User login
An eConsults Program to Improve Patient Access to Specialty Care in an Academic Health System
From the Department of Medicine, University of California, Irvine, Orange, CA.
Abstract
Background: Orange County’s residents have difficulty accessing timely, quality, affordable specialty care services. As the county’s only academic health system, the University of California, Irvine (UCI) aimed to improve specialty care access for the communities it serves by implementing an electronic consultations (eConsults) program that allows primary care providers (PCPs) to efficiently receive specialist recommendations on referral problems that do not require an in-person evaluation.
Objective: To implement an eConsults program at the UCI that enhances access to and the delivery of coordinated specialty care for lower-complexity referral problems.
Methods: We developed custom solutions to integrate eConsults into UCI’s 2 electronic health record platforms. The impact of the eConsults program was assessed by continuously evaluating usage and outcomes. Measures used to track usage included the number of submitted eConsult requests per PCP, the number of completed responses per specialty, and the response time for eConsult requests. Outcome measures included the specialist recommendation (eg, in-office visit, consultation avoided) and physician feedback.
Results: Over 4.5 years, more than 1400 successful eConsults have been completed, and the program has expanded to 17 specialties. The average turnaround time for an eConsult response across all specialties was 1 business day. Moreover, more than 50% of the eConsults received specialty responses within the same day of the eConsult request. Most important, about 80% of eConsult requests were addressed without the need for an in-office visit with a specialist.
Conclusion: The enhanced access to and the delivery of coordinated specialty care provided by eConsults resulted in improved efficiency and specialty access, while likely reducing costs and improving patient satisfaction. The improved communication and collaboration among providers with eConsults has also led to overwhelmingly positive feedback from both PCPs and specialists.
Keywords: electronic consultation; access to care; primary care; specialty referral; telehealth.
Orange County’s growing, aging, and diverse population is driving an increased demand for health care.1 But with the county’s high cost of living and worsening shortage of physicians,1-3 many of its residents are struggling to access timely, quality, affordable care. Access to specialty care services is especially frustrating for many patients and their providers, both primary care providers (PCPs) and specialists, due to problems with the referral process. Many patients experience increased wait times for a visit with a specialist due to poor communication between providers, insufficient guidance on the information or diagnostic results needed by specialists, and lack of care coordination.4-6 One promising approach to overcome these challenges is the use of an electronic consultation, or eConsult, in place of a standard in-person referral. An eConsult is an asynchronous, non-face-to-face, provider-to-provider exchange using a secure electronic communication platform. For appropriate referral problems, the patient is able to receive timely access to specialist expertise through electronic referral by their PCP,7-9 and avoid the time and costs associated with a visit to the specialist,10,11 such as travel, missed work, co-pays, and child-care expenses. Clinical questions addressed using an eConsult system subsequently free up office visit appointment slots, improving access for patients requiring in-office evaluation.8,12
Orange County’s only academic health system, the University of California, Irvine (UCI), serves a population of 3.5 million, and its principal priority is providing the communities in the county (which is the sixth largest in United States) and the surrounding region with the highest quality health care possible. Thus, UCI aimed to improve its referral processes and provide timely access to specialty care for its patients by implementing an eConsults program that allows PCPs to efficiently receive specialist recommendations on referral problems that do not require the specialist to evaluate the patient in person. This report describes our experiences with developing and implementing a custom-built eConsults workflow in UCI’s prior electronic health record (EHR) platform, Allscripts, and subsequently transitioning our mature eConsults program to a new EHR system when UCI adopted Epic. UCI is likely the only academic medical center to have experience in successfully implementing eConsults into 2 different EHR systems.
Setting
UCI’s medical center is a 417-bed acute care hospital providing tertiary and quaternary care, ambulatory and specialty medical clinics, behavioral health care, and rehabilitation services. It is located in Orange, CA, and serves a diverse population of 3.5 million persons with broad health care needs. With more than 400 specialty and primary care physicians, UCI offers a full scope of acute and general care services. It is also the primary teaching location for UCI medical and nursing students, medical residents, and fellows, and is home to Orange County’s only adult Level I and pediatric Level II trauma centers and the regional burn center.
eConsults Program
We designed the initial eConsults program within UCI’s Allscripts EHR platform. Our information technology (IT) build team developed unique “documents-based” eConsults workflows that simplified the process of initiating requests directly from the EHR and facilitated rapid responses from participating specialties. The requesting provider’s eConsults interface was user-friendly, and referring providers were able to initiate an eConsult easily by selecting the customized eConsult icon from the Allscripts main toolbar. To ensure that all relevant information is provided to the specialists, condition-specific templates are embedded in the requesting provider’s eConsults workflow that allow PCPs to enter a focused, patient-specific clinical question and provide guidance on recommended patient information (eg, health history, laboratory results, and digital images) that may help the specialist provide an informed response. The eConsult templates were adapted from standardized forms developed by partner University of California Health Systems in an initiative funded by the University of California Center for Health Quality and Innovation.
To facilitate timely responses from specialists, an innovative notification system was created in the responding provider’s eConsults workflow to automatically send an email to participating specialists when a new eConsult is requested. The responding provider’s workflow also includes an option for the specialist to decline the eConsult if the case is deemed too complex to be addressed electronically. For every completed eConsult that does not result in an in-person patient evaluation, the requesting provider and responding specialist each receives a modest reimbursement, which was initially paid by UCI Health System funds.
Implementation
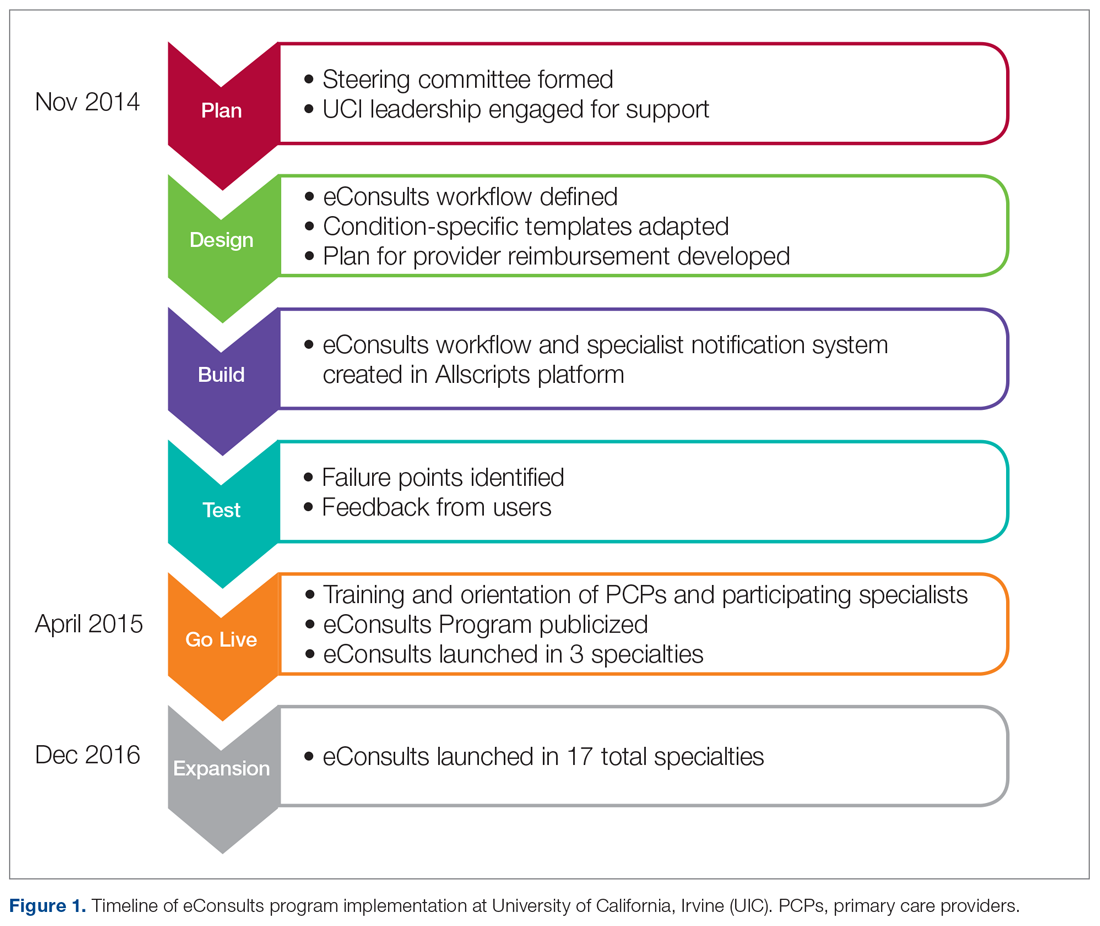
The design and implementation of the eConsults program began in November 2014, and was guided by a steering committee that included the chair of the department of medicine, chief medical information officer, primary care and specialty physician leads, IT build team, and a project manager. Early on, members of this committee engaged UCI leadership to affirm support for the program and obtain the IT resources needed to build the eConsults workflow. Regular steering committee meetings were established to discuss the design of the workflow, adapt the clinical content of the referral templates, and develop a provider reimbursement plan. After completion of the workflow build, the eConsults system was tested to identify failure points and obtain feedback from users. Prior to going live, the eConsults program was publicized by members of the steering committee through meetings with primary care groups and email communications. Committee members also hosted in-person training and orientation sessions with PCPs and participating specialists, and distributed tip sheets summarizing the steps to complete the PCP and specialist eConsult workflows.
The eConsults workflow build, testing, and launch were completed within 5 months (April 2015; Figure 1). eConsults went live in the 3 initial specialties (endocrinology, cardiology, and rheumatology) that were interested in participating in the first wave of the program. UCI’s eConsults service has subsequently expanded to 17 total specialties (allergy, cardiology, dermatology, endocrinology, gastroenterology, geriatrics, gynecology, hematology, hepatology, infectious disease, nephrology, neurology, palliative care, psychiatry, pulmonary, rheumatology, and sports medicine).
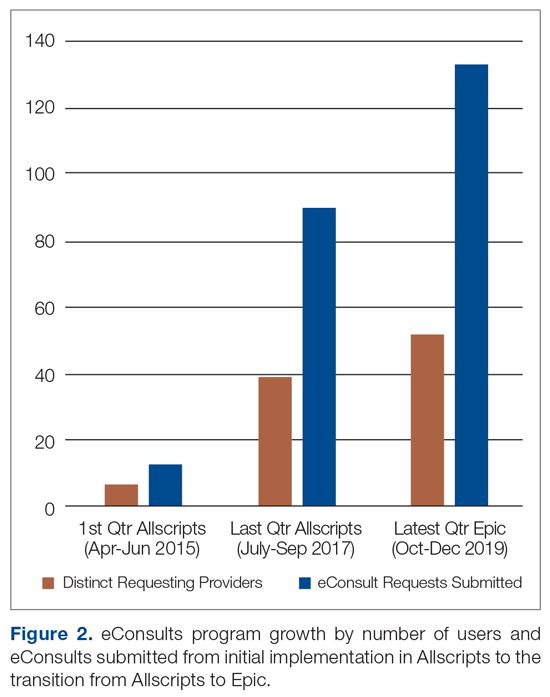
Two and half years after the eConsults program was implemented in Allscripts, UCI adopted a new EHR platform, Epic. By this time, the eConsults service had grown into a mature program with greater numbers of PCP users and submitted eConsults (Figure 2). Using our experience with the Allscripts build, our IT team was able to efficiently transition the eConsults service to the new EHR system. In contrast to the “documents-based” eConsult workflows on Allscripts, our IT team utilized an “orders-based” strategy on Epic, which followed a more traditional approach to requesting a consultation. We re-launched the service in Epic within 3 months (February 2018). However, both platforms utilized user-friendly workflows to achieve similar goals, and the program has continued to grow with respect to the number of users and eConsults.
Measurement/Analysis
The impact of the program was assessed by continuously evaluating usage and outcomes. Measures used to track usage included the number of PCP users, the number of submitted eConsult requests per PCP, and the number of requests per specialty. The response time for eConsult requests and the self-reported amount of time spent by specialists on the response were also tracked. Outcome measures included the specialist recommendation (eg, in-office visit, consultation avoided) and physician feedback. Provider satisfaction was primarily obtained by soliciting feedback from individual eConsult users.
Implementation of this eConsults program constituted a quality improvement activity and did not require Institutional Review Board review.
Results
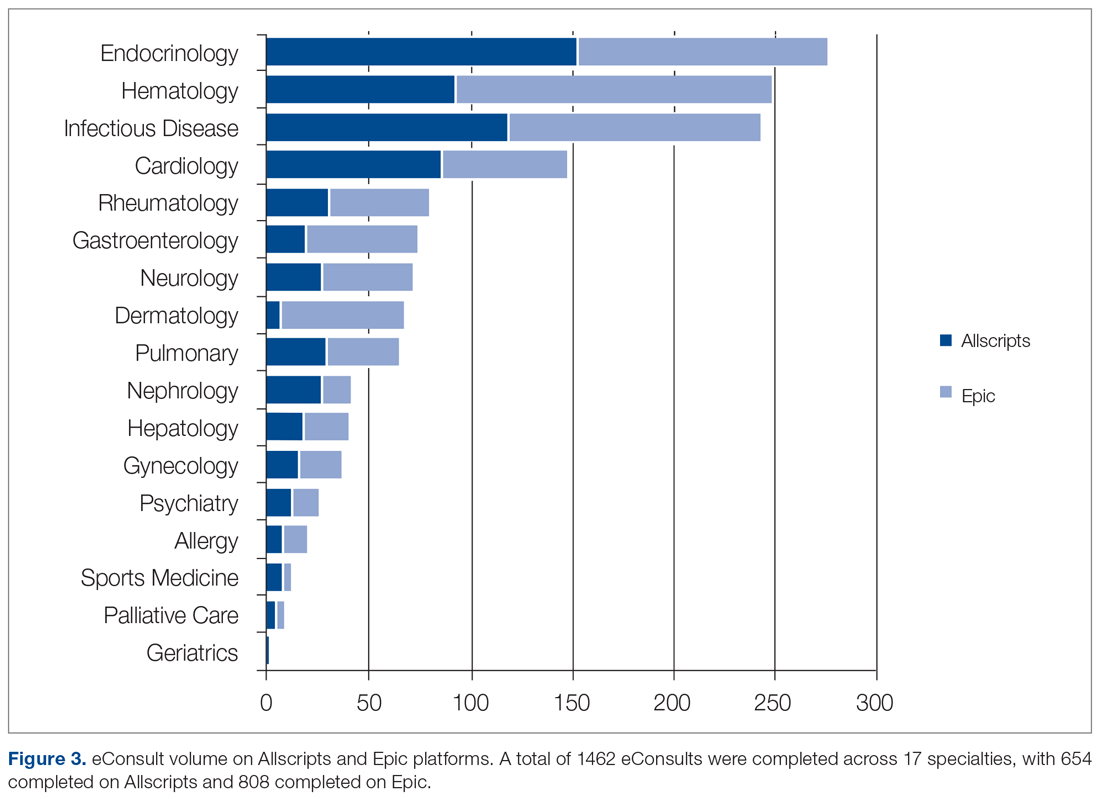
Since the program was launched in April 2015, more than 1400 eConsults have been completed across 17 specialties (Figure 3). There were 654 completed eConsults on the Allscripts platform, and 808 eConsults have been completed using the Epic platform to date. The busiest eConsult specialties were endocrinology (receiving 276, or 19%, of the eConsults requests), hematology (receiving 249 requests, or 17%), infectious disease (receiving 244 requests, or 17% ), and cardiology (receiving 148 requests, or 10%).
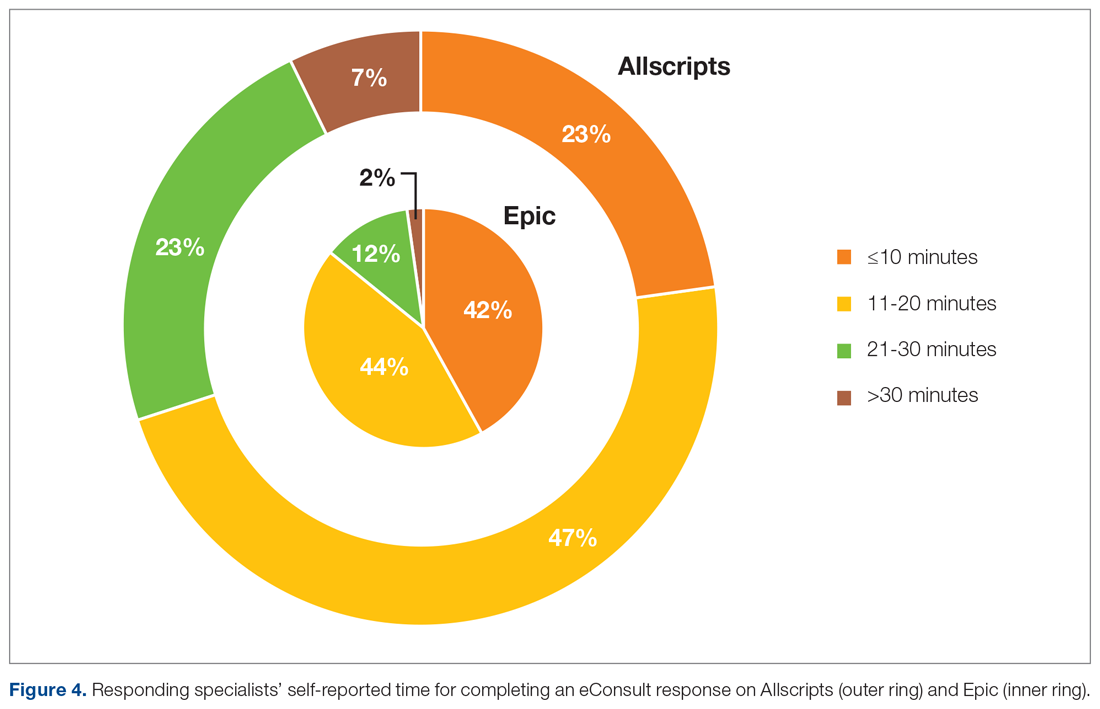
The self-reported amount of time specialists spent on the response was different between the 2 EHR systems (Figure 4). On Allscripts, specialists reported that 23% of eConsults took 10 minutes or less to complete, 47% took 11 to 20 minutes, 23% took 21 to 30 minutes, and 7% took more than 30 minutes. On Epic, specialists reported that 42% of eConsults took 10 minutes or less to complete, 44% took 11 to 20 minutes, 12% took 21 to 30 minutes, and 2% took more than 30 minutes. This difference in time spent fielding eConsults likely represents the subtle nuances between Allscripts’ “documents-based” and Epic’s “orders-based” workflows.
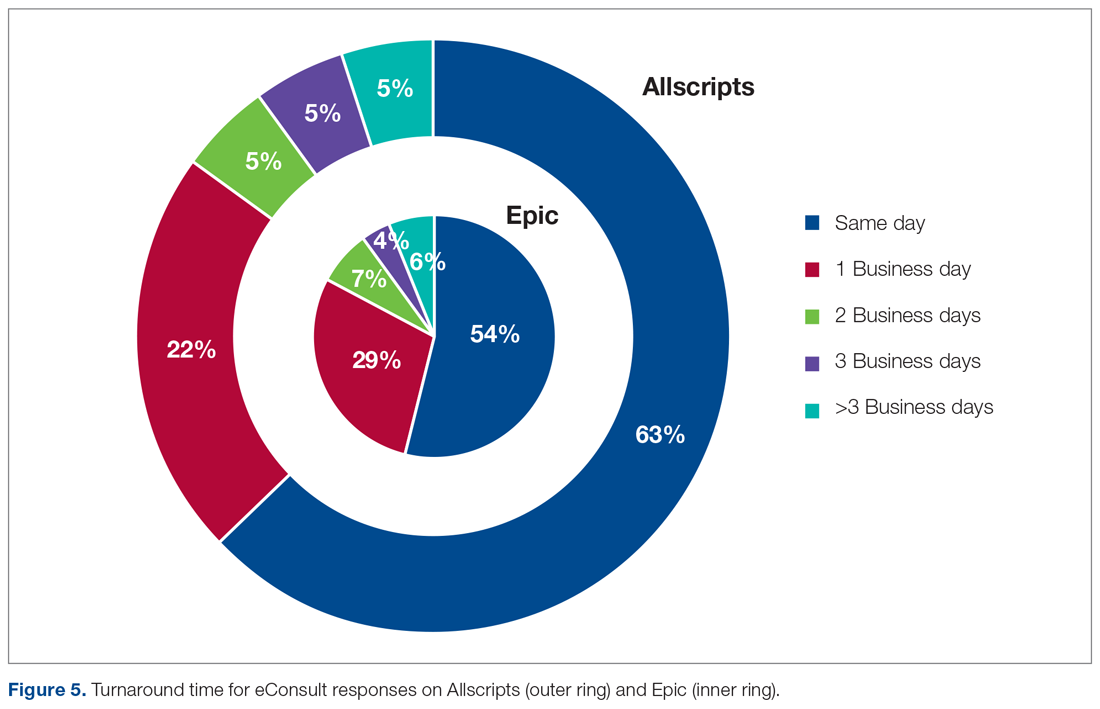
As a result of the automated notification system that was introduced early in the eConsults implementation process on Allscripts, the specialty response times were much faster than the expected 3 business days’ turnaround goal instituted by the Center for Health Quality and Innovation initiative, regardless of the EHR platform used. In fact, the average turnaround time for an eConsult response across all specialties was 1 business day, which was similar for both EHR systems (Figure 5). Furthermore, more than 50% of the eConsults on both EHR systems received specialist responses within the same day of the eConsult request (63% on Allscripts, 54% on Epic). There was a small decrease in the percentage of same-day responses when we transitioned to Epic, likely because the functionality of an automated notification email could not be restored in Epic. Regardless, the specialty response times on Epic remained expeditious, likely because the automated notifications on Allscripts instilled good practices for the specialists, and regularly checking for new eConsult requests became an ingrained behavior.
Our most important finding was that approximately 80% of eConsult requests were addressed without the need for an in-office visit with a specialist. This measure was similar for both EHR platforms (83% on Allscripts and 78% on Epic).
Provider feedback has been overwhelmingly positive. PCPs are impressed with the robust educational content of the eConsult responses, since the goal for specialists is to justify their recommendations. Specialists appreciate the convenience and efficiency that eConsults offer, as well as the improved communication and collaboration among physicians. eConsults have been especially beneficial to PCPs at UCI’s Family Health Centers, who are now able to receive subspecialty consultations from UCI specialists despite insurance barriers.
Discussion
Our eConsults program uniquely contrasts with other programs because UCI is likely the only academic medical center to have experience in successfully incorporating eConsults into 2 different EHR systems: initial development of the eConsults workflow in UCI’s existing Allscripts EHR platform, and subsequently transitioning a mature eConsults program to a new EHR system when the institution adopted Epic.
We measured the impact of the eConsults program on access to care by the response time for eConsult requests and the percentage of eConsults that averted an in-office visit with a specialist. We found that the eConsults program at UCI provided our PCPs access to specialist consultations in a timely manner, with much shorter response times than standard in-person referrals. The average turnaround time for an eConsult response we reported is consistent with findings from other studies.12-15 Additionally, our program was able to address about 80% of its eConsults electronically, helping to reduce unnecessary in-person specialist referrals. In the literature, the percentage of eConsults that avoided an in-person specialist visit varies widely.8,12-16
We reported very positive feedback from both PCPs and specialists on UCI’s eConsults service. Similarly, other studies described PCP satisfaction with their respective eConsults programs to be uniformly high,8,9,13,14,17-19 though some reported that the level of satisfaction among specialists was more varied.18-21
Lessons Learned
The successful design and implementation of our eConsults program began with assembling the right clinical champions and technology partners for our steering committee. Establishing regular steering committee meetings helped maintain an appropriate timeline for completion of different aspects of the project. Engaging support from UCI’s leadership also provided us with a dedicated IT team that helped us with the build, training resources, troubleshooting issues, and reporting for the project.
Our experience with implementing the eConsults program on 2 different EHR systems highlighted the importance of creating efficient, user-friendly workflows to foster provider adoption and achieve sustainability. Allscripts’ open platform gave our IT team the ability to create a homegrown solution to implementing an eConsult model that was simple and easy to use. The Epic platform’s interoperability allowed us to leverage our learnings from the Allscripts build to efficiently implement eConsults in Epic.
We also found that providing modest incentive payments or reimbursements to both PCPs and specialists for each completed eConsult helps with both adoption and program sustainability. Initially, credit for the eConsult work was paid by internal UCI Health System funds. Two payers, UC Care (a preferred provider organization plan created just for the University of California) and more recently, the Centers for Medicare & Medicaid Services, have agreed to reimburse for outpatient eConsults. Securing additional payers for reimbursement of the eConsult service will not only ensure the program’s long-term sustainability, but also represents an acknowledgment of the value of eConsults in supporting access to care.
Applicability
Other health care settings that are experiencing issues with specialty care access can successfully implement their own eConsults program by employing strategies similar to those described in this report—assembling the right team, creating user-friendly workflows, and providing incentives. Our advice for successful implementation is to clearly communicate your goals to all involved, including primary care, specialists, leadership, and IT partners, and establish with these stakeholders the appropriate support and resources needed to facilitate the development of the program and overcome any barriers to adoption.
Current Status and Future Directions
Our future plans include continuing to optimize the Epic eConsult backend build and workflows using our experience in Allscripts. We have implemented eConsult workflows for use by graduate medical education trainees and advanced practice providers, with attending supervision. Further work is in progress to optimize these workflows, which will allow for appropriate education and supervision without delaying care. Furthermore, we plan to expand the program to include inpatient-to-inpatient and emergency department-to-inpatient eConsults. We will continue to expand the eConsults program by offering additional specialties, engage providers to encourage ongoing participation, and maximize PCP use by continuing to market the program through regular newsletters and email communications. Finally, the eConsults has served as an effective, important resource in the current era of COVID-19 in several ways: it allows for optimization of specialty input in patient care delivery without subjecting more health care workers to unnecessary exposure; saves on utilization of precious personal protective equipment; and enhances our ability to deal with a potential surge by providing access to specialists remotely and electronically all hours of the day, thus expanding care to the evening and weekend hours.
Acknowledgment: The authors thank our steering committee members (Dr. Ralph Cygan, Dr. Andrew Reikes, Dr. Byron Allen, Dr. George Lawry) and IT build team (Lori Bocchicchio, Meghan van Witsen, Jaymee Zillgitt, Tanya Sickles, Dennis Hoang, Jeanette Lisak-Phillips) for their contributions in the design and implementation of our eConsults program. We also thank additional team members Kurt McArthur and Neaktisia Lee for their assistance with generating reports, and Kathy LaPierre, Jennifer Rios, and Debra Webb Torres for their guidance with compliance and billing issues.
Corresponding author: Alpesh N. Amin, MD, MBA, University of California, Irvine, 101 The City Drive South, Building 26, Room 1000, ZC-4076H, Orange, CA 92868; anamin@uci.edu.
Financial disclosures: None.
1. County of Orange, Health Care Agency, Public Health Services. Orange County Health Profile 2013.
2. Coffman JM, Fix M Ko, M. California physician supply and distribution: headed for a drought? California Health Care Foundation, June 2018.
3. Spetz J, Coffman J, Geyn I. California’s primary care workforce: forecasted supply, demand, and pipeline of trainees, 2016-2030. Healthforce Center at the University of California, San Francisco, August 2017.
4. Gandhi TK, Sittig DF, Franklin M, et al. Communication breakdown in the outpatient referral process. J Gen Intern Med. 2000;15:626-631.
5. McPhee SJ, Lo B, Saika GY, Meltzer R. How good is communication between primary care physicians and subspecialty consultants? Arch Intern Med. 1984;144:1265-1268.
6. Mehrotra A, Forrest CB, Lin CY. Dropping the baton: specialty referrals in the United States. Milbank Q. 2011;89:39-68.
7. Wrenn K, Catschegn S, Cruz M, et al. Analysis of an electronic consultation program at an academic medical centre: Primary care provider questions, specialist responses, and primary care provider actions. J Telemed Telecare. 2017;23: 217-224.
8. Gleason N, Prasad PA, Ackerman S, et al. Adoption and impact of an eConsult system in a fee-for-service setting. Healthc (Amst). 2017;5(1-2):40-45.
9. Stoves J, Connolly J, Cheung CK, et al. Electronic consultation as an alternative to hospital referral for patients with chronic kidney disease: a novel application for networked electronic health records to improve the accessibility and efficiency of healthcare. Qual Saf Health Care. 2010;19: e54.
10. Datta SK, Warshaw EM, Edison KE, et al. Cost and utility analysis of a store-and-forward teledermatology referral system: a randomized clinical trial. JAMA Dermatol. 2015;151:1323-1329.
11. Liddy C, Drosinis P, Deri Armstrong C, et al. What are the cost savings associated with providing access to specialist care through the Champlain BASE eConsult service? A costing evaluation. BMJ Open. 2016;6:e010920.
12. Barnett ML, Yee HF Jr, Mehrotra A, Giboney P. Los Angeles safety-net program eConsult system was rapidly adopted and decreased wait times to see specialists. Health Aff. 2017;36:492-499.
13. Malagrino GD, Chaudhry R, Gardner M, et al. A study of 6,000 electronic specialty consultations for person-centered care at The Mayo Clinic. Int J Person Centered Med. 2012;2:458-466.
14. Keely E, Liddy C, Afkham A. Utilization, benefits, and impact of an e-consultation service across diverse specialties and primary care providers. Telemed J E Health. 2013;19:733-738.
15. Scherpbier-de Haan ND, van Gelder VA, Van Weel C, et al. Initial implementation of a web-based consultation process for patients with chronic kidney disease. Ann Fam Med. 2013;11:151-156.
16. Palen TE, Price D, Shetterly S, Wallace KB. Comparing virtual consults to traditional consults using an electronic health record: an observational case-control study. BMC Med Inform Decis Mak. 2012;12:65.
17. Liddy C, Afkham A, Drosinis P, et al. Impact of and satisfaction with a new eConsult service: a mixed methods study of primary care providers. J Am Board Fam Med. 2015;28:394-403.
18. Angstman KB, Adamson SC, Furst JW, et al. Provider satisfaction with virtual specialist consultations in a family medicine department. Health Care Manag (Frederick). 2009;28:14-18.
19. McAdams M, Cannavo L, Orlander JD. A medical specialty e-consult program in a VA health care system. Fed Pract. 2014; 31:26–31.
20. Keely E, Williams R, Epstein G, et al. Specialist perspectives on Ontario Provincial electronic consultation services. Telemed J E Health. 2019;25:3-10.
21. Kim-Hwang JE, Chen AH, Bell DS, et al. Evaluating electronic referrals for specialty care at a public hospital. J Gen Intern Med. 2010;25:1123-1128.
From the Department of Medicine, University of California, Irvine, Orange, CA.
Abstract
Background: Orange County’s residents have difficulty accessing timely, quality, affordable specialty care services. As the county’s only academic health system, the University of California, Irvine (UCI) aimed to improve specialty care access for the communities it serves by implementing an electronic consultations (eConsults) program that allows primary care providers (PCPs) to efficiently receive specialist recommendations on referral problems that do not require an in-person evaluation.
Objective: To implement an eConsults program at the UCI that enhances access to and the delivery of coordinated specialty care for lower-complexity referral problems.
Methods: We developed custom solutions to integrate eConsults into UCI’s 2 electronic health record platforms. The impact of the eConsults program was assessed by continuously evaluating usage and outcomes. Measures used to track usage included the number of submitted eConsult requests per PCP, the number of completed responses per specialty, and the response time for eConsult requests. Outcome measures included the specialist recommendation (eg, in-office visit, consultation avoided) and physician feedback.
Results: Over 4.5 years, more than 1400 successful eConsults have been completed, and the program has expanded to 17 specialties. The average turnaround time for an eConsult response across all specialties was 1 business day. Moreover, more than 50% of the eConsults received specialty responses within the same day of the eConsult request. Most important, about 80% of eConsult requests were addressed without the need for an in-office visit with a specialist.
Conclusion: The enhanced access to and the delivery of coordinated specialty care provided by eConsults resulted in improved efficiency and specialty access, while likely reducing costs and improving patient satisfaction. The improved communication and collaboration among providers with eConsults has also led to overwhelmingly positive feedback from both PCPs and specialists.
Keywords: electronic consultation; access to care; primary care; specialty referral; telehealth.
Orange County’s growing, aging, and diverse population is driving an increased demand for health care.1 But with the county’s high cost of living and worsening shortage of physicians,1-3 many of its residents are struggling to access timely, quality, affordable care. Access to specialty care services is especially frustrating for many patients and their providers, both primary care providers (PCPs) and specialists, due to problems with the referral process. Many patients experience increased wait times for a visit with a specialist due to poor communication between providers, insufficient guidance on the information or diagnostic results needed by specialists, and lack of care coordination.4-6 One promising approach to overcome these challenges is the use of an electronic consultation, or eConsult, in place of a standard in-person referral. An eConsult is an asynchronous, non-face-to-face, provider-to-provider exchange using a secure electronic communication platform. For appropriate referral problems, the patient is able to receive timely access to specialist expertise through electronic referral by their PCP,7-9 and avoid the time and costs associated with a visit to the specialist,10,11 such as travel, missed work, co-pays, and child-care expenses. Clinical questions addressed using an eConsult system subsequently free up office visit appointment slots, improving access for patients requiring in-office evaluation.8,12
Orange County’s only academic health system, the University of California, Irvine (UCI), serves a population of 3.5 million, and its principal priority is providing the communities in the county (which is the sixth largest in United States) and the surrounding region with the highest quality health care possible. Thus, UCI aimed to improve its referral processes and provide timely access to specialty care for its patients by implementing an eConsults program that allows PCPs to efficiently receive specialist recommendations on referral problems that do not require the specialist to evaluate the patient in person. This report describes our experiences with developing and implementing a custom-built eConsults workflow in UCI’s prior electronic health record (EHR) platform, Allscripts, and subsequently transitioning our mature eConsults program to a new EHR system when UCI adopted Epic. UCI is likely the only academic medical center to have experience in successfully implementing eConsults into 2 different EHR systems.
Setting
UCI’s medical center is a 417-bed acute care hospital providing tertiary and quaternary care, ambulatory and specialty medical clinics, behavioral health care, and rehabilitation services. It is located in Orange, CA, and serves a diverse population of 3.5 million persons with broad health care needs. With more than 400 specialty and primary care physicians, UCI offers a full scope of acute and general care services. It is also the primary teaching location for UCI medical and nursing students, medical residents, and fellows, and is home to Orange County’s only adult Level I and pediatric Level II trauma centers and the regional burn center.
eConsults Program
We designed the initial eConsults program within UCI’s Allscripts EHR platform. Our information technology (IT) build team developed unique “documents-based” eConsults workflows that simplified the process of initiating requests directly from the EHR and facilitated rapid responses from participating specialties. The requesting provider’s eConsults interface was user-friendly, and referring providers were able to initiate an eConsult easily by selecting the customized eConsult icon from the Allscripts main toolbar. To ensure that all relevant information is provided to the specialists, condition-specific templates are embedded in the requesting provider’s eConsults workflow that allow PCPs to enter a focused, patient-specific clinical question and provide guidance on recommended patient information (eg, health history, laboratory results, and digital images) that may help the specialist provide an informed response. The eConsult templates were adapted from standardized forms developed by partner University of California Health Systems in an initiative funded by the University of California Center for Health Quality and Innovation.
To facilitate timely responses from specialists, an innovative notification system was created in the responding provider’s eConsults workflow to automatically send an email to participating specialists when a new eConsult is requested. The responding provider’s workflow also includes an option for the specialist to decline the eConsult if the case is deemed too complex to be addressed electronically. For every completed eConsult that does not result in an in-person patient evaluation, the requesting provider and responding specialist each receives a modest reimbursement, which was initially paid by UCI Health System funds.
Implementation
The design and implementation of the eConsults program began in November 2014, and was guided by a steering committee that included the chair of the department of medicine, chief medical information officer, primary care and specialty physician leads, IT build team, and a project manager. Early on, members of this committee engaged UCI leadership to affirm support for the program and obtain the IT resources needed to build the eConsults workflow. Regular steering committee meetings were established to discuss the design of the workflow, adapt the clinical content of the referral templates, and develop a provider reimbursement plan. After completion of the workflow build, the eConsults system was tested to identify failure points and obtain feedback from users. Prior to going live, the eConsults program was publicized by members of the steering committee through meetings with primary care groups and email communications. Committee members also hosted in-person training and orientation sessions with PCPs and participating specialists, and distributed tip sheets summarizing the steps to complete the PCP and specialist eConsult workflows.
The eConsults workflow build, testing, and launch were completed within 5 months (April 2015; Figure 1). eConsults went live in the 3 initial specialties (endocrinology, cardiology, and rheumatology) that were interested in participating in the first wave of the program. UCI’s eConsults service has subsequently expanded to 17 total specialties (allergy, cardiology, dermatology, endocrinology, gastroenterology, geriatrics, gynecology, hematology, hepatology, infectious disease, nephrology, neurology, palliative care, psychiatry, pulmonary, rheumatology, and sports medicine).
Two and half years after the eConsults program was implemented in Allscripts, UCI adopted a new EHR platform, Epic. By this time, the eConsults service had grown into a mature program with greater numbers of PCP users and submitted eConsults (Figure 2). Using our experience with the Allscripts build, our IT team was able to efficiently transition the eConsults service to the new EHR system. In contrast to the “documents-based” eConsult workflows on Allscripts, our IT team utilized an “orders-based” strategy on Epic, which followed a more traditional approach to requesting a consultation. We re-launched the service in Epic within 3 months (February 2018). However, both platforms utilized user-friendly workflows to achieve similar goals, and the program has continued to grow with respect to the number of users and eConsults.
Measurement/Analysis
The impact of the program was assessed by continuously evaluating usage and outcomes. Measures used to track usage included the number of PCP users, the number of submitted eConsult requests per PCP, and the number of requests per specialty. The response time for eConsult requests and the self-reported amount of time spent by specialists on the response were also tracked. Outcome measures included the specialist recommendation (eg, in-office visit, consultation avoided) and physician feedback. Provider satisfaction was primarily obtained by soliciting feedback from individual eConsult users.
Implementation of this eConsults program constituted a quality improvement activity and did not require Institutional Review Board review.
Results
Since the program was launched in April 2015, more than 1400 eConsults have been completed across 17 specialties (Figure 3). There were 654 completed eConsults on the Allscripts platform, and 808 eConsults have been completed using the Epic platform to date. The busiest eConsult specialties were endocrinology (receiving 276, or 19%, of the eConsults requests), hematology (receiving 249 requests, or 17%), infectious disease (receiving 244 requests, or 17% ), and cardiology (receiving 148 requests, or 10%).
The self-reported amount of time specialists spent on the response was different between the 2 EHR systems (Figure 4). On Allscripts, specialists reported that 23% of eConsults took 10 minutes or less to complete, 47% took 11 to 20 minutes, 23% took 21 to 30 minutes, and 7% took more than 30 minutes. On Epic, specialists reported that 42% of eConsults took 10 minutes or less to complete, 44% took 11 to 20 minutes, 12% took 21 to 30 minutes, and 2% took more than 30 minutes. This difference in time spent fielding eConsults likely represents the subtle nuances between Allscripts’ “documents-based” and Epic’s “orders-based” workflows.
As a result of the automated notification system that was introduced early in the eConsults implementation process on Allscripts, the specialty response times were much faster than the expected 3 business days’ turnaround goal instituted by the Center for Health Quality and Innovation initiative, regardless of the EHR platform used. In fact, the average turnaround time for an eConsult response across all specialties was 1 business day, which was similar for both EHR systems (Figure 5). Furthermore, more than 50% of the eConsults on both EHR systems received specialist responses within the same day of the eConsult request (63% on Allscripts, 54% on Epic). There was a small decrease in the percentage of same-day responses when we transitioned to Epic, likely because the functionality of an automated notification email could not be restored in Epic. Regardless, the specialty response times on Epic remained expeditious, likely because the automated notifications on Allscripts instilled good practices for the specialists, and regularly checking for new eConsult requests became an ingrained behavior.
Our most important finding was that approximately 80% of eConsult requests were addressed without the need for an in-office visit with a specialist. This measure was similar for both EHR platforms (83% on Allscripts and 78% on Epic).
Provider feedback has been overwhelmingly positive. PCPs are impressed with the robust educational content of the eConsult responses, since the goal for specialists is to justify their recommendations. Specialists appreciate the convenience and efficiency that eConsults offer, as well as the improved communication and collaboration among physicians. eConsults have been especially beneficial to PCPs at UCI’s Family Health Centers, who are now able to receive subspecialty consultations from UCI specialists despite insurance barriers.
Discussion
Our eConsults program uniquely contrasts with other programs because UCI is likely the only academic medical center to have experience in successfully incorporating eConsults into 2 different EHR systems: initial development of the eConsults workflow in UCI’s existing Allscripts EHR platform, and subsequently transitioning a mature eConsults program to a new EHR system when the institution adopted Epic.
We measured the impact of the eConsults program on access to care by the response time for eConsult requests and the percentage of eConsults that averted an in-office visit with a specialist. We found that the eConsults program at UCI provided our PCPs access to specialist consultations in a timely manner, with much shorter response times than standard in-person referrals. The average turnaround time for an eConsult response we reported is consistent with findings from other studies.12-15 Additionally, our program was able to address about 80% of its eConsults electronically, helping to reduce unnecessary in-person specialist referrals. In the literature, the percentage of eConsults that avoided an in-person specialist visit varies widely.8,12-16
We reported very positive feedback from both PCPs and specialists on UCI’s eConsults service. Similarly, other studies described PCP satisfaction with their respective eConsults programs to be uniformly high,8,9,13,14,17-19 though some reported that the level of satisfaction among specialists was more varied.18-21
Lessons Learned
The successful design and implementation of our eConsults program began with assembling the right clinical champions and technology partners for our steering committee. Establishing regular steering committee meetings helped maintain an appropriate timeline for completion of different aspects of the project. Engaging support from UCI’s leadership also provided us with a dedicated IT team that helped us with the build, training resources, troubleshooting issues, and reporting for the project.
Our experience with implementing the eConsults program on 2 different EHR systems highlighted the importance of creating efficient, user-friendly workflows to foster provider adoption and achieve sustainability. Allscripts’ open platform gave our IT team the ability to create a homegrown solution to implementing an eConsult model that was simple and easy to use. The Epic platform’s interoperability allowed us to leverage our learnings from the Allscripts build to efficiently implement eConsults in Epic.
We also found that providing modest incentive payments or reimbursements to both PCPs and specialists for each completed eConsult helps with both adoption and program sustainability. Initially, credit for the eConsult work was paid by internal UCI Health System funds. Two payers, UC Care (a preferred provider organization plan created just for the University of California) and more recently, the Centers for Medicare & Medicaid Services, have agreed to reimburse for outpatient eConsults. Securing additional payers for reimbursement of the eConsult service will not only ensure the program’s long-term sustainability, but also represents an acknowledgment of the value of eConsults in supporting access to care.
Applicability
Other health care settings that are experiencing issues with specialty care access can successfully implement their own eConsults program by employing strategies similar to those described in this report—assembling the right team, creating user-friendly workflows, and providing incentives. Our advice for successful implementation is to clearly communicate your goals to all involved, including primary care, specialists, leadership, and IT partners, and establish with these stakeholders the appropriate support and resources needed to facilitate the development of the program and overcome any barriers to adoption.
Current Status and Future Directions
Our future plans include continuing to optimize the Epic eConsult backend build and workflows using our experience in Allscripts. We have implemented eConsult workflows for use by graduate medical education trainees and advanced practice providers, with attending supervision. Further work is in progress to optimize these workflows, which will allow for appropriate education and supervision without delaying care. Furthermore, we plan to expand the program to include inpatient-to-inpatient and emergency department-to-inpatient eConsults. We will continue to expand the eConsults program by offering additional specialties, engage providers to encourage ongoing participation, and maximize PCP use by continuing to market the program through regular newsletters and email communications. Finally, the eConsults has served as an effective, important resource in the current era of COVID-19 in several ways: it allows for optimization of specialty input in patient care delivery without subjecting more health care workers to unnecessary exposure; saves on utilization of precious personal protective equipment; and enhances our ability to deal with a potential surge by providing access to specialists remotely and electronically all hours of the day, thus expanding care to the evening and weekend hours.
Acknowledgment: The authors thank our steering committee members (Dr. Ralph Cygan, Dr. Andrew Reikes, Dr. Byron Allen, Dr. George Lawry) and IT build team (Lori Bocchicchio, Meghan van Witsen, Jaymee Zillgitt, Tanya Sickles, Dennis Hoang, Jeanette Lisak-Phillips) for their contributions in the design and implementation of our eConsults program. We also thank additional team members Kurt McArthur and Neaktisia Lee for their assistance with generating reports, and Kathy LaPierre, Jennifer Rios, and Debra Webb Torres for their guidance with compliance and billing issues.
Corresponding author: Alpesh N. Amin, MD, MBA, University of California, Irvine, 101 The City Drive South, Building 26, Room 1000, ZC-4076H, Orange, CA 92868; anamin@uci.edu.
Financial disclosures: None.
From the Department of Medicine, University of California, Irvine, Orange, CA.
Abstract
Background: Orange County’s residents have difficulty accessing timely, quality, affordable specialty care services. As the county’s only academic health system, the University of California, Irvine (UCI) aimed to improve specialty care access for the communities it serves by implementing an electronic consultations (eConsults) program that allows primary care providers (PCPs) to efficiently receive specialist recommendations on referral problems that do not require an in-person evaluation.
Objective: To implement an eConsults program at the UCI that enhances access to and the delivery of coordinated specialty care for lower-complexity referral problems.
Methods: We developed custom solutions to integrate eConsults into UCI’s 2 electronic health record platforms. The impact of the eConsults program was assessed by continuously evaluating usage and outcomes. Measures used to track usage included the number of submitted eConsult requests per PCP, the number of completed responses per specialty, and the response time for eConsult requests. Outcome measures included the specialist recommendation (eg, in-office visit, consultation avoided) and physician feedback.
Results: Over 4.5 years, more than 1400 successful eConsults have been completed, and the program has expanded to 17 specialties. The average turnaround time for an eConsult response across all specialties was 1 business day. Moreover, more than 50% of the eConsults received specialty responses within the same day of the eConsult request. Most important, about 80% of eConsult requests were addressed without the need for an in-office visit with a specialist.
Conclusion: The enhanced access to and the delivery of coordinated specialty care provided by eConsults resulted in improved efficiency and specialty access, while likely reducing costs and improving patient satisfaction. The improved communication and collaboration among providers with eConsults has also led to overwhelmingly positive feedback from both PCPs and specialists.
Keywords: electronic consultation; access to care; primary care; specialty referral; telehealth.
Orange County’s growing, aging, and diverse population is driving an increased demand for health care.1 But with the county’s high cost of living and worsening shortage of physicians,1-3 many of its residents are struggling to access timely, quality, affordable care. Access to specialty care services is especially frustrating for many patients and their providers, both primary care providers (PCPs) and specialists, due to problems with the referral process. Many patients experience increased wait times for a visit with a specialist due to poor communication between providers, insufficient guidance on the information or diagnostic results needed by specialists, and lack of care coordination.4-6 One promising approach to overcome these challenges is the use of an electronic consultation, or eConsult, in place of a standard in-person referral. An eConsult is an asynchronous, non-face-to-face, provider-to-provider exchange using a secure electronic communication platform. For appropriate referral problems, the patient is able to receive timely access to specialist expertise through electronic referral by their PCP,7-9 and avoid the time and costs associated with a visit to the specialist,10,11 such as travel, missed work, co-pays, and child-care expenses. Clinical questions addressed using an eConsult system subsequently free up office visit appointment slots, improving access for patients requiring in-office evaluation.8,12
Orange County’s only academic health system, the University of California, Irvine (UCI), serves a population of 3.5 million, and its principal priority is providing the communities in the county (which is the sixth largest in United States) and the surrounding region with the highest quality health care possible. Thus, UCI aimed to improve its referral processes and provide timely access to specialty care for its patients by implementing an eConsults program that allows PCPs to efficiently receive specialist recommendations on referral problems that do not require the specialist to evaluate the patient in person. This report describes our experiences with developing and implementing a custom-built eConsults workflow in UCI’s prior electronic health record (EHR) platform, Allscripts, and subsequently transitioning our mature eConsults program to a new EHR system when UCI adopted Epic. UCI is likely the only academic medical center to have experience in successfully implementing eConsults into 2 different EHR systems.
Setting
UCI’s medical center is a 417-bed acute care hospital providing tertiary and quaternary care, ambulatory and specialty medical clinics, behavioral health care, and rehabilitation services. It is located in Orange, CA, and serves a diverse population of 3.5 million persons with broad health care needs. With more than 400 specialty and primary care physicians, UCI offers a full scope of acute and general care services. It is also the primary teaching location for UCI medical and nursing students, medical residents, and fellows, and is home to Orange County’s only adult Level I and pediatric Level II trauma centers and the regional burn center.
eConsults Program
We designed the initial eConsults program within UCI’s Allscripts EHR platform. Our information technology (IT) build team developed unique “documents-based” eConsults workflows that simplified the process of initiating requests directly from the EHR and facilitated rapid responses from participating specialties. The requesting provider’s eConsults interface was user-friendly, and referring providers were able to initiate an eConsult easily by selecting the customized eConsult icon from the Allscripts main toolbar. To ensure that all relevant information is provided to the specialists, condition-specific templates are embedded in the requesting provider’s eConsults workflow that allow PCPs to enter a focused, patient-specific clinical question and provide guidance on recommended patient information (eg, health history, laboratory results, and digital images) that may help the specialist provide an informed response. The eConsult templates were adapted from standardized forms developed by partner University of California Health Systems in an initiative funded by the University of California Center for Health Quality and Innovation.
To facilitate timely responses from specialists, an innovative notification system was created in the responding provider’s eConsults workflow to automatically send an email to participating specialists when a new eConsult is requested. The responding provider’s workflow also includes an option for the specialist to decline the eConsult if the case is deemed too complex to be addressed electronically. For every completed eConsult that does not result in an in-person patient evaluation, the requesting provider and responding specialist each receives a modest reimbursement, which was initially paid by UCI Health System funds.
Implementation
The design and implementation of the eConsults program began in November 2014, and was guided by a steering committee that included the chair of the department of medicine, chief medical information officer, primary care and specialty physician leads, IT build team, and a project manager. Early on, members of this committee engaged UCI leadership to affirm support for the program and obtain the IT resources needed to build the eConsults workflow. Regular steering committee meetings were established to discuss the design of the workflow, adapt the clinical content of the referral templates, and develop a provider reimbursement plan. After completion of the workflow build, the eConsults system was tested to identify failure points and obtain feedback from users. Prior to going live, the eConsults program was publicized by members of the steering committee through meetings with primary care groups and email communications. Committee members also hosted in-person training and orientation sessions with PCPs and participating specialists, and distributed tip sheets summarizing the steps to complete the PCP and specialist eConsult workflows.
The eConsults workflow build, testing, and launch were completed within 5 months (April 2015; Figure 1). eConsults went live in the 3 initial specialties (endocrinology, cardiology, and rheumatology) that were interested in participating in the first wave of the program. UCI’s eConsults service has subsequently expanded to 17 total specialties (allergy, cardiology, dermatology, endocrinology, gastroenterology, geriatrics, gynecology, hematology, hepatology, infectious disease, nephrology, neurology, palliative care, psychiatry, pulmonary, rheumatology, and sports medicine).
Two and half years after the eConsults program was implemented in Allscripts, UCI adopted a new EHR platform, Epic. By this time, the eConsults service had grown into a mature program with greater numbers of PCP users and submitted eConsults (Figure 2). Using our experience with the Allscripts build, our IT team was able to efficiently transition the eConsults service to the new EHR system. In contrast to the “documents-based” eConsult workflows on Allscripts, our IT team utilized an “orders-based” strategy on Epic, which followed a more traditional approach to requesting a consultation. We re-launched the service in Epic within 3 months (February 2018). However, both platforms utilized user-friendly workflows to achieve similar goals, and the program has continued to grow with respect to the number of users and eConsults.
Measurement/Analysis
The impact of the program was assessed by continuously evaluating usage and outcomes. Measures used to track usage included the number of PCP users, the number of submitted eConsult requests per PCP, and the number of requests per specialty. The response time for eConsult requests and the self-reported amount of time spent by specialists on the response were also tracked. Outcome measures included the specialist recommendation (eg, in-office visit, consultation avoided) and physician feedback. Provider satisfaction was primarily obtained by soliciting feedback from individual eConsult users.
Implementation of this eConsults program constituted a quality improvement activity and did not require Institutional Review Board review.
Results
Since the program was launched in April 2015, more than 1400 eConsults have been completed across 17 specialties (Figure 3). There were 654 completed eConsults on the Allscripts platform, and 808 eConsults have been completed using the Epic platform to date. The busiest eConsult specialties were endocrinology (receiving 276, or 19%, of the eConsults requests), hematology (receiving 249 requests, or 17%), infectious disease (receiving 244 requests, or 17% ), and cardiology (receiving 148 requests, or 10%).
The self-reported amount of time specialists spent on the response was different between the 2 EHR systems (Figure 4). On Allscripts, specialists reported that 23% of eConsults took 10 minutes or less to complete, 47% took 11 to 20 minutes, 23% took 21 to 30 minutes, and 7% took more than 30 minutes. On Epic, specialists reported that 42% of eConsults took 10 minutes or less to complete, 44% took 11 to 20 minutes, 12% took 21 to 30 minutes, and 2% took more than 30 minutes. This difference in time spent fielding eConsults likely represents the subtle nuances between Allscripts’ “documents-based” and Epic’s “orders-based” workflows.
As a result of the automated notification system that was introduced early in the eConsults implementation process on Allscripts, the specialty response times were much faster than the expected 3 business days’ turnaround goal instituted by the Center for Health Quality and Innovation initiative, regardless of the EHR platform used. In fact, the average turnaround time for an eConsult response across all specialties was 1 business day, which was similar for both EHR systems (Figure 5). Furthermore, more than 50% of the eConsults on both EHR systems received specialist responses within the same day of the eConsult request (63% on Allscripts, 54% on Epic). There was a small decrease in the percentage of same-day responses when we transitioned to Epic, likely because the functionality of an automated notification email could not be restored in Epic. Regardless, the specialty response times on Epic remained expeditious, likely because the automated notifications on Allscripts instilled good practices for the specialists, and regularly checking for new eConsult requests became an ingrained behavior.
Our most important finding was that approximately 80% of eConsult requests were addressed without the need for an in-office visit with a specialist. This measure was similar for both EHR platforms (83% on Allscripts and 78% on Epic).
Provider feedback has been overwhelmingly positive. PCPs are impressed with the robust educational content of the eConsult responses, since the goal for specialists is to justify their recommendations. Specialists appreciate the convenience and efficiency that eConsults offer, as well as the improved communication and collaboration among physicians. eConsults have been especially beneficial to PCPs at UCI’s Family Health Centers, who are now able to receive subspecialty consultations from UCI specialists despite insurance barriers.
Discussion
Our eConsults program uniquely contrasts with other programs because UCI is likely the only academic medical center to have experience in successfully incorporating eConsults into 2 different EHR systems: initial development of the eConsults workflow in UCI’s existing Allscripts EHR platform, and subsequently transitioning a mature eConsults program to a new EHR system when the institution adopted Epic.
We measured the impact of the eConsults program on access to care by the response time for eConsult requests and the percentage of eConsults that averted an in-office visit with a specialist. We found that the eConsults program at UCI provided our PCPs access to specialist consultations in a timely manner, with much shorter response times than standard in-person referrals. The average turnaround time for an eConsult response we reported is consistent with findings from other studies.12-15 Additionally, our program was able to address about 80% of its eConsults electronically, helping to reduce unnecessary in-person specialist referrals. In the literature, the percentage of eConsults that avoided an in-person specialist visit varies widely.8,12-16
We reported very positive feedback from both PCPs and specialists on UCI’s eConsults service. Similarly, other studies described PCP satisfaction with their respective eConsults programs to be uniformly high,8,9,13,14,17-19 though some reported that the level of satisfaction among specialists was more varied.18-21
Lessons Learned
The successful design and implementation of our eConsults program began with assembling the right clinical champions and technology partners for our steering committee. Establishing regular steering committee meetings helped maintain an appropriate timeline for completion of different aspects of the project. Engaging support from UCI’s leadership also provided us with a dedicated IT team that helped us with the build, training resources, troubleshooting issues, and reporting for the project.
Our experience with implementing the eConsults program on 2 different EHR systems highlighted the importance of creating efficient, user-friendly workflows to foster provider adoption and achieve sustainability. Allscripts’ open platform gave our IT team the ability to create a homegrown solution to implementing an eConsult model that was simple and easy to use. The Epic platform’s interoperability allowed us to leverage our learnings from the Allscripts build to efficiently implement eConsults in Epic.
We also found that providing modest incentive payments or reimbursements to both PCPs and specialists for each completed eConsult helps with both adoption and program sustainability. Initially, credit for the eConsult work was paid by internal UCI Health System funds. Two payers, UC Care (a preferred provider organization plan created just for the University of California) and more recently, the Centers for Medicare & Medicaid Services, have agreed to reimburse for outpatient eConsults. Securing additional payers for reimbursement of the eConsult service will not only ensure the program’s long-term sustainability, but also represents an acknowledgment of the value of eConsults in supporting access to care.
Applicability
Other health care settings that are experiencing issues with specialty care access can successfully implement their own eConsults program by employing strategies similar to those described in this report—assembling the right team, creating user-friendly workflows, and providing incentives. Our advice for successful implementation is to clearly communicate your goals to all involved, including primary care, specialists, leadership, and IT partners, and establish with these stakeholders the appropriate support and resources needed to facilitate the development of the program and overcome any barriers to adoption.
Current Status and Future Directions
Our future plans include continuing to optimize the Epic eConsult backend build and workflows using our experience in Allscripts. We have implemented eConsult workflows for use by graduate medical education trainees and advanced practice providers, with attending supervision. Further work is in progress to optimize these workflows, which will allow for appropriate education and supervision without delaying care. Furthermore, we plan to expand the program to include inpatient-to-inpatient and emergency department-to-inpatient eConsults. We will continue to expand the eConsults program by offering additional specialties, engage providers to encourage ongoing participation, and maximize PCP use by continuing to market the program through regular newsletters and email communications. Finally, the eConsults has served as an effective, important resource in the current era of COVID-19 in several ways: it allows for optimization of specialty input in patient care delivery without subjecting more health care workers to unnecessary exposure; saves on utilization of precious personal protective equipment; and enhances our ability to deal with a potential surge by providing access to specialists remotely and electronically all hours of the day, thus expanding care to the evening and weekend hours.
Acknowledgment: The authors thank our steering committee members (Dr. Ralph Cygan, Dr. Andrew Reikes, Dr. Byron Allen, Dr. George Lawry) and IT build team (Lori Bocchicchio, Meghan van Witsen, Jaymee Zillgitt, Tanya Sickles, Dennis Hoang, Jeanette Lisak-Phillips) for their contributions in the design and implementation of our eConsults program. We also thank additional team members Kurt McArthur and Neaktisia Lee for their assistance with generating reports, and Kathy LaPierre, Jennifer Rios, and Debra Webb Torres for their guidance with compliance and billing issues.
Corresponding author: Alpesh N. Amin, MD, MBA, University of California, Irvine, 101 The City Drive South, Building 26, Room 1000, ZC-4076H, Orange, CA 92868; anamin@uci.edu.
Financial disclosures: None.
1. County of Orange, Health Care Agency, Public Health Services. Orange County Health Profile 2013.
2. Coffman JM, Fix M Ko, M. California physician supply and distribution: headed for a drought? California Health Care Foundation, June 2018.
3. Spetz J, Coffman J, Geyn I. California’s primary care workforce: forecasted supply, demand, and pipeline of trainees, 2016-2030. Healthforce Center at the University of California, San Francisco, August 2017.
4. Gandhi TK, Sittig DF, Franklin M, et al. Communication breakdown in the outpatient referral process. J Gen Intern Med. 2000;15:626-631.
5. McPhee SJ, Lo B, Saika GY, Meltzer R. How good is communication between primary care physicians and subspecialty consultants? Arch Intern Med. 1984;144:1265-1268.
6. Mehrotra A, Forrest CB, Lin CY. Dropping the baton: specialty referrals in the United States. Milbank Q. 2011;89:39-68.
7. Wrenn K, Catschegn S, Cruz M, et al. Analysis of an electronic consultation program at an academic medical centre: Primary care provider questions, specialist responses, and primary care provider actions. J Telemed Telecare. 2017;23: 217-224.
8. Gleason N, Prasad PA, Ackerman S, et al. Adoption and impact of an eConsult system in a fee-for-service setting. Healthc (Amst). 2017;5(1-2):40-45.
9. Stoves J, Connolly J, Cheung CK, et al. Electronic consultation as an alternative to hospital referral for patients with chronic kidney disease: a novel application for networked electronic health records to improve the accessibility and efficiency of healthcare. Qual Saf Health Care. 2010;19: e54.
10. Datta SK, Warshaw EM, Edison KE, et al. Cost and utility analysis of a store-and-forward teledermatology referral system: a randomized clinical trial. JAMA Dermatol. 2015;151:1323-1329.
11. Liddy C, Drosinis P, Deri Armstrong C, et al. What are the cost savings associated with providing access to specialist care through the Champlain BASE eConsult service? A costing evaluation. BMJ Open. 2016;6:e010920.
12. Barnett ML, Yee HF Jr, Mehrotra A, Giboney P. Los Angeles safety-net program eConsult system was rapidly adopted and decreased wait times to see specialists. Health Aff. 2017;36:492-499.
13. Malagrino GD, Chaudhry R, Gardner M, et al. A study of 6,000 electronic specialty consultations for person-centered care at The Mayo Clinic. Int J Person Centered Med. 2012;2:458-466.
14. Keely E, Liddy C, Afkham A. Utilization, benefits, and impact of an e-consultation service across diverse specialties and primary care providers. Telemed J E Health. 2013;19:733-738.
15. Scherpbier-de Haan ND, van Gelder VA, Van Weel C, et al. Initial implementation of a web-based consultation process for patients with chronic kidney disease. Ann Fam Med. 2013;11:151-156.
16. Palen TE, Price D, Shetterly S, Wallace KB. Comparing virtual consults to traditional consults using an electronic health record: an observational case-control study. BMC Med Inform Decis Mak. 2012;12:65.
17. Liddy C, Afkham A, Drosinis P, et al. Impact of and satisfaction with a new eConsult service: a mixed methods study of primary care providers. J Am Board Fam Med. 2015;28:394-403.
18. Angstman KB, Adamson SC, Furst JW, et al. Provider satisfaction with virtual specialist consultations in a family medicine department. Health Care Manag (Frederick). 2009;28:14-18.
19. McAdams M, Cannavo L, Orlander JD. A medical specialty e-consult program in a VA health care system. Fed Pract. 2014; 31:26–31.
20. Keely E, Williams R, Epstein G, et al. Specialist perspectives on Ontario Provincial electronic consultation services. Telemed J E Health. 2019;25:3-10.
21. Kim-Hwang JE, Chen AH, Bell DS, et al. Evaluating electronic referrals for specialty care at a public hospital. J Gen Intern Med. 2010;25:1123-1128.
1. County of Orange, Health Care Agency, Public Health Services. Orange County Health Profile 2013.
2. Coffman JM, Fix M Ko, M. California physician supply and distribution: headed for a drought? California Health Care Foundation, June 2018.
3. Spetz J, Coffman J, Geyn I. California’s primary care workforce: forecasted supply, demand, and pipeline of trainees, 2016-2030. Healthforce Center at the University of California, San Francisco, August 2017.
4. Gandhi TK, Sittig DF, Franklin M, et al. Communication breakdown in the outpatient referral process. J Gen Intern Med. 2000;15:626-631.
5. McPhee SJ, Lo B, Saika GY, Meltzer R. How good is communication between primary care physicians and subspecialty consultants? Arch Intern Med. 1984;144:1265-1268.
6. Mehrotra A, Forrest CB, Lin CY. Dropping the baton: specialty referrals in the United States. Milbank Q. 2011;89:39-68.
7. Wrenn K, Catschegn S, Cruz M, et al. Analysis of an electronic consultation program at an academic medical centre: Primary care provider questions, specialist responses, and primary care provider actions. J Telemed Telecare. 2017;23: 217-224.
8. Gleason N, Prasad PA, Ackerman S, et al. Adoption and impact of an eConsult system in a fee-for-service setting. Healthc (Amst). 2017;5(1-2):40-45.
9. Stoves J, Connolly J, Cheung CK, et al. Electronic consultation as an alternative to hospital referral for patients with chronic kidney disease: a novel application for networked electronic health records to improve the accessibility and efficiency of healthcare. Qual Saf Health Care. 2010;19: e54.
10. Datta SK, Warshaw EM, Edison KE, et al. Cost and utility analysis of a store-and-forward teledermatology referral system: a randomized clinical trial. JAMA Dermatol. 2015;151:1323-1329.
11. Liddy C, Drosinis P, Deri Armstrong C, et al. What are the cost savings associated with providing access to specialist care through the Champlain BASE eConsult service? A costing evaluation. BMJ Open. 2016;6:e010920.
12. Barnett ML, Yee HF Jr, Mehrotra A, Giboney P. Los Angeles safety-net program eConsult system was rapidly adopted and decreased wait times to see specialists. Health Aff. 2017;36:492-499.
13. Malagrino GD, Chaudhry R, Gardner M, et al. A study of 6,000 electronic specialty consultations for person-centered care at The Mayo Clinic. Int J Person Centered Med. 2012;2:458-466.
14. Keely E, Liddy C, Afkham A. Utilization, benefits, and impact of an e-consultation service across diverse specialties and primary care providers. Telemed J E Health. 2013;19:733-738.
15. Scherpbier-de Haan ND, van Gelder VA, Van Weel C, et al. Initial implementation of a web-based consultation process for patients with chronic kidney disease. Ann Fam Med. 2013;11:151-156.
16. Palen TE, Price D, Shetterly S, Wallace KB. Comparing virtual consults to traditional consults using an electronic health record: an observational case-control study. BMC Med Inform Decis Mak. 2012;12:65.
17. Liddy C, Afkham A, Drosinis P, et al. Impact of and satisfaction with a new eConsult service: a mixed methods study of primary care providers. J Am Board Fam Med. 2015;28:394-403.
18. Angstman KB, Adamson SC, Furst JW, et al. Provider satisfaction with virtual specialist consultations in a family medicine department. Health Care Manag (Frederick). 2009;28:14-18.
19. McAdams M, Cannavo L, Orlander JD. A medical specialty e-consult program in a VA health care system. Fed Pract. 2014; 31:26–31.
20. Keely E, Williams R, Epstein G, et al. Specialist perspectives on Ontario Provincial electronic consultation services. Telemed J E Health. 2019;25:3-10.
21. Kim-Hwang JE, Chen AH, Bell DS, et al. Evaluating electronic referrals for specialty care at a public hospital. J Gen Intern Med. 2010;25:1123-1128.
Outcomes in CVC Occlusions
Long‐term central venous catheters (CVCs) facilitate care for patients with chronic illness by providing easy venous access for laboratory tests, administration of medication, and parenteral nutrition. However, several complications resulting from the use of CVCs, including sepsis, extravasation of infusions, and venous thrombosis, can increase associated morbidity and mortality. These complications can also interrupt and delay treatment for the underlying disease and thereby affect outcomes. One of the most common CVC complications is catheter occlusion.[1]
Catheter occlusion occurs in 14% to 36% of patients within 1 to 2 years of catheter placement.[2, 3, 4, 5, 6, 7, 8] A catheter occlusion can be partial or complete, and can occur secondary to a variety of mechanical problems, including an uncommon, but potentially life‐threatening, pinch‐off syndrome. Medication or parenteral nutrition can also cause occlusion, which can be acute or gradual, with increasingly sluggish flow through the catheter. Inappropriate concentrations or incompatible mixtures can cause medications to precipitate within the catheter lumen.
Occlusions are either thrombotic or nonthrombotic. One autopsy study of patients with a long‐term CVC found that a fibrin sheath encased the catheter tip in every case.[9] An occluded catheter may compromise patient care[9, 10]; it may cause cancellation or delay of procedures, it potentially interrupts administration of critical therapies including vesicants, it may result in risk of infection, and it potentially leads to catheter replacement. This can further complicate care, leading to increased length of stay (LOS) and hospital costs.
To better understand resource utilization, LOS, and cost implications of alteplase compared with catheter replacement, we conducted a preplanned, retrospective analysis of hospitalized patients captured between January 2006 and December 2011 in the database maintained by Premier. The Premier database is a large, US hospital‐based, service‐level, all‐payer, comparative database, with information collected primarily from nearly 600 geographically diverse, nonprofit, nongovernment community and teaching hospitals.
METHODS
Data Sources
The Premier database contains information on over 42 million hospital discharges (mean 5.5 million discharges/year)one‐fifth of all US hospitalizationsfrom the year 2000 to the present. The database contains data from standard hospital discharge files, including patient demographic information and disease state. Patients can be tracked, with a unique identifier, across the inpatient and hospital‐based outpatient settings, as well as across visits. In addition to the data elements available in most of the standard hospital discharge files, the Premier database also contains a date‐stamped log of billed items, including procedures, medications, and laboratory, diagnostic, and therapeutic services at the individual patient level. Drug utilization information is available by day of stay and includes quantity, dosing, strength used, and cost.
The Premier database has been used extensively to benchmark hospital clinical and financial performance as well as by the US Food and Drug Administration (FDA) for drug surveillance and by the Centers for Medicare and Medicaid Services to evaluate next‐generation payment models. Preliminary comparisons between patient and hospital characteristics for hospitals that submit data to Premier and those of the probability sample of hospitals and patients selected for the National Hospital Discharge Survey suggest that the patient populations are similar with regard to patient age, gender, LOS, mortality, primary discharge diagnosis, and primary procedure groups.
Patient Population
In this retrospective observational database analysis, inpatients of all ages were initially identified who were discharged from a hospital between January 1, 2006 and December 31, 2011 and whose records contained 1 or more International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9) procedural codes or Current Procedural Terminology (CPT‐4) codes signifying CVC placement. The catheter replacement group comprised patients having a catheter replacement during the hospitalization. The alteplase treatment group was identified through patient billing records and by computing the dose administered (2 mg) during the index hospitalization period. Healthcare Common Procedure Coding System J‐codes (J2996, alteplase recombinant injection 10 mg; J2997, alteplase recombinant 1 mg) were also evaluated during the analysis to supplement the search string identification. To account for and eliminate catheter replacement due to mechanical failure rather than occlusion, patients with ICD‐9 diagnosis code 996.1 for mechanical failure were excluded. Patients with an ICD‐9 diagnosis code for infection or who received antibiotics on the day of replacement were excluded as an additional way to narrow the study to patients with occlusion as the reason for catheter replacement. In addition, patients receiving kidney dialysis, a chronic condition prone to greater‐than‐usual risk of catheter occlusion, were excluded. When a patient had multiple hospital stays with CVC insertions or placement during the study period, the first hospitalization with insertions or placement was used in our analyses.
Of the CVC patient population (N=574,252), 36,680 patient discharges resulted in the need for CVC replacement, alteplase therapy, or both. Patients receiving both replacement and alteplase (N=144) were excluded from analysis, resulting in 33,551 patient discharges with alteplase and 1028 patient discharges with CVC replacement.
Outcome Measures
The main outcomes of interest were LOS and hospital costs after occlusion, and readmissions at 30 and 90 days. Secondary measures, as they were thought to play a role in influencing outcomes, included LOS and costs before occlusion, as well as departmental costs such as pharmacy, radiology, and days in the intensive care unit (ICU).
Statistical Analysis
Univariate descriptive statistics were used to characterize the patient population by patient, clinical, and hospital attributes. In addition, subgroup analyses were performed among patients with any cardiology diagnosis (using ICD‐9 diagnosis or procedure codes), heart failure, myocardial infarction, and cancer, which were potentially overlapping categories chosen prior to initiating the analyses. Data measured on a continuous scale were expressed as mean, standard deviation, range, and median. Categorical data were expressed as count/percentages in the categories. In addition, categorical costs were also examined before and after occlusion. Tables of results included P values comparing patients who received CVC replacement with those who received alteplase across all measures. The [2] tests were used to test for differences in categorical variables, and t tests were utilized for differences in continuous variables.
Multivariable regression modeling was conducted to better compare outcomes associated with catheter replacement versus alteplase treatment. Linear regression models were performed to evaluate hospital costs and LOS during the initial hospital discharge. Logistic regression models were performed to evaluate the odds of readmission at 30 and 90 days following discharge. All multivariable models controlled for factors found to be statistically significant in univariate analysis. The covariates varied by model, but generally included age, race, sex, cancer, 3M All Patient Refined Diagnosis Related Group risk of mortality and severity of illness, cerebrovascular disease, renal disease, payer, myocardial infarction, hemiplegia/paraplegia, chronic or acute diabetes, peripheral vascular disease, complication, admission source, admission type, congestive heart failure, dementia, metastatic solid tumor, rheumatic disease, peptic ulcer disease, chronic pulmonary disease, hospital teaching status, urban/rural location, US Census region, and number of hospital beds. Certain of these variables, such as 3M measures of severity and risk, as well as measures of LOS and costs before occlusion, were considered as ways to understand differences in risk of increased costs among patients. For each multivariable model, covariates eligible for inclusion in the models were selected using a backward selection method (logistic used stepwise) until all variables remaining in the model were significant at P0.2.
RESULTS
This study included 34,579 patients who first had a CVC insertion and then were treated for a CVC occlusion by receiving a replacement CVC (n=1028) or by receiving alteplase (2 mg) administration (n=33,551) during the same hospitalization (Table 1). Patients who received alteplase tended to be younger (6019 vs 6220 years old). More than 50% were at least 65 years of age. Twelve percent of alteplase patients were black, whereas 18.5% of catheter‐replacement patients were black.
| Catheter Replacement, n=1,028 | Alteplase Treatment, n=33,551 | |
|---|---|---|
| ||
| Age group, ya | ||
| Under 18 | 29 (2.8%) | 984 (2.9%) |
| 1834 | 84 (8.2%) | 2,479 (7.4%) |
| 3544 | 73 (7.1%) | 2,826 (8.4%) |
| 4554 | 116 (11.3%) | 5,217 (15.5%) |
| 5564 | 210 (20.4%) | 6,761 (20.1%) |
| 6574 | 203 (19.7%) | 6,741 (20.1%) |
| 75+ | 313 (30.4%) | 8,543 (25.5%) |
| Mean (SD) | 62 (20) | 60 (19) |
| Sex | ||
| Female | 565 (55.0%) | 18,172 (54.2%) |
| Male | 463 (45.0%) | 15,378 (45.8%) |
| Unknown | 0 (0%) | 1 (0%) |
| Race/ethnicitya | ||
| Black | 190 (18.5%) | 4,057 (12.1%) |
| Hispanic | 40 (3.9%) | 1,098 (3.3%) |
| Other | 126 (12.3%) | 6,250 (18.6%) |
| White | 672 (65.4%) | 22,146 (66.0%) |
| Comorbid conditions | ||
| Myocardial infarction | 96 (9.3%) | 3,746 (11.2%) |
| Congestive heart failure | 258 (25.1%) | 8,210 (24.5%) |
| Peripheral vascular disease | 104 (10.1%) | 3,451 (10.3%) |
| Cerebrovascular disease | 115 (11.2%) | 3,528 (10.5%) |
| Dementia | 33 (3.2%) | 838 (2.5%) |
| Chronic pulmonary diseasea | 264 (25.7%) | 10,495 (31.3%) |
| Rheumatic disease | 37 (3.6%) | 1,344 (4.0%) |
| Peptic ulcer disease | 41 (4.0%) | 1,068 (3.2%) |
| Mild liver diseasea | 94 (9.1%) | 2,392 (7.1%) |
| Moderate/severe liver diseasea | 29 (2.8%) | 531 (1.6%) |
| Acute diabetes | 255 (24.8%) | 9,185 (27.4%) |
| Chronic diabetesa | 44 (4.3%) | 2,327 (6.9%) |
| Hemiplegia paraplegia | 51 (5.0%) | 1,909 (5.7%) |
| Renal diseasea | 209 (20.3%) | 5,351 (16.0%) |
| Cancera | 207 (20.1%) | 5,685 (16.9%) |
| Metastatic solid tumora | 100 (9.7%) | 2,441 (7.3%) |
| AIDS/HIV | 4 (0.4%) | 244 (0.7%) |
| 3M APR‐DRG Severity of Illnessa | ||
| 1‐minor | 36 (3.5%) | 769 (2.3%) |
| 2‐moderate | 172 (16.7%) | 4,109 (12.2%) |
| 3‐major | 384 (37.3%) | 12,175 (36.3%) |
| 4‐extreme | 436 (42.4%) | 16,497 (49.2%) |
| Unknown | 0 (0%) | 1 (0%) |
| 3M APR‐DRG Risk of Mortalitya | ||
| 1‐minor | 159 (15.5%) | 4,716 (14.1%) |
| 2‐moderate | 253 (24.6%) | 6,746 (20.1%) |
| 3‐major | 313 (30.4%) | 10,569 (31.5%) |
| 4‐mxtreme | 303 (29.5%) | 11,519 (34.3%) |
| Unknown | 0 (0%) | 1 (0%) |
Alteplase patients were significantly more likely to have a diagnosis of chronic pulmonary disease, liver disease, renal disease, chronic diabetes (ie, diabetes with complications), and cancer. There was an equivalent number of urban and rural hospitals across the 2 groups of patients (Table 2); however, there were regional differences including a higher proportion of catheter‐replacement patients from the East North Central and Middle Atlantic areas and a lower proportion of catheter‐replacement patients from Mountain and Pacific states. Catheter‐replacement patients more frequently were treated in teaching hospitals and in hospitals of larger size.
| Catheter Replacement, n=1,028 | Alteplase Treatment, n=33,551 | |
|---|---|---|
| ||
| Provider regiona | ||
| New England | 28 (2.7%) | 976 (2.9%) |
| Middle Atlantic | 227 (22.1%) | 1,944 (5.8%) |
| South Atlantic | 247 (24.0%) | 8,047 (24.0%) |
| East North Central | 153 (14.9%) | 3,015 (9.0%) |
| East South Central | 14 (1.4%) | 1,345 (4.0%) |
| West North Central | 98 (9.5%) | 3,590 (10.7%) |
| West South Central | 112 (10.9%) | 5,096 (15.2%) |
| Mountain | 48 (4.7%) | 3,339 (9.9%) |
| Pacific | 94 (9.1%) | 6,083 (18.1%) |
| Unknown | 7 (0.7%) | 116 (0.3%) |
| Population served | ||
| Rural | 56 (5.4%) | 1,838 (5.5%) |
| Urban | 972 (94.6%) | 31,713 (94.5%) |
| Teaching statusa | ||
| Nonteaching | 431 (41.9%) | 18,598 (55.4%) |
| Teaching | 597 (58.1%) | 14,953 (4.6%) |
| Hospital size, no. of bedsa | ||
| <100 | 4 (0.4%) | 475 (1.4%) |
| 100199 | 56 (5.4%) | 1,725 (5.1%) |
| 200299 | 124 (12.1%) | 5,907 (17.6%) |
| 300499 | 432 (42.0%) | 13,790 (41.1%) |
| 500+ | 412 (40.1%) | 11,654 (34.7%) |
| Primary payora | ||
| Commercial | 50 (4.9%) | 1,779 (5.3%) |
| Managed care | 221 (21.5%) | 6,888 (20.5%) |
| Medicaid | 132 (12.8%) | 4,146 (12.4%) |
| Medicare | 572 (55.6%) | 17,226 (51.3%) |
| Other government programs | 9 (0.9%) | 439 (1.3%) |
| Any other payor | 44 (4.3%) | 3,073 (9.2%) |
| Admission sourcea | ||
| Emergency department | 424 (41.2%) | 12,741 (38.0%) |
| Physician referral | 390 (37.9%) | 14,502 (43.2%) |
| Transfer from another health facility | 154 (15.0%) | 4,109 (12.2%) |
| Unknown | 60 (5.8%) | 2,199 (6.5%) |
| Admission typea | ||
| Elective | 205 (19.9%) | 5,872 (17.5%) |
| Emergency | 613 (59.6%) | 19,660 (58.6%) |
| Newborn | 9 (0.9%) | 37 (0.1%) |
| Trauma center | 3 (0.3%) | 279 (0.8%) |
| Urgent | 192 (18.7%) | 7,573 (22.6%) |
| Unknown | 6 (0.6%) | 130 (0.4%) |
After covariate adjustment for baseline measurements significantly related to each outcome, average daily post occlusion costs were estimated to be $317 lower for alteplase recipients than for patients who received catheter replacement ($317; 95% confidence interval [CI]: $238‐$392; P<0.0001) (Table 3). Average adjusted total post occlusion costs were $1419 lower for alteplase recipients than for patients who received catheter replacement ($1418; 95% CI: $307‐$2458; P=0.012).
| CVC Replace Only, n=1,028 | Alteplase Only, n=33,551 | |
|---|---|---|
| ||
| 30‐day readmission | 24.6% | 23.7% |
| 90‐day readmission | 35.1% | 33.9% |
| Preocclusion | ||
| Mean (SD) length of stay, days | 3.8 (6.7) | 7.3 (6.9) |
| Mean (SD) total cost | $10,485 ($29,088) | $18,546 ($22,658) |
| Mean (SD) cost per day | $2,876 ($3,046) | $2,637 ($1,783) |
| Postocclusion | ||
| Mean (SD) length of stay, days | 8.8 (11.0) | 8.8 (10.0) |
| Mean (SD) total cost | $18,714 ($32,189) | $16,765 ($29,966) |
| Mean (SD) cost per day | $2,146 ($2,995) | $2,058 ($6,585) |
Contributing to the lower cost were certain revenue‐center specific costs (Table 4). Total room and board costs were different between the alteplase and catheter‐replacement groups in both the pre‐ and postocclusion periods; this was related to the difference between the 2 comparison groups in postocclusion LOS of about 0.3 days (Table 5). However, the differences favored alteplase use over catheter replacement. Cardiology/electrocardiography costs were lower for catheter replacement in the preocclusion period but lower for alteplase use in the postocclusion period. Emergency department costs were higher for catheter replacement in both periods, as were respiratory costs in the same manner. Additionally, costs for laboratory tests, nursing, operating room/surgery, pharmacy, radiology, supplies, and ICU room and board were lower in the preocclusion period but higher in the postocclusion period for catheter‐replacement patients. It was unclear why the pharmacy costs after catheter replacement would have increased for catheter‐replacement patients in contrast to the decrease for alteplase‐treated patients, but because this occurred at an average daily basis as well, it appeared that catheter‐replacement patients may have received additional medications. Average adjusted postocclusion LOS was similar for alteplase and catheter‐replacement recipients (P=0.24), suggesting that decreased total costs were due to reasons other than shorter LOS.
| Preocclusiona | Postocclusiona | |||
|---|---|---|---|---|
| CVC Replacement Only, n=1,028 | Alteplase Only, n=33,551 | CVC Replacement Only, n=1,028 | Alteplase Onlyn=33,551 | |
| ||||
| Total room and board cost | ||||
| Mean (SD) total cost | $4,380 ($9,545) | $8,535 ($10,175)b | $8,394 ($14,393) | $8,437 ($18,341)b |
| Mean (SD) cost per day | $693 ($734) | $1,097 ($724)b | $751 ($536) | $983 ($3,250) |
| Cardiology/ECG cost | ||||
| Mean (SD) total cost | $82 ($806) | $154 ($605)b | $124 ($540) | $107 ($735)b |
| Mean (SD) cost per day | $17 ($96) | $26 ($131)b | $17 ($93) | $19 ($217)b |
| Emergency department cost | ||||
| Mean (SD) total cost | $10 ($91) | $36 ($284)b | $10 ($67) | $12 ($195) |
| Mean (SD) cost per day | $4 ($32) | $8 ($65)b | $2 ($19) | $6 ($76) |
| Laboratory cost | ||||
| Mean (SD) total cost | $864 ($2,538) | $1,425 ($3,622)b | $1,471 ($5,614) | $1,175 ($3,961) |
| Mean (SD) cost per day | $140 ($314) | $180 ($269)b | $139 ($313) | $142 ($465)b |
| Nursing Cost | ||||
| Mean (SD) total cost | $218 ($1,497) | $224 ($2,364)b | $432 ($2,538) | $231 ($2,785) |
| Mean (SD) cost per day | $39 ($166) | $24 ($127)b | $35 ($140) | $21 ($112) |
| OR/surgery cost | ||||
| Mean (SD) total cost | $902 ($4,743) | $1,602 ($3,597)b | $1,437 ($3,029) | $847 ($2,701)b |
| Mean (SD) cost per day | $207 ($495) | $267 ($513)b | $302 ($646) | $130 ($827)b |
| Pharmacy cost | ||||
| Mean (SD) total cost | $2,085 ($20,338) | $3,014 ($6,408)b | $3,200 ($16,396) | $2,914 ($8,383)b |
| Mean (SD) cost per day | $263 ($1,509) | $368 ($583)b | $362 ($2,427) | $347 ($853)b |
| Radiology cost | ||||
| Mean (SD) total cost | $470 ($869) | $782 ($1,031)b | $731 ($1,160) | $505 ($1,550)b |
| Mean (SD) cost per day | $133 ($362) | $130 ($189)b | $144 ($293) | $83 ($469)b |
| Respiratory cost | ||||
| Mean (SD) total cost | $391 ($1,442) | $895 ($2,160)b | $673 ($2,209) | $783 ($2,297)b |
| Mean (SD) cost per day | $51 ($121) | $104 ($170)b | $61 ($115) | $81 ($280)b |
| Supply cost | ||||
| Mean (SD) total cost | $834 ($3,221) | $1,408 ($5,871)b | $1,636 ($7,250) | $1,117 ($4,477)b |
| Mean (SD) cost per day | $208 ($1,244) | $211 ($789)b | $264 ($871) | $165 ($1,529)b |
| Other therapy cost | ||||
| Mean (SD) total cost | $179 ($702) | $355 ($815)b | $436 ($837) | $509 ($1,263)b |
| Mean (SD) cost per day | $30 ($81) | $46 ($98)b | $51 ($106) | $66 ($481)b |
| Other departments cost | ||||
| Mean (SD) total cost | $26 ($710) | $1 ($36) | $74 ($1,127) | $3 ($144) b |
| Mean (SD) cost per day | $3 ($56) | $0 ($5) | $6 ($86) | $0 ($13)b |
| Fees cost | ||||
| Mean (SD) total cost | $38 ($370) | $82 ($969)b | $67 ($340) | $86 ($2,704) |
| Mean (SD) cost per day | $7 ($47) | $12 ($77)b | $12 ($120) | $12 ($843) |
| Healthcare services cost | ||||
| Mean (SD) total cost | $5 ($53) | $31 ($1,052)b | $29 ($515) | $35 ($1,162) |
| Mean (SD) cost per day | $1 ($10) | $3 ($65)b | $2 ($11) | $3 ($54) |
| ICU room and board cost | ||||
| Mean (SD) total cost | $2,085 ($7,700) | $4,333 ($8,826)b | $3,158 ($10,767) | $2,884 ($15,863) |
| Mean (SD) cost per day | $293 ($677) | $543 ($854)b | $222 ($512) | $323 ($2,330) |
| Model | Parameter Estimate | Summary Statistic | Estimate (95% CI) |
|---|---|---|---|
| |||
| 30‐day readmissiona | 0.0234 | Odds ratio | 1.048 (0.899 to 1.221) |
| 90‐day readmissionb | 0.0248 | Odds ratio | 1.051 (0.915 to 1.207) |
| Postocclusion total costsc | 0.0842 | Mean differenced | $1,418.69 ($2,458.12 to $307.27)f |
| Postocclusion total cost per daye | 0.1857 | Mean differenced | $317.20 ($392.24 to $238.22)f |
| Post occlusion length of stayg | 0.0313 | Mean differenced | 0.299 (0.196 to 0.820) |
Unadjusted 30‐ and 90‐day readmission rates were 24.6% and 35.1% for CVC replacement and slightly lower at 23.7% and 33.9% for alteplase (Table 3), respectively. Odds of readmission after adjusting for patient and hospital factors were not significantly different at 30 days (odds ratio [OR]: 1.048, 95% CI: 0.899‐1.221; P=0.55) or at 90 days (OR: 1.051, 95% CI: 0.915‐1.207; P=0.48) (Table 5). Subgroup analyses for patients with a diagnosis of heart failure, myocardial infarction, and cancer revealed similar results.
