics
Drugs with anticholinergic properties are commonly prescribed in the elderly for conditions such as muscle spasm, overactive bladder, psychiatric disorders, insomnia, extrapyramidal symptoms, vertigo, pruritus, peptic ulcer disease, seasonal allergies, and even the common cold,15 and many of the drugs often prescribed have strong anticholinergic properties (Table 1). Taking multiple medications with anticholinergic properties results in a high “anticholinergic burden,” which is associated with falls, impulsive behavior, poor physical performance, loss of independence, dementia, delirium, and brain atrophy.15–18
The 2014 American College of Physicians guideline on nonsurgical management of urinary incontinence in women recommends pharmacologic treatment for urgency and stress urinary incontinence after failure of nonpharmacologic therapy,19 and many drugs for these urinary symptoms have anticholinergic properties. If an anticholinergic is necessary, an agent that results in a lower anticholinergic burden should be considered in older patients.
A pharmacist-initiated medication review and intervention may be another way to adjust medications to reduce the patient’s anticholinergic burden.20,21 The common use of anticholinergic drugs in older adults reminds us to monitor their use closely.22
Benzodiazepines and nonbenzodiazepines
Benzodiazepines are among the most commonly prescribed psychotropics in developed countries and are prescribed mainly by primary care physicians rather than psychiatrists.23
In 2008, 5.2% of US adults ages 18 to 80 used a benzodiazepine, and long-term use was more prevalent in older patients (ages 65–80).23
Benzodiazepines are prescribed for anxiety,24 insomnia,25 and agitation. They can cause withdrawal26 and have potential for abuse.27 Benzodiazepines are associated with cognitive decline,28 impaired driving,29 falls,30 and hip fractures31 in older adults.
In addition, use of nonbenzodiazepine hypnotics (eg, zolpidem) is on the rise,32 and these drugs are known to increase the risk of hip fracture in nursing home residents.33
The American Geriatrics Society, through the American Board of Internal Medicine’s Choosing Wisely campaign, recommends avoiding benzodiazepines as a first-line treatment for insomnia, agitation, or delirium in older adults.34 Yet prescribing practices with these drugs in primary care settings conflict with guidelines, partly due to lack of training in constructive strategies regarding appropriate use of benzodiazepines.35 Educating patients on the risks and benefits of benzodiazepine treatment, especially long-term use, has been shown to reduce the rate of benzodiazepine-associated secondary events.36
Antipsychotics
Off-label use of antipsychotics is common and is increasing in the United States. In 2008, off-label use of antipsychotic drugs accounted for an estimated $6 billion.37 A common off-label use is to manage behavioral symptoms of dementia, despite a black-box warning about an increased risk of death in patients with dementia who are treated with antipsychotics.38,39 The Choosing Wisely campaign recommends against prescribing antipsychotics as a first-line treatment of behavioral and psychological symptoms of dementia.34
Antipsychotic drugs are associated with risk of acute kidney injury,40 as well as increased risk of falls and fractures (eg, a 52% higher risk of a serious fall, and a 50% higher risk of a nonvertebral osteoporotic fracture).41
Patients with dementia often exhibit aggression, resistance to care, and other challenging or disruptive behaviors. In such instances, antipsychotic drugs are often prescribed, but they provide limited and inconsistent benefits, while causing oversedation and worsening of cognitive function and increasing the likelihood of falling, stroke, and death.38,39,41
Because pharmacologic treatments for dementia are only modestly effective, have notable risks, and do not treat some of the behaviors that family members and caregivers find most distressing, nonpharmacologic measures are recommended as first-line treatment.42 These include caregiver education and support, training in problem-solving, and targeted therapy directed at the underlying causes of specific behaviors (eg, implementing nighttime routines to address sleep disturbances).42 Nonpharmacologic management of behavioral symptoms in dementia can significantly improve quality of life for patients and caregivers.42 Use of antipsychotic drugs in patients with dementia should be limited to cases in which nonpharmacologic measures have failed and patients pose an imminent threat to themselves or others.43
Proton pump inhibitors
Proton pump inhibitors are among the most commonly prescribed medications in the United States, and their use has increased significantly over the decade. It has been estimated that between 25% and 70% of these prescriptions have no appropriate indication.44
There is considerable excess use of acid suppressants in both inpatient and outpatient settings.45,46 In one study, at discharge from an internal medicine service, almost half of patients were taking a proton pump inhibitor.47
Evidence-based guidelines recommend these drugs to treat gastroesophageal reflux disease, nonerosive reflux disease, erosive esophagitis, dyspepsia, and peptic ulcer disease. However, long-term use (ie, beyond 8 weeks) is recommended only for patients with erosive esophagitis, Barrett esophagus, a pathologic hypersecretory condition, or a demonstrated need for maintenance treatment for reflux disease.48
Although proton pump inhibitors are highly effective and have low toxicity, there are reports of an association with Clostridium difficile infection,49 community-acquired pneumonia,50 hip fracture,51 vitamin B12 deficiency,52 atrophic gastritis,53 kidney disease,54 and dementia.55
Nondrug therapies such as weight loss and elevation of the head of the bed may improve esophageal pH levels and reflux symptoms.56
Deprescribing.org has practical advice for healthcare providers, patients, and caregivers on how to discontinue proton pump inhibitors, including videos, algorithms, and guidelines.
TOOLS TO EVALUATE APPROPRIATE DRUG THERAPY
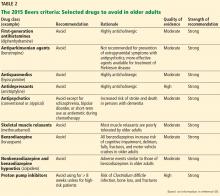
Beers criteria
The Beers criteria (Table 2), developed in 1991 by a geriatrician as an approach to safer, more effective drug therapy in frail elderly nursing home patients,57 were updated by the American Geriatrics Society in 2015 for use in any clinical setting.58 (The criteria are also available as a smartphone application through the American Geriatrics Society at www.americangeriatrics.org.)
The Beers criteria offer evidence-based recommendations on drugs to avoid in the elderly, along with the rationale for use, the quality of evidence behind the recommendation, and the graded strength of the recommendation. The Beers criteria should be viewed through the lens of clinical judgment to offer safer nonpharmacologic and pharmacologic treatments.
The Joint Commission recommends medication reconciliation at every transition of care.59 The Beers criteria are a good starting point for a comprehensive medication review.
STOPP/START criteria
Another tool to aid safe prescribing in older adults is the Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions (STOPP), used in conjuction with the Screening Tool to Alert Doctors to Right Treatment (START). The STOPP/START criteria60,61 are based on an up-to-date literature review and consensus (Table 3).
THE BOTTOM LINE
Physicians caring for older adults need to diligently weigh the benefits of drug therapy and consider the patient’s care goals, current level of functioning, life expectancy, values, and preferences. Statin therapy for primary prevention, anticholinergics, benzodiazepines, antipsychotics, and proton pump inhibitors are widely used without proper indications, pointing to the need for a periodic comprehensive review of medications to reevaluate the risks vs the benefits of the patient’s medications. The Beers criteria and the STOPP/ START criteria can be useful tools for this purpose.
- Steinman MA. Polypharmacy—time to get beyond numbers. JAMA Intern Med 2016; 176:482–483.
- Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA 2015; 314:1818–1831.
- Tinetti ME, Bogardus ST Jr, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med 2004; 351:2870–2874.
- Qato DM, Wilder J, Schumm LP, Gillet V, Alexander GC. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med 2016; 176:473–482.
- Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA 2005; 294:716–724.
- Atkin PA, Veitch PC, Veitch EM, Ogle SJ. The epidemiology of serious adverse drug reactions among the elderly. Drugs Aging 1999; 14:141–152.
- Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016; 338:2532–2561.
- Gurwitz JH, Go AS, Fortman SP. Statins for primary prevention in older adults: uncertainty and the need for more evidence. JAMA 2016; 316:1971–1972.
- Chokshi NP, Messerli FH, Sutin D, Supariwala AA, Shah NR. Appropriateness of statins in patients aged ≥ 80 years and comparison to other age groups. Am J Cardiol 2012; 110:1477–1481.
- Stone NJ, Robinson J, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(suppl 2):S1–S45.
- Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013; 1:CD004816.
- Chatzizisis YS, Koskinas KC, Misirli G, Vaklavas C, Hatzitolios A, Giannoglou GD. Risk factors and drug interactions predisposing to statin-induced myopathy: implications for risk assessment, prevention and treatment. Drug Saf 2010; 33:171–187.
- Odden MC, Pletcher MJ, Coxson PG, et al. Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann Intern Med 2015; 162:533–541.
- Han BH, Sutin D, Williamson JD, et al. Effect of statin treatment vs usual care on primary cardiovascular prevention among older adults. The ALLHAT-LLT randomized clinical trial. JAMA Intern Med 2017; 177:955–965.
- Gray SL, Anderson ML, Dublin S, et al. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med 2015; 175:401–407.
- Rudolph JL, Salow MJ, Angelini MC, McGlinchey RE. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med 2008; 168:508–513.
- Hilmer SN, Mager DE, Simonsick EM, et al. A drug burden index to define the functional burden of medications in older people. Arch Intern Med 2007; 167:781–787.
- Risacher SL, McDonald BC, Tallman EF, et al; Alzheimer’s Disease Neuroimaging Initiative. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol 2016; 73:721–732.
- Qaseem A, Dallas P, Forciea MA, Starkey M, Denberg TD, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Nonsurgical management of urinary incontinence in women: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2014; 161:429–440.
- Efjestad AS, Molden E, Oksengard AR. Pharmacist-initiated management of antagonistic interactions between anticholinergic drugs and acetyl cholinesterase inhibitors in individuals with dementia. J Am Geriatr Soc 2013; 61:1624–1625.
- Kersten H, Molden E, Tolo IK, Skovlund E, Engedal K, Wyller TB. Cognitive effects of reducing anticholinergic drug burden in a frail elderly population: a randomized controlled trial. J Gerontol A Biol Sci Med Sci 2013; 68:271–278.
- Curtis LH, Østbye T, Sendersky V, et al. Inappropriate prescribing for elderly Americans in a large outpatient population. Arch Intern Med 2004; 164:1621–1625.
- Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry 2015; 72:136–142.
- Martin JL, Sainz-Pardo M, Furukawa TA, Martín-Sánchez E, Seoane T, Galán C. Benzodiazepines in generalized anxiety disorder: heterogeneity of outcomes based on a systematic review and meta-analysis of clinical trials. J Psychopharmacol 2007; 21:774–782.
- Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med 2007; 22:1335–1350.
- Rickels K, Schweizer E, Case WG, Greenblatt DJ. Long-term therapeutic use of benzodiazepines, I. Effects of abrupt discontinuation. Arch Gen Psychiatry 1990; 47:899–907.
- Fenton MC, Keyes KM, Martins SS, Hasin DS. The role of a prescription in anxiety medication use, abuse, and dependence. Am J Psychiatry 2010; 167:1247–1253.
- Billoti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ 2014; 349:g5205.
- Smink BE, Egberts AC, Lusthof KJ, Uges DR, de Gier JJ. The relationship between benzodiazepine use and traffic accidents: a systemic literature review. CNS Drugs 2010; 24:639–653.
- Tinett, ME, Speechley M, Ginter S. Risk factors for falls among elderly persons living in the community. N Engl J Med 1988; 319:1701–1707.
- Zint K, Haefeli WE, Glynn RJ, Mogun H, Avorn J, Stürmer T. Impact of drug interactions, dosage, and duration of therapy on the risk of hip fracture associated with benzodiazepine use in older adults. Pharmacoepidemiol Drug Saf 2010; 19:1248–1255.
- Briesacher BA, Soumerai SB, Field TS, Fouayzi H, Gurwitz JH. Medicare Part D’s exclusion of benzodiazepines and fracture risk in nursing homes. Arch Intern Med 2010; 170:693–698.
- Berry SD, Lee Y, Cai S, Dore DD. Nonbenzodiazepine sleep medication use and hip fractures in nursing home residents. JAMA Intern Med 2013; 173:754–761.
- American Geriatrics Society. Choosing Wisely. Ten things clinicians and patients should question. www.choosingwisely.org/societies/american-geriatrics-society/. Accessed December 3, 2017.
- Cook JM, Marshall R, Masci C, Coyne JC. Physicians’ perspectives on prescribing benzodiazepines for older adults: a qualitative study. J Gen Intern Med 2007; 22:303–307.
- Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med 2014; 174:890–898.
- Alexander GC, Gallagher SA, Mascola A, Moloney RM, Stafford RS. Increasing off-label use of antipsychotic medications in the United States, 1995–2008. Phamacoepidemiol Drug Saf 2011; 20:177–184.
- Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med 2007; 146:775–786.
- US Food and Drug Administration (FDA). Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm053171.htm. Accessed December 4, 2017.
- Hwang YJ, Dixon SN, Reiss JP, et al. Atypical antipsychotic drugs and the risk for acute kidney injury and other adverse outcomes in older adults. Ann Intern Med 2014; 161:242–248.
- Fraser L, Liu K, Naylor KL, et al. Falls and fractures with atypical antipsychotic medication use: a population-based cohort study. JAMA Intern Med 2015; 175:450–452.
- Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA 2012; 308:2020–2029.
- Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 2006; 355:1525–1538.
- Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ 2008; 336:2–3.
- Mazer-Amirshahi M, Mullins PM, van den Anker J, Meltzer A, Pines JM. Rising rates of proton pump inhibitor prescribing in US emergency departments. Am J Emerg Med 2014; 32:618–622.
- Heidelbaugh JJ, Goldberg KL, Inadomi JM. Magnitude and economic effect of overuse of antisecretory therapy in the ambulatory care setting. Am J Manag Care 2010; 16:e228–e324.
- Pham CQ, Regal RE, Bostwich TR, Knauf KS. Acid suppressive therapy used on an inpatient internal medicine service. Ann Pharmacother 2006; 40:1261–1266.
- Kahrilas PJ, Shaheen NJ, Vaezi MF, et al; American Gastroenterological Association. American Gastroenterological Association medical position statement on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135:1383–1391.e1–e5.
- Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med 2010; 170:784–790.
- Gulmez SE, Holm A, Frederiksen H, Jensen TG, Pedersen C, Hallas J. Use of proton pump inhibitors and the risk of community-acquired pneumonia: a population-based case-control study. Arch Intern Med 2007; 167:950–955.
- Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006; 296:2947–2953.
- Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA 2013; 310:2435–2442.
- Kuipers EJ, Lundell L, Klinkenberg-Knol EC, et al. Atrophic gastritis and Helicobacter pylori infection in patients with reflux esophagitis treated with omeprazole or fundoplication. N Engl J Med 1996; 334:1018–1022.
- Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med 2016; 176:238–246.
- Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol 2016; 73:410–416.
- Kaltenbach T, Crockett S, Gerson LB. Are lifestyle measures effective in patients with gastroesophageal reflux disease? An evidence-based approach. Arch Intern Med 2006; 166:965–971.
- Beers MH, Ouslander JG, Rollingher I, Reuben DB, Brooks J, Beck JC. Explicit criteria for determining inappropriate medication use in nursing home residents. Arch Intern Med 1991; 151:1825–1832.
- American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63:2227–2246.
- Joint Commission. Sentinel event alert, Issue 35: using medication reconciliation to prevent errors. www.jointcommission.org/sentinel_event_alert_issue_35_using_medication_reconciliation_to_prevent_errors/. Accessed August 18, 2017.
- Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther 2008; 46:72–83.
- O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 2015; 44:213–218.
Medications started for appropriate indications in middle age may need to be monitored more closely as the patient ages. Some drugs may become unnecessary or even dangerous as the patient ages, functional status and renal function decline, and goals of care change.
Older adults tend to have multiple illnesses and therefore take more drugs, and polypharmacy increases the risk of poor outcomes. The number of medications a person uses is a risk factor for adverse drug reactions, nonadherence, financial burden, drug-drug interactions, and worse outcomes.1
The prevalence of polypharmacy increased from an estimated 8.2% to 15% from 1999 to 2011 based on the National Health and Nutrition Examination Survey.2 Guideline-based therapy for specific diseases may lead to the addition of more medications to reach disease targets.3 Most older adults in the United States compound the risk of prescribed medications by also taking over-the-counter medications and dietary supplements.4
In addition, medications are often used in older adults based on studies of younger persons without significant comorbidities. Applying clinical guidelines based on these studies to older adults with comorbidity and functional impairment is challenging.5 Age-related pharmacokinetic and pharmacodynamic changes increase the risk of adverse drug reactions.6
In this article, we review commonly used medications that are potentially inappropriate based on clinical practice. We also review tools to evaluate appropriate drug therapy in older adults.
DRUGS THAT ARE COMMONLY USED, BUT POTENTIALLY INAPPROPRIATE
Statins
Statins are effective when used as secondary prevention in older adults,7 but their efficacy when used as primary prevention of atherosclerotic cardiovascular disease in people age 75 and older is questionable.8 Nevertheless, they are widely used for this purpose. For example, before the 2013 joint guidelines of the American College of Cardiology and the American Heart Association (ACC/AHA) were released, 22% of patients age 80 and older in the Geisinger health system were taking a statin for primary prevention.9
The 2013 ACC/AHA guidelines included a limited recommendation for statins for primary prevention of atherosclerotic cardiovascular disease in adults age 75 and older.10 The guideline noted, however, that few data were available to support this recommendation.10
In a systematic review of 18 randomized clinical trials of statins for primary prevention of atherosclerotic cardiovascular disease, the mean age was 57, yet conclusions were extrapolated to an older patient population.11 The estimated 10-year risk of atherosclerotic cardiovascular disease based on pooled cohort risk equations of adults age 75 and older always exceeds the 7.5% treatment threshold recommended by the guidelines.8
Myopathy is a common adverse effect of statins. In addition, statins interact with other drugs that inhibit the cytochrome P450 3A4 isoenzyme, such as amlodipine, amiodarone, and diltiazem.8,12 If statin therapy caused no functional limitation due to muscle pain or weakness, statins for primary prevention would be cost-effective, but even a small increase in adverse effects in an elderly patient can offset the cardiovascular benefit.13 A recent post hoc secondary analysis found no benefit of pravastatin for primary prevention in adults age 75 and older.14
Thus, statin treatment for primary prevention in older patients should be individualized, based on life expectancy, function, and cardiovascular risk. Statin therapy does not replace modification of other risk factors.
Anticholinergics
Drugs with anticholinergic properties are commonly prescribed in the elderly for conditions such as muscle spasm, overactive bladder, psychiatric disorders, insomnia, extrapyramidal symptoms, vertigo, pruritus, peptic ulcer disease, seasonal allergies, and even the common cold,15 and many of the drugs often prescribed have strong anticholinergic properties (Table 1). Taking multiple medications with anticholinergic properties results in a high “anticholinergic burden,” which is associated with falls, impulsive behavior, poor physical performance, loss of independence, dementia, delirium, and brain atrophy.15–18
The 2014 American College of Physicians guideline on nonsurgical management of urinary incontinence in women recommends pharmacologic treatment for urgency and stress urinary incontinence after failure of nonpharmacologic therapy,19 and many drugs for these urinary symptoms have anticholinergic properties. If an anticholinergic is necessary, an agent that results in a lower anticholinergic burden should be considered in older patients.
A pharmacist-initiated medication review and intervention may be another way to adjust medications to reduce the patient’s anticholinergic burden.20,21 The common use of anticholinergic drugs in older adults reminds us to monitor their use closely.22
Benzodiazepines and nonbenzodiazepines
Benzodiazepines are among the most commonly prescribed psychotropics in developed countries and are prescribed mainly by primary care physicians rather than psychiatrists.23
In 2008, 5.2% of US adults ages 18 to 80 used a benzodiazepine, and long-term use was more prevalent in older patients (ages 65–80).23
Benzodiazepines are prescribed for anxiety,24 insomnia,25 and agitation. They can cause withdrawal26 and have potential for abuse.27 Benzodiazepines are associated with cognitive decline,28 impaired driving,29 falls,30 and hip fractures31 in older adults.
In addition, use of nonbenzodiazepine hypnotics (eg, zolpidem) is on the rise,32 and these drugs are known to increase the risk of hip fracture in nursing home residents.33
The American Geriatrics Society, through the American Board of Internal Medicine’s Choosing Wisely campaign, recommends avoiding benzodiazepines as a first-line treatment for insomnia, agitation, or delirium in older adults.34 Yet prescribing practices with these drugs in primary care settings conflict with guidelines, partly due to lack of training in constructive strategies regarding appropriate use of benzodiazepines.35 Educating patients on the risks and benefits of benzodiazepine treatment, especially long-term use, has been shown to reduce the rate of benzodiazepine-associated secondary events.36
Antipsychotics
Off-label use of antipsychotics is common and is increasing in the United States. In 2008, off-label use of antipsychotic drugs accounted for an estimated $6 billion.37 A common off-label use is to manage behavioral symptoms of dementia, despite a black-box warning about an increased risk of death in patients with dementia who are treated with antipsychotics.38,39 The Choosing Wisely campaign recommends against prescribing antipsychotics as a first-line treatment of behavioral and psychological symptoms of dementia.34
Antipsychotic drugs are associated with risk of acute kidney injury,40 as well as increased risk of falls and fractures (eg, a 52% higher risk of a serious fall, and a 50% higher risk of a nonvertebral osteoporotic fracture).41
Patients with dementia often exhibit aggression, resistance to care, and other challenging or disruptive behaviors. In such instances, antipsychotic drugs are often prescribed, but they provide limited and inconsistent benefits, while causing oversedation and worsening of cognitive function and increasing the likelihood of falling, stroke, and death.38,39,41
Because pharmacologic treatments for dementia are only modestly effective, have notable risks, and do not treat some of the behaviors that family members and caregivers find most distressing, nonpharmacologic measures are recommended as first-line treatment.42 These include caregiver education and support, training in problem-solving, and targeted therapy directed at the underlying causes of specific behaviors (eg, implementing nighttime routines to address sleep disturbances).42 Nonpharmacologic management of behavioral symptoms in dementia can significantly improve quality of life for patients and caregivers.42 Use of antipsychotic drugs in patients with dementia should be limited to cases in which nonpharmacologic measures have failed and patients pose an imminent threat to themselves or others.43
Proton pump inhibitors
Proton pump inhibitors are among the most commonly prescribed medications in the United States, and their use has increased significantly over the decade. It has been estimated that between 25% and 70% of these prescriptions have no appropriate indication.44
There is considerable excess use of acid suppressants in both inpatient and outpatient settings.45,46 In one study, at discharge from an internal medicine service, almost half of patients were taking a proton pump inhibitor.47
Evidence-based guidelines recommend these drugs to treat gastroesophageal reflux disease, nonerosive reflux disease, erosive esophagitis, dyspepsia, and peptic ulcer disease. However, long-term use (ie, beyond 8 weeks) is recommended only for patients with erosive esophagitis, Barrett esophagus, a pathologic hypersecretory condition, or a demonstrated need for maintenance treatment for reflux disease.48
Although proton pump inhibitors are highly effective and have low toxicity, there are reports of an association with Clostridium difficile infection,49 community-acquired pneumonia,50 hip fracture,51 vitamin B12 deficiency,52 atrophic gastritis,53 kidney disease,54 and dementia.55
Nondrug therapies such as weight loss and elevation of the head of the bed may improve esophageal pH levels and reflux symptoms.56
Deprescribing.org has practical advice for healthcare providers, patients, and caregivers on how to discontinue proton pump inhibitors, including videos, algorithms, and guidelines.
TOOLS TO EVALUATE APPROPRIATE DRUG THERAPY
Beers criteria
The Beers criteria (Table 2), developed in 1991 by a geriatrician as an approach to safer, more effective drug therapy in frail elderly nursing home patients,57 were updated by the American Geriatrics Society in 2015 for use in any clinical setting.58 (The criteria are also available as a smartphone application through the American Geriatrics Society at www.americangeriatrics.org.)
The Beers criteria offer evidence-based recommendations on drugs to avoid in the elderly, along with the rationale for use, the quality of evidence behind the recommendation, and the graded strength of the recommendation. The Beers criteria should be viewed through the lens of clinical judgment to offer safer nonpharmacologic and pharmacologic treatments.
The Joint Commission recommends medication reconciliation at every transition of care.59 The Beers criteria are a good starting point for a comprehensive medication review.
STOPP/START criteria
Another tool to aid safe prescribing in older adults is the Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions (STOPP), used in conjuction with the Screening Tool to Alert Doctors to Right Treatment (START). The STOPP/START criteria60,61 are based on an up-to-date literature review and consensus (Table 3).
THE BOTTOM LINE
Physicians caring for older adults need to diligently weigh the benefits of drug therapy and consider the patient’s care goals, current level of functioning, life expectancy, values, and preferences. Statin therapy for primary prevention, anticholinergics, benzodiazepines, antipsychotics, and proton pump inhibitors are widely used without proper indications, pointing to the need for a periodic comprehensive review of medications to reevaluate the risks vs the benefits of the patient’s medications. The Beers criteria and the STOPP/ START criteria can be useful tools for this purpose.
Medications started for appropriate indications in middle age may need to be monitored more closely as the patient ages. Some drugs may become unnecessary or even dangerous as the patient ages, functional status and renal function decline, and goals of care change.
Older adults tend to have multiple illnesses and therefore take more drugs, and polypharmacy increases the risk of poor outcomes. The number of medications a person uses is a risk factor for adverse drug reactions, nonadherence, financial burden, drug-drug interactions, and worse outcomes.1
The prevalence of polypharmacy increased from an estimated 8.2% to 15% from 1999 to 2011 based on the National Health and Nutrition Examination Survey.2 Guideline-based therapy for specific diseases may lead to the addition of more medications to reach disease targets.3 Most older adults in the United States compound the risk of prescribed medications by also taking over-the-counter medications and dietary supplements.4
In addition, medications are often used in older adults based on studies of younger persons without significant comorbidities. Applying clinical guidelines based on these studies to older adults with comorbidity and functional impairment is challenging.5 Age-related pharmacokinetic and pharmacodynamic changes increase the risk of adverse drug reactions.6
In this article, we review commonly used medications that are potentially inappropriate based on clinical practice. We also review tools to evaluate appropriate drug therapy in older adults.
DRUGS THAT ARE COMMONLY USED, BUT POTENTIALLY INAPPROPRIATE
Statins
Statins are effective when used as secondary prevention in older adults,7 but their efficacy when used as primary prevention of atherosclerotic cardiovascular disease in people age 75 and older is questionable.8 Nevertheless, they are widely used for this purpose. For example, before the 2013 joint guidelines of the American College of Cardiology and the American Heart Association (ACC/AHA) were released, 22% of patients age 80 and older in the Geisinger health system were taking a statin for primary prevention.9
The 2013 ACC/AHA guidelines included a limited recommendation for statins for primary prevention of atherosclerotic cardiovascular disease in adults age 75 and older.10 The guideline noted, however, that few data were available to support this recommendation.10
In a systematic review of 18 randomized clinical trials of statins for primary prevention of atherosclerotic cardiovascular disease, the mean age was 57, yet conclusions were extrapolated to an older patient population.11 The estimated 10-year risk of atherosclerotic cardiovascular disease based on pooled cohort risk equations of adults age 75 and older always exceeds the 7.5% treatment threshold recommended by the guidelines.8
Myopathy is a common adverse effect of statins. In addition, statins interact with other drugs that inhibit the cytochrome P450 3A4 isoenzyme, such as amlodipine, amiodarone, and diltiazem.8,12 If statin therapy caused no functional limitation due to muscle pain or weakness, statins for primary prevention would be cost-effective, but even a small increase in adverse effects in an elderly patient can offset the cardiovascular benefit.13 A recent post hoc secondary analysis found no benefit of pravastatin for primary prevention in adults age 75 and older.14
Thus, statin treatment for primary prevention in older patients should be individualized, based on life expectancy, function, and cardiovascular risk. Statin therapy does not replace modification of other risk factors.
Anticholinergics
Drugs with anticholinergic properties are commonly prescribed in the elderly for conditions such as muscle spasm, overactive bladder, psychiatric disorders, insomnia, extrapyramidal symptoms, vertigo, pruritus, peptic ulcer disease, seasonal allergies, and even the common cold,15 and many of the drugs often prescribed have strong anticholinergic properties (Table 1). Taking multiple medications with anticholinergic properties results in a high “anticholinergic burden,” which is associated with falls, impulsive behavior, poor physical performance, loss of independence, dementia, delirium, and brain atrophy.15–18
The 2014 American College of Physicians guideline on nonsurgical management of urinary incontinence in women recommends pharmacologic treatment for urgency and stress urinary incontinence after failure of nonpharmacologic therapy,19 and many drugs for these urinary symptoms have anticholinergic properties. If an anticholinergic is necessary, an agent that results in a lower anticholinergic burden should be considered in older patients.
A pharmacist-initiated medication review and intervention may be another way to adjust medications to reduce the patient’s anticholinergic burden.20,21 The common use of anticholinergic drugs in older adults reminds us to monitor their use closely.22
Benzodiazepines and nonbenzodiazepines
Benzodiazepines are among the most commonly prescribed psychotropics in developed countries and are prescribed mainly by primary care physicians rather than psychiatrists.23
In 2008, 5.2% of US adults ages 18 to 80 used a benzodiazepine, and long-term use was more prevalent in older patients (ages 65–80).23
Benzodiazepines are prescribed for anxiety,24 insomnia,25 and agitation. They can cause withdrawal26 and have potential for abuse.27 Benzodiazepines are associated with cognitive decline,28 impaired driving,29 falls,30 and hip fractures31 in older adults.
