User login
Actinic keratosis (AK), also referred to as solar keratosis or senile keratosis, is an intraepidermal proliferation of dysplastic keratinocytes that develops in response to chronic exposure to UV radiation. Actinic keratoses are among the most commonly encountered lesions seen by dermatologists, and it has been estimated that 60% of predisposed individuals older than 40 years have at least one AK.1,2 Prevalence is notably higher in light-skinned individuals and increases with age, presumably from higher cumulative sun exposure and decreased effectiveness of the immune system.1,3 It remains a point of contention as to whether or not AKs actually represent squamous cell carcinoma (SCC) in situ, but the potential for progression to invasive disease has been well demonstrated, as the majority of SCCs develop from preexisting AKs.4-6 The risk for progression to invasive disease for an individual AK has been estimated to range from 0.025% to 16% per year, with an average of approximately 8% in immunocompetent patients.7
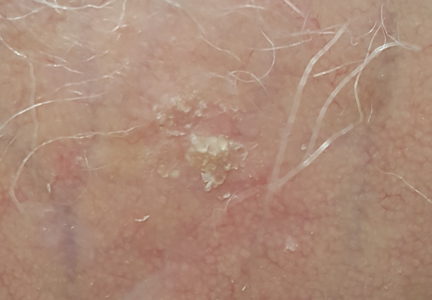
The clinical morphology of AK can vary widely, but the most common presentation is an erythematous scaly macule, papule, or plaque on sun-exposed skin. The skin surrounding AKs typically shows evidence of solar damage with deep wrinkling, mottled pigmentation, scattered telangiectases, purpura, or xerosis (Figure). A variety of clinical variants with unique presentations exist, including atrophic, hypertrophic, acantholytic, lichenoid, bowenoid, and pigmented subtypes. Because more than 80% of AKs occur on highly visible areas such as the head, neck, back of the hands, and forearms, AKs can have an obvious detrimental effect on cosmetic appearance. Studies also have shown a strong association between AKs and decreased overall quality of life (QOL).3,8,9

Because of the risk for AK progression to invasive cancer along with its negative impact on cosmesis and QOL, clinicians generally opt to treat AKs. Numerous different treatment options exist, including topical medications, procedural modalities, and light-based therapies. Here, we review the efficacy of the most commonly utilized treatments and discuss the relevant cosmetic considerations and outcomes.
Topical Treatments
5-Fluorouracil
5-Fluorouracil (5-FU) is a US Food and Drug Administration (FDA)–approved, topically applied pyrimidine analogue that inhibits thymidylate synthase. The resulting suppression of DNA and RNA synthesis induces cell death with a preference for mitotically active cells.10 5-Fluorouracil has been used for more than 50 years as a treatment of AK and its efficacy is well established. A systematic review of 5 randomized controlled studies of topical 5-FU reported an average of 49% of 423 patients achieving complete lesion clearance with 5-FU cream 5% applied once or twice daily for up to 7 weeks.11 Some notable drawbacks of 5-FU, however, are application-site erythema, blistering, pruritus, necrosis, erosion, and pain. These effects often lead to premature cessation of therapy, but newer formulations of 5-FU cream 0.5% have shown good efficacy with better tolerability.12 A randomized, double-blind, multicenter, parallel-group study of 177 patients using 5-FU cream 0.5% once daily for either 1, 2, or 4 weeks demonstrated significant (P<.001) efficacy over vehicle gel in all treatment arms.13 The most effective therapy was 4 weeks of treatment, which achieved a mean 91.7% reduction in lesion count as assessed 1 month after cessation of therapy. The primary adverse effect (AE) reported in this trial was mild to moderate facial irritation, which generally resolved within 18 to 21 days after treatment cessation.13 Overall, 5-FU is a highly effective therapy for treating AKs that also can improve signs of photoaging, but patients should be aware of cosmetically unappealing effects that generally occur throughout therapy and during the immediate posttreatment period.14
Chemical Peels
Chemical peels traditionally employ acidic compounds to strip away outer layers of skin to variable depths depending on the concentration of the agent being applied. For treatment of AK, trichloroacetic acid (TCA) is a commonly employed cauterant that has shown efficacy comparable to topical 5-FU as well as ablative CO2 laser resurfacing.15 Trichloroacetic acid peels also are a convenient therapy, as good results can be achieved after a single treatment session. A split-face study of 15 patients treated with either a single application of 35% TCA and Jessner solution or twice-daily application of 5-FU cream 5% for 3 weeks demonstrated a reduction in 75% of visible AKs in both treatment arms over a 1-year follow-up period.16 Although 80% of patients self-reported considerable cosmetic improvement with both therapies, patient preference was reported to be in favor of the TCA peel, given its quick results and relatively mild side effects as compared to 5-FU. Treatment with chemical peels will result in temporary erythema and mild desquamation that usually resolves within 2 weeks; however, there are cases in which erythema has been reported to persist for several months.16 Adverse effects such as permanent scarring or pigmentation changes rarely are seen with TCA concentrations less than 45%.17 Caution should be used in patients with a history of herpes simplex virus, keloids, postinflammatory hyperpigmentation, radiation exposure, immunosuppression, and those unable or unwilling to use sunscreen and avoid sun exposure in the immediate posttreatment period.
Diclofenac Sodium
Diclofenac sodium (DFS) is an FDA-approved topical, nonsteroidal,
anti-inflammatory drug whose mechanism of action in the treatment of AK is thought to involve inhibition of the cyclooxygenase 2 enzyme.18 The resulting reduction of prostaglandins is believed to inhibit tumor angiogenesis, induce apoptosis, and inhibit cell differentiation.19-22 In a multicenter, double-blind, placebo-controlled study of 195 patients, application of DFS 3% in hyaluronan gel 2.5% twice daily for 60 days showed significant (P<.05) efficacy over placebo in achieving complete resolution of target lesions during a 30-day follow-up period (31% vs 10%). Furthermore, qualitative patient assessment of complete global improvement also was significantly (P<.05) higher in the active treatment group as compared to placebo (31% vs 10%).23 Additional studies of DFS 3% in hyaluronan gel 2.5% applied twice daily for 90 days have shown even higher rates of success, with complete resolution of target lesions in 40% to 58% of cases.24,25 This therapy also has been reported to substantially improve QOL following treatment completion.26 The most frequently cited AEs include pruritus, rash, dry skin, erythema, and application-site reactions. Overall, DFS is a
well-tolerated therapy with efficacy comparable to that of 5-FU but with a lower incidence of AEs
and higher patient satisfaction as determined in
2 head-to-head studies.27,28
ImiquimodImiquimod (IMQ) is an FDA-approved topical agent that functions as an immune response modifier via agonism of toll-like receptor 7.18 The resulting cytokine production and release enhances the innate and acquired immune responses leading to anticancer activity.29 The efficacy of IMQ for treatment of AK has been demonstrated in numerous well-designed clinical trials. A
meta-analysis of 5 randomized, double-blind trials including 1293 patients treated with IMQ cream 5%
2 to 3 times per week for 12 to 16 weeks reported complete clearance of AKs in 50% of patients treated with IMQ as compared to 5% of patients treated with vehicle.30 The most frequently reported AEs with this therapy include erythema, scabbing, flaking, and erosion. These effects generally resolve following cessation of treatment, and therapy is considered to be well tolerated; however, there are case reports of IMQ triggering or exacerbating existing inflammatory conditions.31 Imiquimod cream also is approved at 2.5% and 3.75% concentrations, which have demonstrated significant (P<.001) efficacy over placebo and a reduced incidence of AEs; complete clearance rates have been reported as 30.6% and 35.6%, respectively.32 Notably, a study comparing 75 patients randomized to either IMQ cream 5%
3 times per week for 4 weeks, 1 or 2 courses of cryosurgery, or 5-FU ointment 5% twice daily for 4 weeks reported that IMQ achieved significantly (P<.01) superior sustained clearance rates during a 12-month follow-up period over cryosurgery and 5-FU
(73% vs 4% vs 33%).33 Additionally, cosmetic outcomes as determined by both participants and investigators were reported as excellent at 12 months posttreatment in more than 80% of participants treated with IMQ. These excellent, long-lasting cosmetic outcomes also were determined to be significantly (P<.0001) superior to the cosmetic outcomes of 5-FU and cryotherapy, which both reported excellent outcomes in less than 10% of cases.33
Ingenol MebutateIngenol mebutate (IM) is a macrocyclic diterpene ester derived from the Euphorbia peplus plant that is FDA approved for the treatment of AK.1 Ingenol mebutate’s mechanism of action is thought to involve induction of cell death via disruption of the plasma membrane and mitochondria in addition to production of an inflammatory response, which produces tumor-specific antibodies and a large influx of neutrophils.34,35 The overall evidence for the efficacy of IM is strong. A combined analysis of 4 multicenter, randomized, double-blind studies of 1005 participants reported that IM gel 0.015% applied once daily for 3 days to the face or scalp was significantly superior (P<.001) to placebo in achieving complete clearance as assessed 54 days after completion of therapy (42.2% vs 3.7%) and that IM gel 0.05% applied once daily for 2 days to the trunk or extremities also was significantly superior (P<.001) to placebo in achieving complete clearance as determined 55 days after completion of therapy (34.1% vs 4.7%).36 A follow-up report to this study indicated that IM also appears to achieve long-lasting effects with an overall 87% decrease in total AKs at 12 months follow-up in both trial groups.37 Additionally, it has been recently reported that treatment with IM in these trials was associated with significantly higher overall treatment satisfaction (P<.001) and improved QOL (P<.001) as compared to vehicle.38 Cosmetic outcomes of IM therapy have been assessed in a trial analyzing the efficacy of IM gel 0.025% for 3 days or IM gel 0.05% for 2 or 3 days on nonfacial AKs. This study reported significantly (P<.0001) higher patient satisfaction with the cosmetic outcome at 8 weeks after therapy as compared to vehicle.34 Studies performed in mice have demonstrated that IM is able to promote collagen matrix turnover and impose dermal elasticity, which may contribute to these good cosmetic outcomes.39 The most common AEs of IM therapy are erythema, crusting, and flaking; these effects generally occur 3 to 8 days after starting treatment. These effects, however, generally are short lived and resolve within 2 weeks of treatment cessation when IM is applied to the face or scalp or 4 weeks when applied to the trunk or extremities.40 Overall, IM is a useful therapeutic option given its relatively short treatment course as compared to other topically applied agents, as well as its lasting efficacy, mild AEs, and good cosmetic outcomes.
Procedural Modalities
Surgical Procedures
Surgical approaches for the treatment of AK include excision, curettage with or without electrodesiccation, and dermabrasion. In the past, these modalities were used with greater frequency, but the advent of effective topical medications with lower risks of AEs has largely reduced their use.41 Excision may still be indicated in cases where SCC is suspected, and curettage can be used for treatment of thicker hypertrophic AKs.42 Although these approaches have not been evaluated in clinical trials, they are generally effective but require the use of local anesthetics and come with substantial risk for infection, permanent scarring, and hypopigmentation. Dermabrasion employs the use of a motorized device equipped with an abrasive material to physically remove superficial layers of the skin. Studies are limited, but this method has been reported as an effective treatment in a retrospective review of 23 participants in which 96% remained free of AKs at 1 year, 83% at 2 years, 64% at 4 years, and 54% at 5 years posttherapy.43 Notably, one split-face study of 40 participants treated with dermabrasion followed by 25% TCA on one side and either Jessner solution and 35% TCA or dermabrasion alone on the other side reported that the combination of dermabrasion with 25% TCA consistently produced excellent cosmetic results with nearly complete eradication of AKs.44 In general, however, cosmetic outcomes with dermabrasion are variable, as the technique is highly operator dependent and treatment is associated with notable discomfort as well as risk for scarring and permanent pigmentation alteration.
