User login
Differentiating DNI From DNR
Since the introduction of defibrillation and closed chest cardiopulmonary resuscitation (CPR) in the 1950s, the ability to revive an arrested heart has been a realized possibility. Around the same time, endotracheal intubation with mechanical ventilation (MV) came into wide practice, allowing doctors to augment or even replace their patients' breathing. But just as the 1950s and 1960s saw the rise of these enhanced medical techniques, they also saw the increased importance of medical ethicsin particular, patient autonomy. A natural reaction to medicine's use of CPR and MV was the advent of advance directives and more specific do‐not‐resuscitate (DNR) and do‐not‐intubate (DNI) orders meant to protect a patient's ability to remain autonomous with their end of life decisions.[1]
Unfortunately, the code status discussions that lead to these orders often collapse cardiac arrest with prearrest respiratory failure and CPR with MV.[2, 3] This is a problem for a number of reasons. First, cardiac arrest and prearrest respiratory failure are unique end points, and though their respective treatments (CPR and MV) are often required simultaneously for an individual patient, they are distinct medical interventions with different goals, indications, and associated disease states. Although MV is typically a part of the cadre of interventions meant to ensure continued tissue oxygenation in the setting of a cardiac arrest, this accounts for <2% of indications for MV.[4] The vast majority of MV is used to treat prearrest causes of respiratory failure, such as pneumonia, congestive heart failure, acute exacerbations of chronic obstructive pulmonary disease, and following surgery.[4]
We do not believe these differences are adequately reflected in typical code status discussions.[2, 3] One study using audio‐recorded admission encounters included transcripts of hospitalist‐led code status discussions that all resembled the following: Physician: [I]f an emergency were to happenand your heart would (stop) or your breathing became so difficult that you needed to be attached to machines, would you want the nurses and doctors to attempt heroic measures to try to restart your heart and attach you to a breathing machine?[2] It would come as little surprise if a patient hearing this assumed that just 1 question were being asked and that decisions relating to any cause of respiratory failure (including prearrest causes) were being made. In practice, many physicians then extrapolate DNR orders to other treatment decision (including MV) and interpret them as precluding intubation, even for prearrest states.[5, 6, 7]
A second issue is that the mortality associated with cardiopulmonary arrest requiring CPR and prearrest respiratory failure requiring MV are not equal. Though the mortality after in‐hospital cardiac arrest has decreased over the last decade, it remains >75%.[8] The outcomes for MV for isolated respiratory failure, on the other hand, are not as grim; studies of the general population typically report mortality rates <40%. Despite this, descriptions of outcomes are often left out of goals of care discussions.[9, 10] For example, Sharma et al. recently reported that only one‐third of residents, including those who had undergone training on goals of care discussions, discussed outcomes.[9] And when outcomes are included, they are typically for CPR but not MV as an independent intervention for prearrest respiratory failure.[10] Given that many of the conditions that lead to respiratory failure are among the most common reasons for hospitalization,[11] distinguishing between decisions regarding CPR and prearrest MV with discussion of their associated outcomes is of particular importance to hospitalists. Failing to do so impedes patients from making informed autonomous decisions that incorporate an accurate understanding of the treatments being discussed.
Imagine you are caring for a 75‐year‐old man with a history of coronary artery disease and congestive heart failure now admitted with pneumonia. Given his age, admitting diagnosis, and comorbidities, you feel it would be appropriate to engage him in a discussion of goals of care. His chances of survival with near return to baseline after a cardiac arrest requiring CPR are not the same as his chances of surviving an episode of worsening pneumonia requiring MV. To discuss cardiac arrest and prearrest respiratory failure in the same breath, without acknowledging the differences, is misleading. Based on his goals and values, this patient may see a trial of MV as acceptable. One recent study supports this hypothesis, as 28% of hospitalized patients with a combined DNR/DNI order would accept a trial of MV for pneumonia.[12] If the genesis of these orders was our desire to ensure that patients' autonomous preferences are respected, we must actually know those preferences, and those preferences should be based on adequate information about the expected outcomes, highlighting the differences outlined above.
Some may consider separating CPR from MVtherefore allowing for more clearly separate DNR and DNI ordersproblematic, as it may result in a menu of choices for patients. However, although CPR and MV may be performed at the same time for the same patient, they do not overlap in 100% of their occurrences. This is conceptually different from discussing whether to use epinephrine versus vasopressin, for example, or offering options such as chest compressions alone. More clearly separating CPR from MV would not be dissimilar to what is done with renal dialysis; a patient may wish to be DNR while still electing to undergo dialysis for failing kidneys. Though the discussions surrounding renal dialysis are less urgent, this alone does not adequately explain why the topic is not routinely collapsed into the discussion of CPR. Instead, renal dialysis is an intervention with unique indications, goals, and outcomes; this is what prompts the separation. The same is true of MV.
No matter the situation, code status discussions should focus on determining an individual patient's values and goals of care and should guide physicians in provision (or omission) of certain interventions. For the patient with pneumonia described above, his goal may be to promote quality of life over extension of life. Although this may prompt a recommendation to forego CPR, (if it were felt that his quality of life, even after successful return of spontaneous circulation, would be low), it may not be inconsistent for him to accept a trial of MV were his pneumonia to get worse (if it were felt that he could quickly improve and return to a quality of life close to what he experienced before the episode of pneumonia). We recommend that when discussing options with patients, the indications for and outcomes of CPR and MV be more clearly separated. It may be as simple as saying, there are 2 different situations I would like to discuss with you, followed by a discussion of the associated scenarios and likely outcomes in the best judgment of the care team. For a hospitalist, framing the discussion of MV around anticipated causes of pre‐arrest respiratory failure (eg, pneumonia, acute pulmonary edema) is essential.
In conclusion, if DNR and DNI orders are going to meet their promise of ensuring patients make informed decisions congruent with their goals, then the discussions from which they follow will need to more clearly acknowledge the important differences in indications and outcomes. Although a patient's goals should still be the framework upon which decisions regarding interventions are made, an important distinction should be made between cardiopulmonary arrest and prearrest respiratory failure, with a more explicit accompanying discussion of how the corresponding interventions fit within the patient's overall goals of care.
Acknowledgements
The authors thank Rafael Campo, MD, and Sharon H. Chou, MD, for their suggestions and critical reading of this manuscript.
Disclosures:
Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging.
- , , . Orders not to resuscitate. N Engl J Med. 1976;295(7):364–366.
- , , , , . Code status discussions between attending hospitalist physicians and medical patients at hospital admission. J Gen Intern Med. 2010;26(4):359–366.
- , , . How do medical residents discuss resuscitation with patients? J Gen Intern Med. 1995;10(8):436–442.
- , , , et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28‐day international study. JAMA. 2002;287(3):345–355.
- , . The effect of do‐not‐resuscitate orders on physician decision‐making. J Am Geriatr Soc. 2002;50(12):2057–2061.
- , , . Hospital do‐not‐resuscitate orders: why they have failed and how to fix them. J Gen Intern Med. 2011;26(7):791–797.
- , , . Clinician perspectives regarding the do‐not‐resuscitate order. JAMA Pediatr. 2013;167(10):954–958.
- , , , , , . Trends in survival after in‐hospital cardiac arrest. N Engl J Med. 2012;367(20):1912–1920.
- , , , , , . Unpacking resident‐led code status discussions: results from a mixed methods study. J Gen Intern Med. 2014;29(5):750–7.
- , , , et al. If asked, hospitalized patients will choose whether to receive life‐sustaining therapies. J Hosp Med. 2006;1(3):161–167.
- Healthcare Cost and Utilization Project (HCUP). HCUP Facts and Figures: Statistics on Hospital‐Based Care in the United States, 2009. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
- , , , et al. Preferences for resuscitation and intubation among patients with do‐not‐resuscitate/do‐not‐intubate orders. Mayo Clin Proceed. 2013;88(7):658–665.
Since the introduction of defibrillation and closed chest cardiopulmonary resuscitation (CPR) in the 1950s, the ability to revive an arrested heart has been a realized possibility. Around the same time, endotracheal intubation with mechanical ventilation (MV) came into wide practice, allowing doctors to augment or even replace their patients' breathing. But just as the 1950s and 1960s saw the rise of these enhanced medical techniques, they also saw the increased importance of medical ethicsin particular, patient autonomy. A natural reaction to medicine's use of CPR and MV was the advent of advance directives and more specific do‐not‐resuscitate (DNR) and do‐not‐intubate (DNI) orders meant to protect a patient's ability to remain autonomous with their end of life decisions.[1]
Unfortunately, the code status discussions that lead to these orders often collapse cardiac arrest with prearrest respiratory failure and CPR with MV.[2, 3] This is a problem for a number of reasons. First, cardiac arrest and prearrest respiratory failure are unique end points, and though their respective treatments (CPR and MV) are often required simultaneously for an individual patient, they are distinct medical interventions with different goals, indications, and associated disease states. Although MV is typically a part of the cadre of interventions meant to ensure continued tissue oxygenation in the setting of a cardiac arrest, this accounts for <2% of indications for MV.[4] The vast majority of MV is used to treat prearrest causes of respiratory failure, such as pneumonia, congestive heart failure, acute exacerbations of chronic obstructive pulmonary disease, and following surgery.[4]
We do not believe these differences are adequately reflected in typical code status discussions.[2, 3] One study using audio‐recorded admission encounters included transcripts of hospitalist‐led code status discussions that all resembled the following: Physician: [I]f an emergency were to happenand your heart would (stop) or your breathing became so difficult that you needed to be attached to machines, would you want the nurses and doctors to attempt heroic measures to try to restart your heart and attach you to a breathing machine?[2] It would come as little surprise if a patient hearing this assumed that just 1 question were being asked and that decisions relating to any cause of respiratory failure (including prearrest causes) were being made. In practice, many physicians then extrapolate DNR orders to other treatment decision (including MV) and interpret them as precluding intubation, even for prearrest states.[5, 6, 7]
A second issue is that the mortality associated with cardiopulmonary arrest requiring CPR and prearrest respiratory failure requiring MV are not equal. Though the mortality after in‐hospital cardiac arrest has decreased over the last decade, it remains >75%.[8] The outcomes for MV for isolated respiratory failure, on the other hand, are not as grim; studies of the general population typically report mortality rates <40%. Despite this, descriptions of outcomes are often left out of goals of care discussions.[9, 10] For example, Sharma et al. recently reported that only one‐third of residents, including those who had undergone training on goals of care discussions, discussed outcomes.[9] And when outcomes are included, they are typically for CPR but not MV as an independent intervention for prearrest respiratory failure.[10] Given that many of the conditions that lead to respiratory failure are among the most common reasons for hospitalization,[11] distinguishing between decisions regarding CPR and prearrest MV with discussion of their associated outcomes is of particular importance to hospitalists. Failing to do so impedes patients from making informed autonomous decisions that incorporate an accurate understanding of the treatments being discussed.
Imagine you are caring for a 75‐year‐old man with a history of coronary artery disease and congestive heart failure now admitted with pneumonia. Given his age, admitting diagnosis, and comorbidities, you feel it would be appropriate to engage him in a discussion of goals of care. His chances of survival with near return to baseline after a cardiac arrest requiring CPR are not the same as his chances of surviving an episode of worsening pneumonia requiring MV. To discuss cardiac arrest and prearrest respiratory failure in the same breath, without acknowledging the differences, is misleading. Based on his goals and values, this patient may see a trial of MV as acceptable. One recent study supports this hypothesis, as 28% of hospitalized patients with a combined DNR/DNI order would accept a trial of MV for pneumonia.[12] If the genesis of these orders was our desire to ensure that patients' autonomous preferences are respected, we must actually know those preferences, and those preferences should be based on adequate information about the expected outcomes, highlighting the differences outlined above.
Some may consider separating CPR from MVtherefore allowing for more clearly separate DNR and DNI ordersproblematic, as it may result in a menu of choices for patients. However, although CPR and MV may be performed at the same time for the same patient, they do not overlap in 100% of their occurrences. This is conceptually different from discussing whether to use epinephrine versus vasopressin, for example, or offering options such as chest compressions alone. More clearly separating CPR from MV would not be dissimilar to what is done with renal dialysis; a patient may wish to be DNR while still electing to undergo dialysis for failing kidneys. Though the discussions surrounding renal dialysis are less urgent, this alone does not adequately explain why the topic is not routinely collapsed into the discussion of CPR. Instead, renal dialysis is an intervention with unique indications, goals, and outcomes; this is what prompts the separation. The same is true of MV.
No matter the situation, code status discussions should focus on determining an individual patient's values and goals of care and should guide physicians in provision (or omission) of certain interventions. For the patient with pneumonia described above, his goal may be to promote quality of life over extension of life. Although this may prompt a recommendation to forego CPR, (if it were felt that his quality of life, even after successful return of spontaneous circulation, would be low), it may not be inconsistent for him to accept a trial of MV were his pneumonia to get worse (if it were felt that he could quickly improve and return to a quality of life close to what he experienced before the episode of pneumonia). We recommend that when discussing options with patients, the indications for and outcomes of CPR and MV be more clearly separated. It may be as simple as saying, there are 2 different situations I would like to discuss with you, followed by a discussion of the associated scenarios and likely outcomes in the best judgment of the care team. For a hospitalist, framing the discussion of MV around anticipated causes of pre‐arrest respiratory failure (eg, pneumonia, acute pulmonary edema) is essential.
In conclusion, if DNR and DNI orders are going to meet their promise of ensuring patients make informed decisions congruent with their goals, then the discussions from which they follow will need to more clearly acknowledge the important differences in indications and outcomes. Although a patient's goals should still be the framework upon which decisions regarding interventions are made, an important distinction should be made between cardiopulmonary arrest and prearrest respiratory failure, with a more explicit accompanying discussion of how the corresponding interventions fit within the patient's overall goals of care.
Acknowledgements
The authors thank Rafael Campo, MD, and Sharon H. Chou, MD, for their suggestions and critical reading of this manuscript.
Disclosures:
Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging.
Since the introduction of defibrillation and closed chest cardiopulmonary resuscitation (CPR) in the 1950s, the ability to revive an arrested heart has been a realized possibility. Around the same time, endotracheal intubation with mechanical ventilation (MV) came into wide practice, allowing doctors to augment or even replace their patients' breathing. But just as the 1950s and 1960s saw the rise of these enhanced medical techniques, they also saw the increased importance of medical ethicsin particular, patient autonomy. A natural reaction to medicine's use of CPR and MV was the advent of advance directives and more specific do‐not‐resuscitate (DNR) and do‐not‐intubate (DNI) orders meant to protect a patient's ability to remain autonomous with their end of life decisions.[1]
Unfortunately, the code status discussions that lead to these orders often collapse cardiac arrest with prearrest respiratory failure and CPR with MV.[2, 3] This is a problem for a number of reasons. First, cardiac arrest and prearrest respiratory failure are unique end points, and though their respective treatments (CPR and MV) are often required simultaneously for an individual patient, they are distinct medical interventions with different goals, indications, and associated disease states. Although MV is typically a part of the cadre of interventions meant to ensure continued tissue oxygenation in the setting of a cardiac arrest, this accounts for <2% of indications for MV.[4] The vast majority of MV is used to treat prearrest causes of respiratory failure, such as pneumonia, congestive heart failure, acute exacerbations of chronic obstructive pulmonary disease, and following surgery.[4]
We do not believe these differences are adequately reflected in typical code status discussions.[2, 3] One study using audio‐recorded admission encounters included transcripts of hospitalist‐led code status discussions that all resembled the following: Physician: [I]f an emergency were to happenand your heart would (stop) or your breathing became so difficult that you needed to be attached to machines, would you want the nurses and doctors to attempt heroic measures to try to restart your heart and attach you to a breathing machine?[2] It would come as little surprise if a patient hearing this assumed that just 1 question were being asked and that decisions relating to any cause of respiratory failure (including prearrest causes) were being made. In practice, many physicians then extrapolate DNR orders to other treatment decision (including MV) and interpret them as precluding intubation, even for prearrest states.[5, 6, 7]
A second issue is that the mortality associated with cardiopulmonary arrest requiring CPR and prearrest respiratory failure requiring MV are not equal. Though the mortality after in‐hospital cardiac arrest has decreased over the last decade, it remains >75%.[8] The outcomes for MV for isolated respiratory failure, on the other hand, are not as grim; studies of the general population typically report mortality rates <40%. Despite this, descriptions of outcomes are often left out of goals of care discussions.[9, 10] For example, Sharma et al. recently reported that only one‐third of residents, including those who had undergone training on goals of care discussions, discussed outcomes.[9] And when outcomes are included, they are typically for CPR but not MV as an independent intervention for prearrest respiratory failure.[10] Given that many of the conditions that lead to respiratory failure are among the most common reasons for hospitalization,[11] distinguishing between decisions regarding CPR and prearrest MV with discussion of their associated outcomes is of particular importance to hospitalists. Failing to do so impedes patients from making informed autonomous decisions that incorporate an accurate understanding of the treatments being discussed.
Imagine you are caring for a 75‐year‐old man with a history of coronary artery disease and congestive heart failure now admitted with pneumonia. Given his age, admitting diagnosis, and comorbidities, you feel it would be appropriate to engage him in a discussion of goals of care. His chances of survival with near return to baseline after a cardiac arrest requiring CPR are not the same as his chances of surviving an episode of worsening pneumonia requiring MV. To discuss cardiac arrest and prearrest respiratory failure in the same breath, without acknowledging the differences, is misleading. Based on his goals and values, this patient may see a trial of MV as acceptable. One recent study supports this hypothesis, as 28% of hospitalized patients with a combined DNR/DNI order would accept a trial of MV for pneumonia.[12] If the genesis of these orders was our desire to ensure that patients' autonomous preferences are respected, we must actually know those preferences, and those preferences should be based on adequate information about the expected outcomes, highlighting the differences outlined above.
Some may consider separating CPR from MVtherefore allowing for more clearly separate DNR and DNI ordersproblematic, as it may result in a menu of choices for patients. However, although CPR and MV may be performed at the same time for the same patient, they do not overlap in 100% of their occurrences. This is conceptually different from discussing whether to use epinephrine versus vasopressin, for example, or offering options such as chest compressions alone. More clearly separating CPR from MV would not be dissimilar to what is done with renal dialysis; a patient may wish to be DNR while still electing to undergo dialysis for failing kidneys. Though the discussions surrounding renal dialysis are less urgent, this alone does not adequately explain why the topic is not routinely collapsed into the discussion of CPR. Instead, renal dialysis is an intervention with unique indications, goals, and outcomes; this is what prompts the separation. The same is true of MV.
No matter the situation, code status discussions should focus on determining an individual patient's values and goals of care and should guide physicians in provision (or omission) of certain interventions. For the patient with pneumonia described above, his goal may be to promote quality of life over extension of life. Although this may prompt a recommendation to forego CPR, (if it were felt that his quality of life, even after successful return of spontaneous circulation, would be low), it may not be inconsistent for him to accept a trial of MV were his pneumonia to get worse (if it were felt that he could quickly improve and return to a quality of life close to what he experienced before the episode of pneumonia). We recommend that when discussing options with patients, the indications for and outcomes of CPR and MV be more clearly separated. It may be as simple as saying, there are 2 different situations I would like to discuss with you, followed by a discussion of the associated scenarios and likely outcomes in the best judgment of the care team. For a hospitalist, framing the discussion of MV around anticipated causes of pre‐arrest respiratory failure (eg, pneumonia, acute pulmonary edema) is essential.
In conclusion, if DNR and DNI orders are going to meet their promise of ensuring patients make informed decisions congruent with their goals, then the discussions from which they follow will need to more clearly acknowledge the important differences in indications and outcomes. Although a patient's goals should still be the framework upon which decisions regarding interventions are made, an important distinction should be made between cardiopulmonary arrest and prearrest respiratory failure, with a more explicit accompanying discussion of how the corresponding interventions fit within the patient's overall goals of care.
Acknowledgements
The authors thank Rafael Campo, MD, and Sharon H. Chou, MD, for their suggestions and critical reading of this manuscript.
Disclosures:
Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging.
- , , . Orders not to resuscitate. N Engl J Med. 1976;295(7):364–366.
- , , , , . Code status discussions between attending hospitalist physicians and medical patients at hospital admission. J Gen Intern Med. 2010;26(4):359–366.
- , , . How do medical residents discuss resuscitation with patients? J Gen Intern Med. 1995;10(8):436–442.
- , , , et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28‐day international study. JAMA. 2002;287(3):345–355.
- , . The effect of do‐not‐resuscitate orders on physician decision‐making. J Am Geriatr Soc. 2002;50(12):2057–2061.
- , , . Hospital do‐not‐resuscitate orders: why they have failed and how to fix them. J Gen Intern Med. 2011;26(7):791–797.
- , , . Clinician perspectives regarding the do‐not‐resuscitate order. JAMA Pediatr. 2013;167(10):954–958.
- , , , , , . Trends in survival after in‐hospital cardiac arrest. N Engl J Med. 2012;367(20):1912–1920.
- , , , , , . Unpacking resident‐led code status discussions: results from a mixed methods study. J Gen Intern Med. 2014;29(5):750–7.
- , , , et al. If asked, hospitalized patients will choose whether to receive life‐sustaining therapies. J Hosp Med. 2006;1(3):161–167.
- Healthcare Cost and Utilization Project (HCUP). HCUP Facts and Figures: Statistics on Hospital‐Based Care in the United States, 2009. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
- , , , et al. Preferences for resuscitation and intubation among patients with do‐not‐resuscitate/do‐not‐intubate orders. Mayo Clin Proceed. 2013;88(7):658–665.
- , , . Orders not to resuscitate. N Engl J Med. 1976;295(7):364–366.
- , , , , . Code status discussions between attending hospitalist physicians and medical patients at hospital admission. J Gen Intern Med. 2010;26(4):359–366.
- , , . How do medical residents discuss resuscitation with patients? J Gen Intern Med. 1995;10(8):436–442.
- , , , et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28‐day international study. JAMA. 2002;287(3):345–355.
- , . The effect of do‐not‐resuscitate orders on physician decision‐making. J Am Geriatr Soc. 2002;50(12):2057–2061.
- , , . Hospital do‐not‐resuscitate orders: why they have failed and how to fix them. J Gen Intern Med. 2011;26(7):791–797.
- , , . Clinician perspectives regarding the do‐not‐resuscitate order. JAMA Pediatr. 2013;167(10):954–958.
- , , , , , . Trends in survival after in‐hospital cardiac arrest. N Engl J Med. 2012;367(20):1912–1920.
- , , , , , . Unpacking resident‐led code status discussions: results from a mixed methods study. J Gen Intern Med. 2014;29(5):750–7.
- , , , et al. If asked, hospitalized patients will choose whether to receive life‐sustaining therapies. J Hosp Med. 2006;1(3):161–167.
- Healthcare Cost and Utilization Project (HCUP). HCUP Facts and Figures: Statistics on Hospital‐Based Care in the United States, 2009. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
- , , , et al. Preferences for resuscitation and intubation among patients with do‐not‐resuscitate/do‐not‐intubate orders. Mayo Clin Proceed. 2013;88(7):658–665.
Hospital Readmissions and Preventability
Hospital readmissions cost Medicare $15 to $17 billion per year.[1, 2] In 2010, the Hospital Readmission Reduction Program (HRRP), created by the Patient Protection and Affordable Care Act, authorized the Centers for Medicare and Medicaid Services (CMS) to penalize hospitals with higher‐than‐expected readmission rates for certain index conditions.[3] Other payers may follow suit, so hospitals and health systems nationwide are devoting significant resources to reducing readmissions.[4, 5, 6]
Implicit in these efforts are the assumptions that a significant proportion of readmissions are preventable, and that preventable readmissions can be identified. Unfortunately, estimates of preventability vary widely.[7, 8] In this article, we examine how preventable readmissions have been defined, measured, and calculated, and explore the associated implications for readmission reduction efforts.
THE MEDICARE READMISSION METRIC
The medical literature reveals substantial heterogeneity in how readmissions are assessed. Time periods range from 14 days to 4 years, and readmissions may be counted differently depending on whether they are to the same hospital or to any hospital, whether they are for the same (or a related) condition or for any condition, whether a patient is allowed to count only once during the follow‐up period, how mortality is treated, and whether observation stays are considered.[9]
Despite a lack of consensus in the literature, the approach adopted by CMS is endorsed by the National Quality Forum (NQF)[10] and has become the de facto standard for calculating readmission rates. CMS derives risk‐standardized readmission rates for acute myocardial infarction (AMI), heart failure (HF), and pneumonia (PN), using administrative claims data for each Medicare fee‐for‐service beneficiary 65 years or older.[11, 12, 13, 14] CMS counts the first readmission (but not subsequent ones) for any cause within 30 days of the index discharge, including readmissions to other facilities. Certain planned readmissions for revascularization are excluded, as are patients who left against medical advice, transferred to another acute‐care hospital, or died during the index admission. Admissions to psychiatric, rehabilitation, cancer specialty, and children's hospitals[12] are also excluded, as well as patients classified as observation status for either hospital stay.[15] Only administrative data are used in readmission calculations (ie, there are no chart reviews or interviews with healthcare personnel or patients). Details are published online and updated at least annually.[15]
EFFECTS AND LIMITATIONS OF THE HRRP AND THE CMS READMISSION METRIC
Penalizing hospitals for higher‐than‐expected readmission rates based on the CMS metric has been successful in the sense that hospitals now feel more accountable for patient outcomes after discharge; they are implementing transitional care programs, improving communication, and building relationships with community programs.[4, 5, 16] Early data suggest a small decline in readmission rates of Medicare beneficiaries nationally.[17] Previously, such readmission rates were constant.[18]
Nevertheless, significant concerns with the current approach have surfaced.[19, 20, 21] First, why choose 30 days? This time horizon was believed to be long enough to identify readmissions attributable to an index admission and short enough to reflect hospital‐delivered care and transitions to the outpatient setting, and it allows for collaboration between hospitals and their communities to reduce readmissions.[3] However, some have argued that this time horizon has little scientific basis,[22] and that hospitals are unfairly held accountable for a timeframe when outcomes may largely be influenced by the quality of outpatient care or the development of new problems.[23, 24] Approximately one‐third of 30‐day readmissions occur within the first 7 days, and more than half (55.7%) occur within the first 14 days[22, 25]; such time frames may be more appropriate for hospital accountability.[26]
Second, spurred by the focus of CMS penalties, efforts to reduce readmissions have largely concerned patients admitted for HF, AMI, or PN, although these 3 medical conditions account for only 10% of Medicare hospitalizations.[18] Programs focused on a narrow patient population may not benefit other patients with high readmission rates, such as those with gastrointestinal or psychiatric problems,[2] or lead to improvements in the underlying processes of care that could benefit patients in additional ways. Indeed, research suggests that low readmission rates may not be related to other measures of hospital quality.[27, 28]
Third, public reporting and hospital penalties are based on 3‐year historical performance, in part to accumulate a large enough sample size for each diagnosis. Hospitals that seek real‐time performance monitoring are limited to tracking surrogate outcomes, such as readmissions back to their own facility.[29, 30] Moreover, because of the long performance time frame, hospitals that achieve rapid improvement may endure penalties precisely when they are attempting to sustain their achievements.
Fourth, the CMS approach utilizes a complex risk‐standardization methodology, which has only modest ability to predict readmissions and allow hospital comparisons.[9] There is no adjustment for community characteristics, even though practice patterns are significantly associated with readmission rates,[9, 31] and more than half of the variation in readmission rates across hospitals can be explained by characteristics of the community such as access to care.[32] Moreover, patient factors, such as race and socioeconomic status, are currently not included in an attempt to hold hospitals to similar standards regardless of their patient population. This is hotly contested, however, and critics note this policy penalizes hospitals for factors outside of their control, such as patients' ability to afford medications.[33] Indeed, the June 2013 Medicare Payment Advisory Committee (MedPAC) report to Congress recommended evaluating hospital performance against facilities with a like percentage of low‐income patients as a way to take into account socioeconomic status.[34]
Fifth, observation stays are excluded, so patients who remain in observation status during their index or subsequent hospitalization cannot be counted as a readmission. Prevalence of observation care has increased, raising concerns that inpatient admissions are being shifted to observation status, producing an artificial decline in readmissions.[35] Fortunately, recent population‐level data provide some reassuring evidence to the contrary.[36]
Finally, and perhaps most significantly, the current readmission metric does not consider preventability. Recent reviews have demonstrated that estimates of preventability vary widely in individual studies, ranging from 5% to 79%, depending on study methodology and setting.[7, 8] Across these studies, on average, only 23% of 30‐day readmissions appear to be avoidable.[8] Another way to consider the preventability of hospital readmissions is by noting that the most effective multimodal care‐transition interventions reduce readmission rates by only about 30%, and most interventions are much less effective.[26] The likely fact that only 23% to 30% of readmissions are preventable has profound implications for the anticipated results of hospital readmission reduction efforts. Interventions that are 75% effective in reducing preventable readmissions should be expected to produce only an 18% to 22% reduction in overall readmission rates.[37]
FOCUSING ON PREVENTABLE READMISSIONS
A greater focus on identifying and targeting preventable readmissions would offer a number of advantages over the present approach. First, it is more meaningful to compare hospitals based on their percentage of discharges resulting in a preventable readmission, than on the basis of highly complex risk standardization procedures for selected conditions. Second, a focus on preventable readmissions more clearly identifies and permits hospitals to target opportunities for improvement. Third, if the focus were on preventable readmissions for a large number of conditions, the necessary sample size could be obtained over a shorter period of time. Overall, such a preventable readmissions metric could serve as a more agile and undiluted performance indicator, as opposed to the present 3‐year rolling average rate of all‐cause readmissions for certain conditions, the majority of which are probably not preventable.
DEFINING PREVENTABILITY
Defining a preventable readmission is critically important. However, neither a consensus definition nor a validated standard for assessing preventable hospital readmissions exists. Different conceptual frameworks and terms (eg, avoidable, potentially preventable, or urgent readmission) complicate the issue.[38, 39, 40]
Although the CMS measure does not address preventability, it is helpful to consider whether other readmission metrics incorporate this concept. The United Health Group's (UHG, formerly Pacificare) All‐Cause Readmission Index, University HealthSystem Consortium's 30‐Day Readmission Rate (all cause), and 3M Health Information Systems' (3M) Potentially Preventable Readmissions (PPR) are 3 commonly used measures.
Of these, only the 3M PPR metric includes the concept of preventability. 3M created a proprietary matrix of 98,000 readmission‐index admission All Patient Refined Diagnosis Related Group pairs based on the review of several physicians and the logical assumption that a readmission for a clinically related diagnosis is potentially preventable.[24, 41] Readmission and index admissions are considered clinically related if any of the following occur: (1) medical readmission for continuation or recurrence of an initial, or closely related, condition; (2) medical readmission for acute decompensation of a chronic condition that was not the reason for the index admission but was plausibly related to care during or immediately afterward (eg, readmission for diabetes in a patient whose index admission was AMI); (3) medical readmission for acute complication plausibly related to care during index admission; (4) readmission for surgical procedure for continuation or recurrence of initial problem (eg, readmission for appendectomy following admission for abdominal pain and fever); or (5) readmission for surgical procedure to address complication resulting from care during index admission.[24, 41] The readmission time frame is not standardized and may be set by the user. Though conceptually appealing in some ways, CMS and the NQF have expressed concern about this specific approach because of the uncertain reliability of the relatedness of the admission‐readmission diagnosis dyads.[3]
In the research literature, only a few studies have examined the 3M PPR or other preventability assessments that rely on the relatedness of diagnostic codes.[8] Using the 3M PPR, a study showed that 78% of readmissions were classified as potentially preventable,[42] which explains why the 3M PPR and all‐cause readmission metric may correlate highly.[43] Others have demonstrated that ratings of hospital performance on readmission rates vary by a moderate to large amount, depending on whether the 3M PPR, CMS, or UHG methodology is used.[43, 44] An algorithm called SQLape[45, 46] is used in Switzerland to benchmark hospitals and defines potentially avoidable readmissions as being related to index diagnoses or complications of those conditions. It has recently been tested in the United States in a single‐center study,[47] and a multihospital study is underway.
Aside from these algorithms using related diagnosis codes, most ratings of preventability have relied on subjective assessments made primarily through a review of hospital records, and approximately one‐third also included data from clinic visits or interviews with the treating medical team or patients/families.[8] Unfortunately, these reports provide insufficient detail on how to apply their preventability criteria to subsequent readmission reviews. Studies did, however, provide categories of preventability into which readmissions could be organized (see Supporting Information, Appendix Table 1, in the online version of this article for details from a subset of studies cited in van Walraven's reviews that illustrate this point).
Assessment of preventability by clinician review can be challenging. In general, such assessments have considered readmissions resulting from factors within the hospital's control to be avoidable (eg, providing appropriate discharge instructions, reconciling medications, arranging timely postdischarge follow‐up appointments), whereas readmissions resulting from factors not within the hospital's control are unavoidable (eg, patient socioeconomic status, social support, disease progression). However, readmissions resulting from patient behaviors or social reasons could potentially be classified as avoidable or unavoidable depending on the circumstances. For example, if a patient decides not to take a prescribed antibiotic and is readmitted with worsening infection, this could be classified as an unavoidable readmission from the hospital's perspective. Alternatively, if the physician prescribing the antibiotic was inattentive to the cost of the medication and the patient would have taken a less expensive medication had it been prescribed, this could be classified as an avoidable readmission. Differing interpretations of contextual factors may partially account for the variability in clinical assessments of preventability.
Indeed, despite the lack of consensus around a standard method of defining preventability, hospitals and health systems are moving forward to address the issue and reduce readmissions. A recent survey by America's Essential Hospitals (previously the National Association of Public Hospitals and Health Systems), indicated that: (1) reducing readmissions was a high priority for the majority (86%) of members, (2) most had established interdisciplinary teams to address the issue, and (3) over half had a formal process for determining which readmissions were potentially preventable. Of the survey respondents, just over one‐third rely on staff review of individual patient charts or patient and family interviews, and slightly less than one‐third rely on other mechanisms such as external consultants, criteria developed by other entities, or the Institute for Clinical Systems Improvement methodology.[48] Approximately one‐fifth make use of 3M's PPR product, and slightly fewer use the list of the Agency for Healthcare Research and Quality's ambulatory care sensitive conditions (ACSCs). These are medical conditions for which it is believed that good outpatient care could prevent the need for hospitalization (eg, asthma, congestive heart failure, diabetes) or for which early intervention minimizes complications.[49] Hospitalization rates for ACSCs may represent a good measure of excess hospitalization, with a focus on the quality of outpatient care.
RECOMMENDATIONS
We recommend that reporting of hospital readmission rates be based on preventable or potentially preventable readmissions. Although we acknowledge the challenges in doing so, the advantages are notable. At minimum, a preventable readmission rate would more accurately reflect the true gap in care and therefore hospitals' real opportunity for improvement, without being obscured by readmissions that are not preventable.
Because readmission rates are used for public reporting and financial penalties for hospitals, we favor a measure of preventability that reflects the readmissions that the hospital or hospital system has the ability to prevent. This would not penalize hospitals for factors that are under the control of others, namely patients and caregivers, community supports, or society at large. We further recommend that this measure apply to a broader composite of unplanned care, inclusive of both inpatient and observation stays, which have little distinction in patients' eyes, and both represent potentially unnecessary utilization of acute‐care resources.[50] Such a measure would require development, validation, and appropriate vetting before it is implemented.
The first step is for researchers and policy makers to agree on how a measure of preventable or potentially preventable readmissions could be defined. A common element of preventability assessment is to identify the degree to which the reasons for readmission are related to the diagnoses of the index hospitalization. To be reliable and scalable, this measure will need to be based on algorithms that relate the index and readmission diagnoses, most likely using claims data. Choosing common medical and surgical conditions and developing a consensus‐based list of related readmission diagnoses is an important first step. It would also be important to include some less common conditions, because they may reflect very different aspects of hospital care.
An approach based on a list of related diagnoses would represent potentially preventable rehospitalizations. Generally, clinical review is required to determine actual preventability, taking into account patient factors such as a high level of illness or functional impairment that leads to clinical decompensation in spite of excellent management.[51, 52] Clinical review, like a root cause analysis, also provides greater insight into hospital processes that may warrant improvement. Therefore, even if an administrative measure of potentially preventable readmissions is implemented, hospitals may wish to continue performing detailed clinical review of some readmissions for quality improvement purposes. When clinical review becomes more standardized,[53] a combined approach that uses administrative data plus clinical verification and arbitration may be feasible, as with hospital‐acquired infections.
Similar work to develop related sets of admission and readmission diagnoses has already been undertaken in development of the 3M PPR and SQLape measures.[41, 46] However, the 3M PPR is a proprietary system that has low specificity and a high false‐positive rate for identifying preventable readmissions when compared to clinical review.[42] Moreover, neither measure has yet achieved the consensus required for widespread adoption in the United States. What is needed is a nonproprietary listing of related admission and readmission diagnoses, developed with the engagement of relevant stakeholders, that goes through a period of public comment and vetting by a body such as the NQF.
Until a validated measure of potentially preventable readmission can be developed, how could the current approach evolve toward preventability? The most feasible, rapidly implementable change would be to alter the readmission time horizon from 30 days to 7 or 15 days. A 30‐day period holds hospitals accountable for complications of outpatient care or new problems that may develop weeks after discharge. Even though this may foster shared accountability and collaboration among hospitals and outpatient or community settings, research has demonstrated that early readmissions (eg, within 715 days of discharge) are more likely preventable.[54] Second, consideration of the socioeconomic status of hospital patients, as recommended by MedPAC,[34] would improve on the current model by comparing hospitals to like facilities when determining penalties for excess readmission rates. Finally, adjustment for community factors, such as practice patterns and access to care, would enable readmission metrics to better reflect factors under the hospital's control.[32]
CONCLUSION
Holding hospitals accountable for the quality of acute and transitional care is an important policy initiative that has accelerated many improvements in discharge planning and care coordination. Optimally, the policies, public reporting, and penalties should target preventable readmissions, which may represent as little as one‐quarter of all readmissions. By summarizing some of the issues in defining preventability, we hope to foster continued refinement of quality metrics used in this arena.
Acknowledgements
We thank Eduard Vasilevskis, MD, MPH, for feedback on an earlier draft of this article. This manuscript was informed by a special report titled Preventable Readmissions, written by Julia Lavenberg, Joel Betesh, David Goldmann, Craig Kean, and Kendal Williams of the Penn Medicine Center for Evidence‐based Practice. The review was performed at the request of the Penn Medicine Chief Medical Officer Patrick J. Brennan to inform the development of local readmission prevention metrics, and is available at
Disclosures
Dr. Umscheid's contribution to this project was supported in part by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1TR000003. Dr. Kripalani receives support from the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number R01HL109388, and from the Centers for Medicare and Medicaid Services under awards 1C1CMS331006‐01 and 1C1CMS330979‐01. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Centers for Medicare and Medicaid Services.
- , . Physician Visits After Hospital Discharge: Implications for Reducing Readmissions. Washington, DC: National Institute for Health Care Reform; 2011. Report no. 6.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- Centers for Medicare and Medicaid Services, US Department of Health and Human Services. Medicare program: hospital inpatient prospective payment systems for acute care hospitals and the long‐term care hospital prospective payment system and FY 2012 rates. Fed Regist. 2011;76(160):51476–51846.
- , , , , , . Quality collaboratives and campaigns to reduce readmissions: what strategies are hospitals using? J Hosp Med. 2013;8:601–608.
- , , , , . Contemporary data about hospital strategies to reduce unplanned readmissions: what has changed [research letter]? JAMA Intern Med. 2014;174(1):154–156.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , . Comparing methods to calculate hospital‐specific rates of early death or urgent readmission. CMAJ. 2012;184(15):E810–E817.
- , , , , . Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183(7):E391–E402.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- National Quality Forum. Patient outcomes: all‐cause readmissions expedited review 2011. Available at: http://www.qualityforum.org/WorkArea/linkit.aspx?LinkIdentifier=id60(7):607–614.
- , , , , , . Data shows reduction in Medicare hospital readmission rates during 2012. Medicare Medicaid Res Rev. 2013;3(2):E1–E11.
- , . Thirty‐day readmissions—truth and consequences. N Engl J Med. 2012;366(15):1366–1369.
- , , , . Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2013;8(2):102–109.
- , . A path forward on Medicare readmissions. N Engl J Med. 2013;368(13):1175–1177.
- American Hospital Association. TrendWatch: examining the drivers of readmissions and reducing unnecessary readmissions for better patient care. Washington, DC: American Hospital Association; 2011.
- , , , et al. Diagnoses and timing of 30‐day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309(4):355–363.
- , . Characteristics of hospitals receiving penalties under the hospital readmissions reduction program. JAMA. 2013;309(4):342–343.
- , , , , , , et al. Identifying potentially preventable readmissions. Health Care Financ Rev. 2008;30(1):75–91.
- , , , et al. Use of hospital‐based acute care among patients recently discharged from the hospital. JAMA. 2013;309(4):364–371.
- , , , . Reducing hospital readmission rates: current strategies and future directions. Annu Rev Med. 2014;65:471–485.
- , , , et al. Relationship between hospital readmission and mortality rates for patients hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA. 2013;309(6):587–593.
- , , , et al. Hospital performance measures and 30‐day readmission rates. J Gen Intern Med. 2013;28(3):377–385.
- , , , . Limitations of using same‐hospital readmission metrics. Int J Qual Health Care. 2013;25(6):633–639.
- , , , et al. Is same‐hospital readmission rate a good surrogate for all‐hospital readmission rate? Med Care. 2010;48(5):477–481.
- , , . The relationship between hospital admission rates and rehospitalizations. N Engl J Med. 2011;365(24):2287–2295.
- . Community factors and hospital readmission rates [published online April 9, 2014]. Health Serv Res. doi: 10.1111/1475–6773.12177.
- American Hospital Association. Hospital readmissions reduction program: factsheet. American Hospital Association. Available at: http://www.aha.org/content/13/fs‐readmissions.pdf. Published April 14, 2014. Accessed May 5, 2014.
- Medicare Payment Advisory Commission. Report to the congress: Medicare and the health care delivery system. Available at: http://www.medpac.gov/documents/Jun13_EntireReport.pdf. Published June 14, 2013. Accessed May 5, 2014.
- , , . Sharp rise in Medicare enrollees being held in hospitals for observation raises concerns about causes and consequences. Health Aff (Millwood). 2012;31(6):1251–1259.
- , , . Quality improvement of care transitions and the trend of composite hospital care. JAMA. 2014;311(10):1013–1014.
- , . When projecting required effectiveness of interventions for hospital readmission reduction, the percentage that is potentially avoidable must be considered. J Clin Epidemiol. 2013;66(6):688–690.
- , , . Urgent readmission rates can be used to infer differences in avoidable readmission rates between hospitals. J Clin Epidemiol. 2012;65(10):1124–1130.
- , , , , . Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183(7):E391–E402.
- , , , , , . Measuring and preventing potentially avoidable hospital readmissions: a review of the literature. Hong Kong Med J. 2010;16(5):383–389.
- 3M Health Information Systems. Potentially preventable readmissions classification system methodology: overview. 3M Health Information Systems; May 2008. Report No.: GRP‐139. Available at: http://multimedia.3m.com/mws/mediawebserver?66666UuZjcFSLXTtNXMtmxMEEVuQEcuZgVs6EVs6E666666‐‐. Accessed June 8, 2014.
- , , , , , . Manual and automated methods for identifying potentially preventable readmissions: a comparison in a large healthcare system. BMC Med Inform Decis Mak. 2014;14:28.
- , , , , , , et al. Comparing 2 methods of assessing 30‐day readmissions: what is the impact on hospital profiling in the Veterans Health Administration? Med Care. 2013;51(7):589–596.
- , . It's not six of one, half‐dozen the other: a comparative analysis of 3 rehospitalization measurement systems for Massachusetts. Academy Health Annual Research Meeting. Seattle, WA. 2011. Available at: http://www.academyhealth.org/files/2011/tuesday/boutwell.pdf. Accessed May 9, 2014.
- , , , , , . Validation of the potentially avoidable hospital readmission rate as a routine indicator of the quality of hospital care. Med Care. 2006;44(11):972–981.
- , , , , , . Measuring potentially avoidable hospital readmissions. J Clin Epidemiol. 2002;55:573–587.
- , , , . Potentially avoidable 30‐day hospital readmissions in medical patients: derivation and validation of a prediction model. JAMA Intern Med. 2013;173(8):632–638.
- National Association of Public Hospitals and Health Systems. NAPH members focus on reducing readmissions. Available at: www.naph.org. Published June 2011. Accessed October 19, 2011.
- Agency for Healthcare Research and Quality. AHRQ quality indicators: prevention quality indicators. Available at: http://www.qualityindicators.ahrq.gov/Modules/pqi_resources.aspx. Accessed February 11, 2014.
- , , , , , . Shifting the dialogue from hospital readmissions to unplanned care. Am J Manag Care. 2013;19(6):450–453.
- . Post‐hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100–102.
- , . The hospital‐dependent patient. N Engl J Med. 2014;370(8):694–697.
- , , , et al. The hospital medicine reengineering network (HOMERuN): a learning organization focused on improving hospital care. Acad Med. 2014;89(3):415–420.
- , , , et al. Incidence of potentially avoidable urgent readmissions and their relation to all‐cause urgent readmissions. CMAJ. 2011;183(14):E1067–E1072.
Hospital readmissions cost Medicare $15 to $17 billion per year.[1, 2] In 2010, the Hospital Readmission Reduction Program (HRRP), created by the Patient Protection and Affordable Care Act, authorized the Centers for Medicare and Medicaid Services (CMS) to penalize hospitals with higher‐than‐expected readmission rates for certain index conditions.[3] Other payers may follow suit, so hospitals and health systems nationwide are devoting significant resources to reducing readmissions.[4, 5, 6]
Implicit in these efforts are the assumptions that a significant proportion of readmissions are preventable, and that preventable readmissions can be identified. Unfortunately, estimates of preventability vary widely.[7, 8] In this article, we examine how preventable readmissions have been defined, measured, and calculated, and explore the associated implications for readmission reduction efforts.
THE MEDICARE READMISSION METRIC
The medical literature reveals substantial heterogeneity in how readmissions are assessed. Time periods range from 14 days to 4 years, and readmissions may be counted differently depending on whether they are to the same hospital or to any hospital, whether they are for the same (or a related) condition or for any condition, whether a patient is allowed to count only once during the follow‐up period, how mortality is treated, and whether observation stays are considered.[9]
Despite a lack of consensus in the literature, the approach adopted by CMS is endorsed by the National Quality Forum (NQF)[10] and has become the de facto standard for calculating readmission rates. CMS derives risk‐standardized readmission rates for acute myocardial infarction (AMI), heart failure (HF), and pneumonia (PN), using administrative claims data for each Medicare fee‐for‐service beneficiary 65 years or older.[11, 12, 13, 14] CMS counts the first readmission (but not subsequent ones) for any cause within 30 days of the index discharge, including readmissions to other facilities. Certain planned readmissions for revascularization are excluded, as are patients who left against medical advice, transferred to another acute‐care hospital, or died during the index admission. Admissions to psychiatric, rehabilitation, cancer specialty, and children's hospitals[12] are also excluded, as well as patients classified as observation status for either hospital stay.[15] Only administrative data are used in readmission calculations (ie, there are no chart reviews or interviews with healthcare personnel or patients). Details are published online and updated at least annually.[15]
EFFECTS AND LIMITATIONS OF THE HRRP AND THE CMS READMISSION METRIC
Penalizing hospitals for higher‐than‐expected readmission rates based on the CMS metric has been successful in the sense that hospitals now feel more accountable for patient outcomes after discharge; they are implementing transitional care programs, improving communication, and building relationships with community programs.[4, 5, 16] Early data suggest a small decline in readmission rates of Medicare beneficiaries nationally.[17] Previously, such readmission rates were constant.[18]
Nevertheless, significant concerns with the current approach have surfaced.[19, 20, 21] First, why choose 30 days? This time horizon was believed to be long enough to identify readmissions attributable to an index admission and short enough to reflect hospital‐delivered care and transitions to the outpatient setting, and it allows for collaboration between hospitals and their communities to reduce readmissions.[3] However, some have argued that this time horizon has little scientific basis,[22] and that hospitals are unfairly held accountable for a timeframe when outcomes may largely be influenced by the quality of outpatient care or the development of new problems.[23, 24] Approximately one‐third of 30‐day readmissions occur within the first 7 days, and more than half (55.7%) occur within the first 14 days[22, 25]; such time frames may be more appropriate for hospital accountability.[26]
Second, spurred by the focus of CMS penalties, efforts to reduce readmissions have largely concerned patients admitted for HF, AMI, or PN, although these 3 medical conditions account for only 10% of Medicare hospitalizations.[18] Programs focused on a narrow patient population may not benefit other patients with high readmission rates, such as those with gastrointestinal or psychiatric problems,[2] or lead to improvements in the underlying processes of care that could benefit patients in additional ways. Indeed, research suggests that low readmission rates may not be related to other measures of hospital quality.[27, 28]
Third, public reporting and hospital penalties are based on 3‐year historical performance, in part to accumulate a large enough sample size for each diagnosis. Hospitals that seek real‐time performance monitoring are limited to tracking surrogate outcomes, such as readmissions back to their own facility.[29, 30] Moreover, because of the long performance time frame, hospitals that achieve rapid improvement may endure penalties precisely when they are attempting to sustain their achievements.
Fourth, the CMS approach utilizes a complex risk‐standardization methodology, which has only modest ability to predict readmissions and allow hospital comparisons.[9] There is no adjustment for community characteristics, even though practice patterns are significantly associated with readmission rates,[9, 31] and more than half of the variation in readmission rates across hospitals can be explained by characteristics of the community such as access to care.[32] Moreover, patient factors, such as race and socioeconomic status, are currently not included in an attempt to hold hospitals to similar standards regardless of their patient population. This is hotly contested, however, and critics note this policy penalizes hospitals for factors outside of their control, such as patients' ability to afford medications.[33] Indeed, the June 2013 Medicare Payment Advisory Committee (MedPAC) report to Congress recommended evaluating hospital performance against facilities with a like percentage of low‐income patients as a way to take into account socioeconomic status.[34]
Fifth, observation stays are excluded, so patients who remain in observation status during their index or subsequent hospitalization cannot be counted as a readmission. Prevalence of observation care has increased, raising concerns that inpatient admissions are being shifted to observation status, producing an artificial decline in readmissions.[35] Fortunately, recent population‐level data provide some reassuring evidence to the contrary.[36]
Finally, and perhaps most significantly, the current readmission metric does not consider preventability. Recent reviews have demonstrated that estimates of preventability vary widely in individual studies, ranging from 5% to 79%, depending on study methodology and setting.[7, 8] Across these studies, on average, only 23% of 30‐day readmissions appear to be avoidable.[8] Another way to consider the preventability of hospital readmissions is by noting that the most effective multimodal care‐transition interventions reduce readmission rates by only about 30%, and most interventions are much less effective.[26] The likely fact that only 23% to 30% of readmissions are preventable has profound implications for the anticipated results of hospital readmission reduction efforts. Interventions that are 75% effective in reducing preventable readmissions should be expected to produce only an 18% to 22% reduction in overall readmission rates.[37]
FOCUSING ON PREVENTABLE READMISSIONS
A greater focus on identifying and targeting preventable readmissions would offer a number of advantages over the present approach. First, it is more meaningful to compare hospitals based on their percentage of discharges resulting in a preventable readmission, than on the basis of highly complex risk standardization procedures for selected conditions. Second, a focus on preventable readmissions more clearly identifies and permits hospitals to target opportunities for improvement. Third, if the focus were on preventable readmissions for a large number of conditions, the necessary sample size could be obtained over a shorter period of time. Overall, such a preventable readmissions metric could serve as a more agile and undiluted performance indicator, as opposed to the present 3‐year rolling average rate of all‐cause readmissions for certain conditions, the majority of which are probably not preventable.
DEFINING PREVENTABILITY
Defining a preventable readmission is critically important. However, neither a consensus definition nor a validated standard for assessing preventable hospital readmissions exists. Different conceptual frameworks and terms (eg, avoidable, potentially preventable, or urgent readmission) complicate the issue.[38, 39, 40]
Although the CMS measure does not address preventability, it is helpful to consider whether other readmission metrics incorporate this concept. The United Health Group's (UHG, formerly Pacificare) All‐Cause Readmission Index, University HealthSystem Consortium's 30‐Day Readmission Rate (all cause), and 3M Health Information Systems' (3M) Potentially Preventable Readmissions (PPR) are 3 commonly used measures.
Of these, only the 3M PPR metric includes the concept of preventability. 3M created a proprietary matrix of 98,000 readmission‐index admission All Patient Refined Diagnosis Related Group pairs based on the review of several physicians and the logical assumption that a readmission for a clinically related diagnosis is potentially preventable.[24, 41] Readmission and index admissions are considered clinically related if any of the following occur: (1) medical readmission for continuation or recurrence of an initial, or closely related, condition; (2) medical readmission for acute decompensation of a chronic condition that was not the reason for the index admission but was plausibly related to care during or immediately afterward (eg, readmission for diabetes in a patient whose index admission was AMI); (3) medical readmission for acute complication plausibly related to care during index admission; (4) readmission for surgical procedure for continuation or recurrence of initial problem (eg, readmission for appendectomy following admission for abdominal pain and fever); or (5) readmission for surgical procedure to address complication resulting from care during index admission.[24, 41] The readmission time frame is not standardized and may be set by the user. Though conceptually appealing in some ways, CMS and the NQF have expressed concern about this specific approach because of the uncertain reliability of the relatedness of the admission‐readmission diagnosis dyads.[3]
In the research literature, only a few studies have examined the 3M PPR or other preventability assessments that rely on the relatedness of diagnostic codes.[8] Using the 3M PPR, a study showed that 78% of readmissions were classified as potentially preventable,[42] which explains why the 3M PPR and all‐cause readmission metric may correlate highly.[43] Others have demonstrated that ratings of hospital performance on readmission rates vary by a moderate to large amount, depending on whether the 3M PPR, CMS, or UHG methodology is used.[43, 44] An algorithm called SQLape[45, 46] is used in Switzerland to benchmark hospitals and defines potentially avoidable readmissions as being related to index diagnoses or complications of those conditions. It has recently been tested in the United States in a single‐center study,[47] and a multihospital study is underway.
Aside from these algorithms using related diagnosis codes, most ratings of preventability have relied on subjective assessments made primarily through a review of hospital records, and approximately one‐third also included data from clinic visits or interviews with the treating medical team or patients/families.[8] Unfortunately, these reports provide insufficient detail on how to apply their preventability criteria to subsequent readmission reviews. Studies did, however, provide categories of preventability into which readmissions could be organized (see Supporting Information, Appendix Table 1, in the online version of this article for details from a subset of studies cited in van Walraven's reviews that illustrate this point).
Assessment of preventability by clinician review can be challenging. In general, such assessments have considered readmissions resulting from factors within the hospital's control to be avoidable (eg, providing appropriate discharge instructions, reconciling medications, arranging timely postdischarge follow‐up appointments), whereas readmissions resulting from factors not within the hospital's control are unavoidable (eg, patient socioeconomic status, social support, disease progression). However, readmissions resulting from patient behaviors or social reasons could potentially be classified as avoidable or unavoidable depending on the circumstances. For example, if a patient decides not to take a prescribed antibiotic and is readmitted with worsening infection, this could be classified as an unavoidable readmission from the hospital's perspective. Alternatively, if the physician prescribing the antibiotic was inattentive to the cost of the medication and the patient would have taken a less expensive medication had it been prescribed, this could be classified as an avoidable readmission. Differing interpretations of contextual factors may partially account for the variability in clinical assessments of preventability.
Indeed, despite the lack of consensus around a standard method of defining preventability, hospitals and health systems are moving forward to address the issue and reduce readmissions. A recent survey by America's Essential Hospitals (previously the National Association of Public Hospitals and Health Systems), indicated that: (1) reducing readmissions was a high priority for the majority (86%) of members, (2) most had established interdisciplinary teams to address the issue, and (3) over half had a formal process for determining which readmissions were potentially preventable. Of the survey respondents, just over one‐third rely on staff review of individual patient charts or patient and family interviews, and slightly less than one‐third rely on other mechanisms such as external consultants, criteria developed by other entities, or the Institute for Clinical Systems Improvement methodology.[48] Approximately one‐fifth make use of 3M's PPR product, and slightly fewer use the list of the Agency for Healthcare Research and Quality's ambulatory care sensitive conditions (ACSCs). These are medical conditions for which it is believed that good outpatient care could prevent the need for hospitalization (eg, asthma, congestive heart failure, diabetes) or for which early intervention minimizes complications.[49] Hospitalization rates for ACSCs may represent a good measure of excess hospitalization, with a focus on the quality of outpatient care.
RECOMMENDATIONS
We recommend that reporting of hospital readmission rates be based on preventable or potentially preventable readmissions. Although we acknowledge the challenges in doing so, the advantages are notable. At minimum, a preventable readmission rate would more accurately reflect the true gap in care and therefore hospitals' real opportunity for improvement, without being obscured by readmissions that are not preventable.
Because readmission rates are used for public reporting and financial penalties for hospitals, we favor a measure of preventability that reflects the readmissions that the hospital or hospital system has the ability to prevent. This would not penalize hospitals for factors that are under the control of others, namely patients and caregivers, community supports, or society at large. We further recommend that this measure apply to a broader composite of unplanned care, inclusive of both inpatient and observation stays, which have little distinction in patients' eyes, and both represent potentially unnecessary utilization of acute‐care resources.[50] Such a measure would require development, validation, and appropriate vetting before it is implemented.
The first step is for researchers and policy makers to agree on how a measure of preventable or potentially preventable readmissions could be defined. A common element of preventability assessment is to identify the degree to which the reasons for readmission are related to the diagnoses of the index hospitalization. To be reliable and scalable, this measure will need to be based on algorithms that relate the index and readmission diagnoses, most likely using claims data. Choosing common medical and surgical conditions and developing a consensus‐based list of related readmission diagnoses is an important first step. It would also be important to include some less common conditions, because they may reflect very different aspects of hospital care.
An approach based on a list of related diagnoses would represent potentially preventable rehospitalizations. Generally, clinical review is required to determine actual preventability, taking into account patient factors such as a high level of illness or functional impairment that leads to clinical decompensation in spite of excellent management.[51, 52] Clinical review, like a root cause analysis, also provides greater insight into hospital processes that may warrant improvement. Therefore, even if an administrative measure of potentially preventable readmissions is implemented, hospitals may wish to continue performing detailed clinical review of some readmissions for quality improvement purposes. When clinical review becomes more standardized,[53] a combined approach that uses administrative data plus clinical verification and arbitration may be feasible, as with hospital‐acquired infections.
Similar work to develop related sets of admission and readmission diagnoses has already been undertaken in development of the 3M PPR and SQLape measures.[41, 46] However, the 3M PPR is a proprietary system that has low specificity and a high false‐positive rate for identifying preventable readmissions when compared to clinical review.[42] Moreover, neither measure has yet achieved the consensus required for widespread adoption in the United States. What is needed is a nonproprietary listing of related admission and readmission diagnoses, developed with the engagement of relevant stakeholders, that goes through a period of public comment and vetting by a body such as the NQF.
Until a validated measure of potentially preventable readmission can be developed, how could the current approach evolve toward preventability? The most feasible, rapidly implementable change would be to alter the readmission time horizon from 30 days to 7 or 15 days. A 30‐day period holds hospitals accountable for complications of outpatient care or new problems that may develop weeks after discharge. Even though this may foster shared accountability and collaboration among hospitals and outpatient or community settings, research has demonstrated that early readmissions (eg, within 715 days of discharge) are more likely preventable.[54] Second, consideration of the socioeconomic status of hospital patients, as recommended by MedPAC,[34] would improve on the current model by comparing hospitals to like facilities when determining penalties for excess readmission rates. Finally, adjustment for community factors, such as practice patterns and access to care, would enable readmission metrics to better reflect factors under the hospital's control.[32]
CONCLUSION
Holding hospitals accountable for the quality of acute and transitional care is an important policy initiative that has accelerated many improvements in discharge planning and care coordination. Optimally, the policies, public reporting, and penalties should target preventable readmissions, which may represent as little as one‐quarter of all readmissions. By summarizing some of the issues in defining preventability, we hope to foster continued refinement of quality metrics used in this arena.
Acknowledgements
We thank Eduard Vasilevskis, MD, MPH, for feedback on an earlier draft of this article. This manuscript was informed by a special report titled Preventable Readmissions, written by Julia Lavenberg, Joel Betesh, David Goldmann, Craig Kean, and Kendal Williams of the Penn Medicine Center for Evidence‐based Practice. The review was performed at the request of the Penn Medicine Chief Medical Officer Patrick J. Brennan to inform the development of local readmission prevention metrics, and is available at
Disclosures
Dr. Umscheid's contribution to this project was supported in part by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1TR000003. Dr. Kripalani receives support from the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number R01HL109388, and from the Centers for Medicare and Medicaid Services under awards 1C1CMS331006‐01 and 1C1CMS330979‐01. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Centers for Medicare and Medicaid Services.
Hospital readmissions cost Medicare $15 to $17 billion per year.[1, 2] In 2010, the Hospital Readmission Reduction Program (HRRP), created by the Patient Protection and Affordable Care Act, authorized the Centers for Medicare and Medicaid Services (CMS) to penalize hospitals with higher‐than‐expected readmission rates for certain index conditions.[3] Other payers may follow suit, so hospitals and health systems nationwide are devoting significant resources to reducing readmissions.[4, 5, 6]
Implicit in these efforts are the assumptions that a significant proportion of readmissions are preventable, and that preventable readmissions can be identified. Unfortunately, estimates of preventability vary widely.[7, 8] In this article, we examine how preventable readmissions have been defined, measured, and calculated, and explore the associated implications for readmission reduction efforts.
THE MEDICARE READMISSION METRIC
The medical literature reveals substantial heterogeneity in how readmissions are assessed. Time periods range from 14 days to 4 years, and readmissions may be counted differently depending on whether they are to the same hospital or to any hospital, whether they are for the same (or a related) condition or for any condition, whether a patient is allowed to count only once during the follow‐up period, how mortality is treated, and whether observation stays are considered.[9]
Despite a lack of consensus in the literature, the approach adopted by CMS is endorsed by the National Quality Forum (NQF)[10] and has become the de facto standard for calculating readmission rates. CMS derives risk‐standardized readmission rates for acute myocardial infarction (AMI), heart failure (HF), and pneumonia (PN), using administrative claims data for each Medicare fee‐for‐service beneficiary 65 years or older.[11, 12, 13, 14] CMS counts the first readmission (but not subsequent ones) for any cause within 30 days of the index discharge, including readmissions to other facilities. Certain planned readmissions for revascularization are excluded, as are patients who left against medical advice, transferred to another acute‐care hospital, or died during the index admission. Admissions to psychiatric, rehabilitation, cancer specialty, and children's hospitals[12] are also excluded, as well as patients classified as observation status for either hospital stay.[15] Only administrative data are used in readmission calculations (ie, there are no chart reviews or interviews with healthcare personnel or patients). Details are published online and updated at least annually.[15]
EFFECTS AND LIMITATIONS OF THE HRRP AND THE CMS READMISSION METRIC
Penalizing hospitals for higher‐than‐expected readmission rates based on the CMS metric has been successful in the sense that hospitals now feel more accountable for patient outcomes after discharge; they are implementing transitional care programs, improving communication, and building relationships with community programs.[4, 5, 16] Early data suggest a small decline in readmission rates of Medicare beneficiaries nationally.[17] Previously, such readmission rates were constant.[18]
Nevertheless, significant concerns with the current approach have surfaced.[19, 20, 21] First, why choose 30 days? This time horizon was believed to be long enough to identify readmissions attributable to an index admission and short enough to reflect hospital‐delivered care and transitions to the outpatient setting, and it allows for collaboration between hospitals and their communities to reduce readmissions.[3] However, some have argued that this time horizon has little scientific basis,[22] and that hospitals are unfairly held accountable for a timeframe when outcomes may largely be influenced by the quality of outpatient care or the development of new problems.[23, 24] Approximately one‐third of 30‐day readmissions occur within the first 7 days, and more than half (55.7%) occur within the first 14 days[22, 25]; such time frames may be more appropriate for hospital accountability.[26]
Second, spurred by the focus of CMS penalties, efforts to reduce readmissions have largely concerned patients admitted for HF, AMI, or PN, although these 3 medical conditions account for only 10% of Medicare hospitalizations.[18] Programs focused on a narrow patient population may not benefit other patients with high readmission rates, such as those with gastrointestinal or psychiatric problems,[2] or lead to improvements in the underlying processes of care that could benefit patients in additional ways. Indeed, research suggests that low readmission rates may not be related to other measures of hospital quality.[27, 28]
Third, public reporting and hospital penalties are based on 3‐year historical performance, in part to accumulate a large enough sample size for each diagnosis. Hospitals that seek real‐time performance monitoring are limited to tracking surrogate outcomes, such as readmissions back to their own facility.[29, 30] Moreover, because of the long performance time frame, hospitals that achieve rapid improvement may endure penalties precisely when they are attempting to sustain their achievements.
