User login
Study Finds Mace Risk Remains High in Patients with Psoriasis, Dyslipidemia
Over a period of 5 years, the, even after adjusting for covariates, results from a large retrospective study showed.
“It is well-established that psoriasis is an independent risk factor for the development of MACE, with cardiometabolic risk factors being more prevalent and incident among patients with psoriasis,” the study’s first author Ana Ormaza Vera, MD, a dermatology research fellow at Eastern Virginia Medical School, Norfolk, said in an interview after the annual meeting of the Society for Investigational Dermatology, where the study was presented during a late-breaking abstract session.
Current guidelines from the joint American Academy of Dermatology/National Psoriasis Foundation and the American Academy of Cardiology/American Heart Association Task Force recommend statins, a lipid-lowering and anti-inflammatory therapy, “for patients with psoriasis who have additional risk-enhancing factors, similar to recommendations made for the general population without psoriasis,” she noted. But how the incidence of MACE differs between patients with and without psoriasis while on statin therapy “has not been explored in real-world settings,” she added.
To address this question, the researchers used real-world data from the TriNetX health research network to identify individuals aged 18-90 years with a diagnosis of both psoriasis and lipid disorders who were undergoing treatment with statins. Those with a prior history of MACE were excluded from the analysis. Patients with lipid disorders on statin therapy, but without psoriatic disease, were matched 1:1 by age, sex, race, ethnicity, common risk factors for MACE, and medications shown to reduce MACE risk. The researchers then assessed the cohorts 5 years following their first statin prescription and used the TriNetX analytics tool to calculate the odds ratio (OR) with 95% CI to evaluate the likelihood of MACE in the presence of statin therapy.
Dr. Ormaza Vera and colleagues identified 20,660 patients with psoriasis and 2,768,429 patients without psoriasis who met the criteria for analysis. After propensity score matching, each cohort included 20,660 patients with a mean age of 60 years. During the 5-year observation period, 2725 patients in the psoriasis cohort experienced MACE compared with 2203 patients in the non-psoriasis cohort (OR, 1.40; 95% CI, 1.317-1.488).
“This was an unexpected outcome that challenges the current understanding and highlights the need for further research into tailored treatments for cardiovascular risk in psoriasis patients,” Dr. Ormaza Vera told this news organization.
She acknowledged certain limitations of the study, including its retrospective design, the inherent limitations of an observational study, and the use of electronic medical record data.
Lawrence J. Green, MD, clinical professor of dermatology, George Washington University, Washington, who was asked to comment on the study results, said that the findings imply that there is more than statin use alone to protect someone with psoriasis from having an increased risk for MACE. “This is not really surprising because statin use alone is only part of a prevention strategy in someone with psoriasis who usually has multiple comorbidities,” Dr. Green said. “On the other hand, the study only went out for 5 years and cardiovascular disease is a long accumulating process, so it could also be too early to demonstrate MACE prevention.”
The study was funded by a grant from the American Skin Association. Dr. Ormaza Vera and her coauthors reported having no relevant disclosures. Dr. Green disclosed that he is a speaker, consultant, or investigator for many pharmaceutical companies.
A version of this article appeared on Medscape.com .
Over a period of 5 years, the, even after adjusting for covariates, results from a large retrospective study showed.
“It is well-established that psoriasis is an independent risk factor for the development of MACE, with cardiometabolic risk factors being more prevalent and incident among patients with psoriasis,” the study’s first author Ana Ormaza Vera, MD, a dermatology research fellow at Eastern Virginia Medical School, Norfolk, said in an interview after the annual meeting of the Society for Investigational Dermatology, where the study was presented during a late-breaking abstract session.
Current guidelines from the joint American Academy of Dermatology/National Psoriasis Foundation and the American Academy of Cardiology/American Heart Association Task Force recommend statins, a lipid-lowering and anti-inflammatory therapy, “for patients with psoriasis who have additional risk-enhancing factors, similar to recommendations made for the general population without psoriasis,” she noted. But how the incidence of MACE differs between patients with and without psoriasis while on statin therapy “has not been explored in real-world settings,” she added.
To address this question, the researchers used real-world data from the TriNetX health research network to identify individuals aged 18-90 years with a diagnosis of both psoriasis and lipid disorders who were undergoing treatment with statins. Those with a prior history of MACE were excluded from the analysis. Patients with lipid disorders on statin therapy, but without psoriatic disease, were matched 1:1 by age, sex, race, ethnicity, common risk factors for MACE, and medications shown to reduce MACE risk. The researchers then assessed the cohorts 5 years following their first statin prescription and used the TriNetX analytics tool to calculate the odds ratio (OR) with 95% CI to evaluate the likelihood of MACE in the presence of statin therapy.
Dr. Ormaza Vera and colleagues identified 20,660 patients with psoriasis and 2,768,429 patients without psoriasis who met the criteria for analysis. After propensity score matching, each cohort included 20,660 patients with a mean age of 60 years. During the 5-year observation period, 2725 patients in the psoriasis cohort experienced MACE compared with 2203 patients in the non-psoriasis cohort (OR, 1.40; 95% CI, 1.317-1.488).
“This was an unexpected outcome that challenges the current understanding and highlights the need for further research into tailored treatments for cardiovascular risk in psoriasis patients,” Dr. Ormaza Vera told this news organization.
She acknowledged certain limitations of the study, including its retrospective design, the inherent limitations of an observational study, and the use of electronic medical record data.
Lawrence J. Green, MD, clinical professor of dermatology, George Washington University, Washington, who was asked to comment on the study results, said that the findings imply that there is more than statin use alone to protect someone with psoriasis from having an increased risk for MACE. “This is not really surprising because statin use alone is only part of a prevention strategy in someone with psoriasis who usually has multiple comorbidities,” Dr. Green said. “On the other hand, the study only went out for 5 years and cardiovascular disease is a long accumulating process, so it could also be too early to demonstrate MACE prevention.”
The study was funded by a grant from the American Skin Association. Dr. Ormaza Vera and her coauthors reported having no relevant disclosures. Dr. Green disclosed that he is a speaker, consultant, or investigator for many pharmaceutical companies.
A version of this article appeared on Medscape.com .
Over a period of 5 years, the, even after adjusting for covariates, results from a large retrospective study showed.
“It is well-established that psoriasis is an independent risk factor for the development of MACE, with cardiometabolic risk factors being more prevalent and incident among patients with psoriasis,” the study’s first author Ana Ormaza Vera, MD, a dermatology research fellow at Eastern Virginia Medical School, Norfolk, said in an interview after the annual meeting of the Society for Investigational Dermatology, where the study was presented during a late-breaking abstract session.
Current guidelines from the joint American Academy of Dermatology/National Psoriasis Foundation and the American Academy of Cardiology/American Heart Association Task Force recommend statins, a lipid-lowering and anti-inflammatory therapy, “for patients with psoriasis who have additional risk-enhancing factors, similar to recommendations made for the general population without psoriasis,” she noted. But how the incidence of MACE differs between patients with and without psoriasis while on statin therapy “has not been explored in real-world settings,” she added.
To address this question, the researchers used real-world data from the TriNetX health research network to identify individuals aged 18-90 years with a diagnosis of both psoriasis and lipid disorders who were undergoing treatment with statins. Those with a prior history of MACE were excluded from the analysis. Patients with lipid disorders on statin therapy, but without psoriatic disease, were matched 1:1 by age, sex, race, ethnicity, common risk factors for MACE, and medications shown to reduce MACE risk. The researchers then assessed the cohorts 5 years following their first statin prescription and used the TriNetX analytics tool to calculate the odds ratio (OR) with 95% CI to evaluate the likelihood of MACE in the presence of statin therapy.
Dr. Ormaza Vera and colleagues identified 20,660 patients with psoriasis and 2,768,429 patients without psoriasis who met the criteria for analysis. After propensity score matching, each cohort included 20,660 patients with a mean age of 60 years. During the 5-year observation period, 2725 patients in the psoriasis cohort experienced MACE compared with 2203 patients in the non-psoriasis cohort (OR, 1.40; 95% CI, 1.317-1.488).
“This was an unexpected outcome that challenges the current understanding and highlights the need for further research into tailored treatments for cardiovascular risk in psoriasis patients,” Dr. Ormaza Vera told this news organization.
She acknowledged certain limitations of the study, including its retrospective design, the inherent limitations of an observational study, and the use of electronic medical record data.
Lawrence J. Green, MD, clinical professor of dermatology, George Washington University, Washington, who was asked to comment on the study results, said that the findings imply that there is more than statin use alone to protect someone with psoriasis from having an increased risk for MACE. “This is not really surprising because statin use alone is only part of a prevention strategy in someone with psoriasis who usually has multiple comorbidities,” Dr. Green said. “On the other hand, the study only went out for 5 years and cardiovascular disease is a long accumulating process, so it could also be too early to demonstrate MACE prevention.”
The study was funded by a grant from the American Skin Association. Dr. Ormaza Vera and her coauthors reported having no relevant disclosures. Dr. Green disclosed that he is a speaker, consultant, or investigator for many pharmaceutical companies.
A version of this article appeared on Medscape.com .
FROM SID 2024
Recent Evidence for Home Phototherapy Benefits May Improve Access for Patients with Psoriasis
Supporters of home phototherapy for patients with plaque and guttate psoriasis had plenty to cheer about at the annual meeting of the American Academy of Dermatology (AAD) in March. There, Joel M. Gelfand, MD, professor of dermatology and epidemiology at the University of Pennsylvania in Philadelphia, presented results from the LITE study, a trial that tested the hypothesis that narrowband ultraviolet B phototherapy of psoriasis at home is noninferior to office treatment, based on outcomes that matter to patients, clinicians, and payers. While smaller studies have drawn similar conclusions,
The co-primary outcomes in the LITE study were a Physician’s Global Assessment (PGA) score of 0/1 (clear, almost clear) and a Dermatology Life Quality Index (DLQI) score of 5 or less (small, no effect on health-related quality of life).
Dr. Gelfand and colleagues at 42 sites in the United States enrolled 783 patients aged 12 years and older who had plaque or guttate psoriasis and were candidates for phototherapy at home or in an office setting. Following 12 weeks of treatment, 25.6% of patients in the office-based phototherapy group achieved a PGA score of 0/1 compared with 32.8% of patients in the home-based phototherapy group (P > .0001 for noninferiority, non-response imputation for missing data). Similarly, 33.6% of patients in the office-based phototherapy group achieved a DLQI score of 5 or less compared with 52.4% of patients in the home-based phototherapy group (P > .0001 for noninferiority, non-response imputation for missing data).
A Safe and Effective Option
“I think that it’s important for physicians, insurance companies, and patients with psoriasis to understand that this is a very safe and effective form of therapy,” Craig A. Elmets, MD, professor of dermatology at The University of Alabama at Birmingham, said in an interview. “For people who are not interested in systemic medications or who have contraindications to systemic medications, phototherapy would be ideal,” added Dr. Elmets, first author of the joint AAD–National Psoriasis Foundation (NPF) guidelines for the management and treatment of psoriasis with phototherapy, published in 2019.
Factors beyond efficacy support the role of home phototherapy, Dr. Gelfand said, including the fact that it costs 10-100 times less than biologics for psoriasis and that office-based phototherapy is not available in 90% of counties in the United States. However, insurance coverage of home phototherapy “is highly variable because until the LITE study, there was no large-scale US data to support its use,” he told this news organization.
“Also, insurance companies are broken up into two parts: Durable medical goods and the medical side such as pharmacy costs, and they are siloed. The durable medical goods side views phototherapy as expensive, while the pharmacy side views it as dirt cheap. This is part of the problem with our health system. A lot of things are siloed and don’t make any sense,” said Dr. Gelfand, director of the Psoriasis and Phototherapy Treatment Center at the University of Pennsylvania. By working with the NPF and payers, he added, “we’re hoping ... to transform the way insurance companies think about covering home phototherapy.”
In the meantime, he and Dr. Elmets shared practical ways to optimize access to home phototherapy for psoriasis patients:
Have the discussion. Patients “rarely bring this up as an option,” Dr. Elmets said, so the onus is on clinicians to talk about it. In his view, the ideal candidate “is averse to using systemic agents but whose disease is beyond the point where topical medicines alone will work. One of the advantages of phototherapy is that it doesn’t have immunosuppressive effects.”
Clinicians and patients can learn about the efficacy and safety of phototherapy for psoriasis, including home-based options, on the NPF’s web site and by reading the 2019 joint AAD-NPF guidelines.
Shared decision-making is key. “When a patient comes in, I’ll discuss what their treatment options are and [we] will decide upon a course of action based on their unique needs and preferences [and] if it’s medically appropriate, meaning they have the type of psoriasis likely to respond to phototherapy,” Dr. Gelfand said. A patient with psoriasis mainly on the fingernails or genitals “is not a good candidate for phototherapy. If it’s on the trunk or extremities, that patient would be a good candidate.”
Home phototherapy candidates also must be willing and able to operate a machine and have dedicated space in their dwelling for it (most units are about the size of a door). Patients also have to be reliable, follow directions, and come back in person for follow-up appointments “so we can assess their response to treatment and fine-tune things as necessary and make sure they’re not developing any skin damage,” Dr. Gelfand said.
Educate yourself about existing options. Home phototherapy units from manufacturers such as Daavlin, National Biological Corporation, and SolRx range between $1200 and $6000 in cost, Dr. Gelfand said. He and his colleagues used the Daavlin 7 series in the LITE study. That unit features an integrated dosimetry system that delivers the correct dose of energy based on parameters that the prescribing clinician recommends. Settings are based on the patient’s skin type and how much the prescriber wants to increase the dose for each treatment. “The machine does the rest,” he said. “It knows what dose to give, so they get the same dosing as they would in an office situation.”
Smaller home-based phototherapy units designed to treat the hands and feet are available. So are handheld units to treat the scalp. “These can be a nice option for patients who have a few spots, but if the disease is moderate to severe, then it’s going to be pretty laborious to [use them],” Dr. Elmets said.
Remember that phototherapy is not a cure-all. According to the joint AAD-NPF guidelines, most phototherapy regimens require treatments two to three times per week for 10-14 weeks. Once patients achieve their home phototherapy treatment goal, Dr. Elmets often recommends treatments one to two times per week for maintenance.
“Patients with psoriasis have a lifetime condition,” he noted. “There are certainly cases where people have gone on phototherapy, cleared, and then stopped for a period of time. If they flare up, they can always go back to phototherapy. Usually, people who are on phototherapy use some type of topical agents to touch up areas that are resistant.”
Expect pushback from insurers on coverage. While Medicare and some integrated health plans cover home phototherapy, expect to spend time writing letters or placing phone calls to insurance companies to convince them why they should cover home phototherapy for candidate psoriasis patients. “Usually there’s a lot of letter writing and a long delay in getting approval,” Dr. Elmets said.
Dr. Elmets and Dr. Gelfand reported no relevant financial relationships. The LITE study was funded by the Patient-Centered Outcomes Research Institute. Research partners included the National Psoriasis Foundation and Daavlin, which provided the home phototherapy machines and covered the cost of shipping the devices.
A version of this article appeared on Medscape.com.