Cryotherapy
Cryotherapy remains one of the most commonly utilized treatments of AK and involves the delivery of liquid nitrogen via a spray device or a cotton tip applicator to rapidly freeze cells, thus causing cellular destruction via ice crystal formation and protein denaturation.45 Efficacy with this technique has been reported to be as high as 98.8% at 12 months follow-up, but more recent studies cite lower rates of success.46 A prospective multicenter study of 90 participants with 421 AKs on the face or scalp treated with a single freeze-thaw cycle of liquid nitrogen reported an overall complete response rate of 67.2% at 3 months posttherapy. Additionally, higher complete response rates were associated with longer freeze times, and cosmetic outcomes were reported as good to excellent in 94% of complete response lesions.47 Similar results were reported in an open-label, prospective, randomized, controlled clinical trial of 200 participants with 543 AKs, which compared a single freeze-thaw cycle with liquid nitrogen to a single session of CO2 laser ablation in the treatment of isolated AKs of the face and scalp.48 At 3 months posttherapy, complete clearance was observed in 71.6% of participants treated with cryotherapy and in 65.3% of participants treated with laser ablation (P=.532). At 12 months posttherapy, participants who originally showed complete response at 3 months were assessed for relapse. Complete clearance was preserved in 72.6% of participants treated with cryotherapy versus 21.9% of participants treated with laser ablation (P<.0001), and cosmetic outcomes were reported by participants as good or excellent at 3 months follow-up in more than 93% of participants for both treatment arms.48 Possible AEs of cryotherapy include pain during treatment, blister formation with possible hemorrhage, infection, scarring, and permanent pigmentary changes.47,48 Notably, the risk for hypopigmentation increases with longer freezing times, thus requiring clinicians to consider the balance between improved efficacy and reduced cosmetic outcomes.47
Light-Based Therapies
Laser Therapy
Ablative laser resurfacing with either the CO2 or erbium-doped:YAG (Er:YAG) laser utilizes light of specific wavelengths to selectively induce thermolysis and destruction of the epidermal layer. Both lasers have been studied as treatments of AK, but there is a lack of large, well-designed studies. In one small study of 14 participants treated with 1 to 2 passes of the CO2 laser, complete clearance was reported in all cases without any recurrences during a follow-up period of 6 to 24 months. Additionally, all participants in this study reported satisfaction with the cosmetic outcome.49 The CO2 laser also has demonstrated efficacy comparable to that of the TCA peel and 5-FU therapy in a prospective randomized trial of 34 patients with facial or scalp AKs who received either CO2 laser with 2 passes, 30% TCA peel, or 5-FU cream 5% twice daily for 3 weeks.15 Reduction in mean AK counts at 3 months posttherapy was significantly (P<.03) higher in all treatment arms as compared to the control group (92% for CO2 laser, 89% for TCA peel, and 83% for 5-FU cream). No significant (P=.31) difference in outcomes was noted among the different treatment arms.15 Similar results were reported for the Er:YAG laser in a small prospective study of 5 participants treated with 2 to 3 passes with the Er:YAG laser in which reduction in mean AK counts was reported as ranging from 86% to 96% at 3 months posttherapy.50 The Er:YAG laser in combination with the CO2 laser has shown notable long-term efficacy in achieving higher lesion clearance rates and sustained complete clearance rates over treatment with topical 5-FU.51 In a prospective randomized study of 55 par-ticipants with multiple AKs on the face or scalp, participants were assigned to receive either combination laser ablation with the Er:YAG and CO2 lasers down to the level of the papillary dermis or 5-FU cream 5% applied twice daily for 2 to 7 weeks until an appropriate clinical inflammatory response was achieved. At 12 months follow-up, the laser treatment group achieved significantly (P=.048) higher mean lesion clearance rates (91.1%) as compared to the 5-FU arm (76.6%) and significantly (P=.003) higher sustained complete clearance rates (59.3%) as compared to 5-FU (29.2%). The proportion of participants with an improvement in photoaging score at 12 months follow-up approached statistical significance (P=.07), with 74% of the laser-treated group showing improvement as compared to 43% of the 5-FU–treated group. Long-term, cosmetically unappealing side effects such as erythema and hypopigmentation occurred notably more often in the laser-treated group as compared to the 5-FU group.51 In summary, ablative lasers appear to be a highly effective therapy for AK but at the cost of increased risk for AEs such as permanent pigmentary changes, prolonged erythema lasting up to several months, and scarring.50,52-55
Fractional photothermolysis is a relatively new advancement in the field of laser therapy that has received FDA approval for the treatment of AK.56 This treatment works by creating multiple noncontiguous microscopic columns of thermal injury while sparing adjacent zones of viable tissue.57 Although there are limited studies involving the use of such lasers in the treatment of AK, initial findings suggest that 1927-nm thulium lasers may be more effective than 1550-nm erbium lasers in achieving lesion clearance. A trial of 14 participants who received 5 laser treatments with a 1550-nm fractionated erbium-doped fiber laser reported an average reduction in AK counts of 66.2% at 3 months follow-up and a 55.6% reduction at 6 months follow-up. A participant-determined marked or very significant improvement of lesions was reported in 83% of participants at 1 month posttreatment but only in 44% of participants at 6 months posttreatment.58 A similar trial of 24 participants treated with up to 4 treatment sessions of the fractionated 1927-nm thulium laser reported an 87.3% reduction in number of AKs at 3 months follow-up and an 86.6% reduction at 6 months follow-up.56 The primary advantage of fractional laser therapy is a faster recovery period generally lasting only 2 or 3 days as compared to 2 weeks or more with traditional ablative lasers, thus limiting the amount of time a patient must tolerate cosmetically unappealing erythema.59,60 The quick recovery time has been attributed to the fractional laser’s ability to preserve the stratum corneum and skin barrier, which also helps reduce the risk for other AEs such as scarring and infection.56,59-61 Additional studies are needed to better assess the true efficacy of fractional laser therapy, but treatment with the fractional 1927-nm thulium laser appears to be a promising and well-tolerated therapeutic option for treatment of AK with similar efficacy to traditional ablative lasers but with a lower risk of AEs.
Photodynamic TherapyPhotodynamic therapy (PDT) is an FDA-approved treatment that involves the use of a topical photosensitizing agent such as 5-aminolevulinic acid (ALA) or methyl aminovulinate (MAL) before exposure to an activating light source to generate reactive oxygen species that lead to cell death.62-65 Multiple PDT regimens with varying combinations of photosensitizers, incubation time, and light sources have been studied, but a 2012 Cochrane review determined that treatment with conventional formulations of MAL and ALA with either blue- or red-light PDT were similarly efficacious for treatment of individual AKs as compared to vehicle with blue- or red-light PDT. One exception was that longer incubation time (ie, 4 hours) with ALA resulted in better results than shorter incubation times (ie, 0.5, 1, 2 hours) with ALA.66
Standard PDT treatment with MAL also has consistently demonstrated superior efficacy in achieving complete clearance rates in addition to superior cosmetic outcomes over treatment with either cryotherapy, DFS, or 5-FU.67-73 Three studies in particular noted an excellent or good investigator-determined cosmetic outcome in 96% to 98% of participants treated with MAL-PDT.69,71,74 Photodynamic therapy with ALA also has been reported as superior over CO2 laser ablation for AK reduction as well as both patient and investigator overall satisfaction.75
More recently, several methods of improving photosensitizer delivery have been studied, which have demonstrated remarkable efficacy at achieving lesion clearance over standard cream formulations or application routines. One such method involves the use of gentle heating to increase photosensitizer uptake. In a split-extremity study of 20 participants who were treated with 20% ALA under occlusion for 1 hour with one side heated to 38.8°C, the heated side demonstrated significant (P<.0001) efficacy at achieving higher median clearance rates over control when evaluated at 2 and 6 months posttherapy.76 Notably, occlusion of ALA in itself during the incubation period also has been demonstrated to significantly (P<.0001) improve clearance rates.77 Another method involves the use of a new nanoemulsion-based formulation of ALA gel, known as BF-200 ALA, which has demonstrated remarkable efficacy over standard MAL cream and placebo in a long-term follow-up analysis of 2 prospective, randomized, controlled trials.78 In a similar vein, 3 prospective randomized trials with a minimum follow-up time of 3 months demonstrated that MAL-PDT in combination with fractional ablative laser pretreatment has significant (P<.02 in all trials) efficacy over MAL-PDT without pretreatment in achieving complete AK clearance. Although the cosmetic outcomes were good or excellent in 87% to 100% of patients, they were not significantly different from stand-alone MAL-PDT treatment in any of the trials.79-81 However, pretreatment with microneedling in MAL-PDT has been shown to achieve superior cosmetic outcomes over MAL-PDT without microneedling, according to one small split-face study of 10 participants.82
Overall, PDT is an excellent therapeutic option that is able to provide efficacious clearance of AKs as well as superior cosmetic outcomes. Common AEs of PDT include burning, itching, and stinging during therapy, but pain intensity decreases dramatically upon termination of illumination, with cessation of most symptoms by 12 hours posttherapy.73 Permanent pigmentation changes have been reported to occasionally occur following PDT therapy.81
Conclusion
When determining which therapy to use in a patient, clinicians must take into account a variety of factors such as patient preference, cost of treatment, availability, tolerance for AEs, and the need for field therapy. Although all therapies discussed within this article are effective and reasonable treatment choices, patients who are particularly concerned about cosmetic outcomes would most likely benefit from either IMQ or PDT, as the data for cosmetic outcomes with these therapies are the strongest. Combination or sequential treatments may be required in some cases and all patients should be monitored for lesion recurrence regardless of treatment choice. A summary of the therapies and key studies discussed here is available in the PDF.
- Lebwohl M. Actinic keratosis: epidemiology and progression to squamous cell carcinoma. Br J Dermatol. 2003;149(suppl 66):31-33.
- Drake LA, Ceilley RI, Cornelison RL, et al. Guidelines of care for actinic keratoses. Committee on Guidelines of Care. J Am Acad Dermatol. 1995;32:95-98.
- Salasche SJ. Epidemiology of actinic keratoses and squamous cell carcinoma. J Am Acad Dermatol. 2000;42(1, pt 2):4-7.
- Ackerman AB, Mones JM. Solar (actinic) keratosis is squamous cell carcinoma. Br J Dermatol. 2006;155:9-22.
- Anwar J, Wrone DA, Kimyai-Asadi A, et al. The development of actinic keratosis into invasive squamous cell carcinoma: evidence and evolving classification schemes. Clin Dermatol. 2004;22:189-196.
- Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
- Glogau RG. The risk of progression to invasive disease. J Am Acad Dermatol. 2000;42(1, pt 2):23-24.
- Esmann S, Jemec GB. Management of actinic keratosis patients: a qualitative study. J Dermatolog Treat. 2007;18:53-58.
- Weinstock MA, Lee KC, Chren MM, et al. Quality of life in the actinic neoplasia syndrome: the VA Topical Tretinoin Chemoprevention (VATTC) trial. J Am Acad Dermatol. 2009;61:207-215.
- Berman B, Villa AM, Ramirez CC. Mechanisms of action of new treatment modalities for actinic keratosis. J Drugs Dermatol. 2006;5:167-173.
- Askew DA, Mickan SM, Soyer HP. Effectiveness of 5-fluorouracil treatment for actinic keratosis: a systematic review of randomized controlled trials. Int J Dermatol. 2009;46:452-463.
- Levy S, Furst K, Chern W. A pharmacokinetic evaluation of 0.5% and 5% fluorouracil topical cream in patients with actinic keratosis. Clin Ther. 2001;23:908-920.
- Jorizzo J, Stewart D, Bucko A, et al. Randomized trial evaluating a new 0.5% fluorouracil formulation demonstrates efficacy after 1-, 2-, or 4-week treatment in patients with actinic keratosis. Cutis. 2002;70:335-359.
- Sachs DL, Kang S, Hammerberg C, et al. Topical fluorouracil for actinic keratoses and photoaging: a clinical and molecular analysis. Arch Dermatol. 2009;145:659-666.
- Hantash BM, Stewart DB, Cooper ZA, et al. Facial resurfacing for nonmelanoma skin cancer prophylaxis. Arch Dermatol. 2006;142:976-982.
- Lawrence N, Cox SE, Cockerell CJ, et al. A comparison of the efficacy and safety of Jessner’s solution and 35% trichloroacetic acid vs 5% fluorouracil in the treatment of widespread facial actinic keratoses. Arch Dermatol. 1995;131:176-181.
- Monheit GD. The Jessner’s + TCA peel: a medium-depth chemical peel. J Dermatol Surg Oncol. 1989;15:945-950.
- Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196-200.
- Adamson DJ, Frew D, Tatoud R, et al. Diclofenac antagonizes peroxisome proliferator-activated receptor-gamma signaling. Mol Pharmacol. 2002;61:7-12.
- Alam CA, Seed MP, Willoughby DA. Angiostasis and vascular regression in chronic granulomatous inflammation induced by diclofenac in combination with hyaluronan in mice. J Pharm Pharmacol. 1995;47:407-411.
- Lu X, Xie W, Reed D, et al. Nonsteroidal antiinflammatory drugs cause apoptosis and induce cyclooxygenases in chicken embryo fibroblasts. Proc Natl Acad Sci USA. 1995;92:7961-7965.
- Seed MP, Brown JR, Freemantle CN, et al. The inhibition of colon-26 adenocarcinoma development and angiogenesis by topical diclofenac in 2.5% hyaluronan. Cancer Res. 1997;57:1625-1629.
- Rivers JK, Arlette J, Shear N, et al. Topical treatment of actinic keratoses with 3.0% diclofenac in 2.5% hyaluronan gel. Br J Dermatol. 2002;146:94-100.
- Wolf JE, Taylor JR, Tschen E, et al. Topical 3.0% diclo-fenac in 2.5% hyaluronan gel in the treatment of actinic keratoses. Int J Dermatol. 2001;40:709-713.
- Nelson C, Rigel D, Smith S, et al. Phase IV, open-label assessment of the treatment of actinic keratosis with 3.0% diclofenac sodium topical gel (Solaraze). J Drugs Dermatol. 2004;3:401-407.
- Pflugfelder A, Welter AK, Leiter U, et al. Open label randomized study comparing 3 months vs. 6 months treatment of actinic keratoses with 3% diclofenac in 2.5% hyaluronic acid gel: a trial of the German Dermatologic Cooperative Oncology Group. J Eur Acad Dermatol Venereol. 2012;26:48-53.
- Smith SR, Morhenn VB, Piacquadio DJ. Bilateral comparison of the efficacy and tolerability of 3% diclofenac sodium gel and 5% 5-fluorouracil cream in the treatment of actinic keratoses of the face and scalp. J Drugs Dermatol. 2006;5:156-159.
- Segatto MM, Dornelles SI, Silveira VB, et al. Comparative study of actinic keratosis treatment with 3% diclo- fenac sodium and 5% 5-fluorouracil. An Bras Dermatol. 2013;88:732-738.
- Vidal D. Topical imiquimod: mechanism of action and clinical applications. Mini Rev Med Chem. 2006;6:499-503.
- Hadley G, Derry S, Moore RA. Imiquimod for actinic keratosis: systematic review and meta-analysis. J Invest Dermatol. 2006;126:1251-1255.
- Caperton C, Berman B. Safety, efficacy, and patient acceptability of imiquimod for topical treatment of actinic keratoses. Clin Cosmet Investig Dermatol. 2011;4:35-40.
- Swanson N, Smith CC, Kaur M, et al. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: two phase 3, multicenter, randomized, double-blind, placebo-controlled studies. J Drugs Dermatol. 2014;13:166-169.
- Krawtchenko N, Roewert-Huber J, Ulrich M, et al. A randomised study of topical 5% imiquimod vs. topical 5-fluorouracil vs. cryosurgery in immunocompetent patients with actinic keratoses: a comparison of clinical and histological outcomes including 1-year follow-up. Br J Dermatol. 2007;157(suppl 2):34-40.
- Anderson L, Schmieder GJ, Werschler WP, et al. Randomized, double-blind, double-dummy, vehicle-controlled study of ingenol mebutate gel 0.025% and 0.05% for actinic keratosis. J Am Acad Dermatol. 2009;60:934-943.
- Ogbourne SM, Suhrbier A, Jones B, et al. Antitumor activity of 3-ingenyl angelate: plasma membrane and mitochondrial disruption and necrotic cell death. Cancer Res. 2004;64:2833-2839.
- Lebwohl M, Swanson N, Anderson LL, et al. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366:1010-1019.
