User login
Microneedling Therapy With and Without Platelet-Rich Plasma
Microneedling therapy, also known as collagen induction therapy or percutaneous collagen induction, is an increasingly popular treatment modality for skin rejuvenation. The approach employs small needles to puncture the skin and stimulate local collagen production in a minimally invasive manner. Recently, clinicians have incorporated the use of platelet-rich plasma (PRP) with the aim of augmenting cosmetic outcomes. In this article, we examine the utility of this approach by reviewing comparison studies of microneedling therapy with and without the application of PRP.
Dr. Gary Goldenberg demonstrates microneedling with platelet-rich plasma in a procedural video available here.
Microneedling Therapy
The use of microneedling first gained attention in the 1990s. Initially, Camirand and Doucet1 described tattooing without pigment for the treatment of achromatic and hypertrophic scars. Fernandes2 evolved this concept and developed a drum-shaped device with fine protruding needles to puncture the skin. Microneedling devices have expanded in recent years and now include both cord- and battery-powered pens and rollers, with needles ranging in length from 0.25 to 3.0 mm.
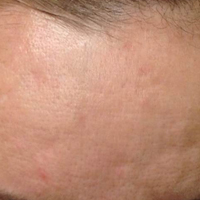
Treatment with microneedling promotes skin rejuvenation by creating small puncture wounds in the epidermis and dermis. This injury triggers the wound healing cascade and alters the modulation of growth factors to promote regenerative effects.3,4 Following microneedling therapy, increases occur in elastic fiber formation, collagen deposition, and dermal thickness (Figure).5 Of interesting histologic note, collagen is deposited in the normal lattice pattern following this treatment rather than in the parallel bundles typical of scars.6 Microneedling preserves the overall integrity of the epidermal layers and basement membrane, allowing the epidermis to heal without abnormality, verified on histology by a normal stratum corneum, enhanced stratum granulosum, and normal rete ridges.7
Microneedling has demonstrated several uses beyond general skin rejuvenation. In patients with atrophic acne scars, therapy can lead to improved scar appearance, skin texture, and patient satisfaction.8,9 Hypertrophic and dyspigmented burn scars on the body, face, arms, and legs have shown to be receptive to repeated treatments.10 Microneedling also has shown promise in treating androgenic alopecia, increasing hair regrowth in patients who previously showed poor response to conventional therapy with minoxidil and finasteride.11,12
Platelet-Rich Plasma
Platelet-rich plasma is developed by enriching blood with an autologous concentration of platelets. The preparation of PRP begins with whole blood, commonly obtained peripherally by venipuncture. Samples undergo centrifugation to allow separation of the blood into 3 layers: platelet-poor plasma, PRP, and erythrocytes.13 The typical platelet count of whole blood is approximately 200,000/µL; PRP aims to prepare a platelet count of at least 1,000,000/µL in a 5-mL volume.14
An attractive component of PRP is its high concentration of growth factors, including platelet-derived growth factor, transforming growth factor, vascular endothelial growth factor, and epithelial growth factor.15 Because of the regenerative effects of these proteins, PRP has been investigated as a modality to augment wound healing in a variety of clinical areas, such as maxillofacial surgery, orthopedics, cardiovascular surgery, and treatment of soft tissue ulcers.16
Combination Use of Microneedling and PRP
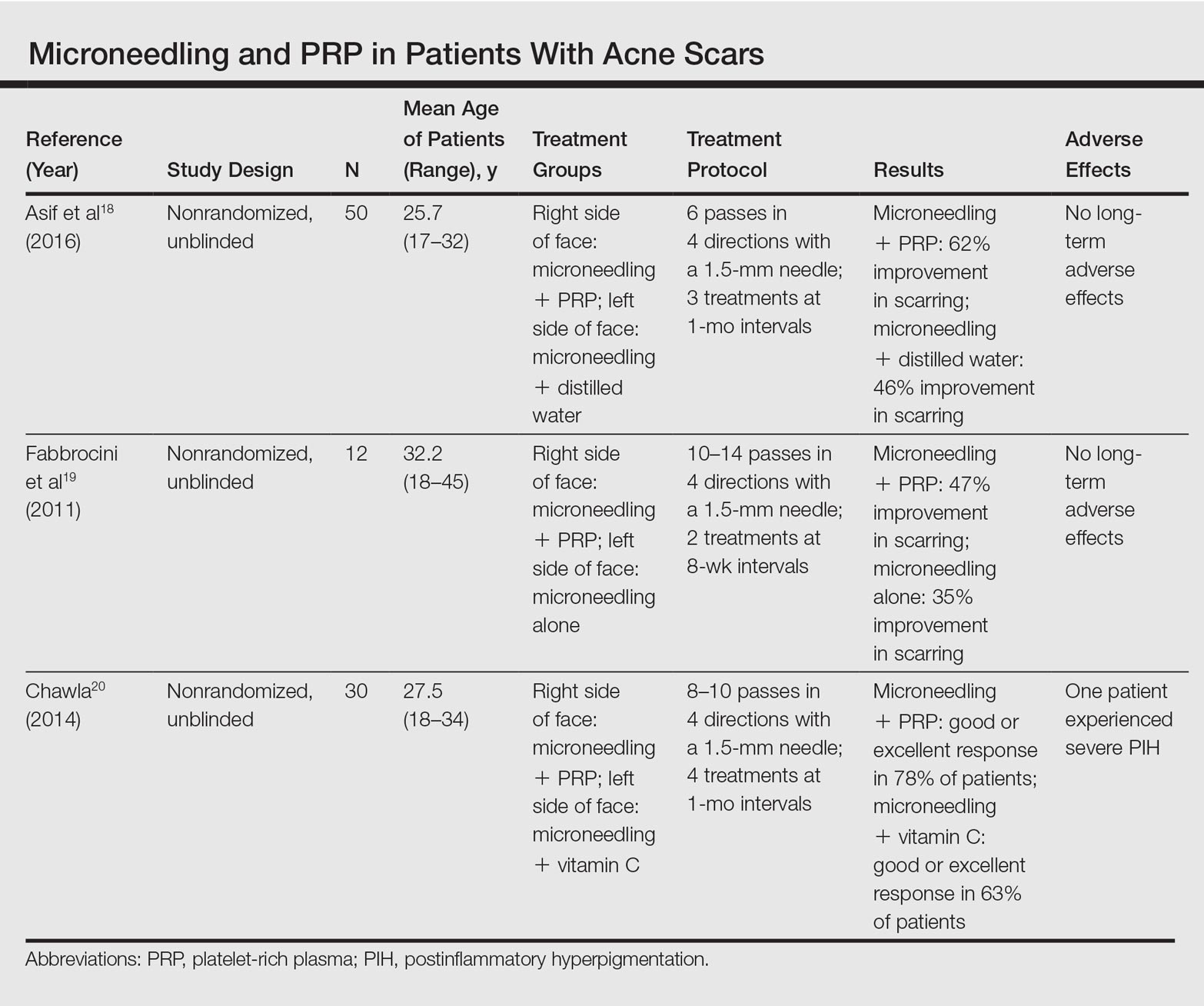
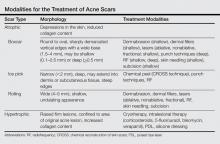
Several studies have compared the effects of microneedling with and without the application of PRP (Table).17-20 In an animal model, Akcal et al17 examined the effects of microneedling and PRP on skin flap survival. Eight rats were randomly divided into 5 groups: sham, control, microneedling alone, microneedling plus PRP, and microneedling plus platelet-poor plasma. Treatments were applied to skin flaps after 4 hours of induced ischemia. The surviving flap area was measured, with results demonstrating significantly higher viable areas in the microneedling plus PRP group relative to all other groups (P<.01). On histologic examination, the microneedling plus PRP group showed well-organized epidermal layers and a dermal integrity that matched the dermis of the sham group.17
Asif et al18 performed a split-face comparison study of 50 patients with atrophic acne scars. On the right side, microneedling was performed followed by intradermal injections and topical application of PRP. On the left side, microneedling was performed followed by intradermal injections of distilled water. The study included 3 treatment sessions with 1 month between each session. Scars were assessed using the Goodman and Baron scale,21 which is designed to grade the morphology of postacne scarring. Scars on the right side improved by 62.2% and scars on the left side improved by 45.8%; prior to treatment, both sides demonstrated similar severity scores, but final severity scores were significantly reduced in the microneedling plus PRP group relative to the microneedling plus distilled water group (P<.00001). No residual side effects from treatment were reported.18
Examining the degree of improvement more carefully, microneedling plus PRP yielded excellent improvement in 40% (20/50) of patients and good improvement in 60% (30/50).18 Microneedling plus distilled water led to excellent improvement in 10% (5/50) and good improvement in 84% (42/50). Given that microneedling plus distilled water still provided good to excellent results in 94% of patients, the addition of PRP was helpful though not necessary in achieving meaningful benefit.18
In another split-face study, Fabbrocini et al19 evaluated 12 adult patients with acne scars. The right side of the face received microneedling plus PRP, while the left side received microneedling alone. Two treatments were performed 8 weeks apart. Severity scores (0=no lesions; 10=maximum severity) were used to assess patient outcomes throughout the study. Acne scars improved on both sides of the face following the treatment period, but the reduction in scar severity with microneedling plus PRP (3.5 points) was significantly greater than with microneedling alone (2.6 points)(P<.05). Patients tended to experience2 to 3 days of mild swelling and erythema after treatment regardless of PRP addition. With only 12 patients, the study was limited by a small sample size. The 10-point grading system differed from the Goodman and Baron scale in that it lacked corresponding qualitative markers, likely decreasing reproducibility.19
Chawla20 compared the effectiveness of combination therapy with microneedling plus PRP versus microneedling and vitamin C application. In a split-face study of 30 patients with atrophic acne scars, the right side of the face was treated with microneedling plus PRP and the left side was treated with microneedling plus vitamin C. Four sessions were performed with an interval of 1 month in between treatments. The Goodman and Baron Scale was used to assess treatment efficacy. Overall, both treatments led to improved outcomes, but in categorizing patients who demonstrated poor responses, a significantly larger percentage existed in the microneedling plus vitamin C group (37% [10/27]) versus the microneedling plus PRP group (22% [6/27])(P=.021). Additionally, aggregate patient satisfaction scores were higher with microneedling plus PRP relative to microneedling plus vitamin C (P=.01). Of note, assessments of improvement were performed by the treating physician and patient satisfaction reports were completed with knowledge of the therapies and cost factor, which may have influenced results.20
Conclusion
Microneedling therapy continues to evolve with a range of applications now emerging in dermatology. As PRP has gained popularity, there has been increased interest in its utilization to amplify the regenerative effects of microneedling. Although the number of direct comparisons examining microneedling with and without PRP is limited, the available evidence indicates that the addition of PRP may improve cosmetic outcomes. These results have been demonstrated primarily in the management of acne scars, but favorable effects may extend to other indications. Continued study is warranted to further quantify the degree of these benefits and to elucidate optimal treatment schedules.
In addition, it is important to consider a cost-benefit analysis of PRP. The price of PRP varies depending on the clinical site but in certain cases may double the cost of a microneedling treatment session. Although studies have demonstrated a statistically significant benefit to PRP, the clinical significance of this supplementary treatment must be weighed against the increased expense. A discussion should take place with the consideration that microneedling alone can provide a satisfactory result for some patients.
- Camirand A, Doucet J. Needle dermabrasion. Aesthetic Plast Surg. 1997;21:48-51.
- Fernandes D. Percutaneous collagen induction: an alternative to laser resurfacing. Aesthet Surg J. 2002;22:307-309.
- Fabbrocini G, Fardella N, Monfrecola A, et al. Acne scarring treatment using skin needling. Clin Exp Dermatol. 2009;34:874-879.
- Zeitter S, Sikora Z, Jahn S, et al. Microneedling: matching the results of medical needling and repetitive treatments to maximize potential for skin regeneration [published online February 7, 2014]. Burns. 2014;40:966-973.
- Schwarz M, Laaff H. A prospective controlled assessment of microneedling with the Dermaroller device. Plast Reconstr Surg. 2011;127:E146-E148.
- Fernandes D, Signorini M. Combating photoaging with percutaneous collagen induction. Clin Dermatol. 2008;26:192-199.
- Aust MC, Fernandes D, Kolokythas P, et al. Percutaneous collagen induction therapy: an alternative treatment for scars, wrinkles, and skin laxity. Plast Reconstr Surg. 2008;121:1421-1429.
- El-Domyati M, Barakat M, Awad S, et al. Microneedling therapy for atrophic acne scars: an objective evaluation. J Clin Aesthet Dermatol. 2015;8:36-42.
- Leheta T, El Tawdy A, Abdel Hay R, et al. Percutaneous collagen induction versus full-concentration trichloroacetic acid in the treatment of atrophic acne scars. Dermatol Surg. 2011;37:207-216.
- Aust MC, Knobloch K, Reimers K, et al. Percutaneous collagen induction therapy: an alternative treatment for burn scars. Burns. 2010;36:836-843.
- Dhurat R, Mathapati S. Response to microneedling treatment in men with androgenetic alopecia who failed to respond to conventional therapy. Indian J Dermatol. 2015;60:260-263.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013;5:6-11.
- Wang HL, Avila G. Platelet rich plasma: myth or reality? Eur J Dent. 2007;1:192-194.
- Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10:225-228.
- Lubkowska A, Dolegowska B, Banfi G. Growth factor content in PRP and their applicability in medicine. J Biol Regul Homeost Agents. 2012;26(2 suppl 1):3S-22S.
- Pietrzak WS, Eppley BL. Platelet rich plasma: biology and new technology. J Craniofac Surg. 2005;16:1043-1054.
- Akcal A, Savas SA, Gorgulu T, et al. The effect of platelete rich plasma combined with microneedling on full venous outflow compromise in a rat skin flap model. Plast Reconstr Surg. 2015;136(4 suppl):71-72.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study [published online January 8, 2016]. J Cosmet Dermatol. 2016;15:434-443.
- Fabbrocini G, De Vita V, Pastore F, et al. Combined use of skin needling and platelet-rich plasma in acne scarring treatment. Cosmet Dermatol. 2011;24:177-183.
- Chawla S. Split face comparative study of microneedling with PRP versus microneedling with vitamin C in treating atrophic post acne scars. J Cutan Aesthet Surg. 2014;7:209-212.
- Goodman GJ, Baron JA. Postacne scarring: a qualitative global scarring grading system. Dermatol Surg. 2006;32:1458-1466.
Microneedling therapy, also known as collagen induction therapy or percutaneous collagen induction, is an increasingly popular treatment modality for skin rejuvenation. The approach employs small needles to puncture the skin and stimulate local collagen production in a minimally invasive manner. Recently, clinicians have incorporated the use of platelet-rich plasma (PRP) with the aim of augmenting cosmetic outcomes. In this article, we examine the utility of this approach by reviewing comparison studies of microneedling therapy with and without the application of PRP.
Dr. Gary Goldenberg demonstrates microneedling with platelet-rich plasma in a procedural video available here.
Microneedling Therapy
The use of microneedling first gained attention in the 1990s. Initially, Camirand and Doucet1 described tattooing without pigment for the treatment of achromatic and hypertrophic scars. Fernandes2 evolved this concept and developed a drum-shaped device with fine protruding needles to puncture the skin. Microneedling devices have expanded in recent years and now include both cord- and battery-powered pens and rollers, with needles ranging in length from 0.25 to 3.0 mm.
Treatment with microneedling promotes skin rejuvenation by creating small puncture wounds in the epidermis and dermis. This injury triggers the wound healing cascade and alters the modulation of growth factors to promote regenerative effects.3,4 Following microneedling therapy, increases occur in elastic fiber formation, collagen deposition, and dermal thickness (Figure).5 Of interesting histologic note, collagen is deposited in the normal lattice pattern following this treatment rather than in the parallel bundles typical of scars.6 Microneedling preserves the overall integrity of the epidermal layers and basement membrane, allowing the epidermis to heal without abnormality, verified on histology by a normal stratum corneum, enhanced stratum granulosum, and normal rete ridges.7

Microneedling has demonstrated several uses beyond general skin rejuvenation. In patients with atrophic acne scars, therapy can lead to improved scar appearance, skin texture, and patient satisfaction.8,9 Hypertrophic and dyspigmented burn scars on the body, face, arms, and legs have shown to be receptive to repeated treatments.10 Microneedling also has shown promise in treating androgenic alopecia, increasing hair regrowth in patients who previously showed poor response to conventional therapy with minoxidil and finasteride.11,12
Platelet-Rich Plasma
Platelet-rich plasma is developed by enriching blood with an autologous concentration of platelets. The preparation of PRP begins with whole blood, commonly obtained peripherally by venipuncture. Samples undergo centrifugation to allow separation of the blood into 3 layers: platelet-poor plasma, PRP, and erythrocytes.13 The typical platelet count of whole blood is approximately 200,000/µL; PRP aims to prepare a platelet count of at least 1,000,000/µL in a 5-mL volume.14
An attractive component of PRP is its high concentration of growth factors, including platelet-derived growth factor, transforming growth factor, vascular endothelial growth factor, and epithelial growth factor.15 Because of the regenerative effects of these proteins, PRP has been investigated as a modality to augment wound healing in a variety of clinical areas, such as maxillofacial surgery, orthopedics, cardiovascular surgery, and treatment of soft tissue ulcers.16
Combination Use of Microneedling and PRP
Several studies have compared the effects of microneedling with and without the application of PRP (Table).17-20 In an animal model, Akcal et al17 examined the effects of microneedling and PRP on skin flap survival. Eight rats were randomly divided into 5 groups: sham, control, microneedling alone, microneedling plus PRP, and microneedling plus platelet-poor plasma. Treatments were applied to skin flaps after 4 hours of induced ischemia. The surviving flap area was measured, with results demonstrating significantly higher viable areas in the microneedling plus PRP group relative to all other groups (P<.01). On histologic examination, the microneedling plus PRP group showed well-organized epidermal layers and a dermal integrity that matched the dermis of the sham group.17
Asif et al18 performed a split-face comparison study of 50 patients with atrophic acne scars. On the right side, microneedling was performed followed by intradermal injections and topical application of PRP. On the left side, microneedling was performed followed by intradermal injections of distilled water. The study included 3 treatment sessions with 1 month between each session. Scars were assessed using the Goodman and Baron scale,21 which is designed to grade the morphology of postacne scarring. Scars on the right side improved by 62.2% and scars on the left side improved by 45.8%; prior to treatment, both sides demonstrated similar severity scores, but final severity scores were significantly reduced in the microneedling plus PRP group relative to the microneedling plus distilled water group (P<.00001). No residual side effects from treatment were reported.18
Examining the degree of improvement more carefully, microneedling plus PRP yielded excellent improvement in 40% (20/50) of patients and good improvement in 60% (30/50).18 Microneedling plus distilled water led to excellent improvement in 10% (5/50) and good improvement in 84% (42/50). Given that microneedling plus distilled water still provided good to excellent results in 94% of patients, the addition of PRP was helpful though not necessary in achieving meaningful benefit.18
In another split-face study, Fabbrocini et al19 evaluated 12 adult patients with acne scars. The right side of the face received microneedling plus PRP, while the left side received microneedling alone. Two treatments were performed 8 weeks apart. Severity scores (0=no lesions; 10=maximum severity) were used to assess patient outcomes throughout the study. Acne scars improved on both sides of the face following the treatment period, but the reduction in scar severity with microneedling plus PRP (3.5 points) was significantly greater than with microneedling alone (2.6 points)(P<.05). Patients tended to experience2 to 3 days of mild swelling and erythema after treatment regardless of PRP addition. With only 12 patients, the study was limited by a small sample size. The 10-point grading system differed from the Goodman and Baron scale in that it lacked corresponding qualitative markers, likely decreasing reproducibility.19
Chawla20 compared the effectiveness of combination therapy with microneedling plus PRP versus microneedling and vitamin C application. In a split-face study of 30 patients with atrophic acne scars, the right side of the face was treated with microneedling plus PRP and the left side was treated with microneedling plus vitamin C. Four sessions were performed with an interval of 1 month in between treatments. The Goodman and Baron Scale was used to assess treatment efficacy. Overall, both treatments led to improved outcomes, but in categorizing patients who demonstrated poor responses, a significantly larger percentage existed in the microneedling plus vitamin C group (37% [10/27]) versus the microneedling plus PRP group (22% [6/27])(P=.021). Additionally, aggregate patient satisfaction scores were higher with microneedling plus PRP relative to microneedling plus vitamin C (P=.01). Of note, assessments of improvement were performed by the treating physician and patient satisfaction reports were completed with knowledge of the therapies and cost factor, which may have influenced results.20
Conclusion
Microneedling therapy continues to evolve with a range of applications now emerging in dermatology. As PRP has gained popularity, there has been increased interest in its utilization to amplify the regenerative effects of microneedling. Although the number of direct comparisons examining microneedling with and without PRP is limited, the available evidence indicates that the addition of PRP may improve cosmetic outcomes. These results have been demonstrated primarily in the management of acne scars, but favorable effects may extend to other indications. Continued study is warranted to further quantify the degree of these benefits and to elucidate optimal treatment schedules.
In addition, it is important to consider a cost-benefit analysis of PRP. The price of PRP varies depending on the clinical site but in certain cases may double the cost of a microneedling treatment session. Although studies have demonstrated a statistically significant benefit to PRP, the clinical significance of this supplementary treatment must be weighed against the increased expense. A discussion should take place with the consideration that microneedling alone can provide a satisfactory result for some patients.
Microneedling therapy, also known as collagen induction therapy or percutaneous collagen induction, is an increasingly popular treatment modality for skin rejuvenation. The approach employs small needles to puncture the skin and stimulate local collagen production in a minimally invasive manner. Recently, clinicians have incorporated the use of platelet-rich plasma (PRP) with the aim of augmenting cosmetic outcomes. In this article, we examine the utility of this approach by reviewing comparison studies of microneedling therapy with and without the application of PRP.
Dr. Gary Goldenberg demonstrates microneedling with platelet-rich plasma in a procedural video available here.
Microneedling Therapy
The use of microneedling first gained attention in the 1990s. Initially, Camirand and Doucet1 described tattooing without pigment for the treatment of achromatic and hypertrophic scars. Fernandes2 evolved this concept and developed a drum-shaped device with fine protruding needles to puncture the skin. Microneedling devices have expanded in recent years and now include both cord- and battery-powered pens and rollers, with needles ranging in length from 0.25 to 3.0 mm.
Treatment with microneedling promotes skin rejuvenation by creating small puncture wounds in the epidermis and dermis. This injury triggers the wound healing cascade and alters the modulation of growth factors to promote regenerative effects.3,4 Following microneedling therapy, increases occur in elastic fiber formation, collagen deposition, and dermal thickness (Figure).5 Of interesting histologic note, collagen is deposited in the normal lattice pattern following this treatment rather than in the parallel bundles typical of scars.6 Microneedling preserves the overall integrity of the epidermal layers and basement membrane, allowing the epidermis to heal without abnormality, verified on histology by a normal stratum corneum, enhanced stratum granulosum, and normal rete ridges.7

Microneedling has demonstrated several uses beyond general skin rejuvenation. In patients with atrophic acne scars, therapy can lead to improved scar appearance, skin texture, and patient satisfaction.8,9 Hypertrophic and dyspigmented burn scars on the body, face, arms, and legs have shown to be receptive to repeated treatments.10 Microneedling also has shown promise in treating androgenic alopecia, increasing hair regrowth in patients who previously showed poor response to conventional therapy with minoxidil and finasteride.11,12
Platelet-Rich Plasma
Platelet-rich plasma is developed by enriching blood with an autologous concentration of platelets. The preparation of PRP begins with whole blood, commonly obtained peripherally by venipuncture. Samples undergo centrifugation to allow separation of the blood into 3 layers: platelet-poor plasma, PRP, and erythrocytes.13 The typical platelet count of whole blood is approximately 200,000/µL; PRP aims to prepare a platelet count of at least 1,000,000/µL in a 5-mL volume.14
An attractive component of PRP is its high concentration of growth factors, including platelet-derived growth factor, transforming growth factor, vascular endothelial growth factor, and epithelial growth factor.15 Because of the regenerative effects of these proteins, PRP has been investigated as a modality to augment wound healing in a variety of clinical areas, such as maxillofacial surgery, orthopedics, cardiovascular surgery, and treatment of soft tissue ulcers.16
Combination Use of Microneedling and PRP
Several studies have compared the effects of microneedling with and without the application of PRP (Table).17-20 In an animal model, Akcal et al17 examined the effects of microneedling and PRP on skin flap survival. Eight rats were randomly divided into 5 groups: sham, control, microneedling alone, microneedling plus PRP, and microneedling plus platelet-poor plasma. Treatments were applied to skin flaps after 4 hours of induced ischemia. The surviving flap area was measured, with results demonstrating significantly higher viable areas in the microneedling plus PRP group relative to all other groups (P<.01). On histologic examination, the microneedling plus PRP group showed well-organized epidermal layers and a dermal integrity that matched the dermis of the sham group.17
Asif et al18 performed a split-face comparison study of 50 patients with atrophic acne scars. On the right side, microneedling was performed followed by intradermal injections and topical application of PRP. On the left side, microneedling was performed followed by intradermal injections of distilled water. The study included 3 treatment sessions with 1 month between each session. Scars were assessed using the Goodman and Baron scale,21 which is designed to grade the morphology of postacne scarring. Scars on the right side improved by 62.2% and scars on the left side improved by 45.8%; prior to treatment, both sides demonstrated similar severity scores, but final severity scores were significantly reduced in the microneedling plus PRP group relative to the microneedling plus distilled water group (P<.00001). No residual side effects from treatment were reported.18
Examining the degree of improvement more carefully, microneedling plus PRP yielded excellent improvement in 40% (20/50) of patients and good improvement in 60% (30/50).18 Microneedling plus distilled water led to excellent improvement in 10% (5/50) and good improvement in 84% (42/50). Given that microneedling plus distilled water still provided good to excellent results in 94% of patients, the addition of PRP was helpful though not necessary in achieving meaningful benefit.18
In another split-face study, Fabbrocini et al19 evaluated 12 adult patients with acne scars. The right side of the face received microneedling plus PRP, while the left side received microneedling alone. Two treatments were performed 8 weeks apart. Severity scores (0=no lesions; 10=maximum severity) were used to assess patient outcomes throughout the study. Acne scars improved on both sides of the face following the treatment period, but the reduction in scar severity with microneedling plus PRP (3.5 points) was significantly greater than with microneedling alone (2.6 points)(P<.05). Patients tended to experience2 to 3 days of mild swelling and erythema after treatment regardless of PRP addition. With only 12 patients, the study was limited by a small sample size. The 10-point grading system differed from the Goodman and Baron scale in that it lacked corresponding qualitative markers, likely decreasing reproducibility.19
Chawla20 compared the effectiveness of combination therapy with microneedling plus PRP versus microneedling and vitamin C application. In a split-face study of 30 patients with atrophic acne scars, the right side of the face was treated with microneedling plus PRP and the left side was treated with microneedling plus vitamin C. Four sessions were performed with an interval of 1 month in between treatments. The Goodman and Baron Scale was used to assess treatment efficacy. Overall, both treatments led to improved outcomes, but in categorizing patients who demonstrated poor responses, a significantly larger percentage existed in the microneedling plus vitamin C group (37% [10/27]) versus the microneedling plus PRP group (22% [6/27])(P=.021). Additionally, aggregate patient satisfaction scores were higher with microneedling plus PRP relative to microneedling plus vitamin C (P=.01). Of note, assessments of improvement were performed by the treating physician and patient satisfaction reports were completed with knowledge of the therapies and cost factor, which may have influenced results.20
Conclusion
Microneedling therapy continues to evolve with a range of applications now emerging in dermatology. As PRP has gained popularity, there has been increased interest in its utilization to amplify the regenerative effects of microneedling. Although the number of direct comparisons examining microneedling with and without PRP is limited, the available evidence indicates that the addition of PRP may improve cosmetic outcomes. These results have been demonstrated primarily in the management of acne scars, but favorable effects may extend to other indications. Continued study is warranted to further quantify the degree of these benefits and to elucidate optimal treatment schedules.
In addition, it is important to consider a cost-benefit analysis of PRP. The price of PRP varies depending on the clinical site but in certain cases may double the cost of a microneedling treatment session. Although studies have demonstrated a statistically significant benefit to PRP, the clinical significance of this supplementary treatment must be weighed against the increased expense. A discussion should take place with the consideration that microneedling alone can provide a satisfactory result for some patients.
- Camirand A, Doucet J. Needle dermabrasion. Aesthetic Plast Surg. 1997;21:48-51.
- Fernandes D. Percutaneous collagen induction: an alternative to laser resurfacing. Aesthet Surg J. 2002;22:307-309.
- Fabbrocini G, Fardella N, Monfrecola A, et al. Acne scarring treatment using skin needling. Clin Exp Dermatol. 2009;34:874-879.
- Zeitter S, Sikora Z, Jahn S, et al. Microneedling: matching the results of medical needling and repetitive treatments to maximize potential for skin regeneration [published online February 7, 2014]. Burns. 2014;40:966-973.
- Schwarz M, Laaff H. A prospective controlled assessment of microneedling with the Dermaroller device. Plast Reconstr Surg. 2011;127:E146-E148.
- Fernandes D, Signorini M. Combating photoaging with percutaneous collagen induction. Clin Dermatol. 2008;26:192-199.
- Aust MC, Fernandes D, Kolokythas P, et al. Percutaneous collagen induction therapy: an alternative treatment for scars, wrinkles, and skin laxity. Plast Reconstr Surg. 2008;121:1421-1429.
- El-Domyati M, Barakat M, Awad S, et al. Microneedling therapy for atrophic acne scars: an objective evaluation. J Clin Aesthet Dermatol. 2015;8:36-42.
- Leheta T, El Tawdy A, Abdel Hay R, et al. Percutaneous collagen induction versus full-concentration trichloroacetic acid in the treatment of atrophic acne scars. Dermatol Surg. 2011;37:207-216.
- Aust MC, Knobloch K, Reimers K, et al. Percutaneous collagen induction therapy: an alternative treatment for burn scars. Burns. 2010;36:836-843.
- Dhurat R, Mathapati S. Response to microneedling treatment in men with androgenetic alopecia who failed to respond to conventional therapy. Indian J Dermatol. 2015;60:260-263.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013;5:6-11.
- Wang HL, Avila G. Platelet rich plasma: myth or reality? Eur J Dent. 2007;1:192-194.
- Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10:225-228.
- Lubkowska A, Dolegowska B, Banfi G. Growth factor content in PRP and their applicability in medicine. J Biol Regul Homeost Agents. 2012;26(2 suppl 1):3S-22S.
- Pietrzak WS, Eppley BL. Platelet rich plasma: biology and new technology. J Craniofac Surg. 2005;16:1043-1054.
- Akcal A, Savas SA, Gorgulu T, et al. The effect of platelete rich plasma combined with microneedling on full venous outflow compromise in a rat skin flap model. Plast Reconstr Surg. 2015;136(4 suppl):71-72.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study [published online January 8, 2016]. J Cosmet Dermatol. 2016;15:434-443.
- Fabbrocini G, De Vita V, Pastore F, et al. Combined use of skin needling and platelet-rich plasma in acne scarring treatment. Cosmet Dermatol. 2011;24:177-183.
- Chawla S. Split face comparative study of microneedling with PRP versus microneedling with vitamin C in treating atrophic post acne scars. J Cutan Aesthet Surg. 2014;7:209-212.
- Goodman GJ, Baron JA. Postacne scarring: a qualitative global scarring grading system. Dermatol Surg. 2006;32:1458-1466.
- Camirand A, Doucet J. Needle dermabrasion. Aesthetic Plast Surg. 1997;21:48-51.
- Fernandes D. Percutaneous collagen induction: an alternative to laser resurfacing. Aesthet Surg J. 2002;22:307-309.
- Fabbrocini G, Fardella N, Monfrecola A, et al. Acne scarring treatment using skin needling. Clin Exp Dermatol. 2009;34:874-879.
- Zeitter S, Sikora Z, Jahn S, et al. Microneedling: matching the results of medical needling and repetitive treatments to maximize potential for skin regeneration [published online February 7, 2014]. Burns. 2014;40:966-973.
- Schwarz M, Laaff H. A prospective controlled assessment of microneedling with the Dermaroller device. Plast Reconstr Surg. 2011;127:E146-E148.
- Fernandes D, Signorini M. Combating photoaging with percutaneous collagen induction. Clin Dermatol. 2008;26:192-199.
- Aust MC, Fernandes D, Kolokythas P, et al. Percutaneous collagen induction therapy: an alternative treatment for scars, wrinkles, and skin laxity. Plast Reconstr Surg. 2008;121:1421-1429.
- El-Domyati M, Barakat M, Awad S, et al. Microneedling therapy for atrophic acne scars: an objective evaluation. J Clin Aesthet Dermatol. 2015;8:36-42.
- Leheta T, El Tawdy A, Abdel Hay R, et al. Percutaneous collagen induction versus full-concentration trichloroacetic acid in the treatment of atrophic acne scars. Dermatol Surg. 2011;37:207-216.
- Aust MC, Knobloch K, Reimers K, et al. Percutaneous collagen induction therapy: an alternative treatment for burn scars. Burns. 2010;36:836-843.
- Dhurat R, Mathapati S. Response to microneedling treatment in men with androgenetic alopecia who failed to respond to conventional therapy. Indian J Dermatol. 2015;60:260-263.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013;5:6-11.
- Wang HL, Avila G. Platelet rich plasma: myth or reality? Eur J Dent. 2007;1:192-194.
- Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10:225-228.
- Lubkowska A, Dolegowska B, Banfi G. Growth factor content in PRP and their applicability in medicine. J Biol Regul Homeost Agents. 2012;26(2 suppl 1):3S-22S.
- Pietrzak WS, Eppley BL. Platelet rich plasma: biology and new technology. J Craniofac Surg. 2005;16:1043-1054.
- Akcal A, Savas SA, Gorgulu T, et al. The effect of platelete rich plasma combined with microneedling on full venous outflow compromise in a rat skin flap model. Plast Reconstr Surg. 2015;136(4 suppl):71-72.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study [published online January 8, 2016]. J Cosmet Dermatol. 2016;15:434-443.
- Fabbrocini G, De Vita V, Pastore F, et al. Combined use of skin needling and platelet-rich plasma in acne scarring treatment. Cosmet Dermatol. 2011;24:177-183.
- Chawla S. Split face comparative study of microneedling with PRP versus microneedling with vitamin C in treating atrophic post acne scars. J Cutan Aesthet Surg. 2014;7:209-212.
- Goodman GJ, Baron JA. Postacne scarring: a qualitative global scarring grading system. Dermatol Surg. 2006;32:1458-1466.
Practice Points
- Microneedling is an effective therapy for skin rejuvenation.
- Preliminary evidence indicates that the addition of platelet-rich plasma to microneedling improves cosmetic outcomes.
Advances in Minimally Invasive and Noninvasive Treatments for Submental Fat
Submental fat (SMF) accumulation within the subcutaneous (preplatysmal) or subplatysmal fat compartment of the cervical anatomy results in an obtuse cervicomental angle and loss of mandibular and cervical contours. It is a common cosmetic concern due to its aesthetic association with weight gain and aging.1 Minimally invasive or noninvasive submental lipolytic agents and techniques are sought for patients who are not candidates for surgery or prefer more conservative cosmetic treatments. These methods typically are only effective in addressing preplatysmal SMF, as subplatysmal SMF requires more surgical methods due to its less-accessible location. The pathology of SMF should initially be assessed by clinical examination or ultrasonography. In this article, we review the most relevant clinical and safety data on minimally invasive and noninvasive treatments for SMF, including laser-assisted lipolysis (LAL), radiofrequency (RF)–assisted lipolysis, deoxycholic acid (DCA), and cryolipolysis.
MINIMALLY INVASIVE MODALITIES
Traditional, or tumescent, liposuction is still widely considered the most effective method for removal of large masses of adiposity. Laser- and RF-assisted adjuncts have been more recently developed to improve patient side effects and recovery time and reduce the manual effort of surgeons. Of note, these adjuncts, with some exceptions, still require the same invasiveness as traditional liposuction, involving submental stab incisions of up to 2.4 mm.
Laser-Assisted Lipolysis
Laser-assisted lipolysis produces a similar effect as suction-assisted lipoplasty by focusing pulses of laser energy through a 1-mm wide fiber optic cannula and inducing thermally mediated adipolysis. The directed laser results in adipocyte rupturing with added benefits of skin retraction and small vessel coagulation, thus lessening intraoperative blood loss.2 This technique typically requires smaller incisions than traditional liposuction. The most common laser lipolysis systems used in cosmetic dermatology are the 920- to 980-nm diode lasers and 1064- to 1440-nm Nd:YAG lasers. The 924-nm diode, 1064-nm Nd:YAG, and 1064/1320-nm Nd:YAG have been best characterized in clinical trials, as reviewed by Fakhouri et al,3 with demonstrated efficacy in reducing SMF density.
The first randomized prospective trial comparing LAL (using 1064-nm Nd:YAG) and traditional liposuction in various anatomical areas on 25 patients showed no difference in cosmetic results, ecchymoses, edema, or retraction, and significantly lower postoperative pain ratings (P<.0001) in LAL.4 A more recent prospective randomized comparison of LAL (980-nm diode laser; 6–8 W) and traditional liposuction of the submental area in 40 female patients showed greater reduction in SMF thickness in the LAL group compared to the liposuction group at 2-month follow-up (6.2 vs 8.22 unspecified units; P<.001) with significant improvement from baseline in both groups (P<.001).5 However, the cosmetic benefit of LAL over traditional liposuction remains controversial and has not been unequivocally established in the literature.
Common adverse events (AEs) are postoperative swelling, ecchymoses, and pain, and complications of interest are nodularity, skin infections, burns, and nerve damage.6 In one retrospective investigation (N=537), these complications occurred at a rate of less than 1% (4 burns and 1 skin infection).6 Patients treated with LAL may report fewer AEs, especially pain and bleeding, compared to liposuction-treated patients.3
RF-Assisted Lipolysis
Radiofrequency-assisted lipolysis is one of the newest technologies in lipocontouring. NeckTite (Invasix Aesthetic Solutions) is effective for treatment of preplatysmal adiposity and cervicomental lipocontouring; a 2.4-mm bipolar probe that is inserted into the subdermal space and connected with an external electrode emits RF energy and simultaneously coagulates and aspirates adipose tissue. NeckTite also may be used in conjunction with FaceTite (Invasix Aesthetic Solutions), which promotes fibroseptal network remodeling and dermal contraction.2
In the first published investigation of the efficacy and safety of NeckTite, 47 of 55 patients received treatment of slight to moderate SMF (average body mass index [BMI], 25 kg/m2) with NeckTite and FaceTite or NeckTite alone.7 At 6-month follow-up, 87% (48/55) of patients subjectively rated treatment efficacy as satisfactory, and 2 independent physicians rated the improvement between before-and-after frontal and lateral photographs of the submental area as moderate to excellent in 95% (52/55) of all cases. Reported complications in this study were full-thickness burns resulting in minor scarring (2/55 [4%]), neck tissue hardness that resolved with daily massage after 3 months (5/55 [9%]), and transient facial nerve paresis of the mandibular branch that resolved after 2 months (1/55 [2%]).7
NONINVASIVE MODALITIES
RF-Assisted Contouring
Another exciting development in RF technology is truSculpt (Cutera), a noninvasive contouring device that is placed over the epidermis and emits RF energy that preferentially heats fat more than other tissue types. In a single-center prospective trial of efficacy and safety in the treatment of SMF, 17 patients received 2 treatments with truSculpt administered 1 month apart.8 At 1- and 6-month follow-up, 82.3% (14/17) and 52.9% (9/17) of patients showed improvement on physician assessment. Submental circumference and ultrasonographic fat thickness reductions at 1-month follow-up were 1.4 cm (5.7% of pretreatment circumference [P<.001]) and 5.4 mm (9.7% of pretreatment fat thickness [P=.005]), respectively. At further longer-term follow-up to 6 months, submental circumference was 0.9 cm (3.8% of pretreatment circumference [P<.001]) and ultrasonographic fat reduction was 6.8 mm (10.5% of pretreatment fat thickness [P=.006]). Commonly reported AEs were pain (rate not given), erythema (8/17 [47%]), edema (1/17 [6%]), and vesicle formation (1/17 [6%]); all were self-resolving. Erythema usually subsided within 6 hours posttreatment. No other AEs or complications were reported.8
Deoxycholic Acid
Deoxycholic acid (DCA)(formerly ATX-101) is an injectable liquid formulation of synthetic DCA that was approved by the US Food and Drug Administration (FDA) in 2015 for moderate to severe SMF. Deoxycholic acid exists endogenously as a bile salt emulsifier and has been shown to cause dose-dependent adipocyte lysis, necrosis, disruption and dissolution of fat architecture, and inflammatory targeting of adipocytes by immune cells.9,10 Thus, DCA causes targeted adipocytolysis and is a novel medical agent in the treatment of SMF. Supplied in 2-mL vials, clinicians may inject 10 mL at each treatment for up to 6 treatments administered 1 month apart.11
Efficacy
REFINE-1, a pivotal North American–based phase 3 trial, investigated the efficacy and safety of DCA.12 A total of 506 participants with scores of 2 (moderate) or 3 (severe) on the Clinician-Reported Submental Fat Rating Scale (CR-SMFRS) and a mean BMI of 29 kg/m2 were randomized to receive preplatysmal fat injections of 2 mg/cm2 of DCA (n=256) or placebo (n=250). Participants received up to 10 mL of product (mean total of 25 mL of DCA across all visits) at each treatment session for up to 6 sessions depending on individual efficacy, with approximately 28 days between sessions. Sixty-four percent of the treatment group received all 6 treatments. At 12-week follow-up after the last treatment session, 70% of DCA-treated participants versus 18.6% of placebo-treated participants (P<.001) improved by 1 grade or more on the CR-SMFRS and 13.4% versus 0% (P<.001) improved by 2 grades or more. Skin laxity was unchanged or improved in 92.7% of the DCA group and 87.6% of the placebo group.12
REFINE-2, the second of the North American phase 3 trials, had parallel inclusionary criteria and study design and established efficacy of 2 mg/cm2 DCA over placebo in 516 participants (randomized 1:1).13 At 12 weeks posttreatment, 66.5% of DCA-treated participants versus 22.2% of placebo-treated participants improved by 1 grade or more according to the CR-SMFRS (P<.001) and 18.6% versus 3% improved by 2 grades or more in SMF (P<.001). Magnetic resonance imaging analysis of participants in the DCA (n=113) and placebo groups (n=112) showed that 40.2% versus 5.2% (P<.001) exhibited 10% or more reduction in submental volume, with similar comparative rates of SMF thickness reduction via caliper measurements.13
Safety
Safety data from REFINE-1 showed higher rates of treatment-related AEs in DCA-treated participants compared to placebo, including hematoma (70% vs 67.3%), anesthesia (66.9% vs 4.4%), pain (65.4% vs 23.4%), edema (52.9% vs 21.8%), induration (18.3% vs 1.6%), paresthesia (12.8% vs 3.2%), nodule formation (12.5% vs 0.8%), and pruritus (8.6% vs 3.6%).12 In this trial, 11 of 258 cases (4.3%) of marginal mandibular nerve paresis and asymmetric smile occurred, all in DCA-treated participants and with a median duration of 31 days. Dysphagia resolving in a median duration of 4 days occurred in 1.6% (4/258) of DCA-treated participants.12 REFINE-2 exhibited similar rates of common AEs. Complications of note were 14 cases of marginal mandibular nerve paresis (11 in DCA group, 3 in placebo group) attributed to injection technique, 1 case of skin ulceration possibly related to accidental injection into dermis, and 6 cases of dysphagia in DCA participants attributed to higher volume treatment sessions and postinjection swelling. Dysphagia lasted a median of 2.5 days and resolved without sequelae.13
Overall, DCA demonstrated high rates of minor injection-site AEs that resolved without sequelae and could be mitigated by comfort therapies (eg, lidocaine, nonsteroidal anti-inflammatory drugs) as well as understanding the anatomy of the submental region. Adverse effects of particular interest included marginal mandibular nerve palsy, skin ulceration, and dysphagia.12,13
Cryolipolysis
Cryolipolysis is an advancement that utilizes the application of noninvasive cooling temperatures to the skin’s surface to destroy underlying adipocytes based on the concept that lipid-filled cells are more susceptible to cold-induced injury than water-filled cells. Thus, cryolipolysis selectively targets adipose tissue, leading to cell death without harm to surrounding cells and without the need for surgery or injections.14
Cryolipolysis typically is delivered via a vacuum applicator (CoolMini, Zeltiq Aesthetics Inc), which applies temperatures of –10°C (14°F) to the skin in cycles of 60 minutes each. Initially approved by the FDA for treatment of flank adiposity in 2010, cryolipolysis has since been approved for treatment of the abdomen, thighs, and submental area.14 An advantage of cryolipolysis is that it does not require frequent treatment sessions for maximal efficacy.
Efficacy
The efficacy of cryolipolysis in the treatment of SMF was established in a multicenter device investigation resulting in its FDA approval for the submental region.15 Sixty participants with a mean BMI of 31.8 kg/m2 received 1 (1/60) or 2 (59/60) treatment sessions of the submental area administered 6 weeks apart. Primary efficacy assessments included analysis by 3 blinded reviewers who viewed photographs of each participant at baseline, immediately posttreatment, 6 weeks posttreatment, and 12 weeks posttreatment; ultrasonographic measurements of SMF thickness; and a 12-point patient satisfaction questionnaire. Blinded reviewers correctly identified baseline images in 91.4% (55/60) of cases. Ultrasonography confirmed a mean reduction in SMF of 2 mm (P<.0001) or 20% of fat thickness at 12 weeks posttreatment. On subjective patient satisfaction surveys, 83% (50/60) of participants were satisfied with the procedure and 77% (46/60) reported a visible reduction in fat and perceived an improvement in appearance.15
Safety
The most common immediate posttreatment AEs were erythema/purpura (100%), numbness (90%), edema (62%), tingling (30%), blanching (25%), and bruising (3%) at the site of cryolipolysis with resolution within 1 week posttreatment, except for numbness.15 At 6-week follow-up, all AEs had resolved, except continued numbness in 4 participants that resolved by 12-week follow-up. A further event of note was fullness in the throat in 1 participant that was attributed to swelling and resolved at 40 days posttreatment without incident. No serious AEs were reported in this trial.15
A particularly concerning but rare complication that is increasing in awareness is paradoxical adipose hyperplasia following cryolipolysis. Patients may develop firm painless areas of soft tissue enlargements in the area of cryolipolysis typically 3 to 6 months posttreatment.16 The largest published report recorded an incidence rate of 0.46% (n=2, all males) at a single-center institution of 422 cryolipolysis treatments.16 Other incidence rates reported are 0.0051% and 0.78%.17 Causes and associations are not known, though male gender is speculated to increase risk.
Conclusion
This article highlights the available information on advances in minimally invasive and noninvasive treatments for SMF accumulation. The efficacy and safety trials varied in quality and in different methods of end point analysis of SMF reduction. Further, few trials have featured head-to-head comparisons of treatments.
Although liposuction and adjuncts remain the gold standard in large-mass lipid removal, these procedures are invasive and exhibit typical risks of surgery. Given its sensitive location, the submental area may require the use of more delicate therapeutic methods, including completely noninvasive devices such as truSculpt and cryolipolysis. Regardless of the chosen treatment, the most important factors in yielding patient satisfaction and SMF improvement are proper patient selection and an understanding of the anatomical source of adiposity to be addressed with the therapeutic modalities.
[polldaddy:9711250]
- Hatef DA, Koshy JC, Sandoval SE, et al. The submental fat compartment of the neck. Semin Plast Surg. 2009;23:288-291.
- Mulholland RS. Nonexcisional, minimally invasive rejuvenation of the neck. Clin Plast Surg. 2014;41:11-31.
- Fakhouri TM, El Tal AK, Abrou AE, et al. Laser-assisted lipolysis: a review. Dermatol Surg. 2012;38:155-169.
- Prado A, Andrades P, Danilla S, et al. A prospective, randomized, double-blind, controlled clinical trial comparing laser-assisted lipoplasty with suction-assisted lipoplasty. Plast Reconstr Surg. 2006;118:1032-1045.
- Valizadeh N, Jalaly NY, Zarghampour M, et al. Evaluation of safety and efficacy of 980-nm diode laser-assisted lipolysis versus traditional liposuction for submental rejuvenation: a randomized clinical trial. J Cosmet Laser Ther. 2016;18:41-45.
- Katz B, McBean J. Laser-assisted lipolysis: a report on complications. J Cosmet Laser Ther. 2008;10:231-233.
- Keramidas E, Rodopoulou S. Radiofrequency-assisted liposuction for neck and lower face adipodermal remodeling and contouring. Plast Reconstr Surg Glob Open. 2016;4:e850.
- Park JH, Kim JI, Park HJ, et al. Evaluation of safety and efficacy of noninvasive radiofrequency technology for submental rejuvenation [published online July 12, 2016]. Lasers Med Sci. 2016;31:1599-1605.
- Yagima Odo ME, Cucé LC, Odo LM, et al. Action of sodium deoxycholate on subcutaneous human tissue: local and systemic effects. Dermatol Surg. 2007;33:178-188; discussion 188-189.
- Rotunda AM, Suzuki H, Moy RL, et al. Detergent effects of sodium deoxycholate are a major feature of an injectable phosphatidylcholine formulation used for localized fat dissolution. Dermatol Surg. 2004;30:1001-1008.
- Kybella [package insert]. Westlake Village, CA: Kythera Biopharmaceuticals, Inc; 2015.
- Jones DH, Carruthers J, Joseph JH, et al. REFINE-1, a multicenter, randomized, double-blind, placebo-controlled, phase 3 trial with ATX-101, an injectable drug for submental fat reduction. Dermatol Surg. 2016;42:38-49.
- Humphrey S, Sykes J, Kantor J, et al. ATX-101 for reduction of submental fat: a phase III randomized controlled trial [published online July 16, 2016]. J Am Acad Dermatol. 2016;75:788-797.e7.
- Manstein D, Laubach H, Watanabe K, et al. Selective cryolysis: a novel method of non-invasive fat removal. Lasers Surg Med. 2008;40:595-604.
- Kilmer SL, Burns AJ, Zelickson BD. Safety and efficacy of cryolipolysis for non-invasive reduction of submental fat. Lasers Surg Med. 2016;48:3-13.
- Singh SM, Geddes ER, Boutrous SG, et al. Paradoxical adipose hyperplasia secondary to cryolipolysis: an underreported entity? Lasers Surg Med. 2015;47:476-478.
- Kelly E, Rodriguez-Feliz J, Kelly ME. Paradoxical adipose hyperplasia after cryolipolysis: a report on incidence and common factors identified in 510 patients. Plast Reconst Surg. 2016;137:639e-640e.
Submental fat (SMF) accumulation within the subcutaneous (preplatysmal) or subplatysmal fat compartment of the cervical anatomy results in an obtuse cervicomental angle and loss of mandibular and cervical contours. It is a common cosmetic concern due to its aesthetic association with weight gain and aging.1 Minimally invasive or noninvasive submental lipolytic agents and techniques are sought for patients who are not candidates for surgery or prefer more conservative cosmetic treatments. These methods typically are only effective in addressing preplatysmal SMF, as subplatysmal SMF requires more surgical methods due to its less-accessible location. The pathology of SMF should initially be assessed by clinical examination or ultrasonography. In this article, we review the most relevant clinical and safety data on minimally invasive and noninvasive treatments for SMF, including laser-assisted lipolysis (LAL), radiofrequency (RF)–assisted lipolysis, deoxycholic acid (DCA), and cryolipolysis.
MINIMALLY INVASIVE MODALITIES
Traditional, or tumescent, liposuction is still widely considered the most effective method for removal of large masses of adiposity. Laser- and RF-assisted adjuncts have been more recently developed to improve patient side effects and recovery time and reduce the manual effort of surgeons. Of note, these adjuncts, with some exceptions, still require the same invasiveness as traditional liposuction, involving submental stab incisions of up to 2.4 mm.
Laser-Assisted Lipolysis
Laser-assisted lipolysis produces a similar effect as suction-assisted lipoplasty by focusing pulses of laser energy through a 1-mm wide fiber optic cannula and inducing thermally mediated adipolysis. The directed laser results in adipocyte rupturing with added benefits of skin retraction and small vessel coagulation, thus lessening intraoperative blood loss.2 This technique typically requires smaller incisions than traditional liposuction. The most common laser lipolysis systems used in cosmetic dermatology are the 920- to 980-nm diode lasers and 1064- to 1440-nm Nd:YAG lasers. The 924-nm diode, 1064-nm Nd:YAG, and 1064/1320-nm Nd:YAG have been best characterized in clinical trials, as reviewed by Fakhouri et al,3 with demonstrated efficacy in reducing SMF density.
The first randomized prospective trial comparing LAL (using 1064-nm Nd:YAG) and traditional liposuction in various anatomical areas on 25 patients showed no difference in cosmetic results, ecchymoses, edema, or retraction, and significantly lower postoperative pain ratings (P<.0001) in LAL.4 A more recent prospective randomized comparison of LAL (980-nm diode laser; 6–8 W) and traditional liposuction of the submental area in 40 female patients showed greater reduction in SMF thickness in the LAL group compared to the liposuction group at 2-month follow-up (6.2 vs 8.22 unspecified units; P<.001) with significant improvement from baseline in both groups (P<.001).5 However, the cosmetic benefit of LAL over traditional liposuction remains controversial and has not been unequivocally established in the literature.
Common adverse events (AEs) are postoperative swelling, ecchymoses, and pain, and complications of interest are nodularity, skin infections, burns, and nerve damage.6 In one retrospective investigation (N=537), these complications occurred at a rate of less than 1% (4 burns and 1 skin infection).6 Patients treated with LAL may report fewer AEs, especially pain and bleeding, compared to liposuction-treated patients.3
RF-Assisted Lipolysis
Radiofrequency-assisted lipolysis is one of the newest technologies in lipocontouring. NeckTite (Invasix Aesthetic Solutions) is effective for treatment of preplatysmal adiposity and cervicomental lipocontouring; a 2.4-mm bipolar probe that is inserted into the subdermal space and connected with an external electrode emits RF energy and simultaneously coagulates and aspirates adipose tissue. NeckTite also may be used in conjunction with FaceTite (Invasix Aesthetic Solutions), which promotes fibroseptal network remodeling and dermal contraction.2
In the first published investigation of the efficacy and safety of NeckTite, 47 of 55 patients received treatment of slight to moderate SMF (average body mass index [BMI], 25 kg/m2) with NeckTite and FaceTite or NeckTite alone.7 At 6-month follow-up, 87% (48/55) of patients subjectively rated treatment efficacy as satisfactory, and 2 independent physicians rated the improvement between before-and-after frontal and lateral photographs of the submental area as moderate to excellent in 95% (52/55) of all cases. Reported complications in this study were full-thickness burns resulting in minor scarring (2/55 [4%]), neck tissue hardness that resolved with daily massage after 3 months (5/55 [9%]), and transient facial nerve paresis of the mandibular branch that resolved after 2 months (1/55 [2%]).7
NONINVASIVE MODALITIES
RF-Assisted Contouring
Another exciting development in RF technology is truSculpt (Cutera), a noninvasive contouring device that is placed over the epidermis and emits RF energy that preferentially heats fat more than other tissue types. In a single-center prospective trial of efficacy and safety in the treatment of SMF, 17 patients received 2 treatments with truSculpt administered 1 month apart.8 At 1- and 6-month follow-up, 82.3% (14/17) and 52.9% (9/17) of patients showed improvement on physician assessment. Submental circumference and ultrasonographic fat thickness reductions at 1-month follow-up were 1.4 cm (5.7% of pretreatment circumference [P<.001]) and 5.4 mm (9.7% of pretreatment fat thickness [P=.005]), respectively. At further longer-term follow-up to 6 months, submental circumference was 0.9 cm (3.8% of pretreatment circumference [P<.001]) and ultrasonographic fat reduction was 6.8 mm (10.5% of pretreatment fat thickness [P=.006]). Commonly reported AEs were pain (rate not given), erythema (8/17 [47%]), edema (1/17 [6%]), and vesicle formation (1/17 [6%]); all were self-resolving. Erythema usually subsided within 6 hours posttreatment. No other AEs or complications were reported.8
Deoxycholic Acid
Deoxycholic acid (DCA)(formerly ATX-101) is an injectable liquid formulation of synthetic DCA that was approved by the US Food and Drug Administration (FDA) in 2015 for moderate to severe SMF. Deoxycholic acid exists endogenously as a bile salt emulsifier and has been shown to cause dose-dependent adipocyte lysis, necrosis, disruption and dissolution of fat architecture, and inflammatory targeting of adipocytes by immune cells.9,10 Thus, DCA causes targeted adipocytolysis and is a novel medical agent in the treatment of SMF. Supplied in 2-mL vials, clinicians may inject 10 mL at each treatment for up to 6 treatments administered 1 month apart.11
Efficacy
REFINE-1, a pivotal North American–based phase 3 trial, investigated the efficacy and safety of DCA.12 A total of 506 participants with scores of 2 (moderate) or 3 (severe) on the Clinician-Reported Submental Fat Rating Scale (CR-SMFRS) and a mean BMI of 29 kg/m2 were randomized to receive preplatysmal fat injections of 2 mg/cm2 of DCA (n=256) or placebo (n=250). Participants received up to 10 mL of product (mean total of 25 mL of DCA across all visits) at each treatment session for up to 6 sessions depending on individual efficacy, with approximately 28 days between sessions. Sixty-four percent of the treatment group received all 6 treatments. At 12-week follow-up after the last treatment session, 70% of DCA-treated participants versus 18.6% of placebo-treated participants (P<.001) improved by 1 grade or more on the CR-SMFRS and 13.4% versus 0% (P<.001) improved by 2 grades or more. Skin laxity was unchanged or improved in 92.7% of the DCA group and 87.6% of the placebo group.12
REFINE-2, the second of the North American phase 3 trials, had parallel inclusionary criteria and study design and established efficacy of 2 mg/cm2 DCA over placebo in 516 participants (randomized 1:1).13 At 12 weeks posttreatment, 66.5% of DCA-treated participants versus 22.2% of placebo-treated participants improved by 1 grade or more according to the CR-SMFRS (P<.001) and 18.6% versus 3% improved by 2 grades or more in SMF (P<.001). Magnetic resonance imaging analysis of participants in the DCA (n=113) and placebo groups (n=112) showed that 40.2% versus 5.2% (P<.001) exhibited 10% or more reduction in submental volume, with similar comparative rates of SMF thickness reduction via caliper measurements.13
Safety
Safety data from REFINE-1 showed higher rates of treatment-related AEs in DCA-treated participants compared to placebo, including hematoma (70% vs 67.3%), anesthesia (66.9% vs 4.4%), pain (65.4% vs 23.4%), edema (52.9% vs 21.8%), induration (18.3% vs 1.6%), paresthesia (12.8% vs 3.2%), nodule formation (12.5% vs 0.8%), and pruritus (8.6% vs 3.6%).12 In this trial, 11 of 258 cases (4.3%) of marginal mandibular nerve paresis and asymmetric smile occurred, all in DCA-treated participants and with a median duration of 31 days. Dysphagia resolving in a median duration of 4 days occurred in 1.6% (4/258) of DCA-treated participants.12 REFINE-2 exhibited similar rates of common AEs. Complications of note were 14 cases of marginal mandibular nerve paresis (11 in DCA group, 3 in placebo group) attributed to injection technique, 1 case of skin ulceration possibly related to accidental injection into dermis, and 6 cases of dysphagia in DCA participants attributed to higher volume treatment sessions and postinjection swelling. Dysphagia lasted a median of 2.5 days and resolved without sequelae.13
Overall, DCA demonstrated high rates of minor injection-site AEs that resolved without sequelae and could be mitigated by comfort therapies (eg, lidocaine, nonsteroidal anti-inflammatory drugs) as well as understanding the anatomy of the submental region. Adverse effects of particular interest included marginal mandibular nerve palsy, skin ulceration, and dysphagia.12,13
Cryolipolysis
Cryolipolysis is an advancement that utilizes the application of noninvasive cooling temperatures to the skin’s surface to destroy underlying adipocytes based on the concept that lipid-filled cells are more susceptible to cold-induced injury than water-filled cells. Thus, cryolipolysis selectively targets adipose tissue, leading to cell death without harm to surrounding cells and without the need for surgery or injections.14
Cryolipolysis typically is delivered via a vacuum applicator (CoolMini, Zeltiq Aesthetics Inc), which applies temperatures of –10°C (14°F) to the skin in cycles of 60 minutes each. Initially approved by the FDA for treatment of flank adiposity in 2010, cryolipolysis has since been approved for treatment of the abdomen, thighs, and submental area.14 An advantage of cryolipolysis is that it does not require frequent treatment sessions for maximal efficacy.
Efficacy
The efficacy of cryolipolysis in the treatment of SMF was established in a multicenter device investigation resulting in its FDA approval for the submental region.15 Sixty participants with a mean BMI of 31.8 kg/m2 received 1 (1/60) or 2 (59/60) treatment sessions of the submental area administered 6 weeks apart. Primary efficacy assessments included analysis by 3 blinded reviewers who viewed photographs of each participant at baseline, immediately posttreatment, 6 weeks posttreatment, and 12 weeks posttreatment; ultrasonographic measurements of SMF thickness; and a 12-point patient satisfaction questionnaire. Blinded reviewers correctly identified baseline images in 91.4% (55/60) of cases. Ultrasonography confirmed a mean reduction in SMF of 2 mm (P<.0001) or 20% of fat thickness at 12 weeks posttreatment. On subjective patient satisfaction surveys, 83% (50/60) of participants were satisfied with the procedure and 77% (46/60) reported a visible reduction in fat and perceived an improvement in appearance.15
Safety
The most common immediate posttreatment AEs were erythema/purpura (100%), numbness (90%), edema (62%), tingling (30%), blanching (25%), and bruising (3%) at the site of cryolipolysis with resolution within 1 week posttreatment, except for numbness.15 At 6-week follow-up, all AEs had resolved, except continued numbness in 4 participants that resolved by 12-week follow-up. A further event of note was fullness in the throat in 1 participant that was attributed to swelling and resolved at 40 days posttreatment without incident. No serious AEs were reported in this trial.15
A particularly concerning but rare complication that is increasing in awareness is paradoxical adipose hyperplasia following cryolipolysis. Patients may develop firm painless areas of soft tissue enlargements in the area of cryolipolysis typically 3 to 6 months posttreatment.16 The largest published report recorded an incidence rate of 0.46% (n=2, all males) at a single-center institution of 422 cryolipolysis treatments.16 Other incidence rates reported are 0.0051% and 0.78%.17 Causes and associations are not known, though male gender is speculated to increase risk.
Conclusion
This article highlights the available information on advances in minimally invasive and noninvasive treatments for SMF accumulation. The efficacy and safety trials varied in quality and in different methods of end point analysis of SMF reduction. Further, few trials have featured head-to-head comparisons of treatments.
Although liposuction and adjuncts remain the gold standard in large-mass lipid removal, these procedures are invasive and exhibit typical risks of surgery. Given its sensitive location, the submental area may require the use of more delicate therapeutic methods, including completely noninvasive devices such as truSculpt and cryolipolysis. Regardless of the chosen treatment, the most important factors in yielding patient satisfaction and SMF improvement are proper patient selection and an understanding of the anatomical source of adiposity to be addressed with the therapeutic modalities.
[polldaddy:9711250]
Submental fat (SMF) accumulation within the subcutaneous (preplatysmal) or subplatysmal fat compartment of the cervical anatomy results in an obtuse cervicomental angle and loss of mandibular and cervical contours. It is a common cosmetic concern due to its aesthetic association with weight gain and aging.1 Minimally invasive or noninvasive submental lipolytic agents and techniques are sought for patients who are not candidates for surgery or prefer more conservative cosmetic treatments. These methods typically are only effective in addressing preplatysmal SMF, as subplatysmal SMF requires more surgical methods due to its less-accessible location. The pathology of SMF should initially be assessed by clinical examination or ultrasonography. In this article, we review the most relevant clinical and safety data on minimally invasive and noninvasive treatments for SMF, including laser-assisted lipolysis (LAL), radiofrequency (RF)–assisted lipolysis, deoxycholic acid (DCA), and cryolipolysis.
MINIMALLY INVASIVE MODALITIES
Traditional, or tumescent, liposuction is still widely considered the most effective method for removal of large masses of adiposity. Laser- and RF-assisted adjuncts have been more recently developed to improve patient side effects and recovery time and reduce the manual effort of surgeons. Of note, these adjuncts, with some exceptions, still require the same invasiveness as traditional liposuction, involving submental stab incisions of up to 2.4 mm.
Laser-Assisted Lipolysis
Laser-assisted lipolysis produces a similar effect as suction-assisted lipoplasty by focusing pulses of laser energy through a 1-mm wide fiber optic cannula and inducing thermally mediated adipolysis. The directed laser results in adipocyte rupturing with added benefits of skin retraction and small vessel coagulation, thus lessening intraoperative blood loss.2 This technique typically requires smaller incisions than traditional liposuction. The most common laser lipolysis systems used in cosmetic dermatology are the 920- to 980-nm diode lasers and 1064- to 1440-nm Nd:YAG lasers. The 924-nm diode, 1064-nm Nd:YAG, and 1064/1320-nm Nd:YAG have been best characterized in clinical trials, as reviewed by Fakhouri et al,3 with demonstrated efficacy in reducing SMF density.
The first randomized prospective trial comparing LAL (using 1064-nm Nd:YAG) and traditional liposuction in various anatomical areas on 25 patients showed no difference in cosmetic results, ecchymoses, edema, or retraction, and significantly lower postoperative pain ratings (P<.0001) in LAL.4 A more recent prospective randomized comparison of LAL (980-nm diode laser; 6–8 W) and traditional liposuction of the submental area in 40 female patients showed greater reduction in SMF thickness in the LAL group compared to the liposuction group at 2-month follow-up (6.2 vs 8.22 unspecified units; P<.001) with significant improvement from baseline in both groups (P<.001).5 However, the cosmetic benefit of LAL over traditional liposuction remains controversial and has not been unequivocally established in the literature.
