User login
Photoprotection: Benefits of Sunscreens With Iron Oxide
CHICAGO — One of the more recent developments in sunscreen technology is the addition of iron oxide to mineral sunscreens.
Iron oxide is “an excellent pigment” that absorbs and blocks visible light, which is particularly important in individuals with Fitzpatrick skin types III-VI, Zoe D. Draelos, MD, consulting professor of dermatology at Duke University, Durham, North Carolina, said at the Pigmentation Disorders Exchange symposium.
Susan C. Taylor, MD, professor of dermatology at the University of Pennsylvania, Philadelphia, who spoke at the conference, also recommended tinted sunscreen with iron oxide for patients with skin of color. “It still needs to be broad spectrum,” she said, “and at least an SPF [Sun Protection Factor] 30.”
When blended with mineral sunscreens, iron oxide can reduce transmission of visible light by 90% and can protect patients from hyperpigmentation. Iron oxide comes in different colors blended together for various degrees of tinting.
Dr. Taylor noted that iron oxide is listed under the inactive ingredients. “The literature indicates a 3% concentration to aim for, but we don’t know the concentration in most of the products,” she added.
During her presentation, Dr. Draelos noted that inorganic sunscreens, such as zinc oxide and titanium oxides, are highly effective but make the skin white and pasty. To address this issue, many companies are now grinding these materials into such small particles that they are transparent.
“That’s great, except the smaller the particle is, the less UV [ultraviolet] radiation it reflects and that lowers the [SPF],” she said.
In addition to providing photoprotection, sunscreens in general provide protection from nanoparticles in tobacco and combustion, such as traffic exhaust, which can harm skin over time. “Moisturizers and sunscreens are the best way to protect against pollution and tobacco nanoparticle damage, which can contribute to inflammation,” she noted. They create a film over the skin and trap the nanoparticles.
Start the Patient Visit With a Photoprotection Talk
At the meeting, Dr. Taylor recommended that for all patients with hypopigmentation and hyperpigmentation disorders, “treatment really begins with photoprotection.”
She acknowledged that photoprotection discussions, including the basics of seeking shade, wearing protective clothing, and avoiding midday sun, often come at the end of the patient visit but she urged dermatologists to make that the first topic instead.
Dr. Taylor said a question often asked of patients of color about prolonged sun exposure — whether their skin turns bright red after too much sun — may get a negative reply. The better question is whether the patient has experienced tender skin after too much sun — which can signify a sunburn, she said.
Dr. Draelos reported no relevant financial relationships. Dr. Taylor reported financial relationships and grant support from multiple pharmaceutical companies.
A version of this article first appeared on Medscape.com.
CHICAGO — One of the more recent developments in sunscreen technology is the addition of iron oxide to mineral sunscreens.
Iron oxide is “an excellent pigment” that absorbs and blocks visible light, which is particularly important in individuals with Fitzpatrick skin types III-VI, Zoe D. Draelos, MD, consulting professor of dermatology at Duke University, Durham, North Carolina, said at the Pigmentation Disorders Exchange symposium.
Susan C. Taylor, MD, professor of dermatology at the University of Pennsylvania, Philadelphia, who spoke at the conference, also recommended tinted sunscreen with iron oxide for patients with skin of color. “It still needs to be broad spectrum,” she said, “and at least an SPF [Sun Protection Factor] 30.”
When blended with mineral sunscreens, iron oxide can reduce transmission of visible light by 90% and can protect patients from hyperpigmentation. Iron oxide comes in different colors blended together for various degrees of tinting.
Dr. Taylor noted that iron oxide is listed under the inactive ingredients. “The literature indicates a 3% concentration to aim for, but we don’t know the concentration in most of the products,” she added.
During her presentation, Dr. Draelos noted that inorganic sunscreens, such as zinc oxide and titanium oxides, are highly effective but make the skin white and pasty. To address this issue, many companies are now grinding these materials into such small particles that they are transparent.
“That’s great, except the smaller the particle is, the less UV [ultraviolet] radiation it reflects and that lowers the [SPF],” she said.
In addition to providing photoprotection, sunscreens in general provide protection from nanoparticles in tobacco and combustion, such as traffic exhaust, which can harm skin over time. “Moisturizers and sunscreens are the best way to protect against pollution and tobacco nanoparticle damage, which can contribute to inflammation,” she noted. They create a film over the skin and trap the nanoparticles.
Start the Patient Visit With a Photoprotection Talk
At the meeting, Dr. Taylor recommended that for all patients with hypopigmentation and hyperpigmentation disorders, “treatment really begins with photoprotection.”
She acknowledged that photoprotection discussions, including the basics of seeking shade, wearing protective clothing, and avoiding midday sun, often come at the end of the patient visit but she urged dermatologists to make that the first topic instead.
Dr. Taylor said a question often asked of patients of color about prolonged sun exposure — whether their skin turns bright red after too much sun — may get a negative reply. The better question is whether the patient has experienced tender skin after too much sun — which can signify a sunburn, she said.
Dr. Draelos reported no relevant financial relationships. Dr. Taylor reported financial relationships and grant support from multiple pharmaceutical companies.
A version of this article first appeared on Medscape.com.
CHICAGO — One of the more recent developments in sunscreen technology is the addition of iron oxide to mineral sunscreens.
Iron oxide is “an excellent pigment” that absorbs and blocks visible light, which is particularly important in individuals with Fitzpatrick skin types III-VI, Zoe D. Draelos, MD, consulting professor of dermatology at Duke University, Durham, North Carolina, said at the Pigmentation Disorders Exchange symposium.
Susan C. Taylor, MD, professor of dermatology at the University of Pennsylvania, Philadelphia, who spoke at the conference, also recommended tinted sunscreen with iron oxide for patients with skin of color. “It still needs to be broad spectrum,” she said, “and at least an SPF [Sun Protection Factor] 30.”
When blended with mineral sunscreens, iron oxide can reduce transmission of visible light by 90% and can protect patients from hyperpigmentation. Iron oxide comes in different colors blended together for various degrees of tinting.
Dr. Taylor noted that iron oxide is listed under the inactive ingredients. “The literature indicates a 3% concentration to aim for, but we don’t know the concentration in most of the products,” she added.
During her presentation, Dr. Draelos noted that inorganic sunscreens, such as zinc oxide and titanium oxides, are highly effective but make the skin white and pasty. To address this issue, many companies are now grinding these materials into such small particles that they are transparent.
“That’s great, except the smaller the particle is, the less UV [ultraviolet] radiation it reflects and that lowers the [SPF],” she said.
In addition to providing photoprotection, sunscreens in general provide protection from nanoparticles in tobacco and combustion, such as traffic exhaust, which can harm skin over time. “Moisturizers and sunscreens are the best way to protect against pollution and tobacco nanoparticle damage, which can contribute to inflammation,” she noted. They create a film over the skin and trap the nanoparticles.
Start the Patient Visit With a Photoprotection Talk
At the meeting, Dr. Taylor recommended that for all patients with hypopigmentation and hyperpigmentation disorders, “treatment really begins with photoprotection.”
She acknowledged that photoprotection discussions, including the basics of seeking shade, wearing protective clothing, and avoiding midday sun, often come at the end of the patient visit but she urged dermatologists to make that the first topic instead.
Dr. Taylor said a question often asked of patients of color about prolonged sun exposure — whether their skin turns bright red after too much sun — may get a negative reply. The better question is whether the patient has experienced tender skin after too much sun — which can signify a sunburn, she said.
Dr. Draelos reported no relevant financial relationships. Dr. Taylor reported financial relationships and grant support from multiple pharmaceutical companies.
A version of this article first appeared on Medscape.com.
FDA Expands Repotrectinib Label to All NTRK Gene Fusion+ Solid Tumors
The approval is a label expansion for the tyrosine kinase inhibitor (TKI), which received initial clearance in November 2023 for locally advanced or metastatic ROS1-positive non–small cell lung cancer.
NTRK gene fusions are genetic abnormalities wherein part of the NTRK gene fuses with an unrelated gene. The abnormal gene can then produce an oncogenic protein. Although rare, these mutations are found in many cancer types.
The approval, for adult and pediatric patients aged 12 years or older, was based on the single-arm open-label TRIDENT-1 trial in 88 adults with locally advanced or metastatic NTRK gene fusion solid tumors.
In the 40 patients who were TKI-naive, the overall response rate was 58%, and the median duration of response was not estimable. In the 48 patients who had a TKI previously, the overall response rate was 50% and median duration of response was 9.9 months.
In 20% or more of participants, treatment caused dizziness, dysgeusia, peripheral neuropathy, constipation, dyspnea, fatigue, ataxia, cognitive impairment, muscular weakness, and nausea.
Labeling warns of central nervous system reactions, interstitial lung disease/pneumonitis, hepatotoxicity, myalgia with creatine phosphokinase elevation, hyperuricemia, bone fractures, and embryo-fetal toxicity.
The recommended dose is 160 mg orally once daily for 14 days then increased to 160 mg twice daily until disease progression or unacceptable toxicity.
Sixty 40-mg capsules cost around $7,644, according to drugs.com.
A version of this article appeared on Medscape.com.
The approval is a label expansion for the tyrosine kinase inhibitor (TKI), which received initial clearance in November 2023 for locally advanced or metastatic ROS1-positive non–small cell lung cancer.
NTRK gene fusions are genetic abnormalities wherein part of the NTRK gene fuses with an unrelated gene. The abnormal gene can then produce an oncogenic protein. Although rare, these mutations are found in many cancer types.
The approval, for adult and pediatric patients aged 12 years or older, was based on the single-arm open-label TRIDENT-1 trial in 88 adults with locally advanced or metastatic NTRK gene fusion solid tumors.
In the 40 patients who were TKI-naive, the overall response rate was 58%, and the median duration of response was not estimable. In the 48 patients who had a TKI previously, the overall response rate was 50% and median duration of response was 9.9 months.
In 20% or more of participants, treatment caused dizziness, dysgeusia, peripheral neuropathy, constipation, dyspnea, fatigue, ataxia, cognitive impairment, muscular weakness, and nausea.
Labeling warns of central nervous system reactions, interstitial lung disease/pneumonitis, hepatotoxicity, myalgia with creatine phosphokinase elevation, hyperuricemia, bone fractures, and embryo-fetal toxicity.
The recommended dose is 160 mg orally once daily for 14 days then increased to 160 mg twice daily until disease progression or unacceptable toxicity.
Sixty 40-mg capsules cost around $7,644, according to drugs.com.
A version of this article appeared on Medscape.com.
The approval is a label expansion for the tyrosine kinase inhibitor (TKI), which received initial clearance in November 2023 for locally advanced or metastatic ROS1-positive non–small cell lung cancer.
NTRK gene fusions are genetic abnormalities wherein part of the NTRK gene fuses with an unrelated gene. The abnormal gene can then produce an oncogenic protein. Although rare, these mutations are found in many cancer types.
The approval, for adult and pediatric patients aged 12 years or older, was based on the single-arm open-label TRIDENT-1 trial in 88 adults with locally advanced or metastatic NTRK gene fusion solid tumors.
In the 40 patients who were TKI-naive, the overall response rate was 58%, and the median duration of response was not estimable. In the 48 patients who had a TKI previously, the overall response rate was 50% and median duration of response was 9.9 months.
In 20% or more of participants, treatment caused dizziness, dysgeusia, peripheral neuropathy, constipation, dyspnea, fatigue, ataxia, cognitive impairment, muscular weakness, and nausea.
Labeling warns of central nervous system reactions, interstitial lung disease/pneumonitis, hepatotoxicity, myalgia with creatine phosphokinase elevation, hyperuricemia, bone fractures, and embryo-fetal toxicity.
The recommended dose is 160 mg orally once daily for 14 days then increased to 160 mg twice daily until disease progression or unacceptable toxicity.
Sixty 40-mg capsules cost around $7,644, according to drugs.com.
A version of this article appeared on Medscape.com.
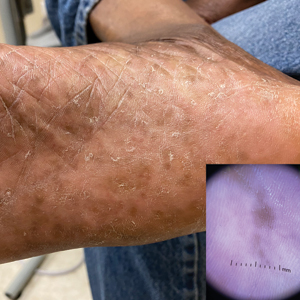
Plantar Hyperpigmentation
The Comparison
Plantar hyperpigmentation (also known as plantar melanosis [increased melanin], volar pigmented macules, benign racial melanosis, acral pigmentation, acral ethnic melanosis, or mottled hyperpigmentation of the plantar surface) is a benign finding in many individuals and is especially prevalent in those with darker skin tones. Acral refers to manifestation on the hands and feet, volar on the palms and soles, and plantar on the soles only. Here, we focus on plantar hyperpigmentation. We use the terms ethnic and racial interchangeably.
It is critically important to differentiate benign hyperpigmentation, which is common in patients with skin of color, from melanoma. Although rare, Black patients in the United States experience high morbidity and mortality from acral melanoma, which often is diagnosed late in the disease course.1
There are many causes of hyperpigmentation on the plantar surfaces, including benign ethnic melanosis, nevi, melanoma, infections such as syphilis and tinea nigra, conditions such as Peutz-Jeghers syndrome and Laugier-Hunziker syndrome, and postinflammatory hyperpigmentation secondary to atopic dermatitis and psoriasis. We focus on the most common causes, ethnic melanosis and nevi, as well as melanoma, which is the deadliest cause.
Epidemiology
In a 1980 study (N=251), Black Americans had a high incidence of plantar hyperpigmentation, with 52% of affected patients having dark brown skin and 31% having light brown skin.2
The epidemiology of melanoma varies by race/ethnicity. Melanoma in Black individuals is relatively rare, with an annual incidence of approximately 1 in 100,000 individuals.3 However, when individuals with skin of color develop melanoma, they are more likely than their White counterparts to have acral melanoma (acral lentiginous melanoma), one of the deadliest types.1 In a case series of Black patients with melanoma (N=48) from 2 tertiary care centers in Texas, 30 of 40 primary cutaneous melanomas (75%) were located on acral skin.4 Overall, 13 patients developed stage IV disease and 12 died due to disease progression. All patients who developed distant metastases or died of melanoma had acral melanoma.4 Individuals of Asian descent also have a high incidence of acral melanoma, as shown in research from Japan.5-9
Key clinical features in individuals with darker skin tones
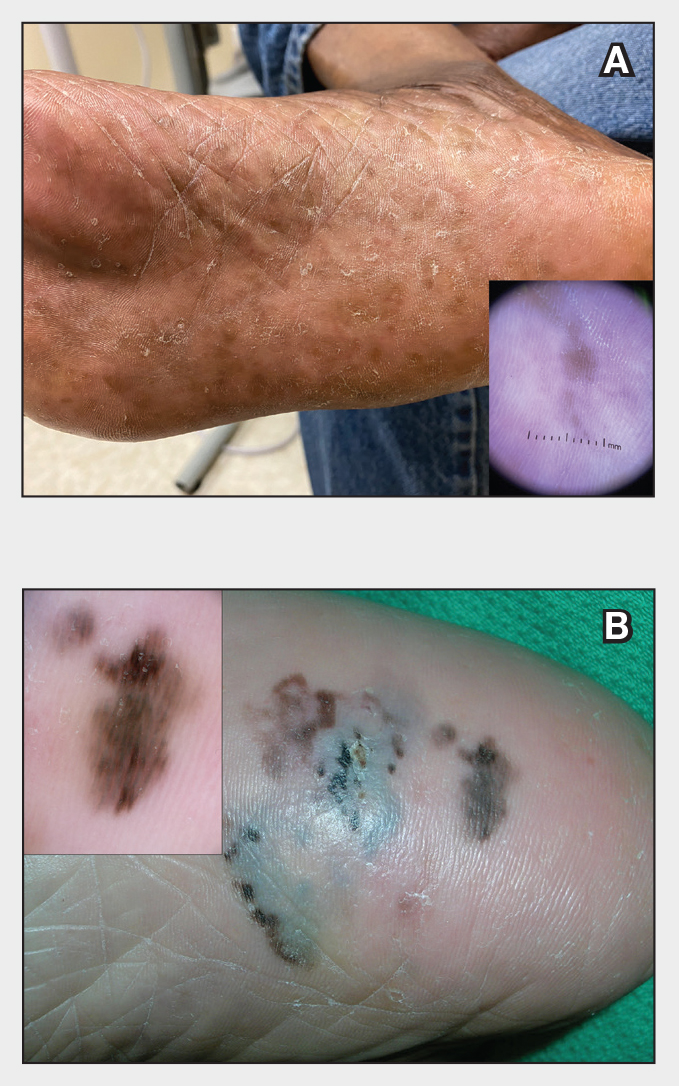
Dermoscopy is an evidence-based clinical examination method for earlier diagnosis of cutaneous melanoma, including on acral skin.10,11 Benign nevi on the volar skin as well as the palms and soles tend to have one of these 3 dermoscopic patterns: parallel furrow, lattice, or irregular fibrillar. The pattern that is most predictive of volar melanoma is the parallel ridge pattern (PRP) (Figures A and B [insets]), which showed a high specificity (99.0%) and very high negative predictive value (97.7%) for malignant melanoma in a Japanese population.7 The PRP data from this study cannot be applied reliably to Black individuals, especially because benign ethnic melanosis and other benign conditions can demonstrate PRP.12 Reliance on the PRP as a diagnostic clue could result in unneccessary biopsies in as many as 50% of Black patients with benign plantar hyperpigmentation.2 Furthermore, biopsies of the plantar surface can be painful and cause pain while walking.