DISCUSSION
The cost of healthcare in the United States has risen at an outstanding rate compared with other countries. Our percentage of gross national product spent on healthcare is on the order of 16% to 18%, almost twice as much as the next most industrialized country in terms of healthcare expenditure.[11] In the current era, finding opportunities to reduce healthcare costs without negatively impacting quality of care is the name of the game. Professional societies have come together under the campaign of Choosing Wisely: An Initiative of the ABIM (American Board of Internal Medicine) Foundation to help educate clinicians and patients on cost‐containment strategies.[12] Research that demonstrates opportunities to reduce cost will help healthcare providers choose wisely among diagnostic and therapeutic options for patients. Our study demonstrated that the use of a drug such as alteplase in clearing CVC catheter obstruction was significantly less costly to the hospital than catheter replacement.
Cathflo Activase (alteplase: Genentech, South San Francisco, CA), the only FDA‐approved thrombolytic for the restoration of central venous catheter function, is the current standard treatment for catheter occlusions in the United States. A dose of 2 mg in 2 mL is instilled in patients weighing 30 kg or 110% of the internal lumen volume of the catheter not to exceed 2 mg in 2 mL for those patients weighing <30 kg. Haire et al. showed that a 2‐mg dose of alteplase was more effective than urokinase (5000 IU) for treating radiographically proven thrombotic occlusion of a CVC after a dwell time of 120 minutes.[13] In the Cardiovascular thrombolytic used to Open Occluded Lines (COOL) trial, one 2‐mg dose of alteplase cleared the catheter occlusion after 120 minutes in 74% of patients, compared with only 17% of patients who received a placebo. Studies have confirmed the safety and efficacy of alteplase administered at various time intervals in different long‐term catheters, including peripherally inserted central catheters, with major hemorrhage reported in 0.3% of patients.[14, 15, 16]
Adding to the knowledge of patient outcomes from clinical studies, many health outcomes studies have demonstrated benefit in cost containment through decreasing LOS, which one can argue is just shifting the cost to an earlier part of the stay. Even though this is highly beneficial, it does not address the core resource utilization within the hospital. Our study found its cost benefit not in the LOS, but in decreasing core resource utilization such as radiology, lab, nursing, and supplies. If patients are admitted for a noncardiovascular condition and have CVC occlusion, using alteplase to clear the CVC occlusion along with implementing strategies to manage the underlying disease to reduce the LOS becomes a powerful opportunity to impact cost. Among patients who may come to the hospital for just the CVC occlusion, the LOS should be short. There may be no significant opportunity to reduce the LOS in those cases, but opportunities to decrease core hospital resource utilization with alteplase make this approach beneficial if the patient can tolerate it.
Limitations of the study include the retrospective and administrative nature of the database used, which is unable to provide certain clinical measures as would be available at the patient's bedside when treatment choices are being made. Had they been available, we might have included them in our assessment of whether patients who underwent CVC replacement were significantly different from those who received alteplase. In addition, not all hospitals in the database had charge masters that facilitated identification of CVC replacements or reinsertions, requiring the use of CPT‐4 codes and evidence of new CVCs being placed or inserted. Certain patients were excluded if there was conflicting information about whether the CVC was new within the hospital stay or dwelling in the patient prior to admission. Also, dialysis patients were excluded because they were not part of any approved indication for alteplase 2 mg, and this group is particularly prone to catheter obstruction. As such, they represent more complicated cases than the norm; this exclusion may have limited the overall generalizability of the study. The study also relied on charge master (billing) data to identify the use of alteplase and other treatments, where there is the potential, albeit minimal, for inaccuracies in the data. Of greater importance, the study relied on ICD‐9 coding to identify comorbid conditions, and as in other studies using similar data sources, such methods are subject to coding errors and omissions. However, many of the listed limitations above were not thought to be different between the comparison groups or more problematic for this study than for other studies based on similar data sources.
CONCLUSION
Among patients treated for an occluded CVC, alteplase‐treated patients had lower daily postocclusion costs and lower total postocclusion costs than patients who received catheter replacement. Differences in costs did not appear to be driven by differences in postocclusion LOS. Readmissions at 30‐ and 90‐day periods were found to be similar between alteplase recipients and catheter‐replacement patients.
Acknowledgements
The authors thank W. Kenne Mountford for his editorial assistance with a prior version of the article and Dima Qato for assistance with the analyses. Additional editorial assistance was provided by Steve Melvin. This assistance was funded by Genentech, Inc.
Disclosures: This study was funded by Genentech, Inc. F. R. Ernst and C. Lipkin are employees of Premier, which was contracted by Genentech to conduct the study covered in this article. E. Chen is an employee of and holds stock in Genentech. D. Tayama is an employee of Genentech. A. N. Amin received research funding from Premier, which was contracted by Genentech to conduct the study covered in this article.
- , , , , et al. Management of occlusion and thrombosis associated with long‐term indwelling central venous catheters. Lancet. 2009;374:159–169.
- , , , , , ; Children's Oncology Group. Prophylactic urokinase in the management of long‐term venous access devices in children; a Children's Oncology Group study. J Clin Oncol. 2004;22:2718–2723.
- , , , et al. Central venous catheter‐related complications in children with oncological/hematological diseases: an observational study of 418 devices. Ann Oncol. 1985;16:648–654.
- , , , et al. A cross‐sectional study evaluating post‐thrombotic syndrome in children. Thromb Res. 2003;111:227–233.
- , , , . Complications and management of implanted venous access catheters. J Clin Oncol. 1985;3:710–717.
- . Local installation of small doses of streptokinase for treatment of thrombotic occlusions of long‐term access catheters. J Clin Oncol. 1983;1:572–573.
- , , . Are clinical signs accurate indicators of the cause of central venous catheter occlusion? J Parenter Enteral Nutr. 1995;19:75–79.
- , . Mechanism and management of persistent withdrawal occlusion. Am Surg. 1988;54:326–328.
- . Catheter‐related thrombosis in pediatrics. Pediatr Nurs. 2002;28:97–102, 105–106.
- . Reopen the pipeline. Nursing. 2005;35:54–61.
- , . Chemotherapy extravasation: a consequence of fibrin sheath formation around venous access devices. Oncol Nurs Forum. 1995;22:675–680.
- Organisation for Economic Co‐operation and Development (OECD). Statistics. OECD Health Data 2010, June 2010. http://www.oecd.org/unitedstates/Briefing‐Note‐USA‐2013.pdf. Accessed May 8, 2014.
- ABIM Foundation. Choosing Wisely: An Initiative of the ABIM Foundation. (2013). Available at: http://www.choosingwisely.org. Accessed April 6, 2013.
- , , , . Urokinase versus recombinant tissue plasminogen activator in thrombosed central venous catheters: a double‐blinded, randomized trial. Thromb Haemost. 1994;72:543–547.
- , , , et al. Recombinant tissue plasminogen activator (alteplase) for restoration of flow in occluded central venous access devices: a double‐blind placebo‐controlled trial—the Cardiovascular Thrombolytic to Open Occluded Lines (COOL) efficacy trial. J Vasc Interv Radiol. 2001;12:951–955.
- , , , et al. Safety and efficacy of alteplase for restoring function in occluded central venous catheters: results of the cardiovascular thrombolytic to open occluded lines trial. J Clin Oncol. 2002;20:317–324.
- , , , . Alteplase for treatment of occluded peripherally inserted central catheters: safety and efficacy in 240 patients. J Vasc Interv Radiol. 2004;15:45–49.
Long‐term central venous catheters (CVCs) facilitate care for patients with chronic illness by providing easy venous access for laboratory tests, administration of medication, and parenteral nutrition. However, several complications resulting from the use of CVCs, including sepsis, extravasation of infusions, and venous thrombosis, can increase associated morbidity and mortality. These complications can also interrupt and delay treatment for the underlying disease and thereby affect outcomes. One of the most common CVC complications is catheter occlusion.[1]
Catheter occlusion occurs in 14% to 36% of patients within 1 to 2 years of catheter placement.[2, 3, 4, 5, 6, 7, 8] A catheter occlusion can be partial or complete, and can occur secondary to a variety of mechanical problems, including an uncommon, but potentially life‐threatening, pinch‐off syndrome. Medication or parenteral nutrition can also cause occlusion, which can be acute or gradual, with increasingly sluggish flow through the catheter. Inappropriate concentrations or incompatible mixtures can cause medications to precipitate within the catheter lumen.
Occlusions are either thrombotic or nonthrombotic. One autopsy study of patients with a long‐term CVC found that a fibrin sheath encased the catheter tip in every case.[9] An occluded catheter may compromise patient care[9, 10]; it may cause cancellation or delay of procedures, it potentially interrupts administration of critical therapies including vesicants, it may result in risk of infection, and it potentially leads to catheter replacement. This can further complicate care, leading to increased length of stay (LOS) and hospital costs.
To better understand resource utilization, LOS, and cost implications of alteplase compared with catheter replacement, we conducted a preplanned, retrospective analysis of hospitalized patients captured between January 2006 and December 2011 in the database maintained by Premier. The Premier database is a large, US hospital‐based, service‐level, all‐payer, comparative database, with information collected primarily from nearly 600 geographically diverse, nonprofit, nongovernment community and teaching hospitals.
METHODS
Data Sources
The Premier database contains information on over 42 million hospital discharges (mean 5.5 million discharges/year)one‐fifth of all US hospitalizationsfrom the year 2000 to the present. The database contains data from standard hospital discharge files, including patient demographic information and disease state. Patients can be tracked, with a unique identifier, across the inpatient and hospital‐based outpatient settings, as well as across visits. In addition to the data elements available in most of the standard hospital discharge files, the Premier database also contains a date‐stamped log of billed items, including procedures, medications, and laboratory, diagnostic, and therapeutic services at the individual patient level. Drug utilization information is available by day of stay and includes quantity, dosing, strength used, and cost.
The Premier database has been used extensively to benchmark hospital clinical and financial performance as well as by the US Food and Drug Administration (FDA) for drug surveillance and by the Centers for Medicare and Medicaid Services to evaluate next‐generation payment models. Preliminary comparisons between patient and hospital characteristics for hospitals that submit data to Premier and those of the probability sample of hospitals and patients selected for the National Hospital Discharge Survey suggest that the patient populations are similar with regard to patient age, gender, LOS, mortality, primary discharge diagnosis, and primary procedure groups.
Patient Population
In this retrospective observational database analysis, inpatients of all ages were initially identified who were discharged from a hospital between January 1, 2006 and December 31, 2011 and whose records contained 1 or more International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9) procedural codes or Current Procedural Terminology (CPT‐4) codes signifying CVC placement. The catheter replacement group comprised patients having a catheter replacement during the hospitalization. The alteplase treatment group was identified through patient billing records and by computing the dose administered (2 mg) during the index hospitalization period. Healthcare Common Procedure Coding System J‐codes (J2996, alteplase recombinant injection 10 mg; J2997, alteplase recombinant 1 mg) were also evaluated during the analysis to supplement the search string identification. To account for and eliminate catheter replacement due to mechanical failure rather than occlusion, patients with ICD‐9 diagnosis code 996.1 for mechanical failure were excluded. Patients with an ICD‐9 diagnosis code for infection or who received antibiotics on the day of replacement were excluded as an additional way to narrow the study to patients with occlusion as the reason for catheter replacement. In addition, patients receiving kidney dialysis, a chronic condition prone to greater‐than‐usual risk of catheter occlusion, were excluded. When a patient had multiple hospital stays with CVC insertions or placement during the study period, the first hospitalization with insertions or placement was used in our analyses.
Of the CVC patient population (N=574,252), 36,680 patient discharges resulted in the need for CVC replacement, alteplase therapy, or both. Patients receiving both replacement and alteplase (N=144) were excluded from analysis, resulting in 33,551 patient discharges with alteplase and 1028 patient discharges with CVC replacement.
Outcome Measures
The main outcomes of interest were LOS and hospital costs after occlusion, and readmissions at 30 and 90 days. Secondary measures, as they were thought to play a role in influencing outcomes, included LOS and costs before occlusion, as well as departmental costs such as pharmacy, radiology, and days in the intensive care unit (ICU).
Statistical Analysis
Univariate descriptive statistics were used to characterize the patient population by patient, clinical, and hospital attributes. In addition, subgroup analyses were performed among patients with any cardiology diagnosis (using ICD‐9 diagnosis or procedure codes), heart failure, myocardial infarction, and cancer, which were potentially overlapping categories chosen prior to initiating the analyses. Data measured on a continuous scale were expressed as mean, standard deviation, range, and median. Categorical data were expressed as count/percentages in the categories. In addition, categorical costs were also examined before and after occlusion. Tables of results included P values comparing patients who received CVC replacement with those who received alteplase across all measures. The [2] tests were used to test for differences in categorical variables, and t tests were utilized for differences in continuous variables.
Multivariable regression modeling was conducted to better compare outcomes associated with catheter replacement versus alteplase treatment. Linear regression models were performed to evaluate hospital costs and LOS during the initial hospital discharge. Logistic regression models were performed to evaluate the odds of readmission at 30 and 90 days following discharge. All multivariable models controlled for factors found to be statistically significant in univariate analysis. The covariates varied by model, but generally included age, race, sex, cancer, 3M All Patient Refined Diagnosis Related Group risk of mortality and severity of illness, cerebrovascular disease, renal disease, payer, myocardial infarction, hemiplegia/paraplegia, chronic or acute diabetes, peripheral vascular disease, complication, admission source, admission type, congestive heart failure, dementia, metastatic solid tumor, rheumatic disease, peptic ulcer disease, chronic pulmonary disease, hospital teaching status, urban/rural location, US Census region, and number of hospital beds. Certain of these variables, such as 3M measures of severity and risk, as well as measures of LOS and costs before occlusion, were considered as ways to understand differences in risk of increased costs among patients. For each multivariable model, covariates eligible for inclusion in the models were selected using a backward selection method (logistic used stepwise) until all variables remaining in the model were significant at P0.2.
RESULTS
This study included 34,579 patients who first had a CVC insertion and then were treated for a CVC occlusion by receiving a replacement CVC (n=1028) or by receiving alteplase (2 mg) administration (n=33,551) during the same hospitalization (Table 1). Patients who received alteplase tended to be younger (6019 vs 6220 years old). More than 50% were at least 65 years of age. Twelve percent of alteplase patients were black, whereas 18.5% of catheter‐replacement patients were black.
| Catheter Replacement, n=1,028 | Alteplase Treatment, n=33,551 | |
|---|---|---|
| ||
| Age group, ya | ||
| Under 18 | 29 (2.8%) | 984 (2.9%) |
| 1834 | 84 (8.2%) | 2,479 (7.4%) |
| 3544 | 73 (7.1%) | 2,826 (8.4%) |
| 4554 | 116 (11.3%) | 5,217 (15.5%) |
| 5564 | 210 (20.4%) | 6,761 (20.1%) |
| 6574 | 203 (19.7%) | 6,741 (20.1%) |
| 75+ | 313 (30.4%) | 8,543 (25.5%) |
| Mean (SD) | 62 (20) | 60 (19) |
| Sex | ||
| Female | 565 (55.0%) | 18,172 (54.2%) |
| Male | 463 (45.0%) | 15,378 (45.8%) |
| Unknown | 0 (0%) | 1 (0%) |
| Race/ethnicitya | ||
| Black | 190 (18.5%) | 4,057 (12.1%) |
| Hispanic | 40 (3.9%) | 1,098 (3.3%) |
| Other | 126 (12.3%) | 6,250 (18.6%) |
| White | 672 (65.4%) | 22,146 (66.0%) |
| Comorbid conditions | ||
| Myocardial infarction | 96 (9.3%) | 3,746 (11.2%) |
| Congestive heart failure | 258 (25.1%) | 8,210 (24.5%) |
| Peripheral vascular disease | 104 (10.1%) | 3,451 (10.3%) |
| Cerebrovascular disease | 115 (11.2%) | 3,528 (10.5%) |
| Dementia | 33 (3.2%) | 838 (2.5%) |
| Chronic pulmonary diseasea | 264 (25.7%) | 10,495 (31.3%) |
| Rheumatic disease | 37 (3.6%) | 1,344 (4.0%) |
| Peptic ulcer disease | 41 (4.0%) | 1,068 (3.2%) |
| Mild liver diseasea | 94 (9.1%) | 2,392 (7.1%) |
| Moderate/severe liver diseasea | 29 (2.8%) | 531 (1.6%) |
| Acute diabetes | 255 (24.8%) | 9,185 (27.4%) |
| Chronic diabetesa | 44 (4.3%) | 2,327 (6.9%) |
| Hemiplegia paraplegia | 51 (5.0%) | 1,909 (5.7%) |
| Renal diseasea | 209 (20.3%) | 5,351 (16.0%) |
| Cancera | 207 (20.1%) | 5,685 (16.9%) |
| Metastatic solid tumora | 100 (9.7%) | 2,441 (7.3%) |
| AIDS/HIV | 4 (0.4%) | 244 (0.7%) |
| 3M APR‐DRG Severity of Illnessa | ||
| 1‐minor | 36 (3.5%) | 769 (2.3%) |
| 2‐moderate | 172 (16.7%) | 4,109 (12.2%) |
| 3‐major | 384 (37.3%) | 12,175 (36.3%) |
| 4‐extreme | 436 (42.4%) | 16,497 (49.2%) |
| Unknown | 0 (0%) | 1 (0%) |
| 3M APR‐DRG Risk of Mortalitya | ||
| 1‐minor | 159 (15.5%) | 4,716 (14.1%) |
| 2‐moderate | 253 (24.6%) | 6,746 (20.1%) |
| 3‐major | 313 (30.4%) | 10,569 (31.5%) |
| 4‐mxtreme | 303 (29.5%) | 11,519 (34.3%) |
| Unknown | 0 (0%) | 1 (0%) |
Alteplase patients were significantly more likely to have a diagnosis of chronic pulmonary disease, liver disease, renal disease, chronic diabetes (ie, diabetes with complications), and cancer. There was an equivalent number of urban and rural hospitals across the 2 groups of patients (Table 2); however, there were regional differences including a higher proportion of catheter‐replacement patients from the East North Central and Middle Atlantic areas and a lower proportion of catheter‐replacement patients from Mountain and Pacific states. Catheter‐replacement patients more frequently were treated in teaching hospitals and in hospitals of larger size.
| Catheter Replacement, n=1,028 | Alteplase Treatment, n=33,551 | |
|---|---|---|
| ||
| Provider regiona | ||
| New England | 28 (2.7%) | 976 (2.9%) |
| Middle Atlantic | 227 (22.1%) | 1,944 (5.8%) |
| South Atlantic | 247 (24.0%) | 8,047 (24.0%) |
| East North Central | 153 (14.9%) | 3,015 (9.0%) |
| East South Central | 14 (1.4%) | 1,345 (4.0%) |
| West North Central | 98 (9.5%) | 3,590 (10.7%) |
| West South Central | 112 (10.9%) | 5,096 (15.2%) |
| Mountain | 48 (4.7%) | 3,339 (9.9%) |
| Pacific | 94 (9.1%) | 6,083 (18.1%) |
| Unknown | 7 (0.7%) | 116 (0.3%) |
| Population served | ||
| Rural | 56 (5.4%) | 1,838 (5.5%) |
| Urban | 972 (94.6%) | 31,713 (94.5%) |
| Teaching statusa | ||
| Nonteaching | 431 (41.9%) | 18,598 (55.4%) |
| Teaching | 597 (58.1%) | 14,953 (4.6%) |
| Hospital size, no. of bedsa | ||
| <100 | 4 (0.4%) | 475 (1.4%) |
| 100199 | 56 (5.4%) | 1,725 (5.1%) |
| 200299 | 124 (12.1%) | 5,907 (17.6%) |
| 300499 | 432 (42.0%) | 13,790 (41.1%) |
| 500+ | 412 (40.1%) | 11,654 (34.7%) |
| Primary payora | ||
| Commercial | 50 (4.9%) | 1,779 (5.3%) |
| Managed care | 221 (21.5%) | 6,888 (20.5%) |
| Medicaid | 132 (12.8%) | 4,146 (12.4%) |
| Medicare | 572 (55.6%) | 17,226 (51.3%) |
| Other government programs | 9 (0.9%) | 439 (1.3%) |
| Any other payor | 44 (4.3%) | 3,073 (9.2%) |
| Admission sourcea | ||
| Emergency department | 424 (41.2%) | 12,741 (38.0%) |
| Physician referral | 390 (37.9%) | 14,502 (43.2%) |
| Transfer from another health facility | 154 (15.0%) | 4,109 (12.2%) |
| Unknown | 60 (5.8%) | 2,199 (6.5%) |
| Admission typea | ||
| Elective | 205 (19.9%) | 5,872 (17.5%) |
| Emergency | 613 (59.6%) | 19,660 (58.6%) |
| Newborn | 9 (0.9%) | 37 (0.1%) |
| Trauma center | 3 (0.3%) | 279 (0.8%) |
| Urgent | 192 (18.7%) | 7,573 (22.6%) |
| Unknown | 6 (0.6%) | 130 (0.4%) |
After covariate adjustment for baseline measurements significantly related to each outcome, average daily post occlusion costs were estimated to be $317 lower for alteplase recipients than for patients who received catheter replacement ($317; 95% confidence interval [CI]: $238‐$392; P<0.0001) (Table 3). Average adjusted total post occlusion costs were $1419 lower for alteplase recipients than for patients who received catheter replacement ($1418; 95% CI: $307‐$2458; P=0.012).
| CVC Replace Only, n=1,028 | Alteplase Only, n=33,551 | |
|---|---|---|
| ||
| 30‐day readmission | 24.6% | 23.7% |
| 90‐day readmission | 35.1% | 33.9% |
| Preocclusion | ||
| Mean (SD) length of stay, days | 3.8 (6.7) | 7.3 (6.9) |
| Mean (SD) total cost | $10,485 ($29,088) | $18,546 ($22,658) |
| Mean (SD) cost per day | $2,876 ($3,046) | $2,637 ($1,783) |
| Postocclusion | ||
| Mean (SD) length of stay, days | 8.8 (11.0) | 8.8 (10.0) |
| Mean (SD) total cost | $18,714 ($32,189) | $16,765 ($29,966) |
| Mean (SD) cost per day | $2,146 ($2,995) | $2,058 ($6,585) |
Contributing to the lower cost were certain revenue‐center specific costs (Table 4). Total room and board costs were different between the alteplase and catheter‐replacement groups in both the pre‐ and postocclusion periods; this was related to the difference between the 2 comparison groups in postocclusion LOS of about 0.3 days (Table 5). However, the differences favored alteplase use over catheter replacement. Cardiology/electrocardiography costs were lower for catheter replacement in the preocclusion period but lower for alteplase use in the postocclusion period. Emergency department costs were higher for catheter replacement in both periods, as were respiratory costs in the same manner. Additionally, costs for laboratory tests, nursing, operating room/surgery, pharmacy, radiology, supplies, and ICU room and board were lower in the preocclusion period but higher in the postocclusion period for catheter‐replacement patients. It was unclear why the pharmacy costs after catheter replacement would have increased for catheter‐replacement patients in contrast to the decrease for alteplase‐treated patients, but because this occurred at an average daily basis as well, it appeared that catheter‐replacement patients may have received additional medications. Average adjusted postocclusion LOS was similar for alteplase and catheter‐replacement recipients (P=0.24), suggesting that decreased total costs were due to reasons other than shorter LOS.
| Preocclusiona | Postocclusiona | |||
|---|---|---|---|---|
| CVC Replacement Only, n=1,028 | Alteplase Only, n=33,551 | CVC Replacement Only, n=1,028 | Alteplase Onlyn=33,551 | |
| ||||
| Total room and board cost | ||||
| Mean (SD) total cost | $4,380 ($9,545) | $8,535 ($10,175)b | $8,394 ($14,393) | $8,437 ($18,341)b |
| Mean (SD) cost per day | $693 ($734) | $1,097 ($724)b | $751 ($536) | $983 ($3,250) |
| Cardiology/ECG cost | ||||
| Mean (SD) total cost | $82 ($806) | $154 ($605)b | $124 ($540) | $107 ($735)b |
| Mean (SD) cost per day | $17 ($96) | $26 ($131)b | $17 ($93) | $19 ($217)b |
| Emergency department cost | ||||
| Mean (SD) total cost | $10 ($91) | $36 ($284)b | $10 ($67) | $12 ($195) |
| Mean (SD) cost per day | $4 ($32) | $8 ($65)b | $2 ($19) | $6 ($76) |
| Laboratory cost | ||||
| Mean (SD) total cost | $864 ($2,538) | $1,425 ($3,622)b | $1,471 ($5,614) | $1,175 ($3,961) |
| Mean (SD) cost per day | $140 ($314) | $180 ($269)b | $139 ($313) | $142 ($465)b |
| Nursing Cost | ||||
| Mean (SD) total cost | $218 ($1,497) | $224 ($2,364)b | $432 ($2,538) | $231 ($2,785) |
| Mean (SD) cost per day | $39 ($166) | $24 ($127)b | $35 ($140) | $21 ($112) |
| OR/surgery cost | ||||
| Mean (SD) total cost | $902 ($4,743) | $1,602 ($3,597)b | $1,437 ($3,029) | $847 ($2,701)b |
| Mean (SD) cost per day | $207 ($495) | $267 ($513)b | $302 ($646) | $130 ($827)b |
| Pharmacy cost | ||||
| Mean (SD) total cost | $2,085 ($20,338) | $3,014 ($6,408)b | $3,200 ($16,396) | $2,914 ($8,383)b |
| Mean (SD) cost per day | $263 ($1,509) | $368 ($583)b | $362 ($2,427) | $347 ($853)b |
| Radiology cost | ||||
| Mean (SD) total cost | $470 ($869) | $782 ($1,031)b | $731 ($1,160) | $505 ($1,550)b |
| Mean (SD) cost per day | $133 ($362) | $130 ($189)b | $144 ($293) | $83 ($469)b |
| Respiratory cost | ||||
| Mean (SD) total cost | $391 ($1,442) | $895 ($2,160)b | $673 ($2,209) | $783 ($2,297)b |
| Mean (SD) cost per day | $51 ($121) | $104 ($170)b | $61 ($115) | $81 ($280)b |
| Supply cost | ||||
| Mean (SD) total cost | $834 ($3,221) | $1,408 ($5,871)b | $1,636 ($7,250) | $1,117 ($4,477)b |
| Mean (SD) cost per day | $208 ($1,244) | $211 ($789)b | $264 ($871) | $165 ($1,529)b |
| Other therapy cost | ||||
| Mean (SD) total cost | $179 ($702) | $355 ($815)b | $436 ($837) | $509 ($1,263)b |
| Mean (SD) cost per day | $30 ($81) | $46 ($98)b | $51 ($106) | $66 ($481)b |
| Other departments cost | ||||
| Mean (SD) total cost | $26 ($710) | $1 ($36) | $74 ($1,127) | $3 ($144) b |
| Mean (SD) cost per day | $3 ($56) | $0 ($5) | $6 ($86) | $0 ($13)b |
| Fees cost | ||||
| Mean (SD) total cost | $38 ($370) | $82 ($969)b | $67 ($340) | $86 ($2,704) |
| Mean (SD) cost per day | $7 ($47) | $12 ($77)b | $12 ($120) | $12 ($843) |
| Healthcare services cost | ||||
| Mean (SD) total cost | $5 ($53) | $31 ($1,052)b | $29 ($515) | $35 ($1,162) |
| Mean (SD) cost per day | $1 ($10) | $3 ($65)b | $2 ($11) | $3 ($54) |
| ICU room and board cost | ||||
| Mean (SD) total cost | $2,085 ($7,700) | $4,333 ($8,826)b | $3,158 ($10,767) | $2,884 ($15,863) |
| Mean (SD) cost per day | $293 ($677) | $543 ($854)b | $222 ($512) | $323 ($2,330) |
| Model | Parameter Estimate | Summary Statistic | Estimate (95% CI) |
|---|---|---|---|
| |||
| 30‐day readmissiona | 0.0234 | Odds ratio | 1.048 (0.899 to 1.221) |
| 90‐day readmissionb | 0.0248 | Odds ratio | 1.051 (0.915 to 1.207) |
| Postocclusion total costsc | 0.0842 | Mean differenced | $1,418.69 ($2,458.12 to $307.27)f |
| Postocclusion total cost per daye | 0.1857 | Mean differenced | $317.20 ($392.24 to $238.22)f |
| Post occlusion length of stayg | 0.0313 | Mean differenced | 0.299 (0.196 to 0.820) |
Unadjusted 30‐ and 90‐day readmission rates were 24.6% and 35.1% for CVC replacement and slightly lower at 23.7% and 33.9% for alteplase (Table 3), respectively. Odds of readmission after adjusting for patient and hospital factors were not significantly different at 30 days (odds ratio [OR]: 1.048, 95% CI: 0.899‐1.221; P=0.55) or at 90 days (OR: 1.051, 95% CI: 0.915‐1.207; P=0.48) (Table 5). Subgroup analyses for patients with a diagnosis of heart failure, myocardial infarction, and cancer revealed similar results.
DISCUSSION
The cost of healthcare in the United States has risen at an outstanding rate compared with other countries. Our percentage of gross national product spent on healthcare is on the order of 16% to 18%, almost twice as much as the next most industrialized country in terms of healthcare expenditure.[11] In the current era, finding opportunities to reduce healthcare costs without negatively impacting quality of care is the name of the game. Professional societies have come together under the campaign of Choosing Wisely: An Initiative of the ABIM (American Board of Internal Medicine) Foundation to help educate clinicians and patients on cost‐containment strategies.[12] Research that demonstrates opportunities to reduce cost will help healthcare providers choose wisely among diagnostic and therapeutic options for patients. Our study demonstrated that the use of a drug such as alteplase in clearing CVC catheter obstruction was significantly less costly to the hospital than catheter replacement.
Cathflo Activase (alteplase: Genentech, South San Francisco, CA), the only FDA‐approved thrombolytic for the restoration of central venous catheter function, is the current standard treatment for catheter occlusions in the United States. A dose of 2 mg in 2 mL is instilled in patients weighing 30 kg or 110% of the internal lumen volume of the catheter not to exceed 2 mg in 2 mL for those patients weighing <30 kg. Haire et al. showed that a 2‐mg dose of alteplase was more effective than urokinase (5000 IU) for treating radiographically proven thrombotic occlusion of a CVC after a dwell time of 120 minutes.[13] In the Cardiovascular thrombolytic used to Open Occluded Lines (COOL) trial, one 2‐mg dose of alteplase cleared the catheter occlusion after 120 minutes in 74% of patients, compared with only 17% of patients who received a placebo. Studies have confirmed the safety and efficacy of alteplase administered at various time intervals in different long‐term catheters, including peripherally inserted central catheters, with major hemorrhage reported in 0.3% of patients.[14, 15, 16]
Adding to the knowledge of patient outcomes from clinical studies, many health outcomes studies have demonstrated benefit in cost containment through decreasing LOS, which one can argue is just shifting the cost to an earlier part of the stay. Even though this is highly beneficial, it does not address the core resource utilization within the hospital. Our study found its cost benefit not in the LOS, but in decreasing core resource utilization such as radiology, lab, nursing, and supplies. If patients are admitted for a noncardiovascular condition and have CVC occlusion, using alteplase to clear the CVC occlusion along with implementing strategies to manage the underlying disease to reduce the LOS becomes a powerful opportunity to impact cost. Among patients who may come to the hospital for just the CVC occlusion, the LOS should be short. There may be no significant opportunity to reduce the LOS in those cases, but opportunities to decrease core hospital resource utilization with alteplase make this approach beneficial if the patient can tolerate it.
Limitations of the study include the retrospective and administrative nature of the database used, which is unable to provide certain clinical measures as would be available at the patient's bedside when treatment choices are being made. Had they been available, we might have included them in our assessment of whether patients who underwent CVC replacement were significantly different from those who received alteplase. In addition, not all hospitals in the database had charge masters that facilitated identification of CVC replacements or reinsertions, requiring the use of CPT‐4 codes and evidence of new CVCs being placed or inserted. Certain patients were excluded if there was conflicting information about whether the CVC was new within the hospital stay or dwelling in the patient prior to admission. Also, dialysis patients were excluded because they were not part of any approved indication for alteplase 2 mg, and this group is particularly prone to catheter obstruction. As such, they represent more complicated cases than the norm; this exclusion may have limited the overall generalizability of the study. The study also relied on charge master (billing) data to identify the use of alteplase and other treatments, where there is the potential, albeit minimal, for inaccuracies in the data. Of greater importance, the study relied on ICD‐9 coding to identify comorbid conditions, and as in other studies using similar data sources, such methods are subject to coding errors and omissions. However, many of the listed limitations above were not thought to be different between the comparison groups or more problematic for this study than for other studies based on similar data sources.
CONCLUSION
Among patients treated for an occluded CVC, alteplase‐treated patients had lower daily postocclusion costs and lower total postocclusion costs than patients who received catheter replacement. Differences in costs did not appear to be driven by differences in postocclusion LOS. Readmissions at 30‐ and 90‐day periods were found to be similar between alteplase recipients and catheter‐replacement patients.
Acknowledgements
The authors thank W. Kenne Mountford for his editorial assistance with a prior version of the article and Dima Qato for assistance with the analyses. Additional editorial assistance was provided by Steve Melvin. This assistance was funded by Genentech, Inc.
Disclosures: This study was funded by Genentech, Inc. F. R. Ernst and C. Lipkin are employees of Premier, which was contracted by Genentech to conduct the study covered in this article. E. Chen is an employee of and holds stock in Genentech. D. Tayama is an employee of Genentech. A. N. Amin received research funding from Premier, which was contracted by Genentech to conduct the study covered in this article.
Long‐term central venous catheters (CVCs) facilitate care for patients with chronic illness by providing easy venous access for laboratory tests, administration of medication, and parenteral nutrition. However, several complications resulting from the use of CVCs, including sepsis, extravasation of infusions, and venous thrombosis, can increase associated morbidity and mortality. These complications can also interrupt and delay treatment for the underlying disease and thereby affect outcomes. One of the most common CVC complications is catheter occlusion.[1]
Catheter occlusion occurs in 14% to 36% of patients within 1 to 2 years of catheter placement.[2, 3, 4, 5, 6, 7, 8] A catheter occlusion can be partial or complete, and can occur secondary to a variety of mechanical problems, including an uncommon, but potentially life‐threatening, pinch‐off syndrome. Medication or parenteral nutrition can also cause occlusion, which can be acute or gradual, with increasingly sluggish flow through the catheter. Inappropriate concentrations or incompatible mixtures can cause medications to precipitate within the catheter lumen.
Occlusions are either thrombotic or nonthrombotic. One autopsy study of patients with a long‐term CVC found that a fibrin sheath encased the catheter tip in every case.[9] An occluded catheter may compromise patient care[9, 10]; it may cause cancellation or delay of procedures, it potentially interrupts administration of critical therapies including vesicants, it may result in risk of infection, and it potentially leads to catheter replacement. This can further complicate care, leading to increased length of stay (LOS) and hospital costs.
To better understand resource utilization, LOS, and cost implications of alteplase compared with catheter replacement, we conducted a preplanned, retrospective analysis of hospitalized patients captured between January 2006 and December 2011 in the database maintained by Premier. The Premier database is a large, US hospital‐based, service‐level, all‐payer, comparative database, with information collected primarily from nearly 600 geographically diverse, nonprofit, nongovernment community and teaching hospitals.
METHODS
Data Sources
The Premier database contains information on over 42 million hospital discharges (mean 5.5 million discharges/year)one‐fifth of all US hospitalizationsfrom the year 2000 to the present. The database contains data from standard hospital discharge files, including patient demographic information and disease state. Patients can be tracked, with a unique identifier, across the inpatient and hospital‐based outpatient settings, as well as across visits. In addition to the data elements available in most of the standard hospital discharge files, the Premier database also contains a date‐stamped log of billed items, including procedures, medications, and laboratory, diagnostic, and therapeutic services at the individual patient level. Drug utilization information is available by day of stay and includes quantity, dosing, strength used, and cost.
The Premier database has been used extensively to benchmark hospital clinical and financial performance as well as by the US Food and Drug Administration (FDA) for drug surveillance and by the Centers for Medicare and Medicaid Services to evaluate next‐generation payment models. Preliminary comparisons between patient and hospital characteristics for hospitals that submit data to Premier and those of the probability sample of hospitals and patients selected for the National Hospital Discharge Survey suggest that the patient populations are similar with regard to patient age, gender, LOS, mortality, primary discharge diagnosis, and primary procedure groups.
Patient Population
In this retrospective observational database analysis, inpatients of all ages were initially identified who were discharged from a hospital between January 1, 2006 and December 31, 2011 and whose records contained 1 or more International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9) procedural codes or Current Procedural Terminology (CPT‐4) codes signifying CVC placement. The catheter replacement group comprised patients having a catheter replacement during the hospitalization. The alteplase treatment group was identified through patient billing records and by computing the dose administered (2 mg) during the index hospitalization period. Healthcare Common Procedure Coding System J‐codes (J2996, alteplase recombinant injection 10 mg; J2997, alteplase recombinant 1 mg) were also evaluated during the analysis to supplement the search string identification. To account for and eliminate catheter replacement due to mechanical failure rather than occlusion, patients with ICD‐9 diagnosis code 996.1 for mechanical failure were excluded. Patients with an ICD‐9 diagnosis code for infection or who received antibiotics on the day of replacement were excluded as an additional way to narrow the study to patients with occlusion as the reason for catheter replacement. In addition, patients receiving kidney dialysis, a chronic condition prone to greater‐than‐usual risk of catheter occlusion, were excluded. When a patient had multiple hospital stays with CVC insertions or placement during the study period, the first hospitalization with insertions or placement was used in our analyses.
Of the CVC patient population (N=574,252), 36,680 patient discharges resulted in the need for CVC replacement, alteplase therapy, or both. Patients receiving both replacement and alteplase (N=144) were excluded from analysis, resulting in 33,551 patient discharges with alteplase and 1028 patient discharges with CVC replacement.
Outcome Measures
The main outcomes of interest were LOS and hospital costs after occlusion, and readmissions at 30 and 90 days. Secondary measures, as they were thought to play a role in influencing outcomes, included LOS and costs before occlusion, as well as departmental costs such as pharmacy, radiology, and days in the intensive care unit (ICU).
Statistical Analysis
Univariate descriptive statistics were used to characterize the patient population by patient, clinical, and hospital attributes. In addition, subgroup analyses were performed among patients with any cardiology diagnosis (using ICD‐9 diagnosis or procedure codes), heart failure, myocardial infarction, and cancer, which were potentially overlapping categories chosen prior to initiating the analyses. Data measured on a continuous scale were expressed as mean, standard deviation, range, and median. Categorical data were expressed as count/percentages in the categories. In addition, categorical costs were also examined before and after occlusion. Tables of results included P values comparing patients who received CVC replacement with those who received alteplase across all measures. The [2] tests were used to test for differences in categorical variables, and t tests were utilized for differences in continuous variables.
Multivariable regression modeling was conducted to better compare outcomes associated with catheter replacement versus alteplase treatment. Linear regression models were performed to evaluate hospital costs and LOS during the initial hospital discharge. Logistic regression models were performed to evaluate the odds of readmission at 30 and 90 days following discharge. All multivariable models controlled for factors found to be statistically significant in univariate analysis. The covariates varied by model, but generally included age, race, sex, cancer, 3M All Patient Refined Diagnosis Related Group risk of mortality and severity of illness, cerebrovascular disease, renal disease, payer, myocardial infarction, hemiplegia/paraplegia, chronic or acute diabetes, peripheral vascular disease, complication, admission source, admission type, congestive heart failure, dementia, metastatic solid tumor, rheumatic disease, peptic ulcer disease, chronic pulmonary disease, hospital teaching status, urban/rural location, US Census region, and number of hospital beds. Certain of these variables, such as 3M measures of severity and risk, as well as measures of LOS and costs before occlusion, were considered as ways to understand differences in risk of increased costs among patients. For each multivariable model, covariates eligible for inclusion in the models were selected using a backward selection method (logistic used stepwise) until all variables remaining in the model were significant at P0.2.
RESULTS
This study included 34,579 patients who first had a CVC insertion and then were treated for a CVC occlusion by receiving a replacement CVC (n=1028) or by receiving alteplase (2 mg) administration (n=33,551) during the same hospitalization (Table 1). Patients who received alteplase tended to be younger (6019 vs 6220 years old). More than 50% were at least 65 years of age. Twelve percent of alteplase patients were black, whereas 18.5% of catheter‐replacement patients were black.
| Catheter Replacement, n=1,028 | Alteplase Treatment, n=33,551 | |
|---|---|---|
| ||
| Age group, ya | ||
| Under 18 | 29 (2.8%) | 984 (2.9%) |
| 1834 | 84 (8.2%) | 2,479 (7.4%) |
| 3544 | 73 (7.1%) | 2,826 (8.4%) |
| 4554 | 116 (11.3%) | 5,217 (15.5%) |
| 5564 | 210 (20.4%) | 6,761 (20.1%) |
| 6574 | 203 (19.7%) | 6,741 (20.1%) |
| 75+ | 313 (30.4%) | 8,543 (25.5%) |
| Mean (SD) | 62 (20) | 60 (19) |
| Sex | ||
| Female | 565 (55.0%) | 18,172 (54.2%) |
| Male | 463 (45.0%) | 15,378 (45.8%) |
| Unknown | 0 (0%) | 1 (0%) |
| Race/ethnicitya | ||
| Black | 190 (18.5%) | 4,057 (12.1%) |
| Hispanic | 40 (3.9%) | 1,098 (3.3%) |
| Other | 126 (12.3%) | 6,250 (18.6%) |
| White | 672 (65.4%) | 22,146 (66.0%) |
| Comorbid conditions | ||
| Myocardial infarction | 96 (9.3%) | 3,746 (11.2%) |
| Congestive heart failure | 258 (25.1%) | 8,210 (24.5%) |
| Peripheral vascular disease | 104 (10.1%) | 3,451 (10.3%) |
| Cerebrovascular disease | 115 (11.2%) | 3,528 (10.5%) |
| Dementia | 33 (3.2%) | 838 (2.5%) |
| Chronic pulmonary diseasea | 264 (25.7%) | 10,495 (31.3%) |
| Rheumatic disease | 37 (3.6%) | 1,344 (4.0%) |
| Peptic ulcer disease | 41 (4.0%) | 1,068 (3.2%) |
| Mild liver diseasea | 94 (9.1%) | 2,392 (7.1%) |
| Moderate/severe liver diseasea | 29 (2.8%) | 531 (1.6%) |
| Acute diabetes | 255 (24.8%) | 9,185 (27.4%) |
| Chronic diabetesa | 44 (4.3%) | 2,327 (6.9%) |
| Hemiplegia paraplegia | 51 (5.0%) | 1,909 (5.7%) |
| Renal diseasea | 209 (20.3%) | 5,351 (16.0%) |
| Cancera | 207 (20.1%) | 5,685 (16.9%) |
| Metastatic solid tumora | 100 (9.7%) | 2,441 (7.3%) |
| AIDS/HIV | 4 (0.4%) | 244 (0.7%) |
| 3M APR‐DRG Severity of Illnessa | ||
| 1‐minor | 36 (3.5%) | 769 (2.3%) |
| 2‐moderate | 172 (16.7%) | 4,109 (12.2%) |
| 3‐major | 384 (37.3%) | 12,175 (36.3%) |
| 4‐extreme | 436 (42.4%) | 16,497 (49.2%) |
| Unknown | 0 (0%) | 1 (0%) |
| 3M APR‐DRG Risk of Mortalitya | ||
| 1‐minor | 159 (15.5%) | 4,716 (14.1%) |
| 2‐moderate | 253 (24.6%) | 6,746 (20.1%) |
| 3‐major | 313 (30.4%) | 10,569 (31.5%) |
| 4‐mxtreme | 303 (29.5%) | 11,519 (34.3%) |
| Unknown | 0 (0%) | 1 (0%) |
Alteplase patients were significantly more likely to have a diagnosis of chronic pulmonary disease, liver disease, renal disease, chronic diabetes (ie, diabetes with complications), and cancer. There was an equivalent number of urban and rural hospitals across the 2 groups of patients (Table 2); however, there were regional differences including a higher proportion of catheter‐replacement patients from the East North Central and Middle Atlantic areas and a lower proportion of catheter‐replacement patients from Mountain and Pacific states. Catheter‐replacement patients more frequently were treated in teaching hospitals and in hospitals of larger size.
| Catheter Replacement, n=1,028 | Alteplase Treatment, n=33,551 | |
|---|---|---|
| ||
| Provider regiona | ||
| New England | 28 (2.7%) | 976 (2.9%) |
| Middle Atlantic | 227 (22.1%) | 1,944 (5.8%) |
| South Atlantic | 247 (24.0%) | 8,047 (24.0%) |
| East North Central | 153 (14.9%) | 3,015 (9.0%) |
| East South Central | 14 (1.4%) | 1,345 (4.0%) |
| West North Central | 98 (9.5%) | 3,590 (10.7%) |
| West South Central | 112 (10.9%) | 5,096 (15.2%) |
| Mountain | 48 (4.7%) | 3,339 (9.9%) |
| Pacific | 94 (9.1%) | 6,083 (18.1%) |
| Unknown | 7 (0.7%) | 116 (0.3%) |
| Population served | ||
| Rural | 56 (5.4%) | 1,838 (5.5%) |
| Urban | 972 (94.6%) | 31,713 (94.5%) |
| Teaching statusa | ||
| Nonteaching | 431 (41.9%) | 18,598 (55.4%) |
| Teaching | 597 (58.1%) | 14,953 (4.6%) |
| Hospital size, no. of bedsa | ||
| <100 | 4 (0.4%) | 475 (1.4%) |
| 100199 | 56 (5.4%) | 1,725 (5.1%) |
| 200299 | 124 (12.1%) | 5,907 (17.6%) |
| 300499 | 432 (42.0%) | 13,790 (41.1%) |
| 500+ | 412 (40.1%) | 11,654 (34.7%) |
| Primary payora | ||
| Commercial | 50 (4.9%) | 1,779 (5.3%) |
| Managed care | 221 (21.5%) | 6,888 (20.5%) |
| Medicaid | 132 (12.8%) | 4,146 (12.4%) |
| Medicare | 572 (55.6%) | 17,226 (51.3%) |
| Other government programs | 9 (0.9%) | 439 (1.3%) |
| Any other payor | 44 (4.3%) | 3,073 (9.2%) |
| Admission sourcea | ||
| Emergency department | 424 (41.2%) | 12,741 (38.0%) |
| Physician referral | 390 (37.9%) | 14,502 (43.2%) |
| Transfer from another health facility | 154 (15.0%) | 4,109 (12.2%) |
| Unknown | 60 (5.8%) | 2,199 (6.5%) |
| Admission typea | ||
| Elective | 205 (19.9%) | 5,872 (17.5%) |
| Emergency | 613 (59.6%) | 19,660 (58.6%) |
| Newborn | 9 (0.9%) | 37 (0.1%) |
| Trauma center | 3 (0.3%) | 279 (0.8%) |
| Urgent | 192 (18.7%) | 7,573 (22.6%) |
| Unknown | 6 (0.6%) | 130 (0.4%) |
After covariate adjustment for baseline measurements significantly related to each outcome, average daily post occlusion costs were estimated to be $317 lower for alteplase recipients than for patients who received catheter replacement ($317; 95% confidence interval [CI]: $238‐$392; P<0.0001) (Table 3). Average adjusted total post occlusion costs were $1419 lower for alteplase recipients than for patients who received catheter replacement ($1418; 95% CI: $307‐$2458; P=0.012).
| CVC Replace Only, n=1,028 | Alteplase Only, n=33,551 | |
|---|---|---|
| ||
| 30‐day readmission | 24.6% | 23.7% |
| 90‐day readmission | 35.1% | 33.9% |
| Preocclusion | ||
| Mean (SD) length of stay, days | 3.8 (6.7) | 7.3 (6.9) |
| Mean (SD) total cost | $10,485 ($29,088) | $18,546 ($22,658) |
| Mean (SD) cost per day | $2,876 ($3,046) | $2,637 ($1,783) |
| Postocclusion | ||
| Mean (SD) length of stay, days | 8.8 (11.0) | 8.8 (10.0) |
| Mean (SD) total cost | $18,714 ($32,189) | $16,765 ($29,966) |
| Mean (SD) cost per day | $2,146 ($2,995) | $2,058 ($6,585) |
Contributing to the lower cost were certain revenue‐center specific costs (Table 4). Total room and board costs were different between the alteplase and catheter‐replacement groups in both the pre‐ and postocclusion periods; this was related to the difference between the 2 comparison groups in postocclusion LOS of about 0.3 days (Table 5). However, the differences favored alteplase use over catheter replacement. Cardiology/electrocardiography costs were lower for catheter replacement in the preocclusion period but lower for alteplase use in the postocclusion period. Emergency department costs were higher for catheter replacement in both periods, as were respiratory costs in the same manner. Additionally, costs for laboratory tests, nursing, operating room/surgery, pharmacy, radiology, supplies, and ICU room and board were lower in the preocclusion period but higher in the postocclusion period for catheter‐replacement patients. It was unclear why the pharmacy costs after catheter replacement would have increased for catheter‐replacement patients in contrast to the decrease for alteplase‐treated patients, but because this occurred at an average daily basis as well, it appeared that catheter‐replacement patients may have received additional medications. Average adjusted postocclusion LOS was similar for alteplase and catheter‐replacement recipients (P=0.24), suggesting that decreased total costs were due to reasons other than shorter LOS.
| Preocclusiona | Postocclusiona | |||
|---|---|---|---|---|
| CVC Replacement Only, n=1,028 | Alteplase Only, n=33,551 | CVC Replacement Only, n=1,028 | Alteplase Onlyn=33,551 | |
| ||||
| Total room and board cost | ||||
| Mean (SD) total cost | $4,380 ($9,545) | $8,535 ($10,175)b | $8,394 ($14,393) | $8,437 ($18,341)b |
| Mean (SD) cost per day | $693 ($734) | $1,097 ($724)b | $751 ($536) | $983 ($3,250) |
| Cardiology/ECG cost | ||||
| Mean (SD) total cost | $82 ($806) | $154 ($605)b | $124 ($540) | $107 ($735)b |
| Mean (SD) cost per day | $17 ($96) | $26 ($131)b | $17 ($93) | $19 ($217)b |
| Emergency department cost | ||||
| Mean (SD) total cost | $10 ($91) | $36 ($284)b | $10 ($67) | $12 ($195) |
| Mean (SD) cost per day | $4 ($32) | $8 ($65)b | $2 ($19) | $6 ($76) |
| Laboratory cost | ||||
| Mean (SD) total cost | $864 ($2,538) | $1,425 ($3,622)b | $1,471 ($5,614) | $1,175 ($3,961) |
| Mean (SD) cost per day | $140 ($314) | $180 ($269)b | $139 ($313) | $142 ($465)b |
| Nursing Cost | ||||
| Mean (SD) total cost | $218 ($1,497) | $224 ($2,364)b | $432 ($2,538) | $231 ($2,785) |
| Mean (SD) cost per day | $39 ($166) | $24 ($127)b | $35 ($140) | $21 ($112) |
| OR/surgery cost | ||||
| Mean (SD) total cost | $902 ($4,743) | $1,602 ($3,597)b | $1,437 ($3,029) | $847 ($2,701)b |
| Mean (SD) cost per day | $207 ($495) | $267 ($513)b | $302 ($646) | $130 ($827)b |
| Pharmacy cost | ||||
| Mean (SD) total cost | $2,085 ($20,338) | $3,014 ($6,408)b | $3,200 ($16,396) | $2,914 ($8,383)b |
| Mean (SD) cost per day | $263 ($1,509) | $368 ($583)b | $362 ($2,427) | $347 ($853)b |
| Radiology cost | ||||
| Mean (SD) total cost | $470 ($869) | $782 ($1,031)b | $731 ($1,160) | $505 ($1,550)b |
| Mean (SD) cost per day | $133 ($362) | $130 ($189)b | $144 ($293) | $83 ($469)b |
| Respiratory cost | ||||
| Mean (SD) total cost | $391 ($1,442) | $895 ($2,160)b | $673 ($2,209) | $783 ($2,297)b |
| Mean (SD) cost per day | $51 ($121) | $104 ($170)b | $61 ($115) | $81 ($280)b |
| Supply cost | ||||
| Mean (SD) total cost | $834 ($3,221) | $1,408 ($5,871)b | $1,636 ($7,250) | $1,117 ($4,477)b |
| Mean (SD) cost per day | $208 ($1,244) | $211 ($789)b | $264 ($871) | $165 ($1,529)b |
| Other therapy cost | ||||
| Mean (SD) total cost | $179 ($702) | $355 ($815)b | $436 ($837) | $509 ($1,263)b |
| Mean (SD) cost per day | $30 ($81) | $46 ($98)b | $51 ($106) | $66 ($481)b |
| Other departments cost | ||||
| Mean (SD) total cost | $26 ($710) | $1 ($36) | $74 ($1,127) | $3 ($144) b |
| Mean (SD) cost per day | $3 ($56) | $0 ($5) | $6 ($86) | $0 ($13)b |
| Fees cost | ||||
| Mean (SD) total cost | $38 ($370) | $82 ($969)b | $67 ($340) | $86 ($2,704) |
| Mean (SD) cost per day | $7 ($47) | $12 ($77)b | $12 ($120) | $12 ($843) |
| Healthcare services cost | ||||
| Mean (SD) total cost | $5 ($53) | $31 ($1,052)b | $29 ($515) | $35 ($1,162) |
| Mean (SD) cost per day | $1 ($10) | $3 ($65)b | $2 ($11) | $3 ($54) |
| ICU room and board cost | ||||
| Mean (SD) total cost | $2,085 ($7,700) | $4,333 ($8,826)b | $3,158 ($10,767) | $2,884 ($15,863) |
| Mean (SD) cost per day | $293 ($677) | $543 ($854)b | $222 ($512) | $323 ($2,330) |
| Model | Parameter Estimate | Summary Statistic | Estimate (95% CI) |
|---|---|---|---|
| |||
| 30‐day readmissiona | 0.0234 | Odds ratio | 1.048 (0.899 to 1.221) |
| 90‐day readmissionb | 0.0248 | Odds ratio | 1.051 (0.915 to 1.207) |
| Postocclusion total costsc | 0.0842 | Mean differenced | $1,418.69 ($2,458.12 to $307.27)f |
| Postocclusion total cost per daye | 0.1857 | Mean differenced | $317.20 ($392.24 to $238.22)f |
| Post occlusion length of stayg | 0.0313 | Mean differenced | 0.299 (0.196 to 0.820) |
Unadjusted 30‐ and 90‐day readmission rates were 24.6% and 35.1% for CVC replacement and slightly lower at 23.7% and 33.9% for alteplase (Table 3), respectively. Odds of readmission after adjusting for patient and hospital factors were not significantly different at 30 days (odds ratio [OR]: 1.048, 95% CI: 0.899‐1.221; P=0.55) or at 90 days (OR: 1.051, 95% CI: 0.915‐1.207; P=0.48) (Table 5). Subgroup analyses for patients with a diagnosis of heart failure, myocardial infarction, and cancer revealed similar results.
DISCUSSION
The cost of healthcare in the United States has risen at an outstanding rate compared with other countries. Our percentage of gross national product spent on healthcare is on the order of 16% to 18%, almost twice as much as the next most industrialized country in terms of healthcare expenditure.[11] In the current era, finding opportunities to reduce healthcare costs without negatively impacting quality of care is the name of the game. Professional societies have come together under the campaign of Choosing Wisely: An Initiative of the ABIM (American Board of Internal Medicine) Foundation to help educate clinicians and patients on cost‐containment strategies.[12] Research that demonstrates opportunities to reduce cost will help healthcare providers choose wisely among diagnostic and therapeutic options for patients. Our study demonstrated that the use of a drug such as alteplase in clearing CVC catheter obstruction was significantly less costly to the hospital than catheter replacement.
Cathflo Activase (alteplase: Genentech, South San Francisco, CA), the only FDA‐approved thrombolytic for the restoration of central venous catheter function, is the current standard treatment for catheter occlusions in the United States. A dose of 2 mg in 2 mL is instilled in patients weighing 30 kg or 110% of the internal lumen volume of the catheter not to exceed 2 mg in 2 mL for those patients weighing <30 kg. Haire et al. showed that a 2‐mg dose of alteplase was more effective than urokinase (5000 IU) for treating radiographically proven thrombotic occlusion of a CVC after a dwell time of 120 minutes.[13] In the Cardiovascular thrombolytic used to Open Occluded Lines (COOL) trial, one 2‐mg dose of alteplase cleared the catheter occlusion after 120 minutes in 74% of patients, compared with only 17% of patients who received a placebo. Studies have confirmed the safety and efficacy of alteplase administered at various time intervals in different long‐term catheters, including peripherally inserted central catheters, with major hemorrhage reported in 0.3% of patients.[14, 15, 16]
Adding to the knowledge of patient outcomes from clinical studies, many health outcomes studies have demonstrated benefit in cost containment through decreasing LOS, which one can argue is just shifting the cost to an earlier part of the stay. Even though this is highly beneficial, it does not address the core resource utilization within the hospital. Our study found its cost benefit not in the LOS, but in decreasing core resource utilization such as radiology, lab, nursing, and supplies. If patients are admitted for a noncardiovascular condition and have CVC occlusion, using alteplase to clear the CVC occlusion along with implementing strategies to manage the underlying disease to reduce the LOS becomes a powerful opportunity to impact cost. Among patients who may come to the hospital for just the CVC occlusion, the LOS should be short. There may be no significant opportunity to reduce the LOS in those cases, but opportunities to decrease core hospital resource utilization with alteplase make this approach beneficial if the patient can tolerate it.
Limitations of the study include the retrospective and administrative nature of the database used, which is unable to provide certain clinical measures as would be available at the patient's bedside when treatment choices are being made. Had they been available, we might have included them in our assessment of whether patients who underwent CVC replacement were significantly different from those who received alteplase. In addition, not all hospitals in the database had charge masters that facilitated identification of CVC replacements or reinsertions, requiring the use of CPT‐4 codes and evidence of new CVCs being placed or inserted. Certain patients were excluded if there was conflicting information about whether the CVC was new within the hospital stay or dwelling in the patient prior to admission. Also, dialysis patients were excluded because they were not part of any approved indication for alteplase 2 mg, and this group is particularly prone to catheter obstruction. As such, they represent more complicated cases than the norm; this exclusion may have limited the overall generalizability of the study. The study also relied on charge master (billing) data to identify the use of alteplase and other treatments, where there is the potential, albeit minimal, for inaccuracies in the data. Of greater importance, the study relied on ICD‐9 coding to identify comorbid conditions, and as in other studies using similar data sources, such methods are subject to coding errors and omissions. However, many of the listed limitations above were not thought to be different between the comparison groups or more problematic for this study than for other studies based on similar data sources.
CONCLUSION
Among patients treated for an occluded CVC, alteplase‐treated patients had lower daily postocclusion costs and lower total postocclusion costs than patients who received catheter replacement. Differences in costs did not appear to be driven by differences in postocclusion LOS. Readmissions at 30‐ and 90‐day periods were found to be similar between alteplase recipients and catheter‐replacement patients.
Acknowledgements
The authors thank W. Kenne Mountford for his editorial assistance with a prior version of the article and Dima Qato for assistance with the analyses. Additional editorial assistance was provided by Steve Melvin. This assistance was funded by Genentech, Inc.
Disclosures: This study was funded by Genentech, Inc. F. R. Ernst and C. Lipkin are employees of Premier, which was contracted by Genentech to conduct the study covered in this article. E. Chen is an employee of and holds stock in Genentech. D. Tayama is an employee of Genentech. A. N. Amin received research funding from Premier, which was contracted by Genentech to conduct the study covered in this article.
- , , , , et al. Management of occlusion and thrombosis associated with long‐term indwelling central venous catheters. Lancet. 2009;374:159–169.
- , , , , , ; Children's Oncology Group. Prophylactic urokinase in the management of long‐term venous access devices in children; a Children's Oncology Group study. J Clin Oncol. 2004;22:2718–2723.
- , , , et al. Central venous catheter‐related complications in children with oncological/hematological diseases: an observational study of 418 devices. Ann Oncol. 1985;16:648–654.
- , , , et al. A cross‐sectional study evaluating post‐thrombotic syndrome in children. Thromb Res. 2003;111:227–233.
- , , , . Complications and management of implanted venous access catheters. J Clin Oncol. 1985;3:710–717.
- . Local installation of small doses of streptokinase for treatment of thrombotic occlusions of long‐term access catheters. J Clin Oncol. 1983;1:572–573.
- , , . Are clinical signs accurate indicators of the cause of central venous catheter occlusion? J Parenter Enteral Nutr. 1995;19:75–79.
- , . Mechanism and management of persistent withdrawal occlusion. Am Surg. 1988;54:326–328.
- . Catheter‐related thrombosis in pediatrics. Pediatr Nurs. 2002;28:97–102, 105–106.
- . Reopen the pipeline. Nursing. 2005;35:54–61.
- , . Chemotherapy extravasation: a consequence of fibrin sheath formation around venous access devices. Oncol Nurs Forum. 1995;22:675–680.
- Organisation for Economic Co‐operation and Development (OECD). Statistics. OECD Health Data 2010, June 2010. http://www.oecd.org/unitedstates/Briefing‐Note‐USA‐2013.pdf. Accessed May 8, 2014.
- ABIM Foundation. Choosing Wisely: An Initiative of the ABIM Foundation. (2013). Available at: http://www.choosingwisely.org. Accessed April 6, 2013.
- , , , . Urokinase versus recombinant tissue plasminogen activator in thrombosed central venous catheters: a double‐blinded, randomized trial. Thromb Haemost. 1994;72:543–547.
- , , , et al. Recombinant tissue plasminogen activator (alteplase) for restoration of flow in occluded central venous access devices: a double‐blind placebo‐controlled trial—the Cardiovascular Thrombolytic to Open Occluded Lines (COOL) efficacy trial. J Vasc Interv Radiol. 2001;12:951–955.
- , , , et al. Safety and efficacy of alteplase for restoring function in occluded central venous catheters: results of the cardiovascular thrombolytic to open occluded lines trial. J Clin Oncol. 2002;20:317–324.
- , , , . Alteplase for treatment of occluded peripherally inserted central catheters: safety and efficacy in 240 patients. J Vasc Interv Radiol. 2004;15:45–49.
- , , , , et al. Management of occlusion and thrombosis associated with long‐term indwelling central venous catheters. Lancet. 2009;374:159–169.
- , , , , , ; Children's Oncology Group. Prophylactic urokinase in the management of long‐term venous access devices in children; a Children's Oncology Group study. J Clin Oncol. 2004;22:2718–2723.
- , , , et al. Central venous catheter‐related complications in children with oncological/hematological diseases: an observational study of 418 devices. Ann Oncol. 1985;16:648–654.
- , , , et al. A cross‐sectional study evaluating post‐thrombotic syndrome in children. Thromb Res. 2003;111:227–233.
- , , , . Complications and management of implanted venous access catheters. J Clin Oncol. 1985;3:710–717.
- . Local installation of small doses of streptokinase for treatment of thrombotic occlusions of long‐term access catheters. J Clin Oncol. 1983;1:572–573.
- , , . Are clinical signs accurate indicators of the cause of central venous catheter occlusion? J Parenter Enteral Nutr. 1995;19:75–79.
- , . Mechanism and management of persistent withdrawal occlusion. Am Surg. 1988;54:326–328.
- . Catheter‐related thrombosis in pediatrics. Pediatr Nurs. 2002;28:97–102, 105–106.
- . Reopen the pipeline. Nursing. 2005;35:54–61.
- , . Chemotherapy extravasation: a consequence of fibrin sheath formation around venous access devices. Oncol Nurs Forum. 1995;22:675–680.
- Organisation for Economic Co‐operation and Development (OECD). Statistics. OECD Health Data 2010, June 2010. http://www.oecd.org/unitedstates/Briefing‐Note‐USA‐2013.pdf. Accessed May 8, 2014.
- ABIM Foundation. Choosing Wisely: An Initiative of the ABIM Foundation. (2013). Available at: http://www.choosingwisely.org. Accessed April 6, 2013.
- , , , . Urokinase versus recombinant tissue plasminogen activator in thrombosed central venous catheters: a double‐blinded, randomized trial. Thromb Haemost. 1994;72:543–547.
- , , , et al. Recombinant tissue plasminogen activator (alteplase) for restoration of flow in occluded central venous access devices: a double‐blind placebo‐controlled trial—the Cardiovascular Thrombolytic to Open Occluded Lines (COOL) efficacy trial. J Vasc Interv Radiol. 2001;12:951–955.
- , , , et al. Safety and efficacy of alteplase for restoring function in occluded central venous catheters: results of the cardiovascular thrombolytic to open occluded lines trial. J Clin Oncol. 2002;20:317–324.
- , , , . Alteplase for treatment of occluded peripherally inserted central catheters: safety and efficacy in 240 patients. J Vasc Interv Radiol. 2004;15:45–49.
Published 2014. The Authors Journal of Hospital Medicine published by Wiley Periodicals, Inc. on behalf of Society of Hospital Medicine
Thromboprophylaxis use in U.S. Hospitals
Venous thromboembolism (VTE) is the third most prevalent cardiovascular disease in the United States, only surpassed by myocardial infarction and stroke.1 There are an estimated 600,000 symptomatic VTE events and 300,000 VTE‐related deaths per year in the United States, with two‐thirds of VTE events being acquired in‐hospital.2
High rates of VTE remain despite evidence from clinical trials showing that VTE can be safely and effectively reduced by VTE prophylaxis in at‐risk medical and surgical patients.1, 3, 4 A cohort study in 2001 suggested that as many as 1 in 6 VTE cases could have been prevented by adequate VTE prophylaxis, amounting to approximately 100,000 preventable cases per year in the United States.5 Clinical guidelines are available to guide the practitioner in the choice of prophylaxis regimen and provide evidence‐based recommendations on the choice of prophylaxis for each risk‐category group.1, 6
Awareness of the importance of preventing VTE is growing in the United States. The improvement of VTE prevention and VTE treatment has been identified as a key goal for hospitals in the United States by The Joint Commission and the National Quality Forum.7, 8 Furthermore, a 2006 U.S. Surgeon General's workshop discussed the issues surrounding deep‐vein thrombosis (DVT) prevention, and released a summary of the priority areas for action.9 The Surgical Care Improvement Project (SCIP), a national partnership initiative for improving surgical care, has also recently released 2 VTE‐focused initiatives (SCIP VTE‐1 and SCIP VTE‐2) to help reduce preventable causes of mortality and morbidity in surgical patients.10
Such quality‐assurance initiatives are likely related to the body of data on inadequate use of VTE prophylaxis in U.S. hospitals, including a number of cohort and registry studies that demonstrated low prophylaxis use compared with evidence‐based guideline recommendations.5, 1115 Although these studies provide an insight into the low levels of use of VTE prophylaxis, recent studies have suggested that the rates of appropriate VTE prophylaxis are even lower when one defines appropriate prophylaxis to include the type or choice of VTE prophylaxis (pharmacological and/or mechanical), drug dose, and duration of prophylaxis.16, 17
In these real‐world studies of U.S. hospital prophylaxis practices from 2002 to 2005, 61.8% of medical patients and 72.9% of surgical patients received at least 1 dose of prophylaxis agent, but only 33.9% of medical patients and 32.3% of surgical patients received an appropriate VTE prophylaxis course.16, 17 These data suggest that the lack of appropriate prophylaxis is not only due to physicians not being aware of the need for VTE prophylaxis, but also due to a lack of understanding of the guideline recommendations.
The seventh update of the American College of Chest Physicians (ACCP) guidelines, released in September 2004, provides more specific recommendations than in previous guidelines. With this publication, and the recent efforts to encourage greater awareness and attention to VTE prophylaxis practices, it is important to see if the combination of increased awareness activities and more specific recommendations have led to an increase in appropriate prophylaxis use compared with previous studies that assessed appropriate prophylaxis with the sixth ACCP guidelines.16, 17 This study will therefore build on studies from 2001 to 2004 to compare appropriate inpatient prophylaxis use, in accordance with the seventh ACCP guidelines, in a wide range of U.S. medical and surgical patients from January 2005 to December 2006.
Materials and Methods
Data Source
The Premier Perspective database is a patient‐level dataset of administrative, billing, and discharge information used for comparative analysis of clinical performance. This database contains approximately 5.5 million patient discharges per year from nearly 500 not‐for‐profit, nongovernmental, community, and teaching hospitals and health systems. Hospitals submit data to the Premier Perspective database on a monthly basis, with the data undergoing numerous quality and validation checks on entry.
Data Collection
All patient records used in this study were di‐dentified in compliance with the Health Insurance Portability and Accountability Act (HIPAA) of 1996 (
Patient Discharge Selection Criteria
The selected study population of patients at high risk of VTE was derived from discharge records of patients discharged between January 1, 2005 and December 31, 2006 that met all inclusion criteria. Data were obtained from 429 hospitals across 39 states in the United States that submitted detailed patient hospitalization information by day of stay in monthly reports to the Premier Perspective database.
Inclusion criteria were:
40 years old.
Minimum hospital length of stay of 6 days (based on the inclusion criteria from the Prophylaxis in Medical Patients with Enoxaparin (MEDENOX) trial, which first demonstrated a reduction in 14‐day VTE rates in medical patients receiving low‐molecular weight heparin (LMWH) compared with placebo.3
Deemed at‐risk of VTE due to the presence of 1 or more of the VTE risk factors identified by the seventh ACCP guidelines.1
Principal medical diagnosis or surgical procedure (based on the International Classification of Diseases, ninth Revision, Clinical Modification [ICD‐9 CM] coding system) belonging to an acute medical illness or a surgical procedure group. Medical diagnoses were as follows: heart failure, burns, severe lung disease, cancer (with or without surgery), acute spinal cord injury (without surgery), and trauma (without surgery). Surgical procedure groups were as follows: major orthopedic surgery, general surgery, gynecological surgery, laparoscopic surgery, urological surgery, and neurological surgery. A principal diagnosis or procedure code was assigned to each patient by the hospital. Where multiple surgeries were performed, the principal procedure assigned to the discharge was used. Discharges with both a principal medical diagnosis and a principal surgical procedure were excluded to eliminate uncertainty about which ACCP guideline recommendation should be applied, except in the cancer group where prophylaxis recommendations for a surgical procedure took priority if present. A final group, the critical care group, was also studied. The critical care group consisted of any discharge from the above medical and surgical groups that was flagged for the critical care unit. Appropriate prophylaxis in this group was defined by their principal medical or surgical diagnosis.
Absence of any contraindication that required‐modification to ACCP‐recommended anticoagulant therapy. Patient discharges were excluded if they had ICD‐9 CM codes for active peptic ulcer disease, malignant hypertension, blood disease (iron deficiency and other anemias, hereditary hemolytic anemias, hereditary elliptocytosis, anemias due to disorders of glutathione metabolism, thalassemias, sickle‐cell trait and disease, other hemoglobinopathies, acquired hemolytic anemias, aplastic and other unspecified anemias, coagulation defects, purpura, and other hemorrhagic conditions), human immunodeficiency virus (HIV) infection, pregnancy, VTE present on admission, intubations of the gastrointestinal and respiratory tracts, liver disease, thrombocytopenia, or insufficient renal function (severe or moderate renal insufficiency).19
Any and Appropriate VTE Prophylaxis Definitions
The use of guideline‐recommended pharmacological prophylaxis (unfractionated heparin, enoxaparin, dalteparin, tinzaparin, fondaparinux, or warfarin) or mechanical prophylaxis (intermittent pneumatic compression or elastic stockings) was collected and measured for each patient discharge included in the study.
Any prophylaxis was defined as the discharge receiving at least 1 order for pharmacological or mechanical prophylaxis during the hospital stay. Appropriate prophylaxis was defined as the discharge receiving VTE prophylaxis that was in accordance with the recommendations for that discharge's principal diagnosis in the seventh ACCP guidelines.1 In order for a discharge to have received appropriate prophylaxis in this study, a detailed examination of the hospital administrative records had to show that the discharge received a guideline‐recommended VTE prophylaxis regimen (pharmacological or mechanical) at the appropriate dose (if a pharmacological regimen was recommended) and for the appropriate duration. The regimens that were derived from the seventh ACCP guidelines and considered as appropriate prophylaxis in this study can be seen in Appendix A. All VTE prophylaxis had to be provided daily for the length of the discharge's hospital stay minus 2 days. The allowance for 2 missing days was to accommodate for the possibility of partial days of stay occurring at admission and discharge, or for the possibility of an invasive procedure occurring during hospitalization for which anticoagulation is not recommended on the day of the procedure. Due to the short hospital length of stay in orthopedic surgery discharges, the duration of prophylaxis had to reach a minimum of length of stay minus 2 days or 7 days total in order to be deemed appropriate.
Levels of any and appropriate prophylaxis were compared between the medical and surgical discharge groups. Furthermore, the influence of factors that may have affected the levels of appropriate prophylaxis such as admission source, geographical region, and hospital type, size, and location was studied. In discharges where VTE prophylaxis did not meet the criteria for appropriate prophylaxis, the reasons were collected and compared between discharge groups. Potential reasons for not receiving appropriate prophylaxis were receiving no pharmacological prophylaxis when prophylaxis was recommended, receiving mechanical prophylaxis alone when pharmacological prophylaxis was recommended, receiving an insufficient dose of pharmacological prophylaxis, or receiving an insufficient duration of prophylaxis.
Results
Among the 2,353,287 discharges in the database during the study period, 390,024 (16.6%) discharges were included in this analysis. Of these discharges, 201,224 (51.6%) were in acute medical illness groups and 188,800 (48.4%) were in surgical procedure groups (Table 1). The medical and surgical groups containing the highest numbers of discharges were critical care (97,022 discharges) and vascular surgery (90,727 discharges), respectively (Table 1).
| Diagnostic Group | Number of Discharges |
|---|---|
| |
| Medical Groups | |
| Acute spinal cord injury | 229 |
| Burns | 973 |
| Cancer | 57,792 |
| Trauma | 21,119 |
| Heart failure | 34,286 |
| Severe lung disease | 86,825 |
| Critical care* | 97,022 |
| Total medical | 201,224 |
| Surgical Groups | |
| General surgery | 61,157 |
| Gynecological surgery | 601 |
| Laparoscopic surgery | 23,341 |
| Major orthopedic surgery | 4021 |
| Elective hip arthroplasty | 1071 |
| Elective knee arthroplasty | 2616 |
| Emergency knee arthroplasty | 13 |
| Hip fracture surgery | 51 |
| Elective spinal surgery | 270 |
| Urological surgery | 4142 |
| Neurological surgery | 4811 |
| Vascular surgery | 90,727 |
| Total surgical | 188,800 |
The total rate of any prophylaxis in this analysis was 71.6%, meaning that nearly 3 in every 4 discharges that were eligible for VTE prophylaxis received at least 1 order for pharmacological or mechanical VTE prophylaxis (Table 2). Rates of any prophylaxis were lower for medical discharges at 65.9%, compared with 77.7% in surgical discharges. Variation was observed within individual discharge diagnosis groups, with the highest rate of any prophylaxis being 93.8% in the major orthopedic surgery group and the lowest rate being 36.8% in the burns group (Table 2).
| Discharge Group | Any Prophylaxis (%) | Appropriate Prophylaxis (%) |
|---|---|---|
| ||
| Medical groups | 65.9 | 12.7 |
| Acute spinal injury | 81.2 | 10.0 |
| Burns | 36.8 | 4.7 |
| Cancer | 69.4 | 12.5 |
| Trauma | 69.4 | 17.5 |
| Heart failure | 79.8 | 15.9 |
| Severe lung disease | 51.8 | 10.5 |
| Surgical groups | 77.7 | 16.4 |
| General | 66.4 | 13.3 |
| Gynecological | 89.7 | 7.7 |
| Laparoscopic | 79.5 | 11.3 |
| Orthopedic | 93.8 | 48.6 |
| Urological | 66.8 | 6.3 |
| Neurological | 69.8 | 5.7 |
| Vascular | 85.0 | 19.5 |
| Critical care* | 89.9 | 15.7 |
| Total | 71.6 | 14.5 |
However, when the recommendations of the seventh ACCP guidelines were applied for prophylaxis type, dose, and duration, only 14.5% of all patients received appropriate prophylaxis (Table 2). Medical discharges also received lower levels of appropriate prophylaxis at 12.7% than surgical discharges (16.4%). Large variations in the rates of appropriate prophylaxis were observed between discharge groups in both the medical and surgical populations. In the medical groups, the highest rate of appropriate prophylaxis was 17.5% in trauma discharges, and the lowest rate was 4.7% in burns discharges. In the surgical groups, the highest rate of appropriate prophylaxis was 48.6% in major orthopedic surgery discharges, and the lowest rate was 5.7% in neurological surgery discharges.
Further examination of the individual discharge records reveals that the primary reason that discharges in the medical diagnosis groups did not receive appropriate prophylaxis was due to no pharmacological prophylaxis being provided, despite the lack of a contraindication to anticoagulant therapy (Table 3). A total of 34.1% of all medical discharges received no pharmacological prophylaxis when indicated. Other reasons for medical discharges not receiving appropriate prophylaxis were receiving pharmacological prophylaxis at an incorrect dose (lower than the guideline‐recommended daily total; 22.7%), receiving prophylaxis for an insufficient duration (missing at least 1 day of prophylaxis that was not the admission or discharge date; 22.1%), and receiving mechanical prophylaxis alone when pharmacological prophylaxis was recommended (8.4%). Variation in the reasons for not receiving appropriate prophylaxis was observed between medical diagnosis groups, with the primary reason being mechanical prophylaxis alone in acute spinal injury discharges, and no prophylaxis in burns, cancer, and trauma discharges (Table 3).
| Inappropriate Dose (%) | Insufficient Duration (%) | Mechanical Prophylaxis Only (%) | No prophylaxis Ordered (%) | |
|---|---|---|---|---|
| ||||
| Medical groups | 22.7 | 22.1 | 8.4 | 34.1 |
| Acute spinal injury | 15.3 | 26.2 | 29.7 | 18.8 |
| Burns* | 14.9 | 12.0 | 5.1 | 63.2 |
| Cancer | 18.3 | 22.3 | 16.3 | 30.6 |
| Trauma* | 12.6 | 19.7 | 19.6 | 30.6 |
| Heart failure | 22.1 | 39.3 | 1.6 | 21.1 |
| Severe lung disease | 19.5 | 17.5 | 3.0 | 50.0 |
| Surgical groups | 13.7 | 36.1 | 11.5 | 22.3 |
| General | 10.9 | 24.5 | 17.6 | 33.6 |
| Gynecological | 11.8 | 23.6 | 46.6 | 10.3 |
| Laparoscopic | 21.4 | 19.7 | 27.1 | 20.5 |
| Orthopedic | 39.8 | 1.6 | 3.7 | 6.2 |
| Urological | 12.6 | 24.8 | 23.0 | 33.2 |
| Neurological | 15.2 | 16.2 | 32.7 | 30.2 |
| Vascular | 12.4 | 51.2 | 1.9 | 15.0 |
| Critical care | 14.2 | 49.4 | 10.5 | 10.1 |
In the total surgical discharge population, an insufficient duration of prophylaxis was the main reason for not receiving appropriate prophylaxis, with 36.1% of all surgical discharges receiving an insufficient duration of prophylaxis (Table 3). Other reasons for surgical discharges not receiving appropriate prophylaxis were receiving no pharmacological prophylaxis (22.3%), receiving pharmacological prophylaxis at an incorrect dose (13.7%), and receiving mechanical prophylaxis alone (11.5%). Variation in the reasons for not receiving appropriate prophylaxis was also observed between surgical diagnosis groups, with the primary reason being inappropriate duration in vascular surgery discharges, mechanical prophylaxis alone in gynecological, laparoscopic, and neurological surgery discharges, inappropriate dosage in orthopedic surgery discharges, and no prophylaxis provided in general and urological surgery discharges (Table 3). In medical and surgical discharges that had a critical care unit stay during their hospitalization, only 15.7% received appropriate prophylaxis, with nearly one‐half of all critical care discharges receiving an insufficient duration of VTE prophylaxis (Table 3).
Analysis of the mean rates of appropriate prophylaxis by hospital factors suggests that trends exist toward increased use of appropriate prophylaxis in larger hospitals, in urban hospitals compared with rural hospitals, and in teaching compared with nonteaching hospitals (Table 4).
| Medical Discharges (%) | Surgical Discharges (%) | |
|---|---|---|
| Hospital size (number of beds) | ||
| 0‒99 | 7.5 | 11.0 |
| 100‒299 | 9.6 | 13.4 |
| 300‒499 | 13.1 | 16.2 |
| 500+ | 14.9 | 18.5 |
| Teaching status | ||
| Teaching | 16.5 | 18.9 |
| Nonteaching | 9.9 | 14.2 |
| Location | ||
| Urban | 13.2 | 16.7 |
| Rural | 8.9 | 14.0 |
| Admission source | ||
| Emergency department | 12.4 | 15.4 |
| Physician referral | 12.2 | 17.2 |
| Other | 22.2 | 19.9 |
| Primary payor | ||
| Commercial | 13.3 | 16.5 |
| Managed care | 13.6 | 16.8 |
| Medicaid | 11.7 | 12.7 |
| Medicare | 12.3 | 17.0 |
| Other payors | 13.7 | 15.6 |
| Geographical region | ||
| East North Central | 19.2 | 25.1 |
| East South Central | 8.3 | 14.0 |
| Middle Atlantic | 20.3 | 21.1 |
| Mountain | 15.7 | 19.4 |
| New England | 10.9 | 12.3 |
| Pacific | 11.0 | 11.7 |
| South Atlantic | 10.5 | 14.3 |
| West North Central | 7.5 | 13.2 |
| West South Central | 8.9 | 15.0 |
Discussion
This study suggests that appropriate prophylaxis, as defined in current practice guidelines for the prevention of VTE in specific at‐risk groups, is not widely applied in a selected cohort of hospitalized patients with known risks for VTE. Current ACCP guidelines provide specific direction on safe and effective prophylaxis regimens. These recommendations include the appropriate dosing and appropriate duration of prophylaxis, according to the specific risk in defined medical and surgical risk groups. However, in nearly 400,000 medical and surgical discharges at risk for VTE in U.S. hospitals, only 14.5% of discharges received VTE prophylaxis that met the recommendations of the seventh ACCP guidelines for prophylaxis type, dose, and duration. Although 71.6% of discharges received some form of prophylaxis during hospitalization, the majority of these discharges did not receive appropriate prophylaxis. Furthermore, nearly 30% of patients who should have received prophylaxis did not have a single order for prophylaxis during their hospitalization.
The ACCP has regularly updated its VTE prevention guidelines from the first release of the guidelines in 1986 to the most recent seventh guidelines in 2008.20, 21 These updates have been in line with emerging literature for both patient populations that are at risk for VTE, and for VTE prophylaxis regimens that are safe and effective in these patients. The main changes between the two most recent guidelines in VTE prevention (sixth and seventh) were to introduce risk assessment within patient groups, resulting in a greater number of more stringent recommendations.1, 11 The combination of the more stringent recommendations, and the recently growing national focus on the need for improved VTE prevention from groups such as The Joint Commission and the National Quality Forum would suggest that the levels of appropriate VTE prophylaxis in U.S. hospitals should be increasing.7, 8
The number of patients, including both medical and surgical discharges, in this study that were eligible for prophylaxis was approximately 16.6%. This number is substantially lower than previously reported in a recent U.S. study that found that 31% of U.S. hospital discharges in 2003 were at risk of VTE.22 It is likely that the discrepancy between the 2 studies is due to the more stringent length of stay criteria (6 days) in our study compared to 2 days in the Anderson et al.22 study. This length of stay criteria will have likely selected for complicated, higher‐risk patients and as such the results of this study may be more applicable to patients at higher risk of VTE than to the general population.
However, when the results of this study are compared to similar studies of appropriate prophylaxis with the sixth ACCP guidelines during the period of 2002 to 2005, the level of appropriate prophylaxis appears to have decreased.16, 17 Although strong conclusions can not be drawn from the comparison of the analyses, the appropriate prophylaxis in medical patients during the timeframe of the sixth ACCP guidelines occurred in 33.9% of patients, compared with only 13.7% in the present study. Two of the categories with the highest rates of appropriate VTE prophylaxis in the analysis of the sixth ACCP guidelines were not included in our study (acute myocardial infarction and ischemic strokedue to these patients being likely to receive treatment dose anticoagulants), but the categories that were included in both studies, ie, acute spinal cord injury, cancer, heart failure, and severe lung disease, have a 50% to 66% decrease in appropriate prophylaxis rates in the current study.16 Only trauma patients have similar rates between studies. Interestingly, the rates of any prophylaxis have increased in the current study, with 65.9% of medical patients receiving some form of prophylaxis in this analysis, compared with 61.8% in the prior study. Similar results are observed when comparing the surgical population in this analysis to prior data on surgical discharges, with the rate of any prophylaxis being higher with the seventh ACCP guidelines than the sixth ACCP guidelines (77.7% vs. 72.9%, respectively), but the rate of appropriate prophylaxis being lower (16.4% vs. 32.3%, respectively).17
The combination of an increase in any prophylaxis, but a decrease in appropriate prophylaxis may suggest that the overall national awareness of the need for VTE prophylaxis in at‐risk patients is increasing. However, the combination of more stringent guideline recommendations, and perhaps a lack of awareness as to the guideline recommendations themselves, has actually led to a decrease in the amount of appropriate prophylaxis being prescribed. Despite this, there still remain approximately 30% of patients who receive no prophylaxis at all. To this end, it is important that awareness initiatives and quality improvement programs address both the need for prophylaxis, and the most safe and effective way to provide appropriate prophylaxis in specific patient populations. The use of electronic or manual alerts and order forms for VTE prophylaxis is one effective way of increasing appropriate prophylaxis, and ultimately reducing the incidence of hospital‐acquired VTE.23 A pivotal study by Kucher et al.23 studied over 2500 patients who were randomly assigned to an electronic intervention group or a control group. In the intervention group, the physician received an electronic alert of the patients' VTE risk, whereas in the control group no alert was issued. The study found that, compared to control, both pharmacological prophylaxis (23.6% vs. 13.0%, P < 0.001) and mechanical prophylaxis (10.0% vs. 1.5%, P < 0.001) were prescribed more frequently in the intervention group. Furthermore, this led to a significant reduction in the incidence of clinically diagnosed, objectively confirmed deep‐vein thrombosis or pulmonary embolism at 90 days, with an incidence of 4.9% in the intervention group compared with 8.2% in the control group (P < 0.001). As this study has found that prophylaxis is inappropriately provided due to insufficient prescribing, insufficient duration, and inappropriate dosing, it would be interesting to identify the educational or procedural interventions that have the biggest impact on each factor. This would allow hospitals to create multicomponent initiatives with a greater chance of increasing the rates of appropriate prophylaxis.
