User login
Therapies for Actinic Keratosis With a Focus on Cosmetic Outcomes
Actinic keratosis (AK), also referred to as solar keratosis or senile keratosis, is an intraepidermal proliferation of dysplastic keratinocytes that develops in response to chronic exposure to UV radiation. Actinic keratoses are among the most commonly encountered lesions seen by dermatologists, and it has been estimated that 60% of predisposed individuals older than 40 years have at least one AK.1,2 Prevalence is notably higher in light-skinned individuals and increases with age, presumably from higher cumulative sun exposure and decreased effectiveness of the immune system.1,3 It remains a point of contention as to whether or not AKs actually represent squamous cell carcinoma (SCC) in situ, but the potential for progression to invasive disease has been well demonstrated, as the majority of SCCs develop from preexisting AKs.4-6 The risk for progression to invasive disease for an individual AK has been estimated to range from 0.025% to 16% per year, with an average of approximately 8% in immunocompetent patients.7
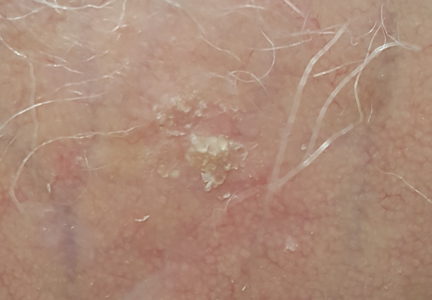
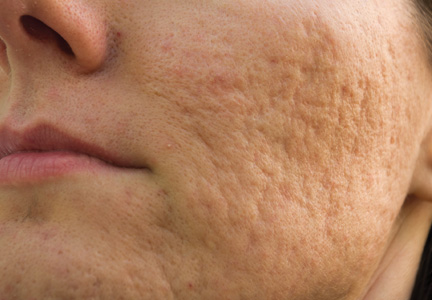
The clinical morphology of AK can vary widely, but the most common presentation is an erythematous scaly macule, papule, or plaque on sun-exposed skin. The skin surrounding AKs typically shows evidence of solar damage with deep wrinkling, mottled pigmentation, scattered telangiectases, purpura, or xerosis (Figure). A variety of clinical variants with unique presentations exist, including atrophic, hypertrophic, acantholytic, lichenoid, bowenoid, and pigmented subtypes. Because more than 80% of AKs occur on highly visible areas such as the head, neck, back of the hands, and forearms, AKs can have an obvious detrimental effect on cosmetic appearance. Studies also have shown a strong association between AKs and decreased overall quality of life (QOL).3,8,9

Because of the risk for AK progression to invasive cancer along with its negative impact on cosmesis and QOL, clinicians generally opt to treat AKs. Numerous different treatment options exist, including topical medications, procedural modalities, and light-based therapies. Here, we review the efficacy of the most commonly utilized treatments and discuss the relevant cosmetic considerations and outcomes.
Topical Treatments
5-Fluorouracil
5-Fluorouracil (5-FU) is a US Food and Drug Administration (FDA)–approved, topically applied pyrimidine analogue that inhibits thymidylate synthase. The resulting suppression of DNA and RNA synthesis induces cell death with a preference for mitotically active cells.10 5-Fluorouracil has been used for more than 50 years as a treatment of AK and its efficacy is well established. A systematic review of 5 randomized controlled studies of topical 5-FU reported an average of 49% of 423 patients achieving complete lesion clearance with 5-FU cream 5% applied once or twice daily for up to 7 weeks.11 Some notable drawbacks of 5-FU, however, are application-site erythema, blistering, pruritus, necrosis, erosion, and pain. These effects often lead to premature cessation of therapy, but newer formulations of 5-FU cream 0.5% have shown good efficacy with better tolerability.12 A randomized, double-blind, multicenter, parallel-group study of 177 patients using 5-FU cream 0.5% once daily for either 1, 2, or 4 weeks demonstrated significant (P<.001) efficacy over vehicle gel in all treatment arms.13 The most effective therapy was 4 weeks of treatment, which achieved a mean 91.7% reduction in lesion count as assessed 1 month after cessation of therapy. The primary adverse effect (AE) reported in this trial was mild to moderate facial irritation, which generally resolved within 18 to 21 days after treatment cessation.13 Overall, 5-FU is a highly effective therapy for treating AKs that also can improve signs of photoaging, but patients should be aware of cosmetically unappealing effects that generally occur throughout therapy and during the immediate posttreatment period.14
Chemical Peels
Chemical peels traditionally employ acidic compounds to strip away outer layers of skin to variable depths depending on the concentration of the agent being applied. For treatment of AK, trichloroacetic acid (TCA) is a commonly employed cauterant that has shown efficacy comparable to topical 5-FU as well as ablative CO2 laser resurfacing.15 Trichloroacetic acid peels also are a convenient therapy, as good results can be achieved after a single treatment session. A split-face study of 15 patients treated with either a single application of 35% TCA and Jessner solution or twice-daily application of 5-FU cream 5% for 3 weeks demonstrated a reduction in 75% of visible AKs in both treatment arms over a 1-year follow-up period.16 Although 80% of patients self-reported considerable cosmetic improvement with both therapies, patient preference was reported to be in favor of the TCA peel, given its quick results and relatively mild side effects as compared to 5-FU. Treatment with chemical peels will result in temporary erythema and mild desquamation that usually resolves within 2 weeks; however, there are cases in which erythema has been reported to persist for several months.16 Adverse effects such as permanent scarring or pigmentation changes rarely are seen with TCA concentrations less than 45%.17 Caution should be used in patients with a history of herpes simplex virus, keloids, postinflammatory hyperpigmentation, radiation exposure, immunosuppression, and those unable or unwilling to use sunscreen and avoid sun exposure in the immediate posttreatment period.
Diclofenac Sodium
Diclofenac sodium (DFS) is an FDA-approved topical, nonsteroidal,
anti-inflammatory drug whose mechanism of action in the treatment of AK is thought to involve inhibition of the cyclooxygenase 2 enzyme.18 The resulting reduction of prostaglandins is believed to inhibit tumor angiogenesis, induce apoptosis, and inhibit cell differentiation.19-22 In a multicenter, double-blind, placebo-controlled study of 195 patients, application of DFS 3% in hyaluronan gel 2.5% twice daily for 60 days showed significant (P<.05) efficacy over placebo in achieving complete resolution of target lesions during a 30-day follow-up period (31% vs 10%). Furthermore, qualitative patient assessment of complete global improvement also was significantly (P<.05) higher in the active treatment group as compared to placebo (31% vs 10%).23 Additional studies of DFS 3% in hyaluronan gel 2.5% applied twice daily for 90 days have shown even higher rates of success, with complete resolution of target lesions in 40% to 58% of cases.24,25 This therapy also has been reported to substantially improve QOL following treatment completion.26 The most frequently cited AEs include pruritus, rash, dry skin, erythema, and application-site reactions. Overall, DFS is a
well-tolerated therapy with efficacy comparable to that of 5-FU but with a lower incidence of AEs
and higher patient satisfaction as determined in
2 head-to-head studies.27,28
ImiquimodImiquimod (IMQ) is an FDA-approved topical agent that functions as an immune response modifier via agonism of toll-like receptor 7.18 The resulting cytokine production and release enhances the innate and acquired immune responses leading to anticancer activity.29 The efficacy of IMQ for treatment of AK has been demonstrated in numerous well-designed clinical trials. A
meta-analysis of 5 randomized, double-blind trials including 1293 patients treated with IMQ cream 5%
2 to 3 times per week for 12 to 16 weeks reported complete clearance of AKs in 50% of patients treated with IMQ as compared to 5% of patients treated with vehicle.30 The most frequently reported AEs with this therapy include erythema, scabbing, flaking, and erosion. These effects generally resolve following cessation of treatment, and therapy is considered to be well tolerated; however, there are case reports of IMQ triggering or exacerbating existing inflammatory conditions.31 Imiquimod cream also is approved at 2.5% and 3.75% concentrations, which have demonstrated significant (P<.001) efficacy over placebo and a reduced incidence of AEs; complete clearance rates have been reported as 30.6% and 35.6%, respectively.32 Notably, a study comparing 75 patients randomized to either IMQ cream 5%
3 times per week for 4 weeks, 1 or 2 courses of cryosurgery, or 5-FU ointment 5% twice daily for 4 weeks reported that IMQ achieved significantly (P<.01) superior sustained clearance rates during a 12-month follow-up period over cryosurgery and 5-FU
(73% vs 4% vs 33%).33 Additionally, cosmetic outcomes as determined by both participants and investigators were reported as excellent at 12 months posttreatment in more than 80% of participants treated with IMQ. These excellent, long-lasting cosmetic outcomes also were determined to be significantly (P<.0001) superior to the cosmetic outcomes of 5-FU and cryotherapy, which both reported excellent outcomes in less than 10% of cases.33
Ingenol MebutateIngenol mebutate (IM) is a macrocyclic diterpene ester derived from the Euphorbia peplus plant that is FDA approved for the treatment of AK.1 Ingenol mebutate’s mechanism of action is thought to involve induction of cell death via disruption of the plasma membrane and mitochondria in addition to production of an inflammatory response, which produces tumor-specific antibodies and a large influx of neutrophils.34,35 The overall evidence for the efficacy of IM is strong. A combined analysis of 4 multicenter, randomized, double-blind studies of 1005 participants reported that IM gel 0.015% applied once daily for 3 days to the face or scalp was significantly superior (P<.001) to placebo in achieving complete clearance as assessed 54 days after completion of therapy (42.2% vs 3.7%) and that IM gel 0.05% applied once daily for 2 days to the trunk or extremities also was significantly superior (P<.001) to placebo in achieving complete clearance as determined 55 days after completion of therapy (34.1% vs 4.7%).36 A follow-up report to this study indicated that IM also appears to achieve long-lasting effects with an overall 87% decrease in total AKs at 12 months follow-up in both trial groups.37 Additionally, it has been recently reported that treatment with IM in these trials was associated with significantly higher overall treatment satisfaction (P<.001) and improved QOL (P<.001) as compared to vehicle.38 Cosmetic outcomes of IM therapy have been assessed in a trial analyzing the efficacy of IM gel 0.025% for 3 days or IM gel 0.05% for 2 or 3 days on nonfacial AKs. This study reported significantly (P<.0001) higher patient satisfaction with the cosmetic outcome at 8 weeks after therapy as compared to vehicle.34 Studies performed in mice have demonstrated that IM is able to promote collagen matrix turnover and impose dermal elasticity, which may contribute to these good cosmetic outcomes.39 The most common AEs of IM therapy are erythema, crusting, and flaking; these effects generally occur 3 to 8 days after starting treatment. These effects, however, generally are short lived and resolve within 2 weeks of treatment cessation when IM is applied to the face or scalp or 4 weeks when applied to the trunk or extremities.40 Overall, IM is a useful therapeutic option given its relatively short treatment course as compared to other topically applied agents, as well as its lasting efficacy, mild AEs, and good cosmetic outcomes.
Procedural Modalities
Surgical Procedures
Surgical approaches for the treatment of AK include excision, curettage with or without electrodesiccation, and dermabrasion. In the past, these modalities were used with greater frequency, but the advent of effective topical medications with lower risks of AEs has largely reduced their use.41 Excision may still be indicated in cases where SCC is suspected, and curettage can be used for treatment of thicker hypertrophic AKs.42 Although these approaches have not been evaluated in clinical trials, they are generally effective but require the use of local anesthetics and come with substantial risk for infection, permanent scarring, and hypopigmentation. Dermabrasion employs the use of a motorized device equipped with an abrasive material to physically remove superficial layers of the skin. Studies are limited, but this method has been reported as an effective treatment in a retrospective review of 23 participants in which 96% remained free of AKs at 1 year, 83% at 2 years, 64% at 4 years, and 54% at 5 years posttherapy.43 Notably, one split-face study of 40 participants treated with dermabrasion followed by 25% TCA on one side and either Jessner solution and 35% TCA or dermabrasion alone on the other side reported that the combination of dermabrasion with 25% TCA consistently produced excellent cosmetic results with nearly complete eradication of AKs.44 In general, however, cosmetic outcomes with dermabrasion are variable, as the technique is highly operator dependent and treatment is associated with notable discomfort as well as risk for scarring and permanent pigmentation alteration.
Cryotherapy
Cryotherapy remains one of the most commonly utilized treatments of AK and involves the delivery of liquid nitrogen via a spray device or a cotton tip applicator to rapidly freeze cells, thus causing cellular destruction via ice crystal formation and protein denaturation.45 Efficacy with this technique has been reported to be as high as 98.8% at 12 months follow-up, but more recent studies cite lower rates of success.46 A prospective multicenter study of 90 participants with 421 AKs on the face or scalp treated with a single freeze-thaw cycle of liquid nitrogen reported an overall complete response rate of 67.2% at 3 months posttherapy. Additionally, higher complete response rates were associated with longer freeze times, and cosmetic outcomes were reported as good to excellent in 94% of complete response lesions.47 Similar results were reported in an open-label, prospective, randomized, controlled clinical trial of 200 participants with 543 AKs, which compared a single freeze-thaw cycle with liquid nitrogen to a single session of CO2 laser ablation in the treatment of isolated AKs of the face and scalp.48 At 3 months posttherapy, complete clearance was observed in 71.6% of participants treated with cryotherapy and in 65.3% of participants treated with laser ablation (P=.532). At 12 months posttherapy, participants who originally showed complete response at 3 months were assessed for relapse. Complete clearance was preserved in 72.6% of participants treated with cryotherapy versus 21.9% of participants treated with laser ablation (P<.0001), and cosmetic outcomes were reported by participants as good or excellent at 3 months follow-up in more than 93% of participants for both treatment arms.48 Possible AEs of cryotherapy include pain during treatment, blister formation with possible hemorrhage, infection, scarring, and permanent pigmentary changes.47,48 Notably, the risk for hypopigmentation increases with longer freezing times, thus requiring clinicians to consider the balance between improved efficacy and reduced cosmetic outcomes.47
Light-Based Therapies
Laser Therapy
Ablative laser resurfacing with either the CO2 or erbium-doped:YAG (Er:YAG) laser utilizes light of specific wavelengths to selectively induce thermolysis and destruction of the epidermal layer. Both lasers have been studied as treatments of AK, but there is a lack of large, well-designed studies. In one small study of 14 participants treated with 1 to 2 passes of the CO2 laser, complete clearance was reported in all cases without any recurrences during a follow-up period of 6 to 24 months. Additionally, all participants in this study reported satisfaction with the cosmetic outcome.49 The CO2 laser also has demonstrated efficacy comparable to that of the TCA peel and 5-FU therapy in a prospective randomized trial of 34 patients with facial or scalp AKs who received either CO2 laser with 2 passes, 30% TCA peel, or 5-FU cream 5% twice daily for 3 weeks.15 Reduction in mean AK counts at 3 months posttherapy was significantly (P<.03) higher in all treatment arms as compared to the control group (92% for CO2 laser, 89% for TCA peel, and 83% for 5-FU cream). No significant (P=.31) difference in outcomes was noted among the different treatment arms.15 Similar results were reported for the Er:YAG laser in a small prospective study of 5 participants treated with 2 to 3 passes with the Er:YAG laser in which reduction in mean AK counts was reported as ranging from 86% to 96% at 3 months posttherapy.50 The Er:YAG laser in combination with the CO2 laser has shown notable long-term efficacy in achieving higher lesion clearance rates and sustained complete clearance rates over treatment with topical 5-FU.51 In a prospective randomized study of 55 par-ticipants with multiple AKs on the face or scalp, participants were assigned to receive either combination laser ablation with the Er:YAG and CO2 lasers down to the level of the papillary dermis or 5-FU cream 5% applied twice daily for 2 to 7 weeks until an appropriate clinical inflammatory response was achieved. At 12 months follow-up, the laser treatment group achieved significantly (P=.048) higher mean lesion clearance rates (91.1%) as compared to the 5-FU arm (76.6%) and significantly (P=.003) higher sustained complete clearance rates (59.3%) as compared to 5-FU (29.2%). The proportion of participants with an improvement in photoaging score at 12 months follow-up approached statistical significance (P=.07), with 74% of the laser-treated group showing improvement as compared to 43% of the 5-FU–treated group. Long-term, cosmetically unappealing side effects such as erythema and hypopigmentation occurred notably more often in the laser-treated group as compared to the 5-FU group.51 In summary, ablative lasers appear to be a highly effective therapy for AK but at the cost of increased risk for AEs such as permanent pigmentary changes, prolonged erythema lasting up to several months, and scarring.50,52-55
Fractional photothermolysis is a relatively new advancement in the field of laser therapy that has received FDA approval for the treatment of AK.56 This treatment works by creating multiple noncontiguous microscopic columns of thermal injury while sparing adjacent zones of viable tissue.57 Although there are limited studies involving the use of such lasers in the treatment of AK, initial findings suggest that 1927-nm thulium lasers may be more effective than 1550-nm erbium lasers in achieving lesion clearance. A trial of 14 participants who received 5 laser treatments with a 1550-nm fractionated erbium-doped fiber laser reported an average reduction in AK counts of 66.2% at 3 months follow-up and a 55.6% reduction at 6 months follow-up. A participant-determined marked or very significant improvement of lesions was reported in 83% of participants at 1 month posttreatment but only in 44% of participants at 6 months posttreatment.58 A similar trial of 24 participants treated with up to 4 treatment sessions of the fractionated 1927-nm thulium laser reported an 87.3% reduction in number of AKs at 3 months follow-up and an 86.6% reduction at 6 months follow-up.56 The primary advantage of fractional laser therapy is a faster recovery period generally lasting only 2 or 3 days as compared to 2 weeks or more with traditional ablative lasers, thus limiting the amount of time a patient must tolerate cosmetically unappealing erythema.59,60 The quick recovery time has been attributed to the fractional laser’s ability to preserve the stratum corneum and skin barrier, which also helps reduce the risk for other AEs such as scarring and infection.56,59-61 Additional studies are needed to better assess the true efficacy of fractional laser therapy, but treatment with the fractional 1927-nm thulium laser appears to be a promising and well-tolerated therapeutic option for treatment of AK with similar efficacy to traditional ablative lasers but with a lower risk of AEs.
Photodynamic TherapyPhotodynamic therapy (PDT) is an FDA-approved treatment that involves the use of a topical photosensitizing agent such as 5-aminolevulinic acid (ALA) or methyl aminovulinate (MAL) before exposure to an activating light source to generate reactive oxygen species that lead to cell death.62-65 Multiple PDT regimens with varying combinations of photosensitizers, incubation time, and light sources have been studied, but a 2012 Cochrane review determined that treatment with conventional formulations of MAL and ALA with either blue- or red-light PDT were similarly efficacious for treatment of individual AKs as compared to vehicle with blue- or red-light PDT. One exception was that longer incubation time (ie, 4 hours) with ALA resulted in better results than shorter incubation times (ie, 0.5, 1, 2 hours) with ALA.66
Standard PDT treatment with MAL also has consistently demonstrated superior efficacy in achieving complete clearance rates in addition to superior cosmetic outcomes over treatment with either cryotherapy, DFS, or 5-FU.67-73 Three studies in particular noted an excellent or good investigator-determined cosmetic outcome in 96% to 98% of participants treated with MAL-PDT.69,71,74 Photodynamic therapy with ALA also has been reported as superior over CO2 laser ablation for AK reduction as well as both patient and investigator overall satisfaction.75
More recently, several methods of improving photosensitizer delivery have been studied, which have demonstrated remarkable efficacy at achieving lesion clearance over standard cream formulations or application routines. One such method involves the use of gentle heating to increase photosensitizer uptake. In a split-extremity study of 20 participants who were treated with 20% ALA under occlusion for 1 hour with one side heated to 38.8°C, the heated side demonstrated significant (P<.0001) efficacy at achieving higher median clearance rates over control when evaluated at 2 and 6 months posttherapy.76 Notably, occlusion of ALA in itself during the incubation period also has been demonstrated to significantly (P<.0001) improve clearance rates.77 Another method involves the use of a new nanoemulsion-based formulation of ALA gel, known as BF-200 ALA, which has demonstrated remarkable efficacy over standard MAL cream and placebo in a long-term follow-up analysis of 2 prospective, randomized, controlled trials.78 In a similar vein, 3 prospective randomized trials with a minimum follow-up time of 3 months demonstrated that MAL-PDT in combination with fractional ablative laser pretreatment has significant (P<.02 in all trials) efficacy over MAL-PDT without pretreatment in achieving complete AK clearance. Although the cosmetic outcomes were good or excellent in 87% to 100% of patients, they were not significantly different from stand-alone MAL-PDT treatment in any of the trials.79-81 However, pretreatment with microneedling in MAL-PDT has been shown to achieve superior cosmetic outcomes over MAL-PDT without microneedling, according to one small split-face study of 10 participants.82
Overall, PDT is an excellent therapeutic option that is able to provide efficacious clearance of AKs as well as superior cosmetic outcomes. Common AEs of PDT include burning, itching, and stinging during therapy, but pain intensity decreases dramatically upon termination of illumination, with cessation of most symptoms by 12 hours posttherapy.73 Permanent pigmentation changes have been reported to occasionally occur following PDT therapy.81
Conclusion
When determining which therapy to use in a patient, clinicians must take into account a variety of factors such as patient preference, cost of treatment, availability, tolerance for AEs, and the need for field therapy. Although all therapies discussed within this article are effective and reasonable treatment choices, patients who are particularly concerned about cosmetic outcomes would most likely benefit from either IMQ or PDT, as the data for cosmetic outcomes with these therapies are the strongest. Combination or sequential treatments may be required in some cases and all patients should be monitored for lesion recurrence regardless of treatment choice. A summary of the therapies and key studies discussed here is available in the PDF.
- Lebwohl M. Actinic keratosis: epidemiology and progression to squamous cell carcinoma. Br J Dermatol. 2003;149(suppl 66):31-33.
- Drake LA, Ceilley RI, Cornelison RL, et al. Guidelines of care for actinic keratoses. Committee on Guidelines of Care. J Am Acad Dermatol. 1995;32:95-98.
- Salasche SJ. Epidemiology of actinic keratoses and squamous cell carcinoma. J Am Acad Dermatol. 2000;42(1, pt 2):4-7.
- Ackerman AB, Mones JM. Solar (actinic) keratosis is squamous cell carcinoma. Br J Dermatol. 2006;155:9-22.
- Anwar J, Wrone DA, Kimyai-Asadi A, et al. The development of actinic keratosis into invasive squamous cell carcinoma: evidence and evolving classification schemes. Clin Dermatol. 2004;22:189-196.
- Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
- Glogau RG. The risk of progression to invasive disease. J Am Acad Dermatol. 2000;42(1, pt 2):23-24.
- Esmann S, Jemec GB. Management of actinic keratosis patients: a qualitative study. J Dermatolog Treat. 2007;18:53-58.
- Weinstock MA, Lee KC, Chren MM, et al. Quality of life in the actinic neoplasia syndrome: the VA Topical Tretinoin Chemoprevention (VATTC) trial. J Am Acad Dermatol. 2009;61:207-215.
- Berman B, Villa AM, Ramirez CC. Mechanisms of action of new treatment modalities for actinic keratosis. J Drugs Dermatol. 2006;5:167-173.
- Askew DA, Mickan SM, Soyer HP. Effectiveness of 5-fluorouracil treatment for actinic keratosis: a systematic review of randomized controlled trials. Int J Dermatol. 2009;46:452-463.
