User login
A Clinical Review of Eslicarbazepine Acetate
This supplement reviews Eslicarbazepine Acetate and its effectiveness as a first-line or later adjunctive therapy in patients with partial-onset seizures.
This supplement reviews Eslicarbazepine Acetate and its effectiveness as a first-line or later adjunctive therapy in patients with partial-onset seizures.
This supplement reviews Eslicarbazepine Acetate and its effectiveness as a first-line or later adjunctive therapy in patients with partial-onset seizures.
Disparities in cardiovascular care: Past, present, and solutions
Cardiovascular disease became the leading cause of death in the United States in the early 20th century, and it accounts for nearly half of all deaths in industrialized nations.1 The mortality it inflicts was thought to be shared equally between both sexes and among all age groups and races.2 The cardiology community implemented innovative epidemiologic research, through which risk factors for cardiovascular disease were established.1 The development of coronary care units reduced in-hospital mortality from acute myocardial infarction from 30% to 15%.2–5 Further advances in pharmacology, revascularization, and imaging have aided in the detection and treatment of cardiovascular disease.6 Though cardiovascular disease remains the number-one cause of death worldwide, rates are on the decline.7
For several decades, health disparities have been recognized as a source of pathology in cardiovascular medicine, resulting in inequity of care administration among select populations. In this review, we examine whether the same forward thinking that has resulted in a decline in cardiovascular disease has had an impact on the pervasive disparities in cardiovascular medicine.
DISPARITIES DEFINED
Compared with whites, members of minority groups have a higher burden of chronic diseases, receive lower quality care, and have less access to medical care. Recognizing the potential public health ramifications, in 1999 the US Congress tasked the Institute of Medicine to study and assess the extent of healthcare disparities. This led to the landmark publication, Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care.8
The Institute of Medicine defines disparities in healthcare as racial or ethnic differences in the quality of healthcare that are not due to access-related factors, clinical needs, preferences, and appropriateness of intervention.8 Disparities can also exist according to socioeconomic status and sex.9
In an early study documenting the concept of disparities in cardiovascular disease, Stone and Vanzant10 concluded that heart disease was more common in African Americans than in whites, and that hypertension was the principal cause of cardiovascular disease mortality in African Americans.
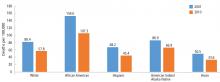
Although avoidable deaths from heart disease, stroke, and hypertensive disease declined between 2001 and 2010, African Americans still have a higher mortality rate than other racial and ethnic groups (Figure 1).11
DISPARITIES AND CARDIOVASCULAR HEALTH
The concept of cardiovascular health was established by the American Heart Association (AHA) in efforts to achieve an additional 20% reduction in cardiovascular disease-related mortality by 2020.7 Cardiovascular health is defined as the absence of clinically manifest cardiovascular disease and is measured by 7 components:
- Not smoking or abstaining from smoking for at least 1 year
- A normal body weight, defined as a body mass index less than 25 kg/m2
- Optimal physical activity, defined as 75 minutes of vigorous physical activity or 150 minutes of moderate-intensity physical activity per week
- Regular consumption of a healthy diet
- Total cholesterol below 200 mg/dL
- Blood pressure less than 120/80 mm Hg
- Fasting blood sugar below 100 mg/dL.
Nearly 70% of the US population can claim 2, 3, or 4 of these components, but differences exist according to race,12 and 60% of adult white Americans are limited to achieving no more than 3 of these healthy metrics, compared with 70% of adult African Americans and Hispanic Americans.
Smoking
Smoking is a major risk factor for cardiovascular disease.12–14
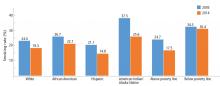
During adolescence, white males are more likely to smoke than African American and Hispanic males,12 but this trend reverses in adulthood, when African American men have a higher prevalence of smoking than white men (21.4% vs 19%).7 Rates of lifetime use are highest among American Indian or Alaskan natives and whites (75.9%), followed by African Americans (58.4%), native Hawaiians (56.8%), and Hispanics (56.7%).15 Trends for current smoking are similar (Figure 2).16 Moreover, households with lower socioeconomic status have a higher prevalence of smoking.7
Physical activity
People with a sedentary lifestyle are more likely to die of cardiovascular disease. As many as 250,000 deaths annually in the United States are attributed to lack of regular physical activity.17
Recognizing the potential public health ramifications, the AHA and the 2018 Federal Guidelines on Physical Activity recommend that children engage in 60 minutes of daily physical activity and that adults participate in 150 minutes of moderate-intensity or 75 minutes of vigorous physical activity weekly.18,19
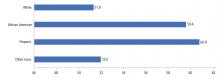
In the United States, 15.2% of adolescents reported being physically inactive, according to data published in 2016.7 Similar to most cardiovascular risk factors, minority populations and those of lower socioeconomic status had the worst profiles. The prevalence of physical inactivity was highest in African Americans and Hispanics (Figure 3).20
Studies have shown an association between screen-based sedentary behavior (computers, television, and video games) and cardiovascular disease.21–23 In the United States, 41% of adolescents used computers for activities other than homework for more than 3 hours per day on a school day.7 The pattern of use was highest in African American boys and African American girls, followed by Hispanic girls and Hispanic boys.18 Trends were similar with regard to watching television for more than 3 hours per day.
Sedentary behavior persists into adulthood, with rates of inactivity of 38.3% in African Americans, 40.1% in Hispanics, and 26.3% in white adults.7
Nutrition and obesity
Nutrition plays a major role in cardiovascular disease, specifically in the pathogenesis of atherosclerotic disease and hypertension.24 Most Americans do not meet dietary recommendations, with minority communities performing worse in specific metrics.7
Dietary patterns are reflected in the rate of obesity in this nation. Studies have shown a direct correlation between obesity and cardiovascular disease such as coronary artery disease, heart failure, and atrial fibrillation.25–28 According to data from the National Health and Nutrition Examination Survey (NHANES), 31% of children between the ages of 2 and 19 years are classified as obese or overweight. The highest rates of obesity are seen in Hispanic and African American boys and girls. The obesity epidemic is disproportionately rampant in children living in households with low income, low education, and high unemployment rates.7,29–31
Despite the risks associated with obesity, only 64.8% of obese adults report being informed by a doctor or health professional that they were overweight. The proportion of obese adults informed that they were overweight was significantly lower for African Americans and Hispanics compared with whites. Similar differences are seen based on socioeconomic status, as middle-income patients were less likely to be informed than those in the higher income strata (62.4% vs 70.6%).7,31
Blood pressure
Hypertension is a well-established risk factor for cardiovascular disease and stroke, and a blood pressure of 120/80 mm Hg or lower is identified as a component of ideal cardiovascular health.
In the United States the prevalence of hypertension in adults older than 20 is 32%.7 The prevalence of hypertension in African Americans is among the highest in the world.32,33 African Americans develop high blood pressure at earlier ages, and their average resting blood pressures are higher than in whites.34,35 For a 45-year-old without hypertension, the 40-year risk of developing hypertension is 92.7% for African Americans and 86% for whites.35 Hypertension is a major risk factor for stroke, and African Americans have a 1.8 times greater rate of fatal stroke than whites.7
In 2013 there were 71,942 deaths attributable to high blood pressure, and the 2011 death rate associated with hypertension was 18.9 per 100,000. By race, the death rate was 17.6 per 100,000 for white males and an alarming 47.1 per 100,000 for African American males; rates were 15.2 per 100,000 for white females and 35.1 per 100,000 for African American females.7
It is unclear what accounts for the racial difference in prevalence in hypertension. Studies have shown that African Americans are more likely than whites to have been told on more than 2 occasions that they have hypertension. And 85.7% of African Americans are aware that they have high blood pressure, compared with 82.7% of whites.14
African Americans and Hispanics have poorer hypertension control compared with whites.36,37 These observed differences cannot be attributed to access alone, as African Americans were more likely to be on higher-intensity blood pressure therapy, whereas Hispanics were more likely to be undertreated.36,38 In a meta-analysis of 13 trials, Peck et al39 showed that African Americans showed a lesser reduction in systolic and diastolic blood pressure when treated with angiotensin-converting enzyme (ACE) inhibitors.
The 2017 American College of Cardiology (ACC) and AHA guidelines for the prevention, detection, evaluation, and management of high blood pressure in adults40 identifies 4 drug classes as reducing cardiovascular disease morbidity and mortality: thiazide diuretics, ACE inhibitors, angiotensin II receptor blockers (ARBs), and calcium channel blockers. Of these 4 classes, thiazide diuretics and calcium channel blockers have been shown to lower blood pressure more effectively in African Americans than renin-angiotensin-aldosterone inhibition with ACE inhibitors or ARBs.
Glycemic control
Type 2 diabetes mellitus secondary to insulin resistance disproportionately affects minority groups, as the prevalence of diabetes mellitus in African Americans is almost twice as high as that in whites, and 35% higher in Hispanics compared with whites.7,41 Based on NHANES data between 1984 and 2004, the prevalence of diabetes mellitus is expected to increase by 99% in whites, 107% in African Americans, and 127% in Hispanics by 2050. Alarmingly, African Americans over age 75 are expected to experience a 606% increase by 2050.42
With regard to mortality, 21.7 deaths per 100,000 population were attributable to diabetes mellitus according to reports by the AHA in 2016. The death rate in white males was 24.3 per 100,000 compared with 44.9 per 100,000 for African Americans males. The associated mortality rate for white women was 16.2 per 100,000, and 35.8 per 100,000 for African American females.7
DISPARITIES AND CORONARY ARTERY DISEASE CARE
The management of coronary artery disease has evolved from prolonged bed rest to surgical, pharmacologic, and percutaneous revascularization.2,5 Coronary revascularization procedures are now relatively common: 950,000 percutaneous coronary interventions and 397,000 coronary artery bypass procedures were performed in 2010.7
Nevertheless, despite similar clinical presentations, African Americans with acute myocardial infarction were less likely to be referred for coronary artery bypass grafting than whites.43–46 They were also less likely to be given thrombolytics47 and less likely to undergo coronary angiography with percutaneous coronary intervention.48 Similar differences have been reported when comparing Hispanics with whites.49
Some suggest that healthcare access is a key mediator of health disparities.50 In 2009, Hispanics and African Americans accounted for more than 50% of those without health insurance.51 Improved access to healthcare might mitigate the disparity in revascularizations.
Massachusetts was one of the first states to mandate that all residents obtain health insurance. As a result, the uninsured rates declined in African Americans and Hispanics in Massachusetts, but a disparity in revascularization persisted. African Americans and Hispanics were 27% and 16% less likely to undergo revascularization procedures (coronary artery bypass grafting or percutaneous coronary intervention) than whites,51 suggesting that disparities in revascularization are not solely secondary to healthcare access.
These findings are consistent with a 2004 Veterans Administration study,52 in which healthcare access was equal among races. The study showed that African Americans received fewer cardiac procedures after an acute myocardial infarction compared with whites.
Have we made progress? The largest disparity between African Americans and whites in coronary artery disease mortality existed in 1990. The disparity persisted to 2012, and although decreased, it is projected to persist to 2030.53
DISPARITIES IN HEART FAILURE
An estimated 5.7 million Americans have heart failure, and 915,000 new cases are diagnosed annually.7 Unlike coronary artery disease, heart failure is expected to increase in prevalence by 46%, to 8 million Americans with heart failure by 2030.7,54
Our knowledge of disparities in the area of heart failure is derived primarily from epidemiologic studies. The Multi-Ethnic Study of Atherosclerosis55 showed that African Americans (4.6 per 1,000), followed by Hispanics (3.5 per 1,000) had a higher risk of developing heart failure compared with whites (2.4 per 1,000).The higher risk is in part due to disparities in socioeconomic status and prevalence of hypertension, as African Americans accounted for 75% of cases of nonischemic-related heart failure.55 African Americans also have a higher 5-year mortality rate than whites.55
Even though the 5-year mortality rate in heart failure is still 50%, the past 30 years have seen innovations in pharmacologic and device therapy and thus improved outcomes in heart failure patients. Still, significant gaps in the use of guideline-recommended therapies, quality of care, and clinical outcomes persist in contemporary practice for racial minorities with heart failure.
Disparities in inpatient care for heart failure
Patients admitted for heart failure and cared for by a cardiologist are more likely to be discharged on guideline-directed medical therapy, have fewer heart failure readmissions, and lower mortality.56,57 Breathett et al,58 in a study of 104,835 patients hospitalized in an intensive care unit for heart failure, found that primary intensive care by a cardiologist was associated with higher survival in both races. However, in the same study, white patients had a higher odds of receiving care from a cardiologist than African American patients.
Disparities and cardiac resynchronization therapy devices
In one-third of patients with heart failure, conduction delays result in dyssynchronous left ventricular contraction.59 Dyssynchrony leads to reduced cardiac performance, left ventricular remodeling, and increased mortality.56
Cardiac resynchronization therapy (CRT) was approved for clinical use in 2001, and studies have shown that it improves quality of life, exercise tolerance, cardiac performance, and morbidity and mortality rates.59–66 The 2013 ACC/AHA guidelines for the management of heart failure give a class IA recommendation (the highest) for its use in patients with a left ventricular ejection fraction of 35% or less, sinus rhythm, left bundle branch block and a QRS duration of 150 ms or greater, and New York Heart Association class II, III, or ambulatory IV symptoms while on guideline-directed medical therapy.67
Despite these recommendations, racial differences are observed. A study using the Nationwide Inpatient Sample database59 showed that between 2002 and 2010, a total of 374,202 CRT devices were implanted, averaging 41,578 annually. After adjusting for heart failure admissions, the study showed that CRT use was favored in men and in whites.
Another study, using the National Cardiovascular Data Registry,68 looked at patients who received implantable cardiac defibrillators (ICDs) and were eligible to receive CRT. It found that African Americans and Hispanics were less likely than whites to receive CRT, even though they were more likely to meet established criteria.
Disparities and left ventricular assist devices
The Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart failure (REMATCH) trial and Heart Mate II trial demonstrated that left ventricular assist devices (LVADs) were durable options for long-term support for patients with end-stage heart failure.69,70 Studies that examined the role of race and clinical outcomes after LVAD implantation have reported mixed findings.71,72 Few studies have looked at the role racial differences play in accessing LVAD therapy.
Joyce et al73 reviewed data from the Nationwide Inpatient Sample from 2002 to 2003 on patients admitted to the hospital with a primary diagnosis of heart failure or cardiogenic shock. A total of 297,866 patients were included in the study, of whom only 291 underwent LVAD implantation. A multivariate analysis found that factors such as age over 65, female sex, admission to a nonacademic center, geographic region, and African American race adversely influenced access to LVAD therapy.
Breathett et al74 evaluated racial differences in LVAD implantations from 2012 to 2015, a period that corresponds to increased health insurance expansion, and found LVAD implantations increased among African American patients with advanced heart failure, but no other racial or ethnic group.
Disparities and heart transplant
For patients with end-stage heart failure, orthotopic heart transplant is the most definitive and durable option for long-term survival. According to data from the United Network for Organ Sharing, 62,508 heart transplants were performed from January 1, 1988 to December 31, 2015. Compared with transplants of other solid organs, heart transplant occurs in significantly infrequent rates.
Barriers to transplant include lack of health insurance, considered a surrogate for low socioeconomic status. Hispanics and African Americans are less likely to have private health insurance than non-Hispanic whites, and this difference is magnified among the working poor.
Despite these perceived barriers, Kilic et al75 found that African Americans comprised 16.4% of heart transplant recipients, although they make up only approximately 13% of the US population. They also had significantly shorter wait-list times than whites. On the negative side, African Americans had a higher unadjusted mortality rate than whites (15% vs 12% P = .002). African Americans also tended to receive their transplants at centers with lower transplant volumes and higher transplant mortality rates.
Several other studies also showed that African Americans compared to whites have significantly worse outcomes after transplant.76–79 What accounts for this difference? Kilic et al75 showed that African Americans had the lowest proportion of blood type matching and lowest human leukocyte antigen matching, were younger (because African Americans develop more advanced heart failure at younger ages), had higher serum creatinine levels, and were more often bridged to transplant with an LVAD.
DISPARITIES IN CARDIOVASCULAR RESEARCH
Although the United States has the most sophisticated and robust medical system in the world, select groups have significant differences in delivery and healthcare outcomes. There are many explanations for these differences, but a contributing factor may be the paucity of research dedicated to understand racial and ethnic differences.80
Differences observed in epidemiologic studies may be secondary to pathophysiology, genetic differences, environment, and lifestyle choices. Historically, clinical trials were conducted in homogeneous populations with respect to age (middle-aged), sex (male), and race (white), and the results were generalized to heterogeneous populations.80
Disparities in research have implications in clinical practice. Overall, the primary cause of heart failure is ischemia; however, in African Americans, the primary cause is hypertensive heart disease.81 Studies in hypertension have shown that African Americans have less of a response to neurohormonal blockade with ACE inhibitors and beta-blockers than non-African Americans.82 Nevertheless, neurohormonal blockade has become the cornerstone of heart failure treatment.
Retrospective analysis of the Vasodilator-Heart Failure trials83 showed that treatment with isosorbide dinitrate plus hydralazine, compared with placebo, conferred a survival benefit for African Americans but not whites.80 No survival advantage was noted when isosorbide dinitrate/hydralazine was compared to enalapril in African Americans, although enalapril was superior to isosorbide dinitrate in whites.45 These observations were recognized 10 to 15 years after trial completion, and were only possible because the trials included sufficient numbers of African American patients to complete analysis.
In 1993, the US Congress passed the National Institutes of Health (NIH) Revitalization Act, which established guidelines requiring NIH grant applicants to include minorities in human subject research, as they were historically underrepresented in clinical research trials.84,85
In 2001, the Beta-Blocker Evaluation of Survival Trial86 reported its results investigating whether bucindolol, a nonselective beta-blocker, would reduce mortality in patients with advanced heart failure (New York Heart Association class III or IV). This was one of the first trials to prospectively investigate racial and ethnic differences in response to treatment. Though it showed no overall benefit in the use of bucindolol in the treatment of advanced heart failure, subgroup analysis showed that whites did enjoy a benefit in terms of lower mortality, whereas African Americans did not.
Results of the Vasodilator-Heart Failure trials led to further population-directed research, most notably the African American Heart Failure Trial,87 a double-blind, placebo-controlled, randomized trial in patients who identified as African American. Patients who were randomized to receive a fixed dose of hydralazine and isosorbide dinitrate had a 43% lower mortality rate, a 33% lower hospitalization rate for heart failure, and better quality of life than patients in the placebo group, leading to early termination of the trial. The outcomes suggested that the combination of isosorbide dinitrate and hydralazine treats heart failure in a manner independent of pure neurohormonal blockade.
CHALLENGES IN STUDY PARTICIPATION
Recruitment of minority participants in biomedical research is a challenging task for clinical investigators.88,89 Some of the factors thought to pose potential barriers for racial and ethnic minority participation in health research include poor access to primary medical care, failure of researchers to recruit minority populations actively, and language and cultural barriers.90
Further, it is widely claimed that African Americans are less willing than nonminority individuals to participate in clinical research trials due to general distrust of the medical community as a result of the Tuskegee Syphilis Experiment.91 That infamous study, conducted by the US Public Health Service between 1932 and 1972, sought to record the natural progression of untreated syphilis in poor African American men in Alabama. The participants were not informed of the true purpose of the study, and they were under the impression that they were simply receiving free healthcare from the US government. Further, they were denied appropriate treatment even after it became readily available, in order for researchers to observe the progression of the disease.
While the 1993 mandate did in fact increase pressure on researchers to develop strategies to overcome participation barriers, the issue of underrepresentation of racial minorities in clinical research, including cardiovascular research, has not been resolved and continues to be a problem today.
The overall goal of clinical research is to determine the best strategies to prevent and treat disease. But if the study population is not representative of the affected population at large, the results cannot be generalized to underrepresented subgroups. The implications of underrepresentation in research are far-reaching, and can further contribute to disparate care of minority patients such as African Americans, who have a higher prevalence of cardiovascular risk factors and greater burden of heart failure.
PROPOSING SOLUTIONS
Between 1986 and 2018, according to a PUBMED search, 10,462 articles highlighted the presence of a health-related disparity. Solutions to address and ultimately eradicate disparities will need to eliminate healthcare bias, increase patient access, and increase diversity and inclusion in the physician work force.
Eliminating bias
Implicit bias refers to attitudes, thoughts, and feelings that exist outside of the conscious awareness.92 These biases can be triggered by race, gender, or socioeconomic status. They have manifested in society as stereotypes that men are more competent than women, women are more verbal than men, and African Americans are more athletic than whites.93
The concept of implicit bias is important, in that the populations that experience the greatest health disparities also suffer from negative cultural stereotypes.94 Healthcare professionals are not inoculated against implicit bias.95 Studies have shown that most healthcare providers have implicit biases that reflect positive attitudes toward whites and negative attitudes toward people of color.92,94,96–98
The Implicit Association Test, introduced in 1998, is widely used to measure implicit bias. It measures response time of subjects to match particular social groups to particular attributes.99 Green et al,99 using this test, showed that although physicians reported no explicit preference for white vs African American patients or differences in perceived cooperativeness, the test revealed implicit preference favoring white Americans and implicit stereotypes of African Americans as less cooperative for medical procedures and in general. This also manifested in clinical decision-making, as white Americans were more likely, and African Americans less likely, to be treated with thrombolysis.99
Sabin et al100 showed that implicit bias was present among pediatricians, although less than in society as a whole and in other healthcare professionals.
But how does one change feelings that exist outside of the conscious awareness? Green et al99 showed that making physicians aware of their susceptibility to bias changed their behavior. A subset of physicians who were made aware that bias was a focus of the study were more likely to refer African Americans for thrombolysis even if they had a high degree of implicit pro-white bias.94,100 Perhaps mandating that all healthcare providers take a self-administered and confidentially reported Implicit Association Test will lead to awareness of implicit bias and minimize healthcare behaviors that contribute to the current state of disparities.
Improving access
Common indicators of access to healthcare include health insurance status, having a usual source of healthcare, and having a regular physician.101 Health insurance does offer protection from the costs associated with illness and health maintenance.101 It is also a major contributing factor in racial and ethnic disparities.
Chen et al102 examined the effects of the Affordable Care Act and found that it was associated with reduction in the probability of being uninsured, delaying necessary care, and forgoing necessary care, and increased probability of having a physician. However, earlier studies showed that access to health insurance by itself does not equate to equitable care.103,104
Diversifying the work force
African Americans comprise 4% of physicians and Hispanic Americans 5%, despite accounting for 13% and 16% of the US population.105 This underrepresentation has led to African American and Hispanic American patients being more likely than white patients to be treated by a physician from a dissimilar racial or ethnic background.106 Studies have shown that minority patients in a race- or ethnic-concordant relationship are more likely to use needed health services, less likely to postpone seeking care, and report greater satisfaction.106,107 Minority physicians often locate and practice in neighborhoods with high minority populations, and they disproportionately care for disadvantaged patients of lower socioeconomic status and poorer health.106,108
WE ARE STILL IN THE TUNNEL, BUT THERE IS LIGHT AT THE END
The cardiovascular community has faced tremendous challenges in the past and responded with innovative research that has led to imaging that aids in the diagnosis of subclinical cardiovascular disease and invasive and pharmacologic strategies that have improved cardiovascular outcomes. One may say that there is light at the end of the tunnel; however, the existence of disparate care reminds us that we are still in the tunnel.
Disparities in cardiovascular disease management present a unique challenge for the community. There is no drug, device, or invasive procedure to eliminate this pathology. However, by acknowledging the problem and implementing changes at the system, provider, and patient level, the cardiovascular community can achieve yet another momentous achievement: the end of cardiovascular health disparities. Cardiovascular disease makes no distinction in race, sex, age, or socioeconomic status, and neither should the medical community.