A strength of this study is that this is the largest database analysis of hospital discharges and seventh ACCP guideline‐recommended VTE prophylaxis use to date, giving insights into real‐world clinical practice in the United States with the most recent guidelines. This will provide a checkpoint for improvements in advance of the 2008 guidelines being released. A limitation of this study is that we have utilized a conservative approach to selecting patients who were clearly at risk of VTE. Patients were required to have a length of stay 6 days. This may have both excluded a number of orthopedic surgery and medical patients despite their requirements for VTE prophylaxis and likely have selected a cohort of sicker patients at high‐risk for VTE. It is possible that this will have created a bias for specific patient or hospital characteristics (eg, complex patients or hospitals with less efficient systems) that we cannot adjust for, and this may have affected the results of the study. Due to the use of hospital records alone, we are also unable to examine whether discharges continued to receive appropriate prophylaxis following discharge. As some orthopedic surgery patients are recommended to receive prophylaxis for up to 28 to 35 days following surgery,1 this limitation is likely to have resulted in an overestimation of appropriate prophylaxis rates in the current study. However, it is important to note that the appropriate prophylaxis rate was extremely low, even in this selected higher‐risk population. Furthermore, the use of length of stay minus 2 days as the criteria for appropriate duration may have led to a slight underestimation of appropriate prophylaxis, especially as the reasons for any interruption of prophylaxis by the physician during the hospital stay are unknown. An additional limitation is that the study uses retrospective discharge record data that cannot fully evaluate whether the prophylaxis was appropriate in a complex individual patient. For example, contraindications to anticoagulant prophylaxis are not always documented and may not have been identified in the hospital coding exclusion criteria. In addition, we are only able to assess whether mechanical prophylaxis was ordered, and not whether it was appropriately used. Another limitation is that basing assignment of prophylaxis on the principal diagnosis increases the likelihood that clinical decisions on prophylaxis were based on the primary reason for admission, when in reality there may have been multiple factors affecting the patient's risk assessment and the physician's prophylaxis decision. In this analysis, we used the ACCP guidelines as these are currently the most long‐standing VTE prophylaxis guidelines available, as well as being the most comprehensive for multiple patient groups. However, it is important to acknowledge that specific specialties, such as oncologists and orthopedic surgeons, also have their own specialized guidelines which may have different recommendations. This may therefore have led to an underestimation of appropriate prophylaxis. In addition, the ACCP guidelines have been updated in 2008, providing physicians with a revised set of recommendations for VTE prophylaxis.21 We utilized the 2004 guidelines in our analysis as we feel that it is important to assess whether the prophylaxis provided was appropriate by the standards of care during the timeframe within which the data were collected. However, we acknowledge that applying the new guidelines may impact the results of the study. One final consideration that would make an interesting follow‐up study is an assessment of whether appropriate or inappropriate prophylaxis impacts the clinical outcomes. For example, do patients with appropriate prophylaxis have fewer VTE events and improved mortality compared with those without prophylaxis or with inappropriate prophylaxis.
In summary, this work identifies that, in the United States, there is still considerable underutilization of appropriate VTE prophylaxis across a broad range of diagnostic groups with known VTE risk. While nearly three‐quarters of patients do receive at least 1 order for VTE prophylaxis during their hospitalization, only approximately 1 in 7 patients receive appropriate prophylaxis that matches evidence‐based recommendations for type, dose, and duration. Physician awareness of both the need for VTE prophylaxis, and more specifically what constitutes appropriate prophylaxis in certain patient groups, needs to be increased. The current national performance initiatives will provide a framework for this improvement, but it is the responsibility of individual hospitals to improve their VTE prophylaxis practices. Such an improvement across hospitals will lead to a sizeable reduction in the incidence and economic burden of VTE on the U.S. healthcare system.
Acknowledgements
Editorial and financial support for this publication was provided by sanofi‐aventis U.S., Inc. The authors, however, are fully responsible for the content and editorial decisions for this work. A.A. is a research consultant and on the speakers bureau for sanofi‐aventis U.S., Inc. S.S. and G.Y. work for Premier, Inc., and received funding to carry out this work from sanofi‐aventis U.S., Inc. J.L. is an employee of sanofi‐aventis U.S., Inc.
- , , , et al.Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126:338S–400S.
- , , .Estimated annual number of incident and recurrent, non‐fatal and fatal venous thromboembolism events in the US.Blood.2005;106:Abstract910.
- , , , et al.A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group.N Engl J Med.1999;341:793–800.
- ENOXACAN Study Group.Efficacy and safety of enoxaparin versus unfractionated heparin for prevention of deep vein thrombosis in elective cancer surgery: a double‐blind randomized multicentre trial with venographic assessment. ENOXACAN Study Group.Br J Surg.1997;84:1099–1103.
- , , .Missed opportunities for prevention of venous thromboembolism: an evaluation of the use of thromboprophylaxis guidelines.Chest.2001;120:1964–1971.
- Cardiovascular Disease Educational and Research Trust;Cyprus Cardiovascular Disease Educational and Research Trust;European Venous Forum;International Surgical Thrombosis, Forum;International Union of Angiology;Union Internationale de Phlebologie.Prevention and treatment of venous thromboembolism. International Consensus Statement (Guidelines According to Scientific Evidence).Int Angiol.2006;25:101–161.
- .Preparing for DVT core measures. Healthcare leaders should begin preparing for new deep vein thrombosis prevention standards.Healthc Exec.2005;20:66–67.
- Joint Commission on Accreditation of Healthcare Organizations (JCAHO). Available at: http://www.jointcommission.org. Accessed May 2009.
- U.S. Surgeon General. Summary and consideration of priority areas for action: Surgeon General's workshop on deep‐vein thrombosis. Available at: http://www.surgeongeneral.gov/topics/deepvein/workshop/presentations/summary.pdf. Accessed May 2009.
- Medicare Quality Improvement Committee. SCIP Project Information. Available at: http://www.qualitynet.org/dcs/ContentServer?c=MQParents 119:132S–175S.
- , , , .Use of venous thromboprophylaxis and adherence to guideline recommendations: a cross‐sectional study.Thromb J.2004;2:3–9.
- , , , .Pharmacological thromboembolic prophylaxis in a medical ward: room for improvement.J Gen Intern Med.2002;17:788–791.
- , .A prospective registry of 5,451 patients with ultrasound‐confirmed deep vein thrombosis.Am J Cardiol.2004;93:259–262.
- , , , et al.Antithrombotic therapy practices in US hospitals in an era of practice guidelines.Arch Intern Med.2005;165:1458–1464.
- , , , .Thromboprophylaxis rates in US medical centers: success or failure?J Thromb Haemost.2007;5:1610–1616.
- , , , .Preventing venous thromboembolism in US hospitals: are surgical patients receiving appropriate prophylaxis?Thromb Haemost.2008;99:796–797.
- U.S. Department of Health and Human Services. Policy for Protection of Human Research Subjects. Available at: http://www.hhs. gov/ohrp/humansubjects/guidance/45cfr46.htm#46.101. Accessed: May 2009.
- , .Retrospective database analysis of the prevention of venous thromboembolism with low‐molecular‐weight heparin in acutely III medical inpatients in community practice.Clin Ther.2004;26:419–430.
- ACCP/NHLBI National Conference on Antithrombotic Therapy.Chest.1986;89:1S–106S.
- , , , et al.Prevention of venous thromboembolism: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th edition).Chest.2008;133:381S–453S.
- , , , , .Estimated annual numbers of US acute‐care hospital patients at risk for venous thromboembolism.Am J Hematol.2007;82:777–782.
- , , , et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352:969–977.
Venous thromboembolism (VTE) is the third most prevalent cardiovascular disease in the United States, only surpassed by myocardial infarction and stroke.1 There are an estimated 600,000 symptomatic VTE events and 300,000 VTE‐related deaths per year in the United States, with two‐thirds of VTE events being acquired in‐hospital.2
High rates of VTE remain despite evidence from clinical trials showing that VTE can be safely and effectively reduced by VTE prophylaxis in at‐risk medical and surgical patients.1, 3, 4 A cohort study in 2001 suggested that as many as 1 in 6 VTE cases could have been prevented by adequate VTE prophylaxis, amounting to approximately 100,000 preventable cases per year in the United States.5 Clinical guidelines are available to guide the practitioner in the choice of prophylaxis regimen and provide evidence‐based recommendations on the choice of prophylaxis for each risk‐category group.1, 6
Awareness of the importance of preventing VTE is growing in the United States. The improvement of VTE prevention and VTE treatment has been identified as a key goal for hospitals in the United States by The Joint Commission and the National Quality Forum.7, 8 Furthermore, a 2006 U.S. Surgeon General's workshop discussed the issues surrounding deep‐vein thrombosis (DVT) prevention, and released a summary of the priority areas for action.9 The Surgical Care Improvement Project (SCIP), a national partnership initiative for improving surgical care, has also recently released 2 VTE‐focused initiatives (SCIP VTE‐1 and SCIP VTE‐2) to help reduce preventable causes of mortality and morbidity in surgical patients.10
Such quality‐assurance initiatives are likely related to the body of data on inadequate use of VTE prophylaxis in U.S. hospitals, including a number of cohort and registry studies that demonstrated low prophylaxis use compared with evidence‐based guideline recommendations.5, 1115 Although these studies provide an insight into the low levels of use of VTE prophylaxis, recent studies have suggested that the rates of appropriate VTE prophylaxis are even lower when one defines appropriate prophylaxis to include the type or choice of VTE prophylaxis (pharmacological and/or mechanical), drug dose, and duration of prophylaxis.16, 17
In these real‐world studies of U.S. hospital prophylaxis practices from 2002 to 2005, 61.8% of medical patients and 72.9% of surgical patients received at least 1 dose of prophylaxis agent, but only 33.9% of medical patients and 32.3% of surgical patients received an appropriate VTE prophylaxis course.16, 17 These data suggest that the lack of appropriate prophylaxis is not only due to physicians not being aware of the need for VTE prophylaxis, but also due to a lack of understanding of the guideline recommendations.
The seventh update of the American College of Chest Physicians (ACCP) guidelines, released in September 2004, provides more specific recommendations than in previous guidelines. With this publication, and the recent efforts to encourage greater awareness and attention to VTE prophylaxis practices, it is important to see if the combination of increased awareness activities and more specific recommendations have led to an increase in appropriate prophylaxis use compared with previous studies that assessed appropriate prophylaxis with the sixth ACCP guidelines.16, 17 This study will therefore build on studies from 2001 to 2004 to compare appropriate inpatient prophylaxis use, in accordance with the seventh ACCP guidelines, in a wide range of U.S. medical and surgical patients from January 2005 to December 2006.
Materials and Methods
Data Source
The Premier Perspective database is a patient‐level dataset of administrative, billing, and discharge information used for comparative analysis of clinical performance. This database contains approximately 5.5 million patient discharges per year from nearly 500 not‐for‐profit, nongovernmental, community, and teaching hospitals and health systems. Hospitals submit data to the Premier Perspective database on a monthly basis, with the data undergoing numerous quality and validation checks on entry.
Data Collection
All patient records used in this study were di‐dentified in compliance with the Health Insurance Portability and Accountability Act (HIPAA) of 1996 (
Patient Discharge Selection Criteria
The selected study population of patients at high risk of VTE was derived from discharge records of patients discharged between January 1, 2005 and December 31, 2006 that met all inclusion criteria. Data were obtained from 429 hospitals across 39 states in the United States that submitted detailed patient hospitalization information by day of stay in monthly reports to the Premier Perspective database.
Inclusion criteria were:
40 years old.
Minimum hospital length of stay of 6 days (based on the inclusion criteria from the Prophylaxis in Medical Patients with Enoxaparin (MEDENOX) trial, which first demonstrated a reduction in 14‐day VTE rates in medical patients receiving low‐molecular weight heparin (LMWH) compared with placebo.3
Deemed at‐risk of VTE due to the presence of 1 or more of the VTE risk factors identified by the seventh ACCP guidelines.1
Principal medical diagnosis or surgical procedure (based on the International Classification of Diseases, ninth Revision, Clinical Modification [ICD‐9 CM] coding system) belonging to an acute medical illness or a surgical procedure group. Medical diagnoses were as follows: heart failure, burns, severe lung disease, cancer (with or without surgery), acute spinal cord injury (without surgery), and trauma (without surgery). Surgical procedure groups were as follows: major orthopedic surgery, general surgery, gynecological surgery, laparoscopic surgery, urological surgery, and neurological surgery. A principal diagnosis or procedure code was assigned to each patient by the hospital. Where multiple surgeries were performed, the principal procedure assigned to the discharge was used. Discharges with both a principal medical diagnosis and a principal surgical procedure were excluded to eliminate uncertainty about which ACCP guideline recommendation should be applied, except in the cancer group where prophylaxis recommendations for a surgical procedure took priority if present. A final group, the critical care group, was also studied. The critical care group consisted of any discharge from the above medical and surgical groups that was flagged for the critical care unit. Appropriate prophylaxis in this group was defined by their principal medical or surgical diagnosis.
Absence of any contraindication that required‐modification to ACCP‐recommended anticoagulant therapy. Patient discharges were excluded if they had ICD‐9 CM codes for active peptic ulcer disease, malignant hypertension, blood disease (iron deficiency and other anemias, hereditary hemolytic anemias, hereditary elliptocytosis, anemias due to disorders of glutathione metabolism, thalassemias, sickle‐cell trait and disease, other hemoglobinopathies, acquired hemolytic anemias, aplastic and other unspecified anemias, coagulation defects, purpura, and other hemorrhagic conditions), human immunodeficiency virus (HIV) infection, pregnancy, VTE present on admission, intubations of the gastrointestinal and respiratory tracts, liver disease, thrombocytopenia, or insufficient renal function (severe or moderate renal insufficiency).19
Any and Appropriate VTE Prophylaxis Definitions
The use of guideline‐recommended pharmacological prophylaxis (unfractionated heparin, enoxaparin, dalteparin, tinzaparin, fondaparinux, or warfarin) or mechanical prophylaxis (intermittent pneumatic compression or elastic stockings) was collected and measured for each patient discharge included in the study.
Any prophylaxis was defined as the discharge receiving at least 1 order for pharmacological or mechanical prophylaxis during the hospital stay. Appropriate prophylaxis was defined as the discharge receiving VTE prophylaxis that was in accordance with the recommendations for that discharge's principal diagnosis in the seventh ACCP guidelines.1 In order for a discharge to have received appropriate prophylaxis in this study, a detailed examination of the hospital administrative records had to show that the discharge received a guideline‐recommended VTE prophylaxis regimen (pharmacological or mechanical) at the appropriate dose (if a pharmacological regimen was recommended) and for the appropriate duration. The regimens that were derived from the seventh ACCP guidelines and considered as appropriate prophylaxis in this study can be seen in Appendix A. All VTE prophylaxis had to be provided daily for the length of the discharge's hospital stay minus 2 days. The allowance for 2 missing days was to accommodate for the possibility of partial days of stay occurring at admission and discharge, or for the possibility of an invasive procedure occurring during hospitalization for which anticoagulation is not recommended on the day of the procedure. Due to the short hospital length of stay in orthopedic surgery discharges, the duration of prophylaxis had to reach a minimum of length of stay minus 2 days or 7 days total in order to be deemed appropriate.
Levels of any and appropriate prophylaxis were compared between the medical and surgical discharge groups. Furthermore, the influence of factors that may have affected the levels of appropriate prophylaxis such as admission source, geographical region, and hospital type, size, and location was studied. In discharges where VTE prophylaxis did not meet the criteria for appropriate prophylaxis, the reasons were collected and compared between discharge groups. Potential reasons for not receiving appropriate prophylaxis were receiving no pharmacological prophylaxis when prophylaxis was recommended, receiving mechanical prophylaxis alone when pharmacological prophylaxis was recommended, receiving an insufficient dose of pharmacological prophylaxis, or receiving an insufficient duration of prophylaxis.
Results
Among the 2,353,287 discharges in the database during the study period, 390,024 (16.6%) discharges were included in this analysis. Of these discharges, 201,224 (51.6%) were in acute medical illness groups and 188,800 (48.4%) were in surgical procedure groups (Table 1). The medical and surgical groups containing the highest numbers of discharges were critical care (97,022 discharges) and vascular surgery (90,727 discharges), respectively (Table 1).
| Diagnostic Group | Number of Discharges |
|---|---|
| |
| Medical Groups | |
| Acute spinal cord injury | 229 |
| Burns | 973 |
| Cancer | 57,792 |
| Trauma | 21,119 |
| Heart failure | 34,286 |
| Severe lung disease | 86,825 |
| Critical care* | 97,022 |
| Total medical | 201,224 |
| Surgical Groups | |
| General surgery | 61,157 |
| Gynecological surgery | 601 |
| Laparoscopic surgery | 23,341 |
| Major orthopedic surgery | 4021 |
| Elective hip arthroplasty | 1071 |
| Elective knee arthroplasty | 2616 |
| Emergency knee arthroplasty | 13 |
| Hip fracture surgery | 51 |
| Elective spinal surgery | 270 |
| Urological surgery | 4142 |
| Neurological surgery | 4811 |
| Vascular surgery | 90,727 |
| Total surgical | 188,800 |
The total rate of any prophylaxis in this analysis was 71.6%, meaning that nearly 3 in every 4 discharges that were eligible for VTE prophylaxis received at least 1 order for pharmacological or mechanical VTE prophylaxis (Table 2). Rates of any prophylaxis were lower for medical discharges at 65.9%, compared with 77.7% in surgical discharges. Variation was observed within individual discharge diagnosis groups, with the highest rate of any prophylaxis being 93.8% in the major orthopedic surgery group and the lowest rate being 36.8% in the burns group (Table 2).
| Discharge Group | Any Prophylaxis (%) | Appropriate Prophylaxis (%) |
|---|---|---|
| ||
| Medical groups | 65.9 | 12.7 |
| Acute spinal injury | 81.2 | 10.0 |
| Burns | 36.8 | 4.7 |
| Cancer | 69.4 | 12.5 |
| Trauma | 69.4 | 17.5 |
| Heart failure | 79.8 | 15.9 |
| Severe lung disease | 51.8 | 10.5 |
| Surgical groups | 77.7 | 16.4 |
| General | 66.4 | 13.3 |
| Gynecological | 89.7 | 7.7 |
| Laparoscopic | 79.5 | 11.3 |
| Orthopedic | 93.8 | 48.6 |
| Urological | 66.8 | 6.3 |
| Neurological | 69.8 | 5.7 |
| Vascular | 85.0 | 19.5 |
| Critical care* | 89.9 | 15.7 |
| Total | 71.6 | 14.5 |
However, when the recommendations of the seventh ACCP guidelines were applied for prophylaxis type, dose, and duration, only 14.5% of all patients received appropriate prophylaxis (Table 2). Medical discharges also received lower levels of appropriate prophylaxis at 12.7% than surgical discharges (16.4%). Large variations in the rates of appropriate prophylaxis were observed between discharge groups in both the medical and surgical populations. In the medical groups, the highest rate of appropriate prophylaxis was 17.5% in trauma discharges, and the lowest rate was 4.7% in burns discharges. In the surgical groups, the highest rate of appropriate prophylaxis was 48.6% in major orthopedic surgery discharges, and the lowest rate was 5.7% in neurological surgery discharges.
Further examination of the individual discharge records reveals that the primary reason that discharges in the medical diagnosis groups did not receive appropriate prophylaxis was due to no pharmacological prophylaxis being provided, despite the lack of a contraindication to anticoagulant therapy (Table 3). A total of 34.1% of all medical discharges received no pharmacological prophylaxis when indicated. Other reasons for medical discharges not receiving appropriate prophylaxis were receiving pharmacological prophylaxis at an incorrect dose (lower than the guideline‐recommended daily total; 22.7%), receiving prophylaxis for an insufficient duration (missing at least 1 day of prophylaxis that was not the admission or discharge date; 22.1%), and receiving mechanical prophylaxis alone when pharmacological prophylaxis was recommended (8.4%). Variation in the reasons for not receiving appropriate prophylaxis was observed between medical diagnosis groups, with the primary reason being mechanical prophylaxis alone in acute spinal injury discharges, and no prophylaxis in burns, cancer, and trauma discharges (Table 3).
| Inappropriate Dose (%) | Insufficient Duration (%) | Mechanical Prophylaxis Only (%) | No prophylaxis Ordered (%) | |
|---|---|---|---|---|
| ||||
| Medical groups | 22.7 | 22.1 | 8.4 | 34.1 |
| Acute spinal injury | 15.3 | 26.2 | 29.7 | 18.8 |
| Burns* | 14.9 | 12.0 | 5.1 | 63.2 |
| Cancer | 18.3 | 22.3 | 16.3 | 30.6 |
| Trauma* | 12.6 | 19.7 | 19.6 | 30.6 |
| Heart failure | 22.1 | 39.3 | 1.6 | 21.1 |
| Severe lung disease | 19.5 | 17.5 | 3.0 | 50.0 |
| Surgical groups | 13.7 | 36.1 | 11.5 | 22.3 |
| General | 10.9 | 24.5 | 17.6 | 33.6 |
| Gynecological | 11.8 | 23.6 | 46.6 | 10.3 |
| Laparoscopic | 21.4 | 19.7 | 27.1 | 20.5 |
| Orthopedic | 39.8 | 1.6 | 3.7 | 6.2 |
| Urological | 12.6 | 24.8 | 23.0 | 33.2 |
| Neurological | 15.2 | 16.2 | 32.7 | 30.2 |
| Vascular | 12.4 | 51.2 | 1.9 | 15.0 |
| Critical care | 14.2 | 49.4 | 10.5 | 10.1 |
In the total surgical discharge population, an insufficient duration of prophylaxis was the main reason for not receiving appropriate prophylaxis, with 36.1% of all surgical discharges receiving an insufficient duration of prophylaxis (Table 3). Other reasons for surgical discharges not receiving appropriate prophylaxis were receiving no pharmacological prophylaxis (22.3%), receiving pharmacological prophylaxis at an incorrect dose (13.7%), and receiving mechanical prophylaxis alone (11.5%). Variation in the reasons for not receiving appropriate prophylaxis was also observed between surgical diagnosis groups, with the primary reason being inappropriate duration in vascular surgery discharges, mechanical prophylaxis alone in gynecological, laparoscopic, and neurological surgery discharges, inappropriate dosage in orthopedic surgery discharges, and no prophylaxis provided in general and urological surgery discharges (Table 3). In medical and surgical discharges that had a critical care unit stay during their hospitalization, only 15.7% received appropriate prophylaxis, with nearly one‐half of all critical care discharges receiving an insufficient duration of VTE prophylaxis (Table 3).
Analysis of the mean rates of appropriate prophylaxis by hospital factors suggests that trends exist toward increased use of appropriate prophylaxis in larger hospitals, in urban hospitals compared with rural hospitals, and in teaching compared with nonteaching hospitals (Table 4).
| Medical Discharges (%) | Surgical Discharges (%) | |
|---|---|---|
| Hospital size (number of beds) | ||
| 0‒99 | 7.5 | 11.0 |
| 100‒299 | 9.6 | 13.4 |
| 300‒499 | 13.1 | 16.2 |
| 500+ | 14.9 | 18.5 |
| Teaching status | ||
| Teaching | 16.5 | 18.9 |
| Nonteaching | 9.9 | 14.2 |
| Location | ||
| Urban | 13.2 | 16.7 |
| Rural | 8.9 | 14.0 |
| Admission source | ||
| Emergency department | 12.4 | 15.4 |
| Physician referral | 12.2 | 17.2 |
| Other | 22.2 | 19.9 |
| Primary payor | ||
| Commercial | 13.3 | 16.5 |
| Managed care | 13.6 | 16.8 |
| Medicaid | 11.7 | 12.7 |
| Medicare | 12.3 | 17.0 |
| Other payors | 13.7 | 15.6 |
| Geographical region | ||
| East North Central | 19.2 | 25.1 |
| East South Central | 8.3 | 14.0 |
| Middle Atlantic | 20.3 | 21.1 |
| Mountain | 15.7 | 19.4 |
| New England | 10.9 | 12.3 |
| Pacific | 11.0 | 11.7 |
| South Atlantic | 10.5 | 14.3 |
| West North Central | 7.5 | 13.2 |
| West South Central | 8.9 | 15.0 |
Discussion
This study suggests that appropriate prophylaxis, as defined in current practice guidelines for the prevention of VTE in specific at‐risk groups, is not widely applied in a selected cohort of hospitalized patients with known risks for VTE. Current ACCP guidelines provide specific direction on safe and effective prophylaxis regimens. These recommendations include the appropriate dosing and appropriate duration of prophylaxis, according to the specific risk in defined medical and surgical risk groups. However, in nearly 400,000 medical and surgical discharges at risk for VTE in U.S. hospitals, only 14.5% of discharges received VTE prophylaxis that met the recommendations of the seventh ACCP guidelines for prophylaxis type, dose, and duration. Although 71.6% of discharges received some form of prophylaxis during hospitalization, the majority of these discharges did not receive appropriate prophylaxis. Furthermore, nearly 30% of patients who should have received prophylaxis did not have a single order for prophylaxis during their hospitalization.
The ACCP has regularly updated its VTE prevention guidelines from the first release of the guidelines in 1986 to the most recent seventh guidelines in 2008.20, 21 These updates have been in line with emerging literature for both patient populations that are at risk for VTE, and for VTE prophylaxis regimens that are safe and effective in these patients. The main changes between the two most recent guidelines in VTE prevention (sixth and seventh) were to introduce risk assessment within patient groups, resulting in a greater number of more stringent recommendations.1, 11 The combination of the more stringent recommendations, and the recently growing national focus on the need for improved VTE prevention from groups such as The Joint Commission and the National Quality Forum would suggest that the levels of appropriate VTE prophylaxis in U.S. hospitals should be increasing.7, 8
The number of patients, including both medical and surgical discharges, in this study that were eligible for prophylaxis was approximately 16.6%. This number is substantially lower than previously reported in a recent U.S. study that found that 31% of U.S. hospital discharges in 2003 were at risk of VTE.22 It is likely that the discrepancy between the 2 studies is due to the more stringent length of stay criteria (6 days) in our study compared to 2 days in the Anderson et al.22 study. This length of stay criteria will have likely selected for complicated, higher‐risk patients and as such the results of this study may be more applicable to patients at higher risk of VTE than to the general population.
However, when the results of this study are compared to similar studies of appropriate prophylaxis with the sixth ACCP guidelines during the period of 2002 to 2005, the level of appropriate prophylaxis appears to have decreased.16, 17 Although strong conclusions can not be drawn from the comparison of the analyses, the appropriate prophylaxis in medical patients during the timeframe of the sixth ACCP guidelines occurred in 33.9% of patients, compared with only 13.7% in the present study. Two of the categories with the highest rates of appropriate VTE prophylaxis in the analysis of the sixth ACCP guidelines were not included in our study (acute myocardial infarction and ischemic strokedue to these patients being likely to receive treatment dose anticoagulants), but the categories that were included in both studies, ie, acute spinal cord injury, cancer, heart failure, and severe lung disease, have a 50% to 66% decrease in appropriate prophylaxis rates in the current study.16 Only trauma patients have similar rates between studies. Interestingly, the rates of any prophylaxis have increased in the current study, with 65.9% of medical patients receiving some form of prophylaxis in this analysis, compared with 61.8% in the prior study. Similar results are observed when comparing the surgical population in this analysis to prior data on surgical discharges, with the rate of any prophylaxis being higher with the seventh ACCP guidelines than the sixth ACCP guidelines (77.7% vs. 72.9%, respectively), but the rate of appropriate prophylaxis being lower (16.4% vs. 32.3%, respectively).17
The combination of an increase in any prophylaxis, but a decrease in appropriate prophylaxis may suggest that the overall national awareness of the need for VTE prophylaxis in at‐risk patients is increasing. However, the combination of more stringent guideline recommendations, and perhaps a lack of awareness as to the guideline recommendations themselves, has actually led to a decrease in the amount of appropriate prophylaxis being prescribed. Despite this, there still remain approximately 30% of patients who receive no prophylaxis at all. To this end, it is important that awareness initiatives and quality improvement programs address both the need for prophylaxis, and the most safe and effective way to provide appropriate prophylaxis in specific patient populations. The use of electronic or manual alerts and order forms for VTE prophylaxis is one effective way of increasing appropriate prophylaxis, and ultimately reducing the incidence of hospital‐acquired VTE.23 A pivotal study by Kucher et al.23 studied over 2500 patients who were randomly assigned to an electronic intervention group or a control group. In the intervention group, the physician received an electronic alert of the patients' VTE risk, whereas in the control group no alert was issued. The study found that, compared to control, both pharmacological prophylaxis (23.6% vs. 13.0%, P < 0.001) and mechanical prophylaxis (10.0% vs. 1.5%, P < 0.001) were prescribed more frequently in the intervention group. Furthermore, this led to a significant reduction in the incidence of clinically diagnosed, objectively confirmed deep‐vein thrombosis or pulmonary embolism at 90 days, with an incidence of 4.9% in the intervention group compared with 8.2% in the control group (P < 0.001). As this study has found that prophylaxis is inappropriately provided due to insufficient prescribing, insufficient duration, and inappropriate dosing, it would be interesting to identify the educational or procedural interventions that have the biggest impact on each factor. This would allow hospitals to create multicomponent initiatives with a greater chance of increasing the rates of appropriate prophylaxis.
A strength of this study is that this is the largest database analysis of hospital discharges and seventh ACCP guideline‐recommended VTE prophylaxis use to date, giving insights into real‐world clinical practice in the United States with the most recent guidelines. This will provide a checkpoint for improvements in advance of the 2008 guidelines being released. A limitation of this study is that we have utilized a conservative approach to selecting patients who were clearly at risk of VTE. Patients were required to have a length of stay 6 days. This may have both excluded a number of orthopedic surgery and medical patients despite their requirements for VTE prophylaxis and likely have selected a cohort of sicker patients at high‐risk for VTE. It is possible that this will have created a bias for specific patient or hospital characteristics (eg, complex patients or hospitals with less efficient systems) that we cannot adjust for, and this may have affected the results of the study. Due to the use of hospital records alone, we are also unable to examine whether discharges continued to receive appropriate prophylaxis following discharge. As some orthopedic surgery patients are recommended to receive prophylaxis for up to 28 to 35 days following surgery,1 this limitation is likely to have resulted in an overestimation of appropriate prophylaxis rates in the current study. However, it is important to note that the appropriate prophylaxis rate was extremely low, even in this selected higher‐risk population. Furthermore, the use of length of stay minus 2 days as the criteria for appropriate duration may have led to a slight underestimation of appropriate prophylaxis, especially as the reasons for any interruption of prophylaxis by the physician during the hospital stay are unknown. An additional limitation is that the study uses retrospective discharge record data that cannot fully evaluate whether the prophylaxis was appropriate in a complex individual patient. For example, contraindications to anticoagulant prophylaxis are not always documented and may not have been identified in the hospital coding exclusion criteria. In addition, we are only able to assess whether mechanical prophylaxis was ordered, and not whether it was appropriately used. Another limitation is that basing assignment of prophylaxis on the principal diagnosis increases the likelihood that clinical decisions on prophylaxis were based on the primary reason for admission, when in reality there may have been multiple factors affecting the patient's risk assessment and the physician's prophylaxis decision. In this analysis, we used the ACCP guidelines as these are currently the most long‐standing VTE prophylaxis guidelines available, as well as being the most comprehensive for multiple patient groups. However, it is important to acknowledge that specific specialties, such as oncologists and orthopedic surgeons, also have their own specialized guidelines which may have different recommendations. This may therefore have led to an underestimation of appropriate prophylaxis. In addition, the ACCP guidelines have been updated in 2008, providing physicians with a revised set of recommendations for VTE prophylaxis.21 We utilized the 2004 guidelines in our analysis as we feel that it is important to assess whether the prophylaxis provided was appropriate by the standards of care during the timeframe within which the data were collected. However, we acknowledge that applying the new guidelines may impact the results of the study. One final consideration that would make an interesting follow‐up study is an assessment of whether appropriate or inappropriate prophylaxis impacts the clinical outcomes. For example, do patients with appropriate prophylaxis have fewer VTE events and improved mortality compared with those without prophylaxis or with inappropriate prophylaxis.
In summary, this work identifies that, in the United States, there is still considerable underutilization of appropriate VTE prophylaxis across a broad range of diagnostic groups with known VTE risk. While nearly three‐quarters of patients do receive at least 1 order for VTE prophylaxis during their hospitalization, only approximately 1 in 7 patients receive appropriate prophylaxis that matches evidence‐based recommendations for type, dose, and duration. Physician awareness of both the need for VTE prophylaxis, and more specifically what constitutes appropriate prophylaxis in certain patient groups, needs to be increased. The current national performance initiatives will provide a framework for this improvement, but it is the responsibility of individual hospitals to improve their VTE prophylaxis practices. Such an improvement across hospitals will lead to a sizeable reduction in the incidence and economic burden of VTE on the U.S. healthcare system.
Acknowledgements
Editorial and financial support for this publication was provided by sanofi‐aventis U.S., Inc. The authors, however, are fully responsible for the content and editorial decisions for this work. A.A. is a research consultant and on the speakers bureau for sanofi‐aventis U.S., Inc. S.S. and G.Y. work for Premier, Inc., and received funding to carry out this work from sanofi‐aventis U.S., Inc. J.L. is an employee of sanofi‐aventis U.S., Inc.
Venous thromboembolism (VTE) is the third most prevalent cardiovascular disease in the United States, only surpassed by myocardial infarction and stroke.1 There are an estimated 600,000 symptomatic VTE events and 300,000 VTE‐related deaths per year in the United States, with two‐thirds of VTE events being acquired in‐hospital.2
High rates of VTE remain despite evidence from clinical trials showing that VTE can be safely and effectively reduced by VTE prophylaxis in at‐risk medical and surgical patients.1, 3, 4 A cohort study in 2001 suggested that as many as 1 in 6 VTE cases could have been prevented by adequate VTE prophylaxis, amounting to approximately 100,000 preventable cases per year in the United States.5 Clinical guidelines are available to guide the practitioner in the choice of prophylaxis regimen and provide evidence‐based recommendations on the choice of prophylaxis for each risk‐category group.1, 6
Awareness of the importance of preventing VTE is growing in the United States. The improvement of VTE prevention and VTE treatment has been identified as a key goal for hospitals in the United States by The Joint Commission and the National Quality Forum.7, 8 Furthermore, a 2006 U.S. Surgeon General's workshop discussed the issues surrounding deep‐vein thrombosis (DVT) prevention, and released a summary of the priority areas for action.9 The Surgical Care Improvement Project (SCIP), a national partnership initiative for improving surgical care, has also recently released 2 VTE‐focused initiatives (SCIP VTE‐1 and SCIP VTE‐2) to help reduce preventable causes of mortality and morbidity in surgical patients.10
Such quality‐assurance initiatives are likely related to the body of data on inadequate use of VTE prophylaxis in U.S. hospitals, including a number of cohort and registry studies that demonstrated low prophylaxis use compared with evidence‐based guideline recommendations.5, 1115 Although these studies provide an insight into the low levels of use of VTE prophylaxis, recent studies have suggested that the rates of appropriate VTE prophylaxis are even lower when one defines appropriate prophylaxis to include the type or choice of VTE prophylaxis (pharmacological and/or mechanical), drug dose, and duration of prophylaxis.16, 17
In these real‐world studies of U.S. hospital prophylaxis practices from 2002 to 2005, 61.8% of medical patients and 72.9% of surgical patients received at least 1 dose of prophylaxis agent, but only 33.9% of medical patients and 32.3% of surgical patients received an appropriate VTE prophylaxis course.16, 17 These data suggest that the lack of appropriate prophylaxis is not only due to physicians not being aware of the need for VTE prophylaxis, but also due to a lack of understanding of the guideline recommendations.
The seventh update of the American College of Chest Physicians (ACCP) guidelines, released in September 2004, provides more specific recommendations than in previous guidelines. With this publication, and the recent efforts to encourage greater awareness and attention to VTE prophylaxis practices, it is important to see if the combination of increased awareness activities and more specific recommendations have led to an increase in appropriate prophylaxis use compared with previous studies that assessed appropriate prophylaxis with the sixth ACCP guidelines.16, 17 This study will therefore build on studies from 2001 to 2004 to compare appropriate inpatient prophylaxis use, in accordance with the seventh ACCP guidelines, in a wide range of U.S. medical and surgical patients from January 2005 to December 2006.
Materials and Methods
Data Source
The Premier Perspective database is a patient‐level dataset of administrative, billing, and discharge information used for comparative analysis of clinical performance. This database contains approximately 5.5 million patient discharges per year from nearly 500 not‐for‐profit, nongovernmental, community, and teaching hospitals and health systems. Hospitals submit data to the Premier Perspective database on a monthly basis, with the data undergoing numerous quality and validation checks on entry.
Data Collection
All patient records used in this study were di‐dentified in compliance with the Health Insurance Portability and Accountability Act (HIPAA) of 1996 (
Patient Discharge Selection Criteria
The selected study population of patients at high risk of VTE was derived from discharge records of patients discharged between January 1, 2005 and December 31, 2006 that met all inclusion criteria. Data were obtained from 429 hospitals across 39 states in the United States that submitted detailed patient hospitalization information by day of stay in monthly reports to the Premier Perspective database.
Inclusion criteria were:
40 years old.
Minimum hospital length of stay of 6 days (based on the inclusion criteria from the Prophylaxis in Medical Patients with Enoxaparin (MEDENOX) trial, which first demonstrated a reduction in 14‐day VTE rates in medical patients receiving low‐molecular weight heparin (LMWH) compared with placebo.3
Deemed at‐risk of VTE due to the presence of 1 or more of the VTE risk factors identified by the seventh ACCP guidelines.1
Principal medical diagnosis or surgical procedure (based on the International Classification of Diseases, ninth Revision, Clinical Modification [ICD‐9 CM] coding system) belonging to an acute medical illness or a surgical procedure group. Medical diagnoses were as follows: heart failure, burns, severe lung disease, cancer (with or without surgery), acute spinal cord injury (without surgery), and trauma (without surgery). Surgical procedure groups were as follows: major orthopedic surgery, general surgery, gynecological surgery, laparoscopic surgery, urological surgery, and neurological surgery. A principal diagnosis or procedure code was assigned to each patient by the hospital. Where multiple surgeries were performed, the principal procedure assigned to the discharge was used. Discharges with both a principal medical diagnosis and a principal surgical procedure were excluded to eliminate uncertainty about which ACCP guideline recommendation should be applied, except in the cancer group where prophylaxis recommendations for a surgical procedure took priority if present. A final group, the critical care group, was also studied. The critical care group consisted of any discharge from the above medical and surgical groups that was flagged for the critical care unit. Appropriate prophylaxis in this group was defined by their principal medical or surgical diagnosis.
Absence of any contraindication that required‐modification to ACCP‐recommended anticoagulant therapy. Patient discharges were excluded if they had ICD‐9 CM codes for active peptic ulcer disease, malignant hypertension, blood disease (iron deficiency and other anemias, hereditary hemolytic anemias, hereditary elliptocytosis, anemias due to disorders of glutathione metabolism, thalassemias, sickle‐cell trait and disease, other hemoglobinopathies, acquired hemolytic anemias, aplastic and other unspecified anemias, coagulation defects, purpura, and other hemorrhagic conditions), human immunodeficiency virus (HIV) infection, pregnancy, VTE present on admission, intubations of the gastrointestinal and respiratory tracts, liver disease, thrombocytopenia, or insufficient renal function (severe or moderate renal insufficiency).19
Any and Appropriate VTE Prophylaxis Definitions
The use of guideline‐recommended pharmacological prophylaxis (unfractionated heparin, enoxaparin, dalteparin, tinzaparin, fondaparinux, or warfarin) or mechanical prophylaxis (intermittent pneumatic compression or elastic stockings) was collected and measured for each patient discharge included in the study.
Any prophylaxis was defined as the discharge receiving at least 1 order for pharmacological or mechanical prophylaxis during the hospital stay. Appropriate prophylaxis was defined as the discharge receiving VTE prophylaxis that was in accordance with the recommendations for that discharge's principal diagnosis in the seventh ACCP guidelines.1 In order for a discharge to have received appropriate prophylaxis in this study, a detailed examination of the hospital administrative records had to show that the discharge received a guideline‐recommended VTE prophylaxis regimen (pharmacological or mechanical) at the appropriate dose (if a pharmacological regimen was recommended) and for the appropriate duration. The regimens that were derived from the seventh ACCP guidelines and considered as appropriate prophylaxis in this study can be seen in Appendix A. All VTE prophylaxis had to be provided daily for the length of the discharge's hospital stay minus 2 days. The allowance for 2 missing days was to accommodate for the possibility of partial days of stay occurring at admission and discharge, or for the possibility of an invasive procedure occurring during hospitalization for which anticoagulation is not recommended on the day of the procedure. Due to the short hospital length of stay in orthopedic surgery discharges, the duration of prophylaxis had to reach a minimum of length of stay minus 2 days or 7 days total in order to be deemed appropriate.
Levels of any and appropriate prophylaxis were compared between the medical and surgical discharge groups. Furthermore, the influence of factors that may have affected the levels of appropriate prophylaxis such as admission source, geographical region, and hospital type, size, and location was studied. In discharges where VTE prophylaxis did not meet the criteria for appropriate prophylaxis, the reasons were collected and compared between discharge groups. Potential reasons for not receiving appropriate prophylaxis were receiving no pharmacological prophylaxis when prophylaxis was recommended, receiving mechanical prophylaxis alone when pharmacological prophylaxis was recommended, receiving an insufficient dose of pharmacological prophylaxis, or receiving an insufficient duration of prophylaxis.
Results
Among the 2,353,287 discharges in the database during the study period, 390,024 (16.6%) discharges were included in this analysis. Of these discharges, 201,224 (51.6%) were in acute medical illness groups and 188,800 (48.4%) were in surgical procedure groups (Table 1). The medical and surgical groups containing the highest numbers of discharges were critical care (97,022 discharges) and vascular surgery (90,727 discharges), respectively (Table 1).
| Diagnostic Group | Number of Discharges |
|---|---|
| |
| Medical Groups | |
| Acute spinal cord injury | 229 |
| Burns | 973 |
| Cancer | 57,792 |
| Trauma | 21,119 |
| Heart failure | 34,286 |
| Severe lung disease | 86,825 |
| Critical care* | 97,022 |
| Total medical | 201,224 |
| Surgical Groups | |
| General surgery | 61,157 |
| Gynecological surgery | 601 |
| Laparoscopic surgery | 23,341 |
| Major orthopedic surgery | 4021 |
| Elective hip arthroplasty | 1071 |
| Elective knee arthroplasty | 2616 |
| Emergency knee arthroplasty | 13 |
| Hip fracture surgery | 51 |
| Elective spinal surgery | 270 |
| Urological surgery | 4142 |
| Neurological surgery | 4811 |
| Vascular surgery | 90,727 |
| Total surgical | 188,800 |
The total rate of any prophylaxis in this analysis was 71.6%, meaning that nearly 3 in every 4 discharges that were eligible for VTE prophylaxis received at least 1 order for pharmacological or mechanical VTE prophylaxis (Table 2). Rates of any prophylaxis were lower for medical discharges at 65.9%, compared with 77.7% in surgical discharges. Variation was observed within individual discharge diagnosis groups, with the highest rate of any prophylaxis being 93.8% in the major orthopedic surgery group and the lowest rate being 36.8% in the burns group (Table 2).
| Discharge Group | Any Prophylaxis (%) | Appropriate Prophylaxis (%) |
|---|---|---|
| ||
| Medical groups | 65.9 | 12.7 |
| Acute spinal injury | 81.2 | 10.0 |
| Burns | 36.8 | 4.7 |
| Cancer | 69.4 | 12.5 |
| Trauma | 69.4 | 17.5 |
| Heart failure | 79.8 | 15.9 |
| Severe lung disease | 51.8 | 10.5 |
| Surgical groups | 77.7 | 16.4 |
| General | 66.4 | 13.3 |
| Gynecological | 89.7 | 7.7 |
| Laparoscopic | 79.5 | 11.3 |
| Orthopedic | 93.8 | 48.6 |
| Urological | 66.8 | 6.3 |
| Neurological | 69.8 | 5.7 |
| Vascular | 85.0 | 19.5 |
| Critical care* | 89.9 | 15.7 |
| Total | 71.6 | 14.5 |
However, when the recommendations of the seventh ACCP guidelines were applied for prophylaxis type, dose, and duration, only 14.5% of all patients received appropriate prophylaxis (Table 2). Medical discharges also received lower levels of appropriate prophylaxis at 12.7% than surgical discharges (16.4%). Large variations in the rates of appropriate prophylaxis were observed between discharge groups in both the medical and surgical populations. In the medical groups, the highest rate of appropriate prophylaxis was 17.5% in trauma discharges, and the lowest rate was 4.7% in burns discharges. In the surgical groups, the highest rate of appropriate prophylaxis was 48.6% in major orthopedic surgery discharges, and the lowest rate was 5.7% in neurological surgery discharges.
Further examination of the individual discharge records reveals that the primary reason that discharges in the medical diagnosis groups did not receive appropriate prophylaxis was due to no pharmacological prophylaxis being provided, despite the lack of a contraindication to anticoagulant therapy (Table 3). A total of 34.1% of all medical discharges received no pharmacological prophylaxis when indicated. Other reasons for medical discharges not receiving appropriate prophylaxis were receiving pharmacological prophylaxis at an incorrect dose (lower than the guideline‐recommended daily total; 22.7%), receiving prophylaxis for an insufficient duration (missing at least 1 day of prophylaxis that was not the admission or discharge date; 22.1%), and receiving mechanical prophylaxis alone when pharmacological prophylaxis was recommended (8.4%). Variation in the reasons for not receiving appropriate prophylaxis was observed between medical diagnosis groups, with the primary reason being mechanical prophylaxis alone in acute spinal injury discharges, and no prophylaxis in burns, cancer, and trauma discharges (Table 3).
| Inappropriate Dose (%) | Insufficient Duration (%) | Mechanical Prophylaxis Only (%) | No prophylaxis Ordered (%) | |
|---|---|---|---|---|
| ||||
| Medical groups | 22.7 | 22.1 | 8.4 | 34.1 |
| Acute spinal injury | 15.3 | 26.2 | 29.7 | 18.8 |
| Burns* | 14.9 | 12.0 | 5.1 | 63.2 |
| Cancer | 18.3 | 22.3 | 16.3 | 30.6 |
| Trauma* | 12.6 | 19.7 | 19.6 | 30.6 |
| Heart failure | 22.1 | 39.3 | 1.6 | 21.1 |
| Severe lung disease | 19.5 | 17.5 | 3.0 | 50.0 |
| Surgical groups | 13.7 | 36.1 | 11.5 | 22.3 |
| General | 10.9 | 24.5 | 17.6 | 33.6 |
| Gynecological | 11.8 | 23.6 | 46.6 | 10.3 |
| Laparoscopic | 21.4 | 19.7 | 27.1 | 20.5 |
| Orthopedic | 39.8 | 1.6 | 3.7 | 6.2 |
| Urological | 12.6 | 24.8 | 23.0 | 33.2 |
| Neurological | 15.2 | 16.2 | 32.7 | 30.2 |
| Vascular | 12.4 | 51.2 | 1.9 | 15.0 |
| Critical care | 14.2 | 49.4 | 10.5 | 10.1 |
In the total surgical discharge population, an insufficient duration of prophylaxis was the main reason for not receiving appropriate prophylaxis, with 36.1% of all surgical discharges receiving an insufficient duration of prophylaxis (Table 3). Other reasons for surgical discharges not receiving appropriate prophylaxis were receiving no pharmacological prophylaxis (22.3%), receiving pharmacological prophylaxis at an incorrect dose (13.7%), and receiving mechanical prophylaxis alone (11.5%). Variation in the reasons for not receiving appropriate prophylaxis was also observed between surgical diagnosis groups, with the primary reason being inappropriate duration in vascular surgery discharges, mechanical prophylaxis alone in gynecological, laparoscopic, and neurological surgery discharges, inappropriate dosage in orthopedic surgery discharges, and no prophylaxis provided in general and urological surgery discharges (Table 3). In medical and surgical discharges that had a critical care unit stay during their hospitalization, only 15.7% received appropriate prophylaxis, with nearly one‐half of all critical care discharges receiving an insufficient duration of VTE prophylaxis (Table 3).
Analysis of the mean rates of appropriate prophylaxis by hospital factors suggests that trends exist toward increased use of appropriate prophylaxis in larger hospitals, in urban hospitals compared with rural hospitals, and in teaching compared with nonteaching hospitals (Table 4).
| Medical Discharges (%) | Surgical Discharges (%) | |
|---|---|---|
| Hospital size (number of beds) | ||
| 0‒99 | 7.5 | 11.0 |
| 100‒299 | 9.6 | 13.4 |
| 300‒499 | 13.1 | 16.2 |
| 500+ | 14.9 | 18.5 |
| Teaching status | ||
| Teaching | 16.5 | 18.9 |
| Nonteaching | 9.9 | 14.2 |
| Location | ||
| Urban | 13.2 | 16.7 |
| Rural | 8.9 | 14.0 |
| Admission source | ||
| Emergency department | 12.4 | 15.4 |
| Physician referral | 12.2 | 17.2 |
| Other | 22.2 | 19.9 |
| Primary payor | ||
| Commercial | 13.3 | 16.5 |
| Managed care | 13.6 | 16.8 |
| Medicaid | 11.7 | 12.7 |
| Medicare | 12.3 | 17.0 |
| Other payors | 13.7 | 15.6 |
| Geographical region | ||
| East North Central | 19.2 | 25.1 |
| East South Central | 8.3 | 14.0 |
| Middle Atlantic | 20.3 | 21.1 |
| Mountain | 15.7 | 19.4 |
| New England | 10.9 | 12.3 |
| Pacific | 11.0 | 11.7 |
| South Atlantic | 10.5 | 14.3 |
| West North Central | 7.5 | 13.2 |
| West South Central | 8.9 | 15.0 |
Discussion
This study suggests that appropriate prophylaxis, as defined in current practice guidelines for the prevention of VTE in specific at‐risk groups, is not widely applied in a selected cohort of hospitalized patients with known risks for VTE. Current ACCP guidelines provide specific direction on safe and effective prophylaxis regimens. These recommendations include the appropriate dosing and appropriate duration of prophylaxis, according to the specific risk in defined medical and surgical risk groups. However, in nearly 400,000 medical and surgical discharges at risk for VTE in U.S. hospitals, only 14.5% of discharges received VTE prophylaxis that met the recommendations of the seventh ACCP guidelines for prophylaxis type, dose, and duration. Although 71.6% of discharges received some form of prophylaxis during hospitalization, the majority of these discharges did not receive appropriate prophylaxis. Furthermore, nearly 30% of patients who should have received prophylaxis did not have a single order for prophylaxis during their hospitalization.
The ACCP has regularly updated its VTE prevention guidelines from the first release of the guidelines in 1986 to the most recent seventh guidelines in 2008.20, 21 These updates have been in line with emerging literature for both patient populations that are at risk for VTE, and for VTE prophylaxis regimens that are safe and effective in these patients. The main changes between the two most recent guidelines in VTE prevention (sixth and seventh) were to introduce risk assessment within patient groups, resulting in a greater number of more stringent recommendations.1, 11 The combination of the more stringent recommendations, and the recently growing national focus on the need for improved VTE prevention from groups such as The Joint Commission and the National Quality Forum would suggest that the levels of appropriate VTE prophylaxis in U.S. hospitals should be increasing.7, 8
The number of patients, including both medical and surgical discharges, in this study that were eligible for prophylaxis was approximately 16.6%. This number is substantially lower than previously reported in a recent U.S. study that found that 31% of U.S. hospital discharges in 2003 were at risk of VTE.22 It is likely that the discrepancy between the 2 studies is due to the more stringent length of stay criteria (6 days) in our study compared to 2 days in the Anderson et al.22 study. This length of stay criteria will have likely selected for complicated, higher‐risk patients and as such the results of this study may be more applicable to patients at higher risk of VTE than to the general population.
However, when the results of this study are compared to similar studies of appropriate prophylaxis with the sixth ACCP guidelines during the period of 2002 to 2005, the level of appropriate prophylaxis appears to have decreased.16, 17 Although strong conclusions can not be drawn from the comparison of the analyses, the appropriate prophylaxis in medical patients during the timeframe of the sixth ACCP guidelines occurred in 33.9% of patients, compared with only 13.7% in the present study. Two of the categories with the highest rates of appropriate VTE prophylaxis in the analysis of the sixth ACCP guidelines were not included in our study (acute myocardial infarction and ischemic strokedue to these patients being likely to receive treatment dose anticoagulants), but the categories that were included in both studies, ie, acute spinal cord injury, cancer, heart failure, and severe lung disease, have a 50% to 66% decrease in appropriate prophylaxis rates in the current study.16 Only trauma patients have similar rates between studies. Interestingly, the rates of any prophylaxis have increased in the current study, with 65.9% of medical patients receiving some form of prophylaxis in this analysis, compared with 61.8% in the prior study. Similar results are observed when comparing the surgical population in this analysis to prior data on surgical discharges, with the rate of any prophylaxis being higher with the seventh ACCP guidelines than the sixth ACCP guidelines (77.7% vs. 72.9%, respectively), but the rate of appropriate prophylaxis being lower (16.4% vs. 32.3%, respectively).17
The combination of an increase in any prophylaxis, but a decrease in appropriate prophylaxis may suggest that the overall national awareness of the need for VTE prophylaxis in at‐risk patients is increasing. However, the combination of more stringent guideline recommendations, and perhaps a lack of awareness as to the guideline recommendations themselves, has actually led to a decrease in the amount of appropriate prophylaxis being prescribed. Despite this, there still remain approximately 30% of patients who receive no prophylaxis at all. To this end, it is important that awareness initiatives and quality improvement programs address both the need for prophylaxis, and the most safe and effective way to provide appropriate prophylaxis in specific patient populations. The use of electronic or manual alerts and order forms for VTE prophylaxis is one effective way of increasing appropriate prophylaxis, and ultimately reducing the incidence of hospital‐acquired VTE.23 A pivotal study by Kucher et al.23 studied over 2500 patients who were randomly assigned to an electronic intervention group or a control group. In the intervention group, the physician received an electronic alert of the patients' VTE risk, whereas in the control group no alert was issued. The study found that, compared to control, both pharmacological prophylaxis (23.6% vs. 13.0%, P < 0.001) and mechanical prophylaxis (10.0% vs. 1.5%, P < 0.001) were prescribed more frequently in the intervention group. Furthermore, this led to a significant reduction in the incidence of clinically diagnosed, objectively confirmed deep‐vein thrombosis or pulmonary embolism at 90 days, with an incidence of 4.9% in the intervention group compared with 8.2% in the control group (P < 0.001). As this study has found that prophylaxis is inappropriately provided due to insufficient prescribing, insufficient duration, and inappropriate dosing, it would be interesting to identify the educational or procedural interventions that have the biggest impact on each factor. This would allow hospitals to create multicomponent initiatives with a greater chance of increasing the rates of appropriate prophylaxis.
A strength of this study is that this is the largest database analysis of hospital discharges and seventh ACCP guideline‐recommended VTE prophylaxis use to date, giving insights into real‐world clinical practice in the United States with the most recent guidelines. This will provide a checkpoint for improvements in advance of the 2008 guidelines being released. A limitation of this study is that we have utilized a conservative approach to selecting patients who were clearly at risk of VTE. Patients were required to have a length of stay 6 days. This may have both excluded a number of orthopedic surgery and medical patients despite their requirements for VTE prophylaxis and likely have selected a cohort of sicker patients at high‐risk for VTE. It is possible that this will have created a bias for specific patient or hospital characteristics (eg, complex patients or hospitals with less efficient systems) that we cannot adjust for, and this may have affected the results of the study. Due to the use of hospital records alone, we are also unable to examine whether discharges continued to receive appropriate prophylaxis following discharge. As some orthopedic surgery patients are recommended to receive prophylaxis for up to 28 to 35 days following surgery,1 this limitation is likely to have resulted in an overestimation of appropriate prophylaxis rates in the current study. However, it is important to note that the appropriate prophylaxis rate was extremely low, even in this selected higher‐risk population. Furthermore, the use of length of stay minus 2 days as the criteria for appropriate duration may have led to a slight underestimation of appropriate prophylaxis, especially as the reasons for any interruption of prophylaxis by the physician during the hospital stay are unknown. An additional limitation is that the study uses retrospective discharge record data that cannot fully evaluate whether the prophylaxis was appropriate in a complex individual patient. For example, contraindications to anticoagulant prophylaxis are not always documented and may not have been identified in the hospital coding exclusion criteria. In addition, we are only able to assess whether mechanical prophylaxis was ordered, and not whether it was appropriately used. Another limitation is that basing assignment of prophylaxis on the principal diagnosis increases the likelihood that clinical decisions on prophylaxis were based on the primary reason for admission, when in reality there may have been multiple factors affecting the patient's risk assessment and the physician's prophylaxis decision. In this analysis, we used the ACCP guidelines as these are currently the most long‐standing VTE prophylaxis guidelines available, as well as being the most comprehensive for multiple patient groups. However, it is important to acknowledge that specific specialties, such as oncologists and orthopedic surgeons, also have their own specialized guidelines which may have different recommendations. This may therefore have led to an underestimation of appropriate prophylaxis. In addition, the ACCP guidelines have been updated in 2008, providing physicians with a revised set of recommendations for VTE prophylaxis.21 We utilized the 2004 guidelines in our analysis as we feel that it is important to assess whether the prophylaxis provided was appropriate by the standards of care during the timeframe within which the data were collected. However, we acknowledge that applying the new guidelines may impact the results of the study. One final consideration that would make an interesting follow‐up study is an assessment of whether appropriate or inappropriate prophylaxis impacts the clinical outcomes. For example, do patients with appropriate prophylaxis have fewer VTE events and improved mortality compared with those without prophylaxis or with inappropriate prophylaxis.
In summary, this work identifies that, in the United States, there is still considerable underutilization of appropriate VTE prophylaxis across a broad range of diagnostic groups with known VTE risk. While nearly three‐quarters of patients do receive at least 1 order for VTE prophylaxis during their hospitalization, only approximately 1 in 7 patients receive appropriate prophylaxis that matches evidence‐based recommendations for type, dose, and duration. Physician awareness of both the need for VTE prophylaxis, and more specifically what constitutes appropriate prophylaxis in certain patient groups, needs to be increased. The current national performance initiatives will provide a framework for this improvement, but it is the responsibility of individual hospitals to improve their VTE prophylaxis practices. Such an improvement across hospitals will lead to a sizeable reduction in the incidence and economic burden of VTE on the U.S. healthcare system.
Acknowledgements
Editorial and financial support for this publication was provided by sanofi‐aventis U.S., Inc. The authors, however, are fully responsible for the content and editorial decisions for this work. A.A. is a research consultant and on the speakers bureau for sanofi‐aventis U.S., Inc. S.S. and G.Y. work for Premier, Inc., and received funding to carry out this work from sanofi‐aventis U.S., Inc. J.L. is an employee of sanofi‐aventis U.S., Inc.
- , , , et al.Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126:338S–400S.
- , , .Estimated annual number of incident and recurrent, non‐fatal and fatal venous thromboembolism events in the US.Blood.2005;106:Abstract910.
- , , , et al.A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group.N Engl J Med.1999;341:793–800.
- ENOXACAN Study Group.Efficacy and safety of enoxaparin versus unfractionated heparin for prevention of deep vein thrombosis in elective cancer surgery: a double‐blind randomized multicentre trial with venographic assessment. ENOXACAN Study Group.Br J Surg.1997;84:1099–1103.
- , , .Missed opportunities for prevention of venous thromboembolism: an evaluation of the use of thromboprophylaxis guidelines.Chest.2001;120:1964–1971.
- Cardiovascular Disease Educational and Research Trust;Cyprus Cardiovascular Disease Educational and Research Trust;European Venous Forum;International Surgical Thrombosis, Forum;International Union of Angiology;Union Internationale de Phlebologie.Prevention and treatment of venous thromboembolism. International Consensus Statement (Guidelines According to Scientific Evidence).Int Angiol.2006;25:101–161.
- .Preparing for DVT core measures. Healthcare leaders should begin preparing for new deep vein thrombosis prevention standards.Healthc Exec.2005;20:66–67.
- Joint Commission on Accreditation of Healthcare Organizations (JCAHO). Available at: http://www.jointcommission.org. Accessed May 2009.
- U.S. Surgeon General. Summary and consideration of priority areas for action: Surgeon General's workshop on deep‐vein thrombosis. Available at: http://www.surgeongeneral.gov/topics/deepvein/workshop/presentations/summary.pdf. Accessed May 2009.
- Medicare Quality Improvement Committee. SCIP Project Information. Available at: http://www.qualitynet.org/dcs/ContentServer?c=MQParents 119:132S–175S.
- , , , .Use of venous thromboprophylaxis and adherence to guideline recommendations: a cross‐sectional study.Thromb J.2004;2:3–9.
- , , , .Pharmacological thromboembolic prophylaxis in a medical ward: room for improvement.J Gen Intern Med.2002;17:788–791.
- , .A prospective registry of 5,451 patients with ultrasound‐confirmed deep vein thrombosis.Am J Cardiol.2004;93:259–262.
- , , , et al.Antithrombotic therapy practices in US hospitals in an era of practice guidelines.Arch Intern Med.2005;165:1458–1464.
- , , , .Thromboprophylaxis rates in US medical centers: success or failure?J Thromb Haemost.2007;5:1610–1616.
- , , , .Preventing venous thromboembolism in US hospitals: are surgical patients receiving appropriate prophylaxis?Thromb Haemost.2008;99:796–797.
- U.S. Department of Health and Human Services. Policy for Protection of Human Research Subjects. Available at: http://www.hhs. gov/ohrp/humansubjects/guidance/45cfr46.htm#46.101. Accessed: May 2009.
- , .Retrospective database analysis of the prevention of venous thromboembolism with low‐molecular‐weight heparin in acutely III medical inpatients in community practice.Clin Ther.2004;26:419–430.
- ACCP/NHLBI National Conference on Antithrombotic Therapy.Chest.1986;89:1S–106S.
- , , , et al.Prevention of venous thromboembolism: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th edition).Chest.2008;133:381S–453S.
- , , , , .Estimated annual numbers of US acute‐care hospital patients at risk for venous thromboembolism.Am J Hematol.2007;82:777–782.
- , , , et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352:969–977.
- , , , et al.Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126:338S–400S.
- , , .Estimated annual number of incident and recurrent, non‐fatal and fatal venous thromboembolism events in the US.Blood.2005;106:Abstract910.
- , , , et al.A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group.N Engl J Med.1999;341:793–800.
- ENOXACAN Study Group.Efficacy and safety of enoxaparin versus unfractionated heparin for prevention of deep vein thrombosis in elective cancer surgery: a double‐blind randomized multicentre trial with venographic assessment. ENOXACAN Study Group.Br J Surg.1997;84:1099–1103.
- , , .Missed opportunities for prevention of venous thromboembolism: an evaluation of the use of thromboprophylaxis guidelines.Chest.2001;120:1964–1971.
- Cardiovascular Disease Educational and Research Trust;Cyprus Cardiovascular Disease Educational and Research Trust;European Venous Forum;International Surgical Thrombosis, Forum;International Union of Angiology;Union Internationale de Phlebologie.Prevention and treatment of venous thromboembolism. International Consensus Statement (Guidelines According to Scientific Evidence).Int Angiol.2006;25:101–161.
- .Preparing for DVT core measures. Healthcare leaders should begin preparing for new deep vein thrombosis prevention standards.Healthc Exec.2005;20:66–67.
- Joint Commission on Accreditation of Healthcare Organizations (JCAHO). Available at: http://www.jointcommission.org. Accessed May 2009.
- U.S. Surgeon General. Summary and consideration of priority areas for action: Surgeon General's workshop on deep‐vein thrombosis. Available at: http://www.surgeongeneral.gov/topics/deepvein/workshop/presentations/summary.pdf. Accessed May 2009.
- Medicare Quality Improvement Committee. SCIP Project Information. Available at: http://www.qualitynet.org/dcs/ContentServer?c=MQParents 119:132S–175S.
- , , , .Use of venous thromboprophylaxis and adherence to guideline recommendations: a cross‐sectional study.Thromb J.2004;2:3–9.
- , , , .Pharmacological thromboembolic prophylaxis in a medical ward: room for improvement.J Gen Intern Med.2002;17:788–791.
- , .A prospective registry of 5,451 patients with ultrasound‐confirmed deep vein thrombosis.Am J Cardiol.2004;93:259–262.
- , , , et al.Antithrombotic therapy practices in US hospitals in an era of practice guidelines.Arch Intern Med.2005;165:1458–1464.
- , , , .Thromboprophylaxis rates in US medical centers: success or failure?J Thromb Haemost.2007;5:1610–1616.
- , , , .Preventing venous thromboembolism in US hospitals: are surgical patients receiving appropriate prophylaxis?Thromb Haemost.2008;99:796–797.
- U.S. Department of Health and Human Services. Policy for Protection of Human Research Subjects. Available at: http://www.hhs. gov/ohrp/humansubjects/guidance/45cfr46.htm#46.101. Accessed: May 2009.
- , .Retrospective database analysis of the prevention of venous thromboembolism with low‐molecular‐weight heparin in acutely III medical inpatients in community practice.Clin Ther.2004;26:419–430.
- ACCP/NHLBI National Conference on Antithrombotic Therapy.Chest.1986;89:1S–106S.
- , , , et al.Prevention of venous thromboembolism: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th edition).Chest.2008;133:381S–453S.
- , , , , .Estimated annual numbers of US acute‐care hospital patients at risk for venous thromboembolism.Am J Hematol.2007;82:777–782.
- , , , et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352:969–977.
Copyright © 2009 Society of Hospital Medicine
Transitions in Inpatient Hyperglycemia
Professional and patient safety organizations have recognized the importance of safe transitions as patients move through the health care system, and such attention is even more critical when attempting to achieve glycemic control.14 Since the publication of the Diabetes Control and Complications Trial (DCCT)5 and the United Kingdom Prospective Diabetes Study (UKPDS),6 we have known that intensive glycemic control in the ambulatory setting prevents complications in both type 1 and type 2 diabetes mellitus (DM). Despite the increased risk of hypoglycemia, these trials changed practice patterns in the outpatient settings in favor of intensification of diabetes therapy. In the same way, randomized, prospective trials using intravenous (IV) insulin therapy have revolutionized our thinking about inpatient care by showing that tight glycemic control in the critically ill7 and patients with acute myocardial infarction8 reduces mortality and morbidity. These, as well as additional observational studies associating hyperglycemia with poor outcomes in a variety of medical and surgical patients,915 have led to increased attention on glycemic control in all venues of care.16, 17 Concerns over excessive hypoglycemia and a nonsignificant increase in mortality in certain populations of medical intensive care unit (ICU) patients have raised questions over whether the initial studies can be reproduced or generalized to other groups of inpatients.18, 19 Additional studies are underway to clarify these questions but consensus exists that blood glucose values should at least be less than 180 mg/dL and that the traditional practice of ignoring hyperglycemia is no longer acceptable.
While a uniform focus on glycemic control will allow our patients to receive a consistent message about diabetes, the unique limitations inherent to each practice setting requires different therapeutic regimens and intentional focus on the risks as patients transition from one care area to another. This work addresses several areas of care transition that are particularly important in safely achieving glycemic control including: transition into the hospital for patients on a variety of home regimens, transitions within the hospital (related to changes in dietary intake, change from IV to subcutaneous [SC] therapy, and the perioperative setting), and the transition from the hospital to home or another healthcare facility.
TRANSITION INTO THE HOSPITAL
Until recently, most patients with diabetes admitted to the hospital were managed with sliding‐scale‐only regimens.20, 21 Unfortunately, this led to a variety of complications, including hyperglycemia, hypoglycemia, iatrogenic ketoacidosis, and an inconsistent message to patients on the importance of glycemic control.22 Some outpatient clinicians and patients combated this tradition by creating in‐hospital glucose control plans with orders, which patients would bring with them to the hospital.23 This practice continues to be a helpful way to guide inpatient therapy and is encouraged when available. Glycemic‐controlrelated documents from outpatient clinicians should include the most recent glycosylated hemoglobin (HbA1c) value, diagnosis and known complications, current names and doses of medications, and other patient‐specific preferences or needs (eg, compliance, financial, fear of needles). If the last HbA1c was performed more than 30 days before admission or is not available, one should be obtained upon hospital admission to help guide discharge therapy.24 By knowing the HbA1c, one can determine the level of diabetic control achieved with the current regimen and can help the inpatient team (clinician and patient) determine if a more aggressive glycemic control regimen is necessary at the time of discharge. It is important to note that if the patient has received a transfusion of red blood cells prior to HbA1c measurement or has a hemoglobinopathy, the HbA1c value may not be accurate.25, 26
In general, the outpatient regimen will need to be modified at admission to achieve the appropriate flexibility needed for the changing nutritional intake and insulin requirements that invariably accompany hospitalization. Sulfonylureas and dipeptidyl peptidase 4 inhibitors (DPP4), such as sitagliptin, have most of their effect immediately, but the other oral antihyperglycemic agents have a relatively long delay between treatment and effect, thus they are not a flexible enough method to achieve glycemic control in the hospital. Additionally, inpatients may have transient contraindications to their prior oral antihyperglycemic medications. Metformin is almost always on hold in the hospital setting, at least initially, due to concerns about lactic acidosis. Sulfonylureas can cause hypoglycemia in the setting of worsening renal function or reduced oral intake. Thiazoladinediones (TZDs) are often withheld due to concerns about fluid retention and should be avoided in patients admitted with heart failure. There is little experience in the hospital with the use of newer agents like exenatide, pramlintide, glinides, and DPP4 inhibitors.
Overall, it is generally recommended that oral antihyperglycemic agents be discontinued upon hospital admission and replaced with insulin infusions or scheduled SC insulin. An estimate of 0.4 to 0.5 units/kg of body weight provides a conservative starting point for the total daily dose of insulin (TDD) for most patients. This TDD should then be divided into basal and nutritional components to match the patients' caloric intake. Additional correction doses of insulin should be prescribed to cover episodes of hyperglycemia that develop despite the provision of anticipatory‐physiologic insulin. Further discussion of insulin dosing and SC regimens is available in detail elsewhere.27, 28 The recommendation for these insulin‐only regimens is made regardless of the glycemic control in the outpatient setting and is not meant to imply that they should be continued at discharge. In fact, most patients will return to their home regimen or to one that is intensified but less labor intensive than the basal‐nutritional‐correction insulin used in the hospital. The antihyperglycemic regimen planned for discharge should be anticipated as early as possible and clearly communicated to the patient and/or caregivers to allow for optimal education.
Outpatient insulin regimens that have a high percentage of basal insulin need to be modified during hospital admission to avoid hypoglycemia that may occur from variable nutritional intake. While hospitalized, the basal portion of the estimated TDD generally should not be more than 50% to 60%. The total number of units of all types of insulin used daily as an outpatient can be used as a starting point for determining the inpatient TDD by a 1:1 conversion. Adjustments up or down based on glycemic control, nutritional intake, and other factors are then necessary. If patients are on regimens with insulin plus oral agents at home, the inpatient TDD should either be the home insulin dose or the dose calculated based on their weight, whichever is greater. Patients who use carbohydrate counting to determine nutritional insulin doses as an outpatient might be continued on this regimen if they have a strong understanding of the methods, they are coherent enough to determine their doses, nursing staff are well educated, and dietary services provides the carbohydrate content for the hospital menu. If patients are on insulin pumps at home, these should be managed according to a uniform hospital policy to assure safety. If conversion to multiple daily injections is needed, the same 1:1 conversion is safe.29
Transitions Within the Hospital
General Issues
Within the hospital itself, there are several transitions that have important quality and safety implications regarding glycemic control. The handoffs between providers should follow a standardized format.4, 30, 31 Essential information will vary depending on the setting but should universally include recent hypoglycemia, insulin type and doses, and hypoglycemic risk factors such as changes in insulin doses, the development of renal insufficiency, inability of the patient to self‐report symptoms, tapering of steroids, and cessation or interruption of nutritional intake.32
One of the greatest risks for hypoglycemia in the hospital comes from the unpredictable nutritional interruptions that occur. Unplanned changes are best handled by nurses having an existing order to hold scheduled nutritional insulin if patients are classified nothing by mouth (NPO) or eat <50% of their meal. Additionally, nursing staff should have orders or policies that allow flexibility in the time of administering scheduled rapid‐acting nutritional insulin so that it may be given during or immediately following the meal in patients at higher risk for poor oral intake. Tube feedings also place patients at high risk for hypoglycemia because the tube may become dislodged or they may begin to have feeding intolerance. For these reasons, a measure of safety would be to have standing orders to substitute IV 10% dextrose in water (D10W) at the same rate as the prior tube feeds, hold nutritional insulin, and begin more frequent monitoring whenever tube feeds are stopped.33 Orders that rely on nursing staff to notify a physician when tube feedings are stopped are generally not directive enough because providers may be distracted by other changes or forget the patient is on long‐acting insulin. The need for this flexibility around nutritional dosing emphasizes the importance of avoiding excessive doses of basal insulin. If the total dose of basal insulin is 40% to 50% of the TDD, it can safely be continued at its usual dose despite changing nutritional intake. The only exception is neutral protamine Hagedorn (NPH) insulin, which should be reduced when patients are NPO due to its peak. Generally, a 50% reduction in NPH is recommended for morning doses, but bedtime doses may be given with little to no reduction. Because of the complexity of these issues, standardized order sets are the best way to reliably communicate all the necessary standing orders to nursing staff (Table 1).
|
| Nutritional insulin |
| Hold if patients are NPO or eat less than 50% of their meal. |
| Administer scheduled rapid acting nutritional insulin during or immediately following the meal if oral intake is questionable (ie, nausea, emesis, or newly advancing diet). |
| Tube feedings: When tube feeds are stopped unexpectedly |
| Start dextrose containing IV fluids (many institutions use D10W at the same rate as the prior tube feeds). |
| Hold scheduled nutritional insulin. |
| Notify physician. |
| Basal insulin |
| Continue if NPO. |
| Reduce morning dose of NPH by 50% if NPO and may need to reduce the dose of bedtime NPH. |
| IV to subcutaneous transition |
| Timing for discontinuing IV infusion in relation to first dose of subcutaneous insulin. |
| Prompts for verbal communication between ICU and general ward staff. |
Transitioning the Patient Off of IV Insulin
The strongest evidence for tight glycemic control derives from studies in the surgical ICU.7 Many hospitals have robust, effective IV‐insulin protocols. The frequency of monitoring and rapidity of action of IV insulin allow quick achievement of blood glucose control. As patients begin to eat, the layering of SC nutritional insulin on top of the insulin infusion may reduce the lability of the infusion rate and prevent excursions in glycemic control. When the patient is ready to leave the ICU or start a full oral diet, it is recommended that they transition off of the IV insulin to a basal‐nutritional‐correction regimen.33, 34
The amount of insulin needed with IV infusion is a useful estimate of the TDD of insulin.28, 33, 35, 36 There are important general steps to take when making this transition; but, due to the lack of conclusive data proving the advantage of one regimen over another, there are a variety of acceptable specific protocols (Table 2).3739 First, it should be determined if patients are expected to require ongoing scheduled SC insulin or not. Certainly, all patients with type 1 DM will require scheduled SC insulin, but patients with type 2 DM on low insulin infusion rates or some patients with new hyperglycemia can appropriately be managed with sliding‐scale alone. Next, the average hourly rate of the infusion over the preceding 6 to 8 hours should be determined because it most accurately reflects current insulin needs during the changing stress, nutrition, and medications in critical care patients. This hourly rate will then be converted to a TDD using a safety factor to anticipate decreasing insulin requirements. Some portion of this daily total will then be assigned to be basal insulin. As patients' clinical conditions approach baseline, so will their insulin requirements, and the dose will need to be revised.24
|
| Step 1: Is patient stable enough for transition? Hypotension, active sepsis, vasopressors, and intubation are contraindications to transition due to unreliable subcutaneous insulin absorption and continued need for the most flexible dosing due to frequently changing insulin requirements. |
| Step 2: Does this patient need a transition to scheduled subcutaneous (SC) insulin? |
| Yes |
| All patients with type 1 DM. |
| Type 2 DM patients on insulin as outpatient. |
| Type 2 DM patients with a recent mean infusion rate of 0.5 units/hour.* |
| No |
| Type 2 DM patients with infusion rate <0.5 units/hour.* |
| Stress hyperglycemia or previously unrecognized DM if infusion rate <1 unit/hour, or if HbA1c near normal. |
| Some institutions exclude all stress hyperglycemia patients from transition to a SC insulin regimen, regardless of drip rate. |
| Step 3: If transition is needed, calculate a total daily dose (TDD) of insulin. The TDD is an estimate of the 24‐hour insulin requirement when the patient is receiving full nutrition. |
| Determine mean insulin infusion rate from last 6 to 8 hours. |
| Calculate 24‐hour insulin dose based on this, and reduce this 24‐hour dose by some safety factor. There are several options for this step. |
| Multiply hourly rate by 24, then multiply by 0.7 or 0.8 to arrive at a safety‐adjusted 24 hour insulin dose. |
| OR |
| Multiply hourly infusion rate by 20 (80% of 24). |
| Determine if this total is the TDD or basal dose based on current nutrition. There are several options for this step for you or your institution to choose. |
| If infusion was serving basal AND nutritional needs of patient (such as a patient on 24‐hour tube feedings) this will be your TDD. |
| OR |
| If the infusion insulin was not covering significant nutrition, this could be the BASAL insulin dose. |
| Step 4: Construct a regimen tailored to the patient's nutritional situation, building in safeguards for any changes in nutritional intake and uncertainties about reliability of intake. Several options are again available. |
| Basal: should be ordered as basal glargine or detemir (these are preferred by SHM GCTF but NPH is also an option). |
| Dose is 40% to 50% of TDD. |
| OR |
| Adjusted 24‐hour IV requirement given all as basal. |
| Nutritional: The remainder of the TDD is scheduled nutritional insulin in divided doses. In general, these doses need to be adjusted down for <100% nutritional intake and the orders should allow for administering nutritional insulin just AFTER observed meals to allow an assessment of intake. There are several options for estimating the initial doses: |
| Use 50% of the TDD as nutritional coverage and divide this amount by 3 to determine the scheduled meal dose. Hold if they do not eat more than 50% of their meal. |
| Use a more conservative start of 10% to 20% of the basal dose scheduled with each meal. |
| Use carbohydrate counting to cover nutritional intake. |
| Step 5: Be sure to give SC insulin BEFORE the infusion stops |
| Basal glargine or detemir are ideally given at least 2 hours before infusion is discontinued. |
| Shorter lead times (30 minutes) are possible if rapid acting insulin is given with basal insulin. |
SC insulin should be given before the drip is discontinued to allow an overlap that takes into consideration the onset of action. The first dose of basal insulin should be given 2 hours before the insulin infusion is discontinued.24, 40 However, because this is not always feasible, (ie, the patient needs to leave the ICU sooner), another option is to turn off the drip and give 10% of the basal dose as rapid acting insulin along with the basal dose.39 The timing of subsequent doses will depend on the specific basal insulin that is ordered as well as institutional consideration of usual care delivery and nursing workflow. Given that there are several options to achieve this important overlap between IV and SC insulin, it is best for a multidisciplinary team to choose some preferred way that is the institutional standard. Having a standard allows targeted education and tracking of adherence to best practices.
Because conversion to SC insulin is a complex task and the opportunity may arise while physicians are busy with other clinical priorities, there are several options to assure that the necessary steps take place. Some institutions may build a protocol for this transition on paper or computerized order entry, build cues and dosing charts into order sets, and/or develop nursing documentation and nursing process to influence physician and nurse behavior. This critical juncture is also a good place to focus expertise with a glycemic control team, pharmacist, specially trained nurses, or some other dedicated team to take over this transition for all patients.36 The complexity and aggressiveness of the specific institutional protocol used will depend on the confidence and experience of those individuals responsible for determining the transition doses.
The transition from IV to SC insulin often coincides with a change in patient location, (ie, from the ICU to general medical ward). It is imperative that appropriate communication occurs between the transferring and receiving nurses and physicians to continue with the care plan for glycemic management. This communication can be encouraged through provider education and automated into the standardized order process.
Perioperative Transitions
Patients undergoing surgery present a special challenge. They are faced with not only the physiologic and mental stress of surgery but also the hazards of multiple handoffs across several care teams, all with different priorities and cultures. As in other areas, standardized protocols specific to this area of transition are important in assuring safe and effective perioperative glycemic control. Procedures should preferably be scheduled for the early morning to have the least impact on insulin dosing. Patients who are admitted only for the procedure will have to manage this transition on their own and need to be given specific instructions along with the general preoperative orders.24, 41 In general, the usual dose of glargine can be given the day prior to the procedure if it is approximately 50% of their TDD. This is an important caution because some outpatient regimens use large doses of glargine, which essentially provide both basal and nutritional coverage. In those patients, the glargine dose should be reduced by 20% to 50% to provide a safety margin. As with any patient who is NPO, the morning dose of NPH should be one‐half of the usual dose, scheduled nutritional insulin should be held, and the usual doses of correction insulin should be reduced. The appropriate preoperative dose adjustments also depend on whether the individual patient is ketosis‐prone and how tight their glycemic control is as an outpatient.
Upon arrival to the hospital or during the time that the inpatient is NPO, dextrose containing IV fluids should be administered to minimize the risk of hypoglycemia and prevent ketosis. Given the risks for wide variation, blood glucose monitoring should occur every 1 to 2 hours before, during, and initially after the procedure. Infusion insulin allows the most rapid titration and reliable delivery (compared with SC infusions or injections) and is therefore the preferred regimen for major surgery requiring prolonged NPO status or prolonged surgery in patients with type 1 diabetes. Basal‐nutritional‐correction SC insulin is preferred in other surgical inpatients because their nutritional intake is variable and the stress of surgery affects insulin requirements.
Oral antihyperglycemic agents should be held around the time of surgery. If patients are on an oral agent that can result in hypoglycemia, (ie, sulfonylurea or other insulin secretagogue), it should be held on the day of the procedure. Metformin must be held for safety concerns, given the possible decrease in renal function around surgery. It should be held beginning on the day of the procedure or the day before in the case of the sustained‐release formulation. It can then be resumed 48 hours postoperation after normal renal function is secured and the patient is discharged home. Alpha‐glucosidase inhibitors should be held whenever patients are NPO because they only work when taken with meals. Thiazoladinediones have a long duration of action and so can be continued or stopped around surgery. Finally, glucagon‐like peptide (GLP‐1) agonists (exenatide) should be held until the patient is eating normally and discharged home due to the high incidence of gastrointestinal side effects.
TRANSITIONING FROM THE HOSPITAL
The final but perhaps most important transition is the one from the hospital. With much attention on glycemic control in the hospital, it will become clear to many clinicians that the outpatient regimen needs to be modified. However, any changes in medications increase the chances of hypoglycemia and the possibility of error. The postdischarge time frame has been poorly studied and was specifically identified by the Association for Clinical Endocrinologists (ACE) and American Diabetes Association (ADA) as an area in need of future research.36
Patients may be discharged to a nursing home, hospice, or home, and numerous factors need to be considered to determine the optimal discharge regimen. Important considerations are the HbA1c at admission, home medications, medication interactions, current medical problems, nutritional status, physical disabilities, frequency of self‐monitoring, hypoglycemic risk factors, contraindications to oral medications, goals of care/life expectancy, and financial and other resources. If there are temporary physical or self‐care limitations, then a visiting nurse may need to be arranged to assure a safe transition home with the optimal therapy. If patients are going to a skilled nursing facility or other acute care hospital, the formulary, processes, and staffing issues of that facility will be additional important considerations in determining whether therapy is the same as in the hospital or more like what it will be at home.
An algorithm for outpatient therapy for type 2 DM was recommended in a consensus statement from the ADA and European Association for the Study of Diabetes.42, 43 This has been modified using additional recommendations from the AACE44 and is depicted in Figure 1. While the delineation of these steps is helpful, it must be emphasized that both the choice of regimen and dose will need to be individualized. Prescribing the ideal frequently falls short if there is no way for the patient to implement the recommendations. Intensive insulin therapy requires training in food intake/emnsulin matching, motivation of the patient and outpatient clinician, 4 times daily self‐monitoring of blood glucose, and considerable expense. Some patients may be temporarily continued on basal‐nutritional‐correction regimens as their insulin requirements are rapidly changing and later converted to regimens that involve less frequent insulin doses, (ie, twice daily premixed insulin or basal insulin with oral agents or oral agents alone).45, 46 Other patients who may be medically appropriate for intensive insulin therapy may first need to gain confidence with more simple insulin regimens. There are numerous additional resources on initiating insulin that the reader is referred to for more detail.4448
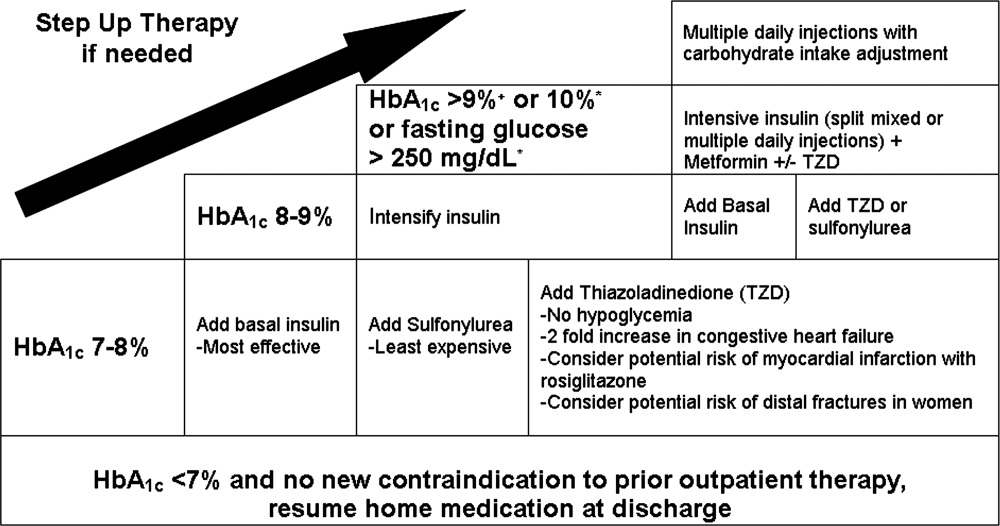
Oral antihyperglycemic drugs are usually held while a patient is admitted to the hospital but once medical conditions are improved, oral intake is established, and renal function stabilized, these drugs can be restarted. If a patient has a new contraindication to metformin or sulfonylureas but does not need insulin, a TZD or DPP4 inhibitor should be considered. Elderly patients and those with renal or liver disease are at increased risk for developing hypoglycemia.49, 50 Glyburide should be avoided, and doses of other sulfonylureas may need to be adjusted. Other options that may be considered in this situation include sitagliptin and exenatide.51 When patients will be discharged on oral diabetic medications alone, discontinue the basal insulin 12 to 24 hours before and the scheduled nutritional insulin at the same time oral agents are restarted. Sulfonylureas, metformin, DPP4 inhibitors, and exenatide will have most of their effect in the first day, but TZDs have a delayed onset and may not be a good bridge for immediate control at discharge.
