User login
Autoeczematization: A Strange Id Reaction of the Skin
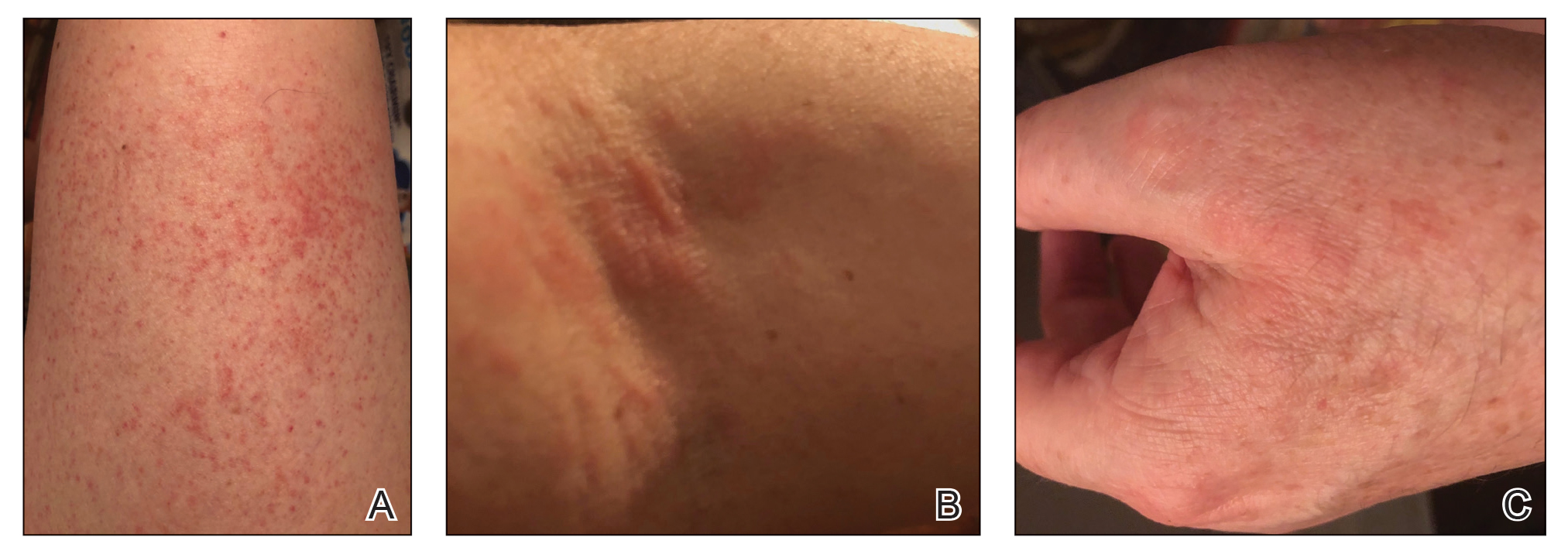
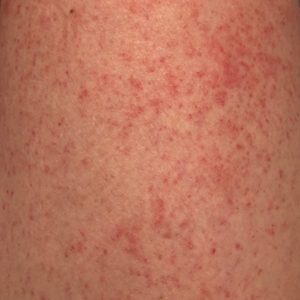
Autoeczematization (AE), or id reaction, is a disseminated eczematous reaction that occurs days or weeks after exposure to a primary stimulus, resulting from a release of antigen(s). Whitfield1 first described AE in 1921, when he postulated that the id reaction was due to sensitization of the skin after a primary stimulus. He called it “a form of auto-intoxication derived from changes in the patient’s own tissues.”1 The exact prevalence of id reactions is unknown; one study showed that 17% of patients with dermatophyte infections developed an id reaction, typically tinea pedis linked with vesicles on the palms.2 Tinea capitis is one of the most common causes of AE in children, which is frequently misdiagnosed as a drug reaction. Approximately 37% of patients diagnosed with stasis dermatitis develop an id reaction (Figure 1). A history of contact dermatitis is common in patients presenting with AE.2-6
Pathophysiology of Id Reactions
An abnormal immune response against autologous skin antigens may be responsible for the development of AE. Shelley5 postulated that hair follicles play an important role in id reactions, as Sharquie et al6 recently emphasized for many skin disorders. The pathogenesis of AE is uncertain, but circulating T lymphocytes play a role in this reaction. Normally, T cells are activated by a release of antigens after a primary exposure to a stimulus. However, overactivation of these T cells induces autoimmune reactions such as AE.7 Activated T lymphocytes express HLA-DR and IL-2 receptor, markers elevated in the peripheral blood of patients undergoing id reactions. After treatment, the levels of activated T lymphocytes decline. An increase in the number of CD25+ T cells and a decrease in the number of suppressor T cells in the blood may occur during an id reaction.7-9 Keratinocytes produce proinflammatory cytokines, such as thymic stromal erythropoietin, IL-25, and IL-33, that activate T cells.10-12 Therefore, the most likely pathogenesis of an id reaction is that T lymphocytes are activated at the primary reaction site due to proinflammatory cytokines released by keratinocytes. These activated T cells then travel systemically via hematogenous dissemination. The spread of activated T lymphocytes produces an eczematous reaction at secondary locations distant to the primary site.9
Clinical and Histopathological Features of Id Reactions
Clinically, AE is first evident as a vesicular dissemination that groups to form papules or nummular patches and usually is present on the legs, feet, arms, and/or trunk (Figure 2). The primary dermatitis is localized to the area that was the site of contact to the offending stimuli. This localized eczematous eruption begins with an acute or subacute onset. It has the appearance of small crusted vesicles with erythema (Figure 1). The first sign of AE is vesicles presenting near the primary site on flexural surfaces or on the hands and feet. A classic example is tinea pedis linked with vesicles on the palms and sides of the fingers, resembling dyshidrotic eczema. Sites of prior cutaneous trauma, such as dermatoses, scars, and burns, are common locations for early AE. In later stages, vesicles disseminate to the legs, arms, and trunk, where they group to form papules and nummular patches in a symmetrical pattern.5,13-15 These lesions may be extremely pruritic. The pruritus may be so intense that it interrupts daily activities and disrupts the ability to fall or stay asleep.16
Histologically, biopsy specimens show psoriasiform spongiotic dermatitis with mononuclear cells contained in the vesicles. Interstitial edema and perivascular lymphohistiocytic infiltrates are evident. Eosinophils also may be present. This pattern is not unique toid reactions.17-19 Although AE is a reaction pattern that may be due to a fungal or bacterial infection, the etiologic agent is not evident microscopically within the eczema itself.
Etiology of Id Reactions
Id reactions most commonly occur from either stasis dermatitis or tinea pedis, although a wide variety of other causes should be considered. Evaluation of the primary site rather than the id reaction may identify an infectious or parasitic agent. Sometimes the AE reaction is specifically named: dermatophytid with dermatophytosis, bacterid with a bacterial infectious process, and tuberculid with tuberculosis. Similarly, there may be reactions to underlying candidiasis, sporotrichosis, histoplasmosis, and other fungal infections that can cause a cutaneous id reaction.18,20-22Mycobacterium species, Pseudomonas, Staphylococcus, and Streptococcus are bacterial causes of AE.15,23-26 Viral infections that can cause an id reaction are herpes simplex virus and molluscum contagiosum.27-29 Scabies, leishmaniasis, and pediculosis capitis are parasitic infections that may be etiologic.14,30,31 In addition, noninfectious stimuli besides stasis dermatitis that can produce id reactions include medications, topical creams, tattoo ink, sutures, radiotherapy, and dyshidrotic eczema. The primary reaction to these agents is a localized dermatitis followed by the immunological response that induces a secondary reaction distant from the primary site.17,18,32-38
Differential Diagnoses
Differential diagnoses include other types of eczema and some vesicular eruptions. Irritant contact dermatitis is another dermatosis that presents as a widespread vesicular eruption due to repetitive exposure to toxic irritants. The rash is erythematous with pustules, blisters, and crusts. It is only found in areas directly exposed to irritants, as opposed to AE, which spreads to areas distant to the primary reaction site. Irritant contact dermatitis presents with more of a burning sensation, whereas AE is more pruritic.39,40 Allergic contact dermatitis presents with erythematous vesicles and papules and sometimes with bullae. There is edema and crust formation, which often can spread past the point of contact in later stages. Similar to AE, there is intense pruritus. However, allergic contact dermatitis most commonly is caused by exposure to metals, cosmetics, and fragrances, whereas infectious agents and stasis dermatitis are the most common causes of AE.40,41 It may be challenging to distinguish AE from other causes of widespread eczematous dissemination. Vesicular eruptions sometimes require distinction from AE, including herpetic infections, insect bite reactions, and drug eruptions.18,42
Treatment
The underlying condition should be treated to mitigate the inflammatory response causing the id reaction. If not skillfully orchestrated, the id reaction can reoccur. For infectious causes of AE, an antifungal, antibacterial, antiviral, or antiparasitic should be given. If stasis dermatitis is responsible for the id reaction, compression stockings and leg elevation are indicated. The id reaction itself is treated with systemic or topical corticosteroids and wet compresses if acute. The goal of these treatments is to reduce patient discomfort caused by the inflammation and pruritus.18,43
Conclusion
Id reactions are an unusual phenomenon that commonly occurs after fungal skin infections and stasis dermatitis. T lymphocytes and keratinocytes may play a key role in this reaction, with newer research further delineating the process and possibly providing enhanced treatment options. Therapy focuses on treating the underlying condition, supplemented with corticosteroids for the autoeczema.
