User login
Christopher Palmer has been an associate editor at MDedge News since 2017. When he's not tidying grammar, he writes short pieces about breaking FDA announcements and approvals, as well as journal articles. He proudly holds a BA in English and philosophy. Follow him on Twitter @cmacmpalm.
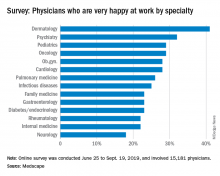
Survey queries pulmonologists' happiness at work
Only 26% of pulmonologists report that they are happy at work, with about twice as many happy outside of work, according to Medscape’s Pulmonologist Lifestyle, Happiness & Burnout Report 2020. Dermatologists are the happiest at work, at 41%, and neurologists are the least happy, at 18%. 
According to the report, which surveyed more than 15,000 physicians from various specialties, 29% of pulmonologists report feeling burned out, with 5% reporting feeling depressed and 12% both depressed and burned out. An overabundance of bureaucratic tasks is the lead contributor to burnout (52%), according to pulmonologists, followed by lack of respect from administrators, employers, colleagues, and staff (38%) and spending too many hours at work (35%).
Pulmonologists report that exercise is the biggest way they cope with burnout (47%), compared with neurologists, for example, who ranked it third at 40%. Other ways they deal with burnout include isolating themselves from others (43%) and playing or listening to music (38%).
Among depressed or burned-out pulmonologists, 70% reported not planning to seek professional help or seeking it in the past, while 12% reported currently seeking professional help. Furthermore, almost half of pulmonologists (48%) say they’re unlikely to participate in workplace programs.
When asked for reasons they wouldn’t seek professional help, 60% said they deal with it without professional help and 49% didn’t think their symptoms were severe enough, while 31% were simply too busy.
The slideshow of the full report is available on Medscape.com.
Only 26% of pulmonologists report that they are happy at work, with about twice as many happy outside of work, according to Medscape’s Pulmonologist Lifestyle, Happiness & Burnout Report 2020. Dermatologists are the happiest at work, at 41%, and neurologists are the least happy, at 18%. 
According to the report, which surveyed more than 15,000 physicians from various specialties, 29% of pulmonologists report feeling burned out, with 5% reporting feeling depressed and 12% both depressed and burned out. An overabundance of bureaucratic tasks is the lead contributor to burnout (52%), according to pulmonologists, followed by lack of respect from administrators, employers, colleagues, and staff (38%) and spending too many hours at work (35%).
Pulmonologists report that exercise is the biggest way they cope with burnout (47%), compared with neurologists, for example, who ranked it third at 40%. Other ways they deal with burnout include isolating themselves from others (43%) and playing or listening to music (38%).
Among depressed or burned-out pulmonologists, 70% reported not planning to seek professional help or seeking it in the past, while 12% reported currently seeking professional help. Furthermore, almost half of pulmonologists (48%) say they’re unlikely to participate in workplace programs.
When asked for reasons they wouldn’t seek professional help, 60% said they deal with it without professional help and 49% didn’t think their symptoms were severe enough, while 31% were simply too busy.
The slideshow of the full report is available on Medscape.com.
Only 26% of pulmonologists report that they are happy at work, with about twice as many happy outside of work, according to Medscape’s Pulmonologist Lifestyle, Happiness & Burnout Report 2020. Dermatologists are the happiest at work, at 41%, and neurologists are the least happy, at 18%. 
According to the report, which surveyed more than 15,000 physicians from various specialties, 29% of pulmonologists report feeling burned out, with 5% reporting feeling depressed and 12% both depressed and burned out. An overabundance of bureaucratic tasks is the lead contributor to burnout (52%), according to pulmonologists, followed by lack of respect from administrators, employers, colleagues, and staff (38%) and spending too many hours at work (35%).
Pulmonologists report that exercise is the biggest way they cope with burnout (47%), compared with neurologists, for example, who ranked it third at 40%. Other ways they deal with burnout include isolating themselves from others (43%) and playing or listening to music (38%).
Among depressed or burned-out pulmonologists, 70% reported not planning to seek professional help or seeking it in the past, while 12% reported currently seeking professional help. Furthermore, almost half of pulmonologists (48%) say they’re unlikely to participate in workplace programs.
When asked for reasons they wouldn’t seek professional help, 60% said they deal with it without professional help and 49% didn’t think their symptoms were severe enough, while 31% were simply too busy.
The slideshow of the full report is available on Medscape.com.
Less than a quarter of endocrinologists happy at work
according to Medscape’s Endocrinologist Lifestyle, Happiness, and Burnout Report 2020.
The report, which surveyed more than 15,000 physicians from various specialties, found that 31% of endocrinologists are burned out, 2% are depressed, and 16% are both depressed and burned out. Among them, 71% report having too many bureaucratic tasks as the biggest contributor, followed by insufficient reimbursement/compensation at 46% and increasing computerization of tasks (including EHR) at 34%.
The top way endocrinologists cope is by talking with family and close friends (42%), with almost equal amounts coping by either eating junk food or exercising (39% and 37%, respectively). Regarding professional help, 60% said they would not and have not previously sought professional help, while 12% said they were currently seeking professional help. Although 40% said they would engage a workplace program, 31% said they would not.
