User login
FDA: No oral ketoconazole for skin, nail fungus
The Food and Drug Administration is warning health care professionals not to prescribe oral ketoconazole for patients with fungal infections of the skin and nails, because of "the risks of serious liver damage, adrenal gland problems, and harmful interactions with other medicines that outweigh its benefit in treating these conditions."
The advisory, issued on May 19, points out that oral ketoconazole (Nizoral) is no longer approved for treating nail or skin fungal infections. Topical forms of ketoconazole have not been associated with liver damage, adrenal problems, or drug interactions, the advisory adds.
"Health care professionals should use ketoconazole tablets only to treat serious fungal infections when no other antifungal therapies are available," according to the FDA. "Skin and nail fungal infections in otherwise healthy persons are not life-threatening, and so the risks associated with oral ketoconazole outweigh the benefits. Other treatment options are available over-the-counter and by prescription, but are also associated with risks that should be weighed against their benefits."
The advisory updates one issued in July 2013 when the drug's label was changed to reflect these safety concerns, including dropping the nail and skin infections from the approved indications. Since then, the FDA has received one report of a patient who died of liver failure associated with oral ketoconazole used to treat nail fungus. Furthermore, a survey of office-based physicians found that in the 18 months ending in June 2015, "skin and nail fungal infections were the only diagnoses cited for the use of oral ketoconazole."
Serious adverse events associated with oral ketoconazole should be reported to the FDA's MedWatch program online or call 800-332-1088.
The Food and Drug Administration is warning health care professionals not to prescribe oral ketoconazole for patients with fungal infections of the skin and nails, because of "the risks of serious liver damage, adrenal gland problems, and harmful interactions with other medicines that outweigh its benefit in treating these conditions."
The advisory, issued on May 19, points out that oral ketoconazole (Nizoral) is no longer approved for treating nail or skin fungal infections. Topical forms of ketoconazole have not been associated with liver damage, adrenal problems, or drug interactions, the advisory adds.
"Health care professionals should use ketoconazole tablets only to treat serious fungal infections when no other antifungal therapies are available," according to the FDA. "Skin and nail fungal infections in otherwise healthy persons are not life-threatening, and so the risks associated with oral ketoconazole outweigh the benefits. Other treatment options are available over-the-counter and by prescription, but are also associated with risks that should be weighed against their benefits."
The advisory updates one issued in July 2013 when the drug's label was changed to reflect these safety concerns, including dropping the nail and skin infections from the approved indications. Since then, the FDA has received one report of a patient who died of liver failure associated with oral ketoconazole used to treat nail fungus. Furthermore, a survey of office-based physicians found that in the 18 months ending in June 2015, "skin and nail fungal infections were the only diagnoses cited for the use of oral ketoconazole."
Serious adverse events associated with oral ketoconazole should be reported to the FDA's MedWatch program online or call 800-332-1088.
The Food and Drug Administration is warning health care professionals not to prescribe oral ketoconazole for patients with fungal infections of the skin and nails, because of "the risks of serious liver damage, adrenal gland problems, and harmful interactions with other medicines that outweigh its benefit in treating these conditions."
The advisory, issued on May 19, points out that oral ketoconazole (Nizoral) is no longer approved for treating nail or skin fungal infections. Topical forms of ketoconazole have not been associated with liver damage, adrenal problems, or drug interactions, the advisory adds.
"Health care professionals should use ketoconazole tablets only to treat serious fungal infections when no other antifungal therapies are available," according to the FDA. "Skin and nail fungal infections in otherwise healthy persons are not life-threatening, and so the risks associated with oral ketoconazole outweigh the benefits. Other treatment options are available over-the-counter and by prescription, but are also associated with risks that should be weighed against their benefits."
The advisory updates one issued in July 2013 when the drug's label was changed to reflect these safety concerns, including dropping the nail and skin infections from the approved indications. Since then, the FDA has received one report of a patient who died of liver failure associated with oral ketoconazole used to treat nail fungus. Furthermore, a survey of office-based physicians found that in the 18 months ending in June 2015, "skin and nail fungal infections were the only diagnoses cited for the use of oral ketoconazole."
Serious adverse events associated with oral ketoconazole should be reported to the FDA's MedWatch program online or call 800-332-1088.
Fungi may exacerbate asthma, chronic sinusitis
LOS ANGELES – Fungi might play a far larger role in asthma and chronic sinusitis than previously thought, according to investigators at Baylor College of Medicine in Houston.
With the help of a special culturing technique to wash antifungal elements out of sputum samples, six or more fungal colony-forming units grew out of the sputum of 112 of 134 patients (83.5%) at the Houston Veterans Affairs Medical Center; about a third of the patients had asthma, a third had chronic sinusitis, and a third had both. Although Aspergillus and Candida species were common, more than 30 fungal species were identified. Only a handful of patients had positive results on IgE testing.
Of 62 patients treated with standard-dose voriconazole or terbinafine, sometimes for more than a year, 54 (87%) reported symptomatic benefit including 31 (50%) with decreased sputum production, 24 (39%) with improved breathing, 20 (32%) with less cough, and nine (14.5%) with less rescue inhaler use.
At Baylor, prescribing antifungals for patients with recalcitrant asthma and chronic sinusitis “has evolved into something we pretty much do all the time now regardless of sensitivity results. I’m pretty certain we are the only institution that does this,” said allergy and immunology fellow Dr. Evan Li.
“Fungi, we think, are important initiating factors in many cases of asthma. They set up chronic mucosal infection. Our [treatment] experience is extremely positive; it may be in the future that if you have significant asthma or sinusitis, you just go on an antifungal, but more research and clinical trials are needed,” said senior investigator Dr. David Corry, professor and chief of medical immunology, allergy, and rheumatology at Baylor.
“The standard culture techniques that have been used for 100 years are inadequate when it comes to culturing fungi from sputum, and why results almost invariably come back negative,” Dr. Corry said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The problem is that “almost everything in sputum” – eosinophils, macrophages, cytokines, and so on – “is designed to kill fungi.” Those elements have to be removed before plating. At Baylor, “we solubilize [sputum] with the reducing agent dithiothreitol, and vigorously stir the mixture to disperse the organisms and wash away the cellular elements and the other things.” The process leaves behind “a sandy material that’s basically fibrin clots mixed with a lot of fungal elements. You spread that on a plate, and it grows like wildfire,” he said.
“It’s easy to do, but time consuming. People are actually shipping their samples to us now from around the country, and we are happy to do those cultures,” Dr. Corry said. There’s no patent on the technique because “we want the community to use it. We want people to be helped,” he said.
Voriconazole seems to be the most effective option, and the team opts for it when possible, Dr. Corry noted. Terbinafine is the go-to drug for patients who can’t tolerate voriconazole. Fluconazole is sometimes added when monotherapy doesn’t seem to be doing the trick.
The work began as a search for household proteases. “One of our first discoveries was that” most are fungal. “The twist is that you are not inhaling the proteases, you are inhaling the fungus,” Dr. Corry said.
There have been both positive and negative results from the few prior investigations of antifungals for asthma. The team suspects that negative findings were a result of patients not being treated long enough, among other reasons.
The Baylor team is looking for funding for a prospective trial. The investigators hope to develop a protocol for diagnosis and treatment of fungal airway disease, but “there’s a lot of work that needs to get done,” Dr. Corry said.
The investigators had no relevant financial disclosures, and there was no outside funding for the work.
LOS ANGELES – Fungi might play a far larger role in asthma and chronic sinusitis than previously thought, according to investigators at Baylor College of Medicine in Houston.
With the help of a special culturing technique to wash antifungal elements out of sputum samples, six or more fungal colony-forming units grew out of the sputum of 112 of 134 patients (83.5%) at the Houston Veterans Affairs Medical Center; about a third of the patients had asthma, a third had chronic sinusitis, and a third had both. Although Aspergillus and Candida species were common, more than 30 fungal species were identified. Only a handful of patients had positive results on IgE testing.
Of 62 patients treated with standard-dose voriconazole or terbinafine, sometimes for more than a year, 54 (87%) reported symptomatic benefit including 31 (50%) with decreased sputum production, 24 (39%) with improved breathing, 20 (32%) with less cough, and nine (14.5%) with less rescue inhaler use.
At Baylor, prescribing antifungals for patients with recalcitrant asthma and chronic sinusitis “has evolved into something we pretty much do all the time now regardless of sensitivity results. I’m pretty certain we are the only institution that does this,” said allergy and immunology fellow Dr. Evan Li.
“Fungi, we think, are important initiating factors in many cases of asthma. They set up chronic mucosal infection. Our [treatment] experience is extremely positive; it may be in the future that if you have significant asthma or sinusitis, you just go on an antifungal, but more research and clinical trials are needed,” said senior investigator Dr. David Corry, professor and chief of medical immunology, allergy, and rheumatology at Baylor.
“The standard culture techniques that have been used for 100 years are inadequate when it comes to culturing fungi from sputum, and why results almost invariably come back negative,” Dr. Corry said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The problem is that “almost everything in sputum” – eosinophils, macrophages, cytokines, and so on – “is designed to kill fungi.” Those elements have to be removed before plating. At Baylor, “we solubilize [sputum] with the reducing agent dithiothreitol, and vigorously stir the mixture to disperse the organisms and wash away the cellular elements and the other things.” The process leaves behind “a sandy material that’s basically fibrin clots mixed with a lot of fungal elements. You spread that on a plate, and it grows like wildfire,” he said.
“It’s easy to do, but time consuming. People are actually shipping their samples to us now from around the country, and we are happy to do those cultures,” Dr. Corry said. There’s no patent on the technique because “we want the community to use it. We want people to be helped,” he said.