Supporters of home phototherapy for patients with plaque and guttate psoriasis had plenty to cheer about at the annual meeting of the American Academy of Dermatology (AAD) in March. There, Joel M. Gelfand, MD, professor of dermatology and epidemiology at the University of Pennsylvania in Philadelphia, presented results from the LITE study, a trial that tested the hypothesis that narrowband ultraviolet B phototherapy of psoriasis at home is noninferior to office treatment, based on outcomes that matter to patients, clinicians, and payers. While smaller studies have drawn similar conclusions,
The co-primary outcomes in the LITE study were a Physician’s Global Assessment (PGA) score of 0/1 (clear, almost clear) and a Dermatology Life Quality Index (DLQI) score of 5 or less (small, no effect on health-related quality of life).
Dr. Gelfand and colleagues at 42 sites in the United States enrolled 783 patients aged 12 years and older who had plaque or guttate psoriasis and were candidates for phototherapy at home or in an office setting. Following 12 weeks of treatment, 25.6% of patients in the office-based phototherapy group achieved a PGA score of 0/1 compared with 32.8% of patients in the home-based phototherapy group (P > .0001 for noninferiority, non-response imputation for missing data). Similarly, 33.6% of patients in the office-based phototherapy group achieved a DLQI score of 5 or less compared with 52.4% of patients in the home-based phototherapy group (P > .0001 for noninferiority, non-response imputation for missing data).
A Safe and Effective Option
“I think that it’s important for physicians, insurance companies, and patients with psoriasis to understand that this is a very safe and effective form of therapy,” Craig A. Elmets, MD, professor of dermatology at The University of Alabama at Birmingham, said in an interview. “For people who are not interested in systemic medications or who have contraindications to systemic medications, phototherapy would be ideal,” added Dr. Elmets, first author of the joint AAD–National Psoriasis Foundation (NPF) guidelines for the management and treatment of psoriasis with phototherapy, published in 2019.
Factors beyond efficacy support the role of home phototherapy, Dr. Gelfand said, including the fact that it costs 10-100 times less than biologics for psoriasis and that office-based phototherapy is not available in 90% of counties in the United States. However, insurance coverage of home phototherapy “is highly variable because until the LITE study, there was no large-scale US data to support its use,” he told this news organization.
“Also, insurance companies are broken up into two parts: Durable medical goods and the medical side such as pharmacy costs, and they are siloed. The durable medical goods side views phototherapy as expensive, while the pharmacy side views it as dirt cheap. This is part of the problem with our health system. A lot of things are siloed and don’t make any sense,” said Dr. Gelfand, director of the Psoriasis and Phototherapy Treatment Center at the University of Pennsylvania. By working with the NPF and payers, he added, “we’re hoping ... to transform the way insurance companies think about covering home phototherapy.”
In the meantime, he and Dr. Elmets shared practical ways to optimize access to home phototherapy for psoriasis patients:
Have the discussion. Patients “rarely bring this up as an option,” Dr. Elmets said, so the onus is on clinicians to talk about it. In his view, the ideal candidate “is averse to using systemic agents but whose disease is beyond the point where topical medicines alone will work. One of the advantages of phototherapy is that it doesn’t have immunosuppressive effects.”
Clinicians and patients can learn about the efficacy and safety of phototherapy for psoriasis, including home-based options, on the NPF’s web site and by reading the 2019 joint AAD-NPF guidelines.
Shared decision-making is key. “When a patient comes in, I’ll discuss what their treatment options are and [we] will decide upon a course of action based on their unique needs and preferences [and] if it’s medically appropriate, meaning they have the type of psoriasis likely to respond to phototherapy,” Dr. Gelfand said. A patient with psoriasis mainly on the fingernails or genitals “is not a good candidate for phototherapy. If it’s on the trunk or extremities, that patient would be a good candidate.”
Home phototherapy candidates also must be willing and able to operate a machine and have dedicated space in their dwelling for it (most units are about the size of a door). Patients also have to be reliable, follow directions, and come back in person for follow-up appointments “so we can assess their response to treatment and fine-tune things as necessary and make sure they’re not developing any skin damage,” Dr. Gelfand said.
Educate yourself about existing options. Home phototherapy units from manufacturers such as Daavlin, National Biological Corporation, and SolRx range between $1200 and $6000 in cost, Dr. Gelfand said. He and his colleagues used the Daavlin 7 series in the LITE study. That unit features an integrated dosimetry system that delivers the correct dose of energy based on parameters that the prescribing clinician recommends. Settings are based on the patient’s skin type and how much the prescriber wants to increase the dose for each treatment. “The machine does the rest,” he said. “It knows what dose to give, so they get the same dosing as they would in an office situation.”
Smaller home-based phototherapy units designed to treat the hands and feet are available. So are handheld units to treat the scalp. “These can be a nice option for patients who have a few spots, but if the disease is moderate to severe, then it’s going to be pretty laborious to [use them],” Dr. Elmets said.
Remember that phototherapy is not a cure-all. According to the joint AAD-NPF guidelines, most phototherapy regimens require treatments two to three times per week for 10-14 weeks. Once patients achieve their home phototherapy treatment goal, Dr. Elmets often recommends treatments one to two times per week for maintenance.
“Patients with psoriasis have a lifetime condition,” he noted. “There are certainly cases where people have gone on phototherapy, cleared, and then stopped for a period of time. If they flare up, they can always go back to phototherapy. Usually, people who are on phototherapy use some type of topical agents to touch up areas that are resistant.”
Expect pushback from insurers on coverage. While Medicare and some integrated health plans cover home phototherapy, expect to spend time writing letters or placing phone calls to insurance companies to convince them why they should cover home phototherapy for candidate psoriasis patients. “Usually there’s a lot of letter writing and a long delay in getting approval,” Dr. Elmets said.
Dr. Elmets and Dr. Gelfand reported no relevant financial relationships. The LITE study was funded by the Patient-Centered Outcomes Research Institute. Research partners included the National Psoriasis Foundation and Daavlin, which provided the home phototherapy machines and covered the cost of shipping the devices.
A version of this article appeared on Medscape.com.
Supporters of home phototherapy for patients with plaque and guttate psoriasis had plenty to cheer about at the annual meeting of the American Academy of Dermatology (AAD) in March. There, Joel M. Gelfand, MD, professor of dermatology and epidemiology at the University of Pennsylvania in Philadelphia, presented results from the LITE study, a trial that tested the hypothesis that narrowband ultraviolet B phototherapy of psoriasis at home is noninferior to office treatment, based on outcomes that matter to patients, clinicians, and payers. While smaller studies have drawn similar conclusions,
The co-primary outcomes in the LITE study were a Physician’s Global Assessment (PGA) score of 0/1 (clear, almost clear) and a Dermatology Life Quality Index (DLQI) score of 5 or less (small, no effect on health-related quality of life).
Dr. Gelfand and colleagues at 42 sites in the United States enrolled 783 patients aged 12 years and older who had plaque or guttate psoriasis and were candidates for phototherapy at home or in an office setting. Following 12 weeks of treatment, 25.6% of patients in the office-based phototherapy group achieved a PGA score of 0/1 compared with 32.8% of patients in the home-based phototherapy group (P > .0001 for noninferiority, non-response imputation for missing data). Similarly, 33.6% of patients in the office-based phototherapy group achieved a DLQI score of 5 or less compared with 52.4% of patients in the home-based phototherapy group (P > .0001 for noninferiority, non-response imputation for missing data).
A Safe and Effective Option
“I think that it’s important for physicians, insurance companies, and patients with psoriasis to understand that this is a very safe and effective form of therapy,” Craig A. Elmets, MD, professor of dermatology at The University of Alabama at Birmingham, said in an interview. “For people who are not interested in systemic medications or who have contraindications to systemic medications, phototherapy would be ideal,” added Dr. Elmets, first author of the joint AAD–National Psoriasis Foundation (NPF) guidelines for the management and treatment of psoriasis with phototherapy, published in 2019.
Factors beyond efficacy support the role of home phototherapy, Dr. Gelfand said, including the fact that it costs 10-100 times less than biologics for psoriasis and that office-based phototherapy is not available in 90% of counties in the United States. However, insurance coverage of home phototherapy “is highly variable because until the LITE study, there was no large-scale US data to support its use,” he told this news organization.
“Also, insurance companies are broken up into two parts: Durable medical goods and the medical side such as pharmacy costs, and they are siloed. The durable medical goods side views phototherapy as expensive, while the pharmacy side views it as dirt cheap. This is part of the problem with our health system. A lot of things are siloed and don’t make any sense,” said Dr. Gelfand, director of the Psoriasis and Phototherapy Treatment Center at the University of Pennsylvania. By working with the NPF and payers, he added, “we’re hoping ... to transform the way insurance companies think about covering home phototherapy.”
In the meantime, he and Dr. Elmets shared practical ways to optimize access to home phototherapy for psoriasis patients:
Have the discussion. Patients “rarely bring this up as an option,” Dr. Elmets said, so the onus is on clinicians to talk about it. In his view, the ideal candidate “is averse to using systemic agents but whose disease is beyond the point where topical medicines alone will work. One of the advantages of phototherapy is that it doesn’t have immunosuppressive effects.”
Clinicians and patients can learn about the efficacy and safety of phototherapy for psoriasis, including home-based options, on the NPF’s web site and by reading the 2019 joint AAD-NPF guidelines.
Shared decision-making is key. “When a patient comes in, I’ll discuss what their treatment options are and [we] will decide upon a course of action based on their unique needs and preferences [and] if it’s medically appropriate, meaning they have the type of psoriasis likely to respond to phototherapy,” Dr. Gelfand said. A patient with psoriasis mainly on the fingernails or genitals “is not a good candidate for phototherapy. If it’s on the trunk or extremities, that patient would be a good candidate.”
Home phototherapy candidates also must be willing and able to operate a machine and have dedicated space in their dwelling for it (most units are about the size of a door). Patients also have to be reliable, follow directions, and come back in person for follow-up appointments “so we can assess their response to treatment and fine-tune things as necessary and make sure they’re not developing any skin damage,” Dr. Gelfand said.
Educate yourself about existing options. Home phototherapy units from manufacturers such as Daavlin, National Biological Corporation, and SolRx range between $1200 and $6000 in cost, Dr. Gelfand said. He and his colleagues used the Daavlin 7 series in the LITE study. That unit features an integrated dosimetry system that delivers the correct dose of energy based on parameters that the prescribing clinician recommends. Settings are based on the patient’s skin type and how much the prescriber wants to increase the dose for each treatment. “The machine does the rest,” he said. “It knows what dose to give, so they get the same dosing as they would in an office situation.”
Smaller home-based phototherapy units designed to treat the hands and feet are available. So are handheld units to treat the scalp. “These can be a nice option for patients who have a few spots, but if the disease is moderate to severe, then it’s going to be pretty laborious to [use them],” Dr. Elmets said.
Remember that phototherapy is not a cure-all. According to the joint AAD-NPF guidelines, most phototherapy regimens require treatments two to three times per week for 10-14 weeks. Once patients achieve their home phototherapy treatment goal, Dr. Elmets often recommends treatments one to two times per week for maintenance.
“Patients with psoriasis have a lifetime condition,” he noted. “There are certainly cases where people have gone on phototherapy, cleared, and then stopped for a period of time. If they flare up, they can always go back to phototherapy. Usually, people who are on phototherapy use some type of topical agents to touch up areas that are resistant.”
Expect pushback from insurers on coverage. While Medicare and some integrated health plans cover home phototherapy, expect to spend time writing letters or placing phone calls to insurance companies to convince them why they should cover home phototherapy for candidate psoriasis patients. “Usually there’s a lot of letter writing and a long delay in getting approval,” Dr. Elmets said.
Dr. Elmets and Dr. Gelfand reported no relevant financial relationships. The LITE study was funded by the Patient-Centered Outcomes Research Institute. Research partners included the National Psoriasis Foundation and Daavlin, which provided the home phototherapy machines and covered the cost of shipping the devices.
A version of this article appeared on Medscape.com.
Specialists Are ‘Underwater’ With Some Insurance-Preferred Biosimilars
Editor’s note: This article is adapted from an explanatory statement that Dr. Feldman wrote for the Coalition of State Rheumatology Organizations (CSRO).
According to the Guinness Book of World records, the longest time someone has held their breath underwater voluntarily is 24 minutes and 37.36 seconds. While certainly an amazing feat, UnitedHealthcare, many of the Blues, and other national “payers” are expecting rheumatologists and other specialists to live “underwater” in order to take care of their patients. In other words, these insurance companies are mandating that specialists use certain provider-administered biosimilars whose acquisition cost is higher than what the insurance company is willing to reimburse them. Essentially, the insurance companies expect the rheumatologists to pay them to take care of their patients. Because of the substantial and destabilizing financial losses incurred, many practices and free-standing infusion centers have been forced to cease offering these biosimilars. Most rheumatologists will provide patients with appropriate alternatives when available and permitted by the insurer; otherwise, they must refer patients to hospital-based infusion centers. That results in delayed care and increased costs for patients and the system, because hospital-based infusion typically costs more than twice what office-based infusion costs.
Quantifying the Problem
To help quantify the magnitude of this issue, the Coalition of State Rheumatology Organizations (CSRO) recently conducted a survey of its membership. A shocking 97% of respondents reported that their practice had been affected by reimbursement rates for some biosimilars being lower than acquisition costs, with 91% of respondents stating that this issue is more pronounced for certain biosimilars than others. Across the board, respondents most frequently identified Inflectra (infliximab-dyyb) and Avsola (infliximab-axxq) as being especially affected: Over 88% and over 85% of respondents identified these two products, respectively, as being underwater. These results support the ongoing anecdotal reports CSRO continues to receive from rheumatology practices.
However, the survey results indicated that this issue is by no means confined to those two biosimilars. Truxima (rituximab-abbs) — a biosimilar for Rituxan — was frequently mentioned as well. Notably, respondents almost uniformly identified biosimilars in the infliximab and rituximab families, which illustrates that this issue is no longer confined to one or two early-to-market biosimilars but has almost become a hallmark of this particular biosimilars market. Remarkably, one respondent commented that the brand products are now cheaper to acquire than the biosimilars. Furthermore, the survey included respondents from across the country, indicating that this issue is not confined to a particular region.
How Did This Happen?
Biosimilars held promise for increasing availability and decreasing biologic costs for patients but, thus far, no patients have seen their cost go down. It appears that the only biosimilars that have made it to “preferred” status on the formulary are the ones that have made more money for the middlemen in the drug supply chain, particularly those that construct formularies. Now, we have provider-administered biosimilars whose acquisition cost exceeds the reimbursement for these drugs. This disparity was ultimately created by biosimilar manufacturers “over-rebating” their drugs to health insurance companies to gain “fail-first” status on the formulary.
For example, the manufacturer of Inflectra offered substantial rebates to health insurers for preferred formulary placement. These rebates are factored into the sales price of the medication, which then results in a rapidly declining average sales price (ASP) for the biosimilar. Unfortunately, the acquisition cost for the drug does not experience commensurate reductions, resulting in physicians being reimbursed far less for the drug than it costs to acquire. The financial losses for physicians put them underwater as a result of the acquisition costs for the preferred drugs far surpassing the reimbursement from the health insurance company that constructed the formulary.
While various factors affect ASPs and acquisition costs, this particular consequence of formulary placement based on price concessions is a major driver of the underwater situation in which physicians have found themselves with many biosimilars. Not only does that lead to a lower uptake of biosimilars, but it also results in patients being referred to the hospital outpatient infusion sites to receive this care, as freestanding infusion centers cannot treat these patients either. Hospitals incur higher costs because of facility fees and elevated rates, and this makes private rheumatology in-office infusion centers a much lower-cost option. Similarly, home infusion services, while convenient, are marginally more expensive than private practices and, in cases of biologic infusions, it is important to note that physicians’ offices have a greater safety profile than home infusion of biologics. The overall result of these “fail-first underwater drugs” is delayed and more costly care for the patient and the “system,” particularly self-insured employers.