- Whitfield A. Lumleian Lectures on Some Points in the Aetiology of Skin Diseases. Delivered before the Royal College of Physicians of London on March 10th, 15th, and 17th, 1921. Lecture II. Lancet. 1921;2:122-127.
- Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128:E453-E457.
- Schrom KP, Kobs A, Nedorost S. Clinical psoriasiform dermatitis following dupilumab use for autoeczematization secondary to chronic stasis dermatitis. Cureus. 2020;12:e7831. doi:10.7759/cureus.7831
- Templeton HJ, Lunsford CJ, Allington HV. Autosensitization dermatitis; report of five cases and protocol of an experiment. Arch Derm Syphilol. 1949;59:68-77.
- Shelley WB. Id reaction. In: Consultations in Dermatology. Saunders; 1972:262-267.
- Sharquie KE, Noaimi AA, Flayih RA. Clinical and histopathological findings in patients with follicular dermatoses: all skin diseases starts in the hair follicles as new hypothesis. Am J Clin Res Rev. 2020;4:17.
- Kasteler JS, Petersen MJ, Vance JE, et al. Circulating activated T lymphocytes in autoeczematization. Arch Dermatol. 1992;128:795-798.
- González-Amaro R, Baranda L, Abud-Mendoza C, et al. Autoeczematization is associated with abnormal immune recognition of autologous skin antigens. J Am Acad Dermatol. 1993;28:56-60.
- Cunningham MJ, Zone JJ, Petersen MJ, et al. Circulating activated (DR-positive) T lymphocytes in a patient with autoeczematization. J Am Acad Dermatol. 1986;14:1039-1041.
- Furue M, Ulzii D, Vu YH, et al. Pathogenesis of atopic dermatitis: current paradigm. Iran J Immunol. 2019;16:97-107.
- Uchi H, Terao H, Koga T, et al. Cytokines and chemokines in the epidermis. J Dermatol Sci. 2000;24(suppl 1):S29-S38.
- Bos JD, Kapsenberg ML. The skin immune system: progress in cutaneous biology. Immunol Today. 1993;14:75-78.
- Young AW Jr. Dynamics of autosensitization dermatitis; a clinical and microscopic concept of autoeczematization. AMA Arch Derm. 1958;77:495-502.
- Brenner S, Wolf R, Landau M. Scabid: an unusual id reaction to scabies. Int J Dermatol. 1993;32:128-129.
- Yamany T, Schwartz RA. Infectious eczematoid dermatitis: a comprehensive review. J Eur Acad Dermatol Venereol. 2015;29:203-208.
- Wang X, Li L, Shi X, et al. Itching and its related factors in subtypes of eczema: a cross-sectional multicenter study in tertiary hospitals of China. Sci Rep. 2018;8:10754.
- Price A, Tavazoie M, Meehan SA, et al. Id reaction associated with red tattoo ink. Cutis. 2018;102:E32-E34.
- Ilkit M, Durdu M, Karaks¸ M. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol. 2012;38:191-202.
- Kaner SR. Dermatitis venenata of the feet with a generalized “id” reaction. J Am Podiatry Assoc. 1970;60:199-204.
- Jordan L, Jackson NA, Carter-Snell B, et al. Pustular tinea id reaction. Cutis. 2019;103:E3-E4.
- Crum N, Hardaway C, Graham B. Development of an idlike reaction during treatment for acute pulmonary histoplasmosis: a new cutaneous manifestation in histoplasmosis. J Am Acad Dermatol. 2003;48(2 suppl):S5-S6.
- Chirac A, Brzezinski P, Chiriac AE, et al. Autosensitisation (autoeczematisation) reactions in a case of diaper dermatitis candidiasis. Niger Med J. 2014;55:274-275.
- Singh PY, Sinha P, Baveja S, et al. Immune-mediated tuberculous uveitis—a rare association with papulonecrotic tuberculid. Indian J Ophthalmol. 2019;67:1207-1209.
- Urso B, Georgesen C, Harp J. Papulonecrotic tuberculid secondary to Mycobacterium avium complex. Cutis. 2019;104:E11-E13.
- Choudhri SH, Magro CM, Crowson AN, et al. An id reaction to Mycobacterium leprae: first documented case. Cutis. 1994;54:282-286.
- Park JW, Jeong GJ, Seo SJ, et al. Pseudomonas toe web infection and autosensitisation dermatitis: diagnostic and therapeutic challenge. Int Wound J. 2020;17:1543-1544. doi:10.1111/iwj.13386
- Netchiporouk E, Cohen BA. Recognizing and managing eczematous id reactions to molluscum contagiosum virus in children. Pediatrics. 2012;129:E1072-E1075.
- Aurelian L, Ono F, Burnett J. Herpes simplex virus (HSV)-associated erythema multiforme (HAEM): a viral disease with an autoimmune component. Dermatol Online J. 2003;9:1.
- Rocamora V, Romaní J, Puig L, et al. Id reaction to molluscum contagiosum. Pediatr Dermatol. 1996;13:349-350.
- Yes¸ilova Y, Özbilgin A, Turan E, et al. Clinical exacerbation developing during treatment of cutaneous leishmaniasis: an id reaction? Turkiye Parazitol Derg. 2014;38:281-282.
- Connor CJ, Selby JC, Wanat KA. Severe pediculosis capitus: a case of “crusted lice” with autoeczematization. Dermatol Online J. 2016;22:13030/qt7c91z913.
- Shelley WB. The autoimmune mechanism in clinical dermatology. Arch Dermatol. 1962;86:27-34.
- Bosworth A, Hull PR. Disseminated eczema following radiotherapy: a case report. J Cutan Med Surg. 2018;22:353-355.
- Lowther C, Miedler JD, Cockerell CJ. Id-like reaction to BCG therapy for bladder cancer. Cutis. 2013;91:145-151.
- Huerth KA, Glick PL, Glick ZR. Cutaneous id reaction after using cyanoacrylate for wound closure. Cutis. 2020;105:E11-E13.
- Amini S, Burdick AE, Janniger CK. Dyshidrotic eczema (pompholyx). Updated April 22, 2020. Accessed August 23, 2021. https://emedicine.medscape.com/article/1122527-overview
- Sundaresan S, Migden MR, Silapunt S. Stasis dermatitis: pathophysiology, evaluation, and management. Am J Clin Dermatol. 2017;18:383-390.
- Hughes JDM, Pratt MD. Allergic contact dermatitis and autoeczematization to proctosedyl® cream and proctomyxin® cream. Case Rep Dermatol. 2018;10:238-246.
- Bains SN, Nash P, Fonacier L. Irritant contact dermatitis. Clin Rev Allergy Immunol. 2019;56:99-109.
- Novak-Bilic´ G, Vucˇic´ M, Japundžic´ I, et al. Irritant and allergic contact dermatitis—skin lesion characteristics. Acta Clin Croat. 2018;57:713-720.
- Nassau S, Fonacier L. Allergic contact dermatitis. Med Clin North Am. 2020;104:61-76.
- Lewis DJ, Schlichte MJ, Dao H Jr. Atypical disseminated herpes zoster: management guidelines in immunocompromised patients. Cutis. 2017;100:321-330.
- Nedorost S, White S, Rowland DY, et al. Development and implementation of an order set to improve value of care for patients with severe stasis dermatitis. J Am Acad Dermatol. 2019;80:815-817.
Autoeczematization (AE), or id reaction, is a disseminated eczematous reaction that occurs days or weeks after exposure to a primary stimulus, resulting from a release of antigen(s). Whitfield1 first described AE in 1921, when he postulated that the id reaction was due to sensitization of the skin after a primary stimulus. He called it “a form of auto-intoxication derived from changes in the patient’s own tissues.”1 The exact prevalence of id reactions is unknown; one study showed that 17% of patients with dermatophyte infections developed an id reaction, typically tinea pedis linked with vesicles on the palms.2 Tinea capitis is one of the most common causes of AE in children, which is frequently misdiagnosed as a drug reaction. Approximately 37% of patients diagnosed with stasis dermatitis develop an id reaction (Figure 1). A history of contact dermatitis is common in patients presenting with AE.2-6
Pathophysiology of Id Reactions
An abnormal immune response against autologous skin antigens may be responsible for the development of AE. Shelley5 postulated that hair follicles play an important role in id reactions, as Sharquie et al6 recently emphasized for many skin disorders. The pathogenesis of AE is uncertain, but circulating T lymphocytes play a role in this reaction. Normally, T cells are activated by a release of antigens after a primary exposure to a stimulus. However, overactivation of these T cells induces autoimmune reactions such as AE.7 Activated T lymphocytes express HLA-DR and IL-2 receptor, markers elevated in the peripheral blood of patients undergoing id reactions. After treatment, the levels of activated T lymphocytes decline. An increase in the number of CD25+ T cells and a decrease in the number of suppressor T cells in the blood may occur during an id reaction.7-9 Keratinocytes produce proinflammatory cytokines, such as thymic stromal erythropoietin, IL-25, and IL-33, that activate T cells.10-12 Therefore, the most likely pathogenesis of an id reaction is that T lymphocytes are activated at the primary reaction site due to proinflammatory cytokines released by keratinocytes. These activated T cells then travel systemically via hematogenous dissemination. The spread of activated T lymphocytes produces an eczematous reaction at secondary locations distant to the primary site.9
Clinical and Histopathological Features of Id Reactions
Clinically, AE is first evident as a vesicular dissemination that groups to form papules or nummular patches and usually is present on the legs, feet, arms, and/or trunk (Figure 2). The primary dermatitis is localized to the area that was the site of contact to the offending stimuli. This localized eczematous eruption begins with an acute or subacute onset. It has the appearance of small crusted vesicles with erythema (Figure 1). The first sign of AE is vesicles presenting near the primary site on flexural surfaces or on the hands and feet. A classic example is tinea pedis linked with vesicles on the palms and sides of the fingers, resembling dyshidrotic eczema. Sites of prior cutaneous trauma, such as dermatoses, scars, and burns, are common locations for early AE. In later stages, vesicles disseminate to the legs, arms, and trunk, where they group to form papules and nummular patches in a symmetrical pattern.5,13-15 These lesions may be extremely pruritic. The pruritus may be so intense that it interrupts daily activities and disrupts the ability to fall or stay asleep.16
Histologically, biopsy specimens show psoriasiform spongiotic dermatitis with mononuclear cells contained in the vesicles. Interstitial edema and perivascular lymphohistiocytic infiltrates are evident. Eosinophils also may be present. This pattern is not unique toid reactions.17-19 Although AE is a reaction pattern that may be due to a fungal or bacterial infection, the etiologic agent is not evident microscopically within the eczema itself.