It has been suggested that PRP seen on dermoscopy in benign hyperpigmentation such as ethnic melanosis and nevi may preserve the acrosyringia (eccrine gland openings on the ridge), whereas PRP in melanoma may obliterate the acrosyringia.13 This observation is based on case reports only and needs further study. However, if validated, it could be a useful diagnostic clue.
Worth noting
In a retrospective cohort study of skin cancer in Black individuals (n=165) at a New York City–based cancer center from 2000 to 2020, 68% of patients were diagnosed with melanomas—80% were the acral subtype and 75% displayed a PRP. However, the surrounding uninvolved background skin, which was visible in most cases, also demonstrated a PRP.14 Because of the high morbidity and mortality rates of acral melanoma, clinicians should biopsy or immediately refer patients with concerning plantar hyperpigmentation to a dermatologist.
Health disparity highlight
The mortality rate for acral melanoma in Black patients is disproportionately high for the following reasons15,16:
- Patients and health care providers do not expect to see melanoma in Black patients (it truly is rare!), so screening and education on sun protection are limited.
- Benign ethnic melanosis makes it more difficult to distinguish between early acral melanoma and benign skin changes.
- Black patients and other US patient populations with skin of color may be less likely to have health insurance, which contributes to inequities in access to health care. As of 2022, the uninsured rates for nonelderly American Indian and Alaska Native, Hispanic, Native Hawaiian and Other Pacific Islander, Black, and White individuals were 19.1%, 18.0%, 12.7%, 10.0%, and 6.6%, respectively.17
Multi-institutional registries could improve understanding of acral melanoma in Black patients.4 More studies are needed to help differentiate between the dermoscopic finding of PRP in benign ethnic melanosis vs malignant melanoma.
- Huang K, Fan J, Misra S. Acral lentiginous melanoma: incidence and survival in the United States, 2006-2015: an analysis of the SEER registry. J Surg Res. 2020;251:329-339. doi:10.1016/j.jss.2020.02.010
- Coleman WP, Gately LE, Krementz AB, et al. Nevi, lentigines, and melanomas in blacks. Arch Dermatol. 1980;116:548-551.
- Centers for Disease Control and Prevention. Melanoma Incidence and Mortality, United States: 2012-2016. USCS Data Brief, no. 9. Centers for Disease Control and Prevention, US Department of Health and Human Services; 2019. https://www.cdc.gov/cancer/uscs/about/data-briefs/no9-melanoma-incidence-mortality-UnitedStates-2012-2016.htm
- Wix SN, Brown AB, Heberton M, et al. Clinical features and outcomes of black patients with melanoma. JAMA Dermatol. 2024;160:328-333. doi:10.1001/jamadermatol.2023.5789
- Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch Dermatol. 2007;143:1423-1426. doi:10.1001/archderm.143.11.1423
- Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38:25-34. doi:10.1111/j.1346-8138.2010.01174.x
- Saida T, Miyazaki A, Oguchi S. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140:1233-1238. doi:10.1001/archderm.140.10.1233
- Saida T, Koga H, Uhara H. Dermoscopy for acral melanocytic lesions: revision of the 3-step algorithm and refined definition of the regular and irregular fibrillar pattern. Dermatol Pract Concept. 2022;12:e2022123. doi:10.5826/dpc.1203a123
- Heath CR, Usatine RP. Melanoma. Cutis. 2022;109:284-285.doi:10.12788/cutis.0513.
- Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Visual inspection and dermoscopy, alone or in combination, for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018; 12:CD011901. doi:10.1002/14651858.CD011901.pub2
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked-eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi:10.1111/j.1365-2133.2008.08713.x
- Phan A, Dalle S, Marcilly MC, et al. Benign dermoscopic parallel ridge pattern variants. Arch Dermatol. 2011;147:634. doi:10.1001/archdermatol.2011.47
- Fracaroli TS, Lavorato FG, Maceira JP, et al. Parallel ridge pattern on dermoscopy: observation in non-melanoma cases. An Bras Dermatol. 2013;88:646-648. doi:10.1590/abd1806-4841.20132058
- Manci RN, Dauscher M, Marchetti MA, et al. Features of skin cancer in black individuals: a single-institution retrospective cohort study. Dermatol Pract Concept. 2022;12:e2022075. doi:10.5826/dpc.1202a75
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
- Ingrassia JP, Stein JA, Levine A, et al. Diagnosis and management of acral pigmented lesions. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2023;49:926-931. doi:10.1097/DSS.0000000000003891
- Hill L, Artiga S, Damico A. Health coverage by race and ethnicity, 2010-2022. Kaiser Family Foundation. Published January 11, 2024. Accessed May 9, 2024. https://www.kff.org/racial-equity-and-health-policy/issue-brief/health-coverage-by-race-and-ethnicity
The Comparison

Plantar hyperpigmentation (also known as plantar melanosis [increased melanin], volar pigmented macules, benign racial melanosis, acral pigmentation, acral ethnic melanosis, or mottled hyperpigmentation of the plantar surface) is a benign finding in many individuals and is especially prevalent in those with darker skin tones. Acral refers to manifestation on the hands and feet, volar on the palms and soles, and plantar on the soles only. Here, we focus on plantar hyperpigmentation. We use the terms ethnic and racial interchangeably.
It is critically important to differentiate benign hyperpigmentation, which is common in patients with skin of color, from melanoma. Although rare, Black patients in the United States experience high morbidity and mortality from acral melanoma, which often is diagnosed late in the disease course.1
There are many causes of hyperpigmentation on the plantar surfaces, including benign ethnic melanosis, nevi, melanoma, infections such as syphilis and tinea nigra, conditions such as Peutz-Jeghers syndrome and Laugier-Hunziker syndrome, and postinflammatory hyperpigmentation secondary to atopic dermatitis and psoriasis. We focus on the most common causes, ethnic melanosis and nevi, as well as melanoma, which is the deadliest cause.
Epidemiology
In a 1980 study (N=251), Black Americans had a high incidence of plantar hyperpigmentation, with 52% of affected patients having dark brown skin and 31% having light brown skin.2
The epidemiology of melanoma varies by race/ethnicity. Melanoma in Black individuals is relatively rare, with an annual incidence of approximately 1 in 100,000 individuals.3 However, when individuals with skin of color develop melanoma, they are more likely than their White counterparts to have acral melanoma (acral lentiginous melanoma), one of the deadliest types.1 In a case series of Black patients with melanoma (N=48) from 2 tertiary care centers in Texas, 30 of 40 primary cutaneous melanomas (75%) were located on acral skin.4 Overall, 13 patients developed stage IV disease and 12 died due to disease progression. All patients who developed distant metastases or died of melanoma had acral melanoma.4 Individuals of Asian descent also have a high incidence of acral melanoma, as shown in research from Japan.5-9
Key clinical features in individuals with darker skin tones
Dermoscopy is an evidence-based clinical examination method for earlier diagnosis of cutaneous melanoma, including on acral skin.10,11 Benign nevi on the volar skin as well as the palms and soles tend to have one of these 3 dermoscopic patterns: parallel furrow, lattice, or irregular fibrillar. The pattern that is most predictive of volar melanoma is the parallel ridge pattern (PRP) (Figures A and B [insets]), which showed a high specificity (99.0%) and very high negative predictive value (97.7%) for malignant melanoma in a Japanese population.7 The PRP data from this study cannot be applied reliably to Black individuals, especially because benign ethnic melanosis and other benign conditions can demonstrate PRP.12 Reliance on the PRP as a diagnostic clue could result in unneccessary biopsies in as many as 50% of Black patients with benign plantar hyperpigmentation.2 Furthermore, biopsies of the plantar surface can be painful and cause pain while walking.
It has been suggested that PRP seen on dermoscopy in benign hyperpigmentation such as ethnic melanosis and nevi may preserve the acrosyringia (eccrine gland openings on the ridge), whereas PRP in melanoma may obliterate the acrosyringia.13 This observation is based on case reports only and needs further study. However, if validated, it could be a useful diagnostic clue.
Worth noting
In a retrospective cohort study of skin cancer in Black individuals (n=165) at a New York City–based cancer center from 2000 to 2020, 68% of patients were diagnosed with melanomas—80% were the acral subtype and 75% displayed a PRP. However, the surrounding uninvolved background skin, which was visible in most cases, also demonstrated a PRP.14 Because of the high morbidity and mortality rates of acral melanoma, clinicians should biopsy or immediately refer patients with concerning plantar hyperpigmentation to a dermatologist.
Health disparity highlight
The mortality rate for acral melanoma in Black patients is disproportionately high for the following reasons15,16:
- Patients and health care providers do not expect to see melanoma in Black patients (it truly is rare!), so screening and education on sun protection are limited.
- Benign ethnic melanosis makes it more difficult to distinguish between early acral melanoma and benign skin changes.
- Black patients and other US patient populations with skin of color may be less likely to have health insurance, which contributes to inequities in access to health care. As of 2022, the uninsured rates for nonelderly American Indian and Alaska Native, Hispanic, Native Hawaiian and Other Pacific Islander, Black, and White individuals were 19.1%, 18.0%, 12.7%, 10.0%, and 6.6%, respectively.17
Multi-institutional registries could improve understanding of acral melanoma in Black patients.4 More studies are needed to help differentiate between the dermoscopic finding of PRP in benign ethnic melanosis vs malignant melanoma.
The Comparison

Plantar hyperpigmentation (also known as plantar melanosis [increased melanin], volar pigmented macules, benign racial melanosis, acral pigmentation, acral ethnic melanosis, or mottled hyperpigmentation of the plantar surface) is a benign finding in many individuals and is especially prevalent in those with darker skin tones. Acral refers to manifestation on the hands and feet, volar on the palms and soles, and plantar on the soles only. Here, we focus on plantar hyperpigmentation. We use the terms ethnic and racial interchangeably.
It is critically important to differentiate benign hyperpigmentation, which is common in patients with skin of color, from melanoma. Although rare, Black patients in the United States experience high morbidity and mortality from acral melanoma, which often is diagnosed late in the disease course.1
There are many causes of hyperpigmentation on the plantar surfaces, including benign ethnic melanosis, nevi, melanoma, infections such as syphilis and tinea nigra, conditions such as Peutz-Jeghers syndrome and Laugier-Hunziker syndrome, and postinflammatory hyperpigmentation secondary to atopic dermatitis and psoriasis. We focus on the most common causes, ethnic melanosis and nevi, as well as melanoma, which is the deadliest cause.
Epidemiology
In a 1980 study (N=251), Black Americans had a high incidence of plantar hyperpigmentation, with 52% of affected patients having dark brown skin and 31% having light brown skin.2
The epidemiology of melanoma varies by race/ethnicity. Melanoma in Black individuals is relatively rare, with an annual incidence of approximately 1 in 100,000 individuals.3 However, when individuals with skin of color develop melanoma, they are more likely than their White counterparts to have acral melanoma (acral lentiginous melanoma), one of the deadliest types.1 In a case series of Black patients with melanoma (N=48) from 2 tertiary care centers in Texas, 30 of 40 primary cutaneous melanomas (75%) were located on acral skin.4 Overall, 13 patients developed stage IV disease and 12 died due to disease progression. All patients who developed distant metastases or died of melanoma had acral melanoma.4 Individuals of Asian descent also have a high incidence of acral melanoma, as shown in research from Japan.5-9
Key clinical features in individuals with darker skin tones
Dermoscopy is an evidence-based clinical examination method for earlier diagnosis of cutaneous melanoma, including on acral skin.10,11 Benign nevi on the volar skin as well as the palms and soles tend to have one of these 3 dermoscopic patterns: parallel furrow, lattice, or irregular fibrillar. The pattern that is most predictive of volar melanoma is the parallel ridge pattern (PRP) (Figures A and B [insets]), which showed a high specificity (99.0%) and very high negative predictive value (97.7%) for malignant melanoma in a Japanese population.7 The PRP data from this study cannot be applied reliably to Black individuals, especially because benign ethnic melanosis and other benign conditions can demonstrate PRP.12 Reliance on the PRP as a diagnostic clue could result in unneccessary biopsies in as many as 50% of Black patients with benign plantar hyperpigmentation.2 Furthermore, biopsies of the plantar surface can be painful and cause pain while walking.
It has been suggested that PRP seen on dermoscopy in benign hyperpigmentation such as ethnic melanosis and nevi may preserve the acrosyringia (eccrine gland openings on the ridge), whereas PRP in melanoma may obliterate the acrosyringia.13 This observation is based on case reports only and needs further study. However, if validated, it could be a useful diagnostic clue.
Worth noting
In a retrospective cohort study of skin cancer in Black individuals (n=165) at a New York City–based cancer center from 2000 to 2020, 68% of patients were diagnosed with melanomas—80% were the acral subtype and 75% displayed a PRP. However, the surrounding uninvolved background skin, which was visible in most cases, also demonstrated a PRP.14 Because of the high morbidity and mortality rates of acral melanoma, clinicians should biopsy or immediately refer patients with concerning plantar hyperpigmentation to a dermatologist.
Health disparity highlight
The mortality rate for acral melanoma in Black patients is disproportionately high for the following reasons15,16:
- Patients and health care providers do not expect to see melanoma in Black patients (it truly is rare!), so screening and education on sun protection are limited.
- Benign ethnic melanosis makes it more difficult to distinguish between early acral melanoma and benign skin changes.
- Black patients and other US patient populations with skin of color may be less likely to have health insurance, which contributes to inequities in access to health care. As of 2022, the uninsured rates for nonelderly American Indian and Alaska Native, Hispanic, Native Hawaiian and Other Pacific Islander, Black, and White individuals were 19.1%, 18.0%, 12.7%, 10.0%, and 6.6%, respectively.17
Multi-institutional registries could improve understanding of acral melanoma in Black patients.4 More studies are needed to help differentiate between the dermoscopic finding of PRP in benign ethnic melanosis vs malignant melanoma.
- Huang K, Fan J, Misra S. Acral lentiginous melanoma: incidence and survival in the United States, 2006-2015: an analysis of the SEER registry. J Surg Res. 2020;251:329-339. doi:10.1016/j.jss.2020.02.010
- Coleman WP, Gately LE, Krementz AB, et al. Nevi, lentigines, and melanomas in blacks. Arch Dermatol. 1980;116:548-551.