What Is Being Done to Correct This?
Since ASPs are updated quarterly, it is possible that acquisition costs and reimbursements might stabilize over time, making the drugs affordable again to practices. However, that does not appear to be happening in the near future, so that possibility does not offer immediate relief to struggling practices. It doesn’t promise a favorable outlook for future biosimilar entries of provider-administered medications if formularies continue to prefer the highest-rebated medication.
This dynamic between ASP and acquisition cost does not happen on the pharmacy side because the price concessions on specific drug rebates and fees are proprietary. There appears to be no equivalent to a publicly known ASP on the pharmacy side, which has led to myriad pricing definitions and manipulation on the pharmacy benefit side of medications. In any event, the savings from rebates and other manufacturer price concessions on pharmacy drugs do not influence ASPs of medical benefit drugs.
The Inflation Reduction Act provided a temporary increase in the add-on payment for biosimilars from ASP+6% to ASP+8%, but as long as the biosimilar’s ASP is lower than the reference brand’s ASP, that temporary increase does not appear to make up for the large differential between ASP and acquisition cost. It should be noted that any federal attempt to artificially lower the ASP of a provider-administered drug without a pathway assuring that the acquisition cost for the provider is less than the reimbursement is going to result in loss of access for patients to those medications and/or higher hospital site of care costs.
A Few Partial Fixes, But Most Complaints Go Ignored
Considering the higher costs of hospital-based infusion, insurers should be motivated to keep patients within private practices. Perhaps through insurers’ recognition of that fact, some practices have successfully negotiated exceptions for specific patients by discussing this situation with insurers. From the feedback that CSRO has received from rheumatology practices, it appears that most insurers have been ignoring the complaints from physicians. The few who have responded have resulted in only partial fixes, with some of the biosimilars still left underwater.
Ultimate Solution?
This issue is a direct result of the “rebate game,” whereby price concessions from drug manufacturers drive formulary placement. For provider-administered medications, this results in an artificially lowered ASP, not as a consequence of free-market incentives that benefit the patient, but as a result of misaligned incentives created by Safe Harbor–protected “kickbacks,” distorting the free market and paradoxically reducing access to these medications, delaying care, and increasing prices for patients and the healthcare system.
While federal and state governments are not likely to address this particular situation in the biosimilars market, CSRO is highlighting this issue as a prime example of why the current formulary construction system urgently requires federal reform. At this time, the biosimilars most affected are Inflectra and Avsola, but if nothing changes, more and more biosimilars will fall victim to the short-sighted pricing strategy of aggressive rebating to gain formulary position, with physician purchasers and patients left to navigate the aftermath. The existing system, which necessitates drug companies purchasing formulary access from pharmacy benefit managers, has led to delayed and even denied patient access to certain provider-administered drugs. Moreover, it now appears to be hindering the adoption of biosimilars.
To address this, a multifaceted approach is required. It not only involves reevaluating the rebate system and its impact on formulary construction and ASP, but also ensuring that acquisition costs for providers are aligned with reimbursement rates. Insurers must recognize the economic and clinical value of maintaining infusions within private practices and immediately update their policies to ensure that physician in-office infusion is financially feasible for these “fail-first” biosimilars.
Ultimately, the goal should be to create a sustainable model that promotes the use of affordable biosimilars, enhances patient access to affordable care, and supports the financial viability of medical practices. Concerted efforts to reform the current formulary construction system are required to achieve a healthcare environment that is both cost effective and patient centric.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of advocacy and government affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.
Editor’s note: This article is adapted from an explanatory statement that Dr. Feldman wrote for the Coalition of State Rheumatology Organizations (CSRO).
According to the Guinness Book of World records, the longest time someone has held their breath underwater voluntarily is 24 minutes and 37.36 seconds. While certainly an amazing feat, UnitedHealthcare, many of the Blues, and other national “payers” are expecting rheumatologists and other specialists to live “underwater” in order to take care of their patients. In other words, these insurance companies are mandating that specialists use certain provider-administered biosimilars whose acquisition cost is higher than what the insurance company is willing to reimburse them. Essentially, the insurance companies expect the rheumatologists to pay them to take care of their patients. Because of the substantial and destabilizing financial losses incurred, many practices and free-standing infusion centers have been forced to cease offering these biosimilars. Most rheumatologists will provide patients with appropriate alternatives when available and permitted by the insurer; otherwise, they must refer patients to hospital-based infusion centers. That results in delayed care and increased costs for patients and the system, because hospital-based infusion typically costs more than twice what office-based infusion costs.
Quantifying the Problem
To help quantify the magnitude of this issue, the Coalition of State Rheumatology Organizations (CSRO) recently conducted a survey of its membership. A shocking 97% of respondents reported that their practice had been affected by reimbursement rates for some biosimilars being lower than acquisition costs, with 91% of respondents stating that this issue is more pronounced for certain biosimilars than others. Across the board, respondents most frequently identified Inflectra (infliximab-dyyb) and Avsola (infliximab-axxq) as being especially affected: Over 88% and over 85% of respondents identified these two products, respectively, as being underwater. These results support the ongoing anecdotal reports CSRO continues to receive from rheumatology practices.
However, the survey results indicated that this issue is by no means confined to those two biosimilars. Truxima (rituximab-abbs) — a biosimilar for Rituxan — was frequently mentioned as well. Notably, respondents almost uniformly identified biosimilars in the infliximab and rituximab families, which illustrates that this issue is no longer confined to one or two early-to-market biosimilars but has almost become a hallmark of this particular biosimilars market. Remarkably, one respondent commented that the brand products are now cheaper to acquire than the biosimilars. Furthermore, the survey included respondents from across the country, indicating that this issue is not confined to a particular region.
How Did This Happen?
Biosimilars held promise for increasing availability and decreasing biologic costs for patients but, thus far, no patients have seen their cost go down. It appears that the only biosimilars that have made it to “preferred” status on the formulary are the ones that have made more money for the middlemen in the drug supply chain, particularly those that construct formularies. Now, we have provider-administered biosimilars whose acquisition cost exceeds the reimbursement for these drugs. This disparity was ultimately created by biosimilar manufacturers “over-rebating” their drugs to health insurance companies to gain “fail-first” status on the formulary.
For example, the manufacturer of Inflectra offered substantial rebates to health insurers for preferred formulary placement. These rebates are factored into the sales price of the medication, which then results in a rapidly declining average sales price (ASP) for the biosimilar. Unfortunately, the acquisition cost for the drug does not experience commensurate reductions, resulting in physicians being reimbursed far less for the drug than it costs to acquire. The financial losses for physicians put them underwater as a result of the acquisition costs for the preferred drugs far surpassing the reimbursement from the health insurance company that constructed the formulary.
While various factors affect ASPs and acquisition costs, this particular consequence of formulary placement based on price concessions is a major driver of the underwater situation in which physicians have found themselves with many biosimilars. Not only does that lead to a lower uptake of biosimilars, but it also results in patients being referred to the hospital outpatient infusion sites to receive this care, as freestanding infusion centers cannot treat these patients either. Hospitals incur higher costs because of facility fees and elevated rates, and this makes private rheumatology in-office infusion centers a much lower-cost option. Similarly, home infusion services, while convenient, are marginally more expensive than private practices and, in cases of biologic infusions, it is important to note that physicians’ offices have a greater safety profile than home infusion of biologics. The overall result of these “fail-first underwater drugs” is delayed and more costly care for the patient and the “system,” particularly self-insured employers.
What Is Being Done to Correct This?
Since ASPs are updated quarterly, it is possible that acquisition costs and reimbursements might stabilize over time, making the drugs affordable again to practices. However, that does not appear to be happening in the near future, so that possibility does not offer immediate relief to struggling practices. It doesn’t promise a favorable outlook for future biosimilar entries of provider-administered medications if formularies continue to prefer the highest-rebated medication.
This dynamic between ASP and acquisition cost does not happen on the pharmacy side because the price concessions on specific drug rebates and fees are proprietary. There appears to be no equivalent to a publicly known ASP on the pharmacy side, which has led to myriad pricing definitions and manipulation on the pharmacy benefit side of medications. In any event, the savings from rebates and other manufacturer price concessions on pharmacy drugs do not influence ASPs of medical benefit drugs.
The Inflation Reduction Act provided a temporary increase in the add-on payment for biosimilars from ASP+6% to ASP+8%, but as long as the biosimilar’s ASP is lower than the reference brand’s ASP, that temporary increase does not appear to make up for the large differential between ASP and acquisition cost. It should be noted that any federal attempt to artificially lower the ASP of a provider-administered drug without a pathway assuring that the acquisition cost for the provider is less than the reimbursement is going to result in loss of access for patients to those medications and/or higher hospital site of care costs.
A Few Partial Fixes, But Most Complaints Go Ignored
Considering the higher costs of hospital-based infusion, insurers should be motivated to keep patients within private practices. Perhaps through insurers’ recognition of that fact, some practices have successfully negotiated exceptions for specific patients by discussing this situation with insurers. From the feedback that CSRO has received from rheumatology practices, it appears that most insurers have been ignoring the complaints from physicians. The few who have responded have resulted in only partial fixes, with some of the biosimilars still left underwater.
Ultimate Solution?
This issue is a direct result of the “rebate game,” whereby price concessions from drug manufacturers drive formulary placement. For provider-administered medications, this results in an artificially lowered ASP, not as a consequence of free-market incentives that benefit the patient, but as a result of misaligned incentives created by Safe Harbor–protected “kickbacks,” distorting the free market and paradoxically reducing access to these medications, delaying care, and increasing prices for patients and the healthcare system.
While federal and state governments are not likely to address this particular situation in the biosimilars market, CSRO is highlighting this issue as a prime example of why the current formulary construction system urgently requires federal reform. At this time, the biosimilars most affected are Inflectra and Avsola, but if nothing changes, more and more biosimilars will fall victim to the short-sighted pricing strategy of aggressive rebating to gain formulary position, with physician purchasers and patients left to navigate the aftermath. The existing system, which necessitates drug companies purchasing formulary access from pharmacy benefit managers, has led to delayed and even denied patient access to certain provider-administered drugs. Moreover, it now appears to be hindering the adoption of biosimilars.
To address this, a multifaceted approach is required. It not only involves reevaluating the rebate system and its impact on formulary construction and ASP, but also ensuring that acquisition costs for providers are aligned with reimbursement rates. Insurers must recognize the economic and clinical value of maintaining infusions within private practices and immediately update their policies to ensure that physician in-office infusion is financially feasible for these “fail-first” biosimilars.
Ultimately, the goal should be to create a sustainable model that promotes the use of affordable biosimilars, enhances patient access to affordable care, and supports the financial viability of medical practices. Concerted efforts to reform the current formulary construction system are required to achieve a healthcare environment that is both cost effective and patient centric.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of advocacy and government affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.
Editor’s note: This article is adapted from an explanatory statement that Dr. Feldman wrote for the Coalition of State Rheumatology Organizations (CSRO).
According to the Guinness Book of World records, the longest time someone has held their breath underwater voluntarily is 24 minutes and 37.36 seconds. While certainly an amazing feat, UnitedHealthcare, many of the Blues, and other national “payers” are expecting rheumatologists and other specialists to live “underwater” in order to take care of their patients. In other words, these insurance companies are mandating that specialists use certain provider-administered biosimilars whose acquisition cost is higher than what the insurance company is willing to reimburse them. Essentially, the insurance companies expect the rheumatologists to pay them to take care of their patients. Because of the substantial and destabilizing financial losses incurred, many practices and free-standing infusion centers have been forced to cease offering these biosimilars. Most rheumatologists will provide patients with appropriate alternatives when available and permitted by the insurer; otherwise, they must refer patients to hospital-based infusion centers. That results in delayed care and increased costs for patients and the system, because hospital-based infusion typically costs more than twice what office-based infusion costs.
Quantifying the Problem
To help quantify the magnitude of this issue, the Coalition of State Rheumatology Organizations (CSRO) recently conducted a survey of its membership. A shocking 97% of respondents reported that their practice had been affected by reimbursement rates for some biosimilars being lower than acquisition costs, with 91% of respondents stating that this issue is more pronounced for certain biosimilars than others. Across the board, respondents most frequently identified Inflectra (infliximab-dyyb) and Avsola (infliximab-axxq) as being especially affected: Over 88% and over 85% of respondents identified these two products, respectively, as being underwater. These results support the ongoing anecdotal reports CSRO continues to receive from rheumatology practices.
However, the survey results indicated that this issue is by no means confined to those two biosimilars. Truxima (rituximab-abbs) — a biosimilar for Rituxan — was frequently mentioned as well. Notably, respondents almost uniformly identified biosimilars in the infliximab and rituximab families, which illustrates that this issue is no longer confined to one or two early-to-market biosimilars but has almost become a hallmark of this particular biosimilars market. Remarkably, one respondent commented that the brand products are now cheaper to acquire than the biosimilars. Furthermore, the survey included respondents from across the country, indicating that this issue is not confined to a particular region.
How Did This Happen?
Biosimilars held promise for increasing availability and decreasing biologic costs for patients but, thus far, no patients have seen their cost go down. It appears that the only biosimilars that have made it to “preferred” status on the formulary are the ones that have made more money for the middlemen in the drug supply chain, particularly those that construct formularies. Now, we have provider-administered biosimilars whose acquisition cost exceeds the reimbursement for these drugs. This disparity was ultimately created by biosimilar manufacturers “over-rebating” their drugs to health insurance companies to gain “fail-first” status on the formulary.
For example, the manufacturer of Inflectra offered substantial rebates to health insurers for preferred formulary placement. These rebates are factored into the sales price of the medication, which then results in a rapidly declining average sales price (ASP) for the biosimilar. Unfortunately, the acquisition cost for the drug does not experience commensurate reductions, resulting in physicians being reimbursed far less for the drug than it costs to acquire. The financial losses for physicians put them underwater as a result of the acquisition costs for the preferred drugs far surpassing the reimbursement from the health insurance company that constructed the formulary.
While various factors affect ASPs and acquisition costs, this particular consequence of formulary placement based on price concessions is a major driver of the underwater situation in which physicians have found themselves with many biosimilars. Not only does that lead to a lower uptake of biosimilars, but it also results in patients being referred to the hospital outpatient infusion sites to receive this care, as freestanding infusion centers cannot treat these patients either. Hospitals incur higher costs because of facility fees and elevated rates, and this makes private rheumatology in-office infusion centers a much lower-cost option. Similarly, home infusion services, while convenient, are marginally more expensive than private practices and, in cases of biologic infusions, it is important to note that physicians’ offices have a greater safety profile than home infusion of biologics. The overall result of these “fail-first underwater drugs” is delayed and more costly care for the patient and the “system,” particularly self-insured employers.
What Is Being Done to Correct This?
Since ASPs are updated quarterly, it is possible that acquisition costs and reimbursements might stabilize over time, making the drugs affordable again to practices. However, that does not appear to be happening in the near future, so that possibility does not offer immediate relief to struggling practices. It doesn’t promise a favorable outlook for future biosimilar entries of provider-administered medications if formularies continue to prefer the highest-rebated medication.