Voriconazole seems to be the most effective option, and the team opts for it when possible, Dr. Corry noted. Terbinafine is the go-to drug for patients who can’t tolerate voriconazole. Fluconazole is sometimes added when monotherapy doesn’t seem to be doing the trick.
The work began as a search for household proteases. “One of our first discoveries was that” most are fungal. “The twist is that you are not inhaling the proteases, you are inhaling the fungus,” Dr. Corry said.
There have been both positive and negative results from the few prior investigations of antifungals for asthma. The team suspects that negative findings were a result of patients not being treated long enough, among other reasons.
The Baylor team is looking for funding for a prospective trial. The investigators hope to develop a protocol for diagnosis and treatment of fungal airway disease, but “there’s a lot of work that needs to get done,” Dr. Corry said.
The investigators had no relevant financial disclosures, and there was no outside funding for the work.
LOS ANGELES – Fungi might play a far larger role in asthma and chronic sinusitis than previously thought, according to investigators at Baylor College of Medicine in Houston.
With the help of a special culturing technique to wash antifungal elements out of sputum samples, six or more fungal colony-forming units grew out of the sputum of 112 of 134 patients (83.5%) at the Houston Veterans Affairs Medical Center; about a third of the patients had asthma, a third had chronic sinusitis, and a third had both. Although Aspergillus and Candida species were common, more than 30 fungal species were identified. Only a handful of patients had positive results on IgE testing.
Of 62 patients treated with standard-dose voriconazole or terbinafine, sometimes for more than a year, 54 (87%) reported symptomatic benefit including 31 (50%) with decreased sputum production, 24 (39%) with improved breathing, 20 (32%) with less cough, and nine (14.5%) with less rescue inhaler use.
At Baylor, prescribing antifungals for patients with recalcitrant asthma and chronic sinusitis “has evolved into something we pretty much do all the time now regardless of sensitivity results. I’m pretty certain we are the only institution that does this,” said allergy and immunology fellow Dr. Evan Li.
“Fungi, we think, are important initiating factors in many cases of asthma. They set up chronic mucosal infection. Our [treatment] experience is extremely positive; it may be in the future that if you have significant asthma or sinusitis, you just go on an antifungal, but more research and clinical trials are needed,” said senior investigator Dr. David Corry, professor and chief of medical immunology, allergy, and rheumatology at Baylor.
“The standard culture techniques that have been used for 100 years are inadequate when it comes to culturing fungi from sputum, and why results almost invariably come back negative,” Dr. Corry said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The problem is that “almost everything in sputum” – eosinophils, macrophages, cytokines, and so on – “is designed to kill fungi.” Those elements have to be removed before plating. At Baylor, “we solubilize [sputum] with the reducing agent dithiothreitol, and vigorously stir the mixture to disperse the organisms and wash away the cellular elements and the other things.” The process leaves behind “a sandy material that’s basically fibrin clots mixed with a lot of fungal elements. You spread that on a plate, and it grows like wildfire,” he said.
“It’s easy to do, but time consuming. People are actually shipping their samples to us now from around the country, and we are happy to do those cultures,” Dr. Corry said. There’s no patent on the technique because “we want the community to use it. We want people to be helped,” he said.
Voriconazole seems to be the most effective option, and the team opts for it when possible, Dr. Corry noted. Terbinafine is the go-to drug for patients who can’t tolerate voriconazole. Fluconazole is sometimes added when monotherapy doesn’t seem to be doing the trick.
The work began as a search for household proteases. “One of our first discoveries was that” most are fungal. “The twist is that you are not inhaling the proteases, you are inhaling the fungus,” Dr. Corry said.
There have been both positive and negative results from the few prior investigations of antifungals for asthma. The team suspects that negative findings were a result of patients not being treated long enough, among other reasons.
The Baylor team is looking for funding for a prospective trial. The investigators hope to develop a protocol for diagnosis and treatment of fungal airway disease, but “there’s a lot of work that needs to get done,” Dr. Corry said.
The investigators had no relevant financial disclosures, and there was no outside funding for the work.
AT AAAAI
Key clinical point: Consider antifungal therapy if asthma or chronic sinusitis patients don’t respond well to conventional treatment.
Major finding: With the help of a special culturing technique to wash antifungal elements out of sputum samples, six or more fungal colony-forming units grew out of the sputum of 112 of 134 patients (83.5%) at the Houston Veterans Affairs Medical Center.
Data source: A single-center case review.
Disclosures: The investigators had no relevant financial disclosures, and there was no outside funding for the work.
Serious infections are increasing among psoriasis inpatients
SCOTTSDALE, ARIZ. – From enterocolitis to MRSA, serious infections are on the rise among inpatients with psoriasis, and psoriasis is an independent risk factor for serious infections, according to findings from large retrospective studies from the United States and the United Kingdom.
Inpatients with psoriasis in the United States also were at greater risk of serious infections, compared with nonpsoriatic inpatients at every time point studied, and serious infections were associated with increased hospital costs, length of stay, and risk of mortality, reported Derek Hsu, a medical student at Northwestern University, Chicago, and his associates. “Research is needed to determine how to reduce the risk of serious infections in patients with psoriasis,” the investigators emphasized.
Psoriasis affects some 7 million adults in the United States. Biologics, which are transforming the treatment landscape for moderate-to-severe psoriasis, “should reduce inherent infectious risk by controlling the inflammatory process and reducing disease severity, [but] these effects may be immunosuppressing and increase the risk of infection in other ways,” according to Mr. Hsu and his associates. For their study, they analyzed data for 2002-2012 from the Nationwide Inpatient Sample, which covers 20% of hospitalizations in the United States. They extracted validated ICD-9 codes for psoriasis and serious infections, and calculated costs of care after adjusting for 2014 inflation, based on the United States Consumer Price Index.
Overall rates of serious infection and rates of pneumonia, MRSA, septicemia, diverticulitis, enterocolitis, encephalitis, and any viral or fungal infection rose significantly among inpatients with psoriasis between 2002 and 2012 (all P-values less than .05). Predictors of serious infections among inpatients with psoriasis included diabetes mellitus, obesity, and being of non-Caucasian race or ethnicity, female, older than 60 years, and on Medicare or Medicaid, the researchers reported at the annual meeting of the Society for Investigative Dermatology.
Furthermore, after controlling for age, sex, and race, psoriasis was a significant risk factor for many different types of serious infections. Among these were cellulitis, herpes simplex virus, infectious arthritis, osteomyelitis, meningitis, influenza, encephalitis, septicemia, enterocolitis, MRSA, methicillin-sensitive Staphylococcus aureus infections, and Clostridium difficile. Further, inpatients with psoriasis were more prone to urinary tract infection, peritonitis or intestinal abscess, appendicitis, tuberculosis, and viral and fungal infections (all P-values less than .05). The average cost of hospital stay for inpatients with psoriasis was more than $2,200 greater when they were diagnosed with one or more serious infections than otherwise, and their average length of hospital stay was 2 days longer.
The study in the United Kingdom included nearly 200,000 patients with psoriasis and almost 1 million patients without psoriasis from The Health Improvement Network electronic medical record database. Between 2002 and 2013, patients without psoriasis developed an estimated 78.5 serious infections per 100,000 person-years, compared with 88.9, 85.7, and 145.7 serious infections per 100,000 person-years, respectively, for all psoriasis patients, patients with mild disease, and patients with severe disease requiring systemic or phototherapy, said Dr. Junko Takeshita and her colleagues at the University of Pennsylvania in Philadelphia. After controlling for many potential demographic and clinical confounders, psoriasis increased the risk of serious infection by about 21% (hazard ratio, 1.21; 95% confidence interval, 1.18-1.23). Patients with severe psoriasis had a 63% greater risk of infection than patients without psoriasis, compared with an 18% increase for patients with mild psoriasis.
The findings show “serious infection, particularly respiratory and skin or soft tissue infections, to be an important and common cause of morbidity among patients with psoriasis, especially those with more severe disease,” Dr. Takeshita and her associates said. Notably, the link between psoriasis and risk of serious infection persisted after excluding patients on immunosuppressive therapies, suggesting “that the greater infection risk is at least partially attributable to more severe psoriasis, itself,” they added.
The analysis of Nationwide Inpatient Sample data was funded by the Agency for Healthcare Research and Quality and by the Dermatology Foundation. The analysis of Health Improvement Network data was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, which is part of the National Institutes of Health, and by the Dermatology Foundation. None of the investigators reported conflicts of interest.
SCOTTSDALE, ARIZ. – From enterocolitis to MRSA, serious infections are on the rise among inpatients with psoriasis, and psoriasis is an independent risk factor for serious infections, according to findings from large retrospective studies from the United States and the United Kingdom.
Inpatients with psoriasis in the United States also were at greater risk of serious infections, compared with nonpsoriatic inpatients at every time point studied, and serious infections were associated with increased hospital costs, length of stay, and risk of mortality, reported Derek Hsu, a medical student at Northwestern University, Chicago, and his associates. “Research is needed to determine how to reduce the risk of serious infections in patients with psoriasis,” the investigators emphasized.
Psoriasis affects some 7 million adults in the United States. Biologics, which are transforming the treatment landscape for moderate-to-severe psoriasis, “should reduce inherent infectious risk by controlling the inflammatory process and reducing disease severity, [but] these effects may be immunosuppressing and increase the risk of infection in other ways,” according to Mr. Hsu and his associates. For their study, they analyzed data for 2002-2012 from the Nationwide Inpatient Sample, which covers 20% of hospitalizations in the United States. They extracted validated ICD-9 codes for psoriasis and serious infections, and calculated costs of care after adjusting for 2014 inflation, based on the United States Consumer Price Index.