Among the reasons they gave for not seeking professional help, 45% felt they were too busy, 40% didn’t think their symptoms were severe enough, and 36% said they could handle it without help from professionals.
A slideshow of the full report can be found on Medscape.com.
according to Medscape’s Endocrinologist Lifestyle, Happiness, and Burnout Report 2020.
The report, which surveyed more than 15,000 physicians from various specialties, found that 31% of endocrinologists are burned out, 2% are depressed, and 16% are both depressed and burned out. Among them, 71% report having too many bureaucratic tasks as the biggest contributor, followed by insufficient reimbursement/compensation at 46% and increasing computerization of tasks (including EHR) at 34%.
The top way endocrinologists cope is by talking with family and close friends (42%), with almost equal amounts coping by either eating junk food or exercising (39% and 37%, respectively). Regarding professional help, 60% said they would not and have not previously sought professional help, while 12% said they were currently seeking professional help. Although 40% said they would engage a workplace program, 31% said they would not.
Among the reasons they gave for not seeking professional help, 45% felt they were too busy, 40% didn’t think their symptoms were severe enough, and 36% said they could handle it without help from professionals.
A slideshow of the full report can be found on Medscape.com.
according to Medscape’s Endocrinologist Lifestyle, Happiness, and Burnout Report 2020.
The report, which surveyed more than 15,000 physicians from various specialties, found that 31% of endocrinologists are burned out, 2% are depressed, and 16% are both depressed and burned out. Among them, 71% report having too many bureaucratic tasks as the biggest contributor, followed by insufficient reimbursement/compensation at 46% and increasing computerization of tasks (including EHR) at 34%.
The top way endocrinologists cope is by talking with family and close friends (42%), with almost equal amounts coping by either eating junk food or exercising (39% and 37%, respectively). Regarding professional help, 60% said they would not and have not previously sought professional help, while 12% said they were currently seeking professional help. Although 40% said they would engage a workplace program, 31% said they would not.
Among the reasons they gave for not seeking professional help, 45% felt they were too busy, 40% didn’t think their symptoms were severe enough, and 36% said they could handle it without help from professionals.
A slideshow of the full report can be found on Medscape.com.
FDA issues MiniMed600 insulin pump recall
according to a Food and Drug Administration MedWatch release.
A class I recall such as this indicates that there is reasonable probability that using a defective pump will cause serious adverse health consequences or death, the agency said in the recall notice. It said the company has received more than 26,000 complaints regarding this problem and is aware of 2,175 injuries and 1 death so far. In all, 322,005 devices have been recalled.
If the pumps in question – Model 630G (distributed September 2016 to October 2019) and 670G (June 2017 to August 2019) – have broken or missing retainer rings, the insulin cartridge can end up loose in the reservoir compartment, which can lead to incorrect dosing and therefore potentially to hyperglycemia or hypoglycemia, according to the statement.
Model 630G was approved by the FDA in August 2016, and the 670G in September that same year.
On Nov. 21, 2019, Medtronic advised patients with type 1 diabetes who use the pumps to:
- Examine the retainer ring to see if it is loose, broken, or missing.
- Stop using the pump if the reservoir does not lock correctly or if the retainer ring is loose, damaged, or missing. Patients should contact Medtronic for a replacement pump and follow their doctor’s recommendations and perform manual insulin injections.
- Continue using the pump if the reservoir locks in place correctly.
- Check pump and retainer ring if the pump is dropped by accident, and stop use if it is damaged.
- Check the pump and retainer ring every set change to verify the reservoir is locked correctly.
More information regarding this recall, including how to contact Medtronic Technical Support, can be found on the FDA website.
according to a Food and Drug Administration MedWatch release.
A class I recall such as this indicates that there is reasonable probability that using a defective pump will cause serious adverse health consequences or death, the agency said in the recall notice. It said the company has received more than 26,000 complaints regarding this problem and is aware of 2,175 injuries and 1 death so far. In all, 322,005 devices have been recalled.
If the pumps in question – Model 630G (distributed September 2016 to October 2019) and 670G (June 2017 to August 2019) – have broken or missing retainer rings, the insulin cartridge can end up loose in the reservoir compartment, which can lead to incorrect dosing and therefore potentially to hyperglycemia or hypoglycemia, according to the statement.
Model 630G was approved by the FDA in August 2016, and the 670G in September that same year.
On Nov. 21, 2019, Medtronic advised patients with type 1 diabetes who use the pumps to:
- Examine the retainer ring to see if it is loose, broken, or missing.
- Stop using the pump if the reservoir does not lock correctly or if the retainer ring is loose, damaged, or missing. Patients should contact Medtronic for a replacement pump and follow their doctor’s recommendations and perform manual insulin injections.
- Continue using the pump if the reservoir locks in place correctly.
- Check pump and retainer ring if the pump is dropped by accident, and stop use if it is damaged.
- Check the pump and retainer ring every set change to verify the reservoir is locked correctly.
More information regarding this recall, including how to contact Medtronic Technical Support, can be found on the FDA website.
according to a Food and Drug Administration MedWatch release.