This dynamic between ASP and acquisition cost does not happen on the pharmacy side because the price concessions on specific drug rebates and fees are proprietary. There appears to be no equivalent to a publicly known ASP on the pharmacy side, which has led to myriad pricing definitions and manipulation on the pharmacy benefit side of medications. In any event, the savings from rebates and other manufacturer price concessions on pharmacy drugs do not influence ASPs of medical benefit drugs.
The Inflation Reduction Act provided a temporary increase in the add-on payment for biosimilars from ASP+6% to ASP+8%, but as long as the biosimilar’s ASP is lower than the reference brand’s ASP, that temporary increase does not appear to make up for the large differential between ASP and acquisition cost. It should be noted that any federal attempt to artificially lower the ASP of a provider-administered drug without a pathway assuring that the acquisition cost for the provider is less than the reimbursement is going to result in loss of access for patients to those medications and/or higher hospital site of care costs.
A Few Partial Fixes, But Most Complaints Go Ignored
Considering the higher costs of hospital-based infusion, insurers should be motivated to keep patients within private practices. Perhaps through insurers’ recognition of that fact, some practices have successfully negotiated exceptions for specific patients by discussing this situation with insurers. From the feedback that CSRO has received from rheumatology practices, it appears that most insurers have been ignoring the complaints from physicians. The few who have responded have resulted in only partial fixes, with some of the biosimilars still left underwater.
Ultimate Solution?
This issue is a direct result of the “rebate game,” whereby price concessions from drug manufacturers drive formulary placement. For provider-administered medications, this results in an artificially lowered ASP, not as a consequence of free-market incentives that benefit the patient, but as a result of misaligned incentives created by Safe Harbor–protected “kickbacks,” distorting the free market and paradoxically reducing access to these medications, delaying care, and increasing prices for patients and the healthcare system.
While federal and state governments are not likely to address this particular situation in the biosimilars market, CSRO is highlighting this issue as a prime example of why the current formulary construction system urgently requires federal reform. At this time, the biosimilars most affected are Inflectra and Avsola, but if nothing changes, more and more biosimilars will fall victim to the short-sighted pricing strategy of aggressive rebating to gain formulary position, with physician purchasers and patients left to navigate the aftermath. The existing system, which necessitates drug companies purchasing formulary access from pharmacy benefit managers, has led to delayed and even denied patient access to certain provider-administered drugs. Moreover, it now appears to be hindering the adoption of biosimilars.
To address this, a multifaceted approach is required. It not only involves reevaluating the rebate system and its impact on formulary construction and ASP, but also ensuring that acquisition costs for providers are aligned with reimbursement rates. Insurers must recognize the economic and clinical value of maintaining infusions within private practices and immediately update their policies to ensure that physician in-office infusion is financially feasible for these “fail-first” biosimilars.
Ultimately, the goal should be to create a sustainable model that promotes the use of affordable biosimilars, enhances patient access to affordable care, and supports the financial viability of medical practices. Concerted efforts to reform the current formulary construction system are required to achieve a healthcare environment that is both cost effective and patient centric.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of advocacy and government affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.
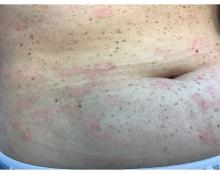
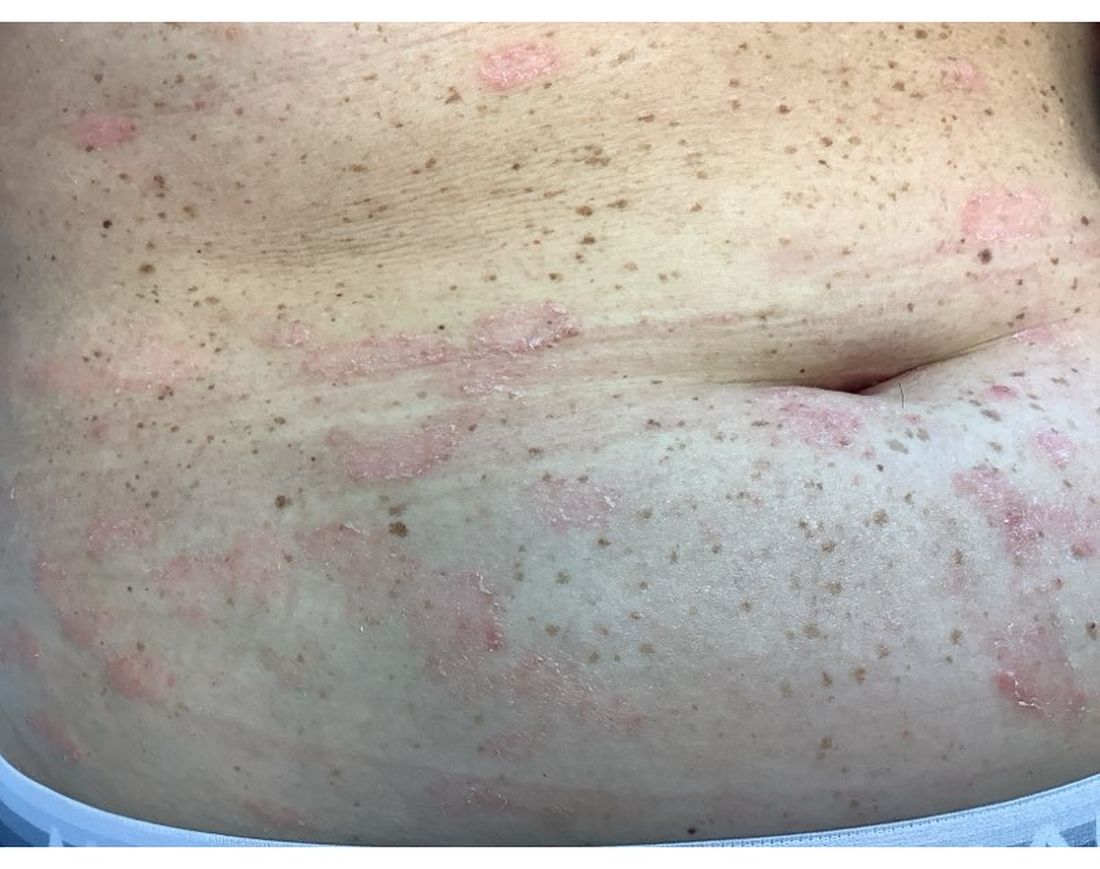
Positive Phase 2 Results Reported for TYK2 Inhibitor for Patients With Moderate to Severe Psoriasis
, in a phase 2 study presented during a late breaker presentation at the annual meeting of the American Academy of Dermatology.
ESK-001 is a highly-selective TYK2 inhibitor that “reduces signaling through several cytokine receptors including receptors for interleukin (IL)-12, IL-23, and interferon (IFN)-alpha,” according to Alumis, the company developing ESK-001.
The STRIDE trial enrolled 228 adults (mean age, 48 years) with a mean PASI score of 17.8 at baseline, randomized to one of five doses of ESK-001, or placebo. Nearly 70% of those enrolled were men, 82.5% were White, nearly 6% were Asian, and about 4% were Black.
The proportion of patients achieving at least a PASI-75 at 12 weeks, the primary endpoint, ranged from 19.4% among those on the lower dose (10 mg a day) to 56.4% among those on 20 mg twice a day and those on 40 mg once a day, and 64.1% among those on 40 mg twice a day, the highest dose evaluated, compared with 0% of those on placebo.
In addition, 38.5% and 15.4% of those on the highest dose achieved a PASI 90 and PASI 100, respectively, at 12 weeks, according to results presented at the meeting by Kim A. Papp, MD.
Improvements in static Physician’s Global Assessment (sPGA) scores, a secondary endpoint, at 12 weeks were also observed; including 59% of those on the highest dose (40 mg twice a day) achieving an sPGA of 0/1 at 12 weeks, according to Dr. Papp, president of Probity Medical Research, in Waterloo, Ontario, and a study investigator.
Treatment was “generally safe and well tolerated” at all doses, and most treatment-emergent adverse events were “mild to moderate” and self-limited, he said.
The most frequent treatment emergent adverse events included headache and nasopharyngitis (also reported in the placebo group), and upper respiratory tract infections. No cases of treatment-related serious adverse events were reported, and there were no cases of MACE (major adverse cardiovascular events), serious infections, thromboses related to treatment, or lab or ECG findings of concern.
In an ongoing open label extension study, 165 patients on 40 mg once a day and those on 40 mg twice a day are continuing to be evaluated, and at week 16, have continued to show significant efficacy, with a favorable risk-benefit profile to date, according to Dr. Papp.
ESK-001 is also being evaluated in a phase 2b study of patients with systemic lupus erythematosus, and a phase 2 proof-of-concept study of patients with non-infectious uveitis, according to Alumis.
In addition to being an investigator for Alumis, Dr. Papp disclosed serving as an adviser, consultant, investigator, and/or speaker for multiple other pharmaceutical companies.
, in a phase 2 study presented during a late breaker presentation at the annual meeting of the American Academy of Dermatology.
ESK-001 is a highly-selective TYK2 inhibitor that “reduces signaling through several cytokine receptors including receptors for interleukin (IL)-12, IL-23, and interferon (IFN)-alpha,” according to Alumis, the company developing ESK-001.
The STRIDE trial enrolled 228 adults (mean age, 48 years) with a mean PASI score of 17.8 at baseline, randomized to one of five doses of ESK-001, or placebo. Nearly 70% of those enrolled were men, 82.5% were White, nearly 6% were Asian, and about 4% were Black.
The proportion of patients achieving at least a PASI-75 at 12 weeks, the primary endpoint, ranged from 19.4% among those on the lower dose (10 mg a day) to 56.4% among those on 20 mg twice a day and those on 40 mg once a day, and 64.1% among those on 40 mg twice a day, the highest dose evaluated, compared with 0% of those on placebo.
In addition, 38.5% and 15.4% of those on the highest dose achieved a PASI 90 and PASI 100, respectively, at 12 weeks, according to results presented at the meeting by Kim A. Papp, MD.
Improvements in static Physician’s Global Assessment (sPGA) scores, a secondary endpoint, at 12 weeks were also observed; including 59% of those on the highest dose (40 mg twice a day) achieving an sPGA of 0/1 at 12 weeks, according to Dr. Papp, president of Probity Medical Research, in Waterloo, Ontario, and a study investigator.
Treatment was “generally safe and well tolerated” at all doses, and most treatment-emergent adverse events were “mild to moderate” and self-limited, he said.
The most frequent treatment emergent adverse events included headache and nasopharyngitis (also reported in the placebo group), and upper respiratory tract infections. No cases of treatment-related serious adverse events were reported, and there were no cases of MACE (major adverse cardiovascular events), serious infections, thromboses related to treatment, or lab or ECG findings of concern.
In an ongoing open label extension study, 165 patients on 40 mg once a day and those on 40 mg twice a day are continuing to be evaluated, and at week 16, have continued to show significant efficacy, with a favorable risk-benefit profile to date, according to Dr. Papp.
ESK-001 is also being evaluated in a phase 2b study of patients with systemic lupus erythematosus, and a phase 2 proof-of-concept study of patients with non-infectious uveitis, according to Alumis.
In addition to being an investigator for Alumis, Dr. Papp disclosed serving as an adviser, consultant, investigator, and/or speaker for multiple other pharmaceutical companies.
, in a phase 2 study presented during a late breaker presentation at the annual meeting of the American Academy of Dermatology.
ESK-001 is a highly-selective TYK2 inhibitor that “reduces signaling through several cytokine receptors including receptors for interleukin (IL)-12, IL-23, and interferon (IFN)-alpha,” according to Alumis, the company developing ESK-001.
The STRIDE trial enrolled 228 adults (mean age, 48 years) with a mean PASI score of 17.8 at baseline, randomized to one of five doses of ESK-001, or placebo. Nearly 70% of those enrolled were men, 82.5% were White, nearly 6% were Asian, and about 4% were Black.
The proportion of patients achieving at least a PASI-75 at 12 weeks, the primary endpoint, ranged from 19.4% among those on the lower dose (10 mg a day) to 56.4% among those on 20 mg twice a day and those on 40 mg once a day, and 64.1% among those on 40 mg twice a day, the highest dose evaluated, compared with 0% of those on placebo.
In addition, 38.5% and 15.4% of those on the highest dose achieved a PASI 90 and PASI 100, respectively, at 12 weeks, according to results presented at the meeting by Kim A. Papp, MD.
Improvements in static Physician’s Global Assessment (sPGA) scores, a secondary endpoint, at 12 weeks were also observed; including 59% of those on the highest dose (40 mg twice a day) achieving an sPGA of 0/1 at 12 weeks, according to Dr. Papp, president of Probity Medical Research, in Waterloo, Ontario, and a study investigator.
Treatment was “generally safe and well tolerated” at all doses, and most treatment-emergent adverse events were “mild to moderate” and self-limited, he said.
The most frequent treatment emergent adverse events included headache and nasopharyngitis (also reported in the placebo group), and upper respiratory tract infections. No cases of treatment-related serious adverse events were reported, and there were no cases of MACE (major adverse cardiovascular events), serious infections, thromboses related to treatment, or lab or ECG findings of concern.
In an ongoing open label extension study, 165 patients on 40 mg once a day and those on 40 mg twice a day are continuing to be evaluated, and at week 16, have continued to show significant efficacy, with a favorable risk-benefit profile to date, according to Dr. Papp.
ESK-001 is also being evaluated in a phase 2b study of patients with systemic lupus erythematosus, and a phase 2 proof-of-concept study of patients with non-infectious uveitis, according to Alumis.
In addition to being an investigator for Alumis, Dr. Papp disclosed serving as an adviser, consultant, investigator, and/or speaker for multiple other pharmaceutical companies.
FROM AAD 2024
Combined Pediatric Derm-Rheum Clinics Supported by Survey Respondents
TOPLINE:
.
METHODOLOGY:
- Combined pediatric dermatology-rheumatology clinics can improve patient outcomes and experiences, particularly for pediatric autoimmune conditions presenting with both cutaneous and systemic manifestations.
- The researchers surveyed 208 pediatric dermatologists working in combined pediatric dermatology-rheumatology clinics.
- A total of 13 member responses were recorded from three countries: 10 from the United States, two from Mexico, and one from Canada.
TAKEAWAY:
- Perceived benefits of combined clinics were improved patient care through coordinated treatment decisions and timely communication between providers.
- Patient satisfaction was favorable, and patients and families endorsed the combined clinic approach.
- Barriers to clinic establishment included differences in the pace between dermatology and rheumatology clinic flow, the need to generate more relative value units, resistance from colleagues, and limited time.
- Areas that needed improvement included more time for patient visits, dedicated research assistants, new patient referrals, additional patient rooms, resources for research, and patient care infrastructure.
IN PRACTICE:
The insights from this survey “will hopefully inspire further development of these combined clinics,” the authors wrote.
SOURCE:
The investigation, led by Olga S. Cherepakhin, BS, University of Washington, Seattle, Washington, was published in Pediatric Dermatology.
LIMITATIONS:
Limitations included the subjective nature, lack of some information, selection bias, and small number of respondents, and the survey reflected the perspective of the pediatric dermatologists only.
DISCLOSURES:
The study was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health. One author reported full-time employment at Janssen R&D, and the other authors had no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- Combined pediatric dermatology-rheumatology clinics can improve patient outcomes and experiences, particularly for pediatric autoimmune conditions presenting with both cutaneous and systemic manifestations.
