User login
Venous thromboembolism (VTE)—which comprises both deep vein thrombosis (DVT) and pulmonary embolism (PE), which can result from DVT—is the third leading cause of cardiovascular death in the United States, after myocardial infarction and stroke. The annual incidence of DVT approaches 2 million.1 Silent PE constitutes approximately half of DVT cases, as suggested by studies using ventilation perfusion scanning. The true incidence of PE is not known but is estimated to be 600,000 cases annually,1 with approximately one third of these cases leading to death.2
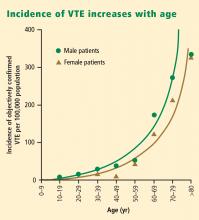
Notably, the incidence of VTE rises at an exponential rate with increasing age after the second decade of life, as shown in Figure 1.2 Given the aging of the US population, this suggests that the clinical and economic impact of VTE will only increase in the years ahead.
DESPITE ESTABLISHED BENEFITS, VTE PROPHYLAXIS REMAINS UNDERUSED
The frequency, clinical impact, and economic impact of VTE make a strong case for VTE prevention. In a 2001 analysis of patient safety practices, the Agency for Healthcare Research and Quality listed appropriate VTE prophylaxis in at-risk patients first in a rating of safety practices with the greatest strength of evidence for impact and effectiveness.4
Despite this recognition of the importance and benefit of VTE prophylaxis, prophylaxis remains highly underutilized. This has been demonstrated in numerous studies; the large epidemiologic investigation by Goldhaber et al using the DVT Free Registry is illustrative.5 This prospective multicenter study enrolled 5,451 consecutive patients with acute DVT documented by venous ultrasonography over a 6month period. Patients were classified as either outpatients or inpatients: outpatients were those who came to the emergency room and were diagnosed with DVT; inpatients were those who developed DVT in the hospital. Of the 2,726 inpatients in the registry, only 42% had received prophylaxis within 30 days prior to their diagnosis of DVT.
Risk extends to the outpatient setting
In a recent population-based analysis, Spencer et al found a similarly low rate of VTE prophylaxis— 42.8%—among 516 patients who had recently been hospitalized and subsequently developed VTE.6 This study also found that VTE was three times as likely in the outpatient setting as in the inpatient setting, and that almost half of the outpatients with VTE had been recently hospitalized. Taken together, these findings indicate that VTE prevention efforts are inadequate both in the hospital and at the time of discharge, when patients’ risk for VTE is still elevated.6,7
VTE PROPHYLAXIS AS AN EMERGING QUALITY MEASURE
Increased recognition of the impact of VTE has prompted accreditation and quality organizations to take interest in VTE risk assessment and prophylaxis as a measure for institutional performance ratings and even reimbursement.
The Joint Commission on Accreditation of Healthcare Organizations and the National Quality Forum have launched a joint project to develop a set of standardized inpatient measures to evaluate hospitals’ practices for the prevention and treatment of VTE.8 The project has pilot-tested several proposed performance measures in dozens of volunteer hospitals, including measures of whether VTE risk assessment is performed and VTE prophylaxis is initiated (if indicated) within 24 hours of admission to the hospital or to the intensive care unit. Hospitals participating in the pilot program are required to report their rates of potentially preventable hospital-acquired VTE.
Similarly, the ongoing Surgical Care Improvement Incidence of VTE increases with age Project (SCIP) has targeted VTE prophylaxis as one of a handful of priority areas for reducing surgical complications. As a national quality partnership of organizations sponsored by the Centers for Medicare and Medicaid Services (CMS), SCIP set a national goal in 2005 to reduce preventable surgical morbidity and mortality by 25% by 2010.9
The stakes of the SCIP initiative are high in both clinical and financial terms. CMS mandated that hospitals report on three SCIP quality measures in 2007 in order to receive full Medicare reimbursement in 2008. Of the three measures, two involved VTE prophylaxis: (1) how often VTE prophylaxis was ordered for surgical patients when indicated, and (2) how often appropriate surgical patients received prophylaxis postoperatively. Moreover, beginning October 1, 2008, CMS will no longer reimburse hospitals for cetain preventable conditions, and DVT and PE are being considered for inclusion in this list of conditions excluded from reimbursement.10
PROPHYLAXIS RATES CAN BE IMPROVED
Fortunately, there is evidence that interventions to increase awareness may increase the rate of VTE prophylaxis. Stinnett et al reported that education, in the form of hospital-specific data on VTE rates and implementation of risk-stratification guidelines, increased the use of VTE prophylaxis in high-risk hospitalized medical patients at a tertiary care center from a preintervention rate of 43% to a postintervention rate of 72%.11
In addition to educational interventions, formalized risk-assessment tools, in the form of electronic alerts, offer another strategy that may increase rates of VTE prophylaxis. The promise of this approach was demonstrated in a study at Brigham and Women’s Hospital in Boston, in which 2,506 hospitalized patients at risk for VTE were randomly assigned to either an intervention group, in which physicians received a computer alert about the patient’s VTE risk, or a control group, in which no alert was issued.12 The rate of VTE prophylaxis was more than twice as high in the intervention group as in the control group (33.5% vs 14.5%; P < .001), and the 90-day incidence of VTE was reduced from 8.2% in the control group to 4.9% in the intervention group (P = .001).