Etiology of Id Reactions
Id reactions most commonly occur from either stasis dermatitis or tinea pedis, although a wide variety of other causes should be considered. Evaluation of the primary site rather than the id reaction may identify an infectious or parasitic agent. Sometimes the AE reaction is specifically named: dermatophytid with dermatophytosis, bacterid with a bacterial infectious process, and tuberculid with tuberculosis. Similarly, there may be reactions to underlying candidiasis, sporotrichosis, histoplasmosis, and other fungal infections that can cause a cutaneous id reaction.18,20-22Mycobacterium species, Pseudomonas, Staphylococcus, and Streptococcus are bacterial causes of AE.15,23-26 Viral infections that can cause an id reaction are herpes simplex virus and molluscum contagiosum.27-29 Scabies, leishmaniasis, and pediculosis capitis are parasitic infections that may be etiologic.14,30,31 In addition, noninfectious stimuli besides stasis dermatitis that can produce id reactions include medications, topical creams, tattoo ink, sutures, radiotherapy, and dyshidrotic eczema. The primary reaction to these agents is a localized dermatitis followed by the immunological response that induces a secondary reaction distant from the primary site.17,18,32-38
Differential Diagnoses
Differential diagnoses include other types of eczema and some vesicular eruptions. Irritant contact dermatitis is another dermatosis that presents as a widespread vesicular eruption due to repetitive exposure to toxic irritants. The rash is erythematous with pustules, blisters, and crusts. It is only found in areas directly exposed to irritants, as opposed to AE, which spreads to areas distant to the primary reaction site. Irritant contact dermatitis presents with more of a burning sensation, whereas AE is more pruritic.39,40 Allergic contact dermatitis presents with erythematous vesicles and papules and sometimes with bullae. There is edema and crust formation, which often can spread past the point of contact in later stages. Similar to AE, there is intense pruritus. However, allergic contact dermatitis most commonly is caused by exposure to metals, cosmetics, and fragrances, whereas infectious agents and stasis dermatitis are the most common causes of AE.40,41 It may be challenging to distinguish AE from other causes of widespread eczematous dissemination. Vesicular eruptions sometimes require distinction from AE, including herpetic infections, insect bite reactions, and drug eruptions.18,42
Treatment
The underlying condition should be treated to mitigate the inflammatory response causing the id reaction. If not skillfully orchestrated, the id reaction can reoccur. For infectious causes of AE, an antifungal, antibacterial, antiviral, or antiparasitic should be given. If stasis dermatitis is responsible for the id reaction, compression stockings and leg elevation are indicated. The id reaction itself is treated with systemic or topical corticosteroids and wet compresses if acute. The goal of these treatments is to reduce patient discomfort caused by the inflammation and pruritus.18,43
Conclusion
Id reactions are an unusual phenomenon that commonly occurs after fungal skin infections and stasis dermatitis. T lymphocytes and keratinocytes may play a key role in this reaction, with newer research further delineating the process and possibly providing enhanced treatment options. Therapy focuses on treating the underlying condition, supplemented with corticosteroids for the autoeczema.
Autoeczematization (AE), or id reaction, is a disseminated eczematous reaction that occurs days or weeks after exposure to a primary stimulus, resulting from a release of antigen(s). Whitfield1 first described AE in 1921, when he postulated that the id reaction was due to sensitization of the skin after a primary stimulus. He called it “a form of auto-intoxication derived from changes in the patient’s own tissues.”1 The exact prevalence of id reactions is unknown; one study showed that 17% of patients with dermatophyte infections developed an id reaction, typically tinea pedis linked with vesicles on the palms.2 Tinea capitis is one of the most common causes of AE in children, which is frequently misdiagnosed as a drug reaction. Approximately 37% of patients diagnosed with stasis dermatitis develop an id reaction (Figure 1). A history of contact dermatitis is common in patients presenting with AE.2-6
Pathophysiology of Id Reactions
An abnormal immune response against autologous skin antigens may be responsible for the development of AE. Shelley5 postulated that hair follicles play an important role in id reactions, as Sharquie et al6 recently emphasized for many skin disorders. The pathogenesis of AE is uncertain, but circulating T lymphocytes play a role in this reaction. Normally, T cells are activated by a release of antigens after a primary exposure to a stimulus. However, overactivation of these T cells induces autoimmune reactions such as AE.7 Activated T lymphocytes express HLA-DR and IL-2 receptor, markers elevated in the peripheral blood of patients undergoing id reactions. After treatment, the levels of activated T lymphocytes decline. An increase in the number of CD25+ T cells and a decrease in the number of suppressor T cells in the blood may occur during an id reaction.7-9 Keratinocytes produce proinflammatory cytokines, such as thymic stromal erythropoietin, IL-25, and IL-33, that activate T cells.10-12 Therefore, the most likely pathogenesis of an id reaction is that T lymphocytes are activated at the primary reaction site due to proinflammatory cytokines released by keratinocytes. These activated T cells then travel systemically via hematogenous dissemination. The spread of activated T lymphocytes produces an eczematous reaction at secondary locations distant to the primary site.9
Clinical and Histopathological Features of Id Reactions
Clinically, AE is first evident as a vesicular dissemination that groups to form papules or nummular patches and usually is present on the legs, feet, arms, and/or trunk (Figure 2). The primary dermatitis is localized to the area that was the site of contact to the offending stimuli. This localized eczematous eruption begins with an acute or subacute onset. It has the appearance of small crusted vesicles with erythema (Figure 1). The first sign of AE is vesicles presenting near the primary site on flexural surfaces or on the hands and feet. A classic example is tinea pedis linked with vesicles on the palms and sides of the fingers, resembling dyshidrotic eczema. Sites of prior cutaneous trauma, such as dermatoses, scars, and burns, are common locations for early AE. In later stages, vesicles disseminate to the legs, arms, and trunk, where they group to form papules and nummular patches in a symmetrical pattern.5,13-15 These lesions may be extremely pruritic. The pruritus may be so intense that it interrupts daily activities and disrupts the ability to fall or stay asleep.16
Histologically, biopsy specimens show psoriasiform spongiotic dermatitis with mononuclear cells contained in the vesicles. Interstitial edema and perivascular lymphohistiocytic infiltrates are evident. Eosinophils also may be present. This pattern is not unique toid reactions.17-19 Although AE is a reaction pattern that may be due to a fungal or bacterial infection, the etiologic agent is not evident microscopically within the eczema itself.
Etiology of Id Reactions
Id reactions most commonly occur from either stasis dermatitis or tinea pedis, although a wide variety of other causes should be considered. Evaluation of the primary site rather than the id reaction may identify an infectious or parasitic agent. Sometimes the AE reaction is specifically named: dermatophytid with dermatophytosis, bacterid with a bacterial infectious process, and tuberculid with tuberculosis. Similarly, there may be reactions to underlying candidiasis, sporotrichosis, histoplasmosis, and other fungal infections that can cause a cutaneous id reaction.18,20-22Mycobacterium species, Pseudomonas, Staphylococcus, and Streptococcus are bacterial causes of AE.15,23-26 Viral infections that can cause an id reaction are herpes simplex virus and molluscum contagiosum.27-29 Scabies, leishmaniasis, and pediculosis capitis are parasitic infections that may be etiologic.14,30,31 In addition, noninfectious stimuli besides stasis dermatitis that can produce id reactions include medications, topical creams, tattoo ink, sutures, radiotherapy, and dyshidrotic eczema. The primary reaction to these agents is a localized dermatitis followed by the immunological response that induces a secondary reaction distant from the primary site.17,18,32-38
Differential Diagnoses
Differential diagnoses include other types of eczema and some vesicular eruptions. Irritant contact dermatitis is another dermatosis that presents as a widespread vesicular eruption due to repetitive exposure to toxic irritants. The rash is erythematous with pustules, blisters, and crusts. It is only found in areas directly exposed to irritants, as opposed to AE, which spreads to areas distant to the primary reaction site. Irritant contact dermatitis presents with more of a burning sensation, whereas AE is more pruritic.39,40 Allergic contact dermatitis presents with erythematous vesicles and papules and sometimes with bullae. There is edema and crust formation, which often can spread past the point of contact in later stages. Similar to AE, there is intense pruritus. However, allergic contact dermatitis most commonly is caused by exposure to metals, cosmetics, and fragrances, whereas infectious agents and stasis dermatitis are the most common causes of AE.40,41 It may be challenging to distinguish AE from other causes of widespread eczematous dissemination. Vesicular eruptions sometimes require distinction from AE, including herpetic infections, insect bite reactions, and drug eruptions.18,42
Treatment
The underlying condition should be treated to mitigate the inflammatory response causing the id reaction. If not skillfully orchestrated, the id reaction can reoccur. For infectious causes of AE, an antifungal, antibacterial, antiviral, or antiparasitic should be given. If stasis dermatitis is responsible for the id reaction, compression stockings and leg elevation are indicated. The id reaction itself is treated with systemic or topical corticosteroids and wet compresses if acute. The goal of these treatments is to reduce patient discomfort caused by the inflammation and pruritus.18,43
Conclusion
Id reactions are an unusual phenomenon that commonly occurs after fungal skin infections and stasis dermatitis. T lymphocytes and keratinocytes may play a key role in this reaction, with newer research further delineating the process and possibly providing enhanced treatment options. Therapy focuses on treating the underlying condition, supplemented with corticosteroids for the autoeczema.
