User login
Facial rejuvenation has become increasingly popular, with nonsurgical and noninvasive procedures comprising a large part of aesthetic practice. According to the American Society for Aesthetic Plastic Surgery, Americans spent $12 billion on cosmetic procedures in 2014, with more than 10 million surgical and nonsurgical procedures performed. The top 5 nonsurgical procedures for both men and women combined were botulinum toxin, hyaluronic acid (HA), hair removal, chemical peel, and microdermabrasion.1
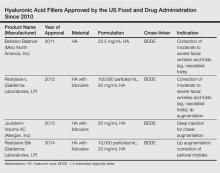
The first dermal filler used was bovine collagen, which was approved by the US Food and Drug Administration (FDA) in 1981. Despite its efficacy in the correction of facial rhytides, bovine collagen required allergy testing prior to use and was discontinued in 2010. Dermal fillers have evolved over the years, and newer products that are superior to earlier fillers with regard to longevity, safety, and tolerability and that do not require allergy testing have become available; however, advances in the use of dermal fillers are not only related to the development of newer products but also to evolving injection techniques. Initially, the aim of treatment with dermal fillers was to correct lines and wrinkles, but an increased understanding of the complex changes that occur with aging have changed our approach to one of volume replacement, with an emphasis on volume restoration in the midface. This approach requires an in-depth understanding of facial anatomy as well as the interactions of the skin, soft tissue, muscle, and bone. Furthermore, placement of filler in specific fat compartments can provide a more natural appearance and an all-around youthful face.2 In this article, we discuss HA fillers that have gained FDA approval within the last 5 years (Table).
Overview of HA Fillers
Hyaluronic acid is a naturally occurring linear glycosaminoglycan with a disaccharide unit, which repeats several thousand times.3 Hyaluronic acid is an essential part of the extracellular matrix of many tissues including the dermis and plays an important role in tissue growth, development, and wound healing. Hyaluronic acid is hygroscopic and absorbs water extensively, thus creating volume.4 Treatment with HA fillers is popular, as they are biocompatible and have a low potential for allergic reactions. They also are easy to use and reversible.4 The first HA filler to gain FDA approval was Restylane (Galderma Laboratories, LP). Currently, several HA fillers are approved in the United States, and each product differs from the others in polymer chain length, degree of HA concentration, particle size, gel consistency, gel hardness, gel viscosity, and degree of water solubility, as well as amount and degree of cross-linking. Cross-linking is essential to avoid enzymatic degradation by endogenous hyaluronidase when injected into the skin and thus to prolong the product’s half-life.5 Cross-linkers used to manufacture HA fillers include 1,4-butanediol diglycidyl ether and divinyl sulfone. More concentrated products with a greater degree of cross-linking provide increased longevity, but they are associated with a higher risk for inflammation and nodule formation. The elastic modulus (G′) is a measure of the firmness of dermal fillers, describing their resistance to deformation. Materials with a higher G′ are stiffer and are meant for deeper injections. Hyaluronic acid fillers can be further classified as biphasic or monophasic. Biphasic fillers (eg, Restylane, Perlane [Galderma Laboratories, LP]) contain a range of microsphere sizes, while monophasic fillers (eg, Juvéderm [Allergan, Inc], Belotero Balance [Merz North America, Inc]) contain homogeneous microspheres. Although randomized clinical trials have reported comparable efficacy and durability of biphasic and monophasic fillers when used to treat the nasolabial folds,6-8 monophasic HA fillers are more cohesive and may not migrate as much following injection.
Restylane Family
Restylane was the first FDA-approved HA filler, gaining its approval in 2003. Restylane is a nonanimal stabilized HA (NASHA) that is produced from the fermentation of equine streptococci. It is cross-linked with 1,4-butanediol diglycidyl ether with a 1% degree of cross-linking. Restylane has an HA concentration of 20 mg/mL. The particle size range of Restylane and Restylane-L is 330 to 430 mm. Restylane (and also Perlane) get passed through sizing screens via sieves and are quantified by their size. The longevity of HA fillers is approximately 6 months; however, various factors affect the product’s longevity, such as the degree of cross-linking, treatment area, and the patient’s metabolism. Restylane-L, which was FDA approved in 2012, is a newer product with 0.3% lidocaine incorporated into the syringe itself. It was the first product from the Restylane range to be approved for lip augmentation. The addition of lidocaine (designated by the L in the product name) does not affect the longevity of a filler.9
The newest FDA-approved HA filler was Restylane Silk (approved in 2014), which has been specifically designed for lip augmentation and correction of perioral rhytides. To avoid postprocedural swelling, it generally is recommended that Restylane Silk be injected slowly. If required, a short course of oral prednisone may be administered after the procedure to treat any edema. Restylane Silk is less viscous than Restylane and requires less pressure to inject. Therefore, it is more suited for treatment of fine perioral lines, as it flows more easily. Because it contains 0.3% lidocaine, discomfort usually is minimal, with treatment lasting approximately 30 to 60 minutes. In the author’s experience (G.G.), Restylane Silk provides a softer correction, though one has to be careful to inject slowly to avoid postinjection swelling. Restylane Silk also may be suitable for neck rejuvenation (off label), but several treatment sessions usually are required. Patients should be warned that they are likely to experience ecchymoses. In our experience, the effects of Restylane Silk injections last approximately 6 to 9 months.