Overall rates of serious infection and rates of pneumonia, MRSA, septicemia, diverticulitis, enterocolitis, encephalitis, and any viral or fungal infection rose significantly among inpatients with psoriasis between 2002 and 2012 (all P-values less than .05). Predictors of serious infections among inpatients with psoriasis included diabetes mellitus, obesity, and being of non-Caucasian race or ethnicity, female, older than 60 years, and on Medicare or Medicaid, the researchers reported at the annual meeting of the Society for Investigative Dermatology.
Furthermore, after controlling for age, sex, and race, psoriasis was a significant risk factor for many different types of serious infections. Among these were cellulitis, herpes simplex virus, infectious arthritis, osteomyelitis, meningitis, influenza, encephalitis, septicemia, enterocolitis, MRSA, methicillin-sensitive Staphylococcus aureus infections, and Clostridium difficile. Further, inpatients with psoriasis were more prone to urinary tract infection, peritonitis or intestinal abscess, appendicitis, tuberculosis, and viral and fungal infections (all P-values less than .05). The average cost of hospital stay for inpatients with psoriasis was more than $2,200 greater when they were diagnosed with one or more serious infections than otherwise, and their average length of hospital stay was 2 days longer.
The study in the United Kingdom included nearly 200,000 patients with psoriasis and almost 1 million patients without psoriasis from The Health Improvement Network electronic medical record database. Between 2002 and 2013, patients without psoriasis developed an estimated 78.5 serious infections per 100,000 person-years, compared with 88.9, 85.7, and 145.7 serious infections per 100,000 person-years, respectively, for all psoriasis patients, patients with mild disease, and patients with severe disease requiring systemic or phototherapy, said Dr. Junko Takeshita and her colleagues at the University of Pennsylvania in Philadelphia. After controlling for many potential demographic and clinical confounders, psoriasis increased the risk of serious infection by about 21% (hazard ratio, 1.21; 95% confidence interval, 1.18-1.23). Patients with severe psoriasis had a 63% greater risk of infection than patients without psoriasis, compared with an 18% increase for patients with mild psoriasis.
The findings show “serious infection, particularly respiratory and skin or soft tissue infections, to be an important and common cause of morbidity among patients with psoriasis, especially those with more severe disease,” Dr. Takeshita and her associates said. Notably, the link between psoriasis and risk of serious infection persisted after excluding patients on immunosuppressive therapies, suggesting “that the greater infection risk is at least partially attributable to more severe psoriasis, itself,” they added.
The analysis of Nationwide Inpatient Sample data was funded by the Agency for Healthcare Research and Quality and by the Dermatology Foundation. The analysis of Health Improvement Network data was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, which is part of the National Institutes of Health, and by the Dermatology Foundation. None of the investigators reported conflicts of interest.
SCOTTSDALE, ARIZ. – From enterocolitis to MRSA, serious infections are on the rise among inpatients with psoriasis, and psoriasis is an independent risk factor for serious infections, according to findings from large retrospective studies from the United States and the United Kingdom.
Inpatients with psoriasis in the United States also were at greater risk of serious infections, compared with nonpsoriatic inpatients at every time point studied, and serious infections were associated with increased hospital costs, length of stay, and risk of mortality, reported Derek Hsu, a medical student at Northwestern University, Chicago, and his associates. “Research is needed to determine how to reduce the risk of serious infections in patients with psoriasis,” the investigators emphasized.
Psoriasis affects some 7 million adults in the United States. Biologics, which are transforming the treatment landscape for moderate-to-severe psoriasis, “should reduce inherent infectious risk by controlling the inflammatory process and reducing disease severity, [but] these effects may be immunosuppressing and increase the risk of infection in other ways,” according to Mr. Hsu and his associates. For their study, they analyzed data for 2002-2012 from the Nationwide Inpatient Sample, which covers 20% of hospitalizations in the United States. They extracted validated ICD-9 codes for psoriasis and serious infections, and calculated costs of care after adjusting for 2014 inflation, based on the United States Consumer Price Index.
Overall rates of serious infection and rates of pneumonia, MRSA, septicemia, diverticulitis, enterocolitis, encephalitis, and any viral or fungal infection rose significantly among inpatients with psoriasis between 2002 and 2012 (all P-values less than .05). Predictors of serious infections among inpatients with psoriasis included diabetes mellitus, obesity, and being of non-Caucasian race or ethnicity, female, older than 60 years, and on Medicare or Medicaid, the researchers reported at the annual meeting of the Society for Investigative Dermatology.
Furthermore, after controlling for age, sex, and race, psoriasis was a significant risk factor for many different types of serious infections. Among these were cellulitis, herpes simplex virus, infectious arthritis, osteomyelitis, meningitis, influenza, encephalitis, septicemia, enterocolitis, MRSA, methicillin-sensitive Staphylococcus aureus infections, and Clostridium difficile. Further, inpatients with psoriasis were more prone to urinary tract infection, peritonitis or intestinal abscess, appendicitis, tuberculosis, and viral and fungal infections (all P-values less than .05). The average cost of hospital stay for inpatients with psoriasis was more than $2,200 greater when they were diagnosed with one or more serious infections than otherwise, and their average length of hospital stay was 2 days longer.
The study in the United Kingdom included nearly 200,000 patients with psoriasis and almost 1 million patients without psoriasis from The Health Improvement Network electronic medical record database. Between 2002 and 2013, patients without psoriasis developed an estimated 78.5 serious infections per 100,000 person-years, compared with 88.9, 85.7, and 145.7 serious infections per 100,000 person-years, respectively, for all psoriasis patients, patients with mild disease, and patients with severe disease requiring systemic or phototherapy, said Dr. Junko Takeshita and her colleagues at the University of Pennsylvania in Philadelphia. After controlling for many potential demographic and clinical confounders, psoriasis increased the risk of serious infection by about 21% (hazard ratio, 1.21; 95% confidence interval, 1.18-1.23). Patients with severe psoriasis had a 63% greater risk of infection than patients without psoriasis, compared with an 18% increase for patients with mild psoriasis.
The findings show “serious infection, particularly respiratory and skin or soft tissue infections, to be an important and common cause of morbidity among patients with psoriasis, especially those with more severe disease,” Dr. Takeshita and her associates said. Notably, the link between psoriasis and risk of serious infection persisted after excluding patients on immunosuppressive therapies, suggesting “that the greater infection risk is at least partially attributable to more severe psoriasis, itself,” they added.
The analysis of Nationwide Inpatient Sample data was funded by the Agency for Healthcare Research and Quality and by the Dermatology Foundation. The analysis of Health Improvement Network data was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, which is part of the National Institutes of Health, and by the Dermatology Foundation. None of the investigators reported conflicts of interest.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: Psoriasis is an independent risk factor for serious infections, and serious infections are increasing among inpatients with psoriasis.
Major finding: Overall rates of serious infection and rates of pneumonia, MRSA, septicemia, diverticulitis, enterocolitis, encephalitis, and any viral or fungal infection rose significantly among inpatients in the United States with psoriasis between 2002 and 2012 (all P-values less than .05). In the United Kingdom during the same time period, patients with severe psoriasis had a 63% greater risk of serious infection than patients without psoriasis.
Data source: Analyses of data from the Nationwide Inpatient Sample for 2002 through 2012, and from The Health Improvement Network for 2003 through 2012.
Disclosures: The Nationwide Inpatient Sample analysis was funded by the Agency for Healthcare Research and Quality and the Dermatology Foundation. The analysis of The Health Improvement Network was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, which is part of the National Institutes of Health, and by the Dermatology Foundation. None of the investigators reported conflicts of interest.
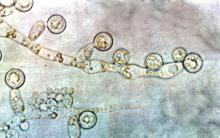
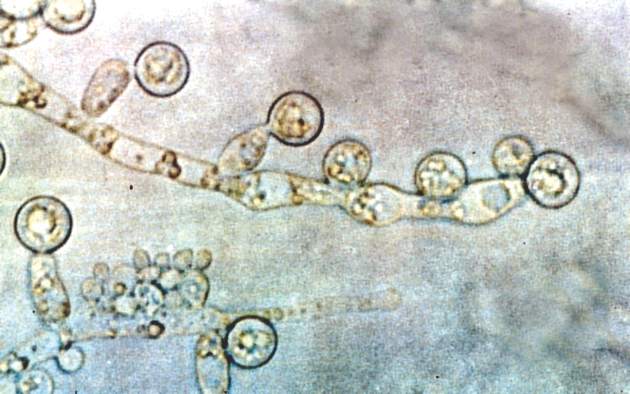
Candida linked to sex-specific schizophrenia symptoms
Exposure to Candida albicans significantly increased the odds of a schizophrenia diagnosis in men, according to case-control data from two cohorts of 947 adults.
“Fungal pathogens have not been extensively evaluated in studies of psychiatric disorders,” wrote Dr. Emily G. Severance of Johns Hopkins University in Baltimore, and her colleagues, in njp (Nature Partner Journals) Schizophrenia.
The researchers compared C. albicans IgG levels of individuals with schizophrenia and bipolar disorder with controls and found no diagnostic differences. However, stratification by sex showed a significantly increased risk of schizophrenia in men with elevated C. albicans IgG levels, with a ninefold increase in risk at the highest of three levels of seropositivity (odds ratio, 9.53). Elevated C. albicans levels in males with bipolar disorder were attributed to a history of homelessness.
C. albicans antibodies in women were not significantly different between groups. However, C. albicans was significantly associated with cognitive impairment in women with schizophrenia vs. control women (OR, 1.12), but no such difference was noted in men.
High levels of C. albicans antibodies were associated with comorbid gastrointestinal, genitourinary, and neoplastic conditions among individuals with psychiatric disorders overall.