A class I recall such as this indicates that there is reasonable probability that using a defective pump will cause serious adverse health consequences or death, the agency said in the recall notice. It said the company has received more than 26,000 complaints regarding this problem and is aware of 2,175 injuries and 1 death so far. In all, 322,005 devices have been recalled.
If the pumps in question – Model 630G (distributed September 2016 to October 2019) and 670G (June 2017 to August 2019) – have broken or missing retainer rings, the insulin cartridge can end up loose in the reservoir compartment, which can lead to incorrect dosing and therefore potentially to hyperglycemia or hypoglycemia, according to the statement.
Model 630G was approved by the FDA in August 2016, and the 670G in September that same year.
On Nov. 21, 2019, Medtronic advised patients with type 1 diabetes who use the pumps to:
- Examine the retainer ring to see if it is loose, broken, or missing.
- Stop using the pump if the reservoir does not lock correctly or if the retainer ring is loose, damaged, or missing. Patients should contact Medtronic for a replacement pump and follow their doctor’s recommendations and perform manual insulin injections.
- Continue using the pump if the reservoir locks in place correctly.
- Check pump and retainer ring if the pump is dropped by accident, and stop use if it is damaged.
- Check the pump and retainer ring every set change to verify the reservoir is locked correctly.
More information regarding this recall, including how to contact Medtronic Technical Support, can be found on the FDA website.
FDA: Cell phones still look safe
according to a review by the Food and Drug Administration.
The FDA reviewed the published literature from 2008 to 2018 and concluded that the data don’t support any quantifiable adverse health risks from RFR. However, the evidence is not without limitations.
The FDA’s evaluation included evidence from in vivo animal studies from Jan. 1, 2008, to Aug. 1, 2018, and epidemiologic studies in humans from Jan. 1, 2008, to May 8, 2018. Both kinds of evidence had limitations, but neither produced strong indications of any causal risks from cell phone use.
The FDA noted that in vivo animal studies are limited by variability of methods and RFR exposure, which make comparisons of results difficult. These studies are also impacted by the indirect effects of temperature increases (the only currently established biological effect of RFR) and stress experienced by the animals, which make teasing out the direct effects of RFR difficult.
The FDA noted that strong epidemiologic studies can provide more relevant and accurate information than in vivo studies, but epidemiologic studies are not without limitations. For example, most have participants track and self-report their cell phone use. There’s also no way to directly track certain factors of RFR exposure, such as frequency, duration, or intensity.
Even with those caveats in mind, the FDA wrote that, “based on the studies that are described in detail in this report, there is insufficient evidence to support a causal association between RFR exposure and tumorigenesis. There is a lack of clear dose-response relationship, a lack of consistent findings or specificity, and a lack of biological mechanistic plausibility.”
The full review is available on the FDA website.
according to a review by the Food and Drug Administration.
The FDA reviewed the published literature from 2008 to 2018 and concluded that the data don’t support any quantifiable adverse health risks from RFR. However, the evidence is not without limitations.
The FDA’s evaluation included evidence from in vivo animal studies from Jan. 1, 2008, to Aug. 1, 2018, and epidemiologic studies in humans from Jan. 1, 2008, to May 8, 2018. Both kinds of evidence had limitations, but neither produced strong indications of any causal risks from cell phone use.
The FDA noted that in vivo animal studies are limited by variability of methods and RFR exposure, which make comparisons of results difficult. These studies are also impacted by the indirect effects of temperature increases (the only currently established biological effect of RFR) and stress experienced by the animals, which make teasing out the direct effects of RFR difficult.
The FDA noted that strong epidemiologic studies can provide more relevant and accurate information than in vivo studies, but epidemiologic studies are not without limitations. For example, most have participants track and self-report their cell phone use. There’s also no way to directly track certain factors of RFR exposure, such as frequency, duration, or intensity.
Even with those caveats in mind, the FDA wrote that, “based on the studies that are described in detail in this report, there is insufficient evidence to support a causal association between RFR exposure and tumorigenesis. There is a lack of clear dose-response relationship, a lack of consistent findings or specificity, and a lack of biological mechanistic plausibility.”
The full review is available on the FDA website.
according to a review by the Food and Drug Administration.
The FDA reviewed the published literature from 2008 to 2018 and concluded that the data don’t support any quantifiable adverse health risks from RFR. However, the evidence is not without limitations.
The FDA’s evaluation included evidence from in vivo animal studies from Jan. 1, 2008, to Aug. 1, 2018, and epidemiologic studies in humans from Jan. 1, 2008, to May 8, 2018. Both kinds of evidence had limitations, but neither produced strong indications of any causal risks from cell phone use.
The FDA noted that in vivo animal studies are limited by variability of methods and RFR exposure, which make comparisons of results difficult. These studies are also impacted by the indirect effects of temperature increases (the only currently established biological effect of RFR) and stress experienced by the animals, which make teasing out the direct effects of RFR difficult.
The FDA noted that strong epidemiologic studies can provide more relevant and accurate information than in vivo studies, but epidemiologic studies are not without limitations. For example, most have participants track and self-report their cell phone use. There’s also no way to directly track certain factors of RFR exposure, such as frequency, duration, or intensity.