Juvéderm Family
The first Juvéderm product was approved by the FDA in 2006. Juvéderm is a bacterium-derived NASHA. Injectable gel formulations of Juvéderm, including Juvéderm Ultra and Juvéderm Ultra Plus, are FDA approved for the correction of moderate to severe facial wrinkles and folds. The first products in the Juvéderm line were produced using a technology called Hylacross technology, with cohesive molecules of cross-linked HA. In contrast to the sizing technology used by Restylane and Perlane, the Hylacross technology does not break up the cross-linked HA by passing the product through sizing screens via sieves, but instead produces monophasic gels. These Juvéderm products have a high concentration of cross-linked HAs, which accounts for their longevity, and they are soft and easy to use.
Juvéderm Voluma XC was FDA approved in 2013. It is a 20-mg/mL, smooth, highly cohesive, viscous HA, gel that is manufactured using Vycross technology, a combination of low- and high-molecular-weight HA, and it is the only HA filler that is indicated for deep injection for cheek augmentation,10 as it creates a lift due to its higher G′ and a low swelling capacity, with results lasting up to 2 years. The mean volume administered over the initial 4-week period of one study was 5.1 mL.11
Belotero Balance
Belotero Balance was approved by the FDA in 2011 for the correction of moderate to severe facial wrinkles and folds (eg, nasolabial folds).12 Belotero Balance is an HA filler with a cohesive polydensified matrix technology and low elasticity and viscosity. It has the lowest G′ of the currently available dermal fillers12 and therefore is associated with increased injection precision. Belotero Balance is ideal for superficial injections,13 such as forehead lines, vermilion border, tear trough, atrophic scars, and neck lines (off label). Some clinicians reconstitute Belotero Balance with lidocaine (off label) to provide a more pain-free procedure. In our experience, results typically last at least 6 to 8 months.
Complications
Hyaluronic acid fillers share the same adverse events across the product lines. The most common reactions include erythema, swelling, and bruising, which often are unavoidable and may be considered expected effects. Less-frequent events include contour irregularities; product migration; bluish discoloration known as the Tyndall effect, which is more likely to occur with superficial injections; nodules; infection at the injection site; scarring; and vascular occlusion, potentially leading to blindness.14 These more severe complications often can be avoided. Appropriate skin preparation and a sterile technique are critical in preventing infections, while deep placement of filler material reduces the risk for Tyndall effect, nodules, and scarring. Skin necrosis occurs by external compression of the blood supply by the product or occlusion via direct injection into a vessel. Aspirating prior to injection, administering lower volumes, and tenting the skin to inject more superficially can reduce the risk for skin necrosis. Every clinician needs to be able to rapidly recognize the signs of necrosis and to administer urgent therapy, such as the application of warm gauze and nitroglycerin paste, tapping the area to facilitate vasodilatation, and injecting hyaluronidase when required.
On the Horizon
Other dermal fillers that may gain FDA approval in the next few years include Teosyal (Laboratories Teoxane Geneva),6 a new range of monophasic NASHA products that provide high viscosity and elasticity with results lasting 6 to 9 months, and potentially Juvéderm Volbella, a smooth, nonparticle, viscous HA gel developed specifically for the lip area with results lasting up to 1 year.15
Conclusion
Over the last decade, the popularity of dermal fillers has steadily increased, and fillers have become a cornerstone of aesthetic medicine. The increased number of available products necessitates thorough knowledge by the treating physician to ensure optimal outcomes. There is no universal filler that can achieve ideal outcomes in all anatomic sites or in all patients. Often a combination approach may be ideal, such as the use of a product with a higher G′ for re-volumization, followed by a filler with a lower G′ for superficial injection. Some patients may also benefit from a combination of both dermal fillers and neurotoxin injections, either on the same day or at separate visits, which may increase the longevity of the filler.