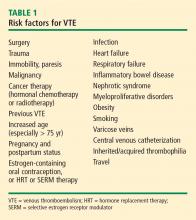
WHO’S AT RISK FOR VTE?
OPTIONS FOR VTE PROPHYLAXIS
An ideal therapy for VTE prophylaxis would be one that is effective, safe, inexpensive, and easy to administer and monitor, and that has few side effects or complications.
Mechanical prophylaxis
Mechanical forms of VTE prevention carry no risk of bleeding, are inexpensive because they can be reused, and are often effective when used properly. Mechanical forms include graduated compression stockings, intermittent pneumatic compression devices, and venous foot pumps.
The American College of Chest Physicians (ACCP), in its Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy, published in 2004,13 recommends that mechanical methods be used primarily in two settings:
- In patients with a high risk of bleeding (in whom pharmacologic prophylaxis is contraindicated)
- As an adjunct to pharmacologic prophylaxis.
Because the use of mechanical forms of prophylaxis in hospitalized medical patients is not evidence-based, mechanical prophylaxis should be reserved for those medical patients at risk for VTE who have a contraindication to pharmacologic prophylaxis.
To be effective, mechanical forms of prophylaxis must be used in accordance with the device manufacturer’s guidelines, which is frequently not what happens in clinical practice. In clinical trials in which the efficacy of intermittent pneumatic compression devices was demonstrated, patients wore their devices for 14 to 15 hours per day.
Pharmacologic options
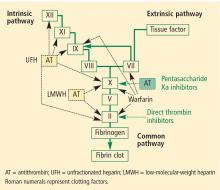
Unfractionated heparin (UFH) inhibits factor Xa and factor IIa equally. Because it is a large heterogeneous molecule, UFH is not well absorbed in subcutaneous tissue. Its anticoagulant response is variable because of its short half-life. It must be dosed two or three times daily subcutaneously for VTE prophylaxis, and must be given intravenously for treatment of VTE. The rate of heparin-induced thrombocytopenia, a potentially catastrophic adverse drug event, is considerably higher with UFH than with low-molecular-weight heparins (3% vs 1%).14 Osteopenia can develop with the use of UFH over even short periods, and osteoporosis can occur with long-term use.
Low-molecular-weight heparins (LMWHs) preferentially inhibit factor Xa compared to factor IIa. The LMWHs (ie, enoxaparin [Lovenox], dalteparin [Fragmin]) are derived from UFH through a chemical depolymerization and defractionation process that results in a much smaller molecule. LMWHs are well absorbed from subcutaneous tissue and have a predictable dose response attributable to their longer half-life (relative to UFH), which allows for once-daily or twice-daily subcutaneous dosing. As noted above, LMWHs carry a much lower rate of heparin-induced thrombocytopenia compared with UFH. Because LMWHs are predominantly cleared by the kidneys, dose adjustment may be needed in patients with renal impairment.
Fondaparinux (Arixtra) is a synthetic pentasaccha-ride that acts as a pure inhibitor of factor Xa. It binds antithrombin III, causing a conformational change by which it inhibits factor Xa and thereby inhibits coagulation further downstream. Fondaparinux has a long half-life (18 to 19 hours), which enables once-daily subcutaneous dosing but which also may require administration of the costly activated factor VII (NovoSeven) to reverse its effects in cases of bleeding. Because fondaparinux is cleared entirely by the kidneys, it is contraindicated in patients with severe renal impairment (creatinine clearance < 30 mL/min). It is also contraindicated in patients who weigh less than 50 kg, due to increased bleeding risk.