- Centers for Disease Control and Prevention. Melanoma Incidence and Mortality, United States: 2012-2016. USCS Data Brief, no. 9. Centers for Disease Control and Prevention, US Department of Health and Human Services; 2019. https://www.cdc.gov/cancer/uscs/about/data-briefs/no9-melanoma-incidence-mortality-UnitedStates-2012-2016.htm
- Wix SN, Brown AB, Heberton M, et al. Clinical features and outcomes of black patients with melanoma. JAMA Dermatol. 2024;160:328-333. doi:10.1001/jamadermatol.2023.5789
- Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch Dermatol. 2007;143:1423-1426. doi:10.1001/archderm.143.11.1423
- Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38:25-34. doi:10.1111/j.1346-8138.2010.01174.x
- Saida T, Miyazaki A, Oguchi S. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140:1233-1238. doi:10.1001/archderm.140.10.1233
- Saida T, Koga H, Uhara H. Dermoscopy for acral melanocytic lesions: revision of the 3-step algorithm and refined definition of the regular and irregular fibrillar pattern. Dermatol Pract Concept. 2022;12:e2022123. doi:10.5826/dpc.1203a123
- Heath CR, Usatine RP. Melanoma. Cutis. 2022;109:284-285.doi:10.12788/cutis.0513.
- Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Visual inspection and dermoscopy, alone or in combination, for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018; 12:CD011901. doi:10.1002/14651858.CD011901.pub2
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked-eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi:10.1111/j.1365-2133.2008.08713.x
- Phan A, Dalle S, Marcilly MC, et al. Benign dermoscopic parallel ridge pattern variants. Arch Dermatol. 2011;147:634. doi:10.1001/archdermatol.2011.47
- Fracaroli TS, Lavorato FG, Maceira JP, et al. Parallel ridge pattern on dermoscopy: observation in non-melanoma cases. An Bras Dermatol. 2013;88:646-648. doi:10.1590/abd1806-4841.20132058
- Manci RN, Dauscher M, Marchetti MA, et al. Features of skin cancer in black individuals: a single-institution retrospective cohort study. Dermatol Pract Concept. 2022;12:e2022075. doi:10.5826/dpc.1202a75
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
- Ingrassia JP, Stein JA, Levine A, et al. Diagnosis and management of acral pigmented lesions. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2023;49:926-931. doi:10.1097/DSS.0000000000003891
- Hill L, Artiga S, Damico A. Health coverage by race and ethnicity, 2010-2022. Kaiser Family Foundation. Published January 11, 2024. Accessed May 9, 2024. https://www.kff.org/racial-equity-and-health-policy/issue-brief/health-coverage-by-race-and-ethnicity
- Huang K, Fan J, Misra S. Acral lentiginous melanoma: incidence and survival in the United States, 2006-2015: an analysis of the SEER registry. J Surg Res. 2020;251:329-339. doi:10.1016/j.jss.2020.02.010
- Coleman WP, Gately LE, Krementz AB, et al. Nevi, lentigines, and melanomas in blacks. Arch Dermatol. 1980;116:548-551.
- Centers for Disease Control and Prevention. Melanoma Incidence and Mortality, United States: 2012-2016. USCS Data Brief, no. 9. Centers for Disease Control and Prevention, US Department of Health and Human Services; 2019. https://www.cdc.gov/cancer/uscs/about/data-briefs/no9-melanoma-incidence-mortality-UnitedStates-2012-2016.htm
- Wix SN, Brown AB, Heberton M, et al. Clinical features and outcomes of black patients with melanoma. JAMA Dermatol. 2024;160:328-333. doi:10.1001/jamadermatol.2023.5789
- Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch Dermatol. 2007;143:1423-1426. doi:10.1001/archderm.143.11.1423
- Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38:25-34. doi:10.1111/j.1346-8138.2010.01174.x
- Saida T, Miyazaki A, Oguchi S. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140:1233-1238. doi:10.1001/archderm.140.10.1233
- Saida T, Koga H, Uhara H. Dermoscopy for acral melanocytic lesions: revision of the 3-step algorithm and refined definition of the regular and irregular fibrillar pattern. Dermatol Pract Concept. 2022;12:e2022123. doi:10.5826/dpc.1203a123
- Heath CR, Usatine RP. Melanoma. Cutis. 2022;109:284-285.doi:10.12788/cutis.0513.
- Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Visual inspection and dermoscopy, alone or in combination, for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018; 12:CD011901. doi:10.1002/14651858.CD011901.pub2
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked-eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi:10.1111/j.1365-2133.2008.08713.x
- Phan A, Dalle S, Marcilly MC, et al. Benign dermoscopic parallel ridge pattern variants. Arch Dermatol. 2011;147:634. doi:10.1001/archdermatol.2011.47
- Fracaroli TS, Lavorato FG, Maceira JP, et al. Parallel ridge pattern on dermoscopy: observation in non-melanoma cases. An Bras Dermatol. 2013;88:646-648. doi:10.1590/abd1806-4841.20132058
- Manci RN, Dauscher M, Marchetti MA, et al. Features of skin cancer in black individuals: a single-institution retrospective cohort study. Dermatol Pract Concept. 2022;12:e2022075. doi:10.5826/dpc.1202a75
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
- Ingrassia JP, Stein JA, Levine A, et al. Diagnosis and management of acral pigmented lesions. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2023;49:926-931. doi:10.1097/DSS.0000000000003891
- Hill L, Artiga S, Damico A. Health coverage by race and ethnicity, 2010-2022. Kaiser Family Foundation. Published January 11, 2024. Accessed May 9, 2024. https://www.kff.org/racial-equity-and-health-policy/issue-brief/health-coverage-by-race-and-ethnicity
Study Highlights Melanoma Survival Disparities in Rural vs Urban Settings
, results from an analysis of data from the National Cancer Institute showed.
“Melanoma is currently the fifth most common malignancy in the United States, with approximately 106,000 new cases and 7180 reported deaths occurring in 2021,” the study’s first author, Mitchell Taylor, MD, a dermatology research fellow at the University of Nebraska, Omaha, and colleagues wrote in the abstract, which was presented during a poster session at the annual meeting of the Society for Investigative Dermatology. “Rural areas have been shown to bear a higher melanoma disease burden, yet there is a paucity of national-level studies examining these disparities.”
To characterize the rural population diagnosed with cutaneous melanoma and assess associated disparities in the United States, the researchers queried the NCI’s Surveillance, Epidemiology, and End Results database to identify individuals diagnosed with cutaneous melanoma from 2000 to 2020 (International Classification of Diseases, 3rd Edition, 8720/3 — 8780/3; Primary Site codes C44.0-C44.9). They drew from US Office of Management and Budget terminology to define and categorize rural and urban communities.
Among 391,047 patients included during the study period, binary logistic regression analysis revealed that patients in rural areas had a greater odds of being older, from ages 50 to 75 years (odds ratio [OR], 1.10; P < .001); had annual incomes < $70,000 (OR, 16.80; P < .001); had tumors located on the head and neck (OR, 1.24; P < .001); and presented with regional/distant disease (OR, 1.13; P < .001).
As for disease-specific survival, patients living in rural areas had significantly reduced survival compared with those living in urban areas (a mean of 207.3 vs 216.3 months, respectively; P < .001). Multivariate Cox regression revealed that living in a rural setting was significantly associated with reduced disease-specific survival (hazard ratio [HR], 1.10; P < .001), as was having head and neck tumors (HR, 1.41; P < .001).“Overall, this study underscores a significant decrease in disease-specific survival among rural patients diagnosed with cutaneous melanoma and establishes a significant association between rural living and high-risk primary tumor locations, particularly the head and neck,” the authors concluded.
Lucinda Kohn, MD, assistant professor of dermatology in the Centers for American Indian and Alaska Native Health at the University of Colorado at Denver, Aurora, Colorado, who was asked to comment on the results, said the findings echo the results of a recent study which characterized melanoma rates among non-Hispanic American Indian/Alaska Native individuals from 1999 to 2019.
“I suspect this decreased disease-specific survival highlights the issues our rural-residing patients face with access to dermatology care,” Dr. Kohn told this news organization. “Dermatologists are able to detect thinner melanomas than patients [and] are preferentially concentrated in metropolitan areas. Dermatologists are also the most skilled and knowledgeable to screen, diagnose, and manage melanomas. Having fewer dermatologists in rural areas impedes melanoma care for our rural-residing patients.”
Neither the researchers nor Dr. Kohn reported any relevant disclosures.
A version of this article first appeared on Medscape.com.
, results from an analysis of data from the National Cancer Institute showed.
“Melanoma is currently the fifth most common malignancy in the United States, with approximately 106,000 new cases and 7180 reported deaths occurring in 2021,” the study’s first author, Mitchell Taylor, MD, a dermatology research fellow at the University of Nebraska, Omaha, and colleagues wrote in the abstract, which was presented during a poster session at the annual meeting of the Society for Investigative Dermatology. “Rural areas have been shown to bear a higher melanoma disease burden, yet there is a paucity of national-level studies examining these disparities.”
To characterize the rural population diagnosed with cutaneous melanoma and assess associated disparities in the United States, the researchers queried the NCI’s Surveillance, Epidemiology, and End Results database to identify individuals diagnosed with cutaneous melanoma from 2000 to 2020 (International Classification of Diseases, 3rd Edition, 8720/3 — 8780/3; Primary Site codes C44.0-C44.9). They drew from US Office of Management and Budget terminology to define and categorize rural and urban communities.
Among 391,047 patients included during the study period, binary logistic regression analysis revealed that patients in rural areas had a greater odds of being older, from ages 50 to 75 years (odds ratio [OR], 1.10; P < .001); had annual incomes < $70,000 (OR, 16.80; P < .001); had tumors located on the head and neck (OR, 1.24; P < .001); and presented with regional/distant disease (OR, 1.13; P < .001).
As for disease-specific survival, patients living in rural areas had significantly reduced survival compared with those living in urban areas (a mean of 207.3 vs 216.3 months, respectively; P < .001). Multivariate Cox regression revealed that living in a rural setting was significantly associated with reduced disease-specific survival (hazard ratio [HR], 1.10; P < .001), as was having head and neck tumors (HR, 1.41; P < .001).“Overall, this study underscores a significant decrease in disease-specific survival among rural patients diagnosed with cutaneous melanoma and establishes a significant association between rural living and high-risk primary tumor locations, particularly the head and neck,” the authors concluded.
Lucinda Kohn, MD, assistant professor of dermatology in the Centers for American Indian and Alaska Native Health at the University of Colorado at Denver, Aurora, Colorado, who was asked to comment on the results, said the findings echo the results of a recent study which characterized melanoma rates among non-Hispanic American Indian/Alaska Native individuals from 1999 to 2019.
“I suspect this decreased disease-specific survival highlights the issues our rural-residing patients face with access to dermatology care,” Dr. Kohn told this news organization. “Dermatologists are able to detect thinner melanomas than patients [and] are preferentially concentrated in metropolitan areas. Dermatologists are also the most skilled and knowledgeable to screen, diagnose, and manage melanomas. Having fewer dermatologists in rural areas impedes melanoma care for our rural-residing patients.”
Neither the researchers nor Dr. Kohn reported any relevant disclosures.
A version of this article first appeared on Medscape.com.
, results from an analysis of data from the National Cancer Institute showed.
“Melanoma is currently the fifth most common malignancy in the United States, with approximately 106,000 new cases and 7180 reported deaths occurring in 2021,” the study’s first author, Mitchell Taylor, MD, a dermatology research fellow at the University of Nebraska, Omaha, and colleagues wrote in the abstract, which was presented during a poster session at the annual meeting of the Society for Investigative Dermatology. “Rural areas have been shown to bear a higher melanoma disease burden, yet there is a paucity of national-level studies examining these disparities.”
To characterize the rural population diagnosed with cutaneous melanoma and assess associated disparities in the United States, the researchers queried the NCI’s Surveillance, Epidemiology, and End Results database to identify individuals diagnosed with cutaneous melanoma from 2000 to 2020 (International Classification of Diseases, 3rd Edition, 8720/3 — 8780/3; Primary Site codes C44.0-C44.9). They drew from US Office of Management and Budget terminology to define and categorize rural and urban communities.
Among 391,047 patients included during the study period, binary logistic regression analysis revealed that patients in rural areas had a greater odds of being older, from ages 50 to 75 years (odds ratio [OR], 1.10; P < .001); had annual incomes < $70,000 (OR, 16.80; P < .001); had tumors located on the head and neck (OR, 1.24; P < .001); and presented with regional/distant disease (OR, 1.13; P < .001).
As for disease-specific survival, patients living in rural areas had significantly reduced survival compared with those living in urban areas (a mean of 207.3 vs 216.3 months, respectively; P < .001). Multivariate Cox regression revealed that living in a rural setting was significantly associated with reduced disease-specific survival (hazard ratio [HR], 1.10; P < .001), as was having head and neck tumors (HR, 1.41; P < .001).“Overall, this study underscores a significant decrease in disease-specific survival among rural patients diagnosed with cutaneous melanoma and establishes a significant association between rural living and high-risk primary tumor locations, particularly the head and neck,” the authors concluded.
Lucinda Kohn, MD, assistant professor of dermatology in the Centers for American Indian and Alaska Native Health at the University of Colorado at Denver, Aurora, Colorado, who was asked to comment on the results, said the findings echo the results of a recent study which characterized melanoma rates among non-Hispanic American Indian/Alaska Native individuals from 1999 to 2019.
“I suspect this decreased disease-specific survival highlights the issues our rural-residing patients face with access to dermatology care,” Dr. Kohn told this news organization. “Dermatologists are able to detect thinner melanomas than patients [and] are preferentially concentrated in metropolitan areas. Dermatologists are also the most skilled and knowledgeable to screen, diagnose, and manage melanomas. Having fewer dermatologists in rural areas impedes melanoma care for our rural-residing patients.”
Neither the researchers nor Dr. Kohn reported any relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM SID 2024
Are Children Born Through ART at Higher Risk for Cancer?
The results of a large French study comparing the cancer risk in children conceived through assisted reproductive technology (ART) with that of naturally conceived children were published recently in JAMA Network Open. This study is one of the largest to date on this subject: It included 8,526,306 children born in France between 2010 and 2021, of whom 260,236 (3%) were conceived through ART, and followed them up to a median age of 6.7 years.
Motivations for the Study
ART (including artificial insemination, in vitro fertilization [IVF], or intracytoplasmic sperm injection [ICSI] with fresh or frozen embryo transfer) accounts for about 1 in 30 births in France. However, limited and heterogeneous data have suggested an increased risk for certain health disorders, including cancer, among children conceived through ART. Therefore, a large-scale evaluation of cancer risk in these children is important.
No Overall Increase
In all, 9256 children developed cancer, including 292 who were conceived through ART. Thus, Nevertheless, a slight increase in the risk for leukemia was observed in children conceived through IVF or ICSI. The investigators observed approximately one additional case for every 5000 newborns conceived through IVF or ICSI who reached age 10 years.
Epidemiological monitoring should be continued to better evaluate long-term risks and see whether the risk for leukemia is confirmed. If it is, then it will be useful to investigate the mechanisms related to ART techniques or the fertility disorders of parents that could lead to an increased risk for leukemia.
This story was translated from Univadis France, which is part of the Medscape Professional Network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The results of a large French study comparing the cancer risk in children conceived through assisted reproductive technology (ART) with that of naturally conceived children were published recently in JAMA Network Open. This study is one of the largest to date on this subject: It included 8,526,306 children born in France between 2010 and 2021, of whom 260,236 (3%) were conceived through ART, and followed them up to a median age of 6.7 years.