Common adverse events (AEs) are postoperative swelling, ecchymoses, and pain, and complications of interest are nodularity, skin infections, burns, and nerve damage.6 In one retrospective investigation (N=537), these complications occurred at a rate of less than 1% (4 burns and 1 skin infection).6 Patients treated with LAL may report fewer AEs, especially pain and bleeding, compared to liposuction-treated patients.3
RF-Assisted Lipolysis
Radiofrequency-assisted lipolysis is one of the newest technologies in lipocontouring. NeckTite (Invasix Aesthetic Solutions) is effective for treatment of preplatysmal adiposity and cervicomental lipocontouring; a 2.4-mm bipolar probe that is inserted into the subdermal space and connected with an external electrode emits RF energy and simultaneously coagulates and aspirates adipose tissue. NeckTite also may be used in conjunction with FaceTite (Invasix Aesthetic Solutions), which promotes fibroseptal network remodeling and dermal contraction.2
In the first published investigation of the efficacy and safety of NeckTite, 47 of 55 patients received treatment of slight to moderate SMF (average body mass index [BMI], 25 kg/m2) with NeckTite and FaceTite or NeckTite alone.7 At 6-month follow-up, 87% (48/55) of patients subjectively rated treatment efficacy as satisfactory, and 2 independent physicians rated the improvement between before-and-after frontal and lateral photographs of the submental area as moderate to excellent in 95% (52/55) of all cases. Reported complications in this study were full-thickness burns resulting in minor scarring (2/55 [4%]), neck tissue hardness that resolved with daily massage after 3 months (5/55 [9%]), and transient facial nerve paresis of the mandibular branch that resolved after 2 months (1/55 [2%]).7
NONINVASIVE MODALITIES
RF-Assisted Contouring
Another exciting development in RF technology is truSculpt (Cutera), a noninvasive contouring device that is placed over the epidermis and emits RF energy that preferentially heats fat more than other tissue types. In a single-center prospective trial of efficacy and safety in the treatment of SMF, 17 patients received 2 treatments with truSculpt administered 1 month apart.8 At 1- and 6-month follow-up, 82.3% (14/17) and 52.9% (9/17) of patients showed improvement on physician assessment. Submental circumference and ultrasonographic fat thickness reductions at 1-month follow-up were 1.4 cm (5.7% of pretreatment circumference [P<.001]) and 5.4 mm (9.7% of pretreatment fat thickness [P=.005]), respectively. At further longer-term follow-up to 6 months, submental circumference was 0.9 cm (3.8% of pretreatment circumference [P<.001]) and ultrasonographic fat reduction was 6.8 mm (10.5% of pretreatment fat thickness [P=.006]). Commonly reported AEs were pain (rate not given), erythema (8/17 [47%]), edema (1/17 [6%]), and vesicle formation (1/17 [6%]); all were self-resolving. Erythema usually subsided within 6 hours posttreatment. No other AEs or complications were reported.8
Deoxycholic Acid
Deoxycholic acid (DCA)(formerly ATX-101) is an injectable liquid formulation of synthetic DCA that was approved by the US Food and Drug Administration (FDA) in 2015 for moderate to severe SMF. Deoxycholic acid exists endogenously as a bile salt emulsifier and has been shown to cause dose-dependent adipocyte lysis, necrosis, disruption and dissolution of fat architecture, and inflammatory targeting of adipocytes by immune cells.9,10 Thus, DCA causes targeted adipocytolysis and is a novel medical agent in the treatment of SMF. Supplied in 2-mL vials, clinicians may inject 10 mL at each treatment for up to 6 treatments administered 1 month apart.11
Efficacy
REFINE-1, a pivotal North American–based phase 3 trial, investigated the efficacy and safety of DCA.12 A total of 506 participants with scores of 2 (moderate) or 3 (severe) on the Clinician-Reported Submental Fat Rating Scale (CR-SMFRS) and a mean BMI of 29 kg/m2 were randomized to receive preplatysmal fat injections of 2 mg/cm2 of DCA (n=256) or placebo (n=250). Participants received up to 10 mL of product (mean total of 25 mL of DCA across all visits) at each treatment session for up to 6 sessions depending on individual efficacy, with approximately 28 days between sessions. Sixty-four percent of the treatment group received all 6 treatments. At 12-week follow-up after the last treatment session, 70% of DCA-treated participants versus 18.6% of placebo-treated participants (P<.001) improved by 1 grade or more on the CR-SMFRS and 13.4% versus 0% (P<.001) improved by 2 grades or more. Skin laxity was unchanged or improved in 92.7% of the DCA group and 87.6% of the placebo group.12
REFINE-2, the second of the North American phase 3 trials, had parallel inclusionary criteria and study design and established efficacy of 2 mg/cm2 DCA over placebo in 516 participants (randomized 1:1).13 At 12 weeks posttreatment, 66.5% of DCA-treated participants versus 22.2% of placebo-treated participants improved by 1 grade or more according to the CR-SMFRS (P<.001) and 18.6% versus 3% improved by 2 grades or more in SMF (P<.001). Magnetic resonance imaging analysis of participants in the DCA (n=113) and placebo groups (n=112) showed that 40.2% versus 5.2% (P<.001) exhibited 10% or more reduction in submental volume, with similar comparative rates of SMF thickness reduction via caliper measurements.13
Safety
Safety data from REFINE-1 showed higher rates of treatment-related AEs in DCA-treated participants compared to placebo, including hematoma (70% vs 67.3%), anesthesia (66.9% vs 4.4%), pain (65.4% vs 23.4%), edema (52.9% vs 21.8%), induration (18.3% vs 1.6%), paresthesia (12.8% vs 3.2%), nodule formation (12.5% vs 0.8%), and pruritus (8.6% vs 3.6%).12 In this trial, 11 of 258 cases (4.3%) of marginal mandibular nerve paresis and asymmetric smile occurred, all in DCA-treated participants and with a median duration of 31 days. Dysphagia resolving in a median duration of 4 days occurred in 1.6% (4/258) of DCA-treated participants.12 REFINE-2 exhibited similar rates of common AEs. Complications of note were 14 cases of marginal mandibular nerve paresis (11 in DCA group, 3 in placebo group) attributed to injection technique, 1 case of skin ulceration possibly related to accidental injection into dermis, and 6 cases of dysphagia in DCA participants attributed to higher volume treatment sessions and postinjection swelling. Dysphagia lasted a median of 2.5 days and resolved without sequelae.13
Overall, DCA demonstrated high rates of minor injection-site AEs that resolved without sequelae and could be mitigated by comfort therapies (eg, lidocaine, nonsteroidal anti-inflammatory drugs) as well as understanding the anatomy of the submental region. Adverse effects of particular interest included marginal mandibular nerve palsy, skin ulceration, and dysphagia.12,13
Cryolipolysis
Cryolipolysis is an advancement that utilizes the application of noninvasive cooling temperatures to the skin’s surface to destroy underlying adipocytes based on the concept that lipid-filled cells are more susceptible to cold-induced injury than water-filled cells. Thus, cryolipolysis selectively targets adipose tissue, leading to cell death without harm to surrounding cells and without the need for surgery or injections.14
Cryolipolysis typically is delivered via a vacuum applicator (CoolMini, Zeltiq Aesthetics Inc), which applies temperatures of –10°C (14°F) to the skin in cycles of 60 minutes each. Initially approved by the FDA for treatment of flank adiposity in 2010, cryolipolysis has since been approved for treatment of the abdomen, thighs, and submental area.14 An advantage of cryolipolysis is that it does not require frequent treatment sessions for maximal efficacy.
Efficacy
The efficacy of cryolipolysis in the treatment of SMF was established in a multicenter device investigation resulting in its FDA approval for the submental region.15 Sixty participants with a mean BMI of 31.8 kg/m2 received 1 (1/60) or 2 (59/60) treatment sessions of the submental area administered 6 weeks apart. Primary efficacy assessments included analysis by 3 blinded reviewers who viewed photographs of each participant at baseline, immediately posttreatment, 6 weeks posttreatment, and 12 weeks posttreatment; ultrasonographic measurements of SMF thickness; and a 12-point patient satisfaction questionnaire. Blinded reviewers correctly identified baseline images in 91.4% (55/60) of cases. Ultrasonography confirmed a mean reduction in SMF of 2 mm (P<.0001) or 20% of fat thickness at 12 weeks posttreatment. On subjective patient satisfaction surveys, 83% (50/60) of participants were satisfied with the procedure and 77% (46/60) reported a visible reduction in fat and perceived an improvement in appearance.15
Safety
The most common immediate posttreatment AEs were erythema/purpura (100%), numbness (90%), edema (62%), tingling (30%), blanching (25%), and bruising (3%) at the site of cryolipolysis with resolution within 1 week posttreatment, except for numbness.15 At 6-week follow-up, all AEs had resolved, except continued numbness in 4 participants that resolved by 12-week follow-up. A further event of note was fullness in the throat in 1 participant that was attributed to swelling and resolved at 40 days posttreatment without incident. No serious AEs were reported in this trial.15
A particularly concerning but rare complication that is increasing in awareness is paradoxical adipose hyperplasia following cryolipolysis. Patients may develop firm painless areas of soft tissue enlargements in the area of cryolipolysis typically 3 to 6 months posttreatment.16 The largest published report recorded an incidence rate of 0.46% (n=2, all males) at a single-center institution of 422 cryolipolysis treatments.16 Other incidence rates reported are 0.0051% and 0.78%.17 Causes and associations are not known, though male gender is speculated to increase risk.
Conclusion
This article highlights the available information on advances in minimally invasive and noninvasive treatments for SMF accumulation. The efficacy and safety trials varied in quality and in different methods of end point analysis of SMF reduction. Further, few trials have featured head-to-head comparisons of treatments.
Although liposuction and adjuncts remain the gold standard in large-mass lipid removal, these procedures are invasive and exhibit typical risks of surgery. Given its sensitive location, the submental area may require the use of more delicate therapeutic methods, including completely noninvasive devices such as truSculpt and cryolipolysis. Regardless of the chosen treatment, the most important factors in yielding patient satisfaction and SMF improvement are proper patient selection and an understanding of the anatomical source of adiposity to be addressed with the therapeutic modalities.
[polldaddy:9711250]
- Hatef DA, Koshy JC, Sandoval SE, et al. The submental fat compartment of the neck. Semin Plast Surg. 2009;23:288-291.
- Mulholland RS. Nonexcisional, minimally invasive rejuvenation of the neck. Clin Plast Surg. 2014;41:11-31.
- Fakhouri TM, El Tal AK, Abrou AE, et al. Laser-assisted lipolysis: a review. Dermatol Surg. 2012;38:155-169.
- Prado A, Andrades P, Danilla S, et al. A prospective, randomized, double-blind, controlled clinical trial comparing laser-assisted lipoplasty with suction-assisted lipoplasty. Plast Reconstr Surg. 2006;118:1032-1045.
- Valizadeh N, Jalaly NY, Zarghampour M, et al. Evaluation of safety and efficacy of 980-nm diode laser-assisted lipolysis versus traditional liposuction for submental rejuvenation: a randomized clinical trial. J Cosmet Laser Ther. 2016;18:41-45.
- Katz B, McBean J. Laser-assisted lipolysis: a report on complications. J Cosmet Laser Ther. 2008;10:231-233.
- Keramidas E, Rodopoulou S. Radiofrequency-assisted liposuction for neck and lower face adipodermal remodeling and contouring. Plast Reconstr Surg Glob Open. 2016;4:e850.
- Park JH, Kim JI, Park HJ, et al. Evaluation of safety and efficacy of noninvasive radiofrequency technology for submental rejuvenation [published online July 12, 2016]. Lasers Med Sci. 2016;31:1599-1605.
- Yagima Odo ME, Cucé LC, Odo LM, et al. Action of sodium deoxycholate on subcutaneous human tissue: local and systemic effects. Dermatol Surg. 2007;33:178-188; discussion 188-189.
- Rotunda AM, Suzuki H, Moy RL, et al. Detergent effects of sodium deoxycholate are a major feature of an injectable phosphatidylcholine formulation used for localized fat dissolution. Dermatol Surg. 2004;30:1001-1008.
- Kybella [package insert]. Westlake Village, CA: Kythera Biopharmaceuticals, Inc; 2015.
- Jones DH, Carruthers J, Joseph JH, et al. REFINE-1, a multicenter, randomized, double-blind, placebo-controlled, phase 3 trial with ATX-101, an injectable drug for submental fat reduction. Dermatol Surg. 2016;42:38-49.
- Humphrey S, Sykes J, Kantor J, et al. ATX-101 for reduction of submental fat: a phase III randomized controlled trial [published online July 16, 2016]. J Am Acad Dermatol. 2016;75:788-797.e7.
- Manstein D, Laubach H, Watanabe K, et al. Selective cryolysis: a novel method of non-invasive fat removal. Lasers Surg Med. 2008;40:595-604.
- Kilmer SL, Burns AJ, Zelickson BD. Safety and efficacy of cryolipolysis for non-invasive reduction of submental fat. Lasers Surg Med. 2016;48:3-13.
- Singh SM, Geddes ER, Boutrous SG, et al. Paradoxical adipose hyperplasia secondary to cryolipolysis: an underreported entity? Lasers Surg Med. 2015;47:476-478.
- Kelly E, Rodriguez-Feliz J, Kelly ME. Paradoxical adipose hyperplasia after cryolipolysis: a report on incidence and common factors identified in 510 patients. Plast Reconst Surg. 2016;137:639e-640e.
- Hatef DA, Koshy JC, Sandoval SE, et al. The submental fat compartment of the neck. Semin Plast Surg. 2009;23:288-291.
- Mulholland RS. Nonexcisional, minimally invasive rejuvenation of the neck. Clin Plast Surg. 2014;41:11-31.
- Fakhouri TM, El Tal AK, Abrou AE, et al. Laser-assisted lipolysis: a review. Dermatol Surg. 2012;38:155-169.
- Prado A, Andrades P, Danilla S, et al. A prospective, randomized, double-blind, controlled clinical trial comparing laser-assisted lipoplasty with suction-assisted lipoplasty. Plast Reconstr Surg. 2006;118:1032-1045.
- Valizadeh N, Jalaly NY, Zarghampour M, et al. Evaluation of safety and efficacy of 980-nm diode laser-assisted lipolysis versus traditional liposuction for submental rejuvenation: a randomized clinical trial. J Cosmet Laser Ther. 2016;18:41-45.
- Katz B, McBean J. Laser-assisted lipolysis: a report on complications. J Cosmet Laser Ther. 2008;10:231-233.
- Keramidas E, Rodopoulou S. Radiofrequency-assisted liposuction for neck and lower face adipodermal remodeling and contouring. Plast Reconstr Surg Glob Open. 2016;4:e850.
- Park JH, Kim JI, Park HJ, et al. Evaluation of safety and efficacy of noninvasive radiofrequency technology for submental rejuvenation [published online July 12, 2016]. Lasers Med Sci. 2016;31:1599-1605.
- Yagima Odo ME, Cucé LC, Odo LM, et al. Action of sodium deoxycholate on subcutaneous human tissue: local and systemic effects. Dermatol Surg. 2007;33:178-188; discussion 188-189.
- Rotunda AM, Suzuki H, Moy RL, et al. Detergent effects of sodium deoxycholate are a major feature of an injectable phosphatidylcholine formulation used for localized fat dissolution. Dermatol Surg. 2004;30:1001-1008.
- Kybella [package insert]. Westlake Village, CA: Kythera Biopharmaceuticals, Inc; 2015.
- Jones DH, Carruthers J, Joseph JH, et al. REFINE-1, a multicenter, randomized, double-blind, placebo-controlled, phase 3 trial with ATX-101, an injectable drug for submental fat reduction. Dermatol Surg. 2016;42:38-49.
- Humphrey S, Sykes J, Kantor J, et al. ATX-101 for reduction of submental fat: a phase III randomized controlled trial [published online July 16, 2016]. J Am Acad Dermatol. 2016;75:788-797.e7.
- Manstein D, Laubach H, Watanabe K, et al. Selective cryolysis: a novel method of non-invasive fat removal. Lasers Surg Med. 2008;40:595-604.
- Kilmer SL, Burns AJ, Zelickson BD. Safety and efficacy of cryolipolysis for non-invasive reduction of submental fat. Lasers Surg Med. 2016;48:3-13.
- Singh SM, Geddes ER, Boutrous SG, et al. Paradoxical adipose hyperplasia secondary to cryolipolysis: an underreported entity? Lasers Surg Med. 2015;47:476-478.
- Kelly E, Rodriguez-Feliz J, Kelly ME. Paradoxical adipose hyperplasia after cryolipolysis: a report on incidence and common factors identified in 510 patients. Plast Reconst Surg. 2016;137:639e-640e.
Practice Points
- New developments in minimally invasive techniques for treating submental adiposity include laser-assisted and radiofrequency-assisted lipoplasty with demonstrated clinical benefit and acceptable safety.
- Noninvasive treatments for submental adiposity include radiofrequency-assisted contouring devices, deoxycholic acid, and cryolipolysis, which offer an alternative to more invasive procedures such as lipoplasty.
- There are no comparative studies to date to suggest noninferiority of these noninvasive treatments compared to lipoplasty.
Efficacy and Safety of New Dermal Fillers
Facial aging is the result of the interplay between loss of skin elasticity, changes in subcutaneous fat and other soft-tissue layers, and skeletal remodeling with chronological age.1 Dermal fillers are effective for the treatment of rhytides, facial scars, and lipoatrophy, as well as facial contouring and augmentation. Given that multiple filler options exist, updated reviews are necessary to inform clinicians of the choices that are available. We provide a detailed review of the clinical efficacy and safety of the dermal fillers with the most recent approvals by the US Food and Drug Administration (FDA).
Polymethylmethacrylate
Polymethylmethacrylate (PMMA) microspheres suspended in bovine collagen and lidocaine 0.3% were approved in 2006 for use in nasolabial folds (NLFs) and in 2014 for acne scars. Now branded as Bellafill (Suneva Medical, Inc), it is the only permanent injectable filler currently available. Once injected, the particles are not reabsorbed and can only be removed by procedural extraction (eg, liposuction of the surrounding fat); however, the permanence of PMMA does not extend to facial rejuvenation, which can last up to 5 years. Prior to use, skin testing for bovine collagen reaction is necessary. In a clinical trial of 147 patients with moderate to severe acne scarring, patients were randomized to receive PMMA in collagen (n=97) or saline (n=50).2 Injections were administered using a linear threading or serial puncture technique, and patients were reevaluated after 4 weeks for touch-up injections. After 6 months, 64% of patients treated with PMMA in collagen achieved improvement in acne scars by 2 points or more on the acne scar rating scale versus 33% of the control group (P=.0005).2
Treatment-related adverse events (AEs) include injection-site pain, bruising, swelling, erythema, and more rarely pruritus and lumps/granulomas.3 A 5-year longitudinal safety investigation of 871 patients initially treated with PMMA in collagen for NLF correction revealed that 17 patients (2.0%) had biopsy-confirmed granulomas with half of these retained at study end.4 Fifteen of these patients were treated with intralesional corticosteroids alone or in combination with intralesional 5-fluorouracil, oral antibiotics, or topical calcineurin inhibitors; 1 patient was untreated and another used topical corticosteroids. The authors noted no correlation between treatment method and granuloma response.4 Polymethylmethacrylate in collagen is contraindicated in patients with lidocaine or bovine collagen sensitivity and is not indicated for use in lip augmentation due to high rates of nodule formation.3
Hyaluronic Acid
Hyaluronic acid (HA) is a naturally occurring glycosaminoglycan polymer found in the extracellular matrix of the dermis. Hyaluronic acid fillers are bacteria derived and come in gel form. A useful advantage of HA fillers compared to other dermal fillers is the commercial availability of hyaluronidase to correct injections. Preinjection skin testing is not necessary.5
This category of nonpermanent dermal fillers has the most robust market choices. Older HA dermal fillers with reliable and proven efficacy are Restylane (Galderma Laboratories, LP)(facial rhytides, lip augmentation), Juvéderm (Ultra/Ultra XC/Ultra Plus/Ultra Plus XC [Allergan, Inc])(facial rhytides, lip augmentation), Hydrelle (Anika Therapeutics, Inc)(facial rhytides), and Prevelle Silk (Mentor Corporation)(facial rhytides); they will not be reviewed here. Newer agents include Belotero Balance (Merz Aesthetics), Juvéderm Voluma XC (Allergan, Inc), Restylane Silk (Galderma Laboratories, LP), and Restylane Lyft (Galderma Laboratories, LP).
Belotero Balance
Belotero Balance is used to treat fine lines and wrinkles, especially NLFs.6 The initial pivotal studies that led to FDA approval in 2011 demonstrated noninferiority and superiority to bovine collagen for use in the treatment of NLFs.7,8 One hundred eighteen patients with bilateral NLFs that were rated as 2 (moderate) or 3 (severe) on the wrinkle severity rating scale (WSRS) were randomized to split-face injection of Belotero Balance in one NLF and bovine collagen in the contralateral NLF.7 An additional injection at week 2 was allowed for optimal correction. Belotero Balance was noninferior to bovine collagen at week 2, with mean improvement in WSRS of 1.52 versus 1.57 (P=.50). Belotero Balance was superior to bovine collagen in mean WSRS improvement at weeks 12 (1.25 vs 0.26; P<.001), 16 (1.09 vs 0.66; P<.001), and 24 (1.08 vs 0.50; P<.001).7 In a subsequent open-label extension study, which included 95 of 118 patients who received Belotero Balance injections in both NLFs at week 24, 80.2% of patients showed sustained improvement in WSRS from baseline for 48 weeks without further injection.8
The first comparative study of Belotero Balance with other established HA fillers at the time—Restylane and Juvéderm Ultra 3/Ultra Plus XC—to treat NLFs demonstrated noninferiority.9 Forty patients with bilateral, moderate to severe NLFs (rated 3 or 4 on the Merz severity scale) were randomized to split-face groups of Belotero Balance versus Restylane or Belotero Balance versus Juvéderm. At 12 months, NLF severity improved from 2.3 to 1.5 in the Restylane group and from 2.3 to 1.6 in the Juvéderm group.9
Belotero Balance has been compared to Juvéderm Ultra XC for use in perioral lines.10 The study included 136 patients with moderate to severe perioral lines, according to the perioral lines severity scale, who were randomized (1:1 ratio) to receive injections of Belotero Balance or Juvéderm Ultra XC to correct upper and lower perioral lines, with assessment at week 2 for optimization. After 6 months, 87% of Juvéderm-treated patients compared to 72% of Belotero Balance–treated patients had 1-point improvement in perioral lines (P<.04). Juvéderm-treated patients also reported significantly less pain than Belotero Balance–treated patients (P<.001).10
Treatment-related AEs are described in the Table, with the majority occurring at lower rates compared to a collagen control group and self-resolving within 2 weeks.7
Juvéderm Voluma XC
Juvéderm Voluma XC was FDA approved in 2013 for cheek augmentation to correct age-related volume deficit restoration by subcutaneous or subperiosteal injections. In its landmark multicenter investigation, 282 patients with moderate to severe midface (eg, zygomaticomalar, anteromedial cheek, submalar regions) volume deficit measured on a validated midface volume deficit scale (MFVDS) were treated with Juvéderm Voluma XC (n=235) or control (n=47).11 Patients were reevaluated at 30 days and 81.9% received touch-up injections. At a 6-month primary evaluation, 86% of the Juvéderm-treated patients versus 39% of the control patients showed 1-point improvement on the MFVDS (P<.001). At 24-months’ follow-up, 44.6% of patients sustained efficacy.11 Of these aforementioned patients, 167 received repeat treatment due to lost correction or patient request and 91.1% improved by 1 point or more on the MFVDS on evaluation 12 months after repeat treatment.12 For this same population of patients, a 2-year extended follow-up of patient-reported outcomes revealed that 49% of patients felt fulfilled in their treatment goals 2 years after treatment and 79% of patients rated improvement from baseline based on the global aesthetic improvement scale.13 Efficacy studies involving Juvéderm Voluma XC are currently ongoing for facial temporal aging (registered at www.clinicaltrials.gov with the identifier NCT02437903) and recruiting for mandibular hypoplasia (NCT02330016).
Common treatment-related AEs are detailed in the Table. Two patients required treatment with hyaluronidase for chronic lumpiness and nodularity following non–treatment-related cellulitis.11 The product is contraindicated in patients with allergy to lidocaine.
Restylane Silk
Restylane Silk was approved in 2014 for lip augmentation and perioral rhytides. Efficacy and safety was demonstrated in a large multicenter randomized investigation in which 221 patients seeking lip augmentation received either Restylane Silk (n=177) injected submucosally for treatment of the upper and lower lips and/or intradermally for perioral rhytides or no treatment (n=44).14 Restylane treatment group patients optionally received touch-up at 2 weeks for optimization. All patients, including the control group, received injections at 6 months. At the 2-month primary end point, 80.2% of the treatment group exhibited at least 1-point improvement in upper lip fullness on the Medicis lip fullness scale compared to 11.9% (P<.001) of the control group; response rates for the lower lips were 84.2% versus 18.4% (P<.001). Patients in the treatment group receiving injections for perioral rhytides showed significant improvement in perioral rhytides through week 24 compared to patients treated for lip augmentation only (P<.001).14 Restylane Silk currently is undergoing investigation for cheek rejuvenation (NCT02636894, NCT02679924) and treatment of hand photoaging (NCT02780258).
The most common AEs are listed in the Table. No lip disorders were considered clinically concerning on evaluation. Concomitant lip augmentation and treatment of perioral rhytides yielded similar rates of AEs.14 Restylane Silk is not to be used in patients with known lidocaine allergy.
Restylane Lyft
Restylane Lyft (formerly known as Perlane-L) was approved in 2010 for use in facial rhytides, including NLFs, and gained approval in 2015 for use in cheek augmentation and midface contouring. Only its efficacy and safety for the more recent indication will be reviewed here.
In an evaluator-blinded investigation of 200 patients with mild to substantial bilateral midface deficiency based on the Medicis midface volume scale (MMVS), patients were randomized to receive supraperiosteal and subcutaneous treatment with Restylane Lyft (n=150) or no treatment (n=50).15 Touch-up injections at week 2 or month 12 were available to treatment group patients and all patients were given either an initial treatment or retreatment at 12 months. Primary end point evaluation at week 8 showed that 89% of treatment group patients had at least 1 grade MMVS improvement compared to 16% of the control group (P<.001). Although the percentage of these MMVS responders in the treatment group decreased with each follow-up period to 54.3% at month 12, retreatment was effective in reproducing a similar MMVS response rate as with initial treatment.15 Restylane Lyft is under ongoing investigation for dorsal hand rejuvenation (NCT02650921).
In addition to the common treatment-related AEs listed in the Table, 2 patients reported serious AEs, including bilateral implant-site inflammation and unilateral implant-site hematoma and infection (organism not described), all of which resolved with unspecified treatment.15 Lidocaine allergies are contraindications for use.
Conclusion
Several new options in dermal fillers have been approved in recent years and have demonstrated efficacy and acceptable safety in various cosmetic rejuvenation applications. Restylane Silk and Restylane Lyft are undergoing further studies to evaluate use in hand rejuvenation, an area that currently has few cosmetic filler treatment options. As technology continues to progress and new formulations of dermal fillers with varied properties and benefits are available, clinicians should expect multiple options for use in rhytides, volume deficits, and contouring.
ADDENDUM
After the manuscript was accepted for publication, Juvéderm Volbella XC (Allergan, Inc) was approved by the FDA for use in lip augmentation and thus is not included in this review.
- Fitzgerald R, Graivier MH, Kane M, et al. Update on facial aging. Aesthet Surg J. 2010;30(suppl):S11-S24.
- Karnik J, Baumann L, Bruce S, et al. A double-blind, randomized, multicenter, controlled trial of suspended polymethylmethacrylate microspheres for the correction of atrophic facial acne scars. J Am Acad Dermatol. 2014;71:77-83.
- Bellafill [package insert]. San Diego, CA: Suneva Medical, Inc; 2015.
- Cohen S, Dover J, Monheit G, et al. Five-year safety and satisfaction study of PMMA-collagen in the correction of nasolabial folds. Dermatol Surg. 2015;41(suppl 1):S302-S313.
- Greene JJ, Sidle DM. The hyaluronic acid fillers: current understanding of the tissue device interface. Facial Plast Surg Clin North Am. 2015;23:423-432.
- Lorenc ZP, Fagien S, Flynn TC, et al. Review of key Belotero Balance safety and efficacy trials. Plast Reconstr Surg. 2013;132(4, suppl 2):33S-40S.
- Narins RS, Coleman W, Donofrio L, et al. Nonanimal sourced hyaluronic acid–based dermal filler using a cohesive polydensified matrix technology is superior to bovine collagen in the correction of moderate to severe nasolabial folds: results from a 6-month, randomized, blinded, controlled, multicenter study. Dermatol Surg. 2010;36(suppl 1):730-740.
- Narins RS, Coleman WP 3rd, Donofrio LM, et al. Improvement in nasolabial folds with a hyaluronic acid filler using a cohesive polydensified matrix technology: results from an 18-month open-label extension trial. Dermatol Surg. 2010;36(suppl 3):1800-1808.
- Prager W, Wissmueller E, Havermann I, et al. A prospective, split-face, randomized, comparative study of safety and 12-month longevity of three formulations of hyaluronic acid dermal filler for treatment of nasolabial folds. Dermatol Surg. 2012;38(7, pt 2):1143-1150.
- Butterwick K, Marmur E, Narurkar V, et al. HYC-24L demonstrates greater effectiveness with less pain than CPM-22.5 for treatment of perioral lines in a randomized controlled trial. Dermatol Surg. 2015;41:1351-1360.
- Jones D, Murphy DK. Volumizing hyaluronic acid filler for midface volume deficit: 2-year results from a pivotal single-blind randomized controlled study. Dermatol Surg. 2013;39:1602-1612.
- Baumann L, Narins RS, Beer K, et al. Volumizing hyaluronic acid filler for midface volume deficit: results after repeat treatment. Dermatol Surg. 2015;41(suppl 1):S284-S292.
- Few J, Cox SE, Paradkar-Mitragotri D, et al. A multicenter, single-blind randomized, controlled study of a volumizing hyaluronic acid filler for midface volume deficit: patient-reported outcomes at 2 years. Aesthet Surg J. 2015;35:589-599.
- Beer K, Glogau RG, Dover JS, et al. A randomized, evaluator-blinded, controlled study of effectiveness and safety of small particle hyaluronic acid plus lidocaine for lip augmentation and perioral rhytides. Dermatol Surg. 2015;41(suppl 1):S127-S136.
- Weiss RA, Moradi A, Bank D, et al. Effectiveness and safety of large gel particle hyaluronic acid with lidocaine for correction of midface volume deficit or contour deficiency. Dermatol Surg. 2016;42:699-709.
Facial aging is the result of the interplay between loss of skin elasticity, changes in subcutaneous fat and other soft-tissue layers, and skeletal remodeling with chronological age.1 Dermal fillers are effective for the treatment of rhytides, facial scars, and lipoatrophy, as well as facial contouring and augmentation. Given that multiple filler options exist, updated reviews are necessary to inform clinicians of the choices that are available. We provide a detailed review of the clinical efficacy and safety of the dermal fillers with the most recent approvals by the US Food and Drug Administration (FDA).
Polymethylmethacrylate
Polymethylmethacrylate (PMMA) microspheres suspended in bovine collagen and lidocaine 0.3% were approved in 2006 for use in nasolabial folds (NLFs) and in 2014 for acne scars. Now branded as Bellafill (Suneva Medical, Inc), it is the only permanent injectable filler currently available. Once injected, the particles are not reabsorbed and can only be removed by procedural extraction (eg, liposuction of the surrounding fat); however, the permanence of PMMA does not extend to facial rejuvenation, which can last up to 5 years. Prior to use, skin testing for bovine collagen reaction is necessary. In a clinical trial of 147 patients with moderate to severe acne scarring, patients were randomized to receive PMMA in collagen (n=97) or saline (n=50).2 Injections were administered using a linear threading or serial puncture technique, and patients were reevaluated after 4 weeks for touch-up injections. After 6 months, 64% of patients treated with PMMA in collagen achieved improvement in acne scars by 2 points or more on the acne scar rating scale versus 33% of the control group (P=.0005).2
Treatment-related adverse events (AEs) include injection-site pain, bruising, swelling, erythema, and more rarely pruritus and lumps/granulomas.3 A 5-year longitudinal safety investigation of 871 patients initially treated with PMMA in collagen for NLF correction revealed that 17 patients (2.0%) had biopsy-confirmed granulomas with half of these retained at study end.4 Fifteen of these patients were treated with intralesional corticosteroids alone or in combination with intralesional 5-fluorouracil, oral antibiotics, or topical calcineurin inhibitors; 1 patient was untreated and another used topical corticosteroids. The authors noted no correlation between treatment method and granuloma response.4 Polymethylmethacrylate in collagen is contraindicated in patients with lidocaine or bovine collagen sensitivity and is not indicated for use in lip augmentation due to high rates of nodule formation.3
Hyaluronic Acid
Hyaluronic acid (HA) is a naturally occurring glycosaminoglycan polymer found in the extracellular matrix of the dermis. Hyaluronic acid fillers are bacteria derived and come in gel form. A useful advantage of HA fillers compared to other dermal fillers is the commercial availability of hyaluronidase to correct injections. Preinjection skin testing is not necessary.5
This category of nonpermanent dermal fillers has the most robust market choices. Older HA dermal fillers with reliable and proven efficacy are Restylane (Galderma Laboratories, LP)(facial rhytides, lip augmentation), Juvéderm (Ultra/Ultra XC/Ultra Plus/Ultra Plus XC [Allergan, Inc])(facial rhytides, lip augmentation), Hydrelle (Anika Therapeutics, Inc)(facial rhytides), and Prevelle Silk (Mentor Corporation)(facial rhytides); they will not be reviewed here. Newer agents include Belotero Balance (Merz Aesthetics), Juvéderm Voluma XC (Allergan, Inc), Restylane Silk (Galderma Laboratories, LP), and Restylane Lyft (Galderma Laboratories, LP).
Belotero Balance
Belotero Balance is used to treat fine lines and wrinkles, especially NLFs.6 The initial pivotal studies that led to FDA approval in 2011 demonstrated noninferiority and superiority to bovine collagen for use in the treatment of NLFs.7,8 One hundred eighteen patients with bilateral NLFs that were rated as 2 (moderate) or 3 (severe) on the wrinkle severity rating scale (WSRS) were randomized to split-face injection of Belotero Balance in one NLF and bovine collagen in the contralateral NLF.7 An additional injection at week 2 was allowed for optimal correction. Belotero Balance was noninferior to bovine collagen at week 2, with mean improvement in WSRS of 1.52 versus 1.57 (P=.50). Belotero Balance was superior to bovine collagen in mean WSRS improvement at weeks 12 (1.25 vs 0.26; P<.001), 16 (1.09 vs 0.66; P<.001), and 24 (1.08 vs 0.50; P<.001).7 In a subsequent open-label extension study, which included 95 of 118 patients who received Belotero Balance injections in both NLFs at week 24, 80.2% of patients showed sustained improvement in WSRS from baseline for 48 weeks without further injection.8
The first comparative study of Belotero Balance with other established HA fillers at the time—Restylane and Juvéderm Ultra 3/Ultra Plus XC—to treat NLFs demonstrated noninferiority.9 Forty patients with bilateral, moderate to severe NLFs (rated 3 or 4 on the Merz severity scale) were randomized to split-face groups of Belotero Balance versus Restylane or Belotero Balance versus Juvéderm. At 12 months, NLF severity improved from 2.3 to 1.5 in the Restylane group and from 2.3 to 1.6 in the Juvéderm group.9
Belotero Balance has been compared to Juvéderm Ultra XC for use in perioral lines.10 The study included 136 patients with moderate to severe perioral lines, according to the perioral lines severity scale, who were randomized (1:1 ratio) to receive injections of Belotero Balance or Juvéderm Ultra XC to correct upper and lower perioral lines, with assessment at week 2 for optimization. After 6 months, 87% of Juvéderm-treated patients compared to 72% of Belotero Balance–treated patients had 1-point improvement in perioral lines (P<.04). Juvéderm-treated patients also reported significantly less pain than Belotero Balance–treated patients (P<.001).10
Treatment-related AEs are described in the Table, with the majority occurring at lower rates compared to a collagen control group and self-resolving within 2 weeks.7
Juvéderm Voluma XC
Juvéderm Voluma XC was FDA approved in 2013 for cheek augmentation to correct age-related volume deficit restoration by subcutaneous or subperiosteal injections. In its landmark multicenter investigation, 282 patients with moderate to severe midface (eg, zygomaticomalar, anteromedial cheek, submalar regions) volume deficit measured on a validated midface volume deficit scale (MFVDS) were treated with Juvéderm Voluma XC (n=235) or control (n=47).11 Patients were reevaluated at 30 days and 81.9% received touch-up injections. At a 6-month primary evaluation, 86% of the Juvéderm-treated patients versus 39% of the control patients showed 1-point improvement on the MFVDS (P<.001). At 24-months’ follow-up, 44.6% of patients sustained efficacy.11 Of these aforementioned patients, 167 received repeat treatment due to lost correction or patient request and 91.1% improved by 1 point or more on the MFVDS on evaluation 12 months after repeat treatment.12 For this same population of patients, a 2-year extended follow-up of patient-reported outcomes revealed that 49% of patients felt fulfilled in their treatment goals 2 years after treatment and 79% of patients rated improvement from baseline based on the global aesthetic improvement scale.13 Efficacy studies involving Juvéderm Voluma XC are currently ongoing for facial temporal aging (registered at www.clinicaltrials.gov with the identifier NCT02437903) and recruiting for mandibular hypoplasia (NCT02330016).
Common treatment-related AEs are detailed in the Table. Two patients required treatment with hyaluronidase for chronic lumpiness and nodularity following non–treatment-related cellulitis.11 The product is contraindicated in patients with allergy to lidocaine.
Restylane Silk
Restylane Silk was approved in 2014 for lip augmentation and perioral rhytides. Efficacy and safety was demonstrated in a large multicenter randomized investigation in which 221 patients seeking lip augmentation received either Restylane Silk (n=177) injected submucosally for treatment of the upper and lower lips and/or intradermally for perioral rhytides or no treatment (n=44).14 Restylane treatment group patients optionally received touch-up at 2 weeks for optimization. All patients, including the control group, received injections at 6 months. At the 2-month primary end point, 80.2% of the treatment group exhibited at least 1-point improvement in upper lip fullness on the Medicis lip fullness scale compared to 11.9% (P<.001) of the control group; response rates for the lower lips were 84.2% versus 18.4% (P<.001). Patients in the treatment group receiving injections for perioral rhytides showed significant improvement in perioral rhytides through week 24 compared to patients treated for lip augmentation only (P<.001).14 Restylane Silk currently is undergoing investigation for cheek rejuvenation (NCT02636894, NCT02679924) and treatment of hand photoaging (NCT02780258).
The most common AEs are listed in the Table. No lip disorders were considered clinically concerning on evaluation. Concomitant lip augmentation and treatment of perioral rhytides yielded similar rates of AEs.14 Restylane Silk is not to be used in patients with known lidocaine allergy.
Restylane Lyft
Restylane Lyft (formerly known as Perlane-L) was approved in 2010 for use in facial rhytides, including NLFs, and gained approval in 2015 for use in cheek augmentation and midface contouring. Only its efficacy and safety for the more recent indication will be reviewed here.
In an evaluator-blinded investigation of 200 patients with mild to substantial bilateral midface deficiency based on the Medicis midface volume scale (MMVS), patients were randomized to receive supraperiosteal and subcutaneous treatment with Restylane Lyft (n=150) or no treatment (n=50).15 Touch-up injections at week 2 or month 12 were available to treatment group patients and all patients were given either an initial treatment or retreatment at 12 months. Primary end point evaluation at week 8 showed that 89% of treatment group patients had at least 1 grade MMVS improvement compared to 16% of the control group (P<.001). Although the percentage of these MMVS responders in the treatment group decreased with each follow-up period to 54.3% at month 12, retreatment was effective in reproducing a similar MMVS response rate as with initial treatment.15 Restylane Lyft is under ongoing investigation for dorsal hand rejuvenation (NCT02650921).
In addition to the common treatment-related AEs listed in the Table, 2 patients reported serious AEs, including bilateral implant-site inflammation and unilateral implant-site hematoma and infection (organism not described), all of which resolved with unspecified treatment.15 Lidocaine allergies are contraindications for use.
Conclusion
Several new options in dermal fillers have been approved in recent years and have demonstrated efficacy and acceptable safety in various cosmetic rejuvenation applications. Restylane Silk and Restylane Lyft are undergoing further studies to evaluate use in hand rejuvenation, an area that currently has few cosmetic filler treatment options. As technology continues to progress and new formulations of dermal fillers with varied properties and benefits are available, clinicians should expect multiple options for use in rhytides, volume deficits, and contouring.
ADDENDUM
After the manuscript was accepted for publication, Juvéderm Volbella XC (Allergan, Inc) was approved by the FDA for use in lip augmentation and thus is not included in this review.
Facial aging is the result of the interplay between loss of skin elasticity, changes in subcutaneous fat and other soft-tissue layers, and skeletal remodeling with chronological age.1 Dermal fillers are effective for the treatment of rhytides, facial scars, and lipoatrophy, as well as facial contouring and augmentation. Given that multiple filler options exist, updated reviews are necessary to inform clinicians of the choices that are available. We provide a detailed review of the clinical efficacy and safety of the dermal fillers with the most recent approvals by the US Food and Drug Administration (FDA).
Polymethylmethacrylate
Polymethylmethacrylate (PMMA) microspheres suspended in bovine collagen and lidocaine 0.3% were approved in 2006 for use in nasolabial folds (NLFs) and in 2014 for acne scars. Now branded as Bellafill (Suneva Medical, Inc), it is the only permanent injectable filler currently available. Once injected, the particles are not reabsorbed and can only be removed by procedural extraction (eg, liposuction of the surrounding fat); however, the permanence of PMMA does not extend to facial rejuvenation, which can last up to 5 years. Prior to use, skin testing for bovine collagen reaction is necessary. In a clinical trial of 147 patients with moderate to severe acne scarring, patients were randomized to receive PMMA in collagen (n=97) or saline (n=50).2 Injections were administered using a linear threading or serial puncture technique, and patients were reevaluated after 4 weeks for touch-up injections. After 6 months, 64% of patients treated with PMMA in collagen achieved improvement in acne scars by 2 points or more on the acne scar rating scale versus 33% of the control group (P=.0005).2
Treatment-related adverse events (AEs) include injection-site pain, bruising, swelling, erythema, and more rarely pruritus and lumps/granulomas.3 A 5-year longitudinal safety investigation of 871 patients initially treated with PMMA in collagen for NLF correction revealed that 17 patients (2.0%) had biopsy-confirmed granulomas with half of these retained at study end.4 Fifteen of these patients were treated with intralesional corticosteroids alone or in combination with intralesional 5-fluorouracil, oral antibiotics, or topical calcineurin inhibitors; 1 patient was untreated and another used topical corticosteroids. The authors noted no correlation between treatment method and granuloma response.4 Polymethylmethacrylate in collagen is contraindicated in patients with lidocaine or bovine collagen sensitivity and is not indicated for use in lip augmentation due to high rates of nodule formation.3
Hyaluronic Acid
Hyaluronic acid (HA) is a naturally occurring glycosaminoglycan polymer found in the extracellular matrix of the dermis. Hyaluronic acid fillers are bacteria derived and come in gel form. A useful advantage of HA fillers compared to other dermal fillers is the commercial availability of hyaluronidase to correct injections. Preinjection skin testing is not necessary.5
This category of nonpermanent dermal fillers has the most robust market choices. Older HA dermal fillers with reliable and proven efficacy are Restylane (Galderma Laboratories, LP)(facial rhytides, lip augmentation), Juvéderm (Ultra/Ultra XC/Ultra Plus/Ultra Plus XC [Allergan, Inc])(facial rhytides, lip augmentation), Hydrelle (Anika Therapeutics, Inc)(facial rhytides), and Prevelle Silk (Mentor Corporation)(facial rhytides); they will not be reviewed here. Newer agents include Belotero Balance (Merz Aesthetics), Juvéderm Voluma XC (Allergan, Inc), Restylane Silk (Galderma Laboratories, LP), and Restylane Lyft (Galderma Laboratories, LP).
Belotero Balance
Belotero Balance is used to treat fine lines and wrinkles, especially NLFs.6 The initial pivotal studies that led to FDA approval in 2011 demonstrated noninferiority and superiority to bovine collagen for use in the treatment of NLFs.7,8 One hundred eighteen patients with bilateral NLFs that were rated as 2 (moderate) or 3 (severe) on the wrinkle severity rating scale (WSRS) were randomized to split-face injection of Belotero Balance in one NLF and bovine collagen in the contralateral NLF.7 An additional injection at week 2 was allowed for optimal correction. Belotero Balance was noninferior to bovine collagen at week 2, with mean improvement in WSRS of 1.52 versus 1.57 (P=.50). Belotero Balance was superior to bovine collagen in mean WSRS improvement at weeks 12 (1.25 vs 0.26; P<.001), 16 (1.09 vs 0.66; P<.001), and 24 (1.08 vs 0.50; P<.001).7 In a subsequent open-label extension study, which included 95 of 118 patients who received Belotero Balance injections in both NLFs at week 24, 80.2% of patients showed sustained improvement in WSRS from baseline for 48 weeks without further injection.8
The first comparative study of Belotero Balance with other established HA fillers at the time—Restylane and Juvéderm Ultra 3/Ultra Plus XC—to treat NLFs demonstrated noninferiority.9 Forty patients with bilateral, moderate to severe NLFs (rated 3 or 4 on the Merz severity scale) were randomized to split-face groups of Belotero Balance versus Restylane or Belotero Balance versus Juvéderm. At 12 months, NLF severity improved from 2.3 to 1.5 in the Restylane group and from 2.3 to 1.6 in the Juvéderm group.9
Belotero Balance has been compared to Juvéderm Ultra XC for use in perioral lines.10 The study included 136 patients with moderate to severe perioral lines, according to the perioral lines severity scale, who were randomized (1:1 ratio) to receive injections of Belotero Balance or Juvéderm Ultra XC to correct upper and lower perioral lines, with assessment at week 2 for optimization. After 6 months, 87% of Juvéderm-treated patients compared to 72% of Belotero Balance–treated patients had 1-point improvement in perioral lines (P<.04). Juvéderm-treated patients also reported significantly less pain than Belotero Balance–treated patients (P<.001).10
Treatment-related AEs are described in the Table, with the majority occurring at lower rates compared to a collagen control group and self-resolving within 2 weeks.7
Juvéderm Voluma XC
Juvéderm Voluma XC was FDA approved in 2013 for cheek augmentation to correct age-related volume deficit restoration by subcutaneous or subperiosteal injections. In its landmark multicenter investigation, 282 patients with moderate to severe midface (eg, zygomaticomalar, anteromedial cheek, submalar regions) volume deficit measured on a validated midface volume deficit scale (MFVDS) were treated with Juvéderm Voluma XC (n=235) or control (n=47).11 Patients were reevaluated at 30 days and 81.9% received touch-up injections. At a 6-month primary evaluation, 86% of the Juvéderm-treated patients versus 39% of the control patients showed 1-point improvement on the MFVDS (P<.001). At 24-months’ follow-up, 44.6% of patients sustained efficacy.11 Of these aforementioned patients, 167 received repeat treatment due to lost correction or patient request and 91.1% improved by 1 point or more on the MFVDS on evaluation 12 months after repeat treatment.12 For this same population of patients, a 2-year extended follow-up of patient-reported outcomes revealed that 49% of patients felt fulfilled in their treatment goals 2 years after treatment and 79% of patients rated improvement from baseline based on the global aesthetic improvement scale.13 Efficacy studies involving Juvéderm Voluma XC are currently ongoing for facial temporal aging (registered at www.clinicaltrials.gov with the identifier NCT02437903) and recruiting for mandibular hypoplasia (NCT02330016).
Common treatment-related AEs are detailed in the Table. Two patients required treatment with hyaluronidase for chronic lumpiness and nodularity following non–treatment-related cellulitis.11 The product is contraindicated in patients with allergy to lidocaine.
Restylane Silk
Restylane Silk was approved in 2014 for lip augmentation and perioral rhytides. Efficacy and safety was demonstrated in a large multicenter randomized investigation in which 221 patients seeking lip augmentation received either Restylane Silk (n=177) injected submucosally for treatment of the upper and lower lips and/or intradermally for perioral rhytides or no treatment (n=44).14 Restylane treatment group patients optionally received touch-up at 2 weeks for optimization. All patients, including the control group, received injections at 6 months. At the 2-month primary end point, 80.2% of the treatment group exhibited at least 1-point improvement in upper lip fullness on the Medicis lip fullness scale compared to 11.9% (P<.001) of the control group; response rates for the lower lips were 84.2% versus 18.4% (P<.001). Patients in the treatment group receiving injections for perioral rhytides showed significant improvement in perioral rhytides through week 24 compared to patients treated for lip augmentation only (P<.001).14 Restylane Silk currently is undergoing investigation for cheek rejuvenation (NCT02636894, NCT02679924) and treatment of hand photoaging (NCT02780258).
The most common AEs are listed in the Table. No lip disorders were considered clinically concerning on evaluation. Concomitant lip augmentation and treatment of perioral rhytides yielded similar rates of AEs.14 Restylane Silk is not to be used in patients with known lidocaine allergy.
Restylane Lyft
Restylane Lyft (formerly known as Perlane-L) was approved in 2010 for use in facial rhytides, including NLFs, and gained approval in 2015 for use in cheek augmentation and midface contouring. Only its efficacy and safety for the more recent indication will be reviewed here.
In an evaluator-blinded investigation of 200 patients with mild to substantial bilateral midface deficiency based on the Medicis midface volume scale (MMVS), patients were randomized to receive supraperiosteal and subcutaneous treatment with Restylane Lyft (n=150) or no treatment (n=50).15 Touch-up injections at week 2 or month 12 were available to treatment group patients and all patients were given either an initial treatment or retreatment at 12 months. Primary end point evaluation at week 8 showed that 89% of treatment group patients had at least 1 grade MMVS improvement compared to 16% of the control group (P<.001). Although the percentage of these MMVS responders in the treatment group decreased with each follow-up period to 54.3% at month 12, retreatment was effective in reproducing a similar MMVS response rate as with initial treatment.15 Restylane Lyft is under ongoing investigation for dorsal hand rejuvenation (NCT02650921).
In addition to the common treatment-related AEs listed in the Table, 2 patients reported serious AEs, including bilateral implant-site inflammation and unilateral implant-site hematoma and infection (organism not described), all of which resolved with unspecified treatment.15 Lidocaine allergies are contraindications for use.
Conclusion
Several new options in dermal fillers have been approved in recent years and have demonstrated efficacy and acceptable safety in various cosmetic rejuvenation applications. Restylane Silk and Restylane Lyft are undergoing further studies to evaluate use in hand rejuvenation, an area that currently has few cosmetic filler treatment options. As technology continues to progress and new formulations of dermal fillers with varied properties and benefits are available, clinicians should expect multiple options for use in rhytides, volume deficits, and contouring.
ADDENDUM
After the manuscript was accepted for publication, Juvéderm Volbella XC (Allergan, Inc) was approved by the FDA for use in lip augmentation and thus is not included in this review.
- Fitzgerald R, Graivier MH, Kane M, et al. Update on facial aging. Aesthet Surg J. 2010;30(suppl):S11-S24.
- Karnik J, Baumann L, Bruce S, et al. A double-blind, randomized, multicenter, controlled trial of suspended polymethylmethacrylate microspheres for the correction of atrophic facial acne scars. J Am Acad Dermatol. 2014;71:77-83.
- Bellafill [package insert]. San Diego, CA: Suneva Medical, Inc; 2015.
- Cohen S, Dover J, Monheit G, et al. Five-year safety and satisfaction study of PMMA-collagen in the correction of nasolabial folds. Dermatol Surg. 2015;41(suppl 1):S302-S313.
- Greene JJ, Sidle DM. The hyaluronic acid fillers: current understanding of the tissue device interface. Facial Plast Surg Clin North Am. 2015;23:423-432.
- Lorenc ZP, Fagien S, Flynn TC, et al. Review of key Belotero Balance safety and efficacy trials. Plast Reconstr Surg. 2013;132(4, suppl 2):33S-40S.
- Narins RS, Coleman W, Donofrio L, et al. Nonanimal sourced hyaluronic acid–based dermal filler using a cohesive polydensified matrix technology is superior to bovine collagen in the correction of moderate to severe nasolabial folds: results from a 6-month, randomized, blinded, controlled, multicenter study. Dermatol Surg. 2010;36(suppl 1):730-740.
- Narins RS, Coleman WP 3rd, Donofrio LM, et al. Improvement in nasolabial folds with a hyaluronic acid filler using a cohesive polydensified matrix technology: results from an 18-month open-label extension trial. Dermatol Surg. 2010;36(suppl 3):1800-1808.
- Prager W, Wissmueller E, Havermann I, et al. A prospective, split-face, randomized, comparative study of safety and 12-month longevity of three formulations of hyaluronic acid dermal filler for treatment of nasolabial folds. Dermatol Surg. 2012;38(7, pt 2):1143-1150.
- Butterwick K, Marmur E, Narurkar V, et al. HYC-24L demonstrates greater effectiveness with less pain than CPM-22.5 for treatment of perioral lines in a randomized controlled trial. Dermatol Surg. 2015;41:1351-1360.
- Jones D, Murphy DK. Volumizing hyaluronic acid filler for midface volume deficit: 2-year results from a pivotal single-blind randomized controlled study. Dermatol Surg. 2013;39:1602-1612.
- Baumann L, Narins RS, Beer K, et al. Volumizing hyaluronic acid filler for midface volume deficit: results after repeat treatment. Dermatol Surg. 2015;41(suppl 1):S284-S292.
- Few J, Cox SE, Paradkar-Mitragotri D, et al. A multicenter, single-blind randomized, controlled study of a volumizing hyaluronic acid filler for midface volume deficit: patient-reported outcomes at 2 years. Aesthet Surg J. 2015;35:589-599.
- Beer K, Glogau RG, Dover JS, et al. A randomized, evaluator-blinded, controlled study of effectiveness and safety of small particle hyaluronic acid plus lidocaine for lip augmentation and perioral rhytides. Dermatol Surg. 2015;41(suppl 1):S127-S136.
- Weiss RA, Moradi A, Bank D, et al. Effectiveness and safety of large gel particle hyaluronic acid with lidocaine for correction of midface volume deficit or contour deficiency. Dermatol Surg. 2016;42:699-709.
- Fitzgerald R, Graivier MH, Kane M, et al. Update on facial aging. Aesthet Surg J. 2010;30(suppl):S11-S24.
- Karnik J, Baumann L, Bruce S, et al. A double-blind, randomized, multicenter, controlled trial of suspended polymethylmethacrylate microspheres for the correction of atrophic facial acne scars. J Am Acad Dermatol. 2014;71:77-83.
- Bellafill [package insert]. San Diego, CA: Suneva Medical, Inc; 2015.
- Cohen S, Dover J, Monheit G, et al. Five-year safety and satisfaction study of PMMA-collagen in the correction of nasolabial folds. Dermatol Surg. 2015;41(suppl 1):S302-S313.
- Greene JJ, Sidle DM. The hyaluronic acid fillers: current understanding of the tissue device interface. Facial Plast Surg Clin North Am. 2015;23:423-432.
- Lorenc ZP, Fagien S, Flynn TC, et al. Review of key Belotero Balance safety and efficacy trials. Plast Reconstr Surg. 2013;132(4, suppl 2):33S-40S.
- Narins RS, Coleman W, Donofrio L, et al. Nonanimal sourced hyaluronic acid–based dermal filler using a cohesive polydensified matrix technology is superior to bovine collagen in the correction of moderate to severe nasolabial folds: results from a 6-month, randomized, blinded, controlled, multicenter study. Dermatol Surg. 2010;36(suppl 1):730-740.
- Narins RS, Coleman WP 3rd, Donofrio LM, et al. Improvement in nasolabial folds with a hyaluronic acid filler using a cohesive polydensified matrix technology: results from an 18-month open-label extension trial. Dermatol Surg. 2010;36(suppl 3):1800-1808.
- Prager W, Wissmueller E, Havermann I, et al. A prospective, split-face, randomized, comparative study of safety and 12-month longevity of three formulations of hyaluronic acid dermal filler for treatment of nasolabial folds. Dermatol Surg. 2012;38(7, pt 2):1143-1150.
- Butterwick K, Marmur E, Narurkar V, et al. HYC-24L demonstrates greater effectiveness with less pain than CPM-22.5 for treatment of perioral lines in a randomized controlled trial. Dermatol Surg. 2015;41:1351-1360.
- Jones D, Murphy DK. Volumizing hyaluronic acid filler for midface volume deficit: 2-year results from a pivotal single-blind randomized controlled study. Dermatol Surg. 2013;39:1602-1612.
- Baumann L, Narins RS, Beer K, et al. Volumizing hyaluronic acid filler for midface volume deficit: results after repeat treatment. Dermatol Surg. 2015;41(suppl 1):S284-S292.
- Few J, Cox SE, Paradkar-Mitragotri D, et al. A multicenter, single-blind randomized, controlled study of a volumizing hyaluronic acid filler for midface volume deficit: patient-reported outcomes at 2 years. Aesthet Surg J. 2015;35:589-599.
- Beer K, Glogau RG, Dover JS, et al. A randomized, evaluator-blinded, controlled study of effectiveness and safety of small particle hyaluronic acid plus lidocaine for lip augmentation and perioral rhytides. Dermatol Surg. 2015;41(suppl 1):S127-S136.
- Weiss RA, Moradi A, Bank D, et al. Effectiveness and safety of large gel particle hyaluronic acid with lidocaine for correction of midface volume deficit or contour deficiency. Dermatol Surg. 2016;42:699-709.
Practice Points
- The merits of new dermal fillers approved by the US Food and Drug Administration should be weighed with an understanding of aesthetic indications of use, duration of efficacy, and common adverse effects, in line with patient preference.
- The most common adverse effects are injection-site contusion, swelling, and pain, usually self-resolving within days to 2 weeks. Patient quality of care can be improved with forewarning and emphasis on alleviating symptoms.
An Update on Neurotoxin Products and Administration Methods
The first botulinum neurotoxin (BoNT) approved by the US Food and Drug Administration (FDA) was onabotulinumtoxinA in 1989 for the treatment of strabismus and blepharospasm. It was not until 1992, however, that the aesthetic benefits of BoNT were first reported in the medical literature by Carruthers and Carruthers,1 and a cosmetic indication was not approved by the FDA until 2002. Since that time, the popularity of BoNT products has grown rapidly with a nearly 6500% increase in popularity from 1997 to 2015 in addition to the introduction of a variety of new BoNT formulations to the market.2 It is estimated by the American Society for Aesthetic Plastic Surgery that there were at least 4,000,000 BoNT injections performed in 2015 alone, making it the most popular nonsurgical aesthetic procedure available.2 As the demand for minimally invasive cosmetic procedures continues to increase, we will continue to see the introduction of additional formulations of BoNT products as well as novel administration techniques and delivery devices. In this article, we provide an update on current and upcoming BoNT products and also review the literature on novel administration methods based on studies published from January 1, 2014, to December 31, 2015.
Current Products
To date, there are only 4 FDA-approved formulations of BoNT available for clinical use (eg, cervical dystonia, strabismus, blepharospasm, headache, urinary incontinence) in the United States: abobotulinumtoxinA, incobotulinumtoxinA, onabotulinumtoxinA, and rimabotulinumtoxinB.The FDA-approved dermatologic indications (eg, moderate to severe glabellar or canthal lines, severe axillary hyperhidrosis) for these products are provided in the Table. On a global scale, there are several other commonly utilized formulations of BoNT, including a Korean serotype resembling onabotulinumtoxinA and a Chinese botulinum toxin type A.3 Although there is some evidence to demonstrate comparable efficacy and safety of these latter products, the literature is relatively lacking in comparison to the FDA-approved products.4,5
Upcoming Products
Currently, there are several new BoNT formulations being studied for clinical use. RT 002 (Revance Therapeutics, Inc) is a novel injectable formulation of onabotulinumtoxinA that consists of the purified neurotoxin in combination with patented TransMTS peptides that have been shown to provide high-binding avidity for the neurotoxin, and thus the product is designed to reduce diffusion to adjacent muscles and diminish unwanted effects. With a reduced level of neurotoxin dissemination, it is theorized that a higher administration of targeted doses can be injected, which may lead to a longer duration of desired effects.6 A clinical pilot study done to establish the safety and efficacy of RT 002 for treatment of moderate to severe glabellar lines evaluated 4 equally sized cohorts of 12 participants, each receiving single-dose administration of RT 002 ranging in potency equivalent to 25 U, 50 U, 75 U, and 100 U of abobotulinumtoxinA as determined by the gelatin phosphate method.6 It was concluded that RT 002 is both safe and efficacious with an extended duration of action, with a median duration of effect of 7 months observed in the highest dose group (dose equivalent to 100 U of abobotulinumtoxinA). Notably, 80% of all 48 participants maintained a minimum 1-point improvement in investigator-determined glabellar line severity scores at the 6-month time point and 60% achieved wrinkle scores of none or mild at 6 months posttreatment.6
DWP 450 (Daewoong Pharmaceutical Co, Ltd) is derived from the wild-type Clostridium botulinum and is reported to be of higher purity than standard onabotulinumtoxinA. An initial 16-week pilot study demonstrated that 20 U of DWP 450 is noninferior and of comparable efficacy and safety to 20 U of onabotulinumtoxinA in the treatment of glabellar lines.7
NTC (Botulax [Hugel, Inc]) is the name of the toxin derived from the C botulinum strain CBFC26, which has already been approved in many Asian, European, and Latin American countries for the treatment of blepharospasm. This formulation has demonstrated noninferiority to onabotulinumtoxinA at equivalent 20-U doses for the treatment of moderate to severe glabellar lines in a double-blind, randomized, multicenter, phase 3 trial of 272 participants with a 16-week follow-up.8
MT 10109L (Medytox Inc) is a unique product in that it is distributed as a liquid type A botulinum toxin rather than the standard freeze-dried formulation; thus, a major advantage of this product is its convenience, as it does not need reconstitution or dilution prior to administration. In a double-blind, randomized, active drug–controlled, phase 3 study of 168 participants, it was determined that MT 10109L (20 U) is comparable in efficacy to onabotulinumtoxinA (20 U) for the treatment of moderate to severe glabellar lines. No significant difference was seen between the 2 treatment groups when glabellar lines were assessed at rest at 4 and 16 weeks after treatment, but a significantly greater improvement in glabellar lines was seen at maximum frown in the MT 10109L group at the 16-week follow-up (P=.0064).9
Administration Techniques
With regard to safe and effective BoNT product administration techniques, a variety of studies have provided insight into optimal practice methods. A 2015 expert consensus statement formed by an American Society for Dermatologic Surgery task force reviewed data from 42 papers and unanimously determined that for all current type A BoNT products available in the United States, a vial of BoNT reconstituted appropriately for the purpose of facial injections can be reconstituted at least 4 weeks prior to administration without contamination risk or decrease in efficacy and that multiple patients can be treated with the same vial.Although the statement was not explicit on whether or not preserved or unpreserved saline is to be used, it is considered routine practice to use preservative-containing saline to reconstitute BoNT, as it has been shown to reduce patient discomfort and is not associated with adverse effects.10
Pain Minimization
With respect to minimizing the pain associated with BoNT injections, several studies have assessed administration techniques to minimize patient discomfort. A split-face, double-blind study of 20 participants demonstrated that the use of a 32-gauge needle has a significantly greater chance of reducing clinically significant levels of pain as compared to a 30-gauge needle when performing facial injections (P=.04). Overall, however, injections of the face and arms were on average only nominally and not significantly more painful with 30-gauge needles compared to 32-gauge needles.11
Another technique that has been found to reduce patient discomfort is the application of cold packs prior to injection. A study of patients with chronic facial palsy observed a significant reduction in pain with the administration of a cold (3°C–5°C) gel pack for 1 minute compared to a room temperature (20°C) gel pack prior to the administration of onabotulinumtoxinA into the platysma (P<.001).12 In the matter of injection with rimabotulinumtoxinB, which has been shown to be considerably more painful to receive than its more popularly administered counterpart onabotulinumtoxinA, a split-face pilot study examined the effect of increasing the pH of rimabotulinumtoxinB to 7.5 with sodium bicarbonate from the usual pH of 5.6.13,14 Pain was reported to be considerably less in the higher pH group and no reduction of efficacy was seen over the 10-week follow-up period.14
Delivery Methods
Several preliminary studies also have examined novel delivery techniques to identify minimally painful yet effective methods for administering BoNT. It has been reported that standard BoNT formulations are not effective as topical agents in a comparison study in which onabotulinumtoxinA injection was compared to topically applied onabotulinumtoxinA.15 However, a follow-up prospective study by the same authors has demonstrated efficacy of topical onabotulinumtoxinA following pretreatment with a fractional ablative CO2 laser for treatment of crow’s-feet. In this randomized, split-face, controlled trial (N=10), participants were first pretreated with topical lidocaine 30% before receiving a single pass of fractional ablative CO2 laser with no overlap and a pulse energy of 100 mJ. Within 60 seconds of laser treatment, participants then received either 100 U of abobotulinumtoxinA diluted in 0.1 mL of saline or simple normal saline applied topically. A clinically significant improvement in periorbital wrinkles was seen both at 1-week and 1-month posttreatment in the laser and onabotulinumtoxinA–treated group compared to the laser and topical saline–treated group (P<.02).15
Another unique administration method studied, albeit with less successful results, involves the use of iontophoresis to deliver BoNT painlessly in a transdermal fashion with the assistance of an electrical current.16 This prospective, randomized, assessor-blinded, split-axilla, controlled trial of 11 participants compared the effectiveness of administering onabotulinumtoxinA via iontophoresis to traditional injection with onabotulinumtoxinA (250 U). Iontophoresis was accomplished with a single electrode pad soaked with 250 U of onabotulinumtoxinA applied directly to the axilla and a second electrode pad soaked in 0.9% saline applied to the hand to complete the circuit. An alternating electrical current was slowly increased for 30 minutes to a maximum current of 15 mA with a voltage of 12 V. Among the 11 participants recruited, the side treated with traditional injection showed a significantly greater percentage reduction in baseline sweating at the 1-week, 1-month, and 6-month posttreatment evaluations compared to iontophoresis (84%, 76%, and 50%, respectively vs 73%, 22%, and 32%, respectively)(P<.05). Despite being less efficacious than standard injection therapy, participants reported that iontophoresis delivery was significantly less painful (P<.05).16
A high-pressure oxygen delivery device, which utilizes a powerful jet of microdroplets containing water, the drug, air, and oxygen to deliver medication onto the skin surface, also has been studied as a means of delivery of BoNT in a minimally painful manner. In this study, the device was used to assess the efficacy of transdermal delivery of BoNT via jet nebulization in the treatment of primary palmar, plantar, and axillary hyperhidrosis.17 The 20 participants included in the study were randomized to receive either a combination of lidocaine and onabotulinumtoxinA (50 U) administered through the device or lidocaine delivered through the device followed by multiple transcutaneous injections of onabotulinumtoxinA (100 U). Both treatments significantly reduced sweating compared to baseline as measured by a visual analogue scale at 3-month follow-up (P<.001), but the combination delivery of lidocaine and onabotulinumtoxinA via the device resulted in significantly less procedure-related pain and sweating (P<.001) as well as significantly greater patient satisfaction (P<.001).17
Optimizing Aesthetic Outcomes
A frequent concern of patients receiving BoNT for cosmetic purposes is a desire to avoid a “frozen” or expressionless look. As such, many clinicians have attempted a variety of techniques to achieve more natural aesthetic results. One such method is known as the multipoint and multilevel injection technique, which consists of utilizing multiple injection sites at varying depths (intramuscular, subcutaneous, or intradermal) and doses (2–6 U) depending on the degree of contractility of the targeted muscle. In a preliminary study of 223 participants using this technique with a total dose of 125 U of abobotulinumtoxinA, good and natural results were reported with perseveration of facial emotion in all participants in addition to a mean overall satisfaction rate of 6.4 of 7 on the Facial Line Treatment Satisfaction Questionnaire with the maximum satisfaction rating possible reported in 66% of cases.18 It also has been postulated that injection depth of BoNT can affect brow elevation whereupon deeper injection depths can result in inactivation of the brow depressors and allow for increased elevation of the eyebrows. This technique has been applied in attempts to correct brow height asymmetry. However, a prospective, split-face study of 23 women suggested that this method is not effective.19 Participants received 64 U of onabotulinumtoxinA via 16 injection sites in the glabella, forehead, and lateral canthal area with either all deep or all shallow injections depending on the side treated and whether brow-lift was desired. Results at 4 weeks posttreatment showed no significant difference in brow height, and it was concluded that eyebrow depressor muscles cannot be accurately targeted with deep injection into the muscle belly for correction of eyebrow height discrepancies.19 Conversely, a 5-year retrospective, nonrandomized study of 227 patients with 563 treatments utilizing a “microdroplet” technique reported success at selectively targeting the eyebrow depressors while leaving the brow elevators unaffected.20 Here, a total dose of 33 U of onabotulinumtoxinA was administered via microdroplets of 10 to 20 μL, each with more than 60 to 100 injections into the brow, glabella, and crow’s-feet area. This method of injection resulted in a statistically significant improvement of forehead lines and brow ptosis and furrowing at follow-up between 10 and 45 days after treatment (P<.0001). Additionally, average brow height was significantly increased from 24.6 mm to 25 mm after treatment (P=.02).20
Conclusion
The use of BoNT products for both on- and off-label cosmetic and medical indications has rapidly grown over the past 2 decades. As demonstrated in this review, a variety of promising new products and delivery techniques are being developed. Given the rise in popularity of BoNT products among both physicians and consumers, clinicians should be aware of the current data and ongoing research.
- Carruthers JD, Carruthers JA. Treatment of glabellar frown lines with C. botulinum-A exotoxin. J Dermatol Surg Oncol. 1992;18:17-21.
- American Society for Aesthetic Plastic Surgery. Cosmetic Surgery National Data Bank statistics. American Society for Aesthetic Plastic Surgery website. http://www.surgery.org/sites/default/files/ASAPS-Stats2015.pdf. Accessed June 12, 2016.
- Walker TJ, Dayan SH. Comparison and overview of currently available neurotoxins. J Clin Aesthet Dermatol. 2014;7:31-39.
- Feng Z, Sun Q, He L, et al. Optimal dosage of botulinum toxin type A for treatment of glabellar frown lines: efficacy and safety in a clinical trial. Dermatol Surg. 2015;41(suppl 1):S56-S63.
- Jiang HY, Chen S, Zhou J, et al. Diffusion of two botulinum toxins type A on the forehead: double-blinded, randomized, controlled study. Dermatol Surg. 2014;40:184-192.
- Garcia-Murray E, Velasco Villasenor ML, Acevedo B, et al. Safety and efficacy of RT002, an injectable botulinum toxin type A, for treating glabellar lines: results of a phase 1/2, open-label, sequential dose-escalation study. Dermatol Surg. 2015;41(suppl 1):S47-S55.
- Won CH, Kim HK, Kim BJ, et al. Comparative trial of a novel botulinum neurotoxin type A versus onabotulinumtoxinA in the treatment of glabellar lines: a multicenter, randomized, double-blind, active-controlled study. Int J Dermatol. 2015;54:227-234.
- Kim BJ, Kwon HH, Park SY, et al. Double-blind, randomized non-inferiority trial of a novel botulinum toxin A processed from the strain CBFC26, compared with onabotulinumtoxin A in the treatment of glabellar lines. J Eur Acad Dermatol Venereol. 2014;28:1761-1767.
- Kim JE, Song EJ, Choi GS, et al. The efficacy and safety of liquid-type botulinum toxin type A for the management of moderate to severe glabellar frown lines. Plast Reconstr Surg. 2015;135:732-741.
- Alam M, Bolotin D, Carruthers J, et al. Consensus statement regarding storage and reuse of previously reconstituted neuromodulators. Dermatol Surg. 2015;41:321-326.
- Alam M, Geisler A, Sadhwani D, et al. Effect of needle size on pain perception in patients treated with botulinum toxin type A injections: a randomized clinical trial. JAMA Dermatol. 2015;151:1194-1199.