“It may be premature to list this pathogen as a risk factor for disease causation, but its status as a comorbidity requires clinical attention,” the researchers wrote. “In the long term, more research is required to understand the mechanisms that trigger pathogenicity of fungal commensals and how this might impact brain function in psychiatric disorders.”
The findings were published in npj Schizophrenia. Read the full study here (npj Schizophrenia 2016 May 4. doi: 10.1038/npjschz.2016.18).
Exposure to Candida albicans significantly increased the odds of a schizophrenia diagnosis in men, according to case-control data from two cohorts of 947 adults.
“Fungal pathogens have not been extensively evaluated in studies of psychiatric disorders,” wrote Dr. Emily G. Severance of Johns Hopkins University in Baltimore, and her colleagues, in njp (Nature Partner Journals) Schizophrenia.
The researchers compared C. albicans IgG levels of individuals with schizophrenia and bipolar disorder with controls and found no diagnostic differences. However, stratification by sex showed a significantly increased risk of schizophrenia in men with elevated C. albicans IgG levels, with a ninefold increase in risk at the highest of three levels of seropositivity (odds ratio, 9.53). Elevated C. albicans levels in males with bipolar disorder were attributed to a history of homelessness.
C. albicans antibodies in women were not significantly different between groups. However, C. albicans was significantly associated with cognitive impairment in women with schizophrenia vs. control women (OR, 1.12), but no such difference was noted in men.
High levels of C. albicans antibodies were associated with comorbid gastrointestinal, genitourinary, and neoplastic conditions among individuals with psychiatric disorders overall.
“It may be premature to list this pathogen as a risk factor for disease causation, but its status as a comorbidity requires clinical attention,” the researchers wrote. “In the long term, more research is required to understand the mechanisms that trigger pathogenicity of fungal commensals and how this might impact brain function in psychiatric disorders.”
The findings were published in npj Schizophrenia. Read the full study here (npj Schizophrenia 2016 May 4. doi: 10.1038/npjschz.2016.18).
Exposure to Candida albicans significantly increased the odds of a schizophrenia diagnosis in men, according to case-control data from two cohorts of 947 adults.
“Fungal pathogens have not been extensively evaluated in studies of psychiatric disorders,” wrote Dr. Emily G. Severance of Johns Hopkins University in Baltimore, and her colleagues, in njp (Nature Partner Journals) Schizophrenia.
The researchers compared C. albicans IgG levels of individuals with schizophrenia and bipolar disorder with controls and found no diagnostic differences. However, stratification by sex showed a significantly increased risk of schizophrenia in men with elevated C. albicans IgG levels, with a ninefold increase in risk at the highest of three levels of seropositivity (odds ratio, 9.53). Elevated C. albicans levels in males with bipolar disorder were attributed to a history of homelessness.
C. albicans antibodies in women were not significantly different between groups. However, C. albicans was significantly associated with cognitive impairment in women with schizophrenia vs. control women (OR, 1.12), but no such difference was noted in men.
High levels of C. albicans antibodies were associated with comorbid gastrointestinal, genitourinary, and neoplastic conditions among individuals with psychiatric disorders overall.
“It may be premature to list this pathogen as a risk factor for disease causation, but its status as a comorbidity requires clinical attention,” the researchers wrote. “In the long term, more research is required to understand the mechanisms that trigger pathogenicity of fungal commensals and how this might impact brain function in psychiatric disorders.”
The findings were published in npj Schizophrenia. Read the full study here (npj Schizophrenia 2016 May 4. doi: 10.1038/npjschz.2016.18).
FROM NPJ SCHIZOPHRENIA
FDA evaluating the use of oral fluconazole in pregnancy
The Food and Drug Administration is reviewing the results of a Danish study that concludes there is a possible increased risk of miscarriage with the use of oral fluconazole (Diflucan) in pregnancy, according to a safety alert issued April 26.
The current drug label for oral fluconazole states that data from studies in women does not suggest an increased risk of problems during pregnancy or abnormalities in developing babies when women used a single 150-mg dose to treat vaginal yeast infections. However, reports of abnormalities at birth have resulted from high doses (400-800 mg/day) taken during pregnancy for much longer than a single dose. The Danish study had most pregnant women use one or two doses of 150 mg.
Oral fluconazole is used to treat yeast infections of the vaginal area, mouth, and esophagus. It can also be used to treat a fungal infection of the brain and spinal cord.
The FDA is also evaluating additional data and will make recommendations when the review is complete.
“Until FDA’s review is complete and more is understood about this study and other available data, FDA advises cautious prescribing of oral fluconazole in pregnancy,” the safety alert states.
The FDA also noted that the Centers for Disease Control and Prevention recommends using topical antifungal products only when treating pregnant women with vulvovaginal yeast infections.
Read more about the investigation on the FDA website.
The Food and Drug Administration is reviewing the results of a Danish study that concludes there is a possible increased risk of miscarriage with the use of oral fluconazole (Diflucan) in pregnancy, according to a safety alert issued April 26.
The current drug label for oral fluconazole states that data from studies in women does not suggest an increased risk of problems during pregnancy or abnormalities in developing babies when women used a single 150-mg dose to treat vaginal yeast infections. However, reports of abnormalities at birth have resulted from high doses (400-800 mg/day) taken during pregnancy for much longer than a single dose. The Danish study had most pregnant women use one or two doses of 150 mg.
Oral fluconazole is used to treat yeast infections of the vaginal area, mouth, and esophagus. It can also be used to treat a fungal infection of the brain and spinal cord.
The FDA is also evaluating additional data and will make recommendations when the review is complete.
“Until FDA’s review is complete and more is understood about this study and other available data, FDA advises cautious prescribing of oral fluconazole in pregnancy,” the safety alert states.
The FDA also noted that the Centers for Disease Control and Prevention recommends using topical antifungal products only when treating pregnant women with vulvovaginal yeast infections.
Read more about the investigation on the FDA website.
The Food and Drug Administration is reviewing the results of a Danish study that concludes there is a possible increased risk of miscarriage with the use of oral fluconazole (Diflucan) in pregnancy, according to a safety alert issued April 26.
The current drug label for oral fluconazole states that data from studies in women does not suggest an increased risk of problems during pregnancy or abnormalities in developing babies when women used a single 150-mg dose to treat vaginal yeast infections. However, reports of abnormalities at birth have resulted from high doses (400-800 mg/day) taken during pregnancy for much longer than a single dose. The Danish study had most pregnant women use one or two doses of 150 mg.
Oral fluconazole is used to treat yeast infections of the vaginal area, mouth, and esophagus. It can also be used to treat a fungal infection of the brain and spinal cord.
The FDA is also evaluating additional data and will make recommendations when the review is complete.
“Until FDA’s review is complete and more is understood about this study and other available data, FDA advises cautious prescribing of oral fluconazole in pregnancy,” the safety alert states.
The FDA also noted that the Centers for Disease Control and Prevention recommends using topical antifungal products only when treating pregnant women with vulvovaginal yeast infections.
Read more about the investigation on the FDA website.
Number of U.S. tuberculosis cases increased in 2015
For the first time in 20 years, incidence of tuberculosis in the United States increased slightly in 2015, according to investigators from the Centers for Disease Control and Prevention.
In 2015, 9,563 cases of TB were reported in the United States, up 1.7% from the 9,406 cases reported in 2014. Texas saw the largest total increase in TB cases, going from 1,269 cases in 2014 to 1,334 cases in 2015, followed by South Carolina and Michigan, which both had 25 more TB cases in 2015 than in 2014. Vermont saw the largest relative increase, going from two cases in 2014 to seven cases in 2015, an increase of 250%.
Among U.S.-born patients, the largest number of TB cases were reported in black non-Hispanics, although the incidence rate was highest in Native Hawaiian/other Pacific Islanders at 8.4/100,000 people. For foreign-born patients, Mexico was the most common origin country, followed by the Philippines, India, Vietnam, and China. Incidence rate was significantly higher for patients from Asian countries than from any other region.
“Resuming declines in TB incidence will require more comprehensive public health approaches, both globally and domestically. These include increasing case detection and cure rates globally, reducing TB transmission in institutional settings such as health care settings and correctional facilities, and increasing detection and treatment of preexisting latent TB infection among the U.S. populations most affected by TB,” the CDC investigators said.
Find the full report in the MMWR (doi: 10.15585/mmwr.mm6511a2).
For the first time in 20 years, incidence of tuberculosis in the United States increased slightly in 2015, according to investigators from the Centers for Disease Control and Prevention.
In 2015, 9,563 cases of TB were reported in the United States, up 1.7% from the 9,406 cases reported in 2014. Texas saw the largest total increase in TB cases, going from 1,269 cases in 2014 to 1,334 cases in 2015, followed by South Carolina and Michigan, which both had 25 more TB cases in 2015 than in 2014. Vermont saw the largest relative increase, going from two cases in 2014 to seven cases in 2015, an increase of 250%.
Among U.S.-born patients, the largest number of TB cases were reported in black non-Hispanics, although the incidence rate was highest in Native Hawaiian/other Pacific Islanders at 8.4/100,000 people. For foreign-born patients, Mexico was the most common origin country, followed by the Philippines, India, Vietnam, and China. Incidence rate was significantly higher for patients from Asian countries than from any other region.
“Resuming declines in TB incidence will require more comprehensive public health approaches, both globally and domestically. These include increasing case detection and cure rates globally, reducing TB transmission in institutional settings such as health care settings and correctional facilities, and increasing detection and treatment of preexisting latent TB infection among the U.S. populations most affected by TB,” the CDC investigators said.
