User login
Secondary syphilis
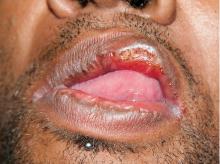
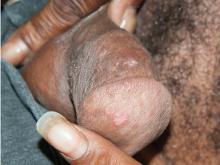
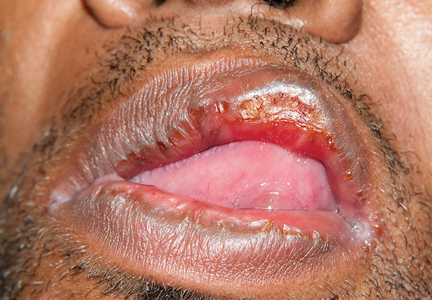
Results of laboratory testing included a positive reactive syphilis immunoglobulin G (IgG) enzyme immunoassay and a positive rapid plasma reagin (RPR) test (titer 1:256). Human immunodeficiency virus (HIV) testing was negative, and serologic testing demonstrated prior immunization to hepatitis B virus. Given the clinical presentation and laboratory findings, secondary syphilis was considered the most probable diagnosis.
The patient was treated with benzathine penicillin G 2.4 million units intramuscularly.
SYPHILIS: A REEMERGING CONDITION
Epidemiology
The rate of reported primary and secondary syphilis cases in the United States has risen since 2001.1 Most cases occur in men who have sex with men.1 Additional risk factors include condomless intercourse and drug use.2
Signs and symptoms of the 3 stages
Primary syphilis begins 2 to 3 weeks after inoculation of a mucosal surface.3 This stage is marked by one or more painless chancres and, in some cases, local nontender lymphadenopathy.3,4 Secondary syphilis presents 4 to 8 weeks later with systemic symptoms including rash, classically involving the palms or soles, lymphadenopathy, myalgia, fever, and weight loss.2,3 Untreated primary and secondary syphilis may progress to latent or asymptomatic disease.5
Tertiary syphilis, defined by the US Centers for Disease Control and Prevention (CDC) as gummas or cardiovascular syphilis, occurs 15 to 30 years after an untreated exposure.4,6 Neurosyphilis can present at any stage of the disease.6
Diagnosis
The diagnosis of syphilis involves a nontreponemal test such as RPR or Venereal Disease Research Laboratory (VDRL) to screen for disease, followed by a treponemal antibody test such as fluorescent treponemal antibody-absorption, Treponema pallidum particle agglutination assay, or syphilis IgG to confirm the diagnosis.5 There is no screening test for tertiary disease in patients previously diagnosed with primary or secondary syphilis, but a cerebrospinal fluid (CSF) examination is recommended if neurologic or ocular manifestations are present.6
Treatment
Treatment of primary, secondary, and early latent syphilis is a single dose of 2.4 million units of benzathine penicillin G given intramuscularly.7 The treatment of late latent and tertiary syphilis is less well defined by the current literature but generally includes penicillin.7
Patients with primary and secondary syphilis undergo serologic and clinical evaluation at 6 and 12 months to be assessed for treatment failure or reinfection.6 Patients with latent disease require serologic follow-up at 6, 12, and 24 months.6 Additionally, CSF analysis should be done if baseline high titers do not fall within 12 to 24 months of treatment or if symptoms suggest syphilis.6 Patients with neurosyphilis often require CSF evaluation every 6 months.6 Follow-up for patients with tertiary syphilis is less well defined.
Patients coinfected with HIV have special needs and considerations, as outlined in the CDC’s 2015 Sexually Transmitted Diseases Treatment Guidelines.6
Sexual contacts of patients with syphilis deserve evaluation. Exposure within 90 days of a patient’s diagnosis with primary, secondary, or latent disease requires treatment regardless of the results of serologic testing.6 Persons exposed more than 90 days before diagnosis may undergo serologic testing; however, if results are not immediately available or follow-up is unlikely, the individual should be treated for early syphilis.6 If serologic testing results are negative, no treatment is required.6
- Centers for Disease Control and Prevention (CDC). 2015 Sexually transmitted disease surveillance. www.cdc.gov/std/stats15. Accessed May 22, 2017.
- Nyatsanza F, Tipple C. Syphilis: presentations in general medicine. Clin Med (Lond) 2016; 16:184–188.
- French P. Syphilis. BMJ 2007; 334:143–147. Erratum in: BMJ 2007; 335:0.
- Centers for Disease Control and Prevention (CDC). Appendix C1. Case definitions for nationally notifiable infectious diseases. www.cdc.gov/std/stats14/appendixc.htm. Accessed May 18, 2017.
- Centers for Disease Control and Prevention (CDC). Syphilis—CDC fact sheet (detailed). www.cdc.gov/std/syphilis/STDFact-Syphilis-detailed.htm. Accessed May 18, 2017.
- Centers for Disease Control and Prevention (CDC). 2015 Sexually transmitted diseases treatment guidelines. Syphilis. www.cdc.gov/std/tg2015/syphilis.htm. Accessed June 15, 2016.
- Clement ME, Okeke NL, Hicks CB. Treatment of syphilis: a systematic review. JAMA 2014; 312:1905–1917.
Results of laboratory testing included a positive reactive syphilis immunoglobulin G (IgG) enzyme immunoassay and a positive rapid plasma reagin (RPR) test (titer 1:256). Human immunodeficiency virus (HIV) testing was negative, and serologic testing demonstrated prior immunization to hepatitis B virus. Given the clinical presentation and laboratory findings, secondary syphilis was considered the most probable diagnosis.
The patient was treated with benzathine penicillin G 2.4 million units intramuscularly.
SYPHILIS: A REEMERGING CONDITION
Epidemiology
The rate of reported primary and secondary syphilis cases in the United States has risen since 2001.1 Most cases occur in men who have sex with men.1 Additional risk factors include condomless intercourse and drug use.2
Signs and symptoms of the 3 stages
Primary syphilis begins 2 to 3 weeks after inoculation of a mucosal surface.3 This stage is marked by one or more painless chancres and, in some cases, local nontender lymphadenopathy.3,4 Secondary syphilis presents 4 to 8 weeks later with systemic symptoms including rash, classically involving the palms or soles, lymphadenopathy, myalgia, fever, and weight loss.2,3 Untreated primary and secondary syphilis may progress to latent or asymptomatic disease.5
Tertiary syphilis, defined by the US Centers for Disease Control and Prevention (CDC) as gummas or cardiovascular syphilis, occurs 15 to 30 years after an untreated exposure.4,6 Neurosyphilis can present at any stage of the disease.6
Diagnosis
The diagnosis of syphilis involves a nontreponemal test such as RPR or Venereal Disease Research Laboratory (VDRL) to screen for disease, followed by a treponemal antibody test such as fluorescent treponemal antibody-absorption, Treponema pallidum particle agglutination assay, or syphilis IgG to confirm the diagnosis.5 There is no screening test for tertiary disease in patients previously diagnosed with primary or secondary syphilis, but a cerebrospinal fluid (CSF) examination is recommended if neurologic or ocular manifestations are present.6
Treatment
Treatment of primary, secondary, and early latent syphilis is a single dose of 2.4 million units of benzathine penicillin G given intramuscularly.7 The treatment of late latent and tertiary syphilis is less well defined by the current literature but generally includes penicillin.7
Patients with primary and secondary syphilis undergo serologic and clinical evaluation at 6 and 12 months to be assessed for treatment failure or reinfection.6 Patients with latent disease require serologic follow-up at 6, 12, and 24 months.6 Additionally, CSF analysis should be done if baseline high titers do not fall within 12 to 24 months of treatment or if symptoms suggest syphilis.6 Patients with neurosyphilis often require CSF evaluation every 6 months.6 Follow-up for patients with tertiary syphilis is less well defined.
Patients coinfected with HIV have special needs and considerations, as outlined in the CDC’s 2015 Sexually Transmitted Diseases Treatment Guidelines.6
Sexual contacts of patients with syphilis deserve evaluation. Exposure within 90 days of a patient’s diagnosis with primary, secondary, or latent disease requires treatment regardless of the results of serologic testing.6 Persons exposed more than 90 days before diagnosis may undergo serologic testing; however, if results are not immediately available or follow-up is unlikely, the individual should be treated for early syphilis.6 If serologic testing results are negative, no treatment is required.6
Results of laboratory testing included a positive reactive syphilis immunoglobulin G (IgG) enzyme immunoassay and a positive rapid plasma reagin (RPR) test (titer 1:256). Human immunodeficiency virus (HIV) testing was negative, and serologic testing demonstrated prior immunization to hepatitis B virus. Given the clinical presentation and laboratory findings, secondary syphilis was considered the most probable diagnosis.
The patient was treated with benzathine penicillin G 2.4 million units intramuscularly.
SYPHILIS: A REEMERGING CONDITION
Epidemiology
The rate of reported primary and secondary syphilis cases in the United States has risen since 2001.1 Most cases occur in men who have sex with men.1 Additional risk factors include condomless intercourse and drug use.2
Signs and symptoms of the 3 stages
Primary syphilis begins 2 to 3 weeks after inoculation of a mucosal surface.3 This stage is marked by one or more painless chancres and, in some cases, local nontender lymphadenopathy.3,4 Secondary syphilis presents 4 to 8 weeks later with systemic symptoms including rash, classically involving the palms or soles, lymphadenopathy, myalgia, fever, and weight loss.2,3 Untreated primary and secondary syphilis may progress to latent or asymptomatic disease.5
Tertiary syphilis, defined by the US Centers for Disease Control and Prevention (CDC) as gummas or cardiovascular syphilis, occurs 15 to 30 years after an untreated exposure.4,6 Neurosyphilis can present at any stage of the disease.6
Diagnosis
The diagnosis of syphilis involves a nontreponemal test such as RPR or Venereal Disease Research Laboratory (VDRL) to screen for disease, followed by a treponemal antibody test such as fluorescent treponemal antibody-absorption, Treponema pallidum particle agglutination assay, or syphilis IgG to confirm the diagnosis.5 There is no screening test for tertiary disease in patients previously diagnosed with primary or secondary syphilis, but a cerebrospinal fluid (CSF) examination is recommended if neurologic or ocular manifestations are present.6
Treatment
Treatment of primary, secondary, and early latent syphilis is a single dose of 2.4 million units of benzathine penicillin G given intramuscularly.7 The treatment of late latent and tertiary syphilis is less well defined by the current literature but generally includes penicillin.7
Patients with primary and secondary syphilis undergo serologic and clinical evaluation at 6 and 12 months to be assessed for treatment failure or reinfection.6 Patients with latent disease require serologic follow-up at 6, 12, and 24 months.6 Additionally, CSF analysis should be done if baseline high titers do not fall within 12 to 24 months of treatment or if symptoms suggest syphilis.6 Patients with neurosyphilis often require CSF evaluation every 6 months.6 Follow-up for patients with tertiary syphilis is less well defined.