- Levy S, Furst K, Chern W. A pharmacokinetic evaluation of 0.5% and 5% fluorouracil topical cream in patients with actinic keratosis. Clin Ther. 2001;23:908-920.
- Jorizzo J, Stewart D, Bucko A, et al. Randomized trial evaluating a new 0.5% fluorouracil formulation demonstrates efficacy after 1-, 2-, or 4-week treatment in patients with actinic keratosis. Cutis. 2002;70:335-359.
- Sachs DL, Kang S, Hammerberg C, et al. Topical fluorouracil for actinic keratoses and photoaging: a clinical and molecular analysis. Arch Dermatol. 2009;145:659-666.
- Hantash BM, Stewart DB, Cooper ZA, et al. Facial resurfacing for nonmelanoma skin cancer prophylaxis. Arch Dermatol. 2006;142:976-982.
- Lawrence N, Cox SE, Cockerell CJ, et al. A comparison of the efficacy and safety of Jessner’s solution and 35% trichloroacetic acid vs 5% fluorouracil in the treatment of widespread facial actinic keratoses. Arch Dermatol. 1995;131:176-181.
- Monheit GD. The Jessner’s + TCA peel: a medium-depth chemical peel. J Dermatol Surg Oncol. 1989;15:945-950.
- Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196-200.
- Adamson DJ, Frew D, Tatoud R, et al. Diclofenac antagonizes peroxisome proliferator-activated receptor-gamma signaling. Mol Pharmacol. 2002;61:7-12.
- Alam CA, Seed MP, Willoughby DA. Angiostasis and vascular regression in chronic granulomatous inflammation induced by diclofenac in combination with hyaluronan in mice. J Pharm Pharmacol. 1995;47:407-411.
- Lu X, Xie W, Reed D, et al. Nonsteroidal antiinflammatory drugs cause apoptosis and induce cyclooxygenases in chicken embryo fibroblasts. Proc Natl Acad Sci USA. 1995;92:7961-7965.
- Seed MP, Brown JR, Freemantle CN, et al. The inhibition of colon-26 adenocarcinoma development and angiogenesis by topical diclofenac in 2.5% hyaluronan. Cancer Res. 1997;57:1625-1629.
- Rivers JK, Arlette J, Shear N, et al. Topical treatment of actinic keratoses with 3.0% diclofenac in 2.5% hyaluronan gel. Br J Dermatol. 2002;146:94-100.
- Wolf JE, Taylor JR, Tschen E, et al. Topical 3.0% diclo-fenac in 2.5% hyaluronan gel in the treatment of actinic keratoses. Int J Dermatol. 2001;40:709-713.
- Nelson C, Rigel D, Smith S, et al. Phase IV, open-label assessment of the treatment of actinic keratosis with 3.0% diclofenac sodium topical gel (Solaraze). J Drugs Dermatol. 2004;3:401-407.
- Pflugfelder A, Welter AK, Leiter U, et al. Open label randomized study comparing 3 months vs. 6 months treatment of actinic keratoses with 3% diclofenac in 2.5% hyaluronic acid gel: a trial of the German Dermatologic Cooperative Oncology Group. J Eur Acad Dermatol Venereol. 2012;26:48-53.
- Smith SR, Morhenn VB, Piacquadio DJ. Bilateral comparison of the efficacy and tolerability of 3% diclofenac sodium gel and 5% 5-fluorouracil cream in the treatment of actinic keratoses of the face and scalp. J Drugs Dermatol. 2006;5:156-159.
- Segatto MM, Dornelles SI, Silveira VB, et al. Comparative study of actinic keratosis treatment with 3% diclo- fenac sodium and 5% 5-fluorouracil. An Bras Dermatol. 2013;88:732-738.
- Vidal D. Topical imiquimod: mechanism of action and clinical applications. Mini Rev Med Chem. 2006;6:499-503.
- Hadley G, Derry S, Moore RA. Imiquimod for actinic keratosis: systematic review and meta-analysis. J Invest Dermatol. 2006;126:1251-1255.
- Caperton C, Berman B. Safety, efficacy, and patient acceptability of imiquimod for topical treatment of actinic keratoses. Clin Cosmet Investig Dermatol. 2011;4:35-40.
- Swanson N, Smith CC, Kaur M, et al. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: two phase 3, multicenter, randomized, double-blind, placebo-controlled studies. J Drugs Dermatol. 2014;13:166-169.
- Krawtchenko N, Roewert-Huber J, Ulrich M, et al. A randomised study of topical 5% imiquimod vs. topical 5-fluorouracil vs. cryosurgery in immunocompetent patients with actinic keratoses: a comparison of clinical and histological outcomes including 1-year follow-up. Br J Dermatol. 2007;157(suppl 2):34-40.
- Anderson L, Schmieder GJ, Werschler WP, et al. Randomized, double-blind, double-dummy, vehicle-controlled study of ingenol mebutate gel 0.025% and 0.05% for actinic keratosis. J Am Acad Dermatol. 2009;60:934-943.
- Ogbourne SM, Suhrbier A, Jones B, et al. Antitumor activity of 3-ingenyl angelate: plasma membrane and mitochondrial disruption and necrotic cell death. Cancer Res. 2004;64:2833-2839.
- Lebwohl M, Swanson N, Anderson LL, et al. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366:1010-1019.
- Lebwohl M, Shumack S, Stein-Gold L, et al. Long-term follow-up study of ingenol mebutate gel for the treatment of actinic keratoses. JAMA Dermatol. 2013;149:666-670.
- Augustin M, Tu JH, Knudsen KM, et al. Ingenol mebutate gel for actinic keratosis: the link between quality of life, treatment satisfaction, and clinical outcomes. J Am Acad Dermatol. 2015;72:816-821.
- Kane-Maguire N, Moseley R, Cozzi S, et al. Modulation of fibroblast phenotype and extracellular matrix composition by ingenol mebutate may be associated with scar resolution and improved dermal cosmesis. J Am Acad Dermatol. 2012;66:AB218.
- Martin G, Swanson N. Clinical findings using ingenol mebutate gel to treat actinic keratoses. J Am Acad Dermatol. 2013;68(1, suppl 1):S39-S48.
- Feldman SR, Fleischer AB, Williford PM, et al. Destructive procedures are the standard of care for treatment of actinic keratoses. J Am Acad Dermatol. 1999;40:43-47.
- Berlin JM. Current and emerging treatment strategies for the treatment of actinic keratosis. Clin Cosmet Investig Dermatol. 2010;3:119-126.
- Coleman WP, Yarborough JM, Mandy SH. Dermabrasion for prophylaxis and treatment of actinic keratoses. Dermatol Surg. 1996;22:17-21.
- Cooley JE, Casey DL, Kauffman CL. Manual resurfacing and trichloroacetic acid for the treatment of patients with widespread actinic damage. clinical and histologic observations. Dermatol Surg. 1997;23:373-379.
- Goldberg LH, Kaplan B, Vergilis-Kalner I, et al. Liquid nitrogen: temperature control in the treatment of actinic keratosis. Dermatol Surg. 2010;36:1956-1961.
- Lubritz RR, Smolewski SA. Cryosurgery cure rate of actinic keratoses. J Am Acad Dermatol. 1982;7:631-632.
- Thai KE, Fergin P, Freeman M, et al. A prospective study of the use of cryosurgery for the treatment of actinic keratoses. Int J Dermatol. 2004;43:687-692.
- Zane C, Facchinetti E, Rossi MT, et al. Cryotherapy is preferable to ablative CO2 laser for the treatment of isolated actinic keratoses of the face and scalp: a randomized clinical trial. Br J Dermatol. 2014;170:1114-1121.
- Trimas SJ, Ellis DA, Metz RD. The carbon dioxide laser. an alternative for the treatment of actinically damaged skin. Dermatol Surg. 1997;23:885-889.
- Jiang SB, Levine VJ, Nehal KS, et al. Er:YAG laser for the treatment of actinic keratoses. Dermatol Surg. 2000;26:437-440.
- Ostertag JU, Quaedvlieg PJ, Van der geer S, et al. A clinical comparison and long-term follow-up of topical 5-fluorouracil versus laser resurfacing in the treatment of widespread actinic keratoses. Lasers Surg Med. 2006;38:731-739.
- Iyer S, Friedli A, Bowes L, et al. Full face laser resurfacing: therapy and prophylaxis for actinic keratoses and non-melanoma skin cancer. Lasers Surg Med. 2004;34:114-119.
- Rubin MG. A peeler’s thoughts on skin improvement with chemical peels and laser resurfacing. Clin Plast Surg. 1997;24:407-409.
- Riggs K, Keller M, Humphreys TR. Ablative laser resurfacing: high-energy pulsed carbon dioxide and erbium:yttrium-aluminum-garnet. Clin Dermatol. 2007;25:462-473.
- Adrian RM. Pulsed carbon dioxide and long pulse 10-ms erbium-YAG laser resurfacing: a comparative clinical and histological study. J Cutan Laser Ther. 1999;1:197-202.
- Weiss ET, Brauer JA, Anolik R, et al. 1927-nm fractional resurfacing of facial actinic keratoses: a promising new therapeutic option. J Am Acad Dermatol. 2013; 68:98-102.
- Manstein D, Herron GS, Sink RK, et al. Fractional photothermolysis: a new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg Med. 2004;34:426-438.
- Katz TM, Goldberg LH, Marquez D, et al. Nonablative fractional photothermolysis for facial actinic keratoses: 6-month follow-up with histologic evaluation. J Am Acad Dermatol. 2011;65:349-356.
- Prens SP, De Vries K, Neumann HA, et al. Non-ablative fractional resurfacing in combination with topical tretinoin cream as a field treatment modality for multiple actinic keratosis: a pilot study and a review of other field treatment modalities. J Dermatolog Treat. 2013;24:227-231.
- Alexiades-Armenakas MR, Dover JS, Arndt KA. The spectrum of laser skin resurfacing: nonablative, fractional, and ablative laser resurfacing. J Am Acad Dermatol. 2008;58:719-737.
- Tannous Z. Fractional resurfacing. Clin Dermatol. 2007;25:480-486.
- Gold MH. Continuing medical education article-skin treatment: photodynamic therapy: indications and treatment. Aesthet Surg J. 2008;28:545-552.
- Juarranz A, Jaén P, Sanz-Rodríguez F, et al. Photodynamic therapy of cancer. basic principles and applications. Clin Transl Oncol. 2008;10:148-154.
- Juzeniene A, Peng Q, Moan J. Milestones in the development of photodynamic therapy and fluorescence diagnosis. Photochem Photobiol Sci. 2007;6:1234-1245.
- Moan J, Berg K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem Photobiol. 1991;53:549-553.
- Gupta AK, Paquet M, Villanueva E, et al. Interventions for actinic keratoses. Cochrane Database Syst Rev. 2012;12:CD004415.
- Patel G, Armstrong AW, Eisen DB. Efficacy of photodynamic therapy vs other interventions in randomized clinical trials for the treatment of actinic keratoses: a systematic review and meta-analysis. JAMA Dermatol. 2014;150:1281-1288.
- Kaufmann R, Spelman L, Weightman W, et al. Multicentre intraindividual randomized trial of topical methyl aminolaevulinate-photodynamic therapy vs. cryotherapy for multiple actinic keratoses on the extremities. Br J Dermatol. 2008;158:994-999.
- Freeman M, Vinciullo C, Francis D, et al. A comparison of photodynamic therapy using topical methyl aminolevulinate (Metvix) with single cycle cryotherapy in patients with actinic keratosis: a prospective, randomized study. J Dermatolog Treat. 2003;14:99-106.
- Morton C, Campbell S, Gupta G, et al. Intraindividual, right-left comparison of topical methyl aminolaevulinate-photodynamic therapy and cryotherapy in subjects with actinic keratoses: a multicentre, randomized controlled study. Br J Dermatol. 2006;155:1029-1036.
- Pariser DM, Lowe NJ, Stewart DM, et al. Photodynamic therapy with topical methyl aminolevulinate for actinic keratosis: results of a prospective randomized multicenter trial. J Am Acad Dermatol. 2003;48:227-232.
- Zane C, Facchinetti E, Rossi MT, et al. A randomized clinical trial of photodynamic therapy with methyl aminolaevulinate vs. diclofenac 3% plus hyaluronic acid gel for the treatment of multiple actinic keratoses of the face and scalp. Br J Dermatol. 2014;170:1143-1150.
- Perrett CM, McGregor JM, Warwick J, et al. Treatment of post-transplant premalignant skin disease: a randomized intrapatient comparative study of 5-fluorouracil cream and topical photodynamic therapy. Br J Dermatol. 2007;156:320-328.
- Szeimies RM, Karrer S, Radakovic-Fijan S, et al. Photodynamic therapy using topical methyl 5-aminolevulinate compared with cryotherapy for actinic keratosis: a prospective, randomized study. J Am Acad Dermatol. 2002; 47:258-262.
- Scola N, Terras S, Georgas D, et al. A randomized, half-side comparative study of aminolaevulinate photodynamic therapy vs. CO(2) laser ablation in immunocompetent patients with multiple actinic keratoses. Br J Dermatol. 2012;167:1366-1373.
- Willey A, Anderson RR, Sakamoto FH. Temperature-modulated photodynamic therapy for the treatment of actinic keratosis on the extremities: a pilot study. Dermatol Surg. 2014;40:1094-1102.
- Pariser DM. Management of Actinic Keratoses: Treatment Selection and Optimizing Outcomes. Presented at: Winter Clinical Dermatology Conference Hawaii; January 18, 2015; Kaanapali, HI.
- Dirschka T, Radny P, Dominicus R, et al. Long-term (6 and 12 months) follow-up of two prospective, randomized, controlled phase III trials of photodynamic therapy with BF-200 ALA and methyl aminolaevulinate for the treatment of actinic keratosis. Br J Dermatol. 2013;168:825-836.
- Choi SH, Kim KH, Song KH. Efficacy of ablative fractional laser-assisted photodynamic therapy with short-incubation time for the treatment of facial and scalp actinic keratosis: 12-month follow-up results of a randomized, prospective, comparative trial. J Eur Acad Dermatol Venereol. 2015;29:1598-1605.
- Ko DY, Jeon SY, Kim KH, et al. Fractional erbium:YAG laser-assisted photodynamic therapy for facial actinic keratoses: a randomized, comparative, prospective study. J Eur Acad Dermatol Venereol. 2014;28:1529-1539.
- Togsverd-Ho K, Haak CS, Thaysen-Petersen D, et al. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol. 2012;166:1262-1269.
- Torezan L, Chaves Y, Niwa A, et al. A pilot split-face study comparing conventional methyl aminolevulinate-photodynamic therapy (PDT) with microneedling-assisted PDT on actinically damaged skin. Dermatol Surg. 2013;39:1197-1201.
Actinic keratosis (AK), also referred to as solar keratosis or senile keratosis, is an intraepidermal proliferation of dysplastic keratinocytes that develops in response to chronic exposure to UV radiation. Actinic keratoses are among the most commonly encountered lesions seen by dermatologists, and it has been estimated that 60% of predisposed individuals older than 40 years have at least one AK.1,2 Prevalence is notably higher in light-skinned individuals and increases with age, presumably from higher cumulative sun exposure and decreased effectiveness of the immune system.1,3 It remains a point of contention as to whether or not AKs actually represent squamous cell carcinoma (SCC) in situ, but the potential for progression to invasive disease has been well demonstrated, as the majority of SCCs develop from preexisting AKs.4-6 The risk for progression to invasive disease for an individual AK has been estimated to range from 0.025% to 16% per year, with an average of approximately 8% in immunocompetent patients.7
The clinical morphology of AK can vary widely, but the most common presentation is an erythematous scaly macule, papule, or plaque on sun-exposed skin. The skin surrounding AKs typically shows evidence of solar damage with deep wrinkling, mottled pigmentation, scattered telangiectases, purpura, or xerosis (Figure). A variety of clinical variants with unique presentations exist, including atrophic, hypertrophic, acantholytic, lichenoid, bowenoid, and pigmented subtypes. Because more than 80% of AKs occur on highly visible areas such as the head, neck, back of the hands, and forearms, AKs can have an obvious detrimental effect on cosmetic appearance. Studies also have shown a strong association between AKs and decreased overall quality of life (QOL).3,8,9

Because of the risk for AK progression to invasive cancer along with its negative impact on cosmesis and QOL, clinicians generally opt to treat AKs. Numerous different treatment options exist, including topical medications, procedural modalities, and light-based therapies. Here, we review the efficacy of the most commonly utilized treatments and discuss the relevant cosmetic considerations and outcomes.
Topical Treatments
5-Fluorouracil
5-Fluorouracil (5-FU) is a US Food and Drug Administration (FDA)–approved, topically applied pyrimidine analogue that inhibits thymidylate synthase. The resulting suppression of DNA and RNA synthesis induces cell death with a preference for mitotically active cells.10 5-Fluorouracil has been used for more than 50 years as a treatment of AK and its efficacy is well established. A systematic review of 5 randomized controlled studies of topical 5-FU reported an average of 49% of 423 patients achieving complete lesion clearance with 5-FU cream 5% applied once or twice daily for up to 7 weeks.11 Some notable drawbacks of 5-FU, however, are application-site erythema, blistering, pruritus, necrosis, erosion, and pain. These effects often lead to premature cessation of therapy, but newer formulations of 5-FU cream 0.5% have shown good efficacy with better tolerability.12 A randomized, double-blind, multicenter, parallel-group study of 177 patients using 5-FU cream 0.5% once daily for either 1, 2, or 4 weeks demonstrated significant (P<.001) efficacy over vehicle gel in all treatment arms.13 The most effective therapy was 4 weeks of treatment, which achieved a mean 91.7% reduction in lesion count as assessed 1 month after cessation of therapy. The primary adverse effect (AE) reported in this trial was mild to moderate facial irritation, which generally resolved within 18 to 21 days after treatment cessation.13 Overall, 5-FU is a highly effective therapy for treating AKs that also can improve signs of photoaging, but patients should be aware of cosmetically unappealing effects that generally occur throughout therapy and during the immediate posttreatment period.14
Chemical Peels
Chemical peels traditionally employ acidic compounds to strip away outer layers of skin to variable depths depending on the concentration of the agent being applied. For treatment of AK, trichloroacetic acid (TCA) is a commonly employed cauterant that has shown efficacy comparable to topical 5-FU as well as ablative CO2 laser resurfacing.15 Trichloroacetic acid peels also are a convenient therapy, as good results can be achieved after a single treatment session. A split-face study of 15 patients treated with either a single application of 35% TCA and Jessner solution or twice-daily application of 5-FU cream 5% for 3 weeks demonstrated a reduction in 75% of visible AKs in both treatment arms over a 1-year follow-up period.16 Although 80% of patients self-reported considerable cosmetic improvement with both therapies, patient preference was reported to be in favor of the TCA peel, given its quick results and relatively mild side effects as compared to 5-FU. Treatment with chemical peels will result in temporary erythema and mild desquamation that usually resolves within 2 weeks; however, there are cases in which erythema has been reported to persist for several months.16 Adverse effects such as permanent scarring or pigmentation changes rarely are seen with TCA concentrations less than 45%.17 Caution should be used in patients with a history of herpes simplex virus, keloids, postinflammatory hyperpigmentation, radiation exposure, immunosuppression, and those unable or unwilling to use sunscreen and avoid sun exposure in the immediate posttreatment period.
Diclofenac Sodium
Diclofenac sodium (DFS) is an FDA-approved topical, nonsteroidal,
anti-inflammatory drug whose mechanism of action in the treatment of AK is thought to involve inhibition of the cyclooxygenase 2 enzyme.18 The resulting reduction of prostaglandins is believed to inhibit tumor angiogenesis, induce apoptosis, and inhibit cell differentiation.19-22 In a multicenter, double-blind, placebo-controlled study of 195 patients, application of DFS 3% in hyaluronan gel 2.5% twice daily for 60 days showed significant (P<.05) efficacy over placebo in achieving complete resolution of target lesions during a 30-day follow-up period (31% vs 10%). Furthermore, qualitative patient assessment of complete global improvement also was significantly (P<.05) higher in the active treatment group as compared to placebo (31% vs 10%).23 Additional studies of DFS 3% in hyaluronan gel 2.5% applied twice daily for 90 days have shown even higher rates of success, with complete resolution of target lesions in 40% to 58% of cases.24,25 This therapy also has been reported to substantially improve QOL following treatment completion.26 The most frequently cited AEs include pruritus, rash, dry skin, erythema, and application-site reactions. Overall, DFS is a
well-tolerated therapy with efficacy comparable to that of 5-FU but with a lower incidence of AEs
and higher patient satisfaction as determined in
2 head-to-head studies.27,28
ImiquimodImiquimod (IMQ) is an FDA-approved topical agent that functions as an immune response modifier via agonism of toll-like receptor 7.18 The resulting cytokine production and release enhances the innate and acquired immune responses leading to anticancer activity.29 The efficacy of IMQ for treatment of AK has been demonstrated in numerous well-designed clinical trials. A
meta-analysis of 5 randomized, double-blind trials including 1293 patients treated with IMQ cream 5%
2 to 3 times per week for 12 to 16 weeks reported complete clearance of AKs in 50% of patients treated with IMQ as compared to 5% of patients treated with vehicle.30 The most frequently reported AEs with this therapy include erythema, scabbing, flaking, and erosion. These effects generally resolve following cessation of treatment, and therapy is considered to be well tolerated; however, there are case reports of IMQ triggering or exacerbating existing inflammatory conditions.31 Imiquimod cream also is approved at 2.5% and 3.75% concentrations, which have demonstrated significant (P<.001) efficacy over placebo and a reduced incidence of AEs; complete clearance rates have been reported as 30.6% and 35.6%, respectively.32 Notably, a study comparing 75 patients randomized to either IMQ cream 5%
3 times per week for 4 weeks, 1 or 2 courses of cryosurgery, or 5-FU ointment 5% twice daily for 4 weeks reported that IMQ achieved significantly (P<.01) superior sustained clearance rates during a 12-month follow-up period over cryosurgery and 5-FU
(73% vs 4% vs 33%).33 Additionally, cosmetic outcomes as determined by both participants and investigators were reported as excellent at 12 months posttreatment in more than 80% of participants treated with IMQ. These excellent, long-lasting cosmetic outcomes also were determined to be significantly (P<.0001) superior to the cosmetic outcomes of 5-FU and cryotherapy, which both reported excellent outcomes in less than 10% of cases.33
Ingenol MebutateIngenol mebutate (IM) is a macrocyclic diterpene ester derived from the Euphorbia peplus plant that is FDA approved for the treatment of AK.1 Ingenol mebutate’s mechanism of action is thought to involve induction of cell death via disruption of the plasma membrane and mitochondria in addition to production of an inflammatory response, which produces tumor-specific antibodies and a large influx of neutrophils.34,35 The overall evidence for the efficacy of IM is strong. A combined analysis of 4 multicenter, randomized, double-blind studies of 1005 participants reported that IM gel 0.015% applied once daily for 3 days to the face or scalp was significantly superior (P<.001) to placebo in achieving complete clearance as assessed 54 days after completion of therapy (42.2% vs 3.7%) and that IM gel 0.05% applied once daily for 2 days to the trunk or extremities also was significantly superior (P<.001) to placebo in achieving complete clearance as determined 55 days after completion of therapy (34.1% vs 4.7%).36 A follow-up report to this study indicated that IM also appears to achieve long-lasting effects with an overall 87% decrease in total AKs at 12 months follow-up in both trial groups.37 Additionally, it has been recently reported that treatment with IM in these trials was associated with significantly higher overall treatment satisfaction (P<.001) and improved QOL (P<.001) as compared to vehicle.38 Cosmetic outcomes of IM therapy have been assessed in a trial analyzing the efficacy of IM gel 0.025% for 3 days or IM gel 0.05% for 2 or 3 days on nonfacial AKs. This study reported significantly (P<.0001) higher patient satisfaction with the cosmetic outcome at 8 weeks after therapy as compared to vehicle.34 Studies performed in mice have demonstrated that IM is able to promote collagen matrix turnover and impose dermal elasticity, which may contribute to these good cosmetic outcomes.39 The most common AEs of IM therapy are erythema, crusting, and flaking; these effects generally occur 3 to 8 days after starting treatment. These effects, however, generally are short lived and resolve within 2 weeks of treatment cessation when IM is applied to the face or scalp or 4 weeks when applied to the trunk or extremities.40 Overall, IM is a useful therapeutic option given its relatively short treatment course as compared to other topically applied agents, as well as its lasting efficacy, mild AEs, and good cosmetic outcomes.