If patients are going to be discharged on basal insulin in addition to oral agents, several options exist for determining the dose. Because of the risk of hypoglycemia after discharge, it is advised to either reduce the doses of oral agents or choose more conservative insulin starting doses.52 One possibility is to discontinue the nutritional and correction doses, continue the hospital dose of basal insulin, and restart the oral antidiabetes medications. If the dose of basal insulin was more than 50% of the TDD of insulin, it may need to be reduced. A more conservative option for patients at a higher risk of hypoglycemia is to start 0.2 units/kg or 10 units of NPH, glargine, or detemir at bedtime (Figure 2). Once discharged, blood glucose should be measured 1 to 4 times a day and the basal dose titrated by several different validated methods.53, 54 Appropriate orders for necessary supplies for insulin therapy include a meter with test strips, lancets, syringes, needles, and glucagon kit.55
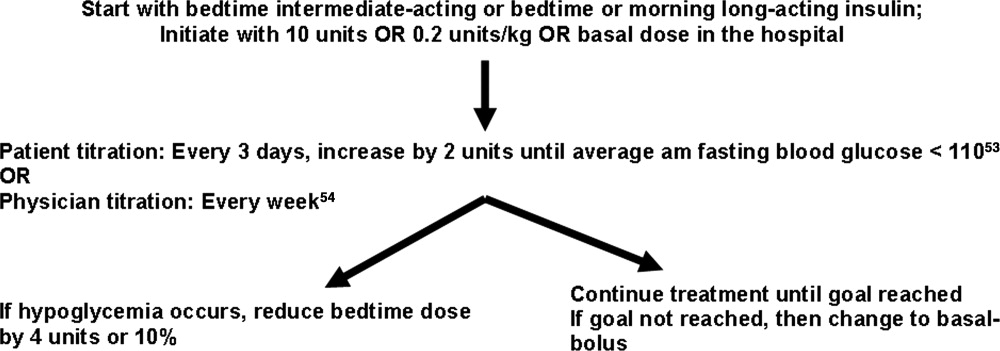
With a large number of patients with diabetes remaining undiagnosed, it is important to use the information available during hospitalization to identify previously unrecognized diabetes or prediabetes.24 Because there are no unique criteria for the diagnosis of DM in the stressed state, patients may have a presumptive diagnosis made in the hospital and/or follow‐up testing with fasting glucose or an oral glucose tolerance test. No ADA diagnostic thresholds for the HbA1c currently exist, but it can be a useful marker in making this distinction.56 Among patients with new hyperglycemia, an HbA1c of 6% or greater was 100% specific for predicting a future diagnosis of diabetes in the small prospective cohort study by Greci et al.,57 but many endocrinologists use a cutoff of 7%. For all hyperglycemic patients, lifestyle interventions that promote weight loss and increased activity levels should be encouraged. New hyperglycemia should be clearly identified as a diagnosis in discharge communication.
There are many barriers to diabetes self‐management education in the inpatient setting but there are also numerous resources and opportunities. New information will be available regarding patients' understanding of their disease and glycemic control and there may be plans for changes in the home medication regimen. Most of the focus of inpatient education sessions is on survival skills such as taking medications, performing blood glucose monitoring, basic meal planning, identification and treatment of hypoglycemia, sick‐day management, how to access further diabetes education as an outpatient, and when to call the healthcare team.58 The most effective way to accomplish all of this is to identify the discharge regimen early and include nurses and staff in a plan to educate all patients. An inpatient diabetes educator can provide additional help with newly‐diagnosed or uncontrolled patients. Dividing the material over the hospitalization makes it less overwhelming for patients, reinforces previously taught concepts, spreads the responsibility to more providers, and offers it in conjunction with the correlating clinical care. Throughout their hospital stay, patients can begin to practice new skills, including blood glucose monitoring and logbook use, drawing up and administering insulin, sharps disposal, basic diabetic diet information, and sick‐day management. The specific topics addressed in each session can be tracked as part of an interdisciplinary education record that allows coordination among the individuals involved in teaching.59 It is important to give patients the basics, support them with minimal written information, and provide them appropriate follow‐up diabetes education.60 Furthermore, the inpatient team should view the patient's glycemic control education as something that needs to continue across the continuum of care and develop communication strategies that connect with the follow‐up clinical team.
At the time of discharge, it is essential that written documentation and communication with outpatient care providers be completed.61, 62 The more standardized the inpatient insulin regimens are, the more likely the patient is to be on a much different glycemic control regimen than the one on admission; therefore, it is even more important to assure that the admission medication list is accurate and reconciled completely with the modified list at discharge. Discharge check lists and tools for assessing patient acceptance of the discharge plan help with this process.63 Follow‐up with the primary care physician should occur within 7 to 14 days if patients are new to insulin, had medication changes, or are elderly. An increased likelihood of keeping posthospitalization appointments with a diabetes specialty clinic has been associated with being discharged on insulin, a new diagnosis of diabetes, and direct referral.64 Additional attention should be paid to barriers to follow‐up, including lack of health insurance, prior difficulty with follow‐up, and transportation problems.65
SUMMARY
A variety of factors have contributed to difficulty in achieving inpatient and outpatient glucose control. These include care complexity, the lack of standardized protocols, limited knowledge about glucose control, and clinical inertia. Inpatient clinicians have a tendency toward keeping patients on their home regimen in hopes that they might test its effectiveness. Furthermore, there has been the notion of why optimize the glycemic regimen of inpatients because their diabetic needs will change in the outpatient setting. However, because the insulin requirements during acute illness are different and nutritional intake is variable, nearly all inpatients should be placed on multiple daily doses of scheduled insulin or IV insulin to allow the necessary flexibility for rapid titration and abrupt changes in nutrition. This intensive regimen is only appropriate for a minority of outpatients. This difference illustrates that a regimen that works perfectly in one clinical setting will not necessarily be optimal in the next. The patient's outpatient treatment regimen should be reassessed based on HbA1c, self‐monitoring prior to admission, and new contraindications based on medical issues. If a change is indicated and the inpatient physician is motivated, there are numerous helpful resources to aid in addressing all the necessary factors surrounding intensification of therapy.
Despite requiring different glycemic control regimens, the information gained from the needs in each setting guide the next, making communication and planning paramount. Important transitions that must be given attention are: (1) admission to the hospital; (2) in‐hospital transitions, including the perioperative period and IV‐to‐SC insulin; and (3) the hospital to outpatient transition. The complexity of such frequent transitions requires planning, education, and clear communication that are best handled with a systems approach and the development of standardized protocols and order sets. Hospitalists, endocrinologists, and other members of the healthcare team should take an aggressive role in developing systems and facilitating optimal transitions to maximize glycemic control. Further studies are needed to determine the best practices among the variety of options discussed in this article.
- ACE/ADA Task Force on Inpatient Diabetes.American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control.Endocr Pract.2006;12:458–468.
- American Board of Internal Medicine Foundation Stepping Up to the Plate Alliance. Available at: http://www.abimfoundation.org/quality/suttp.shtm. Accessed November2007.
- National Transitions of Care Coalition. Available at: http://www.ntocc.org. Accessed November2007.
- JCAHO 2008 National Patient Safety Goals. Availableat: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/08_hap_npsgs.htm. Accessed November2007.
- Diabetes Control and Complications Trial Research Group.The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus.N Engl J Med.1993;329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group.Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes: UK Prospective Diabetes Study (UKPDS) Group.Lancet.1998;352:837–853.
- ,,, et al.,Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,,.Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long‐term results from the Diabetes and Insulin‐Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study.Circulation.1999;99:2626–2632.
- ,,,,.Relationship of early hyperglycemia to mortality in trauma patients.J Trauma.2004;56:1058–1062.
- ,,,,.Early peri‐operative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321–1324.
- ,,, et al.Relation between the duration of remission and hyperglycemia in induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone/methotrexate cytarabine regimen.Cancer.2004;100:1179–1185.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,,,.Diabetes and outcome of community‐acquired pneumococcal bacteriemia.Diabetes Care.2004;27:70–76.
- ,,.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.JPEN J Parenter Enteral Nutr.1998;22:77–81.
- ,,,,.Early post‐operative glucose levels are an independent risk factor for infection after peripheral vascular surgery. A retrospective study.Eur J Vasc Endovasc Surg.2004;28:520–525.
- American Diabetes Association.Standards of medical care in diabetes, 2006.Diabetes Care.2006;29(suppl 1):s4–s42.
- American College of Endocrinology Task Force on Inpatient Diabetes and Metabolic Control.American College of Endocrinology Position Statement on Inpatient Diabetes and Metabolic Control.Endocr Pract.2004;10:77–82.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461
- ,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis.N Engl J Med.2008;358(2):125–139.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.,Diabetes care in hospitalized noncritically ill patients: More evidence for clinical inertia and negative therapeutic momentum.J Hosp Med.2007;2:203–211.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,.Hospital management of hyperglycemia.Clin Diabetes.2004;22:81–88.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals. [Erratum appears in Diabetes Care. 2005; 28: 1990. Dosage error in text].Diabetes Care.2004;27:553–591.
- ,.Hemoglobinopathies and HbA(1c) measurement.Diabetes Care.2000;23(8):1197–1198.
- ,,,,,.Evaluation of HbA1c determination methods in patients with hemoglobinopathiesDiabetes Care.2000;23(3):339–344.
- ,,,.Subcutaneous insulin order sets and protocols: effective design and implementation strategies.J Hosp Med.2008;3.
- ,,,,.Management of diabetes and hyperglycemia in the hospital: a practical guide to subcutaneous insulin use in the non‐critically ill, adult patient.J Hosp Med.2008;3.PMID:8675920.
- ,,,.Switch to multiple daily injections with insulin glargine and insulin lispro from continuous subcutaneous insulin infusion with insulin lispro: a randomized, open‐label study using a continuous glucose monitoring system.Endocr Pract.2005;11:157–164.
- SBAR technique for communication: a situational briefing model. Available at: http://www.ihi.org/IHI/Topics/PatientSafety/SafetyGeneral/Tools/SBARTechniqueforCommunicationASituationalBriefingModel.htm. Accessed December2007.
- . Promising quality improvement initiatives: reports from the field. AHRQ Summit—Improving Health Care Quality for All Americans: Celebrating Success, Measuring Progress, Moving Forward 2004. Available at: http://www.ahrq.gov/qual/qsummit/qsummit4.htm#sentara. Accessed December2007.
- ,,, et al.Hospital hypoglycemia: not only treatment but also prevention.Endocr Pract.2004;10(suppl 2):89–99.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Available at: http://resources.aace.com/PDF/Section_05‐Final‐Inpatient_Non‐ICU/Hyperglycemia_Non‐ICU_Protocols/Transition_from_ Intravenous_to_Subcutaneous_Insulin.PDF. Accessed November2007.
- Recommendations for safe use of insulin in hospitals. American Society of Health System Pharmacists and the Hospital and Health System Association of Pennsylvania. 2005. Available at: http://www.premierinc.com/safety/safety‐share/01–06‐downloads/01‐safe‐use‐insulin‐ashp.pdf. Accessed December2007.
- ,,, for the Society of Hospital Medicine Glycemic Control Taskforce. Glycemic control resource room: improving reliability of care across transitions and in the perioperative setting. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/html/07Layer_Inter/06_Transitions.cfm. Accessed August2008.
- ACE/ADA Task Force on Inpatient Diabetes American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control: a call to action.Diabetes Care.2006;29:1955–1962.
- ,,, et al.Conversion of intravenous insulin infusions to subcutaneously administered insulin glargine in patients with hyperglycemia.Endocr Pract.2006;12:641–650.
- ,,,.Intravenous insulin infusion therapy: indications, methods, and transition to subcutaneous insulin therapy.Endocr Pract.2004;10(suppl 2):71–80.
- ,,, et al.Inpatient management of hyperglycemia: the northwestern experience.Endocr Pract.2006;12(5):491–505.
- American Diabetes Association.Position statement: standards of medical care in diabetes‐2007.Diabetes Care.2007;30(suppl 1):S4–S41.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Available at: http://resources.aace.com/PDF/Section_05‐Final‐Inpatient_Non‐ICU/Hyperglycemia_Non‐ICU_Protocols/Pre‐Operative_Instructions_for_Patients_with_Diabetes.PDF Accessed November2007.
- ,,, et al.Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes.Diabetes Care.2006;29:1963–1972.
- ,,, et al.Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: update regarding thiazoladinediones.Diabetes Care.2008;31:173–175.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Challenges in Effective Discharge Planning for Hospitalized Patients with Diabetes. Available at: http://resources.aace.com/PDF/Section_07‐Final‐Transition‐Inpatient_to_Outpatient/Challenges_in_Effective_Discharge_for_Diabetes_Patients.PPT. Accessed December2007.
- ,,.Initiating insulin therapy in type 2 diabetes: a comparison of biphasic and basal insulin analogs.Diabetes Care.2005;28:260–265.
- ,,, et al.Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes.N Engl J Med.2007;357:1716–1730.
- ,,.Narrative review: a rational approach to starting insulin therapy.Ann Intern Med.2006;145:125–134.
- ,,,,.A real‐world approach to insulin therapy in primary care practice.Clin Diabetes.2005;23:78–86.
- ,,,.Individual sulfonylureas and serious hypoglycemia in older persons.J Am Geriatr Soc.1996;44:751–755.
- ,,,.Incidence and risk factors for serious hypoglycemia in older persons using insulin or sulfonylureas.Arch Intern Med.1997;157(15):1681–1686.
- ,,, et al.Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial.Ann Intern Med.2005;143:559–569.
- .The transition from insulin infusions to long‐term diabetes therapy: the argument for insulin analogs.Semin Thorac Cardiovasc Surg.2006;18:366–378.
- ,,,,.ATLANTUS Study Group. Improvement of glycemic control in subjects with poorly controlled type 2 diabetes.Diabetes Care.2005;28:1282–1288.
- ,,.Investigators Insulin Glargine 4002 Study. The Treat‐to Target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetes patients.Diabetes Care.2003;26:3080–3086.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Available at: http://resources.aace.com/PDF/Section_07‐Final‐Transition‐Inpatient_to_Outpatient/Effective_Discharge_Planning‐Sample_Discharge_Plans/Inpatient_Diabetes_Discharge_Prescription.PDF. Accessed November2007.
- American Diabetes Association.Diagnosis and classification of diabetes mellitus.Diabetes Care.2007;30(suppl):S42–S47.
- ,,, et al.Utility of HbA1c levels for diabetes case finding in hospitalized patients with hyperglycemia.Diabetes Care.2003;26:1064–1068.
- ,,, et al.National standards for diabetes self‐management education.Diabetes Care.2006;29(suppl 1):S78–S85.
- Society of Hospital Medicine Glycemic Control Task Force. Workbook for improvement: improving glycemic control, preventing hypoglycemia and optimizing care of the inpatient with diabetes and hyperglycemia. page 105. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/pdf/GC_Workbook.pdf. Accessed December,2007.
- Joslin Diabetes Center. EZ Start Patient Information Handouts. Available at: http://www.joslin.org/ezstart. Accessed December2007.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2:314–323.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- Society of Hospital Medicine On‐line Clinical Tools. Ideal discharge for the elderly patient: a hospitalist checklist. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=QI_Clinical_Toolsemplate=/CM/ContentDisplay.cfmContentID=10303. Accessed December2007.
- ,,, et al.Inpatient to outpatient transfer of care in urban patients with diabetes: patterns and determinants of immediate post‐discharge follow‐up.Arch Intern Med.2004;164:447–453.
- ,,,,,.Inpatient to outpatient transfer of diabetes care: perceptions of barriers to postdischarge follow‐up in urban African American patients.Ethn Dis.2007;17:238–243.
Professional and patient safety organizations have recognized the importance of safe transitions as patients move through the health care system, and such attention is even more critical when attempting to achieve glycemic control.14 Since the publication of the Diabetes Control and Complications Trial (DCCT)5 and the United Kingdom Prospective Diabetes Study (UKPDS),6 we have known that intensive glycemic control in the ambulatory setting prevents complications in both type 1 and type 2 diabetes mellitus (DM). Despite the increased risk of hypoglycemia, these trials changed practice patterns in the outpatient settings in favor of intensification of diabetes therapy. In the same way, randomized, prospective trials using intravenous (IV) insulin therapy have revolutionized our thinking about inpatient care by showing that tight glycemic control in the critically ill7 and patients with acute myocardial infarction8 reduces mortality and morbidity. These, as well as additional observational studies associating hyperglycemia with poor outcomes in a variety of medical and surgical patients,915 have led to increased attention on glycemic control in all venues of care.16, 17 Concerns over excessive hypoglycemia and a nonsignificant increase in mortality in certain populations of medical intensive care unit (ICU) patients have raised questions over whether the initial studies can be reproduced or generalized to other groups of inpatients.18, 19 Additional studies are underway to clarify these questions but consensus exists that blood glucose values should at least be less than 180 mg/dL and that the traditional practice of ignoring hyperglycemia is no longer acceptable.
While a uniform focus on glycemic control will allow our patients to receive a consistent message about diabetes, the unique limitations inherent to each practice setting requires different therapeutic regimens and intentional focus on the risks as patients transition from one care area to another. This work addresses several areas of care transition that are particularly important in safely achieving glycemic control including: transition into the hospital for patients on a variety of home regimens, transitions within the hospital (related to changes in dietary intake, change from IV to subcutaneous [SC] therapy, and the perioperative setting), and the transition from the hospital to home or another healthcare facility.
TRANSITION INTO THE HOSPITAL
Until recently, most patients with diabetes admitted to the hospital were managed with sliding‐scale‐only regimens.20, 21 Unfortunately, this led to a variety of complications, including hyperglycemia, hypoglycemia, iatrogenic ketoacidosis, and an inconsistent message to patients on the importance of glycemic control.22 Some outpatient clinicians and patients combated this tradition by creating in‐hospital glucose control plans with orders, which patients would bring with them to the hospital.23 This practice continues to be a helpful way to guide inpatient therapy and is encouraged when available. Glycemic‐controlrelated documents from outpatient clinicians should include the most recent glycosylated hemoglobin (HbA1c) value, diagnosis and known complications, current names and doses of medications, and other patient‐specific preferences or needs (eg, compliance, financial, fear of needles). If the last HbA1c was performed more than 30 days before admission or is not available, one should be obtained upon hospital admission to help guide discharge therapy.24 By knowing the HbA1c, one can determine the level of diabetic control achieved with the current regimen and can help the inpatient team (clinician and patient) determine if a more aggressive glycemic control regimen is necessary at the time of discharge. It is important to note that if the patient has received a transfusion of red blood cells prior to HbA1c measurement or has a hemoglobinopathy, the HbA1c value may not be accurate.25, 26
In general, the outpatient regimen will need to be modified at admission to achieve the appropriate flexibility needed for the changing nutritional intake and insulin requirements that invariably accompany hospitalization. Sulfonylureas and dipeptidyl peptidase 4 inhibitors (DPP4), such as sitagliptin, have most of their effect immediately, but the other oral antihyperglycemic agents have a relatively long delay between treatment and effect, thus they are not a flexible enough method to achieve glycemic control in the hospital. Additionally, inpatients may have transient contraindications to their prior oral antihyperglycemic medications. Metformin is almost always on hold in the hospital setting, at least initially, due to concerns about lactic acidosis. Sulfonylureas can cause hypoglycemia in the setting of worsening renal function or reduced oral intake. Thiazoladinediones (TZDs) are often withheld due to concerns about fluid retention and should be avoided in patients admitted with heart failure. There is little experience in the hospital with the use of newer agents like exenatide, pramlintide, glinides, and DPP4 inhibitors.
Overall, it is generally recommended that oral antihyperglycemic agents be discontinued upon hospital admission and replaced with insulin infusions or scheduled SC insulin. An estimate of 0.4 to 0.5 units/kg of body weight provides a conservative starting point for the total daily dose of insulin (TDD) for most patients. This TDD should then be divided into basal and nutritional components to match the patients' caloric intake. Additional correction doses of insulin should be prescribed to cover episodes of hyperglycemia that develop despite the provision of anticipatory‐physiologic insulin. Further discussion of insulin dosing and SC regimens is available in detail elsewhere.27, 28 The recommendation for these insulin‐only regimens is made regardless of the glycemic control in the outpatient setting and is not meant to imply that they should be continued at discharge. In fact, most patients will return to their home regimen or to one that is intensified but less labor intensive than the basal‐nutritional‐correction insulin used in the hospital. The antihyperglycemic regimen planned for discharge should be anticipated as early as possible and clearly communicated to the patient and/or caregivers to allow for optimal education.
Outpatient insulin regimens that have a high percentage of basal insulin need to be modified during hospital admission to avoid hypoglycemia that may occur from variable nutritional intake. While hospitalized, the basal portion of the estimated TDD generally should not be more than 50% to 60%. The total number of units of all types of insulin used daily as an outpatient can be used as a starting point for determining the inpatient TDD by a 1:1 conversion. Adjustments up or down based on glycemic control, nutritional intake, and other factors are then necessary. If patients are on regimens with insulin plus oral agents at home, the inpatient TDD should either be the home insulin dose or the dose calculated based on their weight, whichever is greater. Patients who use carbohydrate counting to determine nutritional insulin doses as an outpatient might be continued on this regimen if they have a strong understanding of the methods, they are coherent enough to determine their doses, nursing staff are well educated, and dietary services provides the carbohydrate content for the hospital menu. If patients are on insulin pumps at home, these should be managed according to a uniform hospital policy to assure safety. If conversion to multiple daily injections is needed, the same 1:1 conversion is safe.29
Transitions Within the Hospital
General Issues
Within the hospital itself, there are several transitions that have important quality and safety implications regarding glycemic control. The handoffs between providers should follow a standardized format.4, 30, 31 Essential information will vary depending on the setting but should universally include recent hypoglycemia, insulin type and doses, and hypoglycemic risk factors such as changes in insulin doses, the development of renal insufficiency, inability of the patient to self‐report symptoms, tapering of steroids, and cessation or interruption of nutritional intake.32
One of the greatest risks for hypoglycemia in the hospital comes from the unpredictable nutritional interruptions that occur. Unplanned changes are best handled by nurses having an existing order to hold scheduled nutritional insulin if patients are classified nothing by mouth (NPO) or eat <50% of their meal. Additionally, nursing staff should have orders or policies that allow flexibility in the time of administering scheduled rapid‐acting nutritional insulin so that it may be given during or immediately following the meal in patients at higher risk for poor oral intake. Tube feedings also place patients at high risk for hypoglycemia because the tube may become dislodged or they may begin to have feeding intolerance. For these reasons, a measure of safety would be to have standing orders to substitute IV 10% dextrose in water (D10W) at the same rate as the prior tube feeds, hold nutritional insulin, and begin more frequent monitoring whenever tube feeds are stopped.33 Orders that rely on nursing staff to notify a physician when tube feedings are stopped are generally not directive enough because providers may be distracted by other changes or forget the patient is on long‐acting insulin. The need for this flexibility around nutritional dosing emphasizes the importance of avoiding excessive doses of basal insulin. If the total dose of basal insulin is 40% to 50% of the TDD, it can safely be continued at its usual dose despite changing nutritional intake. The only exception is neutral protamine Hagedorn (NPH) insulin, which should be reduced when patients are NPO due to its peak. Generally, a 50% reduction in NPH is recommended for morning doses, but bedtime doses may be given with little to no reduction. Because of the complexity of these issues, standardized order sets are the best way to reliably communicate all the necessary standing orders to nursing staff (Table 1).
|
| Nutritional insulin |
| Hold if patients are NPO or eat less than 50% of their meal. |
| Administer scheduled rapid acting nutritional insulin during or immediately following the meal if oral intake is questionable (ie, nausea, emesis, or newly advancing diet). |
| Tube feedings: When tube feeds are stopped unexpectedly |
| Start dextrose containing IV fluids (many institutions use D10W at the same rate as the prior tube feeds). |
| Hold scheduled nutritional insulin. |
| Notify physician. |
| Basal insulin |
| Continue if NPO. |
| Reduce morning dose of NPH by 50% if NPO and may need to reduce the dose of bedtime NPH. |
| IV to subcutaneous transition |
| Timing for discontinuing IV infusion in relation to first dose of subcutaneous insulin. |
| Prompts for verbal communication between ICU and general ward staff. |
Transitioning the Patient Off of IV Insulin
The strongest evidence for tight glycemic control derives from studies in the surgical ICU.7 Many hospitals have robust, effective IV‐insulin protocols. The frequency of monitoring and rapidity of action of IV insulin allow quick achievement of blood glucose control. As patients begin to eat, the layering of SC nutritional insulin on top of the insulin infusion may reduce the lability of the infusion rate and prevent excursions in glycemic control. When the patient is ready to leave the ICU or start a full oral diet, it is recommended that they transition off of the IV insulin to a basal‐nutritional‐correction regimen.33, 34
The amount of insulin needed with IV infusion is a useful estimate of the TDD of insulin.28, 33, 35, 36 There are important general steps to take when making this transition; but, due to the lack of conclusive data proving the advantage of one regimen over another, there are a variety of acceptable specific protocols (Table 2).3739 First, it should be determined if patients are expected to require ongoing scheduled SC insulin or not. Certainly, all patients with type 1 DM will require scheduled SC insulin, but patients with type 2 DM on low insulin infusion rates or some patients with new hyperglycemia can appropriately be managed with sliding‐scale alone. Next, the average hourly rate of the infusion over the preceding 6 to 8 hours should be determined because it most accurately reflects current insulin needs during the changing stress, nutrition, and medications in critical care patients. This hourly rate will then be converted to a TDD using a safety factor to anticipate decreasing insulin requirements. Some portion of this daily total will then be assigned to be basal insulin. As patients' clinical conditions approach baseline, so will their insulin requirements, and the dose will need to be revised.24
|
| Step 1: Is patient stable enough for transition? Hypotension, active sepsis, vasopressors, and intubation are contraindications to transition due to unreliable subcutaneous insulin absorption and continued need for the most flexible dosing due to frequently changing insulin requirements. |
| Step 2: Does this patient need a transition to scheduled subcutaneous (SC) insulin? |
| Yes |
| All patients with type 1 DM. |
| Type 2 DM patients on insulin as outpatient. |
| Type 2 DM patients with a recent mean infusion rate of 0.5 units/hour.* |
| No |
| Type 2 DM patients with infusion rate <0.5 units/hour.* |
| Stress hyperglycemia or previously unrecognized DM if infusion rate <1 unit/hour, or if HbA1c near normal. |
| Some institutions exclude all stress hyperglycemia patients from transition to a SC insulin regimen, regardless of drip rate. |
| Step 3: If transition is needed, calculate a total daily dose (TDD) of insulin. The TDD is an estimate of the 24‐hour insulin requirement when the patient is receiving full nutrition. |
| Determine mean insulin infusion rate from last 6 to 8 hours. |
| Calculate 24‐hour insulin dose based on this, and reduce this 24‐hour dose by some safety factor. There are several options for this step. |
| Multiply hourly rate by 24, then multiply by 0.7 or 0.8 to arrive at a safety‐adjusted 24 hour insulin dose. |
| OR |
| Multiply hourly infusion rate by 20 (80% of 24). |
| Determine if this total is the TDD or basal dose based on current nutrition. There are several options for this step for you or your institution to choose. |
| If infusion was serving basal AND nutritional needs of patient (such as a patient on 24‐hour tube feedings) this will be your TDD. |
| OR |
| If the infusion insulin was not covering significant nutrition, this could be the BASAL insulin dose. |
| Step 4: Construct a regimen tailored to the patient's nutritional situation, building in safeguards for any changes in nutritional intake and uncertainties about reliability of intake. Several options are again available. |
| Basal: should be ordered as basal glargine or detemir (these are preferred by SHM GCTF but NPH is also an option). |
| Dose is 40% to 50% of TDD. |
| OR |
| Adjusted 24‐hour IV requirement given all as basal. |
| Nutritional: The remainder of the TDD is scheduled nutritional insulin in divided doses. In general, these doses need to be adjusted down for <100% nutritional intake and the orders should allow for administering nutritional insulin just AFTER observed meals to allow an assessment of intake. There are several options for estimating the initial doses: |
| Use 50% of the TDD as nutritional coverage and divide this amount by 3 to determine the scheduled meal dose. Hold if they do not eat more than 50% of their meal. |
| Use a more conservative start of 10% to 20% of the basal dose scheduled with each meal. |
| Use carbohydrate counting to cover nutritional intake. |
| Step 5: Be sure to give SC insulin BEFORE the infusion stops |
| Basal glargine or detemir are ideally given at least 2 hours before infusion is discontinued. |
| Shorter lead times (30 minutes) are possible if rapid acting insulin is given with basal insulin. |
SC insulin should be given before the drip is discontinued to allow an overlap that takes into consideration the onset of action. The first dose of basal insulin should be given 2 hours before the insulin infusion is discontinued.24, 40 However, because this is not always feasible, (ie, the patient needs to leave the ICU sooner), another option is to turn off the drip and give 10% of the basal dose as rapid acting insulin along with the basal dose.39 The timing of subsequent doses will depend on the specific basal insulin that is ordered as well as institutional consideration of usual care delivery and nursing workflow. Given that there are several options to achieve this important overlap between IV and SC insulin, it is best for a multidisciplinary team to choose some preferred way that is the institutional standard. Having a standard allows targeted education and tracking of adherence to best practices.
Because conversion to SC insulin is a complex task and the opportunity may arise while physicians are busy with other clinical priorities, there are several options to assure that the necessary steps take place. Some institutions may build a protocol for this transition on paper or computerized order entry, build cues and dosing charts into order sets, and/or develop nursing documentation and nursing process to influence physician and nurse behavior. This critical juncture is also a good place to focus expertise with a glycemic control team, pharmacist, specially trained nurses, or some other dedicated team to take over this transition for all patients.36 The complexity and aggressiveness of the specific institutional protocol used will depend on the confidence and experience of those individuals responsible for determining the transition doses.
The transition from IV to SC insulin often coincides with a change in patient location, (ie, from the ICU to general medical ward). It is imperative that appropriate communication occurs between the transferring and receiving nurses and physicians to continue with the care plan for glycemic management. This communication can be encouraged through provider education and automated into the standardized order process.
Perioperative Transitions
Patients undergoing surgery present a special challenge. They are faced with not only the physiologic and mental stress of surgery but also the hazards of multiple handoffs across several care teams, all with different priorities and cultures. As in other areas, standardized protocols specific to this area of transition are important in assuring safe and effective perioperative glycemic control. Procedures should preferably be scheduled for the early morning to have the least impact on insulin dosing. Patients who are admitted only for the procedure will have to manage this transition on their own and need to be given specific instructions along with the general preoperative orders.24, 41 In general, the usual dose of glargine can be given the day prior to the procedure if it is approximately 50% of their TDD. This is an important caution because some outpatient regimens use large doses of glargine, which essentially provide both basal and nutritional coverage. In those patients, the glargine dose should be reduced by 20% to 50% to provide a safety margin. As with any patient who is NPO, the morning dose of NPH should be one‐half of the usual dose, scheduled nutritional insulin should be held, and the usual doses of correction insulin should be reduced. The appropriate preoperative dose adjustments also depend on whether the individual patient is ketosis‐prone and how tight their glycemic control is as an outpatient.
Upon arrival to the hospital or during the time that the inpatient is NPO, dextrose containing IV fluids should be administered to minimize the risk of hypoglycemia and prevent ketosis. Given the risks for wide variation, blood glucose monitoring should occur every 1 to 2 hours before, during, and initially after the procedure. Infusion insulin allows the most rapid titration and reliable delivery (compared with SC infusions or injections) and is therefore the preferred regimen for major surgery requiring prolonged NPO status or prolonged surgery in patients with type 1 diabetes. Basal‐nutritional‐correction SC insulin is preferred in other surgical inpatients because their nutritional intake is variable and the stress of surgery affects insulin requirements.
Oral antihyperglycemic agents should be held around the time of surgery. If patients are on an oral agent that can result in hypoglycemia, (ie, sulfonylurea or other insulin secretagogue), it should be held on the day of the procedure. Metformin must be held for safety concerns, given the possible decrease in renal function around surgery. It should be held beginning on the day of the procedure or the day before in the case of the sustained‐release formulation. It can then be resumed 48 hours postoperation after normal renal function is secured and the patient is discharged home. Alpha‐glucosidase inhibitors should be held whenever patients are NPO because they only work when taken with meals. Thiazoladinediones have a long duration of action and so can be continued or stopped around surgery. Finally, glucagon‐like peptide (GLP‐1) agonists (exenatide) should be held until the patient is eating normally and discharged home due to the high incidence of gastrointestinal side effects.
TRANSITIONING FROM THE HOSPITAL
The final but perhaps most important transition is the one from the hospital. With much attention on glycemic control in the hospital, it will become clear to many clinicians that the outpatient regimen needs to be modified. However, any changes in medications increase the chances of hypoglycemia and the possibility of error. The postdischarge time frame has been poorly studied and was specifically identified by the Association for Clinical Endocrinologists (ACE) and American Diabetes Association (ADA) as an area in need of future research.36
Patients may be discharged to a nursing home, hospice, or home, and numerous factors need to be considered to determine the optimal discharge regimen. Important considerations are the HbA1c at admission, home medications, medication interactions, current medical problems, nutritional status, physical disabilities, frequency of self‐monitoring, hypoglycemic risk factors, contraindications to oral medications, goals of care/life expectancy, and financial and other resources. If there are temporary physical or self‐care limitations, then a visiting nurse may need to be arranged to assure a safe transition home with the optimal therapy. If patients are going to a skilled nursing facility or other acute care hospital, the formulary, processes, and staffing issues of that facility will be additional important considerations in determining whether therapy is the same as in the hospital or more like what it will be at home.
An algorithm for outpatient therapy for type 2 DM was recommended in a consensus statement from the ADA and European Association for the Study of Diabetes.42, 43 This has been modified using additional recommendations from the AACE44 and is depicted in Figure 1. While the delineation of these steps is helpful, it must be emphasized that both the choice of regimen and dose will need to be individualized. Prescribing the ideal frequently falls short if there is no way for the patient to implement the recommendations. Intensive insulin therapy requires training in food intake/emnsulin matching, motivation of the patient and outpatient clinician, 4 times daily self‐monitoring of blood glucose, and considerable expense. Some patients may be temporarily continued on basal‐nutritional‐correction regimens as their insulin requirements are rapidly changing and later converted to regimens that involve less frequent insulin doses, (ie, twice daily premixed insulin or basal insulin with oral agents or oral agents alone).45, 46 Other patients who may be medically appropriate for intensive insulin therapy may first need to gain confidence with more simple insulin regimens. There are numerous additional resources on initiating insulin that the reader is referred to for more detail.4448

Oral antihyperglycemic drugs are usually held while a patient is admitted to the hospital but once medical conditions are improved, oral intake is established, and renal function stabilized, these drugs can be restarted. If a patient has a new contraindication to metformin or sulfonylureas but does not need insulin, a TZD or DPP4 inhibitor should be considered. Elderly patients and those with renal or liver disease are at increased risk for developing hypoglycemia.49, 50 Glyburide should be avoided, and doses of other sulfonylureas may need to be adjusted. Other options that may be considered in this situation include sitagliptin and exenatide.51 When patients will be discharged on oral diabetic medications alone, discontinue the basal insulin 12 to 24 hours before and the scheduled nutritional insulin at the same time oral agents are restarted. Sulfonylureas, metformin, DPP4 inhibitors, and exenatide will have most of their effect in the first day, but TZDs have a delayed onset and may not be a good bridge for immediate control at discharge.
If patients are going to be discharged on basal insulin in addition to oral agents, several options exist for determining the dose. Because of the risk of hypoglycemia after discharge, it is advised to either reduce the doses of oral agents or choose more conservative insulin starting doses.52 One possibility is to discontinue the nutritional and correction doses, continue the hospital dose of basal insulin, and restart the oral antidiabetes medications. If the dose of basal insulin was more than 50% of the TDD of insulin, it may need to be reduced. A more conservative option for patients at a higher risk of hypoglycemia is to start 0.2 units/kg or 10 units of NPH, glargine, or detemir at bedtime (Figure 2). Once discharged, blood glucose should be measured 1 to 4 times a day and the basal dose titrated by several different validated methods.53, 54 Appropriate orders for necessary supplies for insulin therapy include a meter with test strips, lancets, syringes, needles, and glucagon kit.55

With a large number of patients with diabetes remaining undiagnosed, it is important to use the information available during hospitalization to identify previously unrecognized diabetes or prediabetes.24 Because there are no unique criteria for the diagnosis of DM in the stressed state, patients may have a presumptive diagnosis made in the hospital and/or follow‐up testing with fasting glucose or an oral glucose tolerance test. No ADA diagnostic thresholds for the HbA1c currently exist, but it can be a useful marker in making this distinction.56 Among patients with new hyperglycemia, an HbA1c of 6% or greater was 100% specific for predicting a future diagnosis of diabetes in the small prospective cohort study by Greci et al.,57 but many endocrinologists use a cutoff of 7%. For all hyperglycemic patients, lifestyle interventions that promote weight loss and increased activity levels should be encouraged. New hyperglycemia should be clearly identified as a diagnosis in discharge communication.
There are many barriers to diabetes self‐management education in the inpatient setting but there are also numerous resources and opportunities. New information will be available regarding patients' understanding of their disease and glycemic control and there may be plans for changes in the home medication regimen. Most of the focus of inpatient education sessions is on survival skills such as taking medications, performing blood glucose monitoring, basic meal planning, identification and treatment of hypoglycemia, sick‐day management, how to access further diabetes education as an outpatient, and when to call the healthcare team.58 The most effective way to accomplish all of this is to identify the discharge regimen early and include nurses and staff in a plan to educate all patients. An inpatient diabetes educator can provide additional help with newly‐diagnosed or uncontrolled patients. Dividing the material over the hospitalization makes it less overwhelming for patients, reinforces previously taught concepts, spreads the responsibility to more providers, and offers it in conjunction with the correlating clinical care. Throughout their hospital stay, patients can begin to practice new skills, including blood glucose monitoring and logbook use, drawing up and administering insulin, sharps disposal, basic diabetic diet information, and sick‐day management. The specific topics addressed in each session can be tracked as part of an interdisciplinary education record that allows coordination among the individuals involved in teaching.59 It is important to give patients the basics, support them with minimal written information, and provide them appropriate follow‐up diabetes education.60 Furthermore, the inpatient team should view the patient's glycemic control education as something that needs to continue across the continuum of care and develop communication strategies that connect with the follow‐up clinical team.
At the time of discharge, it is essential that written documentation and communication with outpatient care providers be completed.61, 62 The more standardized the inpatient insulin regimens are, the more likely the patient is to be on a much different glycemic control regimen than the one on admission; therefore, it is even more important to assure that the admission medication list is accurate and reconciled completely with the modified list at discharge. Discharge check lists and tools for assessing patient acceptance of the discharge plan help with this process.63 Follow‐up with the primary care physician should occur within 7 to 14 days if patients are new to insulin, had medication changes, or are elderly. An increased likelihood of keeping posthospitalization appointments with a diabetes specialty clinic has been associated with being discharged on insulin, a new diagnosis of diabetes, and direct referral.64 Additional attention should be paid to barriers to follow‐up, including lack of health insurance, prior difficulty with follow‐up, and transportation problems.65
SUMMARY
A variety of factors have contributed to difficulty in achieving inpatient and outpatient glucose control. These include care complexity, the lack of standardized protocols, limited knowledge about glucose control, and clinical inertia. Inpatient clinicians have a tendency toward keeping patients on their home regimen in hopes that they might test its effectiveness. Furthermore, there has been the notion of why optimize the glycemic regimen of inpatients because their diabetic needs will change in the outpatient setting. However, because the insulin requirements during acute illness are different and nutritional intake is variable, nearly all inpatients should be placed on multiple daily doses of scheduled insulin or IV insulin to allow the necessary flexibility for rapid titration and abrupt changes in nutrition. This intensive regimen is only appropriate for a minority of outpatients. This difference illustrates that a regimen that works perfectly in one clinical setting will not necessarily be optimal in the next. The patient's outpatient treatment regimen should be reassessed based on HbA1c, self‐monitoring prior to admission, and new contraindications based on medical issues. If a change is indicated and the inpatient physician is motivated, there are numerous helpful resources to aid in addressing all the necessary factors surrounding intensification of therapy.
Despite requiring different glycemic control regimens, the information gained from the needs in each setting guide the next, making communication and planning paramount. Important transitions that must be given attention are: (1) admission to the hospital; (2) in‐hospital transitions, including the perioperative period and IV‐to‐SC insulin; and (3) the hospital to outpatient transition. The complexity of such frequent transitions requires planning, education, and clear communication that are best handled with a systems approach and the development of standardized protocols and order sets. Hospitalists, endocrinologists, and other members of the healthcare team should take an aggressive role in developing systems and facilitating optimal transitions to maximize glycemic control. Further studies are needed to determine the best practices among the variety of options discussed in this article.
Professional and patient safety organizations have recognized the importance of safe transitions as patients move through the health care system, and such attention is even more critical when attempting to achieve glycemic control.14 Since the publication of the Diabetes Control and Complications Trial (DCCT)5 and the United Kingdom Prospective Diabetes Study (UKPDS),6 we have known that intensive glycemic control in the ambulatory setting prevents complications in both type 1 and type 2 diabetes mellitus (DM). Despite the increased risk of hypoglycemia, these trials changed practice patterns in the outpatient settings in favor of intensification of diabetes therapy. In the same way, randomized, prospective trials using intravenous (IV) insulin therapy have revolutionized our thinking about inpatient care by showing that tight glycemic control in the critically ill7 and patients with acute myocardial infarction8 reduces mortality and morbidity. These, as well as additional observational studies associating hyperglycemia with poor outcomes in a variety of medical and surgical patients,915 have led to increased attention on glycemic control in all venues of care.16, 17 Concerns over excessive hypoglycemia and a nonsignificant increase in mortality in certain populations of medical intensive care unit (ICU) patients have raised questions over whether the initial studies can be reproduced or generalized to other groups of inpatients.18, 19 Additional studies are underway to clarify these questions but consensus exists that blood glucose values should at least be less than 180 mg/dL and that the traditional practice of ignoring hyperglycemia is no longer acceptable.
While a uniform focus on glycemic control will allow our patients to receive a consistent message about diabetes, the unique limitations inherent to each practice setting requires different therapeutic regimens and intentional focus on the risks as patients transition from one care area to another. This work addresses several areas of care transition that are particularly important in safely achieving glycemic control including: transition into the hospital for patients on a variety of home regimens, transitions within the hospital (related to changes in dietary intake, change from IV to subcutaneous [SC] therapy, and the perioperative setting), and the transition from the hospital to home or another healthcare facility.
TRANSITION INTO THE HOSPITAL
Until recently, most patients with diabetes admitted to the hospital were managed with sliding‐scale‐only regimens.20, 21 Unfortunately, this led to a variety of complications, including hyperglycemia, hypoglycemia, iatrogenic ketoacidosis, and an inconsistent message to patients on the importance of glycemic control.22 Some outpatient clinicians and patients combated this tradition by creating in‐hospital glucose control plans with orders, which patients would bring with them to the hospital.23 This practice continues to be a helpful way to guide inpatient therapy and is encouraged when available. Glycemic‐controlrelated documents from outpatient clinicians should include the most recent glycosylated hemoglobin (HbA1c) value, diagnosis and known complications, current names and doses of medications, and other patient‐specific preferences or needs (eg, compliance, financial, fear of needles). If the last HbA1c was performed more than 30 days before admission or is not available, one should be obtained upon hospital admission to help guide discharge therapy.24 By knowing the HbA1c, one can determine the level of diabetic control achieved with the current regimen and can help the inpatient team (clinician and patient) determine if a more aggressive glycemic control regimen is necessary at the time of discharge. It is important to note that if the patient has received a transfusion of red blood cells prior to HbA1c measurement or has a hemoglobinopathy, the HbA1c value may not be accurate.25, 26
In general, the outpatient regimen will need to be modified at admission to achieve the appropriate flexibility needed for the changing nutritional intake and insulin requirements that invariably accompany hospitalization. Sulfonylureas and dipeptidyl peptidase 4 inhibitors (DPP4), such as sitagliptin, have most of their effect immediately, but the other oral antihyperglycemic agents have a relatively long delay between treatment and effect, thus they are not a flexible enough method to achieve glycemic control in the hospital. Additionally, inpatients may have transient contraindications to their prior oral antihyperglycemic medications. Metformin is almost always on hold in the hospital setting, at least initially, due to concerns about lactic acidosis. Sulfonylureas can cause hypoglycemia in the setting of worsening renal function or reduced oral intake. Thiazoladinediones (TZDs) are often withheld due to concerns about fluid retention and should be avoided in patients admitted with heart failure. There is little experience in the hospital with the use of newer agents like exenatide, pramlintide, glinides, and DPP4 inhibitors.
Overall, it is generally recommended that oral antihyperglycemic agents be discontinued upon hospital admission and replaced with insulin infusions or scheduled SC insulin. An estimate of 0.4 to 0.5 units/kg of body weight provides a conservative starting point for the total daily dose of insulin (TDD) for most patients. This TDD should then be divided into basal and nutritional components to match the patients' caloric intake. Additional correction doses of insulin should be prescribed to cover episodes of hyperglycemia that develop despite the provision of anticipatory‐physiologic insulin. Further discussion of insulin dosing and SC regimens is available in detail elsewhere.27, 28 The recommendation for these insulin‐only regimens is made regardless of the glycemic control in the outpatient setting and is not meant to imply that they should be continued at discharge. In fact, most patients will return to their home regimen or to one that is intensified but less labor intensive than the basal‐nutritional‐correction insulin used in the hospital. The antihyperglycemic regimen planned for discharge should be anticipated as early as possible and clearly communicated to the patient and/or caregivers to allow for optimal education.
Outpatient insulin regimens that have a high percentage of basal insulin need to be modified during hospital admission to avoid hypoglycemia that may occur from variable nutritional intake. While hospitalized, the basal portion of the estimated TDD generally should not be more than 50% to 60%. The total number of units of all types of insulin used daily as an outpatient can be used as a starting point for determining the inpatient TDD by a 1:1 conversion. Adjustments up or down based on glycemic control, nutritional intake, and other factors are then necessary. If patients are on regimens with insulin plus oral agents at home, the inpatient TDD should either be the home insulin dose or the dose calculated based on their weight, whichever is greater. Patients who use carbohydrate counting to determine nutritional insulin doses as an outpatient might be continued on this regimen if they have a strong understanding of the methods, they are coherent enough to determine their doses, nursing staff are well educated, and dietary services provides the carbohydrate content for the hospital menu. If patients are on insulin pumps at home, these should be managed according to a uniform hospital policy to assure safety. If conversion to multiple daily injections is needed, the same 1:1 conversion is safe.29
Transitions Within the Hospital
General Issues
Within the hospital itself, there are several transitions that have important quality and safety implications regarding glycemic control. The handoffs between providers should follow a standardized format.4, 30, 31 Essential information will vary depending on the setting but should universally include recent hypoglycemia, insulin type and doses, and hypoglycemic risk factors such as changes in insulin doses, the development of renal insufficiency, inability of the patient to self‐report symptoms, tapering of steroids, and cessation or interruption of nutritional intake.32
One of the greatest risks for hypoglycemia in the hospital comes from the unpredictable nutritional interruptions that occur. Unplanned changes are best handled by nurses having an existing order to hold scheduled nutritional insulin if patients are classified nothing by mouth (NPO) or eat <50% of their meal. Additionally, nursing staff should have orders or policies that allow flexibility in the time of administering scheduled rapid‐acting nutritional insulin so that it may be given during or immediately following the meal in patients at higher risk for poor oral intake. Tube feedings also place patients at high risk for hypoglycemia because the tube may become dislodged or they may begin to have feeding intolerance. For these reasons, a measure of safety would be to have standing orders to substitute IV 10% dextrose in water (D10W) at the same rate as the prior tube feeds, hold nutritional insulin, and begin more frequent monitoring whenever tube feeds are stopped.33 Orders that rely on nursing staff to notify a physician when tube feedings are stopped are generally not directive enough because providers may be distracted by other changes or forget the patient is on long‐acting insulin. The need for this flexibility around nutritional dosing emphasizes the importance of avoiding excessive doses of basal insulin. If the total dose of basal insulin is 40% to 50% of the TDD, it can safely be continued at its usual dose despite changing nutritional intake. The only exception is neutral protamine Hagedorn (NPH) insulin, which should be reduced when patients are NPO due to its peak. Generally, a 50% reduction in NPH is recommended for morning doses, but bedtime doses may be given with little to no reduction. Because of the complexity of these issues, standardized order sets are the best way to reliably communicate all the necessary standing orders to nursing staff (Table 1).
|
| Nutritional insulin |
| Hold if patients are NPO or eat less than 50% of their meal. |
| Administer scheduled rapid acting nutritional insulin during or immediately following the meal if oral intake is questionable (ie, nausea, emesis, or newly advancing diet). |
| Tube feedings: When tube feeds are stopped unexpectedly |
| Start dextrose containing IV fluids (many institutions use D10W at the same rate as the prior tube feeds). |
| Hold scheduled nutritional insulin. |
| Notify physician. |
| Basal insulin |
| Continue if NPO. |
| Reduce morning dose of NPH by 50% if NPO and may need to reduce the dose of bedtime NPH. |
| IV to subcutaneous transition |
| Timing for discontinuing IV infusion in relation to first dose of subcutaneous insulin. |
| Prompts for verbal communication between ICU and general ward staff. |
Transitioning the Patient Off of IV Insulin
The strongest evidence for tight glycemic control derives from studies in the surgical ICU.7 Many hospitals have robust, effective IV‐insulin protocols. The frequency of monitoring and rapidity of action of IV insulin allow quick achievement of blood glucose control. As patients begin to eat, the layering of SC nutritional insulin on top of the insulin infusion may reduce the lability of the infusion rate and prevent excursions in glycemic control. When the patient is ready to leave the ICU or start a full oral diet, it is recommended that they transition off of the IV insulin to a basal‐nutritional‐correction regimen.33, 34
The amount of insulin needed with IV infusion is a useful estimate of the TDD of insulin.28, 33, 35, 36 There are important general steps to take when making this transition; but, due to the lack of conclusive data proving the advantage of one regimen over another, there are a variety of acceptable specific protocols (Table 2).3739 First, it should be determined if patients are expected to require ongoing scheduled SC insulin or not. Certainly, all patients with type 1 DM will require scheduled SC insulin, but patients with type 2 DM on low insulin infusion rates or some patients with new hyperglycemia can appropriately be managed with sliding‐scale alone. Next, the average hourly rate of the infusion over the preceding 6 to 8 hours should be determined because it most accurately reflects current insulin needs during the changing stress, nutrition, and medications in critical care patients. This hourly rate will then be converted to a TDD using a safety factor to anticipate decreasing insulin requirements. Some portion of this daily total will then be assigned to be basal insulin. As patients' clinical conditions approach baseline, so will their insulin requirements, and the dose will need to be revised.24
|
| Step 1: Is patient stable enough for transition? Hypotension, active sepsis, vasopressors, and intubation are contraindications to transition due to unreliable subcutaneous insulin absorption and continued need for the most flexible dosing due to frequently changing insulin requirements. |
| Step 2: Does this patient need a transition to scheduled subcutaneous (SC) insulin? |
| Yes |
| All patients with type 1 DM. |
| Type 2 DM patients on insulin as outpatient. |
| Type 2 DM patients with a recent mean infusion rate of 0.5 units/hour.* |
| No |
| Type 2 DM patients with infusion rate <0.5 units/hour.* |
| Stress hyperglycemia or previously unrecognized DM if infusion rate <1 unit/hour, or if HbA1c near normal. |
| Some institutions exclude all stress hyperglycemia patients from transition to a SC insulin regimen, regardless of drip rate. |
| Step 3: If transition is needed, calculate a total daily dose (TDD) of insulin. The TDD is an estimate of the 24‐hour insulin requirement when the patient is receiving full nutrition. |
| Determine mean insulin infusion rate from last 6 to 8 hours. |
| Calculate 24‐hour insulin dose based on this, and reduce this 24‐hour dose by some safety factor. There are several options for this step. |
| Multiply hourly rate by 24, then multiply by 0.7 or 0.8 to arrive at a safety‐adjusted 24 hour insulin dose. |
| OR |
| Multiply hourly infusion rate by 20 (80% of 24). |
| Determine if this total is the TDD or basal dose based on current nutrition. There are several options for this step for you or your institution to choose. |
| If infusion was serving basal AND nutritional needs of patient (such as a patient on 24‐hour tube feedings) this will be your TDD. |
| OR |
| If the infusion insulin was not covering significant nutrition, this could be the BASAL insulin dose. |
| Step 4: Construct a regimen tailored to the patient's nutritional situation, building in safeguards for any changes in nutritional intake and uncertainties about reliability of intake. Several options are again available. |
| Basal: should be ordered as basal glargine or detemir (these are preferred by SHM GCTF but NPH is also an option). |
| Dose is 40% to 50% of TDD. |
| OR |
| Adjusted 24‐hour IV requirement given all as basal. |
| Nutritional: The remainder of the TDD is scheduled nutritional insulin in divided doses. In general, these doses need to be adjusted down for <100% nutritional intake and the orders should allow for administering nutritional insulin just AFTER observed meals to allow an assessment of intake. There are several options for estimating the initial doses: |
| Use 50% of the TDD as nutritional coverage and divide this amount by 3 to determine the scheduled meal dose. Hold if they do not eat more than 50% of their meal. |
| Use a more conservative start of 10% to 20% of the basal dose scheduled with each meal. |
| Use carbohydrate counting to cover nutritional intake. |
| Step 5: Be sure to give SC insulin BEFORE the infusion stops |
| Basal glargine or detemir are ideally given at least 2 hours before infusion is discontinued. |
| Shorter lead times (30 minutes) are possible if rapid acting insulin is given with basal insulin. |
SC insulin should be given before the drip is discontinued to allow an overlap that takes into consideration the onset of action. The first dose of basal insulin should be given 2 hours before the insulin infusion is discontinued.24, 40 However, because this is not always feasible, (ie, the patient needs to leave the ICU sooner), another option is to turn off the drip and give 10% of the basal dose as rapid acting insulin along with the basal dose.39 The timing of subsequent doses will depend on the specific basal insulin that is ordered as well as institutional consideration of usual care delivery and nursing workflow. Given that there are several options to achieve this important overlap between IV and SC insulin, it is best for a multidisciplinary team to choose some preferred way that is the institutional standard. Having a standard allows targeted education and tracking of adherence to best practices.
Because conversion to SC insulin is a complex task and the opportunity may arise while physicians are busy with other clinical priorities, there are several options to assure that the necessary steps take place. Some institutions may build a protocol for this transition on paper or computerized order entry, build cues and dosing charts into order sets, and/or develop nursing documentation and nursing process to influence physician and nurse behavior. This critical juncture is also a good place to focus expertise with a glycemic control team, pharmacist, specially trained nurses, or some other dedicated team to take over this transition for all patients.36 The complexity and aggressiveness of the specific institutional protocol used will depend on the confidence and experience of those individuals responsible for determining the transition doses.
The transition from IV to SC insulin often coincides with a change in patient location, (ie, from the ICU to general medical ward). It is imperative that appropriate communication occurs between the transferring and receiving nurses and physicians to continue with the care plan for glycemic management. This communication can be encouraged through provider education and automated into the standardized order process.
Perioperative Transitions
Patients undergoing surgery present a special challenge. They are faced with not only the physiologic and mental stress of surgery but also the hazards of multiple handoffs across several care teams, all with different priorities and cultures. As in other areas, standardized protocols specific to this area of transition are important in assuring safe and effective perioperative glycemic control. Procedures should preferably be scheduled for the early morning to have the least impact on insulin dosing. Patients who are admitted only for the procedure will have to manage this transition on their own and need to be given specific instructions along with the general preoperative orders.24, 41 In general, the usual dose of glargine can be given the day prior to the procedure if it is approximately 50% of their TDD. This is an important caution because some outpatient regimens use large doses of glargine, which essentially provide both basal and nutritional coverage. In those patients, the glargine dose should be reduced by 20% to 50% to provide a safety margin. As with any patient who is NPO, the morning dose of NPH should be one‐half of the usual dose, scheduled nutritional insulin should be held, and the usual doses of correction insulin should be reduced. The appropriate preoperative dose adjustments also depend on whether the individual patient is ketosis‐prone and how tight their glycemic control is as an outpatient.
Upon arrival to the hospital or during the time that the inpatient is NPO, dextrose containing IV fluids should be administered to minimize the risk of hypoglycemia and prevent ketosis. Given the risks for wide variation, blood glucose monitoring should occur every 1 to 2 hours before, during, and initially after the procedure. Infusion insulin allows the most rapid titration and reliable delivery (compared with SC infusions or injections) and is therefore the preferred regimen for major surgery requiring prolonged NPO status or prolonged surgery in patients with type 1 diabetes. Basal‐nutritional‐correction SC insulin is preferred in other surgical inpatients because their nutritional intake is variable and the stress of surgery affects insulin requirements.
Oral antihyperglycemic agents should be held around the time of surgery. If patients are on an oral agent that can result in hypoglycemia, (ie, sulfonylurea or other insulin secretagogue), it should be held on the day of the procedure. Metformin must be held for safety concerns, given the possible decrease in renal function around surgery. It should be held beginning on the day of the procedure or the day before in the case of the sustained‐release formulation. It can then be resumed 48 hours postoperation after normal renal function is secured and the patient is discharged home. Alpha‐glucosidase inhibitors should be held whenever patients are NPO because they only work when taken with meals. Thiazoladinediones have a long duration of action and so can be continued or stopped around surgery. Finally, glucagon‐like peptide (GLP‐1) agonists (exenatide) should be held until the patient is eating normally and discharged home due to the high incidence of gastrointestinal side effects.
TRANSITIONING FROM THE HOSPITAL
The final but perhaps most important transition is the one from the hospital. With much attention on glycemic control in the hospital, it will become clear to many clinicians that the outpatient regimen needs to be modified. However, any changes in medications increase the chances of hypoglycemia and the possibility of error. The postdischarge time frame has been poorly studied and was specifically identified by the Association for Clinical Endocrinologists (ACE) and American Diabetes Association (ADA) as an area in need of future research.36
Patients may be discharged to a nursing home, hospice, or home, and numerous factors need to be considered to determine the optimal discharge regimen. Important considerations are the HbA1c at admission, home medications, medication interactions, current medical problems, nutritional status, physical disabilities, frequency of self‐monitoring, hypoglycemic risk factors, contraindications to oral medications, goals of care/life expectancy, and financial and other resources. If there are temporary physical or self‐care limitations, then a visiting nurse may need to be arranged to assure a safe transition home with the optimal therapy. If patients are going to a skilled nursing facility or other acute care hospital, the formulary, processes, and staffing issues of that facility will be additional important considerations in determining whether therapy is the same as in the hospital or more like what it will be at home.
An algorithm for outpatient therapy for type 2 DM was recommended in a consensus statement from the ADA and European Association for the Study of Diabetes.42, 43 This has been modified using additional recommendations from the AACE44 and is depicted in Figure 1. While the delineation of these steps is helpful, it must be emphasized that both the choice of regimen and dose will need to be individualized. Prescribing the ideal frequently falls short if there is no way for the patient to implement the recommendations. Intensive insulin therapy requires training in food intake/emnsulin matching, motivation of the patient and outpatient clinician, 4 times daily self‐monitoring of blood glucose, and considerable expense. Some patients may be temporarily continued on basal‐nutritional‐correction regimens as their insulin requirements are rapidly changing and later converted to regimens that involve less frequent insulin doses, (ie, twice daily premixed insulin or basal insulin with oral agents or oral agents alone).45, 46 Other patients who may be medically appropriate for intensive insulin therapy may first need to gain confidence with more simple insulin regimens. There are numerous additional resources on initiating insulin that the reader is referred to for more detail.4448

Oral antihyperglycemic drugs are usually held while a patient is admitted to the hospital but once medical conditions are improved, oral intake is established, and renal function stabilized, these drugs can be restarted. If a patient has a new contraindication to metformin or sulfonylureas but does not need insulin, a TZD or DPP4 inhibitor should be considered. Elderly patients and those with renal or liver disease are at increased risk for developing hypoglycemia.49, 50 Glyburide should be avoided, and doses of other sulfonylureas may need to be adjusted. Other options that may be considered in this situation include sitagliptin and exenatide.51 When patients will be discharged on oral diabetic medications alone, discontinue the basal insulin 12 to 24 hours before and the scheduled nutritional insulin at the same time oral agents are restarted. Sulfonylureas, metformin, DPP4 inhibitors, and exenatide will have most of their effect in the first day, but TZDs have a delayed onset and may not be a good bridge for immediate control at discharge.
If patients are going to be discharged on basal insulin in addition to oral agents, several options exist for determining the dose. Because of the risk of hypoglycemia after discharge, it is advised to either reduce the doses of oral agents or choose more conservative insulin starting doses.52 One possibility is to discontinue the nutritional and correction doses, continue the hospital dose of basal insulin, and restart the oral antidiabetes medications. If the dose of basal insulin was more than 50% of the TDD of insulin, it may need to be reduced. A more conservative option for patients at a higher risk of hypoglycemia is to start 0.2 units/kg or 10 units of NPH, glargine, or detemir at bedtime (Figure 2). Once discharged, blood glucose should be measured 1 to 4 times a day and the basal dose titrated by several different validated methods.53, 54 Appropriate orders for necessary supplies for insulin therapy include a meter with test strips, lancets, syringes, needles, and glucagon kit.55

With a large number of patients with diabetes remaining undiagnosed, it is important to use the information available during hospitalization to identify previously unrecognized diabetes or prediabetes.24 Because there are no unique criteria for the diagnosis of DM in the stressed state, patients may have a presumptive diagnosis made in the hospital and/or follow‐up testing with fasting glucose or an oral glucose tolerance test. No ADA diagnostic thresholds for the HbA1c currently exist, but it can be a useful marker in making this distinction.56 Among patients with new hyperglycemia, an HbA1c of 6% or greater was 100% specific for predicting a future diagnosis of diabetes in the small prospective cohort study by Greci et al.,57 but many endocrinologists use a cutoff of 7%. For all hyperglycemic patients, lifestyle interventions that promote weight loss and increased activity levels should be encouraged. New hyperglycemia should be clearly identified as a diagnosis in discharge communication.
There are many barriers to diabetes self‐management education in the inpatient setting but there are also numerous resources and opportunities. New information will be available regarding patients' understanding of their disease and glycemic control and there may be plans for changes in the home medication regimen. Most of the focus of inpatient education sessions is on survival skills such as taking medications, performing blood glucose monitoring, basic meal planning, identification and treatment of hypoglycemia, sick‐day management, how to access further diabetes education as an outpatient, and when to call the healthcare team.58 The most effective way to accomplish all of this is to identify the discharge regimen early and include nurses and staff in a plan to educate all patients. An inpatient diabetes educator can provide additional help with newly‐diagnosed or uncontrolled patients. Dividing the material over the hospitalization makes it less overwhelming for patients, reinforces previously taught concepts, spreads the responsibility to more providers, and offers it in conjunction with the correlating clinical care. Throughout their hospital stay, patients can begin to practice new skills, including blood glucose monitoring and logbook use, drawing up and administering insulin, sharps disposal, basic diabetic diet information, and sick‐day management. The specific topics addressed in each session can be tracked as part of an interdisciplinary education record that allows coordination among the individuals involved in teaching.59 It is important to give patients the basics, support them with minimal written information, and provide them appropriate follow‐up diabetes education.60 Furthermore, the inpatient team should view the patient's glycemic control education as something that needs to continue across the continuum of care and develop communication strategies that connect with the follow‐up clinical team.
At the time of discharge, it is essential that written documentation and communication with outpatient care providers be completed.61, 62 The more standardized the inpatient insulin regimens are, the more likely the patient is to be on a much different glycemic control regimen than the one on admission; therefore, it is even more important to assure that the admission medication list is accurate and reconciled completely with the modified list at discharge. Discharge check lists and tools for assessing patient acceptance of the discharge plan help with this process.63 Follow‐up with the primary care physician should occur within 7 to 14 days if patients are new to insulin, had medication changes, or are elderly. An increased likelihood of keeping posthospitalization appointments with a diabetes specialty clinic has been associated with being discharged on insulin, a new diagnosis of diabetes, and direct referral.64 Additional attention should be paid to barriers to follow‐up, including lack of health insurance, prior difficulty with follow‐up, and transportation problems.65
SUMMARY
A variety of factors have contributed to difficulty in achieving inpatient and outpatient glucose control. These include care complexity, the lack of standardized protocols, limited knowledge about glucose control, and clinical inertia. Inpatient clinicians have a tendency toward keeping patients on their home regimen in hopes that they might test its effectiveness. Furthermore, there has been the notion of why optimize the glycemic regimen of inpatients because their diabetic needs will change in the outpatient setting. However, because the insulin requirements during acute illness are different and nutritional intake is variable, nearly all inpatients should be placed on multiple daily doses of scheduled insulin or IV insulin to allow the necessary flexibility for rapid titration and abrupt changes in nutrition. This intensive regimen is only appropriate for a minority of outpatients. This difference illustrates that a regimen that works perfectly in one clinical setting will not necessarily be optimal in the next. The patient's outpatient treatment regimen should be reassessed based on HbA1c, self‐monitoring prior to admission, and new contraindications based on medical issues. If a change is indicated and the inpatient physician is motivated, there are numerous helpful resources to aid in addressing all the necessary factors surrounding intensification of therapy.
Despite requiring different glycemic control regimens, the information gained from the needs in each setting guide the next, making communication and planning paramount. Important transitions that must be given attention are: (1) admission to the hospital; (2) in‐hospital transitions, including the perioperative period and IV‐to‐SC insulin; and (3) the hospital to outpatient transition. The complexity of such frequent transitions requires planning, education, and clear communication that are best handled with a systems approach and the development of standardized protocols and order sets. Hospitalists, endocrinologists, and other members of the healthcare team should take an aggressive role in developing systems and facilitating optimal transitions to maximize glycemic control. Further studies are needed to determine the best practices among the variety of options discussed in this article.
- ACE/ADA Task Force on Inpatient Diabetes.American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control.Endocr Pract.2006;12:458–468.
- American Board of Internal Medicine Foundation Stepping Up to the Plate Alliance. Available at: http://www.abimfoundation.org/quality/suttp.shtm. Accessed November2007.
- National Transitions of Care Coalition. Available at: http://www.ntocc.org. Accessed November2007.
- JCAHO 2008 National Patient Safety Goals. Availableat: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/08_hap_npsgs.htm. Accessed November2007.
- Diabetes Control and Complications Trial Research Group.The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus.N Engl J Med.1993;329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group.Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes: UK Prospective Diabetes Study (UKPDS) Group.Lancet.1998;352:837–853.
- ,,, et al.,Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,,.Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long‐term results from the Diabetes and Insulin‐Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study.Circulation.1999;99:2626–2632.
- ,,,,.Relationship of early hyperglycemia to mortality in trauma patients.J Trauma.2004;56:1058–1062.
- ,,,,.Early peri‐operative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321–1324.
- ,,, et al.Relation between the duration of remission and hyperglycemia in induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone/methotrexate cytarabine regimen.Cancer.2004;100:1179–1185.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,,,.Diabetes and outcome of community‐acquired pneumococcal bacteriemia.Diabetes Care.2004;27:70–76.
- ,,.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.JPEN J Parenter Enteral Nutr.1998;22:77–81.
- ,,,,.Early post‐operative glucose levels are an independent risk factor for infection after peripheral vascular surgery. A retrospective study.Eur J Vasc Endovasc Surg.2004;28:520–525.
- American Diabetes Association.Standards of medical care in diabetes, 2006.Diabetes Care.2006;29(suppl 1):s4–s42.
- American College of Endocrinology Task Force on Inpatient Diabetes and Metabolic Control.American College of Endocrinology Position Statement on Inpatient Diabetes and Metabolic Control.Endocr Pract.2004;10:77–82.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461
- ,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis.N Engl J Med.2008;358(2):125–139.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.,Diabetes care in hospitalized noncritically ill patients: More evidence for clinical inertia and negative therapeutic momentum.J Hosp Med.2007;2:203–211.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,.Hospital management of hyperglycemia.Clin Diabetes.2004;22:81–88.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals. [Erratum appears in Diabetes Care. 2005; 28: 1990. Dosage error in text].Diabetes Care.2004;27:553–591.
- ,.Hemoglobinopathies and HbA(1c) measurement.Diabetes Care.2000;23(8):1197–1198.
- ,,,,,.Evaluation of HbA1c determination methods in patients with hemoglobinopathiesDiabetes Care.2000;23(3):339–344.
- ,,,.Subcutaneous insulin order sets and protocols: effective design and implementation strategies.J Hosp Med.2008;3.
- ,,,,.Management of diabetes and hyperglycemia in the hospital: a practical guide to subcutaneous insulin use in the non‐critically ill, adult patient.J Hosp Med.2008;3.PMID:8675920.
- ,,,.Switch to multiple daily injections with insulin glargine and insulin lispro from continuous subcutaneous insulin infusion with insulin lispro: a randomized, open‐label study using a continuous glucose monitoring system.Endocr Pract.2005;11:157–164.
- SBAR technique for communication: a situational briefing model. Available at: http://www.ihi.org/IHI/Topics/PatientSafety/SafetyGeneral/Tools/SBARTechniqueforCommunicationASituationalBriefingModel.htm. Accessed December2007.
- . Promising quality improvement initiatives: reports from the field. AHRQ Summit—Improving Health Care Quality for All Americans: Celebrating Success, Measuring Progress, Moving Forward 2004. Available at: http://www.ahrq.gov/qual/qsummit/qsummit4.htm#sentara. Accessed December2007.
- ,,, et al.Hospital hypoglycemia: not only treatment but also prevention.Endocr Pract.2004;10(suppl 2):89–99.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Available at: http://resources.aace.com/PDF/Section_05‐Final‐Inpatient_Non‐ICU/Hyperglycemia_Non‐ICU_Protocols/Transition_from_ Intravenous_to_Subcutaneous_Insulin.PDF. Accessed November2007.
- Recommendations for safe use of insulin in hospitals. American Society of Health System Pharmacists and the Hospital and Health System Association of Pennsylvania. 2005. Available at: http://www.premierinc.com/safety/safety‐share/01–06‐downloads/01‐safe‐use‐insulin‐ashp.pdf. Accessed December2007.
- ,,, for the Society of Hospital Medicine Glycemic Control Taskforce. Glycemic control resource room: improving reliability of care across transitions and in the perioperative setting. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/html/07Layer_Inter/06_Transitions.cfm. Accessed August2008.
- ACE/ADA Task Force on Inpatient Diabetes American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control: a call to action.Diabetes Care.2006;29:1955–1962.
- ,,, et al.Conversion of intravenous insulin infusions to subcutaneously administered insulin glargine in patients with hyperglycemia.Endocr Pract.2006;12:641–650.
- ,,,.Intravenous insulin infusion therapy: indications, methods, and transition to subcutaneous insulin therapy.Endocr Pract.2004;10(suppl 2):71–80.
- ,,, et al.Inpatient management of hyperglycemia: the northwestern experience.Endocr Pract.2006;12(5):491–505.
- American Diabetes Association.Position statement: standards of medical care in diabetes‐2007.Diabetes Care.2007;30(suppl 1):S4–S41.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Available at: http://resources.aace.com/PDF/Section_05‐Final‐Inpatient_Non‐ICU/Hyperglycemia_Non‐ICU_Protocols/Pre‐Operative_Instructions_for_Patients_with_Diabetes.PDF Accessed November2007.
- ,,, et al.Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes.Diabetes Care.2006;29:1963–1972.
- ,,, et al.Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: update regarding thiazoladinediones.Diabetes Care.2008;31:173–175.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Challenges in Effective Discharge Planning for Hospitalized Patients with Diabetes. Available at: http://resources.aace.com/PDF/Section_07‐Final‐Transition‐Inpatient_to_Outpatient/Challenges_in_Effective_Discharge_for_Diabetes_Patients.PPT. Accessed December2007.
- ,,.Initiating insulin therapy in type 2 diabetes: a comparison of biphasic and basal insulin analogs.Diabetes Care.2005;28:260–265.
- ,,, et al.Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes.N Engl J Med.2007;357:1716–1730.
- ,,.Narrative review: a rational approach to starting insulin therapy.Ann Intern Med.2006;145:125–134.
- ,,,,.A real‐world approach to insulin therapy in primary care practice.Clin Diabetes.2005;23:78–86.
- ,,,.Individual sulfonylureas and serious hypoglycemia in older persons.J Am Geriatr Soc.1996;44:751–755.
- ,,,.Incidence and risk factors for serious hypoglycemia in older persons using insulin or sulfonylureas.Arch Intern Med.1997;157(15):1681–1686.
- ,,, et al.Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial.Ann Intern Med.2005;143:559–569.
- .The transition from insulin infusions to long‐term diabetes therapy: the argument for insulin analogs.Semin Thorac Cardiovasc Surg.2006;18:366–378.
- ,,,,.ATLANTUS Study Group. Improvement of glycemic control in subjects with poorly controlled type 2 diabetes.Diabetes Care.2005;28:1282–1288.
- ,,.Investigators Insulin Glargine 4002 Study. The Treat‐to Target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetes patients.Diabetes Care.2003;26:3080–3086.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Available at: http://resources.aace.com/PDF/Section_07‐Final‐Transition‐Inpatient_to_Outpatient/Effective_Discharge_Planning‐Sample_Discharge_Plans/Inpatient_Diabetes_Discharge_Prescription.PDF. Accessed November2007.
- American Diabetes Association.Diagnosis and classification of diabetes mellitus.Diabetes Care.2007;30(suppl):S42–S47.
- ,,, et al.Utility of HbA1c levels for diabetes case finding in hospitalized patients with hyperglycemia.Diabetes Care.2003;26:1064–1068.
- ,,, et al.National standards for diabetes self‐management education.Diabetes Care.2006;29(suppl 1):S78–S85.
- Society of Hospital Medicine Glycemic Control Task Force. Workbook for improvement: improving glycemic control, preventing hypoglycemia and optimizing care of the inpatient with diabetes and hyperglycemia. page 105. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/pdf/GC_Workbook.pdf. Accessed December,2007.
- Joslin Diabetes Center. EZ Start Patient Information Handouts. Available at: http://www.joslin.org/ezstart. Accessed December2007.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2:314–323.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- Society of Hospital Medicine On‐line Clinical Tools. Ideal discharge for the elderly patient: a hospitalist checklist. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=QI_Clinical_Toolsemplate=/CM/ContentDisplay.cfmContentID=10303. Accessed December2007.
- ,,, et al.Inpatient to outpatient transfer of care in urban patients with diabetes: patterns and determinants of immediate post‐discharge follow‐up.Arch Intern Med.2004;164:447–453.
- ,,,,,.Inpatient to outpatient transfer of diabetes care: perceptions of barriers to postdischarge follow‐up in urban African American patients.Ethn Dis.2007;17:238–243.
- ACE/ADA Task Force on Inpatient Diabetes.American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control.Endocr Pract.2006;12:458–468.
- American Board of Internal Medicine Foundation Stepping Up to the Plate Alliance. Available at: http://www.abimfoundation.org/quality/suttp.shtm. Accessed November2007.
- National Transitions of Care Coalition. Available at: http://www.ntocc.org. Accessed November2007.
- JCAHO 2008 National Patient Safety Goals. Availableat: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/08_hap_npsgs.htm. Accessed November2007.
- Diabetes Control and Complications Trial Research Group.The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus.N Engl J Med.1993;329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group.Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes: UK Prospective Diabetes Study (UKPDS) Group.Lancet.1998;352:837–853.
- ,,, et al.,Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,,.Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long‐term results from the Diabetes and Insulin‐Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study.Circulation.1999;99:2626–2632.
- ,,,,.Relationship of early hyperglycemia to mortality in trauma patients.J Trauma.2004;56:1058–1062.
- ,,,,.Early peri‐operative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321–1324.
- ,,, et al.Relation between the duration of remission and hyperglycemia in induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone/methotrexate cytarabine regimen.Cancer.2004;100:1179–1185.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,,,.Diabetes and outcome of community‐acquired pneumococcal bacteriemia.Diabetes Care.2004;27:70–76.
- ,,.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.JPEN J Parenter Enteral Nutr.1998;22:77–81.
- ,,,,.Early post‐operative glucose levels are an independent risk factor for infection after peripheral vascular surgery. A retrospective study.Eur J Vasc Endovasc Surg.2004;28:520–525.
- American Diabetes Association.Standards of medical care in diabetes, 2006.Diabetes Care.2006;29(suppl 1):s4–s42.
- American College of Endocrinology Task Force on Inpatient Diabetes and Metabolic Control.American College of Endocrinology Position Statement on Inpatient Diabetes and Metabolic Control.Endocr Pract.2004;10:77–82.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461
- ,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis.N Engl J Med.2008;358(2):125–139.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.,Diabetes care in hospitalized noncritically ill patients: More evidence for clinical inertia and negative therapeutic momentum.J Hosp Med.2007;2:203–211.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,.Hospital management of hyperglycemia.Clin Diabetes.2004;22:81–88.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals. [Erratum appears in Diabetes Care. 2005; 28: 1990. Dosage error in text].Diabetes Care.2004;27:553–591.
- ,.Hemoglobinopathies and HbA(1c) measurement.Diabetes Care.2000;23(8):1197–1198.
- ,,,,,.Evaluation of HbA1c determination methods in patients with hemoglobinopathiesDiabetes Care.2000;23(3):339–344.
- ,,,.Subcutaneous insulin order sets and protocols: effective design and implementation strategies.J Hosp Med.2008;3.
- ,,,,.Management of diabetes and hyperglycemia in the hospital: a practical guide to subcutaneous insulin use in the non‐critically ill, adult patient.J Hosp Med.2008;3.PMID:8675920.
- ,,,.Switch to multiple daily injections with insulin glargine and insulin lispro from continuous subcutaneous insulin infusion with insulin lispro: a randomized, open‐label study using a continuous glucose monitoring system.Endocr Pract.2005;11:157–164.
- SBAR technique for communication: a situational briefing model. Available at: http://www.ihi.org/IHI/Topics/PatientSafety/SafetyGeneral/Tools/SBARTechniqueforCommunicationASituationalBriefingModel.htm. Accessed December2007.
- . Promising quality improvement initiatives: reports from the field. AHRQ Summit—Improving Health Care Quality for All Americans: Celebrating Success, Measuring Progress, Moving Forward 2004. Available at: http://www.ahrq.gov/qual/qsummit/qsummit4.htm#sentara. Accessed December2007.
- ,,, et al.Hospital hypoglycemia: not only treatment but also prevention.Endocr Pract.2004;10(suppl 2):89–99.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Available at: http://resources.aace.com/PDF/Section_05‐Final‐Inpatient_Non‐ICU/Hyperglycemia_Non‐ICU_Protocols/Transition_from_ Intravenous_to_Subcutaneous_Insulin.PDF. Accessed November2007.
- Recommendations for safe use of insulin in hospitals. American Society of Health System Pharmacists and the Hospital and Health System Association of Pennsylvania. 2005. Available at: http://www.premierinc.com/safety/safety‐share/01–06‐downloads/01‐safe‐use‐insulin‐ashp.pdf. Accessed December2007.
- ,,, for the Society of Hospital Medicine Glycemic Control Taskforce. Glycemic control resource room: improving reliability of care across transitions and in the perioperative setting. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/html/07Layer_Inter/06_Transitions.cfm. Accessed August2008.
- ACE/ADA Task Force on Inpatient Diabetes American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control: a call to action.Diabetes Care.2006;29:1955–1962.
- ,,, et al.Conversion of intravenous insulin infusions to subcutaneously administered insulin glargine in patients with hyperglycemia.Endocr Pract.2006;12:641–650.
- ,,,.Intravenous insulin infusion therapy: indications, methods, and transition to subcutaneous insulin therapy.Endocr Pract.2004;10(suppl 2):71–80.
- ,,, et al.Inpatient management of hyperglycemia: the northwestern experience.Endocr Pract.2006;12(5):491–505.
- American Diabetes Association.Position statement: standards of medical care in diabetes‐2007.Diabetes Care.2007;30(suppl 1):S4–S41.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Available at: http://resources.aace.com/PDF/Section_05‐Final‐Inpatient_Non‐ICU/Hyperglycemia_Non‐ICU_Protocols/Pre‐Operative_Instructions_for_Patients_with_Diabetes.PDF Accessed November2007.
- ,,, et al.Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes.Diabetes Care.2006;29:1963–1972.
- ,,, et al.Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: update regarding thiazoladinediones.Diabetes Care.2008;31:173–175.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Challenges in Effective Discharge Planning for Hospitalized Patients with Diabetes. Available at: http://resources.aace.com/PDF/Section_07‐Final‐Transition‐Inpatient_to_Outpatient/Challenges_in_Effective_Discharge_for_Diabetes_Patients.PPT. Accessed December2007.
- ,,.Initiating insulin therapy in type 2 diabetes: a comparison of biphasic and basal insulin analogs.Diabetes Care.2005;28:260–265.
- ,,, et al.Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes.N Engl J Med.2007;357:1716–1730.
- ,,.Narrative review: a rational approach to starting insulin therapy.Ann Intern Med.2006;145:125–134.
- ,,,,.A real‐world approach to insulin therapy in primary care practice.Clin Diabetes.2005;23:78–86.
- ,,,.Individual sulfonylureas and serious hypoglycemia in older persons.J Am Geriatr Soc.1996;44:751–755.
- ,,,.Incidence and risk factors for serious hypoglycemia in older persons using insulin or sulfonylureas.Arch Intern Med.1997;157(15):1681–1686.
- ,,, et al.Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial.Ann Intern Med.2005;143:559–569.
- .The transition from insulin infusions to long‐term diabetes therapy: the argument for insulin analogs.Semin Thorac Cardiovasc Surg.2006;18:366–378.
- ,,,,.ATLANTUS Study Group. Improvement of glycemic control in subjects with poorly controlled type 2 diabetes.Diabetes Care.2005;28:1282–1288.
- ,,.Investigators Insulin Glargine 4002 Study. The Treat‐to Target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetes patients.Diabetes Care.2003;26:3080–3086.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Available at: http://resources.aace.com/PDF/Section_07‐Final‐Transition‐Inpatient_to_Outpatient/Effective_Discharge_Planning‐Sample_Discharge_Plans/Inpatient_Diabetes_Discharge_Prescription.PDF. Accessed November2007.
- American Diabetes Association.Diagnosis and classification of diabetes mellitus.Diabetes Care.2007;30(suppl):S42–S47.
- ,,, et al.Utility of HbA1c levels for diabetes case finding in hospitalized patients with hyperglycemia.Diabetes Care.2003;26:1064–1068.
- ,,, et al.National standards for diabetes self‐management education.Diabetes Care.2006;29(suppl 1):S78–S85.
- Society of Hospital Medicine Glycemic Control Task Force. Workbook for improvement: improving glycemic control, preventing hypoglycemia and optimizing care of the inpatient with diabetes and hyperglycemia. page 105. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/pdf/GC_Workbook.pdf. Accessed December,2007.
- Joslin Diabetes Center. EZ Start Patient Information Handouts. Available at: http://www.joslin.org/ezstart. Accessed December2007.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2:314–323.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- Society of Hospital Medicine On‐line Clinical Tools. Ideal discharge for the elderly patient: a hospitalist checklist. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=QI_Clinical_Toolsemplate=/CM/ContentDisplay.cfmContentID=10303. Accessed December2007.
- ,,, et al.Inpatient to outpatient transfer of care in urban patients with diabetes: patterns and determinants of immediate post‐discharge follow‐up.Arch Intern Med.2004;164:447–453.
- ,,,,,.Inpatient to outpatient transfer of diabetes care: perceptions of barriers to postdischarge follow‐up in urban African American patients.Ethn Dis.2007;17:238–243.
Prevention of venous thromboembolism in the hospitalized medical patient
The need for prophylaxis of venous thromboembolism (VTE) in hospitalized acutely ill medical patients is well established. Without prophylaxis, hospitalized medical patients develop VTE at a rate of 5% to 15%.1–3 Moreover, pulmonary embolism (PE) occurs more frequently in hospitalized medical patients than in nonmedical patients, and is a leading cause of sudden death in hospitalized medical patients.4,5 Without appropriate prophylaxis, 1 in 20 hospitalized medical patients may suffer a fatal PE.4
PROPHYLAXIS IN MEDICAL PATIENTS: UNDERUSED AND OFTEN INAPPROPRIATE
Despite these risks and the clear indications for VTE prophylaxis in hospitalized medical patients, prophylaxis of VTE is omitted more often in these patients than in hospitalized surgical patients.5 Even when prophylaxis is given, it is often used inappropriately in the medical population. So concludes a recent analysis of data from 196,104 patients with acute medical conditions who were discharged from 227 US hospitals from January 2002 to September 2005.6 Criteria for inclusion in the analysis were patient age of 40 years or older, a hospital stay of 6 days or greater, and an absence of contraindications to anticoagulation. Appropriate prophylaxis was defined in accordance with the Sixth American College of Chest Physicians (ACCP) Consensus Conference on Antithrombotic Therapy.7
The analysis revealed an overall VTE prophylaxis rate of 61.8%, but the rate of appropriate prophylaxis was only 33.9%, meaning that two-thirds of discharged patients did not receive prophylaxis in accordance with ACCP guidelines. When temporal trends were analyzed according to groups based on patients’ diagnosis at admission (acute myocardial infarction, severe lung disease, ischemic stroke, cancer, heart failure, or trauma), the rate of appropriate prophylaxis remained essentially flat from the beginning to the end of the study period for virtually all diagnosis groups.6
Similar findings have emerged from the International Medical Prevention Registry on Venous Thromboembolism (IMPROVE), an ongoing international registry of acutely ill medical patients.8 Data from the first 15,156 patients, enrolled from July 2002 through September 2006, reveal that 50% of patients received pharmacologic and/or mechanical VTE prophylaxis in the hospital, and only 60% of patients who met established criteria for VTE prophylaxis actually received it.
Analysis of the US portion of the IMPROVE data shows that 54% of the US patient sample received some form of VTE prophylaxis; 22% of US patients received intermittent pneumatic compression, 21% received unfractionated heparin (UFH), 14% received low-molecular-weight heparin (LMWH), and 3% wore compression stockings.8 Thus, despite a paucity of data supporting a benefit of intermittent pneumatic compression in this population,9 it was the most frequently used form of prophylaxis in US patients.
CLINICAL TRIALS OF PHARMACOLOGIC PROPHYLAXIS IN MEDICAL PATIENTS
The Prophylaxis in Medical Patients with Enoxaparin (MEDENOX) trial1 randomized 1,102 hospitalized patients to one of two doses of the LMWH enoxaparin (20 mg or 40 mg once daily subcutaneously) or placebo for 6 to 14 days. Compared with placebo, the 40-mg dose of enoxaparin was associated with a 63% reduction in risk of VTE over 3 months of follow-up (P < .001) (Figure 1).