Patients coinfected with HIV have special needs and considerations, as outlined in the CDC’s 2015 Sexually Transmitted Diseases Treatment Guidelines.6
Sexual contacts of patients with syphilis deserve evaluation. Exposure within 90 days of a patient’s diagnosis with primary, secondary, or latent disease requires treatment regardless of the results of serologic testing.6 Persons exposed more than 90 days before diagnosis may undergo serologic testing; however, if results are not immediately available or follow-up is unlikely, the individual should be treated for early syphilis.6 If serologic testing results are negative, no treatment is required.6
- Centers for Disease Control and Prevention (CDC). 2015 Sexually transmitted disease surveillance. www.cdc.gov/std/stats15. Accessed May 22, 2017.
- Nyatsanza F, Tipple C. Syphilis: presentations in general medicine. Clin Med (Lond) 2016; 16:184–188.
- French P. Syphilis. BMJ 2007; 334:143–147. Erratum in: BMJ 2007; 335:0.
- Centers for Disease Control and Prevention (CDC). Appendix C1. Case definitions for nationally notifiable infectious diseases. www.cdc.gov/std/stats14/appendixc.htm. Accessed May 18, 2017.
- Centers for Disease Control and Prevention (CDC). Syphilis—CDC fact sheet (detailed). www.cdc.gov/std/syphilis/STDFact-Syphilis-detailed.htm. Accessed May 18, 2017.
- Centers for Disease Control and Prevention (CDC). 2015 Sexually transmitted diseases treatment guidelines. Syphilis. www.cdc.gov/std/tg2015/syphilis.htm. Accessed June 15, 2016.
- Clement ME, Okeke NL, Hicks CB. Treatment of syphilis: a systematic review. JAMA 2014; 312:1905–1917.
- Centers for Disease Control and Prevention (CDC). 2015 Sexually transmitted disease surveillance. www.cdc.gov/std/stats15. Accessed May 22, 2017.
- Nyatsanza F, Tipple C. Syphilis: presentations in general medicine. Clin Med (Lond) 2016; 16:184–188.
- French P. Syphilis. BMJ 2007; 334:143–147. Erratum in: BMJ 2007; 335:0.
- Centers for Disease Control and Prevention (CDC). Appendix C1. Case definitions for nationally notifiable infectious diseases. www.cdc.gov/std/stats14/appendixc.htm. Accessed May 18, 2017.
- Centers for Disease Control and Prevention (CDC). Syphilis—CDC fact sheet (detailed). www.cdc.gov/std/syphilis/STDFact-Syphilis-detailed.htm. Accessed May 18, 2017.
- Centers for Disease Control and Prevention (CDC). 2015 Sexually transmitted diseases treatment guidelines. Syphilis. www.cdc.gov/std/tg2015/syphilis.htm. Accessed June 15, 2016.
- Clement ME, Okeke NL, Hicks CB. Treatment of syphilis: a systematic review. JAMA 2014; 312:1905–1917.
Starting insulin in patients with type 2 diabetes: An individualized approach
Insulin therapy is one of the most effective tools clinicians can use to help patients reach their individualized hemoglobin A1c target. However, decisions about when and how to start insulin therapy have to be individualized to the needs and goals of each patient. Many insulin options are available, one of the most common being the addition of basal insulin to oral antidiabetic drugs. Although patients are often reluctant to start insulin, this reluctance can be overcome through patient education and hands-on training.
Here, we review hemoglobin A1c targets, factors that determine when to start insulin therapy, and the different regimens that can be used.
MOST PATIENTS EVENTUALLY NEED INSULIN
Type 2 diabetes mellitus is a chronic progressive disease associated with insulin resistance, beta-cell dysfunction, and decreased insulin secretion. Consequently, most patients eventually require insulin therapy to reduce the risk of long-term complications.
The efficacy of therapy can be assessed by measuring hemoglobin A1c, an important marker of the chronic hyperglycemic state. The hemoglobin A1c value can be reported as a ratio (%) standardized against the results of the Diabetes Control and Complications Trial,1 or as International Federation of Clinical Chemistry units (mmol/mol).2 Table 1 shows the relationship between hemoglobin A1c and average glucose values.3
WHAT IS AN APPROPRIATE HEMOGLOBIN A1c TARGET?
The short answer is, “It depends.”
Currently, the American Association of Clinical Endocrinologists (AACE) supports a hemoglobin A1c goal of less than 6.5% for otherwise healthy patients but states that the goal should be individualized for patients with concurrent illnesses or at risk of hypoglycemia.4
On the other hand, the American Diabetes Association (ADA) recommends a higher hemoglobin A1c target of less than 7% for most adults with type 2 diabetes mellitus.5 This value was shown to be associated with a reduction in the microvascular and macrovascular complications of diabetes.
Yet when three large trials6–8 recently compared intensive and standard glucose control regimens, tighter glucose control failed to improve cardiovascular outcomes. Moreover, in one of the trials,7 patients receiving intensive treatment had a higher rate of all-cause mortality. Details:
- Action in Diabetes and Vascular Disease (ADVANCE): 11,140 patients; average hemoglobin A1c levels 6.5% vs 7.3%6
- Action to Control Cardiovascular Risk in Diabetes (ACCORD): 10,251 patients; average hemoglobin A1c levels 6.4% vs 7.5%7
- Veterans Affairs Diabetes Trial (VADT): 1,791 patients; average hemoglobin A1c levels 6.9% vs 8.4%.8
Similarly, a 2013 Cochrane review9 that included 28 randomized controlled trials concluded that intensive control (in 18,717 patients) did not decrease all-cause and cardiovascular mortality rates compared with traditional glucose control (in 16,195 patients), and it increased the risk of hypoglycemia and serious adverse events.
As a result, the ADA5 states that a hemoglobin A1c target less than 6.5% is optional for patients with a long life expectancy, short duration of diabetes, low risk of hypoglycemia, and no significant cardiovascular disease. The ADA further defines a hemoglobin A1c goal of less than 8% for patients with a history of severe hypoglycemia, limited life expectancy, advanced microvascular or macrovascular complications, extensive comorbid conditions, and long-standing diabetes.
Therefore, the AACE and ADA are moving away from “one-size-fits-all” goals and toward individualizing their recommendations.
WHEN SHOULD INSULIN BE STARTED?
Physicians should consider the needs and preferences of each patient and individualize the treatment. The most recent recommendations from the ADA5 stress the importance of a patient-centered approach, with multiple factors taken into account. These include the patient’s attitude, expected compliance with treatment, risk of hypoglycemia, disease duration, life expectancy, and comorbidities, and the side effects of oral medications and insulin.
Compared with previous guidelines, there are fewer rules on how and when to start insulin therapy. But absolute and relative indications for insulin therapy should be considered in patients with the following:
Absolute indications for insulin
- Ketoacidosis or catabolic symptoms, including ketonuria
- Newly diagnosed type 2 diabetes with pronounced hyperglycemia (glucose ≥ 300 mg/dL or hemoglobin A1c ≥ 10.0%) with or without severe symptoms, including weight loss, polyuria, or polydipsia10
- Uncontrolled type 2 diabetes mellitus despite using one, two, or more oral antidiabetic drugs or glucagon-like peptide 1 (GLP-1) receptor agonists
- Gestational diabetes
- Preference for insulin.
Relative indications for insulin
- Hospitalized for surgery or acute illnesses
- Advanced renal or hepatic disease
- Inability to afford the cost or tolerate the side effects of oral antidiabetic drugs and GLP-1 receptor agonists.
Depending on the situation, blood glucose is measured fasting, before meals, or after meals after initiating or adjusting insulin regimens (Table 2).
WHAT ARE THE INSULIN REGIMENS?
Basal insulin
In the early stages of type 2 diabetes, metformin alone or in combination with another oral antidiabetic drug or with a GLP-1 receptor agonist is often used along with healthy eating, weight control, and increased physical activity.
When the target hemoglobin A1c cannot be achieved with one or two noninsulin drugs, the ADA suggests basal insulin be added to metformin or a two-medication regimen that includes metformin (Table 3). However, recent evidence suggests that combining a GLP-1 receptor agonist with basal insulin, in a regimen without metformin, is safe and improves glycemic control without hypoglycemia or weight gain.11
While a total daily dose of insulin of 0.1 to 0.2 units/kg could be initially used in patients with a hemoglobin A1c level less than 8%, a higher dose of 0.2 to 0.3 units/kg is required if the hemoglobin A1c level is between 8% and 10%. The dose can be titrated once or twice weekly if the fasting glucose is above the target level (usually < 130 mg/dL). If hypoglycemia develops (glucose < 70 mg/dL), the insulin dose should be reduced by 10% to 20%.10
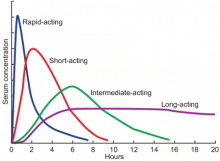
Available basal insulins include glargine, detemir, and neutral protamine Hagedorn (NPH) (Table 4).12–14 Because glargine and detemir offer better pharmacokinetic properties, less variability in response, and less risk of hypoglycemia, they are preferred over NPH. Glargine has a relatively constant plasma concentration over 24 hours, allowing once-daily dosing at any time during the day (Figure 1).15 The dose should be taken at the same time every day. Detemir and NPH are usually taken once or twice daily.