Fourth, the CMS approach utilizes a complex risk‐standardization methodology, which has only modest ability to predict readmissions and allow hospital comparisons.[9] There is no adjustment for community characteristics, even though practice patterns are significantly associated with readmission rates,[9, 31] and more than half of the variation in readmission rates across hospitals can be explained by characteristics of the community such as access to care.[32] Moreover, patient factors, such as race and socioeconomic status, are currently not included in an attempt to hold hospitals to similar standards regardless of their patient population. This is hotly contested, however, and critics note this policy penalizes hospitals for factors outside of their control, such as patients' ability to afford medications.[33] Indeed, the June 2013 Medicare Payment Advisory Committee (MedPAC) report to Congress recommended evaluating hospital performance against facilities with a like percentage of low‐income patients as a way to take into account socioeconomic status.[34]
Fifth, observation stays are excluded, so patients who remain in observation status during their index or subsequent hospitalization cannot be counted as a readmission. Prevalence of observation care has increased, raising concerns that inpatient admissions are being shifted to observation status, producing an artificial decline in readmissions.[35] Fortunately, recent population‐level data provide some reassuring evidence to the contrary.[36]
Finally, and perhaps most significantly, the current readmission metric does not consider preventability. Recent reviews have demonstrated that estimates of preventability vary widely in individual studies, ranging from 5% to 79%, depending on study methodology and setting.[7, 8] Across these studies, on average, only 23% of 30‐day readmissions appear to be avoidable.[8] Another way to consider the preventability of hospital readmissions is by noting that the most effective multimodal care‐transition interventions reduce readmission rates by only about 30%, and most interventions are much less effective.[26] The likely fact that only 23% to 30% of readmissions are preventable has profound implications for the anticipated results of hospital readmission reduction efforts. Interventions that are 75% effective in reducing preventable readmissions should be expected to produce only an 18% to 22% reduction in overall readmission rates.[37]
FOCUSING ON PREVENTABLE READMISSIONS
A greater focus on identifying and targeting preventable readmissions would offer a number of advantages over the present approach. First, it is more meaningful to compare hospitals based on their percentage of discharges resulting in a preventable readmission, than on the basis of highly complex risk standardization procedures for selected conditions. Second, a focus on preventable readmissions more clearly identifies and permits hospitals to target opportunities for improvement. Third, if the focus were on preventable readmissions for a large number of conditions, the necessary sample size could be obtained over a shorter period of time. Overall, such a preventable readmissions metric could serve as a more agile and undiluted performance indicator, as opposed to the present 3‐year rolling average rate of all‐cause readmissions for certain conditions, the majority of which are probably not preventable.
DEFINING PREVENTABILITY
Defining a preventable readmission is critically important. However, neither a consensus definition nor a validated standard for assessing preventable hospital readmissions exists. Different conceptual frameworks and terms (eg, avoidable, potentially preventable, or urgent readmission) complicate the issue.[38, 39, 40]
Although the CMS measure does not address preventability, it is helpful to consider whether other readmission metrics incorporate this concept. The United Health Group's (UHG, formerly Pacificare) All‐Cause Readmission Index, University HealthSystem Consortium's 30‐Day Readmission Rate (all cause), and 3M Health Information Systems' (3M) Potentially Preventable Readmissions (PPR) are 3 commonly used measures.
Of these, only the 3M PPR metric includes the concept of preventability. 3M created a proprietary matrix of 98,000 readmission‐index admission All Patient Refined Diagnosis Related Group pairs based on the review of several physicians and the logical assumption that a readmission for a clinically related diagnosis is potentially preventable.[24, 41] Readmission and index admissions are considered clinically related if any of the following occur: (1) medical readmission for continuation or recurrence of an initial, or closely related, condition; (2) medical readmission for acute decompensation of a chronic condition that was not the reason for the index admission but was plausibly related to care during or immediately afterward (eg, readmission for diabetes in a patient whose index admission was AMI); (3) medical readmission for acute complication plausibly related to care during index admission; (4) readmission for surgical procedure for continuation or recurrence of initial problem (eg, readmission for appendectomy following admission for abdominal pain and fever); or (5) readmission for surgical procedure to address complication resulting from care during index admission.[24, 41] The readmission time frame is not standardized and may be set by the user. Though conceptually appealing in some ways, CMS and the NQF have expressed concern about this specific approach because of the uncertain reliability of the relatedness of the admission‐readmission diagnosis dyads.[3]
In the research literature, only a few studies have examined the 3M PPR or other preventability assessments that rely on the relatedness of diagnostic codes.[8] Using the 3M PPR, a study showed that 78% of readmissions were classified as potentially preventable,[42] which explains why the 3M PPR and all‐cause readmission metric may correlate highly.[43] Others have demonstrated that ratings of hospital performance on readmission rates vary by a moderate to large amount, depending on whether the 3M PPR, CMS, or UHG methodology is used.[43, 44] An algorithm called SQLape[45, 46] is used in Switzerland to benchmark hospitals and defines potentially avoidable readmissions as being related to index diagnoses or complications of those conditions. It has recently been tested in the United States in a single‐center study,[47] and a multihospital study is underway.
Aside from these algorithms using related diagnosis codes, most ratings of preventability have relied on subjective assessments made primarily through a review of hospital records, and approximately one‐third also included data from clinic visits or interviews with the treating medical team or patients/families.[8] Unfortunately, these reports provide insufficient detail on how to apply their preventability criteria to subsequent readmission reviews. Studies did, however, provide categories of preventability into which readmissions could be organized (see Supporting Information, Appendix Table 1, in the online version of this article for details from a subset of studies cited in van Walraven's reviews that illustrate this point).
Assessment of preventability by clinician review can be challenging. In general, such assessments have considered readmissions resulting from factors within the hospital's control to be avoidable (eg, providing appropriate discharge instructions, reconciling medications, arranging timely postdischarge follow‐up appointments), whereas readmissions resulting from factors not within the hospital's control are unavoidable (eg, patient socioeconomic status, social support, disease progression). However, readmissions resulting from patient behaviors or social reasons could potentially be classified as avoidable or unavoidable depending on the circumstances. For example, if a patient decides not to take a prescribed antibiotic and is readmitted with worsening infection, this could be classified as an unavoidable readmission from the hospital's perspective. Alternatively, if the physician prescribing the antibiotic was inattentive to the cost of the medication and the patient would have taken a less expensive medication had it been prescribed, this could be classified as an avoidable readmission. Differing interpretations of contextual factors may partially account for the variability in clinical assessments of preventability.
Indeed, despite the lack of consensus around a standard method of defining preventability, hospitals and health systems are moving forward to address the issue and reduce readmissions. A recent survey by America's Essential Hospitals (previously the National Association of Public Hospitals and Health Systems), indicated that: (1) reducing readmissions was a high priority for the majority (86%) of members, (2) most had established interdisciplinary teams to address the issue, and (3) over half had a formal process for determining which readmissions were potentially preventable. Of the survey respondents, just over one‐third rely on staff review of individual patient charts or patient and family interviews, and slightly less than one‐third rely on other mechanisms such as external consultants, criteria developed by other entities, or the Institute for Clinical Systems Improvement methodology.[48] Approximately one‐fifth make use of 3M's PPR product, and slightly fewer use the list of the Agency for Healthcare Research and Quality's ambulatory care sensitive conditions (ACSCs). These are medical conditions for which it is believed that good outpatient care could prevent the need for hospitalization (eg, asthma, congestive heart failure, diabetes) or for which early intervention minimizes complications.[49] Hospitalization rates for ACSCs may represent a good measure of excess hospitalization, with a focus on the quality of outpatient care.
RECOMMENDATIONS
We recommend that reporting of hospital readmission rates be based on preventable or potentially preventable readmissions. Although we acknowledge the challenges in doing so, the advantages are notable. At minimum, a preventable readmission rate would more accurately reflect the true gap in care and therefore hospitals' real opportunity for improvement, without being obscured by readmissions that are not preventable.
Because readmission rates are used for public reporting and financial penalties for hospitals, we favor a measure of preventability that reflects the readmissions that the hospital or hospital system has the ability to prevent. This would not penalize hospitals for factors that are under the control of others, namely patients and caregivers, community supports, or society at large. We further recommend that this measure apply to a broader composite of unplanned care, inclusive of both inpatient and observation stays, which have little distinction in patients' eyes, and both represent potentially unnecessary utilization of acute‐care resources.[50] Such a measure would require development, validation, and appropriate vetting before it is implemented.
The first step is for researchers and policy makers to agree on how a measure of preventable or potentially preventable readmissions could be defined. A common element of preventability assessment is to identify the degree to which the reasons for readmission are related to the diagnoses of the index hospitalization. To be reliable and scalable, this measure will need to be based on algorithms that relate the index and readmission diagnoses, most likely using claims data. Choosing common medical and surgical conditions and developing a consensus‐based list of related readmission diagnoses is an important first step. It would also be important to include some less common conditions, because they may reflect very different aspects of hospital care.
An approach based on a list of related diagnoses would represent potentially preventable rehospitalizations. Generally, clinical review is required to determine actual preventability, taking into account patient factors such as a high level of illness or functional impairment that leads to clinical decompensation in spite of excellent management.[51, 52] Clinical review, like a root cause analysis, also provides greater insight into hospital processes that may warrant improvement. Therefore, even if an administrative measure of potentially preventable readmissions is implemented, hospitals may wish to continue performing detailed clinical review of some readmissions for quality improvement purposes. When clinical review becomes more standardized,[53] a combined approach that uses administrative data plus clinical verification and arbitration may be feasible, as with hospital‐acquired infections.
Similar work to develop related sets of admission and readmission diagnoses has already been undertaken in development of the 3M PPR and SQLape measures.[41, 46] However, the 3M PPR is a proprietary system that has low specificity and a high false‐positive rate for identifying preventable readmissions when compared to clinical review.[42] Moreover, neither measure has yet achieved the consensus required for widespread adoption in the United States. What is needed is a nonproprietary listing of related admission and readmission diagnoses, developed with the engagement of relevant stakeholders, that goes through a period of public comment and vetting by a body such as the NQF.
Until a validated measure of potentially preventable readmission can be developed, how could the current approach evolve toward preventability? The most feasible, rapidly implementable change would be to alter the readmission time horizon from 30 days to 7 or 15 days. A 30‐day period holds hospitals accountable for complications of outpatient care or new problems that may develop weeks after discharge. Even though this may foster shared accountability and collaboration among hospitals and outpatient or community settings, research has demonstrated that early readmissions (eg, within 715 days of discharge) are more likely preventable.[54] Second, consideration of the socioeconomic status of hospital patients, as recommended by MedPAC,[34] would improve on the current model by comparing hospitals to like facilities when determining penalties for excess readmission rates. Finally, adjustment for community factors, such as practice patterns and access to care, would enable readmission metrics to better reflect factors under the hospital's control.[32]
CONCLUSION
Holding hospitals accountable for the quality of acute and transitional care is an important policy initiative that has accelerated many improvements in discharge planning and care coordination. Optimally, the policies, public reporting, and penalties should target preventable readmissions, which may represent as little as one‐quarter of all readmissions. By summarizing some of the issues in defining preventability, we hope to foster continued refinement of quality metrics used in this arena.
Acknowledgements
We thank Eduard Vasilevskis, MD, MPH, for feedback on an earlier draft of this article. This manuscript was informed by a special report titled Preventable Readmissions, written by Julia Lavenberg, Joel Betesh, David Goldmann, Craig Kean, and Kendal Williams of the Penn Medicine Center for Evidence‐based Practice. The review was performed at the request of the Penn Medicine Chief Medical Officer Patrick J. Brennan to inform the development of local readmission prevention metrics, and is available at
Disclosures
Dr. Umscheid's contribution to this project was supported in part by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1TR000003. Dr. Kripalani receives support from the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number R01HL109388, and from the Centers for Medicare and Medicaid Services under awards 1C1CMS331006‐01 and 1C1CMS330979‐01. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Centers for Medicare and Medicaid Services.
- , . Physician Visits After Hospital Discharge: Implications for Reducing Readmissions. Washington, DC: National Institute for Health Care Reform; 2011. Report no. 6.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- Centers for Medicare and Medicaid Services, US Department of Health and Human Services. Medicare program: hospital inpatient prospective payment systems for acute care hospitals and the long‐term care hospital prospective payment system and FY 2012 rates. Fed Regist. 2011;76(160):51476–51846.
- , , , , , . Quality collaboratives and campaigns to reduce readmissions: what strategies are hospitals using? J Hosp Med. 2013;8:601–608.
- , , , , . Contemporary data about hospital strategies to reduce unplanned readmissions: what has changed [research letter]? JAMA Intern Med. 2014;174(1):154–156.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , . Comparing methods to calculate hospital‐specific rates of early death or urgent readmission. CMAJ. 2012;184(15):E810–E817.
- , , , , . Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183(7):E391–E402.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- National Quality Forum. Patient outcomes: all‐cause readmissions expedited review 2011. Available at: http://www.qualityforum.org/WorkArea/linkit.aspx?LinkIdentifier=id60(7):607–614.
- , , , , , . Data shows reduction in Medicare hospital readmission rates during 2012. Medicare Medicaid Res Rev. 2013;3(2):E1–E11.
- , . Thirty‐day readmissions—truth and consequences. N Engl J Med. 2012;366(15):1366–1369.
- , , , . Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2013;8(2):102–109.
- , . A path forward on Medicare readmissions. N Engl J Med. 2013;368(13):1175–1177.
- American Hospital Association. TrendWatch: examining the drivers of readmissions and reducing unnecessary readmissions for better patient care. Washington, DC: American Hospital Association; 2011.
- , , , et al. Diagnoses and timing of 30‐day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309(4):355–363.
- , . Characteristics of hospitals receiving penalties under the hospital readmissions reduction program. JAMA. 2013;309(4):342–343.
- , , , , , , et al. Identifying potentially preventable readmissions. Health Care Financ Rev. 2008;30(1):75–91.
- , , , et al. Use of hospital‐based acute care among patients recently discharged from the hospital. JAMA. 2013;309(4):364–371.
- , , , . Reducing hospital readmission rates: current strategies and future directions. Annu Rev Med. 2014;65:471–485.
- , , , et al. Relationship between hospital readmission and mortality rates for patients hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA. 2013;309(6):587–593.
- , , , et al. Hospital performance measures and 30‐day readmission rates. J Gen Intern Med. 2013;28(3):377–385.
- , , , . Limitations of using same‐hospital readmission metrics. Int J Qual Health Care. 2013;25(6):633–639.
- , , , et al. Is same‐hospital readmission rate a good surrogate for all‐hospital readmission rate? Med Care. 2010;48(5):477–481.
- , , . The relationship between hospital admission rates and rehospitalizations. N Engl J Med. 2011;365(24):2287–2295.
- . Community factors and hospital readmission rates [published online April 9, 2014]. Health Serv Res. doi: 10.1111/1475–6773.12177.
- American Hospital Association. Hospital readmissions reduction program: factsheet. American Hospital Association. Available at: http://www.aha.org/content/13/fs‐readmissions.pdf. Published April 14, 2014. Accessed May 5, 2014.
- Medicare Payment Advisory Commission. Report to the congress: Medicare and the health care delivery system. Available at: http://www.medpac.gov/documents/Jun13_EntireReport.pdf. Published June 14, 2013. Accessed May 5, 2014.
- , , . Sharp rise in Medicare enrollees being held in hospitals for observation raises concerns about causes and consequences. Health Aff (Millwood). 2012;31(6):1251–1259.
- , , . Quality improvement of care transitions and the trend of composite hospital care. JAMA. 2014;311(10):1013–1014.
- , . When projecting required effectiveness of interventions for hospital readmission reduction, the percentage that is potentially avoidable must be considered. J Clin Epidemiol. 2013;66(6):688–690.
- , , . Urgent readmission rates can be used to infer differences in avoidable readmission rates between hospitals. J Clin Epidemiol. 2012;65(10):1124–1130.
- , , , , . Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183(7):E391–E402.
- , , , , , . Measuring and preventing potentially avoidable hospital readmissions: a review of the literature. Hong Kong Med J. 2010;16(5):383–389.
- 3M Health Information Systems. Potentially preventable readmissions classification system methodology: overview. 3M Health Information Systems; May 2008. Report No.: GRP‐139. Available at: http://multimedia.3m.com/mws/mediawebserver?66666UuZjcFSLXTtNXMtmxMEEVuQEcuZgVs6EVs6E666666‐‐. Accessed June 8, 2014.
- , , , , , . Manual and automated methods for identifying potentially preventable readmissions: a comparison in a large healthcare system. BMC Med Inform Decis Mak. 2014;14:28.
- , , , , , , et al. Comparing 2 methods of assessing 30‐day readmissions: what is the impact on hospital profiling in the Veterans Health Administration? Med Care. 2013;51(7):589–596.
- , . It's not six of one, half‐dozen the other: a comparative analysis of 3 rehospitalization measurement systems for Massachusetts. Academy Health Annual Research Meeting. Seattle, WA. 2011. Available at: http://www.academyhealth.org/files/2011/tuesday/boutwell.pdf. Accessed May 9, 2014.
- , , , , , . Validation of the potentially avoidable hospital readmission rate as a routine indicator of the quality of hospital care. Med Care. 2006;44(11):972–981.
- , , , , , . Measuring potentially avoidable hospital readmissions. J Clin Epidemiol. 2002;55:573–587.
- , , , . Potentially avoidable 30‐day hospital readmissions in medical patients: derivation and validation of a prediction model. JAMA Intern Med. 2013;173(8):632–638.
- National Association of Public Hospitals and Health Systems. NAPH members focus on reducing readmissions. Available at: www.naph.org. Published June 2011. Accessed October 19, 2011.
- Agency for Healthcare Research and Quality. AHRQ quality indicators: prevention quality indicators. Available at: http://www.qualityindicators.ahrq.gov/Modules/pqi_resources.aspx. Accessed February 11, 2014.
- , , , , , . Shifting the dialogue from hospital readmissions to unplanned care. Am J Manag Care. 2013;19(6):450–453.
- . Post‐hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100–102.
- , . The hospital‐dependent patient. N Engl J Med. 2014;370(8):694–697.
- , , , et al. The hospital medicine reengineering network (HOMERuN): a learning organization focused on improving hospital care. Acad Med. 2014;89(3):415–420.
- , , , et al. Incidence of potentially avoidable urgent readmissions and their relation to all‐cause urgent readmissions. CMAJ. 2011;183(14):E1067–E1072.
- , . Physician Visits After Hospital Discharge: Implications for Reducing Readmissions. Washington, DC: National Institute for Health Care Reform; 2011. Report no. 6.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- Centers for Medicare and Medicaid Services, US Department of Health and Human Services. Medicare program: hospital inpatient prospective payment systems for acute care hospitals and the long‐term care hospital prospective payment system and FY 2012 rates. Fed Regist. 2011;76(160):51476–51846.
- , , , , , . Quality collaboratives and campaigns to reduce readmissions: what strategies are hospitals using? J Hosp Med. 2013;8:601–608.
- , , , , . Contemporary data about hospital strategies to reduce unplanned readmissions: what has changed [research letter]? JAMA Intern Med. 2014;174(1):154–156.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , . Comparing methods to calculate hospital‐specific rates of early death or urgent readmission. CMAJ. 2012;184(15):E810–E817.
- , , , , . Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183(7):E391–E402.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- National Quality Forum. Patient outcomes: all‐cause readmissions expedited review 2011. Available at: http://www.qualityforum.org/WorkArea/linkit.aspx?LinkIdentifier=id60(7):607–614.
- , , , , , . Data shows reduction in Medicare hospital readmission rates during 2012. Medicare Medicaid Res Rev. 2013;3(2):E1–E11.
- , . Thirty‐day readmissions—truth and consequences. N Engl J Med. 2012;366(15):1366–1369.
- , , , . Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2013;8(2):102–109.
- , . A path forward on Medicare readmissions. N Engl J Med. 2013;368(13):1175–1177.
- American Hospital Association. TrendWatch: examining the drivers of readmissions and reducing unnecessary readmissions for better patient care. Washington, DC: American Hospital Association; 2011.
- , , , et al. Diagnoses and timing of 30‐day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309(4):355–363.
- , . Characteristics of hospitals receiving penalties under the hospital readmissions reduction program. JAMA. 2013;309(4):342–343.
- , , , , , , et al. Identifying potentially preventable readmissions. Health Care Financ Rev. 2008;30(1):75–91.
- , , , et al. Use of hospital‐based acute care among patients recently discharged from the hospital. JAMA. 2013;309(4):364–371.
- , , , . Reducing hospital readmission rates: current strategies and future directions. Annu Rev Med. 2014;65:471–485.
- , , , et al. Relationship between hospital readmission and mortality rates for patients hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA. 2013;309(6):587–593.
- , , , et al. Hospital performance measures and 30‐day readmission rates. J Gen Intern Med. 2013;28(3):377–385.
- , , , . Limitations of using same‐hospital readmission metrics. Int J Qual Health Care. 2013;25(6):633–639.
- , , , et al. Is same‐hospital readmission rate a good surrogate for all‐hospital readmission rate? Med Care. 2010;48(5):477–481.
- , , . The relationship between hospital admission rates and rehospitalizations. N Engl J Med. 2011;365(24):2287–2295.
- . Community factors and hospital readmission rates [published online April 9, 2014]. Health Serv Res. doi: 10.1111/1475–6773.12177.
- American Hospital Association. Hospital readmissions reduction program: factsheet. American Hospital Association. Available at: http://www.aha.org/content/13/fs‐readmissions.pdf. Published April 14, 2014. Accessed May 5, 2014.
- Medicare Payment Advisory Commission. Report to the congress: Medicare and the health care delivery system. Available at: http://www.medpac.gov/documents/Jun13_EntireReport.pdf. Published June 14, 2013. Accessed May 5, 2014.
- , , . Sharp rise in Medicare enrollees being held in hospitals for observation raises concerns about causes and consequences. Health Aff (Millwood). 2012;31(6):1251–1259.
- , , . Quality improvement of care transitions and the trend of composite hospital care. JAMA. 2014;311(10):1013–1014.
- , . When projecting required effectiveness of interventions for hospital readmission reduction, the percentage that is potentially avoidable must be considered. J Clin Epidemiol. 2013;66(6):688–690.
- , , . Urgent readmission rates can be used to infer differences in avoidable readmission rates between hospitals. J Clin Epidemiol. 2012;65(10):1124–1130.
- , , , , . Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183(7):E391–E402.
- , , , , , . Measuring and preventing potentially avoidable hospital readmissions: a review of the literature. Hong Kong Med J. 2010;16(5):383–389.
- 3M Health Information Systems. Potentially preventable readmissions classification system methodology: overview. 3M Health Information Systems; May 2008. Report No.: GRP‐139. Available at: http://multimedia.3m.com/mws/mediawebserver?66666UuZjcFSLXTtNXMtmxMEEVuQEcuZgVs6EVs6E666666‐‐. Accessed June 8, 2014.
- , , , , , . Manual and automated methods for identifying potentially preventable readmissions: a comparison in a large healthcare system. BMC Med Inform Decis Mak. 2014;14:28.
- , , , , , , et al. Comparing 2 methods of assessing 30‐day readmissions: what is the impact on hospital profiling in the Veterans Health Administration? Med Care. 2013;51(7):589–596.
- , . It's not six of one, half‐dozen the other: a comparative analysis of 3 rehospitalization measurement systems for Massachusetts. Academy Health Annual Research Meeting. Seattle, WA. 2011. Available at: http://www.academyhealth.org/files/2011/tuesday/boutwell.pdf. Accessed May 9, 2014.
- , , , , , . Validation of the potentially avoidable hospital readmission rate as a routine indicator of the quality of hospital care. Med Care. 2006;44(11):972–981.
- , , , , , . Measuring potentially avoidable hospital readmissions. J Clin Epidemiol. 2002;55:573–587.
- , , , . Potentially avoidable 30‐day hospital readmissions in medical patients: derivation and validation of a prediction model. JAMA Intern Med. 2013;173(8):632–638.
- National Association of Public Hospitals and Health Systems. NAPH members focus on reducing readmissions. Available at: www.naph.org. Published June 2011. Accessed October 19, 2011.
- Agency for Healthcare Research and Quality. AHRQ quality indicators: prevention quality indicators. Available at: http://www.qualityindicators.ahrq.gov/Modules/pqi_resources.aspx. Accessed February 11, 2014.
- , , , , , . Shifting the dialogue from hospital readmissions to unplanned care. Am J Manag Care. 2013;19(6):450–453.
- . Post‐hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100–102.
- , . The hospital‐dependent patient. N Engl J Med. 2014;370(8):694–697.
- , , , et al. The hospital medicine reengineering network (HOMERuN): a learning organization focused on improving hospital care. Acad Med. 2014;89(3):415–420.
- , , , et al. Incidence of potentially avoidable urgent readmissions and their relation to all‐cause urgent readmissions. CMAJ. 2011;183(14):E1067–E1072.
Hospital Unit‐Based Leadership Models
Hospital‐based care has become more complex over time. Patients are sicker, with more chronic comorbid conditions requiring greater collaboration to provide coordinated patient care.[1, 2] Care coordination requires an interdisciplinary approach during hospitalization and especially during transitions of care.[3, 4] In addition, hospitals are tasked with managing and improving clinical workflow efficiencies, and implementing electronic health records (EHR)[5] that require healthcare professionals to learn new systems of care and technology. Payment models have also started to shift toward an incentive and penalty‐based structure in the form of value‐based purchasing, readmission penalties, hospital‐acquired conditions, and meaningful use.[4, 6]
In response to these pressures, hospitals are searching for ways to reliably deliver quality care that is safe, effective, patient centered, timely, efficient, and equitable.[7] Previous efforts to improve quality in the general medical inpatient setting have included redesign of the clinical work environment and new workflows through the use of checklists and whiteboards to enhance communication, patient‐centered bedside rounds, standardized protocols and handovers, and integrated clinical decision support using health information technology.[8, 9, 10, 11, 12, 13] Although each of these care coordination activities has potential value, integrating them at the unit level often remains a challenge. Some hospitals have addressed this challenge by establishing and supporting a unit‐based leadership model, where a medical director and nurse manager work together to assess and improve the quality, safety, efficiency, and patient experience‐based mission of the organization.[14, 15] However, there are few descriptions of this leadership model in the current literature. Herein, we present the unit‐based leadership model that has been developed and implemented at 6 hospitals.
MODELS OF UNIT‐BASED LEADERSHIP
The unit‐based leadership model is grounded on the idea that culture and clinical care are products of frontline structure, process, and relationships, and that leaders at the site of care can have the greatest influence on the local work environment.[16, 17] The objective is to influence care and culture at the bedside and the unit, where care is delivered and where alignment with organizational vision and mission must occur. The concept of the inpatient unit medical director is not new, and hospitals in the past have recruited physician leaders to become clinical champions for quality improvement and help establish a collaborative work environment for physicians and unit‐based staff.[18, 19, 20, 21, 22] These studies report on the challenges and benefits of incorporating a medical director to inpatient psychiatry or general care units, but do not provide specific details about the recruitment and responsibilities for unit‐based dyad partnerships, which are critical factors for success on multidisciplinary inpatient care units.
There are several logistical matters to consider when instituting a unit‐based leadership model. These include the composition of the leadership team, selection process of the leaders, the presence of trainees and permanent faculty, and whether the units are able to geographically cohort patients. Other considerations include a clear role description with established shared goals and expectations, and a compensation model that includes effort and incentives. In addition, there should be a clearly established reporting structure to senior leadership, and the unit leaders should be given opportunities for professional growth and development. Table 1 provides a summary overview of 6 hospitals' experiences to date.
| Structure | Hospital of the University of Pennsylvania | Northwestern Memorial Hospital | Emory University Hospital | University of Michigan Health System | Christiana Care Health System | St. Joseph Mercy Health System/Integrated Health Associates |
|---|---|---|---|---|---|---|
| ||||||
| Description of hospital(s) | Academic medical center, 784 beds, 40,000 annual admissions | Academic medical center, 897 beds, 53,000 annual admissions | Academic medical center, 579 beds, 24,000 annual admissions | Academic medical center, 839 beds, 45,000 annual admissions | Independent academic medical center, 1,100 beds, 53,000 annual admissions | Tertiary community hospital that is part of a larger health care system (Trinity Health), 579 beds, 33,000 annual admissions |
| Unit leadership model | Triad of medical director, nurse manager, and quality improvement specialist/project manager | Dyad of medical director and nurse manager | Dyad of medical director and nurse manager | Dyad of medical director and nurse manager | Dyad of medical director and nurse manager | Dyad of medical director and nurse manager |
| Percent effort time supported for unit medical director | 10% | 17% | 10% | 20% | 20% | 10% |
| Incentives built into unit leaders' performance in outcomes metrics | No | Yes | No | No | No | Yes |
| Professional development/leadership training | Quality improvement method: PDSA, Six Sigma, Lean Healthcare | Quality improvement method: Six Sigma | Situational leadership training with 1:1 mentoring | Quality improvement method: Lean Healthcare, service excellence program | Quality Improvement method: Six Sigma, Lean Healthcare | Quality improvement method: Six Sigma |
| Additional leadership development through Penn Medicine Leadership Academy and Wharton Executive Education | Additional leadership development through Northwestern's professional development center and simulation training center | Conflict resolution skill development | Attend patient and Family Centered Care conference | Additional leadership development through Christiana Care Learning Institute | Attend educational course on Crucial Conversations | |
| Personality profile with coaching | Additional leadership development through University of Michigan Health System's human resources group | |||||
| Outcomes metrics monitored | Patient satisfaction | Patient satisfaction | Patient satisfaction | Patient satisfaction | Patient satisfaction | Patient satisfaction |
| Efficiency of multidisciplinary rounds | Teamwork climate (survey) | Teamwork and implementation of structured interdisciplinary bedside rounds | Multidisciplinary rounds | Interdisciplinary rounds | Participation in interdisciplinary rounds | |
| RNMD work environment surveys | Adverse events | Unit‐based patient safety culture survey | Patient‐centered, bedside rounds | Readmission rates | ||
| Hospital‐acquired conditions (CAUTI, CLABSI, VAP, DVT, pressure ulcers) | Hospital‐acquired conditions (fall rates, pressure ulcers | Hospital‐acquired conditions (CAUTI, CLABSI, fall rates, pressure ulcers) | Hospital‐acquired conditions (CAUTI) | Hospital‐acquired conditions (fall rates, pressure ulcers) | Core measures | |
| Readmission rates | Readmission rates | Mortality | Readmission rates | Readmission rates | Medication reconciliation | |
| Core measures, patient safety indicators | Core measures | Length of stay | DVT prophylaxis | Hand hygiene | Discharge by 11 am | |
| Mortality (observed to expected, transfer, inpatient) | Hand hygiene | Glycemic control | Meeting attendance | Length of stay | Use of patient teach‐back | |
| Medication reconciliation | Restraint use | Communication with PCPs | ||||
| Home care, hospice, post‐acute care referral rates | ||||||
| Organizational leadership structure support for clinical unit partnership program | CMO, CNO, vice president of quality/patient safety, directors of medical and surgical nursing | Associate chair of medicine, director of medicine nursing; all medical directors are members of the department of medicine quality management committee | CMO, CNO, CEO, CQO | CMO, CNO | All teams report to and are supported by 3 overarching, system‐wide committees: (1) safety first, (2) think of yourself as a patient, (3) clinical excellence. Those committees, in turn, report up to the senior management quality/safety coordinating council. | Director of hospitalist program (reports to CMO); nursing director of acute care (reports to CNO) |
DISCUSSION
In reviewing our 6 organization's collective experiences, we identified several common themes and some notable differences across sites. The core of the leadership team was primarily composed of the medical director and nurse manager on the unit. Across all 6 organizations, medical directors had a portion of their effort supported for their leadership work on the unit. Leadership development training was provided at all of our sites, with particular emphasis on quality improvement (QI) methods such as Six‐Sigma, Lean, or Plan, Do, Study, Act (PDSA). Additional leadership development sessions were provided through the organization's human resources or affiliated university. Common outcome measures of interest include patient satisfaction, interdisciplinary practice, and collaboration on the unit, and some hospital‐acquired condition measures. Last, there is a direct reporting relationship to a chief or senior nurse or physician leader within each organization. These commonalities and variances are further detailed below.
Establishing the Unit‐Based Leadership Model
The composition of the unit‐based leadership model in our 6 organizations is predominantly a dyad partnership of medical directors and nurse managers. Although informal physician‐nurse collaborative practices have likely been in existence at many hospitals, formalizing this dyad partnership is an important step to fostering collaborative efforts to improve quality of care. It is also essential for hospital leadership to clearly articulate the need for this unit‐based leadership model. Whether the motivation for change is from a previously untenable practice environment, or part of an ongoing improvement program, the model should be presented in a manner that supports the organization's commitment to improve collaborative practices for better patient care. One of our 6 hospitals initiated this leadership model based on troubling relationships between nurses and physicians on some of their inpatient care units, which threatened to stall the organization's Magnet application. Implementation of the leadership model at the unit level yielded improvements in nursephysician interactions, patient satisfaction, and staff turnover.[15, 23] Another of the hospitals first evaluated why a previous attempt at this model did not deliver the intended outcomes, and redesigned the model based on its analysis.[14]
Across all of the organizations featured here, a common driver behind the adoption of the unit‐based leadership model was to bridge the divide between physician services and nursing and other allied health providers. We found that many of the physicians routinely had patients on multiple units, limiting the quantity and quality of collaborative practices between unit‐based staff and physician teams. The unit‐based dyad leaders are ideally positioned to build and foster a culture of collaboration, and our organizations have been inclusive to ensure the participation of a multidisciplinary group of providers, including representatives from pharmacy, environmental services, physical therapy, respiratory therapy, social work, case management, and nutrition at leadership meetings or in daily patient‐care discussions. In addition, 2 of the organizations have added quality improvement specialist/project managers to their teams to support the physiciannurse manager leaders on the unit.
Selection Process and Professional Development
The traditional approach to hiring a physician leader or a nurse manager has been an isolated process of drafting a job description for each position and hiring within their respective departments. For the dyad partnership to be successful, there should be established goals and expectations that require shared responsibilities between the 2 partners, which should guide the selection of these leaders. Other leadership attributes and essential character traits that should be modeled by the unit‐based leaders include good communication skills, respect among coworkers, and a collaborative approach to decision making and action. In addition, both physician leaders and nurse managers in these roles should have the ability to take a system's view, recognizing that within the complex network of healthcare providers and processes on their unit, these elements interact with each other, which lead to the outcomes achieved on their units.[24, 25] Table 2 lists some general shared responsibilities, highlighting specific activities that can be used to achieve the established outcomes. As the unit's dyad leadership works together to address these shared responsibilities, they should keep their sights focused on the overall strategic goals of the healthcare organization. Bohmer has defined 4 habits of the high‐value healthcare organization that in turn can be reflected through the inpatient unit leadership model to capture these activities at the local level: (1) planning care for specific patient populations, (2) microsystem design, (3) measurement and oversight, and (4) self‐study.[26] In determining specific shared responsibilities for each dyad partner, it is important for these leaders to understand the clinical microsystem of their unit such as their patient population, interdisciplinary care team, approach to process improvement, and performance patterns over time.[27]
| General Shared Responsibilities of Physician and Nurse Unit Directors | Examples of Specific Activities |
|---|---|
| |
| Serve as management partners to enhance culture of the unit | Co‐craft and deliver consistent leadership message |
| Co‐establish and enforce unit processes and protocols | |
| Co‐lead recruitment and retention efforts | |
| Co‐orient trainees and faculty rotating through unit | |
| Co‐educate on the management of common medical and surgical conditions | |
| Facilitate interstaff conflict resolution sessions | |
| Regular leadership meetings | |
| Actively manage unit processes and outcomes | Quality: improve core quality measure performance |
| Safety: improve culture of patient safety within the unit as measured by surveys and incident reporting systems | |
| Efficiency: reduce unnecessary length of stay and variability in resource use | |
| Patient experience: focus on improving patient‐family experience with targeted outcomes in patient experience metrics (eg, HCAHPS) | |
| Education: develop trainee and staff clinical and teamwork competencies | |
| Continuous process improvement initiatives (eg, PDSA cycles) | Improve the discharge transitions process, tailoring the process to each individual patient's identified risk factors |
| Focus improvement efforts on reduction in specific hospital acquired conditions such as CAUTI, VTE, CLABSI, pressure ulcers, falls | |
| Measure, analyze, reassess, and improve in all described areas of shared responsibilities | |
| Perform unit level chart reviews to evaluate readmissions and LOS and identify improvement opportunities | |
In our collective experience, the dyad leaders bring passion and commitment to improving care; however, many (the medical directors in particular) have minimal prior formal training in leadership, quality improvement, or hospital management. Recognizing that unit leaders require specialized knowledge and skills, each of our organizations has enrolled unit medical directors and nurse managers in leadership development courses or educational programs. Many healthcare organizations have become more grounded in a QI methodology including Six‐Sigma, Lean Healthcare, PDSA, and other scientifically based methods, and the unit‐based leaders should receive advanced training in the preferred methods of their institution. Additional training in quality improvement, patient safety, and physician leadership can also be obtained through supplemental coursework specifically designed to train hospital leaders, with some programs leading to a certification or additional credentials.[28]
Beyond such formal educational opportunities, hospitals should not overlook the opportunity to learn from and share experiences with the other dyad leadership units within the hospital. One of the organizations described here holds monthly meetings with all of the unit dyad leaders, and 2 other organizations conduct quarterly meetings to share experiences and best practices related to specific improvement initiatives in a learning network model. Those units with more experience in specific initiatives are asked to share their lessons learned with others, as well as support each other in their efforts to collectively meet the strategic goals of the hospital.
Time and Organizational Support
In addition to leadership development, hospitals and the clinical department leadership need to support the medical directors with dedicated time away from their usual clinical duties. Some organizations in this report are providing up to 20% effort for the medical director's unit‐based leadership work; however, there is some variation in practice with regard to physician effort across sites. The University of Pennsylvania has a smaller effort support at 10%; however, some of that effort differential may be offset through the allocation of the quality improvement specialist/project manager assigned to work with the medical director and nurse manager dyad. St. Joseph Mercy Hospital also has a lower allocation, as there is additional financial compensation for the role that is at risk and not included in this 10% allocation.
It is also important to assure that the medical directors have institutional support to carry out their work in partnership with their nursing leadership. The 6 health systems described here report that although most of the physicians have appointments within a physician group or clinical department, there is hospital leadership oversight from a chief medical, nursing, or operating officer. This organizational structure may be an important aspect of the model as the unit‐based leaders seek to align their efforts with that of the hospital. Further, this form of organizational oversight can ensure that the unit leaders will receive timely and essential unit‐ and hospital‐based performance measures to manage local improvement efforts. These measures may include some components of patient experiences as reported in the Hospital Consumer Assessment of Healthcare Providers and Systems survey, readmission rates, hospital‐acquired condition rates, length of stay, observed to expected mortality rates, and results of staff satisfaction and safety culture surveys. As highlighted by several studies and commentaries, our collective experiences also identified interdisciplinary teamwork, collaboration, and communication as desirable outcome measures through the unit‐based leadership structure.[21, 22, 24, 29, 30] The medical director and nurse manager dyads can prioritize their improvement efforts based on the data provided to them, and mobilize the appropriate group of multidisciplinary practitioners and support staff on the unit.
OTHER CONSIDERATIONS
Other infrastructure variables that may increase the effectiveness of the unit leadership dyad include unit‐based clinical services (geographic localization), engaging the frontline team members in the design and implementation of change innovations, a commitment to patient and family centered practices on the unit, and enhancing clinical workflow through the support of EHR functions such as concurrent documentation and provider order entry. Geographic localization, placing the fewest possible clinical service providers on the unit to work alongside unit‐based staff, allows for a cohesive interdisciplinary unit‐based team to develop under the dyad leadership, and has been shown to improve communication practices.[9, 31] Beyond geographic localization of patients, it is critical to ensure team members are committed to the changes in workflow by directly involving them through the design and implementation of new models of care taking place on the unit. This commitment starts from the top senior nurse and physician leaders in the organization, and extends to the unit‐based dyad partners, and down to each individual interdisciplinary team member on the unit.[1] Thus, it is critical to clarify roles and responsibilities and how team members on the unit will interact with each other. For some situations, conflict management training will be helpful to the unit‐based leaders to resolve issues. To appreciate potential barriers to successful rollout of this unit leadership model, a phased implementation of pilot units, followed by successive waves, should be considered. Many of the units that instituted unit‐based interdisciplinary team rounds solicited and implemented direct feedback from frontline team members in efforts to improve communication and be more patient centered. Conversely, there are also likely to be situations where the unit‐based leaders will be confronted with hindrances to their unit‐based collaborative improvement efforts. To help prepare the dyad leaders, many of our unit‐based leaders have received specific training on how to coach and conduct difficult conversations with individuals who have performance gaps or are perceived to be hindering the progress of the unit's work. These crucial negotiation skills are not innate among most managers and should be explicitly provided to new leaders across organizations.
The goals and merits of patient‐ and family‐centered care (PFCC) have been well described.[32, 33, 34] Organizational support to teach and disseminate PFCC practices throughout all settings of care may help the leadership dyads implement rounding strategies that engage all staff, patients, and family members throughout the hospital course and during the transitions out of the hospital.
Clinical workflow has become heavily dependent on the EHR systems. For those organizations that have yet to adopt a particular EHR system, the leadership dyads should be involved throughout the EHR design process to help ensure that the technological solutions will be built to assist the clinical workflow, and once the system has been built, the leadership dyad should monitor and enhance the interface between workflow and EHR system so that it can support the creation and advancement of interdisciplinary plans of care on the unit.
CONCLUSION
The care of the hospitalized patient has become more complex over time. Interdisciplinary teamwork needs to be improved at the unit level to achieve the strategic goals of the hospital. Although quality improvement is an organizational goal, change takes place locally. Physician leaders, in partnership with nurse managers, are needed now more than ever to take on this task to improve the hospital‐care experience for patients by functioning as the primary effector arms for changing the landscape of hospital‐based care. We have described characteristics of unit‐based leadership programs adopted across 6 organizations. Hospitalists with clinical experience as the principal providers of inpatient‐based care and quality improvement experience and training, have been key participants in the development and implementation of the local leadership models in each of these hospital systems. We hope the comparison of the various models featured in this article serves as a valuable reference to hospitals and healthcare organizations who are contemplating the incorporation of this model into their strategic plan.
- , , , et al. Organizational predictors of coordination in inpatient medicine [published online ahead of print February 26, 2014]. Health Care Manage Rev. doi: 10.1097/HMR.0000000000000004.
- . Trends in case‐mix in the medicare population. Paper presented at: American Hospital Association, Federation of American Hospitals, Association of American Medical Colleges; http://www.aha.org/content/00‐10/100715‐CMItrends.pdf. July 15, 2010.
- . A requirement to reduce readmissions: take care of the patient, not just the disease. JAMA. 2013;309(4):394–396.
- , . Value‐based purchasing—national programs to move from volume to value. N Engl J Med. 2012;367(4):292–295.
- Medicare and Medicaid programs; electronic health record incentive program. Final rule. Fed Regist. 2010;75(144):44313–44588.
- . The Center for Medicare and Medicaid innovation's blueprint for rapid‐cycle evaluation of new care and payment models. Health Aff (Millwood). 2013;32(4):807–812.
- Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a medical teaching unit. J Gen Intern Med. 2010;25(8):826–832.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24(11):1223–1227.
- , , , . A review on systematic reviews of health information system studies. J Am Med Inform Assoc. 2010;17(6):637–645.
- , , , , . Patient whiteboards as a communication tool in the hospital setting: a survey of practices and recommendations. J Hosp Med. Apr 2010;5(4):234–239.
- , , . Development of a checklist for documenting team and collaborative behaviors during multidisciplinary bedside rounds. J Nurs Adm. 2013;43(5):280–285.
- , , , , . Assessment of teamwork during structured interdisciplinary rounds on medical units. J Hosp Med. 2012;7(9):679–683.
- , , , et al. Leadership at the front line: a clinical partnership model on general care inpatient units. Am J Med Qual. 2012;27(2):106–111.
- , . AHRQ health care innovations exchange: improvement projects led by unit‐based teams of nurse, physician, and quality leaders reduce infections, lower costs, improve patient satisfaction, and nurse‐physician communication. Available at: http://www.innovations.ahrq.gov/content.aspx?id=2719. Published April 14, 2010. Accessed November 26, 2011.
- , , , , , . Microsystems in health care: part 8. Developing people and improving work life: what front‐line staff told us. Jt Comm J Qual Saf. 2003;29(10):512–522.
- , , , et al. Microsystems in health care: part 5. How leaders are leading. Jt Comm J Qual Saf. 2003;29(6):297–308.
- , , . The academic dilemma of the inpatient unit director. Am J Psychiatry. 1989;146(1):73–76.
- , , , , . Improving and sustaining core measure performance through effective accountability of clinical microsystems in an academic medical center. Jt Comm J Qual Patient Saf. 2010;36(9):387–398.
- , , . Physician leadership and quality improvement in the acute child and adolescent psychiatric care setting. Child Adolesc Psychiatr Clin N Am. 2010;19(1):1–19; table of contents.
- , , , . Effect of a multidisciplinary intervention on communication and collaboration among physicians and nurses. Am J Crit Care. 2005;14(1):71–77.
- , . Nurse‐physician leadership: insights into interprofessional collaboration. J Nurs Adm. 2013;43(12):653–659.
- The Advisory Board. University of Pennsylvania Health System pilots unit clinical leadership model to spur quality gains. Nurs Exec Watch. 2008;9(2):4–6.
- , . Physicians as leaders in improving health care: a new series in Annals of Internal Medicine. Ann Intern Med. 1998;128(4):289–292.
- . Understanding medical systems. Ann Intern Med. 1998;128(4):293–298.
- . The four habits of high‐value health care organizations. N Engl J Med. 2011;365(22):2045–2047.
- , , , et al. Microsystems in health care: Part 1. Learning from high‐performing front‐line clinical units. Jt Comm J Qual Improv. 2002;28(9):472–493.
- , , , et al. The quality and safety educators academy: fulfilling an unmet need for faculty development. Am J Med Qual. 2014;29(1):5–12.
- , , , . Cooperation: the foundation of improvement. Ann Intern Med. 1998;128(12 pt 1):1004–1009.
- , , , , , . Ten principles of good interdisciplinary team work. Hum Resour Health 2013;11(1):19.
- , , , et al. Impact of localizing general medical teams to a single nursing unit. J Hosp Med. 2012;7(7):551–556.
- , , , . Integrating patient‐ and family‐centered care with health policy: four proposed policy approaches. Qual Manag Health Care. 2013;22(2):137–145.
- , , . Incorporating patient‐ and family‐centered care into resident education: approaches, benefits, and challenges. J Grad Med Educ. 2011;3(2):272–278.
- Smith M, Saunders R, Stuckhardt L, McGinnis JM, eds. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; 2012.
Hospital‐based care has become more complex over time. Patients are sicker, with more chronic comorbid conditions requiring greater collaboration to provide coordinated patient care.[1, 2] Care coordination requires an interdisciplinary approach during hospitalization and especially during transitions of care.[3, 4] In addition, hospitals are tasked with managing and improving clinical workflow efficiencies, and implementing electronic health records (EHR)[5] that require healthcare professionals to learn new systems of care and technology. Payment models have also started to shift toward an incentive and penalty‐based structure in the form of value‐based purchasing, readmission penalties, hospital‐acquired conditions, and meaningful use.[4, 6]
In response to these pressures, hospitals are searching for ways to reliably deliver quality care that is safe, effective, patient centered, timely, efficient, and equitable.[7] Previous efforts to improve quality in the general medical inpatient setting have included redesign of the clinical work environment and new workflows through the use of checklists and whiteboards to enhance communication, patient‐centered bedside rounds, standardized protocols and handovers, and integrated clinical decision support using health information technology.[8, 9, 10, 11, 12, 13] Although each of these care coordination activities has potential value, integrating them at the unit level often remains a challenge. Some hospitals have addressed this challenge by establishing and supporting a unit‐based leadership model, where a medical director and nurse manager work together to assess and improve the quality, safety, efficiency, and patient experience‐based mission of the organization.[14, 15] However, there are few descriptions of this leadership model in the current literature. Herein, we present the unit‐based leadership model that has been developed and implemented at 6 hospitals.
MODELS OF UNIT‐BASED LEADERSHIP
The unit‐based leadership model is grounded on the idea that culture and clinical care are products of frontline structure, process, and relationships, and that leaders at the site of care can have the greatest influence on the local work environment.[16, 17] The objective is to influence care and culture at the bedside and the unit, where care is delivered and where alignment with organizational vision and mission must occur. The concept of the inpatient unit medical director is not new, and hospitals in the past have recruited physician leaders to become clinical champions for quality improvement and help establish a collaborative work environment for physicians and unit‐based staff.[18, 19, 20, 21, 22] These studies report on the challenges and benefits of incorporating a medical director to inpatient psychiatry or general care units, but do not provide specific details about the recruitment and responsibilities for unit‐based dyad partnerships, which are critical factors for success on multidisciplinary inpatient care units.
There are several logistical matters to consider when instituting a unit‐based leadership model. These include the composition of the leadership team, selection process of the leaders, the presence of trainees and permanent faculty, and whether the units are able to geographically cohort patients. Other considerations include a clear role description with established shared goals and expectations, and a compensation model that includes effort and incentives. In addition, there should be a clearly established reporting structure to senior leadership, and the unit leaders should be given opportunities for professional growth and development. Table 1 provides a summary overview of 6 hospitals' experiences to date.
| Structure | Hospital of the University of Pennsylvania | Northwestern Memorial Hospital | Emory University Hospital | University of Michigan Health System | Christiana Care Health System | St. Joseph Mercy Health System/Integrated Health Associates |
|---|---|---|---|---|---|---|
| ||||||
| Description of hospital(s) | Academic medical center, 784 beds, 40,000 annual admissions | Academic medical center, 897 beds, 53,000 annual admissions | Academic medical center, 579 beds, 24,000 annual admissions | Academic medical center, 839 beds, 45,000 annual admissions | Independent academic medical center, 1,100 beds, 53,000 annual admissions | Tertiary community hospital that is part of a larger health care system (Trinity Health), 579 beds, 33,000 annual admissions |
| Unit leadership model | Triad of medical director, nurse manager, and quality improvement specialist/project manager | Dyad of medical director and nurse manager | Dyad of medical director and nurse manager | Dyad of medical director and nurse manager | Dyad of medical director and nurse manager | Dyad of medical director and nurse manager |
| Percent effort time supported for unit medical director | 10% | 17% | 10% | 20% | 20% | 10% |
| Incentives built into unit leaders' performance in outcomes metrics | No | Yes | No | No | No | Yes |
| Professional development/leadership training | Quality improvement method: PDSA, Six Sigma, Lean Healthcare | Quality improvement method: Six Sigma | Situational leadership training with 1:1 mentoring | Quality improvement method: Lean Healthcare, service excellence program | Quality Improvement method: Six Sigma, Lean Healthcare | Quality improvement method: Six Sigma |
| Additional leadership development through Penn Medicine Leadership Academy and Wharton Executive Education | Additional leadership development through Northwestern's professional development center and simulation training center | Conflict resolution skill development | Attend patient and Family Centered Care conference | Additional leadership development through Christiana Care Learning Institute | Attend educational course on Crucial Conversations | |
| Personality profile with coaching | Additional leadership development through University of Michigan Health System's human resources group | |||||
| Outcomes metrics monitored | Patient satisfaction | Patient satisfaction | Patient satisfaction | Patient satisfaction | Patient satisfaction | Patient satisfaction |
| Efficiency of multidisciplinary rounds | Teamwork climate (survey) | Teamwork and implementation of structured interdisciplinary bedside rounds | Multidisciplinary rounds | Interdisciplinary rounds | Participation in interdisciplinary rounds | |
| RNMD work environment surveys | Adverse events | Unit‐based patient safety culture survey | Patient‐centered, bedside rounds | Readmission rates | ||
| Hospital‐acquired conditions (CAUTI, CLABSI, VAP, DVT, pressure ulcers) | Hospital‐acquired conditions (fall rates, pressure ulcers | Hospital‐acquired conditions (CAUTI, CLABSI, fall rates, pressure ulcers) | Hospital‐acquired conditions (CAUTI) | Hospital‐acquired conditions (fall rates, pressure ulcers) | Core measures | |
| Readmission rates | Readmission rates | Mortality | Readmission rates | Readmission rates | Medication reconciliation | |
| Core measures, patient safety indicators | Core measures | Length of stay | DVT prophylaxis | Hand hygiene | Discharge by 11 am | |
| Mortality (observed to expected, transfer, inpatient) | Hand hygiene | Glycemic control | Meeting attendance | Length of stay | Use of patient teach‐back | |
| Medication reconciliation | Restraint use | Communication with PCPs | ||||
| Home care, hospice, post‐acute care referral rates | ||||||
| Organizational leadership structure support for clinical unit partnership program | CMO, CNO, vice president of quality/patient safety, directors of medical and surgical nursing | Associate chair of medicine, director of medicine nursing; all medical directors are members of the department of medicine quality management committee | CMO, CNO, CEO, CQO | CMO, CNO | All teams report to and are supported by 3 overarching, system‐wide committees: (1) safety first, (2) think of yourself as a patient, (3) clinical excellence. Those committees, in turn, report up to the senior management quality/safety coordinating council. | Director of hospitalist program (reports to CMO); nursing director of acute care (reports to CNO) |
DISCUSSION
In reviewing our 6 organization's collective experiences, we identified several common themes and some notable differences across sites. The core of the leadership team was primarily composed of the medical director and nurse manager on the unit. Across all 6 organizations, medical directors had a portion of their effort supported for their leadership work on the unit. Leadership development training was provided at all of our sites, with particular emphasis on quality improvement (QI) methods such as Six‐Sigma, Lean, or Plan, Do, Study, Act (PDSA). Additional leadership development sessions were provided through the organization's human resources or affiliated university. Common outcome measures of interest include patient satisfaction, interdisciplinary practice, and collaboration on the unit, and some hospital‐acquired condition measures. Last, there is a direct reporting relationship to a chief or senior nurse or physician leader within each organization. These commonalities and variances are further detailed below.
Establishing the Unit‐Based Leadership Model
The composition of the unit‐based leadership model in our 6 organizations is predominantly a dyad partnership of medical directors and nurse managers. Although informal physician‐nurse collaborative practices have likely been in existence at many hospitals, formalizing this dyad partnership is an important step to fostering collaborative efforts to improve quality of care. It is also essential for hospital leadership to clearly articulate the need for this unit‐based leadership model. Whether the motivation for change is from a previously untenable practice environment, or part of an ongoing improvement program, the model should be presented in a manner that supports the organization's commitment to improve collaborative practices for better patient care. One of our 6 hospitals initiated this leadership model based on troubling relationships between nurses and physicians on some of their inpatient care units, which threatened to stall the organization's Magnet application. Implementation of the leadership model at the unit level yielded improvements in nursephysician interactions, patient satisfaction, and staff turnover.[15, 23] Another of the hospitals first evaluated why a previous attempt at this model did not deliver the intended outcomes, and redesigned the model based on its analysis.[14]
Across all of the organizations featured here, a common driver behind the adoption of the unit‐based leadership model was to bridge the divide between physician services and nursing and other allied health providers. We found that many of the physicians routinely had patients on multiple units, limiting the quantity and quality of collaborative practices between unit‐based staff and physician teams. The unit‐based dyad leaders are ideally positioned to build and foster a culture of collaboration, and our organizations have been inclusive to ensure the participation of a multidisciplinary group of providers, including representatives from pharmacy, environmental services, physical therapy, respiratory therapy, social work, case management, and nutrition at leadership meetings or in daily patient‐care discussions. In addition, 2 of the organizations have added quality improvement specialist/project managers to their teams to support the physiciannurse manager leaders on the unit.
Selection Process and Professional Development
The traditional approach to hiring a physician leader or a nurse manager has been an isolated process of drafting a job description for each position and hiring within their respective departments. For the dyad partnership to be successful, there should be established goals and expectations that require shared responsibilities between the 2 partners, which should guide the selection of these leaders. Other leadership attributes and essential character traits that should be modeled by the unit‐based leaders include good communication skills, respect among coworkers, and a collaborative approach to decision making and action. In addition, both physician leaders and nurse managers in these roles should have the ability to take a system's view, recognizing that within the complex network of healthcare providers and processes on their unit, these elements interact with each other, which lead to the outcomes achieved on their units.[24, 25] Table 2 lists some general shared responsibilities, highlighting specific activities that can be used to achieve the established outcomes. As the unit's dyad leadership works together to address these shared responsibilities, they should keep their sights focused on the overall strategic goals of the healthcare organization. Bohmer has defined 4 habits of the high‐value healthcare organization that in turn can be reflected through the inpatient unit leadership model to capture these activities at the local level: (1) planning care for specific patient populations, (2) microsystem design, (3) measurement and oversight, and (4) self‐study.[26] In determining specific shared responsibilities for each dyad partner, it is important for these leaders to understand the clinical microsystem of their unit such as their patient population, interdisciplinary care team, approach to process improvement, and performance patterns over time.[27]
| General Shared Responsibilities of Physician and Nurse Unit Directors | Examples of Specific Activities |
|---|---|
| |
| Serve as management partners to enhance culture of the unit | Co‐craft and deliver consistent leadership message |
| Co‐establish and enforce unit processes and protocols | |
| Co‐lead recruitment and retention efforts | |
| Co‐orient trainees and faculty rotating through unit | |
| Co‐educate on the management of common medical and surgical conditions | |
| Facilitate interstaff conflict resolution sessions | |
| Regular leadership meetings | |
| Actively manage unit processes and outcomes | Quality: improve core quality measure performance |
| Safety: improve culture of patient safety within the unit as measured by surveys and incident reporting systems | |
| Efficiency: reduce unnecessary length of stay and variability in resource use | |
| Patient experience: focus on improving patient‐family experience with targeted outcomes in patient experience metrics (eg, HCAHPS) | |
| Education: develop trainee and staff clinical and teamwork competencies | |
| Continuous process improvement initiatives (eg, PDSA cycles) | Improve the discharge transitions process, tailoring the process to each individual patient's identified risk factors |
| Focus improvement efforts on reduction in specific hospital acquired conditions such as CAUTI, VTE, CLABSI, pressure ulcers, falls | |
| Measure, analyze, reassess, and improve in all described areas of shared responsibilities | |
| Perform unit level chart reviews to evaluate readmissions and LOS and identify improvement opportunities | |
In our collective experience, the dyad leaders bring passion and commitment to improving care; however, many (the medical directors in particular) have minimal prior formal training in leadership, quality improvement, or hospital management. Recognizing that unit leaders require specialized knowledge and skills, each of our organizations has enrolled unit medical directors and nurse managers in leadership development courses or educational programs. Many healthcare organizations have become more grounded in a QI methodology including Six‐Sigma, Lean Healthcare, PDSA, and other scientifically based methods, and the unit‐based leaders should receive advanced training in the preferred methods of their institution. Additional training in quality improvement, patient safety, and physician leadership can also be obtained through supplemental coursework specifically designed to train hospital leaders, with some programs leading to a certification or additional credentials.[28]
Beyond such formal educational opportunities, hospitals should not overlook the opportunity to learn from and share experiences with the other dyad leadership units within the hospital. One of the organizations described here holds monthly meetings with all of the unit dyad leaders, and 2 other organizations conduct quarterly meetings to share experiences and best practices related to specific improvement initiatives in a learning network model. Those units with more experience in specific initiatives are asked to share their lessons learned with others, as well as support each other in their efforts to collectively meet the strategic goals of the hospital.
Time and Organizational Support
In addition to leadership development, hospitals and the clinical department leadership need to support the medical directors with dedicated time away from their usual clinical duties. Some organizations in this report are providing up to 20% effort for the medical director's unit‐based leadership work; however, there is some variation in practice with regard to physician effort across sites. The University of Pennsylvania has a smaller effort support at 10%; however, some of that effort differential may be offset through the allocation of the quality improvement specialist/project manager assigned to work with the medical director and nurse manager dyad. St. Joseph Mercy Hospital also has a lower allocation, as there is additional financial compensation for the role that is at risk and not included in this 10% allocation.
It is also important to assure that the medical directors have institutional support to carry out their work in partnership with their nursing leadership. The 6 health systems described here report that although most of the physicians have appointments within a physician group or clinical department, there is hospital leadership oversight from a chief medical, nursing, or operating officer. This organizational structure may be an important aspect of the model as the unit‐based leaders seek to align their efforts with that of the hospital. Further, this form of organizational oversight can ensure that the unit leaders will receive timely and essential unit‐ and hospital‐based performance measures to manage local improvement efforts. These measures may include some components of patient experiences as reported in the Hospital Consumer Assessment of Healthcare Providers and Systems survey, readmission rates, hospital‐acquired condition rates, length of stay, observed to expected mortality rates, and results of staff satisfaction and safety culture surveys. As highlighted by several studies and commentaries, our collective experiences also identified interdisciplinary teamwork, collaboration, and communication as desirable outcome measures through the unit‐based leadership structure.[21, 22, 24, 29, 30] The medical director and nurse manager dyads can prioritize their improvement efforts based on the data provided to them, and mobilize the appropriate group of multidisciplinary practitioners and support staff on the unit.
OTHER CONSIDERATIONS
Other infrastructure variables that may increase the effectiveness of the unit leadership dyad include unit‐based clinical services (geographic localization), engaging the frontline team members in the design and implementation of change innovations, a commitment to patient and family centered practices on the unit, and enhancing clinical workflow through the support of EHR functions such as concurrent documentation and provider order entry. Geographic localization, placing the fewest possible clinical service providers on the unit to work alongside unit‐based staff, allows for a cohesive interdisciplinary unit‐based team to develop under the dyad leadership, and has been shown to improve communication practices.[9, 31] Beyond geographic localization of patients, it is critical to ensure team members are committed to the changes in workflow by directly involving them through the design and implementation of new models of care taking place on the unit. This commitment starts from the top senior nurse and physician leaders in the organization, and extends to the unit‐based dyad partners, and down to each individual interdisciplinary team member on the unit.[1] Thus, it is critical to clarify roles and responsibilities and how team members on the unit will interact with each other. For some situations, conflict management training will be helpful to the unit‐based leaders to resolve issues. To appreciate potential barriers to successful rollout of this unit leadership model, a phased implementation of pilot units, followed by successive waves, should be considered. Many of the units that instituted unit‐based interdisciplinary team rounds solicited and implemented direct feedback from frontline team members in efforts to improve communication and be more patient centered. Conversely, there are also likely to be situations where the unit‐based leaders will be confronted with hindrances to their unit‐based collaborative improvement efforts. To help prepare the dyad leaders, many of our unit‐based leaders have received specific training on how to coach and conduct difficult conversations with individuals who have performance gaps or are perceived to be hindering the progress of the unit's work. These crucial negotiation skills are not innate among most managers and should be explicitly provided to new leaders across organizations.
The goals and merits of patient‐ and family‐centered care (PFCC) have been well described.[32, 33, 34] Organizational support to teach and disseminate PFCC practices throughout all settings of care may help the leadership dyads implement rounding strategies that engage all staff, patients, and family members throughout the hospital course and during the transitions out of the hospital.
Clinical workflow has become heavily dependent on the EHR systems. For those organizations that have yet to adopt a particular EHR system, the leadership dyads should be involved throughout the EHR design process to help ensure that the technological solutions will be built to assist the clinical workflow, and once the system has been built, the leadership dyad should monitor and enhance the interface between workflow and EHR system so that it can support the creation and advancement of interdisciplinary plans of care on the unit.
CONCLUSION
The care of the hospitalized patient has become more complex over time. Interdisciplinary teamwork needs to be improved at the unit level to achieve the strategic goals of the hospital. Although quality improvement is an organizational goal, change takes place locally. Physician leaders, in partnership with nurse managers, are needed now more than ever to take on this task to improve the hospital‐care experience for patients by functioning as the primary effector arms for changing the landscape of hospital‐based care. We have described characteristics of unit‐based leadership programs adopted across 6 organizations. Hospitalists with clinical experience as the principal providers of inpatient‐based care and quality improvement experience and training, have been key participants in the development and implementation of the local leadership models in each of these hospital systems. We hope the comparison of the various models featured in this article serves as a valuable reference to hospitals and healthcare organizations who are contemplating the incorporation of this model into their strategic plan.
Hospital‐based care has become more complex over time. Patients are sicker, with more chronic comorbid conditions requiring greater collaboration to provide coordinated patient care.[1, 2] Care coordination requires an interdisciplinary approach during hospitalization and especially during transitions of care.[3, 4] In addition, hospitals are tasked with managing and improving clinical workflow efficiencies, and implementing electronic health records (EHR)[5] that require healthcare professionals to learn new systems of care and technology. Payment models have also started to shift toward an incentive and penalty‐based structure in the form of value‐based purchasing, readmission penalties, hospital‐acquired conditions, and meaningful use.[4, 6]
In response to these pressures, hospitals are searching for ways to reliably deliver quality care that is safe, effective, patient centered, timely, efficient, and equitable.[7] Previous efforts to improve quality in the general medical inpatient setting have included redesign of the clinical work environment and new workflows through the use of checklists and whiteboards to enhance communication, patient‐centered bedside rounds, standardized protocols and handovers, and integrated clinical decision support using health information technology.[8, 9, 10, 11, 12, 13] Although each of these care coordination activities has potential value, integrating them at the unit level often remains a challenge. Some hospitals have addressed this challenge by establishing and supporting a unit‐based leadership model, where a medical director and nurse manager work together to assess and improve the quality, safety, efficiency, and patient experience‐based mission of the organization.[14, 15] However, there are few descriptions of this leadership model in the current literature. Herein, we present the unit‐based leadership model that has been developed and implemented at 6 hospitals.
MODELS OF UNIT‐BASED LEADERSHIP
The unit‐based leadership model is grounded on the idea that culture and clinical care are products of frontline structure, process, and relationships, and that leaders at the site of care can have the greatest influence on the local work environment.[16, 17] The objective is to influence care and culture at the bedside and the unit, where care is delivered and where alignment with organizational vision and mission must occur. The concept of the inpatient unit medical director is not new, and hospitals in the past have recruited physician leaders to become clinical champions for quality improvement and help establish a collaborative work environment for physicians and unit‐based staff.[18, 19, 20, 21, 22] These studies report on the challenges and benefits of incorporating a medical director to inpatient psychiatry or general care units, but do not provide specific details about the recruitment and responsibilities for unit‐based dyad partnerships, which are critical factors for success on multidisciplinary inpatient care units.