Details on the efficacy of these agents for VTE prophylaxis in various patient groups are provided in the subsequent articles in this supplement.
Investigational anticoagulants
The above pharmacologic options may soon be joined by several experimental anticoagulants that are currently in phase 3 trials for VTE prophylaxis—oral factor Xa inhibitors such as rivaroxaban and apixaban, and oral factor IIa (thrombin) inhibitors such as dabigatran.
- Hirsh J, Hoak J. Management of deep vein thrombosis and pulmonary embolism. A statement for healthcare professionals from the Council on Thrombosis (in consultation with the Council on Cardiovascular Radiology), American Heart Association. Circulation 1996; 93:2212–2245.
- Anderson FA Jr, Wheeler HB, Goldberg RJ, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med 1991; 151:933–938.
- Dimick JB, Chen SL, Taheri PA, Henderson WG, Khuri SF, Campbell DA Jr. Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg 2004; 199:531–537.
- Agency for Healthcare Research and Quality. Making Health Care Safer: A Critical Analysis of Patient Safety Practices. Evidence Report/Technology/Assessment: Number 43. AHRQ Publication No. 01-E058. Rockville, MD: Agency for Healthcare Research and Quality; July 2001:620. http://www.ahrq.gov/clinic/ptsafety. Accessed December 4, 2007.
- Goldhaber SZ, Tapson VF; DVT FREE Steering Committee. A prospective registry of 5,451 patients with ultrasound-confirmed deep vein thrombosis. Am J Cardiol 2004; 93:259–262.
- Spencer FA, Lessard D, Emery C, Reed G, Goldberg RJ. Venous thromboembolism in the outpatient setting. Arch Intern Med 2007; 167:1471–1475.
- Goldhaber SZ. Outpatient venous thromboembolism: a common but often preventable public health threat. Arch Intern Med 2007; 167:1451–1452.
- National Consensus Standards for Prevention and Care of Venous Thromboembolism (VTE). The Joint Commission Web site. http://www.jointcommission.org/PerformanceMeasurement/ PerformanceMeasurement/VTE.htm. Accessed January 8, 2008.
- Surgical Care Improvement Project. MedQIC Web site. http://www.medqic.org/scip. Accessed January 8, 2008.
- US Department of Health and Human Services. Medicare program; changes to the hospital inpatient prospective payment systems and fiscal year 2008 rates. http://www.cms.hhs.gov/AcuteInpatientPPS/ downloads/CMS-1533-FC.pdf. Accessed December 4, 2007.
- Stinnett JM, Pendleton R, Skordos L, Wheeler M, Rodgers GM. Venous thromboembolism prophylaxis in medically ill patients and the development of strategies to improve prophylaxis rates. Am J Hematol 2005; 78:167–172.
- Kucher N, Koo S, Quiroz R, et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med 2005; 352:969–977.
- Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Anti-thrombotic and Thrombolytic Therapy. Chest 2004; 126(3 Suppl):338S–400S.
- Warkentin TE, Levine MN, Hirsh J, et al. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. N Engl J Med 1995; 332:1330–1335.
Venous thromboembolism (VTE)—which comprises both deep vein thrombosis (DVT) and pulmonary embolism (PE), which can result from DVT—is the third leading cause of cardiovascular death in the United States, after myocardial infarction and stroke. The annual incidence of DVT approaches 2 million.1 Silent PE constitutes approximately half of DVT cases, as suggested by studies using ventilation perfusion scanning. The true incidence of PE is not known but is estimated to be 600,000 cases annually,1 with approximately one third of these cases leading to death.2
Notably, the incidence of VTE rises at an exponential rate with increasing age after the second decade of life, as shown in Figure 1.2 Given the aging of the US population, this suggests that the clinical and economic impact of VTE will only increase in the years ahead.