Even with those caveats in mind, the FDA wrote that, “based on the studies that are described in detail in this report, there is insufficient evidence to support a causal association between RFR exposure and tumorigenesis. There is a lack of clear dose-response relationship, a lack of consistent findings or specificity, and a lack of biological mechanistic plausibility.”
The full review is available on the FDA website.
Fast-acting, mealtime insulin aspart is approved for kids
making it the first fast-acting mealtime insulin injection that does not come with a premeal dosing recommendation, according to a release.
The injection is now available in various dosing options for both adult and pediatric patients with diabetes. Fast-acting mealtime insulin was approved in September 2017 for adults with type 1 or 2 disease, and in October 2019, it was approved for use in insulin pumps for adults.
The most recent approval was based on findings from the onset 7 trial, a 26-week, phase 3b, partially double-blind, treat-to-target trial that included 777 patients aged 1-18 years and demonstrated noninferiority to ordinary, non–fast-acting insulin aspart (Diabetes Care. 2019 Jul;42[7]:1255-62).
Removal of the premeal dosing requirement could help better manage mealtime insulin needs in children, according to the release from Novo Nordisk.
Use of the mealtime insulin injection comes with concerns of serious side effects, such as hypoglycemia, hypokalemia, serious allergic reactions, and heart failure. Common side effects can include skin problems (such as rash, itching, and swelling), injection-site reactions, and weight gain.
making it the first fast-acting mealtime insulin injection that does not come with a premeal dosing recommendation, according to a release.
The injection is now available in various dosing options for both adult and pediatric patients with diabetes. Fast-acting mealtime insulin was approved in September 2017 for adults with type 1 or 2 disease, and in October 2019, it was approved for use in insulin pumps for adults.
The most recent approval was based on findings from the onset 7 trial, a 26-week, phase 3b, partially double-blind, treat-to-target trial that included 777 patients aged 1-18 years and demonstrated noninferiority to ordinary, non–fast-acting insulin aspart (Diabetes Care. 2019 Jul;42[7]:1255-62).
Removal of the premeal dosing requirement could help better manage mealtime insulin needs in children, according to the release from Novo Nordisk.
Use of the mealtime insulin injection comes with concerns of serious side effects, such as hypoglycemia, hypokalemia, serious allergic reactions, and heart failure. Common side effects can include skin problems (such as rash, itching, and swelling), injection-site reactions, and weight gain.
making it the first fast-acting mealtime insulin injection that does not come with a premeal dosing recommendation, according to a release.
The injection is now available in various dosing options for both adult and pediatric patients with diabetes. Fast-acting mealtime insulin was approved in September 2017 for adults with type 1 or 2 disease, and in October 2019, it was approved for use in insulin pumps for adults.
The most recent approval was based on findings from the onset 7 trial, a 26-week, phase 3b, partially double-blind, treat-to-target trial that included 777 patients aged 1-18 years and demonstrated noninferiority to ordinary, non–fast-acting insulin aspart (Diabetes Care. 2019 Jul;42[7]:1255-62).
Removal of the premeal dosing requirement could help better manage mealtime insulin needs in children, according to the release from Novo Nordisk.
Use of the mealtime insulin injection comes with concerns of serious side effects, such as hypoglycemia, hypokalemia, serious allergic reactions, and heart failure. Common side effects can include skin problems (such as rash, itching, and swelling), injection-site reactions, and weight gain.
Intersubject correlation analyses can be used to pinpoint neural patterns in ADHD
Functional MRI-based intersubject correlations (ISCs) hold promise for studying the neural bases of ADHD’s heterogeneous symptoms in situations that reflect real-world difficulties, new research shows.
“The present results provide the first evidence of a connection between symptom scales and brain activity recorded when the participants have been involved in a situation that is similar to the ones where their difficulties typically occur,” wrote Juha Salmi, of the department of neuroscience and biomedical engineering at Aalto University in Espoo, Finland, and associates. The study was published in NeuroImage.
Many imaging studies in ADHD are too narrow and fail to reflect real-world situations and distractions, the investigators wrote. For the current study, the investigators conducted fMRI scans of participants in “cocktail party” situations. During fMRI, the participants viewed excerpts from the 2008 Finnish film “Three Wise Men,” in which three men were talking.
“There were periods when no additional distractors were presented and periods when the film was embedded with irrelevant distractors that the participants were told to ignore. The three different distractors (white noise, green; jazz music, red; speech, magenta) and nondistracted periods (blue) were presented in a pseudo-randomized order so that all other distractor types had to occur before the same distractor type was presented again,” the authors wrote. Each distractor lasted for 15 seconds.
The ISC approach sought to gauge how much neural networks in each group, 51 with ADHD and 29 without, “ticked together” – that is, synchronized and coordinated in a feature-specific manner.
As expected, across the film, ISCs of those in the ADHD group were weaker than were those in the control group in multiple brain areas, including the left precuneus, bilateral medial occipital cortices, left lateral occipital cortex, left temporoparietal junction, and medial and posterior parts of the left superior temporal cortex. Likewise, when the other distractors occurred – with the exception of constant white noise – weaker ISCs were found among the ADHD group. In fact, there were no brain regions in which healthy controls had weaker ISCs than did those with ADHD.