In addition, use of nonbenzodiazepine hypnotics (eg, zolpidem) is on the rise,32 and these drugs are known to increase the risk of hip fracture in nursing home residents.33
The American Geriatrics Society, through the American Board of Internal Medicine’s Choosing Wisely campaign, recommends avoiding benzodiazepines as a first-line treatment for insomnia, agitation, or delirium in older adults.34 Yet prescribing practices with these drugs in primary care settings conflict with guidelines, partly due to lack of training in constructive strategies regarding appropriate use of benzodiazepines.35 Educating patients on the risks and benefits of benzodiazepine treatment, especially long-term use, has been shown to reduce the rate of benzodiazepine-associated secondary events.36
Antipsychotics
Off-label use of antipsychotics is common and is increasing in the United States. In 2008, off-label use of antipsychotic drugs accounted for an estimated $6 billion.37 A common off-label use is to manage behavioral symptoms of dementia, despite a black-box warning about an increased risk of death in patients with dementia who are treated with antipsychotics.38,39 The Choosing Wisely campaign recommends against prescribing antipsychotics as a first-line treatment of behavioral and psychological symptoms of dementia.34
Antipsychotic drugs are associated with risk of acute kidney injury,40 as well as increased risk of falls and fractures (eg, a 52% higher risk of a serious fall, and a 50% higher risk of a nonvertebral osteoporotic fracture).41
Patients with dementia often exhibit aggression, resistance to care, and other challenging or disruptive behaviors. In such instances, antipsychotic drugs are often prescribed, but they provide limited and inconsistent benefits, while causing oversedation and worsening of cognitive function and increasing the likelihood of falling, stroke, and death.38,39,41
Because pharmacologic treatments for dementia are only modestly effective, have notable risks, and do not treat some of the behaviors that family members and caregivers find most distressing, nonpharmacologic measures are recommended as first-line treatment.42 These include caregiver education and support, training in problem-solving, and targeted therapy directed at the underlying causes of specific behaviors (eg, implementing nighttime routines to address sleep disturbances).42 Nonpharmacologic management of behavioral symptoms in dementia can significantly improve quality of life for patients and caregivers.42 Use of antipsychotic drugs in patients with dementia should be limited to cases in which nonpharmacologic measures have failed and patients pose an imminent threat to themselves or others.43
Proton pump inhibitors
Proton pump inhibitors are among the most commonly prescribed medications in the United States, and their use has increased significantly over the decade. It has been estimated that between 25% and 70% of these prescriptions have no appropriate indication.44
There is considerable excess use of acid suppressants in both inpatient and outpatient settings.45,46 In one study, at discharge from an internal medicine service, almost half of patients were taking a proton pump inhibitor.47
Evidence-based guidelines recommend these drugs to treat gastroesophageal reflux disease, nonerosive reflux disease, erosive esophagitis, dyspepsia, and peptic ulcer disease. However, long-term use (ie, beyond 8 weeks) is recommended only for patients with erosive esophagitis, Barrett esophagus, a pathologic hypersecretory condition, or a demonstrated need for maintenance treatment for reflux disease.48
Although proton pump inhibitors are highly effective and have low toxicity, there are reports of an association with Clostridium difficile infection,49 community-acquired pneumonia,50 hip fracture,51 vitamin B12 deficiency,52 atrophic gastritis,53 kidney disease,54 and dementia.55
Nondrug therapies such as weight loss and elevation of the head of the bed may improve esophageal pH levels and reflux symptoms.56
Deprescribing.org has practical advice for healthcare providers, patients, and caregivers on how to discontinue proton pump inhibitors, including videos, algorithms, and guidelines.
TOOLS TO EVALUATE APPROPRIATE DRUG THERAPY
Beers criteria
The Beers criteria (Table 2), developed in 1991 by a geriatrician as an approach to safer, more effective drug therapy in frail elderly nursing home patients,57 were updated by the American Geriatrics Society in 2015 for use in any clinical setting.58 (The criteria are also available as a smartphone application through the American Geriatrics Society at www.americangeriatrics.org.)
The Beers criteria offer evidence-based recommendations on drugs to avoid in the elderly, along with the rationale for use, the quality of evidence behind the recommendation, and the graded strength of the recommendation. The Beers criteria should be viewed through the lens of clinical judgment to offer safer nonpharmacologic and pharmacologic treatments.
The Joint Commission recommends medication reconciliation at every transition of care.59 The Beers criteria are a good starting point for a comprehensive medication review.
STOPP/START criteria
Another tool to aid safe prescribing in older adults is the Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions (STOPP), used in conjuction with the Screening Tool to Alert Doctors to Right Treatment (START). The STOPP/START criteria60,61 are based on an up-to-date literature review and consensus (Table 3).
THE BOTTOM LINE
Physicians caring for older adults need to diligently weigh the benefits of drug therapy and consider the patient’s care goals, current level of functioning, life expectancy, values, and preferences. Statin therapy for primary prevention, anticholinergics, benzodiazepines, antipsychotics, and proton pump inhibitors are widely used without proper indications, pointing to the need for a periodic comprehensive review of medications to reevaluate the risks vs the benefits of the patient’s medications. The Beers criteria and the STOPP/ START criteria can be useful tools for this purpose.
- Steinman MA. Polypharmacy—time to get beyond numbers. JAMA Intern Med 2016; 176:482–483.
- Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA 2015; 314:1818–1831.
- Tinetti ME, Bogardus ST Jr, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med 2004; 351:2870–2874.
- Qato DM, Wilder J, Schumm LP, Gillet V, Alexander GC. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med 2016; 176:473–482.
- Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA 2005; 294:716–724.
- Atkin PA, Veitch PC, Veitch EM, Ogle SJ. The epidemiology of serious adverse drug reactions among the elderly. Drugs Aging 1999; 14:141–152.
- Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016; 338:2532–2561.
- Gurwitz JH, Go AS, Fortman SP. Statins for primary prevention in older adults: uncertainty and the need for more evidence. JAMA 2016; 316:1971–1972.
- Chokshi NP, Messerli FH, Sutin D, Supariwala AA, Shah NR. Appropriateness of statins in patients aged ≥ 80 years and comparison to other age groups. Am J Cardiol 2012; 110:1477–1481.
- Stone NJ, Robinson J, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(suppl 2):S1–S45.
- Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013; 1:CD004816.
- Chatzizisis YS, Koskinas KC, Misirli G, Vaklavas C, Hatzitolios A, Giannoglou GD. Risk factors and drug interactions predisposing to statin-induced myopathy: implications for risk assessment, prevention and treatment. Drug Saf 2010; 33:171–187.
- Odden MC, Pletcher MJ, Coxson PG, et al. Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann Intern Med 2015; 162:533–541.
- Han BH, Sutin D, Williamson JD, et al. Effect of statin treatment vs usual care on primary cardiovascular prevention among older adults. The ALLHAT-LLT randomized clinical trial. JAMA Intern Med 2017; 177:955–965.
- Gray SL, Anderson ML, Dublin S, et al. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med 2015; 175:401–407.
- Rudolph JL, Salow MJ, Angelini MC, McGlinchey RE. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med 2008; 168:508–513.
- Hilmer SN, Mager DE, Simonsick EM, et al. A drug burden index to define the functional burden of medications in older people. Arch Intern Med 2007; 167:781–787.
- Risacher SL, McDonald BC, Tallman EF, et al; Alzheimer’s Disease Neuroimaging Initiative. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol 2016; 73:721–732.
- Qaseem A, Dallas P, Forciea MA, Starkey M, Denberg TD, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Nonsurgical management of urinary incontinence in women: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2014; 161:429–440.
- Efjestad AS, Molden E, Oksengard AR. Pharmacist-initiated management of antagonistic interactions between anticholinergic drugs and acetyl cholinesterase inhibitors in individuals with dementia. J Am Geriatr Soc 2013; 61:1624–1625.
- Kersten H, Molden E, Tolo IK, Skovlund E, Engedal K, Wyller TB. Cognitive effects of reducing anticholinergic drug burden in a frail elderly population: a randomized controlled trial. J Gerontol A Biol Sci Med Sci 2013; 68:271–278.
- Curtis LH, Østbye T, Sendersky V, et al. Inappropriate prescribing for elderly Americans in a large outpatient population. Arch Intern Med 2004; 164:1621–1625.
- Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry 2015; 72:136–142.
- Martin JL, Sainz-Pardo M, Furukawa TA, Martín-Sánchez E, Seoane T, Galán C. Benzodiazepines in generalized anxiety disorder: heterogeneity of outcomes based on a systematic review and meta-analysis of clinical trials. J Psychopharmacol 2007; 21:774–782.
- Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med 2007; 22:1335–1350.
- Rickels K, Schweizer E, Case WG, Greenblatt DJ. Long-term therapeutic use of benzodiazepines, I. Effects of abrupt discontinuation. Arch Gen Psychiatry 1990; 47:899–907.
- Fenton MC, Keyes KM, Martins SS, Hasin DS. The role of a prescription in anxiety medication use, abuse, and dependence. Am J Psychiatry 2010; 167:1247–1253.
- Billoti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ 2014; 349:g5205.
- Smink BE, Egberts AC, Lusthof KJ, Uges DR, de Gier JJ. The relationship between benzodiazepine use and traffic accidents: a systemic literature review. CNS Drugs 2010; 24:639–653.
- Tinett, ME, Speechley M, Ginter S. Risk factors for falls among elderly persons living in the community. N Engl J Med 1988; 319:1701–1707.
- Zint K, Haefeli WE, Glynn RJ, Mogun H, Avorn J, Stürmer T. Impact of drug interactions, dosage, and duration of therapy on the risk of hip fracture associated with benzodiazepine use in older adults. Pharmacoepidemiol Drug Saf 2010; 19:1248–1255.
- Briesacher BA, Soumerai SB, Field TS, Fouayzi H, Gurwitz JH. Medicare Part D’s exclusion of benzodiazepines and fracture risk in nursing homes. Arch Intern Med 2010; 170:693–698.
- Berry SD, Lee Y, Cai S, Dore DD. Nonbenzodiazepine sleep medication use and hip fractures in nursing home residents. JAMA Intern Med 2013; 173:754–761.
- American Geriatrics Society. Choosing Wisely. Ten things clinicians and patients should question. www.choosingwisely.org/societies/american-geriatrics-society/. Accessed December 3, 2017.
- Cook JM, Marshall R, Masci C, Coyne JC. Physicians’ perspectives on prescribing benzodiazepines for older adults: a qualitative study. J Gen Intern Med 2007; 22:303–307.
- Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med 2014; 174:890–898.
- Alexander GC, Gallagher SA, Mascola A, Moloney RM, Stafford RS. Increasing off-label use of antipsychotic medications in the United States, 1995–2008. Phamacoepidemiol Drug Saf 2011; 20:177–184.
- Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med 2007; 146:775–786.
- US Food and Drug Administration (FDA). Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm053171.htm. Accessed December 4, 2017.
- Hwang YJ, Dixon SN, Reiss JP, et al. Atypical antipsychotic drugs and the risk for acute kidney injury and other adverse outcomes in older adults. Ann Intern Med 2014; 161:242–248.
- Fraser L, Liu K, Naylor KL, et al. Falls and fractures with atypical antipsychotic medication use: a population-based cohort study. JAMA Intern Med 2015; 175:450–452.
- Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA 2012; 308:2020–2029.
- Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 2006; 355:1525–1538.
- Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ 2008; 336:2–3.
- Mazer-Amirshahi M, Mullins PM, van den Anker J, Meltzer A, Pines JM. Rising rates of proton pump inhibitor prescribing in US emergency departments. Am J Emerg Med 2014; 32:618–622.
- Heidelbaugh JJ, Goldberg KL, Inadomi JM. Magnitude and economic effect of overuse of antisecretory therapy in the ambulatory care setting. Am J Manag Care 2010; 16:e228–e324.
- Pham CQ, Regal RE, Bostwich TR, Knauf KS. Acid suppressive therapy used on an inpatient internal medicine service. Ann Pharmacother 2006; 40:1261–1266.
- Kahrilas PJ, Shaheen NJ, Vaezi MF, et al; American Gastroenterological Association. American Gastroenterological Association medical position statement on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135:1383–1391.e1–e5.
- Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med 2010; 170:784–790.
- Gulmez SE, Holm A, Frederiksen H, Jensen TG, Pedersen C, Hallas J. Use of proton pump inhibitors and the risk of community-acquired pneumonia: a population-based case-control study. Arch Intern Med 2007; 167:950–955.
- Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006; 296:2947–2953.
- Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA 2013; 310:2435–2442.
- Kuipers EJ, Lundell L, Klinkenberg-Knol EC, et al. Atrophic gastritis and Helicobacter pylori infection in patients with reflux esophagitis treated with omeprazole or fundoplication. N Engl J Med 1996; 334:1018–1022.
- Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med 2016; 176:238–246.
- Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol 2016; 73:410–416.
- Kaltenbach T, Crockett S, Gerson LB. Are lifestyle measures effective in patients with gastroesophageal reflux disease? An evidence-based approach. Arch Intern Med 2006; 166:965–971.
- Beers MH, Ouslander JG, Rollingher I, Reuben DB, Brooks J, Beck JC. Explicit criteria for determining inappropriate medication use in nursing home residents. Arch Intern Med 1991; 151:1825–1832.
- American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63:2227–2246.
- Joint Commission. Sentinel event alert, Issue 35: using medication reconciliation to prevent errors. www.jointcommission.org/sentinel_event_alert_issue_35_using_medication_reconciliation_to_prevent_errors/. Accessed August 18, 2017.
- Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther 2008; 46:72–83.
- O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 2015; 44:213–218.
- Steinman MA. Polypharmacy—time to get beyond numbers. JAMA Intern Med 2016; 176:482–483.
- Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA 2015; 314:1818–1831.
- Tinetti ME, Bogardus ST Jr, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med 2004; 351:2870–2874.
- Qato DM, Wilder J, Schumm LP, Gillet V, Alexander GC. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med 2016; 176:473–482.
- Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA 2005; 294:716–724.
- Atkin PA, Veitch PC, Veitch EM, Ogle SJ. The epidemiology of serious adverse drug reactions among the elderly. Drugs Aging 1999; 14:141–152.
- Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016; 338:2532–2561.
- Gurwitz JH, Go AS, Fortman SP. Statins for primary prevention in older adults: uncertainty and the need for more evidence. JAMA 2016; 316:1971–1972.
- Chokshi NP, Messerli FH, Sutin D, Supariwala AA, Shah NR. Appropriateness of statins in patients aged ≥ 80 years and comparison to other age groups. Am J Cardiol 2012; 110:1477–1481.
- Stone NJ, Robinson J, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(suppl 2):S1–S45.
- Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013; 1:CD004816.
- Chatzizisis YS, Koskinas KC, Misirli G, Vaklavas C, Hatzitolios A, Giannoglou GD. Risk factors and drug interactions predisposing to statin-induced myopathy: implications for risk assessment, prevention and treatment. Drug Saf 2010; 33:171–187.
- Odden MC, Pletcher MJ, Coxson PG, et al. Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann Intern Med 2015; 162:533–541.
- Han BH, Sutin D, Williamson JD, et al. Effect of statin treatment vs usual care on primary cardiovascular prevention among older adults. The ALLHAT-LLT randomized clinical trial. JAMA Intern Med 2017; 177:955–965.
- Gray SL, Anderson ML, Dublin S, et al. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med 2015; 175:401–407.
- Rudolph JL, Salow MJ, Angelini MC, McGlinchey RE. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med 2008; 168:508–513.
- Hilmer SN, Mager DE, Simonsick EM, et al. A drug burden index to define the functional burden of medications in older people. Arch Intern Med 2007; 167:781–787.
- Risacher SL, McDonald BC, Tallman EF, et al; Alzheimer’s Disease Neuroimaging Initiative. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol 2016; 73:721–732.
- Qaseem A, Dallas P, Forciea MA, Starkey M, Denberg TD, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Nonsurgical management of urinary incontinence in women: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2014; 161:429–440.
- Efjestad AS, Molden E, Oksengard AR. Pharmacist-initiated management of antagonistic interactions between anticholinergic drugs and acetyl cholinesterase inhibitors in individuals with dementia. J Am Geriatr Soc 2013; 61:1624–1625.
- Kersten H, Molden E, Tolo IK, Skovlund E, Engedal K, Wyller TB. Cognitive effects of reducing anticholinergic drug burden in a frail elderly population: a randomized controlled trial. J Gerontol A Biol Sci Med Sci 2013; 68:271–278.
- Curtis LH, Østbye T, Sendersky V, et al. Inappropriate prescribing for elderly Americans in a large outpatient population. Arch Intern Med 2004; 164:1621–1625.
- Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry 2015; 72:136–142.
- Martin JL, Sainz-Pardo M, Furukawa TA, Martín-Sánchez E, Seoane T, Galán C. Benzodiazepines in generalized anxiety disorder: heterogeneity of outcomes based on a systematic review and meta-analysis of clinical trials. J Psychopharmacol 2007; 21:774–782.
- Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med 2007; 22:1335–1350.
- Rickels K, Schweizer E, Case WG, Greenblatt DJ. Long-term therapeutic use of benzodiazepines, I. Effects of abrupt discontinuation. Arch Gen Psychiatry 1990; 47:899–907.
- Fenton MC, Keyes KM, Martins SS, Hasin DS. The role of a prescription in anxiety medication use, abuse, and dependence. Am J Psychiatry 2010; 167:1247–1253.
- Billoti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ 2014; 349:g5205.
- Smink BE, Egberts AC, Lusthof KJ, Uges DR, de Gier JJ. The relationship between benzodiazepine use and traffic accidents: a systemic literature review. CNS Drugs 2010; 24:639–653.
- Tinett, ME, Speechley M, Ginter S. Risk factors for falls among elderly persons living in the community. N Engl J Med 1988; 319:1701–1707.
- Zint K, Haefeli WE, Glynn RJ, Mogun H, Avorn J, Stürmer T. Impact of drug interactions, dosage, and duration of therapy on the risk of hip fracture associated with benzodiazepine use in older adults. Pharmacoepidemiol Drug Saf 2010; 19:1248–1255.
- Briesacher BA, Soumerai SB, Field TS, Fouayzi H, Gurwitz JH. Medicare Part D’s exclusion of benzodiazepines and fracture risk in nursing homes. Arch Intern Med 2010; 170:693–698.
- Berry SD, Lee Y, Cai S, Dore DD. Nonbenzodiazepine sleep medication use and hip fractures in nursing home residents. JAMA Intern Med 2013; 173:754–761.
- American Geriatrics Society. Choosing Wisely. Ten things clinicians and patients should question. www.choosingwisely.org/societies/american-geriatrics-society/. Accessed December 3, 2017.
- Cook JM, Marshall R, Masci C, Coyne JC. Physicians’ perspectives on prescribing benzodiazepines for older adults: a qualitative study. J Gen Intern Med 2007; 22:303–307.
- Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med 2014; 174:890–898.
- Alexander GC, Gallagher SA, Mascola A, Moloney RM, Stafford RS. Increasing off-label use of antipsychotic medications in the United States, 1995–2008. Phamacoepidemiol Drug Saf 2011; 20:177–184.
- Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med 2007; 146:775–786.
- US Food and Drug Administration (FDA). Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm053171.htm. Accessed December 4, 2017.
- Hwang YJ, Dixon SN, Reiss JP, et al. Atypical antipsychotic drugs and the risk for acute kidney injury and other adverse outcomes in older adults. Ann Intern Med 2014; 161:242–248.
- Fraser L, Liu K, Naylor KL, et al. Falls and fractures with atypical antipsychotic medication use: a population-based cohort study. JAMA Intern Med 2015; 175:450–452.
- Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA 2012; 308:2020–2029.
- Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 2006; 355:1525–1538.
- Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ 2008; 336:2–3.
- Mazer-Amirshahi M, Mullins PM, van den Anker J, Meltzer A, Pines JM. Rising rates of proton pump inhibitor prescribing in US emergency departments. Am J Emerg Med 2014; 32:618–622.
- Heidelbaugh JJ, Goldberg KL, Inadomi JM. Magnitude and economic effect of overuse of antisecretory therapy in the ambulatory care setting. Am J Manag Care 2010; 16:e228–e324.
- Pham CQ, Regal RE, Bostwich TR, Knauf KS. Acid suppressive therapy used on an inpatient internal medicine service. Ann Pharmacother 2006; 40:1261–1266.
- Kahrilas PJ, Shaheen NJ, Vaezi MF, et al; American Gastroenterological Association. American Gastroenterological Association medical position statement on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135:1383–1391.e1–e5.
- Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med 2010; 170:784–790.
- Gulmez SE, Holm A, Frederiksen H, Jensen TG, Pedersen C, Hallas J. Use of proton pump inhibitors and the risk of community-acquired pneumonia: a population-based case-control study. Arch Intern Med 2007; 167:950–955.
- Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006; 296:2947–2953.
- Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA 2013; 310:2435–2442.
- Kuipers EJ, Lundell L, Klinkenberg-Knol EC, et al. Atrophic gastritis and Helicobacter pylori infection in patients with reflux esophagitis treated with omeprazole or fundoplication. N Engl J Med 1996; 334:1018–1022.
- Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med 2016; 176:238–246.
- Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol 2016; 73:410–416.
- Kaltenbach T, Crockett S, Gerson LB. Are lifestyle measures effective in patients with gastroesophageal reflux disease? An evidence-based approach. Arch Intern Med 2006; 166:965–971.
- Beers MH, Ouslander JG, Rollingher I, Reuben DB, Brooks J, Beck JC. Explicit criteria for determining inappropriate medication use in nursing home residents. Arch Intern Med 1991; 151:1825–1832.
- American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63:2227–2246.
- Joint Commission. Sentinel event alert, Issue 35: using medication reconciliation to prevent errors. www.jointcommission.org/sentinel_event_alert_issue_35_using_medication_reconciliation_to_prevent_errors/. Accessed August 18, 2017.
- Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther 2008; 46:72–83.
- O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 2015; 44:213–218.
KEY POINTS
- Statins, anticholinergics, benzodiazepines, antipsychotics, and proton pump inhibitors are widely prescribed.
- In older patients, a periodic comprehensive medication review is needed to reevaluate the risks and the benefits of current medications in light of goals of care, life expectancy, and the patient’s preferences.
- The Beers criteria and the Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions provide valuable guidance for safe prescribing in older adults.
In reply: Starting insulin therapy
In Reply: We thank Dr. Weiss for his insightful comments and for the opportunity to clarify a number of points from our article.
We agree that controlling the fasting glucose should not take months. As mentioned in our article, adjusting the basal insulin dose should be done with 2 to 4 units every 2 to 3 days in order to reach the fasting glycemic goal. Applying this approach and systematically titrating the NPH, glargine, or detemir insulin will smoothly decrease the fasting glucose within 12 weeks, as described in the 24-week1 and 52-week2 treat-to-target trials in which basal insulin was added to the oral therapy in patients with type 2 diabetes.
When basal insulin is no longer sufficient to reach a target hemoglobin A1c, a glucagon-like peptide-1 receptor agonist or prandial insulin can be used. The basal-bolus or twice-daily premixed insulin analogues can also be considered as the initial therapy, depending on the patient, disease, and drug characteristics.3 We agree that once a prandial insulin regimen is initiated, the dose titration can be done based on preprandial or postprandial blood glucose measurements, as shown in Table 2 in our article. However, adding the prandial insulin without first optimizing the basal therapy was considered a limitation of the Orals Plus Apidra and Lantus (OPAL) study,4 which investigated the addition of one prandial insulin injection to basal glargine insulin.5 As a consequence, the subsequent studies investigating the effects of initiating and titrating the preprandial rapid-acting insulin (as a single dose or using a stepwise approach) in patients inadequately controlled with once-daily basal insulin and oral antidiabetic drugs had run-in periods of 12 to 14 weeks, in order to optimize the basal insulin dosage and achieve target fasting blood glucose levels of 110 mg/dL or less. This approach had the additional benefit of achieving a target hemoglobin A1c level of less than 7% in a significant number of patients (up to 37%),6 before starting the preprandial insulin.6–8
Regardless of the regimen selected, titration of the insulin doses can only be achieved with understanding the pharmacodynamic characteristics of each type of insulin used.9
- Riddle MC, Rosenstock J, Gerich J; Insulin Glargine 4002 Study Investigators. The Treat-to-Target Trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003; 26:3080–3086.
- Rosenstock J, Davies M, Home PD, Larsen J, Koenen C, Schernthaner G. A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetologia 2008; 51:408–416.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centered approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2015; 58:429–442.
- Owens DR. Stepwise intensification of insulin therapy in type 2 diabetes management—exploring the concept of the basal-plus approach in clinical practice. Diabet Med 2013; 30:276–288.
- Lankisch MR, Ferlinz KC, Leahy JL, Scherbaum WA; Orals Plus Apidra and Lantus (OPAL) Study Group. Introducing a simplified approach to insulin therapy in type 2 diabetes: a comparison of two single-dose regimens of insulin glulisine plus insulin glargine and oral antidiabetic drugs. Diabetes Obes Metab 2008; 10:1178–1185.
- Davidson MB, Raskin P, Tanenberg RJ, Vlajnic A, Hollander P. A stepwise approach to insulin therapy in patients with type 2 diabetes mellitus and basal insulin treatment failure. Endocr Pract 2011; 17:395–403.
- Meneghini L, Mersebach H, Kumar S, Svendsen AL, Hermansen K. Comparison of 2 intensification regimens with rapid-acting insulin aspart in type 2 diabetes mellitus inadequately controlled by once-daily insulin detemir and oral antidiabetes drugs: the Step-Wise Randomized Study. Endocrine Practice 2011; 17:727–736.
- Owens DR, Luzio SD, Sert-Langeron C, Riddle MC. Effects of initiation and titration of a single pre-prandial dose of insulin glulisine while continuing titrated insulin glargine in type 2 diabetes: a 6-month ‘proof-of-concept’ study. Diabetes Obes Metab 2011; 13:1020–1027.
- American Diabetes Association. 7. Approaches to glycemic treatment. Diabetes Care 2015; 38(suppl):S41–S48.
In Reply: We thank Dr. Weiss for his insightful comments and for the opportunity to clarify a number of points from our article.
We agree that controlling the fasting glucose should not take months. As mentioned in our article, adjusting the basal insulin dose should be done with 2 to 4 units every 2 to 3 days in order to reach the fasting glycemic goal. Applying this approach and systematically titrating the NPH, glargine, or detemir insulin will smoothly decrease the fasting glucose within 12 weeks, as described in the 24-week1 and 52-week2 treat-to-target trials in which basal insulin was added to the oral therapy in patients with type 2 diabetes.
When basal insulin is no longer sufficient to reach a target hemoglobin A1c, a glucagon-like peptide-1 receptor agonist or prandial insulin can be used. The basal-bolus or twice-daily premixed insulin analogues can also be considered as the initial therapy, depending on the patient, disease, and drug characteristics.3 We agree that once a prandial insulin regimen is initiated, the dose titration can be done based on preprandial or postprandial blood glucose measurements, as shown in Table 2 in our article. However, adding the prandial insulin without first optimizing the basal therapy was considered a limitation of the Orals Plus Apidra and Lantus (OPAL) study,4 which investigated the addition of one prandial insulin injection to basal glargine insulin.5 As a consequence, the subsequent studies investigating the effects of initiating and titrating the preprandial rapid-acting insulin (as a single dose or using a stepwise approach) in patients inadequately controlled with once-daily basal insulin and oral antidiabetic drugs had run-in periods of 12 to 14 weeks, in order to optimize the basal insulin dosage and achieve target fasting blood glucose levels of 110 mg/dL or less. This approach had the additional benefit of achieving a target hemoglobin A1c level of less than 7% in a significant number of patients (up to 37%),6 before starting the preprandial insulin.6–8
Regardless of the regimen selected, titration of the insulin doses can only be achieved with understanding the pharmacodynamic characteristics of each type of insulin used.9
In Reply: We thank Dr. Weiss for his insightful comments and for the opportunity to clarify a number of points from our article.
We agree that controlling the fasting glucose should not take months. As mentioned in our article, adjusting the basal insulin dose should be done with 2 to 4 units every 2 to 3 days in order to reach the fasting glycemic goal. Applying this approach and systematically titrating the NPH, glargine, or detemir insulin will smoothly decrease the fasting glucose within 12 weeks, as described in the 24-week1 and 52-week2 treat-to-target trials in which basal insulin was added to the oral therapy in patients with type 2 diabetes.
When basal insulin is no longer sufficient to reach a target hemoglobin A1c, a glucagon-like peptide-1 receptor agonist or prandial insulin can be used. The basal-bolus or twice-daily premixed insulin analogues can also be considered as the initial therapy, depending on the patient, disease, and drug characteristics.3 We agree that once a prandial insulin regimen is initiated, the dose titration can be done based on preprandial or postprandial blood glucose measurements, as shown in Table 2 in our article. However, adding the prandial insulin without first optimizing the basal therapy was considered a limitation of the Orals Plus Apidra and Lantus (OPAL) study,4 which investigated the addition of one prandial insulin injection to basal glargine insulin.5 As a consequence, the subsequent studies investigating the effects of initiating and titrating the preprandial rapid-acting insulin (as a single dose or using a stepwise approach) in patients inadequately controlled with once-daily basal insulin and oral antidiabetic drugs had run-in periods of 12 to 14 weeks, in order to optimize the basal insulin dosage and achieve target fasting blood glucose levels of 110 mg/dL or less. This approach had the additional benefit of achieving a target hemoglobin A1c level of less than 7% in a significant number of patients (up to 37%),6 before starting the preprandial insulin.6–8
Regardless of the regimen selected, titration of the insulin doses can only be achieved with understanding the pharmacodynamic characteristics of each type of insulin used.9
- Riddle MC, Rosenstock J, Gerich J; Insulin Glargine 4002 Study Investigators. The Treat-to-Target Trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003; 26:3080–3086.
- Rosenstock J, Davies M, Home PD, Larsen J, Koenen C, Schernthaner G. A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetologia 2008; 51:408–416.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centered approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2015; 58:429–442.
- Owens DR. Stepwise intensification of insulin therapy in type 2 diabetes management—exploring the concept of the basal-plus approach in clinical practice. Diabet Med 2013; 30:276–288.
- Lankisch MR, Ferlinz KC, Leahy JL, Scherbaum WA; Orals Plus Apidra and Lantus (OPAL) Study Group. Introducing a simplified approach to insulin therapy in type 2 diabetes: a comparison of two single-dose regimens of insulin glulisine plus insulin glargine and oral antidiabetic drugs. Diabetes Obes Metab 2008; 10:1178–1185.
- Davidson MB, Raskin P, Tanenberg RJ, Vlajnic A, Hollander P. A stepwise approach to insulin therapy in patients with type 2 diabetes mellitus and basal insulin treatment failure. Endocr Pract 2011; 17:395–403.
- Meneghini L, Mersebach H, Kumar S, Svendsen AL, Hermansen K. Comparison of 2 intensification regimens with rapid-acting insulin aspart in type 2 diabetes mellitus inadequately controlled by once-daily insulin detemir and oral antidiabetes drugs: the Step-Wise Randomized Study. Endocrine Practice 2011; 17:727–736.
- Owens DR, Luzio SD, Sert-Langeron C, Riddle MC. Effects of initiation and titration of a single pre-prandial dose of insulin glulisine while continuing titrated insulin glargine in type 2 diabetes: a 6-month ‘proof-of-concept’ study. Diabetes Obes Metab 2011; 13:1020–1027.
- American Diabetes Association. 7. Approaches to glycemic treatment. Diabetes Care 2015; 38(suppl):S41–S48.