- Nabel EG, Braunwald E. A tale of coronary artery disease and myocardial infarction. N Engl J Med 2012; 366(1):54–63. doi:10.1056/NEJMra1112570
- Braunwald E. Evolution of the management of acute myocardial infarction: a 20th century saga. Lancet 1998; 352(9142):1771–1774. doi:10.1016/S0140-6736(98)03212-7
- Julian DG. The history of coronary care units. Br Heart J 1987; 57(6):497–502. doi:10.1136/hrt.57.6.497
- Caswell JE. A brief history of coronary care units. Public Health Rep 1967; 82(12):1105–1111. pmid:19316519
- Day HW. History of coronary care units. Am J Cardiol 1972; 30(4):405–407. pmid:4560377
- Braunwald E. Shattuck lecture—cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med 1997; 337(19):1360–1369. doi:10.1056/NEJM199711063371906
- Mozaffarian D, Benjamin EJ, Go AS, et al; American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation 2016; 133(4):e38–e360. doi:10.1161/CIR.0000000000000350
- Nelson A. Unequal treatment: confronting racial and ethnic disparities in health care. J Natl Med Assoc 2002; 94(8):666–668. pmid:12152921
- McGuire TG, Alegria M, Cook BL, Wells KB, Zaslavsky AM. Implementing the Institute of Medicine definition of disparities: an application to mental health care. Health Serv Res 2006; 41(5):1979–2005. doi:10.1111/j.1475-6773.2006.00583.x
- Stone CT, Vanzant FR. Heart disease as seen in a southern clinic: a clinical and pathologic survey. JAMA 1927; 89(18):1473–1480. doi:10.1001/jama.1927.02690180005002
- Centers For Disease Control And Prevention (CDC). Vital signs: avoidable deaths from heart disease, stroke, and hypertensive disease—United States, 2001–2010. MMWR Morb Mortal Wkly Rep 2013; 62(35):727–727. pmid:PMC4585625
- Mozaffarian D, Benjamin EJ, Go AS, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 2015; 131(4):e29–e322. doi:10.1161/CIR.0000000000000152
- Ockene IS, Miller NH. Cigarette smoking, cardiovascular disease, and stroke: a statement for healthcare professionals from the American Heart Association. American Heart Association Task Force on Risk Reduction. Circulation 1997; 96(9):3243–3247. pmid:9386200
- Messner B, Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol 2014; 34(3):509–515. doi:10.1161/ATVBAHA.113.300156
- Benjamin EJ, Virani SS, Callaway CW, et al; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation 2018; 137(12):e67–e492. doi:10.1161/CIR.0000000000000558
- Jamal A, Homa DM, O’Conner E, et al. Current cigarette smoking among adults—United States, 2005–2014. MMWR 2015; 64(44):1233–1240. doi:10.15585/mmwr.mm6444a2
- Myers J. Cardiology patient pages. Exercise and cardiovascular health. Circulation 2003; 107(1):e2–e5. pmid:12515760
- Shiroma EJ, Lee IM. Physical activity and cardiovascular health: lessons learned from epidemiological studies across age, gender, and race/ethnicity. Circulation 2010; 122(7):743–752. doi:10.1161/CIRCULATIONAHA.109.914721
- Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA 2018; 320(19):2020–2028. doi:10.1001/jama.2018.14854
- Omura JD, Carlson SA, Paul P, et al. Adults eligible for cardiovascular disease prevention counseling and participation in aerobic physical activity—United States, 2013. MMWR 2015; 64(37):1047–1051. doi:10.15585/mmwr.mm6437a4
- Dunstan DW, Barr EL, Healy GN, et al. Television viewing time and mortality: the Australian diabetes, obesity and lifestyle study (AusDiab). Circulation 2010; 121(3):384–391. doi:10.1161/CIRCULATIONAHA.109.894824
- Warren TY, Barry V, Hooker SP, Sui X, Church TS, Blair SN. Sedentary behaviors increase risk of cardiovascular disease mortality in men. Med Sci Sports Exerc 2010; 42(5):879–885. doi:10.1249/MSS.0b013e3181c3aa7e
- Byun W, Dowda M, Pate RR. Associations between screen-based sedentary behavior and cardiovascular disease risk factors in Korean youth. J Korean Med Sci 2012; 27(4):388–394. doi:10.3346/jkms.2012.27.4.388
- Getz GS, Reardon CA. Nutrition and cardiovascular disease. Arterioscler Thromb Vasc Biol 2007; 27(12):2499–2506. doi:10.1161/ATVBAHA.107.155853
- Eckel RH. Obesity and heart disease: a statement for healthcare professionals from the Nutrition Committee, American Heart Association. Circulation 1997; 96(9):3248–3250. pmid:9386201
- Rimm EB, Stampfer MJ, Giovannucci E, et al. Body size and fat distribution as predictors of coronary heart disease among middle-aged and older US men. Am J Epidemiol 1995; 141(12):1117–1127. pmid:7771450
- Duflou J, Virmani R, Rabin I, Burke A, Farb A, Smialek J. Sudden death as a result of heart disease in morbid obesity. Am Heart J 1995; 130(2):306–313. pmid:7631612
- Poirier P, Giles TD, Bray GA, et al; American Heart Association; Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006; 113(6):898–918. doi:10.1161/CIRCULATIONAHA.106.171016
- Wang Y. Disparities in pediatric obesity in the United States. Adv Nutr 2011; 2(1):23–31. doi:10.3945/an.110.000083
- Taveras EM, Gillman MW, Kleinman KP, Rich-Edwards JW, Rifas-Shiman SL. Reducing racial/ethnic disparities in childhood obesity: the role of early life risk factors. JAMA Pediatr 2013; 167(8):731–738. doi:10.1001/jamapediatrics.2013.85
- Powell-Wiley TM, Ayers CR, Banks-Richard K, et al. Disparities in counseling for lifestyle modification among obese adults: insights from the Dallas heart study. Obesity (Silver Spring) 2012; 20(4):849–855. doi:10.1038/oby.2011.242
- Fuchs FD. Why do black Americans have higher prevalence of hypertension?: an enigma still unsolved. Hypertension 2011; 57(3):379–380. doi:10.1161/HYPERTENSIONAHA.110.163196
- Ferdinand KC, Armani AM. The management of hypertension in African Americans. Crit Pathw Cardiol 2007; 6(2):67–71. doi:10.1097/HPC.0b013e318053da59
- Voors AW, Webber LS, Berenson GS. Time course study of blood pressure in children over a three-year period. Bogalusa Heart Study. Hypertension 1980; 2(4 Pt 2):102–108. pmid:7399641
- Carson AP, Howard G, Burke GL, Shea S, Levitan EB, Muntner P. Ethnic differences in hypertension incidence among middle-aged and older adults: the multi-ethnic study of atherosclerosis. Hypertension 2011; 57(6):1101–1107. doi:10.1161/HYPERTENSIONAHA.110.168005
- Gu A, Yue Y, Desai RP, Argulian E. Racial and ethnic differences in antihypertensive medication use and blood pressure control among US adults with hypertension: the National Health and Nutrition Examination Survey, 2003 to 2012. Circ Cardiovasc Qual Outcomes 2017; 10(1). pii:e003166. doi:10.1161/CIRCOUTCOMES.116.003166
- Lackland DT. Racial differences in hypertension: implications for high blood pressure management. Am J Med Sci 2014; 348(2):135–138. doi:10.1097/MAJ.0000000000000308
- Bosworth HB, Dudley T, Olsen MK, et al. Racial differences in blood pressure control: potential explanatory factors. Am J Med 2006; 119(1):70.e9–e15. doi:10.1016/j.amjmed.2005.08.019
- Peck RN, Smart LR, Beier R, et al. Difference in blood pressure response to ACE-Inhibitor monotherapy between black and white adults with arterial hypertension: a meta-analysis of 13 clinical trials. BMC Nephrol 2013; 14:201. doi:10.1186/1471-2369-14-201
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2018; 138(17):e484–e594. doi:10.1161/CIR.0000000000000596
- Peek ME, Cargill A, Huang ES. Diabetes health disparities: a systematic review of health care interventions. Med Care Res Rev 2007; 64(5 suppl):101S–156S. doi:10.1177/1077558707305409
- Narayan KM, Boyle JP, Geiss LS, Saaddine JB, Thompson TJ. Impact of recent increase in incidence on future diabetes burden: US, 2005–2050. Diabetes Care 2006; 29(9):2114–2116. doi:10.2337/dc06-1136
- Johnson PA, Lee TH, Cook EF, Rouan GW, Goldman L. Effect of race on the presentation and management of patients with acute chest pain. Ann Intern Med 1993; 118(8):593–601. pmid:8452325
- Hannan EL, van Ryn M, Burke J, et al. Access to coronary artery bypass surgery by race/ethnicity and gender among patients who are appropriate for surgery. Med Care 1999; 37(1):68–77. pmid:10413394
- Peterson ED, Shaw LK, DeLong ER, Pryor DB, Califf RM, Mark DB. Racial variation in the use of coronary-revascularization procedures. Are the differences real? Do they matter? N Engl J Med 1997; 336(7):480–486. doi:10.1056/NEJM199702133360706
- Wenneker MB, Epstein AM. Racial inequalities in the use of procedures for patients with ischemic heart disease in Massachusetts. JAMA 1989; 261(2):253–257. pmid:2521191
- Allison JJ, Kiefe CI, Centor RM, Box JB, Farmer RM. Racial differences in the medical treatment of elderly Medicare patients with acute myocardial infarction. J Gen Intern Med 1996; 11(12):736–743. pmid:9016420
- Mickelson JK, Blum CM, Geraci JM. Acute myocardial infarction: clinical characteristics, management and outcome in a metropolitan Veterans Affairs Medical Center teaching hospital. J Am Coll Cardiol 1997; 29(5):915–925. pmid:9120176
- Yarzebski J, Bujor CF, Lessard D, Gore JM, Goldberg RJ. Recent and temporal trends (1975 to 1999) in the treatment, hospital, and long-term outcomes of Hispanic and non-Hispanic white patients hospitalized with acute myocardial infarction: a population-based perspective. Am Heart J 2004; 147(4):690–697. doi:10.1016/j.ahj.2003.10.023
- Riley WJ. Health disparities: gaps in access, quality and affordability of medical care. Trans Am Clin Climatol Assoc 2012; 123:167–172. pmid:23303983
- Albert MA, Ayanian JZ, Silbaugh TS, et al. Early results of Massachusetts healthcare reform on racial, ethnic, and socioeconomic disparities in cardiovascular care. Circulation 2014; 129(24):2528–2538. doi:10.1161/CIRCULATIONAHA.113.005231
- Peterson ED, Wright SM, Daley J, Thibault GE. Racial variation in cardiac procedure use and survival following acute myocardial infarction in the Department of Veterans Affairs. JAMA 1994; 271(15):1175–1180. pmid:8151875
- Pearson-Stuttard J, Guzman-Castillo M, Penalvo JL, et al. Modeling future cardiovascular disease mortality in the United States: national trends and racial and ethnic disparities. Circulation 2016; 133(10):967–978. doi:10.1161/CIRCULATIONAHA.115.019904
- Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013; 6(3):606–619. doi:10.1161/HHF.0b013e318291329a
- Bahrami H, Kronmal R, Bluemke DA, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med 2008; 168(19):2138–2145. doi:10.1001/archinte.168.19.2138
- Parmar KR, Xiu PY, Chowdhury MR, Patel E, Cohen M. In-hospital treatment and outcomes of heart failure in specialist and non-specialist services: a retrospective cohort study in the elderly. Open Heart 2015; 2(1):e000095. doi:10.1136/openhrt-2014-000095
- Avaldi VM, Lenzi J, Urbinati S, et al. Effect of cardiologist care on 6-month outcomes in patients discharged with heart failure: results from an observational study based on administrative data. BMJ Open 2017; 7(11):e018243. doi:10.1136/bmjopen-2017-018243
- Breathett K, Liu WG, Allen LA, et al. African Americans are less likely to receive care by a cardiologist during an intensive care unit admission for heart failure. JACC Heart Fail 2018; 6(5):413–420. doi:10.1016/j.jchf.2018.02.015
- Sridhar AR, Yarlagadda V, Parasa S, et al. Cardiac resynchronization therapy: US trends and disparities in utilization and outcomes. Circ Arrhythm Electrophysiol 2016; 9(3):e003108. doi:10.1161/CIRCEP.115.003108
- Abraham WT, Fisher WG, Smith AL, et al; MIRACLE Study Group. Multicenter insync randomized clinical evaluation. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002; 346(24):1845–1853. doi:10.1056/NEJMoa013168
- Auricchio A, Stellbrink C, Sack S, et al; Pacing Therapies in Congestive Heart Failure (PATH-CHF) Study Group. Long-term clinical effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay. J Am Coll Cardiol 2002; 39(12):2026–2033. pmid:12084604
- Cazeau S, Leclercq C, Lavergne T, et al; Multisite Stimulation in Cardiomyopathies (MUSTIC) Study Investigators. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med 2001; 344(12):873–880. doi:10.1056/NEJM200103223441202
- Higgins SL, Hummel JD, Niazi IK, et al. Cardiac resynchronization therapy for the treatment of heart failure in patients with intraventricular conduction delay and malignant ventricular tachyarrhythmias. J Am Coll Cardiol 2003; 42(8):1454–1459. pmid:14563591
- Young JB, Abraham WT, Smith AL, et al; Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE ICD) Trial Investigators. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA 2003; 289(20):2685–2694. doi:10.1001/jama.289.20.2685
- Sutton MG, Plappert T, Hilpisch KE, Abraham WT, Hayes DL, Chinchoy E. Sustained reverse left ventricular structural remodeling with cardiac resynchronization at one year is a function of etiology: quantitative Doppler echocardiographic evidence from the Multicenter InSync Randomized Clinical Evaluation (MIRACLE). Circulation 2006; 113(2):266–272. doi:10.1161/CIRCULATIONAHA.104.520817
- Cleland JG, Daubert JC, Erdmann E, et al; Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005; 352(15):1539–1549. doi:10.1056/NEJMoa050496
- Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013; 128(16):e240–e327. doi:10.1161/CIR.0b013e31829e8776
- Farmer SA, Kirkpatrick JN, Heidenreich PA, Curtis JP, Wang Y, Groeneveld PW. Ethnic and racial disparities in cardiac resynchronization therapy. Heart Rhythm 2009; 6(3):325–331. doi:10.1016/j.hrthm.2008.12.018
- Rose EA, Gelijns AC, Moskowitz AJ, et al; Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001; 345(20):1435–1443. doi:10.1056/NEJMoa012175
- Slaughter MS, Rogers JG, Milano CA, et al; HeartMate II Investigators. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009; 361(23):2241–2251. doi:10.1056/NEJMoa0909938
- Tsiouris A, Brewer RJ, Borgi J, Nemeh H, Paone G, Morgan JA. Continuous-flow left ventricular assist device implantation as a bridge to transplantation or destination therapy: racial disparities in outcomes. J Heart Lung Transplant 2013; 2(3):299–304. doi:10.1016/j.healun.2012.11.017
- Stulak JM, Deo S, Cowger J, et al. Do racial and sex disparities exist in clinical characteristics and outcomes for patients undergoing left ventricular assist device implantation? J Heart Lung Transplant 2013; 32(45):S279–S280.
- Joyce DL, Conte JV, Russell SD, Joyce LD, Chang DC. Disparities in access to left ventricular assist device therapy. J Surg Res 2009; 152(1):111–117. doi:10.1016/j.jss.2008.02.065
- Breathett K, Allen LA, Helmkamp L, et al. Temporal trends in contemporary use of ventricular assist devices by race and ethnicity. Circ Heart Fail 2018; 11(8):e005008. doi:10.1161/CIRCHEARTFAILURE.118.005008
- Kilic A, Higgins RS, Whitson BA, Kilic A. Racial disparities in outcomes of adult heart transplantation. Circulation 2015; 131(10):882–889. doi:10.1161/CIRCULATIONAHA.114.011676
- Liu V, Bhattacharya J, Weill D, Hlatky MA. Persistent racial disparities in survival after heart transplantation. Circulation 2011; 123(15):1642–1649. doi:10.1161/CIRCULATIONAHA.110.976811
- Mahle WT, Kanter KR, Vincent RN. Disparities in outcome for black patients after pediatric heart transplantation. J Pediatr 2005; 147(6):739–743. doi:10.1016/j.jpeds.2005.07.018
- Park MH, Tolman DE, Kimball PM. The impact of race and HLA matching on long-term survival following cardiac transplantation. Transplant Proc 1997; 29(1–2):1460–1463. pmid:9123381
- Higgins RS, Fishman JA. Disparities in solid organ transplantation for ethnic minorities: facts and solutions. Am J Transplant 2006; 6(11):2556–2562. doi:10.1111/j.1600-6143.2006.01514.x
- Taylor AL, Wright JT Jr. Should ethnicity serve as the basis for clinical trial design? Importance of race/ethnicity in clinical trials: lessons from the African-American Heart Failure Trial (A-HeFT), the African-American Study of Kidney Disease and Hypertension (AASK), and the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Circulation 2005; 112(23):3654–3660. doi:10.1161/CIRCULATIONAHA.105.540443
- Yancy CW. Heart failure in African Americans: a cardiovascular engima. J Card Fail 2000; 6(3):183–186. pmid:10997742
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA 2003; 289(19):2560–2572. doi:10.1001/jama.289.19.2560
- Cohn JN, Archibald DG, Ziesche S, et al. Effect of vasodilator therapy on mortality in chronic congestive heart failure. Results of a Veterans Administration cooperative study. N Engl J Med 1986; 314(24):1547–1552. doi:10.1056/NEJM198606123142404
- Chen MS Jr, Lara PN, Dang JH, Paterniti DA, Kelly K. Twenty years post-NIH Revitalization Act: enhancing minority participation in clinical trials (EMPaCT): laying the groundwork for improving minority clinical trial accrual: renewing the case for enhancing minority participation in cancer clinical trials. Cancer 2014;120(suppl 7):1091–1096. doi:10.1002/cncr.28575
- Geller SE, Koch A, Pellettieri B, Carnes M. Inclusion, analysis, and reporting of sex and race/ethnicity in clinical trials: have we made progress? J Womens Health (Larchmt) 2011; 20(3):315–320. doi:10.1089/jwh.2010.2469
- Beta-Blocker Evaluation of Survival Trial Investigators; Eichhorn EJ, Domanski MJ, Krause-Steinrauf H, Bristow MR, Lavori PW. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med 2001; 344(22):1659–1667. doi:10.1056/NEJM200105313442202
- Taylor AL, Ziesche S, Yancy C, et al; African-American Heart Failure Trial Investigators. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med 2004; 351(20):2049–2057. doi:10.1056/NEJMoa042934
- Corbie-Smith G, Thomas SB, Williams MV, Moody-Ayers S. Attitudes and beliefs of African Americans toward participation in medical research. J Gen Intern Med 1999; 14(9):537–546. pmid:10491242
- Swanson GM, Ward AJ. Recruiting minorities into clinical trials: toward a participant-friendly system. J Natl Cancer Inst 1995; 87(23):1747–1759. doi:10.1093/jnci/87.23.1747
- Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care; Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: National Academies Press (US); 2003. https://www.ncbi.nlm.nih.gov/books/NBK220358/. Accessed May 13, 2019.
- Fisher JA, Kalbaugh CA. Challenging assumptions about minority participation in US clinical research. Am J Public Health 2011; 101(12):2217–2222. doi:10.2105/AJPH.2011.300279
- Hall WJ, Chapman MV, Lee KM, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. Am J Public Health 2015; 105(12):e60–e76. doi:10.2105/AJPH.2015.302903
- Biernat M, Manis M. Shifting standards and stereotype-based judgments. J Pers Soc Psychol 1994; 66(1):5–20. pmid:8126651
- Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med 2013; 28(11):1504–1510. doi:10.1007/s11606-013-2441-1
- FitzGerald C, Hurst S. Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics 2017; 18(1):19. doi:10.1186/s12910-017-0179-8
- van Ryn M, Burke J. The effect of patient race and socio-economic status on physicians’ perceptions of patients. Soc Sci Med 2000; 50(6):813–828. pmid:10695979
- Mayo RM, Sherrill WW, Sundareswaran P, Crew L. Attitudes and perceptions of Hispanic patients and health care providers in the treatment of Hispanic patients: a review of the literature. Hisp Health Care Int 2007; 5(2):64–72.
- Blair IV, Steiner JF, Havranek EP. Unconscious (implicit) bias and health disparities: where do we go from here? Perm J 2011; 15(2):71–78. pmid:21841929
- Green AR, Carney DR, Pallin DJ, et al. Implicit bias among physicians and its prediction of thrombolysis decisions for black and white patients. J Gen Intern Med 2007; 22(9):1231–1238. doi:10.1007/s11606-007-0258-5
- Sabin JA, Rivara FP, Greenwald AG. Physician implicit attitudes and stereotypes about race and quality of medical care. Med Care 2008; 46(7):678–685. doi:10.1097/MLR.0b013e3181653d58
- Smedley BD, Stith AY, Colburn L, et al; Institute of Medicine. The Right Thing to Do, The Smart Thing to Do: Enhancing Diversity in the Health Professions: Summary of the Symposium on Diversity in Health Professions in Honor of Herbert W. Nickens, MD. Washington, DC: National Academies Press; 2001. https://www.ncbi.nlm.nih.gov/books/NBK223633/. Accessed May 13, 2019.
- Chen J, Vargas-Bustamante A, Mortensen K, Ortega AN. Racial and ethnic disparities in health care access and utilization under the Affordable Care Act. Med Care 2016; 54(2):140–146. doi:10.1097/MLR.0000000000000467
- Saha S, Freeman M, Toure J, Tippens KM, Weeks C, Ibrahim S. Racial and ethnic disparities in the VA health care system: a systematic review. J Gen Intern Med 2008; 23(5):654–671. doi:10.1007/s11606-008-0521-4
- McCormick D, Sayah A, Lokko H, Woolhandler S, Nardin R. Access to care after Massachusetts’ health care reform: a safety net hospital patient survey. J Gen Intern Med 2012; 27(11):1548–1554. doi:10.1007/s11606-012-2173-7
- Burgos JL, Yee D, Csordas T, et al. Supporting the minority physician pipeline: providing global health experiences to undergraduate students in the United States-Mexico border region. Med Educ Online 2015; 20:27260. doi:10.3402/meo.v20.27260
- Traylor AH, Schmittdiel JA, Uratsu CS, Mangione CM, Subramanian U. The predictors of patient–physician race and ethnic concordance: a medical facility fixed-effects approach. Health Serv Res 2010; 45(3):792–805. doi:10.1111/j.1475-6773.2010.01086.x
- LaVeist TA, Nuru-Jeter A. Is doctor-patient race concordance associated with greater satisfaction with care? J Health Soc Behav 2002; 43(3):296–306. pmid:12467254
- Marrast LM, Zallman L, Woolhandler S, Bor DH, McCormick D. Minority physicians’ role in the care of underserved patients: diversifying the physician workforce may be key in addressing health disparities. JAMA Intern Med 2014; 174(2):289–291. doi:10.1001/jamainternmed.2013.12756
Cardiovascular disease became the leading cause of death in the United States in the early 20th century, and it accounts for nearly half of all deaths in industrialized nations.1 The mortality it inflicts was thought to be shared equally between both sexes and among all age groups and races.2 The cardiology community implemented innovative epidemiologic research, through which risk factors for cardiovascular disease were established.1 The development of coronary care units reduced in-hospital mortality from acute myocardial infarction from 30% to 15%.2–5 Further advances in pharmacology, revascularization, and imaging have aided in the detection and treatment of cardiovascular disease.6 Though cardiovascular disease remains the number-one cause of death worldwide, rates are on the decline.7
For several decades, health disparities have been recognized as a source of pathology in cardiovascular medicine, resulting in inequity of care administration among select populations. In this review, we examine whether the same forward thinking that has resulted in a decline in cardiovascular disease has had an impact on the pervasive disparities in cardiovascular medicine.
DISPARITIES DEFINED
Compared with whites, members of minority groups have a higher burden of chronic diseases, receive lower quality care, and have less access to medical care. Recognizing the potential public health ramifications, in 1999 the US Congress tasked the Institute of Medicine to study and assess the extent of healthcare disparities. This led to the landmark publication, Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care.8
The Institute of Medicine defines disparities in healthcare as racial or ethnic differences in the quality of healthcare that are not due to access-related factors, clinical needs, preferences, and appropriateness of intervention.8 Disparities can also exist according to socioeconomic status and sex.9
In an early study documenting the concept of disparities in cardiovascular disease, Stone and Vanzant10 concluded that heart disease was more common in African Americans than in whites, and that hypertension was the principal cause of cardiovascular disease mortality in African Americans.
Although avoidable deaths from heart disease, stroke, and hypertensive disease declined between 2001 and 2010, African Americans still have a higher mortality rate than other racial and ethnic groups (Figure 1).11
DISPARITIES AND CARDIOVASCULAR HEALTH
The concept of cardiovascular health was established by the American Heart Association (AHA) in efforts to achieve an additional 20% reduction in cardiovascular disease-related mortality by 2020.7 Cardiovascular health is defined as the absence of clinically manifest cardiovascular disease and is measured by 7 components:
- Not smoking or abstaining from smoking for at least 1 year
- A normal body weight, defined as a body mass index less than 25 kg/m2
- Optimal physical activity, defined as 75 minutes of vigorous physical activity or 150 minutes of moderate-intensity physical activity per week
- Regular consumption of a healthy diet
- Total cholesterol below 200 mg/dL
- Blood pressure less than 120/80 mm Hg
- Fasting blood sugar below 100 mg/dL.
Nearly 70% of the US population can claim 2, 3, or 4 of these components, but differences exist according to race,12 and 60% of adult white Americans are limited to achieving no more than 3 of these healthy metrics, compared with 70% of adult African Americans and Hispanic Americans.
Smoking
Smoking is a major risk factor for cardiovascular disease.12–14
During adolescence, white males are more likely to smoke than African American and Hispanic males,12 but this trend reverses in adulthood, when African American men have a higher prevalence of smoking than white men (21.4% vs 19%).7 Rates of lifetime use are highest among American Indian or Alaskan natives and whites (75.9%), followed by African Americans (58.4%), native Hawaiians (56.8%), and Hispanics (56.7%).15 Trends for current smoking are similar (Figure 2).16 Moreover, households with lower socioeconomic status have a higher prevalence of smoking.7
Physical activity
People with a sedentary lifestyle are more likely to die of cardiovascular disease. As many as 250,000 deaths annually in the United States are attributed to lack of regular physical activity.17
Recognizing the potential public health ramifications, the AHA and the 2018 Federal Guidelines on Physical Activity recommend that children engage in 60 minutes of daily physical activity and that adults participate in 150 minutes of moderate-intensity or 75 minutes of vigorous physical activity weekly.18,19
In the United States, 15.2% of adolescents reported being physically inactive, according to data published in 2016.7 Similar to most cardiovascular risk factors, minority populations and those of lower socioeconomic status had the worst profiles. The prevalence of physical inactivity was highest in African Americans and Hispanics (Figure 3).20
Studies have shown an association between screen-based sedentary behavior (computers, television, and video games) and cardiovascular disease.21–23 In the United States, 41% of adolescents used computers for activities other than homework for more than 3 hours per day on a school day.7 The pattern of use was highest in African American boys and African American girls, followed by Hispanic girls and Hispanic boys.18 Trends were similar with regard to watching television for more than 3 hours per day.
Sedentary behavior persists into adulthood, with rates of inactivity of 38.3% in African Americans, 40.1% in Hispanics, and 26.3% in white adults.7
Nutrition and obesity
Nutrition plays a major role in cardiovascular disease, specifically in the pathogenesis of atherosclerotic disease and hypertension.24 Most Americans do not meet dietary recommendations, with minority communities performing worse in specific metrics.7
Dietary patterns are reflected in the rate of obesity in this nation. Studies have shown a direct correlation between obesity and cardiovascular disease such as coronary artery disease, heart failure, and atrial fibrillation.25–28 According to data from the National Health and Nutrition Examination Survey (NHANES), 31% of children between the ages of 2 and 19 years are classified as obese or overweight. The highest rates of obesity are seen in Hispanic and African American boys and girls. The obesity epidemic is disproportionately rampant in children living in households with low income, low education, and high unemployment rates.7,29–31
Despite the risks associated with obesity, only 64.8% of obese adults report being informed by a doctor or health professional that they were overweight. The proportion of obese adults informed that they were overweight was significantly lower for African Americans and Hispanics compared with whites. Similar differences are seen based on socioeconomic status, as middle-income patients were less likely to be informed than those in the higher income strata (62.4% vs 70.6%).7,31
Blood pressure
Hypertension is a well-established risk factor for cardiovascular disease and stroke, and a blood pressure of 120/80 mm Hg or lower is identified as a component of ideal cardiovascular health.
In the United States the prevalence of hypertension in adults older than 20 is 32%.7 The prevalence of hypertension in African Americans is among the highest in the world.32,33 African Americans develop high blood pressure at earlier ages, and their average resting blood pressures are higher than in whites.34,35 For a 45-year-old without hypertension, the 40-year risk of developing hypertension is 92.7% for African Americans and 86% for whites.35 Hypertension is a major risk factor for stroke, and African Americans have a 1.8 times greater rate of fatal stroke than whites.7
In 2013 there were 71,942 deaths attributable to high blood pressure, and the 2011 death rate associated with hypertension was 18.9 per 100,000. By race, the death rate was 17.6 per 100,000 for white males and an alarming 47.1 per 100,000 for African American males; rates were 15.2 per 100,000 for white females and 35.1 per 100,000 for African American females.7
It is unclear what accounts for the racial difference in prevalence in hypertension. Studies have shown that African Americans are more likely than whites to have been told on more than 2 occasions that they have hypertension. And 85.7% of African Americans are aware that they have high blood pressure, compared with 82.7% of whites.14
African Americans and Hispanics have poorer hypertension control compared with whites.36,37 These observed differences cannot be attributed to access alone, as African Americans were more likely to be on higher-intensity blood pressure therapy, whereas Hispanics were more likely to be undertreated.36,38 In a meta-analysis of 13 trials, Peck et al39 showed that African Americans showed a lesser reduction in systolic and diastolic blood pressure when treated with angiotensin-converting enzyme (ACE) inhibitors.
The 2017 American College of Cardiology (ACC) and AHA guidelines for the prevention, detection, evaluation, and management of high blood pressure in adults40 identifies 4 drug classes as reducing cardiovascular disease morbidity and mortality: thiazide diuretics, ACE inhibitors, angiotensin II receptor blockers (ARBs), and calcium channel blockers. Of these 4 classes, thiazide diuretics and calcium channel blockers have been shown to lower blood pressure more effectively in African Americans than renin-angiotensin-aldosterone inhibition with ACE inhibitors or ARBs.
Glycemic control
Type 2 diabetes mellitus secondary to insulin resistance disproportionately affects minority groups, as the prevalence of diabetes mellitus in African Americans is almost twice as high as that in whites, and 35% higher in Hispanics compared with whites.7,41 Based on NHANES data between 1984 and 2004, the prevalence of diabetes mellitus is expected to increase by 99% in whites, 107% in African Americans, and 127% in Hispanics by 2050. Alarmingly, African Americans over age 75 are expected to experience a 606% increase by 2050.42
With regard to mortality, 21.7 deaths per 100,000 population were attributable to diabetes mellitus according to reports by the AHA in 2016. The death rate in white males was 24.3 per 100,000 compared with 44.9 per 100,000 for African Americans males. The associated mortality rate for white women was 16.2 per 100,000, and 35.8 per 100,000 for African American females.7
DISPARITIES AND CORONARY ARTERY DISEASE CARE
The management of coronary artery disease has evolved from prolonged bed rest to surgical, pharmacologic, and percutaneous revascularization.2,5 Coronary revascularization procedures are now relatively common: 950,000 percutaneous coronary interventions and 397,000 coronary artery bypass procedures were performed in 2010.7
Nevertheless, despite similar clinical presentations, African Americans with acute myocardial infarction were less likely to be referred for coronary artery bypass grafting than whites.43–46 They were also less likely to be given thrombolytics47 and less likely to undergo coronary angiography with percutaneous coronary intervention.48 Similar differences have been reported when comparing Hispanics with whites.49
Some suggest that healthcare access is a key mediator of health disparities.50 In 2009, Hispanics and African Americans accounted for more than 50% of those without health insurance.51 Improved access to healthcare might mitigate the disparity in revascularizations.