- Whitfield A. Lumleian Lectures on Some Points in the Aetiology of Skin Diseases. Delivered before the Royal College of Physicians of London on March 10th, 15th, and 17th, 1921. Lecture II. Lancet. 1921;2:122-127.
- Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128:E453-E457.
- Schrom KP, Kobs A, Nedorost S. Clinical psoriasiform dermatitis following dupilumab use for autoeczematization secondary to chronic stasis dermatitis. Cureus. 2020;12:e7831. doi:10.7759/cureus.7831
- Templeton HJ, Lunsford CJ, Allington HV. Autosensitization dermatitis; report of five cases and protocol of an experiment. Arch Derm Syphilol. 1949;59:68-77.
- Shelley WB. Id reaction. In: Consultations in Dermatology. Saunders; 1972:262-267.
- Sharquie KE, Noaimi AA, Flayih RA. Clinical and histopathological findings in patients with follicular dermatoses: all skin diseases starts in the hair follicles as new hypothesis. Am J Clin Res Rev. 2020;4:17.
- Kasteler JS, Petersen MJ, Vance JE, et al. Circulating activated T lymphocytes in autoeczematization. Arch Dermatol. 1992;128:795-798.
- González-Amaro R, Baranda L, Abud-Mendoza C, et al. Autoeczematization is associated with abnormal immune recognition of autologous skin antigens. J Am Acad Dermatol. 1993;28:56-60.
- Cunningham MJ, Zone JJ, Petersen MJ, et al. Circulating activated (DR-positive) T lymphocytes in a patient with autoeczematization. J Am Acad Dermatol. 1986;14:1039-1041.
- Furue M, Ulzii D, Vu YH, et al. Pathogenesis of atopic dermatitis: current paradigm. Iran J Immunol. 2019;16:97-107.
- Uchi H, Terao H, Koga T, et al. Cytokines and chemokines in the epidermis. J Dermatol Sci. 2000;24(suppl 1):S29-S38.
- Bos JD, Kapsenberg ML. The skin immune system: progress in cutaneous biology. Immunol Today. 1993;14:75-78.
- Young AW Jr. Dynamics of autosensitization dermatitis; a clinical and microscopic concept of autoeczematization. AMA Arch Derm. 1958;77:495-502.
- Brenner S, Wolf R, Landau M. Scabid: an unusual id reaction to scabies. Int J Dermatol. 1993;32:128-129.
- Yamany T, Schwartz RA. Infectious eczematoid dermatitis: a comprehensive review. J Eur Acad Dermatol Venereol. 2015;29:203-208.
- Wang X, Li L, Shi X, et al. Itching and its related factors in subtypes of eczema: a cross-sectional multicenter study in tertiary hospitals of China. Sci Rep. 2018;8:10754.
- Price A, Tavazoie M, Meehan SA, et al. Id reaction associated with red tattoo ink. Cutis. 2018;102:E32-E34.
- Ilkit M, Durdu M, Karaks¸ M. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol. 2012;38:191-202.
- Kaner SR. Dermatitis venenata of the feet with a generalized “id” reaction. J Am Podiatry Assoc. 1970;60:199-204.
- Jordan L, Jackson NA, Carter-Snell B, et al. Pustular tinea id reaction. Cutis. 2019;103:E3-E4.
- Crum N, Hardaway C, Graham B. Development of an idlike reaction during treatment for acute pulmonary histoplasmosis: a new cutaneous manifestation in histoplasmosis. J Am Acad Dermatol. 2003;48(2 suppl):S5-S6.
- Chirac A, Brzezinski P, Chiriac AE, et al. Autosensitisation (autoeczematisation) reactions in a case of diaper dermatitis candidiasis. Niger Med J. 2014;55:274-275.
- Singh PY, Sinha P, Baveja S, et al. Immune-mediated tuberculous uveitis—a rare association with papulonecrotic tuberculid. Indian J Ophthalmol. 2019;67:1207-1209.
- Urso B, Georgesen C, Harp J. Papulonecrotic tuberculid secondary to Mycobacterium avium complex. Cutis. 2019;104:E11-E13.
- Choudhri SH, Magro CM, Crowson AN, et al. An id reaction to Mycobacterium leprae: first documented case. Cutis. 1994;54:282-286.
- Park JW, Jeong GJ, Seo SJ, et al. Pseudomonas toe web infection and autosensitisation dermatitis: diagnostic and therapeutic challenge. Int Wound J. 2020;17:1543-1544. doi:10.1111/iwj.13386
- Netchiporouk E, Cohen BA. Recognizing and managing eczematous id reactions to molluscum contagiosum virus in children. Pediatrics. 2012;129:E1072-E1075.
- Aurelian L, Ono F, Burnett J. Herpes simplex virus (HSV)-associated erythema multiforme (HAEM): a viral disease with an autoimmune component. Dermatol Online J. 2003;9:1.
- Rocamora V, Romaní J, Puig L, et al. Id reaction to molluscum contagiosum. Pediatr Dermatol. 1996;13:349-350.
- Yes¸ilova Y, Özbilgin A, Turan E, et al. Clinical exacerbation developing during treatment of cutaneous leishmaniasis: an id reaction? Turkiye Parazitol Derg. 2014;38:281-282.
- Connor CJ, Selby JC, Wanat KA. Severe pediculosis capitus: a case of “crusted lice” with autoeczematization. Dermatol Online J. 2016;22:13030/qt7c91z913.
- Shelley WB. The autoimmune mechanism in clinical dermatology. Arch Dermatol. 1962;86:27-34.
- Bosworth A, Hull PR. Disseminated eczema following radiotherapy: a case report. J Cutan Med Surg. 2018;22:353-355.
- Lowther C, Miedler JD, Cockerell CJ. Id-like reaction to BCG therapy for bladder cancer. Cutis. 2013;91:145-151.
- Huerth KA, Glick PL, Glick ZR. Cutaneous id reaction after using cyanoacrylate for wound closure. Cutis. 2020;105:E11-E13.
- Amini S, Burdick AE, Janniger CK. Dyshidrotic eczema (pompholyx). Updated April 22, 2020. Accessed August 23, 2021. https://emedicine.medscape.com/article/1122527-overview
- Sundaresan S, Migden MR, Silapunt S. Stasis dermatitis: pathophysiology, evaluation, and management. Am J Clin Dermatol. 2017;18:383-390.
- Hughes JDM, Pratt MD. Allergic contact dermatitis and autoeczematization to proctosedyl® cream and proctomyxin® cream. Case Rep Dermatol. 2018;10:238-246.
- Bains SN, Nash P, Fonacier L. Irritant contact dermatitis. Clin Rev Allergy Immunol. 2019;56:99-109.
- Novak-Bilic´ G, Vucˇic´ M, Japundžic´ I, et al. Irritant and allergic contact dermatitis—skin lesion characteristics. Acta Clin Croat. 2018;57:713-720.
- Nassau S, Fonacier L. Allergic contact dermatitis. Med Clin North Am. 2020;104:61-76.
- Lewis DJ, Schlichte MJ, Dao H Jr. Atypical disseminated herpes zoster: management guidelines in immunocompromised patients. Cutis. 2017;100:321-330.
- Nedorost S, White S, Rowland DY, et al. Development and implementation of an order set to improve value of care for patients with severe stasis dermatitis. J Am Acad Dermatol. 2019;80:815-817.
- Whitfield A. Lumleian Lectures on Some Points in the Aetiology of Skin Diseases. Delivered before the Royal College of Physicians of London on March 10th, 15th, and 17th, 1921. Lecture II. Lancet. 1921;2:122-127.
- Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128:E453-E457.
- Schrom KP, Kobs A, Nedorost S. Clinical psoriasiform dermatitis following dupilumab use for autoeczematization secondary to chronic stasis dermatitis. Cureus. 2020;12:e7831. doi:10.7759/cureus.7831
- Templeton HJ, Lunsford CJ, Allington HV. Autosensitization dermatitis; report of five cases and protocol of an experiment. Arch Derm Syphilol. 1949;59:68-77.
- Shelley WB. Id reaction. In: Consultations in Dermatology. Saunders; 1972:262-267.
- Sharquie KE, Noaimi AA, Flayih RA. Clinical and histopathological findings in patients with follicular dermatoses: all skin diseases starts in the hair follicles as new hypothesis. Am J Clin Res Rev. 2020;4:17.
- Kasteler JS, Petersen MJ, Vance JE, et al. Circulating activated T lymphocytes in autoeczematization. Arch Dermatol. 1992;128:795-798.
- González-Amaro R, Baranda L, Abud-Mendoza C, et al. Autoeczematization is associated with abnormal immune recognition of autologous skin antigens. J Am Acad Dermatol. 1993;28:56-60.