- Riddle MC, Rosenstock J, Gerich J; Insulin Glargine 4002 Study Investigators. The Treat-to-Target Trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003; 26:3080–3086.
- Rosenstock J, Davies M, Home PD, Larsen J, Koenen C, Schernthaner G. A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetologia 2008; 51:408–416.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centered approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2015; 58:429–442.
- Owens DR. Stepwise intensification of insulin therapy in type 2 diabetes management—exploring the concept of the basal-plus approach in clinical practice. Diabet Med 2013; 30:276–288.
- Lankisch MR, Ferlinz KC, Leahy JL, Scherbaum WA; Orals Plus Apidra and Lantus (OPAL) Study Group. Introducing a simplified approach to insulin therapy in type 2 diabetes: a comparison of two single-dose regimens of insulin glulisine plus insulin glargine and oral antidiabetic drugs. Diabetes Obes Metab 2008; 10:1178–1185.
- Davidson MB, Raskin P, Tanenberg RJ, Vlajnic A, Hollander P. A stepwise approach to insulin therapy in patients with type 2 diabetes mellitus and basal insulin treatment failure. Endocr Pract 2011; 17:395–403.
- Meneghini L, Mersebach H, Kumar S, Svendsen AL, Hermansen K. Comparison of 2 intensification regimens with rapid-acting insulin aspart in type 2 diabetes mellitus inadequately controlled by once-daily insulin detemir and oral antidiabetes drugs: the Step-Wise Randomized Study. Endocrine Practice 2011; 17:727–736.
- Owens DR, Luzio SD, Sert-Langeron C, Riddle MC. Effects of initiation and titration of a single pre-prandial dose of insulin glulisine while continuing titrated insulin glargine in type 2 diabetes: a 6-month ‘proof-of-concept’ study. Diabetes Obes Metab 2011; 13:1020–1027.
- American Diabetes Association. 7. Approaches to glycemic treatment. Diabetes Care 2015; 38(suppl):S41–S48.
Starting insulin in patients with type 2 diabetes: An individualized approach
Insulin therapy is one of the most effective tools clinicians can use to help patients reach their individualized hemoglobin A1c target. However, decisions about when and how to start insulin therapy have to be individualized to the needs and goals of each patient. Many insulin options are available, one of the most common being the addition of basal insulin to oral antidiabetic drugs. Although patients are often reluctant to start insulin, this reluctance can be overcome through patient education and hands-on training.
Here, we review hemoglobin A1c targets, factors that determine when to start insulin therapy, and the different regimens that can be used.
MOST PATIENTS EVENTUALLY NEED INSULIN
Type 2 diabetes mellitus is a chronic progressive disease associated with insulin resistance, beta-cell dysfunction, and decreased insulin secretion. Consequently, most patients eventually require insulin therapy to reduce the risk of long-term complications.
The efficacy of therapy can be assessed by measuring hemoglobin A1c, an important marker of the chronic hyperglycemic state. The hemoglobin A1c value can be reported as a ratio (%) standardized against the results of the Diabetes Control and Complications Trial,1 or as International Federation of Clinical Chemistry units (mmol/mol).2 Table 1 shows the relationship between hemoglobin A1c and average glucose values.3
WHAT IS AN APPROPRIATE HEMOGLOBIN A1c TARGET?
The short answer is, “It depends.”
Currently, the American Association of Clinical Endocrinologists (AACE) supports a hemoglobin A1c goal of less than 6.5% for otherwise healthy patients but states that the goal should be individualized for patients with concurrent illnesses or at risk of hypoglycemia.4
On the other hand, the American Diabetes Association (ADA) recommends a higher hemoglobin A1c target of less than 7% for most adults with type 2 diabetes mellitus.5 This value was shown to be associated with a reduction in the microvascular and macrovascular complications of diabetes.
Yet when three large trials6–8 recently compared intensive and standard glucose control regimens, tighter glucose control failed to improve cardiovascular outcomes. Moreover, in one of the trials,7 patients receiving intensive treatment had a higher rate of all-cause mortality. Details:
- Action in Diabetes and Vascular Disease (ADVANCE): 11,140 patients; average hemoglobin A1c levels 6.5% vs 7.3%6
- Action to Control Cardiovascular Risk in Diabetes (ACCORD): 10,251 patients; average hemoglobin A1c levels 6.4% vs 7.5%7
- Veterans Affairs Diabetes Trial (VADT): 1,791 patients; average hemoglobin A1c levels 6.9% vs 8.4%.8
Similarly, a 2013 Cochrane review9 that included 28 randomized controlled trials concluded that intensive control (in 18,717 patients) did not decrease all-cause and cardiovascular mortality rates compared with traditional glucose control (in 16,195 patients), and it increased the risk of hypoglycemia and serious adverse events.
As a result, the ADA5 states that a hemoglobin A1c target less than 6.5% is optional for patients with a long life expectancy, short duration of diabetes, low risk of hypoglycemia, and no significant cardiovascular disease. The ADA further defines a hemoglobin A1c goal of less than 8% for patients with a history of severe hypoglycemia, limited life expectancy, advanced microvascular or macrovascular complications, extensive comorbid conditions, and long-standing diabetes.
Therefore, the AACE and ADA are moving away from “one-size-fits-all” goals and toward individualizing their recommendations.
WHEN SHOULD INSULIN BE STARTED?
Physicians should consider the needs and preferences of each patient and individualize the treatment. The most recent recommendations from the ADA5 stress the importance of a patient-centered approach, with multiple factors taken into account. These include the patient’s attitude, expected compliance with treatment, risk of hypoglycemia, disease duration, life expectancy, and comorbidities, and the side effects of oral medications and insulin.
Compared with previous guidelines, there are fewer rules on how and when to start insulin therapy. But absolute and relative indications for insulin therapy should be considered in patients with the following:
Absolute indications for insulin
- Ketoacidosis or catabolic symptoms, including ketonuria
- Newly diagnosed type 2 diabetes with pronounced hyperglycemia (glucose ≥ 300 mg/dL or hemoglobin A1c ≥ 10.0%) with or without severe symptoms, including weight loss, polyuria, or polydipsia10
- Uncontrolled type 2 diabetes mellitus despite using one, two, or more oral antidiabetic drugs or glucagon-like peptide 1 (GLP-1) receptor agonists
- Gestational diabetes
- Preference for insulin.
Relative indications for insulin
- Hospitalized for surgery or acute illnesses
- Advanced renal or hepatic disease
- Inability to afford the cost or tolerate the side effects of oral antidiabetic drugs and GLP-1 receptor agonists.
Depending on the situation, blood glucose is measured fasting, before meals, or after meals after initiating or adjusting insulin regimens (Table 2).
WHAT ARE THE INSULIN REGIMENS?
Basal insulin
In the early stages of type 2 diabetes, metformin alone or in combination with another oral antidiabetic drug or with a GLP-1 receptor agonist is often used along with healthy eating, weight control, and increased physical activity.
When the target hemoglobin A1c cannot be achieved with one or two noninsulin drugs, the ADA suggests basal insulin be added to metformin or a two-medication regimen that includes metformin (Table 3). However, recent evidence suggests that combining a GLP-1 receptor agonist with basal insulin, in a regimen without metformin, is safe and improves glycemic control without hypoglycemia or weight gain.11
While a total daily dose of insulin of 0.1 to 0.2 units/kg could be initially used in patients with a hemoglobin A1c level less than 8%, a higher dose of 0.2 to 0.3 units/kg is required if the hemoglobin A1c level is between 8% and 10%. The dose can be titrated once or twice weekly if the fasting glucose is above the target level (usually < 130 mg/dL). If hypoglycemia develops (glucose < 70 mg/dL), the insulin dose should be reduced by 10% to 20%.10
Available basal insulins include glargine, detemir, and neutral protamine Hagedorn (NPH) (Table 4).12–14 Because glargine and detemir offer better pharmacokinetic properties, less variability in response, and less risk of hypoglycemia, they are preferred over NPH. Glargine has a relatively constant plasma concentration over 24 hours, allowing once-daily dosing at any time during the day (Figure 1).15 The dose should be taken at the same time every day. Detemir and NPH are usually taken once or twice daily.
Patients treated once daily should take the dose with the evening meal or at bedtime. Patients who require a twice-daily regimen can take the first dose with breakfast and the second one with the evening meal, at bedtime, or 12 hours after the morning dose.
The randomized Treat-to-Target trial,16 in 756 patients, showed that both glargine and NPH, when added to oral therapy in patients with type 2 diabetes, achieve the target hemoglobin A1c, but NPH is associated with more episodes of nocturnal hypoglycemia. Similar results were found when NPH was compared with detemir insulin.17
A Cochrane review18 suggested that glargine and detemir are similar in efficacy and safety. However, detemir often needs to be injected twice daily, in a higher dose, and is associated with less weight gain. Furthermore, a meta-analysis of 46 randomized clinical trials19 showed that the weight increase at 1 year is less in patients treated with basal than with twice-daily or prandial regimens.
A noninterventional longitudinal study20 in 2,179 patients newly started on insulin showed that the mean weight increase at 1 year was 1.78 kg, and 24% of patients gained more than 5 kg. However, the factors independently associated with the weight gain were a higher hemoglobin A1c at baseline, a higher insulin dose at baseline and at 1 year, and a lower baseline body mass index, but not the type of insulin regimen.
Currently, a new class of ultralong-acting basal insulins is being studied. Insulins in this class are approved in other countries, but the US Food and Drug Administration requires additional data for approval. Ultralong-acting insulins are expected to reduce the risk of hypoglycemia, specifically the risk of nocturnal episodes. Also, given their longer duration of action and stable steady-state pharmacokinetics, they will offer flexibility in the dose timing.21
Basal-bolus regimens
Basal insulin often does not control postprandial hyperglycemia. The need for multiple doses of insulin (including one or more preprandial doses) is suggested by postprandial glucose values above target (usually > 180 mg/dL) or by a hemoglobin A1c above goal despite well-controlled fasting glucose levels. This usually becomes evident when the total daily dose of basal insulin exceeds 0.5 units/kg. Patients newly diagnosed with diabetes who have a hemoglobin A1c higher than 10% may also respond better to an initial basal-bolus regimen.
Available bolus insulins include lispro, aspart, glulisine, regular insulin, and the newly approved Technosphere inhaled regular insulin (Table 4).12–14 They can be taken before each meal, and the total bolus dose usually represents 50% of the total daily dose.22 Rapid-acting insulins have faster onset, shorter duration of action, and more predictable pharmacokinetics, which makes them preferable to regular insulin (Figure 1).15 Inhaled insulin is another option, but it is contraindicated in patients with chronic obstructive pulmonary disease or asthma because of the increased risk of acute bronchospasm.12
Alternatively, the transition to a basal-bolus regimen can be accomplished with a single dose of bolus insulin before the main meal, using a dose that represents approximately 10% of the total daily dose. Additional bolus doses can be added later based on the glycemic control. The adjustment of the preprandial insulin dose is done once or twice weekly, based on the postprandial glucose levels.10
Premixed combinations of long- and short-acting insulins in ratios of 50% to 50%, 70% to 30%, or 75% to 25% can be considered in patients who cannot adhere to a complex insulin regimen. A propensity-matched comparison of different insulin regimens (basal, premixed, mealtime plus basal, and mealtime) in patients with type 2 diabetes revealed that the hemoglobin A1c reduction was similar between the different groups.23 However, the number of hypoglycemic episodes was higher in the premixed insulin group, and the weight gain was less in the basal insulin group.
While premixed insulins require fewer injections, they do not provide dosing flexibility. In other words, dose adjustments for premixed insulins lead to increases in both basal and bolus amounts even though a dose adjustment is needed for only one insulin type. Thus, this is a common reason for increased hypoglycemic episodes.
Continuous subcutaneous insulin infusion
A meta-analysis showed that continuous subcutaneous insulin infusion (ie, use of an insulin pump) was similar to intensive therapy with multiple daily insulin injections in terms of glycemic control and hypoglycemia.24 Since both options can lead to similar glucose control, additional factors to consider when initiating insulin infusion include lifestyle and technical expertise. Some patients may or may not prefer having a pump attached for nearly all daily activities. Additionally, this type of therapy is complex and requires significant training to ensure efficacy and safety.25
WHAT IS THE COST OF INSULIN THERAPY?
A final factor to keep in mind when initiating insulin is cost (Table 4).12–14 Asking patients to check their prescription insurance formulary is important to ensure that an affordable option is selected. If patients do not have prescription insurance, medication assistance programs could be an option. However, if a patient is considering an insulin pump, insurance coverage is essential. Depending on the manufacturer, insulin pumps cost about $6,000 to $7,000, and the additional monthly supplies for the pump are also expensive.
If patients are engaged when considering and selecting insulin therapy, the likelihood of treatment success is greater.26–28
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- Hanas R, John WG; International HbA1c Consensus Committee. 2013 Update on the worldwide standardization of the hemoglobin A1c measurement. Pediatr Diabetes 2014; 15:e1–e2.
- Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ; A1c-Derived Average Glucose Study Group. Translating the A1C assay into estimated average glucose values. Diabetes Care 2008; 31:1473–1478.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al; American Association of Clinical Endocrinologists. AACE comprehensive diabetes management algorithm 2013. Endocr Pract 2013; 19:327–336.
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care 2014; 37(suppl 1):S14–S80.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Hemmingsen B, Lund SS, Gluud C, et al. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev 2013; 11:CD008143.
- Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35:1364–1379.
- Vora J, Bain SC, Damci T, et al. Incretin-based therapy in combination with basal insulin: a promising tactic for the treatment of type 2 diabetes. Diabetes Metab 2013; 39:6–15.
- Nuffer W, Trujillo JM, Ellis SL. Technosphere insulin (Afrezza): a new, inhaled prandial insulin. Ann Pharmacother 2015; 49:99–106.
- Pharmacist’s Letter/Prescriber’s Letter. Comparison of insulins and injectable diabetes meds. PL Detail-Document #281107 November 2012. www.PharmacistsLetter.com. Accessed July 2, 2015
- Lexicomp Online. www.wolterskluwercdi.com/lexicomp-online/. Accessed July 2, 2015.
- Hirsch IB. Insulin analogues. N Engl J Med 2005; 352:174-183.
- Riddle MC, Rosenstock J, Gerich J; Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003; 26:3080–3086.
- Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care 2006; 29:1269–1274.
- Swinnen SG, Simon AC, Holleman F, Hoekstra JB, Devries JH. Insulin detemir versus insulin glargine for type 2 diabetes mellitus. Cochrane Database Syst Rev 2011; 7:CD006383.
- Pontiroli AE, Miele L, Morabito A. Increase of body weight during the first year of intensive insulin treatment in type 2 diabetes: systematic review and meta-analysis. Diabetes Obes Metab 2011; 13:1008–1019.
- Balkau B, Home PD, Vincent M, Marre M, Freemantle N. Factors associated with weight gain in people with type 2 diabetes starting on insulin. Diabetes Care 2014; 37:2108–2113.
- Garber AJ. Will the next generation of basal insulins offer clinical advantages? Diabetes Obes Metab 2014; 16:483–491.
- Tamaki M, Shimizu T, Kanazawa A, et al. Effects of changes in basal/total daily insulin ratio in type 2 diabetes patients on intensive insulin therapy including insulin glargine (JUN-LAN Study 6). Diabetes Res Clin Pract 2008; 81:e1–e3.
- Freemantle N, Balkau B, Home PD. A propensity score matched comparison of different insulin regimens 1 year after beginning insulin in people with type 2 diabetes. Diabetes Obes Metab 2013; 15:1120–1127.
- Yeh HC, Brown TT, Maruthur N, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med 2012; 157:336–347.
- Schade DS, Valentine V. To pump or not to pump. Diabetes Care 2002; 25:2100–2102.
- Liu L, Lee MJ, Brateanu A. Improved A1C and lipid profile in patients referred to diabetes education programs in a wide health care network: a retrospective study. Diabetes Spectr 2014; 27:297–303.
- Funnell MM, Kruger DF, Spencer M. Self-management support for insulin therapy in type 2 diabetes. Diabetes Educ 2004; 30:274–280.
- Norris SL, Engelgau MM, Narayan KM. Effectiveness of self-management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care 2001; 24:561–587.
Insulin therapy is one of the most effective tools clinicians can use to help patients reach their individualized hemoglobin A1c target. However, decisions about when and how to start insulin therapy have to be individualized to the needs and goals of each patient. Many insulin options are available, one of the most common being the addition of basal insulin to oral antidiabetic drugs. Although patients are often reluctant to start insulin, this reluctance can be overcome through patient education and hands-on training.
Here, we review hemoglobin A1c targets, factors that determine when to start insulin therapy, and the different regimens that can be used.
MOST PATIENTS EVENTUALLY NEED INSULIN
Type 2 diabetes mellitus is a chronic progressive disease associated with insulin resistance, beta-cell dysfunction, and decreased insulin secretion. Consequently, most patients eventually require insulin therapy to reduce the risk of long-term complications.
The efficacy of therapy can be assessed by measuring hemoglobin A1c, an important marker of the chronic hyperglycemic state. The hemoglobin A1c value can be reported as a ratio (%) standardized against the results of the Diabetes Control and Complications Trial,1 or as International Federation of Clinical Chemistry units (mmol/mol).2 Table 1 shows the relationship between hemoglobin A1c and average glucose values.3
WHAT IS AN APPROPRIATE HEMOGLOBIN A1c TARGET?
The short answer is, “It depends.”
Currently, the American Association of Clinical Endocrinologists (AACE) supports a hemoglobin A1c goal of less than 6.5% for otherwise healthy patients but states that the goal should be individualized for patients with concurrent illnesses or at risk of hypoglycemia.4
On the other hand, the American Diabetes Association (ADA) recommends a higher hemoglobin A1c target of less than 7% for most adults with type 2 diabetes mellitus.5 This value was shown to be associated with a reduction in the microvascular and macrovascular complications of diabetes.
Yet when three large trials6–8 recently compared intensive and standard glucose control regimens, tighter glucose control failed to improve cardiovascular outcomes. Moreover, in one of the trials,7 patients receiving intensive treatment had a higher rate of all-cause mortality. Details:
- Action in Diabetes and Vascular Disease (ADVANCE): 11,140 patients; average hemoglobin A1c levels 6.5% vs 7.3%6
- Action to Control Cardiovascular Risk in Diabetes (ACCORD): 10,251 patients; average hemoglobin A1c levels 6.4% vs 7.5%7
- Veterans Affairs Diabetes Trial (VADT): 1,791 patients; average hemoglobin A1c levels 6.9% vs 8.4%.8
Similarly, a 2013 Cochrane review9 that included 28 randomized controlled trials concluded that intensive control (in 18,717 patients) did not decrease all-cause and cardiovascular mortality rates compared with traditional glucose control (in 16,195 patients), and it increased the risk of hypoglycemia and serious adverse events.
As a result, the ADA5 states that a hemoglobin A1c target less than 6.5% is optional for patients with a long life expectancy, short duration of diabetes, low risk of hypoglycemia, and no significant cardiovascular disease. The ADA further defines a hemoglobin A1c goal of less than 8% for patients with a history of severe hypoglycemia, limited life expectancy, advanced microvascular or macrovascular complications, extensive comorbid conditions, and long-standing diabetes.
Therefore, the AACE and ADA are moving away from “one-size-fits-all” goals and toward individualizing their recommendations.
WHEN SHOULD INSULIN BE STARTED?
Physicians should consider the needs and preferences of each patient and individualize the treatment. The most recent recommendations from the ADA5 stress the importance of a patient-centered approach, with multiple factors taken into account. These include the patient’s attitude, expected compliance with treatment, risk of hypoglycemia, disease duration, life expectancy, and comorbidities, and the side effects of oral medications and insulin.
Compared with previous guidelines, there are fewer rules on how and when to start insulin therapy. But absolute and relative indications for insulin therapy should be considered in patients with the following:
Absolute indications for insulin
- Ketoacidosis or catabolic symptoms, including ketonuria
- Newly diagnosed type 2 diabetes with pronounced hyperglycemia (glucose ≥ 300 mg/dL or hemoglobin A1c ≥ 10.0%) with or without severe symptoms, including weight loss, polyuria, or polydipsia10
- Uncontrolled type 2 diabetes mellitus despite using one, two, or more oral antidiabetic drugs or glucagon-like peptide 1 (GLP-1) receptor agonists
- Gestational diabetes
- Preference for insulin.
Relative indications for insulin
- Hospitalized for surgery or acute illnesses
- Advanced renal or hepatic disease
- Inability to afford the cost or tolerate the side effects of oral antidiabetic drugs and GLP-1 receptor agonists.
Depending on the situation, blood glucose is measured fasting, before meals, or after meals after initiating or adjusting insulin regimens (Table 2).
WHAT ARE THE INSULIN REGIMENS?
Basal insulin
In the early stages of type 2 diabetes, metformin alone or in combination with another oral antidiabetic drug or with a GLP-1 receptor agonist is often used along with healthy eating, weight control, and increased physical activity.
When the target hemoglobin A1c cannot be achieved with one or two noninsulin drugs, the ADA suggests basal insulin be added to metformin or a two-medication regimen that includes metformin (Table 3). However, recent evidence suggests that combining a GLP-1 receptor agonist with basal insulin, in a regimen without metformin, is safe and improves glycemic control without hypoglycemia or weight gain.11
While a total daily dose of insulin of 0.1 to 0.2 units/kg could be initially used in patients with a hemoglobin A1c level less than 8%, a higher dose of 0.2 to 0.3 units/kg is required if the hemoglobin A1c level is between 8% and 10%. The dose can be titrated once or twice weekly if the fasting glucose is above the target level (usually < 130 mg/dL). If hypoglycemia develops (glucose < 70 mg/dL), the insulin dose should be reduced by 10% to 20%.10
Available basal insulins include glargine, detemir, and neutral protamine Hagedorn (NPH) (Table 4).12–14 Because glargine and detemir offer better pharmacokinetic properties, less variability in response, and less risk of hypoglycemia, they are preferred over NPH. Glargine has a relatively constant plasma concentration over 24 hours, allowing once-daily dosing at any time during the day (Figure 1).15 The dose should be taken at the same time every day. Detemir and NPH are usually taken once or twice daily.
Patients treated once daily should take the dose with the evening meal or at bedtime. Patients who require a twice-daily regimen can take the first dose with breakfast and the second one with the evening meal, at bedtime, or 12 hours after the morning dose.
The randomized Treat-to-Target trial,16 in 756 patients, showed that both glargine and NPH, when added to oral therapy in patients with type 2 diabetes, achieve the target hemoglobin A1c, but NPH is associated with more episodes of nocturnal hypoglycemia. Similar results were found when NPH was compared with detemir insulin.17
A Cochrane review18 suggested that glargine and detemir are similar in efficacy and safety. However, detemir often needs to be injected twice daily, in a higher dose, and is associated with less weight gain. Furthermore, a meta-analysis of 46 randomized clinical trials19 showed that the weight increase at 1 year is less in patients treated with basal than with twice-daily or prandial regimens.
A noninterventional longitudinal study20 in 2,179 patients newly started on insulin showed that the mean weight increase at 1 year was 1.78 kg, and 24% of patients gained more than 5 kg. However, the factors independently associated with the weight gain were a higher hemoglobin A1c at baseline, a higher insulin dose at baseline and at 1 year, and a lower baseline body mass index, but not the type of insulin regimen.
Currently, a new class of ultralong-acting basal insulins is being studied. Insulins in this class are approved in other countries, but the US Food and Drug Administration requires additional data for approval. Ultralong-acting insulins are expected to reduce the risk of hypoglycemia, specifically the risk of nocturnal episodes. Also, given their longer duration of action and stable steady-state pharmacokinetics, they will offer flexibility in the dose timing.21
Basal-bolus regimens
Basal insulin often does not control postprandial hyperglycemia. The need for multiple doses of insulin (including one or more preprandial doses) is suggested by postprandial glucose values above target (usually > 180 mg/dL) or by a hemoglobin A1c above goal despite well-controlled fasting glucose levels. This usually becomes evident when the total daily dose of basal insulin exceeds 0.5 units/kg. Patients newly diagnosed with diabetes who have a hemoglobin A1c higher than 10% may also respond better to an initial basal-bolus regimen.
Available bolus insulins include lispro, aspart, glulisine, regular insulin, and the newly approved Technosphere inhaled regular insulin (Table 4).12–14 They can be taken before each meal, and the total bolus dose usually represents 50% of the total daily dose.22 Rapid-acting insulins have faster onset, shorter duration of action, and more predictable pharmacokinetics, which makes them preferable to regular insulin (Figure 1).15 Inhaled insulin is another option, but it is contraindicated in patients with chronic obstructive pulmonary disease or asthma because of the increased risk of acute bronchospasm.12
Alternatively, the transition to a basal-bolus regimen can be accomplished with a single dose of bolus insulin before the main meal, using a dose that represents approximately 10% of the total daily dose. Additional bolus doses can be added later based on the glycemic control. The adjustment of the preprandial insulin dose is done once or twice weekly, based on the postprandial glucose levels.10
Premixed combinations of long- and short-acting insulins in ratios of 50% to 50%, 70% to 30%, or 75% to 25% can be considered in patients who cannot adhere to a complex insulin regimen. A propensity-matched comparison of different insulin regimens (basal, premixed, mealtime plus basal, and mealtime) in patients with type 2 diabetes revealed that the hemoglobin A1c reduction was similar between the different groups.23 However, the number of hypoglycemic episodes was higher in the premixed insulin group, and the weight gain was less in the basal insulin group.
While premixed insulins require fewer injections, they do not provide dosing flexibility. In other words, dose adjustments for premixed insulins lead to increases in both basal and bolus amounts even though a dose adjustment is needed for only one insulin type. Thus, this is a common reason for increased hypoglycemic episodes.
Continuous subcutaneous insulin infusion
A meta-analysis showed that continuous subcutaneous insulin infusion (ie, use of an insulin pump) was similar to intensive therapy with multiple daily insulin injections in terms of glycemic control and hypoglycemia.24 Since both options can lead to similar glucose control, additional factors to consider when initiating insulin infusion include lifestyle and technical expertise. Some patients may or may not prefer having a pump attached for nearly all daily activities. Additionally, this type of therapy is complex and requires significant training to ensure efficacy and safety.25
WHAT IS THE COST OF INSULIN THERAPY?
A final factor to keep in mind when initiating insulin is cost (Table 4).12–14 Asking patients to check their prescription insurance formulary is important to ensure that an affordable option is selected. If patients do not have prescription insurance, medication assistance programs could be an option. However, if a patient is considering an insulin pump, insurance coverage is essential. Depending on the manufacturer, insulin pumps cost about $6,000 to $7,000, and the additional monthly supplies for the pump are also expensive.
If patients are engaged when considering and selecting insulin therapy, the likelihood of treatment success is greater.26–28
Insulin therapy is one of the most effective tools clinicians can use to help patients reach their individualized hemoglobin A1c target. However, decisions about when and how to start insulin therapy have to be individualized to the needs and goals of each patient. Many insulin options are available, one of the most common being the addition of basal insulin to oral antidiabetic drugs. Although patients are often reluctant to start insulin, this reluctance can be overcome through patient education and hands-on training.
Here, we review hemoglobin A1c targets, factors that determine when to start insulin therapy, and the different regimens that can be used.
MOST PATIENTS EVENTUALLY NEED INSULIN
Type 2 diabetes mellitus is a chronic progressive disease associated with insulin resistance, beta-cell dysfunction, and decreased insulin secretion. Consequently, most patients eventually require insulin therapy to reduce the risk of long-term complications.
The efficacy of therapy can be assessed by measuring hemoglobin A1c, an important marker of the chronic hyperglycemic state. The hemoglobin A1c value can be reported as a ratio (%) standardized against the results of the Diabetes Control and Complications Trial,1 or as International Federation of Clinical Chemistry units (mmol/mol).2 Table 1 shows the relationship between hemoglobin A1c and average glucose values.3
WHAT IS AN APPROPRIATE HEMOGLOBIN A1c TARGET?
The short answer is, “It depends.”
Currently, the American Association of Clinical Endocrinologists (AACE) supports a hemoglobin A1c goal of less than 6.5% for otherwise healthy patients but states that the goal should be individualized for patients with concurrent illnesses or at risk of hypoglycemia.4
On the other hand, the American Diabetes Association (ADA) recommends a higher hemoglobin A1c target of less than 7% for most adults with type 2 diabetes mellitus.5 This value was shown to be associated with a reduction in the microvascular and macrovascular complications of diabetes.
Yet when three large trials6–8 recently compared intensive and standard glucose control regimens, tighter glucose control failed to improve cardiovascular outcomes. Moreover, in one of the trials,7 patients receiving intensive treatment had a higher rate of all-cause mortality. Details:
- Action in Diabetes and Vascular Disease (ADVANCE): 11,140 patients; average hemoglobin A1c levels 6.5% vs 7.3%6
- Action to Control Cardiovascular Risk in Diabetes (ACCORD): 10,251 patients; average hemoglobin A1c levels 6.4% vs 7.5%7
- Veterans Affairs Diabetes Trial (VADT): 1,791 patients; average hemoglobin A1c levels 6.9% vs 8.4%.8
Similarly, a 2013 Cochrane review9 that included 28 randomized controlled trials concluded that intensive control (in 18,717 patients) did not decrease all-cause and cardiovascular mortality rates compared with traditional glucose control (in 16,195 patients), and it increased the risk of hypoglycemia and serious adverse events.
As a result, the ADA5 states that a hemoglobin A1c target less than 6.5% is optional for patients with a long life expectancy, short duration of diabetes, low risk of hypoglycemia, and no significant cardiovascular disease. The ADA further defines a hemoglobin A1c goal of less than 8% for patients with a history of severe hypoglycemia, limited life expectancy, advanced microvascular or macrovascular complications, extensive comorbid conditions, and long-standing diabetes.
Therefore, the AACE and ADA are moving away from “one-size-fits-all” goals and toward individualizing their recommendations.
WHEN SHOULD INSULIN BE STARTED?
Physicians should consider the needs and preferences of each patient and individualize the treatment. The most recent recommendations from the ADA5 stress the importance of a patient-centered approach, with multiple factors taken into account. These include the patient’s attitude, expected compliance with treatment, risk of hypoglycemia, disease duration, life expectancy, and comorbidities, and the side effects of oral medications and insulin.