- Lebwohl M, Shumack S, Stein-Gold L, et al. Long-term follow-up study of ingenol mebutate gel for the treatment of actinic keratoses. JAMA Dermatol. 2013;149:666-670.
- Augustin M, Tu JH, Knudsen KM, et al. Ingenol mebutate gel for actinic keratosis: the link between quality of life, treatment satisfaction, and clinical outcomes. J Am Acad Dermatol. 2015;72:816-821.
- Kane-Maguire N, Moseley R, Cozzi S, et al. Modulation of fibroblast phenotype and extracellular matrix composition by ingenol mebutate may be associated with scar resolution and improved dermal cosmesis. J Am Acad Dermatol. 2012;66:AB218.
- Martin G, Swanson N. Clinical findings using ingenol mebutate gel to treat actinic keratoses. J Am Acad Dermatol. 2013;68(1, suppl 1):S39-S48.
- Feldman SR, Fleischer AB, Williford PM, et al. Destructive procedures are the standard of care for treatment of actinic keratoses. J Am Acad Dermatol. 1999;40:43-47.
- Berlin JM. Current and emerging treatment strategies for the treatment of actinic keratosis. Clin Cosmet Investig Dermatol. 2010;3:119-126.
- Coleman WP, Yarborough JM, Mandy SH. Dermabrasion for prophylaxis and treatment of actinic keratoses. Dermatol Surg. 1996;22:17-21.
- Cooley JE, Casey DL, Kauffman CL. Manual resurfacing and trichloroacetic acid for the treatment of patients with widespread actinic damage. clinical and histologic observations. Dermatol Surg. 1997;23:373-379.
- Goldberg LH, Kaplan B, Vergilis-Kalner I, et al. Liquid nitrogen: temperature control in the treatment of actinic keratosis. Dermatol Surg. 2010;36:1956-1961.
- Lubritz RR, Smolewski SA. Cryosurgery cure rate of actinic keratoses. J Am Acad Dermatol. 1982;7:631-632.
- Thai KE, Fergin P, Freeman M, et al. A prospective study of the use of cryosurgery for the treatment of actinic keratoses. Int J Dermatol. 2004;43:687-692.
- Zane C, Facchinetti E, Rossi MT, et al. Cryotherapy is preferable to ablative CO2 laser for the treatment of isolated actinic keratoses of the face and scalp: a randomized clinical trial. Br J Dermatol. 2014;170:1114-1121.
- Trimas SJ, Ellis DA, Metz RD. The carbon dioxide laser. an alternative for the treatment of actinically damaged skin. Dermatol Surg. 1997;23:885-889.
- Jiang SB, Levine VJ, Nehal KS, et al. Er:YAG laser for the treatment of actinic keratoses. Dermatol Surg. 2000;26:437-440.
- Ostertag JU, Quaedvlieg PJ, Van der geer S, et al. A clinical comparison and long-term follow-up of topical 5-fluorouracil versus laser resurfacing in the treatment of widespread actinic keratoses. Lasers Surg Med. 2006;38:731-739.
- Iyer S, Friedli A, Bowes L, et al. Full face laser resurfacing: therapy and prophylaxis for actinic keratoses and non-melanoma skin cancer. Lasers Surg Med. 2004;34:114-119.
- Rubin MG. A peeler’s thoughts on skin improvement with chemical peels and laser resurfacing. Clin Plast Surg. 1997;24:407-409.
- Riggs K, Keller M, Humphreys TR. Ablative laser resurfacing: high-energy pulsed carbon dioxide and erbium:yttrium-aluminum-garnet. Clin Dermatol. 2007;25:462-473.
- Adrian RM. Pulsed carbon dioxide and long pulse 10-ms erbium-YAG laser resurfacing: a comparative clinical and histological study. J Cutan Laser Ther. 1999;1:197-202.
- Weiss ET, Brauer JA, Anolik R, et al. 1927-nm fractional resurfacing of facial actinic keratoses: a promising new therapeutic option. J Am Acad Dermatol. 2013; 68:98-102.
- Manstein D, Herron GS, Sink RK, et al. Fractional photothermolysis: a new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg Med. 2004;34:426-438.
- Katz TM, Goldberg LH, Marquez D, et al. Nonablative fractional photothermolysis for facial actinic keratoses: 6-month follow-up with histologic evaluation. J Am Acad Dermatol. 2011;65:349-356.
- Prens SP, De Vries K, Neumann HA, et al. Non-ablative fractional resurfacing in combination with topical tretinoin cream as a field treatment modality for multiple actinic keratosis: a pilot study and a review of other field treatment modalities. J Dermatolog Treat. 2013;24:227-231.
- Alexiades-Armenakas MR, Dover JS, Arndt KA. The spectrum of laser skin resurfacing: nonablative, fractional, and ablative laser resurfacing. J Am Acad Dermatol. 2008;58:719-737.
- Tannous Z. Fractional resurfacing. Clin Dermatol. 2007;25:480-486.
- Gold MH. Continuing medical education article-skin treatment: photodynamic therapy: indications and treatment. Aesthet Surg J. 2008;28:545-552.
- Juarranz A, Jaén P, Sanz-Rodríguez F, et al. Photodynamic therapy of cancer. basic principles and applications. Clin Transl Oncol. 2008;10:148-154.
- Juzeniene A, Peng Q, Moan J. Milestones in the development of photodynamic therapy and fluorescence diagnosis. Photochem Photobiol Sci. 2007;6:1234-1245.
- Moan J, Berg K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem Photobiol. 1991;53:549-553.
- Gupta AK, Paquet M, Villanueva E, et al. Interventions for actinic keratoses. Cochrane Database Syst Rev. 2012;12:CD004415.
- Patel G, Armstrong AW, Eisen DB. Efficacy of photodynamic therapy vs other interventions in randomized clinical trials for the treatment of actinic keratoses: a systematic review and meta-analysis. JAMA Dermatol. 2014;150:1281-1288.
- Kaufmann R, Spelman L, Weightman W, et al. Multicentre intraindividual randomized trial of topical methyl aminolaevulinate-photodynamic therapy vs. cryotherapy for multiple actinic keratoses on the extremities. Br J Dermatol. 2008;158:994-999.
- Freeman M, Vinciullo C, Francis D, et al. A comparison of photodynamic therapy using topical methyl aminolevulinate (Metvix) with single cycle cryotherapy in patients with actinic keratosis: a prospective, randomized study. J Dermatolog Treat. 2003;14:99-106.
- Morton C, Campbell S, Gupta G, et al. Intraindividual, right-left comparison of topical methyl aminolaevulinate-photodynamic therapy and cryotherapy in subjects with actinic keratoses: a multicentre, randomized controlled study. Br J Dermatol. 2006;155:1029-1036.
- Pariser DM, Lowe NJ, Stewart DM, et al. Photodynamic therapy with topical methyl aminolevulinate for actinic keratosis: results of a prospective randomized multicenter trial. J Am Acad Dermatol. 2003;48:227-232.
- Zane C, Facchinetti E, Rossi MT, et al. A randomized clinical trial of photodynamic therapy with methyl aminolaevulinate vs. diclofenac 3% plus hyaluronic acid gel for the treatment of multiple actinic keratoses of the face and scalp. Br J Dermatol. 2014;170:1143-1150.
- Perrett CM, McGregor JM, Warwick J, et al. Treatment of post-transplant premalignant skin disease: a randomized intrapatient comparative study of 5-fluorouracil cream and topical photodynamic therapy. Br J Dermatol. 2007;156:320-328.
- Szeimies RM, Karrer S, Radakovic-Fijan S, et al. Photodynamic therapy using topical methyl 5-aminolevulinate compared with cryotherapy for actinic keratosis: a prospective, randomized study. J Am Acad Dermatol. 2002; 47:258-262.
- Scola N, Terras S, Georgas D, et al. A randomized, half-side comparative study of aminolaevulinate photodynamic therapy vs. CO(2) laser ablation in immunocompetent patients with multiple actinic keratoses. Br J Dermatol. 2012;167:1366-1373.
- Willey A, Anderson RR, Sakamoto FH. Temperature-modulated photodynamic therapy for the treatment of actinic keratosis on the extremities: a pilot study. Dermatol Surg. 2014;40:1094-1102.
- Pariser DM. Management of Actinic Keratoses: Treatment Selection and Optimizing Outcomes. Presented at: Winter Clinical Dermatology Conference Hawaii; January 18, 2015; Kaanapali, HI.
- Dirschka T, Radny P, Dominicus R, et al. Long-term (6 and 12 months) follow-up of two prospective, randomized, controlled phase III trials of photodynamic therapy with BF-200 ALA and methyl aminolaevulinate for the treatment of actinic keratosis. Br J Dermatol. 2013;168:825-836.
- Choi SH, Kim KH, Song KH. Efficacy of ablative fractional laser-assisted photodynamic therapy with short-incubation time for the treatment of facial and scalp actinic keratosis: 12-month follow-up results of a randomized, prospective, comparative trial. J Eur Acad Dermatol Venereol. 2015;29:1598-1605.
- Ko DY, Jeon SY, Kim KH, et al. Fractional erbium:YAG laser-assisted photodynamic therapy for facial actinic keratoses: a randomized, comparative, prospective study. J Eur Acad Dermatol Venereol. 2014;28:1529-1539.
- Togsverd-Ho K, Haak CS, Thaysen-Petersen D, et al. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol. 2012;166:1262-1269.
- Torezan L, Chaves Y, Niwa A, et al. A pilot split-face study comparing conventional methyl aminolevulinate-photodynamic therapy (PDT) with microneedling-assisted PDT on actinically damaged skin. Dermatol Surg. 2013;39:1197-1201.
Actinic keratosis (AK), also referred to as solar keratosis or senile keratosis, is an intraepidermal proliferation of dysplastic keratinocytes that develops in response to chronic exposure to UV radiation. Actinic keratoses are among the most commonly encountered lesions seen by dermatologists, and it has been estimated that 60% of predisposed individuals older than 40 years have at least one AK.1,2 Prevalence is notably higher in light-skinned individuals and increases with age, presumably from higher cumulative sun exposure and decreased effectiveness of the immune system.1,3 It remains a point of contention as to whether or not AKs actually represent squamous cell carcinoma (SCC) in situ, but the potential for progression to invasive disease has been well demonstrated, as the majority of SCCs develop from preexisting AKs.4-6 The risk for progression to invasive disease for an individual AK has been estimated to range from 0.025% to 16% per year, with an average of approximately 8% in immunocompetent patients.7
The clinical morphology of AK can vary widely, but the most common presentation is an erythematous scaly macule, papule, or plaque on sun-exposed skin. The skin surrounding AKs typically shows evidence of solar damage with deep wrinkling, mottled pigmentation, scattered telangiectases, purpura, or xerosis (Figure). A variety of clinical variants with unique presentations exist, including atrophic, hypertrophic, acantholytic, lichenoid, bowenoid, and pigmented subtypes. Because more than 80% of AKs occur on highly visible areas such as the head, neck, back of the hands, and forearms, AKs can have an obvious detrimental effect on cosmetic appearance. Studies also have shown a strong association between AKs and decreased overall quality of life (QOL).3,8,9

Because of the risk for AK progression to invasive cancer along with its negative impact on cosmesis and QOL, clinicians generally opt to treat AKs. Numerous different treatment options exist, including topical medications, procedural modalities, and light-based therapies. Here, we review the efficacy of the most commonly utilized treatments and discuss the relevant cosmetic considerations and outcomes.
Topical Treatments
5-Fluorouracil
5-Fluorouracil (5-FU) is a US Food and Drug Administration (FDA)–approved, topically applied pyrimidine analogue that inhibits thymidylate synthase. The resulting suppression of DNA and RNA synthesis induces cell death with a preference for mitotically active cells.10 5-Fluorouracil has been used for more than 50 years as a treatment of AK and its efficacy is well established. A systematic review of 5 randomized controlled studies of topical 5-FU reported an average of 49% of 423 patients achieving complete lesion clearance with 5-FU cream 5% applied once or twice daily for up to 7 weeks.11 Some notable drawbacks of 5-FU, however, are application-site erythema, blistering, pruritus, necrosis, erosion, and pain. These effects often lead to premature cessation of therapy, but newer formulations of 5-FU cream 0.5% have shown good efficacy with better tolerability.12 A randomized, double-blind, multicenter, parallel-group study of 177 patients using 5-FU cream 0.5% once daily for either 1, 2, or 4 weeks demonstrated significant (P<.001) efficacy over vehicle gel in all treatment arms.13 The most effective therapy was 4 weeks of treatment, which achieved a mean 91.7% reduction in lesion count as assessed 1 month after cessation of therapy. The primary adverse effect (AE) reported in this trial was mild to moderate facial irritation, which generally resolved within 18 to 21 days after treatment cessation.13 Overall, 5-FU is a highly effective therapy for treating AKs that also can improve signs of photoaging, but patients should be aware of cosmetically unappealing effects that generally occur throughout therapy and during the immediate posttreatment period.14
Chemical Peels
Chemical peels traditionally employ acidic compounds to strip away outer layers of skin to variable depths depending on the concentration of the agent being applied. For treatment of AK, trichloroacetic acid (TCA) is a commonly employed cauterant that has shown efficacy comparable to topical 5-FU as well as ablative CO2 laser resurfacing.15 Trichloroacetic acid peels also are a convenient therapy, as good results can be achieved after a single treatment session. A split-face study of 15 patients treated with either a single application of 35% TCA and Jessner solution or twice-daily application of 5-FU cream 5% for 3 weeks demonstrated a reduction in 75% of visible AKs in both treatment arms over a 1-year follow-up period.16 Although 80% of patients self-reported considerable cosmetic improvement with both therapies, patient preference was reported to be in favor of the TCA peel, given its quick results and relatively mild side effects as compared to 5-FU. Treatment with chemical peels will result in temporary erythema and mild desquamation that usually resolves within 2 weeks; however, there are cases in which erythema has been reported to persist for several months.16 Adverse effects such as permanent scarring or pigmentation changes rarely are seen with TCA concentrations less than 45%.17 Caution should be used in patients with a history of herpes simplex virus, keloids, postinflammatory hyperpigmentation, radiation exposure, immunosuppression, and those unable or unwilling to use sunscreen and avoid sun exposure in the immediate posttreatment period.