- Cunningham MJ, Zone JJ, Petersen MJ, et al. Circulating activated (DR-positive) T lymphocytes in a patient with autoeczematization. J Am Acad Dermatol. 1986;14:1039-1041.
- Furue M, Ulzii D, Vu YH, et al. Pathogenesis of atopic dermatitis: current paradigm. Iran J Immunol. 2019;16:97-107.
- Uchi H, Terao H, Koga T, et al. Cytokines and chemokines in the epidermis. J Dermatol Sci. 2000;24(suppl 1):S29-S38.
- Bos JD, Kapsenberg ML. The skin immune system: progress in cutaneous biology. Immunol Today. 1993;14:75-78.
- Young AW Jr. Dynamics of autosensitization dermatitis; a clinical and microscopic concept of autoeczematization. AMA Arch Derm. 1958;77:495-502.
- Brenner S, Wolf R, Landau M. Scabid: an unusual id reaction to scabies. Int J Dermatol. 1993;32:128-129.
- Yamany T, Schwartz RA. Infectious eczematoid dermatitis: a comprehensive review. J Eur Acad Dermatol Venereol. 2015;29:203-208.
- Wang X, Li L, Shi X, et al. Itching and its related factors in subtypes of eczema: a cross-sectional multicenter study in tertiary hospitals of China. Sci Rep. 2018;8:10754.
- Price A, Tavazoie M, Meehan SA, et al. Id reaction associated with red tattoo ink. Cutis. 2018;102:E32-E34.
- Ilkit M, Durdu M, Karaks¸ M. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol. 2012;38:191-202.
- Kaner SR. Dermatitis venenata of the feet with a generalized “id” reaction. J Am Podiatry Assoc. 1970;60:199-204.
- Jordan L, Jackson NA, Carter-Snell B, et al. Pustular tinea id reaction. Cutis. 2019;103:E3-E4.
- Crum N, Hardaway C, Graham B. Development of an idlike reaction during treatment for acute pulmonary histoplasmosis: a new cutaneous manifestation in histoplasmosis. J Am Acad Dermatol. 2003;48(2 suppl):S5-S6.
- Chirac A, Brzezinski P, Chiriac AE, et al. Autosensitisation (autoeczematisation) reactions in a case of diaper dermatitis candidiasis. Niger Med J. 2014;55:274-275.
- Singh PY, Sinha P, Baveja S, et al. Immune-mediated tuberculous uveitis—a rare association with papulonecrotic tuberculid. Indian J Ophthalmol. 2019;67:1207-1209.
- Urso B, Georgesen C, Harp J. Papulonecrotic tuberculid secondary to Mycobacterium avium complex. Cutis. 2019;104:E11-E13.
- Choudhri SH, Magro CM, Crowson AN, et al. An id reaction to Mycobacterium leprae: first documented case. Cutis. 1994;54:282-286.
- Park JW, Jeong GJ, Seo SJ, et al. Pseudomonas toe web infection and autosensitisation dermatitis: diagnostic and therapeutic challenge. Int Wound J. 2020;17:1543-1544. doi:10.1111/iwj.13386
- Netchiporouk E, Cohen BA. Recognizing and managing eczematous id reactions to molluscum contagiosum virus in children. Pediatrics. 2012;129:E1072-E1075.
- Aurelian L, Ono F, Burnett J. Herpes simplex virus (HSV)-associated erythema multiforme (HAEM): a viral disease with an autoimmune component. Dermatol Online J. 2003;9:1.
- Rocamora V, Romaní J, Puig L, et al. Id reaction to molluscum contagiosum. Pediatr Dermatol. 1996;13:349-350.
- Yes¸ilova Y, Özbilgin A, Turan E, et al. Clinical exacerbation developing during treatment of cutaneous leishmaniasis: an id reaction? Turkiye Parazitol Derg. 2014;38:281-282.
- Connor CJ, Selby JC, Wanat KA. Severe pediculosis capitus: a case of “crusted lice” with autoeczematization. Dermatol Online J. 2016;22:13030/qt7c91z913.
- Shelley WB. The autoimmune mechanism in clinical dermatology. Arch Dermatol. 1962;86:27-34.
- Bosworth A, Hull PR. Disseminated eczema following radiotherapy: a case report. J Cutan Med Surg. 2018;22:353-355.
- Lowther C, Miedler JD, Cockerell CJ. Id-like reaction to BCG therapy for bladder cancer. Cutis. 2013;91:145-151.
- Huerth KA, Glick PL, Glick ZR. Cutaneous id reaction after using cyanoacrylate for wound closure. Cutis. 2020;105:E11-E13.
- Amini S, Burdick AE, Janniger CK. Dyshidrotic eczema (pompholyx). Updated April 22, 2020. Accessed August 23, 2021. https://emedicine.medscape.com/article/1122527-overview
- Sundaresan S, Migden MR, Silapunt S. Stasis dermatitis: pathophysiology, evaluation, and management. Am J Clin Dermatol. 2017;18:383-390.
- Hughes JDM, Pratt MD. Allergic contact dermatitis and autoeczematization to proctosedyl® cream and proctomyxin® cream. Case Rep Dermatol. 2018;10:238-246.
- Bains SN, Nash P, Fonacier L. Irritant contact dermatitis. Clin Rev Allergy Immunol. 2019;56:99-109.
- Novak-Bilic´ G, Vucˇic´ M, Japundžic´ I, et al. Irritant and allergic contact dermatitis—skin lesion characteristics. Acta Clin Croat. 2018;57:713-720.
- Nassau S, Fonacier L. Allergic contact dermatitis. Med Clin North Am. 2020;104:61-76.
- Lewis DJ, Schlichte MJ, Dao H Jr. Atypical disseminated herpes zoster: management guidelines in immunocompromised patients. Cutis. 2017;100:321-330.
- Nedorost S, White S, Rowland DY, et al. Development and implementation of an order set to improve value of care for patients with severe stasis dermatitis. J Am Acad Dermatol. 2019;80:815-817.
Practice Points
- Autoeczematization, or id reaction, is a disseminated reaction of the skin occurring at a site distant to a primary cutaneous infection or stimulus.
- T lymphocytes and keratinocytes are postulated to be involved in the pathogenesis of id reactions.
- Therapy includes treating the underlying pathology while providing topical corticosteroids for the autoeczematous lesions.
Neonatal and Infantile Acne Vulgaris: An Update
Acne vulgaris typically is associated with adolescence and young adulthood; however, it also can affect neonates, infants, and small children.1 Acne neonatorum occurs in up to 20% of newborns. The clinical importance of neonatal acne lies in its differentiation from infectious diseases, the exclusion of virilization as its underlying cause, and the possible implication of severe acne in adolescence.2 Neonatal acne also must be distinguished from acne that is induced by application of topical oils and ointments (acne venenata) and from acneform eruptions induced by acnegenic maternal medications such as hydantoin (fetal hydantoin syndrome) and lithium.3
Neonatal Acne (Acne Neonatorum)
Clinical Presentation
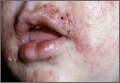
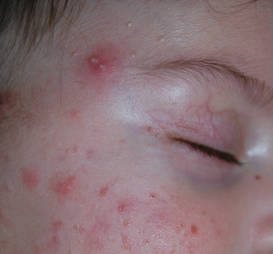
Neonatal acne (acne neonatorum) typically presents as small closed comedones on the forehead, nose, and cheeks (Figure 1).4 Accompanying sebaceous hyperplasia often is noted.5 Less frequently, open comedones, inflammatory papules, and pustules may develop.6 Neonatal acne may be evident at birth or appear during the first 4 weeks of life7 and is more commonly seen in boys.8
Etiology
Several factors may be pivotal in the etiology of neonatal acne, including increased sebum excretion, stimulation of the sebaceous glands by maternal or neonatal androgens,4 and colonization of sebaceous glands by Malassezia species.2 Increased sebum excretion occurs during the neonatal period due to enlarged sebaceous glands,2 which may result from the substantial production of β-hydroxysteroids from the relatively large adrenal glands.9,10 After 6 months of age, the size of the sebaceous glands and the sebum excretion rate decrease.9,10
Both maternal and neonatal androgens have been implicated in the stimulation of sebaceous glands in neonatal acne.2 The neonatal adrenal gland produces high levels of dehydroepiandrosterone,2 which stimulate sebaceous glands until around 1 year of age when dehydroepiandrosterone levels drop off as a consequence of involution of the neonatal adrenal gland.11 Testicular androgens provide additional stimulation to the sebaceous glands, which may explain why neonatal acne is more common in boys.1 Neonatal acne may be an inflammatory response to Malassezia species; however, Malassezia was not isolated in a series of patients,12 suggesting that neonatal acne is an early presentation of comedonal acne and not a response to Malassezia.2,12
Differential Diagnosis