- The researchers surveyed 208 pediatric dermatologists working in combined pediatric dermatology-rheumatology clinics.
- A total of 13 member responses were recorded from three countries: 10 from the United States, two from Mexico, and one from Canada.
TAKEAWAY:
- Perceived benefits of combined clinics were improved patient care through coordinated treatment decisions and timely communication between providers.
- Patient satisfaction was favorable, and patients and families endorsed the combined clinic approach.
- Barriers to clinic establishment included differences in the pace between dermatology and rheumatology clinic flow, the need to generate more relative value units, resistance from colleagues, and limited time.
- Areas that needed improvement included more time for patient visits, dedicated research assistants, new patient referrals, additional patient rooms, resources for research, and patient care infrastructure.
IN PRACTICE:
The insights from this survey “will hopefully inspire further development of these combined clinics,” the authors wrote.
SOURCE:
The investigation, led by Olga S. Cherepakhin, BS, University of Washington, Seattle, Washington, was published in Pediatric Dermatology.
LIMITATIONS:
Limitations included the subjective nature, lack of some information, selection bias, and small number of respondents, and the survey reflected the perspective of the pediatric dermatologists only.
DISCLOSURES:
The study was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health. One author reported full-time employment at Janssen R&D, and the other authors had no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- Combined pediatric dermatology-rheumatology clinics can improve patient outcomes and experiences, particularly for pediatric autoimmune conditions presenting with both cutaneous and systemic manifestations.
- The researchers surveyed 208 pediatric dermatologists working in combined pediatric dermatology-rheumatology clinics.
- A total of 13 member responses were recorded from three countries: 10 from the United States, two from Mexico, and one from Canada.
TAKEAWAY:
- Perceived benefits of combined clinics were improved patient care through coordinated treatment decisions and timely communication between providers.
- Patient satisfaction was favorable, and patients and families endorsed the combined clinic approach.
- Barriers to clinic establishment included differences in the pace between dermatology and rheumatology clinic flow, the need to generate more relative value units, resistance from colleagues, and limited time.
- Areas that needed improvement included more time for patient visits, dedicated research assistants, new patient referrals, additional patient rooms, resources for research, and patient care infrastructure.
IN PRACTICE:
The insights from this survey “will hopefully inspire further development of these combined clinics,” the authors wrote.
SOURCE:
The investigation, led by Olga S. Cherepakhin, BS, University of Washington, Seattle, Washington, was published in Pediatric Dermatology.
LIMITATIONS:
Limitations included the subjective nature, lack of some information, selection bias, and small number of respondents, and the survey reflected the perspective of the pediatric dermatologists only.
DISCLOSURES:
The study was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health. One author reported full-time employment at Janssen R&D, and the other authors had no disclosures.
A version of this article appeared on Medscape.com.
Second Ustekinumab Biosimilar Gets FDA Approval
The US Food and Drug Administration (FDA) has approved the biosimilar ustekinumab-aekn (Selarsdi) for the treatment of moderate to severe plaque psoriasis and psoriatic arthritis in adults and pediatric patients aged 6 years or older.
This is the second ustekinumab biosimilar approved by the regulatory agency and is the second biosimilar approval in the United States for the Icelandic pharmaceutical company Alvotech in partnership with Teva Pharmaceuticals.
Ustekinumab (Stelara) is a human monoclonal antibody targeting interleukin (IL)–12 and IL-23. The drug, manufactured by Johnson & Johnson, totaled nearly $7 billion in sales in 2023 alone, according a press release.
“Bringing Selarsdi to market in the US early next year presents a significant opportunity to improve patient access to a vital biologic in inflammatory disease and contribute to the reduction of inflationary pressure in healthcare costs,” the chairman and CEO of Alvotech said in the release.
The first ustekinumab biosimilar, ustekinumab-auub (Wezlana), was approved by the FDA in on October 31, 2023 and is interchangeable with the reference product. This allows pharmacists to substitute the biosimilar for the reference product without involving the prescribing clinician (according to state law). Besides psoriasis and psoriatic arthritis, ustekinumab-auub was also approved for treating moderate to severely active Crohn’s disease and ulcerative colitis. Ustekinumab-aekn does not have an interchangeability designation and was not approved for Crohn’s disease or ulcerative colitis.
The approval of ustekinumab-aekn was based on two clinical studies. A randomized, double blind, multicenter, 52-week study of 581 patients with moderate to severe plaque psoriasis demonstrated that the biosimilar was as effective as the reference product, with equivalent safety and immunogenicity profiles. A phase 1, randomized, double-blind, single-dose, parallel-group, three-arm study also compared the pharmacokinetic profile of the biosimilar to ustekinumab in 294 healthy adults.
Ustekinumab-aekn is expected to be marketed in the United States on or after February 21, 2025 per a settlement and license agreement with Johnson & Johnson.
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved the biosimilar ustekinumab-aekn (Selarsdi) for the treatment of moderate to severe plaque psoriasis and psoriatic arthritis in adults and pediatric patients aged 6 years or older.
This is the second ustekinumab biosimilar approved by the regulatory agency and is the second biosimilar approval in the United States for the Icelandic pharmaceutical company Alvotech in partnership with Teva Pharmaceuticals.
Ustekinumab (Stelara) is a human monoclonal antibody targeting interleukin (IL)–12 and IL-23. The drug, manufactured by Johnson & Johnson, totaled nearly $7 billion in sales in 2023 alone, according a press release.
“Bringing Selarsdi to market in the US early next year presents a significant opportunity to improve patient access to a vital biologic in inflammatory disease and contribute to the reduction of inflationary pressure in healthcare costs,” the chairman and CEO of Alvotech said in the release.
The first ustekinumab biosimilar, ustekinumab-auub (Wezlana), was approved by the FDA in on October 31, 2023 and is interchangeable with the reference product. This allows pharmacists to substitute the biosimilar for the reference product without involving the prescribing clinician (according to state law). Besides psoriasis and psoriatic arthritis, ustekinumab-auub was also approved for treating moderate to severely active Crohn’s disease and ulcerative colitis. Ustekinumab-aekn does not have an interchangeability designation and was not approved for Crohn’s disease or ulcerative colitis.
The approval of ustekinumab-aekn was based on two clinical studies. A randomized, double blind, multicenter, 52-week study of 581 patients with moderate to severe plaque psoriasis demonstrated that the biosimilar was as effective as the reference product, with equivalent safety and immunogenicity profiles. A phase 1, randomized, double-blind, single-dose, parallel-group, three-arm study also compared the pharmacokinetic profile of the biosimilar to ustekinumab in 294 healthy adults.
Ustekinumab-aekn is expected to be marketed in the United States on or after February 21, 2025 per a settlement and license agreement with Johnson & Johnson.
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved the biosimilar ustekinumab-aekn (Selarsdi) for the treatment of moderate to severe plaque psoriasis and psoriatic arthritis in adults and pediatric patients aged 6 years or older.
This is the second ustekinumab biosimilar approved by the regulatory agency and is the second biosimilar approval in the United States for the Icelandic pharmaceutical company Alvotech in partnership with Teva Pharmaceuticals.
Ustekinumab (Stelara) is a human monoclonal antibody targeting interleukin (IL)–12 and IL-23. The drug, manufactured by Johnson & Johnson, totaled nearly $7 billion in sales in 2023 alone, according a press release.
“Bringing Selarsdi to market in the US early next year presents a significant opportunity to improve patient access to a vital biologic in inflammatory disease and contribute to the reduction of inflationary pressure in healthcare costs,” the chairman and CEO of Alvotech said in the release.
The first ustekinumab biosimilar, ustekinumab-auub (Wezlana), was approved by the FDA in on October 31, 2023 and is interchangeable with the reference product. This allows pharmacists to substitute the biosimilar for the reference product without involving the prescribing clinician (according to state law). Besides psoriasis and psoriatic arthritis, ustekinumab-auub was also approved for treating moderate to severely active Crohn’s disease and ulcerative colitis. Ustekinumab-aekn does not have an interchangeability designation and was not approved for Crohn’s disease or ulcerative colitis.
The approval of ustekinumab-aekn was based on two clinical studies. A randomized, double blind, multicenter, 52-week study of 581 patients with moderate to severe plaque psoriasis demonstrated that the biosimilar was as effective as the reference product, with equivalent safety and immunogenicity profiles. A phase 1, randomized, double-blind, single-dose, parallel-group, three-arm study also compared the pharmacokinetic profile of the biosimilar to ustekinumab in 294 healthy adults.
Ustekinumab-aekn is expected to be marketed in the United States on or after February 21, 2025 per a settlement and license agreement with Johnson & Johnson.
A version of this article appeared on Medscape.com.
A 30-Year-Old White Female Presented With a 4-Month History of Scaly, Erythematous Patches and Plaques on Her Trunk and Extremities
Tumor necrosis factor (TNF)-alpha inhibitors are used to treat a variety of autoimmune conditions including psoriasis, psoriatic arthritis, rheumatoid arthritis (RA), spondyloarthritis, and inflammatory bowel disease (IBD). Interestingly, they have also been observed to cause paradoxical psoriasis with an incidence between 0.6%-5.3%, most commonly occurring in patients with underlying Crohn’s disease and rheumatoid arthritis (RA). Infliximab is the most common TNF inhibitor associated with this condition (52.6%-62.6% of cases) followed by etanercept (12%-29%). .
Psoriasis is traditionally divided into two types. Patients with type I psoriasis have a family history, develop symptoms before the age of 40 and are often positive for HLA-Cw6. Type II psoriasis is not related to HLA-Cw6, lacks a family history, and typically manifests after age 40. Psoriatic lesions are well-defined, erythematous plaques with silvery scales most commonly appearing on extensor surfaces and the scalp. Variants include nail psoriasis, pustular psoriasis, inverse psoriasis, and guttate psoriasis.
Although psoriasis is typically a clinical diagnosis, histologic examination may be used to differentiate from other dermatoses if necessary. The lesions of TNF inhibitor-induced psoriasis characteristically display patterns similar to primary psoriasis, including parakeratosis, microabscesses, and rete ridges. Eosinophilic hypersensitivity reactions and features overlapping with eczematous hypersensitivity (psoriasiform dermatitis) may also be present.
The pathogenesis of this condition is not well understood, but theories include a variety of immune processes including interferon overproduction, interleukin and T-cell activation, and the presence of an infectious nidus. Classical psoriasis is related to type 1 interferon release, so theoretically, immunosuppression caused by TNF inhibitor treatment may permit uncontrolled production of interferons, resulting in psoriatic lesions. Another theory is that interleukin (IL)-23, a pro-inflammatory cytokine, promotes activation of T-helper 17 (Th17) cells. Th17 cells are part of the pathogenesis of primary psoriasis and other inflammatory conditions, such as RA and inflammatory bowel disease. Of note, individuals with gastrointestinal inflammatory diseases are already known to be at a greater risk for developing psoriasis. Immunosuppression caused by a TNF inhibitor may leave patients more susceptible to other infections, which may induce psoriatic plaques.
There are multiple approaches to treatment depending on the severity of the disease. If the psoriatic eruption is mild, the medication may be continued. This “treat-through” method is often considered when stopping the current immunotherapy would cause the patient significant issues. Moderate to severe cases of TNF inhibitor-induced psoriasis may warrant switching TNF inhibitor therapy or completely changing the drug class used in the treatment of the underlying autoimmune condition. Additional treatments include topical and oral steroids, UV therapy, methotrexate, cyclosporine, and acitretin.
This case and the photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Leon S. Maratchi, MD, Gastro Health, Hollywood, Florida. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Li SJ et al. J Psoriasis Psoriatic Arthritis. 2019 Apr;4(2):70-80. doi: 10.1177/2475530318810851.
2. Lu J and Lu Y. J Transl Autoimmun. 2023 Sep 6:7:100211. doi: 10.1016/j.jtauto.2023.100211.
3. Nair PA and Badri T. Psoriasis. [Updated 2023 Apr 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK448194/
Tumor necrosis factor (TNF)-alpha inhibitors are used to treat a variety of autoimmune conditions including psoriasis, psoriatic arthritis, rheumatoid arthritis (RA), spondyloarthritis, and inflammatory bowel disease (IBD). Interestingly, they have also been observed to cause paradoxical psoriasis with an incidence between 0.6%-5.3%, most commonly occurring in patients with underlying Crohn’s disease and rheumatoid arthritis (RA). Infliximab is the most common TNF inhibitor associated with this condition (52.6%-62.6% of cases) followed by etanercept (12%-29%). .
Psoriasis is traditionally divided into two types. Patients with type I psoriasis have a family history, develop symptoms before the age of 40 and are often positive for HLA-Cw6. Type II psoriasis is not related to HLA-Cw6, lacks a family history, and typically manifests after age 40. Psoriatic lesions are well-defined, erythematous plaques with silvery scales most commonly appearing on extensor surfaces and the scalp. Variants include nail psoriasis, pustular psoriasis, inverse psoriasis, and guttate psoriasis.
Although psoriasis is typically a clinical diagnosis, histologic examination may be used to differentiate from other dermatoses if necessary. The lesions of TNF inhibitor-induced psoriasis characteristically display patterns similar to primary psoriasis, including parakeratosis, microabscesses, and rete ridges. Eosinophilic hypersensitivity reactions and features overlapping with eczematous hypersensitivity (psoriasiform dermatitis) may also be present.
The pathogenesis of this condition is not well understood, but theories include a variety of immune processes including interferon overproduction, interleukin and T-cell activation, and the presence of an infectious nidus. Classical psoriasis is related to type 1 interferon release, so theoretically, immunosuppression caused by TNF inhibitor treatment may permit uncontrolled production of interferons, resulting in psoriatic lesions. Another theory is that interleukin (IL)-23, a pro-inflammatory cytokine, promotes activation of T-helper 17 (Th17) cells. Th17 cells are part of the pathogenesis of primary psoriasis and other inflammatory conditions, such as RA and inflammatory bowel disease. Of note, individuals with gastrointestinal inflammatory diseases are already known to be at a greater risk for developing psoriasis. Immunosuppression caused by a TNF inhibitor may leave patients more susceptible to other infections, which may induce psoriatic plaques.
There are multiple approaches to treatment depending on the severity of the disease. If the psoriatic eruption is mild, the medication may be continued. This “treat-through” method is often considered when stopping the current immunotherapy would cause the patient significant issues. Moderate to severe cases of TNF inhibitor-induced psoriasis may warrant switching TNF inhibitor therapy or completely changing the drug class used in the treatment of the underlying autoimmune condition. Additional treatments include topical and oral steroids, UV therapy, methotrexate, cyclosporine, and acitretin.
This case and the photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Leon S. Maratchi, MD, Gastro Health, Hollywood, Florida. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Li SJ et al. J Psoriasis Psoriatic Arthritis. 2019 Apr;4(2):70-80. doi: 10.1177/2475530318810851.
2. Lu J and Lu Y. J Transl Autoimmun. 2023 Sep 6:7:100211. doi: 10.1016/j.jtauto.2023.100211.
3. Nair PA and Badri T. Psoriasis. [Updated 2023 Apr 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK448194/
Tumor necrosis factor (TNF)-alpha inhibitors are used to treat a variety of autoimmune conditions including psoriasis, psoriatic arthritis, rheumatoid arthritis (RA), spondyloarthritis, and inflammatory bowel disease (IBD). Interestingly, they have also been observed to cause paradoxical psoriasis with an incidence between 0.6%-5.3%, most commonly occurring in patients with underlying Crohn’s disease and rheumatoid arthritis (RA). Infliximab is the most common TNF inhibitor associated with this condition (52.6%-62.6% of cases) followed by etanercept (12%-29%). .