Diclofenac Sodium
Diclofenac sodium (DFS) is an FDA-approved topical, nonsteroidal,
anti-inflammatory drug whose mechanism of action in the treatment of AK is thought to involve inhibition of the cyclooxygenase 2 enzyme.18 The resulting reduction of prostaglandins is believed to inhibit tumor angiogenesis, induce apoptosis, and inhibit cell differentiation.19-22 In a multicenter, double-blind, placebo-controlled study of 195 patients, application of DFS 3% in hyaluronan gel 2.5% twice daily for 60 days showed significant (P<.05) efficacy over placebo in achieving complete resolution of target lesions during a 30-day follow-up period (31% vs 10%). Furthermore, qualitative patient assessment of complete global improvement also was significantly (P<.05) higher in the active treatment group as compared to placebo (31% vs 10%).23 Additional studies of DFS 3% in hyaluronan gel 2.5% applied twice daily for 90 days have shown even higher rates of success, with complete resolution of target lesions in 40% to 58% of cases.24,25 This therapy also has been reported to substantially improve QOL following treatment completion.26 The most frequently cited AEs include pruritus, rash, dry skin, erythema, and application-site reactions. Overall, DFS is a
well-tolerated therapy with efficacy comparable to that of 5-FU but with a lower incidence of AEs
and higher patient satisfaction as determined in
2 head-to-head studies.27,28
ImiquimodImiquimod (IMQ) is an FDA-approved topical agent that functions as an immune response modifier via agonism of toll-like receptor 7.18 The resulting cytokine production and release enhances the innate and acquired immune responses leading to anticancer activity.29 The efficacy of IMQ for treatment of AK has been demonstrated in numerous well-designed clinical trials. A
meta-analysis of 5 randomized, double-blind trials including 1293 patients treated with IMQ cream 5%
2 to 3 times per week for 12 to 16 weeks reported complete clearance of AKs in 50% of patients treated with IMQ as compared to 5% of patients treated with vehicle.30 The most frequently reported AEs with this therapy include erythema, scabbing, flaking, and erosion. These effects generally resolve following cessation of treatment, and therapy is considered to be well tolerated; however, there are case reports of IMQ triggering or exacerbating existing inflammatory conditions.31 Imiquimod cream also is approved at 2.5% and 3.75% concentrations, which have demonstrated significant (P<.001) efficacy over placebo and a reduced incidence of AEs; complete clearance rates have been reported as 30.6% and 35.6%, respectively.32 Notably, a study comparing 75 patients randomized to either IMQ cream 5%
3 times per week for 4 weeks, 1 or 2 courses of cryosurgery, or 5-FU ointment 5% twice daily for 4 weeks reported that IMQ achieved significantly (P<.01) superior sustained clearance rates during a 12-month follow-up period over cryosurgery and 5-FU
(73% vs 4% vs 33%).33 Additionally, cosmetic outcomes as determined by both participants and investigators were reported as excellent at 12 months posttreatment in more than 80% of participants treated with IMQ. These excellent, long-lasting cosmetic outcomes also were determined to be significantly (P<.0001) superior to the cosmetic outcomes of 5-FU and cryotherapy, which both reported excellent outcomes in less than 10% of cases.33
Ingenol MebutateIngenol mebutate (IM) is a macrocyclic diterpene ester derived from the Euphorbia peplus plant that is FDA approved for the treatment of AK.1 Ingenol mebutate’s mechanism of action is thought to involve induction of cell death via disruption of the plasma membrane and mitochondria in addition to production of an inflammatory response, which produces tumor-specific antibodies and a large influx of neutrophils.34,35 The overall evidence for the efficacy of IM is strong. A combined analysis of 4 multicenter, randomized, double-blind studies of 1005 participants reported that IM gel 0.015% applied once daily for 3 days to the face or scalp was significantly superior (P<.001) to placebo in achieving complete clearance as assessed 54 days after completion of therapy (42.2% vs 3.7%) and that IM gel 0.05% applied once daily for 2 days to the trunk or extremities also was significantly superior (P<.001) to placebo in achieving complete clearance as determined 55 days after completion of therapy (34.1% vs 4.7%).36 A follow-up report to this study indicated that IM also appears to achieve long-lasting effects with an overall 87% decrease in total AKs at 12 months follow-up in both trial groups.37 Additionally, it has been recently reported that treatment with IM in these trials was associated with significantly higher overall treatment satisfaction (P<.001) and improved QOL (P<.001) as compared to vehicle.38 Cosmetic outcomes of IM therapy have been assessed in a trial analyzing the efficacy of IM gel 0.025% for 3 days or IM gel 0.05% for 2 or 3 days on nonfacial AKs. This study reported significantly (P<.0001) higher patient satisfaction with the cosmetic outcome at 8 weeks after therapy as compared to vehicle.34 Studies performed in mice have demonstrated that IM is able to promote collagen matrix turnover and impose dermal elasticity, which may contribute to these good cosmetic outcomes.39 The most common AEs of IM therapy are erythema, crusting, and flaking; these effects generally occur 3 to 8 days after starting treatment. These effects, however, generally are short lived and resolve within 2 weeks of treatment cessation when IM is applied to the face or scalp or 4 weeks when applied to the trunk or extremities.40 Overall, IM is a useful therapeutic option given its relatively short treatment course as compared to other topically applied agents, as well as its lasting efficacy, mild AEs, and good cosmetic outcomes.
Procedural Modalities
Surgical Procedures
Surgical approaches for the treatment of AK include excision, curettage with or without electrodesiccation, and dermabrasion. In the past, these modalities were used with greater frequency, but the advent of effective topical medications with lower risks of AEs has largely reduced their use.41 Excision may still be indicated in cases where SCC is suspected, and curettage can be used for treatment of thicker hypertrophic AKs.42 Although these approaches have not been evaluated in clinical trials, they are generally effective but require the use of local anesthetics and come with substantial risk for infection, permanent scarring, and hypopigmentation. Dermabrasion employs the use of a motorized device equipped with an abrasive material to physically remove superficial layers of the skin. Studies are limited, but this method has been reported as an effective treatment in a retrospective review of 23 participants in which 96% remained free of AKs at 1 year, 83% at 2 years, 64% at 4 years, and 54% at 5 years posttherapy.43 Notably, one split-face study of 40 participants treated with dermabrasion followed by 25% TCA on one side and either Jessner solution and 35% TCA or dermabrasion alone on the other side reported that the combination of dermabrasion with 25% TCA consistently produced excellent cosmetic results with nearly complete eradication of AKs.44 In general, however, cosmetic outcomes with dermabrasion are variable, as the technique is highly operator dependent and treatment is associated with notable discomfort as well as risk for scarring and permanent pigmentation alteration.
Cryotherapy
Cryotherapy remains one of the most commonly utilized treatments of AK and involves the delivery of liquid nitrogen via a spray device or a cotton tip applicator to rapidly freeze cells, thus causing cellular destruction via ice crystal formation and protein denaturation.45 Efficacy with this technique has been reported to be as high as 98.8% at 12 months follow-up, but more recent studies cite lower rates of success.46 A prospective multicenter study of 90 participants with 421 AKs on the face or scalp treated with a single freeze-thaw cycle of liquid nitrogen reported an overall complete response rate of 67.2% at 3 months posttherapy. Additionally, higher complete response rates were associated with longer freeze times, and cosmetic outcomes were reported as good to excellent in 94% of complete response lesions.47 Similar results were reported in an open-label, prospective, randomized, controlled clinical trial of 200 participants with 543 AKs, which compared a single freeze-thaw cycle with liquid nitrogen to a single session of CO2 laser ablation in the treatment of isolated AKs of the face and scalp.48 At 3 months posttherapy, complete clearance was observed in 71.6% of participants treated with cryotherapy and in 65.3% of participants treated with laser ablation (P=.532). At 12 months posttherapy, participants who originally showed complete response at 3 months were assessed for relapse. Complete clearance was preserved in 72.6% of participants treated with cryotherapy versus 21.9% of participants treated with laser ablation (P<.0001), and cosmetic outcomes were reported by participants as good or excellent at 3 months follow-up in more than 93% of participants for both treatment arms.48 Possible AEs of cryotherapy include pain during treatment, blister formation with possible hemorrhage, infection, scarring, and permanent pigmentary changes.47,48 Notably, the risk for hypopigmentation increases with longer freezing times, thus requiring clinicians to consider the balance between improved efficacy and reduced cosmetic outcomes.47
Light-Based Therapies
Laser Therapy
Ablative laser resurfacing with either the CO2 or erbium-doped:YAG (Er:YAG) laser utilizes light of specific wavelengths to selectively induce thermolysis and destruction of the epidermal layer. Both lasers have been studied as treatments of AK, but there is a lack of large, well-designed studies. In one small study of 14 participants treated with 1 to 2 passes of the CO2 laser, complete clearance was reported in all cases without any recurrences during a follow-up period of 6 to 24 months. Additionally, all participants in this study reported satisfaction with the cosmetic outcome.49 The CO2 laser also has demonstrated efficacy comparable to that of the TCA peel and 5-FU therapy in a prospective randomized trial of 34 patients with facial or scalp AKs who received either CO2 laser with 2 passes, 30% TCA peel, or 5-FU cream 5% twice daily for 3 weeks.15 Reduction in mean AK counts at 3 months posttherapy was significantly (P<.03) higher in all treatment arms as compared to the control group (92% for CO2 laser, 89% for TCA peel, and 83% for 5-FU cream). No significant (P=.31) difference in outcomes was noted among the different treatment arms.15 Similar results were reported for the Er:YAG laser in a small prospective study of 5 participants treated with 2 to 3 passes with the Er:YAG laser in which reduction in mean AK counts was reported as ranging from 86% to 96% at 3 months posttherapy.50 The Er:YAG laser in combination with the CO2 laser has shown notable long-term efficacy in achieving higher lesion clearance rates and sustained complete clearance rates over treatment with topical 5-FU.51 In a prospective randomized study of 55 par-ticipants with multiple AKs on the face or scalp, participants were assigned to receive either combination laser ablation with the Er:YAG and CO2 lasers down to the level of the papillary dermis or 5-FU cream 5% applied twice daily for 2 to 7 weeks until an appropriate clinical inflammatory response was achieved. At 12 months follow-up, the laser treatment group achieved significantly (P=.048) higher mean lesion clearance rates (91.1%) as compared to the 5-FU arm (76.6%) and significantly (P=.003) higher sustained complete clearance rates (59.3%) as compared to 5-FU (29.2%). The proportion of participants with an improvement in photoaging score at 12 months follow-up approached statistical significance (P=.07), with 74% of the laser-treated group showing improvement as compared to 43% of the 5-FU–treated group. Long-term, cosmetically unappealing side effects such as erythema and hypopigmentation occurred notably more often in the laser-treated group as compared to the 5-FU group.51 In summary, ablative lasers appear to be a highly effective therapy for AK but at the cost of increased risk for AEs such as permanent pigmentary changes, prolonged erythema lasting up to several months, and scarring.50,52-55
Fractional photothermolysis is a relatively new advancement in the field of laser therapy that has received FDA approval for the treatment of AK.56 This treatment works by creating multiple noncontiguous microscopic columns of thermal injury while sparing adjacent zones of viable tissue.57 Although there are limited studies involving the use of such lasers in the treatment of AK, initial findings suggest that 1927-nm thulium lasers may be more effective than 1550-nm erbium lasers in achieving lesion clearance. A trial of 14 participants who received 5 laser treatments with a 1550-nm fractionated erbium-doped fiber laser reported an average reduction in AK counts of 66.2% at 3 months follow-up and a 55.6% reduction at 6 months follow-up. A participant-determined marked or very significant improvement of lesions was reported in 83% of participants at 1 month posttreatment but only in 44% of participants at 6 months posttreatment.58 A similar trial of 24 participants treated with up to 4 treatment sessions of the fractionated 1927-nm thulium laser reported an 87.3% reduction in number of AKs at 3 months follow-up and an 86.6% reduction at 6 months follow-up.56 The primary advantage of fractional laser therapy is a faster recovery period generally lasting only 2 or 3 days as compared to 2 weeks or more with traditional ablative lasers, thus limiting the amount of time a patient must tolerate cosmetically unappealing erythema.59,60 The quick recovery time has been attributed to the fractional laser’s ability to preserve the stratum corneum and skin barrier, which also helps reduce the risk for other AEs such as scarring and infection.56,59-61 Additional studies are needed to better assess the true efficacy of fractional laser therapy, but treatment with the fractional 1927-nm thulium laser appears to be a promising and well-tolerated therapeutic option for treatment of AK with similar efficacy to traditional ablative lasers but with a lower risk of AEs.