DESPITE ESTABLISHED BENEFITS, VTE PROPHYLAXIS REMAINS UNDERUSED
The frequency, clinical impact, and economic impact of VTE make a strong case for VTE prevention. In a 2001 analysis of patient safety practices, the Agency for Healthcare Research and Quality listed appropriate VTE prophylaxis in at-risk patients first in a rating of safety practices with the greatest strength of evidence for impact and effectiveness.4
Despite this recognition of the importance and benefit of VTE prophylaxis, prophylaxis remains highly underutilized. This has been demonstrated in numerous studies; the large epidemiologic investigation by Goldhaber et al using the DVT Free Registry is illustrative.5 This prospective multicenter study enrolled 5,451 consecutive patients with acute DVT documented by venous ultrasonography over a 6month period. Patients were classified as either outpatients or inpatients: outpatients were those who came to the emergency room and were diagnosed with DVT; inpatients were those who developed DVT in the hospital. Of the 2,726 inpatients in the registry, only 42% had received prophylaxis within 30 days prior to their diagnosis of DVT.
Risk extends to the outpatient setting
In a recent population-based analysis, Spencer et al found a similarly low rate of VTE prophylaxis— 42.8%—among 516 patients who had recently been hospitalized and subsequently developed VTE.6 This study also found that VTE was three times as likely in the outpatient setting as in the inpatient setting, and that almost half of the outpatients with VTE had been recently hospitalized. Taken together, these findings indicate that VTE prevention efforts are inadequate both in the hospital and at the time of discharge, when patients’ risk for VTE is still elevated.6,7
VTE PROPHYLAXIS AS AN EMERGING QUALITY MEASURE
Increased recognition of the impact of VTE has prompted accreditation and quality organizations to take interest in VTE risk assessment and prophylaxis as a measure for institutional performance ratings and even reimbursement.
The Joint Commission on Accreditation of Healthcare Organizations and the National Quality Forum have launched a joint project to develop a set of standardized inpatient measures to evaluate hospitals’ practices for the prevention and treatment of VTE.8 The project has pilot-tested several proposed performance measures in dozens of volunteer hospitals, including measures of whether VTE risk assessment is performed and VTE prophylaxis is initiated (if indicated) within 24 hours of admission to the hospital or to the intensive care unit. Hospitals participating in the pilot program are required to report their rates of potentially preventable hospital-acquired VTE.
Similarly, the ongoing Surgical Care Improvement Incidence of VTE increases with age Project (SCIP) has targeted VTE prophylaxis as one of a handful of priority areas for reducing surgical complications. As a national quality partnership of organizations sponsored by the Centers for Medicare and Medicaid Services (CMS), SCIP set a national goal in 2005 to reduce preventable surgical morbidity and mortality by 25% by 2010.9
The stakes of the SCIP initiative are high in both clinical and financial terms. CMS mandated that hospitals report on three SCIP quality measures in 2007 in order to receive full Medicare reimbursement in 2008. Of the three measures, two involved VTE prophylaxis: (1) how often VTE prophylaxis was ordered for surgical patients when indicated, and (2) how often appropriate surgical patients received prophylaxis postoperatively. Moreover, beginning October 1, 2008, CMS will no longer reimburse hospitals for cetain preventable conditions, and DVT and PE are being considered for inclusion in this list of conditions excluded from reimbursement.10
PROPHYLAXIS RATES CAN BE IMPROVED
Fortunately, there is evidence that interventions to increase awareness may increase the rate of VTE prophylaxis. Stinnett et al reported that education, in the form of hospital-specific data on VTE rates and implementation of risk-stratification guidelines, increased the use of VTE prophylaxis in high-risk hospitalized medical patients at a tertiary care center from a preintervention rate of 43% to a postintervention rate of 72%.11
In addition to educational interventions, formalized risk-assessment tools, in the form of electronic alerts, offer another strategy that may increase rates of VTE prophylaxis. The promise of this approach was demonstrated in a study at Brigham and Women’s Hospital in Boston, in which 2,506 hospitalized patients at risk for VTE were randomly assigned to either an intervention group, in which physicians received a computer alert about the patient’s VTE risk, or a control group, in which no alert was issued.12 The rate of VTE prophylaxis was more than twice as high in the intervention group as in the control group (33.5% vs 14.5%; P < .001), and the 90-day incidence of VTE was reduced from 8.2% in the control group to 4.9% in the intervention group (P = .001).
WHO’S AT RISK FOR VTE?
OPTIONS FOR VTE PROPHYLAXIS
An ideal therapy for VTE prophylaxis would be one that is effective, safe, inexpensive, and easy to administer and monitor, and that has few side effects or complications.