“ the investigators wrote. “At least theoretically, this approach could be used to identify neural patterns reflecting specific symptoms in complex, dynamic situations.”
One limitation of naturalistic studies is that the inferences are by their nature more general than they might be in more conventional, discrete experiments. Bridging the gap between naturalistic studies such as this one and other more conventional designs are needed, the investigators wrote, because dove-tailing their findings could provide significant insights.
The study was supported by the Academy of Finland and the Åbo Akademi University Endowment for the BrainTrain project. None of the authors have any biomedical financial interests or potential conflicts of interest.
SOURCE: Salmi J et al. Neuroimage. 2019 Nov 12. doi: 10.1016/j.neuroimage.2019.116352.
Functional MRI-based intersubject correlations (ISCs) hold promise for studying the neural bases of ADHD’s heterogeneous symptoms in situations that reflect real-world difficulties, new research shows.
“The present results provide the first evidence of a connection between symptom scales and brain activity recorded when the participants have been involved in a situation that is similar to the ones where their difficulties typically occur,” wrote Juha Salmi, of the department of neuroscience and biomedical engineering at Aalto University in Espoo, Finland, and associates. The study was published in NeuroImage.
Many imaging studies in ADHD are too narrow and fail to reflect real-world situations and distractions, the investigators wrote. For the current study, the investigators conducted fMRI scans of participants in “cocktail party” situations. During fMRI, the participants viewed excerpts from the 2008 Finnish film “Three Wise Men,” in which three men were talking.
“There were periods when no additional distractors were presented and periods when the film was embedded with irrelevant distractors that the participants were told to ignore. The three different distractors (white noise, green; jazz music, red; speech, magenta) and nondistracted periods (blue) were presented in a pseudo-randomized order so that all other distractor types had to occur before the same distractor type was presented again,” the authors wrote. Each distractor lasted for 15 seconds.
The ISC approach sought to gauge how much neural networks in each group, 51 with ADHD and 29 without, “ticked together” – that is, synchronized and coordinated in a feature-specific manner.
As expected, across the film, ISCs of those in the ADHD group were weaker than were those in the control group in multiple brain areas, including the left precuneus, bilateral medial occipital cortices, left lateral occipital cortex, left temporoparietal junction, and medial and posterior parts of the left superior temporal cortex. Likewise, when the other distractors occurred – with the exception of constant white noise – weaker ISCs were found among the ADHD group. In fact, there were no brain regions in which healthy controls had weaker ISCs than did those with ADHD.
“ the investigators wrote. “At least theoretically, this approach could be used to identify neural patterns reflecting specific symptoms in complex, dynamic situations.”
One limitation of naturalistic studies is that the inferences are by their nature more general than they might be in more conventional, discrete experiments. Bridging the gap between naturalistic studies such as this one and other more conventional designs are needed, the investigators wrote, because dove-tailing their findings could provide significant insights.
The study was supported by the Academy of Finland and the Åbo Akademi University Endowment for the BrainTrain project. None of the authors have any biomedical financial interests or potential conflicts of interest.
SOURCE: Salmi J et al. Neuroimage. 2019 Nov 12. doi: 10.1016/j.neuroimage.2019.116352.
Functional MRI-based intersubject correlations (ISCs) hold promise for studying the neural bases of ADHD’s heterogeneous symptoms in situations that reflect real-world difficulties, new research shows.
“The present results provide the first evidence of a connection between symptom scales and brain activity recorded when the participants have been involved in a situation that is similar to the ones where their difficulties typically occur,” wrote Juha Salmi, of the department of neuroscience and biomedical engineering at Aalto University in Espoo, Finland, and associates. The study was published in NeuroImage.
Many imaging studies in ADHD are too narrow and fail to reflect real-world situations and distractions, the investigators wrote. For the current study, the investigators conducted fMRI scans of participants in “cocktail party” situations. During fMRI, the participants viewed excerpts from the 2008 Finnish film “Three Wise Men,” in which three men were talking.
“There were periods when no additional distractors were presented and periods when the film was embedded with irrelevant distractors that the participants were told to ignore. The three different distractors (white noise, green; jazz music, red; speech, magenta) and nondistracted periods (blue) were presented in a pseudo-randomized order so that all other distractor types had to occur before the same distractor type was presented again,” the authors wrote. Each distractor lasted for 15 seconds.
The ISC approach sought to gauge how much neural networks in each group, 51 with ADHD and 29 without, “ticked together” – that is, synchronized and coordinated in a feature-specific manner.
As expected, across the film, ISCs of those in the ADHD group were weaker than were those in the control group in multiple brain areas, including the left precuneus, bilateral medial occipital cortices, left lateral occipital cortex, left temporoparietal junction, and medial and posterior parts of the left superior temporal cortex. Likewise, when the other distractors occurred – with the exception of constant white noise – weaker ISCs were found among the ADHD group. In fact, there were no brain regions in which healthy controls had weaker ISCs than did those with ADHD.
“ the investigators wrote. “At least theoretically, this approach could be used to identify neural patterns reflecting specific symptoms in complex, dynamic situations.”