Find the full report in the MMWR (doi: 10.15585/mmwr.mm6511a2).
For the first time in 20 years, incidence of tuberculosis in the United States increased slightly in 2015, according to investigators from the Centers for Disease Control and Prevention.
In 2015, 9,563 cases of TB were reported in the United States, up 1.7% from the 9,406 cases reported in 2014. Texas saw the largest total increase in TB cases, going from 1,269 cases in 2014 to 1,334 cases in 2015, followed by South Carolina and Michigan, which both had 25 more TB cases in 2015 than in 2014. Vermont saw the largest relative increase, going from two cases in 2014 to seven cases in 2015, an increase of 250%.
Among U.S.-born patients, the largest number of TB cases were reported in black non-Hispanics, although the incidence rate was highest in Native Hawaiian/other Pacific Islanders at 8.4/100,000 people. For foreign-born patients, Mexico was the most common origin country, followed by the Philippines, India, Vietnam, and China. Incidence rate was significantly higher for patients from Asian countries than from any other region.
“Resuming declines in TB incidence will require more comprehensive public health approaches, both globally and domestically. These include increasing case detection and cure rates globally, reducing TB transmission in institutional settings such as health care settings and correctional facilities, and increasing detection and treatment of preexisting latent TB infection among the U.S. populations most affected by TB,” the CDC investigators said.
Find the full report in the MMWR (doi: 10.15585/mmwr.mm6511a2).
FROM THE MMWR
FDA approves first generic form of oxiconazole nitrate cream
A generic formulation of oxiconazole nitrate cream, 1% has been approved by the Food and Drug Administration, for the treatment of tinea pedis, tinea cruris, tinea corporis due to Trichophyton rubrum, Trichophyton mentagrophytes, or Epidermophyton floccosum) and tinea (pityriasis) versicolor due to Malassezia furfur.
This is the first generic version of Oxistat to be approved, according to the FDA’s statement announcing the approval.
The label for the generic, manufactured by Taro Pharmaceuticals U.S.A. is available here.
A generic formulation of oxiconazole nitrate cream, 1% has been approved by the Food and Drug Administration, for the treatment of tinea pedis, tinea cruris, tinea corporis due to Trichophyton rubrum, Trichophyton mentagrophytes, or Epidermophyton floccosum) and tinea (pityriasis) versicolor due to Malassezia furfur.
This is the first generic version of Oxistat to be approved, according to the FDA’s statement announcing the approval.
The label for the generic, manufactured by Taro Pharmaceuticals U.S.A. is available here.
A generic formulation of oxiconazole nitrate cream, 1% has been approved by the Food and Drug Administration, for the treatment of tinea pedis, tinea cruris, tinea corporis due to Trichophyton rubrum, Trichophyton mentagrophytes, or Epidermophyton floccosum) and tinea (pityriasis) versicolor due to Malassezia furfur.
This is the first generic version of Oxistat to be approved, according to the FDA’s statement announcing the approval.
The label for the generic, manufactured by Taro Pharmaceuticals U.S.A. is available here.
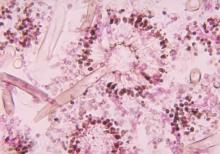
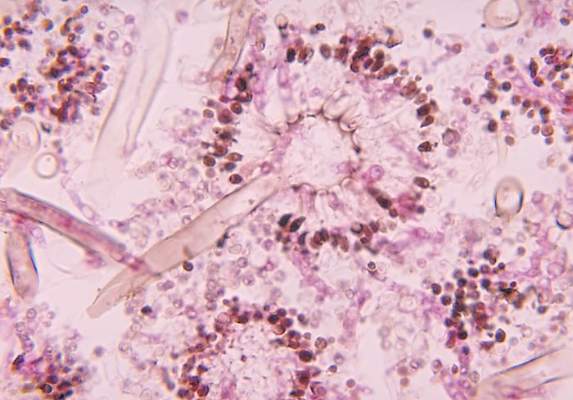
When toenail onychomycosis can turn deadly
WAIKOLOA, HAWAII – Toenail onychomycosis is a common condition in the general population, but it’s three- to fourfold more prevalent in certain at risk populations where it can have serious and even life-threatening consequences, Dr. Theodore Rosen observed at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
He cited a recent systematic review led by Dr. Aditya K. Gupta, professor of dermatology at the University of Toronto, whom Dr. Rosen hailed as one of the world’s great fungal disease authorities. Dr. Gupta and coworkers concluded that while the prevalence of dermatophyte toenail onychomycosis is 3.2% worldwide in the general population, it climbs to 8.8% in diabetics, 10.2% in psoriatics, 10.3% in the elderly, 11.9% in dialysis patients, 5.2% in renal transplant recipients, and 10.4% in HIV-positive individuals. The highest prevalence of onychomycosis due to non-dermatophyte molds was seen in psoriasis patients, at 2.5%, while elderly patients had the highest prevalence of onychomycosis caused by yeasts, at 6.1% (J Eur Acad Dermatol Venereol. 2015 Jun;29[6]:1039-44).
“Onychomycosis is especially important in those who are immunocompromised and immunosuppressed, for two reasons. One is that really odd organisms that aren’t Trichophyton rubrum or T. interdigitale can be involved: saprophytes like Scopulariopsis, Acremonium, Aspergillus, and Paecilomyces. And some of these saprophytes, like Fusarium, can get from the nail and nail bed into the bloodstream and can kill,” explained Dr. Rosen, professor of dermatology at Baylor College of Medicine in Houston.
“Onychomycosis, aside from the fact that it looks bad and often leads to pain, can also lead to breaks in the skin which then result in secondary bacterial infections. In fact, after motor vehicle accidents, onychomycosis and tinea pedis combined are the most common cause of lower extremity cellulitis leading to hospitalization in the United States,” he continued.
The go-to treatments for onychomycosis in patients with a bad prognostic factor are oral itraconazole (Sporanox) and terbinafine. Don’t be unduly swayed by the complete cure rates reported in clinical trials and cited in the product package inserts; they don’t tell the full story because of important differences in study design, according to Dr. Rosen.
He recommended that physicians familiarize themselves with posaconazole (Noxafil) as an antifungal to consider for second-line therapy in difficult-to-cure cases of onychomycosis in immunosuppressed patients. This is off-label therapy. The approved indications for this triazole antifungal agent are prophylaxis of invasive Aspergillus and Candida infections in severely immunocompromised patients, as well as treatment of oropharyngeal candidiasis. But this is a potent agent that provides broad-spectrum coverage coupled with a favorable safety profile. It performed well in a phase IIb randomized, placebo- and active-controlled, multicenter, investigator-blinded study of 218 adults with toenail onychomycosis (Br J Dermatol. 2012 Feb;166[2]:389-98).
Dr. Rosen reported serving on scientific advisory boards for Anacor, Merz, and Valeant.
SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Toenail onychomycosis is a common condition in the general population, but it’s three- to fourfold more prevalent in certain at risk populations where it can have serious and even life-threatening consequences, Dr. Theodore Rosen observed at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
He cited a recent systematic review led by Dr. Aditya K. Gupta, professor of dermatology at the University of Toronto, whom Dr. Rosen hailed as one of the world’s great fungal disease authorities. Dr. Gupta and coworkers concluded that while the prevalence of dermatophyte toenail onychomycosis is 3.2% worldwide in the general population, it climbs to 8.8% in diabetics, 10.2% in psoriatics, 10.3% in the elderly, 11.9% in dialysis patients, 5.2% in renal transplant recipients, and 10.4% in HIV-positive individuals. The highest prevalence of onychomycosis due to non-dermatophyte molds was seen in psoriasis patients, at 2.5%, while elderly patients had the highest prevalence of onychomycosis caused by yeasts, at 6.1% (J Eur Acad Dermatol Venereol. 2015 Jun;29[6]:1039-44).
“Onychomycosis is especially important in those who are immunocompromised and immunosuppressed, for two reasons. One is that really odd organisms that aren’t Trichophyton rubrum or T. interdigitale can be involved: saprophytes like Scopulariopsis, Acremonium, Aspergillus, and Paecilomyces. And some of these saprophytes, like Fusarium, can get from the nail and nail bed into the bloodstream and can kill,” explained Dr. Rosen, professor of dermatology at Baylor College of Medicine in Houston.
“Onychomycosis, aside from the fact that it looks bad and often leads to pain, can also lead to breaks in the skin which then result in secondary bacterial infections. In fact, after motor vehicle accidents, onychomycosis and tinea pedis combined are the most common cause of lower extremity cellulitis leading to hospitalization in the United States,” he continued.
The go-to treatments for onychomycosis in patients with a bad prognostic factor are oral itraconazole (Sporanox) and terbinafine. Don’t be unduly swayed by the complete cure rates reported in clinical trials and cited in the product package inserts; they don’t tell the full story because of important differences in study design, according to Dr. Rosen.
He recommended that physicians familiarize themselves with posaconazole (Noxafil) as an antifungal to consider for second-line therapy in difficult-to-cure cases of onychomycosis in immunosuppressed patients. This is off-label therapy. The approved indications for this triazole antifungal agent are prophylaxis of invasive Aspergillus and Candida infections in severely immunocompromised patients, as well as treatment of oropharyngeal candidiasis. But this is a potent agent that provides broad-spectrum coverage coupled with a favorable safety profile. It performed well in a phase IIb randomized, placebo- and active-controlled, multicenter, investigator-blinded study of 218 adults with toenail onychomycosis (Br J Dermatol. 2012 Feb;166[2]:389-98).
Dr. Rosen reported serving on scientific advisory boards for Anacor, Merz, and Valeant.
SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Toenail onychomycosis is a common condition in the general population, but it’s three- to fourfold more prevalent in certain at risk populations where it can have serious and even life-threatening consequences, Dr. Theodore Rosen observed at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
He cited a recent systematic review led by Dr. Aditya K. Gupta, professor of dermatology at the University of Toronto, whom Dr. Rosen hailed as one of the world’s great fungal disease authorities. Dr. Gupta and coworkers concluded that while the prevalence of dermatophyte toenail onychomycosis is 3.2% worldwide in the general population, it climbs to 8.8% in diabetics, 10.2% in psoriatics, 10.3% in the elderly, 11.9% in dialysis patients, 5.2% in renal transplant recipients, and 10.4% in HIV-positive individuals. The highest prevalence of onychomycosis due to non-dermatophyte molds was seen in psoriasis patients, at 2.5%, while elderly patients had the highest prevalence of onychomycosis caused by yeasts, at 6.1% (J Eur Acad Dermatol Venereol. 2015 Jun;29[6]:1039-44).
“Onychomycosis is especially important in those who are immunocompromised and immunosuppressed, for two reasons. One is that really odd organisms that aren’t Trichophyton rubrum or T. interdigitale can be involved: saprophytes like Scopulariopsis, Acremonium, Aspergillus, and Paecilomyces. And some of these saprophytes, like Fusarium, can get from the nail and nail bed into the bloodstream and can kill,” explained Dr. Rosen, professor of dermatology at Baylor College of Medicine in Houston.
“Onychomycosis, aside from the fact that it looks bad and often leads to pain, can also lead to breaks in the skin which then result in secondary bacterial infections. In fact, after motor vehicle accidents, onychomycosis and tinea pedis combined are the most common cause of lower extremity cellulitis leading to hospitalization in the United States,” he continued.
The go-to treatments for onychomycosis in patients with a bad prognostic factor are oral itraconazole (Sporanox) and terbinafine. Don’t be unduly swayed by the complete cure rates reported in clinical trials and cited in the product package inserts; they don’t tell the full story because of important differences in study design, according to Dr. Rosen.
He recommended that physicians familiarize themselves with posaconazole (Noxafil) as an antifungal to consider for second-line therapy in difficult-to-cure cases of onychomycosis in immunosuppressed patients. This is off-label therapy. The approved indications for this triazole antifungal agent are prophylaxis of invasive Aspergillus and Candida infections in severely immunocompromised patients, as well as treatment of oropharyngeal candidiasis. But this is a potent agent that provides broad-spectrum coverage coupled with a favorable safety profile. It performed well in a phase IIb randomized, placebo- and active-controlled, multicenter, investigator-blinded study of 218 adults with toenail onychomycosis (Br J Dermatol. 2012 Feb;166[2]:389-98).
Dr. Rosen reported serving on scientific advisory boards for Anacor, Merz, and Valeant.
SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Maximizing bang in topical onychomycosis therapy
WAIKOLOA, HAWAII – Two recent studies highlight several key points regarding topical therapy for onychomycosis: Treat it early for best results, and if concomitant tinea pedis is present, be sure to treat that, too, Dr. Theodore Rosen said at the Hawaii Dermatology Seminar.
The studies were separate secondary analyses of the pooled results of two large, double blind, vehicle-controlled, 48-week, phase III randomized trials of efinaconazole 10% topical solution (Jublia) for onychomycosis. But the same lessons probably apply to any topical antifungal, according to Dr. Rosen, professor of dermatology at Baylor College of Medicine, Houston.
Early treatment: This makes a big difference in outcome, as demonstrated in Dr. Phoebe Rich’s analysis of 1,655 patients in the phase III studies. Dr. Rich, director of the nail disorders clinic at Oregon Health and Science University, Portland, divided participants into three groups based upon disease duration: less than a year, 1-5 years, or more than 5 years. The complete cure rate was much better in the group with less than 1 year of onychomycosis, even though the extent of nail involvement of the target toenail didn’t differ significantly between the three groups (J Drugs Dermatol. 2015;Jan 14[1]:58-62).
“Now we have data: Don’t wait to treat until it has been there for 35 years. It’s easier to treat if it’s early,” Dr. Rosen commented at the seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
When onychomycosis and tinea pedis coexist, treat both: Dr. Leon H. Kircik of Indiana University, Indianapolis, and associates reported in a poster at the Hawaii Dermatology Seminar that one in five participants in the two phase III trials had tinea pedis as well as onychomycosis, and nearly half of them were treated for their athlete’s foot using their physician’s choice of topical antifungals.
The primary endpoint in the two trials was the week 53 complete cure rate, defined as no clinical involvement of the target toenail, a negative potassium hydroxide exam, and a negative fungal culture. Among subjects with concomitant onychomycosis and tinea pedis, the onychomycosis complete cure rate was 28.2% if they received efinaconazole for their onychomycosis and got treatment for their tinea pedis, compared with 20.9% if they got efinaconazole but no treatment for their tinea pedis. The complete/almost complete cure rate was 35.5% with dual therapy versus 29.6% if they only received efinaconazole. Both differences were significant.
“Doesn’t that make logical sense? If you leave the fungus on the foot or between the toes, it’s going to say, ‘Wow, that’s steak up there on the nail. That’s real food. I’m just going to crawl back onto the nail because all my brothers up there are dead and there’s wide-open space,” Dr. Rosen explained.
He added that the reverse is also true: if a patient presents seeking treatment for athlete’s foot but also has onychomycosis, the best treatment results for the tinea pedis are obtained by also treating the nail infection.
Dr. Rosen offered a money-saving tip for effective OTC therapy for tinea pedis. Two words: Lotrimin Ultra. That’s the brand name for butenafine cream 1%, not to be confused with plain old Lotrimin, which is clotrimazole.
“Clotrimazole has been around since the dawn of man, and it’s not very effective. Many of the fungi are actually resistant to it. But they’re not resistant to butenafine, which is a very good topical antifungal now available over the counter. It costs $9 or $10 dollars for a tube the size of a baseball bat. It’s a good, effective, cheap way of treating concomitant tinea pedis,” he said.
Dr. Rosen reported serving on scientific advisory boards for Anacor, Merz, and Valeant.
SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Two recent studies highlight several key points regarding topical therapy for onychomycosis: Treat it early for best results, and if concomitant tinea pedis is present, be sure to treat that, too, Dr. Theodore Rosen said at the Hawaii Dermatology Seminar.
The studies were separate secondary analyses of the pooled results of two large, double blind, vehicle-controlled, 48-week, phase III randomized trials of efinaconazole 10% topical solution (Jublia) for onychomycosis. But the same lessons probably apply to any topical antifungal, according to Dr. Rosen, professor of dermatology at Baylor College of Medicine, Houston.
Early treatment: This makes a big difference in outcome, as demonstrated in Dr. Phoebe Rich’s analysis of 1,655 patients in the phase III studies. Dr. Rich, director of the nail disorders clinic at Oregon Health and Science University, Portland, divided participants into three groups based upon disease duration: less than a year, 1-5 years, or more than 5 years. The complete cure rate was much better in the group with less than 1 year of onychomycosis, even though the extent of nail involvement of the target toenail didn’t differ significantly between the three groups (J Drugs Dermatol. 2015;Jan 14[1]:58-62).
“Now we have data: Don’t wait to treat until it has been there for 35 years. It’s easier to treat if it’s early,” Dr. Rosen commented at the seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
When onychomycosis and tinea pedis coexist, treat both: Dr. Leon H. Kircik of Indiana University, Indianapolis, and associates reported in a poster at the Hawaii Dermatology Seminar that one in five participants in the two phase III trials had tinea pedis as well as onychomycosis, and nearly half of them were treated for their athlete’s foot using their physician’s choice of topical antifungals.
The primary endpoint in the two trials was the week 53 complete cure rate, defined as no clinical involvement of the target toenail, a negative potassium hydroxide exam, and a negative fungal culture. Among subjects with concomitant onychomycosis and tinea pedis, the onychomycosis complete cure rate was 28.2% if they received efinaconazole for their onychomycosis and got treatment for their tinea pedis, compared with 20.9% if they got efinaconazole but no treatment for their tinea pedis. The complete/almost complete cure rate was 35.5% with dual therapy versus 29.6% if they only received efinaconazole. Both differences were significant.
“Doesn’t that make logical sense? If you leave the fungus on the foot or between the toes, it’s going to say, ‘Wow, that’s steak up there on the nail. That’s real food. I’m just going to crawl back onto the nail because all my brothers up there are dead and there’s wide-open space,” Dr. Rosen explained.
He added that the reverse is also true: if a patient presents seeking treatment for athlete’s foot but also has onychomycosis, the best treatment results for the tinea pedis are obtained by also treating the nail infection.
Dr. Rosen offered a money-saving tip for effective OTC therapy for tinea pedis. Two words: Lotrimin Ultra. That’s the brand name for butenafine cream 1%, not to be confused with plain old Lotrimin, which is clotrimazole.
“Clotrimazole has been around since the dawn of man, and it’s not very effective. Many of the fungi are actually resistant to it. But they’re not resistant to butenafine, which is a very good topical antifungal now available over the counter. It costs $9 or $10 dollars for a tube the size of a baseball bat. It’s a good, effective, cheap way of treating concomitant tinea pedis,” he said.
Dr. Rosen reported serving on scientific advisory boards for Anacor, Merz, and Valeant.
SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Two recent studies highlight several key points regarding topical therapy for onychomycosis: Treat it early for best results, and if concomitant tinea pedis is present, be sure to treat that, too, Dr. Theodore Rosen said at the Hawaii Dermatology Seminar.