Massachusetts was one of the first states to mandate that all residents obtain health insurance. As a result, the uninsured rates declined in African Americans and Hispanics in Massachusetts, but a disparity in revascularization persisted. African Americans and Hispanics were 27% and 16% less likely to undergo revascularization procedures (coronary artery bypass grafting or percutaneous coronary intervention) than whites,51 suggesting that disparities in revascularization are not solely secondary to healthcare access.
These findings are consistent with a 2004 Veterans Administration study,52 in which healthcare access was equal among races. The study showed that African Americans received fewer cardiac procedures after an acute myocardial infarction compared with whites.
Have we made progress? The largest disparity between African Americans and whites in coronary artery disease mortality existed in 1990. The disparity persisted to 2012, and although decreased, it is projected to persist to 2030.53
DISPARITIES IN HEART FAILURE
An estimated 5.7 million Americans have heart failure, and 915,000 new cases are diagnosed annually.7 Unlike coronary artery disease, heart failure is expected to increase in prevalence by 46%, to 8 million Americans with heart failure by 2030.7,54
Our knowledge of disparities in the area of heart failure is derived primarily from epidemiologic studies. The Multi-Ethnic Study of Atherosclerosis55 showed that African Americans (4.6 per 1,000), followed by Hispanics (3.5 per 1,000) had a higher risk of developing heart failure compared with whites (2.4 per 1,000).The higher risk is in part due to disparities in socioeconomic status and prevalence of hypertension, as African Americans accounted for 75% of cases of nonischemic-related heart failure.55 African Americans also have a higher 5-year mortality rate than whites.55
Even though the 5-year mortality rate in heart failure is still 50%, the past 30 years have seen innovations in pharmacologic and device therapy and thus improved outcomes in heart failure patients. Still, significant gaps in the use of guideline-recommended therapies, quality of care, and clinical outcomes persist in contemporary practice for racial minorities with heart failure.
Disparities in inpatient care for heart failure
Patients admitted for heart failure and cared for by a cardiologist are more likely to be discharged on guideline-directed medical therapy, have fewer heart failure readmissions, and lower mortality.56,57 Breathett et al,58 in a study of 104,835 patients hospitalized in an intensive care unit for heart failure, found that primary intensive care by a cardiologist was associated with higher survival in both races. However, in the same study, white patients had a higher odds of receiving care from a cardiologist than African American patients.
Disparities and cardiac resynchronization therapy devices
In one-third of patients with heart failure, conduction delays result in dyssynchronous left ventricular contraction.59 Dyssynchrony leads to reduced cardiac performance, left ventricular remodeling, and increased mortality.56
Cardiac resynchronization therapy (CRT) was approved for clinical use in 2001, and studies have shown that it improves quality of life, exercise tolerance, cardiac performance, and morbidity and mortality rates.59–66 The 2013 ACC/AHA guidelines for the management of heart failure give a class IA recommendation (the highest) for its use in patients with a left ventricular ejection fraction of 35% or less, sinus rhythm, left bundle branch block and a QRS duration of 150 ms or greater, and New York Heart Association class II, III, or ambulatory IV symptoms while on guideline-directed medical therapy.67
Despite these recommendations, racial differences are observed. A study using the Nationwide Inpatient Sample database59 showed that between 2002 and 2010, a total of 374,202 CRT devices were implanted, averaging 41,578 annually. After adjusting for heart failure admissions, the study showed that CRT use was favored in men and in whites.
Another study, using the National Cardiovascular Data Registry,68 looked at patients who received implantable cardiac defibrillators (ICDs) and were eligible to receive CRT. It found that African Americans and Hispanics were less likely than whites to receive CRT, even though they were more likely to meet established criteria.
Disparities and left ventricular assist devices
The Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart failure (REMATCH) trial and Heart Mate II trial demonstrated that left ventricular assist devices (LVADs) were durable options for long-term support for patients with end-stage heart failure.69,70 Studies that examined the role of race and clinical outcomes after LVAD implantation have reported mixed findings.71,72 Few studies have looked at the role racial differences play in accessing LVAD therapy.
Joyce et al73 reviewed data from the Nationwide Inpatient Sample from 2002 to 2003 on patients admitted to the hospital with a primary diagnosis of heart failure or cardiogenic shock. A total of 297,866 patients were included in the study, of whom only 291 underwent LVAD implantation. A multivariate analysis found that factors such as age over 65, female sex, admission to a nonacademic center, geographic region, and African American race adversely influenced access to LVAD therapy.
Breathett et al74 evaluated racial differences in LVAD implantations from 2012 to 2015, a period that corresponds to increased health insurance expansion, and found LVAD implantations increased among African American patients with advanced heart failure, but no other racial or ethnic group.
Disparities and heart transplant
For patients with end-stage heart failure, orthotopic heart transplant is the most definitive and durable option for long-term survival. According to data from the United Network for Organ Sharing, 62,508 heart transplants were performed from January 1, 1988 to December 31, 2015. Compared with transplants of other solid organs, heart transplant occurs in significantly infrequent rates.
Barriers to transplant include lack of health insurance, considered a surrogate for low socioeconomic status. Hispanics and African Americans are less likely to have private health insurance than non-Hispanic whites, and this difference is magnified among the working poor.
Despite these perceived barriers, Kilic et al75 found that African Americans comprised 16.4% of heart transplant recipients, although they make up only approximately 13% of the US population. They also had significantly shorter wait-list times than whites. On the negative side, African Americans had a higher unadjusted mortality rate than whites (15% vs 12% P = .002). African Americans also tended to receive their transplants at centers with lower transplant volumes and higher transplant mortality rates.
Several other studies also showed that African Americans compared to whites have significantly worse outcomes after transplant.76–79 What accounts for this difference? Kilic et al75 showed that African Americans had the lowest proportion of blood type matching and lowest human leukocyte antigen matching, were younger (because African Americans develop more advanced heart failure at younger ages), had higher serum creatinine levels, and were more often bridged to transplant with an LVAD.
DISPARITIES IN CARDIOVASCULAR RESEARCH
Although the United States has the most sophisticated and robust medical system in the world, select groups have significant differences in delivery and healthcare outcomes. There are many explanations for these differences, but a contributing factor may be the paucity of research dedicated to understand racial and ethnic differences.80
Differences observed in epidemiologic studies may be secondary to pathophysiology, genetic differences, environment, and lifestyle choices. Historically, clinical trials were conducted in homogeneous populations with respect to age (middle-aged), sex (male), and race (white), and the results were generalized to heterogeneous populations.80
Disparities in research have implications in clinical practice. Overall, the primary cause of heart failure is ischemia; however, in African Americans, the primary cause is hypertensive heart disease.81 Studies in hypertension have shown that African Americans have less of a response to neurohormonal blockade with ACE inhibitors and beta-blockers than non-African Americans.82 Nevertheless, neurohormonal blockade has become the cornerstone of heart failure treatment.
Retrospective analysis of the Vasodilator-Heart Failure trials83 showed that treatment with isosorbide dinitrate plus hydralazine, compared with placebo, conferred a survival benefit for African Americans but not whites.80 No survival advantage was noted when isosorbide dinitrate/hydralazine was compared to enalapril in African Americans, although enalapril was superior to isosorbide dinitrate in whites.45 These observations were recognized 10 to 15 years after trial completion, and were only possible because the trials included sufficient numbers of African American patients to complete analysis.
In 1993, the US Congress passed the National Institutes of Health (NIH) Revitalization Act, which established guidelines requiring NIH grant applicants to include minorities in human subject research, as they were historically underrepresented in clinical research trials.84,85
In 2001, the Beta-Blocker Evaluation of Survival Trial86 reported its results investigating whether bucindolol, a nonselective beta-blocker, would reduce mortality in patients with advanced heart failure (New York Heart Association class III or IV). This was one of the first trials to prospectively investigate racial and ethnic differences in response to treatment. Though it showed no overall benefit in the use of bucindolol in the treatment of advanced heart failure, subgroup analysis showed that whites did enjoy a benefit in terms of lower mortality, whereas African Americans did not.
Results of the Vasodilator-Heart Failure trials led to further population-directed research, most notably the African American Heart Failure Trial,87 a double-blind, placebo-controlled, randomized trial in patients who identified as African American. Patients who were randomized to receive a fixed dose of hydralazine and isosorbide dinitrate had a 43% lower mortality rate, a 33% lower hospitalization rate for heart failure, and better quality of life than patients in the placebo group, leading to early termination of the trial. The outcomes suggested that the combination of isosorbide dinitrate and hydralazine treats heart failure in a manner independent of pure neurohormonal blockade.
CHALLENGES IN STUDY PARTICIPATION
Recruitment of minority participants in biomedical research is a challenging task for clinical investigators.88,89 Some of the factors thought to pose potential barriers for racial and ethnic minority participation in health research include poor access to primary medical care, failure of researchers to recruit minority populations actively, and language and cultural barriers.90
Further, it is widely claimed that African Americans are less willing than nonminority individuals to participate in clinical research trials due to general distrust of the medical community as a result of the Tuskegee Syphilis Experiment.91 That infamous study, conducted by the US Public Health Service between 1932 and 1972, sought to record the natural progression of untreated syphilis in poor African American men in Alabama. The participants were not informed of the true purpose of the study, and they were under the impression that they were simply receiving free healthcare from the US government. Further, they were denied appropriate treatment even after it became readily available, in order for researchers to observe the progression of the disease.
While the 1993 mandate did in fact increase pressure on researchers to develop strategies to overcome participation barriers, the issue of underrepresentation of racial minorities in clinical research, including cardiovascular research, has not been resolved and continues to be a problem today.
The overall goal of clinical research is to determine the best strategies to prevent and treat disease. But if the study population is not representative of the affected population at large, the results cannot be generalized to underrepresented subgroups. The implications of underrepresentation in research are far-reaching, and can further contribute to disparate care of minority patients such as African Americans, who have a higher prevalence of cardiovascular risk factors and greater burden of heart failure.
PROPOSING SOLUTIONS
Between 1986 and 2018, according to a PUBMED search, 10,462 articles highlighted the presence of a health-related disparity. Solutions to address and ultimately eradicate disparities will need to eliminate healthcare bias, increase patient access, and increase diversity and inclusion in the physician work force.
Eliminating bias
Implicit bias refers to attitudes, thoughts, and feelings that exist outside of the conscious awareness.92 These biases can be triggered by race, gender, or socioeconomic status. They have manifested in society as stereotypes that men are more competent than women, women are more verbal than men, and African Americans are more athletic than whites.93
The concept of implicit bias is important, in that the populations that experience the greatest health disparities also suffer from negative cultural stereotypes.94 Healthcare professionals are not inoculated against implicit bias.95 Studies have shown that most healthcare providers have implicit biases that reflect positive attitudes toward whites and negative attitudes toward people of color.92,94,96–98
The Implicit Association Test, introduced in 1998, is widely used to measure implicit bias. It measures response time of subjects to match particular social groups to particular attributes.99 Green et al,99 using this test, showed that although physicians reported no explicit preference for white vs African American patients or differences in perceived cooperativeness, the test revealed implicit preference favoring white Americans and implicit stereotypes of African Americans as less cooperative for medical procedures and in general. This also manifested in clinical decision-making, as white Americans were more likely, and African Americans less likely, to be treated with thrombolysis.99
Sabin et al100 showed that implicit bias was present among pediatricians, although less than in society as a whole and in other healthcare professionals.
But how does one change feelings that exist outside of the conscious awareness? Green et al99 showed that making physicians aware of their susceptibility to bias changed their behavior. A subset of physicians who were made aware that bias was a focus of the study were more likely to refer African Americans for thrombolysis even if they had a high degree of implicit pro-white bias.94,100 Perhaps mandating that all healthcare providers take a self-administered and confidentially reported Implicit Association Test will lead to awareness of implicit bias and minimize healthcare behaviors that contribute to the current state of disparities.
Improving access
Common indicators of access to healthcare include health insurance status, having a usual source of healthcare, and having a regular physician.101 Health insurance does offer protection from the costs associated with illness and health maintenance.101 It is also a major contributing factor in racial and ethnic disparities.
Chen et al102 examined the effects of the Affordable Care Act and found that it was associated with reduction in the probability of being uninsured, delaying necessary care, and forgoing necessary care, and increased probability of having a physician. However, earlier studies showed that access to health insurance by itself does not equate to equitable care.103,104
Diversifying the work force
African Americans comprise 4% of physicians and Hispanic Americans 5%, despite accounting for 13% and 16% of the US population.105 This underrepresentation has led to African American and Hispanic American patients being more likely than white patients to be treated by a physician from a dissimilar racial or ethnic background.106 Studies have shown that minority patients in a race- or ethnic-concordant relationship are more likely to use needed health services, less likely to postpone seeking care, and report greater satisfaction.106,107 Minority physicians often locate and practice in neighborhoods with high minority populations, and they disproportionately care for disadvantaged patients of lower socioeconomic status and poorer health.106,108
WE ARE STILL IN THE TUNNEL, BUT THERE IS LIGHT AT THE END
The cardiovascular community has faced tremendous challenges in the past and responded with innovative research that has led to imaging that aids in the diagnosis of subclinical cardiovascular disease and invasive and pharmacologic strategies that have improved cardiovascular outcomes. One may say that there is light at the end of the tunnel; however, the existence of disparate care reminds us that we are still in the tunnel.
Disparities in cardiovascular disease management present a unique challenge for the community. There is no drug, device, or invasive procedure to eliminate this pathology. However, by acknowledging the problem and implementing changes at the system, provider, and patient level, the cardiovascular community can achieve yet another momentous achievement: the end of cardiovascular health disparities. Cardiovascular disease makes no distinction in race, sex, age, or socioeconomic status, and neither should the medical community.
Cardiovascular disease became the leading cause of death in the United States in the early 20th century, and it accounts for nearly half of all deaths in industrialized nations.1 The mortality it inflicts was thought to be shared equally between both sexes and among all age groups and races.2 The cardiology community implemented innovative epidemiologic research, through which risk factors for cardiovascular disease were established.1 The development of coronary care units reduced in-hospital mortality from acute myocardial infarction from 30% to 15%.2–5 Further advances in pharmacology, revascularization, and imaging have aided in the detection and treatment of cardiovascular disease.6 Though cardiovascular disease remains the number-one cause of death worldwide, rates are on the decline.7
For several decades, health disparities have been recognized as a source of pathology in cardiovascular medicine, resulting in inequity of care administration among select populations. In this review, we examine whether the same forward thinking that has resulted in a decline in cardiovascular disease has had an impact on the pervasive disparities in cardiovascular medicine.
DISPARITIES DEFINED
Compared with whites, members of minority groups have a higher burden of chronic diseases, receive lower quality care, and have less access to medical care. Recognizing the potential public health ramifications, in 1999 the US Congress tasked the Institute of Medicine to study and assess the extent of healthcare disparities. This led to the landmark publication, Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care.8
The Institute of Medicine defines disparities in healthcare as racial or ethnic differences in the quality of healthcare that are not due to access-related factors, clinical needs, preferences, and appropriateness of intervention.8 Disparities can also exist according to socioeconomic status and sex.9
In an early study documenting the concept of disparities in cardiovascular disease, Stone and Vanzant10 concluded that heart disease was more common in African Americans than in whites, and that hypertension was the principal cause of cardiovascular disease mortality in African Americans.
Although avoidable deaths from heart disease, stroke, and hypertensive disease declined between 2001 and 2010, African Americans still have a higher mortality rate than other racial and ethnic groups (Figure 1).11
DISPARITIES AND CARDIOVASCULAR HEALTH
The concept of cardiovascular health was established by the American Heart Association (AHA) in efforts to achieve an additional 20% reduction in cardiovascular disease-related mortality by 2020.7 Cardiovascular health is defined as the absence of clinically manifest cardiovascular disease and is measured by 7 components:
- Not smoking or abstaining from smoking for at least 1 year
- A normal body weight, defined as a body mass index less than 25 kg/m2
- Optimal physical activity, defined as 75 minutes of vigorous physical activity or 150 minutes of moderate-intensity physical activity per week
- Regular consumption of a healthy diet
- Total cholesterol below 200 mg/dL
- Blood pressure less than 120/80 mm Hg
- Fasting blood sugar below 100 mg/dL.
Nearly 70% of the US population can claim 2, 3, or 4 of these components, but differences exist according to race,12 and 60% of adult white Americans are limited to achieving no more than 3 of these healthy metrics, compared with 70% of adult African Americans and Hispanic Americans.
Smoking
Smoking is a major risk factor for cardiovascular disease.12–14
During adolescence, white males are more likely to smoke than African American and Hispanic males,12 but this trend reverses in adulthood, when African American men have a higher prevalence of smoking than white men (21.4% vs 19%).7 Rates of lifetime use are highest among American Indian or Alaskan natives and whites (75.9%), followed by African Americans (58.4%), native Hawaiians (56.8%), and Hispanics (56.7%).15 Trends for current smoking are similar (Figure 2).16 Moreover, households with lower socioeconomic status have a higher prevalence of smoking.7
Physical activity
People with a sedentary lifestyle are more likely to die of cardiovascular disease. As many as 250,000 deaths annually in the United States are attributed to lack of regular physical activity.17
Recognizing the potential public health ramifications, the AHA and the 2018 Federal Guidelines on Physical Activity recommend that children engage in 60 minutes of daily physical activity and that adults participate in 150 minutes of moderate-intensity or 75 minutes of vigorous physical activity weekly.18,19
In the United States, 15.2% of adolescents reported being physically inactive, according to data published in 2016.7 Similar to most cardiovascular risk factors, minority populations and those of lower socioeconomic status had the worst profiles. The prevalence of physical inactivity was highest in African Americans and Hispanics (Figure 3).20
Studies have shown an association between screen-based sedentary behavior (computers, television, and video games) and cardiovascular disease.21–23 In the United States, 41% of adolescents used computers for activities other than homework for more than 3 hours per day on a school day.7 The pattern of use was highest in African American boys and African American girls, followed by Hispanic girls and Hispanic boys.18 Trends were similar with regard to watching television for more than 3 hours per day.
Sedentary behavior persists into adulthood, with rates of inactivity of 38.3% in African Americans, 40.1% in Hispanics, and 26.3% in white adults.7
Nutrition and obesity
Nutrition plays a major role in cardiovascular disease, specifically in the pathogenesis of atherosclerotic disease and hypertension.24 Most Americans do not meet dietary recommendations, with minority communities performing worse in specific metrics.7
Dietary patterns are reflected in the rate of obesity in this nation. Studies have shown a direct correlation between obesity and cardiovascular disease such as coronary artery disease, heart failure, and atrial fibrillation.25–28 According to data from the National Health and Nutrition Examination Survey (NHANES), 31% of children between the ages of 2 and 19 years are classified as obese or overweight. The highest rates of obesity are seen in Hispanic and African American boys and girls. The obesity epidemic is disproportionately rampant in children living in households with low income, low education, and high unemployment rates.7,29–31
Despite the risks associated with obesity, only 64.8% of obese adults report being informed by a doctor or health professional that they were overweight. The proportion of obese adults informed that they were overweight was significantly lower for African Americans and Hispanics compared with whites. Similar differences are seen based on socioeconomic status, as middle-income patients were less likely to be informed than those in the higher income strata (62.4% vs 70.6%).7,31
Blood pressure
Hypertension is a well-established risk factor for cardiovascular disease and stroke, and a blood pressure of 120/80 mm Hg or lower is identified as a component of ideal cardiovascular health.
In the United States the prevalence of hypertension in adults older than 20 is 32%.7 The prevalence of hypertension in African Americans is among the highest in the world.32,33 African Americans develop high blood pressure at earlier ages, and their average resting blood pressures are higher than in whites.34,35 For a 45-year-old without hypertension, the 40-year risk of developing hypertension is 92.7% for African Americans and 86% for whites.35 Hypertension is a major risk factor for stroke, and African Americans have a 1.8 times greater rate of fatal stroke than whites.7
In 2013 there were 71,942 deaths attributable to high blood pressure, and the 2011 death rate associated with hypertension was 18.9 per 100,000. By race, the death rate was 17.6 per 100,000 for white males and an alarming 47.1 per 100,000 for African American males; rates were 15.2 per 100,000 for white females and 35.1 per 100,000 for African American females.7
It is unclear what accounts for the racial difference in prevalence in hypertension. Studies have shown that African Americans are more likely than whites to have been told on more than 2 occasions that they have hypertension. And 85.7% of African Americans are aware that they have high blood pressure, compared with 82.7% of whites.14
African Americans and Hispanics have poorer hypertension control compared with whites.36,37 These observed differences cannot be attributed to access alone, as African Americans were more likely to be on higher-intensity blood pressure therapy, whereas Hispanics were more likely to be undertreated.36,38 In a meta-analysis of 13 trials, Peck et al39 showed that African Americans showed a lesser reduction in systolic and diastolic blood pressure when treated with angiotensin-converting enzyme (ACE) inhibitors.
The 2017 American College of Cardiology (ACC) and AHA guidelines for the prevention, detection, evaluation, and management of high blood pressure in adults40 identifies 4 drug classes as reducing cardiovascular disease morbidity and mortality: thiazide diuretics, ACE inhibitors, angiotensin II receptor blockers (ARBs), and calcium channel blockers. Of these 4 classes, thiazide diuretics and calcium channel blockers have been shown to lower blood pressure more effectively in African Americans than renin-angiotensin-aldosterone inhibition with ACE inhibitors or ARBs.
Glycemic control
Type 2 diabetes mellitus secondary to insulin resistance disproportionately affects minority groups, as the prevalence of diabetes mellitus in African Americans is almost twice as high as that in whites, and 35% higher in Hispanics compared with whites.7,41 Based on NHANES data between 1984 and 2004, the prevalence of diabetes mellitus is expected to increase by 99% in whites, 107% in African Americans, and 127% in Hispanics by 2050. Alarmingly, African Americans over age 75 are expected to experience a 606% increase by 2050.42
With regard to mortality, 21.7 deaths per 100,000 population were attributable to diabetes mellitus according to reports by the AHA in 2016. The death rate in white males was 24.3 per 100,000 compared with 44.9 per 100,000 for African Americans males. The associated mortality rate for white women was 16.2 per 100,000, and 35.8 per 100,000 for African American females.7
DISPARITIES AND CORONARY ARTERY DISEASE CARE
The management of coronary artery disease has evolved from prolonged bed rest to surgical, pharmacologic, and percutaneous revascularization.2,5 Coronary revascularization procedures are now relatively common: 950,000 percutaneous coronary interventions and 397,000 coronary artery bypass procedures were performed in 2010.7
Nevertheless, despite similar clinical presentations, African Americans with acute myocardial infarction were less likely to be referred for coronary artery bypass grafting than whites.43–46 They were also less likely to be given thrombolytics47 and less likely to undergo coronary angiography with percutaneous coronary intervention.48 Similar differences have been reported when comparing Hispanics with whites.49
Some suggest that healthcare access is a key mediator of health disparities.50 In 2009, Hispanics and African Americans accounted for more than 50% of those without health insurance.51 Improved access to healthcare might mitigate the disparity in revascularizations.
Massachusetts was one of the first states to mandate that all residents obtain health insurance. As a result, the uninsured rates declined in African Americans and Hispanics in Massachusetts, but a disparity in revascularization persisted. African Americans and Hispanics were 27% and 16% less likely to undergo revascularization procedures (coronary artery bypass grafting or percutaneous coronary intervention) than whites,51 suggesting that disparities in revascularization are not solely secondary to healthcare access.
These findings are consistent with a 2004 Veterans Administration study,52 in which healthcare access was equal among races. The study showed that African Americans received fewer cardiac procedures after an acute myocardial infarction compared with whites.
Have we made progress? The largest disparity between African Americans and whites in coronary artery disease mortality existed in 1990. The disparity persisted to 2012, and although decreased, it is projected to persist to 2030.53
DISPARITIES IN HEART FAILURE
An estimated 5.7 million Americans have heart failure, and 915,000 new cases are diagnosed annually.7 Unlike coronary artery disease, heart failure is expected to increase in prevalence by 46%, to 8 million Americans with heart failure by 2030.7,54
Our knowledge of disparities in the area of heart failure is derived primarily from epidemiologic studies. The Multi-Ethnic Study of Atherosclerosis55 showed that African Americans (4.6 per 1,000), followed by Hispanics (3.5 per 1,000) had a higher risk of developing heart failure compared with whites (2.4 per 1,000).The higher risk is in part due to disparities in socioeconomic status and prevalence of hypertension, as African Americans accounted for 75% of cases of nonischemic-related heart failure.55 African Americans also have a higher 5-year mortality rate than whites.55
Even though the 5-year mortality rate in heart failure is still 50%, the past 30 years have seen innovations in pharmacologic and device therapy and thus improved outcomes in heart failure patients. Still, significant gaps in the use of guideline-recommended therapies, quality of care, and clinical outcomes persist in contemporary practice for racial minorities with heart failure.
Disparities in inpatient care for heart failure
Patients admitted for heart failure and cared for by a cardiologist are more likely to be discharged on guideline-directed medical therapy, have fewer heart failure readmissions, and lower mortality.56,57 Breathett et al,58 in a study of 104,835 patients hospitalized in an intensive care unit for heart failure, found that primary intensive care by a cardiologist was associated with higher survival in both races. However, in the same study, white patients had a higher odds of receiving care from a cardiologist than African American patients.
Disparities and cardiac resynchronization therapy devices
In one-third of patients with heart failure, conduction delays result in dyssynchronous left ventricular contraction.59 Dyssynchrony leads to reduced cardiac performance, left ventricular remodeling, and increased mortality.56
Cardiac resynchronization therapy (CRT) was approved for clinical use in 2001, and studies have shown that it improves quality of life, exercise tolerance, cardiac performance, and morbidity and mortality rates.59–66 The 2013 ACC/AHA guidelines for the management of heart failure give a class IA recommendation (the highest) for its use in patients with a left ventricular ejection fraction of 35% or less, sinus rhythm, left bundle branch block and a QRS duration of 150 ms or greater, and New York Heart Association class II, III, or ambulatory IV symptoms while on guideline-directed medical therapy.67
Despite these recommendations, racial differences are observed. A study using the Nationwide Inpatient Sample database59 showed that between 2002 and 2010, a total of 374,202 CRT devices were implanted, averaging 41,578 annually. After adjusting for heart failure admissions, the study showed that CRT use was favored in men and in whites.
Another study, using the National Cardiovascular Data Registry,68 looked at patients who received implantable cardiac defibrillators (ICDs) and were eligible to receive CRT. It found that African Americans and Hispanics were less likely than whites to receive CRT, even though they were more likely to meet established criteria.
Disparities and left ventricular assist devices
The Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart failure (REMATCH) trial and Heart Mate II trial demonstrated that left ventricular assist devices (LVADs) were durable options for long-term support for patients with end-stage heart failure.69,70 Studies that examined the role of race and clinical outcomes after LVAD implantation have reported mixed findings.71,72 Few studies have looked at the role racial differences play in accessing LVAD therapy.
Joyce et al73 reviewed data from the Nationwide Inpatient Sample from 2002 to 2003 on patients admitted to the hospital with a primary diagnosis of heart failure or cardiogenic shock. A total of 297,866 patients were included in the study, of whom only 291 underwent LVAD implantation. A multivariate analysis found that factors such as age over 65, female sex, admission to a nonacademic center, geographic region, and African American race adversely influenced access to LVAD therapy.