Motivations for the Study
ART (including artificial insemination, in vitro fertilization [IVF], or intracytoplasmic sperm injection [ICSI] with fresh or frozen embryo transfer) accounts for about 1 in 30 births in France. However, limited and heterogeneous data have suggested an increased risk for certain health disorders, including cancer, among children conceived through ART. Therefore, a large-scale evaluation of cancer risk in these children is important.
No Overall Increase
In all, 9256 children developed cancer, including 292 who were conceived through ART. Thus, Nevertheless, a slight increase in the risk for leukemia was observed in children conceived through IVF or ICSI. The investigators observed approximately one additional case for every 5000 newborns conceived through IVF or ICSI who reached age 10 years.
Epidemiological monitoring should be continued to better evaluate long-term risks and see whether the risk for leukemia is confirmed. If it is, then it will be useful to investigate the mechanisms related to ART techniques or the fertility disorders of parents that could lead to an increased risk for leukemia.
This story was translated from Univadis France, which is part of the Medscape Professional Network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The results of a large French study comparing the cancer risk in children conceived through assisted reproductive technology (ART) with that of naturally conceived children were published recently in JAMA Network Open. This study is one of the largest to date on this subject: It included 8,526,306 children born in France between 2010 and 2021, of whom 260,236 (3%) were conceived through ART, and followed them up to a median age of 6.7 years.
Motivations for the Study
ART (including artificial insemination, in vitro fertilization [IVF], or intracytoplasmic sperm injection [ICSI] with fresh or frozen embryo transfer) accounts for about 1 in 30 births in France. However, limited and heterogeneous data have suggested an increased risk for certain health disorders, including cancer, among children conceived through ART. Therefore, a large-scale evaluation of cancer risk in these children is important.
No Overall Increase
In all, 9256 children developed cancer, including 292 who were conceived through ART. Thus, Nevertheless, a slight increase in the risk for leukemia was observed in children conceived through IVF or ICSI. The investigators observed approximately one additional case for every 5000 newborns conceived through IVF or ICSI who reached age 10 years.
Epidemiological monitoring should be continued to better evaluate long-term risks and see whether the risk for leukemia is confirmed. If it is, then it will be useful to investigate the mechanisms related to ART techniques or the fertility disorders of parents that could lead to an increased risk for leukemia.
This story was translated from Univadis France, which is part of the Medscape Professional Network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Neoadjuvant Checkpoint Inhibition Study Sets New Standard of Care in Melanoma
These results set a new standard of care in this patient population, the study’s lead author, Christian U. Blank, MD, PhD, reported at the annual meeting of the American Society of Clinical Oncology in Chicago.
Dr. Blank, a hematologist/oncologist from the Netherlands Cancer Institute in Amsterdam, called the result “very special,” noting that the trial included an active comparator, rather than a placebo control.
“When we treat these patients with surgery only, the outcome … is very bad: The 5-year relapse-free survival is only 30% and the overall survival is only 50%. Adjuvant therapy improves relapse-free survival but not overall survival ...Thus, there is an urgent need for these patients for novel therapy approaches,” he said during a press conference at the meeting.
Study Methods and Results
The study included 423 patients with stage III de novo or recurrent pathologically proven resectable melanoma with at least 1 lymph node metastasis. Patients were randomized to either the experimental neoadjuvant arm (n = 212), or the standard treatment control arm (n = 211), which consisted of therapeutic lymph node dissection (TLND) followed by 12 cycles of adjuvant nivolumab (NIVO 480 mg every 4 weeks).
Patients in the experimental arm received two cycles of neoadjuvant ipilimumab (IPI 80 mg every 3 weeks) plus NIVO 240 mg for 3 weeks followed by TLND. Those with a major pathologic response (MPR), defined as less than 10% vital tumor cells in the post-neoadjuvant resection specimen, went straight to follow-up.
Those without an MPR received adjuvant therapy. For patients with BRAF wild-type, this involved 11 cycles of adjuvant NIVO (480 mg every 4 weeks), while BRAF-mutated patients received dabrafenib plus trametinib (150 mg b.i.d./2 mg once a day; 46 weeks).
The study met its primary endpoint — event-free survival (EFS) — at the first interim analysis. After a median follow-up of 9.9 months, the estimated EFS was 83.7% for neoadjuvant immunotherapy versus 57.2% for standard of care, (P less than .0001, hazard ratio [HR] = 0.32).
“When we look into the subgroups, for example BRAF-mutated status or BRAF-wild-type status ... you see for both groups also a highly statistically significant outcome favoring the neoadjuvant therapy with hazard ratios of 0.29 and 0.35,” said Dr. Blank.
In total, 59% of patients in the experimental arm had an MPR needing no further treatment. “This is important, because the patients that achieve a major pathologic response have excellent outcomes, with an EFS of 95%,” said Dr. Blank.
He added that those with a partial response had an EFS of 76%, and among those who had “nonresponse,” the EFS was 57% — the same as that of patients in the control arm.
Toxicities were considered transient and acceptable, with systemic treatment-related grade 3 or 4 events in 29.7% of the neoadjuvant arm and 14.7% of the adjuvant arm.
NADINA is the first neoadjuvant checkpoint inhibitor phase 3 study in melanoma and the first phase 3 trial in oncology testing a checkpoint inhibitor without chemotherapy, noted Dr. Blank.
“At the moment we see only additions of immunotherapy to the chemotherapy neoadjuvant arms, but here you see that we can also treat patients with pure immunotherapy.”
Neoadjuvant Therapy Defined as Standard of Care
When considered along with evidence from the phase 2 SWOG 1801 study (N Engl J Med. 2023;388:813-8), “NADINA defines neoadjuvant therapy as the new standard of care for macroscopic stage III melanoma “which means that all trials currently ongoing need to be amended from adjuvant comparators to neoadjuvant comparators,” he said.
Dr. Blank called the trial a “new template for other malignancies implementing a neoadjuvant immunotherapy regimen followed by a response-driven adjuvant therapy.
“I think we see at the moment only sandwich designs, and this is more sales driven than patient driven, because what we have seen is that if a patient achieves a really deep response, the patient doesn’t need an adjuvant part,” he said.
Commenting during the press conference, Michael Lowe, MD, said the result “confirms and shows for the first time in a phase 3 study that giving immunotherapy before surgery results in superior outcomes to giving immunotherapy only after surgery.”
Dr. Lowe, associate professor in the Division of Surgical Oncology, at Emory University School of Medicine, Atlanta, added that the study “also confirms that giving two immunotherapy drugs before surgery results in excellent responses.”
However, he cautioned that “we cannot make comparisons to trials in which patients only got one immunotherapy. But this study confirms that consistency that patients who receive ipilimumab and nivolumab have superior responses compared to single-agent immunotherapy.”
He noted that all of the patients in the new study had all of their lymph nodes removed and called for doing that to remain the standard of care in terms of surgical approach.
“With short follow-up, it is too early to tell if some patients may have benefited from that adjuvant therapy. However, NADINA confirms that immunotherapy should be given to all patients with advanced melanoma before surgery, when possible, and establishes dual therapy with nivolumab and ipilimumab, as the standard of care in the appropriate patient,” Dr. Lowe said.
EFS Improvement Exceeds Expectations
In an interview, Rodabe N. Amaria, MD, a medical oncologist and professor at The University of Texas MD Anderson Cancer Center in Houston, agreed with Dr. Lowe’s assessment of the findings.
“For years we have been doing neoadjuvant immunotherapy trials, all with favorable results, but all relatively small, with data that was intriguing, but not necessarily definitive,” she said. “I see the data from the NADINA trial as being definitive and true evidence of the many advantages of neoadjuvant immunotherapy for clinical stage 3 melanoma ... This work builds on the data from the SWOG 1801 trial but also exceeds expectations with the 68% improvement in EFS appreciated with the dual combination immunotherapy regimen compared to adjuvant nivolumab.”
Additionally, the approximately 30% grade 3 or higher immune-mediated toxicity is reasonable and in keeping with known data, and this trial demonstrates clearly that neoadjuvant immunotherapy does not increase the rate of surgical complications, she said.
Dr. Amaria also considered that 59% of patients who achieved a major pathologic response were observed in the neoadjuvant setting to be a key finding.
This indicates thats “over half the patients could be spared additional immunotherapy and risk of further immune-mediated toxicities by having only two doses of neoadjuvant immunotherapy, she said.
The results “demonstrate the superiority of a neoadjuvant combination immunotherapy approach for patients with clinical stage III melanoma,” she added.
The study was funded by Bristol Myers-Squibb and the Australian government.
Dr. Blank disclosed ties with Immagene, Signature Oncology, AstraZeneca, Bristol-Myers Squibb, GenMab, GlaxoSmithKline, Lilly, MSD Oncology, Novartis, Pfizer, Pierre Fabre, Roche/Genentech, Third Rock Ventures, 4SC, NanoString Technologies, WO 2021/177822 A1, and Freshfields Bruckhaus Deringer. No other experts reported any relevant disclosures.
These results set a new standard of care in this patient population, the study’s lead author, Christian U. Blank, MD, PhD, reported at the annual meeting of the American Society of Clinical Oncology in Chicago.
Dr. Blank, a hematologist/oncologist from the Netherlands Cancer Institute in Amsterdam, called the result “very special,” noting that the trial included an active comparator, rather than a placebo control.
“When we treat these patients with surgery only, the outcome … is very bad: The 5-year relapse-free survival is only 30% and the overall survival is only 50%. Adjuvant therapy improves relapse-free survival but not overall survival ...Thus, there is an urgent need for these patients for novel therapy approaches,” he said during a press conference at the meeting.
Study Methods and Results
The study included 423 patients with stage III de novo or recurrent pathologically proven resectable melanoma with at least 1 lymph node metastasis. Patients were randomized to either the experimental neoadjuvant arm (n = 212), or the standard treatment control arm (n = 211), which consisted of therapeutic lymph node dissection (TLND) followed by 12 cycles of adjuvant nivolumab (NIVO 480 mg every 4 weeks).
Patients in the experimental arm received two cycles of neoadjuvant ipilimumab (IPI 80 mg every 3 weeks) plus NIVO 240 mg for 3 weeks followed by TLND. Those with a major pathologic response (MPR), defined as less than 10% vital tumor cells in the post-neoadjuvant resection specimen, went straight to follow-up.
Those without an MPR received adjuvant therapy. For patients with BRAF wild-type, this involved 11 cycles of adjuvant NIVO (480 mg every 4 weeks), while BRAF-mutated patients received dabrafenib plus trametinib (150 mg b.i.d./2 mg once a day; 46 weeks).
The study met its primary endpoint — event-free survival (EFS) — at the first interim analysis. After a median follow-up of 9.9 months, the estimated EFS was 83.7% for neoadjuvant immunotherapy versus 57.2% for standard of care, (P less than .0001, hazard ratio [HR] = 0.32).
“When we look into the subgroups, for example BRAF-mutated status or BRAF-wild-type status ... you see for both groups also a highly statistically significant outcome favoring the neoadjuvant therapy with hazard ratios of 0.29 and 0.35,” said Dr. Blank.
In total, 59% of patients in the experimental arm had an MPR needing no further treatment. “This is important, because the patients that achieve a major pathologic response have excellent outcomes, with an EFS of 95%,” said Dr. Blank.
He added that those with a partial response had an EFS of 76%, and among those who had “nonresponse,” the EFS was 57% — the same as that of patients in the control arm.
Toxicities were considered transient and acceptable, with systemic treatment-related grade 3 or 4 events in 29.7% of the neoadjuvant arm and 14.7% of the adjuvant arm.
NADINA is the first neoadjuvant checkpoint inhibitor phase 3 study in melanoma and the first phase 3 trial in oncology testing a checkpoint inhibitor without chemotherapy, noted Dr. Blank.
“At the moment we see only additions of immunotherapy to the chemotherapy neoadjuvant arms, but here you see that we can also treat patients with pure immunotherapy.”
Neoadjuvant Therapy Defined as Standard of Care
When considered along with evidence from the phase 2 SWOG 1801 study (N Engl J Med. 2023;388:813-8), “NADINA defines neoadjuvant therapy as the new standard of care for macroscopic stage III melanoma “which means that all trials currently ongoing need to be amended from adjuvant comparators to neoadjuvant comparators,” he said.
Dr. Blank called the trial a “new template for other malignancies implementing a neoadjuvant immunotherapy regimen followed by a response-driven adjuvant therapy.
“I think we see at the moment only sandwich designs, and this is more sales driven than patient driven, because what we have seen is that if a patient achieves a really deep response, the patient doesn’t need an adjuvant part,” he said.
Commenting during the press conference, Michael Lowe, MD, said the result “confirms and shows for the first time in a phase 3 study that giving immunotherapy before surgery results in superior outcomes to giving immunotherapy only after surgery.”
Dr. Lowe, associate professor in the Division of Surgical Oncology, at Emory University School of Medicine, Atlanta, added that the study “also confirms that giving two immunotherapy drugs before surgery results in excellent responses.”
However, he cautioned that “we cannot make comparisons to trials in which patients only got one immunotherapy. But this study confirms that consistency that patients who receive ipilimumab and nivolumab have superior responses compared to single-agent immunotherapy.”
He noted that all of the patients in the new study had all of their lymph nodes removed and called for doing that to remain the standard of care in terms of surgical approach.
“With short follow-up, it is too early to tell if some patients may have benefited from that adjuvant therapy. However, NADINA confirms that immunotherapy should be given to all patients with advanced melanoma before surgery, when possible, and establishes dual therapy with nivolumab and ipilimumab, as the standard of care in the appropriate patient,” Dr. Lowe said.
EFS Improvement Exceeds Expectations
In an interview, Rodabe N. Amaria, MD, a medical oncologist and professor at The University of Texas MD Anderson Cancer Center in Houston, agreed with Dr. Lowe’s assessment of the findings.
“For years we have been doing neoadjuvant immunotherapy trials, all with favorable results, but all relatively small, with data that was intriguing, but not necessarily definitive,” she said. “I see the data from the NADINA trial as being definitive and true evidence of the many advantages of neoadjuvant immunotherapy for clinical stage 3 melanoma ... This work builds on the data from the SWOG 1801 trial but also exceeds expectations with the 68% improvement in EFS appreciated with the dual combination immunotherapy regimen compared to adjuvant nivolumab.”
Additionally, the approximately 30% grade 3 or higher immune-mediated toxicity is reasonable and in keeping with known data, and this trial demonstrates clearly that neoadjuvant immunotherapy does not increase the rate of surgical complications, she said.
Dr. Amaria also considered that 59% of patients who achieved a major pathologic response were observed in the neoadjuvant setting to be a key finding.
This indicates thats “over half the patients could be spared additional immunotherapy and risk of further immune-mediated toxicities by having only two doses of neoadjuvant immunotherapy, she said.
The results “demonstrate the superiority of a neoadjuvant combination immunotherapy approach for patients with clinical stage III melanoma,” she added.
The study was funded by Bristol Myers-Squibb and the Australian government.
Dr. Blank disclosed ties with Immagene, Signature Oncology, AstraZeneca, Bristol-Myers Squibb, GenMab, GlaxoSmithKline, Lilly, MSD Oncology, Novartis, Pfizer, Pierre Fabre, Roche/Genentech, Third Rock Ventures, 4SC, NanoString Technologies, WO 2021/177822 A1, and Freshfields Bruckhaus Deringer. No other experts reported any relevant disclosures.
These results set a new standard of care in this patient population, the study’s lead author, Christian U. Blank, MD, PhD, reported at the annual meeting of the American Society of Clinical Oncology in Chicago.
Dr. Blank, a hematologist/oncologist from the Netherlands Cancer Institute in Amsterdam, called the result “very special,” noting that the trial included an active comparator, rather than a placebo control.
“When we treat these patients with surgery only, the outcome … is very bad: The 5-year relapse-free survival is only 30% and the overall survival is only 50%. Adjuvant therapy improves relapse-free survival but not overall survival ...Thus, there is an urgent need for these patients for novel therapy approaches,” he said during a press conference at the meeting.