Compared with previous guidelines, there are fewer rules on how and when to start insulin therapy. But absolute and relative indications for insulin therapy should be considered in patients with the following:
Absolute indications for insulin
- Ketoacidosis or catabolic symptoms, including ketonuria
- Newly diagnosed type 2 diabetes with pronounced hyperglycemia (glucose ≥ 300 mg/dL or hemoglobin A1c ≥ 10.0%) with or without severe symptoms, including weight loss, polyuria, or polydipsia10
- Uncontrolled type 2 diabetes mellitus despite using one, two, or more oral antidiabetic drugs or glucagon-like peptide 1 (GLP-1) receptor agonists
- Gestational diabetes
- Preference for insulin.
Relative indications for insulin
- Hospitalized for surgery or acute illnesses
- Advanced renal or hepatic disease
- Inability to afford the cost or tolerate the side effects of oral antidiabetic drugs and GLP-1 receptor agonists.
Depending on the situation, blood glucose is measured fasting, before meals, or after meals after initiating or adjusting insulin regimens (Table 2).
WHAT ARE THE INSULIN REGIMENS?
Basal insulin
In the early stages of type 2 diabetes, metformin alone or in combination with another oral antidiabetic drug or with a GLP-1 receptor agonist is often used along with healthy eating, weight control, and increased physical activity.
When the target hemoglobin A1c cannot be achieved with one or two noninsulin drugs, the ADA suggests basal insulin be added to metformin or a two-medication regimen that includes metformin (Table 3). However, recent evidence suggests that combining a GLP-1 receptor agonist with basal insulin, in a regimen without metformin, is safe and improves glycemic control without hypoglycemia or weight gain.11
While a total daily dose of insulin of 0.1 to 0.2 units/kg could be initially used in patients with a hemoglobin A1c level less than 8%, a higher dose of 0.2 to 0.3 units/kg is required if the hemoglobin A1c level is between 8% and 10%. The dose can be titrated once or twice weekly if the fasting glucose is above the target level (usually < 130 mg/dL). If hypoglycemia develops (glucose < 70 mg/dL), the insulin dose should be reduced by 10% to 20%.10
Available basal insulins include glargine, detemir, and neutral protamine Hagedorn (NPH) (Table 4).12–14 Because glargine and detemir offer better pharmacokinetic properties, less variability in response, and less risk of hypoglycemia, they are preferred over NPH. Glargine has a relatively constant plasma concentration over 24 hours, allowing once-daily dosing at any time during the day (Figure 1).15 The dose should be taken at the same time every day. Detemir and NPH are usually taken once or twice daily.
Patients treated once daily should take the dose with the evening meal or at bedtime. Patients who require a twice-daily regimen can take the first dose with breakfast and the second one with the evening meal, at bedtime, or 12 hours after the morning dose.
The randomized Treat-to-Target trial,16 in 756 patients, showed that both glargine and NPH, when added to oral therapy in patients with type 2 diabetes, achieve the target hemoglobin A1c, but NPH is associated with more episodes of nocturnal hypoglycemia. Similar results were found when NPH was compared with detemir insulin.17
A Cochrane review18 suggested that glargine and detemir are similar in efficacy and safety. However, detemir often needs to be injected twice daily, in a higher dose, and is associated with less weight gain. Furthermore, a meta-analysis of 46 randomized clinical trials19 showed that the weight increase at 1 year is less in patients treated with basal than with twice-daily or prandial regimens.
A noninterventional longitudinal study20 in 2,179 patients newly started on insulin showed that the mean weight increase at 1 year was 1.78 kg, and 24% of patients gained more than 5 kg. However, the factors independently associated with the weight gain were a higher hemoglobin A1c at baseline, a higher insulin dose at baseline and at 1 year, and a lower baseline body mass index, but not the type of insulin regimen.
Currently, a new class of ultralong-acting basal insulins is being studied. Insulins in this class are approved in other countries, but the US Food and Drug Administration requires additional data for approval. Ultralong-acting insulins are expected to reduce the risk of hypoglycemia, specifically the risk of nocturnal episodes. Also, given their longer duration of action and stable steady-state pharmacokinetics, they will offer flexibility in the dose timing.21
Basal-bolus regimens
Basal insulin often does not control postprandial hyperglycemia. The need for multiple doses of insulin (including one or more preprandial doses) is suggested by postprandial glucose values above target (usually > 180 mg/dL) or by a hemoglobin A1c above goal despite well-controlled fasting glucose levels. This usually becomes evident when the total daily dose of basal insulin exceeds 0.5 units/kg. Patients newly diagnosed with diabetes who have a hemoglobin A1c higher than 10% may also respond better to an initial basal-bolus regimen.
Available bolus insulins include lispro, aspart, glulisine, regular insulin, and the newly approved Technosphere inhaled regular insulin (Table 4).12–14 They can be taken before each meal, and the total bolus dose usually represents 50% of the total daily dose.22 Rapid-acting insulins have faster onset, shorter duration of action, and more predictable pharmacokinetics, which makes them preferable to regular insulin (Figure 1).15 Inhaled insulin is another option, but it is contraindicated in patients with chronic obstructive pulmonary disease or asthma because of the increased risk of acute bronchospasm.12
Alternatively, the transition to a basal-bolus regimen can be accomplished with a single dose of bolus insulin before the main meal, using a dose that represents approximately 10% of the total daily dose. Additional bolus doses can be added later based on the glycemic control. The adjustment of the preprandial insulin dose is done once or twice weekly, based on the postprandial glucose levels.10
Premixed combinations of long- and short-acting insulins in ratios of 50% to 50%, 70% to 30%, or 75% to 25% can be considered in patients who cannot adhere to a complex insulin regimen. A propensity-matched comparison of different insulin regimens (basal, premixed, mealtime plus basal, and mealtime) in patients with type 2 diabetes revealed that the hemoglobin A1c reduction was similar between the different groups.23 However, the number of hypoglycemic episodes was higher in the premixed insulin group, and the weight gain was less in the basal insulin group.
While premixed insulins require fewer injections, they do not provide dosing flexibility. In other words, dose adjustments for premixed insulins lead to increases in both basal and bolus amounts even though a dose adjustment is needed for only one insulin type. Thus, this is a common reason for increased hypoglycemic episodes.
Continuous subcutaneous insulin infusion
A meta-analysis showed that continuous subcutaneous insulin infusion (ie, use of an insulin pump) was similar to intensive therapy with multiple daily insulin injections in terms of glycemic control and hypoglycemia.24 Since both options can lead to similar glucose control, additional factors to consider when initiating insulin infusion include lifestyle and technical expertise. Some patients may or may not prefer having a pump attached for nearly all daily activities. Additionally, this type of therapy is complex and requires significant training to ensure efficacy and safety.25
WHAT IS THE COST OF INSULIN THERAPY?
A final factor to keep in mind when initiating insulin is cost (Table 4).12–14 Asking patients to check their prescription insurance formulary is important to ensure that an affordable option is selected. If patients do not have prescription insurance, medication assistance programs could be an option. However, if a patient is considering an insulin pump, insurance coverage is essential. Depending on the manufacturer, insulin pumps cost about $6,000 to $7,000, and the additional monthly supplies for the pump are also expensive.
If patients are engaged when considering and selecting insulin therapy, the likelihood of treatment success is greater.26–28
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- Hanas R, John WG; International HbA1c Consensus Committee. 2013 Update on the worldwide standardization of the hemoglobin A1c measurement. Pediatr Diabetes 2014; 15:e1–e2.
- Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ; A1c-Derived Average Glucose Study Group. Translating the A1C assay into estimated average glucose values. Diabetes Care 2008; 31:1473–1478.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al; American Association of Clinical Endocrinologists. AACE comprehensive diabetes management algorithm 2013. Endocr Pract 2013; 19:327–336.
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care 2014; 37(suppl 1):S14–S80.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Hemmingsen B, Lund SS, Gluud C, et al. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev 2013; 11:CD008143.
- Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35:1364–1379.
- Vora J, Bain SC, Damci T, et al. Incretin-based therapy in combination with basal insulin: a promising tactic for the treatment of type 2 diabetes. Diabetes Metab 2013; 39:6–15.
- Nuffer W, Trujillo JM, Ellis SL. Technosphere insulin (Afrezza): a new, inhaled prandial insulin. Ann Pharmacother 2015; 49:99–106.
- Pharmacist’s Letter/Prescriber’s Letter. Comparison of insulins and injectable diabetes meds. PL Detail-Document #281107 November 2012. www.PharmacistsLetter.com. Accessed July 2, 2015
- Lexicomp Online. www.wolterskluwercdi.com/lexicomp-online/. Accessed July 2, 2015.
- Hirsch IB. Insulin analogues. N Engl J Med 2005; 352:174-183.
- Riddle MC, Rosenstock J, Gerich J; Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003; 26:3080–3086.
- Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care 2006; 29:1269–1274.
- Swinnen SG, Simon AC, Holleman F, Hoekstra JB, Devries JH. Insulin detemir versus insulin glargine for type 2 diabetes mellitus. Cochrane Database Syst Rev 2011; 7:CD006383.
- Pontiroli AE, Miele L, Morabito A. Increase of body weight during the first year of intensive insulin treatment in type 2 diabetes: systematic review and meta-analysis. Diabetes Obes Metab 2011; 13:1008–1019.
- Balkau B, Home PD, Vincent M, Marre M, Freemantle N. Factors associated with weight gain in people with type 2 diabetes starting on insulin. Diabetes Care 2014; 37:2108–2113.
- Garber AJ. Will the next generation of basal insulins offer clinical advantages? Diabetes Obes Metab 2014; 16:483–491.
- Tamaki M, Shimizu T, Kanazawa A, et al. Effects of changes in basal/total daily insulin ratio in type 2 diabetes patients on intensive insulin therapy including insulin glargine (JUN-LAN Study 6). Diabetes Res Clin Pract 2008; 81:e1–e3.
- Freemantle N, Balkau B, Home PD. A propensity score matched comparison of different insulin regimens 1 year after beginning insulin in people with type 2 diabetes. Diabetes Obes Metab 2013; 15:1120–1127.
- Yeh HC, Brown TT, Maruthur N, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med 2012; 157:336–347.
- Schade DS, Valentine V. To pump or not to pump. Diabetes Care 2002; 25:2100–2102.
- Liu L, Lee MJ, Brateanu A. Improved A1C and lipid profile in patients referred to diabetes education programs in a wide health care network: a retrospective study. Diabetes Spectr 2014; 27:297–303.
- Funnell MM, Kruger DF, Spencer M. Self-management support for insulin therapy in type 2 diabetes. Diabetes Educ 2004; 30:274–280.
- Norris SL, Engelgau MM, Narayan KM. Effectiveness of self-management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care 2001; 24:561–587.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- Hanas R, John WG; International HbA1c Consensus Committee. 2013 Update on the worldwide standardization of the hemoglobin A1c measurement. Pediatr Diabetes 2014; 15:e1–e2.
- Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ; A1c-Derived Average Glucose Study Group. Translating the A1C assay into estimated average glucose values. Diabetes Care 2008; 31:1473–1478.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al; American Association of Clinical Endocrinologists. AACE comprehensive diabetes management algorithm 2013. Endocr Pract 2013; 19:327–336.
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care 2014; 37(suppl 1):S14–S80.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Hemmingsen B, Lund SS, Gluud C, et al. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev 2013; 11:CD008143.
- Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35:1364–1379.
- Vora J, Bain SC, Damci T, et al. Incretin-based therapy in combination with basal insulin: a promising tactic for the treatment of type 2 diabetes. Diabetes Metab 2013; 39:6–15.
- Nuffer W, Trujillo JM, Ellis SL. Technosphere insulin (Afrezza): a new, inhaled prandial insulin. Ann Pharmacother 2015; 49:99–106.
- Pharmacist’s Letter/Prescriber’s Letter. Comparison of insulins and injectable diabetes meds. PL Detail-Document #281107 November 2012. www.PharmacistsLetter.com. Accessed July 2, 2015
- Lexicomp Online. www.wolterskluwercdi.com/lexicomp-online/. Accessed July 2, 2015.
- Hirsch IB. Insulin analogues. N Engl J Med 2005; 352:174-183.
- Riddle MC, Rosenstock J, Gerich J; Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003; 26:3080–3086.
- Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care 2006; 29:1269–1274.
- Swinnen SG, Simon AC, Holleman F, Hoekstra JB, Devries JH. Insulin detemir versus insulin glargine for type 2 diabetes mellitus. Cochrane Database Syst Rev 2011; 7:CD006383.
- Pontiroli AE, Miele L, Morabito A. Increase of body weight during the first year of intensive insulin treatment in type 2 diabetes: systematic review and meta-analysis. Diabetes Obes Metab 2011; 13:1008–1019.
- Balkau B, Home PD, Vincent M, Marre M, Freemantle N. Factors associated with weight gain in people with type 2 diabetes starting on insulin. Diabetes Care 2014; 37:2108–2113.
- Garber AJ. Will the next generation of basal insulins offer clinical advantages? Diabetes Obes Metab 2014; 16:483–491.
- Tamaki M, Shimizu T, Kanazawa A, et al. Effects of changes in basal/total daily insulin ratio in type 2 diabetes patients on intensive insulin therapy including insulin glargine (JUN-LAN Study 6). Diabetes Res Clin Pract 2008; 81:e1–e3.
- Freemantle N, Balkau B, Home PD. A propensity score matched comparison of different insulin regimens 1 year after beginning insulin in people with type 2 diabetes. Diabetes Obes Metab 2013; 15:1120–1127.
- Yeh HC, Brown TT, Maruthur N, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med 2012; 157:336–347.
- Schade DS, Valentine V. To pump or not to pump. Diabetes Care 2002; 25:2100–2102.
- Liu L, Lee MJ, Brateanu A. Improved A1C and lipid profile in patients referred to diabetes education programs in a wide health care network: a retrospective study. Diabetes Spectr 2014; 27:297–303.
- Funnell MM, Kruger DF, Spencer M. Self-management support for insulin therapy in type 2 diabetes. Diabetes Educ 2004; 30:274–280.
- Norris SL, Engelgau MM, Narayan KM. Effectiveness of self-management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care 2001; 24:561–587.
KEY POINTS
- In deciding a patient’s hemoglobin A1c goal and whether it is time to start insulin therapy, one should take into account the patient’s age, life expectancy, concurrent illnesses, risk of hypoglycemia, and other factors.
- When the target hemoglobin A1c is not achieved with metformin or a two-drug regimen that includes metformin, the American Diabetes Association recommends adding a daily dose of basal insulin.
- Eventually, preprandial bolus doses may need to be added to the insulin regimen to control postprandial blood glucose levels and hemoglobin A1c.
Six screening tests for adults: What’s recommended? What’s controversial?
A 68-year-old man with a history of hyperlipidemia is evaluated during a routine examination. He has a 25-pack-year cigarette smoking history but quit 12 years ago. He has no history of hypertension, diabetes mellitus, or stroke. A review of systems is unremarkable, and he has no family history of heart disease or cancer. He has noted no change in his bowel movements, and his most recent screening colonoscopy, done at age 60, was normal. His only current medication is lovastatin.
Physical examination reveals no abnormalities. His blood pressure is 130/82 mm Hg, and his body mass index is 24 kg/m2. His total cholesterol level is 213 mg/dL, and his high-density lipoprotein level is 48 mg/dL.
Which screening tests, if any, would be appropriate for this patient?
The advent in recent years of several new screening tests, along with changing and conflicting screening recommendations, has made it a challenge to manage this aspect of patient care. This article reviews six common screening tests and presents the current recommendations for their use (Table 1).
SCREENING CAN HARM
Screening is used to detect a disease in people who have no signs or symptoms of that disease; if signs or symptoms are present, diagnostic testing is indicated instead. Ideally, screening allows for early treatment to reduce the risk of illness and death associated with a disease.
Problems with screening relate to lead-time bias (detection of disease earlier in its course without actually affecting survival time), length-time bias (detection of indolent and benign cancers rather than aggressive ones), and overdiagnosis (detection of abnormalities that would not cause a problem in the patient’s lifetime, causing unnecessary concern, cost, or treatment).
The leading advisory groups on screening are the US Preventive Services Task Force (USPSTF),1 which is stringently evidence-based in its recommendations, and subspecialty societies, which often rely on expert opinion.2,3
ULTRASONOGRAPHY FOR ABDOMINAL AORTIC ANEURYSM
In 2005, the USPSTF gave a grade-B recommendation (recommended; benefit outweighs harm) for one-time ultrasonographic screening for abdominal aortic aneurysm in men ages 65 to 75 who have ever smoked at least 100 cigarettes over a lifetime. For men in the same age range who have never smoked, they gave a grade-C recommendation (no recommendation; small net benefit). The USPSTF updated its recommendation in 2014. For women ages 65 to 75 who smoke, the USPSTF thinks the evidence is insufficient to recommend for or against screening (grade-I recommendation).
Our patient described above—male, age 68, and with a 25 pack-year smoking history—is a candidate for screening for abdominal aortic aneurysm.
CT SCREENING FOR LUNG CANCER
In December 2013, the USPSTF gave a B-grade recommendation for annual screening for lung cancer with low-dose computed tomography (CT) for adults ages 55 to 80 who have a 30-pack-year smoking history and currently smoke or have quit within the past 15 years. Screening should be discontinued once a person has not smoked for 15 years or develops a health problem that limits life expectancy or the ability to undergo curative lung surgery.
These recommendations were based on the outcomes of the National Lung Screening Trial.4 However, whereas this trial was in people ages 55 to 74, the USPSTF boosted the upper age limit to 80 based on computer modeling, a decision that was somewhat controversial.
Patz et al5 analyzed data from the National Lung Screening Trial and found that about 18% of lung cancers detected by low-dose CT appeared to be indolent and were unlikely to become clinically apparent during the patient’s lifetime. The authors concluded that overdiagnosis should be considered when guidelines for mass screening programs are developed.
Our 68-year-old patient would not qualify for CT screening for lung cancer, since his smoking history is less than 30 pack-years.
COLORECTAL CANCER SCREENING AND PREVENTION
Unlike other cancer screening tests, colorectal cancer screening can also be a preventive measure; removing polyps found during screening with colonoscopy or sigmoidoscopy is an effective strategy in preventing colon cancer.
The USPSTF last updated its colorectal screening recommendations in 2008, giving a grade-A recommendation (strongly recommended; benefit far outweighs harm) to screening using fecal occult blood testing, sigmoidoscopy, or colonoscopy for adults ages 50 to 75. The risks and benefits of these screening methods vary. For adults ages 76 to 85, the task force recommends against routine screening but gives a grade-C recommendation for screening in that age group in some circumstances. They give a grade-D recommendation for screening after age 85.
The USPSTF concluded that the evidence is insufficient to assess the benefits and harms of CT colonography and fecal DNA testing for colorectal cancer screening.
The American Cancer Society issued similar guidelines in 2013, recommending that starting at age 50, men and women at low risk of colorectal cancer should be screened using one of the following schedules (the first four methods help detect both polyps and cancers, and the others detect only cancer)6:
- Colonoscopy every 10 years
- Flexible sigmoidoscopy every 5 years
- A double-contrast barium enema every 5 years
- CT colonography (“virtual colonoscopy”) every 5 years
- A guaiac-based fecal occult blood test annually
- A fecal immunochemical test annually.
Those at moderate or high risk of colorectal cancer are advised to talk with a doctor about a different testing schedule. (eg, colonoscopy every 5 years in patients with a significant family history of colon cancer).
Our patient last underwent colonoscopy 8 years ago and so does not need to be screened again for another 2 years.
CERVICAL CANCER SCREENING: MOVING TOWARD HPV TESTING FIRST?
Cervical cancer screening recommendations are fairly uniform across the major guideline-setting organizations.7 In general, they are:
- Ages 21–29: Check cytology every 3 years
- Ages 30–65: Cytology plus human papillomavirus (HPV) testing every 5 years (or cytology alone every 3 years)
- After age 65: Stop screening if prior screenings have been adequate and negative over the past 20 years.
Women who have been vaccinated against HPV have the same screening recommendations as above. Women who have had a hysterectomy for benign reasons do not need further screening.
The future of cervical cancer screening may be “reflex testing.” Rather than checking cervical samples for cytologic study (Papanicolaou smear) and HPV status together, we may one day screen samples first for HPV and, if that is positive, follow up with cytologic study. Easy-to-use home tests for HPV will likely be developed and should increase screening rates.
PROSTATE CANCER SCREENING: A SHARED DECISION
Prostate cancer screening remains controversial. Different guideline-setting bodies have different recommendations, creating confusion for patients. Physicians must follow what fits their own practice and beliefs.
The USPSTF in 2012 gave a grade-D recommendation to prostate-specific antigen (PSA) testing to screen for prostate cancer, stating that it did more harm than good. However, some men continue to be screened for PSA.
The American Cancer Society in 2013 recommended against routine testing for prostate cancer without a full discussion between physician and patient of the pros and cons of testing.8 If screening is decided upon, it should be done with annual PSA measurement or digital rectal examination, or both, starting at age 50. Men at high risk (ie, African American men, and men with a first-degree relative diagnosed with prostate cancer before age 65) should begin screening at age 45.
The American College of Physicians in 2013 issued a statement that clinicians should inform men between the ages of 50 and 69 about the limited potential benefits and substantial harms of prostate cancer screening.9 They recommended against PSA screening in men of average risk who are younger than age 50 or older than age 69, or those whose life expectancy is less than 10 to 15 years.
The American Urological Association in 2013 advised that10:
- PSA screening is not recommended in men younger than 40.
- Routine screening is not recommended in men between ages 40 and 54 at average risk.
- In men ages 55 to 69, decisions about PSA screening should be shared and based on each patient’s values and preferences. The decision to undergo PSA screening involves weighing the benefits of preventing death from prostate cancer in 1 man for every 1,000 men screened over a decade against the known potential harms associated with screening and treatment.
- To reduce the harm of screening, a routine interval of 2 years may be chosen over annual screening; such a schedule may preserve most benefits and reduce overdiagnosis and false-positive results.
- Routine PSA screening is not recommended in men ages 70 and older or with less than a 10- to 15-year life expectancy.
Shared decision-making. Many of the guidelines for prostate cancer screening are based on the concept of shared decision-making. However, studies indicate that many patients do not receive a full discussion of the issue,11 and in any event, patient education may make little difference in PSA testing rates.12,13
On the horizon for prostate cancer screening is the hope of finding a more predictable test. There is also discussion of using the PSA test earlier: some evidence shows that a very low result at age 45 predicts a less than 1% chance of developing metastatic prostate cancer by age 75, so it is possible that screening could stop in that population.
BREAST CANCER SCREENING: DIVERGENT RECOMMENDATIONS
The USPSTF created considerable controversy a few years ago when it recommended screening mammography from ages 50 to 74, and then only every 2 years—a departure from the traditional practice of starting screening at age 40. Few doctors heed the USPSTF guideline: most of the other guideline-setting organizations (eg, the American Cancer Society, the American Congress of Obstetricians and Gynecologists) recommend annual mammography for women starting at age 40.
Overdiagnosis is an especially pertinent issue with screening mammography for breast cancer because some cancers are indolent and will not cause a problem during a lifetime. Falk et al14 analyzed a Norwegian breast cancer screening program and found that overdiagnosis occurred in 10% to 20% of cases. Welch and Passow15 quantified the benefits and harms of screening mammography in 50-year-old women in the United States and found that of 1,000 women screened annually for a decade, 0.3 to 3.2 will avoid a breast cancer death, 490 to 670 will have at least one false alarm, and 3 to 14 will be overdiagnosed and treated needlessly.
Mammography screening for breast cancer will likely stay controversial for some time as we await additional data.
OTHER CANCERS: SCREENING NOT RECOMMENDED
The USPSTF currently does not recommend screening for ovarian cancer (guideline issued in 2012), pancreatic cancer (2004), or testicular cancer (2011), giving each a grade-D recommendation, indicating that screening does more harm than good. It also stated that there is insufficient evidence to recommend screening for oral cancer (2013), skin cancer (2009), and bladder cancer (2011).
- US Preventive Services Task Force. www.uspreventiveservicestask-force.org. Accessed August 11, 2014.
- Tricoci P, Allen JM, Kramer JM, Califf RM, Smith SC Jr. Scientific evidence underlying the ACC/AHA clinical practice guidelines. JAMA 2009; 301:831–841. Erratum in: JAMA 2009; 301:1544.
- Lee DH, Vielemeyer O. Analysis of overall level of evidence behind Infectious Diseases Society of America practice guidelines. Arch Intern Med 2011; 171:18–22.
- National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365:395–409.
- Patz EF, Pinsky P, Gatsonis C, et al; NLST Overdiagnosis Manuscript Writing Team. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med 2014; 174:269–274.
- American Cancer Society. Colorectal cancer screening and surveillance guidelines. www.cancer.org/healthy/informationforhealth-careprofessionals/colonmdclinicansinformationsource/colorec-talcancerscreeningandsurveillanceguidelines/index. Accessed August 11, 2014.
- Jin XW, Lipold L, McKenzie M, Sikon A. Cervical cancer screening: what’s new and what’s coming? Cleve Clin J Med 2013; 80:153–160.
- American Cancer Society. Prostate cancer screening guidelines. www.cancer.org/healthy/informationforhealthcareprofessionals/pros-tatemdcliniciansinformationsource/prostatecancerscreeningguide-lines/index. Accessed August 11, 2014.
- Qaseem A, Barry MJ, Denberg TD, Owens DK, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Screening for prostate cancer: a guidance statement from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med 2013; 158:761–769.
- Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA guideline. www.auanet.org/common/pdf/education/clinical-guidance/Prostate-Cancer-Detection.pdf. Accessed September 5, 2014.
- Han PK, Kobrin S, Breen N, et al. National evidence on the use of shared decision making in prostate-specific antigen screening. Ann Fam Med 2013; 11:306–314.
- Taylor KL, Williams RM, Davis K, et al. Decision making in prostate cancer screening using decision aids vs usual care: a randomized clinical trial. JAMA Intern Med 2013; 173:1704–1712.
- Landrey AR, Matlock DD, Andrews L, Bronsert M, Denberg T. Shared decision making in prostate-specific antigen testing: the effect of a mailed patient flyer prior to an annual exam. J Prim Care Community Health 2013; 4:67–74.
- Falk RS, Hofvind S, Skaane P, Haldorsen T. Overdiagnosis among women attending a population-based mammography screening program. Int J Cancer 2013; 133:705–712.
- Welch HG, Passow HJ. Quantifying the benefits and harms of screening mammography. JAMA Intern Med 2014; 174:448–454.
A 68-year-old man with a history of hyperlipidemia is evaluated during a routine examination. He has a 25-pack-year cigarette smoking history but quit 12 years ago. He has no history of hypertension, diabetes mellitus, or stroke. A review of systems is unremarkable, and he has no family history of heart disease or cancer. He has noted no change in his bowel movements, and his most recent screening colonoscopy, done at age 60, was normal. His only current medication is lovastatin.
Physical examination reveals no abnormalities. His blood pressure is 130/82 mm Hg, and his body mass index is 24 kg/m2. His total cholesterol level is 213 mg/dL, and his high-density lipoprotein level is 48 mg/dL.
Which screening tests, if any, would be appropriate for this patient?
The advent in recent years of several new screening tests, along with changing and conflicting screening recommendations, has made it a challenge to manage this aspect of patient care. This article reviews six common screening tests and presents the current recommendations for their use (Table 1).
SCREENING CAN HARM
Screening is used to detect a disease in people who have no signs or symptoms of that disease; if signs or symptoms are present, diagnostic testing is indicated instead. Ideally, screening allows for early treatment to reduce the risk of illness and death associated with a disease.
Problems with screening relate to lead-time bias (detection of disease earlier in its course without actually affecting survival time), length-time bias (detection of indolent and benign cancers rather than aggressive ones), and overdiagnosis (detection of abnormalities that would not cause a problem in the patient’s lifetime, causing unnecessary concern, cost, or treatment).
The leading advisory groups on screening are the US Preventive Services Task Force (USPSTF),1 which is stringently evidence-based in its recommendations, and subspecialty societies, which often rely on expert opinion.2,3
ULTRASONOGRAPHY FOR ABDOMINAL AORTIC ANEURYSM
In 2005, the USPSTF gave a grade-B recommendation (recommended; benefit outweighs harm) for one-time ultrasonographic screening for abdominal aortic aneurysm in men ages 65 to 75 who have ever smoked at least 100 cigarettes over a lifetime. For men in the same age range who have never smoked, they gave a grade-C recommendation (no recommendation; small net benefit). The USPSTF updated its recommendation in 2014. For women ages 65 to 75 who smoke, the USPSTF thinks the evidence is insufficient to recommend for or against screening (grade-I recommendation).
Our patient described above—male, age 68, and with a 25 pack-year smoking history—is a candidate for screening for abdominal aortic aneurysm.
CT SCREENING FOR LUNG CANCER
In December 2013, the USPSTF gave a B-grade recommendation for annual screening for lung cancer with low-dose computed tomography (CT) for adults ages 55 to 80 who have a 30-pack-year smoking history and currently smoke or have quit within the past 15 years. Screening should be discontinued once a person has not smoked for 15 years or develops a health problem that limits life expectancy or the ability to undergo curative lung surgery.
These recommendations were based on the outcomes of the National Lung Screening Trial.4 However, whereas this trial was in people ages 55 to 74, the USPSTF boosted the upper age limit to 80 based on computer modeling, a decision that was somewhat controversial.
Patz et al5 analyzed data from the National Lung Screening Trial and found that about 18% of lung cancers detected by low-dose CT appeared to be indolent and were unlikely to become clinically apparent during the patient’s lifetime. The authors concluded that overdiagnosis should be considered when guidelines for mass screening programs are developed.
Our 68-year-old patient would not qualify for CT screening for lung cancer, since his smoking history is less than 30 pack-years.
COLORECTAL CANCER SCREENING AND PREVENTION
Unlike other cancer screening tests, colorectal cancer screening can also be a preventive measure; removing polyps found during screening with colonoscopy or sigmoidoscopy is an effective strategy in preventing colon cancer.
The USPSTF last updated its colorectal screening recommendations in 2008, giving a grade-A recommendation (strongly recommended; benefit far outweighs harm) to screening using fecal occult blood testing, sigmoidoscopy, or colonoscopy for adults ages 50 to 75. The risks and benefits of these screening methods vary. For adults ages 76 to 85, the task force recommends against routine screening but gives a grade-C recommendation for screening in that age group in some circumstances. They give a grade-D recommendation for screening after age 85.
The USPSTF concluded that the evidence is insufficient to assess the benefits and harms of CT colonography and fecal DNA testing for colorectal cancer screening.