Procedural Modalities
Surgical Procedures
Surgical approaches for the treatment of AK include excision, curettage with or without electrodesiccation, and dermabrasion. In the past, these modalities were used with greater frequency, but the advent of effective topical medications with lower risks of AEs has largely reduced their use.41 Excision may still be indicated in cases where SCC is suspected, and curettage can be used for treatment of thicker hypertrophic AKs.42 Although these approaches have not been evaluated in clinical trials, they are generally effective but require the use of local anesthetics and come with substantial risk for infection, permanent scarring, and hypopigmentation. Dermabrasion employs the use of a motorized device equipped with an abrasive material to physically remove superficial layers of the skin. Studies are limited, but this method has been reported as an effective treatment in a retrospective review of 23 participants in which 96% remained free of AKs at 1 year, 83% at 2 years, 64% at 4 years, and 54% at 5 years posttherapy.43 Notably, one split-face study of 40 participants treated with dermabrasion followed by 25% TCA on one side and either Jessner solution and 35% TCA or dermabrasion alone on the other side reported that the combination of dermabrasion with 25% TCA consistently produced excellent cosmetic results with nearly complete eradication of AKs.44 In general, however, cosmetic outcomes with dermabrasion are variable, as the technique is highly operator dependent and treatment is associated with notable discomfort as well as risk for scarring and permanent pigmentation alteration.
Cryotherapy
Cryotherapy remains one of the most commonly utilized treatments of AK and involves the delivery of liquid nitrogen via a spray device or a cotton tip applicator to rapidly freeze cells, thus causing cellular destruction via ice crystal formation and protein denaturation.45 Efficacy with this technique has been reported to be as high as 98.8% at 12 months follow-up, but more recent studies cite lower rates of success.46 A prospective multicenter study of 90 participants with 421 AKs on the face or scalp treated with a single freeze-thaw cycle of liquid nitrogen reported an overall complete response rate of 67.2% at 3 months posttherapy. Additionally, higher complete response rates were associated with longer freeze times, and cosmetic outcomes were reported as good to excellent in 94% of complete response lesions.47 Similar results were reported in an open-label, prospective, randomized, controlled clinical trial of 200 participants with 543 AKs, which compared a single freeze-thaw cycle with liquid nitrogen to a single session of CO2 laser ablation in the treatment of isolated AKs of the face and scalp.48 At 3 months posttherapy, complete clearance was observed in 71.6% of participants treated with cryotherapy and in 65.3% of participants treated with laser ablation (P=.532). At 12 months posttherapy, participants who originally showed complete response at 3 months were assessed for relapse. Complete clearance was preserved in 72.6% of participants treated with cryotherapy versus 21.9% of participants treated with laser ablation (P<.0001), and cosmetic outcomes were reported by participants as good or excellent at 3 months follow-up in more than 93% of participants for both treatment arms.48 Possible AEs of cryotherapy include pain during treatment, blister formation with possible hemorrhage, infection, scarring, and permanent pigmentary changes.47,48 Notably, the risk for hypopigmentation increases with longer freezing times, thus requiring clinicians to consider the balance between improved efficacy and reduced cosmetic outcomes.47
Light-Based Therapies
Laser Therapy
Ablative laser resurfacing with either the CO2 or erbium-doped:YAG (Er:YAG) laser utilizes light of specific wavelengths to selectively induce thermolysis and destruction of the epidermal layer. Both lasers have been studied as treatments of AK, but there is a lack of large, well-designed studies. In one small study of 14 participants treated with 1 to 2 passes of the CO2 laser, complete clearance was reported in all cases without any recurrences during a follow-up period of 6 to 24 months. Additionally, all participants in this study reported satisfaction with the cosmetic outcome.49 The CO2 laser also has demonstrated efficacy comparable to that of the TCA peel and 5-FU therapy in a prospective randomized trial of 34 patients with facial or scalp AKs who received either CO2 laser with 2 passes, 30% TCA peel, or 5-FU cream 5% twice daily for 3 weeks.15 Reduction in mean AK counts at 3 months posttherapy was significantly (P<.03) higher in all treatment arms as compared to the control group (92% for CO2 laser, 89% for TCA peel, and 83% for 5-FU cream). No significant (P=.31) difference in outcomes was noted among the different treatment arms.15 Similar results were reported for the Er:YAG laser in a small prospective study of 5 participants treated with 2 to 3 passes with the Er:YAG laser in which reduction in mean AK counts was reported as ranging from 86% to 96% at 3 months posttherapy.50 The Er:YAG laser in combination with the CO2 laser has shown notable long-term efficacy in achieving higher lesion clearance rates and sustained complete clearance rates over treatment with topical 5-FU.51 In a prospective randomized study of 55 par-ticipants with multiple AKs on the face or scalp, participants were assigned to receive either combination laser ablation with the Er:YAG and CO2 lasers down to the level of the papillary dermis or 5-FU cream 5% applied twice daily for 2 to 7 weeks until an appropriate clinical inflammatory response was achieved. At 12 months follow-up, the laser treatment group achieved significantly (P=.048) higher mean lesion clearance rates (91.1%) as compared to the 5-FU arm (76.6%) and significantly (P=.003) higher sustained complete clearance rates (59.3%) as compared to 5-FU (29.2%). The proportion of participants with an improvement in photoaging score at 12 months follow-up approached statistical significance (P=.07), with 74% of the laser-treated group showing improvement as compared to 43% of the 5-FU–treated group. Long-term, cosmetically unappealing side effects such as erythema and hypopigmentation occurred notably more often in the laser-treated group as compared to the 5-FU group.51 In summary, ablative lasers appear to be a highly effective therapy for AK but at the cost of increased risk for AEs such as permanent pigmentary changes, prolonged erythema lasting up to several months, and scarring.50,52-55
Fractional photothermolysis is a relatively new advancement in the field of laser therapy that has received FDA approval for the treatment of AK.56 This treatment works by creating multiple noncontiguous microscopic columns of thermal injury while sparing adjacent zones of viable tissue.57 Although there are limited studies involving the use of such lasers in the treatment of AK, initial findings suggest that 1927-nm thulium lasers may be more effective than 1550-nm erbium lasers in achieving lesion clearance. A trial of 14 participants who received 5 laser treatments with a 1550-nm fractionated erbium-doped fiber laser reported an average reduction in AK counts of 66.2% at 3 months follow-up and a 55.6% reduction at 6 months follow-up. A participant-determined marked or very significant improvement of lesions was reported in 83% of participants at 1 month posttreatment but only in 44% of participants at 6 months posttreatment.58 A similar trial of 24 participants treated with up to 4 treatment sessions of the fractionated 1927-nm thulium laser reported an 87.3% reduction in number of AKs at 3 months follow-up and an 86.6% reduction at 6 months follow-up.56 The primary advantage of fractional laser therapy is a faster recovery period generally lasting only 2 or 3 days as compared to 2 weeks or more with traditional ablative lasers, thus limiting the amount of time a patient must tolerate cosmetically unappealing erythema.59,60 The quick recovery time has been attributed to the fractional laser’s ability to preserve the stratum corneum and skin barrier, which also helps reduce the risk for other AEs such as scarring and infection.56,59-61 Additional studies are needed to better assess the true efficacy of fractional laser therapy, but treatment with the fractional 1927-nm thulium laser appears to be a promising and well-tolerated therapeutic option for treatment of AK with similar efficacy to traditional ablative lasers but with a lower risk of AEs.
Photodynamic TherapyPhotodynamic therapy (PDT) is an FDA-approved treatment that involves the use of a topical photosensitizing agent such as 5-aminolevulinic acid (ALA) or methyl aminovulinate (MAL) before exposure to an activating light source to generate reactive oxygen species that lead to cell death.62-65 Multiple PDT regimens with varying combinations of photosensitizers, incubation time, and light sources have been studied, but a 2012 Cochrane review determined that treatment with conventional formulations of MAL and ALA with either blue- or red-light PDT were similarly efficacious for treatment of individual AKs as compared to vehicle with blue- or red-light PDT. One exception was that longer incubation time (ie, 4 hours) with ALA resulted in better results than shorter incubation times (ie, 0.5, 1, 2 hours) with ALA.66
Standard PDT treatment with MAL also has consistently demonstrated superior efficacy in achieving complete clearance rates in addition to superior cosmetic outcomes over treatment with either cryotherapy, DFS, or 5-FU.67-73 Three studies in particular noted an excellent or good investigator-determined cosmetic outcome in 96% to 98% of participants treated with MAL-PDT.69,71,74 Photodynamic therapy with ALA also has been reported as superior over CO2 laser ablation for AK reduction as well as both patient and investigator overall satisfaction.75
More recently, several methods of improving photosensitizer delivery have been studied, which have demonstrated remarkable efficacy at achieving lesion clearance over standard cream formulations or application routines. One such method involves the use of gentle heating to increase photosensitizer uptake. In a split-extremity study of 20 participants who were treated with 20% ALA under occlusion for 1 hour with one side heated to 38.8°C, the heated side demonstrated significant (P<.0001) efficacy at achieving higher median clearance rates over control when evaluated at 2 and 6 months posttherapy.76 Notably, occlusion of ALA in itself during the incubation period also has been demonstrated to significantly (P<.0001) improve clearance rates.77 Another method involves the use of a new nanoemulsion-based formulation of ALA gel, known as BF-200 ALA, which has demonstrated remarkable efficacy over standard MAL cream and placebo in a long-term follow-up analysis of 2 prospective, randomized, controlled trials.78 In a similar vein, 3 prospective randomized trials with a minimum follow-up time of 3 months demonstrated that MAL-PDT in combination with fractional ablative laser pretreatment has significant (P<.02 in all trials) efficacy over MAL-PDT without pretreatment in achieving complete AK clearance. Although the cosmetic outcomes were good or excellent in 87% to 100% of patients, they were not significantly different from stand-alone MAL-PDT treatment in any of the trials.79-81 However, pretreatment with microneedling in MAL-PDT has been shown to achieve superior cosmetic outcomes over MAL-PDT without microneedling, according to one small split-face study of 10 participants.82
Overall, PDT is an excellent therapeutic option that is able to provide efficacious clearance of AKs as well as superior cosmetic outcomes. Common AEs of PDT include burning, itching, and stinging during therapy, but pain intensity decreases dramatically upon termination of illumination, with cessation of most symptoms by 12 hours posttherapy.73 Permanent pigmentation changes have been reported to occasionally occur following PDT therapy.81
Conclusion
When determining which therapy to use in a patient, clinicians must take into account a variety of factors such as patient preference, cost of treatment, availability, tolerance for AEs, and the need for field therapy. Although all therapies discussed within this article are effective and reasonable treatment choices, patients who are particularly concerned about cosmetic outcomes would most likely benefit from either IMQ or PDT, as the data for cosmetic outcomes with these therapies are the strongest. Combination or sequential treatments may be required in some cases and all patients should be monitored for lesion recurrence regardless of treatment choice. A summary of the therapies and key studies discussed here is available in the PDF.
Actinic keratosis (AK), also referred to as solar keratosis or senile keratosis, is an intraepidermal proliferation of dysplastic keratinocytes that develops in response to chronic exposure to UV radiation. Actinic keratoses are among the most commonly encountered lesions seen by dermatologists, and it has been estimated that 60% of predisposed individuals older than 40 years have at least one AK.1,2 Prevalence is notably higher in light-skinned individuals and increases with age, presumably from higher cumulative sun exposure and decreased effectiveness of the immune system.1,3 It remains a point of contention as to whether or not AKs actually represent squamous cell carcinoma (SCC) in situ, but the potential for progression to invasive disease has been well demonstrated, as the majority of SCCs develop from preexisting AKs.4-6 The risk for progression to invasive disease for an individual AK has been estimated to range from 0.025% to 16% per year, with an average of approximately 8% in immunocompetent patients.7
The clinical morphology of AK can vary widely, but the most common presentation is an erythematous scaly macule, papule, or plaque on sun-exposed skin. The skin surrounding AKs typically shows evidence of solar damage with deep wrinkling, mottled pigmentation, scattered telangiectases, purpura, or xerosis (Figure). A variety of clinical variants with unique presentations exist, including atrophic, hypertrophic, acantholytic, lichenoid, bowenoid, and pigmented subtypes. Because more than 80% of AKs occur on highly visible areas such as the head, neck, back of the hands, and forearms, AKs can have an obvious detrimental effect on cosmetic appearance. Studies also have shown a strong association between AKs and decreased overall quality of life (QOL).3,8,9

Because of the risk for AK progression to invasive cancer along with its negative impact on cosmesis and QOL, clinicians generally opt to treat AKs. Numerous different treatment options exist, including topical medications, procedural modalities, and light-based therapies. Here, we review the efficacy of the most commonly utilized treatments and discuss the relevant cosmetic considerations and outcomes.
Topical Treatments
5-Fluorouracil
5-Fluorouracil (5-FU) is a US Food and Drug Administration (FDA)–approved, topically applied pyrimidine analogue that inhibits thymidylate synthase. The resulting suppression of DNA and RNA synthesis induces cell death with a preference for mitotically active cells.10 5-Fluorouracil has been used for more than 50 years as a treatment of AK and its efficacy is well established. A systematic review of 5 randomized controlled studies of topical 5-FU reported an average of 49% of 423 patients achieving complete lesion clearance with 5-FU cream 5% applied once or twice daily for up to 7 weeks.11 Some notable drawbacks of 5-FU, however, are application-site erythema, blistering, pruritus, necrosis, erosion, and pain. These effects often lead to premature cessation of therapy, but newer formulations of 5-FU cream 0.5% have shown good efficacy with better tolerability.12 A randomized, double-blind, multicenter, parallel-group study of 177 patients using 5-FU cream 0.5% once daily for either 1, 2, or 4 weeks demonstrated significant (P<.001) efficacy over vehicle gel in all treatment arms.13 The most effective therapy was 4 weeks of treatment, which achieved a mean 91.7% reduction in lesion count as assessed 1 month after cessation of therapy. The primary adverse effect (AE) reported in this trial was mild to moderate facial irritation, which generally resolved within 18 to 21 days after treatment cessation.13 Overall, 5-FU is a highly effective therapy for treating AKs that also can improve signs of photoaging, but patients should be aware of cosmetically unappealing effects that generally occur throughout therapy and during the immediate posttreatment period.14
Chemical Peels
Chemical peels traditionally employ acidic compounds to strip away outer layers of skin to variable depths depending on the concentration of the agent being applied. For treatment of AK, trichloroacetic acid (TCA) is a commonly employed cauterant that has shown efficacy comparable to topical 5-FU as well as ablative CO2 laser resurfacing.15 Trichloroacetic acid peels also are a convenient therapy, as good results can be achieved after a single treatment session. A split-face study of 15 patients treated with either a single application of 35% TCA and Jessner solution or twice-daily application of 5-FU cream 5% for 3 weeks demonstrated a reduction in 75% of visible AKs in both treatment arms over a 1-year follow-up period.16 Although 80% of patients self-reported considerable cosmetic improvement with both therapies, patient preference was reported to be in favor of the TCA peel, given its quick results and relatively mild side effects as compared to 5-FU. Treatment with chemical peels will result in temporary erythema and mild desquamation that usually resolves within 2 weeks; however, there are cases in which erythema has been reported to persist for several months.16 Adverse effects such as permanent scarring or pigmentation changes rarely are seen with TCA concentrations less than 45%.17 Caution should be used in patients with a history of herpes simplex virus, keloids, postinflammatory hyperpigmentation, radiation exposure, immunosuppression, and those unable or unwilling to use sunscreen and avoid sun exposure in the immediate posttreatment period.
Diclofenac Sodium
Diclofenac sodium (DFS) is an FDA-approved topical, nonsteroidal,
anti-inflammatory drug whose mechanism of action in the treatment of AK is thought to involve inhibition of the cyclooxygenase 2 enzyme.18 The resulting reduction of prostaglandins is believed to inhibit tumor angiogenesis, induce apoptosis, and inhibit cell differentiation.19-22 In a multicenter, double-blind, placebo-controlled study of 195 patients, application of DFS 3% in hyaluronan gel 2.5% twice daily for 60 days showed significant (P<.05) efficacy over placebo in achieving complete resolution of target lesions during a 30-day follow-up period (31% vs 10%). Furthermore, qualitative patient assessment of complete global improvement also was significantly (P<.05) higher in the active treatment group as compared to placebo (31% vs 10%).23 Additional studies of DFS 3% in hyaluronan gel 2.5% applied twice daily for 90 days have shown even higher rates of success, with complete resolution of target lesions in 40% to 58% of cases.24,25 This therapy also has been reported to substantially improve QOL following treatment completion.26 The most frequently cited AEs include pruritus, rash, dry skin, erythema, and application-site reactions. Overall, DFS is a
well-tolerated therapy with efficacy comparable to that of 5-FU but with a lower incidence of AEs
and higher patient satisfaction as determined in
2 head-to-head studies.27,28
ImiquimodImiquimod (IMQ) is an FDA-approved topical agent that functions as an immune response modifier via agonism of toll-like receptor 7.18 The resulting cytokine production and release enhances the innate and acquired immune responses leading to anticancer activity.29 The efficacy of IMQ for treatment of AK has been demonstrated in numerous well-designed clinical trials. A
meta-analysis of 5 randomized, double-blind trials including 1293 patients treated with IMQ cream 5%
2 to 3 times per week for 12 to 16 weeks reported complete clearance of AKs in 50% of patients treated with IMQ as compared to 5% of patients treated with vehicle.30 The most frequently reported AEs with this therapy include erythema, scabbing, flaking, and erosion. These effects generally resolve following cessation of treatment, and therapy is considered to be well tolerated; however, there are case reports of IMQ triggering or exacerbating existing inflammatory conditions.31 Imiquimod cream also is approved at 2.5% and 3.75% concentrations, which have demonstrated significant (P<.001) efficacy over placebo and a reduced incidence of AEs; complete clearance rates have been reported as 30.6% and 35.6%, respectively.32 Notably, a study comparing 75 patients randomized to either IMQ cream 5%
3 times per week for 4 weeks, 1 or 2 courses of cryosurgery, or 5-FU ointment 5% twice daily for 4 weeks reported that IMQ achieved significantly (P<.01) superior sustained clearance rates during a 12-month follow-up period over cryosurgery and 5-FU
(73% vs 4% vs 33%).33 Additionally, cosmetic outcomes as determined by both participants and investigators were reported as excellent at 12 months posttreatment in more than 80% of participants treated with IMQ. These excellent, long-lasting cosmetic outcomes also were determined to be significantly (P<.0001) superior to the cosmetic outcomes of 5-FU and cryotherapy, which both reported excellent outcomes in less than 10% of cases.33
Ingenol MebutateIngenol mebutate (IM) is a macrocyclic diterpene ester derived from the Euphorbia peplus plant that is FDA approved for the treatment of AK.1 Ingenol mebutate’s mechanism of action is thought to involve induction of cell death via disruption of the plasma membrane and mitochondria in addition to production of an inflammatory response, which produces tumor-specific antibodies and a large influx of neutrophils.34,35 The overall evidence for the efficacy of IM is strong. A combined analysis of 4 multicenter, randomized, double-blind studies of 1005 participants reported that IM gel 0.015% applied once daily for 3 days to the face or scalp was significantly superior (P<.001) to placebo in achieving complete clearance as assessed 54 days after completion of therapy (42.2% vs 3.7%) and that IM gel 0.05% applied once daily for 2 days to the trunk or extremities also was significantly superior (P<.001) to placebo in achieving complete clearance as determined 55 days after completion of therapy (34.1% vs 4.7%).36 A follow-up report to this study indicated that IM also appears to achieve long-lasting effects with an overall 87% decrease in total AKs at 12 months follow-up in both trial groups.37 Additionally, it has been recently reported that treatment with IM in these trials was associated with significantly higher overall treatment satisfaction (P<.001) and improved QOL (P<.001) as compared to vehicle.38 Cosmetic outcomes of IM therapy have been assessed in a trial analyzing the efficacy of IM gel 0.025% for 3 days or IM gel 0.05% for 2 or 3 days on nonfacial AKs. This study reported significantly (P<.0001) higher patient satisfaction with the cosmetic outcome at 8 weeks after therapy as compared to vehicle.34 Studies performed in mice have demonstrated that IM is able to promote collagen matrix turnover and impose dermal elasticity, which may contribute to these good cosmetic outcomes.39 The most common AEs of IM therapy are erythema, crusting, and flaking; these effects generally occur 3 to 8 days after starting treatment. These effects, however, generally are short lived and resolve within 2 weeks of treatment cessation when IM is applied to the face or scalp or 4 weeks when applied to the trunk or extremities.40 Overall, IM is a useful therapeutic option given its relatively short treatment course as compared to other topically applied agents, as well as its lasting efficacy, mild AEs, and good cosmetic outcomes.
Procedural Modalities
Surgical Procedures
Surgical approaches for the treatment of AK include excision, curettage with or without electrodesiccation, and dermabrasion. In the past, these modalities were used with greater frequency, but the advent of effective topical medications with lower risks of AEs has largely reduced their use.41 Excision may still be indicated in cases where SCC is suspected, and curettage can be used for treatment of thicker hypertrophic AKs.42 Although these approaches have not been evaluated in clinical trials, they are generally effective but require the use of local anesthetics and come with substantial risk for infection, permanent scarring, and hypopigmentation. Dermabrasion employs the use of a motorized device equipped with an abrasive material to physically remove superficial layers of the skin. Studies are limited, but this method has been reported as an effective treatment in a retrospective review of 23 participants in which 96% remained free of AKs at 1 year, 83% at 2 years, 64% at 4 years, and 54% at 5 years posttherapy.43 Notably, one split-face study of 40 participants treated with dermabrasion followed by 25% TCA on one side and either Jessner solution and 35% TCA or dermabrasion alone on the other side reported that the combination of dermabrasion with 25% TCA consistently produced excellent cosmetic results with nearly complete eradication of AKs.44 In general, however, cosmetic outcomes with dermabrasion are variable, as the technique is highly operator dependent and treatment is associated with notable discomfort as well as risk for scarring and permanent pigmentation alteration.