Breathett et al74 evaluated racial differences in LVAD implantations from 2012 to 2015, a period that corresponds to increased health insurance expansion, and found LVAD implantations increased among African American patients with advanced heart failure, but no other racial or ethnic group.
Disparities and heart transplant
For patients with end-stage heart failure, orthotopic heart transplant is the most definitive and durable option for long-term survival. According to data from the United Network for Organ Sharing, 62,508 heart transplants were performed from January 1, 1988 to December 31, 2015. Compared with transplants of other solid organs, heart transplant occurs in significantly infrequent rates.
Barriers to transplant include lack of health insurance, considered a surrogate for low socioeconomic status. Hispanics and African Americans are less likely to have private health insurance than non-Hispanic whites, and this difference is magnified among the working poor.
Despite these perceived barriers, Kilic et al75 found that African Americans comprised 16.4% of heart transplant recipients, although they make up only approximately 13% of the US population. They also had significantly shorter wait-list times than whites. On the negative side, African Americans had a higher unadjusted mortality rate than whites (15% vs 12% P = .002). African Americans also tended to receive their transplants at centers with lower transplant volumes and higher transplant mortality rates.
Several other studies also showed that African Americans compared to whites have significantly worse outcomes after transplant.76–79 What accounts for this difference? Kilic et al75 showed that African Americans had the lowest proportion of blood type matching and lowest human leukocyte antigen matching, were younger (because African Americans develop more advanced heart failure at younger ages), had higher serum creatinine levels, and were more often bridged to transplant with an LVAD.
DISPARITIES IN CARDIOVASCULAR RESEARCH
Although the United States has the most sophisticated and robust medical system in the world, select groups have significant differences in delivery and healthcare outcomes. There are many explanations for these differences, but a contributing factor may be the paucity of research dedicated to understand racial and ethnic differences.80
Differences observed in epidemiologic studies may be secondary to pathophysiology, genetic differences, environment, and lifestyle choices. Historically, clinical trials were conducted in homogeneous populations with respect to age (middle-aged), sex (male), and race (white), and the results were generalized to heterogeneous populations.80
Disparities in research have implications in clinical practice. Overall, the primary cause of heart failure is ischemia; however, in African Americans, the primary cause is hypertensive heart disease.81 Studies in hypertension have shown that African Americans have less of a response to neurohormonal blockade with ACE inhibitors and beta-blockers than non-African Americans.82 Nevertheless, neurohormonal blockade has become the cornerstone of heart failure treatment.
Retrospective analysis of the Vasodilator-Heart Failure trials83 showed that treatment with isosorbide dinitrate plus hydralazine, compared with placebo, conferred a survival benefit for African Americans but not whites.80 No survival advantage was noted when isosorbide dinitrate/hydralazine was compared to enalapril in African Americans, although enalapril was superior to isosorbide dinitrate in whites.45 These observations were recognized 10 to 15 years after trial completion, and were only possible because the trials included sufficient numbers of African American patients to complete analysis.
In 1993, the US Congress passed the National Institutes of Health (NIH) Revitalization Act, which established guidelines requiring NIH grant applicants to include minorities in human subject research, as they were historically underrepresented in clinical research trials.84,85
In 2001, the Beta-Blocker Evaluation of Survival Trial86 reported its results investigating whether bucindolol, a nonselective beta-blocker, would reduce mortality in patients with advanced heart failure (New York Heart Association class III or IV). This was one of the first trials to prospectively investigate racial and ethnic differences in response to treatment. Though it showed no overall benefit in the use of bucindolol in the treatment of advanced heart failure, subgroup analysis showed that whites did enjoy a benefit in terms of lower mortality, whereas African Americans did not.
Results of the Vasodilator-Heart Failure trials led to further population-directed research, most notably the African American Heart Failure Trial,87 a double-blind, placebo-controlled, randomized trial in patients who identified as African American. Patients who were randomized to receive a fixed dose of hydralazine and isosorbide dinitrate had a 43% lower mortality rate, a 33% lower hospitalization rate for heart failure, and better quality of life than patients in the placebo group, leading to early termination of the trial. The outcomes suggested that the combination of isosorbide dinitrate and hydralazine treats heart failure in a manner independent of pure neurohormonal blockade.
CHALLENGES IN STUDY PARTICIPATION
Recruitment of minority participants in biomedical research is a challenging task for clinical investigators.88,89 Some of the factors thought to pose potential barriers for racial and ethnic minority participation in health research include poor access to primary medical care, failure of researchers to recruit minority populations actively, and language and cultural barriers.90
Further, it is widely claimed that African Americans are less willing than nonminority individuals to participate in clinical research trials due to general distrust of the medical community as a result of the Tuskegee Syphilis Experiment.91 That infamous study, conducted by the US Public Health Service between 1932 and 1972, sought to record the natural progression of untreated syphilis in poor African American men in Alabama. The participants were not informed of the true purpose of the study, and they were under the impression that they were simply receiving free healthcare from the US government. Further, they were denied appropriate treatment even after it became readily available, in order for researchers to observe the progression of the disease.
While the 1993 mandate did in fact increase pressure on researchers to develop strategies to overcome participation barriers, the issue of underrepresentation of racial minorities in clinical research, including cardiovascular research, has not been resolved and continues to be a problem today.
The overall goal of clinical research is to determine the best strategies to prevent and treat disease. But if the study population is not representative of the affected population at large, the results cannot be generalized to underrepresented subgroups. The implications of underrepresentation in research are far-reaching, and can further contribute to disparate care of minority patients such as African Americans, who have a higher prevalence of cardiovascular risk factors and greater burden of heart failure.
PROPOSING SOLUTIONS
Between 1986 and 2018, according to a PUBMED search, 10,462 articles highlighted the presence of a health-related disparity. Solutions to address and ultimately eradicate disparities will need to eliminate healthcare bias, increase patient access, and increase diversity and inclusion in the physician work force.
Eliminating bias
Implicit bias refers to attitudes, thoughts, and feelings that exist outside of the conscious awareness.92 These biases can be triggered by race, gender, or socioeconomic status. They have manifested in society as stereotypes that men are more competent than women, women are more verbal than men, and African Americans are more athletic than whites.93
The concept of implicit bias is important, in that the populations that experience the greatest health disparities also suffer from negative cultural stereotypes.94 Healthcare professionals are not inoculated against implicit bias.95 Studies have shown that most healthcare providers have implicit biases that reflect positive attitudes toward whites and negative attitudes toward people of color.92,94,96–98
The Implicit Association Test, introduced in 1998, is widely used to measure implicit bias. It measures response time of subjects to match particular social groups to particular attributes.99 Green et al,99 using this test, showed that although physicians reported no explicit preference for white vs African American patients or differences in perceived cooperativeness, the test revealed implicit preference favoring white Americans and implicit stereotypes of African Americans as less cooperative for medical procedures and in general. This also manifested in clinical decision-making, as white Americans were more likely, and African Americans less likely, to be treated with thrombolysis.99
Sabin et al100 showed that implicit bias was present among pediatricians, although less than in society as a whole and in other healthcare professionals.
But how does one change feelings that exist outside of the conscious awareness? Green et al99 showed that making physicians aware of their susceptibility to bias changed their behavior. A subset of physicians who were made aware that bias was a focus of the study were more likely to refer African Americans for thrombolysis even if they had a high degree of implicit pro-white bias.94,100 Perhaps mandating that all healthcare providers take a self-administered and confidentially reported Implicit Association Test will lead to awareness of implicit bias and minimize healthcare behaviors that contribute to the current state of disparities.
Improving access
Common indicators of access to healthcare include health insurance status, having a usual source of healthcare, and having a regular physician.101 Health insurance does offer protection from the costs associated with illness and health maintenance.101 It is also a major contributing factor in racial and ethnic disparities.
Chen et al102 examined the effects of the Affordable Care Act and found that it was associated with reduction in the probability of being uninsured, delaying necessary care, and forgoing necessary care, and increased probability of having a physician. However, earlier studies showed that access to health insurance by itself does not equate to equitable care.103,104
Diversifying the work force
African Americans comprise 4% of physicians and Hispanic Americans 5%, despite accounting for 13% and 16% of the US population.105 This underrepresentation has led to African American and Hispanic American patients being more likely than white patients to be treated by a physician from a dissimilar racial or ethnic background.106 Studies have shown that minority patients in a race- or ethnic-concordant relationship are more likely to use needed health services, less likely to postpone seeking care, and report greater satisfaction.106,107 Minority physicians often locate and practice in neighborhoods with high minority populations, and they disproportionately care for disadvantaged patients of lower socioeconomic status and poorer health.106,108
WE ARE STILL IN THE TUNNEL, BUT THERE IS LIGHT AT THE END
The cardiovascular community has faced tremendous challenges in the past and responded with innovative research that has led to imaging that aids in the diagnosis of subclinical cardiovascular disease and invasive and pharmacologic strategies that have improved cardiovascular outcomes. One may say that there is light at the end of the tunnel; however, the existence of disparate care reminds us that we are still in the tunnel.
Disparities in cardiovascular disease management present a unique challenge for the community. There is no drug, device, or invasive procedure to eliminate this pathology. However, by acknowledging the problem and implementing changes at the system, provider, and patient level, the cardiovascular community can achieve yet another momentous achievement: the end of cardiovascular health disparities. Cardiovascular disease makes no distinction in race, sex, age, or socioeconomic status, and neither should the medical community.
- Nabel EG, Braunwald E. A tale of coronary artery disease and myocardial infarction. N Engl J Med 2012; 366(1):54–63. doi:10.1056/NEJMra1112570
- Braunwald E. Evolution of the management of acute myocardial infarction: a 20th century saga. Lancet 1998; 352(9142):1771–1774. doi:10.1016/S0140-6736(98)03212-7
- Julian DG. The history of coronary care units. Br Heart J 1987; 57(6):497–502. doi:10.1136/hrt.57.6.497
- Caswell JE. A brief history of coronary care units. Public Health Rep 1967; 82(12):1105–1111. pmid:19316519
- Day HW. History of coronary care units. Am J Cardiol 1972; 30(4):405–407. pmid:4560377
- Braunwald E. Shattuck lecture—cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med 1997; 337(19):1360–1369. doi:10.1056/NEJM199711063371906
- Mozaffarian D, Benjamin EJ, Go AS, et al; American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation 2016; 133(4):e38–e360. doi:10.1161/CIR.0000000000000350
- Nelson A. Unequal treatment: confronting racial and ethnic disparities in health care. J Natl Med Assoc 2002; 94(8):666–668. pmid:12152921
- McGuire TG, Alegria M, Cook BL, Wells KB, Zaslavsky AM. Implementing the Institute of Medicine definition of disparities: an application to mental health care. Health Serv Res 2006; 41(5):1979–2005. doi:10.1111/j.1475-6773.2006.00583.x
- Stone CT, Vanzant FR. Heart disease as seen in a southern clinic: a clinical and pathologic survey. JAMA 1927; 89(18):1473–1480. doi:10.1001/jama.1927.02690180005002
- Centers For Disease Control And Prevention (CDC). Vital signs: avoidable deaths from heart disease, stroke, and hypertensive disease—United States, 2001–2010. MMWR Morb Mortal Wkly Rep 2013; 62(35):727–727. pmid:PMC4585625
- Mozaffarian D, Benjamin EJ, Go AS, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 2015; 131(4):e29–e322. doi:10.1161/CIR.0000000000000152
- Ockene IS, Miller NH. Cigarette smoking, cardiovascular disease, and stroke: a statement for healthcare professionals from the American Heart Association. American Heart Association Task Force on Risk Reduction. Circulation 1997; 96(9):3243–3247. pmid:9386200
- Messner B, Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol 2014; 34(3):509–515. doi:10.1161/ATVBAHA.113.300156
- Benjamin EJ, Virani SS, Callaway CW, et al; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation 2018; 137(12):e67–e492. doi:10.1161/CIR.0000000000000558
- Jamal A, Homa DM, O’Conner E, et al. Current cigarette smoking among adults—United States, 2005–2014. MMWR 2015; 64(44):1233–1240. doi:10.15585/mmwr.mm6444a2
- Myers J. Cardiology patient pages. Exercise and cardiovascular health. Circulation 2003; 107(1):e2–e5. pmid:12515760
- Shiroma EJ, Lee IM. Physical activity and cardiovascular health: lessons learned from epidemiological studies across age, gender, and race/ethnicity. Circulation 2010; 122(7):743–752. doi:10.1161/CIRCULATIONAHA.109.914721
- Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA 2018; 320(19):2020–2028. doi:10.1001/jama.2018.14854
- Omura JD, Carlson SA, Paul P, et al. Adults eligible for cardiovascular disease prevention counseling and participation in aerobic physical activity—United States, 2013. MMWR 2015; 64(37):1047–1051. doi:10.15585/mmwr.mm6437a4
- Dunstan DW, Barr EL, Healy GN, et al. Television viewing time and mortality: the Australian diabetes, obesity and lifestyle study (AusDiab). Circulation 2010; 121(3):384–391. doi:10.1161/CIRCULATIONAHA.109.894824
- Warren TY, Barry V, Hooker SP, Sui X, Church TS, Blair SN. Sedentary behaviors increase risk of cardiovascular disease mortality in men. Med Sci Sports Exerc 2010; 42(5):879–885. doi:10.1249/MSS.0b013e3181c3aa7e
- Byun W, Dowda M, Pate RR. Associations between screen-based sedentary behavior and cardiovascular disease risk factors in Korean youth. J Korean Med Sci 2012; 27(4):388–394. doi:10.3346/jkms.2012.27.4.388
- Getz GS, Reardon CA. Nutrition and cardiovascular disease. Arterioscler Thromb Vasc Biol 2007; 27(12):2499–2506. doi:10.1161/ATVBAHA.107.155853
- Eckel RH. Obesity and heart disease: a statement for healthcare professionals from the Nutrition Committee, American Heart Association. Circulation 1997; 96(9):3248–3250. pmid:9386201
- Rimm EB, Stampfer MJ, Giovannucci E, et al. Body size and fat distribution as predictors of coronary heart disease among middle-aged and older US men. Am J Epidemiol 1995; 141(12):1117–1127. pmid:7771450
- Duflou J, Virmani R, Rabin I, Burke A, Farb A, Smialek J. Sudden death as a result of heart disease in morbid obesity. Am Heart J 1995; 130(2):306–313. pmid:7631612
- Poirier P, Giles TD, Bray GA, et al; American Heart Association; Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006; 113(6):898–918. doi:10.1161/CIRCULATIONAHA.106.171016
- Wang Y. Disparities in pediatric obesity in the United States. Adv Nutr 2011; 2(1):23–31. doi:10.3945/an.110.000083
- Taveras EM, Gillman MW, Kleinman KP, Rich-Edwards JW, Rifas-Shiman SL. Reducing racial/ethnic disparities in childhood obesity: the role of early life risk factors. JAMA Pediatr 2013; 167(8):731–738. doi:10.1001/jamapediatrics.2013.85
- Powell-Wiley TM, Ayers CR, Banks-Richard K, et al. Disparities in counseling for lifestyle modification among obese adults: insights from the Dallas heart study. Obesity (Silver Spring) 2012; 20(4):849–855. doi:10.1038/oby.2011.242
- Fuchs FD. Why do black Americans have higher prevalence of hypertension?: an enigma still unsolved. Hypertension 2011; 57(3):379–380. doi:10.1161/HYPERTENSIONAHA.110.163196
- Ferdinand KC, Armani AM. The management of hypertension in African Americans. Crit Pathw Cardiol 2007; 6(2):67–71. doi:10.1097/HPC.0b013e318053da59
- Voors AW, Webber LS, Berenson GS. Time course study of blood pressure in children over a three-year period. Bogalusa Heart Study. Hypertension 1980; 2(4 Pt 2):102–108. pmid:7399641
- Carson AP, Howard G, Burke GL, Shea S, Levitan EB, Muntner P. Ethnic differences in hypertension incidence among middle-aged and older adults: the multi-ethnic study of atherosclerosis. Hypertension 2011; 57(6):1101–1107. doi:10.1161/HYPERTENSIONAHA.110.168005
- Gu A, Yue Y, Desai RP, Argulian E. Racial and ethnic differences in antihypertensive medication use and blood pressure control among US adults with hypertension: the National Health and Nutrition Examination Survey, 2003 to 2012. Circ Cardiovasc Qual Outcomes 2017; 10(1). pii:e003166. doi:10.1161/CIRCOUTCOMES.116.003166
- Lackland DT. Racial differences in hypertension: implications for high blood pressure management. Am J Med Sci 2014; 348(2):135–138. doi:10.1097/MAJ.0000000000000308
- Bosworth HB, Dudley T, Olsen MK, et al. Racial differences in blood pressure control: potential explanatory factors. Am J Med 2006; 119(1):70.e9–e15. doi:10.1016/j.amjmed.2005.08.019
- Peck RN, Smart LR, Beier R, et al. Difference in blood pressure response to ACE-Inhibitor monotherapy between black and white adults with arterial hypertension: a meta-analysis of 13 clinical trials. BMC Nephrol 2013; 14:201. doi:10.1186/1471-2369-14-201
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2018; 138(17):e484–e594. doi:10.1161/CIR.0000000000000596
- Peek ME, Cargill A, Huang ES. Diabetes health disparities: a systematic review of health care interventions. Med Care Res Rev 2007; 64(5 suppl):101S–156S. doi:10.1177/1077558707305409
- Narayan KM, Boyle JP, Geiss LS, Saaddine JB, Thompson TJ. Impact of recent increase in incidence on future diabetes burden: US, 2005–2050. Diabetes Care 2006; 29(9):2114–2116. doi:10.2337/dc06-1136
- Johnson PA, Lee TH, Cook EF, Rouan GW, Goldman L. Effect of race on the presentation and management of patients with acute chest pain. Ann Intern Med 1993; 118(8):593–601. pmid:8452325
- Hannan EL, van Ryn M, Burke J, et al. Access to coronary artery bypass surgery by race/ethnicity and gender among patients who are appropriate for surgery. Med Care 1999; 37(1):68–77. pmid:10413394
- Peterson ED, Shaw LK, DeLong ER, Pryor DB, Califf RM, Mark DB. Racial variation in the use of coronary-revascularization procedures. Are the differences real? Do they matter? N Engl J Med 1997; 336(7):480–486. doi:10.1056/NEJM199702133360706
- Wenneker MB, Epstein AM. Racial inequalities in the use of procedures for patients with ischemic heart disease in Massachusetts. JAMA 1989; 261(2):253–257. pmid:2521191
- Allison JJ, Kiefe CI, Centor RM, Box JB, Farmer RM. Racial differences in the medical treatment of elderly Medicare patients with acute myocardial infarction. J Gen Intern Med 1996; 11(12):736–743. pmid:9016420
- Mickelson JK, Blum CM, Geraci JM. Acute myocardial infarction: clinical characteristics, management and outcome in a metropolitan Veterans Affairs Medical Center teaching hospital. J Am Coll Cardiol 1997; 29(5):915–925. pmid:9120176
- Yarzebski J, Bujor CF, Lessard D, Gore JM, Goldberg RJ. Recent and temporal trends (1975 to 1999) in the treatment, hospital, and long-term outcomes of Hispanic and non-Hispanic white patients hospitalized with acute myocardial infarction: a population-based perspective. Am Heart J 2004; 147(4):690–697. doi:10.1016/j.ahj.2003.10.023
- Riley WJ. Health disparities: gaps in access, quality and affordability of medical care. Trans Am Clin Climatol Assoc 2012; 123:167–172. pmid:23303983
- Albert MA, Ayanian JZ, Silbaugh TS, et al. Early results of Massachusetts healthcare reform on racial, ethnic, and socioeconomic disparities in cardiovascular care. Circulation 2014; 129(24):2528–2538. doi:10.1161/CIRCULATIONAHA.113.005231
- Peterson ED, Wright SM, Daley J, Thibault GE. Racial variation in cardiac procedure use and survival following acute myocardial infarction in the Department of Veterans Affairs. JAMA 1994; 271(15):1175–1180. pmid:8151875
- Pearson-Stuttard J, Guzman-Castillo M, Penalvo JL, et al. Modeling future cardiovascular disease mortality in the United States: national trends and racial and ethnic disparities. Circulation 2016; 133(10):967–978. doi:10.1161/CIRCULATIONAHA.115.019904
- Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013; 6(3):606–619. doi:10.1161/HHF.0b013e318291329a
- Bahrami H, Kronmal R, Bluemke DA, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med 2008; 168(19):2138–2145. doi:10.1001/archinte.168.19.2138
- Parmar KR, Xiu PY, Chowdhury MR, Patel E, Cohen M. In-hospital treatment and outcomes of heart failure in specialist and non-specialist services: a retrospective cohort study in the elderly. Open Heart 2015; 2(1):e000095. doi:10.1136/openhrt-2014-000095
- Avaldi VM, Lenzi J, Urbinati S, et al. Effect of cardiologist care on 6-month outcomes in patients discharged with heart failure: results from an observational study based on administrative data. BMJ Open 2017; 7(11):e018243. doi:10.1136/bmjopen-2017-018243
- Breathett K, Liu WG, Allen LA, et al. African Americans are less likely to receive care by a cardiologist during an intensive care unit admission for heart failure. JACC Heart Fail 2018; 6(5):413–420. doi:10.1016/j.jchf.2018.02.015
- Sridhar AR, Yarlagadda V, Parasa S, et al. Cardiac resynchronization therapy: US trends and disparities in utilization and outcomes. Circ Arrhythm Electrophysiol 2016; 9(3):e003108. doi:10.1161/CIRCEP.115.003108
- Abraham WT, Fisher WG, Smith AL, et al; MIRACLE Study Group. Multicenter insync randomized clinical evaluation. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002; 346(24):1845–1853. doi:10.1056/NEJMoa013168
- Auricchio A, Stellbrink C, Sack S, et al; Pacing Therapies in Congestive Heart Failure (PATH-CHF) Study Group. Long-term clinical effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay. J Am Coll Cardiol 2002; 39(12):2026–2033. pmid:12084604
- Cazeau S, Leclercq C, Lavergne T, et al; Multisite Stimulation in Cardiomyopathies (MUSTIC) Study Investigators. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med 2001; 344(12):873–880. doi:10.1056/NEJM200103223441202
- Higgins SL, Hummel JD, Niazi IK, et al. Cardiac resynchronization therapy for the treatment of heart failure in patients with intraventricular conduction delay and malignant ventricular tachyarrhythmias. J Am Coll Cardiol 2003; 42(8):1454–1459. pmid:14563591
- Young JB, Abraham WT, Smith AL, et al; Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE ICD) Trial Investigators. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA 2003; 289(20):2685–2694. doi:10.1001/jama.289.20.2685
- Sutton MG, Plappert T, Hilpisch KE, Abraham WT, Hayes DL, Chinchoy E. Sustained reverse left ventricular structural remodeling with cardiac resynchronization at one year is a function of etiology: quantitative Doppler echocardiographic evidence from the Multicenter InSync Randomized Clinical Evaluation (MIRACLE). Circulation 2006; 113(2):266–272. doi:10.1161/CIRCULATIONAHA.104.520817
- Cleland JG, Daubert JC, Erdmann E, et al; Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005; 352(15):1539–1549. doi:10.1056/NEJMoa050496
- Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013; 128(16):e240–e327. doi:10.1161/CIR.0b013e31829e8776
- Farmer SA, Kirkpatrick JN, Heidenreich PA, Curtis JP, Wang Y, Groeneveld PW. Ethnic and racial disparities in cardiac resynchronization therapy. Heart Rhythm 2009; 6(3):325–331. doi:10.1016/j.hrthm.2008.12.018
- Rose EA, Gelijns AC, Moskowitz AJ, et al; Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001; 345(20):1435–1443. doi:10.1056/NEJMoa012175
- Slaughter MS, Rogers JG, Milano CA, et al; HeartMate II Investigators. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009; 361(23):2241–2251. doi:10.1056/NEJMoa0909938
- Tsiouris A, Brewer RJ, Borgi J, Nemeh H, Paone G, Morgan JA. Continuous-flow left ventricular assist device implantation as a bridge to transplantation or destination therapy: racial disparities in outcomes. J Heart Lung Transplant 2013; 2(3):299–304. doi:10.1016/j.healun.2012.11.017
- Stulak JM, Deo S, Cowger J, et al. Do racial and sex disparities exist in clinical characteristics and outcomes for patients undergoing left ventricular assist device implantation? J Heart Lung Transplant 2013; 32(45):S279–S280.
- Joyce DL, Conte JV, Russell SD, Joyce LD, Chang DC. Disparities in access to left ventricular assist device therapy. J Surg Res 2009; 152(1):111–117. doi:10.1016/j.jss.2008.02.065
- Breathett K, Allen LA, Helmkamp L, et al. Temporal trends in contemporary use of ventricular assist devices by race and ethnicity. Circ Heart Fail 2018; 11(8):e005008. doi:10.1161/CIRCHEARTFAILURE.118.005008
- Kilic A, Higgins RS, Whitson BA, Kilic A. Racial disparities in outcomes of adult heart transplantation. Circulation 2015; 131(10):882–889. doi:10.1161/CIRCULATIONAHA.114.011676
- Liu V, Bhattacharya J, Weill D, Hlatky MA. Persistent racial disparities in survival after heart transplantation. Circulation 2011; 123(15):1642–1649. doi:10.1161/CIRCULATIONAHA.110.976811
- Mahle WT, Kanter KR, Vincent RN. Disparities in outcome for black patients after pediatric heart transplantation. J Pediatr 2005; 147(6):739–743. doi:10.1016/j.jpeds.2005.07.018
- Park MH, Tolman DE, Kimball PM. The impact of race and HLA matching on long-term survival following cardiac transplantation. Transplant Proc 1997; 29(1–2):1460–1463. pmid:9123381
- Higgins RS, Fishman JA. Disparities in solid organ transplantation for ethnic minorities: facts and solutions. Am J Transplant 2006; 6(11):2556–2562. doi:10.1111/j.1600-6143.2006.01514.x
- Taylor AL, Wright JT Jr. Should ethnicity serve as the basis for clinical trial design? Importance of race/ethnicity in clinical trials: lessons from the African-American Heart Failure Trial (A-HeFT), the African-American Study of Kidney Disease and Hypertension (AASK), and the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Circulation 2005; 112(23):3654–3660. doi:10.1161/CIRCULATIONAHA.105.540443
- Yancy CW. Heart failure in African Americans: a cardiovascular engima. J Card Fail 2000; 6(3):183–186. pmid:10997742
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA 2003; 289(19):2560–2572. doi:10.1001/jama.289.19.2560
- Cohn JN, Archibald DG, Ziesche S, et al. Effect of vasodilator therapy on mortality in chronic congestive heart failure. Results of a Veterans Administration cooperative study. N Engl J Med 1986; 314(24):1547–1552. doi:10.1056/NEJM198606123142404
- Chen MS Jr, Lara PN, Dang JH, Paterniti DA, Kelly K. Twenty years post-NIH Revitalization Act: enhancing minority participation in clinical trials (EMPaCT): laying the groundwork for improving minority clinical trial accrual: renewing the case for enhancing minority participation in cancer clinical trials. Cancer 2014;120(suppl 7):1091–1096. doi:10.1002/cncr.28575
- Geller SE, Koch A, Pellettieri B, Carnes M. Inclusion, analysis, and reporting of sex and race/ethnicity in clinical trials: have we made progress? J Womens Health (Larchmt) 2011; 20(3):315–320. doi:10.1089/jwh.2010.2469
- Beta-Blocker Evaluation of Survival Trial Investigators; Eichhorn EJ, Domanski MJ, Krause-Steinrauf H, Bristow MR, Lavori PW. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med 2001; 344(22):1659–1667. doi:10.1056/NEJM200105313442202
- Taylor AL, Ziesche S, Yancy C, et al; African-American Heart Failure Trial Investigators. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med 2004; 351(20):2049–2057. doi:10.1056/NEJMoa042934
- Corbie-Smith G, Thomas SB, Williams MV, Moody-Ayers S. Attitudes and beliefs of African Americans toward participation in medical research. J Gen Intern Med 1999; 14(9):537–546. pmid:10491242
- Swanson GM, Ward AJ. Recruiting minorities into clinical trials: toward a participant-friendly system. J Natl Cancer Inst 1995; 87(23):1747–1759. doi:10.1093/jnci/87.23.1747
- Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care; Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: National Academies Press (US); 2003. https://www.ncbi.nlm.nih.gov/books/NBK220358/. Accessed May 13, 2019.