The Prospective Evaluation of Dalteparin Efficacy for Prevention of VTE in Immobilized Patients Trial (PREVENT)2 was a multicenter, randomized, double-blind study comparing the LMWH dalteparin (5,000 IU daily given subcutaneously for 14 days) with placebo in 3,706 acutely ill medical patients. Over 90 days of follow-up, the risk of VTE was reduced by 44% in patients assigned to dalteparin compared with those assigned to placebo (P = .0015) (Figure 1).
The Arixtra for Thromboembolism Prevention in a Medical Indications Study (ARTEMIS)3 randomized 849 medical patients 60 years or older to 6 to 14 days of therapy with the selective factor Xa inhibitor fondaparinux (2.5 mg once daily subcutaneously) or placebo. Compared with the placebo group, fondaparinux recipients had a 47% lower risk of developing VTE by day 15 (P = .029) (Figure 1).
Fewer events and fatal PEs, but no effect on all-cause mortality
A recent meta-analysis by Dentali et al10 further demonstrates the efficacy of anticoagulant therapy for preventing symptomatic VTE in hospitalized medical patients. This analysis included several other trials in addition to the three reviewed above,1–3 for a total of nine randomized studies (seven of which were dou-ble-blind) comprising 19,958 patients. Across the nine studies, anticoagulant prophylaxis was clearly superior to placebo in preventing fatal PE (relative risk, 0.38 [95% CI, 0.21 to 0.69]). There was a strong trend toward a reduction in symptomatic deep vein thrombosis (DVT) with prophylaxis but no effect on all-cause mortality. The meta-analysis also provided reassurance that prophylaxis does not increase the rate of major bleeding.
HOW DO THE PROPHYLAXIS OPTIONS STACK UP?
What the ACCP recommends
Current ACCP guidelines recommend the use of either LMWH or low-dose UFH (5,000 U subcutaneously two or three times daily) as a Grade 1A recommendation for VTE prophylaxis in patients with medical conditions and risk factors for VTE.9 This represents the guidelines’ highest level of recommendation, ie, one that is based on randomized controlled trials (RCTs) without important limitations. In contrast, the 2006 International Consensus Statement, developed as a collaborative effort among expert bodies on VTE, specified a more narrow dosing recommendation for UFH in this patient population (5,000 U three times daily, not twice daily) as well as specifying 40 mg once daily as the recommended dose of enoxaparin and 5,000 IU once daily as the recommended dose of dalteparin.11
For medical patients with risk factors for VTE who have a contraindication to anticoagulant prophylaxis, the ACCP guidelines recommend the use of graduated compression stockings or intermittent pneumatic compression devices as a Grade 1C+ recommendation (“no RCTs but strong RCT results can be unequivocally extrapolated, or overwhelming evidence from observational studies”9).
Current ACCP guidelines do not address the use of fondaparinux in their recommendations for VTE prophylaxis in medical patients.
Getting a handle on bleeding risk
Patient characteristics that exclude pharmacologic thromboprophylaxis due to bleeding risk are generally limited to active bleeding or coagulopathy, as demonstrated by a platelet count less than 50,000 cells/µL or an international normalized ratio greater than 1.5. Additionally, bleeding risk should be carefully assessed if an invasive procedure is planned during a patient’s hospital stay.
It is worth noting that the anticoagulant doses used for VTE prophylaxis are a fraction of those used for treatment of VTE. Thus, if a patient would be treated with full-dose anticoagulation if VTE developed, then that patient should be eligible for VTE prophylaxis.
Because the use of mechanical forms of prophylaxis in medical patients is not truly evidence-based, mechanical prophylaxis should be reserved for medical patients who have a contraindication to anticoagulants, or for use in combination with anticoagulants in patients at very high risk of VTE.
UFH vs LMWH
Two meta-analyses have compared UFH with LMWH for VTE prevention in medical patients.12,13 In a recent analysis that included 10 trials directly comparing the two therapies, 14 trials comparing UFH with control, and 11 trials comparing LMWH with control, Wein et al found a lower risk of DVT with LMWH than with UFH (relative risk, 0.68 [95% CI, 0.52 to 0.88]) but no difference between the therapies in mortality or bleeding risk.12 In an earlier and smaller analysis, Mismetti et al found no significant differences between UFH and LMWH in preventing DVT or death but did find a significant reduction in major bleeding episodes with LMWH versus three-times-daily UFH (52% relative reduction; P = .049).13
Randomized trials also reveal that enoxaparin 40 mg once daily is as efficacious as UFH 5,000 U three times daily for VTE prevention in medical patients.14,15 The above analysis by Wein et al12 and an additional meta-analysis by King and colleagues16 found that three-times-daily dosing of UFH is more efficacious than twice-daily dosing of UFH, but at the expense of more bleeding, including major bleeding.
Economic considerations
Because of differences in drug acquisition costs between UFH and the LMWH agents, several economic evaluations have compared the use of these therapies for prophylaxis in medical patients at risk of VTE.
In an analysis of hospital costs for medical patients receiving VTE prophylaxis from more than 330 US hospitals for the period 2001–2004, Burleigh et al found that mean total hospital costs were higher for patients who received UFH than for those who received LMWH ($7,615 vs $6,866) even though mean drug costs were higher for LMWH ($791 vs $569 for UFH).17 A reduction in hospital length of stay appeared to contribute to the overall savings with LMWH; other contributors may have included costs associated with heparin-induced thrombocytopenia (HIT) in UFH recipients or the extra nursing time required for administering UFH in two or three daily doses.
Leykum et al used a decision analysis model to estimate the economic effect of substituting enoxaparin for UFH in hospitalized medical patients for whom VTE prophylaxis is indicated.18 Cost data were based on Medicare reimbursement rates as well as drug and laboratory costs for a multi-institutional health system. The model assumed HIT incidence rates of 2.7% with UFH and 0.3% with enoxaparin. It also assumed the cost of a daily dose to be $4 for UFH versus $84 for enoxaparin. From the payer perspective, the model showed that substituting enoxaparin for UFH would reduce the overall cost of care by $28.61 per day on a per-patient basis, despite enoxaparin’s higher acquisition cost, and would save $4,550 per quality-adjusted life-year by reducing the incidence of HIT.
Another cost analysis confirms the association between HIT and increased hospital costs. Creekmore et al retrospectively analyzed data from 10,121 adult medical patients who received VTE prophylaxis at the University of Utah Hospital in Salt Lake City from August 2000 to November 2004.19 They found that an admission during which HIT developed incurred a mean cost of $56,364, compared with $15,231 for an admission without HIT. Because LMWH was associated with a lower incidence of HIT compared with UFH (0.084% vs 0.51%, respectively), LMWH reduced the incremental cost of VTE prophylaxis by $13.88 per patient compared with UFH.
THE EXCLAIM TRIAL: IS THERE A ROLE FOR EXTENDED PROPHYLAXIS?
Although the previously discussed studies have clearly demonstrated the benefit of in-hospital VTE prophylaxis for acutely ill medical patients, none has rigorously examined extended-duration out-of-hospital prophylaxis in these patients. This represents an important gap in the literature, since a substantial proportion of VTE develops in the outpatient setting within 3 months of a hospitalization, and most outpatient VTE episodes occur within 1 month of a preceding hospitalization.20
To begin to fill this gap, the Extended Clinical Prophylaxis in Acutely Ill Medical Patients (EXCLAIM) trial was conducted to compare extended-duration LMWH prophylaxis with a standard LMWH prophylaxis regimen in acutely ill medical patients using a prospective, multicenter, randomized, double-blind, placebo-controlled design.21
Patients and study design
Patients were eligible for enrollment if they were aged 40 years or older and had recent immobilization (≤ 3 days), a predefined acute medical illness, and either level 1 mobility (total bed rest or sedentary state) or level 2 mobility (level 1 with bathroom privileges). The predefined acute medical illnesses consisted of New York Heart Association class III/IV heart failure, acute respiratory insufficiency, or other acute medical conditions, including post-acute ischemic stroke, acute infection without septic shock, and active cancer.
All patients received open-label enoxaparin 40 mg subcutaneously once daily for 10 ± 4 days, after which they were randomized to either enoxaparin 40 mg subcutaneously once daily or placebo for an additional 28 ± 4 days.
The primary efficacy end point was the incidence of VTE events, defined as asymptomatic DVT documented by mandatory ultrasonography at the end of the double-blind treatment period (28 ± 4 days) or as symptomatic DVT, symptomatic PE, or fatal PE at any time during the double-blind period. Symptomatic DVT was confirmed by objective tests; PE was confirmed by ventilation-perfusion scan, computed tomography, angiography, or autopsy.
Secondary efficacy end points were mortality at the end of the double-blind period, at 3 months, and at 6 months, as well as the incidence of VTE at 3 months.
The primary safety outcome measure was the incidence of major hemorrhage during the double-blind period; secondary safety measures were rates of major and minor hemorrhage, minor hemorrhage, HIT, and serious adverse events.
Population amended at planned interim analysis
After approximately half of the patients were enrolled, a planned and blinded interim analysis for futility concluded that the study was unlikely to show a statistically significant advantage of enoxaparin over placebo. The trial’s steering committee followed the suggestion of its data safety monitoring board to redefine the inclusion criteria to refocus enrollment on patients with a high risk of VTE. A blinded analysis was performed to identify this subgroup.
The resulting amended inclusion criteria were the same as above except that level 2 mobility had to be accompanied by at least one of three additional high-risk criteria: (1) age greater than 75 years, (2) history of prior VTE, or (3) diagnosis of cancer.
The trial’s main exclusion criteria were evidence of active bleeding, a contraindication to anticoagulation, receipt of prophylactic LMWH or UFH more than 72 hours prior to enrollment, treatment with an oral anticoagulant within 72 hours of enrollment, major surgery within the prior 3 months, cerebral stroke with bleeding, and persistent renal failure (creatinine clearance < 30 mL/min).


Results
The amended study population included 5,105 patients, 5,049 of whom received open-label enoxaparin. Of this group, 2,013 were randomized to active extended prophylaxis with enoxparain and 2,027 to placebo. Baseline characteristics, including level of mobility, were similar between the two groups.
The efficacy of extended prophylaxis with enoxaparin was enduring, as the cumulative incidence of VTE events at day 90 was significantly lower in enoxaparin recipients than in placebo recipients (3.0% vs 5.2%; relative reduction of 42%; P = .0115).
There was no difference in all-cause mortality at 6 months between the enoxaparin and placebo groups (10.1% vs 8.9%, respectively; P = .179).
Safety. Major hemorrhage was significantly more frequent in the enoxaparin arm, occurring in 0.60% of enoxaparin recipients compared with 0.15% of placebo recipients (P = .019). Minor bleeding was also more common with enoxaparin (5.20% vs 3.70%; P = .024).
Conclusions
The EXCLAIM trial found that an extended-duration (38-day) enoxaparin regimen significantly reduced the overall incidence of VTE relative to a 10-day enoxaparin regimen in acutely ill medical patients with reduced mobility. At the same time, the extended regimen was associated with a significant increase in the rate of major bleeding, although the incidence of major bleeding was low. The investigators concluded that the net clinical effect of extended-duration prophylaxis with enoxaparin is favorable, as only 46 patients would need to be treated to prevent one VTE event, whereas 224 patients would need to be treated to result in one major bleeding event.21
For this reason, it is reasonable to consider extended prophylaxis for hospitalized medical patients after identifying these patients’ risk factors. In keeping with the trial’s amended inclusion criteria, patients older than age 75 and those with cancer or prior VTE should receive special consideration for extended prophylaxis.
RECOMMENDED APPROACH TO VTE PREVENTION IN HOSPITALIZED MEDICAL PATIENTS
- All hospitalized medical patients should be screened at the time of admission, and patients at risk for VTE should receive prophylaxis.
- All patients with reduced mobility and one or more other risk factors for VTE are candidates for prophylaxis.
- Patients should be reassessed daily for the development of VTE risk factors during their hospitalization if risk factors are absent on admission.
- If screening or reassessment reveals any VTE risk factors, pharmacologic prophylaxis is the mainstay of therapy. If exclusion criteria for pharmacologic prophylaxis are present, mechanical prophylaxis with graduated compression stockings and intermittent compression devices should be used. For very high-risk medical patients without a contraindication to anticoagulants, combination prophylaxis with both an anticoagulant and mechanical devices is preferred.
- In this patient population, LMWH agents are preferred as pharmacologic prophylaxis over UFH and over fondaparinux (which is not currently approved by the US Food and Drug Administration for this population).
- If UFH is to be used in this patient population, 5,000 U three times daily is the preferred dosage.
- Extended pharmacologic prophylaxis should be considered in patients older than age 75 and in patients with a cancer diagnosis or a prior VTE episode.
DISCUSSION: ADDITIONAL PERSPECTIVES FROM THE AUTHORS
Dr. Jaffer: Dr. Spyropoulos, are there any guidelines, other than those from the ACCP, that speak to VTE prophylaxis in hospitalized medical patients? If so, what are their take-home messages and how do they differ from the ACCP guidelines?
Dr. Spyropoulos: I was part of the group that developed the International Consensus Statement (ICS) published in International Angiology in 2006,11 which is more recent than the latest ACCP guidelines, which were published in 2004. The ICS drew on much of the same data that the ACCP did, but we did an updated review of clinical trials.
For VTE prophylaxis in hospitalized medical patients, the ICS recommendations are more specific with regard to the type, dose, and dosing frequency of anticoagulant agents. First, they specify doses for both LMWH agents in this patient setting: 40 mg once daily for enoxaparin, and 5,000 IU once daily for dalteparin.
The ICS document also states that if UFH is the choice for prophylaxis, a regimen of 5,000 U three times daily should be considered. In the past year alone, two analyses suggest that three-times-daily dosing of UFH in medical patients provides superior efficacy to twice-daily dosing, although perhaps at the expense of more bleeding episodes.12,16 It is important to remember that no large placebo-controlled trial supports the efficacy of a UFH regimen of 5,000 U twice daily in this population.
Finally, the ICS document states that fondaparinux 2.5 mg once daily is a viable option for prophylaxis in medical patients, based on the ARTEMIS trial,3 even though this represents an off-label use.
Dr. Jaffer: Real-world use of VTE prophylaxis is far from optimal, especially in medical patients, and this is partly a result of system-of-care issues. I’d like to conclude by asking each of my colleagues to offer your perspectives on how your own institutions have improved their systems of care to promote better use of VTE prophylaxis and what lessons might be shared with others. Dr. McKean, you work at Brigham and Women’s Hospital, which recently reported impressive results with an electronic alert system designed to increase clinicians’ consideration of VTE risk assessment and use of prophylaxis.24 Please tell us about that study and the alert system.
Dr. McKean: Despite many educational initiatives at Brigham and Women’s Hospital, there were still some patients at high risk for VTE who were not receiving appropriate prophylaxis. What Dr. Samuel Goldhaber and his colleagues wanted to determine was whether changing the system of care could result in a reduced incidence of VTE.24 They devised a computer software program linked to the patient database that used eight common risk factors to determine each hospitalized patient’s risk profile for VTE. Each risk factor was weighted according to a point scale, with major risk factors (cancer, prior VTE, or hypercoagulability) assigned 3 points, the intermediate risk factor of surgery assigned 2 points, and minor risk factors (advanced age, obesity, immobility, or use of hormone replacement therapy or oral contraceptives) assigned 1 point. For patients with a total risk score of 4 or greater, the computer screen generates a color-coded VTE risk alert that requires the physician to acknowledge the alert and choose one of three options: order prophylaxis as appropriate, review a 60-page document on the computer to learn more about prophylaxis, or do nothing.
The study found that hospitalized patients who were randomized to treatment under the computer alert system were significantly more likely to receive VTE prophylaxis and significantly less likely to develop VTE than were patients randomized to a control group. The alert system reduced the risk of DVT or PE at 90 days by 41% in patients considered to be at high risk. It was particularly interesting that the incidence of VTE was lower in the intervention group even when physicians chose not to use prophylaxis, which suggests that simply having this alert system in place improved outcomes, perhaps by raising awareness of the risk of VTE.24
Additional studies are needed to better understand physicians’ behavior and determine why they seem to have a disproportionate fear of the risk of bleeding relative to the risk of clotting, including fatal PE, because that is really the heart of the matter. When patients are not given prophylaxis, often it is because of fear of bleeding. It is not clear, however, why some of these patients did not receive mechanical devices as an alternative form of prophylaxis, but this seems to be the case worldwide, as shown recently by the multinational ENDORSE study.22 Meanwhile, as we await studies to better understand physician perceptions and behaviors regarding prophylaxis, we need to work hard to change the system of care.
Dr. Deitelzweig: Over the past couple of years, the Ochsner Clinic has grown from a one-hospital teaching organization to a seven-hospital system with a mix of closed and open medical staff. The challenge is how to take a process that worked well in the one center, where appropriate prophylaxis was used about 90% of the time, and transfer it to the other centers in the larger system. We have endorsed several types of performance tools, such as the change-acceleration processes used by General Electric. The aim is to share a vision of heightening awareness. To do that, we have worked to mobilize the key stakeholders, at least half of them, to build algorithms that they all will endorse. It is easier said than done, however, and we have found it essential to involve both physicians and non-physician colleagues from pharmacy and nursing who have political and organizational clout.
Dr. Brotman: At Johns Hopkins, I took a bit more draconian approach to this issue because I thought that hospitalists often knew that they should be using VTE prophylaxis but sometimes weren’t, and I am not convinced that clinicians always look at prompts. So we came up with a system that incorporates both billing and documentation simultaneously. We put a hard stop on users’ documentation so that they could not sign off on a note or bill for their care until they checked off the kind of VTE prophylaxis they were using. Since hospitalists ultimately care about billing for their work, this system has at least ensured that everybody has considered and documented VTE prophylaxis on a daily basis. There are other hard stops that can be implemented in computer order-entry systems as well, and we are considering ways to roll them out on a broader scale.
However, all of these systems can have problems because patient situations change from day to day. For instance, VTE prophylaxis is not necessarily indicated in a 38-year-old ambulatory patient who comes in with a sickle cell crisis, but you will need to reconsider if the patient ends up in acute chest syndrome in the intensive care unit. I do not yet have a good way to ensure that this is being done on a daily basis with all patients.
Dr. Amin: At the University of California, Irvine, we implemented an electronic alert system, but we locked users in so that they could not complete their admission orders until they answered questions about VTE prevention. This practice increased our VTE prophylaxis rates tremendously. Because we are a level I trauma center, we allow users to bypass the screens one time, but the next time they log in, even to get a simple lab result, they have to answer the questions about VTE prevention.
With any system you develop, you also have to continue with the education process, because clinicians sometimes get into bad habits or simply forget things.
Dr. Spyropolous: At Lovelace Medical Center, we didn’t have the sophistication of an electronic order-entry system, but we had an experienced clinical pharmacist (the director of inpatient pharmacy) who helped to develop and champion VTE prevention guidelines that have then been used throughout the system in close conjunction with our hospitalists’ rounds. This system has been used successfully for the past 7 years.
- Samama MM, Cohen AT, Darmon JY, et al. A comparison of enoxaparin with placebo for the prevention of venous thrombo-embolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med 1999; 341:793–800.
- Leizorovicz A, Cohen AT, Turpie AG, et al. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation 2004; 110:874–879.
- Cohen AT, Davidson BL, Gallus AS, et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ 2006; 332:325–329.
- Baglin TP, White K, Charles A. Fatal pulmonary embolism in hos-pitalised medical patients. J Clin Pathol 1997; 50:609–610.
- Piazza G, Seddighzadeh A, Goldhaber SZ. Double trouble for 2,609 hospitalized medical patients who developed deep vein thrombosis: prophylaxis omitted more often and pulmonary embolism more frequent. Chest 2007; 132:554–561.
- Amin A, Stemkowski S, Lin J, Yang G. Thromboprophylaxis rates in US medical centers: success or failure? J Thromb Haemost 2007; 5:1610–1616.
- Geerts WH, Heit JA, Clagett GP, et al. Prevention of venous thromboembolism. Chest 2001; 119(1 Suppl):132S–175S.
- Tapson VF, Decousus H, Pini M, et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest 2007; 132:936–945.
- Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thrombo-embolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126(3 Suppl):338S–400S.
- Dentali F, Douketis JD, Gianni M, Lim W, Crowther MA. Meta-analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med 2007; 146:278–288.
- Cardiovascular Disease Educational and Research Trust; Cyprus Cardiovascular Disease Educational and Research Trust; European Venous Forum; International Surgical Thrombosis Forum; International Union of Angiology; Union Internationale de Phlébologie. Prevention and treatment of venous thromboembolism. International Consensus Statement (guidelines according to scientific evidence). Int Angiol 2006; 25:101–161.
- Wein L, Wein S, Haas SJ, Shaw J, Krum H. Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta-analysis of randomized controlled trials. Arch Intern Med 2007; 167:1476–1486.
- Mismetti P, Laporte-Simitsidis S, Tardy B, et al. Prevention of venous thromboembolism in internal medicine with unfractionated or low-molecular-weight heparins: a meta-analysis of randomised clinical trials. Thromb Haemost 2000; 83:14–19.
- Lechler E, Schramm W, Flosbach CW. The venous thrombotic risk in non-surgical patients: epidemiological data and efficacy/safety profile of a low-molecular-weight heparin (enoxaparin). The Prime Study Group. Haemostasis 1996; 26(Suppl 2):49–56.
- Kleber FX, Witt C, Vogel G, et al; the PRINCE Study Group. Randomized comparison of enoxaparin with unfractionated heparin for the prevention of venous thromboembolism in medical patients with heart failure or severe respiratory disease. Am Heart J 2003; 145:614–621.
- King CS, Holley AB, Jackson JL, Shorr AF, Moores LK. Twice vs three times daily heparin dosing for thromboembolism prophylaxis in the general medical population: a meta-analysis. Chest 2007; 131:507–516.
- Burleigh E, Wang C, Foster D, et al. Thromboprophylaxis in medically ill patients at risk for venous thromboembolism. Am J Health Syst Pharm 2006; 63(20 Suppl 6):S23–S29.
- Leykum L, Pugh J, Diuguid D, Papadopoulos K. Cost utility of substituting enoxaparin for unfractionated heparin for prophylaxis of venous thrombosis in the hospitalized medical patient. J Hosp Med 2006; 1:168–176.
- Creekmore FM, Oderda GM, Pendleton RC, Brixner DI. Incidence and economic implications of heparin-induced thrombocytopenia in medical patients receiving prophylaxis for venous thromboembolism. Pharmacotherapy 2006; 26:1438–1445.
- Spencer FA, Lessard D, Emery C, Reed G, Goldberg RJ. Venous thromboembolism in the outpatient setting. Arch Intern Med 2007; 167:1471–1475.
- Hull RD, Schellong SM Tapson VF, et al. Extended-duration venous thromboembolism (VTE) prophylaxis in acutely ill medical patients with recent reduced mobility: the EXCLAIM study. Presentation at: International Society on Thrombosis and Haemo-stasis XXIst Congress; July 6–12, 2007; Geneva, Switzerland.
- Cohen AT, Tapson VF, Bergmann JF, et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet 2008; 371:387–394.
- Kahn SR, Panju A, Geerts W, et al; CURVE study investigators. Multicenter evaluation of the use of venous thromboembolism prophylaxis in acutely ill medical patients in Canada. Thromb Res 2007; 119:145–155.
- Kucher N, Koo S, Quiroz R, et al. Electronic alerts to prevent thromboembolism among hospitalized patients. N Engl J Med 2005; 352:969–977.
- Anderson FA Jr, Wheeler HB, Goldberg RJ, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med 1991; 151:933–938.
- Howell MD, Geraci JM, Knowlton AA. Congestive heart failure and outpatient risk of venous thromboembolism: a retrospective, case-control study. J Clin Epidemiol 2001; 54:810–816.
- Smeeth L, Cook C, Thomas S, Hall AJ, Hubbard R, Vallance P. Risk of deep vein thrombosis and pulmonary embolism after acute infection in a community setting. Lancet 2006; 367:1075–1079.
- Alikhan R, Cohen AT, Combe S, et al. Prevention of venous thromboembolism in medical patients with enoxaparin: a subgroup analysis of the MEDENOX study. Blood Coagul Firbinolysis 2003; 14:341–346.
- Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation 2003; 107(23 Suppl 1):I9–I16.
- Greinacher A, Warkentin TE. Recognition, treatment, and prevention of heparin-induced thrombocytopenia: review and update. Thromb Res 2006; 118:165–176.
- Martel N, Lee J, Wells PS. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood 2005; 106:2710–2715.
- Sanderink GJ, Guimart CG, Ozoux ML, Jariwala NU, Shukla UA, Boutouyrie BX. Pharmacokinetics and pharmacodynamics of the prophylactic dose of enoxaparin once daily over 4 days in patients with renal impairment. Thromb Res 2002; 105:225–231.
- Douketis J, Cook D, Zytaruk N, et al. Dalteparin thromboprophylaxis in critically ill patients with severe renal insufficiency: the DIRECT study [abstract]. J Thromb Haemost 2007; 5(Suppl 2): P-S-680.
- Scholten DJ, Hoedema RM, Scholten SE. A comparison of two different prophylactic dose regimens of low molecular weight heparin in bariatric surgery. Obes Surg 2002; 12:19–24.
The need for prophylaxis of venous thromboembolism (VTE) in hospitalized acutely ill medical patients is well established. Without prophylaxis, hospitalized medical patients develop VTE at a rate of 5% to 15%.1–3 Moreover, pulmonary embolism (PE) occurs more frequently in hospitalized medical patients than in nonmedical patients, and is a leading cause of sudden death in hospitalized medical patients.4,5 Without appropriate prophylaxis, 1 in 20 hospitalized medical patients may suffer a fatal PE.4
PROPHYLAXIS IN MEDICAL PATIENTS: UNDERUSED AND OFTEN INAPPROPRIATE
Despite these risks and the clear indications for VTE prophylaxis in hospitalized medical patients, prophylaxis of VTE is omitted more often in these patients than in hospitalized surgical patients.5 Even when prophylaxis is given, it is often used inappropriately in the medical population. So concludes a recent analysis of data from 196,104 patients with acute medical conditions who were discharged from 227 US hospitals from January 2002 to September 2005.6 Criteria for inclusion in the analysis were patient age of 40 years or older, a hospital stay of 6 days or greater, and an absence of contraindications to anticoagulation. Appropriate prophylaxis was defined in accordance with the Sixth American College of Chest Physicians (ACCP) Consensus Conference on Antithrombotic Therapy.7
The analysis revealed an overall VTE prophylaxis rate of 61.8%, but the rate of appropriate prophylaxis was only 33.9%, meaning that two-thirds of discharged patients did not receive prophylaxis in accordance with ACCP guidelines. When temporal trends were analyzed according to groups based on patients’ diagnosis at admission (acute myocardial infarction, severe lung disease, ischemic stroke, cancer, heart failure, or trauma), the rate of appropriate prophylaxis remained essentially flat from the beginning to the end of the study period for virtually all diagnosis groups.6
Similar findings have emerged from the International Medical Prevention Registry on Venous Thromboembolism (IMPROVE), an ongoing international registry of acutely ill medical patients.8 Data from the first 15,156 patients, enrolled from July 2002 through September 2006, reveal that 50% of patients received pharmacologic and/or mechanical VTE prophylaxis in the hospital, and only 60% of patients who met established criteria for VTE prophylaxis actually received it.
Analysis of the US portion of the IMPROVE data shows that 54% of the US patient sample received some form of VTE prophylaxis; 22% of US patients received intermittent pneumatic compression, 21% received unfractionated heparin (UFH), 14% received low-molecular-weight heparin (LMWH), and 3% wore compression stockings.8 Thus, despite a paucity of data supporting a benefit of intermittent pneumatic compression in this population,9 it was the most frequently used form of prophylaxis in US patients.
CLINICAL TRIALS OF PHARMACOLOGIC PROPHYLAXIS IN MEDICAL PATIENTS
The Prophylaxis in Medical Patients with Enoxaparin (MEDENOX) trial1 randomized 1,102 hospitalized patients to one of two doses of the LMWH enoxaparin (20 mg or 40 mg once daily subcutaneously) or placebo for 6 to 14 days. Compared with placebo, the 40-mg dose of enoxaparin was associated with a 63% reduction in risk of VTE over 3 months of follow-up (P < .001) (Figure 1).
The Prospective Evaluation of Dalteparin Efficacy for Prevention of VTE in Immobilized Patients Trial (PREVENT)2 was a multicenter, randomized, double-blind study comparing the LMWH dalteparin (5,000 IU daily given subcutaneously for 14 days) with placebo in 3,706 acutely ill medical patients. Over 90 days of follow-up, the risk of VTE was reduced by 44% in patients assigned to dalteparin compared with those assigned to placebo (P = .0015) (Figure 1).
The Arixtra for Thromboembolism Prevention in a Medical Indications Study (ARTEMIS)3 randomized 849 medical patients 60 years or older to 6 to 14 days of therapy with the selective factor Xa inhibitor fondaparinux (2.5 mg once daily subcutaneously) or placebo. Compared with the placebo group, fondaparinux recipients had a 47% lower risk of developing VTE by day 15 (P = .029) (Figure 1).
Fewer events and fatal PEs, but no effect on all-cause mortality
A recent meta-analysis by Dentali et al10 further demonstrates the efficacy of anticoagulant therapy for preventing symptomatic VTE in hospitalized medical patients. This analysis included several other trials in addition to the three reviewed above,1–3 for a total of nine randomized studies (seven of which were dou-ble-blind) comprising 19,958 patients. Across the nine studies, anticoagulant prophylaxis was clearly superior to placebo in preventing fatal PE (relative risk, 0.38 [95% CI, 0.21 to 0.69]). There was a strong trend toward a reduction in symptomatic deep vein thrombosis (DVT) with prophylaxis but no effect on all-cause mortality. The meta-analysis also provided reassurance that prophylaxis does not increase the rate of major bleeding.
HOW DO THE PROPHYLAXIS OPTIONS STACK UP?
What the ACCP recommends
Current ACCP guidelines recommend the use of either LMWH or low-dose UFH (5,000 U subcutaneously two or three times daily) as a Grade 1A recommendation for VTE prophylaxis in patients with medical conditions and risk factors for VTE.9 This represents the guidelines’ highest level of recommendation, ie, one that is based on randomized controlled trials (RCTs) without important limitations. In contrast, the 2006 International Consensus Statement, developed as a collaborative effort among expert bodies on VTE, specified a more narrow dosing recommendation for UFH in this patient population (5,000 U three times daily, not twice daily) as well as specifying 40 mg once daily as the recommended dose of enoxaparin and 5,000 IU once daily as the recommended dose of dalteparin.11
For medical patients with risk factors for VTE who have a contraindication to anticoagulant prophylaxis, the ACCP guidelines recommend the use of graduated compression stockings or intermittent pneumatic compression devices as a Grade 1C+ recommendation (“no RCTs but strong RCT results can be unequivocally extrapolated, or overwhelming evidence from observational studies”9).
Current ACCP guidelines do not address the use of fondaparinux in their recommendations for VTE prophylaxis in medical patients.
Getting a handle on bleeding risk
Patient characteristics that exclude pharmacologic thromboprophylaxis due to bleeding risk are generally limited to active bleeding or coagulopathy, as demonstrated by a platelet count less than 50,000 cells/µL or an international normalized ratio greater than 1.5. Additionally, bleeding risk should be carefully assessed if an invasive procedure is planned during a patient’s hospital stay.
It is worth noting that the anticoagulant doses used for VTE prophylaxis are a fraction of those used for treatment of VTE. Thus, if a patient would be treated with full-dose anticoagulation if VTE developed, then that patient should be eligible for VTE prophylaxis.
Because the use of mechanical forms of prophylaxis in medical patients is not truly evidence-based, mechanical prophylaxis should be reserved for medical patients who have a contraindication to anticoagulants, or for use in combination with anticoagulants in patients at very high risk of VTE.
UFH vs LMWH
Two meta-analyses have compared UFH with LMWH for VTE prevention in medical patients.12,13 In a recent analysis that included 10 trials directly comparing the two therapies, 14 trials comparing UFH with control, and 11 trials comparing LMWH with control, Wein et al found a lower risk of DVT with LMWH than with UFH (relative risk, 0.68 [95% CI, 0.52 to 0.88]) but no difference between the therapies in mortality or bleeding risk.12 In an earlier and smaller analysis, Mismetti et al found no significant differences between UFH and LMWH in preventing DVT or death but did find a significant reduction in major bleeding episodes with LMWH versus three-times-daily UFH (52% relative reduction; P = .049).13
Randomized trials also reveal that enoxaparin 40 mg once daily is as efficacious as UFH 5,000 U three times daily for VTE prevention in medical patients.14,15 The above analysis by Wein et al12 and an additional meta-analysis by King and colleagues16 found that three-times-daily dosing of UFH is more efficacious than twice-daily dosing of UFH, but at the expense of more bleeding, including major bleeding.
Economic considerations
Because of differences in drug acquisition costs between UFH and the LMWH agents, several economic evaluations have compared the use of these therapies for prophylaxis in medical patients at risk of VTE.
In an analysis of hospital costs for medical patients receiving VTE prophylaxis from more than 330 US hospitals for the period 2001–2004, Burleigh et al found that mean total hospital costs were higher for patients who received UFH than for those who received LMWH ($7,615 vs $6,866) even though mean drug costs were higher for LMWH ($791 vs $569 for UFH).17 A reduction in hospital length of stay appeared to contribute to the overall savings with LMWH; other contributors may have included costs associated with heparin-induced thrombocytopenia (HIT) in UFH recipients or the extra nursing time required for administering UFH in two or three daily doses.
Leykum et al used a decision analysis model to estimate the economic effect of substituting enoxaparin for UFH in hospitalized medical patients for whom VTE prophylaxis is indicated.18 Cost data were based on Medicare reimbursement rates as well as drug and laboratory costs for a multi-institutional health system. The model assumed HIT incidence rates of 2.7% with UFH and 0.3% with enoxaparin. It also assumed the cost of a daily dose to be $4 for UFH versus $84 for enoxaparin. From the payer perspective, the model showed that substituting enoxaparin for UFH would reduce the overall cost of care by $28.61 per day on a per-patient basis, despite enoxaparin’s higher acquisition cost, and would save $4,550 per quality-adjusted life-year by reducing the incidence of HIT.
Another cost analysis confirms the association between HIT and increased hospital costs. Creekmore et al retrospectively analyzed data from 10,121 adult medical patients who received VTE prophylaxis at the University of Utah Hospital in Salt Lake City from August 2000 to November 2004.19 They found that an admission during which HIT developed incurred a mean cost of $56,364, compared with $15,231 for an admission without HIT. Because LMWH was associated with a lower incidence of HIT compared with UFH (0.084% vs 0.51%, respectively), LMWH reduced the incremental cost of VTE prophylaxis by $13.88 per patient compared with UFH.
THE EXCLAIM TRIAL: IS THERE A ROLE FOR EXTENDED PROPHYLAXIS?
Although the previously discussed studies have clearly demonstrated the benefit of in-hospital VTE prophylaxis for acutely ill medical patients, none has rigorously examined extended-duration out-of-hospital prophylaxis in these patients. This represents an important gap in the literature, since a substantial proportion of VTE develops in the outpatient setting within 3 months of a hospitalization, and most outpatient VTE episodes occur within 1 month of a preceding hospitalization.20
To begin to fill this gap, the Extended Clinical Prophylaxis in Acutely Ill Medical Patients (EXCLAIM) trial was conducted to compare extended-duration LMWH prophylaxis with a standard LMWH prophylaxis regimen in acutely ill medical patients using a prospective, multicenter, randomized, double-blind, placebo-controlled design.21
Patients and study design
Patients were eligible for enrollment if they were aged 40 years or older and had recent immobilization (≤ 3 days), a predefined acute medical illness, and either level 1 mobility (total bed rest or sedentary state) or level 2 mobility (level 1 with bathroom privileges). The predefined acute medical illnesses consisted of New York Heart Association class III/IV heart failure, acute respiratory insufficiency, or other acute medical conditions, including post-acute ischemic stroke, acute infection without septic shock, and active cancer.
All patients received open-label enoxaparin 40 mg subcutaneously once daily for 10 ± 4 days, after which they were randomized to either enoxaparin 40 mg subcutaneously once daily or placebo for an additional 28 ± 4 days.
The primary efficacy end point was the incidence of VTE events, defined as asymptomatic DVT documented by mandatory ultrasonography at the end of the double-blind treatment period (28 ± 4 days) or as symptomatic DVT, symptomatic PE, or fatal PE at any time during the double-blind period. Symptomatic DVT was confirmed by objective tests; PE was confirmed by ventilation-perfusion scan, computed tomography, angiography, or autopsy.
Secondary efficacy end points were mortality at the end of the double-blind period, at 3 months, and at 6 months, as well as the incidence of VTE at 3 months.
The primary safety outcome measure was the incidence of major hemorrhage during the double-blind period; secondary safety measures were rates of major and minor hemorrhage, minor hemorrhage, HIT, and serious adverse events.
Population amended at planned interim analysis
After approximately half of the patients were enrolled, a planned and blinded interim analysis for futility concluded that the study was unlikely to show a statistically significant advantage of enoxaparin over placebo. The trial’s steering committee followed the suggestion of its data safety monitoring board to redefine the inclusion criteria to refocus enrollment on patients with a high risk of VTE. A blinded analysis was performed to identify this subgroup.
The resulting amended inclusion criteria were the same as above except that level 2 mobility had to be accompanied by at least one of three additional high-risk criteria: (1) age greater than 75 years, (2) history of prior VTE, or (3) diagnosis of cancer.
The trial’s main exclusion criteria were evidence of active bleeding, a contraindication to anticoagulation, receipt of prophylactic LMWH or UFH more than 72 hours prior to enrollment, treatment with an oral anticoagulant within 72 hours of enrollment, major surgery within the prior 3 months, cerebral stroke with bleeding, and persistent renal failure (creatinine clearance < 30 mL/min).


Results
The amended study population included 5,105 patients, 5,049 of whom received open-label enoxaparin. Of this group, 2,013 were randomized to active extended prophylaxis with enoxparain and 2,027 to placebo. Baseline characteristics, including level of mobility, were similar between the two groups.
The efficacy of extended prophylaxis with enoxaparin was enduring, as the cumulative incidence of VTE events at day 90 was significantly lower in enoxaparin recipients than in placebo recipients (3.0% vs 5.2%; relative reduction of 42%; P = .0115).
There was no difference in all-cause mortality at 6 months between the enoxaparin and placebo groups (10.1% vs 8.9%, respectively; P = .179).
Safety. Major hemorrhage was significantly more frequent in the enoxaparin arm, occurring in 0.60% of enoxaparin recipients compared with 0.15% of placebo recipients (P = .019). Minor bleeding was also more common with enoxaparin (5.20% vs 3.70%; P = .024).
Conclusions
The EXCLAIM trial found that an extended-duration (38-day) enoxaparin regimen significantly reduced the overall incidence of VTE relative to a 10-day enoxaparin regimen in acutely ill medical patients with reduced mobility. At the same time, the extended regimen was associated with a significant increase in the rate of major bleeding, although the incidence of major bleeding was low. The investigators concluded that the net clinical effect of extended-duration prophylaxis with enoxaparin is favorable, as only 46 patients would need to be treated to prevent one VTE event, whereas 224 patients would need to be treated to result in one major bleeding event.21
For this reason, it is reasonable to consider extended prophylaxis for hospitalized medical patients after identifying these patients’ risk factors. In keeping with the trial’s amended inclusion criteria, patients older than age 75 and those with cancer or prior VTE should receive special consideration for extended prophylaxis.
RECOMMENDED APPROACH TO VTE PREVENTION IN HOSPITALIZED MEDICAL PATIENTS
- All hospitalized medical patients should be screened at the time of admission, and patients at risk for VTE should receive prophylaxis.
- All patients with reduced mobility and one or more other risk factors for VTE are candidates for prophylaxis.
- Patients should be reassessed daily for the development of VTE risk factors during their hospitalization if risk factors are absent on admission.
- If screening or reassessment reveals any VTE risk factors, pharmacologic prophylaxis is the mainstay of therapy. If exclusion criteria for pharmacologic prophylaxis are present, mechanical prophylaxis with graduated compression stockings and intermittent compression devices should be used. For very high-risk medical patients without a contraindication to anticoagulants, combination prophylaxis with both an anticoagulant and mechanical devices is preferred.
- In this patient population, LMWH agents are preferred as pharmacologic prophylaxis over UFH and over fondaparinux (which is not currently approved by the US Food and Drug Administration for this population).
- If UFH is to be used in this patient population, 5,000 U three times daily is the preferred dosage.
- Extended pharmacologic prophylaxis should be considered in patients older than age 75 and in patients with a cancer diagnosis or a prior VTE episode.
DISCUSSION: ADDITIONAL PERSPECTIVES FROM THE AUTHORS
Dr. Jaffer: Dr. Spyropoulos, are there any guidelines, other than those from the ACCP, that speak to VTE prophylaxis in hospitalized medical patients? If so, what are their take-home messages and how do they differ from the ACCP guidelines?
Dr. Spyropoulos: I was part of the group that developed the International Consensus Statement (ICS) published in International Angiology in 2006,11 which is more recent than the latest ACCP guidelines, which were published in 2004. The ICS drew on much of the same data that the ACCP did, but we did an updated review of clinical trials.
For VTE prophylaxis in hospitalized medical patients, the ICS recommendations are more specific with regard to the type, dose, and dosing frequency of anticoagulant agents. First, they specify doses for both LMWH agents in this patient setting: 40 mg once daily for enoxaparin, and 5,000 IU once daily for dalteparin.
The ICS document also states that if UFH is the choice for prophylaxis, a regimen of 5,000 U three times daily should be considered. In the past year alone, two analyses suggest that three-times-daily dosing of UFH in medical patients provides superior efficacy to twice-daily dosing, although perhaps at the expense of more bleeding episodes.12,16 It is important to remember that no large placebo-controlled trial supports the efficacy of a UFH regimen of 5,000 U twice daily in this population.
Finally, the ICS document states that fondaparinux 2.5 mg once daily is a viable option for prophylaxis in medical patients, based on the ARTEMIS trial,3 even though this represents an off-label use.
Dr. Jaffer: Real-world use of VTE prophylaxis is far from optimal, especially in medical patients, and this is partly a result of system-of-care issues. I’d like to conclude by asking each of my colleagues to offer your perspectives on how your own institutions have improved their systems of care to promote better use of VTE prophylaxis and what lessons might be shared with others. Dr. McKean, you work at Brigham and Women’s Hospital, which recently reported impressive results with an electronic alert system designed to increase clinicians’ consideration of VTE risk assessment and use of prophylaxis.24 Please tell us about that study and the alert system.
Dr. McKean: Despite many educational initiatives at Brigham and Women’s Hospital, there were still some patients at high risk for VTE who were not receiving appropriate prophylaxis. What Dr. Samuel Goldhaber and his colleagues wanted to determine was whether changing the system of care could result in a reduced incidence of VTE.24 They devised a computer software program linked to the patient database that used eight common risk factors to determine each hospitalized patient’s risk profile for VTE. Each risk factor was weighted according to a point scale, with major risk factors (cancer, prior VTE, or hypercoagulability) assigned 3 points, the intermediate risk factor of surgery assigned 2 points, and minor risk factors (advanced age, obesity, immobility, or use of hormone replacement therapy or oral contraceptives) assigned 1 point. For patients with a total risk score of 4 or greater, the computer screen generates a color-coded VTE risk alert that requires the physician to acknowledge the alert and choose one of three options: order prophylaxis as appropriate, review a 60-page document on the computer to learn more about prophylaxis, or do nothing.
The study found that hospitalized patients who were randomized to treatment under the computer alert system were significantly more likely to receive VTE prophylaxis and significantly less likely to develop VTE than were patients randomized to a control group. The alert system reduced the risk of DVT or PE at 90 days by 41% in patients considered to be at high risk. It was particularly interesting that the incidence of VTE was lower in the intervention group even when physicians chose not to use prophylaxis, which suggests that simply having this alert system in place improved outcomes, perhaps by raising awareness of the risk of VTE.24
Additional studies are needed to better understand physicians’ behavior and determine why they seem to have a disproportionate fear of the risk of bleeding relative to the risk of clotting, including fatal PE, because that is really the heart of the matter. When patients are not given prophylaxis, often it is because of fear of bleeding. It is not clear, however, why some of these patients did not receive mechanical devices as an alternative form of prophylaxis, but this seems to be the case worldwide, as shown recently by the multinational ENDORSE study.22 Meanwhile, as we await studies to better understand physician perceptions and behaviors regarding prophylaxis, we need to work hard to change the system of care.
Dr. Deitelzweig: Over the past couple of years, the Ochsner Clinic has grown from a one-hospital teaching organization to a seven-hospital system with a mix of closed and open medical staff. The challenge is how to take a process that worked well in the one center, where appropriate prophylaxis was used about 90% of the time, and transfer it to the other centers in the larger system. We have endorsed several types of performance tools, such as the change-acceleration processes used by General Electric. The aim is to share a vision of heightening awareness. To do that, we have worked to mobilize the key stakeholders, at least half of them, to build algorithms that they all will endorse. It is easier said than done, however, and we have found it essential to involve both physicians and non-physician colleagues from pharmacy and nursing who have political and organizational clout.
Dr. Brotman: At Johns Hopkins, I took a bit more draconian approach to this issue because I thought that hospitalists often knew that they should be using VTE prophylaxis but sometimes weren’t, and I am not convinced that clinicians always look at prompts. So we came up with a system that incorporates both billing and documentation simultaneously. We put a hard stop on users’ documentation so that they could not sign off on a note or bill for their care until they checked off the kind of VTE prophylaxis they were using. Since hospitalists ultimately care about billing for their work, this system has at least ensured that everybody has considered and documented VTE prophylaxis on a daily basis. There are other hard stops that can be implemented in computer order-entry systems as well, and we are considering ways to roll them out on a broader scale.
However, all of these systems can have problems because patient situations change from day to day. For instance, VTE prophylaxis is not necessarily indicated in a 38-year-old ambulatory patient who comes in with a sickle cell crisis, but you will need to reconsider if the patient ends up in acute chest syndrome in the intensive care unit. I do not yet have a good way to ensure that this is being done on a daily basis with all patients.
Dr. Amin: At the University of California, Irvine, we implemented an electronic alert system, but we locked users in so that they could not complete their admission orders until they answered questions about VTE prevention. This practice increased our VTE prophylaxis rates tremendously. Because we are a level I trauma center, we allow users to bypass the screens one time, but the next time they log in, even to get a simple lab result, they have to answer the questions about VTE prevention.
With any system you develop, you also have to continue with the education process, because clinicians sometimes get into bad habits or simply forget things.
Dr. Spyropolous: At Lovelace Medical Center, we didn’t have the sophistication of an electronic order-entry system, but we had an experienced clinical pharmacist (the director of inpatient pharmacy) who helped to develop and champion VTE prevention guidelines that have then been used throughout the system in close conjunction with our hospitalists’ rounds. This system has been used successfully for the past 7 years.
The need for prophylaxis of venous thromboembolism (VTE) in hospitalized acutely ill medical patients is well established. Without prophylaxis, hospitalized medical patients develop VTE at a rate of 5% to 15%.1–3 Moreover, pulmonary embolism (PE) occurs more frequently in hospitalized medical patients than in nonmedical patients, and is a leading cause of sudden death in hospitalized medical patients.4,5 Without appropriate prophylaxis, 1 in 20 hospitalized medical patients may suffer a fatal PE.4
PROPHYLAXIS IN MEDICAL PATIENTS: UNDERUSED AND OFTEN INAPPROPRIATE
Despite these risks and the clear indications for VTE prophylaxis in hospitalized medical patients, prophylaxis of VTE is omitted more often in these patients than in hospitalized surgical patients.5 Even when prophylaxis is given, it is often used inappropriately in the medical population. So concludes a recent analysis of data from 196,104 patients with acute medical conditions who were discharged from 227 US hospitals from January 2002 to September 2005.6 Criteria for inclusion in the analysis were patient age of 40 years or older, a hospital stay of 6 days or greater, and an absence of contraindications to anticoagulation. Appropriate prophylaxis was defined in accordance with the Sixth American College of Chest Physicians (ACCP) Consensus Conference on Antithrombotic Therapy.7
The analysis revealed an overall VTE prophylaxis rate of 61.8%, but the rate of appropriate prophylaxis was only 33.9%, meaning that two-thirds of discharged patients did not receive prophylaxis in accordance with ACCP guidelines. When temporal trends were analyzed according to groups based on patients’ diagnosis at admission (acute myocardial infarction, severe lung disease, ischemic stroke, cancer, heart failure, or trauma), the rate of appropriate prophylaxis remained essentially flat from the beginning to the end of the study period for virtually all diagnosis groups.6
Similar findings have emerged from the International Medical Prevention Registry on Venous Thromboembolism (IMPROVE), an ongoing international registry of acutely ill medical patients.8 Data from the first 15,156 patients, enrolled from July 2002 through September 2006, reveal that 50% of patients received pharmacologic and/or mechanical VTE prophylaxis in the hospital, and only 60% of patients who met established criteria for VTE prophylaxis actually received it.
Analysis of the US portion of the IMPROVE data shows that 54% of the US patient sample received some form of VTE prophylaxis; 22% of US patients received intermittent pneumatic compression, 21% received unfractionated heparin (UFH), 14% received low-molecular-weight heparin (LMWH), and 3% wore compression stockings.8 Thus, despite a paucity of data supporting a benefit of intermittent pneumatic compression in this population,9 it was the most frequently used form of prophylaxis in US patients.
CLINICAL TRIALS OF PHARMACOLOGIC PROPHYLAXIS IN MEDICAL PATIENTS
The Prophylaxis in Medical Patients with Enoxaparin (MEDENOX) trial1 randomized 1,102 hospitalized patients to one of two doses of the LMWH enoxaparin (20 mg or 40 mg once daily subcutaneously) or placebo for 6 to 14 days. Compared with placebo, the 40-mg dose of enoxaparin was associated with a 63% reduction in risk of VTE over 3 months of follow-up (P < .001) (Figure 1).
The Prospective Evaluation of Dalteparin Efficacy for Prevention of VTE in Immobilized Patients Trial (PREVENT)2 was a multicenter, randomized, double-blind study comparing the LMWH dalteparin (5,000 IU daily given subcutaneously for 14 days) with placebo in 3,706 acutely ill medical patients. Over 90 days of follow-up, the risk of VTE was reduced by 44% in patients assigned to dalteparin compared with those assigned to placebo (P = .0015) (Figure 1).
The Arixtra for Thromboembolism Prevention in a Medical Indications Study (ARTEMIS)3 randomized 849 medical patients 60 years or older to 6 to 14 days of therapy with the selective factor Xa inhibitor fondaparinux (2.5 mg once daily subcutaneously) or placebo. Compared with the placebo group, fondaparinux recipients had a 47% lower risk of developing VTE by day 15 (P = .029) (Figure 1).
Fewer events and fatal PEs, but no effect on all-cause mortality
A recent meta-analysis by Dentali et al10 further demonstrates the efficacy of anticoagulant therapy for preventing symptomatic VTE in hospitalized medical patients. This analysis included several other trials in addition to the three reviewed above,1–3 for a total of nine randomized studies (seven of which were dou-ble-blind) comprising 19,958 patients. Across the nine studies, anticoagulant prophylaxis was clearly superior to placebo in preventing fatal PE (relative risk, 0.38 [95% CI, 0.21 to 0.69]). There was a strong trend toward a reduction in symptomatic deep vein thrombosis (DVT) with prophylaxis but no effect on all-cause mortality. The meta-analysis also provided reassurance that prophylaxis does not increase the rate of major bleeding.
HOW DO THE PROPHYLAXIS OPTIONS STACK UP?
What the ACCP recommends
Current ACCP guidelines recommend the use of either LMWH or low-dose UFH (5,000 U subcutaneously two or three times daily) as a Grade 1A recommendation for VTE prophylaxis in patients with medical conditions and risk factors for VTE.9 This represents the guidelines’ highest level of recommendation, ie, one that is based on randomized controlled trials (RCTs) without important limitations. In contrast, the 2006 International Consensus Statement, developed as a collaborative effort among expert bodies on VTE, specified a more narrow dosing recommendation for UFH in this patient population (5,000 U three times daily, not twice daily) as well as specifying 40 mg once daily as the recommended dose of enoxaparin and 5,000 IU once daily as the recommended dose of dalteparin.11
For medical patients with risk factors for VTE who have a contraindication to anticoagulant prophylaxis, the ACCP guidelines recommend the use of graduated compression stockings or intermittent pneumatic compression devices as a Grade 1C+ recommendation (“no RCTs but strong RCT results can be unequivocally extrapolated, or overwhelming evidence from observational studies”9).
Current ACCP guidelines do not address the use of fondaparinux in their recommendations for VTE prophylaxis in medical patients.
Getting a handle on bleeding risk
Patient characteristics that exclude pharmacologic thromboprophylaxis due to bleeding risk are generally limited to active bleeding or coagulopathy, as demonstrated by a platelet count less than 50,000 cells/µL or an international normalized ratio greater than 1.5. Additionally, bleeding risk should be carefully assessed if an invasive procedure is planned during a patient’s hospital stay.
It is worth noting that the anticoagulant doses used for VTE prophylaxis are a fraction of those used for treatment of VTE. Thus, if a patient would be treated with full-dose anticoagulation if VTE developed, then that patient should be eligible for VTE prophylaxis.
Because the use of mechanical forms of prophylaxis in medical patients is not truly evidence-based, mechanical prophylaxis should be reserved for medical patients who have a contraindication to anticoagulants, or for use in combination with anticoagulants in patients at very high risk of VTE.
UFH vs LMWH
Two meta-analyses have compared UFH with LMWH for VTE prevention in medical patients.12,13 In a recent analysis that included 10 trials directly comparing the two therapies, 14 trials comparing UFH with control, and 11 trials comparing LMWH with control, Wein et al found a lower risk of DVT with LMWH than with UFH (relative risk, 0.68 [95% CI, 0.52 to 0.88]) but no difference between the therapies in mortality or bleeding risk.12 In an earlier and smaller analysis, Mismetti et al found no significant differences between UFH and LMWH in preventing DVT or death but did find a significant reduction in major bleeding episodes with LMWH versus three-times-daily UFH (52% relative reduction; P = .049).13
Randomized trials also reveal that enoxaparin 40 mg once daily is as efficacious as UFH 5,000 U three times daily for VTE prevention in medical patients.14,15 The above analysis by Wein et al12 and an additional meta-analysis by King and colleagues16 found that three-times-daily dosing of UFH is more efficacious than twice-daily dosing of UFH, but at the expense of more bleeding, including major bleeding.
Economic considerations
Because of differences in drug acquisition costs between UFH and the LMWH agents, several economic evaluations have compared the use of these therapies for prophylaxis in medical patients at risk of VTE.
In an analysis of hospital costs for medical patients receiving VTE prophylaxis from more than 330 US hospitals for the period 2001–2004, Burleigh et al found that mean total hospital costs were higher for patients who received UFH than for those who received LMWH ($7,615 vs $6,866) even though mean drug costs were higher for LMWH ($791 vs $569 for UFH).17 A reduction in hospital length of stay appeared to contribute to the overall savings with LMWH; other contributors may have included costs associated with heparin-induced thrombocytopenia (HIT) in UFH recipients or the extra nursing time required for administering UFH in two or three daily doses.
Leykum et al used a decision analysis model to estimate the economic effect of substituting enoxaparin for UFH in hospitalized medical patients for whom VTE prophylaxis is indicated.18 Cost data were based on Medicare reimbursement rates as well as drug and laboratory costs for a multi-institutional health system. The model assumed HIT incidence rates of 2.7% with UFH and 0.3% with enoxaparin. It also assumed the cost of a daily dose to be $4 for UFH versus $84 for enoxaparin. From the payer perspective, the model showed that substituting enoxaparin for UFH would reduce the overall cost of care by $28.61 per day on a per-patient basis, despite enoxaparin’s higher acquisition cost, and would save $4,550 per quality-adjusted life-year by reducing the incidence of HIT.
Another cost analysis confirms the association between HIT and increased hospital costs. Creekmore et al retrospectively analyzed data from 10,121 adult medical patients who received VTE prophylaxis at the University of Utah Hospital in Salt Lake City from August 2000 to November 2004.19 They found that an admission during which HIT developed incurred a mean cost of $56,364, compared with $15,231 for an admission without HIT. Because LMWH was associated with a lower incidence of HIT compared with UFH (0.084% vs 0.51%, respectively), LMWH reduced the incremental cost of VTE prophylaxis by $13.88 per patient compared with UFH.
THE EXCLAIM TRIAL: IS THERE A ROLE FOR EXTENDED PROPHYLAXIS?
Although the previously discussed studies have clearly demonstrated the benefit of in-hospital VTE prophylaxis for acutely ill medical patients, none has rigorously examined extended-duration out-of-hospital prophylaxis in these patients. This represents an important gap in the literature, since a substantial proportion of VTE develops in the outpatient setting within 3 months of a hospitalization, and most outpatient VTE episodes occur within 1 month of a preceding hospitalization.20
To begin to fill this gap, the Extended Clinical Prophylaxis in Acutely Ill Medical Patients (EXCLAIM) trial was conducted to compare extended-duration LMWH prophylaxis with a standard LMWH prophylaxis regimen in acutely ill medical patients using a prospective, multicenter, randomized, double-blind, placebo-controlled design.21
Patients and study design
Patients were eligible for enrollment if they were aged 40 years or older and had recent immobilization (≤ 3 days), a predefined acute medical illness, and either level 1 mobility (total bed rest or sedentary state) or level 2 mobility (level 1 with bathroom privileges). The predefined acute medical illnesses consisted of New York Heart Association class III/IV heart failure, acute respiratory insufficiency, or other acute medical conditions, including post-acute ischemic stroke, acute infection without septic shock, and active cancer.
All patients received open-label enoxaparin 40 mg subcutaneously once daily for 10 ± 4 days, after which they were randomized to either enoxaparin 40 mg subcutaneously once daily or placebo for an additional 28 ± 4 days.
The primary efficacy end point was the incidence of VTE events, defined as asymptomatic DVT documented by mandatory ultrasonography at the end of the double-blind treatment period (28 ± 4 days) or as symptomatic DVT, symptomatic PE, or fatal PE at any time during the double-blind period. Symptomatic DVT was confirmed by objective tests; PE was confirmed by ventilation-perfusion scan, computed tomography, angiography, or autopsy.
Secondary efficacy end points were mortality at the end of the double-blind period, at 3 months, and at 6 months, as well as the incidence of VTE at 3 months.
The primary safety outcome measure was the incidence of major hemorrhage during the double-blind period; secondary safety measures were rates of major and minor hemorrhage, minor hemorrhage, HIT, and serious adverse events.
Population amended at planned interim analysis
After approximately half of the patients were enrolled, a planned and blinded interim analysis for futility concluded that the study was unlikely to show a statistically significant advantage of enoxaparin over placebo. The trial’s steering committee followed the suggestion of its data safety monitoring board to redefine the inclusion criteria to refocus enrollment on patients with a high risk of VTE. A blinded analysis was performed to identify this subgroup.
The resulting amended inclusion criteria were the same as above except that level 2 mobility had to be accompanied by at least one of three additional high-risk criteria: (1) age greater than 75 years, (2) history of prior VTE, or (3) diagnosis of cancer.
The trial’s main exclusion criteria were evidence of active bleeding, a contraindication to anticoagulation, receipt of prophylactic LMWH or UFH more than 72 hours prior to enrollment, treatment with an oral anticoagulant within 72 hours of enrollment, major surgery within the prior 3 months, cerebral stroke with bleeding, and persistent renal failure (creatinine clearance < 30 mL/min).


Results
The amended study population included 5,105 patients, 5,049 of whom received open-label enoxaparin. Of this group, 2,013 were randomized to active extended prophylaxis with enoxparain and 2,027 to placebo. Baseline characteristics, including level of mobility, were similar between the two groups.
The efficacy of extended prophylaxis with enoxaparin was enduring, as the cumulative incidence of VTE events at day 90 was significantly lower in enoxaparin recipients than in placebo recipients (3.0% vs 5.2%; relative reduction of 42%; P = .0115).
There was no difference in all-cause mortality at 6 months between the enoxaparin and placebo groups (10.1% vs 8.9%, respectively; P = .179).
Safety. Major hemorrhage was significantly more frequent in the enoxaparin arm, occurring in 0.60% of enoxaparin recipients compared with 0.15% of placebo recipients (P = .019). Minor bleeding was also more common with enoxaparin (5.20% vs 3.70%; P = .024).
Conclusions
The EXCLAIM trial found that an extended-duration (38-day) enoxaparin regimen significantly reduced the overall incidence of VTE relative to a 10-day enoxaparin regimen in acutely ill medical patients with reduced mobility. At the same time, the extended regimen was associated with a significant increase in the rate of major bleeding, although the incidence of major bleeding was low. The investigators concluded that the net clinical effect of extended-duration prophylaxis with enoxaparin is favorable, as only 46 patients would need to be treated to prevent one VTE event, whereas 224 patients would need to be treated to result in one major bleeding event.21
For this reason, it is reasonable to consider extended prophylaxis for hospitalized medical patients after identifying these patients’ risk factors. In keeping with the trial’s amended inclusion criteria, patients older than age 75 and those with cancer or prior VTE should receive special consideration for extended prophylaxis.
RECOMMENDED APPROACH TO VTE PREVENTION IN HOSPITALIZED MEDICAL PATIENTS
- All hospitalized medical patients should be screened at the time of admission, and patients at risk for VTE should receive prophylaxis.
- All patients with reduced mobility and one or more other risk factors for VTE are candidates for prophylaxis.
- Patients should be reassessed daily for the development of VTE risk factors during their hospitalization if risk factors are absent on admission.
- If screening or reassessment reveals any VTE risk factors, pharmacologic prophylaxis is the mainstay of therapy. If exclusion criteria for pharmacologic prophylaxis are present, mechanical prophylaxis with graduated compression stockings and intermittent compression devices should be used. For very high-risk medical patients without a contraindication to anticoagulants, combination prophylaxis with both an anticoagulant and mechanical devices is preferred.
- In this patient population, LMWH agents are preferred as pharmacologic prophylaxis over UFH and over fondaparinux (which is not currently approved by the US Food and Drug Administration for this population).
- If UFH is to be used in this patient population, 5,000 U three times daily is the preferred dosage.
- Extended pharmacologic prophylaxis should be considered in patients older than age 75 and in patients with a cancer diagnosis or a prior VTE episode.
DISCUSSION: ADDITIONAL PERSPECTIVES FROM THE AUTHORS
Dr. Jaffer: Dr. Spyropoulos, are there any guidelines, other than those from the ACCP, that speak to VTE prophylaxis in hospitalized medical patients? If so, what are their take-home messages and how do they differ from the ACCP guidelines?
Dr. Spyropoulos: I was part of the group that developed the International Consensus Statement (ICS) published in International Angiology in 2006,11 which is more recent than the latest ACCP guidelines, which were published in 2004. The ICS drew on much of the same data that the ACCP did, but we did an updated review of clinical trials.
For VTE prophylaxis in hospitalized medical patients, the ICS recommendations are more specific with regard to the type, dose, and dosing frequency of anticoagulant agents. First, they specify doses for both LMWH agents in this patient setting: 40 mg once daily for enoxaparin, and 5,000 IU once daily for dalteparin.
The ICS document also states that if UFH is the choice for prophylaxis, a regimen of 5,000 U three times daily should be considered. In the past year alone, two analyses suggest that three-times-daily dosing of UFH in medical patients provides superior efficacy to twice-daily dosing, although perhaps at the expense of more bleeding episodes.12,16 It is important to remember that no large placebo-controlled trial supports the efficacy of a UFH regimen of 5,000 U twice daily in this population.
Finally, the ICS document states that fondaparinux 2.5 mg once daily is a viable option for prophylaxis in medical patients, based on the ARTEMIS trial,3 even though this represents an off-label use.
Dr. Jaffer: Real-world use of VTE prophylaxis is far from optimal, especially in medical patients, and this is partly a result of system-of-care issues. I’d like to conclude by asking each of my colleagues to offer your perspectives on how your own institutions have improved their systems of care to promote better use of VTE prophylaxis and what lessons might be shared with others. Dr. McKean, you work at Brigham and Women’s Hospital, which recently reported impressive results with an electronic alert system designed to increase clinicians’ consideration of VTE risk assessment and use of prophylaxis.24 Please tell us about that study and the alert system.
Dr. McKean: Despite many educational initiatives at Brigham and Women’s Hospital, there were still some patients at high risk for VTE who were not receiving appropriate prophylaxis. What Dr. Samuel Goldhaber and his colleagues wanted to determine was whether changing the system of care could result in a reduced incidence of VTE.24 They devised a computer software program linked to the patient database that used eight common risk factors to determine each hospitalized patient’s risk profile for VTE. Each risk factor was weighted according to a point scale, with major risk factors (cancer, prior VTE, or hypercoagulability) assigned 3 points, the intermediate risk factor of surgery assigned 2 points, and minor risk factors (advanced age, obesity, immobility, or use of hormone replacement therapy or oral contraceptives) assigned 1 point. For patients with a total risk score of 4 or greater, the computer screen generates a color-coded VTE risk alert that requires the physician to acknowledge the alert and choose one of three options: order prophylaxis as appropriate, review a 60-page document on the computer to learn more about prophylaxis, or do nothing.
The study found that hospitalized patients who were randomized to treatment under the computer alert system were significantly more likely to receive VTE prophylaxis and significantly less likely to develop VTE than were patients randomized to a control group. The alert system reduced the risk of DVT or PE at 90 days by 41% in patients considered to be at high risk. It was particularly interesting that the incidence of VTE was lower in the intervention group even when physicians chose not to use prophylaxis, which suggests that simply having this alert system in place improved outcomes, perhaps by raising awareness of the risk of VTE.24
Additional studies are needed to better understand physicians’ behavior and determine why they seem to have a disproportionate fear of the risk of bleeding relative to the risk of clotting, including fatal PE, because that is really the heart of the matter. When patients are not given prophylaxis, often it is because of fear of bleeding. It is not clear, however, why some of these patients did not receive mechanical devices as an alternative form of prophylaxis, but this seems to be the case worldwide, as shown recently by the multinational ENDORSE study.22 Meanwhile, as we await studies to better understand physician perceptions and behaviors regarding prophylaxis, we need to work hard to change the system of care.
Dr. Deitelzweig: Over the past couple of years, the Ochsner Clinic has grown from a one-hospital teaching organization to a seven-hospital system with a mix of closed and open medical staff. The challenge is how to take a process that worked well in the one center, where appropriate prophylaxis was used about 90% of the time, and transfer it to the other centers in the larger system. We have endorsed several types of performance tools, such as the change-acceleration processes used by General Electric. The aim is to share a vision of heightening awareness. To do that, we have worked to mobilize the key stakeholders, at least half of them, to build algorithms that they all will endorse. It is easier said than done, however, and we have found it essential to involve both physicians and non-physician colleagues from pharmacy and nursing who have political and organizational clout.
Dr. Brotman: At Johns Hopkins, I took a bit more draconian approach to this issue because I thought that hospitalists often knew that they should be using VTE prophylaxis but sometimes weren’t, and I am not convinced that clinicians always look at prompts. So we came up with a system that incorporates both billing and documentation simultaneously. We put a hard stop on users’ documentation so that they could not sign off on a note or bill for their care until they checked off the kind of VTE prophylaxis they were using. Since hospitalists ultimately care about billing for their work, this system has at least ensured that everybody has considered and documented VTE prophylaxis on a daily basis. There are other hard stops that can be implemented in computer order-entry systems as well, and we are considering ways to roll them out on a broader scale.
However, all of these systems can have problems because patient situations change from day to day. For instance, VTE prophylaxis is not necessarily indicated in a 38-year-old ambulatory patient who comes in with a sickle cell crisis, but you will need to reconsider if the patient ends up in acute chest syndrome in the intensive care unit. I do not yet have a good way to ensure that this is being done on a daily basis with all patients.
Dr. Amin: At the University of California, Irvine, we implemented an electronic alert system, but we locked users in so that they could not complete their admission orders until they answered questions about VTE prevention. This practice increased our VTE prophylaxis rates tremendously. Because we are a level I trauma center, we allow users to bypass the screens one time, but the next time they log in, even to get a simple lab result, they have to answer the questions about VTE prevention.
With any system you develop, you also have to continue with the education process, because clinicians sometimes get into bad habits or simply forget things.
Dr. Spyropolous: At Lovelace Medical Center, we didn’t have the sophistication of an electronic order-entry system, but we had an experienced clinical pharmacist (the director of inpatient pharmacy) who helped to develop and champion VTE prevention guidelines that have then been used throughout the system in close conjunction with our hospitalists’ rounds. This system has been used successfully for the past 7 years.
- Samama MM, Cohen AT, Darmon JY, et al. A comparison of enoxaparin with placebo for the prevention of venous thrombo-embolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med 1999; 341:793–800.
- Leizorovicz A, Cohen AT, Turpie AG, et al. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation 2004; 110:874–879.
- Cohen AT, Davidson BL, Gallus AS, et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ 2006; 332:325–329.
- Baglin TP, White K, Charles A. Fatal pulmonary embolism in hos-pitalised medical patients. J Clin Pathol 1997; 50:609–610.
- Piazza G, Seddighzadeh A, Goldhaber SZ. Double trouble for 2,609 hospitalized medical patients who developed deep vein thrombosis: prophylaxis omitted more often and pulmonary embolism more frequent. Chest 2007; 132:554–561.
- Amin A, Stemkowski S, Lin J, Yang G. Thromboprophylaxis rates in US medical centers: success or failure? J Thromb Haemost 2007; 5:1610–1616.
- Geerts WH, Heit JA, Clagett GP, et al. Prevention of venous thromboembolism. Chest 2001; 119(1 Suppl):132S–175S.
- Tapson VF, Decousus H, Pini M, et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest 2007; 132:936–945.
- Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thrombo-embolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126(3 Suppl):338S–400S.
- Dentali F, Douketis JD, Gianni M, Lim W, Crowther MA. Meta-analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med 2007; 146:278–288.
- Cardiovascular Disease Educational and Research Trust; Cyprus Cardiovascular Disease Educational and Research Trust; European Venous Forum; International Surgical Thrombosis Forum; International Union of Angiology; Union Internationale de Phlébologie. Prevention and treatment of venous thromboembolism. International Consensus Statement (guidelines according to scientific evidence). Int Angiol 2006; 25:101–161.
- Wein L, Wein S, Haas SJ, Shaw J, Krum H. Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta-analysis of randomized controlled trials. Arch Intern Med 2007; 167:1476–1486.
- Mismetti P, Laporte-Simitsidis S, Tardy B, et al. Prevention of venous thromboembolism in internal medicine with unfractionated or low-molecular-weight heparins: a meta-analysis of randomised clinical trials. Thromb Haemost 2000; 83:14–19.
- Lechler E, Schramm W, Flosbach CW. The venous thrombotic risk in non-surgical patients: epidemiological data and efficacy/safety profile of a low-molecular-weight heparin (enoxaparin). The Prime Study Group. Haemostasis 1996; 26(Suppl 2):49–56.
- Kleber FX, Witt C, Vogel G, et al; the PRINCE Study Group. Randomized comparison of enoxaparin with unfractionated heparin for the prevention of venous thromboembolism in medical patients with heart failure or severe respiratory disease. Am Heart J 2003; 145:614–621.
- King CS, Holley AB, Jackson JL, Shorr AF, Moores LK. Twice vs three times daily heparin dosing for thromboembolism prophylaxis in the general medical population: a meta-analysis. Chest 2007; 131:507–516.
- Burleigh E, Wang C, Foster D, et al. Thromboprophylaxis in medically ill patients at risk for venous thromboembolism. Am J Health Syst Pharm 2006; 63(20 Suppl 6):S23–S29.
- Leykum L, Pugh J, Diuguid D, Papadopoulos K. Cost utility of substituting enoxaparin for unfractionated heparin for prophylaxis of venous thrombosis in the hospitalized medical patient. J Hosp Med 2006; 1:168–176.
- Creekmore FM, Oderda GM, Pendleton RC, Brixner DI. Incidence and economic implications of heparin-induced thrombocytopenia in medical patients receiving prophylaxis for venous thromboembolism. Pharmacotherapy 2006; 26:1438–1445.
- Spencer FA, Lessard D, Emery C, Reed G, Goldberg RJ. Venous thromboembolism in the outpatient setting. Arch Intern Med 2007; 167:1471–1475.
- Hull RD, Schellong SM Tapson VF, et al. Extended-duration venous thromboembolism (VTE) prophylaxis in acutely ill medical patients with recent reduced mobility: the EXCLAIM study. Presentation at: International Society on Thrombosis and Haemo-stasis XXIst Congress; July 6–12, 2007; Geneva, Switzerland.
- Cohen AT, Tapson VF, Bergmann JF, et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet 2008; 371:387–394.
- Kahn SR, Panju A, Geerts W, et al; CURVE study investigators. Multicenter evaluation of the use of venous thromboembolism prophylaxis in acutely ill medical patients in Canada. Thromb Res 2007; 119:145–155.
- Kucher N, Koo S, Quiroz R, et al. Electronic alerts to prevent thromboembolism among hospitalized patients. N Engl J Med 2005; 352:969–977.
- Anderson FA Jr, Wheeler HB, Goldberg RJ, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med 1991; 151:933–938.
- Howell MD, Geraci JM, Knowlton AA. Congestive heart failure and outpatient risk of venous thromboembolism: a retrospective, case-control study. J Clin Epidemiol 2001; 54:810–816.
- Smeeth L, Cook C, Thomas S, Hall AJ, Hubbard R, Vallance P. Risk of deep vein thrombosis and pulmonary embolism after acute infection in a community setting. Lancet 2006; 367:1075–1079.
- Alikhan R, Cohen AT, Combe S, et al. Prevention of venous thromboembolism in medical patients with enoxaparin: a subgroup analysis of the MEDENOX study. Blood Coagul Firbinolysis 2003; 14:341–346.
- Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation 2003; 107(23 Suppl 1):I9–I16.
- Greinacher A, Warkentin TE. Recognition, treatment, and prevention of heparin-induced thrombocytopenia: review and update. Thromb Res 2006; 118:165–176.
- Martel N, Lee J, Wells PS. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood 2005; 106:2710–2715.
- Sanderink GJ, Guimart CG, Ozoux ML, Jariwala NU, Shukla UA, Boutouyrie BX. Pharmacokinetics and pharmacodynamics of the prophylactic dose of enoxaparin once daily over 4 days in patients with renal impairment. Thromb Res 2002; 105:225–231.
- Douketis J, Cook D, Zytaruk N, et al. Dalteparin thromboprophylaxis in critically ill patients with severe renal insufficiency: the DIRECT study [abstract]. J Thromb Haemost 2007; 5(Suppl 2): P-S-680.
- Scholten DJ, Hoedema RM, Scholten SE. A comparison of two different prophylactic dose regimens of low molecular weight heparin in bariatric surgery. Obes Surg 2002; 12:19–24.
- Samama MM, Cohen AT, Darmon JY, et al. A comparison of enoxaparin with placebo for the prevention of venous thrombo-embolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med 1999; 341:793–800.
- Leizorovicz A, Cohen AT, Turpie AG, et al. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation 2004; 110:874–879.
- Cohen AT, Davidson BL, Gallus AS, et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ 2006; 332:325–329.
- Baglin TP, White K, Charles A. Fatal pulmonary embolism in hos-pitalised medical patients. J Clin Pathol 1997; 50:609–610.
- Piazza G, Seddighzadeh A, Goldhaber SZ. Double trouble for 2,609 hospitalized medical patients who developed deep vein thrombosis: prophylaxis omitted more often and pulmonary embolism more frequent. Chest 2007; 132:554–561.
- Amin A, Stemkowski S, Lin J, Yang G. Thromboprophylaxis rates in US medical centers: success or failure? J Thromb Haemost 2007; 5:1610–1616.
- Geerts WH, Heit JA, Clagett GP, et al. Prevention of venous thromboembolism. Chest 2001; 119(1 Suppl):132S–175S.
- Tapson VF, Decousus H, Pini M, et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest 2007; 132:936–945.
- Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thrombo-embolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126(3 Suppl):338S–400S.
- Dentali F, Douketis JD, Gianni M, Lim W, Crowther MA. Meta-analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med 2007; 146:278–288.
- Cardiovascular Disease Educational and Research Trust; Cyprus Cardiovascular Disease Educational and Research Trust; European Venous Forum; International Surgical Thrombosis Forum; International Union of Angiology; Union Internationale de Phlébologie. Prevention and treatment of venous thromboembolism. International Consensus Statement (guidelines according to scientific evidence). Int Angiol 2006; 25:101–161.
- Wein L, Wein S, Haas SJ, Shaw J, Krum H. Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta-analysis of randomized controlled trials. Arch Intern Med 2007; 167:1476–1486.
- Mismetti P, Laporte-Simitsidis S, Tardy B, et al. Prevention of venous thromboembolism in internal medicine with unfractionated or low-molecular-weight heparins: a meta-analysis of randomised clinical trials. Thromb Haemost 2000; 83:14–19.
- Lechler E, Schramm W, Flosbach CW. The venous thrombotic risk in non-surgical patients: epidemiological data and efficacy/safety profile of a low-molecular-weight heparin (enoxaparin). The Prime Study Group. Haemostasis 1996; 26(Suppl 2):49–56.
- Kleber FX, Witt C, Vogel G, et al; the PRINCE Study Group. Randomized comparison of enoxaparin with unfractionated heparin for the prevention of venous thromboembolism in medical patients with heart failure or severe respiratory disease. Am Heart J 2003; 145:614–621.
- King CS, Holley AB, Jackson JL, Shorr AF, Moores LK. Twice vs three times daily heparin dosing for thromboembolism prophylaxis in the general medical population: a meta-analysis. Chest 2007; 131:507–516.
- Burleigh E, Wang C, Foster D, et al. Thromboprophylaxis in medically ill patients at risk for venous thromboembolism. Am J Health Syst Pharm 2006; 63(20 Suppl 6):S23–S29.
- Leykum L, Pugh J, Diuguid D, Papadopoulos K. Cost utility of substituting enoxaparin for unfractionated heparin for prophylaxis of venous thrombosis in the hospitalized medical patient. J Hosp Med 2006; 1:168–176.
- Creekmore FM, Oderda GM, Pendleton RC, Brixner DI. Incidence and economic implications of heparin-induced thrombocytopenia in medical patients receiving prophylaxis for venous thromboembolism. Pharmacotherapy 2006; 26:1438–1445.
- Spencer FA, Lessard D, Emery C, Reed G, Goldberg RJ. Venous thromboembolism in the outpatient setting. Arch Intern Med 2007; 167:1471–1475.
- Hull RD, Schellong SM Tapson VF, et al. Extended-duration venous thromboembolism (VTE) prophylaxis in acutely ill medical patients with recent reduced mobility: the EXCLAIM study. Presentation at: International Society on Thrombosis and Haemo-stasis XXIst Congress; July 6–12, 2007; Geneva, Switzerland.
- Cohen AT, Tapson VF, Bergmann JF, et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet 2008; 371:387–394.
- Kahn SR, Panju A, Geerts W, et al; CURVE study investigators. Multicenter evaluation of the use of venous thromboembolism prophylaxis in acutely ill medical patients in Canada. Thromb Res 2007; 119:145–155.
- Kucher N, Koo S, Quiroz R, et al. Electronic alerts to prevent thromboembolism among hospitalized patients. N Engl J Med 2005; 352:969–977.
- Anderson FA Jr, Wheeler HB, Goldberg RJ, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med 1991; 151:933–938.
- Howell MD, Geraci JM, Knowlton AA. Congestive heart failure and outpatient risk of venous thromboembolism: a retrospective, case-control study. J Clin Epidemiol 2001; 54:810–816.
- Smeeth L, Cook C, Thomas S, Hall AJ, Hubbard R, Vallance P. Risk of deep vein thrombosis and pulmonary embolism after acute infection in a community setting. Lancet 2006; 367:1075–1079.
- Alikhan R, Cohen AT, Combe S, et al. Prevention of venous thromboembolism in medical patients with enoxaparin: a subgroup analysis of the MEDENOX study. Blood Coagul Firbinolysis 2003; 14:341–346.
- Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation 2003; 107(23 Suppl 1):I9–I16.
- Greinacher A, Warkentin TE. Recognition, treatment, and prevention of heparin-induced thrombocytopenia: review and update. Thromb Res 2006; 118:165–176.
- Martel N, Lee J, Wells PS. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood 2005; 106:2710–2715.
- Sanderink GJ, Guimart CG, Ozoux ML, Jariwala NU, Shukla UA, Boutouyrie BX. Pharmacokinetics and pharmacodynamics of the prophylactic dose of enoxaparin once daily over 4 days in patients with renal impairment. Thromb Res 2002; 105:225–231.
- Douketis J, Cook D, Zytaruk N, et al. Dalteparin thromboprophylaxis in critically ill patients with severe renal insufficiency: the DIRECT study [abstract]. J Thromb Haemost 2007; 5(Suppl 2): P-S-680.
- Scholten DJ, Hoedema RM, Scholten SE. A comparison of two different prophylactic dose regimens of low molecular weight heparin in bariatric surgery. Obes Surg 2002; 12:19–24.
Prevention of venous thromboembolism in the cancer surgery patient
Venous thromboembolism (VTE) is a major complication of cancer, occurring in 4% to 20% of patients,1 and is one of the leading causes of death in cancer patients, although these figures are believed to be underestimates, given the low autopsy rates among cancer patients.2 In hospitalized cancer patients specifically, VTE is the second leading cause of death.3,4 The risk of VTE in cancer patients undergoing surgery is three to five times greater than that in surgical patients without cancer.4 Moreover, cancer patients with symptomatic deep vein thrombosis (DVT) exhibit a high risk of recurrent VTE that may persist for many years after the index event.5
VTE PREVENTION POSES PARTICULAR CHALLENGES IN CANCER PATIENTS
Until recently, data on VTE prevention specific to cancer patients have been sparse. Cancer patients have represented only a small subset (< 20%) of participants in most of the largest clinical trials of VTE prophylaxis. Until the past 2 or 3 years, clinicians largely have had to extrapolate their approach to VTE prophylaxis in cancer patients from data in patients without cancer, bearing in mind that cancer patients are among the populations at highest risk of developing VTE.
High rates of VTE, even with prophylaxis
Further insights have come from the @RISTOS project, a Web-based prospective registry of patients undergoing general, urologic, or gynecologic surgery for cancer at multiple centers in Italy.8 Of the 2,372 patients tracked in this study, 82% received in-hospital VTE prophylaxis and 31% received prophylaxis following discharge. Despite this relatively high frequency of prophylaxis, however, the incidence of clinically overt VTE was 2.1% and the incidence of fatal VTE was 0.8%. Notably, most VTE events occurred after hospital discharge, and VTE was the most common cause of 30-day postoperative death in this registry.
RISK FACTORS: CANCER TYPE AND TREATMENT LOOM LARGE
Both the type and stage of a patient’s cancer are important in assessing the risk of VTE. For men, cancers of the prostate, colon, brain, and lung have been associated with an increased risk of VTE. Among women, cancers of the breast, ovary, and lung have been especially implicated as risk factors for VTE.9,10
The type of cancer therapy also influences VTE risk:
- Surgery. Among patients who undergo cancer-related surgery, the rate of proximal DVT is 10% to 20%, the rate of clinically evident PE is 4% to 10%, and the incidence of fatal PE is 0.2% to 5%.8,11
- Systemic treatments, including chemotherapy and hormone therapy, are also associated with an increased risk of VTE.12–15
- Central venous catheters. Approximately 4% of cancer patients who have central venous catheters placed develop clinically relevant VTE.16,17
In addition to the above risks related to cancer treatments, the following have been identified as risk factors for VTE in surgical oncology patients:
- Age greater than 40 years (risk also increases steeply after age 60 and again after age 75)
- Cancer procoagulants
- Thrombophilia
- Length and complications of cancer surgery (ie, often involving tissue trauma and immobilization)
- Debilitation and slow recovery.
Another risk factor worth noting is perioperative transfusion, as illustrated in a recent study of 14,104 adults undergoing colorectal cancer resection.18 The overall incidence of VTE in these patients was 1.0%, and the risk of death was nearly four times as great in patients who developed VTE as in those who did not. Notably, the need for transfusion was a marker of increased risk of VTE, particularly in women: women who received perioperative transfusions had almost double the risk of developing VTE compared with women who did not receive transfusions (P = .004).
CLINICAL TRIALS OF PROPHYLAXIS IN CANCER SURGERY PATIENTS
LMWH vs UFH for in-hospital prophylaxis
Two large randomized, double-blind trials have compared low-molecular-weight heparin (LMWH) with low-dose unfractionated heparin (UFH) for VTE prophylaxis in surgical patients with cancer—the Enoxaparin and Cancer (ENOXACAN) study19 and the Canadian Colorectal Surgery DVT Prophylaxis Trial.20 Patients in these studies underwent surgery for abdominal or pelvic cancer (mostly colorectal cancer). Both studies compared 40 mg of the LMWH enoxaparin given once daily with 5,000 U of UFH given three times daily for 7 to 10 days postoperatively. Outcome measures were the presence of DVT determined by venography on day 7 to 10 and the incidence of symptomatic VTE. Rates of VTE were statistically equivalent between the two treatment arms in both ENOXACAN (14.7% with LMWH vs 18.2% with UFH) and the Canadian Colorectal Surgery study (9.4% with both therapies), as were rates of major bleeding (4.1% with LMWH vs 2.9% with UFH in ENOXACAN; 2.7% with LMWH vs 1.5% with UFH in the Canadian study).
These findings are consistent with a 2001 meta-analysis by Mismetti et al of all available randomized trials comparing LMWH with placebo or with UFH for VTE prophylaxis in general surgery.21 This analysis found no differences in rates of asymptomatic DVT, clinical PE, clinical thromboembolism, death, major hemorrhage, total hemorrhage, wound hematoma, or need for transfusion between LMWH and UFH in patients undergoing either cancer-related surgery or surgery not related to cancer.
Fondaparinux for in-hospital prophylaxis
Subgroup analysis of the large randomized trial known as PEGASUS22 sheds some light on the efficacy of the factor Xa inhibitor fondaparinux relative to LMWH for thromboprophylaxis in cancer surgery patients. PEGASUS compared fondaparinux 2.5 mg once daily with the LMWH dalteparin 5,000 IU once daily for 5 to 9 days in patients undergoing high-risk abdominal surgery. Among the study’s 1,408 patients undergoing surgery for cancer, rates of VTE were 4.7% in the fondaparinux group compared with 7.7% in the LMWH group, a relative risk reduction of 38.6% with fondaparinux (95% CI, 6.7% to 59.6%). In contrast, in the rest of the PEGASUS population (patients undergoing abdominal surgery for reasons other than cancer), LMWH was nonsignificantly more efficacious at preventing VTE than was fondaparinux. Rates of major bleeding in this cancer subgroup were comparable between the two treatments.