Mechanical prophylaxis
Mechanical forms of VTE prevention carry no risk of bleeding, are inexpensive because they can be reused, and are often effective when used properly. Mechanical forms include graduated compression stockings, intermittent pneumatic compression devices, and venous foot pumps.
The American College of Chest Physicians (ACCP), in its Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy, published in 2004,13 recommends that mechanical methods be used primarily in two settings:
- In patients with a high risk of bleeding (in whom pharmacologic prophylaxis is contraindicated)
- As an adjunct to pharmacologic prophylaxis.
Because the use of mechanical forms of prophylaxis in hospitalized medical patients is not evidence-based, mechanical prophylaxis should be reserved for those medical patients at risk for VTE who have a contraindication to pharmacologic prophylaxis.
To be effective, mechanical forms of prophylaxis must be used in accordance with the device manufacturer’s guidelines, which is frequently not what happens in clinical practice. In clinical trials in which the efficacy of intermittent pneumatic compression devices was demonstrated, patients wore their devices for 14 to 15 hours per day.
Pharmacologic options
Unfractionated heparin (UFH) inhibits factor Xa and factor IIa equally. Because it is a large heterogeneous molecule, UFH is not well absorbed in subcutaneous tissue. Its anticoagulant response is variable because of its short half-life. It must be dosed two or three times daily subcutaneously for VTE prophylaxis, and must be given intravenously for treatment of VTE. The rate of heparin-induced thrombocytopenia, a potentially catastrophic adverse drug event, is considerably higher with UFH than with low-molecular-weight heparins (3% vs 1%).14 Osteopenia can develop with the use of UFH over even short periods, and osteoporosis can occur with long-term use.
Low-molecular-weight heparins (LMWHs) preferentially inhibit factor Xa compared to factor IIa. The LMWHs (ie, enoxaparin [Lovenox], dalteparin [Fragmin]) are derived from UFH through a chemical depolymerization and defractionation process that results in a much smaller molecule. LMWHs are well absorbed from subcutaneous tissue and have a predictable dose response attributable to their longer half-life (relative to UFH), which allows for once-daily or twice-daily subcutaneous dosing. As noted above, LMWHs carry a much lower rate of heparin-induced thrombocytopenia compared with UFH. Because LMWHs are predominantly cleared by the kidneys, dose adjustment may be needed in patients with renal impairment.
Fondaparinux (Arixtra) is a synthetic pentasaccha-ride that acts as a pure inhibitor of factor Xa. It binds antithrombin III, causing a conformational change by which it inhibits factor Xa and thereby inhibits coagulation further downstream. Fondaparinux has a long half-life (18 to 19 hours), which enables once-daily subcutaneous dosing but which also may require administration of the costly activated factor VII (NovoSeven) to reverse its effects in cases of bleeding. Because fondaparinux is cleared entirely by the kidneys, it is contraindicated in patients with severe renal impairment (creatinine clearance < 30 mL/min). It is also contraindicated in patients who weigh less than 50 kg, due to increased bleeding risk.
Details on the efficacy of these agents for VTE prophylaxis in various patient groups are provided in the subsequent articles in this supplement.
Investigational anticoagulants
The above pharmacologic options may soon be joined by several experimental anticoagulants that are currently in phase 3 trials for VTE prophylaxis—oral factor Xa inhibitors such as rivaroxaban and apixaban, and oral factor IIa (thrombin) inhibitors such as dabigatran.
Venous thromboembolism (VTE)—which comprises both deep vein thrombosis (DVT) and pulmonary embolism (PE), which can result from DVT—is the third leading cause of cardiovascular death in the United States, after myocardial infarction and stroke. The annual incidence of DVT approaches 2 million.1 Silent PE constitutes approximately half of DVT cases, as suggested by studies using ventilation perfusion scanning. The true incidence of PE is not known but is estimated to be 600,000 cases annually,1 with approximately one third of these cases leading to death.2
Notably, the incidence of VTE rises at an exponential rate with increasing age after the second decade of life, as shown in Figure 1.2 Given the aging of the US population, this suggests that the clinical and economic impact of VTE will only increase in the years ahead.