Patients treated once daily should take the dose with the evening meal or at bedtime. Patients who require a twice-daily regimen can take the first dose with breakfast and the second one with the evening meal, at bedtime, or 12 hours after the morning dose.
The randomized Treat-to-Target trial,16 in 756 patients, showed that both glargine and NPH, when added to oral therapy in patients with type 2 diabetes, achieve the target hemoglobin A1c, but NPH is associated with more episodes of nocturnal hypoglycemia. Similar results were found when NPH was compared with detemir insulin.17
A Cochrane review18 suggested that glargine and detemir are similar in efficacy and safety. However, detemir often needs to be injected twice daily, in a higher dose, and is associated with less weight gain. Furthermore, a meta-analysis of 46 randomized clinical trials19 showed that the weight increase at 1 year is less in patients treated with basal than with twice-daily or prandial regimens.
A noninterventional longitudinal study20 in 2,179 patients newly started on insulin showed that the mean weight increase at 1 year was 1.78 kg, and 24% of patients gained more than 5 kg. However, the factors independently associated with the weight gain were a higher hemoglobin A1c at baseline, a higher insulin dose at baseline and at 1 year, and a lower baseline body mass index, but not the type of insulin regimen.
Currently, a new class of ultralong-acting basal insulins is being studied. Insulins in this class are approved in other countries, but the US Food and Drug Administration requires additional data for approval. Ultralong-acting insulins are expected to reduce the risk of hypoglycemia, specifically the risk of nocturnal episodes. Also, given their longer duration of action and stable steady-state pharmacokinetics, they will offer flexibility in the dose timing.21
Basal-bolus regimens
Basal insulin often does not control postprandial hyperglycemia. The need for multiple doses of insulin (including one or more preprandial doses) is suggested by postprandial glucose values above target (usually > 180 mg/dL) or by a hemoglobin A1c above goal despite well-controlled fasting glucose levels. This usually becomes evident when the total daily dose of basal insulin exceeds 0.5 units/kg. Patients newly diagnosed with diabetes who have a hemoglobin A1c higher than 10% may also respond better to an initial basal-bolus regimen.
Available bolus insulins include lispro, aspart, glulisine, regular insulin, and the newly approved Technosphere inhaled regular insulin (Table 4).12–14 They can be taken before each meal, and the total bolus dose usually represents 50% of the total daily dose.22 Rapid-acting insulins have faster onset, shorter duration of action, and more predictable pharmacokinetics, which makes them preferable to regular insulin (Figure 1).15 Inhaled insulin is another option, but it is contraindicated in patients with chronic obstructive pulmonary disease or asthma because of the increased risk of acute bronchospasm.12
Alternatively, the transition to a basal-bolus regimen can be accomplished with a single dose of bolus insulin before the main meal, using a dose that represents approximately 10% of the total daily dose. Additional bolus doses can be added later based on the glycemic control. The adjustment of the preprandial insulin dose is done once or twice weekly, based on the postprandial glucose levels.10
Premixed combinations of long- and short-acting insulins in ratios of 50% to 50%, 70% to 30%, or 75% to 25% can be considered in patients who cannot adhere to a complex insulin regimen. A propensity-matched comparison of different insulin regimens (basal, premixed, mealtime plus basal, and mealtime) in patients with type 2 diabetes revealed that the hemoglobin A1c reduction was similar between the different groups.23 However, the number of hypoglycemic episodes was higher in the premixed insulin group, and the weight gain was less in the basal insulin group.
While premixed insulins require fewer injections, they do not provide dosing flexibility. In other words, dose adjustments for premixed insulins lead to increases in both basal and bolus amounts even though a dose adjustment is needed for only one insulin type. Thus, this is a common reason for increased hypoglycemic episodes.
Continuous subcutaneous insulin infusion
A meta-analysis showed that continuous subcutaneous insulin infusion (ie, use of an insulin pump) was similar to intensive therapy with multiple daily insulin injections in terms of glycemic control and hypoglycemia.24 Since both options can lead to similar glucose control, additional factors to consider when initiating insulin infusion include lifestyle and technical expertise. Some patients may or may not prefer having a pump attached for nearly all daily activities. Additionally, this type of therapy is complex and requires significant training to ensure efficacy and safety.25
WHAT IS THE COST OF INSULIN THERAPY?
A final factor to keep in mind when initiating insulin is cost (Table 4).12–14 Asking patients to check their prescription insurance formulary is important to ensure that an affordable option is selected. If patients do not have prescription insurance, medication assistance programs could be an option. However, if a patient is considering an insulin pump, insurance coverage is essential. Depending on the manufacturer, insulin pumps cost about $6,000 to $7,000, and the additional monthly supplies for the pump are also expensive.
If patients are engaged when considering and selecting insulin therapy, the likelihood of treatment success is greater.26–28
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- Hanas R, John WG; International HbA1c Consensus Committee. 2013 Update on the worldwide standardization of the hemoglobin A1c measurement. Pediatr Diabetes 2014; 15:e1–e2.
- Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ; A1c-Derived Average Glucose Study Group. Translating the A1C assay into estimated average glucose values. Diabetes Care 2008; 31:1473–1478.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al; American Association of Clinical Endocrinologists. AACE comprehensive diabetes management algorithm 2013. Endocr Pract 2013; 19:327–336.
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care 2014; 37(suppl 1):S14–S80.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Hemmingsen B, Lund SS, Gluud C, et al. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev 2013; 11:CD008143.
- Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35:1364–1379.
- Vora J, Bain SC, Damci T, et al. Incretin-based therapy in combination with basal insulin: a promising tactic for the treatment of type 2 diabetes. Diabetes Metab 2013; 39:6–15.
- Nuffer W, Trujillo JM, Ellis SL. Technosphere insulin (Afrezza): a new, inhaled prandial insulin. Ann Pharmacother 2015; 49:99–106.
- Pharmacist’s Letter/Prescriber’s Letter. Comparison of insulins and injectable diabetes meds. PL Detail-Document #281107 November 2012. www.PharmacistsLetter.com. Accessed July 2, 2015
- Lexicomp Online. www.wolterskluwercdi.com/lexicomp-online/. Accessed July 2, 2015.
- Hirsch IB. Insulin analogues. N Engl J Med 2005; 352:174-183.
- Riddle MC, Rosenstock J, Gerich J; Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003; 26:3080–3086.
- Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care 2006; 29:1269–1274.
- Swinnen SG, Simon AC, Holleman F, Hoekstra JB, Devries JH. Insulin detemir versus insulin glargine for type 2 diabetes mellitus. Cochrane Database Syst Rev 2011; 7:CD006383.
- Pontiroli AE, Miele L, Morabito A. Increase of body weight during the first year of intensive insulin treatment in type 2 diabetes: systematic review and meta-analysis. Diabetes Obes Metab 2011; 13:1008–1019.
- Balkau B, Home PD, Vincent M, Marre M, Freemantle N. Factors associated with weight gain in people with type 2 diabetes starting on insulin. Diabetes Care 2014; 37:2108–2113.
- Garber AJ. Will the next generation of basal insulins offer clinical advantages? Diabetes Obes Metab 2014; 16:483–491.
- Tamaki M, Shimizu T, Kanazawa A, et al. Effects of changes in basal/total daily insulin ratio in type 2 diabetes patients on intensive insulin therapy including insulin glargine (JUN-LAN Study 6). Diabetes Res Clin Pract 2008; 81:e1–e3.
- Freemantle N, Balkau B, Home PD. A propensity score matched comparison of different insulin regimens 1 year after beginning insulin in people with type 2 diabetes. Diabetes Obes Metab 2013; 15:1120–1127.
- Yeh HC, Brown TT, Maruthur N, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med 2012; 157:336–347.
- Schade DS, Valentine V. To pump or not to pump. Diabetes Care 2002; 25:2100–2102.
- Liu L, Lee MJ, Brateanu A. Improved A1C and lipid profile in patients referred to diabetes education programs in a wide health care network: a retrospective study. Diabetes Spectr 2014; 27:297–303.
- Funnell MM, Kruger DF, Spencer M. Self-management support for insulin therapy in type 2 diabetes. Diabetes Educ 2004; 30:274–280.
- Norris SL, Engelgau MM, Narayan KM. Effectiveness of self-management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care 2001; 24:561–587.
Insulin therapy is one of the most effective tools clinicians can use to help patients reach their individualized hemoglobin A1c target. However, decisions about when and how to start insulin therapy have to be individualized to the needs and goals of each patient. Many insulin options are available, one of the most common being the addition of basal insulin to oral antidiabetic drugs. Although patients are often reluctant to start insulin, this reluctance can be overcome through patient education and hands-on training.
Here, we review hemoglobin A1c targets, factors that determine when to start insulin therapy, and the different regimens that can be used.
MOST PATIENTS EVENTUALLY NEED INSULIN
Type 2 diabetes mellitus is a chronic progressive disease associated with insulin resistance, beta-cell dysfunction, and decreased insulin secretion. Consequently, most patients eventually require insulin therapy to reduce the risk of long-term complications.
The efficacy of therapy can be assessed by measuring hemoglobin A1c, an important marker of the chronic hyperglycemic state. The hemoglobin A1c value can be reported as a ratio (%) standardized against the results of the Diabetes Control and Complications Trial,1 or as International Federation of Clinical Chemistry units (mmol/mol).2 Table 1 shows the relationship between hemoglobin A1c and average glucose values.3
WHAT IS AN APPROPRIATE HEMOGLOBIN A1c TARGET?
The short answer is, “It depends.”
Currently, the American Association of Clinical Endocrinologists (AACE) supports a hemoglobin A1c goal of less than 6.5% for otherwise healthy patients but states that the goal should be individualized for patients with concurrent illnesses or at risk of hypoglycemia.4
On the other hand, the American Diabetes Association (ADA) recommends a higher hemoglobin A1c target of less than 7% for most adults with type 2 diabetes mellitus.5 This value was shown to be associated with a reduction in the microvascular and macrovascular complications of diabetes.