Cryotherapy
Cryotherapy remains one of the most commonly utilized treatments of AK and involves the delivery of liquid nitrogen via a spray device or a cotton tip applicator to rapidly freeze cells, thus causing cellular destruction via ice crystal formation and protein denaturation.45 Efficacy with this technique has been reported to be as high as 98.8% at 12 months follow-up, but more recent studies cite lower rates of success.46 A prospective multicenter study of 90 participants with 421 AKs on the face or scalp treated with a single freeze-thaw cycle of liquid nitrogen reported an overall complete response rate of 67.2% at 3 months posttherapy. Additionally, higher complete response rates were associated with longer freeze times, and cosmetic outcomes were reported as good to excellent in 94% of complete response lesions.47 Similar results were reported in an open-label, prospective, randomized, controlled clinical trial of 200 participants with 543 AKs, which compared a single freeze-thaw cycle with liquid nitrogen to a single session of CO2 laser ablation in the treatment of isolated AKs of the face and scalp.48 At 3 months posttherapy, complete clearance was observed in 71.6% of participants treated with cryotherapy and in 65.3% of participants treated with laser ablation (P=.532). At 12 months posttherapy, participants who originally showed complete response at 3 months were assessed for relapse. Complete clearance was preserved in 72.6% of participants treated with cryotherapy versus 21.9% of participants treated with laser ablation (P<.0001), and cosmetic outcomes were reported by participants as good or excellent at 3 months follow-up in more than 93% of participants for both treatment arms.48 Possible AEs of cryotherapy include pain during treatment, blister formation with possible hemorrhage, infection, scarring, and permanent pigmentary changes.47,48 Notably, the risk for hypopigmentation increases with longer freezing times, thus requiring clinicians to consider the balance between improved efficacy and reduced cosmetic outcomes.47
Light-Based Therapies
Laser Therapy
Ablative laser resurfacing with either the CO2 or erbium-doped:YAG (Er:YAG) laser utilizes light of specific wavelengths to selectively induce thermolysis and destruction of the epidermal layer. Both lasers have been studied as treatments of AK, but there is a lack of large, well-designed studies. In one small study of 14 participants treated with 1 to 2 passes of the CO2 laser, complete clearance was reported in all cases without any recurrences during a follow-up period of 6 to 24 months. Additionally, all participants in this study reported satisfaction with the cosmetic outcome.49 The CO2 laser also has demonstrated efficacy comparable to that of the TCA peel and 5-FU therapy in a prospective randomized trial of 34 patients with facial or scalp AKs who received either CO2 laser with 2 passes, 30% TCA peel, or 5-FU cream 5% twice daily for 3 weeks.15 Reduction in mean AK counts at 3 months posttherapy was significantly (P<.03) higher in all treatment arms as compared to the control group (92% for CO2 laser, 89% for TCA peel, and 83% for 5-FU cream). No significant (P=.31) difference in outcomes was noted among the different treatment arms.15 Similar results were reported for the Er:YAG laser in a small prospective study of 5 participants treated with 2 to 3 passes with the Er:YAG laser in which reduction in mean AK counts was reported as ranging from 86% to 96% at 3 months posttherapy.50 The Er:YAG laser in combination with the CO2 laser has shown notable long-term efficacy in achieving higher lesion clearance rates and sustained complete clearance rates over treatment with topical 5-FU.51 In a prospective randomized study of 55 par-ticipants with multiple AKs on the face or scalp, participants were assigned to receive either combination laser ablation with the Er:YAG and CO2 lasers down to the level of the papillary dermis or 5-FU cream 5% applied twice daily for 2 to 7 weeks until an appropriate clinical inflammatory response was achieved. At 12 months follow-up, the laser treatment group achieved significantly (P=.048) higher mean lesion clearance rates (91.1%) as compared to the 5-FU arm (76.6%) and significantly (P=.003) higher sustained complete clearance rates (59.3%) as compared to 5-FU (29.2%). The proportion of participants with an improvement in photoaging score at 12 months follow-up approached statistical significance (P=.07), with 74% of the laser-treated group showing improvement as compared to 43% of the 5-FU–treated group. Long-term, cosmetically unappealing side effects such as erythema and hypopigmentation occurred notably more often in the laser-treated group as compared to the 5-FU group.51 In summary, ablative lasers appear to be a highly effective therapy for AK but at the cost of increased risk for AEs such as permanent pigmentary changes, prolonged erythema lasting up to several months, and scarring.50,52-55
Fractional photothermolysis is a relatively new advancement in the field of laser therapy that has received FDA approval for the treatment of AK.56 This treatment works by creating multiple noncontiguous microscopic columns of thermal injury while sparing adjacent zones of viable tissue.57 Although there are limited studies involving the use of such lasers in the treatment of AK, initial findings suggest that 1927-nm thulium lasers may be more effective than 1550-nm erbium lasers in achieving lesion clearance. A trial of 14 participants who received 5 laser treatments with a 1550-nm fractionated erbium-doped fiber laser reported an average reduction in AK counts of 66.2% at 3 months follow-up and a 55.6% reduction at 6 months follow-up. A participant-determined marked or very significant improvement of lesions was reported in 83% of participants at 1 month posttreatment but only in 44% of participants at 6 months posttreatment.58 A similar trial of 24 participants treated with up to 4 treatment sessions of the fractionated 1927-nm thulium laser reported an 87.3% reduction in number of AKs at 3 months follow-up and an 86.6% reduction at 6 months follow-up.56 The primary advantage of fractional laser therapy is a faster recovery period generally lasting only 2 or 3 days as compared to 2 weeks or more with traditional ablative lasers, thus limiting the amount of time a patient must tolerate cosmetically unappealing erythema.59,60 The quick recovery time has been attributed to the fractional laser’s ability to preserve the stratum corneum and skin barrier, which also helps reduce the risk for other AEs such as scarring and infection.56,59-61 Additional studies are needed to better assess the true efficacy of fractional laser therapy, but treatment with the fractional 1927-nm thulium laser appears to be a promising and well-tolerated therapeutic option for treatment of AK with similar efficacy to traditional ablative lasers but with a lower risk of AEs.
Photodynamic TherapyPhotodynamic therapy (PDT) is an FDA-approved treatment that involves the use of a topical photosensitizing agent such as 5-aminolevulinic acid (ALA) or methyl aminovulinate (MAL) before exposure to an activating light source to generate reactive oxygen species that lead to cell death.62-65 Multiple PDT regimens with varying combinations of photosensitizers, incubation time, and light sources have been studied, but a 2012 Cochrane review determined that treatment with conventional formulations of MAL and ALA with either blue- or red-light PDT were similarly efficacious for treatment of individual AKs as compared to vehicle with blue- or red-light PDT. One exception was that longer incubation time (ie, 4 hours) with ALA resulted in better results than shorter incubation times (ie, 0.5, 1, 2 hours) with ALA.66
Standard PDT treatment with MAL also has consistently demonstrated superior efficacy in achieving complete clearance rates in addition to superior cosmetic outcomes over treatment with either cryotherapy, DFS, or 5-FU.67-73 Three studies in particular noted an excellent or good investigator-determined cosmetic outcome in 96% to 98% of participants treated with MAL-PDT.69,71,74 Photodynamic therapy with ALA also has been reported as superior over CO2 laser ablation for AK reduction as well as both patient and investigator overall satisfaction.75
More recently, several methods of improving photosensitizer delivery have been studied, which have demonstrated remarkable efficacy at achieving lesion clearance over standard cream formulations or application routines. One such method involves the use of gentle heating to increase photosensitizer uptake. In a split-extremity study of 20 participants who were treated with 20% ALA under occlusion for 1 hour with one side heated to 38.8°C, the heated side demonstrated significant (P<.0001) efficacy at achieving higher median clearance rates over control when evaluated at 2 and 6 months posttherapy.76 Notably, occlusion of ALA in itself during the incubation period also has been demonstrated to significantly (P<.0001) improve clearance rates.77 Another method involves the use of a new nanoemulsion-based formulation of ALA gel, known as BF-200 ALA, which has demonstrated remarkable efficacy over standard MAL cream and placebo in a long-term follow-up analysis of 2 prospective, randomized, controlled trials.78 In a similar vein, 3 prospective randomized trials with a minimum follow-up time of 3 months demonstrated that MAL-PDT in combination with fractional ablative laser pretreatment has significant (P<.02 in all trials) efficacy over MAL-PDT without pretreatment in achieving complete AK clearance. Although the cosmetic outcomes were good or excellent in 87% to 100% of patients, they were not significantly different from stand-alone MAL-PDT treatment in any of the trials.79-81 However, pretreatment with microneedling in MAL-PDT has been shown to achieve superior cosmetic outcomes over MAL-PDT without microneedling, according to one small split-face study of 10 participants.82
Overall, PDT is an excellent therapeutic option that is able to provide efficacious clearance of AKs as well as superior cosmetic outcomes. Common AEs of PDT include burning, itching, and stinging during therapy, but pain intensity decreases dramatically upon termination of illumination, with cessation of most symptoms by 12 hours posttherapy.73 Permanent pigmentation changes have been reported to occasionally occur following PDT therapy.81
Conclusion
When determining which therapy to use in a patient, clinicians must take into account a variety of factors such as patient preference, cost of treatment, availability, tolerance for AEs, and the need for field therapy. Although all therapies discussed within this article are effective and reasonable treatment choices, patients who are particularly concerned about cosmetic outcomes would most likely benefit from either IMQ or PDT, as the data for cosmetic outcomes with these therapies are the strongest. Combination or sequential treatments may be required in some cases and all patients should be monitored for lesion recurrence regardless of treatment choice. A summary of the therapies and key studies discussed here is available in the PDF.
- Lebwohl M. Actinic keratosis: epidemiology and progression to squamous cell carcinoma. Br J Dermatol. 2003;149(suppl 66):31-33.
- Drake LA, Ceilley RI, Cornelison RL, et al. Guidelines of care for actinic keratoses. Committee on Guidelines of Care. J Am Acad Dermatol. 1995;32:95-98.
- Salasche SJ. Epidemiology of actinic keratoses and squamous cell carcinoma. J Am Acad Dermatol. 2000;42(1, pt 2):4-7.
- Ackerman AB, Mones JM. Solar (actinic) keratosis is squamous cell carcinoma. Br J Dermatol. 2006;155:9-22.
- Anwar J, Wrone DA, Kimyai-Asadi A, et al. The development of actinic keratosis into invasive squamous cell carcinoma: evidence and evolving classification schemes. Clin Dermatol. 2004;22:189-196.
- Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
- Glogau RG. The risk of progression to invasive disease. J Am Acad Dermatol. 2000;42(1, pt 2):23-24.
- Esmann S, Jemec GB. Management of actinic keratosis patients: a qualitative study. J Dermatolog Treat. 2007;18:53-58.
- Weinstock MA, Lee KC, Chren MM, et al. Quality of life in the actinic neoplasia syndrome: the VA Topical Tretinoin Chemoprevention (VATTC) trial. J Am Acad Dermatol. 2009;61:207-215.
- Berman B, Villa AM, Ramirez CC. Mechanisms of action of new treatment modalities for actinic keratosis. J Drugs Dermatol. 2006;5:167-173.
- Askew DA, Mickan SM, Soyer HP. Effectiveness of 5-fluorouracil treatment for actinic keratosis: a systematic review of randomized controlled trials. Int J Dermatol. 2009;46:452-463.
- Levy S, Furst K, Chern W. A pharmacokinetic evaluation of 0.5% and 5% fluorouracil topical cream in patients with actinic keratosis. Clin Ther. 2001;23:908-920.
- Jorizzo J, Stewart D, Bucko A, et al. Randomized trial evaluating a new 0.5% fluorouracil formulation demonstrates efficacy after 1-, 2-, or 4-week treatment in patients with actinic keratosis. Cutis. 2002;70:335-359.
- Sachs DL, Kang S, Hammerberg C, et al. Topical fluorouracil for actinic keratoses and photoaging: a clinical and molecular analysis. Arch Dermatol. 2009;145:659-666.
- Hantash BM, Stewart DB, Cooper ZA, et al. Facial resurfacing for nonmelanoma skin cancer prophylaxis. Arch Dermatol. 2006;142:976-982.
- Lawrence N, Cox SE, Cockerell CJ, et al. A comparison of the efficacy and safety of Jessner’s solution and 35% trichloroacetic acid vs 5% fluorouracil in the treatment of widespread facial actinic keratoses. Arch Dermatol. 1995;131:176-181.
- Monheit GD. The Jessner’s + TCA peel: a medium-depth chemical peel. J Dermatol Surg Oncol. 1989;15:945-950.
- Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196-200.
- Adamson DJ, Frew D, Tatoud R, et al. Diclofenac antagonizes peroxisome proliferator-activated receptor-gamma signaling. Mol Pharmacol. 2002;61:7-12.
- Alam CA, Seed MP, Willoughby DA. Angiostasis and vascular regression in chronic granulomatous inflammation induced by diclofenac in combination with hyaluronan in mice. J Pharm Pharmacol. 1995;47:407-411.
- Lu X, Xie W, Reed D, et al. Nonsteroidal antiinflammatory drugs cause apoptosis and induce cyclooxygenases in chicken embryo fibroblasts. Proc Natl Acad Sci USA. 1995;92:7961-7965.
- Seed MP, Brown JR, Freemantle CN, et al. The inhibition of colon-26 adenocarcinoma development and angiogenesis by topical diclofenac in 2.5% hyaluronan. Cancer Res. 1997;57:1625-1629.
- Rivers JK, Arlette J, Shear N, et al. Topical treatment of actinic keratoses with 3.0% diclofenac in 2.5% hyaluronan gel. Br J Dermatol. 2002;146:94-100.
- Wolf JE, Taylor JR, Tschen E, et al. Topical 3.0% diclo-fenac in 2.5% hyaluronan gel in the treatment of actinic keratoses. Int J Dermatol. 2001;40:709-713.
- Nelson C, Rigel D, Smith S, et al. Phase IV, open-label assessment of the treatment of actinic keratosis with 3.0% diclofenac sodium topical gel (Solaraze). J Drugs Dermatol. 2004;3:401-407.
- Pflugfelder A, Welter AK, Leiter U, et al. Open label randomized study comparing 3 months vs. 6 months treatment of actinic keratoses with 3% diclofenac in 2.5% hyaluronic acid gel: a trial of the German Dermatologic Cooperative Oncology Group. J Eur Acad Dermatol Venereol. 2012;26:48-53.
- Smith SR, Morhenn VB, Piacquadio DJ. Bilateral comparison of the efficacy and tolerability of 3% diclofenac sodium gel and 5% 5-fluorouracil cream in the treatment of actinic keratoses of the face and scalp. J Drugs Dermatol. 2006;5:156-159.
- Segatto MM, Dornelles SI, Silveira VB, et al. Comparative study of actinic keratosis treatment with 3% diclo- fenac sodium and 5% 5-fluorouracil. An Bras Dermatol. 2013;88:732-738.
- Vidal D. Topical imiquimod: mechanism of action and clinical applications. Mini Rev Med Chem. 2006;6:499-503.
- Hadley G, Derry S, Moore RA. Imiquimod for actinic keratosis: systematic review and meta-analysis. J Invest Dermatol. 2006;126:1251-1255.
- Caperton C, Berman B. Safety, efficacy, and patient acceptability of imiquimod for topical treatment of actinic keratoses. Clin Cosmet Investig Dermatol. 2011;4:35-40.
- Swanson N, Smith CC, Kaur M, et al. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: two phase 3, multicenter, randomized, double-blind, placebo-controlled studies. J Drugs Dermatol. 2014;13:166-169.
- Krawtchenko N, Roewert-Huber J, Ulrich M, et al. A randomised study of topical 5% imiquimod vs. topical 5-fluorouracil vs. cryosurgery in immunocompetent patients with actinic keratoses: a comparison of clinical and histological outcomes including 1-year follow-up. Br J Dermatol. 2007;157(suppl 2):34-40.
- Anderson L, Schmieder GJ, Werschler WP, et al. Randomized, double-blind, double-dummy, vehicle-controlled study of ingenol mebutate gel 0.025% and 0.05% for actinic keratosis. J Am Acad Dermatol. 2009;60:934-943.
- Ogbourne SM, Suhrbier A, Jones B, et al. Antitumor activity of 3-ingenyl angelate: plasma membrane and mitochondrial disruption and necrotic cell death. Cancer Res. 2004;64:2833-2839.
- Lebwohl M, Swanson N, Anderson LL, et al. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366:1010-1019.
- Lebwohl M, Shumack S, Stein-Gold L, et al. Long-term follow-up study of ingenol mebutate gel for the treatment of actinic keratoses. JAMA Dermatol. 2013;149:666-670.
- Augustin M, Tu JH, Knudsen KM, et al. Ingenol mebutate gel for actinic keratosis: the link between quality of life, treatment satisfaction, and clinical outcomes. J Am Acad Dermatol. 2015;72:816-821.
- Kane-Maguire N, Moseley R, Cozzi S, et al. Modulation of fibroblast phenotype and extracellular matrix composition by ingenol mebutate may be associated with scar resolution and improved dermal cosmesis. J Am Acad Dermatol. 2012;66:AB218.
- Martin G, Swanson N. Clinical findings using ingenol mebutate gel to treat actinic keratoses. J Am Acad Dermatol. 2013;68(1, suppl 1):S39-S48.
- Feldman SR, Fleischer AB, Williford PM, et al. Destructive procedures are the standard of care for treatment of actinic keratoses. J Am Acad Dermatol. 1999;40:43-47.
- Berlin JM. Current and emerging treatment strategies for the treatment of actinic keratosis. Clin Cosmet Investig Dermatol. 2010;3:119-126.
- Coleman WP, Yarborough JM, Mandy SH. Dermabrasion for prophylaxis and treatment of actinic keratoses. Dermatol Surg. 1996;22:17-21.
- Cooley JE, Casey DL, Kauffman CL. Manual resurfacing and trichloroacetic acid for the treatment of patients with widespread actinic damage. clinical and histologic observations. Dermatol Surg. 1997;23:373-379.
- Goldberg LH, Kaplan B, Vergilis-Kalner I, et al. Liquid nitrogen: temperature control in the treatment of actinic keratosis. Dermatol Surg. 2010;36:1956-1961.
- Lubritz RR, Smolewski SA. Cryosurgery cure rate of actinic keratoses. J Am Acad Dermatol. 1982;7:631-632.
- Thai KE, Fergin P, Freeman M, et al. A prospective study of the use of cryosurgery for the treatment of actinic keratoses. Int J Dermatol. 2004;43:687-692.
- Zane C, Facchinetti E, Rossi MT, et al. Cryotherapy is preferable to ablative CO2 laser for the treatment of isolated actinic keratoses of the face and scalp: a randomized clinical trial. Br J Dermatol. 2014;170:1114-1121.
- Trimas SJ, Ellis DA, Metz RD. The carbon dioxide laser. an alternative for the treatment of actinically damaged skin. Dermatol Surg. 1997;23:885-889.
- Jiang SB, Levine VJ, Nehal KS, et al. Er:YAG laser for the treatment of actinic keratoses. Dermatol Surg. 2000;26:437-440.
- Ostertag JU, Quaedvlieg PJ, Van der geer S, et al. A clinical comparison and long-term follow-up of topical 5-fluorouracil versus laser resurfacing in the treatment of widespread actinic keratoses. Lasers Surg Med. 2006;38:731-739.
- Iyer S, Friedli A, Bowes L, et al. Full face laser resurfacing: therapy and prophylaxis for actinic keratoses and non-melanoma skin cancer. Lasers Surg Med. 2004;34:114-119.
- Rubin MG. A peeler’s thoughts on skin improvement with chemical peels and laser resurfacing. Clin Plast Surg. 1997;24:407-409.
- Riggs K, Keller M, Humphreys TR. Ablative laser resurfacing: high-energy pulsed carbon dioxide and erbium:yttrium-aluminum-garnet. Clin Dermatol. 2007;25:462-473.
- Adrian RM. Pulsed carbon dioxide and long pulse 10-ms erbium-YAG laser resurfacing: a comparative clinical and histological study. J Cutan Laser Ther. 1999;1:197-202.
- Weiss ET, Brauer JA, Anolik R, et al. 1927-nm fractional resurfacing of facial actinic keratoses: a promising new therapeutic option. J Am Acad Dermatol. 2013; 68:98-102.
- Manstein D, Herron GS, Sink RK, et al. Fractional photothermolysis: a new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg Med. 2004;34:426-438.
- Katz TM, Goldberg LH, Marquez D, et al. Nonablative fractional photothermolysis for facial actinic keratoses: 6-month follow-up with histologic evaluation. J Am Acad Dermatol. 2011;65:349-356.
- Prens SP, De Vries K, Neumann HA, et al. Non-ablative fractional resurfacing in combination with topical tretinoin cream as a field treatment modality for multiple actinic keratosis: a pilot study and a review of other field treatment modalities. J Dermatolog Treat. 2013;24:227-231.
- Alexiades-Armenakas MR, Dover JS, Arndt KA. The spectrum of laser skin resurfacing: nonablative, fractional, and ablative laser resurfacing. J Am Acad Dermatol. 2008;58:719-737.
- Tannous Z. Fractional resurfacing. Clin Dermatol. 2007;25:480-486.
- Gold MH. Continuing medical education article-skin treatment: photodynamic therapy: indications and treatment. Aesthet Surg J. 2008;28:545-552.
- Juarranz A, Jaén P, Sanz-Rodríguez F, et al. Photodynamic therapy of cancer. basic principles and applications. Clin Transl Oncol. 2008;10:148-154.
- Juzeniene A, Peng Q, Moan J. Milestones in the development of photodynamic therapy and fluorescence diagnosis. Photochem Photobiol Sci. 2007;6:1234-1245.
- Moan J, Berg K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem Photobiol. 1991;53:549-553.
- Gupta AK, Paquet M, Villanueva E, et al. Interventions for actinic keratoses. Cochrane Database Syst Rev. 2012;12:CD004415.
- Patel G, Armstrong AW, Eisen DB. Efficacy of photodynamic therapy vs other interventions in randomized clinical trials for the treatment of actinic keratoses: a systematic review and meta-analysis. JAMA Dermatol. 2014;150:1281-1288.
- Kaufmann R, Spelman L, Weightman W, et al. Multicentre intraindividual randomized trial of topical methyl aminolaevulinate-photodynamic therapy vs. cryotherapy for multiple actinic keratoses on the extremities. Br J Dermatol. 2008;158:994-999.
- Freeman M, Vinciullo C, Francis D, et al. A comparison of photodynamic therapy using topical methyl aminolevulinate (Metvix) with single cycle cryotherapy in patients with actinic keratosis: a prospective, randomized study. J Dermatolog Treat. 2003;14:99-106.
- Morton C, Campbell S, Gupta G, et al. Intraindividual, right-left comparison of topical methyl aminolaevulinate-photodynamic therapy and cryotherapy in subjects with actinic keratoses: a multicentre, randomized controlled study. Br J Dermatol. 2006;155:1029-1036.
- Pariser DM, Lowe NJ, Stewart DM, et al. Photodynamic therapy with topical methyl aminolevulinate for actinic keratosis: results of a prospective randomized multicenter trial. J Am Acad Dermatol. 2003;48:227-232.
- Zane C, Facchinetti E, Rossi MT, et al. A randomized clinical trial of photodynamic therapy with methyl aminolaevulinate vs. diclofenac 3% plus hyaluronic acid gel for the treatment of multiple actinic keratoses of the face and scalp. Br J Dermatol. 2014;170:1143-1150.
- Perrett CM, McGregor JM, Warwick J, et al. Treatment of post-transplant premalignant skin disease: a randomized intrapatient comparative study of 5-fluorouracil cream and topical photodynamic therapy. Br J Dermatol. 2007;156:320-328.