Study Methods and Results
The study included 423 patients with stage III de novo or recurrent pathologically proven resectable melanoma with at least 1 lymph node metastasis. Patients were randomized to either the experimental neoadjuvant arm (n = 212), or the standard treatment control arm (n = 211), which consisted of therapeutic lymph node dissection (TLND) followed by 12 cycles of adjuvant nivolumab (NIVO 480 mg every 4 weeks).
Patients in the experimental arm received two cycles of neoadjuvant ipilimumab (IPI 80 mg every 3 weeks) plus NIVO 240 mg for 3 weeks followed by TLND. Those with a major pathologic response (MPR), defined as less than 10% vital tumor cells in the post-neoadjuvant resection specimen, went straight to follow-up.
Those without an MPR received adjuvant therapy. For patients with BRAF wild-type, this involved 11 cycles of adjuvant NIVO (480 mg every 4 weeks), while BRAF-mutated patients received dabrafenib plus trametinib (150 mg b.i.d./2 mg once a day; 46 weeks).
The study met its primary endpoint — event-free survival (EFS) — at the first interim analysis. After a median follow-up of 9.9 months, the estimated EFS was 83.7% for neoadjuvant immunotherapy versus 57.2% for standard of care, (P less than .0001, hazard ratio [HR] = 0.32).
“When we look into the subgroups, for example BRAF-mutated status or BRAF-wild-type status ... you see for both groups also a highly statistically significant outcome favoring the neoadjuvant therapy with hazard ratios of 0.29 and 0.35,” said Dr. Blank.
In total, 59% of patients in the experimental arm had an MPR needing no further treatment. “This is important, because the patients that achieve a major pathologic response have excellent outcomes, with an EFS of 95%,” said Dr. Blank.
He added that those with a partial response had an EFS of 76%, and among those who had “nonresponse,” the EFS was 57% — the same as that of patients in the control arm.
Toxicities were considered transient and acceptable, with systemic treatment-related grade 3 or 4 events in 29.7% of the neoadjuvant arm and 14.7% of the adjuvant arm.
NADINA is the first neoadjuvant checkpoint inhibitor phase 3 study in melanoma and the first phase 3 trial in oncology testing a checkpoint inhibitor without chemotherapy, noted Dr. Blank.
“At the moment we see only additions of immunotherapy to the chemotherapy neoadjuvant arms, but here you see that we can also treat patients with pure immunotherapy.”
Neoadjuvant Therapy Defined as Standard of Care
When considered along with evidence from the phase 2 SWOG 1801 study (N Engl J Med. 2023;388:813-8), “NADINA defines neoadjuvant therapy as the new standard of care for macroscopic stage III melanoma “which means that all trials currently ongoing need to be amended from adjuvant comparators to neoadjuvant comparators,” he said.
Dr. Blank called the trial a “new template for other malignancies implementing a neoadjuvant immunotherapy regimen followed by a response-driven adjuvant therapy.
“I think we see at the moment only sandwich designs, and this is more sales driven than patient driven, because what we have seen is that if a patient achieves a really deep response, the patient doesn’t need an adjuvant part,” he said.
Commenting during the press conference, Michael Lowe, MD, said the result “confirms and shows for the first time in a phase 3 study that giving immunotherapy before surgery results in superior outcomes to giving immunotherapy only after surgery.”
Dr. Lowe, associate professor in the Division of Surgical Oncology, at Emory University School of Medicine, Atlanta, added that the study “also confirms that giving two immunotherapy drugs before surgery results in excellent responses.”
However, he cautioned that “we cannot make comparisons to trials in which patients only got one immunotherapy. But this study confirms that consistency that patients who receive ipilimumab and nivolumab have superior responses compared to single-agent immunotherapy.”
He noted that all of the patients in the new study had all of their lymph nodes removed and called for doing that to remain the standard of care in terms of surgical approach.
“With short follow-up, it is too early to tell if some patients may have benefited from that adjuvant therapy. However, NADINA confirms that immunotherapy should be given to all patients with advanced melanoma before surgery, when possible, and establishes dual therapy with nivolumab and ipilimumab, as the standard of care in the appropriate patient,” Dr. Lowe said.
EFS Improvement Exceeds Expectations
In an interview, Rodabe N. Amaria, MD, a medical oncologist and professor at The University of Texas MD Anderson Cancer Center in Houston, agreed with Dr. Lowe’s assessment of the findings.
“For years we have been doing neoadjuvant immunotherapy trials, all with favorable results, but all relatively small, with data that was intriguing, but not necessarily definitive,” she said. “I see the data from the NADINA trial as being definitive and true evidence of the many advantages of neoadjuvant immunotherapy for clinical stage 3 melanoma ... This work builds on the data from the SWOG 1801 trial but also exceeds expectations with the 68% improvement in EFS appreciated with the dual combination immunotherapy regimen compared to adjuvant nivolumab.”
Additionally, the approximately 30% grade 3 or higher immune-mediated toxicity is reasonable and in keeping with known data, and this trial demonstrates clearly that neoadjuvant immunotherapy does not increase the rate of surgical complications, she said.
Dr. Amaria also considered that 59% of patients who achieved a major pathologic response were observed in the neoadjuvant setting to be a key finding.
This indicates thats “over half the patients could be spared additional immunotherapy and risk of further immune-mediated toxicities by having only two doses of neoadjuvant immunotherapy, she said.
The results “demonstrate the superiority of a neoadjuvant combination immunotherapy approach for patients with clinical stage III melanoma,” she added.
The study was funded by Bristol Myers-Squibb and the Australian government.
Dr. Blank disclosed ties with Immagene, Signature Oncology, AstraZeneca, Bristol-Myers Squibb, GenMab, GlaxoSmithKline, Lilly, MSD Oncology, Novartis, Pfizer, Pierre Fabre, Roche/Genentech, Third Rock Ventures, 4SC, NanoString Technologies, WO 2021/177822 A1, and Freshfields Bruckhaus Deringer. No other experts reported any relevant disclosures.
FROM ASCO 2024
Plantar Hyperpigmentation
Plantar hyperpigmentation (also known as plantar melanosis [increased melanin], volar pigmented macules, benign racial melanosis, acral pigmentation, acral ethnic melanosis, or mottled hyperpigmentation of the plantar surface) is a benign finding in many individuals and is especially prevalent in those with darker skin tones. Acral refers to manifestation on the hands and feet, volar on the palms and soles, and plantar on the soles only. Here, we focus on plantar hyper-pigmentation. We use the terms ethnic and racial interchangeably.
It is critically important to differentiate benign hyperpigmentation, which is common in patients with skin of color, from melanoma. Although rare, Black patients in the United States experience high morbidity and mortality from acral melanoma, which often is diagnosed late in the disease course.1
There are many causes of hyperpigmentation on the plantar surfaces, including benign ethnic melanosis, nevi, melanoma, infections such as syphilis and tinea nigra, conditions such as Peutz-Jeghers syndrome and Laugier-Hunziker syndrome, and postinflammatory hyperpigmentation secondary to atopic dermatitis and psoriasis. We focus on the most common causes, ethnic melanosis and nevi, as well as melanoma, which is the deadliest cause.
Epidemiology
In a 1980 study (N=251), Black Americans had a high incidence of plantar hyperpigmentation, with 52% of affected patients having dark brown skin and 31% having light brown skin.2
The epidemiology of melanoma varies by race/ethnicity. Melanoma in Black individuals is relatively rare, with an annual incidence of approximately 1 in 100,000 individuals.3 However, when individuals with skin of color develop melanoma, they are more likely than their White counterparts to have acral melanoma (acral lentiginous melanoma), one of the deadliest types.1 In a case series of Black patients with melanoma (N=48) from 2 tertiary care centers in Texas, 30 of 40 primary cutaneous melanomas (75%) were located on acral skin.4 Overall, 13 patients developed stage IV disease and 12 died due to disease progression. All patients who developed distant metastases or died of melanoma had acral melanoma.4 Individuals of Asian descent also have a high incidence of acral melanoma, as shown in research from Japan.5-9
Key Clinical Features in Individuals With Darker Skin Tones
Dermoscopy is an evidence-based clinical examination method for earlier diagnosis of cutaneous melanoma, including on acral skin.10,11 Benign nevi on the volar skin as well as the palms and soles tend to have one of these 3 dermoscopic patterns: parallel furrow, lattice, or irregular fibrillar. The pattern that is most predictive of volar melanoma is the parallel ridge pattern (PRP) (Figures A and B [insets]), which showed a high specificity (99.0%) and very high negative predictive value (97.7%) for malignant melanoma in a Japanese population.7 The PRP data from this study cannot be applied reliably to Black individuals, especially because benign ethnic melanosis and other benign conditions can demonstrate PRP.12 Reliance on the PRP as a diagnostic clue could result in unneccessary biopsies in as many as 50% of Black patients with benign plantar hyperpigmentation.2 Furthermore, biopsies of the plantar surface can be painful and cause pain while walking.
It has been suggested that PRP seen on dermoscopy in benign hyperpigmentation such as ethnic melanosis and nevi may preserve the acrosyringia (eccrine gland openings on the ridge), whereas PRP in melanoma may obliterate the acrosyringia.13 This observation is based on case reports only and needs further study. However, if validated, it could be a useful diagnostic clue.
Worth noting
In a retrospective cohort study of skin cancer in Black individuals (n=165) at a New York City–based cancer center from 2000 to 2020, 68% of patients were diagnosed with melanomas—80% were the acral subtype and 75% displayed a PRP. However, the surrounding uninvolved background skin, which was visible in most cases, also demonstrated a PRP.14 Because of the high morbidity and mortality rates of acral melanoma, clinicians should biopsy or immediately refer patients with concerning plantar hyperpigmentation to a dermatologist.
Health disparity highlight
The mortality rate for acral melanoma in Black patients is disproportionately high for the following reasons15,16:
• Patients and health care providers do not expect to see melanoma in Black patients (it truly is rare!), so screening and education on sun protection are limited.
• Benign ethnic melanosis makes it more difficult to distinguish between early acral melanoma and benign skin changes.
• Black patients and other US patient populations with skin of color may be less likely to have health insurance, which contributes to inequities in access to health care. As of 2022, the uninsured rates for nonelderly American Indian and Alaska Native, Hispanic, Native Hawaiian and Other Pacific Islander, Black, and White individuals were 19.1%, 18.0%, 12.7%, 10.0%, and 6.6%, respectively.17
Multi-institutional registries could improve understanding of acral melanoma in Black patients.4 More studies are needed to help differentiate between the dermoscopic finding of PRP in benign ethnic melanosis vs malignant melanoma.
1. Huang K, Fan J, Misra S. Acral lentiginous melanoma: incidence and survival in the United States, 2006-2015: an analysis of the SEER registry. J Surg Res. 2020;251:329-339. doi:10.1016/j.jss.2020.02.010
2. Coleman WP, Gately LE, Krementz AB, et al. Nevi, lentigines, and melanomas in blacks. Arch Dermatol. 1980;116:548-551.
3. Centers for Disease Control and Prevention. Melanoma Incidence and Mortality, United States: 2012-2016. USCS Data Brief, no. 9. Centers for Disease Control and Prevention, US Department of Health and Human Services; 2019. https://www.cdc.gov/cancer/uscs/about/data-briefs/no9-melanoma-incidence-mortality-UnitedStates-2012-2016.htm
4. Wix SN, Brown AB, Heberton M, et al. Clinical features and outcomes of black patients with melanoma. JAMA Dermatol. 2024;160:328-333. doi:10.1001/jamadermatol.2023.5789
5. Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch Dermatol. 2007;143:1423-1426. doi:10.1001/archderm.143.11.1423
6. Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38:25-34. doi:10.1111/j.1346-8138.2010.01174.x
7. Saida T, Miyazaki A, Oguchi S. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140:1233-1238. doi:10.1001/archderm.140.10.1233
8. Saida T, Koga H, Uhara H. Dermoscopy for acral melanocytic lesions: revision of the 3-step algorithm and refined definition of the regular and irregular fibrillar pattern. Dermatol Pract Concept. 2022;12:e2022123. doi:10.5826/dpc.1203a123
9. Heath CR, Usatine RP. Melanoma. Cutis. 2022;109:284-285. doi:10.12788/cutis.0513.
10. Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Visual inspection and dermoscopy, alone or in combination, for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018; 12:CD011901. doi:10.1002/14651858.CD011901.pub2
11. Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked-eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi:10.1111/j.1365-2133.2008.08713.x
12. Phan A, Dalle S, Marcilly MC, et al. Benign dermoscopic parallel ridge pattern variants. Arch Dermatol. 2011;147:634. doi:10.1001/archdermatol.2011.47
13. Fracaroli TS, Lavorato FG, Maceira JP, et al. Parallel ridge pattern on dermoscopy: observation in non-melanoma cases. An Bras Dermatol. 2013;88:646-648. doi:10.1590/abd1806-4841.20132058
14. Manci RN, Dauscher M, Marchetti MA, et al. Features of skin cancer in black individuals: a single-institution retrospective cohort study. Dermatol Pract Concept. 2022;12:e2022075. doi:10.5826/dpc.1202a75
15. Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dematol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
16. Ingrassia JP, Stein JA, Levine A, et al. Diagnosis and management of acral pigmented lesions. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2023;49:926-931. doi:10.1097/DSS.0000000000003891
17. Hill L, Artiga S, Damico A. Health coverage by race and ethnicity, 2010-2022. Kaiser Family Foundation. Published January 11, 2024. Accessed May 9, 2024. https://www.kff.org/racial-equity-and-health-policy/issue-brief/health-coverage-by-race-and-ethnicity
Plantar hyperpigmentation (also known as plantar melanosis [increased melanin], volar pigmented macules, benign racial melanosis, acral pigmentation, acral ethnic melanosis, or mottled hyperpigmentation of the plantar surface) is a benign finding in many individuals and is especially prevalent in those with darker skin tones. Acral refers to manifestation on the hands and feet, volar on the palms and soles, and plantar on the soles only. Here, we focus on plantar hyper-pigmentation. We use the terms ethnic and racial interchangeably.
It is critically important to differentiate benign hyperpigmentation, which is common in patients with skin of color, from melanoma. Although rare, Black patients in the United States experience high morbidity and mortality from acral melanoma, which often is diagnosed late in the disease course.1
There are many causes of hyperpigmentation on the plantar surfaces, including benign ethnic melanosis, nevi, melanoma, infections such as syphilis and tinea nigra, conditions such as Peutz-Jeghers syndrome and Laugier-Hunziker syndrome, and postinflammatory hyperpigmentation secondary to atopic dermatitis and psoriasis. We focus on the most common causes, ethnic melanosis and nevi, as well as melanoma, which is the deadliest cause.
Epidemiology
In a 1980 study (N=251), Black Americans had a high incidence of plantar hyperpigmentation, with 52% of affected patients having dark brown skin and 31% having light brown skin.2
The epidemiology of melanoma varies by race/ethnicity. Melanoma in Black individuals is relatively rare, with an annual incidence of approximately 1 in 100,000 individuals.3 However, when individuals with skin of color develop melanoma, they are more likely than their White counterparts to have acral melanoma (acral lentiginous melanoma), one of the deadliest types.1 In a case series of Black patients with melanoma (N=48) from 2 tertiary care centers in Texas, 30 of 40 primary cutaneous melanomas (75%) were located on acral skin.4 Overall, 13 patients developed stage IV disease and 12 died due to disease progression. All patients who developed distant metastases or died of melanoma had acral melanoma.4 Individuals of Asian descent also have a high incidence of acral melanoma, as shown in research from Japan.5-9
Key Clinical Features in Individuals With Darker Skin Tones
Dermoscopy is an evidence-based clinical examination method for earlier diagnosis of cutaneous melanoma, including on acral skin.10,11 Benign nevi on the volar skin as well as the palms and soles tend to have one of these 3 dermoscopic patterns: parallel furrow, lattice, or irregular fibrillar. The pattern that is most predictive of volar melanoma is the parallel ridge pattern (PRP) (Figures A and B [insets]), which showed a high specificity (99.0%) and very high negative predictive value (97.7%) for malignant melanoma in a Japanese population.7 The PRP data from this study cannot be applied reliably to Black individuals, especially because benign ethnic melanosis and other benign conditions can demonstrate PRP.12 Reliance on the PRP as a diagnostic clue could result in unneccessary biopsies in as many as 50% of Black patients with benign plantar hyperpigmentation.2 Furthermore, biopsies of the plantar surface can be painful and cause pain while walking.