1. The American Society for Aesthetic Plastic Surgery reports Americans spent more than 12 billion in 2014; procedures for men up 43% over five year period [news release]. New York, NY: American Society for Aesthetic Plastic Surgery; March 11, 2015. http://www.surgery.org/media/news-releases/the-american-society-for-aesthetic-plastic-surgery-reports-americans-spent-more-than-12-billion-in-2014--pro. Accessed July 7, 2015.
2. Fitzgerald R, Rubin AG. Filler placement and the fat compartments. Dermatol Clin. 2014;32:37-50.
3. Cowman MK, Matsuoka S. Experimental approaches to hyaluronan structure. Carbohydr Res. 2005;340:791-809.
4. Lee A, Grummer SE, Kriegel D, et al. Hyaluronidase. Dermatol Surg. 2010;36:1071-1077.
5. Kablik J, Monheit GD, Yu L, et al. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol Surg. 2009;35(suppl 1):302-312.
6. Nast A, Reytan N, Hartmann V, et al. Efficacy and durability of two hyaluronic acid-based fillers in the correction of nasolabial folds: results of a prospective, randomized, double-blind, actively controlled clinical pilot study. Dermatol Surg. 2011;37:768-775.
7. Ascher B, Bayerl C, Brun P, et al. Efficacy and safety of a new hyaluronic acid dermal filler in the treatment of severe nasolabial lines: 6-month interim results of a randomized, evaluator-blinded, intra-individual comparison study. J Cosmet Dermatol. 2011;10:94-98.
8. Rzany B, Bayerl C, Bodokh I, et al. Efficacy and safety of a new hyaluronic acid dermal filler in the treatment of moderate nasolabial folds: 6-month interim results of a randomized, evaluator-blinded, intra-individual comparison study. J Cosmet Laser Ther. 2011;13:107-112.
9. Lupo MP, Swetman G, Waller W. The effect of lidocaine when mixed with large gel particle hyaluronic acid filler tolerability and longevity: a six-month trial. J Drugs Dermatol. 2010;9:1097-1100.
10. Ho D, Jagdeo J. Biological properties of a new volumizing hyaluronic acid filler: a systematic review. J Drugs Dermatol. 2015;14:50-54.
11. Callan P, Goodman GJ, Carlisle I, et al. Efficacy and safety of a hyaluronic acid filler in subjects treated for correction of midface volume deficiency: a 24 month study. Clin Cosmet Investig Dermatol. 2013;6:81-89.
12. Hevia O, Cohen BH, Howell DJ. Safety and efficacy of a cohesive polydensified matrix hyaluronic acid for the correction of infraorbital hollow: an observational study with results at 40 weeks. J Drugs Dermatol. 2014;13:1030-1036.
13. Lorenc ZP, Fagien S, Flynn TC, et al. Clinical application and assessment of Belotero: a roundtable discussion. Plast Reconstr Surg. 2013;132(4, suppl 2):69S-76S.
14. Carruthers JD, Fagien S, Rohrich RJ, et al. Blindness caused by cosmetic filler injection: a review of cause and therapy. Plast Reconstr Surg. 2014;134:1197-1201.
15. Eccleston D, Murphy DK. Juvéderm(®) Volbella™ in the perioral area: a 12-month prospective, multicenter, open-label study. Clin Cosmet Investig Dermatol. 2012;5:167-172.
Facial rejuvenation has become increasingly popular, with nonsurgical and noninvasive procedures comprising a large part of aesthetic practice. According to the American Society for Aesthetic Plastic Surgery, Americans spent $12 billion on cosmetic procedures in 2014, with more than 10 million surgical and nonsurgical procedures performed. The top 5 nonsurgical procedures for both men and women combined were botulinum toxin, hyaluronic acid (HA), hair removal, chemical peel, and microdermabrasion.1
The first dermal filler used was bovine collagen, which was approved by the US Food and Drug Administration (FDA) in 1981. Despite its efficacy in the correction of facial rhytides, bovine collagen required allergy testing prior to use and was discontinued in 2010. Dermal fillers have evolved over the years, and newer products that are superior to earlier fillers with regard to longevity, safety, and tolerability and that do not require allergy testing have become available; however, advances in the use of dermal fillers are not only related to the development of newer products but also to evolving injection techniques. Initially, the aim of treatment with dermal fillers was to correct lines and wrinkles, but an increased understanding of the complex changes that occur with aging have changed our approach to one of volume replacement, with an emphasis on volume restoration in the midface. This approach requires an in-depth understanding of facial anatomy as well as the interactions of the skin, soft tissue, muscle, and bone. Furthermore, placement of filler in specific fat compartments can provide a more natural appearance and an all-around youthful face.2 In this article, we discuss HA fillers that have gained FDA approval within the last 5 years (Table).