Extended prophylaxis
Two additional randomized trials have evaluated extended prophylaxis with LMWH in surgical cancer patients—ENOXACAN II23 and the Fragmin After Major Abdominal Surgery (FAME) study.24
In ENOXACAN II, patients undergoing surgery for abdominal or pelvic cancer first received 6 to 10 days of prophylaxis with enoxaparin 40 mg once daily and then were randomized in a double-blind fashion to an additional 21 days of enoxaparin or placebo.23 Among 332 patients in the intent-to-treat analysis, the rate of VTE at the end of the double-blind phase was reduced from 12.0% with placebo to 4.8% with extended-duration enoxaparin (P = .02), an effect that was maintained at 3-month follow-up (P = .01). There was no significant difference between the two groups in rates of major bleeding events or any bleeding events.
In FAME, patients received 5,000 IU of dalteparin once daily for 1 week following major abdominal surgery and then were randomized in open-label fashion to either placebo or extended prophylaxis with dalteparin for 3 more weeks; a subanalysis examined outcomes in the 198 FAME participants whose abdominal surgery was for cancer.24 Among these 198 cancer surgery patients, the rate of venography-documented VTE at 4 weeks was reduced from 19.6% with placebo to 8.8% with extended-duration dalteparin, a relative reduction of 55% (P = .03). The rate of proximal DVT was reduced from 10.4% to 2.2% with extended prophylaxis, a relative reduction of 79% (P = .02).
The number needed to treat with extended LMWH prophylaxis to prevent one VTE event was 14 in ENOXACAN II23 and 9 in the FAME subanalysis of cancer surgery patients.24
New systematic review of relevant trials
Leonardi et al recently published a systematic review of 26 randomized controlled trials of DVT prophylaxis in 7,639 cancer surgery patients.25 They found the overall incidence of DVT to be 12.7% in those who received pharmacologic prophylaxis compared with 35.2% in controls. They also found high-dose LMWH therapy (> 3,400 U daily) to be associated with a significantly lower incidence of DVT than low-dose LMWH therapy (≤ 3,400 U daily) (7.9% vs 14.5%, respectively; P < .01). No differences were demonstrated between LMWH and UFH in preventing DVT, DVT location, or bleeding. Bleeding complications requiring discontinuation of pharmacologic prophylaxis occurred in 3% of patients overall.
Implications of HIT
The sequelae of heparin-induced thrombocytopenia (HIT) can have major consequences for cancer surgery patients. The incidence of HIT is markedly lower with LMWH than with UFH, as demonstrated in a nested case-control study by Creekmore et al.26 These researchers also found that the average cost of an admission during which HIT developed was nearly four times as great as the average cost of an admission during which UFH or LMWH was given without development of HIT ($56,364 vs $15,231; P < .001).
EVIDENCE IN SPECIFIC ONCOLOGIC POPULATIONS
Most of the patients in the trials reviewed above underwent abdominal surgery for malignancy. Although studies of VTE prophylaxis in patients undergoing nonabdominal cancer surgery are relatively few, some data are available for a few other specific oncologic populations, as reviewed below.
Surgery for gynecologic cancer
There is a paucity of randomized controlled trials or prospective observational studies on VTE and its prevention in the gynecologic cancer surgery population. Based on small historical studies, the postoperative risk of VTE in this population varies from 12% to 35%.27,28 Twice-daily administration of UFH 5,000 U appears to be ineffective as VTE prophylaxis in this population, but increasing the frequency to three times daily reduces VTE risk by 50% to 60% compared with placebo. Once-daily LMWH is comparable to three-times-daily UFH in efficacy and safety in this population.
A systematic Cochrane review of eight randomized controlled trials in patients undergoing major gynecologic surgery revealed that heparin prophylaxis (either UFH or LMWH) reduces the risk of DVT by 70% compared with no prophylaxis, with an identical risk reduction specifically among women with malignancy (odds ratio, 0.30; 95% CI, 0.10 to 0.89).29 This review found no evidence that anticoagulation reduces the risk of PE following major gynecologic surgery. LMWH and UFH were similar in efficacy for preventing DVT and had a comparable risk of bleeding complications.
Surgery for urologic cancer
The risk of VTE and the benefits of thromboprophylaxis also are poorly studied in patients undergoing surgery for urologic cancer.
The risk of VTE varies with the type of urologic surgery and the method used to diagnose VTE. For instance, patients undergoing radical retropubic prostatectomy have been reported to develop DVT at rates of 1% to 3%, PE at rates of 1% to 3%, and fatal PE at a rate of 0.6%, whereas the incidences of these events are somewhat higher in patients undergoing cystectomy: 8% for DVT, 2% to 4% for PE, and 2% for fatal PE. Radiologic diagnosis of thromboembolism in pelvic surgery patients has yielded higher incidences, with DVT rates of 21% to 51% and PE rates of 11% to 22%.30
Small studies suggest that prophylaxis with either low-dose UFH or LMWH is both effective in reducing VTE risk and safe in urologic cancer surgery patients, although pharmacologic prophylaxis poses a possible increased risk of pelvic hematoma and lymphocele formation in this population.30
Neurosurgery
Most neurosurgical procedures are performed for malignancies. The risk of venography-confirmed VTE in patients undergoing neurosurgery is approximately 30% to 40%.31,32 Likewise, the risks of intracranial or intraspinal hemorrhage in these patients are high. For this reason, mechanical methods of VTE prophylaxis are preferred in these patients. The use of anticoagulant prophylaxis remains controversial in this setting, although more recent data suggest that it might be safer than previously recognized.
GUIDELINES FOR VTE PROPHYLAXIS IN THE CANCER SURGERY PATIENT
American College of Chest Physicians
National Comprehensive Cancer Network
The National Comprehensive Cancer Network (NCCN) recently published clinical practice guidelines on venous thromboembolic disease in cancer patients.35 The defined at-risk population for these guidelines is the adult cancer inpatient with a diagnosis of (or clinical suspicion for) cancer. The guidelines recommend prophylactic anticoagulation (category 1 recommendation) with or without a sequential compression device as initial prophylaxis, unless the patient has a relative contraindication to anticoagulation, in which case mechanical prophylaxis (sequential compression device or graduated compression stockings) is recommended. (A category 1 recommendation indicates “uniform NCCN consensus, based on high-level evidence.”)
The NCCN guidelines include a specific recommended risk-factor assessment, which includes noting the patient’s age (VTE risk increases beginning at age 40 and then steeply again at age 75), any prior VTE, the presence of familial thrombophilia or active cancer, the use of medications associated with increased VTE risk (chemotherapy, exogenous estrogen compounds, and thalidomide or lenalidomide), and a number of other risk factors for VTE as outlined in the prior two articles in this supplement. The NCCN guidelines explicitly call for assessment of modifiable risk factors for VTE (ie, smoking or other tobacco use, obesity, and a low level of activity or lack of exercise) and call for active patient education on these factors.
American Society of Clinical Oncology
Our recommended algorithm
LINGERING CHALLENGE OF UNDERUTILIZATION
Despite this consensus on ways to reduce thromboembolic risk in this population and the clear evidence of the benefit of VTE prophylaxis in patients with cancer, data from several registries confirm a persistently low utilization of prophylaxis in patients with cancer.36–38 The global Fundamental Research in Oncology and Thrombosis (FRONTLINE) study surveyed 3,891 clinicians who treat cancer patients regarding their practices with respect to VTE in those patients.36 The survey found that only 52% of respondents routinely used thromboprophylaxis for their surgical patients with cancer. More striking, however, was the finding that most respondents routinely considered thrombo-prophylaxis in only 5% of their medical oncology patients. These data are echoed by findings of other retrospective medical record reviews in patients undergoing major abdominal or abdominothoracic surgery (in many cases for cancer), with VTE prophylaxis rates ranging from 38% to 75%.37,38
SUMMARY
Patients undergoing surgery for cancer have an increased risk of VTE and fatal PE, even when thromboprophylaxis is used. Nevertheless, prophylaxis with either LMWH or UFH does reduce venographic VTE event rates in these patients. If UFH is chosen for prophylaxis, a three-times-daily regimen should be used in this population. In specific surgical cancer populations, especially those undergoing abdominal surgery, out-of-hospital prophylaxis with once-daily LMWH is warranted. Current registries reveal that compliance with established guidelines for VTE prophylaxis in this population is low.


Dr. Jaffer: Dr. Amin, based on your study on thrombo-prophylaxis rates in US medical centers, will you comment on rates of prophylaxis for cancer surgery patients?
Dr. Amin: The overall study included approximately 200,000 medical patients and about 80,000 surgical patients enrolled over more than a 3-year period between 2002 and 2005.39,40 Our goal was to assess rates of prophylaxis and, when it was provided, whether it was appropriate (in terms of type, dosage, and duration) based on the ACCP guidelines. A subanalysis assessed medical cancer patients and surgical cancer patients separately. Medical cancer patients received thromboprophylaxis 56% of the time but received appropriate prophylaxis only 28% of the time. Among surgical cancer patients, appropriate prophylaxis was given only about 24% of the time for those undergoing gynecologic surgery and about 12% of the time for those undergoing neurosurgery. These percentages are consistent with data from other national registries, such as the IMPROVE registry, which documented prophylaxis rates on the order of 45% in medical patients with cancer.41 We also analyzed the data according to individual practitioners and found that medical oncologists use prophylaxis about 25% of the time, which is relatively consistent with other providers, such as internists and surgeons.
So there is a huge opportunity to improve rates of prophylaxis for this group of patients that national guidelines say are at high risk. Why is prophylaxis so underutilized in the cancer population? One factor may be a misperception about the risk of bleeding with anticoagulants. Yet several studies have shown that the rate of bleeding from prophylaxis is extremely low, whether LMWH or UFH is used, so more awareness of actual bleeding risk is needed. Another factor is the obvious focus among internists and oncologists on treating the patient, with perhaps a reduced consideration of prophylaxis and prevention. A third factor may be a concern about thrombocytopenia. However, in our study of prophylaxis rates in US medical centers, we excluded patients who had thrombocytopenia, yet rates of prophylaxis were still low. Nothing in the literature indicates that anticoagulants cannot be used in patients with platelet counts of 50,000 to 150,000 cells/µL or higher, so this suggests that we need to do more education.
Dr. Jaffer: Dr. Brotman, can you tell us more about how clinicians in practice should use prophylaxis in their neurosurgery patients, such as those undergoing craniotomy or spine surgery for cancer? What is the safest and most efficacious way to prevent DVT in these patients?
Dr. Brotman: First, it’s important to recognize that some sort of prophylaxis needs to be used. Neurosurgery patients are at an extremely high risk for thromboembolic events, and such events are often fatal in these patients. Having said that, the jury is still out on whether the prophylaxis in these patients should be compression devices or anticoagulation. This gives physicians some latitude in their decisions. They can decide not to use pharmacologic prophylaxis so long as they use pneumatic compression devices consistently, perhaps even starting during the operation and certainly throughout hospitalization when the patient is immobilized.
Certainly, the concerns about using full-dose anticoagulation in the immediate postoperative setting in neurosurgery patients are valid. Yet these patients are at very high risk for thromboembolic events, and if we take too cautious an approach to prophylaxis in the immediate perioperative setting, more patients are going to have thromboembolic events, at which point management decisions become much more difficult. The risk of intracranial bleeding with anticoagulation to treat a patient who develops a DVT at postoperative day 10 will certainly be higher than it would have been with lower-dose perioperative prophylactic anticoagulation. Plus, if you put in a filter at that point, the outcomes tend to be poor. Therefore, I believe there is some degree of risk that we should be willing to take with regard to perioperative bleeding, even in neurosurgery patients.
Dr. McKean: I’d like to make a point about combination prophylaxis. At many institutions, compression stockings and sequential compression devices are used preoperatively and intraoperatively, and then pharmacologic prophylaxis—for example, twice-daily UFH—is used postoperatively. There is concern that these patients are hypercoagulable, and most clinicians believe that mechanical prophylaxis alone, even with sequential compression devices plus compression stockings, is not aggressive enough in these high-risk patients.
Dr. Jaffer: Dr. Spyropoulos, what is the optimal duration of pharmacologic prophylaxis for cancer surgery patients?
Dr. Spyropoulos: First let’s consider in-hospital prophylaxis. The supportive data for in-hospital prophylaxis are strong, and the duration of therapy used in the major in-hospital prophylaxis trials was 7 to 10 days. With regard to extended prophylaxis, we have at least two moderately sized randomized controlled trials, ENOXACAN II23 and the substudy of FAME,24 that demonstrated that extending prophylaxis with LMWH at doses of 3,400 U once daily (5,000 IU of dalteparin; 40 mg of enoxaparin) reduces VTE risk at postoperative day 30. Also, recent data from the @RISTOS registry show that in cancer surgery patients, especially those having abdominal or pelvic procedures, the leading cause of 30-day mortality was VTE.8 This registry also shows that despite prophylaxis, the rate of symptomatic VTE can be as high as 2%, with the rate of fatal VTE approaching 1%. Thus, in cancer patients undergoing abdominal or pelvic surgery, physicians should strongly consider prophylaxis of up to 30 days’ duration.
Dr. Jaffer: One striking finding from the @RISTOS registry was that 40% of VTE events in these cancer surgery patients occurred after postoperative day 21. This really underscores the need to consider prophylaxis for at least 4 weeks in these patients in real-world practice.
Dr. Brotman: The other striking finding from that registry was that the in-hospital prophylaxis rate was quite high, about 80%, and the rate of extended prophylaxis approached 35%. These are rates that are rarely achieved in clinical practice. Yet despite these high levels of prophylaxis, patients in this registry still had a high incidence of morbidity and mortality from VTE. This suggests that we need to improve our out-of-hospital VTE prevention paradigms.
Dr. Jaffer: Dr. Deitelzweig, oncologists and internists are often unsure about whether their ambulatory cancer patients who are receiving chemotherapy should be on any form of prophylaxis. What is your opinion?
Dr. Deitelzweig: That question comes up regularly because these patients are encountered across many medical specialties. At this point, all of the large organizations, including ASCO and NCCN, are advocating that prophylaxis is not indicated for such patients.
- Lyman GH, Khorana AA, Falanga A, et al. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol 2007; 25:5490–5505.
- Khorana AA, Francis CW, Culakova E, et al. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost 2007; 5:632–634.
- Ambrus JL, Ambrus CM, Mink IB, Pickren JW. Causes of death in cancer patients. J Med 1975; 6:61–64.
- Donati MB. Cancer and thrombosis. Haemostasis 1994; 24:128–131.
- Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med 1996; 125:1–7.
- Haas S, Wolf H, Kakkar AK, Fareed J, Encke A. Prevention of fatal pulmonary embolism and mortality in surgical patients: a randomized double-blind comparison of LMWH with unfractionated heparin. Thromb Haemost 2005; 94:814–819.
- Kakkar AK, Haas S, Wolf H, Encke A. Evaluation of perioperative fatal pulmonary embolism and death in cancer surgical patients: the MC-4 cancer substudy. Thromb Haemost 2005; 94:867–871.
- Agnelli G, Bolis G, Capussotti L, et al. A clinical outcome-based prospective study on venous thromboembolism after cancer surgery: the @RISTOS project. Ann Surg 2006; 243:89–95.
- Levitan N, Dowlati A, Remick SC, et al. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy: risk analysis using Medicare claims data. Medicine (Baltimore) 1999; 78:285–291.
- Levine M, Gent M, Hirsh J, et al. A comparison of low-molecular-weight heparin administered primarily at home with unfractionated heparin administered in the hospital for proximal deep-vein thrombosis. N Engl J Med 1996; 334:677–681.
- Bergqvist D. Risk of venous thromboembolism in patients undergoing cancer surgery and options for thromboprophylaxis. J Surg Oncol 2007; 95:167–174.
- Heit JA, Silverstein MD, Mohr DN, et al. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med 2000; 160:809–815.
- Sallah S, Wan JY, Nguyen NP. Venous thrombosis in patients with solid tumors: determination of frequency and characteristics. Thromb Haemost 2002; 87:575–579.
- Kröger K, Weiland D, Ose C, et al. Risk factors for venous thromboembolic events in cancer patients. Ann Oncol 2006; 17:297–303.
- Blom JW, Vanderschoot JP, Oostindiër MJ, et al. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: results of a record linkage study. J Thromb Haemost 2006; 4:529–535.
- Couban S, Simpson DR, Barnett MJ, et al. A randomized multicenter comparison of bone marrow and peripheral blood in recipients of matched sibling allogeneic transplants for myeloid malignancies. Blood 2002; 100:1525–1531.
- Walshe LJ, Malak SF, Eagan J, Sepkowitz KA. Complication rates among cancer patients with peripherally inserted central catheters. J Clin Oncol 2002; 20:3276–3281.
- Nilsson KR, Berenholtz SM, Garrett-Mayer E, et al. Association between venous thromboembolism and perioperative allogeneic transfusion. Arch Surg 2007; 142:126–133.
- Efficacy and safety of enoxaparin versus unfractionated heparin for prevention of deep vein thrombosis in elective cancer surgery: a double-blind randomized multicentre trial with venographic assessment. ENOXACAN Study Group. Br J Surg 1997; 84:1099–1103.
- McLeod RS, Geerts WH, Sniderman KW, et al. Subcutaneous heparin versus low-molecular-weight heparin as thromboprophylaxis in patients undergoing colorectal surgery: results of the Canadian Colorectal DVT Prophylaxis Trial: a randomized, double-blind trial. Ann Surg 2001; 233:438–444.
- Mismetti P, Laporte S, Darmon JY, Buchmüller A, Decousus H. Meta-analysis of low molecular weight heparin in the prevention of venous thromboembolism in general surgery. Br J Surg 2001; 88:913–930.
- Agnelli G, Bergqvist D, Cohen AT, et al, on behalf of the PEGASUS investigators. Randomized clinical trial of postoperative fondaparinux versus perioperative dalteparin for prevention of venous thromboembolism in high-risk abdominal surgery. Br J Surg 2005; 92:1212–1220.
- Bergqvist D, Agnelli G, Cohen AT, et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med 2002; 346:975–980.
- Rasmussen MS, Wille-Jorgensen P, Jorgensen LN, et al. Prolonged thromboprophylaxis with low molecular weight heparin (dalteparin) following major abdominal surgery for malignancy [abstract 186]. Blood 2003; 102:56a.
- Leonardi MJ, McGory ML, Ko CY. A systematic review of deep venous thrombosis prophylaxis in cancer patients: implications for improving quality. Ann Surg Oncol 2007; 14:929–936.
- Creekmore FM, Oderda GM, Pendleton RC, Brixner DI. Incidence and economic implications of heparin-induced thrombocytopenia in medical patients receiving prophylaxis for venous thromboembolism. Pharmacotherapy 2006; 26:1438–1445.
- Walsh JJ, Bonnar J, Wright FW. A study of pulmonary embolism and deep leg vein thrombosis after major gynaecological surgery using labeled fibrinogen-phlebography and lung scanning. J Obstet Gynaecol Br Commonw 1974; 81:311–316.
- Clarke-Pearson DL, Synan IS, Coleman RE, et al. The natural history of postoperative venous thromboemboli in gynecologic oncology: a prospective study of 382 patients. Am J Obstet Gynecol 1984; 148:1051–1054.
- Oates-Whitehead RM, D’Angelo A, Mol B. Anticoagulant and aspirin prophylaxis for preventing thromboembolism after major gynaecological surgery. Cochrane Database Syst Rev 2003; (4):CD003679.
- Kibel AS, Loughlin KR. Pathogenesis and prophylaxis of postoperative thromboembolic disease in urological pelvic surgery. J Urol 1995; 153:1763–1774.
- Agnelli G, Piovella F, Buoncristiani P, et al. Enoxaparin plus compression stockings compared with compression stockings alone in the prevention of venous thromboembolism after elective neurosurgery. N Engl J Med 1998; 339:80–85.
- Semrad TJ, O’Donnell R, Wun T, et al. Epidemiology of venous thromboembolism in 9489 patients with malignant glioma. J Neurosurg 2007; 106:601–608.
- Iorio A, Agnelli G. Low-molecular-weight and unfractionated heparin for prevention of venous thromboembolism in neurosurgery: a meta-analysis. Arch Intern Med 2000; 160:2327–2332.
- Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126(3 Suppl):338S–400S.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Venous thromboembolic disease. V.1.2007. http://www.nccn.org/professionals/physician_gls/PDF/vte.pdf. Accessed December 5, 2007.
- Kakkar AK, Levine M, Pinedo HM, Wolff R, Wong J. Venous thrombosis in cancer patients: insights from the FRONTLINE survey. Oncologist 2003; 8:381–388.
- Stratton MA, Anderson FA, Bussey HI, et al. Prevention of venous thromboembolism: adherence to the 1995 American College of Chest Physicians consensus guidelines for surgical patients. Arch Intern Med 2000; 160:334–340.
- Bratzler DW, Raskob GE, Murray CK, Bumpus LJ, Piatt DS. Underuse of venous thromboembolism prophylaxis for general surgery patients: physician practices in the community hospital setting. Arch Intern Med 1998; 158:1909–1912.
- Amin A, Stemkowski S, Lin J, et al. Thromboprophylaxis rates in US medical centers: success or failure? J Thromb Haemost 2007; 5:1610–1616.
- Amin A, Stemkowski S, Lin J, Yang G. Thromboprophylaxis in US hospitals: adherence to the 6th American College of Chest Physicians’ recommendations for at-risk medical and surgical patients. Abstract presented at: 41st Midyear Clinical Meeting of the American Society of Health-System Pharmacists; December 3–7, 2006; Anaheim, CA.
- Tapson VF, Decousus H, Pini M, et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest 2007; 132:936–945.
- Clarke-Pearson DL, Synan IS, Dodge R, et al. A randomized trial of low-dose heparin and intermittent pneumatic calf compression for the prevention of deep venous thrombosis after gynecologic oncology surgery. Am J Obstet Gynecol 1993; 168:1146–1154.
- Einstein MH, Pritts EA, Hartenbach EM. Venous thromboembolism prevention in gynecologic cancer surgery: a systematic review. Gynecol Oncol 2007; 105:813–819.
- Clarke-Pearson DL, Synan IS, Hinshaw WM, Coleman RE, Creasman WT. Prevention of postoperative venous thromboembolism by external pneumatic calf compression in patients with gynecologic malignancy. Obstet Gynecol 1984; 63:92–98.
- Ruff RL, Posner JB. Incidence and treatment of peripheral venous thrombosis in patients with glioma. Ann Neurol 1983; 13:334–336.
- Levin JM, Schiff D, Loeffler JS, Fine HA, Black PM, Wen PY. Complications of therapy for venous thromboembolic disease in patients with brain tumors. Neurology 1993; 43:1111–1114.
Venous thromboembolism (VTE) is a major complication of cancer, occurring in 4% to 20% of patients,1 and is one of the leading causes of death in cancer patients, although these figures are believed to be underestimates, given the low autopsy rates among cancer patients.2 In hospitalized cancer patients specifically, VTE is the second leading cause of death.3,4 The risk of VTE in cancer patients undergoing surgery is three to five times greater than that in surgical patients without cancer.4 Moreover, cancer patients with symptomatic deep vein thrombosis (DVT) exhibit a high risk of recurrent VTE that may persist for many years after the index event.5
VTE PREVENTION POSES PARTICULAR CHALLENGES IN CANCER PATIENTS
Until recently, data on VTE prevention specific to cancer patients have been sparse. Cancer patients have represented only a small subset (< 20%) of participants in most of the largest clinical trials of VTE prophylaxis. Until the past 2 or 3 years, clinicians largely have had to extrapolate their approach to VTE prophylaxis in cancer patients from data in patients without cancer, bearing in mind that cancer patients are among the populations at highest risk of developing VTE.
High rates of VTE, even with prophylaxis
Further insights have come from the @RISTOS project, a Web-based prospective registry of patients undergoing general, urologic, or gynecologic surgery for cancer at multiple centers in Italy.8 Of the 2,372 patients tracked in this study, 82% received in-hospital VTE prophylaxis and 31% received prophylaxis following discharge. Despite this relatively high frequency of prophylaxis, however, the incidence of clinically overt VTE was 2.1% and the incidence of fatal VTE was 0.8%. Notably, most VTE events occurred after hospital discharge, and VTE was the most common cause of 30-day postoperative death in this registry.
RISK FACTORS: CANCER TYPE AND TREATMENT LOOM LARGE
Both the type and stage of a patient’s cancer are important in assessing the risk of VTE. For men, cancers of the prostate, colon, brain, and lung have been associated with an increased risk of VTE. Among women, cancers of the breast, ovary, and lung have been especially implicated as risk factors for VTE.9,10
The type of cancer therapy also influences VTE risk:
- Surgery. Among patients who undergo cancer-related surgery, the rate of proximal DVT is 10% to 20%, the rate of clinically evident PE is 4% to 10%, and the incidence of fatal PE is 0.2% to 5%.8,11
- Systemic treatments, including chemotherapy and hormone therapy, are also associated with an increased risk of VTE.12–15
- Central venous catheters. Approximately 4% of cancer patients who have central venous catheters placed develop clinically relevant VTE.16,17
In addition to the above risks related to cancer treatments, the following have been identified as risk factors for VTE in surgical oncology patients:
- Age greater than 40 years (risk also increases steeply after age 60 and again after age 75)
- Cancer procoagulants
- Thrombophilia
- Length and complications of cancer surgery (ie, often involving tissue trauma and immobilization)
- Debilitation and slow recovery.
Another risk factor worth noting is perioperative transfusion, as illustrated in a recent study of 14,104 adults undergoing colorectal cancer resection.18 The overall incidence of VTE in these patients was 1.0%, and the risk of death was nearly four times as great in patients who developed VTE as in those who did not. Notably, the need for transfusion was a marker of increased risk of VTE, particularly in women: women who received perioperative transfusions had almost double the risk of developing VTE compared with women who did not receive transfusions (P = .004).
CLINICAL TRIALS OF PROPHYLAXIS IN CANCER SURGERY PATIENTS
LMWH vs UFH for in-hospital prophylaxis
Two large randomized, double-blind trials have compared low-molecular-weight heparin (LMWH) with low-dose unfractionated heparin (UFH) for VTE prophylaxis in surgical patients with cancer—the Enoxaparin and Cancer (ENOXACAN) study19 and the Canadian Colorectal Surgery DVT Prophylaxis Trial.20 Patients in these studies underwent surgery for abdominal or pelvic cancer (mostly colorectal cancer). Both studies compared 40 mg of the LMWH enoxaparin given once daily with 5,000 U of UFH given three times daily for 7 to 10 days postoperatively. Outcome measures were the presence of DVT determined by venography on day 7 to 10 and the incidence of symptomatic VTE. Rates of VTE were statistically equivalent between the two treatment arms in both ENOXACAN (14.7% with LMWH vs 18.2% with UFH) and the Canadian Colorectal Surgery study (9.4% with both therapies), as were rates of major bleeding (4.1% with LMWH vs 2.9% with UFH in ENOXACAN; 2.7% with LMWH vs 1.5% with UFH in the Canadian study).
These findings are consistent with a 2001 meta-analysis by Mismetti et al of all available randomized trials comparing LMWH with placebo or with UFH for VTE prophylaxis in general surgery.21 This analysis found no differences in rates of asymptomatic DVT, clinical PE, clinical thromboembolism, death, major hemorrhage, total hemorrhage, wound hematoma, or need for transfusion between LMWH and UFH in patients undergoing either cancer-related surgery or surgery not related to cancer.
Fondaparinux for in-hospital prophylaxis
Subgroup analysis of the large randomized trial known as PEGASUS22 sheds some light on the efficacy of the factor Xa inhibitor fondaparinux relative to LMWH for thromboprophylaxis in cancer surgery patients. PEGASUS compared fondaparinux 2.5 mg once daily with the LMWH dalteparin 5,000 IU once daily for 5 to 9 days in patients undergoing high-risk abdominal surgery. Among the study’s 1,408 patients undergoing surgery for cancer, rates of VTE were 4.7% in the fondaparinux group compared with 7.7% in the LMWH group, a relative risk reduction of 38.6% with fondaparinux (95% CI, 6.7% to 59.6%). In contrast, in the rest of the PEGASUS population (patients undergoing abdominal surgery for reasons other than cancer), LMWH was nonsignificantly more efficacious at preventing VTE than was fondaparinux. Rates of major bleeding in this cancer subgroup were comparable between the two treatments.
Extended prophylaxis
Two additional randomized trials have evaluated extended prophylaxis with LMWH in surgical cancer patients—ENOXACAN II23 and the Fragmin After Major Abdominal Surgery (FAME) study.24
In ENOXACAN II, patients undergoing surgery for abdominal or pelvic cancer first received 6 to 10 days of prophylaxis with enoxaparin 40 mg once daily and then were randomized in a double-blind fashion to an additional 21 days of enoxaparin or placebo.23 Among 332 patients in the intent-to-treat analysis, the rate of VTE at the end of the double-blind phase was reduced from 12.0% with placebo to 4.8% with extended-duration enoxaparin (P = .02), an effect that was maintained at 3-month follow-up (P = .01). There was no significant difference between the two groups in rates of major bleeding events or any bleeding events.
In FAME, patients received 5,000 IU of dalteparin once daily for 1 week following major abdominal surgery and then were randomized in open-label fashion to either placebo or extended prophylaxis with dalteparin for 3 more weeks; a subanalysis examined outcomes in the 198 FAME participants whose abdominal surgery was for cancer.24 Among these 198 cancer surgery patients, the rate of venography-documented VTE at 4 weeks was reduced from 19.6% with placebo to 8.8% with extended-duration dalteparin, a relative reduction of 55% (P = .03). The rate of proximal DVT was reduced from 10.4% to 2.2% with extended prophylaxis, a relative reduction of 79% (P = .02).
The number needed to treat with extended LMWH prophylaxis to prevent one VTE event was 14 in ENOXACAN II23 and 9 in the FAME subanalysis of cancer surgery patients.24
New systematic review of relevant trials
Leonardi et al recently published a systematic review of 26 randomized controlled trials of DVT prophylaxis in 7,639 cancer surgery patients.25 They found the overall incidence of DVT to be 12.7% in those who received pharmacologic prophylaxis compared with 35.2% in controls. They also found high-dose LMWH therapy (> 3,400 U daily) to be associated with a significantly lower incidence of DVT than low-dose LMWH therapy (≤ 3,400 U daily) (7.9% vs 14.5%, respectively; P < .01). No differences were demonstrated between LMWH and UFH in preventing DVT, DVT location, or bleeding. Bleeding complications requiring discontinuation of pharmacologic prophylaxis occurred in 3% of patients overall.
Implications of HIT
The sequelae of heparin-induced thrombocytopenia (HIT) can have major consequences for cancer surgery patients. The incidence of HIT is markedly lower with LMWH than with UFH, as demonstrated in a nested case-control study by Creekmore et al.26 These researchers also found that the average cost of an admission during which HIT developed was nearly four times as great as the average cost of an admission during which UFH or LMWH was given without development of HIT ($56,364 vs $15,231; P < .001).
EVIDENCE IN SPECIFIC ONCOLOGIC POPULATIONS
Most of the patients in the trials reviewed above underwent abdominal surgery for malignancy. Although studies of VTE prophylaxis in patients undergoing nonabdominal cancer surgery are relatively few, some data are available for a few other specific oncologic populations, as reviewed below.
Surgery for gynecologic cancer
There is a paucity of randomized controlled trials or prospective observational studies on VTE and its prevention in the gynecologic cancer surgery population. Based on small historical studies, the postoperative risk of VTE in this population varies from 12% to 35%.27,28 Twice-daily administration of UFH 5,000 U appears to be ineffective as VTE prophylaxis in this population, but increasing the frequency to three times daily reduces VTE risk by 50% to 60% compared with placebo. Once-daily LMWH is comparable to three-times-daily UFH in efficacy and safety in this population.
A systematic Cochrane review of eight randomized controlled trials in patients undergoing major gynecologic surgery revealed that heparin prophylaxis (either UFH or LMWH) reduces the risk of DVT by 70% compared with no prophylaxis, with an identical risk reduction specifically among women with malignancy (odds ratio, 0.30; 95% CI, 0.10 to 0.89).29 This review found no evidence that anticoagulation reduces the risk of PE following major gynecologic surgery. LMWH and UFH were similar in efficacy for preventing DVT and had a comparable risk of bleeding complications.
Surgery for urologic cancer
The risk of VTE and the benefits of thromboprophylaxis also are poorly studied in patients undergoing surgery for urologic cancer.
The risk of VTE varies with the type of urologic surgery and the method used to diagnose VTE. For instance, patients undergoing radical retropubic prostatectomy have been reported to develop DVT at rates of 1% to 3%, PE at rates of 1% to 3%, and fatal PE at a rate of 0.6%, whereas the incidences of these events are somewhat higher in patients undergoing cystectomy: 8% for DVT, 2% to 4% for PE, and 2% for fatal PE. Radiologic diagnosis of thromboembolism in pelvic surgery patients has yielded higher incidences, with DVT rates of 21% to 51% and PE rates of 11% to 22%.30
Small studies suggest that prophylaxis with either low-dose UFH or LMWH is both effective in reducing VTE risk and safe in urologic cancer surgery patients, although pharmacologic prophylaxis poses a possible increased risk of pelvic hematoma and lymphocele formation in this population.30
Neurosurgery
Most neurosurgical procedures are performed for malignancies. The risk of venography-confirmed VTE in patients undergoing neurosurgery is approximately 30% to 40%.31,32 Likewise, the risks of intracranial or intraspinal hemorrhage in these patients are high. For this reason, mechanical methods of VTE prophylaxis are preferred in these patients. The use of anticoagulant prophylaxis remains controversial in this setting, although more recent data suggest that it might be safer than previously recognized.
GUIDELINES FOR VTE PROPHYLAXIS IN THE CANCER SURGERY PATIENT
American College of Chest Physicians
National Comprehensive Cancer Network
The National Comprehensive Cancer Network (NCCN) recently published clinical practice guidelines on venous thromboembolic disease in cancer patients.35 The defined at-risk population for these guidelines is the adult cancer inpatient with a diagnosis of (or clinical suspicion for) cancer. The guidelines recommend prophylactic anticoagulation (category 1 recommendation) with or without a sequential compression device as initial prophylaxis, unless the patient has a relative contraindication to anticoagulation, in which case mechanical prophylaxis (sequential compression device or graduated compression stockings) is recommended. (A category 1 recommendation indicates “uniform NCCN consensus, based on high-level evidence.”)
The NCCN guidelines include a specific recommended risk-factor assessment, which includes noting the patient’s age (VTE risk increases beginning at age 40 and then steeply again at age 75), any prior VTE, the presence of familial thrombophilia or active cancer, the use of medications associated with increased VTE risk (chemotherapy, exogenous estrogen compounds, and thalidomide or lenalidomide), and a number of other risk factors for VTE as outlined in the prior two articles in this supplement. The NCCN guidelines explicitly call for assessment of modifiable risk factors for VTE (ie, smoking or other tobacco use, obesity, and a low level of activity or lack of exercise) and call for active patient education on these factors.
American Society of Clinical Oncology
Our recommended algorithm
LINGERING CHALLENGE OF UNDERUTILIZATION
Despite this consensus on ways to reduce thromboembolic risk in this population and the clear evidence of the benefit of VTE prophylaxis in patients with cancer, data from several registries confirm a persistently low utilization of prophylaxis in patients with cancer.36–38 The global Fundamental Research in Oncology and Thrombosis (FRONTLINE) study surveyed 3,891 clinicians who treat cancer patients regarding their practices with respect to VTE in those patients.36 The survey found that only 52% of respondents routinely used thromboprophylaxis for their surgical patients with cancer. More striking, however, was the finding that most respondents routinely considered thrombo-prophylaxis in only 5% of their medical oncology patients. These data are echoed by findings of other retrospective medical record reviews in patients undergoing major abdominal or abdominothoracic surgery (in many cases for cancer), with VTE prophylaxis rates ranging from 38% to 75%.37,38
SUMMARY
Patients undergoing surgery for cancer have an increased risk of VTE and fatal PE, even when thromboprophylaxis is used. Nevertheless, prophylaxis with either LMWH or UFH does reduce venographic VTE event rates in these patients. If UFH is chosen for prophylaxis, a three-times-daily regimen should be used in this population. In specific surgical cancer populations, especially those undergoing abdominal surgery, out-of-hospital prophylaxis with once-daily LMWH is warranted. Current registries reveal that compliance with established guidelines for VTE prophylaxis in this population is low.


Dr. Jaffer: Dr. Amin, based on your study on thrombo-prophylaxis rates in US medical centers, will you comment on rates of prophylaxis for cancer surgery patients?
Dr. Amin: The overall study included approximately 200,000 medical patients and about 80,000 surgical patients enrolled over more than a 3-year period between 2002 and 2005.39,40 Our goal was to assess rates of prophylaxis and, when it was provided, whether it was appropriate (in terms of type, dosage, and duration) based on the ACCP guidelines. A subanalysis assessed medical cancer patients and surgical cancer patients separately. Medical cancer patients received thromboprophylaxis 56% of the time but received appropriate prophylaxis only 28% of the time. Among surgical cancer patients, appropriate prophylaxis was given only about 24% of the time for those undergoing gynecologic surgery and about 12% of the time for those undergoing neurosurgery. These percentages are consistent with data from other national registries, such as the IMPROVE registry, which documented prophylaxis rates on the order of 45% in medical patients with cancer.41 We also analyzed the data according to individual practitioners and found that medical oncologists use prophylaxis about 25% of the time, which is relatively consistent with other providers, such as internists and surgeons.
So there is a huge opportunity to improve rates of prophylaxis for this group of patients that national guidelines say are at high risk. Why is prophylaxis so underutilized in the cancer population? One factor may be a misperception about the risk of bleeding with anticoagulants. Yet several studies have shown that the rate of bleeding from prophylaxis is extremely low, whether LMWH or UFH is used, so more awareness of actual bleeding risk is needed. Another factor is the obvious focus among internists and oncologists on treating the patient, with perhaps a reduced consideration of prophylaxis and prevention. A third factor may be a concern about thrombocytopenia. However, in our study of prophylaxis rates in US medical centers, we excluded patients who had thrombocytopenia, yet rates of prophylaxis were still low. Nothing in the literature indicates that anticoagulants cannot be used in patients with platelet counts of 50,000 to 150,000 cells/µL or higher, so this suggests that we need to do more education.
Dr. Jaffer: Dr. Brotman, can you tell us more about how clinicians in practice should use prophylaxis in their neurosurgery patients, such as those undergoing craniotomy or spine surgery for cancer? What is the safest and most efficacious way to prevent DVT in these patients?
Dr. Brotman: First, it’s important to recognize that some sort of prophylaxis needs to be used. Neurosurgery patients are at an extremely high risk for thromboembolic events, and such events are often fatal in these patients. Having said that, the jury is still out on whether the prophylaxis in these patients should be compression devices or anticoagulation. This gives physicians some latitude in their decisions. They can decide not to use pharmacologic prophylaxis so long as they use pneumatic compression devices consistently, perhaps even starting during the operation and certainly throughout hospitalization when the patient is immobilized.
Certainly, the concerns about using full-dose anticoagulation in the immediate postoperative setting in neurosurgery patients are valid. Yet these patients are at very high risk for thromboembolic events, and if we take too cautious an approach to prophylaxis in the immediate perioperative setting, more patients are going to have thromboembolic events, at which point management decisions become much more difficult. The risk of intracranial bleeding with anticoagulation to treat a patient who develops a DVT at postoperative day 10 will certainly be higher than it would have been with lower-dose perioperative prophylactic anticoagulation. Plus, if you put in a filter at that point, the outcomes tend to be poor. Therefore, I believe there is some degree of risk that we should be willing to take with regard to perioperative bleeding, even in neurosurgery patients.
Dr. McKean: I’d like to make a point about combination prophylaxis. At many institutions, compression stockings and sequential compression devices are used preoperatively and intraoperatively, and then pharmacologic prophylaxis—for example, twice-daily UFH—is used postoperatively. There is concern that these patients are hypercoagulable, and most clinicians believe that mechanical prophylaxis alone, even with sequential compression devices plus compression stockings, is not aggressive enough in these high-risk patients.
Dr. Jaffer: Dr. Spyropoulos, what is the optimal duration of pharmacologic prophylaxis for cancer surgery patients?
Dr. Spyropoulos: First let’s consider in-hospital prophylaxis. The supportive data for in-hospital prophylaxis are strong, and the duration of therapy used in the major in-hospital prophylaxis trials was 7 to 10 days. With regard to extended prophylaxis, we have at least two moderately sized randomized controlled trials, ENOXACAN II23 and the substudy of FAME,24 that demonstrated that extending prophylaxis with LMWH at doses of 3,400 U once daily (5,000 IU of dalteparin; 40 mg of enoxaparin) reduces VTE risk at postoperative day 30. Also, recent data from the @RISTOS registry show that in cancer surgery patients, especially those having abdominal or pelvic procedures, the leading cause of 30-day mortality was VTE.8 This registry also shows that despite prophylaxis, the rate of symptomatic VTE can be as high as 2%, with the rate of fatal VTE approaching 1%. Thus, in cancer patients undergoing abdominal or pelvic surgery, physicians should strongly consider prophylaxis of up to 30 days’ duration.
Dr. Jaffer: One striking finding from the @RISTOS registry was that 40% of VTE events in these cancer surgery patients occurred after postoperative day 21. This really underscores the need to consider prophylaxis for at least 4 weeks in these patients in real-world practice.
Dr. Brotman: The other striking finding from that registry was that the in-hospital prophylaxis rate was quite high, about 80%, and the rate of extended prophylaxis approached 35%. These are rates that are rarely achieved in clinical practice. Yet despite these high levels of prophylaxis, patients in this registry still had a high incidence of morbidity and mortality from VTE. This suggests that we need to improve our out-of-hospital VTE prevention paradigms.
Dr. Jaffer: Dr. Deitelzweig, oncologists and internists are often unsure about whether their ambulatory cancer patients who are receiving chemotherapy should be on any form of prophylaxis. What is your opinion?
Dr. Deitelzweig: That question comes up regularly because these patients are encountered across many medical specialties. At this point, all of the large organizations, including ASCO and NCCN, are advocating that prophylaxis is not indicated for such patients.
Venous thromboembolism (VTE) is a major complication of cancer, occurring in 4% to 20% of patients,1 and is one of the leading causes of death in cancer patients, although these figures are believed to be underestimates, given the low autopsy rates among cancer patients.2 In hospitalized cancer patients specifically, VTE is the second leading cause of death.3,4 The risk of VTE in cancer patients undergoing surgery is three to five times greater than that in surgical patients without cancer.4 Moreover, cancer patients with symptomatic deep vein thrombosis (DVT) exhibit a high risk of recurrent VTE that may persist for many years after the index event.5
VTE PREVENTION POSES PARTICULAR CHALLENGES IN CANCER PATIENTS
Until recently, data on VTE prevention specific to cancer patients have been sparse. Cancer patients have represented only a small subset (< 20%) of participants in most of the largest clinical trials of VTE prophylaxis. Until the past 2 or 3 years, clinicians largely have had to extrapolate their approach to VTE prophylaxis in cancer patients from data in patients without cancer, bearing in mind that cancer patients are among the populations at highest risk of developing VTE.
High rates of VTE, even with prophylaxis
Further insights have come from the @RISTOS project, a Web-based prospective registry of patients undergoing general, urologic, or gynecologic surgery for cancer at multiple centers in Italy.8 Of the 2,372 patients tracked in this study, 82% received in-hospital VTE prophylaxis and 31% received prophylaxis following discharge. Despite this relatively high frequency of prophylaxis, however, the incidence of clinically overt VTE was 2.1% and the incidence of fatal VTE was 0.8%. Notably, most VTE events occurred after hospital discharge, and VTE was the most common cause of 30-day postoperative death in this registry.
RISK FACTORS: CANCER TYPE AND TREATMENT LOOM LARGE
Both the type and stage of a patient’s cancer are important in assessing the risk of VTE. For men, cancers of the prostate, colon, brain, and lung have been associated with an increased risk of VTE. Among women, cancers of the breast, ovary, and lung have been especially implicated as risk factors for VTE.9,10
The type of cancer therapy also influences VTE risk:
- Surgery. Among patients who undergo cancer-related surgery, the rate of proximal DVT is 10% to 20%, the rate of clinically evident PE is 4% to 10%, and the incidence of fatal PE is 0.2% to 5%.8,11
- Systemic treatments, including chemotherapy and hormone therapy, are also associated with an increased risk of VTE.12–15
- Central venous catheters. Approximately 4% of cancer patients who have central venous catheters placed develop clinically relevant VTE.16,17
In addition to the above risks related to cancer treatments, the following have been identified as risk factors for VTE in surgical oncology patients:
- Age greater than 40 years (risk also increases steeply after age 60 and again after age 75)
- Cancer procoagulants
- Thrombophilia
- Length and complications of cancer surgery (ie, often involving tissue trauma and immobilization)
- Debilitation and slow recovery.
Another risk factor worth noting is perioperative transfusion, as illustrated in a recent study of 14,104 adults undergoing colorectal cancer resection.18 The overall incidence of VTE in these patients was 1.0%, and the risk of death was nearly four times as great in patients who developed VTE as in those who did not. Notably, the need for transfusion was a marker of increased risk of VTE, particularly in women: women who received perioperative transfusions had almost double the risk of developing VTE compared with women who did not receive transfusions (P = .004).
CLINICAL TRIALS OF PROPHYLAXIS IN CANCER SURGERY PATIENTS
LMWH vs UFH for in-hospital prophylaxis
Two large randomized, double-blind trials have compared low-molecular-weight heparin (LMWH) with low-dose unfractionated heparin (UFH) for VTE prophylaxis in surgical patients with cancer—the Enoxaparin and Cancer (ENOXACAN) study19 and the Canadian Colorectal Surgery DVT Prophylaxis Trial.20 Patients in these studies underwent surgery for abdominal or pelvic cancer (mostly colorectal cancer). Both studies compared 40 mg of the LMWH enoxaparin given once daily with 5,000 U of UFH given three times daily for 7 to 10 days postoperatively. Outcome measures were the presence of DVT determined by venography on day 7 to 10 and the incidence of symptomatic VTE. Rates of VTE were statistically equivalent between the two treatment arms in both ENOXACAN (14.7% with LMWH vs 18.2% with UFH) and the Canadian Colorectal Surgery study (9.4% with both therapies), as were rates of major bleeding (4.1% with LMWH vs 2.9% with UFH in ENOXACAN; 2.7% with LMWH vs 1.5% with UFH in the Canadian study).
These findings are consistent with a 2001 meta-analysis by Mismetti et al of all available randomized trials comparing LMWH with placebo or with UFH for VTE prophylaxis in general surgery.21 This analysis found no differences in rates of asymptomatic DVT, clinical PE, clinical thromboembolism, death, major hemorrhage, total hemorrhage, wound hematoma, or need for transfusion between LMWH and UFH in patients undergoing either cancer-related surgery or surgery not related to cancer.
Fondaparinux for in-hospital prophylaxis
Subgroup analysis of the large randomized trial known as PEGASUS22 sheds some light on the efficacy of the factor Xa inhibitor fondaparinux relative to LMWH for thromboprophylaxis in cancer surgery patients. PEGASUS compared fondaparinux 2.5 mg once daily with the LMWH dalteparin 5,000 IU once daily for 5 to 9 days in patients undergoing high-risk abdominal surgery. Among the study’s 1,408 patients undergoing surgery for cancer, rates of VTE were 4.7% in the fondaparinux group compared with 7.7% in the LMWH group, a relative risk reduction of 38.6% with fondaparinux (95% CI, 6.7% to 59.6%). In contrast, in the rest of the PEGASUS population (patients undergoing abdominal surgery for reasons other than cancer), LMWH was nonsignificantly more efficacious at preventing VTE than was fondaparinux. Rates of major bleeding in this cancer subgroup were comparable between the two treatments.
Extended prophylaxis
Two additional randomized trials have evaluated extended prophylaxis with LMWH in surgical cancer patients—ENOXACAN II23 and the Fragmin After Major Abdominal Surgery (FAME) study.24
In ENOXACAN II, patients undergoing surgery for abdominal or pelvic cancer first received 6 to 10 days of prophylaxis with enoxaparin 40 mg once daily and then were randomized in a double-blind fashion to an additional 21 days of enoxaparin or placebo.23 Among 332 patients in the intent-to-treat analysis, the rate of VTE at the end of the double-blind phase was reduced from 12.0% with placebo to 4.8% with extended-duration enoxaparin (P = .02), an effect that was maintained at 3-month follow-up (P = .01). There was no significant difference between the two groups in rates of major bleeding events or any bleeding events.
In FAME, patients received 5,000 IU of dalteparin once daily for 1 week following major abdominal surgery and then were randomized in open-label fashion to either placebo or extended prophylaxis with dalteparin for 3 more weeks; a subanalysis examined outcomes in the 198 FAME participants whose abdominal surgery was for cancer.24 Among these 198 cancer surgery patients, the rate of venography-documented VTE at 4 weeks was reduced from 19.6% with placebo to 8.8% with extended-duration dalteparin, a relative reduction of 55% (P = .03). The rate of proximal DVT was reduced from 10.4% to 2.2% with extended prophylaxis, a relative reduction of 79% (P = .02).
The number needed to treat with extended LMWH prophylaxis to prevent one VTE event was 14 in ENOXACAN II23 and 9 in the FAME subanalysis of cancer surgery patients.24
New systematic review of relevant trials
Leonardi et al recently published a systematic review of 26 randomized controlled trials of DVT prophylaxis in 7,639 cancer surgery patients.25 They found the overall incidence of DVT to be 12.7% in those who received pharmacologic prophylaxis compared with 35.2% in controls. They also found high-dose LMWH therapy (> 3,400 U daily) to be associated with a significantly lower incidence of DVT than low-dose LMWH therapy (≤ 3,400 U daily) (7.9% vs 14.5%, respectively; P < .01). No differences were demonstrated between LMWH and UFH in preventing DVT, DVT location, or bleeding. Bleeding complications requiring discontinuation of pharmacologic prophylaxis occurred in 3% of patients overall.
Implications of HIT
The sequelae of heparin-induced thrombocytopenia (HIT) can have major consequences for cancer surgery patients. The incidence of HIT is markedly lower with LMWH than with UFH, as demonstrated in a nested case-control study by Creekmore et al.26 These researchers also found that the average cost of an admission during which HIT developed was nearly four times as great as the average cost of an admission during which UFH or LMWH was given without development of HIT ($56,364 vs $15,231; P < .001).
EVIDENCE IN SPECIFIC ONCOLOGIC POPULATIONS
Most of the patients in the trials reviewed above underwent abdominal surgery for malignancy. Although studies of VTE prophylaxis in patients undergoing nonabdominal cancer surgery are relatively few, some data are available for a few other specific oncologic populations, as reviewed below.
Surgery for gynecologic cancer
There is a paucity of randomized controlled trials or prospective observational studies on VTE and its prevention in the gynecologic cancer surgery population. Based on small historical studies, the postoperative risk of VTE in this population varies from 12% to 35%.27,28 Twice-daily administration of UFH 5,000 U appears to be ineffective as VTE prophylaxis in this population, but increasing the frequency to three times daily reduces VTE risk by 50% to 60% compared with placebo. Once-daily LMWH is comparable to three-times-daily UFH in efficacy and safety in this population.
A systematic Cochrane review of eight randomized controlled trials in patients undergoing major gynecologic surgery revealed that heparin prophylaxis (either UFH or LMWH) reduces the risk of DVT by 70% compared with no prophylaxis, with an identical risk reduction specifically among women with malignancy (odds ratio, 0.30; 95% CI, 0.10 to 0.89).29 This review found no evidence that anticoagulation reduces the risk of PE following major gynecologic surgery. LMWH and UFH were similar in efficacy for preventing DVT and had a comparable risk of bleeding complications.
Surgery for urologic cancer
The risk of VTE and the benefits of thromboprophylaxis also are poorly studied in patients undergoing surgery for urologic cancer.
The risk of VTE varies with the type of urologic surgery and the method used to diagnose VTE. For instance, patients undergoing radical retropubic prostatectomy have been reported to develop DVT at rates of 1% to 3%, PE at rates of 1% to 3%, and fatal PE at a rate of 0.6%, whereas the incidences of these events are somewhat higher in patients undergoing cystectomy: 8% for DVT, 2% to 4% for PE, and 2% for fatal PE. Radiologic diagnosis of thromboembolism in pelvic surgery patients has yielded higher incidences, with DVT rates of 21% to 51% and PE rates of 11% to 22%.30
Small studies suggest that prophylaxis with either low-dose UFH or LMWH is both effective in reducing VTE risk and safe in urologic cancer surgery patients, although pharmacologic prophylaxis poses a possible increased risk of pelvic hematoma and lymphocele formation in this population.30
Neurosurgery
Most neurosurgical procedures are performed for malignancies. The risk of venography-confirmed VTE in patients undergoing neurosurgery is approximately 30% to 40%.31,32 Likewise, the risks of intracranial or intraspinal hemorrhage in these patients are high. For this reason, mechanical methods of VTE prophylaxis are preferred in these patients. The use of anticoagulant prophylaxis remains controversial in this setting, although more recent data suggest that it might be safer than previously recognized.
GUIDELINES FOR VTE PROPHYLAXIS IN THE CANCER SURGERY PATIENT
American College of Chest Physicians
National Comprehensive Cancer Network
The National Comprehensive Cancer Network (NCCN) recently published clinical practice guidelines on venous thromboembolic disease in cancer patients.35 The defined at-risk population for these guidelines is the adult cancer inpatient with a diagnosis of (or clinical suspicion for) cancer. The guidelines recommend prophylactic anticoagulation (category 1 recommendation) with or without a sequential compression device as initial prophylaxis, unless the patient has a relative contraindication to anticoagulation, in which case mechanical prophylaxis (sequential compression device or graduated compression stockings) is recommended. (A category 1 recommendation indicates “uniform NCCN consensus, based on high-level evidence.”)
The NCCN guidelines include a specific recommended risk-factor assessment, which includes noting the patient’s age (VTE risk increases beginning at age 40 and then steeply again at age 75), any prior VTE, the presence of familial thrombophilia or active cancer, the use of medications associated with increased VTE risk (chemotherapy, exogenous estrogen compounds, and thalidomide or lenalidomide), and a number of other risk factors for VTE as outlined in the prior two articles in this supplement. The NCCN guidelines explicitly call for assessment of modifiable risk factors for VTE (ie, smoking or other tobacco use, obesity, and a low level of activity or lack of exercise) and call for active patient education on these factors.
American Society of Clinical Oncology
Our recommended algorithm
LINGERING CHALLENGE OF UNDERUTILIZATION
Despite this consensus on ways to reduce thromboembolic risk in this population and the clear evidence of the benefit of VTE prophylaxis in patients with cancer, data from several registries confirm a persistently low utilization of prophylaxis in patients with cancer.36–38 The global Fundamental Research in Oncology and Thrombosis (FRONTLINE) study surveyed 3,891 clinicians who treat cancer patients regarding their practices with respect to VTE in those patients.36 The survey found that only 52% of respondents routinely used thromboprophylaxis for their surgical patients with cancer. More striking, however, was the finding that most respondents routinely considered thrombo-prophylaxis in only 5% of their medical oncology patients. These data are echoed by findings of other retrospective medical record reviews in patients undergoing major abdominal or abdominothoracic surgery (in many cases for cancer), with VTE prophylaxis rates ranging from 38% to 75%.37,38
SUMMARY
Patients undergoing surgery for cancer have an increased risk of VTE and fatal PE, even when thromboprophylaxis is used. Nevertheless, prophylaxis with either LMWH or UFH does reduce venographic VTE event rates in these patients. If UFH is chosen for prophylaxis, a three-times-daily regimen should be used in this population. In specific surgical cancer populations, especially those undergoing abdominal surgery, out-of-hospital prophylaxis with once-daily LMWH is warranted. Current registries reveal that compliance with established guidelines for VTE prophylaxis in this population is low.


Dr. Jaffer: Dr. Amin, based on your study on thrombo-prophylaxis rates in US medical centers, will you comment on rates of prophylaxis for cancer surgery patients?
Dr. Amin: The overall study included approximately 200,000 medical patients and about 80,000 surgical patients enrolled over more than a 3-year period between 2002 and 2005.39,40 Our goal was to assess rates of prophylaxis and, when it was provided, whether it was appropriate (in terms of type, dosage, and duration) based on the ACCP guidelines. A subanalysis assessed medical cancer patients and surgical cancer patients separately. Medical cancer patients received thromboprophylaxis 56% of the time but received appropriate prophylaxis only 28% of the time. Among surgical cancer patients, appropriate prophylaxis was given only about 24% of the time for those undergoing gynecologic surgery and about 12% of the time for those undergoing neurosurgery. These percentages are consistent with data from other national registries, such as the IMPROVE registry, which documented prophylaxis rates on the order of 45% in medical patients with cancer.41 We also analyzed the data according to individual practitioners and found that medical oncologists use prophylaxis about 25% of the time, which is relatively consistent with other providers, such as internists and surgeons.
So there is a huge opportunity to improve rates of prophylaxis for this group of patients that national guidelines say are at high risk. Why is prophylaxis so underutilized in the cancer population? One factor may be a misperception about the risk of bleeding with anticoagulants. Yet several studies have shown that the rate of bleeding from prophylaxis is extremely low, whether LMWH or UFH is used, so more awareness of actual bleeding risk is needed. Another factor is the obvious focus among internists and oncologists on treating the patient, with perhaps a reduced consideration of prophylaxis and prevention. A third factor may be a concern about thrombocytopenia. However, in our study of prophylaxis rates in US medical centers, we excluded patients who had thrombocytopenia, yet rates of prophylaxis were still low. Nothing in the literature indicates that anticoagulants cannot be used in patients with platelet counts of 50,000 to 150,000 cells/µL or higher, so this suggests that we need to do more education.
Dr. Jaffer: Dr. Brotman, can you tell us more about how clinicians in practice should use prophylaxis in their neurosurgery patients, such as those undergoing craniotomy or spine surgery for cancer? What is the safest and most efficacious way to prevent DVT in these patients?
Dr. Brotman: First, it’s important to recognize that some sort of prophylaxis needs to be used. Neurosurgery patients are at an extremely high risk for thromboembolic events, and such events are often fatal in these patients. Having said that, the jury is still out on whether the prophylaxis in these patients should be compression devices or anticoagulation. This gives physicians some latitude in their decisions. They can decide not to use pharmacologic prophylaxis so long as they use pneumatic compression devices consistently, perhaps even starting during the operation and certainly throughout hospitalization when the patient is immobilized.
Certainly, the concerns about using full-dose anticoagulation in the immediate postoperative setting in neurosurgery patients are valid. Yet these patients are at very high risk for thromboembolic events, and if we take too cautious an approach to prophylaxis in the immediate perioperative setting, more patients are going to have thromboembolic events, at which point management decisions become much more difficult. The risk of intracranial bleeding with anticoagulation to treat a patient who develops a DVT at postoperative day 10 will certainly be higher than it would have been with lower-dose perioperative prophylactic anticoagulation. Plus, if you put in a filter at that point, the outcomes tend to be poor. Therefore, I believe there is some degree of risk that we should be willing to take with regard to perioperative bleeding, even in neurosurgery patients.
Dr. McKean: I’d like to make a point about combination prophylaxis. At many institutions, compression stockings and sequential compression devices are used preoperatively and intraoperatively, and then pharmacologic prophylaxis—for example, twice-daily UFH—is used postoperatively. There is concern that these patients are hypercoagulable, and most clinicians believe that mechanical prophylaxis alone, even with sequential compression devices plus compression stockings, is not aggressive enough in these high-risk patients.
Dr. Jaffer: Dr. Spyropoulos, what is the optimal duration of pharmacologic prophylaxis for cancer surgery patients?
Dr. Spyropoulos: First let’s consider in-hospital prophylaxis. The supportive data for in-hospital prophylaxis are strong, and the duration of therapy used in the major in-hospital prophylaxis trials was 7 to 10 days. With regard to extended prophylaxis, we have at least two moderately sized randomized controlled trials, ENOXACAN II23 and the substudy of FAME,24 that demonstrated that extending prophylaxis with LMWH at doses of 3,400 U once daily (5,000 IU of dalteparin; 40 mg of enoxaparin) reduces VTE risk at postoperative day 30. Also, recent data from the @RISTOS registry show that in cancer surgery patients, especially those having abdominal or pelvic procedures, the leading cause of 30-day mortality was VTE.8 This registry also shows that despite prophylaxis, the rate of symptomatic VTE can be as high as 2%, with the rate of fatal VTE approaching 1%. Thus, in cancer patients undergoing abdominal or pelvic surgery, physicians should strongly consider prophylaxis of up to 30 days’ duration.
Dr. Jaffer: One striking finding from the @RISTOS registry was that 40% of VTE events in these cancer surgery patients occurred after postoperative day 21. This really underscores the need to consider prophylaxis for at least 4 weeks in these patients in real-world practice.
Dr. Brotman: The other striking finding from that registry was that the in-hospital prophylaxis rate was quite high, about 80%, and the rate of extended prophylaxis approached 35%. These are rates that are rarely achieved in clinical practice. Yet despite these high levels of prophylaxis, patients in this registry still had a high incidence of morbidity and mortality from VTE. This suggests that we need to improve our out-of-hospital VTE prevention paradigms.
Dr. Jaffer: Dr. Deitelzweig, oncologists and internists are often unsure about whether their ambulatory cancer patients who are receiving chemotherapy should be on any form of prophylaxis. What is your opinion?
Dr. Deitelzweig: That question comes up regularly because these patients are encountered across many medical specialties. At this point, all of the large organizations, including ASCO and NCCN, are advocating that prophylaxis is not indicated for such patients.
- Lyman GH, Khorana AA, Falanga A, et al. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol 2007; 25:5490–5505.
- Khorana AA, Francis CW, Culakova E, et al. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost 2007; 5:632–634.
- Ambrus JL, Ambrus CM, Mink IB, Pickren JW. Causes of death in cancer patients. J Med 1975; 6:61–64.
- Donati MB. Cancer and thrombosis. Haemostasis 1994; 24:128–131.
- Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med 1996; 125:1–7.
- Haas S, Wolf H, Kakkar AK, Fareed J, Encke A. Prevention of fatal pulmonary embolism and mortality in surgical patients: a randomized double-blind comparison of LMWH with unfractionated heparin. Thromb Haemost 2005; 94:814–819.
- Kakkar AK, Haas S, Wolf H, Encke A. Evaluation of perioperative fatal pulmonary embolism and death in cancer surgical patients: the MC-4 cancer substudy. Thromb Haemost 2005; 94:867–871.
- Agnelli G, Bolis G, Capussotti L, et al. A clinical outcome-based prospective study on venous thromboembolism after cancer surgery: the @RISTOS project. Ann Surg 2006; 243:89–95.
- Levitan N, Dowlati A, Remick SC, et al. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy: risk analysis using Medicare claims data. Medicine (Baltimore) 1999; 78:285–291.
- Levine M, Gent M, Hirsh J, et al. A comparison of low-molecular-weight heparin administered primarily at home with unfractionated heparin administered in the hospital for proximal deep-vein thrombosis. N Engl J Med 1996; 334:677–681.
- Bergqvist D. Risk of venous thromboembolism in patients undergoing cancer surgery and options for thromboprophylaxis. J Surg Oncol 2007; 95:167–174.
- Heit JA, Silverstein MD, Mohr DN, et al. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med 2000; 160:809–815.
- Sallah S, Wan JY, Nguyen NP. Venous thrombosis in patients with solid tumors: determination of frequency and characteristics. Thromb Haemost 2002; 87:575–579.
- Kröger K, Weiland D, Ose C, et al. Risk factors for venous thromboembolic events in cancer patients. Ann Oncol 2006; 17:297–303.
- Blom JW, Vanderschoot JP, Oostindiër MJ, et al. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: results of a record linkage study. J Thromb Haemost 2006; 4:529–535.
- Couban S, Simpson DR, Barnett MJ, et al. A randomized multicenter comparison of bone marrow and peripheral blood in recipients of matched sibling allogeneic transplants for myeloid malignancies. Blood 2002; 100:1525–1531.
- Walshe LJ, Malak SF, Eagan J, Sepkowitz KA. Complication rates among cancer patients with peripherally inserted central catheters. J Clin Oncol 2002; 20:3276–3281.
- Nilsson KR, Berenholtz SM, Garrett-Mayer E, et al. Association between venous thromboembolism and perioperative allogeneic transfusion. Arch Surg 2007; 142:126–133.
- Efficacy and safety of enoxaparin versus unfractionated heparin for prevention of deep vein thrombosis in elective cancer surgery: a double-blind randomized multicentre trial with venographic assessment. ENOXACAN Study Group. Br J Surg 1997; 84:1099–1103.
- McLeod RS, Geerts WH, Sniderman KW, et al. Subcutaneous heparin versus low-molecular-weight heparin as thromboprophylaxis in patients undergoing colorectal surgery: results of the Canadian Colorectal DVT Prophylaxis Trial: a randomized, double-blind trial. Ann Surg 2001; 233:438–444.
- Mismetti P, Laporte S, Darmon JY, Buchmüller A, Decousus H. Meta-analysis of low molecular weight heparin in the prevention of venous thromboembolism in general surgery. Br J Surg 2001; 88:913–930.
- Agnelli G, Bergqvist D, Cohen AT, et al, on behalf of the PEGASUS investigators. Randomized clinical trial of postoperative fondaparinux versus perioperative dalteparin for prevention of venous thromboembolism in high-risk abdominal surgery. Br J Surg 2005; 92:1212–1220.
- Bergqvist D, Agnelli G, Cohen AT, et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med 2002; 346:975–980.
- Rasmussen MS, Wille-Jorgensen P, Jorgensen LN, et al. Prolonged thromboprophylaxis with low molecular weight heparin (dalteparin) following major abdominal surgery for malignancy [abstract 186]. Blood 2003; 102:56a.
- Leonardi MJ, McGory ML, Ko CY. A systematic review of deep venous thrombosis prophylaxis in cancer patients: implications for improving quality. Ann Surg Oncol 2007; 14:929–936.
- Creekmore FM, Oderda GM, Pendleton RC, Brixner DI. Incidence and economic implications of heparin-induced thrombocytopenia in medical patients receiving prophylaxis for venous thromboembolism. Pharmacotherapy 2006; 26:1438–1445.
- Walsh JJ, Bonnar J, Wright FW. A study of pulmonary embolism and deep leg vein thrombosis after major gynaecological surgery using labeled fibrinogen-phlebography and lung scanning. J Obstet Gynaecol Br Commonw 1974; 81:311–316.
- Clarke-Pearson DL, Synan IS, Coleman RE, et al. The natural history of postoperative venous thromboemboli in gynecologic oncology: a prospective study of 382 patients. Am J Obstet Gynecol 1984; 148:1051–1054.
- Oates-Whitehead RM, D’Angelo A, Mol B. Anticoagulant and aspirin prophylaxis for preventing thromboembolism after major gynaecological surgery. Cochrane Database Syst Rev 2003; (4):CD003679.
- Kibel AS, Loughlin KR. Pathogenesis and prophylaxis of postoperative thromboembolic disease in urological pelvic surgery. J Urol 1995; 153:1763–1774.
- Agnelli G, Piovella F, Buoncristiani P, et al. Enoxaparin plus compression stockings compared with compression stockings alone in the prevention of venous thromboembolism after elective neurosurgery. N Engl J Med 1998; 339:80–85.
- Semrad TJ, O’Donnell R, Wun T, et al. Epidemiology of venous thromboembolism in 9489 patients with malignant glioma. J Neurosurg 2007; 106:601–608.
- Iorio A, Agnelli G. Low-molecular-weight and unfractionated heparin for prevention of venous thromboembolism in neurosurgery: a meta-analysis. Arch Intern Med 2000; 160:2327–2332.
- Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126(3 Suppl):338S–400S.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Venous thromboembolic disease. V.1.2007. http://www.nccn.org/professionals/physician_gls/PDF/vte.pdf. Accessed December 5, 2007.
- Kakkar AK, Levine M, Pinedo HM, Wolff R, Wong J. Venous thrombosis in cancer patients: insights from the FRONTLINE survey. Oncologist 2003; 8:381–388.
- Stratton MA, Anderson FA, Bussey HI, et al. Prevention of venous thromboembolism: adherence to the 1995 American College of Chest Physicians consensus guidelines for surgical patients. Arch Intern Med 2000; 160:334–340.
- Bratzler DW, Raskob GE, Murray CK, Bumpus LJ, Piatt DS. Underuse of venous thromboembolism prophylaxis for general surgery patients: physician practices in the community hospital setting. Arch Intern Med 1998; 158:1909–1912.
- Amin A, Stemkowski S, Lin J, et al. Thromboprophylaxis rates in US medical centers: success or failure? J Thromb Haemost 2007; 5:1610–1616.
- Amin A, Stemkowski S, Lin J, Yang G. Thromboprophylaxis in US hospitals: adherence to the 6th American College of Chest Physicians’ recommendations for at-risk medical and surgical patients. Abstract presented at: 41st Midyear Clinical Meeting of the American Society of Health-System Pharmacists; December 3–7, 2006; Anaheim, CA.
- Tapson VF, Decousus H, Pini M, et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest 2007; 132:936–945.
- Clarke-Pearson DL, Synan IS, Dodge R, et al. A randomized trial of low-dose heparin and intermittent pneumatic calf compression for the prevention of deep venous thrombosis after gynecologic oncology surgery. Am J Obstet Gynecol 1993; 168:1146–1154.
- Einstein MH, Pritts EA, Hartenbach EM. Venous thromboembolism prevention in gynecologic cancer surgery: a systematic review. Gynecol Oncol 2007; 105:813–819.
- Clarke-Pearson DL, Synan IS, Hinshaw WM, Coleman RE, Creasman WT. Prevention of postoperative venous thromboembolism by external pneumatic calf compression in patients with gynecologic malignancy. Obstet Gynecol 1984; 63:92–98.
- Ruff RL, Posner JB. Incidence and treatment of peripheral venous thrombosis in patients with glioma. Ann Neurol 1983; 13:334–336.
- Levin JM, Schiff D, Loeffler JS, Fine HA, Black PM, Wen PY. Complications of therapy for venous thromboembolic disease in patients with brain tumors. Neurology 1993; 43:1111–1114.
- Lyman GH, Khorana AA, Falanga A, et al. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol 2007; 25:5490–5505.
- Khorana AA, Francis CW, Culakova E, et al. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost 2007; 5:632–634.
- Ambrus JL, Ambrus CM, Mink IB, Pickren JW. Causes of death in cancer patients. J Med 1975; 6:61–64.
- Donati MB. Cancer and thrombosis. Haemostasis 1994; 24:128–131.
- Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med 1996; 125:1–7.
- Haas S, Wolf H, Kakkar AK, Fareed J, Encke A. Prevention of fatal pulmonary embolism and mortality in surgical patients: a randomized double-blind comparison of LMWH with unfractionated heparin. Thromb Haemost 2005; 94:814–819.
- Kakkar AK, Haas S, Wolf H, Encke A. Evaluation of perioperative fatal pulmonary embolism and death in cancer surgical patients: the MC-4 cancer substudy. Thromb Haemost 2005; 94:867–871.
- Agnelli G, Bolis G, Capussotti L, et al. A clinical outcome-based prospective study on venous thromboembolism after cancer surgery: the @RISTOS project. Ann Surg 2006; 243:89–95.
- Levitan N, Dowlati A, Remick SC, et al. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy: risk analysis using Medicare claims data. Medicine (Baltimore) 1999; 78:285–291.
- Levine M, Gent M, Hirsh J, et al. A comparison of low-molecular-weight heparin administered primarily at home with unfractionated heparin administered in the hospital for proximal deep-vein thrombosis. N Engl J Med 1996; 334:677–681.
- Bergqvist D. Risk of venous thromboembolism in patients undergoing cancer surgery and options for thromboprophylaxis. J Surg Oncol 2007; 95:167–174.
- Heit JA, Silverstein MD, Mohr DN, et al. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med 2000; 160:809–815.
- Sallah S, Wan JY, Nguyen NP. Venous thrombosis in patients with solid tumors: determination of frequency and characteristics. Thromb Haemost 2002; 87:575–579.
- Kröger K, Weiland D, Ose C, et al. Risk factors for venous thromboembolic events in cancer patients. Ann Oncol 2006; 17:297–303.
- Blom JW, Vanderschoot JP, Oostindiër MJ, et al. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: results of a record linkage study. J Thromb Haemost 2006; 4:529–535.
- Couban S, Simpson DR, Barnett MJ, et al. A randomized multicenter comparison of bone marrow and peripheral blood in recipients of matched sibling allogeneic transplants for myeloid malignancies. Blood 2002; 100:1525–1531.
- Walshe LJ, Malak SF, Eagan J, Sepkowitz KA. Complication rates among cancer patients with peripherally inserted central catheters. J Clin Oncol 2002; 20:3276–3281.
- Nilsson KR, Berenholtz SM, Garrett-Mayer E, et al. Association between venous thromboembolism and perioperative allogeneic transfusion. Arch Surg 2007; 142:126–133.
- Efficacy and safety of enoxaparin versus unfractionated heparin for prevention of deep vein thrombosis in elective cancer surgery: a double-blind randomized multicentre trial with venographic assessment. ENOXACAN Study Group. Br J Surg 1997; 84:1099–1103.
- McLeod RS, Geerts WH, Sniderman KW, et al. Subcutaneous heparin versus low-molecular-weight heparin as thromboprophylaxis in patients undergoing colorectal surgery: results of the Canadian Colorectal DVT Prophylaxis Trial: a randomized, double-blind trial. Ann Surg 2001; 233:438–444.
- Mismetti P, Laporte S, Darmon JY, Buchmüller A, Decousus H. Meta-analysis of low molecular weight heparin in the prevention of venous thromboembolism in general surgery. Br J Surg 2001; 88:913–930.
- Agnelli G, Bergqvist D, Cohen AT, et al, on behalf of the PEGASUS investigators. Randomized clinical trial of postoperative fondaparinux versus perioperative dalteparin for prevention of venous thromboembolism in high-risk abdominal surgery. Br J Surg 2005; 92:1212–1220.
- Bergqvist D, Agnelli G, Cohen AT, et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med 2002; 346:975–980.
- Rasmussen MS, Wille-Jorgensen P, Jorgensen LN, et al. Prolonged thromboprophylaxis with low molecular weight heparin (dalteparin) following major abdominal surgery for malignancy [abstract 186]. Blood 2003; 102:56a.
- Leonardi MJ, McGory ML, Ko CY. A systematic review of deep venous thrombosis prophylaxis in cancer patients: implications for improving quality. Ann Surg Oncol 2007; 14:929–936.
- Creekmore FM, Oderda GM, Pendleton RC, Brixner DI. Incidence and economic implications of heparin-induced thrombocytopenia in medical patients receiving prophylaxis for venous thromboembolism. Pharmacotherapy 2006; 26:1438–1445.
- Walsh JJ, Bonnar J, Wright FW. A study of pulmonary embolism and deep leg vein thrombosis after major gynaecological surgery using labeled fibrinogen-phlebography and lung scanning. J Obstet Gynaecol Br Commonw 1974; 81:311–316.
- Clarke-Pearson DL, Synan IS, Coleman RE, et al. The natural history of postoperative venous thromboemboli in gynecologic oncology: a prospective study of 382 patients. Am J Obstet Gynecol 1984; 148:1051–1054.
- Oates-Whitehead RM, D’Angelo A, Mol B. Anticoagulant and aspirin prophylaxis for preventing thromboembolism after major gynaecological surgery. Cochrane Database Syst Rev 2003; (4):CD003679.
- Kibel AS, Loughlin KR. Pathogenesis and prophylaxis of postoperative thromboembolic disease in urological pelvic surgery. J Urol 1995; 153:1763–1774.
- Agnelli G, Piovella F, Buoncristiani P, et al. Enoxaparin plus compression stockings compared with compression stockings alone in the prevention of venous thromboembolism after elective neurosurgery. N Engl J Med 1998; 339:80–85.
- Semrad TJ, O’Donnell R, Wun T, et al. Epidemiology of venous thromboembolism in 9489 patients with malignant glioma. J Neurosurg 2007; 106:601–608.
- Iorio A, Agnelli G. Low-molecular-weight and unfractionated heparin for prevention of venous thromboembolism in neurosurgery: a meta-analysis. Arch Intern Med 2000; 160:2327–2332.
- Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126(3 Suppl):338S–400S.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Venous thromboembolic disease. V.1.2007. http://www.nccn.org/professionals/physician_gls/PDF/vte.pdf. Accessed December 5, 2007.
- Kakkar AK, Levine M, Pinedo HM, Wolff R, Wong J. Venous thrombosis in cancer patients: insights from the FRONTLINE survey. Oncologist 2003; 8:381–388.
- Stratton MA, Anderson FA, Bussey HI, et al. Prevention of venous thromboembolism: adherence to the 1995 American College of Chest Physicians consensus guidelines for surgical patients. Arch Intern Med 2000; 160:334–340.
- Bratzler DW, Raskob GE, Murray CK, Bumpus LJ, Piatt DS. Underuse of venous thromboembolism prophylaxis for general surgery patients: physician practices in the community hospital setting. Arch Intern Med 1998; 158:1909–1912.
- Amin A, Stemkowski S, Lin J, et al. Thromboprophylaxis rates in US medical centers: success or failure? J Thromb Haemost 2007; 5:1610–1616.
- Amin A, Stemkowski S, Lin J, Yang G. Thromboprophylaxis in US hospitals: adherence to the 6th American College of Chest Physicians’ recommendations for at-risk medical and surgical patients. Abstract presented at: 41st Midyear Clinical Meeting of the American Society of Health-System Pharmacists; December 3–7, 2006; Anaheim, CA.
- Tapson VF, Decousus H, Pini M, et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest 2007; 132:936–945.
- Clarke-Pearson DL, Synan IS, Dodge R, et al. A randomized trial of low-dose heparin and intermittent pneumatic calf compression for the prevention of deep venous thrombosis after gynecologic oncology surgery. Am J Obstet Gynecol 1993; 168:1146–1154.
- Einstein MH, Pritts EA, Hartenbach EM. Venous thromboembolism prevention in gynecologic cancer surgery: a systematic review. Gynecol Oncol 2007; 105:813–819.
- Clarke-Pearson DL, Synan IS, Hinshaw WM, Coleman RE, Creasman WT. Prevention of postoperative venous thromboembolism by external pneumatic calf compression in patients with gynecologic malignancy. Obstet Gynecol 1984; 63:92–98.
- Ruff RL, Posner JB. Incidence and treatment of peripheral venous thrombosis in patients with glioma. Ann Neurol 1983; 13:334–336.
- Levin JM, Schiff D, Loeffler JS, Fine HA, Black PM, Wen PY. Complications of therapy for venous thromboembolic disease in patients with brain tumors. Neurology 1993; 43:1111–1114.
Prevention of venous thromboembolism in the orthopedic surgery patient
Nearly half of orthopedic surgery patients do not receive appropriate prophylaxis for venous thromboembolism (VTE), as defined by American College of Chest Physicians (ACCP) consensus guidelines, according to a recent analysis of a nationwide database of hospital admissions.1 Even in teaching hospitals, compliance with consensus guidelines for thromboprophylaxis is suboptimal. In a study of adherence to the ACCP guidelines for VTE prevention among 1,907 surgical patients at 10 teaching hospitals, only 45.2% of hip fracture patients received optimal VTE prophylaxis.2 Rates of optimal prophylaxis were higher among patients undergoing hip arthroplasty and knee arthroplasty—84.3% and 75.9%, respectively—but were still in need of improvement.2
GROWING INTEREST IN POSTOPERATIVE VTE PROPHYLAXIS AS A QUALITY INDICATOR
As noted in the introductory article in this supplement, the Joint Commission on Accreditation of Healthcare Organizations has taken notice of these shortcomings and has proposed national consensus standards for VTE prevention and treatment.3 Among its proposed standards are two related to risk assessment and prophylaxis: whether risk assessment/prophylaxis is ordered within 24 hours of hospital admission and within 24 hours of transfer to the intensive care unit.
Other quality-monitoring initiatives are focused specifically on VTE in the surgical population. The Surgical Care Improvement Project (SCIP) has approved two quality measures with respect to VTE prevention: (1) the proportion of surgical patients for whom recommended VTE prophylaxis is ordered, and (2) the proportion of patients who receive appropriate VTE prophylaxis (based on ACCP guideline recommendations) within 24 hours before or after surgery.4
In the future, two other VTE-related quality measures from SCIP may be implemented by the Centers for Medicare and Medicaid Services: (1) how often intra- or postoperative pulmonary embolism (PE) is diagnosed during the index hospitalization and within 30 days of surgery, and (2) how often intra- or postoperative deep vein thrombosis (DVT) is diagnosed during the index hospitalization and within 30 days of surgery.5
VTE RISK IN ORTHOPEDIC SURGERY
Surgical patients can be stratified into four VTE risk levels—low, moderate, high, and highest—based on age, surgery type, surgery duration, duration of immobilization, and other risk factors.6 For patients undergoing orthopedic surgery, these levels may be defined according to the following patient and surgical characteristics:
- Low risk—surgery duration of less than 30 minutes, age less than 40 years, repair of small fractures
- Moderate risk—age of 40 to 60 years, arthroscopy or repair of lower leg fractures, postoperative plaster cast
- High risk—age greater than 60 years, or age 40 to 60 years with additional VTE risk factors, or immobilization for greater than 4 days
- Highest risk—hip or knee arthroplasty, hip fracture repair, repair of open lower leg fractures, major trauma or spinal cord injury, or multiple risk factors for VTE (age > 40 years, prior VTE, cancer, or hypercoagulable state).
For patients in the low-risk category, no specific prophylaxis is indicated beyond early and aggressive ambulation.6 For those in all other risk categories, prophylaxis with pharmacologic anticoagulant agents and/or mechanical devices is indicated, as reviewed below.
All major orthopedic procedures confer highest risk level
Notably, the “highest risk” category includes any patient undergoing hip or knee arthroplasty or hip fracture repair. Among orthopedic surgery patients in this highest-risk category, rates of VTE events in the absence of prophylaxis are as follows:6
- Calf DVT, 40% to 80%
- Proximal DVT, 10% to 20%
- Clinical PE, 4% to 10%
- Fatal PE, 0.2% to 5%.
Hip replacement poses greater risk than knee replacement
Within this overall highest-risk category, thromboembolic risk in the absence of prophylaxis differs among procedures. Although patients undergoing hip replacement and those undergoing knee replacement have similar rates of DVT of any type,6,7 hip replacement is associated with higher rates of the more clinically important events, specifically proximal DVT and PE. In the absence of prophylaxis, proximal DVT occurs in 23% to 36% of hip replacement patients as opposed to 9% to 20% of knee replacement patients; similarly, PE occurs in 0.7% to 30% of hip replacement patients as compared with 1.8% to 7.0% of knee replacement patients.6,7
What about bleeding risk?
For many orthopedic surgeons, the risk of bleeding as a result of anticoagulant prophylaxis of VTE looms larger than the risk of VTE itself. This is likely because bleeding, when it does occur, is likely to occur more acutely than VTE does and may directly compromise the result of the operation. For this reason, orthopedic surgeons may be more likely to actually witness bleeding events than VTE events (especially fatal PEs) while their patients are still under their care, leading to a misperception of the relative risks of anticoagulation-related bleeding and thromboembolism.
In reality, rates of major bleeding with pharmacologic prophylaxis of VTE are a tiny fraction of the above-listed rates of VTE events in the absence of prophylaxis in patients undergoing major orthopedic surgery. Reported 30-day rates of major bleeding in patients receiving VTE prophylaxis with heparins range from 0.2% to 1.7%; these rates barely differ from the rates among placebo recipients in the same VTE prophylaxis trials, which range from 0.2% to 1.5%.8,9 Additionally, within the continuum of risk of major bleeding from various medical interventions, VTE prophylaxis with heparins is one of the lowest-risk interventions, posing far less risk than, for example, the use of warfarin in ischemic stroke patients or in patients older than 75 years.
PHARMACOLOGIC OPTIONS FOR VTE PROPHYLAXIS IN ORTHOPEDIC SURGERY
As reviewed in the introductory article of this supplement, the arsenal of anticoagulants for use in VTE prophylaxis includes low-dose unfractionated heparin (UFH), low-molecular-weight heparin (LMWH) agents such as dalteparin and enoxaparin, and the factor Xa inhibitor fondaparinux. A few additional comments about these and other anticoagulant options is warranted in the specific context of orthopedic surgery.
Fondaparinux. Because most of its formal US indications are for use as VTE prophylaxis in major orthopedic surgery—including hip replacement, knee replacement, and hip fracture repair—fondaparinux has been studied more widely in orthopedic surgery patients than in the other populations reviewed earlier in this supplement. Nevertheless, its use even in these settings has remained somewhat limited. This may be because of concerns over possible increased bleeding risk relative to some other anticoagulants. Because of bleeding risk, fondaparinux is contraindicated in patients who weigh less than 50 kg, and its package insert recommends caution when it is used in the elderly due to an increased risk of bleeding in patients aged 65 or older. Additionally, the Pentasaccharide in Major Knee Surgery (PENTAMAKS) study found fondaparinux to be associated with a significantly higher incidence of major bleeding compared with enoxaparin (2.1% vs 0.2%; P = .006) in major knee surgery, although it was superior to enoxaparin in preventing VTE.10 Other possible reasons for slow adoption of fondaparinux include its long half-life, which results in a sustained antithrombotic effect, its lack of easy reversibility, and a contraindication in patients with renal insufficiency.11
Limited role for UFH. Low-dose UFH has a more limited role in orthopedic surgery than in other settings requiring VTE prophylaxis, as current ACCP guidelines for VTE prevention recognize it only as a possible option in hip fracture surgery and state that it is not to be considered as sole prophylaxis in patients undergoing hip or knee replacement.6
Warfarin. Although not indicated for use in other VTE prophylaxis settings, the vitamin K antagonist warfarin is recommended as an option for all three major orthopedic surgery indications—knee replacement, hip replacement, and hip fracture repair.6
The key to effective prophylaxis with warfarin is achieving the appropriate intensity of anticoagulation. In two separate analyses, Hylek et al demonstrated a balance between safety and efficacy with warfarin therapy targeted to an international normalized ratio (INR) of 2.0 to 3.0.12,13 An INR greater than 4.0 greatly increased the risk of intracranial hemorrhage, whereas thrombosis was not effectively prevented with an INR less than 2.0.12,13 This latter point should be stressed to orthopedic surgeons, who sometimes aim for INR values below 2.0.
Although anticoagulation clinics are superior to usual care at maintaining the INR within the window of 2.0 to 3.0, only about one-third of patients nationally who take warfarin receive care in such clinics.14 Even with optimal care in anticoagulation clinics, some patients will still receive subtherapeutic or supertherapeutic levels of warfarin, which is one of this agent’s limitations.
Aspirin not recommended as sole agent. Although aspirin is still used as thromboprophylaxis in orthopedic surgery patients, current ACCP guidelines recommend against its use as the sole means of VTE prophylaxis in any patient group.6 The limitations of the evidence for aspirin in this setting are illustrated by the Pulmonary Embolism Prevention study, a multicenter randomized trial in patients undergoing hip fracture (n = 13,356) or hip/knee replacement (n = 4,088).15 Patients received aspirin 160 mg/day or placebo for 5 weeks, starting preoperatively, and were evaluated for outcomes at day 35. Among the hip fracture patients, the rate of symptomatic DVT was lower in the aspirin group than in the placebo group (1.0% vs 1.5%; P = .03), as was the rate of PE (0.7% vs 1.2%, respectively; P = .002), but there was no significant difference in outcomes between the groups among the patients undergoing hip or knee replacement. Notably, 40% of patients in the study also received UFH or LMWH. Further confounding the results, some patients received nonpharmacologic VTE prophylaxis modalities, and others received nonsteroidal anti-inflammatory drugs other than aspirin.