DESPITE ESTABLISHED BENEFITS, VTE PROPHYLAXIS REMAINS UNDERUSED
The frequency, clinical impact, and economic impact of VTE make a strong case for VTE prevention. In a 2001 analysis of patient safety practices, the Agency for Healthcare Research and Quality listed appropriate VTE prophylaxis in at-risk patients first in a rating of safety practices with the greatest strength of evidence for impact and effectiveness.4
Despite this recognition of the importance and benefit of VTE prophylaxis, prophylaxis remains highly underutilized. This has been demonstrated in numerous studies; the large epidemiologic investigation by Goldhaber et al using the DVT Free Registry is illustrative.5 This prospective multicenter study enrolled 5,451 consecutive patients with acute DVT documented by venous ultrasonography over a 6month period. Patients were classified as either outpatients or inpatients: outpatients were those who came to the emergency room and were diagnosed with DVT; inpatients were those who developed DVT in the hospital. Of the 2,726 inpatients in the registry, only 42% had received prophylaxis within 30 days prior to their diagnosis of DVT.
Risk extends to the outpatient setting
In a recent population-based analysis, Spencer et al found a similarly low rate of VTE prophylaxis— 42.8%—among 516 patients who had recently been hospitalized and subsequently developed VTE.6 This study also found that VTE was three times as likely in the outpatient setting as in the inpatient setting, and that almost half of the outpatients with VTE had been recently hospitalized. Taken together, these findings indicate that VTE prevention efforts are inadequate both in the hospital and at the time of discharge, when patients’ risk for VTE is still elevated.6,7
VTE PROPHYLAXIS AS AN EMERGING QUALITY MEASURE
Increased recognition of the impact of VTE has prompted accreditation and quality organizations to take interest in VTE risk assessment and prophylaxis as a measure for institutional performance ratings and even reimbursement.
The Joint Commission on Accreditation of Healthcare Organizations and the National Quality Forum have launched a joint project to develop a set of standardized inpatient measures to evaluate hospitals’ practices for the prevention and treatment of VTE.8 The project has pilot-tested several proposed performance measures in dozens of volunteer hospitals, including measures of whether VTE risk assessment is performed and VTE prophylaxis is initiated (if indicated) within 24 hours of admission to the hospital or to the intensive care unit. Hospitals participating in the pilot program are required to report their rates of potentially preventable hospital-acquired VTE.
Similarly, the ongoing Surgical Care Improvement Incidence of VTE increases with age Project (SCIP) has targeted VTE prophylaxis as one of a handful of priority areas for reducing surgical complications. As a national quality partnership of organizations sponsored by the Centers for Medicare and Medicaid Services (CMS), SCIP set a national goal in 2005 to reduce preventable surgical morbidity and mortality by 25% by 2010.9
The stakes of the SCIP initiative are high in both clinical and financial terms. CMS mandated that hospitals report on three SCIP quality measures in 2007 in order to receive full Medicare reimbursement in 2008. Of the three measures, two involved VTE prophylaxis: (1) how often VTE prophylaxis was ordered for surgical patients when indicated, and (2) how often appropriate surgical patients received prophylaxis postoperatively. Moreover, beginning October 1, 2008, CMS will no longer reimburse hospitals for cetain preventable conditions, and DVT and PE are being considered for inclusion in this list of conditions excluded from reimbursement.10
PROPHYLAXIS RATES CAN BE IMPROVED
Fortunately, there is evidence that interventions to increase awareness may increase the rate of VTE prophylaxis. Stinnett et al reported that education, in the form of hospital-specific data on VTE rates and implementation of risk-stratification guidelines, increased the use of VTE prophylaxis in high-risk hospitalized medical patients at a tertiary care center from a preintervention rate of 43% to a postintervention rate of 72%.11
In addition to educational interventions, formalized risk-assessment tools, in the form of electronic alerts, offer another strategy that may increase rates of VTE prophylaxis. The promise of this approach was demonstrated in a study at Brigham and Women’s Hospital in Boston, in which 2,506 hospitalized patients at risk for VTE were randomly assigned to either an intervention group, in which physicians received a computer alert about the patient’s VTE risk, or a control group, in which no alert was issued.12 The rate of VTE prophylaxis was more than twice as high in the intervention group as in the control group (33.5% vs 14.5%; P < .001), and the 90-day incidence of VTE was reduced from 8.2% in the control group to 4.9% in the intervention group (P = .001).
WHO’S AT RISK FOR VTE?
OPTIONS FOR VTE PROPHYLAXIS
An ideal therapy for VTE prophylaxis would be one that is effective, safe, inexpensive, and easy to administer and monitor, and that has few side effects or complications.