There are several logistical matters to consider when instituting a unit‐based leadership model. These include the composition of the leadership team, selection process of the leaders, the presence of trainees and permanent faculty, and whether the units are able to geographically cohort patients. Other considerations include a clear role description with established shared goals and expectations, and a compensation model that includes effort and incentives. In addition, there should be a clearly established reporting structure to senior leadership, and the unit leaders should be given opportunities for professional growth and development. Table 1 provides a summary overview of 6 hospitals' experiences to date.
| Structure | Hospital of the University of Pennsylvania | Northwestern Memorial Hospital | Emory University Hospital | University of Michigan Health System | Christiana Care Health System | St. Joseph Mercy Health System/Integrated Health Associates |
|---|---|---|---|---|---|---|
| ||||||
| Description of hospital(s) | Academic medical center, 784 beds, 40,000 annual admissions | Academic medical center, 897 beds, 53,000 annual admissions | Academic medical center, 579 beds, 24,000 annual admissions | Academic medical center, 839 beds, 45,000 annual admissions | Independent academic medical center, 1,100 beds, 53,000 annual admissions | Tertiary community hospital that is part of a larger health care system (Trinity Health), 579 beds, 33,000 annual admissions |
| Unit leadership model | Triad of medical director, nurse manager, and quality improvement specialist/project manager | Dyad of medical director and nurse manager | Dyad of medical director and nurse manager | Dyad of medical director and nurse manager | Dyad of medical director and nurse manager | Dyad of medical director and nurse manager |
| Percent effort time supported for unit medical director | 10% | 17% | 10% | 20% | 20% | 10% |
| Incentives built into unit leaders' performance in outcomes metrics | No | Yes | No | No | No | Yes |
| Professional development/leadership training | Quality improvement method: PDSA, Six Sigma, Lean Healthcare | Quality improvement method: Six Sigma | Situational leadership training with 1:1 mentoring | Quality improvement method: Lean Healthcare, service excellence program | Quality Improvement method: Six Sigma, Lean Healthcare | Quality improvement method: Six Sigma |
| Additional leadership development through Penn Medicine Leadership Academy and Wharton Executive Education | Additional leadership development through Northwestern's professional development center and simulation training center | Conflict resolution skill development | Attend patient and Family Centered Care conference | Additional leadership development through Christiana Care Learning Institute | Attend educational course on Crucial Conversations | |
| Personality profile with coaching | Additional leadership development through University of Michigan Health System's human resources group | |||||
| Outcomes metrics monitored | Patient satisfaction | Patient satisfaction | Patient satisfaction | Patient satisfaction | Patient satisfaction | Patient satisfaction |
| Efficiency of multidisciplinary rounds | Teamwork climate (survey) | Teamwork and implementation of structured interdisciplinary bedside rounds | Multidisciplinary rounds | Interdisciplinary rounds | Participation in interdisciplinary rounds | |
| RNMD work environment surveys | Adverse events | Unit‐based patient safety culture survey | Patient‐centered, bedside rounds | Readmission rates | ||
| Hospital‐acquired conditions (CAUTI, CLABSI, VAP, DVT, pressure ulcers) | Hospital‐acquired conditions (fall rates, pressure ulcers | Hospital‐acquired conditions (CAUTI, CLABSI, fall rates, pressure ulcers) | Hospital‐acquired conditions (CAUTI) | Hospital‐acquired conditions (fall rates, pressure ulcers) | Core measures | |
| Readmission rates | Readmission rates | Mortality | Readmission rates | Readmission rates | Medication reconciliation | |
| Core measures, patient safety indicators | Core measures | Length of stay | DVT prophylaxis | Hand hygiene | Discharge by 11 am | |
| Mortality (observed to expected, transfer, inpatient) | Hand hygiene | Glycemic control | Meeting attendance | Length of stay | Use of patient teach‐back | |
| Medication reconciliation | Restraint use | Communication with PCPs | ||||
| Home care, hospice, post‐acute care referral rates | ||||||
| Organizational leadership structure support for clinical unit partnership program | CMO, CNO, vice president of quality/patient safety, directors of medical and surgical nursing | Associate chair of medicine, director of medicine nursing; all medical directors are members of the department of medicine quality management committee | CMO, CNO, CEO, CQO | CMO, CNO | All teams report to and are supported by 3 overarching, system‐wide committees: (1) safety first, (2) think of yourself as a patient, (3) clinical excellence. Those committees, in turn, report up to the senior management quality/safety coordinating council. | Director of hospitalist program (reports to CMO); nursing director of acute care (reports to CNO) |
DISCUSSION
In reviewing our 6 organization's collective experiences, we identified several common themes and some notable differences across sites. The core of the leadership team was primarily composed of the medical director and nurse manager on the unit. Across all 6 organizations, medical directors had a portion of their effort supported for their leadership work on the unit. Leadership development training was provided at all of our sites, with particular emphasis on quality improvement (QI) methods such as Six‐Sigma, Lean, or Plan, Do, Study, Act (PDSA). Additional leadership development sessions were provided through the organization's human resources or affiliated university. Common outcome measures of interest include patient satisfaction, interdisciplinary practice, and collaboration on the unit, and some hospital‐acquired condition measures. Last, there is a direct reporting relationship to a chief or senior nurse or physician leader within each organization. These commonalities and variances are further detailed below.
Establishing the Unit‐Based Leadership Model
The composition of the unit‐based leadership model in our 6 organizations is predominantly a dyad partnership of medical directors and nurse managers. Although informal physician‐nurse collaborative practices have likely been in existence at many hospitals, formalizing this dyad partnership is an important step to fostering collaborative efforts to improve quality of care. It is also essential for hospital leadership to clearly articulate the need for this unit‐based leadership model. Whether the motivation for change is from a previously untenable practice environment, or part of an ongoing improvement program, the model should be presented in a manner that supports the organization's commitment to improve collaborative practices for better patient care. One of our 6 hospitals initiated this leadership model based on troubling relationships between nurses and physicians on some of their inpatient care units, which threatened to stall the organization's Magnet application. Implementation of the leadership model at the unit level yielded improvements in nursephysician interactions, patient satisfaction, and staff turnover.[15, 23] Another of the hospitals first evaluated why a previous attempt at this model did not deliver the intended outcomes, and redesigned the model based on its analysis.[14]
Across all of the organizations featured here, a common driver behind the adoption of the unit‐based leadership model was to bridge the divide between physician services and nursing and other allied health providers. We found that many of the physicians routinely had patients on multiple units, limiting the quantity and quality of collaborative practices between unit‐based staff and physician teams. The unit‐based dyad leaders are ideally positioned to build and foster a culture of collaboration, and our organizations have been inclusive to ensure the participation of a multidisciplinary group of providers, including representatives from pharmacy, environmental services, physical therapy, respiratory therapy, social work, case management, and nutrition at leadership meetings or in daily patient‐care discussions. In addition, 2 of the organizations have added quality improvement specialist/project managers to their teams to support the physiciannurse manager leaders on the unit.
Selection Process and Professional Development
The traditional approach to hiring a physician leader or a nurse manager has been an isolated process of drafting a job description for each position and hiring within their respective departments. For the dyad partnership to be successful, there should be established goals and expectations that require shared responsibilities between the 2 partners, which should guide the selection of these leaders. Other leadership attributes and essential character traits that should be modeled by the unit‐based leaders include good communication skills, respect among coworkers, and a collaborative approach to decision making and action. In addition, both physician leaders and nurse managers in these roles should have the ability to take a system's view, recognizing that within the complex network of healthcare providers and processes on their unit, these elements interact with each other, which lead to the outcomes achieved on their units.[24, 25] Table 2 lists some general shared responsibilities, highlighting specific activities that can be used to achieve the established outcomes. As the unit's dyad leadership works together to address these shared responsibilities, they should keep their sights focused on the overall strategic goals of the healthcare organization. Bohmer has defined 4 habits of the high‐value healthcare organization that in turn can be reflected through the inpatient unit leadership model to capture these activities at the local level: (1) planning care for specific patient populations, (2) microsystem design, (3) measurement and oversight, and (4) self‐study.[26] In determining specific shared responsibilities for each dyad partner, it is important for these leaders to understand the clinical microsystem of their unit such as their patient population, interdisciplinary care team, approach to process improvement, and performance patterns over time.[27]
| General Shared Responsibilities of Physician and Nurse Unit Directors | Examples of Specific Activities |
|---|---|
| |
| Serve as management partners to enhance culture of the unit | Co‐craft and deliver consistent leadership message |
| Co‐establish and enforce unit processes and protocols | |
| Co‐lead recruitment and retention efforts | |
| Co‐orient trainees and faculty rotating through unit | |
| Co‐educate on the management of common medical and surgical conditions | |
| Facilitate interstaff conflict resolution sessions | |
| Regular leadership meetings | |
| Actively manage unit processes and outcomes | Quality: improve core quality measure performance |
| Safety: improve culture of patient safety within the unit as measured by surveys and incident reporting systems | |
| Efficiency: reduce unnecessary length of stay and variability in resource use | |
| Patient experience: focus on improving patient‐family experience with targeted outcomes in patient experience metrics (eg, HCAHPS) | |
| Education: develop trainee and staff clinical and teamwork competencies | |
| Continuous process improvement initiatives (eg, PDSA cycles) | Improve the discharge transitions process, tailoring the process to each individual patient's identified risk factors |
| Focus improvement efforts on reduction in specific hospital acquired conditions such as CAUTI, VTE, CLABSI, pressure ulcers, falls | |
| Measure, analyze, reassess, and improve in all described areas of shared responsibilities | |
| Perform unit level chart reviews to evaluate readmissions and LOS and identify improvement opportunities | |
In our collective experience, the dyad leaders bring passion and commitment to improving care; however, many (the medical directors in particular) have minimal prior formal training in leadership, quality improvement, or hospital management. Recognizing that unit leaders require specialized knowledge and skills, each of our organizations has enrolled unit medical directors and nurse managers in leadership development courses or educational programs. Many healthcare organizations have become more grounded in a QI methodology including Six‐Sigma, Lean Healthcare, PDSA, and other scientifically based methods, and the unit‐based leaders should receive advanced training in the preferred methods of their institution. Additional training in quality improvement, patient safety, and physician leadership can also be obtained through supplemental coursework specifically designed to train hospital leaders, with some programs leading to a certification or additional credentials.[28]
Beyond such formal educational opportunities, hospitals should not overlook the opportunity to learn from and share experiences with the other dyad leadership units within the hospital. One of the organizations described here holds monthly meetings with all of the unit dyad leaders, and 2 other organizations conduct quarterly meetings to share experiences and best practices related to specific improvement initiatives in a learning network model. Those units with more experience in specific initiatives are asked to share their lessons learned with others, as well as support each other in their efforts to collectively meet the strategic goals of the hospital.
Time and Organizational Support
In addition to leadership development, hospitals and the clinical department leadership need to support the medical directors with dedicated time away from their usual clinical duties. Some organizations in this report are providing up to 20% effort for the medical director's unit‐based leadership work; however, there is some variation in practice with regard to physician effort across sites. The University of Pennsylvania has a smaller effort support at 10%; however, some of that effort differential may be offset through the allocation of the quality improvement specialist/project manager assigned to work with the medical director and nurse manager dyad. St. Joseph Mercy Hospital also has a lower allocation, as there is additional financial compensation for the role that is at risk and not included in this 10% allocation.
It is also important to assure that the medical directors have institutional support to carry out their work in partnership with their nursing leadership. The 6 health systems described here report that although most of the physicians have appointments within a physician group or clinical department, there is hospital leadership oversight from a chief medical, nursing, or operating officer. This organizational structure may be an important aspect of the model as the unit‐based leaders seek to align their efforts with that of the hospital. Further, this form of organizational oversight can ensure that the unit leaders will receive timely and essential unit‐ and hospital‐based performance measures to manage local improvement efforts. These measures may include some components of patient experiences as reported in the Hospital Consumer Assessment of Healthcare Providers and Systems survey, readmission rates, hospital‐acquired condition rates, length of stay, observed to expected mortality rates, and results of staff satisfaction and safety culture surveys. As highlighted by several studies and commentaries, our collective experiences also identified interdisciplinary teamwork, collaboration, and communication as desirable outcome measures through the unit‐based leadership structure.[21, 22, 24, 29, 30] The medical director and nurse manager dyads can prioritize their improvement efforts based on the data provided to them, and mobilize the appropriate group of multidisciplinary practitioners and support staff on the unit.
OTHER CONSIDERATIONS
Other infrastructure variables that may increase the effectiveness of the unit leadership dyad include unit‐based clinical services (geographic localization), engaging the frontline team members in the design and implementation of change innovations, a commitment to patient and family centered practices on the unit, and enhancing clinical workflow through the support of EHR functions such as concurrent documentation and provider order entry. Geographic localization, placing the fewest possible clinical service providers on the unit to work alongside unit‐based staff, allows for a cohesive interdisciplinary unit‐based team to develop under the dyad leadership, and has been shown to improve communication practices.[9, 31] Beyond geographic localization of patients, it is critical to ensure team members are committed to the changes in workflow by directly involving them through the design and implementation of new models of care taking place on the unit. This commitment starts from the top senior nurse and physician leaders in the organization, and extends to the unit‐based dyad partners, and down to each individual interdisciplinary team member on the unit.[1] Thus, it is critical to clarify roles and responsibilities and how team members on the unit will interact with each other. For some situations, conflict management training will be helpful to the unit‐based leaders to resolve issues. To appreciate potential barriers to successful rollout of this unit leadership model, a phased implementation of pilot units, followed by successive waves, should be considered. Many of the units that instituted unit‐based interdisciplinary team rounds solicited and implemented direct feedback from frontline team members in efforts to improve communication and be more patient centered. Conversely, there are also likely to be situations where the unit‐based leaders will be confronted with hindrances to their unit‐based collaborative improvement efforts. To help prepare the dyad leaders, many of our unit‐based leaders have received specific training on how to coach and conduct difficult conversations with individuals who have performance gaps or are perceived to be hindering the progress of the unit's work. These crucial negotiation skills are not innate among most managers and should be explicitly provided to new leaders across organizations.
The goals and merits of patient‐ and family‐centered care (PFCC) have been well described.[32, 33, 34] Organizational support to teach and disseminate PFCC practices throughout all settings of care may help the leadership dyads implement rounding strategies that engage all staff, patients, and family members throughout the hospital course and during the transitions out of the hospital.
Clinical workflow has become heavily dependent on the EHR systems. For those organizations that have yet to adopt a particular EHR system, the leadership dyads should be involved throughout the EHR design process to help ensure that the technological solutions will be built to assist the clinical workflow, and once the system has been built, the leadership dyad should monitor and enhance the interface between workflow and EHR system so that it can support the creation and advancement of interdisciplinary plans of care on the unit.
CONCLUSION
The care of the hospitalized patient has become more complex over time. Interdisciplinary teamwork needs to be improved at the unit level to achieve the strategic goals of the hospital. Although quality improvement is an organizational goal, change takes place locally. Physician leaders, in partnership with nurse managers, are needed now more than ever to take on this task to improve the hospital‐care experience for patients by functioning as the primary effector arms for changing the landscape of hospital‐based care. We have described characteristics of unit‐based leadership programs adopted across 6 organizations. Hospitalists with clinical experience as the principal providers of inpatient‐based care and quality improvement experience and training, have been key participants in the development and implementation of the local leadership models in each of these hospital systems. We hope the comparison of the various models featured in this article serves as a valuable reference to hospitals and healthcare organizations who are contemplating the incorporation of this model into their strategic plan.
- , , , et al. Organizational predictors of coordination in inpatient medicine [published online ahead of print February 26, 2014]. Health Care Manage Rev. doi: 10.1097/HMR.0000000000000004.
- . Trends in case‐mix in the medicare population. Paper presented at: American Hospital Association, Federation of American Hospitals, Association of American Medical Colleges; http://www.aha.org/content/00‐10/100715‐CMItrends.pdf. July 15, 2010.
- . A requirement to reduce readmissions: take care of the patient, not just the disease. JAMA. 2013;309(4):394–396.
- , . Value‐based purchasing—national programs to move from volume to value. N Engl J Med. 2012;367(4):292–295.
- Medicare and Medicaid programs; electronic health record incentive program. Final rule. Fed Regist. 2010;75(144):44313–44588.
- . The Center for Medicare and Medicaid innovation's blueprint for rapid‐cycle evaluation of new care and payment models. Health Aff (Millwood). 2013;32(4):807–812.
- Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a medical teaching unit. J Gen Intern Med. 2010;25(8):826–832.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24(11):1223–1227.
- , , , . A review on systematic reviews of health information system studies. J Am Med Inform Assoc. 2010;17(6):637–645.
- , , , , . Patient whiteboards as a communication tool in the hospital setting: a survey of practices and recommendations. J Hosp Med. Apr 2010;5(4):234–239.
- , , . Development of a checklist for documenting team and collaborative behaviors during multidisciplinary bedside rounds. J Nurs Adm. 2013;43(5):280–285.
- , , , , . Assessment of teamwork during structured interdisciplinary rounds on medical units. J Hosp Med. 2012;7(9):679–683.
- , , , et al. Leadership at the front line: a clinical partnership model on general care inpatient units. Am J Med Qual. 2012;27(2):106–111.
- , . AHRQ health care innovations exchange: improvement projects led by unit‐based teams of nurse, physician, and quality leaders reduce infections, lower costs, improve patient satisfaction, and nurse‐physician communication. Available at: http://www.innovations.ahrq.gov/content.aspx?id=2719. Published April 14, 2010. Accessed November 26, 2011.
- , , , , , . Microsystems in health care: part 8. Developing people and improving work life: what front‐line staff told us. Jt Comm J Qual Saf. 2003;29(10):512–522.
- , , , et al. Microsystems in health care: part 5. How leaders are leading. Jt Comm J Qual Saf. 2003;29(6):297–308.
- , , . The academic dilemma of the inpatient unit director. Am J Psychiatry. 1989;146(1):73–76.
- , , , , . Improving and sustaining core measure performance through effective accountability of clinical microsystems in an academic medical center. Jt Comm J Qual Patient Saf. 2010;36(9):387–398.
- , , . Physician leadership and quality improvement in the acute child and adolescent psychiatric care setting. Child Adolesc Psychiatr Clin N Am. 2010;19(1):1–19; table of contents.
- , , , . Effect of a multidisciplinary intervention on communication and collaboration among physicians and nurses. Am J Crit Care. 2005;14(1):71–77.
- , . Nurse‐physician leadership: insights into interprofessional collaboration. J Nurs Adm. 2013;43(12):653–659.
- The Advisory Board. University of Pennsylvania Health System pilots unit clinical leadership model to spur quality gains. Nurs Exec Watch. 2008;9(2):4–6.
- , . Physicians as leaders in improving health care: a new series in Annals of Internal Medicine. Ann Intern Med. 1998;128(4):289–292.
- . Understanding medical systems. Ann Intern Med. 1998;128(4):293–298.
- . The four habits of high‐value health care organizations. N Engl J Med. 2011;365(22):2045–2047.
- , , , et al. Microsystems in health care: Part 1. Learning from high‐performing front‐line clinical units. Jt Comm J Qual Improv. 2002;28(9):472–493.
- , , , et al. The quality and safety educators academy: fulfilling an unmet need for faculty development. Am J Med Qual. 2014;29(1):5–12.
- , , , . Cooperation: the foundation of improvement. Ann Intern Med. 1998;128(12 pt 1):1004–1009.
- , , , , , . Ten principles of good interdisciplinary team work. Hum Resour Health 2013;11(1):19.
- , , , et al. Impact of localizing general medical teams to a single nursing unit. J Hosp Med. 2012;7(7):551–556.
- , , , . Integrating patient‐ and family‐centered care with health policy: four proposed policy approaches. Qual Manag Health Care. 2013;22(2):137–145.
- , , . Incorporating patient‐ and family‐centered care into resident education: approaches, benefits, and challenges. J Grad Med Educ. 2011;3(2):272–278.
- Smith M, Saunders R, Stuckhardt L, McGinnis JM, eds. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; 2012.
- , , , et al. Organizational predictors of coordination in inpatient medicine [published online ahead of print February 26, 2014]. Health Care Manage Rev. doi: 10.1097/HMR.0000000000000004.
- . Trends in case‐mix in the medicare population. Paper presented at: American Hospital Association, Federation of American Hospitals, Association of American Medical Colleges; http://www.aha.org/content/00‐10/100715‐CMItrends.pdf. July 15, 2010.
- . A requirement to reduce readmissions: take care of the patient, not just the disease. JAMA. 2013;309(4):394–396.
- , . Value‐based purchasing—national programs to move from volume to value. N Engl J Med. 2012;367(4):292–295.
- Medicare and Medicaid programs; electronic health record incentive program. Final rule. Fed Regist. 2010;75(144):44313–44588.
- . The Center for Medicare and Medicaid innovation's blueprint for rapid‐cycle evaluation of new care and payment models. Health Aff (Millwood). 2013;32(4):807–812.
- Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a medical teaching unit. J Gen Intern Med. 2010;25(8):826–832.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24(11):1223–1227.
- , , , . A review on systematic reviews of health information system studies. J Am Med Inform Assoc. 2010;17(6):637–645.
- , , , , . Patient whiteboards as a communication tool in the hospital setting: a survey of practices and recommendations. J Hosp Med. Apr 2010;5(4):234–239.
- , , . Development of a checklist for documenting team and collaborative behaviors during multidisciplinary bedside rounds. J Nurs Adm. 2013;43(5):280–285.
- , , , , . Assessment of teamwork during structured interdisciplinary rounds on medical units. J Hosp Med. 2012;7(9):679–683.
- , , , et al. Leadership at the front line: a clinical partnership model on general care inpatient units. Am J Med Qual. 2012;27(2):106–111.
- , . AHRQ health care innovations exchange: improvement projects led by unit‐based teams of nurse, physician, and quality leaders reduce infections, lower costs, improve patient satisfaction, and nurse‐physician communication. Available at: http://www.innovations.ahrq.gov/content.aspx?id=2719. Published April 14, 2010. Accessed November 26, 2011.
- , , , , , . Microsystems in health care: part 8. Developing people and improving work life: what front‐line staff told us. Jt Comm J Qual Saf. 2003;29(10):512–522.
- , , , et al. Microsystems in health care: part 5. How leaders are leading. Jt Comm J Qual Saf. 2003;29(6):297–308.
- , , . The academic dilemma of the inpatient unit director. Am J Psychiatry. 1989;146(1):73–76.
- , , , , . Improving and sustaining core measure performance through effective accountability of clinical microsystems in an academic medical center. Jt Comm J Qual Patient Saf. 2010;36(9):387–398.
- , , . Physician leadership and quality improvement in the acute child and adolescent psychiatric care setting. Child Adolesc Psychiatr Clin N Am. 2010;19(1):1–19; table of contents.
- , , , . Effect of a multidisciplinary intervention on communication and collaboration among physicians and nurses. Am J Crit Care. 2005;14(1):71–77.
- , . Nurse‐physician leadership: insights into interprofessional collaboration. J Nurs Adm. 2013;43(12):653–659.
- The Advisory Board. University of Pennsylvania Health System pilots unit clinical leadership model to spur quality gains. Nurs Exec Watch. 2008;9(2):4–6.
- , . Physicians as leaders in improving health care: a new series in Annals of Internal Medicine. Ann Intern Med. 1998;128(4):289–292.
- . Understanding medical systems. Ann Intern Med. 1998;128(4):293–298.
- . The four habits of high‐value health care organizations. N Engl J Med. 2011;365(22):2045–2047.
- , , , et al. Microsystems in health care: Part 1. Learning from high‐performing front‐line clinical units. Jt Comm J Qual Improv. 2002;28(9):472–493.
- , , , et al. The quality and safety educators academy: fulfilling an unmet need for faculty development. Am J Med Qual. 2014;29(1):5–12.
- , , , . Cooperation: the foundation of improvement. Ann Intern Med. 1998;128(12 pt 1):1004–1009.
- , , , , , . Ten principles of good interdisciplinary team work. Hum Resour Health 2013;11(1):19.
- , , , et al. Impact of localizing general medical teams to a single nursing unit. J Hosp Med. 2012;7(7):551–556.
- , , , . Integrating patient‐ and family‐centered care with health policy: four proposed policy approaches. Qual Manag Health Care. 2013;22(2):137–145.
- , , . Incorporating patient‐ and family‐centered care into resident education: approaches, benefits, and challenges. J Grad Med Educ. 2011;3(2):272–278.
- Smith M, Saunders R, Stuckhardt L, McGinnis JM, eds. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; 2012.
Sentinel Hospitalization
As hospitalists now care for expanding numbers of America's aging patients, many of whom have chronic, debilitating illnesses or are near the end of life, there is a burgeoning need for innovative approaches to optimize quality of care and control costs, especially in the last year of life.[1, 2] In the inaugural issue of the Journal of Hospital Medicine, an overview of how hospitalists and palliative care specialists can work hand‐in‐hand to care for these seriously ill, hospitalized patients was presented.[3, 4] This perspective highlighted a symbiotic and mutually beneficial relationship between the 2 specialties based on their shared values, missions, and complementary strengths.[3, 4] Since then, a number of collaborative ventures offering palliative care for seriously ill, hospitalized patients have been developed and examined in a variety of settings.[4, 5, 6]
A key collaborative undertaking for hospitalists and palliative care specialists is the appreciation of the unique trajectory of each chronic illness toward the end of life. For example, patients with cancer or neurodegenerative disease tend to have relatively stable functional status until the final months of rapid deterioration. On the other hand, the courses of patients with chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), end‐stage renal disease, and human immunodeficiency virus/acquired immunodeficiency syndrome tend to be punctuated by episodes of acute exacerbation with often nearly complete return to previous status. Finally, dementia usually follows a slow course of gradual decline leading to death.[7] Ideally, active management of symptoms and discussion of prognosis and goals of care should happen in the early stages of these chronic illnesses, yet most often they are left until an acute hospitalization late in the disease course. The following case illustrates the point.
CASE 1
Mrs. M is an 89‐year‐old woman with Alzheimer's dementia diagnosed 7 years ago who has been cared for at home by family members. She is admitted to the hospital for urinary tract infection and volume depletion. She is bedbound, cachectic, and has a stage III decubitus ulcer. Her daughter describes a 6‐month history of feeding problems, 20‐lb weight loss, and 2 recent hospitalizations for aspiration pneumonia. She improves somewhat with hydration and intravenous antibiotics, and the physical therapist recommends rehabilitation. Mrs. M does not have decision‐making capacity, and her long‐time family physician has not inquired about care preferences or goals. The hospitalist team meets with family members to discuss the trajectory and prognosis of advanced dementia, and recommends against artificial nutrition and hydration, and for initiation of palliative care service at a skilled nursing facility.
In this example, the hospitalist team recognizes the advent of frequent infections and diminished oral intake in advanced dementia as signals of increased morbidity and mortality warranting palliative care intervention.[8] This, we suggest, represents a sentinel hospitalization, a hospitalization in the disease course that heralds a need to reassess prognosis, treatment options and intensity, and goals of care. Hospitalists are well positioned to recognize such transition points in the disease course by considering the patient's recent history of illness, to offer an impartial overview of illness progression, and to optimize patient care using principles of palliative care. Additionally, hospitalists have advantages of geographic convenience, readily available consultants, systemic support, and a detachment from the longitudinal patient‐physician relationship, which may enable more accurate medical prognostication.[9]
There are many ways to identify a sentinel hospitalization. For example, hospitalists can use the surprise question, Would you be surprised if the patient died within 12 months? on admission for the majority of cancer and dialysis patients. The answer No predicts a 3.5‐ to 7‐fold increase in 1‐year mortality.[10, 11] In a powerful predictive model for 1‐year mortality using readily available clinical, laboratory, and functional characteristics, medical inpatients in the highest quartiles have 1‐year mortality exceeding 60%.[12] Recently, several more complicated prognostic models have been derived and validated in large cohorts of medical inpatients, which predict short‐term (30‐day) and long‐term (0.5‐1 year) mortality with great accuracy.[13, 14] There are also many disease‐specific prognostic features (eg, diagnosis of metastatic disease with poor performance status or high symptom burden, progression of chronic kidney disease with consideration of hemodialysis, additional stroke in multi‐infarct dementia, and frequent exacerbation of severe COPD or severe CHF).[15, 16, 17, 18, 19, 20, 21] Finally, frequent readmissions and prolonged hospital or intensive care unit stay can also be used.[17, 19] These criteria are summarized in Table 1 with time frames.
| Common Criteria | Time Frame | References |
|---|---|---|
| ||
| No to the surprise question: Would you be surprised if the patient died in 12 months? | 1 year | [10, 11] |
| Newly diagnosed metastatic solid cancer | Various | [17] |
| Metastatic solid cancer admitted for uncontrolled symptoms | Various | [17] |
| Progressive CKD with consideration for hemodialysis | 1 year | [17, 18] |
| GOLD stage IV COPD with frequent exacerbation | Various | [20] |
| NYHAstage IV CHF with frequent exacerbation | 12 years | [21] |
| Advanced dementia with frequent UTI, aspiration PNA, and feeding problem | 12 years | [8] |
| Overall prognosis of high mortality using available indices | 30 days1 year | [12, 13, 14] |
| More than 3 admissions in last 6 months | 6 months | [17, 19] |
| Prolonged ICU stay (>7 days) | Weeks | [17, 19] |
Once a sentinel hospitalization is identified, hospitalists, with input from the patient's primary care physician and subspecialists, can then develop a comprehensive strategy to evaluate current disease management, to educate patient and family accordingly, and to actively integrate palliative care services as appropriate. The next challenge facing the care team is how to deliver the necessary palliative care since it is unnecessary and improper to ask for palliative care specialist consultation for every sentinel hospitalization. We believe that the best approach is for hospitalists to be the primary deliverers of basic palliative care in the hospital while consulting palliative care specialists for refractory symptoms and complex scenarios.[22] According to this generalist‐specialist palliative care model, physicians of all specialties should define and master a basic palliative care skill set for their patients. For hospitalists, the relevant skill set includes assessing and treating pain and other symptoms such as dyspnea, nausea and vomiting, and constipation, estimating prognosis, and initiating goals of care discussions.[22] The following case illustrates this point.
CASE 2
Ms S, a 21‐year‐old Hispanic woman with advanced, recurrent head and neck cancer, status post multiple surgeries, chemotherapy, and radiation therapy, is admitted to the hospitalist service for aspiration pneumonia, which responds to antibiotics rapidly. However, her cancer‐related somatic and neuropathic pain soon becomes refractory to opioids prescribed by the hospitalist team. She also develops significant dyspnea, xerostomia, depression, anxiety, and existential suffering. With the help of the interdisciplinary palliative care team, her pain is relieved by a patient‐controlled analgesia pump and methadone. A palliative care social worker and chaplain visit her and her family daily to address their distress. Eventually, the care team is able to provide a stable medical regimen for symptom control and to use it across the entire care continuum.
In this example, the hospitalist team, with the support of palliative care specialists, provided basic palliative care and longitudinal integration of palliative practices into the patient's overall treatment scheme. Hospitalists, given their scope of practice and sheer volume of patients, are well positioned to rapidly gain competencies in symptom management, empathic communication, and interdisciplinary teamwork.[23, 24] Hospitalists may benefit from innovative and collaborative palliative care education using interactive online modules, case simulation, communication workshops, and observed evaluation and feedback.[25] Several modes of collaboration between hospital medicine and palliative care have been developed including implementation of palliative care consult triggers on admission, palliative care participation in hospitalist interdisciplinary rounds, and disease specific, integrated management programs.[17, 26] These collaborations are particularly important, as the quality of inpatient care at the end of life is still suboptimal and more appropriate use of palliative care will be beneficial.[27] Recently, some hospitals have developed specialized inpatient palliative care units, combining intensive palliation with inpatient medical surgical level of care, as well as providing hospice care. Staffed by palliative care specialists or hospitalists, they provide efficient, cost‐saving care to patients with advanced chronic illness or terminal disease in need of intensive symptom management.[28] Finally, there is mounting evidence supporting the clinical effectiveness of palliative care in diverse specialties such as oncology, pulmonary and critical care, and nephrology.[29] For example, in the setting of metastatic non‐small cell lung cancer, early initiation of palliative care has been shown to improve symptom control and quality of life, reduce chemotherapy use at the end of life, and interestingly, prolong median survival by almost 3 months.[30] This has led to a position statement from American Society of Clinical Oncology encouraging early integration of palliative care into standard oncologic care for advanced disease.[31]
Recognizing a sentinel hospitalization allows palliative care to be integrated at transitions of care and carried forward. For patients with chronic debilitating illnesses who are approaching the end of life, appropriate care transitions will ensure that their short‐ and long‐term care matches their goals of care, assure timely clinical follow‐ups, and help reduce hospital readmission and healthcare resource utilization.[32] Importantly, timely and compassionate communication is a key to the success of both hospital medicine and palliative care. Many patients with life‐limiting diseases prefer to receive prognostic information and to discuss goals of care.[33] How this information is integrated and communicated through the care continuum is crucial, especially in the era of duty hour limits and frequent handoffs. The information exchange needs to facilitate active participation of primary care physicians who may not be involved in hospital care. Some of the innovative strategies for communication and transfer of palliative care information, such as prognosis, goals of care, family meeting consensus, and symptom control interventions, include a palliative care checklist in the electronic health record, incorporation of prognostic and family meeting information in the discharge summary, and links to the national Physician Orders for Life‐Sustaining Treatment advanced care planning program.[34] Of note, a pilot program in the United Kingdom adopting an electronic palliative care summary has reduced after‐hour emergency room visits and hospital readmissions.[35] The following case illustrates this point.
CASE 3
Mrs. K, an 82‐year‐old Russian‐speaking woman with newly diagnosed metastatic pancreatic cancer, is admitted for worsening obstructive jaundice and a second opinion about treatment. A biliary stent is placed and her jaundice slowly improves. The patient and family have requested chemotherapy. However, the oncologist determines that she would only qualify for a phase I trial given her poor performance status. The hospitalist team requests the help of the palliative care consult team to manage her severe pain, depression, and to provide support to the family. After several family meetings, the patient and family choose not to pursue chemotherapy. Given the lack of adequate support at home, she is discharged to a skilled nursing facility for short‐term rehabilitation with plans to transition to the in‐house hospice program. The hospitalist, palliative care attending physician, and the medical director of the rehabilitation facility have a 3‐way phone conference to confirm the plans of care and to ensure a smooth care transition.
In this case, the hospitalist team recognizes that this is a sentinel hospitalization for Mrs. K that requires extensive palliative care intervention. Often, transitioning to skilled nursing facilities (SNF) is the default pathway for patients needing hospice/palliative care, especially when patients and families are not yet ready to discuss prognosis realistically or to accept hospice, or there is not enough support available at home. A recent large cohort study showed that 30% of patients in their last 6‐month of life had used, and nearly 10% of such patients had died, under Medicare's posthospitalization SNF benefit.[36] Although the worsening disease trajectory may not be apparent at hospital discharge, it is more likely that the financial and practical limitation of the Medicare Hospice Benefit accounts for this observation, which includes limited home health aid hours, lack of coverage for room and board, and lower payments to SNFs.[36] Hospitalists can help address the issue of discharge location for patients needing palliative care. Sometimes this requires extensive communication before and after discharge to help enhance the transition from a rehabilitation facility to hospice/palliative care. Appropriately integrated palliative care at the time of care transitions, in the form of hospice or longitudinal home‐based palliative care rather than just routine clinic follow‐up, has the potential to reduce 30‐day readmission for chronically ill, elderly patients and for patients near the end of life.[37, 38] It is critical that national policy, suitable reimbursement, and financial incentives support this practice. A demonstration project, Better Outcomes by Optimizing Safe Transitions (BOOST), organized by the Society of Hospital Medicine, integrates palliative care evaluation into a comprehensive discharge assessment tool. This intervention has been shown to reduce readmissions to acute care hospitals.[39]
In this article, we define a sentinel hospitalization and suggest that its recognition provides an important opportunity for hospitalists to actively integrate palliative care into patients' chronic disease management programs, with inputs from patients, their families, their primary physicians and subspecialists, as well as palliative care specialists. We also recognize that within nonsentinel hospitalizations, there are important opportunities to discuss prognosis, goals of care, and advanced care planning. This approach allows the fresh eyes of hospitalists to assess the patient's current health status and prognosis, to communicate these relevant clinical issues with the patient and family, and to encourage discussions about goals of care and advanced care planning during the sentinel hospitalization. It also provides a structured vehicle for soliciting the patient's (and family's) perspectives and documenting them in the medical record. A compilation of sample items to guide discussion can be found in Table 2. Hospitalists, equipped with basic palliative care skills and supported by hospital‐ and community‐based palliative care teams, can thrive in this unique position of optimizing the quality of care for these patients.[40] Almost 20 years ago, the field of palliative care rose to national prominence on the findings of the SUPPORT (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments) study, which investigated the suboptimal end‐of‐life experiences of hospitalized adult patients.[41] Since then, the fields of both hospital medicine and palliative care have grown, yet the best is still to come for their collaborative excellence, mutual education, and shared care innovation at the forefront of medicine.
| Patient/family understanding of disease process and treatment outcomes |
| Patient/family understanding of disease prognosis |
| Availability of alternative treatment options including palliative/hospice care |
| Patient/family wishes/goals of care |
| Advanced‐care planning including limitations of care |
| Inventory of symptoms (frequency, severity, modifying factors, timing, and treatments) |
| Social and financial stress |
| Emotional and existential stress |
| Social support system and caregivers |
| Living arrangements |
Disclosure: Nothing to report.
- , , , , , , et al. Evolving practice of hospital medicine and its impact on hospital throughput and efficiencies. J Hosp Med. 2012;7:649–654.
- , , , . Medicare beneficiaries' costs of care in the last year of life. Health Affairs. 2001;20:188–195.
- . Palliative care in hospitals. J Hosp Med. 2006;1:21–28.
- . Palliative care and hospitalists: a partnership for hope. J Hosp Med. 2006;1:5–6.
- , . Palliative care and the hospitalist: an opportunity for cross‐fertilization. Am J Med. 2001;111:10s–14s.
- , . Palliative care. Ann Intern Med. 2012;156:ITC2‐1, TC2‐2–15; quiz TC2‐16.
- , , , , . Patterns of functional decline at the end of life. JAMA. 2003;289:2387–2392.
- , , , et al. The clinical course of advanced dementia. N Engl J Med. 2009;361:1529–1538.
- , . Extent and determinants of error in doctor's prognoses in terminally ill patients: prospective cohort study. BMJ. 2000;320:469–472.
- , , , et al. Prognostic significance of the “surprise” question in cancer patients. J Palliat Med. 2010;13:837–840.
- , , , et al. Utility of the “surprise” question to identify dialysis patients with high mortality. Clin J Am Soc Nephrol. 2008;3:1379–1384.
- , , , et al. Development and validation of a prognostic index for 1‐year mortality in older adults after hospitalization. JAMA. 2001;285:2987–2994.
- , , , , . Mortality predictions on admission as a context for organizing care activities. J Hosp Med. 2013,8:229–235.
- , , , , . Caring about prognosis: a validation study of the CARING criteria to identify hospitalized patients at high risk for death at 1 year. J Hosp Med. 2013,8:696–701.
- , , , , , , et al. Meta‐analysis of survival prediction with palliative performance scale. J Palliat Care. 2007;23:245–254.
- , , , , . Prognostic indices for older adults: a systemic review. JAMA. 2012;307:182–192.
- , . Identifying patients in need of a palliative care assessment in the hospital setting. J Palliat Med. 2011;14:17–23.
- , , , , , . Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361:1539–1547.
- , , . Top 10 things palliative care clinicians wished everyone knew about palliative care. Mayo Clin Proc. 2013;88:859–865.
- . Palliative and end‐of‐life care for patients with severe COPD. Eur Respir J. 2008;32:796–803.
- . Palliative care in congestive heart failure. J Am Coll Cardiol. 2009;54:386–396.
- , . Generalist plus specialist palliative care—creating a more sustainable model. N Engl J Med. 2013;368:1173–1175.
- , , , , , . Common myths about caring for patients with terminal illness: opportunities to improve care in the hospital setting. J Hosp Med. 2007;2:357–365.
- , , , . Opportunity lost: end‐of‐life discussions in cancer patients who die in the hospital. J Hosp Med. 2013;8:334–340.
- , , . Palliative medicine physician education in the United States: a historical review. J Palliat Med. 2013;16:230–236.
- , . Hospitalization as an opportunity to integrate palliative care in heart failure management. Curr Opin Support Palliat Care. 2009;3:247–251.
- , , , et al. The quality of care provided to hospitalized patients at the end of life. Arch Intern Med. 2010;170:1057–1063.
- , , , , , . Outcomes of the acute palliative care unit in an academic medical center [published online ahead of print May 10, 2013]. Am J Hosp Palliat Care. doi: 10.1177/1049909113489164.
- , , , , , . Update in hospital palliative care. J Hosp Med. 2013;12:715–720.
- , , , et al. Early palliative care for patients with metastatic non‐small‐cell lung cancer. N Engl J Med. 2010;363:733–742.
- , , , . Early integration of palliative care services with standard oncology care for patients with advanced cancer. CA Cancer J Clin. 2013;63:349–363.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150:178–187.
- , , , , , . “Knowing is better”: preferences of diverse older adults for discussion prognosis. J Gen Intern Med. 2012;27:568–575.
- , , . POLST, an improvement over traditional advanced directives. Cleve Clin J Med. 2012;79:457–464.
- , , , . Use of a structured palliative care summary in patients with established cancer is associated with reduced hospital admissions by out‐of‐hours general practitioners in Grampian [published online ahead of print January 3, 2013]. BMJ Support Palliat Care. doi:10.1136/bmjspcare‐2012‐000371.
- , , , , , . Use of the Medicare posthospitalization skilled nursing benefit in the last 6 months of life. Arch Intern Med. 2012;172:1573–1579.
- , , , et al. Increased satisfaction with care and lower costs: results of a randomized trial of in‐home palliative care. J Am Geriatr Soc. 2007;55:993–1000.
- , , . 30‐day readmissions among seriously ill older adults. J Palliat Med. 2012;15:1–6.
- , , , et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8:421–427.
- . Ensuring safe, quality care for hospitalized people with advanced illness, a core obligation for hospitalists. J Hosp Med. 2007;2:355–356.
- The SUPPORT Principal Investigators. A controlled trial to improve care for seriously ill hospitalized patients: the Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments (SUPPORT). JAMA. 1995;274:1591–1598.
As hospitalists now care for expanding numbers of America's aging patients, many of whom have chronic, debilitating illnesses or are near the end of life, there is a burgeoning need for innovative approaches to optimize quality of care and control costs, especially in the last year of life.[1, 2] In the inaugural issue of the Journal of Hospital Medicine, an overview of how hospitalists and palliative care specialists can work hand‐in‐hand to care for these seriously ill, hospitalized patients was presented.[3, 4] This perspective highlighted a symbiotic and mutually beneficial relationship between the 2 specialties based on their shared values, missions, and complementary strengths.[3, 4] Since then, a number of collaborative ventures offering palliative care for seriously ill, hospitalized patients have been developed and examined in a variety of settings.[4, 5, 6]
A key collaborative undertaking for hospitalists and palliative care specialists is the appreciation of the unique trajectory of each chronic illness toward the end of life. For example, patients with cancer or neurodegenerative disease tend to have relatively stable functional status until the final months of rapid deterioration. On the other hand, the courses of patients with chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), end‐stage renal disease, and human immunodeficiency virus/acquired immunodeficiency syndrome tend to be punctuated by episodes of acute exacerbation with often nearly complete return to previous status. Finally, dementia usually follows a slow course of gradual decline leading to death.[7] Ideally, active management of symptoms and discussion of prognosis and goals of care should happen in the early stages of these chronic illnesses, yet most often they are left until an acute hospitalization late in the disease course. The following case illustrates the point.
CASE 1
Mrs. M is an 89‐year‐old woman with Alzheimer's dementia diagnosed 7 years ago who has been cared for at home by family members. She is admitted to the hospital for urinary tract infection and volume depletion. She is bedbound, cachectic, and has a stage III decubitus ulcer. Her daughter describes a 6‐month history of feeding problems, 20‐lb weight loss, and 2 recent hospitalizations for aspiration pneumonia. She improves somewhat with hydration and intravenous antibiotics, and the physical therapist recommends rehabilitation. Mrs. M does not have decision‐making capacity, and her long‐time family physician has not inquired about care preferences or goals. The hospitalist team meets with family members to discuss the trajectory and prognosis of advanced dementia, and recommends against artificial nutrition and hydration, and for initiation of palliative care service at a skilled nursing facility.
In this example, the hospitalist team recognizes the advent of frequent infections and diminished oral intake in advanced dementia as signals of increased morbidity and mortality warranting palliative care intervention.[8] This, we suggest, represents a sentinel hospitalization, a hospitalization in the disease course that heralds a need to reassess prognosis, treatment options and intensity, and goals of care. Hospitalists are well positioned to recognize such transition points in the disease course by considering the patient's recent history of illness, to offer an impartial overview of illness progression, and to optimize patient care using principles of palliative care. Additionally, hospitalists have advantages of geographic convenience, readily available consultants, systemic support, and a detachment from the longitudinal patient‐physician relationship, which may enable more accurate medical prognostication.[9]
There are many ways to identify a sentinel hospitalization. For example, hospitalists can use the surprise question, Would you be surprised if the patient died within 12 months? on admission for the majority of cancer and dialysis patients. The answer No predicts a 3.5‐ to 7‐fold increase in 1‐year mortality.[10, 11] In a powerful predictive model for 1‐year mortality using readily available clinical, laboratory, and functional characteristics, medical inpatients in the highest quartiles have 1‐year mortality exceeding 60%.[12] Recently, several more complicated prognostic models have been derived and validated in large cohorts of medical inpatients, which predict short‐term (30‐day) and long‐term (0.5‐1 year) mortality with great accuracy.[13, 14] There are also many disease‐specific prognostic features (eg, diagnosis of metastatic disease with poor performance status or high symptom burden, progression of chronic kidney disease with consideration of hemodialysis, additional stroke in multi‐infarct dementia, and frequent exacerbation of severe COPD or severe CHF).[15, 16, 17, 18, 19, 20, 21] Finally, frequent readmissions and prolonged hospital or intensive care unit stay can also be used.[17, 19] These criteria are summarized in Table 1 with time frames.
| Common Criteria | Time Frame | References |
|---|---|---|
| ||
| No to the surprise question: Would you be surprised if the patient died in 12 months? | 1 year | [10, 11] |
| Newly diagnosed metastatic solid cancer | Various | [17] |
| Metastatic solid cancer admitted for uncontrolled symptoms | Various | [17] |
| Progressive CKD with consideration for hemodialysis | 1 year | [17, 18] |
| GOLD stage IV COPD with frequent exacerbation | Various | [20] |
| NYHAstage IV CHF with frequent exacerbation | 12 years | [21] |
| Advanced dementia with frequent UTI, aspiration PNA, and feeding problem | 12 years | [8] |
| Overall prognosis of high mortality using available indices | 30 days1 year | [12, 13, 14] |
| More than 3 admissions in last 6 months | 6 months | [17, 19] |
| Prolonged ICU stay (>7 days) | Weeks | [17, 19] |
Once a sentinel hospitalization is identified, hospitalists, with input from the patient's primary care physician and subspecialists, can then develop a comprehensive strategy to evaluate current disease management, to educate patient and family accordingly, and to actively integrate palliative care services as appropriate. The next challenge facing the care team is how to deliver the necessary palliative care since it is unnecessary and improper to ask for palliative care specialist consultation for every sentinel hospitalization. We believe that the best approach is for hospitalists to be the primary deliverers of basic palliative care in the hospital while consulting palliative care specialists for refractory symptoms and complex scenarios.[22] According to this generalist‐specialist palliative care model, physicians of all specialties should define and master a basic palliative care skill set for their patients. For hospitalists, the relevant skill set includes assessing and treating pain and other symptoms such as dyspnea, nausea and vomiting, and constipation, estimating prognosis, and initiating goals of care discussions.[22] The following case illustrates this point.
CASE 2
Ms S, a 21‐year‐old Hispanic woman with advanced, recurrent head and neck cancer, status post multiple surgeries, chemotherapy, and radiation therapy, is admitted to the hospitalist service for aspiration pneumonia, which responds to antibiotics rapidly. However, her cancer‐related somatic and neuropathic pain soon becomes refractory to opioids prescribed by the hospitalist team. She also develops significant dyspnea, xerostomia, depression, anxiety, and existential suffering. With the help of the interdisciplinary palliative care team, her pain is relieved by a patient‐controlled analgesia pump and methadone. A palliative care social worker and chaplain visit her and her family daily to address their distress. Eventually, the care team is able to provide a stable medical regimen for symptom control and to use it across the entire care continuum.
In this example, the hospitalist team, with the support of palliative care specialists, provided basic palliative care and longitudinal integration of palliative practices into the patient's overall treatment scheme. Hospitalists, given their scope of practice and sheer volume of patients, are well positioned to rapidly gain competencies in symptom management, empathic communication, and interdisciplinary teamwork.[23, 24] Hospitalists may benefit from innovative and collaborative palliative care education using interactive online modules, case simulation, communication workshops, and observed evaluation and feedback.[25] Several modes of collaboration between hospital medicine and palliative care have been developed including implementation of palliative care consult triggers on admission, palliative care participation in hospitalist interdisciplinary rounds, and disease specific, integrated management programs.[17, 26] These collaborations are particularly important, as the quality of inpatient care at the end of life is still suboptimal and more appropriate use of palliative care will be beneficial.[27] Recently, some hospitals have developed specialized inpatient palliative care units, combining intensive palliation with inpatient medical surgical level of care, as well as providing hospice care. Staffed by palliative care specialists or hospitalists, they provide efficient, cost‐saving care to patients with advanced chronic illness or terminal disease in need of intensive symptom management.[28] Finally, there is mounting evidence supporting the clinical effectiveness of palliative care in diverse specialties such as oncology, pulmonary and critical care, and nephrology.[29] For example, in the setting of metastatic non‐small cell lung cancer, early initiation of palliative care has been shown to improve symptom control and quality of life, reduce chemotherapy use at the end of life, and interestingly, prolong median survival by almost 3 months.[30] This has led to a position statement from American Society of Clinical Oncology encouraging early integration of palliative care into standard oncologic care for advanced disease.[31]
Recognizing a sentinel hospitalization allows palliative care to be integrated at transitions of care and carried forward. For patients with chronic debilitating illnesses who are approaching the end of life, appropriate care transitions will ensure that their short‐ and long‐term care matches their goals of care, assure timely clinical follow‐ups, and help reduce hospital readmission and healthcare resource utilization.[32] Importantly, timely and compassionate communication is a key to the success of both hospital medicine and palliative care. Many patients with life‐limiting diseases prefer to receive prognostic information and to discuss goals of care.[33] How this information is integrated and communicated through the care continuum is crucial, especially in the era of duty hour limits and frequent handoffs. The information exchange needs to facilitate active participation of primary care physicians who may not be involved in hospital care. Some of the innovative strategies for communication and transfer of palliative care information, such as prognosis, goals of care, family meeting consensus, and symptom control interventions, include a palliative care checklist in the electronic health record, incorporation of prognostic and family meeting information in the discharge summary, and links to the national Physician Orders for Life‐Sustaining Treatment advanced care planning program.[34] Of note, a pilot program in the United Kingdom adopting an electronic palliative care summary has reduced after‐hour emergency room visits and hospital readmissions.[35] The following case illustrates this point.
CASE 3
Mrs. K, an 82‐year‐old Russian‐speaking woman with newly diagnosed metastatic pancreatic cancer, is admitted for worsening obstructive jaundice and a second opinion about treatment. A biliary stent is placed and her jaundice slowly improves. The patient and family have requested chemotherapy. However, the oncologist determines that she would only qualify for a phase I trial given her poor performance status. The hospitalist team requests the help of the palliative care consult team to manage her severe pain, depression, and to provide support to the family. After several family meetings, the patient and family choose not to pursue chemotherapy. Given the lack of adequate support at home, she is discharged to a skilled nursing facility for short‐term rehabilitation with plans to transition to the in‐house hospice program. The hospitalist, palliative care attending physician, and the medical director of the rehabilitation facility have a 3‐way phone conference to confirm the plans of care and to ensure a smooth care transition.
In this case, the hospitalist team recognizes that this is a sentinel hospitalization for Mrs. K that requires extensive palliative care intervention. Often, transitioning to skilled nursing facilities (SNF) is the default pathway for patients needing hospice/palliative care, especially when patients and families are not yet ready to discuss prognosis realistically or to accept hospice, or there is not enough support available at home. A recent large cohort study showed that 30% of patients in their last 6‐month of life had used, and nearly 10% of such patients had died, under Medicare's posthospitalization SNF benefit.[36] Although the worsening disease trajectory may not be apparent at hospital discharge, it is more likely that the financial and practical limitation of the Medicare Hospice Benefit accounts for this observation, which includes limited home health aid hours, lack of coverage for room and board, and lower payments to SNFs.[36] Hospitalists can help address the issue of discharge location for patients needing palliative care. Sometimes this requires extensive communication before and after discharge to help enhance the transition from a rehabilitation facility to hospice/palliative care. Appropriately integrated palliative care at the time of care transitions, in the form of hospice or longitudinal home‐based palliative care rather than just routine clinic follow‐up, has the potential to reduce 30‐day readmission for chronically ill, elderly patients and for patients near the end of life.[37, 38] It is critical that national policy, suitable reimbursement, and financial incentives support this practice. A demonstration project, Better Outcomes by Optimizing Safe Transitions (BOOST), organized by the Society of Hospital Medicine, integrates palliative care evaluation into a comprehensive discharge assessment tool. This intervention has been shown to reduce readmissions to acute care hospitals.[39]
In this article, we define a sentinel hospitalization and suggest that its recognition provides an important opportunity for hospitalists to actively integrate palliative care into patients' chronic disease management programs, with inputs from patients, their families, their primary physicians and subspecialists, as well as palliative care specialists. We also recognize that within nonsentinel hospitalizations, there are important opportunities to discuss prognosis, goals of care, and advanced care planning. This approach allows the fresh eyes of hospitalists to assess the patient's current health status and prognosis, to communicate these relevant clinical issues with the patient and family, and to encourage discussions about goals of care and advanced care planning during the sentinel hospitalization. It also provides a structured vehicle for soliciting the patient's (and family's) perspectives and documenting them in the medical record. A compilation of sample items to guide discussion can be found in Table 2. Hospitalists, equipped with basic palliative care skills and supported by hospital‐ and community‐based palliative care teams, can thrive in this unique position of optimizing the quality of care for these patients.[40] Almost 20 years ago, the field of palliative care rose to national prominence on the findings of the SUPPORT (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments) study, which investigated the suboptimal end‐of‐life experiences of hospitalized adult patients.[41] Since then, the fields of both hospital medicine and palliative care have grown, yet the best is still to come for their collaborative excellence, mutual education, and shared care innovation at the forefront of medicine.
| Patient/family understanding of disease process and treatment outcomes |
| Patient/family understanding of disease prognosis |
| Availability of alternative treatment options including palliative/hospice care |
| Patient/family wishes/goals of care |
| Advanced‐care planning including limitations of care |
| Inventory of symptoms (frequency, severity, modifying factors, timing, and treatments) |
| Social and financial stress |
| Emotional and existential stress |
| Social support system and caregivers |
| Living arrangements |
Disclosure: Nothing to report.
As hospitalists now care for expanding numbers of America's aging patients, many of whom have chronic, debilitating illnesses or are near the end of life, there is a burgeoning need for innovative approaches to optimize quality of care and control costs, especially in the last year of life.[1, 2] In the inaugural issue of the Journal of Hospital Medicine, an overview of how hospitalists and palliative care specialists can work hand‐in‐hand to care for these seriously ill, hospitalized patients was presented.[3, 4] This perspective highlighted a symbiotic and mutually beneficial relationship between the 2 specialties based on their shared values, missions, and complementary strengths.[3, 4] Since then, a number of collaborative ventures offering palliative care for seriously ill, hospitalized patients have been developed and examined in a variety of settings.[4, 5, 6]
A key collaborative undertaking for hospitalists and palliative care specialists is the appreciation of the unique trajectory of each chronic illness toward the end of life. For example, patients with cancer or neurodegenerative disease tend to have relatively stable functional status until the final months of rapid deterioration. On the other hand, the courses of patients with chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), end‐stage renal disease, and human immunodeficiency virus/acquired immunodeficiency syndrome tend to be punctuated by episodes of acute exacerbation with often nearly complete return to previous status. Finally, dementia usually follows a slow course of gradual decline leading to death.[7] Ideally, active management of symptoms and discussion of prognosis and goals of care should happen in the early stages of these chronic illnesses, yet most often they are left until an acute hospitalization late in the disease course. The following case illustrates the point.
CASE 1
Mrs. M is an 89‐year‐old woman with Alzheimer's dementia diagnosed 7 years ago who has been cared for at home by family members. She is admitted to the hospital for urinary tract infection and volume depletion. She is bedbound, cachectic, and has a stage III decubitus ulcer. Her daughter describes a 6‐month history of feeding problems, 20‐lb weight loss, and 2 recent hospitalizations for aspiration pneumonia. She improves somewhat with hydration and intravenous antibiotics, and the physical therapist recommends rehabilitation. Mrs. M does not have decision‐making capacity, and her long‐time family physician has not inquired about care preferences or goals. The hospitalist team meets with family members to discuss the trajectory and prognosis of advanced dementia, and recommends against artificial nutrition and hydration, and for initiation of palliative care service at a skilled nursing facility.
In this example, the hospitalist team recognizes the advent of frequent infections and diminished oral intake in advanced dementia as signals of increased morbidity and mortality warranting palliative care intervention.[8] This, we suggest, represents a sentinel hospitalization, a hospitalization in the disease course that heralds a need to reassess prognosis, treatment options and intensity, and goals of care. Hospitalists are well positioned to recognize such transition points in the disease course by considering the patient's recent history of illness, to offer an impartial overview of illness progression, and to optimize patient care using principles of palliative care. Additionally, hospitalists have advantages of geographic convenience, readily available consultants, systemic support, and a detachment from the longitudinal patient‐physician relationship, which may enable more accurate medical prognostication.[9]
There are many ways to identify a sentinel hospitalization. For example, hospitalists can use the surprise question, Would you be surprised if the patient died within 12 months? on admission for the majority of cancer and dialysis patients. The answer No predicts a 3.5‐ to 7‐fold increase in 1‐year mortality.[10, 11] In a powerful predictive model for 1‐year mortality using readily available clinical, laboratory, and functional characteristics, medical inpatients in the highest quartiles have 1‐year mortality exceeding 60%.[12] Recently, several more complicated prognostic models have been derived and validated in large cohorts of medical inpatients, which predict short‐term (30‐day) and long‐term (0.5‐1 year) mortality with great accuracy.[13, 14] There are also many disease‐specific prognostic features (eg, diagnosis of metastatic disease with poor performance status or high symptom burden, progression of chronic kidney disease with consideration of hemodialysis, additional stroke in multi‐infarct dementia, and frequent exacerbation of severe COPD or severe CHF).[15, 16, 17, 18, 19, 20, 21] Finally, frequent readmissions and prolonged hospital or intensive care unit stay can also be used.[17, 19] These criteria are summarized in Table 1 with time frames.
| Common Criteria | Time Frame | References |
|---|---|---|
| ||
| No to the surprise question: Would you be surprised if the patient died in 12 months? | 1 year | [10, 11] |
| Newly diagnosed metastatic solid cancer | Various | [17] |
| Metastatic solid cancer admitted for uncontrolled symptoms | Various | [17] |
| Progressive CKD with consideration for hemodialysis | 1 year | [17, 18] |
| GOLD stage IV COPD with frequent exacerbation | Various | [20] |
| NYHAstage IV CHF with frequent exacerbation | 12 years | [21] |
| Advanced dementia with frequent UTI, aspiration PNA, and feeding problem | 12 years | [8] |
| Overall prognosis of high mortality using available indices | 30 days1 year | [12, 13, 14] |
| More than 3 admissions in last 6 months | 6 months | [17, 19] |
| Prolonged ICU stay (>7 days) | Weeks | [17, 19] |
Once a sentinel hospitalization is identified, hospitalists, with input from the patient's primary care physician and subspecialists, can then develop a comprehensive strategy to evaluate current disease management, to educate patient and family accordingly, and to actively integrate palliative care services as appropriate. The next challenge facing the care team is how to deliver the necessary palliative care since it is unnecessary and improper to ask for palliative care specialist consultation for every sentinel hospitalization. We believe that the best approach is for hospitalists to be the primary deliverers of basic palliative care in the hospital while consulting palliative care specialists for refractory symptoms and complex scenarios.[22] According to this generalist‐specialist palliative care model, physicians of all specialties should define and master a basic palliative care skill set for their patients. For hospitalists, the relevant skill set includes assessing and treating pain and other symptoms such as dyspnea, nausea and vomiting, and constipation, estimating prognosis, and initiating goals of care discussions.[22] The following case illustrates this point.
CASE 2
Ms S, a 21‐year‐old Hispanic woman with advanced, recurrent head and neck cancer, status post multiple surgeries, chemotherapy, and radiation therapy, is admitted to the hospitalist service for aspiration pneumonia, which responds to antibiotics rapidly. However, her cancer‐related somatic and neuropathic pain soon becomes refractory to opioids prescribed by the hospitalist team. She also develops significant dyspnea, xerostomia, depression, anxiety, and existential suffering. With the help of the interdisciplinary palliative care team, her pain is relieved by a patient‐controlled analgesia pump and methadone. A palliative care social worker and chaplain visit her and her family daily to address their distress. Eventually, the care team is able to provide a stable medical regimen for symptom control and to use it across the entire care continuum.
In this example, the hospitalist team, with the support of palliative care specialists, provided basic palliative care and longitudinal integration of palliative practices into the patient's overall treatment scheme. Hospitalists, given their scope of practice and sheer volume of patients, are well positioned to rapidly gain competencies in symptom management, empathic communication, and interdisciplinary teamwork.[23, 24] Hospitalists may benefit from innovative and collaborative palliative care education using interactive online modules, case simulation, communication workshops, and observed evaluation and feedback.[25] Several modes of collaboration between hospital medicine and palliative care have been developed including implementation of palliative care consult triggers on admission, palliative care participation in hospitalist interdisciplinary rounds, and disease specific, integrated management programs.[17, 26] These collaborations are particularly important, as the quality of inpatient care at the end of life is still suboptimal and more appropriate use of palliative care will be beneficial.[27] Recently, some hospitals have developed specialized inpatient palliative care units, combining intensive palliation with inpatient medical surgical level of care, as well as providing hospice care. Staffed by palliative care specialists or hospitalists, they provide efficient, cost‐saving care to patients with advanced chronic illness or terminal disease in need of intensive symptom management.[28] Finally, there is mounting evidence supporting the clinical effectiveness of palliative care in diverse specialties such as oncology, pulmonary and critical care, and nephrology.[29] For example, in the setting of metastatic non‐small cell lung cancer, early initiation of palliative care has been shown to improve symptom control and quality of life, reduce chemotherapy use at the end of life, and interestingly, prolong median survival by almost 3 months.[30] This has led to a position statement from American Society of Clinical Oncology encouraging early integration of palliative care into standard oncologic care for advanced disease.[31]
Recognizing a sentinel hospitalization allows palliative care to be integrated at transitions of care and carried forward. For patients with chronic debilitating illnesses who are approaching the end of life, appropriate care transitions will ensure that their short‐ and long‐term care matches their goals of care, assure timely clinical follow‐ups, and help reduce hospital readmission and healthcare resource utilization.[32] Importantly, timely and compassionate communication is a key to the success of both hospital medicine and palliative care. Many patients with life‐limiting diseases prefer to receive prognostic information and to discuss goals of care.[33] How this information is integrated and communicated through the care continuum is crucial, especially in the era of duty hour limits and frequent handoffs. The information exchange needs to facilitate active participation of primary care physicians who may not be involved in hospital care. Some of the innovative strategies for communication and transfer of palliative care information, such as prognosis, goals of care, family meeting consensus, and symptom control interventions, include a palliative care checklist in the electronic health record, incorporation of prognostic and family meeting information in the discharge summary, and links to the national Physician Orders for Life‐Sustaining Treatment advanced care planning program.[34] Of note, a pilot program in the United Kingdom adopting an electronic palliative care summary has reduced after‐hour emergency room visits and hospital readmissions.[35] The following case illustrates this point.
CASE 3
Mrs. K, an 82‐year‐old Russian‐speaking woman with newly diagnosed metastatic pancreatic cancer, is admitted for worsening obstructive jaundice and a second opinion about treatment. A biliary stent is placed and her jaundice slowly improves. The patient and family have requested chemotherapy. However, the oncologist determines that she would only qualify for a phase I trial given her poor performance status. The hospitalist team requests the help of the palliative care consult team to manage her severe pain, depression, and to provide support to the family. After several family meetings, the patient and family choose not to pursue chemotherapy. Given the lack of adequate support at home, she is discharged to a skilled nursing facility for short‐term rehabilitation with plans to transition to the in‐house hospice program. The hospitalist, palliative care attending physician, and the medical director of the rehabilitation facility have a 3‐way phone conference to confirm the plans of care and to ensure a smooth care transition.
In this case, the hospitalist team recognizes that this is a sentinel hospitalization for Mrs. K that requires extensive palliative care intervention. Often, transitioning to skilled nursing facilities (SNF) is the default pathway for patients needing hospice/palliative care, especially when patients and families are not yet ready to discuss prognosis realistically or to accept hospice, or there is not enough support available at home. A recent large cohort study showed that 30% of patients in their last 6‐month of life had used, and nearly 10% of such patients had died, under Medicare's posthospitalization SNF benefit.[36] Although the worsening disease trajectory may not be apparent at hospital discharge, it is more likely that the financial and practical limitation of the Medicare Hospice Benefit accounts for this observation, which includes limited home health aid hours, lack of coverage for room and board, and lower payments to SNFs.[36] Hospitalists can help address the issue of discharge location for patients needing palliative care. Sometimes this requires extensive communication before and after discharge to help enhance the transition from a rehabilitation facility to hospice/palliative care. Appropriately integrated palliative care at the time of care transitions, in the form of hospice or longitudinal home‐based palliative care rather than just routine clinic follow‐up, has the potential to reduce 30‐day readmission for chronically ill, elderly patients and for patients near the end of life.[37, 38] It is critical that national policy, suitable reimbursement, and financial incentives support this practice. A demonstration project, Better Outcomes by Optimizing Safe Transitions (BOOST), organized by the Society of Hospital Medicine, integrates palliative care evaluation into a comprehensive discharge assessment tool. This intervention has been shown to reduce readmissions to acute care hospitals.[39]
In this article, we define a sentinel hospitalization and suggest that its recognition provides an important opportunity for hospitalists to actively integrate palliative care into patients' chronic disease management programs, with inputs from patients, their families, their primary physicians and subspecialists, as well as palliative care specialists. We also recognize that within nonsentinel hospitalizations, there are important opportunities to discuss prognosis, goals of care, and advanced care planning. This approach allows the fresh eyes of hospitalists to assess the patient's current health status and prognosis, to communicate these relevant clinical issues with the patient and family, and to encourage discussions about goals of care and advanced care planning during the sentinel hospitalization. It also provides a structured vehicle for soliciting the patient's (and family's) perspectives and documenting them in the medical record. A compilation of sample items to guide discussion can be found in Table 2. Hospitalists, equipped with basic palliative care skills and supported by hospital‐ and community‐based palliative care teams, can thrive in this unique position of optimizing the quality of care for these patients.[40] Almost 20 years ago, the field of palliative care rose to national prominence on the findings of the SUPPORT (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments) study, which investigated the suboptimal end‐of‐life experiences of hospitalized adult patients.[41] Since then, the fields of both hospital medicine and palliative care have grown, yet the best is still to come for their collaborative excellence, mutual education, and shared care innovation at the forefront of medicine.
| Patient/family understanding of disease process and treatment outcomes |
| Patient/family understanding of disease prognosis |
| Availability of alternative treatment options including palliative/hospice care |
| Patient/family wishes/goals of care |
| Advanced‐care planning including limitations of care |
| Inventory of symptoms (frequency, severity, modifying factors, timing, and treatments) |
| Social and financial stress |
| Emotional and existential stress |
| Social support system and caregivers |
| Living arrangements |
Disclosure: Nothing to report.