Psoriasis is traditionally divided into two types. Patients with type I psoriasis have a family history, develop symptoms before the age of 40 and are often positive for HLA-Cw6. Type II psoriasis is not related to HLA-Cw6, lacks a family history, and typically manifests after age 40. Psoriatic lesions are well-defined, erythematous plaques with silvery scales most commonly appearing on extensor surfaces and the scalp. Variants include nail psoriasis, pustular psoriasis, inverse psoriasis, and guttate psoriasis.
Although psoriasis is typically a clinical diagnosis, histologic examination may be used to differentiate from other dermatoses if necessary. The lesions of TNF inhibitor-induced psoriasis characteristically display patterns similar to primary psoriasis, including parakeratosis, microabscesses, and rete ridges. Eosinophilic hypersensitivity reactions and features overlapping with eczematous hypersensitivity (psoriasiform dermatitis) may also be present.
The pathogenesis of this condition is not well understood, but theories include a variety of immune processes including interferon overproduction, interleukin and T-cell activation, and the presence of an infectious nidus. Classical psoriasis is related to type 1 interferon release, so theoretically, immunosuppression caused by TNF inhibitor treatment may permit uncontrolled production of interferons, resulting in psoriatic lesions. Another theory is that interleukin (IL)-23, a pro-inflammatory cytokine, promotes activation of T-helper 17 (Th17) cells. Th17 cells are part of the pathogenesis of primary psoriasis and other inflammatory conditions, such as RA and inflammatory bowel disease. Of note, individuals with gastrointestinal inflammatory diseases are already known to be at a greater risk for developing psoriasis. Immunosuppression caused by a TNF inhibitor may leave patients more susceptible to other infections, which may induce psoriatic plaques.
There are multiple approaches to treatment depending on the severity of the disease. If the psoriatic eruption is mild, the medication may be continued. This “treat-through” method is often considered when stopping the current immunotherapy would cause the patient significant issues. Moderate to severe cases of TNF inhibitor-induced psoriasis may warrant switching TNF inhibitor therapy or completely changing the drug class used in the treatment of the underlying autoimmune condition. Additional treatments include topical and oral steroids, UV therapy, methotrexate, cyclosporine, and acitretin.
This case and the photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Leon S. Maratchi, MD, Gastro Health, Hollywood, Florida. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Li SJ et al. J Psoriasis Psoriatic Arthritis. 2019 Apr;4(2):70-80. doi: 10.1177/2475530318810851.
2. Lu J and Lu Y. J Transl Autoimmun. 2023 Sep 6:7:100211. doi: 10.1016/j.jtauto.2023.100211.
3. Nair PA and Badri T. Psoriasis. [Updated 2023 Apr 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK448194/
Lichenoid Dermatosis on the Feet
The Diagnosis: Hypertrophic Lichen Planus
Two biopsies from the left lateral foot revealed hyperkeratosis, wedge-shaped hypergranulosis, irregular acanthosis, and a bandlike lymphocytic infiltrate in the superficial dermis with a classic sawtooth pattern of the rete ridges (Figure 1). Based on the clinical findings and histopathology, the patient was diagnosed with hypertrophic lichen planus (LP) and was treated with clobetasol ointment 0.05%, which resulted in progression of the symptoms. She experienced notable improvement 3 months after adding methotrexate 12.5 mg weekly (Figure 2).
Lichen planus is an idiopathic chronic inflammatory condition of the skin and mucous membranes that classically manifests as pruritic violaceous papules and plaques, which commonly are found on the wrists, lower back, and ankles.1 The most common variants of LP are hypertrophic, linear, mucosal, actinic, follicular, pigmented, annular, atrophic, and guttate.2 The clinical presentation and biopsy results in our patient were consistent with the hypertrophic variant of LP, which is a chronic condition that most often manifests on the lower legs, especially around the ankles, as hyperkeratotic papules, plaques, and nodules.2,3 The exact pathophysiology of hypertrophic LP is unknown, but there is evidence that the immune system plays a role in its development and that the Koebner phenomenon may contribute to its exacerbation.4 There is a well-known association between LP and hepatitis. Patients with chronic LP may develop squamous cell carcinoma.4 The variants of LP can overlap and do not exist independent of one another. Recognizing the overlap in these variants allows for earlier diagnosis and therapeutic intervention of the disease process to limit disease progression and patient clinic visits and to improve patient quality of life.
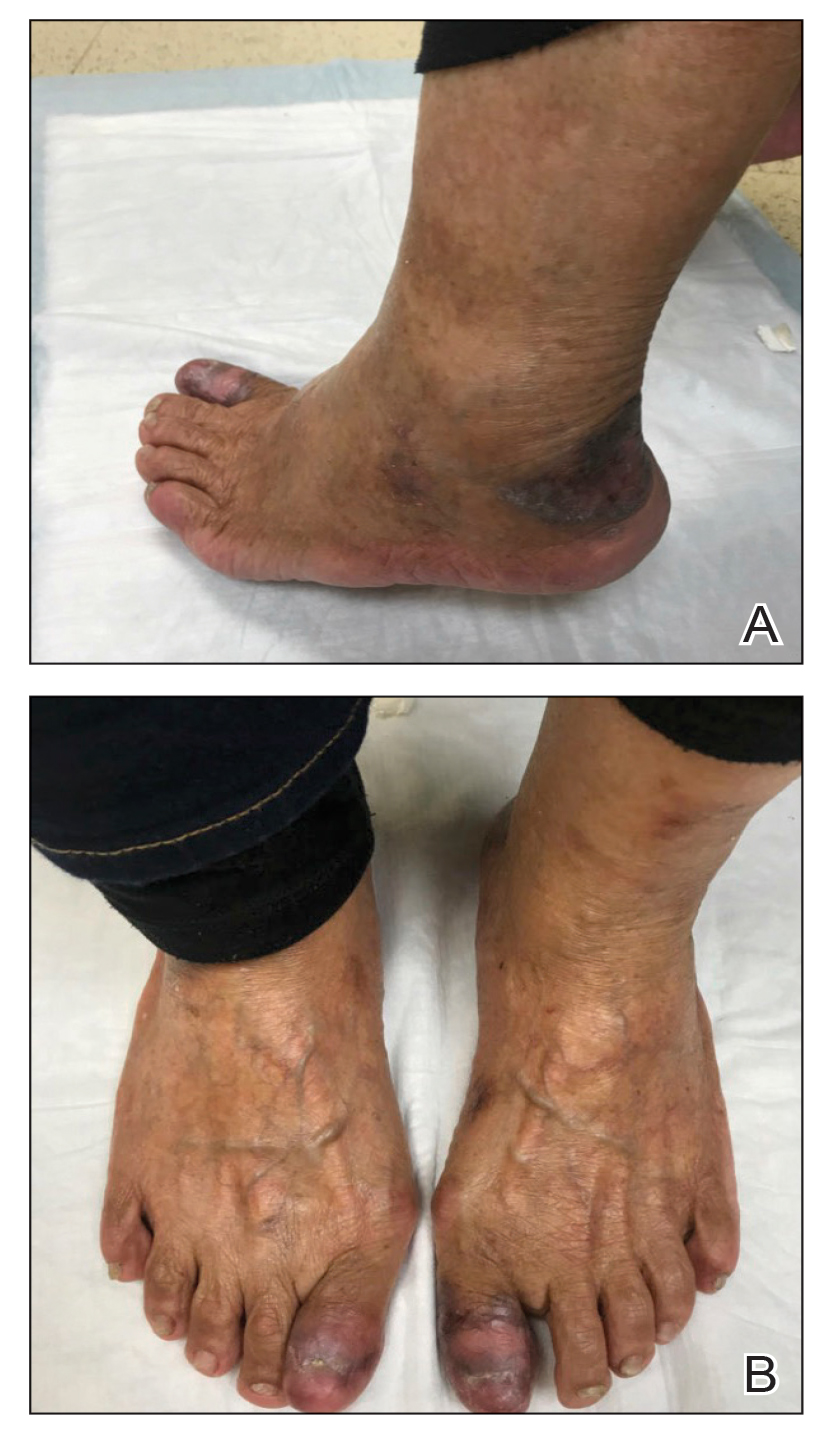
The differential diagnosis for hyperkeratotic plaques of the feet and ankles can be broad and may include keratosis lichenoides chronica, palmoplantar keratoderma, palmoplantar psoriasis, or lichen amyloidosis. These conditions are classified based on various criteria that include extent of disease manifestations, morphology of palmoplantar skin involvement, inheritance patterns, and molecular pathogenesis.5 Keratosis lichenoides chronica is a rare dermatosis that presents as a distinctive seborrheic dermatitis–like facial eruption. The facial eruption is accompanied by violaceous papular and nodular lesions that appear on the extremities and trunk, typically arranged in a linear or reticular pattern.6 Palmoplantar keratoderma represents a group of acquired and hereditary conditions that are characterized by excessive thickening of the palms and soles.5 Palmoplantar psoriasis is a variant of psoriasis that affects the palms and soles and can manifest as hyperkeratosis, pustular, or mixed morphology.7 Lichen amyloidosis is a subtype of primary localized cutaneous amyloidosis that manifests as multiple pruritic, firm, hyperpigmented, hyperkeratotic papules on the shins that later coalesce in a rippled pattern.8,9
The first-line treatment for hypertrophic LP is topical corticosteroids. Alternative therapies include mycophenolate mofetil, acitretin, and intralesional corticosteroid injections.4 Treatment is similar for all of the LP variants.
- Arnold DL, Krishnamurthy K. Lichen planus. In: StatPearls. StatPearls Publishing; 2022.
- Namazi MR, Bahmani M. Diagnosis: hypertrophic lichen planus. Ann Saudi Med. 2008;28:1-2. doi:10.5144/0256-4947.2008.222
- Riahi RR, Cohen PR. Hypertrophic lichen planus mimicking verrucous lupus erythematosus. Cureus. 2018;10:e3555. doi:10.7759 /cureus.3555
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-149. doi:10.1016/j .ijwd.2015.04.001
- Has C, Technau-Hafsi K. Palmoplantar keratodermas: clinical and genetic aspects. J Dtsch Dermatol Ges. 2016;14:123-139; quiz 140. doi:10.1111/ddg.12930
- Konstantinov KN, Søndergaard J, Izuno G, et al. Keratosis lichenoides chronica. J Am Acad Dermatol. 1998;38(2 Pt 2):306-309. doi:10.1016 /s0190-9622(98)70570-5
- Miceli A, Schmieder GJ. Palmoplantar psoriasis. In: StatPearls. StatPearls Publishing; 2023.
- Tay CH, Dacosta JL. Lichen amyloidosis—clinical study of 40 cases. Br J Dermatol. 1970;82:129-136.
- Salim T, Shenoi SD, Balachandran C, et al. Lichen amyloidosis: a study of clinical, histopathologic and immunofluorescence findings in 30 cases. Indian J Dermatol Venereol Leprol. 2005;71:166-169.
The Diagnosis: Hypertrophic Lichen Planus
Two biopsies from the left lateral foot revealed hyperkeratosis, wedge-shaped hypergranulosis, irregular acanthosis, and a bandlike lymphocytic infiltrate in the superficial dermis with a classic sawtooth pattern of the rete ridges (Figure 1). Based on the clinical findings and histopathology, the patient was diagnosed with hypertrophic lichen planus (LP) and was treated with clobetasol ointment 0.05%, which resulted in progression of the symptoms. She experienced notable improvement 3 months after adding methotrexate 12.5 mg weekly (Figure 2).

Lichen planus is an idiopathic chronic inflammatory condition of the skin and mucous membranes that classically manifests as pruritic violaceous papules and plaques, which commonly are found on the wrists, lower back, and ankles.1 The most common variants of LP are hypertrophic, linear, mucosal, actinic, follicular, pigmented, annular, atrophic, and guttate.2 The clinical presentation and biopsy results in our patient were consistent with the hypertrophic variant of LP, which is a chronic condition that most often manifests on the lower legs, especially around the ankles, as hyperkeratotic papules, plaques, and nodules.2,3 The exact pathophysiology of hypertrophic LP is unknown, but there is evidence that the immune system plays a role in its development and that the Koebner phenomenon may contribute to its exacerbation.4 There is a well-known association between LP and hepatitis. Patients with chronic LP may develop squamous cell carcinoma.4 The variants of LP can overlap and do not exist independent of one another. Recognizing the overlap in these variants allows for earlier diagnosis and therapeutic intervention of the disease process to limit disease progression and patient clinic visits and to improve patient quality of life.

The differential diagnosis for hyperkeratotic plaques of the feet and ankles can be broad and may include keratosis lichenoides chronica, palmoplantar keratoderma, palmoplantar psoriasis, or lichen amyloidosis. These conditions are classified based on various criteria that include extent of disease manifestations, morphology of palmoplantar skin involvement, inheritance patterns, and molecular pathogenesis.5 Keratosis lichenoides chronica is a rare dermatosis that presents as a distinctive seborrheic dermatitis–like facial eruption. The facial eruption is accompanied by violaceous papular and nodular lesions that appear on the extremities and trunk, typically arranged in a linear or reticular pattern.6 Palmoplantar keratoderma represents a group of acquired and hereditary conditions that are characterized by excessive thickening of the palms and soles.5 Palmoplantar psoriasis is a variant of psoriasis that affects the palms and soles and can manifest as hyperkeratosis, pustular, or mixed morphology.7 Lichen amyloidosis is a subtype of primary localized cutaneous amyloidosis that manifests as multiple pruritic, firm, hyperpigmented, hyperkeratotic papules on the shins that later coalesce in a rippled pattern.8,9
The first-line treatment for hypertrophic LP is topical corticosteroids. Alternative therapies include mycophenolate mofetil, acitretin, and intralesional corticosteroid injections.4 Treatment is similar for all of the LP variants.
The Diagnosis: Hypertrophic Lichen Planus
Two biopsies from the left lateral foot revealed hyperkeratosis, wedge-shaped hypergranulosis, irregular acanthosis, and a bandlike lymphocytic infiltrate in the superficial dermis with a classic sawtooth pattern of the rete ridges (Figure 1). Based on the clinical findings and histopathology, the patient was diagnosed with hypertrophic lichen planus (LP) and was treated with clobetasol ointment 0.05%, which resulted in progression of the symptoms. She experienced notable improvement 3 months after adding methotrexate 12.5 mg weekly (Figure 2).

Lichen planus is an idiopathic chronic inflammatory condition of the skin and mucous membranes that classically manifests as pruritic violaceous papules and plaques, which commonly are found on the wrists, lower back, and ankles.1 The most common variants of LP are hypertrophic, linear, mucosal, actinic, follicular, pigmented, annular, atrophic, and guttate.2 The clinical presentation and biopsy results in our patient were consistent with the hypertrophic variant of LP, which is a chronic condition that most often manifests on the lower legs, especially around the ankles, as hyperkeratotic papules, plaques, and nodules.2,3 The exact pathophysiology of hypertrophic LP is unknown, but there is evidence that the immune system plays a role in its development and that the Koebner phenomenon may contribute to its exacerbation.4 There is a well-known association between LP and hepatitis. Patients with chronic LP may develop squamous cell carcinoma.4 The variants of LP can overlap and do not exist independent of one another. Recognizing the overlap in these variants allows for earlier diagnosis and therapeutic intervention of the disease process to limit disease progression and patient clinic visits and to improve patient quality of life.