The American Cancer Society issued similar guidelines in 2013, recommending that starting at age 50, men and women at low risk of colorectal cancer should be screened using one of the following schedules (the first four methods help detect both polyps and cancers, and the others detect only cancer)6:
- Colonoscopy every 10 years
- Flexible sigmoidoscopy every 5 years
- A double-contrast barium enema every 5 years
- CT colonography (“virtual colonoscopy”) every 5 years
- A guaiac-based fecal occult blood test annually
- A fecal immunochemical test annually.
Those at moderate or high risk of colorectal cancer are advised to talk with a doctor about a different testing schedule. (eg, colonoscopy every 5 years in patients with a significant family history of colon cancer).
Our patient last underwent colonoscopy 8 years ago and so does not need to be screened again for another 2 years.
CERVICAL CANCER SCREENING: MOVING TOWARD HPV TESTING FIRST?
Cervical cancer screening recommendations are fairly uniform across the major guideline-setting organizations.7 In general, they are:
- Ages 21–29: Check cytology every 3 years
- Ages 30–65: Cytology plus human papillomavirus (HPV) testing every 5 years (or cytology alone every 3 years)
- After age 65: Stop screening if prior screenings have been adequate and negative over the past 20 years.
Women who have been vaccinated against HPV have the same screening recommendations as above. Women who have had a hysterectomy for benign reasons do not need further screening.
The future of cervical cancer screening may be “reflex testing.” Rather than checking cervical samples for cytologic study (Papanicolaou smear) and HPV status together, we may one day screen samples first for HPV and, if that is positive, follow up with cytologic study. Easy-to-use home tests for HPV will likely be developed and should increase screening rates.
PROSTATE CANCER SCREENING: A SHARED DECISION
Prostate cancer screening remains controversial. Different guideline-setting bodies have different recommendations, creating confusion for patients. Physicians must follow what fits their own practice and beliefs.
The USPSTF in 2012 gave a grade-D recommendation to prostate-specific antigen (PSA) testing to screen for prostate cancer, stating that it did more harm than good. However, some men continue to be screened for PSA.
The American Cancer Society in 2013 recommended against routine testing for prostate cancer without a full discussion between physician and patient of the pros and cons of testing.8 If screening is decided upon, it should be done with annual PSA measurement or digital rectal examination, or both, starting at age 50. Men at high risk (ie, African American men, and men with a first-degree relative diagnosed with prostate cancer before age 65) should begin screening at age 45.
The American College of Physicians in 2013 issued a statement that clinicians should inform men between the ages of 50 and 69 about the limited potential benefits and substantial harms of prostate cancer screening.9 They recommended against PSA screening in men of average risk who are younger than age 50 or older than age 69, or those whose life expectancy is less than 10 to 15 years.
The American Urological Association in 2013 advised that10:
- PSA screening is not recommended in men younger than 40.
- Routine screening is not recommended in men between ages 40 and 54 at average risk.
- In men ages 55 to 69, decisions about PSA screening should be shared and based on each patient’s values and preferences. The decision to undergo PSA screening involves weighing the benefits of preventing death from prostate cancer in 1 man for every 1,000 men screened over a decade against the known potential harms associated with screening and treatment.
- To reduce the harm of screening, a routine interval of 2 years may be chosen over annual screening; such a schedule may preserve most benefits and reduce overdiagnosis and false-positive results.
- Routine PSA screening is not recommended in men ages 70 and older or with less than a 10- to 15-year life expectancy.
Shared decision-making. Many of the guidelines for prostate cancer screening are based on the concept of shared decision-making. However, studies indicate that many patients do not receive a full discussion of the issue,11 and in any event, patient education may make little difference in PSA testing rates.12,13
On the horizon for prostate cancer screening is the hope of finding a more predictable test. There is also discussion of using the PSA test earlier: some evidence shows that a very low result at age 45 predicts a less than 1% chance of developing metastatic prostate cancer by age 75, so it is possible that screening could stop in that population.
BREAST CANCER SCREENING: DIVERGENT RECOMMENDATIONS
The USPSTF created considerable controversy a few years ago when it recommended screening mammography from ages 50 to 74, and then only every 2 years—a departure from the traditional practice of starting screening at age 40. Few doctors heed the USPSTF guideline: most of the other guideline-setting organizations (eg, the American Cancer Society, the American Congress of Obstetricians and Gynecologists) recommend annual mammography for women starting at age 40.
Overdiagnosis is an especially pertinent issue with screening mammography for breast cancer because some cancers are indolent and will not cause a problem during a lifetime. Falk et al14 analyzed a Norwegian breast cancer screening program and found that overdiagnosis occurred in 10% to 20% of cases. Welch and Passow15 quantified the benefits and harms of screening mammography in 50-year-old women in the United States and found that of 1,000 women screened annually for a decade, 0.3 to 3.2 will avoid a breast cancer death, 490 to 670 will have at least one false alarm, and 3 to 14 will be overdiagnosed and treated needlessly.
Mammography screening for breast cancer will likely stay controversial for some time as we await additional data.
OTHER CANCERS: SCREENING NOT RECOMMENDED
The USPSTF currently does not recommend screening for ovarian cancer (guideline issued in 2012), pancreatic cancer (2004), or testicular cancer (2011), giving each a grade-D recommendation, indicating that screening does more harm than good. It also stated that there is insufficient evidence to recommend screening for oral cancer (2013), skin cancer (2009), and bladder cancer (2011).
A 68-year-old man with a history of hyperlipidemia is evaluated during a routine examination. He has a 25-pack-year cigarette smoking history but quit 12 years ago. He has no history of hypertension, diabetes mellitus, or stroke. A review of systems is unremarkable, and he has no family history of heart disease or cancer. He has noted no change in his bowel movements, and his most recent screening colonoscopy, done at age 60, was normal. His only current medication is lovastatin.
Physical examination reveals no abnormalities. His blood pressure is 130/82 mm Hg, and his body mass index is 24 kg/m2. His total cholesterol level is 213 mg/dL, and his high-density lipoprotein level is 48 mg/dL.
Which screening tests, if any, would be appropriate for this patient?
The advent in recent years of several new screening tests, along with changing and conflicting screening recommendations, has made it a challenge to manage this aspect of patient care. This article reviews six common screening tests and presents the current recommendations for their use (Table 1).
SCREENING CAN HARM
Screening is used to detect a disease in people who have no signs or symptoms of that disease; if signs or symptoms are present, diagnostic testing is indicated instead. Ideally, screening allows for early treatment to reduce the risk of illness and death associated with a disease.
Problems with screening relate to lead-time bias (detection of disease earlier in its course without actually affecting survival time), length-time bias (detection of indolent and benign cancers rather than aggressive ones), and overdiagnosis (detection of abnormalities that would not cause a problem in the patient’s lifetime, causing unnecessary concern, cost, or treatment).
The leading advisory groups on screening are the US Preventive Services Task Force (USPSTF),1 which is stringently evidence-based in its recommendations, and subspecialty societies, which often rely on expert opinion.2,3
ULTRASONOGRAPHY FOR ABDOMINAL AORTIC ANEURYSM
In 2005, the USPSTF gave a grade-B recommendation (recommended; benefit outweighs harm) for one-time ultrasonographic screening for abdominal aortic aneurysm in men ages 65 to 75 who have ever smoked at least 100 cigarettes over a lifetime. For men in the same age range who have never smoked, they gave a grade-C recommendation (no recommendation; small net benefit). The USPSTF updated its recommendation in 2014. For women ages 65 to 75 who smoke, the USPSTF thinks the evidence is insufficient to recommend for or against screening (grade-I recommendation).
Our patient described above—male, age 68, and with a 25 pack-year smoking history—is a candidate for screening for abdominal aortic aneurysm.
CT SCREENING FOR LUNG CANCER
In December 2013, the USPSTF gave a B-grade recommendation for annual screening for lung cancer with low-dose computed tomography (CT) for adults ages 55 to 80 who have a 30-pack-year smoking history and currently smoke or have quit within the past 15 years. Screening should be discontinued once a person has not smoked for 15 years or develops a health problem that limits life expectancy or the ability to undergo curative lung surgery.
These recommendations were based on the outcomes of the National Lung Screening Trial.4 However, whereas this trial was in people ages 55 to 74, the USPSTF boosted the upper age limit to 80 based on computer modeling, a decision that was somewhat controversial.
Patz et al5 analyzed data from the National Lung Screening Trial and found that about 18% of lung cancers detected by low-dose CT appeared to be indolent and were unlikely to become clinically apparent during the patient’s lifetime. The authors concluded that overdiagnosis should be considered when guidelines for mass screening programs are developed.
Our 68-year-old patient would not qualify for CT screening for lung cancer, since his smoking history is less than 30 pack-years.
COLORECTAL CANCER SCREENING AND PREVENTION
Unlike other cancer screening tests, colorectal cancer screening can also be a preventive measure; removing polyps found during screening with colonoscopy or sigmoidoscopy is an effective strategy in preventing colon cancer.
The USPSTF last updated its colorectal screening recommendations in 2008, giving a grade-A recommendation (strongly recommended; benefit far outweighs harm) to screening using fecal occult blood testing, sigmoidoscopy, or colonoscopy for adults ages 50 to 75. The risks and benefits of these screening methods vary. For adults ages 76 to 85, the task force recommends against routine screening but gives a grade-C recommendation for screening in that age group in some circumstances. They give a grade-D recommendation for screening after age 85.
The USPSTF concluded that the evidence is insufficient to assess the benefits and harms of CT colonography and fecal DNA testing for colorectal cancer screening.
The American Cancer Society issued similar guidelines in 2013, recommending that starting at age 50, men and women at low risk of colorectal cancer should be screened using one of the following schedules (the first four methods help detect both polyps and cancers, and the others detect only cancer)6:
- Colonoscopy every 10 years
- Flexible sigmoidoscopy every 5 years
- A double-contrast barium enema every 5 years
- CT colonography (“virtual colonoscopy”) every 5 years
- A guaiac-based fecal occult blood test annually
- A fecal immunochemical test annually.
Those at moderate or high risk of colorectal cancer are advised to talk with a doctor about a different testing schedule. (eg, colonoscopy every 5 years in patients with a significant family history of colon cancer).
Our patient last underwent colonoscopy 8 years ago and so does not need to be screened again for another 2 years.
CERVICAL CANCER SCREENING: MOVING TOWARD HPV TESTING FIRST?
Cervical cancer screening recommendations are fairly uniform across the major guideline-setting organizations.7 In general, they are:
- Ages 21–29: Check cytology every 3 years
- Ages 30–65: Cytology plus human papillomavirus (HPV) testing every 5 years (or cytology alone every 3 years)
- After age 65: Stop screening if prior screenings have been adequate and negative over the past 20 years.
Women who have been vaccinated against HPV have the same screening recommendations as above. Women who have had a hysterectomy for benign reasons do not need further screening.
The future of cervical cancer screening may be “reflex testing.” Rather than checking cervical samples for cytologic study (Papanicolaou smear) and HPV status together, we may one day screen samples first for HPV and, if that is positive, follow up with cytologic study. Easy-to-use home tests for HPV will likely be developed and should increase screening rates.
PROSTATE CANCER SCREENING: A SHARED DECISION
Prostate cancer screening remains controversial. Different guideline-setting bodies have different recommendations, creating confusion for patients. Physicians must follow what fits their own practice and beliefs.
The USPSTF in 2012 gave a grade-D recommendation to prostate-specific antigen (PSA) testing to screen for prostate cancer, stating that it did more harm than good. However, some men continue to be screened for PSA.
The American Cancer Society in 2013 recommended against routine testing for prostate cancer without a full discussion between physician and patient of the pros and cons of testing.8 If screening is decided upon, it should be done with annual PSA measurement or digital rectal examination, or both, starting at age 50. Men at high risk (ie, African American men, and men with a first-degree relative diagnosed with prostate cancer before age 65) should begin screening at age 45.
The American College of Physicians in 2013 issued a statement that clinicians should inform men between the ages of 50 and 69 about the limited potential benefits and substantial harms of prostate cancer screening.9 They recommended against PSA screening in men of average risk who are younger than age 50 or older than age 69, or those whose life expectancy is less than 10 to 15 years.
The American Urological Association in 2013 advised that10:
- PSA screening is not recommended in men younger than 40.
- Routine screening is not recommended in men between ages 40 and 54 at average risk.
- In men ages 55 to 69, decisions about PSA screening should be shared and based on each patient’s values and preferences. The decision to undergo PSA screening involves weighing the benefits of preventing death from prostate cancer in 1 man for every 1,000 men screened over a decade against the known potential harms associated with screening and treatment.
- To reduce the harm of screening, a routine interval of 2 years may be chosen over annual screening; such a schedule may preserve most benefits and reduce overdiagnosis and false-positive results.
- Routine PSA screening is not recommended in men ages 70 and older or with less than a 10- to 15-year life expectancy.
Shared decision-making. Many of the guidelines for prostate cancer screening are based on the concept of shared decision-making. However, studies indicate that many patients do not receive a full discussion of the issue,11 and in any event, patient education may make little difference in PSA testing rates.12,13
On the horizon for prostate cancer screening is the hope of finding a more predictable test. There is also discussion of using the PSA test earlier: some evidence shows that a very low result at age 45 predicts a less than 1% chance of developing metastatic prostate cancer by age 75, so it is possible that screening could stop in that population.
BREAST CANCER SCREENING: DIVERGENT RECOMMENDATIONS
The USPSTF created considerable controversy a few years ago when it recommended screening mammography from ages 50 to 74, and then only every 2 years—a departure from the traditional practice of starting screening at age 40. Few doctors heed the USPSTF guideline: most of the other guideline-setting organizations (eg, the American Cancer Society, the American Congress of Obstetricians and Gynecologists) recommend annual mammography for women starting at age 40.
Overdiagnosis is an especially pertinent issue with screening mammography for breast cancer because some cancers are indolent and will not cause a problem during a lifetime. Falk et al14 analyzed a Norwegian breast cancer screening program and found that overdiagnosis occurred in 10% to 20% of cases. Welch and Passow15 quantified the benefits and harms of screening mammography in 50-year-old women in the United States and found that of 1,000 women screened annually for a decade, 0.3 to 3.2 will avoid a breast cancer death, 490 to 670 will have at least one false alarm, and 3 to 14 will be overdiagnosed and treated needlessly.
Mammography screening for breast cancer will likely stay controversial for some time as we await additional data.
OTHER CANCERS: SCREENING NOT RECOMMENDED
The USPSTF currently does not recommend screening for ovarian cancer (guideline issued in 2012), pancreatic cancer (2004), or testicular cancer (2011), giving each a grade-D recommendation, indicating that screening does more harm than good. It also stated that there is insufficient evidence to recommend screening for oral cancer (2013), skin cancer (2009), and bladder cancer (2011).
- US Preventive Services Task Force. www.uspreventiveservicestask-force.org. Accessed August 11, 2014.
- Tricoci P, Allen JM, Kramer JM, Califf RM, Smith SC Jr. Scientific evidence underlying the ACC/AHA clinical practice guidelines. JAMA 2009; 301:831–841. Erratum in: JAMA 2009; 301:1544.
- Lee DH, Vielemeyer O. Analysis of overall level of evidence behind Infectious Diseases Society of America practice guidelines. Arch Intern Med 2011; 171:18–22.
- National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365:395–409.
- Patz EF, Pinsky P, Gatsonis C, et al; NLST Overdiagnosis Manuscript Writing Team. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med 2014; 174:269–274.
- American Cancer Society. Colorectal cancer screening and surveillance guidelines. www.cancer.org/healthy/informationforhealth-careprofessionals/colonmdclinicansinformationsource/colorec-talcancerscreeningandsurveillanceguidelines/index. Accessed August 11, 2014.
- Jin XW, Lipold L, McKenzie M, Sikon A. Cervical cancer screening: what’s new and what’s coming? Cleve Clin J Med 2013; 80:153–160.
- American Cancer Society. Prostate cancer screening guidelines. www.cancer.org/healthy/informationforhealthcareprofessionals/pros-tatemdcliniciansinformationsource/prostatecancerscreeningguide-lines/index. Accessed August 11, 2014.
- Qaseem A, Barry MJ, Denberg TD, Owens DK, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Screening for prostate cancer: a guidance statement from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med 2013; 158:761–769.
- Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA guideline. www.auanet.org/common/pdf/education/clinical-guidance/Prostate-Cancer-Detection.pdf. Accessed September 5, 2014.
- Han PK, Kobrin S, Breen N, et al. National evidence on the use of shared decision making in prostate-specific antigen screening. Ann Fam Med 2013; 11:306–314.
- Taylor KL, Williams RM, Davis K, et al. Decision making in prostate cancer screening using decision aids vs usual care: a randomized clinical trial. JAMA Intern Med 2013; 173:1704–1712.
- Landrey AR, Matlock DD, Andrews L, Bronsert M, Denberg T. Shared decision making in prostate-specific antigen testing: the effect of a mailed patient flyer prior to an annual exam. J Prim Care Community Health 2013; 4:67–74.
- Falk RS, Hofvind S, Skaane P, Haldorsen T. Overdiagnosis among women attending a population-based mammography screening program. Int J Cancer 2013; 133:705–712.
- Welch HG, Passow HJ. Quantifying the benefits and harms of screening mammography. JAMA Intern Med 2014; 174:448–454.
- US Preventive Services Task Force. www.uspreventiveservicestask-force.org. Accessed August 11, 2014.
- Tricoci P, Allen JM, Kramer JM, Califf RM, Smith SC Jr. Scientific evidence underlying the ACC/AHA clinical practice guidelines. JAMA 2009; 301:831–841. Erratum in: JAMA 2009; 301:1544.
- Lee DH, Vielemeyer O. Analysis of overall level of evidence behind Infectious Diseases Society of America practice guidelines. Arch Intern Med 2011; 171:18–22.
- National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365:395–409.
- Patz EF, Pinsky P, Gatsonis C, et al; NLST Overdiagnosis Manuscript Writing Team. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med 2014; 174:269–274.
- American Cancer Society. Colorectal cancer screening and surveillance guidelines. www.cancer.org/healthy/informationforhealth-careprofessionals/colonmdclinicansinformationsource/colorec-talcancerscreeningandsurveillanceguidelines/index. Accessed August 11, 2014.
- Jin XW, Lipold L, McKenzie M, Sikon A. Cervical cancer screening: what’s new and what’s coming? Cleve Clin J Med 2013; 80:153–160.
- American Cancer Society. Prostate cancer screening guidelines. www.cancer.org/healthy/informationforhealthcareprofessionals/pros-tatemdcliniciansinformationsource/prostatecancerscreeningguide-lines/index. Accessed August 11, 2014.
- Qaseem A, Barry MJ, Denberg TD, Owens DK, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Screening for prostate cancer: a guidance statement from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med 2013; 158:761–769.
- Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA guideline. www.auanet.org/common/pdf/education/clinical-guidance/Prostate-Cancer-Detection.pdf. Accessed September 5, 2014.
- Han PK, Kobrin S, Breen N, et al. National evidence on the use of shared decision making in prostate-specific antigen screening. Ann Fam Med 2013; 11:306–314.
- Taylor KL, Williams RM, Davis K, et al. Decision making in prostate cancer screening using decision aids vs usual care: a randomized clinical trial. JAMA Intern Med 2013; 173:1704–1712.
- Landrey AR, Matlock DD, Andrews L, Bronsert M, Denberg T. Shared decision making in prostate-specific antigen testing: the effect of a mailed patient flyer prior to an annual exam. J Prim Care Community Health 2013; 4:67–74.
- Falk RS, Hofvind S, Skaane P, Haldorsen T. Overdiagnosis among women attending a population-based mammography screening program. Int J Cancer 2013; 133:705–712.
- Welch HG, Passow HJ. Quantifying the benefits and harms of screening mammography. JAMA Intern Med 2014; 174:448–454.
KEY POINTS
- The USPSTF has stringent standards of evidence and therefore its recommendations tend to be more conservative than those of other organizations that issue guidelines. Recommendations are available at www.uspreventiveservicestaskforce.org.
- Because screening can result in harm as well as benefit, screening should be done after shared decision-making with the patient, especially if the screening is controversial, as is the case with mammography for breast cancer and prostate-specific antigen testing for prostate cancer.
- Screening for lung cancer using low-dose computed tomography is recommended yearly beginning at age 55 for people who have at least a 30-pack-year smoking history.
- In women over age 30, cervical cancer screening with Papanicolaou (Pap) and human papillomavirus (HPV) testing is now recommended every 5 years rather than every 3 years. Testing for HPV infection may soon become the first-line screening test, with Pap testing reserved for patients who have a positive HPV result.
- Although the USPSTF no longer recommends mammography for women ages 40 to 49, other organizations continue to do so.
Keeping up with immunizations for adults
A 58-year-old man with a history of irritable bowel syndrome and diabetes presents for an evaluation in early November. He is taking metformin and insulin glargine 10 units. He smokes 1 pack per day. He believes that his childhood immunizations were completed, but he has no records. He thinks his last “shot” was 15 years ago when he cut his hand on some wood.
Which immunizations, if any, would be most appropriate for this patient?
The explosion of new vaccines, new formulations, and new combinations made available in recent years makes managing immunizations a challenge. This article reviews common immunizations and current recommendations for their appropriate use.
Immunization recommendations (Table 1) are made predominantly by the Advisory Committee on Immunization Practices (ACIP) of the US Centers for Disease Control and Prevention (CDC). The last 15 years have seen the arrival of new vaccines (eg, varicella, hepatitis A, pneumococcal, and human papillomavirus), new formulations (eg, intranasal influenza), and new combinations.
To keep clinicians abreast of new indications, the ACIP issues immunization schedules annually for children and adults, available online and downloadable for easy reference.1 For adults, the ACIP provides schedules based on age and medical condition. The schedule for medical conditions offers specific information regarding immunization and pregnancy, human immunodeficiency virus (HIV) infection, kidney failure, heart disease, asplenia, and other conditions. The ACIP also provides guidance on contraindications; for example, pregnant and immunocompromised patients should not receive the live-attenuated vaccines, ie, zoster, varicella, and combined measles, mumps, and rubella [MMR]).
Adult awareness of vaccines is low, as are vaccination rates: in people older than 60, the vaccination rate is about 70% for influenza, 60% for pneumococcus, 50% for tetanus, and 15% for zoster. The lack of vaccine awareness and the availability of new vaccines and indications have made it difficult to manage immunizations in the primary care setting. The electronic medical record is useful for tracking patient vaccine needs. Ideally, keeping up with immunizations should be a routine part of visits provided by a physician’s care team and does not always require direct physician coordination.
TETANUS, DIPHTHERIA, PERTUSSIS EVERY 10 YEARS
Tetanus (also called “lockjaw”) is a nervous system disorder characterized by muscle spasms. Caused by infection with Clostridium tetani, it is a rare disease in the United States thanks to widespread immunization, and it causes fewer than 50 cases annually. Worldwide, the incidence is about 1 million cases a year with 200,000 to 300,000 deaths.
Diphtheria (formerly sometimes called “throat distemper”) is caused by the gram-positive bacillus Corynebacterium diphtheriae and can occur as a respiratory illness or as a milder cutaneous form. The last outbreak in the United States was in Seattle in the 1970s, with the last reported case in 2003. The ACIP recommends booster shots for tetanus and diphtheria every 10 years following completion of the primary series.
Pertussis or whooping cough, caused by Bordetella pertussis infection, is a highly contagious disease increasingly seen in adults in the United States. It causes few deaths but high morbidity, with coughing that can persist up to 3 months. Coughing can be severe enough to cause vomiting, a characteristic sign.
In July 2012, the CDC reported that the United States was at a 50-year high for pertussis, with 18,000 cases reported and 8 deaths.2 In Washington State alone, more than 2,520 cases had been seen through June 16 of that year, a 1,300% increase over the previous year. Rates were high in older children and adolescents despite previous vaccination, suggesting an early waning of immunity.
The ACIP recommends a single dose of the combination of high-dose tetanus and low-dose diphtheria and pertussis vaccines (Tdap) for all adults regardless of age and for all pregnant women with each pregnancy between 27 and 36 weeks of gestation. A dose of Tdap counts as the tetanus-diphtheria booster shot that is recommended every 10 years.
The patient described above is due for his tetanus-diphtheria booster and so should be given Tdap.
MEASLES, MUMPS, RUBELLA FOR THOSE BORN AFTER 1957
Measles remains a problem in the developing world, with an estimated average of 330 deaths daily. The number of cases fell 99% in the United States following the vaccination program that started in the early 1960s. Before the measles vaccine was available, an estimated 90% of children acquired measles by age 15.
The clinical syndrome consists of fever, conjunctivitis, cough, rash, and the characteristic Koplik spots—small white spots occurring on the inside of cheeks early in the disease course.
During the first 5 months of 2014, the CDC reported 334 cases of measles in the United States in 18 states, with most people affected being unvaccinated.3 In comparison, from 2001 to 2008, the number of cases averaged 56 annually.
Many of the recent cases were associated with infections brought from the Philippines. The increased number of measles cases underscores the need for vaccination to prevent measles and its complications.
Mumps is an acute, self-limited viral syndrome, and parotitis is the hallmark. Vaccination led to a 99% decline in cases in the United States. Although complications are rare, they can be serious and include orchitis (with risk of sterility), meningoencephalitis, and deafness.
Mumps outbreaks still occur, especially in close-contact settings such as schools, colleges, and camps. During the first half of 2014, central Ohio had more than 400 cases linked to The Ohio State University.
Rubella, also known as German measles, is a generally mild infection but is associated with congenital rubella syndrome. If a woman is infected with rubella in the first trimester of pregnancy, the risk of miscarriage is greater than 80%, as is the risk of birth defects, including hearing loss, developmental delay, growth retardation, and cardiac and eye defects.
Recommendations for MMR vaccination. People born before 1957 are considered immune to measles and usually to mumps. Health care workers should document immunity before assuming no vaccination is needed.
People born in 1957 or after should have one dose of MMR vaccine unless immunity is documented or unless there is a contraindication such as immunosuppression. A second dose is recommended for those born in or after 1957 who are considered to be at high risk: eg, health care workers, students entering college, and international travelers. The second dose should be given 4 weeks after the first.
Women of childbearing age should be screened for immunity to rubella. Susceptible women should receive MMR, although not during pregnancy and not if they may get pregnant within 4 weeks.
The patient described above was born before 1957, and so he is probably immune to measles and mumps.
HEPATITIS B FOR THOSE AT RISK
Hepatitis B vaccination is recommended for all adolescents and adults at increased risk: eg, men who have sex with men, intravenous drug users, people with multiple sexual partners, health care workers, patients with end-stage renal disease on hemodialysis, patients with chronic liver disease, and those with diabetes (age < 60).
Immunization consists of a series of three shots (at 0, 1–2, and 4–6 months). Booster doses are not recommended. Postvaccination testing for immunity is available and is recommended for health care workers, patients on hemodialysis, patients with HIV infection or who are otherwise immunocompromised, and sexual partners of people who are positive for hepatitis B surface antigen. Nonresponders should be revaccinated with the entire three-shot schedule. Hepatitis B vaccination is safe in pregnancy.
The patient described above has diabetes and so is a candidate for vaccination.
HEPATITIS A: A SLIGHTLY DIFFERENT RISK GROUP
Hepatitis A vaccination is recommended only for at-risk populations: international travelers; intravenous drug users; men who have sex with men; patients with clotting disorders, chronic liver disease, or hepatitis C infection; international adoptees; and laboratory personnel working with hepatitis A virus. The vaccination is given in two doses with a minimum interval of 6 months between doses.
A hepatitis A and hepatitis B combination vaccine (Twinrix) is also available. It is given in three doses, at 0, 1, and 6 months.
ANNUAL INFLUENZA VACCINE FOR ALL
In 2010, the ACIP recommended a policy of universal annual vaccination for everyone age 6 months and older. Some patients are at especially high risk themselves or are at high risk of exposing others and so are given higher priority during vaccine shortages—ie, patients who are immunosuppressed or have other medical risk factors, health care workers, household members of at-risk patients, and pregnant women after 13 weeks of gestation.
There are few contraindications, so almost everyone should be encouraged to receive the influenza vaccine. The flu shot does not cause the flu, but it may cause soreness at the injection site. Those with severe egg allergy should not receive the standard flu shot; a recombinant vaccine that does not use egg culture is available.
The standard flu shot is an inactivated influenza vaccine. In the past, most formulations were trivalent, but quadrivalent formulations are becoming more common. Special high-dose formulations are believed to elicit a better immune response and can be recommended for people over age 65. Intradermal and intramuscular formulations are available.
An intranasal live-attenuated influenza vaccine is also available and may be used for people ages 2 through 49. It should not be given to immunosuppressed people or to pregnant women.
Our patient should get a flu shot.
PNEUMOCOCCAL VACCINE FOR THOSE AGE 65 AND OLDER OR AT RISK
Two formulations are now available for pneumococcal immunization. The standard is a 23-valent polysaccharide vaccine (PPSV23; Pneumovax) indicated for people age 65 and older.
Patients under age 65 can receive PPSV23 if they have chronic lung disease, chronic cardiovascular disease, diabetes, chronic liver disease, or alcoholism or are a resident of a nursing home or an active smoker.
Our patient is a candidate for PPSV23 since he smokes and has diabetes.
The other formulation is a conjugate 13-valent vaccine (PCV13; Prevnar 13). Patients over age 19 at high risk should be given PCV13 plus the PPSV23 8 weeks later. Those who already received PPSV23 should be given PCV13 vaccine more than 1 year later. Candidates for PCV13 are those with immunocompromising conditions (including chronic renal failure and nephrotic syndrome), functional or anatomic asplenia, cerebrospinal fluid leaks, or cochlear implants.
The current revaccination schedule for PPSV23 is as follows:
- One-time revaccination 5 years after the first dose in patients with chronic renal failure, nephrotic syndrome, asplenia, or an immunosuppressive condition
- One-time revaccination for patients age 65 or older if they were younger than 65 when first immunized (with one or two doses of PPSV23) and 5 years have passed
- No revaccination is needed for people vaccinated with PPSV23 after age 65.
HUMAN PAPILLOMAVIRUS VACCINE
Human papillomavirus is the most common sexually transmitted infection in the United States and is strongly associated with cervical cancer. Immunization is now indicated for both sexes, generally between the ages of 9 and 26. Two vaccines are available: the quadrivalent formulation (Gardasil) for males or females and the bivalent formulation (Cervarix) for females only.
Immunization should be given in three doses: at 0, 1 to 2 months, and 6 months. It can be given to patients who are immunocompromised as a result of infection (including HIV infection), disease, or medications, or who have a history of genital warts, an abnormal Papanicolaou test, or a positive human papillomavirus DNA test.