It has been suggested that PRP seen on dermoscopy in benign hyperpigmentation such as ethnic melanosis and nevi may preserve the acrosyringia (eccrine gland openings on the ridge), whereas PRP in melanoma may obliterate the acrosyringia.13 This observation is based on case reports only and needs further study. However, if validated, it could be a useful diagnostic clue.
Worth noting
In a retrospective cohort study of skin cancer in Black individuals (n=165) at a New York City–based cancer center from 2000 to 2020, 68% of patients were diagnosed with melanomas—80% were the acral subtype and 75% displayed a PRP. However, the surrounding uninvolved background skin, which was visible in most cases, also demonstrated a PRP.14 Because of the high morbidity and mortality rates of acral melanoma, clinicians should biopsy or immediately refer patients with concerning plantar hyperpigmentation to a dermatologist.
Health disparity highlight
The mortality rate for acral melanoma in Black patients is disproportionately high for the following reasons15,16:
• Patients and health care providers do not expect to see melanoma in Black patients (it truly is rare!), so screening and education on sun protection are limited.
• Benign ethnic melanosis makes it more difficult to distinguish between early acral melanoma and benign skin changes.
• Black patients and other US patient populations with skin of color may be less likely to have health insurance, which contributes to inequities in access to health care. As of 2022, the uninsured rates for nonelderly American Indian and Alaska Native, Hispanic, Native Hawaiian and Other Pacific Islander, Black, and White individuals were 19.1%, 18.0%, 12.7%, 10.0%, and 6.6%, respectively.17
Multi-institutional registries could improve understanding of acral melanoma in Black patients.4 More studies are needed to help differentiate between the dermoscopic finding of PRP in benign ethnic melanosis vs malignant melanoma.
Plantar hyperpigmentation (also known as plantar melanosis [increased melanin], volar pigmented macules, benign racial melanosis, acral pigmentation, acral ethnic melanosis, or mottled hyperpigmentation of the plantar surface) is a benign finding in many individuals and is especially prevalent in those with darker skin tones. Acral refers to manifestation on the hands and feet, volar on the palms and soles, and plantar on the soles only. Here, we focus on plantar hyper-pigmentation. We use the terms ethnic and racial interchangeably.
It is critically important to differentiate benign hyperpigmentation, which is common in patients with skin of color, from melanoma. Although rare, Black patients in the United States experience high morbidity and mortality from acral melanoma, which often is diagnosed late in the disease course.1
There are many causes of hyperpigmentation on the plantar surfaces, including benign ethnic melanosis, nevi, melanoma, infections such as syphilis and tinea nigra, conditions such as Peutz-Jeghers syndrome and Laugier-Hunziker syndrome, and postinflammatory hyperpigmentation secondary to atopic dermatitis and psoriasis. We focus on the most common causes, ethnic melanosis and nevi, as well as melanoma, which is the deadliest cause.
Epidemiology
In a 1980 study (N=251), Black Americans had a high incidence of plantar hyperpigmentation, with 52% of affected patients having dark brown skin and 31% having light brown skin.2
The epidemiology of melanoma varies by race/ethnicity. Melanoma in Black individuals is relatively rare, with an annual incidence of approximately 1 in 100,000 individuals.3 However, when individuals with skin of color develop melanoma, they are more likely than their White counterparts to have acral melanoma (acral lentiginous melanoma), one of the deadliest types.1 In a case series of Black patients with melanoma (N=48) from 2 tertiary care centers in Texas, 30 of 40 primary cutaneous melanomas (75%) were located on acral skin.4 Overall, 13 patients developed stage IV disease and 12 died due to disease progression. All patients who developed distant metastases or died of melanoma had acral melanoma.4 Individuals of Asian descent also have a high incidence of acral melanoma, as shown in research from Japan.5-9
Key Clinical Features in Individuals With Darker Skin Tones
Dermoscopy is an evidence-based clinical examination method for earlier diagnosis of cutaneous melanoma, including on acral skin.10,11 Benign nevi on the volar skin as well as the palms and soles tend to have one of these 3 dermoscopic patterns: parallel furrow, lattice, or irregular fibrillar. The pattern that is most predictive of volar melanoma is the parallel ridge pattern (PRP) (Figures A and B [insets]), which showed a high specificity (99.0%) and very high negative predictive value (97.7%) for malignant melanoma in a Japanese population.7 The PRP data from this study cannot be applied reliably to Black individuals, especially because benign ethnic melanosis and other benign conditions can demonstrate PRP.12 Reliance on the PRP as a diagnostic clue could result in unneccessary biopsies in as many as 50% of Black patients with benign plantar hyperpigmentation.2 Furthermore, biopsies of the plantar surface can be painful and cause pain while walking.
It has been suggested that PRP seen on dermoscopy in benign hyperpigmentation such as ethnic melanosis and nevi may preserve the acrosyringia (eccrine gland openings on the ridge), whereas PRP in melanoma may obliterate the acrosyringia.13 This observation is based on case reports only and needs further study. However, if validated, it could be a useful diagnostic clue.
Worth noting
In a retrospective cohort study of skin cancer in Black individuals (n=165) at a New York City–based cancer center from 2000 to 2020, 68% of patients were diagnosed with melanomas—80% were the acral subtype and 75% displayed a PRP. However, the surrounding uninvolved background skin, which was visible in most cases, also demonstrated a PRP.14 Because of the high morbidity and mortality rates of acral melanoma, clinicians should biopsy or immediately refer patients with concerning plantar hyperpigmentation to a dermatologist.
Health disparity highlight
The mortality rate for acral melanoma in Black patients is disproportionately high for the following reasons15,16:
• Patients and health care providers do not expect to see melanoma in Black patients (it truly is rare!), so screening and education on sun protection are limited.
• Benign ethnic melanosis makes it more difficult to distinguish between early acral melanoma and benign skin changes.
• Black patients and other US patient populations with skin of color may be less likely to have health insurance, which contributes to inequities in access to health care. As of 2022, the uninsured rates for nonelderly American Indian and Alaska Native, Hispanic, Native Hawaiian and Other Pacific Islander, Black, and White individuals were 19.1%, 18.0%, 12.7%, 10.0%, and 6.6%, respectively.17
Multi-institutional registries could improve understanding of acral melanoma in Black patients.4 More studies are needed to help differentiate between the dermoscopic finding of PRP in benign ethnic melanosis vs malignant melanoma.
1. Huang K, Fan J, Misra S. Acral lentiginous melanoma: incidence and survival in the United States, 2006-2015: an analysis of the SEER registry. J Surg Res. 2020;251:329-339. doi:10.1016/j.jss.2020.02.010
2. Coleman WP, Gately LE, Krementz AB, et al. Nevi, lentigines, and melanomas in blacks. Arch Dermatol. 1980;116:548-551.
3. Centers for Disease Control and Prevention. Melanoma Incidence and Mortality, United States: 2012-2016. USCS Data Brief, no. 9. Centers for Disease Control and Prevention, US Department of Health and Human Services; 2019. https://www.cdc.gov/cancer/uscs/about/data-briefs/no9-melanoma-incidence-mortality-UnitedStates-2012-2016.htm
4. Wix SN, Brown AB, Heberton M, et al. Clinical features and outcomes of black patients with melanoma. JAMA Dermatol. 2024;160:328-333. doi:10.1001/jamadermatol.2023.5789
5. Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch Dermatol. 2007;143:1423-1426. doi:10.1001/archderm.143.11.1423
6. Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38:25-34. doi:10.1111/j.1346-8138.2010.01174.x
7. Saida T, Miyazaki A, Oguchi S. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140:1233-1238. doi:10.1001/archderm.140.10.1233
8. Saida T, Koga H, Uhara H. Dermoscopy for acral melanocytic lesions: revision of the 3-step algorithm and refined definition of the regular and irregular fibrillar pattern. Dermatol Pract Concept. 2022;12:e2022123. doi:10.5826/dpc.1203a123
9. Heath CR, Usatine RP. Melanoma. Cutis. 2022;109:284-285. doi:10.12788/cutis.0513.
10. Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Visual inspection and dermoscopy, alone or in combination, for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018; 12:CD011901. doi:10.1002/14651858.CD011901.pub2
11. Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked-eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi:10.1111/j.1365-2133.2008.08713.x
12. Phan A, Dalle S, Marcilly MC, et al. Benign dermoscopic parallel ridge pattern variants. Arch Dermatol. 2011;147:634. doi:10.1001/archdermatol.2011.47
13. Fracaroli TS, Lavorato FG, Maceira JP, et al. Parallel ridge pattern on dermoscopy: observation in non-melanoma cases. An Bras Dermatol. 2013;88:646-648. doi:10.1590/abd1806-4841.20132058
14. Manci RN, Dauscher M, Marchetti MA, et al. Features of skin cancer in black individuals: a single-institution retrospective cohort study. Dermatol Pract Concept. 2022;12:e2022075. doi:10.5826/dpc.1202a75
15. Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dematol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
16. Ingrassia JP, Stein JA, Levine A, et al. Diagnosis and management of acral pigmented lesions. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2023;49:926-931. doi:10.1097/DSS.0000000000003891
17. Hill L, Artiga S, Damico A. Health coverage by race and ethnicity, 2010-2022. Kaiser Family Foundation. Published January 11, 2024. Accessed May 9, 2024. https://www.kff.org/racial-equity-and-health-policy/issue-brief/health-coverage-by-race-and-ethnicity
1. Huang K, Fan J, Misra S. Acral lentiginous melanoma: incidence and survival in the United States, 2006-2015: an analysis of the SEER registry. J Surg Res. 2020;251:329-339. doi:10.1016/j.jss.2020.02.010
2. Coleman WP, Gately LE, Krementz AB, et al. Nevi, lentigines, and melanomas in blacks. Arch Dermatol. 1980;116:548-551.
3. Centers for Disease Control and Prevention. Melanoma Incidence and Mortality, United States: 2012-2016. USCS Data Brief, no. 9. Centers for Disease Control and Prevention, US Department of Health and Human Services; 2019. https://www.cdc.gov/cancer/uscs/about/data-briefs/no9-melanoma-incidence-mortality-UnitedStates-2012-2016.htm
4. Wix SN, Brown AB, Heberton M, et al. Clinical features and outcomes of black patients with melanoma. JAMA Dermatol. 2024;160:328-333. doi:10.1001/jamadermatol.2023.5789
5. Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch Dermatol. 2007;143:1423-1426. doi:10.1001/archderm.143.11.1423
6. Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38:25-34. doi:10.1111/j.1346-8138.2010.01174.x
7. Saida T, Miyazaki A, Oguchi S. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140:1233-1238. doi:10.1001/archderm.140.10.1233
8. Saida T, Koga H, Uhara H. Dermoscopy for acral melanocytic lesions: revision of the 3-step algorithm and refined definition of the regular and irregular fibrillar pattern. Dermatol Pract Concept. 2022;12:e2022123. doi:10.5826/dpc.1203a123
9. Heath CR, Usatine RP. Melanoma. Cutis. 2022;109:284-285. doi:10.12788/cutis.0513.
10. Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Visual inspection and dermoscopy, alone or in combination, for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018; 12:CD011901. doi:10.1002/14651858.CD011901.pub2
11. Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked-eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi:10.1111/j.1365-2133.2008.08713.x
12. Phan A, Dalle S, Marcilly MC, et al. Benign dermoscopic parallel ridge pattern variants. Arch Dermatol. 2011;147:634. doi:10.1001/archdermatol.2011.47
13. Fracaroli TS, Lavorato FG, Maceira JP, et al. Parallel ridge pattern on dermoscopy: observation in non-melanoma cases. An Bras Dermatol. 2013;88:646-648. doi:10.1590/abd1806-4841.20132058
14. Manci RN, Dauscher M, Marchetti MA, et al. Features of skin cancer in black individuals: a single-institution retrospective cohort study. Dermatol Pract Concept. 2022;12:e2022075. doi:10.5826/dpc.1202a75
15. Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dematol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
16. Ingrassia JP, Stein JA, Levine A, et al. Diagnosis and management of acral pigmented lesions. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2023;49:926-931. doi:10.1097/DSS.0000000000003891
17. Hill L, Artiga S, Damico A. Health coverage by race and ethnicity, 2010-2022. Kaiser Family Foundation. Published January 11, 2024. Accessed May 9, 2024. https://www.kff.org/racial-equity-and-health-policy/issue-brief/health-coverage-by-race-and-ethnicity
The ASCO Annual Meeting Starts This Week
From its origins in 1964, ASCO’s annual event has grown to become the world’s largest clinical oncology meeting, drawing attendees from across the globe.
More than 7000 abstracts were submitted for this year’s meeting a new record — and over 5000 were selected for presentation.
This year’s chair of the Annual Meeting Education Committee, Thomas William LeBlanc, MD, told us he has been attending the meeting since his training days more than a decade ago.
The event is “just incredibly empowering and energizing,” Dr. LeBlanc said, with opportunities to catch up with old colleagues and meet new ones, learn how far oncology has come and where it’s headed, and hear clinical pearls to take back the clinic.
This year’s theme, selected by ASCO President Lynn M. Schuchter, MD, is “The Art and Science of Cancer Care: From Comfort to Cure.”
Dr. LeBlanc, a blood cancer specialist at Duke University, Durham, North Carolina, said the theme has been woven throughout the abstract and educational sessions. Most sessions will have at least one presentation related to how we support people — not only “when we cure them but also when we can’t cure them,” he said.
Topics will include patient well-being, comfort measures, and survivorship. And for the first time the plenary session will include a palliative care abstract that addresses whether or not palliative care can be delivered effectively through telemedicine. The session is on Sunday, June 2.
Other potentially practice changing plenary abstracts tackle immunotherapy combinations for resectable melanoma, perioperative chemotherapy vs neoadjuvant chemoradiation for esophageal cancer, and osimertinib after definitive chemoradiotherapy for unresectable non–small cell lung cancer.
ASCO is piloting a slightly different format for research presentations this year. Instead of starting with context and background, speakers have been asked to present study results upfront as well as repeat them at the end of the talk. The reason behind the tweak is that engagement and retention tend to be better when results are presented upfront, instead of just at the end of a talk.
A popular session — ASCO Voices — has also been given a more central position in the conference: Friday, May 31. In this session, speakers will give short presentations about their personal experiences as providers, researchers, or patients.
ASCO Voices is a relatively recent addition to the meeting that has grown and gotten better. The talks are usually “very powerful narratives” that remind clinicians about “the importance of what they’re doing each day,” Dr. LeBlanc said.
Snippets of the talks will be played while people wait for sessions to begin at the meeting, so attendees who miss the Friday talks can still hear them.
In terms of educational sessions, Dr. LeBlanc highlighted two that might be of general interest to practicing oncologists: A joint ASCO/American Association for Cancer Research session entitled “Drugging the ‘Undruggable’ Target: Successes, Challenges, and the Road Ahead,” on Sunday morning and “Common Sense Oncology: Equity, Value, and Outcomes That Matter” on Monday morning.
As a blood cancer specialist, he said he is particularly interested in the topline results from the ASC4FIRST trial of asciminib, a newer kinase inhibitor, in newly diagnosed chronic myeloid leukemia, presented on Friday.