- Pucks N, Thomas A, Hallam MJ, et al. Cutaneous cooling to manage botulinum toxin injection-associated pain in patients with facial palsy: a randomised controlled trial. J Plast Reconstr Aesthet Surg. 2015;68:1701-1705.
- Kranz G, Sycha T, Voller B, et al. Pain sensation during intradermal injections of three different botulinum toxin preparations in different doses and dilutions. Dermatol Surg. 2006;32:886-890.
- Lowe PL, Lowe NJ. Botulinum toxin type B: pH change reduces injection pain, retains efficacy. Dermatol Surg. 2014;40:1328-1333.
- Mahmoud BH, Burnett C, Ozog D. Prospective randomized controlled study to determine the effect of topical application of botulinum toxin A for crow’s feet after treatment with ablative fractional CO2 laser. Dermatol Surg. 2015;41(suppl 1):S75-S81.
- Montaser-Kouhsari L, Zartab H, Fanian F, et al. Comparison of intradermal injection with iontophoresis of abo-botulinum toxin A for the treatment of primary axillary hyperhidrosis: a randomized, controlled trial. J Dermatolog Treat. 2014;25:337-341.
- Iannitti T, Palmieri B, Aspiro A, et al. A preliminary study of painless and effective transdermal botulinum toxin A delivery by jet nebulization for treatment of primary hyperhidrosis. Drug Des Devel Ther. 2014;8:931-935.
- Iozzo I, Tengattini V, Antonucci VA. Multipoint and multilevel injection technique of botulinum toxin A in facial aesthetics. J Cosmet Dermatol. 2014;13:135-142.
- Sneath J, Humphrey S, Carruthers A, et al. Injecting botulinum toxin at different depths is not effective for the correction of eyebrow asymmetry. Dermatol Surg. 2015;41(suppl 1):S82-S87.
- Steinsapir KD, Rootman D, Wulc A, et al. Cosmetic microdroplet botulinum toxin A forehead lift: a new treatment paradigm. Ophthal Plast Reconstr Surg. 2015;31:263-268.
The first botulinum neurotoxin (BoNT) approved by the US Food and Drug Administration (FDA) was onabotulinumtoxinA in 1989 for the treatment of strabismus and blepharospasm. It was not until 1992, however, that the aesthetic benefits of BoNT were first reported in the medical literature by Carruthers and Carruthers,1 and a cosmetic indication was not approved by the FDA until 2002. Since that time, the popularity of BoNT products has grown rapidly with a nearly 6500% increase in popularity from 1997 to 2015 in addition to the introduction of a variety of new BoNT formulations to the market.2 It is estimated by the American Society for Aesthetic Plastic Surgery that there were at least 4,000,000 BoNT injections performed in 2015 alone, making it the most popular nonsurgical aesthetic procedure available.2 As the demand for minimally invasive cosmetic procedures continues to increase, we will continue to see the introduction of additional formulations of BoNT products as well as novel administration techniques and delivery devices. In this article, we provide an update on current and upcoming BoNT products and also review the literature on novel administration methods based on studies published from January 1, 2014, to December 31, 2015.
Current Products
To date, there are only 4 FDA-approved formulations of BoNT available for clinical use (eg, cervical dystonia, strabismus, blepharospasm, headache, urinary incontinence) in the United States: abobotulinumtoxinA, incobotulinumtoxinA, onabotulinumtoxinA, and rimabotulinumtoxinB.The FDA-approved dermatologic indications (eg, moderate to severe glabellar or canthal lines, severe axillary hyperhidrosis) for these products are provided in the Table. On a global scale, there are several other commonly utilized formulations of BoNT, including a Korean serotype resembling onabotulinumtoxinA and a Chinese botulinum toxin type A.3 Although there is some evidence to demonstrate comparable efficacy and safety of these latter products, the literature is relatively lacking in comparison to the FDA-approved products.4,5
Upcoming Products
Currently, there are several new BoNT formulations being studied for clinical use. RT 002 (Revance Therapeutics, Inc) is a novel injectable formulation of onabotulinumtoxinA that consists of the purified neurotoxin in combination with patented TransMTS peptides that have been shown to provide high-binding avidity for the neurotoxin, and thus the product is designed to reduce diffusion to adjacent muscles and diminish unwanted effects. With a reduced level of neurotoxin dissemination, it is theorized that a higher administration of targeted doses can be injected, which may lead to a longer duration of desired effects.6 A clinical pilot study done to establish the safety and efficacy of RT 002 for treatment of moderate to severe glabellar lines evaluated 4 equally sized cohorts of 12 participants, each receiving single-dose administration of RT 002 ranging in potency equivalent to 25 U, 50 U, 75 U, and 100 U of abobotulinumtoxinA as determined by the gelatin phosphate method.6 It was concluded that RT 002 is both safe and efficacious with an extended duration of action, with a median duration of effect of 7 months observed in the highest dose group (dose equivalent to 100 U of abobotulinumtoxinA). Notably, 80% of all 48 participants maintained a minimum 1-point improvement in investigator-determined glabellar line severity scores at the 6-month time point and 60% achieved wrinkle scores of none or mild at 6 months posttreatment.6
DWP 450 (Daewoong Pharmaceutical Co, Ltd) is derived from the wild-type Clostridium botulinum and is reported to be of higher purity than standard onabotulinumtoxinA. An initial 16-week pilot study demonstrated that 20 U of DWP 450 is noninferior and of comparable efficacy and safety to 20 U of onabotulinumtoxinA in the treatment of glabellar lines.7
NTC (Botulax [Hugel, Inc]) is the name of the toxin derived from the C botulinum strain CBFC26, which has already been approved in many Asian, European, and Latin American countries for the treatment of blepharospasm. This formulation has demonstrated noninferiority to onabotulinumtoxinA at equivalent 20-U doses for the treatment of moderate to severe glabellar lines in a double-blind, randomized, multicenter, phase 3 trial of 272 participants with a 16-week follow-up.8
MT 10109L (Medytox Inc) is a unique product in that it is distributed as a liquid type A botulinum toxin rather than the standard freeze-dried formulation; thus, a major advantage of this product is its convenience, as it does not need reconstitution or dilution prior to administration. In a double-blind, randomized, active drug–controlled, phase 3 study of 168 participants, it was determined that MT 10109L (20 U) is comparable in efficacy to onabotulinumtoxinA (20 U) for the treatment of moderate to severe glabellar lines. No significant difference was seen between the 2 treatment groups when glabellar lines were assessed at rest at 4 and 16 weeks after treatment, but a significantly greater improvement in glabellar lines was seen at maximum frown in the MT 10109L group at the 16-week follow-up (P=.0064).9
Administration Techniques
With regard to safe and effective BoNT product administration techniques, a variety of studies have provided insight into optimal practice methods. A 2015 expert consensus statement formed by an American Society for Dermatologic Surgery task force reviewed data from 42 papers and unanimously determined that for all current type A BoNT products available in the United States, a vial of BoNT reconstituted appropriately for the purpose of facial injections can be reconstituted at least 4 weeks prior to administration without contamination risk or decrease in efficacy and that multiple patients can be treated with the same vial.Although the statement was not explicit on whether or not preserved or unpreserved saline is to be used, it is considered routine practice to use preservative-containing saline to reconstitute BoNT, as it has been shown to reduce patient discomfort and is not associated with adverse effects.10
Pain Minimization
With respect to minimizing the pain associated with BoNT injections, several studies have assessed administration techniques to minimize patient discomfort. A split-face, double-blind study of 20 participants demonstrated that the use of a 32-gauge needle has a significantly greater chance of reducing clinically significant levels of pain as compared to a 30-gauge needle when performing facial injections (P=.04). Overall, however, injections of the face and arms were on average only nominally and not significantly more painful with 30-gauge needles compared to 32-gauge needles.11
Another technique that has been found to reduce patient discomfort is the application of cold packs prior to injection. A study of patients with chronic facial palsy observed a significant reduction in pain with the administration of a cold (3°C–5°C) gel pack for 1 minute compared to a room temperature (20°C) gel pack prior to the administration of onabotulinumtoxinA into the platysma (P<.001).12 In the matter of injection with rimabotulinumtoxinB, which has been shown to be considerably more painful to receive than its more popularly administered counterpart onabotulinumtoxinA, a split-face pilot study examined the effect of increasing the pH of rimabotulinumtoxinB to 7.5 with sodium bicarbonate from the usual pH of 5.6.13,14 Pain was reported to be considerably less in the higher pH group and no reduction of efficacy was seen over the 10-week follow-up period.14
Delivery Methods
Several preliminary studies also have examined novel delivery techniques to identify minimally painful yet effective methods for administering BoNT. It has been reported that standard BoNT formulations are not effective as topical agents in a comparison study in which onabotulinumtoxinA injection was compared to topically applied onabotulinumtoxinA.15 However, a follow-up prospective study by the same authors has demonstrated efficacy of topical onabotulinumtoxinA following pretreatment with a fractional ablative CO2 laser for treatment of crow’s-feet. In this randomized, split-face, controlled trial (N=10), participants were first pretreated with topical lidocaine 30% before receiving a single pass of fractional ablative CO2 laser with no overlap and a pulse energy of 100 mJ. Within 60 seconds of laser treatment, participants then received either 100 U of abobotulinumtoxinA diluted in 0.1 mL of saline or simple normal saline applied topically. A clinically significant improvement in periorbital wrinkles was seen both at 1-week and 1-month posttreatment in the laser and onabotulinumtoxinA–treated group compared to the laser and topical saline–treated group (P<.02).15
Another unique administration method studied, albeit with less successful results, involves the use of iontophoresis to deliver BoNT painlessly in a transdermal fashion with the assistance of an electrical current.16 This prospective, randomized, assessor-blinded, split-axilla, controlled trial of 11 participants compared the effectiveness of administering onabotulinumtoxinA via iontophoresis to traditional injection with onabotulinumtoxinA (250 U). Iontophoresis was accomplished with a single electrode pad soaked with 250 U of onabotulinumtoxinA applied directly to the axilla and a second electrode pad soaked in 0.9% saline applied to the hand to complete the circuit. An alternating electrical current was slowly increased for 30 minutes to a maximum current of 15 mA with a voltage of 12 V. Among the 11 participants recruited, the side treated with traditional injection showed a significantly greater percentage reduction in baseline sweating at the 1-week, 1-month, and 6-month posttreatment evaluations compared to iontophoresis (84%, 76%, and 50%, respectively vs 73%, 22%, and 32%, respectively)(P<.05). Despite being less efficacious than standard injection therapy, participants reported that iontophoresis delivery was significantly less painful (P<.05).16
A high-pressure oxygen delivery device, which utilizes a powerful jet of microdroplets containing water, the drug, air, and oxygen to deliver medication onto the skin surface, also has been studied as a means of delivery of BoNT in a minimally painful manner. In this study, the device was used to assess the efficacy of transdermal delivery of BoNT via jet nebulization in the treatment of primary palmar, plantar, and axillary hyperhidrosis.17 The 20 participants included in the study were randomized to receive either a combination of lidocaine and onabotulinumtoxinA (50 U) administered through the device or lidocaine delivered through the device followed by multiple transcutaneous injections of onabotulinumtoxinA (100 U). Both treatments significantly reduced sweating compared to baseline as measured by a visual analogue scale at 3-month follow-up (P<.001), but the combination delivery of lidocaine and onabotulinumtoxinA via the device resulted in significantly less procedure-related pain and sweating (P<.001) as well as significantly greater patient satisfaction (P<.001).17
Optimizing Aesthetic Outcomes
A frequent concern of patients receiving BoNT for cosmetic purposes is a desire to avoid a “frozen” or expressionless look. As such, many clinicians have attempted a variety of techniques to achieve more natural aesthetic results. One such method is known as the multipoint and multilevel injection technique, which consists of utilizing multiple injection sites at varying depths (intramuscular, subcutaneous, or intradermal) and doses (2–6 U) depending on the degree of contractility of the targeted muscle. In a preliminary study of 223 participants using this technique with a total dose of 125 U of abobotulinumtoxinA, good and natural results were reported with perseveration of facial emotion in all participants in addition to a mean overall satisfaction rate of 6.4 of 7 on the Facial Line Treatment Satisfaction Questionnaire with the maximum satisfaction rating possible reported in 66% of cases.18 It also has been postulated that injection depth of BoNT can affect brow elevation whereupon deeper injection depths can result in inactivation of the brow depressors and allow for increased elevation of the eyebrows. This technique has been applied in attempts to correct brow height asymmetry. However, a prospective, split-face study of 23 women suggested that this method is not effective.19 Participants received 64 U of onabotulinumtoxinA via 16 injection sites in the glabella, forehead, and lateral canthal area with either all deep or all shallow injections depending on the side treated and whether brow-lift was desired. Results at 4 weeks posttreatment showed no significant difference in brow height, and it was concluded that eyebrow depressor muscles cannot be accurately targeted with deep injection into the muscle belly for correction of eyebrow height discrepancies.19 Conversely, a 5-year retrospective, nonrandomized study of 227 patients with 563 treatments utilizing a “microdroplet” technique reported success at selectively targeting the eyebrow depressors while leaving the brow elevators unaffected.20 Here, a total dose of 33 U of onabotulinumtoxinA was administered via microdroplets of 10 to 20 μL, each with more than 60 to 100 injections into the brow, glabella, and crow’s-feet area. This method of injection resulted in a statistically significant improvement of forehead lines and brow ptosis and furrowing at follow-up between 10 and 45 days after treatment (P<.0001). Additionally, average brow height was significantly increased from 24.6 mm to 25 mm after treatment (P=.02).20
Conclusion
The use of BoNT products for both on- and off-label cosmetic and medical indications has rapidly grown over the past 2 decades. As demonstrated in this review, a variety of promising new products and delivery techniques are being developed. Given the rise in popularity of BoNT products among both physicians and consumers, clinicians should be aware of the current data and ongoing research.
The first botulinum neurotoxin (BoNT) approved by the US Food and Drug Administration (FDA) was onabotulinumtoxinA in 1989 for the treatment of strabismus and blepharospasm. It was not until 1992, however, that the aesthetic benefits of BoNT were first reported in the medical literature by Carruthers and Carruthers,1 and a cosmetic indication was not approved by the FDA until 2002. Since that time, the popularity of BoNT products has grown rapidly with a nearly 6500% increase in popularity from 1997 to 2015 in addition to the introduction of a variety of new BoNT formulations to the market.2 It is estimated by the American Society for Aesthetic Plastic Surgery that there were at least 4,000,000 BoNT injections performed in 2015 alone, making it the most popular nonsurgical aesthetic procedure available.2 As the demand for minimally invasive cosmetic procedures continues to increase, we will continue to see the introduction of additional formulations of BoNT products as well as novel administration techniques and delivery devices. In this article, we provide an update on current and upcoming BoNT products and also review the literature on novel administration methods based on studies published from January 1, 2014, to December 31, 2015.
Current Products
To date, there are only 4 FDA-approved formulations of BoNT available for clinical use (eg, cervical dystonia, strabismus, blepharospasm, headache, urinary incontinence) in the United States: abobotulinumtoxinA, incobotulinumtoxinA, onabotulinumtoxinA, and rimabotulinumtoxinB.The FDA-approved dermatologic indications (eg, moderate to severe glabellar or canthal lines, severe axillary hyperhidrosis) for these products are provided in the Table. On a global scale, there are several other commonly utilized formulations of BoNT, including a Korean serotype resembling onabotulinumtoxinA and a Chinese botulinum toxin type A.3 Although there is some evidence to demonstrate comparable efficacy and safety of these latter products, the literature is relatively lacking in comparison to the FDA-approved products.4,5
Upcoming Products
Currently, there are several new BoNT formulations being studied for clinical use. RT 002 (Revance Therapeutics, Inc) is a novel injectable formulation of onabotulinumtoxinA that consists of the purified neurotoxin in combination with patented TransMTS peptides that have been shown to provide high-binding avidity for the neurotoxin, and thus the product is designed to reduce diffusion to adjacent muscles and diminish unwanted effects. With a reduced level of neurotoxin dissemination, it is theorized that a higher administration of targeted doses can be injected, which may lead to a longer duration of desired effects.6 A clinical pilot study done to establish the safety and efficacy of RT 002 for treatment of moderate to severe glabellar lines evaluated 4 equally sized cohorts of 12 participants, each receiving single-dose administration of RT 002 ranging in potency equivalent to 25 U, 50 U, 75 U, and 100 U of abobotulinumtoxinA as determined by the gelatin phosphate method.6 It was concluded that RT 002 is both safe and efficacious with an extended duration of action, with a median duration of effect of 7 months observed in the highest dose group (dose equivalent to 100 U of abobotulinumtoxinA). Notably, 80% of all 48 participants maintained a minimum 1-point improvement in investigator-determined glabellar line severity scores at the 6-month time point and 60% achieved wrinkle scores of none or mild at 6 months posttreatment.6
DWP 450 (Daewoong Pharmaceutical Co, Ltd) is derived from the wild-type Clostridium botulinum and is reported to be of higher purity than standard onabotulinumtoxinA. An initial 16-week pilot study demonstrated that 20 U of DWP 450 is noninferior and of comparable efficacy and safety to 20 U of onabotulinumtoxinA in the treatment of glabellar lines.7
NTC (Botulax [Hugel, Inc]) is the name of the toxin derived from the C botulinum strain CBFC26, which has already been approved in many Asian, European, and Latin American countries for the treatment of blepharospasm. This formulation has demonstrated noninferiority to onabotulinumtoxinA at equivalent 20-U doses for the treatment of moderate to severe glabellar lines in a double-blind, randomized, multicenter, phase 3 trial of 272 participants with a 16-week follow-up.8
MT 10109L (Medytox Inc) is a unique product in that it is distributed as a liquid type A botulinum toxin rather than the standard freeze-dried formulation; thus, a major advantage of this product is its convenience, as it does not need reconstitution or dilution prior to administration. In a double-blind, randomized, active drug–controlled, phase 3 study of 168 participants, it was determined that MT 10109L (20 U) is comparable in efficacy to onabotulinumtoxinA (20 U) for the treatment of moderate to severe glabellar lines. No significant difference was seen between the 2 treatment groups when glabellar lines were assessed at rest at 4 and 16 weeks after treatment, but a significantly greater improvement in glabellar lines was seen at maximum frown in the MT 10109L group at the 16-week follow-up (P=.0064).9
Administration Techniques
With regard to safe and effective BoNT product administration techniques, a variety of studies have provided insight into optimal practice methods. A 2015 expert consensus statement formed by an American Society for Dermatologic Surgery task force reviewed data from 42 papers and unanimously determined that for all current type A BoNT products available in the United States, a vial of BoNT reconstituted appropriately for the purpose of facial injections can be reconstituted at least 4 weeks prior to administration without contamination risk or decrease in efficacy and that multiple patients can be treated with the same vial.Although the statement was not explicit on whether or not preserved or unpreserved saline is to be used, it is considered routine practice to use preservative-containing saline to reconstitute BoNT, as it has been shown to reduce patient discomfort and is not associated with adverse effects.10
Pain Minimization
With respect to minimizing the pain associated with BoNT injections, several studies have assessed administration techniques to minimize patient discomfort. A split-face, double-blind study of 20 participants demonstrated that the use of a 32-gauge needle has a significantly greater chance of reducing clinically significant levels of pain as compared to a 30-gauge needle when performing facial injections (P=.04). Overall, however, injections of the face and arms were on average only nominally and not significantly more painful with 30-gauge needles compared to 32-gauge needles.11
Another technique that has been found to reduce patient discomfort is the application of cold packs prior to injection. A study of patients with chronic facial palsy observed a significant reduction in pain with the administration of a cold (3°C–5°C) gel pack for 1 minute compared to a room temperature (20°C) gel pack prior to the administration of onabotulinumtoxinA into the platysma (P<.001).12 In the matter of injection with rimabotulinumtoxinB, which has been shown to be considerably more painful to receive than its more popularly administered counterpart onabotulinumtoxinA, a split-face pilot study examined the effect of increasing the pH of rimabotulinumtoxinB to 7.5 with sodium bicarbonate from the usual pH of 5.6.13,14 Pain was reported to be considerably less in the higher pH group and no reduction of efficacy was seen over the 10-week follow-up period.14
Delivery Methods
Several preliminary studies also have examined novel delivery techniques to identify minimally painful yet effective methods for administering BoNT. It has been reported that standard BoNT formulations are not effective as topical agents in a comparison study in which onabotulinumtoxinA injection was compared to topically applied onabotulinumtoxinA.15 However, a follow-up prospective study by the same authors has demonstrated efficacy of topical onabotulinumtoxinA following pretreatment with a fractional ablative CO2 laser for treatment of crow’s-feet. In this randomized, split-face, controlled trial (N=10), participants were first pretreated with topical lidocaine 30% before receiving a single pass of fractional ablative CO2 laser with no overlap and a pulse energy of 100 mJ. Within 60 seconds of laser treatment, participants then received either 100 U of abobotulinumtoxinA diluted in 0.1 mL of saline or simple normal saline applied topically. A clinically significant improvement in periorbital wrinkles was seen both at 1-week and 1-month posttreatment in the laser and onabotulinumtoxinA–treated group compared to the laser and topical saline–treated group (P<.02).15
Another unique administration method studied, albeit with less successful results, involves the use of iontophoresis to deliver BoNT painlessly in a transdermal fashion with the assistance of an electrical current.16 This prospective, randomized, assessor-blinded, split-axilla, controlled trial of 11 participants compared the effectiveness of administering onabotulinumtoxinA via iontophoresis to traditional injection with onabotulinumtoxinA (250 U). Iontophoresis was accomplished with a single electrode pad soaked with 250 U of onabotulinumtoxinA applied directly to the axilla and a second electrode pad soaked in 0.9% saline applied to the hand to complete the circuit. An alternating electrical current was slowly increased for 30 minutes to a maximum current of 15 mA with a voltage of 12 V. Among the 11 participants recruited, the side treated with traditional injection showed a significantly greater percentage reduction in baseline sweating at the 1-week, 1-month, and 6-month posttreatment evaluations compared to iontophoresis (84%, 76%, and 50%, respectively vs 73%, 22%, and 32%, respectively)(P<.05). Despite being less efficacious than standard injection therapy, participants reported that iontophoresis delivery was significantly less painful (P<.05).16
A high-pressure oxygen delivery device, which utilizes a powerful jet of microdroplets containing water, the drug, air, and oxygen to deliver medication onto the skin surface, also has been studied as a means of delivery of BoNT in a minimally painful manner. In this study, the device was used to assess the efficacy of transdermal delivery of BoNT via jet nebulization in the treatment of primary palmar, plantar, and axillary hyperhidrosis.17 The 20 participants included in the study were randomized to receive either a combination of lidocaine and onabotulinumtoxinA (50 U) administered through the device or lidocaine delivered through the device followed by multiple transcutaneous injections of onabotulinumtoxinA (100 U). Both treatments significantly reduced sweating compared to baseline as measured by a visual analogue scale at 3-month follow-up (P<.001), but the combination delivery of lidocaine and onabotulinumtoxinA via the device resulted in significantly less procedure-related pain and sweating (P<.001) as well as significantly greater patient satisfaction (P<.001).17
Optimizing Aesthetic Outcomes
A frequent concern of patients receiving BoNT for cosmetic purposes is a desire to avoid a “frozen” or expressionless look. As such, many clinicians have attempted a variety of techniques to achieve more natural aesthetic results. One such method is known as the multipoint and multilevel injection technique, which consists of utilizing multiple injection sites at varying depths (intramuscular, subcutaneous, or intradermal) and doses (2–6 U) depending on the degree of contractility of the targeted muscle. In a preliminary study of 223 participants using this technique with a total dose of 125 U of abobotulinumtoxinA, good and natural results were reported with perseveration of facial emotion in all participants in addition to a mean overall satisfaction rate of 6.4 of 7 on the Facial Line Treatment Satisfaction Questionnaire with the maximum satisfaction rating possible reported in 66% of cases.18 It also has been postulated that injection depth of BoNT can affect brow elevation whereupon deeper injection depths can result in inactivation of the brow depressors and allow for increased elevation of the eyebrows. This technique has been applied in attempts to correct brow height asymmetry. However, a prospective, split-face study of 23 women suggested that this method is not effective.19 Participants received 64 U of onabotulinumtoxinA via 16 injection sites in the glabella, forehead, and lateral canthal area with either all deep or all shallow injections depending on the side treated and whether brow-lift was desired. Results at 4 weeks posttreatment showed no significant difference in brow height, and it was concluded that eyebrow depressor muscles cannot be accurately targeted with deep injection into the muscle belly for correction of eyebrow height discrepancies.19 Conversely, a 5-year retrospective, nonrandomized study of 227 patients with 563 treatments utilizing a “microdroplet” technique reported success at selectively targeting the eyebrow depressors while leaving the brow elevators unaffected.20 Here, a total dose of 33 U of onabotulinumtoxinA was administered via microdroplets of 10 to 20 μL, each with more than 60 to 100 injections into the brow, glabella, and crow’s-feet area. This method of injection resulted in a statistically significant improvement of forehead lines and brow ptosis and furrowing at follow-up between 10 and 45 days after treatment (P<.0001). Additionally, average brow height was significantly increased from 24.6 mm to 25 mm after treatment (P=.02).20
Conclusion
The use of BoNT products for both on- and off-label cosmetic and medical indications has rapidly grown over the past 2 decades. As demonstrated in this review, a variety of promising new products and delivery techniques are being developed. Given the rise in popularity of BoNT products among both physicians and consumers, clinicians should be aware of the current data and ongoing research.
- Carruthers JD, Carruthers JA. Treatment of glabellar frown lines with C. botulinum-A exotoxin. J Dermatol Surg Oncol. 1992;18:17-21.
- American Society for Aesthetic Plastic Surgery. Cosmetic Surgery National Data Bank statistics. American Society for Aesthetic Plastic Surgery website. http://www.surgery.org/sites/default/files/ASAPS-Stats2015.pdf. Accessed June 12, 2016.
- Walker TJ, Dayan SH. Comparison and overview of currently available neurotoxins. J Clin Aesthet Dermatol. 2014;7:31-39.
- Feng Z, Sun Q, He L, et al. Optimal dosage of botulinum toxin type A for treatment of glabellar frown lines: efficacy and safety in a clinical trial. Dermatol Surg. 2015;41(suppl 1):S56-S63.
- Jiang HY, Chen S, Zhou J, et al. Diffusion of two botulinum toxins type A on the forehead: double-blinded, randomized, controlled study. Dermatol Surg. 2014;40:184-192.
- Garcia-Murray E, Velasco Villasenor ML, Acevedo B, et al. Safety and efficacy of RT002, an injectable botulinum toxin type A, for treating glabellar lines: results of a phase 1/2, open-label, sequential dose-escalation study. Dermatol Surg. 2015;41(suppl 1):S47-S55.
- Won CH, Kim HK, Kim BJ, et al. Comparative trial of a novel botulinum neurotoxin type A versus onabotulinumtoxinA in the treatment of glabellar lines: a multicenter, randomized, double-blind, active-controlled study. Int J Dermatol. 2015;54:227-234.
- Kim BJ, Kwon HH, Park SY, et al. Double-blind, randomized non-inferiority trial of a novel botulinum toxin A processed from the strain CBFC26, compared with onabotulinumtoxin A in the treatment of glabellar lines. J Eur Acad Dermatol Venereol. 2014;28:1761-1767.
- Kim JE, Song EJ, Choi GS, et al. The efficacy and safety of liquid-type botulinum toxin type A for the management of moderate to severe glabellar frown lines. Plast Reconstr Surg. 2015;135:732-741.
- Alam M, Bolotin D, Carruthers J, et al. Consensus statement regarding storage and reuse of previously reconstituted neuromodulators. Dermatol Surg. 2015;41:321-326.
- Alam M, Geisler A, Sadhwani D, et al. Effect of needle size on pain perception in patients treated with botulinum toxin type A injections: a randomized clinical trial. JAMA Dermatol. 2015;151:1194-1199.
- Pucks N, Thomas A, Hallam MJ, et al. Cutaneous cooling to manage botulinum toxin injection-associated pain in patients with facial palsy: a randomised controlled trial. J Plast Reconstr Aesthet Surg. 2015;68:1701-1705.
- Kranz G, Sycha T, Voller B, et al. Pain sensation during intradermal injections of three different botulinum toxin preparations in different doses and dilutions. Dermatol Surg. 2006;32:886-890.
- Lowe PL, Lowe NJ. Botulinum toxin type B: pH change reduces injection pain, retains efficacy. Dermatol Surg. 2014;40:1328-1333.
- Mahmoud BH, Burnett C, Ozog D. Prospective randomized controlled study to determine the effect of topical application of botulinum toxin A for crow’s feet after treatment with ablative fractional CO2 laser. Dermatol Surg. 2015;41(suppl 1):S75-S81.
- Montaser-Kouhsari L, Zartab H, Fanian F, et al. Comparison of intradermal injection with iontophoresis of abo-botulinum toxin A for the treatment of primary axillary hyperhidrosis: a randomized, controlled trial. J Dermatolog Treat. 2014;25:337-341.
- Iannitti T, Palmieri B, Aspiro A, et al. A preliminary study of painless and effective transdermal botulinum toxin A delivery by jet nebulization for treatment of primary hyperhidrosis. Drug Des Devel Ther. 2014;8:931-935.
- Iozzo I, Tengattini V, Antonucci VA. Multipoint and multilevel injection technique of botulinum toxin A in facial aesthetics. J Cosmet Dermatol. 2014;13:135-142.
- Sneath J, Humphrey S, Carruthers A, et al. Injecting botulinum toxin at different depths is not effective for the correction of eyebrow asymmetry. Dermatol Surg. 2015;41(suppl 1):S82-S87.
- Steinsapir KD, Rootman D, Wulc A, et al. Cosmetic microdroplet botulinum toxin A forehead lift: a new treatment paradigm. Ophthal Plast Reconstr Surg. 2015;31:263-268.
- Carruthers JD, Carruthers JA. Treatment of glabellar frown lines with C. botulinum-A exotoxin. J Dermatol Surg Oncol. 1992;18:17-21.
- American Society for Aesthetic Plastic Surgery. Cosmetic Surgery National Data Bank statistics. American Society for Aesthetic Plastic Surgery website. http://www.surgery.org/sites/default/files/ASAPS-Stats2015.pdf. Accessed June 12, 2016.
- Walker TJ, Dayan SH. Comparison and overview of currently available neurotoxins. J Clin Aesthet Dermatol. 2014;7:31-39.
- Feng Z, Sun Q, He L, et al. Optimal dosage of botulinum toxin type A for treatment of glabellar frown lines: efficacy and safety in a clinical trial. Dermatol Surg. 2015;41(suppl 1):S56-S63.
- Jiang HY, Chen S, Zhou J, et al. Diffusion of two botulinum toxins type A on the forehead: double-blinded, randomized, controlled study. Dermatol Surg. 2014;40:184-192.
- Garcia-Murray E, Velasco Villasenor ML, Acevedo B, et al. Safety and efficacy of RT002, an injectable botulinum toxin type A, for treating glabellar lines: results of a phase 1/2, open-label, sequential dose-escalation study. Dermatol Surg. 2015;41(suppl 1):S47-S55.
- Won CH, Kim HK, Kim BJ, et al. Comparative trial of a novel botulinum neurotoxin type A versus onabotulinumtoxinA in the treatment of glabellar lines: a multicenter, randomized, double-blind, active-controlled study. Int J Dermatol. 2015;54:227-234.
- Kim BJ, Kwon HH, Park SY, et al. Double-blind, randomized non-inferiority trial of a novel botulinum toxin A processed from the strain CBFC26, compared with onabotulinumtoxin A in the treatment of glabellar lines. J Eur Acad Dermatol Venereol. 2014;28:1761-1767.
- Kim JE, Song EJ, Choi GS, et al. The efficacy and safety of liquid-type botulinum toxin type A for the management of moderate to severe glabellar frown lines. Plast Reconstr Surg. 2015;135:732-741.
- Alam M, Bolotin D, Carruthers J, et al. Consensus statement regarding storage and reuse of previously reconstituted neuromodulators. Dermatol Surg. 2015;41:321-326.
- Alam M, Geisler A, Sadhwani D, et al. Effect of needle size on pain perception in patients treated with botulinum toxin type A injections: a randomized clinical trial. JAMA Dermatol. 2015;151:1194-1199.
- Pucks N, Thomas A, Hallam MJ, et al. Cutaneous cooling to manage botulinum toxin injection-associated pain in patients with facial palsy: a randomised controlled trial. J Plast Reconstr Aesthet Surg. 2015;68:1701-1705.
- Kranz G, Sycha T, Voller B, et al. Pain sensation during intradermal injections of three different botulinum toxin preparations in different doses and dilutions. Dermatol Surg. 2006;32:886-890.
- Lowe PL, Lowe NJ. Botulinum toxin type B: pH change reduces injection pain, retains efficacy. Dermatol Surg. 2014;40:1328-1333.
- Mahmoud BH, Burnett C, Ozog D. Prospective randomized controlled study to determine the effect of topical application of botulinum toxin A for crow’s feet after treatment with ablative fractional CO2 laser. Dermatol Surg. 2015;41(suppl 1):S75-S81.
- Montaser-Kouhsari L, Zartab H, Fanian F, et al. Comparison of intradermal injection with iontophoresis of abo-botulinum toxin A for the treatment of primary axillary hyperhidrosis: a randomized, controlled trial. J Dermatolog Treat. 2014;25:337-341.
- Iannitti T, Palmieri B, Aspiro A, et al. A preliminary study of painless and effective transdermal botulinum toxin A delivery by jet nebulization for treatment of primary hyperhidrosis. Drug Des Devel Ther. 2014;8:931-935.
- Iozzo I, Tengattini V, Antonucci VA. Multipoint and multilevel injection technique of botulinum toxin A in facial aesthetics. J Cosmet Dermatol. 2014;13:135-142.
- Sneath J, Humphrey S, Carruthers A, et al. Injecting botulinum toxin at different depths is not effective for the correction of eyebrow asymmetry. Dermatol Surg. 2015;41(suppl 1):S82-S87.
- Steinsapir KD, Rootman D, Wulc A, et al. Cosmetic microdroplet botulinum toxin A forehead lift: a new treatment paradigm. Ophthal Plast Reconstr Surg. 2015;31:263-268.
Practice Points
- Botulinum neurotoxin (BoNT) injection is the most popular nonsurgical aesthetic procedure available of which there are currently 4 products approved by the US Food and Drug Administration.
- A variety of new BoNT products with unique properties and formulations are currently being studied, some of which are already available for clinical use in foreign markets.
- Administration technique and novel product delivery methods also can be utilized to minimize pain and maximize aesthetic outcomes.
Enlarged Facial Pores: An Update on Treatments
Enlarged facial pores are superficial skin structures that are visualized as small openings on the skin corresponding to the openings of the pilosebaceous apparatus. These openings may be impacted with horny follicular plugs consisting of sebaceous debris that appear as open comedones.1 Skin pores is a lay term that is poorly defined in the medical literature and often is categorized in terms of arbitrary circular diameters determined through cosmetic skin analyzers.2 The term refers to pilosebaceous follicular enlargements (with or without open comedonal horny impactions) that can be visualized by the naked eye, most commonly occurring on the face and scalp. These enlarged pores remain a pervasive cosmetic concern that impacts patient quality of life. Enlarged pores are difficult to treat, in part due to lack of knowledge of the pathophysiology; thus, we review the currently proposed causes of enlarged pilosebaceous openings and the treatments in the scope of this pathogenesis with a focus on therapeutic efficacy.
Pathogenesis of Enlarged Facial Pores
It is now thought that seborrhea, loss of skin elasticity and tension, and hair follicle size are most clinically relevant to the pathogenesis of enlarged pores.2 Other potential associated and causative factors include genetic predisposition, acne, comedogenic xenobiotics, chronic photodamage, chronic radiodermatitis, and vitamin A deficiency.1,3
The direct relationship between sebum output and pore size has been well established, particularly in men who generally have higher sebum output levels than women, which likely is testosterone driven.4,5 However, there are contradictory data on whether sex affects pore size, as females also exhibit contributory hormonal factors. Sebum output and pore size increase substantially during the ovulation phase of the female menstrual cycle, likely secondary to increased progesterone affecting sebaceous gland activity.2,4 The presence of acne also is associated with enlarged facial pores, though the extent of seborrhea as a confounding factor is unclear. Furthermore, acne severity does not correlate with increased pore size.5 However, the processes of acne and facial pores are interlinked, given the frequent occurrence of open comedones within the pores.
Skin elasticity and tensile strength when defined visually and mechanically has shown a negative correlation with facial pore size and density.5 It is well known that cutaneous aging and chronic photodamage cause perturbation in the collagen and elastin framework that allows for the skin to maintain its resilient properties.6 Aged and photodamaged skin also demonstrates decreased expression of microfibril-associated glycoprotein-1 (MAGP-1), a crucial component in elastic fiber assembly and skin elasticity in the dermis and perifollicular/pore areas.7
Pore density and size appears to range diversely across ethnicities, though Chinese women exhibit notably lower pore size and density across all ages as compared to other ethnicities.8 Black individuals have aberrant epidermal architecture, defined as the presence of stalagmitelike structures at the dermoepidermal junction, correlating with enlarged pore size compared to other ethnicities.2,8
Treating Enlarged Facial Pores
Treatments for enlarged facial pores primarily aim to decrease sebum production, rejuvenate skin, remove hair, and/or decrease follicular size. Evidence-based studies are limited, and many currently used therapies have not been studied with enlarged facial pores as a primary investigative outcome. Here, we include studies that report efficacy in decreasing pore size specifically. It is important to note the lack of a uniform and objective modality with which to report skin pore size. Studies use a wide range of techniques including patient self-reporting, physician observation, and software image analyzers.
Topical Therapies
Topical retinoids are vitamin A derivatives, and they are first-line therapies in reversing the aberrant collagen and elastin-associated epidermal and dermal changes that occur with chronological aging and photoaging. Tretinoin, isotretinoin, and tazarotene have shown efficacy in multiple parameters of skin rejuvenation, including facial pores, skin wrinkling, hyperpigmentation, skin laxity, and sebum production.9 However, it is important to note that retinoids treat keratinocyte atypia in acne, and efficacy in facial pores is confounded by improvement in follicular keratinization. Because studies have not distinctly uncoupled this association, it is erroneous to conclude that retinoids reduce facial pore size and density irrespective of concomitant acne vulgaris.
Tazarotene has been evaluated for use in reducing facial pore size. In one investigation, 568 patients with moderate wrinkling or hyperpigmentation were randomized to receive tazarotene cream 0.1% or placebo once daily for 24 weeks and were evaluated for enlarged facial pores as a secondary outcome using a double-blinded physician 5-point scale.10 At week 24, 42% of tazarotene-treated patients achieved improvement of at least 1 point compared to 20% of placebo-treated patients (P<.001). Adverse events were dermatitic, as can be expected of retinoids, leading to a 4% discontinuation rate in the tazarotene group compared to 1% in the placebo group.10
Tretinoin has long been used off label for antiaging treatments but has only recently shown efficacy for facial pores. In one study, 60 women who had previously sought antiaging procedures were treated with tretinoin cream 0.025% once daily and no other antiaging products or procedures for 90 days.11 Facial pore evaluations were determined by a modified dermatoscope with a polarized analyzer for clinical scoring using a photonumeric scale. Patients improved from a baseline average score of 3.2 in facial pores to a posttreatment average score of 2.0 (P<.05) at day 84. This improvement was sustained from day 28 of treatment and corresponded to patient self-perception. Adverse events included xerosis, desquamation, burning, and erythema, which led to 3 premature discontinuations.11
Various chemical peel formulations are used in skin rejuvenation and have shown application in enlarged facial pores. Chemical peels act at the epidermal or dermal level to induce temporary breakdown and regeneration of healthier cells and improved skin matrix.12 Twenty-two Japanese women applied glycolic acid (30% solution) every 2 weeks for a total of 5 treatments and exhibited reduced appearance of conspicuous, open, and dark pores, defined by surface area and shading as determined through dermatoscopic and software analysis, with mean improvement rates of 34.6%, 11%, and 34.3%, respectively. More than 70% of participants exhibited improvement in enlarged facial pores.13 A study involving a 40% glycolic acid and vitamin C formulation demonstrated significant improvement in facial pores (28.3%; P<.001).14
The newest topical therapies studied for use in minimizing facial pilosebaceous openings are natural plant-derived copper chlorophyllin complex sodium salt (CHLcu) and tetra-hydro-jasmonic acid (LR2412). Clinical trials of these botanicals are limited with small sample sizes but are included here as novel treatments requiring further investigation.
Chlorophyllin copper complex sodium salt is derived from chlorophyll, a green pigment found in plants, and has been investigated as a topical gel in liposomal dispersions for application in photodamaged and aged skin. Chlorophyllin copper complex sodium salt exerts in vitro hyaluronidase inhibitory activity to maintain hyaluronic acid in the extracellular matrix and counteract the structural breakdown of cutaneous aging.15 Two small single-center pilot trials enrolled 10 participants each in a 3-week study of CHLcu 0.1% twice daily and an 8-week study of CHLcu 0.066% twice daily.16,17 After 3 weeks, patients treated with CHLcu 0.1% exhibited a 22.2% improvement in facial pores by clinical assessment grading, though this improvement was not significant on software imaging analysis. Patients improved the most on parameters of facial seborrhea by clinical assessment.16 After 8 weeks, patients treated with CHLcu 0.066% exhibited 25.3% improvement in facial pores by clinical assessment grading.17 Treatments were reported to be well tolerated without noted adverse events in both studies.
Tetra-hydro-jasmonic acid is an analogue of jasmonic acid, a plant hormone derived from linoleic acid. Due to its favorable safety profile and bioavailability, penetration into epidermal and dermal layers, and potential effects in rejuvenating desquamation, LR2412 is currently being assessed for treatment of skin wrinkles, texture, and pores.18 Its effect is thought to relate to stimulation of laminin-5, collagen IV, and fibrillin deposition at the dermoepidermal junction.19 In an open-label trial of a topical preparation of LR2412, 15 participants were treated twice daily for 6 weeks and assessed through investigator clinical assessment scoring.20 Investigator scoring of pores improved by 25.2% from baseline (P<.05) after 6 weeks of treatment. Improvement in pores was seen as early as days 1 and 3. No serious adverse events were reported, though 2 participants developed acne on follow-up.20
Tetra-hydro-jasmonic acid also is formulated with retinol (retinol 0.2%/LR2412 2.0%) and demonstrated cosmetic efficacy in a noninferiority trial with tretinoin cream 0.025%.11 Sixty patients each were randomized to retinol/LR2412 or tretinoin at bedtime and treated for 90 days. At day 84, participants in the retinol/LR2412 group exhibited an improvement in investigator clinical assessment scoring from a baseline of 3.6 to 2.5 (P<.05). There were no significant differences in investigator-assessed efficacy between the treatment arms. Participants reported similar or better results and fewer side effects with retinol/LR2412 on self-questionnaires. Eight participants treated with retinol/LR2412 and 15 participants treated with tretinoin reported various incidences of skin irritation, burning, and desquamation.11
Oral Therapies
The most commonly used oral therapies for enlarged pores are antiandrogens, such as combined oral contraceptives, spironolactone, and cyproterone acetate, which modulate sebum production due to the presence of androgen receptors within sebaceous glands.21 Forty-four white women in an open-label, phase 4 study were treated with combined oral contraceptives containing chlormadinone acetate–ethinyl estradiol for 6 menstrual cycles, with standardized photography taken before and after the treatment period for software analysis. After 6 treatment cycles, 9.1% (4/44) of participants had visibly enlarged pores of the forehead and cheeks compared to 43.2% (19/44) of participants at baseline (P<.0001).22 The effects of other antiandrogens on facial pores have not been studied in this capacity.
Lasers, Radiofrequency, and Ultrasound Devices
The development of various devices that can deliver targeted thermal or ultrasound energy to the skin offers the newest and most robust modality in cosmetic therapy. The mechanism of their efficacy may be due to a combination of induced remodeling of collagen fibers near pilosebaceous openings to increase skin elasticity and decrease sebum production.2,23
Devices with established antiaging effects have been extensively reviewed and include the gold particle 800-nm diode laser, 1450-nm diode laser, microneedle apparatuses, fractional radiofrequency devices, 2790-nm erbium:YAG laser, nonablative 1410-nm fractionated erbium-doped fiber laser, and nonablative 1440-nm fractional laser.2
Literature on the use of these devices for minimizing facial pore size is limited. One treatment of intense focused ultrasound using a 3-mm transducer successfully improved overall pore appearance in 91% of sites at 6-week follow-up on a clinical grading scale.24 Three sessions of nonablative 1410-nm fractionated erbium-doped fiber laser treatments yielded facial skin pore minimization of greater than 51% in 14 of 15 participants.25
The nonablative 1440-nm diode fractional laser received 510(k) clearance by the US Food and Drug Administration in 2011 for aesthetic use in chronologically aged and photoaged skin. Twenty participants treated for 2 weeks and a total of 6 facial treatments with this laser system showed a 17% average improvement in facial pore score on software analysis (P≤.002). Adverse events were mild and included erythema and xerosis.26
Conclusion
The reliability of available literature on efficacy of various treatments in diminishing facial skin pores has been challenging given that most studies are low in power, lack control groups, use nonuniform methods of reporting outcomes, and do not report complete adverse events. Thus, all results should be interpreted with caution.
Overall, it is clear that the pathogenesis of enlarged facial pores is multifactorial and complex, necessitating a similar approach to therapeutics. Topical treatments offer a range of diverse therapies with proven benefit in facial pore reduction. The advent of lasers and devices offers constantly evolving therapeutic options with diffuse antiaging effects. Despite the numerous topical, oral, and device-oriented options, enlarged facial pores remain a challenging cosmetic concern. More robust efficacy studies on new treatments are necessary.
- Uhoda E, Pierard-Franchimont C, Petit L, et al. The conundrum of skin pores in dermocosmetology. Dermatology. 2005;210:3-7.
- Lee SJ, Seok J, Jeong SY, et al. Facial pores: definition, causes, and treatment options. Dermatol Surg. 2016;42:277-285.
- Pierard GE, Pierard-Franchimont C, Marks R, et al. EEMCO guidance for the in vivo assessment of skin greasiness. The EEMCO Group. Skin Pharmacol Appl Skin Physiol. 2000;13:372-389.
- Roh M, Han M, Kim D, et al. Sebum output as a factor contributing to the size of facial pores. Br J Dermatol. 2006;155:890-894.
- Kim BY, Choi JW, Park KC, et al. Sebum, acne, skin elasticity, and gender difference-which is the major influencing factor for facial pores? Skin Res Technol. 2013;19:E45-E53.
- Uitto J. The role of elastin and collagen in cutaneous aging: intrinsic aging versus photoexposure. J Drugs Dermatol. 2008;7(2 suppl):S12-S16.
- Zheng Q, Chen S, Chen Y, et al. Investigation of age-related decline of microfibril-associated glycoprotein-1 in human skin through immunohistochemistry study. Clin Cosmet Investig Dermatol. 2013;6:317-323.
- Sugiyama-Nakagiri Y, Sugata K, Hachiya A, et al. Ethnic differences in the structural properties of facial skin. J Dermatol Sci. 2009;53:135-139.
- Mukherjee S, Date A, Patravale V, et al. Retinoids in the treatment of skin aging: an overview of clinical efficacy and safety. Clin Interv Aging. 2006;1:327-348.
- Kang S, Krueger GG, Tanghetti EA, et al; Tazarotene Cream in Photodamage Study Group. A multicenter, randomized, double-blind trial of tazarotene 0.1% cream in the treatment of photodamage. J Am Acad Dermatol. 2005;52:268-274.
- Bouloc A, Vergnanini AL, Issa MC. A double-blind randomized study comparing the association of retinol and LR2412 with tretinoin 0.025% in photoaged skin. J Cosmet Dermatol. 2015;14:40-46.
- Fischer TC, Perosino E, Poli F, et al. Chemical peels in aesthetic dermatology: an update 2009 [published online September 8, 2009]. J Eur Acad Dermatol Venereol. 2010;24:281-292.
- Kakudo N, Kushida S, Tanaka N, et al. A novel method to measure conspicuous facial pores using computer analysis of digital-camera-captured images: the effect of glycolic acid chemical peeling. Skin Res Technol. 2011;17:427-433.
- Kim WS. Efficacy and safety of a new superficial chemical peel using alpha-hydroxy acid, vitamin C and oxygen for melasma. J Cosmet Laser Ther. 2013;15:21-24.
- McCook JP, Dorogi PL, Vasily DB, et al. In vitro inhibition of hyaluronidase by sodium copper chlorophyllin complex and chlorophyllin analogs. Clin Cosmet Investig Dermatol. 2015;8:443-448.
- Stephens TJ, McCook JP, Herndon JH Jr. Pilot study of topical copper chlorophyllin complex in subjects with facial acne and large pores. J Drugs Dermatol. 2015;14:589-592.
- Sigler ML, Stephens TJ. Assessment of the safety and efficacy of topical copper chlorophyllin in women with photodamaged facial skin. J Drugs Dermatol. 2015;14:401-404.
- Alexiades M. Jasmonates and tetrahydrojasmonic acid: a novel class of anti-aging molecules. J Drugs Dermatol. 2016;15:206-207.
- Tran C, Michelet JF, Simonetti L, et al. In vitro and in vivo studies with tetra-hydro-jasmonic acid (LR2412) reveal its potential to correct signs of skin ageing. J Eur Acad Dermatol Venereol. 2014;28:415-423.
- Alexiades M. Clinical assessment of a novel jasmonate cosmeceutical, LR2412-Cx, for the treatment of skin aging. J Drugs Dermatol. 2016;15:209-215.
- Lam C, Zaenglein AL. Contraceptive use in acne. Clin Dermatol. 2014;32:502-515.
- Kerscher M, Reuther T, Bayrhammer J, et al. Effects of an oral contraceptive containing chlormadinone and ethinylestradiol on acne-prone skin of women of different age groups: an open-label, single-centre, phase IV study. Clin Drug Investig. 2008;28:703-711.
- Schmults CD, Phelps R, Goldberg DJ. Nonablative facial remodeling: erythema reduction and histologic evidence of new collagen formation using a 300-microsecond 1064-nm Nd:YAG laser. Arch Dermatol. 2004;140:1373-1376.
- Lee HJ, Lee KR, Park JY, et al. The efficacy and safety of intense focused ultrasound in the treatment of enlarged facial pores in Asian skin. J Dermatolog Treat. 2015;26:73-77.
- Suh DH, Chang KY, Lee SJ, et al. Treatment of dilated pores with 1410-nm fractional erbium-doped fiber laser. Lasers Med Sci. 2015;30:1135-1139.
- Saedi N, Petrell K, Arndt K, et al. Evaluating facial pores and skin texture after low-energy nonablative fractional 1440-nm laser treatments. J Am Acad Dermatol. 2013;68:113-118.
Enlarged facial pores are superficial skin structures that are visualized as small openings on the skin corresponding to the openings of the pilosebaceous apparatus. These openings may be impacted with horny follicular plugs consisting of sebaceous debris that appear as open comedones.1 Skin pores is a lay term that is poorly defined in the medical literature and often is categorized in terms of arbitrary circular diameters determined through cosmetic skin analyzers.2 The term refers to pilosebaceous follicular enlargements (with or without open comedonal horny impactions) that can be visualized by the naked eye, most commonly occurring on the face and scalp. These enlarged pores remain a pervasive cosmetic concern that impacts patient quality of life. Enlarged pores are difficult to treat, in part due to lack of knowledge of the pathophysiology; thus, we review the currently proposed causes of enlarged pilosebaceous openings and the treatments in the scope of this pathogenesis with a focus on therapeutic efficacy.
Pathogenesis of Enlarged Facial Pores
It is now thought that seborrhea, loss of skin elasticity and tension, and hair follicle size are most clinically relevant to the pathogenesis of enlarged pores.2 Other potential associated and causative factors include genetic predisposition, acne, comedogenic xenobiotics, chronic photodamage, chronic radiodermatitis, and vitamin A deficiency.1,3
The direct relationship between sebum output and pore size has been well established, particularly in men who generally have higher sebum output levels than women, which likely is testosterone driven.4,5 However, there are contradictory data on whether sex affects pore size, as females also exhibit contributory hormonal factors. Sebum output and pore size increase substantially during the ovulation phase of the female menstrual cycle, likely secondary to increased progesterone affecting sebaceous gland activity.2,4 The presence of acne also is associated with enlarged facial pores, though the extent of seborrhea as a confounding factor is unclear. Furthermore, acne severity does not correlate with increased pore size.5 However, the processes of acne and facial pores are interlinked, given the frequent occurrence of open comedones within the pores.
Skin elasticity and tensile strength when defined visually and mechanically has shown a negative correlation with facial pore size and density.5 It is well known that cutaneous aging and chronic photodamage cause perturbation in the collagen and elastin framework that allows for the skin to maintain its resilient properties.6 Aged and photodamaged skin also demonstrates decreased expression of microfibril-associated glycoprotein-1 (MAGP-1), a crucial component in elastic fiber assembly and skin elasticity in the dermis and perifollicular/pore areas.7
Pore density and size appears to range diversely across ethnicities, though Chinese women exhibit notably lower pore size and density across all ages as compared to other ethnicities.8 Black individuals have aberrant epidermal architecture, defined as the presence of stalagmitelike structures at the dermoepidermal junction, correlating with enlarged pore size compared to other ethnicities.2,8
Treating Enlarged Facial Pores
Treatments for enlarged facial pores primarily aim to decrease sebum production, rejuvenate skin, remove hair, and/or decrease follicular size. Evidence-based studies are limited, and many currently used therapies have not been studied with enlarged facial pores as a primary investigative outcome. Here, we include studies that report efficacy in decreasing pore size specifically. It is important to note the lack of a uniform and objective modality with which to report skin pore size. Studies use a wide range of techniques including patient self-reporting, physician observation, and software image analyzers.
Topical Therapies
Topical retinoids are vitamin A derivatives, and they are first-line therapies in reversing the aberrant collagen and elastin-associated epidermal and dermal changes that occur with chronological aging and photoaging. Tretinoin, isotretinoin, and tazarotene have shown efficacy in multiple parameters of skin rejuvenation, including facial pores, skin wrinkling, hyperpigmentation, skin laxity, and sebum production.9 However, it is important to note that retinoids treat keratinocyte atypia in acne, and efficacy in facial pores is confounded by improvement in follicular keratinization. Because studies have not distinctly uncoupled this association, it is erroneous to conclude that retinoids reduce facial pore size and density irrespective of concomitant acne vulgaris.
Tazarotene has been evaluated for use in reducing facial pore size. In one investigation, 568 patients with moderate wrinkling or hyperpigmentation were randomized to receive tazarotene cream 0.1% or placebo once daily for 24 weeks and were evaluated for enlarged facial pores as a secondary outcome using a double-blinded physician 5-point scale.10 At week 24, 42% of tazarotene-treated patients achieved improvement of at least 1 point compared to 20% of placebo-treated patients (P<.001). Adverse events were dermatitic, as can be expected of retinoids, leading to a 4% discontinuation rate in the tazarotene group compared to 1% in the placebo group.10
Tretinoin has long been used off label for antiaging treatments but has only recently shown efficacy for facial pores. In one study, 60 women who had previously sought antiaging procedures were treated with tretinoin cream 0.025% once daily and no other antiaging products or procedures for 90 days.11 Facial pore evaluations were determined by a modified dermatoscope with a polarized analyzer for clinical scoring using a photonumeric scale. Patients improved from a baseline average score of 3.2 in facial pores to a posttreatment average score of 2.0 (P<.05) at day 84. This improvement was sustained from day 28 of treatment and corresponded to patient self-perception. Adverse events included xerosis, desquamation, burning, and erythema, which led to 3 premature discontinuations.11
Various chemical peel formulations are used in skin rejuvenation and have shown application in enlarged facial pores. Chemical peels act at the epidermal or dermal level to induce temporary breakdown and regeneration of healthier cells and improved skin matrix.12 Twenty-two Japanese women applied glycolic acid (30% solution) every 2 weeks for a total of 5 treatments and exhibited reduced appearance of conspicuous, open, and dark pores, defined by surface area and shading as determined through dermatoscopic and software analysis, with mean improvement rates of 34.6%, 11%, and 34.3%, respectively. More than 70% of participants exhibited improvement in enlarged facial pores.13 A study involving a 40% glycolic acid and vitamin C formulation demonstrated significant improvement in facial pores (28.3%; P<.001).14
The newest topical therapies studied for use in minimizing facial pilosebaceous openings are natural plant-derived copper chlorophyllin complex sodium salt (CHLcu) and tetra-hydro-jasmonic acid (LR2412). Clinical trials of these botanicals are limited with small sample sizes but are included here as novel treatments requiring further investigation.
Chlorophyllin copper complex sodium salt is derived from chlorophyll, a green pigment found in plants, and has been investigated as a topical gel in liposomal dispersions for application in photodamaged and aged skin. Chlorophyllin copper complex sodium salt exerts in vitro hyaluronidase inhibitory activity to maintain hyaluronic acid in the extracellular matrix and counteract the structural breakdown of cutaneous aging.15 Two small single-center pilot trials enrolled 10 participants each in a 3-week study of CHLcu 0.1% twice daily and an 8-week study of CHLcu 0.066% twice daily.16,17 After 3 weeks, patients treated with CHLcu 0.1% exhibited a 22.2% improvement in facial pores by clinical assessment grading, though this improvement was not significant on software imaging analysis. Patients improved the most on parameters of facial seborrhea by clinical assessment.16 After 8 weeks, patients treated with CHLcu 0.066% exhibited 25.3% improvement in facial pores by clinical assessment grading.17 Treatments were reported to be well tolerated without noted adverse events in both studies.
Tetra-hydro-jasmonic acid is an analogue of jasmonic acid, a plant hormone derived from linoleic acid. Due to its favorable safety profile and bioavailability, penetration into epidermal and dermal layers, and potential effects in rejuvenating desquamation, LR2412 is currently being assessed for treatment of skin wrinkles, texture, and pores.18 Its effect is thought to relate to stimulation of laminin-5, collagen IV, and fibrillin deposition at the dermoepidermal junction.19 In an open-label trial of a topical preparation of LR2412, 15 participants were treated twice daily for 6 weeks and assessed through investigator clinical assessment scoring.20 Investigator scoring of pores improved by 25.2% from baseline (P<.05) after 6 weeks of treatment. Improvement in pores was seen as early as days 1 and 3. No serious adverse events were reported, though 2 participants developed acne on follow-up.20
Tetra-hydro-jasmonic acid also is formulated with retinol (retinol 0.2%/LR2412 2.0%) and demonstrated cosmetic efficacy in a noninferiority trial with tretinoin cream 0.025%.11 Sixty patients each were randomized to retinol/LR2412 or tretinoin at bedtime and treated for 90 days. At day 84, participants in the retinol/LR2412 group exhibited an improvement in investigator clinical assessment scoring from a baseline of 3.6 to 2.5 (P<.05). There were no significant differences in investigator-assessed efficacy between the treatment arms. Participants reported similar or better results and fewer side effects with retinol/LR2412 on self-questionnaires. Eight participants treated with retinol/LR2412 and 15 participants treated with tretinoin reported various incidences of skin irritation, burning, and desquamation.11
Oral Therapies
The most commonly used oral therapies for enlarged pores are antiandrogens, such as combined oral contraceptives, spironolactone, and cyproterone acetate, which modulate sebum production due to the presence of androgen receptors within sebaceous glands.21 Forty-four white women in an open-label, phase 4 study were treated with combined oral contraceptives containing chlormadinone acetate–ethinyl estradiol for 6 menstrual cycles, with standardized photography taken before and after the treatment period for software analysis. After 6 treatment cycles, 9.1% (4/44) of participants had visibly enlarged pores of the forehead and cheeks compared to 43.2% (19/44) of participants at baseline (P<.0001).22 The effects of other antiandrogens on facial pores have not been studied in this capacity.
Lasers, Radiofrequency, and Ultrasound Devices
The development of various devices that can deliver targeted thermal or ultrasound energy to the skin offers the newest and most robust modality in cosmetic therapy. The mechanism of their efficacy may be due to a combination of induced remodeling of collagen fibers near pilosebaceous openings to increase skin elasticity and decrease sebum production.2,23
Devices with established antiaging effects have been extensively reviewed and include the gold particle 800-nm diode laser, 1450-nm diode laser, microneedle apparatuses, fractional radiofrequency devices, 2790-nm erbium:YAG laser, nonablative 1410-nm fractionated erbium-doped fiber laser, and nonablative 1440-nm fractional laser.2
Literature on the use of these devices for minimizing facial pore size is limited. One treatment of intense focused ultrasound using a 3-mm transducer successfully improved overall pore appearance in 91% of sites at 6-week follow-up on a clinical grading scale.24 Three sessions of nonablative 1410-nm fractionated erbium-doped fiber laser treatments yielded facial skin pore minimization of greater than 51% in 14 of 15 participants.25
The nonablative 1440-nm diode fractional laser received 510(k) clearance by the US Food and Drug Administration in 2011 for aesthetic use in chronologically aged and photoaged skin. Twenty participants treated for 2 weeks and a total of 6 facial treatments with this laser system showed a 17% average improvement in facial pore score on software analysis (P≤.002). Adverse events were mild and included erythema and xerosis.26
Conclusion
The reliability of available literature on efficacy of various treatments in diminishing facial skin pores has been challenging given that most studies are low in power, lack control groups, use nonuniform methods of reporting outcomes, and do not report complete adverse events. Thus, all results should be interpreted with caution.
Overall, it is clear that the pathogenesis of enlarged facial pores is multifactorial and complex, necessitating a similar approach to therapeutics. Topical treatments offer a range of diverse therapies with proven benefit in facial pore reduction. The advent of lasers and devices offers constantly evolving therapeutic options with diffuse antiaging effects. Despite the numerous topical, oral, and device-oriented options, enlarged facial pores remain a challenging cosmetic concern. More robust efficacy studies on new treatments are necessary.
Enlarged facial pores are superficial skin structures that are visualized as small openings on the skin corresponding to the openings of the pilosebaceous apparatus. These openings may be impacted with horny follicular plugs consisting of sebaceous debris that appear as open comedones.1 Skin pores is a lay term that is poorly defined in the medical literature and often is categorized in terms of arbitrary circular diameters determined through cosmetic skin analyzers.2 The term refers to pilosebaceous follicular enlargements (with or without open comedonal horny impactions) that can be visualized by the naked eye, most commonly occurring on the face and scalp. These enlarged pores remain a pervasive cosmetic concern that impacts patient quality of life. Enlarged pores are difficult to treat, in part due to lack of knowledge of the pathophysiology; thus, we review the currently proposed causes of enlarged pilosebaceous openings and the treatments in the scope of this pathogenesis with a focus on therapeutic efficacy.
Pathogenesis of Enlarged Facial Pores
It is now thought that seborrhea, loss of skin elasticity and tension, and hair follicle size are most clinically relevant to the pathogenesis of enlarged pores.2 Other potential associated and causative factors include genetic predisposition, acne, comedogenic xenobiotics, chronic photodamage, chronic radiodermatitis, and vitamin A deficiency.1,3
The direct relationship between sebum output and pore size has been well established, particularly in men who generally have higher sebum output levels than women, which likely is testosterone driven.4,5 However, there are contradictory data on whether sex affects pore size, as females also exhibit contributory hormonal factors. Sebum output and pore size increase substantially during the ovulation phase of the female menstrual cycle, likely secondary to increased progesterone affecting sebaceous gland activity.2,4 The presence of acne also is associated with enlarged facial pores, though the extent of seborrhea as a confounding factor is unclear. Furthermore, acne severity does not correlate with increased pore size.5 However, the processes of acne and facial pores are interlinked, given the frequent occurrence of open comedones within the pores.
Skin elasticity and tensile strength when defined visually and mechanically has shown a negative correlation with facial pore size and density.5 It is well known that cutaneous aging and chronic photodamage cause perturbation in the collagen and elastin framework that allows for the skin to maintain its resilient properties.6 Aged and photodamaged skin also demonstrates decreased expression of microfibril-associated glycoprotein-1 (MAGP-1), a crucial component in elastic fiber assembly and skin elasticity in the dermis and perifollicular/pore areas.7
Pore density and size appears to range diversely across ethnicities, though Chinese women exhibit notably lower pore size and density across all ages as compared to other ethnicities.8 Black individuals have aberrant epidermal architecture, defined as the presence of stalagmitelike structures at the dermoepidermal junction, correlating with enlarged pore size compared to other ethnicities.2,8
Treating Enlarged Facial Pores
Treatments for enlarged facial pores primarily aim to decrease sebum production, rejuvenate skin, remove hair, and/or decrease follicular size. Evidence-based studies are limited, and many currently used therapies have not been studied with enlarged facial pores as a primary investigative outcome. Here, we include studies that report efficacy in decreasing pore size specifically. It is important to note the lack of a uniform and objective modality with which to report skin pore size. Studies use a wide range of techniques including patient self-reporting, physician observation, and software image analyzers.
Topical Therapies
Topical retinoids are vitamin A derivatives, and they are first-line therapies in reversing the aberrant collagen and elastin-associated epidermal and dermal changes that occur with chronological aging and photoaging. Tretinoin, isotretinoin, and tazarotene have shown efficacy in multiple parameters of skin rejuvenation, including facial pores, skin wrinkling, hyperpigmentation, skin laxity, and sebum production.9 However, it is important to note that retinoids treat keratinocyte atypia in acne, and efficacy in facial pores is confounded by improvement in follicular keratinization. Because studies have not distinctly uncoupled this association, it is erroneous to conclude that retinoids reduce facial pore size and density irrespective of concomitant acne vulgaris.
Tazarotene has been evaluated for use in reducing facial pore size. In one investigation, 568 patients with moderate wrinkling or hyperpigmentation were randomized to receive tazarotene cream 0.1% or placebo once daily for 24 weeks and were evaluated for enlarged facial pores as a secondary outcome using a double-blinded physician 5-point scale.10 At week 24, 42% of tazarotene-treated patients achieved improvement of at least 1 point compared to 20% of placebo-treated patients (P<.001). Adverse events were dermatitic, as can be expected of retinoids, leading to a 4% discontinuation rate in the tazarotene group compared to 1% in the placebo group.10
Tretinoin has long been used off label for antiaging treatments but has only recently shown efficacy for facial pores. In one study, 60 women who had previously sought antiaging procedures were treated with tretinoin cream 0.025% once daily and no other antiaging products or procedures for 90 days.11 Facial pore evaluations were determined by a modified dermatoscope with a polarized analyzer for clinical scoring using a photonumeric scale. Patients improved from a baseline average score of 3.2 in facial pores to a posttreatment average score of 2.0 (P<.05) at day 84. This improvement was sustained from day 28 of treatment and corresponded to patient self-perception. Adverse events included xerosis, desquamation, burning, and erythema, which led to 3 premature discontinuations.11
Various chemical peel formulations are used in skin rejuvenation and have shown application in enlarged facial pores. Chemical peels act at the epidermal or dermal level to induce temporary breakdown and regeneration of healthier cells and improved skin matrix.12 Twenty-two Japanese women applied glycolic acid (30% solution) every 2 weeks for a total of 5 treatments and exhibited reduced appearance of conspicuous, open, and dark pores, defined by surface area and shading as determined through dermatoscopic and software analysis, with mean improvement rates of 34.6%, 11%, and 34.3%, respectively. More than 70% of participants exhibited improvement in enlarged facial pores.13 A study involving a 40% glycolic acid and vitamin C formulation demonstrated significant improvement in facial pores (28.3%; P<.001).14
The newest topical therapies studied for use in minimizing facial pilosebaceous openings are natural plant-derived copper chlorophyllin complex sodium salt (CHLcu) and tetra-hydro-jasmonic acid (LR2412). Clinical trials of these botanicals are limited with small sample sizes but are included here as novel treatments requiring further investigation.
Chlorophyllin copper complex sodium salt is derived from chlorophyll, a green pigment found in plants, and has been investigated as a topical gel in liposomal dispersions for application in photodamaged and aged skin. Chlorophyllin copper complex sodium salt exerts in vitro hyaluronidase inhibitory activity to maintain hyaluronic acid in the extracellular matrix and counteract the structural breakdown of cutaneous aging.15 Two small single-center pilot trials enrolled 10 participants each in a 3-week study of CHLcu 0.1% twice daily and an 8-week study of CHLcu 0.066% twice daily.16,17 After 3 weeks, patients treated with CHLcu 0.1% exhibited a 22.2% improvement in facial pores by clinical assessment grading, though this improvement was not significant on software imaging analysis. Patients improved the most on parameters of facial seborrhea by clinical assessment.16 After 8 weeks, patients treated with CHLcu 0.066% exhibited 25.3% improvement in facial pores by clinical assessment grading.17 Treatments were reported to be well tolerated without noted adverse events in both studies.