It is hoped that immunization will lead to a significant decrease in cervical cancer rates. Eradication is unlikely because other papillomavirus strains also can lead to cancer, so cancer screening is still warranted. For men who have sex with men, it is hoped that immunization will prevent condyloma and anal cancer.
CHICKENPOX AND SHINGLES VACCINES
Varicella vaccine (Varivax) contains a live-attenuated virus to protect against chickenpox. It is recommended for all adults who have no evidence of immunity. Immunity is assumed with a history of chickenpox, being born before 1980, or having positive titers. Vaccination should be emphasized for those who come in contact with patients at high risk of severe disease (eg, health care workers, family contacts of immunocompromised patients) and in individuals with a high risk of personal exposure (eg, teachers, day care workers).
The vaccine is given in two doses, 4 to 8 weeks apart. Women who are pregnant or who may get pregnant within 4 weeks should not be vaccinated.
The shingles vaccine (Zostavax) is a larger dose of the varicella vaccine and reduces the incidence of shingles by 50% and postherpetic neuralgia by 66%.4 It was approved by the US Food and Drug Administration in May 2006 for people starting at age 50, but was recommended by ACIP in October 2006 for people age 60 and older; as a result, some insurance companies deny coverage for patients ages 50 through 59.
The shingles vaccine can be given to patients who have already had shingles. Pregnancy and severe immunodeficiency are contraindications.
Our patient, 58 years old, could be considered for shingles vaccine if covered by his insurance company or if he wishes to pay for it.
MENINGOCOCCUS VACCINE
Meningococcal immunization is recommended for people at high risk: college students who plan to live in dormitories, adults without a spleen or with complement deficiencies or HIV infection, or travelers to the “meningitis belt” of sub-Saharan Africa.
Two types of meningococcal vaccine are available: the conjugate quadrivalent vaccine (MCV4) for people age 55 and younger, and the polysaccharide quadrivalent vaccine (MPSV4) for people over age 56.
- US Centers for Disease Control and Prevention (CDC). Adult immunization schedules. www.cdc.gov/vaccines/schedules/hcp/adult.html. Accessed August 21, 2014.
- US Centers for Disease Control and Prevention (CDC). Pertussis epidemic—Washington, 2012. MMWR Morb Mortal Wkly Rep 2012; 61:517–522.
- US Centers for Disease Control and Prevention (CDC). Measles cases and outbreaks, January 1 to August 15, 2014. www.cdc.gov/measles/cases-outbreaks.html. Accessed August 21, 2014.
- Oxman MN, Levin MJ, Johnson MS, et al; for the Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271–2284.
A 58-year-old man with a history of irritable bowel syndrome and diabetes presents for an evaluation in early November. He is taking metformin and insulin glargine 10 units. He smokes 1 pack per day. He believes that his childhood immunizations were completed, but he has no records. He thinks his last “shot” was 15 years ago when he cut his hand on some wood.
Which immunizations, if any, would be most appropriate for this patient?
The explosion of new vaccines, new formulations, and new combinations made available in recent years makes managing immunizations a challenge. This article reviews common immunizations and current recommendations for their appropriate use.
Immunization recommendations (Table 1) are made predominantly by the Advisory Committee on Immunization Practices (ACIP) of the US Centers for Disease Control and Prevention (CDC). The last 15 years have seen the arrival of new vaccines (eg, varicella, hepatitis A, pneumococcal, and human papillomavirus), new formulations (eg, intranasal influenza), and new combinations.
To keep clinicians abreast of new indications, the ACIP issues immunization schedules annually for children and adults, available online and downloadable for easy reference.1 For adults, the ACIP provides schedules based on age and medical condition. The schedule for medical conditions offers specific information regarding immunization and pregnancy, human immunodeficiency virus (HIV) infection, kidney failure, heart disease, asplenia, and other conditions. The ACIP also provides guidance on contraindications; for example, pregnant and immunocompromised patients should not receive the live-attenuated vaccines, ie, zoster, varicella, and combined measles, mumps, and rubella [MMR]).
Adult awareness of vaccines is low, as are vaccination rates: in people older than 60, the vaccination rate is about 70% for influenza, 60% for pneumococcus, 50% for tetanus, and 15% for zoster. The lack of vaccine awareness and the availability of new vaccines and indications have made it difficult to manage immunizations in the primary care setting. The electronic medical record is useful for tracking patient vaccine needs. Ideally, keeping up with immunizations should be a routine part of visits provided by a physician’s care team and does not always require direct physician coordination.
TETANUS, DIPHTHERIA, PERTUSSIS EVERY 10 YEARS
Tetanus (also called “lockjaw”) is a nervous system disorder characterized by muscle spasms. Caused by infection with Clostridium tetani, it is a rare disease in the United States thanks to widespread immunization, and it causes fewer than 50 cases annually. Worldwide, the incidence is about 1 million cases a year with 200,000 to 300,000 deaths.
Diphtheria (formerly sometimes called “throat distemper”) is caused by the gram-positive bacillus Corynebacterium diphtheriae and can occur as a respiratory illness or as a milder cutaneous form. The last outbreak in the United States was in Seattle in the 1970s, with the last reported case in 2003. The ACIP recommends booster shots for tetanus and diphtheria every 10 years following completion of the primary series.
Pertussis or whooping cough, caused by Bordetella pertussis infection, is a highly contagious disease increasingly seen in adults in the United States. It causes few deaths but high morbidity, with coughing that can persist up to 3 months. Coughing can be severe enough to cause vomiting, a characteristic sign.
In July 2012, the CDC reported that the United States was at a 50-year high for pertussis, with 18,000 cases reported and 8 deaths.2 In Washington State alone, more than 2,520 cases had been seen through June 16 of that year, a 1,300% increase over the previous year. Rates were high in older children and adolescents despite previous vaccination, suggesting an early waning of immunity.
The ACIP recommends a single dose of the combination of high-dose tetanus and low-dose diphtheria and pertussis vaccines (Tdap) for all adults regardless of age and for all pregnant women with each pregnancy between 27 and 36 weeks of gestation. A dose of Tdap counts as the tetanus-diphtheria booster shot that is recommended every 10 years.
The patient described above is due for his tetanus-diphtheria booster and so should be given Tdap.
MEASLES, MUMPS, RUBELLA FOR THOSE BORN AFTER 1957
Measles remains a problem in the developing world, with an estimated average of 330 deaths daily. The number of cases fell 99% in the United States following the vaccination program that started in the early 1960s. Before the measles vaccine was available, an estimated 90% of children acquired measles by age 15.
The clinical syndrome consists of fever, conjunctivitis, cough, rash, and the characteristic Koplik spots—small white spots occurring on the inside of cheeks early in the disease course.
During the first 5 months of 2014, the CDC reported 334 cases of measles in the United States in 18 states, with most people affected being unvaccinated.3 In comparison, from 2001 to 2008, the number of cases averaged 56 annually.
Many of the recent cases were associated with infections brought from the Philippines. The increased number of measles cases underscores the need for vaccination to prevent measles and its complications.
Mumps is an acute, self-limited viral syndrome, and parotitis is the hallmark. Vaccination led to a 99% decline in cases in the United States. Although complications are rare, they can be serious and include orchitis (with risk of sterility), meningoencephalitis, and deafness.
Mumps outbreaks still occur, especially in close-contact settings such as schools, colleges, and camps. During the first half of 2014, central Ohio had more than 400 cases linked to The Ohio State University.
Rubella, also known as German measles, is a generally mild infection but is associated with congenital rubella syndrome. If a woman is infected with rubella in the first trimester of pregnancy, the risk of miscarriage is greater than 80%, as is the risk of birth defects, including hearing loss, developmental delay, growth retardation, and cardiac and eye defects.
Recommendations for MMR vaccination. People born before 1957 are considered immune to measles and usually to mumps. Health care workers should document immunity before assuming no vaccination is needed.
People born in 1957 or after should have one dose of MMR vaccine unless immunity is documented or unless there is a contraindication such as immunosuppression. A second dose is recommended for those born in or after 1957 who are considered to be at high risk: eg, health care workers, students entering college, and international travelers. The second dose should be given 4 weeks after the first.
Women of childbearing age should be screened for immunity to rubella. Susceptible women should receive MMR, although not during pregnancy and not if they may get pregnant within 4 weeks.
The patient described above was born before 1957, and so he is probably immune to measles and mumps.
HEPATITIS B FOR THOSE AT RISK
Hepatitis B vaccination is recommended for all adolescents and adults at increased risk: eg, men who have sex with men, intravenous drug users, people with multiple sexual partners, health care workers, patients with end-stage renal disease on hemodialysis, patients with chronic liver disease, and those with diabetes (age < 60).
Immunization consists of a series of three shots (at 0, 1–2, and 4–6 months). Booster doses are not recommended. Postvaccination testing for immunity is available and is recommended for health care workers, patients on hemodialysis, patients with HIV infection or who are otherwise immunocompromised, and sexual partners of people who are positive for hepatitis B surface antigen. Nonresponders should be revaccinated with the entire three-shot schedule. Hepatitis B vaccination is safe in pregnancy.
The patient described above has diabetes and so is a candidate for vaccination.
HEPATITIS A: A SLIGHTLY DIFFERENT RISK GROUP
Hepatitis A vaccination is recommended only for at-risk populations: international travelers; intravenous drug users; men who have sex with men; patients with clotting disorders, chronic liver disease, or hepatitis C infection; international adoptees; and laboratory personnel working with hepatitis A virus. The vaccination is given in two doses with a minimum interval of 6 months between doses.
A hepatitis A and hepatitis B combination vaccine (Twinrix) is also available. It is given in three doses, at 0, 1, and 6 months.
ANNUAL INFLUENZA VACCINE FOR ALL
In 2010, the ACIP recommended a policy of universal annual vaccination for everyone age 6 months and older. Some patients are at especially high risk themselves or are at high risk of exposing others and so are given higher priority during vaccine shortages—ie, patients who are immunosuppressed or have other medical risk factors, health care workers, household members of at-risk patients, and pregnant women after 13 weeks of gestation.
There are few contraindications, so almost everyone should be encouraged to receive the influenza vaccine. The flu shot does not cause the flu, but it may cause soreness at the injection site. Those with severe egg allergy should not receive the standard flu shot; a recombinant vaccine that does not use egg culture is available.
The standard flu shot is an inactivated influenza vaccine. In the past, most formulations were trivalent, but quadrivalent formulations are becoming more common. Special high-dose formulations are believed to elicit a better immune response and can be recommended for people over age 65. Intradermal and intramuscular formulations are available.
An intranasal live-attenuated influenza vaccine is also available and may be used for people ages 2 through 49. It should not be given to immunosuppressed people or to pregnant women.
Our patient should get a flu shot.
PNEUMOCOCCAL VACCINE FOR THOSE AGE 65 AND OLDER OR AT RISK
Two formulations are now available for pneumococcal immunization. The standard is a 23-valent polysaccharide vaccine (PPSV23; Pneumovax) indicated for people age 65 and older.
Patients under age 65 can receive PPSV23 if they have chronic lung disease, chronic cardiovascular disease, diabetes, chronic liver disease, or alcoholism or are a resident of a nursing home or an active smoker.
Our patient is a candidate for PPSV23 since he smokes and has diabetes.
The other formulation is a conjugate 13-valent vaccine (PCV13; Prevnar 13). Patients over age 19 at high risk should be given PCV13 plus the PPSV23 8 weeks later. Those who already received PPSV23 should be given PCV13 vaccine more than 1 year later. Candidates for PCV13 are those with immunocompromising conditions (including chronic renal failure and nephrotic syndrome), functional or anatomic asplenia, cerebrospinal fluid leaks, or cochlear implants.
The current revaccination schedule for PPSV23 is as follows:
- One-time revaccination 5 years after the first dose in patients with chronic renal failure, nephrotic syndrome, asplenia, or an immunosuppressive condition
- One-time revaccination for patients age 65 or older if they were younger than 65 when first immunized (with one or two doses of PPSV23) and 5 years have passed
- No revaccination is needed for people vaccinated with PPSV23 after age 65.
HUMAN PAPILLOMAVIRUS VACCINE
Human papillomavirus is the most common sexually transmitted infection in the United States and is strongly associated with cervical cancer. Immunization is now indicated for both sexes, generally between the ages of 9 and 26. Two vaccines are available: the quadrivalent formulation (Gardasil) for males or females and the bivalent formulation (Cervarix) for females only.
Immunization should be given in three doses: at 0, 1 to 2 months, and 6 months. It can be given to patients who are immunocompromised as a result of infection (including HIV infection), disease, or medications, or who have a history of genital warts, an abnormal Papanicolaou test, or a positive human papillomavirus DNA test.
It is hoped that immunization will lead to a significant decrease in cervical cancer rates. Eradication is unlikely because other papillomavirus strains also can lead to cancer, so cancer screening is still warranted. For men who have sex with men, it is hoped that immunization will prevent condyloma and anal cancer.
CHICKENPOX AND SHINGLES VACCINES
Varicella vaccine (Varivax) contains a live-attenuated virus to protect against chickenpox. It is recommended for all adults who have no evidence of immunity. Immunity is assumed with a history of chickenpox, being born before 1980, or having positive titers. Vaccination should be emphasized for those who come in contact with patients at high risk of severe disease (eg, health care workers, family contacts of immunocompromised patients) and in individuals with a high risk of personal exposure (eg, teachers, day care workers).
The vaccine is given in two doses, 4 to 8 weeks apart. Women who are pregnant or who may get pregnant within 4 weeks should not be vaccinated.
The shingles vaccine (Zostavax) is a larger dose of the varicella vaccine and reduces the incidence of shingles by 50% and postherpetic neuralgia by 66%.4 It was approved by the US Food and Drug Administration in May 2006 for people starting at age 50, but was recommended by ACIP in October 2006 for people age 60 and older; as a result, some insurance companies deny coverage for patients ages 50 through 59.
The shingles vaccine can be given to patients who have already had shingles. Pregnancy and severe immunodeficiency are contraindications.
Our patient, 58 years old, could be considered for shingles vaccine if covered by his insurance company or if he wishes to pay for it.
MENINGOCOCCUS VACCINE
Meningococcal immunization is recommended for people at high risk: college students who plan to live in dormitories, adults without a spleen or with complement deficiencies or HIV infection, or travelers to the “meningitis belt” of sub-Saharan Africa.
Two types of meningococcal vaccine are available: the conjugate quadrivalent vaccine (MCV4) for people age 55 and younger, and the polysaccharide quadrivalent vaccine (MPSV4) for people over age 56.
A 58-year-old man with a history of irritable bowel syndrome and diabetes presents for an evaluation in early November. He is taking metformin and insulin glargine 10 units. He smokes 1 pack per day. He believes that his childhood immunizations were completed, but he has no records. He thinks his last “shot” was 15 years ago when he cut his hand on some wood.
Which immunizations, if any, would be most appropriate for this patient?
The explosion of new vaccines, new formulations, and new combinations made available in recent years makes managing immunizations a challenge. This article reviews common immunizations and current recommendations for their appropriate use.
Immunization recommendations (Table 1) are made predominantly by the Advisory Committee on Immunization Practices (ACIP) of the US Centers for Disease Control and Prevention (CDC). The last 15 years have seen the arrival of new vaccines (eg, varicella, hepatitis A, pneumococcal, and human papillomavirus), new formulations (eg, intranasal influenza), and new combinations.
To keep clinicians abreast of new indications, the ACIP issues immunization schedules annually for children and adults, available online and downloadable for easy reference.1 For adults, the ACIP provides schedules based on age and medical condition. The schedule for medical conditions offers specific information regarding immunization and pregnancy, human immunodeficiency virus (HIV) infection, kidney failure, heart disease, asplenia, and other conditions. The ACIP also provides guidance on contraindications; for example, pregnant and immunocompromised patients should not receive the live-attenuated vaccines, ie, zoster, varicella, and combined measles, mumps, and rubella [MMR]).
Adult awareness of vaccines is low, as are vaccination rates: in people older than 60, the vaccination rate is about 70% for influenza, 60% for pneumococcus, 50% for tetanus, and 15% for zoster. The lack of vaccine awareness and the availability of new vaccines and indications have made it difficult to manage immunizations in the primary care setting. The electronic medical record is useful for tracking patient vaccine needs. Ideally, keeping up with immunizations should be a routine part of visits provided by a physician’s care team and does not always require direct physician coordination.
TETANUS, DIPHTHERIA, PERTUSSIS EVERY 10 YEARS
Tetanus (also called “lockjaw”) is a nervous system disorder characterized by muscle spasms. Caused by infection with Clostridium tetani, it is a rare disease in the United States thanks to widespread immunization, and it causes fewer than 50 cases annually. Worldwide, the incidence is about 1 million cases a year with 200,000 to 300,000 deaths.
Diphtheria (formerly sometimes called “throat distemper”) is caused by the gram-positive bacillus Corynebacterium diphtheriae and can occur as a respiratory illness or as a milder cutaneous form. The last outbreak in the United States was in Seattle in the 1970s, with the last reported case in 2003. The ACIP recommends booster shots for tetanus and diphtheria every 10 years following completion of the primary series.
Pertussis or whooping cough, caused by Bordetella pertussis infection, is a highly contagious disease increasingly seen in adults in the United States. It causes few deaths but high morbidity, with coughing that can persist up to 3 months. Coughing can be severe enough to cause vomiting, a characteristic sign.
In July 2012, the CDC reported that the United States was at a 50-year high for pertussis, with 18,000 cases reported and 8 deaths.2 In Washington State alone, more than 2,520 cases had been seen through June 16 of that year, a 1,300% increase over the previous year. Rates were high in older children and adolescents despite previous vaccination, suggesting an early waning of immunity.
The ACIP recommends a single dose of the combination of high-dose tetanus and low-dose diphtheria and pertussis vaccines (Tdap) for all adults regardless of age and for all pregnant women with each pregnancy between 27 and 36 weeks of gestation. A dose of Tdap counts as the tetanus-diphtheria booster shot that is recommended every 10 years.
The patient described above is due for his tetanus-diphtheria booster and so should be given Tdap.
MEASLES, MUMPS, RUBELLA FOR THOSE BORN AFTER 1957
Measles remains a problem in the developing world, with an estimated average of 330 deaths daily. The number of cases fell 99% in the United States following the vaccination program that started in the early 1960s. Before the measles vaccine was available, an estimated 90% of children acquired measles by age 15.
The clinical syndrome consists of fever, conjunctivitis, cough, rash, and the characteristic Koplik spots—small white spots occurring on the inside of cheeks early in the disease course.
During the first 5 months of 2014, the CDC reported 334 cases of measles in the United States in 18 states, with most people affected being unvaccinated.3 In comparison, from 2001 to 2008, the number of cases averaged 56 annually.
Many of the recent cases were associated with infections brought from the Philippines. The increased number of measles cases underscores the need for vaccination to prevent measles and its complications.
Mumps is an acute, self-limited viral syndrome, and parotitis is the hallmark. Vaccination led to a 99% decline in cases in the United States. Although complications are rare, they can be serious and include orchitis (with risk of sterility), meningoencephalitis, and deafness.
Mumps outbreaks still occur, especially in close-contact settings such as schools, colleges, and camps. During the first half of 2014, central Ohio had more than 400 cases linked to The Ohio State University.
Rubella, also known as German measles, is a generally mild infection but is associated with congenital rubella syndrome. If a woman is infected with rubella in the first trimester of pregnancy, the risk of miscarriage is greater than 80%, as is the risk of birth defects, including hearing loss, developmental delay, growth retardation, and cardiac and eye defects.
Recommendations for MMR vaccination. People born before 1957 are considered immune to measles and usually to mumps. Health care workers should document immunity before assuming no vaccination is needed.
People born in 1957 or after should have one dose of MMR vaccine unless immunity is documented or unless there is a contraindication such as immunosuppression. A second dose is recommended for those born in or after 1957 who are considered to be at high risk: eg, health care workers, students entering college, and international travelers. The second dose should be given 4 weeks after the first.
Women of childbearing age should be screened for immunity to rubella. Susceptible women should receive MMR, although not during pregnancy and not if they may get pregnant within 4 weeks.
The patient described above was born before 1957, and so he is probably immune to measles and mumps.
HEPATITIS B FOR THOSE AT RISK
Hepatitis B vaccination is recommended for all adolescents and adults at increased risk: eg, men who have sex with men, intravenous drug users, people with multiple sexual partners, health care workers, patients with end-stage renal disease on hemodialysis, patients with chronic liver disease, and those with diabetes (age < 60).
Immunization consists of a series of three shots (at 0, 1–2, and 4–6 months). Booster doses are not recommended. Postvaccination testing for immunity is available and is recommended for health care workers, patients on hemodialysis, patients with HIV infection or who are otherwise immunocompromised, and sexual partners of people who are positive for hepatitis B surface antigen. Nonresponders should be revaccinated with the entire three-shot schedule. Hepatitis B vaccination is safe in pregnancy.
The patient described above has diabetes and so is a candidate for vaccination.
HEPATITIS A: A SLIGHTLY DIFFERENT RISK GROUP
Hepatitis A vaccination is recommended only for at-risk populations: international travelers; intravenous drug users; men who have sex with men; patients with clotting disorders, chronic liver disease, or hepatitis C infection; international adoptees; and laboratory personnel working with hepatitis A virus. The vaccination is given in two doses with a minimum interval of 6 months between doses.
A hepatitis A and hepatitis B combination vaccine (Twinrix) is also available. It is given in three doses, at 0, 1, and 6 months.
ANNUAL INFLUENZA VACCINE FOR ALL
In 2010, the ACIP recommended a policy of universal annual vaccination for everyone age 6 months and older. Some patients are at especially high risk themselves or are at high risk of exposing others and so are given higher priority during vaccine shortages—ie, patients who are immunosuppressed or have other medical risk factors, health care workers, household members of at-risk patients, and pregnant women after 13 weeks of gestation.
There are few contraindications, so almost everyone should be encouraged to receive the influenza vaccine. The flu shot does not cause the flu, but it may cause soreness at the injection site. Those with severe egg allergy should not receive the standard flu shot; a recombinant vaccine that does not use egg culture is available.
The standard flu shot is an inactivated influenza vaccine. In the past, most formulations were trivalent, but quadrivalent formulations are becoming more common. Special high-dose formulations are believed to elicit a better immune response and can be recommended for people over age 65. Intradermal and intramuscular formulations are available.
An intranasal live-attenuated influenza vaccine is also available and may be used for people ages 2 through 49. It should not be given to immunosuppressed people or to pregnant women.
Our patient should get a flu shot.
PNEUMOCOCCAL VACCINE FOR THOSE AGE 65 AND OLDER OR AT RISK
Two formulations are now available for pneumococcal immunization. The standard is a 23-valent polysaccharide vaccine (PPSV23; Pneumovax) indicated for people age 65 and older.
Patients under age 65 can receive PPSV23 if they have chronic lung disease, chronic cardiovascular disease, diabetes, chronic liver disease, or alcoholism or are a resident of a nursing home or an active smoker.
Our patient is a candidate for PPSV23 since he smokes and has diabetes.
The other formulation is a conjugate 13-valent vaccine (PCV13; Prevnar 13). Patients over age 19 at high risk should be given PCV13 plus the PPSV23 8 weeks later. Those who already received PPSV23 should be given PCV13 vaccine more than 1 year later. Candidates for PCV13 are those with immunocompromising conditions (including chronic renal failure and nephrotic syndrome), functional or anatomic asplenia, cerebrospinal fluid leaks, or cochlear implants.
The current revaccination schedule for PPSV23 is as follows:
- One-time revaccination 5 years after the first dose in patients with chronic renal failure, nephrotic syndrome, asplenia, or an immunosuppressive condition
- One-time revaccination for patients age 65 or older if they were younger than 65 when first immunized (with one or two doses of PPSV23) and 5 years have passed
- No revaccination is needed for people vaccinated with PPSV23 after age 65.
HUMAN PAPILLOMAVIRUS VACCINE
Human papillomavirus is the most common sexually transmitted infection in the United States and is strongly associated with cervical cancer. Immunization is now indicated for both sexes, generally between the ages of 9 and 26. Two vaccines are available: the quadrivalent formulation (Gardasil) for males or females and the bivalent formulation (Cervarix) for females only.
Immunization should be given in three doses: at 0, 1 to 2 months, and 6 months. It can be given to patients who are immunocompromised as a result of infection (including HIV infection), disease, or medications, or who have a history of genital warts, an abnormal Papanicolaou test, or a positive human papillomavirus DNA test.
It is hoped that immunization will lead to a significant decrease in cervical cancer rates. Eradication is unlikely because other papillomavirus strains also can lead to cancer, so cancer screening is still warranted. For men who have sex with men, it is hoped that immunization will prevent condyloma and anal cancer.
CHICKENPOX AND SHINGLES VACCINES
Varicella vaccine (Varivax) contains a live-attenuated virus to protect against chickenpox. It is recommended for all adults who have no evidence of immunity. Immunity is assumed with a history of chickenpox, being born before 1980, or having positive titers. Vaccination should be emphasized for those who come in contact with patients at high risk of severe disease (eg, health care workers, family contacts of immunocompromised patients) and in individuals with a high risk of personal exposure (eg, teachers, day care workers).
The vaccine is given in two doses, 4 to 8 weeks apart. Women who are pregnant or who may get pregnant within 4 weeks should not be vaccinated.
The shingles vaccine (Zostavax) is a larger dose of the varicella vaccine and reduces the incidence of shingles by 50% and postherpetic neuralgia by 66%.4 It was approved by the US Food and Drug Administration in May 2006 for people starting at age 50, but was recommended by ACIP in October 2006 for people age 60 and older; as a result, some insurance companies deny coverage for patients ages 50 through 59.
The shingles vaccine can be given to patients who have already had shingles. Pregnancy and severe immunodeficiency are contraindications.
Our patient, 58 years old, could be considered for shingles vaccine if covered by his insurance company or if he wishes to pay for it.
MENINGOCOCCUS VACCINE
Meningococcal immunization is recommended for people at high risk: college students who plan to live in dormitories, adults without a spleen or with complement deficiencies or HIV infection, or travelers to the “meningitis belt” of sub-Saharan Africa.
Two types of meningococcal vaccine are available: the conjugate quadrivalent vaccine (MCV4) for people age 55 and younger, and the polysaccharide quadrivalent vaccine (MPSV4) for people over age 56.
- US Centers for Disease Control and Prevention (CDC). Adult immunization schedules. www.cdc.gov/vaccines/schedules/hcp/adult.html. Accessed August 21, 2014.
- US Centers for Disease Control and Prevention (CDC). Pertussis epidemic—Washington, 2012. MMWR Morb Mortal Wkly Rep 2012; 61:517–522.
- US Centers for Disease Control and Prevention (CDC). Measles cases and outbreaks, January 1 to August 15, 2014. www.cdc.gov/measles/cases-outbreaks.html. Accessed August 21, 2014.
- Oxman MN, Levin MJ, Johnson MS, et al; for the Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271–2284.
- US Centers for Disease Control and Prevention (CDC). Adult immunization schedules. www.cdc.gov/vaccines/schedules/hcp/adult.html. Accessed August 21, 2014.
- US Centers for Disease Control and Prevention (CDC). Pertussis epidemic—Washington, 2012. MMWR Morb Mortal Wkly Rep 2012; 61:517–522.
- US Centers for Disease Control and Prevention (CDC). Measles cases and outbreaks, January 1 to August 15, 2014. www.cdc.gov/measles/cases-outbreaks.html. Accessed August 21, 2014.
- Oxman MN, Levin MJ, Johnson MS, et al; for the Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271–2284.
KEY POINTS
- Information on immunization schedules, including an app for mobile devices, is available at www.cdc.gov/vaccines/schedules/hcp/adult.html.
- Vaccination rates in adults are low, and appropriate vaccinations should be encouraged. The electronic medical record can help remind us when vaccinations are due.
- The live-attenuated vaccines, ie, zoster, varicella, and combined measles, mumps, and rubella, are contraindicated during pregnancy and in immunocompromised patients.
Do patients who received only two doses of hepatitis B vaccine need a booster?
The Advisory Committee on Immunization Practices (ACIP) currently recommends that people who have not completed the three-dose vaccination series against hepatitis B virus (HBV) should receive the missed doses: ie, the three-dose regimen does not need to be restarted.1 However, evidence suggests that a two-dose regimen may provide adequate seroprotection for healthy young adults.
As the three-dose regimen has been shown to protect 90% to 100% of adults,2 it has gained widespread acceptance and is now standard clinical practice.2 However, deviating from the three-dose regimen may not leave healthy young adults vulnerable to HBV infection.
RECOMMENDED DOSES AND SCHEDULES
Widespread use of the three-dose regimen for HBV stemmed from the first clinical evaluation of the recombinant vaccine, in which three 10-μg doses were given at 0, 1, and 6 months to healthy, low-risk adult volunteers.3 This regimen was shown to provide seroprotection in over 95% of adolescents and 90% of healthy adults.2
Currently, three HBV vaccines for adults are approved in the United States: Recombivax HB, Engerix-B, and Twinrix (Table 1). While Recombivax has a seroprotection rate of 89% in healthy adults over age 40, it has higher seroprotection rates in younger people: eg, two doses of Recombivax given 4 to 6 months apart provide seroprotection to 99% of children aged 11 to 15.4 On the other hand, patients on hemodialysis require three 40-μg doses of Recombivax or four 40-μg doses of Engerix-B.
Evidence for a two-dose regimen
Since the development of the recombinant HBV vaccine used today, studies have shown that a two-dose regimen offers seroprotection comparable with, if not better than, the three-dose regimen in adolescents and healthy young adults. Marsano et al5 found that with a two-dose regimen, 96% to 99% of young adults attained seroprotection, with immune memory persisting for up to 2 years.5 Moreover, Cassidy et al6 randomized adolescents to a two-dose or a three-dose regimen and found the two regimens to be equally effective in conferring immunogenicity and immunologic memory.6
Other studies in adolescents have confirmed these findings and offered new evidence in support of the two-dose regimen.7,8 For example, studies found that the two-dose regimen conferred seroprotection at even lower doses than previously studied, and that it conferred immune memory lasting at least 5 years.6,7
However, because these studies were conducted in adolescents and healthy young adults, the findings may not hold true for other populations. Studies suggest that the three-dose regimen is best for those over age 40. Moreover, it is advisable to adhere to a three-dose regimen when treating people at high risk of contracting HBV, such as health care workers; people with chronic liver disease, diabetes mellitus, or end-stage renal disease on hemodialysis; people who have multiple sex partners; and men who have sex with men.