- Fisher JA, Kalbaugh CA. Challenging assumptions about minority participation in US clinical research. Am J Public Health 2011; 101(12):2217–2222. doi:10.2105/AJPH.2011.300279
- Hall WJ, Chapman MV, Lee KM, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. Am J Public Health 2015; 105(12):e60–e76. doi:10.2105/AJPH.2015.302903
- Biernat M, Manis M. Shifting standards and stereotype-based judgments. J Pers Soc Psychol 1994; 66(1):5–20. pmid:8126651
- Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med 2013; 28(11):1504–1510. doi:10.1007/s11606-013-2441-1
- FitzGerald C, Hurst S. Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics 2017; 18(1):19. doi:10.1186/s12910-017-0179-8
- van Ryn M, Burke J. The effect of patient race and socio-economic status on physicians’ perceptions of patients. Soc Sci Med 2000; 50(6):813–828. pmid:10695979
- Mayo RM, Sherrill WW, Sundareswaran P, Crew L. Attitudes and perceptions of Hispanic patients and health care providers in the treatment of Hispanic patients: a review of the literature. Hisp Health Care Int 2007; 5(2):64–72.
- Blair IV, Steiner JF, Havranek EP. Unconscious (implicit) bias and health disparities: where do we go from here? Perm J 2011; 15(2):71–78. pmid:21841929
- Green AR, Carney DR, Pallin DJ, et al. Implicit bias among physicians and its prediction of thrombolysis decisions for black and white patients. J Gen Intern Med 2007; 22(9):1231–1238. doi:10.1007/s11606-007-0258-5
- Sabin JA, Rivara FP, Greenwald AG. Physician implicit attitudes and stereotypes about race and quality of medical care. Med Care 2008; 46(7):678–685. doi:10.1097/MLR.0b013e3181653d58
- Smedley BD, Stith AY, Colburn L, et al; Institute of Medicine. The Right Thing to Do, The Smart Thing to Do: Enhancing Diversity in the Health Professions: Summary of the Symposium on Diversity in Health Professions in Honor of Herbert W. Nickens, MD. Washington, DC: National Academies Press; 2001. https://www.ncbi.nlm.nih.gov/books/NBK223633/. Accessed May 13, 2019.
- Chen J, Vargas-Bustamante A, Mortensen K, Ortega AN. Racial and ethnic disparities in health care access and utilization under the Affordable Care Act. Med Care 2016; 54(2):140–146. doi:10.1097/MLR.0000000000000467
- Saha S, Freeman M, Toure J, Tippens KM, Weeks C, Ibrahim S. Racial and ethnic disparities in the VA health care system: a systematic review. J Gen Intern Med 2008; 23(5):654–671. doi:10.1007/s11606-008-0521-4
- McCormick D, Sayah A, Lokko H, Woolhandler S, Nardin R. Access to care after Massachusetts’ health care reform: a safety net hospital patient survey. J Gen Intern Med 2012; 27(11):1548–1554. doi:10.1007/s11606-012-2173-7
- Burgos JL, Yee D, Csordas T, et al. Supporting the minority physician pipeline: providing global health experiences to undergraduate students in the United States-Mexico border region. Med Educ Online 2015; 20:27260. doi:10.3402/meo.v20.27260
- Traylor AH, Schmittdiel JA, Uratsu CS, Mangione CM, Subramanian U. The predictors of patient–physician race and ethnic concordance: a medical facility fixed-effects approach. Health Serv Res 2010; 45(3):792–805. doi:10.1111/j.1475-6773.2010.01086.x
- LaVeist TA, Nuru-Jeter A. Is doctor-patient race concordance associated with greater satisfaction with care? J Health Soc Behav 2002; 43(3):296–306. pmid:12467254
- Marrast LM, Zallman L, Woolhandler S, Bor DH, McCormick D. Minority physicians’ role in the care of underserved patients: diversifying the physician workforce may be key in addressing health disparities. JAMA Intern Med 2014; 174(2):289–291. doi:10.1001/jamainternmed.2013.12756
- Nabel EG, Braunwald E. A tale of coronary artery disease and myocardial infarction. N Engl J Med 2012; 366(1):54–63. doi:10.1056/NEJMra1112570
- Braunwald E. Evolution of the management of acute myocardial infarction: a 20th century saga. Lancet 1998; 352(9142):1771–1774. doi:10.1016/S0140-6736(98)03212-7
- Julian DG. The history of coronary care units. Br Heart J 1987; 57(6):497–502. doi:10.1136/hrt.57.6.497
- Caswell JE. A brief history of coronary care units. Public Health Rep 1967; 82(12):1105–1111. pmid:19316519
- Day HW. History of coronary care units. Am J Cardiol 1972; 30(4):405–407. pmid:4560377
- Braunwald E. Shattuck lecture—cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med 1997; 337(19):1360–1369. doi:10.1056/NEJM199711063371906
- Mozaffarian D, Benjamin EJ, Go AS, et al; American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation 2016; 133(4):e38–e360. doi:10.1161/CIR.0000000000000350
- Nelson A. Unequal treatment: confronting racial and ethnic disparities in health care. J Natl Med Assoc 2002; 94(8):666–668. pmid:12152921
- McGuire TG, Alegria M, Cook BL, Wells KB, Zaslavsky AM. Implementing the Institute of Medicine definition of disparities: an application to mental health care. Health Serv Res 2006; 41(5):1979–2005. doi:10.1111/j.1475-6773.2006.00583.x
- Stone CT, Vanzant FR. Heart disease as seen in a southern clinic: a clinical and pathologic survey. JAMA 1927; 89(18):1473–1480. doi:10.1001/jama.1927.02690180005002
- Centers For Disease Control And Prevention (CDC). Vital signs: avoidable deaths from heart disease, stroke, and hypertensive disease—United States, 2001–2010. MMWR Morb Mortal Wkly Rep 2013; 62(35):727–727. pmid:PMC4585625
- Mozaffarian D, Benjamin EJ, Go AS, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 2015; 131(4):e29–e322. doi:10.1161/CIR.0000000000000152
- Ockene IS, Miller NH. Cigarette smoking, cardiovascular disease, and stroke: a statement for healthcare professionals from the American Heart Association. American Heart Association Task Force on Risk Reduction. Circulation 1997; 96(9):3243–3247. pmid:9386200
- Messner B, Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol 2014; 34(3):509–515. doi:10.1161/ATVBAHA.113.300156
- Benjamin EJ, Virani SS, Callaway CW, et al; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation 2018; 137(12):e67–e492. doi:10.1161/CIR.0000000000000558
- Jamal A, Homa DM, O’Conner E, et al. Current cigarette smoking among adults—United States, 2005–2014. MMWR 2015; 64(44):1233–1240. doi:10.15585/mmwr.mm6444a2
- Myers J. Cardiology patient pages. Exercise and cardiovascular health. Circulation 2003; 107(1):e2–e5. pmid:12515760
- Shiroma EJ, Lee IM. Physical activity and cardiovascular health: lessons learned from epidemiological studies across age, gender, and race/ethnicity. Circulation 2010; 122(7):743–752. doi:10.1161/CIRCULATIONAHA.109.914721
- Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA 2018; 320(19):2020–2028. doi:10.1001/jama.2018.14854
- Omura JD, Carlson SA, Paul P, et al. Adults eligible for cardiovascular disease prevention counseling and participation in aerobic physical activity—United States, 2013. MMWR 2015; 64(37):1047–1051. doi:10.15585/mmwr.mm6437a4
- Dunstan DW, Barr EL, Healy GN, et al. Television viewing time and mortality: the Australian diabetes, obesity and lifestyle study (AusDiab). Circulation 2010; 121(3):384–391. doi:10.1161/CIRCULATIONAHA.109.894824
- Warren TY, Barry V, Hooker SP, Sui X, Church TS, Blair SN. Sedentary behaviors increase risk of cardiovascular disease mortality in men. Med Sci Sports Exerc 2010; 42(5):879–885. doi:10.1249/MSS.0b013e3181c3aa7e
- Byun W, Dowda M, Pate RR. Associations between screen-based sedentary behavior and cardiovascular disease risk factors in Korean youth. J Korean Med Sci 2012; 27(4):388–394. doi:10.3346/jkms.2012.27.4.388
- Getz GS, Reardon CA. Nutrition and cardiovascular disease. Arterioscler Thromb Vasc Biol 2007; 27(12):2499–2506. doi:10.1161/ATVBAHA.107.155853
- Eckel RH. Obesity and heart disease: a statement for healthcare professionals from the Nutrition Committee, American Heart Association. Circulation 1997; 96(9):3248–3250. pmid:9386201
- Rimm EB, Stampfer MJ, Giovannucci E, et al. Body size and fat distribution as predictors of coronary heart disease among middle-aged and older US men. Am J Epidemiol 1995; 141(12):1117–1127. pmid:7771450
- Duflou J, Virmani R, Rabin I, Burke A, Farb A, Smialek J. Sudden death as a result of heart disease in morbid obesity. Am Heart J 1995; 130(2):306–313. pmid:7631612
- Poirier P, Giles TD, Bray GA, et al; American Heart Association; Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006; 113(6):898–918. doi:10.1161/CIRCULATIONAHA.106.171016
- Wang Y. Disparities in pediatric obesity in the United States. Adv Nutr 2011; 2(1):23–31. doi:10.3945/an.110.000083
- Taveras EM, Gillman MW, Kleinman KP, Rich-Edwards JW, Rifas-Shiman SL. Reducing racial/ethnic disparities in childhood obesity: the role of early life risk factors. JAMA Pediatr 2013; 167(8):731–738. doi:10.1001/jamapediatrics.2013.85
- Powell-Wiley TM, Ayers CR, Banks-Richard K, et al. Disparities in counseling for lifestyle modification among obese adults: insights from the Dallas heart study. Obesity (Silver Spring) 2012; 20(4):849–855. doi:10.1038/oby.2011.242
- Fuchs FD. Why do black Americans have higher prevalence of hypertension?: an enigma still unsolved. Hypertension 2011; 57(3):379–380. doi:10.1161/HYPERTENSIONAHA.110.163196
- Ferdinand KC, Armani AM. The management of hypertension in African Americans. Crit Pathw Cardiol 2007; 6(2):67–71. doi:10.1097/HPC.0b013e318053da59
- Voors AW, Webber LS, Berenson GS. Time course study of blood pressure in children over a three-year period. Bogalusa Heart Study. Hypertension 1980; 2(4 Pt 2):102–108. pmid:7399641
- Carson AP, Howard G, Burke GL, Shea S, Levitan EB, Muntner P. Ethnic differences in hypertension incidence among middle-aged and older adults: the multi-ethnic study of atherosclerosis. Hypertension 2011; 57(6):1101–1107. doi:10.1161/HYPERTENSIONAHA.110.168005
- Gu A, Yue Y, Desai RP, Argulian E. Racial and ethnic differences in antihypertensive medication use and blood pressure control among US adults with hypertension: the National Health and Nutrition Examination Survey, 2003 to 2012. Circ Cardiovasc Qual Outcomes 2017; 10(1). pii:e003166. doi:10.1161/CIRCOUTCOMES.116.003166
- Lackland DT. Racial differences in hypertension: implications for high blood pressure management. Am J Med Sci 2014; 348(2):135–138. doi:10.1097/MAJ.0000000000000308
- Bosworth HB, Dudley T, Olsen MK, et al. Racial differences in blood pressure control: potential explanatory factors. Am J Med 2006; 119(1):70.e9–e15. doi:10.1016/j.amjmed.2005.08.019
- Peck RN, Smart LR, Beier R, et al. Difference in blood pressure response to ACE-Inhibitor monotherapy between black and white adults with arterial hypertension: a meta-analysis of 13 clinical trials. BMC Nephrol 2013; 14:201. doi:10.1186/1471-2369-14-201
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2018; 138(17):e484–e594. doi:10.1161/CIR.0000000000000596
- Peek ME, Cargill A, Huang ES. Diabetes health disparities: a systematic review of health care interventions. Med Care Res Rev 2007; 64(5 suppl):101S–156S. doi:10.1177/1077558707305409
- Narayan KM, Boyle JP, Geiss LS, Saaddine JB, Thompson TJ. Impact of recent increase in incidence on future diabetes burden: US, 2005–2050. Diabetes Care 2006; 29(9):2114–2116. doi:10.2337/dc06-1136
- Johnson PA, Lee TH, Cook EF, Rouan GW, Goldman L. Effect of race on the presentation and management of patients with acute chest pain. Ann Intern Med 1993; 118(8):593–601. pmid:8452325
- Hannan EL, van Ryn M, Burke J, et al. Access to coronary artery bypass surgery by race/ethnicity and gender among patients who are appropriate for surgery. Med Care 1999; 37(1):68–77. pmid:10413394
- Peterson ED, Shaw LK, DeLong ER, Pryor DB, Califf RM, Mark DB. Racial variation in the use of coronary-revascularization procedures. Are the differences real? Do they matter? N Engl J Med 1997; 336(7):480–486. doi:10.1056/NEJM199702133360706
- Wenneker MB, Epstein AM. Racial inequalities in the use of procedures for patients with ischemic heart disease in Massachusetts. JAMA 1989; 261(2):253–257. pmid:2521191
- Allison JJ, Kiefe CI, Centor RM, Box JB, Farmer RM. Racial differences in the medical treatment of elderly Medicare patients with acute myocardial infarction. J Gen Intern Med 1996; 11(12):736–743. pmid:9016420
- Mickelson JK, Blum CM, Geraci JM. Acute myocardial infarction: clinical characteristics, management and outcome in a metropolitan Veterans Affairs Medical Center teaching hospital. J Am Coll Cardiol 1997; 29(5):915–925. pmid:9120176
- Yarzebski J, Bujor CF, Lessard D, Gore JM, Goldberg RJ. Recent and temporal trends (1975 to 1999) in the treatment, hospital, and long-term outcomes of Hispanic and non-Hispanic white patients hospitalized with acute myocardial infarction: a population-based perspective. Am Heart J 2004; 147(4):690–697. doi:10.1016/j.ahj.2003.10.023
- Riley WJ. Health disparities: gaps in access, quality and affordability of medical care. Trans Am Clin Climatol Assoc 2012; 123:167–172. pmid:23303983
- Albert MA, Ayanian JZ, Silbaugh TS, et al. Early results of Massachusetts healthcare reform on racial, ethnic, and socioeconomic disparities in cardiovascular care. Circulation 2014; 129(24):2528–2538. doi:10.1161/CIRCULATIONAHA.113.005231
- Peterson ED, Wright SM, Daley J, Thibault GE. Racial variation in cardiac procedure use and survival following acute myocardial infarction in the Department of Veterans Affairs. JAMA 1994; 271(15):1175–1180. pmid:8151875
- Pearson-Stuttard J, Guzman-Castillo M, Penalvo JL, et al. Modeling future cardiovascular disease mortality in the United States: national trends and racial and ethnic disparities. Circulation 2016; 133(10):967–978. doi:10.1161/CIRCULATIONAHA.115.019904
- Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013; 6(3):606–619. doi:10.1161/HHF.0b013e318291329a
- Bahrami H, Kronmal R, Bluemke DA, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med 2008; 168(19):2138–2145. doi:10.1001/archinte.168.19.2138
- Parmar KR, Xiu PY, Chowdhury MR, Patel E, Cohen M. In-hospital treatment and outcomes of heart failure in specialist and non-specialist services: a retrospective cohort study in the elderly. Open Heart 2015; 2(1):e000095. doi:10.1136/openhrt-2014-000095
- Avaldi VM, Lenzi J, Urbinati S, et al. Effect of cardiologist care on 6-month outcomes in patients discharged with heart failure: results from an observational study based on administrative data. BMJ Open 2017; 7(11):e018243. doi:10.1136/bmjopen-2017-018243
- Breathett K, Liu WG, Allen LA, et al. African Americans are less likely to receive care by a cardiologist during an intensive care unit admission for heart failure. JACC Heart Fail 2018; 6(5):413–420. doi:10.1016/j.jchf.2018.02.015
- Sridhar AR, Yarlagadda V, Parasa S, et al. Cardiac resynchronization therapy: US trends and disparities in utilization and outcomes. Circ Arrhythm Electrophysiol 2016; 9(3):e003108. doi:10.1161/CIRCEP.115.003108
- Abraham WT, Fisher WG, Smith AL, et al; MIRACLE Study Group. Multicenter insync randomized clinical evaluation. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002; 346(24):1845–1853. doi:10.1056/NEJMoa013168
- Auricchio A, Stellbrink C, Sack S, et al; Pacing Therapies in Congestive Heart Failure (PATH-CHF) Study Group. Long-term clinical effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay. J Am Coll Cardiol 2002; 39(12):2026–2033. pmid:12084604
- Cazeau S, Leclercq C, Lavergne T, et al; Multisite Stimulation in Cardiomyopathies (MUSTIC) Study Investigators. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med 2001; 344(12):873–880. doi:10.1056/NEJM200103223441202
- Higgins SL, Hummel JD, Niazi IK, et al. Cardiac resynchronization therapy for the treatment of heart failure in patients with intraventricular conduction delay and malignant ventricular tachyarrhythmias. J Am Coll Cardiol 2003; 42(8):1454–1459. pmid:14563591
- Young JB, Abraham WT, Smith AL, et al; Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE ICD) Trial Investigators. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA 2003; 289(20):2685–2694. doi:10.1001/jama.289.20.2685
- Sutton MG, Plappert T, Hilpisch KE, Abraham WT, Hayes DL, Chinchoy E. Sustained reverse left ventricular structural remodeling with cardiac resynchronization at one year is a function of etiology: quantitative Doppler echocardiographic evidence from the Multicenter InSync Randomized Clinical Evaluation (MIRACLE). Circulation 2006; 113(2):266–272. doi:10.1161/CIRCULATIONAHA.104.520817
- Cleland JG, Daubert JC, Erdmann E, et al; Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005; 352(15):1539–1549. doi:10.1056/NEJMoa050496
- Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013; 128(16):e240–e327. doi:10.1161/CIR.0b013e31829e8776
- Farmer SA, Kirkpatrick JN, Heidenreich PA, Curtis JP, Wang Y, Groeneveld PW. Ethnic and racial disparities in cardiac resynchronization therapy. Heart Rhythm 2009; 6(3):325–331. doi:10.1016/j.hrthm.2008.12.018
- Rose EA, Gelijns AC, Moskowitz AJ, et al; Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001; 345(20):1435–1443. doi:10.1056/NEJMoa012175
- Slaughter MS, Rogers JG, Milano CA, et al; HeartMate II Investigators. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009; 361(23):2241–2251. doi:10.1056/NEJMoa0909938
- Tsiouris A, Brewer RJ, Borgi J, Nemeh H, Paone G, Morgan JA. Continuous-flow left ventricular assist device implantation as a bridge to transplantation or destination therapy: racial disparities in outcomes. J Heart Lung Transplant 2013; 2(3):299–304. doi:10.1016/j.healun.2012.11.017
- Stulak JM, Deo S, Cowger J, et al. Do racial and sex disparities exist in clinical characteristics and outcomes for patients undergoing left ventricular assist device implantation? J Heart Lung Transplant 2013; 32(45):S279–S280.
- Joyce DL, Conte JV, Russell SD, Joyce LD, Chang DC. Disparities in access to left ventricular assist device therapy. J Surg Res 2009; 152(1):111–117. doi:10.1016/j.jss.2008.02.065
- Breathett K, Allen LA, Helmkamp L, et al. Temporal trends in contemporary use of ventricular assist devices by race and ethnicity. Circ Heart Fail 2018; 11(8):e005008. doi:10.1161/CIRCHEARTFAILURE.118.005008
- Kilic A, Higgins RS, Whitson BA, Kilic A. Racial disparities in outcomes of adult heart transplantation. Circulation 2015; 131(10):882–889. doi:10.1161/CIRCULATIONAHA.114.011676
- Liu V, Bhattacharya J, Weill D, Hlatky MA. Persistent racial disparities in survival after heart transplantation. Circulation 2011; 123(15):1642–1649. doi:10.1161/CIRCULATIONAHA.110.976811
- Mahle WT, Kanter KR, Vincent RN. Disparities in outcome for black patients after pediatric heart transplantation. J Pediatr 2005; 147(6):739–743. doi:10.1016/j.jpeds.2005.07.018
- Park MH, Tolman DE, Kimball PM. The impact of race and HLA matching on long-term survival following cardiac transplantation. Transplant Proc 1997; 29(1–2):1460–1463. pmid:9123381
- Higgins RS, Fishman JA. Disparities in solid organ transplantation for ethnic minorities: facts and solutions. Am J Transplant 2006; 6(11):2556–2562. doi:10.1111/j.1600-6143.2006.01514.x
- Taylor AL, Wright JT Jr. Should ethnicity serve as the basis for clinical trial design? Importance of race/ethnicity in clinical trials: lessons from the African-American Heart Failure Trial (A-HeFT), the African-American Study of Kidney Disease and Hypertension (AASK), and the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Circulation 2005; 112(23):3654–3660. doi:10.1161/CIRCULATIONAHA.105.540443
- Yancy CW. Heart failure in African Americans: a cardiovascular engima. J Card Fail 2000; 6(3):183–186. pmid:10997742
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA 2003; 289(19):2560–2572. doi:10.1001/jama.289.19.2560
- Cohn JN, Archibald DG, Ziesche S, et al. Effect of vasodilator therapy on mortality in chronic congestive heart failure. Results of a Veterans Administration cooperative study. N Engl J Med 1986; 314(24):1547–1552. doi:10.1056/NEJM198606123142404
- Chen MS Jr, Lara PN, Dang JH, Paterniti DA, Kelly K. Twenty years post-NIH Revitalization Act: enhancing minority participation in clinical trials (EMPaCT): laying the groundwork for improving minority clinical trial accrual: renewing the case for enhancing minority participation in cancer clinical trials. Cancer 2014;120(suppl 7):1091–1096. doi:10.1002/cncr.28575
- Geller SE, Koch A, Pellettieri B, Carnes M. Inclusion, analysis, and reporting of sex and race/ethnicity in clinical trials: have we made progress? J Womens Health (Larchmt) 2011; 20(3):315–320. doi:10.1089/jwh.2010.2469
- Beta-Blocker Evaluation of Survival Trial Investigators; Eichhorn EJ, Domanski MJ, Krause-Steinrauf H, Bristow MR, Lavori PW. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med 2001; 344(22):1659–1667. doi:10.1056/NEJM200105313442202
- Taylor AL, Ziesche S, Yancy C, et al; African-American Heart Failure Trial Investigators. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med 2004; 351(20):2049–2057. doi:10.1056/NEJMoa042934
- Corbie-Smith G, Thomas SB, Williams MV, Moody-Ayers S. Attitudes and beliefs of African Americans toward participation in medical research. J Gen Intern Med 1999; 14(9):537–546. pmid:10491242
- Swanson GM, Ward AJ. Recruiting minorities into clinical trials: toward a participant-friendly system. J Natl Cancer Inst 1995; 87(23):1747–1759. doi:10.1093/jnci/87.23.1747
- Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care; Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: National Academies Press (US); 2003. https://www.ncbi.nlm.nih.gov/books/NBK220358/. Accessed May 13, 2019.
- Fisher JA, Kalbaugh CA. Challenging assumptions about minority participation in US clinical research. Am J Public Health 2011; 101(12):2217–2222. doi:10.2105/AJPH.2011.300279
- Hall WJ, Chapman MV, Lee KM, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. Am J Public Health 2015; 105(12):e60–e76. doi:10.2105/AJPH.2015.302903
- Biernat M, Manis M. Shifting standards and stereotype-based judgments. J Pers Soc Psychol 1994; 66(1):5–20. pmid:8126651
- Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med 2013; 28(11):1504–1510. doi:10.1007/s11606-013-2441-1
- FitzGerald C, Hurst S. Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics 2017; 18(1):19. doi:10.1186/s12910-017-0179-8
- van Ryn M, Burke J. The effect of patient race and socio-economic status on physicians’ perceptions of patients. Soc Sci Med 2000; 50(6):813–828. pmid:10695979
- Mayo RM, Sherrill WW, Sundareswaran P, Crew L. Attitudes and perceptions of Hispanic patients and health care providers in the treatment of Hispanic patients: a review of the literature. Hisp Health Care Int 2007; 5(2):64–72.
- Blair IV, Steiner JF, Havranek EP. Unconscious (implicit) bias and health disparities: where do we go from here? Perm J 2011; 15(2):71–78. pmid:21841929
- Green AR, Carney DR, Pallin DJ, et al. Implicit bias among physicians and its prediction of thrombolysis decisions for black and white patients. J Gen Intern Med 2007; 22(9):1231–1238. doi:10.1007/s11606-007-0258-5
- Sabin JA, Rivara FP, Greenwald AG. Physician implicit attitudes and stereotypes about race and quality of medical care. Med Care 2008; 46(7):678–685. doi:10.1097/MLR.0b013e3181653d58
- Smedley BD, Stith AY, Colburn L, et al; Institute of Medicine. The Right Thing to Do, The Smart Thing to Do: Enhancing Diversity in the Health Professions: Summary of the Symposium on Diversity in Health Professions in Honor of Herbert W. Nickens, MD. Washington, DC: National Academies Press; 2001. https://www.ncbi.nlm.nih.gov/books/NBK223633/. Accessed May 13, 2019.
- Chen J, Vargas-Bustamante A, Mortensen K, Ortega AN. Racial and ethnic disparities in health care access and utilization under the Affordable Care Act. Med Care 2016; 54(2):140–146. doi:10.1097/MLR.0000000000000467
- Saha S, Freeman M, Toure J, Tippens KM, Weeks C, Ibrahim S. Racial and ethnic disparities in the VA health care system: a systematic review. J Gen Intern Med 2008; 23(5):654–671. doi:10.1007/s11606-008-0521-4
- McCormick D, Sayah A, Lokko H, Woolhandler S, Nardin R. Access to care after Massachusetts’ health care reform: a safety net hospital patient survey. J Gen Intern Med 2012; 27(11):1548–1554. doi:10.1007/s11606-012-2173-7
- Burgos JL, Yee D, Csordas T, et al. Supporting the minority physician pipeline: providing global health experiences to undergraduate students in the United States-Mexico border region. Med Educ Online 2015; 20:27260. doi:10.3402/meo.v20.27260
- Traylor AH, Schmittdiel JA, Uratsu CS, Mangione CM, Subramanian U. The predictors of patient–physician race and ethnic concordance: a medical facility fixed-effects approach. Health Serv Res 2010; 45(3):792–805. doi:10.1111/j.1475-6773.2010.01086.x
- LaVeist TA, Nuru-Jeter A. Is doctor-patient race concordance associated with greater satisfaction with care? J Health Soc Behav 2002; 43(3):296–306. pmid:12467254
- Marrast LM, Zallman L, Woolhandler S, Bor DH, McCormick D. Minority physicians’ role in the care of underserved patients: diversifying the physician workforce may be key in addressing health disparities. JAMA Intern Med 2014; 174(2):289–291. doi:10.1001/jamainternmed.2013.12756
KEY POINTS
- Although avoidable deaths from heart disease, stroke, and hypertensive disease have declined overall, African Americans still have a higher mortality rate than other racial and ethnic groups.