Tetra-hydro-jasmonic acid is an analogue of jasmonic acid, a plant hormone derived from linoleic acid. Due to its favorable safety profile and bioavailability, penetration into epidermal and dermal layers, and potential effects in rejuvenating desquamation, LR2412 is currently being assessed for treatment of skin wrinkles, texture, and pores.18 Its effect is thought to relate to stimulation of laminin-5, collagen IV, and fibrillin deposition at the dermoepidermal junction.19 In an open-label trial of a topical preparation of LR2412, 15 participants were treated twice daily for 6 weeks and assessed through investigator clinical assessment scoring.20 Investigator scoring of pores improved by 25.2% from baseline (P<.05) after 6 weeks of treatment. Improvement in pores was seen as early as days 1 and 3. No serious adverse events were reported, though 2 participants developed acne on follow-up.20
Tetra-hydro-jasmonic acid also is formulated with retinol (retinol 0.2%/LR2412 2.0%) and demonstrated cosmetic efficacy in a noninferiority trial with tretinoin cream 0.025%.11 Sixty patients each were randomized to retinol/LR2412 or tretinoin at bedtime and treated for 90 days. At day 84, participants in the retinol/LR2412 group exhibited an improvement in investigator clinical assessment scoring from a baseline of 3.6 to 2.5 (P<.05). There were no significant differences in investigator-assessed efficacy between the treatment arms. Participants reported similar or better results and fewer side effects with retinol/LR2412 on self-questionnaires. Eight participants treated with retinol/LR2412 and 15 participants treated with tretinoin reported various incidences of skin irritation, burning, and desquamation.11
Oral Therapies
The most commonly used oral therapies for enlarged pores are antiandrogens, such as combined oral contraceptives, spironolactone, and cyproterone acetate, which modulate sebum production due to the presence of androgen receptors within sebaceous glands.21 Forty-four white women in an open-label, phase 4 study were treated with combined oral contraceptives containing chlormadinone acetate–ethinyl estradiol for 6 menstrual cycles, with standardized photography taken before and after the treatment period for software analysis. After 6 treatment cycles, 9.1% (4/44) of participants had visibly enlarged pores of the forehead and cheeks compared to 43.2% (19/44) of participants at baseline (P<.0001).22 The effects of other antiandrogens on facial pores have not been studied in this capacity.
Lasers, Radiofrequency, and Ultrasound Devices
The development of various devices that can deliver targeted thermal or ultrasound energy to the skin offers the newest and most robust modality in cosmetic therapy. The mechanism of their efficacy may be due to a combination of induced remodeling of collagen fibers near pilosebaceous openings to increase skin elasticity and decrease sebum production.2,23
Devices with established antiaging effects have been extensively reviewed and include the gold particle 800-nm diode laser, 1450-nm diode laser, microneedle apparatuses, fractional radiofrequency devices, 2790-nm erbium:YAG laser, nonablative 1410-nm fractionated erbium-doped fiber laser, and nonablative 1440-nm fractional laser.2
Literature on the use of these devices for minimizing facial pore size is limited. One treatment of intense focused ultrasound using a 3-mm transducer successfully improved overall pore appearance in 91% of sites at 6-week follow-up on a clinical grading scale.24 Three sessions of nonablative 1410-nm fractionated erbium-doped fiber laser treatments yielded facial skin pore minimization of greater than 51% in 14 of 15 participants.25
The nonablative 1440-nm diode fractional laser received 510(k) clearance by the US Food and Drug Administration in 2011 for aesthetic use in chronologically aged and photoaged skin. Twenty participants treated for 2 weeks and a total of 6 facial treatments with this laser system showed a 17% average improvement in facial pore score on software analysis (P≤.002). Adverse events were mild and included erythema and xerosis.26
Conclusion
The reliability of available literature on efficacy of various treatments in diminishing facial skin pores has been challenging given that most studies are low in power, lack control groups, use nonuniform methods of reporting outcomes, and do not report complete adverse events. Thus, all results should be interpreted with caution.
Overall, it is clear that the pathogenesis of enlarged facial pores is multifactorial and complex, necessitating a similar approach to therapeutics. Topical treatments offer a range of diverse therapies with proven benefit in facial pore reduction. The advent of lasers and devices offers constantly evolving therapeutic options with diffuse antiaging effects. Despite the numerous topical, oral, and device-oriented options, enlarged facial pores remain a challenging cosmetic concern. More robust efficacy studies on new treatments are necessary.
- Uhoda E, Pierard-Franchimont C, Petit L, et al. The conundrum of skin pores in dermocosmetology. Dermatology. 2005;210:3-7.
- Lee SJ, Seok J, Jeong SY, et al. Facial pores: definition, causes, and treatment options. Dermatol Surg. 2016;42:277-285.
- Pierard GE, Pierard-Franchimont C, Marks R, et al. EEMCO guidance for the in vivo assessment of skin greasiness. The EEMCO Group. Skin Pharmacol Appl Skin Physiol. 2000;13:372-389.
- Roh M, Han M, Kim D, et al. Sebum output as a factor contributing to the size of facial pores. Br J Dermatol. 2006;155:890-894.
- Kim BY, Choi JW, Park KC, et al. Sebum, acne, skin elasticity, and gender difference-which is the major influencing factor for facial pores? Skin Res Technol. 2013;19:E45-E53.
- Uitto J. The role of elastin and collagen in cutaneous aging: intrinsic aging versus photoexposure. J Drugs Dermatol. 2008;7(2 suppl):S12-S16.
- Zheng Q, Chen S, Chen Y, et al. Investigation of age-related decline of microfibril-associated glycoprotein-1 in human skin through immunohistochemistry study. Clin Cosmet Investig Dermatol. 2013;6:317-323.
- Sugiyama-Nakagiri Y, Sugata K, Hachiya A, et al. Ethnic differences in the structural properties of facial skin. J Dermatol Sci. 2009;53:135-139.
- Mukherjee S, Date A, Patravale V, et al. Retinoids in the treatment of skin aging: an overview of clinical efficacy and safety. Clin Interv Aging. 2006;1:327-348.
- Kang S, Krueger GG, Tanghetti EA, et al; Tazarotene Cream in Photodamage Study Group. A multicenter, randomized, double-blind trial of tazarotene 0.1% cream in the treatment of photodamage. J Am Acad Dermatol. 2005;52:268-274.
- Bouloc A, Vergnanini AL, Issa MC. A double-blind randomized study comparing the association of retinol and LR2412 with tretinoin 0.025% in photoaged skin. J Cosmet Dermatol. 2015;14:40-46.
- Fischer TC, Perosino E, Poli F, et al. Chemical peels in aesthetic dermatology: an update 2009 [published online September 8, 2009]. J Eur Acad Dermatol Venereol. 2010;24:281-292.
- Kakudo N, Kushida S, Tanaka N, et al. A novel method to measure conspicuous facial pores using computer analysis of digital-camera-captured images: the effect of glycolic acid chemical peeling. Skin Res Technol. 2011;17:427-433.
- Kim WS. Efficacy and safety of a new superficial chemical peel using alpha-hydroxy acid, vitamin C and oxygen for melasma. J Cosmet Laser Ther. 2013;15:21-24.
- McCook JP, Dorogi PL, Vasily DB, et al. In vitro inhibition of hyaluronidase by sodium copper chlorophyllin complex and chlorophyllin analogs. Clin Cosmet Investig Dermatol. 2015;8:443-448.
- Stephens TJ, McCook JP, Herndon JH Jr. Pilot study of topical copper chlorophyllin complex in subjects with facial acne and large pores. J Drugs Dermatol. 2015;14:589-592.
- Sigler ML, Stephens TJ. Assessment of the safety and efficacy of topical copper chlorophyllin in women with photodamaged facial skin. J Drugs Dermatol. 2015;14:401-404.
- Alexiades M. Jasmonates and tetrahydrojasmonic acid: a novel class of anti-aging molecules. J Drugs Dermatol. 2016;15:206-207.
- Tran C, Michelet JF, Simonetti L, et al. In vitro and in vivo studies with tetra-hydro-jasmonic acid (LR2412) reveal its potential to correct signs of skin ageing. J Eur Acad Dermatol Venereol. 2014;28:415-423.
- Alexiades M. Clinical assessment of a novel jasmonate cosmeceutical, LR2412-Cx, for the treatment of skin aging. J Drugs Dermatol. 2016;15:209-215.
- Lam C, Zaenglein AL. Contraceptive use in acne. Clin Dermatol. 2014;32:502-515.
- Kerscher M, Reuther T, Bayrhammer J, et al. Effects of an oral contraceptive containing chlormadinone and ethinylestradiol on acne-prone skin of women of different age groups: an open-label, single-centre, phase IV study. Clin Drug Investig. 2008;28:703-711.
- Schmults CD, Phelps R, Goldberg DJ. Nonablative facial remodeling: erythema reduction and histologic evidence of new collagen formation using a 300-microsecond 1064-nm Nd:YAG laser. Arch Dermatol. 2004;140:1373-1376.
- Lee HJ, Lee KR, Park JY, et al. The efficacy and safety of intense focused ultrasound in the treatment of enlarged facial pores in Asian skin. J Dermatolog Treat. 2015;26:73-77.
- Suh DH, Chang KY, Lee SJ, et al. Treatment of dilated pores with 1410-nm fractional erbium-doped fiber laser. Lasers Med Sci. 2015;30:1135-1139.
- Saedi N, Petrell K, Arndt K, et al. Evaluating facial pores and skin texture after low-energy nonablative fractional 1440-nm laser treatments. J Am Acad Dermatol. 2013;68:113-118.
- Uhoda E, Pierard-Franchimont C, Petit L, et al. The conundrum of skin pores in dermocosmetology. Dermatology. 2005;210:3-7.
- Lee SJ, Seok J, Jeong SY, et al. Facial pores: definition, causes, and treatment options. Dermatol Surg. 2016;42:277-285.
- Pierard GE, Pierard-Franchimont C, Marks R, et al. EEMCO guidance for the in vivo assessment of skin greasiness. The EEMCO Group. Skin Pharmacol Appl Skin Physiol. 2000;13:372-389.
- Roh M, Han M, Kim D, et al. Sebum output as a factor contributing to the size of facial pores. Br J Dermatol. 2006;155:890-894.
- Kim BY, Choi JW, Park KC, et al. Sebum, acne, skin elasticity, and gender difference-which is the major influencing factor for facial pores? Skin Res Technol. 2013;19:E45-E53.
- Uitto J. The role of elastin and collagen in cutaneous aging: intrinsic aging versus photoexposure. J Drugs Dermatol. 2008;7(2 suppl):S12-S16.
- Zheng Q, Chen S, Chen Y, et al. Investigation of age-related decline of microfibril-associated glycoprotein-1 in human skin through immunohistochemistry study. Clin Cosmet Investig Dermatol. 2013;6:317-323.
- Sugiyama-Nakagiri Y, Sugata K, Hachiya A, et al. Ethnic differences in the structural properties of facial skin. J Dermatol Sci. 2009;53:135-139.
- Mukherjee S, Date A, Patravale V, et al. Retinoids in the treatment of skin aging: an overview of clinical efficacy and safety. Clin Interv Aging. 2006;1:327-348.
- Kang S, Krueger GG, Tanghetti EA, et al; Tazarotene Cream in Photodamage Study Group. A multicenter, randomized, double-blind trial of tazarotene 0.1% cream in the treatment of photodamage. J Am Acad Dermatol. 2005;52:268-274.
- Bouloc A, Vergnanini AL, Issa MC. A double-blind randomized study comparing the association of retinol and LR2412 with tretinoin 0.025% in photoaged skin. J Cosmet Dermatol. 2015;14:40-46.
- Fischer TC, Perosino E, Poli F, et al. Chemical peels in aesthetic dermatology: an update 2009 [published online September 8, 2009]. J Eur Acad Dermatol Venereol. 2010;24:281-292.
- Kakudo N, Kushida S, Tanaka N, et al. A novel method to measure conspicuous facial pores using computer analysis of digital-camera-captured images: the effect of glycolic acid chemical peeling. Skin Res Technol. 2011;17:427-433.
- Kim WS. Efficacy and safety of a new superficial chemical peel using alpha-hydroxy acid, vitamin C and oxygen for melasma. J Cosmet Laser Ther. 2013;15:21-24.
- McCook JP, Dorogi PL, Vasily DB, et al. In vitro inhibition of hyaluronidase by sodium copper chlorophyllin complex and chlorophyllin analogs. Clin Cosmet Investig Dermatol. 2015;8:443-448.
- Stephens TJ, McCook JP, Herndon JH Jr. Pilot study of topical copper chlorophyllin complex in subjects with facial acne and large pores. J Drugs Dermatol. 2015;14:589-592.
- Sigler ML, Stephens TJ. Assessment of the safety and efficacy of topical copper chlorophyllin in women with photodamaged facial skin. J Drugs Dermatol. 2015;14:401-404.
- Alexiades M. Jasmonates and tetrahydrojasmonic acid: a novel class of anti-aging molecules. J Drugs Dermatol. 2016;15:206-207.
- Tran C, Michelet JF, Simonetti L, et al. In vitro and in vivo studies with tetra-hydro-jasmonic acid (LR2412) reveal its potential to correct signs of skin ageing. J Eur Acad Dermatol Venereol. 2014;28:415-423.
- Alexiades M. Clinical assessment of a novel jasmonate cosmeceutical, LR2412-Cx, for the treatment of skin aging. J Drugs Dermatol. 2016;15:209-215.
- Lam C, Zaenglein AL. Contraceptive use in acne. Clin Dermatol. 2014;32:502-515.
- Kerscher M, Reuther T, Bayrhammer J, et al. Effects of an oral contraceptive containing chlormadinone and ethinylestradiol on acne-prone skin of women of different age groups: an open-label, single-centre, phase IV study. Clin Drug Investig. 2008;28:703-711.
- Schmults CD, Phelps R, Goldberg DJ. Nonablative facial remodeling: erythema reduction and histologic evidence of new collagen formation using a 300-microsecond 1064-nm Nd:YAG laser. Arch Dermatol. 2004;140:1373-1376.
- Lee HJ, Lee KR, Park JY, et al. The efficacy and safety of intense focused ultrasound in the treatment of enlarged facial pores in Asian skin. J Dermatolog Treat. 2015;26:73-77.
- Suh DH, Chang KY, Lee SJ, et al. Treatment of dilated pores with 1410-nm fractional erbium-doped fiber laser. Lasers Med Sci. 2015;30:1135-1139.
- Saedi N, Petrell K, Arndt K, et al. Evaluating facial pores and skin texture after low-energy nonablative fractional 1440-nm laser treatments. J Am Acad Dermatol. 2013;68:113-118.
Practice Points
- The pathogenesis of enlarged facial pores is speculated to be associated with sebum production, skin aging and photodamage, and hair follicle size, among other factors.
- Current treatment modalities for enlarged facial pores target these factors and include topical retinoids, chemical peels, oral antiandrogens, lasers, radiofrequency, and ultrasound devices, with the latter devices offering the most novel and robust choices.
- New botanically derived topical treatments, specifically copper chlorophyllin complex sodium salt and tetra-hydro-jasmonic acid, are in development with initial positive results, though studies are still limited.
Therapies to Improve the Cosmetic Symptoms of Atopic Dermatitis
Atopic dermatitis (AD), more commonly referred to as eczema, is a chronic pruritic inflammatory skin disease that frequently affects both children and adults. Atopic dermatitis is most common in urban and developed countries, with a prevalence of approximately 11% in the United States.1 The pathophysiology of AD is complex and not fully understood, despite the increasing incidence of the disease.2 A myriad of factors, including genetics, defects in the innate and adaptive immune response, and skin barrier abnormalities all contribute to the pathogenesis.3,4 As a result of these abnormalities, patients with AD are more prone to damage from environmental irritants and allergens.
The diagnosis of AD is made clinically based on patient history and visual assessment of the skin.5 Atopic dermatitis follows a chronic and relapsing course characterized by severe pruritus and visible skin changes including xerosis, redness, blistering, oozing, crusting, scaling, thickening, and color change.6,7 Due to the genetic predisposition to make IgE antibodies in response to common environmental and food antigens, patients also may develop allergic rhinitis, asthma, and food-induced anaphylaxis.8,9 Patients also are susceptible to cutaneous viral, fungal, and bacterial infections, the most common of which is an infection with Staphylococcus aureus.10
Atopic dermatitis can have a substantial impact on quality of life, which has been revealed in studies linking chronic skin conditions to depression, impairment of self-esteem, and financial hardship.11 Because skin appearance impacts how a person is initially perceived by others, patients often report feeling self-conscious about their disease and experience teasing or bullying.12 To improve their physical appearance, patients may incur considerable medical expenses. According to 2 population-based studies comprising more than 60,000 adults aged 18 to 85 years, individuals with AD face substantial financial burdens and utilize the health care system more than those without the disease. On average, patients with AD spend $371 to $489 per year on costly out-of-pocket medical expenses and report more absences from work.13
Although there currently is no cure for AD, treatment is aimed at relieving its symptoms and preventing acute exacerbations as well as improving cosmetic appearance to enhance quality of life. Treatment must follow a stepwise approach, which focuses on hydrating the skin, repairing the dysfunctional epithelial barrier, and controlling inflammation. Thus, the standard of care focuses on avoiding skin irritants and triggers along with the use of moisturizers and topical corticosteroids (TCs). In patients with recurring severe disease, topical calcineurin inhibitors, phototherapy, and systemic agents also may be utilized.14
Avoiding Irritants and Triggers
Atopic dermatitis is worsened by skin contact with physical and chemical irritants. Exacerbating factors in AD include exposure to food allergens, dust, emotional stress, detergents, fragranced soaps, textiles, and ingredients in cosmetic products. Patients should be advised to use mild detergents and fragrance-free soaps and to avoid harsh materials such as wool. However, avoidance of specific ingredients in cosmetic products is not as straightforward because manufacturers are not required to disclose certain ingredients. In general, fragrances such as balsam of Peru and cinnamaldehyde, as well as preservatives such as parabens, isothiazolinones, and formaldehyde, should be avoided when selecting cosmetic products. Patients with AD should purchase fragrance-free products that are specifically formulated for sensitive skin. Additionally, patients should not apply makeup if their skin is irritated or oozing, as the flare may worsen.15
Moisturizers
Due to the impaired skin barrier function in patients with AD, regular application of fragrance-free moisturizers is essential to maintain hydration and to reduce xerosis. Various classes of moisturizers may be prescribed (eg, lotions, creams, gels, ointments) based on disease severity and patient preference. Light preparations such as lotions, creams, and gels have a high water content and generally are more appealing from a cosmetic standpoint because they do not create any residue on the skin. However, these options may require more frequent application because they are absorbed quickly. Heavy preparations such as ointments have longer-lasting effects due to their high oil content but tend to be less cosmetically appealing because of their greasiness.16
Although the amount and frequency of application of moisturizers has not been defined, liberal application several times daily is generally advised to minimize xerosis.17 Most physicians recommend applying moisturizer to the skin immediately after bathing to seal in moisture. Some patients prefer to use lotions and creams during the day because these products make the skin feel smooth and reserve the greasier ointments for nighttime application.
Topical Corticosteroids
Prescribed in conjunction with moisturizers, TCs are the mainstay of anti-inflammatory therapy in AD. Topical corticosteroids are classified into 7 groups based on potency, ranging from superpotent (class 1) to least potent (class 7). For acute AD flares, TCs should be applied daily for up to several weeks. Once the inflammation has resolved, it is recommended to apply TCs once to twice weekly to reduce the rate of relapse.18 Despite their effectiveness in the treatment of acute AD flares, TCs have a considerable side-effect profile. Potential adverse effects include skin atrophy, striae, telangiectasia, hypopigmentation, increased hair growth, steroid acne, growth retardation, and Cushing syndrome. Skin atrophy, which is the most common complication associated with TCs, results in shiny transparent skin, allowing for visualization of veins.19,20 Although many of these side effects will resolve after discontinuing the TCs, they are aesthetically displeasing during treatment, making it crucial for physicians to educate their patients on the proper usage of TCs to prevent negative outcomes.
Topical Calcineurin Inhibitors
Topical calcineurin inhibitors (TCIs) are a class of anti-inflammatories that are used to overcome the adverse effects of TCs. They are approved as alternatives to TCs in patients who have failed to respond to other topical treatments as well as those who have developed cutaneous atrophy from the use of TCs or have AD in sensitive areas such as the face, neck, and/or skin folds. Unlike TCs, TCIs do not cause atrophy, striae, or discoloration of the skin, which makes them more desirable from a cosmetic perspective. Their mechanism of action is distinct from TCs in that they inhibit calcineurin-dependent T-cell activation, thus preventing the transcription of inflammatory cytokines.21 Two TCIs are currently available: tacrolimus ointment 0.03% and 0.1% concentrations for moderate to severe AD and pimecrolimus cream 1% for mild to moderate AD.22 Twice-daily application of TCIs is recommended to decrease inflammation and pruritus associated with AD. Studies also have shown that intermittent use of TCIs 3 times weekly can aid in reducing relapses.23-25
The results from clinical trials demonstrate the rapid and continuous effects of both pimecrolimus and tacrolimus. In a controlled long-term study of adults, pimecrolimus provided significant relief of pruritus as soon as day 3 (P<.001).26,27 Pimecrolimus also provides long-term relief by preventing disease progression to flares, which was exemplified in a study (N=713) with no flares in 51% of pimecrolimus patients at 12 months versus 28% in the conventional treatment group (P<.001).28 Similarly, long-term studies of tacrolimus demonstrated an improvement of all symptoms of AD after 1 week of treatment. Maximal improvement was achieved with continued use of tacrolimus, and up to 1 year of tacrolimus use was found to be safe and effective.29,30 Thus, TCIs have been proven to be an effective choice in maintenance therapy for AD and have a good safety profile. The most common adverse effects of TCIs are local skin reactions, such as stinging and burning at the site of application. Rare cases of skin cancer and lymphoma have been reported; however, a causal relationship has yet to be established.31,32
Additional Therapies
Wet wrap therapy is effective for rapid control of flares and in controlling recalcitrant AD. Wet wraps function via several mechanisms; they provide a mechanical barrier against scratching, increase moisture and soften the skin, and enhance absorption of topical medications.33,34 The following method is employed when using wet wraps: an emollient or TC is applied to the area, a tubular bandage soaked in warm water is wrapped over the area, and dry bandages are used to form the outermost layer. Although wet wrap therapy is beneficial in treating AD, it is labor intensive and may require the expertise of a nurse. Thus, unlike other therapies, which patients can easily apply without interfering with their day, wet wraps must be applied at home or in a hospital setting.
Light therapy is another effective method of controlling AD. Although multiple forms of UV phototherapy are beneficial for symptom control in AD, there is no definitive recommendation regarding the specific type of light therapy due to a lack of comparative studies. Natural sunlight, narrowband UVB, broadband UVB, UVA, oral or topical psoralen plus UVA, as well as UVA and UVB can all be utilized in the treatment of AD. However, similar to natural sunlight, artificial light therapy can cause burning, blistering, hyperpigmentation, dark spots, and wrinkles. Because society places a large emphasis on maintaining a youthful appearance, patients may be hesitant to use a treatment that could potentially advance the skin’s aging process. Thus, it is important that this therapy is properly controlled to prevent further skin damage.35-37
When optimal topical regimens and phototherapy have failed to control AD, systemic immunomodulation therapies may be used. Currently, the most commonly used medications are cyclosporine 150 to 300 mg daily, methotrexate 7.5 to 25 mg weekly, mycophenolate mofetil 0.5 to 3 g daily, and azathioprine 1 to 3 mg/kg daily.38,39 Decisions regarding the specific class of drugs should be based on the patient’s AD status, comorbidities, and personal preference.
Conclusion
Atopic dermatitis is a common chronic condition that can occur at any age and cause substantial physical, psychological, social, and/or emotional stress for patients and their families. Although TCs have been the standard of treatment for many years, ongoing concerns regarding their safety have led to the use of TCIs, which overcome some of the drawbacks of steroid therapy. Phototherapy and systemic immunosuppressant therapy are reserved for patients who have not responded to optimal topical therapies. Although several therapeutic avenues exist for patients, there is a need for the development of more effective and safer drugs. Furthermore, cosmetic products created specifically for patients with AD would be beneficial, as patients often struggle to select products that do not cause more harm than good. Given the complexity of the pathogenesis of AD, further research must focus on defining the specific pathways involved in the disease and targeting these pathways with therapies.
1. Shaw TE, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
2. Deckers IA, McLean S, Linssen S, et al. Investigating international time trends in the incidence and prevalence of atopic eczema 1990-2010: a systematic review of epidemiological studies. PLoS One. 2012;7:e39803.
3. Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242:233-246.
4. Peate I. Eczema: causes, symptoms and treatment in the community. Br J Community Nurs. 2011;16:324, 326-331.
5. Williams HC, Burney PG, Pembroke AC, et al. The U.K. Working Party’s diagnostic criteria for atopic dermatitis. III. independent hospital validation. Br J Dermatol. 1994;131:406-416.
6. Magin P, Adams J, Heading G, et al. Experiences of appearance-related teasing and bullying in skin diseases and their psychological sequelae: results of a qualitative study. Scand J Caring Sci. 2008;22:430-436.
7. Beattie P, Lewis-Jones M. A comparative study of impairment of quality of life in children with skin disease and children with other chronic childhood diseases. Br J Dermatol. 2006;155:145-151.
8. Spergel JM. From atopic dermatitis to asthma: the atopic march [published online January 22, 2010]. Ann Allergy Asthma Immunol. 2010;105:99-106; quiz 107-109, 117.
9. Leung DY. New insights into atopic dermatitis: role of skin barrier and immune dysregulation. Allergol Int. 2013;62:151-161.
10. Balma-Mena A, Lara-Corrales I, Zeller J, et al. Colonization with community-acquired methicillin-resistant Staphylococcus aureus in children with atopic dermatitis: a cross-sectional study. Int J Dermatol. 2011;50:682-688.
11. Strawser MS, Storch EA, Roberti JW. The Teasing Questionnaire-Revised: measurement of childhood teasing in adults. J Anxiety Disord. 2005;19:780-792.
12. Magin P, Adams J, Heading G, et al. Experiences of appearance-related teasing and bullying in skin diseases and their psychological sequelae: results of a qualitative study. Scand J Caring Sci. 2008;22:430-436.
13. Silverberg J. Health care utilization, patient costs, and access to care in US adults with eczema: a population-based study. JAMA Dermatol. 2015;151:743-752.
14. Ellis C, Luger T, Abeck D, et al. International Consensus Conference on Atopic Dermatitis II (ICCAD II): clinical update and current treatment strategies. Br J Dermatol. 2003;148(suppl 63):3-10.
15. Kim K. Influences of environmental chemicals on atopic dermatitis. Toxicol Res. 2015;31:89-96.
16. Ridd M, Redmond N, Hollinghurst S, et al. Choice of Moisturiser for Eczema Treatment (COMET): study protocol for a randomized controlled trial. Trials. 2015;16:304.
17. Hon KL, Ching GK, Leung TF, et al. Estimating emollient usage in patients with eczema. Clin Exp Dermatol. 2010;35:22-26.
18. Hanifin J, Gupta AK, Rajagopalan R. Intermittent dosing of fluticasone propionate cream for reducing the risk of relapse in atopic dermatitis patients. Br J Dermatol. 2002;147:528-537.
19. Hill CJ, Rostenberg A Jr. Adverse effects from topical steroids. Cutis. 1978;21:624-628.
20. Ruiz-Maldonado R, Zapata G, Lourdes T, et al. Cushing’s syndrome after topical application of corticosteroids. Am J Dis Child. 1982;136:274-275.
21. Grassberger M, Baumruker T, Enz A, et al. A novel anti-inflammatory drug, SDZ ASM 981, for the treatment of skin diseases: in vitro pharmacology. Br J Dermatol. 1999;141:264-273.
22. Eichenfield L, Wynnis T, Berger T. Guidelines of care for the management of atopic dermatitis: management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
23. Reitamo S, Harper J, Bos JD, et al. 0.03% Tacrolimus ointment applied once or twice daily is more efficacious than 1% hydrocortisone acetate in children with moderate to severe atopic dermatitis: results of a randomized double-blind controlled trial. Br J Dermatol. 2004;150:554-562.
24. Ruer-Mulard M, Aberer W, Gunstone A, et al. Twice-daily versus once-daily applications of pimecrolimus cream 1% for the prevention of disease relapse in pediatric patients with atopic dermatitis. Pediatr Dermatol. 2009;26:551-558.
25. Breneman D, Fleischer AB Jr, Abramovits W, et al. Intermittent therapy for flare prevention and long-term disease control in stabilized atopic dermatitis: a randomized comparison of 3-times-weekly applications of tacrolimus ointment versus vehicle. J Am Acad Dermatol. 2008;58:990-999.
26. Meurer M, Fölster-Holst R, Wozel G, et al. Pimecrolimus cream 1% (Elidel) provides significant and rapid relief of pruritus and improves disease control and quality of life in atopic dermatitis in adults. J Invest Dermatol. 2002;119:350.
27. Meurer M, Fölster-Holst R, Wozel G, et al. Pimecrolimus cream in the long-term management of atopic dermatitis in adults: a six-month study. Dermatology. 2002;205:271-277.
28. Wahn U, Bos JD, Goodfield M, et al. Efficacy and safety of pimecrolimus cream in the long-term management of atopic dermatitis in children. Pediatrics. 2002;110(1, pt 1):e2.
29. Kang S, Lucky AW, Pariser D, et al. Long-term safety and efficacy of tacrolimus ointment for the treatment of atopic dermatitis in children. J Am Acad Dermatol. 2001;44(suppl 1):S58-S64.
30. Reitamo S, Wollenberg A, Schöpf E, et al. Safety and efficacy of 1 year of tacrolimus ointment monotherapy in adults with atopic dermatitis. the European Tacrolimus Ointment Study Group. Arch Dermatol. 2000;136:999-1006.
31. Frankel HC, Qureshi AA. Comparative effectiveness of topical calcineurin inhibitors in adult patients with atopic dermatitis. Am J Clin Dermatol. 2012;13:113-123.
32. Tennis P, Gelfand JM, Rothman KJ. Evaluation of cancer risk related to atopic dermatitis and use of topical calcineurin inhibitors. Br J Dermatol. 2011;165:465-473.
33. Dabade TS, Davis DM, Wetter DA, et al. Wet dressing therapy in conjunction with topical corticosteroids is effective for rapid control of severe pediatric atopic dermatitis: experience with 218 patients over 30 years at Mayo Clinic. J Am Acad Dermatol. 2012;67:100-106.
34. Devillers AC, Oranje AP. Efficacy and safety of ‘wet-wrap’ dressings as an intervention treatment in children with severe and/or refractory atopic dermatitis: a critical review of the literature. Br J Dermatol. 2006;154:579-585.
35. Meduri NB, Vandergriff T, Rasmussen H, et al. Phototherapy in the management of atopic dermatitis: a systematic review. Photodermatol Photoimmunol Photomed. 2007;23:106-112.
36. Clayton TH, Clark SM, Turner D, et al. The treatment of severe atopic dermatitis in childhood with narrowband ultraviolet B phototherapy. Clin Exp Dermatol. 2007;32:28-33.
37. Jekler J, Larko O. UVB phototherapy of atopic dermatitis. Br J Dermatol. 1988;119:697-705.
38. Roekevisch E, Spuls PI, Kuester D, et al. Efficacy and safety of systemic treatments for moderate-to-severe atopic dermatitis: a systematic review. J Allergy Clin Immunol. 2014;133:429-438.39. Hoare C, Li Wan Po A, Williams H. Systematic review of treatments for atopic eczema. Health Technol Assess. 2000;4:1-191.
39. Hoare C, Li Wan Po A, Williams H. Systematic review of treatments for atopic eczema. Health Technol Assess. 2000;4:1-191.
Atopic dermatitis (AD), more commonly referred to as eczema, is a chronic pruritic inflammatory skin disease that frequently affects both children and adults. Atopic dermatitis is most common in urban and developed countries, with a prevalence of approximately 11% in the United States.1 The pathophysiology of AD is complex and not fully understood, despite the increasing incidence of the disease.2 A myriad of factors, including genetics, defects in the innate and adaptive immune response, and skin barrier abnormalities all contribute to the pathogenesis.3,4 As a result of these abnormalities, patients with AD are more prone to damage from environmental irritants and allergens.
The diagnosis of AD is made clinically based on patient history and visual assessment of the skin.5 Atopic dermatitis follows a chronic and relapsing course characterized by severe pruritus and visible skin changes including xerosis, redness, blistering, oozing, crusting, scaling, thickening, and color change.6,7 Due to the genetic predisposition to make IgE antibodies in response to common environmental and food antigens, patients also may develop allergic rhinitis, asthma, and food-induced anaphylaxis.8,9 Patients also are susceptible to cutaneous viral, fungal, and bacterial infections, the most common of which is an infection with Staphylococcus aureus.10
Atopic dermatitis can have a substantial impact on quality of life, which has been revealed in studies linking chronic skin conditions to depression, impairment of self-esteem, and financial hardship.11 Because skin appearance impacts how a person is initially perceived by others, patients often report feeling self-conscious about their disease and experience teasing or bullying.12 To improve their physical appearance, patients may incur considerable medical expenses. According to 2 population-based studies comprising more than 60,000 adults aged 18 to 85 years, individuals with AD face substantial financial burdens and utilize the health care system more than those without the disease. On average, patients with AD spend $371 to $489 per year on costly out-of-pocket medical expenses and report more absences from work.13
Although there currently is no cure for AD, treatment is aimed at relieving its symptoms and preventing acute exacerbations as well as improving cosmetic appearance to enhance quality of life. Treatment must follow a stepwise approach, which focuses on hydrating the skin, repairing the dysfunctional epithelial barrier, and controlling inflammation. Thus, the standard of care focuses on avoiding skin irritants and triggers along with the use of moisturizers and topical corticosteroids (TCs). In patients with recurring severe disease, topical calcineurin inhibitors, phototherapy, and systemic agents also may be utilized.14
Avoiding Irritants and Triggers
Atopic dermatitis is worsened by skin contact with physical and chemical irritants. Exacerbating factors in AD include exposure to food allergens, dust, emotional stress, detergents, fragranced soaps, textiles, and ingredients in cosmetic products. Patients should be advised to use mild detergents and fragrance-free soaps and to avoid harsh materials such as wool. However, avoidance of specific ingredients in cosmetic products is not as straightforward because manufacturers are not required to disclose certain ingredients. In general, fragrances such as balsam of Peru and cinnamaldehyde, as well as preservatives such as parabens, isothiazolinones, and formaldehyde, should be avoided when selecting cosmetic products. Patients with AD should purchase fragrance-free products that are specifically formulated for sensitive skin. Additionally, patients should not apply makeup if their skin is irritated or oozing, as the flare may worsen.15
Moisturizers
Due to the impaired skin barrier function in patients with AD, regular application of fragrance-free moisturizers is essential to maintain hydration and to reduce xerosis. Various classes of moisturizers may be prescribed (eg, lotions, creams, gels, ointments) based on disease severity and patient preference. Light preparations such as lotions, creams, and gels have a high water content and generally are more appealing from a cosmetic standpoint because they do not create any residue on the skin. However, these options may require more frequent application because they are absorbed quickly. Heavy preparations such as ointments have longer-lasting effects due to their high oil content but tend to be less cosmetically appealing because of their greasiness.16
Although the amount and frequency of application of moisturizers has not been defined, liberal application several times daily is generally advised to minimize xerosis.17 Most physicians recommend applying moisturizer to the skin immediately after bathing to seal in moisture. Some patients prefer to use lotions and creams during the day because these products make the skin feel smooth and reserve the greasier ointments for nighttime application.
Topical Corticosteroids
Prescribed in conjunction with moisturizers, TCs are the mainstay of anti-inflammatory therapy in AD. Topical corticosteroids are classified into 7 groups based on potency, ranging from superpotent (class 1) to least potent (class 7). For acute AD flares, TCs should be applied daily for up to several weeks. Once the inflammation has resolved, it is recommended to apply TCs once to twice weekly to reduce the rate of relapse.18 Despite their effectiveness in the treatment of acute AD flares, TCs have a considerable side-effect profile. Potential adverse effects include skin atrophy, striae, telangiectasia, hypopigmentation, increased hair growth, steroid acne, growth retardation, and Cushing syndrome. Skin atrophy, which is the most common complication associated with TCs, results in shiny transparent skin, allowing for visualization of veins.19,20 Although many of these side effects will resolve after discontinuing the TCs, they are aesthetically displeasing during treatment, making it crucial for physicians to educate their patients on the proper usage of TCs to prevent negative outcomes.
Topical Calcineurin Inhibitors
Topical calcineurin inhibitors (TCIs) are a class of anti-inflammatories that are used to overcome the adverse effects of TCs. They are approved as alternatives to TCs in patients who have failed to respond to other topical treatments as well as those who have developed cutaneous atrophy from the use of TCs or have AD in sensitive areas such as the face, neck, and/or skin folds. Unlike TCs, TCIs do not cause atrophy, striae, or discoloration of the skin, which makes them more desirable from a cosmetic perspective. Their mechanism of action is distinct from TCs in that they inhibit calcineurin-dependent T-cell activation, thus preventing the transcription of inflammatory cytokines.21 Two TCIs are currently available: tacrolimus ointment 0.03% and 0.1% concentrations for moderate to severe AD and pimecrolimus cream 1% for mild to moderate AD.22 Twice-daily application of TCIs is recommended to decrease inflammation and pruritus associated with AD. Studies also have shown that intermittent use of TCIs 3 times weekly can aid in reducing relapses.23-25
The results from clinical trials demonstrate the rapid and continuous effects of both pimecrolimus and tacrolimus. In a controlled long-term study of adults, pimecrolimus provided significant relief of pruritus as soon as day 3 (P<.001).26,27 Pimecrolimus also provides long-term relief by preventing disease progression to flares, which was exemplified in a study (N=713) with no flares in 51% of pimecrolimus patients at 12 months versus 28% in the conventional treatment group (P<.001).28 Similarly, long-term studies of tacrolimus demonstrated an improvement of all symptoms of AD after 1 week of treatment. Maximal improvement was achieved with continued use of tacrolimus, and up to 1 year of tacrolimus use was found to be safe and effective.29,30 Thus, TCIs have been proven to be an effective choice in maintenance therapy for AD and have a good safety profile. The most common adverse effects of TCIs are local skin reactions, such as stinging and burning at the site of application. Rare cases of skin cancer and lymphoma have been reported; however, a causal relationship has yet to be established.31,32
Additional Therapies
Wet wrap therapy is effective for rapid control of flares and in controlling recalcitrant AD. Wet wraps function via several mechanisms; they provide a mechanical barrier against scratching, increase moisture and soften the skin, and enhance absorption of topical medications.33,34 The following method is employed when using wet wraps: an emollient or TC is applied to the area, a tubular bandage soaked in warm water is wrapped over the area, and dry bandages are used to form the outermost layer. Although wet wrap therapy is beneficial in treating AD, it is labor intensive and may require the expertise of a nurse. Thus, unlike other therapies, which patients can easily apply without interfering with their day, wet wraps must be applied at home or in a hospital setting.
Light therapy is another effective method of controlling AD. Although multiple forms of UV phototherapy are beneficial for symptom control in AD, there is no definitive recommendation regarding the specific type of light therapy due to a lack of comparative studies. Natural sunlight, narrowband UVB, broadband UVB, UVA, oral or topical psoralen plus UVA, as well as UVA and UVB can all be utilized in the treatment of AD. However, similar to natural sunlight, artificial light therapy can cause burning, blistering, hyperpigmentation, dark spots, and wrinkles. Because society places a large emphasis on maintaining a youthful appearance, patients may be hesitant to use a treatment that could potentially advance the skin’s aging process. Thus, it is important that this therapy is properly controlled to prevent further skin damage.35-37
When optimal topical regimens and phototherapy have failed to control AD, systemic immunomodulation therapies may be used. Currently, the most commonly used medications are cyclosporine 150 to 300 mg daily, methotrexate 7.5 to 25 mg weekly, mycophenolate mofetil 0.5 to 3 g daily, and azathioprine 1 to 3 mg/kg daily.38,39 Decisions regarding the specific class of drugs should be based on the patient’s AD status, comorbidities, and personal preference.
Conclusion
Atopic dermatitis is a common chronic condition that can occur at any age and cause substantial physical, psychological, social, and/or emotional stress for patients and their families. Although TCs have been the standard of treatment for many years, ongoing concerns regarding their safety have led to the use of TCIs, which overcome some of the drawbacks of steroid therapy. Phototherapy and systemic immunosuppressant therapy are reserved for patients who have not responded to optimal topical therapies. Although several therapeutic avenues exist for patients, there is a need for the development of more effective and safer drugs. Furthermore, cosmetic products created specifically for patients with AD would be beneficial, as patients often struggle to select products that do not cause more harm than good. Given the complexity of the pathogenesis of AD, further research must focus on defining the specific pathways involved in the disease and targeting these pathways with therapies.
Atopic dermatitis (AD), more commonly referred to as eczema, is a chronic pruritic inflammatory skin disease that frequently affects both children and adults. Atopic dermatitis is most common in urban and developed countries, with a prevalence of approximately 11% in the United States.1 The pathophysiology of AD is complex and not fully understood, despite the increasing incidence of the disease.2 A myriad of factors, including genetics, defects in the innate and adaptive immune response, and skin barrier abnormalities all contribute to the pathogenesis.3,4 As a result of these abnormalities, patients with AD are more prone to damage from environmental irritants and allergens.
The diagnosis of AD is made clinically based on patient history and visual assessment of the skin.5 Atopic dermatitis follows a chronic and relapsing course characterized by severe pruritus and visible skin changes including xerosis, redness, blistering, oozing, crusting, scaling, thickening, and color change.6,7 Due to the genetic predisposition to make IgE antibodies in response to common environmental and food antigens, patients also may develop allergic rhinitis, asthma, and food-induced anaphylaxis.8,9 Patients also are susceptible to cutaneous viral, fungal, and bacterial infections, the most common of which is an infection with Staphylococcus aureus.10
Atopic dermatitis can have a substantial impact on quality of life, which has been revealed in studies linking chronic skin conditions to depression, impairment of self-esteem, and financial hardship.11 Because skin appearance impacts how a person is initially perceived by others, patients often report feeling self-conscious about their disease and experience teasing or bullying.12 To improve their physical appearance, patients may incur considerable medical expenses. According to 2 population-based studies comprising more than 60,000 adults aged 18 to 85 years, individuals with AD face substantial financial burdens and utilize the health care system more than those without the disease. On average, patients with AD spend $371 to $489 per year on costly out-of-pocket medical expenses and report more absences from work.13
Although there currently is no cure for AD, treatment is aimed at relieving its symptoms and preventing acute exacerbations as well as improving cosmetic appearance to enhance quality of life. Treatment must follow a stepwise approach, which focuses on hydrating the skin, repairing the dysfunctional epithelial barrier, and controlling inflammation. Thus, the standard of care focuses on avoiding skin irritants and triggers along with the use of moisturizers and topical corticosteroids (TCs). In patients with recurring severe disease, topical calcineurin inhibitors, phototherapy, and systemic agents also may be utilized.14
Avoiding Irritants and Triggers
Atopic dermatitis is worsened by skin contact with physical and chemical irritants. Exacerbating factors in AD include exposure to food allergens, dust, emotional stress, detergents, fragranced soaps, textiles, and ingredients in cosmetic products. Patients should be advised to use mild detergents and fragrance-free soaps and to avoid harsh materials such as wool. However, avoidance of specific ingredients in cosmetic products is not as straightforward because manufacturers are not required to disclose certain ingredients. In general, fragrances such as balsam of Peru and cinnamaldehyde, as well as preservatives such as parabens, isothiazolinones, and formaldehyde, should be avoided when selecting cosmetic products. Patients with AD should purchase fragrance-free products that are specifically formulated for sensitive skin. Additionally, patients should not apply makeup if their skin is irritated or oozing, as the flare may worsen.15
Moisturizers
Due to the impaired skin barrier function in patients with AD, regular application of fragrance-free moisturizers is essential to maintain hydration and to reduce xerosis. Various classes of moisturizers may be prescribed (eg, lotions, creams, gels, ointments) based on disease severity and patient preference. Light preparations such as lotions, creams, and gels have a high water content and generally are more appealing from a cosmetic standpoint because they do not create any residue on the skin. However, these options may require more frequent application because they are absorbed quickly. Heavy preparations such as ointments have longer-lasting effects due to their high oil content but tend to be less cosmetically appealing because of their greasiness.16
Although the amount and frequency of application of moisturizers has not been defined, liberal application several times daily is generally advised to minimize xerosis.17 Most physicians recommend applying moisturizer to the skin immediately after bathing to seal in moisture. Some patients prefer to use lotions and creams during the day because these products make the skin feel smooth and reserve the greasier ointments for nighttime application.
Topical Corticosteroids
Prescribed in conjunction with moisturizers, TCs are the mainstay of anti-inflammatory therapy in AD. Topical corticosteroids are classified into 7 groups based on potency, ranging from superpotent (class 1) to least potent (class 7). For acute AD flares, TCs should be applied daily for up to several weeks. Once the inflammation has resolved, it is recommended to apply TCs once to twice weekly to reduce the rate of relapse.18 Despite their effectiveness in the treatment of acute AD flares, TCs have a considerable side-effect profile. Potential adverse effects include skin atrophy, striae, telangiectasia, hypopigmentation, increased hair growth, steroid acne, growth retardation, and Cushing syndrome. Skin atrophy, which is the most common complication associated with TCs, results in shiny transparent skin, allowing for visualization of veins.19,20 Although many of these side effects will resolve after discontinuing the TCs, they are aesthetically displeasing during treatment, making it crucial for physicians to educate their patients on the proper usage of TCs to prevent negative outcomes.
Topical Calcineurin Inhibitors
Topical calcineurin inhibitors (TCIs) are a class of anti-inflammatories that are used to overcome the adverse effects of TCs. They are approved as alternatives to TCs in patients who have failed to respond to other topical treatments as well as those who have developed cutaneous atrophy from the use of TCs or have AD in sensitive areas such as the face, neck, and/or skin folds. Unlike TCs, TCIs do not cause atrophy, striae, or discoloration of the skin, which makes them more desirable from a cosmetic perspective. Their mechanism of action is distinct from TCs in that they inhibit calcineurin-dependent T-cell activation, thus preventing the transcription of inflammatory cytokines.21 Two TCIs are currently available: tacrolimus ointment 0.03% and 0.1% concentrations for moderate to severe AD and pimecrolimus cream 1% for mild to moderate AD.22 Twice-daily application of TCIs is recommended to decrease inflammation and pruritus associated with AD. Studies also have shown that intermittent use of TCIs 3 times weekly can aid in reducing relapses.23-25
The results from clinical trials demonstrate the rapid and continuous effects of both pimecrolimus and tacrolimus. In a controlled long-term study of adults, pimecrolimus provided significant relief of pruritus as soon as day 3 (P<.001).26,27 Pimecrolimus also provides long-term relief by preventing disease progression to flares, which was exemplified in a study (N=713) with no flares in 51% of pimecrolimus patients at 12 months versus 28% in the conventional treatment group (P<.001).28 Similarly, long-term studies of tacrolimus demonstrated an improvement of all symptoms of AD after 1 week of treatment. Maximal improvement was achieved with continued use of tacrolimus, and up to 1 year of tacrolimus use was found to be safe and effective.29,30 Thus, TCIs have been proven to be an effective choice in maintenance therapy for AD and have a good safety profile. The most common adverse effects of TCIs are local skin reactions, such as stinging and burning at the site of application. Rare cases of skin cancer and lymphoma have been reported; however, a causal relationship has yet to be established.31,32
Additional Therapies
Wet wrap therapy is effective for rapid control of flares and in controlling recalcitrant AD. Wet wraps function via several mechanisms; they provide a mechanical barrier against scratching, increase moisture and soften the skin, and enhance absorption of topical medications.33,34 The following method is employed when using wet wraps: an emollient or TC is applied to the area, a tubular bandage soaked in warm water is wrapped over the area, and dry bandages are used to form the outermost layer. Although wet wrap therapy is beneficial in treating AD, it is labor intensive and may require the expertise of a nurse. Thus, unlike other therapies, which patients can easily apply without interfering with their day, wet wraps must be applied at home or in a hospital setting.
Light therapy is another effective method of controlling AD. Although multiple forms of UV phototherapy are beneficial for symptom control in AD, there is no definitive recommendation regarding the specific type of light therapy due to a lack of comparative studies. Natural sunlight, narrowband UVB, broadband UVB, UVA, oral or topical psoralen plus UVA, as well as UVA and UVB can all be utilized in the treatment of AD. However, similar to natural sunlight, artificial light therapy can cause burning, blistering, hyperpigmentation, dark spots, and wrinkles. Because society places a large emphasis on maintaining a youthful appearance, patients may be hesitant to use a treatment that could potentially advance the skin’s aging process. Thus, it is important that this therapy is properly controlled to prevent further skin damage.35-37
When optimal topical regimens and phototherapy have failed to control AD, systemic immunomodulation therapies may be used. Currently, the most commonly used medications are cyclosporine 150 to 300 mg daily, methotrexate 7.5 to 25 mg weekly, mycophenolate mofetil 0.5 to 3 g daily, and azathioprine 1 to 3 mg/kg daily.38,39 Decisions regarding the specific class of drugs should be based on the patient’s AD status, comorbidities, and personal preference.
Conclusion
Atopic dermatitis is a common chronic condition that can occur at any age and cause substantial physical, psychological, social, and/or emotional stress for patients and their families. Although TCs have been the standard of treatment for many years, ongoing concerns regarding their safety have led to the use of TCIs, which overcome some of the drawbacks of steroid therapy. Phototherapy and systemic immunosuppressant therapy are reserved for patients who have not responded to optimal topical therapies. Although several therapeutic avenues exist for patients, there is a need for the development of more effective and safer drugs. Furthermore, cosmetic products created specifically for patients with AD would be beneficial, as patients often struggle to select products that do not cause more harm than good. Given the complexity of the pathogenesis of AD, further research must focus on defining the specific pathways involved in the disease and targeting these pathways with therapies.
1. Shaw TE, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
2. Deckers IA, McLean S, Linssen S, et al. Investigating international time trends in the incidence and prevalence of atopic eczema 1990-2010: a systematic review of epidemiological studies. PLoS One. 2012;7:e39803.
3. Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242:233-246.
4. Peate I. Eczema: causes, symptoms and treatment in the community. Br J Community Nurs. 2011;16:324, 326-331.
5. Williams HC, Burney PG, Pembroke AC, et al. The U.K. Working Party’s diagnostic criteria for atopic dermatitis. III. independent hospital validation. Br J Dermatol. 1994;131:406-416.
6. Magin P, Adams J, Heading G, et al. Experiences of appearance-related teasing and bullying in skin diseases and their psychological sequelae: results of a qualitative study. Scand J Caring Sci. 2008;22:430-436.
7. Beattie P, Lewis-Jones M. A comparative study of impairment of quality of life in children with skin disease and children with other chronic childhood diseases. Br J Dermatol. 2006;155:145-151.
8. Spergel JM. From atopic dermatitis to asthma: the atopic march [published online January 22, 2010]. Ann Allergy Asthma Immunol. 2010;105:99-106; quiz 107-109, 117.
9. Leung DY. New insights into atopic dermatitis: role of skin barrier and immune dysregulation. Allergol Int. 2013;62:151-161.
10. Balma-Mena A, Lara-Corrales I, Zeller J, et al. Colonization with community-acquired methicillin-resistant Staphylococcus aureus in children with atopic dermatitis: a cross-sectional study. Int J Dermatol. 2011;50:682-688.
11. Strawser MS, Storch EA, Roberti JW. The Teasing Questionnaire-Revised: measurement of childhood teasing in adults. J Anxiety Disord. 2005;19:780-792.
12. Magin P, Adams J, Heading G, et al. Experiences of appearance-related teasing and bullying in skin diseases and their psychological sequelae: results of a qualitative study. Scand J Caring Sci. 2008;22:430-436.
13. Silverberg J. Health care utilization, patient costs, and access to care in US adults with eczema: a population-based study. JAMA Dermatol. 2015;151:743-752.
14. Ellis C, Luger T, Abeck D, et al. International Consensus Conference on Atopic Dermatitis II (ICCAD II): clinical update and current treatment strategies. Br J Dermatol. 2003;148(suppl 63):3-10.
15. Kim K. Influences of environmental chemicals on atopic dermatitis. Toxicol Res. 2015;31:89-96.
16. Ridd M, Redmond N, Hollinghurst S, et al. Choice of Moisturiser for Eczema Treatment (COMET): study protocol for a randomized controlled trial. Trials. 2015;16:304.
17. Hon KL, Ching GK, Leung TF, et al. Estimating emollient usage in patients with eczema. Clin Exp Dermatol. 2010;35:22-26.
18. Hanifin J, Gupta AK, Rajagopalan R. Intermittent dosing of fluticasone propionate cream for reducing the risk of relapse in atopic dermatitis patients. Br J Dermatol. 2002;147:528-537.
19. Hill CJ, Rostenberg A Jr. Adverse effects from topical steroids. Cutis. 1978;21:624-628.
20. Ruiz-Maldonado R, Zapata G, Lourdes T, et al. Cushing’s syndrome after topical application of corticosteroids. Am J Dis Child. 1982;136:274-275.
21. Grassberger M, Baumruker T, Enz A, et al. A novel anti-inflammatory drug, SDZ ASM 981, for the treatment of skin diseases: in vitro pharmacology. Br J Dermatol. 1999;141:264-273.
22. Eichenfield L, Wynnis T, Berger T. Guidelines of care for the management of atopic dermatitis: management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
23. Reitamo S, Harper J, Bos JD, et al. 0.03% Tacrolimus ointment applied once or twice daily is more efficacious than 1% hydrocortisone acetate in children with moderate to severe atopic dermatitis: results of a randomized double-blind controlled trial. Br J Dermatol. 2004;150:554-562.
24. Ruer-Mulard M, Aberer W, Gunstone A, et al. Twice-daily versus once-daily applications of pimecrolimus cream 1% for the prevention of disease relapse in pediatric patients with atopic dermatitis. Pediatr Dermatol. 2009;26:551-558.
25. Breneman D, Fleischer AB Jr, Abramovits W, et al. Intermittent therapy for flare prevention and long-term disease control in stabilized atopic dermatitis: a randomized comparison of 3-times-weekly applications of tacrolimus ointment versus vehicle. J Am Acad Dermatol. 2008;58:990-999.
26. Meurer M, Fölster-Holst R, Wozel G, et al. Pimecrolimus cream 1% (Elidel) provides significant and rapid relief of pruritus and improves disease control and quality of life in atopic dermatitis in adults. J Invest Dermatol. 2002;119:350.
27. Meurer M, Fölster-Holst R, Wozel G, et al. Pimecrolimus cream in the long-term management of atopic dermatitis in adults: a six-month study. Dermatology. 2002;205:271-277.
28. Wahn U, Bos JD, Goodfield M, et al. Efficacy and safety of pimecrolimus cream in the long-term management of atopic dermatitis in children. Pediatrics. 2002;110(1, pt 1):e2.
29. Kang S, Lucky AW, Pariser D, et al. Long-term safety and efficacy of tacrolimus ointment for the treatment of atopic dermatitis in children. J Am Acad Dermatol. 2001;44(suppl 1):S58-S64.
30. Reitamo S, Wollenberg A, Schöpf E, et al. Safety and efficacy of 1 year of tacrolimus ointment monotherapy in adults with atopic dermatitis. the European Tacrolimus Ointment Study Group. Arch Dermatol. 2000;136:999-1006.
31. Frankel HC, Qureshi AA. Comparative effectiveness of topical calcineurin inhibitors in adult patients with atopic dermatitis. Am J Clin Dermatol. 2012;13:113-123.
32. Tennis P, Gelfand JM, Rothman KJ. Evaluation of cancer risk related to atopic dermatitis and use of topical calcineurin inhibitors. Br J Dermatol. 2011;165:465-473.
33. Dabade TS, Davis DM, Wetter DA, et al. Wet dressing therapy in conjunction with topical corticosteroids is effective for rapid control of severe pediatric atopic dermatitis: experience with 218 patients over 30 years at Mayo Clinic. J Am Acad Dermatol. 2012;67:100-106.
34. Devillers AC, Oranje AP. Efficacy and safety of ‘wet-wrap’ dressings as an intervention treatment in children with severe and/or refractory atopic dermatitis: a critical review of the literature. Br J Dermatol. 2006;154:579-585.
35. Meduri NB, Vandergriff T, Rasmussen H, et al. Phototherapy in the management of atopic dermatitis: a systematic review. Photodermatol Photoimmunol Photomed. 2007;23:106-112.
36. Clayton TH, Clark SM, Turner D, et al. The treatment of severe atopic dermatitis in childhood with narrowband ultraviolet B phototherapy. Clin Exp Dermatol. 2007;32:28-33.
37. Jekler J, Larko O. UVB phototherapy of atopic dermatitis. Br J Dermatol. 1988;119:697-705.
38. Roekevisch E, Spuls PI, Kuester D, et al. Efficacy and safety of systemic treatments for moderate-to-severe atopic dermatitis: a systematic review. J Allergy Clin Immunol. 2014;133:429-438.39. Hoare C, Li Wan Po A, Williams H. Systematic review of treatments for atopic eczema. Health Technol Assess. 2000;4:1-191.
39. Hoare C, Li Wan Po A, Williams H. Systematic review of treatments for atopic eczema. Health Technol Assess. 2000;4:1-191.
1. Shaw TE, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
2. Deckers IA, McLean S, Linssen S, et al. Investigating international time trends in the incidence and prevalence of atopic eczema 1990-2010: a systematic review of epidemiological studies. PLoS One. 2012;7:e39803.
3. Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242:233-246.
4. Peate I. Eczema: causes, symptoms and treatment in the community. Br J Community Nurs. 2011;16:324, 326-331.
5. Williams HC, Burney PG, Pembroke AC, et al. The U.K. Working Party’s diagnostic criteria for atopic dermatitis. III. independent hospital validation. Br J Dermatol. 1994;131:406-416.
6. Magin P, Adams J, Heading G, et al. Experiences of appearance-related teasing and bullying in skin diseases and their psychological sequelae: results of a qualitative study. Scand J Caring Sci. 2008;22:430-436.
7. Beattie P, Lewis-Jones M. A comparative study of impairment of quality of life in children with skin disease and children with other chronic childhood diseases. Br J Dermatol. 2006;155:145-151.
8. Spergel JM. From atopic dermatitis to asthma: the atopic march [published online January 22, 2010]. Ann Allergy Asthma Immunol. 2010;105:99-106; quiz 107-109, 117.
9. Leung DY. New insights into atopic dermatitis: role of skin barrier and immune dysregulation. Allergol Int. 2013;62:151-161.
10. Balma-Mena A, Lara-Corrales I, Zeller J, et al. Colonization with community-acquired methicillin-resistant Staphylococcus aureus in children with atopic dermatitis: a cross-sectional study. Int J Dermatol. 2011;50:682-688.
11. Strawser MS, Storch EA, Roberti JW. The Teasing Questionnaire-Revised: measurement of childhood teasing in adults. J Anxiety Disord. 2005;19:780-792.
12. Magin P, Adams J, Heading G, et al. Experiences of appearance-related teasing and bullying in skin diseases and their psychological sequelae: results of a qualitative study. Scand J Caring Sci. 2008;22:430-436.
13. Silverberg J. Health care utilization, patient costs, and access to care in US adults with eczema: a population-based study. JAMA Dermatol. 2015;151:743-752.
14. Ellis C, Luger T, Abeck D, et al. International Consensus Conference on Atopic Dermatitis II (ICCAD II): clinical update and current treatment strategies. Br J Dermatol. 2003;148(suppl 63):3-10.
15. Kim K. Influences of environmental chemicals on atopic dermatitis. Toxicol Res. 2015;31:89-96.
16. Ridd M, Redmond N, Hollinghurst S, et al. Choice of Moisturiser for Eczema Treatment (COMET): study protocol for a randomized controlled trial. Trials. 2015;16:304.
17. Hon KL, Ching GK, Leung TF, et al. Estimating emollient usage in patients with eczema. Clin Exp Dermatol. 2010;35:22-26.
18. Hanifin J, Gupta AK, Rajagopalan R. Intermittent dosing of fluticasone propionate cream for reducing the risk of relapse in atopic dermatitis patients. Br J Dermatol. 2002;147:528-537.
19. Hill CJ, Rostenberg A Jr. Adverse effects from topical steroids. Cutis. 1978;21:624-628.
20. Ruiz-Maldonado R, Zapata G, Lourdes T, et al. Cushing’s syndrome after topical application of corticosteroids. Am J Dis Child. 1982;136:274-275.
21. Grassberger M, Baumruker T, Enz A, et al. A novel anti-inflammatory drug, SDZ ASM 981, for the treatment of skin diseases: in vitro pharmacology. Br J Dermatol. 1999;141:264-273.
22. Eichenfield L, Wynnis T, Berger T. Guidelines of care for the management of atopic dermatitis: management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
23. Reitamo S, Harper J, Bos JD, et al. 0.03% Tacrolimus ointment applied once or twice daily is more efficacious than 1% hydrocortisone acetate in children with moderate to severe atopic dermatitis: results of a randomized double-blind controlled trial. Br J Dermatol. 2004;150:554-562.
24. Ruer-Mulard M, Aberer W, Gunstone A, et al. Twice-daily versus once-daily applications of pimecrolimus cream 1% for the prevention of disease relapse in pediatric patients with atopic dermatitis. Pediatr Dermatol. 2009;26:551-558.
25. Breneman D, Fleischer AB Jr, Abramovits W, et al. Intermittent therapy for flare prevention and long-term disease control in stabilized atopic dermatitis: a randomized comparison of 3-times-weekly applications of tacrolimus ointment versus vehicle. J Am Acad Dermatol. 2008;58:990-999.
26. Meurer M, Fölster-Holst R, Wozel G, et al. Pimecrolimus cream 1% (Elidel) provides significant and rapid relief of pruritus and improves disease control and quality of life in atopic dermatitis in adults. J Invest Dermatol. 2002;119:350.
27. Meurer M, Fölster-Holst R, Wozel G, et al. Pimecrolimus cream in the long-term management of atopic dermatitis in adults: a six-month study. Dermatology. 2002;205:271-277.
28. Wahn U, Bos JD, Goodfield M, et al. Efficacy and safety of pimecrolimus cream in the long-term management of atopic dermatitis in children. Pediatrics. 2002;110(1, pt 1):e2.
29. Kang S, Lucky AW, Pariser D, et al. Long-term safety and efficacy of tacrolimus ointment for the treatment of atopic dermatitis in children. J Am Acad Dermatol. 2001;44(suppl 1):S58-S64.
30. Reitamo S, Wollenberg A, Schöpf E, et al. Safety and efficacy of 1 year of tacrolimus ointment monotherapy in adults with atopic dermatitis. the European Tacrolimus Ointment Study Group. Arch Dermatol. 2000;136:999-1006.
31. Frankel HC, Qureshi AA. Comparative effectiveness of topical calcineurin inhibitors in adult patients with atopic dermatitis. Am J Clin Dermatol. 2012;13:113-123.
32. Tennis P, Gelfand JM, Rothman KJ. Evaluation of cancer risk related to atopic dermatitis and use of topical calcineurin inhibitors. Br J Dermatol. 2011;165:465-473.
33. Dabade TS, Davis DM, Wetter DA, et al. Wet dressing therapy in conjunction with topical corticosteroids is effective for rapid control of severe pediatric atopic dermatitis: experience with 218 patients over 30 years at Mayo Clinic. J Am Acad Dermatol. 2012;67:100-106.
34. Devillers AC, Oranje AP. Efficacy and safety of ‘wet-wrap’ dressings as an intervention treatment in children with severe and/or refractory atopic dermatitis: a critical review of the literature. Br J Dermatol. 2006;154:579-585.
35. Meduri NB, Vandergriff T, Rasmussen H, et al. Phototherapy in the management of atopic dermatitis: a systematic review. Photodermatol Photoimmunol Photomed. 2007;23:106-112.
36. Clayton TH, Clark SM, Turner D, et al. The treatment of severe atopic dermatitis in childhood with narrowband ultraviolet B phototherapy. Clin Exp Dermatol. 2007;32:28-33.
37. Jekler J, Larko O. UVB phototherapy of atopic dermatitis. Br J Dermatol. 1988;119:697-705.
38. Roekevisch E, Spuls PI, Kuester D, et al. Efficacy and safety of systemic treatments for moderate-to-severe atopic dermatitis: a systematic review. J Allergy Clin Immunol. 2014;133:429-438.39. Hoare C, Li Wan Po A, Williams H. Systematic review of treatments for atopic eczema. Health Technol Assess. 2000;4:1-191.
39. Hoare C, Li Wan Po A, Williams H. Systematic review of treatments for atopic eczema. Health Technol Assess. 2000;4:1-191.
Practice Points
- Cosmetic symptoms of atopic dermatitis can have a serious impact on the patient’s quality of life.
- Avoidance of flares and prevention of triggers is an important aspect of care.
- Treatment options range from optimized skin care to topical prescription therapies to systemic medications.
Therapies for Actinic Keratosis With a Focus on Cosmetic Outcomes
Actinic keratosis (AK), also referred to as solar keratosis or senile keratosis, is an intraepidermal proliferation of dysplastic keratinocytes that develops in response to chronic exposure to UV radiation. Actinic keratoses are among the most commonly encountered lesions seen by dermatologists, and it has been estimated that 60% of predisposed individuals older than 40 years have at least one AK.1,2 Prevalence is notably higher in light-skinned individuals and increases with age, presumably from higher cumulative sun exposure and decreased effectiveness of the immune system.1,3 It remains a point of contention as to whether or not AKs actually represent squamous cell carcinoma (SCC) in situ, but the potential for progression to invasive disease has been well demonstrated, as the majority of SCCs develop from preexisting AKs.4-6 The risk for progression to invasive disease for an individual AK has been estimated to range from 0.025% to 16% per year, with an average of approximately 8% in immunocompetent patients.7
The clinical morphology of AK can vary widely, but the most common presentation is an erythematous scaly macule, papule, or plaque on sun-exposed skin. The skin surrounding AKs typically shows evidence of solar damage with deep wrinkling, mottled pigmentation, scattered telangiectases, purpura, or xerosis (Figure). A variety of clinical variants with unique presentations exist, including atrophic, hypertrophic, acantholytic, lichenoid, bowenoid, and pigmented subtypes. Because more than 80% of AKs occur on highly visible areas such as the head, neck, back of the hands, and forearms, AKs can have an obvious detrimental effect on cosmetic appearance. Studies also have shown a strong association between AKs and decreased overall quality of life (QOL).3,8,9

Because of the risk for AK progression to invasive cancer along with its negative impact on cosmesis and QOL, clinicians generally opt to treat AKs. Numerous different treatment options exist, including topical medications, procedural modalities, and light-based therapies. Here, we review the efficacy of the most commonly utilized treatments and discuss the relevant cosmetic considerations and outcomes.
Topical Treatments
5-Fluorouracil
5-Fluorouracil (5-FU) is a US Food and Drug Administration (FDA)–approved, topically applied pyrimidine analogue that inhibits thymidylate synthase. The resulting suppression of DNA and RNA synthesis induces cell death with a preference for mitotically active cells.10 5-Fluorouracil has been used for more than 50 years as a treatment of AK and its efficacy is well established. A systematic review of 5 randomized controlled studies of topical 5-FU reported an average of 49% of 423 patients achieving complete lesion clearance with 5-FU cream 5% applied once or twice daily for up to 7 weeks.11 Some notable drawbacks of 5-FU, however, are application-site erythema, blistering, pruritus, necrosis, erosion, and pain. These effects often lead to premature cessation of therapy, but newer formulations of 5-FU cream 0.5% have shown good efficacy with better tolerability.12 A randomized, double-blind, multicenter, parallel-group study of 177 patients using 5-FU cream 0.5% once daily for either 1, 2, or 4 weeks demonstrated significant (P<.001) efficacy over vehicle gel in all treatment arms.13 The most effective therapy was 4 weeks of treatment, which achieved a mean 91.7% reduction in lesion count as assessed 1 month after cessation of therapy. The primary adverse effect (AE) reported in this trial was mild to moderate facial irritation, which generally resolved within 18 to 21 days after treatment cessation.13 Overall, 5-FU is a highly effective therapy for treating AKs that also can improve signs of photoaging, but patients should be aware of cosmetically unappealing effects that generally occur throughout therapy and during the immediate posttreatment period.14
Chemical Peels
Chemical peels traditionally employ acidic compounds to strip away outer layers of skin to variable depths depending on the concentration of the agent being applied. For treatment of AK, trichloroacetic acid (TCA) is a commonly employed cauterant that has shown efficacy comparable to topical 5-FU as well as ablative CO2 laser resurfacing.15 Trichloroacetic acid peels also are a convenient therapy, as good results can be achieved after a single treatment session. A split-face study of 15 patients treated with either a single application of 35% TCA and Jessner solution or twice-daily application of 5-FU cream 5% for 3 weeks demonstrated a reduction in 75% of visible AKs in both treatment arms over a 1-year follow-up period.16 Although 80% of patients self-reported considerable cosmetic improvement with both therapies, patient preference was reported to be in favor of the TCA peel, given its quick results and relatively mild side effects as compared to 5-FU. Treatment with chemical peels will result in temporary erythema and mild desquamation that usually resolves within 2 weeks; however, there are cases in which erythema has been reported to persist for several months.16 Adverse effects such as permanent scarring or pigmentation changes rarely are seen with TCA concentrations less than 45%.17 Caution should be used in patients with a history of herpes simplex virus, keloids, postinflammatory hyperpigmentation, radiation exposure, immunosuppression, and those unable or unwilling to use sunscreen and avoid sun exposure in the immediate posttreatment period.