Heparin-induced thrombocytopenia. As noted earlier in this supplement, the incidence of heparin-induced thrombocytopenia (HIT) is markedly higher in patients who receive UFH than in those who receive LMWH. This difference in frequency, which constitutes about a sixfold to eightfold differential, is due to the relationship between standard heparin and platelet factor IV, which can induce formation of IgG antibodies.16 A 50% or greater reduction in platelet count in heparin recipients should prompt consideration of HIT.
Oral direct thrombin inhibitors. Although the oral direct thrombin inhibitor ximelagatran was rejected for approval by the US Food and Drug Administration (FDA) and recently withdrawn from the market worldwide as a result of hepatic risks, other oral direct thrombin inhibitors are in phase 3 studies for use in orthopedic surgery and may be commercially available options for postoperative VTE prophylaxis before long.
GUIDELINES FOR VTE PROPHYLAXIS IN ORTHOPEDIC SURGERY
The ACCP guidelines referred to throughout this article are widely recognized as a practice standard for VTE prevention and treatment, and have been regularly updated throughout recent decades. The most recent version, issued in 2004, is formally known as the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.6 Key orthopedic surgery-related recommendations and notable changes from the previous version of the guidelines, issued in 2001, are outlined below, along with pertinent supportive or illustrative studies.
Hip replacement surgery
In a change from the previous guidelines, the Seventh ACCP Conference recommends extended prophylaxis, for up to 28 to 35 days after surgery, for patients undergoing hip replacement or hip fracture surgery. For hip replacement surgery, this is a Grade 1A recommendation for prophylaxis with either LMWH or warfarin and a Grade 1C+ recommendation (“no RCTs but strong RCT results can be unequivocally extrapolated, or overwhelming evidence from observational studies”) for prophylaxis with fondaparinux.6
Knee replacement surgery
The same three anticoagulant options that received Grade 1A recommendations for patients undergoing total hip replacement—LMWH, fondaparinux, and adjusted-dose warfarin—are also given Grade 1A recommendations as routine thromboprophylaxis in patients undergoing elective knee replacement (see Table 1 for dosing). In addition, optimal use of intermittent pneumatic compression devices is recommended as an alternative option to anticoagulant prophylaxis in these patients (Grade 1B, indicating a “strong recommendation” based on RCTs with important limitations). Use of UFH as the sole agent for prophylaxis is recommended against.6
For both hip and knee replacement surgery, the Seventh ACCP Conference does not endorse superiority of any one of its three recommended prophylaxis options—LMWH, fondaparinux, and adjusted-dose warfarin—over the other two. However, at least four large randomized trials have directly compared LMWH and adjusted-dose warfarin in the setting of arthroplasty—two in total hip replacement surgery19,20 and two in total knee replacement surgery.21,22 Each of these four studies found LMWH to be significantly more effective than warfarin in preventing VTE. In three of the four trials, there was no significant difference between the therapies in rates of major bleeding.19,21,22 In the remaining trial, which was conducted in hip replacement surgery patients and compared postoperative warfarin with dalteparin initiated either immediately before or early after surgery, patients who received preoperative dalteparin initiation (but not those who received postoperative dalteparin initiation) had an increased rate of major bleeding compared with warfarin recipients (P = .01).20
Hip fracture surgery
The supportive evidence for anticoagulant prophylaxis in hip fracture surgery is less robust than that in hip and knee replacement surgery. As a result, only fondaparinux has a Grade 1A recommendation as routine prophylaxis in patients undergoing hip fracture surgery. Options with less definitive recommendations are LMWH (Grade 1C+), low-dose UFH (Grade 1B), and adjusted-dose warfarin (Grade 2B, indicating a “weak recommendation” based on RCTs with important limitations) (see Table 1 for dosing of all agents).6
These differing recommendations are supported by the double-blind Pentasaccharide in Hip Fracture Surgery Study (PENTHIFRA) of 1,711 consecutive patients undergoing surgery for hip fracture repair.23 Patients were randomized to at least 5 days of fondaparinux 2.5 mg once daily, initiated postoperatively, or enoxaparin 40 mg once daily, initiated preoperatively. The incidence of DVT or PE by postoperative day 11 was 8.3% in the fondaparinux arm versus 19.1% in the enoxaparin arm, a statistically significant difference (P < .001) in favor of fondaparinux. There were no differences between the groups in rates of death or clinically relevant bleeding.
As noted above, the newly added recommendation in the Seventh ACCP Conference for extended prophylaxis, for up to 28 to 35 days after surgery, applies to patients undergoing hip fracture surgery as well as those undergoing hip replacement surgery. In the setting of hip fracture repair, extended prophylaxis is a Grade 1A recommendation with the use of fondaparinux and a Grade 1C+ recommendation with the use of either LMWH or adjusted-dose warfarin.6
Lower extremity fractures and trauma
Although lower extremity fractures are very common, the risk of DVT has been poorly studied in this setting. For patients with isolated lower extremity fractures, the Seventh ACCP Conference recommends that clinicians not use thromboprophylaxis routinely (Grade 2A, indicating an “intermediate-strength recommendation” based on RCTs without important limitations).6
Trauma patients, in contrast, are well recognized as being at very high risk for DVT and PE. The Seventh ACCP Conference gives a Grade 1A recommendation to thromboprophylaxis for all trauma patients who have at least one risk factor for VTE. LMWH is recommended (Grade 1A) as the agent of choice for this purpose, provided there are no contraindications to its use, and should be administered as soon as safely possible. Mechanical modalities are reserved for trauma patients with active bleeding or high risk for hemorrhage (Grade 1B). The guidelines recommend against use of inferior vena cava (IVC) filters as primary thromboprophylaxis in trauma patients (Grade 1C, indicating an “intermediate-strength recommendation” based on observational studies).6
Use of ultrasonography
Duplex ultrasonographic screening is recommended in orthopedic trauma patients who are at high risk for VTE and have received suboptimal or no prophylaxis (Grade 1C). In contrast, the Seventh ACCP Conference recommends against routine use of duplex ultrasonography to screen for VTE at hospital discharge in asymptomatic patients following major orthopedic surgery (Grade 1A).6
Knee arthroscopy
Arthroscopic knee procedures are increasing in frequency and raise the specter of a potential role for thromboprophylaxis. However, the clinical diagnosis of DVT is unreliable, and even diagnosis by ultrasonography is unreliable following knee arthroscopy, as interpreting scans of veins below the knee is challenging in this setting.24
The Seventh ACCP Conference recommends that clinicians not use routine thromboprophylaxis, other than early mobilization, for patients who undergo knee arthroscopy (Grade 2B). However, for arthroscopy patients who have inherent risk factors for VTE or who undergo a prolonged or complicated arthroscopy procedure, thromboprophylaxis with LMWH is suggested (Grade 2B).6
RECOMMENDED APPROACH TO VTE PROPHYLAXIS IN ORTHOPEDIC SURGERY
Drawing on the ACCP guidelines and the evidence reviewed above, we have outlined our evidence-based recommendations for pharmacologic VTE prophylaxis in patients undergoing orthopedic surgery, as presented in Table 1. All patients undergoing major orthopedic surgical procedures (ie, procedures other than arthroscopy) should routinely receive anticoagulant prophylaxis unless they have contraindications to anticoagulation. Recommended agents and their duration of use vary according to the type of surgery, as detailed in Table 1.
Extended-duration prophylaxis is recommended for patients undergoing total hip replacement and hip fracture surgery. Aspirin is not recommended as the sole agent for prophylaxis in any orthopedic surgery setting.
Importance of a postoperative prophylaxis protocol
In addition to these broad pharmacologic recommendations, it is important that a postoperative VTE prophylaxis protocol be in place at all hospitals.
At the Ochsner Medical Center in New Orleans, where one of us (S.B.D.) practices, postoperative orders include antithrombotic therapy for surgical patients, starting with placement of thigh-high antiembolism stockings on both legs on the day of surgery for patients undergoing hip replacement and on postoperative day 1 in those undergoing knee replacement. Plantar pneumatic compression devices are applied to both legs in the recovery room and kept on except when the patient is walking. The hospitalist team dictates further anticoagulation orders. If extended prophylaxis is prescribed, the discharge planner sets up drug delivery and reimbursement, provides a LMWH discharge kit, and teaches the patient to self-inject. If there is concern about increasing swelling at the surgical site while anticoagulant therapy continues, the protocol calls for prompt notification of the responsible physician. To minimize the risk that spinal or epidural hematomas will develop, all agents that increase bleeding propensity should be recognized and ordered accordingly.
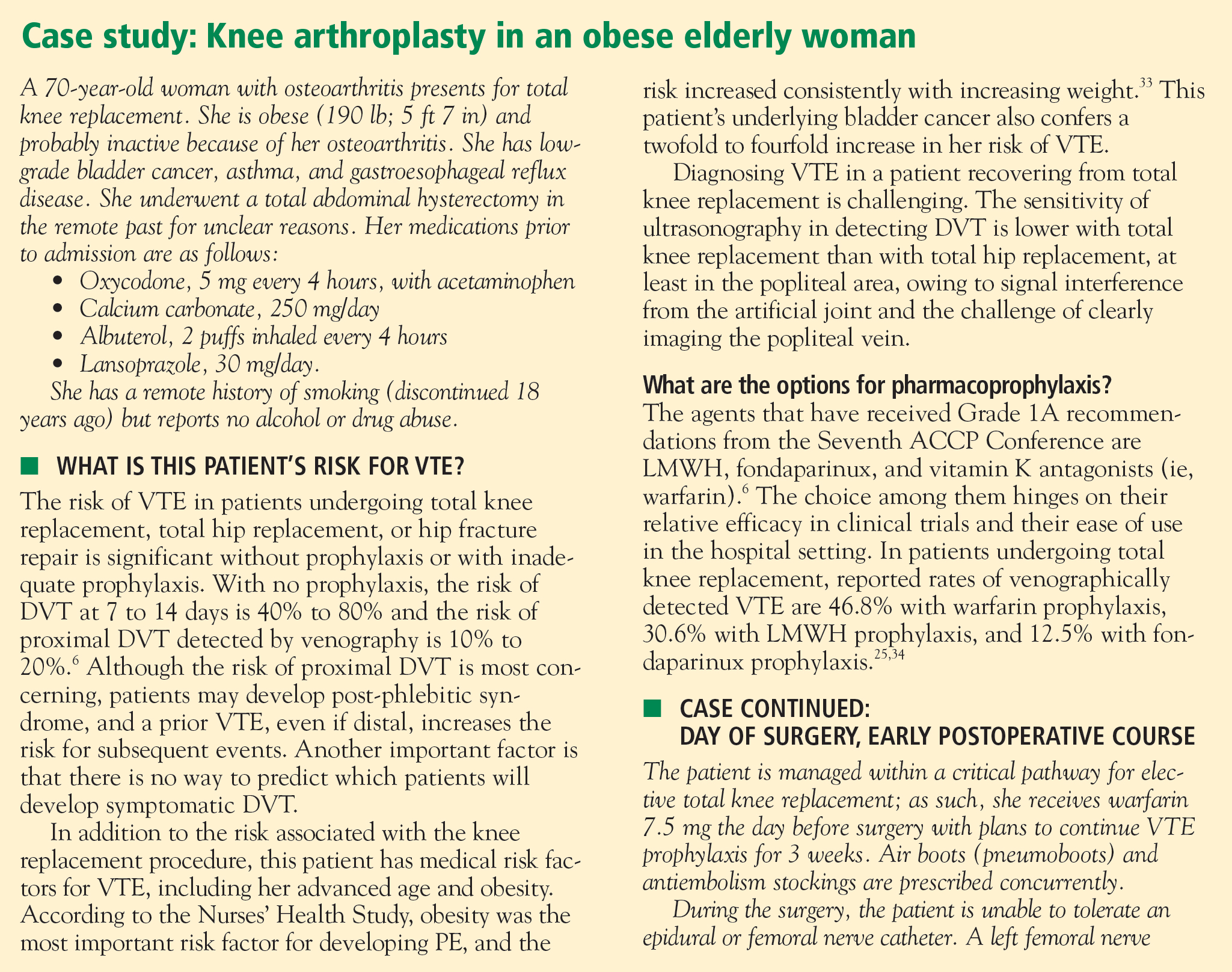


SUMMARY
VTE in patients undergoing major orthopedic surgery is a serious health problem that is highly preventable, yet VTE prophylaxis remains underused in this patient population. Despite the availability of practice guidelines for VTE prevention in the orthopedic surgery setting, recommendations are not widely implemented in clinical practice. Recommended prophylactic options differ somewhat among various orthopedic procedures, and the supportive evidence differs for various anticoagulant options.
DISCUSSION: ADDITIONAL PERSPECTIVES FROM THE AUTHORS
Dr. Jaffer: The ACCP recommends against the routine use of aspirin as primary prophylaxis against VTE in major orthopedic surgery, yet orthopedic surgeons across the country still continue to use aspirin in this setting. What are your thoughts on this, Dr. McKean?
Dr. McKean: We agree with the ACCP’s recommendation against aspirin as primary VTE prophylaxis in orthopedic patients. The percentage of US knee arthroplasty patients who develop VTE after receiving no prophylaxis at all is roughly 64%; this percentage declines only slightly (to 56%) for knee arthroplasty patients who receive prophylaxis with aspirin.25 Since we clearly want to reduce VTE risk as much as possible, I would not use aspirin alone. I would use it only if the patient were already on aspirin, but then I would add either LMWH or fondaparinux.
Dr. Jaffer: Warfarin is another agent that is widely used for prophylaxis in major orthopedic surgery. In fact, the large registries of VTE prevention in major orthopedic surgery suggest that the use of warfarin may be slightly higher than the use of LMWH. If clinicians choose to use warfarin in their practice, what are your recommendations, Dr. Deitelzweig?
Dr. Deitelzweig: As primary prophylaxis for orthopedic surgery patients, warfarin must be dosed to achieve an INR of 2.0 to 3.0; the need for a value in this range is unequivocal. This is a challenging target to attain in the hospital setting.
Dr. Brotman: A study I was involved with a few years ago suggested that warfarin may be inadequate for VTE prevention in the first few days after orthopedic surgery.26 Orthopedic surgeons at the Cleveland Clinic, where I was practicing at the time, routinely used systematic ultrasonography to assess for thrombosis on postoperative day 2 or 3 following hip or knee arthroplasty, so we conducted a secondary analysis of a case-control study in these ultrasonographically screened arthroplasty patients to assess rates of early VTE and look for any associations with the type of prophylaxis used. We found that there was about a tenfold increase in the risk of VTE, both distal and proximal, on postoperative day 2 or 3 among patients who received warfarin compared with those who received LMWH. We concluded that warfarin’s delayed antithrombotic effects may not provide sufficient VTE prophylaxis in the immediate postoperative setting.26
Dr. Deitelzweig: That’s a good point. Although it’s important to achieve a therapeutic level of warfarin, we now have evidence that it takes some time to achieve that level, and in the interim, bad things can happen to patients.
Dr. Jaffer: Orthopedic surgery encompasses several types of procedures. Dr. Amin, which specific orthopedic surgery patients stand to benefit from extended prophylaxis, and how long should extended prophylaxis last?
Dr. Amin: Major orthopedic surgery comprises hip fracture repair, total hip replacement, and total knee replacement. For hip fracture, there are strong data to support the use of extended prophylaxis with fondaparinux 2.5 mg/day, which showed about an 88% relative reduction in the risk of symptomatic VTE compared with standard-duration fondaparinux (6 to 8 days) followed by matching placebo for the extended phase.27 The total duration of fondaparinux therapy in the extended-duration arm was 4 to 5 weeks.
Likewise, data support extended prophylaxis in hip arthroplasty patients, for whom the recommended duration is also 4 to 5 weeks. The systematic review by Hull et al17 demonstrated a 0.41 relative risk of DVT with extended-duration LMWH prophylaxis versus placebo in hip replacement patients (Figure 1), which was a highly statistically significant result.
In contrast, we do not yet have good data to support extended prophylaxis for patients undergoing total knee replacement, which is a bit surprising. In this setting, prophylaxis is recommended for 7 to 14 days but not beyond that.
Dr. Jaffer: Arthroscopy is probably the most common orthopedic procedure performed in the United States today. Dr. Brotman, what is the role of prophylaxis in patients undergoing arthroscopy?
Dr. Brotman: Minor surgery such as arthroscopy can typically be performed safely without routine prophylaxis, other than having the patient ambulate as soon as possible after the procedure. There may be exceptions to this rule, however. I believe that there is potentially a role for pharmacologic prophylaxis in arthroscopy patients who have major risk factors for VTE, such as a personal history of VTE, or who are not expected to become mobile again in a normal rapid fashion after the operation, but prophylaxis has not been studied systematically in such patients.
Dr. Jaffer: Dr. Spyropoulos, there are several new anticoagulants in the pipeline, specifically agents such as the oral direct factor Xa inhibitors and the direct thrombin inhibitors. What do recent clinical trials suggest with regard to the efficacy of these two drug classes for thromboprophylaxis in major orthopedic surgery?
Dr. Spyropoulos: The agents with the most available data are the oral direct factor Xa inhibitors apixaban and rivaroxaban and the oral direct thrombin inhibitor dabigatran. For prophylaxis in orthopedic surgery populations, phase 2 studies have been completed for apixaban and phase 3 trials have been completed for rivaroxaban and dabigatran.
It appears that the factor Xa inhibitors, apixaban and rivaroxaban, are efficacious in comparison with both adjusted-dose warfarin and LMWH, which is the gold standard for this group of patients.28,29 So these indeed appear to be promising agents. Rivaroxaban has been submitted to European regulatory agencies for approval for the prevention of VTE in patients undergoing major orthopedic surgery, and its developer plans to submit it to the FDA in 2008 for a similar indication in the United States.
The data are more equivocal with dabigatran. There have been several positive phase 3 studies in orthopedic surgery comparing two dabigatran dosing schemes, 150 and 220 mg once daily, with the European regimen of enoxaparin (40 mg once daily),30 but a recent study that compared these doses with the North American enoxaparin regimen (30 mg twice daily) failed to meet the criteria for noninferiority.31 Further clinical trial development is necessary for dabigatran, although in January 2008 the European Medicines Agency recommended its marketing approval for thromboprophylaxis in patients undergoing orthopedic procedures.32
I believe that in the next 3 to 5 years our armamentarium will see the addition of at least one, if not more, of these new agents that offer the promise of oral anticoagulation with highly predictable pharmacokinetics and pharmacodynamics and no need for monitoring.
- Yu HT, Dylan ML, Lin J, Dubois RW. Hospitals’ compliance with prophylaxis guidelines for venous thromboembolism. Am J Health Syst Pharm 2007; 64:69–76.
- Stratton MA, Anderson FA, Bussey HI, et al. Prevention of venous thromboembolism: adherence to the 1995 ACCP consensus guidelines for surgical patients. Arch Intern Med 2000; 160:334–340.
- National Consensus Standards for Prevention and Care of Venous Thromboembolism (VTE). The Joint Commission Web site. http://www.jointcommission.org/PerformanceMeasurement/Perform anceMeasurement/VTE.htm. Accessed January 8, 2008.
- Surgical Care Improvement Project. MedQIC Web site. http://www.medqic.org/scip. Accessed January 8, 2008.
- SCIP process and outcome measures, October 2005. MedQIC Web site. http://www.medqic.org. Accessed January 1, 2007.
- Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126(3 Suppl):338S–400S.
- Zimlich RH, Fulbright BM, Friedman RJ. Current status of anticoagulation therapy after total hip and total knee arthroplasty.J Am Acad Orthop Surg 1996; 4:54–62.
- Planes A, Vochelle N, Mazas F, et al. Prevention of postoperative venous thrombosis: a randomized trial comparing unfractionated heparin with low molecular weight heparin in patients undergoing total hip replacement. Thromb Haemost 1988; 60:407–410.
- Colwell CW Jr, Spiro TE, Trowbridge AA, et al. Efficacy and safety of enoxaparin versus unfractionated heparin for prevention of deep venous thrombosis after elective knee arthroplasty. Clin Orthop Relat Res 1995; 321:19–27.
- Bauer KA, Eriksson BI, Lassen MR, Turpie AG. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after elective major knee surgery. N Engl J Med 2001; 345:1305–1310.
- Turpie AGG. Pentasaccharide Org31540/SR90107A clinical trials update: lessons for practice. Am Heart J 2001; 142(Suppl):S9–S15.
- Hylek EM, Singer DE. Risk factors for intracranial hemorrhage in outpatients taking warfarin. Ann Intern Med 1994; 120:897–902.
- Hylek EM, Skates SJ, Sheehan MA, Singer DE. An analysis of the lowest effective intensity of prophylactic anticoagulation for patients with nonrheumatic atrial fibrillation. N Engl J Med 1996; 335:540–546.
- Samsa GP, Matchar DB, Goldstein LB, et al. Quality of anticoagulation management among patients with atrial fibrillation: review of medical records from 2 communities. Arch Intern Med 2000; 160:967–973.
- PEP Trial Collaborative Group. Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary Embolism Prevention (PEP) trial. Lancet 2000; 355:1295–1302.
- Warkentin TE. Heparin-induced thrombocytopenia: pathogenesis and management. Br J Haemotol 2003; 121:535–555.
- Hull RD, Pineo GF, Stein PD, et al. Extended out-of-hospital low-molecular-weight heparin prophylaxis against deep venous thrombosis in patients after elective hip arthroplasty: a systematic review. Ann Intern Med 2001; 135:858–869.
- Haentjens P, De Groote K, Annemans L. Prolonged enoxaparin therapy to prevent venous thromboembolism after primary hip or knee replacement: a cost-utility analysis. Arch Orthop Trauma Surg 2004; 124:507–517.
- Colwell CW Jr, Collis DK, Paulson R, et al. Comparison of enoxaparin and warfarin for the prevention of venous thromboembolic disease after total hip arthroplasty: evaluation during hospitalization and three months after discharge. J Bone Joint Surg Am 1999; 81:932–940.
- Hull RD, Pineo GF, Francis C, et al. Low-molecular-weight heparin prophylaxis using dalteparin in close proximity to surgery vs warfarin in hip arthroplasty patients: a double-blind, randomized comparison. Arch Intern Med 2000; 160:2199–2207.
- Leclerc JR, Geerts WH, Desjardins L, et al. Prevention of venous thromboembolism after knee arthroplasty: a randomized, double-blind trial comparing enoxaparin with warfarin. Ann Intern Med 1996; 124:619–626.
- Fitzgerald RH Jr, Spiro TE, Trowbridge AA, et al. Prevention of venous thromboembolic disease following primary total knee arthroplasty: a randomized, multicenter, open-label, parallel-group comparison of enoxaparin and warfarin. J Bone Joint Surg Am 2001; 83-A:900–906.
- Eriksson BI, Bauer KA, Lassen MR, Turpie AG, Steering Committee of the Pentasaccharide in Hip-Fracture Surgery Study. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after hip-fracture surgery. N Engl J Med 2001; 345:1298–1304.
- Demers C, Marcoux S, Ginsberg JS, Laroche F, Cloutier R, Poulin J. Incidence of venographically proved deep vein thrombosis after knee arthroscopy. Arch Intern Med 1998; 158:47–50.
- Geerts WH, Heit JA, Clagett GP, et al. Prevention of venous thromboembolism. Chest 2001; 119(1 Suppl):132S–175S.
- Brotman DJ, Jaffer AK, Hurbanek JG, Morra N. Warfarin prophylaxis and venous thromboembolism in the first 5 days following hip and knee arthroplasty. Thromb Haemost 2004; 92:1012–1017.
- Eriksson BI, Lassen MR; Pentasaccharide in Hip-Fracture Surgery Plus Investigators. Duration of prophylaxis against venous thromboembolism with fondaparinux after hip fracture surgery: a multicenter, randomized, placebo-controlled, double-blind study. Arch Intern Med 2003; 163:1337–1342.
- The Botticelli Investigators. Late-breaking clinical trial: a dose-finding study of the oral direct factor Xa inhibitor apixaban in the treatment of patients with acute symptomatic deep vein thrombosis [abstract]. Presented at the 21st Congress of the International Society on Thrombosis and Haemostasis; July 2007; Geneva, Switzerland.
- Fisher WD, Eriksson BI, Bauer KA, et al. Rivaroxaban for thromboprophylaxis after orthopaedic surgery: pooled analysis of two studies. Thromb Haemost 2007; 97:931–937.
- Haas S. New oral Xa and IIa inhibitors: updates on clinical trial results. J Thromb Thrombolysis 2008; 25:52–60.
- Friedman RJ, Caprini JA, Comp PC, et al. Dabigatran etexilate vs enoxaparin in preventing venous thromboembolism following total knee arthroplasty. Presented at: 2007 Congress of the International Society on Thrombosis and Haemostasis; July 7–13, 2007; Geneva, Switzerland.
- Committee for Medicinal Products for Human Use summary of positive opinion for Pradaxa [news release]. London, UK: European Medicines Agency. January 24, 2008. http://www.emea.europa.eu/pdfs/human/ opinion/Pradaxa_3503008en.pdf. Accessed February 21, 2008.
- Goldhaber SZ, Grodstein F, Stampfer MJ. A prospective study of risk factors for pulmonary embolism in women. JAMA 1997; 277:642–645.
- Turpie AGG, Bauer KA, Eriksson BI, Lassen MR, for the Steering Committees of the Pentasaccharide Orthopedic Prophylaxis Studies. Fondaparinux vs enoxaparin for the prevention of venous thromboembolism in major orthopedic surgery. Arch Intern Med 2002; 162:1833–1840.
- Stein PD, Beemath A, Matta F, et al. Clinical characteristics of patients with acute pulmonary embolism: data from PIOPED II. Am J Med 2007; 120:871–879.
- Goldhaber SZ. Diagnosis of acute pulmonary embolism: always be vigilant. Am J Med 2007; 120:827–828.
- American Academy of Orthopaedic Surgeons Clinical Guideline on Prevention of Symptomatic Pulmonary Embolism in Patients Undergoing Total Hip or Knee Arthroplasty: Summary of Recommendations. http://www.aaos.org/Research/guidelines/PE_ summary.pdf. Accessed December 10, 2007.
Nearly half of orthopedic surgery patients do not receive appropriate prophylaxis for venous thromboembolism (VTE), as defined by American College of Chest Physicians (ACCP) consensus guidelines, according to a recent analysis of a nationwide database of hospital admissions.1 Even in teaching hospitals, compliance with consensus guidelines for thromboprophylaxis is suboptimal. In a study of adherence to the ACCP guidelines for VTE prevention among 1,907 surgical patients at 10 teaching hospitals, only 45.2% of hip fracture patients received optimal VTE prophylaxis.2 Rates of optimal prophylaxis were higher among patients undergoing hip arthroplasty and knee arthroplasty—84.3% and 75.9%, respectively—but were still in need of improvement.2
GROWING INTEREST IN POSTOPERATIVE VTE PROPHYLAXIS AS A QUALITY INDICATOR
As noted in the introductory article in this supplement, the Joint Commission on Accreditation of Healthcare Organizations has taken notice of these shortcomings and has proposed national consensus standards for VTE prevention and treatment.3 Among its proposed standards are two related to risk assessment and prophylaxis: whether risk assessment/prophylaxis is ordered within 24 hours of hospital admission and within 24 hours of transfer to the intensive care unit.
Other quality-monitoring initiatives are focused specifically on VTE in the surgical population. The Surgical Care Improvement Project (SCIP) has approved two quality measures with respect to VTE prevention: (1) the proportion of surgical patients for whom recommended VTE prophylaxis is ordered, and (2) the proportion of patients who receive appropriate VTE prophylaxis (based on ACCP guideline recommendations) within 24 hours before or after surgery.4
In the future, two other VTE-related quality measures from SCIP may be implemented by the Centers for Medicare and Medicaid Services: (1) how often intra- or postoperative pulmonary embolism (PE) is diagnosed during the index hospitalization and within 30 days of surgery, and (2) how often intra- or postoperative deep vein thrombosis (DVT) is diagnosed during the index hospitalization and within 30 days of surgery.5
VTE RISK IN ORTHOPEDIC SURGERY
Surgical patients can be stratified into four VTE risk levels—low, moderate, high, and highest—based on age, surgery type, surgery duration, duration of immobilization, and other risk factors.6 For patients undergoing orthopedic surgery, these levels may be defined according to the following patient and surgical characteristics:
- Low risk—surgery duration of less than 30 minutes, age less than 40 years, repair of small fractures
- Moderate risk—age of 40 to 60 years, arthroscopy or repair of lower leg fractures, postoperative plaster cast
- High risk—age greater than 60 years, or age 40 to 60 years with additional VTE risk factors, or immobilization for greater than 4 days
- Highest risk—hip or knee arthroplasty, hip fracture repair, repair of open lower leg fractures, major trauma or spinal cord injury, or multiple risk factors for VTE (age > 40 years, prior VTE, cancer, or hypercoagulable state).
For patients in the low-risk category, no specific prophylaxis is indicated beyond early and aggressive ambulation.6 For those in all other risk categories, prophylaxis with pharmacologic anticoagulant agents and/or mechanical devices is indicated, as reviewed below.
All major orthopedic procedures confer highest risk level
Notably, the “highest risk” category includes any patient undergoing hip or knee arthroplasty or hip fracture repair. Among orthopedic surgery patients in this highest-risk category, rates of VTE events in the absence of prophylaxis are as follows:6
- Calf DVT, 40% to 80%
- Proximal DVT, 10% to 20%
- Clinical PE, 4% to 10%
- Fatal PE, 0.2% to 5%.
Hip replacement poses greater risk than knee replacement
Within this overall highest-risk category, thromboembolic risk in the absence of prophylaxis differs among procedures. Although patients undergoing hip replacement and those undergoing knee replacement have similar rates of DVT of any type,6,7 hip replacement is associated with higher rates of the more clinically important events, specifically proximal DVT and PE. In the absence of prophylaxis, proximal DVT occurs in 23% to 36% of hip replacement patients as opposed to 9% to 20% of knee replacement patients; similarly, PE occurs in 0.7% to 30% of hip replacement patients as compared with 1.8% to 7.0% of knee replacement patients.6,7
What about bleeding risk?
For many orthopedic surgeons, the risk of bleeding as a result of anticoagulant prophylaxis of VTE looms larger than the risk of VTE itself. This is likely because bleeding, when it does occur, is likely to occur more acutely than VTE does and may directly compromise the result of the operation. For this reason, orthopedic surgeons may be more likely to actually witness bleeding events than VTE events (especially fatal PEs) while their patients are still under their care, leading to a misperception of the relative risks of anticoagulation-related bleeding and thromboembolism.
In reality, rates of major bleeding with pharmacologic prophylaxis of VTE are a tiny fraction of the above-listed rates of VTE events in the absence of prophylaxis in patients undergoing major orthopedic surgery. Reported 30-day rates of major bleeding in patients receiving VTE prophylaxis with heparins range from 0.2% to 1.7%; these rates barely differ from the rates among placebo recipients in the same VTE prophylaxis trials, which range from 0.2% to 1.5%.8,9 Additionally, within the continuum of risk of major bleeding from various medical interventions, VTE prophylaxis with heparins is one of the lowest-risk interventions, posing far less risk than, for example, the use of warfarin in ischemic stroke patients or in patients older than 75 years.
PHARMACOLOGIC OPTIONS FOR VTE PROPHYLAXIS IN ORTHOPEDIC SURGERY
As reviewed in the introductory article of this supplement, the arsenal of anticoagulants for use in VTE prophylaxis includes low-dose unfractionated heparin (UFH), low-molecular-weight heparin (LMWH) agents such as dalteparin and enoxaparin, and the factor Xa inhibitor fondaparinux. A few additional comments about these and other anticoagulant options is warranted in the specific context of orthopedic surgery.
Fondaparinux. Because most of its formal US indications are for use as VTE prophylaxis in major orthopedic surgery—including hip replacement, knee replacement, and hip fracture repair—fondaparinux has been studied more widely in orthopedic surgery patients than in the other populations reviewed earlier in this supplement. Nevertheless, its use even in these settings has remained somewhat limited. This may be because of concerns over possible increased bleeding risk relative to some other anticoagulants. Because of bleeding risk, fondaparinux is contraindicated in patients who weigh less than 50 kg, and its package insert recommends caution when it is used in the elderly due to an increased risk of bleeding in patients aged 65 or older. Additionally, the Pentasaccharide in Major Knee Surgery (PENTAMAKS) study found fondaparinux to be associated with a significantly higher incidence of major bleeding compared with enoxaparin (2.1% vs 0.2%; P = .006) in major knee surgery, although it was superior to enoxaparin in preventing VTE.10 Other possible reasons for slow adoption of fondaparinux include its long half-life, which results in a sustained antithrombotic effect, its lack of easy reversibility, and a contraindication in patients with renal insufficiency.11
Limited role for UFH. Low-dose UFH has a more limited role in orthopedic surgery than in other settings requiring VTE prophylaxis, as current ACCP guidelines for VTE prevention recognize it only as a possible option in hip fracture surgery and state that it is not to be considered as sole prophylaxis in patients undergoing hip or knee replacement.6
Warfarin. Although not indicated for use in other VTE prophylaxis settings, the vitamin K antagonist warfarin is recommended as an option for all three major orthopedic surgery indications—knee replacement, hip replacement, and hip fracture repair.6
The key to effective prophylaxis with warfarin is achieving the appropriate intensity of anticoagulation. In two separate analyses, Hylek et al demonstrated a balance between safety and efficacy with warfarin therapy targeted to an international normalized ratio (INR) of 2.0 to 3.0.12,13 An INR greater than 4.0 greatly increased the risk of intracranial hemorrhage, whereas thrombosis was not effectively prevented with an INR less than 2.0.12,13 This latter point should be stressed to orthopedic surgeons, who sometimes aim for INR values below 2.0.
Although anticoagulation clinics are superior to usual care at maintaining the INR within the window of 2.0 to 3.0, only about one-third of patients nationally who take warfarin receive care in such clinics.14 Even with optimal care in anticoagulation clinics, some patients will still receive subtherapeutic or supertherapeutic levels of warfarin, which is one of this agent’s limitations.
Aspirin not recommended as sole agent. Although aspirin is still used as thromboprophylaxis in orthopedic surgery patients, current ACCP guidelines recommend against its use as the sole means of VTE prophylaxis in any patient group.6 The limitations of the evidence for aspirin in this setting are illustrated by the Pulmonary Embolism Prevention study, a multicenter randomized trial in patients undergoing hip fracture (n = 13,356) or hip/knee replacement (n = 4,088).15 Patients received aspirin 160 mg/day or placebo for 5 weeks, starting preoperatively, and were evaluated for outcomes at day 35. Among the hip fracture patients, the rate of symptomatic DVT was lower in the aspirin group than in the placebo group (1.0% vs 1.5%; P = .03), as was the rate of PE (0.7% vs 1.2%, respectively; P = .002), but there was no significant difference in outcomes between the groups among the patients undergoing hip or knee replacement. Notably, 40% of patients in the study also received UFH or LMWH. Further confounding the results, some patients received nonpharmacologic VTE prophylaxis modalities, and others received nonsteroidal anti-inflammatory drugs other than aspirin.
Heparin-induced thrombocytopenia. As noted earlier in this supplement, the incidence of heparin-induced thrombocytopenia (HIT) is markedly higher in patients who receive UFH than in those who receive LMWH. This difference in frequency, which constitutes about a sixfold to eightfold differential, is due to the relationship between standard heparin and platelet factor IV, which can induce formation of IgG antibodies.16 A 50% or greater reduction in platelet count in heparin recipients should prompt consideration of HIT.
Oral direct thrombin inhibitors. Although the oral direct thrombin inhibitor ximelagatran was rejected for approval by the US Food and Drug Administration (FDA) and recently withdrawn from the market worldwide as a result of hepatic risks, other oral direct thrombin inhibitors are in phase 3 studies for use in orthopedic surgery and may be commercially available options for postoperative VTE prophylaxis before long.
GUIDELINES FOR VTE PROPHYLAXIS IN ORTHOPEDIC SURGERY
The ACCP guidelines referred to throughout this article are widely recognized as a practice standard for VTE prevention and treatment, and have been regularly updated throughout recent decades. The most recent version, issued in 2004, is formally known as the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.6 Key orthopedic surgery-related recommendations and notable changes from the previous version of the guidelines, issued in 2001, are outlined below, along with pertinent supportive or illustrative studies.
Hip replacement surgery
In a change from the previous guidelines, the Seventh ACCP Conference recommends extended prophylaxis, for up to 28 to 35 days after surgery, for patients undergoing hip replacement or hip fracture surgery. For hip replacement surgery, this is a Grade 1A recommendation for prophylaxis with either LMWH or warfarin and a Grade 1C+ recommendation (“no RCTs but strong RCT results can be unequivocally extrapolated, or overwhelming evidence from observational studies”) for prophylaxis with fondaparinux.6
Knee replacement surgery
The same three anticoagulant options that received Grade 1A recommendations for patients undergoing total hip replacement—LMWH, fondaparinux, and adjusted-dose warfarin—are also given Grade 1A recommendations as routine thromboprophylaxis in patients undergoing elective knee replacement (see Table 1 for dosing). In addition, optimal use of intermittent pneumatic compression devices is recommended as an alternative option to anticoagulant prophylaxis in these patients (Grade 1B, indicating a “strong recommendation” based on RCTs with important limitations). Use of UFH as the sole agent for prophylaxis is recommended against.6
For both hip and knee replacement surgery, the Seventh ACCP Conference does not endorse superiority of any one of its three recommended prophylaxis options—LMWH, fondaparinux, and adjusted-dose warfarin—over the other two. However, at least four large randomized trials have directly compared LMWH and adjusted-dose warfarin in the setting of arthroplasty—two in total hip replacement surgery19,20 and two in total knee replacement surgery.21,22 Each of these four studies found LMWH to be significantly more effective than warfarin in preventing VTE. In three of the four trials, there was no significant difference between the therapies in rates of major bleeding.19,21,22 In the remaining trial, which was conducted in hip replacement surgery patients and compared postoperative warfarin with dalteparin initiated either immediately before or early after surgery, patients who received preoperative dalteparin initiation (but not those who received postoperative dalteparin initiation) had an increased rate of major bleeding compared with warfarin recipients (P = .01).20
Hip fracture surgery
The supportive evidence for anticoagulant prophylaxis in hip fracture surgery is less robust than that in hip and knee replacement surgery. As a result, only fondaparinux has a Grade 1A recommendation as routine prophylaxis in patients undergoing hip fracture surgery. Options with less definitive recommendations are LMWH (Grade 1C+), low-dose UFH (Grade 1B), and adjusted-dose warfarin (Grade 2B, indicating a “weak recommendation” based on RCTs with important limitations) (see Table 1 for dosing of all agents).6
These differing recommendations are supported by the double-blind Pentasaccharide in Hip Fracture Surgery Study (PENTHIFRA) of 1,711 consecutive patients undergoing surgery for hip fracture repair.23 Patients were randomized to at least 5 days of fondaparinux 2.5 mg once daily, initiated postoperatively, or enoxaparin 40 mg once daily, initiated preoperatively. The incidence of DVT or PE by postoperative day 11 was 8.3% in the fondaparinux arm versus 19.1% in the enoxaparin arm, a statistically significant difference (P < .001) in favor of fondaparinux. There were no differences between the groups in rates of death or clinically relevant bleeding.
As noted above, the newly added recommendation in the Seventh ACCP Conference for extended prophylaxis, for up to 28 to 35 days after surgery, applies to patients undergoing hip fracture surgery as well as those undergoing hip replacement surgery. In the setting of hip fracture repair, extended prophylaxis is a Grade 1A recommendation with the use of fondaparinux and a Grade 1C+ recommendation with the use of either LMWH or adjusted-dose warfarin.6
Lower extremity fractures and trauma
Although lower extremity fractures are very common, the risk of DVT has been poorly studied in this setting. For patients with isolated lower extremity fractures, the Seventh ACCP Conference recommends that clinicians not use thromboprophylaxis routinely (Grade 2A, indicating an “intermediate-strength recommendation” based on RCTs without important limitations).6
Trauma patients, in contrast, are well recognized as being at very high risk for DVT and PE. The Seventh ACCP Conference gives a Grade 1A recommendation to thromboprophylaxis for all trauma patients who have at least one risk factor for VTE. LMWH is recommended (Grade 1A) as the agent of choice for this purpose, provided there are no contraindications to its use, and should be administered as soon as safely possible. Mechanical modalities are reserved for trauma patients with active bleeding or high risk for hemorrhage (Grade 1B). The guidelines recommend against use of inferior vena cava (IVC) filters as primary thromboprophylaxis in trauma patients (Grade 1C, indicating an “intermediate-strength recommendation” based on observational studies).6
Use of ultrasonography
Duplex ultrasonographic screening is recommended in orthopedic trauma patients who are at high risk for VTE and have received suboptimal or no prophylaxis (Grade 1C). In contrast, the Seventh ACCP Conference recommends against routine use of duplex ultrasonography to screen for VTE at hospital discharge in asymptomatic patients following major orthopedic surgery (Grade 1A).6
Knee arthroscopy
Arthroscopic knee procedures are increasing in frequency and raise the specter of a potential role for thromboprophylaxis. However, the clinical diagnosis of DVT is unreliable, and even diagnosis by ultrasonography is unreliable following knee arthroscopy, as interpreting scans of veins below the knee is challenging in this setting.24
The Seventh ACCP Conference recommends that clinicians not use routine thromboprophylaxis, other than early mobilization, for patients who undergo knee arthroscopy (Grade 2B). However, for arthroscopy patients who have inherent risk factors for VTE or who undergo a prolonged or complicated arthroscopy procedure, thromboprophylaxis with LMWH is suggested (Grade 2B).6
RECOMMENDED APPROACH TO VTE PROPHYLAXIS IN ORTHOPEDIC SURGERY
Drawing on the ACCP guidelines and the evidence reviewed above, we have outlined our evidence-based recommendations for pharmacologic VTE prophylaxis in patients undergoing orthopedic surgery, as presented in Table 1. All patients undergoing major orthopedic surgical procedures (ie, procedures other than arthroscopy) should routinely receive anticoagulant prophylaxis unless they have contraindications to anticoagulation. Recommended agents and their duration of use vary according to the type of surgery, as detailed in Table 1.
Extended-duration prophylaxis is recommended for patients undergoing total hip replacement and hip fracture surgery. Aspirin is not recommended as the sole agent for prophylaxis in any orthopedic surgery setting.
Importance of a postoperative prophylaxis protocol
In addition to these broad pharmacologic recommendations, it is important that a postoperative VTE prophylaxis protocol be in place at all hospitals.
At the Ochsner Medical Center in New Orleans, where one of us (S.B.D.) practices, postoperative orders include antithrombotic therapy for surgical patients, starting with placement of thigh-high antiembolism stockings on both legs on the day of surgery for patients undergoing hip replacement and on postoperative day 1 in those undergoing knee replacement. Plantar pneumatic compression devices are applied to both legs in the recovery room and kept on except when the patient is walking. The hospitalist team dictates further anticoagulation orders. If extended prophylaxis is prescribed, the discharge planner sets up drug delivery and reimbursement, provides a LMWH discharge kit, and teaches the patient to self-inject. If there is concern about increasing swelling at the surgical site while anticoagulant therapy continues, the protocol calls for prompt notification of the responsible physician. To minimize the risk that spinal or epidural hematomas will develop, all agents that increase bleeding propensity should be recognized and ordered accordingly.



SUMMARY
VTE in patients undergoing major orthopedic surgery is a serious health problem that is highly preventable, yet VTE prophylaxis remains underused in this patient population. Despite the availability of practice guidelines for VTE prevention in the orthopedic surgery setting, recommendations are not widely implemented in clinical practice. Recommended prophylactic options differ somewhat among various orthopedic procedures, and the supportive evidence differs for various anticoagulant options.
DISCUSSION: ADDITIONAL PERSPECTIVES FROM THE AUTHORS
Dr. Jaffer: The ACCP recommends against the routine use of aspirin as primary prophylaxis against VTE in major orthopedic surgery, yet orthopedic surgeons across the country still continue to use aspirin in this setting. What are your thoughts on this, Dr. McKean?
Dr. McKean: We agree with the ACCP’s recommendation against aspirin as primary VTE prophylaxis in orthopedic patients. The percentage of US knee arthroplasty patients who develop VTE after receiving no prophylaxis at all is roughly 64%; this percentage declines only slightly (to 56%) for knee arthroplasty patients who receive prophylaxis with aspirin.25 Since we clearly want to reduce VTE risk as much as possible, I would not use aspirin alone. I would use it only if the patient were already on aspirin, but then I would add either LMWH or fondaparinux.
Dr. Jaffer: Warfarin is another agent that is widely used for prophylaxis in major orthopedic surgery. In fact, the large registries of VTE prevention in major orthopedic surgery suggest that the use of warfarin may be slightly higher than the use of LMWH. If clinicians choose to use warfarin in their practice, what are your recommendations, Dr. Deitelzweig?
Dr. Deitelzweig: As primary prophylaxis for orthopedic surgery patients, warfarin must be dosed to achieve an INR of 2.0 to 3.0; the need for a value in this range is unequivocal. This is a challenging target to attain in the hospital setting.
Dr. Brotman: A study I was involved with a few years ago suggested that warfarin may be inadequate for VTE prevention in the first few days after orthopedic surgery.26 Orthopedic surgeons at the Cleveland Clinic, where I was practicing at the time, routinely used systematic ultrasonography to assess for thrombosis on postoperative day 2 or 3 following hip or knee arthroplasty, so we conducted a secondary analysis of a case-control study in these ultrasonographically screened arthroplasty patients to assess rates of early VTE and look for any associations with the type of prophylaxis used. We found that there was about a tenfold increase in the risk of VTE, both distal and proximal, on postoperative day 2 or 3 among patients who received warfarin compared with those who received LMWH. We concluded that warfarin’s delayed antithrombotic effects may not provide sufficient VTE prophylaxis in the immediate postoperative setting.26
Dr. Deitelzweig: That’s a good point. Although it’s important to achieve a therapeutic level of warfarin, we now have evidence that it takes some time to achieve that level, and in the interim, bad things can happen to patients.
Dr. Jaffer: Orthopedic surgery encompasses several types of procedures. Dr. Amin, which specific orthopedic surgery patients stand to benefit from extended prophylaxis, and how long should extended prophylaxis last?
Dr. Amin: Major orthopedic surgery comprises hip fracture repair, total hip replacement, and total knee replacement. For hip fracture, there are strong data to support the use of extended prophylaxis with fondaparinux 2.5 mg/day, which showed about an 88% relative reduction in the risk of symptomatic VTE compared with standard-duration fondaparinux (6 to 8 days) followed by matching placebo for the extended phase.27 The total duration of fondaparinux therapy in the extended-duration arm was 4 to 5 weeks.
Likewise, data support extended prophylaxis in hip arthroplasty patients, for whom the recommended duration is also 4 to 5 weeks. The systematic review by Hull et al17 demonstrated a 0.41 relative risk of DVT with extended-duration LMWH prophylaxis versus placebo in hip replacement patients (Figure 1), which was a highly statistically significant result.
In contrast, we do not yet have good data to support extended prophylaxis for patients undergoing total knee replacement, which is a bit surprising. In this setting, prophylaxis is recommended for 7 to 14 days but not beyond that.
Dr. Jaffer: Arthroscopy is probably the most common orthopedic procedure performed in the United States today. Dr. Brotman, what is the role of prophylaxis in patients undergoing arthroscopy?
Dr. Brotman: Minor surgery such as arthroscopy can typically be performed safely without routine prophylaxis, other than having the patient ambulate as soon as possible after the procedure. There may be exceptions to this rule, however. I believe that there is potentially a role for pharmacologic prophylaxis in arthroscopy patients who have major risk factors for VTE, such as a personal history of VTE, or who are not expected to become mobile again in a normal rapid fashion after the operation, but prophylaxis has not been studied systematically in such patients.
Dr. Jaffer: Dr. Spyropoulos, there are several new anticoagulants in the pipeline, specifically agents such as the oral direct factor Xa inhibitors and the direct thrombin inhibitors. What do recent clinical trials suggest with regard to the efficacy of these two drug classes for thromboprophylaxis in major orthopedic surgery?
Dr. Spyropoulos: The agents with the most available data are the oral direct factor Xa inhibitors apixaban and rivaroxaban and the oral direct thrombin inhibitor dabigatran. For prophylaxis in orthopedic surgery populations, phase 2 studies have been completed for apixaban and phase 3 trials have been completed for rivaroxaban and dabigatran.
It appears that the factor Xa inhibitors, apixaban and rivaroxaban, are efficacious in comparison with both adjusted-dose warfarin and LMWH, which is the gold standard for this group of patients.28,29 So these indeed appear to be promising agents. Rivaroxaban has been submitted to European regulatory agencies for approval for the prevention of VTE in patients undergoing major orthopedic surgery, and its developer plans to submit it to the FDA in 2008 for a similar indication in the United States.
The data are more equivocal with dabigatran. There have been several positive phase 3 studies in orthopedic surgery comparing two dabigatran dosing schemes, 150 and 220 mg once daily, with the European regimen of enoxaparin (40 mg once daily),30 but a recent study that compared these doses with the North American enoxaparin regimen (30 mg twice daily) failed to meet the criteria for noninferiority.31 Further clinical trial development is necessary for dabigatran, although in January 2008 the European Medicines Agency recommended its marketing approval for thromboprophylaxis in patients undergoing orthopedic procedures.32
I believe that in the next 3 to 5 years our armamentarium will see the addition of at least one, if not more, of these new agents that offer the promise of oral anticoagulation with highly predictable pharmacokinetics and pharmacodynamics and no need for monitoring.
Nearly half of orthopedic surgery patients do not receive appropriate prophylaxis for venous thromboembolism (VTE), as defined by American College of Chest Physicians (ACCP) consensus guidelines, according to a recent analysis of a nationwide database of hospital admissions.1 Even in teaching hospitals, compliance with consensus guidelines for thromboprophylaxis is suboptimal. In a study of adherence to the ACCP guidelines for VTE prevention among 1,907 surgical patients at 10 teaching hospitals, only 45.2% of hip fracture patients received optimal VTE prophylaxis.2 Rates of optimal prophylaxis were higher among patients undergoing hip arthroplasty and knee arthroplasty—84.3% and 75.9%, respectively—but were still in need of improvement.2
GROWING INTEREST IN POSTOPERATIVE VTE PROPHYLAXIS AS A QUALITY INDICATOR
As noted in the introductory article in this supplement, the Joint Commission on Accreditation of Healthcare Organizations has taken notice of these shortcomings and has proposed national consensus standards for VTE prevention and treatment.3 Among its proposed standards are two related to risk assessment and prophylaxis: whether risk assessment/prophylaxis is ordered within 24 hours of hospital admission and within 24 hours of transfer to the intensive care unit.
Other quality-monitoring initiatives are focused specifically on VTE in the surgical population. The Surgical Care Improvement Project (SCIP) has approved two quality measures with respect to VTE prevention: (1) the proportion of surgical patients for whom recommended VTE prophylaxis is ordered, and (2) the proportion of patients who receive appropriate VTE prophylaxis (based on ACCP guideline recommendations) within 24 hours before or after surgery.4
In the future, two other VTE-related quality measures from SCIP may be implemented by the Centers for Medicare and Medicaid Services: (1) how often intra- or postoperative pulmonary embolism (PE) is diagnosed during the index hospitalization and within 30 days of surgery, and (2) how often intra- or postoperative deep vein thrombosis (DVT) is diagnosed during the index hospitalization and within 30 days of surgery.5
VTE RISK IN ORTHOPEDIC SURGERY
Surgical patients can be stratified into four VTE risk levels—low, moderate, high, and highest—based on age, surgery type, surgery duration, duration of immobilization, and other risk factors.6 For patients undergoing orthopedic surgery, these levels may be defined according to the following patient and surgical characteristics:
- Low risk—surgery duration of less than 30 minutes, age less than 40 years, repair of small fractures
- Moderate risk—age of 40 to 60 years, arthroscopy or repair of lower leg fractures, postoperative plaster cast
- High risk—age greater than 60 years, or age 40 to 60 years with additional VTE risk factors, or immobilization for greater than 4 days
- Highest risk—hip or knee arthroplasty, hip fracture repair, repair of open lower leg fractures, major trauma or spinal cord injury, or multiple risk factors for VTE (age > 40 years, prior VTE, cancer, or hypercoagulable state).
For patients in the low-risk category, no specific prophylaxis is indicated beyond early and aggressive ambulation.6 For those in all other risk categories, prophylaxis with pharmacologic anticoagulant agents and/or mechanical devices is indicated, as reviewed below.
All major orthopedic procedures confer highest risk level
Notably, the “highest risk” category includes any patient undergoing hip or knee arthroplasty or hip fracture repair. Among orthopedic surgery patients in this highest-risk category, rates of VTE events in the absence of prophylaxis are as follows:6
- Calf DVT, 40% to 80%
- Proximal DVT, 10% to 20%
- Clinical PE, 4% to 10%
- Fatal PE, 0.2% to 5%.
Hip replacement poses greater risk than knee replacement
Within this overall highest-risk category, thromboembolic risk in the absence of prophylaxis differs among procedures. Although patients undergoing hip replacement and those undergoing knee replacement have similar rates of DVT of any type,6,7 hip replacement is associated with higher rates of the more clinically important events, specifically proximal DVT and PE. In the absence of prophylaxis, proximal DVT occurs in 23% to 36% of hip replacement patients as opposed to 9% to 20% of knee replacement patients; similarly, PE occurs in 0.7% to 30% of hip replacement patients as compared with 1.8% to 7.0% of knee replacement patients.6,7
What about bleeding risk?
For many orthopedic surgeons, the risk of bleeding as a result of anticoagulant prophylaxis of VTE looms larger than the risk of VTE itself. This is likely because bleeding, when it does occur, is likely to occur more acutely than VTE does and may directly compromise the result of the operation. For this reason, orthopedic surgeons may be more likely to actually witness bleeding events than VTE events (especially fatal PEs) while their patients are still under their care, leading to a misperception of the relative risks of anticoagulation-related bleeding and thromboembolism.
In reality, rates of major bleeding with pharmacologic prophylaxis of VTE are a tiny fraction of the above-listed rates of VTE events in the absence of prophylaxis in patients undergoing major orthopedic surgery. Reported 30-day rates of major bleeding in patients receiving VTE prophylaxis with heparins range from 0.2% to 1.7%; these rates barely differ from the rates among placebo recipients in the same VTE prophylaxis trials, which range from 0.2% to 1.5%.8,9 Additionally, within the continuum of risk of major bleeding from various medical interventions, VTE prophylaxis with heparins is one of the lowest-risk interventions, posing far less risk than, for example, the use of warfarin in ischemic stroke patients or in patients older than 75 years.
PHARMACOLOGIC OPTIONS FOR VTE PROPHYLAXIS IN ORTHOPEDIC SURGERY
As reviewed in the introductory article of this supplement, the arsenal of anticoagulants for use in VTE prophylaxis includes low-dose unfractionated heparin (UFH), low-molecular-weight heparin (LMWH) agents such as dalteparin and enoxaparin, and the factor Xa inhibitor fondaparinux. A few additional comments about these and other anticoagulant options is warranted in the specific context of orthopedic surgery.
Fondaparinux. Because most of its formal US indications are for use as VTE prophylaxis in major orthopedic surgery—including hip replacement, knee replacement, and hip fracture repair—fondaparinux has been studied more widely in orthopedic surgery patients than in the other populations reviewed earlier in this supplement. Nevertheless, its use even in these settings has remained somewhat limited. This may be because of concerns over possible increased bleeding risk relative to some other anticoagulants. Because of bleeding risk, fondaparinux is contraindicated in patients who weigh less than 50 kg, and its package insert recommends caution when it is used in the elderly due to an increased risk of bleeding in patients aged 65 or older. Additionally, the Pentasaccharide in Major Knee Surgery (PENTAMAKS) study found fondaparinux to be associated with a significantly higher incidence of major bleeding compared with enoxaparin (2.1% vs 0.2%; P = .006) in major knee surgery, although it was superior to enoxaparin in preventing VTE.10 Other possible reasons for slow adoption of fondaparinux include its long half-life, which results in a sustained antithrombotic effect, its lack of easy reversibility, and a contraindication in patients with renal insufficiency.11
Limited role for UFH. Low-dose UFH has a more limited role in orthopedic surgery than in other settings requiring VTE prophylaxis, as current ACCP guidelines for VTE prevention recognize it only as a possible option in hip fracture surgery and state that it is not to be considered as sole prophylaxis in patients undergoing hip or knee replacement.6
Warfarin. Although not indicated for use in other VTE prophylaxis settings, the vitamin K antagonist warfarin is recommended as an option for all three major orthopedic surgery indications—knee replacement, hip replacement, and hip fracture repair.6
The key to effective prophylaxis with warfarin is achieving the appropriate intensity of anticoagulation. In two separate analyses, Hylek et al demonstrated a balance between safety and efficacy with warfarin therapy targeted to an international normalized ratio (INR) of 2.0 to 3.0.12,13 An INR greater than 4.0 greatly increased the risk of intracranial hemorrhage, whereas thrombosis was not effectively prevented with an INR less than 2.0.12,13 This latter point should be stressed to orthopedic surgeons, who sometimes aim for INR values below 2.0.
Although anticoagulation clinics are superior to usual care at maintaining the INR within the window of 2.0 to 3.0, only about one-third of patients nationally who take warfarin receive care in such clinics.14 Even with optimal care in anticoagulation clinics, some patients will still receive subtherapeutic or supertherapeutic levels of warfarin, which is one of this agent’s limitations.
Aspirin not recommended as sole agent. Although aspirin is still used as thromboprophylaxis in orthopedic surgery patients, current ACCP guidelines recommend against its use as the sole means of VTE prophylaxis in any patient group.6 The limitations of the evidence for aspirin in this setting are illustrated by the Pulmonary Embolism Prevention study, a multicenter randomized trial in patients undergoing hip fracture (n = 13,356) or hip/knee replacement (n = 4,088).15 Patients received aspirin 160 mg/day or placebo for 5 weeks, starting preoperatively, and were evaluated for outcomes at day 35. Among the hip fracture patients, the rate of symptomatic DVT was lower in the aspirin group than in the placebo group (1.0% vs 1.5%; P = .03), as was the rate of PE (0.7% vs 1.2%, respectively; P = .002), but there was no significant difference in outcomes between the groups among the patients undergoing hip or knee replacement. Notably, 40% of patients in the study also received UFH or LMWH. Further confounding the results, some patients received nonpharmacologic VTE prophylaxis modalities, and others received nonsteroidal anti-inflammatory drugs other than aspirin.
Heparin-induced thrombocytopenia. As noted earlier in this supplement, the incidence of heparin-induced thrombocytopenia (HIT) is markedly higher in patients who receive UFH than in those who receive LMWH. This difference in frequency, which constitutes about a sixfold to eightfold differential, is due to the relationship between standard heparin and platelet factor IV, which can induce formation of IgG antibodies.16 A 50% or greater reduction in platelet count in heparin recipients should prompt consideration of HIT.
Oral direct thrombin inhibitors. Although the oral direct thrombin inhibitor ximelagatran was rejected for approval by the US Food and Drug Administration (FDA) and recently withdrawn from the market worldwide as a result of hepatic risks, other oral direct thrombin inhibitors are in phase 3 studies for use in orthopedic surgery and may be commercially available options for postoperative VTE prophylaxis before long.
GUIDELINES FOR VTE PROPHYLAXIS IN ORTHOPEDIC SURGERY
The ACCP guidelines referred to throughout this article are widely recognized as a practice standard for VTE prevention and treatment, and have been regularly updated throughout recent decades. The most recent version, issued in 2004, is formally known as the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.6 Key orthopedic surgery-related recommendations and notable changes from the previous version of the guidelines, issued in 2001, are outlined below, along with pertinent supportive or illustrative studies.
Hip replacement surgery
In a change from the previous guidelines, the Seventh ACCP Conference recommends extended prophylaxis, for up to 28 to 35 days after surgery, for patients undergoing hip replacement or hip fracture surgery. For hip replacement surgery, this is a Grade 1A recommendation for prophylaxis with either LMWH or warfarin and a Grade 1C+ recommendation (“no RCTs but strong RCT results can be unequivocally extrapolated, or overwhelming evidence from observational studies”) for prophylaxis with fondaparinux.6
Knee replacement surgery
The same three anticoagulant options that received Grade 1A recommendations for patients undergoing total hip replacement—LMWH, fondaparinux, and adjusted-dose warfarin—are also given Grade 1A recommendations as routine thromboprophylaxis in patients undergoing elective knee replacement (see Table 1 for dosing). In addition, optimal use of intermittent pneumatic compression devices is recommended as an alternative option to anticoagulant prophylaxis in these patients (Grade 1B, indicating a “strong recommendation” based on RCTs with important limitations). Use of UFH as the sole agent for prophylaxis is recommended against.6
For both hip and knee replacement surgery, the Seventh ACCP Conference does not endorse superiority of any one of its three recommended prophylaxis options—LMWH, fondaparinux, and adjusted-dose warfarin—over the other two. However, at least four large randomized trials have directly compared LMWH and adjusted-dose warfarin in the setting of arthroplasty—two in total hip replacement surgery19,20 and two in total knee replacement surgery.21,22 Each of these four studies found LMWH to be significantly more effective than warfarin in preventing VTE. In three of the four trials, there was no significant difference between the therapies in rates of major bleeding.19,21,22 In the remaining trial, which was conducted in hip replacement surgery patients and compared postoperative warfarin with dalteparin initiated either immediately before or early after surgery, patients who received preoperative dalteparin initiation (but not those who received postoperative dalteparin initiation) had an increased rate of major bleeding compared with warfarin recipients (P = .01).20
Hip fracture surgery
The supportive evidence for anticoagulant prophylaxis in hip fracture surgery is less robust than that in hip and knee replacement surgery. As a result, only fondaparinux has a Grade 1A recommendation as routine prophylaxis in patients undergoing hip fracture surgery. Options with less definitive recommendations are LMWH (Grade 1C+), low-dose UFH (Grade 1B), and adjusted-dose warfarin (Grade 2B, indicating a “weak recommendation” based on RCTs with important limitations) (see Table 1 for dosing of all agents).6
These differing recommendations are supported by the double-blind Pentasaccharide in Hip Fracture Surgery Study (PENTHIFRA) of 1,711 consecutive patients undergoing surgery for hip fracture repair.23 Patients were randomized to at least 5 days of fondaparinux 2.5 mg once daily, initiated postoperatively, or enoxaparin 40 mg once daily, initiated preoperatively. The incidence of DVT or PE by postoperative day 11 was 8.3% in the fondaparinux arm versus 19.1% in the enoxaparin arm, a statistically significant difference (P < .001) in favor of fondaparinux. There were no differences between the groups in rates of death or clinically relevant bleeding.
As noted above, the newly added recommendation in the Seventh ACCP Conference for extended prophylaxis, for up to 28 to 35 days after surgery, applies to patients undergoing hip fracture surgery as well as those undergoing hip replacement surgery. In the setting of hip fracture repair, extended prophylaxis is a Grade 1A recommendation with the use of fondaparinux and a Grade 1C+ recommendation with the use of either LMWH or adjusted-dose warfarin.6
Lower extremity fractures and trauma
Although lower extremity fractures are very common, the risk of DVT has been poorly studied in this setting. For patients with isolated lower extremity fractures, the Seventh ACCP Conference recommends that clinicians not use thromboprophylaxis routinely (Grade 2A, indicating an “intermediate-strength recommendation” based on RCTs without important limitations).6
Trauma patients, in contrast, are well recognized as being at very high risk for DVT and PE. The Seventh ACCP Conference gives a Grade 1A recommendation to thromboprophylaxis for all trauma patients who have at least one risk factor for VTE. LMWH is recommended (Grade 1A) as the agent of choice for this purpose, provided there are no contraindications to its use, and should be administered as soon as safely possible. Mechanical modalities are reserved for trauma patients with active bleeding or high risk for hemorrhage (Grade 1B). The guidelines recommend against use of inferior vena cava (IVC) filters as primary thromboprophylaxis in trauma patients (Grade 1C, indicating an “intermediate-strength recommendation” based on observational studies).6
Use of ultrasonography
Duplex ultrasonographic screening is recommended in orthopedic trauma patients who are at high risk for VTE and have received suboptimal or no prophylaxis (Grade 1C). In contrast, the Seventh ACCP Conference recommends against routine use of duplex ultrasonography to screen for VTE at hospital discharge in asymptomatic patients following major orthopedic surgery (Grade 1A).6
Knee arthroscopy
Arthroscopic knee procedures are increasing in frequency and raise the specter of a potential role for thromboprophylaxis. However, the clinical diagnosis of DVT is unreliable, and even diagnosis by ultrasonography is unreliable following knee arthroscopy, as interpreting scans of veins below the knee is challenging in this setting.24
The Seventh ACCP Conference recommends that clinicians not use routine thromboprophylaxis, other than early mobilization, for patients who undergo knee arthroscopy (Grade 2B). However, for arthroscopy patients who have inherent risk factors for VTE or who undergo a prolonged or complicated arthroscopy procedure, thromboprophylaxis with LMWH is suggested (Grade 2B).6
RECOMMENDED APPROACH TO VTE PROPHYLAXIS IN ORTHOPEDIC SURGERY
Drawing on the ACCP guidelines and the evidence reviewed above, we have outlined our evidence-based recommendations for pharmacologic VTE prophylaxis in patients undergoing orthopedic surgery, as presented in Table 1. All patients undergoing major orthopedic surgical procedures (ie, procedures other than arthroscopy) should routinely receive anticoagulant prophylaxis unless they have contraindications to anticoagulation. Recommended agents and their duration of use vary according to the type of surgery, as detailed in Table 1.
Extended-duration prophylaxis is recommended for patients undergoing total hip replacement and hip fracture surgery. Aspirin is not recommended as the sole agent for prophylaxis in any orthopedic surgery setting.
Importance of a postoperative prophylaxis protocol
In addition to these broad pharmacologic recommendations, it is important that a postoperative VTE prophylaxis protocol be in place at all hospitals.
At the Ochsner Medical Center in New Orleans, where one of us (S.B.D.) practices, postoperative orders include antithrombotic therapy for surgical patients, starting with placement of thigh-high antiembolism stockings on both legs on the day of surgery for patients undergoing hip replacement and on postoperative day 1 in those undergoing knee replacement. Plantar pneumatic compression devices are applied to both legs in the recovery room and kept on except when the patient is walking. The hospitalist team dictates further anticoagulation orders. If extended prophylaxis is prescribed, the discharge planner sets up drug delivery and reimbursement, provides a LMWH discharge kit, and teaches the patient to self-inject. If there is concern about increasing swelling at the surgical site while anticoagulant therapy continues, the protocol calls for prompt notification of the responsible physician. To minimize the risk that spinal or epidural hematomas will develop, all agents that increase bleeding propensity should be recognized and ordered accordingly.



SUMMARY
VTE in patients undergoing major orthopedic surgery is a serious health problem that is highly preventable, yet VTE prophylaxis remains underused in this patient population. Despite the availability of practice guidelines for VTE prevention in the orthopedic surgery setting, recommendations are not widely implemented in clinical practice. Recommended prophylactic options differ somewhat among various orthopedic procedures, and the supportive evidence differs for various anticoagulant options.
DISCUSSION: ADDITIONAL PERSPECTIVES FROM THE AUTHORS
Dr. Jaffer: The ACCP recommends against the routine use of aspirin as primary prophylaxis against VTE in major orthopedic surgery, yet orthopedic surgeons across the country still continue to use aspirin in this setting. What are your thoughts on this, Dr. McKean?
Dr. McKean: We agree with the ACCP’s recommendation against aspirin as primary VTE prophylaxis in orthopedic patients. The percentage of US knee arthroplasty patients who develop VTE after receiving no prophylaxis at all is roughly 64%; this percentage declines only slightly (to 56%) for knee arthroplasty patients who receive prophylaxis with aspirin.25 Since we clearly want to reduce VTE risk as much as possible, I would not use aspirin alone. I would use it only if the patient were already on aspirin, but then I would add either LMWH or fondaparinux.
Dr. Jaffer: Warfarin is another agent that is widely used for prophylaxis in major orthopedic surgery. In fact, the large registries of VTE prevention in major orthopedic surgery suggest that the use of warfarin may be slightly higher than the use of LMWH. If clinicians choose to use warfarin in their practice, what are your recommendations, Dr. Deitelzweig?
Dr. Deitelzweig: As primary prophylaxis for orthopedic surgery patients, warfarin must be dosed to achieve an INR of 2.0 to 3.0; the need for a value in this range is unequivocal. This is a challenging target to attain in the hospital setting.
Dr. Brotman: A study I was involved with a few years ago suggested that warfarin may be inadequate for VTE prevention in the first few days after orthopedic surgery.26 Orthopedic surgeons at the Cleveland Clinic, where I was practicing at the time, routinely used systematic ultrasonography to assess for thrombosis on postoperative day 2 or 3 following hip or knee arthroplasty, so we conducted a secondary analysis of a case-control study in these ultrasonographically screened arthroplasty patients to assess rates of early VTE and look for any associations with the type of prophylaxis used. We found that there was about a tenfold increase in the risk of VTE, both distal and proximal, on postoperative day 2 or 3 among patients who received warfarin compared with those who received LMWH. We concluded that warfarin’s delayed antithrombotic effects may not provide sufficient VTE prophylaxis in the immediate postoperative setting.26
Dr. Deitelzweig: That’s a good point. Although it’s important to achieve a therapeutic level of warfarin, we now have evidence that it takes some time to achieve that level, and in the interim, bad things can happen to patients.
Dr. Jaffer: Orthopedic surgery encompasses several types of procedures. Dr. Amin, which specific orthopedic surgery patients stand to benefit from extended prophylaxis, and how long should extended prophylaxis last?
Dr. Amin: Major orthopedic surgery comprises hip fracture repair, total hip replacement, and total knee replacement. For hip fracture, there are strong data to support the use of extended prophylaxis with fondaparinux 2.5 mg/day, which showed about an 88% relative reduction in the risk of symptomatic VTE compared with standard-duration fondaparinux (6 to 8 days) followed by matching placebo for the extended phase.27 The total duration of fondaparinux therapy in the extended-duration arm was 4 to 5 weeks.
Likewise, data support extended prophylaxis in hip arthroplasty patients, for whom the recommended duration is also 4 to 5 weeks. The systematic review by Hull et al17 demonstrated a 0.41 relative risk of DVT with extended-duration LMWH prophylaxis versus placebo in hip replacement patients (Figure 1), which was a highly statistically significant result.
In contrast, we do not yet have good data to support extended prophylaxis for patients undergoing total knee replacement, which is a bit surprising. In this setting, prophylaxis is recommended for 7 to 14 days but not beyond that.
Dr. Jaffer: Arthroscopy is probably the most common orthopedic procedure performed in the United States today. Dr. Brotman, what is the role of prophylaxis in patients undergoing arthroscopy?
Dr. Brotman: Minor surgery such as arthroscopy can typically be performed safely without routine prophylaxis, other than having the patient ambulate as soon as possible after the procedure. There may be exceptions to this rule, however. I believe that there is potentially a role for pharmacologic prophylaxis in arthroscopy patients who have major risk factors for VTE, such as a personal history of VTE, or who are not expected to become mobile again in a normal rapid fashion after the operation, but prophylaxis has not been studied systematically in such patients.
Dr. Jaffer: Dr. Spyropoulos, there are several new anticoagulants in the pipeline, specifically agents such as the oral direct factor Xa inhibitors and the direct thrombin inhibitors. What do recent clinical trials suggest with regard to the efficacy of these two drug classes for thromboprophylaxis in major orthopedic surgery?
Dr. Spyropoulos: The agents with the most available data are the oral direct factor Xa inhibitors apixaban and rivaroxaban and the oral direct thrombin inhibitor dabigatran. For prophylaxis in orthopedic surgery populations, phase 2 studies have been completed for apixaban and phase 3 trials have been completed for rivaroxaban and dabigatran.
It appears that the factor Xa inhibitors, apixaban and rivaroxaban, are efficacious in comparison with both adjusted-dose warfarin and LMWH, which is the gold standard for this group of patients.28,29 So these indeed appear to be promising agents. Rivaroxaban has been submitted to European regulatory agencies for approval for the prevention of VTE in patients undergoing major orthopedic surgery, and its developer plans to submit it to the FDA in 2008 for a similar indication in the United States.
The data are more equivocal with dabigatran. There have been several positive phase 3 studies in orthopedic surgery comparing two dabigatran dosing schemes, 150 and 220 mg once daily, with the European regimen of enoxaparin (40 mg once daily),30 but a recent study that compared these doses with the North American enoxaparin regimen (30 mg twice daily) failed to meet the criteria for noninferiority.31 Further clinical trial development is necessary for dabigatran, although in January 2008 the European Medicines Agency recommended its marketing approval for thromboprophylaxis in patients undergoing orthopedic procedures.32
I believe that in the next 3 to 5 years our armamentarium will see the addition of at least one, if not more, of these new agents that offer the promise of oral anticoagulation with highly predictable pharmacokinetics and pharmacodynamics and no need for monitoring.
- Yu HT, Dylan ML, Lin J, Dubois RW. Hospitals’ compliance with prophylaxis guidelines for venous thromboembolism. Am J Health Syst Pharm 2007; 64:69–76.
- Stratton MA, Anderson FA, Bussey HI, et al. Prevention of venous thromboembolism: adherence to the 1995 ACCP consensus guidelines for surgical patients. Arch Intern Med 2000; 160:334–340.
- National Consensus Standards for Prevention and Care of Venous Thromboembolism (VTE). The Joint Commission Web site. http://www.jointcommission.org/PerformanceMeasurement/Perform anceMeasurement/VTE.htm. Accessed January 8, 2008.
- Surgical Care Improvement Project. MedQIC Web site. http://www.medqic.org/scip. Accessed January 8, 2008.
- SCIP process and outcome measures, October 2005. MedQIC Web site. http://www.medqic.org. Accessed January 1, 2007.
- Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126(3 Suppl):338S–400S.
- Zimlich RH, Fulbright BM, Friedman RJ. Current status of anticoagulation therapy after total hip and total knee arthroplasty.J Am Acad Orthop Surg 1996; 4:54–62.
- Planes A, Vochelle N, Mazas F, et al. Prevention of postoperative venous thrombosis: a randomized trial comparing unfractionated heparin with low molecular weight heparin in patients undergoing total hip replacement. Thromb Haemost 1988; 60:407–410.
- Colwell CW Jr, Spiro TE, Trowbridge AA, et al. Efficacy and safety of enoxaparin versus unfractionated heparin for prevention of deep venous thrombosis after elective knee arthroplasty. Clin Orthop Relat Res 1995; 321:19–27.
- Bauer KA, Eriksson BI, Lassen MR, Turpie AG. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after elective major knee surgery. N Engl J Med 2001; 345:1305–1310.
- Turpie AGG. Pentasaccharide Org31540/SR90107A clinical trials update: lessons for practice. Am Heart J 2001; 142(Suppl):S9–S15.
- Hylek EM, Singer DE. Risk factors for intracranial hemorrhage in outpatients taking warfarin. Ann Intern Med 1994; 120:897–902.
- Hylek EM, Skates SJ, Sheehan MA, Singer DE. An analysis of the lowest effective intensity of prophylactic anticoagulation for patients with nonrheumatic atrial fibrillation. N Engl J Med 1996; 335:540–546.
- Samsa GP, Matchar DB, Goldstein LB, et al. Quality of anticoagulation management among patients with atrial fibrillation: review of medical records from 2 communities. Arch Intern Med 2000; 160:967–973.
- PEP Trial Collaborative Group. Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary Embolism Prevention (PEP) trial. Lancet 2000; 355:1295–1302.
- Warkentin TE. Heparin-induced thrombocytopenia: pathogenesis and management. Br J Haemotol 2003; 121:535–555.
- Hull RD, Pineo GF, Stein PD, et al. Extended out-of-hospital low-molecular-weight heparin prophylaxis against deep venous thrombosis in patients after elective hip arthroplasty: a systematic review. Ann Intern Med 2001; 135:858–869.
- Haentjens P, De Groote K, Annemans L. Prolonged enoxaparin therapy to prevent venous thromboembolism after primary hip or knee replacement: a cost-utility analysis. Arch Orthop Trauma Surg 2004; 124:507–517.
- Colwell CW Jr, Collis DK, Paulson R, et al. Comparison of enoxaparin and warfarin for the prevention of venous thromboembolic disease after total hip arthroplasty: evaluation during hospitalization and three months after discharge. J Bone Joint Surg Am 1999; 81:932–940.
- Hull RD, Pineo GF, Francis C, et al. Low-molecular-weight heparin prophylaxis using dalteparin in close proximity to surgery vs warfarin in hip arthroplasty patients: a double-blind, randomized comparison. Arch Intern Med 2000; 160:2199–2207.
- Leclerc JR, Geerts WH, Desjardins L, et al. Prevention of venous thromboembolism after knee arthroplasty: a randomized, double-blind trial comparing enoxaparin with warfarin. Ann Intern Med 1996; 124:619–626.
- Fitzgerald RH Jr, Spiro TE, Trowbridge AA, et al. Prevention of venous thromboembolic disease following primary total knee arthroplasty: a randomized, multicenter, open-label, parallel-group comparison of enoxaparin and warfarin. J Bone Joint Surg Am 2001; 83-A:900–906.
- Eriksson BI, Bauer KA, Lassen MR, Turpie AG, Steering Committee of the Pentasaccharide in Hip-Fracture Surgery Study. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after hip-fracture surgery. N Engl J Med 2001; 345:1298–1304.
- Demers C, Marcoux S, Ginsberg JS, Laroche F, Cloutier R, Poulin J. Incidence of venographically proved deep vein thrombosis after knee arthroscopy. Arch Intern Med 1998; 158:47–50.
- Geerts WH, Heit JA, Clagett GP, et al. Prevention of venous thromboembolism. Chest 2001; 119(1 Suppl):132S–175S.
- Brotman DJ, Jaffer AK, Hurbanek JG, Morra N. Warfarin prophylaxis and venous thromboembolism in the first 5 days following hip and knee arthroplasty. Thromb Haemost 2004; 92:1012–1017.
- Eriksson BI, Lassen MR; Pentasaccharide in Hip-Fracture Surgery Plus Investigators. Duration of prophylaxis against venous thromboembolism with fondaparinux after hip fracture surgery: a multicenter, randomized, placebo-controlled, double-blind study. Arch Intern Med 2003; 163:1337–1342.
- The Botticelli Investigators. Late-breaking clinical trial: a dose-finding study of the oral direct factor Xa inhibitor apixaban in the treatment of patients with acute symptomatic deep vein thrombosis [abstract]. Presented at the 21st Congress of the International Society on Thrombosis and Haemostasis; July 2007; Geneva, Switzerland.
- Fisher WD, Eriksson BI, Bauer KA, et al. Rivaroxaban for thromboprophylaxis after orthopaedic surgery: pooled analysis of two studies. Thromb Haemost 2007; 97:931–937.
- Haas S. New oral Xa and IIa inhibitors: updates on clinical trial results. J Thromb Thrombolysis 2008; 25:52–60.
- Friedman RJ, Caprini JA, Comp PC, et al. Dabigatran etexilate vs enoxaparin in preventing venous thromboembolism following total knee arthroplasty. Presented at: 2007 Congress of the International Society on Thrombosis and Haemostasis; July 7–13, 2007; Geneva, Switzerland.
- Committee for Medicinal Products for Human Use summary of positive opinion for Pradaxa [news release]. London, UK: European Medicines Agency. January 24, 2008. http://www.emea.europa.eu/pdfs/human/ opinion/Pradaxa_3503008en.pdf. Accessed February 21, 2008.
- Goldhaber SZ, Grodstein F, Stampfer MJ. A prospective study of risk factors for pulmonary embolism in women. JAMA 1997; 277:642–645.
- Turpie AGG, Bauer KA, Eriksson BI, Lassen MR, for the Steering Committees of the Pentasaccharide Orthopedic Prophylaxis Studies. Fondaparinux vs enoxaparin for the prevention of venous thromboembolism in major orthopedic surgery. Arch Intern Med 2002; 162:1833–1840.
- Stein PD, Beemath A, Matta F, et al. Clinical characteristics of patients with acute pulmonary embolism: data from PIOPED II. Am J Med 2007; 120:871–879.
- Goldhaber SZ. Diagnosis of acute pulmonary embolism: always be vigilant. Am J Med 2007; 120:827–828.
- American Academy of Orthopaedic Surgeons Clinical Guideline on Prevention of Symptomatic Pulmonary Embolism in Patients Undergoing Total Hip or Knee Arthroplasty: Summary of Recommendations. http://www.aaos.org/Research/guidelines/PE_ summary.pdf. Accessed December 10, 2007.
- Yu HT, Dylan ML, Lin J, Dubois RW. Hospitals’ compliance with prophylaxis guidelines for venous thromboembolism. Am J Health Syst Pharm 2007; 64:69–76.
- Stratton MA, Anderson FA, Bussey HI, et al. Prevention of venous thromboembolism: adherence to the 1995 ACCP consensus guidelines for surgical patients. Arch Intern Med 2000; 160:334–340.
- National Consensus Standards for Prevention and Care of Venous Thromboembolism (VTE). The Joint Commission Web site. http://www.jointcommission.org/PerformanceMeasurement/Perform anceMeasurement/VTE.htm. Accessed January 8, 2008.
- Surgical Care Improvement Project. MedQIC Web site. http://www.medqic.org/scip. Accessed January 8, 2008.
- SCIP process and outcome measures, October 2005. MedQIC Web site. http://www.medqic.org. Accessed January 1, 2007.
- Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126(3 Suppl):338S–400S.
- Zimlich RH, Fulbright BM, Friedman RJ. Current status of anticoagulation therapy after total hip and total knee arthroplasty.J Am Acad Orthop Surg 1996; 4:54–62.
- Planes A, Vochelle N, Mazas F, et al. Prevention of postoperative venous thrombosis: a randomized trial comparing unfractionated heparin with low molecular weight heparin in patients undergoing total hip replacement. Thromb Haemost 1988; 60:407–410.
- Colwell CW Jr, Spiro TE, Trowbridge AA, et al. Efficacy and safety of enoxaparin versus unfractionated heparin for prevention of deep venous thrombosis after elective knee arthroplasty. Clin Orthop Relat Res 1995; 321:19–27.
- Bauer KA, Eriksson BI, Lassen MR, Turpie AG. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after elective major knee surgery. N Engl J Med 2001; 345:1305–1310.
- Turpie AGG. Pentasaccharide Org31540/SR90107A clinical trials update: lessons for practice. Am Heart J 2001; 142(Suppl):S9–S15.
- Hylek EM, Singer DE. Risk factors for intracranial hemorrhage in outpatients taking warfarin. Ann Intern Med 1994; 120:897–902.
- Hylek EM, Skates SJ, Sheehan MA, Singer DE. An analysis of the lowest effective intensity of prophylactic anticoagulation for patients with nonrheumatic atrial fibrillation. N Engl J Med 1996; 335:540–546.
- Samsa GP, Matchar DB, Goldstein LB, et al. Quality of anticoagulation management among patients with atrial fibrillation: review of medical records from 2 communities. Arch Intern Med 2000; 160:967–973.
- PEP Trial Collaborative Group. Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary Embolism Prevention (PEP) trial. Lancet 2000; 355:1295–1302.
- Warkentin TE. Heparin-induced thrombocytopenia: pathogenesis and management. Br J Haemotol 2003; 121:535–555.
- Hull RD, Pineo GF, Stein PD, et al. Extended out-of-hospital low-molecular-weight heparin prophylaxis against deep venous thrombosis in patients after elective hip arthroplasty: a systematic review. Ann Intern Med 2001; 135:858–869.
- Haentjens P, De Groote K, Annemans L. Prolonged enoxaparin therapy to prevent venous thromboembolism after primary hip or knee replacement: a cost-utility analysis. Arch Orthop Trauma Surg 2004; 124:507–517.
- Colwell CW Jr, Collis DK, Paulson R, et al. Comparison of enoxaparin and warfarin for the prevention of venous thromboembolic disease after total hip arthroplasty: evaluation during hospitalization and three months after discharge. J Bone Joint Surg Am 1999; 81:932–940.
- Hull RD, Pineo GF, Francis C, et al. Low-molecular-weight heparin prophylaxis using dalteparin in close proximity to surgery vs warfarin in hip arthroplasty patients: a double-blind, randomized comparison. Arch Intern Med 2000; 160:2199–2207.
- Leclerc JR, Geerts WH, Desjardins L, et al. Prevention of venous thromboembolism after knee arthroplasty: a randomized, double-blind trial comparing enoxaparin with warfarin. Ann Intern Med 1996; 124:619–626.
- Fitzgerald RH Jr, Spiro TE, Trowbridge AA, et al. Prevention of venous thromboembolic disease following primary total knee arthroplasty: a randomized, multicenter, open-label, parallel-group comparison of enoxaparin and warfarin. J Bone Joint Surg Am 2001; 83-A:900–906.
- Eriksson BI, Bauer KA, Lassen MR, Turpie AG, Steering Committee of the Pentasaccharide in Hip-Fracture Surgery Study. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after hip-fracture surgery. N Engl J Med 2001; 345:1298–1304.
- Demers C, Marcoux S, Ginsberg JS, Laroche F, Cloutier R, Poulin J. Incidence of venographically proved deep vein thrombosis after knee arthroscopy. Arch Intern Med 1998; 158:47–50.
- Geerts WH, Heit JA, Clagett GP, et al. Prevention of venous thromboembolism. Chest 2001; 119(1 Suppl):132S–175S.
- Brotman DJ, Jaffer AK, Hurbanek JG, Morra N. Warfarin prophylaxis and venous thromboembolism in the first 5 days following hip and knee arthroplasty. Thromb Haemost 2004; 92:1012–1017.
- Eriksson BI, Lassen MR; Pentasaccharide in Hip-Fracture Surgery Plus Investigators. Duration of prophylaxis against venous thromboembolism with fondaparinux after hip fracture surgery: a multicenter, randomized, placebo-controlled, double-blind study. Arch Intern Med 2003; 163:1337–1342.
- The Botticelli Investigators. Late-breaking clinical trial: a dose-finding study of the oral direct factor Xa inhibitor apixaban in the treatment of patients with acute symptomatic deep vein thrombosis [abstract]. Presented at the 21st Congress of the International Society on Thrombosis and Haemostasis; July 2007; Geneva, Switzerland.
- Fisher WD, Eriksson BI, Bauer KA, et al. Rivaroxaban for thromboprophylaxis after orthopaedic surgery: pooled analysis of two studies. Thromb Haemost 2007; 97:931–937.
- Haas S. New oral Xa and IIa inhibitors: updates on clinical trial results. J Thromb Thrombolysis 2008; 25:52–60.
- Friedman RJ, Caprini JA, Comp PC, et al. Dabigatran etexilate vs enoxaparin in preventing venous thromboembolism following total knee arthroplasty. Presented at: 2007 Congress of the International Society on Thrombosis and Haemostasis; July 7–13, 2007; Geneva, Switzerland.
- Committee for Medicinal Products for Human Use summary of positive opinion for Pradaxa [news release]. London, UK: European Medicines Agency. January 24, 2008. http://www.emea.europa.eu/pdfs/human/ opinion/Pradaxa_3503008en.pdf. Accessed February 21, 2008.