- The prevalence of modifiable risk factors for cardiovascular disease is higher in African Americans than in the general US population.
- Disparities in care exist and may persist even with equal access to care.
- Since 1993, studies funded by the National Institutes of Health must include minorities that were historically underrepresented in clinical research trials.
- Solutions to disparities will need to eliminate healthcare bias, increase patient access, and increase diversity and inclusion in the physician work force.
- Cardiovascular disease makes no distinction in race, sex, age, or socioeconomic status, and neither should the medical community.
Liposomal Bupivacaine vs Interscalene Nerve Block for Pain Control After Shoulder Arthroplasty: A Retrospective Cohort Analysis
The annual number of total shoulder arthroplasties (TSAs) is rising with the growing elderly population and development of new technologies such as reverse shoulder arthroplasty.1 In 2008, 47,000 shoulder arthroplasties were performed in the US compared with 19,000 in 1998.1 As of 2011, there were 53,000 shoulder arthroplasties performed annually.2 Pain control after shoulder procedures, particularly TSA, is challenging. 3
Several modalities exist to manage pain after shoulder arthroplasty. The interscalene brachial plexus nerve block is considered the “gold standard” for shoulder analgesia. A new approach is the periarticular injection method, in which the surgeon administers a local anesthetic intraoperatively. Liposomal bupivacaine (Exparel, Pacira Pharmaceuticals, Inc.) is a nonopioid anesthetic that has been shown to improve pain control, shorten hospital stays, and decrease costs for total knee and hip arthroplasty compared with nerve blocks.4-6 Patients who were treated with liposomal bupivacaine consumed less opioid medication than a placebo group.7
Our purpose was to compare intraoperative local liposomal bupivacaine injection with preoperative single-shot interscalene nerve block (ISNB) in terms of pain control, opioid use, and length of hospital stay (LOS) after shoulder arthroplasty. We hypothesized that patients in the liposomal bupivacaine group would have lower pain scores, less opioid use, and shorter LOS compared with patients in the ISNB group.
Methods
A retrospective cohort analysis was conducted with 58 patients who underwent shoulder arthroplasty by 1 surgeon at our academically affiliated community hospital from January 2012 through January 2015. ISNBs were the standard at the beginning of the study period and were used until Exparel became available on the hospital formulary in 2013. We began using Exparel for all shoulder arthroplasties in November 2013. No other changes were made in the perioperative management of our arthroplasty patients during this period. Patients who underwent TSA, reverse TSA, or hemiarthroplasty of the shoulder were included. Patients who underwent revision TSA were excluded. Twenty-one patients received ISNBs and 37 received liposomal bupivacaine injections. This study was approved by our Institutional Review Board.
Baseline data for each patient were age, sex, body mass index, and the American Society of Anesthesiologists (ASA) Physical Status Classification. The primary outcome measure was the numeric rating scale (NRS) pain score at 4 post-operative time intervals. The NRS pain score has a range of 0 to 10, with 10 representing severe pain. Data were gathered from nursing and physical therapy notes in patient charts. The postoperative time intervals were 0 to 1 hour, 8 to 14 hours, 18 to 24 hours, and 27 to 36 hours. Available NRS scores for these time intervals were averaged. Patients were included if they had pain scores for at least 3 of the postoperative time intervals documented in their charts. Secondary outcome measures were LOS and opioid consumption during hospital admission. Intravenous acetaminophen use was also measured in both groups. All data on opioids were converted to oral morphine equivalents using the method described by Schneider and colleagues.8
A board-certified, fellowship-trained anesthesiologist, experienced in regional anesthesia, administered the single-shot ISNB before surgery. The block was administered under ultrasound guidance using a 44-mm, 22-gauge needle with the patient in the supine position. No indwelling catheter was used. The medication consisted of 30 mL of 5% ropivacaine (5 mg/mL). The surgeon injected liposomal bupivacaine (266 mg diluted into 40 mL of injectable saline) near the end of the procedure throughout the pericapsular area and multiple layers of the wound, per manufacturer guidelines.9 A 60-mL syringe with a 20-gauge needle was used. All operations were performed by 1 board-certified, fellowship-trained surgeon using a standard deltopectoral approach with the same surgical equipment. The same postoperative pain protocol was used for all patients, including intravenous acetaminophen and patient-controlled analgesia. Additional oral pain medication was provided as needed for all patients. Physical therapy protocols were identical between groups.
Statistical Analysis
Mean patient ages in the 2 treatment groups were compared using the Student t test. Sex distribution and the ASA scores were compared using a χ2 test and a Fisher exact test, respectively. Arthroplasty types were compared using a Fisher exact test. The medians and interquartile ranges of the NRS scores at each time point measured were tabulated by treatment group, and at each time point the difference between groups was tested using nonparametric rank sum tests.
We tested the longitudinal trajectory of NRS scores over time, accounting for repeated measurements in the same patients using linear mixed model analysis. Treatment group, time period as a categorical variable, and the interaction between treatment and time period were included as fixed effects, and patient identification number was included as the random effect. An initial omnibus test was performed for all treatment and treatment-by-time interaction effects. Subsequently, the treatment-by-time interaction was tested for each of the time periods. The association of day of discharge (as a categorical variable) with treatment was tested using the Fisher exact test. All analyses were conducted using Stata, version 13, software (StataCorp LP). P values <.05 were considered significant.
Sample Size Analysis
We calculated the minimum detectable effect size with 80% power at an alpha level of 0.05 for the nonparametric rank sum test in terms of the proportion of every possible pair of patients treated with the 2 treatments, where the patient treated with liposomal bupivacaine has a lower pain score than the patient treated with ISNB. For pain score at 18 to 24 hours, the sample sizes of 33 patients treated with liposomal bupivacaine and 20 treated with ISNB, the minimum detectable effect size is 73%.
Results
Fifty-eight patient charts (21 in the ISNB group and 37 in the liposomal bupivacaine group) were reviewed for the study. Patient sex distribution, mean age, mean body mass index, and mean baseline ASA scores were not statistically different (Table 1).
The primary outcome measure, NRS pain score, showed no significant differences between groups at 0 to 1 hour after surgery (P = .99) or 8 to 14 hours after surgery (P = .208).
There was no difference in the amount of intravenous acetaminophen given during the hospital stay between groups. There was no significant difference in opioid consumption on postoperative day 1 in the hospital (P = .59) (Figure 2). However, there were significant differences between groups on postoperative days 2 and 3.
Sixteen of 37 patients in the liposomal bupivacaine group and 2 of 21 in the ISNB group were discharged on the day after surgery (P = .010) (Table 3).
There were no major cardiac or respiratory events in either group. No long-term paresthesias or neuropathies were noted. There were no readmissions for either group.
Discussion
Postoperative pain control after shoulder arthroplasty can be challenging, and several modalities have been tried in various combinations to minimize pain and decrease adverse effects of opioid medications. The most common method for pain relief after shoulder arthroplasty is the ISNB. Several studies of ISNBs have shown improved pain control after shoulder arthroplasty with associated decreased opioid consumption and related side effects.10 Patient rehabilitation and satisfaction have improved with the increasing use of peripheral nerve blocks.11
Despite the well-established benefits of ISNBs, several limitations exist. Although the superior portion of the shoulder is well covered by an ISNB, the inferior portion of the brachial plexus can remain uncovered or only partially covered.12 Complications of ISNBs include hemidiaphragmatic paresis, rebound pain 24 hours after surgery,13 chronic neurologic complications,14 and substantial respiratory and cardiovascular events.15 Nerve blocks also require additional time and resources in the perioperative period, including an anesthesiologist with specialized training, assistants, and ultrasonography or nerve stimulation equipment contraindicated in patients taking blood thinners.16
Periarticular injections of local anesthetics have also shown promise in reducing pain after arthroplasty.4 Benefits include an enhanced safety profile because local injection avoids the concurrent blockade of the phrenic nerve and recurrent laryngeal nerve and has not been associated with the risk of peripheral neuropathies. Further, local injection is a simple technique that can be performed during surgery without additional personnel or expertise. A limitation of this approach is the relatively short duration of effectiveness of the local anesthetic and uncertainty regarding the best agent and the ideal volume of injection.6 Liposomal bupivacaine is a new agent (approved by the US Food and Drug Administration in 201117) with a sustained release over 72 to 96 hours.18 The most common adverse effects of liposomal bupivacaine are nausea, vomiting, constipation, pyrexia, dizziness, and headache.19 Chondrotoxicity and granulomatous inflammation are more serious, yet rare, complications of liposomal bupivacaine.20
We found that liposomal bupivacaine injections were associated with lower pain scores compared with ISNB at 18 to 24 hours after surgery. This correlated with less opioid consumption in the liposomal bupivacaine group than in the ISNB group on the second postoperative day. These differences in pain values are consistent with the known pharmacokinetics of liposomal bupivacaine.18 Peak plasma levels normally occur approximately 24 hours after injection, leaving the early postoperative period relatively uncovered by anesthetic agent. This finding of relatively poor pain control early after surgery has also been noted in patients undergoing knee arthroplasty.5 On the basis of the findings of this study, we have added standard bupivacaine injections to our separate liposomal bupivacaine injection to cover early postoperative pain. Opioid consumption was significantly lower in the liposomal bupivacaine group than in the ISNB group on postoperative days 2 and 3. We did not measure adverse events related to opioid consumption, so we cannot comment on whether the decreased opioid consumption was associated with the rate of adverse events. However, other studies21,22 have established this relationship.
We found the liposomal bupivacaine group to have earlier discharges to home. Sixteen of 37 patients in the liposomal bupivacaine group compared with 2 of 21 patients in the ISNB group were discharged on the day after surgery. A mean reduction in LOS of 18 hours for the liposomal bupivacaine group was statistically significant (P = .012). This reduction in LOS has important implications for hospitals and value analysis committees considering whether to keep a new, more expensive local anesthetic on formulary. Savings from reduced LOS and improvements in patient satisfaction may justify the expense (approximately $300 per 266-mg vial) of Exparel.
From a societal cost perspective, liposomal bupivacaine is more economical compared with ISNB, which adds approximately $1500 to the cost of anesthesia per patient.23 Eliminating the costs associated with ISNB administration in shoulder arthroplasties could result in substantial savings to our healthcare system. More research examining time savings and exact costs of each procedure is needed to determine the true cost effectiveness of each approach.
Limitations of our study include the retrospective design, relatively small numbers of patients in each group, missing data for some patients at various time points, variation in the types of procedures in each group, and lack of long-term outcome measures. It is important to note that we did not confirm the success of the nerve block after administration. However, this study reflects the effectiveness of each of the modalities in actual clinical conditions (as opposed to a controlled experimental setting). The actual effectiveness of a nerve block varies, even when performed by an experienced anesthesiologist with ultrasound guidance. Furthermore, immediate postoperative pain scores in the nerve block group are consistent with those of prior research reporting pain values ranging from 4 to 5 and a mean duration of effect ranging from 9 to 14 hours.23,24 Additionally, the patients, surgeon, and nursing team were not blinded to the treatment group. Although we did note a significant difference in the types of procedures between groups, this finding is related to the greater number of hemiarthroplasties performed in the ISNB group (N = 5) compared with the liposomal group (N = 1). Because of this variation and the decreased invasiveness of hemiarthroplasties, the bias is against the liposomal group. Finally, our primary outcome variable was pain, which is a subjective, self-reported measure. However, our opioid consumption data and LOS data corroborate the improved pain scores in the liposomal bupivacaine group.
Limiting the study to a single surgeon may limit external validity. Another limitation is the lack of data on adverse events related to opioid medication use. There was no additional experimental group to determine whether less expensive local anesthetics injected locally would perform similarly to liposomal bupivacaine. In total knee arthroplasty, periarticular injections of liposomal bupivacaine were not as effective as less expensive periarticular injections.25 It is unclear which agents (and in what doses or combinations) should be used for periarticular injections. Finally, we acknowledge that our retrospective study design cannot account for all potential factors affecting discharge time.
This is the first comparative study of liposomal bupivacaine and ISNB in TSA. The study design allowed us to control for variables such as surgical technique, postoperative protocols (including use and type of sling), and use of other pain modalities such as patient-controlled analgesia and intravenous acetaminophen that are likely to affect postoperative pain and LOS. This study provides preliminary data that confirm relative equipoise between liposomal bupivacaine and ISNB, which is needed for the ethical conduct of a randomized controlled trial. Such a trial would allow for a more robust comparison, and this retrospective study provides appropriate pilot data on which to base this design and the clinical information needed to counsel patients during enrollment.
Our results suggest that liposomal bupivacaine may provide superior or similar pain relief compared with ISNB after shoulder arthroplasty. Additionally, the use of liposomal bupivacaine was associated with decreased opioid consumption and earlier discharge to home compared with ISNB. These findings have important implications for pain control after TSA because pain represents a major concern for patients and providers after surgery. In addition to clinical improvements, use of liposomal bupivacaine may save time and eliminate costs associated with administering nerve blocks. Local injection may also be used in patients who are contraindicated for ISNB such as those with obesity, pulmonary disease, or peripheral neuropathy. Although we cannot definitively suggest that liposomal bupivacaine is superior to the current gold standard ISNB for pain control after shoulder arthroplasty, our results suggest a relative clinical equipoise between these modalities. Larger analytical studies, including randomized trials, should be performed to explore the potential benefits of liposomal bupivacaine injections for pain control after shoulder arthroplasty.
Am J Orthop. 2016;45(7):424-430. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
2. American Academy of Orthopaedic Surgeons. Shoulder joint replacement. http://orthoinfo.aaos.org/topic.cfm?topic=A00094. Accessed June 3, 2015.
3. Desai VN, Cheung EV. Postoperative pain associated with orthopedic shoulder and elbow surgery: a prospective study. J Shoulder Elbow Surg. 2012;21(4):441-450.
4. Springer BD. Transition from nerve blocks to periarticular injections and emerging techniques in total joint arthroplasty. Am J Orthop. 2014;43(10 Suppl):S6-S9.
5. Surdam JW, Licini DJ, Baynes NT, Arce BR. The use of exparel (liposomal bupivacaine) to manage postoperative pain in unilateral total knee arthroplasty patients. J Arthroplasty. 2015;30(2):325-329.
6. Tong YC, Kaye AD, Urman RD. Liposomal bupivacaine and clinical outcomes. Best Pract Res Clin Anaesthesiol. 2014;28(1):15-27.
7. Chahar P, Cummings KC 3rd. Liposomal bupivacaine: a review of a new bupivacaine formulation. J Pain Res. 2012;5:257-264.
8. Schneider C, Yale SH, Larson M. Principles of pain management. Clin Med Res. 2003;1(4):337-340.
9. Pacira Pharmaceuticals, Inc. Highlights of prescribing information. http://www.exparel.com/pdf/EXPAREL_Prescribing_Information.pdf. Accessed May 7, 2015.
10. Gohl MR, Moeller RK, Olson RL, Vacchiano CA. The addition of interscalene block to general anesthesia for patients undergoing open shoulder procedures. AANA J. 2001;69(2):105-109.
11. Ironfield CM, Barrington MJ, Kluger R, Sites B. Are patients satisfied after peripheral nerve blockade? Results from an International Registry of Regional Anesthesia. Reg Anesth Pain Med. 2014;39(1):48-55.
12. Srikumaran U, Stein BE, Tan EW, Freehill MT, Wilckens JH. Upper-extremity peripheral nerve blocks in the perioperative pain management of orthopaedic patients: AAOS exhibit selection. J Bone Joint Surg Am. 2013;95(24):e197(1-13).
13. DeMarco JR, Componovo R, Barfield WR, Liles L, Nietert P. Efficacy of augmenting a subacromial continuous-infusion pump with a preoperative interscalene block in outpatient arthroscopic shoulder surgery: a prospective, randomized, blinded, and placebo-controlled study. Arthroscopy. 2011;27(5):603-610.
14. Misamore G, Webb B, McMurray S, Sallay P. A prospective analysis of interscalene brachial plexus blocks performed under general anesthesia. J Shoulder Elbow Surg. 2011;20(2):308-314.
15. Lenters TR, Davies J, Matsen FA 3rd. The types and severity of complications associated with interscalene brachial plexus block anesthesia: local and national evidence. J Shoulder Elbow Surg. 2007;16(4):379-387.
16. Park SK, Choi YS, Choi SW, Song SW. A comparison of three methods for postoperative pain control in patients undergoing arthroscopic shoulder surgery. Korean J Pain. 2015;28(1):45-51.
17. Pacira Pharmaceuticals, Inc. Pacira Pharmaceuticals, Inc. announces U.S. FDA approval of EXPAREL™ for postsurgical pain management. http://investor.pacira.com/phoenix.zhtml?c=220759&p=irol-newsArticle_print&ID=1623529. Published October 31, 2011. Accessed June 3, 2015.
18. White PF, Ardeleanu M, Schooley G, Burch RM. Pharmocokinetics of depobupivacaine following infiltration in patients undergoing two types of surgery and in normal volunteers. Paper presented at: Annual Meeting of the International Anesthesia Research Society; March 14, 2009; San Diego, CA.
19. Bramlett K, Onel E, Viscusi ER, Jones K. A randomized, double-blind, dose-ranging study comparing wound infiltration of DepoFoam bupivacaine, an extended-release liposomal bupivacaine, to bupivacaine HCl for postsurgical analgesia in total knee arthroplasty. Knee. 2012;19(5):530-536.
20. Lambrechts M, O’Brien MJ, Savoie FH, You Z. Liposomal extended-release bupivacaine for postsurgical analgesia. Patient Prefer Adherence. 2013;7:885-890.
21. American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116(2):248-273.
22. Candiotti KA, Sands LR, Lee E, et al. Liposome bupivacaine for postsurgical analgesia in adult patients undergoing laparoscopic colectomy: results from prospective phase IV sequential cohort studies assessing health economic outcomes. Curr Ther Res Clin Exp. 2013;76:1-6.
23. Weber SC, Jain R. Scalene regional anesthesia for shoulder surgery in a community setting: an assessment of risk. J Bone Joint Surg Am. 2002;84-A(5):775-779.
24. Beaudet V, Williams SR, Tétreault P, Perrault MA. Perioperative interscalene block versus intra-articular injection of local anesthetics for postoperative analgesia in shoulder surgery. Reg Anesth Pain Med. 2008;33(2):134-138.
25. Bagsby DT, Ireland PH, Meneghini RM. Liposomal bupivacaine versus traditional periarticular injection for pain control after total knee arthroplasty. J Arthroplasty. 2014;29(8):1687-1690.
The annual number of total shoulder arthroplasties (TSAs) is rising with the growing elderly population and development of new technologies such as reverse shoulder arthroplasty.1 In 2008, 47,000 shoulder arthroplasties were performed in the US compared with 19,000 in 1998.1 As of 2011, there were 53,000 shoulder arthroplasties performed annually.2 Pain control after shoulder procedures, particularly TSA, is challenging. 3
Several modalities exist to manage pain after shoulder arthroplasty. The interscalene brachial plexus nerve block is considered the “gold standard” for shoulder analgesia. A new approach is the periarticular injection method, in which the surgeon administers a local anesthetic intraoperatively. Liposomal bupivacaine (Exparel, Pacira Pharmaceuticals, Inc.) is a nonopioid anesthetic that has been shown to improve pain control, shorten hospital stays, and decrease costs for total knee and hip arthroplasty compared with nerve blocks.4-6 Patients who were treated with liposomal bupivacaine consumed less opioid medication than a placebo group.7
Our purpose was to compare intraoperative local liposomal bupivacaine injection with preoperative single-shot interscalene nerve block (ISNB) in terms of pain control, opioid use, and length of hospital stay (LOS) after shoulder arthroplasty. We hypothesized that patients in the liposomal bupivacaine group would have lower pain scores, less opioid use, and shorter LOS compared with patients in the ISNB group.
Methods
A retrospective cohort analysis was conducted with 58 patients who underwent shoulder arthroplasty by 1 surgeon at our academically affiliated community hospital from January 2012 through January 2015. ISNBs were the standard at the beginning of the study period and were used until Exparel became available on the hospital formulary in 2013. We began using Exparel for all shoulder arthroplasties in November 2013. No other changes were made in the perioperative management of our arthroplasty patients during this period. Patients who underwent TSA, reverse TSA, or hemiarthroplasty of the shoulder were included. Patients who underwent revision TSA were excluded. Twenty-one patients received ISNBs and 37 received liposomal bupivacaine injections. This study was approved by our Institutional Review Board.
Baseline data for each patient were age, sex, body mass index, and the American Society of Anesthesiologists (ASA) Physical Status Classification. The primary outcome measure was the numeric rating scale (NRS) pain score at 4 post-operative time intervals. The NRS pain score has a range of 0 to 10, with 10 representing severe pain. Data were gathered from nursing and physical therapy notes in patient charts. The postoperative time intervals were 0 to 1 hour, 8 to 14 hours, 18 to 24 hours, and 27 to 36 hours. Available NRS scores for these time intervals were averaged. Patients were included if they had pain scores for at least 3 of the postoperative time intervals documented in their charts. Secondary outcome measures were LOS and opioid consumption during hospital admission. Intravenous acetaminophen use was also measured in both groups. All data on opioids were converted to oral morphine equivalents using the method described by Schneider and colleagues.8
A board-certified, fellowship-trained anesthesiologist, experienced in regional anesthesia, administered the single-shot ISNB before surgery. The block was administered under ultrasound guidance using a 44-mm, 22-gauge needle with the patient in the supine position. No indwelling catheter was used. The medication consisted of 30 mL of 5% ropivacaine (5 mg/mL). The surgeon injected liposomal bupivacaine (266 mg diluted into 40 mL of injectable saline) near the end of the procedure throughout the pericapsular area and multiple layers of the wound, per manufacturer guidelines.9 A 60-mL syringe with a 20-gauge needle was used. All operations were performed by 1 board-certified, fellowship-trained surgeon using a standard deltopectoral approach with the same surgical equipment. The same postoperative pain protocol was used for all patients, including intravenous acetaminophen and patient-controlled analgesia. Additional oral pain medication was provided as needed for all patients. Physical therapy protocols were identical between groups.
Statistical Analysis
Mean patient ages in the 2 treatment groups were compared using the Student t test. Sex distribution and the ASA scores were compared using a χ2 test and a Fisher exact test, respectively. Arthroplasty types were compared using a Fisher exact test. The medians and interquartile ranges of the NRS scores at each time point measured were tabulated by treatment group, and at each time point the difference between groups was tested using nonparametric rank sum tests.
We tested the longitudinal trajectory of NRS scores over time, accounting for repeated measurements in the same patients using linear mixed model analysis. Treatment group, time period as a categorical variable, and the interaction between treatment and time period were included as fixed effects, and patient identification number was included as the random effect. An initial omnibus test was performed for all treatment and treatment-by-time interaction effects. Subsequently, the treatment-by-time interaction was tested for each of the time periods. The association of day of discharge (as a categorical variable) with treatment was tested using the Fisher exact test. All analyses were conducted using Stata, version 13, software (StataCorp LP). P values <.05 were considered significant.
Sample Size Analysis
We calculated the minimum detectable effect size with 80% power at an alpha level of 0.05 for the nonparametric rank sum test in terms of the proportion of every possible pair of patients treated with the 2 treatments, where the patient treated with liposomal bupivacaine has a lower pain score than the patient treated with ISNB. For pain score at 18 to 24 hours, the sample sizes of 33 patients treated with liposomal bupivacaine and 20 treated with ISNB, the minimum detectable effect size is 73%.
Results
Fifty-eight patient charts (21 in the ISNB group and 37 in the liposomal bupivacaine group) were reviewed for the study. Patient sex distribution, mean age, mean body mass index, and mean baseline ASA scores were not statistically different (Table 1).
The primary outcome measure, NRS pain score, showed no significant differences between groups at 0 to 1 hour after surgery (P = .99) or 8 to 14 hours after surgery (P = .208).
There was no difference in the amount of intravenous acetaminophen given during the hospital stay between groups. There was no significant difference in opioid consumption on postoperative day 1 in the hospital (P = .59) (Figure 2). However, there were significant differences between groups on postoperative days 2 and 3.
Sixteen of 37 patients in the liposomal bupivacaine group and 2 of 21 in the ISNB group were discharged on the day after surgery (P = .010) (Table 3).
There were no major cardiac or respiratory events in either group. No long-term paresthesias or neuropathies were noted. There were no readmissions for either group.
Discussion
Postoperative pain control after shoulder arthroplasty can be challenging, and several modalities have been tried in various combinations to minimize pain and decrease adverse effects of opioid medications. The most common method for pain relief after shoulder arthroplasty is the ISNB. Several studies of ISNBs have shown improved pain control after shoulder arthroplasty with associated decreased opioid consumption and related side effects.10 Patient rehabilitation and satisfaction have improved with the increasing use of peripheral nerve blocks.11
Despite the well-established benefits of ISNBs, several limitations exist. Although the superior portion of the shoulder is well covered by an ISNB, the inferior portion of the brachial plexus can remain uncovered or only partially covered.12 Complications of ISNBs include hemidiaphragmatic paresis, rebound pain 24 hours after surgery,13 chronic neurologic complications,14 and substantial respiratory and cardiovascular events.15 Nerve blocks also require additional time and resources in the perioperative period, including an anesthesiologist with specialized training, assistants, and ultrasonography or nerve stimulation equipment contraindicated in patients taking blood thinners.16
Periarticular injections of local anesthetics have also shown promise in reducing pain after arthroplasty.4 Benefits include an enhanced safety profile because local injection avoids the concurrent blockade of the phrenic nerve and recurrent laryngeal nerve and has not been associated with the risk of peripheral neuropathies. Further, local injection is a simple technique that can be performed during surgery without additional personnel or expertise. A limitation of this approach is the relatively short duration of effectiveness of the local anesthetic and uncertainty regarding the best agent and the ideal volume of injection.6 Liposomal bupivacaine is a new agent (approved by the US Food and Drug Administration in 201117) with a sustained release over 72 to 96 hours.18 The most common adverse effects of liposomal bupivacaine are nausea, vomiting, constipation, pyrexia, dizziness, and headache.19 Chondrotoxicity and granulomatous inflammation are more serious, yet rare, complications of liposomal bupivacaine.20
We found that liposomal bupivacaine injections were associated with lower pain scores compared with ISNB at 18 to 24 hours after surgery. This correlated with less opioid consumption in the liposomal bupivacaine group than in the ISNB group on the second postoperative day. These differences in pain values are consistent with the known pharmacokinetics of liposomal bupivacaine.18 Peak plasma levels normally occur approximately 24 hours after injection, leaving the early postoperative period relatively uncovered by anesthetic agent. This finding of relatively poor pain control early after surgery has also been noted in patients undergoing knee arthroplasty.5 On the basis of the findings of this study, we have added standard bupivacaine injections to our separate liposomal bupivacaine injection to cover early postoperative pain. Opioid consumption was significantly lower in the liposomal bupivacaine group than in the ISNB group on postoperative days 2 and 3. We did not measure adverse events related to opioid consumption, so we cannot comment on whether the decreased opioid consumption was associated with the rate of adverse events. However, other studies21,22 have established this relationship.
We found the liposomal bupivacaine group to have earlier discharges to home. Sixteen of 37 patients in the liposomal bupivacaine group compared with 2 of 21 patients in the ISNB group were discharged on the day after surgery. A mean reduction in LOS of 18 hours for the liposomal bupivacaine group was statistically significant (P = .012). This reduction in LOS has important implications for hospitals and value analysis committees considering whether to keep a new, more expensive local anesthetic on formulary. Savings from reduced LOS and improvements in patient satisfaction may justify the expense (approximately $300 per 266-mg vial) of Exparel.
From a societal cost perspective, liposomal bupivacaine is more economical compared with ISNB, which adds approximately $1500 to the cost of anesthesia per patient.23 Eliminating the costs associated with ISNB administration in shoulder arthroplasties could result in substantial savings to our healthcare system. More research examining time savings and exact costs of each procedure is needed to determine the true cost effectiveness of each approach.