Diclofenac Sodium
Diclofenac sodium (DFS) is an FDA-approved topical, nonsteroidal,
anti-inflammatory drug whose mechanism of action in the treatment of AK is thought to involve inhibition of the cyclooxygenase 2 enzyme.18 The resulting reduction of prostaglandins is believed to inhibit tumor angiogenesis, induce apoptosis, and inhibit cell differentiation.19-22 In a multicenter, double-blind, placebo-controlled study of 195 patients, application of DFS 3% in hyaluronan gel 2.5% twice daily for 60 days showed significant (P<.05) efficacy over placebo in achieving complete resolution of target lesions during a 30-day follow-up period (31% vs 10%). Furthermore, qualitative patient assessment of complete global improvement also was significantly (P<.05) higher in the active treatment group as compared to placebo (31% vs 10%).23 Additional studies of DFS 3% in hyaluronan gel 2.5% applied twice daily for 90 days have shown even higher rates of success, with complete resolution of target lesions in 40% to 58% of cases.24,25 This therapy also has been reported to substantially improve QOL following treatment completion.26 The most frequently cited AEs include pruritus, rash, dry skin, erythema, and application-site reactions. Overall, DFS is a
well-tolerated therapy with efficacy comparable to that of 5-FU but with a lower incidence of AEs
and higher patient satisfaction as determined in
2 head-to-head studies.27,28
ImiquimodImiquimod (IMQ) is an FDA-approved topical agent that functions as an immune response modifier via agonism of toll-like receptor 7.18 The resulting cytokine production and release enhances the innate and acquired immune responses leading to anticancer activity.29 The efficacy of IMQ for treatment of AK has been demonstrated in numerous well-designed clinical trials. A
meta-analysis of 5 randomized, double-blind trials including 1293 patients treated with IMQ cream 5%
2 to 3 times per week for 12 to 16 weeks reported complete clearance of AKs in 50% of patients treated with IMQ as compared to 5% of patients treated with vehicle.30 The most frequently reported AEs with this therapy include erythema, scabbing, flaking, and erosion. These effects generally resolve following cessation of treatment, and therapy is considered to be well tolerated; however, there are case reports of IMQ triggering or exacerbating existing inflammatory conditions.31 Imiquimod cream also is approved at 2.5% and 3.75% concentrations, which have demonstrated significant (P<.001) efficacy over placebo and a reduced incidence of AEs; complete clearance rates have been reported as 30.6% and 35.6%, respectively.32 Notably, a study comparing 75 patients randomized to either IMQ cream 5%
3 times per week for 4 weeks, 1 or 2 courses of cryosurgery, or 5-FU ointment 5% twice daily for 4 weeks reported that IMQ achieved significantly (P<.01) superior sustained clearance rates during a 12-month follow-up period over cryosurgery and 5-FU
(73% vs 4% vs 33%).33 Additionally, cosmetic outcomes as determined by both participants and investigators were reported as excellent at 12 months posttreatment in more than 80% of participants treated with IMQ. These excellent, long-lasting cosmetic outcomes also were determined to be significantly (P<.0001) superior to the cosmetic outcomes of 5-FU and cryotherapy, which both reported excellent outcomes in less than 10% of cases.33
Ingenol MebutateIngenol mebutate (IM) is a macrocyclic diterpene ester derived from the Euphorbia peplus plant that is FDA approved for the treatment of AK.1 Ingenol mebutate’s mechanism of action is thought to involve induction of cell death via disruption of the plasma membrane and mitochondria in addition to production of an inflammatory response, which produces tumor-specific antibodies and a large influx of neutrophils.34,35 The overall evidence for the efficacy of IM is strong. A combined analysis of 4 multicenter, randomized, double-blind studies of 1005 participants reported that IM gel 0.015% applied once daily for 3 days to the face or scalp was significantly superior (P<.001) to placebo in achieving complete clearance as assessed 54 days after completion of therapy (42.2% vs 3.7%) and that IM gel 0.05% applied once daily for 2 days to the trunk or extremities also was significantly superior (P<.001) to placebo in achieving complete clearance as determined 55 days after completion of therapy (34.1% vs 4.7%).36 A follow-up report to this study indicated that IM also appears to achieve long-lasting effects with an overall 87% decrease in total AKs at 12 months follow-up in both trial groups.37 Additionally, it has been recently reported that treatment with IM in these trials was associated with significantly higher overall treatment satisfaction (P<.001) and improved QOL (P<.001) as compared to vehicle.38 Cosmetic outcomes of IM therapy have been assessed in a trial analyzing the efficacy of IM gel 0.025% for 3 days or IM gel 0.05% for 2 or 3 days on nonfacial AKs. This study reported significantly (P<.0001) higher patient satisfaction with the cosmetic outcome at 8 weeks after therapy as compared to vehicle.34 Studies performed in mice have demonstrated that IM is able to promote collagen matrix turnover and impose dermal elasticity, which may contribute to these good cosmetic outcomes.39 The most common AEs of IM therapy are erythema, crusting, and flaking; these effects generally occur 3 to 8 days after starting treatment. These effects, however, generally are short lived and resolve within 2 weeks of treatment cessation when IM is applied to the face or scalp or 4 weeks when applied to the trunk or extremities.40 Overall, IM is a useful therapeutic option given its relatively short treatment course as compared to other topically applied agents, as well as its lasting efficacy, mild AEs, and good cosmetic outcomes.
Procedural Modalities
Surgical Procedures
Surgical approaches for the treatment of AK include excision, curettage with or without electrodesiccation, and dermabrasion. In the past, these modalities were used with greater frequency, but the advent of effective topical medications with lower risks of AEs has largely reduced their use.41 Excision may still be indicated in cases where SCC is suspected, and curettage can be used for treatment of thicker hypertrophic AKs.42 Although these approaches have not been evaluated in clinical trials, they are generally effective but require the use of local anesthetics and come with substantial risk for infection, permanent scarring, and hypopigmentation. Dermabrasion employs the use of a motorized device equipped with an abrasive material to physically remove superficial layers of the skin. Studies are limited, but this method has been reported as an effective treatment in a retrospective review of 23 participants in which 96% remained free of AKs at 1 year, 83% at 2 years, 64% at 4 years, and 54% at 5 years posttherapy.43 Notably, one split-face study of 40 participants treated with dermabrasion followed by 25% TCA on one side and either Jessner solution and 35% TCA or dermabrasion alone on the other side reported that the combination of dermabrasion with 25% TCA consistently produced excellent cosmetic results with nearly complete eradication of AKs.44 In general, however, cosmetic outcomes with dermabrasion are variable, as the technique is highly operator dependent and treatment is associated with notable discomfort as well as risk for scarring and permanent pigmentation alteration.
Cryotherapy
Cryotherapy remains one of the most commonly utilized treatments of AK and involves the delivery of liquid nitrogen via a spray device or a cotton tip applicator to rapidly freeze cells, thus causing cellular destruction via ice crystal formation and protein denaturation.45 Efficacy with this technique has been reported to be as high as 98.8% at 12 months follow-up, but more recent studies cite lower rates of success.46 A prospective multicenter study of 90 participants with 421 AKs on the face or scalp treated with a single freeze-thaw cycle of liquid nitrogen reported an overall complete response rate of 67.2% at 3 months posttherapy. Additionally, higher complete response rates were associated with longer freeze times, and cosmetic outcomes were reported as good to excellent in 94% of complete response lesions.47 Similar results were reported in an open-label, prospective, randomized, controlled clinical trial of 200 participants with 543 AKs, which compared a single freeze-thaw cycle with liquid nitrogen to a single session of CO2 laser ablation in the treatment of isolated AKs of the face and scalp.48 At 3 months posttherapy, complete clearance was observed in 71.6% of participants treated with cryotherapy and in 65.3% of participants treated with laser ablation (P=.532). At 12 months posttherapy, participants who originally showed complete response at 3 months were assessed for relapse. Complete clearance was preserved in 72.6% of participants treated with cryotherapy versus 21.9% of participants treated with laser ablation (P<.0001), and cosmetic outcomes were reported by participants as good or excellent at 3 months follow-up in more than 93% of participants for both treatment arms.48 Possible AEs of cryotherapy include pain during treatment, blister formation with possible hemorrhage, infection, scarring, and permanent pigmentary changes.47,48 Notably, the risk for hypopigmentation increases with longer freezing times, thus requiring clinicians to consider the balance between improved efficacy and reduced cosmetic outcomes.47
Light-Based Therapies
Laser Therapy
Ablative laser resurfacing with either the CO2 or erbium-doped:YAG (Er:YAG) laser utilizes light of specific wavelengths to selectively induce thermolysis and destruction of the epidermal layer. Both lasers have been studied as treatments of AK, but there is a lack of large, well-designed studies. In one small study of 14 participants treated with 1 to 2 passes of the CO2 laser, complete clearance was reported in all cases without any recurrences during a follow-up period of 6 to 24 months. Additionally, all participants in this study reported satisfaction with the cosmetic outcome.49 The CO2 laser also has demonstrated efficacy comparable to that of the TCA peel and 5-FU therapy in a prospective randomized trial of 34 patients with facial or scalp AKs who received either CO2 laser with 2 passes, 30% TCA peel, or 5-FU cream 5% twice daily for 3 weeks.15 Reduction in mean AK counts at 3 months posttherapy was significantly (P<.03) higher in all treatment arms as compared to the control group (92% for CO2 laser, 89% for TCA peel, and 83% for 5-FU cream). No significant (P=.31) difference in outcomes was noted among the different treatment arms.15 Similar results were reported for the Er:YAG laser in a small prospective study of 5 participants treated with 2 to 3 passes with the Er:YAG laser in which reduction in mean AK counts was reported as ranging from 86% to 96% at 3 months posttherapy.50 The Er:YAG laser in combination with the CO2 laser has shown notable long-term efficacy in achieving higher lesion clearance rates and sustained complete clearance rates over treatment with topical 5-FU.51 In a prospective randomized study of 55 par-ticipants with multiple AKs on the face or scalp, participants were assigned to receive either combination laser ablation with the Er:YAG and CO2 lasers down to the level of the papillary dermis or 5-FU cream 5% applied twice daily for 2 to 7 weeks until an appropriate clinical inflammatory response was achieved. At 12 months follow-up, the laser treatment group achieved significantly (P=.048) higher mean lesion clearance rates (91.1%) as compared to the 5-FU arm (76.6%) and significantly (P=.003) higher sustained complete clearance rates (59.3%) as compared to 5-FU (29.2%). The proportion of participants with an improvement in photoaging score at 12 months follow-up approached statistical significance (P=.07), with 74% of the laser-treated group showing improvement as compared to 43% of the 5-FU–treated group. Long-term, cosmetically unappealing side effects such as erythema and hypopigmentation occurred notably more often in the laser-treated group as compared to the 5-FU group.51 In summary, ablative lasers appear to be a highly effective therapy for AK but at the cost of increased risk for AEs such as permanent pigmentary changes, prolonged erythema lasting up to several months, and scarring.50,52-55
Fractional photothermolysis is a relatively new advancement in the field of laser therapy that has received FDA approval for the treatment of AK.56 This treatment works by creating multiple noncontiguous microscopic columns of thermal injury while sparing adjacent zones of viable tissue.57 Although there are limited studies involving the use of such lasers in the treatment of AK, initial findings suggest that 1927-nm thulium lasers may be more effective than 1550-nm erbium lasers in achieving lesion clearance. A trial of 14 participants who received 5 laser treatments with a 1550-nm fractionated erbium-doped fiber laser reported an average reduction in AK counts of 66.2% at 3 months follow-up and a 55.6% reduction at 6 months follow-up. A participant-determined marked or very significant improvement of lesions was reported in 83% of participants at 1 month posttreatment but only in 44% of participants at 6 months posttreatment.58 A similar trial of 24 participants treated with up to 4 treatment sessions of the fractionated 1927-nm thulium laser reported an 87.3% reduction in number of AKs at 3 months follow-up and an 86.6% reduction at 6 months follow-up.56 The primary advantage of fractional laser therapy is a faster recovery period generally lasting only 2 or 3 days as compared to 2 weeks or more with traditional ablative lasers, thus limiting the amount of time a patient must tolerate cosmetically unappealing erythema.59,60 The quick recovery time has been attributed to the fractional laser’s ability to preserve the stratum corneum and skin barrier, which also helps reduce the risk for other AEs such as scarring and infection.56,59-61 Additional studies are needed to better assess the true efficacy of fractional laser therapy, but treatment with the fractional 1927-nm thulium laser appears to be a promising and well-tolerated therapeutic option for treatment of AK with similar efficacy to traditional ablative lasers but with a lower risk of AEs.
Photodynamic TherapyPhotodynamic therapy (PDT) is an FDA-approved treatment that involves the use of a topical photosensitizing agent such as 5-aminolevulinic acid (ALA) or methyl aminovulinate (MAL) before exposure to an activating light source to generate reactive oxygen species that lead to cell death.62-65 Multiple PDT regimens with varying combinations of photosensitizers, incubation time, and light sources have been studied, but a 2012 Cochrane review determined that treatment with conventional formulations of MAL and ALA with either blue- or red-light PDT were similarly efficacious for treatment of individual AKs as compared to vehicle with blue- or red-light PDT. One exception was that longer incubation time (ie, 4 hours) with ALA resulted in better results than shorter incubation times (ie, 0.5, 1, 2 hours) with ALA.66
Standard PDT treatment with MAL also has consistently demonstrated superior efficacy in achieving complete clearance rates in addition to superior cosmetic outcomes over treatment with either cryotherapy, DFS, or 5-FU.67-73 Three studies in particular noted an excellent or good investigator-determined cosmetic outcome in 96% to 98% of participants treated with MAL-PDT.69,71,74 Photodynamic therapy with ALA also has been reported as superior over CO2 laser ablation for AK reduction as well as both patient and investigator overall satisfaction.75
More recently, several methods of improving photosensitizer delivery have been studied, which have demonstrated remarkable efficacy at achieving lesion clearance over standard cream formulations or application routines. One such method involves the use of gentle heating to increase photosensitizer uptake. In a split-extremity study of 20 participants who were treated with 20% ALA under occlusion for 1 hour with one side heated to 38.8°C, the heated side demonstrated significant (P<.0001) efficacy at achieving higher median clearance rates over control when evaluated at 2 and 6 months posttherapy.76 Notably, occlusion of ALA in itself during the incubation period also has been demonstrated to significantly (P<.0001) improve clearance rates.77 Another method involves the use of a new nanoemulsion-based formulation of ALA gel, known as BF-200 ALA, which has demonstrated remarkable efficacy over standard MAL cream and placebo in a long-term follow-up analysis of 2 prospective, randomized, controlled trials.78 In a similar vein, 3 prospective randomized trials with a minimum follow-up time of 3 months demonstrated that MAL-PDT in combination with fractional ablative laser pretreatment has significant (P<.02 in all trials) efficacy over MAL-PDT without pretreatment in achieving complete AK clearance. Although the cosmetic outcomes were good or excellent in 87% to 100% of patients, they were not significantly different from stand-alone MAL-PDT treatment in any of the trials.79-81 However, pretreatment with microneedling in MAL-PDT has been shown to achieve superior cosmetic outcomes over MAL-PDT without microneedling, according to one small split-face study of 10 participants.82
Overall, PDT is an excellent therapeutic option that is able to provide efficacious clearance of AKs as well as superior cosmetic outcomes. Common AEs of PDT include burning, itching, and stinging during therapy, but pain intensity decreases dramatically upon termination of illumination, with cessation of most symptoms by 12 hours posttherapy.73 Permanent pigmentation changes have been reported to occasionally occur following PDT therapy.81
Conclusion
When determining which therapy to use in a patient, clinicians must take into account a variety of factors such as patient preference, cost of treatment, availability, tolerance for AEs, and the need for field therapy. Although all therapies discussed within this article are effective and reasonable treatment choices, patients who are particularly concerned about cosmetic outcomes would most likely benefit from either IMQ or PDT, as the data for cosmetic outcomes with these therapies are the strongest. Combination or sequential treatments may be required in some cases and all patients should be monitored for lesion recurrence regardless of treatment choice. A summary of the therapies and key studies discussed here is available in the PDF.
- Lebwohl M. Actinic keratosis: epidemiology and progression to squamous cell carcinoma. Br J Dermatol. 2003;149(suppl 66):31-33.
- Drake LA, Ceilley RI, Cornelison RL, et al. Guidelines of care for actinic keratoses. Committee on Guidelines of Care. J Am Acad Dermatol. 1995;32:95-98.
- Salasche SJ. Epidemiology of actinic keratoses and squamous cell carcinoma. J Am Acad Dermatol. 2000;42(1, pt 2):4-7.
- Ackerman AB, Mones JM. Solar (actinic) keratosis is squamous cell carcinoma. Br J Dermatol. 2006;155:9-22.
- Anwar J, Wrone DA, Kimyai-Asadi A, et al. The development of actinic keratosis into invasive squamous cell carcinoma: evidence and evolving classification schemes. Clin Dermatol. 2004;22:189-196.
- Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
- Glogau RG. The risk of progression to invasive disease. J Am Acad Dermatol. 2000;42(1, pt 2):23-24.
- Esmann S, Jemec GB. Management of actinic keratosis patients: a qualitative study. J Dermatolog Treat. 2007;18:53-58.
- Weinstock MA, Lee KC, Chren MM, et al. Quality of life in the actinic neoplasia syndrome: the VA Topical Tretinoin Chemoprevention (VATTC) trial. J Am Acad Dermatol. 2009;61:207-215.
- Berman B, Villa AM, Ramirez CC. Mechanisms of action of new treatment modalities for actinic keratosis. J Drugs Dermatol. 2006;5:167-173.
- Askew DA, Mickan SM, Soyer HP. Effectiveness of 5-fluorouracil treatment for actinic keratosis: a systematic review of randomized controlled trials. Int J Dermatol. 2009;46:452-463.
- Levy S, Furst K, Chern W. A pharmacokinetic evaluation of 0.5% and 5% fluorouracil topical cream in patients with actinic keratosis. Clin Ther. 2001;23:908-920.
- Jorizzo J, Stewart D, Bucko A, et al. Randomized trial evaluating a new 0.5% fluorouracil formulation demonstrates efficacy after 1-, 2-, or 4-week treatment in patients with actinic keratosis. Cutis. 2002;70:335-359.
- Sachs DL, Kang S, Hammerberg C, et al. Topical fluorouracil for actinic keratoses and photoaging: a clinical and molecular analysis. Arch Dermatol. 2009;145:659-666.
- Hantash BM, Stewart DB, Cooper ZA, et al. Facial resurfacing for nonmelanoma skin cancer prophylaxis. Arch Dermatol. 2006;142:976-982.
- Lawrence N, Cox SE, Cockerell CJ, et al. A comparison of the efficacy and safety of Jessner’s solution and 35% trichloroacetic acid vs 5% fluorouracil in the treatment of widespread facial actinic keratoses. Arch Dermatol. 1995;131:176-181.
- Monheit GD. The Jessner’s + TCA peel: a medium-depth chemical peel. J Dermatol Surg Oncol. 1989;15:945-950.
- Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196-200.
- Adamson DJ, Frew D, Tatoud R, et al. Diclofenac antagonizes peroxisome proliferator-activated receptor-gamma signaling. Mol Pharmacol. 2002;61:7-12.
- Alam CA, Seed MP, Willoughby DA. Angiostasis and vascular regression in chronic granulomatous inflammation induced by diclofenac in combination with hyaluronan in mice. J Pharm Pharmacol. 1995;47:407-411.
- Lu X, Xie W, Reed D, et al. Nonsteroidal antiinflammatory drugs cause apoptosis and induce cyclooxygenases in chicken embryo fibroblasts. Proc Natl Acad Sci USA. 1995;92:7961-7965.
- Seed MP, Brown JR, Freemantle CN, et al. The inhibition of colon-26 adenocarcinoma development and angiogenesis by topical diclofenac in 2.5% hyaluronan. Cancer Res. 1997;57:1625-1629.
- Rivers JK, Arlette J, Shear N, et al. Topical treatment of actinic keratoses with 3.0% diclofenac in 2.5% hyaluronan gel. Br J Dermatol. 2002;146:94-100.
- Wolf JE, Taylor JR, Tschen E, et al. Topical 3.0% diclo-fenac in 2.5% hyaluronan gel in the treatment of actinic keratoses. Int J Dermatol. 2001;40:709-713.
- Nelson C, Rigel D, Smith S, et al. Phase IV, open-label assessment of the treatment of actinic keratosis with 3.0% diclofenac sodium topical gel (Solaraze). J Drugs Dermatol. 2004;3:401-407.
- Pflugfelder A, Welter AK, Leiter U, et al. Open label randomized study comparing 3 months vs. 6 months treatment of actinic keratoses with 3% diclofenac in 2.5% hyaluronic acid gel: a trial of the German Dermatologic Cooperative Oncology Group. J Eur Acad Dermatol Venereol. 2012;26:48-53.
- Smith SR, Morhenn VB, Piacquadio DJ. Bilateral comparison of the efficacy and tolerability of 3% diclofenac sodium gel and 5% 5-fluorouracil cream in the treatment of actinic keratoses of the face and scalp. J Drugs Dermatol. 2006;5:156-159.
- Segatto MM, Dornelles SI, Silveira VB, et al. Comparative study of actinic keratosis treatment with 3% diclo- fenac sodium and 5% 5-fluorouracil. An Bras Dermatol. 2013;88:732-738.
- Vidal D. Topical imiquimod: mechanism of action and clinical applications. Mini Rev Med Chem. 2006;6:499-503.
- Hadley G, Derry S, Moore RA. Imiquimod for actinic keratosis: systematic review and meta-analysis. J Invest Dermatol. 2006;126:1251-1255.
- Caperton C, Berman B. Safety, efficacy, and patient acceptability of imiquimod for topical treatment of actinic keratoses. Clin Cosmet Investig Dermatol. 2011;4:35-40.
- Swanson N, Smith CC, Kaur M, et al. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: two phase 3, multicenter, randomized, double-blind, placebo-controlled studies. J Drugs Dermatol. 2014;13:166-169.
- Krawtchenko N, Roewert-Huber J, Ulrich M, et al. A randomised study of topical 5% imiquimod vs. topical 5-fluorouracil vs. cryosurgery in immunocompetent patients with actinic keratoses: a comparison of clinical and histological outcomes including 1-year follow-up. Br J Dermatol. 2007;157(suppl 2):34-40.
- Anderson L, Schmieder GJ, Werschler WP, et al. Randomized, double-blind, double-dummy, vehicle-controlled study of ingenol mebutate gel 0.025% and 0.05% for actinic keratosis. J Am Acad Dermatol. 2009;60:934-943.
- Ogbourne SM, Suhrbier A, Jones B, et al. Antitumor activity of 3-ingenyl angelate: plasma membrane and mitochondrial disruption and necrotic cell death. Cancer Res. 2004;64:2833-2839.
- Lebwohl M, Swanson N, Anderson LL, et al. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366:1010-1019.
- Lebwohl M, Shumack S, Stein-Gold L, et al. Long-term follow-up study of ingenol mebutate gel for the treatment of actinic keratoses. JAMA Dermatol. 2013;149:666-670.
- Augustin M, Tu JH, Knudsen KM, et al. Ingenol mebutate gel for actinic keratosis: the link between quality of life, treatment satisfaction, and clinical outcomes. J Am Acad Dermatol. 2015;72:816-821.
- Kane-Maguire N, Moseley R, Cozzi S, et al. Modulation of fibroblast phenotype and extracellular matrix composition by ingenol mebutate may be associated with scar resolution and improved dermal cosmesis. J Am Acad Dermatol. 2012;66:AB218.
- Martin G, Swanson N. Clinical findings using ingenol mebutate gel to treat actinic keratoses. J Am Acad Dermatol. 2013;68(1, suppl 1):S39-S48.
- Feldman SR, Fleischer AB, Williford PM, et al. Destructive procedures are the standard of care for treatment of actinic keratoses. J Am Acad Dermatol. 1999;40:43-47.
- Berlin JM. Current and emerging treatment strategies for the treatment of actinic keratosis. Clin Cosmet Investig Dermatol. 2010;3:119-126.
- Coleman WP, Yarborough JM, Mandy SH. Dermabrasion for prophylaxis and treatment of actinic keratoses. Dermatol Surg. 1996;22:17-21.
- Cooley JE, Casey DL, Kauffman CL. Manual resurfacing and trichloroacetic acid for the treatment of patients with widespread actinic damage. clinical and histologic observations. Dermatol Surg. 1997;23:373-379.
- Goldberg LH, Kaplan B, Vergilis-Kalner I, et al. Liquid nitrogen: temperature control in the treatment of actinic keratosis. Dermatol Surg. 2010;36:1956-1961.
- Lubritz RR, Smolewski SA. Cryosurgery cure rate of actinic keratoses. J Am Acad Dermatol. 1982;7:631-632.
- Thai KE, Fergin P, Freeman M, et al. A prospective study of the use of cryosurgery for the treatment of actinic keratoses. Int J Dermatol. 2004;43:687-692.
- Zane C, Facchinetti E, Rossi MT, et al. Cryotherapy is preferable to ablative CO2 laser for the treatment of isolated actinic keratoses of the face and scalp: a randomized clinical trial. Br J Dermatol. 2014;170:1114-1121.
- Trimas SJ, Ellis DA, Metz RD. The carbon dioxide laser. an alternative for the treatment of actinically damaged skin. Dermatol Surg. 1997;23:885-889.
- Jiang SB, Levine VJ, Nehal KS, et al. Er:YAG laser for the treatment of actinic keratoses. Dermatol Surg. 2000;26:437-440.
- Ostertag JU, Quaedvlieg PJ, Van der geer S, et al. A clinical comparison and long-term follow-up of topical 5-fluorouracil versus laser resurfacing in the treatment of widespread actinic keratoses. Lasers Surg Med. 2006;38:731-739.
- Iyer S, Friedli A, Bowes L, et al. Full face laser resurfacing: therapy and prophylaxis for actinic keratoses and non-melanoma skin cancer. Lasers Surg Med. 2004;34:114-119.
- Rubin MG. A peeler’s thoughts on skin improvement with chemical peels and laser resurfacing. Clin Plast Surg. 1997;24:407-409.
- Riggs K, Keller M, Humphreys TR. Ablative laser resurfacing: high-energy pulsed carbon dioxide and erbium:yttrium-aluminum-garnet. Clin Dermatol. 2007;25:462-473.
- Adrian RM. Pulsed carbon dioxide and long pulse 10-ms erbium-YAG laser resurfacing: a comparative clinical and histological study. J Cutan Laser Ther. 1999;1:197-202.
- Weiss ET, Brauer JA, Anolik R, et al. 1927-nm fractional resurfacing of facial actinic keratoses: a promising new therapeutic option. J Am Acad Dermatol. 2013; 68:98-102.
- Manstein D, Herron GS, Sink RK, et al. Fractional photothermolysis: a new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg Med. 2004;34:426-438.
- Katz TM, Goldberg LH, Marquez D, et al. Nonablative fractional photothermolysis for facial actinic keratoses: 6-month follow-up with histologic evaluation. J Am Acad Dermatol. 2011;65:349-356.
- Prens SP, De Vries K, Neumann HA, et al. Non-ablative fractional resurfacing in combination with topical tretinoin cream as a field treatment modality for multiple actinic keratosis: a pilot study and a review of other field treatment modalities. J Dermatolog Treat. 2013;24:227-231.
- Alexiades-Armenakas MR, Dover JS, Arndt KA. The spectrum of laser skin resurfacing: nonablative, fractional, and ablative laser resurfacing. J Am Acad Dermatol. 2008;58:719-737.
- Tannous Z. Fractional resurfacing. Clin Dermatol. 2007;25:480-486.
- Gold MH. Continuing medical education article-skin treatment: photodynamic therapy: indications and treatment. Aesthet Surg J. 2008;28:545-552.
- Juarranz A, Jaén P, Sanz-Rodríguez F, et al. Photodynamic therapy of cancer. basic principles and applications. Clin Transl Oncol. 2008;10:148-154.
- Juzeniene A, Peng Q, Moan J. Milestones in the development of photodynamic therapy and fluorescence diagnosis. Photochem Photobiol Sci. 2007;6:1234-1245.
- Moan J, Berg K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem Photobiol. 1991;53:549-553.
- Gupta AK, Paquet M, Villanueva E, et al. Interventions for actinic keratoses. Cochrane Database Syst Rev. 2012;12:CD004415.
- Patel G, Armstrong AW, Eisen DB. Efficacy of photodynamic therapy vs other interventions in randomized clinical trials for the treatment of actinic keratoses: a systematic review and meta-analysis. JAMA Dermatol. 2014;150:1281-1288.
- Kaufmann R, Spelman L, Weightman W, et al. Multicentre intraindividual randomized trial of topical methyl aminolaevulinate-photodynamic therapy vs. cryotherapy for multiple actinic keratoses on the extremities. Br J Dermatol. 2008;158:994-999.
- Freeman M, Vinciullo C, Francis D, et al. A comparison of photodynamic therapy using topical methyl aminolevulinate (Metvix) with single cycle cryotherapy in patients with actinic keratosis: a prospective, randomized study. J Dermatolog Treat. 2003;14:99-106.
- Morton C, Campbell S, Gupta G, et al. Intraindividual, right-left comparison of topical methyl aminolaevulinate-photodynamic therapy and cryotherapy in subjects with actinic keratoses: a multicentre, randomized controlled study. Br J Dermatol. 2006;155:1029-1036.
- Pariser DM, Lowe NJ, Stewart DM, et al. Photodynamic therapy with topical methyl aminolevulinate for actinic keratosis: results of a prospective randomized multicenter trial. J Am Acad Dermatol. 2003;48:227-232.
- Zane C, Facchinetti E, Rossi MT, et al. A randomized clinical trial of photodynamic therapy with methyl aminolaevulinate vs. diclofenac 3% plus hyaluronic acid gel for the treatment of multiple actinic keratoses of the face and scalp. Br J Dermatol. 2014;170:1143-1150.
- Perrett CM, McGregor JM, Warwick J, et al. Treatment of post-transplant premalignant skin disease: a randomized intrapatient comparative study of 5-fluorouracil cream and topical photodynamic therapy. Br J Dermatol. 2007;156:320-328.
- Szeimies RM, Karrer S, Radakovic-Fijan S, et al. Photodynamic therapy using topical methyl 5-aminolevulinate compared with cryotherapy for actinic keratosis: a prospective, randomized study. J Am Acad Dermatol. 2002; 47:258-262.
- Scola N, Terras S, Georgas D, et al. A randomized, half-side comparative study of aminolaevulinate photodynamic therapy vs. CO(2) laser ablation in immunocompetent patients with multiple actinic keratoses. Br J Dermatol. 2012;167:1366-1373.
- Willey A, Anderson RR, Sakamoto FH. Temperature-modulated photodynamic therapy for the treatment of actinic keratosis on the extremities: a pilot study. Dermatol Surg. 2014;40:1094-1102.
- Pariser DM. Management of Actinic Keratoses: Treatment Selection and Optimizing Outcomes. Presented at: Winter Clinical Dermatology Conference Hawaii; January 18, 2015; Kaanapali, HI.
- Dirschka T, Radny P, Dominicus R, et al. Long-term (6 and 12 months) follow-up of two prospective, randomized, controlled phase III trials of photodynamic therapy with BF-200 ALA and methyl aminolaevulinate for the treatment of actinic keratosis. Br J Dermatol. 2013;168:825-836.
- Choi SH, Kim KH, Song KH. Efficacy of ablative fractional laser-assisted photodynamic therapy with short-incubation time for the treatment of facial and scalp actinic keratosis: 12-month follow-up results of a randomized, prospective, comparative trial. J Eur Acad Dermatol Venereol. 2015;29:1598-1605.
- Ko DY, Jeon SY, Kim KH, et al. Fractional erbium:YAG laser-assisted photodynamic therapy for facial actinic keratoses: a randomized, comparative, prospective study. J Eur Acad Dermatol Venereol. 2014;28:1529-1539.
- Togsverd-Ho K, Haak CS, Thaysen-Petersen D, et al. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol. 2012;166:1262-1269.
- Torezan L, Chaves Y, Niwa A, et al. A pilot split-face study comparing conventional methyl aminolevulinate-photodynamic therapy (PDT) with microneedling-assisted PDT on actinically damaged skin. Dermatol Surg. 2013;39:1197-1201.
Actinic keratosis (AK), also referred to as solar keratosis or senile keratosis, is an intraepidermal proliferation of dysplastic keratinocytes that develops in response to chronic exposure to UV radiation. Actinic keratoses are among the most commonly encountered lesions seen by dermatologists, and it has been estimated that 60% of predisposed individuals older than 40 years have at least one AK.1,2 Prevalence is notably higher in light-skinned individuals and increases with age, presumably from higher cumulative sun exposure and decreased effectiveness of the immune system.1,3 It remains a point of contention as to whether or not AKs actually represent squamous cell carcinoma (SCC) in situ, but the potential for progression to invasive disease has been well demonstrated, as the majority of SCCs develop from preexisting AKs.4-6 The risk for progression to invasive disease for an individual AK has been estimated to range from 0.025% to 16% per year, with an average of approximately 8% in immunocompetent patients.7
The clinical morphology of AK can vary widely, but the most common presentation is an erythematous scaly macule, papule, or plaque on sun-exposed skin. The skin surrounding AKs typically shows evidence of solar damage with deep wrinkling, mottled pigmentation, scattered telangiectases, purpura, or xerosis (Figure). A variety of clinical variants with unique presentations exist, including atrophic, hypertrophic, acantholytic, lichenoid, bowenoid, and pigmented subtypes. Because more than 80% of AKs occur on highly visible areas such as the head, neck, back of the hands, and forearms, AKs can have an obvious detrimental effect on cosmetic appearance. Studies also have shown a strong association between AKs and decreased overall quality of life (QOL).3,8,9

Because of the risk for AK progression to invasive cancer along with its negative impact on cosmesis and QOL, clinicians generally opt to treat AKs. Numerous different treatment options exist, including topical medications, procedural modalities, and light-based therapies. Here, we review the efficacy of the most commonly utilized treatments and discuss the relevant cosmetic considerations and outcomes.
Topical Treatments
5-Fluorouracil
5-Fluorouracil (5-FU) is a US Food and Drug Administration (FDA)–approved, topically applied pyrimidine analogue that inhibits thymidylate synthase. The resulting suppression of DNA and RNA synthesis induces cell death with a preference for mitotically active cells.10 5-Fluorouracil has been used for more than 50 years as a treatment of AK and its efficacy is well established. A systematic review of 5 randomized controlled studies of topical 5-FU reported an average of 49% of 423 patients achieving complete lesion clearance with 5-FU cream 5% applied once or twice daily for up to 7 weeks.11 Some notable drawbacks of 5-FU, however, are application-site erythema, blistering, pruritus, necrosis, erosion, and pain. These effects often lead to premature cessation of therapy, but newer formulations of 5-FU cream 0.5% have shown good efficacy with better tolerability.12 A randomized, double-blind, multicenter, parallel-group study of 177 patients using 5-FU cream 0.5% once daily for either 1, 2, or 4 weeks demonstrated significant (P<.001) efficacy over vehicle gel in all treatment arms.13 The most effective therapy was 4 weeks of treatment, which achieved a mean 91.7% reduction in lesion count as assessed 1 month after cessation of therapy. The primary adverse effect (AE) reported in this trial was mild to moderate facial irritation, which generally resolved within 18 to 21 days after treatment cessation.13 Overall, 5-FU is a highly effective therapy for treating AKs that also can improve signs of photoaging, but patients should be aware of cosmetically unappealing effects that generally occur throughout therapy and during the immediate posttreatment period.14
Chemical Peels
Chemical peels traditionally employ acidic compounds to strip away outer layers of skin to variable depths depending on the concentration of the agent being applied. For treatment of AK, trichloroacetic acid (TCA) is a commonly employed cauterant that has shown efficacy comparable to topical 5-FU as well as ablative CO2 laser resurfacing.15 Trichloroacetic acid peels also are a convenient therapy, as good results can be achieved after a single treatment session. A split-face study of 15 patients treated with either a single application of 35% TCA and Jessner solution or twice-daily application of 5-FU cream 5% for 3 weeks demonstrated a reduction in 75% of visible AKs in both treatment arms over a 1-year follow-up period.16 Although 80% of patients self-reported considerable cosmetic improvement with both therapies, patient preference was reported to be in favor of the TCA peel, given its quick results and relatively mild side effects as compared to 5-FU. Treatment with chemical peels will result in temporary erythema and mild desquamation that usually resolves within 2 weeks; however, there are cases in which erythema has been reported to persist for several months.16 Adverse effects such as permanent scarring or pigmentation changes rarely are seen with TCA concentrations less than 45%.17 Caution should be used in patients with a history of herpes simplex virus, keloids, postinflammatory hyperpigmentation, radiation exposure, immunosuppression, and those unable or unwilling to use sunscreen and avoid sun exposure in the immediate posttreatment period.
Diclofenac Sodium
Diclofenac sodium (DFS) is an FDA-approved topical, nonsteroidal,
anti-inflammatory drug whose mechanism of action in the treatment of AK is thought to involve inhibition of the cyclooxygenase 2 enzyme.18 The resulting reduction of prostaglandins is believed to inhibit tumor angiogenesis, induce apoptosis, and inhibit cell differentiation.19-22 In a multicenter, double-blind, placebo-controlled study of 195 patients, application of DFS 3% in hyaluronan gel 2.5% twice daily for 60 days showed significant (P<.05) efficacy over placebo in achieving complete resolution of target lesions during a 30-day follow-up period (31% vs 10%). Furthermore, qualitative patient assessment of complete global improvement also was significantly (P<.05) higher in the active treatment group as compared to placebo (31% vs 10%).23 Additional studies of DFS 3% in hyaluronan gel 2.5% applied twice daily for 90 days have shown even higher rates of success, with complete resolution of target lesions in 40% to 58% of cases.24,25 This therapy also has been reported to substantially improve QOL following treatment completion.26 The most frequently cited AEs include pruritus, rash, dry skin, erythema, and application-site reactions. Overall, DFS is a
well-tolerated therapy with efficacy comparable to that of 5-FU but with a lower incidence of AEs
and higher patient satisfaction as determined in
2 head-to-head studies.27,28
ImiquimodImiquimod (IMQ) is an FDA-approved topical agent that functions as an immune response modifier via agonism of toll-like receptor 7.18 The resulting cytokine production and release enhances the innate and acquired immune responses leading to anticancer activity.29 The efficacy of IMQ for treatment of AK has been demonstrated in numerous well-designed clinical trials. A
meta-analysis of 5 randomized, double-blind trials including 1293 patients treated with IMQ cream 5%
2 to 3 times per week for 12 to 16 weeks reported complete clearance of AKs in 50% of patients treated with IMQ as compared to 5% of patients treated with vehicle.30 The most frequently reported AEs with this therapy include erythema, scabbing, flaking, and erosion. These effects generally resolve following cessation of treatment, and therapy is considered to be well tolerated; however, there are case reports of IMQ triggering or exacerbating existing inflammatory conditions.31 Imiquimod cream also is approved at 2.5% and 3.75% concentrations, which have demonstrated significant (P<.001) efficacy over placebo and a reduced incidence of AEs; complete clearance rates have been reported as 30.6% and 35.6%, respectively.32 Notably, a study comparing 75 patients randomized to either IMQ cream 5%
3 times per week for 4 weeks, 1 or 2 courses of cryosurgery, or 5-FU ointment 5% twice daily for 4 weeks reported that IMQ achieved significantly (P<.01) superior sustained clearance rates during a 12-month follow-up period over cryosurgery and 5-FU
(73% vs 4% vs 33%).33 Additionally, cosmetic outcomes as determined by both participants and investigators were reported as excellent at 12 months posttreatment in more than 80% of participants treated with IMQ. These excellent, long-lasting cosmetic outcomes also were determined to be significantly (P<.0001) superior to the cosmetic outcomes of 5-FU and cryotherapy, which both reported excellent outcomes in less than 10% of cases.33
Ingenol MebutateIngenol mebutate (IM) is a macrocyclic diterpene ester derived from the Euphorbia peplus plant that is FDA approved for the treatment of AK.1 Ingenol mebutate’s mechanism of action is thought to involve induction of cell death via disruption of the plasma membrane and mitochondria in addition to production of an inflammatory response, which produces tumor-specific antibodies and a large influx of neutrophils.34,35 The overall evidence for the efficacy of IM is strong. A combined analysis of 4 multicenter, randomized, double-blind studies of 1005 participants reported that IM gel 0.015% applied once daily for 3 days to the face or scalp was significantly superior (P<.001) to placebo in achieving complete clearance as assessed 54 days after completion of therapy (42.2% vs 3.7%) and that IM gel 0.05% applied once daily for 2 days to the trunk or extremities also was significantly superior (P<.001) to placebo in achieving complete clearance as determined 55 days after completion of therapy (34.1% vs 4.7%).36 A follow-up report to this study indicated that IM also appears to achieve long-lasting effects with an overall 87% decrease in total AKs at 12 months follow-up in both trial groups.37 Additionally, it has been recently reported that treatment with IM in these trials was associated with significantly higher overall treatment satisfaction (P<.001) and improved QOL (P<.001) as compared to vehicle.38 Cosmetic outcomes of IM therapy have been assessed in a trial analyzing the efficacy of IM gel 0.025% for 3 days or IM gel 0.05% for 2 or 3 days on nonfacial AKs. This study reported significantly (P<.0001) higher patient satisfaction with the cosmetic outcome at 8 weeks after therapy as compared to vehicle.34 Studies performed in mice have demonstrated that IM is able to promote collagen matrix turnover and impose dermal elasticity, which may contribute to these good cosmetic outcomes.39 The most common AEs of IM therapy are erythema, crusting, and flaking; these effects generally occur 3 to 8 days after starting treatment. These effects, however, generally are short lived and resolve within 2 weeks of treatment cessation when IM is applied to the face or scalp or 4 weeks when applied to the trunk or extremities.40 Overall, IM is a useful therapeutic option given its relatively short treatment course as compared to other topically applied agents, as well as its lasting efficacy, mild AEs, and good cosmetic outcomes.
Procedural Modalities
Surgical Procedures
Surgical approaches for the treatment of AK include excision, curettage with or without electrodesiccation, and dermabrasion. In the past, these modalities were used with greater frequency, but the advent of effective topical medications with lower risks of AEs has largely reduced their use.41 Excision may still be indicated in cases where SCC is suspected, and curettage can be used for treatment of thicker hypertrophic AKs.42 Although these approaches have not been evaluated in clinical trials, they are generally effective but require the use of local anesthetics and come with substantial risk for infection, permanent scarring, and hypopigmentation. Dermabrasion employs the use of a motorized device equipped with an abrasive material to physically remove superficial layers of the skin. Studies are limited, but this method has been reported as an effective treatment in a retrospective review of 23 participants in which 96% remained free of AKs at 1 year, 83% at 2 years, 64% at 4 years, and 54% at 5 years posttherapy.43 Notably, one split-face study of 40 participants treated with dermabrasion followed by 25% TCA on one side and either Jessner solution and 35% TCA or dermabrasion alone on the other side reported that the combination of dermabrasion with 25% TCA consistently produced excellent cosmetic results with nearly complete eradication of AKs.44 In general, however, cosmetic outcomes with dermabrasion are variable, as the technique is highly operator dependent and treatment is associated with notable discomfort as well as risk for scarring and permanent pigmentation alteration.
Cryotherapy
Cryotherapy remains one of the most commonly utilized treatments of AK and involves the delivery of liquid nitrogen via a spray device or a cotton tip applicator to rapidly freeze cells, thus causing cellular destruction via ice crystal formation and protein denaturation.45 Efficacy with this technique has been reported to be as high as 98.8% at 12 months follow-up, but more recent studies cite lower rates of success.46 A prospective multicenter study of 90 participants with 421 AKs on the face or scalp treated with a single freeze-thaw cycle of liquid nitrogen reported an overall complete response rate of 67.2% at 3 months posttherapy. Additionally, higher complete response rates were associated with longer freeze times, and cosmetic outcomes were reported as good to excellent in 94% of complete response lesions.47 Similar results were reported in an open-label, prospective, randomized, controlled clinical trial of 200 participants with 543 AKs, which compared a single freeze-thaw cycle with liquid nitrogen to a single session of CO2 laser ablation in the treatment of isolated AKs of the face and scalp.48 At 3 months posttherapy, complete clearance was observed in 71.6% of participants treated with cryotherapy and in 65.3% of participants treated with laser ablation (P=.532). At 12 months posttherapy, participants who originally showed complete response at 3 months were assessed for relapse. Complete clearance was preserved in 72.6% of participants treated with cryotherapy versus 21.9% of participants treated with laser ablation (P<.0001), and cosmetic outcomes were reported by participants as good or excellent at 3 months follow-up in more than 93% of participants for both treatment arms.48 Possible AEs of cryotherapy include pain during treatment, blister formation with possible hemorrhage, infection, scarring, and permanent pigmentary changes.47,48 Notably, the risk for hypopigmentation increases with longer freezing times, thus requiring clinicians to consider the balance between improved efficacy and reduced cosmetic outcomes.47
Light-Based Therapies
Laser Therapy
Ablative laser resurfacing with either the CO2 or erbium-doped:YAG (Er:YAG) laser utilizes light of specific wavelengths to selectively induce thermolysis and destruction of the epidermal layer. Both lasers have been studied as treatments of AK, but there is a lack of large, well-designed studies. In one small study of 14 participants treated with 1 to 2 passes of the CO2 laser, complete clearance was reported in all cases without any recurrences during a follow-up period of 6 to 24 months. Additionally, all participants in this study reported satisfaction with the cosmetic outcome.49 The CO2 laser also has demonstrated efficacy comparable to that of the TCA peel and 5-FU therapy in a prospective randomized trial of 34 patients with facial or scalp AKs who received either CO2 laser with 2 passes, 30% TCA peel, or 5-FU cream 5% twice daily for 3 weeks.15 Reduction in mean AK counts at 3 months posttherapy was significantly (P<.03) higher in all treatment arms as compared to the control group (92% for CO2 laser, 89% for TCA peel, and 83% for 5-FU cream). No significant (P=.31) difference in outcomes was noted among the different treatment arms.15 Similar results were reported for the Er:YAG laser in a small prospective study of 5 participants treated with 2 to 3 passes with the Er:YAG laser in which reduction in mean AK counts was reported as ranging from 86% to 96% at 3 months posttherapy.50 The Er:YAG laser in combination with the CO2 laser has shown notable long-term efficacy in achieving higher lesion clearance rates and sustained complete clearance rates over treatment with topical 5-FU.51 In a prospective randomized study of 55 par-ticipants with multiple AKs on the face or scalp, participants were assigned to receive either combination laser ablation with the Er:YAG and CO2 lasers down to the level of the papillary dermis or 5-FU cream 5% applied twice daily for 2 to 7 weeks until an appropriate clinical inflammatory response was achieved. At 12 months follow-up, the laser treatment group achieved significantly (P=.048) higher mean lesion clearance rates (91.1%) as compared to the 5-FU arm (76.6%) and significantly (P=.003) higher sustained complete clearance rates (59.3%) as compared to 5-FU (29.2%). The proportion of participants with an improvement in photoaging score at 12 months follow-up approached statistical significance (P=.07), with 74% of the laser-treated group showing improvement as compared to 43% of the 5-FU–treated group. Long-term, cosmetically unappealing side effects such as erythema and hypopigmentation occurred notably more often in the laser-treated group as compared to the 5-FU group.51 In summary, ablative lasers appear to be a highly effective therapy for AK but at the cost of increased risk for AEs such as permanent pigmentary changes, prolonged erythema lasting up to several months, and scarring.50,52-55
Fractional photothermolysis is a relatively new advancement in the field of laser therapy that has received FDA approval for the treatment of AK.56 This treatment works by creating multiple noncontiguous microscopic columns of thermal injury while sparing adjacent zones of viable tissue.57 Although there are limited studies involving the use of such lasers in the treatment of AK, initial findings suggest that 1927-nm thulium lasers may be more effective than 1550-nm erbium lasers in achieving lesion clearance. A trial of 14 participants who received 5 laser treatments with a 1550-nm fractionated erbium-doped fiber laser reported an average reduction in AK counts of 66.2% at 3 months follow-up and a 55.6% reduction at 6 months follow-up. A participant-determined marked or very significant improvement of lesions was reported in 83% of participants at 1 month posttreatment but only in 44% of participants at 6 months posttreatment.58 A similar trial of 24 participants treated with up to 4 treatment sessions of the fractionated 1927-nm thulium laser reported an 87.3% reduction in number of AKs at 3 months follow-up and an 86.6% reduction at 6 months follow-up.56 The primary advantage of fractional laser therapy is a faster recovery period generally lasting only 2 or 3 days as compared to 2 weeks or more with traditional ablative lasers, thus limiting the amount of time a patient must tolerate cosmetically unappealing erythema.59,60 The quick recovery time has been attributed to the fractional laser’s ability to preserve the stratum corneum and skin barrier, which also helps reduce the risk for other AEs such as scarring and infection.56,59-61 Additional studies are needed to better assess the true efficacy of fractional laser therapy, but treatment with the fractional 1927-nm thulium laser appears to be a promising and well-tolerated therapeutic option for treatment of AK with similar efficacy to traditional ablative lasers but with a lower risk of AEs.
Photodynamic TherapyPhotodynamic therapy (PDT) is an FDA-approved treatment that involves the use of a topical photosensitizing agent such as 5-aminolevulinic acid (ALA) or methyl aminovulinate (MAL) before exposure to an activating light source to generate reactive oxygen species that lead to cell death.62-65 Multiple PDT regimens with varying combinations of photosensitizers, incubation time, and light sources have been studied, but a 2012 Cochrane review determined that treatment with conventional formulations of MAL and ALA with either blue- or red-light PDT were similarly efficacious for treatment of individual AKs as compared to vehicle with blue- or red-light PDT. One exception was that longer incubation time (ie, 4 hours) with ALA resulted in better results than shorter incubation times (ie, 0.5, 1, 2 hours) with ALA.66
Standard PDT treatment with MAL also has consistently demonstrated superior efficacy in achieving complete clearance rates in addition to superior cosmetic outcomes over treatment with either cryotherapy, DFS, or 5-FU.67-73 Three studies in particular noted an excellent or good investigator-determined cosmetic outcome in 96% to 98% of participants treated with MAL-PDT.69,71,74 Photodynamic therapy with ALA also has been reported as superior over CO2 laser ablation for AK reduction as well as both patient and investigator overall satisfaction.75
More recently, several methods of improving photosensitizer delivery have been studied, which have demonstrated remarkable efficacy at achieving lesion clearance over standard cream formulations or application routines. One such method involves the use of gentle heating to increase photosensitizer uptake. In a split-extremity study of 20 participants who were treated with 20% ALA under occlusion for 1 hour with one side heated to 38.8°C, the heated side demonstrated significant (P<.0001) efficacy at achieving higher median clearance rates over control when evaluated at 2 and 6 months posttherapy.76 Notably, occlusion of ALA in itself during the incubation period also has been demonstrated to significantly (P<.0001) improve clearance rates.77 Another method involves the use of a new nanoemulsion-based formulation of ALA gel, known as BF-200 ALA, which has demonstrated remarkable efficacy over standard MAL cream and placebo in a long-term follow-up analysis of 2 prospective, randomized, controlled trials.78 In a similar vein, 3 prospective randomized trials with a minimum follow-up time of 3 months demonstrated that MAL-PDT in combination with fractional ablative laser pretreatment has significant (P<.02 in all trials) efficacy over MAL-PDT without pretreatment in achieving complete AK clearance. Although the cosmetic outcomes were good or excellent in 87% to 100% of patients, they were not significantly different from stand-alone MAL-PDT treatment in any of the trials.79-81 However, pretreatment with microneedling in MAL-PDT has been shown to achieve superior cosmetic outcomes over MAL-PDT without microneedling, according to one small split-face study of 10 participants.82
Overall, PDT is an excellent therapeutic option that is able to provide efficacious clearance of AKs as well as superior cosmetic outcomes. Common AEs of PDT include burning, itching, and stinging during therapy, but pain intensity decreases dramatically upon termination of illumination, with cessation of most symptoms by 12 hours posttherapy.73 Permanent pigmentation changes have been reported to occasionally occur following PDT therapy.81
Conclusion
When determining which therapy to use in a patient, clinicians must take into account a variety of factors such as patient preference, cost of treatment, availability, tolerance for AEs, and the need for field therapy. Although all therapies discussed within this article are effective and reasonable treatment choices, patients who are particularly concerned about cosmetic outcomes would most likely benefit from either IMQ or PDT, as the data for cosmetic outcomes with these therapies are the strongest. Combination or sequential treatments may be required in some cases and all patients should be monitored for lesion recurrence regardless of treatment choice. A summary of the therapies and key studies discussed here is available in the PDF.
Actinic keratosis (AK), also referred to as solar keratosis or senile keratosis, is an intraepidermal proliferation of dysplastic keratinocytes that develops in response to chronic exposure to UV radiation. Actinic keratoses are among the most commonly encountered lesions seen by dermatologists, and it has been estimated that 60% of predisposed individuals older than 40 years have at least one AK.1,2 Prevalence is notably higher in light-skinned individuals and increases with age, presumably from higher cumulative sun exposure and decreased effectiveness of the immune system.1,3 It remains a point of contention as to whether or not AKs actually represent squamous cell carcinoma (SCC) in situ, but the potential for progression to invasive disease has been well demonstrated, as the majority of SCCs develop from preexisting AKs.4-6 The risk for progression to invasive disease for an individual AK has been estimated to range from 0.025% to 16% per year, with an average of approximately 8% in immunocompetent patients.7
The clinical morphology of AK can vary widely, but the most common presentation is an erythematous scaly macule, papule, or plaque on sun-exposed skin. The skin surrounding AKs typically shows evidence of solar damage with deep wrinkling, mottled pigmentation, scattered telangiectases, purpura, or xerosis (Figure). A variety of clinical variants with unique presentations exist, including atrophic, hypertrophic, acantholytic, lichenoid, bowenoid, and pigmented subtypes. Because more than 80% of AKs occur on highly visible areas such as the head, neck, back of the hands, and forearms, AKs can have an obvious detrimental effect on cosmetic appearance. Studies also have shown a strong association between AKs and decreased overall quality of life (QOL).3,8,9

Because of the risk for AK progression to invasive cancer along with its negative impact on cosmesis and QOL, clinicians generally opt to treat AKs. Numerous different treatment options exist, including topical medications, procedural modalities, and light-based therapies. Here, we review the efficacy of the most commonly utilized treatments and discuss the relevant cosmetic considerations and outcomes.
Topical Treatments
5-Fluorouracil
5-Fluorouracil (5-FU) is a US Food and Drug Administration (FDA)–approved, topically applied pyrimidine analogue that inhibits thymidylate synthase. The resulting suppression of DNA and RNA synthesis induces cell death with a preference for mitotically active cells.10 5-Fluorouracil has been used for more than 50 years as a treatment of AK and its efficacy is well established. A systematic review of 5 randomized controlled studies of topical 5-FU reported an average of 49% of 423 patients achieving complete lesion clearance with 5-FU cream 5% applied once or twice daily for up to 7 weeks.11 Some notable drawbacks of 5-FU, however, are application-site erythema, blistering, pruritus, necrosis, erosion, and pain. These effects often lead to premature cessation of therapy, but newer formulations of 5-FU cream 0.5% have shown good efficacy with better tolerability.12 A randomized, double-blind, multicenter, parallel-group study of 177 patients using 5-FU cream 0.5% once daily for either 1, 2, or 4 weeks demonstrated significant (P<.001) efficacy over vehicle gel in all treatment arms.13 The most effective therapy was 4 weeks of treatment, which achieved a mean 91.7% reduction in lesion count as assessed 1 month after cessation of therapy. The primary adverse effect (AE) reported in this trial was mild to moderate facial irritation, which generally resolved within 18 to 21 days after treatment cessation.13 Overall, 5-FU is a highly effective therapy for treating AKs that also can improve signs of photoaging, but patients should be aware of cosmetically unappealing effects that generally occur throughout therapy and during the immediate posttreatment period.14
Chemical Peels
Chemical peels traditionally employ acidic compounds to strip away outer layers of skin to variable depths depending on the concentration of the agent being applied. For treatment of AK, trichloroacetic acid (TCA) is a commonly employed cauterant that has shown efficacy comparable to topical 5-FU as well as ablative CO2 laser resurfacing.15 Trichloroacetic acid peels also are a convenient therapy, as good results can be achieved after a single treatment session. A split-face study of 15 patients treated with either a single application of 35% TCA and Jessner solution or twice-daily application of 5-FU cream 5% for 3 weeks demonstrated a reduction in 75% of visible AKs in both treatment arms over a 1-year follow-up period.16 Although 80% of patients self-reported considerable cosmetic improvement with both therapies, patient preference was reported to be in favor of the TCA peel, given its quick results and relatively mild side effects as compared to 5-FU. Treatment with chemical peels will result in temporary erythema and mild desquamation that usually resolves within 2 weeks; however, there are cases in which erythema has been reported to persist for several months.16 Adverse effects such as permanent scarring or pigmentation changes rarely are seen with TCA concentrations less than 45%.17 Caution should be used in patients with a history of herpes simplex virus, keloids, postinflammatory hyperpigmentation, radiation exposure, immunosuppression, and those unable or unwilling to use sunscreen and avoid sun exposure in the immediate posttreatment period.
Diclofenac Sodium
Diclofenac sodium (DFS) is an FDA-approved topical, nonsteroidal,
anti-inflammatory drug whose mechanism of action in the treatment of AK is thought to involve inhibition of the cyclooxygenase 2 enzyme.18 The resulting reduction of prostaglandins is believed to inhibit tumor angiogenesis, induce apoptosis, and inhibit cell differentiation.19-22 In a multicenter, double-blind, placebo-controlled study of 195 patients, application of DFS 3% in hyaluronan gel 2.5% twice daily for 60 days showed significant (P<.05) efficacy over placebo in achieving complete resolution of target lesions during a 30-day follow-up period (31% vs 10%). Furthermore, qualitative patient assessment of complete global improvement also was significantly (P<.05) higher in the active treatment group as compared to placebo (31% vs 10%).23 Additional studies of DFS 3% in hyaluronan gel 2.5% applied twice daily for 90 days have shown even higher rates of success, with complete resolution of target lesions in 40% to 58% of cases.24,25 This therapy also has been reported to substantially improve QOL following treatment completion.26 The most frequently cited AEs include pruritus, rash, dry skin, erythema, and application-site reactions. Overall, DFS is a
well-tolerated therapy with efficacy comparable to that of 5-FU but with a lower incidence of AEs
and higher patient satisfaction as determined in
2 head-to-head studies.27,28
ImiquimodImiquimod (IMQ) is an FDA-approved topical agent that functions as an immune response modifier via agonism of toll-like receptor 7.18 The resulting cytokine production and release enhances the innate and acquired immune responses leading to anticancer activity.29 The efficacy of IMQ for treatment of AK has been demonstrated in numerous well-designed clinical trials. A
meta-analysis of 5 randomized, double-blind trials including 1293 patients treated with IMQ cream 5%
2 to 3 times per week for 12 to 16 weeks reported complete clearance of AKs in 50% of patients treated with IMQ as compared to 5% of patients treated with vehicle.30 The most frequently reported AEs with this therapy include erythema, scabbing, flaking, and erosion. These effects generally resolve following cessation of treatment, and therapy is considered to be well tolerated; however, there are case reports of IMQ triggering or exacerbating existing inflammatory conditions.31 Imiquimod cream also is approved at 2.5% and 3.75% concentrations, which have demonstrated significant (P<.001) efficacy over placebo and a reduced incidence of AEs; complete clearance rates have been reported as 30.6% and 35.6%, respectively.32 Notably, a study comparing 75 patients randomized to either IMQ cream 5%
3 times per week for 4 weeks, 1 or 2 courses of cryosurgery, or 5-FU ointment 5% twice daily for 4 weeks reported that IMQ achieved significantly (P<.01) superior sustained clearance rates during a 12-month follow-up period over cryosurgery and 5-FU
(73% vs 4% vs 33%).33 Additionally, cosmetic outcomes as determined by both participants and investigators were reported as excellent at 12 months posttreatment in more than 80% of participants treated with IMQ. These excellent, long-lasting cosmetic outcomes also were determined to be significantly (P<.0001) superior to the cosmetic outcomes of 5-FU and cryotherapy, which both reported excellent outcomes in less than 10% of cases.33
Ingenol MebutateIngenol mebutate (IM) is a macrocyclic diterpene ester derived from the Euphorbia peplus plant that is FDA approved for the treatment of AK.1 Ingenol mebutate’s mechanism of action is thought to involve induction of cell death via disruption of the plasma membrane and mitochondria in addition to production of an inflammatory response, which produces tumor-specific antibodies and a large influx of neutrophils.34,35 The overall evidence for the efficacy of IM is strong. A combined analysis of 4 multicenter, randomized, double-blind studies of 1005 participants reported that IM gel 0.015% applied once daily for 3 days to the face or scalp was significantly superior (P<.001) to placebo in achieving complete clearance as assessed 54 days after completion of therapy (42.2% vs 3.7%) and that IM gel 0.05% applied once daily for 2 days to the trunk or extremities also was significantly superior (P<.001) to placebo in achieving complete clearance as determined 55 days after completion of therapy (34.1% vs 4.7%).36 A follow-up report to this study indicated that IM also appears to achieve long-lasting effects with an overall 87% decrease in total AKs at 12 months follow-up in both trial groups.37 Additionally, it has been recently reported that treatment with IM in these trials was associated with significantly higher overall treatment satisfaction (P<.001) and improved QOL (P<.001) as compared to vehicle.38 Cosmetic outcomes of IM therapy have been assessed in a trial analyzing the efficacy of IM gel 0.025% for 3 days or IM gel 0.05% for 2 or 3 days on nonfacial AKs. This study reported significantly (P<.0001) higher patient satisfaction with the cosmetic outcome at 8 weeks after therapy as compared to vehicle.34 Studies performed in mice have demonstrated that IM is able to promote collagen matrix turnover and impose dermal elasticity, which may contribute to these good cosmetic outcomes.39 The most common AEs of IM therapy are erythema, crusting, and flaking; these effects generally occur 3 to 8 days after starting treatment. These effects, however, generally are short lived and resolve within 2 weeks of treatment cessation when IM is applied to the face or scalp or 4 weeks when applied to the trunk or extremities.40 Overall, IM is a useful therapeutic option given its relatively short treatment course as compared to other topically applied agents, as well as its lasting efficacy, mild AEs, and good cosmetic outcomes.
Procedural Modalities
Surgical Procedures
Surgical approaches for the treatment of AK include excision, curettage with or without electrodesiccation, and dermabrasion. In the past, these modalities were used with greater frequency, but the advent of effective topical medications with lower risks of AEs has largely reduced their use.41 Excision may still be indicated in cases where SCC is suspected, and curettage can be used for treatment of thicker hypertrophic AKs.42 Although these approaches have not been evaluated in clinical trials, they are generally effective but require the use of local anesthetics and come with substantial risk for infection, permanent scarring, and hypopigmentation. Dermabrasion employs the use of a motorized device equipped with an abrasive material to physically remove superficial layers of the skin. Studies are limited, but this method has been reported as an effective treatment in a retrospective review of 23 participants in which 96% remained free of AKs at 1 year, 83% at 2 years, 64% at 4 years, and 54% at 5 years posttherapy.43 Notably, one split-face study of 40 participants treated with dermabrasion followed by 25% TCA on one side and either Jessner solution and 35% TCA or dermabrasion alone on the other side reported that the combination of dermabrasion with 25% TCA consistently produced excellent cosmetic results with nearly complete eradication of AKs.44 In general, however, cosmetic outcomes with dermabrasion are variable, as the technique is highly operator dependent and treatment is associated with notable discomfort as well as risk for scarring and permanent pigmentation alteration.
Cryotherapy
Cryotherapy remains one of the most commonly utilized treatments of AK and involves the delivery of liquid nitrogen via a spray device or a cotton tip applicator to rapidly freeze cells, thus causing cellular destruction via ice crystal formation and protein denaturation.45 Efficacy with this technique has been reported to be as high as 98.8% at 12 months follow-up, but more recent studies cite lower rates of success.46 A prospective multicenter study of 90 participants with 421 AKs on the face or scalp treated with a single freeze-thaw cycle of liquid nitrogen reported an overall complete response rate of 67.2% at 3 months posttherapy. Additionally, higher complete response rates were associated with longer freeze times, and cosmetic outcomes were reported as good to excellent in 94% of complete response lesions.47 Similar results were reported in an open-label, prospective, randomized, controlled clinical trial of 200 participants with 543 AKs, which compared a single freeze-thaw cycle with liquid nitrogen to a single session of CO2 laser ablation in the treatment of isolated AKs of the face and scalp.48 At 3 months posttherapy, complete clearance was observed in 71.6% of participants treated with cryotherapy and in 65.3% of participants treated with laser ablation (P=.532). At 12 months posttherapy, participants who originally showed complete response at 3 months were assessed for relapse. Complete clearance was preserved in 72.6% of participants treated with cryotherapy versus 21.9% of participants treated with laser ablation (P<.0001), and cosmetic outcomes were reported by participants as good or excellent at 3 months follow-up in more than 93% of participants for both treatment arms.48 Possible AEs of cryotherapy include pain during treatment, blister formation with possible hemorrhage, infection, scarring, and permanent pigmentary changes.47,48 Notably, the risk for hypopigmentation increases with longer freezing times, thus requiring clinicians to consider the balance between improved efficacy and reduced cosmetic outcomes.47
Light-Based Therapies
Laser Therapy
Ablative laser resurfacing with either the CO2 or erbium-doped:YAG (Er:YAG) laser utilizes light of specific wavelengths to selectively induce thermolysis and destruction of the epidermal layer. Both lasers have been studied as treatments of AK, but there is a lack of large, well-designed studies. In one small study of 14 participants treated with 1 to 2 passes of the CO2 laser, complete clearance was reported in all cases without any recurrences during a follow-up period of 6 to 24 months. Additionally, all participants in this study reported satisfaction with the cosmetic outcome.49 The CO2 laser also has demonstrated efficacy comparable to that of the TCA peel and 5-FU therapy in a prospective randomized trial of 34 patients with facial or scalp AKs who received either CO2 laser with 2 passes, 30% TCA peel, or 5-FU cream 5% twice daily for 3 weeks.15 Reduction in mean AK counts at 3 months posttherapy was significantly (P<.03) higher in all treatment arms as compared to the control group (92% for CO2 laser, 89% for TCA peel, and 83% for 5-FU cream). No significant (P=.31) difference in outcomes was noted among the different treatment arms.15 Similar results were reported for the Er:YAG laser in a small prospective study of 5 participants treated with 2 to 3 passes with the Er:YAG laser in which reduction in mean AK counts was reported as ranging from 86% to 96% at 3 months posttherapy.50 The Er:YAG laser in combination with the CO2 laser has shown notable long-term efficacy in achieving higher lesion clearance rates and sustained complete clearance rates over treatment with topical 5-FU.51 In a prospective randomized study of 55 par-ticipants with multiple AKs on the face or scalp, participants were assigned to receive either combination laser ablation with the Er:YAG and CO2 lasers down to the level of the papillary dermis or 5-FU cream 5% applied twice daily for 2 to 7 weeks until an appropriate clinical inflammatory response was achieved. At 12 months follow-up, the laser treatment group achieved significantly (P=.048) higher mean lesion clearance rates (91.1%) as compared to the 5-FU arm (76.6%) and significantly (P=.003) higher sustained complete clearance rates (59.3%) as compared to 5-FU (29.2%). The proportion of participants with an improvement in photoaging score at 12 months follow-up approached statistical significance (P=.07), with 74% of the laser-treated group showing improvement as compared to 43% of the 5-FU–treated group. Long-term, cosmetically unappealing side effects such as erythema and hypopigmentation occurred notably more often in the laser-treated group as compared to the 5-FU group.51 In summary, ablative lasers appear to be a highly effective therapy for AK but at the cost of increased risk for AEs such as permanent pigmentary changes, prolonged erythema lasting up to several months, and scarring.50,52-55
Fractional photothermolysis is a relatively new advancement in the field of laser therapy that has received FDA approval for the treatment of AK.56 This treatment works by creating multiple noncontiguous microscopic columns of thermal injury while sparing adjacent zones of viable tissue.57 Although there are limited studies involving the use of such lasers in the treatment of AK, initial findings suggest that 1927-nm thulium lasers may be more effective than 1550-nm erbium lasers in achieving lesion clearance. A trial of 14 participants who received 5 laser treatments with a 1550-nm fractionated erbium-doped fiber laser reported an average reduction in AK counts of 66.2% at 3 months follow-up and a 55.6% reduction at 6 months follow-up. A participant-determined marked or very significant improvement of lesions was reported in 83% of participants at 1 month posttreatment but only in 44% of participants at 6 months posttreatment.58 A similar trial of 24 participants treated with up to 4 treatment sessions of the fractionated 1927-nm thulium laser reported an 87.3% reduction in number of AKs at 3 months follow-up and an 86.6% reduction at 6 months follow-up.56 The primary advantage of fractional laser therapy is a faster recovery period generally lasting only 2 or 3 days as compared to 2 weeks or more with traditional ablative lasers, thus limiting the amount of time a patient must tolerate cosmetically unappealing erythema.59,60 The quick recovery time has been attributed to the fractional laser’s ability to preserve the stratum corneum and skin barrier, which also helps reduce the risk for other AEs such as scarring and infection.56,59-61 Additional studies are needed to better assess the true efficacy of fractional laser therapy, but treatment with the fractional 1927-nm thulium laser appears to be a promising and well-tolerated therapeutic option for treatment of AK with similar efficacy to traditional ablative lasers but with a lower risk of AEs.