Overview of HA Fillers
Hyaluronic acid is a naturally occurring linear glycosaminoglycan with a disaccharide unit, which repeats several thousand times.3 Hyaluronic acid is an essential part of the extracellular matrix of many tissues including the dermis and plays an important role in tissue growth, development, and wound healing. Hyaluronic acid is hygroscopic and absorbs water extensively, thus creating volume.4 Treatment with HA fillers is popular, as they are biocompatible and have a low potential for allergic reactions. They also are easy to use and reversible.4 The first HA filler to gain FDA approval was Restylane (Galderma Laboratories, LP). Currently, several HA fillers are approved in the United States, and each product differs from the others in polymer chain length, degree of HA concentration, particle size, gel consistency, gel hardness, gel viscosity, and degree of water solubility, as well as amount and degree of cross-linking. Cross-linking is essential to avoid enzymatic degradation by endogenous hyaluronidase when injected into the skin and thus to prolong the product’s half-life.5 Cross-linkers used to manufacture HA fillers include 1,4-butanediol diglycidyl ether and divinyl sulfone. More concentrated products with a greater degree of cross-linking provide increased longevity, but they are associated with a higher risk for inflammation and nodule formation. The elastic modulus (G′) is a measure of the firmness of dermal fillers, describing their resistance to deformation. Materials with a higher G′ are stiffer and are meant for deeper injections. Hyaluronic acid fillers can be further classified as biphasic or monophasic. Biphasic fillers (eg, Restylane, Perlane [Galderma Laboratories, LP]) contain a range of microsphere sizes, while monophasic fillers (eg, Juvéderm [Allergan, Inc], Belotero Balance [Merz North America, Inc]) contain homogeneous microspheres. Although randomized clinical trials have reported comparable efficacy and durability of biphasic and monophasic fillers when used to treat the nasolabial folds,6-8 monophasic HA fillers are more cohesive and may not migrate as much following injection.
Restylane Family
Restylane was the first FDA-approved HA filler, gaining its approval in 2003. Restylane is a nonanimal stabilized HA (NASHA) that is produced from the fermentation of equine streptococci. It is cross-linked with 1,4-butanediol diglycidyl ether with a 1% degree of cross-linking. Restylane has an HA concentration of 20 mg/mL. The particle size range of Restylane and Restylane-L is 330 to 430 mm. Restylane (and also Perlane) get passed through sizing screens via sieves and are quantified by their size. The longevity of HA fillers is approximately 6 months; however, various factors affect the product’s longevity, such as the degree of cross-linking, treatment area, and the patient’s metabolism. Restylane-L, which was FDA approved in 2012, is a newer product with 0.3% lidocaine incorporated into the syringe itself. It was the first product from the Restylane range to be approved for lip augmentation. The addition of lidocaine (designated by the L in the product name) does not affect the longevity of a filler.9
The newest FDA-approved HA filler was Restylane Silk (approved in 2014), which has been specifically designed for lip augmentation and correction of perioral rhytides. To avoid postprocedural swelling, it generally is recommended that Restylane Silk be injected slowly. If required, a short course of oral prednisone may be administered after the procedure to treat any edema. Restylane Silk is less viscous than Restylane and requires less pressure to inject. Therefore, it is more suited for treatment of fine perioral lines, as it flows more easily. Because it contains 0.3% lidocaine, discomfort usually is minimal, with treatment lasting approximately 30 to 60 minutes. In the author’s experience (G.G.), Restylane Silk provides a softer correction, though one has to be careful to inject slowly to avoid postinjection swelling. Restylane Silk also may be suitable for neck rejuvenation (off label), but several treatment sessions usually are required. Patients should be warned that they are likely to experience ecchymoses. In our experience, the effects of Restylane Silk injections last approximately 6 to 9 months.
Juvéderm Family
The first Juvéderm product was approved by the FDA in 2006. Juvéderm is a bacterium-derived NASHA. Injectable gel formulations of Juvéderm, including Juvéderm Ultra and Juvéderm Ultra Plus, are FDA approved for the correction of moderate to severe facial wrinkles and folds. The first products in the Juvéderm line were produced using a technology called Hylacross technology, with cohesive molecules of cross-linked HA. In contrast to the sizing technology used by Restylane and Perlane, the Hylacross technology does not break up the cross-linked HA by passing the product through sizing screens via sieves, but instead produces monophasic gels. These Juvéderm products have a high concentration of cross-linked HAs, which accounts for their longevity, and they are soft and easy to use.