One limitation of naturalistic studies is that the inferences are by their nature more general than they might be in more conventional, discrete experiments. Bridging the gap between naturalistic studies such as this one and other more conventional designs are needed, the investigators wrote, because dove-tailing their findings could provide significant insights.
The study was supported by the Academy of Finland and the Åbo Akademi University Endowment for the BrainTrain project. None of the authors have any biomedical financial interests or potential conflicts of interest.
SOURCE: Salmi J et al. Neuroimage. 2019 Nov 12. doi: 10.1016/j.neuroimage.2019.116352.
FROM NEUROIMAGE
FDA authorizes customizable automated glycemic controller
The Food and Drug Administration has authorized marketing of the Tandem Diabetes Care Control-IQ Technology, an interoperable automated glycemic controller, for use in a customizable glucose control system, according to a release from the agency.
The move also paves the way for the review and authorization of similar devices in the future.
The Control-IQ Technology controller coordinates with an alternate controller-enabled insulin pump and an integrated continuous glucose monitor, which can be made by other manufacturers as long they are compatible with this modular technology.
The agency reviewed data from a clinical study of 168 patients with type 1 diabetes who were randomized to use either the Control-IQ Technology controller installed on a Tandem t:slim X2 insulin pump, or a continuous glucose monitor and insulin pump without the Control-IQ controller. The findings showed that,
However, the agency noted that, although the system has been assessed for reliability, delays in insulin delivery remain possible and care should be taken when using it.
This authorization comes along with establishment of criteria and regulatory requirements that create a new regulatory classification for this type of device, whereby future devices of the same type and with the same purpose can go through the FDA’s 510(k) premarket process. Such a process would mean that, going forward, similar devices can “obtain marketing authorization by demonstrating substantial equivalence to a predicate device.”
More information can be found in the full release, available on the FDA website.
The Food and Drug Administration has authorized marketing of the Tandem Diabetes Care Control-IQ Technology, an interoperable automated glycemic controller, for use in a customizable glucose control system, according to a release from the agency.
The move also paves the way for the review and authorization of similar devices in the future.
The Control-IQ Technology controller coordinates with an alternate controller-enabled insulin pump and an integrated continuous glucose monitor, which can be made by other manufacturers as long they are compatible with this modular technology.
The agency reviewed data from a clinical study of 168 patients with type 1 diabetes who were randomized to use either the Control-IQ Technology controller installed on a Tandem t:slim X2 insulin pump, or a continuous glucose monitor and insulin pump without the Control-IQ controller. The findings showed that,
However, the agency noted that, although the system has been assessed for reliability, delays in insulin delivery remain possible and care should be taken when using it.
This authorization comes along with establishment of criteria and regulatory requirements that create a new regulatory classification for this type of device, whereby future devices of the same type and with the same purpose can go through the FDA’s 510(k) premarket process. Such a process would mean that, going forward, similar devices can “obtain marketing authorization by demonstrating substantial equivalence to a predicate device.”
More information can be found in the full release, available on the FDA website.
The Food and Drug Administration has authorized marketing of the Tandem Diabetes Care Control-IQ Technology, an interoperable automated glycemic controller, for use in a customizable glucose control system, according to a release from the agency.
The move also paves the way for the review and authorization of similar devices in the future.
The Control-IQ Technology controller coordinates with an alternate controller-enabled insulin pump and an integrated continuous glucose monitor, which can be made by other manufacturers as long they are compatible with this modular technology.
The agency reviewed data from a clinical study of 168 patients with type 1 diabetes who were randomized to use either the Control-IQ Technology controller installed on a Tandem t:slim X2 insulin pump, or a continuous glucose monitor and insulin pump without the Control-IQ controller. The findings showed that,
However, the agency noted that, although the system has been assessed for reliability, delays in insulin delivery remain possible and care should be taken when using it.
This authorization comes along with establishment of criteria and regulatory requirements that create a new regulatory classification for this type of device, whereby future devices of the same type and with the same purpose can go through the FDA’s 510(k) premarket process. Such a process would mean that, going forward, similar devices can “obtain marketing authorization by demonstrating substantial equivalence to a predicate device.”
More information can be found in the full release, available on the FDA website.
Abnormal gaze processing found in patients with bipolar disorder
Patients with bipolar disorder show altered gaze processing on EEG recordings taken during working memory exercises, new study results suggest.
The study, led by Cristina Berchio of the department of basic neurosciences at the University of Geneva, recruited 19 euthymic patients with bipolar I or II from the Mood Disorders Unit at the University Hospital of Geneva and 19 controls matched for age, gender, education level, and handedness. While undergoing high-density EEG recording, participants performed a two-back working memory exercise that involved neutral faces with either direct or averted gazes. The study was published in NeuroImage: Clinical.
; both of those functions are thought to be impaired in patients with bipolar disorder. They suggested that this might reflect early-life dysfunctional parental-infant gaze experiences that could affect how those patients with bipolar disorder learned emotion-regulation strategies. “In this sense, our early gaze experiences might also be considered an environmental risk factor, that might remain as a vulnerability trait in [bipolar patients],” they wrote.