Yet when three large trials6–8 recently compared intensive and standard glucose control regimens, tighter glucose control failed to improve cardiovascular outcomes. Moreover, in one of the trials,7 patients receiving intensive treatment had a higher rate of all-cause mortality. Details:
- Action in Diabetes and Vascular Disease (ADVANCE): 11,140 patients; average hemoglobin A1c levels 6.5% vs 7.3%6
- Action to Control Cardiovascular Risk in Diabetes (ACCORD): 10,251 patients; average hemoglobin A1c levels 6.4% vs 7.5%7
- Veterans Affairs Diabetes Trial (VADT): 1,791 patients; average hemoglobin A1c levels 6.9% vs 8.4%.8
Similarly, a 2013 Cochrane review9 that included 28 randomized controlled trials concluded that intensive control (in 18,717 patients) did not decrease all-cause and cardiovascular mortality rates compared with traditional glucose control (in 16,195 patients), and it increased the risk of hypoglycemia and serious adverse events.
As a result, the ADA5 states that a hemoglobin A1c target less than 6.5% is optional for patients with a long life expectancy, short duration of diabetes, low risk of hypoglycemia, and no significant cardiovascular disease. The ADA further defines a hemoglobin A1c goal of less than 8% for patients with a history of severe hypoglycemia, limited life expectancy, advanced microvascular or macrovascular complications, extensive comorbid conditions, and long-standing diabetes.
Therefore, the AACE and ADA are moving away from “one-size-fits-all” goals and toward individualizing their recommendations.
WHEN SHOULD INSULIN BE STARTED?
Physicians should consider the needs and preferences of each patient and individualize the treatment. The most recent recommendations from the ADA5 stress the importance of a patient-centered approach, with multiple factors taken into account. These include the patient’s attitude, expected compliance with treatment, risk of hypoglycemia, disease duration, life expectancy, and comorbidities, and the side effects of oral medications and insulin.
Compared with previous guidelines, there are fewer rules on how and when to start insulin therapy. But absolute and relative indications for insulin therapy should be considered in patients with the following:
Absolute indications for insulin
- Ketoacidosis or catabolic symptoms, including ketonuria
- Newly diagnosed type 2 diabetes with pronounced hyperglycemia (glucose ≥ 300 mg/dL or hemoglobin A1c ≥ 10.0%) with or without severe symptoms, including weight loss, polyuria, or polydipsia10
- Uncontrolled type 2 diabetes mellitus despite using one, two, or more oral antidiabetic drugs or glucagon-like peptide 1 (GLP-1) receptor agonists
- Gestational diabetes
- Preference for insulin.
Relative indications for insulin
- Hospitalized for surgery or acute illnesses
- Advanced renal or hepatic disease
- Inability to afford the cost or tolerate the side effects of oral antidiabetic drugs and GLP-1 receptor agonists.
Depending on the situation, blood glucose is measured fasting, before meals, or after meals after initiating or adjusting insulin regimens (Table 2).
WHAT ARE THE INSULIN REGIMENS?
Basal insulin
In the early stages of type 2 diabetes, metformin alone or in combination with another oral antidiabetic drug or with a GLP-1 receptor agonist is often used along with healthy eating, weight control, and increased physical activity.
When the target hemoglobin A1c cannot be achieved with one or two noninsulin drugs, the ADA suggests basal insulin be added to metformin or a two-medication regimen that includes metformin (Table 3). However, recent evidence suggests that combining a GLP-1 receptor agonist with basal insulin, in a regimen without metformin, is safe and improves glycemic control without hypoglycemia or weight gain.11
While a total daily dose of insulin of 0.1 to 0.2 units/kg could be initially used in patients with a hemoglobin A1c level less than 8%, a higher dose of 0.2 to 0.3 units/kg is required if the hemoglobin A1c level is between 8% and 10%. The dose can be titrated once or twice weekly if the fasting glucose is above the target level (usually < 130 mg/dL). If hypoglycemia develops (glucose < 70 mg/dL), the insulin dose should be reduced by 10% to 20%.10
Available basal insulins include glargine, detemir, and neutral protamine Hagedorn (NPH) (Table 4).12–14 Because glargine and detemir offer better pharmacokinetic properties, less variability in response, and less risk of hypoglycemia, they are preferred over NPH. Glargine has a relatively constant plasma concentration over 24 hours, allowing once-daily dosing at any time during the day (Figure 1).15 The dose should be taken at the same time every day. Detemir and NPH are usually taken once or twice daily.
Patients treated once daily should take the dose with the evening meal or at bedtime. Patients who require a twice-daily regimen can take the first dose with breakfast and the second one with the evening meal, at bedtime, or 12 hours after the morning dose.
The randomized Treat-to-Target trial,16 in 756 patients, showed that both glargine and NPH, when added to oral therapy in patients with type 2 diabetes, achieve the target hemoglobin A1c, but NPH is associated with more episodes of nocturnal hypoglycemia. Similar results were found when NPH was compared with detemir insulin.17
A Cochrane review18 suggested that glargine and detemir are similar in efficacy and safety. However, detemir often needs to be injected twice daily, in a higher dose, and is associated with less weight gain. Furthermore, a meta-analysis of 46 randomized clinical trials19 showed that the weight increase at 1 year is less in patients treated with basal than with twice-daily or prandial regimens.
A noninterventional longitudinal study20 in 2,179 patients newly started on insulin showed that the mean weight increase at 1 year was 1.78 kg, and 24% of patients gained more than 5 kg. However, the factors independently associated with the weight gain were a higher hemoglobin A1c at baseline, a higher insulin dose at baseline and at 1 year, and a lower baseline body mass index, but not the type of insulin regimen.
Currently, a new class of ultralong-acting basal insulins is being studied. Insulins in this class are approved in other countries, but the US Food and Drug Administration requires additional data for approval. Ultralong-acting insulins are expected to reduce the risk of hypoglycemia, specifically the risk of nocturnal episodes. Also, given their longer duration of action and stable steady-state pharmacokinetics, they will offer flexibility in the dose timing.21
Basal-bolus regimens
Basal insulin often does not control postprandial hyperglycemia. The need for multiple doses of insulin (including one or more preprandial doses) is suggested by postprandial glucose values above target (usually > 180 mg/dL) or by a hemoglobin A1c above goal despite well-controlled fasting glucose levels. This usually becomes evident when the total daily dose of basal insulin exceeds 0.5 units/kg. Patients newly diagnosed with diabetes who have a hemoglobin A1c higher than 10% may also respond better to an initial basal-bolus regimen.
Available bolus insulins include lispro, aspart, glulisine, regular insulin, and the newly approved Technosphere inhaled regular insulin (Table 4).12–14 They can be taken before each meal, and the total bolus dose usually represents 50% of the total daily dose.22 Rapid-acting insulins have faster onset, shorter duration of action, and more predictable pharmacokinetics, which makes them preferable to regular insulin (Figure 1).15 Inhaled insulin is another option, but it is contraindicated in patients with chronic obstructive pulmonary disease or asthma because of the increased risk of acute bronchospasm.12
Alternatively, the transition to a basal-bolus regimen can be accomplished with a single dose of bolus insulin before the main meal, using a dose that represents approximately 10% of the total daily dose. Additional bolus doses can be added later based on the glycemic control. The adjustment of the preprandial insulin dose is done once or twice weekly, based on the postprandial glucose levels.10
Premixed combinations of long- and short-acting insulins in ratios of 50% to 50%, 70% to 30%, or 75% to 25% can be considered in patients who cannot adhere to a complex insulin regimen. A propensity-matched comparison of different insulin regimens (basal, premixed, mealtime plus basal, and mealtime) in patients with type 2 diabetes revealed that the hemoglobin A1c reduction was similar between the different groups.23 However, the number of hypoglycemic episodes was higher in the premixed insulin group, and the weight gain was less in the basal insulin group.
While premixed insulins require fewer injections, they do not provide dosing flexibility. In other words, dose adjustments for premixed insulins lead to increases in both basal and bolus amounts even though a dose adjustment is needed for only one insulin type. Thus, this is a common reason for increased hypoglycemic episodes.
Continuous subcutaneous insulin infusion
A meta-analysis showed that continuous subcutaneous insulin infusion (ie, use of an insulin pump) was similar to intensive therapy with multiple daily insulin injections in terms of glycemic control and hypoglycemia.24 Since both options can lead to similar glucose control, additional factors to consider when initiating insulin infusion include lifestyle and technical expertise. Some patients may or may not prefer having a pump attached for nearly all daily activities. Additionally, this type of therapy is complex and requires significant training to ensure efficacy and safety.25
WHAT IS THE COST OF INSULIN THERAPY?
A final factor to keep in mind when initiating insulin is cost (Table 4).12–14 Asking patients to check their prescription insurance formulary is important to ensure that an affordable option is selected. If patients do not have prescription insurance, medication assistance programs could be an option. However, if a patient is considering an insulin pump, insurance coverage is essential. Depending on the manufacturer, insulin pumps cost about $6,000 to $7,000, and the additional monthly supplies for the pump are also expensive.
If patients are engaged when considering and selecting insulin therapy, the likelihood of treatment success is greater.26–28
Insulin therapy is one of the most effective tools clinicians can use to help patients reach their individualized hemoglobin A1c target. However, decisions about when and how to start insulin therapy have to be individualized to the needs and goals of each patient. Many insulin options are available, one of the most common being the addition of basal insulin to oral antidiabetic drugs. Although patients are often reluctant to start insulin, this reluctance can be overcome through patient education and hands-on training.
Here, we review hemoglobin A1c targets, factors that determine when to start insulin therapy, and the different regimens that can be used.
MOST PATIENTS EVENTUALLY NEED INSULIN
Type 2 diabetes mellitus is a chronic progressive disease associated with insulin resistance, beta-cell dysfunction, and decreased insulin secretion. Consequently, most patients eventually require insulin therapy to reduce the risk of long-term complications.