Photodynamic TherapyPhotodynamic therapy (PDT) is an FDA-approved treatment that involves the use of a topical photosensitizing agent such as 5-aminolevulinic acid (ALA) or methyl aminovulinate (MAL) before exposure to an activating light source to generate reactive oxygen species that lead to cell death.62-65 Multiple PDT regimens with varying combinations of photosensitizers, incubation time, and light sources have been studied, but a 2012 Cochrane review determined that treatment with conventional formulations of MAL and ALA with either blue- or red-light PDT were similarly efficacious for treatment of individual AKs as compared to vehicle with blue- or red-light PDT. One exception was that longer incubation time (ie, 4 hours) with ALA resulted in better results than shorter incubation times (ie, 0.5, 1, 2 hours) with ALA.66
Standard PDT treatment with MAL also has consistently demonstrated superior efficacy in achieving complete clearance rates in addition to superior cosmetic outcomes over treatment with either cryotherapy, DFS, or 5-FU.67-73 Three studies in particular noted an excellent or good investigator-determined cosmetic outcome in 96% to 98% of participants treated with MAL-PDT.69,71,74 Photodynamic therapy with ALA also has been reported as superior over CO2 laser ablation for AK reduction as well as both patient and investigator overall satisfaction.75
More recently, several methods of improving photosensitizer delivery have been studied, which have demonstrated remarkable efficacy at achieving lesion clearance over standard cream formulations or application routines. One such method involves the use of gentle heating to increase photosensitizer uptake. In a split-extremity study of 20 participants who were treated with 20% ALA under occlusion for 1 hour with one side heated to 38.8°C, the heated side demonstrated significant (P<.0001) efficacy at achieving higher median clearance rates over control when evaluated at 2 and 6 months posttherapy.76 Notably, occlusion of ALA in itself during the incubation period also has been demonstrated to significantly (P<.0001) improve clearance rates.77 Another method involves the use of a new nanoemulsion-based formulation of ALA gel, known as BF-200 ALA, which has demonstrated remarkable efficacy over standard MAL cream and placebo in a long-term follow-up analysis of 2 prospective, randomized, controlled trials.78 In a similar vein, 3 prospective randomized trials with a minimum follow-up time of 3 months demonstrated that MAL-PDT in combination with fractional ablative laser pretreatment has significant (P<.02 in all trials) efficacy over MAL-PDT without pretreatment in achieving complete AK clearance. Although the cosmetic outcomes were good or excellent in 87% to 100% of patients, they were not significantly different from stand-alone MAL-PDT treatment in any of the trials.79-81 However, pretreatment with microneedling in MAL-PDT has been shown to achieve superior cosmetic outcomes over MAL-PDT without microneedling, according to one small split-face study of 10 participants.82
Overall, PDT is an excellent therapeutic option that is able to provide efficacious clearance of AKs as well as superior cosmetic outcomes. Common AEs of PDT include burning, itching, and stinging during therapy, but pain intensity decreases dramatically upon termination of illumination, with cessation of most symptoms by 12 hours posttherapy.73 Permanent pigmentation changes have been reported to occasionally occur following PDT therapy.81
Conclusion
When determining which therapy to use in a patient, clinicians must take into account a variety of factors such as patient preference, cost of treatment, availability, tolerance for AEs, and the need for field therapy. Although all therapies discussed within this article are effective and reasonable treatment choices, patients who are particularly concerned about cosmetic outcomes would most likely benefit from either IMQ or PDT, as the data for cosmetic outcomes with these therapies are the strongest. Combination or sequential treatments may be required in some cases and all patients should be monitored for lesion recurrence regardless of treatment choice. A summary of the therapies and key studies discussed here is available in the PDF.
Actinic keratosis (AK), also referred to as solar keratosis or senile keratosis, is an intraepidermal proliferation of dysplastic keratinocytes that develops in response to chronic exposure to UV radiation. Actinic keratoses are among the most commonly encountered lesions seen by dermatologists, and it has been estimated that 60% of predisposed individuals older than 40 years have at least one AK.1,2 Prevalence is notably higher in light-skinned individuals and increases with age, presumably from higher cumulative sun exposure and decreased effectiveness of the immune system.1,3 It remains a point of contention as to whether or not AKs actually represent squamous cell carcinoma (SCC) in situ, but the potential for progression to invasive disease has been well demonstrated, as the majority of SCCs develop from preexisting AKs.4-6 The risk for progression to invasive disease for an individual AK has been estimated to range from 0.025% to 16% per year, with an average of approximately 8% in immunocompetent patients.7
The clinical morphology of AK can vary widely, but the most common presentation is an erythematous scaly macule, papule, or plaque on sun-exposed skin. The skin surrounding AKs typically shows evidence of solar damage with deep wrinkling, mottled pigmentation, scattered telangiectases, purpura, or xerosis (Figure). A variety of clinical variants with unique presentations exist, including atrophic, hypertrophic, acantholytic, lichenoid, bowenoid, and pigmented subtypes. Because more than 80% of AKs occur on highly visible areas such as the head, neck, back of the hands, and forearms, AKs can have an obvious detrimental effect on cosmetic appearance. Studies also have shown a strong association between AKs and decreased overall quality of life (QOL).3,8,9

Because of the risk for AK progression to invasive cancer along with its negative impact on cosmesis and QOL, clinicians generally opt to treat AKs. Numerous different treatment options exist, including topical medications, procedural modalities, and light-based therapies. Here, we review the efficacy of the most commonly utilized treatments and discuss the relevant cosmetic considerations and outcomes.
Topical Treatments
5-Fluorouracil
5-Fluorouracil (5-FU) is a US Food and Drug Administration (FDA)–approved, topically applied pyrimidine analogue that inhibits thymidylate synthase. The resulting suppression of DNA and RNA synthesis induces cell death with a preference for mitotically active cells.10 5-Fluorouracil has been used for more than 50 years as a treatment of AK and its efficacy is well established. A systematic review of 5 randomized controlled studies of topical 5-FU reported an average of 49% of 423 patients achieving complete lesion clearance with 5-FU cream 5% applied once or twice daily for up to 7 weeks.11 Some notable drawbacks of 5-FU, however, are application-site erythema, blistering, pruritus, necrosis, erosion, and pain. These effects often lead to premature cessation of therapy, but newer formulations of 5-FU cream 0.5% have shown good efficacy with better tolerability.12 A randomized, double-blind, multicenter, parallel-group study of 177 patients using 5-FU cream 0.5% once daily for either 1, 2, or 4 weeks demonstrated significant (P<.001) efficacy over vehicle gel in all treatment arms.13 The most effective therapy was 4 weeks of treatment, which achieved a mean 91.7% reduction in lesion count as assessed 1 month after cessation of therapy. The primary adverse effect (AE) reported in this trial was mild to moderate facial irritation, which generally resolved within 18 to 21 days after treatment cessation.13 Overall, 5-FU is a highly effective therapy for treating AKs that also can improve signs of photoaging, but patients should be aware of cosmetically unappealing effects that generally occur throughout therapy and during the immediate posttreatment period.14
Chemical Peels
Chemical peels traditionally employ acidic compounds to strip away outer layers of skin to variable depths depending on the concentration of the agent being applied. For treatment of AK, trichloroacetic acid (TCA) is a commonly employed cauterant that has shown efficacy comparable to topical 5-FU as well as ablative CO2 laser resurfacing.15 Trichloroacetic acid peels also are a convenient therapy, as good results can be achieved after a single treatment session. A split-face study of 15 patients treated with either a single application of 35% TCA and Jessner solution or twice-daily application of 5-FU cream 5% for 3 weeks demonstrated a reduction in 75% of visible AKs in both treatment arms over a 1-year follow-up period.16 Although 80% of patients self-reported considerable cosmetic improvement with both therapies, patient preference was reported to be in favor of the TCA peel, given its quick results and relatively mild side effects as compared to 5-FU. Treatment with chemical peels will result in temporary erythema and mild desquamation that usually resolves within 2 weeks; however, there are cases in which erythema has been reported to persist for several months.16 Adverse effects such as permanent scarring or pigmentation changes rarely are seen with TCA concentrations less than 45%.17 Caution should be used in patients with a history of herpes simplex virus, keloids, postinflammatory hyperpigmentation, radiation exposure, immunosuppression, and those unable or unwilling to use sunscreen and avoid sun exposure in the immediate posttreatment period.
Diclofenac Sodium
Diclofenac sodium (DFS) is an FDA-approved topical, nonsteroidal,
anti-inflammatory drug whose mechanism of action in the treatment of AK is thought to involve inhibition of the cyclooxygenase 2 enzyme.18 The resulting reduction of prostaglandins is believed to inhibit tumor angiogenesis, induce apoptosis, and inhibit cell differentiation.19-22 In a multicenter, double-blind, placebo-controlled study of 195 patients, application of DFS 3% in hyaluronan gel 2.5% twice daily for 60 days showed significant (P<.05) efficacy over placebo in achieving complete resolution of target lesions during a 30-day follow-up period (31% vs 10%). Furthermore, qualitative patient assessment of complete global improvement also was significantly (P<.05) higher in the active treatment group as compared to placebo (31% vs 10%).23 Additional studies of DFS 3% in hyaluronan gel 2.5% applied twice daily for 90 days have shown even higher rates of success, with complete resolution of target lesions in 40% to 58% of cases.24,25 This therapy also has been reported to substantially improve QOL following treatment completion.26 The most frequently cited AEs include pruritus, rash, dry skin, erythema, and application-site reactions. Overall, DFS is a
well-tolerated therapy with efficacy comparable to that of 5-FU but with a lower incidence of AEs
and higher patient satisfaction as determined in
2 head-to-head studies.27,28
ImiquimodImiquimod (IMQ) is an FDA-approved topical agent that functions as an immune response modifier via agonism of toll-like receptor 7.18 The resulting cytokine production and release enhances the innate and acquired immune responses leading to anticancer activity.29 The efficacy of IMQ for treatment of AK has been demonstrated in numerous well-designed clinical trials. A
meta-analysis of 5 randomized, double-blind trials including 1293 patients treated with IMQ cream 5%
2 to 3 times per week for 12 to 16 weeks reported complete clearance of AKs in 50% of patients treated with IMQ as compared to 5% of patients treated with vehicle.30 The most frequently reported AEs with this therapy include erythema, scabbing, flaking, and erosion. These effects generally resolve following cessation of treatment, and therapy is considered to be well tolerated; however, there are case reports of IMQ triggering or exacerbating existing inflammatory conditions.31 Imiquimod cream also is approved at 2.5% and 3.75% concentrations, which have demonstrated significant (P<.001) efficacy over placebo and a reduced incidence of AEs; complete clearance rates have been reported as 30.6% and 35.6%, respectively.32 Notably, a study comparing 75 patients randomized to either IMQ cream 5%
3 times per week for 4 weeks, 1 or 2 courses of cryosurgery, or 5-FU ointment 5% twice daily for 4 weeks reported that IMQ achieved significantly (P<.01) superior sustained clearance rates during a 12-month follow-up period over cryosurgery and 5-FU
(73% vs 4% vs 33%).33 Additionally, cosmetic outcomes as determined by both participants and investigators were reported as excellent at 12 months posttreatment in more than 80% of participants treated with IMQ. These excellent, long-lasting cosmetic outcomes also were determined to be significantly (P<.0001) superior to the cosmetic outcomes of 5-FU and cryotherapy, which both reported excellent outcomes in less than 10% of cases.33
Ingenol MebutateIngenol mebutate (IM) is a macrocyclic diterpene ester derived from the Euphorbia peplus plant that is FDA approved for the treatment of AK.1 Ingenol mebutate’s mechanism of action is thought to involve induction of cell death via disruption of the plasma membrane and mitochondria in addition to production of an inflammatory response, which produces tumor-specific antibodies and a large influx of neutrophils.34,35 The overall evidence for the efficacy of IM is strong. A combined analysis of 4 multicenter, randomized, double-blind studies of 1005 participants reported that IM gel 0.015% applied once daily for 3 days to the face or scalp was significantly superior (P<.001) to placebo in achieving complete clearance as assessed 54 days after completion of therapy (42.2% vs 3.7%) and that IM gel 0.05% applied once daily for 2 days to the trunk or extremities also was significantly superior (P<.001) to placebo in achieving complete clearance as determined 55 days after completion of therapy (34.1% vs 4.7%).36 A follow-up report to this study indicated that IM also appears to achieve long-lasting effects with an overall 87% decrease in total AKs at 12 months follow-up in both trial groups.37 Additionally, it has been recently reported that treatment with IM in these trials was associated with significantly higher overall treatment satisfaction (P<.001) and improved QOL (P<.001) as compared to vehicle.38 Cosmetic outcomes of IM therapy have been assessed in a trial analyzing the efficacy of IM gel 0.025% for 3 days or IM gel 0.05% for 2 or 3 days on nonfacial AKs. This study reported significantly (P<.0001) higher patient satisfaction with the cosmetic outcome at 8 weeks after therapy as compared to vehicle.34 Studies performed in mice have demonstrated that IM is able to promote collagen matrix turnover and impose dermal elasticity, which may contribute to these good cosmetic outcomes.39 The most common AEs of IM therapy are erythema, crusting, and flaking; these effects generally occur 3 to 8 days after starting treatment. These effects, however, generally are short lived and resolve within 2 weeks of treatment cessation when IM is applied to the face or scalp or 4 weeks when applied to the trunk or extremities.40 Overall, IM is a useful therapeutic option given its relatively short treatment course as compared to other topically applied agents, as well as its lasting efficacy, mild AEs, and good cosmetic outcomes.
Procedural Modalities
Surgical Procedures
Surgical approaches for the treatment of AK include excision, curettage with or without electrodesiccation, and dermabrasion. In the past, these modalities were used with greater frequency, but the advent of effective topical medications with lower risks of AEs has largely reduced their use.41 Excision may still be indicated in cases where SCC is suspected, and curettage can be used for treatment of thicker hypertrophic AKs.42 Although these approaches have not been evaluated in clinical trials, they are generally effective but require the use of local anesthetics and come with substantial risk for infection, permanent scarring, and hypopigmentation. Dermabrasion employs the use of a motorized device equipped with an abrasive material to physically remove superficial layers of the skin. Studies are limited, but this method has been reported as an effective treatment in a retrospective review of 23 participants in which 96% remained free of AKs at 1 year, 83% at 2 years, 64% at 4 years, and 54% at 5 years posttherapy.43 Notably, one split-face study of 40 participants treated with dermabrasion followed by 25% TCA on one side and either Jessner solution and 35% TCA or dermabrasion alone on the other side reported that the combination of dermabrasion with 25% TCA consistently produced excellent cosmetic results with nearly complete eradication of AKs.44 In general, however, cosmetic outcomes with dermabrasion are variable, as the technique is highly operator dependent and treatment is associated with notable discomfort as well as risk for scarring and permanent pigmentation alteration.