The impact of long intervals between doses
Although the aforementioned studies focused on a two-dose regimen with a 6-month interval, longer intervals between doses do not impair seroprotection and in some cases may even prove beneficial. Heron et al9 demonstrated that a two-dose regimen with a 12-month interval induces seroprotection as effectively as a standard three-dose or two-dose regimen with a 6-month interval.9 Moreover, studies of the impact of deviating from a three-dose regimen found that intervals of longer than 1 year did not impede seroprotection. Not only may seroprotection be attained with intervals of 5 to 10 years before the final dose, but final antibody levels tend to increase with increasing time between doses.10
Nevertheless, even though an extended interval between doses may prove beneficial after the final dose is received, delaying doses may leave patients unprotected. Indeed, alternative three-dose and even four-dose schedules with shorter intervals between doses exist for certain high-risk populations, such as those recently exposed to HBV and travelers to areas of high prevalence. Therefore, intentionally extending intervals between doses may be inappropriate.
SEROPROTECTION AND PROTECTION AGAINST INFECTION
Legitimate concerns exist about the final antibody level attained with a two-dose regimen, which is typically lower than that attained with a three-dose regimen. As HBV antibody levels decline with time, lower final antibody levels theoretically increase the risk of losing seroprotection. Study of vaccine efficacy has defined seroprotection as antibody levels greater than or equal to 10 mIU/mL.11 Yet evidence suggests that even when antibody levels drop below this level, the risk of symptomatic HBV infection does not increase. Evidence also suggests that immune memory outlasts the presence of seroprotective antibody levels, indicating that true protection against significant infection does not necessarily correlate with, and may even exceed, seroprotection.2 This may relate to HBV’s long incubation period, which allows memory cells time to generate an effective immune response.10 For example, Floreani et al12 showed that even though 15% of adults lost seroprotective antibody levels 10 years after vaccination, none demonstrated hepatitis B antigen reactivity or seroconversion.
POSTVACCINATION TESTING AND ADDITIONAL DOSES
At times, it may be wise to measure antibody levels after the final dose to confirm seroprotection. Seroprotection should be documented when knowledge of the patient’s immune status will affect subsequent management. As recommended by the US Centers for Disease Control and Prevention, health care workers, hemodialysis patients, immunocompromised patients, and sexual partners of patients with chronic HBV infection should undergo antibody testing 1 to 2 months after the completion of a three-dose vaccination regimen. Hemodialysis patients require annual confirmation of seroprotection and should receive booster doses of HBV vaccine if necessary.
Postvaccination testing (quantitative HBV surface antibody testing) costs about the same as a single dose of HBV vaccine. Therefore, if postvaccination testing is considered because of missed vaccine doses, it may be more cost-efficient to simply administer the missed dose.
- Department of Health and Human Services. Appendix A Immunization Management Issues. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5516a2.htm. Accessed April 6, 2014.
- Leuridan E, Van Damme P. Hepatitis B and the need for a booster dose. Clin Infect Dis 2011; 53:68–75.
- Scolnick EM, McLean AA, West DJ, McAleer WJ, Miller WJ, Buynak EB. Clinical evaluation in healthy adults of a hepatitis B vaccine made by recombinant DNA. JAMA 1984; 251:2812–2815.
- Merck and Co, Inc. 1998. Recombivax HB. http://www.merck.com/product/usa/pi_circulars/r/recombivax_hb/re-combivax_pi.pdf. Accessed April 7, 2014.
- Marsano LS, West DJ, Chan I, et al. A two-dose hepatitis B vaccine regimen: proof of priming and memory responses in young adults. Vaccine 1998; 16:624–629.
- Cassidy WM, Watson B, Ioli VA, Williams K, Bird S, West DJ. A randomized trial of alternative two- and three-dose hepatitis B vaccination regimens in adolescents: antibody responses, safety, and immunologic memory. Pediatrics 2001; 107:626–631.
- Van Damme P, Moiseeva A, Marichev I, et al. Five years follow-up following two or three doses of a hepatitis B vaccine in adolescents aged 11–15 years: a randomised controlled study. BMC Infect Dis 2010; 10:357.
- Heron L, Selnikova O, Moiseieva A, et al. Immunogenicity, reactogenicity and safety of two-dose versus three-dose (standard care) hepatitis B immunisation of healthy adolescents aged 11–15 years: a randomised controlled trial. Vaccine 2007; 25:2817–2822.
- Heron LG, Chant KG, Jalaludin BB. A novel hepatitis B vaccination regimen for adolescents: two doses 12 months apart. Vaccine 2002; 20:3472–3476.
- Jackson Y, Chappuis F, Mezger N, Kanappa K, Loutan L. High immunogenicity of delayed third dose of hepatitis B vaccine in travellers. Vaccine 2007; 25:3482–3484.
- Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC. What level of hepatitis B antibody is protective? J Infect Dis 1999; 179:489–492.
- Floreani A, Baldo V, Cristofoletti M, et al. Long-term persistence of anti-HBs after vaccination against HBV: an 18 year experience in health care workers. Vaccine 2004; 22:607–610.
The Advisory Committee on Immunization Practices (ACIP) currently recommends that people who have not completed the three-dose vaccination series against hepatitis B virus (HBV) should receive the missed doses: ie, the three-dose regimen does not need to be restarted.1 However, evidence suggests that a two-dose regimen may provide adequate seroprotection for healthy young adults.
As the three-dose regimen has been shown to protect 90% to 100% of adults,2 it has gained widespread acceptance and is now standard clinical practice.2 However, deviating from the three-dose regimen may not leave healthy young adults vulnerable to HBV infection.
RECOMMENDED DOSES AND SCHEDULES
Widespread use of the three-dose regimen for HBV stemmed from the first clinical evaluation of the recombinant vaccine, in which three 10-μg doses were given at 0, 1, and 6 months to healthy, low-risk adult volunteers.3 This regimen was shown to provide seroprotection in over 95% of adolescents and 90% of healthy adults.2
Currently, three HBV vaccines for adults are approved in the United States: Recombivax HB, Engerix-B, and Twinrix (Table 1). While Recombivax has a seroprotection rate of 89% in healthy adults over age 40, it has higher seroprotection rates in younger people: eg, two doses of Recombivax given 4 to 6 months apart provide seroprotection to 99% of children aged 11 to 15.4 On the other hand, patients on hemodialysis require three 40-μg doses of Recombivax or four 40-μg doses of Engerix-B.
Evidence for a two-dose regimen
Since the development of the recombinant HBV vaccine used today, studies have shown that a two-dose regimen offers seroprotection comparable with, if not better than, the three-dose regimen in adolescents and healthy young adults. Marsano et al5 found that with a two-dose regimen, 96% to 99% of young adults attained seroprotection, with immune memory persisting for up to 2 years.5 Moreover, Cassidy et al6 randomized adolescents to a two-dose or a three-dose regimen and found the two regimens to be equally effective in conferring immunogenicity and immunologic memory.6
Other studies in adolescents have confirmed these findings and offered new evidence in support of the two-dose regimen.7,8 For example, studies found that the two-dose regimen conferred seroprotection at even lower doses than previously studied, and that it conferred immune memory lasting at least 5 years.6,7
However, because these studies were conducted in adolescents and healthy young adults, the findings may not hold true for other populations. Studies suggest that the three-dose regimen is best for those over age 40. Moreover, it is advisable to adhere to a three-dose regimen when treating people at high risk of contracting HBV, such as health care workers; people with chronic liver disease, diabetes mellitus, or end-stage renal disease on hemodialysis; people who have multiple sex partners; and men who have sex with men.
The impact of long intervals between doses
Although the aforementioned studies focused on a two-dose regimen with a 6-month interval, longer intervals between doses do not impair seroprotection and in some cases may even prove beneficial. Heron et al9 demonstrated that a two-dose regimen with a 12-month interval induces seroprotection as effectively as a standard three-dose or two-dose regimen with a 6-month interval.9 Moreover, studies of the impact of deviating from a three-dose regimen found that intervals of longer than 1 year did not impede seroprotection. Not only may seroprotection be attained with intervals of 5 to 10 years before the final dose, but final antibody levels tend to increase with increasing time between doses.10
Nevertheless, even though an extended interval between doses may prove beneficial after the final dose is received, delaying doses may leave patients unprotected. Indeed, alternative three-dose and even four-dose schedules with shorter intervals between doses exist for certain high-risk populations, such as those recently exposed to HBV and travelers to areas of high prevalence. Therefore, intentionally extending intervals between doses may be inappropriate.
SEROPROTECTION AND PROTECTION AGAINST INFECTION
Legitimate concerns exist about the final antibody level attained with a two-dose regimen, which is typically lower than that attained with a three-dose regimen. As HBV antibody levels decline with time, lower final antibody levels theoretically increase the risk of losing seroprotection. Study of vaccine efficacy has defined seroprotection as antibody levels greater than or equal to 10 mIU/mL.11 Yet evidence suggests that even when antibody levels drop below this level, the risk of symptomatic HBV infection does not increase. Evidence also suggests that immune memory outlasts the presence of seroprotective antibody levels, indicating that true protection against significant infection does not necessarily correlate with, and may even exceed, seroprotection.2 This may relate to HBV’s long incubation period, which allows memory cells time to generate an effective immune response.10 For example, Floreani et al12 showed that even though 15% of adults lost seroprotective antibody levels 10 years after vaccination, none demonstrated hepatitis B antigen reactivity or seroconversion.
POSTVACCINATION TESTING AND ADDITIONAL DOSES
At times, it may be wise to measure antibody levels after the final dose to confirm seroprotection. Seroprotection should be documented when knowledge of the patient’s immune status will affect subsequent management. As recommended by the US Centers for Disease Control and Prevention, health care workers, hemodialysis patients, immunocompromised patients, and sexual partners of patients with chronic HBV infection should undergo antibody testing 1 to 2 months after the completion of a three-dose vaccination regimen. Hemodialysis patients require annual confirmation of seroprotection and should receive booster doses of HBV vaccine if necessary.
Postvaccination testing (quantitative HBV surface antibody testing) costs about the same as a single dose of HBV vaccine. Therefore, if postvaccination testing is considered because of missed vaccine doses, it may be more cost-efficient to simply administer the missed dose.
The Advisory Committee on Immunization Practices (ACIP) currently recommends that people who have not completed the three-dose vaccination series against hepatitis B virus (HBV) should receive the missed doses: ie, the three-dose regimen does not need to be restarted.1 However, evidence suggests that a two-dose regimen may provide adequate seroprotection for healthy young adults.
As the three-dose regimen has been shown to protect 90% to 100% of adults,2 it has gained widespread acceptance and is now standard clinical practice.2 However, deviating from the three-dose regimen may not leave healthy young adults vulnerable to HBV infection.
RECOMMENDED DOSES AND SCHEDULES
Widespread use of the three-dose regimen for HBV stemmed from the first clinical evaluation of the recombinant vaccine, in which three 10-μg doses were given at 0, 1, and 6 months to healthy, low-risk adult volunteers.3 This regimen was shown to provide seroprotection in over 95% of adolescents and 90% of healthy adults.2
Currently, three HBV vaccines for adults are approved in the United States: Recombivax HB, Engerix-B, and Twinrix (Table 1). While Recombivax has a seroprotection rate of 89% in healthy adults over age 40, it has higher seroprotection rates in younger people: eg, two doses of Recombivax given 4 to 6 months apart provide seroprotection to 99% of children aged 11 to 15.4 On the other hand, patients on hemodialysis require three 40-μg doses of Recombivax or four 40-μg doses of Engerix-B.
Evidence for a two-dose regimen
Since the development of the recombinant HBV vaccine used today, studies have shown that a two-dose regimen offers seroprotection comparable with, if not better than, the three-dose regimen in adolescents and healthy young adults. Marsano et al5 found that with a two-dose regimen, 96% to 99% of young adults attained seroprotection, with immune memory persisting for up to 2 years.5 Moreover, Cassidy et al6 randomized adolescents to a two-dose or a three-dose regimen and found the two regimens to be equally effective in conferring immunogenicity and immunologic memory.6
Other studies in adolescents have confirmed these findings and offered new evidence in support of the two-dose regimen.7,8 For example, studies found that the two-dose regimen conferred seroprotection at even lower doses than previously studied, and that it conferred immune memory lasting at least 5 years.6,7
However, because these studies were conducted in adolescents and healthy young adults, the findings may not hold true for other populations. Studies suggest that the three-dose regimen is best for those over age 40. Moreover, it is advisable to adhere to a three-dose regimen when treating people at high risk of contracting HBV, such as health care workers; people with chronic liver disease, diabetes mellitus, or end-stage renal disease on hemodialysis; people who have multiple sex partners; and men who have sex with men.
The impact of long intervals between doses
Although the aforementioned studies focused on a two-dose regimen with a 6-month interval, longer intervals between doses do not impair seroprotection and in some cases may even prove beneficial. Heron et al9 demonstrated that a two-dose regimen with a 12-month interval induces seroprotection as effectively as a standard three-dose or two-dose regimen with a 6-month interval.9 Moreover, studies of the impact of deviating from a three-dose regimen found that intervals of longer than 1 year did not impede seroprotection. Not only may seroprotection be attained with intervals of 5 to 10 years before the final dose, but final antibody levels tend to increase with increasing time between doses.10
Nevertheless, even though an extended interval between doses may prove beneficial after the final dose is received, delaying doses may leave patients unprotected. Indeed, alternative three-dose and even four-dose schedules with shorter intervals between doses exist for certain high-risk populations, such as those recently exposed to HBV and travelers to areas of high prevalence. Therefore, intentionally extending intervals between doses may be inappropriate.
SEROPROTECTION AND PROTECTION AGAINST INFECTION
Legitimate concerns exist about the final antibody level attained with a two-dose regimen, which is typically lower than that attained with a three-dose regimen. As HBV antibody levels decline with time, lower final antibody levels theoretically increase the risk of losing seroprotection. Study of vaccine efficacy has defined seroprotection as antibody levels greater than or equal to 10 mIU/mL.11 Yet evidence suggests that even when antibody levels drop below this level, the risk of symptomatic HBV infection does not increase. Evidence also suggests that immune memory outlasts the presence of seroprotective antibody levels, indicating that true protection against significant infection does not necessarily correlate with, and may even exceed, seroprotection.2 This may relate to HBV’s long incubation period, which allows memory cells time to generate an effective immune response.10 For example, Floreani et al12 showed that even though 15% of adults lost seroprotective antibody levels 10 years after vaccination, none demonstrated hepatitis B antigen reactivity or seroconversion.
POSTVACCINATION TESTING AND ADDITIONAL DOSES
At times, it may be wise to measure antibody levels after the final dose to confirm seroprotection. Seroprotection should be documented when knowledge of the patient’s immune status will affect subsequent management. As recommended by the US Centers for Disease Control and Prevention, health care workers, hemodialysis patients, immunocompromised patients, and sexual partners of patients with chronic HBV infection should undergo antibody testing 1 to 2 months after the completion of a three-dose vaccination regimen. Hemodialysis patients require annual confirmation of seroprotection and should receive booster doses of HBV vaccine if necessary.
Postvaccination testing (quantitative HBV surface antibody testing) costs about the same as a single dose of HBV vaccine. Therefore, if postvaccination testing is considered because of missed vaccine doses, it may be more cost-efficient to simply administer the missed dose.
- Department of Health and Human Services. Appendix A Immunization Management Issues. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5516a2.htm. Accessed April 6, 2014.
- Leuridan E, Van Damme P. Hepatitis B and the need for a booster dose. Clin Infect Dis 2011; 53:68–75.
- Scolnick EM, McLean AA, West DJ, McAleer WJ, Miller WJ, Buynak EB. Clinical evaluation in healthy adults of a hepatitis B vaccine made by recombinant DNA. JAMA 1984; 251:2812–2815.
- Merck and Co, Inc. 1998. Recombivax HB. http://www.merck.com/product/usa/pi_circulars/r/recombivax_hb/re-combivax_pi.pdf. Accessed April 7, 2014.
- Marsano LS, West DJ, Chan I, et al. A two-dose hepatitis B vaccine regimen: proof of priming and memory responses in young adults. Vaccine 1998; 16:624–629.
- Cassidy WM, Watson B, Ioli VA, Williams K, Bird S, West DJ. A randomized trial of alternative two- and three-dose hepatitis B vaccination regimens in adolescents: antibody responses, safety, and immunologic memory. Pediatrics 2001; 107:626–631.
- Van Damme P, Moiseeva A, Marichev I, et al. Five years follow-up following two or three doses of a hepatitis B vaccine in adolescents aged 11–15 years: a randomised controlled study. BMC Infect Dis 2010; 10:357.
- Heron L, Selnikova O, Moiseieva A, et al. Immunogenicity, reactogenicity and safety of two-dose versus three-dose (standard care) hepatitis B immunisation of healthy adolescents aged 11–15 years: a randomised controlled trial. Vaccine 2007; 25:2817–2822.
- Heron LG, Chant KG, Jalaludin BB. A novel hepatitis B vaccination regimen for adolescents: two doses 12 months apart. Vaccine 2002; 20:3472–3476.
- Jackson Y, Chappuis F, Mezger N, Kanappa K, Loutan L. High immunogenicity of delayed third dose of hepatitis B vaccine in travellers. Vaccine 2007; 25:3482–3484.
- Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC. What level of hepatitis B antibody is protective? J Infect Dis 1999; 179:489–492.
- Floreani A, Baldo V, Cristofoletti M, et al. Long-term persistence of anti-HBs after vaccination against HBV: an 18 year experience in health care workers. Vaccine 2004; 22:607–610.
- Department of Health and Human Services. Appendix A Immunization Management Issues. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5516a2.htm. Accessed April 6, 2014.
- Leuridan E, Van Damme P. Hepatitis B and the need for a booster dose. Clin Infect Dis 2011; 53:68–75.
- Scolnick EM, McLean AA, West DJ, McAleer WJ, Miller WJ, Buynak EB. Clinical evaluation in healthy adults of a hepatitis B vaccine made by recombinant DNA. JAMA 1984; 251:2812–2815.
- Merck and Co, Inc. 1998. Recombivax HB. http://www.merck.com/product/usa/pi_circulars/r/recombivax_hb/re-combivax_pi.pdf. Accessed April 7, 2014.
- Marsano LS, West DJ, Chan I, et al. A two-dose hepatitis B vaccine regimen: proof of priming and memory responses in young adults. Vaccine 1998; 16:624–629.
- Cassidy WM, Watson B, Ioli VA, Williams K, Bird S, West DJ. A randomized trial of alternative two- and three-dose hepatitis B vaccination regimens in adolescents: antibody responses, safety, and immunologic memory. Pediatrics 2001; 107:626–631.
- Van Damme P, Moiseeva A, Marichev I, et al. Five years follow-up following two or three doses of a hepatitis B vaccine in adolescents aged 11–15 years: a randomised controlled study. BMC Infect Dis 2010; 10:357.
- Heron L, Selnikova O, Moiseieva A, et al. Immunogenicity, reactogenicity and safety of two-dose versus three-dose (standard care) hepatitis B immunisation of healthy adolescents aged 11–15 years: a randomised controlled trial. Vaccine 2007; 25:2817–2822.
- Heron LG, Chant KG, Jalaludin BB. A novel hepatitis B vaccination regimen for adolescents: two doses 12 months apart. Vaccine 2002; 20:3472–3476.
- Jackson Y, Chappuis F, Mezger N, Kanappa K, Loutan L. High immunogenicity of delayed third dose of hepatitis B vaccine in travellers. Vaccine 2007; 25:3482–3484.
- Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC. What level of hepatitis B antibody is protective? J Infect Dis 1999; 179:489–492.
- Floreani A, Baldo V, Cristofoletti M, et al. Long-term persistence of anti-HBs after vaccination against HBV: an 18 year experience in health care workers. Vaccine 2004; 22:607–610.
Ascites in a 42-year-old woman
A 42-year-old woman is admitted to the hospital with worsening shortness of breath on exertion, poor exercise tolerance, leg edema, and swelling of the abdomen. Her symptoms have been getting worse over the last 4 months. She reports no history of fever, chills, night sweats, bleeding disorder, joint pain, weight loss, or loss of appetite.
She has type 2 diabetes mellitus and hypothyroidism. She had rheumatoid arthritis but said it was “inactive,” not requiring treatment for the last 18 years. Three months ago, she underwent a total hysterectomy and salpingo-oophorectomy for a complex adnexal mass, biopsy of which revealed a benign mucinous ovarian cyst.
Her current medications include furosemide, levothyroxine, and metformin. She is an ex-smoker with a 7 pack-year history. She drinks a glass of wine on social occasions only. Her family history is unremarkable.
On examination, she is not in distress and she has no fever. She has jugular venous distention of 5 cm, tense ascites, and marked edema of the legs, as well as hyperpigmented patches and erythematous plaques over both shins. Neck palpation reveals no lymphadenopathy or thyromegaly.
Her liver and the tip of the spleen are palpable following paracentesis, once ascitic fluid is removed.
The cardiovascular examination is normal. Chest auscultation reveals decreased breath sounds at the right lung base with bibasilar crackles. No focal neurologic deficit is noted on clinical examination.
Laboratory testing at the time of hospital admission (Table 1) includes a hepatitis panel (negative for exposure to hepatitis A, B, and C) and ascitic fluid studies. Chest radiography shows a right pleural effusion. Echocardiography demonstrates moderate pericardial effusion without tamponade; left and right ventricular function is normal. Cardiac magnetic resonance imaging finds no evidence of pericardial constriction or restrictive cardiomyopathy. Pressures are normal on pulmonary artery catheterization.
FINDING THE CAUSE OF ASCITES
1. What is the most likely cause of ascites in this patient?
- Cirrhosis
- Recent abdominal surgery
- Congestive heart failure
- Abdominal malignancy
- Nephrotic syndrome
The serum-ascites albumin gradient—ie, the serum albumin concentration minus the ascitic fluid albumin concentration—helps determine whether ascites is related to portal hypertension.1 A high gradient (ie, above 1.1 g/dL) is seen in cirrhosis, alcoholic hepatitis, congestive heart failure, vascular occlusion syndromes (eg, Budd-Chiari syndrome), and metastatic liver disease.
From the values in Table 1, our patient’s gradient is 0.8 g/dL, which is considered low. However, we cannot completely rule out cirrhosis as the cause of her ascites because she was taking a diuretic, and diuretics can falsely decrease the gradient. Heart failure is unlikely, based on the results of echocardiography and catheterization. In addition, the 24-hour urinary protein concentration is normal, as is alpha-1 antitrypsin secretion in the stool, ruling out protein-losing nephropathy or enteropathy as the cause of her low albumin and ascites.
A high triglyceride content in her ascitic fluid (> 150 mg/dL) is consistent with chylous ascites, which is seen in patients with previous abdominal surgery or with lymphatic obstruction due to malignancy. A high neutrophil count in the ascitic fluid and a negative culture are also consistent with chylous ascites. However, in this patient, recent surgery as the cause of chylous ascites does not explain the systemic features of hepatosplenomegaly, anemia, thrombocytosis, and low albumin. Moreover, her high C-reactive protein value suggests an ongoing inflammatory process, although her erythrocyte sedimentation rate is not significantly elevated.
Therefore, the most likely cause of ascites in this patient is abdominal malignancy.
WHAT SHOULD BE DONE NEXT?
2. Which of the following studies is reasonable in this patient at this point?
- Serum protein electrophoresis
- Computed tomography (CT) of the chest, abdomen, and pelvis
- Liver biopsy
- Cytologic study of the ascitic fluid
All of these studies would be reasonable and in fact were done in this patient.
Serum protein electrophoresis (Table 2) identified a monoclonal protein band in the immunoglobulin G (IgG) kappa region.
Cytologic study of the ascitic fluid was negative for malignant cells.
Chest CT revealed bilateral pleural effusions, pericardial effusion, and bilateral axillary lymphadenopathy. CT of the abdomen and pelvis was normal, except for ascites, and no pelvic tumor was noted.
Liver biopsy was done to look for the source of her unexplained ascites with elevated alkaline phosphatase, as all other investigations so far were normal. It revealed mild centrilobular scarring, but the rest of the parenchymal architecture was normal, with no evidence of bridging fibrosis or nodular regenerative hyperplasia (Figure 1).
Transjugular measurement of the hepatic vein pressure revealed a hepatic vein pressure gradient of 9 mm Hg, indicating mild portal hypertension. Venography showed widely patent hepatic and portal veins. Her high inflammatory marker levels could have been caused by smoldering rheumatoid arthritis; however, since the patient has had no joint symptoms for 18 years, this is very unlikely. It is more likely to be caused by a plasma cell disorder, as suggested by a monoclonal protein on electrophoresis.
WHAT IS THE DIAGNOSIS?
3. What is the most likely diagnosis in our patient?
- Rheumatoid arthritis
- Cryoglobulinemia
- Capillary leak syndrome
- Hematologic malignancy
- Syndrome of polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, and skin changes (POEMS syndrome)
Rheumatoid arthritis can present with hepatosplenomegaly, lymphadenopathy, ascites, and skin rash, particularly if antinuclear antibody and rheumatoid factor are elevated. Ascites is known to occur in association with rheumatoid arthritis in the setting of Felty syndrome or nodular regenerative hyperplasia of the liver.2 However, our patient did not have leukopenia or evidence of regenerative hyperplasia on liver biopsy. Moreover, her rheumatoid arthritis had remained clinically inactive for a long time.
Cryoglobulinemia was possible, given her ascites, neuropathy, and splenomegaly, but her serum hepatic antibody and C4 complement values were normal.3 Also, the appearance of her rash was not typical of cryoglobulinemia.
Capillary leak syndrome was ruled out by the absence of hypotensive episodes, edema of the face or upper extremities, or renal failure.4
Lymphoma was excluded by flow cytometry.
A monoclonal protein on serum electrophoresis may suggest multiple myeloma, but this patient had multisystem involvement including organomegaly, endocrinopathy, and skin abnormalities. Thus, POEMS syndrome is the most likely diagnosis.
4. Which test should be done at this time to confirm the diagnosis of POEMS syndrome?
- Bone marrow biopsy
- Vascular endothelial growth factor testing
- Nerve conduction study
- Complete x-ray bone survey
A test for vascular endothelial growth factor should be done. This growth factor is almost always elevated in POEMS, and a positive test helps confirm the diagnosis of POEMS. Our patient’s level was elevated at 1,664 pg/mL (reference range 31–86).
POEMS is thought to be a variant of plasma cell dyscrasia, and all patients with POEMS have a monoclonal protein on electrophoresis. On this background, multiple myeloma is an important consideration.
Our patient underwent bone marrow biopsy, which revealed mild plasmacytosis (< 10%) (Figure 2). A complete bone survey showed generalized osteopenia without blastic or lytic lesions. To complete the workup for POEMS syndrome, a nerve conduction study was done to look for neuropathy; it showed bilateral sensory motor neuropathy with features of both a demyelinating process and axonal loss.
POEMS SYNDROME
POEMS syndrome is a constellation of features such as organomegaly and endocrine and skin abnormalities in association with neuropathy and a monoclonal protein on electrophoresis.5 In 2003, Dispenzieri et al6 described the major and minor diagnostic criteria based on a retrospective analysis of 99 patients with POEMS syndrome.6 Later, elevated vascular endothelial growth factor was added as a confirmatory diagnostic criterion.7 This growth factor is also an indicator of prognosis in POEMS syndrome, and its level can be used to monitor the response to treatment.7
Our patient met both major criteria for POEMS syndrome, ie, polyneuropathy (based on nerve conduction studies) and a monoclonal protein. Polyneuropathy in POEMS syndrome usually occurs as sensorimotor peripheral neuropathy of insidious onset and is seldom painful. Nerve biopsy study reveals demyelination with features of axonal loss. Interestingly, although our patient had neuropathy as diagnosed by electromyography, she remained clinically asymptomatic.
The monoclonal protein in POEMS syndrome is commonly IgA or IgG. Light chains are always present and are mainly the lambda type; kappa light chains are also reported in rare cases. Our patient had IgG kappa light chains.
Our patient met a number of the minor criteria for POEMS syndrome: ie, organomegaly (hepatosplenomegaly, lymphadenopathy), endocrinopathy (hypothyroidism, diabetes), skin changes (hyperpigmentation and plaques of the lower extremities), edema, pleural effusion, and ascites.
Endocrine disorders in POEMS syndrome
The endocrine abnormalities most often described in POEMS syndrome are hypogonadism, hypothyroidism, and diabetes mellitus. But because hypothyroidism and diabetes are common in the general population, it is debatable whether either of these could constitute the endocrine component of POEMS syndrome. Nevertheless, in three large series,6,7 occurrences of these two disorders were common, although less specific than adrenal or pituitary involvement.
In the analysis by Dispenzieri et al,6 67% of patients had at least one endocrine abnormality. Our patient had no evidence of an adrenal disorder.
Skin, skeletal, and other changes
The skin changes in POEMS syndrome are often nonspecific and include hyperpigmentation, sclerodema-like thickening, and plaques.
Skeletal changes are noted in up to 97% of patients. A skeletal survey in our patient revealed generalized osteopenia as opposed to osteosclerotic lesions, which are common in POEMS syndrome.
Anemia and thrombocytosis (as in our patient) are usually seen in POEMS syndrome and are induced by cytokines.6 POEMS syndrome also leads to increased thrombotic complications from the release of inflammatory cytokines.
Hypoalbuminemia and anasarca including ascites are often seen in POEMS syndrome (prevalence 29% to 89%) and are attributed to cytokine-induced increased vascular permeability. In POEMS syndrome, the serum-ascites albumin gradient is usually less than 1.1 g/dL, as in our patient.
Stepani et al8 reported one case of culture-negative neutrocytic ascites with portal hypertension in POEMS syndrome.8 (Culture-negative neutrocytic ascites is defined as an ascitic fluid polymorphonuclear count greater than 250/mm3 and a negative ascitic fluid culture in the absence of previous antibiotic therapy.) Chylous ascites has not yet been described in POEMS syndrome. However, chylous ascites is predominantly lymphocytic, whereas our patient had neutrocytic ascites.
We concluded that the cause of our patient’s ascites was multifactorial and included previous surgery and POEMS syndrome.