Limitations of the study include the working memory exercise’s design, which could have led to misleading anticipatory effects. The small sample size is another limitation that affected the ability to perform certain analyses. The surface nature of EEG also limited evaluation of deeper brain structures that might have proved salient to this exercise.
The study was supported by several entities, including the Swiss National Center of Competence in Research. The authors declared no conflicts of interest.
SOURCE: Berchio C et al. NeuroImage Clin. 2019. doi: 10.1016/j.nicl.2017.09.006.
Patients with bipolar disorder show altered gaze processing on EEG recordings taken during working memory exercises, new study results suggest.
The study, led by Cristina Berchio of the department of basic neurosciences at the University of Geneva, recruited 19 euthymic patients with bipolar I or II from the Mood Disorders Unit at the University Hospital of Geneva and 19 controls matched for age, gender, education level, and handedness. While undergoing high-density EEG recording, participants performed a two-back working memory exercise that involved neutral faces with either direct or averted gazes. The study was published in NeuroImage: Clinical.
; both of those functions are thought to be impaired in patients with bipolar disorder. They suggested that this might reflect early-life dysfunctional parental-infant gaze experiences that could affect how those patients with bipolar disorder learned emotion-regulation strategies. “In this sense, our early gaze experiences might also be considered an environmental risk factor, that might remain as a vulnerability trait in [bipolar patients],” they wrote.
Limitations of the study include the working memory exercise’s design, which could have led to misleading anticipatory effects. The small sample size is another limitation that affected the ability to perform certain analyses. The surface nature of EEG also limited evaluation of deeper brain structures that might have proved salient to this exercise.
The study was supported by several entities, including the Swiss National Center of Competence in Research. The authors declared no conflicts of interest.
SOURCE: Berchio C et al. NeuroImage Clin. 2019. doi: 10.1016/j.nicl.2017.09.006.
Patients with bipolar disorder show altered gaze processing on EEG recordings taken during working memory exercises, new study results suggest.
The study, led by Cristina Berchio of the department of basic neurosciences at the University of Geneva, recruited 19 euthymic patients with bipolar I or II from the Mood Disorders Unit at the University Hospital of Geneva and 19 controls matched for age, gender, education level, and handedness. While undergoing high-density EEG recording, participants performed a two-back working memory exercise that involved neutral faces with either direct or averted gazes. The study was published in NeuroImage: Clinical.
; both of those functions are thought to be impaired in patients with bipolar disorder. They suggested that this might reflect early-life dysfunctional parental-infant gaze experiences that could affect how those patients with bipolar disorder learned emotion-regulation strategies. “In this sense, our early gaze experiences might also be considered an environmental risk factor, that might remain as a vulnerability trait in [bipolar patients],” they wrote.
Limitations of the study include the working memory exercise’s design, which could have led to misleading anticipatory effects. The small sample size is another limitation that affected the ability to perform certain analyses. The surface nature of EEG also limited evaluation of deeper brain structures that might have proved salient to this exercise.
The study was supported by several entities, including the Swiss National Center of Competence in Research. The authors declared no conflicts of interest.
SOURCE: Berchio C et al. NeuroImage Clin. 2019. doi: 10.1016/j.nicl.2017.09.006.
FROM NEUROIMAGE: CLINICAL
Brains of MDD patients show alterations in areas tied to positive emotions
The brains of medication-naive patients with major depressive disorder (MDD) exhibit interhemispheric structural imbalances in areas that regulate positive emotional processes, results of a small cross-sectional study suggest.
“To the best of our knowledge, asymmetric alterations in cortical thickness and subcortical volume in patients with MDD have not yet been reported,” wrote Zhiwei Zuo, of the department of radiology, Southwest Hospital, Army Medical University, Chongqing, China, and associates. “A comprehensive understanding of the cerebral pathophysiology changes in depression is essential and may lead to more targeted approaches for the prevention and treatment of MDD.”
The investigators enrolled 35 medication-naive, untreated patients with MDD from the hospital’s department of psychology, and 35 age-, gender-, and education-matched controls. Using whole-brain analysis, the investigators identified asymmetry in cortical thickness and subcortical volume that was mostly present in the cortical-striatal-pallidal-thalamic circuit. This part of the brain helps translate underlying positive affect processes into conscious feelings of pleasure, the authors reported. in recent years (Clin Psychol Psychother. 2012 Jul-Aug;19[4]:326-40). The current study results were published in NeuroImage: Clinical.
Some limitations of the study include its small sample size and its cross-sectional nature. Nevertheless, they said, the findings could provide possible targets for therapeutic monitoring of the illness.
“These alterations were independent of depressive symptom severity, suggesting that cerebral asymmetry could be an appropriate indicator of morphological variations in mental disease,” the investigators noted.
The study was funded by the National Nature Science Foundation of China, the National Key Research and Development Plan of China, and the Innovative Talents Project of Southwest Hospital. The authors had no conflicts of interest to disclose.
SOURCE: Zuo Z et al. Neuroimage Clin. 2019. doi: 10.1016/j.nicl.2018.101614.