There are a number of acneform eruptions that should be considered in the differential diagnosis,3 including bacterial folliculitis, secondary syphilis,13 herpes simplex virus and varicella zoster virus,14 and skin colonization by fungi of Malassezia species.15 Other neonatal eruptions such as erythema toxicum neonatorum,16 transient neonatal pustular melanosis, and milia and pustular miliaria, as well as a drug eruption associated with hydantoin, lithium, or halogens should be considered.17 The relationship between neonatal acne and neonatal cephalic pustulosis, which is characterized by papules and pustules without comedones, is controversial; some consider them to be 2 different entities,14 while others do not.18
Treatment
Guardians should be reassured that neonatal acne is mild, self-limited, and generally resolves spontaneously without scarring in approximately 1 to 3 months.1,2 In most cases, no treatment is needed.19 If necessary, comedones may be treated with azelaic acid cream 20% or tretinoin cream 0.025% to 0.05%.1,2 For inflammatory lesions, erythromycin solution 2% and benzoyl peroxide gel 2.5% may be used.1,20 Severe or recalcitrant disease warrants a workup for congenital adrenal hyperplasia, a virilizing tumor, or underlying endocrinopathy.19
Infantile Acne Vulgaris
Clinical Presentation
Infantile acne vulgaris shares similarities with neonatal acne21,22 in that they both affect the face, predominantly the cheeks, and have a male predominance (Figure 2).1,10 However, by definition, onset of infantile acne typically occurs later than acne neonatorum, usually at 3 to 6 months of age.1,4 Lesions are more pleomorphic and inflammatory than in neonatal acne. In addition to closed and open comedones, infantile acne may be first evident with papules, pustules, severe nodules, and cysts with scarring potential (Figure 3).1,2,5 Accordingly, treatment may be required. Most cases of infantile acne resolve by 4 or 5 years of age, but some remain active into puberty.1 Patients with a history of infantile acne have an increased incidence of acne vulgaris during adolescence compared to their peers, with greater severity and enhanced risk for scarring.4,23

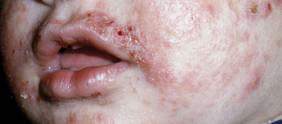
Etiology
The etiology of infantile acne remains unclear.2 Similar to neonatal acne, infantile acne may be a result of elevated androgens produced by the fetal adrenal glands as well as by the testes in males.11 For example, a child with infantile acne had elevated luteinizing hormone, follicle-stimulating hormone, and testosterone levels.24 Therefore, hyperandrogenism should be considered as an etiology. Other causes also have been suggested. Rarely, an adrenocortical tumor may be associated with persistent infantile acne with signs of virilization and rapid development.25Malassezia was implicated in infantile acne in a 6-month-old infant who was successfully treated with ketoconazole cream 2%.26
Differential Diagnosis
Infantile acne often is misdiagnosed because it is rarely considered in the differential diagnosis. When closed comedones predominate, acne venenata induced by topical creams, lotions, or oils may be etiologic. Chloracne also should be considered.14
Treatment
Guardians should be educated about the likely chronicity of infantile acne, which may require long-term treatment, as well as the possibility that acne may recur in severe form during puberty.1 The treatment strategy for infantile acne is similar to treatment of acne at any age, with topical agents including retinoids (eg, tretinoin, benzoyl peroxide) and topical antibacterials (eg, erythromycin). Twice-daily erythromycin 125 to 250 mg is the treatment of choice when oral antibiotics are indicated. Tetracyclines are contraindicated in treatment of neonatal and infantile acne. Intralesional injections with low-concentration triamcinolone acetonide, cryotherapy, or topical corticosteroids for a short period of time can be used to treat deep nodules and cysts.2 Acne that is refractory to treatment with oral antibiotics alone or combined with topical treatments poses a dilemma, given the potential cosmetic sequelae of scarring and quality-of-life concerns. Because reducing or eliminating dairy intake appears beneficial for adolescents with moderate to severe acne,27 this approach may represent a good option for infantile acne.
Conclusion
Neonatal and infantile acne vulgaris may be overlooked or misdiagnosed. It is important to consider and treat. Early childhood acne may represent a virilization syndrome.
- Jansen T, Burgdorf WH, Plewig G. Pathogenesis and treatment of acne in childhood. Pediatr Dermatol. 1997;14:17-21.
- Antoniou C, Dessinioti C, Stratigos AJ, et al. Clinical and therapeutic approach to childhood acne: an update. Pediatr Dermatol. 2009;26:373-380.
- Kuflik JH, Schwartz RA. Acneiform eruptions. Cutis. 2000;66:97-100.
- Barbareschi M, Benardon S, Guanziroli E, et al. Classification and grading. In: Schwartz RA, Micali G, eds. Acne. Gurgaon, India: Nature Publishing Group; 2013:67-75.
- Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol. 2002;3:389-400.
- O’Connor NR, McLaughlin MR, Ham P. Newborn skin: part I. common rashes. Am Fam Physician. 2008;77:47-52.
- Nanda S, Reddy BS, Ramji S, et al. Analytical study of pustular eruptions in neonates. Pediatr Dermatol. 2002;19:210-215.
- Yonkosky DM, Pochi PE. Acne vulgaris in childhood. pathogenesis and management. Dermatol Clin. 1986;4:127-136.
- Agache P, Blanc D, Barrand C, et al. Sebum levels during the first year of life. Br J Dermatol. 1980;103:643-649.
- Herane MI, Ando I. Acne in infancy and acne genetics. Dermatology. 2003;206:24-28.
- Lucky AW. A review of infantile and pediatric acne. Dermatology (Basel, Switzerland). 1998;103:643-649.
- Bernier V, Weill FX, Hirigoyen V, et al. Skin colonization by Malassezia species in neonates: a prospective study and relationship with neonatal cephalic pustulosis. Arch Dermatol. 2002;138:215-218.
- Lambert WC, Bagley MP, Khan Y, et al. Pustular acneiform secondary syphilis. Cutis. 1986;37:69-70.
- Antoniou C, Dessinioti C, Stratigos AJ, et al. Clinical and therapeutic approach to childhood acne: an update. Pediatr Dermatol. 2009;26:373-380.
- Borton LK, Schwartz RA. Pityrosporum folliculitis: a common acneiform condition of middle age. Ariz Med. 1981;38:598-601.
- Morgan AJ, Steen CJ, Schwartz RA, et al. Erythema toxicum neonatorum revisited. Cutis. 2009;83:13-16.
- Brodkin RH, Schwartz RA. Cutaneous signs of dioxin exposure. Am Fam Physician. 1984;30:189-194.
- Mancini AJ, Baldwin HE, Eichenfield LF, et al. Acne life cycle: the spectrum of pediatric disease. Semin Cutan Med Surg. 2011;30(suppl 3):S2-S5.
- Katsambas AD, Katoulis AC, Stavropoulos P. Acne neonatorum: a study of 22 cases. Int J Dermatol. 1999;38:128-130.
- Van Praag MC, Van Rooij RW, Folkers E, et al. Diagnosis and treatment of pustular disorders in the neonate. Pediatr Dermatol. 1997;14:131-143.
- Barnes CJ, Eichenfield LF, Lee J, et al. A practical approach for the use of oral isotretinoin for infantile acne. Pediatr Dermatol. 2005;22:166-169.
- Janniger CK. Neonatal and infantile acne vulgaris. Cutis. 1993;52:16.
- Chew EW, Bingham A, Burrows D. Incidence of acne vulgaris in patients with infantile acne. Clin Exp Dermatol. 1990;15:376-377.
- Duke EM. Infantile acne associated with transient increases in plasma concentrations of luteinising hormone, follicle-stimulating hormone, and testosterone. Br Med J (Clinical Res Ed). 1981;282:1275-1276.
- Mann MW, Ellis SS, Mallory SB. Infantile acne as the initial sign of an adrenocortical tumor [published online ahead of print September 14, 2006]. J Am Acad Dermatol. 2007;56(suppl 2):S15-S18.
- Kang SK, Jee MS, Choi JH, et al. A case of infantile acne due to Pityrosporum. Pediatr Dermatol. 2003;20:68-70.
- Di Landro A, Cazzaniga S, Parazzini F, et al. Family history, body mass index, selected dietary factors, menstrual history, and risk of moderate to severe acne in adolescents and young adults [published online ahead of print March 3, 2012]. J Am Acad Dermatol. 2012;67:1129-1135.