- Goldhaber SZ, Grodstein F, Stampfer MJ. A prospective study of risk factors for pulmonary embolism in women. JAMA 1997; 277:642–645.
- Turpie AGG, Bauer KA, Eriksson BI, Lassen MR, for the Steering Committees of the Pentasaccharide Orthopedic Prophylaxis Studies. Fondaparinux vs enoxaparin for the prevention of venous thromboembolism in major orthopedic surgery. Arch Intern Med 2002; 162:1833–1840.
- Stein PD, Beemath A, Matta F, et al. Clinical characteristics of patients with acute pulmonary embolism: data from PIOPED II. Am J Med 2007; 120:871–879.
- Goldhaber SZ. Diagnosis of acute pulmonary embolism: always be vigilant. Am J Med 2007; 120:827–828.
- American Academy of Orthopaedic Surgeons Clinical Guideline on Prevention of Symptomatic Pulmonary Embolism in Patients Undergoing Total Hip or Knee Arthroplasty: Summary of Recommendations. http://www.aaos.org/Research/guidelines/PE_ summary.pdf. Accessed December 10, 2007.
Infections in hospitalized patients: What is happening and who can help?
Training Opportunities for Academic Hospitalists
There is a growing demand for hospitalists in the United States. In academic settings, hospitalists are called on to perform a variety of duties, from leading quality improvement initiatives to serving on hospital committees to helping to offset restrictions on work hours of the house staff.1 Although hospitalists may be well positioned to take on these roles, obtaining adequate protected time and recognition for such contributions remains a challenge. The existing promotion and tenure processes at academic institutions may not give adequate consideration to such responsibilities. Hospitalists who do not meet the traditional benchmarks of teaching and research may suffer in their career advancement and, ultimately, in their desire to remain in academics. Developing a sustainable and long‐term career in hospital medicine is important not only from a professional developmental standpoint, but also because it may lead to better patient care; evidence from a large multicenter hospitalist study suggests that physician experience is linked to improved patient care and outcomes.2 Thus, it behooves academic medical centers that employ hospitalists to create rewarding hospitalist career paths.
Goldman described academic hospital medicine as comprising periods of systole, during which hospitalists provide clinical care, and periods of diastole, the portion of a hospitalist's time spent in nonclinical activities.3 Far from being a period of relaxation, diastole is an active component of a hospitalist's work, the time devoted to the pursuit of complementary interests, career advancement, and job diversity. A well‐thought‐out plan for the diastolic phase of a hospitalist job description can lead to significant improvement in quality, education, research, and outcomes for an academic medical center.4 A good balance of systole and diastole allows for focus on career development and advancement and has the potential to be very helpful in preventing burnout. This is of particular concern to academic hospitalists, who report working longer hours, feeling more stress, and worrying more about burnout than their nonhospitalist colleagues.5 This suggests the diastolic phase is an important part of creating a sustainable hospitalist job and should be funded as part of an academic hospitalist position.
Although the optimal balance of systole and diastole to prevent burnout is not known, outlining clear expectations is an important strategy for preparing physicians for a sustainable academic hospitalist career. This is an important issue, given the increasing number of residency graduates who are choosing careers in hospital medicine.6 Based on the reported career plans of residents taking internal medicine in‐training exams from 2002 through 2006, the number of residents going into hospital medicine has more than doubled, from 3% (in 2002) to 6.5% (in 2006). The goal of this article is to compare and contrast several career paths that balance systole and diastole in academic hospital medicine. Specifically, we review training opportunities for becoming a successful hospitalist‐educator, hospitalistquality expert, hospitalist‐investigator, and hospitalist‐administrator.
EDUCATION (THE HOSPITALIST‐EDUCATOR)
Hospitalists in academic centers often play central roles as teachers and leaders in medical education. This is not surprising given that most teaching of medical trainees occurs in the inpatient setting.7 Furthermore, several studies have consistently demonstrated that trainee satisfaction with teaching by hospitalists is high, and hospitalists are rated as more effective teachers than traditional subspecialist ward attendings.810
A typical hospitalist‐educator position is 80%‐90% clinical time, with 10%‐20% set aside for teaching. However, academic hospitalists are often expected to teach medical trainees concurrently with their clinical care activities, rather than during a separate, protected time.11 Thus, most hospitalist‐educator responsibilities do not occur during diastole, as may be conceived, but instead are add to the systole. Small amounts of protected diastolic time for a hospitalist‐educator can be used for related administrative activities, such as writing letters of recommendation, mentoring students and residents, doing creative thinking and curriculum development, and conducting educational research, such as evaluating a new educational program or curriculum. Some hospitalist‐educator positions, such as director of the residency program or internal medicine clerkship, are exceptions in that they generally include a greater amount of protected time, which may be earmarked for administrative activities and hands‐on teaching.
Education and Training
One possibility for advanced training in education is the addition of a chief resident year, either at a physician's own institution or at another academic center. Such a year provides an opportunity to consolidate knowledge, build a teaching portfolio, and accumulate expertise in an area such as evidence‐based medicine or perioperative care. Serving as a chief resident can enhance subsequent applications by being able to demonstrate the ability to teach and, more importantly, to assume a leadership role within an organization. These skills can be applied to a number of activities in an academic hospitalist program, such as heading a committee, teaching during inpatient service time, or developing a new course for students, residents, or faculty.
An advanced training program in medical education is also an option (Table 1). Offerings include medical education fellowship training, formal degree‐granting programs (such as a master's in health professions education), or short‐term intensive coursework. Fellowships and degree‐granting programs are generally 2‐year programs designed for health professionals who want to better prepare for educational leadership roles. Core topics include curriculum development, program evaluation, instruction, student assessment, current educational issues, research methods, and leadership. An alternative option for busy clinician‐educators is online or distance learning courses in medical education, which cover similar topics and skill sets. In early 2006 the Society of Hospital Medicine released the Core Competencies in Hospital Medicine, which can serve as a useful framework for developing novel inpatient curricula for faculty, residents, and students.12, 13
| Description | Length of time | Cost | Source/website |
|---|---|---|---|
| Degrees/fellowships | |||
| Master's in health professional education (MHPE): Preparation for educational leadership roles. Typical coursework in curriculum development, program evaluation, instruction, student assessment, current educational issues, research methods, and leadership. | Varies according to program | Tuition ranges from approximately $1500‐$4300 | Example: University of Illinois |
| Fellowship in medical education: Prepares faculty to pursue scholarship in medical education or educational leadership or to become effective teachers through workshops, coursework, and/or a mentored project. Often affiliated with a department of medical education. | Varies according to program. Generally 1 year. | Varies. May be subsidized in certain institutions as part of internal faculty development. | Example: University of Michigan |
| Short‐term coursework | |||
| Harvard Macy Institute: Programs designed to promote leadership and scholarship in medical education | 1‐ or 2‐week programs | Fees for the year 2006 are $4500 USD. | |
| Stanford Faculty Development Center (SFDC): Train‐the‐trainer approach for clinical teaching and professionalism in contemporary practice | 4‐week training sessions | The institutions of faculty selected for the month‐long training programs are asked to pay a fee of $5000. Transportation, housing and food are not included. | |
Short‐term extramural courses offered by institutions such as the Harvard Macy Institute for Medical Educators and the Stanford Faculty Development Program in Teaching can also provide advanced instruction to hospitalist‐educators.14, 15 In addition to these training programs, the Society of General Internal Medicine, along with other professional societies, offers career development workshops for clinician educators on topics such as curriculum development and teaching skills.
Regardless of the type of training, adequate mentorship and resources are critical to the successful application of new skills to the design or evaluation of hospital‐based curricula. Mentorship may be available from institutional leaders in medical education, even those not formally affiliated with the hospitalist program. For instance, medical school leaders, such as deans, division chiefs, chairpersons, program directors, and clerkship directors, can often be helpful in guiding junior faculty in obtaining skills and time for teaching.
We encourage those interested in a career in medical education to begin volunteering at their institution early on. Volunteering to directly teach residents and students (eg, assisting in introduction to clinical medicine, giving lectures to third‐year clerks) can be a valuable way of becoming distinguished as a qualified teacher. Likewise, joining a professional medical society of individuals with similar interests can facilitate mentorship and skill acquisition. Certain professional medical societies, such as the American College of Physicians, promote national recognition through awarding fellowships, an honor for those physicians who have demonstrated superior competence in internal medicine, professional accomplishment, and scholarship.16 Developing concrete examples of expertise in the field, such as through the publication of abstracts and articles on medical education and development of curricula, help lead to advancement in the educational track. Clear focus on a career path, development of an intellectual product, positive learner evaluation of educational activities, and national recognition can all be used by an academic institution to evaluate suitability for promotion.
Rewards and Challenges
One of the rewards of a hospitalist‐educator career is being able to meaningfully interact with a variety of trainees, including medical students and residents. As teaching attendings, hospitalist‐educators are likely to engage students and residents for short‐term but intensive periods, resulting in the ability to influence career choice and professional growth as a physician.17 Hospitalists may be called on by trainees to serve as mentors or advisers and to write letters of recommendation. In addition, with experience, hospitalist‐educators are well positioned to serve in administrative roles in medical education, such as clerkship director or program director.
Burnout is a particular concern for hospitalist‐educators, given the heavy clinical demands of inpatient academic service combined with the additional pressure to be academically productive.5 Because of this, it is important to design academic hospitalist‐educator positions with a diastole that contains time to recover from the heavy clinical demands of inpatient service, in addition to providing time for career development activities.
Successful career development as an educator can be difficult. There are relatively few venues at which educational work can be peer‐evaluated and published, which are keys to successful academic promotion.18 Because some educational journals are highly competitive, one possibility way to get educational work disseminated is through the MedEd Portal, sponsored by the Association of American Medical Colleges, which allows peer review of medical educational materials, including innovative curricula.19 In addition to original research contributions, many scientific meetings and medical education journals also accept descriptions of interesting clinical vignettes and innovations in medical education. New online education journals, such as BMC Medical Education and Seminars in Medical Practice, have expanded publication opportunities.20
Limited opportunities are available to help fund research in medical education. Although funding may be more readily available to educators who focus on a particular clinical entity or patient population, most medical education research is conducted with inadequate funding and requires extensive donated time by committed faculty.21 For this reason, securing advanced training in medical education and having protected time will allow hospitalists on the educator track to compete more successfully for limited educational research dollars and to have sufficient time to produce and publish scholarly work, thus improving their chances of academic success and career satisfaction.
CLINICAL QUALITY AND OPERATIONS IMPROVEMENT (THE HOSPITALISTQUALITY EXPERT)
Hospitalists are increasingly being called on to lead clinical quality and operations improvement at academic teaching hospitals. Benefits to the institution include the consistent presence of a committed physician who is able to plan and execute change in the context of clinical care. This is in contrast to the transient nature of residents and nonhospitalist attending physicians, whose ability to participate in such initiatives is impaired by the scheduling of their rotations. Hospitalists, however, are often able to cultivate long‐standing relationships with nurses, case managers, and hospital administrators, thereby building the institutional clout to lead such initiatives while considering views from all the necessary stakeholders.22 Thus, they are in a good position to serve as physician champions and expedite the adoption of new innovations within hospitalist groups and among other physician groups and clinical staff.23, 24
Education and Training
Being a successful agent of change requires knowledge of the science of quality improvement coupled with the skills necessary to make such changes, such as the ability to perform a needs assessment, to develop measures of performance, to negotiate and motivate others to change behaviors, to adopt new tools and practices, and to implement and test interventions designed to improve care. It is possible for residents or junior faculty members to gain this experience through designing and implementing a quality improvement project during residency training under the direction of a mentor.25, 26 However, given the likely variability in such experience, there is no substitute for formal training in these core areas of hospital medicine.
A broad range of opportunities for advanced training in quality and operations improvement are available (Table 2). Choosing the correct program may depend on baseline expertise, availability, and the desired level of involvement. For example, introductions to these skills can be obtained through precourses or workshops at medical conferences such as the Institute of Healthcare Improvement or the Society of Hospital Medicine. For more in‐depth training, the Advanced Training Program (ATP) in Health Care Delivery Improvement, sponsored by Intermountain Healthcare, offers 12‐ to 21‐day in‐depth minicourses designed to train individuals for leadership positions in quality and safety.27 Lastly, more structured fellowships, such as the Veterans Affairs Quality Scholars Program or the George W. Merck Fellowships in Health Care Improvement, offer junior and midcareer faculty the opportunity to obtain formal training in the science of quality improvement.28, 29 Because early‐career hospitalists may face geographic and financial restrictions, exploration of local or institutional opportunities for advanced education in quality improvement can be particularly important.
| Description | Length of time | Cost | Source/website |
|---|---|---|---|
| Degrees/fellowships | |||
| Veterans Association National Quality Scholars: Fellowship to learn and apply knowledge for improvement of health care | 2 years | No cost, application to fellowship program required | |
| George W. Merck Fellowship: Mentored research or improvement project at Institute of Healthcare Improvement with a plan to return to home institution to execute change | 1 year | No cost, application to fellowship program required | |
| Short‐term coursework | |||
| Intermountain Health Care: Designed to give executives and quality improvement leaders the necessary tools to conduct clinical practice improvement projects. | 20‐ and 12‐day training programs in Salt Lake City, UT | Tuition for the 20‐day program:
| |
Rewards and Challenges
Engaging in successful clinical or process improvement can be very rewarding, both professionally and personally. Professional gains include building new interdisciplinary relationships and infrastructure to continually monitor and improve key performance measures. In addition, a rigorous evaluation of this type of work can result in being able to make presentations at national meetings or to be published in a variety of peer‐reviewed medical journals, including specialty journals for quality improvement work, such as Quality and Safety in Healthcare and the Joint Commission Journal on Quality Improvement. Many national medical meetings, such as the Institute for Healthcare Improvement, the Society of Hospital Medicine and other subspecialty society meetings, also provide an opportunity to showcase innovations in practice.
Despite the potential rewards, it can also be challenging for academic hospitalists to participate in or lead quality improvement projects. One major challenge is ensuring that hospitalists are engaged in improvement work that is aligned with the interests of the hospital. Because most hospital administrators and frontline staff are employed by the hospital, whereas those comprising the academic faculty are employed by the university, this alignment is not always guaranteed. For example, an area of interest to a hospitalist that also could lead to academic productivity and career advancement might not be considered a priority area of improvement for the hospital because of competing clinical or operations improvements. In this scenario, it can be extremely difficult to engage other stakeholders such as nurses or administrative support staff in order to make a meaningful, sustainable change or improvement. To avoid this situation, it can be helpful from the outset to partner with hospital quality leaders in discussing priority areas, with attention to any potential interface in which hospitalist expertise is needed. In the event a potential project or area is identified, a hospitalist is particularly well positioned to serve as a physician champion, which is often key to the success of any hospitalwide initiative. In some cases, hospital funding may be available for these types of initiatives, increasing the likelihood of resource development for sustainable change.
RESEARCH (THE HOSPITALIST‐INVESTIGATOR)
Few hospitalists devote most of their time to clinical research. Having a strong research base is essential for the field of hospital medicine to gain credibility as a distinct specialty.4 Although the initial research in hospital medicine sought to prove the value of the field itself, hospitalists have now begun to focus on quality improvement and outcomes research.3032 Because of their unique position in clinical care, hospitalists are well situated to oversee inpatient data collection and perform research on a variety of conditions ranging from acute coronary syndromes to venous thromboembolism. Another potential area of research for hospitalists is participation in clinical trials focused on the inpatient setting. Although the proportion of time spent in research can vary widely, to become an independently successful clinical researcher typically requires a substantial amount of time be devoted to research. In general, at least 50% protected time, greater if possible, is recommended.
Education and Training
To develop a career around research generally requires advanced training in research methods. The most frequently used option for obtaining such training is through completing a clinical research fellowship in general internal medicine or an equivalent program, such as the fellowships administered by the Robert Wood Johnson Clinical Scholars Program (Table 3).33 Several academic centers also have developed such hospital medicine fellowships, which often can be tailored to provide the desired experience in research ethics, methodology, and statistical analysis.34, 35 In selecting a training program, prospective hospitalist‐researchers should consider the availability of suitable research mentors. Because hospital medicine as a field is relatively new, research mentors within the group of hospitalists may be scarce; if so, researchers should seek appropriate mentorship from established investigators in other programs or departments. Effective mentorship is a strong predictor of future research success.36
| Description | Length of time | Cost | Source/website |
|---|---|---|---|
| Degrees/fellowships | |||
| Hospital or General Medicine Fellowships: Designed to provide clinical research training through mentored projects and coursework with possible master's degree | Generally 2‐year programs | No cost, application to program is required. Stipends vary. No cost, application to program is required | Hospital Medicine: |
| Robert Wood Johnson Clinical‐Scholars Program: Training in health services research with an emphasis on community‐based research and leadership training. | 2 years | Stipends currently range from $48,000 to $50,000 per year, depending on the training site. | Robert Wood Johnson: |
| Short‐term coursework | |||
| University‐based summer programs in clinical research (eg, Harvard University Summer Session for Public Health Studies which features graduate courses in epidemiology, biostatistics, economics, health care management, etc.) | Intensive 3‐week courses in Harvard University Summer Session | 2004 tuition for each 2.5‐credit course was $1830. There is a nonrefundable deposit/registration fee of $125. These fees do not include certain course materials (ie, texts estimated at $60 per course). | Example: Harvard School of Public Health |
Negotiating protected time can be challenging for new investigators, particularly when hospitalist salaries are generated by clinical activity. Some academic programs are willing to provide a few years of departmental support to promising young investigators in order to allow them to develop their research program and obtain additional funding. Several career development awards are available through the National Institutes of Health and through nonfederally funded sources.37, 38 These awards generally protect 3‐5 years of a researcher's time for research and require that a substantial proportion of time be devoted to that purpose, often at least 75%.
To gain visibility as a researcher, it is advantageous to present original findings at national meetings, such as those of the Society of Hospital Medicine, the Society of General Internal Medicine, and other subspecialty meetings.39, 40 These meetings not only increase awareness of a hospitalist's research but also provide opportunities for networking and developing collaboration on research. Many societies, including the Society of Hospital Medicine, have research abstract competitions and offer research grants for investigators that can help to fund projects and support protected time.
Rewards and Challenges
There are many rewards and opportunities for a hospitalist investigator, particularly because the field is young and there are many unanswered research questions related to inpatient medicine. There are also the intrinsic rewards of being devoted to scientific inquiry and having greater autonomy over how time is spent. A hospitalist's schedule can be well suited to research. Although attending on the wards can be very time‐consuming, time off the wards is often free of outpatient duties and can be entirely devoted to research.
There are also several challenges to becoming a successful researcher. The pressure to obtain grant funding and publish high‐quality scientific manuscripts is high. Obtaining sufficient protected time may be difficult in busy clinical departments, and applying for grant funding is both time‐consuming and highly competitive. It is very important to be familiar with the specific criteria for academic promotion at one's institution. Understanding these expectations can help to effectively prioritize activities. Standard requirements generally include number and quality of articles published in peer‐reviewed journals, successful application for research funding, national recognition in the field, service to the institution and research community, and evidence of research independence. One significant challenge is the lack of a single large funding source for hospital‐related research. Although the Agency for Healthcare Research and Quality funds studies related to hospital care, such as on the quality of care or cost effectiveness of various system‐based hospital care interventions, their budget for investigator‐initiated proposals is limited.41 One promising funding source for research in hospital care is from agencies and foundations dedicated to the aging population, such as the National Institute for Aging (NIA), the Hartford Foundation, and the Aetna Foundation, to name a few.42, 43 Yet research on hospital care alone, without detailed attention to issues unique to geriatric‐specific conditions or populations, is unlikely to be funded by these avenues. With few federal grant programs directly suited to the emerging research agenda in hospital medicine, hospitalist‐investigators may be at a disadvantage for obtaining tenure‐track positions, compared with their subspecialist colleagues, who may receive funding from NIH agencies or foundations dedicated to their own field.
ADMINISTRATION (THE HOSPITALIST‐ADMINISTRATOR)
Physician leaders in hospital administration are not new. Many hospitals already include physicians in senior management positions, such as chief medical officer.44 Naturally, a career in hospital administration is another potential path for diastole in academic medical centers.
Education and Training
Although a master of business, health administration, or medical management is not a prerequisite for the physician who wants to move into management, it is an increasingly important credential for senior administrative positions (Table 4). Primarily, it serves as a signal that a physician is committed to management and has a working knowledge of strategic planning, business models, human resources, leadership, and clinical operations. For physicians without formal business training who are interested in management, exploring internal opportunities is a necessary first step. Likewise, getting a business degree is not as important as management experience. The successful application of business skills requires practice, mentoring, and on‐the‐job experience. For hospitalists, this experience could be obtained by volunteering to serve on committees such as utilization review, quality assurance, credentialing, or medical staff executive committees. In lieu of a graduate degree, physicians may wish to participate in one of the many fellowships in health services administration. These programs generally aim to provide practical mentored learning experience in a health care organization and may last up to 2 years.45
| Description | Length of time | Cost | Source/website |
|---|---|---|---|
| Degrees/fellowships | |||
| Master's in business administration (MBA): General management core with option for courses specializing in health care. | Generally 2‐year program | Varies in accordance with each institution. | Directory websites (MBA): |
| Master's in health administration (MHA): Studies in analytic and management needs of health care. | Generally 2‐year program | Varies in accordance with each institution. | Directory websites (MHA): |
| Fellowship in health services administration: Preceptor‐directed program that provides practical learning experience in a health care organization beyond graduate‐level academic instruction. | Usually lasts 1‐2 years. | Compensation varies. Median reported as $39,055. | Directory (American College of Healthcare Executives): |
| Short‐term coursework | |||
| Society of Hospital Medicine Leadership Academy: Instruction for hospitalists in leading change, communicating effectively, handling conflict and negotiation, doing strategic planning, and interpreting hospital business drivers. Held biannually. | 3‐ to 4‐day program | $1400‐$1600. Discounted rate for members of Society of Hospital Medicine | |
For hospitalists and trainees considering a career as an executive, the American College of Physician Executives can serve as a valuable resource.46 This organization, founded in 1975, offers educational resources, including publications, comprehensive CD‐ROM products, and 1‐day courses and master's degree programs in conjunction with several leading business schools in medical management. In addition, the Society of Hospital Medicine offers a Leadership Academy designed to assist practicing hospitalists in evaluating their leadership strengths and applying them to everyday management challenges.47 Such a program also can facilitate the development of a peer network and the mentoring relationships needed to achieve these goals.
Rewards and Challenges
The life of the physician executive can be rewarding, but making the transition may prove challenging. However, if physicians can navigate this transition successfully, they will likely find a wide array of opportunities, as demand for physician‐executives remains high.
One major challenge to becoming a physician‐executive is reconciling the administrative role with the initial desire to enter a career in clinical medicine.48 Physician‐executives who continue to see patients are more likely to be satisfied with their jobs than physician‐executives who do not.49 Physician‐executives also may feel they are being criticized by their purely clinical colleagues for working in the business or management of medicine.50 Actual or perceived lack of support may promote isolation and burnout.51 In addition, the constantly shifting landscape of health care administration results in a much more unstable environment than that found in clinical medicine. For example, the risk of termination for a physician‐executive is 20‐40 times higher than that for a practicing physician.50 The reasons for this higher risk include personal conflict with a boss, reorganization (ie, downsizing, merging, etc.), and immediate departure of a supervisor. Access to mentors, support groups, and the option to practice part time are all potential mechanisms to ensure long‐term success as a physician‐administrator.
CONCLUSIONS
As hospital medicine continues to grow and evolve, designing sustainable and rewarding academic careers will be crucial to the success of the field. Being able to balance clinical systole time with obtaining the skills to support nonclinical diastole time is important to ensuring a successful career as an academic hospitalist. We have described several possible career paths in teaching, research, quality improvement, and administration. By preparing future hospitalists with the knowledge and skills required to assume a variety of roles during their diastolic time, we hope to encourage the growth of hospitalist leaders with well‐developed skill sets. If hospitalists adequately prepare themselves, academic hospital medicine will likely remain sustainable and rewarding, and future generations of trainees will be inspired and prepared to enter the field.
Acknowledgements
We are grateful to Jennifer Higa and Kimberly Alvarez for their assistance in preparing this manuscript.
- ,,.Balancing continuity of care with residents' limited work hours: defining the implications.Acad Med.2005;80:39–43.
- ,,, et al.Effects of inpatient experience on outcomes and costs in a multicenter trial of academic hospitalists.J Gen Intern Med.2005;20(s1):141–142.
- .The hospitalist movement.Ann Intern Med.1999;131:545.
- .The future of hospital medicine: evolution or revolution?Am J Med.2004;117:446–450.
- ,,, et al.Satisfaction and worklife of academic hospitalist and non‐hospitalist attendings on general medical inpatient rotations.J Gen Intern Med.2006;21(s4):128.
- ,,.Career plans for trainees in internal medicine residency programs.Acad Med.2005;80:507–512.
- ,,,,.Closing the gap between internal medicine training and practice: recommendations from recent graduates.Amer J Med.2005;118:680–687.
- ,,, et al..Hospitalists as teachers.J Gen Intern Med.2004;19(1):8–15.
- ,.Implications of the hospitalist model for medical students' education.Acad Med.2001;76:324–330.
- ,,,,,.Resident satisfaction on an academic hospitalist service: time to teach.Am J Med.2002;112:597–601.
- ,.The present and future of appointment, tenure, and compensation policies for medical school clinical faculty.Acad Med.2001;76:993–1004.
- ,,,,.Core competencies in hospital medicine: development and methodology.J Hosp Med.2006;1(1):48–56.
- ,,,,.The core competencies in hospital medicine.J Hosp Med.2006;1(1).
- Harvard Macy Institute. Harvard College. Available at: http://www.harvardmacy.org/programs.asp?DocumentID=1. Accessed October 3,2005.
- Stanford Faculty Development Center. Stanford University. Available at http://sfdc.stanford.edu/. Accessed January 23,2006.
- American College of Physicians. Available at: http://www.acponline.org/college/membership/classes.htm#fellow. Accessed June 10,2006
- ,,, et al.Effect of the inpatient general medicine rotation on student pursuit of a generalist career.J Gen Intern Med.2006;21:471–475.
- ,.Graduate medical education research in the 21st century and JAMA on call.JAMA.2004;292:2913–2915.
- Association of American Medical Colleges. MedEd (PORTAL); Providing Online Resources to Advance Learning in Medical Education. Available at: http://www.aamc.org/meded/mededportal/start.htm. Accessed January 23,2006.
- BioMed Central. BMC Medical Education. Available at: http://www.biomedcentral.com/bmcmededuc/.Accessed January 23,2006.
- ,,,.Costs and funding for published medical education research.JAMA.2005;294(9):1052–7.
- .Teamwork and hospital medicine. A vision for the future.Crit Care Nurse.2003;23(3):8,10–11.
- . (1995)Diffusion of Innovations.4th ed.The Free Press:,Toronto.
- ,,.Clarifying the concepts in knowledge transfer: a literature review.J Adv Nurs.2006;53:691–701.
- ,,,,.Creating a quality improvement elective for medical house officers.J Gen Intern Med.2004;19:861–867.
- ,,.A continuous quality improvement curriculum for residents: addressing core competency, improving systems.Acad Med.2004;79(10 Suppl):S65–7.
- Institute for Healthcare Delivery Research. Advanced Training Program in Health Care Delivery Improvement (ATP). Available at: http://www.ihc.com/xp/ihc/institute/education/atp/. Accessed October 3,2005.
- Veterans Health Administration. VA Quality Scholars Program. Available at: http://www.dartmouth.edu/∼cecs/fellowships/vaqs.html
- Institute for Healthcare Improvement. George W. Merck Fellowships. Available at: http://www.ihi.org/ihi. Accessed October 3,2005.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,.The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- Robert Wood Johnson Clinical Scholars Program. Stanford University (Palo Alto, CA). Available at: http://rwjcsp.stanford.edu/. Accessed October 3,2005.
- ,.Hospital Medicine Fellowship Update.Society of Hospital Medicine.The Hospitalist.2004;8(5):38.
- ,,,.Hospital medicine fellowships: works in progress.Am J Med.2006;119(1):72.e1–e7.
- ,,, et al.Mentorship in Academic General Internal Medicine.J Gen Intern Med.2005;2(34):1–5.
- ,,,,.Getting funded. Career development awards for aspiring clinical investigators.J Gen Intern Med.2004;19(5 Pt 1):472–478.
- K Kiosk—Information about NIH Career Development Awards. Available at: http://grants.nih.gov/training/careerdevelopmentawards.htm. Accessed March 20,2006.
- Research Career Development Awards for Junior Faculty and Fellows in General Internal Medicine. Available at: http://www.sgim.org/careerdevelopment.cfm. Accessed March 24,2006.
- Society of Hospital Medicine. Available at: http://www.hospitalmedicine.org//AM/Template.cfm?Section=Home. Accessed October 4,2005.
- ,.What is an academic general internist? Career options and training pathways.JAMA.2002;288:2045–2048.
- U.S. National Institutes of Health.National Institute on Aging. Available at: http://www.nia.nih.gov/. Accessed January 25,2006.
- .Hartford Foundation. Available from: http://www.jhartfound.org/. Accessed January 25,2006.
- .Why will physicians in this new environment replace MHAs?Physician Exec.1996;22(2):5–10.
- Directory of Fellowships in Health Services Administration. Available at: http://www.ache.org/pgfd/purpose.cfm. Accessed March 24,2006.
- American College of Physician Executives. Available at: http://www.acpe.org/. Accessed October 3,2005.
- Society of Hospital Medicine. Leadership Academy statement. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Search_Advanced_Search6(7):37–40.
- ,,,.Satisfaction higher for physician executives who treat patients, survey finds.Physician Exec.2002;28(3):17–21.
- .Physician executives don't have to go it alone.Managed Care Magazine.2003. Available at: http://www.managedcaremag.com/archives/0307/0307.viewpoint_lazarus.html.Accessed January 25,year="2006"2006.
- .Controlled burn! Physician executives must be ready to handle job burnout, career stress.Physician Exec.2001;27(4):42–45.
There is a growing demand for hospitalists in the United States. In academic settings, hospitalists are called on to perform a variety of duties, from leading quality improvement initiatives to serving on hospital committees to helping to offset restrictions on work hours of the house staff.1 Although hospitalists may be well positioned to take on these roles, obtaining adequate protected time and recognition for such contributions remains a challenge. The existing promotion and tenure processes at academic institutions may not give adequate consideration to such responsibilities. Hospitalists who do not meet the traditional benchmarks of teaching and research may suffer in their career advancement and, ultimately, in their desire to remain in academics. Developing a sustainable and long‐term career in hospital medicine is important not only from a professional developmental standpoint, but also because it may lead to better patient care; evidence from a large multicenter hospitalist study suggests that physician experience is linked to improved patient care and outcomes.2 Thus, it behooves academic medical centers that employ hospitalists to create rewarding hospitalist career paths.
Goldman described academic hospital medicine as comprising periods of systole, during which hospitalists provide clinical care, and periods of diastole, the portion of a hospitalist's time spent in nonclinical activities.3 Far from being a period of relaxation, diastole is an active component of a hospitalist's work, the time devoted to the pursuit of complementary interests, career advancement, and job diversity. A well‐thought‐out plan for the diastolic phase of a hospitalist job description can lead to significant improvement in quality, education, research, and outcomes for an academic medical center.4 A good balance of systole and diastole allows for focus on career development and advancement and has the potential to be very helpful in preventing burnout. This is of particular concern to academic hospitalists, who report working longer hours, feeling more stress, and worrying more about burnout than their nonhospitalist colleagues.5 This suggests the diastolic phase is an important part of creating a sustainable hospitalist job and should be funded as part of an academic hospitalist position.
Although the optimal balance of systole and diastole to prevent burnout is not known, outlining clear expectations is an important strategy for preparing physicians for a sustainable academic hospitalist career. This is an important issue, given the increasing number of residency graduates who are choosing careers in hospital medicine.6 Based on the reported career plans of residents taking internal medicine in‐training exams from 2002 through 2006, the number of residents going into hospital medicine has more than doubled, from 3% (in 2002) to 6.5% (in 2006). The goal of this article is to compare and contrast several career paths that balance systole and diastole in academic hospital medicine. Specifically, we review training opportunities for becoming a successful hospitalist‐educator, hospitalistquality expert, hospitalist‐investigator, and hospitalist‐administrator.
EDUCATION (THE HOSPITALIST‐EDUCATOR)
Hospitalists in academic centers often play central roles as teachers and leaders in medical education. This is not surprising given that most teaching of medical trainees occurs in the inpatient setting.7 Furthermore, several studies have consistently demonstrated that trainee satisfaction with teaching by hospitalists is high, and hospitalists are rated as more effective teachers than traditional subspecialist ward attendings.810
A typical hospitalist‐educator position is 80%‐90% clinical time, with 10%‐20% set aside for teaching. However, academic hospitalists are often expected to teach medical trainees concurrently with their clinical care activities, rather than during a separate, protected time.11 Thus, most hospitalist‐educator responsibilities do not occur during diastole, as may be conceived, but instead are add to the systole. Small amounts of protected diastolic time for a hospitalist‐educator can be used for related administrative activities, such as writing letters of recommendation, mentoring students and residents, doing creative thinking and curriculum development, and conducting educational research, such as evaluating a new educational program or curriculum. Some hospitalist‐educator positions, such as director of the residency program or internal medicine clerkship, are exceptions in that they generally include a greater amount of protected time, which may be earmarked for administrative activities and hands‐on teaching.
Education and Training
One possibility for advanced training in education is the addition of a chief resident year, either at a physician's own institution or at another academic center. Such a year provides an opportunity to consolidate knowledge, build a teaching portfolio, and accumulate expertise in an area such as evidence‐based medicine or perioperative care. Serving as a chief resident can enhance subsequent applications by being able to demonstrate the ability to teach and, more importantly, to assume a leadership role within an organization. These skills can be applied to a number of activities in an academic hospitalist program, such as heading a committee, teaching during inpatient service time, or developing a new course for students, residents, or faculty.
An advanced training program in medical education is also an option (Table 1). Offerings include medical education fellowship training, formal degree‐granting programs (such as a master's in health professions education), or short‐term intensive coursework. Fellowships and degree‐granting programs are generally 2‐year programs designed for health professionals who want to better prepare for educational leadership roles. Core topics include curriculum development, program evaluation, instruction, student assessment, current educational issues, research methods, and leadership. An alternative option for busy clinician‐educators is online or distance learning courses in medical education, which cover similar topics and skill sets. In early 2006 the Society of Hospital Medicine released the Core Competencies in Hospital Medicine, which can serve as a useful framework for developing novel inpatient curricula for faculty, residents, and students.12, 13
| Description | Length of time | Cost | Source/website |
|---|---|---|---|
| Degrees/fellowships | |||
| Master's in health professional education (MHPE): Preparation for educational leadership roles. Typical coursework in curriculum development, program evaluation, instruction, student assessment, current educational issues, research methods, and leadership. | Varies according to program | Tuition ranges from approximately $1500‐$4300 | Example: University of Illinois |
| Fellowship in medical education: Prepares faculty to pursue scholarship in medical education or educational leadership or to become effective teachers through workshops, coursework, and/or a mentored project. Often affiliated with a department of medical education. | Varies according to program. Generally 1 year. | Varies. May be subsidized in certain institutions as part of internal faculty development. | Example: University of Michigan |
| Short‐term coursework | |||
| Harvard Macy Institute: Programs designed to promote leadership and scholarship in medical education | 1‐ or 2‐week programs | Fees for the year 2006 are $4500 USD. | |
| Stanford Faculty Development Center (SFDC): Train‐the‐trainer approach for clinical teaching and professionalism in contemporary practice | 4‐week training sessions | The institutions of faculty selected for the month‐long training programs are asked to pay a fee of $5000. Transportation, housing and food are not included. | |
Short‐term extramural courses offered by institutions such as the Harvard Macy Institute for Medical Educators and the Stanford Faculty Development Program in Teaching can also provide advanced instruction to hospitalist‐educators.14, 15 In addition to these training programs, the Society of General Internal Medicine, along with other professional societies, offers career development workshops for clinician educators on topics such as curriculum development and teaching skills.
Regardless of the type of training, adequate mentorship and resources are critical to the successful application of new skills to the design or evaluation of hospital‐based curricula. Mentorship may be available from institutional leaders in medical education, even those not formally affiliated with the hospitalist program. For instance, medical school leaders, such as deans, division chiefs, chairpersons, program directors, and clerkship directors, can often be helpful in guiding junior faculty in obtaining skills and time for teaching.
We encourage those interested in a career in medical education to begin volunteering at their institution early on. Volunteering to directly teach residents and students (eg, assisting in introduction to clinical medicine, giving lectures to third‐year clerks) can be a valuable way of becoming distinguished as a qualified teacher. Likewise, joining a professional medical society of individuals with similar interests can facilitate mentorship and skill acquisition. Certain professional medical societies, such as the American College of Physicians, promote national recognition through awarding fellowships, an honor for those physicians who have demonstrated superior competence in internal medicine, professional accomplishment, and scholarship.16 Developing concrete examples of expertise in the field, such as through the publication of abstracts and articles on medical education and development of curricula, help lead to advancement in the educational track. Clear focus on a career path, development of an intellectual product, positive learner evaluation of educational activities, and national recognition can all be used by an academic institution to evaluate suitability for promotion.
Rewards and Challenges
One of the rewards of a hospitalist‐educator career is being able to meaningfully interact with a variety of trainees, including medical students and residents. As teaching attendings, hospitalist‐educators are likely to engage students and residents for short‐term but intensive periods, resulting in the ability to influence career choice and professional growth as a physician.17 Hospitalists may be called on by trainees to serve as mentors or advisers and to write letters of recommendation. In addition, with experience, hospitalist‐educators are well positioned to serve in administrative roles in medical education, such as clerkship director or program director.
Burnout is a particular concern for hospitalist‐educators, given the heavy clinical demands of inpatient academic service combined with the additional pressure to be academically productive.5 Because of this, it is important to design academic hospitalist‐educator positions with a diastole that contains time to recover from the heavy clinical demands of inpatient service, in addition to providing time for career development activities.
Successful career development as an educator can be difficult. There are relatively few venues at which educational work can be peer‐evaluated and published, which are keys to successful academic promotion.18 Because some educational journals are highly competitive, one possibility way to get educational work disseminated is through the MedEd Portal, sponsored by the Association of American Medical Colleges, which allows peer review of medical educational materials, including innovative curricula.19 In addition to original research contributions, many scientific meetings and medical education journals also accept descriptions of interesting clinical vignettes and innovations in medical education. New online education journals, such as BMC Medical Education and Seminars in Medical Practice, have expanded publication opportunities.20
Limited opportunities are available to help fund research in medical education. Although funding may be more readily available to educators who focus on a particular clinical entity or patient population, most medical education research is conducted with inadequate funding and requires extensive donated time by committed faculty.21 For this reason, securing advanced training in medical education and having protected time will allow hospitalists on the educator track to compete more successfully for limited educational research dollars and to have sufficient time to produce and publish scholarly work, thus improving their chances of academic success and career satisfaction.
CLINICAL QUALITY AND OPERATIONS IMPROVEMENT (THE HOSPITALISTQUALITY EXPERT)
Hospitalists are increasingly being called on to lead clinical quality and operations improvement at academic teaching hospitals. Benefits to the institution include the consistent presence of a committed physician who is able to plan and execute change in the context of clinical care. This is in contrast to the transient nature of residents and nonhospitalist attending physicians, whose ability to participate in such initiatives is impaired by the scheduling of their rotations. Hospitalists, however, are often able to cultivate long‐standing relationships with nurses, case managers, and hospital administrators, thereby building the institutional clout to lead such initiatives while considering views from all the necessary stakeholders.22 Thus, they are in a good position to serve as physician champions and expedite the adoption of new innovations within hospitalist groups and among other physician groups and clinical staff.23, 24
Education and Training
Being a successful agent of change requires knowledge of the science of quality improvement coupled with the skills necessary to make such changes, such as the ability to perform a needs assessment, to develop measures of performance, to negotiate and motivate others to change behaviors, to adopt new tools and practices, and to implement and test interventions designed to improve care. It is possible for residents or junior faculty members to gain this experience through designing and implementing a quality improvement project during residency training under the direction of a mentor.25, 26 However, given the likely variability in such experience, there is no substitute for formal training in these core areas of hospital medicine.
A broad range of opportunities for advanced training in quality and operations improvement are available (Table 2). Choosing the correct program may depend on baseline expertise, availability, and the desired level of involvement. For example, introductions to these skills can be obtained through precourses or workshops at medical conferences such as the Institute of Healthcare Improvement or the Society of Hospital Medicine. For more in‐depth training, the Advanced Training Program (ATP) in Health Care Delivery Improvement, sponsored by Intermountain Healthcare, offers 12‐ to 21‐day in‐depth minicourses designed to train individuals for leadership positions in quality and safety.27 Lastly, more structured fellowships, such as the Veterans Affairs Quality Scholars Program or the George W. Merck Fellowships in Health Care Improvement, offer junior and midcareer faculty the opportunity to obtain formal training in the science of quality improvement.28, 29 Because early‐career hospitalists may face geographic and financial restrictions, exploration of local or institutional opportunities for advanced education in quality improvement can be particularly important.
| Description | Length of time | Cost | Source/website |
|---|---|---|---|
| Degrees/fellowships | |||
| Veterans Association National Quality Scholars: Fellowship to learn and apply knowledge for improvement of health care | 2 years | No cost, application to fellowship program required | |
| George W. Merck Fellowship: Mentored research or improvement project at Institute of Healthcare Improvement with a plan to return to home institution to execute change | 1 year | No cost, application to fellowship program required | |
| Short‐term coursework | |||
| Intermountain Health Care: Designed to give executives and quality improvement leaders the necessary tools to conduct clinical practice improvement projects. | 20‐ and 12‐day training programs in Salt Lake City, UT | Tuition for the 20‐day program:
| |
Rewards and Challenges
Engaging in successful clinical or process improvement can be very rewarding, both professionally and personally. Professional gains include building new interdisciplinary relationships and infrastructure to continually monitor and improve key performance measures. In addition, a rigorous evaluation of this type of work can result in being able to make presentations at national meetings or to be published in a variety of peer‐reviewed medical journals, including specialty journals for quality improvement work, such as Quality and Safety in Healthcare and the Joint Commission Journal on Quality Improvement. Many national medical meetings, such as the Institute for Healthcare Improvement, the Society of Hospital Medicine and other subspecialty society meetings, also provide an opportunity to showcase innovations in practice.
Despite the potential rewards, it can also be challenging for academic hospitalists to participate in or lead quality improvement projects. One major challenge is ensuring that hospitalists are engaged in improvement work that is aligned with the interests of the hospital. Because most hospital administrators and frontline staff are employed by the hospital, whereas those comprising the academic faculty are employed by the university, this alignment is not always guaranteed. For example, an area of interest to a hospitalist that also could lead to academic productivity and career advancement might not be considered a priority area of improvement for the hospital because of competing clinical or operations improvements. In this scenario, it can be extremely difficult to engage other stakeholders such as nurses or administrative support staff in order to make a meaningful, sustainable change or improvement. To avoid this situation, it can be helpful from the outset to partner with hospital quality leaders in discussing priority areas, with attention to any potential interface in which hospitalist expertise is needed. In the event a potential project or area is identified, a hospitalist is particularly well positioned to serve as a physician champion, which is often key to the success of any hospitalwide initiative. In some cases, hospital funding may be available for these types of initiatives, increasing the likelihood of resource development for sustainable change.
RESEARCH (THE HOSPITALIST‐INVESTIGATOR)
Few hospitalists devote most of their time to clinical research. Having a strong research base is essential for the field of hospital medicine to gain credibility as a distinct specialty.4 Although the initial research in hospital medicine sought to prove the value of the field itself, hospitalists have now begun to focus on quality improvement and outcomes research.3032 Because of their unique position in clinical care, hospitalists are well situated to oversee inpatient data collection and perform research on a variety of conditions ranging from acute coronary syndromes to venous thromboembolism. Another potential area of research for hospitalists is participation in clinical trials focused on the inpatient setting. Although the proportion of time spent in research can vary widely, to become an independently successful clinical researcher typically requires a substantial amount of time be devoted to research. In general, at least 50% protected time, greater if possible, is recommended.
Education and Training
To develop a career around research generally requires advanced training in research methods. The most frequently used option for obtaining such training is through completing a clinical research fellowship in general internal medicine or an equivalent program, such as the fellowships administered by the Robert Wood Johnson Clinical Scholars Program (Table 3).33 Several academic centers also have developed such hospital medicine fellowships, which often can be tailored to provide the desired experience in research ethics, methodology, and statistical analysis.34, 35 In selecting a training program, prospective hospitalist‐researchers should consider the availability of suitable research mentors. Because hospital medicine as a field is relatively new, research mentors within the group of hospitalists may be scarce; if so, researchers should seek appropriate mentorship from established investigators in other programs or departments. Effective mentorship is a strong predictor of future research success.36
| Description | Length of time | Cost | Source/website |
|---|---|---|---|
| Degrees/fellowships | |||
| Hospital or General Medicine Fellowships: Designed to provide clinical research training through mentored projects and coursework with possible master's degree | Generally 2‐year programs | No cost, application to program is required. Stipends vary. No cost, application to program is required | Hospital Medicine: |
| Robert Wood Johnson Clinical‐Scholars Program: Training in health services research with an emphasis on community‐based research and leadership training. | 2 years | Stipends currently range from $48,000 to $50,000 per year, depending on the training site. | Robert Wood Johnson: |
| Short‐term coursework | |||
| University‐based summer programs in clinical research (eg, Harvard University Summer Session for Public Health Studies which features graduate courses in epidemiology, biostatistics, economics, health care management, etc.) | Intensive 3‐week courses in Harvard University Summer Session | 2004 tuition for each 2.5‐credit course was $1830. There is a nonrefundable deposit/registration fee of $125. These fees do not include certain course materials (ie, texts estimated at $60 per course). | Example: Harvard School of Public Health |
Negotiating protected time can be challenging for new investigators, particularly when hospitalist salaries are generated by clinical activity. Some academic programs are willing to provide a few years of departmental support to promising young investigators in order to allow them to develop their research program and obtain additional funding. Several career development awards are available through the National Institutes of Health and through nonfederally funded sources.37, 38 These awards generally protect 3‐5 years of a researcher's time for research and require that a substantial proportion of time be devoted to that purpose, often at least 75%.
To gain visibility as a researcher, it is advantageous to present original findings at national meetings, such as those of the Society of Hospital Medicine, the Society of General Internal Medicine, and other subspecialty meetings.39, 40 These meetings not only increase awareness of a hospitalist's research but also provide opportunities for networking and developing collaboration on research. Many societies, including the Society of Hospital Medicine, have research abstract competitions and offer research grants for investigators that can help to fund projects and support protected time.
Rewards and Challenges
There are many rewards and opportunities for a hospitalist investigator, particularly because the field is young and there are many unanswered research questions related to inpatient medicine. There are also the intrinsic rewards of being devoted to scientific inquiry and having greater autonomy over how time is spent. A hospitalist's schedule can be well suited to research. Although attending on the wards can be very time‐consuming, time off the wards is often free of outpatient duties and can be entirely devoted to research.
There are also several challenges to becoming a successful researcher. The pressure to obtain grant funding and publish high‐quality scientific manuscripts is high. Obtaining sufficient protected time may be difficult in busy clinical departments, and applying for grant funding is both time‐consuming and highly competitive. It is very important to be familiar with the specific criteria for academic promotion at one's institution. Understanding these expectations can help to effectively prioritize activities. Standard requirements generally include number and quality of articles published in peer‐reviewed journals, successful application for research funding, national recognition in the field, service to the institution and research community, and evidence of research independence. One significant challenge is the lack of a single large funding source for hospital‐related research. Although the Agency for Healthcare Research and Quality funds studies related to hospital care, such as on the quality of care or cost effectiveness of various system‐based hospital care interventions, their budget for investigator‐initiated proposals is limited.41 One promising funding source for research in hospital care is from agencies and foundations dedicated to the aging population, such as the National Institute for Aging (NIA), the Hartford Foundation, and the Aetna Foundation, to name a few.42, 43 Yet research on hospital care alone, without detailed attention to issues unique to geriatric‐specific conditions or populations, is unlikely to be funded by these avenues. With few federal grant programs directly suited to the emerging research agenda in hospital medicine, hospitalist‐investigators may be at a disadvantage for obtaining tenure‐track positions, compared with their subspecialist colleagues, who may receive funding from NIH agencies or foundations dedicated to their own field.
ADMINISTRATION (THE HOSPITALIST‐ADMINISTRATOR)
Physician leaders in hospital administration are not new. Many hospitals already include physicians in senior management positions, such as chief medical officer.44 Naturally, a career in hospital administration is another potential path for diastole in academic medical centers.
Education and Training
Although a master of business, health administration, or medical management is not a prerequisite for the physician who wants to move into management, it is an increasingly important credential for senior administrative positions (Table 4). Primarily, it serves as a signal that a physician is committed to management and has a working knowledge of strategic planning, business models, human resources, leadership, and clinical operations. For physicians without formal business training who are interested in management, exploring internal opportunities is a necessary first step. Likewise, getting a business degree is not as important as management experience. The successful application of business skills requires practice, mentoring, and on‐the‐job experience. For hospitalists, this experience could be obtained by volunteering to serve on committees such as utilization review, quality assurance, credentialing, or medical staff executive committees. In lieu of a graduate degree, physicians may wish to participate in one of the many fellowships in health services administration. These programs generally aim to provide practical mentored learning experience in a health care organization and may last up to 2 years.45
| Description | Length of time | Cost | Source/website |
|---|---|---|---|
| Degrees/fellowships | |||
| Master's in business administration (MBA): General management core with option for courses specializing in health care. | Generally 2‐year program | Varies in accordance with each institution. | Directory websites (MBA): |
| Master's in health administration (MHA): Studies in analytic and management needs of health care. | Generally 2‐year program | Varies in accordance with each institution. | Directory websites (MHA): |
| Fellowship in health services administration: Preceptor‐directed program that provides practical learning experience in a health care organization beyond graduate‐level academic instruction. | Usually lasts 1‐2 years. | Compensation varies. Median reported as $39,055. | Directory (American College of Healthcare Executives): |
| Short‐term coursework | |||
| Society of Hospital Medicine Leadership Academy: Instruction for hospitalists in leading change, communicating effectively, handling conflict and negotiation, doing strategic planning, and interpreting hospital business drivers. Held biannually. | 3‐ to 4‐day program | $1400‐$1600. Discounted rate for members of Society of Hospital Medicine | |
For hospitalists and trainees considering a career as an executive, the American College of Physician Executives can serve as a valuable resource.46 This organization, founded in 1975, offers educational resources, including publications, comprehensive CD‐ROM products, and 1‐day courses and master's degree programs in conjunction with several leading business schools in medical management. In addition, the Society of Hospital Medicine offers a Leadership Academy designed to assist practicing hospitalists in evaluating their leadership strengths and applying them to everyday management challenges.47 Such a program also can facilitate the development of a peer network and the mentoring relationships needed to achieve these goals.
Rewards and Challenges
The life of the physician executive can be rewarding, but making the transition may prove challenging. However, if physicians can navigate this transition successfully, they will likely find a wide array of opportunities, as demand for physician‐executives remains high.
One major challenge to becoming a physician‐executive is reconciling the administrative role with the initial desire to enter a career in clinical medicine.48 Physician‐executives who continue to see patients are more likely to be satisfied with their jobs than physician‐executives who do not.49 Physician‐executives also may feel they are being criticized by their purely clinical colleagues for working in the business or management of medicine.50 Actual or perceived lack of support may promote isolation and burnout.51 In addition, the constantly shifting landscape of health care administration results in a much more unstable environment than that found in clinical medicine. For example, the risk of termination for a physician‐executive is 20‐40 times higher than that for a practicing physician.50 The reasons for this higher risk include personal conflict with a boss, reorganization (ie, downsizing, merging, etc.), and immediate departure of a supervisor. Access to mentors, support groups, and the option to practice part time are all potential mechanisms to ensure long‐term success as a physician‐administrator.
CONCLUSIONS
As hospital medicine continues to grow and evolve, designing sustainable and rewarding academic careers will be crucial to the success of the field. Being able to balance clinical systole time with obtaining the skills to support nonclinical diastole time is important to ensuring a successful career as an academic hospitalist. We have described several possible career paths in teaching, research, quality improvement, and administration. By preparing future hospitalists with the knowledge and skills required to assume a variety of roles during their diastolic time, we hope to encourage the growth of hospitalist leaders with well‐developed skill sets. If hospitalists adequately prepare themselves, academic hospital medicine will likely remain sustainable and rewarding, and future generations of trainees will be inspired and prepared to enter the field.
Acknowledgements
We are grateful to Jennifer Higa and Kimberly Alvarez for their assistance in preparing this manuscript.
There is a growing demand for hospitalists in the United States. In academic settings, hospitalists are called on to perform a variety of duties, from leading quality improvement initiatives to serving on hospital committees to helping to offset restrictions on work hours of the house staff.1 Although hospitalists may be well positioned to take on these roles, obtaining adequate protected time and recognition for such contributions remains a challenge. The existing promotion and tenure processes at academic institutions may not give adequate consideration to such responsibilities. Hospitalists who do not meet the traditional benchmarks of teaching and research may suffer in their career advancement and, ultimately, in their desire to remain in academics. Developing a sustainable and long‐term career in hospital medicine is important not only from a professional developmental standpoint, but also because it may lead to better patient care; evidence from a large multicenter hospitalist study suggests that physician experience is linked to improved patient care and outcomes.2 Thus, it behooves academic medical centers that employ hospitalists to create rewarding hospitalist career paths.
Goldman described academic hospital medicine as comprising periods of systole, during which hospitalists provide clinical care, and periods of diastole, the portion of a hospitalist's time spent in nonclinical activities.3 Far from being a period of relaxation, diastole is an active component of a hospitalist's work, the time devoted to the pursuit of complementary interests, career advancement, and job diversity. A well‐thought‐out plan for the diastolic phase of a hospitalist job description can lead to significant improvement in quality, education, research, and outcomes for an academic medical center.4 A good balance of systole and diastole allows for focus on career development and advancement and has the potential to be very helpful in preventing burnout. This is of particular concern to academic hospitalists, who report working longer hours, feeling more stress, and worrying more about burnout than their nonhospitalist colleagues.5 This suggests the diastolic phase is an important part of creating a sustainable hospitalist job and should be funded as part of an academic hospitalist position.
Although the optimal balance of systole and diastole to prevent burnout is not known, outlining clear expectations is an important strategy for preparing physicians for a sustainable academic hospitalist career. This is an important issue, given the increasing number of residency graduates who are choosing careers in hospital medicine.6 Based on the reported career plans of residents taking internal medicine in‐training exams from 2002 through 2006, the number of residents going into hospital medicine has more than doubled, from 3% (in 2002) to 6.5% (in 2006). The goal of this article is to compare and contrast several career paths that balance systole and diastole in academic hospital medicine. Specifically, we review training opportunities for becoming a successful hospitalist‐educator, hospitalistquality expert, hospitalist‐investigator, and hospitalist‐administrator.
EDUCATION (THE HOSPITALIST‐EDUCATOR)
Hospitalists in academic centers often play central roles as teachers and leaders in medical education. This is not surprising given that most teaching of medical trainees occurs in the inpatient setting.7 Furthermore, several studies have consistently demonstrated that trainee satisfaction with teaching by hospitalists is high, and hospitalists are rated as more effective teachers than traditional subspecialist ward attendings.810
A typical hospitalist‐educator position is 80%‐90% clinical time, with 10%‐20% set aside for teaching. However, academic hospitalists are often expected to teach medical trainees concurrently with their clinical care activities, rather than during a separate, protected time.11 Thus, most hospitalist‐educator responsibilities do not occur during diastole, as may be conceived, but instead are add to the systole. Small amounts of protected diastolic time for a hospitalist‐educator can be used for related administrative activities, such as writing letters of recommendation, mentoring students and residents, doing creative thinking and curriculum development, and conducting educational research, such as evaluating a new educational program or curriculum. Some hospitalist‐educator positions, such as director of the residency program or internal medicine clerkship, are exceptions in that they generally include a greater amount of protected time, which may be earmarked for administrative activities and hands‐on teaching.
Education and Training
One possibility for advanced training in education is the addition of a chief resident year, either at a physician's own institution or at another academic center. Such a year provides an opportunity to consolidate knowledge, build a teaching portfolio, and accumulate expertise in an area such as evidence‐based medicine or perioperative care. Serving as a chief resident can enhance subsequent applications by being able to demonstrate the ability to teach and, more importantly, to assume a leadership role within an organization. These skills can be applied to a number of activities in an academic hospitalist program, such as heading a committee, teaching during inpatient service time, or developing a new course for students, residents, or faculty.
An advanced training program in medical education is also an option (Table 1). Offerings include medical education fellowship training, formal degree‐granting programs (such as a master's in health professions education), or short‐term intensive coursework. Fellowships and degree‐granting programs are generally 2‐year programs designed for health professionals who want to better prepare for educational leadership roles. Core topics include curriculum development, program evaluation, instruction, student assessment, current educational issues, research methods, and leadership. An alternative option for busy clinician‐educators is online or distance learning courses in medical education, which cover similar topics and skill sets. In early 2006 the Society of Hospital Medicine released the Core Competencies in Hospital Medicine, which can serve as a useful framework for developing novel inpatient curricula for faculty, residents, and students.12, 13
| Description | Length of time | Cost | Source/website |
|---|---|---|---|
| Degrees/fellowships | |||
| Master's in health professional education (MHPE): Preparation for educational leadership roles. Typical coursework in curriculum development, program evaluation, instruction, student assessment, current educational issues, research methods, and leadership. | Varies according to program | Tuition ranges from approximately $1500‐$4300 | Example: University of Illinois |
| Fellowship in medical education: Prepares faculty to pursue scholarship in medical education or educational leadership or to become effective teachers through workshops, coursework, and/or a mentored project. Often affiliated with a department of medical education. | Varies according to program. Generally 1 year. | Varies. May be subsidized in certain institutions as part of internal faculty development. | Example: University of Michigan |
| Short‐term coursework | |||
| Harvard Macy Institute: Programs designed to promote leadership and scholarship in medical education | 1‐ or 2‐week programs | Fees for the year 2006 are $4500 USD. | |
| Stanford Faculty Development Center (SFDC): Train‐the‐trainer approach for clinical teaching and professionalism in contemporary practice | 4‐week training sessions | The institutions of faculty selected for the month‐long training programs are asked to pay a fee of $5000. Transportation, housing and food are not included. | |
Short‐term extramural courses offered by institutions such as the Harvard Macy Institute for Medical Educators and the Stanford Faculty Development Program in Teaching can also provide advanced instruction to hospitalist‐educators.14, 15 In addition to these training programs, the Society of General Internal Medicine, along with other professional societies, offers career development workshops for clinician educators on topics such as curriculum development and teaching skills.
Regardless of the type of training, adequate mentorship and resources are critical to the successful application of new skills to the design or evaluation of hospital‐based curricula. Mentorship may be available from institutional leaders in medical education, even those not formally affiliated with the hospitalist program. For instance, medical school leaders, such as deans, division chiefs, chairpersons, program directors, and clerkship directors, can often be helpful in guiding junior faculty in obtaining skills and time for teaching.
We encourage those interested in a career in medical education to begin volunteering at their institution early on. Volunteering to directly teach residents and students (eg, assisting in introduction to clinical medicine, giving lectures to third‐year clerks) can be a valuable way of becoming distinguished as a qualified teacher. Likewise, joining a professional medical society of individuals with similar interests can facilitate mentorship and skill acquisition. Certain professional medical societies, such as the American College of Physicians, promote national recognition through awarding fellowships, an honor for those physicians who have demonstrated superior competence in internal medicine, professional accomplishment, and scholarship.16 Developing concrete examples of expertise in the field, such as through the publication of abstracts and articles on medical education and development of curricula, help lead to advancement in the educational track. Clear focus on a career path, development of an intellectual product, positive learner evaluation of educational activities, and national recognition can all be used by an academic institution to evaluate suitability for promotion.
Rewards and Challenges
One of the rewards of a hospitalist‐educator career is being able to meaningfully interact with a variety of trainees, including medical students and residents. As teaching attendings, hospitalist‐educators are likely to engage students and residents for short‐term but intensive periods, resulting in the ability to influence career choice and professional growth as a physician.17 Hospitalists may be called on by trainees to serve as mentors or advisers and to write letters of recommendation. In addition, with experience, hospitalist‐educators are well positioned to serve in administrative roles in medical education, such as clerkship director or program director.
Burnout is a particular concern for hospitalist‐educators, given the heavy clinical demands of inpatient academic service combined with the additional pressure to be academically productive.5 Because of this, it is important to design academic hospitalist‐educator positions with a diastole that contains time to recover from the heavy clinical demands of inpatient service, in addition to providing time for career development activities.
Successful career development as an educator can be difficult. There are relatively few venues at which educational work can be peer‐evaluated and published, which are keys to successful academic promotion.18 Because some educational journals are highly competitive, one possibility way to get educational work disseminated is through the MedEd Portal, sponsored by the Association of American Medical Colleges, which allows peer review of medical educational materials, including innovative curricula.19 In addition to original research contributions, many scientific meetings and medical education journals also accept descriptions of interesting clinical vignettes and innovations in medical education. New online education journals, such as BMC Medical Education and Seminars in Medical Practice, have expanded publication opportunities.20
Limited opportunities are available to help fund research in medical education. Although funding may be more readily available to educators who focus on a particular clinical entity or patient population, most medical education research is conducted with inadequate funding and requires extensive donated time by committed faculty.21 For this reason, securing advanced training in medical education and having protected time will allow hospitalists on the educator track to compete more successfully for limited educational research dollars and to have sufficient time to produce and publish scholarly work, thus improving their chances of academic success and career satisfaction.
CLINICAL QUALITY AND OPERATIONS IMPROVEMENT (THE HOSPITALISTQUALITY EXPERT)
Hospitalists are increasingly being called on to lead clinical quality and operations improvement at academic teaching hospitals. Benefits to the institution include the consistent presence of a committed physician who is able to plan and execute change in the context of clinical care. This is in contrast to the transient nature of residents and nonhospitalist attending physicians, whose ability to participate in such initiatives is impaired by the scheduling of their rotations. Hospitalists, however, are often able to cultivate long‐standing relationships with nurses, case managers, and hospital administrators, thereby building the institutional clout to lead such initiatives while considering views from all the necessary stakeholders.22 Thus, they are in a good position to serve as physician champions and expedite the adoption of new innovations within hospitalist groups and among other physician groups and clinical staff.23, 24
Education and Training
Being a successful agent of change requires knowledge of the science of quality improvement coupled with the skills necessary to make such changes, such as the ability to perform a needs assessment, to develop measures of performance, to negotiate and motivate others to change behaviors, to adopt new tools and practices, and to implement and test interventions designed to improve care. It is possible for residents or junior faculty members to gain this experience through designing and implementing a quality improvement project during residency training under the direction of a mentor.25, 26 However, given the likely variability in such experience, there is no substitute for formal training in these core areas of hospital medicine.
A broad range of opportunities for advanced training in quality and operations improvement are available (Table 2). Choosing the correct program may depend on baseline expertise, availability, and the desired level of involvement. For example, introductions to these skills can be obtained through precourses or workshops at medical conferences such as the Institute of Healthcare Improvement or the Society of Hospital Medicine. For more in‐depth training, the Advanced Training Program (ATP) in Health Care Delivery Improvement, sponsored by Intermountain Healthcare, offers 12‐ to 21‐day in‐depth minicourses designed to train individuals for leadership positions in quality and safety.27 Lastly, more structured fellowships, such as the Veterans Affairs Quality Scholars Program or the George W. Merck Fellowships in Health Care Improvement, offer junior and midcareer faculty the opportunity to obtain formal training in the science of quality improvement.28, 29 Because early‐career hospitalists may face geographic and financial restrictions, exploration of local or institutional opportunities for advanced education in quality improvement can be particularly important.
| Description | Length of time | Cost | Source/website |
|---|---|---|---|
| Degrees/fellowships | |||
| Veterans Association National Quality Scholars: Fellowship to learn and apply knowledge for improvement of health care | 2 years | No cost, application to fellowship program required | |
| George W. Merck Fellowship: Mentored research or improvement project at Institute of Healthcare Improvement with a plan to return to home institution to execute change | 1 year | No cost, application to fellowship program required | |
| Short‐term coursework | |||
| Intermountain Health Care: Designed to give executives and quality improvement leaders the necessary tools to conduct clinical practice improvement projects. | 20‐ and 12‐day training programs in Salt Lake City, UT | Tuition for the 20‐day program:
| |
Rewards and Challenges
Engaging in successful clinical or process improvement can be very rewarding, both professionally and personally. Professional gains include building new interdisciplinary relationships and infrastructure to continually monitor and improve key performance measures. In addition, a rigorous evaluation of this type of work can result in being able to make presentations at national meetings or to be published in a variety of peer‐reviewed medical journals, including specialty journals for quality improvement work, such as Quality and Safety in Healthcare and the Joint Commission Journal on Quality Improvement. Many national medical meetings, such as the Institute for Healthcare Improvement, the Society of Hospital Medicine and other subspecialty society meetings, also provide an opportunity to showcase innovations in practice.
Despite the potential rewards, it can also be challenging for academic hospitalists to participate in or lead quality improvement projects. One major challenge is ensuring that hospitalists are engaged in improvement work that is aligned with the interests of the hospital. Because most hospital administrators and frontline staff are employed by the hospital, whereas those comprising the academic faculty are employed by the university, this alignment is not always guaranteed. For example, an area of interest to a hospitalist that also could lead to academic productivity and career advancement might not be considered a priority area of improvement for the hospital because of competing clinical or operations improvements. In this scenario, it can be extremely difficult to engage other stakeholders such as nurses or administrative support staff in order to make a meaningful, sustainable change or improvement. To avoid this situation, it can be helpful from the outset to partner with hospital quality leaders in discussing priority areas, with attention to any potential interface in which hospitalist expertise is needed. In the event a potential project or area is identified, a hospitalist is particularly well positioned to serve as a physician champion, which is often key to the success of any hospitalwide initiative. In some cases, hospital funding may be available for these types of initiatives, increasing the likelihood of resource development for sustainable change.
RESEARCH (THE HOSPITALIST‐INVESTIGATOR)
Few hospitalists devote most of their time to clinical research. Having a strong research base is essential for the field of hospital medicine to gain credibility as a distinct specialty.4 Although the initial research in hospital medicine sought to prove the value of the field itself, hospitalists have now begun to focus on quality improvement and outcomes research.3032 Because of their unique position in clinical care, hospitalists are well situated to oversee inpatient data collection and perform research on a variety of conditions ranging from acute coronary syndromes to venous thromboembolism. Another potential area of research for hospitalists is participation in clinical trials focused on the inpatient setting. Although the proportion of time spent in research can vary widely, to become an independently successful clinical researcher typically requires a substantial amount of time be devoted to research. In general, at least 50% protected time, greater if possible, is recommended.
Education and Training
To develop a career around research generally requires advanced training in research methods. The most frequently used option for obtaining such training is through completing a clinical research fellowship in general internal medicine or an equivalent program, such as the fellowships administered by the Robert Wood Johnson Clinical Scholars Program (Table 3).33 Several academic centers also have developed such hospital medicine fellowships, which often can be tailored to provide the desired experience in research ethics, methodology, and statistical analysis.34, 35 In selecting a training program, prospective hospitalist‐researchers should consider the availability of suitable research mentors. Because hospital medicine as a field is relatively new, research mentors within the group of hospitalists may be scarce; if so, researchers should seek appropriate mentorship from established investigators in other programs or departments. Effective mentorship is a strong predictor of future research success.36
| Description | Length of time | Cost | Source/website |
|---|---|---|---|
| Degrees/fellowships | |||
| Hospital or General Medicine Fellowships: Designed to provide clinical research training through mentored projects and coursework with possible master's degree | Generally 2‐year programs | No cost, application to program is required. Stipends vary. No cost, application to program is required | Hospital Medicine: |
| Robert Wood Johnson Clinical‐Scholars Program: Training in health services research with an emphasis on community‐based research and leadership training. | 2 years | Stipends currently range from $48,000 to $50,000 per year, depending on the training site. | Robert Wood Johnson: |
| Short‐term coursework | |||
| University‐based summer programs in clinical research (eg, Harvard University Summer Session for Public Health Studies which features graduate courses in epidemiology, biostatistics, economics, health care management, etc.) | Intensive 3‐week courses in Harvard University Summer Session | 2004 tuition for each 2.5‐credit course was $1830. There is a nonrefundable deposit/registration fee of $125. These fees do not include certain course materials (ie, texts estimated at $60 per course). | Example: Harvard School of Public Health |
Negotiating protected time can be challenging for new investigators, particularly when hospitalist salaries are generated by clinical activity. Some academic programs are willing to provide a few years of departmental support to promising young investigators in order to allow them to develop their research program and obtain additional funding. Several career development awards are available through the National Institutes of Health and through nonfederally funded sources.37, 38 These awards generally protect 3‐5 years of a researcher's time for research and require that a substantial proportion of time be devoted to that purpose, often at least 75%.
To gain visibility as a researcher, it is advantageous to present original findings at national meetings, such as those of the Society of Hospital Medicine, the Society of General Internal Medicine, and other subspecialty meetings.39, 40 These meetings not only increase awareness of a hospitalist's research but also provide opportunities for networking and developing collaboration on research. Many societies, including the Society of Hospital Medicine, have research abstract competitions and offer research grants for investigators that can help to fund projects and support protected time.
Rewards and Challenges
There are many rewards and opportunities for a hospitalist investigator, particularly because the field is young and there are many unanswered research questions related to inpatient medicine. There are also the intrinsic rewards of being devoted to scientific inquiry and having greater autonomy over how time is spent. A hospitalist's schedule can be well suited to research. Although attending on the wards can be very time‐consuming, time off the wards is often free of outpatient duties and can be entirely devoted to research.
There are also several challenges to becoming a successful researcher. The pressure to obtain grant funding and publish high‐quality scientific manuscripts is high. Obtaining sufficient protected time may be difficult in busy clinical departments, and applying for grant funding is both time‐consuming and highly competitive. It is very important to be familiar with the specific criteria for academic promotion at one's institution. Understanding these expectations can help to effectively prioritize activities. Standard requirements generally include number and quality of articles published in peer‐reviewed journals, successful application for research funding, national recognition in the field, service to the institution and research community, and evidence of research independence. One significant challenge is the lack of a single large funding source for hospital‐related research. Although the Agency for Healthcare Research and Quality funds studies related to hospital care, such as on the quality of care or cost effectiveness of various system‐based hospital care interventions, their budget for investigator‐initiated proposals is limited.41 One promising funding source for research in hospital care is from agencies and foundations dedicated to the aging population, such as the National Institute for Aging (NIA), the Hartford Foundation, and the Aetna Foundation, to name a few.42, 43 Yet research on hospital care alone, without detailed attention to issues unique to geriatric‐specific conditions or populations, is unlikely to be funded by these avenues. With few federal grant programs directly suited to the emerging research agenda in hospital medicine, hospitalist‐investigators may be at a disadvantage for obtaining tenure‐track positions, compared with their subspecialist colleagues, who may receive funding from NIH agencies or foundations dedicated to their own field.
ADMINISTRATION (THE HOSPITALIST‐ADMINISTRATOR)
Physician leaders in hospital administration are not new. Many hospitals already include physicians in senior management positions, such as chief medical officer.44 Naturally, a career in hospital administration is another potential path for diastole in academic medical centers.
Education and Training
Although a master of business, health administration, or medical management is not a prerequisite for the physician who wants to move into management, it is an increasingly important credential for senior administrative positions (Table 4). Primarily, it serves as a signal that a physician is committed to management and has a working knowledge of strategic planning, business models, human resources, leadership, and clinical operations. For physicians without formal business training who are interested in management, exploring internal opportunities is a necessary first step. Likewise, getting a business degree is not as important as management experience. The successful application of business skills requires practice, mentoring, and on‐the‐job experience. For hospitalists, this experience could be obtained by volunteering to serve on committees such as utilization review, quality assurance, credentialing, or medical staff executive committees. In lieu of a graduate degree, physicians may wish to participate in one of the many fellowships in health services administration. These programs generally aim to provide practical mentored learning experience in a health care organization and may last up to 2 years.45
| Description | Length of time | Cost | Source/website |
|---|---|---|---|
| Degrees/fellowships | |||
| Master's in business administration (MBA): General management core with option for courses specializing in health care. | Generally 2‐year program | Varies in accordance with each institution. | Directory websites (MBA): |
| Master's in health administration (MHA): Studies in analytic and management needs of health care. | Generally 2‐year program | Varies in accordance with each institution. | Directory websites (MHA): |
| Fellowship in health services administration: Preceptor‐directed program that provides practical learning experience in a health care organization beyond graduate‐level academic instruction. | Usually lasts 1‐2 years. | Compensation varies. Median reported as $39,055. | Directory (American College of Healthcare Executives): |
| Short‐term coursework | |||
| Society of Hospital Medicine Leadership Academy: Instruction for hospitalists in leading change, communicating effectively, handling conflict and negotiation, doing strategic planning, and interpreting hospital business drivers. Held biannually. | 3‐ to 4‐day program | $1400‐$1600. Discounted rate for members of Society of Hospital Medicine | |
For hospitalists and trainees considering a career as an executive, the American College of Physician Executives can serve as a valuable resource.46 This organization, founded in 1975, offers educational resources, including publications, comprehensive CD‐ROM products, and 1‐day courses and master's degree programs in conjunction with several leading business schools in medical management. In addition, the Society of Hospital Medicine offers a Leadership Academy designed to assist practicing hospitalists in evaluating their leadership strengths and applying them to everyday management challenges.47 Such a program also can facilitate the development of a peer network and the mentoring relationships needed to achieve these goals.
Rewards and Challenges
The life of the physician executive can be rewarding, but making the transition may prove challenging. However, if physicians can navigate this transition successfully, they will likely find a wide array of opportunities, as demand for physician‐executives remains high.
One major challenge to becoming a physician‐executive is reconciling the administrative role with the initial desire to enter a career in clinical medicine.48 Physician‐executives who continue to see patients are more likely to be satisfied with their jobs than physician‐executives who do not.49 Physician‐executives also may feel they are being criticized by their purely clinical colleagues for working in the business or management of medicine.50 Actual or perceived lack of support may promote isolation and burnout.51 In addition, the constantly shifting landscape of health care administration results in a much more unstable environment than that found in clinical medicine. For example, the risk of termination for a physician‐executive is 20‐40 times higher than that for a practicing physician.50 The reasons for this higher risk include personal conflict with a boss, reorganization (ie, downsizing, merging, etc.), and immediate departure of a supervisor. Access to mentors, support groups, and the option to practice part time are all potential mechanisms to ensure long‐term success as a physician‐administrator.
CONCLUSIONS
As hospital medicine continues to grow and evolve, designing sustainable and rewarding academic careers will be crucial to the success of the field. Being able to balance clinical systole time with obtaining the skills to support nonclinical diastole time is important to ensuring a successful career as an academic hospitalist. We have described several possible career paths in teaching, research, quality improvement, and administration. By preparing future hospitalists with the knowledge and skills required to assume a variety of roles during their diastolic time, we hope to encourage the growth of hospitalist leaders with well‐developed skill sets. If hospitalists adequately prepare themselves, academic hospital medicine will likely remain sustainable and rewarding, and future generations of trainees will be inspired and prepared to enter the field.
Acknowledgements
We are grateful to Jennifer Higa and Kimberly Alvarez for their assistance in preparing this manuscript.
- ,,.Balancing continuity of care with residents' limited work hours: defining the implications.Acad Med.2005;80:39–43.
- ,,, et al.Effects of inpatient experience on outcomes and costs in a multicenter trial of academic hospitalists.J Gen Intern Med.2005;20(s1):141–142.
- .The hospitalist movement.Ann Intern Med.1999;131:545.
- .The future of hospital medicine: evolution or revolution?Am J Med.2004;117:446–450.
- ,,, et al.Satisfaction and worklife of academic hospitalist and non‐hospitalist attendings on general medical inpatient rotations.J Gen Intern Med.2006;21(s4):128.
- ,,.Career plans for trainees in internal medicine residency programs.Acad Med.2005;80:507–512.
- ,,,,.Closing the gap between internal medicine training and practice: recommendations from recent graduates.Amer J Med.2005;118:680–687.
- ,,, et al..Hospitalists as teachers.J Gen Intern Med.2004;19(1):8–15.
- ,.Implications of the hospitalist model for medical students' education.Acad Med.2001;76:324–330.
- ,,,,,.Resident satisfaction on an academic hospitalist service: time to teach.Am J Med.2002;112:597–601.
- ,.The present and future of appointment, tenure, and compensation policies for medical school clinical faculty.Acad Med.2001;76:993–1004.
- ,,,,.Core competencies in hospital medicine: development and methodology.J Hosp Med.2006;1(1):48–56.
- ,,,,.The core competencies in hospital medicine.J Hosp Med.2006;1(1).
- Harvard Macy Institute. Harvard College. Available at: http://www.harvardmacy.org/programs.asp?DocumentID=1. Accessed October 3,2005.
- Stanford Faculty Development Center. Stanford University. Available at http://sfdc.stanford.edu/. Accessed January 23,2006.
- American College of Physicians. Available at: http://www.acponline.org/college/membership/classes.htm#fellow. Accessed June 10,2006
- ,,, et al.Effect of the inpatient general medicine rotation on student pursuit of a generalist career.J Gen Intern Med.2006;21:471–475.
- ,.Graduate medical education research in the 21st century and JAMA on call.JAMA.2004;292:2913–2915.
- Association of American Medical Colleges. MedEd (PORTAL); Providing Online Resources to Advance Learning in Medical Education. Available at: http://www.aamc.org/meded/mededportal/start.htm. Accessed January 23,2006.
- BioMed Central. BMC Medical Education. Available at: http://www.biomedcentral.com/bmcmededuc/.Accessed January 23,2006.
- ,,,.Costs and funding for published medical education research.JAMA.2005;294(9):1052–7.
- .Teamwork and hospital medicine. A vision for the future.Crit Care Nurse.2003;23(3):8,10–11.
- . (1995)Diffusion of Innovations.4th ed.The Free Press:,Toronto.
- ,,.Clarifying the concepts in knowledge transfer: a literature review.J Adv Nurs.2006;53:691–701.
- ,,,,.Creating a quality improvement elective for medical house officers.J Gen Intern Med.2004;19:861–867.
- ,,.A continuous quality improvement curriculum for residents: addressing core competency, improving systems.Acad Med.2004;79(10 Suppl):S65–7.
- Institute for Healthcare Delivery Research. Advanced Training Program in Health Care Delivery Improvement (ATP). Available at: http://www.ihc.com/xp/ihc/institute/education/atp/. Accessed October 3,2005.
- Veterans Health Administration. VA Quality Scholars Program. Available at: http://www.dartmouth.edu/∼cecs/fellowships/vaqs.html
- Institute for Healthcare Improvement. George W. Merck Fellowships. Available at: http://www.ihi.org/ihi. Accessed October 3,2005.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,.The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- Robert Wood Johnson Clinical Scholars Program. Stanford University (Palo Alto, CA). Available at: http://rwjcsp.stanford.edu/. Accessed October 3,2005.
- ,.Hospital Medicine Fellowship Update.Society of Hospital Medicine.The Hospitalist.2004;8(5):38.
- ,,,.Hospital medicine fellowships: works in progress.Am J Med.2006;119(1):72.e1–e7.
- ,,, et al.Mentorship in Academic General Internal Medicine.J Gen Intern Med.2005;2(34):1–5.
- ,,,,.Getting funded. Career development awards for aspiring clinical investigators.J Gen Intern Med.2004;19(5 Pt 1):472–478.
- K Kiosk—Information about NIH Career Development Awards. Available at: http://grants.nih.gov/training/careerdevelopmentawards.htm. Accessed March 20,2006.
- Research Career Development Awards for Junior Faculty and Fellows in General Internal Medicine. Available at: http://www.sgim.org/careerdevelopment.cfm. Accessed March 24,2006.
- Society of Hospital Medicine. Available at: http://www.hospitalmedicine.org//AM/Template.cfm?Section=Home. Accessed October 4,2005.
- ,.What is an academic general internist? Career options and training pathways.JAMA.2002;288:2045–2048.
- U.S. National Institutes of Health.National Institute on Aging. Available at: http://www.nia.nih.gov/. Accessed January 25,2006.
- .Hartford Foundation. Available from: http://www.jhartfound.org/. Accessed January 25,2006.
- .Why will physicians in this new environment replace MHAs?Physician Exec.1996;22(2):5–10.
- Directory of Fellowships in Health Services Administration. Available at: http://www.ache.org/pgfd/purpose.cfm. Accessed March 24,2006.
- American College of Physician Executives. Available at: http://www.acpe.org/. Accessed October 3,2005.
- Society of Hospital Medicine. Leadership Academy statement. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Search_Advanced_Search6(7):37–40.
- ,,,.Satisfaction higher for physician executives who treat patients, survey finds.Physician Exec.2002;28(3):17–21.
- .Physician executives don't have to go it alone.Managed Care Magazine.2003. Available at: http://www.managedcaremag.com/archives/0307/0307.viewpoint_lazarus.html.Accessed January 25,year="2006"2006.
- .Controlled burn! Physician executives must be ready to handle job burnout, career stress.Physician Exec.2001;27(4):42–45.
- ,,.Balancing continuity of care with residents' limited work hours: defining the implications.Acad Med.2005;80:39–43.
- ,,, et al.Effects of inpatient experience on outcomes and costs in a multicenter trial of academic hospitalists.J Gen Intern Med.2005;20(s1):141–142.
- .The hospitalist movement.Ann Intern Med.1999;131:545.
- .The future of hospital medicine: evolution or revolution?Am J Med.2004;117:446–450.
- ,,, et al.Satisfaction and worklife of academic hospitalist and non‐hospitalist attendings on general medical inpatient rotations.J Gen Intern Med.2006;21(s4):128.
- ,,.Career plans for trainees in internal medicine residency programs.Acad Med.2005;80:507–512.
- ,,,,.Closing the gap between internal medicine training and practice: recommendations from recent graduates.Amer J Med.2005;118:680–687.
- ,,, et al..Hospitalists as teachers.J Gen Intern Med.2004;19(1):8–15.
- ,.Implications of the hospitalist model for medical students' education.Acad Med.2001;76:324–330.
- ,,,,,.Resident satisfaction on an academic hospitalist service: time to teach.Am J Med.2002;112:597–601.
- ,.The present and future of appointment, tenure, and compensation policies for medical school clinical faculty.Acad Med.2001;76:993–1004.
- ,,,,.Core competencies in hospital medicine: development and methodology.J Hosp Med.2006;1(1):48–56.
- ,,,,.The core competencies in hospital medicine.J Hosp Med.2006;1(1).
- Harvard Macy Institute. Harvard College. Available at: http://www.harvardmacy.org/programs.asp?DocumentID=1. Accessed October 3,2005.
- Stanford Faculty Development Center. Stanford University. Available at http://sfdc.stanford.edu/. Accessed January 23,2006.
- American College of Physicians. Available at: http://www.acponline.org/college/membership/classes.htm#fellow. Accessed June 10,2006
- ,,, et al.Effect of the inpatient general medicine rotation on student pursuit of a generalist career.J Gen Intern Med.2006;21:471–475.
- ,.Graduate medical education research in the 21st century and JAMA on call.JAMA.2004;292:2913–2915.
- Association of American Medical Colleges. MedEd (PORTAL); Providing Online Resources to Advance Learning in Medical Education. Available at: http://www.aamc.org/meded/mededportal/start.htm. Accessed January 23,2006.
- BioMed Central. BMC Medical Education. Available at: http://www.biomedcentral.com/bmcmededuc/.Accessed January 23,2006.
- ,,,.Costs and funding for published medical education research.JAMA.2005;294(9):1052–7.
- .Teamwork and hospital medicine. A vision for the future.Crit Care Nurse.2003;23(3):8,10–11.
- . (1995)Diffusion of Innovations.4th ed.The Free Press:,Toronto.
- ,,.Clarifying the concepts in knowledge transfer: a literature review.J Adv Nurs.2006;53:691–701.
- ,,,,.Creating a quality improvement elective for medical house officers.J Gen Intern Med.2004;19:861–867.
- ,,.A continuous quality improvement curriculum for residents: addressing core competency, improving systems.Acad Med.2004;79(10 Suppl):S65–7.
- Institute for Healthcare Delivery Research. Advanced Training Program in Health Care Delivery Improvement (ATP). Available at: http://www.ihc.com/xp/ihc/institute/education/atp/. Accessed October 3,2005.
- Veterans Health Administration. VA Quality Scholars Program. Available at: http://www.dartmouth.edu/∼cecs/fellowships/vaqs.html
- Institute for Healthcare Improvement. George W. Merck Fellowships. Available at: http://www.ihi.org/ihi. Accessed October 3,2005.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,.The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- Robert Wood Johnson Clinical Scholars Program. Stanford University (Palo Alto, CA). Available at: http://rwjcsp.stanford.edu/. Accessed October 3,2005.
- ,.Hospital Medicine Fellowship Update.Society of Hospital Medicine.The Hospitalist.2004;8(5):38.
- ,,,.Hospital medicine fellowships: works in progress.Am J Med.2006;119(1):72.e1–e7.
- ,,, et al.Mentorship in Academic General Internal Medicine.J Gen Intern Med.2005;2(34):1–5.
- ,,,,.Getting funded. Career development awards for aspiring clinical investigators.J Gen Intern Med.2004;19(5 Pt 1):472–478.
- K Kiosk—Information about NIH Career Development Awards. Available at: http://grants.nih.gov/training/careerdevelopmentawards.htm. Accessed March 20,2006.
- Research Career Development Awards for Junior Faculty and Fellows in General Internal Medicine. Available at: http://www.sgim.org/careerdevelopment.cfm. Accessed March 24,2006.
- Society of Hospital Medicine. Available at: http://www.hospitalmedicine.org//AM/Template.cfm?Section=Home. Accessed October 4,2005.
- ,.What is an academic general internist? Career options and training pathways.JAMA.2002;288:2045–2048.
- U.S. National Institutes of Health.National Institute on Aging. Available at: http://www.nia.nih.gov/. Accessed January 25,2006.
- .Hartford Foundation. Available from: http://www.jhartfound.org/. Accessed January 25,2006.
- .Why will physicians in this new environment replace MHAs?Physician Exec.1996;22(2):5–10.
- Directory of Fellowships in Health Services Administration. Available at: http://www.ache.org/pgfd/purpose.cfm. Accessed March 24,2006.
- American College of Physician Executives. Available at: http://www.acpe.org/. Accessed October 3,2005.
- Society of Hospital Medicine. Leadership Academy statement. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Search_Advanced_Search6(7):37–40.
- ,,,.Satisfaction higher for physician executives who treat patients, survey finds.Physician Exec.2002;28(3):17–21.
- .Physician executives don't have to go it alone.Managed Care Magazine.2003. Available at: http://www.managedcaremag.com/archives/0307/0307.viewpoint_lazarus.html.Accessed January 25,year="2006"2006.
- .Controlled burn! Physician executives must be ready to handle job burnout, career stress.Physician Exec.2001;27(4):42–45.
Copyright © 2006 Society of Hospital Medicine