Cryotherapy
Cryotherapy remains one of the most commonly utilized treatments of AK and involves the delivery of liquid nitrogen via a spray device or a cotton tip applicator to rapidly freeze cells, thus causing cellular destruction via ice crystal formation and protein denaturation.45 Efficacy with this technique has been reported to be as high as 98.8% at 12 months follow-up, but more recent studies cite lower rates of success.46 A prospective multicenter study of 90 participants with 421 AKs on the face or scalp treated with a single freeze-thaw cycle of liquid nitrogen reported an overall complete response rate of 67.2% at 3 months posttherapy. Additionally, higher complete response rates were associated with longer freeze times, and cosmetic outcomes were reported as good to excellent in 94% of complete response lesions.47 Similar results were reported in an open-label, prospective, randomized, controlled clinical trial of 200 participants with 543 AKs, which compared a single freeze-thaw cycle with liquid nitrogen to a single session of CO2 laser ablation in the treatment of isolated AKs of the face and scalp.48 At 3 months posttherapy, complete clearance was observed in 71.6% of participants treated with cryotherapy and in 65.3% of participants treated with laser ablation (P=.532). At 12 months posttherapy, participants who originally showed complete response at 3 months were assessed for relapse. Complete clearance was preserved in 72.6% of participants treated with cryotherapy versus 21.9% of participants treated with laser ablation (P<.0001), and cosmetic outcomes were reported by participants as good or excellent at 3 months follow-up in more than 93% of participants for both treatment arms.48 Possible AEs of cryotherapy include pain during treatment, blister formation with possible hemorrhage, infection, scarring, and permanent pigmentary changes.47,48 Notably, the risk for hypopigmentation increases with longer freezing times, thus requiring clinicians to consider the balance between improved efficacy and reduced cosmetic outcomes.47
Light-Based Therapies
Laser Therapy
Ablative laser resurfacing with either the CO2 or erbium-doped:YAG (Er:YAG) laser utilizes light of specific wavelengths to selectively induce thermolysis and destruction of the epidermal layer. Both lasers have been studied as treatments of AK, but there is a lack of large, well-designed studies. In one small study of 14 participants treated with 1 to 2 passes of the CO2 laser, complete clearance was reported in all cases without any recurrences during a follow-up period of 6 to 24 months. Additionally, all participants in this study reported satisfaction with the cosmetic outcome.49 The CO2 laser also has demonstrated efficacy comparable to that of the TCA peel and 5-FU therapy in a prospective randomized trial of 34 patients with facial or scalp AKs who received either CO2 laser with 2 passes, 30% TCA peel, or 5-FU cream 5% twice daily for 3 weeks.15 Reduction in mean AK counts at 3 months posttherapy was significantly (P<.03) higher in all treatment arms as compared to the control group (92% for CO2 laser, 89% for TCA peel, and 83% for 5-FU cream). No significant (P=.31) difference in outcomes was noted among the different treatment arms.15 Similar results were reported for the Er:YAG laser in a small prospective study of 5 participants treated with 2 to 3 passes with the Er:YAG laser in which reduction in mean AK counts was reported as ranging from 86% to 96% at 3 months posttherapy.50 The Er:YAG laser in combination with the CO2 laser has shown notable long-term efficacy in achieving higher lesion clearance rates and sustained complete clearance rates over treatment with topical 5-FU.51 In a prospective randomized study of 55 par-ticipants with multiple AKs on the face or scalp, participants were assigned to receive either combination laser ablation with the Er:YAG and CO2 lasers down to the level of the papillary dermis or 5-FU cream 5% applied twice daily for 2 to 7 weeks until an appropriate clinical inflammatory response was achieved. At 12 months follow-up, the laser treatment group achieved significantly (P=.048) higher mean lesion clearance rates (91.1%) as compared to the 5-FU arm (76.6%) and significantly (P=.003) higher sustained complete clearance rates (59.3%) as compared to 5-FU (29.2%). The proportion of participants with an improvement in photoaging score at 12 months follow-up approached statistical significance (P=.07), with 74% of the laser-treated group showing improvement as compared to 43% of the 5-FU–treated group. Long-term, cosmetically unappealing side effects such as erythema and hypopigmentation occurred notably more often in the laser-treated group as compared to the 5-FU group.51 In summary, ablative lasers appear to be a highly effective therapy for AK but at the cost of increased risk for AEs such as permanent pigmentary changes, prolonged erythema lasting up to several months, and scarring.50,52-55
Fractional photothermolysis is a relatively new advancement in the field of laser therapy that has received FDA approval for the treatment of AK.56 This treatment works by creating multiple noncontiguous microscopic columns of thermal injury while sparing adjacent zones of viable tissue.57 Although there are limited studies involving the use of such lasers in the treatment of AK, initial findings suggest that 1927-nm thulium lasers may be more effective than 1550-nm erbium lasers in achieving lesion clearance. A trial of 14 participants who received 5 laser treatments with a 1550-nm fractionated erbium-doped fiber laser reported an average reduction in AK counts of 66.2% at 3 months follow-up and a 55.6% reduction at 6 months follow-up. A participant-determined marked or very significant improvement of lesions was reported in 83% of participants at 1 month posttreatment but only in 44% of participants at 6 months posttreatment.58 A similar trial of 24 participants treated with up to 4 treatment sessions of the fractionated 1927-nm thulium laser reported an 87.3% reduction in number of AKs at 3 months follow-up and an 86.6% reduction at 6 months follow-up.56 The primary advantage of fractional laser therapy is a faster recovery period generally lasting only 2 or 3 days as compared to 2 weeks or more with traditional ablative lasers, thus limiting the amount of time a patient must tolerate cosmetically unappealing erythema.59,60 The quick recovery time has been attributed to the fractional laser’s ability to preserve the stratum corneum and skin barrier, which also helps reduce the risk for other AEs such as scarring and infection.56,59-61 Additional studies are needed to better assess the true efficacy of fractional laser therapy, but treatment with the fractional 1927-nm thulium laser appears to be a promising and well-tolerated therapeutic option for treatment of AK with similar efficacy to traditional ablative lasers but with a lower risk of AEs.
Photodynamic TherapyPhotodynamic therapy (PDT) is an FDA-approved treatment that involves the use of a topical photosensitizing agent such as 5-aminolevulinic acid (ALA) or methyl aminovulinate (MAL) before exposure to an activating light source to generate reactive oxygen species that lead to cell death.62-65 Multiple PDT regimens with varying combinations of photosensitizers, incubation time, and light sources have been studied, but a 2012 Cochrane review determined that treatment with conventional formulations of MAL and ALA with either blue- or red-light PDT were similarly efficacious for treatment of individual AKs as compared to vehicle with blue- or red-light PDT. One exception was that longer incubation time (ie, 4 hours) with ALA resulted in better results than shorter incubation times (ie, 0.5, 1, 2 hours) with ALA.66
Standard PDT treatment with MAL also has consistently demonstrated superior efficacy in achieving complete clearance rates in addition to superior cosmetic outcomes over treatment with either cryotherapy, DFS, or 5-FU.67-73 Three studies in particular noted an excellent or good investigator-determined cosmetic outcome in 96% to 98% of participants treated with MAL-PDT.69,71,74 Photodynamic therapy with ALA also has been reported as superior over CO2 laser ablation for AK reduction as well as both patient and investigator overall satisfaction.75
More recently, several methods of improving photosensitizer delivery have been studied, which have demonstrated remarkable efficacy at achieving lesion clearance over standard cream formulations or application routines. One such method involves the use of gentle heating to increase photosensitizer uptake. In a split-extremity study of 20 participants who were treated with 20% ALA under occlusion for 1 hour with one side heated to 38.8°C, the heated side demonstrated significant (P<.0001) efficacy at achieving higher median clearance rates over control when evaluated at 2 and 6 months posttherapy.76 Notably, occlusion of ALA in itself during the incubation period also has been demonstrated to significantly (P<.0001) improve clearance rates.77 Another method involves the use of a new nanoemulsion-based formulation of ALA gel, known as BF-200 ALA, which has demonstrated remarkable efficacy over standard MAL cream and placebo in a long-term follow-up analysis of 2 prospective, randomized, controlled trials.78 In a similar vein, 3 prospective randomized trials with a minimum follow-up time of 3 months demonstrated that MAL-PDT in combination with fractional ablative laser pretreatment has significant (P<.02 in all trials) efficacy over MAL-PDT without pretreatment in achieving complete AK clearance. Although the cosmetic outcomes were good or excellent in 87% to 100% of patients, they were not significantly different from stand-alone MAL-PDT treatment in any of the trials.79-81 However, pretreatment with microneedling in MAL-PDT has been shown to achieve superior cosmetic outcomes over MAL-PDT without microneedling, according to one small split-face study of 10 participants.82
Overall, PDT is an excellent therapeutic option that is able to provide efficacious clearance of AKs as well as superior cosmetic outcomes. Common AEs of PDT include burning, itching, and stinging during therapy, but pain intensity decreases dramatically upon termination of illumination, with cessation of most symptoms by 12 hours posttherapy.73 Permanent pigmentation changes have been reported to occasionally occur following PDT therapy.81
Conclusion
When determining which therapy to use in a patient, clinicians must take into account a variety of factors such as patient preference, cost of treatment, availability, tolerance for AEs, and the need for field therapy. Although all therapies discussed within this article are effective and reasonable treatment choices, patients who are particularly concerned about cosmetic outcomes would most likely benefit from either IMQ or PDT, as the data for cosmetic outcomes with these therapies are the strongest. Combination or sequential treatments may be required in some cases and all patients should be monitored for lesion recurrence regardless of treatment choice. A summary of the therapies and key studies discussed here is available in the PDF.
- Lebwohl M. Actinic keratosis: epidemiology and progression to squamous cell carcinoma. Br J Dermatol. 2003;149(suppl 66):31-33.
- Drake LA, Ceilley RI, Cornelison RL, et al. Guidelines of care for actinic keratoses. Committee on Guidelines of Care. J Am Acad Dermatol. 1995;32:95-98.
- Salasche SJ. Epidemiology of actinic keratoses and squamous cell carcinoma. J Am Acad Dermatol. 2000;42(1, pt 2):4-7.
- Ackerman AB, Mones JM. Solar (actinic) keratosis is squamous cell carcinoma. Br J Dermatol. 2006;155:9-22.
- Anwar J, Wrone DA, Kimyai-Asadi A, et al. The development of actinic keratosis into invasive squamous cell carcinoma: evidence and evolving classification schemes. Clin Dermatol. 2004;22:189-196.
- Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
- Glogau RG. The risk of progression to invasive disease. J Am Acad Dermatol. 2000;42(1, pt 2):23-24.
- Esmann S, Jemec GB. Management of actinic keratosis patients: a qualitative study. J Dermatolog Treat. 2007;18:53-58.
- Weinstock MA, Lee KC, Chren MM, et al. Quality of life in the actinic neoplasia syndrome: the VA Topical Tretinoin Chemoprevention (VATTC) trial. J Am Acad Dermatol. 2009;61:207-215.
- Berman B, Villa AM, Ramirez CC. Mechanisms of action of new treatment modalities for actinic keratosis. J Drugs Dermatol. 2006;5:167-173.
- Askew DA, Mickan SM, Soyer HP. Effectiveness of 5-fluorouracil treatment for actinic keratosis: a systematic review of randomized controlled trials. Int J Dermatol. 2009;46:452-463.
- Levy S, Furst K, Chern W. A pharmacokinetic evaluation of 0.5% and 5% fluorouracil topical cream in patients with actinic keratosis. Clin Ther. 2001;23:908-920.
- Jorizzo J, Stewart D, Bucko A, et al. Randomized trial evaluating a new 0.5% fluorouracil formulation demonstrates efficacy after 1-, 2-, or 4-week treatment in patients with actinic keratosis. Cutis. 2002;70:335-359.
- Sachs DL, Kang S, Hammerberg C, et al. Topical fluorouracil for actinic keratoses and photoaging: a clinical and molecular analysis. Arch Dermatol. 2009;145:659-666.
- Hantash BM, Stewart DB, Cooper ZA, et al. Facial resurfacing for nonmelanoma skin cancer prophylaxis. Arch Dermatol. 2006;142:976-982.
- Lawrence N, Cox SE, Cockerell CJ, et al. A comparison of the efficacy and safety of Jessner’s solution and 35% trichloroacetic acid vs 5% fluorouracil in the treatment of widespread facial actinic keratoses. Arch Dermatol. 1995;131:176-181.
- Monheit GD. The Jessner’s + TCA peel: a medium-depth chemical peel. J Dermatol Surg Oncol. 1989;15:945-950.
- Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196-200.
- Adamson DJ, Frew D, Tatoud R, et al. Diclofenac antagonizes peroxisome proliferator-activated receptor-gamma signaling. Mol Pharmacol. 2002;61:7-12.
- Alam CA, Seed MP, Willoughby DA. Angiostasis and vascular regression in chronic granulomatous inflammation induced by diclofenac in combination with hyaluronan in mice. J Pharm Pharmacol. 1995;47:407-411.
- Lu X, Xie W, Reed D, et al. Nonsteroidal antiinflammatory drugs cause apoptosis and induce cyclooxygenases in chicken embryo fibroblasts. Proc Natl Acad Sci USA. 1995;92:7961-7965.
- Seed MP, Brown JR, Freemantle CN, et al. The inhibition of colon-26 adenocarcinoma development and angiogenesis by topical diclofenac in 2.5% hyaluronan. Cancer Res. 1997;57:1625-1629.
- Rivers JK, Arlette J, Shear N, et al. Topical treatment of actinic keratoses with 3.0% diclofenac in 2.5% hyaluronan gel. Br J Dermatol. 2002;146:94-100.
- Wolf JE, Taylor JR, Tschen E, et al. Topical 3.0% diclo-fenac in 2.5% hyaluronan gel in the treatment of actinic keratoses. Int J Dermatol. 2001;40:709-713.
- Nelson C, Rigel D, Smith S, et al. Phase IV, open-label assessment of the treatment of actinic keratosis with 3.0% diclofenac sodium topical gel (Solaraze). J Drugs Dermatol. 2004;3:401-407.
- Pflugfelder A, Welter AK, Leiter U, et al. Open label randomized study comparing 3 months vs. 6 months treatment of actinic keratoses with 3% diclofenac in 2.5% hyaluronic acid gel: a trial of the German Dermatologic Cooperative Oncology Group. J Eur Acad Dermatol Venereol. 2012;26:48-53.
- Smith SR, Morhenn VB, Piacquadio DJ. Bilateral comparison of the efficacy and tolerability of 3% diclofenac sodium gel and 5% 5-fluorouracil cream in the treatment of actinic keratoses of the face and scalp. J Drugs Dermatol. 2006;5:156-159.