As in past years, this news organization will be on hand providing coverage with a dedicated team of reporters, editors, and videographers. Stop by our exhibit hall booth — number 26030 — to learn about the tools we offer to support your practice.
A version of this article appeared on Medscape.com .
From its origins in 1964, ASCO’s annual event has grown to become the world’s largest clinical oncology meeting, drawing attendees from across the globe.
More than 7000 abstracts were submitted for this year’s meeting a new record — and over 5000 were selected for presentation.
This year’s chair of the Annual Meeting Education Committee, Thomas William LeBlanc, MD, told us he has been attending the meeting since his training days more than a decade ago.
The event is “just incredibly empowering and energizing,” Dr. LeBlanc said, with opportunities to catch up with old colleagues and meet new ones, learn how far oncology has come and where it’s headed, and hear clinical pearls to take back the clinic.
This year’s theme, selected by ASCO President Lynn M. Schuchter, MD, is “The Art and Science of Cancer Care: From Comfort to Cure.”
Dr. LeBlanc, a blood cancer specialist at Duke University, Durham, North Carolina, said the theme has been woven throughout the abstract and educational sessions. Most sessions will have at least one presentation related to how we support people — not only “when we cure them but also when we can’t cure them,” he said.
Topics will include patient well-being, comfort measures, and survivorship. And for the first time the plenary session will include a palliative care abstract that addresses whether or not palliative care can be delivered effectively through telemedicine. The session is on Sunday, June 2.
Other potentially practice changing plenary abstracts tackle immunotherapy combinations for resectable melanoma, perioperative chemotherapy vs neoadjuvant chemoradiation for esophageal cancer, and osimertinib after definitive chemoradiotherapy for unresectable non–small cell lung cancer.
ASCO is piloting a slightly different format for research presentations this year. Instead of starting with context and background, speakers have been asked to present study results upfront as well as repeat them at the end of the talk. The reason behind the tweak is that engagement and retention tend to be better when results are presented upfront, instead of just at the end of a talk.
A popular session — ASCO Voices — has also been given a more central position in the conference: Friday, May 31. In this session, speakers will give short presentations about their personal experiences as providers, researchers, or patients.
ASCO Voices is a relatively recent addition to the meeting that has grown and gotten better. The talks are usually “very powerful narratives” that remind clinicians about “the importance of what they’re doing each day,” Dr. LeBlanc said.
Snippets of the talks will be played while people wait for sessions to begin at the meeting, so attendees who miss the Friday talks can still hear them.
In terms of educational sessions, Dr. LeBlanc highlighted two that might be of general interest to practicing oncologists: A joint ASCO/American Association for Cancer Research session entitled “Drugging the ‘Undruggable’ Target: Successes, Challenges, and the Road Ahead,” on Sunday morning and “Common Sense Oncology: Equity, Value, and Outcomes That Matter” on Monday morning.
As a blood cancer specialist, he said he is particularly interested in the topline results from the ASC4FIRST trial of asciminib, a newer kinase inhibitor, in newly diagnosed chronic myeloid leukemia, presented on Friday.
As in past years, this news organization will be on hand providing coverage with a dedicated team of reporters, editors, and videographers. Stop by our exhibit hall booth — number 26030 — to learn about the tools we offer to support your practice.
A version of this article appeared on Medscape.com .
From its origins in 1964, ASCO’s annual event has grown to become the world’s largest clinical oncology meeting, drawing attendees from across the globe.
More than 7000 abstracts were submitted for this year’s meeting a new record — and over 5000 were selected for presentation.
This year’s chair of the Annual Meeting Education Committee, Thomas William LeBlanc, MD, told us he has been attending the meeting since his training days more than a decade ago.
The event is “just incredibly empowering and energizing,” Dr. LeBlanc said, with opportunities to catch up with old colleagues and meet new ones, learn how far oncology has come and where it’s headed, and hear clinical pearls to take back the clinic.
This year’s theme, selected by ASCO President Lynn M. Schuchter, MD, is “The Art and Science of Cancer Care: From Comfort to Cure.”
Dr. LeBlanc, a blood cancer specialist at Duke University, Durham, North Carolina, said the theme has been woven throughout the abstract and educational sessions. Most sessions will have at least one presentation related to how we support people — not only “when we cure them but also when we can’t cure them,” he said.
Topics will include patient well-being, comfort measures, and survivorship. And for the first time the plenary session will include a palliative care abstract that addresses whether or not palliative care can be delivered effectively through telemedicine. The session is on Sunday, June 2.
Other potentially practice changing plenary abstracts tackle immunotherapy combinations for resectable melanoma, perioperative chemotherapy vs neoadjuvant chemoradiation for esophageal cancer, and osimertinib after definitive chemoradiotherapy for unresectable non–small cell lung cancer.
ASCO is piloting a slightly different format for research presentations this year. Instead of starting with context and background, speakers have been asked to present study results upfront as well as repeat them at the end of the talk. The reason behind the tweak is that engagement and retention tend to be better when results are presented upfront, instead of just at the end of a talk.
A popular session — ASCO Voices — has also been given a more central position in the conference: Friday, May 31. In this session, speakers will give short presentations about their personal experiences as providers, researchers, or patients.
ASCO Voices is a relatively recent addition to the meeting that has grown and gotten better. The talks are usually “very powerful narratives” that remind clinicians about “the importance of what they’re doing each day,” Dr. LeBlanc said.
Snippets of the talks will be played while people wait for sessions to begin at the meeting, so attendees who miss the Friday talks can still hear them.
In terms of educational sessions, Dr. LeBlanc highlighted two that might be of general interest to practicing oncologists: A joint ASCO/American Association for Cancer Research session entitled “Drugging the ‘Undruggable’ Target: Successes, Challenges, and the Road Ahead,” on Sunday morning and “Common Sense Oncology: Equity, Value, and Outcomes That Matter” on Monday morning.
As a blood cancer specialist, he said he is particularly interested in the topline results from the ASC4FIRST trial of asciminib, a newer kinase inhibitor, in newly diagnosed chronic myeloid leukemia, presented on Friday.
As in past years, this news organization will be on hand providing coverage with a dedicated team of reporters, editors, and videographers. Stop by our exhibit hall booth — number 26030 — to learn about the tools we offer to support your practice.
A version of this article appeared on Medscape.com .
ASTRO Releases New EBRT Guideline for Symptomatic Bone Mets
The guideline was needed to update previous recommendations and incorporate new high-quality evidence for the management of symptomatic bone metastases, Sara Alcorn, MD, PhD, of the University of Minnesota, Minneapolis, and colleagues wrote in Practical Radiation Oncology.
The focus was on the efficacy of EBRT in reducing pain, improving skeletal function, and enhancing quality of life, they wrote in the clinical practice guideline.
In developing their recommendations, the ASTRO task force reviewed evidence from 53 randomized controlled trials (RCTs) and 31 nonrandomized studies, and considered clinical experience.
Indications for Palliative Radiation
EBRT is strongly recommended for reducing pain from osseous metastasis and improving ambulatory status, sphincter function, and reducing pain in patients with spinal metastases causing compression of the spinal cord or cauda equina.
For patients with symptomatic bone metastases and an anticipated life expectancy of at least 4 weeks, EBRT is conditionally recommended to improve quality of life.
Implementation of other Treatments Alongside Palliative Radiation
Instead of RT alone, surgery with postoperative RT is conditionally recommended for patients with compression of the spinal cord or cauda equina.
Postoperative RT is strongly recommended for patients who have undergone surgery for non-spine bone metastases or spine metastases without involving spinal cord or cauda equina compression.
For patients with spinal bone metastases compressing the spinal cord or cauda equina, combining RT with dexamethasone is strongly recommended over RT alone.
Techniques, Dose-Fractionation, and Dose-Constraints for Initial Palliative Radiation
For patients with symptomatic bone metastases undergoing conventional palliative RT, strongly recommended doses are 800 cGy in 1 fraction, 2000 cGy in 5 fractions, 2400 cGy in 6 fractions, or 3000 cGy in 10 fractions.
For patients with spinal bone metastases causing compression of the spinal cord or cauda equina who are not candidates for initial surgical decompression and are treated with conventional palliative RT, strongly recommended doses are 800 cGy in 1 fraction, 1600 cGy in 2 fractions, 2000 cGy in 5 fractions, or 3000 cGy in 10 fractions.
When selecting dose-fractionation, consider patient and disease factors such as prognosis and radiosensitivity, the authors wrote.
Highly conformal planning and delivery techniques, such as intensity-modulated radiation therapy, are conditionally recommended for patients with spinal bone metastases compressing the spinal cord or cauda equina who are receiving dose-escalated palliative RT.
The strongly recommended stereotactic body radiotherapy (SBRT) doses for patients with symptomatic bone metastases are 1200 to 1600 cGy in 1 fraction for non-spine metastases and 2400 cGy in 2 fractions for spine metastases. Other established SBRT dose and fractionation regimens with similar biologically effective doses may be considered based on patient tumor characteristics, normal tissue factors, and physician experience.
For patients with symptomatic bone metastases who have an ECOG PS of 0-2, are not undergoing surgical intervention, and have no neurological symptoms, SBRT is conditionally recommended over conventional palliative RT. Other factors to consider include life expectancy, tumor radiosensitivity, and metastatic disease burden, the guideline says.
Techniques, Dose-Fractionation, and Dose-Constraints for Palliative Reirradiation
For patients with spinal bone metastases requiring reirradiation to the same site, the strongly recommended conventional palliative RT regimens are 800 cGy in 1 fraction, 2000 cGy in 5 fractions, 2400 cGy in 6 fractions, or 2000 cGy in 8 fractions. When determining the RT dose-fractionation, consider the prior RT dose, time interval, and total spinal cord tolerance, the guideline says.
Treatment with SBRT is conditionally recommended for patients with spinal bone metastases needing reirradiation at the same site. When determining if SBRT is appropriate, consider patient factors such as urgency of treatment, prognosis, and radio-resistance. In addition, consider the prior RT dose, time interval, and total spinal cord tolerance when determining the RT dose-fractionation, the authors say.
The strongly recommended options for patients with symptomatic non-spine bone metastases needing reirradiation at the same site are single-fraction RT (800 cGy in 1 fraction) or multifraction conventional palliative RT (2000 cGy in 5 fractions or 2400 cGy in 6 fractions).
Impact of Techniques and Dose-fractionation on Quality of Life and Toxicity
For patients with bone metastases undergoing palliative radiation, it is strongly recommended to use a shared decision-making approach to determine the dose, fractionation, and supportive measures to optimize quality of life.
“Based on published data, the ASTRO task force’s recommendations inform best clinical practices on palliative RT for symptomatic bone metastases,” the guideline panelists said.
Limitations
While the guideline provides comprehensive recommendations, the panelists underscored the importance of individualized treatment approaches. Future research is needed to address gaps in evidence, particularly regarding advanced RT techniques and reirradiation strategies.
Guideline development was funded by ASTRO, with the systematic evidence review funded by the Patient-Centered Outcomes Research Institute. The panelists disclosed relationships with AstraZeneca, Elekta, Teladoc, and others.
The guideline was needed to update previous recommendations and incorporate new high-quality evidence for the management of symptomatic bone metastases, Sara Alcorn, MD, PhD, of the University of Minnesota, Minneapolis, and colleagues wrote in Practical Radiation Oncology.
The focus was on the efficacy of EBRT in reducing pain, improving skeletal function, and enhancing quality of life, they wrote in the clinical practice guideline.
In developing their recommendations, the ASTRO task force reviewed evidence from 53 randomized controlled trials (RCTs) and 31 nonrandomized studies, and considered clinical experience.
Indications for Palliative Radiation
EBRT is strongly recommended for reducing pain from osseous metastasis and improving ambulatory status, sphincter function, and reducing pain in patients with spinal metastases causing compression of the spinal cord or cauda equina.
For patients with symptomatic bone metastases and an anticipated life expectancy of at least 4 weeks, EBRT is conditionally recommended to improve quality of life.
Implementation of other Treatments Alongside Palliative Radiation
Instead of RT alone, surgery with postoperative RT is conditionally recommended for patients with compression of the spinal cord or cauda equina.
Postoperative RT is strongly recommended for patients who have undergone surgery for non-spine bone metastases or spine metastases without involving spinal cord or cauda equina compression.
For patients with spinal bone metastases compressing the spinal cord or cauda equina, combining RT with dexamethasone is strongly recommended over RT alone.
Techniques, Dose-Fractionation, and Dose-Constraints for Initial Palliative Radiation
For patients with symptomatic bone metastases undergoing conventional palliative RT, strongly recommended doses are 800 cGy in 1 fraction, 2000 cGy in 5 fractions, 2400 cGy in 6 fractions, or 3000 cGy in 10 fractions.
For patients with spinal bone metastases causing compression of the spinal cord or cauda equina who are not candidates for initial surgical decompression and are treated with conventional palliative RT, strongly recommended doses are 800 cGy in 1 fraction, 1600 cGy in 2 fractions, 2000 cGy in 5 fractions, or 3000 cGy in 10 fractions.
When selecting dose-fractionation, consider patient and disease factors such as prognosis and radiosensitivity, the authors wrote.
Highly conformal planning and delivery techniques, such as intensity-modulated radiation therapy, are conditionally recommended for patients with spinal bone metastases compressing the spinal cord or cauda equina who are receiving dose-escalated palliative RT.
The strongly recommended stereotactic body radiotherapy (SBRT) doses for patients with symptomatic bone metastases are 1200 to 1600 cGy in 1 fraction for non-spine metastases and 2400 cGy in 2 fractions for spine metastases. Other established SBRT dose and fractionation regimens with similar biologically effective doses may be considered based on patient tumor characteristics, normal tissue factors, and physician experience.
For patients with symptomatic bone metastases who have an ECOG PS of 0-2, are not undergoing surgical intervention, and have no neurological symptoms, SBRT is conditionally recommended over conventional palliative RT. Other factors to consider include life expectancy, tumor radiosensitivity, and metastatic disease burden, the guideline says.
Techniques, Dose-Fractionation, and Dose-Constraints for Palliative Reirradiation
For patients with spinal bone metastases requiring reirradiation to the same site, the strongly recommended conventional palliative RT regimens are 800 cGy in 1 fraction, 2000 cGy in 5 fractions, 2400 cGy in 6 fractions, or 2000 cGy in 8 fractions. When determining the RT dose-fractionation, consider the prior RT dose, time interval, and total spinal cord tolerance, the guideline says.
Treatment with SBRT is conditionally recommended for patients with spinal bone metastases needing reirradiation at the same site. When determining if SBRT is appropriate, consider patient factors such as urgency of treatment, prognosis, and radio-resistance. In addition, consider the prior RT dose, time interval, and total spinal cord tolerance when determining the RT dose-fractionation, the authors say.
The strongly recommended options for patients with symptomatic non-spine bone metastases needing reirradiation at the same site are single-fraction RT (800 cGy in 1 fraction) or multifraction conventional palliative RT (2000 cGy in 5 fractions or 2400 cGy in 6 fractions).
Impact of Techniques and Dose-fractionation on Quality of Life and Toxicity
For patients with bone metastases undergoing palliative radiation, it is strongly recommended to use a shared decision-making approach to determine the dose, fractionation, and supportive measures to optimize quality of life.
“Based on published data, the ASTRO task force’s recommendations inform best clinical practices on palliative RT for symptomatic bone metastases,” the guideline panelists said.
Limitations
While the guideline provides comprehensive recommendations, the panelists underscored the importance of individualized treatment approaches. Future research is needed to address gaps in evidence, particularly regarding advanced RT techniques and reirradiation strategies.
Guideline development was funded by ASTRO, with the systematic evidence review funded by the Patient-Centered Outcomes Research Institute. The panelists disclosed relationships with AstraZeneca, Elekta, Teladoc, and others.