The efficacy of therapy can be assessed by measuring hemoglobin A1c, an important marker of the chronic hyperglycemic state. The hemoglobin A1c value can be reported as a ratio (%) standardized against the results of the Diabetes Control and Complications Trial,1 or as International Federation of Clinical Chemistry units (mmol/mol).2 Table 1 shows the relationship between hemoglobin A1c and average glucose values.3
WHAT IS AN APPROPRIATE HEMOGLOBIN A1c TARGET?
The short answer is, “It depends.”
Currently, the American Association of Clinical Endocrinologists (AACE) supports a hemoglobin A1c goal of less than 6.5% for otherwise healthy patients but states that the goal should be individualized for patients with concurrent illnesses or at risk of hypoglycemia.4
On the other hand, the American Diabetes Association (ADA) recommends a higher hemoglobin A1c target of less than 7% for most adults with type 2 diabetes mellitus.5 This value was shown to be associated with a reduction in the microvascular and macrovascular complications of diabetes.
Yet when three large trials6–8 recently compared intensive and standard glucose control regimens, tighter glucose control failed to improve cardiovascular outcomes. Moreover, in one of the trials,7 patients receiving intensive treatment had a higher rate of all-cause mortality. Details:
- Action in Diabetes and Vascular Disease (ADVANCE): 11,140 patients; average hemoglobin A1c levels 6.5% vs 7.3%6
- Action to Control Cardiovascular Risk in Diabetes (ACCORD): 10,251 patients; average hemoglobin A1c levels 6.4% vs 7.5%7
- Veterans Affairs Diabetes Trial (VADT): 1,791 patients; average hemoglobin A1c levels 6.9% vs 8.4%.8
Similarly, a 2013 Cochrane review9 that included 28 randomized controlled trials concluded that intensive control (in 18,717 patients) did not decrease all-cause and cardiovascular mortality rates compared with traditional glucose control (in 16,195 patients), and it increased the risk of hypoglycemia and serious adverse events.
As a result, the ADA5 states that a hemoglobin A1c target less than 6.5% is optional for patients with a long life expectancy, short duration of diabetes, low risk of hypoglycemia, and no significant cardiovascular disease. The ADA further defines a hemoglobin A1c goal of less than 8% for patients with a history of severe hypoglycemia, limited life expectancy, advanced microvascular or macrovascular complications, extensive comorbid conditions, and long-standing diabetes.
Therefore, the AACE and ADA are moving away from “one-size-fits-all” goals and toward individualizing their recommendations.
WHEN SHOULD INSULIN BE STARTED?
Physicians should consider the needs and preferences of each patient and individualize the treatment. The most recent recommendations from the ADA5 stress the importance of a patient-centered approach, with multiple factors taken into account. These include the patient’s attitude, expected compliance with treatment, risk of hypoglycemia, disease duration, life expectancy, and comorbidities, and the side effects of oral medications and insulin.
Compared with previous guidelines, there are fewer rules on how and when to start insulin therapy. But absolute and relative indications for insulin therapy should be considered in patients with the following:
Absolute indications for insulin
- Ketoacidosis or catabolic symptoms, including ketonuria
- Newly diagnosed type 2 diabetes with pronounced hyperglycemia (glucose ≥ 300 mg/dL or hemoglobin A1c ≥ 10.0%) with or without severe symptoms, including weight loss, polyuria, or polydipsia10
- Uncontrolled type 2 diabetes mellitus despite using one, two, or more oral antidiabetic drugs or glucagon-like peptide 1 (GLP-1) receptor agonists
- Gestational diabetes
- Preference for insulin.
Relative indications for insulin
- Hospitalized for surgery or acute illnesses
- Advanced renal or hepatic disease
- Inability to afford the cost or tolerate the side effects of oral antidiabetic drugs and GLP-1 receptor agonists.
Depending on the situation, blood glucose is measured fasting, before meals, or after meals after initiating or adjusting insulin regimens (Table 2).
WHAT ARE THE INSULIN REGIMENS?
Basal insulin
In the early stages of type 2 diabetes, metformin alone or in combination with another oral antidiabetic drug or with a GLP-1 receptor agonist is often used along with healthy eating, weight control, and increased physical activity.
When the target hemoglobin A1c cannot be achieved with one or two noninsulin drugs, the ADA suggests basal insulin be added to metformin or a two-medication regimen that includes metformin (Table 3). However, recent evidence suggests that combining a GLP-1 receptor agonist with basal insulin, in a regimen without metformin, is safe and improves glycemic control without hypoglycemia or weight gain.11
While a total daily dose of insulin of 0.1 to 0.2 units/kg could be initially used in patients with a hemoglobin A1c level less than 8%, a higher dose of 0.2 to 0.3 units/kg is required if the hemoglobin A1c level is between 8% and 10%. The dose can be titrated once or twice weekly if the fasting glucose is above the target level (usually < 130 mg/dL). If hypoglycemia develops (glucose < 70 mg/dL), the insulin dose should be reduced by 10% to 20%.10
Available basal insulins include glargine, detemir, and neutral protamine Hagedorn (NPH) (Table 4).12–14 Because glargine and detemir offer better pharmacokinetic properties, less variability in response, and less risk of hypoglycemia, they are preferred over NPH. Glargine has a relatively constant plasma concentration over 24 hours, allowing once-daily dosing at any time during the day (Figure 1).15 The dose should be taken at the same time every day. Detemir and NPH are usually taken once or twice daily.
Patients treated once daily should take the dose with the evening meal or at bedtime. Patients who require a twice-daily regimen can take the first dose with breakfast and the second one with the evening meal, at bedtime, or 12 hours after the morning dose.
The randomized Treat-to-Target trial,16 in 756 patients, showed that both glargine and NPH, when added to oral therapy in patients with type 2 diabetes, achieve the target hemoglobin A1c, but NPH is associated with more episodes of nocturnal hypoglycemia. Similar results were found when NPH was compared with detemir insulin.17
A Cochrane review18 suggested that glargine and detemir are similar in efficacy and safety. However, detemir often needs to be injected twice daily, in a higher dose, and is associated with less weight gain. Furthermore, a meta-analysis of 46 randomized clinical trials19 showed that the weight increase at 1 year is less in patients treated with basal than with twice-daily or prandial regimens.
A noninterventional longitudinal study20 in 2,179 patients newly started on insulin showed that the mean weight increase at 1 year was 1.78 kg, and 24% of patients gained more than 5 kg. However, the factors independently associated with the weight gain were a higher hemoglobin A1c at baseline, a higher insulin dose at baseline and at 1 year, and a lower baseline body mass index, but not the type of insulin regimen.
Currently, a new class of ultralong-acting basal insulins is being studied. Insulins in this class are approved in other countries, but the US Food and Drug Administration requires additional data for approval. Ultralong-acting insulins are expected to reduce the risk of hypoglycemia, specifically the risk of nocturnal episodes. Also, given their longer duration of action and stable steady-state pharmacokinetics, they will offer flexibility in the dose timing.21
Basal-bolus regimens
Basal insulin often does not control postprandial hyperglycemia. The need for multiple doses of insulin (including one or more preprandial doses) is suggested by postprandial glucose values above target (usually > 180 mg/dL) or by a hemoglobin A1c above goal despite well-controlled fasting glucose levels. This usually becomes evident when the total daily dose of basal insulin exceeds 0.5 units/kg. Patients newly diagnosed with diabetes who have a hemoglobin A1c higher than 10% may also respond better to an initial basal-bolus regimen.
Available bolus insulins include lispro, aspart, glulisine, regular insulin, and the newly approved Technosphere inhaled regular insulin (Table 4).12–14 They can be taken before each meal, and the total bolus dose usually represents 50% of the total daily dose.22 Rapid-acting insulins have faster onset, shorter duration of action, and more predictable pharmacokinetics, which makes them preferable to regular insulin (Figure 1).15 Inhaled insulin is another option, but it is contraindicated in patients with chronic obstructive pulmonary disease or asthma because of the increased risk of acute bronchospasm.12
Alternatively, the transition to a basal-bolus regimen can be accomplished with a single dose of bolus insulin before the main meal, using a dose that represents approximately 10% of the total daily dose. Additional bolus doses can be added later based on the glycemic control. The adjustment of the preprandial insulin dose is done once or twice weekly, based on the postprandial glucose levels.10
Premixed combinations of long- and short-acting insulins in ratios of 50% to 50%, 70% to 30%, or 75% to 25% can be considered in patients who cannot adhere to a complex insulin regimen. A propensity-matched comparison of different insulin regimens (basal, premixed, mealtime plus basal, and mealtime) in patients with type 2 diabetes revealed that the hemoglobin A1c reduction was similar between the different groups.23 However, the number of hypoglycemic episodes was higher in the premixed insulin group, and the weight gain was less in the basal insulin group.
While premixed insulins require fewer injections, they do not provide dosing flexibility. In other words, dose adjustments for premixed insulins lead to increases in both basal and bolus amounts even though a dose adjustment is needed for only one insulin type. Thus, this is a common reason for increased hypoglycemic episodes.
Continuous subcutaneous insulin infusion
A meta-analysis showed that continuous subcutaneous insulin infusion (ie, use of an insulin pump) was similar to intensive therapy with multiple daily insulin injections in terms of glycemic control and hypoglycemia.24 Since both options can lead to similar glucose control, additional factors to consider when initiating insulin infusion include lifestyle and technical expertise. Some patients may or may not prefer having a pump attached for nearly all daily activities. Additionally, this type of therapy is complex and requires significant training to ensure efficacy and safety.25
WHAT IS THE COST OF INSULIN THERAPY?
A final factor to keep in mind when initiating insulin is cost (Table 4).12–14 Asking patients to check their prescription insurance formulary is important to ensure that an affordable option is selected. If patients do not have prescription insurance, medication assistance programs could be an option. However, if a patient is considering an insulin pump, insurance coverage is essential. Depending on the manufacturer, insulin pumps cost about $6,000 to $7,000, and the additional monthly supplies for the pump are also expensive.
If patients are engaged when considering and selecting insulin therapy, the likelihood of treatment success is greater.26–28
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- Hanas R, John WG; International HbA1c Consensus Committee. 2013 Update on the worldwide standardization of the hemoglobin A1c measurement. Pediatr Diabetes 2014; 15:e1–e2.
- Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ; A1c-Derived Average Glucose Study Group. Translating the A1C assay into estimated average glucose values. Diabetes Care 2008; 31:1473–1478.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al; American Association of Clinical Endocrinologists. AACE comprehensive diabetes management algorithm 2013. Endocr Pract 2013; 19:327–336.
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care 2014; 37(suppl 1):S14–S80.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Hemmingsen B, Lund SS, Gluud C, et al. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev 2013; 11:CD008143.
- Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35:1364–1379.
- Vora J, Bain SC, Damci T, et al. Incretin-based therapy in combination with basal insulin: a promising tactic for the treatment of type 2 diabetes. Diabetes Metab 2013; 39:6–15.
- Nuffer W, Trujillo JM, Ellis SL. Technosphere insulin (Afrezza): a new, inhaled prandial insulin. Ann Pharmacother 2015; 49:99–106.
- Pharmacist’s Letter/Prescriber’s Letter. Comparison of insulins and injectable diabetes meds. PL Detail-Document #281107 November 2012. www.PharmacistsLetter.com. Accessed July 2, 2015
- Lexicomp Online. www.wolterskluwercdi.com/lexicomp-online/. Accessed July 2, 2015.
- Hirsch IB. Insulin analogues. N Engl J Med 2005; 352:174-183.
- Riddle MC, Rosenstock J, Gerich J; Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003; 26:3080–3086.
- Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care 2006; 29:1269–1274.
- Swinnen SG, Simon AC, Holleman F, Hoekstra JB, Devries JH. Insulin detemir versus insulin glargine for type 2 diabetes mellitus. Cochrane Database Syst Rev 2011; 7:CD006383.
- Pontiroli AE, Miele L, Morabito A. Increase of body weight during the first year of intensive insulin treatment in type 2 diabetes: systematic review and meta-analysis. Diabetes Obes Metab 2011; 13:1008–1019.
- Balkau B, Home PD, Vincent M, Marre M, Freemantle N. Factors associated with weight gain in people with type 2 diabetes starting on insulin. Diabetes Care 2014; 37:2108–2113.
- Garber AJ. Will the next generation of basal insulins offer clinical advantages? Diabetes Obes Metab 2014; 16:483–491.
- Tamaki M, Shimizu T, Kanazawa A, et al. Effects of changes in basal/total daily insulin ratio in type 2 diabetes patients on intensive insulin therapy including insulin glargine (JUN-LAN Study 6). Diabetes Res Clin Pract 2008; 81:e1–e3.
- Freemantle N, Balkau B, Home PD. A propensity score matched comparison of different insulin regimens 1 year after beginning insulin in people with type 2 diabetes. Diabetes Obes Metab 2013; 15:1120–1127.
- Yeh HC, Brown TT, Maruthur N, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med 2012; 157:336–347.
- Schade DS, Valentine V. To pump or not to pump. Diabetes Care 2002; 25:2100–2102.
- Liu L, Lee MJ, Brateanu A. Improved A1C and lipid profile in patients referred to diabetes education programs in a wide health care network: a retrospective study. Diabetes Spectr 2014; 27:297–303.
- Funnell MM, Kruger DF, Spencer M. Self-management support for insulin therapy in type 2 diabetes. Diabetes Educ 2004; 30:274–280.
- Norris SL, Engelgau MM, Narayan KM. Effectiveness of self-management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care 2001; 24:561–587.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- Hanas R, John WG; International HbA1c Consensus Committee. 2013 Update on the worldwide standardization of the hemoglobin A1c measurement. Pediatr Diabetes 2014; 15:e1–e2.
- Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ; A1c-Derived Average Glucose Study Group. Translating the A1C assay into estimated average glucose values. Diabetes Care 2008; 31:1473–1478.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al; American Association of Clinical Endocrinologists. AACE comprehensive diabetes management algorithm 2013. Endocr Pract 2013; 19:327–336.
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care 2014; 37(suppl 1):S14–S80.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Hemmingsen B, Lund SS, Gluud C, et al. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev 2013; 11:CD008143.
- Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35:1364–1379.
- Vora J, Bain SC, Damci T, et al. Incretin-based therapy in combination with basal insulin: a promising tactic for the treatment of type 2 diabetes. Diabetes Metab 2013; 39:6–15.
- Nuffer W, Trujillo JM, Ellis SL. Technosphere insulin (Afrezza): a new, inhaled prandial insulin. Ann Pharmacother 2015; 49:99–106.
- Pharmacist’s Letter/Prescriber’s Letter. Comparison of insulins and injectable diabetes meds. PL Detail-Document #281107 November 2012. www.PharmacistsLetter.com. Accessed July 2, 2015
- Lexicomp Online. www.wolterskluwercdi.com/lexicomp-online/. Accessed July 2, 2015.
- Hirsch IB. Insulin analogues. N Engl J Med 2005; 352:174-183.
- Riddle MC, Rosenstock J, Gerich J; Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003; 26:3080–3086.
- Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care 2006; 29:1269–1274.
- Swinnen SG, Simon AC, Holleman F, Hoekstra JB, Devries JH. Insulin detemir versus insulin glargine for type 2 diabetes mellitus. Cochrane Database Syst Rev 2011; 7:CD006383.
- Pontiroli AE, Miele L, Morabito A. Increase of body weight during the first year of intensive insulin treatment in type 2 diabetes: systematic review and meta-analysis. Diabetes Obes Metab 2011; 13:1008–1019.
- Balkau B, Home PD, Vincent M, Marre M, Freemantle N. Factors associated with weight gain in people with type 2 diabetes starting on insulin. Diabetes Care 2014; 37:2108–2113.
- Garber AJ. Will the next generation of basal insulins offer clinical advantages? Diabetes Obes Metab 2014; 16:483–491.
- Tamaki M, Shimizu T, Kanazawa A, et al. Effects of changes in basal/total daily insulin ratio in type 2 diabetes patients on intensive insulin therapy including insulin glargine (JUN-LAN Study 6). Diabetes Res Clin Pract 2008; 81:e1–e3.
- Freemantle N, Balkau B, Home PD. A propensity score matched comparison of different insulin regimens 1 year after beginning insulin in people with type 2 diabetes. Diabetes Obes Metab 2013; 15:1120–1127.
- Yeh HC, Brown TT, Maruthur N, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med 2012; 157:336–347.
- Schade DS, Valentine V. To pump or not to pump. Diabetes Care 2002; 25:2100–2102.
- Liu L, Lee MJ, Brateanu A. Improved A1C and lipid profile in patients referred to diabetes education programs in a wide health care network: a retrospective study. Diabetes Spectr 2014; 27:297–303.
- Funnell MM, Kruger DF, Spencer M. Self-management support for insulin therapy in type 2 diabetes. Diabetes Educ 2004; 30:274–280.
- Norris SL, Engelgau MM, Narayan KM. Effectiveness of self-management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care 2001; 24:561–587.
KEY POINTS
- In deciding a patient’s hemoglobin A1c goal and whether it is time to start insulin therapy, one should take into account the patient’s age, life expectancy, concurrent illnesses, risk of hypoglycemia, and other factors.
- When the target hemoglobin A1c is not achieved with metformin or a two-drug regimen that includes metformin, the American Diabetes Association recommends adding a daily dose of basal insulin.
- Eventually, preprandial bolus doses may need to be added to the insulin regimen to control postprandial blood glucose levels and hemoglobin A1c.
Do patients who received only two doses of hepatitis B vaccine need a booster?
The Advisory Committee on Immunization Practices (ACIP) currently recommends that people who have not completed the three-dose vaccination series against hepatitis B virus (HBV) should receive the missed doses: ie, the three-dose regimen does not need to be restarted.1 However, evidence suggests that a two-dose regimen may provide adequate seroprotection for healthy young adults.
As the three-dose regimen has been shown to protect 90% to 100% of adults,2 it has gained widespread acceptance and is now standard clinical practice.2 However, deviating from the three-dose regimen may not leave healthy young adults vulnerable to HBV infection.
RECOMMENDED DOSES AND SCHEDULES
Widespread use of the three-dose regimen for HBV stemmed from the first clinical evaluation of the recombinant vaccine, in which three 10-μg doses were given at 0, 1, and 6 months to healthy, low-risk adult volunteers.3 This regimen was shown to provide seroprotection in over 95% of adolescents and 90% of healthy adults.2
Currently, three HBV vaccines for adults are approved in the United States: Recombivax HB, Engerix-B, and Twinrix (Table 1). While Recombivax has a seroprotection rate of 89% in healthy adults over age 40, it has higher seroprotection rates in younger people: eg, two doses of Recombivax given 4 to 6 months apart provide seroprotection to 99% of children aged 11 to 15.4 On the other hand, patients on hemodialysis require three 40-μg doses of Recombivax or four 40-μg doses of Engerix-B.
Evidence for a two-dose regimen
Since the development of the recombinant HBV vaccine used today, studies have shown that a two-dose regimen offers seroprotection comparable with, if not better than, the three-dose regimen in adolescents and healthy young adults. Marsano et al5 found that with a two-dose regimen, 96% to 99% of young adults attained seroprotection, with immune memory persisting for up to 2 years.5 Moreover, Cassidy et al6 randomized adolescents to a two-dose or a three-dose regimen and found the two regimens to be equally effective in conferring immunogenicity and immunologic memory.6
Other studies in adolescents have confirmed these findings and offered new evidence in support of the two-dose regimen.7,8 For example, studies found that the two-dose regimen conferred seroprotection at even lower doses than previously studied, and that it conferred immune memory lasting at least 5 years.6,7
However, because these studies were conducted in adolescents and healthy young adults, the findings may not hold true for other populations. Studies suggest that the three-dose regimen is best for those over age 40. Moreover, it is advisable to adhere to a three-dose regimen when treating people at high risk of contracting HBV, such as health care workers; people with chronic liver disease, diabetes mellitus, or end-stage renal disease on hemodialysis; people who have multiple sex partners; and men who have sex with men.