- , , , , , , et al. Evolving practice of hospital medicine and its impact on hospital throughput and efficiencies. J Hosp Med. 2012;7:649–654.
- , , , . Medicare beneficiaries' costs of care in the last year of life. Health Affairs. 2001;20:188–195.
- . Palliative care in hospitals. J Hosp Med. 2006;1:21–28.
- . Palliative care and hospitalists: a partnership for hope. J Hosp Med. 2006;1:5–6.
- , . Palliative care and the hospitalist: an opportunity for cross‐fertilization. Am J Med. 2001;111:10s–14s.
- , . Palliative care. Ann Intern Med. 2012;156:ITC2‐1, TC2‐2–15; quiz TC2‐16.
- , , , , . Patterns of functional decline at the end of life. JAMA. 2003;289:2387–2392.
- , , , et al. The clinical course of advanced dementia. N Engl J Med. 2009;361:1529–1538.
- , . Extent and determinants of error in doctor's prognoses in terminally ill patients: prospective cohort study. BMJ. 2000;320:469–472.
- , , , et al. Prognostic significance of the “surprise” question in cancer patients. J Palliat Med. 2010;13:837–840.
- , , , et al. Utility of the “surprise” question to identify dialysis patients with high mortality. Clin J Am Soc Nephrol. 2008;3:1379–1384.
- , , , et al. Development and validation of a prognostic index for 1‐year mortality in older adults after hospitalization. JAMA. 2001;285:2987–2994.
- , , , , . Mortality predictions on admission as a context for organizing care activities. J Hosp Med. 2013,8:229–235.
- , , , , . Caring about prognosis: a validation study of the CARING criteria to identify hospitalized patients at high risk for death at 1 year. J Hosp Med. 2013,8:696–701.
- , , , , , , et al. Meta‐analysis of survival prediction with palliative performance scale. J Palliat Care. 2007;23:245–254.
- , , , , . Prognostic indices for older adults: a systemic review. JAMA. 2012;307:182–192.
- , . Identifying patients in need of a palliative care assessment in the hospital setting. J Palliat Med. 2011;14:17–23.
- , , , , , . Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361:1539–1547.
- , , . Top 10 things palliative care clinicians wished everyone knew about palliative care. Mayo Clin Proc. 2013;88:859–865.
- . Palliative and end‐of‐life care for patients with severe COPD. Eur Respir J. 2008;32:796–803.
- . Palliative care in congestive heart failure. J Am Coll Cardiol. 2009;54:386–396.
- , . Generalist plus specialist palliative care—creating a more sustainable model. N Engl J Med. 2013;368:1173–1175.
- , , , , , . Common myths about caring for patients with terminal illness: opportunities to improve care in the hospital setting. J Hosp Med. 2007;2:357–365.
- , , , . Opportunity lost: end‐of‐life discussions in cancer patients who die in the hospital. J Hosp Med. 2013;8:334–340.
- , , . Palliative medicine physician education in the United States: a historical review. J Palliat Med. 2013;16:230–236.
- , . Hospitalization as an opportunity to integrate palliative care in heart failure management. Curr Opin Support Palliat Care. 2009;3:247–251.
- , , , et al. The quality of care provided to hospitalized patients at the end of life. Arch Intern Med. 2010;170:1057–1063.
- , , , , , . Outcomes of the acute palliative care unit in an academic medical center [published online ahead of print May 10, 2013]. Am J Hosp Palliat Care. doi: 10.1177/1049909113489164.
- , , , , , . Update in hospital palliative care. J Hosp Med. 2013;12:715–720.
- , , , et al. Early palliative care for patients with metastatic non‐small‐cell lung cancer. N Engl J Med. 2010;363:733–742.
- , , , . Early integration of palliative care services with standard oncology care for patients with advanced cancer. CA Cancer J Clin. 2013;63:349–363.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150:178–187.
- , , , , , . “Knowing is better”: preferences of diverse older adults for discussion prognosis. J Gen Intern Med. 2012;27:568–575.
- , , . POLST, an improvement over traditional advanced directives. Cleve Clin J Med. 2012;79:457–464.
- , , , . Use of a structured palliative care summary in patients with established cancer is associated with reduced hospital admissions by out‐of‐hours general practitioners in Grampian [published online ahead of print January 3, 2013]. BMJ Support Palliat Care. doi:10.1136/bmjspcare‐2012‐000371.
- , , , , , . Use of the Medicare posthospitalization skilled nursing benefit in the last 6 months of life. Arch Intern Med. 2012;172:1573–1579.
- , , , et al. Increased satisfaction with care and lower costs: results of a randomized trial of in‐home palliative care. J Am Geriatr Soc. 2007;55:993–1000.
- , , . 30‐day readmissions among seriously ill older adults. J Palliat Med. 2012;15:1–6.
- , , , et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8:421–427.
- . Ensuring safe, quality care for hospitalized people with advanced illness, a core obligation for hospitalists. J Hosp Med. 2007;2:355–356.
- The SUPPORT Principal Investigators. A controlled trial to improve care for seriously ill hospitalized patients: the Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments (SUPPORT). JAMA. 1995;274:1591–1598.
- , , , , , , et al. Evolving practice of hospital medicine and its impact on hospital throughput and efficiencies. J Hosp Med. 2012;7:649–654.
- , , , . Medicare beneficiaries' costs of care in the last year of life. Health Affairs. 2001;20:188–195.
- . Palliative care in hospitals. J Hosp Med. 2006;1:21–28.
- . Palliative care and hospitalists: a partnership for hope. J Hosp Med. 2006;1:5–6.
- , . Palliative care and the hospitalist: an opportunity for cross‐fertilization. Am J Med. 2001;111:10s–14s.
- , . Palliative care. Ann Intern Med. 2012;156:ITC2‐1, TC2‐2–15; quiz TC2‐16.
- , , , , . Patterns of functional decline at the end of life. JAMA. 2003;289:2387–2392.
- , , , et al. The clinical course of advanced dementia. N Engl J Med. 2009;361:1529–1538.
- , . Extent and determinants of error in doctor's prognoses in terminally ill patients: prospective cohort study. BMJ. 2000;320:469–472.
- , , , et al. Prognostic significance of the “surprise” question in cancer patients. J Palliat Med. 2010;13:837–840.
- , , , et al. Utility of the “surprise” question to identify dialysis patients with high mortality. Clin J Am Soc Nephrol. 2008;3:1379–1384.
- , , , et al. Development and validation of a prognostic index for 1‐year mortality in older adults after hospitalization. JAMA. 2001;285:2987–2994.
- , , , , . Mortality predictions on admission as a context for organizing care activities. J Hosp Med. 2013,8:229–235.
- , , , , . Caring about prognosis: a validation study of the CARING criteria to identify hospitalized patients at high risk for death at 1 year. J Hosp Med. 2013,8:696–701.
- , , , , , , et al. Meta‐analysis of survival prediction with palliative performance scale. J Palliat Care. 2007;23:245–254.
- , , , , . Prognostic indices for older adults: a systemic review. JAMA. 2012;307:182–192.
- , . Identifying patients in need of a palliative care assessment in the hospital setting. J Palliat Med. 2011;14:17–23.
- , , , , , . Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361:1539–1547.
- , , . Top 10 things palliative care clinicians wished everyone knew about palliative care. Mayo Clin Proc. 2013;88:859–865.
- . Palliative and end‐of‐life care for patients with severe COPD. Eur Respir J. 2008;32:796–803.
- . Palliative care in congestive heart failure. J Am Coll Cardiol. 2009;54:386–396.
- , . Generalist plus specialist palliative care—creating a more sustainable model. N Engl J Med. 2013;368:1173–1175.
- , , , , , . Common myths about caring for patients with terminal illness: opportunities to improve care in the hospital setting. J Hosp Med. 2007;2:357–365.
- , , , . Opportunity lost: end‐of‐life discussions in cancer patients who die in the hospital. J Hosp Med. 2013;8:334–340.
- , , . Palliative medicine physician education in the United States: a historical review. J Palliat Med. 2013;16:230–236.
- , . Hospitalization as an opportunity to integrate palliative care in heart failure management. Curr Opin Support Palliat Care. 2009;3:247–251.
- , , , et al. The quality of care provided to hospitalized patients at the end of life. Arch Intern Med. 2010;170:1057–1063.
- , , , , , . Outcomes of the acute palliative care unit in an academic medical center [published online ahead of print May 10, 2013]. Am J Hosp Palliat Care. doi: 10.1177/1049909113489164.
- , , , , , . Update in hospital palliative care. J Hosp Med. 2013;12:715–720.
- , , , et al. Early palliative care for patients with metastatic non‐small‐cell lung cancer. N Engl J Med. 2010;363:733–742.
- , , , . Early integration of palliative care services with standard oncology care for patients with advanced cancer. CA Cancer J Clin. 2013;63:349–363.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150:178–187.
- , , , , , . “Knowing is better”: preferences of diverse older adults for discussion prognosis. J Gen Intern Med. 2012;27:568–575.
- , , . POLST, an improvement over traditional advanced directives. Cleve Clin J Med. 2012;79:457–464.
- , , , . Use of a structured palliative care summary in patients with established cancer is associated with reduced hospital admissions by out‐of‐hours general practitioners in Grampian [published online ahead of print January 3, 2013]. BMJ Support Palliat Care. doi:10.1136/bmjspcare‐2012‐000371.
- , , , , , . Use of the Medicare posthospitalization skilled nursing benefit in the last 6 months of life. Arch Intern Med. 2012;172:1573–1579.
- , , , et al. Increased satisfaction with care and lower costs: results of a randomized trial of in‐home palliative care. J Am Geriatr Soc. 2007;55:993–1000.
- , , . 30‐day readmissions among seriously ill older adults. J Palliat Med. 2012;15:1–6.
- , , , et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8:421–427.
- . Ensuring safe, quality care for hospitalized people with advanced illness, a core obligation for hospitalists. J Hosp Med. 2007;2:355–356.
- The SUPPORT Principal Investigators. A controlled trial to improve care for seriously ill hospitalized patients: the Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments (SUPPORT). JAMA. 1995;274:1591–1598.
Hospitalists' Challenge and Opportunity
Can you explain why Dr. Johnson thinks I should be taking antibiotics, while your note says I shouldn't?
Today you may be surprised by such an inquiry during morning rounds, but such questions are likely coming to your wards. At a time of societal fascination both with transparency and the explosion of health information technologies, a growing number of hospitals are offering, or will soon offer, patients and their family instantaneous access to their doctors' and nurses' notes. What will this new opportunity for patient engagement mean for the hospitalist?
BACKGROUND
Helping patients through highly complicated care processes is no easy feat, and enabling patients and their families to deal successfully with a constantly changing scenario is a particular challenge for hospitalists. Multiple studies show how poorly patients recall information offered them in office visits,[1, 2] and such settings are far less stressful than the rapid fire mixture of procedures, multiple medications, and morbid disease processes that take center stage in so many hospitalizations. And now something new: What is in store for patients and their doctors when patients in a hospital room gain access in real time not only to test results, but also to notes written by their hospitalists, nurses, and consultants?
ENGAGING PATIENTS
With the principal goal of promoting more active patient engagement in care, patient portals designed primarily for ambulatory practice are proliferating rapidly. Not only do they offer patients windows into their records and secure ways to communicate with their providers, their goal is also to automate chores such as reporting results or other administrative tasks that take away from valuable face‐to‐face time between providers and patients. First appearing shortly after the dawn of the Internet, secure electronic portals began to offer patients access to much of their chart.[3] Rapidly evolving beyond limited data feeds over very simple connections, portals today share far more data, are spreading rapidly, and in some cases offer patients access to their entire records. Whether or not 1 record can serve all the traditional users and also the patient and family is a fascinating question,[4] but the fact is that patients can now access their records from their computers, and via smartphones and tablets on the go. While lying in hospital beds, they can gain access to their laboratory and test data as the data evolve, and sometimes the patients see the findings well before their busy clinicians. Moreover, family members, other informal caregivers, or a formally designated health care proxy, will access the patient's record as well, whether through documented proxy functions or by informally peering at the patient's tablet.
MEANINGFUL USE INCENTIVES
Today, state and federal government regulations either encourage or require healthcare providers to grant patients access to their clinical information. But despite the rules embedded in the federal Health Insurance Portability and Accountability Act, patients often face time‐consuming obstacles in their quest for access, and many providers view compliance as a burden. We suggest an alternative view. Over time, we anticipate that inviting patients to review their medical record will reduce risk, increase knowledge, foster active engagement, and help them take more control of their care.
With the goal also of reducing medical errors and improving outcomes, the expansion of portals is accompanied by a combination of incentives, and in the future, sanctions, as the Center for Medicare and Medicaid Services (CMS) refines efforts to promote certified electronic health record technologies that focus on meaningful use (MU), which often include patient engagement tools such as portals. In the fall of 2012, CMS announced stage 2 MU objectives, with several having substantial implications for hospitalists and their patients. One calls for providing patients the ability to view online, download and transmit their health information within 4 business days of the information being available to the provider. Rather than an outpatient‐only requirement, it is a practice‐based requirement, and we can soon expect hospitalist data to appear on portals.
INSIGHTS FROM TRANSPARENCY IN PRIMARY CARE
The OpenNotes trial provides clues as to how such practice will affect both patients and providers.[5, 6] The trial included patients and primary care physicians (PCPs) from 3 diverse settings: Beth Israel Deaconess Medical Center (BIDMC), an urban academic health center in Boston, Massachusetts, and affiliated community practices near Boston; Geisinger Health System, a primarily rural integrated health system in Pennsylvania; and adult medicine and human immunodeficiency virus clinics at Harborview Medical Center, a safety net hospital in Seattle, Washington. More than 100 volunteering PCPs invited 20,000 of their patients enrolled in their institution's portals to read their office visit notes over a 1‐year period. Physicianpatient messaging was tracked to examine impact on physician workloads, and patients and physicians were surveyed before and after the intervention.
The experience generated considerable enthusiasm and potential clinical benefits among the patients, with little adverse impact on patients and providers. Of particular relevance for hospitalists, more than 4 in 5 patients read their notes, with more than 70% reporting they understood their medical conditions better and felt more in control of their care, and two‐thirds reported increased adherence to their medicines, a finding both unanticipated and striking. More than 1 in 5 shared their notes with others. And in spite of doctors' worries, few found their notes confusing (2%8% of patients at the 3 sites), worried more (5%8%), or felt offended by their notes (1%2%). At the end of the year‐long intervention, 99% of patients returning surveys recommended that the practice continue.
PCPs reported virtually no impact on their workflow, although about 1 in 3 reported changing their documentation, given the knowledge that their patients might read their notes. Fewer than 5% of physicians reported visits taking more time, whereas 15% to 20% of physicians reported taking longer to write their notes. Approximately 30% of physicians reported changing the content of their notes to address obesity, substance abuse, mental health, or issues concerning malignancies. Of note, physicians were given an opt out function for any note, but they called on this very rarely during the study. And at the end of the year, not 1 PCP chose to discontinue offering patients his or her notes.
The 3 participating institutions felt that the trial was so successful that they decided to expand this practice aggressively. At BIDMC, OpenNotes will soon extend to all clinical departments and include all notes signed in the online record by doctors (including housestaff and fellows), nurses, social workers, physician assistants, clinical pharmacists, nutritionists, and occupational and physical therapists. The only exceptions will be those notes authored primarily by students, and those the clinician chooses to monitor, thereby blinding access to patients.
With stage 2 MU incentives in place, and the patient engagement movement accelerating, such practice will likely spread rapidly nationwide. We expect that more and more patients will be soon able to read all signed notes by hospitalists in real time. But differences abound among outpatients and inpatients, and PCPs and hospitalists, and inpatient notes are vastly different from those describing office visits. How may this change in practice affect hospitalized patients and their clinicians?
IMPLICATIONS FOR HOSPITALISTS
Most inpatients meet their hospitalists for the first time at admission. During their stay, they may encounter many hospitalists, along with multiple specialty consultants, house officers, nurses, and ancillary providers. Moreover, inpatient notes vary widely in their content and context. They may describe the patient tersely, while spelling out both a broad (and frightening) differential diagnosis, along with options for addressing a range of contingencies. Such notes, written during the acute diagnostic and treatment phase of an admission, tend to focus primarily on acute and discrete issues at hand, in contrast to outpatient notes that may take a more comprehensive approach. Moreover, given the enormous burden and acuity of illness today among many hospitalized patients, a large volume of data is generated in a very short period of time. Due both to time constraints and complexity, decisions are made quickly, often without the patient's input. When did you last ask a hospitalized patient if you could order specific blood tests? Unless a major therapeutic change is anticipated, how often are your patients told their results as a matter of course?
As acutely ill patients suddenly experience a life out of their control, how will they and their families respond to new access to a large volume of information? Should hospitalists expect an avalanche of questions, or might the prime impact be a change in the nature of those questions, as patients and their families move from What was the result? to What is the meaning of this result, given my condition? When the patient sees test results and reads consultant notes before the hospitalist has had a chance to review them, how will this impact the process of care and shape the patient's view of the hospitalist? When questions arise, will they discuss them immediately with their hospitalists, might they try to contact the doctor with whom they have an ongoing relationship, or will they wait until discharge to contact their PCPs? One hopes that offering patients ready access to their hospital record will foster trust and facilitate a positive relationship with hospitalists. But notes could also foster confusion and distrust, particularly if patients feel out of the loop and perceive differing opinions among those caring for them.
We anticipate that transparent records will stimulate hospitalists, PCPs, and other caregivers to improve communication throughout the patient's hospital stay. We know that medical errors occur with alarming frequency in all care settings, and unfortunately electronic medical records make it easier to spread erroneous information widely. As providers we are both morally and legally responsible for eliminating such errors, inviting the patient (and family) to review the chart may help prevent mistakes well before an adverse outcome ensues.
OPPORTUNITIES FOR IMPROVED CARE
Open notes will be viewed by many as a disruptive change, and the best strategy for adapting will be to move proactively to create policies that establish clear guidelines. Consider the following strategies:
- Draw on complex provider notes that may include potentially alarming differential diagnoses as an opportunity for engaging and educating the patient and caregiver.
- Try to avoid jargon and wording that patients may find objectionable, such as patient denies, poor historian, or even obese. Instead, use more situational wording, such as the patient was unclear on his history.
- Avoid abbreviations when possible. They are a frequent source of confusion among clinicians, let alone patients.
- When it is likely that a treatment may not succeed or a diagnosis may prove wrong, address contingency plans in your notes. Where possible, express likelihoods in terms consistent with the patient's level of comfort with numbers.
- Teach trainees to review notes with supervisors before signing.
- Explain to patients and families when they may expect to see your notes.
- Try rephrasing some of the technical content of notes. Move from incr. Cr FeNa=Prerenal, 1L IVF, to Due to dehydration (creatinine rising to 1.8, and fena 0.8), will give 1L IV fluids. Although at first blush this seems like more work, short circuiting need for explanation may save the hospitalist or nurse time later on. And clarity may lead to important additional history from the patient, furnishing perhaps insight into how he or she became dehydrated.
- Expect patients to download, copy, paste, and forward your note. Document with this in mind.
- Discuss with providers concerns about potential medicallegal risks and how to address them.
OpenNotes offers a special opportunity for improving the patient experience after leaving the hospital. For example, providing patients and their families with a medication list may be helpful, but a note adding context to medications may drive the reasoning home and prove vitally important, especially for those faced with complex medical regimens who may have poor health literacy.[7] Moreover, though providers are learning to focus on patient and family education during the discharge transition period in the hope of minimizing rehospitalizations, time spent at the bedside may have little impact.[8] Methods to improve patient/family understanding are often time consuming,[9, 10] and time is a luxury hospitalists rarely have. Providing patients full access to their providers' notes may mitigate confusion about salient aspects of the hospitalization or prompt timely questions, thereby facilitating a safe transition home.
Open access to notes should also help hospitalized patients engage a range of individuals well beyond those directly involved in their care. Patients will be increasingly likely to grant access to surrogates, whether through formal or informal mechanisms. Patients and their families may also forward notes to providers in other institutions, an activity that all too often falls between cracks. But such capabilities create both new opportunities and new challenges for hospitalists. On the 1 hand, they may find themselves more often in the difficult position of trying to arbitrate differences of opinion within a family. Alternatively, family members or friends, including health professionals offering informal consultation, may prove invaluable in helping hospitalists and patients agree on a plan of care developed collaboratively by a wide range of individuals.
FUTURE WORK
Opening hospital notes to patients will affect both clinicians and patients, and the hospital medicine community should begin to consider its options:
- Should we establish a formal curriculum designed to help hospitalists compose notes that will intelligently and efficiently engage patients?
- Can we identify best practice techniques for preparing notes that engage patients and families without overwhelming them?
- How can we use such notes to assure respect for the individual needs of patients and their families? How can we best assure maintaining their dignity?
- How can we use open notes to support patient safety? Can they reduce malpractice claims?
- How should we handle unsolicited second opinions initiated by patients and families who shared open notes with providers and others outside the care team?
- Should we encourage hospitals to offer portal access to all patients, including those who may have only a brief, passing relationship with the institution?
- What patient portal functions could best assist patients and families in understanding the content of inpatient notes?
- In the rapidly changing inpatient environment, how should we deal with patient‐initiated requests for corrections and changes to notes?
- Should all hospital notes be opened? Should clinicians be able to hide specific notes? Clinicians worry about medical record access for patients with mental illness; should patients with these or other specified conditions be exempted, and if so, how can one structure such processes openly and honestly?
The inexorable spread of fully open medical records requires rapid and intense intellectual scrutiny. Benefits will accompany risks, and unforeseen consequences are virtually inevitable. But this expression of transparency may soon constitute the standard of care in hospital medicine. We need to shape it carefully so that in inures to the benefit of both our patients and ourselves. Over time, we expect that inviting patients and their families to read notes openly will improve the quality of care and promote patient safety. We should take full advantage of such opportunity.
- , . New prescriptions: how well do patients remember important information? Fam Med. 2011;43(4):254–259.
- . Do physicians tell patients enough about prescription drugs? Do patients think so? Postgrad Med. 1983;74:169–175.
- , , . Early experiences with personal health records. J Am Med Inform Assoc. 2008;15:1–7.
- , , , et al. Open notes: doctors and patients signing on. Ann Intern Med. 2010;153(2):121–125.
- , , , Vodicka E, Darer JD, Dhanireddy S, Elmore JG, Feldman HJ, Lichtenfeld MJ, Oster N, Ralston JD, Ross S, Delbanco T. Inviting patients to read their doctors' notes: patients and doctors look ahead: patient and physician surveys. Ann Intern Med. 2011;155:811–819.
- , , , Darer JD, Elmore JG, Farag N, Feldman HJ, Mejilla R, Ngo L, Ralston JD, Ross SE, Trivedi N, Vodicka E, Leveille SG. Inviting patients to read their doctors' notes: a quasi‐experimental study and a look ahead. Ann Intern Med. 2012;157(7):461–470.
- , , , , . Hospital quality and patient safety competencies: development, description, and recommendations for use. J Hosp Med. 2011;6(9):530–536.
- , , , , , . The relationship between time spent communicating and communication outcomes on a hospital medicine service. J Gen Intern Med. 2012;27(2):185–189.
- , , , , . Is “teach‐back” associated with knowledge retention and hospital readmission in hospitalized heart failure patients? J Cardiovasc Nurs. 2013;28(2):137–146.
- , , ., “They never told us anything”: postdischarge instruction for families of persons with brain injuries. Rehabil Nurs.2001;26(2):48–53.
Can you explain why Dr. Johnson thinks I should be taking antibiotics, while your note says I shouldn't?
Today you may be surprised by such an inquiry during morning rounds, but such questions are likely coming to your wards. At a time of societal fascination both with transparency and the explosion of health information technologies, a growing number of hospitals are offering, or will soon offer, patients and their family instantaneous access to their doctors' and nurses' notes. What will this new opportunity for patient engagement mean for the hospitalist?
BACKGROUND
Helping patients through highly complicated care processes is no easy feat, and enabling patients and their families to deal successfully with a constantly changing scenario is a particular challenge for hospitalists. Multiple studies show how poorly patients recall information offered them in office visits,[1, 2] and such settings are far less stressful than the rapid fire mixture of procedures, multiple medications, and morbid disease processes that take center stage in so many hospitalizations. And now something new: What is in store for patients and their doctors when patients in a hospital room gain access in real time not only to test results, but also to notes written by their hospitalists, nurses, and consultants?
ENGAGING PATIENTS
With the principal goal of promoting more active patient engagement in care, patient portals designed primarily for ambulatory practice are proliferating rapidly. Not only do they offer patients windows into their records and secure ways to communicate with their providers, their goal is also to automate chores such as reporting results or other administrative tasks that take away from valuable face‐to‐face time between providers and patients. First appearing shortly after the dawn of the Internet, secure electronic portals began to offer patients access to much of their chart.[3] Rapidly evolving beyond limited data feeds over very simple connections, portals today share far more data, are spreading rapidly, and in some cases offer patients access to their entire records. Whether or not 1 record can serve all the traditional users and also the patient and family is a fascinating question,[4] but the fact is that patients can now access their records from their computers, and via smartphones and tablets on the go. While lying in hospital beds, they can gain access to their laboratory and test data as the data evolve, and sometimes the patients see the findings well before their busy clinicians. Moreover, family members, other informal caregivers, or a formally designated health care proxy, will access the patient's record as well, whether through documented proxy functions or by informally peering at the patient's tablet.
MEANINGFUL USE INCENTIVES
Today, state and federal government regulations either encourage or require healthcare providers to grant patients access to their clinical information. But despite the rules embedded in the federal Health Insurance Portability and Accountability Act, patients often face time‐consuming obstacles in their quest for access, and many providers view compliance as a burden. We suggest an alternative view. Over time, we anticipate that inviting patients to review their medical record will reduce risk, increase knowledge, foster active engagement, and help them take more control of their care.
With the goal also of reducing medical errors and improving outcomes, the expansion of portals is accompanied by a combination of incentives, and in the future, sanctions, as the Center for Medicare and Medicaid Services (CMS) refines efforts to promote certified electronic health record technologies that focus on meaningful use (MU), which often include patient engagement tools such as portals. In the fall of 2012, CMS announced stage 2 MU objectives, with several having substantial implications for hospitalists and their patients. One calls for providing patients the ability to view online, download and transmit their health information within 4 business days of the information being available to the provider. Rather than an outpatient‐only requirement, it is a practice‐based requirement, and we can soon expect hospitalist data to appear on portals.
INSIGHTS FROM TRANSPARENCY IN PRIMARY CARE
The OpenNotes trial provides clues as to how such practice will affect both patients and providers.[5, 6] The trial included patients and primary care physicians (PCPs) from 3 diverse settings: Beth Israel Deaconess Medical Center (BIDMC), an urban academic health center in Boston, Massachusetts, and affiliated community practices near Boston; Geisinger Health System, a primarily rural integrated health system in Pennsylvania; and adult medicine and human immunodeficiency virus clinics at Harborview Medical Center, a safety net hospital in Seattle, Washington. More than 100 volunteering PCPs invited 20,000 of their patients enrolled in their institution's portals to read their office visit notes over a 1‐year period. Physicianpatient messaging was tracked to examine impact on physician workloads, and patients and physicians were surveyed before and after the intervention.
The experience generated considerable enthusiasm and potential clinical benefits among the patients, with little adverse impact on patients and providers. Of particular relevance for hospitalists, more than 4 in 5 patients read their notes, with more than 70% reporting they understood their medical conditions better and felt more in control of their care, and two‐thirds reported increased adherence to their medicines, a finding both unanticipated and striking. More than 1 in 5 shared their notes with others. And in spite of doctors' worries, few found their notes confusing (2%8% of patients at the 3 sites), worried more (5%8%), or felt offended by their notes (1%2%). At the end of the year‐long intervention, 99% of patients returning surveys recommended that the practice continue.
PCPs reported virtually no impact on their workflow, although about 1 in 3 reported changing their documentation, given the knowledge that their patients might read their notes. Fewer than 5% of physicians reported visits taking more time, whereas 15% to 20% of physicians reported taking longer to write their notes. Approximately 30% of physicians reported changing the content of their notes to address obesity, substance abuse, mental health, or issues concerning malignancies. Of note, physicians were given an opt out function for any note, but they called on this very rarely during the study. And at the end of the year, not 1 PCP chose to discontinue offering patients his or her notes.
The 3 participating institutions felt that the trial was so successful that they decided to expand this practice aggressively. At BIDMC, OpenNotes will soon extend to all clinical departments and include all notes signed in the online record by doctors (including housestaff and fellows), nurses, social workers, physician assistants, clinical pharmacists, nutritionists, and occupational and physical therapists. The only exceptions will be those notes authored primarily by students, and those the clinician chooses to monitor, thereby blinding access to patients.
With stage 2 MU incentives in place, and the patient engagement movement accelerating, such practice will likely spread rapidly nationwide. We expect that more and more patients will be soon able to read all signed notes by hospitalists in real time. But differences abound among outpatients and inpatients, and PCPs and hospitalists, and inpatient notes are vastly different from those describing office visits. How may this change in practice affect hospitalized patients and their clinicians?
IMPLICATIONS FOR HOSPITALISTS
Most inpatients meet their hospitalists for the first time at admission. During their stay, they may encounter many hospitalists, along with multiple specialty consultants, house officers, nurses, and ancillary providers. Moreover, inpatient notes vary widely in their content and context. They may describe the patient tersely, while spelling out both a broad (and frightening) differential diagnosis, along with options for addressing a range of contingencies. Such notes, written during the acute diagnostic and treatment phase of an admission, tend to focus primarily on acute and discrete issues at hand, in contrast to outpatient notes that may take a more comprehensive approach. Moreover, given the enormous burden and acuity of illness today among many hospitalized patients, a large volume of data is generated in a very short period of time. Due both to time constraints and complexity, decisions are made quickly, often without the patient's input. When did you last ask a hospitalized patient if you could order specific blood tests? Unless a major therapeutic change is anticipated, how often are your patients told their results as a matter of course?
As acutely ill patients suddenly experience a life out of their control, how will they and their families respond to new access to a large volume of information? Should hospitalists expect an avalanche of questions, or might the prime impact be a change in the nature of those questions, as patients and their families move from What was the result? to What is the meaning of this result, given my condition? When the patient sees test results and reads consultant notes before the hospitalist has had a chance to review them, how will this impact the process of care and shape the patient's view of the hospitalist? When questions arise, will they discuss them immediately with their hospitalists, might they try to contact the doctor with whom they have an ongoing relationship, or will they wait until discharge to contact their PCPs? One hopes that offering patients ready access to their hospital record will foster trust and facilitate a positive relationship with hospitalists. But notes could also foster confusion and distrust, particularly if patients feel out of the loop and perceive differing opinions among those caring for them.
We anticipate that transparent records will stimulate hospitalists, PCPs, and other caregivers to improve communication throughout the patient's hospital stay. We know that medical errors occur with alarming frequency in all care settings, and unfortunately electronic medical records make it easier to spread erroneous information widely. As providers we are both morally and legally responsible for eliminating such errors, inviting the patient (and family) to review the chart may help prevent mistakes well before an adverse outcome ensues.
OPPORTUNITIES FOR IMPROVED CARE
Open notes will be viewed by many as a disruptive change, and the best strategy for adapting will be to move proactively to create policies that establish clear guidelines. Consider the following strategies:
- Draw on complex provider notes that may include potentially alarming differential diagnoses as an opportunity for engaging and educating the patient and caregiver.
- Try to avoid jargon and wording that patients may find objectionable, such as patient denies, poor historian, or even obese. Instead, use more situational wording, such as the patient was unclear on his history.
- Avoid abbreviations when possible. They are a frequent source of confusion among clinicians, let alone patients.
- When it is likely that a treatment may not succeed or a diagnosis may prove wrong, address contingency plans in your notes. Where possible, express likelihoods in terms consistent with the patient's level of comfort with numbers.
- Teach trainees to review notes with supervisors before signing.
- Explain to patients and families when they may expect to see your notes.
- Try rephrasing some of the technical content of notes. Move from incr. Cr FeNa=Prerenal, 1L IVF, to Due to dehydration (creatinine rising to 1.8, and fena 0.8), will give 1L IV fluids. Although at first blush this seems like more work, short circuiting need for explanation may save the hospitalist or nurse time later on. And clarity may lead to important additional history from the patient, furnishing perhaps insight into how he or she became dehydrated.
- Expect patients to download, copy, paste, and forward your note. Document with this in mind.
- Discuss with providers concerns about potential medicallegal risks and how to address them.
OpenNotes offers a special opportunity for improving the patient experience after leaving the hospital. For example, providing patients and their families with a medication list may be helpful, but a note adding context to medications may drive the reasoning home and prove vitally important, especially for those faced with complex medical regimens who may have poor health literacy.[7] Moreover, though providers are learning to focus on patient and family education during the discharge transition period in the hope of minimizing rehospitalizations, time spent at the bedside may have little impact.[8] Methods to improve patient/family understanding are often time consuming,[9, 10] and time is a luxury hospitalists rarely have. Providing patients full access to their providers' notes may mitigate confusion about salient aspects of the hospitalization or prompt timely questions, thereby facilitating a safe transition home.
Open access to notes should also help hospitalized patients engage a range of individuals well beyond those directly involved in their care. Patients will be increasingly likely to grant access to surrogates, whether through formal or informal mechanisms. Patients and their families may also forward notes to providers in other institutions, an activity that all too often falls between cracks. But such capabilities create both new opportunities and new challenges for hospitalists. On the 1 hand, they may find themselves more often in the difficult position of trying to arbitrate differences of opinion within a family. Alternatively, family members or friends, including health professionals offering informal consultation, may prove invaluable in helping hospitalists and patients agree on a plan of care developed collaboratively by a wide range of individuals.
FUTURE WORK
Opening hospital notes to patients will affect both clinicians and patients, and the hospital medicine community should begin to consider its options:
- Should we establish a formal curriculum designed to help hospitalists compose notes that will intelligently and efficiently engage patients?
- Can we identify best practice techniques for preparing notes that engage patients and families without overwhelming them?
- How can we use such notes to assure respect for the individual needs of patients and their families? How can we best assure maintaining their dignity?
- How can we use open notes to support patient safety? Can they reduce malpractice claims?
- How should we handle unsolicited second opinions initiated by patients and families who shared open notes with providers and others outside the care team?
- Should we encourage hospitals to offer portal access to all patients, including those who may have only a brief, passing relationship with the institution?
- What patient portal functions could best assist patients and families in understanding the content of inpatient notes?
- In the rapidly changing inpatient environment, how should we deal with patient‐initiated requests for corrections and changes to notes?
- Should all hospital notes be opened? Should clinicians be able to hide specific notes? Clinicians worry about medical record access for patients with mental illness; should patients with these or other specified conditions be exempted, and if so, how can one structure such processes openly and honestly?
The inexorable spread of fully open medical records requires rapid and intense intellectual scrutiny. Benefits will accompany risks, and unforeseen consequences are virtually inevitable. But this expression of transparency may soon constitute the standard of care in hospital medicine. We need to shape it carefully so that in inures to the benefit of both our patients and ourselves. Over time, we expect that inviting patients and their families to read notes openly will improve the quality of care and promote patient safety. We should take full advantage of such opportunity.
Can you explain why Dr. Johnson thinks I should be taking antibiotics, while your note says I shouldn't?
Today you may be surprised by such an inquiry during morning rounds, but such questions are likely coming to your wards. At a time of societal fascination both with transparency and the explosion of health information technologies, a growing number of hospitals are offering, or will soon offer, patients and their family instantaneous access to their doctors' and nurses' notes. What will this new opportunity for patient engagement mean for the hospitalist?
BACKGROUND
Helping patients through highly complicated care processes is no easy feat, and enabling patients and their families to deal successfully with a constantly changing scenario is a particular challenge for hospitalists. Multiple studies show how poorly patients recall information offered them in office visits,[1, 2] and such settings are far less stressful than the rapid fire mixture of procedures, multiple medications, and morbid disease processes that take center stage in so many hospitalizations. And now something new: What is in store for patients and their doctors when patients in a hospital room gain access in real time not only to test results, but also to notes written by their hospitalists, nurses, and consultants?
ENGAGING PATIENTS
With the principal goal of promoting more active patient engagement in care, patient portals designed primarily for ambulatory practice are proliferating rapidly. Not only do they offer patients windows into their records and secure ways to communicate with their providers, their goal is also to automate chores such as reporting results or other administrative tasks that take away from valuable face‐to‐face time between providers and patients. First appearing shortly after the dawn of the Internet, secure electronic portals began to offer patients access to much of their chart.[3] Rapidly evolving beyond limited data feeds over very simple connections, portals today share far more data, are spreading rapidly, and in some cases offer patients access to their entire records. Whether or not 1 record can serve all the traditional users and also the patient and family is a fascinating question,[4] but the fact is that patients can now access their records from their computers, and via smartphones and tablets on the go. While lying in hospital beds, they can gain access to their laboratory and test data as the data evolve, and sometimes the patients see the findings well before their busy clinicians. Moreover, family members, other informal caregivers, or a formally designated health care proxy, will access the patient's record as well, whether through documented proxy functions or by informally peering at the patient's tablet.
MEANINGFUL USE INCENTIVES
Today, state and federal government regulations either encourage or require healthcare providers to grant patients access to their clinical information. But despite the rules embedded in the federal Health Insurance Portability and Accountability Act, patients often face time‐consuming obstacles in their quest for access, and many providers view compliance as a burden. We suggest an alternative view. Over time, we anticipate that inviting patients to review their medical record will reduce risk, increase knowledge, foster active engagement, and help them take more control of their care.
With the goal also of reducing medical errors and improving outcomes, the expansion of portals is accompanied by a combination of incentives, and in the future, sanctions, as the Center for Medicare and Medicaid Services (CMS) refines efforts to promote certified electronic health record technologies that focus on meaningful use (MU), which often include patient engagement tools such as portals. In the fall of 2012, CMS announced stage 2 MU objectives, with several having substantial implications for hospitalists and their patients. One calls for providing patients the ability to view online, download and transmit their health information within 4 business days of the information being available to the provider. Rather than an outpatient‐only requirement, it is a practice‐based requirement, and we can soon expect hospitalist data to appear on portals.
INSIGHTS FROM TRANSPARENCY IN PRIMARY CARE
The OpenNotes trial provides clues as to how such practice will affect both patients and providers.[5, 6] The trial included patients and primary care physicians (PCPs) from 3 diverse settings: Beth Israel Deaconess Medical Center (BIDMC), an urban academic health center in Boston, Massachusetts, and affiliated community practices near Boston; Geisinger Health System, a primarily rural integrated health system in Pennsylvania; and adult medicine and human immunodeficiency virus clinics at Harborview Medical Center, a safety net hospital in Seattle, Washington. More than 100 volunteering PCPs invited 20,000 of their patients enrolled in their institution's portals to read their office visit notes over a 1‐year period. Physicianpatient messaging was tracked to examine impact on physician workloads, and patients and physicians were surveyed before and after the intervention.
The experience generated considerable enthusiasm and potential clinical benefits among the patients, with little adverse impact on patients and providers. Of particular relevance for hospitalists, more than 4 in 5 patients read their notes, with more than 70% reporting they understood their medical conditions better and felt more in control of their care, and two‐thirds reported increased adherence to their medicines, a finding both unanticipated and striking. More than 1 in 5 shared their notes with others. And in spite of doctors' worries, few found their notes confusing (2%8% of patients at the 3 sites), worried more (5%8%), or felt offended by their notes (1%2%). At the end of the year‐long intervention, 99% of patients returning surveys recommended that the practice continue.
PCPs reported virtually no impact on their workflow, although about 1 in 3 reported changing their documentation, given the knowledge that their patients might read their notes. Fewer than 5% of physicians reported visits taking more time, whereas 15% to 20% of physicians reported taking longer to write their notes. Approximately 30% of physicians reported changing the content of their notes to address obesity, substance abuse, mental health, or issues concerning malignancies. Of note, physicians were given an opt out function for any note, but they called on this very rarely during the study. And at the end of the year, not 1 PCP chose to discontinue offering patients his or her notes.
The 3 participating institutions felt that the trial was so successful that they decided to expand this practice aggressively. At BIDMC, OpenNotes will soon extend to all clinical departments and include all notes signed in the online record by doctors (including housestaff and fellows), nurses, social workers, physician assistants, clinical pharmacists, nutritionists, and occupational and physical therapists. The only exceptions will be those notes authored primarily by students, and those the clinician chooses to monitor, thereby blinding access to patients.
With stage 2 MU incentives in place, and the patient engagement movement accelerating, such practice will likely spread rapidly nationwide. We expect that more and more patients will be soon able to read all signed notes by hospitalists in real time. But differences abound among outpatients and inpatients, and PCPs and hospitalists, and inpatient notes are vastly different from those describing office visits. How may this change in practice affect hospitalized patients and their clinicians?
IMPLICATIONS FOR HOSPITALISTS
Most inpatients meet their hospitalists for the first time at admission. During their stay, they may encounter many hospitalists, along with multiple specialty consultants, house officers, nurses, and ancillary providers. Moreover, inpatient notes vary widely in their content and context. They may describe the patient tersely, while spelling out both a broad (and frightening) differential diagnosis, along with options for addressing a range of contingencies. Such notes, written during the acute diagnostic and treatment phase of an admission, tend to focus primarily on acute and discrete issues at hand, in contrast to outpatient notes that may take a more comprehensive approach. Moreover, given the enormous burden and acuity of illness today among many hospitalized patients, a large volume of data is generated in a very short period of time. Due both to time constraints and complexity, decisions are made quickly, often without the patient's input. When did you last ask a hospitalized patient if you could order specific blood tests? Unless a major therapeutic change is anticipated, how often are your patients told their results as a matter of course?
As acutely ill patients suddenly experience a life out of their control, how will they and their families respond to new access to a large volume of information? Should hospitalists expect an avalanche of questions, or might the prime impact be a change in the nature of those questions, as patients and their families move from What was the result? to What is the meaning of this result, given my condition? When the patient sees test results and reads consultant notes before the hospitalist has had a chance to review them, how will this impact the process of care and shape the patient's view of the hospitalist? When questions arise, will they discuss them immediately with their hospitalists, might they try to contact the doctor with whom they have an ongoing relationship, or will they wait until discharge to contact their PCPs? One hopes that offering patients ready access to their hospital record will foster trust and facilitate a positive relationship with hospitalists. But notes could also foster confusion and distrust, particularly if patients feel out of the loop and perceive differing opinions among those caring for them.
We anticipate that transparent records will stimulate hospitalists, PCPs, and other caregivers to improve communication throughout the patient's hospital stay. We know that medical errors occur with alarming frequency in all care settings, and unfortunately electronic medical records make it easier to spread erroneous information widely. As providers we are both morally and legally responsible for eliminating such errors, inviting the patient (and family) to review the chart may help prevent mistakes well before an adverse outcome ensues.
OPPORTUNITIES FOR IMPROVED CARE
Open notes will be viewed by many as a disruptive change, and the best strategy for adapting will be to move proactively to create policies that establish clear guidelines. Consider the following strategies:
- Draw on complex provider notes that may include potentially alarming differential diagnoses as an opportunity for engaging and educating the patient and caregiver.
- Try to avoid jargon and wording that patients may find objectionable, such as patient denies, poor historian, or even obese. Instead, use more situational wording, such as the patient was unclear on his history.
- Avoid abbreviations when possible. They are a frequent source of confusion among clinicians, let alone patients.
- When it is likely that a treatment may not succeed or a diagnosis may prove wrong, address contingency plans in your notes. Where possible, express likelihoods in terms consistent with the patient's level of comfort with numbers.
- Teach trainees to review notes with supervisors before signing.
- Explain to patients and families when they may expect to see your notes.
- Try rephrasing some of the technical content of notes. Move from incr. Cr FeNa=Prerenal, 1L IVF, to Due to dehydration (creatinine rising to 1.8, and fena 0.8), will give 1L IV fluids. Although at first blush this seems like more work, short circuiting need for explanation may save the hospitalist or nurse time later on. And clarity may lead to important additional history from the patient, furnishing perhaps insight into how he or she became dehydrated.
- Expect patients to download, copy, paste, and forward your note. Document with this in mind.
- Discuss with providers concerns about potential medicallegal risks and how to address them.
OpenNotes offers a special opportunity for improving the patient experience after leaving the hospital. For example, providing patients and their families with a medication list may be helpful, but a note adding context to medications may drive the reasoning home and prove vitally important, especially for those faced with complex medical regimens who may have poor health literacy.[7] Moreover, though providers are learning to focus on patient and family education during the discharge transition period in the hope of minimizing rehospitalizations, time spent at the bedside may have little impact.[8] Methods to improve patient/family understanding are often time consuming,[9, 10] and time is a luxury hospitalists rarely have. Providing patients full access to their providers' notes may mitigate confusion about salient aspects of the hospitalization or prompt timely questions, thereby facilitating a safe transition home.
Open access to notes should also help hospitalized patients engage a range of individuals well beyond those directly involved in their care. Patients will be increasingly likely to grant access to surrogates, whether through formal or informal mechanisms. Patients and their families may also forward notes to providers in other institutions, an activity that all too often falls between cracks. But such capabilities create both new opportunities and new challenges for hospitalists. On the 1 hand, they may find themselves more often in the difficult position of trying to arbitrate differences of opinion within a family. Alternatively, family members or friends, including health professionals offering informal consultation, may prove invaluable in helping hospitalists and patients agree on a plan of care developed collaboratively by a wide range of individuals.
FUTURE WORK
Opening hospital notes to patients will affect both clinicians and patients, and the hospital medicine community should begin to consider its options:
- Should we establish a formal curriculum designed to help hospitalists compose notes that will intelligently and efficiently engage patients?
- Can we identify best practice techniques for preparing notes that engage patients and families without overwhelming them?
- How can we use such notes to assure respect for the individual needs of patients and their families? How can we best assure maintaining their dignity?
- How can we use open notes to support patient safety? Can they reduce malpractice claims?
- How should we handle unsolicited second opinions initiated by patients and families who shared open notes with providers and others outside the care team?
- Should we encourage hospitals to offer portal access to all patients, including those who may have only a brief, passing relationship with the institution?
- What patient portal functions could best assist patients and families in understanding the content of inpatient notes?
- In the rapidly changing inpatient environment, how should we deal with patient‐initiated requests for corrections and changes to notes?
- Should all hospital notes be opened? Should clinicians be able to hide specific notes? Clinicians worry about medical record access for patients with mental illness; should patients with these or other specified conditions be exempted, and if so, how can one structure such processes openly and honestly?
The inexorable spread of fully open medical records requires rapid and intense intellectual scrutiny. Benefits will accompany risks, and unforeseen consequences are virtually inevitable. But this expression of transparency may soon constitute the standard of care in hospital medicine. We need to shape it carefully so that in inures to the benefit of both our patients and ourselves. Over time, we expect that inviting patients and their families to read notes openly will improve the quality of care and promote patient safety. We should take full advantage of such opportunity.
- , . New prescriptions: how well do patients remember important information? Fam Med. 2011;43(4):254–259.
- . Do physicians tell patients enough about prescription drugs? Do patients think so? Postgrad Med. 1983;74:169–175.
- , , . Early experiences with personal health records. J Am Med Inform Assoc. 2008;15:1–7.
- , , , et al. Open notes: doctors and patients signing on. Ann Intern Med. 2010;153(2):121–125.
- , , , Vodicka E, Darer JD, Dhanireddy S, Elmore JG, Feldman HJ, Lichtenfeld MJ, Oster N, Ralston JD, Ross S, Delbanco T. Inviting patients to read their doctors' notes: patients and doctors look ahead: patient and physician surveys. Ann Intern Med. 2011;155:811–819.
- , , , Darer JD, Elmore JG, Farag N, Feldman HJ, Mejilla R, Ngo L, Ralston JD, Ross SE, Trivedi N, Vodicka E, Leveille SG. Inviting patients to read their doctors' notes: a quasi‐experimental study and a look ahead. Ann Intern Med. 2012;157(7):461–470.
- , , , , . Hospital quality and patient safety competencies: development, description, and recommendations for use. J Hosp Med. 2011;6(9):530–536.
- , , , , , . The relationship between time spent communicating and communication outcomes on a hospital medicine service. J Gen Intern Med. 2012;27(2):185–189.
- , , , , . Is “teach‐back” associated with knowledge retention and hospital readmission in hospitalized heart failure patients? J Cardiovasc Nurs. 2013;28(2):137–146.
- , , ., “They never told us anything”: postdischarge instruction for families of persons with brain injuries. Rehabil Nurs.2001;26(2):48–53.
- , . New prescriptions: how well do patients remember important information? Fam Med. 2011;43(4):254–259.
- . Do physicians tell patients enough about prescription drugs? Do patients think so? Postgrad Med. 1983;74:169–175.
- , , . Early experiences with personal health records. J Am Med Inform Assoc. 2008;15:1–7.
- , , , et al. Open notes: doctors and patients signing on. Ann Intern Med. 2010;153(2):121–125.
- , , , Vodicka E, Darer JD, Dhanireddy S, Elmore JG, Feldman HJ, Lichtenfeld MJ, Oster N, Ralston JD, Ross S, Delbanco T. Inviting patients to read their doctors' notes: patients and doctors look ahead: patient and physician surveys. Ann Intern Med. 2011;155:811–819.
- , , , Darer JD, Elmore JG, Farag N, Feldman HJ, Mejilla R, Ngo L, Ralston JD, Ross SE, Trivedi N, Vodicka E, Leveille SG. Inviting patients to read their doctors' notes: a quasi‐experimental study and a look ahead. Ann Intern Med. 2012;157(7):461–470.
- , , , , . Hospital quality and patient safety competencies: development, description, and recommendations for use. J Hosp Med. 2011;6(9):530–536.
- , , , , , . The relationship between time spent communicating and communication outcomes on a hospital medicine service. J Gen Intern Med. 2012;27(2):185–189.
- , , , , . Is “teach‐back” associated with knowledge retention and hospital readmission in hospitalized heart failure patients? J Cardiovasc Nurs. 2013;28(2):137–146.
- , , ., “They never told us anything”: postdischarge instruction for families of persons with brain injuries. Rehabil Nurs.2001;26(2):48–53.
Rapid Response Systems
In 2006,[1] we questioned whether rapid response systems (RRSs) were an effective strategy for detecting and managing deteriorating general ward patients. Since then, the implementation of RRSs has flourished, especially in the United States where accreditors (Joint Commission)[2] and patient‐safety organizations (Institute for Healthcare Improvement 100,000 Live Campaign)[3] have strongly supported RRSs. Decades of evidence show that general ward patients often experience unrecognized deterioration and cardiorespiratory arrest (CA). The low sensitivity and accuracy of periodic assessments by staff are thought to be a major reason for these lapses, as are imbalances between patient needs and clinician (primarily nursing) resources. Additionally, a medical culture that punishes speaking up or bypassing the chain of command are also likely contributors to the problem. A system that effectively recognizes the early signs of deterioration and quickly responds should catch problems before they become life threatening. Over the last decade, RRSs have been the primary intervention implemented to do this. The potential for RRSs to improve outcomes has strong face validity, but researchers have struggled to demonstrate consistent improvements in outcomes across institutions. Given this, are RRSs the best intervention to prevent this failure to rescue? In this editorial we examine the progress of RRSs, how they compare to other options, and we consider whether we should continue to question their implementation.
In our 2007 systematic review,[4] we concluded there was weak to moderate evidence supporting RRSs. Since then, 6 other systematic reviews of the effectiveness or implementation of RRSs have been published. One high‐quality review of effectiveness studies published through 2008 by Chan et al.[5] found that RRSs significantly reduced non‐intensive care unit (ICU) CA (relative risk [RR], 0.66; 95% confidence interval [CI], 0.54‐0.80), but not total hospital mortality (RR, 0.96; 95% CI, 0.84‐1.09) in adult inpatients. In pediatric inpatients, RRSs led to significant improvements in both non‐ICU CA (RR, 0.62; 95% CI, 0.46 to 0.84) and total hospital mortality (RR, 0.79; 95% CI, 0.63 to 0.98). Subsequent to 2008, a structured search[6] finds 26 additional studies.[7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30] Although the benefit for CA in both adults and children has remained robust, even more so since Chan's review, mortality reductions in adult patients appear to have had the most notable shift. In aggregate, the point estimate (for those studies providing analyzable data), for adult mortality has strengthened to 0.88, with a confidence interval of 0.82‐0.96 in favor of the RRS strategy.
This change has occurred as the analyzable studies since 2008 have all had favorable point estimates, and 4 have had statistically significant confidence intervals. Prior to 2008, 5 had unfavorable point estimates, and only 2 had favorable confidence intervals. As RRSs expand, the benefits, although not universal (some hospitals still experience no improvement in outcomes), seem to be getting stronger and more consistent. This may be secondary to maturation of the intervention and implementation strategies, or it may be the result of secular trends outside of the RRS intervention, although studies controlling for this found it not to be the case.[10] The factors associated with successful implementation of the RRS or improved outcomes include knowledge of activation criteria, communication, teamwork, lack of criticism for activating the RRS, and better attitudes about the team's positive effect on nurses and patients. Many of these factors relate to an improved safety culture in general. Additionally, activation rates may have increased in more recent studies, as greater utilization is associated with improved outcomes.[31] Finally, RRSs, like other patient‐safety and quality interventions, mature with time, often taking several years before they have a full effect on outcomes.[31, 32]
Despite these more favorable results for RRSs, we still see a large discrepancy between the magnitude of benefit for CA and mortality. This may partly be because the exposure groups are different; most studies examined non‐ICU CA, yet studies reporting mortality used total hospital mortality (ICU and non‐ICU). Additionally, although RRSs may effectively prevent CA, this intervention may have a more limited effect in preventing the patient's ultimate demise (particularly in the ICU).
We also still see that effectiveness reports for RRSs continue to be of low to moderate quality. Many reports give no statistics or denominator data or have missing data. Few control for secular trends in providers, outcomes, and confounders. Outcome measures vary widely, and none conducted blinded outcome assessments. Most studies use a pre‐post design without concurrent controls, substantially increasing the risk of bias. The better‐designed studies that use concurrent controls or cluster randomization (Priestley,[33] Bristow,[34] and the MERIT trial[35]) tend to show lower treatment effects, although interestingly in the MERIT trial, while the cluster‐randomized data showed no benefit, the pre‐post data showed significant improvement in the RRS intervention hospitals. These results have been attributed to the control hospitals using their code teams for RRS activities,[36] negating a comparative improvement in the intervention hospitals.
Can we improve RRS research? Likely, yes. We can begin by being more careful about defining the exposure group. Ideally, studies should not include data from the ICU or the emergency department because these patient populations are not part of the exposure group. Although most studies removed ICU and emergency department data for CA, they did not do so for hospital mortality. ICU mortality is likely biased, because only a small proportion of ICU patients have been exposed to an RRS. Definitions also need to be stringent and uniform. For example, CA may be defined in a variety of ways such as calling the code team versus documented cardiopulmonary resuscitation. Unexpected hospital mortality is often defined as excluding patients with do not resuscitate (DNR) orders, but this may or may not accurately exclude expected deaths. We also need to better attempt to control for confounders and secular trends. Outcomes such as CA and mortality are strongly influenced by changes in patient case‐mix over time, the frequency of care limitation/DNR orders, or by poor triage decisions.[37] Outcomes such as unanticipated ICU admission are indirect and may be heavily influenced by local cultural factors. Finally, authors need to provide robust statistical data and clear numerators and denominators to support their conclusions.
Although we need to do our best to improve the quality of the RRS literature, the near ubiquitous presence of this patient‐safety intervention in North American hospitals raises a crucial question, Do we even need more effectiveness studies and if so what kind? Randomized controlled trials are not likely. It is hard to argue that we still sit at a position of equipoise, and randomizing patients who are deteriorating to standard care versus an RRS is neither practical nor ethical. Finding appropriate concurrent control hospitals that have not implemented some type of RRS would also be very difficult.
We should, however, continue to test the effectiveness of RRSs but in a more diverse manner. RRSs should be more directly compared to other interventions that can improve the problem of failure to rescue such as increased nurse staffing[38, 39, 40] and hospitalist staffing.[41] The low sensitivity and accuracy of monitoring vital signs on general wards by staff is also an area strongly deserving of investigation, as it is likely central to the problem. Researchers have sought to use various combinations of vital signs, including aggregated or weighted scoring systems, and recent data suggest some approaches may be superior to others.[42] Many have advocated for continuous monitoring of a limited set of vital signs similar to the ICU, and there are some recent data indicating that this might be effective.[43, 44] This work is in the early stages, and we do not yet know whether this strategy will affect outcomes. It is conceivable that if the false alarm rate can be kept very low and we can minimize the failure to recognize deteriorating patients (good sensitivity, specificity, and positive predictive value), the need for the RRS response team may be reduced or even eliminated. Additionally, as electronic medical records (EMRs) have expanded, there has been growing interest in leveraging these systems to improve the effectiveness of RRSs.[45] There is a tremendous amount of information within the EMRs that can be used to complement vital‐sign monitoring (manual or continuous), because baseline medical problems, laboratory values, and recent history may have a strong impact on the predictive value of changes in vital signs.
Research should also focus on the possible unintended consequences, costs, and the cost‐effectiveness of RRSs compared with other interventions that can or may reduce the rate of failure to rescue. Certainly, establishing RRSs has costs including staff time and the need to pull staff from other clinical duties to respond. Unintended harm, such as diversion of ICU staff from their usual care, are often mentioned but never rigorously evaluated. Increasing nurse staffing has very substantial costs, but how these costs compare to the costs of the RRS are unclear, although likely the comparison would be very favorable to the RRS, because staffing typically relies on existing employees with expertise in caring for the critically ill as opposed to workforce expansion. Given the current healthcare economic climate, any model that relies on additional employees is not likely to gain support. Establishing continuous monitoring systems have up‐front capital costs, although they may reduce other costs in the long run (eg, staff, medical liability). They also have intangible costs for provider workload if the false alarm rates are too high. Again, this strategy is too new to know the answers to these concerns. As we move forward, such evaluations are needed to guide policy decisions.
We also need more evaluation of RRS implementation science. The optimal way to organize, train, and staff RRSs is unknown. Most programs use physician‐led teams, although some use nurse‐led teams. Few studies have compared the various models, although 1 study that compared a resident‐led to an attending‐led team found no difference.[17] Education is ubiquitous, although actual staff training (simulation for example) is not commonly described. In addition, there is wide variation in the frequency of RRS activation. We know nurses and residents often feel pressured not to activate RRSs, and much of the success of the RRS relies on nurses identifying deteriorating patients and calling the response team. The use of continuous monitoring combined with automatic notification of staff may reduce the barriers to activating RRSs, increasing activation rates, but until then we need more understanding of how to break down these barriers. Family/patient access to activation has also gained ground (1 program demonstrated outcome improvement only after this was established[13]), but is not yet widespread.
The role of the RRS in improving processes of care, such as the appropriate institution of DNR orders, end of life/palliative care discussions, and early goal‐directed therapy for sepsis, have been presented in several studies[46, 47] but remain inadequately evaluated. Here too, there is much to learn about how we might realize the full effectiveness of this patient‐safety strategy beyond outcomes such as CA and hospital mortality. Ideally, if all appropriate patients had DNR orders and we stopped failing to recognize and respond to deteriorating ward patients, CAs on general hospital wards could be nearly eliminated.
RRSs have been described as a band‐aid for a failed model of general ward care.[37] What is clear is that many patients suffer preventable harm from unrecognized deterioration. This needs to be challenged, but are RRSs the best intervention? Despite the Joint Commission's Patient Safety Goal 16, should we still question their implementation? Should we (and the Joint Commission) reconsider our approach and prioritize our efforts elsewhere or should we feel comfortable with the investment that we have made in these systems? Even though there are many unknowns, and the quality of RRS studies needs improvement, the literature is accumulating that RRSs do reduce non‐ICU CA and improve hospital mortality. Without direct comparison studies demonstrating superiority of other expensive strategies, there is little reason to reconsider the RRS concept or question their implementation and our investment. We should instead invest further in this foundational patient‐safety strategy to make it as effective as it can be.
Disclosures: Dr. Pronovost reports the following potential conflicts of interest: grant or contract support from the Agency for Healthcare Research and Quality, and the Gordon and Betty Moore Foundation (research related to patient safety and quality of care), and the National Institutes of Health (acute lung injury research); consulting fees from the Association of Professionals in Infection Control and Epidemiology, Inc.; honoraria from various hospitals, health systems, and the Leigh Bureau to speak on quality and patient safety; book royalties from the Penguin Group; and board membership for the Cantel Medical Group. Dr. Winters reports the following potential conflicts of interest: contract or grant support from Masimo Corporation, honoraria from 3M Corporation and various hospitals and health systems, royalties from Lippincott Williams &Wilkins (UptoDate), and consulting fees from several legal firms for medical legal consulting.
- , , . Rapid response teams: walk, don't run. JAMA. 2006;296:1645–1647.
- Joint Commission requirement: The Joint Commission announces the 2008 National Patient Safety Goals and Requirements. Jt Comm Perspect. 2007;27(7):1–22.
- Institute for Healthcare Improvement. 5 million lives campaign: overview. Available at: http://www.ihi.org/offerings/Initiatives/PastStrategicInitiatives/5MillionLivesCampaign/Pages/default.aspx. Accessed November 28, 2012.
- , , , , , . Rapid response systems: a systematic review. Crit Care Med. 2007;35:1238–1243.
- , , , , . Rapid response teams: a systematic review and meta‐analysis. Arch Intern Med. 2010;170:18–26.
- , , , , , . Rapid‐response systems as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158:417–425.
- , , , , , . Hospital‐wide code rates and mortality before and after implementation of a rapid response team. JAMA. 2008;300:2506–2513.
- , , , . Experience of pediatric rapid response team in a tertiary care hospital in Pakistan. Indian J Pediatr. 2010;77:273–276.
- , , , et al. Rescue me: saving the vulnerable non‐ICU patient population. Jt Comm J Qual Patient Saf. 2009;35:199–205.
- , , , , . Reduction in hospital‐wide mortality after implementation of a rapid response team: a long‐term cohort study. Crit Care. 2011;15:R269.
- , , , , . Using an advanced practice nursing model for a rapid response team. Jt Comm J Qual Patient Saf. 2008;34:743–747.
- , , , , , . Immediate and long‐term impact of medical emergency teams on cardiac arrest prevalence and mortality: a plea for periodic basic life‐support training programs. Crit Care Med. 2009;37:3054–3061.
- , , , , , . Successful implementation of a family and patient activated rapid response team in an adult level 1 trauma center. Resuscitation. 2010;81:1676–1681.
- , , , et al. A reduction in cardiac arrests and duration of clinical instability after implementation of a paediatric rapid response system. Qual Saf Health Care. 2009;18:500–504.
- , , , et al. Implementing a rapid response team to decrease emergencies outside the ICU: one hospital's experience. Medsurg Nurs. 2009;18:84–90, 126.
- , , , et al. Sustained effectiveness of a primary‐team‐based rapid response system. Crit Care Med. 2012;40:2562–2568.
- , , , . Association between implementation of an intensivist‐led medical emergency team and mortality. BMJ Qual Saf. 2012;21:152–159.
- , , , , , . Reducing in‐hospital cardiac arrests and hospital mortality by introducing a medical emergency team. Intensive Care Med. 2010;36:100–106.
- , , , et al. Implementation of a multicenter rapid response system in pediatric academic hospitals is effective. Pediatrics. 2011;128:72–78.
- , . The impact of medical emergency teams on ICU admission rates, cardiopulmonary arrests and mortality in a regional hospital. Resuscitation. 2011;82:707–712.
- , , , . Introduction of a rapid response system at a United States veterans affairs hospital reduced cardiac arrests. Anesth Analg. 2010;111:679–686.
- , , , . The effect of the medical emergency team on unexpected cardiac arrest and death at the VA Caribbean healthcare system: a retrospective study. Crit Care Shock. 2010;13:98–105.
- , , , , . Four years' experience with a hospitalist‐led medical emergency team: an interrupted time series. J Hosp Med. 2012;7:98–103.
- , , . Changing cardiac arrest and hospital mortality rates through a medical emergency team takes time and constant review. Crit Care Med. 2010;38:445–450.
- , , , et al. Clinical emergencies and outcomes in patients admitted to a surgical versus medical service. Resuscitation. 2011;82:415–418.
- , , , . Evaluating a new rapid response team: NP‐led versus intensivist‐led comparisons. AACN Adv Crit Care. 2012;23:32–42.
- , . Implementation of a rapid response team: a success story. Crit Care Nurse. 2009;29:66–75.
- , , , . Rapid response team in an academic institution: does it make a difference? Chest. 2011;139:1361–1367.