Limitations of our study include the retrospective design, relatively small numbers of patients in each group, missing data for some patients at various time points, variation in the types of procedures in each group, and lack of long-term outcome measures. It is important to note that we did not confirm the success of the nerve block after administration. However, this study reflects the effectiveness of each of the modalities in actual clinical conditions (as opposed to a controlled experimental setting). The actual effectiveness of a nerve block varies, even when performed by an experienced anesthesiologist with ultrasound guidance. Furthermore, immediate postoperative pain scores in the nerve block group are consistent with those of prior research reporting pain values ranging from 4 to 5 and a mean duration of effect ranging from 9 to 14 hours.23,24 Additionally, the patients, surgeon, and nursing team were not blinded to the treatment group. Although we did note a significant difference in the types of procedures between groups, this finding is related to the greater number of hemiarthroplasties performed in the ISNB group (N = 5) compared with the liposomal group (N = 1). Because of this variation and the decreased invasiveness of hemiarthroplasties, the bias is against the liposomal group. Finally, our primary outcome variable was pain, which is a subjective, self-reported measure. However, our opioid consumption data and LOS data corroborate the improved pain scores in the liposomal bupivacaine group.
Limiting the study to a single surgeon may limit external validity. Another limitation is the lack of data on adverse events related to opioid medication use. There was no additional experimental group to determine whether less expensive local anesthetics injected locally would perform similarly to liposomal bupivacaine. In total knee arthroplasty, periarticular injections of liposomal bupivacaine were not as effective as less expensive periarticular injections.25 It is unclear which agents (and in what doses or combinations) should be used for periarticular injections. Finally, we acknowledge that our retrospective study design cannot account for all potential factors affecting discharge time.
This is the first comparative study of liposomal bupivacaine and ISNB in TSA. The study design allowed us to control for variables such as surgical technique, postoperative protocols (including use and type of sling), and use of other pain modalities such as patient-controlled analgesia and intravenous acetaminophen that are likely to affect postoperative pain and LOS. This study provides preliminary data that confirm relative equipoise between liposomal bupivacaine and ISNB, which is needed for the ethical conduct of a randomized controlled trial. Such a trial would allow for a more robust comparison, and this retrospective study provides appropriate pilot data on which to base this design and the clinical information needed to counsel patients during enrollment.
Our results suggest that liposomal bupivacaine may provide superior or similar pain relief compared with ISNB after shoulder arthroplasty. Additionally, the use of liposomal bupivacaine was associated with decreased opioid consumption and earlier discharge to home compared with ISNB. These findings have important implications for pain control after TSA because pain represents a major concern for patients and providers after surgery. In addition to clinical improvements, use of liposomal bupivacaine may save time and eliminate costs associated with administering nerve blocks. Local injection may also be used in patients who are contraindicated for ISNB such as those with obesity, pulmonary disease, or peripheral neuropathy. Although we cannot definitively suggest that liposomal bupivacaine is superior to the current gold standard ISNB for pain control after shoulder arthroplasty, our results suggest a relative clinical equipoise between these modalities. Larger analytical studies, including randomized trials, should be performed to explore the potential benefits of liposomal bupivacaine injections for pain control after shoulder arthroplasty.
Am J Orthop. 2016;45(7):424-430. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
The annual number of total shoulder arthroplasties (TSAs) is rising with the growing elderly population and development of new technologies such as reverse shoulder arthroplasty.1 In 2008, 47,000 shoulder arthroplasties were performed in the US compared with 19,000 in 1998.1 As of 2011, there were 53,000 shoulder arthroplasties performed annually.2 Pain control after shoulder procedures, particularly TSA, is challenging. 3
Several modalities exist to manage pain after shoulder arthroplasty. The interscalene brachial plexus nerve block is considered the “gold standard” for shoulder analgesia. A new approach is the periarticular injection method, in which the surgeon administers a local anesthetic intraoperatively. Liposomal bupivacaine (Exparel, Pacira Pharmaceuticals, Inc.) is a nonopioid anesthetic that has been shown to improve pain control, shorten hospital stays, and decrease costs for total knee and hip arthroplasty compared with nerve blocks.4-6 Patients who were treated with liposomal bupivacaine consumed less opioid medication than a placebo group.7
Our purpose was to compare intraoperative local liposomal bupivacaine injection with preoperative single-shot interscalene nerve block (ISNB) in terms of pain control, opioid use, and length of hospital stay (LOS) after shoulder arthroplasty. We hypothesized that patients in the liposomal bupivacaine group would have lower pain scores, less opioid use, and shorter LOS compared with patients in the ISNB group.
Methods
A retrospective cohort analysis was conducted with 58 patients who underwent shoulder arthroplasty by 1 surgeon at our academically affiliated community hospital from January 2012 through January 2015. ISNBs were the standard at the beginning of the study period and were used until Exparel became available on the hospital formulary in 2013. We began using Exparel for all shoulder arthroplasties in November 2013. No other changes were made in the perioperative management of our arthroplasty patients during this period. Patients who underwent TSA, reverse TSA, or hemiarthroplasty of the shoulder were included. Patients who underwent revision TSA were excluded. Twenty-one patients received ISNBs and 37 received liposomal bupivacaine injections. This study was approved by our Institutional Review Board.
Baseline data for each patient were age, sex, body mass index, and the American Society of Anesthesiologists (ASA) Physical Status Classification. The primary outcome measure was the numeric rating scale (NRS) pain score at 4 post-operative time intervals. The NRS pain score has a range of 0 to 10, with 10 representing severe pain. Data were gathered from nursing and physical therapy notes in patient charts. The postoperative time intervals were 0 to 1 hour, 8 to 14 hours, 18 to 24 hours, and 27 to 36 hours. Available NRS scores for these time intervals were averaged. Patients were included if they had pain scores for at least 3 of the postoperative time intervals documented in their charts. Secondary outcome measures were LOS and opioid consumption during hospital admission. Intravenous acetaminophen use was also measured in both groups. All data on opioids were converted to oral morphine equivalents using the method described by Schneider and colleagues.8
A board-certified, fellowship-trained anesthesiologist, experienced in regional anesthesia, administered the single-shot ISNB before surgery. The block was administered under ultrasound guidance using a 44-mm, 22-gauge needle with the patient in the supine position. No indwelling catheter was used. The medication consisted of 30 mL of 5% ropivacaine (5 mg/mL). The surgeon injected liposomal bupivacaine (266 mg diluted into 40 mL of injectable saline) near the end of the procedure throughout the pericapsular area and multiple layers of the wound, per manufacturer guidelines.9 A 60-mL syringe with a 20-gauge needle was used. All operations were performed by 1 board-certified, fellowship-trained surgeon using a standard deltopectoral approach with the same surgical equipment. The same postoperative pain protocol was used for all patients, including intravenous acetaminophen and patient-controlled analgesia. Additional oral pain medication was provided as needed for all patients. Physical therapy protocols were identical between groups.
Statistical Analysis
Mean patient ages in the 2 treatment groups were compared using the Student t test. Sex distribution and the ASA scores were compared using a χ2 test and a Fisher exact test, respectively. Arthroplasty types were compared using a Fisher exact test. The medians and interquartile ranges of the NRS scores at each time point measured were tabulated by treatment group, and at each time point the difference between groups was tested using nonparametric rank sum tests.
We tested the longitudinal trajectory of NRS scores over time, accounting for repeated measurements in the same patients using linear mixed model analysis. Treatment group, time period as a categorical variable, and the interaction between treatment and time period were included as fixed effects, and patient identification number was included as the random effect. An initial omnibus test was performed for all treatment and treatment-by-time interaction effects. Subsequently, the treatment-by-time interaction was tested for each of the time periods. The association of day of discharge (as a categorical variable) with treatment was tested using the Fisher exact test. All analyses were conducted using Stata, version 13, software (StataCorp LP). P values <.05 were considered significant.
Sample Size Analysis
We calculated the minimum detectable effect size with 80% power at an alpha level of 0.05 for the nonparametric rank sum test in terms of the proportion of every possible pair of patients treated with the 2 treatments, where the patient treated with liposomal bupivacaine has a lower pain score than the patient treated with ISNB. For pain score at 18 to 24 hours, the sample sizes of 33 patients treated with liposomal bupivacaine and 20 treated with ISNB, the minimum detectable effect size is 73%.
Results
Fifty-eight patient charts (21 in the ISNB group and 37 in the liposomal bupivacaine group) were reviewed for the study. Patient sex distribution, mean age, mean body mass index, and mean baseline ASA scores were not statistically different (Table 1).
The primary outcome measure, NRS pain score, showed no significant differences between groups at 0 to 1 hour after surgery (P = .99) or 8 to 14 hours after surgery (P = .208).
There was no difference in the amount of intravenous acetaminophen given during the hospital stay between groups. There was no significant difference in opioid consumption on postoperative day 1 in the hospital (P = .59) (Figure 2). However, there were significant differences between groups on postoperative days 2 and 3.
Sixteen of 37 patients in the liposomal bupivacaine group and 2 of 21 in the ISNB group were discharged on the day after surgery (P = .010) (Table 3).
There were no major cardiac or respiratory events in either group. No long-term paresthesias or neuropathies were noted. There were no readmissions for either group.
Discussion
Postoperative pain control after shoulder arthroplasty can be challenging, and several modalities have been tried in various combinations to minimize pain and decrease adverse effects of opioid medications. The most common method for pain relief after shoulder arthroplasty is the ISNB. Several studies of ISNBs have shown improved pain control after shoulder arthroplasty with associated decreased opioid consumption and related side effects.10 Patient rehabilitation and satisfaction have improved with the increasing use of peripheral nerve blocks.11
Despite the well-established benefits of ISNBs, several limitations exist. Although the superior portion of the shoulder is well covered by an ISNB, the inferior portion of the brachial plexus can remain uncovered or only partially covered.12 Complications of ISNBs include hemidiaphragmatic paresis, rebound pain 24 hours after surgery,13 chronic neurologic complications,14 and substantial respiratory and cardiovascular events.15 Nerve blocks also require additional time and resources in the perioperative period, including an anesthesiologist with specialized training, assistants, and ultrasonography or nerve stimulation equipment contraindicated in patients taking blood thinners.16
Periarticular injections of local anesthetics have also shown promise in reducing pain after arthroplasty.4 Benefits include an enhanced safety profile because local injection avoids the concurrent blockade of the phrenic nerve and recurrent laryngeal nerve and has not been associated with the risk of peripheral neuropathies. Further, local injection is a simple technique that can be performed during surgery without additional personnel or expertise. A limitation of this approach is the relatively short duration of effectiveness of the local anesthetic and uncertainty regarding the best agent and the ideal volume of injection.6 Liposomal bupivacaine is a new agent (approved by the US Food and Drug Administration in 201117) with a sustained release over 72 to 96 hours.18 The most common adverse effects of liposomal bupivacaine are nausea, vomiting, constipation, pyrexia, dizziness, and headache.19 Chondrotoxicity and granulomatous inflammation are more serious, yet rare, complications of liposomal bupivacaine.20
We found that liposomal bupivacaine injections were associated with lower pain scores compared with ISNB at 18 to 24 hours after surgery. This correlated with less opioid consumption in the liposomal bupivacaine group than in the ISNB group on the second postoperative day. These differences in pain values are consistent with the known pharmacokinetics of liposomal bupivacaine.18 Peak plasma levels normally occur approximately 24 hours after injection, leaving the early postoperative period relatively uncovered by anesthetic agent. This finding of relatively poor pain control early after surgery has also been noted in patients undergoing knee arthroplasty.5 On the basis of the findings of this study, we have added standard bupivacaine injections to our separate liposomal bupivacaine injection to cover early postoperative pain. Opioid consumption was significantly lower in the liposomal bupivacaine group than in the ISNB group on postoperative days 2 and 3. We did not measure adverse events related to opioid consumption, so we cannot comment on whether the decreased opioid consumption was associated with the rate of adverse events. However, other studies21,22 have established this relationship.
We found the liposomal bupivacaine group to have earlier discharges to home. Sixteen of 37 patients in the liposomal bupivacaine group compared with 2 of 21 patients in the ISNB group were discharged on the day after surgery. A mean reduction in LOS of 18 hours for the liposomal bupivacaine group was statistically significant (P = .012). This reduction in LOS has important implications for hospitals and value analysis committees considering whether to keep a new, more expensive local anesthetic on formulary. Savings from reduced LOS and improvements in patient satisfaction may justify the expense (approximately $300 per 266-mg vial) of Exparel.
From a societal cost perspective, liposomal bupivacaine is more economical compared with ISNB, which adds approximately $1500 to the cost of anesthesia per patient.23 Eliminating the costs associated with ISNB administration in shoulder arthroplasties could result in substantial savings to our healthcare system. More research examining time savings and exact costs of each procedure is needed to determine the true cost effectiveness of each approach.
Limitations of our study include the retrospective design, relatively small numbers of patients in each group, missing data for some patients at various time points, variation in the types of procedures in each group, and lack of long-term outcome measures. It is important to note that we did not confirm the success of the nerve block after administration. However, this study reflects the effectiveness of each of the modalities in actual clinical conditions (as opposed to a controlled experimental setting). The actual effectiveness of a nerve block varies, even when performed by an experienced anesthesiologist with ultrasound guidance. Furthermore, immediate postoperative pain scores in the nerve block group are consistent with those of prior research reporting pain values ranging from 4 to 5 and a mean duration of effect ranging from 9 to 14 hours.23,24 Additionally, the patients, surgeon, and nursing team were not blinded to the treatment group. Although we did note a significant difference in the types of procedures between groups, this finding is related to the greater number of hemiarthroplasties performed in the ISNB group (N = 5) compared with the liposomal group (N = 1). Because of this variation and the decreased invasiveness of hemiarthroplasties, the bias is against the liposomal group. Finally, our primary outcome variable was pain, which is a subjective, self-reported measure. However, our opioid consumption data and LOS data corroborate the improved pain scores in the liposomal bupivacaine group.
Limiting the study to a single surgeon may limit external validity. Another limitation is the lack of data on adverse events related to opioid medication use. There was no additional experimental group to determine whether less expensive local anesthetics injected locally would perform similarly to liposomal bupivacaine. In total knee arthroplasty, periarticular injections of liposomal bupivacaine were not as effective as less expensive periarticular injections.25 It is unclear which agents (and in what doses or combinations) should be used for periarticular injections. Finally, we acknowledge that our retrospective study design cannot account for all potential factors affecting discharge time.
This is the first comparative study of liposomal bupivacaine and ISNB in TSA. The study design allowed us to control for variables such as surgical technique, postoperative protocols (including use and type of sling), and use of other pain modalities such as patient-controlled analgesia and intravenous acetaminophen that are likely to affect postoperative pain and LOS. This study provides preliminary data that confirm relative equipoise between liposomal bupivacaine and ISNB, which is needed for the ethical conduct of a randomized controlled trial. Such a trial would allow for a more robust comparison, and this retrospective study provides appropriate pilot data on which to base this design and the clinical information needed to counsel patients during enrollment.
Our results suggest that liposomal bupivacaine may provide superior or similar pain relief compared with ISNB after shoulder arthroplasty. Additionally, the use of liposomal bupivacaine was associated with decreased opioid consumption and earlier discharge to home compared with ISNB. These findings have important implications for pain control after TSA because pain represents a major concern for patients and providers after surgery. In addition to clinical improvements, use of liposomal bupivacaine may save time and eliminate costs associated with administering nerve blocks. Local injection may also be used in patients who are contraindicated for ISNB such as those with obesity, pulmonary disease, or peripheral neuropathy. Although we cannot definitively suggest that liposomal bupivacaine is superior to the current gold standard ISNB for pain control after shoulder arthroplasty, our results suggest a relative clinical equipoise between these modalities. Larger analytical studies, including randomized trials, should be performed to explore the potential benefits of liposomal bupivacaine injections for pain control after shoulder arthroplasty.
Am J Orthop. 2016;45(7):424-430. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
2. American Academy of Orthopaedic Surgeons. Shoulder joint replacement. http://orthoinfo.aaos.org/topic.cfm?topic=A00094. Accessed June 3, 2015.
3. Desai VN, Cheung EV. Postoperative pain associated with orthopedic shoulder and elbow surgery: a prospective study. J Shoulder Elbow Surg. 2012;21(4):441-450.
4. Springer BD. Transition from nerve blocks to periarticular injections and emerging techniques in total joint arthroplasty. Am J Orthop. 2014;43(10 Suppl):S6-S9.
5. Surdam JW, Licini DJ, Baynes NT, Arce BR. The use of exparel (liposomal bupivacaine) to manage postoperative pain in unilateral total knee arthroplasty patients. J Arthroplasty. 2015;30(2):325-329.
6. Tong YC, Kaye AD, Urman RD. Liposomal bupivacaine and clinical outcomes. Best Pract Res Clin Anaesthesiol. 2014;28(1):15-27.
7. Chahar P, Cummings KC 3rd. Liposomal bupivacaine: a review of a new bupivacaine formulation. J Pain Res. 2012;5:257-264.
8. Schneider C, Yale SH, Larson M. Principles of pain management. Clin Med Res. 2003;1(4):337-340.
9. Pacira Pharmaceuticals, Inc. Highlights of prescribing information. http://www.exparel.com/pdf/EXPAREL_Prescribing_Information.pdf. Accessed May 7, 2015.
10. Gohl MR, Moeller RK, Olson RL, Vacchiano CA. The addition of interscalene block to general anesthesia for patients undergoing open shoulder procedures. AANA J. 2001;69(2):105-109.
11. Ironfield CM, Barrington MJ, Kluger R, Sites B. Are patients satisfied after peripheral nerve blockade? Results from an International Registry of Regional Anesthesia. Reg Anesth Pain Med. 2014;39(1):48-55.
12. Srikumaran U, Stein BE, Tan EW, Freehill MT, Wilckens JH. Upper-extremity peripheral nerve blocks in the perioperative pain management of orthopaedic patients: AAOS exhibit selection. J Bone Joint Surg Am. 2013;95(24):e197(1-13).
13. DeMarco JR, Componovo R, Barfield WR, Liles L, Nietert P. Efficacy of augmenting a subacromial continuous-infusion pump with a preoperative interscalene block in outpatient arthroscopic shoulder surgery: a prospective, randomized, blinded, and placebo-controlled study. Arthroscopy. 2011;27(5):603-610.
14. Misamore G, Webb B, McMurray S, Sallay P. A prospective analysis of interscalene brachial plexus blocks performed under general anesthesia. J Shoulder Elbow Surg. 2011;20(2):308-314.
15. Lenters TR, Davies J, Matsen FA 3rd. The types and severity of complications associated with interscalene brachial plexus block anesthesia: local and national evidence. J Shoulder Elbow Surg. 2007;16(4):379-387.
16. Park SK, Choi YS, Choi SW, Song SW. A comparison of three methods for postoperative pain control in patients undergoing arthroscopic shoulder surgery. Korean J Pain. 2015;28(1):45-51.
17. Pacira Pharmaceuticals, Inc. Pacira Pharmaceuticals, Inc. announces U.S. FDA approval of EXPAREL™ for postsurgical pain management. http://investor.pacira.com/phoenix.zhtml?c=220759&p=irol-newsArticle_print&ID=1623529. Published October 31, 2011. Accessed June 3, 2015.
18. White PF, Ardeleanu M, Schooley G, Burch RM. Pharmocokinetics of depobupivacaine following infiltration in patients undergoing two types of surgery and in normal volunteers. Paper presented at: Annual Meeting of the International Anesthesia Research Society; March 14, 2009; San Diego, CA.
19. Bramlett K, Onel E, Viscusi ER, Jones K. A randomized, double-blind, dose-ranging study comparing wound infiltration of DepoFoam bupivacaine, an extended-release liposomal bupivacaine, to bupivacaine HCl for postsurgical analgesia in total knee arthroplasty. Knee. 2012;19(5):530-536.
20. Lambrechts M, O’Brien MJ, Savoie FH, You Z. Liposomal extended-release bupivacaine for postsurgical analgesia. Patient Prefer Adherence. 2013;7:885-890.
21. American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116(2):248-273.
22. Candiotti KA, Sands LR, Lee E, et al. Liposome bupivacaine for postsurgical analgesia in adult patients undergoing laparoscopic colectomy: results from prospective phase IV sequential cohort studies assessing health economic outcomes. Curr Ther Res Clin Exp. 2013;76:1-6.
23. Weber SC, Jain R. Scalene regional anesthesia for shoulder surgery in a community setting: an assessment of risk. J Bone Joint Surg Am. 2002;84-A(5):775-779.
24. Beaudet V, Williams SR, Tétreault P, Perrault MA. Perioperative interscalene block versus intra-articular injection of local anesthetics for postoperative analgesia in shoulder surgery. Reg Anesth Pain Med. 2008;33(2):134-138.
25. Bagsby DT, Ireland PH, Meneghini RM. Liposomal bupivacaine versus traditional periarticular injection for pain control after total knee arthroplasty. J Arthroplasty. 2014;29(8):1687-1690.
1. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
2. American Academy of Orthopaedic Surgeons. Shoulder joint replacement. http://orthoinfo.aaos.org/topic.cfm?topic=A00094. Accessed June 3, 2015.
3. Desai VN, Cheung EV. Postoperative pain associated with orthopedic shoulder and elbow surgery: a prospective study. J Shoulder Elbow Surg. 2012;21(4):441-450.
4. Springer BD. Transition from nerve blocks to periarticular injections and emerging techniques in total joint arthroplasty. Am J Orthop. 2014;43(10 Suppl):S6-S9.
5. Surdam JW, Licini DJ, Baynes NT, Arce BR. The use of exparel (liposomal bupivacaine) to manage postoperative pain in unilateral total knee arthroplasty patients. J Arthroplasty. 2015;30(2):325-329.
6. Tong YC, Kaye AD, Urman RD. Liposomal bupivacaine and clinical outcomes. Best Pract Res Clin Anaesthesiol. 2014;28(1):15-27.
7. Chahar P, Cummings KC 3rd. Liposomal bupivacaine: a review of a new bupivacaine formulation. J Pain Res. 2012;5:257-264.
8. Schneider C, Yale SH, Larson M. Principles of pain management. Clin Med Res. 2003;1(4):337-340.
9. Pacira Pharmaceuticals, Inc. Highlights of prescribing information. http://www.exparel.com/pdf/EXPAREL_Prescribing_Information.pdf. Accessed May 7, 2015.
10. Gohl MR, Moeller RK, Olson RL, Vacchiano CA. The addition of interscalene block to general anesthesia for patients undergoing open shoulder procedures. AANA J. 2001;69(2):105-109.
11. Ironfield CM, Barrington MJ, Kluger R, Sites B. Are patients satisfied after peripheral nerve blockade? Results from an International Registry of Regional Anesthesia. Reg Anesth Pain Med. 2014;39(1):48-55.
12. Srikumaran U, Stein BE, Tan EW, Freehill MT, Wilckens JH. Upper-extremity peripheral nerve blocks in the perioperative pain management of orthopaedic patients: AAOS exhibit selection. J Bone Joint Surg Am. 2013;95(24):e197(1-13).
13. DeMarco JR, Componovo R, Barfield WR, Liles L, Nietert P. Efficacy of augmenting a subacromial continuous-infusion pump with a preoperative interscalene block in outpatient arthroscopic shoulder surgery: a prospective, randomized, blinded, and placebo-controlled study. Arthroscopy. 2011;27(5):603-610.
14. Misamore G, Webb B, McMurray S, Sallay P. A prospective analysis of interscalene brachial plexus blocks performed under general anesthesia. J Shoulder Elbow Surg. 2011;20(2):308-314.
15. Lenters TR, Davies J, Matsen FA 3rd. The types and severity of complications associated with interscalene brachial plexus block anesthesia: local and national evidence. J Shoulder Elbow Surg. 2007;16(4):379-387.
16. Park SK, Choi YS, Choi SW, Song SW. A comparison of three methods for postoperative pain control in patients undergoing arthroscopic shoulder surgery. Korean J Pain. 2015;28(1):45-51.
17. Pacira Pharmaceuticals, Inc. Pacira Pharmaceuticals, Inc. announces U.S. FDA approval of EXPAREL™ for postsurgical pain management. http://investor.pacira.com/phoenix.zhtml?c=220759&p=irol-newsArticle_print&ID=1623529. Published October 31, 2011. Accessed June 3, 2015.
18. White PF, Ardeleanu M, Schooley G, Burch RM. Pharmocokinetics of depobupivacaine following infiltration in patients undergoing two types of surgery and in normal volunteers. Paper presented at: Annual Meeting of the International Anesthesia Research Society; March 14, 2009; San Diego, CA.
19. Bramlett K, Onel E, Viscusi ER, Jones K. A randomized, double-blind, dose-ranging study comparing wound infiltration of DepoFoam bupivacaine, an extended-release liposomal bupivacaine, to bupivacaine HCl for postsurgical analgesia in total knee arthroplasty. Knee. 2012;19(5):530-536.
20. Lambrechts M, O’Brien MJ, Savoie FH, You Z. Liposomal extended-release bupivacaine for postsurgical analgesia. Patient Prefer Adherence. 2013;7:885-890.
21. American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116(2):248-273.
22. Candiotti KA, Sands LR, Lee E, et al. Liposome bupivacaine for postsurgical analgesia in adult patients undergoing laparoscopic colectomy: results from prospective phase IV sequential cohort studies assessing health economic outcomes. Curr Ther Res Clin Exp. 2013;76:1-6.
23. Weber SC, Jain R. Scalene regional anesthesia for shoulder surgery in a community setting: an assessment of risk. J Bone Joint Surg Am. 2002;84-A(5):775-779.
24. Beaudet V, Williams SR, Tétreault P, Perrault MA. Perioperative interscalene block versus intra-articular injection of local anesthetics for postoperative analgesia in shoulder surgery. Reg Anesth Pain Med. 2008;33(2):134-138.
25. Bagsby DT, Ireland PH, Meneghini RM. Liposomal bupivacaine versus traditional periarticular injection for pain control after total knee arthroplasty. J Arthroplasty. 2014;29(8):1687-1690.
Bazex Syndrome (Paraneoplastic Acrokeratosis)
Psoriasiform dermatitis seen with Bazex syndrome may involve the nose and the helices of the ears in addition to the palms and soles. In most reported cases, the appearance of the characteristic psoriasiform lesions preceded the diagnosis of the associated underlying malignancy. Skin scrapings for potassium hydroxide and fungal cultures should be performed, and skin biopsy of keratotic plaques is recommended to exclude psoriasis.
Case Report
A 70-year-old man with no personal or family history of psoriasis or other skin diseases developed psoriasiform dermatitis of the fingers, toes, and helices of the ears over a period of 3 months. He reported a history of cigarette smoking (1 pack per day) with significant consumption of alcoholic beverages over a period of 30 years. Results from a review of systems revealed progressive hoarseness and dysphagia, with a recent history of a 15-lb weight loss. On physical examination, psoriasiform plaques were seen on the palms and soles, as well as on the helices of the ears (Figure 1) and the tip and dorsum of the nose. There was a yellowish discoloration and dystrophy of all the fingernails and toenails (Figure 2). Results from potassium hydroxide preparations from scrapings of the palms and soles were negative, and fungal culture did not grow any pathogenic fungi.
Six weeks later, the patient developed bilateral cervical lymphadenopathy. Otolaryngologic examination consisted of direct laryngoscopy; imaging studies including magnetic resonance imaging and computed tomography scans; and laryngeal biopsy, which revealed a stage IV squamous cell carcinoma (SCC) confined to the head and neck area. Although the patient did not return for follow-up, management of the laryngeal SCC with surgery and postoperative chemotherapy completely cleared his skin and nail lesions without adjunct dermatologic treatments.