The differential diagnosis for hyperkeratotic plaques of the feet and ankles can be broad and may include keratosis lichenoides chronica, palmoplantar keratoderma, palmoplantar psoriasis, or lichen amyloidosis. These conditions are classified based on various criteria that include extent of disease manifestations, morphology of palmoplantar skin involvement, inheritance patterns, and molecular pathogenesis.5 Keratosis lichenoides chronica is a rare dermatosis that presents as a distinctive seborrheic dermatitis–like facial eruption. The facial eruption is accompanied by violaceous papular and nodular lesions that appear on the extremities and trunk, typically arranged in a linear or reticular pattern.6 Palmoplantar keratoderma represents a group of acquired and hereditary conditions that are characterized by excessive thickening of the palms and soles.5 Palmoplantar psoriasis is a variant of psoriasis that affects the palms and soles and can manifest as hyperkeratosis, pustular, or mixed morphology.7 Lichen amyloidosis is a subtype of primary localized cutaneous amyloidosis that manifests as multiple pruritic, firm, hyperpigmented, hyperkeratotic papules on the shins that later coalesce in a rippled pattern.8,9
The first-line treatment for hypertrophic LP is topical corticosteroids. Alternative therapies include mycophenolate mofetil, acitretin, and intralesional corticosteroid injections.4 Treatment is similar for all of the LP variants.
- Arnold DL, Krishnamurthy K. Lichen planus. In: StatPearls. StatPearls Publishing; 2022.
- Namazi MR, Bahmani M. Diagnosis: hypertrophic lichen planus. Ann Saudi Med. 2008;28:1-2. doi:10.5144/0256-4947.2008.222
- Riahi RR, Cohen PR. Hypertrophic lichen planus mimicking verrucous lupus erythematosus. Cureus. 2018;10:e3555. doi:10.7759 /cureus.3555
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-149. doi:10.1016/j .ijwd.2015.04.001
- Has C, Technau-Hafsi K. Palmoplantar keratodermas: clinical and genetic aspects. J Dtsch Dermatol Ges. 2016;14:123-139; quiz 140. doi:10.1111/ddg.12930
- Konstantinov KN, Søndergaard J, Izuno G, et al. Keratosis lichenoides chronica. J Am Acad Dermatol. 1998;38(2 Pt 2):306-309. doi:10.1016 /s0190-9622(98)70570-5
- Miceli A, Schmieder GJ. Palmoplantar psoriasis. In: StatPearls. StatPearls Publishing; 2023.
- Tay CH, Dacosta JL. Lichen amyloidosis—clinical study of 40 cases. Br J Dermatol. 1970;82:129-136.
- Salim T, Shenoi SD, Balachandran C, et al. Lichen amyloidosis: a study of clinical, histopathologic and immunofluorescence findings in 30 cases. Indian J Dermatol Venereol Leprol. 2005;71:166-169.
- Arnold DL, Krishnamurthy K. Lichen planus. In: StatPearls. StatPearls Publishing; 2022.
- Namazi MR, Bahmani M. Diagnosis: hypertrophic lichen planus. Ann Saudi Med. 2008;28:1-2. doi:10.5144/0256-4947.2008.222
- Riahi RR, Cohen PR. Hypertrophic lichen planus mimicking verrucous lupus erythematosus. Cureus. 2018;10:e3555. doi:10.7759 /cureus.3555
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-149. doi:10.1016/j .ijwd.2015.04.001
- Has C, Technau-Hafsi K. Palmoplantar keratodermas: clinical and genetic aspects. J Dtsch Dermatol Ges. 2016;14:123-139; quiz 140. doi:10.1111/ddg.12930
- Konstantinov KN, Søndergaard J, Izuno G, et al. Keratosis lichenoides chronica. J Am Acad Dermatol. 1998;38(2 Pt 2):306-309. doi:10.1016 /s0190-9622(98)70570-5
- Miceli A, Schmieder GJ. Palmoplantar psoriasis. In: StatPearls. StatPearls Publishing; 2023.
- Tay CH, Dacosta JL. Lichen amyloidosis—clinical study of 40 cases. Br J Dermatol. 1970;82:129-136.
- Salim T, Shenoi SD, Balachandran C, et al. Lichen amyloidosis: a study of clinical, histopathologic and immunofluorescence findings in 30 cases. Indian J Dermatol Venereol Leprol. 2005;71:166-169.
An 83-year-old woman presented for evaluation of hyperkeratotic plaques on the medial and lateral aspects of the left heel (top). Physical examination also revealed onychodystrophy of the toenails on the halluces (bottom). A crusted friable plaque on the lower lip and white plaques with peripheral reticulation and erosions on the buccal mucosa also were present. The patient had a history of nummular eczema, stasis dermatitis, and hand dermatitis. She denied a history of cold sores.
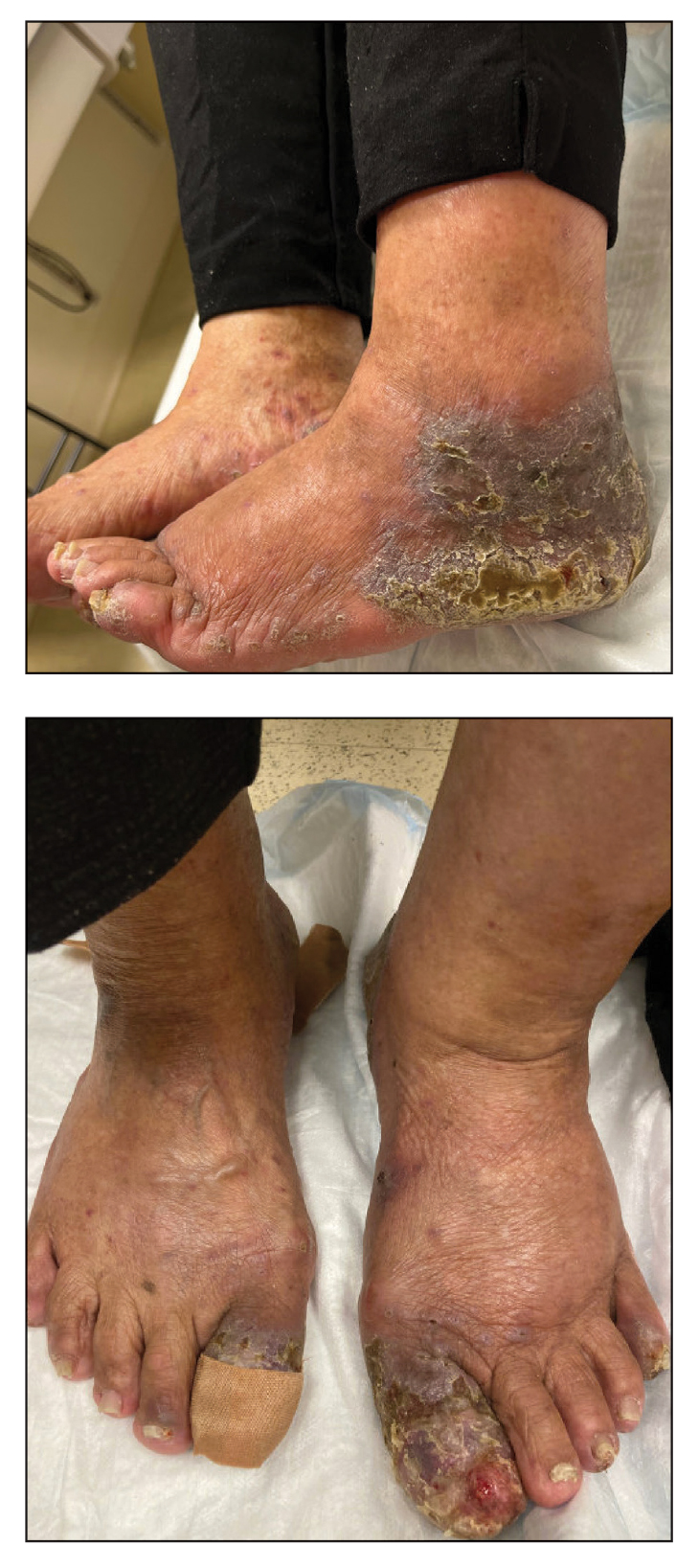
Approval of Spesolimab for Generalized Pustular Psoriasis Expanded
The in adults and in pediatric patients aged ≥ 12 years who weigh ≥ 40 kg, according to an announcement from the manufacturer.
This is an expanded indication for spesolimab-sbzo, first approved in September 2022 for treating GPP flares. Developed by Boehringer Ingelheim and marketed under the name Spevigo, the product is an injectable antibody that blocks the IL-36 receptor, a key part of the pathway shown to be involved in the cause of GPP, which is rare and is a potentially-life-threatening disease.
According to a press release from the company, the FDA’s approval of the expanded indication was based on the results of a 48-week clinical trial of 123 patients (Effisayil 2), which showed that individuals who received spesolimab experienced a significant 84% reduction in GPP flares compared with those who received placebo. Among 30 study participants who received a high treatment dose, no flares were observed after week 4. Among all patients who received spesolimab-sbzo, treatment was associated with an increased incidence (defined as ≥ 9 cases per 100 patient-years) of injection site reactions, urinary tract infections, arthralgia, and pruritus compared with placebo.
Spesolimab-sbzo is currently available in 48 countries, according to the Boehringer Ingelheim release, which states that the approval makes it the first targeted therapy that is available for the acute and chronic treatment of patients with GPP.
A version of this article appeared on Medscape.com.
The in adults and in pediatric patients aged ≥ 12 years who weigh ≥ 40 kg, according to an announcement from the manufacturer.
This is an expanded indication for spesolimab-sbzo, first approved in September 2022 for treating GPP flares. Developed by Boehringer Ingelheim and marketed under the name Spevigo, the product is an injectable antibody that blocks the IL-36 receptor, a key part of the pathway shown to be involved in the cause of GPP, which is rare and is a potentially-life-threatening disease.
According to a press release from the company, the FDA’s approval of the expanded indication was based on the results of a 48-week clinical trial of 123 patients (Effisayil 2), which showed that individuals who received spesolimab experienced a significant 84% reduction in GPP flares compared with those who received placebo. Among 30 study participants who received a high treatment dose, no flares were observed after week 4. Among all patients who received spesolimab-sbzo, treatment was associated with an increased incidence (defined as ≥ 9 cases per 100 patient-years) of injection site reactions, urinary tract infections, arthralgia, and pruritus compared with placebo.
Spesolimab-sbzo is currently available in 48 countries, according to the Boehringer Ingelheim release, which states that the approval makes it the first targeted therapy that is available for the acute and chronic treatment of patients with GPP.
A version of this article appeared on Medscape.com.
The in adults and in pediatric patients aged ≥ 12 years who weigh ≥ 40 kg, according to an announcement from the manufacturer.
This is an expanded indication for spesolimab-sbzo, first approved in September 2022 for treating GPP flares. Developed by Boehringer Ingelheim and marketed under the name Spevigo, the product is an injectable antibody that blocks the IL-36 receptor, a key part of the pathway shown to be involved in the cause of GPP, which is rare and is a potentially-life-threatening disease.
According to a press release from the company, the FDA’s approval of the expanded indication was based on the results of a 48-week clinical trial of 123 patients (Effisayil 2), which showed that individuals who received spesolimab experienced a significant 84% reduction in GPP flares compared with those who received placebo. Among 30 study participants who received a high treatment dose, no flares were observed after week 4. Among all patients who received spesolimab-sbzo, treatment was associated with an increased incidence (defined as ≥ 9 cases per 100 patient-years) of injection site reactions, urinary tract infections, arthralgia, and pruritus compared with placebo.
Spesolimab-sbzo is currently available in 48 countries, according to the Boehringer Ingelheim release, which states that the approval makes it the first targeted therapy that is available for the acute and chronic treatment of patients with GPP.
A version of this article appeared on Medscape.com.
LITE Study Provides Encouraging Data on Home-Based Phototherapy for Psoriasis
SAN DIEGO — and Dermatology Life Quality Index (DLQI) scores, results from a pragmatic, multicenter study showed.
“In 2024, we have a lot of ways to treat moderate-to-severe psoriasis, and phototherapy remains relevant,” lead investigator Joel M. Gelfand, MD, professor of dermatology and epidemiology at the University of Pennsylvania in Philadelphia, told attendees of a late-breaking abstract session at the annual meeting of the American Academy of Dermatology.
“Office phototherapy is 10 to 100 times less expensive than biologics for psoriasis, and in head-to-head trials, it’s about as effective as adalimumab and achieves better patient-reported outcomes. It may have some cardiovascular benefits by lowering IL-6 and improving HDL-P,” he said. “And, compared to secukinumab, it has no risk of infection.”
Although phototherapy is a preferred as a treatment by patients with psoriasis, he continued, inconvenience of traveling to a clinician’s office for the treatment and lack of coverage by health insurance plans remain major barriers to this option. According to Dr. Gelfand, office-based phototherapy is not available in 90% of counties in the United States, “and a lack of US data has resulted in many insurance companies not covering home phototherapy. As a result, many providers are uncertain about prescribing it.”
LITE Study Data
In 2019, Dr. Gelfand and colleagues Light Treatment Effectiveness (LITE) study, a patient-centered study that tested the hypothesis that narrowband UVB phototherapy of psoriasis at home is non-inferior to office treatment, based on outcomes that matter to patients, clinicians, and payers. The co-primary outcomes were a PGA score of 0/1 (clear, almost clear) and a DLQI score of 5 or less (small, no effect on health-related quality of life).
Dr. Gelfand and colleagues at 42 sites in the United States enrolled 783 patients aged 12 years and older who had plaque or guttate psoriasis and were candidates for phototherapy at home or in an office setting. New or established patients to the practices were accepted into the trial, while those treated with phototherapy within 14 days before the baseline visit were not. These entry criteria “are highly pragmatic and reflect routine clinical practice,” he said.
The researchers evenly stratified patients by skin types I and II, III and IV, and V and VI. They collected data from medical records or from an app on the patient’s cell phone, which captured the DLQI data. Study participants were randomly assigned 1:1 to office- or home-based phototherapy for 12 weeks at doses recommended in the 2019 AAD-National Psoriasis Foundation guidelines. This was followed by a 12-week observation period, which ended at 24 weeks.
At baseline, the mean DLQI score of patients was 12.2, the mean PGA score was 3, and their mean body surface area affected was 12.5%. “These patients had pretty severe disease, long-standing disease, and about 12% were on biologics or nonbiologic systemic therapy during the study,” said Dr. Gelfand, also the director of the Psoriasis and Phototherapy Treatment Center at Penn. In addition, he said, “the average round-trip to receive phototherapy in the office was about 60 minutes.”
An Improvement in Health Equity
Following treatment at 12 weeks, 25.6% of patients in the office-based phototherapy group achieved a PGA of 0/1, compared with 32.8% of patients in the home-based phototherapy group (P >.0001 for non-inferiority). Similarly, 33.6% of patients in the office-based phototherapy group achieved a score of 5 or less on the DLQI, compared with 52.4% of patients in the home-based phototherapy group (P >.0001 for non-inferiority).