Acne vulgaris typically is associated with adolescence and young adulthood; however, it also can affect neonates, infants, and small children.1 Acne neonatorum occurs in up to 20% of newborns. The clinical importance of neonatal acne lies in its differentiation from infectious diseases, the exclusion of virilization as its underlying cause, and the possible implication of severe acne in adolescence.2 Neonatal acne also must be distinguished from acne that is induced by application of topical oils and ointments (acne venenata) and from acneform eruptions induced by acnegenic maternal medications such as hydantoin (fetal hydantoin syndrome) and lithium.3
Neonatal Acne (Acne Neonatorum)
Clinical Presentation
Neonatal acne (acne neonatorum) typically presents as small closed comedones on the forehead, nose, and cheeks (Figure 1).4 Accompanying sebaceous hyperplasia often is noted.5 Less frequently, open comedones, inflammatory papules, and pustules may develop.6 Neonatal acne may be evident at birth or appear during the first 4 weeks of life7 and is more commonly seen in boys.8

Etiology
Several factors may be pivotal in the etiology of neonatal acne, including increased sebum excretion, stimulation of the sebaceous glands by maternal or neonatal androgens,4 and colonization of sebaceous glands by Malassezia species.2 Increased sebum excretion occurs during the neonatal period due to enlarged sebaceous glands,2 which may result from the substantial production of β-hydroxysteroids from the relatively large adrenal glands.9,10 After 6 months of age, the size of the sebaceous glands and the sebum excretion rate decrease.9,10
Both maternal and neonatal androgens have been implicated in the stimulation of sebaceous glands in neonatal acne.2 The neonatal adrenal gland produces high levels of dehydroepiandrosterone,2 which stimulate sebaceous glands until around 1 year of age when dehydroepiandrosterone levels drop off as a consequence of involution of the neonatal adrenal gland.11 Testicular androgens provide additional stimulation to the sebaceous glands, which may explain why neonatal acne is more common in boys.1 Neonatal acne may be an inflammatory response to Malassezia species; however, Malassezia was not isolated in a series of patients,12 suggesting that neonatal acne is an early presentation of comedonal acne and not a response to Malassezia.2,12
Differential Diagnosis
There are a number of acneform eruptions that should be considered in the differential diagnosis,3 including bacterial folliculitis, secondary syphilis,13 herpes simplex virus and varicella zoster virus,14 and skin colonization by fungi of Malassezia species.15 Other neonatal eruptions such as erythema toxicum neonatorum,16 transient neonatal pustular melanosis, and milia and pustular miliaria, as well as a drug eruption associated with hydantoin, lithium, or halogens should be considered.17 The relationship between neonatal acne and neonatal cephalic pustulosis, which is characterized by papules and pustules without comedones, is controversial; some consider them to be 2 different entities,14 while others do not.18
Treatment
Guardians should be reassured that neonatal acne is mild, self-limited, and generally resolves spontaneously without scarring in approximately 1 to 3 months.1,2 In most cases, no treatment is needed.19 If necessary, comedones may be treated with azelaic acid cream 20% or tretinoin cream 0.025% to 0.05%.1,2 For inflammatory lesions, erythromycin solution 2% and benzoyl peroxide gel 2.5% may be used.1,20 Severe or recalcitrant disease warrants a workup for congenital adrenal hyperplasia, a virilizing tumor, or underlying endocrinopathy.19
Infantile Acne Vulgaris
Clinical Presentation
Infantile acne vulgaris shares similarities with neonatal acne21,22 in that they both affect the face, predominantly the cheeks, and have a male predominance (Figure 2).1,10 However, by definition, onset of infantile acne typically occurs later than acne neonatorum, usually at 3 to 6 months of age.1,4 Lesions are more pleomorphic and inflammatory than in neonatal acne. In addition to closed and open comedones, infantile acne may be first evident with papules, pustules, severe nodules, and cysts with scarring potential (Figure 3).1,2,5 Accordingly, treatment may be required. Most cases of infantile acne resolve by 4 or 5 years of age, but some remain active into puberty.1 Patients with a history of infantile acne have an increased incidence of acne vulgaris during adolescence compared to their peers, with greater severity and enhanced risk for scarring.4,23


Etiology
The etiology of infantile acne remains unclear.2 Similar to neonatal acne, infantile acne may be a result of elevated androgens produced by the fetal adrenal glands as well as by the testes in males.11 For example, a child with infantile acne had elevated luteinizing hormone, follicle-stimulating hormone, and testosterone levels.24 Therefore, hyperandrogenism should be considered as an etiology. Other causes also have been suggested. Rarely, an adrenocortical tumor may be associated with persistent infantile acne with signs of virilization and rapid development.25Malassezia was implicated in infantile acne in a 6-month-old infant who was successfully treated with ketoconazole cream 2%.26
Differential Diagnosis
Infantile acne often is misdiagnosed because it is rarely considered in the differential diagnosis. When closed comedones predominate, acne venenata induced by topical creams, lotions, or oils may be etiologic. Chloracne also should be considered.14
Treatment
Guardians should be educated about the likely chronicity of infantile acne, which may require long-term treatment, as well as the possibility that acne may recur in severe form during puberty.1 The treatment strategy for infantile acne is similar to treatment of acne at any age, with topical agents including retinoids (eg, tretinoin, benzoyl peroxide) and topical antibacterials (eg, erythromycin). Twice-daily erythromycin 125 to 250 mg is the treatment of choice when oral antibiotics are indicated. Tetracyclines are contraindicated in treatment of neonatal and infantile acne. Intralesional injections with low-concentration triamcinolone acetonide, cryotherapy, or topical corticosteroids for a short period of time can be used to treat deep nodules and cysts.2 Acne that is refractory to treatment with oral antibiotics alone or combined with topical treatments poses a dilemma, given the potential cosmetic sequelae of scarring and quality-of-life concerns. Because reducing or eliminating dairy intake appears beneficial for adolescents with moderate to severe acne,27 this approach may represent a good option for infantile acne.
Conclusion
Neonatal and infantile acne vulgaris may be overlooked or misdiagnosed. It is important to consider and treat. Early childhood acne may represent a virilization syndrome.
Acne vulgaris typically is associated with adolescence and young adulthood; however, it also can affect neonates, infants, and small children.1 Acne neonatorum occurs in up to 20% of newborns. The clinical importance of neonatal acne lies in its differentiation from infectious diseases, the exclusion of virilization as its underlying cause, and the possible implication of severe acne in adolescence.2 Neonatal acne also must be distinguished from acne that is induced by application of topical oils and ointments (acne venenata) and from acneform eruptions induced by acnegenic maternal medications such as hydantoin (fetal hydantoin syndrome) and lithium.3
Neonatal Acne (Acne Neonatorum)
Clinical Presentation
Neonatal acne (acne neonatorum) typically presents as small closed comedones on the forehead, nose, and cheeks (Figure 1).4 Accompanying sebaceous hyperplasia often is noted.5 Less frequently, open comedones, inflammatory papules, and pustules may develop.6 Neonatal acne may be evident at birth or appear during the first 4 weeks of life7 and is more commonly seen in boys.8

Etiology
Several factors may be pivotal in the etiology of neonatal acne, including increased sebum excretion, stimulation of the sebaceous glands by maternal or neonatal androgens,4 and colonization of sebaceous glands by Malassezia species.2 Increased sebum excretion occurs during the neonatal period due to enlarged sebaceous glands,2 which may result from the substantial production of β-hydroxysteroids from the relatively large adrenal glands.9,10 After 6 months of age, the size of the sebaceous glands and the sebum excretion rate decrease.9,10
Both maternal and neonatal androgens have been implicated in the stimulation of sebaceous glands in neonatal acne.2 The neonatal adrenal gland produces high levels of dehydroepiandrosterone,2 which stimulate sebaceous glands until around 1 year of age when dehydroepiandrosterone levels drop off as a consequence of involution of the neonatal adrenal gland.11 Testicular androgens provide additional stimulation to the sebaceous glands, which may explain why neonatal acne is more common in boys.1 Neonatal acne may be an inflammatory response to Malassezia species; however, Malassezia was not isolated in a series of patients,12 suggesting that neonatal acne is an early presentation of comedonal acne and not a response to Malassezia.2,12
Differential Diagnosis
There are a number of acneform eruptions that should be considered in the differential diagnosis,3 including bacterial folliculitis, secondary syphilis,13 herpes simplex virus and varicella zoster virus,14 and skin colonization by fungi of Malassezia species.15 Other neonatal eruptions such as erythema toxicum neonatorum,16 transient neonatal pustular melanosis, and milia and pustular miliaria, as well as a drug eruption associated with hydantoin, lithium, or halogens should be considered.17 The relationship between neonatal acne and neonatal cephalic pustulosis, which is characterized by papules and pustules without comedones, is controversial; some consider them to be 2 different entities,14 while others do not.18
Treatment
Guardians should be reassured that neonatal acne is mild, self-limited, and generally resolves spontaneously without scarring in approximately 1 to 3 months.1,2 In most cases, no treatment is needed.19 If necessary, comedones may be treated with azelaic acid cream 20% or tretinoin cream 0.025% to 0.05%.1,2 For inflammatory lesions, erythromycin solution 2% and benzoyl peroxide gel 2.5% may be used.1,20 Severe or recalcitrant disease warrants a workup for congenital adrenal hyperplasia, a virilizing tumor, or underlying endocrinopathy.19
Infantile Acne Vulgaris
Clinical Presentation
Infantile acne vulgaris shares similarities with neonatal acne21,22 in that they both affect the face, predominantly the cheeks, and have a male predominance (Figure 2).1,10 However, by definition, onset of infantile acne typically occurs later than acne neonatorum, usually at 3 to 6 months of age.1,4 Lesions are more pleomorphic and inflammatory than in neonatal acne. In addition to closed and open comedones, infantile acne may be first evident with papules, pustules, severe nodules, and cysts with scarring potential (Figure 3).1,2,5 Accordingly, treatment may be required. Most cases of infantile acne resolve by 4 or 5 years of age, but some remain active into puberty.1 Patients with a history of infantile acne have an increased incidence of acne vulgaris during adolescence compared to their peers, with greater severity and enhanced risk for scarring.4,23


Etiology
The etiology of infantile acne remains unclear.2 Similar to neonatal acne, infantile acne may be a result of elevated androgens produced by the fetal adrenal glands as well as by the testes in males.11 For example, a child with infantile acne had elevated luteinizing hormone, follicle-stimulating hormone, and testosterone levels.24 Therefore, hyperandrogenism should be considered as an etiology. Other causes also have been suggested. Rarely, an adrenocortical tumor may be associated with persistent infantile acne with signs of virilization and rapid development.25Malassezia was implicated in infantile acne in a 6-month-old infant who was successfully treated with ketoconazole cream 2%.26
Differential Diagnosis
Infantile acne often is misdiagnosed because it is rarely considered in the differential diagnosis. When closed comedones predominate, acne venenata induced by topical creams, lotions, or oils may be etiologic. Chloracne also should be considered.14
Treatment
Guardians should be educated about the likely chronicity of infantile acne, which may require long-term treatment, as well as the possibility that acne may recur in severe form during puberty.1 The treatment strategy for infantile acne is similar to treatment of acne at any age, with topical agents including retinoids (eg, tretinoin, benzoyl peroxide) and topical antibacterials (eg, erythromycin). Twice-daily erythromycin 125 to 250 mg is the treatment of choice when oral antibiotics are indicated. Tetracyclines are contraindicated in treatment of neonatal and infantile acne. Intralesional injections with low-concentration triamcinolone acetonide, cryotherapy, or topical corticosteroids for a short period of time can be used to treat deep nodules and cysts.2 Acne that is refractory to treatment with oral antibiotics alone or combined with topical treatments poses a dilemma, given the potential cosmetic sequelae of scarring and quality-of-life concerns. Because reducing or eliminating dairy intake appears beneficial for adolescents with moderate to severe acne,27 this approach may represent a good option for infantile acne.