The studies were separate secondary analyses of the pooled results of two large, double blind, vehicle-controlled, 48-week, phase III randomized trials of efinaconazole 10% topical solution (Jublia) for onychomycosis. But the same lessons probably apply to any topical antifungal, according to Dr. Rosen, professor of dermatology at Baylor College of Medicine, Houston.
Early treatment: This makes a big difference in outcome, as demonstrated in Dr. Phoebe Rich’s analysis of 1,655 patients in the phase III studies. Dr. Rich, director of the nail disorders clinic at Oregon Health and Science University, Portland, divided participants into three groups based upon disease duration: less than a year, 1-5 years, or more than 5 years. The complete cure rate was much better in the group with less than 1 year of onychomycosis, even though the extent of nail involvement of the target toenail didn’t differ significantly between the three groups (J Drugs Dermatol. 2015;Jan 14[1]:58-62).
“Now we have data: Don’t wait to treat until it has been there for 35 years. It’s easier to treat if it’s early,” Dr. Rosen commented at the seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
When onychomycosis and tinea pedis coexist, treat both: Dr. Leon H. Kircik of Indiana University, Indianapolis, and associates reported in a poster at the Hawaii Dermatology Seminar that one in five participants in the two phase III trials had tinea pedis as well as onychomycosis, and nearly half of them were treated for their athlete’s foot using their physician’s choice of topical antifungals.
The primary endpoint in the two trials was the week 53 complete cure rate, defined as no clinical involvement of the target toenail, a negative potassium hydroxide exam, and a negative fungal culture. Among subjects with concomitant onychomycosis and tinea pedis, the onychomycosis complete cure rate was 28.2% if they received efinaconazole for their onychomycosis and got treatment for their tinea pedis, compared with 20.9% if they got efinaconazole but no treatment for their tinea pedis. The complete/almost complete cure rate was 35.5% with dual therapy versus 29.6% if they only received efinaconazole. Both differences were significant.
“Doesn’t that make logical sense? If you leave the fungus on the foot or between the toes, it’s going to say, ‘Wow, that’s steak up there on the nail. That’s real food. I’m just going to crawl back onto the nail because all my brothers up there are dead and there’s wide-open space,” Dr. Rosen explained.
He added that the reverse is also true: if a patient presents seeking treatment for athlete’s foot but also has onychomycosis, the best treatment results for the tinea pedis are obtained by also treating the nail infection.
Dr. Rosen offered a money-saving tip for effective OTC therapy for tinea pedis. Two words: Lotrimin Ultra. That’s the brand name for butenafine cream 1%, not to be confused with plain old Lotrimin, which is clotrimazole.
“Clotrimazole has been around since the dawn of man, and it’s not very effective. Many of the fungi are actually resistant to it. But they’re not resistant to butenafine, which is a very good topical antifungal now available over the counter. It costs $9 or $10 dollars for a tube the size of a baseball bat. It’s a good, effective, cheap way of treating concomitant tinea pedis,” he said.
Dr. Rosen reported serving on scientific advisory boards for Anacor, Merz, and Valeant.
SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
New drug comparable to voriconazole for aspergillosis
The broad-spectrum triazole isavuconazole was as effective as voriconazole in patients with suspected invasive mold disease and caused significantly fewer drug-related adverse events, particularly those of the skin, eyes, and hepatobiliary system, a randomized double-blind study of 516 adults has shown.
The findings suggest that the newer agent “could allow safer therapy” for the primary treatment of invasive aspergillosis and other mold disease than standard therapy with voriconazole, researchers for the phase III, industry-sponsored SECURE trial say in a report published in the Lancet.
The researchers assessed the safety and efficacy of isavuconazole versus voriconazole in patients with invasive mold infection. Patients were recruited from 102 centers across 26 countries over a 7-year period and were randomized to receive either drug.
In the study group of 516 adults with suspected invasive mold infection who received at least one dose of either antifungal drug, isavuconazole proved to be noninferior to voriconazole, by the primary endpoint of all-cause mortality at 6 weeks.
All-cause mortality at 6 weeks in this intention-to-treat group, of whom more than 80% had hematologic malignant disease, was 19% in the isavuconazole group (48 of 258) and 20% (52 of 258) in the voriconazole group.
This primary endpoint was chosen because “it provides the most objective and reproducible effect of therapy, and approximates best the attributable mortality, because deaths due to competing causes occur increasingly after 6 weeks,” Dr. Johan A. Maertensof the UZ Leuven (Belgium), and his associates wrote.
Secondary endpoints included overall response at the end of treatment among patients who were determined by an independent review committee to have proven or probable invasive mold disease – the study’s modified intention-to-treat population – as well as all-cause mortality at day 42 and day 84.
All-cause mortality in this modified intention-to-treat group, as well as in the group of patients found to have proven or probable invasive aspergillosis, specifically, supported the study’s primary findings (Lancet 2016 Feb:387:760-9).
Nearly all patients in the study had at least one treatment-emergent adverse event, and the proportion with serious treatment-emergent adverse events was similar between the treatment groups. However, patients treated with isavuconazole had a significantly lower frequency of hepatobiliary disorders, eye disorders, and skin or subcutaneous disorders.
And overall, significantly fewer patients reported drug-related adverse events with isavuconazole (42% of patients) than with voriconazole (60% of patients). Discontinuation from adverse events, moreover, was significantly less common among isavuconazole-treated patients.
Of the 516 patients in the intention-to-treat group, approximately 53% were confirmed to have proven or probable invasive mold disease, and more than 80% of the mycologically documented cases were Aspergillus infections. Enrollment of patients with possible invasive mold disease at the start “reflects the real-life strategy of early initiation of antifungal treatment,” the investigators say.
Isavuconazonium sulfate was approved in 2015 by the FDA for the treatment of invasive aspergillosis and invasive mucormycosis.
Voriconazole is the current gold standard for the primary treatment of invasive aspergillosis and is recommended for some other mold infections as well, but it is not active against mucormycosis and has “highly variable nonlinear pharmacokinetics in adults,” which has triggered recommendations for drug monitoring, Dr. Maertens and his associates say.
Therapeutic monitoring aimed at individualizing dosage regimes in order to improve response and prevent adverse events became the standard of care in some institutions during the study period (2007-2013). The study used the labeled dose of voriconazole, however, and did not address the efficacy of either drug with therapeutic drug monitoring.
The study also excluded patients with AIDS, abnormal liver or renal function, and those receiving antifungal prophylaxis with a mold-active triazole – factors that may limit generalizability of the findings, the investigators note.
Funding for the study was provided by Astellas Pharma Global Development and Basilea Pharmaceutica International.
Dr. Maertens disclosed receiving grants and fees from Bio-Rad, personal fees and nonfinancial support from Astellas and Basilea, and grants, fees and support from Gilead Sciences, Merck Sharp and Dohme, and Pfizer during the study.
The advantages of isavuconazole over voriconazole include its broader spectrum of activity, linear pharmacokinetics, once-daily dosing after the loading dose, and fewer CYP enzyme-mediated drug-drug interactions. This trial represents important progress in widening the therapeutic options for mold infections.
Numerous issues require further evaluation, including the effectiveness of isavuconazole after mold-active triazole prophylaxis, which is a common practice in patients at risk for mold infection, and the agent’s effectiveness against molds other than Aspergillus.
In addition, experience in a more varied patient population will be required to be certain that therapeutic drug monitoring is unnecessary.
Cost-effectiveness must also be explored. Isavuconazole will probably achieve an equivalent recommendation as voriconazole for initial treatment of aspergillosis in clinical guidelines, but voriconazole will soon come off patent in many countries and new formulations of posaconazole are now available.
That the finding that 42-day mortality in both treatment groups (isavuconazole and voriconazole) was no different than the mortality seen in research done 15 years ago on voriconazole treatment of aspergillosis is disappointing and suggests that we need to do better with the prevention and early detection of mold infection in vulnerable patients.
Dr. Monica A. Slavin and Dr. Karin A. Thursky are affiliated with the Peter MacCallum Cancer Centre, East Melbourne, Australia. Their comments are excerpted from an accompanying editorial in the Lancet. Dr. Slavin reported receiving grants from Merck, Gilead, and Pfizer. Dr. Thursky reported no disclosures.
The advantages of isavuconazole over voriconazole include its broader spectrum of activity, linear pharmacokinetics, once-daily dosing after the loading dose, and fewer CYP enzyme-mediated drug-drug interactions. This trial represents important progress in widening the therapeutic options for mold infections.
Numerous issues require further evaluation, including the effectiveness of isavuconazole after mold-active triazole prophylaxis, which is a common practice in patients at risk for mold infection, and the agent’s effectiveness against molds other than Aspergillus.
In addition, experience in a more varied patient population will be required to be certain that therapeutic drug monitoring is unnecessary.
Cost-effectiveness must also be explored. Isavuconazole will probably achieve an equivalent recommendation as voriconazole for initial treatment of aspergillosis in clinical guidelines, but voriconazole will soon come off patent in many countries and new formulations of posaconazole are now available.
That the finding that 42-day mortality in both treatment groups (isavuconazole and voriconazole) was no different than the mortality seen in research done 15 years ago on voriconazole treatment of aspergillosis is disappointing and suggests that we need to do better with the prevention and early detection of mold infection in vulnerable patients.
Dr. Monica A. Slavin and Dr. Karin A. Thursky are affiliated with the Peter MacCallum Cancer Centre, East Melbourne, Australia. Their comments are excerpted from an accompanying editorial in the Lancet. Dr. Slavin reported receiving grants from Merck, Gilead, and Pfizer. Dr. Thursky reported no disclosures.
The advantages of isavuconazole over voriconazole include its broader spectrum of activity, linear pharmacokinetics, once-daily dosing after the loading dose, and fewer CYP enzyme-mediated drug-drug interactions. This trial represents important progress in widening the therapeutic options for mold infections.