Juvéderm Voluma XC was FDA approved in 2013. It is a 20-mg/mL, smooth, highly cohesive, viscous HA, gel that is manufactured using Vycross technology, a combination of low- and high-molecular-weight HA, and it is the only HA filler that is indicated for deep injection for cheek augmentation,10 as it creates a lift due to its higher G′ and a low swelling capacity, with results lasting up to 2 years. The mean volume administered over the initial 4-week period of one study was 5.1 mL.11
Belotero Balance
Belotero Balance was approved by the FDA in 2011 for the correction of moderate to severe facial wrinkles and folds (eg, nasolabial folds).12 Belotero Balance is an HA filler with a cohesive polydensified matrix technology and low elasticity and viscosity. It has the lowest G′ of the currently available dermal fillers12 and therefore is associated with increased injection precision. Belotero Balance is ideal for superficial injections,13 such as forehead lines, vermilion border, tear trough, atrophic scars, and neck lines (off label). Some clinicians reconstitute Belotero Balance with lidocaine (off label) to provide a more pain-free procedure. In our experience, results typically last at least 6 to 8 months.
Complications
Hyaluronic acid fillers share the same adverse events across the product lines. The most common reactions include erythema, swelling, and bruising, which often are unavoidable and may be considered expected effects. Less-frequent events include contour irregularities; product migration; bluish discoloration known as the Tyndall effect, which is more likely to occur with superficial injections; nodules; infection at the injection site; scarring; and vascular occlusion, potentially leading to blindness.14 These more severe complications often can be avoided. Appropriate skin preparation and a sterile technique are critical in preventing infections, while deep placement of filler material reduces the risk for Tyndall effect, nodules, and scarring. Skin necrosis occurs by external compression of the blood supply by the product or occlusion via direct injection into a vessel. Aspirating prior to injection, administering lower volumes, and tenting the skin to inject more superficially can reduce the risk for skin necrosis. Every clinician needs to be able to rapidly recognize the signs of necrosis and to administer urgent therapy, such as the application of warm gauze and nitroglycerin paste, tapping the area to facilitate vasodilatation, and injecting hyaluronidase when required.
On the Horizon
Other dermal fillers that may gain FDA approval in the next few years include Teosyal (Laboratories Teoxane Geneva),6 a new range of monophasic NASHA products that provide high viscosity and elasticity with results lasting 6 to 9 months, and potentially Juvéderm Volbella, a smooth, nonparticle, viscous HA gel developed specifically for the lip area with results lasting up to 1 year.15
Conclusion
Over the last decade, the popularity of dermal fillers has steadily increased, and fillers have become a cornerstone of aesthetic medicine. The increased number of available products necessitates thorough knowledge by the treating physician to ensure optimal outcomes. There is no universal filler that can achieve ideal outcomes in all anatomic sites or in all patients. Often a combination approach may be ideal, such as the use of a product with a higher G′ for re-volumization, followed by a filler with a lower G′ for superficial injection. Some patients may also benefit from a combination of both dermal fillers and neurotoxin injections, either on the same day or at separate visits, which may increase the longevity of the filler.
Facial rejuvenation has become increasingly popular, with nonsurgical and noninvasive procedures comprising a large part of aesthetic practice. According to the American Society for Aesthetic Plastic Surgery, Americans spent $12 billion on cosmetic procedures in 2014, with more than 10 million surgical and nonsurgical procedures performed. The top 5 nonsurgical procedures for both men and women combined were botulinum toxin, hyaluronic acid (HA), hair removal, chemical peel, and microdermabrasion.1
The first dermal filler used was bovine collagen, which was approved by the US Food and Drug Administration (FDA) in 1981. Despite its efficacy in the correction of facial rhytides, bovine collagen required allergy testing prior to use and was discontinued in 2010. Dermal fillers have evolved over the years, and newer products that are superior to earlier fillers with regard to longevity, safety, and tolerability and that do not require allergy testing have become available; however, advances in the use of dermal fillers are not only related to the development of newer products but also to evolving injection techniques. Initially, the aim of treatment with dermal fillers was to correct lines and wrinkles, but an increased understanding of the complex changes that occur with aging have changed our approach to one of volume replacement, with an emphasis on volume restoration in the midface. This approach requires an in-depth understanding of facial anatomy as well as the interactions of the skin, soft tissue, muscle, and bone. Furthermore, placement of filler in specific fat compartments can provide a more natural appearance and an all-around youthful face.2 In this article, we discuss HA fillers that have gained FDA approval within the last 5 years (Table).