The impact of long intervals between doses
Although the aforementioned studies focused on a two-dose regimen with a 6-month interval, longer intervals between doses do not impair seroprotection and in some cases may even prove beneficial. Heron et al9 demonstrated that a two-dose regimen with a 12-month interval induces seroprotection as effectively as a standard three-dose or two-dose regimen with a 6-month interval.9 Moreover, studies of the impact of deviating from a three-dose regimen found that intervals of longer than 1 year did not impede seroprotection. Not only may seroprotection be attained with intervals of 5 to 10 years before the final dose, but final antibody levels tend to increase with increasing time between doses.10
Nevertheless, even though an extended interval between doses may prove beneficial after the final dose is received, delaying doses may leave patients unprotected. Indeed, alternative three-dose and even four-dose schedules with shorter intervals between doses exist for certain high-risk populations, such as those recently exposed to HBV and travelers to areas of high prevalence. Therefore, intentionally extending intervals between doses may be inappropriate.
SEROPROTECTION AND PROTECTION AGAINST INFECTION
Legitimate concerns exist about the final antibody level attained with a two-dose regimen, which is typically lower than that attained with a three-dose regimen. As HBV antibody levels decline with time, lower final antibody levels theoretically increase the risk of losing seroprotection. Study of vaccine efficacy has defined seroprotection as antibody levels greater than or equal to 10 mIU/mL.11 Yet evidence suggests that even when antibody levels drop below this level, the risk of symptomatic HBV infection does not increase. Evidence also suggests that immune memory outlasts the presence of seroprotective antibody levels, indicating that true protection against significant infection does not necessarily correlate with, and may even exceed, seroprotection.2 This may relate to HBV’s long incubation period, which allows memory cells time to generate an effective immune response.10 For example, Floreani et al12 showed that even though 15% of adults lost seroprotective antibody levels 10 years after vaccination, none demonstrated hepatitis B antigen reactivity or seroconversion.
POSTVACCINATION TESTING AND ADDITIONAL DOSES
At times, it may be wise to measure antibody levels after the final dose to confirm seroprotection. Seroprotection should be documented when knowledge of the patient’s immune status will affect subsequent management. As recommended by the US Centers for Disease Control and Prevention, health care workers, hemodialysis patients, immunocompromised patients, and sexual partners of patients with chronic HBV infection should undergo antibody testing 1 to 2 months after the completion of a three-dose vaccination regimen. Hemodialysis patients require annual confirmation of seroprotection and should receive booster doses of HBV vaccine if necessary.
Postvaccination testing (quantitative HBV surface antibody testing) costs about the same as a single dose of HBV vaccine. Therefore, if postvaccination testing is considered because of missed vaccine doses, it may be more cost-efficient to simply administer the missed dose.
- Department of Health and Human Services. Appendix A Immunization Management Issues. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5516a2.htm. Accessed April 6, 2014.
- Leuridan E, Van Damme P. Hepatitis B and the need for a booster dose. Clin Infect Dis 2011; 53:68–75.
- Scolnick EM, McLean AA, West DJ, McAleer WJ, Miller WJ, Buynak EB. Clinical evaluation in healthy adults of a hepatitis B vaccine made by recombinant DNA. JAMA 1984; 251:2812–2815.
- Merck and Co, Inc. 1998. Recombivax HB. http://www.merck.com/product/usa/pi_circulars/r/recombivax_hb/re-combivax_pi.pdf. Accessed April 7, 2014.
- Marsano LS, West DJ, Chan I, et al. A two-dose hepatitis B vaccine regimen: proof of priming and memory responses in young adults. Vaccine 1998; 16:624–629.
- Cassidy WM, Watson B, Ioli VA, Williams K, Bird S, West DJ. A randomized trial of alternative two- and three-dose hepatitis B vaccination regimens in adolescents: antibody responses, safety, and immunologic memory. Pediatrics 2001; 107:626–631.
- Van Damme P, Moiseeva A, Marichev I, et al. Five years follow-up following two or three doses of a hepatitis B vaccine in adolescents aged 11–15 years: a randomised controlled study. BMC Infect Dis 2010; 10:357.
- Heron L, Selnikova O, Moiseieva A, et al. Immunogenicity, reactogenicity and safety of two-dose versus three-dose (standard care) hepatitis B immunisation of healthy adolescents aged 11–15 years: a randomised controlled trial. Vaccine 2007; 25:2817–2822.
- Heron LG, Chant KG, Jalaludin BB. A novel hepatitis B vaccination regimen for adolescents: two doses 12 months apart. Vaccine 2002; 20:3472–3476.
- Jackson Y, Chappuis F, Mezger N, Kanappa K, Loutan L. High immunogenicity of delayed third dose of hepatitis B vaccine in travellers. Vaccine 2007; 25:3482–3484.
- Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC. What level of hepatitis B antibody is protective? J Infect Dis 1999; 179:489–492.
- Floreani A, Baldo V, Cristofoletti M, et al. Long-term persistence of anti-HBs after vaccination against HBV: an 18 year experience in health care workers. Vaccine 2004; 22:607–610.
The Advisory Committee on Immunization Practices (ACIP) currently recommends that people who have not completed the three-dose vaccination series against hepatitis B virus (HBV) should receive the missed doses: ie, the three-dose regimen does not need to be restarted.1 However, evidence suggests that a two-dose regimen may provide adequate seroprotection for healthy young adults.
As the three-dose regimen has been shown to protect 90% to 100% of adults,2 it has gained widespread acceptance and is now standard clinical practice.2 However, deviating from the three-dose regimen may not leave healthy young adults vulnerable to HBV infection.
RECOMMENDED DOSES AND SCHEDULES
Widespread use of the three-dose regimen for HBV stemmed from the first clinical evaluation of the recombinant vaccine, in which three 10-μg doses were given at 0, 1, and 6 months to healthy, low-risk adult volunteers.3 This regimen was shown to provide seroprotection in over 95% of adolescents and 90% of healthy adults.2
Currently, three HBV vaccines for adults are approved in the United States: Recombivax HB, Engerix-B, and Twinrix (Table 1). While Recombivax has a seroprotection rate of 89% in healthy adults over age 40, it has higher seroprotection rates in younger people: eg, two doses of Recombivax given 4 to 6 months apart provide seroprotection to 99% of children aged 11 to 15.4 On the other hand, patients on hemodialysis require three 40-μg doses of Recombivax or four 40-μg doses of Engerix-B.
Evidence for a two-dose regimen
Since the development of the recombinant HBV vaccine used today, studies have shown that a two-dose regimen offers seroprotection comparable with, if not better than, the three-dose regimen in adolescents and healthy young adults. Marsano et al5 found that with a two-dose regimen, 96% to 99% of young adults attained seroprotection, with immune memory persisting for up to 2 years.5 Moreover, Cassidy et al6 randomized adolescents to a two-dose or a three-dose regimen and found the two regimens to be equally effective in conferring immunogenicity and immunologic memory.6
Other studies in adolescents have confirmed these findings and offered new evidence in support of the two-dose regimen.7,8 For example, studies found that the two-dose regimen conferred seroprotection at even lower doses than previously studied, and that it conferred immune memory lasting at least 5 years.6,7
However, because these studies were conducted in adolescents and healthy young adults, the findings may not hold true for other populations. Studies suggest that the three-dose regimen is best for those over age 40. Moreover, it is advisable to adhere to a three-dose regimen when treating people at high risk of contracting HBV, such as health care workers; people with chronic liver disease, diabetes mellitus, or end-stage renal disease on hemodialysis; people who have multiple sex partners; and men who have sex with men.
The impact of long intervals between doses
Although the aforementioned studies focused on a two-dose regimen with a 6-month interval, longer intervals between doses do not impair seroprotection and in some cases may even prove beneficial. Heron et al9 demonstrated that a two-dose regimen with a 12-month interval induces seroprotection as effectively as a standard three-dose or two-dose regimen with a 6-month interval.9 Moreover, studies of the impact of deviating from a three-dose regimen found that intervals of longer than 1 year did not impede seroprotection. Not only may seroprotection be attained with intervals of 5 to 10 years before the final dose, but final antibody levels tend to increase with increasing time between doses.10
Nevertheless, even though an extended interval between doses may prove beneficial after the final dose is received, delaying doses may leave patients unprotected. Indeed, alternative three-dose and even four-dose schedules with shorter intervals between doses exist for certain high-risk populations, such as those recently exposed to HBV and travelers to areas of high prevalence. Therefore, intentionally extending intervals between doses may be inappropriate.
SEROPROTECTION AND PROTECTION AGAINST INFECTION
Legitimate concerns exist about the final antibody level attained with a two-dose regimen, which is typically lower than that attained with a three-dose regimen. As HBV antibody levels decline with time, lower final antibody levels theoretically increase the risk of losing seroprotection. Study of vaccine efficacy has defined seroprotection as antibody levels greater than or equal to 10 mIU/mL.11 Yet evidence suggests that even when antibody levels drop below this level, the risk of symptomatic HBV infection does not increase. Evidence also suggests that immune memory outlasts the presence of seroprotective antibody levels, indicating that true protection against significant infection does not necessarily correlate with, and may even exceed, seroprotection.2 This may relate to HBV’s long incubation period, which allows memory cells time to generate an effective immune response.10 For example, Floreani et al12 showed that even though 15% of adults lost seroprotective antibody levels 10 years after vaccination, none demonstrated hepatitis B antigen reactivity or seroconversion.
POSTVACCINATION TESTING AND ADDITIONAL DOSES
At times, it may be wise to measure antibody levels after the final dose to confirm seroprotection. Seroprotection should be documented when knowledge of the patient’s immune status will affect subsequent management. As recommended by the US Centers for Disease Control and Prevention, health care workers, hemodialysis patients, immunocompromised patients, and sexual partners of patients with chronic HBV infection should undergo antibody testing 1 to 2 months after the completion of a three-dose vaccination regimen. Hemodialysis patients require annual confirmation of seroprotection and should receive booster doses of HBV vaccine if necessary.