- , . Reduction of hospital mortality and of preventable cardiac arrest and death on introduction of a pediatric medical emergency team. Ped Crit Care Med. 2009;10:306–312.
- , . Medical emergency teams are associated with reduced mortality across a major metropolitan health network after two years service: a retrospective study using government administrative data. Crit Care. 2012;16:R210.
- , , , , et al. Long term effect of a medical emergency team on cardiac arrests in a teaching hospital. Crit Care. 2005;9:R808–R815.
- , , , . Six year audit of cardiac arrests and medical emergency team calls in an Australian outer metropolitan teaching hospital. BMJ. 2007;335:1210–1212.
- , , , et al. Introducing Critical Care Outreach: a ward‐randomised trial of phased introduction in a general hospital. Intensive Care Med. 2004;30:1398–1404.
- , , , et al. Rates of in‐hospital arrests, deaths, and intensive care admissions: the effect of a medical emergency team. Med J Aust. 2000;173:236–240.
- , , , et al. Introduction of the medical emergency team (MET) system: a cluster randomised controlled trial. Lancet. 2005;365:2091–2097.
- , , , , , . The effectiveness of implementation of the medical emergency team (MET) system and factors associated with use during the MERIT study. Crit Care Resusc. 2007;9:206–212.
- , . Rethinking rapid response teams. JAMA. 2010;304:1375–1376.
- , , . Lower mortality for abdominal aortic aneurysm repair in high‐volume hospitals is contingent upon nurse staffing [published online ahead of print October 22, 2012]. Health Serv Res. doi: 10.1111/1475–6773.12004.
- , , , , . Nurse‐staffing levels and the quality of care in hospitals. N Engl J Med. 2002;346:1715–1722.
- . The association of registered nurse staffing levels and patient outcomes: systematic review and meta‐analysis. Med Care. 2007;45:1195–1204.
- , , , , , . Outcomes of care by hospitalists, general internists, and family physicians. N Engl J Med. 2007;357:2589–2600.
- , , , , . The ability of the National Early Warning Score (NEWS) to discriminate patients at risk of early cardiac arrest, unanticipated intensive care unit admission, and death. Resuscitation. 2013;84:465–470.
- , , , . Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before‐and‐after concurrence study. Anesthesiology. 2010;112:282–287.
- , , , et al. A controlled trial of electronic automated advisory vital signs monitoring in general hospital wards. Crit Care Med. 2012;40:2349–2361.
- Agency for Healthcare Research and Quality. Early warning scoring system proactively identifies patients at risk of deterioration, leading to fewer cardiopulmonary emergencies and deaths. Available at: http://www.innovations.ahrq.gov/content.aspx?id=2607. Accessed March 26, 2013.
- , , , et al. Effect of a rapid response system for patients in shock on time to treatment and mortality during 5 years. Crit Care Med. 2007;35:2568–2575.
- , , , , , . The medical emergency team and end‐of‐life care: a pilot study. Crit Care Resusc. 2007;9:151–156.
In 2006,[1] we questioned whether rapid response systems (RRSs) were an effective strategy for detecting and managing deteriorating general ward patients. Since then, the implementation of RRSs has flourished, especially in the United States where accreditors (Joint Commission)[2] and patient‐safety organizations (Institute for Healthcare Improvement 100,000 Live Campaign)[3] have strongly supported RRSs. Decades of evidence show that general ward patients often experience unrecognized deterioration and cardiorespiratory arrest (CA). The low sensitivity and accuracy of periodic assessments by staff are thought to be a major reason for these lapses, as are imbalances between patient needs and clinician (primarily nursing) resources. Additionally, a medical culture that punishes speaking up or bypassing the chain of command are also likely contributors to the problem. A system that effectively recognizes the early signs of deterioration and quickly responds should catch problems before they become life threatening. Over the last decade, RRSs have been the primary intervention implemented to do this. The potential for RRSs to improve outcomes has strong face validity, but researchers have struggled to demonstrate consistent improvements in outcomes across institutions. Given this, are RRSs the best intervention to prevent this failure to rescue? In this editorial we examine the progress of RRSs, how they compare to other options, and we consider whether we should continue to question their implementation.
In our 2007 systematic review,[4] we concluded there was weak to moderate evidence supporting RRSs. Since then, 6 other systematic reviews of the effectiveness or implementation of RRSs have been published. One high‐quality review of effectiveness studies published through 2008 by Chan et al.[5] found that RRSs significantly reduced non‐intensive care unit (ICU) CA (relative risk [RR], 0.66; 95% confidence interval [CI], 0.54‐0.80), but not total hospital mortality (RR, 0.96; 95% CI, 0.84‐1.09) in adult inpatients. In pediatric inpatients, RRSs led to significant improvements in both non‐ICU CA (RR, 0.62; 95% CI, 0.46 to 0.84) and total hospital mortality (RR, 0.79; 95% CI, 0.63 to 0.98). Subsequent to 2008, a structured search[6] finds 26 additional studies.[7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30] Although the benefit for CA in both adults and children has remained robust, even more so since Chan's review, mortality reductions in adult patients appear to have had the most notable shift. In aggregate, the point estimate (for those studies providing analyzable data), for adult mortality has strengthened to 0.88, with a confidence interval of 0.82‐0.96 in favor of the RRS strategy.
This change has occurred as the analyzable studies since 2008 have all had favorable point estimates, and 4 have had statistically significant confidence intervals. Prior to 2008, 5 had unfavorable point estimates, and only 2 had favorable confidence intervals. As RRSs expand, the benefits, although not universal (some hospitals still experience no improvement in outcomes), seem to be getting stronger and more consistent. This may be secondary to maturation of the intervention and implementation strategies, or it may be the result of secular trends outside of the RRS intervention, although studies controlling for this found it not to be the case.[10] The factors associated with successful implementation of the RRS or improved outcomes include knowledge of activation criteria, communication, teamwork, lack of criticism for activating the RRS, and better attitudes about the team's positive effect on nurses and patients. Many of these factors relate to an improved safety culture in general. Additionally, activation rates may have increased in more recent studies, as greater utilization is associated with improved outcomes.[31] Finally, RRSs, like other patient‐safety and quality interventions, mature with time, often taking several years before they have a full effect on outcomes.[31, 32]
Despite these more favorable results for RRSs, we still see a large discrepancy between the magnitude of benefit for CA and mortality. This may partly be because the exposure groups are different; most studies examined non‐ICU CA, yet studies reporting mortality used total hospital mortality (ICU and non‐ICU). Additionally, although RRSs may effectively prevent CA, this intervention may have a more limited effect in preventing the patient's ultimate demise (particularly in the ICU).
We also still see that effectiveness reports for RRSs continue to be of low to moderate quality. Many reports give no statistics or denominator data or have missing data. Few control for secular trends in providers, outcomes, and confounders. Outcome measures vary widely, and none conducted blinded outcome assessments. Most studies use a pre‐post design without concurrent controls, substantially increasing the risk of bias. The better‐designed studies that use concurrent controls or cluster randomization (Priestley,[33] Bristow,[34] and the MERIT trial[35]) tend to show lower treatment effects, although interestingly in the MERIT trial, while the cluster‐randomized data showed no benefit, the pre‐post data showed significant improvement in the RRS intervention hospitals. These results have been attributed to the control hospitals using their code teams for RRS activities,[36] negating a comparative improvement in the intervention hospitals.
Can we improve RRS research? Likely, yes. We can begin by being more careful about defining the exposure group. Ideally, studies should not include data from the ICU or the emergency department because these patient populations are not part of the exposure group. Although most studies removed ICU and emergency department data for CA, they did not do so for hospital mortality. ICU mortality is likely biased, because only a small proportion of ICU patients have been exposed to an RRS. Definitions also need to be stringent and uniform. For example, CA may be defined in a variety of ways such as calling the code team versus documented cardiopulmonary resuscitation. Unexpected hospital mortality is often defined as excluding patients with do not resuscitate (DNR) orders, but this may or may not accurately exclude expected deaths. We also need to better attempt to control for confounders and secular trends. Outcomes such as CA and mortality are strongly influenced by changes in patient case‐mix over time, the frequency of care limitation/DNR orders, or by poor triage decisions.[37] Outcomes such as unanticipated ICU admission are indirect and may be heavily influenced by local cultural factors. Finally, authors need to provide robust statistical data and clear numerators and denominators to support their conclusions.
Although we need to do our best to improve the quality of the RRS literature, the near ubiquitous presence of this patient‐safety intervention in North American hospitals raises a crucial question, Do we even need more effectiveness studies and if so what kind? Randomized controlled trials are not likely. It is hard to argue that we still sit at a position of equipoise, and randomizing patients who are deteriorating to standard care versus an RRS is neither practical nor ethical. Finding appropriate concurrent control hospitals that have not implemented some type of RRS would also be very difficult.
We should, however, continue to test the effectiveness of RRSs but in a more diverse manner. RRSs should be more directly compared to other interventions that can improve the problem of failure to rescue such as increased nurse staffing[38, 39, 40] and hospitalist staffing.[41] The low sensitivity and accuracy of monitoring vital signs on general wards by staff is also an area strongly deserving of investigation, as it is likely central to the problem. Researchers have sought to use various combinations of vital signs, including aggregated or weighted scoring systems, and recent data suggest some approaches may be superior to others.[42] Many have advocated for continuous monitoring of a limited set of vital signs similar to the ICU, and there are some recent data indicating that this might be effective.[43, 44] This work is in the early stages, and we do not yet know whether this strategy will affect outcomes. It is conceivable that if the false alarm rate can be kept very low and we can minimize the failure to recognize deteriorating patients (good sensitivity, specificity, and positive predictive value), the need for the RRS response team may be reduced or even eliminated. Additionally, as electronic medical records (EMRs) have expanded, there has been growing interest in leveraging these systems to improve the effectiveness of RRSs.[45] There is a tremendous amount of information within the EMRs that can be used to complement vital‐sign monitoring (manual or continuous), because baseline medical problems, laboratory values, and recent history may have a strong impact on the predictive value of changes in vital signs.
Research should also focus on the possible unintended consequences, costs, and the cost‐effectiveness of RRSs compared with other interventions that can or may reduce the rate of failure to rescue. Certainly, establishing RRSs has costs including staff time and the need to pull staff from other clinical duties to respond. Unintended harm, such as diversion of ICU staff from their usual care, are often mentioned but never rigorously evaluated. Increasing nurse staffing has very substantial costs, but how these costs compare to the costs of the RRS are unclear, although likely the comparison would be very favorable to the RRS, because staffing typically relies on existing employees with expertise in caring for the critically ill as opposed to workforce expansion. Given the current healthcare economic climate, any model that relies on additional employees is not likely to gain support. Establishing continuous monitoring systems have up‐front capital costs, although they may reduce other costs in the long run (eg, staff, medical liability). They also have intangible costs for provider workload if the false alarm rates are too high. Again, this strategy is too new to know the answers to these concerns. As we move forward, such evaluations are needed to guide policy decisions.
We also need more evaluation of RRS implementation science. The optimal way to organize, train, and staff RRSs is unknown. Most programs use physician‐led teams, although some use nurse‐led teams. Few studies have compared the various models, although 1 study that compared a resident‐led to an attending‐led team found no difference.[17] Education is ubiquitous, although actual staff training (simulation for example) is not commonly described. In addition, there is wide variation in the frequency of RRS activation. We know nurses and residents often feel pressured not to activate RRSs, and much of the success of the RRS relies on nurses identifying deteriorating patients and calling the response team. The use of continuous monitoring combined with automatic notification of staff may reduce the barriers to activating RRSs, increasing activation rates, but until then we need more understanding of how to break down these barriers. Family/patient access to activation has also gained ground (1 program demonstrated outcome improvement only after this was established[13]), but is not yet widespread.
The role of the RRS in improving processes of care, such as the appropriate institution of DNR orders, end of life/palliative care discussions, and early goal‐directed therapy for sepsis, have been presented in several studies[46, 47] but remain inadequately evaluated. Here too, there is much to learn about how we might realize the full effectiveness of this patient‐safety strategy beyond outcomes such as CA and hospital mortality. Ideally, if all appropriate patients had DNR orders and we stopped failing to recognize and respond to deteriorating ward patients, CAs on general hospital wards could be nearly eliminated.
RRSs have been described as a band‐aid for a failed model of general ward care.[37] What is clear is that many patients suffer preventable harm from unrecognized deterioration. This needs to be challenged, but are RRSs the best intervention? Despite the Joint Commission's Patient Safety Goal 16, should we still question their implementation? Should we (and the Joint Commission) reconsider our approach and prioritize our efforts elsewhere or should we feel comfortable with the investment that we have made in these systems? Even though there are many unknowns, and the quality of RRS studies needs improvement, the literature is accumulating that RRSs do reduce non‐ICU CA and improve hospital mortality. Without direct comparison studies demonstrating superiority of other expensive strategies, there is little reason to reconsider the RRS concept or question their implementation and our investment. We should instead invest further in this foundational patient‐safety strategy to make it as effective as it can be.
Disclosures: Dr. Pronovost reports the following potential conflicts of interest: grant or contract support from the Agency for Healthcare Research and Quality, and the Gordon and Betty Moore Foundation (research related to patient safety and quality of care), and the National Institutes of Health (acute lung injury research); consulting fees from the Association of Professionals in Infection Control and Epidemiology, Inc.; honoraria from various hospitals, health systems, and the Leigh Bureau to speak on quality and patient safety; book royalties from the Penguin Group; and board membership for the Cantel Medical Group. Dr. Winters reports the following potential conflicts of interest: contract or grant support from Masimo Corporation, honoraria from 3M Corporation and various hospitals and health systems, royalties from Lippincott Williams &Wilkins (UptoDate), and consulting fees from several legal firms for medical legal consulting.
In 2006,[1] we questioned whether rapid response systems (RRSs) were an effective strategy for detecting and managing deteriorating general ward patients. Since then, the implementation of RRSs has flourished, especially in the United States where accreditors (Joint Commission)[2] and patient‐safety organizations (Institute for Healthcare Improvement 100,000 Live Campaign)[3] have strongly supported RRSs. Decades of evidence show that general ward patients often experience unrecognized deterioration and cardiorespiratory arrest (CA). The low sensitivity and accuracy of periodic assessments by staff are thought to be a major reason for these lapses, as are imbalances between patient needs and clinician (primarily nursing) resources. Additionally, a medical culture that punishes speaking up or bypassing the chain of command are also likely contributors to the problem. A system that effectively recognizes the early signs of deterioration and quickly responds should catch problems before they become life threatening. Over the last decade, RRSs have been the primary intervention implemented to do this. The potential for RRSs to improve outcomes has strong face validity, but researchers have struggled to demonstrate consistent improvements in outcomes across institutions. Given this, are RRSs the best intervention to prevent this failure to rescue? In this editorial we examine the progress of RRSs, how they compare to other options, and we consider whether we should continue to question their implementation.
In our 2007 systematic review,[4] we concluded there was weak to moderate evidence supporting RRSs. Since then, 6 other systematic reviews of the effectiveness or implementation of RRSs have been published. One high‐quality review of effectiveness studies published through 2008 by Chan et al.[5] found that RRSs significantly reduced non‐intensive care unit (ICU) CA (relative risk [RR], 0.66; 95% confidence interval [CI], 0.54‐0.80), but not total hospital mortality (RR, 0.96; 95% CI, 0.84‐1.09) in adult inpatients. In pediatric inpatients, RRSs led to significant improvements in both non‐ICU CA (RR, 0.62; 95% CI, 0.46 to 0.84) and total hospital mortality (RR, 0.79; 95% CI, 0.63 to 0.98). Subsequent to 2008, a structured search[6] finds 26 additional studies.[7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30] Although the benefit for CA in both adults and children has remained robust, even more so since Chan's review, mortality reductions in adult patients appear to have had the most notable shift. In aggregate, the point estimate (for those studies providing analyzable data), for adult mortality has strengthened to 0.88, with a confidence interval of 0.82‐0.96 in favor of the RRS strategy.
This change has occurred as the analyzable studies since 2008 have all had favorable point estimates, and 4 have had statistically significant confidence intervals. Prior to 2008, 5 had unfavorable point estimates, and only 2 had favorable confidence intervals. As RRSs expand, the benefits, although not universal (some hospitals still experience no improvement in outcomes), seem to be getting stronger and more consistent. This may be secondary to maturation of the intervention and implementation strategies, or it may be the result of secular trends outside of the RRS intervention, although studies controlling for this found it not to be the case.[10] The factors associated with successful implementation of the RRS or improved outcomes include knowledge of activation criteria, communication, teamwork, lack of criticism for activating the RRS, and better attitudes about the team's positive effect on nurses and patients. Many of these factors relate to an improved safety culture in general. Additionally, activation rates may have increased in more recent studies, as greater utilization is associated with improved outcomes.[31] Finally, RRSs, like other patient‐safety and quality interventions, mature with time, often taking several years before they have a full effect on outcomes.[31, 32]
Despite these more favorable results for RRSs, we still see a large discrepancy between the magnitude of benefit for CA and mortality. This may partly be because the exposure groups are different; most studies examined non‐ICU CA, yet studies reporting mortality used total hospital mortality (ICU and non‐ICU). Additionally, although RRSs may effectively prevent CA, this intervention may have a more limited effect in preventing the patient's ultimate demise (particularly in the ICU).
We also still see that effectiveness reports for RRSs continue to be of low to moderate quality. Many reports give no statistics or denominator data or have missing data. Few control for secular trends in providers, outcomes, and confounders. Outcome measures vary widely, and none conducted blinded outcome assessments. Most studies use a pre‐post design without concurrent controls, substantially increasing the risk of bias. The better‐designed studies that use concurrent controls or cluster randomization (Priestley,[33] Bristow,[34] and the MERIT trial[35]) tend to show lower treatment effects, although interestingly in the MERIT trial, while the cluster‐randomized data showed no benefit, the pre‐post data showed significant improvement in the RRS intervention hospitals. These results have been attributed to the control hospitals using their code teams for RRS activities,[36] negating a comparative improvement in the intervention hospitals.
Can we improve RRS research? Likely, yes. We can begin by being more careful about defining the exposure group. Ideally, studies should not include data from the ICU or the emergency department because these patient populations are not part of the exposure group. Although most studies removed ICU and emergency department data for CA, they did not do so for hospital mortality. ICU mortality is likely biased, because only a small proportion of ICU patients have been exposed to an RRS. Definitions also need to be stringent and uniform. For example, CA may be defined in a variety of ways such as calling the code team versus documented cardiopulmonary resuscitation. Unexpected hospital mortality is often defined as excluding patients with do not resuscitate (DNR) orders, but this may or may not accurately exclude expected deaths. We also need to better attempt to control for confounders and secular trends. Outcomes such as CA and mortality are strongly influenced by changes in patient case‐mix over time, the frequency of care limitation/DNR orders, or by poor triage decisions.[37] Outcomes such as unanticipated ICU admission are indirect and may be heavily influenced by local cultural factors. Finally, authors need to provide robust statistical data and clear numerators and denominators to support their conclusions.
Although we need to do our best to improve the quality of the RRS literature, the near ubiquitous presence of this patient‐safety intervention in North American hospitals raises a crucial question, Do we even need more effectiveness studies and if so what kind? Randomized controlled trials are not likely. It is hard to argue that we still sit at a position of equipoise, and randomizing patients who are deteriorating to standard care versus an RRS is neither practical nor ethical. Finding appropriate concurrent control hospitals that have not implemented some type of RRS would also be very difficult.
We should, however, continue to test the effectiveness of RRSs but in a more diverse manner. RRSs should be more directly compared to other interventions that can improve the problem of failure to rescue such as increased nurse staffing[38, 39, 40] and hospitalist staffing.[41] The low sensitivity and accuracy of monitoring vital signs on general wards by staff is also an area strongly deserving of investigation, as it is likely central to the problem. Researchers have sought to use various combinations of vital signs, including aggregated or weighted scoring systems, and recent data suggest some approaches may be superior to others.[42] Many have advocated for continuous monitoring of a limited set of vital signs similar to the ICU, and there are some recent data indicating that this might be effective.[43, 44] This work is in the early stages, and we do not yet know whether this strategy will affect outcomes. It is conceivable that if the false alarm rate can be kept very low and we can minimize the failure to recognize deteriorating patients (good sensitivity, specificity, and positive predictive value), the need for the RRS response team may be reduced or even eliminated. Additionally, as electronic medical records (EMRs) have expanded, there has been growing interest in leveraging these systems to improve the effectiveness of RRSs.[45] There is a tremendous amount of information within the EMRs that can be used to complement vital‐sign monitoring (manual or continuous), because baseline medical problems, laboratory values, and recent history may have a strong impact on the predictive value of changes in vital signs.
Research should also focus on the possible unintended consequences, costs, and the cost‐effectiveness of RRSs compared with other interventions that can or may reduce the rate of failure to rescue. Certainly, establishing RRSs has costs including staff time and the need to pull staff from other clinical duties to respond. Unintended harm, such as diversion of ICU staff from their usual care, are often mentioned but never rigorously evaluated. Increasing nurse staffing has very substantial costs, but how these costs compare to the costs of the RRS are unclear, although likely the comparison would be very favorable to the RRS, because staffing typically relies on existing employees with expertise in caring for the critically ill as opposed to workforce expansion. Given the current healthcare economic climate, any model that relies on additional employees is not likely to gain support. Establishing continuous monitoring systems have up‐front capital costs, although they may reduce other costs in the long run (eg, staff, medical liability). They also have intangible costs for provider workload if the false alarm rates are too high. Again, this strategy is too new to know the answers to these concerns. As we move forward, such evaluations are needed to guide policy decisions.
We also need more evaluation of RRS implementation science. The optimal way to organize, train, and staff RRSs is unknown. Most programs use physician‐led teams, although some use nurse‐led teams. Few studies have compared the various models, although 1 study that compared a resident‐led to an attending‐led team found no difference.[17] Education is ubiquitous, although actual staff training (simulation for example) is not commonly described. In addition, there is wide variation in the frequency of RRS activation. We know nurses and residents often feel pressured not to activate RRSs, and much of the success of the RRS relies on nurses identifying deteriorating patients and calling the response team. The use of continuous monitoring combined with automatic notification of staff may reduce the barriers to activating RRSs, increasing activation rates, but until then we need more understanding of how to break down these barriers. Family/patient access to activation has also gained ground (1 program demonstrated outcome improvement only after this was established[13]), but is not yet widespread.
The role of the RRS in improving processes of care, such as the appropriate institution of DNR orders, end of life/palliative care discussions, and early goal‐directed therapy for sepsis, have been presented in several studies[46, 47] but remain inadequately evaluated. Here too, there is much to learn about how we might realize the full effectiveness of this patient‐safety strategy beyond outcomes such as CA and hospital mortality. Ideally, if all appropriate patients had DNR orders and we stopped failing to recognize and respond to deteriorating ward patients, CAs on general hospital wards could be nearly eliminated.
RRSs have been described as a band‐aid for a failed model of general ward care.[37] What is clear is that many patients suffer preventable harm from unrecognized deterioration. This needs to be challenged, but are RRSs the best intervention? Despite the Joint Commission's Patient Safety Goal 16, should we still question their implementation? Should we (and the Joint Commission) reconsider our approach and prioritize our efforts elsewhere or should we feel comfortable with the investment that we have made in these systems? Even though there are many unknowns, and the quality of RRS studies needs improvement, the literature is accumulating that RRSs do reduce non‐ICU CA and improve hospital mortality. Without direct comparison studies demonstrating superiority of other expensive strategies, there is little reason to reconsider the RRS concept or question their implementation and our investment. We should instead invest further in this foundational patient‐safety strategy to make it as effective as it can be.
Disclosures: Dr. Pronovost reports the following potential conflicts of interest: grant or contract support from the Agency for Healthcare Research and Quality, and the Gordon and Betty Moore Foundation (research related to patient safety and quality of care), and the National Institutes of Health (acute lung injury research); consulting fees from the Association of Professionals in Infection Control and Epidemiology, Inc.; honoraria from various hospitals, health systems, and the Leigh Bureau to speak on quality and patient safety; book royalties from the Penguin Group; and board membership for the Cantel Medical Group. Dr. Winters reports the following potential conflicts of interest: contract or grant support from Masimo Corporation, honoraria from 3M Corporation and various hospitals and health systems, royalties from Lippincott Williams &Wilkins (UptoDate), and consulting fees from several legal firms for medical legal consulting.
- , , . Rapid response teams: walk, don't run. JAMA. 2006;296:1645–1647.
- Joint Commission requirement: The Joint Commission announces the 2008 National Patient Safety Goals and Requirements. Jt Comm Perspect. 2007;27(7):1–22.
- Institute for Healthcare Improvement. 5 million lives campaign: overview. Available at: http://www.ihi.org/offerings/Initiatives/PastStrategicInitiatives/5MillionLivesCampaign/Pages/default.aspx. Accessed November 28, 2012.
- , , , , , . Rapid response systems: a systematic review. Crit Care Med. 2007;35:1238–1243.
- , , , , . Rapid response teams: a systematic review and meta‐analysis. Arch Intern Med. 2010;170:18–26.
- , , , , , . Rapid‐response systems as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158:417–425.
- , , , , , . Hospital‐wide code rates and mortality before and after implementation of a rapid response team. JAMA. 2008;300:2506–2513.
- , , , . Experience of pediatric rapid response team in a tertiary care hospital in Pakistan. Indian J Pediatr. 2010;77:273–276.
- , , , et al. Rescue me: saving the vulnerable non‐ICU patient population. Jt Comm J Qual Patient Saf. 2009;35:199–205.
- , , , , . Reduction in hospital‐wide mortality after implementation of a rapid response team: a long‐term cohort study. Crit Care. 2011;15:R269.
- , , , , . Using an advanced practice nursing model for a rapid response team. Jt Comm J Qual Patient Saf. 2008;34:743–747.
- , , , , , . Immediate and long‐term impact of medical emergency teams on cardiac arrest prevalence and mortality: a plea for periodic basic life‐support training programs. Crit Care Med. 2009;37:3054–3061.
- , , , , , . Successful implementation of a family and patient activated rapid response team in an adult level 1 trauma center. Resuscitation. 2010;81:1676–1681.
- , , , et al. A reduction in cardiac arrests and duration of clinical instability after implementation of a paediatric rapid response system. Qual Saf Health Care. 2009;18:500–504.
- , , , et al. Implementing a rapid response team to decrease emergencies outside the ICU: one hospital's experience. Medsurg Nurs. 2009;18:84–90, 126.
- , , , et al. Sustained effectiveness of a primary‐team‐based rapid response system. Crit Care Med. 2012;40:2562–2568.
- , , , . Association between implementation of an intensivist‐led medical emergency team and mortality. BMJ Qual Saf. 2012;21:152–159.
- , , , , , . Reducing in‐hospital cardiac arrests and hospital mortality by introducing a medical emergency team. Intensive Care Med. 2010;36:100–106.
- , , , et al. Implementation of a multicenter rapid response system in pediatric academic hospitals is effective. Pediatrics. 2011;128:72–78.
- , . The impact of medical emergency teams on ICU admission rates, cardiopulmonary arrests and mortality in a regional hospital. Resuscitation. 2011;82:707–712.
- , , , . Introduction of a rapid response system at a United States veterans affairs hospital reduced cardiac arrests. Anesth Analg. 2010;111:679–686.
- , , , . The effect of the medical emergency team on unexpected cardiac arrest and death at the VA Caribbean healthcare system: a retrospective study. Crit Care Shock. 2010;13:98–105.
- , , , , . Four years' experience with a hospitalist‐led medical emergency team: an interrupted time series. J Hosp Med. 2012;7:98–103.
- , , . Changing cardiac arrest and hospital mortality rates through a medical emergency team takes time and constant review. Crit Care Med. 2010;38:445–450.
- , , , et al. Clinical emergencies and outcomes in patients admitted to a surgical versus medical service. Resuscitation. 2011;82:415–418.
- , , , . Evaluating a new rapid response team: NP‐led versus intensivist‐led comparisons. AACN Adv Crit Care. 2012;23:32–42.
- , . Implementation of a rapid response team: a success story. Crit Care Nurse. 2009;29:66–75.
- , , , . Rapid response team in an academic institution: does it make a difference? Chest. 2011;139:1361–1367.
- , . Reduction of hospital mortality and of preventable cardiac arrest and death on introduction of a pediatric medical emergency team. Ped Crit Care Med. 2009;10:306–312.
- , . Medical emergency teams are associated with reduced mortality across a major metropolitan health network after two years service: a retrospective study using government administrative data. Crit Care. 2012;16:R210.
- , , , , et al. Long term effect of a medical emergency team on cardiac arrests in a teaching hospital. Crit Care. 2005;9:R808–R815.
- , , , . Six year audit of cardiac arrests and medical emergency team calls in an Australian outer metropolitan teaching hospital. BMJ. 2007;335:1210–1212.
- , , , et al. Introducing Critical Care Outreach: a ward‐randomised trial of phased introduction in a general hospital. Intensive Care Med. 2004;30:1398–1404.
- , , , et al. Rates of in‐hospital arrests, deaths, and intensive care admissions: the effect of a medical emergency team. Med J Aust. 2000;173:236–240.
- , , , et al. Introduction of the medical emergency team (MET) system: a cluster randomised controlled trial. Lancet. 2005;365:2091–2097.
- , , , , , . The effectiveness of implementation of the medical emergency team (MET) system and factors associated with use during the MERIT study. Crit Care Resusc. 2007;9:206–212.
- , . Rethinking rapid response teams. JAMA. 2010;304:1375–1376.
- , , . Lower mortality for abdominal aortic aneurysm repair in high‐volume hospitals is contingent upon nurse staffing [published online ahead of print October 22, 2012]. Health Serv Res. doi: 10.1111/1475–6773.12004.
- , , , , . Nurse‐staffing levels and the quality of care in hospitals. N Engl J Med. 2002;346:1715–1722.
- . The association of registered nurse staffing levels and patient outcomes: systematic review and meta‐analysis. Med Care. 2007;45:1195–1204.
- , , , , , . Outcomes of care by hospitalists, general internists, and family physicians. N Engl J Med. 2007;357:2589–2600.
- , , , , . The ability of the National Early Warning Score (NEWS) to discriminate patients at risk of early cardiac arrest, unanticipated intensive care unit admission, and death. Resuscitation. 2013;84:465–470.
- , , , . Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before‐and‐after concurrence study. Anesthesiology. 2010;112:282–287.
- , , , et al. A controlled trial of electronic automated advisory vital signs monitoring in general hospital wards. Crit Care Med. 2012;40:2349–2361.
- Agency for Healthcare Research and Quality. Early warning scoring system proactively identifies patients at risk of deterioration, leading to fewer cardiopulmonary emergencies and deaths. Available at: http://www.innovations.ahrq.gov/content.aspx?id=2607. Accessed March 26, 2013.
- , , , et al. Effect of a rapid response system for patients in shock on time to treatment and mortality during 5 years. Crit Care Med. 2007;35:2568–2575.
- , , , , , . The medical emergency team and end‐of‐life care: a pilot study. Crit Care Resusc. 2007;9:151–156.
- , , . Rapid response teams: walk, don't run. JAMA. 2006;296:1645–1647.
- Joint Commission requirement: The Joint Commission announces the 2008 National Patient Safety Goals and Requirements. Jt Comm Perspect. 2007;27(7):1–22.
- Institute for Healthcare Improvement. 5 million lives campaign: overview. Available at: http://www.ihi.org/offerings/Initiatives/PastStrategicInitiatives/5MillionLivesCampaign/Pages/default.aspx. Accessed November 28, 2012.
- , , , , , . Rapid response systems: a systematic review. Crit Care Med. 2007;35:1238–1243.
- , , , , . Rapid response teams: a systematic review and meta‐analysis. Arch Intern Med. 2010;170:18–26.
- , , , , , . Rapid‐response systems as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158:417–425.
- , , , , , . Hospital‐wide code rates and mortality before and after implementation of a rapid response team. JAMA. 2008;300:2506–2513.
- , , , . Experience of pediatric rapid response team in a tertiary care hospital in Pakistan. Indian J Pediatr. 2010;77:273–276.
- , , , et al. Rescue me: saving the vulnerable non‐ICU patient population. Jt Comm J Qual Patient Saf. 2009;35:199–205.
- , , , , . Reduction in hospital‐wide mortality after implementation of a rapid response team: a long‐term cohort study. Crit Care. 2011;15:R269.
- , , , , . Using an advanced practice nursing model for a rapid response team. Jt Comm J Qual Patient Saf. 2008;34:743–747.
- , , , , , . Immediate and long‐term impact of medical emergency teams on cardiac arrest prevalence and mortality: a plea for periodic basic life‐support training programs. Crit Care Med. 2009;37:3054–3061.
- , , , , , . Successful implementation of a family and patient activated rapid response team in an adult level 1 trauma center. Resuscitation. 2010;81:1676–1681.
- , , , et al. A reduction in cardiac arrests and duration of clinical instability after implementation of a paediatric rapid response system. Qual Saf Health Care. 2009;18:500–504.
- , , , et al. Implementing a rapid response team to decrease emergencies outside the ICU: one hospital's experience. Medsurg Nurs. 2009;18:84–90, 126.
- , , , et al. Sustained effectiveness of a primary‐team‐based rapid response system. Crit Care Med. 2012;40:2562–2568.
- , , , . Association between implementation of an intensivist‐led medical emergency team and mortality. BMJ Qual Saf. 2012;21:152–159.
- , , , , , . Reducing in‐hospital cardiac arrests and hospital mortality by introducing a medical emergency team. Intensive Care Med. 2010;36:100–106.
- , , , et al. Implementation of a multicenter rapid response system in pediatric academic hospitals is effective. Pediatrics. 2011;128:72–78.
- , . The impact of medical emergency teams on ICU admission rates, cardiopulmonary arrests and mortality in a regional hospital. Resuscitation. 2011;82:707–712.
- , , , . Introduction of a rapid response system at a United States veterans affairs hospital reduced cardiac arrests. Anesth Analg. 2010;111:679–686.
- , , , . The effect of the medical emergency team on unexpected cardiac arrest and death at the VA Caribbean healthcare system: a retrospective study. Crit Care Shock. 2010;13:98–105.
- , , , , . Four years' experience with a hospitalist‐led medical emergency team: an interrupted time series. J Hosp Med. 2012;7:98–103.
- , , . Changing cardiac arrest and hospital mortality rates through a medical emergency team takes time and constant review. Crit Care Med. 2010;38:445–450.
- , , , et al. Clinical emergencies and outcomes in patients admitted to a surgical versus medical service. Resuscitation. 2011;82:415–418.
- , , , . Evaluating a new rapid response team: NP‐led versus intensivist‐led comparisons. AACN Adv Crit Care. 2012;23:32–42.
- , . Implementation of a rapid response team: a success story. Crit Care Nurse. 2009;29:66–75.
- , , , . Rapid response team in an academic institution: does it make a difference? Chest. 2011;139:1361–1367.
- , . Reduction of hospital mortality and of preventable cardiac arrest and death on introduction of a pediatric medical emergency team. Ped Crit Care Med. 2009;10:306–312.
- , . Medical emergency teams are associated with reduced mortality across a major metropolitan health network after two years service: a retrospective study using government administrative data. Crit Care. 2012;16:R210.
- , , , , et al. Long term effect of a medical emergency team on cardiac arrests in a teaching hospital. Crit Care. 2005;9:R808–R815.
- , , , . Six year audit of cardiac arrests and medical emergency team calls in an Australian outer metropolitan teaching hospital. BMJ. 2007;335:1210–1212.
- , , , et al. Introducing Critical Care Outreach: a ward‐randomised trial of phased introduction in a general hospital. Intensive Care Med. 2004;30:1398–1404.
- , , , et al. Rates of in‐hospital arrests, deaths, and intensive care admissions: the effect of a medical emergency team. Med J Aust. 2000;173:236–240.
- , , , et al. Introduction of the medical emergency team (MET) system: a cluster randomised controlled trial. Lancet. 2005;365:2091–2097.
- , , , , , . The effectiveness of implementation of the medical emergency team (MET) system and factors associated with use during the MERIT study. Crit Care Resusc. 2007;9:206–212.
- , . Rethinking rapid response teams. JAMA. 2010;304:1375–1376.
- , , . Lower mortality for abdominal aortic aneurysm repair in high‐volume hospitals is contingent upon nurse staffing [published online ahead of print October 22, 2012]. Health Serv Res. doi: 10.1111/1475–6773.12004.
- , , , , . Nurse‐staffing levels and the quality of care in hospitals. N Engl J Med. 2002;346:1715–1722.
- . The association of registered nurse staffing levels and patient outcomes: systematic review and meta‐analysis. Med Care. 2007;45:1195–1204.
- , , , , , . Outcomes of care by hospitalists, general internists, and family physicians. N Engl J Med. 2007;357:2589–2600.
- , , , , . The ability of the National Early Warning Score (NEWS) to discriminate patients at risk of early cardiac arrest, unanticipated intensive care unit admission, and death. Resuscitation. 2013;84:465–470.
- , , , . Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before‐and‐after concurrence study. Anesthesiology. 2010;112:282–287.
- , , , et al. A controlled trial of electronic automated advisory vital signs monitoring in general hospital wards. Crit Care Med. 2012;40:2349–2361.
- Agency for Healthcare Research and Quality. Early warning scoring system proactively identifies patients at risk of deterioration, leading to fewer cardiopulmonary emergencies and deaths. Available at: http://www.innovations.ahrq.gov/content.aspx?id=2607. Accessed March 26, 2013.
- , , , et al. Effect of a rapid response system for patients in shock on time to treatment and mortality during 5 years. Crit Care Med. 2007;35:2568–2575.
- , , , , , . The medical emergency team and end‐of‐life care: a pilot study. Crit Care Resusc. 2007;9:151–156.
Moving Beyond Readmission Penalties
Containing the rise of healthcare costs has taken on a new sense of urgency in the wake of the recent economic recession and continued growth in the cost of healthcare. Accordingly, many stakeholders seek solutions to improve value (reducing costs while improving care)[1]; hospital readmissions, which are common and costly,[2] have emerged as a key target. The Centers for Medicare and Medicaid Services (CMS) have instituted several programs intended to reduce readmissions, including funding for community‐based, care‐transition programs; penalties for hospitals with elevated risk‐adjusted readmission rates for selected diagnoses; pioneer Accountable Care Organizations (ACOs) with incentives to reduce global costs of care; and Hospital Engagement Networks (HENs) through the Partnership for Patients.[3] A primary aim of these initiatives is to enhance the quality of care transitions as patients are discharged from the hospital.
Though the recent focus on hospital readmissions has appropriately drawn attention to transitions in care, some have expressed concerns. Among these are questions about: 1) the extent to which readmissions truly reflect the quality of hospital care[4]; 2) the preventability of readmissions[5]; 3) limitations in risk‐adjustment techniques[6]; and 4) best practices for preventing readmissions.[7] We believe these concerns stem in part from deficiencies in the state of the science of transitional care, and that future efforts in this area will be hindered without a clear vision of an ideal transition in care. We propose the key components of an ideal transition in care and discuss the implications of this concept as it pertains to hospital readmissions.
THE IDEAL TRANSITION IN CARE
We propose the key components of an ideal transition in care in Figure 1 and Table 1. Figure 1 represents 10 domains described more fully below as structural supports of the bridge patients must cross from one care environment to another during a care transition. This figure highlights key domains and suggests that lack of a domain makes the bridge weaker and more prone to gaps in care and poor outcomes. It also implies that the more components are missing, the less safe is the bridge or transition. Those domains that mainly take place prior to discharge are placed closer to the hospital side of the bridge, those that mainly take place after discharge are placed closer to the community side of the bridge, while those that take place both prior to and after discharge are in the middle. Table 1 provides descriptions of the key content for each of these domains, as well as guidance about which personnel might be involved and where in the transition process that domain should be implemented. We support these domains with supporting evidence where available.
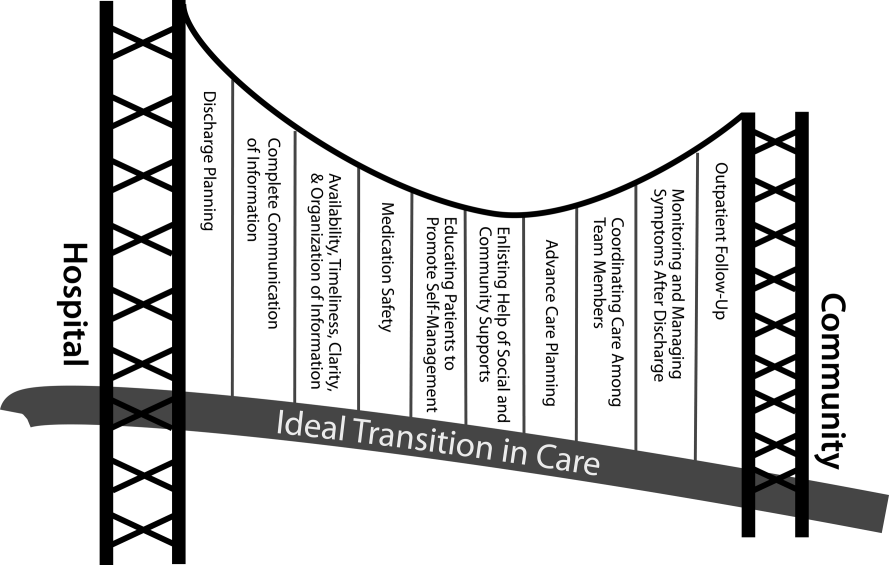
| Domain | Who | When | References |
|---|---|---|---|
| |||
| Discharge planning | |||
| Use a multidisciplinary team to create a discharge plan | Discharging clinician | Predischarge | 911 |
| Collaborate with PCP regarding discharge and follow‐up plan | Care managers/discharge planners | ||
| Arrange follow‐up appointments prior to discharge | Nurses | ||
| Make timely appointments for follow‐up care | |||
| Make appointments that take patient and caregiver's schedules and transportation needs into account | |||
| Complete communication of information | |||
| Includes: | Discharging clinician | Time of discharge | 1214 |
| Patient's full name | |||
| Age | |||
| Dates of admission and discharge | |||
| Names of responsible hospital physicians | |||
| Name of physician preparing discharge summary | |||
| Name of PCP | |||
| Main diagnosis | |||
| Other relevant diagnoses, procedures, and complications | |||
| Relevant findings at admission | |||
| Treatment and response for each active problem | |||
| Results of procedures and abnormal laboratory test results | |||
| Recommendations of any subspecialty consultants | |||
| Patient's functional status at discharge | |||
| Discharge medications | |||
| Follow‐up appointments made and those to be made | |||
| Tests to be ordered and pending tests to be followed‐up | |||
| Counseling provided to patient and caregiver, when applicable | |||
| Contingency planning | |||
| Code status | |||
| Availability, timeliness, clarity, and organization of information | |||
| Timely communication with postdischarge providers verbally (preferred) or by fax/e‐mail | Discharging clinician | Time of discharge | 1214 |
| Timely completion of discharge summary and reliable transmission to postdischarge providers | |||
| Availability of information in medical record | |||
| Use of a structured template with subheadings in discharge communication | |||
| Medication safety | |||
| Take an accurate preadmission medication history | Clinicians | Admission | 1521 |
| Reconcile preadmission medications with all ordered medications at all transfers in care, including discharge | Pharmacists | Throughout hospitalization | |
| Communicate discharge medications to all outpatient providers, including all changes and rationale for those changes | Nurses | Time of discharge | |
| Educating patients, promoting self‐management | |||
| Focus discharge counseling on major diagnoses, medication changes, dates of follow‐up appointments, self‐care instructions, warning signs and symptoms, and who to contact for problems | Clinicians | Daily | 911, 2228, 30 |
| Include caregivers as appropriate | Nurses | Time of discharge | |
| Ensure staff members provide consistent messages | Care managers/discharge planners | Postdischarge | |
| Provide simply written patient‐centered materials with instructions | Transition coaches | ||
| Use teach‐back methods to confirm understanding | |||
| Encourage questions | |||
| Continue teaching during postdischarge follow‐up | |||
| Use transition coaches in high‐risk patients: focus on medication management, keeping a personal medical record, follow‐up appointments, and knowledge of red flags | |||
| Enlisting help of social and community supports | |||
| Assess needs and appropriately arrange for home services | Clinicians | Predischarge and postdischarge | 29, 30 |
| Enlist help of caregivers | Nurses | ||
| Enlist help of community supports | Care managers | ||
| Home health staff | |||
| Advanced care planning | |||
| Establish healthcare proxy | Clinicians | Predischarge and postdischarge | 31, 32 |
| Discuss goals of care | Palliative care staff | ||
| Palliative care consultation (if appropriate) | Social workers | ||
| Enlist hospice services (if appropriate) | Nurses | ||
| Hospice workers | |||
| Coordinating care among team members | |||
| Share medical records | Clinicians | Predischarge and postdischarge | 33 |
| Communicate involving all team members | Nurses | ||
| Optimize continuity of providers and formal handoffs of care | Office personnel | ||
| IT staff | |||
| Monitoring and managing symptoms after discharge | |||
| Monitor for: | Clinicians | Postdischarge | 1113, 28, 3436 |
| Worsening disease control | Nurses | ||
| Medication side effects, discrepancies, nonadherence | Pharmacists | ||
| Therapeutic drug monitoring | Care managers | ||
| Inability to manage conditions at home | Visiting nurses and other home health staff | ||
| Via: | |||
| Postdischarge phone calls | |||
| Home visits | |||
| Postdischarge clinic visits | |||
| Patient hotline | |||
| Availability of inpatient providers after discharge | |||
| Follow‐up with outpatient providers | |||
| Within an appropriate time frame (eg, 7 d or sooner for high‐risk patients) | Clinicians | Postdischarge | 3740 |
| With appropriate providers (eg, most related to reasons for hospitalization, who manage least stable conditions, and/or PCP) | Nurses Pharmacists | ||
| Utilize multidisciplinary teams as appropriate | Care managers | ||
| Ensure appropriate progress along plan of care and safe transition | Office personnel | ||
| Other clinical staff as appropriate | |||
Our concept of an ideal transition in care began with work by Naylor, who described several important components of a safe transition in care, including complete communication of information, patient education, enlisting the help of social and community supports, ensuring continuity of care, and coordinating care among team members.[8] It is supplemented by the Transitions of Care Consensus Policy Statement proposed by representatives from hospital medicine, primary care, and emergency medicine, which emphasized aspects of timeliness and content of communication between providers.[9] Our present articulation of these key components includes 10 organizing domains.
The Discharge Planning domain highlights the important principle of planning ahead for hospital discharge while the patient is still being treated in the hospital, a paradigm espoused by Project RED[10] and other successful care transitions interventions.[11, 12] Collaborating with the outpatient provider and taking the patient and caregiver's preferences for appointment scheduling into account can help ensure optimal outpatient follow‐up.
Complete Communication of Information refers to the content that should be included in discharge summaries and other means of information transfer from hospital to postdischarge care. The specific content areas are based on the Society of Hospital Medicine and Society of General Internal Medicine Continuity of Care Task Force systematic review and recommendations,[13] which takes into account information requested by primary care physicians after discharge.
Availability, Timeliness, Clarity, and Organization of that information is as important as the content because postdischarge providers must be able to access and quickly understand the information they have been provided before assuming care of the patient.[14, 15]
The Medication Safety domain is of central importance because medications are responsible for most postdischarge adverse events.[16] Taking an accurate medication history,[17] reconciling changes throughout the hospitalization,[18] and communicating the reconciled medication regimen to patients and providers across transitions of care can reduce medication errors and improve patient safety.[19, 20, 21, 22]
The Patient Education and Promotion of Self‐Management domain involves teaching patients and their caregivers about the main hospital diagnoses and instructions for self‐care, including medication changes, appointments, and whom to contact if issues arise. Confirming comprehension of instructions through assessments of acute (delirium) and chronic (dementia) cognitive impairments[23, 24, 25, 26] and teach‐back from the patient (or caregiver) is an important aspect of such counseling, as is providing patients and caregivers with educational materials that are appropriate for their level of health literacy and preferred language.[14] High‐risk patients may benefit from patient coaching to improve their self‐management skills.[12] These recommendations are based on years of health literacy research,[27, 28, 29] and such elements are generally included in effective interventions (including Project RED,[10] Naylor and colleagues' Transitional Care Model,[11] and Coleman and colleagues' Care Transitions Intervention[12]).
Enlisting the help of Social and Community Supports is an important adjunct to medical care and is the rationale for the recent increase in CMS funding for community‐based, care‐transition programs. These programs are crucial for assisting patients with household activities, meals, and other necessities during the period of recovery, though they should be distinguished from care management or care coordination interventions, which have not been found to be helpful in preventing readmissions unless high touch in nature.[30, 31]
The Advanced Care Planning domain may begin in the hospital or outpatient setting, and involves establishing goals of care and healthcare proxies, as well as engaging with palliative care or hospice services if appropriate. Emerging evidence supports the intuitive conclusion that this approach prevents readmissions, particularly in patients who do not benefit from hospital readmission.[32, 33]
Attention to the Coordinating Care Among Team Members domain is needed to synchronize efforts across settings and providers. Clearly, many healthcare professionals as well as other involved parties can be involved in helping a single patient during transitions in care. It is vital that they coordinate information, assessments, and plans as a team.[34]
We recognize the domain of Monitoring and Managing Symptoms After Discharge as increasingly crucial as reflected in our growing understanding of the reasons for readmission, especially among patients with fragile conditions such as heart failure, chronic lung disease, gastrointestinal disorders, dementia,[23, 24, 25, 26] and vascular disease.[35] Monitoring for new or worsening symptoms; medication side effects, discrepancies, or nonadherence; and other self‐management challenges will allow problems to be detected and addressed early, before they result in unplanned healthcare utilization. It is noteworthy that successful interventions in this regard rely on in‐home evaluation[13, 14, 29] by nurses rather than telemonitoring, which in isolation has not been effective to date.[36, 37]
Finally, optimal Outpatient Follow‐Up with appropriate postdischarge providers is crucial for providing ideal transitions. These appointments need to be prompt[38, 39] (eg, within 7 days if not sooner for high‐risk patients) and with providers who have a longitudinal relationship to the patient, as prior work has shown increased readmissions when the provider is unfamiliar with the patient.[40] The advantages and disadvantages of hospitalist‐run postdischarge clinics as one way to increase access and expedite follow‐up are currently being explored. Although the optimal content of a postdischarge visit has not been defined, logical tasks to be completed are myriad and imply the need for checklists, adequate time, and a multidisciplinary team of providers.[41]
IMPLICATIONS OF THE IDEAL TRANSITION IN CARE
Our conceptualization of an ideal transition in care provides insight for hospital and healthcare system leadership, policymakers, researchers, clinicians, and educators seeking to improve transitions of care and reduce hospital readmissions. In the sections below, we briefly review commonly cited concerns about the recent focus on readmissions as a quality measure, illustrate how the Ideal Transition in Care addresses these concerns, and propose fruitful areas for future work.
How Does the Framework Address the Extent to Which Readmissions Reflect Hospital Quality?
One of the chief problems with readmissionrates as a hospital quality measure is that many of the factors that influence readmission may not currently be under the hospital's control. The healthcare environment to which a patient is being discharged (and was admitted from in the first place) is an important determinant of readmission.[42] In this context, it is noteworthy that successful interventions to reduce readmission are generally those that focus on outpatient follow‐up, while inpatient‐only interventions have had less success.[7] This is reflected in our framework above, informed by the literature, highlighting the importance of coordination between inpatient and outpatient providers and the importance of postdischarge care, including monitoring and managing symptoms after discharge, prompt follow‐up appointments, the continuation of patient self‐management activities, monitoring for drug‐related problems after discharge, and the effective utilization of community supports. Accountable care organizations, once established, would be responsible for several components of this environment, including the provision of prompt and effective follow‐up care.
The implication of the framework is that if a hospital does not have control over most of the factors that influence its readmission rate, it should see financial incentives to reduce readmission rates as an opportunity to invest in relationships with the outpatient environment from which their patients are admitted and to which they are discharged. One can envision hospitals growing ever‐closer relationships with their network of primary care physician groups, community agencies, and home health services, rehabilitation facilities, and nursing homes through coordinated discharge planning, medication management, patient education, shared electronic medical records, structured handoffs in care, and systems of intensive outpatient monitoring. Our proposed framework, in other words, emphasizes that hospitals cannot reduce their readmission rates by focusing on aspects of care within their walls. They must forge new and stronger relationships with their communities if they are to be successful.
How Does the Framework Help Us Understand Which Readmissions Are Preventable?
Public reporting and financial penalties are currently tied to all‐cause readmission, but preventable readmissions are a more appealing outcome to target. In one study, the ranking of hospitals by all‐cause readmission rate had very little correlation with the ranking by preventable readmission rate.[5] However, researchers have struggled to establish standardized, valid, and reliable measures for determining what proportion of readmissions are in fact preventable, with estimates ranging from 5% to 79% in the published literature.[43]
The difficulty of accurately determining preventability stems from an inadequate understanding of the roles that patient comorbidities, transitional processes of care, individual patient behaviors, and social and environmental determinants of health play in the complex process of hospital recidivism. Our proposed elements of an ideal transition in care provide a structure to frame this discussion and suggest future research opportunities to allow a more accurate and reliable understanding of the spectrum of preventability. Care system leadership can use the framework to rigorously evaluate their readmissions and determine the extent to which the transitions process approached the ideal. For example, if a readmission occurs despite care processes that addressed most of the domains with high fidelity, it becomes much less likely that the readmission was preventable. It should be noted that the converse is not always true: When a transition falls well short of the ideal, it does not always imply that provision of a more ideal transition would necessarily have prevented the readmission, but it does make it more likely.
For educators, the framework may provide insights for trainees into the complexity of the transitions process and vulnerability of patients during this time, highlighting preventable aspects of readmissions that are within the grasp of the discharging clinician or team. It highlights the importance of medication reconciliation, synchronous communication, and predischarge teaching, which are measurable and teachable skills for non‐physician providers, housestaff, and medical students. It also may allow for more structured feedback, for example, on the quality of discharge summaries produced by trainees.
How Could the Framework Improve Risk Adjustment for Between‐Hospital Comparisons?
Under the Patient Protection and Affordable Care Act (PPACA), hospitals will be compared to one another using risk‐standardized readmission rates as a way to penalize poorly performing hospitals. However, risk‐adjustment models have only modest ability to predict hospital readmission.[6] Moreover, current approaches predominantly adjust for patients' medical comorbidities (which are easily measurable), but they do not adequately take into account the growing literature on other factors that influence readmission rates, including a patient's health literacy, visual or cognitive impairment, functional status, language barriers, and community‐level factors such as social supports.[44, 45]
The Ideal Transition of Care provides a comprehensive framework of hospital discharge quality that provides additional process measures on which hospitals could be compared rather than focusing solely on (inadequately) risk‐adjusted readmission rates. Indeed, most other quality and safety measures (such as the National Quality Forum's Safe Practices[46] and The Joint Commission's National Patient Safety Goals),[47] emphasize process over outcome, in part because of issues of fairness. Process measures are less subject to differences in patient populations and also change the focus from simply reducing readmissions to improving transitional care more broadly. These process measures should be based on our framework and should attempt to capture as many dimensions of an optimal care transition as possible.
Possible examples of process measures include: the accuracy of medication reconciliation at admission and discharge; provision of prompt outpatient follow‐up; provision of adequate systems to monitor and manage symptoms after discharge; advanced care planning in appropriate patients; and the quality of discharge education, incorporating measurements of the patient's understanding and ability to self‐manage their illness. At least some of these could be used now as part of a performance measurement set that highlights opportunities for immediate system change and can serve as performance milestones.
The framework could also be used to validate risk‐adjustment techniques. After accounting for patient factors, the remaining variability in outcomes should be accounted for by processes of care that are in the transitions framework. Once these processes are accurately measured, one can determine if indeed the remaining variability is due to transitions processes, or rather unaccounted factors that are not being measured and that hospitals may have little control over. Such work can lead to iterative refinement of patient risk‐adjustment models.
What Does the Framework Imply About Best Practices for Reducing Readmission Rates?
Despite the limitations of readmission rates as a quality measure noted above, hospitals presently face potentially large financial penalties for readmissions and are allocating resources to readmission reduction efforts. However, hospitals currently may not have enough guidance to know what actions to take to reduce readmissions, and thus could be spending money inefficiently and reducing the value proposition of focusing on readmissions.
A recent systematic review of interventions hospitals could employ to reduce readmissions identified several positive studies, but also many negative studies, and there were significant barriers to understanding what works to reduce readmissions.[7] For example, most of the interventions described in both positive and negative studies were multifaceted, and the authors were unable to identify which components of the intervention were most effective. Also, while several studies have identified risk factors for readmission,[6, 48, 49] very few studies have identified which subgroups of patients benefit most from specific interventions. Few of the studies described key contextual factors that may have led to successful or failed implementation, or the fidelity with which the intervention was implemented.[50, 51, 52]
Few if any of the studies were guided by a concept of the ideal transition in care.[10] Such a framework will better guide development of multifaceted interventions and provide an improved means for interpreting the results. Clearly, rigorously conducted, multicenter studies of readmission prevention interventions are needed to move the field forward. These studies should: 1) correlate implementation of specific intervention components with reductions in readmission rates to better understand the most effective components; 2) be adequately powered to show effect modification, ie, which patients benefit most from these interventions; and 3) rigorously measure environmental context and intervention fidelity, and employ mixed methods to better understand predictors of implementation success and failure.
Our framework can be used in the design and evaluation of such interventions. For example, interventions could be designed that incorporate as many of the domains of an ideal transition as possible, in particular those that span the inpatient and outpatient settings. Processes of care metrics can be developed that measure the extent to which each domain is delivered, analogous to the way the Joint Commission might aggregate individual scores on the 10 items in Acute Myocardial Infarction Core Measure Set[53] to provide a composite of the quality of care provided to patients with this diagnosis. These can be used to correlate certain intervention components with success in reducing readmissions and also in measuring intervention fidelity.
NEXT STEPS
For hospital and healthcare system leaders, who need to take action now to avoid financial penalties, we recommend starting with proven, high‐touch interventions such as Project RED and the Care Transitions Intervention, which are durable, cost‐effective, robustly address multiple domains of the Ideal Transition in Care, and have been implemented at numerous sites.[54, 55] Each hospital or group will need to decide on a bundle of interventions and customize them based on local workflow, resources, and culture.
Risk‐stratification, to match the intensity of the intervention to the risk of readmission of the patient, will undoubtedly be a key component for the efficient use of resources. We anticipate future research will allow risk stratification to be a robust part of any implementation plan. However, as noted above, current risk prediction models are imperfect,[6] and more work is needed to determine which patients benefit most from which interventions. Few if any studies have described interventions tailored to risk for this reason.
Based on our ideal transition in care, our collective experience, and published evidence,[7, 10, 11, 12] potential elements to start with include: early discharge planning; medication reconciliation[56]; patient/caregiver education using health literacy principles, cognitive assessments, and teach‐back to confirm understanding; synchronous communication (eg, by phone) between inpatient and postdischarge providers; follow‐up phone calls to patients within 72 hours of discharge; 24/7 availability of a responsible inpatient provider to address questions and problems (both from the patient/caregiver and from postdischarge providers); and prompt appointments for patients discharged home. High‐risk patients will likely require additional interventions, including in‐home assessments, disease‐monitoring programs, and/or patient coaching. Lastly, patients with certain conditions prone to readmission (such as heart failure and chronic obstructive pulmonary disease) may benefit from disease‐specific programs, including patient education, outpatient disease management, and monitoring protocols.
It is likely that the most effective interventions are those that come from combined, coordinated interventions shared between inpatient and outpatient settings, and are intensive in nature. We expect that the more domains in the framework that are addressed, the safer and more seamless transitions in care will be, with improvement in patient outcomes. To the extent that fragmentation of care has been a barrier to the implementation of these types of interventions in the past, ACOs, perhaps with imbedded Patient‐Centered Medical Homes, may be in the best position to take advantage of newly aligned financial incentives to design comprehensive transitional care. Indeed, we anticipate that Figure 1 may provide substrate for a discussion of postdischarge care and division of responsibilities between inpatient and outpatient care teams at the time of transition, so effort is not duplicated and multiple domains are addressed.
Other barriers to implementation of ideal transitions in care will continue to be an issue for most healthcare systems. Financial constraints that have been a barrier up until now will be partially overcome by penalties for high readmission rates and by ACOs, bundled payments, and alternative care contracts (ie, global payments), but the extent to which each institution feels rewarded for investing in transitional interventions will vary greatly. Healthcare leadership that sees the value of improving transitions in care will be critical to overcoming this barrier. Competing demands (such as lowering hospital length of stay and carrying out other patient care responsibilities),[57] lack of coordination and diffusion of responsibility among various clinical personnel, and lack of standards are other barriers[58] that will require clear prioritization from leadership, policy changes, team‐based care, provider education and feedback, and adequate allocation of personnel resources. In short, process redesign using continuous quality improvement efforts and effective tools will be required to maximize the possibility of success.
CONCLUSIONS
Readmissions are costly and undesirable. Intuition suggests they are a marker of poor care and that hospitals should be capable of reducing them, thereby improving care and decreasing costs. In a potential future world of ACOs based on global payments, financial incentives would be aligned for each system to reduce readmissions below their current baseline, therefore obviating the need for external financial rewards and penalties. In the meantime, financial penalties do exist, and controversy exists over their fairness and likelihood of driving appropriate behavior. To address these controversies and promote better transitional care, we call for the development and use of multifaceted, collaborative transitions interventions that span settings, risk‐adjustment models that allow for fairer comparisons among hospitals, better and more widespread measurement of processes of transitional care, a better understanding of what interventions are most effective and in whom, and better guidance in how to implement these interventions. Our conceptualization of an ideal transition of care serves as a guide and provides a common vocabulary for these efforts. Such research is likely to produce the knowledge needed for healthcare systems to improve transitions in care, reduce readmissions, and reduce costs.
Disclosure
Funding for Dr Vasilevskis has been provided by the National Institutes of Health (K23AG040157) and the VA Tennessee Valley Geriatric Research, Education and Clinical Center (GRECC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging, the National Institutes of Health, or the US Department of Veterans Affairs.
- , . Redefining Health Care: Creating Value‐Based Competition on Results. Boston, MA:Harvard Business School Press;2006.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- Patient Protection and Affordable Care Act (PPACA). Public Law 111–148; 2010. Available at: http://www.gpo.gov/fdsys/pkg/PLAW‐111publ148/pdf/PLAW‐111publ148.pdf. Accessed on June 4, 2012.
- , . Hospital readmission as an accountability measure. JAMA. 2011;305(5):504–505.
- , , , et al. Incidence of potentially avoidable urgent readmissions and their relation to all‐cause urgent readmissions. Can Med Assoc J. 2011;183(14):E1067–E1072.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- . A decade of transitional care research with vulnerable elders. J Cardiovasc Nurs. 2000;14(3):1–14.
- , , , et al;for the American College of Physicians; Society of General Internal Medicine; Society of Hospital Medicine; American Geriatrics Society; American College of Emergency Physicians; Society of Academic Emergency Medicine. Transitions of Care Consensus Policy Statement American College of Physicians–Society of General Internal Medicine–Society of Hospital Medicine–American Geriatrics Society–American College of Emergency Physicians–Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24(8):971–976.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187.
- , , , et al. Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281(7):613–620.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , . Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314–323.
- , , , , , . Discharge documentation of patients discharged to subacute facilities: a three‐year quality improvement process across an integrated health care system. Jt Comm J Qual Patient Saf. 2010;36(6):243–251.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , , , . Frequency, type and clinical importance of medication history errors at admission to hospital: a systematic review. Can Med Assoc J. 2005;173(5):510–515.
- , , , et al. Classifying and predicting errors of inpatient medication reconciliation. J Gen Intern Med. 2008;23(9):1414–1422.
- , , et al;for the PILL‐CVD (Pharmacist Intervention for Low Literacy in Cardiovascular Disease) Study Group. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med. 2012;157(1):1–10.
- , , , . Hospital‐based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057–1069.
- , , , et al. Effect of an electronic medication reconciliation application and process redesign on potential adverse drug events: a cluster‐randomized trial. Arch Intern Med. 2009;169(8):771–780.
- , , , et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166(5):565–571.
- , , , , . Insufficient help for activity of daily living disabilities and risk of all–cause hospitalization. J Am Geriatr Soc. 2012;60(5):927–933.
- , , , et al. Transitions in care for older adults with and without dementia. J Am Geriatr Soc. 2012;60(5):813–820.
- , , , , . Association of incident dementia with hospitalizations. JAMA. 2012;307(2):165–172.
- , , , et al. Potentially avoidable hospitalizations of dually eligible Medicare and Medicaid beneficiaries from nursing facility and home– and community–based services waiver programs. J Am Geriatr Soc. 2012;60(5):821–829.
- , . Teaching about health literacy and clear communication. J Gen Intern Med. 2006;21(8):888–890.
- , , , et al. Health literacy and outcomes among patients with heart failure. JAMA. 2011;305(16):1695–1701.
- , , , , . Patient experiences of transitioning from hospital to home: an ethnographic quality improvement project. J Hosp Med. 2012;7(5):382–387.
- , , , . Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries: 15 randomized trials. JAMA. 2009;301(6):603–618.
- , , , , . How changes in Washington University's Medicare coordinated care demonstration pilot ultimately achieved savings. Health Aff (Millwood). 2012;31(6):1216–1226.
- , , , et al. Quality of care and rehospitalization rate in the last stage of disease in brain tumor patients assisted at home: a cost effectiveness study. J Palliat Med. 2012;15(2):225–227.
- , , , , . Inpatient palliative care consults and the probability of hospital readmission. Perm J. 2011;15(2):48–51.
- , , , et al. TeamSTEPPS™: team strategies and tools to enhance performance and patient safety. In: Henriksen K, Battles JB, Keyes MA, Grady ML, ed. Advances in Patient Safety: New Directions and Alternative Approaches. Vol 3: Performance and Tools. Rockville, MD:Agency for Healthcare Research and Quality; August2008.
- , , , et al. Factors contributing to all‐cause 30‐day readmissions: a structured case series across 18 hospitals. Med Care. 2012;50(7):599–605.
- , , , et al. Telemonitoring in patients with heart failure [erratum, N Engl J Med. 2011;364(5):490]. N Engl J Med. 2010;363(24):2301–2309.