- Szeimies RM, Karrer S, Radakovic-Fijan S, et al. Photodynamic therapy using topical methyl 5-aminolevulinate compared with cryotherapy for actinic keratosis: a prospective, randomized study. J Am Acad Dermatol. 2002; 47:258-262.
- Scola N, Terras S, Georgas D, et al. A randomized, half-side comparative study of aminolaevulinate photodynamic therapy vs. CO(2) laser ablation in immunocompetent patients with multiple actinic keratoses. Br J Dermatol. 2012;167:1366-1373.
- Willey A, Anderson RR, Sakamoto FH. Temperature-modulated photodynamic therapy for the treatment of actinic keratosis on the extremities: a pilot study. Dermatol Surg. 2014;40:1094-1102.
- Pariser DM. Management of Actinic Keratoses: Treatment Selection and Optimizing Outcomes. Presented at: Winter Clinical Dermatology Conference Hawaii; January 18, 2015; Kaanapali, HI.
- Dirschka T, Radny P, Dominicus R, et al. Long-term (6 and 12 months) follow-up of two prospective, randomized, controlled phase III trials of photodynamic therapy with BF-200 ALA and methyl aminolaevulinate for the treatment of actinic keratosis. Br J Dermatol. 2013;168:825-836.
- Choi SH, Kim KH, Song KH. Efficacy of ablative fractional laser-assisted photodynamic therapy with short-incubation time for the treatment of facial and scalp actinic keratosis: 12-month follow-up results of a randomized, prospective, comparative trial. J Eur Acad Dermatol Venereol. 2015;29:1598-1605.
- Ko DY, Jeon SY, Kim KH, et al. Fractional erbium:YAG laser-assisted photodynamic therapy for facial actinic keratoses: a randomized, comparative, prospective study. J Eur Acad Dermatol Venereol. 2014;28:1529-1539.
- Togsverd-Ho K, Haak CS, Thaysen-Petersen D, et al. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol. 2012;166:1262-1269.
- Torezan L, Chaves Y, Niwa A, et al. A pilot split-face study comparing conventional methyl aminolevulinate-photodynamic therapy (PDT) with microneedling-assisted PDT on actinically damaged skin. Dermatol Surg. 2013;39:1197-1201.
- Lebwohl M. Actinic keratosis: epidemiology and progression to squamous cell carcinoma. Br J Dermatol. 2003;149(suppl 66):31-33.
- Drake LA, Ceilley RI, Cornelison RL, et al. Guidelines of care for actinic keratoses. Committee on Guidelines of Care. J Am Acad Dermatol. 1995;32:95-98.
- Salasche SJ. Epidemiology of actinic keratoses and squamous cell carcinoma. J Am Acad Dermatol. 2000;42(1, pt 2):4-7.
- Ackerman AB, Mones JM. Solar (actinic) keratosis is squamous cell carcinoma. Br J Dermatol. 2006;155:9-22.
- Anwar J, Wrone DA, Kimyai-Asadi A, et al. The development of actinic keratosis into invasive squamous cell carcinoma: evidence and evolving classification schemes. Clin Dermatol. 2004;22:189-196.
- Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
- Glogau RG. The risk of progression to invasive disease. J Am Acad Dermatol. 2000;42(1, pt 2):23-24.
- Esmann S, Jemec GB. Management of actinic keratosis patients: a qualitative study. J Dermatolog Treat. 2007;18:53-58.
- Weinstock MA, Lee KC, Chren MM, et al. Quality of life in the actinic neoplasia syndrome: the VA Topical Tretinoin Chemoprevention (VATTC) trial. J Am Acad Dermatol. 2009;61:207-215.
- Berman B, Villa AM, Ramirez CC. Mechanisms of action of new treatment modalities for actinic keratosis. J Drugs Dermatol. 2006;5:167-173.
- Askew DA, Mickan SM, Soyer HP. Effectiveness of 5-fluorouracil treatment for actinic keratosis: a systematic review of randomized controlled trials. Int J Dermatol. 2009;46:452-463.
- Levy S, Furst K, Chern W. A pharmacokinetic evaluation of 0.5% and 5% fluorouracil topical cream in patients with actinic keratosis. Clin Ther. 2001;23:908-920.
- Jorizzo J, Stewart D, Bucko A, et al. Randomized trial evaluating a new 0.5% fluorouracil formulation demonstrates efficacy after 1-, 2-, or 4-week treatment in patients with actinic keratosis. Cutis. 2002;70:335-359.
- Sachs DL, Kang S, Hammerberg C, et al. Topical fluorouracil for actinic keratoses and photoaging: a clinical and molecular analysis. Arch Dermatol. 2009;145:659-666.
- Hantash BM, Stewart DB, Cooper ZA, et al. Facial resurfacing for nonmelanoma skin cancer prophylaxis. Arch Dermatol. 2006;142:976-982.
- Lawrence N, Cox SE, Cockerell CJ, et al. A comparison of the efficacy and safety of Jessner’s solution and 35% trichloroacetic acid vs 5% fluorouracil in the treatment of widespread facial actinic keratoses. Arch Dermatol. 1995;131:176-181.
- Monheit GD. The Jessner’s + TCA peel: a medium-depth chemical peel. J Dermatol Surg Oncol. 1989;15:945-950.
- Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196-200.
- Adamson DJ, Frew D, Tatoud R, et al. Diclofenac antagonizes peroxisome proliferator-activated receptor-gamma signaling. Mol Pharmacol. 2002;61:7-12.
- Alam CA, Seed MP, Willoughby DA. Angiostasis and vascular regression in chronic granulomatous inflammation induced by diclofenac in combination with hyaluronan in mice. J Pharm Pharmacol. 1995;47:407-411.
- Lu X, Xie W, Reed D, et al. Nonsteroidal antiinflammatory drugs cause apoptosis and induce cyclooxygenases in chicken embryo fibroblasts. Proc Natl Acad Sci USA. 1995;92:7961-7965.
- Seed MP, Brown JR, Freemantle CN, et al. The inhibition of colon-26 adenocarcinoma development and angiogenesis by topical diclofenac in 2.5% hyaluronan. Cancer Res. 1997;57:1625-1629.
- Rivers JK, Arlette J, Shear N, et al. Topical treatment of actinic keratoses with 3.0% diclofenac in 2.5% hyaluronan gel. Br J Dermatol. 2002;146:94-100.
- Wolf JE, Taylor JR, Tschen E, et al. Topical 3.0% diclo-fenac in 2.5% hyaluronan gel in the treatment of actinic keratoses. Int J Dermatol. 2001;40:709-713.
- Nelson C, Rigel D, Smith S, et al. Phase IV, open-label assessment of the treatment of actinic keratosis with 3.0% diclofenac sodium topical gel (Solaraze). J Drugs Dermatol. 2004;3:401-407.
- Pflugfelder A, Welter AK, Leiter U, et al. Open label randomized study comparing 3 months vs. 6 months treatment of actinic keratoses with 3% diclofenac in 2.5% hyaluronic acid gel: a trial of the German Dermatologic Cooperative Oncology Group. J Eur Acad Dermatol Venereol. 2012;26:48-53.
- Smith SR, Morhenn VB, Piacquadio DJ. Bilateral comparison of the efficacy and tolerability of 3% diclofenac sodium gel and 5% 5-fluorouracil cream in the treatment of actinic keratoses of the face and scalp. J Drugs Dermatol. 2006;5:156-159.
- Segatto MM, Dornelles SI, Silveira VB, et al. Comparative study of actinic keratosis treatment with 3% diclo- fenac sodium and 5% 5-fluorouracil. An Bras Dermatol. 2013;88:732-738.
- Vidal D. Topical imiquimod: mechanism of action and clinical applications. Mini Rev Med Chem. 2006;6:499-503.
- Hadley G, Derry S, Moore RA. Imiquimod for actinic keratosis: systematic review and meta-analysis. J Invest Dermatol. 2006;126:1251-1255.
- Caperton C, Berman B. Safety, efficacy, and patient acceptability of imiquimod for topical treatment of actinic keratoses. Clin Cosmet Investig Dermatol. 2011;4:35-40.
- Swanson N, Smith CC, Kaur M, et al. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: two phase 3, multicenter, randomized, double-blind, placebo-controlled studies. J Drugs Dermatol. 2014;13:166-169.
- Krawtchenko N, Roewert-Huber J, Ulrich M, et al. A randomised study of topical 5% imiquimod vs. topical 5-fluorouracil vs. cryosurgery in immunocompetent patients with actinic keratoses: a comparison of clinical and histological outcomes including 1-year follow-up. Br J Dermatol. 2007;157(suppl 2):34-40.
- Anderson L, Schmieder GJ, Werschler WP, et al. Randomized, double-blind, double-dummy, vehicle-controlled study of ingenol mebutate gel 0.025% and 0.05% for actinic keratosis. J Am Acad Dermatol. 2009;60:934-943.
- Ogbourne SM, Suhrbier A, Jones B, et al. Antitumor activity of 3-ingenyl angelate: plasma membrane and mitochondrial disruption and necrotic cell death. Cancer Res. 2004;64:2833-2839.
- Lebwohl M, Swanson N, Anderson LL, et al. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366:1010-1019.
- Lebwohl M, Shumack S, Stein-Gold L, et al. Long-term follow-up study of ingenol mebutate gel for the treatment of actinic keratoses. JAMA Dermatol. 2013;149:666-670.
- Augustin M, Tu JH, Knudsen KM, et al. Ingenol mebutate gel for actinic keratosis: the link between quality of life, treatment satisfaction, and clinical outcomes. J Am Acad Dermatol. 2015;72:816-821.
- Kane-Maguire N, Moseley R, Cozzi S, et al. Modulation of fibroblast phenotype and extracellular matrix composition by ingenol mebutate may be associated with scar resolution and improved dermal cosmesis. J Am Acad Dermatol. 2012;66:AB218.
- Martin G, Swanson N. Clinical findings using ingenol mebutate gel to treat actinic keratoses. J Am Acad Dermatol. 2013;68(1, suppl 1):S39-S48.
- Feldman SR, Fleischer AB, Williford PM, et al. Destructive procedures are the standard of care for treatment of actinic keratoses. J Am Acad Dermatol. 1999;40:43-47.
- Berlin JM. Current and emerging treatment strategies for the treatment of actinic keratosis. Clin Cosmet Investig Dermatol. 2010;3:119-126.
- Coleman WP, Yarborough JM, Mandy SH. Dermabrasion for prophylaxis and treatment of actinic keratoses. Dermatol Surg. 1996;22:17-21.
- Cooley JE, Casey DL, Kauffman CL. Manual resurfacing and trichloroacetic acid for the treatment of patients with widespread actinic damage. clinical and histologic observations. Dermatol Surg. 1997;23:373-379.
- Goldberg LH, Kaplan B, Vergilis-Kalner I, et al. Liquid nitrogen: temperature control in the treatment of actinic keratosis. Dermatol Surg. 2010;36:1956-1961.
- Lubritz RR, Smolewski SA. Cryosurgery cure rate of actinic keratoses. J Am Acad Dermatol. 1982;7:631-632.
- Thai KE, Fergin P, Freeman M, et al. A prospective study of the use of cryosurgery for the treatment of actinic keratoses. Int J Dermatol. 2004;43:687-692.
- Zane C, Facchinetti E, Rossi MT, et al. Cryotherapy is preferable to ablative CO2 laser for the treatment of isolated actinic keratoses of the face and scalp: a randomized clinical trial. Br J Dermatol. 2014;170:1114-1121.
- Trimas SJ, Ellis DA, Metz RD. The carbon dioxide laser. an alternative for the treatment of actinically damaged skin. Dermatol Surg. 1997;23:885-889.
- Jiang SB, Levine VJ, Nehal KS, et al. Er:YAG laser for the treatment of actinic keratoses. Dermatol Surg. 2000;26:437-440.
- Ostertag JU, Quaedvlieg PJ, Van der geer S, et al. A clinical comparison and long-term follow-up of topical 5-fluorouracil versus laser resurfacing in the treatment of widespread actinic keratoses. Lasers Surg Med. 2006;38:731-739.
- Iyer S, Friedli A, Bowes L, et al. Full face laser resurfacing: therapy and prophylaxis for actinic keratoses and non-melanoma skin cancer. Lasers Surg Med. 2004;34:114-119.
- Rubin MG. A peeler’s thoughts on skin improvement with chemical peels and laser resurfacing. Clin Plast Surg. 1997;24:407-409.
- Riggs K, Keller M, Humphreys TR. Ablative laser resurfacing: high-energy pulsed carbon dioxide and erbium:yttrium-aluminum-garnet. Clin Dermatol. 2007;25:462-473.
- Adrian RM. Pulsed carbon dioxide and long pulse 10-ms erbium-YAG laser resurfacing: a comparative clinical and histological study. J Cutan Laser Ther. 1999;1:197-202.
- Weiss ET, Brauer JA, Anolik R, et al. 1927-nm fractional resurfacing of facial actinic keratoses: a promising new therapeutic option. J Am Acad Dermatol. 2013; 68:98-102.
- Manstein D, Herron GS, Sink RK, et al. Fractional photothermolysis: a new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg Med. 2004;34:426-438.
- Katz TM, Goldberg LH, Marquez D, et al. Nonablative fractional photothermolysis for facial actinic keratoses: 6-month follow-up with histologic evaluation. J Am Acad Dermatol. 2011;65:349-356.
- Prens SP, De Vries K, Neumann HA, et al. Non-ablative fractional resurfacing in combination with topical tretinoin cream as a field treatment modality for multiple actinic keratosis: a pilot study and a review of other field treatment modalities. J Dermatolog Treat. 2013;24:227-231.
- Alexiades-Armenakas MR, Dover JS, Arndt KA. The spectrum of laser skin resurfacing: nonablative, fractional, and ablative laser resurfacing. J Am Acad Dermatol. 2008;58:719-737.
- Tannous Z. Fractional resurfacing. Clin Dermatol. 2007;25:480-486.
- Gold MH. Continuing medical education article-skin treatment: photodynamic therapy: indications and treatment. Aesthet Surg J. 2008;28:545-552.
- Juarranz A, Jaén P, Sanz-Rodríguez F, et al. Photodynamic therapy of cancer. basic principles and applications. Clin Transl Oncol. 2008;10:148-154.
- Juzeniene A, Peng Q, Moan J. Milestones in the development of photodynamic therapy and fluorescence diagnosis. Photochem Photobiol Sci. 2007;6:1234-1245.
- Moan J, Berg K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem Photobiol. 1991;53:549-553.
- Gupta AK, Paquet M, Villanueva E, et al. Interventions for actinic keratoses. Cochrane Database Syst Rev. 2012;12:CD004415.
- Patel G, Armstrong AW, Eisen DB. Efficacy of photodynamic therapy vs other interventions in randomized clinical trials for the treatment of actinic keratoses: a systematic review and meta-analysis. JAMA Dermatol. 2014;150:1281-1288.
- Kaufmann R, Spelman L, Weightman W, et al. Multicentre intraindividual randomized trial of topical methyl aminolaevulinate-photodynamic therapy vs. cryotherapy for multiple actinic keratoses on the extremities. Br J Dermatol. 2008;158:994-999.
- Freeman M, Vinciullo C, Francis D, et al. A comparison of photodynamic therapy using topical methyl aminolevulinate (Metvix) with single cycle cryotherapy in patients with actinic keratosis: a prospective, randomized study. J Dermatolog Treat. 2003;14:99-106.
- Morton C, Campbell S, Gupta G, et al. Intraindividual, right-left comparison of topical methyl aminolaevulinate-photodynamic therapy and cryotherapy in subjects with actinic keratoses: a multicentre, randomized controlled study. Br J Dermatol. 2006;155:1029-1036.
- Pariser DM, Lowe NJ, Stewart DM, et al. Photodynamic therapy with topical methyl aminolevulinate for actinic keratosis: results of a prospective randomized multicenter trial. J Am Acad Dermatol. 2003;48:227-232.
- Zane C, Facchinetti E, Rossi MT, et al. A randomized clinical trial of photodynamic therapy with methyl aminolaevulinate vs. diclofenac 3% plus hyaluronic acid gel for the treatment of multiple actinic keratoses of the face and scalp. Br J Dermatol. 2014;170:1143-1150.
- Perrett CM, McGregor JM, Warwick J, et al. Treatment of post-transplant premalignant skin disease: a randomized intrapatient comparative study of 5-fluorouracil cream and topical photodynamic therapy. Br J Dermatol. 2007;156:320-328.
- Szeimies RM, Karrer S, Radakovic-Fijan S, et al. Photodynamic therapy using topical methyl 5-aminolevulinate compared with cryotherapy for actinic keratosis: a prospective, randomized study. J Am Acad Dermatol. 2002; 47:258-262.
- Scola N, Terras S, Georgas D, et al. A randomized, half-side comparative study of aminolaevulinate photodynamic therapy vs. CO(2) laser ablation in immunocompetent patients with multiple actinic keratoses. Br J Dermatol. 2012;167:1366-1373.
- Willey A, Anderson RR, Sakamoto FH. Temperature-modulated photodynamic therapy for the treatment of actinic keratosis on the extremities: a pilot study. Dermatol Surg. 2014;40:1094-1102.
- Pariser DM. Management of Actinic Keratoses: Treatment Selection and Optimizing Outcomes. Presented at: Winter Clinical Dermatology Conference Hawaii; January 18, 2015; Kaanapali, HI.
- Dirschka T, Radny P, Dominicus R, et al. Long-term (6 and 12 months) follow-up of two prospective, randomized, controlled phase III trials of photodynamic therapy with BF-200 ALA and methyl aminolaevulinate for the treatment of actinic keratosis. Br J Dermatol. 2013;168:825-836.
- Choi SH, Kim KH, Song KH. Efficacy of ablative fractional laser-assisted photodynamic therapy with short-incubation time for the treatment of facial and scalp actinic keratosis: 12-month follow-up results of a randomized, prospective, comparative trial. J Eur Acad Dermatol Venereol. 2015;29:1598-1605.
- Ko DY, Jeon SY, Kim KH, et al. Fractional erbium:YAG laser-assisted photodynamic therapy for facial actinic keratoses: a randomized, comparative, prospective study. J Eur Acad Dermatol Venereol. 2014;28:1529-1539.
- Togsverd-Ho K, Haak CS, Thaysen-Petersen D, et al. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol. 2012;166:1262-1269.
- Torezan L, Chaves Y, Niwa A, et al. A pilot split-face study comparing conventional methyl aminolevulinate-photodynamic therapy (PDT) with microneedling-assisted PDT on actinically damaged skin. Dermatol Surg. 2013;39:1197-1201.
Practice Points
- In addition to their risk for progression to malignancy, actinic keratoses (AKs) can have negative impacts on cosmetic appearance and quality of life.
- A variety of topical medications, procedural modalities, and light-based therapies are available for treatment of AKs, which offer varying degrees of efficacy for clearance of lesions and cosmetic outcomes. Based on the current data, imiquimod and photodynamic therapy are the treatments most likely to provide an excellent cosmetic outcome.
Therapies to Improve the Cosmetic Symptoms of Rosacea
Rosacea is a commonly encountered chronic inflammatory skin disease that affects an estimated 16 million Americans and exhibits a particular predilection for the convexities of the central face (eg, forehead, cheeks, nose, chin).1,2 The pathophysiology of rosacea remains poorly understood despite the relatively high prevalence of the disease and substantial ongoing research.3 The current paradigm suggests a complex multifactorial interplay involving aberrations of the innate and adaptive immune system, neurovascular dysregulation, blood and lymphatic vessel changes, genetic predispositions, and overgrowth of commensal organisms such as Demodex.3 Additionally, a variety of external factors may exacerbate clinical symptoms (eg, UV radiation, heat exposure, spicy food, alcohol, stress).
The diagnosis of rosacea is made clinically and rarely requires histologic confirmation. Although rosacea can present with a wide range of clinical features that often wax and wane over time, a near universal finding is diffuse centrofacial erythema.4 This centrofacial redness may symptomatically worsen during a flare period, causing flushing, but it often persists nontransiently between flares as background erythema. Other variable findings of rosacea include the presence of telangiectases, edema, plaques, phymatous changes, dry skin, ocular manifestations, and inflammatory lesions in the form of papules and pustules.5 Patients also may report a stinging or burning sensation in affected areas. It is important to note that most patients will only exhibit some of these clinical features and that symptoms often vary in the timing of their emergence or regression.5 A classification system has been developed for rosacea that categorizes the disease into 4 subtypes (erythematotelangiectatic, papulopustular, phymatous, and ocular) and one variant (granulomatous).6 These categories are determined by the grouping of clinical features present, but it is not uncommon for patients to exhibit clinical manifestations of more than 1 subtype.7
The detrimental cosmetic effects of rosacea are obvious given its chronic nature and tendency to affect highly visible areas such as the face. As such, rosacea can have a devastating impact on patients’ quality of life.8 Patients with rosacea have been reported to have higher incidence rates of low self-esteem, embarrassment, social anxiety, and depression as compared to the rest of the population. Effective treatment, however, can improve cosmetic appearance and mitigate the negative psychosocial impacts of the disease.8
Treatment of rosacea focuses on relieving cosmetic symptoms, as no curative therapy currently exists. Treatment comes in a wide variety of forms, including topical medications, systemic pharmacologic therapies, light-based modalities, and procedural interventions. Choice of therapy should be determined on a case-by-case basis as guided by the clinical features present, and combination or sequential therapies often are required to achieve optimal cosmetic results. In this article, we review both existing and emerging treatments of rosacea and assess their ability to improve the cosmetic symptoms of rosacea (Table).
Skin Care
Proper skin care is an important aspect of treatment for all patients with rosacea and thus includes the use of over-the-counter cleansers, moisturizers, and sunscreens.9 The choice of skin care products is an important consideration given the often hypersensitive skin of rosacea patients. Moisturizers and cleansers should have an acidic to neutral pH, similar to normal skin. They should not contain emulsifiers that strip moisture from the skin or protective lipids and proteins from the stratum corneum.10 Moisturizers without irritants, abrasives, or allergens should be used following skin cleansing. Protection from UV radiation with sunscreen, ideally with a sun protection factor greater than 30, is particularly important, as it can prevent UV-induced rosacea flares as well as photodamage that can cause additional erythema and telangiectasia.4 Rosacea patients also may find green-tinted makeup to be useful in concealing areas of erythema.8
Topical Therapy
Currently, there are only 5 US Food and Drug Administration (FDA)–approved topical medications for the treatment of rosacea: metronidazole (MTZ) gel 0.75% and 1%, azelaic acid (AzA) gel 15%, sodium sulfacetamide (SS) 10%–sulfur 5% lotion and cream, brimonidine tartrate (BT) gel 0.5%, and the most recently approved ivermectin (IVM) cream 1%.7 Metronidazole, AzA, and SS primarily are used to treat the inflammatory papules and pustules of rosacea, while BT is used to treat persistent background erythema. The exact mechanisms of action by which MTZ, AzA, and SS treat rosacea are unclear, but they are thought to reduce inflammation and/or immune response. Metronidazole and AzA both have demonstrated favorable safety profiles and significant (P<.05) efficacy over vehicle in reducing inflammatory lesions in numerous well-controlled randomized clinical studies.4,11,12 There is some evidence that AzA may be more effective than MTZ; one 15-week multicenter, double-blind, randomized, parallel-group study demonstrated that twice-daily AzA gel 15% showed significant superiority (P=.02) over twice-daily MTZ gel 0.75% in improving the inflammatory lesions and erythema of rosacea.13 Sodium sulfacetamide also has shown good efficacy in the treatment of inflammatory lesions and performed significantly better (P=.04) than MTZ according to one multicenter, investigator-blinded, randomized, parallel-group study,14 but the overall evidence is not as strong as MTZ and AzA.4,11,15 The most common adverse effect for MTZ, AzA, and SS is application-site irritation, but overall most patients report good tolerance to these topical medications.4 Azelaic acid is unique in that patients may report stinging, tingling, or burning after application, but these effects are not associated with visible skin changes and usually are transient, generally remitting after 1 to 2 weeks.4
Brimonidine tartrate is a highly selective α2-adrenergic receptor agonist whose mechanism of action in the treatment of rosacea is thought to involve vasoconstriction of superficial skin vasculature and to a lesser extent anti-inflammatory effects.16 In a double-blind, randomized, vehicle-controlled phase 3 trial, application of BT gel 0.5% once daily for 4 weeks demonstrated significant efficacy over vehicle (P<.001) in treating persistent nontransient facial erythema in 553 adult patients with 2 or fewer papulopustular lesions as evaluated over 12 hours on days 1, 15, and 29.17 Notably, a substantial difference in cosmetic appearance was observed in another study as early as 30 minutes after the first gel application on day 1.18 The results of this phase 3 trial17 mirrored those of the phase 2 dose-optimization and safety studies of similar design.18 In addition to another long-term, 1-year, open-label study,19 both phase 2 and 3 studies have shown favorable safety profiles with no reports of tachyphylaxis, rebound erythema, or aggravation of other disease features such as telangiectases or inflammatory lesions.17,18 Recently, however, there have been some reports of considerable rebound erythema with BT use and thus patients should be made aware of this possibility.20,21 Case reports of successful treatment of background erythema and flushing with other topically applied adrenergic receptor modifiers such as oxymetazoline and xylometazoline have been published in the literature,22,23 but additional research will be necessary to validate these claims.