- Segatto MM, Dornelles SI, Silveira VB, et al. Comparative study of actinic keratosis treatment with 3% diclo- fenac sodium and 5% 5-fluorouracil. An Bras Dermatol. 2013;88:732-738.
- Vidal D. Topical imiquimod: mechanism of action and clinical applications. Mini Rev Med Chem. 2006;6:499-503.
- Hadley G, Derry S, Moore RA. Imiquimod for actinic keratosis: systematic review and meta-analysis. J Invest Dermatol. 2006;126:1251-1255.
- Caperton C, Berman B. Safety, efficacy, and patient acceptability of imiquimod for topical treatment of actinic keratoses. Clin Cosmet Investig Dermatol. 2011;4:35-40.
- Swanson N, Smith CC, Kaur M, et al. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: two phase 3, multicenter, randomized, double-blind, placebo-controlled studies. J Drugs Dermatol. 2014;13:166-169.
- Krawtchenko N, Roewert-Huber J, Ulrich M, et al. A randomised study of topical 5% imiquimod vs. topical 5-fluorouracil vs. cryosurgery in immunocompetent patients with actinic keratoses: a comparison of clinical and histological outcomes including 1-year follow-up. Br J Dermatol. 2007;157(suppl 2):34-40.
- Anderson L, Schmieder GJ, Werschler WP, et al. Randomized, double-blind, double-dummy, vehicle-controlled study of ingenol mebutate gel 0.025% and 0.05% for actinic keratosis. J Am Acad Dermatol. 2009;60:934-943.
- Ogbourne SM, Suhrbier A, Jones B, et al. Antitumor activity of 3-ingenyl angelate: plasma membrane and mitochondrial disruption and necrotic cell death. Cancer Res. 2004;64:2833-2839.
- Lebwohl M, Swanson N, Anderson LL, et al. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366:1010-1019.
- Lebwohl M, Shumack S, Stein-Gold L, et al. Long-term follow-up study of ingenol mebutate gel for the treatment of actinic keratoses. JAMA Dermatol. 2013;149:666-670.
- Augustin M, Tu JH, Knudsen KM, et al. Ingenol mebutate gel for actinic keratosis: the link between quality of life, treatment satisfaction, and clinical outcomes. J Am Acad Dermatol. 2015;72:816-821.
- Kane-Maguire N, Moseley R, Cozzi S, et al. Modulation of fibroblast phenotype and extracellular matrix composition by ingenol mebutate may be associated with scar resolution and improved dermal cosmesis. J Am Acad Dermatol. 2012;66:AB218.
- Martin G, Swanson N. Clinical findings using ingenol mebutate gel to treat actinic keratoses. J Am Acad Dermatol. 2013;68(1, suppl 1):S39-S48.
- Feldman SR, Fleischer AB, Williford PM, et al. Destructive procedures are the standard of care for treatment of actinic keratoses. J Am Acad Dermatol. 1999;40:43-47.
- Berlin JM. Current and emerging treatment strategies for the treatment of actinic keratosis. Clin Cosmet Investig Dermatol. 2010;3:119-126.
- Coleman WP, Yarborough JM, Mandy SH. Dermabrasion for prophylaxis and treatment of actinic keratoses. Dermatol Surg. 1996;22:17-21.
- Cooley JE, Casey DL, Kauffman CL. Manual resurfacing and trichloroacetic acid for the treatment of patients with widespread actinic damage. clinical and histologic observations. Dermatol Surg. 1997;23:373-379.
- Goldberg LH, Kaplan B, Vergilis-Kalner I, et al. Liquid nitrogen: temperature control in the treatment of actinic keratosis. Dermatol Surg. 2010;36:1956-1961.
- Lubritz RR, Smolewski SA. Cryosurgery cure rate of actinic keratoses. J Am Acad Dermatol. 1982;7:631-632.
- Thai KE, Fergin P, Freeman M, et al. A prospective study of the use of cryosurgery for the treatment of actinic keratoses. Int J Dermatol. 2004;43:687-692.
- Zane C, Facchinetti E, Rossi MT, et al. Cryotherapy is preferable to ablative CO2 laser for the treatment of isolated actinic keratoses of the face and scalp: a randomized clinical trial. Br J Dermatol. 2014;170:1114-1121.
- Trimas SJ, Ellis DA, Metz RD. The carbon dioxide laser. an alternative for the treatment of actinically damaged skin. Dermatol Surg. 1997;23:885-889.
- Jiang SB, Levine VJ, Nehal KS, et al. Er:YAG laser for the treatment of actinic keratoses. Dermatol Surg. 2000;26:437-440.
- Ostertag JU, Quaedvlieg PJ, Van der geer S, et al. A clinical comparison and long-term follow-up of topical 5-fluorouracil versus laser resurfacing in the treatment of widespread actinic keratoses. Lasers Surg Med. 2006;38:731-739.
- Iyer S, Friedli A, Bowes L, et al. Full face laser resurfacing: therapy and prophylaxis for actinic keratoses and non-melanoma skin cancer. Lasers Surg Med. 2004;34:114-119.
- Rubin MG. A peeler’s thoughts on skin improvement with chemical peels and laser resurfacing. Clin Plast Surg. 1997;24:407-409.
- Riggs K, Keller M, Humphreys TR. Ablative laser resurfacing: high-energy pulsed carbon dioxide and erbium:yttrium-aluminum-garnet. Clin Dermatol. 2007;25:462-473.
- Adrian RM. Pulsed carbon dioxide and long pulse 10-ms erbium-YAG laser resurfacing: a comparative clinical and histological study. J Cutan Laser Ther. 1999;1:197-202.
- Weiss ET, Brauer JA, Anolik R, et al. 1927-nm fractional resurfacing of facial actinic keratoses: a promising new therapeutic option. J Am Acad Dermatol. 2013; 68:98-102.
- Manstein D, Herron GS, Sink RK, et al. Fractional photothermolysis: a new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg Med. 2004;34:426-438.
- Katz TM, Goldberg LH, Marquez D, et al. Nonablative fractional photothermolysis for facial actinic keratoses: 6-month follow-up with histologic evaluation. J Am Acad Dermatol. 2011;65:349-356.
- Prens SP, De Vries K, Neumann HA, et al. Non-ablative fractional resurfacing in combination with topical tretinoin cream as a field treatment modality for multiple actinic keratosis: a pilot study and a review of other field treatment modalities. J Dermatolog Treat. 2013;24:227-231.
- Alexiades-Armenakas MR, Dover JS, Arndt KA. The spectrum of laser skin resurfacing: nonablative, fractional, and ablative laser resurfacing. J Am Acad Dermatol. 2008;58:719-737.
- Tannous Z. Fractional resurfacing. Clin Dermatol. 2007;25:480-486.
- Gold MH. Continuing medical education article-skin treatment: photodynamic therapy: indications and treatment. Aesthet Surg J. 2008;28:545-552.
- Juarranz A, Jaén P, Sanz-Rodríguez F, et al. Photodynamic therapy of cancer. basic principles and applications. Clin Transl Oncol. 2008;10:148-154.
- Juzeniene A, Peng Q, Moan J. Milestones in the development of photodynamic therapy and fluorescence diagnosis. Photochem Photobiol Sci. 2007;6:1234-1245.
- Moan J, Berg K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem Photobiol. 1991;53:549-553.
- Gupta AK, Paquet M, Villanueva E, et al. Interventions for actinic keratoses. Cochrane Database Syst Rev. 2012;12:CD004415.
- Patel G, Armstrong AW, Eisen DB. Efficacy of photodynamic therapy vs other interventions in randomized clinical trials for the treatment of actinic keratoses: a systematic review and meta-analysis. JAMA Dermatol. 2014;150:1281-1288.
- Kaufmann R, Spelman L, Weightman W, et al. Multicentre intraindividual randomized trial of topical methyl aminolaevulinate-photodynamic therapy vs. cryotherapy for multiple actinic keratoses on the extremities. Br J Dermatol. 2008;158:994-999.
- Freeman M, Vinciullo C, Francis D, et al. A comparison of photodynamic therapy using topical methyl aminolevulinate (Metvix) with single cycle cryotherapy in patients with actinic keratosis: a prospective, randomized study. J Dermatolog Treat. 2003;14:99-106.
- Morton C, Campbell S, Gupta G, et al. Intraindividual, right-left comparison of topical methyl aminolaevulinate-photodynamic therapy and cryotherapy in subjects with actinic keratoses: a multicentre, randomized controlled study. Br J Dermatol. 2006;155:1029-1036.
- Pariser DM, Lowe NJ, Stewart DM, et al. Photodynamic therapy with topical methyl aminolevulinate for actinic keratosis: results of a prospective randomized multicenter trial. J Am Acad Dermatol. 2003;48:227-232.
- Zane C, Facchinetti E, Rossi MT, et al. A randomized clinical trial of photodynamic therapy with methyl aminolaevulinate vs. diclofenac 3% plus hyaluronic acid gel for the treatment of multiple actinic keratoses of the face and scalp. Br J Dermatol. 2014;170:1143-1150.
- Perrett CM, McGregor JM, Warwick J, et al. Treatment of post-transplant premalignant skin disease: a randomized intrapatient comparative study of 5-fluorouracil cream and topical photodynamic therapy. Br J Dermatol. 2007;156:320-328.
- Szeimies RM, Karrer S, Radakovic-Fijan S, et al. Photodynamic therapy using topical methyl 5-aminolevulinate compared with cryotherapy for actinic keratosis: a prospective, randomized study. J Am Acad Dermatol. 2002; 47:258-262.
- Scola N, Terras S, Georgas D, et al. A randomized, half-side comparative study of aminolaevulinate photodynamic therapy vs. CO(2) laser ablation in immunocompetent patients with multiple actinic keratoses. Br J Dermatol. 2012;167:1366-1373.
- Willey A, Anderson RR, Sakamoto FH. Temperature-modulated photodynamic therapy for the treatment of actinic keratosis on the extremities: a pilot study. Dermatol Surg. 2014;40:1094-1102.
- Pariser DM. Management of Actinic Keratoses: Treatment Selection and Optimizing Outcomes. Presented at: Winter Clinical Dermatology Conference Hawaii; January 18, 2015; Kaanapali, HI.
- Dirschka T, Radny P, Dominicus R, et al. Long-term (6 and 12 months) follow-up of two prospective, randomized, controlled phase III trials of photodynamic therapy with BF-200 ALA and methyl aminolaevulinate for the treatment of actinic keratosis. Br J Dermatol. 2013;168:825-836.
- Choi SH, Kim KH, Song KH. Efficacy of ablative fractional laser-assisted photodynamic therapy with short-incubation time for the treatment of facial and scalp actinic keratosis: 12-month follow-up results of a randomized, prospective, comparative trial. J Eur Acad Dermatol Venereol. 2015;29:1598-1605.
- Ko DY, Jeon SY, Kim KH, et al. Fractional erbium:YAG laser-assisted photodynamic therapy for facial actinic keratoses: a randomized, comparative, prospective study. J Eur Acad Dermatol Venereol. 2014;28:1529-1539.
- Togsverd-Ho K, Haak CS, Thaysen-Petersen D, et al. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol. 2012;166:1262-1269.
- Torezan L, Chaves Y, Niwa A, et al. A pilot split-face study comparing conventional methyl aminolevulinate-photodynamic therapy (PDT) with microneedling-assisted PDT on actinically damaged skin. Dermatol Surg. 2013;39:1197-1201.
- Lebwohl M. Actinic keratosis: epidemiology and progression to squamous cell carcinoma. Br J Dermatol. 2003;149(suppl 66):31-33.
- Drake LA, Ceilley RI, Cornelison RL, et al. Guidelines of care for actinic keratoses. Committee on Guidelines of Care. J Am Acad Dermatol. 1995;32:95-98.
- Salasche SJ. Epidemiology of actinic keratoses and squamous cell carcinoma. J Am Acad Dermatol. 2000;42(1, pt 2):4-7.
- Ackerman AB, Mones JM. Solar (actinic) keratosis is squamous cell carcinoma. Br J Dermatol. 2006;155:9-22.
- Anwar J, Wrone DA, Kimyai-Asadi A, et al. The development of actinic keratosis into invasive squamous cell carcinoma: evidence and evolving classification schemes. Clin Dermatol. 2004;22:189-196.
- Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
- Glogau RG. The risk of progression to invasive disease. J Am Acad Dermatol. 2000;42(1, pt 2):23-24.
- Esmann S, Jemec GB. Management of actinic keratosis patients: a qualitative study. J Dermatolog Treat. 2007;18:53-58.
- Weinstock MA, Lee KC, Chren MM, et al. Quality of life in the actinic neoplasia syndrome: the VA Topical Tretinoin Chemoprevention (VATTC) trial. J Am Acad Dermatol. 2009;61:207-215.
- Berman B, Villa AM, Ramirez CC. Mechanisms of action of new treatment modalities for actinic keratosis. J Drugs Dermatol. 2006;5:167-173.
- Askew DA, Mickan SM, Soyer HP. Effectiveness of 5-fluorouracil treatment for actinic keratosis: a systematic review of randomized controlled trials. Int J Dermatol. 2009;46:452-463.
- Levy S, Furst K, Chern W. A pharmacokinetic evaluation of 0.5% and 5% fluorouracil topical cream in patients with actinic keratosis. Clin Ther. 2001;23:908-920.
- Jorizzo J, Stewart D, Bucko A, et al. Randomized trial evaluating a new 0.5% fluorouracil formulation demonstrates efficacy after 1-, 2-, or 4-week treatment in patients with actinic keratosis. Cutis. 2002;70:335-359.
- Sachs DL, Kang S, Hammerberg C, et al. Topical fluorouracil for actinic keratoses and photoaging: a clinical and molecular analysis. Arch Dermatol. 2009;145:659-666.
- Hantash BM, Stewart DB, Cooper ZA, et al. Facial resurfacing for nonmelanoma skin cancer prophylaxis. Arch Dermatol. 2006;142:976-982.
- Lawrence N, Cox SE, Cockerell CJ, et al. A comparison of the efficacy and safety of Jessner’s solution and 35% trichloroacetic acid vs 5% fluorouracil in the treatment of widespread facial actinic keratoses. Arch Dermatol. 1995;131:176-181.