- , , , et al. A randomized controlled trial of telemonitoring in older adults with multiple health issues to prevent hospitalizations and emergency department visits. Arch Intern Med. 2012;172(10):773–779.
- , , , et al. Relationship between early physician follow‐up and 30‐day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303(17):1716–1722.
- , , . Post‐hospitalization transitions: examining the effects of timing of primary care provider follow‐up. J Hosp Med. 2010;5(7):392–397.
- , , . Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on Primary Care and Hospital Readmission. N Engl J Med. 1996;334(22):1441–1447.
- . The Post‐Hospital Follow‐Up Visit: A Physician Checklist to Reduce Readmissions. California Healthcare Foundation; October 2010. Available at: http://www.chcf.org/publications/2010/10/the‐post‐hospital‐follow‐up‐visit‐a‐physician‐checklist. Accessed on January 10, 2012.
- , , . Thirty‐day readmission rates for Medicare beneficiaries by race and site of care. JAMA. 2011;305(7):675–681.
- , , , , . Proportion of hospital readmissions deemed avoidable: a systematic review. Can Med Assoc J. 2011;183(7):E391–E402.
- , , , et al. Postdischarge environmental and socioeconomic factors and the likelihood of early hospital readmission among community‐dwelling Medicare beneficiaries. Gerontologist. 2008;48(4):495–504.
- , , , , . Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155(2):97–107.
- National Quality Forum. Safe Practices for Better Healthcare—2010 Update: A Consensus Report. Washington, DC;2010.
- Joint Commission on Accreditation of Healthcare Organizations. Accreditation Program: Hospital 2010 National Patient Safety Goals (NPSGs). 2010. Available at: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/. Accessed on March 20, 2012.
- , , , et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25(3):211–219.
- , , , et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. Can Med Assoc J. 2010;182(6):551–557.
- , . Evaluating service delivery interventions to enhance patient safety. BMJ. 2008;337:a2764.
- , , . Assessing the Evidence for Context‐Sensitive Effectiveness and Safety of Patient Safety Practices: Developing Criteria. Rockville, MD:Agency for Healthcare Research and Quality; December2010.
- , , , et al. Advancing the science of patient safety. Ann Intern Med. 2011;154(10):693–696.
- The Joint Commission. Acute Myocardial Infarction Core Measure Set. Available at: http://www.jointcommission.org/assets/1/6/Acute%20Myocardial%20Infarction.pdf. Accessed August 20,2012.
- , , , , , . The care transitions intervention: translating from efficacy to effectiveness. Arch Intern Med. 2011;171(14):1232–1237.
- Project RED toolkit, AHRQ Innovations Exchange. Available at:http://www.innovations.ahrq.gov/content.aspx?id=2180. Accessed on July 2, 2012.
- , , , et al. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: a randomized controlled trial. Arch Intern Med. 2009;169(9):894–900.
- , . Thirty‐day readmissions—truth and consequences. N Engl J Med. 2012;366(15):1366–1369.
- , , , , . “Out of sight, out of mind”: housestaff perceptions of quality‐limiting factors in discharge care at teaching hospitals. J Hosp Med. 2012;7(5):376–381.
Containing the rise of healthcare costs has taken on a new sense of urgency in the wake of the recent economic recession and continued growth in the cost of healthcare. Accordingly, many stakeholders seek solutions to improve value (reducing costs while improving care)[1]; hospital readmissions, which are common and costly,[2] have emerged as a key target. The Centers for Medicare and Medicaid Services (CMS) have instituted several programs intended to reduce readmissions, including funding for community‐based, care‐transition programs; penalties for hospitals with elevated risk‐adjusted readmission rates for selected diagnoses; pioneer Accountable Care Organizations (ACOs) with incentives to reduce global costs of care; and Hospital Engagement Networks (HENs) through the Partnership for Patients.[3] A primary aim of these initiatives is to enhance the quality of care transitions as patients are discharged from the hospital.
Though the recent focus on hospital readmissions has appropriately drawn attention to transitions in care, some have expressed concerns. Among these are questions about: 1) the extent to which readmissions truly reflect the quality of hospital care[4]; 2) the preventability of readmissions[5]; 3) limitations in risk‐adjustment techniques[6]; and 4) best practices for preventing readmissions.[7] We believe these concerns stem in part from deficiencies in the state of the science of transitional care, and that future efforts in this area will be hindered without a clear vision of an ideal transition in care. We propose the key components of an ideal transition in care and discuss the implications of this concept as it pertains to hospital readmissions.
THE IDEAL TRANSITION IN CARE
We propose the key components of an ideal transition in care in Figure 1 and Table 1. Figure 1 represents 10 domains described more fully below as structural supports of the bridge patients must cross from one care environment to another during a care transition. This figure highlights key domains and suggests that lack of a domain makes the bridge weaker and more prone to gaps in care and poor outcomes. It also implies that the more components are missing, the less safe is the bridge or transition. Those domains that mainly take place prior to discharge are placed closer to the hospital side of the bridge, those that mainly take place after discharge are placed closer to the community side of the bridge, while those that take place both prior to and after discharge are in the middle. Table 1 provides descriptions of the key content for each of these domains, as well as guidance about which personnel might be involved and where in the transition process that domain should be implemented. We support these domains with supporting evidence where available.

| Domain | Who | When | References |
|---|---|---|---|
| |||
| Discharge planning | |||
| Use a multidisciplinary team to create a discharge plan | Discharging clinician | Predischarge | 911 |
| Collaborate with PCP regarding discharge and follow‐up plan | Care managers/discharge planners | ||
| Arrange follow‐up appointments prior to discharge | Nurses | ||
| Make timely appointments for follow‐up care | |||
| Make appointments that take patient and caregiver's schedules and transportation needs into account | |||
| Complete communication of information | |||
| Includes: | Discharging clinician | Time of discharge | 1214 |
| Patient's full name | |||
| Age | |||
| Dates of admission and discharge | |||
| Names of responsible hospital physicians | |||
| Name of physician preparing discharge summary | |||
| Name of PCP | |||
| Main diagnosis | |||
| Other relevant diagnoses, procedures, and complications | |||
| Relevant findings at admission | |||
| Treatment and response for each active problem | |||
| Results of procedures and abnormal laboratory test results | |||
| Recommendations of any subspecialty consultants | |||
| Patient's functional status at discharge | |||
| Discharge medications | |||
| Follow‐up appointments made and those to be made | |||
| Tests to be ordered and pending tests to be followed‐up | |||
| Counseling provided to patient and caregiver, when applicable | |||
| Contingency planning | |||
| Code status | |||
| Availability, timeliness, clarity, and organization of information | |||
| Timely communication with postdischarge providers verbally (preferred) or by fax/e‐mail | Discharging clinician | Time of discharge | 1214 |
| Timely completion of discharge summary and reliable transmission to postdischarge providers | |||
| Availability of information in medical record | |||
| Use of a structured template with subheadings in discharge communication | |||
| Medication safety | |||
| Take an accurate preadmission medication history | Clinicians | Admission | 1521 |
| Reconcile preadmission medications with all ordered medications at all transfers in care, including discharge | Pharmacists | Throughout hospitalization | |
| Communicate discharge medications to all outpatient providers, including all changes and rationale for those changes | Nurses | Time of discharge | |
| Educating patients, promoting self‐management | |||
| Focus discharge counseling on major diagnoses, medication changes, dates of follow‐up appointments, self‐care instructions, warning signs and symptoms, and who to contact for problems | Clinicians | Daily | 911, 2228, 30 |
| Include caregivers as appropriate | Nurses | Time of discharge | |
| Ensure staff members provide consistent messages | Care managers/discharge planners | Postdischarge | |
| Provide simply written patient‐centered materials with instructions | Transition coaches | ||
| Use teach‐back methods to confirm understanding | |||
| Encourage questions | |||
| Continue teaching during postdischarge follow‐up | |||
| Use transition coaches in high‐risk patients: focus on medication management, keeping a personal medical record, follow‐up appointments, and knowledge of red flags | |||
| Enlisting help of social and community supports | |||
| Assess needs and appropriately arrange for home services | Clinicians | Predischarge and postdischarge | 29, 30 |
| Enlist help of caregivers | Nurses | ||
| Enlist help of community supports | Care managers | ||
| Home health staff | |||
| Advanced care planning | |||
| Establish healthcare proxy | Clinicians | Predischarge and postdischarge | 31, 32 |
| Discuss goals of care | Palliative care staff | ||
| Palliative care consultation (if appropriate) | Social workers | ||
| Enlist hospice services (if appropriate) | Nurses | ||
| Hospice workers | |||
| Coordinating care among team members | |||
| Share medical records | Clinicians | Predischarge and postdischarge | 33 |
| Communicate involving all team members | Nurses | ||
| Optimize continuity of providers and formal handoffs of care | Office personnel | ||
| IT staff | |||
| Monitoring and managing symptoms after discharge | |||
| Monitor for: | Clinicians | Postdischarge | 1113, 28, 3436 |
| Worsening disease control | Nurses | ||
| Medication side effects, discrepancies, nonadherence | Pharmacists | ||
| Therapeutic drug monitoring | Care managers | ||
| Inability to manage conditions at home | Visiting nurses and other home health staff | ||
| Via: | |||
| Postdischarge phone calls | |||
| Home visits | |||
| Postdischarge clinic visits | |||
| Patient hotline | |||
| Availability of inpatient providers after discharge | |||
| Follow‐up with outpatient providers | |||
| Within an appropriate time frame (eg, 7 d or sooner for high‐risk patients) | Clinicians | Postdischarge | 3740 |
| With appropriate providers (eg, most related to reasons for hospitalization, who manage least stable conditions, and/or PCP) | Nurses Pharmacists | ||
| Utilize multidisciplinary teams as appropriate | Care managers | ||
| Ensure appropriate progress along plan of care and safe transition | Office personnel | ||
| Other clinical staff as appropriate | |||
Our concept of an ideal transition in care began with work by Naylor, who described several important components of a safe transition in care, including complete communication of information, patient education, enlisting the help of social and community supports, ensuring continuity of care, and coordinating care among team members.[8] It is supplemented by the Transitions of Care Consensus Policy Statement proposed by representatives from hospital medicine, primary care, and emergency medicine, which emphasized aspects of timeliness and content of communication between providers.[9] Our present articulation of these key components includes 10 organizing domains.
The Discharge Planning domain highlights the important principle of planning ahead for hospital discharge while the patient is still being treated in the hospital, a paradigm espoused by Project RED[10] and other successful care transitions interventions.[11, 12] Collaborating with the outpatient provider and taking the patient and caregiver's preferences for appointment scheduling into account can help ensure optimal outpatient follow‐up.
Complete Communication of Information refers to the content that should be included in discharge summaries and other means of information transfer from hospital to postdischarge care. The specific content areas are based on the Society of Hospital Medicine and Society of General Internal Medicine Continuity of Care Task Force systematic review and recommendations,[13] which takes into account information requested by primary care physicians after discharge.
Availability, Timeliness, Clarity, and Organization of that information is as important as the content because postdischarge providers must be able to access and quickly understand the information they have been provided before assuming care of the patient.[14, 15]
The Medication Safety domain is of central importance because medications are responsible for most postdischarge adverse events.[16] Taking an accurate medication history,[17] reconciling changes throughout the hospitalization,[18] and communicating the reconciled medication regimen to patients and providers across transitions of care can reduce medication errors and improve patient safety.[19, 20, 21, 22]
The Patient Education and Promotion of Self‐Management domain involves teaching patients and their caregivers about the main hospital diagnoses and instructions for self‐care, including medication changes, appointments, and whom to contact if issues arise. Confirming comprehension of instructions through assessments of acute (delirium) and chronic (dementia) cognitive impairments[23, 24, 25, 26] and teach‐back from the patient (or caregiver) is an important aspect of such counseling, as is providing patients and caregivers with educational materials that are appropriate for their level of health literacy and preferred language.[14] High‐risk patients may benefit from patient coaching to improve their self‐management skills.[12] These recommendations are based on years of health literacy research,[27, 28, 29] and such elements are generally included in effective interventions (including Project RED,[10] Naylor and colleagues' Transitional Care Model,[11] and Coleman and colleagues' Care Transitions Intervention[12]).
Enlisting the help of Social and Community Supports is an important adjunct to medical care and is the rationale for the recent increase in CMS funding for community‐based, care‐transition programs. These programs are crucial for assisting patients with household activities, meals, and other necessities during the period of recovery, though they should be distinguished from care management or care coordination interventions, which have not been found to be helpful in preventing readmissions unless high touch in nature.[30, 31]
The Advanced Care Planning domain may begin in the hospital or outpatient setting, and involves establishing goals of care and healthcare proxies, as well as engaging with palliative care or hospice services if appropriate. Emerging evidence supports the intuitive conclusion that this approach prevents readmissions, particularly in patients who do not benefit from hospital readmission.[32, 33]
Attention to the Coordinating Care Among Team Members domain is needed to synchronize efforts across settings and providers. Clearly, many healthcare professionals as well as other involved parties can be involved in helping a single patient during transitions in care. It is vital that they coordinate information, assessments, and plans as a team.[34]
We recognize the domain of Monitoring and Managing Symptoms After Discharge as increasingly crucial as reflected in our growing understanding of the reasons for readmission, especially among patients with fragile conditions such as heart failure, chronic lung disease, gastrointestinal disorders, dementia,[23, 24, 25, 26] and vascular disease.[35] Monitoring for new or worsening symptoms; medication side effects, discrepancies, or nonadherence; and other self‐management challenges will allow problems to be detected and addressed early, before they result in unplanned healthcare utilization. It is noteworthy that successful interventions in this regard rely on in‐home evaluation[13, 14, 29] by nurses rather than telemonitoring, which in isolation has not been effective to date.[36, 37]
Finally, optimal Outpatient Follow‐Up with appropriate postdischarge providers is crucial for providing ideal transitions. These appointments need to be prompt[38, 39] (eg, within 7 days if not sooner for high‐risk patients) and with providers who have a longitudinal relationship to the patient, as prior work has shown increased readmissions when the provider is unfamiliar with the patient.[40] The advantages and disadvantages of hospitalist‐run postdischarge clinics as one way to increase access and expedite follow‐up are currently being explored. Although the optimal content of a postdischarge visit has not been defined, logical tasks to be completed are myriad and imply the need for checklists, adequate time, and a multidisciplinary team of providers.[41]
IMPLICATIONS OF THE IDEAL TRANSITION IN CARE
Our conceptualization of an ideal transition in care provides insight for hospital and healthcare system leadership, policymakers, researchers, clinicians, and educators seeking to improve transitions of care and reduce hospital readmissions. In the sections below, we briefly review commonly cited concerns about the recent focus on readmissions as a quality measure, illustrate how the Ideal Transition in Care addresses these concerns, and propose fruitful areas for future work.
How Does the Framework Address the Extent to Which Readmissions Reflect Hospital Quality?
One of the chief problems with readmissionrates as a hospital quality measure is that many of the factors that influence readmission may not currently be under the hospital's control. The healthcare environment to which a patient is being discharged (and was admitted from in the first place) is an important determinant of readmission.[42] In this context, it is noteworthy that successful interventions to reduce readmission are generally those that focus on outpatient follow‐up, while inpatient‐only interventions have had less success.[7] This is reflected in our framework above, informed by the literature, highlighting the importance of coordination between inpatient and outpatient providers and the importance of postdischarge care, including monitoring and managing symptoms after discharge, prompt follow‐up appointments, the continuation of patient self‐management activities, monitoring for drug‐related problems after discharge, and the effective utilization of community supports. Accountable care organizations, once established, would be responsible for several components of this environment, including the provision of prompt and effective follow‐up care.
The implication of the framework is that if a hospital does not have control over most of the factors that influence its readmission rate, it should see financial incentives to reduce readmission rates as an opportunity to invest in relationships with the outpatient environment from which their patients are admitted and to which they are discharged. One can envision hospitals growing ever‐closer relationships with their network of primary care physician groups, community agencies, and home health services, rehabilitation facilities, and nursing homes through coordinated discharge planning, medication management, patient education, shared electronic medical records, structured handoffs in care, and systems of intensive outpatient monitoring. Our proposed framework, in other words, emphasizes that hospitals cannot reduce their readmission rates by focusing on aspects of care within their walls. They must forge new and stronger relationships with their communities if they are to be successful.
How Does the Framework Help Us Understand Which Readmissions Are Preventable?
Public reporting and financial penalties are currently tied to all‐cause readmission, but preventable readmissions are a more appealing outcome to target. In one study, the ranking of hospitals by all‐cause readmission rate had very little correlation with the ranking by preventable readmission rate.[5] However, researchers have struggled to establish standardized, valid, and reliable measures for determining what proportion of readmissions are in fact preventable, with estimates ranging from 5% to 79% in the published literature.[43]
The difficulty of accurately determining preventability stems from an inadequate understanding of the roles that patient comorbidities, transitional processes of care, individual patient behaviors, and social and environmental determinants of health play in the complex process of hospital recidivism. Our proposed elements of an ideal transition in care provide a structure to frame this discussion and suggest future research opportunities to allow a more accurate and reliable understanding of the spectrum of preventability. Care system leadership can use the framework to rigorously evaluate their readmissions and determine the extent to which the transitions process approached the ideal. For example, if a readmission occurs despite care processes that addressed most of the domains with high fidelity, it becomes much less likely that the readmission was preventable. It should be noted that the converse is not always true: When a transition falls well short of the ideal, it does not always imply that provision of a more ideal transition would necessarily have prevented the readmission, but it does make it more likely.
For educators, the framework may provide insights for trainees into the complexity of the transitions process and vulnerability of patients during this time, highlighting preventable aspects of readmissions that are within the grasp of the discharging clinician or team. It highlights the importance of medication reconciliation, synchronous communication, and predischarge teaching, which are measurable and teachable skills for non‐physician providers, housestaff, and medical students. It also may allow for more structured feedback, for example, on the quality of discharge summaries produced by trainees.
How Could the Framework Improve Risk Adjustment for Between‐Hospital Comparisons?
Under the Patient Protection and Affordable Care Act (PPACA), hospitals will be compared to one another using risk‐standardized readmission rates as a way to penalize poorly performing hospitals. However, risk‐adjustment models have only modest ability to predict hospital readmission.[6] Moreover, current approaches predominantly adjust for patients' medical comorbidities (which are easily measurable), but they do not adequately take into account the growing literature on other factors that influence readmission rates, including a patient's health literacy, visual or cognitive impairment, functional status, language barriers, and community‐level factors such as social supports.[44, 45]
The Ideal Transition of Care provides a comprehensive framework of hospital discharge quality that provides additional process measures on which hospitals could be compared rather than focusing solely on (inadequately) risk‐adjusted readmission rates. Indeed, most other quality and safety measures (such as the National Quality Forum's Safe Practices[46] and The Joint Commission's National Patient Safety Goals),[47] emphasize process over outcome, in part because of issues of fairness. Process measures are less subject to differences in patient populations and also change the focus from simply reducing readmissions to improving transitional care more broadly. These process measures should be based on our framework and should attempt to capture as many dimensions of an optimal care transition as possible.
Possible examples of process measures include: the accuracy of medication reconciliation at admission and discharge; provision of prompt outpatient follow‐up; provision of adequate systems to monitor and manage symptoms after discharge; advanced care planning in appropriate patients; and the quality of discharge education, incorporating measurements of the patient's understanding and ability to self‐manage their illness. At least some of these could be used now as part of a performance measurement set that highlights opportunities for immediate system change and can serve as performance milestones.
The framework could also be used to validate risk‐adjustment techniques. After accounting for patient factors, the remaining variability in outcomes should be accounted for by processes of care that are in the transitions framework. Once these processes are accurately measured, one can determine if indeed the remaining variability is due to transitions processes, or rather unaccounted factors that are not being measured and that hospitals may have little control over. Such work can lead to iterative refinement of patient risk‐adjustment models.
What Does the Framework Imply About Best Practices for Reducing Readmission Rates?
Despite the limitations of readmission rates as a quality measure noted above, hospitals presently face potentially large financial penalties for readmissions and are allocating resources to readmission reduction efforts. However, hospitals currently may not have enough guidance to know what actions to take to reduce readmissions, and thus could be spending money inefficiently and reducing the value proposition of focusing on readmissions.
A recent systematic review of interventions hospitals could employ to reduce readmissions identified several positive studies, but also many negative studies, and there were significant barriers to understanding what works to reduce readmissions.[7] For example, most of the interventions described in both positive and negative studies were multifaceted, and the authors were unable to identify which components of the intervention were most effective. Also, while several studies have identified risk factors for readmission,[6, 48, 49] very few studies have identified which subgroups of patients benefit most from specific interventions. Few of the studies described key contextual factors that may have led to successful or failed implementation, or the fidelity with which the intervention was implemented.[50, 51, 52]
Few if any of the studies were guided by a concept of the ideal transition in care.[10] Such a framework will better guide development of multifaceted interventions and provide an improved means for interpreting the results. Clearly, rigorously conducted, multicenter studies of readmission prevention interventions are needed to move the field forward. These studies should: 1) correlate implementation of specific intervention components with reductions in readmission rates to better understand the most effective components; 2) be adequately powered to show effect modification, ie, which patients benefit most from these interventions; and 3) rigorously measure environmental context and intervention fidelity, and employ mixed methods to better understand predictors of implementation success and failure.
Our framework can be used in the design and evaluation of such interventions. For example, interventions could be designed that incorporate as many of the domains of an ideal transition as possible, in particular those that span the inpatient and outpatient settings. Processes of care metrics can be developed that measure the extent to which each domain is delivered, analogous to the way the Joint Commission might aggregate individual scores on the 10 items in Acute Myocardial Infarction Core Measure Set[53] to provide a composite of the quality of care provided to patients with this diagnosis. These can be used to correlate certain intervention components with success in reducing readmissions and also in measuring intervention fidelity.
NEXT STEPS
For hospital and healthcare system leaders, who need to take action now to avoid financial penalties, we recommend starting with proven, high‐touch interventions such as Project RED and the Care Transitions Intervention, which are durable, cost‐effective, robustly address multiple domains of the Ideal Transition in Care, and have been implemented at numerous sites.[54, 55] Each hospital or group will need to decide on a bundle of interventions and customize them based on local workflow, resources, and culture.
Risk‐stratification, to match the intensity of the intervention to the risk of readmission of the patient, will undoubtedly be a key component for the efficient use of resources. We anticipate future research will allow risk stratification to be a robust part of any implementation plan. However, as noted above, current risk prediction models are imperfect,[6] and more work is needed to determine which patients benefit most from which interventions. Few if any studies have described interventions tailored to risk for this reason.
Based on our ideal transition in care, our collective experience, and published evidence,[7, 10, 11, 12] potential elements to start with include: early discharge planning; medication reconciliation[56]; patient/caregiver education using health literacy principles, cognitive assessments, and teach‐back to confirm understanding; synchronous communication (eg, by phone) between inpatient and postdischarge providers; follow‐up phone calls to patients within 72 hours of discharge; 24/7 availability of a responsible inpatient provider to address questions and problems (both from the patient/caregiver and from postdischarge providers); and prompt appointments for patients discharged home. High‐risk patients will likely require additional interventions, including in‐home assessments, disease‐monitoring programs, and/or patient coaching. Lastly, patients with certain conditions prone to readmission (such as heart failure and chronic obstructive pulmonary disease) may benefit from disease‐specific programs, including patient education, outpatient disease management, and monitoring protocols.
It is likely that the most effective interventions are those that come from combined, coordinated interventions shared between inpatient and outpatient settings, and are intensive in nature. We expect that the more domains in the framework that are addressed, the safer and more seamless transitions in care will be, with improvement in patient outcomes. To the extent that fragmentation of care has been a barrier to the implementation of these types of interventions in the past, ACOs, perhaps with imbedded Patient‐Centered Medical Homes, may be in the best position to take advantage of newly aligned financial incentives to design comprehensive transitional care. Indeed, we anticipate that Figure 1 may provide substrate for a discussion of postdischarge care and division of responsibilities between inpatient and outpatient care teams at the time of transition, so effort is not duplicated and multiple domains are addressed.
Other barriers to implementation of ideal transitions in care will continue to be an issue for most healthcare systems. Financial constraints that have been a barrier up until now will be partially overcome by penalties for high readmission rates and by ACOs, bundled payments, and alternative care contracts (ie, global payments), but the extent to which each institution feels rewarded for investing in transitional interventions will vary greatly. Healthcare leadership that sees the value of improving transitions in care will be critical to overcoming this barrier. Competing demands (such as lowering hospital length of stay and carrying out other patient care responsibilities),[57] lack of coordination and diffusion of responsibility among various clinical personnel, and lack of standards are other barriers[58] that will require clear prioritization from leadership, policy changes, team‐based care, provider education and feedback, and adequate allocation of personnel resources. In short, process redesign using continuous quality improvement efforts and effective tools will be required to maximize the possibility of success.
CONCLUSIONS
Readmissions are costly and undesirable. Intuition suggests they are a marker of poor care and that hospitals should be capable of reducing them, thereby improving care and decreasing costs. In a potential future world of ACOs based on global payments, financial incentives would be aligned for each system to reduce readmissions below their current baseline, therefore obviating the need for external financial rewards and penalties. In the meantime, financial penalties do exist, and controversy exists over their fairness and likelihood of driving appropriate behavior. To address these controversies and promote better transitional care, we call for the development and use of multifaceted, collaborative transitions interventions that span settings, risk‐adjustment models that allow for fairer comparisons among hospitals, better and more widespread measurement of processes of transitional care, a better understanding of what interventions are most effective and in whom, and better guidance in how to implement these interventions. Our conceptualization of an ideal transition of care serves as a guide and provides a common vocabulary for these efforts. Such research is likely to produce the knowledge needed for healthcare systems to improve transitions in care, reduce readmissions, and reduce costs.
Disclosure
Funding for Dr Vasilevskis has been provided by the National Institutes of Health (K23AG040157) and the VA Tennessee Valley Geriatric Research, Education and Clinical Center (GRECC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging, the National Institutes of Health, or the US Department of Veterans Affairs.
Containing the rise of healthcare costs has taken on a new sense of urgency in the wake of the recent economic recession and continued growth in the cost of healthcare. Accordingly, many stakeholders seek solutions to improve value (reducing costs while improving care)[1]; hospital readmissions, which are common and costly,[2] have emerged as a key target. The Centers for Medicare and Medicaid Services (CMS) have instituted several programs intended to reduce readmissions, including funding for community‐based, care‐transition programs; penalties for hospitals with elevated risk‐adjusted readmission rates for selected diagnoses; pioneer Accountable Care Organizations (ACOs) with incentives to reduce global costs of care; and Hospital Engagement Networks (HENs) through the Partnership for Patients.[3] A primary aim of these initiatives is to enhance the quality of care transitions as patients are discharged from the hospital.
Though the recent focus on hospital readmissions has appropriately drawn attention to transitions in care, some have expressed concerns. Among these are questions about: 1) the extent to which readmissions truly reflect the quality of hospital care[4]; 2) the preventability of readmissions[5]; 3) limitations in risk‐adjustment techniques[6]; and 4) best practices for preventing readmissions.[7] We believe these concerns stem in part from deficiencies in the state of the science of transitional care, and that future efforts in this area will be hindered without a clear vision of an ideal transition in care. We propose the key components of an ideal transition in care and discuss the implications of this concept as it pertains to hospital readmissions.
THE IDEAL TRANSITION IN CARE
We propose the key components of an ideal transition in care in Figure 1 and Table 1. Figure 1 represents 10 domains described more fully below as structural supports of the bridge patients must cross from one care environment to another during a care transition. This figure highlights key domains and suggests that lack of a domain makes the bridge weaker and more prone to gaps in care and poor outcomes. It also implies that the more components are missing, the less safe is the bridge or transition. Those domains that mainly take place prior to discharge are placed closer to the hospital side of the bridge, those that mainly take place after discharge are placed closer to the community side of the bridge, while those that take place both prior to and after discharge are in the middle. Table 1 provides descriptions of the key content for each of these domains, as well as guidance about which personnel might be involved and where in the transition process that domain should be implemented. We support these domains with supporting evidence where available.

| Domain | Who | When | References |
|---|---|---|---|
| |||
| Discharge planning | |||
| Use a multidisciplinary team to create a discharge plan | Discharging clinician | Predischarge | 911 |
| Collaborate with PCP regarding discharge and follow‐up plan | Care managers/discharge planners | ||
| Arrange follow‐up appointments prior to discharge | Nurses | ||
| Make timely appointments for follow‐up care | |||
| Make appointments that take patient and caregiver's schedules and transportation needs into account | |||
| Complete communication of information | |||
| Includes: | Discharging clinician | Time of discharge | 1214 |
| Patient's full name | |||
| Age | |||
| Dates of admission and discharge | |||
| Names of responsible hospital physicians | |||
| Name of physician preparing discharge summary | |||
| Name of PCP | |||
| Main diagnosis | |||
| Other relevant diagnoses, procedures, and complications | |||
| Relevant findings at admission | |||
| Treatment and response for each active problem | |||
| Results of procedures and abnormal laboratory test results | |||
| Recommendations of any subspecialty consultants | |||
| Patient's functional status at discharge | |||
| Discharge medications | |||
| Follow‐up appointments made and those to be made | |||
| Tests to be ordered and pending tests to be followed‐up | |||
| Counseling provided to patient and caregiver, when applicable | |||
| Contingency planning | |||
| Code status | |||
| Availability, timeliness, clarity, and organization of information | |||
| Timely communication with postdischarge providers verbally (preferred) or by fax/e‐mail | Discharging clinician | Time of discharge | 1214 |
| Timely completion of discharge summary and reliable transmission to postdischarge providers | |||
| Availability of information in medical record | |||
| Use of a structured template with subheadings in discharge communication | |||
| Medication safety | |||
| Take an accurate preadmission medication history | Clinicians | Admission | 1521 |
| Reconcile preadmission medications with all ordered medications at all transfers in care, including discharge | Pharmacists | Throughout hospitalization | |
| Communicate discharge medications to all outpatient providers, including all changes and rationale for those changes | Nurses | Time of discharge | |
| Educating patients, promoting self‐management | |||
| Focus discharge counseling on major diagnoses, medication changes, dates of follow‐up appointments, self‐care instructions, warning signs and symptoms, and who to contact for problems | Clinicians | Daily | 911, 2228, 30 |
| Include caregivers as appropriate | Nurses | Time of discharge | |
| Ensure staff members provide consistent messages | Care managers/discharge planners | Postdischarge | |
| Provide simply written patient‐centered materials with instructions | Transition coaches | ||
| Use teach‐back methods to confirm understanding | |||
| Encourage questions | |||
| Continue teaching during postdischarge follow‐up | |||
| Use transition coaches in high‐risk patients: focus on medication management, keeping a personal medical record, follow‐up appointments, and knowledge of red flags | |||
| Enlisting help of social and community supports | |||
| Assess needs and appropriately arrange for home services | Clinicians | Predischarge and postdischarge | 29, 30 |
| Enlist help of caregivers | Nurses | ||
| Enlist help of community supports | Care managers | ||
| Home health staff | |||
| Advanced care planning | |||
| Establish healthcare proxy | Clinicians | Predischarge and postdischarge | 31, 32 |
| Discuss goals of care | Palliative care staff | ||
| Palliative care consultation (if appropriate) | Social workers | ||
| Enlist hospice services (if appropriate) | Nurses | ||
| Hospice workers | |||
| Coordinating care among team members | |||
| Share medical records | Clinicians | Predischarge and postdischarge | 33 |
| Communicate involving all team members | Nurses | ||
| Optimize continuity of providers and formal handoffs of care | Office personnel | ||
| IT staff | |||
| Monitoring and managing symptoms after discharge | |||
| Monitor for: | Clinicians | Postdischarge | 1113, 28, 3436 |
| Worsening disease control | Nurses | ||
| Medication side effects, discrepancies, nonadherence | Pharmacists | ||
| Therapeutic drug monitoring | Care managers | ||
| Inability to manage conditions at home | Visiting nurses and other home health staff | ||
| Via: | |||
| Postdischarge phone calls | |||
| Home visits | |||
| Postdischarge clinic visits | |||
| Patient hotline | |||
| Availability of inpatient providers after discharge | |||
| Follow‐up with outpatient providers | |||
| Within an appropriate time frame (eg, 7 d or sooner for high‐risk patients) | Clinicians | Postdischarge | 3740 |
| With appropriate providers (eg, most related to reasons for hospitalization, who manage least stable conditions, and/or PCP) | Nurses Pharmacists | ||
| Utilize multidisciplinary teams as appropriate | Care managers | ||
| Ensure appropriate progress along plan of care and safe transition | Office personnel | ||
| Other clinical staff as appropriate | |||
Our concept of an ideal transition in care began with work by Naylor, who described several important components of a safe transition in care, including complete communication of information, patient education, enlisting the help of social and community supports, ensuring continuity of care, and coordinating care among team members.[8] It is supplemented by the Transitions of Care Consensus Policy Statement proposed by representatives from hospital medicine, primary care, and emergency medicine, which emphasized aspects of timeliness and content of communication between providers.[9] Our present articulation of these key components includes 10 organizing domains.
The Discharge Planning domain highlights the important principle of planning ahead for hospital discharge while the patient is still being treated in the hospital, a paradigm espoused by Project RED[10] and other successful care transitions interventions.[11, 12] Collaborating with the outpatient provider and taking the patient and caregiver's preferences for appointment scheduling into account can help ensure optimal outpatient follow‐up.
Complete Communication of Information refers to the content that should be included in discharge summaries and other means of information transfer from hospital to postdischarge care. The specific content areas are based on the Society of Hospital Medicine and Society of General Internal Medicine Continuity of Care Task Force systematic review and recommendations,[13] which takes into account information requested by primary care physicians after discharge.
Availability, Timeliness, Clarity, and Organization of that information is as important as the content because postdischarge providers must be able to access and quickly understand the information they have been provided before assuming care of the patient.[14, 15]
The Medication Safety domain is of central importance because medications are responsible for most postdischarge adverse events.[16] Taking an accurate medication history,[17] reconciling changes throughout the hospitalization,[18] and communicating the reconciled medication regimen to patients and providers across transitions of care can reduce medication errors and improve patient safety.[19, 20, 21, 22]
The Patient Education and Promotion of Self‐Management domain involves teaching patients and their caregivers about the main hospital diagnoses and instructions for self‐care, including medication changes, appointments, and whom to contact if issues arise. Confirming comprehension of instructions through assessments of acute (delirium) and chronic (dementia) cognitive impairments[23, 24, 25, 26] and teach‐back from the patient (or caregiver) is an important aspect of such counseling, as is providing patients and caregivers with educational materials that are appropriate for their level of health literacy and preferred language.[14] High‐risk patients may benefit from patient coaching to improve their self‐management skills.[12] These recommendations are based on years of health literacy research,[27, 28, 29] and such elements are generally included in effective interventions (including Project RED,[10] Naylor and colleagues' Transitional Care Model,[11] and Coleman and colleagues' Care Transitions Intervention[12]).
Enlisting the help of Social and Community Supports is an important adjunct to medical care and is the rationale for the recent increase in CMS funding for community‐based, care‐transition programs. These programs are crucial for assisting patients with household activities, meals, and other necessities during the period of recovery, though they should be distinguished from care management or care coordination interventions, which have not been found to be helpful in preventing readmissions unless high touch in nature.[30, 31]
The Advanced Care Planning domain may begin in the hospital or outpatient setting, and involves establishing goals of care and healthcare proxies, as well as engaging with palliative care or hospice services if appropriate. Emerging evidence supports the intuitive conclusion that this approach prevents readmissions, particularly in patients who do not benefit from hospital readmission.[32, 33]
Attention to the Coordinating Care Among Team Members domain is needed to synchronize efforts across settings and providers. Clearly, many healthcare professionals as well as other involved parties can be involved in helping a single patient during transitions in care. It is vital that they coordinate information, assessments, and plans as a team.[34]
We recognize the domain of Monitoring and Managing Symptoms After Discharge as increasingly crucial as reflected in our growing understanding of the reasons for readmission, especially among patients with fragile conditions such as heart failure, chronic lung disease, gastrointestinal disorders, dementia,[23, 24, 25, 26] and vascular disease.[35] Monitoring for new or worsening symptoms; medication side effects, discrepancies, or nonadherence; and other self‐management challenges will allow problems to be detected and addressed early, before they result in unplanned healthcare utilization. It is noteworthy that successful interventions in this regard rely on in‐home evaluation[13, 14, 29] by nurses rather than telemonitoring, which in isolation has not been effective to date.[36, 37]
Finally, optimal Outpatient Follow‐Up with appropriate postdischarge providers is crucial for providing ideal transitions. These appointments need to be prompt[38, 39] (eg, within 7 days if not sooner for high‐risk patients) and with providers who have a longitudinal relationship to the patient, as prior work has shown increased readmissions when the provider is unfamiliar with the patient.[40] The advantages and disadvantages of hospitalist‐run postdischarge clinics as one way to increase access and expedite follow‐up are currently being explored. Although the optimal content of a postdischarge visit has not been defined, logical tasks to be completed are myriad and imply the need for checklists, adequate time, and a multidisciplinary team of providers.[41]
IMPLICATIONS OF THE IDEAL TRANSITION IN CARE
Our conceptualization of an ideal transition in care provides insight for hospital and healthcare system leadership, policymakers, researchers, clinicians, and educators seeking to improve transitions of care and reduce hospital readmissions. In the sections below, we briefly review commonly cited concerns about the recent focus on readmissions as a quality measure, illustrate how the Ideal Transition in Care addresses these concerns, and propose fruitful areas for future work.
How Does the Framework Address the Extent to Which Readmissions Reflect Hospital Quality?
One of the chief problems with readmissionrates as a hospital quality measure is that many of the factors that influence readmission may not currently be under the hospital's control. The healthcare environment to which a patient is being discharged (and was admitted from in the first place) is an important determinant of readmission.[42] In this context, it is noteworthy that successful interventions to reduce readmission are generally those that focus on outpatient follow‐up, while inpatient‐only interventions have had less success.[7] This is reflected in our framework above, informed by the literature, highlighting the importance of coordination between inpatient and outpatient providers and the importance of postdischarge care, including monitoring and managing symptoms after discharge, prompt follow‐up appointments, the continuation of patient self‐management activities, monitoring for drug‐related problems after discharge, and the effective utilization of community supports. Accountable care organizations, once established, would be responsible for several components of this environment, including the provision of prompt and effective follow‐up care.
The implication of the framework is that if a hospital does not have control over most of the factors that influence its readmission rate, it should see financial incentives to reduce readmission rates as an opportunity to invest in relationships with the outpatient environment from which their patients are admitted and to which they are discharged. One can envision hospitals growing ever‐closer relationships with their network of primary care physician groups, community agencies, and home health services, rehabilitation facilities, and nursing homes through coordinated discharge planning, medication management, patient education, shared electronic medical records, structured handoffs in care, and systems of intensive outpatient monitoring. Our proposed framework, in other words, emphasizes that hospitals cannot reduce their readmission rates by focusing on aspects of care within their walls. They must forge new and stronger relationships with their communities if they are to be successful.
How Does the Framework Help Us Understand Which Readmissions Are Preventable?
Public reporting and financial penalties are currently tied to all‐cause readmission, but preventable readmissions are a more appealing outcome to target. In one study, the ranking of hospitals by all‐cause readmission rate had very little correlation with the ranking by preventable readmission rate.[5] However, researchers have struggled to establish standardized, valid, and reliable measures for determining what proportion of readmissions are in fact preventable, with estimates ranging from 5% to 79% in the published literature.[43]
The difficulty of accurately determining preventability stems from an inadequate understanding of the roles that patient comorbidities, transitional processes of care, individual patient behaviors, and social and environmental determinants of health play in the complex process of hospital recidivism. Our proposed elements of an ideal transition in care provide a structure to frame this discussion and suggest future research opportunities to allow a more accurate and reliable understanding of the spectrum of preventability. Care system leadership can use the framework to rigorously evaluate their readmissions and determine the extent to which the transitions process approached the ideal. For example, if a readmission occurs despite care processes that addressed most of the domains with high fidelity, it becomes much less likely that the readmission was preventable. It should be noted that the converse is not always true: When a transition falls well short of the ideal, it does not always imply that provision of a more ideal transition would necessarily have prevented the readmission, but it does make it more likely.
For educators, the framework may provide insights for trainees into the complexity of the transitions process and vulnerability of patients during this time, highlighting preventable aspects of readmissions that are within the grasp of the discharging clinician or team. It highlights the importance of medication reconciliation, synchronous communication, and predischarge teaching, which are measurable and teachable skills for non‐physician providers, housestaff, and medical students. It also may allow for more structured feedback, for example, on the quality of discharge summaries produced by trainees.
How Could the Framework Improve Risk Adjustment for Between‐Hospital Comparisons?
Under the Patient Protection and Affordable Care Act (PPACA), hospitals will be compared to one another using risk‐standardized readmission rates as a way to penalize poorly performing hospitals. However, risk‐adjustment models have only modest ability to predict hospital readmission.[6] Moreover, current approaches predominantly adjust for patients' medical comorbidities (which are easily measurable), but they do not adequately take into account the growing literature on other factors that influence readmission rates, including a patient's health literacy, visual or cognitive impairment, functional status, language barriers, and community‐level factors such as social supports.[44, 45]
The Ideal Transition of Care provides a comprehensive framework of hospital discharge quality that provides additional process measures on which hospitals could be compared rather than focusing solely on (inadequately) risk‐adjusted readmission rates. Indeed, most other quality and safety measures (such as the National Quality Forum's Safe Practices[46] and The Joint Commission's National Patient Safety Goals),[47] emphasize process over outcome, in part because of issues of fairness. Process measures are less subject to differences in patient populations and also change the focus from simply reducing readmissions to improving transitional care more broadly. These process measures should be based on our framework and should attempt to capture as many dimensions of an optimal care transition as possible.
Possible examples of process measures include: the accuracy of medication reconciliation at admission and discharge; provision of prompt outpatient follow‐up; provision of adequate systems to monitor and manage symptoms after discharge; advanced care planning in appropriate patients; and the quality of discharge education, incorporating measurements of the patient's understanding and ability to self‐manage their illness. At least some of these could be used now as part of a performance measurement set that highlights opportunities for immediate system change and can serve as performance milestones.
The framework could also be used to validate risk‐adjustment techniques. After accounting for patient factors, the remaining variability in outcomes should be accounted for by processes of care that are in the transitions framework. Once these processes are accurately measured, one can determine if indeed the remaining variability is due to transitions processes, or rather unaccounted factors that are not being measured and that hospitals may have little control over. Such work can lead to iterative refinement of patient risk‐adjustment models.
What Does the Framework Imply About Best Practices for Reducing Readmission Rates?
Despite the limitations of readmission rates as a quality measure noted above, hospitals presently face potentially large financial penalties for readmissions and are allocating resources to readmission reduction efforts. However, hospitals currently may not have enough guidance to know what actions to take to reduce readmissions, and thus could be spending money inefficiently and reducing the value proposition of focusing on readmissions.
A recent systematic review of interventions hospitals could employ to reduce readmissions identified several positive studies, but also many negative studies, and there were significant barriers to understanding what works to reduce readmissions.[7] For example, most of the interventions described in both positive and negative studies were multifaceted, and the authors were unable to identify which components of the intervention were most effective. Also, while several studies have identified risk factors for readmission,[6, 48, 49] very few studies have identified which subgroups of patients benefit most from specific interventions. Few of the studies described key contextual factors that may have led to successful or failed implementation, or the fidelity with which the intervention was implemented.[50, 51, 52]
Few if any of the studies were guided by a concept of the ideal transition in care.[10] Such a framework will better guide development of multifaceted interventions and provide an improved means for interpreting the results. Clearly, rigorously conducted, multicenter studies of readmission prevention interventions are needed to move the field forward. These studies should: 1) correlate implementation of specific intervention components with reductions in readmission rates to better understand the most effective components; 2) be adequately powered to show effect modification, ie, which patients benefit most from these interventions; and 3) rigorously measure environmental context and intervention fidelity, and employ mixed methods to better understand predictors of implementation success and failure.
Our framework can be used in the design and evaluation of such interventions. For example, interventions could be designed that incorporate as many of the domains of an ideal transition as possible, in particular those that span the inpatient and outpatient settings. Processes of care metrics can be developed that measure the extent to which each domain is delivered, analogous to the way the Joint Commission might aggregate individual scores on the 10 items in Acute Myocardial Infarction Core Measure Set[53] to provide a composite of the quality of care provided to patients with this diagnosis. These can be used to correlate certain intervention components with success in reducing readmissions and also in measuring intervention fidelity.
NEXT STEPS
For hospital and healthcare system leaders, who need to take action now to avoid financial penalties, we recommend starting with proven, high‐touch interventions such as Project RED and the Care Transitions Intervention, which are durable, cost‐effective, robustly address multiple domains of the Ideal Transition in Care, and have been implemented at numerous sites.[54, 55] Each hospital or group will need to decide on a bundle of interventions and customize them based on local workflow, resources, and culture.
Risk‐stratification, to match the intensity of the intervention to the risk of readmission of the patient, will undoubtedly be a key component for the efficient use of resources. We anticipate future research will allow risk stratification to be a robust part of any implementation plan. However, as noted above, current risk prediction models are imperfect,[6] and more work is needed to determine which patients benefit most from which interventions. Few if any studies have described interventions tailored to risk for this reason.
Based on our ideal transition in care, our collective experience, and published evidence,[7, 10, 11, 12] potential elements to start with include: early discharge planning; medication reconciliation[56]; patient/caregiver education using health literacy principles, cognitive assessments, and teach‐back to confirm understanding; synchronous communication (eg, by phone) between inpatient and postdischarge providers; follow‐up phone calls to patients within 72 hours of discharge; 24/7 availability of a responsible inpatient provider to address questions and problems (both from the patient/caregiver and from postdischarge providers); and prompt appointments for patients discharged home. High‐risk patients will likely require additional interventions, including in‐home assessments, disease‐monitoring programs, and/or patient coaching. Lastly, patients with certain conditions prone to readmission (such as heart failure and chronic obstructive pulmonary disease) may benefit from disease‐specific programs, including patient education, outpatient disease management, and monitoring protocols.
It is likely that the most effective interventions are those that come from combined, coordinated interventions shared between inpatient and outpatient settings, and are intensive in nature. We expect that the more domains in the framework that are addressed, the safer and more seamless transitions in care will be, with improvement in patient outcomes. To the extent that fragmentation of care has been a barrier to the implementation of these types of interventions in the past, ACOs, perhaps with imbedded Patient‐Centered Medical Homes, may be in the best position to take advantage of newly aligned financial incentives to design comprehensive transitional care. Indeed, we anticipate that Figure 1 may provide substrate for a discussion of postdischarge care and division of responsibilities between inpatient and outpatient care teams at the time of transition, so effort is not duplicated and multiple domains are addressed.
Other barriers to implementation of ideal transitions in care will continue to be an issue for most healthcare systems. Financial constraints that have been a barrier up until now will be partially overcome by penalties for high readmission rates and by ACOs, bundled payments, and alternative care contracts (ie, global payments), but the extent to which each institution feels rewarded for investing in transitional interventions will vary greatly. Healthcare leadership that sees the value of improving transitions in care will be critical to overcoming this barrier. Competing demands (such as lowering hospital length of stay and carrying out other patient care responsibilities),[57] lack of coordination and diffusion of responsibility among various clinical personnel, and lack of standards are other barriers[58] that will require clear prioritization from leadership, policy changes, team‐based care, provider education and feedback, and adequate allocation of personnel resources. In short, process redesign using continuous quality improvement efforts and effective tools will be required to maximize the possibility of success.
CONCLUSIONS
Readmissions are costly and undesirable. Intuition suggests they are a marker of poor care and that hospitals should be capable of reducing them, thereby improving care and decreasing costs. In a potential future world of ACOs based on global payments, financial incentives would be aligned for each system to reduce readmissions below their current baseline, therefore obviating the need for external financial rewards and penalties. In the meantime, financial penalties do exist, and controversy exists over their fairness and likelihood of driving appropriate behavior. To address these controversies and promote better transitional care, we call for the development and use of multifaceted, collaborative transitions interventions that span settings, risk‐adjustment models that allow for fairer comparisons among hospitals, better and more widespread measurement of processes of transitional care, a better understanding of what interventions are most effective and in whom, and better guidance in how to implement these interventions. Our conceptualization of an ideal transition of care serves as a guide and provides a common vocabulary for these efforts. Such research is likely to produce the knowledge needed for healthcare systems to improve transitions in care, reduce readmissions, and reduce costs.
Disclosure
Funding for Dr Vasilevskis has been provided by the National Institutes of Health (K23AG040157) and the VA Tennessee Valley Geriatric Research, Education and Clinical Center (GRECC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging, the National Institutes of Health, or the US Department of Veterans Affairs.
- , . Redefining Health Care: Creating Value‐Based Competition on Results. Boston, MA:Harvard Business School Press;2006.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- Patient Protection and Affordable Care Act (PPACA). Public Law 111–148; 2010. Available at: http://www.gpo.gov/fdsys/pkg/PLAW‐111publ148/pdf/PLAW‐111publ148.pdf. Accessed on June 4, 2012.
- , . Hospital readmission as an accountability measure. JAMA. 2011;305(5):504–505.
- , , , et al. Incidence of potentially avoidable urgent readmissions and their relation to all‐cause urgent readmissions. Can Med Assoc J. 2011;183(14):E1067–E1072.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- . A decade of transitional care research with vulnerable elders. J Cardiovasc Nurs. 2000;14(3):1–14.
- , , , et al;for the American College of Physicians; Society of General Internal Medicine; Society of Hospital Medicine; American Geriatrics Society; American College of Emergency Physicians; Society of Academic Emergency Medicine. Transitions of Care Consensus Policy Statement American College of Physicians–Society of General Internal Medicine–Society of Hospital Medicine–American Geriatrics Society–American College of Emergency Physicians–Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24(8):971–976.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187.
- , , , et al. Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281(7):613–620.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , . Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314–323.
- , , , , , . Discharge documentation of patients discharged to subacute facilities: a three‐year quality improvement process across an integrated health care system. Jt Comm J Qual Patient Saf. 2010;36(6):243–251.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , , , . Frequency, type and clinical importance of medication history errors at admission to hospital: a systematic review. Can Med Assoc J. 2005;173(5):510–515.
- , , , et al. Classifying and predicting errors of inpatient medication reconciliation. J Gen Intern Med. 2008;23(9):1414–1422.
- , , et al;for the PILL‐CVD (Pharmacist Intervention for Low Literacy in Cardiovascular Disease) Study Group. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med. 2012;157(1):1–10.
- , , , . Hospital‐based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057–1069.
- , , , et al. Effect of an electronic medication reconciliation application and process redesign on potential adverse drug events: a cluster‐randomized trial. Arch Intern Med. 2009;169(8):771–780.
- , , , et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166(5):565–571.
- , , , , . Insufficient help for activity of daily living disabilities and risk of all–cause hospitalization. J Am Geriatr Soc. 2012;60(5):927–933.
- , , , et al. Transitions in care for older adults with and without dementia. J Am Geriatr Soc. 2012;60(5):813–820.
- , , , , . Association of incident dementia with hospitalizations. JAMA. 2012;307(2):165–172.
- , , , et al. Potentially avoidable hospitalizations of dually eligible Medicare and Medicaid beneficiaries from nursing facility and home– and community–based services waiver programs. J Am Geriatr Soc. 2012;60(5):821–829.
- , . Teaching about health literacy and clear communication. J Gen Intern Med. 2006;21(8):888–890.
- , , , et al. Health literacy and outcomes among patients with heart failure. JAMA. 2011;305(16):1695–1701.
- , , , , . Patient experiences of transitioning from hospital to home: an ethnographic quality improvement project. J Hosp Med. 2012;7(5):382–387.
- , , , . Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries: 15 randomized trials. JAMA. 2009;301(6):603–618.
- , , , , . How changes in Washington University's Medicare coordinated care demonstration pilot ultimately achieved savings. Health Aff (Millwood). 2012;31(6):1216–1226.
- , , , et al. Quality of care and rehospitalization rate in the last stage of disease in brain tumor patients assisted at home: a cost effectiveness study. J Palliat Med. 2012;15(2):225–227.
- , , , , . Inpatient palliative care consults and the probability of hospital readmission. Perm J. 2011;15(2):48–51.
- , , , et al. TeamSTEPPS™: team strategies and tools to enhance performance and patient safety. In: Henriksen K, Battles JB, Keyes MA, Grady ML, ed. Advances in Patient Safety: New Directions and Alternative Approaches. Vol 3: Performance and Tools. Rockville, MD:Agency for Healthcare Research and Quality; August2008.
- , , , et al. Factors contributing to all‐cause 30‐day readmissions: a structured case series across 18 hospitals. Med Care. 2012;50(7):599–605.
- , , , et al. Telemonitoring in patients with heart failure [erratum, N Engl J Med. 2011;364(5):490]. N Engl J Med. 2010;363(24):2301–2309.
- , , , et al. A randomized controlled trial of telemonitoring in older adults with multiple health issues to prevent hospitalizations and emergency department visits. Arch Intern Med. 2012;172(10):773–779.
- , , , et al. Relationship between early physician follow‐up and 30‐day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303(17):1716–1722.
- , , . Post‐hospitalization transitions: examining the effects of timing of primary care provider follow‐up. J Hosp Med. 2010;5(7):392–397.
- , , . Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on Primary Care and Hospital Readmission. N Engl J Med. 1996;334(22):1441–1447.
- . The Post‐Hospital Follow‐Up Visit: A Physician Checklist to Reduce Readmissions. California Healthcare Foundation; October 2010. Available at: http://www.chcf.org/publications/2010/10/the‐post‐hospital‐follow‐up‐visit‐a‐physician‐checklist. Accessed on January 10, 2012.
- , , . Thirty‐day readmission rates for Medicare beneficiaries by race and site of care. JAMA. 2011;305(7):675–681.
- , , , , . Proportion of hospital readmissions deemed avoidable: a systematic review. Can Med Assoc J. 2011;183(7):E391–E402.
- , , , et al. Postdischarge environmental and socioeconomic factors and the likelihood of early hospital readmission among community‐dwelling Medicare beneficiaries. Gerontologist. 2008;48(4):495–504.
- , , , , . Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155(2):97–107.
- National Quality Forum. Safe Practices for Better Healthcare—2010 Update: A Consensus Report. Washington, DC;2010.
- Joint Commission on Accreditation of Healthcare Organizations. Accreditation Program: Hospital 2010 National Patient Safety Goals (NPSGs). 2010. Available at: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/. Accessed on March 20, 2012.
- , , , et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25(3):211–219.
- , , , et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. Can Med Assoc J. 2010;182(6):551–557.
- , . Evaluating service delivery interventions to enhance patient safety. BMJ. 2008;337:a2764.
- , , . Assessing the Evidence for Context‐Sensitive Effectiveness and Safety of Patient Safety Practices: Developing Criteria. Rockville, MD:Agency for Healthcare Research and Quality; December2010.
- , , , et al. Advancing the science of patient safety. Ann Intern Med. 2011;154(10):693–696.
- The Joint Commission. Acute Myocardial Infarction Core Measure Set. Available at: http://www.jointcommission.org/assets/1/6/Acute%20Myocardial%20Infarction.pdf. Accessed August 20,2012.
- , , , , , . The care transitions intervention: translating from efficacy to effectiveness. Arch Intern Med. 2011;171(14):1232–1237.
- Project RED toolkit, AHRQ Innovations Exchange. Available at:http://www.innovations.ahrq.gov/content.aspx?id=2180. Accessed on July 2, 2012.
- , , , et al. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: a randomized controlled trial. Arch Intern Med. 2009;169(9):894–900.
- , . Thirty‐day readmissions—truth and consequences. N Engl J Med. 2012;366(15):1366–1369.
- , , , , . “Out of sight, out of mind”: housestaff perceptions of quality‐limiting factors in discharge care at teaching hospitals. J Hosp Med. 2012;7(5):376–381.
- , . Redefining Health Care: Creating Value‐Based Competition on Results. Boston, MA:Harvard Business School Press;2006.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- Patient Protection and Affordable Care Act (PPACA). Public Law 111–148; 2010. Available at: http://www.gpo.gov/fdsys/pkg/PLAW‐111publ148/pdf/PLAW‐111publ148.pdf. Accessed on June 4, 2012.
- , . Hospital readmission as an accountability measure. JAMA. 2011;305(5):504–505.
- , , , et al. Incidence of potentially avoidable urgent readmissions and their relation to all‐cause urgent readmissions. Can Med Assoc J. 2011;183(14):E1067–E1072.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- . A decade of transitional care research with vulnerable elders. J Cardiovasc Nurs. 2000;14(3):1–14.
- , , , et al;for the American College of Physicians; Society of General Internal Medicine; Society of Hospital Medicine; American Geriatrics Society; American College of Emergency Physicians; Society of Academic Emergency Medicine. Transitions of Care Consensus Policy Statement American College of Physicians–Society of General Internal Medicine–Society of Hospital Medicine–American Geriatrics Society–American College of Emergency Physicians–Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24(8):971–976.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187.
- , , , et al. Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281(7):613–620.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , . Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314–323.
- , , , , , . Discharge documentation of patients discharged to subacute facilities: a three‐year quality improvement process across an integrated health care system. Jt Comm J Qual Patient Saf. 2010;36(6):243–251.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , , , . Frequency, type and clinical importance of medication history errors at admission to hospital: a systematic review. Can Med Assoc J. 2005;173(5):510–515.
- , , , et al. Classifying and predicting errors of inpatient medication reconciliation. J Gen Intern Med. 2008;23(9):1414–1422.
- , , et al;for the PILL‐CVD (Pharmacist Intervention for Low Literacy in Cardiovascular Disease) Study Group. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med. 2012;157(1):1–10.
- , , , . Hospital‐based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057–1069.
- , , , et al. Effect of an electronic medication reconciliation application and process redesign on potential adverse drug events: a cluster‐randomized trial. Arch Intern Med. 2009;169(8):771–780.
- , , , et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166(5):565–571.
- , , , , . Insufficient help for activity of daily living disabilities and risk of all–cause hospitalization. J Am Geriatr Soc. 2012;60(5):927–933.
- , , , et al. Transitions in care for older adults with and without dementia. J Am Geriatr Soc. 2012;60(5):813–820.
- , , , , . Association of incident dementia with hospitalizations. JAMA. 2012;307(2):165–172.
- , , , et al. Potentially avoidable hospitalizations of dually eligible Medicare and Medicaid beneficiaries from nursing facility and home– and community–based services waiver programs. J Am Geriatr Soc. 2012;60(5):821–829.
- , . Teaching about health literacy and clear communication. J Gen Intern Med. 2006;21(8):888–890.
- , , , et al. Health literacy and outcomes among patients with heart failure. JAMA. 2011;305(16):1695–1701.
- , , , , . Patient experiences of transitioning from hospital to home: an ethnographic quality improvement project. J Hosp Med. 2012;7(5):382–387.
- , , , . Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries: 15 randomized trials. JAMA. 2009;301(6):603–618.
- , , , , . How changes in Washington University's Medicare coordinated care demonstration pilot ultimately achieved savings. Health Aff (Millwood). 2012;31(6):1216–1226.
- , , , et al. Quality of care and rehospitalization rate in the last stage of disease in brain tumor patients assisted at home: a cost effectiveness study. J Palliat Med. 2012;15(2):225–227.
- , , , , . Inpatient palliative care consults and the probability of hospital readmission. Perm J. 2011;15(2):48–51.
- , , , et al. TeamSTEPPS™: team strategies and tools to enhance performance and patient safety. In: Henriksen K, Battles JB, Keyes MA, Grady ML, ed. Advances in Patient Safety: New Directions and Alternative Approaches. Vol 3: Performance and Tools. Rockville, MD:Agency for Healthcare Research and Quality; August2008.
- , , , et al. Factors contributing to all‐cause 30‐day readmissions: a structured case series across 18 hospitals. Med Care. 2012;50(7):599–605.
- , , , et al. Telemonitoring in patients with heart failure [erratum, N Engl J Med. 2011;364(5):490]. N Engl J Med. 2010;363(24):2301–2309.
- , , , et al. A randomized controlled trial of telemonitoring in older adults with multiple health issues to prevent hospitalizations and emergency department visits. Arch Intern Med. 2012;172(10):773–779.
- , , , et al. Relationship between early physician follow‐up and 30‐day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303(17):1716–1722.
- , , . Post‐hospitalization transitions: examining the effects of timing of primary care provider follow‐up. J Hosp Med. 2010;5(7):392–397.
- , , . Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on Primary Care and Hospital Readmission. N Engl J Med. 1996;334(22):1441–1447.
- . The Post‐Hospital Follow‐Up Visit: A Physician Checklist to Reduce Readmissions. California Healthcare Foundation; October 2010. Available at: http://www.chcf.org/publications/2010/10/the‐post‐hospital‐follow‐up‐visit‐a‐physician‐checklist. Accessed on January 10, 2012.
- , , . Thirty‐day readmission rates for Medicare beneficiaries by race and site of care. JAMA. 2011;305(7):675–681.
- , , , , . Proportion of hospital readmissions deemed avoidable: a systematic review. Can Med Assoc J. 2011;183(7):E391–E402.
- , , , et al. Postdischarge environmental and socioeconomic factors and the likelihood of early hospital readmission among community‐dwelling Medicare beneficiaries. Gerontologist. 2008;48(4):495–504.
- , , , , . Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155(2):97–107.
- National Quality Forum. Safe Practices for Better Healthcare—2010 Update: A Consensus Report. Washington, DC;2010.
- Joint Commission on Accreditation of Healthcare Organizations. Accreditation Program: Hospital 2010 National Patient Safety Goals (NPSGs). 2010. Available at: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/. Accessed on March 20, 2012.
- , , , et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25(3):211–219.
- , , , et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. Can Med Assoc J. 2010;182(6):551–557.
- , . Evaluating service delivery interventions to enhance patient safety. BMJ. 2008;337:a2764.
- , , . Assessing the Evidence for Context‐Sensitive Effectiveness and Safety of Patient Safety Practices: Developing Criteria. Rockville, MD:Agency for Healthcare Research and Quality; December2010.
- , , , et al. Advancing the science of patient safety. Ann Intern Med. 2011;154(10):693–696.
- The Joint Commission. Acute Myocardial Infarction Core Measure Set. Available at: http://www.jointcommission.org/assets/1/6/Acute%20Myocardial%20Infarction.pdf. Accessed August 20,2012.
- , , , , , . The care transitions intervention: translating from efficacy to effectiveness. Arch Intern Med. 2011;171(14):1232–1237.
- Project RED toolkit, AHRQ Innovations Exchange. Available at:http://www.innovations.ahrq.gov/content.aspx?id=2180. Accessed on July 2, 2012.
- , , , et al. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: a randomized controlled trial. Arch Intern Med. 2009;169(9):894–900.
- , . Thirty‐day readmissions—truth and consequences. N Engl J Med. 2012;366(15):1366–1369.
- , , , , . “Out of sight, out of mind”: housestaff perceptions of quality‐limiting factors in discharge care at teaching hospitals. J Hosp Med. 2012;7(5):376–381.
Rethinking Cardiac Risk Reduction
Noncardiac surgery is frequently associated with major adverse cardiac events. Prevention of these events has traditionally focused on risk estimation and targeted interventions prior to surgical intervention.1 Cardiac risk is assessed through clinical prediction rules, such as the revised cardiac risk index (RCRI) or the American Society of Anesthesiology (ASA) classification. In patients deemed to be at high risk of adverse cardiac events, discretionary preoperative testing, medical treatments, and interventions are implemented.2 However, even when executed optimally, this approach fails to protect all patients. Thus, many patients undergoing noncardiac surgery continue to experience perioperative myocardial infarction (MI) and death.3
Recently, a prospective international study involving 15,133 patients reported that cardiac troponin levels measured within 3 days of noncardiac surgery were strongly associated with 30‐day mortality.4 This study is but the latest in a series of investigations that have concluded that postoperative measurement of cardiac troponin is a better predictor of cardiac outcomes than preoperative‐risk algorithms.48 Intuitively, this link is not surprising. Surgery represents the ultimate physiologic stress test. In the setting of hemodynamic changes and sustained oxygen supply:demand mismatch, it is hardly shocking that those with significant epicardial coronary stenosis or major subendocardial ischemia suffer poorer outcomes. What is perhaps most important about the association between troponin release and clinical outcomes, however, is the foundation it provides for a novel framework to improve perioperative care: using troponin measurement to guide treatment and interventions in the postoperative setting.
MODEL TO IMPROVE POSTOPERATIVE CARDIAC OUTCOMES
We hypothesize that the postoperative period can be seized as a golden moment to ameliorate cardiac risk and improve clinical outcomes for 3 reasons. First, those who release cardiac troponin during surgery declare themselves as harboring myocardial territories in jeopardy. In effect, the peripheral presence of troponin amounts to a cry for help in a population where many cardiac events are silent and thus remain clinically unnoticed.9, 10 Routine postoperative monitoring for this biomarker can thus help identify a population that would ordinarily escape detection until the precipitation of a major clinical event. Second, as the pathophysiology of cardiac events centers around rupture of vulnerable plaque (type‐1 MI), supply:demand mismatch (type‐2 MI), or both of these phenomena, escalation or institution of important medical risk‐reducing treatments (such as aspirin, beta‐blockers, or statins), can be established to improve outcomes. The ability to initiate these treatments in a monitored setting may offset and address many of the risks feared with these therapies, including hypotension, stroke, or rhabdomyolysis. Third, as patients convalesce from surgery, timely and discretionary cardiac testing or interventions can be undertaken in those identified as being at elevated cardiovascular risk prior to discharge. In sum, the trifecta of clearly recognizing those at risk, instituting interventions in controlled settings, and performing well‐timed interventions could translate into greater odds of survival in this cohort.