Ivermectin, a decades-old antiparasitic, has recently shown promising results as a treatment of rosacea patients with moderate to severe papulopustular lesions. Its therapeutic effect is believed to be mediated by its activity against Demodex, a natural skin mite that has been found at increased concentrations in a subset of patients with rosacea, as well as by its natural anti-inflammatory properties.24 In 2 identically designed, randomized, double-blind, controlled trials of IVM cream 1% applied once daily for 12 weeks, a significantly larger proportion of patients in the IVM groups achieved an investigator global assessment of clear or almost clear as compared to vehicle (IVM: 38.4% and 40.1%, respectively; vehicle: 11.6% and 18.8%, respectively; P<.001). Both trials also demonstrated that IVM was significantly superior to vehicle in the reduction of inflammatory lesion counts measured at week 12 as compared to baseline (IVM: 76.0% and 75.0%, respectively; vehicle: 50.0% and 50.0%, respectively; P<.001).24 An extension of these original trials demonstrated long-term safety with up to 52 weeks of topical IVM use and reported a low incidence rate of adverse effects, most commonly transient skin burning, pruritus, and dryness. Notably, the incidence rate of these adverse effects was lower than a comparison group receiving AzA gel 15% once daily.25 Once-daily application of IVM cream 1% also has recently demonstrated superiority over twice-daily MTZ cream 0.75% for 16 weeks in a phase 3 investigator-blinded, randomized, parallel-group study. The IVM group was significantly superior to MTZ in the reduction of inflammatory lesions as compared to baseline (83.0% vs 73.7%) and in the number of participants who achieved an investigator global assessment score of clear or almost clear (84.9% vs 75.4%)(both P<.001).26 There also is limited evidence for the use of other antiparasitic topical medications such as crotamiton 10% and permethrin 5%, but such agents frequently cause irritation and may not be well tolerated in rosacea patients.27-29
There are a variety of other non–FDA-approved topical medications that have been used with varying success in the literature, including cyclosporine, macrolides, benzoyl peroxide, retinoids, and calcineurin inhibitors such as tacrolimus and pimecrolimus. Evidence for the use of these medications generally is limited to a few studies with small numbers of patients and will not be discussed further in this article.4,11,30 These agents, however, may be useful in select cases when first-line regimens have failed and also may be good targets for future research.
Systemic Therapy
The mainstay of systemic treatment of rosacea centers around the tetracyclines, a group of antibiotics that have been used off label for rosacea since the 1950s.31 The therapeutic effects of tetracyclines in the treatment of rosacea are thought to revolve around their anti-inflammatory effects rather than their antibacterial properties.32 Currently, the only FDA-approved oral agent for treatment of the inflammatory lesions of rosacea is doxycycline 40-mg modified-release capsules taken once daily. These modified capsules allow for instant release of 30 mg and delayed release of 10 mg of doxycycline. This dosing is considered to be anti-inflammatory rather than antimicrobial, as it does not produce antibiotic selection pressure even with prolonged use.33 Efficacy of 40-mg subantimicrobial-dose doxycycline (SDD) has been demonstrated in 2 phase 3 multicenter, parallel-group, randomized, double-blind, placebo-controlled studies in which SDD demonstrated a significantly greater reduction in the number of total inflammatory lesions at week 16 compared to placebo (P<.001).34 Subantimicrobial-dose doxycycline also has been shown to be equally as efficacious in reducing inflammatory lesions as traditional-dose doxycycline.35 There also is some evidence for the efficacy of SDD in reducing overall erythema, as demonstrated by one open-label, community-based study in which SDD monotherapy resulted in clinician erythema assessment scores of mild or no erythema in 75% of patients with mild to severe rosacea at baseline after 12 weeks of therapy.35 Additionally, SDD is considered to be safe and well-tolerated and does not generally result in the adverse effects that may be seen in antibiotic-level doses of doxycycline (eg, gastrointestinal upset, vaginal candidiasis, photosensitivity).34,36,37 Other antibiotics such as clarithromycin, azithromycin, and MTZ also have been studied as treatments of papulopustular rosacea at antibiotic-level doses with good therapeutic effect.38-40 These therapies, however, generally are not used unless there are contraindications for use of tetracycline antibiotics, such as pregnancy or allergy, as the overall evidence is not as strong and there may be increased risks for serious adverse effects.30
Although it is not FDA approved, isotretinoin is an important therapeutic option for select rosacea patients, as it is the only pharmacologic agent that has shown efficacy for the phymatous changes of rosacea. Its efficacy, however, is limited to early-stage rhinophyma that has not yet progressed to the fibrotic or mucinous stages of disease in which it has been shown to reduce the size and number of cutaneous sebaceous glands.30,41 Isotretinoin at 0.3 mg/kg daily also has shown noninferiority in treatment of the inflammatory papules and pustules of rosacea as compared to antibiotic dosing of doxycycline in one large-scale, placebo-controlled, randomized, 12-week multicenter study.42 Unfortunately, recurrence is highly likely after isotretinoin therapy is discontinued.30,41 However, continuous “microdose” isotretinoin at 0.03 to 0.17 mg/kg daily has shown evidence for efficacy in treatment of recalcitrant papulopustular disease.43 Such dosing may have the added benefit of reduced risk for radiographic changes associated with long-term isotretinoin use.43
Light-Based Therapy
Light-based modalities are an important tool set in the management of rosacea symptoms, as they can treat telangiectases for which medical therapy is not generally effective.9 To a lesser extent, light-based modalities also can help alleviate background erythema. The most commonly used light-based modalities include the pulsed dye laser (PDL)(Figure), potassium titanyl phosphate (KTP) laser, Nd:YAG laser, intense pulsed light, photodynamic therapy, CO2 laser, and erbium-doped YAG (Er:YAG) laser. These treatments produce clinical results by targeting specific chromophores such as oxyhemoglobin, deoxyhemoglobin, methemoglobin, and clotted blood with light of specific wavelengths to induce thermolysis of vasculature while sparing collateral tissue.44 Generally, larger telangiectatic vessels are more amenable to therapy than smaller vessels, which usually require higher energy to be delivered in a shorter period of time, thus predisposing the patient to the development of purpura that may last for 1 to 2 weeks.44
  |
Historically, PDL used a light wavelength of 577 nm and was classically associated with posttherapy purpura; however, modern PDLs use wavelengths of 585 or 595 nm and are associated with a reduced risk for purpura through the use of longer pulse durations (ie, 10–40 millisecond), multiple minipulses, multiple passes, and advanced epidermal cooling methods.9,44 In a small, prospective, randomized, controlled, nonblinded study, PDL therapy with fluence sufficiently high enough to induce purpura achieved an approximate 50% improvement in telangiectasia grading scores in most patients after a single treatment.45 Notably, PDL therapy at purpura-inducing settings was reported to be much more efficacious than settings that did not induce purpura (purpura free), especially in the treatment of thicker telangiectases.45
Potassium titanyl phosphate lasers make use of shorter wavelengths (532 nm) than PDL and thus are better able to target superficial vasculature, which translates into a reduced risk for purpura and faster healing times. However, KTP laser therapy typically is only reserved for patients with lighter skin types, as this wavelength of light is more likely to result in higher melanin absorption and possible postinflammatory hyperpigmentation.44 A split-face study comparing the KTP laser with PDL determined that the KTP laser was able to achieve 62% clearing after the first treatment and 85% clearance after the third treatment versus 49% and 75% for PDL treatment, respectively; however, the KTP laser had higher rates of posttherapy erythema lasting at least 1 day (58% vs 8%).46
Conversely, the Nd:YAG laser uses longer wavelengths (1064 nm) and can achieve deeper skin penetration, which may be effective for larger, recalcitrant, or deeper blue-tinted vessels. A split-face, double-blind, randomized, controlled trial found Nd:YAG laser therapy to be an effective treatment of facial erythema, though it was observed to be less effective than purpura-free PDL therapy in reducing redness after 4 treatments (34% vs 52% improvement, respectively); however, treatment with the Nd:YAG laser was found to be significantly (P=.0028) less painful.47
Intense pulsed light is unique from the previously discussed light-based therapies in that it uses noncoherent light with wavelengths between 500 and 1200 nm. Cutoff filters may be used to allow for more selective tissue damage depending on the depth of penetration desired. Intense pulsed light has been shown to be equally as efficacious as purpura-free PDL therapy in the treatment of erythema and telangiectasia in a randomized, controlled, single-blind, split-face trial.48 Additionally, a study of 200 patients with facial vascular lesions, of whom 74 patients had rosacea, showed that intense pulsed light therapy resulted in a 75% to 100% improvement of lesions in 174 of 188 (92.5%) patients who returned for follow-up. Treatment often required at least 2 sessions, but overall adverse effects were reported to be minimal.49
Photodynamic therapy is a well-studied and often utilized treatment of a variety of skin conditions, but there have only been a few studies regarding its use in rosacea. Photodynamic therapy involves the use of topically applied photosensitizing agents such as 5-aminolevulinic acid or methyl aminolevulinate before exposure to red or blue light. This process generates reactive oxygen species, though the exact mechanism of action through which patients achieve cosmetic improvement in rosacea is unclear. In one study of 17 patients with varying rosacea subtypes treated with methyl aminolevulinate and red light, drastic relief of symptoms was seen in 10 (58.8%) patients, marked improvement in 4 (23.5%) patients, and no response in 3 (17.6%) patients. Most patients report a transient irritant skin reaction at the site of therapy.50
Ablative lasers such as the CO2 (10,600 nm) and Er:YAG (2940 nm) lasers also have been shown to be useful in the treatment of rosacea, specifically for the management of rhinophymatous features. Excellent results have been achieved with these lasers given their ability to provide near-bloodless surgical fields. In a 13-year review of 124 patients with rhinophyma receiving a single CO2 laser treatment, good to excellent results were achieved in 118 (95.2%) of patients when evaluated at 3 months posttreatment.51 Patient satisfaction also is reported to be high with few adverse effects reported. The evidence for the Er:YAG laser is not as strong, but the current reports indicate efficacy and safety similar to that of the CO2 laser.52
Procedural Therapies
Procedural therapies in rosacea generally are reserved for management of rhinophyma and include electrocautery, cryotherapy, radiotherapy, dermabrasion, scalpel excisions, flap reconstruction, and skin grafts.30,53 The details and evidence for these methods is beyond the scope of this paper, but it is important to be aware of such modalities. As with most surgical procedures, operator skill and experience may affect treatment outcomes, and there also are definite risks for postprocedural scarring, swelling, erythema, and pigmentation changes. Recently, anecdotal evidence has shown that botulinum toxin injections may be effective for patients with refractory flushing and erythema, but larger studies will be necessary to better assess these claims.54,55
Conclusion
Although recent advances in pharmacology and laser technology have provided physicians with new and effective treatment modalities for rosacea, it remains a poorly understood disease without a definitive cure. The negative impact of rosacea on patients’ quality of life can be substantial, but effective management of cosmetic symptoms can minimize such deleterious effects. Therapy should be individualized and directed at treating the symptoms that are most bothersome to the patient. Additionally, effective treatment often will require a combination of modalities or sequential therapies to achieve optimal cosmetic outcomes.
1. Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6 suppl 1):S27-S35.
2. Rosacea prevalence map. National Rosacea Society Web site. http://rosacea.org/press/prevalencemap. Accessed June 16, 2015.
3. Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 suppl 1):S15-S26.
4. Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 1: a status report on the disease state, general measures, and adjunctive skin care. Cutis. 2013;92:234-240.
5. Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004;50:907-912.
6. Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society expert committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
7. Feldman SR, Huang WW, Huynh TT. Current drug therapies for rosacea: a chronic vascular and inflammatory skin disease. J Manag Care Spec Pharm. 2014;20:623-629.
8. Moustafa F, Lewallen RS, Feldman SR. The psychological impact of rosacea and the influence of current management options. J Am Acad Dermatol. 2014;71:973-980.
9. Mansouri Y, Goldenberg G. Devices and topical agents for rosacea management. Cutis. 2014;94:21-25.
10. Levin J, Miller R. A guide to the ingredients and potential benefits of over-the-counter cleansers and moisturizers for rosacea patients. J Clin Aesthet Dermatol. 2011;4:31-49.
11. van Zuuren EJ, Kramer S, Carter B, et al. Interventions for rosacea. Cochrane Database Syst Rev. 2011;3:CD003262.
12. Liu RH, Smith MK, Basta SA, et al. Azelaic acid in the treatment of papulopustular rosacea: a systematic review of randomized controlled trials. Arch Dermatol. 2006;142:1047-1052.
13. Elewski B, Fleischer AB Jr, Pariser DM. A comparison of 15% azelaic acid gel and 0.75% metronidazole gel in the topical treatment of papulopustular rosacea: results of a randomized trial. Arch Dermatol. 2003;139:1444-1450.
14. Torok HM, Webster G, Dunlap FE, et al. Combination sodium sulfacetamide 10% and sulfur 5% cream with sunscreens versus metronidazole 0.75% cream for rosacea. Cutis. 2005;75:357-363.
15. Trumbore MW, Goldstein JA, Gurge RM. Treatment of papulopustular rosacea with sodium sulfacetamide 10%/sulfur 5% emollient foam. J Drugs Dermatol. 2009;8:299-304.
16. Piwnica D, Rosignoli C, de Menonville ST, et al. Vasoconstriction and anti-inflammatory properties of the selective alpha-adrenergic receptor agonist brimonidine. J Dermatol Sci. 2014;75:49-54.
17. Fowler J Jr, Jackson M, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12:650-656.
18. Fowler J, Jarratt M, Moore A, et al. Once-daily topical brimonidine tartrate gel 0.5% is a novel treatment of moderate to severe facial erythema of rosacea: results of two multicenter, randomized and vehicle-controlled studies. Br J Dermatol. 2012;166:633-641.
19. Moore A, Kempers S, Murakawa G, et al. Long-term safety and efficacy of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of a 1-year open-label study. J Drugs Dermatol. 2014;13:56-61.
20. Routt ET, Levitt JO. Rebound erythema and burning sensation from a new topical brimonidine tartrate gel 0.33%. J Am Acad Dermatol. 2014;70:e37-e38.
21. Ilkovitch D, Pomerantz RG. Brimonidine effective but may lead to significant rebound erythema. J Am Acad Dermatol. 2014;70:e109-e110.
22. Kim JH, Oh YS, Ji JH, et al. Rosacea (erythematotelangiectatic type) effectively improved by topical xylometazoline. J Dermatol. 2011;38:510-513.
23. Shanler SD, Ondo AL. Successful treatment of erythema and flushing of rosacea using a topically applied selective alpha1-adrenergic receptor antagonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
24. Stein-Gold L, Kircik L, Fowler J, et al. Efficacy and safety of ivermectin 1% cream in treatment of papulopustular rosacea: results of two randomized, double-blind, vehicle-controlled pivotal studies. J Drugs Dermatol. 2014;13:316-323.
25. Stein-Gold L, Kircik L, Fowler J, et al. Long-term safety of ivermectin 1% cream vs azelaic acid 15% gel in treating inflammatory lesions of rosacea: results of two 40-week controlled, investigator-blinded trials. J Drugs Dermatol. 2014;13:1380-1386.
26. Taieb A, Ortonne JP, Ruzicka T, et al. Superiority of ivermectin 1% cream over metronidazole 0.75% cream in treating inflammatory lesions of rosacea: a randomized, investigator-blinded trial. Br J Dermatol. 2015;172:1103-1110.
27. Koçak M, Ya˘gli S, Vahapo˘glu G, et al. Permethrin 5% cream versus metronidazole 0.75% gel for the treatment of papulopustular rosacea. a randomized double-blind placebo-controlled study. Dermatology (Basel). 2002;205:265-270.
28. Bikowski JB, Del Rosso JQ. Demodex dermatitis: a retrospective analysis of clinical diagnosis and successful treatment with topical crotamiton. J Clin Aesthet Dermatol. 2009;2:20-25.
29. Layton A, Thiboutot D. Emerging therapies in rosacea. J Am Acad Dermatol. 2013;69(6 suppl 1):S57-S65.
30. Pelle MT, Crawford GH, James WD. Rosacea: II. therapy. J Am Acad Dermatol. 2004;51:499-512, quiz 513-514.
31. Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006;54:258-265.
32. Korting HC, Schöllmann C. Tetracycline actions relevant to rosacea treatment. Skin Pharmacol Physiol. 2009;22:287-294.
33. Thomas J, Walker C, Bradshaw M. Long-term use of subantimicrobial dose doxycycline does not lead to changes in antimicrobial susceptibility. J Periodontol. 2000;71:1472-1483.
34. Del Rosso JQ, Webster GF, Jackson M, et al. Two randomized phase III clinical trials evaluating anti-inflammatory dose doxycycline (40-mg doxycycline, USP capsules) administered once daily for treatment of rosacea. J Am Acad Dermatol. 2007;56:791-802.
35. Webster GF. An open-label, community-based, 12-week assessment of the effectiveness and safety of monotherapy with doxycycline 40 mg (30-mg immediate-release and 10-mg delayed-release beads). Cutis. 2010;86(suppl 5):7-15.
36. Del Rosso JQ, Schlessinger J, Werschler P. Comparison of anti-inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. J Drugs Dermatol. 2008;7:573-576.
37. Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 3: a status report on systemic therapies. Cutis. 2014;93:18-28.
38. Torresani C. Clarithromycin: a new perspective in rosacea treatment. Int J Dermatol. 1998;37:347-349.
39. Bakar O, Demircay Z, Gürbüz O. Therapeutic potential of azithromycin in rosacea. Int J Dermatol. 2004;43:151-154.
40. Saihan EM, Burton JL. A double-blind trial of metronidazole versus oxytetracycline therapy for rosacea. Br J Dermatol. 1980;102:443-445.
41. Park H, Del Rosso JQ. Use of oral isotretinoin in the management of rosacea. J Clin Aesthet Dermatol. 2011;4:54-61.
42. Gollnick H, Blume-Peytavi U, Szabo EL, et al. Systemic isotretinoin in the treatment of rosacea—doxycycline-and placebo-controlled, randomized clinical study. J Dtsch Dermatol Ges. 2010;8:505-515.
43. Hofer T. Continuous “microdose” isotretinoin in adult recalcitrant rosacea. Clin Exp Dermatol. 2004;29:204-205.
44. Tanghetti E, Del Rosso JQ, Thiboutot D, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 4: a status report on physical modalities and devices. Cutis. 2014;93:71-76.
45. Alam M, Dover JS, Arndt KA. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol Surg. 2003;29:681-684.
46. Uebelhoer NS, Bogle MA, Stewart B, et al. A split-face comparison study of pulsed 532-nm KTP laser and 595-nm pulsed dye laser in the treatment of facial telangiectasias and diffuse telangiectatic facial erythema. Dermatol Surg. 2007;33:441-448.
47. Alam M, Voravutinon N, Warycha M, et al. Comparative effectiveness of nonpurpuragenic 595-nm pulsed dye laser and microsecond 1064-nm neodymium:yttrium-aluminum-garnet laser for treatment of diffuse facial erythema: a double-blind randomized controlled trial. J Am Acad Dermatol. 2013;69:438-443.
48. Neuhaus IM, Zane LT, Tope WD. Comparative efficacy of nonpurpuragenic pulsed dye laser and intense pulsed light for erythematotelangiectatic rosacea. Dermatol Surg. 2009;35:920-928.
49. Angermeier MC. Treatment of facial vascular lesions with intense pulsed light. J Cutan Laser Ther. 1999;1:95-100.
50. Bryld LE, Jemec GB. Photodynamic therapy in a series of rosacea patients. J Eur Acad Dermatol Venereol. 2007;21:1199-1202.
51. Maden V, Ferguson JE, August PJ. Carbon dioxide laser treatment of rhinophyma: a review of 124 patients. Br J Dermatol. 2009;161:814-818.
52. Fincher EF, Gladstone HB. Use of a dual-mode erbium:YAG laser for the surgical correction of rhinophyma. Arch Facial Plast Surg. 2004;6:267-271.
53. Lloyd KM. Surgical correction of rhinophyma. Arch Dermatol. 1990;126:721-723.
54. Dayan SH, Pritzker RN, Arkins JP. A new treatment regimen for rosacea: onabotulinumtoxinA. J Drugs Dermatol. 2012;11:e76-e79.
55. Park KY, Hyun MY, Jeong SY, et al. Botulinum toxin for the treatment of refractory erythema and flushing of rosacea. Dermatology. 2015;230:299-301.
Rosacea is a commonly encountered chronic inflammatory skin disease that affects an estimated 16 million Americans and exhibits a particular predilection for the convexities of the central face (eg, forehead, cheeks, nose, chin).1,2 The pathophysiology of rosacea remains poorly understood despite the relatively high prevalence of the disease and substantial ongoing research.3 The current paradigm suggests a complex multifactorial interplay involving aberrations of the innate and adaptive immune system, neurovascular dysregulation, blood and lymphatic vessel changes, genetic predispositions, and overgrowth of commensal organisms such as Demodex.3 Additionally, a variety of external factors may exacerbate clinical symptoms (eg, UV radiation, heat exposure, spicy food, alcohol, stress).