Overview of HA Fillers
Hyaluronic acid is a naturally occurring linear glycosaminoglycan with a disaccharide unit, which repeats several thousand times.3 Hyaluronic acid is an essential part of the extracellular matrix of many tissues including the dermis and plays an important role in tissue growth, development, and wound healing. Hyaluronic acid is hygroscopic and absorbs water extensively, thus creating volume.4 Treatment with HA fillers is popular, as they are biocompatible and have a low potential for allergic reactions. They also are easy to use and reversible.4 The first HA filler to gain FDA approval was Restylane (Galderma Laboratories, LP). Currently, several HA fillers are approved in the United States, and each product differs from the others in polymer chain length, degree of HA concentration, particle size, gel consistency, gel hardness, gel viscosity, and degree of water solubility, as well as amount and degree of cross-linking. Cross-linking is essential to avoid enzymatic degradation by endogenous hyaluronidase when injected into the skin and thus to prolong the product’s half-life.5 Cross-linkers used to manufacture HA fillers include 1,4-butanediol diglycidyl ether and divinyl sulfone. More concentrated products with a greater degree of cross-linking provide increased longevity, but they are associated with a higher risk for inflammation and nodule formation. The elastic modulus (G′) is a measure of the firmness of dermal fillers, describing their resistance to deformation. Materials with a higher G′ are stiffer and are meant for deeper injections. Hyaluronic acid fillers can be further classified as biphasic or monophasic. Biphasic fillers (eg, Restylane, Perlane [Galderma Laboratories, LP]) contain a range of microsphere sizes, while monophasic fillers (eg, Juvéderm [Allergan, Inc], Belotero Balance [Merz North America, Inc]) contain homogeneous microspheres. Although randomized clinical trials have reported comparable efficacy and durability of biphasic and monophasic fillers when used to treat the nasolabial folds,6-8 monophasic HA fillers are more cohesive and may not migrate as much following injection.
Restylane Family
Restylane was the first FDA-approved HA filler, gaining its approval in 2003. Restylane is a nonanimal stabilized HA (NASHA) that is produced from the fermentation of equine streptococci. It is cross-linked with 1,4-butanediol diglycidyl ether with a 1% degree of cross-linking. Restylane has an HA concentration of 20 mg/mL. The particle size range of Restylane and Restylane-L is 330 to 430 mm. Restylane (and also Perlane) get passed through sizing screens via sieves and are quantified by their size. The longevity of HA fillers is approximately 6 months; however, various factors affect the product’s longevity, such as the degree of cross-linking, treatment area, and the patient’s metabolism. Restylane-L, which was FDA approved in 2012, is a newer product with 0.3% lidocaine incorporated into the syringe itself. It was the first product from the Restylane range to be approved for lip augmentation. The addition of lidocaine (designated by the L in the product name) does not affect the longevity of a filler.9
The newest FDA-approved HA filler was Restylane Silk (approved in 2014), which has been specifically designed for lip augmentation and correction of perioral rhytides. To avoid postprocedural swelling, it generally is recommended that Restylane Silk be injected slowly. If required, a short course of oral prednisone may be administered after the procedure to treat any edema. Restylane Silk is less viscous than Restylane and requires less pressure to inject. Therefore, it is more suited for treatment of fine perioral lines, as it flows more easily. Because it contains 0.3% lidocaine, discomfort usually is minimal, with treatment lasting approximately 30 to 60 minutes. In the author’s experience (G.G.), Restylane Silk provides a softer correction, though one has to be careful to inject slowly to avoid postinjection swelling. Restylane Silk also may be suitable for neck rejuvenation (off label), but several treatment sessions usually are required. Patients should be warned that they are likely to experience ecchymoses. In our experience, the effects of Restylane Silk injections last approximately 6 to 9 months.
Juvéderm Family
The first Juvéderm product was approved by the FDA in 2006. Juvéderm is a bacterium-derived NASHA. Injectable gel formulations of Juvéderm, including Juvéderm Ultra and Juvéderm Ultra Plus, are FDA approved for the correction of moderate to severe facial wrinkles and folds. The first products in the Juvéderm line were produced using a technology called Hylacross technology, with cohesive molecules of cross-linked HA. In contrast to the sizing technology used by Restylane and Perlane, the Hylacross technology does not break up the cross-linked HA by passing the product through sizing screens via sieves, but instead produces monophasic gels. These Juvéderm products have a high concentration of cross-linked HAs, which accounts for their longevity, and they are soft and easy to use.
Juvéderm Voluma XC was FDA approved in 2013. It is a 20-mg/mL, smooth, highly cohesive, viscous HA, gel that is manufactured using Vycross technology, a combination of low- and high-molecular-weight HA, and it is the only HA filler that is indicated for deep injection for cheek augmentation,10 as it creates a lift due to its higher G′ and a low swelling capacity, with results lasting up to 2 years. The mean volume administered over the initial 4-week period of one study was 5.1 mL.11
Belotero Balance
Belotero Balance was approved by the FDA in 2011 for the correction of moderate to severe facial wrinkles and folds (eg, nasolabial folds).12 Belotero Balance is an HA filler with a cohesive polydensified matrix technology and low elasticity and viscosity. It has the lowest G′ of the currently available dermal fillers12 and therefore is associated with increased injection precision. Belotero Balance is ideal for superficial injections,13 such as forehead lines, vermilion border, tear trough, atrophic scars, and neck lines (off label). Some clinicians reconstitute Belotero Balance with lidocaine (off label) to provide a more pain-free procedure. In our experience, results typically last at least 6 to 8 months.