PLEASE REFER TO THE PDF TO VIEW THE FIGURES
Comment
Bazex syndrome (paraneoplastic acrokeratosis) was first described as a clinical entity by Gougerot and Rupp1 more than 40 years prior to the coining of the disease's current widely used eponym of Bazex syndrome. In 1922, Gougerot and Grupper1 described a patient with hyperkeratotic lesions on the nose, ears, palms, and soles in conjunction with an SCC on the tongue. Years later, Bazex and colleagues2 described a patient with an SCC of the pyriform fossa and an associated psoriasiform dermatosis. Since that report, more than 110 cases of Bazex syndrome have been reported, most of which describe the condition as a cutaneous paraneoplastic syndrome characterized by psoriasiform lesions associated with an underlying malignancy of the upper aerodigestive tract (oropharynx, larynx, or esophagus), most often of the SCC subtype.3-7
Bazex syndrome can be classified among the cutaneous paraneoplastic disorders that also include acanthosis nigricans maligna, erythema gyratum, necrolytic migratory erythema, and hypertrichosis lanuginosa acquisita.7 The cutaneous manifestations of Bazex syndrome are paraneoplastic in that the developing skin changes coevolve with an underlying malignancy; these cutaneous hallmarks of the syndrome do not, however, represent metastatic extensions of this malignancy. On the contrary, they may actually serve as harbingers of future oncologic progression.
The cutaneous changes observed in Bazex syndrome have been classified into 3 stages.3 In the first stage, psoriasiform changes of the fingers, toes, auricular helix, and nose are noted. In addition, the earliest stage of the syndrome is characterized by nail changes, including horizontal and vertical ridging, subungual hyperkeratosis, yellow discoloration, and nail dystrophy. During this stage, the primary tumor is considered asymptomatic. The second stage is primarily typified by proximal extension of the cutaneous changes observed in the first stage to involve the dorsum of the hands and feet, as well as the malar regions of the face. Local symptoms secondary to growth of the primary tumor also may surface during this stage. The third stage in the course of the syndrome is defined by progressive centripetal extension of the cutaneous disease process to affect regions of the arms and legs (nails, hands, elbows, knees, and feet), scalp, and trunk.3-7 Other cutaneous changes that have been reported include hyperpigmentation, particularly in individuals with darker skin pigmentation,6 and development of bullous lesions.5,8,9
Based on the initial dermatologic manifestations of Bazex syndrome, it is not surprising that the condition is often misdiagnosed as psoriasis or chronic dermatitis. Indeed, histopathologic examination of skin lesions in the syndrome is nonspecific and may mimic psoriasis or other more common dermatoses, demonstrating hyperkeratosis, parakeratosis, acanthosis, vacuolar degeneration of keratinocytes, and/or perivascular lymphohistiocytic infiltrate.6,7,10 One potential distinguishing feature of Bazex syndrome, however, is specific psoriasiform involvement of the helix of the ear, as opposed to the entire ear, as would be more commonly expected in psoriasis.7 The tip of the nose also is involved in Bazex syndrome, which is an unusual location for psoriasis.
Extensive reviews of the literature reporting cases of Bazex syndrome demonstrate that most patients have been Caucasian, male, of French descent, and older than 40 years.6,7 SCCs have accounted for nearly 60% of tumors found in patients with this syndrome, and adenocarcinomas have accounted for less than 10% of malignancies. Furthermore, the majority of the neoplasms have involved the oropharynx and larynx.7 These neoplasms may be silent and only present with lymph node metastases. Less commonly, primary tumors may occur in the lungs and esophagus. Rare cases of neoplasms of the prostate, liver, stomach, thymus, uterus, vulva, and lymphoid tissues also have been reported.11 Numerous cases have been described in which the primary tumor could not be identified, and affected patients were diagnosed on the basis of metastases to cervical lymph nodes. In the vast majority of reported cases, the appearance of the characteristic psoriasiform lesions preceded the diagnosis of the associated malignancy.6,7 Finally, the skin lesions either markedly improved or completely resolved in the great preponderance of patients in whom the underlying malignancy was either treated with chemotherapy and/or radiation therapy or surgical excision.6,7,10-12 This was true of the patient presented in this report.
The pathogenesis of Bazex syndrome remains a mystery, though several authors have suggested an autoimmune etiology based on the common histologic finding of inflammatory infiltrates along the basal cell layer of affected skin regions.5,8,9 The immune reaction may be humoral or cellular; the proposed mechanism states that cross reactivity between skin and tumor antigens may produce the characteristic cutaneous changes observed, because antitumor antibodies cross reacting with the epidermis or basement membrane zone could elicit an immunologic response resulting in basal cell layer damage.13,14 Several authors also have proposed that the tumors may produce a host of growth factors that collectively lead to hyperkeratotic skin changes.14,15
Ideal treatment of Bazex syndrome is eradication of the underlying malignancy. Unresectable or treatment-resistant tumors, however, pose a significant challenge for the clinician. Numerous studies have been conducted demonstrating equivocal efficacies of various standard dermatological therapies in the treatment of skin lesions occurring in this syndrome. Unfortunately, in the vast majority of patients, such treatment options as topical tar, topical and systemic corticosteroids, UVB irradiation, antifungals, and antibiotics have proven to be of little use.6,7 Gill and colleagues9 have reported that oral psoralen–UVA phototherapy may offer some promise of effective treatment in these patients. However, larger studies are required to further investigate the therapeutic benefits of this treatment option. Although the management of treatment-resistant cutaneous lesions in Bazex syndrome may prove problematic, it is clear that the clinician must be astute in recognizing this disease process in its earlier stages to identify and effectively treat any underlying malignancy as expeditiously as possible.
Acknowledgment—The authors wish to thank Dr. Eric Ehrsam for his assistance with the preparation of this manuscript.
References
- Gougerot H, Grupper C. Dermatose érythémato-squameuse avec hyperkératose palmoplantaire, porectasies digitales et cancer de la langue latent. Paris Méd. 1922;43:234-237.
- Bazex A, Salvador R, Dupré A, et al. Syndrome paranéoplasique à type d'hyperkératose des extrémités. Guérison après le traitment del'épthélioma laryngé [letter]. Bull Soc Fr Dermatol Syphiligr. 1965;72:182.
- Bazex A, Griffiths A. Acrokeratosis paraneoplastica: a new cutaneous marker of malignancy. Br J Dermatol. 1980;103:801-805.
- O'Brien TJ. Bazex syndrome (acrokeratosis paraneoplastica). Australas J Dermatol. 1995;36:91-93.
- Bolognia JL, Brewer YP, Cooper DL. Bazex syndrome (acrokeratosis paraneoplastica): an analytic review. Medicine (Baltimore). 1991;70:269-280.
- Bolognia JL. Bazex syndrome: acrokeratosis paraneoplastica. Semin Dermatol. 1995;14:84-89.
- Sarkar B, Knecht R, Sarkar C, et al. Bazex syndrome (acrokeratosis paraneoplastica). Eur Arch Otorhinolaryngol. 1998;255:205-210.
- Handfield-Jones SE, Matthews CAN, Ellis JP, et al. Acrokeratosis paraneoplastica of Bazex. J R Soc Med. 1992;85:548-550.
- Gill D, Fergin P, Kelly J. Bullous lesions in Bazex syndrome and successful treatment with oral psoralen phototherapy. Australas J Dermatol. 2001;42:278-280.
- Wareing MJ, Vaughan-Jones SA, McGibbon DH. Acrokeratosis paraneoplastica: Bazex syndrome. J Laryngol Otol. 1996;110:899-900.
- Buxtorf K, Hübscher E, Panizzon R. Bazex syndrome. Dermatology. 2001;202:350-352.
- Hsu YS, Lien GS, Lai HH, et al. Acrokeratosis paraneoplastica (Bazex syndrome) with adenocarcinoma of the colon: report of a case and review of the literature. J Gastroenterol. 2000;35:460-464.
- Pecora AL, Landsman L, Imgrund SP, et al. Acrokeratosis paraneoplastica: report of a case and review of the literature. Arch Dermatol. 1983;119:820-826.
- Jean LB, Yvelise PB, Dennis LC. Bazex syndrome (acrokeratosis paraneoplastica): an analytic review. Medicine. 1991;70:269-280.
- Politi Y, Ophir J, Brenner S. Cutaneous paraneoplastic syndromes. Acta Derm Venereol (Stockh). 1993;73:161-170.
Psoriasiform dermatitis seen with Bazex syndrome may involve the nose and the helices of the ears in addition to the palms and soles. In most reported cases, the appearance of the characteristic psoriasiform lesions preceded the diagnosis of the associated underlying malignancy. Skin scrapings for potassium hydroxide and fungal cultures should be performed, and skin biopsy of keratotic plaques is recommended to exclude psoriasis.
Case Report
A 70-year-old man with no personal or family history of psoriasis or other skin diseases developed psoriasiform dermatitis of the fingers, toes, and helices of the ears over a period of 3 months. He reported a history of cigarette smoking (1 pack per day) with significant consumption of alcoholic beverages over a period of 30 years. Results from a review of systems revealed progressive hoarseness and dysphagia, with a recent history of a 15-lb weight loss. On physical examination, psoriasiform plaques were seen on the palms and soles, as well as on the helices of the ears (Figure 1) and the tip and dorsum of the nose. There was a yellowish discoloration and dystrophy of all the fingernails and toenails (Figure 2). Results from potassium hydroxide preparations from scrapings of the palms and soles were negative, and fungal culture did not grow any pathogenic fungi.
Six weeks later, the patient developed bilateral cervical lymphadenopathy. Otolaryngologic examination consisted of direct laryngoscopy; imaging studies including magnetic resonance imaging and computed tomography scans; and laryngeal biopsy, which revealed a stage IV squamous cell carcinoma (SCC) confined to the head and neck area. Although the patient did not return for follow-up, management of the laryngeal SCC with surgery and postoperative chemotherapy completely cleared his skin and nail lesions without adjunct dermatologic treatments.
PLEASE REFER TO THE PDF TO VIEW THE FIGURES
Comment
Bazex syndrome (paraneoplastic acrokeratosis) was first described as a clinical entity by Gougerot and Rupp1 more than 40 years prior to the coining of the disease's current widely used eponym of Bazex syndrome. In 1922, Gougerot and Grupper1 described a patient with hyperkeratotic lesions on the nose, ears, palms, and soles in conjunction with an SCC on the tongue. Years later, Bazex and colleagues2 described a patient with an SCC of the pyriform fossa and an associated psoriasiform dermatosis. Since that report, more than 110 cases of Bazex syndrome have been reported, most of which describe the condition as a cutaneous paraneoplastic syndrome characterized by psoriasiform lesions associated with an underlying malignancy of the upper aerodigestive tract (oropharynx, larynx, or esophagus), most often of the SCC subtype.3-7
Bazex syndrome can be classified among the cutaneous paraneoplastic disorders that also include acanthosis nigricans maligna, erythema gyratum, necrolytic migratory erythema, and hypertrichosis lanuginosa acquisita.7 The cutaneous manifestations of Bazex syndrome are paraneoplastic in that the developing skin changes coevolve with an underlying malignancy; these cutaneous hallmarks of the syndrome do not, however, represent metastatic extensions of this malignancy. On the contrary, they may actually serve as harbingers of future oncologic progression.
The cutaneous changes observed in Bazex syndrome have been classified into 3 stages.3 In the first stage, psoriasiform changes of the fingers, toes, auricular helix, and nose are noted. In addition, the earliest stage of the syndrome is characterized by nail changes, including horizontal and vertical ridging, subungual hyperkeratosis, yellow discoloration, and nail dystrophy. During this stage, the primary tumor is considered asymptomatic. The second stage is primarily typified by proximal extension of the cutaneous changes observed in the first stage to involve the dorsum of the hands and feet, as well as the malar regions of the face. Local symptoms secondary to growth of the primary tumor also may surface during this stage. The third stage in the course of the syndrome is defined by progressive centripetal extension of the cutaneous disease process to affect regions of the arms and legs (nails, hands, elbows, knees, and feet), scalp, and trunk.3-7 Other cutaneous changes that have been reported include hyperpigmentation, particularly in individuals with darker skin pigmentation,6 and development of bullous lesions.5,8,9
Based on the initial dermatologic manifestations of Bazex syndrome, it is not surprising that the condition is often misdiagnosed as psoriasis or chronic dermatitis. Indeed, histopathologic examination of skin lesions in the syndrome is nonspecific and may mimic psoriasis or other more common dermatoses, demonstrating hyperkeratosis, parakeratosis, acanthosis, vacuolar degeneration of keratinocytes, and/or perivascular lymphohistiocytic infiltrate.6,7,10 One potential distinguishing feature of Bazex syndrome, however, is specific psoriasiform involvement of the helix of the ear, as opposed to the entire ear, as would be more commonly expected in psoriasis.7 The tip of the nose also is involved in Bazex syndrome, which is an unusual location for psoriasis.
Extensive reviews of the literature reporting cases of Bazex syndrome demonstrate that most patients have been Caucasian, male, of French descent, and older than 40 years.6,7 SCCs have accounted for nearly 60% of tumors found in patients with this syndrome, and adenocarcinomas have accounted for less than 10% of malignancies. Furthermore, the majority of the neoplasms have involved the oropharynx and larynx.7 These neoplasms may be silent and only present with lymph node metastases. Less commonly, primary tumors may occur in the lungs and esophagus. Rare cases of neoplasms of the prostate, liver, stomach, thymus, uterus, vulva, and lymphoid tissues also have been reported.11 Numerous cases have been described in which the primary tumor could not be identified, and affected patients were diagnosed on the basis of metastases to cervical lymph nodes. In the vast majority of reported cases, the appearance of the characteristic psoriasiform lesions preceded the diagnosis of the associated malignancy.6,7 Finally, the skin lesions either markedly improved or completely resolved in the great preponderance of patients in whom the underlying malignancy was either treated with chemotherapy and/or radiation therapy or surgical excision.6,7,10-12 This was true of the patient presented in this report.
The pathogenesis of Bazex syndrome remains a mystery, though several authors have suggested an autoimmune etiology based on the common histologic finding of inflammatory infiltrates along the basal cell layer of affected skin regions.5,8,9 The immune reaction may be humoral or cellular; the proposed mechanism states that cross reactivity between skin and tumor antigens may produce the characteristic cutaneous changes observed, because antitumor antibodies cross reacting with the epidermis or basement membrane zone could elicit an immunologic response resulting in basal cell layer damage.13,14 Several authors also have proposed that the tumors may produce a host of growth factors that collectively lead to hyperkeratotic skin changes.14,15
Ideal treatment of Bazex syndrome is eradication of the underlying malignancy. Unresectable or treatment-resistant tumors, however, pose a significant challenge for the clinician. Numerous studies have been conducted demonstrating equivocal efficacies of various standard dermatological therapies in the treatment of skin lesions occurring in this syndrome. Unfortunately, in the vast majority of patients, such treatment options as topical tar, topical and systemic corticosteroids, UVB irradiation, antifungals, and antibiotics have proven to be of little use.6,7 Gill and colleagues9 have reported that oral psoralen–UVA phototherapy may offer some promise of effective treatment in these patients. However, larger studies are required to further investigate the therapeutic benefits of this treatment option. Although the management of treatment-resistant cutaneous lesions in Bazex syndrome may prove problematic, it is clear that the clinician must be astute in recognizing this disease process in its earlier stages to identify and effectively treat any underlying malignancy as expeditiously as possible.
Acknowledgment—The authors wish to thank Dr. Eric Ehrsam for his assistance with the preparation of this manuscript.
Psoriasiform dermatitis seen with Bazex syndrome may involve the nose and the helices of the ears in addition to the palms and soles. In most reported cases, the appearance of the characteristic psoriasiform lesions preceded the diagnosis of the associated underlying malignancy. Skin scrapings for potassium hydroxide and fungal cultures should be performed, and skin biopsy of keratotic plaques is recommended to exclude psoriasis.
Case Report
A 70-year-old man with no personal or family history of psoriasis or other skin diseases developed psoriasiform dermatitis of the fingers, toes, and helices of the ears over a period of 3 months. He reported a history of cigarette smoking (1 pack per day) with significant consumption of alcoholic beverages over a period of 30 years. Results from a review of systems revealed progressive hoarseness and dysphagia, with a recent history of a 15-lb weight loss. On physical examination, psoriasiform plaques were seen on the palms and soles, as well as on the helices of the ears (Figure 1) and the tip and dorsum of the nose. There was a yellowish discoloration and dystrophy of all the fingernails and toenails (Figure 2). Results from potassium hydroxide preparations from scrapings of the palms and soles were negative, and fungal culture did not grow any pathogenic fungi.
Six weeks later, the patient developed bilateral cervical lymphadenopathy. Otolaryngologic examination consisted of direct laryngoscopy; imaging studies including magnetic resonance imaging and computed tomography scans; and laryngeal biopsy, which revealed a stage IV squamous cell carcinoma (SCC) confined to the head and neck area. Although the patient did not return for follow-up, management of the laryngeal SCC with surgery and postoperative chemotherapy completely cleared his skin and nail lesions without adjunct dermatologic treatments.
PLEASE REFER TO THE PDF TO VIEW THE FIGURES
Comment
Bazex syndrome (paraneoplastic acrokeratosis) was first described as a clinical entity by Gougerot and Rupp1 more than 40 years prior to the coining of the disease's current widely used eponym of Bazex syndrome. In 1922, Gougerot and Grupper1 described a patient with hyperkeratotic lesions on the nose, ears, palms, and soles in conjunction with an SCC on the tongue. Years later, Bazex and colleagues2 described a patient with an SCC of the pyriform fossa and an associated psoriasiform dermatosis. Since that report, more than 110 cases of Bazex syndrome have been reported, most of which describe the condition as a cutaneous paraneoplastic syndrome characterized by psoriasiform lesions associated with an underlying malignancy of the upper aerodigestive tract (oropharynx, larynx, or esophagus), most often of the SCC subtype.3-7
Bazex syndrome can be classified among the cutaneous paraneoplastic disorders that also include acanthosis nigricans maligna, erythema gyratum, necrolytic migratory erythema, and hypertrichosis lanuginosa acquisita.7 The cutaneous manifestations of Bazex syndrome are paraneoplastic in that the developing skin changes coevolve with an underlying malignancy; these cutaneous hallmarks of the syndrome do not, however, represent metastatic extensions of this malignancy. On the contrary, they may actually serve as harbingers of future oncologic progression.
The cutaneous changes observed in Bazex syndrome have been classified into 3 stages.3 In the first stage, psoriasiform changes of the fingers, toes, auricular helix, and nose are noted. In addition, the earliest stage of the syndrome is characterized by nail changes, including horizontal and vertical ridging, subungual hyperkeratosis, yellow discoloration, and nail dystrophy. During this stage, the primary tumor is considered asymptomatic. The second stage is primarily typified by proximal extension of the cutaneous changes observed in the first stage to involve the dorsum of the hands and feet, as well as the malar regions of the face. Local symptoms secondary to growth of the primary tumor also may surface during this stage. The third stage in the course of the syndrome is defined by progressive centripetal extension of the cutaneous disease process to affect regions of the arms and legs (nails, hands, elbows, knees, and feet), scalp, and trunk.3-7 Other cutaneous changes that have been reported include hyperpigmentation, particularly in individuals with darker skin pigmentation,6 and development of bullous lesions.5,8,9
Based on the initial dermatologic manifestations of Bazex syndrome, it is not surprising that the condition is often misdiagnosed as psoriasis or chronic dermatitis. Indeed, histopathologic examination of skin lesions in the syndrome is nonspecific and may mimic psoriasis or other more common dermatoses, demonstrating hyperkeratosis, parakeratosis, acanthosis, vacuolar degeneration of keratinocytes, and/or perivascular lymphohistiocytic infiltrate.6,7,10 One potential distinguishing feature of Bazex syndrome, however, is specific psoriasiform involvement of the helix of the ear, as opposed to the entire ear, as would be more commonly expected in psoriasis.7 The tip of the nose also is involved in Bazex syndrome, which is an unusual location for psoriasis.
Extensive reviews of the literature reporting cases of Bazex syndrome demonstrate that most patients have been Caucasian, male, of French descent, and older than 40 years.6,7 SCCs have accounted for nearly 60% of tumors found in patients with this syndrome, and adenocarcinomas have accounted for less than 10% of malignancies. Furthermore, the majority of the neoplasms have involved the oropharynx and larynx.7 These neoplasms may be silent and only present with lymph node metastases. Less commonly, primary tumors may occur in the lungs and esophagus. Rare cases of neoplasms of the prostate, liver, stomach, thymus, uterus, vulva, and lymphoid tissues also have been reported.11 Numerous cases have been described in which the primary tumor could not be identified, and affected patients were diagnosed on the basis of metastases to cervical lymph nodes. In the vast majority of reported cases, the appearance of the characteristic psoriasiform lesions preceded the diagnosis of the associated malignancy.6,7 Finally, the skin lesions either markedly improved or completely resolved in the great preponderance of patients in whom the underlying malignancy was either treated with chemotherapy and/or radiation therapy or surgical excision.6,7,10-12 This was true of the patient presented in this report.
The pathogenesis of Bazex syndrome remains a mystery, though several authors have suggested an autoimmune etiology based on the common histologic finding of inflammatory infiltrates along the basal cell layer of affected skin regions.5,8,9 The immune reaction may be humoral or cellular; the proposed mechanism states that cross reactivity between skin and tumor antigens may produce the characteristic cutaneous changes observed, because antitumor antibodies cross reacting with the epidermis or basement membrane zone could elicit an immunologic response resulting in basal cell layer damage.13,14 Several authors also have proposed that the tumors may produce a host of growth factors that collectively lead to hyperkeratotic skin changes.14,15
Ideal treatment of Bazex syndrome is eradication of the underlying malignancy. Unresectable or treatment-resistant tumors, however, pose a significant challenge for the clinician. Numerous studies have been conducted demonstrating equivocal efficacies of various standard dermatological therapies in the treatment of skin lesions occurring in this syndrome. Unfortunately, in the vast majority of patients, such treatment options as topical tar, topical and systemic corticosteroids, UVB irradiation, antifungals, and antibiotics have proven to be of little use.6,7 Gill and colleagues9 have reported that oral psoralen–UVA phototherapy may offer some promise of effective treatment in these patients. However, larger studies are required to further investigate the therapeutic benefits of this treatment option. Although the management of treatment-resistant cutaneous lesions in Bazex syndrome may prove problematic, it is clear that the clinician must be astute in recognizing this disease process in its earlier stages to identify and effectively treat any underlying malignancy as expeditiously as possible.
Acknowledgment—The authors wish to thank Dr. Eric Ehrsam for his assistance with the preparation of this manuscript.
References
- Gougerot H, Grupper C. Dermatose érythémato-squameuse avec hyperkératose palmoplantaire, porectasies digitales et cancer de la langue latent. Paris Méd. 1922;43:234-237.
- Bazex A, Salvador R, Dupré A, et al. Syndrome paranéoplasique à type d'hyperkératose des extrémités. Guérison après le traitment del'épthélioma laryngé [letter]. Bull Soc Fr Dermatol Syphiligr. 1965;72:182.
- Bazex A, Griffiths A. Acrokeratosis paraneoplastica: a new cutaneous marker of malignancy. Br J Dermatol. 1980;103:801-805.
- O'Brien TJ. Bazex syndrome (acrokeratosis paraneoplastica). Australas J Dermatol. 1995;36:91-93.
- Bolognia JL, Brewer YP, Cooper DL. Bazex syndrome (acrokeratosis paraneoplastica): an analytic review. Medicine (Baltimore). 1991;70:269-280.
- Bolognia JL. Bazex syndrome: acrokeratosis paraneoplastica. Semin Dermatol. 1995;14:84-89.
- Sarkar B, Knecht R, Sarkar C, et al. Bazex syndrome (acrokeratosis paraneoplastica). Eur Arch Otorhinolaryngol. 1998;255:205-210.
- Handfield-Jones SE, Matthews CAN, Ellis JP, et al. Acrokeratosis paraneoplastica of Bazex. J R Soc Med. 1992;85:548-550.
- Gill D, Fergin P, Kelly J. Bullous lesions in Bazex syndrome and successful treatment with oral psoralen phototherapy. Australas J Dermatol. 2001;42:278-280.
- Wareing MJ, Vaughan-Jones SA, McGibbon DH. Acrokeratosis paraneoplastica: Bazex syndrome. J Laryngol Otol. 1996;110:899-900.
- Buxtorf K, Hübscher E, Panizzon R. Bazex syndrome. Dermatology. 2001;202:350-352.
- Hsu YS, Lien GS, Lai HH, et al. Acrokeratosis paraneoplastica (Bazex syndrome) with adenocarcinoma of the colon: report of a case and review of the literature. J Gastroenterol. 2000;35:460-464.
- Pecora AL, Landsman L, Imgrund SP, et al. Acrokeratosis paraneoplastica: report of a case and review of the literature. Arch Dermatol. 1983;119:820-826.
- Jean LB, Yvelise PB, Dennis LC. Bazex syndrome (acrokeratosis paraneoplastica): an analytic review. Medicine. 1991;70:269-280.
- Politi Y, Ophir J, Brenner S. Cutaneous paraneoplastic syndromes. Acta Derm Venereol (Stockh). 1993;73:161-170.
References
- Gougerot H, Grupper C. Dermatose érythémato-squameuse avec hyperkératose palmoplantaire, porectasies digitales et cancer de la langue latent. Paris Méd. 1922;43:234-237.
- Bazex A, Salvador R, Dupré A, et al. Syndrome paranéoplasique à type d'hyperkératose des extrémités. Guérison après le traitment del'épthélioma laryngé [letter]. Bull Soc Fr Dermatol Syphiligr. 1965;72:182.
- Bazex A, Griffiths A. Acrokeratosis paraneoplastica: a new cutaneous marker of malignancy. Br J Dermatol. 1980;103:801-805.
- O'Brien TJ. Bazex syndrome (acrokeratosis paraneoplastica). Australas J Dermatol. 1995;36:91-93.
- Bolognia JL, Brewer YP, Cooper DL. Bazex syndrome (acrokeratosis paraneoplastica): an analytic review. Medicine (Baltimore). 1991;70:269-280.
- Bolognia JL. Bazex syndrome: acrokeratosis paraneoplastica. Semin Dermatol. 1995;14:84-89.
- Sarkar B, Knecht R, Sarkar C, et al. Bazex syndrome (acrokeratosis paraneoplastica). Eur Arch Otorhinolaryngol. 1998;255:205-210.
- Handfield-Jones SE, Matthews CAN, Ellis JP, et al. Acrokeratosis paraneoplastica of Bazex. J R Soc Med. 1992;85:548-550.
- Gill D, Fergin P, Kelly J. Bullous lesions in Bazex syndrome and successful treatment with oral psoralen phototherapy. Australas J Dermatol. 2001;42:278-280.
- Wareing MJ, Vaughan-Jones SA, McGibbon DH. Acrokeratosis paraneoplastica: Bazex syndrome. J Laryngol Otol. 1996;110:899-900.
- Buxtorf K, Hübscher E, Panizzon R. Bazex syndrome. Dermatology. 2001;202:350-352.
- Hsu YS, Lien GS, Lai HH, et al. Acrokeratosis paraneoplastica (Bazex syndrome) with adenocarcinoma of the colon: report of a case and review of the literature. J Gastroenterol. 2000;35:460-464.
- Pecora AL, Landsman L, Imgrund SP, et al. Acrokeratosis paraneoplastica: report of a case and review of the literature. Arch Dermatol. 1983;119:820-826.
- Jean LB, Yvelise PB, Dennis LC. Bazex syndrome (acrokeratosis paraneoplastica): an analytic review. Medicine. 1991;70:269-280.
- Politi Y, Ophir J, Brenner S. Cutaneous paraneoplastic syndromes. Acta Derm Venereol (Stockh). 1993;73:161-170.