In subgroup analyses, patients with darkly pigmented skin did especially well on home phototherapy relative to office treatment. “This finding is an example of how the LITE study was specifically designed to improve health equity through an intentionally inclusive approach,” Dr. Gelfand said. Perhaps not surprisingly, patients in the home-based phototherapy arm were more adherent to treatment compared with those in the office-based arm (a mean of 26.8 sessions during the study period, compared with a mean of 17.9, respectively; P < .0001). “They also had higher cumulative doses of phototherapy and therefore higher episodes of treatments with erythema,” he noted.
Among patients who reported “itchy, sore, painful, or stinging” skin in the previous week, 63% characterized the degree of discomfort as “not at all or a little,” while 28% said “a lot,” and 9% said “very much.” No patients withdrew or stopped phototherapy during the trial because of treatment-related side effects, “so it’s very well tolerated,” Dr. Gelfand said.
“If a patient never had phototherapy before, they did just as well at home as they did in the office. This suggests that there’s no reason to insist that a patient use office-based phototherapy before using home phototherapy.”
The researchers studied the efficacy of narrow-band UVB in patients who had at least two treatments per week for 12 weeks. In this subgroup of patients, 60% achieved clear or almost clear skin and nearly 50% achieved the equivalent of a Psoriasis Area and Severity Index (PASI) 90 score.
“Home phototherapy is clearly non-inferior to office-based phototherapy across all skin types and both primary outcomes, PGA and DLQI, and both have excellent effectiveness and safety in real-world settings,” Dr. Gelfand concluded. “These data support the use of home phototherapy as a first-line treatment option for psoriasis, including those with no prior phototherapy experience.”
LITE Study Described as “Groundbreaking”
One of the session moderators, dermatologist Andrew Blauvelt, MD, MBA, of the Oregon Medical Research Center, Portland, asked about the impact that lockdowns during the early phase of the COVID-19 pandemic had on the trial. “The study shut down for a couple weeks during the initial lockdown, but we got back up and running pretty quickly,” Dr. Gelfand responded. “We didn’t study that specific period of time, but the study was going on well before COVID and well after COVID restrictions were lifted. We’ll have to analyze that period of time you question but I suspect that it’s not driving the results we see.”
Asked to comment, Henry W. Lim, MD, a dermatologist with Henry Ford Health in Detroit, characterized the findings of the study as “groundbreaking, because it looked at a real-life situation in the use of phototherapy at home vs in the office, showing that the home phototherapy is not inferior to office-based phototherapy.”
This is important, he continued, “because it can inform payers to approve home phototherapy equipment for patients, because it’s much more convenient and it definitely works. The other strong point of the study is that it included patients of different skin types,” he said in an interview at the meeting.
The study was funded by the Patient-Centered Outcomes Research Institute. Research partners included the National Psoriasis Foundation and Daavlin, which provided the home phototherapy machines and covered the cost of shipping the devices. Dr. Gelfand reported no relevant financial relationships. Dr. Blauvelt disclosed conflicts of interest from many pharmaceutical companies. Dr. Lim disclosed conflicts of interest from many pharmaceutical companies.
A version of this article appeared on Medscape.com.
SAN DIEGO — and Dermatology Life Quality Index (DLQI) scores, results from a pragmatic, multicenter study showed.
“In 2024, we have a lot of ways to treat moderate-to-severe psoriasis, and phototherapy remains relevant,” lead investigator Joel M. Gelfand, MD, professor of dermatology and epidemiology at the University of Pennsylvania in Philadelphia, told attendees of a late-breaking abstract session at the annual meeting of the American Academy of Dermatology.
“Office phototherapy is 10 to 100 times less expensive than biologics for psoriasis, and in head-to-head trials, it’s about as effective as adalimumab and achieves better patient-reported outcomes. It may have some cardiovascular benefits by lowering IL-6 and improving HDL-P,” he said. “And, compared to secukinumab, it has no risk of infection.”
Although phototherapy is a preferred as a treatment by patients with psoriasis, he continued, inconvenience of traveling to a clinician’s office for the treatment and lack of coverage by health insurance plans remain major barriers to this option. According to Dr. Gelfand, office-based phototherapy is not available in 90% of counties in the United States, “and a lack of US data has resulted in many insurance companies not covering home phototherapy. As a result, many providers are uncertain about prescribing it.”
LITE Study Data
In 2019, Dr. Gelfand and colleagues Light Treatment Effectiveness (LITE) study, a patient-centered study that tested the hypothesis that narrowband UVB phototherapy of psoriasis at home is non-inferior to office treatment, based on outcomes that matter to patients, clinicians, and payers. The co-primary outcomes were a PGA score of 0/1 (clear, almost clear) and a DLQI score of 5 or less (small, no effect on health-related quality of life).
Dr. Gelfand and colleagues at 42 sites in the United States enrolled 783 patients aged 12 years and older who had plaque or guttate psoriasis and were candidates for phototherapy at home or in an office setting. New or established patients to the practices were accepted into the trial, while those treated with phototherapy within 14 days before the baseline visit were not. These entry criteria “are highly pragmatic and reflect routine clinical practice,” he said.
The researchers evenly stratified patients by skin types I and II, III and IV, and V and VI. They collected data from medical records or from an app on the patient’s cell phone, which captured the DLQI data. Study participants were randomly assigned 1:1 to office- or home-based phototherapy for 12 weeks at doses recommended in the 2019 AAD-National Psoriasis Foundation guidelines. This was followed by a 12-week observation period, which ended at 24 weeks.
At baseline, the mean DLQI score of patients was 12.2, the mean PGA score was 3, and their mean body surface area affected was 12.5%. “These patients had pretty severe disease, long-standing disease, and about 12% were on biologics or nonbiologic systemic therapy during the study,” said Dr. Gelfand, also the director of the Psoriasis and Phototherapy Treatment Center at Penn. In addition, he said, “the average round-trip to receive phototherapy in the office was about 60 minutes.”
An Improvement in Health Equity
Following treatment at 12 weeks, 25.6% of patients in the office-based phototherapy group achieved a PGA of 0/1, compared with 32.8% of patients in the home-based phototherapy group (P >.0001 for non-inferiority). Similarly, 33.6% of patients in the office-based phototherapy group achieved a score of 5 or less on the DLQI, compared with 52.4% of patients in the home-based phototherapy group (P >.0001 for non-inferiority).
In subgroup analyses, patients with darkly pigmented skin did especially well on home phototherapy relative to office treatment. “This finding is an example of how the LITE study was specifically designed to improve health equity through an intentionally inclusive approach,” Dr. Gelfand said. Perhaps not surprisingly, patients in the home-based phototherapy arm were more adherent to treatment compared with those in the office-based arm (a mean of 26.8 sessions during the study period, compared with a mean of 17.9, respectively; P < .0001). “They also had higher cumulative doses of phototherapy and therefore higher episodes of treatments with erythema,” he noted.
Among patients who reported “itchy, sore, painful, or stinging” skin in the previous week, 63% characterized the degree of discomfort as “not at all or a little,” while 28% said “a lot,” and 9% said “very much.” No patients withdrew or stopped phototherapy during the trial because of treatment-related side effects, “so it’s very well tolerated,” Dr. Gelfand said.
“If a patient never had phototherapy before, they did just as well at home as they did in the office. This suggests that there’s no reason to insist that a patient use office-based phototherapy before using home phototherapy.”
The researchers studied the efficacy of narrow-band UVB in patients who had at least two treatments per week for 12 weeks. In this subgroup of patients, 60% achieved clear or almost clear skin and nearly 50% achieved the equivalent of a Psoriasis Area and Severity Index (PASI) 90 score.
“Home phototherapy is clearly non-inferior to office-based phototherapy across all skin types and both primary outcomes, PGA and DLQI, and both have excellent effectiveness and safety in real-world settings,” Dr. Gelfand concluded. “These data support the use of home phototherapy as a first-line treatment option for psoriasis, including those with no prior phototherapy experience.”
LITE Study Described as “Groundbreaking”
One of the session moderators, dermatologist Andrew Blauvelt, MD, MBA, of the Oregon Medical Research Center, Portland, asked about the impact that lockdowns during the early phase of the COVID-19 pandemic had on the trial. “The study shut down for a couple weeks during the initial lockdown, but we got back up and running pretty quickly,” Dr. Gelfand responded. “We didn’t study that specific period of time, but the study was going on well before COVID and well after COVID restrictions were lifted. We’ll have to analyze that period of time you question but I suspect that it’s not driving the results we see.”
Asked to comment, Henry W. Lim, MD, a dermatologist with Henry Ford Health in Detroit, characterized the findings of the study as “groundbreaking, because it looked at a real-life situation in the use of phototherapy at home vs in the office, showing that the home phototherapy is not inferior to office-based phototherapy.”
This is important, he continued, “because it can inform payers to approve home phototherapy equipment for patients, because it’s much more convenient and it definitely works. The other strong point of the study is that it included patients of different skin types,” he said in an interview at the meeting.
The study was funded by the Patient-Centered Outcomes Research Institute. Research partners included the National Psoriasis Foundation and Daavlin, which provided the home phototherapy machines and covered the cost of shipping the devices. Dr. Gelfand reported no relevant financial relationships. Dr. Blauvelt disclosed conflicts of interest from many pharmaceutical companies. Dr. Lim disclosed conflicts of interest from many pharmaceutical companies.
A version of this article appeared on Medscape.com.
SAN DIEGO — and Dermatology Life Quality Index (DLQI) scores, results from a pragmatic, multicenter study showed.
“In 2024, we have a lot of ways to treat moderate-to-severe psoriasis, and phototherapy remains relevant,” lead investigator Joel M. Gelfand, MD, professor of dermatology and epidemiology at the University of Pennsylvania in Philadelphia, told attendees of a late-breaking abstract session at the annual meeting of the American Academy of Dermatology.
“Office phototherapy is 10 to 100 times less expensive than biologics for psoriasis, and in head-to-head trials, it’s about as effective as adalimumab and achieves better patient-reported outcomes. It may have some cardiovascular benefits by lowering IL-6 and improving HDL-P,” he said. “And, compared to secukinumab, it has no risk of infection.”
Although phototherapy is a preferred as a treatment by patients with psoriasis, he continued, inconvenience of traveling to a clinician’s office for the treatment and lack of coverage by health insurance plans remain major barriers to this option. According to Dr. Gelfand, office-based phototherapy is not available in 90% of counties in the United States, “and a lack of US data has resulted in many insurance companies not covering home phototherapy. As a result, many providers are uncertain about prescribing it.”
LITE Study Data
In 2019, Dr. Gelfand and colleagues Light Treatment Effectiveness (LITE) study, a patient-centered study that tested the hypothesis that narrowband UVB phototherapy of psoriasis at home is non-inferior to office treatment, based on outcomes that matter to patients, clinicians, and payers. The co-primary outcomes were a PGA score of 0/1 (clear, almost clear) and a DLQI score of 5 or less (small, no effect on health-related quality of life).
Dr. Gelfand and colleagues at 42 sites in the United States enrolled 783 patients aged 12 years and older who had plaque or guttate psoriasis and were candidates for phototherapy at home or in an office setting. New or established patients to the practices were accepted into the trial, while those treated with phototherapy within 14 days before the baseline visit were not. These entry criteria “are highly pragmatic and reflect routine clinical practice,” he said.
The researchers evenly stratified patients by skin types I and II, III and IV, and V and VI. They collected data from medical records or from an app on the patient’s cell phone, which captured the DLQI data. Study participants were randomly assigned 1:1 to office- or home-based phototherapy for 12 weeks at doses recommended in the 2019 AAD-National Psoriasis Foundation guidelines. This was followed by a 12-week observation period, which ended at 24 weeks.
At baseline, the mean DLQI score of patients was 12.2, the mean PGA score was 3, and their mean body surface area affected was 12.5%. “These patients had pretty severe disease, long-standing disease, and about 12% were on biologics or nonbiologic systemic therapy during the study,” said Dr. Gelfand, also the director of the Psoriasis and Phototherapy Treatment Center at Penn. In addition, he said, “the average round-trip to receive phototherapy in the office was about 60 minutes.”
An Improvement in Health Equity
Following treatment at 12 weeks, 25.6% of patients in the office-based phototherapy group achieved a PGA of 0/1, compared with 32.8% of patients in the home-based phototherapy group (P >.0001 for non-inferiority). Similarly, 33.6% of patients in the office-based phototherapy group achieved a score of 5 or less on the DLQI, compared with 52.4% of patients in the home-based phototherapy group (P >.0001 for non-inferiority).
In subgroup analyses, patients with darkly pigmented skin did especially well on home phototherapy relative to office treatment. “This finding is an example of how the LITE study was specifically designed to improve health equity through an intentionally inclusive approach,” Dr. Gelfand said. Perhaps not surprisingly, patients in the home-based phototherapy arm were more adherent to treatment compared with those in the office-based arm (a mean of 26.8 sessions during the study period, compared with a mean of 17.9, respectively; P < .0001). “They also had higher cumulative doses of phototherapy and therefore higher episodes of treatments with erythema,” he noted.
Among patients who reported “itchy, sore, painful, or stinging” skin in the previous week, 63% characterized the degree of discomfort as “not at all or a little,” while 28% said “a lot,” and 9% said “very much.” No patients withdrew or stopped phototherapy during the trial because of treatment-related side effects, “so it’s very well tolerated,” Dr. Gelfand said.
“If a patient never had phototherapy before, they did just as well at home as they did in the office. This suggests that there’s no reason to insist that a patient use office-based phototherapy before using home phototherapy.”
The researchers studied the efficacy of narrow-band UVB in patients who had at least two treatments per week for 12 weeks. In this subgroup of patients, 60% achieved clear or almost clear skin and nearly 50% achieved the equivalent of a Psoriasis Area and Severity Index (PASI) 90 score.
“Home phototherapy is clearly non-inferior to office-based phototherapy across all skin types and both primary outcomes, PGA and DLQI, and both have excellent effectiveness and safety in real-world settings,” Dr. Gelfand concluded. “These data support the use of home phototherapy as a first-line treatment option for psoriasis, including those with no prior phototherapy experience.”
LITE Study Described as “Groundbreaking”
One of the session moderators, dermatologist Andrew Blauvelt, MD, MBA, of the Oregon Medical Research Center, Portland, asked about the impact that lockdowns during the early phase of the COVID-19 pandemic had on the trial. “The study shut down for a couple weeks during the initial lockdown, but we got back up and running pretty quickly,” Dr. Gelfand responded. “We didn’t study that specific period of time, but the study was going on well before COVID and well after COVID restrictions were lifted. We’ll have to analyze that period of time you question but I suspect that it’s not driving the results we see.”
Asked to comment, Henry W. Lim, MD, a dermatologist with Henry Ford Health in Detroit, characterized the findings of the study as “groundbreaking, because it looked at a real-life situation in the use of phototherapy at home vs in the office, showing that the home phototherapy is not inferior to office-based phototherapy.”
This is important, he continued, “because it can inform payers to approve home phototherapy equipment for patients, because it’s much more convenient and it definitely works. The other strong point of the study is that it included patients of different skin types,” he said in an interview at the meeting.
The study was funded by the Patient-Centered Outcomes Research Institute. Research partners included the National Psoriasis Foundation and Daavlin, which provided the home phototherapy machines and covered the cost of shipping the devices. Dr. Gelfand reported no relevant financial relationships. Dr. Blauvelt disclosed conflicts of interest from many pharmaceutical companies. Dr. Lim disclosed conflicts of interest from many pharmaceutical companies.
A version of this article appeared on Medscape.com.
FROM AAD 2024