Complications
Hyaluronic acid fillers share the same adverse events across the product lines. The most common reactions include erythema, swelling, and bruising, which often are unavoidable and may be considered expected effects. Less-frequent events include contour irregularities; product migration; bluish discoloration known as the Tyndall effect, which is more likely to occur with superficial injections; nodules; infection at the injection site; scarring; and vascular occlusion, potentially leading to blindness.14 These more severe complications often can be avoided. Appropriate skin preparation and a sterile technique are critical in preventing infections, while deep placement of filler material reduces the risk for Tyndall effect, nodules, and scarring. Skin necrosis occurs by external compression of the blood supply by the product or occlusion via direct injection into a vessel. Aspirating prior to injection, administering lower volumes, and tenting the skin to inject more superficially can reduce the risk for skin necrosis. Every clinician needs to be able to rapidly recognize the signs of necrosis and to administer urgent therapy, such as the application of warm gauze and nitroglycerin paste, tapping the area to facilitate vasodilatation, and injecting hyaluronidase when required.
On the Horizon
Other dermal fillers that may gain FDA approval in the next few years include Teosyal (Laboratories Teoxane Geneva),6 a new range of monophasic NASHA products that provide high viscosity and elasticity with results lasting 6 to 9 months, and potentially Juvéderm Volbella, a smooth, nonparticle, viscous HA gel developed specifically for the lip area with results lasting up to 1 year.15
Conclusion
Over the last decade, the popularity of dermal fillers has steadily increased, and fillers have become a cornerstone of aesthetic medicine. The increased number of available products necessitates thorough knowledge by the treating physician to ensure optimal outcomes. There is no universal filler that can achieve ideal outcomes in all anatomic sites or in all patients. Often a combination approach may be ideal, such as the use of a product with a higher G′ for re-volumization, followed by a filler with a lower G′ for superficial injection. Some patients may also benefit from a combination of both dermal fillers and neurotoxin injections, either on the same day or at separate visits, which may increase the longevity of the filler.
1. The American Society for Aesthetic Plastic Surgery reports Americans spent more than 12 billion in 2014; procedures for men up 43% over five year period [news release]. New York, NY: American Society for Aesthetic Plastic Surgery; March 11, 2015. http://www.surgery.org/media/news-releases/the-american-society-for-aesthetic-plastic-surgery-reports-americans-spent-more-than-12-billion-in-2014--pro. Accessed July 7, 2015.
2. Fitzgerald R, Rubin AG. Filler placement and the fat compartments. Dermatol Clin. 2014;32:37-50.
3. Cowman MK, Matsuoka S. Experimental approaches to hyaluronan structure. Carbohydr Res. 2005;340:791-809.
4. Lee A, Grummer SE, Kriegel D, et al. Hyaluronidase. Dermatol Surg. 2010;36:1071-1077.
5. Kablik J, Monheit GD, Yu L, et al. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol Surg. 2009;35(suppl 1):302-312.
6. Nast A, Reytan N, Hartmann V, et al. Efficacy and durability of two hyaluronic acid-based fillers in the correction of nasolabial folds: results of a prospective, randomized, double-blind, actively controlled clinical pilot study. Dermatol Surg. 2011;37:768-775.
7. Ascher B, Bayerl C, Brun P, et al. Efficacy and safety of a new hyaluronic acid dermal filler in the treatment of severe nasolabial lines: 6-month interim results of a randomized, evaluator-blinded, intra-individual comparison study. J Cosmet Dermatol. 2011;10:94-98.
8. Rzany B, Bayerl C, Bodokh I, et al. Efficacy and safety of a new hyaluronic acid dermal filler in the treatment of moderate nasolabial folds: 6-month interim results of a randomized, evaluator-blinded, intra-individual comparison study. J Cosmet Laser Ther. 2011;13:107-112.
9. Lupo MP, Swetman G, Waller W. The effect of lidocaine when mixed with large gel particle hyaluronic acid filler tolerability and longevity: a six-month trial. J Drugs Dermatol. 2010;9:1097-1100.
10. Ho D, Jagdeo J. Biological properties of a new volumizing hyaluronic acid filler: a systematic review. J Drugs Dermatol. 2015;14:50-54.