Owing to their intimate involvement with patients in the perioperative setting, hospitalists are uniquely suited for implementing a troponin surveillance paradigm. Yet, how may such a schema be realized? In Figure 1, we outline a pathway with which to implement this approach. This model replicates perioperative management workflow, calling for 3 discrete interventionspreoperative assessment, perioperative surveillance, and postoperative risk stratification:
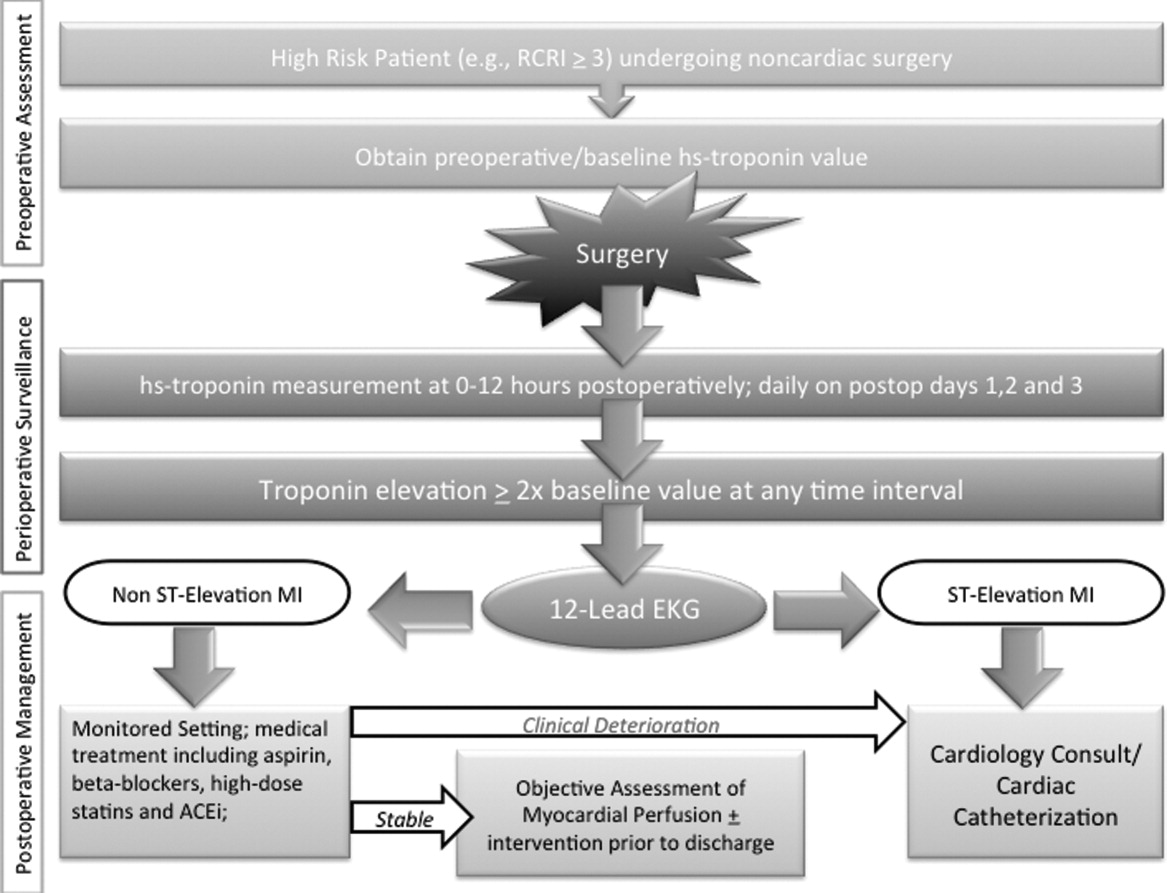
-
Preoperative assessment: Patients estimated to be at high risk of perioperative cardiac events (eg, RCRI 3) should undergo baseline, preoperative high‐sensitivity troponin measurement, as the frequency of major adverse cardiac outcomes is significant this group (11%).11 The importance of a baseline measurement of troponin cannot be overstated, as biologic variation of this marker can impact subsequent management.12, 13 Hospitalists can obtain these assays in preoperative clinics at the time of initial review and risk‐estimation.
-
Perioperative surveillance: Following the model implemented by a recent study, serial troponin surveillance should commence 612 hours after surgery, then daily on the first, second, and third day.4 As the optimal cardiac troponin threshold for the diagnosis of perioperative ischemia remains uncertain, doubling of baseline troponin levels may serve as a logical signal for further evaluation.14 The electrocardiographic pattern may be used as a branch point for immediate management at this stage: ST‐segment elevation MI may be treated aggressively with an early invasive strategy and cardiac catheterization when apropos (much as in the nonoperative context). However, as most perioperative cardiac events are non‐ST elevation MIs, treatment of this subset should center on initiation or escalation of medical therapy. Thus, achieving heart‐rate control in order to preserve coronary perfusion by careful titration of beta‐blockers, and initiation/up‐titration of statins to temper unstable atherosclerotic plaques represent cornerstones of this approach. Other potential therapeutic entities such as initiation of angiotensin‐converting‐enzyme (ACE)‐inhibitors in the context of left‐ventricular dysfunction, and/or antiplatelet agents such as aspirin, may also be relevant risk‐reducing tools.
-
Postoperative risk stratification: Prior to hospital discharge, objective assessment of myocardial perfusion and viability should occur in selected individuals with troponin elevations. Modalities that may be utilized in this context include either noninvasive imaging or occasional coronary angiography in patients with unstable coronary syndromes. Defining an optimal approach must be contextual, as interventions that may subsequently mandate uninterrupted antiplatelet treatment may be logistically challenging in this setting.
LIMITATIONS
Although this implementation model is plausible, it generates many questions that must be tested through the lens of a randomized controlled study. For instance, what is the optimal strategy for intervention in the postoperative setting? Though medical treatment with statins and beta‐blockade may represent the mainstay of treatment, are these therapies safe during potential clinical instability? Can net benefit be realized through judicious use of coronary angiography and revascularization? Can the artifact of heightened awareness and reporting of postoperative cardiac events mar the reported quality of a hospital? Finally, though cardiac troponin has been shown to be a strong and independent predictor of mortality, which troponin assay, reference range, and troponometric standard to use remain unclear.15 Whether or not these interventions translate into lesser mortality and improved clinical outcomes represents the raison d'etre for this experimental approach. As no alternative approach to define and target patients at high risk of adverse outcome after seemingly uneventful surgery currently exists, we believe that this hypothetical paradigm is worthy of further investigation.
CONCLUSION
For decades, perioperative practitioners have searched for a divining rod to reduce risk during surgery. While our efforts have been focused on the preoperative setting, the postoperative setting represents an as yet untapped and potentially profound period in the quest to improving surgical outcomes. Through our proximity to patients in the perioperative setting, hospitalists are ideal agents to test, deliver, and bring about this change. It is time for a postoperative carpe diem.
- , , , et al. Predictors of cardiac events after major vascular surgery: role of clinical characteristics, dobutamine echocardiography, and beta‐blocker therapy. JAMA. 2001;285(14):1865–1873.
- , , , et al. Combining clinical and thallium data optimizes preoperative assessment of cardiac risk before major vascular surgery. Ann Intern Med. 1989;110(11):859–866.
- , , , et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet. 2008;372(9633):139–144.
- VISION Study Investigators. Association between postoperative troponin levels and 30‐day mortlality among patients undergoing noncardiac surgery. JAMA. 2012;307(21):2295–2304.
- , , , et al. Postoperative levels of cardiac troponin versus CK‐MB and high‐sensitivity C‐reactive protein for the prediction of 1‐year cardiovascular outcome in patients undergoing vascular surgery. Coron Artery Dis. 2011;22(6):428–434.
- , , , et al. Peak postoperative troponin levels outperform preoperative cardiac risk indices as predictors of long‐term mortality after vascular surgery Troponins and postoperative outcomes. J Crit Care. 2012;27(1):66–72.
- , , , et al. Prognostic value of troponin and creatine kinase muscle and brain isoenzyme measurement after noncardiac surgery: a systematic review and meta‐analysis. Anesthesiology. 2011;114(4):796–806.
- , , . Outcomes in vascular surgical patients with isolated postoperative troponin leak: a meta‐analysis. Anaesthesia. 2011;66(7):604–610.
- , , , et al. Characteristics and short‐term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med. 2011;154(8):523–528.
- , , , , . Perioperative myocardial necrosis in patients at high cardiovascular risk undergoing elective non‐cardiac surgery. Heart. 2012;98(10):792–798.
- , , , et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043–1049.
- , , , , . Biologic variation of a novel cardiac troponin I assay. Clin Chem. 2011;57(7):1080–1081.
- , , , , . Short‐ and long‐term biological variation in cardiac troponin I measured with a high‐sensitivity assay: implications for clinical practice. Clin Chem. 2009;55(1):52–58.
- , , , et al. Relationship between perioperative troponin elevation and other indicators of myocardial injury in vascular surgery patients. Br J Anaesth. 2006;96(3):303–309.
- , , , et al. Which troponometric best predicts midterm outcome after coronary artery bypass graft surgery? Ann Thorac Surg. 2011;91(6):1860–1867.
Noncardiac surgery is frequently associated with major adverse cardiac events. Prevention of these events has traditionally focused on risk estimation and targeted interventions prior to surgical intervention.1 Cardiac risk is assessed through clinical prediction rules, such as the revised cardiac risk index (RCRI) or the American Society of Anesthesiology (ASA) classification. In patients deemed to be at high risk of adverse cardiac events, discretionary preoperative testing, medical treatments, and interventions are implemented.2 However, even when executed optimally, this approach fails to protect all patients. Thus, many patients undergoing noncardiac surgery continue to experience perioperative myocardial infarction (MI) and death.3
Recently, a prospective international study involving 15,133 patients reported that cardiac troponin levels measured within 3 days of noncardiac surgery were strongly associated with 30‐day mortality.4 This study is but the latest in a series of investigations that have concluded that postoperative measurement of cardiac troponin is a better predictor of cardiac outcomes than preoperative‐risk algorithms.48 Intuitively, this link is not surprising. Surgery represents the ultimate physiologic stress test. In the setting of hemodynamic changes and sustained oxygen supply:demand mismatch, it is hardly shocking that those with significant epicardial coronary stenosis or major subendocardial ischemia suffer poorer outcomes. What is perhaps most important about the association between troponin release and clinical outcomes, however, is the foundation it provides for a novel framework to improve perioperative care: using troponin measurement to guide treatment and interventions in the postoperative setting.
MODEL TO IMPROVE POSTOPERATIVE CARDIAC OUTCOMES
We hypothesize that the postoperative period can be seized as a golden moment to ameliorate cardiac risk and improve clinical outcomes for 3 reasons. First, those who release cardiac troponin during surgery declare themselves as harboring myocardial territories in jeopardy. In effect, the peripheral presence of troponin amounts to a cry for help in a population where many cardiac events are silent and thus remain clinically unnoticed.9, 10 Routine postoperative monitoring for this biomarker can thus help identify a population that would ordinarily escape detection until the precipitation of a major clinical event. Second, as the pathophysiology of cardiac events centers around rupture of vulnerable plaque (type‐1 MI), supply:demand mismatch (type‐2 MI), or both of these phenomena, escalation or institution of important medical risk‐reducing treatments (such as aspirin, beta‐blockers, or statins), can be established to improve outcomes. The ability to initiate these treatments in a monitored setting may offset and address many of the risks feared with these therapies, including hypotension, stroke, or rhabdomyolysis. Third, as patients convalesce from surgery, timely and discretionary cardiac testing or interventions can be undertaken in those identified as being at elevated cardiovascular risk prior to discharge. In sum, the trifecta of clearly recognizing those at risk, instituting interventions in controlled settings, and performing well‐timed interventions could translate into greater odds of survival in this cohort.
Owing to their intimate involvement with patients in the perioperative setting, hospitalists are uniquely suited for implementing a troponin surveillance paradigm. Yet, how may such a schema be realized? In Figure 1, we outline a pathway with which to implement this approach. This model replicates perioperative management workflow, calling for 3 discrete interventionspreoperative assessment, perioperative surveillance, and postoperative risk stratification:

-
Preoperative assessment: Patients estimated to be at high risk of perioperative cardiac events (eg, RCRI 3) should undergo baseline, preoperative high‐sensitivity troponin measurement, as the frequency of major adverse cardiac outcomes is significant this group (11%).11 The importance of a baseline measurement of troponin cannot be overstated, as biologic variation of this marker can impact subsequent management.12, 13 Hospitalists can obtain these assays in preoperative clinics at the time of initial review and risk‐estimation.
-
Perioperative surveillance: Following the model implemented by a recent study, serial troponin surveillance should commence 612 hours after surgery, then daily on the first, second, and third day.4 As the optimal cardiac troponin threshold for the diagnosis of perioperative ischemia remains uncertain, doubling of baseline troponin levels may serve as a logical signal for further evaluation.14 The electrocardiographic pattern may be used as a branch point for immediate management at this stage: ST‐segment elevation MI may be treated aggressively with an early invasive strategy and cardiac catheterization when apropos (much as in the nonoperative context). However, as most perioperative cardiac events are non‐ST elevation MIs, treatment of this subset should center on initiation or escalation of medical therapy. Thus, achieving heart‐rate control in order to preserve coronary perfusion by careful titration of beta‐blockers, and initiation/up‐titration of statins to temper unstable atherosclerotic plaques represent cornerstones of this approach. Other potential therapeutic entities such as initiation of angiotensin‐converting‐enzyme (ACE)‐inhibitors in the context of left‐ventricular dysfunction, and/or antiplatelet agents such as aspirin, may also be relevant risk‐reducing tools.
-
Postoperative risk stratification: Prior to hospital discharge, objective assessment of myocardial perfusion and viability should occur in selected individuals with troponin elevations. Modalities that may be utilized in this context include either noninvasive imaging or occasional coronary angiography in patients with unstable coronary syndromes. Defining an optimal approach must be contextual, as interventions that may subsequently mandate uninterrupted antiplatelet treatment may be logistically challenging in this setting.
LIMITATIONS
Although this implementation model is plausible, it generates many questions that must be tested through the lens of a randomized controlled study. For instance, what is the optimal strategy for intervention in the postoperative setting? Though medical treatment with statins and beta‐blockade may represent the mainstay of treatment, are these therapies safe during potential clinical instability? Can net benefit be realized through judicious use of coronary angiography and revascularization? Can the artifact of heightened awareness and reporting of postoperative cardiac events mar the reported quality of a hospital? Finally, though cardiac troponin has been shown to be a strong and independent predictor of mortality, which troponin assay, reference range, and troponometric standard to use remain unclear.15 Whether or not these interventions translate into lesser mortality and improved clinical outcomes represents the raison d'etre for this experimental approach. As no alternative approach to define and target patients at high risk of adverse outcome after seemingly uneventful surgery currently exists, we believe that this hypothetical paradigm is worthy of further investigation.
CONCLUSION
For decades, perioperative practitioners have searched for a divining rod to reduce risk during surgery. While our efforts have been focused on the preoperative setting, the postoperative setting represents an as yet untapped and potentially profound period in the quest to improving surgical outcomes. Through our proximity to patients in the perioperative setting, hospitalists are ideal agents to test, deliver, and bring about this change. It is time for a postoperative carpe diem.
Noncardiac surgery is frequently associated with major adverse cardiac events. Prevention of these events has traditionally focused on risk estimation and targeted interventions prior to surgical intervention.1 Cardiac risk is assessed through clinical prediction rules, such as the revised cardiac risk index (RCRI) or the American Society of Anesthesiology (ASA) classification. In patients deemed to be at high risk of adverse cardiac events, discretionary preoperative testing, medical treatments, and interventions are implemented.2 However, even when executed optimally, this approach fails to protect all patients. Thus, many patients undergoing noncardiac surgery continue to experience perioperative myocardial infarction (MI) and death.3
Recently, a prospective international study involving 15,133 patients reported that cardiac troponin levels measured within 3 days of noncardiac surgery were strongly associated with 30‐day mortality.4 This study is but the latest in a series of investigations that have concluded that postoperative measurement of cardiac troponin is a better predictor of cardiac outcomes than preoperative‐risk algorithms.48 Intuitively, this link is not surprising. Surgery represents the ultimate physiologic stress test. In the setting of hemodynamic changes and sustained oxygen supply:demand mismatch, it is hardly shocking that those with significant epicardial coronary stenosis or major subendocardial ischemia suffer poorer outcomes. What is perhaps most important about the association between troponin release and clinical outcomes, however, is the foundation it provides for a novel framework to improve perioperative care: using troponin measurement to guide treatment and interventions in the postoperative setting.
MODEL TO IMPROVE POSTOPERATIVE CARDIAC OUTCOMES
We hypothesize that the postoperative period can be seized as a golden moment to ameliorate cardiac risk and improve clinical outcomes for 3 reasons. First, those who release cardiac troponin during surgery declare themselves as harboring myocardial territories in jeopardy. In effect, the peripheral presence of troponin amounts to a cry for help in a population where many cardiac events are silent and thus remain clinically unnoticed.9, 10 Routine postoperative monitoring for this biomarker can thus help identify a population that would ordinarily escape detection until the precipitation of a major clinical event. Second, as the pathophysiology of cardiac events centers around rupture of vulnerable plaque (type‐1 MI), supply:demand mismatch (type‐2 MI), or both of these phenomena, escalation or institution of important medical risk‐reducing treatments (such as aspirin, beta‐blockers, or statins), can be established to improve outcomes. The ability to initiate these treatments in a monitored setting may offset and address many of the risks feared with these therapies, including hypotension, stroke, or rhabdomyolysis. Third, as patients convalesce from surgery, timely and discretionary cardiac testing or interventions can be undertaken in those identified as being at elevated cardiovascular risk prior to discharge. In sum, the trifecta of clearly recognizing those at risk, instituting interventions in controlled settings, and performing well‐timed interventions could translate into greater odds of survival in this cohort.
Owing to their intimate involvement with patients in the perioperative setting, hospitalists are uniquely suited for implementing a troponin surveillance paradigm. Yet, how may such a schema be realized? In Figure 1, we outline a pathway with which to implement this approach. This model replicates perioperative management workflow, calling for 3 discrete interventionspreoperative assessment, perioperative surveillance, and postoperative risk stratification:

-
Preoperative assessment: Patients estimated to be at high risk of perioperative cardiac events (eg, RCRI 3) should undergo baseline, preoperative high‐sensitivity troponin measurement, as the frequency of major adverse cardiac outcomes is significant this group (11%).11 The importance of a baseline measurement of troponin cannot be overstated, as biologic variation of this marker can impact subsequent management.12, 13 Hospitalists can obtain these assays in preoperative clinics at the time of initial review and risk‐estimation.
-
Perioperative surveillance: Following the model implemented by a recent study, serial troponin surveillance should commence 612 hours after surgery, then daily on the first, second, and third day.4 As the optimal cardiac troponin threshold for the diagnosis of perioperative ischemia remains uncertain, doubling of baseline troponin levels may serve as a logical signal for further evaluation.14 The electrocardiographic pattern may be used as a branch point for immediate management at this stage: ST‐segment elevation MI may be treated aggressively with an early invasive strategy and cardiac catheterization when apropos (much as in the nonoperative context). However, as most perioperative cardiac events are non‐ST elevation MIs, treatment of this subset should center on initiation or escalation of medical therapy. Thus, achieving heart‐rate control in order to preserve coronary perfusion by careful titration of beta‐blockers, and initiation/up‐titration of statins to temper unstable atherosclerotic plaques represent cornerstones of this approach. Other potential therapeutic entities such as initiation of angiotensin‐converting‐enzyme (ACE)‐inhibitors in the context of left‐ventricular dysfunction, and/or antiplatelet agents such as aspirin, may also be relevant risk‐reducing tools.
-
Postoperative risk stratification: Prior to hospital discharge, objective assessment of myocardial perfusion and viability should occur in selected individuals with troponin elevations. Modalities that may be utilized in this context include either noninvasive imaging or occasional coronary angiography in patients with unstable coronary syndromes. Defining an optimal approach must be contextual, as interventions that may subsequently mandate uninterrupted antiplatelet treatment may be logistically challenging in this setting.
LIMITATIONS
Although this implementation model is plausible, it generates many questions that must be tested through the lens of a randomized controlled study. For instance, what is the optimal strategy for intervention in the postoperative setting? Though medical treatment with statins and beta‐blockade may represent the mainstay of treatment, are these therapies safe during potential clinical instability? Can net benefit be realized through judicious use of coronary angiography and revascularization? Can the artifact of heightened awareness and reporting of postoperative cardiac events mar the reported quality of a hospital? Finally, though cardiac troponin has been shown to be a strong and independent predictor of mortality, which troponin assay, reference range, and troponometric standard to use remain unclear.15 Whether or not these interventions translate into lesser mortality and improved clinical outcomes represents the raison d'etre for this experimental approach. As no alternative approach to define and target patients at high risk of adverse outcome after seemingly uneventful surgery currently exists, we believe that this hypothetical paradigm is worthy of further investigation.
CONCLUSION
For decades, perioperative practitioners have searched for a divining rod to reduce risk during surgery. While our efforts have been focused on the preoperative setting, the postoperative setting represents an as yet untapped and potentially profound period in the quest to improving surgical outcomes. Through our proximity to patients in the perioperative setting, hospitalists are ideal agents to test, deliver, and bring about this change. It is time for a postoperative carpe diem.
- , , , et al. Predictors of cardiac events after major vascular surgery: role of clinical characteristics, dobutamine echocardiography, and beta‐blocker therapy. JAMA. 2001;285(14):1865–1873.
- , , , et al. Combining clinical and thallium data optimizes preoperative assessment of cardiac risk before major vascular surgery. Ann Intern Med. 1989;110(11):859–866.
- , , , et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet. 2008;372(9633):139–144.
- VISION Study Investigators. Association between postoperative troponin levels and 30‐day mortlality among patients undergoing noncardiac surgery. JAMA. 2012;307(21):2295–2304.
- , , , et al. Postoperative levels of cardiac troponin versus CK‐MB and high‐sensitivity C‐reactive protein for the prediction of 1‐year cardiovascular outcome in patients undergoing vascular surgery. Coron Artery Dis. 2011;22(6):428–434.
- , , , et al. Peak postoperative troponin levels outperform preoperative cardiac risk indices as predictors of long‐term mortality after vascular surgery Troponins and postoperative outcomes. J Crit Care. 2012;27(1):66–72.
- , , , et al. Prognostic value of troponin and creatine kinase muscle and brain isoenzyme measurement after noncardiac surgery: a systematic review and meta‐analysis. Anesthesiology. 2011;114(4):796–806.
- , , . Outcomes in vascular surgical patients with isolated postoperative troponin leak: a meta‐analysis. Anaesthesia. 2011;66(7):604–610.
- , , , et al. Characteristics and short‐term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med. 2011;154(8):523–528.
- , , , , . Perioperative myocardial necrosis in patients at high cardiovascular risk undergoing elective non‐cardiac surgery. Heart. 2012;98(10):792–798.
- , , , et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043–1049.
- , , , , . Biologic variation of a novel cardiac troponin I assay. Clin Chem. 2011;57(7):1080–1081.
- , , , , . Short‐ and long‐term biological variation in cardiac troponin I measured with a high‐sensitivity assay: implications for clinical practice. Clin Chem. 2009;55(1):52–58.
- , , , et al. Relationship between perioperative troponin elevation and other indicators of myocardial injury in vascular surgery patients. Br J Anaesth. 2006;96(3):303–309.
- , , , et al. Which troponometric best predicts midterm outcome after coronary artery bypass graft surgery? Ann Thorac Surg. 2011;91(6):1860–1867.
- , , , et al. Predictors of cardiac events after major vascular surgery: role of clinical characteristics, dobutamine echocardiography, and beta‐blocker therapy. JAMA. 2001;285(14):1865–1873.
- , , , et al. Combining clinical and thallium data optimizes preoperative assessment of cardiac risk before major vascular surgery. Ann Intern Med. 1989;110(11):859–866.
- , , , et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet. 2008;372(9633):139–144.
- VISION Study Investigators. Association between postoperative troponin levels and 30‐day mortlality among patients undergoing noncardiac surgery. JAMA. 2012;307(21):2295–2304.
- , , , et al. Postoperative levels of cardiac troponin versus CK‐MB and high‐sensitivity C‐reactive protein for the prediction of 1‐year cardiovascular outcome in patients undergoing vascular surgery. Coron Artery Dis. 2011;22(6):428–434.
- , , , et al. Peak postoperative troponin levels outperform preoperative cardiac risk indices as predictors of long‐term mortality after vascular surgery Troponins and postoperative outcomes. J Crit Care. 2012;27(1):66–72.
- , , , et al. Prognostic value of troponin and creatine kinase muscle and brain isoenzyme measurement after noncardiac surgery: a systematic review and meta‐analysis. Anesthesiology. 2011;114(4):796–806.
- , , . Outcomes in vascular surgical patients with isolated postoperative troponin leak: a meta‐analysis. Anaesthesia. 2011;66(7):604–610.
- , , , et al. Characteristics and short‐term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med. 2011;154(8):523–528.
- , , , , . Perioperative myocardial necrosis in patients at high cardiovascular risk undergoing elective non‐cardiac surgery. Heart. 2012;98(10):792–798.
- , , , et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043–1049.
- , , , , . Biologic variation of a novel cardiac troponin I assay. Clin Chem. 2011;57(7):1080–1081.
- , , , , . Short‐ and long‐term biological variation in cardiac troponin I measured with a high‐sensitivity assay: implications for clinical practice. Clin Chem. 2009;55(1):52–58.
- , , , et al. Relationship between perioperative troponin elevation and other indicators of myocardial injury in vascular surgery patients. Br J Anaesth. 2006;96(3):303–309.
- , , , et al. Which troponometric best predicts midterm outcome after coronary artery bypass graft surgery? Ann Thorac Surg. 2011;91(6):1860–1867.
Acute Medicine in the United Kingdom
Hospital medicine has emerged in the United States (US) to address the complexity of hospital care and over the last 15 years has become the fastest growing specialty in US history.1 The field has been shaped by societal, financial, and clinical factors within American health care, several of which also exist elsewhere in the world.2, 3 Outside the US, analogs of hospital medicine have evolved; in the United Kingdom (UK), where the term and concept of a hospitalist is widely unknown, the specialty of acute medicine has evolved to meet the complex needs of the acutely unwell medical patient in the modern health care environment. The similarities are notable, as are the differences. Our objective in this brief communication is to introduce the UK model of acute medicine to counterparts in the US. We trace the development of acute medicine in the UK, describe current practice, and note features of the model potentially applicable to hospital medicine in the US. We use UK terminology but provide equivalent terms from the US, as shown in Table 1.
| UK Term | US Term |
|---|---|
| |
| General practitioner | Family practice physician |
| Consultant physician* | Attending physician (including all general internists) |
| Postgraduate trainee physicians | Interns, residents, or fellows |
| Respiratory service | Pulmonary service |
| Medicine of the elderly service | Geriatric service |
| Accident and emergency department | Emergency department |
| High‐dependency unit | Step‐down unit |
Background and Factors Contributing to the Rise of Acute Medicine in the UK
Patient care in the UK National Health Service (NHS) is separated into inpatient and outpatient care. Generally, outpatient care is provided by general practitioners (GPs). GP clinics are independent structures and interact with local NHS‐funded services via contract, in contrast to NHS hospitals that are directly controlled by their local NHS municipal‐based body. GPs have no independent admission rights to hospitals, and (with few exceptions) do not participate in direct inpatient care. Consequently, patients in GP clinics requiring hospital admission have been referred to hospital‐based providers who assume all responsibility for inpatient care. The inpatient medical physician body in the UK is comprised of consultants, each usually trained in both general internal medicine and a medical specialty very similar to US internal medicinebased subspecialists, such as endocrinology or infectious disease. Prior to the advent of acute medicine, each consultant shared responsibility for admission of medical patients with consultants from other specialties, according to a call schedule. Generalist‐focused care would be initiated by postgraduate trainee physicians at the time of admission, and continued by the accepting consultant who often conducted subspecialty inpatient and outpatient work simultaneously. Due to advances in medical care at the turn of the century, inpatient care became more specialized; as a result, a general trend developed where the contribution of some specialties to generalist‐focused care grew (respiratory and medicine of the elderly), while other specialties began to focus on specialty‐specific interventions at the expense of practice and training in the generalist approach to care (cardiology, nephrology). Consequently, interservice disparity in provision of generalist‐focused care grew, especially in larger UK teaching hospitals. These trends have manifested as recent changes in UK medical training; presently, all UK medical specialty training programs require concomitant training in general internal medicine competencies, but for some specialties, general internal medicine training is truncated (either by the training program or by allowed choice of trainees) to provide less training than what is required for recognition as a specialist in general internal medicine.
In the UK, the majority of direct clinical care is provided via supervision of postgraduate trainee physicians. Over the last 20 years, limits on resident duty‐hours have been applied, much as has happened over the previous decade in the US.4 In 1991, the NHS and the British Medical Association negotiated a compensation package for physicians in training, termed the New Deal for Junior Doctors, which called for limitation of actual work hours for postgraduate trainee physicians to 56 hours per week. Enforcement of New Deal work guidelines was implemented over the next 12 years; with the introduction of the European Working Time Directive in 2000, work hours were further limited to 48 hours a week by 2009 for consultant and trainee physicians alike. Many UK consultants had already been devoting a higher percentage of time to subspecialty‐based hospital work and, with the reduced availability of the postgraduate trainee physician resource, the quality of generalist‐focused care (for conditions out with a consultant's given specialty) became more disparate between medical specialties, with some specialties providing little generalist input during the admission process. Simultaneously, and in the context of evolving demographic and regulatory pressures (Table 2), the admission procedure required an increasingly specific set of competencies. A subset of consultants from many different specialties began to focus specifically on management of the admission process and to informally self‐identify as specialists in acute medicine.
|
| Advances in medical care leading to increased specialization |
| Increasing numbers of elderly patients with complex medical needs |
| UK‐wide targets to limit emergency department patient stays to <4 hours |
| New limits to postgraduate trainee physician work hours |
| Increased standards of supervision of trainee physicians by consultants |
| Deficiencies in availability of outpatient out‐of‐hours care |
| Locally led reconfigurations of health care resources to favor community‐based care over inpatient‐based care |
Concerns were published about the quality of initial care for the acutely unwell patient in the UK.5 The UK Royal Colleges were concerned that patients with acute medical illnesses should receive high‐quality clinical care and commissioned a number of working groups to determine how acute medicine should best be delivered. Although initial reports suggested that acute care should be delivered by physicians who maintained an organ‐specific specialty focus, subsequent reports suggested that acute medicine should be delivered by specifically trained individuals capable of managing both the acutely ill medical patient and the administration of an acute medical unit (AMU).6, 7 In response to these trends, in 2003 the Royal Colleges of Physicians Joint Committee for Higher Medical Training, now known as the Joint Royal College of Physicians Training Board (JRCPTB), introduced a training curriculum for acute medicine as a subspecialty of general medicine.8 In 2007, the Royal College of Physicians convened an Acute Medicine Task Force that published further recommendations on the purpose and design of acute medicine services.9 Application by the JRCPTB to the regulatory bodies for medical education and training in the UK led to recognition in 2009 of acute internal medicine as a separate and distinct specialty from all other specialties, including general medicine.10
Acute Medicine in Practice: the Admission Process and Prevention of Prolonged Hospital Admission
The defining characteristic of an acute medical service in the UK is the sole dedication of a team of physician, nursing, and allied health care support staff (such as therapists, pharmacists, and social workers) to the task of admission and initial care of medical inpatients during their work shifts. Admission activity usually takes place in a dedicated physical area: the AMU. The AMU is commonly located near an accident and emergency (AE) department and is often colocated with radiology services, an intensive care unit, and/or a high‐dependency unit. Patients may be admitted to the AMU from the AE department, or directly from GP clinics. Generally, an AMU is responsible for a spectrum of medical conditions identical to the conditions potentially managed by a US‐based hospitalist. Unlike general and subspecialty medical wards, where consultant bedside input may be available as infrequently as 2 to 3 times per week, twice‐daily consultant bedside input into AMU patient care is the recommended standard. AMUs provide consultant bedside input via multiple rounds during the day, or alternatively in a continuous, per‐admission rolling pattern. Existing data suggest that AMUs with daily consultant input shorten hospital length of stay and increase same‐day discharges without affecting readmissions or mortality.11 Outside the US, observational studies associate AMUs with improved hospital mortality, shortened length of stay, decreased emergency department waiting times, and improved patient satisfaction.12
Three major models of acute medicine practice have evolved in the UK, as outlined in Table 3. The model adopted by each AMU varies depending on availability of staff, AMU bed capacity, the number and variability of patients requiring admission, and even hospital philosophy regarding division of responsibility between acute medicine physicians and those of other specialties. AMUs also vary in critical care capability, with many providing noninvasive ventilation or invasive hemodynamic monitoring. Admitted medical inpatients may bypass an AMU altogether if the AMU staff are unable to provide a procedure (eg, hemodialysis), if a patient requires no further diagnostic clarification or stabilization (eg, routine chemotherapy), or if an AMU admission would delay provision of time‐sensitive care (eg, percutaneous coronary intervention for ST‐elevation myocardial infarction). In all AMUs, patients requiring inpatient care outside of the AMU will be admitted to a medical specialty ward (cardiology, general internal medicine, neurology, etc). Generalist‐focused care is then provided by postgraduate trainee physicians on the medical specialty ward, based on guidance generated by AMU physicians, per guidance form their supervising specialty consultant physician (if possible), or through the advice given by other specialty services. Whether AMU physicians continue to be responsible for the care of AMU patients transferred to a general internal medicine ward depends on arrangements based on the particular AMU model and hospital staffing factors.
| Acute Medicine Models | Acute Medicine Team Focus |
|---|---|
| |
| Triage | Inpatient care rapidly transitioned to specialty medical ward with minimal stay in AMU |
| Short stay | Short‐term inpatient care (<72 hours) provided in AMU, including extensive assessment (eg, physical therapy, sequential radiologic imaging), multispecialty bedside input, medical therapy, and either coordination of postdischarge follow‐up or transition of care to specialty medical ward |
| Hybrid | Subset of patients rapidly transitioned to specialty medical ward, while others receive care in AMU for up to 72 hours; mix dependent on patient needs and available hospital/AMU resources |
Weaknesses and Strengths of Acute Medicine Model Applicable to US‐Based Hospital Medicine
The acute medicine model of care does instantiate potential risks. Utilization of an acute medicine team hardwires fragmentation of care, necessitating handovers. In the context of US hospital medicine practice, this fragmentation may compromise safety or throughput; however, no such deficit has been detected to date in the context of acute medicine practice in the UK.13 Mismatch between AMU bed or staff capacity and the number or rate of hospital admissions can generate safety risks or give away efficiency gains. Further inefficiencies can develop if hospital‐wide processes of handover, medical decision making, patient transport, and discharge are not synchronized with AMU outflow and intake. Evidence of AMU throughput failure is most often manifest by the premature transfer of patients from AMU to the main hospital ward areas, or by delay of admissions from the emergency department into the AMU (UK standards until recently mandated that 98% of AE patients complete their AE stay in 4 hours). Although some successful UK AMUs have minimized these failures, such problems are still experienced by many acute medicine services throughout the UK. Ongoing debate, both local and national, persists within the acute medicine community about how best to address these challenges.
The strengths of the acute medicine model appear to be clinically meaningful, however. The admission process is complex and occurs at a time when patients are sickest and potentially the most vulnerable. Effective management of this period offers significant opportunity to improve value for patients, hospitals, and health systems. When applied in the context of US hospitalist programs, instances of successful short stay units and active bed management do exist.1417 These documented successes represent partial application of UK‐style acute medicine activity in a US hospital setting. A multidisciplinary health care team dedicated to streamlining admissions, short stays, and follow‐up care offers many potential benefits. Standardization and accountability of admission process, especially important for quality improvement and research activity applicable to the initial portion of a hospital stay, may be more readily realized if embedded into the practice of a discrete cohort of hospital staff. In the UK, several hospital processes fall within the exclusive remit of an acute medicine service (Table 4). Optimization of several of these processes of care can reduce hospital morbidity, mortality, and length of stay.1821 As health care financing reform arrives in the US, the ability of American hospitals to manage admission‐specific processes of care with reliability will become more vital.3 In the US, programs that force hospitalists to make ad hoc, moment‐to‐moment prioritizations about when and where to perform admissions, discharges, and daily ward care may do so at the expense of system predictability, standardization, and patient‐centeredness. Where hospitalists are forced to juggle these geographically and substantively disparate care duties, data suggest significant opportunities to reduce variability and improve efficiency.22, 23
| Initiation of time‐sensitive acute care bundles (eg, stroke, sepsis, myocardial infarction) |
| Initiation of disease‐specific protocols (eg, venous thromboembolism prophylaxis, glycemic control) |
| Outpatient‐inpatient information reconciliation (medicines, code status, etc) |
| Outpatient‐to‐inpatient consultation (general practitioner phone consultation, telemedicine) |
| Stewardship of empiric antimicrobial therapy |
| Early involvement of discharge planning apparatus |
| Provision of follow‐up ambulatory care (medical assessment unit discharge with next‐day hospital follow‐up) |
| Outpatient intravenous antibiotic services |
| Frequent patient admission policies |
Integrated into US hospital medicine practices, the UK acute medicine model might capture otherwise elusive quality and efficiency gains.14 By the same token, integrating portions of the US hospital medicine model into a UK acute medicine model could be beneficial as well. For instance, when compared with the interservice handover common in UK AMUs, intraservice handover (acute care hospitalist‐to‐ward hospitalist) may promote standardization of the handover process and potentially fewer instances of failed communication. What seems certain is that greater attention should be focused on an exchange of ideas between acute medicine and hospital medicine.
Acknowledgements
The authors thank Valery Akopov for review of the manuscript.
Conflicts of Interest: Drs. Smith and Jones are employed as acute medicine physicians by NHS Lothian, and both have received reimbursement for public speaking related to acute medicine. Dr. Jones has received reimbursement for curriculum design activity for the acute medicine specialty in the UK.
- Society of Hospital Medicine. Growth of Hospital Medicine Nationwide. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/Media/GrowthofHospitalMedicineNationwide/Growth_of_Hospital_M.htm. Accessed May 15, 2011.
- . The hospitalist field turns 15: new opportunities and challenges. J Hosp Med. 2011;6:E1–E4.
- . Value‐driven healthcare: implications for hospitals and hospitalists. J Hosp Med. 2009;4:507–511.
- , , . ACGME Work Group on Resident Duty Hours. Accreditation Council for Graduate Medical Education. New requirements for resident duty hours. JAMA. 2002;288:1112–1114.
- , , , et al. Confidential inquiry into quality of care before admission to intensive care. BMJ. 1998;316:1853–1858.
- Federation of Medical Royal Colleges. Acute Medicine: The Physician's Role: Proposals for the Future. A Working Party Report of the Federation of Medical Royal Colleges. London, UK: Federation of Medical Royal Colleges; 2000.
- Federation of Medical Royal Colleges. Acute Medicine: Making it Work for Patients. A Blueprint for Organization and Training. Report of a Working Party. London, UK: Federation of Medical Royal Colleges; 2004.
- Joint Royal College of Physicians Training Board. Higher medical training curriculum for subspecialty training in acute medicine for general (internal) medicine NTN holders. July 2003.
- College of Physicians, London. Acute medical care: the right person, in the right setting—first time. Report of the Acute Medicine Task Force. October 2007.
- Joint Royal College of Physicians Training Board. Specialty training curriculum for acute internal medicine. August 2009.
- , , , . What is the effect of a consultant presence in an acute medical unit? Clin Med. 2009;9:214–218.
- , , . Effectiveness of acute medical units in hospitals: a systematic review. Int J Qual Health Care. 2009;21:397–407.
- , , , , . The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5:335–338.
- , , . Implementation of a hospitalist‐run observation unit and impact on length of stay (LOS): a brief report. J Hosp Med. 2010;5:E2–E5.
- , , , , , . Active bed management by hospitalists and emergency department throughput. Ann Intern Med. 2008;149:804–811.
- , , , . Hospitalist bed management effecting throughput from the emergency department to the intensive care unit. J Crit Care. 2001;25:184–189.
- , . MSSU: a multidisciplinary approach to finding cost effective and efficient care for observation patients. Quality and Safety Fall Forum, University HealthSystem Consortium Conference; 2009.
- , , , , . Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta‐analysis of randomized controlled trials. Arch Intern Med. 2007;167:1476–1486.
- , , , et al. Results of the Medications at Transitions and Clinical Handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. J Gen Intern Med. 2010;25:441–447.
- , , , . Antiplatelet therapy for acute ischaemic stroke. Cochrane Database Syst Rev. 2008;16:CD000029.
- , , , et al. Early goal‐directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377.
- , , , et al. Where did the day go?—A time motion study of hospitalists. J Hosp Med. 2010;5:323–328.
- , , , , . Hospitalist time usage and cyclicality: opportunities to improve efficiency. J Hosp Med. 2010;5:329–334.
Hospital medicine has emerged in the United States (US) to address the complexity of hospital care and over the last 15 years has become the fastest growing specialty in US history.1 The field has been shaped by societal, financial, and clinical factors within American health care, several of which also exist elsewhere in the world.2, 3 Outside the US, analogs of hospital medicine have evolved; in the United Kingdom (UK), where the term and concept of a hospitalist is widely unknown, the specialty of acute medicine has evolved to meet the complex needs of the acutely unwell medical patient in the modern health care environment. The similarities are notable, as are the differences. Our objective in this brief communication is to introduce the UK model of acute medicine to counterparts in the US. We trace the development of acute medicine in the UK, describe current practice, and note features of the model potentially applicable to hospital medicine in the US. We use UK terminology but provide equivalent terms from the US, as shown in Table 1.
| UK Term | US Term |
|---|---|
| |
| General practitioner | Family practice physician |
| Consultant physician* | Attending physician (including all general internists) |
| Postgraduate trainee physicians | Interns, residents, or fellows |
| Respiratory service | Pulmonary service |
| Medicine of the elderly service | Geriatric service |
| Accident and emergency department | Emergency department |
| High‐dependency unit | Step‐down unit |
Background and Factors Contributing to the Rise of Acute Medicine in the UK
Patient care in the UK National Health Service (NHS) is separated into inpatient and outpatient care. Generally, outpatient care is provided by general practitioners (GPs). GP clinics are independent structures and interact with local NHS‐funded services via contract, in contrast to NHS hospitals that are directly controlled by their local NHS municipal‐based body. GPs have no independent admission rights to hospitals, and (with few exceptions) do not participate in direct inpatient care. Consequently, patients in GP clinics requiring hospital admission have been referred to hospital‐based providers who assume all responsibility for inpatient care. The inpatient medical physician body in the UK is comprised of consultants, each usually trained in both general internal medicine and a medical specialty very similar to US internal medicinebased subspecialists, such as endocrinology or infectious disease. Prior to the advent of acute medicine, each consultant shared responsibility for admission of medical patients with consultants from other specialties, according to a call schedule. Generalist‐focused care would be initiated by postgraduate trainee physicians at the time of admission, and continued by the accepting consultant who often conducted subspecialty inpatient and outpatient work simultaneously. Due to advances in medical care at the turn of the century, inpatient care became more specialized; as a result, a general trend developed where the contribution of some specialties to generalist‐focused care grew (respiratory and medicine of the elderly), while other specialties began to focus on specialty‐specific interventions at the expense of practice and training in the generalist approach to care (cardiology, nephrology). Consequently, interservice disparity in provision of generalist‐focused care grew, especially in larger UK teaching hospitals. These trends have manifested as recent changes in UK medical training; presently, all UK medical specialty training programs require concomitant training in general internal medicine competencies, but for some specialties, general internal medicine training is truncated (either by the training program or by allowed choice of trainees) to provide less training than what is required for recognition as a specialist in general internal medicine.
In the UK, the majority of direct clinical care is provided via supervision of postgraduate trainee physicians. Over the last 20 years, limits on resident duty‐hours have been applied, much as has happened over the previous decade in the US.4 In 1991, the NHS and the British Medical Association negotiated a compensation package for physicians in training, termed the New Deal for Junior Doctors, which called for limitation of actual work hours for postgraduate trainee physicians to 56 hours per week. Enforcement of New Deal work guidelines was implemented over the next 12 years; with the introduction of the European Working Time Directive in 2000, work hours were further limited to 48 hours a week by 2009 for consultant and trainee physicians alike. Many UK consultants had already been devoting a higher percentage of time to subspecialty‐based hospital work and, with the reduced availability of the postgraduate trainee physician resource, the quality of generalist‐focused care (for conditions out with a consultant's given specialty) became more disparate between medical specialties, with some specialties providing little generalist input during the admission process. Simultaneously, and in the context of evolving demographic and regulatory pressures (Table 2), the admission procedure required an increasingly specific set of competencies. A subset of consultants from many different specialties began to focus specifically on management of the admission process and to informally self‐identify as specialists in acute medicine.
|
| Advances in medical care leading to increased specialization |
| Increasing numbers of elderly patients with complex medical needs |
| UK‐wide targets to limit emergency department patient stays to <4 hours |
| New limits to postgraduate trainee physician work hours |
| Increased standards of supervision of trainee physicians by consultants |
| Deficiencies in availability of outpatient out‐of‐hours care |
| Locally led reconfigurations of health care resources to favor community‐based care over inpatient‐based care |
Concerns were published about the quality of initial care for the acutely unwell patient in the UK.5 The UK Royal Colleges were concerned that patients with acute medical illnesses should receive high‐quality clinical care and commissioned a number of working groups to determine how acute medicine should best be delivered. Although initial reports suggested that acute care should be delivered by physicians who maintained an organ‐specific specialty focus, subsequent reports suggested that acute medicine should be delivered by specifically trained individuals capable of managing both the acutely ill medical patient and the administration of an acute medical unit (AMU).6, 7 In response to these trends, in 2003 the Royal Colleges of Physicians Joint Committee for Higher Medical Training, now known as the Joint Royal College of Physicians Training Board (JRCPTB), introduced a training curriculum for acute medicine as a subspecialty of general medicine.8 In 2007, the Royal College of Physicians convened an Acute Medicine Task Force that published further recommendations on the purpose and design of acute medicine services.9 Application by the JRCPTB to the regulatory bodies for medical education and training in the UK led to recognition in 2009 of acute internal medicine as a separate and distinct specialty from all other specialties, including general medicine.10
Acute Medicine in Practice: the Admission Process and Prevention of Prolonged Hospital Admission
The defining characteristic of an acute medical service in the UK is the sole dedication of a team of physician, nursing, and allied health care support staff (such as therapists, pharmacists, and social workers) to the task of admission and initial care of medical inpatients during their work shifts. Admission activity usually takes place in a dedicated physical area: the AMU. The AMU is commonly located near an accident and emergency (AE) department and is often colocated with radiology services, an intensive care unit, and/or a high‐dependency unit. Patients may be admitted to the AMU from the AE department, or directly from GP clinics. Generally, an AMU is responsible for a spectrum of medical conditions identical to the conditions potentially managed by a US‐based hospitalist. Unlike general and subspecialty medical wards, where consultant bedside input may be available as infrequently as 2 to 3 times per week, twice‐daily consultant bedside input into AMU patient care is the recommended standard. AMUs provide consultant bedside input via multiple rounds during the day, or alternatively in a continuous, per‐admission rolling pattern. Existing data suggest that AMUs with daily consultant input shorten hospital length of stay and increase same‐day discharges without affecting readmissions or mortality.11 Outside the US, observational studies associate AMUs with improved hospital mortality, shortened length of stay, decreased emergency department waiting times, and improved patient satisfaction.12
Three major models of acute medicine practice have evolved in the UK, as outlined in Table 3. The model adopted by each AMU varies depending on availability of staff, AMU bed capacity, the number and variability of patients requiring admission, and even hospital philosophy regarding division of responsibility between acute medicine physicians and those of other specialties. AMUs also vary in critical care capability, with many providing noninvasive ventilation or invasive hemodynamic monitoring. Admitted medical inpatients may bypass an AMU altogether if the AMU staff are unable to provide a procedure (eg, hemodialysis), if a patient requires no further diagnostic clarification or stabilization (eg, routine chemotherapy), or if an AMU admission would delay provision of time‐sensitive care (eg, percutaneous coronary intervention for ST‐elevation myocardial infarction). In all AMUs, patients requiring inpatient care outside of the AMU will be admitted to a medical specialty ward (cardiology, general internal medicine, neurology, etc). Generalist‐focused care is then provided by postgraduate trainee physicians on the medical specialty ward, based on guidance generated by AMU physicians, per guidance form their supervising specialty consultant physician (if possible), or through the advice given by other specialty services. Whether AMU physicians continue to be responsible for the care of AMU patients transferred to a general internal medicine ward depends on arrangements based on the particular AMU model and hospital staffing factors.
| Acute Medicine Models | Acute Medicine Team Focus |
|---|---|
| |
| Triage | Inpatient care rapidly transitioned to specialty medical ward with minimal stay in AMU |
| Short stay | Short‐term inpatient care (<72 hours) provided in AMU, including extensive assessment (eg, physical therapy, sequential radiologic imaging), multispecialty bedside input, medical therapy, and either coordination of postdischarge follow‐up or transition of care to specialty medical ward |
| Hybrid | Subset of patients rapidly transitioned to specialty medical ward, while others receive care in AMU for up to 72 hours; mix dependent on patient needs and available hospital/AMU resources |
Weaknesses and Strengths of Acute Medicine Model Applicable to US‐Based Hospital Medicine
The acute medicine model of care does instantiate potential risks. Utilization of an acute medicine team hardwires fragmentation of care, necessitating handovers. In the context of US hospital medicine practice, this fragmentation may compromise safety or throughput; however, no such deficit has been detected to date in the context of acute medicine practice in the UK.13 Mismatch between AMU bed or staff capacity and the number or rate of hospital admissions can generate safety risks or give away efficiency gains. Further inefficiencies can develop if hospital‐wide processes of handover, medical decision making, patient transport, and discharge are not synchronized with AMU outflow and intake. Evidence of AMU throughput failure is most often manifest by the premature transfer of patients from AMU to the main hospital ward areas, or by delay of admissions from the emergency department into the AMU (UK standards until recently mandated that 98% of AE patients complete their AE stay in 4 hours). Although some successful UK AMUs have minimized these failures, such problems are still experienced by many acute medicine services throughout the UK. Ongoing debate, both local and national, persists within the acute medicine community about how best to address these challenges.
The strengths of the acute medicine model appear to be clinically meaningful, however. The admission process is complex and occurs at a time when patients are sickest and potentially the most vulnerable. Effective management of this period offers significant opportunity to improve value for patients, hospitals, and health systems. When applied in the context of US hospitalist programs, instances of successful short stay units and active bed management do exist.1417 These documented successes represent partial application of UK‐style acute medicine activity in a US hospital setting. A multidisciplinary health care team dedicated to streamlining admissions, short stays, and follow‐up care offers many potential benefits. Standardization and accountability of admission process, especially important for quality improvement and research activity applicable to the initial portion of a hospital stay, may be more readily realized if embedded into the practice of a discrete cohort of hospital staff. In the UK, several hospital processes fall within the exclusive remit of an acute medicine service (Table 4). Optimization of several of these processes of care can reduce hospital morbidity, mortality, and length of stay.1821 As health care financing reform arrives in the US, the ability of American hospitals to manage admission‐specific processes of care with reliability will become more vital.3 In the US, programs that force hospitalists to make ad hoc, moment‐to‐moment prioritizations about when and where to perform admissions, discharges, and daily ward care may do so at the expense of system predictability, standardization, and patient‐centeredness. Where hospitalists are forced to juggle these geographically and substantively disparate care duties, data suggest significant opportunities to reduce variability and improve efficiency.22, 23
| Initiation of time‐sensitive acute care bundles (eg, stroke, sepsis, myocardial infarction) |
| Initiation of disease‐specific protocols (eg, venous thromboembolism prophylaxis, glycemic control) |
| Outpatient‐inpatient information reconciliation (medicines, code status, etc) |
| Outpatient‐to‐inpatient consultation (general practitioner phone consultation, telemedicine) |
| Stewardship of empiric antimicrobial therapy |
| Early involvement of discharge planning apparatus |
| Provision of follow‐up ambulatory care (medical assessment unit discharge with next‐day hospital follow‐up) |
| Outpatient intravenous antibiotic services |
| Frequent patient admission policies |
Integrated into US hospital medicine practices, the UK acute medicine model might capture otherwise elusive quality and efficiency gains.14 By the same token, integrating portions of the US hospital medicine model into a UK acute medicine model could be beneficial as well. For instance, when compared with the interservice handover common in UK AMUs, intraservice handover (acute care hospitalist‐to‐ward hospitalist) may promote standardization of the handover process and potentially fewer instances of failed communication. What seems certain is that greater attention should be focused on an exchange of ideas between acute medicine and hospital medicine.
Acknowledgements
The authors thank Valery Akopov for review of the manuscript.
Conflicts of Interest: Drs. Smith and Jones are employed as acute medicine physicians by NHS Lothian, and both have received reimbursement for public speaking related to acute medicine. Dr. Jones has received reimbursement for curriculum design activity for the acute medicine specialty in the UK.
Hospital medicine has emerged in the United States (US) to address the complexity of hospital care and over the last 15 years has become the fastest growing specialty in US history.1 The field has been shaped by societal, financial, and clinical factors within American health care, several of which also exist elsewhere in the world.2, 3 Outside the US, analogs of hospital medicine have evolved; in the United Kingdom (UK), where the term and concept of a hospitalist is widely unknown, the specialty of acute medicine has evolved to meet the complex needs of the acutely unwell medical patient in the modern health care environment. The similarities are notable, as are the differences. Our objective in this brief communication is to introduce the UK model of acute medicine to counterparts in the US. We trace the development of acute medicine in the UK, describe current practice, and note features of the model potentially applicable to hospital medicine in the US. We use UK terminology but provide equivalent terms from the US, as shown in Table 1.
| UK Term | US Term |
|---|---|
| |
| General practitioner | Family practice physician |
| Consultant physician* | Attending physician (including all general internists) |
| Postgraduate trainee physicians | Interns, residents, or fellows |
| Respiratory service | Pulmonary service |
| Medicine of the elderly service | Geriatric service |
| Accident and emergency department | Emergency department |
| High‐dependency unit | Step‐down unit |
Background and Factors Contributing to the Rise of Acute Medicine in the UK
Patient care in the UK National Health Service (NHS) is separated into inpatient and outpatient care. Generally, outpatient care is provided by general practitioners (GPs). GP clinics are independent structures and interact with local NHS‐funded services via contract, in contrast to NHS hospitals that are directly controlled by their local NHS municipal‐based body. GPs have no independent admission rights to hospitals, and (with few exceptions) do not participate in direct inpatient care. Consequently, patients in GP clinics requiring hospital admission have been referred to hospital‐based providers who assume all responsibility for inpatient care. The inpatient medical physician body in the UK is comprised of consultants, each usually trained in both general internal medicine and a medical specialty very similar to US internal medicinebased subspecialists, such as endocrinology or infectious disease. Prior to the advent of acute medicine, each consultant shared responsibility for admission of medical patients with consultants from other specialties, according to a call schedule. Generalist‐focused care would be initiated by postgraduate trainee physicians at the time of admission, and continued by the accepting consultant who often conducted subspecialty inpatient and outpatient work simultaneously. Due to advances in medical care at the turn of the century, inpatient care became more specialized; as a result, a general trend developed where the contribution of some specialties to generalist‐focused care grew (respiratory and medicine of the elderly), while other specialties began to focus on specialty‐specific interventions at the expense of practice and training in the generalist approach to care (cardiology, nephrology). Consequently, interservice disparity in provision of generalist‐focused care grew, especially in larger UK teaching hospitals. These trends have manifested as recent changes in UK medical training; presently, all UK medical specialty training programs require concomitant training in general internal medicine competencies, but for some specialties, general internal medicine training is truncated (either by the training program or by allowed choice of trainees) to provide less training than what is required for recognition as a specialist in general internal medicine.
In the UK, the majority of direct clinical care is provided via supervision of postgraduate trainee physicians. Over the last 20 years, limits on resident duty‐hours have been applied, much as has happened over the previous decade in the US.4 In 1991, the NHS and the British Medical Association negotiated a compensation package for physicians in training, termed the New Deal for Junior Doctors, which called for limitation of actual work hours for postgraduate trainee physicians to 56 hours per week. Enforcement of New Deal work guidelines was implemented over the next 12 years; with the introduction of the European Working Time Directive in 2000, work hours were further limited to 48 hours a week by 2009 for consultant and trainee physicians alike. Many UK consultants had already been devoting a higher percentage of time to subspecialty‐based hospital work and, with the reduced availability of the postgraduate trainee physician resource, the quality of generalist‐focused care (for conditions out with a consultant's given specialty) became more disparate between medical specialties, with some specialties providing little generalist input during the admission process. Simultaneously, and in the context of evolving demographic and regulatory pressures (Table 2), the admission procedure required an increasingly specific set of competencies. A subset of consultants from many different specialties began to focus specifically on management of the admission process and to informally self‐identify as specialists in acute medicine.
|
| Advances in medical care leading to increased specialization |
| Increasing numbers of elderly patients with complex medical needs |
| UK‐wide targets to limit emergency department patient stays to <4 hours |
| New limits to postgraduate trainee physician work hours |
| Increased standards of supervision of trainee physicians by consultants |
| Deficiencies in availability of outpatient out‐of‐hours care |
| Locally led reconfigurations of health care resources to favor community‐based care over inpatient‐based care |
Concerns were published about the quality of initial care for the acutely unwell patient in the UK.5 The UK Royal Colleges were concerned that patients with acute medical illnesses should receive high‐quality clinical care and commissioned a number of working groups to determine how acute medicine should best be delivered. Although initial reports suggested that acute care should be delivered by physicians who maintained an organ‐specific specialty focus, subsequent reports suggested that acute medicine should be delivered by specifically trained individuals capable of managing both the acutely ill medical patient and the administration of an acute medical unit (AMU).6, 7 In response to these trends, in 2003 the Royal Colleges of Physicians Joint Committee for Higher Medical Training, now known as the Joint Royal College of Physicians Training Board (JRCPTB), introduced a training curriculum for acute medicine as a subspecialty of general medicine.8 In 2007, the Royal College of Physicians convened an Acute Medicine Task Force that published further recommendations on the purpose and design of acute medicine services.9 Application by the JRCPTB to the regulatory bodies for medical education and training in the UK led to recognition in 2009 of acute internal medicine as a separate and distinct specialty from all other specialties, including general medicine.10
Acute Medicine in Practice: the Admission Process and Prevention of Prolonged Hospital Admission
The defining characteristic of an acute medical service in the UK is the sole dedication of a team of physician, nursing, and allied health care support staff (such as therapists, pharmacists, and social workers) to the task of admission and initial care of medical inpatients during their work shifts. Admission activity usually takes place in a dedicated physical area: the AMU. The AMU is commonly located near an accident and emergency (AE) department and is often colocated with radiology services, an intensive care unit, and/or a high‐dependency unit. Patients may be admitted to the AMU from the AE department, or directly from GP clinics. Generally, an AMU is responsible for a spectrum of medical conditions identical to the conditions potentially managed by a US‐based hospitalist. Unlike general and subspecialty medical wards, where consultant bedside input may be available as infrequently as 2 to 3 times per week, twice‐daily consultant bedside input into AMU patient care is the recommended standard. AMUs provide consultant bedside input via multiple rounds during the day, or alternatively in a continuous, per‐admission rolling pattern. Existing data suggest that AMUs with daily consultant input shorten hospital length of stay and increase same‐day discharges without affecting readmissions or mortality.11 Outside the US, observational studies associate AMUs with improved hospital mortality, shortened length of stay, decreased emergency department waiting times, and improved patient satisfaction.12
Three major models of acute medicine practice have evolved in the UK, as outlined in Table 3. The model adopted by each AMU varies depending on availability of staff, AMU bed capacity, the number and variability of patients requiring admission, and even hospital philosophy regarding division of responsibility between acute medicine physicians and those of other specialties. AMUs also vary in critical care capability, with many providing noninvasive ventilation or invasive hemodynamic monitoring. Admitted medical inpatients may bypass an AMU altogether if the AMU staff are unable to provide a procedure (eg, hemodialysis), if a patient requires no further diagnostic clarification or stabilization (eg, routine chemotherapy), or if an AMU admission would delay provision of time‐sensitive care (eg, percutaneous coronary intervention for ST‐elevation myocardial infarction). In all AMUs, patients requiring inpatient care outside of the AMU will be admitted to a medical specialty ward (cardiology, general internal medicine, neurology, etc). Generalist‐focused care is then provided by postgraduate trainee physicians on the medical specialty ward, based on guidance generated by AMU physicians, per guidance form their supervising specialty consultant physician (if possible), or through the advice given by other specialty services. Whether AMU physicians continue to be responsible for the care of AMU patients transferred to a general internal medicine ward depends on arrangements based on the particular AMU model and hospital staffing factors.
| Acute Medicine Models | Acute Medicine Team Focus |
|---|---|
| |
| Triage | Inpatient care rapidly transitioned to specialty medical ward with minimal stay in AMU |
| Short stay | Short‐term inpatient care (<72 hours) provided in AMU, including extensive assessment (eg, physical therapy, sequential radiologic imaging), multispecialty bedside input, medical therapy, and either coordination of postdischarge follow‐up or transition of care to specialty medical ward |
| Hybrid | Subset of patients rapidly transitioned to specialty medical ward, while others receive care in AMU for up to 72 hours; mix dependent on patient needs and available hospital/AMU resources |
Weaknesses and Strengths of Acute Medicine Model Applicable to US‐Based Hospital Medicine
The acute medicine model of care does instantiate potential risks. Utilization of an acute medicine team hardwires fragmentation of care, necessitating handovers. In the context of US hospital medicine practice, this fragmentation may compromise safety or throughput; however, no such deficit has been detected to date in the context of acute medicine practice in the UK.13 Mismatch between AMU bed or staff capacity and the number or rate of hospital admissions can generate safety risks or give away efficiency gains. Further inefficiencies can develop if hospital‐wide processes of handover, medical decision making, patient transport, and discharge are not synchronized with AMU outflow and intake. Evidence of AMU throughput failure is most often manifest by the premature transfer of patients from AMU to the main hospital ward areas, or by delay of admissions from the emergency department into the AMU (UK standards until recently mandated that 98% of AE patients complete their AE stay in 4 hours). Although some successful UK AMUs have minimized these failures, such problems are still experienced by many acute medicine services throughout the UK. Ongoing debate, both local and national, persists within the acute medicine community about how best to address these challenges.
The strengths of the acute medicine model appear to be clinically meaningful, however. The admission process is complex and occurs at a time when patients are sickest and potentially the most vulnerable. Effective management of this period offers significant opportunity to improve value for patients, hospitals, and health systems. When applied in the context of US hospitalist programs, instances of successful short stay units and active bed management do exist.1417 These documented successes represent partial application of UK‐style acute medicine activity in a US hospital setting. A multidisciplinary health care team dedicated to streamlining admissions, short stays, and follow‐up care offers many potential benefits. Standardization and accountability of admission process, especially important for quality improvement and research activity applicable to the initial portion of a hospital stay, may be more readily realized if embedded into the practice of a discrete cohort of hospital staff. In the UK, several hospital processes fall within the exclusive remit of an acute medicine service (Table 4). Optimization of several of these processes of care can reduce hospital morbidity, mortality, and length of stay.1821 As health care financing reform arrives in the US, the ability of American hospitals to manage admission‐specific processes of care with reliability will become more vital.3 In the US, programs that force hospitalists to make ad hoc, moment‐to‐moment prioritizations about when and where to perform admissions, discharges, and daily ward care may do so at the expense of system predictability, standardization, and patient‐centeredness. Where hospitalists are forced to juggle these geographically and substantively disparate care duties, data suggest significant opportunities to reduce variability and improve efficiency.22, 23
| Initiation of time‐sensitive acute care bundles (eg, stroke, sepsis, myocardial infarction) |
| Initiation of disease‐specific protocols (eg, venous thromboembolism prophylaxis, glycemic control) |
| Outpatient‐inpatient information reconciliation (medicines, code status, etc) |
| Outpatient‐to‐inpatient consultation (general practitioner phone consultation, telemedicine) |
| Stewardship of empiric antimicrobial therapy |
| Early involvement of discharge planning apparatus |
| Provision of follow‐up ambulatory care (medical assessment unit discharge with next‐day hospital follow‐up) |
| Outpatient intravenous antibiotic services |
| Frequent patient admission policies |
Integrated into US hospital medicine practices, the UK acute medicine model might capture otherwise elusive quality and efficiency gains.14 By the same token, integrating portions of the US hospital medicine model into a UK acute medicine model could be beneficial as well. For instance, when compared with the interservice handover common in UK AMUs, intraservice handover (acute care hospitalist‐to‐ward hospitalist) may promote standardization of the handover process and potentially fewer instances of failed communication. What seems certain is that greater attention should be focused on an exchange of ideas between acute medicine and hospital medicine.
Acknowledgements
The authors thank Valery Akopov for review of the manuscript.
Conflicts of Interest: Drs. Smith and Jones are employed as acute medicine physicians by NHS Lothian, and both have received reimbursement for public speaking related to acute medicine. Dr. Jones has received reimbursement for curriculum design activity for the acute medicine specialty in the UK.
- Society of Hospital Medicine. Growth of Hospital Medicine Nationwide. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/Media/GrowthofHospitalMedicineNationwide/Growth_of_Hospital_M.htm. Accessed May 15, 2011.
- . The hospitalist field turns 15: new opportunities and challenges. J Hosp Med. 2011;6:E1–E4.
- . Value‐driven healthcare: implications for hospitals and hospitalists. J Hosp Med. 2009;4:507–511.
- , , . ACGME Work Group on Resident Duty Hours. Accreditation Council for Graduate Medical Education. New requirements for resident duty hours. JAMA. 2002;288:1112–1114.
- , , , et al. Confidential inquiry into quality of care before admission to intensive care. BMJ. 1998;316:1853–1858.
- Federation of Medical Royal Colleges. Acute Medicine: The Physician's Role: Proposals for the Future. A Working Party Report of the Federation of Medical Royal Colleges. London, UK: Federation of Medical Royal Colleges; 2000.
- Federation of Medical Royal Colleges. Acute Medicine: Making it Work for Patients. A Blueprint for Organization and Training. Report of a Working Party. London, UK: Federation of Medical Royal Colleges; 2004.
- Joint Royal College of Physicians Training Board. Higher medical training curriculum for subspecialty training in acute medicine for general (internal) medicine NTN holders. July 2003.