Photodynamic TherapyPhotodynamic therapy (PDT) is an FDA-approved treatment that involves the use of a topical photosensitizing agent such as 5-aminolevulinic acid (ALA) or methyl aminovulinate (MAL) before exposure to an activating light source to generate reactive oxygen species that lead to cell death.62-65 Multiple PDT regimens with varying combinations of photosensitizers, incubation time, and light sources have been studied, but a 2012 Cochrane review determined that treatment with conventional formulations of MAL and ALA with either blue- or red-light PDT were similarly efficacious for treatment of individual AKs as compared to vehicle with blue- or red-light PDT. One exception was that longer incubation time (ie, 4 hours) with ALA resulted in better results than shorter incubation times (ie, 0.5, 1, 2 hours) with ALA.66
Standard PDT treatment with MAL also has consistently demonstrated superior efficacy in achieving complete clearance rates in addition to superior cosmetic outcomes over treatment with either cryotherapy, DFS, or 5-FU.67-73 Three studies in particular noted an excellent or good investigator-determined cosmetic outcome in 96% to 98% of participants treated with MAL-PDT.69,71,74 Photodynamic therapy with ALA also has been reported as superior over CO2 laser ablation for AK reduction as well as both patient and investigator overall satisfaction.75
More recently, several methods of improving photosensitizer delivery have been studied, which have demonstrated remarkable efficacy at achieving lesion clearance over standard cream formulations or application routines. One such method involves the use of gentle heating to increase photosensitizer uptake. In a split-extremity study of 20 participants who were treated with 20% ALA under occlusion for 1 hour with one side heated to 38.8°C, the heated side demonstrated significant (P<.0001) efficacy at achieving higher median clearance rates over control when evaluated at 2 and 6 months posttherapy.76 Notably, occlusion of ALA in itself during the incubation period also has been demonstrated to significantly (P<.0001) improve clearance rates.77 Another method involves the use of a new nanoemulsion-based formulation of ALA gel, known as BF-200 ALA, which has demonstrated remarkable efficacy over standard MAL cream and placebo in a long-term follow-up analysis of 2 prospective, randomized, controlled trials.78 In a similar vein, 3 prospective randomized trials with a minimum follow-up time of 3 months demonstrated that MAL-PDT in combination with fractional ablative laser pretreatment has significant (P<.02 in all trials) efficacy over MAL-PDT without pretreatment in achieving complete AK clearance. Although the cosmetic outcomes were good or excellent in 87% to 100% of patients, they were not significantly different from stand-alone MAL-PDT treatment in any of the trials.79-81 However, pretreatment with microneedling in MAL-PDT has been shown to achieve superior cosmetic outcomes over MAL-PDT without microneedling, according to one small split-face study of 10 participants.82
Overall, PDT is an excellent therapeutic option that is able to provide efficacious clearance of AKs as well as superior cosmetic outcomes. Common AEs of PDT include burning, itching, and stinging during therapy, but pain intensity decreases dramatically upon termination of illumination, with cessation of most symptoms by 12 hours posttherapy.73 Permanent pigmentation changes have been reported to occasionally occur following PDT therapy.81
Conclusion
When determining which therapy to use in a patient, clinicians must take into account a variety of factors such as patient preference, cost of treatment, availability, tolerance for AEs, and the need for field therapy. Although all therapies discussed within this article are effective and reasonable treatment choices, patients who are particularly concerned about cosmetic outcomes would most likely benefit from either IMQ or PDT, as the data for cosmetic outcomes with these therapies are the strongest. Combination or sequential treatments may be required in some cases and all patients should be monitored for lesion recurrence regardless of treatment choice. A summary of the therapies and key studies discussed here is available in the PDF.
- Lebwohl M. Actinic keratosis: epidemiology and progression to squamous cell carcinoma. Br J Dermatol. 2003;149(suppl 66):31-33.
- Drake LA, Ceilley RI, Cornelison RL, et al. Guidelines of care for actinic keratoses. Committee on Guidelines of Care. J Am Acad Dermatol. 1995;32:95-98.
- Salasche SJ. Epidemiology of actinic keratoses and squamous cell carcinoma. J Am Acad Dermatol. 2000;42(1, pt 2):4-7.
- Ackerman AB, Mones JM. Solar (actinic) keratosis is squamous cell carcinoma. Br J Dermatol. 2006;155:9-22.
- Anwar J, Wrone DA, Kimyai-Asadi A, et al. The development of actinic keratosis into invasive squamous cell carcinoma: evidence and evolving classification schemes. Clin Dermatol. 2004;22:189-196.
- Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
- Glogau RG. The risk of progression to invasive disease. J Am Acad Dermatol. 2000;42(1, pt 2):23-24.
- Esmann S, Jemec GB. Management of actinic keratosis patients: a qualitative study. J Dermatolog Treat. 2007;18:53-58.
- Weinstock MA, Lee KC, Chren MM, et al. Quality of life in the actinic neoplasia syndrome: the VA Topical Tretinoin Chemoprevention (VATTC) trial. J Am Acad Dermatol. 2009;61:207-215.
- Berman B, Villa AM, Ramirez CC. Mechanisms of action of new treatment modalities for actinic keratosis. J Drugs Dermatol. 2006;5:167-173.
- Askew DA, Mickan SM, Soyer HP. Effectiveness of 5-fluorouracil treatment for actinic keratosis: a systematic review of randomized controlled trials. Int J Dermatol. 2009;46:452-463.
- Levy S, Furst K, Chern W. A pharmacokinetic evaluation of 0.5% and 5% fluorouracil topical cream in patients with actinic keratosis. Clin Ther. 2001;23:908-920.
- Jorizzo J, Stewart D, Bucko A, et al. Randomized trial evaluating a new 0.5% fluorouracil formulation demonstrates efficacy after 1-, 2-, or 4-week treatment in patients with actinic keratosis. Cutis. 2002;70:335-359.
- Sachs DL, Kang S, Hammerberg C, et al. Topical fluorouracil for actinic keratoses and photoaging: a clinical and molecular analysis. Arch Dermatol. 2009;145:659-666.
- Hantash BM, Stewart DB, Cooper ZA, et al. Facial resurfacing for nonmelanoma skin cancer prophylaxis. Arch Dermatol. 2006;142:976-982.
- Lawrence N, Cox SE, Cockerell CJ, et al. A comparison of the efficacy and safety of Jessner’s solution and 35% trichloroacetic acid vs 5% fluorouracil in the treatment of widespread facial actinic keratoses. Arch Dermatol. 1995;131:176-181.
- Monheit GD. The Jessner’s + TCA peel: a medium-depth chemical peel. J Dermatol Surg Oncol. 1989;15:945-950.
- Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196-200.
- Adamson DJ, Frew D, Tatoud R, et al. Diclofenac antagonizes peroxisome proliferator-activated receptor-gamma signaling. Mol Pharmacol. 2002;61:7-12.
- Alam CA, Seed MP, Willoughby DA. Angiostasis and vascular regression in chronic granulomatous inflammation induced by diclofenac in combination with hyaluronan in mice. J Pharm Pharmacol. 1995;47:407-411.
- Lu X, Xie W, Reed D, et al. Nonsteroidal antiinflammatory drugs cause apoptosis and induce cyclooxygenases in chicken embryo fibroblasts. Proc Natl Acad Sci USA. 1995;92:7961-7965.
- Seed MP, Brown JR, Freemantle CN, et al. The inhibition of colon-26 adenocarcinoma development and angiogenesis by topical diclofenac in 2.5% hyaluronan. Cancer Res. 1997;57:1625-1629.
- Rivers JK, Arlette J, Shear N, et al. Topical treatment of actinic keratoses with 3.0% diclofenac in 2.5% hyaluronan gel. Br J Dermatol. 2002;146:94-100.
- Wolf JE, Taylor JR, Tschen E, et al. Topical 3.0% diclo-fenac in 2.5% hyaluronan gel in the treatment of actinic keratoses. Int J Dermatol. 2001;40:709-713.
- Nelson C, Rigel D, Smith S, et al. Phase IV, open-label assessment of the treatment of actinic keratosis with 3.0% diclofenac sodium topical gel (Solaraze). J Drugs Dermatol. 2004;3:401-407.
- Pflugfelder A, Welter AK, Leiter U, et al. Open label randomized study comparing 3 months vs. 6 months treatment of actinic keratoses with 3% diclofenac in 2.5% hyaluronic acid gel: a trial of the German Dermatologic Cooperative Oncology Group. J Eur Acad Dermatol Venereol. 2012;26:48-53.
- Smith SR, Morhenn VB, Piacquadio DJ. Bilateral comparison of the efficacy and tolerability of 3% diclofenac sodium gel and 5% 5-fluorouracil cream in the treatment of actinic keratoses of the face and scalp. J Drugs Dermatol. 2006;5:156-159.
- Segatto MM, Dornelles SI, Silveira VB, et al. Comparative study of actinic keratosis treatment with 3% diclo- fenac sodium and 5% 5-fluorouracil. An Bras Dermatol. 2013;88:732-738.
- Vidal D. Topical imiquimod: mechanism of action and clinical applications. Mini Rev Med Chem. 2006;6:499-503.
- Hadley G, Derry S, Moore RA. Imiquimod for actinic keratosis: systematic review and meta-analysis. J Invest Dermatol. 2006;126:1251-1255.
- Caperton C, Berman B. Safety, efficacy, and patient acceptability of imiquimod for topical treatment of actinic keratoses. Clin Cosmet Investig Dermatol. 2011;4:35-40.
- Swanson N, Smith CC, Kaur M, et al. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: two phase 3, multicenter, randomized, double-blind, placebo-controlled studies. J Drugs Dermatol. 2014;13:166-169.
- Krawtchenko N, Roewert-Huber J, Ulrich M, et al. A randomised study of topical 5% imiquimod vs. topical 5-fluorouracil vs. cryosurgery in immunocompetent patients with actinic keratoses: a comparison of clinical and histological outcomes including 1-year follow-up. Br J Dermatol. 2007;157(suppl 2):34-40.
- Anderson L, Schmieder GJ, Werschler WP, et al. Randomized, double-blind, double-dummy, vehicle-controlled study of ingenol mebutate gel 0.025% and 0.05% for actinic keratosis. J Am Acad Dermatol. 2009;60:934-943.
- Ogbourne SM, Suhrbier A, Jones B, et al. Antitumor activity of 3-ingenyl angelate: plasma membrane and mitochondrial disruption and necrotic cell death. Cancer Res. 2004;64:2833-2839.
- Lebwohl M, Swanson N, Anderson LL, et al. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366:1010-1019.
- Lebwohl M, Shumack S, Stein-Gold L, et al. Long-term follow-up study of ingenol mebutate gel for the treatment of actinic keratoses. JAMA Dermatol. 2013;149:666-670.
- Augustin M, Tu JH, Knudsen KM, et al. Ingenol mebutate gel for actinic keratosis: the link between quality of life, treatment satisfaction, and clinical outcomes. J Am Acad Dermatol. 2015;72:816-821.
- Kane-Maguire N, Moseley R, Cozzi S, et al. Modulation of fibroblast phenotype and extracellular matrix composition by ingenol mebutate may be associated with scar resolution and improved dermal cosmesis. J Am Acad Dermatol. 2012;66:AB218.
- Martin G, Swanson N. Clinical findings using ingenol mebutate gel to treat actinic keratoses. J Am Acad Dermatol. 2013;68(1, suppl 1):S39-S48.
- Feldman SR, Fleischer AB, Williford PM, et al. Destructive procedures are the standard of care for treatment of actinic keratoses. J Am Acad Dermatol. 1999;40:43-47.
- Berlin JM. Current and emerging treatment strategies for the treatment of actinic keratosis. Clin Cosmet Investig Dermatol. 2010;3:119-126.
- Coleman WP, Yarborough JM, Mandy SH. Dermabrasion for prophylaxis and treatment of actinic keratoses. Dermatol Surg. 1996;22:17-21.
- Cooley JE, Casey DL, Kauffman CL. Manual resurfacing and trichloroacetic acid for the treatment of patients with widespread actinic damage. clinical and histologic observations. Dermatol Surg. 1997;23:373-379.
- Goldberg LH, Kaplan B, Vergilis-Kalner I, et al. Liquid nitrogen: temperature control in the treatment of actinic keratosis. Dermatol Surg. 2010;36:1956-1961.
- Lubritz RR, Smolewski SA. Cryosurgery cure rate of actinic keratoses. J Am Acad Dermatol. 1982;7:631-632.
- Thai KE, Fergin P, Freeman M, et al. A prospective study of the use of cryosurgery for the treatment of actinic keratoses. Int J Dermatol. 2004;43:687-692.
- Zane C, Facchinetti E, Rossi MT, et al. Cryotherapy is preferable to ablative CO2 laser for the treatment of isolated actinic keratoses of the face and scalp: a randomized clinical trial. Br J Dermatol. 2014;170:1114-1121.
- Trimas SJ, Ellis DA, Metz RD. The carbon dioxide laser. an alternative for the treatment of actinically damaged skin. Dermatol Surg. 1997;23:885-889.
- Jiang SB, Levine VJ, Nehal KS, et al. Er:YAG laser for the treatment of actinic keratoses. Dermatol Surg. 2000;26:437-440.
- Ostertag JU, Quaedvlieg PJ, Van der geer S, et al. A clinical comparison and long-term follow-up of topical 5-fluorouracil versus laser resurfacing in the treatment of widespread actinic keratoses. Lasers Surg Med. 2006;38:731-739.
- Iyer S, Friedli A, Bowes L, et al. Full face laser resurfacing: therapy and prophylaxis for actinic keratoses and non-melanoma skin cancer. Lasers Surg Med. 2004;34:114-119.
- Rubin MG. A peeler’s thoughts on skin improvement with chemical peels and laser resurfacing. Clin Plast Surg. 1997;24:407-409.
- Riggs K, Keller M, Humphreys TR. Ablative laser resurfacing: high-energy pulsed carbon dioxide and erbium:yttrium-aluminum-garnet. Clin Dermatol. 2007;25:462-473.
- Adrian RM. Pulsed carbon dioxide and long pulse 10-ms erbium-YAG laser resurfacing: a comparative clinical and histological study. J Cutan Laser Ther. 1999;1:197-202.
- Weiss ET, Brauer JA, Anolik R, et al. 1927-nm fractional resurfacing of facial actinic keratoses: a promising new therapeutic option. J Am Acad Dermatol. 2013; 68:98-102.
- Manstein D, Herron GS, Sink RK, et al. Fractional photothermolysis: a new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg Med. 2004;34:426-438.
- Katz TM, Goldberg LH, Marquez D, et al. Nonablative fractional photothermolysis for facial actinic keratoses: 6-month follow-up with histologic evaluation. J Am Acad Dermatol. 2011;65:349-356.
- Prens SP, De Vries K, Neumann HA, et al. Non-ablative fractional resurfacing in combination with topical tretinoin cream as a field treatment modality for multiple actinic keratosis: a pilot study and a review of other field treatment modalities. J Dermatolog Treat. 2013;24:227-231.
- Alexiades-Armenakas MR, Dover JS, Arndt KA. The spectrum of laser skin resurfacing: nonablative, fractional, and ablative laser resurfacing. J Am Acad Dermatol. 2008;58:719-737.
- Tannous Z. Fractional resurfacing. Clin Dermatol. 2007;25:480-486.
- Gold MH. Continuing medical education article-skin treatment: photodynamic therapy: indications and treatment. Aesthet Surg J. 2008;28:545-552.
- Juarranz A, Jaén P, Sanz-Rodríguez F, et al. Photodynamic therapy of cancer. basic principles and applications. Clin Transl Oncol. 2008;10:148-154.
- Juzeniene A, Peng Q, Moan J. Milestones in the development of photodynamic therapy and fluorescence diagnosis. Photochem Photobiol Sci. 2007;6:1234-1245.
- Moan J, Berg K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem Photobiol. 1991;53:549-553.
- Gupta AK, Paquet M, Villanueva E, et al. Interventions for actinic keratoses. Cochrane Database Syst Rev. 2012;12:CD004415.
- Patel G, Armstrong AW, Eisen DB. Efficacy of photodynamic therapy vs other interventions in randomized clinical trials for the treatment of actinic keratoses: a systematic review and meta-analysis. JAMA Dermatol. 2014;150:1281-1288.
- Kaufmann R, Spelman L, Weightman W, et al. Multicentre intraindividual randomized trial of topical methyl aminolaevulinate-photodynamic therapy vs. cryotherapy for multiple actinic keratoses on the extremities. Br J Dermatol. 2008;158:994-999.
- Freeman M, Vinciullo C, Francis D, et al. A comparison of photodynamic therapy using topical methyl aminolevulinate (Metvix) with single cycle cryotherapy in patients with actinic keratosis: a prospective, randomized study. J Dermatolog Treat. 2003;14:99-106.
- Morton C, Campbell S, Gupta G, et al. Intraindividual, right-left comparison of topical methyl aminolaevulinate-photodynamic therapy and cryotherapy in subjects with actinic keratoses: a multicentre, randomized controlled study. Br J Dermatol. 2006;155:1029-1036.
- Pariser DM, Lowe NJ, Stewart DM, et al. Photodynamic therapy with topical methyl aminolevulinate for actinic keratosis: results of a prospective randomized multicenter trial. J Am Acad Dermatol. 2003;48:227-232.
- Zane C, Facchinetti E, Rossi MT, et al. A randomized clinical trial of photodynamic therapy with methyl aminolaevulinate vs. diclofenac 3% plus hyaluronic acid gel for the treatment of multiple actinic keratoses of the face and scalp. Br J Dermatol. 2014;170:1143-1150.
- Perrett CM, McGregor JM, Warwick J, et al. Treatment of post-transplant premalignant skin disease: a randomized intrapatient comparative study of 5-fluorouracil cream and topical photodynamic therapy. Br J Dermatol. 2007;156:320-328.
- Szeimies RM, Karrer S, Radakovic-Fijan S, et al. Photodynamic therapy using topical methyl 5-aminolevulinate compared with cryotherapy for actinic keratosis: a prospective, randomized study. J Am Acad Dermatol. 2002; 47:258-262.
- Scola N, Terras S, Georgas D, et al. A randomized, half-side comparative study of aminolaevulinate photodynamic therapy vs. CO(2) laser ablation in immunocompetent patients with multiple actinic keratoses. Br J Dermatol. 2012;167:1366-1373.
- Willey A, Anderson RR, Sakamoto FH. Temperature-modulated photodynamic therapy for the treatment of actinic keratosis on the extremities: a pilot study. Dermatol Surg. 2014;40:1094-1102.
- Pariser DM. Management of Actinic Keratoses: Treatment Selection and Optimizing Outcomes. Presented at: Winter Clinical Dermatology Conference Hawaii; January 18, 2015; Kaanapali, HI.
- Dirschka T, Radny P, Dominicus R, et al. Long-term (6 and 12 months) follow-up of two prospective, randomized, controlled phase III trials of photodynamic therapy with BF-200 ALA and methyl aminolaevulinate for the treatment of actinic keratosis. Br J Dermatol. 2013;168:825-836.
- Choi SH, Kim KH, Song KH. Efficacy of ablative fractional laser-assisted photodynamic therapy with short-incubation time for the treatment of facial and scalp actinic keratosis: 12-month follow-up results of a randomized, prospective, comparative trial. J Eur Acad Dermatol Venereol. 2015;29:1598-1605.
- Ko DY, Jeon SY, Kim KH, et al. Fractional erbium:YAG laser-assisted photodynamic therapy for facial actinic keratoses: a randomized, comparative, prospective study. J Eur Acad Dermatol Venereol. 2014;28:1529-1539.
- Togsverd-Ho K, Haak CS, Thaysen-Petersen D, et al. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol. 2012;166:1262-1269.
- Torezan L, Chaves Y, Niwa A, et al. A pilot split-face study comparing conventional methyl aminolevulinate-photodynamic therapy (PDT) with microneedling-assisted PDT on actinically damaged skin. Dermatol Surg. 2013;39:1197-1201.
- Lebwohl M. Actinic keratosis: epidemiology and progression to squamous cell carcinoma. Br J Dermatol. 2003;149(suppl 66):31-33.
- Drake LA, Ceilley RI, Cornelison RL, et al. Guidelines of care for actinic keratoses. Committee on Guidelines of Care. J Am Acad Dermatol. 1995;32:95-98.
- Salasche SJ. Epidemiology of actinic keratoses and squamous cell carcinoma. J Am Acad Dermatol. 2000;42(1, pt 2):4-7.
- Ackerman AB, Mones JM. Solar (actinic) keratosis is squamous cell carcinoma. Br J Dermatol. 2006;155:9-22.
- Anwar J, Wrone DA, Kimyai-Asadi A, et al. The development of actinic keratosis into invasive squamous cell carcinoma: evidence and evolving classification schemes. Clin Dermatol. 2004;22:189-196.
- Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
- Glogau RG. The risk of progression to invasive disease. J Am Acad Dermatol. 2000;42(1, pt 2):23-24.
- Esmann S, Jemec GB. Management of actinic keratosis patients: a qualitative study. J Dermatolog Treat. 2007;18:53-58.
- Weinstock MA, Lee KC, Chren MM, et al. Quality of life in the actinic neoplasia syndrome: the VA Topical Tretinoin Chemoprevention (VATTC) trial. J Am Acad Dermatol. 2009;61:207-215.
- Berman B, Villa AM, Ramirez CC. Mechanisms of action of new treatment modalities for actinic keratosis. J Drugs Dermatol. 2006;5:167-173.
- Askew DA, Mickan SM, Soyer HP. Effectiveness of 5-fluorouracil treatment for actinic keratosis: a systematic review of randomized controlled trials. Int J Dermatol. 2009;46:452-463.
- Levy S, Furst K, Chern W. A pharmacokinetic evaluation of 0.5% and 5% fluorouracil topical cream in patients with actinic keratosis. Clin Ther. 2001;23:908-920.
- Jorizzo J, Stewart D, Bucko A, et al. Randomized trial evaluating a new 0.5% fluorouracil formulation demonstrates efficacy after 1-, 2-, or 4-week treatment in patients with actinic keratosis. Cutis. 2002;70:335-359.
- Sachs DL, Kang S, Hammerberg C, et al. Topical fluorouracil for actinic keratoses and photoaging: a clinical and molecular analysis. Arch Dermatol. 2009;145:659-666.
- Hantash BM, Stewart DB, Cooper ZA, et al. Facial resurfacing for nonmelanoma skin cancer prophylaxis. Arch Dermatol. 2006;142:976-982.
- Lawrence N, Cox SE, Cockerell CJ, et al. A comparison of the efficacy and safety of Jessner’s solution and 35% trichloroacetic acid vs 5% fluorouracil in the treatment of widespread facial actinic keratoses. Arch Dermatol. 1995;131:176-181.
- Monheit GD. The Jessner’s + TCA peel: a medium-depth chemical peel. J Dermatol Surg Oncol. 1989;15:945-950.
- Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196-200.
- Adamson DJ, Frew D, Tatoud R, et al. Diclofenac antagonizes peroxisome proliferator-activated receptor-gamma signaling. Mol Pharmacol. 2002;61:7-12.
- Alam CA, Seed MP, Willoughby DA. Angiostasis and vascular regression in chronic granulomatous inflammation induced by diclofenac in combination with hyaluronan in mice. J Pharm Pharmacol. 1995;47:407-411.
- Lu X, Xie W, Reed D, et al. Nonsteroidal antiinflammatory drugs cause apoptosis and induce cyclooxygenases in chicken embryo fibroblasts. Proc Natl Acad Sci USA. 1995;92:7961-7965.
- Seed MP, Brown JR, Freemantle CN, et al. The inhibition of colon-26 adenocarcinoma development and angiogenesis by topical diclofenac in 2.5% hyaluronan. Cancer Res. 1997;57:1625-1629.
- Rivers JK, Arlette J, Shear N, et al. Topical treatment of actinic keratoses with 3.0% diclofenac in 2.5% hyaluronan gel. Br J Dermatol. 2002;146:94-100.
- Wolf JE, Taylor JR, Tschen E, et al. Topical 3.0% diclo-fenac in 2.5% hyaluronan gel in the treatment of actinic keratoses. Int J Dermatol. 2001;40:709-713.
- Nelson C, Rigel D, Smith S, et al. Phase IV, open-label assessment of the treatment of actinic keratosis with 3.0% diclofenac sodium topical gel (Solaraze). J Drugs Dermatol. 2004;3:401-407.
- Pflugfelder A, Welter AK, Leiter U, et al. Open label randomized study comparing 3 months vs. 6 months treatment of actinic keratoses with 3% diclofenac in 2.5% hyaluronic acid gel: a trial of the German Dermatologic Cooperative Oncology Group. J Eur Acad Dermatol Venereol. 2012;26:48-53.
- Smith SR, Morhenn VB, Piacquadio DJ. Bilateral comparison of the efficacy and tolerability of 3% diclofenac sodium gel and 5% 5-fluorouracil cream in the treatment of actinic keratoses of the face and scalp. J Drugs Dermatol. 2006;5:156-159.
- Segatto MM, Dornelles SI, Silveira VB, et al. Comparative study of actinic keratosis treatment with 3% diclo- fenac sodium and 5% 5-fluorouracil. An Bras Dermatol. 2013;88:732-738.
- Vidal D. Topical imiquimod: mechanism of action and clinical applications. Mini Rev Med Chem. 2006;6:499-503.
- Hadley G, Derry S, Moore RA. Imiquimod for actinic keratosis: systematic review and meta-analysis. J Invest Dermatol. 2006;126:1251-1255.
- Caperton C, Berman B. Safety, efficacy, and patient acceptability of imiquimod for topical treatment of actinic keratoses. Clin Cosmet Investig Dermatol. 2011;4:35-40.
- Swanson N, Smith CC, Kaur M, et al. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: two phase 3, multicenter, randomized, double-blind, placebo-controlled studies. J Drugs Dermatol. 2014;13:166-169.
- Krawtchenko N, Roewert-Huber J, Ulrich M, et al. A randomised study of topical 5% imiquimod vs. topical 5-fluorouracil vs. cryosurgery in immunocompetent patients with actinic keratoses: a comparison of clinical and histological outcomes including 1-year follow-up. Br J Dermatol. 2007;157(suppl 2):34-40.
- Anderson L, Schmieder GJ, Werschler WP, et al. Randomized, double-blind, double-dummy, vehicle-controlled study of ingenol mebutate gel 0.025% and 0.05% for actinic keratosis. J Am Acad Dermatol. 2009;60:934-943.
- Ogbourne SM, Suhrbier A, Jones B, et al. Antitumor activity of 3-ingenyl angelate: plasma membrane and mitochondrial disruption and necrotic cell death. Cancer Res. 2004;64:2833-2839.
- Lebwohl M, Swanson N, Anderson LL, et al. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366:1010-1019.
- Lebwohl M, Shumack S, Stein-Gold L, et al. Long-term follow-up study of ingenol mebutate gel for the treatment of actinic keratoses. JAMA Dermatol. 2013;149:666-670.
- Augustin M, Tu JH, Knudsen KM, et al. Ingenol mebutate gel for actinic keratosis: the link between quality of life, treatment satisfaction, and clinical outcomes. J Am Acad Dermatol. 2015;72:816-821.
- Kane-Maguire N, Moseley R, Cozzi S, et al. Modulation of fibroblast phenotype and extracellular matrix composition by ingenol mebutate may be associated with scar resolution and improved dermal cosmesis. J Am Acad Dermatol. 2012;66:AB218.
- Martin G, Swanson N. Clinical findings using ingenol mebutate gel to treat actinic keratoses. J Am Acad Dermatol. 2013;68(1, suppl 1):S39-S48.
- Feldman SR, Fleischer AB, Williford PM, et al. Destructive procedures are the standard of care for treatment of actinic keratoses. J Am Acad Dermatol. 1999;40:43-47.
- Berlin JM. Current and emerging treatment strategies for the treatment of actinic keratosis. Clin Cosmet Investig Dermatol. 2010;3:119-126.
- Coleman WP, Yarborough JM, Mandy SH. Dermabrasion for prophylaxis and treatment of actinic keratoses. Dermatol Surg. 1996;22:17-21.
- Cooley JE, Casey DL, Kauffman CL. Manual resurfacing and trichloroacetic acid for the treatment of patients with widespread actinic damage. clinical and histologic observations. Dermatol Surg. 1997;23:373-379.
- Goldberg LH, Kaplan B, Vergilis-Kalner I, et al. Liquid nitrogen: temperature control in the treatment of actinic keratosis. Dermatol Surg. 2010;36:1956-1961.
- Lubritz RR, Smolewski SA. Cryosurgery cure rate of actinic keratoses. J Am Acad Dermatol. 1982;7:631-632.
- Thai KE, Fergin P, Freeman M, et al. A prospective study of the use of cryosurgery for the treatment of actinic keratoses. Int J Dermatol. 2004;43:687-692.
- Zane C, Facchinetti E, Rossi MT, et al. Cryotherapy is preferable to ablative CO2 laser for the treatment of isolated actinic keratoses of the face and scalp: a randomized clinical trial. Br J Dermatol. 2014;170:1114-1121.
- Trimas SJ, Ellis DA, Metz RD. The carbon dioxide laser. an alternative for the treatment of actinically damaged skin. Dermatol Surg. 1997;23:885-889.
- Jiang SB, Levine VJ, Nehal KS, et al. Er:YAG laser for the treatment of actinic keratoses. Dermatol Surg. 2000;26:437-440.
- Ostertag JU, Quaedvlieg PJ, Van der geer S, et al. A clinical comparison and long-term follow-up of topical 5-fluorouracil versus laser resurfacing in the treatment of widespread actinic keratoses. Lasers Surg Med. 2006;38:731-739.
- Iyer S, Friedli A, Bowes L, et al. Full face laser resurfacing: therapy and prophylaxis for actinic keratoses and non-melanoma skin cancer. Lasers Surg Med. 2004;34:114-119.
- Rubin MG. A peeler’s thoughts on skin improvement with chemical peels and laser resurfacing. Clin Plast Surg. 1997;24:407-409.
- Riggs K, Keller M, Humphreys TR. Ablative laser resurfacing: high-energy pulsed carbon dioxide and erbium:yttrium-aluminum-garnet. Clin Dermatol. 2007;25:462-473.
- Adrian RM. Pulsed carbon dioxide and long pulse 10-ms erbium-YAG laser resurfacing: a comparative clinical and histological study. J Cutan Laser Ther. 1999;1:197-202.
- Weiss ET, Brauer JA, Anolik R, et al. 1927-nm fractional resurfacing of facial actinic keratoses: a promising new therapeutic option. J Am Acad Dermatol. 2013; 68:98-102.
- Manstein D, Herron GS, Sink RK, et al. Fractional photothermolysis: a new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg Med. 2004;34:426-438.
- Katz TM, Goldberg LH, Marquez D, et al. Nonablative fractional photothermolysis for facial actinic keratoses: 6-month follow-up with histologic evaluation. J Am Acad Dermatol. 2011;65:349-356.
- Prens SP, De Vries K, Neumann HA, et al. Non-ablative fractional resurfacing in combination with topical tretinoin cream as a field treatment modality for multiple actinic keratosis: a pilot study and a review of other field treatment modalities. J Dermatolog Treat. 2013;24:227-231.
- Alexiades-Armenakas MR, Dover JS, Arndt KA. The spectrum of laser skin resurfacing: nonablative, fractional, and ablative laser resurfacing. J Am Acad Dermatol. 2008;58:719-737.
- Tannous Z. Fractional resurfacing. Clin Dermatol. 2007;25:480-486.
- Gold MH. Continuing medical education article-skin treatment: photodynamic therapy: indications and treatment. Aesthet Surg J. 2008;28:545-552.
- Juarranz A, Jaén P, Sanz-Rodríguez F, et al. Photodynamic therapy of cancer. basic principles and applications. Clin Transl Oncol. 2008;10:148-154.
- Juzeniene A, Peng Q, Moan J. Milestones in the development of photodynamic therapy and fluorescence diagnosis. Photochem Photobiol Sci. 2007;6:1234-1245.
- Moan J, Berg K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem Photobiol. 1991;53:549-553.
- Gupta AK, Paquet M, Villanueva E, et al. Interventions for actinic keratoses. Cochrane Database Syst Rev. 2012;12:CD004415.
- Patel G, Armstrong AW, Eisen DB. Efficacy of photodynamic therapy vs other interventions in randomized clinical trials for the treatment of actinic keratoses: a systematic review and meta-analysis. JAMA Dermatol. 2014;150:1281-1288.
- Kaufmann R, Spelman L, Weightman W, et al. Multicentre intraindividual randomized trial of topical methyl aminolaevulinate-photodynamic therapy vs. cryotherapy for multiple actinic keratoses on the extremities. Br J Dermatol. 2008;158:994-999.
- Freeman M, Vinciullo C, Francis D, et al. A comparison of photodynamic therapy using topical methyl aminolevulinate (Metvix) with single cycle cryotherapy in patients with actinic keratosis: a prospective, randomized study. J Dermatolog Treat. 2003;14:99-106.
- Morton C, Campbell S, Gupta G, et al. Intraindividual, right-left comparison of topical methyl aminolaevulinate-photodynamic therapy and cryotherapy in subjects with actinic keratoses: a multicentre, randomized controlled study. Br J Dermatol. 2006;155:1029-1036.
- Pariser DM, Lowe NJ, Stewart DM, et al. Photodynamic therapy with topical methyl aminolevulinate for actinic keratosis: results of a prospective randomized multicenter trial. J Am Acad Dermatol. 2003;48:227-232.
- Zane C, Facchinetti E, Rossi MT, et al. A randomized clinical trial of photodynamic therapy with methyl aminolaevulinate vs. diclofenac 3% plus hyaluronic acid gel for the treatment of multiple actinic keratoses of the face and scalp. Br J Dermatol. 2014;170:1143-1150.
- Perrett CM, McGregor JM, Warwick J, et al. Treatment of post-transplant premalignant skin disease: a randomized intrapatient comparative study of 5-fluorouracil cream and topical photodynamic therapy. Br J Dermatol. 2007;156:320-328.
- Szeimies RM, Karrer S, Radakovic-Fijan S, et al. Photodynamic therapy using topical methyl 5-aminolevulinate compared with cryotherapy for actinic keratosis: a prospective, randomized study. J Am Acad Dermatol. 2002; 47:258-262.
- Scola N, Terras S, Georgas D, et al. A randomized, half-side comparative study of aminolaevulinate photodynamic therapy vs. CO(2) laser ablation in immunocompetent patients with multiple actinic keratoses. Br J Dermatol. 2012;167:1366-1373.
- Willey A, Anderson RR, Sakamoto FH. Temperature-modulated photodynamic therapy for the treatment of actinic keratosis on the extremities: a pilot study. Dermatol Surg. 2014;40:1094-1102.
- Pariser DM. Management of Actinic Keratoses: Treatment Selection and Optimizing Outcomes. Presented at: Winter Clinical Dermatology Conference Hawaii; January 18, 2015; Kaanapali, HI.
- Dirschka T, Radny P, Dominicus R, et al. Long-term (6 and 12 months) follow-up of two prospective, randomized, controlled phase III trials of photodynamic therapy with BF-200 ALA and methyl aminolaevulinate for the treatment of actinic keratosis. Br J Dermatol. 2013;168:825-836.
- Choi SH, Kim KH, Song KH. Efficacy of ablative fractional laser-assisted photodynamic therapy with short-incubation time for the treatment of facial and scalp actinic keratosis: 12-month follow-up results of a randomized, prospective, comparative trial. J Eur Acad Dermatol Venereol. 2015;29:1598-1605.
- Ko DY, Jeon SY, Kim KH, et al. Fractional erbium:YAG laser-assisted photodynamic therapy for facial actinic keratoses: a randomized, comparative, prospective study. J Eur Acad Dermatol Venereol. 2014;28:1529-1539.
- Togsverd-Ho K, Haak CS, Thaysen-Petersen D, et al. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol. 2012;166:1262-1269.
- Torezan L, Chaves Y, Niwa A, et al. A pilot split-face study comparing conventional methyl aminolevulinate-photodynamic therapy (PDT) with microneedling-assisted PDT on actinically damaged skin. Dermatol Surg. 2013;39:1197-1201.
Practice Points
- In addition to their risk for progression to malignancy, actinic keratoses (AKs) can have negative impacts on cosmetic appearance and quality of life.
- A variety of topical medications, procedural modalities, and light-based therapies are available for treatment of AKs, which offer varying degrees of efficacy for clearance of lesions and cosmetic outcomes. Based on the current data, imiquimod and photodynamic therapy are the treatments most likely to provide an excellent cosmetic outcome.
Update on Hyaluronic Acid Fillers for Facial Rejuvenation
Facial rejuvenation has become increasingly popular, with nonsurgical and noninvasive procedures comprising a large part of aesthetic practice. According to the American Society for Aesthetic Plastic Surgery, Americans spent $12 billion on cosmetic procedures in 2014, with more than 10 million surgical and nonsurgical procedures performed. The top 5 nonsurgical procedures for both men and women combined were botulinum toxin, hyaluronic acid (HA), hair removal, chemical peel, and microdermabrasion.1
The first dermal filler used was bovine collagen, which was approved by the US Food and Drug Administration (FDA) in 1981. Despite its efficacy in the correction of facial rhytides, bovine collagen required allergy testing prior to use and was discontinued in 2010. Dermal fillers have evolved over the years, and newer products that are superior to earlier fillers with regard to longevity, safety, and tolerability and that do not require allergy testing have become available; however, advances in the use of dermal fillers are not only related to the development of newer products but also to evolving injection techniques. Initially, the aim of treatment with dermal fillers was to correct lines and wrinkles, but an increased understanding of the complex changes that occur with aging have changed our approach to one of volume replacement, with an emphasis on volume restoration in the midface. This approach requires an in-depth understanding of facial anatomy as well as the interactions of the skin, soft tissue, muscle, and bone. Furthermore, placement of filler in specific fat compartments can provide a more natural appearance and an all-around youthful face.2 In this article, we discuss HA fillers that have gained FDA approval within the last 5 years (Table).
Overview of HA Fillers
Hyaluronic acid is a naturally occurring linear glycosaminoglycan with a disaccharide unit, which repeats several thousand times.3 Hyaluronic acid is an essential part of the extracellular matrix of many tissues including the dermis and plays an important role in tissue growth, development, and wound healing. Hyaluronic acid is hygroscopic and absorbs water extensively, thus creating volume.4 Treatment with HA fillers is popular, as they are biocompatible and have a low potential for allergic reactions. They also are easy to use and reversible.4 The first HA filler to gain FDA approval was Restylane (Galderma Laboratories, LP). Currently, several HA fillers are approved in the United States, and each product differs from the others in polymer chain length, degree of HA concentration, particle size, gel consistency, gel hardness, gel viscosity, and degree of water solubility, as well as amount and degree of cross-linking. Cross-linking is essential to avoid enzymatic degradation by endogenous hyaluronidase when injected into the skin and thus to prolong the product’s half-life.5 Cross-linkers used to manufacture HA fillers include 1,4-butanediol diglycidyl ether and divinyl sulfone. More concentrated products with a greater degree of cross-linking provide increased longevity, but they are associated with a higher risk for inflammation and nodule formation. The elastic modulus (G′) is a measure of the firmness of dermal fillers, describing their resistance to deformation. Materials with a higher G′ are stiffer and are meant for deeper injections. Hyaluronic acid fillers can be further classified as biphasic or monophasic. Biphasic fillers (eg, Restylane, Perlane [Galderma Laboratories, LP]) contain a range of microsphere sizes, while monophasic fillers (eg, Juvéderm [Allergan, Inc], Belotero Balance [Merz North America, Inc]) contain homogeneous microspheres. Although randomized clinical trials have reported comparable efficacy and durability of biphasic and monophasic fillers when used to treat the nasolabial folds,6-8 monophasic HA fillers are more cohesive and may not migrate as much following injection.
Restylane Family
Restylane was the first FDA-approved HA filler, gaining its approval in 2003. Restylane is a nonanimal stabilized HA (NASHA) that is produced from the fermentation of equine streptococci. It is cross-linked with 1,4-butanediol diglycidyl ether with a 1% degree of cross-linking. Restylane has an HA concentration of 20 mg/mL. The particle size range of Restylane and Restylane-L is 330 to 430 mm. Restylane (and also Perlane) get passed through sizing screens via sieves and are quantified by their size. The longevity of HA fillers is approximately 6 months; however, various factors affect the product’s longevity, such as the degree of cross-linking, treatment area, and the patient’s metabolism. Restylane-L, which was FDA approved in 2012, is a newer product with 0.3% lidocaine incorporated into the syringe itself. It was the first product from the Restylane range to be approved for lip augmentation. The addition of lidocaine (designated by the L in the product name) does not affect the longevity of a filler.9
The newest FDA-approved HA filler was Restylane Silk (approved in 2014), which has been specifically designed for lip augmentation and correction of perioral rhytides. To avoid postprocedural swelling, it generally is recommended that Restylane Silk be injected slowly. If required, a short course of oral prednisone may be administered after the procedure to treat any edema. Restylane Silk is less viscous than Restylane and requires less pressure to inject. Therefore, it is more suited for treatment of fine perioral lines, as it flows more easily. Because it contains 0.3% lidocaine, discomfort usually is minimal, with treatment lasting approximately 30 to 60 minutes. In the author’s experience (G.G.), Restylane Silk provides a softer correction, though one has to be careful to inject slowly to avoid postinjection swelling. Restylane Silk also may be suitable for neck rejuvenation (off label), but several treatment sessions usually are required. Patients should be warned that they are likely to experience ecchymoses. In our experience, the effects of Restylane Silk injections last approximately 6 to 9 months.
Juvéderm Family
The first Juvéderm product was approved by the FDA in 2006. Juvéderm is a bacterium-derived NASHA. Injectable gel formulations of Juvéderm, including Juvéderm Ultra and Juvéderm Ultra Plus, are FDA approved for the correction of moderate to severe facial wrinkles and folds. The first products in the Juvéderm line were produced using a technology called Hylacross technology, with cohesive molecules of cross-linked HA. In contrast to the sizing technology used by Restylane and Perlane, the Hylacross technology does not break up the cross-linked HA by passing the product through sizing screens via sieves, but instead produces monophasic gels. These Juvéderm products have a high concentration of cross-linked HAs, which accounts for their longevity, and they are soft and easy to use.
Juvéderm Voluma XC was FDA approved in 2013. It is a 20-mg/mL, smooth, highly cohesive, viscous HA, gel that is manufactured using Vycross technology, a combination of low- and high-molecular-weight HA, and it is the only HA filler that is indicated for deep injection for cheek augmentation,10 as it creates a lift due to its higher G′ and a low swelling capacity, with results lasting up to 2 years. The mean volume administered over the initial 4-week period of one study was 5.1 mL.11
Belotero Balance
Belotero Balance was approved by the FDA in 2011 for the correction of moderate to severe facial wrinkles and folds (eg, nasolabial folds).12 Belotero Balance is an HA filler with a cohesive polydensified matrix technology and low elasticity and viscosity. It has the lowest G′ of the currently available dermal fillers12 and therefore is associated with increased injection precision. Belotero Balance is ideal for superficial injections,13 such as forehead lines, vermilion border, tear trough, atrophic scars, and neck lines (off label). Some clinicians reconstitute Belotero Balance with lidocaine (off label) to provide a more pain-free procedure. In our experience, results typically last at least 6 to 8 months.
Complications
Hyaluronic acid fillers share the same adverse events across the product lines. The most common reactions include erythema, swelling, and bruising, which often are unavoidable and may be considered expected effects. Less-frequent events include contour irregularities; product migration; bluish discoloration known as the Tyndall effect, which is more likely to occur with superficial injections; nodules; infection at the injection site; scarring; and vascular occlusion, potentially leading to blindness.14 These more severe complications often can be avoided. Appropriate skin preparation and a sterile technique are critical in preventing infections, while deep placement of filler material reduces the risk for Tyndall effect, nodules, and scarring. Skin necrosis occurs by external compression of the blood supply by the product or occlusion via direct injection into a vessel. Aspirating prior to injection, administering lower volumes, and tenting the skin to inject more superficially can reduce the risk for skin necrosis. Every clinician needs to be able to rapidly recognize the signs of necrosis and to administer urgent therapy, such as the application of warm gauze and nitroglycerin paste, tapping the area to facilitate vasodilatation, and injecting hyaluronidase when required.
On the Horizon
Other dermal fillers that may gain FDA approval in the next few years include Teosyal (Laboratories Teoxane Geneva),6 a new range of monophasic NASHA products that provide high viscosity and elasticity with results lasting 6 to 9 months, and potentially Juvéderm Volbella, a smooth, nonparticle, viscous HA gel developed specifically for the lip area with results lasting up to 1 year.15
Conclusion
Over the last decade, the popularity of dermal fillers has steadily increased, and fillers have become a cornerstone of aesthetic medicine. The increased number of available products necessitates thorough knowledge by the treating physician to ensure optimal outcomes. There is no universal filler that can achieve ideal outcomes in all anatomic sites or in all patients. Often a combination approach may be ideal, such as the use of a product with a higher G′ for re-volumization, followed by a filler with a lower G′ for superficial injection. Some patients may also benefit from a combination of both dermal fillers and neurotoxin injections, either on the same day or at separate visits, which may increase the longevity of the filler.
1. The American Society for Aesthetic Plastic Surgery reports Americans spent more than 12 billion in 2014; procedures for men up 43% over five year period [news release]. New York, NY: American Society for Aesthetic Plastic Surgery; March 11, 2015. http://www.surgery.org/media/news-releases/the-american-society-for-aesthetic-plastic-surgery-reports-americans-spent-more-than-12-billion-in-2014--pro. Accessed July 7, 2015.
2. Fitzgerald R, Rubin AG. Filler placement and the fat compartments. Dermatol Clin. 2014;32:37-50.
3. Cowman MK, Matsuoka S. Experimental approaches to hyaluronan structure. Carbohydr Res. 2005;340:791-809.
4. Lee A, Grummer SE, Kriegel D, et al. Hyaluronidase. Dermatol Surg. 2010;36:1071-1077.
5. Kablik J, Monheit GD, Yu L, et al. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol Surg. 2009;35(suppl 1):302-312.
6. Nast A, Reytan N, Hartmann V, et al. Efficacy and durability of two hyaluronic acid-based fillers in the correction of nasolabial folds: results of a prospective, randomized, double-blind, actively controlled clinical pilot study. Dermatol Surg. 2011;37:768-775.
7. Ascher B, Bayerl C, Brun P, et al. Efficacy and safety of a new hyaluronic acid dermal filler in the treatment of severe nasolabial lines: 6-month interim results of a randomized, evaluator-blinded, intra-individual comparison study. J Cosmet Dermatol. 2011;10:94-98.
8. Rzany B, Bayerl C, Bodokh I, et al. Efficacy and safety of a new hyaluronic acid dermal filler in the treatment of moderate nasolabial folds: 6-month interim results of a randomized, evaluator-blinded, intra-individual comparison study. J Cosmet Laser Ther. 2011;13:107-112.
9. Lupo MP, Swetman G, Waller W. The effect of lidocaine when mixed with large gel particle hyaluronic acid filler tolerability and longevity: a six-month trial. J Drugs Dermatol. 2010;9:1097-1100.
10. Ho D, Jagdeo J. Biological properties of a new volumizing hyaluronic acid filler: a systematic review. J Drugs Dermatol. 2015;14:50-54.
11. Callan P, Goodman GJ, Carlisle I, et al. Efficacy and safety of a hyaluronic acid filler in subjects treated for correction of midface volume deficiency: a 24 month study. Clin Cosmet Investig Dermatol. 2013;6:81-89.
12. Hevia O, Cohen BH, Howell DJ. Safety and efficacy of a cohesive polydensified matrix hyaluronic acid for the correction of infraorbital hollow: an observational study with results at 40 weeks. J Drugs Dermatol. 2014;13:1030-1036.
13. Lorenc ZP, Fagien S, Flynn TC, et al. Clinical application and assessment of Belotero: a roundtable discussion. Plast Reconstr Surg. 2013;132(4, suppl 2):69S-76S.
14. Carruthers JD, Fagien S, Rohrich RJ, et al. Blindness caused by cosmetic filler injection: a review of cause and therapy. Plast Reconstr Surg. 2014;134:1197-1201.
15. Eccleston D, Murphy DK. Juvéderm(®) Volbella™ in the perioral area: a 12-month prospective, multicenter, open-label study. Clin Cosmet Investig Dermatol. 2012;5:167-172.
Facial rejuvenation has become increasingly popular, with nonsurgical and noninvasive procedures comprising a large part of aesthetic practice. According to the American Society for Aesthetic Plastic Surgery, Americans spent $12 billion on cosmetic procedures in 2014, with more than 10 million surgical and nonsurgical procedures performed. The top 5 nonsurgical procedures for both men and women combined were botulinum toxin, hyaluronic acid (HA), hair removal, chemical peel, and microdermabrasion.1
The first dermal filler used was bovine collagen, which was approved by the US Food and Drug Administration (FDA) in 1981. Despite its efficacy in the correction of facial rhytides, bovine collagen required allergy testing prior to use and was discontinued in 2010. Dermal fillers have evolved over the years, and newer products that are superior to earlier fillers with regard to longevity, safety, and tolerability and that do not require allergy testing have become available; however, advances in the use of dermal fillers are not only related to the development of newer products but also to evolving injection techniques. Initially, the aim of treatment with dermal fillers was to correct lines and wrinkles, but an increased understanding of the complex changes that occur with aging have changed our approach to one of volume replacement, with an emphasis on volume restoration in the midface. This approach requires an in-depth understanding of facial anatomy as well as the interactions of the skin, soft tissue, muscle, and bone. Furthermore, placement of filler in specific fat compartments can provide a more natural appearance and an all-around youthful face.2 In this article, we discuss HA fillers that have gained FDA approval within the last 5 years (Table).
Overview of HA Fillers
Hyaluronic acid is a naturally occurring linear glycosaminoglycan with a disaccharide unit, which repeats several thousand times.3 Hyaluronic acid is an essential part of the extracellular matrix of many tissues including the dermis and plays an important role in tissue growth, development, and wound healing. Hyaluronic acid is hygroscopic and absorbs water extensively, thus creating volume.4 Treatment with HA fillers is popular, as they are biocompatible and have a low potential for allergic reactions. They also are easy to use and reversible.4 The first HA filler to gain FDA approval was Restylane (Galderma Laboratories, LP). Currently, several HA fillers are approved in the United States, and each product differs from the others in polymer chain length, degree of HA concentration, particle size, gel consistency, gel hardness, gel viscosity, and degree of water solubility, as well as amount and degree of cross-linking. Cross-linking is essential to avoid enzymatic degradation by endogenous hyaluronidase when injected into the skin and thus to prolong the product’s half-life.5 Cross-linkers used to manufacture HA fillers include 1,4-butanediol diglycidyl ether and divinyl sulfone. More concentrated products with a greater degree of cross-linking provide increased longevity, but they are associated with a higher risk for inflammation and nodule formation. The elastic modulus (G′) is a measure of the firmness of dermal fillers, describing their resistance to deformation. Materials with a higher G′ are stiffer and are meant for deeper injections. Hyaluronic acid fillers can be further classified as biphasic or monophasic. Biphasic fillers (eg, Restylane, Perlane [Galderma Laboratories, LP]) contain a range of microsphere sizes, while monophasic fillers (eg, Juvéderm [Allergan, Inc], Belotero Balance [Merz North America, Inc]) contain homogeneous microspheres. Although randomized clinical trials have reported comparable efficacy and durability of biphasic and monophasic fillers when used to treat the nasolabial folds,6-8 monophasic HA fillers are more cohesive and may not migrate as much following injection.
Restylane Family
Restylane was the first FDA-approved HA filler, gaining its approval in 2003. Restylane is a nonanimal stabilized HA (NASHA) that is produced from the fermentation of equine streptococci. It is cross-linked with 1,4-butanediol diglycidyl ether with a 1% degree of cross-linking. Restylane has an HA concentration of 20 mg/mL. The particle size range of Restylane and Restylane-L is 330 to 430 mm. Restylane (and also Perlane) get passed through sizing screens via sieves and are quantified by their size. The longevity of HA fillers is approximately 6 months; however, various factors affect the product’s longevity, such as the degree of cross-linking, treatment area, and the patient’s metabolism. Restylane-L, which was FDA approved in 2012, is a newer product with 0.3% lidocaine incorporated into the syringe itself. It was the first product from the Restylane range to be approved for lip augmentation. The addition of lidocaine (designated by the L in the product name) does not affect the longevity of a filler.9
The newest FDA-approved HA filler was Restylane Silk (approved in 2014), which has been specifically designed for lip augmentation and correction of perioral rhytides. To avoid postprocedural swelling, it generally is recommended that Restylane Silk be injected slowly. If required, a short course of oral prednisone may be administered after the procedure to treat any edema. Restylane Silk is less viscous than Restylane and requires less pressure to inject. Therefore, it is more suited for treatment of fine perioral lines, as it flows more easily. Because it contains 0.3% lidocaine, discomfort usually is minimal, with treatment lasting approximately 30 to 60 minutes. In the author’s experience (G.G.), Restylane Silk provides a softer correction, though one has to be careful to inject slowly to avoid postinjection swelling. Restylane Silk also may be suitable for neck rejuvenation (off label), but several treatment sessions usually are required. Patients should be warned that they are likely to experience ecchymoses. In our experience, the effects of Restylane Silk injections last approximately 6 to 9 months.
Juvéderm Family
The first Juvéderm product was approved by the FDA in 2006. Juvéderm is a bacterium-derived NASHA. Injectable gel formulations of Juvéderm, including Juvéderm Ultra and Juvéderm Ultra Plus, are FDA approved for the correction of moderate to severe facial wrinkles and folds. The first products in the Juvéderm line were produced using a technology called Hylacross technology, with cohesive molecules of cross-linked HA. In contrast to the sizing technology used by Restylane and Perlane, the Hylacross technology does not break up the cross-linked HA by passing the product through sizing screens via sieves, but instead produces monophasic gels. These Juvéderm products have a high concentration of cross-linked HAs, which accounts for their longevity, and they are soft and easy to use.
Juvéderm Voluma XC was FDA approved in 2013. It is a 20-mg/mL, smooth, highly cohesive, viscous HA, gel that is manufactured using Vycross technology, a combination of low- and high-molecular-weight HA, and it is the only HA filler that is indicated for deep injection for cheek augmentation,10 as it creates a lift due to its higher G′ and a low swelling capacity, with results lasting up to 2 years. The mean volume administered over the initial 4-week period of one study was 5.1 mL.11
Belotero Balance
Belotero Balance was approved by the FDA in 2011 for the correction of moderate to severe facial wrinkles and folds (eg, nasolabial folds).12 Belotero Balance is an HA filler with a cohesive polydensified matrix technology and low elasticity and viscosity. It has the lowest G′ of the currently available dermal fillers12 and therefore is associated with increased injection precision. Belotero Balance is ideal for superficial injections,13 such as forehead lines, vermilion border, tear trough, atrophic scars, and neck lines (off label). Some clinicians reconstitute Belotero Balance with lidocaine (off label) to provide a more pain-free procedure. In our experience, results typically last at least 6 to 8 months.
Complications
Hyaluronic acid fillers share the same adverse events across the product lines. The most common reactions include erythema, swelling, and bruising, which often are unavoidable and may be considered expected effects. Less-frequent events include contour irregularities; product migration; bluish discoloration known as the Tyndall effect, which is more likely to occur with superficial injections; nodules; infection at the injection site; scarring; and vascular occlusion, potentially leading to blindness.14 These more severe complications often can be avoided. Appropriate skin preparation and a sterile technique are critical in preventing infections, while deep placement of filler material reduces the risk for Tyndall effect, nodules, and scarring. Skin necrosis occurs by external compression of the blood supply by the product or occlusion via direct injection into a vessel. Aspirating prior to injection, administering lower volumes, and tenting the skin to inject more superficially can reduce the risk for skin necrosis. Every clinician needs to be able to rapidly recognize the signs of necrosis and to administer urgent therapy, such as the application of warm gauze and nitroglycerin paste, tapping the area to facilitate vasodilatation, and injecting hyaluronidase when required.
On the Horizon
Other dermal fillers that may gain FDA approval in the next few years include Teosyal (Laboratories Teoxane Geneva),6 a new range of monophasic NASHA products that provide high viscosity and elasticity with results lasting 6 to 9 months, and potentially Juvéderm Volbella, a smooth, nonparticle, viscous HA gel developed specifically for the lip area with results lasting up to 1 year.15
Conclusion
Over the last decade, the popularity of dermal fillers has steadily increased, and fillers have become a cornerstone of aesthetic medicine. The increased number of available products necessitates thorough knowledge by the treating physician to ensure optimal outcomes. There is no universal filler that can achieve ideal outcomes in all anatomic sites or in all patients. Often a combination approach may be ideal, such as the use of a product with a higher G′ for re-volumization, followed by a filler with a lower G′ for superficial injection. Some patients may also benefit from a combination of both dermal fillers and neurotoxin injections, either on the same day or at separate visits, which may increase the longevity of the filler.
Facial rejuvenation has become increasingly popular, with nonsurgical and noninvasive procedures comprising a large part of aesthetic practice. According to the American Society for Aesthetic Plastic Surgery, Americans spent $12 billion on cosmetic procedures in 2014, with more than 10 million surgical and nonsurgical procedures performed. The top 5 nonsurgical procedures for both men and women combined were botulinum toxin, hyaluronic acid (HA), hair removal, chemical peel, and microdermabrasion.1
The first dermal filler used was bovine collagen, which was approved by the US Food and Drug Administration (FDA) in 1981. Despite its efficacy in the correction of facial rhytides, bovine collagen required allergy testing prior to use and was discontinued in 2010. Dermal fillers have evolved over the years, and newer products that are superior to earlier fillers with regard to longevity, safety, and tolerability and that do not require allergy testing have become available; however, advances in the use of dermal fillers are not only related to the development of newer products but also to evolving injection techniques. Initially, the aim of treatment with dermal fillers was to correct lines and wrinkles, but an increased understanding of the complex changes that occur with aging have changed our approach to one of volume replacement, with an emphasis on volume restoration in the midface. This approach requires an in-depth understanding of facial anatomy as well as the interactions of the skin, soft tissue, muscle, and bone. Furthermore, placement of filler in specific fat compartments can provide a more natural appearance and an all-around youthful face.2 In this article, we discuss HA fillers that have gained FDA approval within the last 5 years (Table).
Overview of HA Fillers
Hyaluronic acid is a naturally occurring linear glycosaminoglycan with a disaccharide unit, which repeats several thousand times.3 Hyaluronic acid is an essential part of the extracellular matrix of many tissues including the dermis and plays an important role in tissue growth, development, and wound healing. Hyaluronic acid is hygroscopic and absorbs water extensively, thus creating volume.4 Treatment with HA fillers is popular, as they are biocompatible and have a low potential for allergic reactions. They also are easy to use and reversible.4 The first HA filler to gain FDA approval was Restylane (Galderma Laboratories, LP). Currently, several HA fillers are approved in the United States, and each product differs from the others in polymer chain length, degree of HA concentration, particle size, gel consistency, gel hardness, gel viscosity, and degree of water solubility, as well as amount and degree of cross-linking. Cross-linking is essential to avoid enzymatic degradation by endogenous hyaluronidase when injected into the skin and thus to prolong the product’s half-life.5 Cross-linkers used to manufacture HA fillers include 1,4-butanediol diglycidyl ether and divinyl sulfone. More concentrated products with a greater degree of cross-linking provide increased longevity, but they are associated with a higher risk for inflammation and nodule formation. The elastic modulus (G′) is a measure of the firmness of dermal fillers, describing their resistance to deformation. Materials with a higher G′ are stiffer and are meant for deeper injections. Hyaluronic acid fillers can be further classified as biphasic or monophasic. Biphasic fillers (eg, Restylane, Perlane [Galderma Laboratories, LP]) contain a range of microsphere sizes, while monophasic fillers (eg, Juvéderm [Allergan, Inc], Belotero Balance [Merz North America, Inc]) contain homogeneous microspheres. Although randomized clinical trials have reported comparable efficacy and durability of biphasic and monophasic fillers when used to treat the nasolabial folds,6-8 monophasic HA fillers are more cohesive and may not migrate as much following injection.
Restylane Family
Restylane was the first FDA-approved HA filler, gaining its approval in 2003. Restylane is a nonanimal stabilized HA (NASHA) that is produced from the fermentation of equine streptococci. It is cross-linked with 1,4-butanediol diglycidyl ether with a 1% degree of cross-linking. Restylane has an HA concentration of 20 mg/mL. The particle size range of Restylane and Restylane-L is 330 to 430 mm. Restylane (and also Perlane) get passed through sizing screens via sieves and are quantified by their size. The longevity of HA fillers is approximately 6 months; however, various factors affect the product’s longevity, such as the degree of cross-linking, treatment area, and the patient’s metabolism. Restylane-L, which was FDA approved in 2012, is a newer product with 0.3% lidocaine incorporated into the syringe itself. It was the first product from the Restylane range to be approved for lip augmentation. The addition of lidocaine (designated by the L in the product name) does not affect the longevity of a filler.9
The newest FDA-approved HA filler was Restylane Silk (approved in 2014), which has been specifically designed for lip augmentation and correction of perioral rhytides. To avoid postprocedural swelling, it generally is recommended that Restylane Silk be injected slowly. If required, a short course of oral prednisone may be administered after the procedure to treat any edema. Restylane Silk is less viscous than Restylane and requires less pressure to inject. Therefore, it is more suited for treatment of fine perioral lines, as it flows more easily. Because it contains 0.3% lidocaine, discomfort usually is minimal, with treatment lasting approximately 30 to 60 minutes. In the author’s experience (G.G.), Restylane Silk provides a softer correction, though one has to be careful to inject slowly to avoid postinjection swelling. Restylane Silk also may be suitable for neck rejuvenation (off label), but several treatment sessions usually are required. Patients should be warned that they are likely to experience ecchymoses. In our experience, the effects of Restylane Silk injections last approximately 6 to 9 months.
Juvéderm Family
The first Juvéderm product was approved by the FDA in 2006. Juvéderm is a bacterium-derived NASHA. Injectable gel formulations of Juvéderm, including Juvéderm Ultra and Juvéderm Ultra Plus, are FDA approved for the correction of moderate to severe facial wrinkles and folds. The first products in the Juvéderm line were produced using a technology called Hylacross technology, with cohesive molecules of cross-linked HA. In contrast to the sizing technology used by Restylane and Perlane, the Hylacross technology does not break up the cross-linked HA by passing the product through sizing screens via sieves, but instead produces monophasic gels. These Juvéderm products have a high concentration of cross-linked HAs, which accounts for their longevity, and they are soft and easy to use.
Juvéderm Voluma XC was FDA approved in 2013. It is a 20-mg/mL, smooth, highly cohesive, viscous HA, gel that is manufactured using Vycross technology, a combination of low- and high-molecular-weight HA, and it is the only HA filler that is indicated for deep injection for cheek augmentation,10 as it creates a lift due to its higher G′ and a low swelling capacity, with results lasting up to 2 years. The mean volume administered over the initial 4-week period of one study was 5.1 mL.11
Belotero Balance
Belotero Balance was approved by the FDA in 2011 for the correction of moderate to severe facial wrinkles and folds (eg, nasolabial folds).12 Belotero Balance is an HA filler with a cohesive polydensified matrix technology and low elasticity and viscosity. It has the lowest G′ of the currently available dermal fillers12 and therefore is associated with increased injection precision. Belotero Balance is ideal for superficial injections,13 such as forehead lines, vermilion border, tear trough, atrophic scars, and neck lines (off label). Some clinicians reconstitute Belotero Balance with lidocaine (off label) to provide a more pain-free procedure. In our experience, results typically last at least 6 to 8 months.
Complications
Hyaluronic acid fillers share the same adverse events across the product lines. The most common reactions include erythema, swelling, and bruising, which often are unavoidable and may be considered expected effects. Less-frequent events include contour irregularities; product migration; bluish discoloration known as the Tyndall effect, which is more likely to occur with superficial injections; nodules; infection at the injection site; scarring; and vascular occlusion, potentially leading to blindness.14 These more severe complications often can be avoided. Appropriate skin preparation and a sterile technique are critical in preventing infections, while deep placement of filler material reduces the risk for Tyndall effect, nodules, and scarring. Skin necrosis occurs by external compression of the blood supply by the product or occlusion via direct injection into a vessel. Aspirating prior to injection, administering lower volumes, and tenting the skin to inject more superficially can reduce the risk for skin necrosis. Every clinician needs to be able to rapidly recognize the signs of necrosis and to administer urgent therapy, such as the application of warm gauze and nitroglycerin paste, tapping the area to facilitate vasodilatation, and injecting hyaluronidase when required.
On the Horizon
Other dermal fillers that may gain FDA approval in the next few years include Teosyal (Laboratories Teoxane Geneva),6 a new range of monophasic NASHA products that provide high viscosity and elasticity with results lasting 6 to 9 months, and potentially Juvéderm Volbella, a smooth, nonparticle, viscous HA gel developed specifically for the lip area with results lasting up to 1 year.15
Conclusion
Over the last decade, the popularity of dermal fillers has steadily increased, and fillers have become a cornerstone of aesthetic medicine. The increased number of available products necessitates thorough knowledge by the treating physician to ensure optimal outcomes. There is no universal filler that can achieve ideal outcomes in all anatomic sites or in all patients. Often a combination approach may be ideal, such as the use of a product with a higher G′ for re-volumization, followed by a filler with a lower G′ for superficial injection. Some patients may also benefit from a combination of both dermal fillers and neurotoxin injections, either on the same day or at separate visits, which may increase the longevity of the filler.
1. The American Society for Aesthetic Plastic Surgery reports Americans spent more than 12 billion in 2014; procedures for men up 43% over five year period [news release]. New York, NY: American Society for Aesthetic Plastic Surgery; March 11, 2015. http://www.surgery.org/media/news-releases/the-american-society-for-aesthetic-plastic-surgery-reports-americans-spent-more-than-12-billion-in-2014--pro. Accessed July 7, 2015.
2. Fitzgerald R, Rubin AG. Filler placement and the fat compartments. Dermatol Clin. 2014;32:37-50.
3. Cowman MK, Matsuoka S. Experimental approaches to hyaluronan structure. Carbohydr Res. 2005;340:791-809.
4. Lee A, Grummer SE, Kriegel D, et al. Hyaluronidase. Dermatol Surg. 2010;36:1071-1077.
5. Kablik J, Monheit GD, Yu L, et al. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol Surg. 2009;35(suppl 1):302-312.
6. Nast A, Reytan N, Hartmann V, et al. Efficacy and durability of two hyaluronic acid-based fillers in the correction of nasolabial folds: results of a prospective, randomized, double-blind, actively controlled clinical pilot study. Dermatol Surg. 2011;37:768-775.
7. Ascher B, Bayerl C, Brun P, et al. Efficacy and safety of a new hyaluronic acid dermal filler in the treatment of severe nasolabial lines: 6-month interim results of a randomized, evaluator-blinded, intra-individual comparison study. J Cosmet Dermatol. 2011;10:94-98.
8. Rzany B, Bayerl C, Bodokh I, et al. Efficacy and safety of a new hyaluronic acid dermal filler in the treatment of moderate nasolabial folds: 6-month interim results of a randomized, evaluator-blinded, intra-individual comparison study. J Cosmet Laser Ther. 2011;13:107-112.
9. Lupo MP, Swetman G, Waller W. The effect of lidocaine when mixed with large gel particle hyaluronic acid filler tolerability and longevity: a six-month trial. J Drugs Dermatol. 2010;9:1097-1100.
10. Ho D, Jagdeo J. Biological properties of a new volumizing hyaluronic acid filler: a systematic review. J Drugs Dermatol. 2015;14:50-54.
11. Callan P, Goodman GJ, Carlisle I, et al. Efficacy and safety of a hyaluronic acid filler in subjects treated for correction of midface volume deficiency: a 24 month study. Clin Cosmet Investig Dermatol. 2013;6:81-89.
12. Hevia O, Cohen BH, Howell DJ. Safety and efficacy of a cohesive polydensified matrix hyaluronic acid for the correction of infraorbital hollow: an observational study with results at 40 weeks. J Drugs Dermatol. 2014;13:1030-1036.
13. Lorenc ZP, Fagien S, Flynn TC, et al. Clinical application and assessment of Belotero: a roundtable discussion. Plast Reconstr Surg. 2013;132(4, suppl 2):69S-76S.
14. Carruthers JD, Fagien S, Rohrich RJ, et al. Blindness caused by cosmetic filler injection: a review of cause and therapy. Plast Reconstr Surg. 2014;134:1197-1201.
15. Eccleston D, Murphy DK. Juvéderm(®) Volbella™ in the perioral area: a 12-month prospective, multicenter, open-label study. Clin Cosmet Investig Dermatol. 2012;5:167-172.