- Monheit GD. The Jessner’s + TCA peel: a medium-depth chemical peel. J Dermatol Surg Oncol. 1989;15:945-950.
- Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196-200.
- Adamson DJ, Frew D, Tatoud R, et al. Diclofenac antagonizes peroxisome proliferator-activated receptor-gamma signaling. Mol Pharmacol. 2002;61:7-12.
- Alam CA, Seed MP, Willoughby DA. Angiostasis and vascular regression in chronic granulomatous inflammation induced by diclofenac in combination with hyaluronan in mice. J Pharm Pharmacol. 1995;47:407-411.
- Lu X, Xie W, Reed D, et al. Nonsteroidal antiinflammatory drugs cause apoptosis and induce cyclooxygenases in chicken embryo fibroblasts. Proc Natl Acad Sci USA. 1995;92:7961-7965.
- Seed MP, Brown JR, Freemantle CN, et al. The inhibition of colon-26 adenocarcinoma development and angiogenesis by topical diclofenac in 2.5% hyaluronan. Cancer Res. 1997;57:1625-1629.
- Rivers JK, Arlette J, Shear N, et al. Topical treatment of actinic keratoses with 3.0% diclofenac in 2.5% hyaluronan gel. Br J Dermatol. 2002;146:94-100.
- Wolf JE, Taylor JR, Tschen E, et al. Topical 3.0% diclo-fenac in 2.5% hyaluronan gel in the treatment of actinic keratoses. Int J Dermatol. 2001;40:709-713.
- Nelson C, Rigel D, Smith S, et al. Phase IV, open-label assessment of the treatment of actinic keratosis with 3.0% diclofenac sodium topical gel (Solaraze). J Drugs Dermatol. 2004;3:401-407.
- Pflugfelder A, Welter AK, Leiter U, et al. Open label randomized study comparing 3 months vs. 6 months treatment of actinic keratoses with 3% diclofenac in 2.5% hyaluronic acid gel: a trial of the German Dermatologic Cooperative Oncology Group. J Eur Acad Dermatol Venereol. 2012;26:48-53.
- Smith SR, Morhenn VB, Piacquadio DJ. Bilateral comparison of the efficacy and tolerability of 3% diclofenac sodium gel and 5% 5-fluorouracil cream in the treatment of actinic keratoses of the face and scalp. J Drugs Dermatol. 2006;5:156-159.
- Segatto MM, Dornelles SI, Silveira VB, et al. Comparative study of actinic keratosis treatment with 3% diclo- fenac sodium and 5% 5-fluorouracil. An Bras Dermatol. 2013;88:732-738.
- Vidal D. Topical imiquimod: mechanism of action and clinical applications. Mini Rev Med Chem. 2006;6:499-503.
- Hadley G, Derry S, Moore RA. Imiquimod for actinic keratosis: systematic review and meta-analysis. J Invest Dermatol. 2006;126:1251-1255.
- Caperton C, Berman B. Safety, efficacy, and patient acceptability of imiquimod for topical treatment of actinic keratoses. Clin Cosmet Investig Dermatol. 2011;4:35-40.
- Swanson N, Smith CC, Kaur M, et al. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: two phase 3, multicenter, randomized, double-blind, placebo-controlled studies. J Drugs Dermatol. 2014;13:166-169.
- Krawtchenko N, Roewert-Huber J, Ulrich M, et al. A randomised study of topical 5% imiquimod vs. topical 5-fluorouracil vs. cryosurgery in immunocompetent patients with actinic keratoses: a comparison of clinical and histological outcomes including 1-year follow-up. Br J Dermatol. 2007;157(suppl 2):34-40.
- Anderson L, Schmieder GJ, Werschler WP, et al. Randomized, double-blind, double-dummy, vehicle-controlled study of ingenol mebutate gel 0.025% and 0.05% for actinic keratosis. J Am Acad Dermatol. 2009;60:934-943.
- Ogbourne SM, Suhrbier A, Jones B, et al. Antitumor activity of 3-ingenyl angelate: plasma membrane and mitochondrial disruption and necrotic cell death. Cancer Res. 2004;64:2833-2839.
- Lebwohl M, Swanson N, Anderson LL, et al. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366:1010-1019.
- Lebwohl M, Shumack S, Stein-Gold L, et al. Long-term follow-up study of ingenol mebutate gel for the treatment of actinic keratoses. JAMA Dermatol. 2013;149:666-670.
- Augustin M, Tu JH, Knudsen KM, et al. Ingenol mebutate gel for actinic keratosis: the link between quality of life, treatment satisfaction, and clinical outcomes. J Am Acad Dermatol. 2015;72:816-821.
- Kane-Maguire N, Moseley R, Cozzi S, et al. Modulation of fibroblast phenotype and extracellular matrix composition by ingenol mebutate may be associated with scar resolution and improved dermal cosmesis. J Am Acad Dermatol. 2012;66:AB218.
- Martin G, Swanson N. Clinical findings using ingenol mebutate gel to treat actinic keratoses. J Am Acad Dermatol. 2013;68(1, suppl 1):S39-S48.
- Feldman SR, Fleischer AB, Williford PM, et al. Destructive procedures are the standard of care for treatment of actinic keratoses. J Am Acad Dermatol. 1999;40:43-47.
- Berlin JM. Current and emerging treatment strategies for the treatment of actinic keratosis. Clin Cosmet Investig Dermatol. 2010;3:119-126.
- Coleman WP, Yarborough JM, Mandy SH. Dermabrasion for prophylaxis and treatment of actinic keratoses. Dermatol Surg. 1996;22:17-21.
- Cooley JE, Casey DL, Kauffman CL. Manual resurfacing and trichloroacetic acid for the treatment of patients with widespread actinic damage. clinical and histologic observations. Dermatol Surg. 1997;23:373-379.
- Goldberg LH, Kaplan B, Vergilis-Kalner I, et al. Liquid nitrogen: temperature control in the treatment of actinic keratosis. Dermatol Surg. 2010;36:1956-1961.
- Lubritz RR, Smolewski SA. Cryosurgery cure rate of actinic keratoses. J Am Acad Dermatol. 1982;7:631-632.
- Thai KE, Fergin P, Freeman M, et al. A prospective study of the use of cryosurgery for the treatment of actinic keratoses. Int J Dermatol. 2004;43:687-692.
- Zane C, Facchinetti E, Rossi MT, et al. Cryotherapy is preferable to ablative CO2 laser for the treatment of isolated actinic keratoses of the face and scalp: a randomized clinical trial. Br J Dermatol. 2014;170:1114-1121.
- Trimas SJ, Ellis DA, Metz RD. The carbon dioxide laser. an alternative for the treatment of actinically damaged skin. Dermatol Surg. 1997;23:885-889.
- Jiang SB, Levine VJ, Nehal KS, et al. Er:YAG laser for the treatment of actinic keratoses. Dermatol Surg. 2000;26:437-440.
- Ostertag JU, Quaedvlieg PJ, Van der geer S, et al. A clinical comparison and long-term follow-up of topical 5-fluorouracil versus laser resurfacing in the treatment of widespread actinic keratoses. Lasers Surg Med. 2006;38:731-739.
- Iyer S, Friedli A, Bowes L, et al. Full face laser resurfacing: therapy and prophylaxis for actinic keratoses and non-melanoma skin cancer. Lasers Surg Med. 2004;34:114-119.
- Rubin MG. A peeler’s thoughts on skin improvement with chemical peels and laser resurfacing. Clin Plast Surg. 1997;24:407-409.
- Riggs K, Keller M, Humphreys TR. Ablative laser resurfacing: high-energy pulsed carbon dioxide and erbium:yttrium-aluminum-garnet. Clin Dermatol. 2007;25:462-473.
- Adrian RM. Pulsed carbon dioxide and long pulse 10-ms erbium-YAG laser resurfacing: a comparative clinical and histological study. J Cutan Laser Ther. 1999;1:197-202.
- Weiss ET, Brauer JA, Anolik R, et al. 1927-nm fractional resurfacing of facial actinic keratoses: a promising new therapeutic option. J Am Acad Dermatol. 2013; 68:98-102.
- Manstein D, Herron GS, Sink RK, et al. Fractional photothermolysis: a new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg Med. 2004;34:426-438.
- Katz TM, Goldberg LH, Marquez D, et al. Nonablative fractional photothermolysis for facial actinic keratoses: 6-month follow-up with histologic evaluation. J Am Acad Dermatol. 2011;65:349-356.
- Prens SP, De Vries K, Neumann HA, et al. Non-ablative fractional resurfacing in combination with topical tretinoin cream as a field treatment modality for multiple actinic keratosis: a pilot study and a review of other field treatment modalities. J Dermatolog Treat. 2013;24:227-231.
- Alexiades-Armenakas MR, Dover JS, Arndt KA. The spectrum of laser skin resurfacing: nonablative, fractional, and ablative laser resurfacing. J Am Acad Dermatol. 2008;58:719-737.
- Tannous Z. Fractional resurfacing. Clin Dermatol. 2007;25:480-486.
- Gold MH. Continuing medical education article-skin treatment: photodynamic therapy: indications and treatment. Aesthet Surg J. 2008;28:545-552.
- Juarranz A, Jaén P, Sanz-Rodríguez F, et al. Photodynamic therapy of cancer. basic principles and applications. Clin Transl Oncol. 2008;10:148-154.
- Juzeniene A, Peng Q, Moan J. Milestones in the development of photodynamic therapy and fluorescence diagnosis. Photochem Photobiol Sci. 2007;6:1234-1245.
- Moan J, Berg K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem Photobiol. 1991;53:549-553.
- Gupta AK, Paquet M, Villanueva E, et al. Interventions for actinic keratoses. Cochrane Database Syst Rev. 2012;12:CD004415.
- Patel G, Armstrong AW, Eisen DB. Efficacy of photodynamic therapy vs other interventions in randomized clinical trials for the treatment of actinic keratoses: a systematic review and meta-analysis. JAMA Dermatol. 2014;150:1281-1288.
- Kaufmann R, Spelman L, Weightman W, et al. Multicentre intraindividual randomized trial of topical methyl aminolaevulinate-photodynamic therapy vs. cryotherapy for multiple actinic keratoses on the extremities. Br J Dermatol. 2008;158:994-999.
- Freeman M, Vinciullo C, Francis D, et al. A comparison of photodynamic therapy using topical methyl aminolevulinate (Metvix) with single cycle cryotherapy in patients with actinic keratosis: a prospective, randomized study. J Dermatolog Treat. 2003;14:99-106.
- Morton C, Campbell S, Gupta G, et al. Intraindividual, right-left comparison of topical methyl aminolaevulinate-photodynamic therapy and cryotherapy in subjects with actinic keratoses: a multicentre, randomized controlled study. Br J Dermatol. 2006;155:1029-1036.
- Pariser DM, Lowe NJ, Stewart DM, et al. Photodynamic therapy with topical methyl aminolevulinate for actinic keratosis: results of a prospective randomized multicenter trial. J Am Acad Dermatol. 2003;48:227-232.
- Zane C, Facchinetti E, Rossi MT, et al. A randomized clinical trial of photodynamic therapy with methyl aminolaevulinate vs. diclofenac 3% plus hyaluronic acid gel for the treatment of multiple actinic keratoses of the face and scalp. Br J Dermatol. 2014;170:1143-1150.
- Perrett CM, McGregor JM, Warwick J, et al. Treatment of post-transplant premalignant skin disease: a randomized intrapatient comparative study of 5-fluorouracil cream and topical photodynamic therapy. Br J Dermatol. 2007;156:320-328.
- Szeimies RM, Karrer S, Radakovic-Fijan S, et al. Photodynamic therapy using topical methyl 5-aminolevulinate compared with cryotherapy for actinic keratosis: a prospective, randomized study. J Am Acad Dermatol. 2002; 47:258-262.
- Scola N, Terras S, Georgas D, et al. A randomized, half-side comparative study of aminolaevulinate photodynamic therapy vs. CO(2) laser ablation in immunocompetent patients with multiple actinic keratoses. Br J Dermatol. 2012;167:1366-1373.
- Willey A, Anderson RR, Sakamoto FH. Temperature-modulated photodynamic therapy for the treatment of actinic keratosis on the extremities: a pilot study. Dermatol Surg. 2014;40:1094-1102.
- Pariser DM. Management of Actinic Keratoses: Treatment Selection and Optimizing Outcomes. Presented at: Winter Clinical Dermatology Conference Hawaii; January 18, 2015; Kaanapali, HI.
- Dirschka T, Radny P, Dominicus R, et al. Long-term (6 and 12 months) follow-up of two prospective, randomized, controlled phase III trials of photodynamic therapy with BF-200 ALA and methyl aminolaevulinate for the treatment of actinic keratosis. Br J Dermatol. 2013;168:825-836.
- Choi SH, Kim KH, Song KH. Efficacy of ablative fractional laser-assisted photodynamic therapy with short-incubation time for the treatment of facial and scalp actinic keratosis: 12-month follow-up results of a randomized, prospective, comparative trial. J Eur Acad Dermatol Venereol. 2015;29:1598-1605.
- Ko DY, Jeon SY, Kim KH, et al. Fractional erbium:YAG laser-assisted photodynamic therapy for facial actinic keratoses: a randomized, comparative, prospective study. J Eur Acad Dermatol Venereol. 2014;28:1529-1539.
- Togsverd-Ho K, Haak CS, Thaysen-Petersen D, et al. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol. 2012;166:1262-1269.
- Torezan L, Chaves Y, Niwa A, et al. A pilot split-face study comparing conventional methyl aminolevulinate-photodynamic therapy (PDT) with microneedling-assisted PDT on actinically damaged skin. Dermatol Surg. 2013;39:1197-1201.
Practice Points
- In addition to their risk for progression to malignancy, actinic keratoses (AKs) can have negative impacts on cosmetic appearance and quality of life.
- A variety of topical medications, procedural modalities, and light-based therapies are available for treatment of AKs, which offer varying degrees of efficacy for clearance of lesions and cosmetic outcomes. Based on the current data, imiquimod and photodynamic therapy are the treatments most likely to provide an excellent cosmetic outcome.