The guideline was needed to update previous recommendations and incorporate new high-quality evidence for the management of symptomatic bone metastases, Sara Alcorn, MD, PhD, of the University of Minnesota, Minneapolis, and colleagues wrote in Practical Radiation Oncology.
The focus was on the efficacy of EBRT in reducing pain, improving skeletal function, and enhancing quality of life, they wrote in the clinical practice guideline.
In developing their recommendations, the ASTRO task force reviewed evidence from 53 randomized controlled trials (RCTs) and 31 nonrandomized studies, and considered clinical experience.
Indications for Palliative Radiation
EBRT is strongly recommended for reducing pain from osseous metastasis and improving ambulatory status, sphincter function, and reducing pain in patients with spinal metastases causing compression of the spinal cord or cauda equina.
For patients with symptomatic bone metastases and an anticipated life expectancy of at least 4 weeks, EBRT is conditionally recommended to improve quality of life.
Implementation of other Treatments Alongside Palliative Radiation
Instead of RT alone, surgery with postoperative RT is conditionally recommended for patients with compression of the spinal cord or cauda equina.
Postoperative RT is strongly recommended for patients who have undergone surgery for non-spine bone metastases or spine metastases without involving spinal cord or cauda equina compression.
For patients with spinal bone metastases compressing the spinal cord or cauda equina, combining RT with dexamethasone is strongly recommended over RT alone.
Techniques, Dose-Fractionation, and Dose-Constraints for Initial Palliative Radiation
For patients with symptomatic bone metastases undergoing conventional palliative RT, strongly recommended doses are 800 cGy in 1 fraction, 2000 cGy in 5 fractions, 2400 cGy in 6 fractions, or 3000 cGy in 10 fractions.
For patients with spinal bone metastases causing compression of the spinal cord or cauda equina who are not candidates for initial surgical decompression and are treated with conventional palliative RT, strongly recommended doses are 800 cGy in 1 fraction, 1600 cGy in 2 fractions, 2000 cGy in 5 fractions, or 3000 cGy in 10 fractions.
When selecting dose-fractionation, consider patient and disease factors such as prognosis and radiosensitivity, the authors wrote.
Highly conformal planning and delivery techniques, such as intensity-modulated radiation therapy, are conditionally recommended for patients with spinal bone metastases compressing the spinal cord or cauda equina who are receiving dose-escalated palliative RT.
The strongly recommended stereotactic body radiotherapy (SBRT) doses for patients with symptomatic bone metastases are 1200 to 1600 cGy in 1 fraction for non-spine metastases and 2400 cGy in 2 fractions for spine metastases. Other established SBRT dose and fractionation regimens with similar biologically effective doses may be considered based on patient tumor characteristics, normal tissue factors, and physician experience.
For patients with symptomatic bone metastases who have an ECOG PS of 0-2, are not undergoing surgical intervention, and have no neurological symptoms, SBRT is conditionally recommended over conventional palliative RT. Other factors to consider include life expectancy, tumor radiosensitivity, and metastatic disease burden, the guideline says.
Techniques, Dose-Fractionation, and Dose-Constraints for Palliative Reirradiation
For patients with spinal bone metastases requiring reirradiation to the same site, the strongly recommended conventional palliative RT regimens are 800 cGy in 1 fraction, 2000 cGy in 5 fractions, 2400 cGy in 6 fractions, or 2000 cGy in 8 fractions. When determining the RT dose-fractionation, consider the prior RT dose, time interval, and total spinal cord tolerance, the guideline says.
Treatment with SBRT is conditionally recommended for patients with spinal bone metastases needing reirradiation at the same site. When determining if SBRT is appropriate, consider patient factors such as urgency of treatment, prognosis, and radio-resistance. In addition, consider the prior RT dose, time interval, and total spinal cord tolerance when determining the RT dose-fractionation, the authors say.
The strongly recommended options for patients with symptomatic non-spine bone metastases needing reirradiation at the same site are single-fraction RT (800 cGy in 1 fraction) or multifraction conventional palliative RT (2000 cGy in 5 fractions or 2400 cGy in 6 fractions).
Impact of Techniques and Dose-fractionation on Quality of Life and Toxicity
For patients with bone metastases undergoing palliative radiation, it is strongly recommended to use a shared decision-making approach to determine the dose, fractionation, and supportive measures to optimize quality of life.
“Based on published data, the ASTRO task force’s recommendations inform best clinical practices on palliative RT for symptomatic bone metastases,” the guideline panelists said.
Limitations
While the guideline provides comprehensive recommendations, the panelists underscored the importance of individualized treatment approaches. Future research is needed to address gaps in evidence, particularly regarding advanced RT techniques and reirradiation strategies.
Guideline development was funded by ASTRO, with the systematic evidence review funded by the Patient-Centered Outcomes Research Institute. The panelists disclosed relationships with AstraZeneca, Elekta, Teladoc, and others.
FROM PRACTICAL RADIATION ONCOLOGY
Study Finds Injuries, Stress Levels Increased Among Mohs Surgeons
PHOENIX — In addition, most surgeons did not feel prepared to manage or prevent these symptoms.
“Our study highlights the need to implement ergonomic training and emotion-focused coping skills, as part of fellowship training and the continuing medical education curriculum, to alleviate and prevent emotional burnout,” said lead author Eduardo A. Michelen-Gómez, MD, a dermatology resident at the University of Puerto Rico School of Medicine, San Juano. “This interaction also must be designed to cater to generation and sex specific needs.”
Dr. Michelen-Gómez presented the findings at the annual meeting of the American College of Mohs Surgery.
Mohs is a demanding procedure that involves repetitive motion, strict attention to detail, and high practice efficiency, all of which must be balanced with the need to prioritize patient safety and well-being. “All of these factors predispose Mohs surgeons to be at an increased risk of physical and emotional stress,” he said.
Despite these concerns, however, the literature is limited concerning work-associated stressors among Mohs surgeons. To further explore this issue, Dr. Michelen-Gómez and colleagues conducted a survey study of ACMS members to investigate not only the prevalence of emotional and physical stressors associated with being a Mohs surgeon but also what specific actions physicians were taking to prevent and/or treat these stressors.
They designed a 21-question cross-sectional electronic survey that was sent to all active ACMS members in 2023. Outcomes evaluated were gender, years of practice, concern for and prevalence of occupational musculoskeletal disorders, emotional stress and burnout, and surgeon’s knowledge and training to manage these symptoms. A total of 473 Mohs surgeons responded.
High Prevalence of Injury and Burnout
“Almost 90% of respondents reported moderate to severe concern for occupational musculoskeletal injuries,” said Dr. Michelen-Gómez. “The prevalence of these injuries was 68%, with neck injuries being the most common complaint. Of the entire cohort, 67% have adopted ergonomic practices patterns.”
Female surgeons had a higher prevalence of musculoskeletal injuries than men, and there was no correlation between years of practice and prevalence of these injuries.
Their results also showed that 70% of respondents reported experiencing psychological and emotional stress or burnout associated with being a Mohs surgeon. The cause of emotional stress differed between men and women. “In males, the most common cause was patient care–related anxiety, while in females, it was finding an adequate work-life balance,” he said.
Surgeons with fewer years of experience were more likely to experience emotional stress (P = .01), and female surgeons had a higher prevalence of burnout and musculoskeletal disorders (71.0% and 71.4%, respectively) than male surgeons (67.7% and 65.2%, respectively).
To prevent or manage musculoskeletal injury, respondents reported using interventions such as physical therapy, yoga/stretching/Pilates, massage therapy, cupping, and using a physical trainer. Specific actions for preventing or managing emotional stress and burnout included engaging with a therapist, working with a life coach, practicing meditation or mindfulness, journaling, relying on religion or spirituality, and exercise.
However, among those who reported musculoskeletal disorders or emotional stress, only 40.56% and 46.67%, respectively, felt they had sufficient knowledge and the resources to manage them appropriately.
“In addition, we found a positive correlation between the development of psychological stress and physical issues,” said Dr. Michelen-Gómez. Future studies can include determining the most effective methods to address the emotional and physical stressors of practicing Mohs Surgery.”
Asked to comment on the study findings, Jesse M. Lewin, MD, chief of Mohs micrographic and dermatologic surgery and vice chair of surgical operations at the Icahn School of Medicine at Mount Sinai, New York City, said that the real-world take-home messages from this study are twofold.
“It is important to focus on physician wellness and prevention of burnout and physical injury to protect our physician workforce, and two, we should equip physicians-in-training with tools to protect their physical and emotional health,” he said.
Dr. Michelen-Gómez and Dr. Lewin, who was not involved with the study, had no relevant disclosures.
A version of this article appeared on Medscape.com.
PHOENIX — In addition, most surgeons did not feel prepared to manage or prevent these symptoms.
“Our study highlights the need to implement ergonomic training and emotion-focused coping skills, as part of fellowship training and the continuing medical education curriculum, to alleviate and prevent emotional burnout,” said lead author Eduardo A. Michelen-Gómez, MD, a dermatology resident at the University of Puerto Rico School of Medicine, San Juano. “This interaction also must be designed to cater to generation and sex specific needs.”
Dr. Michelen-Gómez presented the findings at the annual meeting of the American College of Mohs Surgery.
Mohs is a demanding procedure that involves repetitive motion, strict attention to detail, and high practice efficiency, all of which must be balanced with the need to prioritize patient safety and well-being. “All of these factors predispose Mohs surgeons to be at an increased risk of physical and emotional stress,” he said.
Despite these concerns, however, the literature is limited concerning work-associated stressors among Mohs surgeons. To further explore this issue, Dr. Michelen-Gómez and colleagues conducted a survey study of ACMS members to investigate not only the prevalence of emotional and physical stressors associated with being a Mohs surgeon but also what specific actions physicians were taking to prevent and/or treat these stressors.
They designed a 21-question cross-sectional electronic survey that was sent to all active ACMS members in 2023. Outcomes evaluated were gender, years of practice, concern for and prevalence of occupational musculoskeletal disorders, emotional stress and burnout, and surgeon’s knowledge and training to manage these symptoms. A total of 473 Mohs surgeons responded.
High Prevalence of Injury and Burnout
“Almost 90% of respondents reported moderate to severe concern for occupational musculoskeletal injuries,” said Dr. Michelen-Gómez. “The prevalence of these injuries was 68%, with neck injuries being the most common complaint. Of the entire cohort, 67% have adopted ergonomic practices patterns.”
Female surgeons had a higher prevalence of musculoskeletal injuries than men, and there was no correlation between years of practice and prevalence of these injuries.
Their results also showed that 70% of respondents reported experiencing psychological and emotional stress or burnout associated with being a Mohs surgeon. The cause of emotional stress differed between men and women. “In males, the most common cause was patient care–related anxiety, while in females, it was finding an adequate work-life balance,” he said.
Surgeons with fewer years of experience were more likely to experience emotional stress (P = .01), and female surgeons had a higher prevalence of burnout and musculoskeletal disorders (71.0% and 71.4%, respectively) than male surgeons (67.7% and 65.2%, respectively).
To prevent or manage musculoskeletal injury, respondents reported using interventions such as physical therapy, yoga/stretching/Pilates, massage therapy, cupping, and using a physical trainer. Specific actions for preventing or managing emotional stress and burnout included engaging with a therapist, working with a life coach, practicing meditation or mindfulness, journaling, relying on religion or spirituality, and exercise.
However, among those who reported musculoskeletal disorders or emotional stress, only 40.56% and 46.67%, respectively, felt they had sufficient knowledge and the resources to manage them appropriately.
“In addition, we found a positive correlation between the development of psychological stress and physical issues,” said Dr. Michelen-Gómez. Future studies can include determining the most effective methods to address the emotional and physical stressors of practicing Mohs Surgery.”
Asked to comment on the study findings, Jesse M. Lewin, MD, chief of Mohs micrographic and dermatologic surgery and vice chair of surgical operations at the Icahn School of Medicine at Mount Sinai, New York City, said that the real-world take-home messages from this study are twofold.
“It is important to focus on physician wellness and prevention of burnout and physical injury to protect our physician workforce, and two, we should equip physicians-in-training with tools to protect their physical and emotional health,” he said.
Dr. Michelen-Gómez and Dr. Lewin, who was not involved with the study, had no relevant disclosures.
A version of this article appeared on Medscape.com.
PHOENIX — In addition, most surgeons did not feel prepared to manage or prevent these symptoms.
“Our study highlights the need to implement ergonomic training and emotion-focused coping skills, as part of fellowship training and the continuing medical education curriculum, to alleviate and prevent emotional burnout,” said lead author Eduardo A. Michelen-Gómez, MD, a dermatology resident at the University of Puerto Rico School of Medicine, San Juano. “This interaction also must be designed to cater to generation and sex specific needs.”
Dr. Michelen-Gómez presented the findings at the annual meeting of the American College of Mohs Surgery.
Mohs is a demanding procedure that involves repetitive motion, strict attention to detail, and high practice efficiency, all of which must be balanced with the need to prioritize patient safety and well-being. “All of these factors predispose Mohs surgeons to be at an increased risk of physical and emotional stress,” he said.
Despite these concerns, however, the literature is limited concerning work-associated stressors among Mohs surgeons. To further explore this issue, Dr. Michelen-Gómez and colleagues conducted a survey study of ACMS members to investigate not only the prevalence of emotional and physical stressors associated with being a Mohs surgeon but also what specific actions physicians were taking to prevent and/or treat these stressors.
They designed a 21-question cross-sectional electronic survey that was sent to all active ACMS members in 2023. Outcomes evaluated were gender, years of practice, concern for and prevalence of occupational musculoskeletal disorders, emotional stress and burnout, and surgeon’s knowledge and training to manage these symptoms. A total of 473 Mohs surgeons responded.
High Prevalence of Injury and Burnout
“Almost 90% of respondents reported moderate to severe concern for occupational musculoskeletal injuries,” said Dr. Michelen-Gómez. “The prevalence of these injuries was 68%, with neck injuries being the most common complaint. Of the entire cohort, 67% have adopted ergonomic practices patterns.”
Female surgeons had a higher prevalence of musculoskeletal injuries than men, and there was no correlation between years of practice and prevalence of these injuries.
Their results also showed that 70% of respondents reported experiencing psychological and emotional stress or burnout associated with being a Mohs surgeon. The cause of emotional stress differed between men and women. “In males, the most common cause was patient care–related anxiety, while in females, it was finding an adequate work-life balance,” he said.
Surgeons with fewer years of experience were more likely to experience emotional stress (P = .01), and female surgeons had a higher prevalence of burnout and musculoskeletal disorders (71.0% and 71.4%, respectively) than male surgeons (67.7% and 65.2%, respectively).
To prevent or manage musculoskeletal injury, respondents reported using interventions such as physical therapy, yoga/stretching/Pilates, massage therapy, cupping, and using a physical trainer. Specific actions for preventing or managing emotional stress and burnout included engaging with a therapist, working with a life coach, practicing meditation or mindfulness, journaling, relying on religion or spirituality, and exercise.
However, among those who reported musculoskeletal disorders or emotional stress, only 40.56% and 46.67%, respectively, felt they had sufficient knowledge and the resources to manage them appropriately.
“In addition, we found a positive correlation between the development of psychological stress and physical issues,” said Dr. Michelen-Gómez. Future studies can include determining the most effective methods to address the emotional and physical stressors of practicing Mohs Surgery.”
Asked to comment on the study findings, Jesse M. Lewin, MD, chief of Mohs micrographic and dermatologic surgery and vice chair of surgical operations at the Icahn School of Medicine at Mount Sinai, New York City, said that the real-world take-home messages from this study are twofold.
“It is important to focus on physician wellness and prevention of burnout and physical injury to protect our physician workforce, and two, we should equip physicians-in-training with tools to protect their physical and emotional health,” he said.
Dr. Michelen-Gómez and Dr. Lewin, who was not involved with the study, had no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM ACMS 2024