Postvaccination testing (quantitative HBV surface antibody testing) costs about the same as a single dose of HBV vaccine. Therefore, if postvaccination testing is considered because of missed vaccine doses, it may be more cost-efficient to simply administer the missed dose.
The Advisory Committee on Immunization Practices (ACIP) currently recommends that people who have not completed the three-dose vaccination series against hepatitis B virus (HBV) should receive the missed doses: ie, the three-dose regimen does not need to be restarted.1 However, evidence suggests that a two-dose regimen may provide adequate seroprotection for healthy young adults.
As the three-dose regimen has been shown to protect 90% to 100% of adults,2 it has gained widespread acceptance and is now standard clinical practice.2 However, deviating from the three-dose regimen may not leave healthy young adults vulnerable to HBV infection.
RECOMMENDED DOSES AND SCHEDULES
Widespread use of the three-dose regimen for HBV stemmed from the first clinical evaluation of the recombinant vaccine, in which three 10-μg doses were given at 0, 1, and 6 months to healthy, low-risk adult volunteers.3 This regimen was shown to provide seroprotection in over 95% of adolescents and 90% of healthy adults.2
Currently, three HBV vaccines for adults are approved in the United States: Recombivax HB, Engerix-B, and Twinrix (Table 1). While Recombivax has a seroprotection rate of 89% in healthy adults over age 40, it has higher seroprotection rates in younger people: eg, two doses of Recombivax given 4 to 6 months apart provide seroprotection to 99% of children aged 11 to 15.4 On the other hand, patients on hemodialysis require three 40-μg doses of Recombivax or four 40-μg doses of Engerix-B.
Evidence for a two-dose regimen
Since the development of the recombinant HBV vaccine used today, studies have shown that a two-dose regimen offers seroprotection comparable with, if not better than, the three-dose regimen in adolescents and healthy young adults. Marsano et al5 found that with a two-dose regimen, 96% to 99% of young adults attained seroprotection, with immune memory persisting for up to 2 years.5 Moreover, Cassidy et al6 randomized adolescents to a two-dose or a three-dose regimen and found the two regimens to be equally effective in conferring immunogenicity and immunologic memory.6
Other studies in adolescents have confirmed these findings and offered new evidence in support of the two-dose regimen.7,8 For example, studies found that the two-dose regimen conferred seroprotection at even lower doses than previously studied, and that it conferred immune memory lasting at least 5 years.6,7
However, because these studies were conducted in adolescents and healthy young adults, the findings may not hold true for other populations. Studies suggest that the three-dose regimen is best for those over age 40. Moreover, it is advisable to adhere to a three-dose regimen when treating people at high risk of contracting HBV, such as health care workers; people with chronic liver disease, diabetes mellitus, or end-stage renal disease on hemodialysis; people who have multiple sex partners; and men who have sex with men.
The impact of long intervals between doses
Although the aforementioned studies focused on a two-dose regimen with a 6-month interval, longer intervals between doses do not impair seroprotection and in some cases may even prove beneficial. Heron et al9 demonstrated that a two-dose regimen with a 12-month interval induces seroprotection as effectively as a standard three-dose or two-dose regimen with a 6-month interval.9 Moreover, studies of the impact of deviating from a three-dose regimen found that intervals of longer than 1 year did not impede seroprotection. Not only may seroprotection be attained with intervals of 5 to 10 years before the final dose, but final antibody levels tend to increase with increasing time between doses.10
Nevertheless, even though an extended interval between doses may prove beneficial after the final dose is received, delaying doses may leave patients unprotected. Indeed, alternative three-dose and even four-dose schedules with shorter intervals between doses exist for certain high-risk populations, such as those recently exposed to HBV and travelers to areas of high prevalence. Therefore, intentionally extending intervals between doses may be inappropriate.
SEROPROTECTION AND PROTECTION AGAINST INFECTION
Legitimate concerns exist about the final antibody level attained with a two-dose regimen, which is typically lower than that attained with a three-dose regimen. As HBV antibody levels decline with time, lower final antibody levels theoretically increase the risk of losing seroprotection. Study of vaccine efficacy has defined seroprotection as antibody levels greater than or equal to 10 mIU/mL.11 Yet evidence suggests that even when antibody levels drop below this level, the risk of symptomatic HBV infection does not increase. Evidence also suggests that immune memory outlasts the presence of seroprotective antibody levels, indicating that true protection against significant infection does not necessarily correlate with, and may even exceed, seroprotection.2 This may relate to HBV’s long incubation period, which allows memory cells time to generate an effective immune response.10 For example, Floreani et al12 showed that even though 15% of adults lost seroprotective antibody levels 10 years after vaccination, none demonstrated hepatitis B antigen reactivity or seroconversion.
POSTVACCINATION TESTING AND ADDITIONAL DOSES
At times, it may be wise to measure antibody levels after the final dose to confirm seroprotection. Seroprotection should be documented when knowledge of the patient’s immune status will affect subsequent management. As recommended by the US Centers for Disease Control and Prevention, health care workers, hemodialysis patients, immunocompromised patients, and sexual partners of patients with chronic HBV infection should undergo antibody testing 1 to 2 months after the completion of a three-dose vaccination regimen. Hemodialysis patients require annual confirmation of seroprotection and should receive booster doses of HBV vaccine if necessary.
Postvaccination testing (quantitative HBV surface antibody testing) costs about the same as a single dose of HBV vaccine. Therefore, if postvaccination testing is considered because of missed vaccine doses, it may be more cost-efficient to simply administer the missed dose.
- Department of Health and Human Services. Appendix A Immunization Management Issues. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5516a2.htm. Accessed April 6, 2014.
- Leuridan E, Van Damme P. Hepatitis B and the need for a booster dose. Clin Infect Dis 2011; 53:68–75.
- Scolnick EM, McLean AA, West DJ, McAleer WJ, Miller WJ, Buynak EB. Clinical evaluation in healthy adults of a hepatitis B vaccine made by recombinant DNA. JAMA 1984; 251:2812–2815.
- Merck and Co, Inc. 1998. Recombivax HB. http://www.merck.com/product/usa/pi_circulars/r/recombivax_hb/re-combivax_pi.pdf. Accessed April 7, 2014.
- Marsano LS, West DJ, Chan I, et al. A two-dose hepatitis B vaccine regimen: proof of priming and memory responses in young adults. Vaccine 1998; 16:624–629.
- Cassidy WM, Watson B, Ioli VA, Williams K, Bird S, West DJ. A randomized trial of alternative two- and three-dose hepatitis B vaccination regimens in adolescents: antibody responses, safety, and immunologic memory. Pediatrics 2001; 107:626–631.
- Van Damme P, Moiseeva A, Marichev I, et al. Five years follow-up following two or three doses of a hepatitis B vaccine in adolescents aged 11–15 years: a randomised controlled study. BMC Infect Dis 2010; 10:357.
- Heron L, Selnikova O, Moiseieva A, et al. Immunogenicity, reactogenicity and safety of two-dose versus three-dose (standard care) hepatitis B immunisation of healthy adolescents aged 11–15 years: a randomised controlled trial. Vaccine 2007; 25:2817–2822.
- Heron LG, Chant KG, Jalaludin BB. A novel hepatitis B vaccination regimen for adolescents: two doses 12 months apart. Vaccine 2002; 20:3472–3476.
- Jackson Y, Chappuis F, Mezger N, Kanappa K, Loutan L. High immunogenicity of delayed third dose of hepatitis B vaccine in travellers. Vaccine 2007; 25:3482–3484.
- Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC. What level of hepatitis B antibody is protective? J Infect Dis 1999; 179:489–492.
- Floreani A, Baldo V, Cristofoletti M, et al. Long-term persistence of anti-HBs after vaccination against HBV: an 18 year experience in health care workers. Vaccine 2004; 22:607–610.
- Department of Health and Human Services. Appendix A Immunization Management Issues. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5516a2.htm. Accessed April 6, 2014.
- Leuridan E, Van Damme P. Hepatitis B and the need for a booster dose. Clin Infect Dis 2011; 53:68–75.
- Scolnick EM, McLean AA, West DJ, McAleer WJ, Miller WJ, Buynak EB. Clinical evaluation in healthy adults of a hepatitis B vaccine made by recombinant DNA. JAMA 1984; 251:2812–2815.
- Merck and Co, Inc. 1998. Recombivax HB. http://www.merck.com/product/usa/pi_circulars/r/recombivax_hb/re-combivax_pi.pdf. Accessed April 7, 2014.
- Marsano LS, West DJ, Chan I, et al. A two-dose hepatitis B vaccine regimen: proof of priming and memory responses in young adults. Vaccine 1998; 16:624–629.
- Cassidy WM, Watson B, Ioli VA, Williams K, Bird S, West DJ. A randomized trial of alternative two- and three-dose hepatitis B vaccination regimens in adolescents: antibody responses, safety, and immunologic memory. Pediatrics 2001; 107:626–631.
- Van Damme P, Moiseeva A, Marichev I, et al. Five years follow-up following two or three doses of a hepatitis B vaccine in adolescents aged 11–15 years: a randomised controlled study. BMC Infect Dis 2010; 10:357.
- Heron L, Selnikova O, Moiseieva A, et al. Immunogenicity, reactogenicity and safety of two-dose versus three-dose (standard care) hepatitis B immunisation of healthy adolescents aged 11–15 years: a randomised controlled trial. Vaccine 2007; 25:2817–2822.
- Heron LG, Chant KG, Jalaludin BB. A novel hepatitis B vaccination regimen for adolescents: two doses 12 months apart. Vaccine 2002; 20:3472–3476.
- Jackson Y, Chappuis F, Mezger N, Kanappa K, Loutan L. High immunogenicity of delayed third dose of hepatitis B vaccine in travellers. Vaccine 2007; 25:3482–3484.
- Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC. What level of hepatitis B antibody is protective? J Infect Dis 1999; 179:489–492.
- Floreani A, Baldo V, Cristofoletti M, et al. Long-term persistence of anti-HBs after vaccination against HBV: an 18 year experience in health care workers. Vaccine 2004; 22:607–610.