The diagnosis of rosacea is made clinically and rarely requires histologic confirmation. Although rosacea can present with a wide range of clinical features that often wax and wane over time, a near universal finding is diffuse centrofacial erythema.4 This centrofacial redness may symptomatically worsen during a flare period, causing flushing, but it often persists nontransiently between flares as background erythema. Other variable findings of rosacea include the presence of telangiectases, edema, plaques, phymatous changes, dry skin, ocular manifestations, and inflammatory lesions in the form of papules and pustules.5 Patients also may report a stinging or burning sensation in affected areas. It is important to note that most patients will only exhibit some of these clinical features and that symptoms often vary in the timing of their emergence or regression.5 A classification system has been developed for rosacea that categorizes the disease into 4 subtypes (erythematotelangiectatic, papulopustular, phymatous, and ocular) and one variant (granulomatous).6 These categories are determined by the grouping of clinical features present, but it is not uncommon for patients to exhibit clinical manifestations of more than 1 subtype.7
The detrimental cosmetic effects of rosacea are obvious given its chronic nature and tendency to affect highly visible areas such as the face. As such, rosacea can have a devastating impact on patients’ quality of life.8 Patients with rosacea have been reported to have higher incidence rates of low self-esteem, embarrassment, social anxiety, and depression as compared to the rest of the population. Effective treatment, however, can improve cosmetic appearance and mitigate the negative psychosocial impacts of the disease.8
Treatment of rosacea focuses on relieving cosmetic symptoms, as no curative therapy currently exists. Treatment comes in a wide variety of forms, including topical medications, systemic pharmacologic therapies, light-based modalities, and procedural interventions. Choice of therapy should be determined on a case-by-case basis as guided by the clinical features present, and combination or sequential therapies often are required to achieve optimal cosmetic results. In this article, we review both existing and emerging treatments of rosacea and assess their ability to improve the cosmetic symptoms of rosacea (Table).
Skin Care
Proper skin care is an important aspect of treatment for all patients with rosacea and thus includes the use of over-the-counter cleansers, moisturizers, and sunscreens.9 The choice of skin care products is an important consideration given the often hypersensitive skin of rosacea patients. Moisturizers and cleansers should have an acidic to neutral pH, similar to normal skin. They should not contain emulsifiers that strip moisture from the skin or protective lipids and proteins from the stratum corneum.10 Moisturizers without irritants, abrasives, or allergens should be used following skin cleansing. Protection from UV radiation with sunscreen, ideally with a sun protection factor greater than 30, is particularly important, as it can prevent UV-induced rosacea flares as well as photodamage that can cause additional erythema and telangiectasia.4 Rosacea patients also may find green-tinted makeup to be useful in concealing areas of erythema.8
Topical Therapy
Currently, there are only 5 US Food and Drug Administration (FDA)–approved topical medications for the treatment of rosacea: metronidazole (MTZ) gel 0.75% and 1%, azelaic acid (AzA) gel 15%, sodium sulfacetamide (SS) 10%–sulfur 5% lotion and cream, brimonidine tartrate (BT) gel 0.5%, and the most recently approved ivermectin (IVM) cream 1%.7 Metronidazole, AzA, and SS primarily are used to treat the inflammatory papules and pustules of rosacea, while BT is used to treat persistent background erythema. The exact mechanisms of action by which MTZ, AzA, and SS treat rosacea are unclear, but they are thought to reduce inflammation and/or immune response. Metronidazole and AzA both have demonstrated favorable safety profiles and significant (P<.05) efficacy over vehicle in reducing inflammatory lesions in numerous well-controlled randomized clinical studies.4,11,12 There is some evidence that AzA may be more effective than MTZ; one 15-week multicenter, double-blind, randomized, parallel-group study demonstrated that twice-daily AzA gel 15% showed significant superiority (P=.02) over twice-daily MTZ gel 0.75% in improving the inflammatory lesions and erythema of rosacea.13 Sodium sulfacetamide also has shown good efficacy in the treatment of inflammatory lesions and performed significantly better (P=.04) than MTZ according to one multicenter, investigator-blinded, randomized, parallel-group study,14 but the overall evidence is not as strong as MTZ and AzA.4,11,15 The most common adverse effect for MTZ, AzA, and SS is application-site irritation, but overall most patients report good tolerance to these topical medications.4 Azelaic acid is unique in that patients may report stinging, tingling, or burning after application, but these effects are not associated with visible skin changes and usually are transient, generally remitting after 1 to 2 weeks.4
Brimonidine tartrate is a highly selective α2-adrenergic receptor agonist whose mechanism of action in the treatment of rosacea is thought to involve vasoconstriction of superficial skin vasculature and to a lesser extent anti-inflammatory effects.16 In a double-blind, randomized, vehicle-controlled phase 3 trial, application of BT gel 0.5% once daily for 4 weeks demonstrated significant efficacy over vehicle (P<.001) in treating persistent nontransient facial erythema in 553 adult patients with 2 or fewer papulopustular lesions as evaluated over 12 hours on days 1, 15, and 29.17 Notably, a substantial difference in cosmetic appearance was observed in another study as early as 30 minutes after the first gel application on day 1.18 The results of this phase 3 trial17 mirrored those of the phase 2 dose-optimization and safety studies of similar design.18 In addition to another long-term, 1-year, open-label study,19 both phase 2 and 3 studies have shown favorable safety profiles with no reports of tachyphylaxis, rebound erythema, or aggravation of other disease features such as telangiectases or inflammatory lesions.17,18 Recently, however, there have been some reports of considerable rebound erythema with BT use and thus patients should be made aware of this possibility.20,21 Case reports of successful treatment of background erythema and flushing with other topically applied adrenergic receptor modifiers such as oxymetazoline and xylometazoline have been published in the literature,22,23 but additional research will be necessary to validate these claims.
Ivermectin, a decades-old antiparasitic, has recently shown promising results as a treatment of rosacea patients with moderate to severe papulopustular lesions. Its therapeutic effect is believed to be mediated by its activity against Demodex, a natural skin mite that has been found at increased concentrations in a subset of patients with rosacea, as well as by its natural anti-inflammatory properties.24 In 2 identically designed, randomized, double-blind, controlled trials of IVM cream 1% applied once daily for 12 weeks, a significantly larger proportion of patients in the IVM groups achieved an investigator global assessment of clear or almost clear as compared to vehicle (IVM: 38.4% and 40.1%, respectively; vehicle: 11.6% and 18.8%, respectively; P<.001). Both trials also demonstrated that IVM was significantly superior to vehicle in the reduction of inflammatory lesion counts measured at week 12 as compared to baseline (IVM: 76.0% and 75.0%, respectively; vehicle: 50.0% and 50.0%, respectively; P<.001).24 An extension of these original trials demonstrated long-term safety with up to 52 weeks of topical IVM use and reported a low incidence rate of adverse effects, most commonly transient skin burning, pruritus, and dryness. Notably, the incidence rate of these adverse effects was lower than a comparison group receiving AzA gel 15% once daily.25 Once-daily application of IVM cream 1% also has recently demonstrated superiority over twice-daily MTZ cream 0.75% for 16 weeks in a phase 3 investigator-blinded, randomized, parallel-group study. The IVM group was significantly superior to MTZ in the reduction of inflammatory lesions as compared to baseline (83.0% vs 73.7%) and in the number of participants who achieved an investigator global assessment score of clear or almost clear (84.9% vs 75.4%)(both P<.001).26 There also is limited evidence for the use of other antiparasitic topical medications such as crotamiton 10% and permethrin 5%, but such agents frequently cause irritation and may not be well tolerated in rosacea patients.27-29
There are a variety of other non–FDA-approved topical medications that have been used with varying success in the literature, including cyclosporine, macrolides, benzoyl peroxide, retinoids, and calcineurin inhibitors such as tacrolimus and pimecrolimus. Evidence for the use of these medications generally is limited to a few studies with small numbers of patients and will not be discussed further in this article.4,11,30 These agents, however, may be useful in select cases when first-line regimens have failed and also may be good targets for future research.
Systemic Therapy
The mainstay of systemic treatment of rosacea centers around the tetracyclines, a group of antibiotics that have been used off label for rosacea since the 1950s.31 The therapeutic effects of tetracyclines in the treatment of rosacea are thought to revolve around their anti-inflammatory effects rather than their antibacterial properties.32 Currently, the only FDA-approved oral agent for treatment of the inflammatory lesions of rosacea is doxycycline 40-mg modified-release capsules taken once daily. These modified capsules allow for instant release of 30 mg and delayed release of 10 mg of doxycycline. This dosing is considered to be anti-inflammatory rather than antimicrobial, as it does not produce antibiotic selection pressure even with prolonged use.33 Efficacy of 40-mg subantimicrobial-dose doxycycline (SDD) has been demonstrated in 2 phase 3 multicenter, parallel-group, randomized, double-blind, placebo-controlled studies in which SDD demonstrated a significantly greater reduction in the number of total inflammatory lesions at week 16 compared to placebo (P<.001).34 Subantimicrobial-dose doxycycline also has been shown to be equally as efficacious in reducing inflammatory lesions as traditional-dose doxycycline.35 There also is some evidence for the efficacy of SDD in reducing overall erythema, as demonstrated by one open-label, community-based study in which SDD monotherapy resulted in clinician erythema assessment scores of mild or no erythema in 75% of patients with mild to severe rosacea at baseline after 12 weeks of therapy.35 Additionally, SDD is considered to be safe and well-tolerated and does not generally result in the adverse effects that may be seen in antibiotic-level doses of doxycycline (eg, gastrointestinal upset, vaginal candidiasis, photosensitivity).34,36,37 Other antibiotics such as clarithromycin, azithromycin, and MTZ also have been studied as treatments of papulopustular rosacea at antibiotic-level doses with good therapeutic effect.38-40 These therapies, however, generally are not used unless there are contraindications for use of tetracycline antibiotics, such as pregnancy or allergy, as the overall evidence is not as strong and there may be increased risks for serious adverse effects.30
Although it is not FDA approved, isotretinoin is an important therapeutic option for select rosacea patients, as it is the only pharmacologic agent that has shown efficacy for the phymatous changes of rosacea. Its efficacy, however, is limited to early-stage rhinophyma that has not yet progressed to the fibrotic or mucinous stages of disease in which it has been shown to reduce the size and number of cutaneous sebaceous glands.30,41 Isotretinoin at 0.3 mg/kg daily also has shown noninferiority in treatment of the inflammatory papules and pustules of rosacea as compared to antibiotic dosing of doxycycline in one large-scale, placebo-controlled, randomized, 12-week multicenter study.42 Unfortunately, recurrence is highly likely after isotretinoin therapy is discontinued.30,41 However, continuous “microdose” isotretinoin at 0.03 to 0.17 mg/kg daily has shown evidence for efficacy in treatment of recalcitrant papulopustular disease.43 Such dosing may have the added benefit of reduced risk for radiographic changes associated with long-term isotretinoin use.43
Light-Based Therapy
Light-based modalities are an important tool set in the management of rosacea symptoms, as they can treat telangiectases for which medical therapy is not generally effective.9 To a lesser extent, light-based modalities also can help alleviate background erythema. The most commonly used light-based modalities include the pulsed dye laser (PDL)(Figure), potassium titanyl phosphate (KTP) laser, Nd:YAG laser, intense pulsed light, photodynamic therapy, CO2 laser, and erbium-doped YAG (Er:YAG) laser. These treatments produce clinical results by targeting specific chromophores such as oxyhemoglobin, deoxyhemoglobin, methemoglobin, and clotted blood with light of specific wavelengths to induce thermolysis of vasculature while sparing collateral tissue.44 Generally, larger telangiectatic vessels are more amenable to therapy than smaller vessels, which usually require higher energy to be delivered in a shorter period of time, thus predisposing the patient to the development of purpura that may last for 1 to 2 weeks.44
  |
Historically, PDL used a light wavelength of 577 nm and was classically associated with posttherapy purpura; however, modern PDLs use wavelengths of 585 or 595 nm and are associated with a reduced risk for purpura through the use of longer pulse durations (ie, 10–40 millisecond), multiple minipulses, multiple passes, and advanced epidermal cooling methods.9,44 In a small, prospective, randomized, controlled, nonblinded study, PDL therapy with fluence sufficiently high enough to induce purpura achieved an approximate 50% improvement in telangiectasia grading scores in most patients after a single treatment.45 Notably, PDL therapy at purpura-inducing settings was reported to be much more efficacious than settings that did not induce purpura (purpura free), especially in the treatment of thicker telangiectases.45
Potassium titanyl phosphate lasers make use of shorter wavelengths (532 nm) than PDL and thus are better able to target superficial vasculature, which translates into a reduced risk for purpura and faster healing times. However, KTP laser therapy typically is only reserved for patients with lighter skin types, as this wavelength of light is more likely to result in higher melanin absorption and possible postinflammatory hyperpigmentation.44 A split-face study comparing the KTP laser with PDL determined that the KTP laser was able to achieve 62% clearing after the first treatment and 85% clearance after the third treatment versus 49% and 75% for PDL treatment, respectively; however, the KTP laser had higher rates of posttherapy erythema lasting at least 1 day (58% vs 8%).46
Conversely, the Nd:YAG laser uses longer wavelengths (1064 nm) and can achieve deeper skin penetration, which may be effective for larger, recalcitrant, or deeper blue-tinted vessels. A split-face, double-blind, randomized, controlled trial found Nd:YAG laser therapy to be an effective treatment of facial erythema, though it was observed to be less effective than purpura-free PDL therapy in reducing redness after 4 treatments (34% vs 52% improvement, respectively); however, treatment with the Nd:YAG laser was found to be significantly (P=.0028) less painful.47
Intense pulsed light is unique from the previously discussed light-based therapies in that it uses noncoherent light with wavelengths between 500 and 1200 nm. Cutoff filters may be used to allow for more selective tissue damage depending on the depth of penetration desired. Intense pulsed light has been shown to be equally as efficacious as purpura-free PDL therapy in the treatment of erythema and telangiectasia in a randomized, controlled, single-blind, split-face trial.48 Additionally, a study of 200 patients with facial vascular lesions, of whom 74 patients had rosacea, showed that intense pulsed light therapy resulted in a 75% to 100% improvement of lesions in 174 of 188 (92.5%) patients who returned for follow-up. Treatment often required at least 2 sessions, but overall adverse effects were reported to be minimal.49
Photodynamic therapy is a well-studied and often utilized treatment of a variety of skin conditions, but there have only been a few studies regarding its use in rosacea. Photodynamic therapy involves the use of topically applied photosensitizing agents such as 5-aminolevulinic acid or methyl aminolevulinate before exposure to red or blue light. This process generates reactive oxygen species, though the exact mechanism of action through which patients achieve cosmetic improvement in rosacea is unclear. In one study of 17 patients with varying rosacea subtypes treated with methyl aminolevulinate and red light, drastic relief of symptoms was seen in 10 (58.8%) patients, marked improvement in 4 (23.5%) patients, and no response in 3 (17.6%) patients. Most patients report a transient irritant skin reaction at the site of therapy.50
Ablative lasers such as the CO2 (10,600 nm) and Er:YAG (2940 nm) lasers also have been shown to be useful in the treatment of rosacea, specifically for the management of rhinophymatous features. Excellent results have been achieved with these lasers given their ability to provide near-bloodless surgical fields. In a 13-year review of 124 patients with rhinophyma receiving a single CO2 laser treatment, good to excellent results were achieved in 118 (95.2%) of patients when evaluated at 3 months posttreatment.51 Patient satisfaction also is reported to be high with few adverse effects reported. The evidence for the Er:YAG laser is not as strong, but the current reports indicate efficacy and safety similar to that of the CO2 laser.52
Procedural Therapies
Procedural therapies in rosacea generally are reserved for management of rhinophyma and include electrocautery, cryotherapy, radiotherapy, dermabrasion, scalpel excisions, flap reconstruction, and skin grafts.30,53 The details and evidence for these methods is beyond the scope of this paper, but it is important to be aware of such modalities. As with most surgical procedures, operator skill and experience may affect treatment outcomes, and there also are definite risks for postprocedural scarring, swelling, erythema, and pigmentation changes. Recently, anecdotal evidence has shown that botulinum toxin injections may be effective for patients with refractory flushing and erythema, but larger studies will be necessary to better assess these claims.54,55
Conclusion
Although recent advances in pharmacology and laser technology have provided physicians with new and effective treatment modalities for rosacea, it remains a poorly understood disease without a definitive cure. The negative impact of rosacea on patients’ quality of life can be substantial, but effective management of cosmetic symptoms can minimize such deleterious effects. Therapy should be individualized and directed at treating the symptoms that are most bothersome to the patient. Additionally, effective treatment often will require a combination of modalities or sequential therapies to achieve optimal cosmetic outcomes.
Rosacea is a commonly encountered chronic inflammatory skin disease that affects an estimated 16 million Americans and exhibits a particular predilection for the convexities of the central face (eg, forehead, cheeks, nose, chin).1,2 The pathophysiology of rosacea remains poorly understood despite the relatively high prevalence of the disease and substantial ongoing research.3 The current paradigm suggests a complex multifactorial interplay involving aberrations of the innate and adaptive immune system, neurovascular dysregulation, blood and lymphatic vessel changes, genetic predispositions, and overgrowth of commensal organisms such as Demodex.3 Additionally, a variety of external factors may exacerbate clinical symptoms (eg, UV radiation, heat exposure, spicy food, alcohol, stress).
The diagnosis of rosacea is made clinically and rarely requires histologic confirmation. Although rosacea can present with a wide range of clinical features that often wax and wane over time, a near universal finding is diffuse centrofacial erythema.4 This centrofacial redness may symptomatically worsen during a flare period, causing flushing, but it often persists nontransiently between flares as background erythema. Other variable findings of rosacea include the presence of telangiectases, edema, plaques, phymatous changes, dry skin, ocular manifestations, and inflammatory lesions in the form of papules and pustules.5 Patients also may report a stinging or burning sensation in affected areas. It is important to note that most patients will only exhibit some of these clinical features and that symptoms often vary in the timing of their emergence or regression.5 A classification system has been developed for rosacea that categorizes the disease into 4 subtypes (erythematotelangiectatic, papulopustular, phymatous, and ocular) and one variant (granulomatous).6 These categories are determined by the grouping of clinical features present, but it is not uncommon for patients to exhibit clinical manifestations of more than 1 subtype.7
The detrimental cosmetic effects of rosacea are obvious given its chronic nature and tendency to affect highly visible areas such as the face. As such, rosacea can have a devastating impact on patients’ quality of life.8 Patients with rosacea have been reported to have higher incidence rates of low self-esteem, embarrassment, social anxiety, and depression as compared to the rest of the population. Effective treatment, however, can improve cosmetic appearance and mitigate the negative psychosocial impacts of the disease.8
Treatment of rosacea focuses on relieving cosmetic symptoms, as no curative therapy currently exists. Treatment comes in a wide variety of forms, including topical medications, systemic pharmacologic therapies, light-based modalities, and procedural interventions. Choice of therapy should be determined on a case-by-case basis as guided by the clinical features present, and combination or sequential therapies often are required to achieve optimal cosmetic results. In this article, we review both existing and emerging treatments of rosacea and assess their ability to improve the cosmetic symptoms of rosacea (Table).
Skin Care
Proper skin care is an important aspect of treatment for all patients with rosacea and thus includes the use of over-the-counter cleansers, moisturizers, and sunscreens.9 The choice of skin care products is an important consideration given the often hypersensitive skin of rosacea patients. Moisturizers and cleansers should have an acidic to neutral pH, similar to normal skin. They should not contain emulsifiers that strip moisture from the skin or protective lipids and proteins from the stratum corneum.10 Moisturizers without irritants, abrasives, or allergens should be used following skin cleansing. Protection from UV radiation with sunscreen, ideally with a sun protection factor greater than 30, is particularly important, as it can prevent UV-induced rosacea flares as well as photodamage that can cause additional erythema and telangiectasia.4 Rosacea patients also may find green-tinted makeup to be useful in concealing areas of erythema.8
Topical Therapy
Currently, there are only 5 US Food and Drug Administration (FDA)–approved topical medications for the treatment of rosacea: metronidazole (MTZ) gel 0.75% and 1%, azelaic acid (AzA) gel 15%, sodium sulfacetamide (SS) 10%–sulfur 5% lotion and cream, brimonidine tartrate (BT) gel 0.5%, and the most recently approved ivermectin (IVM) cream 1%.7 Metronidazole, AzA, and SS primarily are used to treat the inflammatory papules and pustules of rosacea, while BT is used to treat persistent background erythema. The exact mechanisms of action by which MTZ, AzA, and SS treat rosacea are unclear, but they are thought to reduce inflammation and/or immune response. Metronidazole and AzA both have demonstrated favorable safety profiles and significant (P<.05) efficacy over vehicle in reducing inflammatory lesions in numerous well-controlled randomized clinical studies.4,11,12 There is some evidence that AzA may be more effective than MTZ; one 15-week multicenter, double-blind, randomized, parallel-group study demonstrated that twice-daily AzA gel 15% showed significant superiority (P=.02) over twice-daily MTZ gel 0.75% in improving the inflammatory lesions and erythema of rosacea.13 Sodium sulfacetamide also has shown good efficacy in the treatment of inflammatory lesions and performed significantly better (P=.04) than MTZ according to one multicenter, investigator-blinded, randomized, parallel-group study,14 but the overall evidence is not as strong as MTZ and AzA.4,11,15 The most common adverse effect for MTZ, AzA, and SS is application-site irritation, but overall most patients report good tolerance to these topical medications.4 Azelaic acid is unique in that patients may report stinging, tingling, or burning after application, but these effects are not associated with visible skin changes and usually are transient, generally remitting after 1 to 2 weeks.4
Brimonidine tartrate is a highly selective α2-adrenergic receptor agonist whose mechanism of action in the treatment of rosacea is thought to involve vasoconstriction of superficial skin vasculature and to a lesser extent anti-inflammatory effects.16 In a double-blind, randomized, vehicle-controlled phase 3 trial, application of BT gel 0.5% once daily for 4 weeks demonstrated significant efficacy over vehicle (P<.001) in treating persistent nontransient facial erythema in 553 adult patients with 2 or fewer papulopustular lesions as evaluated over 12 hours on days 1, 15, and 29.17 Notably, a substantial difference in cosmetic appearance was observed in another study as early as 30 minutes after the first gel application on day 1.18 The results of this phase 3 trial17 mirrored those of the phase 2 dose-optimization and safety studies of similar design.18 In addition to another long-term, 1-year, open-label study,19 both phase 2 and 3 studies have shown favorable safety profiles with no reports of tachyphylaxis, rebound erythema, or aggravation of other disease features such as telangiectases or inflammatory lesions.17,18 Recently, however, there have been some reports of considerable rebound erythema with BT use and thus patients should be made aware of this possibility.20,21 Case reports of successful treatment of background erythema and flushing with other topically applied adrenergic receptor modifiers such as oxymetazoline and xylometazoline have been published in the literature,22,23 but additional research will be necessary to validate these claims.