Mechanical prophylaxis
Mechanical forms of VTE prevention carry no risk of bleeding, are inexpensive because they can be reused, and are often effective when used properly. Mechanical forms include graduated compression stockings, intermittent pneumatic compression devices, and venous foot pumps.
The American College of Chest Physicians (ACCP), in its Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy, published in 2004,13 recommends that mechanical methods be used primarily in two settings:
- In patients with a high risk of bleeding (in whom pharmacologic prophylaxis is contraindicated)
- As an adjunct to pharmacologic prophylaxis.
Because the use of mechanical forms of prophylaxis in hospitalized medical patients is not evidence-based, mechanical prophylaxis should be reserved for those medical patients at risk for VTE who have a contraindication to pharmacologic prophylaxis.
To be effective, mechanical forms of prophylaxis must be used in accordance with the device manufacturer’s guidelines, which is frequently not what happens in clinical practice. In clinical trials in which the efficacy of intermittent pneumatic compression devices was demonstrated, patients wore their devices for 14 to 15 hours per day.
Pharmacologic options
Unfractionated heparin (UFH) inhibits factor Xa and factor IIa equally. Because it is a large heterogeneous molecule, UFH is not well absorbed in subcutaneous tissue. Its anticoagulant response is variable because of its short half-life. It must be dosed two or three times daily subcutaneously for VTE prophylaxis, and must be given intravenously for treatment of VTE. The rate of heparin-induced thrombocytopenia, a potentially catastrophic adverse drug event, is considerably higher with UFH than with low-molecular-weight heparins (3% vs 1%).14 Osteopenia can develop with the use of UFH over even short periods, and osteoporosis can occur with long-term use.
Low-molecular-weight heparins (LMWHs) preferentially inhibit factor Xa compared to factor IIa. The LMWHs (ie, enoxaparin [Lovenox], dalteparin [Fragmin]) are derived from UFH through a chemical depolymerization and defractionation process that results in a much smaller molecule. LMWHs are well absorbed from subcutaneous tissue and have a predictable dose response attributable to their longer half-life (relative to UFH), which allows for once-daily or twice-daily subcutaneous dosing. As noted above, LMWHs carry a much lower rate of heparin-induced thrombocytopenia compared with UFH. Because LMWHs are predominantly cleared by the kidneys, dose adjustment may be needed in patients with renal impairment.
Fondaparinux (Arixtra) is a synthetic pentasaccha-ride that acts as a pure inhibitor of factor Xa. It binds antithrombin III, causing a conformational change by which it inhibits factor Xa and thereby inhibits coagulation further downstream. Fondaparinux has a long half-life (18 to 19 hours), which enables once-daily subcutaneous dosing but which also may require administration of the costly activated factor VII (NovoSeven) to reverse its effects in cases of bleeding. Because fondaparinux is cleared entirely by the kidneys, it is contraindicated in patients with severe renal impairment (creatinine clearance < 30 mL/min). It is also contraindicated in patients who weigh less than 50 kg, due to increased bleeding risk.
Details on the efficacy of these agents for VTE prophylaxis in various patient groups are provided in the subsequent articles in this supplement.
Investigational anticoagulants
The above pharmacologic options may soon be joined by several experimental anticoagulants that are currently in phase 3 trials for VTE prophylaxis—oral factor Xa inhibitors such as rivaroxaban and apixaban, and oral factor IIa (thrombin) inhibitors such as dabigatran.
- Hirsh J, Hoak J. Management of deep vein thrombosis and pulmonary embolism. A statement for healthcare professionals from the Council on Thrombosis (in consultation with the Council on Cardiovascular Radiology), American Heart Association. Circulation 1996; 93:2212–2245.
- Anderson FA Jr, Wheeler HB, Goldberg RJ, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med 1991; 151:933–938.
- Dimick JB, Chen SL, Taheri PA, Henderson WG, Khuri SF, Campbell DA Jr. Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg 2004; 199:531–537.
- Agency for Healthcare Research and Quality. Making Health Care Safer: A Critical Analysis of Patient Safety Practices. Evidence Report/Technology/Assessment: Number 43. AHRQ Publication No. 01-E058. Rockville, MD: Agency for Healthcare Research and Quality; July 2001:620. http://www.ahrq.gov/clinic/ptsafety. Accessed December 4, 2007.