Nonclassic presentation
In addition to its classic presentation, POEMS syndrome is often reported in association with other “unusual features” such as cardiomyopathy, pulmonary hypertension, and cryoglobulinemia.6
So far, very few cases of portal hypertension in POEMS syndrome have been reported. Stepani et al8 described a patient who had POEMS syndrome and portal hypertension with extensive portal fibrosis without cirrhosis on liver biopsy. Inoue et al9 reported a liver biopsy feature consistent with idiopathic portal hypertension, also noting a case with mild fibrosis and few lymphocytic infiltrates in the portal tract.9
The etiopathogenesis of POEMS syndrome is attributed to proangiogenic vascular endothelial growth factor, and other inflammatory cytokines (interleukin 6, interleukin 1 beta, tumor necrosis factor alpha) also play a key role in pulmonary hypertension.10,11 A similar pathogenesis could also contribute to the development of portal hypertension (Figure 3).
CASE CONCLUDED
We started our patient on oral prednisone 60 mg daily for a month, tapered to a maintenance dose of 15 mg to suppress clonal proliferation of plasma cells. Her symptoms improved. Her vascular endothelial growth factor level decreased from 1,664 to 624 pg/mL. She was enrolled in a National Institutes of Health study to evaluate the effect of a potential new immunomodulator treatment for POEMS syndrome.
In conclusion, POEMS syndrome is rare and can present with many atypical features. A high index of suspicion is needed to detect it in a patient who has noncirrhotic portal hypertension with ascites and multisystem involvement.
- Runyon BA, Montano AA, Akriviadis EA, Antillon MR, Irving MA, McHutchison JG. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med 1992; 117:215–220.
- Harris M, Rash RM, Dymock IW. Nodular, non-cirrhotic liver associated with portal hypertension in a patient with rheumatoid arthritis. J Clin Pathol 1974; 27:963–966.
- Ramos-Casals M, Stone JH, Cid MC, Bosch X. The cryoglobulinaemias. Lancet 2012; 379:348–360.
- Druey KM, Greipp PR. Narrative review: the systemic capillary leak syndrome. Ann Intern Med 2010; 153:90–98.
- Bardwick PA, Zvaifler NJ, Gill GN, Newman D, Greenway GD, Resnick DL. Plasma cell dyscrasia with polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes: the POEMS syndrome. Report on two cases and a review of the literature. Medicine (Baltimore) 1980; 59:311–322.
- Dispenzieri A, Kyle RA, Lacy MQ, et al. POEMS syndrome: definitions and long-term outcome. Blood 2003; 101:2496–2506.
- Dispenzieri A. POEMS syndrome. Blood Rev 2007; 21:285–299.
- Stepani P, Courouble Y, Postel P, et al. Portal hypertension and neutrocytic ascites in POEMS syndrome. Gastroenterol Clin Biol 1998; 22:1095–1097. Article in French.
- Inoue R, Nakazawa A, Tsukada N, et al. POEMS syndrome with idiopathic portal hypertension: autopsy case and review of the literature. Pathol Int 2010; 60:316–320.
- Gherardi RK, Bélec L, Soubrier M, et al. Overproduction of proinflammatory cytokines imbalanced by their antagonists in POEMS syndrome. Blood 1996; 87:1458–1465.
- Mukerjee D, Kingdon E, Vanderpump M, Coghlan JG. Pathophysiological insights from a case of reversible pulmonary arterial hypertension. J R Soc Med 2003; 96:403–404.
A 42-year-old woman is admitted to the hospital with worsening shortness of breath on exertion, poor exercise tolerance, leg edema, and swelling of the abdomen. Her symptoms have been getting worse over the last 4 months. She reports no history of fever, chills, night sweats, bleeding disorder, joint pain, weight loss, or loss of appetite.
She has type 2 diabetes mellitus and hypothyroidism. She had rheumatoid arthritis but said it was “inactive,” not requiring treatment for the last 18 years. Three months ago, she underwent a total hysterectomy and salpingo-oophorectomy for a complex adnexal mass, biopsy of which revealed a benign mucinous ovarian cyst.
Her current medications include furosemide, levothyroxine, and metformin. She is an ex-smoker with a 7 pack-year history. She drinks a glass of wine on social occasions only. Her family history is unremarkable.
On examination, she is not in distress and she has no fever. She has jugular venous distention of 5 cm, tense ascites, and marked edema of the legs, as well as hyperpigmented patches and erythematous plaques over both shins. Neck palpation reveals no lymphadenopathy or thyromegaly.
Her liver and the tip of the spleen are palpable following paracentesis, once ascitic fluid is removed.
The cardiovascular examination is normal. Chest auscultation reveals decreased breath sounds at the right lung base with bibasilar crackles. No focal neurologic deficit is noted on clinical examination.
Laboratory testing at the time of hospital admission (Table 1) includes a hepatitis panel (negative for exposure to hepatitis A, B, and C) and ascitic fluid studies. Chest radiography shows a right pleural effusion. Echocardiography demonstrates moderate pericardial effusion without tamponade; left and right ventricular function is normal. Cardiac magnetic resonance imaging finds no evidence of pericardial constriction or restrictive cardiomyopathy. Pressures are normal on pulmonary artery catheterization.
FINDING THE CAUSE OF ASCITES
1. What is the most likely cause of ascites in this patient?
- Cirrhosis
- Recent abdominal surgery
- Congestive heart failure
- Abdominal malignancy
- Nephrotic syndrome
The serum-ascites albumin gradient—ie, the serum albumin concentration minus the ascitic fluid albumin concentration—helps determine whether ascites is related to portal hypertension.1 A high gradient (ie, above 1.1 g/dL) is seen in cirrhosis, alcoholic hepatitis, congestive heart failure, vascular occlusion syndromes (eg, Budd-Chiari syndrome), and metastatic liver disease.
From the values in Table 1, our patient’s gradient is 0.8 g/dL, which is considered low. However, we cannot completely rule out cirrhosis as the cause of her ascites because she was taking a diuretic, and diuretics can falsely decrease the gradient. Heart failure is unlikely, based on the results of echocardiography and catheterization. In addition, the 24-hour urinary protein concentration is normal, as is alpha-1 antitrypsin secretion in the stool, ruling out protein-losing nephropathy or enteropathy as the cause of her low albumin and ascites.
A high triglyceride content in her ascitic fluid (> 150 mg/dL) is consistent with chylous ascites, which is seen in patients with previous abdominal surgery or with lymphatic obstruction due to malignancy. A high neutrophil count in the ascitic fluid and a negative culture are also consistent with chylous ascites. However, in this patient, recent surgery as the cause of chylous ascites does not explain the systemic features of hepatosplenomegaly, anemia, thrombocytosis, and low albumin. Moreover, her high C-reactive protein value suggests an ongoing inflammatory process, although her erythrocyte sedimentation rate is not significantly elevated.
Therefore, the most likely cause of ascites in this patient is abdominal malignancy.
WHAT SHOULD BE DONE NEXT?
2. Which of the following studies is reasonable in this patient at this point?
- Serum protein electrophoresis
- Computed tomography (CT) of the chest, abdomen, and pelvis
- Liver biopsy
- Cytologic study of the ascitic fluid
All of these studies would be reasonable and in fact were done in this patient.
Serum protein electrophoresis (Table 2) identified a monoclonal protein band in the immunoglobulin G (IgG) kappa region.
Cytologic study of the ascitic fluid was negative for malignant cells.
Chest CT revealed bilateral pleural effusions, pericardial effusion, and bilateral axillary lymphadenopathy. CT of the abdomen and pelvis was normal, except for ascites, and no pelvic tumor was noted.
Liver biopsy was done to look for the source of her unexplained ascites with elevated alkaline phosphatase, as all other investigations so far were normal. It revealed mild centrilobular scarring, but the rest of the parenchymal architecture was normal, with no evidence of bridging fibrosis or nodular regenerative hyperplasia (Figure 1).
Transjugular measurement of the hepatic vein pressure revealed a hepatic vein pressure gradient of 9 mm Hg, indicating mild portal hypertension. Venography showed widely patent hepatic and portal veins. Her high inflammatory marker levels could have been caused by smoldering rheumatoid arthritis; however, since the patient has had no joint symptoms for 18 years, this is very unlikely. It is more likely to be caused by a plasma cell disorder, as suggested by a monoclonal protein on electrophoresis.
WHAT IS THE DIAGNOSIS?
3. What is the most likely diagnosis in our patient?
- Rheumatoid arthritis
- Cryoglobulinemia
- Capillary leak syndrome
- Hematologic malignancy
- Syndrome of polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, and skin changes (POEMS syndrome)
Rheumatoid arthritis can present with hepatosplenomegaly, lymphadenopathy, ascites, and skin rash, particularly if antinuclear antibody and rheumatoid factor are elevated. Ascites is known to occur in association with rheumatoid arthritis in the setting of Felty syndrome or nodular regenerative hyperplasia of the liver.2 However, our patient did not have leukopenia or evidence of regenerative hyperplasia on liver biopsy. Moreover, her rheumatoid arthritis had remained clinically inactive for a long time.
Cryoglobulinemia was possible, given her ascites, neuropathy, and splenomegaly, but her serum hepatic antibody and C4 complement values were normal.3 Also, the appearance of her rash was not typical of cryoglobulinemia.
Capillary leak syndrome was ruled out by the absence of hypotensive episodes, edema of the face or upper extremities, or renal failure.4
Lymphoma was excluded by flow cytometry.
A monoclonal protein on serum electrophoresis may suggest multiple myeloma, but this patient had multisystem involvement including organomegaly, endocrinopathy, and skin abnormalities. Thus, POEMS syndrome is the most likely diagnosis.
4. Which test should be done at this time to confirm the diagnosis of POEMS syndrome?
- Bone marrow biopsy
- Vascular endothelial growth factor testing
- Nerve conduction study
- Complete x-ray bone survey
A test for vascular endothelial growth factor should be done. This growth factor is almost always elevated in POEMS, and a positive test helps confirm the diagnosis of POEMS. Our patient’s level was elevated at 1,664 pg/mL (reference range 31–86).
POEMS is thought to be a variant of plasma cell dyscrasia, and all patients with POEMS have a monoclonal protein on electrophoresis. On this background, multiple myeloma is an important consideration.
Our patient underwent bone marrow biopsy, which revealed mild plasmacytosis (< 10%) (Figure 2). A complete bone survey showed generalized osteopenia without blastic or lytic lesions. To complete the workup for POEMS syndrome, a nerve conduction study was done to look for neuropathy; it showed bilateral sensory motor neuropathy with features of both a demyelinating process and axonal loss.
POEMS SYNDROME
POEMS syndrome is a constellation of features such as organomegaly and endocrine and skin abnormalities in association with neuropathy and a monoclonal protein on electrophoresis.5 In 2003, Dispenzieri et al6 described the major and minor diagnostic criteria based on a retrospective analysis of 99 patients with POEMS syndrome.6 Later, elevated vascular endothelial growth factor was added as a confirmatory diagnostic criterion.7 This growth factor is also an indicator of prognosis in POEMS syndrome, and its level can be used to monitor the response to treatment.7
Our patient met both major criteria for POEMS syndrome, ie, polyneuropathy (based on nerve conduction studies) and a monoclonal protein. Polyneuropathy in POEMS syndrome usually occurs as sensorimotor peripheral neuropathy of insidious onset and is seldom painful. Nerve biopsy study reveals demyelination with features of axonal loss. Interestingly, although our patient had neuropathy as diagnosed by electromyography, she remained clinically asymptomatic.
The monoclonal protein in POEMS syndrome is commonly IgA or IgG. Light chains are always present and are mainly the lambda type; kappa light chains are also reported in rare cases. Our patient had IgG kappa light chains.
Our patient met a number of the minor criteria for POEMS syndrome: ie, organomegaly (hepatosplenomegaly, lymphadenopathy), endocrinopathy (hypothyroidism, diabetes), skin changes (hyperpigmentation and plaques of the lower extremities), edema, pleural effusion, and ascites.
Endocrine disorders in POEMS syndrome
The endocrine abnormalities most often described in POEMS syndrome are hypogonadism, hypothyroidism, and diabetes mellitus. But because hypothyroidism and diabetes are common in the general population, it is debatable whether either of these could constitute the endocrine component of POEMS syndrome. Nevertheless, in three large series,6,7 occurrences of these two disorders were common, although less specific than adrenal or pituitary involvement.
In the analysis by Dispenzieri et al,6 67% of patients had at least one endocrine abnormality. Our patient had no evidence of an adrenal disorder.
Skin, skeletal, and other changes
The skin changes in POEMS syndrome are often nonspecific and include hyperpigmentation, sclerodema-like thickening, and plaques.
Skeletal changes are noted in up to 97% of patients. A skeletal survey in our patient revealed generalized osteopenia as opposed to osteosclerotic lesions, which are common in POEMS syndrome.
Anemia and thrombocytosis (as in our patient) are usually seen in POEMS syndrome and are induced by cytokines.6 POEMS syndrome also leads to increased thrombotic complications from the release of inflammatory cytokines.
Hypoalbuminemia and anasarca including ascites are often seen in POEMS syndrome (prevalence 29% to 89%) and are attributed to cytokine-induced increased vascular permeability. In POEMS syndrome, the serum-ascites albumin gradient is usually less than 1.1 g/dL, as in our patient.
Stepani et al8 reported one case of culture-negative neutrocytic ascites with portal hypertension in POEMS syndrome.8 (Culture-negative neutrocytic ascites is defined as an ascitic fluid polymorphonuclear count greater than 250/mm3 and a negative ascitic fluid culture in the absence of previous antibiotic therapy.) Chylous ascites has not yet been described in POEMS syndrome. However, chylous ascites is predominantly lymphocytic, whereas our patient had neutrocytic ascites.
We concluded that the cause of our patient’s ascites was multifactorial and included previous surgery and POEMS syndrome.
Nonclassic presentation
In addition to its classic presentation, POEMS syndrome is often reported in association with other “unusual features” such as cardiomyopathy, pulmonary hypertension, and cryoglobulinemia.6
So far, very few cases of portal hypertension in POEMS syndrome have been reported. Stepani et al8 described a patient who had POEMS syndrome and portal hypertension with extensive portal fibrosis without cirrhosis on liver biopsy. Inoue et al9 reported a liver biopsy feature consistent with idiopathic portal hypertension, also noting a case with mild fibrosis and few lymphocytic infiltrates in the portal tract.9
The etiopathogenesis of POEMS syndrome is attributed to proangiogenic vascular endothelial growth factor, and other inflammatory cytokines (interleukin 6, interleukin 1 beta, tumor necrosis factor alpha) also play a key role in pulmonary hypertension.10,11 A similar pathogenesis could also contribute to the development of portal hypertension (Figure 3).
CASE CONCLUDED
We started our patient on oral prednisone 60 mg daily for a month, tapered to a maintenance dose of 15 mg to suppress clonal proliferation of plasma cells. Her symptoms improved. Her vascular endothelial growth factor level decreased from 1,664 to 624 pg/mL. She was enrolled in a National Institutes of Health study to evaluate the effect of a potential new immunomodulator treatment for POEMS syndrome.
In conclusion, POEMS syndrome is rare and can present with many atypical features. A high index of suspicion is needed to detect it in a patient who has noncirrhotic portal hypertension with ascites and multisystem involvement.
A 42-year-old woman is admitted to the hospital with worsening shortness of breath on exertion, poor exercise tolerance, leg edema, and swelling of the abdomen. Her symptoms have been getting worse over the last 4 months. She reports no history of fever, chills, night sweats, bleeding disorder, joint pain, weight loss, or loss of appetite.
She has type 2 diabetes mellitus and hypothyroidism. She had rheumatoid arthritis but said it was “inactive,” not requiring treatment for the last 18 years. Three months ago, she underwent a total hysterectomy and salpingo-oophorectomy for a complex adnexal mass, biopsy of which revealed a benign mucinous ovarian cyst.
Her current medications include furosemide, levothyroxine, and metformin. She is an ex-smoker with a 7 pack-year history. She drinks a glass of wine on social occasions only. Her family history is unremarkable.
On examination, she is not in distress and she has no fever. She has jugular venous distention of 5 cm, tense ascites, and marked edema of the legs, as well as hyperpigmented patches and erythematous plaques over both shins. Neck palpation reveals no lymphadenopathy or thyromegaly.
Her liver and the tip of the spleen are palpable following paracentesis, once ascitic fluid is removed.
The cardiovascular examination is normal. Chest auscultation reveals decreased breath sounds at the right lung base with bibasilar crackles. No focal neurologic deficit is noted on clinical examination.
Laboratory testing at the time of hospital admission (Table 1) includes a hepatitis panel (negative for exposure to hepatitis A, B, and C) and ascitic fluid studies. Chest radiography shows a right pleural effusion. Echocardiography demonstrates moderate pericardial effusion without tamponade; left and right ventricular function is normal. Cardiac magnetic resonance imaging finds no evidence of pericardial constriction or restrictive cardiomyopathy. Pressures are normal on pulmonary artery catheterization.
FINDING THE CAUSE OF ASCITES
1. What is the most likely cause of ascites in this patient?
- Cirrhosis
- Recent abdominal surgery
- Congestive heart failure
- Abdominal malignancy
- Nephrotic syndrome
The serum-ascites albumin gradient—ie, the serum albumin concentration minus the ascitic fluid albumin concentration—helps determine whether ascites is related to portal hypertension.1 A high gradient (ie, above 1.1 g/dL) is seen in cirrhosis, alcoholic hepatitis, congestive heart failure, vascular occlusion syndromes (eg, Budd-Chiari syndrome), and metastatic liver disease.
From the values in Table 1, our patient’s gradient is 0.8 g/dL, which is considered low. However, we cannot completely rule out cirrhosis as the cause of her ascites because she was taking a diuretic, and diuretics can falsely decrease the gradient. Heart failure is unlikely, based on the results of echocardiography and catheterization. In addition, the 24-hour urinary protein concentration is normal, as is alpha-1 antitrypsin secretion in the stool, ruling out protein-losing nephropathy or enteropathy as the cause of her low albumin and ascites.
A high triglyceride content in her ascitic fluid (> 150 mg/dL) is consistent with chylous ascites, which is seen in patients with previous abdominal surgery or with lymphatic obstruction due to malignancy. A high neutrophil count in the ascitic fluid and a negative culture are also consistent with chylous ascites. However, in this patient, recent surgery as the cause of chylous ascites does not explain the systemic features of hepatosplenomegaly, anemia, thrombocytosis, and low albumin. Moreover, her high C-reactive protein value suggests an ongoing inflammatory process, although her erythrocyte sedimentation rate is not significantly elevated.
Therefore, the most likely cause of ascites in this patient is abdominal malignancy.
WHAT SHOULD BE DONE NEXT?
2. Which of the following studies is reasonable in this patient at this point?
- Serum protein electrophoresis
- Computed tomography (CT) of the chest, abdomen, and pelvis
- Liver biopsy
- Cytologic study of the ascitic fluid
All of these studies would be reasonable and in fact were done in this patient.
Serum protein electrophoresis (Table 2) identified a monoclonal protein band in the immunoglobulin G (IgG) kappa region.
Cytologic study of the ascitic fluid was negative for malignant cells.
Chest CT revealed bilateral pleural effusions, pericardial effusion, and bilateral axillary lymphadenopathy. CT of the abdomen and pelvis was normal, except for ascites, and no pelvic tumor was noted.
Liver biopsy was done to look for the source of her unexplained ascites with elevated alkaline phosphatase, as all other investigations so far were normal. It revealed mild centrilobular scarring, but the rest of the parenchymal architecture was normal, with no evidence of bridging fibrosis or nodular regenerative hyperplasia (Figure 1).
Transjugular measurement of the hepatic vein pressure revealed a hepatic vein pressure gradient of 9 mm Hg, indicating mild portal hypertension. Venography showed widely patent hepatic and portal veins. Her high inflammatory marker levels could have been caused by smoldering rheumatoid arthritis; however, since the patient has had no joint symptoms for 18 years, this is very unlikely. It is more likely to be caused by a plasma cell disorder, as suggested by a monoclonal protein on electrophoresis.
WHAT IS THE DIAGNOSIS?
3. What is the most likely diagnosis in our patient?
- Rheumatoid arthritis
- Cryoglobulinemia
- Capillary leak syndrome
- Hematologic malignancy
- Syndrome of polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, and skin changes (POEMS syndrome)
Rheumatoid arthritis can present with hepatosplenomegaly, lymphadenopathy, ascites, and skin rash, particularly if antinuclear antibody and rheumatoid factor are elevated. Ascites is known to occur in association with rheumatoid arthritis in the setting of Felty syndrome or nodular regenerative hyperplasia of the liver.2 However, our patient did not have leukopenia or evidence of regenerative hyperplasia on liver biopsy. Moreover, her rheumatoid arthritis had remained clinically inactive for a long time.
Cryoglobulinemia was possible, given her ascites, neuropathy, and splenomegaly, but her serum hepatic antibody and C4 complement values were normal.3 Also, the appearance of her rash was not typical of cryoglobulinemia.
Capillary leak syndrome was ruled out by the absence of hypotensive episodes, edema of the face or upper extremities, or renal failure.4
Lymphoma was excluded by flow cytometry.
A monoclonal protein on serum electrophoresis may suggest multiple myeloma, but this patient had multisystem involvement including organomegaly, endocrinopathy, and skin abnormalities. Thus, POEMS syndrome is the most likely diagnosis.
4. Which test should be done at this time to confirm the diagnosis of POEMS syndrome?
- Bone marrow biopsy
- Vascular endothelial growth factor testing
- Nerve conduction study
- Complete x-ray bone survey
A test for vascular endothelial growth factor should be done. This growth factor is almost always elevated in POEMS, and a positive test helps confirm the diagnosis of POEMS. Our patient’s level was elevated at 1,664 pg/mL (reference range 31–86).
POEMS is thought to be a variant of plasma cell dyscrasia, and all patients with POEMS have a monoclonal protein on electrophoresis. On this background, multiple myeloma is an important consideration.
Our patient underwent bone marrow biopsy, which revealed mild plasmacytosis (< 10%) (Figure 2). A complete bone survey showed generalized osteopenia without blastic or lytic lesions. To complete the workup for POEMS syndrome, a nerve conduction study was done to look for neuropathy; it showed bilateral sensory motor neuropathy with features of both a demyelinating process and axonal loss.
POEMS SYNDROME
POEMS syndrome is a constellation of features such as organomegaly and endocrine and skin abnormalities in association with neuropathy and a monoclonal protein on electrophoresis.5 In 2003, Dispenzieri et al6 described the major and minor diagnostic criteria based on a retrospective analysis of 99 patients with POEMS syndrome.6 Later, elevated vascular endothelial growth factor was added as a confirmatory diagnostic criterion.7 This growth factor is also an indicator of prognosis in POEMS syndrome, and its level can be used to monitor the response to treatment.7
Our patient met both major criteria for POEMS syndrome, ie, polyneuropathy (based on nerve conduction studies) and a monoclonal protein. Polyneuropathy in POEMS syndrome usually occurs as sensorimotor peripheral neuropathy of insidious onset and is seldom painful. Nerve biopsy study reveals demyelination with features of axonal loss. Interestingly, although our patient had neuropathy as diagnosed by electromyography, she remained clinically asymptomatic.
The monoclonal protein in POEMS syndrome is commonly IgA or IgG. Light chains are always present and are mainly the lambda type; kappa light chains are also reported in rare cases. Our patient had IgG kappa light chains.
Our patient met a number of the minor criteria for POEMS syndrome: ie, organomegaly (hepatosplenomegaly, lymphadenopathy), endocrinopathy (hypothyroidism, diabetes), skin changes (hyperpigmentation and plaques of the lower extremities), edema, pleural effusion, and ascites.
Endocrine disorders in POEMS syndrome
The endocrine abnormalities most often described in POEMS syndrome are hypogonadism, hypothyroidism, and diabetes mellitus. But because hypothyroidism and diabetes are common in the general population, it is debatable whether either of these could constitute the endocrine component of POEMS syndrome. Nevertheless, in three large series,6,7 occurrences of these two disorders were common, although less specific than adrenal or pituitary involvement.
In the analysis by Dispenzieri et al,6 67% of patients had at least one endocrine abnormality. Our patient had no evidence of an adrenal disorder.
Skin, skeletal, and other changes
The skin changes in POEMS syndrome are often nonspecific and include hyperpigmentation, sclerodema-like thickening, and plaques.
Skeletal changes are noted in up to 97% of patients. A skeletal survey in our patient revealed generalized osteopenia as opposed to osteosclerotic lesions, which are common in POEMS syndrome.
Anemia and thrombocytosis (as in our patient) are usually seen in POEMS syndrome and are induced by cytokines.6 POEMS syndrome also leads to increased thrombotic complications from the release of inflammatory cytokines.
Hypoalbuminemia and anasarca including ascites are often seen in POEMS syndrome (prevalence 29% to 89%) and are attributed to cytokine-induced increased vascular permeability. In POEMS syndrome, the serum-ascites albumin gradient is usually less than 1.1 g/dL, as in our patient.
Stepani et al8 reported one case of culture-negative neutrocytic ascites with portal hypertension in POEMS syndrome.8 (Culture-negative neutrocytic ascites is defined as an ascitic fluid polymorphonuclear count greater than 250/mm3 and a negative ascitic fluid culture in the absence of previous antibiotic therapy.) Chylous ascites has not yet been described in POEMS syndrome. However, chylous ascites is predominantly lymphocytic, whereas our patient had neutrocytic ascites.
We concluded that the cause of our patient’s ascites was multifactorial and included previous surgery and POEMS syndrome.
Nonclassic presentation
In addition to its classic presentation, POEMS syndrome is often reported in association with other “unusual features” such as cardiomyopathy, pulmonary hypertension, and cryoglobulinemia.6
So far, very few cases of portal hypertension in POEMS syndrome have been reported. Stepani et al8 described a patient who had POEMS syndrome and portal hypertension with extensive portal fibrosis without cirrhosis on liver biopsy. Inoue et al9 reported a liver biopsy feature consistent with idiopathic portal hypertension, also noting a case with mild fibrosis and few lymphocytic infiltrates in the portal tract.9
The etiopathogenesis of POEMS syndrome is attributed to proangiogenic vascular endothelial growth factor, and other inflammatory cytokines (interleukin 6, interleukin 1 beta, tumor necrosis factor alpha) also play a key role in pulmonary hypertension.10,11 A similar pathogenesis could also contribute to the development of portal hypertension (Figure 3).
CASE CONCLUDED
We started our patient on oral prednisone 60 mg daily for a month, tapered to a maintenance dose of 15 mg to suppress clonal proliferation of plasma cells. Her symptoms improved. Her vascular endothelial growth factor level decreased from 1,664 to 624 pg/mL. She was enrolled in a National Institutes of Health study to evaluate the effect of a potential new immunomodulator treatment for POEMS syndrome.
In conclusion, POEMS syndrome is rare and can present with many atypical features. A high index of suspicion is needed to detect it in a patient who has noncirrhotic portal hypertension with ascites and multisystem involvement.
- Runyon BA, Montano AA, Akriviadis EA, Antillon MR, Irving MA, McHutchison JG. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med 1992; 117:215–220.
- Harris M, Rash RM, Dymock IW. Nodular, non-cirrhotic liver associated with portal hypertension in a patient with rheumatoid arthritis. J Clin Pathol 1974; 27:963–966.
- Ramos-Casals M, Stone JH, Cid MC, Bosch X. The cryoglobulinaemias. Lancet 2012; 379:348–360.
- Druey KM, Greipp PR. Narrative review: the systemic capillary leak syndrome. Ann Intern Med 2010; 153:90–98.
- Bardwick PA, Zvaifler NJ, Gill GN, Newman D, Greenway GD, Resnick DL. Plasma cell dyscrasia with polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes: the POEMS syndrome. Report on two cases and a review of the literature. Medicine (Baltimore) 1980; 59:311–322.
- Dispenzieri A, Kyle RA, Lacy MQ, et al. POEMS syndrome: definitions and long-term outcome. Blood 2003; 101:2496–2506.
- Dispenzieri A. POEMS syndrome. Blood Rev 2007; 21:285–299.
- Stepani P, Courouble Y, Postel P, et al. Portal hypertension and neutrocytic ascites in POEMS syndrome. Gastroenterol Clin Biol 1998; 22:1095–1097. Article in French.
- Inoue R, Nakazawa A, Tsukada N, et al. POEMS syndrome with idiopathic portal hypertension: autopsy case and review of the literature. Pathol Int 2010; 60:316–320.
- Gherardi RK, Bélec L, Soubrier M, et al. Overproduction of proinflammatory cytokines imbalanced by their antagonists in POEMS syndrome. Blood 1996; 87:1458–1465.
- Mukerjee D, Kingdon E, Vanderpump M, Coghlan JG. Pathophysiological insights from a case of reversible pulmonary arterial hypertension. J R Soc Med 2003; 96:403–404.
- Runyon BA, Montano AA, Akriviadis EA, Antillon MR, Irving MA, McHutchison JG. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med 1992; 117:215–220.
- Harris M, Rash RM, Dymock IW. Nodular, non-cirrhotic liver associated with portal hypertension in a patient with rheumatoid arthritis. J Clin Pathol 1974; 27:963–966.
- Ramos-Casals M, Stone JH, Cid MC, Bosch X. The cryoglobulinaemias. Lancet 2012; 379:348–360.
- Druey KM, Greipp PR. Narrative review: the systemic capillary leak syndrome. Ann Intern Med 2010; 153:90–98.
- Bardwick PA, Zvaifler NJ, Gill GN, Newman D, Greenway GD, Resnick DL. Plasma cell dyscrasia with polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes: the POEMS syndrome. Report on two cases and a review of the literature. Medicine (Baltimore) 1980; 59:311–322.
- Dispenzieri A, Kyle RA, Lacy MQ, et al. POEMS syndrome: definitions and long-term outcome. Blood 2003; 101:2496–2506.
- Dispenzieri A. POEMS syndrome. Blood Rev 2007; 21:285–299.
- Stepani P, Courouble Y, Postel P, et al. Portal hypertension and neutrocytic ascites in POEMS syndrome. Gastroenterol Clin Biol 1998; 22:1095–1097. Article in French.
- Inoue R, Nakazawa A, Tsukada N, et al. POEMS syndrome with idiopathic portal hypertension: autopsy case and review of the literature. Pathol Int 2010; 60:316–320.
- Gherardi RK, Bélec L, Soubrier M, et al. Overproduction of proinflammatory cytokines imbalanced by their antagonists in POEMS syndrome. Blood 1996; 87:1458–1465.
- Mukerjee D, Kingdon E, Vanderpump M, Coghlan JG. Pathophysiological insights from a case of reversible pulmonary arterial hypertension. J R Soc Med 2003; 96:403–404.