The brains of medication-naive patients with major depressive disorder (MDD) exhibit interhemispheric structural imbalances in areas that regulate positive emotional processes, results of a small cross-sectional study suggest.
“To the best of our knowledge, asymmetric alterations in cortical thickness and subcortical volume in patients with MDD have not yet been reported,” wrote Zhiwei Zuo, of the department of radiology, Southwest Hospital, Army Medical University, Chongqing, China, and associates. “A comprehensive understanding of the cerebral pathophysiology changes in depression is essential and may lead to more targeted approaches for the prevention and treatment of MDD.”
The investigators enrolled 35 medication-naive, untreated patients with MDD from the hospital’s department of psychology, and 35 age-, gender-, and education-matched controls. Using whole-brain analysis, the investigators identified asymmetry in cortical thickness and subcortical volume that was mostly present in the cortical-striatal-pallidal-thalamic circuit. This part of the brain helps translate underlying positive affect processes into conscious feelings of pleasure, the authors reported. in recent years (Clin Psychol Psychother. 2012 Jul-Aug;19[4]:326-40). The current study results were published in NeuroImage: Clinical.
Some limitations of the study include its small sample size and its cross-sectional nature. Nevertheless, they said, the findings could provide possible targets for therapeutic monitoring of the illness.
“These alterations were independent of depressive symptom severity, suggesting that cerebral asymmetry could be an appropriate indicator of morphological variations in mental disease,” the investigators noted.
The study was funded by the National Nature Science Foundation of China, the National Key Research and Development Plan of China, and the Innovative Talents Project of Southwest Hospital. The authors had no conflicts of interest to disclose.
SOURCE: Zuo Z et al. Neuroimage Clin. 2019. doi: 10.1016/j.nicl.2018.101614.
The brains of medication-naive patients with major depressive disorder (MDD) exhibit interhemispheric structural imbalances in areas that regulate positive emotional processes, results of a small cross-sectional study suggest.
“To the best of our knowledge, asymmetric alterations in cortical thickness and subcortical volume in patients with MDD have not yet been reported,” wrote Zhiwei Zuo, of the department of radiology, Southwest Hospital, Army Medical University, Chongqing, China, and associates. “A comprehensive understanding of the cerebral pathophysiology changes in depression is essential and may lead to more targeted approaches for the prevention and treatment of MDD.”
The investigators enrolled 35 medication-naive, untreated patients with MDD from the hospital’s department of psychology, and 35 age-, gender-, and education-matched controls. Using whole-brain analysis, the investigators identified asymmetry in cortical thickness and subcortical volume that was mostly present in the cortical-striatal-pallidal-thalamic circuit. This part of the brain helps translate underlying positive affect processes into conscious feelings of pleasure, the authors reported. in recent years (Clin Psychol Psychother. 2012 Jul-Aug;19[4]:326-40). The current study results were published in NeuroImage: Clinical.
Some limitations of the study include its small sample size and its cross-sectional nature. Nevertheless, they said, the findings could provide possible targets for therapeutic monitoring of the illness.
“These alterations were independent of depressive symptom severity, suggesting that cerebral asymmetry could be an appropriate indicator of morphological variations in mental disease,” the investigators noted.
The study was funded by the National Nature Science Foundation of China, the National Key Research and Development Plan of China, and the Innovative Talents Project of Southwest Hospital. The authors had no conflicts of interest to disclose.
SOURCE: Zuo Z et al. Neuroimage Clin. 2019. doi: 10.1016/j.nicl.2018.101614.
FROM NEUROIMAGE: CLINICAL
FDA approves infliximab-axxq for numerous indications
The Food and Drug Administration has approved the biosimilar infliximab-axxq (Avsola) for various indications, making it the fourth biosimilar of infliximab (Remicade) to be cleared for marketing by the agency.
The tumor necrosis factor inhibitor is indicated for patients with Crohn’s disease or ulcerative colitis who are aged 6 years and older, RA in combination with methotrexate, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis. The approval is based on numerous trials. The most common adverse reactions are infections, infusion-related reactions, headache, and abdominal pain.
Full prescribing information can be found on the FDA website, as can more information about biosimilars.
The Food and Drug Administration has approved the biosimilar infliximab-axxq (Avsola) for various indications, making it the fourth biosimilar of infliximab (Remicade) to be cleared for marketing by the agency.
The tumor necrosis factor inhibitor is indicated for patients with Crohn’s disease or ulcerative colitis who are aged 6 years and older, RA in combination with methotrexate, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis. The approval is based on numerous trials. The most common adverse reactions are infections, infusion-related reactions, headache, and abdominal pain.
Full prescribing information can be found on the FDA website, as can more information about biosimilars.
The Food and Drug Administration has approved the biosimilar infliximab-axxq (Avsola) for various indications, making it the fourth biosimilar of infliximab (Remicade) to be cleared for marketing by the agency.
The tumor necrosis factor inhibitor is indicated for patients with Crohn’s disease or ulcerative colitis who are aged 6 years and older, RA in combination with methotrexate, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis. The approval is based on numerous trials. The most common adverse reactions are infections, infusion-related reactions, headache, and abdominal pain.
Full prescribing information can be found on the FDA website, as can more information about biosimilars.