- Goldhaber SZ, Tapson VF; DVT FREE Steering Committee. A prospective registry of 5,451 patients with ultrasound-confirmed deep vein thrombosis. Am J Cardiol 2004; 93:259–262.
- Spencer FA, Lessard D, Emery C, Reed G, Goldberg RJ. Venous thromboembolism in the outpatient setting. Arch Intern Med 2007; 167:1471–1475.
- Goldhaber SZ. Outpatient venous thromboembolism: a common but often preventable public health threat. Arch Intern Med 2007; 167:1451–1452.
- National Consensus Standards for Prevention and Care of Venous Thromboembolism (VTE). The Joint Commission Web site. http://www.jointcommission.org/PerformanceMeasurement/ PerformanceMeasurement/VTE.htm. Accessed January 8, 2008.
- Surgical Care Improvement Project. MedQIC Web site. http://www.medqic.org/scip. Accessed January 8, 2008.
- US Department of Health and Human Services. Medicare program; changes to the hospital inpatient prospective payment systems and fiscal year 2008 rates. http://www.cms.hhs.gov/AcuteInpatientPPS/ downloads/CMS-1533-FC.pdf. Accessed December 4, 2007.
- Stinnett JM, Pendleton R, Skordos L, Wheeler M, Rodgers GM. Venous thromboembolism prophylaxis in medically ill patients and the development of strategies to improve prophylaxis rates. Am J Hematol 2005; 78:167–172.
- Kucher N, Koo S, Quiroz R, et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med 2005; 352:969–977.
- Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Anti-thrombotic and Thrombolytic Therapy. Chest 2004; 126(3 Suppl):338S–400S.
- Warkentin TE, Levine MN, Hirsh J, et al. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. N Engl J Med 1995; 332:1330–1335.
- Hirsh J, Hoak J. Management of deep vein thrombosis and pulmonary embolism. A statement for healthcare professionals from the Council on Thrombosis (in consultation with the Council on Cardiovascular Radiology), American Heart Association. Circulation 1996; 93:2212–2245.
- Anderson FA Jr, Wheeler HB, Goldberg RJ, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med 1991; 151:933–938.
- Dimick JB, Chen SL, Taheri PA, Henderson WG, Khuri SF, Campbell DA Jr. Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg 2004; 199:531–537.
- Agency for Healthcare Research and Quality. Making Health Care Safer: A Critical Analysis of Patient Safety Practices. Evidence Report/Technology/Assessment: Number 43. AHRQ Publication No. 01-E058. Rockville, MD: Agency for Healthcare Research and Quality; July 2001:620. http://www.ahrq.gov/clinic/ptsafety. Accessed December 4, 2007.
- Goldhaber SZ, Tapson VF; DVT FREE Steering Committee. A prospective registry of 5,451 patients with ultrasound-confirmed deep vein thrombosis. Am J Cardiol 2004; 93:259–262.
- Spencer FA, Lessard D, Emery C, Reed G, Goldberg RJ. Venous thromboembolism in the outpatient setting. Arch Intern Med 2007; 167:1471–1475.
- Goldhaber SZ. Outpatient venous thromboembolism: a common but often preventable public health threat. Arch Intern Med 2007; 167:1451–1452.
- National Consensus Standards for Prevention and Care of Venous Thromboembolism (VTE). The Joint Commission Web site. http://www.jointcommission.org/PerformanceMeasurement/ PerformanceMeasurement/VTE.htm. Accessed January 8, 2008.
- Surgical Care Improvement Project. MedQIC Web site. http://www.medqic.org/scip. Accessed January 8, 2008.
- US Department of Health and Human Services. Medicare program; changes to the hospital inpatient prospective payment systems and fiscal year 2008 rates. http://www.cms.hhs.gov/AcuteInpatientPPS/ downloads/CMS-1533-FC.pdf. Accessed December 4, 2007.
- Stinnett JM, Pendleton R, Skordos L, Wheeler M, Rodgers GM. Venous thromboembolism prophylaxis in medically ill patients and the development of strategies to improve prophylaxis rates. Am J Hematol 2005; 78:167–172.
- Kucher N, Koo S, Quiroz R, et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med 2005; 352:969–977.
- Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Anti-thrombotic and Thrombolytic Therapy. Chest 2004; 126(3 Suppl):338S–400S.
- Warkentin TE, Levine MN, Hirsh J, et al. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. N Engl J Med 1995; 332:1330–1335.