11. Callan P, Goodman GJ, Carlisle I, et al. Efficacy and safety of a hyaluronic acid filler in subjects treated for correction of midface volume deficiency: a 24 month study. Clin Cosmet Investig Dermatol. 2013;6:81-89.
12. Hevia O, Cohen BH, Howell DJ. Safety and efficacy of a cohesive polydensified matrix hyaluronic acid for the correction of infraorbital hollow: an observational study with results at 40 weeks. J Drugs Dermatol. 2014;13:1030-1036.
13. Lorenc ZP, Fagien S, Flynn TC, et al. Clinical application and assessment of Belotero: a roundtable discussion. Plast Reconstr Surg. 2013;132(4, suppl 2):69S-76S.
14. Carruthers JD, Fagien S, Rohrich RJ, et al. Blindness caused by cosmetic filler injection: a review of cause and therapy. Plast Reconstr Surg. 2014;134:1197-1201.
15. Eccleston D, Murphy DK. Juvéderm(®) Volbella™ in the perioral area: a 12-month prospective, multicenter, open-label study. Clin Cosmet Investig Dermatol. 2012;5:167-172.
1. The American Society for Aesthetic Plastic Surgery reports Americans spent more than 12 billion in 2014; procedures for men up 43% over five year period [news release]. New York, NY: American Society for Aesthetic Plastic Surgery; March 11, 2015. http://www.surgery.org/media/news-releases/the-american-society-for-aesthetic-plastic-surgery-reports-americans-spent-more-than-12-billion-in-2014--pro. Accessed July 7, 2015.
2. Fitzgerald R, Rubin AG. Filler placement and the fat compartments. Dermatol Clin. 2014;32:37-50.
3. Cowman MK, Matsuoka S. Experimental approaches to hyaluronan structure. Carbohydr Res. 2005;340:791-809.
4. Lee A, Grummer SE, Kriegel D, et al. Hyaluronidase. Dermatol Surg. 2010;36:1071-1077.
5. Kablik J, Monheit GD, Yu L, et al. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol Surg. 2009;35(suppl 1):302-312.
6. Nast A, Reytan N, Hartmann V, et al. Efficacy and durability of two hyaluronic acid-based fillers in the correction of nasolabial folds: results of a prospective, randomized, double-blind, actively controlled clinical pilot study. Dermatol Surg. 2011;37:768-775.
7. Ascher B, Bayerl C, Brun P, et al. Efficacy and safety of a new hyaluronic acid dermal filler in the treatment of severe nasolabial lines: 6-month interim results of a randomized, evaluator-blinded, intra-individual comparison study. J Cosmet Dermatol. 2011;10:94-98.
8. Rzany B, Bayerl C, Bodokh I, et al. Efficacy and safety of a new hyaluronic acid dermal filler in the treatment of moderate nasolabial folds: 6-month interim results of a randomized, evaluator-blinded, intra-individual comparison study. J Cosmet Laser Ther. 2011;13:107-112.
9. Lupo MP, Swetman G, Waller W. The effect of lidocaine when mixed with large gel particle hyaluronic acid filler tolerability and longevity: a six-month trial. J Drugs Dermatol. 2010;9:1097-1100.
10. Ho D, Jagdeo J. Biological properties of a new volumizing hyaluronic acid filler: a systematic review. J Drugs Dermatol. 2015;14:50-54.
11. Callan P, Goodman GJ, Carlisle I, et al. Efficacy and safety of a hyaluronic acid filler in subjects treated for correction of midface volume deficiency: a 24 month study. Clin Cosmet Investig Dermatol. 2013;6:81-89.
12. Hevia O, Cohen BH, Howell DJ. Safety and efficacy of a cohesive polydensified matrix hyaluronic acid for the correction of infraorbital hollow: an observational study with results at 40 weeks. J Drugs Dermatol. 2014;13:1030-1036.
13. Lorenc ZP, Fagien S, Flynn TC, et al. Clinical application and assessment of Belotero: a roundtable discussion. Plast Reconstr Surg. 2013;132(4, suppl 2):69S-76S.
14. Carruthers JD, Fagien S, Rohrich RJ, et al. Blindness caused by cosmetic filler injection: a review of cause and therapy. Plast Reconstr Surg. 2014;134:1197-1201.
15. Eccleston D, Murphy DK. Juvéderm(®) Volbella™ in the perioral area: a 12-month prospective, multicenter, open-label study. Clin Cosmet Investig Dermatol. 2012;5:167-172.
Practice Points
- Restylane Silk is useful for the treatment of fine perioral lines.
- Juvéderm Voluma XC is a newer product in the Juvéderm range and is indicated for cheek augmentation.
- Belotero Balance has the lowest G′ of the currently available dermal fillers and allows greater precision.