Conclusion
Neonatal and infantile acne vulgaris may be overlooked or misdiagnosed. It is important to consider and treat. Early childhood acne may represent a virilization syndrome.
- Jansen T, Burgdorf WH, Plewig G. Pathogenesis and treatment of acne in childhood. Pediatr Dermatol. 1997;14:17-21.
- Antoniou C, Dessinioti C, Stratigos AJ, et al. Clinical and therapeutic approach to childhood acne: an update. Pediatr Dermatol. 2009;26:373-380.
- Kuflik JH, Schwartz RA. Acneiform eruptions. Cutis. 2000;66:97-100.
- Barbareschi M, Benardon S, Guanziroli E, et al. Classification and grading. In: Schwartz RA, Micali G, eds. Acne. Gurgaon, India: Nature Publishing Group; 2013:67-75.
- Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol. 2002;3:389-400.
- O’Connor NR, McLaughlin MR, Ham P. Newborn skin: part I. common rashes. Am Fam Physician. 2008;77:47-52.
- Nanda S, Reddy BS, Ramji S, et al. Analytical study of pustular eruptions in neonates. Pediatr Dermatol. 2002;19:210-215.
- Yonkosky DM, Pochi PE. Acne vulgaris in childhood. pathogenesis and management. Dermatol Clin. 1986;4:127-136.
- Agache P, Blanc D, Barrand C, et al. Sebum levels during the first year of life. Br J Dermatol. 1980;103:643-649.
- Herane MI, Ando I. Acne in infancy and acne genetics. Dermatology. 2003;206:24-28.
- Lucky AW. A review of infantile and pediatric acne. Dermatology (Basel, Switzerland). 1998;103:643-649.
- Bernier V, Weill FX, Hirigoyen V, et al. Skin colonization by Malassezia species in neonates: a prospective study and relationship with neonatal cephalic pustulosis. Arch Dermatol. 2002;138:215-218.
- Lambert WC, Bagley MP, Khan Y, et al. Pustular acneiform secondary syphilis. Cutis. 1986;37:69-70.
- Antoniou C, Dessinioti C, Stratigos AJ, et al. Clinical and therapeutic approach to childhood acne: an update. Pediatr Dermatol. 2009;26:373-380.
- Borton LK, Schwartz RA. Pityrosporum folliculitis: a common acneiform condition of middle age. Ariz Med. 1981;38:598-601.
- Morgan AJ, Steen CJ, Schwartz RA, et al. Erythema toxicum neonatorum revisited. Cutis. 2009;83:13-16.
- Brodkin RH, Schwartz RA. Cutaneous signs of dioxin exposure. Am Fam Physician. 1984;30:189-194.
- Mancini AJ, Baldwin HE, Eichenfield LF, et al. Acne life cycle: the spectrum of pediatric disease. Semin Cutan Med Surg. 2011;30(suppl 3):S2-S5.
- Katsambas AD, Katoulis AC, Stavropoulos P. Acne neonatorum: a study of 22 cases. Int J Dermatol. 1999;38:128-130.
- Van Praag MC, Van Rooij RW, Folkers E, et al. Diagnosis and treatment of pustular disorders in the neonate. Pediatr Dermatol. 1997;14:131-143.
- Barnes CJ, Eichenfield LF, Lee J, et al. A practical approach for the use of oral isotretinoin for infantile acne. Pediatr Dermatol. 2005;22:166-169.
- Janniger CK. Neonatal and infantile acne vulgaris. Cutis. 1993;52:16.
- Chew EW, Bingham A, Burrows D. Incidence of acne vulgaris in patients with infantile acne. Clin Exp Dermatol. 1990;15:376-377.
- Duke EM. Infantile acne associated with transient increases in plasma concentrations of luteinising hormone, follicle-stimulating hormone, and testosterone. Br Med J (Clinical Res Ed). 1981;282:1275-1276.
- Mann MW, Ellis SS, Mallory SB. Infantile acne as the initial sign of an adrenocortical tumor [published online ahead of print September 14, 2006]. J Am Acad Dermatol. 2007;56(suppl 2):S15-S18.
- Kang SK, Jee MS, Choi JH, et al. A case of infantile acne due to Pityrosporum. Pediatr Dermatol. 2003;20:68-70.
- Di Landro A, Cazzaniga S, Parazzini F, et al. Family history, body mass index, selected dietary factors, menstrual history, and risk of moderate to severe acne in adolescents and young adults [published online ahead of print March 3, 2012]. J Am Acad Dermatol. 2012;67:1129-1135.
- Jansen T, Burgdorf WH, Plewig G. Pathogenesis and treatment of acne in childhood. Pediatr Dermatol. 1997;14:17-21.
- Antoniou C, Dessinioti C, Stratigos AJ, et al. Clinical and therapeutic approach to childhood acne: an update. Pediatr Dermatol. 2009;26:373-380.
- Kuflik JH, Schwartz RA. Acneiform eruptions. Cutis. 2000;66:97-100.
- Barbareschi M, Benardon S, Guanziroli E, et al. Classification and grading. In: Schwartz RA, Micali G, eds. Acne. Gurgaon, India: Nature Publishing Group; 2013:67-75.
- Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol. 2002;3:389-400.
- O’Connor NR, McLaughlin MR, Ham P. Newborn skin: part I. common rashes. Am Fam Physician. 2008;77:47-52.
- Nanda S, Reddy BS, Ramji S, et al. Analytical study of pustular eruptions in neonates. Pediatr Dermatol. 2002;19:210-215.
- Yonkosky DM, Pochi PE. Acne vulgaris in childhood. pathogenesis and management. Dermatol Clin. 1986;4:127-136.
- Agache P, Blanc D, Barrand C, et al. Sebum levels during the first year of life. Br J Dermatol. 1980;103:643-649.
- Herane MI, Ando I. Acne in infancy and acne genetics. Dermatology. 2003;206:24-28.
- Lucky AW. A review of infantile and pediatric acne. Dermatology (Basel, Switzerland). 1998;103:643-649.
- Bernier V, Weill FX, Hirigoyen V, et al. Skin colonization by Malassezia species in neonates: a prospective study and relationship with neonatal cephalic pustulosis. Arch Dermatol. 2002;138:215-218.
- Lambert WC, Bagley MP, Khan Y, et al. Pustular acneiform secondary syphilis. Cutis. 1986;37:69-70.
- Antoniou C, Dessinioti C, Stratigos AJ, et al. Clinical and therapeutic approach to childhood acne: an update. Pediatr Dermatol. 2009;26:373-380.
- Borton LK, Schwartz RA. Pityrosporum folliculitis: a common acneiform condition of middle age. Ariz Med. 1981;38:598-601.
- Morgan AJ, Steen CJ, Schwartz RA, et al. Erythema toxicum neonatorum revisited. Cutis. 2009;83:13-16.
- Brodkin RH, Schwartz RA. Cutaneous signs of dioxin exposure. Am Fam Physician. 1984;30:189-194.
- Mancini AJ, Baldwin HE, Eichenfield LF, et al. Acne life cycle: the spectrum of pediatric disease. Semin Cutan Med Surg. 2011;30(suppl 3):S2-S5.
- Katsambas AD, Katoulis AC, Stavropoulos P. Acne neonatorum: a study of 22 cases. Int J Dermatol. 1999;38:128-130.
- Van Praag MC, Van Rooij RW, Folkers E, et al. Diagnosis and treatment of pustular disorders in the neonate. Pediatr Dermatol. 1997;14:131-143.
- Barnes CJ, Eichenfield LF, Lee J, et al. A practical approach for the use of oral isotretinoin for infantile acne. Pediatr Dermatol. 2005;22:166-169.
- Janniger CK. Neonatal and infantile acne vulgaris. Cutis. 1993;52:16.
- Chew EW, Bingham A, Burrows D. Incidence of acne vulgaris in patients with infantile acne. Clin Exp Dermatol. 1990;15:376-377.
- Duke EM. Infantile acne associated with transient increases in plasma concentrations of luteinising hormone, follicle-stimulating hormone, and testosterone. Br Med J (Clinical Res Ed). 1981;282:1275-1276.
- Mann MW, Ellis SS, Mallory SB. Infantile acne as the initial sign of an adrenocortical tumor [published online ahead of print September 14, 2006]. J Am Acad Dermatol. 2007;56(suppl 2):S15-S18.
- Kang SK, Jee MS, Choi JH, et al. A case of infantile acne due to Pityrosporum. Pediatr Dermatol. 2003;20:68-70.
- Di Landro A, Cazzaniga S, Parazzini F, et al. Family history, body mass index, selected dietary factors, menstrual history, and risk of moderate to severe acne in adolescents and young adults [published online ahead of print March 3, 2012]. J Am Acad Dermatol. 2012;67:1129-1135.
Practice Points
- Infantile acne needs to be recognized and treated.
- Acne in early childhood may represent virilization.
Ulerythema Ophryogenes: Updates and Insights
Test your knowledge on ulerythema ophryogenes with MD-IQ: the medical intelligence quiz. Click here to answer 5 questions.