Numerous issues require further evaluation, including the effectiveness of isavuconazole after mold-active triazole prophylaxis, which is a common practice in patients at risk for mold infection, and the agent’s effectiveness against molds other than Aspergillus.
In addition, experience in a more varied patient population will be required to be certain that therapeutic drug monitoring is unnecessary.
Cost-effectiveness must also be explored. Isavuconazole will probably achieve an equivalent recommendation as voriconazole for initial treatment of aspergillosis in clinical guidelines, but voriconazole will soon come off patent in many countries and new formulations of posaconazole are now available.
That the finding that 42-day mortality in both treatment groups (isavuconazole and voriconazole) was no different than the mortality seen in research done 15 years ago on voriconazole treatment of aspergillosis is disappointing and suggests that we need to do better with the prevention and early detection of mold infection in vulnerable patients.
Dr. Monica A. Slavin and Dr. Karin A. Thursky are affiliated with the Peter MacCallum Cancer Centre, East Melbourne, Australia. Their comments are excerpted from an accompanying editorial in the Lancet. Dr. Slavin reported receiving grants from Merck, Gilead, and Pfizer. Dr. Thursky reported no disclosures.
The broad-spectrum triazole isavuconazole was as effective as voriconazole in patients with suspected invasive mold disease and caused significantly fewer drug-related adverse events, particularly those of the skin, eyes, and hepatobiliary system, a randomized double-blind study of 516 adults has shown.
The findings suggest that the newer agent “could allow safer therapy” for the primary treatment of invasive aspergillosis and other mold disease than standard therapy with voriconazole, researchers for the phase III, industry-sponsored SECURE trial say in a report published in the Lancet.
The researchers assessed the safety and efficacy of isavuconazole versus voriconazole in patients with invasive mold infection. Patients were recruited from 102 centers across 26 countries over a 7-year period and were randomized to receive either drug.
In the study group of 516 adults with suspected invasive mold infection who received at least one dose of either antifungal drug, isavuconazole proved to be noninferior to voriconazole, by the primary endpoint of all-cause mortality at 6 weeks.
All-cause mortality at 6 weeks in this intention-to-treat group, of whom more than 80% had hematologic malignant disease, was 19% in the isavuconazole group (48 of 258) and 20% (52 of 258) in the voriconazole group.
This primary endpoint was chosen because “it provides the most objective and reproducible effect of therapy, and approximates best the attributable mortality, because deaths due to competing causes occur increasingly after 6 weeks,” Dr. Johan A. Maertensof the UZ Leuven (Belgium), and his associates wrote.
Secondary endpoints included overall response at the end of treatment among patients who were determined by an independent review committee to have proven or probable invasive mold disease – the study’s modified intention-to-treat population – as well as all-cause mortality at day 42 and day 84.
All-cause mortality in this modified intention-to-treat group, as well as in the group of patients found to have proven or probable invasive aspergillosis, specifically, supported the study’s primary findings (Lancet 2016 Feb:387:760-9).
Nearly all patients in the study had at least one treatment-emergent adverse event, and the proportion with serious treatment-emergent adverse events was similar between the treatment groups. However, patients treated with isavuconazole had a significantly lower frequency of hepatobiliary disorders, eye disorders, and skin or subcutaneous disorders.
And overall, significantly fewer patients reported drug-related adverse events with isavuconazole (42% of patients) than with voriconazole (60% of patients). Discontinuation from adverse events, moreover, was significantly less common among isavuconazole-treated patients.
Of the 516 patients in the intention-to-treat group, approximately 53% were confirmed to have proven or probable invasive mold disease, and more than 80% of the mycologically documented cases were Aspergillus infections. Enrollment of patients with possible invasive mold disease at the start “reflects the real-life strategy of early initiation of antifungal treatment,” the investigators say.
Isavuconazonium sulfate was approved in 2015 by the FDA for the treatment of invasive aspergillosis and invasive mucormycosis.
Voriconazole is the current gold standard for the primary treatment of invasive aspergillosis and is recommended for some other mold infections as well, but it is not active against mucormycosis and has “highly variable nonlinear pharmacokinetics in adults,” which has triggered recommendations for drug monitoring, Dr. Maertens and his associates say.
Therapeutic monitoring aimed at individualizing dosage regimes in order to improve response and prevent adverse events became the standard of care in some institutions during the study period (2007-2013). The study used the labeled dose of voriconazole, however, and did not address the efficacy of either drug with therapeutic drug monitoring.
The study also excluded patients with AIDS, abnormal liver or renal function, and those receiving antifungal prophylaxis with a mold-active triazole – factors that may limit generalizability of the findings, the investigators note.
Funding for the study was provided by Astellas Pharma Global Development and Basilea Pharmaceutica International.
Dr. Maertens disclosed receiving grants and fees from Bio-Rad, personal fees and nonfinancial support from Astellas and Basilea, and grants, fees and support from Gilead Sciences, Merck Sharp and Dohme, and Pfizer during the study.
The broad-spectrum triazole isavuconazole was as effective as voriconazole in patients with suspected invasive mold disease and caused significantly fewer drug-related adverse events, particularly those of the skin, eyes, and hepatobiliary system, a randomized double-blind study of 516 adults has shown.
The findings suggest that the newer agent “could allow safer therapy” for the primary treatment of invasive aspergillosis and other mold disease than standard therapy with voriconazole, researchers for the phase III, industry-sponsored SECURE trial say in a report published in the Lancet.
The researchers assessed the safety and efficacy of isavuconazole versus voriconazole in patients with invasive mold infection. Patients were recruited from 102 centers across 26 countries over a 7-year period and were randomized to receive either drug.
In the study group of 516 adults with suspected invasive mold infection who received at least one dose of either antifungal drug, isavuconazole proved to be noninferior to voriconazole, by the primary endpoint of all-cause mortality at 6 weeks.
All-cause mortality at 6 weeks in this intention-to-treat group, of whom more than 80% had hematologic malignant disease, was 19% in the isavuconazole group (48 of 258) and 20% (52 of 258) in the voriconazole group.
This primary endpoint was chosen because “it provides the most objective and reproducible effect of therapy, and approximates best the attributable mortality, because deaths due to competing causes occur increasingly after 6 weeks,” Dr. Johan A. Maertensof the UZ Leuven (Belgium), and his associates wrote.
Secondary endpoints included overall response at the end of treatment among patients who were determined by an independent review committee to have proven or probable invasive mold disease – the study’s modified intention-to-treat population – as well as all-cause mortality at day 42 and day 84.
All-cause mortality in this modified intention-to-treat group, as well as in the group of patients found to have proven or probable invasive aspergillosis, specifically, supported the study’s primary findings (Lancet 2016 Feb:387:760-9).
Nearly all patients in the study had at least one treatment-emergent adverse event, and the proportion with serious treatment-emergent adverse events was similar between the treatment groups. However, patients treated with isavuconazole had a significantly lower frequency of hepatobiliary disorders, eye disorders, and skin or subcutaneous disorders.
And overall, significantly fewer patients reported drug-related adverse events with isavuconazole (42% of patients) than with voriconazole (60% of patients). Discontinuation from adverse events, moreover, was significantly less common among isavuconazole-treated patients.
Of the 516 patients in the intention-to-treat group, approximately 53% were confirmed to have proven or probable invasive mold disease, and more than 80% of the mycologically documented cases were Aspergillus infections. Enrollment of patients with possible invasive mold disease at the start “reflects the real-life strategy of early initiation of antifungal treatment,” the investigators say.
Isavuconazonium sulfate was approved in 2015 by the FDA for the treatment of invasive aspergillosis and invasive mucormycosis.
Voriconazole is the current gold standard for the primary treatment of invasive aspergillosis and is recommended for some other mold infections as well, but it is not active against mucormycosis and has “highly variable nonlinear pharmacokinetics in adults,” which has triggered recommendations for drug monitoring, Dr. Maertens and his associates say.
Therapeutic monitoring aimed at individualizing dosage regimes in order to improve response and prevent adverse events became the standard of care in some institutions during the study period (2007-2013). The study used the labeled dose of voriconazole, however, and did not address the efficacy of either drug with therapeutic drug monitoring.
The study also excluded patients with AIDS, abnormal liver or renal function, and those receiving antifungal prophylaxis with a mold-active triazole – factors that may limit generalizability of the findings, the investigators note.
Funding for the study was provided by Astellas Pharma Global Development and Basilea Pharmaceutica International.
Dr. Maertens disclosed receiving grants and fees from Bio-Rad, personal fees and nonfinancial support from Astellas and Basilea, and grants, fees and support from Gilead Sciences, Merck Sharp and Dohme, and Pfizer during the study.
FROM THE LANCET
Key clinical point: Isavuconazole is an appropriate alternative for primary treatment of suspected invasive aspergillosis.
Major finding: All-cause mortality at 6 weeks in the intention-to-treat group of 516 patients was 19% with isavuconazole and 20% with voriconazole. Fewer drug-related adverse events were reported with isavuconazole, however (42% vs. 60% of patients).
Data source: A phase III randomized, double-blind noninferiority trial – the SECURE trial – comparing the safety and efficacy of intravenous and oral formulations of isavuconazole and voriconazole for the primary treatment of invasive aspergillosis and disease caused by other molds.
Disclosures: Funding for the study was provided by Astellas Pharma Global Development and Basilea Pharmaceutica International. Dr. Maertens disclosed receiving grants and fees from Bio-Rad, personal fees and nonfinancial support from Astellas and Basilea, and grants, fees, and support from Gilead Sciences, Merck Sharp and Dohme, and Pfizer, during the study.