1. The American Society for Aesthetic Plastic Surgery reports Americans spent more than 12 billion in 2014; procedures for men up 43% over five year period [news release]. New York, NY: American Society for Aesthetic Plastic Surgery; March 11, 2015. http://www.surgery.org/media/news-releases/the-american-society-for-aesthetic-plastic-surgery-reports-americans-spent-more-than-12-billion-in-2014--pro. Accessed July 7, 2015.
2. Fitzgerald R, Rubin AG. Filler placement and the fat compartments. Dermatol Clin. 2014;32:37-50.
3. Cowman MK, Matsuoka S. Experimental approaches to hyaluronan structure. Carbohydr Res. 2005;340:791-809.
4. Lee A, Grummer SE, Kriegel D, et al. Hyaluronidase. Dermatol Surg. 2010;36:1071-1077.
5. Kablik J, Monheit GD, Yu L, et al. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol Surg. 2009;35(suppl 1):302-312.
6. Nast A, Reytan N, Hartmann V, et al. Efficacy and durability of two hyaluronic acid-based fillers in the correction of nasolabial folds: results of a prospective, randomized, double-blind, actively controlled clinical pilot study. Dermatol Surg. 2011;37:768-775.
7. Ascher B, Bayerl C, Brun P, et al. Efficacy and safety of a new hyaluronic acid dermal filler in the treatment of severe nasolabial lines: 6-month interim results of a randomized, evaluator-blinded, intra-individual comparison study. J Cosmet Dermatol. 2011;10:94-98.
8. Rzany B, Bayerl C, Bodokh I, et al. Efficacy and safety of a new hyaluronic acid dermal filler in the treatment of moderate nasolabial folds: 6-month interim results of a randomized, evaluator-blinded, intra-individual comparison study. J Cosmet Laser Ther. 2011;13:107-112.
9. Lupo MP, Swetman G, Waller W. The effect of lidocaine when mixed with large gel particle hyaluronic acid filler tolerability and longevity: a six-month trial. J Drugs Dermatol. 2010;9:1097-1100.
10. Ho D, Jagdeo J. Biological properties of a new volumizing hyaluronic acid filler: a systematic review. J Drugs Dermatol. 2015;14:50-54.
11. Callan P, Goodman GJ, Carlisle I, et al. Efficacy and safety of a hyaluronic acid filler in subjects treated for correction of midface volume deficiency: a 24 month study. Clin Cosmet Investig Dermatol. 2013;6:81-89.
12. Hevia O, Cohen BH, Howell DJ. Safety and efficacy of a cohesive polydensified matrix hyaluronic acid for the correction of infraorbital hollow: an observational study with results at 40 weeks. J Drugs Dermatol. 2014;13:1030-1036.
13. Lorenc ZP, Fagien S, Flynn TC, et al. Clinical application and assessment of Belotero: a roundtable discussion. Plast Reconstr Surg. 2013;132(4, suppl 2):69S-76S.
14. Carruthers JD, Fagien S, Rohrich RJ, et al. Blindness caused by cosmetic filler injection: a review of cause and therapy. Plast Reconstr Surg. 2014;134:1197-1201.
15. Eccleston D, Murphy DK. Juvéderm(®) Volbella™ in the perioral area: a 12-month prospective, multicenter, open-label study. Clin Cosmet Investig Dermatol. 2012;5:167-172.
Practice Points
- Restylane Silk is useful for the treatment of fine perioral lines.
- Juvéderm Voluma XC is a newer product in the Juvéderm range and is indicated for cheek augmentation.
- Belotero Balance has the lowest G′ of the currently available dermal fillers and allows greater precision.
Therapies to Improve the Cosmetic Symptoms of Rosacea
Rosacea is a commonly encountered chronic inflammatory skin disease that affects an estimated 16 million Americans and exhibits a particular predilection for the convexities of the central face (eg, forehead, cheeks, nose, chin).1,2 The pathophysiology of rosacea remains poorly understood despite the relatively high prevalence of the disease and substantial ongoing research.3 The current paradigm suggests a complex multifactorial interplay involving aberrations of the innate and adaptive immune system, neurovascular dysregulation, blood and lymphatic vessel changes, genetic predispositions, and overgrowth of commensal organisms such as Demodex.3 Additionally, a variety of external factors may exacerbate clinical symptoms (eg, UV radiation, heat exposure, spicy food, alcohol, stress).
The diagnosis of rosacea is made clinically and rarely requires histologic confirmation. Although rosacea can present with a wide range of clinical features that often wax and wane over time, a near universal finding is diffuse centrofacial erythema.4 This centrofacial redness may symptomatically worsen during a flare period, causing flushing, but it often persists nontransiently between flares as background erythema. Other variable findings of rosacea include the presence of telangiectases, edema, plaques, phymatous changes, dry skin, ocular manifestations, and inflammatory lesions in the form of papules and pustules.5 Patients also may report a stinging or burning sensation in affected areas. It is important to note that most patients will only exhibit some of these clinical features and that symptoms often vary in the timing of their emergence or regression.5 A classification system has been developed for rosacea that categorizes the disease into 4 subtypes (erythematotelangiectatic, papulopustular, phymatous, and ocular) and one variant (granulomatous).6 These categories are determined by the grouping of clinical features present, but it is not uncommon for patients to exhibit clinical manifestations of more than 1 subtype.7
The detrimental cosmetic effects of rosacea are obvious given its chronic nature and tendency to affect highly visible areas such as the face. As such, rosacea can have a devastating impact on patients’ quality of life.8 Patients with rosacea have been reported to have higher incidence rates of low self-esteem, embarrassment, social anxiety, and depression as compared to the rest of the population. Effective treatment, however, can improve cosmetic appearance and mitigate the negative psychosocial impacts of the disease.8
Treatment of rosacea focuses on relieving cosmetic symptoms, as no curative therapy currently exists. Treatment comes in a wide variety of forms, including topical medications, systemic pharmacologic therapies, light-based modalities, and procedural interventions. Choice of therapy should be determined on a case-by-case basis as guided by the clinical features present, and combination or sequential therapies often are required to achieve optimal cosmetic results. In this article, we review both existing and emerging treatments of rosacea and assess their ability to improve the cosmetic symptoms of rosacea (Table).
Skin Care
Proper skin care is an important aspect of treatment for all patients with rosacea and thus includes the use of over-the-counter cleansers, moisturizers, and sunscreens.9 The choice of skin care products is an important consideration given the often hypersensitive skin of rosacea patients. Moisturizers and cleansers should have an acidic to neutral pH, similar to normal skin. They should not contain emulsifiers that strip moisture from the skin or protective lipids and proteins from the stratum corneum.10 Moisturizers without irritants, abrasives, or allergens should be used following skin cleansing. Protection from UV radiation with sunscreen, ideally with a sun protection factor greater than 30, is particularly important, as it can prevent UV-induced rosacea flares as well as photodamage that can cause additional erythema and telangiectasia.4 Rosacea patients also may find green-tinted makeup to be useful in concealing areas of erythema.8
Topical Therapy
Currently, there are only 5 US Food and Drug Administration (FDA)–approved topical medications for the treatment of rosacea: metronidazole (MTZ) gel 0.75% and 1%, azelaic acid (AzA) gel 15%, sodium sulfacetamide (SS) 10%–sulfur 5% lotion and cream, brimonidine tartrate (BT) gel 0.5%, and the most recently approved ivermectin (IVM) cream 1%.7 Metronidazole, AzA, and SS primarily are used to treat the inflammatory papules and pustules of rosacea, while BT is used to treat persistent background erythema. The exact mechanisms of action by which MTZ, AzA, and SS treat rosacea are unclear, but they are thought to reduce inflammation and/or immune response. Metronidazole and AzA both have demonstrated favorable safety profiles and significant (P<.05) efficacy over vehicle in reducing inflammatory lesions in numerous well-controlled randomized clinical studies.4,11,12 There is some evidence that AzA may be more effective than MTZ; one 15-week multicenter, double-blind, randomized, parallel-group study demonstrated that twice-daily AzA gel 15% showed significant superiority (P=.02) over twice-daily MTZ gel 0.75% in improving the inflammatory lesions and erythema of rosacea.13 Sodium sulfacetamide also has shown good efficacy in the treatment of inflammatory lesions and performed significantly better (P=.04) than MTZ according to one multicenter, investigator-blinded, randomized, parallel-group study,14 but the overall evidence is not as strong as MTZ and AzA.4,11,15 The most common adverse effect for MTZ, AzA, and SS is application-site irritation, but overall most patients report good tolerance to these topical medications.4 Azelaic acid is unique in that patients may report stinging, tingling, or burning after application, but these effects are not associated with visible skin changes and usually are transient, generally remitting after 1 to 2 weeks.4
Brimonidine tartrate is a highly selective α2-adrenergic receptor agonist whose mechanism of action in the treatment of rosacea is thought to involve vasoconstriction of superficial skin vasculature and to a lesser extent anti-inflammatory effects.16 In a double-blind, randomized, vehicle-controlled phase 3 trial, application of BT gel 0.5% once daily for 4 weeks demonstrated significant efficacy over vehicle (P<.001) in treating persistent nontransient facial erythema in 553 adult patients with 2 or fewer papulopustular lesions as evaluated over 12 hours on days 1, 15, and 29.17 Notably, a substantial difference in cosmetic appearance was observed in another study as early as 30 minutes after the first gel application on day 1.18 The results of this phase 3 trial17 mirrored those of the phase 2 dose-optimization and safety studies of similar design.18 In addition to another long-term, 1-year, open-label study,19 both phase 2 and 3 studies have shown favorable safety profiles with no reports of tachyphylaxis, rebound erythema, or aggravation of other disease features such as telangiectases or inflammatory lesions.17,18 Recently, however, there have been some reports of considerable rebound erythema with BT use and thus patients should be made aware of this possibility.20,21 Case reports of successful treatment of background erythema and flushing with other topically applied adrenergic receptor modifiers such as oxymetazoline and xylometazoline have been published in the literature,22,23 but additional research will be necessary to validate these claims.
Ivermectin, a decades-old antiparasitic, has recently shown promising results as a treatment of rosacea patients with moderate to severe papulopustular lesions. Its therapeutic effect is believed to be mediated by its activity against Demodex, a natural skin mite that has been found at increased concentrations in a subset of patients with rosacea, as well as by its natural anti-inflammatory properties.24 In 2 identically designed, randomized, double-blind, controlled trials of IVM cream 1% applied once daily for 12 weeks, a significantly larger proportion of patients in the IVM groups achieved an investigator global assessment of clear or almost clear as compared to vehicle (IVM: 38.4% and 40.1%, respectively; vehicle: 11.6% and 18.8%, respectively; P<.001). Both trials also demonstrated that IVM was significantly superior to vehicle in the reduction of inflammatory lesion counts measured at week 12 as compared to baseline (IVM: 76.0% and 75.0%, respectively; vehicle: 50.0% and 50.0%, respectively; P<.001).24 An extension of these original trials demonstrated long-term safety with up to 52 weeks of topical IVM use and reported a low incidence rate of adverse effects, most commonly transient skin burning, pruritus, and dryness. Notably, the incidence rate of these adverse effects was lower than a comparison group receiving AzA gel 15% once daily.25 Once-daily application of IVM cream 1% also has recently demonstrated superiority over twice-daily MTZ cream 0.75% for 16 weeks in a phase 3 investigator-blinded, randomized, parallel-group study. The IVM group was significantly superior to MTZ in the reduction of inflammatory lesions as compared to baseline (83.0% vs 73.7%) and in the number of participants who achieved an investigator global assessment score of clear or almost clear (84.9% vs 75.4%)(both P<.001).26 There also is limited evidence for the use of other antiparasitic topical medications such as crotamiton 10% and permethrin 5%, but such agents frequently cause irritation and may not be well tolerated in rosacea patients.27-29
There are a variety of other non–FDA-approved topical medications that have been used with varying success in the literature, including cyclosporine, macrolides, benzoyl peroxide, retinoids, and calcineurin inhibitors such as tacrolimus and pimecrolimus. Evidence for the use of these medications generally is limited to a few studies with small numbers of patients and will not be discussed further in this article.4,11,30 These agents, however, may be useful in select cases when first-line regimens have failed and also may be good targets for future research.
Systemic Therapy
The mainstay of systemic treatment of rosacea centers around the tetracyclines, a group of antibiotics that have been used off label for rosacea since the 1950s.31 The therapeutic effects of tetracyclines in the treatment of rosacea are thought to revolve around their anti-inflammatory effects rather than their antibacterial properties.32 Currently, the only FDA-approved oral agent for treatment of the inflammatory lesions of rosacea is doxycycline 40-mg modified-release capsules taken once daily. These modified capsules allow for instant release of 30 mg and delayed release of 10 mg of doxycycline. This dosing is considered to be anti-inflammatory rather than antimicrobial, as it does not produce antibiotic selection pressure even with prolonged use.33 Efficacy of 40-mg subantimicrobial-dose doxycycline (SDD) has been demonstrated in 2 phase 3 multicenter, parallel-group, randomized, double-blind, placebo-controlled studies in which SDD demonstrated a significantly greater reduction in the number of total inflammatory lesions at week 16 compared to placebo (P<.001).34 Subantimicrobial-dose doxycycline also has been shown to be equally as efficacious in reducing inflammatory lesions as traditional-dose doxycycline.35 There also is some evidence for the efficacy of SDD in reducing overall erythema, as demonstrated by one open-label, community-based study in which SDD monotherapy resulted in clinician erythema assessment scores of mild or no erythema in 75% of patients with mild to severe rosacea at baseline after 12 weeks of therapy.35 Additionally, SDD is considered to be safe and well-tolerated and does not generally result in the adverse effects that may be seen in antibiotic-level doses of doxycycline (eg, gastrointestinal upset, vaginal candidiasis, photosensitivity).34,36,37 Other antibiotics such as clarithromycin, azithromycin, and MTZ also have been studied as treatments of papulopustular rosacea at antibiotic-level doses with good therapeutic effect.38-40 These therapies, however, generally are not used unless there are contraindications for use of tetracycline antibiotics, such as pregnancy or allergy, as the overall evidence is not as strong and there may be increased risks for serious adverse effects.30
Although it is not FDA approved, isotretinoin is an important therapeutic option for select rosacea patients, as it is the only pharmacologic agent that has shown efficacy for the phymatous changes of rosacea. Its efficacy, however, is limited to early-stage rhinophyma that has not yet progressed to the fibrotic or mucinous stages of disease in which it has been shown to reduce the size and number of cutaneous sebaceous glands.30,41 Isotretinoin at 0.3 mg/kg daily also has shown noninferiority in treatment of the inflammatory papules and pustules of rosacea as compared to antibiotic dosing of doxycycline in one large-scale, placebo-controlled, randomized, 12-week multicenter study.42 Unfortunately, recurrence is highly likely after isotretinoin therapy is discontinued.30,41 However, continuous “microdose” isotretinoin at 0.03 to 0.17 mg/kg daily has shown evidence for efficacy in treatment of recalcitrant papulopustular disease.43 Such dosing may have the added benefit of reduced risk for radiographic changes associated with long-term isotretinoin use.43
Light-Based Therapy
Light-based modalities are an important tool set in the management of rosacea symptoms, as they can treat telangiectases for which medical therapy is not generally effective.9 To a lesser extent, light-based modalities also can help alleviate background erythema. The most commonly used light-based modalities include the pulsed dye laser (PDL)(Figure), potassium titanyl phosphate (KTP) laser, Nd:YAG laser, intense pulsed light, photodynamic therapy, CO2 laser, and erbium-doped YAG (Er:YAG) laser. These treatments produce clinical results by targeting specific chromophores such as oxyhemoglobin, deoxyhemoglobin, methemoglobin, and clotted blood with light of specific wavelengths to induce thermolysis of vasculature while sparing collateral tissue.44 Generally, larger telangiectatic vessels are more amenable to therapy than smaller vessels, which usually require higher energy to be delivered in a shorter period of time, thus predisposing the patient to the development of purpura that may last for 1 to 2 weeks.44
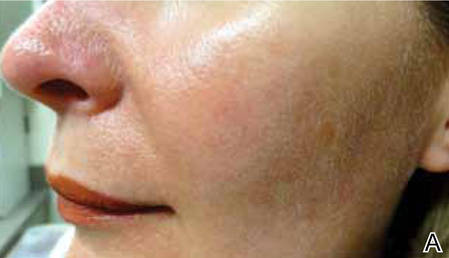 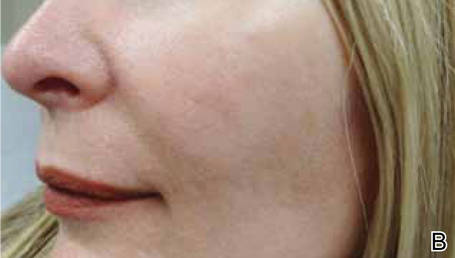 |
Historically, PDL used a light wavelength of 577 nm and was classically associated with posttherapy purpura; however, modern PDLs use wavelengths of 585 or 595 nm and are associated with a reduced risk for purpura through the use of longer pulse durations (ie, 10–40 millisecond), multiple minipulses, multiple passes, and advanced epidermal cooling methods.9,44 In a small, prospective, randomized, controlled, nonblinded study, PDL therapy with fluence sufficiently high enough to induce purpura achieved an approximate 50% improvement in telangiectasia grading scores in most patients after a single treatment.45 Notably, PDL therapy at purpura-inducing settings was reported to be much more efficacious than settings that did not induce purpura (purpura free), especially in the treatment of thicker telangiectases.45
Potassium titanyl phosphate lasers make use of shorter wavelengths (532 nm) than PDL and thus are better able to target superficial vasculature, which translates into a reduced risk for purpura and faster healing times. However, KTP laser therapy typically is only reserved for patients with lighter skin types, as this wavelength of light is more likely to result in higher melanin absorption and possible postinflammatory hyperpigmentation.44 A split-face study comparing the KTP laser with PDL determined that the KTP laser was able to achieve 62% clearing after the first treatment and 85% clearance after the third treatment versus 49% and 75% for PDL treatment, respectively; however, the KTP laser had higher rates of posttherapy erythema lasting at least 1 day (58% vs 8%).46
Conversely, the Nd:YAG laser uses longer wavelengths (1064 nm) and can achieve deeper skin penetration, which may be effective for larger, recalcitrant, or deeper blue-tinted vessels. A split-face, double-blind, randomized, controlled trial found Nd:YAG laser therapy to be an effective treatment of facial erythema, though it was observed to be less effective than purpura-free PDL therapy in reducing redness after 4 treatments (34% vs 52% improvement, respectively); however, treatment with the Nd:YAG laser was found to be significantly (P=.0028) less painful.47
Intense pulsed light is unique from the previously discussed light-based therapies in that it uses noncoherent light with wavelengths between 500 and 1200 nm. Cutoff filters may be used to allow for more selective tissue damage depending on the depth of penetration desired. Intense pulsed light has been shown to be equally as efficacious as purpura-free PDL therapy in the treatment of erythema and telangiectasia in a randomized, controlled, single-blind, split-face trial.48 Additionally, a study of 200 patients with facial vascular lesions, of whom 74 patients had rosacea, showed that intense pulsed light therapy resulted in a 75% to 100% improvement of lesions in 174 of 188 (92.5%) patients who returned for follow-up. Treatment often required at least 2 sessions, but overall adverse effects were reported to be minimal.49
Photodynamic therapy is a well-studied and often utilized treatment of a variety of skin conditions, but there have only been a few studies regarding its use in rosacea. Photodynamic therapy involves the use of topically applied photosensitizing agents such as 5-aminolevulinic acid or methyl aminolevulinate before exposure to red or blue light. This process generates reactive oxygen species, though the exact mechanism of action through which patients achieve cosmetic improvement in rosacea is unclear. In one study of 17 patients with varying rosacea subtypes treated with methyl aminolevulinate and red light, drastic relief of symptoms was seen in 10 (58.8%) patients, marked improvement in 4 (23.5%) patients, and no response in 3 (17.6%) patients. Most patients report a transient irritant skin reaction at the site of therapy.50
Ablative lasers such as the CO2 (10,600 nm) and Er:YAG (2940 nm) lasers also have been shown to be useful in the treatment of rosacea, specifically for the management of rhinophymatous features. Excellent results have been achieved with these lasers given their ability to provide near-bloodless surgical fields. In a 13-year review of 124 patients with rhinophyma receiving a single CO2 laser treatment, good to excellent results were achieved in 118 (95.2%) of patients when evaluated at 3 months posttreatment.51 Patient satisfaction also is reported to be high with few adverse effects reported. The evidence for the Er:YAG laser is not as strong, but the current reports indicate efficacy and safety similar to that of the CO2 laser.52
Procedural Therapies
Procedural therapies in rosacea generally are reserved for management of rhinophyma and include electrocautery, cryotherapy, radiotherapy, dermabrasion, scalpel excisions, flap reconstruction, and skin grafts.30,53 The details and evidence for these methods is beyond the scope of this paper, but it is important to be aware of such modalities. As with most surgical procedures, operator skill and experience may affect treatment outcomes, and there also are definite risks for postprocedural scarring, swelling, erythema, and pigmentation changes. Recently, anecdotal evidence has shown that botulinum toxin injections may be effective for patients with refractory flushing and erythema, but larger studies will be necessary to better assess these claims.54,55
Conclusion
Although recent advances in pharmacology and laser technology have provided physicians with new and effective treatment modalities for rosacea, it remains a poorly understood disease without a definitive cure. The negative impact of rosacea on patients’ quality of life can be substantial, but effective management of cosmetic symptoms can minimize such deleterious effects. Therapy should be individualized and directed at treating the symptoms that are most bothersome to the patient. Additionally, effective treatment often will require a combination of modalities or sequential therapies to achieve optimal cosmetic outcomes.
1. Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6 suppl 1):S27-S35.
2. Rosacea prevalence map. National Rosacea Society Web site. http://rosacea.org/press/prevalencemap. Accessed June 16, 2015.
3. Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 suppl 1):S15-S26.
4. Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 1: a status report on the disease state, general measures, and adjunctive skin care. Cutis. 2013;92:234-240.
5. Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004;50:907-912.
6. Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society expert committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
7. Feldman SR, Huang WW, Huynh TT. Current drug therapies for rosacea: a chronic vascular and inflammatory skin disease. J Manag Care Spec Pharm. 2014;20:623-629.
8. Moustafa F, Lewallen RS, Feldman SR. The psychological impact of rosacea and the influence of current management options. J Am Acad Dermatol. 2014;71:973-980.
9. Mansouri Y, Goldenberg G. Devices and topical agents for rosacea management. Cutis. 2014;94:21-25.
10. Levin J, Miller R. A guide to the ingredients and potential benefits of over-the-counter cleansers and moisturizers for rosacea patients. J Clin Aesthet Dermatol. 2011;4:31-49.
11. van Zuuren EJ, Kramer S, Carter B, et al. Interventions for rosacea. Cochrane Database Syst Rev. 2011;3:CD003262.
12. Liu RH, Smith MK, Basta SA, et al. Azelaic acid in the treatment of papulopustular rosacea: a systematic review of randomized controlled trials. Arch Dermatol. 2006;142:1047-1052.
13. Elewski B, Fleischer AB Jr, Pariser DM. A comparison of 15% azelaic acid gel and 0.75% metronidazole gel in the topical treatment of papulopustular rosacea: results of a randomized trial. Arch Dermatol. 2003;139:1444-1450.
14. Torok HM, Webster G, Dunlap FE, et al. Combination sodium sulfacetamide 10% and sulfur 5% cream with sunscreens versus metronidazole 0.75% cream for rosacea. Cutis. 2005;75:357-363.
15. Trumbore MW, Goldstein JA, Gurge RM. Treatment of papulopustular rosacea with sodium sulfacetamide 10%/sulfur 5% emollient foam. J Drugs Dermatol. 2009;8:299-304.
16. Piwnica D, Rosignoli C, de Menonville ST, et al. Vasoconstriction and anti-inflammatory properties of the selective alpha-adrenergic receptor agonist brimonidine. J Dermatol Sci. 2014;75:49-54.
17. Fowler J Jr, Jackson M, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12:650-656.
18. Fowler J, Jarratt M, Moore A, et al. Once-daily topical brimonidine tartrate gel 0.5% is a novel treatment of moderate to severe facial erythema of rosacea: results of two multicenter, randomized and vehicle-controlled studies. Br J Dermatol. 2012;166:633-641.
19. Moore A, Kempers S, Murakawa G, et al. Long-term safety and efficacy of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of a 1-year open-label study. J Drugs Dermatol. 2014;13:56-61.
20. Routt ET, Levitt JO. Rebound erythema and burning sensation from a new topical brimonidine tartrate gel 0.33%. J Am Acad Dermatol. 2014;70:e37-e38.
21. Ilkovitch D, Pomerantz RG. Brimonidine effective but may lead to significant rebound erythema. J Am Acad Dermatol. 2014;70:e109-e110.
22. Kim JH, Oh YS, Ji JH, et al. Rosacea (erythematotelangiectatic type) effectively improved by topical xylometazoline. J Dermatol. 2011;38:510-513.
23. Shanler SD, Ondo AL. Successful treatment of erythema and flushing of rosacea using a topically applied selective alpha1-adrenergic receptor antagonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
24. Stein-Gold L, Kircik L, Fowler J, et al. Efficacy and safety of ivermectin 1% cream in treatment of papulopustular rosacea: results of two randomized, double-blind, vehicle-controlled pivotal studies. J Drugs Dermatol. 2014;13:316-323.
25. Stein-Gold L, Kircik L, Fowler J, et al. Long-term safety of ivermectin 1% cream vs azelaic acid 15% gel in treating inflammatory lesions of rosacea: results of two 40-week controlled, investigator-blinded trials. J Drugs Dermatol. 2014;13:1380-1386.
26. Taieb A, Ortonne JP, Ruzicka T, et al. Superiority of ivermectin 1% cream over metronidazole 0.75% cream in treating inflammatory lesions of rosacea: a randomized, investigator-blinded trial. Br J Dermatol. 2015;172:1103-1110.
27. Koçak M, Ya˘gli S, Vahapo˘glu G, et al. Permethrin 5% cream versus metronidazole 0.75% gel for the treatment of papulopustular rosacea. a randomized double-blind placebo-controlled study. Dermatology (Basel). 2002;205:265-270.
28. Bikowski JB, Del Rosso JQ. Demodex dermatitis: a retrospective analysis of clinical diagnosis and successful treatment with topical crotamiton. J Clin Aesthet Dermatol. 2009;2:20-25.
29. Layton A, Thiboutot D. Emerging therapies in rosacea. J Am Acad Dermatol. 2013;69(6 suppl 1):S57-S65.
30. Pelle MT, Crawford GH, James WD. Rosacea: II. therapy. J Am Acad Dermatol. 2004;51:499-512, quiz 513-514.
31. Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006;54:258-265.
32. Korting HC, Schöllmann C. Tetracycline actions relevant to rosacea treatment. Skin Pharmacol Physiol. 2009;22:287-294.
33. Thomas J, Walker C, Bradshaw M. Long-term use of subantimicrobial dose doxycycline does not lead to changes in antimicrobial susceptibility. J Periodontol. 2000;71:1472-1483.
34. Del Rosso JQ, Webster GF, Jackson M, et al. Two randomized phase III clinical trials evaluating anti-inflammatory dose doxycycline (40-mg doxycycline, USP capsules) administered once daily for treatment of rosacea. J Am Acad Dermatol. 2007;56:791-802.
35. Webster GF. An open-label, community-based, 12-week assessment of the effectiveness and safety of monotherapy with doxycycline 40 mg (30-mg immediate-release and 10-mg delayed-release beads). Cutis. 2010;86(suppl 5):7-15.
36. Del Rosso JQ, Schlessinger J, Werschler P. Comparison of anti-inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. J Drugs Dermatol. 2008;7:573-576.
37. Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 3: a status report on systemic therapies. Cutis. 2014;93:18-28.
38. Torresani C. Clarithromycin: a new perspective in rosacea treatment. Int J Dermatol. 1998;37:347-349.
39. Bakar O, Demircay Z, Gürbüz O. Therapeutic potential of azithromycin in rosacea. Int J Dermatol. 2004;43:151-154.
40. Saihan EM, Burton JL. A double-blind trial of metronidazole versus oxytetracycline therapy for rosacea. Br J Dermatol. 1980;102:443-445.
41. Park H, Del Rosso JQ. Use of oral isotretinoin in the management of rosacea. J Clin Aesthet Dermatol. 2011;4:54-61.
42. Gollnick H, Blume-Peytavi U, Szabo EL, et al. Systemic isotretinoin in the treatment of rosacea—doxycycline-and placebo-controlled, randomized clinical study. J Dtsch Dermatol Ges. 2010;8:505-515.
43. Hofer T. Continuous “microdose” isotretinoin in adult recalcitrant rosacea. Clin Exp Dermatol. 2004;29:204-205.
44. Tanghetti E, Del Rosso JQ, Thiboutot D, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 4: a status report on physical modalities and devices. Cutis. 2014;93:71-76.
45. Alam M, Dover JS, Arndt KA. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol Surg. 2003;29:681-684.
46. Uebelhoer NS, Bogle MA, Stewart B, et al. A split-face comparison study of pulsed 532-nm KTP laser and 595-nm pulsed dye laser in the treatment of facial telangiectasias and diffuse telangiectatic facial erythema. Dermatol Surg. 2007;33:441-448.
47. Alam M, Voravutinon N, Warycha M, et al. Comparative effectiveness of nonpurpuragenic 595-nm pulsed dye laser and microsecond 1064-nm neodymium:yttrium-aluminum-garnet laser for treatment of diffuse facial erythema: a double-blind randomized controlled trial. J Am Acad Dermatol. 2013;69:438-443.
48. Neuhaus IM, Zane LT, Tope WD. Comparative efficacy of nonpurpuragenic pulsed dye laser and intense pulsed light for erythematotelangiectatic rosacea. Dermatol Surg. 2009;35:920-928.
49. Angermeier MC. Treatment of facial vascular lesions with intense pulsed light. J Cutan Laser Ther. 1999;1:95-100.
50. Bryld LE, Jemec GB. Photodynamic therapy in a series of rosacea patients. J Eur Acad Dermatol Venereol. 2007;21:1199-1202.
51. Maden V, Ferguson JE, August PJ. Carbon dioxide laser treatment of rhinophyma: a review of 124 patients. Br J Dermatol. 2009;161:814-818.
52. Fincher EF, Gladstone HB. Use of a dual-mode erbium:YAG laser for the surgical correction of rhinophyma. Arch Facial Plast Surg. 2004;6:267-271.
53. Lloyd KM. Surgical correction of rhinophyma. Arch Dermatol. 1990;126:721-723.
54. Dayan SH, Pritzker RN, Arkins JP. A new treatment regimen for rosacea: onabotulinumtoxinA. J Drugs Dermatol. 2012;11:e76-e79.
55. Park KY, Hyun MY, Jeong SY, et al. Botulinum toxin for the treatment of refractory erythema and flushing of rosacea. Dermatology. 2015;230:299-301.
Rosacea is a commonly encountered chronic inflammatory skin disease that affects an estimated 16 million Americans and exhibits a particular predilection for the convexities of the central face (eg, forehead, cheeks, nose, chin).1,2 The pathophysiology of rosacea remains poorly understood despite the relatively high prevalence of the disease and substantial ongoing research.3 The current paradigm suggests a complex multifactorial interplay involving aberrations of the innate and adaptive immune system, neurovascular dysregulation, blood and lymphatic vessel changes, genetic predispositions, and overgrowth of commensal organisms such as Demodex.3 Additionally, a variety of external factors may exacerbate clinical symptoms (eg, UV radiation, heat exposure, spicy food, alcohol, stress).
The diagnosis of rosacea is made clinically and rarely requires histologic confirmation. Although rosacea can present with a wide range of clinical features that often wax and wane over time, a near universal finding is diffuse centrofacial erythema.4 This centrofacial redness may symptomatically worsen during a flare period, causing flushing, but it often persists nontransiently between flares as background erythema. Other variable findings of rosacea include the presence of telangiectases, edema, plaques, phymatous changes, dry skin, ocular manifestations, and inflammatory lesions in the form of papules and pustules.5 Patients also may report a stinging or burning sensation in affected areas. It is important to note that most patients will only exhibit some of these clinical features and that symptoms often vary in the timing of their emergence or regression.5 A classification system has been developed for rosacea that categorizes the disease into 4 subtypes (erythematotelangiectatic, papulopustular, phymatous, and ocular) and one variant (granulomatous).6 These categories are determined by the grouping of clinical features present, but it is not uncommon for patients to exhibit clinical manifestations of more than 1 subtype.7
The detrimental cosmetic effects of rosacea are obvious given its chronic nature and tendency to affect highly visible areas such as the face. As such, rosacea can have a devastating impact on patients’ quality of life.8 Patients with rosacea have been reported to have higher incidence rates of low self-esteem, embarrassment, social anxiety, and depression as compared to the rest of the population. Effective treatment, however, can improve cosmetic appearance and mitigate the negative psychosocial impacts of the disease.8
Treatment of rosacea focuses on relieving cosmetic symptoms, as no curative therapy currently exists. Treatment comes in a wide variety of forms, including topical medications, systemic pharmacologic therapies, light-based modalities, and procedural interventions. Choice of therapy should be determined on a case-by-case basis as guided by the clinical features present, and combination or sequential therapies often are required to achieve optimal cosmetic results. In this article, we review both existing and emerging treatments of rosacea and assess their ability to improve the cosmetic symptoms of rosacea (Table).
Skin Care
Proper skin care is an important aspect of treatment for all patients with rosacea and thus includes the use of over-the-counter cleansers, moisturizers, and sunscreens.9 The choice of skin care products is an important consideration given the often hypersensitive skin of rosacea patients. Moisturizers and cleansers should have an acidic to neutral pH, similar to normal skin. They should not contain emulsifiers that strip moisture from the skin or protective lipids and proteins from the stratum corneum.10 Moisturizers without irritants, abrasives, or allergens should be used following skin cleansing. Protection from UV radiation with sunscreen, ideally with a sun protection factor greater than 30, is particularly important, as it can prevent UV-induced rosacea flares as well as photodamage that can cause additional erythema and telangiectasia.4 Rosacea patients also may find green-tinted makeup to be useful in concealing areas of erythema.8
Topical Therapy
Currently, there are only 5 US Food and Drug Administration (FDA)–approved topical medications for the treatment of rosacea: metronidazole (MTZ) gel 0.75% and 1%, azelaic acid (AzA) gel 15%, sodium sulfacetamide (SS) 10%–sulfur 5% lotion and cream, brimonidine tartrate (BT) gel 0.5%, and the most recently approved ivermectin (IVM) cream 1%.7 Metronidazole, AzA, and SS primarily are used to treat the inflammatory papules and pustules of rosacea, while BT is used to treat persistent background erythema. The exact mechanisms of action by which MTZ, AzA, and SS treat rosacea are unclear, but they are thought to reduce inflammation and/or immune response. Metronidazole and AzA both have demonstrated favorable safety profiles and significant (P<.05) efficacy over vehicle in reducing inflammatory lesions in numerous well-controlled randomized clinical studies.4,11,12 There is some evidence that AzA may be more effective than MTZ; one 15-week multicenter, double-blind, randomized, parallel-group study demonstrated that twice-daily AzA gel 15% showed significant superiority (P=.02) over twice-daily MTZ gel 0.75% in improving the inflammatory lesions and erythema of rosacea.13 Sodium sulfacetamide also has shown good efficacy in the treatment of inflammatory lesions and performed significantly better (P=.04) than MTZ according to one multicenter, investigator-blinded, randomized, parallel-group study,14 but the overall evidence is not as strong as MTZ and AzA.4,11,15 The most common adverse effect for MTZ, AzA, and SS is application-site irritation, but overall most patients report good tolerance to these topical medications.4 Azelaic acid is unique in that patients may report stinging, tingling, or burning after application, but these effects are not associated with visible skin changes and usually are transient, generally remitting after 1 to 2 weeks.4
Brimonidine tartrate is a highly selective α2-adrenergic receptor agonist whose mechanism of action in the treatment of rosacea is thought to involve vasoconstriction of superficial skin vasculature and to a lesser extent anti-inflammatory effects.16 In a double-blind, randomized, vehicle-controlled phase 3 trial, application of BT gel 0.5% once daily for 4 weeks demonstrated significant efficacy over vehicle (P<.001) in treating persistent nontransient facial erythema in 553 adult patients with 2 or fewer papulopustular lesions as evaluated over 12 hours on days 1, 15, and 29.17 Notably, a substantial difference in cosmetic appearance was observed in another study as early as 30 minutes after the first gel application on day 1.18 The results of this phase 3 trial17 mirrored those of the phase 2 dose-optimization and safety studies of similar design.18 In addition to another long-term, 1-year, open-label study,19 both phase 2 and 3 studies have shown favorable safety profiles with no reports of tachyphylaxis, rebound erythema, or aggravation of other disease features such as telangiectases or inflammatory lesions.17,18 Recently, however, there have been some reports of considerable rebound erythema with BT use and thus patients should be made aware of this possibility.20,21 Case reports of successful treatment of background erythema and flushing with other topically applied adrenergic receptor modifiers such as oxymetazoline and xylometazoline have been published in the literature,22,23 but additional research will be necessary to validate these claims.
Ivermectin, a decades-old antiparasitic, has recently shown promising results as a treatment of rosacea patients with moderate to severe papulopustular lesions. Its therapeutic effect is believed to be mediated by its activity against Demodex, a natural skin mite that has been found at increased concentrations in a subset of patients with rosacea, as well as by its natural anti-inflammatory properties.24 In 2 identically designed, randomized, double-blind, controlled trials of IVM cream 1% applied once daily for 12 weeks, a significantly larger proportion of patients in the IVM groups achieved an investigator global assessment of clear or almost clear as compared to vehicle (IVM: 38.4% and 40.1%, respectively; vehicle: 11.6% and 18.8%, respectively; P<.001). Both trials also demonstrated that IVM was significantly superior to vehicle in the reduction of inflammatory lesion counts measured at week 12 as compared to baseline (IVM: 76.0% and 75.0%, respectively; vehicle: 50.0% and 50.0%, respectively; P<.001).24 An extension of these original trials demonstrated long-term safety with up to 52 weeks of topical IVM use and reported a low incidence rate of adverse effects, most commonly transient skin burning, pruritus, and dryness. Notably, the incidence rate of these adverse effects was lower than a comparison group receiving AzA gel 15% once daily.25 Once-daily application of IVM cream 1% also has recently demonstrated superiority over twice-daily MTZ cream 0.75% for 16 weeks in a phase 3 investigator-blinded, randomized, parallel-group study. The IVM group was significantly superior to MTZ in the reduction of inflammatory lesions as compared to baseline (83.0% vs 73.7%) and in the number of participants who achieved an investigator global assessment score of clear or almost clear (84.9% vs 75.4%)(both P<.001).26 There also is limited evidence for the use of other antiparasitic topical medications such as crotamiton 10% and permethrin 5%, but such agents frequently cause irritation and may not be well tolerated in rosacea patients.27-29
There are a variety of other non–FDA-approved topical medications that have been used with varying success in the literature, including cyclosporine, macrolides, benzoyl peroxide, retinoids, and calcineurin inhibitors such as tacrolimus and pimecrolimus. Evidence for the use of these medications generally is limited to a few studies with small numbers of patients and will not be discussed further in this article.4,11,30 These agents, however, may be useful in select cases when first-line regimens have failed and also may be good targets for future research.
Systemic Therapy
The mainstay of systemic treatment of rosacea centers around the tetracyclines, a group of antibiotics that have been used off label for rosacea since the 1950s.31 The therapeutic effects of tetracyclines in the treatment of rosacea are thought to revolve around their anti-inflammatory effects rather than their antibacterial properties.32 Currently, the only FDA-approved oral agent for treatment of the inflammatory lesions of rosacea is doxycycline 40-mg modified-release capsules taken once daily. These modified capsules allow for instant release of 30 mg and delayed release of 10 mg of doxycycline. This dosing is considered to be anti-inflammatory rather than antimicrobial, as it does not produce antibiotic selection pressure even with prolonged use.33 Efficacy of 40-mg subantimicrobial-dose doxycycline (SDD) has been demonstrated in 2 phase 3 multicenter, parallel-group, randomized, double-blind, placebo-controlled studies in which SDD demonstrated a significantly greater reduction in the number of total inflammatory lesions at week 16 compared to placebo (P<.001).34 Subantimicrobial-dose doxycycline also has been shown to be equally as efficacious in reducing inflammatory lesions as traditional-dose doxycycline.35 There also is some evidence for the efficacy of SDD in reducing overall erythema, as demonstrated by one open-label, community-based study in which SDD monotherapy resulted in clinician erythema assessment scores of mild or no erythema in 75% of patients with mild to severe rosacea at baseline after 12 weeks of therapy.35 Additionally, SDD is considered to be safe and well-tolerated and does not generally result in the adverse effects that may be seen in antibiotic-level doses of doxycycline (eg, gastrointestinal upset, vaginal candidiasis, photosensitivity).34,36,37 Other antibiotics such as clarithromycin, azithromycin, and MTZ also have been studied as treatments of papulopustular rosacea at antibiotic-level doses with good therapeutic effect.38-40 These therapies, however, generally are not used unless there are contraindications for use of tetracycline antibiotics, such as pregnancy or allergy, as the overall evidence is not as strong and there may be increased risks for serious adverse effects.30
Although it is not FDA approved, isotretinoin is an important therapeutic option for select rosacea patients, as it is the only pharmacologic agent that has shown efficacy for the phymatous changes of rosacea. Its efficacy, however, is limited to early-stage rhinophyma that has not yet progressed to the fibrotic or mucinous stages of disease in which it has been shown to reduce the size and number of cutaneous sebaceous glands.30,41 Isotretinoin at 0.3 mg/kg daily also has shown noninferiority in treatment of the inflammatory papules and pustules of rosacea as compared to antibiotic dosing of doxycycline in one large-scale, placebo-controlled, randomized, 12-week multicenter study.42 Unfortunately, recurrence is highly likely after isotretinoin therapy is discontinued.30,41 However, continuous “microdose” isotretinoin at 0.03 to 0.17 mg/kg daily has shown evidence for efficacy in treatment of recalcitrant papulopustular disease.43 Such dosing may have the added benefit of reduced risk for radiographic changes associated with long-term isotretinoin use.43
Light-Based Therapy
Light-based modalities are an important tool set in the management of rosacea symptoms, as they can treat telangiectases for which medical therapy is not generally effective.9 To a lesser extent, light-based modalities also can help alleviate background erythema. The most commonly used light-based modalities include the pulsed dye laser (PDL)(Figure), potassium titanyl phosphate (KTP) laser, Nd:YAG laser, intense pulsed light, photodynamic therapy, CO2 laser, and erbium-doped YAG (Er:YAG) laser. These treatments produce clinical results by targeting specific chromophores such as oxyhemoglobin, deoxyhemoglobin, methemoglobin, and clotted blood with light of specific wavelengths to induce thermolysis of vasculature while sparing collateral tissue.44 Generally, larger telangiectatic vessels are more amenable to therapy than smaller vessels, which usually require higher energy to be delivered in a shorter period of time, thus predisposing the patient to the development of purpura that may last for 1 to 2 weeks.44
  |
Historically, PDL used a light wavelength of 577 nm and was classically associated with posttherapy purpura; however, modern PDLs use wavelengths of 585 or 595 nm and are associated with a reduced risk for purpura through the use of longer pulse durations (ie, 10–40 millisecond), multiple minipulses, multiple passes, and advanced epidermal cooling methods.9,44 In a small, prospective, randomized, controlled, nonblinded study, PDL therapy with fluence sufficiently high enough to induce purpura achieved an approximate 50% improvement in telangiectasia grading scores in most patients after a single treatment.45 Notably, PDL therapy at purpura-inducing settings was reported to be much more efficacious than settings that did not induce purpura (purpura free), especially in the treatment of thicker telangiectases.45
Potassium titanyl phosphate lasers make use of shorter wavelengths (532 nm) than PDL and thus are better able to target superficial vasculature, which translates into a reduced risk for purpura and faster healing times. However, KTP laser therapy typically is only reserved for patients with lighter skin types, as this wavelength of light is more likely to result in higher melanin absorption and possible postinflammatory hyperpigmentation.44 A split-face study comparing the KTP laser with PDL determined that the KTP laser was able to achieve 62% clearing after the first treatment and 85% clearance after the third treatment versus 49% and 75% for PDL treatment, respectively; however, the KTP laser had higher rates of posttherapy erythema lasting at least 1 day (58% vs 8%).46
Conversely, the Nd:YAG laser uses longer wavelengths (1064 nm) and can achieve deeper skin penetration, which may be effective for larger, recalcitrant, or deeper blue-tinted vessels. A split-face, double-blind, randomized, controlled trial found Nd:YAG laser therapy to be an effective treatment of facial erythema, though it was observed to be less effective than purpura-free PDL therapy in reducing redness after 4 treatments (34% vs 52% improvement, respectively); however, treatment with the Nd:YAG laser was found to be significantly (P=.0028) less painful.47
Intense pulsed light is unique from the previously discussed light-based therapies in that it uses noncoherent light with wavelengths between 500 and 1200 nm. Cutoff filters may be used to allow for more selective tissue damage depending on the depth of penetration desired. Intense pulsed light has been shown to be equally as efficacious as purpura-free PDL therapy in the treatment of erythema and telangiectasia in a randomized, controlled, single-blind, split-face trial.48 Additionally, a study of 200 patients with facial vascular lesions, of whom 74 patients had rosacea, showed that intense pulsed light therapy resulted in a 75% to 100% improvement of lesions in 174 of 188 (92.5%) patients who returned for follow-up. Treatment often required at least 2 sessions, but overall adverse effects were reported to be minimal.49
Photodynamic therapy is a well-studied and often utilized treatment of a variety of skin conditions, but there have only been a few studies regarding its use in rosacea. Photodynamic therapy involves the use of topically applied photosensitizing agents such as 5-aminolevulinic acid or methyl aminolevulinate before exposure to red or blue light. This process generates reactive oxygen species, though the exact mechanism of action through which patients achieve cosmetic improvement in rosacea is unclear. In one study of 17 patients with varying rosacea subtypes treated with methyl aminolevulinate and red light, drastic relief of symptoms was seen in 10 (58.8%) patients, marked improvement in 4 (23.5%) patients, and no response in 3 (17.6%) patients. Most patients report a transient irritant skin reaction at the site of therapy.50
Ablative lasers such as the CO2 (10,600 nm) and Er:YAG (2940 nm) lasers also have been shown to be useful in the treatment of rosacea, specifically for the management of rhinophymatous features. Excellent results have been achieved with these lasers given their ability to provide near-bloodless surgical fields. In a 13-year review of 124 patients with rhinophyma receiving a single CO2 laser treatment, good to excellent results were achieved in 118 (95.2%) of patients when evaluated at 3 months posttreatment.51 Patient satisfaction also is reported to be high with few adverse effects reported. The evidence for the Er:YAG laser is not as strong, but the current reports indicate efficacy and safety similar to that of the CO2 laser.52
Procedural Therapies
Procedural therapies in rosacea generally are reserved for management of rhinophyma and include electrocautery, cryotherapy, radiotherapy, dermabrasion, scalpel excisions, flap reconstruction, and skin grafts.30,53 The details and evidence for these methods is beyond the scope of this paper, but it is important to be aware of such modalities. As with most surgical procedures, operator skill and experience may affect treatment outcomes, and there also are definite risks for postprocedural scarring, swelling, erythema, and pigmentation changes. Recently, anecdotal evidence has shown that botulinum toxin injections may be effective for patients with refractory flushing and erythema, but larger studies will be necessary to better assess these claims.54,55
Conclusion
Although recent advances in pharmacology and laser technology have provided physicians with new and effective treatment modalities for rosacea, it remains a poorly understood disease without a definitive cure. The negative impact of rosacea on patients’ quality of life can be substantial, but effective management of cosmetic symptoms can minimize such deleterious effects. Therapy should be individualized and directed at treating the symptoms that are most bothersome to the patient. Additionally, effective treatment often will require a combination of modalities or sequential therapies to achieve optimal cosmetic outcomes.
Rosacea is a commonly encountered chronic inflammatory skin disease that affects an estimated 16 million Americans and exhibits a particular predilection for the convexities of the central face (eg, forehead, cheeks, nose, chin).1,2 The pathophysiology of rosacea remains poorly understood despite the relatively high prevalence of the disease and substantial ongoing research.3 The current paradigm suggests a complex multifactorial interplay involving aberrations of the innate and adaptive immune system, neurovascular dysregulation, blood and lymphatic vessel changes, genetic predispositions, and overgrowth of commensal organisms such as Demodex.3 Additionally, a variety of external factors may exacerbate clinical symptoms (eg, UV radiation, heat exposure, spicy food, alcohol, stress).
The diagnosis of rosacea is made clinically and rarely requires histologic confirmation. Although rosacea can present with a wide range of clinical features that often wax and wane over time, a near universal finding is diffuse centrofacial erythema.4 This centrofacial redness may symptomatically worsen during a flare period, causing flushing, but it often persists nontransiently between flares as background erythema. Other variable findings of rosacea include the presence of telangiectases, edema, plaques, phymatous changes, dry skin, ocular manifestations, and inflammatory lesions in the form of papules and pustules.5 Patients also may report a stinging or burning sensation in affected areas. It is important to note that most patients will only exhibit some of these clinical features and that symptoms often vary in the timing of their emergence or regression.5 A classification system has been developed for rosacea that categorizes the disease into 4 subtypes (erythematotelangiectatic, papulopustular, phymatous, and ocular) and one variant (granulomatous).6 These categories are determined by the grouping of clinical features present, but it is not uncommon for patients to exhibit clinical manifestations of more than 1 subtype.7
The detrimental cosmetic effects of rosacea are obvious given its chronic nature and tendency to affect highly visible areas such as the face. As such, rosacea can have a devastating impact on patients’ quality of life.8 Patients with rosacea have been reported to have higher incidence rates of low self-esteem, embarrassment, social anxiety, and depression as compared to the rest of the population. Effective treatment, however, can improve cosmetic appearance and mitigate the negative psychosocial impacts of the disease.8
Treatment of rosacea focuses on relieving cosmetic symptoms, as no curative therapy currently exists. Treatment comes in a wide variety of forms, including topical medications, systemic pharmacologic therapies, light-based modalities, and procedural interventions. Choice of therapy should be determined on a case-by-case basis as guided by the clinical features present, and combination or sequential therapies often are required to achieve optimal cosmetic results. In this article, we review both existing and emerging treatments of rosacea and assess their ability to improve the cosmetic symptoms of rosacea (Table).
Skin Care
Proper skin care is an important aspect of treatment for all patients with rosacea and thus includes the use of over-the-counter cleansers, moisturizers, and sunscreens.9 The choice of skin care products is an important consideration given the often hypersensitive skin of rosacea patients. Moisturizers and cleansers should have an acidic to neutral pH, similar to normal skin. They should not contain emulsifiers that strip moisture from the skin or protective lipids and proteins from the stratum corneum.10 Moisturizers without irritants, abrasives, or allergens should be used following skin cleansing. Protection from UV radiation with sunscreen, ideally with a sun protection factor greater than 30, is particularly important, as it can prevent UV-induced rosacea flares as well as photodamage that can cause additional erythema and telangiectasia.4 Rosacea patients also may find green-tinted makeup to be useful in concealing areas of erythema.8
Topical Therapy
Currently, there are only 5 US Food and Drug Administration (FDA)–approved topical medications for the treatment of rosacea: metronidazole (MTZ) gel 0.75% and 1%, azelaic acid (AzA) gel 15%, sodium sulfacetamide (SS) 10%–sulfur 5% lotion and cream, brimonidine tartrate (BT) gel 0.5%, and the most recently approved ivermectin (IVM) cream 1%.7 Metronidazole, AzA, and SS primarily are used to treat the inflammatory papules and pustules of rosacea, while BT is used to treat persistent background erythema. The exact mechanisms of action by which MTZ, AzA, and SS treat rosacea are unclear, but they are thought to reduce inflammation and/or immune response. Metronidazole and AzA both have demonstrated favorable safety profiles and significant (P<.05) efficacy over vehicle in reducing inflammatory lesions in numerous well-controlled randomized clinical studies.4,11,12 There is some evidence that AzA may be more effective than MTZ; one 15-week multicenter, double-blind, randomized, parallel-group study demonstrated that twice-daily AzA gel 15% showed significant superiority (P=.02) over twice-daily MTZ gel 0.75% in improving the inflammatory lesions and erythema of rosacea.13 Sodium sulfacetamide also has shown good efficacy in the treatment of inflammatory lesions and performed significantly better (P=.04) than MTZ according to one multicenter, investigator-blinded, randomized, parallel-group study,14 but the overall evidence is not as strong as MTZ and AzA.4,11,15 The most common adverse effect for MTZ, AzA, and SS is application-site irritation, but overall most patients report good tolerance to these topical medications.4 Azelaic acid is unique in that patients may report stinging, tingling, or burning after application, but these effects are not associated with visible skin changes and usually are transient, generally remitting after 1 to 2 weeks.4
Brimonidine tartrate is a highly selective α2-adrenergic receptor agonist whose mechanism of action in the treatment of rosacea is thought to involve vasoconstriction of superficial skin vasculature and to a lesser extent anti-inflammatory effects.16 In a double-blind, randomized, vehicle-controlled phase 3 trial, application of BT gel 0.5% once daily for 4 weeks demonstrated significant efficacy over vehicle (P<.001) in treating persistent nontransient facial erythema in 553 adult patients with 2 or fewer papulopustular lesions as evaluated over 12 hours on days 1, 15, and 29.17 Notably, a substantial difference in cosmetic appearance was observed in another study as early as 30 minutes after the first gel application on day 1.18 The results of this phase 3 trial17 mirrored those of the phase 2 dose-optimization and safety studies of similar design.18 In addition to another long-term, 1-year, open-label study,19 both phase 2 and 3 studies have shown favorable safety profiles with no reports of tachyphylaxis, rebound erythema, or aggravation of other disease features such as telangiectases or inflammatory lesions.17,18 Recently, however, there have been some reports of considerable rebound erythema with BT use and thus patients should be made aware of this possibility.20,21 Case reports of successful treatment of background erythema and flushing with other topically applied adrenergic receptor modifiers such as oxymetazoline and xylometazoline have been published in the literature,22,23 but additional research will be necessary to validate these claims.
Ivermectin, a decades-old antiparasitic, has recently shown promising results as a treatment of rosacea patients with moderate to severe papulopustular lesions. Its therapeutic effect is believed to be mediated by its activity against Demodex, a natural skin mite that has been found at increased concentrations in a subset of patients with rosacea, as well as by its natural anti-inflammatory properties.24 In 2 identically designed, randomized, double-blind, controlled trials of IVM cream 1% applied once daily for 12 weeks, a significantly larger proportion of patients in the IVM groups achieved an investigator global assessment of clear or almost clear as compared to vehicle (IVM: 38.4% and 40.1%, respectively; vehicle: 11.6% and 18.8%, respectively; P<.001). Both trials also demonstrated that IVM was significantly superior to vehicle in the reduction of inflammatory lesion counts measured at week 12 as compared to baseline (IVM: 76.0% and 75.0%, respectively; vehicle: 50.0% and 50.0%, respectively; P<.001).24 An extension of these original trials demonstrated long-term safety with up to 52 weeks of topical IVM use and reported a low incidence rate of adverse effects, most commonly transient skin burning, pruritus, and dryness. Notably, the incidence rate of these adverse effects was lower than a comparison group receiving AzA gel 15% once daily.25 Once-daily application of IVM cream 1% also has recently demonstrated superiority over twice-daily MTZ cream 0.75% for 16 weeks in a phase 3 investigator-blinded, randomized, parallel-group study. The IVM group was significantly superior to MTZ in the reduction of inflammatory lesions as compared to baseline (83.0% vs 73.7%) and in the number of participants who achieved an investigator global assessment score of clear or almost clear (84.9% vs 75.4%)(both P<.001).26 There also is limited evidence for the use of other antiparasitic topical medications such as crotamiton 10% and permethrin 5%, but such agents frequently cause irritation and may not be well tolerated in rosacea patients.27-29
There are a variety of other non–FDA-approved topical medications that have been used with varying success in the literature, including cyclosporine, macrolides, benzoyl peroxide, retinoids, and calcineurin inhibitors such as tacrolimus and pimecrolimus. Evidence for the use of these medications generally is limited to a few studies with small numbers of patients and will not be discussed further in this article.4,11,30 These agents, however, may be useful in select cases when first-line regimens have failed and also may be good targets for future research.
Systemic Therapy
The mainstay of systemic treatment of rosacea centers around the tetracyclines, a group of antibiotics that have been used off label for rosacea since the 1950s.31 The therapeutic effects of tetracyclines in the treatment of rosacea are thought to revolve around their anti-inflammatory effects rather than their antibacterial properties.32 Currently, the only FDA-approved oral agent for treatment of the inflammatory lesions of rosacea is doxycycline 40-mg modified-release capsules taken once daily. These modified capsules allow for instant release of 30 mg and delayed release of 10 mg of doxycycline. This dosing is considered to be anti-inflammatory rather than antimicrobial, as it does not produce antibiotic selection pressure even with prolonged use.33 Efficacy of 40-mg subantimicrobial-dose doxycycline (SDD) has been demonstrated in 2 phase 3 multicenter, parallel-group, randomized, double-blind, placebo-controlled studies in which SDD demonstrated a significantly greater reduction in the number of total inflammatory lesions at week 16 compared to placebo (P<.001).34 Subantimicrobial-dose doxycycline also has been shown to be equally as efficacious in reducing inflammatory lesions as traditional-dose doxycycline.35 There also is some evidence for the efficacy of SDD in reducing overall erythema, as demonstrated by one open-label, community-based study in which SDD monotherapy resulted in clinician erythema assessment scores of mild or no erythema in 75% of patients with mild to severe rosacea at baseline after 12 weeks of therapy.35 Additionally, SDD is considered to be safe and well-tolerated and does not generally result in the adverse effects that may be seen in antibiotic-level doses of doxycycline (eg, gastrointestinal upset, vaginal candidiasis, photosensitivity).34,36,37 Other antibiotics such as clarithromycin, azithromycin, and MTZ also have been studied as treatments of papulopustular rosacea at antibiotic-level doses with good therapeutic effect.38-40 These therapies, however, generally are not used unless there are contraindications for use of tetracycline antibiotics, such as pregnancy or allergy, as the overall evidence is not as strong and there may be increased risks for serious adverse effects.30
Although it is not FDA approved, isotretinoin is an important therapeutic option for select rosacea patients, as it is the only pharmacologic agent that has shown efficacy for the phymatous changes of rosacea. Its efficacy, however, is limited to early-stage rhinophyma that has not yet progressed to the fibrotic or mucinous stages of disease in which it has been shown to reduce the size and number of cutaneous sebaceous glands.30,41 Isotretinoin at 0.3 mg/kg daily also has shown noninferiority in treatment of the inflammatory papules and pustules of rosacea as compared to antibiotic dosing of doxycycline in one large-scale, placebo-controlled, randomized, 12-week multicenter study.42 Unfortunately, recurrence is highly likely after isotretinoin therapy is discontinued.30,41 However, continuous “microdose” isotretinoin at 0.03 to 0.17 mg/kg daily has shown evidence for efficacy in treatment of recalcitrant papulopustular disease.43 Such dosing may have the added benefit of reduced risk for radiographic changes associated with long-term isotretinoin use.43
Light-Based Therapy
Light-based modalities are an important tool set in the management of rosacea symptoms, as they can treat telangiectases for which medical therapy is not generally effective.9 To a lesser extent, light-based modalities also can help alleviate background erythema. The most commonly used light-based modalities include the pulsed dye laser (PDL)(Figure), potassium titanyl phosphate (KTP) laser, Nd:YAG laser, intense pulsed light, photodynamic therapy, CO2 laser, and erbium-doped YAG (Er:YAG) laser. These treatments produce clinical results by targeting specific chromophores such as oxyhemoglobin, deoxyhemoglobin, methemoglobin, and clotted blood with light of specific wavelengths to induce thermolysis of vasculature while sparing collateral tissue.44 Generally, larger telangiectatic vessels are more amenable to therapy than smaller vessels, which usually require higher energy to be delivered in a shorter period of time, thus predisposing the patient to the development of purpura that may last for 1 to 2 weeks.44
  |
Historically, PDL used a light wavelength of 577 nm and was classically associated with posttherapy purpura; however, modern PDLs use wavelengths of 585 or 595 nm and are associated with a reduced risk for purpura through the use of longer pulse durations (ie, 10–40 millisecond), multiple minipulses, multiple passes, and advanced epidermal cooling methods.9,44 In a small, prospective, randomized, controlled, nonblinded study, PDL therapy with fluence sufficiently high enough to induce purpura achieved an approximate 50% improvement in telangiectasia grading scores in most patients after a single treatment.45 Notably, PDL therapy at purpura-inducing settings was reported to be much more efficacious than settings that did not induce purpura (purpura free), especially in the treatment of thicker telangiectases.45
Potassium titanyl phosphate lasers make use of shorter wavelengths (532 nm) than PDL and thus are better able to target superficial vasculature, which translates into a reduced risk for purpura and faster healing times. However, KTP laser therapy typically is only reserved for patients with lighter skin types, as this wavelength of light is more likely to result in higher melanin absorption and possible postinflammatory hyperpigmentation.44 A split-face study comparing the KTP laser with PDL determined that the KTP laser was able to achieve 62% clearing after the first treatment and 85% clearance after the third treatment versus 49% and 75% for PDL treatment, respectively; however, the KTP laser had higher rates of posttherapy erythema lasting at least 1 day (58% vs 8%).46
Conversely, the Nd:YAG laser uses longer wavelengths (1064 nm) and can achieve deeper skin penetration, which may be effective for larger, recalcitrant, or deeper blue-tinted vessels. A split-face, double-blind, randomized, controlled trial found Nd:YAG laser therapy to be an effective treatment of facial erythema, though it was observed to be less effective than purpura-free PDL therapy in reducing redness after 4 treatments (34% vs 52% improvement, respectively); however, treatment with the Nd:YAG laser was found to be significantly (P=.0028) less painful.47
Intense pulsed light is unique from the previously discussed light-based therapies in that it uses noncoherent light with wavelengths between 500 and 1200 nm. Cutoff filters may be used to allow for more selective tissue damage depending on the depth of penetration desired. Intense pulsed light has been shown to be equally as efficacious as purpura-free PDL therapy in the treatment of erythema and telangiectasia in a randomized, controlled, single-blind, split-face trial.48 Additionally, a study of 200 patients with facial vascular lesions, of whom 74 patients had rosacea, showed that intense pulsed light therapy resulted in a 75% to 100% improvement of lesions in 174 of 188 (92.5%) patients who returned for follow-up. Treatment often required at least 2 sessions, but overall adverse effects were reported to be minimal.49
Photodynamic therapy is a well-studied and often utilized treatment of a variety of skin conditions, but there have only been a few studies regarding its use in rosacea. Photodynamic therapy involves the use of topically applied photosensitizing agents such as 5-aminolevulinic acid or methyl aminolevulinate before exposure to red or blue light. This process generates reactive oxygen species, though the exact mechanism of action through which patients achieve cosmetic improvement in rosacea is unclear. In one study of 17 patients with varying rosacea subtypes treated with methyl aminolevulinate and red light, drastic relief of symptoms was seen in 10 (58.8%) patients, marked improvement in 4 (23.5%) patients, and no response in 3 (17.6%) patients. Most patients report a transient irritant skin reaction at the site of therapy.50
Ablative lasers such as the CO2 (10,600 nm) and Er:YAG (2940 nm) lasers also have been shown to be useful in the treatment of rosacea, specifically for the management of rhinophymatous features. Excellent results have been achieved with these lasers given their ability to provide near-bloodless surgical fields. In a 13-year review of 124 patients with rhinophyma receiving a single CO2 laser treatment, good to excellent results were achieved in 118 (95.2%) of patients when evaluated at 3 months posttreatment.51 Patient satisfaction also is reported to be high with few adverse effects reported. The evidence for the Er:YAG laser is not as strong, but the current reports indicate efficacy and safety similar to that of the CO2 laser.52
Procedural Therapies
Procedural therapies in rosacea generally are reserved for management of rhinophyma and include electrocautery, cryotherapy, radiotherapy, dermabrasion, scalpel excisions, flap reconstruction, and skin grafts.30,53 The details and evidence for these methods is beyond the scope of this paper, but it is important to be aware of such modalities. As with most surgical procedures, operator skill and experience may affect treatment outcomes, and there also are definite risks for postprocedural scarring, swelling, erythema, and pigmentation changes. Recently, anecdotal evidence has shown that botulinum toxin injections may be effective for patients with refractory flushing and erythema, but larger studies will be necessary to better assess these claims.54,55
Conclusion
Although recent advances in pharmacology and laser technology have provided physicians with new and effective treatment modalities for rosacea, it remains a poorly understood disease without a definitive cure. The negative impact of rosacea on patients’ quality of life can be substantial, but effective management of cosmetic symptoms can minimize such deleterious effects. Therapy should be individualized and directed at treating the symptoms that are most bothersome to the patient. Additionally, effective treatment often will require a combination of modalities or sequential therapies to achieve optimal cosmetic outcomes.
1. Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6 suppl 1):S27-S35.
2. Rosacea prevalence map. National Rosacea Society Web site. http://rosacea.org/press/prevalencemap. Accessed June 16, 2015.
3. Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 suppl 1):S15-S26.
4. Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 1: a status report on the disease state, general measures, and adjunctive skin care. Cutis. 2013;92:234-240.
5. Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004;50:907-912.
6. Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society expert committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
7. Feldman SR, Huang WW, Huynh TT. Current drug therapies for rosacea: a chronic vascular and inflammatory skin disease. J Manag Care Spec Pharm. 2014;20:623-629.
8. Moustafa F, Lewallen RS, Feldman SR. The psychological impact of rosacea and the influence of current management options. J Am Acad Dermatol. 2014;71:973-980.
9. Mansouri Y, Goldenberg G. Devices and topical agents for rosacea management. Cutis. 2014;94:21-25.
10. Levin J, Miller R. A guide to the ingredients and potential benefits of over-the-counter cleansers and moisturizers for rosacea patients. J Clin Aesthet Dermatol. 2011;4:31-49.
11. van Zuuren EJ, Kramer S, Carter B, et al. Interventions for rosacea. Cochrane Database Syst Rev. 2011;3:CD003262.
12. Liu RH, Smith MK, Basta SA, et al. Azelaic acid in the treatment of papulopustular rosacea: a systematic review of randomized controlled trials. Arch Dermatol. 2006;142:1047-1052.
13. Elewski B, Fleischer AB Jr, Pariser DM. A comparison of 15% azelaic acid gel and 0.75% metronidazole gel in the topical treatment of papulopustular rosacea: results of a randomized trial. Arch Dermatol. 2003;139:1444-1450.
14. Torok HM, Webster G, Dunlap FE, et al. Combination sodium sulfacetamide 10% and sulfur 5% cream with sunscreens versus metronidazole 0.75% cream for rosacea. Cutis. 2005;75:357-363.
15. Trumbore MW, Goldstein JA, Gurge RM. Treatment of papulopustular rosacea with sodium sulfacetamide 10%/sulfur 5% emollient foam. J Drugs Dermatol. 2009;8:299-304.
16. Piwnica D, Rosignoli C, de Menonville ST, et al. Vasoconstriction and anti-inflammatory properties of the selective alpha-adrenergic receptor agonist brimonidine. J Dermatol Sci. 2014;75:49-54.
17. Fowler J Jr, Jackson M, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12:650-656.
18. Fowler J, Jarratt M, Moore A, et al. Once-daily topical brimonidine tartrate gel 0.5% is a novel treatment of moderate to severe facial erythema of rosacea: results of two multicenter, randomized and vehicle-controlled studies. Br J Dermatol. 2012;166:633-641.
19. Moore A, Kempers S, Murakawa G, et al. Long-term safety and efficacy of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of a 1-year open-label study. J Drugs Dermatol. 2014;13:56-61.
20. Routt ET, Levitt JO. Rebound erythema and burning sensation from a new topical brimonidine tartrate gel 0.33%. J Am Acad Dermatol. 2014;70:e37-e38.
21. Ilkovitch D, Pomerantz RG. Brimonidine effective but may lead to significant rebound erythema. J Am Acad Dermatol. 2014;70:e109-e110.
22. Kim JH, Oh YS, Ji JH, et al. Rosacea (erythematotelangiectatic type) effectively improved by topical xylometazoline. J Dermatol. 2011;38:510-513.
23. Shanler SD, Ondo AL. Successful treatment of erythema and flushing of rosacea using a topically applied selective alpha1-adrenergic receptor antagonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
24. Stein-Gold L, Kircik L, Fowler J, et al. Efficacy and safety of ivermectin 1% cream in treatment of papulopustular rosacea: results of two randomized, double-blind, vehicle-controlled pivotal studies. J Drugs Dermatol. 2014;13:316-323.
25. Stein-Gold L, Kircik L, Fowler J, et al. Long-term safety of ivermectin 1% cream vs azelaic acid 15% gel in treating inflammatory lesions of rosacea: results of two 40-week controlled, investigator-blinded trials. J Drugs Dermatol. 2014;13:1380-1386.
26. Taieb A, Ortonne JP, Ruzicka T, et al. Superiority of ivermectin 1% cream over metronidazole 0.75% cream in treating inflammatory lesions of rosacea: a randomized, investigator-blinded trial. Br J Dermatol. 2015;172:1103-1110.