- College of Physicians, London. Acute medical care: the right person, in the right setting—first time. Report of the Acute Medicine Task Force. October 2007.
- Joint Royal College of Physicians Training Board. Specialty training curriculum for acute internal medicine. August 2009.
- , , , . What is the effect of a consultant presence in an acute medical unit? Clin Med. 2009;9:214–218.
- , , . Effectiveness of acute medical units in hospitals: a systematic review. Int J Qual Health Care. 2009;21:397–407.
- , , , , . The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5:335–338.
- , , . Implementation of a hospitalist‐run observation unit and impact on length of stay (LOS): a brief report. J Hosp Med. 2010;5:E2–E5.
- , , , , , . Active bed management by hospitalists and emergency department throughput. Ann Intern Med. 2008;149:804–811.
- , , , . Hospitalist bed management effecting throughput from the emergency department to the intensive care unit. J Crit Care. 2001;25:184–189.
- , . MSSU: a multidisciplinary approach to finding cost effective and efficient care for observation patients. Quality and Safety Fall Forum, University HealthSystem Consortium Conference; 2009.
- , , , , . Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta‐analysis of randomized controlled trials. Arch Intern Med. 2007;167:1476–1486.
- , , , et al. Results of the Medications at Transitions and Clinical Handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. J Gen Intern Med. 2010;25:441–447.
- , , , . Antiplatelet therapy for acute ischaemic stroke. Cochrane Database Syst Rev. 2008;16:CD000029.
- , , , et al. Early goal‐directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377.
- , , , et al. Where did the day go?—A time motion study of hospitalists. J Hosp Med. 2010;5:323–328.
- , , , , . Hospitalist time usage and cyclicality: opportunities to improve efficiency. J Hosp Med. 2010;5:329–334.
- Society of Hospital Medicine. Growth of Hospital Medicine Nationwide. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/Media/GrowthofHospitalMedicineNationwide/Growth_of_Hospital_M.htm. Accessed May 15, 2011.
- . The hospitalist field turns 15: new opportunities and challenges. J Hosp Med. 2011;6:E1–E4.
- . Value‐driven healthcare: implications for hospitals and hospitalists. J Hosp Med. 2009;4:507–511.
- , , . ACGME Work Group on Resident Duty Hours. Accreditation Council for Graduate Medical Education. New requirements for resident duty hours. JAMA. 2002;288:1112–1114.
- , , , et al. Confidential inquiry into quality of care before admission to intensive care. BMJ. 1998;316:1853–1858.
- Federation of Medical Royal Colleges. Acute Medicine: The Physician's Role: Proposals for the Future. A Working Party Report of the Federation of Medical Royal Colleges. London, UK: Federation of Medical Royal Colleges; 2000.
- Federation of Medical Royal Colleges. Acute Medicine: Making it Work for Patients. A Blueprint for Organization and Training. Report of a Working Party. London, UK: Federation of Medical Royal Colleges; 2004.
- Joint Royal College of Physicians Training Board. Higher medical training curriculum for subspecialty training in acute medicine for general (internal) medicine NTN holders. July 2003.
- College of Physicians, London. Acute medical care: the right person, in the right setting—first time. Report of the Acute Medicine Task Force. October 2007.
- Joint Royal College of Physicians Training Board. Specialty training curriculum for acute internal medicine. August 2009.
- , , , . What is the effect of a consultant presence in an acute medical unit? Clin Med. 2009;9:214–218.
- , , . Effectiveness of acute medical units in hospitals: a systematic review. Int J Qual Health Care. 2009;21:397–407.
- , , , , . The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5:335–338.
- , , . Implementation of a hospitalist‐run observation unit and impact on length of stay (LOS): a brief report. J Hosp Med. 2010;5:E2–E5.
- , , , , , . Active bed management by hospitalists and emergency department throughput. Ann Intern Med. 2008;149:804–811.
- , , , . Hospitalist bed management effecting throughput from the emergency department to the intensive care unit. J Crit Care. 2001;25:184–189.
- , . MSSU: a multidisciplinary approach to finding cost effective and efficient care for observation patients. Quality and Safety Fall Forum, University HealthSystem Consortium Conference; 2009.
- , , , , . Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta‐analysis of randomized controlled trials. Arch Intern Med. 2007;167:1476–1486.
- , , , et al. Results of the Medications at Transitions and Clinical Handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. J Gen Intern Med. 2010;25:441–447.
- , , , . Antiplatelet therapy for acute ischaemic stroke. Cochrane Database Syst Rev. 2008;16:CD000029.
- , , , et al. Early goal‐directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377.
- , , , et al. Where did the day go?—A time motion study of hospitalists. J Hosp Med. 2010;5:323–328.
- , , , , . Hospitalist time usage and cyclicality: opportunities to improve efficiency. J Hosp Med. 2010;5:329–334.
Why Surgeons Can Say “No”
Each day, hospitalists interact with a variety of specialists and sub‐specialists to provide consultative or procedural assistance in care of their patients. Physicians have a duty to practice beneficently and to simultaneously respect patients' autonomy.1 Whether to offer a treatment is a function of many variables, but when benefits approach zero, or when risks substantially outweigh benefits, physicians may justifiably withhold therapies without assent or consent of patients.2 The purpose of this article is to explore why it is accepted practice in the United States to permit unilateral withholding of some potentially life‐prolonging treatments (eg, surgery as the paradigm), while it is not common practice for other critical care procedures (eg, cardiopulmonary resuscitation [CPR]). We offer that these examples demonstrate the tension of 2 pillars of medical ethical conduct, namely beneficence and respect of autonomy.1
Consider 2 real cases that demonstrated a juxtaposition of diametrically opposing views of thoughtful, capable surgeons asked to provide life‐saving surgery to critically ill patients.
CASE 1
A 33‐year‐old man, with a history of obesity, presents with mild epigastric pain and hematemesis of a day's duration. Endoscopic evaluation demonstrates a deep gastric ulcer with visible vessel that is injected with epinephrine. He is transferred to the medical intensive care unit (ICU) for monitoring and has an uneventful first 24 hours. On his second hospital day, he develops severe epigastric pain of sudden onset, accompanied by light‐headedness. He is diaphoretic and dyspneic, sitting bolt upright. His body mass index (BMI) is 40 kg/m2, and his vital signs are: 130/min, 140/80 mmHg, 30/min, 99.0F. Examination is normal except for severe upper abdominal tenderness, absent bowel sounds, and voluntary guarding. Abdominal computed tomography demonstrates a perforation, free air, and a loculated collection adjacent to the original ulcer. He is treated with 4 liters of crystalloids, oxygen, and an emergent surgical consultation is performed. The examining surgeon confirms the diagnosis of acute perforation, but asserts that his operative risk of mortality, due to obesity, is excessive. He will never get off the ventilator. He advises watchful waiting. The medical intensivist believes the patient will die without surgery; he asks for a second opinion. A more senior colleague assesses the patient and reiterates the first surgeon's opinion. The intensivist begins preparations to transfer the patient to the nearest tertiary care center for a third opinion, when the surgeons reverse themselves. The patient is taken to surgery where the collection is removed, with partial gastrectomy. He is extubated in the recovery room, spends 12 hours in the ICU, and is transferred to the wards where he undergoes an uneventful recovery.
CASE 2
A 50‐year‐old man, with a history of end‐stage alcoholic cirrhosis, presents to the intensive care unit with respiratory embarrassment associated with tense ascites, complicated by pneumococcal pneumonia. He responds to antibiotics but has rapidly reaccumulating ascites, where large volume paracentesis (of 4‐5 liters of transudative fluid) is required every 3 to 4 days to promote weaning trials. On his 20th hospital day, the patient develops fulminant septic shock, and work‐up reveals free air in the abdomen. A Board‐certified critical care surgeon meets with the family and informs them that he is willing to attempt exploratory laparotomy, but that operative mortality exceeds 95%. However, he was willing to try because the alternative otherwise is >99% mortality. The family asks for surgery, which reveals a small perforation, but the patient dies shortly thereafter.
In both cases, patients were very likely, if not certain, to die without operative procedures. Equally certain, the (critical care) surgeon in the second case might find case 1s surgeons neglectful. And they might consider operating on case 2with >95% preoperative mortalitymalpractice.
WHY IS SURGERY DIFFERENT FROM CPR? BENEFICENCE VERSUS AUTONOMY MODELS
Why can surgeons withhold potentially life‐saving surgery, whereas most US physicianssurgeons or internistsdo not (routinely) unilaterally withhold CPR or mechanical ventilation?3 A variety of possible reasons may underlie this asymmetry. First, to compel a surgeon to cut another human against his/her judgment would violate the surgeon's autonomy. But why is the act of cutting viewed differently from the act of intubating and ventilating, or compressing and shocking? The bodily integrity of the patient is violated in both. Nobody would take issue with a surgeon who assesses a 2% chance of survival and so does not offer surgery. Yet physicians struggle often with patients/surrogates who demand CPR/mechanical ventilation for similar prognoses.4 In the United States, CPR has crept into general acceptance (ie, when the only other option is death) as a system‐wide default. In the case of surgery, the judgment of the physician is accepted both by patients and the medical establishment, whereas for CPRwith hypothetically identical consequencesthe patient must opt out. Neither model is right or wrong; but the focus in the balance of decision‐making (paternalism/beneficence vs autonomy) is different.
Albert Jonsen introduced the rule of rescue which suggests that we have an instinctive response to rescue the doomed.5 Surgeons can make the reasonable argument that, in some cases, surgery is only likely to hasten death, and so beneficence requires that they not provide it. The same argument cannot be made for CPR; we do not provide it until patients have already died. And some (albeit small) fraction of the sickest patients survive. For example, 6.4% of those on 2 or more vasopressors who arrest, survive hospitalization.4 Another distinction between CPR and surgery is that when a physician does not withhold CPR for a patient who he thinks is not likely to benefit, he is ordinarily not the party providing the CPR. Most hospitals have teams of individuals who may or may not know the patient and the precise pathophysiology and ethics of their case. So there is greater physical distance (than with surgery) between making the decision and performing the procedure. Moreover, the process of informed consent is temporally proximate and prior to the need for surgery, whereas informed consent is not uniformly obtained a priori, and never after cardiac arrest in a patient who has not previously opted out.
PROBLEMS INHERENT IN BOTH EXTREMES
Viewed through the prism of ethical principlism,1 the ability to withhold surgery may be viewed as beneficence‐strong/autonomy‐weak (BS/AW) whereas prohibiting physicians from withholding CPR when it is only likely to prolong death is beneficence‐weak/autonomy‐strong (BW/AS). These extremes have definable risks that can be named and minimized.
Risks of Beneficence at the Expense of Autonomy
All physicians routinely assess patients to determine whether the risk of a particular intervention (eg, surgery or CPR) outweighs potential benefits. Since unilateral withholding of CPR has not been studied, we can only examine what is known about factors that may impact decisions to withhold surgery. While an elegant study demonstrated substantial interoperator variability of surgeons' opinions for elective cases,1 no similar studies have been performed to quantify or qualify this problem for emergency cases. Nonetheless, some factors that may contribute include:
-
Knowledge and heuristicsWe only know what we know. So the surgeon's knowledge about a particular surgical problem and heuristics are sure to contribute to the result of the calculus preceding whether to offer surgery.610 Unilateral withholding of any potentially life‐saving therapy (surgery or CPR) should be predicated on near‐certitude. Unfortunately, clinicians of all specialties are not particularly good at prognosticating. All available evidence suggests that doctors are very poor at predicting which severely ill patients will live or die, and when.1113 In a study that calls into serious question the accuracy of prognostication of critically ill patients, Meadow and colleagues showed that only half of patients with a prediction of death before discharge actually died in hospital.11 So the clinical judgment upon which risk estimates are predicated, are themselves imprecise and vulnerable to a multitude of heuristics.8
-
Risk aversionRisk proclivity is inherent in all medical disciplines, and is likely impacted by a multitude of factors, including genetics,14, 15 upbringing, moral beliefs, fear of litigation (even if reduced by informed consent), and effect of bad outcomes on reputation and morale. A review demonstrates the epidemiology of risk‐taking across various disciplines, but there is very little data regarding the impact of risk and ambiguity on surgeons' practice.16 Medical culture can also impact risk aversion. Morbidity and Mortality Conference (M&M) could serve as a disincentive to undertaking risky care, but such fears can be attenuated by minimizing cultures of blame.17
-
ExperienceThere is scarce data on the effects of years of experience on surgeons' practice. It is plausible that surgeons with greater experiencewith a more extensive personal library of casesare more comfortable or certain about outcomes. There is data to support that older surgeons are more risk‐averse, but the reasons have not been deciphered.18
-
Death by omission or commissionEthicists argue that if the result is the same (ie, the patient is very likely to die irrespective), acts of commission are not morally distinguishable from those of omission. Yet, clinicians in various fields are predisposed to omission bias, that is, when faced with the choice of action or inaction, when the result is likely to be the same, we often favor inaction.1921 So it is not surprising that some surgeons, when faced with difficult, lifedeath decisions regarding surgery, favor omission, because to actto perform surgery and the patient dies nonethelessincludes the possibility that their action could have caused the death, whereas the result from the alternative (ie, no surgery) is unknown.20 The reciprocal is also true, but omission bias allows the surgeon to attribute death entirely to the disease (even if there was a small chance that surgery could have changed the course). If the chances of success of surgery are small, and the chances of death and/or prolonging suffering are substantially larger, beneficence (and non‐malfeasance) is certainly an appropriate consideration.2 But the thresholds, that is, percent likelihood of success versus percent likelihood of failure defined as death or prolonged suffering, at which surgeons withhold (ie, omit consideration; don't offer surgery) will vary based on their own views of professional and moral obligation,22 and some of the factors (ie, knowledge, heuristics, risk aversion) suggested above.
Withholding CPR does not cause the death of the patient, who has already died. We may have hard‐wired survival bias that CPR will not harm a dead personbecause success entails life. There is an intrinsic (biological or value‐laden) presumption that life is always preferable to death, so there is nothing to lose. Yet many patients don't want CPR after they've learned the risks, benefits, and alternatives.23 And beyond issues of patient autonomy, CPR by default has a number of additional negative consequences, including reinforcement of false optimism,24 prolongation of dying in many initial survivors, and distress to clinicians who administer this invasive therapy to some patients who are highly unlikely to benefit. But, as Pope articulates, there is currently a now supposed right of patients to make requests for non‐indicated CPR.24
-
OtherMedical decision‐making is an extremely complex process and is certainly impacted by a multitude of variables. Even nonmedical or logistic exigencies, not considered here, couldin theoryaffect or frame decisions. Surgery often involves hours of hard work and a large emotional investment, whereas CPR is a relatively impersonal procedure, most often performed on an individual we don't know, and seldom lasting for more than an hour. So it is possible that differences in operators' personal/emotional investment impact the apparent inconsistency (of why surgeons can say no, while it is rare to unilaterally withhold CPR).
Other psychological factors, including patients' expectations and physicians' fears may also play a role. Popular culture has (mis‐)shapen patients' understanding of CPR, grossly overestimating success of the procedure.25 Misunderstanding is coupled to creep of CPR from a procedure initially introduced for highly selected cardiac care patients, to a default/right for all Americans. Patients simply don't expect life‐saving surgery on demand; whether it's the mystery of the OR, or some other factor, they're more willing to rely on the surgeon's clinical judgment.
We offer the 4 possibilities discussed above, not as an exhaustive list, but rather to spur greater consideration and discourse on this subject. Even a survey, similar to that undertaken by Rutkow and colleagues to examine elective surgery decisions,6 would be a first step to answering this question with more precision and detail.
RECOMMENDATIONS FOR MINIMIZING ETHICAL RISKS
Life‐Saving Surgery
The inherent ethical risks of extremes (eg, BS/AW as with withholding surgery vs BW/AS as with CPR) can be attenuated. Those who are highly uncomfortable with high risk could make it known, and their exposure to covering in situations where high‐risk patients are likely to be encountered could be minimized wherever possible. In recent years, acute care surgeons have been self‐selected and trained to deal with critically ill patients.26 It stands to reason that ranges of risk aversion are likely to exist among surgeonsand that those who select acute care surgery will have greater facility and comfort with high‐risk critically ill patients. Since there are insufficient acute care surgeons in the country, even if they were preferable (which is unproven) for high‐risk critical care surgery, general surgeons would still be required to fill the manpower gap to staff acute care hospitals appropriately for these problems.26, 27 Surgery, like all of Medicine, will always remain as much art as science, and variability is sure to impact what decisions are made in the care of acutely ill patients; it is a premise of being human. Those who know that they are risk‐averse, but are in a situation of assessing a case with very high but not 100% risk, could acknowledge this in their assessments and offer opportunities for second opinions using validated prognostic tools where possible.28
As some have suggested,9 metacognition, that is, greater attention to thinking about how we think, should be included in all medical curricula. If we consider carefully is there no chance of survival or only small chance of survival, then an optimal model of shared decision‐making can result. For those where they estimate no chance: It is my best professional opinion that your loved one will certainly die if surgery is performed, so I cannot provide it in good faith. But since this decision involves such finality, I'm glad to help you obtain a second opinion if it will help your peace of mind. Or: It is my best professional opinion that your loved one will die without surgery. While there may be a very remote possibility of a miracle, surgery is only likely to prolong death and suffering; the likelihood of survival is very low and the quality of that survival is likely to be very poor what would he want? Such an approach acknowledges the imprecision of medical science, and fully respects autonomy of patients. Beneficence, non‐malfeasance, and respect of autonomy can be served simultaneously without unilateral withholding, in those cases where perioperative mortality is not believed to be 100%.
Additionally, metacognition is a deliberate method for increasing the likelihood that our conclusions are predicated on sound medical science and judgment, and not on biases (eg, heuristics), exigencies related to the healthcare system (eg, resource/personnel availability), fear of litigation, or patient traits. To the extent that socioeconomic variables impact the quality and quantity of care provided to American citizens,29 it is particularly imperative that unconscious, value‐laden effectors of behavior not impact life and death decisions.
Surgical leaders should provide psychological safety30 for surgeons who offer surgery that is not futile, but highly unlikely to succeed, if proper care is taken to quantify and share risks, benefits, and alternatives with patients/surrogates.
Finally, medical physicians who request surgical consultations should always communicate directly, whenever possible, with surgical colleagues. Not infrequently, details are clarified that permit the most accurate costbenefit ratio. If a surgeon feels that surgery will only prolong dying or cause immediate death, and the internist is not so sure (as in case 1 above), a second opinion can be requested respectfully.
Withholding Cardiopulmonary Resuscitation
Unilateral withholding of CPR is a more difficult problem. Since some (albeit a small percentage) of even the most critically ill patients survive, it would be difficult to assert that CPR would be futile in the preponderance of very ill patients.4 There is simply no tool that pre‐defines with certainty successes and failure. There are patients with end‐stage diseases (eg, widely metastatic cancer, end‐stage dementia, or heart disease) where the short‐term prognosis without cardiac arrest is abysmal, and survival after CPR is only likely to extend a patient's suffering. To date, some medical cultures, notably the United States, have not allowed physicians to act beneficently to withhold CPR in such circumstances, requiring instead consent or assent of the patient or surrogate.31, 32 For those who practice in this model, there is room for greater beneficence at the expense of autonomy, but such will come only if accepted norms of conduct change in this medical culture. Medical norms in other countries permit physicians greater latitude to withhold CPR in such situations,33 whereas it is not common in the United States. The risk, of course, is that CPR is withheld unilaterally for patients who otherwise would have wanted it and survived. Nonetheless, perhaps greater emphasis on truly informed consent for CPR increases our duty to beneficence and reduces the likelihood that a patient will insist on CPR that is contrary to their best (medical) interests. There is abundant evidence that patients do not fully understand the risks, benefits, and alternatives of CPR, but when apprised, many opt out.23 The improbable likelihood of survival and the long‐term prognosis (including quality of life) following CPR, and the resulting stay in the critical care unit, should be included in truly informed consent for this procedure. Then, beneficence can be served more fully, albeit short of unilateral withholding. Importantly, while informed consent for CPR may respect patient autonomy, it does not address the (arguably incorrect) notion that CPR is a right.24 Such a shift in views/practicesof both clinicians and laypersonsmight require substantial investment by professional societies and policy‐makers to engage citizens. It has taken 50 years for CPR to be viewed as a right in the United States, and it is likely to require considerable focus and effort to modify that expectation.
Our acutely and critically ill patients are most vulnerable and at the highest risk of adverse and irreversible consequences resulting from medical decisionswhether for surgical or nonsurgical treatments. We will never eliminate entirely interprovider variability of skills and behaviors. But to the extent possible, we might acknowledge and attenuate, where possible, human and systems features that contribute to inconsistent care. It is worth stressing here that while this discussion has been focused through the prism of surgical care, these concepts apply to all medical disciplines. A transparent, mindful approachthat applies shared, rather than unilateral decision‐making, whenever possiblemay simultaneously protect the autonomy of both physicians and patients.
Postscript
Interested readers can explore this topic in greater detail in: Lo B. Resolving Ethical Dilemmas: A Guide for Clinicians. Philadelphia, PA: Lippincott Williams & Wilkins; 2009.
- Medical professionalism in the new millennium: a physician charter.Ann Intern Med.2002;136:243–246.
- Opinion 2.035: Futile Care. AMA Code of Medical Ethics. Available at: http://www.ama‐assn.org/ama/pub/physician‐resources/medical‐ethics/code‐medical‐ethics/opinion2035.page. Accessed March 23,2011.
- .The Texas advance directives act is ethically flawed: medical futility disputes must be resolved by a fair process.Chest.2009;136:971–973.
- ,,, et al.Outcomes of critically ill patients who received cardiopulmonary resuscitation.Am J Respir Crit Care Med.2010;182:501–506.
- .Bentham in a box: technology assessment and health care allocation.Law Med Health Care.1986;14:172–174.
- ,,.Surgical decision making. The reliability of clinical judgment.Ann Surg.1979;190:409–419.
- ,,.Risk taking and tolerance of uncertainty: implications for surgeons.J Surg Res.2006;131:1–6.
- .Simple inference heuristics versus complex decision machines.Minds and Machines.1999;9:461–477.
- ,,.Surgeons and cognitive processes.Br J Surg.2003;90:1–6.
- ,,,,,.Evaluation of patients with severe symptomatic aortic stenosis who do not undergo aortic valve replacement: the potential role of subjectively overestimated operative risk.Circ Cardiovasc Qual Outcomes.2009;2:533–539.
- ,,, et al.Power and limitations of daily prognostications of death in the medical intensive care unit.Crit Care Med.2011;39:474–479.
- ,.Extent and determinants of error in doctors' prognoses in terminally ill patients: prospective cohort study.BMJ.2000;320:469–473.
- ,.Prognostic disclosure to patients with cancer near the end of life.Ann Intern Med.2001;134:1096–1105.
- ,,,.Genetic and environmental influences on disordered gambling in men and women.Arch Gen Psychiatry.2010;67:624–630.
- ,,,,.The dopamine transporter gene, a spectrum of most common risky behaviors, and the legal status of the behaviors.PLoS ONE.2010;22:e935216..
- ,,, et al.An international comparison of physicians' judgments of outcome rates of cardiac procedures and attitudes toward risk, uncertainty, justifiability and regret.Med Decis Making.1998;18:131–140.
- ,,,,.Educational quality improvement report: outcomes from a revised morbidity and mortality format that emphasized patient safety.Qual Saf Health Care.2007;16:422–427.
- ,,,.Risk attitudes of anesthesiologists and surgeons in clinical decision making with expected years of life.J Clin Anesthesia.2000;12:146–150.
- .Intention and the omission bias: omissions perceived as nondecisions.Acta Psychol.1996;93:161–172.
- ,,, et al.An objective analysis of process errors in trauma resuscitations.Acad Emerg Med.2000;1303–1310.
- ,,.Omission bias and decision making in pulmonary and critical care medicine.Chest.2005;128:1497–1505.
- .Professing ethically. On the place of ethics in defining decisions.JAMA.1983;249:1305–1310.
- ,,, et al.The influence of the probability of survival on patients' preferences regarding cardiopulmonary resuscitation.N Engl J Med.1994;330:545–549.
- .Restricting CPR to patients who provide informed consent will not permit physicians to unilaterally refuse requested CPR.Am J Bioethics.2010;10:82–83.
- ,,.Cardiopulmonary resuscitation on television. Miracles and misinformation.N Engl J Med.1996;334:1578–1582.
- ,.Acute care surgery in evolution.Crit Care Med.2010;38:S405–S410.
- ,,,.Acute care surgery survey: opinion of surgeons about a new training paradigm.Arch Surg.2011;146:101–106.
- ,,,.ASA classification and perioperative variables as predictors of postoperative outcome.Br J Anaesth.1996;77:217–222.
- ,.Multicultural Medicine and Health Disparities.New York, NY:McGraw‐Hill;2006.
- ,.Making it safe: the effects of leader inclusiveness and professional status on psychological safety and improvement efforts in health care teams.J Organiz Behav.2006;27:941–966.
- .Counterpoint: is it ethical to order “do not resuscitation” without patient consent?Chest.2007;132:751–754.
- ,.Point: the ethics of unilateral “do not resuscitate” orders: the role of “informed assent.”Chest.2007;132:748–751.
- ,,,.Reviving the conversation around CPR/DNR.Am J Bioethics.2010;10:61–67.
Each day, hospitalists interact with a variety of specialists and sub‐specialists to provide consultative or procedural assistance in care of their patients. Physicians have a duty to practice beneficently and to simultaneously respect patients' autonomy.1 Whether to offer a treatment is a function of many variables, but when benefits approach zero, or when risks substantially outweigh benefits, physicians may justifiably withhold therapies without assent or consent of patients.2 The purpose of this article is to explore why it is accepted practice in the United States to permit unilateral withholding of some potentially life‐prolonging treatments (eg, surgery as the paradigm), while it is not common practice for other critical care procedures (eg, cardiopulmonary resuscitation [CPR]). We offer that these examples demonstrate the tension of 2 pillars of medical ethical conduct, namely beneficence and respect of autonomy.1
Consider 2 real cases that demonstrated a juxtaposition of diametrically opposing views of thoughtful, capable surgeons asked to provide life‐saving surgery to critically ill patients.
CASE 1
A 33‐year‐old man, with a history of obesity, presents with mild epigastric pain and hematemesis of a day's duration. Endoscopic evaluation demonstrates a deep gastric ulcer with visible vessel that is injected with epinephrine. He is transferred to the medical intensive care unit (ICU) for monitoring and has an uneventful first 24 hours. On his second hospital day, he develops severe epigastric pain of sudden onset, accompanied by light‐headedness. He is diaphoretic and dyspneic, sitting bolt upright. His body mass index (BMI) is 40 kg/m2, and his vital signs are: 130/min, 140/80 mmHg, 30/min, 99.0F. Examination is normal except for severe upper abdominal tenderness, absent bowel sounds, and voluntary guarding. Abdominal computed tomography demonstrates a perforation, free air, and a loculated collection adjacent to the original ulcer. He is treated with 4 liters of crystalloids, oxygen, and an emergent surgical consultation is performed. The examining surgeon confirms the diagnosis of acute perforation, but asserts that his operative risk of mortality, due to obesity, is excessive. He will never get off the ventilator. He advises watchful waiting. The medical intensivist believes the patient will die without surgery; he asks for a second opinion. A more senior colleague assesses the patient and reiterates the first surgeon's opinion. The intensivist begins preparations to transfer the patient to the nearest tertiary care center for a third opinion, when the surgeons reverse themselves. The patient is taken to surgery where the collection is removed, with partial gastrectomy. He is extubated in the recovery room, spends 12 hours in the ICU, and is transferred to the wards where he undergoes an uneventful recovery.
CASE 2
A 50‐year‐old man, with a history of end‐stage alcoholic cirrhosis, presents to the intensive care unit with respiratory embarrassment associated with tense ascites, complicated by pneumococcal pneumonia. He responds to antibiotics but has rapidly reaccumulating ascites, where large volume paracentesis (of 4‐5 liters of transudative fluid) is required every 3 to 4 days to promote weaning trials. On his 20th hospital day, the patient develops fulminant septic shock, and work‐up reveals free air in the abdomen. A Board‐certified critical care surgeon meets with the family and informs them that he is willing to attempt exploratory laparotomy, but that operative mortality exceeds 95%. However, he was willing to try because the alternative otherwise is >99% mortality. The family asks for surgery, which reveals a small perforation, but the patient dies shortly thereafter.
In both cases, patients were very likely, if not certain, to die without operative procedures. Equally certain, the (critical care) surgeon in the second case might find case 1s surgeons neglectful. And they might consider operating on case 2with >95% preoperative mortalitymalpractice.
WHY IS SURGERY DIFFERENT FROM CPR? BENEFICENCE VERSUS AUTONOMY MODELS
Why can surgeons withhold potentially life‐saving surgery, whereas most US physicianssurgeons or internistsdo not (routinely) unilaterally withhold CPR or mechanical ventilation?3 A variety of possible reasons may underlie this asymmetry. First, to compel a surgeon to cut another human against his/her judgment would violate the surgeon's autonomy. But why is the act of cutting viewed differently from the act of intubating and ventilating, or compressing and shocking? The bodily integrity of the patient is violated in both. Nobody would take issue with a surgeon who assesses a 2% chance of survival and so does not offer surgery. Yet physicians struggle often with patients/surrogates who demand CPR/mechanical ventilation for similar prognoses.4 In the United States, CPR has crept into general acceptance (ie, when the only other option is death) as a system‐wide default. In the case of surgery, the judgment of the physician is accepted both by patients and the medical establishment, whereas for CPRwith hypothetically identical consequencesthe patient must opt out. Neither model is right or wrong; but the focus in the balance of decision‐making (paternalism/beneficence vs autonomy) is different.
Albert Jonsen introduced the rule of rescue which suggests that we have an instinctive response to rescue the doomed.5 Surgeons can make the reasonable argument that, in some cases, surgery is only likely to hasten death, and so beneficence requires that they not provide it. The same argument cannot be made for CPR; we do not provide it until patients have already died. And some (albeit small) fraction of the sickest patients survive. For example, 6.4% of those on 2 or more vasopressors who arrest, survive hospitalization.4 Another distinction between CPR and surgery is that when a physician does not withhold CPR for a patient who he thinks is not likely to benefit, he is ordinarily not the party providing the CPR. Most hospitals have teams of individuals who may or may not know the patient and the precise pathophysiology and ethics of their case. So there is greater physical distance (than with surgery) between making the decision and performing the procedure. Moreover, the process of informed consent is temporally proximate and prior to the need for surgery, whereas informed consent is not uniformly obtained a priori, and never after cardiac arrest in a patient who has not previously opted out.
PROBLEMS INHERENT IN BOTH EXTREMES
Viewed through the prism of ethical principlism,1 the ability to withhold surgery may be viewed as beneficence‐strong/autonomy‐weak (BS/AW) whereas prohibiting physicians from withholding CPR when it is only likely to prolong death is beneficence‐weak/autonomy‐strong (BW/AS). These extremes have definable risks that can be named and minimized.
Risks of Beneficence at the Expense of Autonomy
All physicians routinely assess patients to determine whether the risk of a particular intervention (eg, surgery or CPR) outweighs potential benefits. Since unilateral withholding of CPR has not been studied, we can only examine what is known about factors that may impact decisions to withhold surgery. While an elegant study demonstrated substantial interoperator variability of surgeons' opinions for elective cases,1 no similar studies have been performed to quantify or qualify this problem for emergency cases. Nonetheless, some factors that may contribute include:
-
Knowledge and heuristicsWe only know what we know. So the surgeon's knowledge about a particular surgical problem and heuristics are sure to contribute to the result of the calculus preceding whether to offer surgery.610 Unilateral withholding of any potentially life‐saving therapy (surgery or CPR) should be predicated on near‐certitude. Unfortunately, clinicians of all specialties are not particularly good at prognosticating. All available evidence suggests that doctors are very poor at predicting which severely ill patients will live or die, and when.1113 In a study that calls into serious question the accuracy of prognostication of critically ill patients, Meadow and colleagues showed that only half of patients with a prediction of death before discharge actually died in hospital.11 So the clinical judgment upon which risk estimates are predicated, are themselves imprecise and vulnerable to a multitude of heuristics.8
-
Risk aversionRisk proclivity is inherent in all medical disciplines, and is likely impacted by a multitude of factors, including genetics,14, 15 upbringing, moral beliefs, fear of litigation (even if reduced by informed consent), and effect of bad outcomes on reputation and morale. A review demonstrates the epidemiology of risk‐taking across various disciplines, but there is very little data regarding the impact of risk and ambiguity on surgeons' practice.16 Medical culture can also impact risk aversion. Morbidity and Mortality Conference (M&M) could serve as a disincentive to undertaking risky care, but such fears can be attenuated by minimizing cultures of blame.17
-
ExperienceThere is scarce data on the effects of years of experience on surgeons' practice. It is plausible that surgeons with greater experiencewith a more extensive personal library of casesare more comfortable or certain about outcomes. There is data to support that older surgeons are more risk‐averse, but the reasons have not been deciphered.18
-
Death by omission or commissionEthicists argue that if the result is the same (ie, the patient is very likely to die irrespective), acts of commission are not morally distinguishable from those of omission. Yet, clinicians in various fields are predisposed to omission bias, that is, when faced with the choice of action or inaction, when the result is likely to be the same, we often favor inaction.1921 So it is not surprising that some surgeons, when faced with difficult, lifedeath decisions regarding surgery, favor omission, because to actto perform surgery and the patient dies nonethelessincludes the possibility that their action could have caused the death, whereas the result from the alternative (ie, no surgery) is unknown.20 The reciprocal is also true, but omission bias allows the surgeon to attribute death entirely to the disease (even if there was a small chance that surgery could have changed the course). If the chances of success of surgery are small, and the chances of death and/or prolonging suffering are substantially larger, beneficence (and non‐malfeasance) is certainly an appropriate consideration.2 But the thresholds, that is, percent likelihood of success versus percent likelihood of failure defined as death or prolonged suffering, at which surgeons withhold (ie, omit consideration; don't offer surgery) will vary based on their own views of professional and moral obligation,22 and some of the factors (ie, knowledge, heuristics, risk aversion) suggested above.
Withholding CPR does not cause the death of the patient, who has already died. We may have hard‐wired survival bias that CPR will not harm a dead personbecause success entails life. There is an intrinsic (biological or value‐laden) presumption that life is always preferable to death, so there is nothing to lose. Yet many patients don't want CPR after they've learned the risks, benefits, and alternatives.23 And beyond issues of patient autonomy, CPR by default has a number of additional negative consequences, including reinforcement of false optimism,24 prolongation of dying in many initial survivors, and distress to clinicians who administer this invasive therapy to some patients who are highly unlikely to benefit. But, as Pope articulates, there is currently a now supposed right of patients to make requests for non‐indicated CPR.24
-
OtherMedical decision‐making is an extremely complex process and is certainly impacted by a multitude of variables. Even nonmedical or logistic exigencies, not considered here, couldin theoryaffect or frame decisions. Surgery often involves hours of hard work and a large emotional investment, whereas CPR is a relatively impersonal procedure, most often performed on an individual we don't know, and seldom lasting for more than an hour. So it is possible that differences in operators' personal/emotional investment impact the apparent inconsistency (of why surgeons can say no, while it is rare to unilaterally withhold CPR).
Other psychological factors, including patients' expectations and physicians' fears may also play a role. Popular culture has (mis‐)shapen patients' understanding of CPR, grossly overestimating success of the procedure.25 Misunderstanding is coupled to creep of CPR from a procedure initially introduced for highly selected cardiac care patients, to a default/right for all Americans. Patients simply don't expect life‐saving surgery on demand; whether it's the mystery of the OR, or some other factor, they're more willing to rely on the surgeon's clinical judgment.
We offer the 4 possibilities discussed above, not as an exhaustive list, but rather to spur greater consideration and discourse on this subject. Even a survey, similar to that undertaken by Rutkow and colleagues to examine elective surgery decisions,6 would be a first step to answering this question with more precision and detail.
RECOMMENDATIONS FOR MINIMIZING ETHICAL RISKS
Life‐Saving Surgery
The inherent ethical risks of extremes (eg, BS/AW as with withholding surgery vs BW/AS as with CPR) can be attenuated. Those who are highly uncomfortable with high risk could make it known, and their exposure to covering in situations where high‐risk patients are likely to be encountered could be minimized wherever possible. In recent years, acute care surgeons have been self‐selected and trained to deal with critically ill patients.26 It stands to reason that ranges of risk aversion are likely to exist among surgeonsand that those who select acute care surgery will have greater facility and comfort with high‐risk critically ill patients. Since there are insufficient acute care surgeons in the country, even if they were preferable (which is unproven) for high‐risk critical care surgery, general surgeons would still be required to fill the manpower gap to staff acute care hospitals appropriately for these problems.26, 27 Surgery, like all of Medicine, will always remain as much art as science, and variability is sure to impact what decisions are made in the care of acutely ill patients; it is a premise of being human. Those who know that they are risk‐averse, but are in a situation of assessing a case with very high but not 100% risk, could acknowledge this in their assessments and offer opportunities for second opinions using validated prognostic tools where possible.28
As some have suggested,9 metacognition, that is, greater attention to thinking about how we think, should be included in all medical curricula. If we consider carefully is there no chance of survival or only small chance of survival, then an optimal model of shared decision‐making can result. For those where they estimate no chance: It is my best professional opinion that your loved one will certainly die if surgery is performed, so I cannot provide it in good faith. But since this decision involves such finality, I'm glad to help you obtain a second opinion if it will help your peace of mind. Or: It is my best professional opinion that your loved one will die without surgery. While there may be a very remote possibility of a miracle, surgery is only likely to prolong death and suffering; the likelihood of survival is very low and the quality of that survival is likely to be very poor what would he want? Such an approach acknowledges the imprecision of medical science, and fully respects autonomy of patients. Beneficence, non‐malfeasance, and respect of autonomy can be served simultaneously without unilateral withholding, in those cases where perioperative mortality is not believed to be 100%.
Additionally, metacognition is a deliberate method for increasing the likelihood that our conclusions are predicated on sound medical science and judgment, and not on biases (eg, heuristics), exigencies related to the healthcare system (eg, resource/personnel availability), fear of litigation, or patient traits. To the extent that socioeconomic variables impact the quality and quantity of care provided to American citizens,29 it is particularly imperative that unconscious, value‐laden effectors of behavior not impact life and death decisions.
Surgical leaders should provide psychological safety30 for surgeons who offer surgery that is not futile, but highly unlikely to succeed, if proper care is taken to quantify and share risks, benefits, and alternatives with patients/surrogates.
Finally, medical physicians who request surgical consultations should always communicate directly, whenever possible, with surgical colleagues. Not infrequently, details are clarified that permit the most accurate costbenefit ratio. If a surgeon feels that surgery will only prolong dying or cause immediate death, and the internist is not so sure (as in case 1 above), a second opinion can be requested respectfully.
Withholding Cardiopulmonary Resuscitation
Unilateral withholding of CPR is a more difficult problem. Since some (albeit a small percentage) of even the most critically ill patients survive, it would be difficult to assert that CPR would be futile in the preponderance of very ill patients.4 There is simply no tool that pre‐defines with certainty successes and failure. There are patients with end‐stage diseases (eg, widely metastatic cancer, end‐stage dementia, or heart disease) where the short‐term prognosis without cardiac arrest is abysmal, and survival after CPR is only likely to extend a patient's suffering. To date, some medical cultures, notably the United States, have not allowed physicians to act beneficently to withhold CPR in such circumstances, requiring instead consent or assent of the patient or surrogate.31, 32 For those who practice in this model, there is room for greater beneficence at the expense of autonomy, but such will come only if accepted norms of conduct change in this medical culture. Medical norms in other countries permit physicians greater latitude to withhold CPR in such situations,33 whereas it is not common in the United States. The risk, of course, is that CPR is withheld unilaterally for patients who otherwise would have wanted it and survived. Nonetheless, perhaps greater emphasis on truly informed consent for CPR increases our duty to beneficence and reduces the likelihood that a patient will insist on CPR that is contrary to their best (medical) interests. There is abundant evidence that patients do not fully understand the risks, benefits, and alternatives of CPR, but when apprised, many opt out.23 The improbable likelihood of survival and the long‐term prognosis (including quality of life) following CPR, and the resulting stay in the critical care unit, should be included in truly informed consent for this procedure. Then, beneficence can be served more fully, albeit short of unilateral withholding. Importantly, while informed consent for CPR may respect patient autonomy, it does not address the (arguably incorrect) notion that CPR is a right.24 Such a shift in views/practicesof both clinicians and laypersonsmight require substantial investment by professional societies and policy‐makers to engage citizens. It has taken 50 years for CPR to be viewed as a right in the United States, and it is likely to require considerable focus and effort to modify that expectation.
Our acutely and critically ill patients are most vulnerable and at the highest risk of adverse and irreversible consequences resulting from medical decisionswhether for surgical or nonsurgical treatments. We will never eliminate entirely interprovider variability of skills and behaviors. But to the extent possible, we might acknowledge and attenuate, where possible, human and systems features that contribute to inconsistent care. It is worth stressing here that while this discussion has been focused through the prism of surgical care, these concepts apply to all medical disciplines. A transparent, mindful approachthat applies shared, rather than unilateral decision‐making, whenever possiblemay simultaneously protect the autonomy of both physicians and patients.
Postscript
Interested readers can explore this topic in greater detail in: Lo B. Resolving Ethical Dilemmas: A Guide for Clinicians. Philadelphia, PA: Lippincott Williams & Wilkins; 2009.
Each day, hospitalists interact with a variety of specialists and sub‐specialists to provide consultative or procedural assistance in care of their patients. Physicians have a duty to practice beneficently and to simultaneously respect patients' autonomy.1 Whether to offer a treatment is a function of many variables, but when benefits approach zero, or when risks substantially outweigh benefits, physicians may justifiably withhold therapies without assent or consent of patients.2 The purpose of this article is to explore why it is accepted practice in the United States to permit unilateral withholding of some potentially life‐prolonging treatments (eg, surgery as the paradigm), while it is not common practice for other critical care procedures (eg, cardiopulmonary resuscitation [CPR]). We offer that these examples demonstrate the tension of 2 pillars of medical ethical conduct, namely beneficence and respect of autonomy.1
Consider 2 real cases that demonstrated a juxtaposition of diametrically opposing views of thoughtful, capable surgeons asked to provide life‐saving surgery to critically ill patients.
CASE 1
A 33‐year‐old man, with a history of obesity, presents with mild epigastric pain and hematemesis of a day's duration. Endoscopic evaluation demonstrates a deep gastric ulcer with visible vessel that is injected with epinephrine. He is transferred to the medical intensive care unit (ICU) for monitoring and has an uneventful first 24 hours. On his second hospital day, he develops severe epigastric pain of sudden onset, accompanied by light‐headedness. He is diaphoretic and dyspneic, sitting bolt upright. His body mass index (BMI) is 40 kg/m2, and his vital signs are: 130/min, 140/80 mmHg, 30/min, 99.0F. Examination is normal except for severe upper abdominal tenderness, absent bowel sounds, and voluntary guarding. Abdominal computed tomography demonstrates a perforation, free air, and a loculated collection adjacent to the original ulcer. He is treated with 4 liters of crystalloids, oxygen, and an emergent surgical consultation is performed. The examining surgeon confirms the diagnosis of acute perforation, but asserts that his operative risk of mortality, due to obesity, is excessive. He will never get off the ventilator. He advises watchful waiting. The medical intensivist believes the patient will die without surgery; he asks for a second opinion. A more senior colleague assesses the patient and reiterates the first surgeon's opinion. The intensivist begins preparations to transfer the patient to the nearest tertiary care center for a third opinion, when the surgeons reverse themselves. The patient is taken to surgery where the collection is removed, with partial gastrectomy. He is extubated in the recovery room, spends 12 hours in the ICU, and is transferred to the wards where he undergoes an uneventful recovery.
CASE 2
A 50‐year‐old man, with a history of end‐stage alcoholic cirrhosis, presents to the intensive care unit with respiratory embarrassment associated with tense ascites, complicated by pneumococcal pneumonia. He responds to antibiotics but has rapidly reaccumulating ascites, where large volume paracentesis (of 4‐5 liters of transudative fluid) is required every 3 to 4 days to promote weaning trials. On his 20th hospital day, the patient develops fulminant septic shock, and work‐up reveals free air in the abdomen. A Board‐certified critical care surgeon meets with the family and informs them that he is willing to attempt exploratory laparotomy, but that operative mortality exceeds 95%. However, he was willing to try because the alternative otherwise is >99% mortality. The family asks for surgery, which reveals a small perforation, but the patient dies shortly thereafter.
In both cases, patients were very likely, if not certain, to die without operative procedures. Equally certain, the (critical care) surgeon in the second case might find case 1s surgeons neglectful. And they might consider operating on case 2with >95% preoperative mortalitymalpractice.
WHY IS SURGERY DIFFERENT FROM CPR? BENEFICENCE VERSUS AUTONOMY MODELS
Why can surgeons withhold potentially life‐saving surgery, whereas most US physicianssurgeons or internistsdo not (routinely) unilaterally withhold CPR or mechanical ventilation?3 A variety of possible reasons may underlie this asymmetry. First, to compel a surgeon to cut another human against his/her judgment would violate the surgeon's autonomy. But why is the act of cutting viewed differently from the act of intubating and ventilating, or compressing and shocking? The bodily integrity of the patient is violated in both. Nobody would take issue with a surgeon who assesses a 2% chance of survival and so does not offer surgery. Yet physicians struggle often with patients/surrogates who demand CPR/mechanical ventilation for similar prognoses.4 In the United States, CPR has crept into general acceptance (ie, when the only other option is death) as a system‐wide default. In the case of surgery, the judgment of the physician is accepted both by patients and the medical establishment, whereas for CPRwith hypothetically identical consequencesthe patient must opt out. Neither model is right or wrong; but the focus in the balance of decision‐making (paternalism/beneficence vs autonomy) is different.
Albert Jonsen introduced the rule of rescue which suggests that we have an instinctive response to rescue the doomed.5 Surgeons can make the reasonable argument that, in some cases, surgery is only likely to hasten death, and so beneficence requires that they not provide it. The same argument cannot be made for CPR; we do not provide it until patients have already died. And some (albeit small) fraction of the sickest patients survive. For example, 6.4% of those on 2 or more vasopressors who arrest, survive hospitalization.4 Another distinction between CPR and surgery is that when a physician does not withhold CPR for a patient who he thinks is not likely to benefit, he is ordinarily not the party providing the CPR. Most hospitals have teams of individuals who may or may not know the patient and the precise pathophysiology and ethics of their case. So there is greater physical distance (than with surgery) between making the decision and performing the procedure. Moreover, the process of informed consent is temporally proximate and prior to the need for surgery, whereas informed consent is not uniformly obtained a priori, and never after cardiac arrest in a patient who has not previously opted out.
PROBLEMS INHERENT IN BOTH EXTREMES
Viewed through the prism of ethical principlism,1 the ability to withhold surgery may be viewed as beneficence‐strong/autonomy‐weak (BS/AW) whereas prohibiting physicians from withholding CPR when it is only likely to prolong death is beneficence‐weak/autonomy‐strong (BW/AS). These extremes have definable risks that can be named and minimized.
Risks of Beneficence at the Expense of Autonomy
All physicians routinely assess patients to determine whether the risk of a particular intervention (eg, surgery or CPR) outweighs potential benefits. Since unilateral withholding of CPR has not been studied, we can only examine what is known about factors that may impact decisions to withhold surgery. While an elegant study demonstrated substantial interoperator variability of surgeons' opinions for elective cases,1 no similar studies have been performed to quantify or qualify this problem for emergency cases. Nonetheless, some factors that may contribute include:
-
Knowledge and heuristicsWe only know what we know. So the surgeon's knowledge about a particular surgical problem and heuristics are sure to contribute to the result of the calculus preceding whether to offer surgery.610 Unilateral withholding of any potentially life‐saving therapy (surgery or CPR) should be predicated on near‐certitude. Unfortunately, clinicians of all specialties are not particularly good at prognosticating. All available evidence suggests that doctors are very poor at predicting which severely ill patients will live or die, and when.1113 In a study that calls into serious question the accuracy of prognostication of critically ill patients, Meadow and colleagues showed that only half of patients with a prediction of death before discharge actually died in hospital.11 So the clinical judgment upon which risk estimates are predicated, are themselves imprecise and vulnerable to a multitude of heuristics.8
-
Risk aversionRisk proclivity is inherent in all medical disciplines, and is likely impacted by a multitude of factors, including genetics,14, 15 upbringing, moral beliefs, fear of litigation (even if reduced by informed consent), and effect of bad outcomes on reputation and morale. A review demonstrates the epidemiology of risk‐taking across various disciplines, but there is very little data regarding the impact of risk and ambiguity on surgeons' practice.16 Medical culture can also impact risk aversion. Morbidity and Mortality Conference (M&M) could serve as a disincentive to undertaking risky care, but such fears can be attenuated by minimizing cultures of blame.17
-
ExperienceThere is scarce data on the effects of years of experience on surgeons' practice. It is plausible that surgeons with greater experiencewith a more extensive personal library of casesare more comfortable or certain about outcomes. There is data to support that older surgeons are more risk‐averse, but the reasons have not been deciphered.18
-
Death by omission or commissionEthicists argue that if the result is the same (ie, the patient is very likely to die irrespective), acts of commission are not morally distinguishable from those of omission. Yet, clinicians in various fields are predisposed to omission bias, that is, when faced with the choice of action or inaction, when the result is likely to be the same, we often favor inaction.1921 So it is not surprising that some surgeons, when faced with difficult, lifedeath decisions regarding surgery, favor omission, because to actto perform surgery and the patient dies nonethelessincludes the possibility that their action could have caused the death, whereas the result from the alternative (ie, no surgery) is unknown.20 The reciprocal is also true, but omission bias allows the surgeon to attribute death entirely to the disease (even if there was a small chance that surgery could have changed the course). If the chances of success of surgery are small, and the chances of death and/or prolonging suffering are substantially larger, beneficence (and non‐malfeasance) is certainly an appropriate consideration.2 But the thresholds, that is, percent likelihood of success versus percent likelihood of failure defined as death or prolonged suffering, at which surgeons withhold (ie, omit consideration; don't offer surgery) will vary based on their own views of professional and moral obligation,22 and some of the factors (ie, knowledge, heuristics, risk aversion) suggested above.
Withholding CPR does not cause the death of the patient, who has already died. We may have hard‐wired survival bias that CPR will not harm a dead personbecause success entails life. There is an intrinsic (biological or value‐laden) presumption that life is always preferable to death, so there is nothing to lose. Yet many patients don't want CPR after they've learned the risks, benefits, and alternatives.23 And beyond issues of patient autonomy, CPR by default has a number of additional negative consequences, including reinforcement of false optimism,24 prolongation of dying in many initial survivors, and distress to clinicians who administer this invasive therapy to some patients who are highly unlikely to benefit. But, as Pope articulates, there is currently a now supposed right of patients to make requests for non‐indicated CPR.24
-
OtherMedical decision‐making is an extremely complex process and is certainly impacted by a multitude of variables. Even nonmedical or logistic exigencies, not considered here, couldin theoryaffect or frame decisions. Surgery often involves hours of hard work and a large emotional investment, whereas CPR is a relatively impersonal procedure, most often performed on an individual we don't know, and seldom lasting for more than an hour. So it is possible that differences in operators' personal/emotional investment impact the apparent inconsistency (of why surgeons can say no, while it is rare to unilaterally withhold CPR).
Other psychological factors, including patients' expectations and physicians' fears may also play a role. Popular culture has (mis‐)shapen patients' understanding of CPR, grossly overestimating success of the procedure.25 Misunderstanding is coupled to creep of CPR from a procedure initially introduced for highly selected cardiac care patients, to a default/right for all Americans. Patients simply don't expect life‐saving surgery on demand; whether it's the mystery of the OR, or some other factor, they're more willing to rely on the surgeon's clinical judgment.
We offer the 4 possibilities discussed above, not as an exhaustive list, but rather to spur greater consideration and discourse on this subject. Even a survey, similar to that undertaken by Rutkow and colleagues to examine elective surgery decisions,6 would be a first step to answering this question with more precision and detail.
RECOMMENDATIONS FOR MINIMIZING ETHICAL RISKS
Life‐Saving Surgery
The inherent ethical risks of extremes (eg, BS/AW as with withholding surgery vs BW/AS as with CPR) can be attenuated. Those who are highly uncomfortable with high risk could make it known, and their exposure to covering in situations where high‐risk patients are likely to be encountered could be minimized wherever possible. In recent years, acute care surgeons have been self‐selected and trained to deal with critically ill patients.26 It stands to reason that ranges of risk aversion are likely to exist among surgeonsand that those who select acute care surgery will have greater facility and comfort with high‐risk critically ill patients. Since there are insufficient acute care surgeons in the country, even if they were preferable (which is unproven) for high‐risk critical care surgery, general surgeons would still be required to fill the manpower gap to staff acute care hospitals appropriately for these problems.26, 27 Surgery, like all of Medicine, will always remain as much art as science, and variability is sure to impact what decisions are made in the care of acutely ill patients; it is a premise of being human. Those who know that they are risk‐averse, but are in a situation of assessing a case with very high but not 100% risk, could acknowledge this in their assessments and offer opportunities for second opinions using validated prognostic tools where possible.28
As some have suggested,9 metacognition, that is, greater attention to thinking about how we think, should be included in all medical curricula. If we consider carefully is there no chance of survival or only small chance of survival, then an optimal model of shared decision‐making can result. For those where they estimate no chance: It is my best professional opinion that your loved one will certainly die if surgery is performed, so I cannot provide it in good faith. But since this decision involves such finality, I'm glad to help you obtain a second opinion if it will help your peace of mind. Or: It is my best professional opinion that your loved one will die without surgery. While there may be a very remote possibility of a miracle, surgery is only likely to prolong death and suffering; the likelihood of survival is very low and the quality of that survival is likely to be very poor what would he want? Such an approach acknowledges the imprecision of medical science, and fully respects autonomy of patients. Beneficence, non‐malfeasance, and respect of autonomy can be served simultaneously without unilateral withholding, in those cases where perioperative mortality is not believed to be 100%.
Additionally, metacognition is a deliberate method for increasing the likelihood that our conclusions are predicated on sound medical science and judgment, and not on biases (eg, heuristics), exigencies related to the healthcare system (eg, resource/personnel availability), fear of litigation, or patient traits. To the extent that socioeconomic variables impact the quality and quantity of care provided to American citizens,29 it is particularly imperative that unconscious, value‐laden effectors of behavior not impact life and death decisions.
Surgical leaders should provide psychological safety30 for surgeons who offer surgery that is not futile, but highly unlikely to succeed, if proper care is taken to quantify and share risks, benefits, and alternatives with patients/surrogates.
Finally, medical physicians who request surgical consultations should always communicate directly, whenever possible, with surgical colleagues. Not infrequently, details are clarified that permit the most accurate costbenefit ratio. If a surgeon feels that surgery will only prolong dying or cause immediate death, and the internist is not so sure (as in case 1 above), a second opinion can be requested respectfully.
Withholding Cardiopulmonary Resuscitation
Unilateral withholding of CPR is a more difficult problem. Since some (albeit a small percentage) of even the most critically ill patients survive, it would be difficult to assert that CPR would be futile in the preponderance of very ill patients.4 There is simply no tool that pre‐defines with certainty successes and failure. There are patients with end‐stage diseases (eg, widely metastatic cancer, end‐stage dementia, or heart disease) where the short‐term prognosis without cardiac arrest is abysmal, and survival after CPR is only likely to extend a patient's suffering. To date, some medical cultures, notably the United States, have not allowed physicians to act beneficently to withhold CPR in such circumstances, requiring instead consent or assent of the patient or surrogate.31, 32 For those who practice in this model, there is room for greater beneficence at the expense of autonomy, but such will come only if accepted norms of conduct change in this medical culture. Medical norms in other countries permit physicians greater latitude to withhold CPR in such situations,33 whereas it is not common in the United States. The risk, of course, is that CPR is withheld unilaterally for patients who otherwise would have wanted it and survived. Nonetheless, perhaps greater emphasis on truly informed consent for CPR increases our duty to beneficence and reduces the likelihood that a patient will insist on CPR that is contrary to their best (medical) interests. There is abundant evidence that patients do not fully understand the risks, benefits, and alternatives of CPR, but when apprised, many opt out.23 The improbable likelihood of survival and the long‐term prognosis (including quality of life) following CPR, and the resulting stay in the critical care unit, should be included in truly informed consent for this procedure. Then, beneficence can be served more fully, albeit short of unilateral withholding. Importantly, while informed consent for CPR may respect patient autonomy, it does not address the (arguably incorrect) notion that CPR is a right.24 Such a shift in views/practicesof both clinicians and laypersonsmight require substantial investment by professional societies and policy‐makers to engage citizens. It has taken 50 years for CPR to be viewed as a right in the United States, and it is likely to require considerable focus and effort to modify that expectation.
Our acutely and critically ill patients are most vulnerable and at the highest risk of adverse and irreversible consequences resulting from medical decisionswhether for surgical or nonsurgical treatments. We will never eliminate entirely interprovider variability of skills and behaviors. But to the extent possible, we might acknowledge and attenuate, where possible, human and systems features that contribute to inconsistent care. It is worth stressing here that while this discussion has been focused through the prism of surgical care, these concepts apply to all medical disciplines. A transparent, mindful approachthat applies shared, rather than unilateral decision‐making, whenever possiblemay simultaneously protect the autonomy of both physicians and patients.
Postscript
Interested readers can explore this topic in greater detail in: Lo B. Resolving Ethical Dilemmas: A Guide for Clinicians. Philadelphia, PA: Lippincott Williams & Wilkins; 2009.
- Medical professionalism in the new millennium: a physician charter.Ann Intern Med.2002;136:243–246.
- Opinion 2.035: Futile Care. AMA Code of Medical Ethics. Available at: http://www.ama‐assn.org/ama/pub/physician‐resources/medical‐ethics/code‐medical‐ethics/opinion2035.page. Accessed March 23,2011.
- .The Texas advance directives act is ethically flawed: medical futility disputes must be resolved by a fair process.Chest.2009;136:971–973.
- ,,, et al.Outcomes of critically ill patients who received cardiopulmonary resuscitation.Am J Respir Crit Care Med.2010;182:501–506.
- .Bentham in a box: technology assessment and health care allocation.Law Med Health Care.1986;14:172–174.
- ,,.Surgical decision making. The reliability of clinical judgment.Ann Surg.1979;190:409–419.
- ,,.Risk taking and tolerance of uncertainty: implications for surgeons.J Surg Res.2006;131:1–6.
- .Simple inference heuristics versus complex decision machines.Minds and Machines.1999;9:461–477.
- ,,.Surgeons and cognitive processes.Br J Surg.2003;90:1–6.
- ,,,,,.Evaluation of patients with severe symptomatic aortic stenosis who do not undergo aortic valve replacement: the potential role of subjectively overestimated operative risk.Circ Cardiovasc Qual Outcomes.2009;2:533–539.
- ,,, et al.Power and limitations of daily prognostications of death in the medical intensive care unit.Crit Care Med.2011;39:474–479.
- ,.Extent and determinants of error in doctors' prognoses in terminally ill patients: prospective cohort study.BMJ.2000;320:469–473.
- ,.Prognostic disclosure to patients with cancer near the end of life.Ann Intern Med.2001;134:1096–1105.
- ,,,.Genetic and environmental influences on disordered gambling in men and women.Arch Gen Psychiatry.2010;67:624–630.
- ,,,,.The dopamine transporter gene, a spectrum of most common risky behaviors, and the legal status of the behaviors.PLoS ONE.2010;22:e935216..
- ,,, et al.An international comparison of physicians' judgments of outcome rates of cardiac procedures and attitudes toward risk, uncertainty, justifiability and regret.Med Decis Making.1998;18:131–140.
- ,,,,.Educational quality improvement report: outcomes from a revised morbidity and mortality format that emphasized patient safety.Qual Saf Health Care.2007;16:422–427.
- ,,,.Risk attitudes of anesthesiologists and surgeons in clinical decision making with expected years of life.J Clin Anesthesia.2000;12:146–150.
- .Intention and the omission bias: omissions perceived as nondecisions.Acta Psychol.1996;93:161–172.
- ,,, et al.An objective analysis of process errors in trauma resuscitations.Acad Emerg Med.2000;1303–1310.
- ,,.Omission bias and decision making in pulmonary and critical care medicine.Chest.2005;128:1497–1505.
- .Professing ethically. On the place of ethics in defining decisions.JAMA.1983;249:1305–1310.
- ,,, et al.The influence of the probability of survival on patients' preferences regarding cardiopulmonary resuscitation.N Engl J Med.1994;330:545–549.
- .Restricting CPR to patients who provide informed consent will not permit physicians to unilaterally refuse requested CPR.Am J Bioethics.2010;10:82–83.
- ,,.Cardiopulmonary resuscitation on television. Miracles and misinformation.N Engl J Med.1996;334:1578–1582.
- ,.Acute care surgery in evolution.Crit Care Med.2010;38:S405–S410.
- ,,,.Acute care surgery survey: opinion of surgeons about a new training paradigm.Arch Surg.2011;146:101–106.
- ,,,.ASA classification and perioperative variables as predictors of postoperative outcome.Br J Anaesth.1996;77:217–222.
- ,.Multicultural Medicine and Health Disparities.New York, NY:McGraw‐Hill;2006.
- ,.Making it safe: the effects of leader inclusiveness and professional status on psychological safety and improvement efforts in health care teams.J Organiz Behav.2006;27:941–966.
- .Counterpoint: is it ethical to order “do not resuscitation” without patient consent?Chest.2007;132:751–754.
- ,.Point: the ethics of unilateral “do not resuscitate” orders: the role of “informed assent.”Chest.2007;132:748–751.
- ,,,.Reviving the conversation around CPR/DNR.Am J Bioethics.2010;10:61–67.
- Medical professionalism in the new millennium: a physician charter.Ann Intern Med.2002;136:243–246.
- Opinion 2.035: Futile Care. AMA Code of Medical Ethics. Available at: http://www.ama‐assn.org/ama/pub/physician‐resources/medical‐ethics/code‐medical‐ethics/opinion2035.page. Accessed March 23,2011.
- .The Texas advance directives act is ethically flawed: medical futility disputes must be resolved by a fair process.Chest.2009;136:971–973.
- ,,, et al.Outcomes of critically ill patients who received cardiopulmonary resuscitation.Am J Respir Crit Care Med.2010;182:501–506.
- .Bentham in a box: technology assessment and health care allocation.Law Med Health Care.1986;14:172–174.
- ,,.Surgical decision making. The reliability of clinical judgment.Ann Surg.1979;190:409–419.
- ,,.Risk taking and tolerance of uncertainty: implications for surgeons.J Surg Res.2006;131:1–6.
- .Simple inference heuristics versus complex decision machines.Minds and Machines.1999;9:461–477.
- ,,.Surgeons and cognitive processes.Br J Surg.2003;90:1–6.
- ,,,,,.Evaluation of patients with severe symptomatic aortic stenosis who do not undergo aortic valve replacement: the potential role of subjectively overestimated operative risk.Circ Cardiovasc Qual Outcomes.2009;2:533–539.
- ,,, et al.Power and limitations of daily prognostications of death in the medical intensive care unit.Crit Care Med.2011;39:474–479.
- ,.Extent and determinants of error in doctors' prognoses in terminally ill patients: prospective cohort study.BMJ.2000;320:469–473.
- ,.Prognostic disclosure to patients with cancer near the end of life.Ann Intern Med.2001;134:1096–1105.
- ,,,.Genetic and environmental influences on disordered gambling in men and women.Arch Gen Psychiatry.2010;67:624–630.
- ,,,,.The dopamine transporter gene, a spectrum of most common risky behaviors, and the legal status of the behaviors.PLoS ONE.2010;22:e935216..
- ,,, et al.An international comparison of physicians' judgments of outcome rates of cardiac procedures and attitudes toward risk, uncertainty, justifiability and regret.Med Decis Making.1998;18:131–140.
- ,,,,.Educational quality improvement report: outcomes from a revised morbidity and mortality format that emphasized patient safety.Qual Saf Health Care.2007;16:422–427.
- ,,,.Risk attitudes of anesthesiologists and surgeons in clinical decision making with expected years of life.J Clin Anesthesia.2000;12:146–150.
- .Intention and the omission bias: omissions perceived as nondecisions.Acta Psychol.1996;93:161–172.
- ,,, et al.An objective analysis of process errors in trauma resuscitations.Acad Emerg Med.2000;1303–1310.
- ,,.Omission bias and decision making in pulmonary and critical care medicine.Chest.2005;128:1497–1505.
- .Professing ethically. On the place of ethics in defining decisions.JAMA.1983;249:1305–1310.
- ,,, et al.The influence of the probability of survival on patients' preferences regarding cardiopulmonary resuscitation.N Engl J Med.1994;330:545–549.
- .Restricting CPR to patients who provide informed consent will not permit physicians to unilaterally refuse requested CPR.Am J Bioethics.2010;10:82–83.
- ,,.Cardiopulmonary resuscitation on television. Miracles and misinformation.N Engl J Med.1996;334:1578–1582.
- ,.Acute care surgery in evolution.Crit Care Med.2010;38:S405–S410.
- ,,,.Acute care surgery survey: opinion of surgeons about a new training paradigm.Arch Surg.2011;146:101–106.
- ,,,.ASA classification and perioperative variables as predictors of postoperative outcome.Br J Anaesth.1996;77:217–222.
- ,.Multicultural Medicine and Health Disparities.New York, NY:McGraw‐Hill;2006.
- ,.Making it safe: the effects of leader inclusiveness and professional status on psychological safety and improvement efforts in health care teams.J Organiz Behav.2006;27:941–966.
- .Counterpoint: is it ethical to order “do not resuscitation” without patient consent?Chest.2007;132:751–754.
- ,.Point: the ethics of unilateral “do not resuscitate” orders: the role of “informed assent.”Chest.2007;132:748–751.
- ,,,.Reviving the conversation around CPR/DNR.Am J Bioethics.2010;10:61–67.