27. Koçak M, Ya˘gli S, Vahapo˘glu G, et al. Permethrin 5% cream versus metronidazole 0.75% gel for the treatment of papulopustular rosacea. a randomized double-blind placebo-controlled study. Dermatology (Basel). 2002;205:265-270.
28. Bikowski JB, Del Rosso JQ. Demodex dermatitis: a retrospective analysis of clinical diagnosis and successful treatment with topical crotamiton. J Clin Aesthet Dermatol. 2009;2:20-25.
29. Layton A, Thiboutot D. Emerging therapies in rosacea. J Am Acad Dermatol. 2013;69(6 suppl 1):S57-S65.
30. Pelle MT, Crawford GH, James WD. Rosacea: II. therapy. J Am Acad Dermatol. 2004;51:499-512, quiz 513-514.
31. Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006;54:258-265.
32. Korting HC, Schöllmann C. Tetracycline actions relevant to rosacea treatment. Skin Pharmacol Physiol. 2009;22:287-294.
33. Thomas J, Walker C, Bradshaw M. Long-term use of subantimicrobial dose doxycycline does not lead to changes in antimicrobial susceptibility. J Periodontol. 2000;71:1472-1483.
34. Del Rosso JQ, Webster GF, Jackson M, et al. Two randomized phase III clinical trials evaluating anti-inflammatory dose doxycycline (40-mg doxycycline, USP capsules) administered once daily for treatment of rosacea. J Am Acad Dermatol. 2007;56:791-802.
35. Webster GF. An open-label, community-based, 12-week assessment of the effectiveness and safety of monotherapy with doxycycline 40 mg (30-mg immediate-release and 10-mg delayed-release beads). Cutis. 2010;86(suppl 5):7-15.
36. Del Rosso JQ, Schlessinger J, Werschler P. Comparison of anti-inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. J Drugs Dermatol. 2008;7:573-576.
37. Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 3: a status report on systemic therapies. Cutis. 2014;93:18-28.
38. Torresani C. Clarithromycin: a new perspective in rosacea treatment. Int J Dermatol. 1998;37:347-349.
39. Bakar O, Demircay Z, Gürbüz O. Therapeutic potential of azithromycin in rosacea. Int J Dermatol. 2004;43:151-154.
40. Saihan EM, Burton JL. A double-blind trial of metronidazole versus oxytetracycline therapy for rosacea. Br J Dermatol. 1980;102:443-445.
41. Park H, Del Rosso JQ. Use of oral isotretinoin in the management of rosacea. J Clin Aesthet Dermatol. 2011;4:54-61.
42. Gollnick H, Blume-Peytavi U, Szabo EL, et al. Systemic isotretinoin in the treatment of rosacea—doxycycline-and placebo-controlled, randomized clinical study. J Dtsch Dermatol Ges. 2010;8:505-515.
43. Hofer T. Continuous “microdose” isotretinoin in adult recalcitrant rosacea. Clin Exp Dermatol. 2004;29:204-205.
44. Tanghetti E, Del Rosso JQ, Thiboutot D, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 4: a status report on physical modalities and devices. Cutis. 2014;93:71-76.
45. Alam M, Dover JS, Arndt KA. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol Surg. 2003;29:681-684.
46. Uebelhoer NS, Bogle MA, Stewart B, et al. A split-face comparison study of pulsed 532-nm KTP laser and 595-nm pulsed dye laser in the treatment of facial telangiectasias and diffuse telangiectatic facial erythema. Dermatol Surg. 2007;33:441-448.
47. Alam M, Voravutinon N, Warycha M, et al. Comparative effectiveness of nonpurpuragenic 595-nm pulsed dye laser and microsecond 1064-nm neodymium:yttrium-aluminum-garnet laser for treatment of diffuse facial erythema: a double-blind randomized controlled trial. J Am Acad Dermatol. 2013;69:438-443.
48. Neuhaus IM, Zane LT, Tope WD. Comparative efficacy of nonpurpuragenic pulsed dye laser and intense pulsed light for erythematotelangiectatic rosacea. Dermatol Surg. 2009;35:920-928.
49. Angermeier MC. Treatment of facial vascular lesions with intense pulsed light. J Cutan Laser Ther. 1999;1:95-100.
50. Bryld LE, Jemec GB. Photodynamic therapy in a series of rosacea patients. J Eur Acad Dermatol Venereol. 2007;21:1199-1202.
51. Maden V, Ferguson JE, August PJ. Carbon dioxide laser treatment of rhinophyma: a review of 124 patients. Br J Dermatol. 2009;161:814-818.
52. Fincher EF, Gladstone HB. Use of a dual-mode erbium:YAG laser for the surgical correction of rhinophyma. Arch Facial Plast Surg. 2004;6:267-271.
53. Lloyd KM. Surgical correction of rhinophyma. Arch Dermatol. 1990;126:721-723.
54. Dayan SH, Pritzker RN, Arkins JP. A new treatment regimen for rosacea: onabotulinumtoxinA. J Drugs Dermatol. 2012;11:e76-e79.
55. Park KY, Hyun MY, Jeong SY, et al. Botulinum toxin for the treatment of refractory erythema and flushing of rosacea. Dermatology. 2015;230:299-301.
1. Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6 suppl 1):S27-S35.
2. Rosacea prevalence map. National Rosacea Society Web site. http://rosacea.org/press/prevalencemap. Accessed June 16, 2015.
3. Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 suppl 1):S15-S26.
4. Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 1: a status report on the disease state, general measures, and adjunctive skin care. Cutis. 2013;92:234-240.
5. Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004;50:907-912.
6. Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society expert committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
7. Feldman SR, Huang WW, Huynh TT. Current drug therapies for rosacea: a chronic vascular and inflammatory skin disease. J Manag Care Spec Pharm. 2014;20:623-629.
8. Moustafa F, Lewallen RS, Feldman SR. The psychological impact of rosacea and the influence of current management options. J Am Acad Dermatol. 2014;71:973-980.
9. Mansouri Y, Goldenberg G. Devices and topical agents for rosacea management. Cutis. 2014;94:21-25.
10. Levin J, Miller R. A guide to the ingredients and potential benefits of over-the-counter cleansers and moisturizers for rosacea patients. J Clin Aesthet Dermatol. 2011;4:31-49.
11. van Zuuren EJ, Kramer S, Carter B, et al. Interventions for rosacea. Cochrane Database Syst Rev. 2011;3:CD003262.
12. Liu RH, Smith MK, Basta SA, et al. Azelaic acid in the treatment of papulopustular rosacea: a systematic review of randomized controlled trials. Arch Dermatol. 2006;142:1047-1052.
13. Elewski B, Fleischer AB Jr, Pariser DM. A comparison of 15% azelaic acid gel and 0.75% metronidazole gel in the topical treatment of papulopustular rosacea: results of a randomized trial. Arch Dermatol. 2003;139:1444-1450.
14. Torok HM, Webster G, Dunlap FE, et al. Combination sodium sulfacetamide 10% and sulfur 5% cream with sunscreens versus metronidazole 0.75% cream for rosacea. Cutis. 2005;75:357-363.
15. Trumbore MW, Goldstein JA, Gurge RM. Treatment of papulopustular rosacea with sodium sulfacetamide 10%/sulfur 5% emollient foam. J Drugs Dermatol. 2009;8:299-304.
16. Piwnica D, Rosignoli C, de Menonville ST, et al. Vasoconstriction and anti-inflammatory properties of the selective alpha-adrenergic receptor agonist brimonidine. J Dermatol Sci. 2014;75:49-54.
17. Fowler J Jr, Jackson M, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12:650-656.
18. Fowler J, Jarratt M, Moore A, et al. Once-daily topical brimonidine tartrate gel 0.5% is a novel treatment of moderate to severe facial erythema of rosacea: results of two multicenter, randomized and vehicle-controlled studies. Br J Dermatol. 2012;166:633-641.
19. Moore A, Kempers S, Murakawa G, et al. Long-term safety and efficacy of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of a 1-year open-label study. J Drugs Dermatol. 2014;13:56-61.
20. Routt ET, Levitt JO. Rebound erythema and burning sensation from a new topical brimonidine tartrate gel 0.33%. J Am Acad Dermatol. 2014;70:e37-e38.
21. Ilkovitch D, Pomerantz RG. Brimonidine effective but may lead to significant rebound erythema. J Am Acad Dermatol. 2014;70:e109-e110.
22. Kim JH, Oh YS, Ji JH, et al. Rosacea (erythematotelangiectatic type) effectively improved by topical xylometazoline. J Dermatol. 2011;38:510-513.
23. Shanler SD, Ondo AL. Successful treatment of erythema and flushing of rosacea using a topically applied selective alpha1-adrenergic receptor antagonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
24. Stein-Gold L, Kircik L, Fowler J, et al. Efficacy and safety of ivermectin 1% cream in treatment of papulopustular rosacea: results of two randomized, double-blind, vehicle-controlled pivotal studies. J Drugs Dermatol. 2014;13:316-323.
25. Stein-Gold L, Kircik L, Fowler J, et al. Long-term safety of ivermectin 1% cream vs azelaic acid 15% gel in treating inflammatory lesions of rosacea: results of two 40-week controlled, investigator-blinded trials. J Drugs Dermatol. 2014;13:1380-1386.
26. Taieb A, Ortonne JP, Ruzicka T, et al. Superiority of ivermectin 1% cream over metronidazole 0.75% cream in treating inflammatory lesions of rosacea: a randomized, investigator-blinded trial. Br J Dermatol. 2015;172:1103-1110.
27. Koçak M, Ya˘gli S, Vahapo˘glu G, et al. Permethrin 5% cream versus metronidazole 0.75% gel for the treatment of papulopustular rosacea. a randomized double-blind placebo-controlled study. Dermatology (Basel). 2002;205:265-270.
28. Bikowski JB, Del Rosso JQ. Demodex dermatitis: a retrospective analysis of clinical diagnosis and successful treatment with topical crotamiton. J Clin Aesthet Dermatol. 2009;2:20-25.
29. Layton A, Thiboutot D. Emerging therapies in rosacea. J Am Acad Dermatol. 2013;69(6 suppl 1):S57-S65.
30. Pelle MT, Crawford GH, James WD. Rosacea: II. therapy. J Am Acad Dermatol. 2004;51:499-512, quiz 513-514.
31. Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006;54:258-265.
32. Korting HC, Schöllmann C. Tetracycline actions relevant to rosacea treatment. Skin Pharmacol Physiol. 2009;22:287-294.
33. Thomas J, Walker C, Bradshaw M. Long-term use of subantimicrobial dose doxycycline does not lead to changes in antimicrobial susceptibility. J Periodontol. 2000;71:1472-1483.
34. Del Rosso JQ, Webster GF, Jackson M, et al. Two randomized phase III clinical trials evaluating anti-inflammatory dose doxycycline (40-mg doxycycline, USP capsules) administered once daily for treatment of rosacea. J Am Acad Dermatol. 2007;56:791-802.
35. Webster GF. An open-label, community-based, 12-week assessment of the effectiveness and safety of monotherapy with doxycycline 40 mg (30-mg immediate-release and 10-mg delayed-release beads). Cutis. 2010;86(suppl 5):7-15.
36. Del Rosso JQ, Schlessinger J, Werschler P. Comparison of anti-inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. J Drugs Dermatol. 2008;7:573-576.
37. Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 3: a status report on systemic therapies. Cutis. 2014;93:18-28.
38. Torresani C. Clarithromycin: a new perspective in rosacea treatment. Int J Dermatol. 1998;37:347-349.
39. Bakar O, Demircay Z, Gürbüz O. Therapeutic potential of azithromycin in rosacea. Int J Dermatol. 2004;43:151-154.
40. Saihan EM, Burton JL. A double-blind trial of metronidazole versus oxytetracycline therapy for rosacea. Br J Dermatol. 1980;102:443-445.
41. Park H, Del Rosso JQ. Use of oral isotretinoin in the management of rosacea. J Clin Aesthet Dermatol. 2011;4:54-61.
42. Gollnick H, Blume-Peytavi U, Szabo EL, et al. Systemic isotretinoin in the treatment of rosacea—doxycycline-and placebo-controlled, randomized clinical study. J Dtsch Dermatol Ges. 2010;8:505-515.
43. Hofer T. Continuous “microdose” isotretinoin in adult recalcitrant rosacea. Clin Exp Dermatol. 2004;29:204-205.
44. Tanghetti E, Del Rosso JQ, Thiboutot D, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 4: a status report on physical modalities and devices. Cutis. 2014;93:71-76.
45. Alam M, Dover JS, Arndt KA. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol Surg. 2003;29:681-684.
46. Uebelhoer NS, Bogle MA, Stewart B, et al. A split-face comparison study of pulsed 532-nm KTP laser and 595-nm pulsed dye laser in the treatment of facial telangiectasias and diffuse telangiectatic facial erythema. Dermatol Surg. 2007;33:441-448.
47. Alam M, Voravutinon N, Warycha M, et al. Comparative effectiveness of nonpurpuragenic 595-nm pulsed dye laser and microsecond 1064-nm neodymium:yttrium-aluminum-garnet laser for treatment of diffuse facial erythema: a double-blind randomized controlled trial. J Am Acad Dermatol. 2013;69:438-443.
48. Neuhaus IM, Zane LT, Tope WD. Comparative efficacy of nonpurpuragenic pulsed dye laser and intense pulsed light for erythematotelangiectatic rosacea. Dermatol Surg. 2009;35:920-928.
49. Angermeier MC. Treatment of facial vascular lesions with intense pulsed light. J Cutan Laser Ther. 1999;1:95-100.
50. Bryld LE, Jemec GB. Photodynamic therapy in a series of rosacea patients. J Eur Acad Dermatol Venereol. 2007;21:1199-1202.
51. Maden V, Ferguson JE, August PJ. Carbon dioxide laser treatment of rhinophyma: a review of 124 patients. Br J Dermatol. 2009;161:814-818.
52. Fincher EF, Gladstone HB. Use of a dual-mode erbium:YAG laser for the surgical correction of rhinophyma. Arch Facial Plast Surg. 2004;6:267-271.
53. Lloyd KM. Surgical correction of rhinophyma. Arch Dermatol. 1990;126:721-723.
54. Dayan SH, Pritzker RN, Arkins JP. A new treatment regimen for rosacea: onabotulinumtoxinA. J Drugs Dermatol. 2012;11:e76-e79.
55. Park KY, Hyun MY, Jeong SY, et al. Botulinum toxin for the treatment of refractory erythema and flushing of rosacea. Dermatology. 2015;230:299-301.
Practice Points
- As no definitive cure for rosacea exists, effective treatment is aimed at improving the cosmetic symptoms.
- Choice of therapy should be determined on a case-by-case basis as guided by the clinical features most bothersome to the patient.
- A combination of modalities and/or sequential therapy often is required to achieve optimal cosmetic outcomes.
Acne Scarring: A Review of Cosmetic Therapies
Acne vulgaris is one of the most common inflammatory dermatoses affecting nearly all adolescents and a large proportion of adults.1 Incidence rates trend downward with age, but prevalence has been reported to be as high as 51% in individuals aged 20 to 29 years.2 Notably, recent evidence suggests there is an increasing incidence rate of acne among postadolescent women, with the severity associated with the menstrual cycle.3,4 Scarring is a common result of acne and may even occur in the setting of appropriate medical therapy. In particular, some form of facial scarring has been reported to occur in up to 95% of acne patients, with severe scarring in 30% of these patients.5 The detrimental effects of acne scarring are not only limited to impaired cosmetic appearance, as it also has been associated with depression symptoms, suicidal ideation, mental health problems, and general social impairment.6 Given the negative impact of acne scarring on overall health and well-being as well as its permanent nature, early and effective treatment is essential to maximize cosmetic outcomes and minimize long-term deleterious effects.
Acne scarring can be broadly divided into 2 major categories: atrophic and hypertrophic. Atrophic scarring is more common and is characterized by an overall localized reduction in collagen content. Clinically, atrophic scars present as depressions in the skin secondary to inflammatory fibrous contractions induced by acne. This type of scarring can be further divided into various subtypes based on morphologic criteria (eg, size, depth), such as boxcar, ice pick, and rolling scars.7 Conversely, hypertrophic scarring is characterized by an overall increase in collagen content and presents as firm raised lesions. Hypertrophic scars should be distinguished from keloid scars, as the former will not outgrow the margins of the original wound while the latter will.8 Treatment of acne scarring is based on scar type and can be accomplished through a variety of medical and surgical modalities (Table). In this article, we review some of the most commonly utilized therapies for both atrophic and hypertrophic acne scarring with a focus on cosmetic outcomes. It is important to keep in mind, however, that the best treatment is to prevent the occurrence of acne scarring through early and proactive treatment of acne.9
Dermabrasion
Dermabrasion is a decades-old technique that employs the use of a motorized device equipped with an abrasive material to physically remove the superficial layers of the skin, thus inducing the wound-healing process with subsequent formation of new collagen.10 In the same vein, microdermabrasion utilizes aluminum oxide crystals ejected from a nozzle to induce superficial microlacerations.11 This technique is most successful when used to soften scar edges in superficial atrophic scars of the rolling or boxcar subtypes.12 Dermabrasion has been shown to be equally as effective as laser therapy in the treatment of facial scars but is reported to have a much greater risk for adverse effects (AEs)(eg, erythema, edema) that may last for several weeks posttherapy.13,14 Dermabrasion is a particularly operator-dependent technique for which outcomes may vary depending on operator experience. As such, it is not generally recommended as a first-line therapy given its risks and relatively modest results; however, dermabrasion can be a useful adjunct when performed in the right setting. This technique, in addition to laser resurfacing, should be used with caution in patients who have recently taken or currently are taking isotretinoin, as several case series have reported postprocedural development of hypertrophic or keloid scars,15-17 but these findings subsequently were questioned in the literature.18
Laser Therapy
Laser technology has advanced tremendously over the last few decades and there are now a multitude of available lasers that are capable of variable depth penetration and energy delivery patterns. Common to all, however, is the ability to induce localized thermal damage with eventual collagen remodeling. Lasers can be divided into 2 major categories: ablative and nonablative. Ablative lasers cause epidermal destruction, while nonablative lasers are able to selectively target dermal layers without disrupting the overlying epithelium. Generally speaking, ablative lasers are more effective than nonablative lasers in the treatment of atrophic scars, with reported mean improvements of up to 81%.19 This increased efficacy comes with an increased risk for AEs such as postinflammatory hyperpigmentation, prolonged posttreatment erythema, and formation of additional scarring.20 Both ablative and nonablative lasers can be applied in the more recently developed technology of fractional photothermolysis. With this method, noncontiguous microscopic columns of thermal injury surrounded by zones of viable tissue are created, which is in contrast to the traditional manner of inducing broad thermal injury. Fractional ablative lasers can achieve efficacy rates similar to traditional ablative lasers with a reduced risk for permanent scarring or dispigmentation.21 Notably, recent studies have shown promising results for the use of fractional ablative lasers as a mechanism to enhance drug delivery of topically applied medications such as poly-L-lactic acid and triamcinolone acetonide in the treatment of atrophic and hypertrophic scars, respectively.22,23
Lasers also play a role in the treatment of hypertrophic acne scars with the use of nonablative pulsed dye lasers. These lasers cause selective thermolysis of dermal vasculature, and average clinical improvements in hypertrophic scars of 67.5% after a single treatment have been reported.24 Temporary postoperative purpura and long-term hyperpigmentation are reported outcomes of this therapy.20
Radiofrequency
Nonablative radiofrequency (RF) is a relatively novel technique that creates an electric current in the dermis at preset depths to induce thermal damage and eventual collagen synthesis. There are a variety of modalities for which RF can be applied, but microneedle bipolar RF and fractional bipolar RF treatments offer the best results for atrophic acne scars. Improvements in scar appearance of 25% to 75% have been reported after several treatment sessions.25 Better results have been reported in the treatment of ice pick scars as compared to more superficial scars,26 but additional studies will be necessary to validate this claim. Adverse effects are largely limited to temporary erythema and posttreatment scabbing.27
Subcision
Subcision is a more physically intensive technique useful for treatment of superficial atrophic acne scars. This method involves the use of a small needle that is inserted into the periphery of a scar before being moved in a back-and-forth manner underneath the base of the scar to loosen the fibrotic adhesions that result in the depressed appearance of the scar. Additionally, loosening of the tissue and resultant bleeding creates a potential space for future collagen deposition during the subsequent wound-healing phase. Subcision has a reported success rate of 50% to 60% in the treatment of rolling scars, and prospective, randomized, split-face trials have indicated that the short-term outcomes of subcision are superior to dermal fillers while being equally effective long-term.28,29 Of note, a small percentage of patients may develop a localized nodule at the site of treatment, which can be resolved with intralesional steroids.11
Skin Needling
Skin needling, also referred to as collagen induction therapy, utilizes vertical needle punctures rather than the horizontally directed punctures that are used in subcision and can be used to treat rolling and boxcar scars. Traditionally, a small roller equipped with rows of small needles typically ranging in size from 0.5 to 3.0 mm in length is passed over the skin using gentle pressure, puncturing the superficial layers of the skin to loosen fibrotic adhesions and induce collagen synthesis. This procedure may be repeated several times within a single session or over multiple sessions depending on the depth and quality of the scars. This technique has been reported to reduce scar depth up to 25% after 2 sessions.30
Punch Techniques
Punch techniques are useful for treatment of deeper atrophic acne scarring, for which most other treatment modalities are not particularly effective. A punch excision approximately equal to the scar size is first performed, which may then be followed by either removal of the scar tissue with subsequent suturing, graft replacement of the removed tissue, or elevation of the already established scar tissue to the level of surrounding skin where it is then held in place by sutures or adhesive skin closure material. Success rates with this method are largely limited to case series, but punch techniques are reported to be efficacious, especially for treatment of ice pick scars. Risks for this method include graft failure, graft depression, and formation of sinus tracts.31
Chemical Peels
Chemicals peels traditionally employ the use of acidic compounds to strip away the outer layers of skin to variable depths depending on the concentration of the agent being applied. Chemical peels are not generally recommended for application in a nonspecific manner in the treatment of acne scars given the relatively mild cosmetic improvements seen and the high rate of AEs such as pigmentary alterations and additional scar formation.12 Rather, clinicians should employ the CROSS (chemical reconstruction of skin scars) technique, in which peel agents such as trichloroacetic acid are applied in high concentrations only to areas of atrophic scarring. Use of this method can minimize AEs while simultaneously achieving high success rates, with excellent results in 100% (32/32) of patients after 5 to 6 treatment sessions.32 This method has been successful for hard-to-treat ice pick scars.33
Soft-Tissue Augmentation
Soft-tissue augmentation is another effective treatment of superficial atrophic acne scarring that utilizes injections of collagen fillers such as hyaluronic acid, calcium hydroxylapatite, poly-L-lactic acid, silicone, and even autologous fat to replace lost tissue volume while simultaneously inducing collagen production via stretching of dermal fibroblasts.34 These treatments may require multiple sessions for cosmetic improvement but have shown considerable efficacy in the treatment of atrophic acne scars. Hyaluronic acid has been reported to be particularly effective for rolling scars.12 However, these compounds only provide temporary results, thus requiring repeated treatments to maintain cosmetic outcomes. Permanent options include the recently US Food and Drug Administration–approved polymethylmethacrylate microspheres suspended in bovine collagen as well as the novel technique of autologous fibroblast transfer. These options are relatively new, but initial double-blind, randomized, controlled trials have shown minimal AEs with substantial improvements in 64% to 100% of atrophic scars treated.35,36
Intralesional Therapy
Intralesional corticosteroid injections are a mainstay treatment of hypertrophic acne scarring and are believed to exert their effects by decreasing fibroblast proliferation and promoting collagen degradation.37 Treatment with steroids generally is effective, with reported improvement in 75% (6/8) of patients and complete flattening in 50% (4/8) of lesions according to one study.38 Development of hypopigmentation, dermal atrophy, and telangiectasia are potential sequelae of this treatment.37
5-Fluorouracil, bleomycin, and verapamil also have been used with good results as intralesional treatments of hypertrophic scars, but these agents typically are reserved for cases of corticosteroid failure. Such compounds are thought to mediate their effects through inhibition of dermal fibroblast proliferation.39 Results with these therapies are varied, but greater than 75% improvement is seen in most cases. Adverse effects include injection-site ulceration and hyperpigmentation.39
Cryotherapy
Contact cryotherapy has been studied as treatment of hypertrophic acne scars. The exact mechanism through which scars are reduced is unclear, but it is hypothesized that the physical damage caused by freezing and thrombosis lead to collagen restructuring. According to one study, cryotherapy was reported to achieve good or excellent results in 76% (29/38) of cases.40 Permanent pigmentary alterations are a possible AE.
Silicone Dressings
Silicone dressings are a reasonable treatment option for hypertrophic acne scarring given their proven efficacy and minimal risk for AEs. Thin sheets of silicone gels or membranes are applied daily in a topical manner to acne scars and are believed to be therapeutic through a combination of pressure and hydration, which subsequently inhibits fibroblast production of collagen. Notable reductions in scar appearance and size are seen in 60% to 80% of individuals using this method.41 Adverse effects are limited to pruritus and local skin maceration. Patient noncompliance may be an issue, as the silicone dressings may be applied on highly visible areas such as the face. Patients may apply the dressings at night, but efficacy may be reduced.
Conclusion
When determining which treatment options to use in a patient with acne scarring, it is important to first determine the patient’s treatment goals while simultaneously establishing realistic expectations. Important factors to consider are the patient’s preferences regarding treatment risk, duration, and permanence, as well as budget and social or work requirements. As such, treatment plans for each patient should be determined on a case-by-case basis. It also is important to note that a combination of different treatment modalities often is necessary and superior to monotherapy in achieving satisfactory cosmetic outcomes.
1. Ghodsi SZ, Orawa H, Zouboulis CC. Prevalence, severity, and severity risk factors of acne in high school pupils: a community-based study. J Invest Dermatol. 2009;129:2136-2141.
2. Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59.
3. Kim GK, Michaels BB. Post-adolescent acne in women: more common and more clinical considerations. J Drugs Dermatol. 2012;11:708-713.
4. Geller L, Rosen J, Frankel A, et al. Perimenstrual flare of adult acne. J Clin Aesthet Dermatol. 2014;7:30-34.
5. Layton AM, Henderson CA, Cunliffe WJ. A clinical evaluation of acne scarring and its incidence. Clin Exp Dermatol. 1994;19:303-308.
6. Halvorsen JA, Stern RS, Dalgard F, et al. Suicidal ideation, mental health problems, and social impairment are increased in adolescents with acne: a population-based study. J Invest Dermatol. 2011;131:363-370.
7. Jacob CI, Dover JS, Kaminer MS. Acne scarring: a classification system and review of treatment options. J Am Acad Dermatol. 2001;45:109-117.
8. Rivera AE. Acne scarring: a review and current treatment modalities. J Am Acad Dermatol. 2008;59:659-676.
9. Goodman GJ. Acne and acne scarring: why should we treat? Med J Aust. 1999;171:62-63.
10. Frank W. Therapeutic dermabrasion. back to the future. Arch Dermatol. 1994;130:1187-1189.
11. Goodman GJ. Postacne scarring: a review of its pathophysiology and treatment. Dermatol Surg. 2000;26:857-871.
12. Hession MT, Graber EM. Atrophic acne scarring: a review of treatment options. J Clin Aesthet Dermatol. 2015;8:50-58.
13. Levy LL, Zeichner JA. Management of acne scarring, part II: a comparative review of non-laser-based, minimally invasive approaches. Am J Clin Dermatol. 2012;13:331-340.
14. Christophel JJ, Elm C, Endrizzi BT, et al. A randomized controlled trial of fractional laser therapy and dermabrasion for scar resurfacing. Dermatol Surg. 2012;38:595-602.
15. Katz BE, McFarlane DF. Atypical facial scarring after isotretinoin therapy in a patient with previous dermabrasion. J Am Acad Dermatol. 1994;30:852-853.
16. Bernestein LJ, Geronemus RG. Keloid formation with the 585-nm pulsed dye laser during isotretinoin treatment. Arch Dermatol. 1997;133:111-112.
17. Zachariae H. Delayed wound healing and keloid formation following argon laser treatment or dermabrasion during isotretinoin treatment. Br J Dermatol. 1988;118:703-706.
18. Wootton CI, Cartwright RP, Manning P, et al. Should isotretinoin be stopped prior to surgery? a critically appraised topic. Br J Dermatol. 2014;170:239-244.
19. Alster TS, West TB. Resurfacing of atrophic facial acne scars with a high-energy, pulsed carbon dioxide laser. Dermatol Surg. 1996;22:151-155.
20. Sobanko JF, Alster TS. Management of acne scarring, part I: a comparative review of laser surgical approaches. Am J Clin Dermatol. 2012;13:319-330.
21. Cho SB, Lee SJ, Oh SH, et al. Non-ablative 1550nm erbium-glass and ablative 10,600nm carbon dioxide fractional lasers for acne scar: a randomized split-face study with blinded response evaluation. J Eur Acad Dermatol Venereol. 2010;24:921-925.
22. Rkein A, Ozog D, Waibel JS. Treatment of atrophic scars with fractionated CO2 laser facilitating delivery of topically applied poly-L-lactic acid. Dermatol Surg. 2014;40:624-631.
23. Waibel JS, Wulkan AJ, Shumaker PR. Treatment of hypertrophic scars using laser and laser assisted corticosteroid delivery. Lasers Surg Med. 2013;45:135-140.
24. Alster TS, McMeekin TO. Improvement of facial acne scars by the 585-nm flashlamp-pumped pulsed dye laser. J Am Acad Dermatol. 1996;35:79-81.
25. Simmons BJ, Griffith RD, Falto-Aizpurua LA, et al. Use of radiofrequency in cosmetic dermatology: focus on nonablative treatment of acne scars. Clin Cosmet Investig Dermatol. 2014;7:335-339.
26. Ramesh M, Gopal M, Kumar S, et al. Novel technology in the treatment of acne scars: the matrix-tunable radiofrequency technology. J Cutan Aesthet Surg. 2010;3:97-101.
27. Johnson WC. Treatment of pitted scars; punch transplant technique. J Dermatol Surg Oncol. 1986;12:260-265.
28. Alam M, Omura N, Kaminer MS. Subcision for acne scarring: technique and outcomes in 40 patients. Dermatol Surg. 2005;31:310-317.
29. Sage R, Lopiccolo M, Liu A, et al. Subcuticular incision versus naturally sourced porcine collagen filler for acne scars: a randomized split-face comparison. Dermatol Surg. 2011;37:426-431.
30. Fabbrocini G, Annunziata MC, D’arco V, et al. Acne scars: pathogenesis, classification and treatment [published online ahead of print October 14, 2010]. Dermatol Res Pract. 2010;2010:893080.
31. Johnson WC. Treatment of pitted scars: punch transplant technique. J Dermatol Surg Oncol. 1986;12:260-265.
32. Lee JB, Chung WG, Kwahck H, et al. Focal treatment of acne scars with trichloroacetic acid: chemical reconstruction of skin scars method. Dermatol Surg. 2002;28:1017-1021.
33. Bhardwaj D, Khunger N. An assessment of the efficacy and safety of CROSS technique with 100% TCA in the management of ice pick acne scars. J Cutan Aesthet Surg. 2010;3:93-96.
34. Wang F, Garza LA, Kang S, et al. In vivo stimulation of de novo collagen production caused by cross-linked hyaluronic acid dermal filler injections in photodamaged human skin. Arch Dermatol. 2007;143:155-163.
35. Karnik J, Baumann L, Bruce S, et al. A double-blind, randomized, multicenter, controlled trial of suspended polymethylmethacrylate microspheres for the correction of atrophic facial acne scars. J Am Acad Dermatol. 2014;71:77-83.
36. Munavalli GS, Smith S, Maslowski JM, et al. Successful treatment of depressed, distensible acne scars using autologous fibroblasts: a multi-site, prospective, double blind, placebo-controlled clinical trial. Dermatol Surg. 2013;39:1226-1236.
37. Leventhal D, Furr M, Reiter D. Treatment of keloids and hypertrophic scars: a meta-analysis and review of the literature. Arch Facial Plast Surg. 2006;8:362-368.
38. Darzi MA, Chowdri NA, Kaul SK, et al. Evaluation of various methods of treating keloids and hypertrophic scars: a 10-year follow-up study. Br J Plast Surg. 1992;45:374-379.
39. Ledon JA, Savas J, Franca K, et al. Intralesional treatment for keloids and hypertrophic scars: a review. Dermatol Surg. 2013;39:1745-1757.
40. Zouboulis CC, Blume U, Büttner P, et al. Outcomes of cryosurgery in keloids and hypertrophic scars. a prospective consecutive trial of case series. Arch Dermatol. 1993;129:1146-1151.
41. Puri N, Talwar A. The efficacy of silicone gel for the treatment of hypertrophic scars and keloids. J Cutan Aesthet Surg. 2009;2:104-106.
Acne vulgaris is one of the most common inflammatory dermatoses affecting nearly all adolescents and a large proportion of adults.1 Incidence rates trend downward with age, but prevalence has been reported to be as high as 51% in individuals aged 20 to 29 years.2 Notably, recent evidence suggests there is an increasing incidence rate of acne among postadolescent women, with the severity associated with the menstrual cycle.3,4 Scarring is a common result of acne and may even occur in the setting of appropriate medical therapy. In particular, some form of facial scarring has been reported to occur in up to 95% of acne patients, with severe scarring in 30% of these patients.5 The detrimental effects of acne scarring are not only limited to impaired cosmetic appearance, as it also has been associated with depression symptoms, suicidal ideation, mental health problems, and general social impairment.6 Given the negative impact of acne scarring on overall health and well-being as well as its permanent nature, early and effective treatment is essential to maximize cosmetic outcomes and minimize long-term deleterious effects.
Acne scarring can be broadly divided into 2 major categories: atrophic and hypertrophic. Atrophic scarring is more common and is characterized by an overall localized reduction in collagen content. Clinically, atrophic scars present as depressions in the skin secondary to inflammatory fibrous contractions induced by acne. This type of scarring can be further divided into various subtypes based on morphologic criteria (eg, size, depth), such as boxcar, ice pick, and rolling scars.7 Conversely, hypertrophic scarring is characterized by an overall increase in collagen content and presents as firm raised lesions. Hypertrophic scars should be distinguished from keloid scars, as the former will not outgrow the margins of the original wound while the latter will.8 Treatment of acne scarring is based on scar type and can be accomplished through a variety of medical and surgical modalities (Table). In this article, we review some of the most commonly utilized therapies for both atrophic and hypertrophic acne scarring with a focus on cosmetic outcomes. It is important to keep in mind, however, that the best treatment is to prevent the occurrence of acne scarring through early and proactive treatment of acne.9
Dermabrasion
Dermabrasion is a decades-old technique that employs the use of a motorized device equipped with an abrasive material to physically remove the superficial layers of the skin, thus inducing the wound-healing process with subsequent formation of new collagen.10 In the same vein, microdermabrasion utilizes aluminum oxide crystals ejected from a nozzle to induce superficial microlacerations.11 This technique is most successful when used to soften scar edges in superficial atrophic scars of the rolling or boxcar subtypes.12 Dermabrasion has been shown to be equally as effective as laser therapy in the treatment of facial scars but is reported to have a much greater risk for adverse effects (AEs)(eg, erythema, edema) that may last for several weeks posttherapy.13,14 Dermabrasion is a particularly operator-dependent technique for which outcomes may vary depending on operator experience. As such, it is not generally recommended as a first-line therapy given its risks and relatively modest results; however, dermabrasion can be a useful adjunct when performed in the right setting. This technique, in addition to laser resurfacing, should be used with caution in patients who have recently taken or currently are taking isotretinoin, as several case series have reported postprocedural development of hypertrophic or keloid scars,15-17 but these findings subsequently were questioned in the literature.18
Laser Therapy
Laser technology has advanced tremendously over the last few decades and there are now a multitude of available lasers that are capable of variable depth penetration and energy delivery patterns. Common to all, however, is the ability to induce localized thermal damage with eventual collagen remodeling. Lasers can be divided into 2 major categories: ablative and nonablative. Ablative lasers cause epidermal destruction, while nonablative lasers are able to selectively target dermal layers without disrupting the overlying epithelium. Generally speaking, ablative lasers are more effective than nonablative lasers in the treatment of atrophic scars, with reported mean improvements of up to 81%.19 This increased efficacy comes with an increased risk for AEs such as postinflammatory hyperpigmentation, prolonged posttreatment erythema, and formation of additional scarring.20 Both ablative and nonablative lasers can be applied in the more recently developed technology of fractional photothermolysis. With this method, noncontiguous microscopic columns of thermal injury surrounded by zones of viable tissue are created, which is in contrast to the traditional manner of inducing broad thermal injury. Fractional ablative lasers can achieve efficacy rates similar to traditional ablative lasers with a reduced risk for permanent scarring or dispigmentation.21 Notably, recent studies have shown promising results for the use of fractional ablative lasers as a mechanism to enhance drug delivery of topically applied medications such as poly-L-lactic acid and triamcinolone acetonide in the treatment of atrophic and hypertrophic scars, respectively.22,23
Lasers also play a role in the treatment of hypertrophic acne scars with the use of nonablative pulsed dye lasers. These lasers cause selective thermolysis of dermal vasculature, and average clinical improvements in hypertrophic scars of 67.5% after a single treatment have been reported.24 Temporary postoperative purpura and long-term hyperpigmentation are reported outcomes of this therapy.20
Radiofrequency
Nonablative radiofrequency (RF) is a relatively novel technique that creates an electric current in the dermis at preset depths to induce thermal damage and eventual collagen synthesis. There are a variety of modalities for which RF can be applied, but microneedle bipolar RF and fractional bipolar RF treatments offer the best results for atrophic acne scars. Improvements in scar appearance of 25% to 75% have been reported after several treatment sessions.25 Better results have been reported in the treatment of ice pick scars as compared to more superficial scars,26 but additional studies will be necessary to validate this claim. Adverse effects are largely limited to temporary erythema and posttreatment scabbing.27
Subcision
Subcision is a more physically intensive technique useful for treatment of superficial atrophic acne scars. This method involves the use of a small needle that is inserted into the periphery of a scar before being moved in a back-and-forth manner underneath the base of the scar to loosen the fibrotic adhesions that result in the depressed appearance of the scar. Additionally, loosening of the tissue and resultant bleeding creates a potential space for future collagen deposition during the subsequent wound-healing phase. Subcision has a reported success rate of 50% to 60% in the treatment of rolling scars, and prospective, randomized, split-face trials have indicated that the short-term outcomes of subcision are superior to dermal fillers while being equally effective long-term.28,29 Of note, a small percentage of patients may develop a localized nodule at the site of treatment, which can be resolved with intralesional steroids.11
Skin Needling
Skin needling, also referred to as collagen induction therapy, utilizes vertical needle punctures rather than the horizontally directed punctures that are used in subcision and can be used to treat rolling and boxcar scars. Traditionally, a small roller equipped with rows of small needles typically ranging in size from 0.5 to 3.0 mm in length is passed over the skin using gentle pressure, puncturing the superficial layers of the skin to loosen fibrotic adhesions and induce collagen synthesis. This procedure may be repeated several times within a single session or over multiple sessions depending on the depth and quality of the scars. This technique has been reported to reduce scar depth up to 25% after 2 sessions.30
Punch Techniques
Punch techniques are useful for treatment of deeper atrophic acne scarring, for which most other treatment modalities are not particularly effective. A punch excision approximately equal to the scar size is first performed, which may then be followed by either removal of the scar tissue with subsequent suturing, graft replacement of the removed tissue, or elevation of the already established scar tissue to the level of surrounding skin where it is then held in place by sutures or adhesive skin closure material. Success rates with this method are largely limited to case series, but punch techniques are reported to be efficacious, especially for treatment of ice pick scars. Risks for this method include graft failure, graft depression, and formation of sinus tracts.31
Chemical Peels
Chemicals peels traditionally employ the use of acidic compounds to strip away the outer layers of skin to variable depths depending on the concentration of the agent being applied. Chemical peels are not generally recommended for application in a nonspecific manner in the treatment of acne scars given the relatively mild cosmetic improvements seen and the high rate of AEs such as pigmentary alterations and additional scar formation.12 Rather, clinicians should employ the CROSS (chemical reconstruction of skin scars) technique, in which peel agents such as trichloroacetic acid are applied in high concentrations only to areas of atrophic scarring. Use of this method can minimize AEs while simultaneously achieving high success rates, with excellent results in 100% (32/32) of patients after 5 to 6 treatment sessions.32 This method has been successful for hard-to-treat ice pick scars.33
Soft-Tissue Augmentation
Soft-tissue augmentation is another effective treatment of superficial atrophic acne scarring that utilizes injections of collagen fillers such as hyaluronic acid, calcium hydroxylapatite, poly-L-lactic acid, silicone, and even autologous fat to replace lost tissue volume while simultaneously inducing collagen production via stretching of dermal fibroblasts.34 These treatments may require multiple sessions for cosmetic improvement but have shown considerable efficacy in the treatment of atrophic acne scars. Hyaluronic acid has been reported to be particularly effective for rolling scars.12 However, these compounds only provide temporary results, thus requiring repeated treatments to maintain cosmetic outcomes. Permanent options include the recently US Food and Drug Administration–approved polymethylmethacrylate microspheres suspended in bovine collagen as well as the novel technique of autologous fibroblast transfer. These options are relatively new, but initial double-blind, randomized, controlled trials have shown minimal AEs with substantial improvements in 64% to 100% of atrophic scars treated.35,36
Intralesional Therapy
Intralesional corticosteroid injections are a mainstay treatment of hypertrophic acne scarring and are believed to exert their effects by decreasing fibroblast proliferation and promoting collagen degradation.37 Treatment with steroids generally is effective, with reported improvement in 75% (6/8) of patients and complete flattening in 50% (4/8) of lesions according to one study.38 Development of hypopigmentation, dermal atrophy, and telangiectasia are potential sequelae of this treatment.37
5-Fluorouracil, bleomycin, and verapamil also have been used with good results as intralesional treatments of hypertrophic scars, but these agents typically are reserved for cases of corticosteroid failure. Such compounds are thought to mediate their effects through inhibition of dermal fibroblast proliferation.39 Results with these therapies are varied, but greater than 75% improvement is seen in most cases. Adverse effects include injection-site ulceration and hyperpigmentation.39
Cryotherapy
Contact cryotherapy has been studied as treatment of hypertrophic acne scars. The exact mechanism through which scars are reduced is unclear, but it is hypothesized that the physical damage caused by freezing and thrombosis lead to collagen restructuring. According to one study, cryotherapy was reported to achieve good or excellent results in 76% (29/38) of cases.40 Permanent pigmentary alterations are a possible AE.
Silicone Dressings
Silicone dressings are a reasonable treatment option for hypertrophic acne scarring given their proven efficacy and minimal risk for AEs. Thin sheets of silicone gels or membranes are applied daily in a topical manner to acne scars and are believed to be therapeutic through a combination of pressure and hydration, which subsequently inhibits fibroblast production of collagen. Notable reductions in scar appearance and size are seen in 60% to 80% of individuals using this method.41 Adverse effects are limited to pruritus and local skin maceration. Patient noncompliance may be an issue, as the silicone dressings may be applied on highly visible areas such as the face. Patients may apply the dressings at night, but efficacy may be reduced.
Conclusion
When determining which treatment options to use in a patient with acne scarring, it is important to first determine the patient’s treatment goals while simultaneously establishing realistic expectations. Important factors to consider are the patient’s preferences regarding treatment risk, duration, and permanence, as well as budget and social or work requirements. As such, treatment plans for each patient should be determined on a case-by-case basis. It also is important to note that a combination of different treatment modalities often is necessary and superior to monotherapy in achieving satisfactory cosmetic outcomes.
Acne vulgaris is one of the most common inflammatory dermatoses affecting nearly all adolescents and a large proportion of adults.1 Incidence rates trend downward with age, but prevalence has been reported to be as high as 51% in individuals aged 20 to 29 years.2 Notably, recent evidence suggests there is an increasing incidence rate of acne among postadolescent women, with the severity associated with the menstrual cycle.3,4 Scarring is a common result of acne and may even occur in the setting of appropriate medical therapy. In particular, some form of facial scarring has been reported to occur in up to 95% of acne patients, with severe scarring in 30% of these patients.5 The detrimental effects of acne scarring are not only limited to impaired cosmetic appearance, as it also has been associated with depression symptoms, suicidal ideation, mental health problems, and general social impairment.6 Given the negative impact of acne scarring on overall health and well-being as well as its permanent nature, early and effective treatment is essential to maximize cosmetic outcomes and minimize long-term deleterious effects.
Acne scarring can be broadly divided into 2 major categories: atrophic and hypertrophic. Atrophic scarring is more common and is characterized by an overall localized reduction in collagen content. Clinically, atrophic scars present as depressions in the skin secondary to inflammatory fibrous contractions induced by acne. This type of scarring can be further divided into various subtypes based on morphologic criteria (eg, size, depth), such as boxcar, ice pick, and rolling scars.7 Conversely, hypertrophic scarring is characterized by an overall increase in collagen content and presents as firm raised lesions. Hypertrophic scars should be distinguished from keloid scars, as the former will not outgrow the margins of the original wound while the latter will.8 Treatment of acne scarring is based on scar type and can be accomplished through a variety of medical and surgical modalities (Table). In this article, we review some of the most commonly utilized therapies for both atrophic and hypertrophic acne scarring with a focus on cosmetic outcomes. It is important to keep in mind, however, that the best treatment is to prevent the occurrence of acne scarring through early and proactive treatment of acne.9
Dermabrasion
Dermabrasion is a decades-old technique that employs the use of a motorized device equipped with an abrasive material to physically remove the superficial layers of the skin, thus inducing the wound-healing process with subsequent formation of new collagen.10 In the same vein, microdermabrasion utilizes aluminum oxide crystals ejected from a nozzle to induce superficial microlacerations.11 This technique is most successful when used to soften scar edges in superficial atrophic scars of the rolling or boxcar subtypes.12 Dermabrasion has been shown to be equally as effective as laser therapy in the treatment of facial scars but is reported to have a much greater risk for adverse effects (AEs)(eg, erythema, edema) that may last for several weeks posttherapy.13,14 Dermabrasion is a particularly operator-dependent technique for which outcomes may vary depending on operator experience. As such, it is not generally recommended as a first-line therapy given its risks and relatively modest results; however, dermabrasion can be a useful adjunct when performed in the right setting. This technique, in addition to laser resurfacing, should be used with caution in patients who have recently taken or currently are taking isotretinoin, as several case series have reported postprocedural development of hypertrophic or keloid scars,15-17 but these findings subsequently were questioned in the literature.18
Laser Therapy
Laser technology has advanced tremendously over the last few decades and there are now a multitude of available lasers that are capable of variable depth penetration and energy delivery patterns. Common to all, however, is the ability to induce localized thermal damage with eventual collagen remodeling. Lasers can be divided into 2 major categories: ablative and nonablative. Ablative lasers cause epidermal destruction, while nonablative lasers are able to selectively target dermal layers without disrupting the overlying epithelium. Generally speaking, ablative lasers are more effective than nonablative lasers in the treatment of atrophic scars, with reported mean improvements of up to 81%.19 This increased efficacy comes with an increased risk for AEs such as postinflammatory hyperpigmentation, prolonged posttreatment erythema, and formation of additional scarring.20 Both ablative and nonablative lasers can be applied in the more recently developed technology of fractional photothermolysis. With this method, noncontiguous microscopic columns of thermal injury surrounded by zones of viable tissue are created, which is in contrast to the traditional manner of inducing broad thermal injury. Fractional ablative lasers can achieve efficacy rates similar to traditional ablative lasers with a reduced risk for permanent scarring or dispigmentation.21 Notably, recent studies have shown promising results for the use of fractional ablative lasers as a mechanism to enhance drug delivery of topically applied medications such as poly-L-lactic acid and triamcinolone acetonide in the treatment of atrophic and hypertrophic scars, respectively.22,23
Lasers also play a role in the treatment of hypertrophic acne scars with the use of nonablative pulsed dye lasers. These lasers cause selective thermolysis of dermal vasculature, and average clinical improvements in hypertrophic scars of 67.5% after a single treatment have been reported.24 Temporary postoperative purpura and long-term hyperpigmentation are reported outcomes of this therapy.20
Radiofrequency
Nonablative radiofrequency (RF) is a relatively novel technique that creates an electric current in the dermis at preset depths to induce thermal damage and eventual collagen synthesis. There are a variety of modalities for which RF can be applied, but microneedle bipolar RF and fractional bipolar RF treatments offer the best results for atrophic acne scars. Improvements in scar appearance of 25% to 75% have been reported after several treatment sessions.25 Better results have been reported in the treatment of ice pick scars as compared to more superficial scars,26 but additional studies will be necessary to validate this claim. Adverse effects are largely limited to temporary erythema and posttreatment scabbing.27
Subcision
Subcision is a more physically intensive technique useful for treatment of superficial atrophic acne scars. This method involves the use of a small needle that is inserted into the periphery of a scar before being moved in a back-and-forth manner underneath the base of the scar to loosen the fibrotic adhesions that result in the depressed appearance of the scar. Additionally, loosening of the tissue and resultant bleeding creates a potential space for future collagen deposition during the subsequent wound-healing phase. Subcision has a reported success rate of 50% to 60% in the treatment of rolling scars, and prospective, randomized, split-face trials have indicated that the short-term outcomes of subcision are superior to dermal fillers while being equally effective long-term.28,29 Of note, a small percentage of patients may develop a localized nodule at the site of treatment, which can be resolved with intralesional steroids.11
Skin Needling
Skin needling, also referred to as collagen induction therapy, utilizes vertical needle punctures rather than the horizontally directed punctures that are used in subcision and can be used to treat rolling and boxcar scars. Traditionally, a small roller equipped with rows of small needles typically ranging in size from 0.5 to 3.0 mm in length is passed over the skin using gentle pressure, puncturing the superficial layers of the skin to loosen fibrotic adhesions and induce collagen synthesis. This procedure may be repeated several times within a single session or over multiple sessions depending on the depth and quality of the scars. This technique has been reported to reduce scar depth up to 25% after 2 sessions.30
Punch Techniques
Punch techniques are useful for treatment of deeper atrophic acne scarring, for which most other treatment modalities are not particularly effective. A punch excision approximately equal to the scar size is first performed, which may then be followed by either removal of the scar tissue with subsequent suturing, graft replacement of the removed tissue, or elevation of the already established scar tissue to the level of surrounding skin where it is then held in place by sutures or adhesive skin closure material. Success rates with this method are largely limited to case series, but punch techniques are reported to be efficacious, especially for treatment of ice pick scars. Risks for this method include graft failure, graft depression, and formation of sinus tracts.31
Chemical Peels
Chemicals peels traditionally employ the use of acidic compounds to strip away the outer layers of skin to variable depths depending on the concentration of the agent being applied. Chemical peels are not generally recommended for application in a nonspecific manner in the treatment of acne scars given the relatively mild cosmetic improvements seen and the high rate of AEs such as pigmentary alterations and additional scar formation.12 Rather, clinicians should employ the CROSS (chemical reconstruction of skin scars) technique, in which peel agents such as trichloroacetic acid are applied in high concentrations only to areas of atrophic scarring. Use of this method can minimize AEs while simultaneously achieving high success rates, with excellent results in 100% (32/32) of patients after 5 to 6 treatment sessions.32 This method has been successful for hard-to-treat ice pick scars.33
Soft-Tissue Augmentation
Soft-tissue augmentation is another effective treatment of superficial atrophic acne scarring that utilizes injections of collagen fillers such as hyaluronic acid, calcium hydroxylapatite, poly-L-lactic acid, silicone, and even autologous fat to replace lost tissue volume while simultaneously inducing collagen production via stretching of dermal fibroblasts.34 These treatments may require multiple sessions for cosmetic improvement but have shown considerable efficacy in the treatment of atrophic acne scars. Hyaluronic acid has been reported to be particularly effective for rolling scars.12 However, these compounds only provide temporary results, thus requiring repeated treatments to maintain cosmetic outcomes. Permanent options include the recently US Food and Drug Administration–approved polymethylmethacrylate microspheres suspended in bovine collagen as well as the novel technique of autologous fibroblast transfer. These options are relatively new, but initial double-blind, randomized, controlled trials have shown minimal AEs with substantial improvements in 64% to 100% of atrophic scars treated.35,36
Intralesional Therapy
Intralesional corticosteroid injections are a mainstay treatment of hypertrophic acne scarring and are believed to exert their effects by decreasing fibroblast proliferation and promoting collagen degradation.37 Treatment with steroids generally is effective, with reported improvement in 75% (6/8) of patients and complete flattening in 50% (4/8) of lesions according to one study.38 Development of hypopigmentation, dermal atrophy, and telangiectasia are potential sequelae of this treatment.37
5-Fluorouracil, bleomycin, and verapamil also have been used with good results as intralesional treatments of hypertrophic scars, but these agents typically are reserved for cases of corticosteroid failure. Such compounds are thought to mediate their effects through inhibition of dermal fibroblast proliferation.39 Results with these therapies are varied, but greater than 75% improvement is seen in most cases. Adverse effects include injection-site ulceration and hyperpigmentation.39
Cryotherapy
Contact cryotherapy has been studied as treatment of hypertrophic acne scars. The exact mechanism through which scars are reduced is unclear, but it is hypothesized that the physical damage caused by freezing and thrombosis lead to collagen restructuring. According to one study, cryotherapy was reported to achieve good or excellent results in 76% (29/38) of cases.40 Permanent pigmentary alterations are a possible AE.
Silicone Dressings
Silicone dressings are a reasonable treatment option for hypertrophic acne scarring given their proven efficacy and minimal risk for AEs. Thin sheets of silicone gels or membranes are applied daily in a topical manner to acne scars and are believed to be therapeutic through a combination of pressure and hydration, which subsequently inhibits fibroblast production of collagen. Notable reductions in scar appearance and size are seen in 60% to 80% of individuals using this method.41 Adverse effects are limited to pruritus and local skin maceration. Patient noncompliance may be an issue, as the silicone dressings may be applied on highly visible areas such as the face. Patients may apply the dressings at night, but efficacy may be reduced.
Conclusion
When determining which treatment options to use in a patient with acne scarring, it is important to first determine the patient’s treatment goals while simultaneously establishing realistic expectations. Important factors to consider are the patient’s preferences regarding treatment risk, duration, and permanence, as well as budget and social or work requirements. As such, treatment plans for each patient should be determined on a case-by-case basis. It also is important to note that a combination of different treatment modalities often is necessary and superior to monotherapy in achieving satisfactory cosmetic outcomes.
1. Ghodsi SZ, Orawa H, Zouboulis CC. Prevalence, severity, and severity risk factors of acne in high school pupils: a community-based study. J Invest Dermatol. 2009;129:2136-2141.
2. Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59.
3. Kim GK, Michaels BB. Post-adolescent acne in women: more common and more clinical considerations. J Drugs Dermatol. 2012;11:708-713.
4. Geller L, Rosen J, Frankel A, et al. Perimenstrual flare of adult acne. J Clin Aesthet Dermatol. 2014;7:30-34.
5. Layton AM, Henderson CA, Cunliffe WJ. A clinical evaluation of acne scarring and its incidence. Clin Exp Dermatol. 1994;19:303-308.
6. Halvorsen JA, Stern RS, Dalgard F, et al. Suicidal ideation, mental health problems, and social impairment are increased in adolescents with acne: a population-based study. J Invest Dermatol. 2011;131:363-370.
7. Jacob CI, Dover JS, Kaminer MS. Acne scarring: a classification system and review of treatment options. J Am Acad Dermatol. 2001;45:109-117.
8. Rivera AE. Acne scarring: a review and current treatment modalities. J Am Acad Dermatol. 2008;59:659-676.
9. Goodman GJ. Acne and acne scarring: why should we treat? Med J Aust. 1999;171:62-63.
10. Frank W. Therapeutic dermabrasion. back to the future. Arch Dermatol. 1994;130:1187-1189.
11. Goodman GJ. Postacne scarring: a review of its pathophysiology and treatment. Dermatol Surg. 2000;26:857-871.
12. Hession MT, Graber EM. Atrophic acne scarring: a review of treatment options. J Clin Aesthet Dermatol. 2015;8:50-58.
13. Levy LL, Zeichner JA. Management of acne scarring, part II: a comparative review of non-laser-based, minimally invasive approaches. Am J Clin Dermatol. 2012;13:331-340.
14. Christophel JJ, Elm C, Endrizzi BT, et al. A randomized controlled trial of fractional laser therapy and dermabrasion for scar resurfacing. Dermatol Surg. 2012;38:595-602.
15. Katz BE, McFarlane DF. Atypical facial scarring after isotretinoin therapy in a patient with previous dermabrasion. J Am Acad Dermatol. 1994;30:852-853.
16. Bernestein LJ, Geronemus RG. Keloid formation with the 585-nm pulsed dye laser during isotretinoin treatment. Arch Dermatol. 1997;133:111-112.
17. Zachariae H. Delayed wound healing and keloid formation following argon laser treatment or dermabrasion during isotretinoin treatment. Br J Dermatol. 1988;118:703-706.
18. Wootton CI, Cartwright RP, Manning P, et al. Should isotretinoin be stopped prior to surgery? a critically appraised topic. Br J Dermatol. 2014;170:239-244.
19. Alster TS, West TB. Resurfacing of atrophic facial acne scars with a high-energy, pulsed carbon dioxide laser. Dermatol Surg. 1996;22:151-155.
20. Sobanko JF, Alster TS. Management of acne scarring, part I: a comparative review of laser surgical approaches. Am J Clin Dermatol. 2012;13:319-330.
21. Cho SB, Lee SJ, Oh SH, et al. Non-ablative 1550nm erbium-glass and ablative 10,600nm carbon dioxide fractional lasers for acne scar: a randomized split-face study with blinded response evaluation. J Eur Acad Dermatol Venereol. 2010;24:921-925.
22. Rkein A, Ozog D, Waibel JS. Treatment of atrophic scars with fractionated CO2 laser facilitating delivery of topically applied poly-L-lactic acid. Dermatol Surg. 2014;40:624-631.
23. Waibel JS, Wulkan AJ, Shumaker PR. Treatment of hypertrophic scars using laser and laser assisted corticosteroid delivery. Lasers Surg Med. 2013;45:135-140.
24. Alster TS, McMeekin TO. Improvement of facial acne scars by the 585-nm flashlamp-pumped pulsed dye laser. J Am Acad Dermatol. 1996;35:79-81.
25. Simmons BJ, Griffith RD, Falto-Aizpurua LA, et al. Use of radiofrequency in cosmetic dermatology: focus on nonablative treatment of acne scars. Clin Cosmet Investig Dermatol. 2014;7:335-339.
26. Ramesh M, Gopal M, Kumar S, et al. Novel technology in the treatment of acne scars: the matrix-tunable radiofrequency technology. J Cutan Aesthet Surg. 2010;3:97-101.
27. Johnson WC. Treatment of pitted scars; punch transplant technique. J Dermatol Surg Oncol. 1986;12:260-265.
28. Alam M, Omura N, Kaminer MS. Subcision for acne scarring: technique and outcomes in 40 patients. Dermatol Surg. 2005;31:310-317.
29. Sage R, Lopiccolo M, Liu A, et al. Subcuticular incision versus naturally sourced porcine collagen filler for acne scars: a randomized split-face comparison. Dermatol Surg. 2011;37:426-431.
30. Fabbrocini G, Annunziata MC, D’arco V, et al. Acne scars: pathogenesis, classification and treatment [published online ahead of print October 14, 2010]. Dermatol Res Pract. 2010;2010:893080.
31. Johnson WC. Treatment of pitted scars: punch transplant technique. J Dermatol Surg Oncol. 1986;12:260-265.
32. Lee JB, Chung WG, Kwahck H, et al. Focal treatment of acne scars with trichloroacetic acid: chemical reconstruction of skin scars method. Dermatol Surg. 2002;28:1017-1021.
33. Bhardwaj D, Khunger N. An assessment of the efficacy and safety of CROSS technique with 100% TCA in the management of ice pick acne scars. J Cutan Aesthet Surg. 2010;3:93-96.
34. Wang F, Garza LA, Kang S, et al. In vivo stimulation of de novo collagen production caused by cross-linked hyaluronic acid dermal filler injections in photodamaged human skin. Arch Dermatol. 2007;143:155-163.
35. Karnik J, Baumann L, Bruce S, et al. A double-blind, randomized, multicenter, controlled trial of suspended polymethylmethacrylate microspheres for the correction of atrophic facial acne scars. J Am Acad Dermatol. 2014;71:77-83.
36. Munavalli GS, Smith S, Maslowski JM, et al. Successful treatment of depressed, distensible acne scars using autologous fibroblasts: a multi-site, prospective, double blind, placebo-controlled clinical trial. Dermatol Surg. 2013;39:1226-1236.
37. Leventhal D, Furr M, Reiter D. Treatment of keloids and hypertrophic scars: a meta-analysis and review of the literature. Arch Facial Plast Surg. 2006;8:362-368.
38. Darzi MA, Chowdri NA, Kaul SK, et al. Evaluation of various methods of treating keloids and hypertrophic scars: a 10-year follow-up study. Br J Plast Surg. 1992;45:374-379.
39. Ledon JA, Savas J, Franca K, et al. Intralesional treatment for keloids and hypertrophic scars: a review. Dermatol Surg. 2013;39:1745-1757.
40. Zouboulis CC, Blume U, Büttner P, et al. Outcomes of cryosurgery in keloids and hypertrophic scars. a prospective consecutive trial of case series. Arch Dermatol. 1993;129:1146-1151.
41. Puri N, Talwar A. The efficacy of silicone gel for the treatment of hypertrophic scars and keloids. J Cutan Aesthet Surg. 2009;2:104-106.
1. Ghodsi SZ, Orawa H, Zouboulis CC. Prevalence, severity, and severity risk factors of acne in high school pupils: a community-based study. J Invest Dermatol. 2009;129:2136-2141.
2. Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59.
3. Kim GK, Michaels BB. Post-adolescent acne in women: more common and more clinical considerations. J Drugs Dermatol. 2012;11:708-713.
4. Geller L, Rosen J, Frankel A, et al. Perimenstrual flare of adult acne. J Clin Aesthet Dermatol. 2014;7:30-34.
5. Layton AM, Henderson CA, Cunliffe WJ. A clinical evaluation of acne scarring and its incidence. Clin Exp Dermatol. 1994;19:303-308.
6. Halvorsen JA, Stern RS, Dalgard F, et al. Suicidal ideation, mental health problems, and social impairment are increased in adolescents with acne: a population-based study. J Invest Dermatol. 2011;131:363-370.
7. Jacob CI, Dover JS, Kaminer MS. Acne scarring: a classification system and review of treatment options. J Am Acad Dermatol. 2001;45:109-117.
8. Rivera AE. Acne scarring: a review and current treatment modalities. J Am Acad Dermatol. 2008;59:659-676.
9. Goodman GJ. Acne and acne scarring: why should we treat? Med J Aust. 1999;171:62-63.
10. Frank W. Therapeutic dermabrasion. back to the future. Arch Dermatol. 1994;130:1187-1189.
11. Goodman GJ. Postacne scarring: a review of its pathophysiology and treatment. Dermatol Surg. 2000;26:857-871.
12. Hession MT, Graber EM. Atrophic acne scarring: a review of treatment options. J Clin Aesthet Dermatol. 2015;8:50-58.
13. Levy LL, Zeichner JA. Management of acne scarring, part II: a comparative review of non-laser-based, minimally invasive approaches. Am J Clin Dermatol. 2012;13:331-340.
14. Christophel JJ, Elm C, Endrizzi BT, et al. A randomized controlled trial of fractional laser therapy and dermabrasion for scar resurfacing. Dermatol Surg. 2012;38:595-602.
15. Katz BE, McFarlane DF. Atypical facial scarring after isotretinoin therapy in a patient with previous dermabrasion. J Am Acad Dermatol. 1994;30:852-853.
16. Bernestein LJ, Geronemus RG. Keloid formation with the 585-nm pulsed dye laser during isotretinoin treatment. Arch Dermatol. 1997;133:111-112.
17. Zachariae H. Delayed wound healing and keloid formation following argon laser treatment or dermabrasion during isotretinoin treatment. Br J Dermatol. 1988;118:703-706.
18. Wootton CI, Cartwright RP, Manning P, et al. Should isotretinoin be stopped prior to surgery? a critically appraised topic. Br J Dermatol. 2014;170:239-244.
19. Alster TS, West TB. Resurfacing of atrophic facial acne scars with a high-energy, pulsed carbon dioxide laser. Dermatol Surg. 1996;22:151-155.
20. Sobanko JF, Alster TS. Management of acne scarring, part I: a comparative review of laser surgical approaches. Am J Clin Dermatol. 2012;13:319-330.
21. Cho SB, Lee SJ, Oh SH, et al. Non-ablative 1550nm erbium-glass and ablative 10,600nm carbon dioxide fractional lasers for acne scar: a randomized split-face study with blinded response evaluation. J Eur Acad Dermatol Venereol. 2010;24:921-925.
22. Rkein A, Ozog D, Waibel JS. Treatment of atrophic scars with fractionated CO2 laser facilitating delivery of topically applied poly-L-lactic acid. Dermatol Surg. 2014;40:624-631.
23. Waibel JS, Wulkan AJ, Shumaker PR. Treatment of hypertrophic scars using laser and laser assisted corticosteroid delivery. Lasers Surg Med. 2013;45:135-140.
24. Alster TS, McMeekin TO. Improvement of facial acne scars by the 585-nm flashlamp-pumped pulsed dye laser. J Am Acad Dermatol. 1996;35:79-81.
25. Simmons BJ, Griffith RD, Falto-Aizpurua LA, et al. Use of radiofrequency in cosmetic dermatology: focus on nonablative treatment of acne scars. Clin Cosmet Investig Dermatol. 2014;7:335-339.
26. Ramesh M, Gopal M, Kumar S, et al. Novel technology in the treatment of acne scars: the matrix-tunable radiofrequency technology. J Cutan Aesthet Surg. 2010;3:97-101.
27. Johnson WC. Treatment of pitted scars; punch transplant technique. J Dermatol Surg Oncol. 1986;12:260-265.
28. Alam M, Omura N, Kaminer MS. Subcision for acne scarring: technique and outcomes in 40 patients. Dermatol Surg. 2005;31:310-317.
29. Sage R, Lopiccolo M, Liu A, et al. Subcuticular incision versus naturally sourced porcine collagen filler for acne scars: a randomized split-face comparison. Dermatol Surg. 2011;37:426-431.
30. Fabbrocini G, Annunziata MC, D’arco V, et al. Acne scars: pathogenesis, classification and treatment [published online ahead of print October 14, 2010]. Dermatol Res Pract. 2010;2010:893080.
31. Johnson WC. Treatment of pitted scars: punch transplant technique. J Dermatol Surg Oncol. 1986;12:260-265.
32. Lee JB, Chung WG, Kwahck H, et al. Focal treatment of acne scars with trichloroacetic acid: chemical reconstruction of skin scars method. Dermatol Surg. 2002;28:1017-1021.
33. Bhardwaj D, Khunger N. An assessment of the efficacy and safety of CROSS technique with 100% TCA in the management of ice pick acne scars. J Cutan Aesthet Surg. 2010;3:93-96.
34. Wang F, Garza LA, Kang S, et al. In vivo stimulation of de novo collagen production caused by cross-linked hyaluronic acid dermal filler injections in photodamaged human skin. Arch Dermatol. 2007;143:155-163.
35. Karnik J, Baumann L, Bruce S, et al. A double-blind, randomized, multicenter, controlled trial of suspended polymethylmethacrylate microspheres for the correction of atrophic facial acne scars. J Am Acad Dermatol. 2014;71:77-83.
36. Munavalli GS, Smith S, Maslowski JM, et al. Successful treatment of depressed, distensible acne scars using autologous fibroblasts: a multi-site, prospective, double blind, placebo-controlled clinical trial. Dermatol Surg. 2013;39:1226-1236.
37. Leventhal D, Furr M, Reiter D. Treatment of keloids and hypertrophic scars: a meta-analysis and review of the literature. Arch Facial Plast Surg. 2006;8:362-368.
38. Darzi MA, Chowdri NA, Kaul SK, et al. Evaluation of various methods of treating keloids and hypertrophic scars: a 10-year follow-up study. Br J Plast Surg. 1992;45:374-379.
39. Ledon JA, Savas J, Franca K, et al. Intralesional treatment for keloids and hypertrophic scars: a review. Dermatol Surg. 2013;39:1745-1757.
40. Zouboulis CC, Blume U, Büttner P, et al. Outcomes of cryosurgery in keloids and hypertrophic scars. a prospective consecutive trial of case series. Arch Dermatol. 1993;129:1146-1151.
41. Puri N, Talwar A. The efficacy of silicone gel for the treatment of hypertrophic scars and keloids. J Cutan Aesthet Surg. 2009;2:104-106.
Practice Points
- Scarring is a common and undesirable outcome of acne vulgaris that can occur even in the setting of appropriate medical management.
- Acne scars can be classified into several different types based on scar quality and appearance. The choice of treatment with medical or surgical measures should be made with respect to the type of scar present.
- A combination of therapeutic modalities often is necessary to achieve optimal cosmetic outcomes in the treatment of both atrophic and hypertrophic acne scars.