Ivermectin, a decades-old antiparasitic, has recently shown promising results as a treatment of rosacea patients with moderate to severe papulopustular lesions. Its therapeutic effect is believed to be mediated by its activity against Demodex, a natural skin mite that has been found at increased concentrations in a subset of patients with rosacea, as well as by its natural anti-inflammatory properties.24 In 2 identically designed, randomized, double-blind, controlled trials of IVM cream 1% applied once daily for 12 weeks, a significantly larger proportion of patients in the IVM groups achieved an investigator global assessment of clear or almost clear as compared to vehicle (IVM: 38.4% and 40.1%, respectively; vehicle: 11.6% and 18.8%, respectively; P<.001). Both trials also demonstrated that IVM was significantly superior to vehicle in the reduction of inflammatory lesion counts measured at week 12 as compared to baseline (IVM: 76.0% and 75.0%, respectively; vehicle: 50.0% and 50.0%, respectively; P<.001).24 An extension of these original trials demonstrated long-term safety with up to 52 weeks of topical IVM use and reported a low incidence rate of adverse effects, most commonly transient skin burning, pruritus, and dryness. Notably, the incidence rate of these adverse effects was lower than a comparison group receiving AzA gel 15% once daily.25 Once-daily application of IVM cream 1% also has recently demonstrated superiority over twice-daily MTZ cream 0.75% for 16 weeks in a phase 3 investigator-blinded, randomized, parallel-group study. The IVM group was significantly superior to MTZ in the reduction of inflammatory lesions as compared to baseline (83.0% vs 73.7%) and in the number of participants who achieved an investigator global assessment score of clear or almost clear (84.9% vs 75.4%)(both P<.001).26 There also is limited evidence for the use of other antiparasitic topical medications such as crotamiton 10% and permethrin 5%, but such agents frequently cause irritation and may not be well tolerated in rosacea patients.27-29
There are a variety of other non–FDA-approved topical medications that have been used with varying success in the literature, including cyclosporine, macrolides, benzoyl peroxide, retinoids, and calcineurin inhibitors such as tacrolimus and pimecrolimus. Evidence for the use of these medications generally is limited to a few studies with small numbers of patients and will not be discussed further in this article.4,11,30 These agents, however, may be useful in select cases when first-line regimens have failed and also may be good targets for future research.
Systemic Therapy
The mainstay of systemic treatment of rosacea centers around the tetracyclines, a group of antibiotics that have been used off label for rosacea since the 1950s.31 The therapeutic effects of tetracyclines in the treatment of rosacea are thought to revolve around their anti-inflammatory effects rather than their antibacterial properties.32 Currently, the only FDA-approved oral agent for treatment of the inflammatory lesions of rosacea is doxycycline 40-mg modified-release capsules taken once daily. These modified capsules allow for instant release of 30 mg and delayed release of 10 mg of doxycycline. This dosing is considered to be anti-inflammatory rather than antimicrobial, as it does not produce antibiotic selection pressure even with prolonged use.33 Efficacy of 40-mg subantimicrobial-dose doxycycline (SDD) has been demonstrated in 2 phase 3 multicenter, parallel-group, randomized, double-blind, placebo-controlled studies in which SDD demonstrated a significantly greater reduction in the number of total inflammatory lesions at week 16 compared to placebo (P<.001).34 Subantimicrobial-dose doxycycline also has been shown to be equally as efficacious in reducing inflammatory lesions as traditional-dose doxycycline.35 There also is some evidence for the efficacy of SDD in reducing overall erythema, as demonstrated by one open-label, community-based study in which SDD monotherapy resulted in clinician erythema assessment scores of mild or no erythema in 75% of patients with mild to severe rosacea at baseline after 12 weeks of therapy.35 Additionally, SDD is considered to be safe and well-tolerated and does not generally result in the adverse effects that may be seen in antibiotic-level doses of doxycycline (eg, gastrointestinal upset, vaginal candidiasis, photosensitivity).34,36,37 Other antibiotics such as clarithromycin, azithromycin, and MTZ also have been studied as treatments of papulopustular rosacea at antibiotic-level doses with good therapeutic effect.38-40 These therapies, however, generally are not used unless there are contraindications for use of tetracycline antibiotics, such as pregnancy or allergy, as the overall evidence is not as strong and there may be increased risks for serious adverse effects.30
Although it is not FDA approved, isotretinoin is an important therapeutic option for select rosacea patients, as it is the only pharmacologic agent that has shown efficacy for the phymatous changes of rosacea. Its efficacy, however, is limited to early-stage rhinophyma that has not yet progressed to the fibrotic or mucinous stages of disease in which it has been shown to reduce the size and number of cutaneous sebaceous glands.30,41 Isotretinoin at 0.3 mg/kg daily also has shown noninferiority in treatment of the inflammatory papules and pustules of rosacea as compared to antibiotic dosing of doxycycline in one large-scale, placebo-controlled, randomized, 12-week multicenter study.42 Unfortunately, recurrence is highly likely after isotretinoin therapy is discontinued.30,41 However, continuous “microdose” isotretinoin at 0.03 to 0.17 mg/kg daily has shown evidence for efficacy in treatment of recalcitrant papulopustular disease.43 Such dosing may have the added benefit of reduced risk for radiographic changes associated with long-term isotretinoin use.43
Light-Based Therapy
Light-based modalities are an important tool set in the management of rosacea symptoms, as they can treat telangiectases for which medical therapy is not generally effective.9 To a lesser extent, light-based modalities also can help alleviate background erythema. The most commonly used light-based modalities include the pulsed dye laser (PDL)(Figure), potassium titanyl phosphate (KTP) laser, Nd:YAG laser, intense pulsed light, photodynamic therapy, CO2 laser, and erbium-doped YAG (Er:YAG) laser. These treatments produce clinical results by targeting specific chromophores such as oxyhemoglobin, deoxyhemoglobin, methemoglobin, and clotted blood with light of specific wavelengths to induce thermolysis of vasculature while sparing collateral tissue.44 Generally, larger telangiectatic vessels are more amenable to therapy than smaller vessels, which usually require higher energy to be delivered in a shorter period of time, thus predisposing the patient to the development of purpura that may last for 1 to 2 weeks.44
  |
Historically, PDL used a light wavelength of 577 nm and was classically associated with posttherapy purpura; however, modern PDLs use wavelengths of 585 or 595 nm and are associated with a reduced risk for purpura through the use of longer pulse durations (ie, 10–40 millisecond), multiple minipulses, multiple passes, and advanced epidermal cooling methods.9,44 In a small, prospective, randomized, controlled, nonblinded study, PDL therapy with fluence sufficiently high enough to induce purpura achieved an approximate 50% improvement in telangiectasia grading scores in most patients after a single treatment.45 Notably, PDL therapy at purpura-inducing settings was reported to be much more efficacious than settings that did not induce purpura (purpura free), especially in the treatment of thicker telangiectases.45
Potassium titanyl phosphate lasers make use of shorter wavelengths (532 nm) than PDL and thus are better able to target superficial vasculature, which translates into a reduced risk for purpura and faster healing times. However, KTP laser therapy typically is only reserved for patients with lighter skin types, as this wavelength of light is more likely to result in higher melanin absorption and possible postinflammatory hyperpigmentation.44 A split-face study comparing the KTP laser with PDL determined that the KTP laser was able to achieve 62% clearing after the first treatment and 85% clearance after the third treatment versus 49% and 75% for PDL treatment, respectively; however, the KTP laser had higher rates of posttherapy erythema lasting at least 1 day (58% vs 8%).46
Conversely, the Nd:YAG laser uses longer wavelengths (1064 nm) and can achieve deeper skin penetration, which may be effective for larger, recalcitrant, or deeper blue-tinted vessels. A split-face, double-blind, randomized, controlled trial found Nd:YAG laser therapy to be an effective treatment of facial erythema, though it was observed to be less effective than purpura-free PDL therapy in reducing redness after 4 treatments (34% vs 52% improvement, respectively); however, treatment with the Nd:YAG laser was found to be significantly (P=.0028) less painful.47
Intense pulsed light is unique from the previously discussed light-based therapies in that it uses noncoherent light with wavelengths between 500 and 1200 nm. Cutoff filters may be used to allow for more selective tissue damage depending on the depth of penetration desired. Intense pulsed light has been shown to be equally as efficacious as purpura-free PDL therapy in the treatment of erythema and telangiectasia in a randomized, controlled, single-blind, split-face trial.48 Additionally, a study of 200 patients with facial vascular lesions, of whom 74 patients had rosacea, showed that intense pulsed light therapy resulted in a 75% to 100% improvement of lesions in 174 of 188 (92.5%) patients who returned for follow-up. Treatment often required at least 2 sessions, but overall adverse effects were reported to be minimal.49
Photodynamic therapy is a well-studied and often utilized treatment of a variety of skin conditions, but there have only been a few studies regarding its use in rosacea. Photodynamic therapy involves the use of topically applied photosensitizing agents such as 5-aminolevulinic acid or methyl aminolevulinate before exposure to red or blue light. This process generates reactive oxygen species, though the exact mechanism of action through which patients achieve cosmetic improvement in rosacea is unclear. In one study of 17 patients with varying rosacea subtypes treated with methyl aminolevulinate and red light, drastic relief of symptoms was seen in 10 (58.8%) patients, marked improvement in 4 (23.5%) patients, and no response in 3 (17.6%) patients. Most patients report a transient irritant skin reaction at the site of therapy.50
Ablative lasers such as the CO2 (10,600 nm) and Er:YAG (2940 nm) lasers also have been shown to be useful in the treatment of rosacea, specifically for the management of rhinophymatous features. Excellent results have been achieved with these lasers given their ability to provide near-bloodless surgical fields. In a 13-year review of 124 patients with rhinophyma receiving a single CO2 laser treatment, good to excellent results were achieved in 118 (95.2%) of patients when evaluated at 3 months posttreatment.51 Patient satisfaction also is reported to be high with few adverse effects reported. The evidence for the Er:YAG laser is not as strong, but the current reports indicate efficacy and safety similar to that of the CO2 laser.52
Procedural Therapies
Procedural therapies in rosacea generally are reserved for management of rhinophyma and include electrocautery, cryotherapy, radiotherapy, dermabrasion, scalpel excisions, flap reconstruction, and skin grafts.30,53 The details and evidence for these methods is beyond the scope of this paper, but it is important to be aware of such modalities. As with most surgical procedures, operator skill and experience may affect treatment outcomes, and there also are definite risks for postprocedural scarring, swelling, erythema, and pigmentation changes. Recently, anecdotal evidence has shown that botulinum toxin injections may be effective for patients with refractory flushing and erythema, but larger studies will be necessary to better assess these claims.54,55
Conclusion
Although recent advances in pharmacology and laser technology have provided physicians with new and effective treatment modalities for rosacea, it remains a poorly understood disease without a definitive cure. The negative impact of rosacea on patients’ quality of life can be substantial, but effective management of cosmetic symptoms can minimize such deleterious effects. Therapy should be individualized and directed at treating the symptoms that are most bothersome to the patient. Additionally, effective treatment often will require a combination of modalities or sequential therapies to achieve optimal cosmetic outcomes.
1. Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6 suppl 1):S27-S35.
2. Rosacea prevalence map. National Rosacea Society Web site. http://rosacea.org/press/prevalencemap. Accessed June 16, 2015.
3. Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 suppl 1):S15-S26.
4. Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 1: a status report on the disease state, general measures, and adjunctive skin care. Cutis. 2013;92:234-240.
5. Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004;50:907-912.
6. Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society expert committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
7. Feldman SR, Huang WW, Huynh TT. Current drug therapies for rosacea: a chronic vascular and inflammatory skin disease. J Manag Care Spec Pharm. 2014;20:623-629.
8. Moustafa F, Lewallen RS, Feldman SR. The psychological impact of rosacea and the influence of current management options. J Am Acad Dermatol. 2014;71:973-980.
9. Mansouri Y, Goldenberg G. Devices and topical agents for rosacea management. Cutis. 2014;94:21-25.
10. Levin J, Miller R. A guide to the ingredients and potential benefits of over-the-counter cleansers and moisturizers for rosacea patients. J Clin Aesthet Dermatol. 2011;4:31-49.
11. van Zuuren EJ, Kramer S, Carter B, et al. Interventions for rosacea. Cochrane Database Syst Rev. 2011;3:CD003262.
12. Liu RH, Smith MK, Basta SA, et al. Azelaic acid in the treatment of papulopustular rosacea: a systematic review of randomized controlled trials. Arch Dermatol. 2006;142:1047-1052.
13. Elewski B, Fleischer AB Jr, Pariser DM. A comparison of 15% azelaic acid gel and 0.75% metronidazole gel in the topical treatment of papulopustular rosacea: results of a randomized trial. Arch Dermatol. 2003;139:1444-1450.
14. Torok HM, Webster G, Dunlap FE, et al. Combination sodium sulfacetamide 10% and sulfur 5% cream with sunscreens versus metronidazole 0.75% cream for rosacea. Cutis. 2005;75:357-363.
15. Trumbore MW, Goldstein JA, Gurge RM. Treatment of papulopustular rosacea with sodium sulfacetamide 10%/sulfur 5% emollient foam. J Drugs Dermatol. 2009;8:299-304.
16. Piwnica D, Rosignoli C, de Menonville ST, et al. Vasoconstriction and anti-inflammatory properties of the selective alpha-adrenergic receptor agonist brimonidine. J Dermatol Sci. 2014;75:49-54.
17. Fowler J Jr, Jackson M, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12:650-656.
18. Fowler J, Jarratt M, Moore A, et al. Once-daily topical brimonidine tartrate gel 0.5% is a novel treatment of moderate to severe facial erythema of rosacea: results of two multicenter, randomized and vehicle-controlled studies. Br J Dermatol. 2012;166:633-641.
19. Moore A, Kempers S, Murakawa G, et al. Long-term safety and efficacy of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of a 1-year open-label study. J Drugs Dermatol. 2014;13:56-61.
20. Routt ET, Levitt JO. Rebound erythema and burning sensation from a new topical brimonidine tartrate gel 0.33%. J Am Acad Dermatol. 2014;70:e37-e38.
21. Ilkovitch D, Pomerantz RG. Brimonidine effective but may lead to significant rebound erythema. J Am Acad Dermatol. 2014;70:e109-e110.
22. Kim JH, Oh YS, Ji JH, et al. Rosacea (erythematotelangiectatic type) effectively improved by topical xylometazoline. J Dermatol. 2011;38:510-513.
23. Shanler SD, Ondo AL. Successful treatment of erythema and flushing of rosacea using a topically applied selective alpha1-adrenergic receptor antagonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
24. Stein-Gold L, Kircik L, Fowler J, et al. Efficacy and safety of ivermectin 1% cream in treatment of papulopustular rosacea: results of two randomized, double-blind, vehicle-controlled pivotal studies. J Drugs Dermatol. 2014;13:316-323.
25. Stein-Gold L, Kircik L, Fowler J, et al. Long-term safety of ivermectin 1% cream vs azelaic acid 15% gel in treating inflammatory lesions of rosacea: results of two 40-week controlled, investigator-blinded trials. J Drugs Dermatol. 2014;13:1380-1386.
26. Taieb A, Ortonne JP, Ruzicka T, et al. Superiority of ivermectin 1% cream over metronidazole 0.75% cream in treating inflammatory lesions of rosacea: a randomized, investigator-blinded trial. Br J Dermatol. 2015;172:1103-1110.
27. Koçak M, Ya˘gli S, Vahapo˘glu G, et al. Permethrin 5% cream versus metronidazole 0.75% gel for the treatment of papulopustular rosacea. a randomized double-blind placebo-controlled study. Dermatology (Basel). 2002;205:265-270.
28. Bikowski JB, Del Rosso JQ. Demodex dermatitis: a retrospective analysis of clinical diagnosis and successful treatment with topical crotamiton. J Clin Aesthet Dermatol. 2009;2:20-25.
29. Layton A, Thiboutot D. Emerging therapies in rosacea. J Am Acad Dermatol. 2013;69(6 suppl 1):S57-S65.
30. Pelle MT, Crawford GH, James WD. Rosacea: II. therapy. J Am Acad Dermatol. 2004;51:499-512, quiz 513-514.
31. Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006;54:258-265.
32. Korting HC, Schöllmann C. Tetracycline actions relevant to rosacea treatment. Skin Pharmacol Physiol. 2009;22:287-294.
33. Thomas J, Walker C, Bradshaw M. Long-term use of subantimicrobial dose doxycycline does not lead to changes in antimicrobial susceptibility. J Periodontol. 2000;71:1472-1483.
34. Del Rosso JQ, Webster GF, Jackson M, et al. Two randomized phase III clinical trials evaluating anti-inflammatory dose doxycycline (40-mg doxycycline, USP capsules) administered once daily for treatment of rosacea. J Am Acad Dermatol. 2007;56:791-802.
35. Webster GF. An open-label, community-based, 12-week assessment of the effectiveness and safety of monotherapy with doxycycline 40 mg (30-mg immediate-release and 10-mg delayed-release beads). Cutis. 2010;86(suppl 5):7-15.
36. Del Rosso JQ, Schlessinger J, Werschler P. Comparison of anti-inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. J Drugs Dermatol. 2008;7:573-576.
37. Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 3: a status report on systemic therapies. Cutis. 2014;93:18-28.
38. Torresani C. Clarithromycin: a new perspective in rosacea treatment. Int J Dermatol. 1998;37:347-349.
39. Bakar O, Demircay Z, Gürbüz O. Therapeutic potential of azithromycin in rosacea. Int J Dermatol. 2004;43:151-154.
40. Saihan EM, Burton JL. A double-blind trial of metronidazole versus oxytetracycline therapy for rosacea. Br J Dermatol. 1980;102:443-445.
41. Park H, Del Rosso JQ. Use of oral isotretinoin in the management of rosacea. J Clin Aesthet Dermatol. 2011;4:54-61.
42. Gollnick H, Blume-Peytavi U, Szabo EL, et al. Systemic isotretinoin in the treatment of rosacea—doxycycline-and placebo-controlled, randomized clinical study. J Dtsch Dermatol Ges. 2010;8:505-515.
43. Hofer T. Continuous “microdose” isotretinoin in adult recalcitrant rosacea. Clin Exp Dermatol. 2004;29:204-205.
44. Tanghetti E, Del Rosso JQ, Thiboutot D, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 4: a status report on physical modalities and devices. Cutis. 2014;93:71-76.
45. Alam M, Dover JS, Arndt KA. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol Surg. 2003;29:681-684.
46. Uebelhoer NS, Bogle MA, Stewart B, et al. A split-face comparison study of pulsed 532-nm KTP laser and 595-nm pulsed dye laser in the treatment of facial telangiectasias and diffuse telangiectatic facial erythema. Dermatol Surg. 2007;33:441-448.
47. Alam M, Voravutinon N, Warycha M, et al. Comparative effectiveness of nonpurpuragenic 595-nm pulsed dye laser and microsecond 1064-nm neodymium:yttrium-aluminum-garnet laser for treatment of diffuse facial erythema: a double-blind randomized controlled trial. J Am Acad Dermatol. 2013;69:438-443.
48. Neuhaus IM, Zane LT, Tope WD. Comparative efficacy of nonpurpuragenic pulsed dye laser and intense pulsed light for erythematotelangiectatic rosacea. Dermatol Surg. 2009;35:920-928.
49. Angermeier MC. Treatment of facial vascular lesions with intense pulsed light. J Cutan Laser Ther. 1999;1:95-100.
50. Bryld LE, Jemec GB. Photodynamic therapy in a series of rosacea patients. J Eur Acad Dermatol Venereol. 2007;21:1199-1202.
51. Maden V, Ferguson JE, August PJ. Carbon dioxide laser treatment of rhinophyma: a review of 124 patients. Br J Dermatol. 2009;161:814-818.
52. Fincher EF, Gladstone HB. Use of a dual-mode erbium:YAG laser for the surgical correction of rhinophyma. Arch Facial Plast Surg. 2004;6:267-271.
53. Lloyd KM. Surgical correction of rhinophyma. Arch Dermatol. 1990;126:721-723.
54. Dayan SH, Pritzker RN, Arkins JP. A new treatment regimen for rosacea: onabotulinumtoxinA. J Drugs Dermatol. 2012;11:e76-e79.
55. Park KY, Hyun MY, Jeong SY, et al. Botulinum toxin for the treatment of refractory erythema and flushing of rosacea. Dermatology. 2015;230:299-301.
1. Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6 suppl 1):S27-S35.
2. Rosacea prevalence map. National Rosacea Society Web site. http://rosacea.org/press/prevalencemap. Accessed June 16, 2015.
3. Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 suppl 1):S15-S26.
4. Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 1: a status report on the disease state, general measures, and adjunctive skin care. Cutis. 2013;92:234-240.
5. Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004;50:907-912.
6. Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society expert committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
7. Feldman SR, Huang WW, Huynh TT. Current drug therapies for rosacea: a chronic vascular and inflammatory skin disease. J Manag Care Spec Pharm. 2014;20:623-629.
8. Moustafa F, Lewallen RS, Feldman SR. The psychological impact of rosacea and the influence of current management options. J Am Acad Dermatol. 2014;71:973-980.
9. Mansouri Y, Goldenberg G. Devices and topical agents for rosacea management. Cutis. 2014;94:21-25.
10. Levin J, Miller R. A guide to the ingredients and potential benefits of over-the-counter cleansers and moisturizers for rosacea patients. J Clin Aesthet Dermatol. 2011;4:31-49.
11. van Zuuren EJ, Kramer S, Carter B, et al. Interventions for rosacea. Cochrane Database Syst Rev. 2011;3:CD003262.
12. Liu RH, Smith MK, Basta SA, et al. Azelaic acid in the treatment of papulopustular rosacea: a systematic review of randomized controlled trials. Arch Dermatol. 2006;142:1047-1052.
13. Elewski B, Fleischer AB Jr, Pariser DM. A comparison of 15% azelaic acid gel and 0.75% metronidazole gel in the topical treatment of papulopustular rosacea: results of a randomized trial. Arch Dermatol. 2003;139:1444-1450.
14. Torok HM, Webster G, Dunlap FE, et al. Combination sodium sulfacetamide 10% and sulfur 5% cream with sunscreens versus metronidazole 0.75% cream for rosacea. Cutis. 2005;75:357-363.
15. Trumbore MW, Goldstein JA, Gurge RM. Treatment of papulopustular rosacea with sodium sulfacetamide 10%/sulfur 5% emollient foam. J Drugs Dermatol. 2009;8:299-304.
16. Piwnica D, Rosignoli C, de Menonville ST, et al. Vasoconstriction and anti-inflammatory properties of the selective alpha-adrenergic receptor agonist brimonidine. J Dermatol Sci. 2014;75:49-54.
17. Fowler J Jr, Jackson M, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12:650-656.
18. Fowler J, Jarratt M, Moore A, et al. Once-daily topical brimonidine tartrate gel 0.5% is a novel treatment of moderate to severe facial erythema of rosacea: results of two multicenter, randomized and vehicle-controlled studies. Br J Dermatol. 2012;166:633-641.
19. Moore A, Kempers S, Murakawa G, et al. Long-term safety and efficacy of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of a 1-year open-label study. J Drugs Dermatol. 2014;13:56-61.
20. Routt ET, Levitt JO. Rebound erythema and burning sensation from a new topical brimonidine tartrate gel 0.33%. J Am Acad Dermatol. 2014;70:e37-e38.
21. Ilkovitch D, Pomerantz RG. Brimonidine effective but may lead to significant rebound erythema. J Am Acad Dermatol. 2014;70:e109-e110.
22. Kim JH, Oh YS, Ji JH, et al. Rosacea (erythematotelangiectatic type) effectively improved by topical xylometazoline. J Dermatol. 2011;38:510-513.
23. Shanler SD, Ondo AL. Successful treatment of erythema and flushing of rosacea using a topically applied selective alpha1-adrenergic receptor antagonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
24. Stein-Gold L, Kircik L, Fowler J, et al. Efficacy and safety of ivermectin 1% cream in treatment of papulopustular rosacea: results of two randomized, double-blind, vehicle-controlled pivotal studies. J Drugs Dermatol. 2014;13:316-323.
25. Stein-Gold L, Kircik L, Fowler J, et al. Long-term safety of ivermectin 1% cream vs azelaic acid 15% gel in treating inflammatory lesions of rosacea: results of two 40-week controlled, investigator-blinded trials. J Drugs Dermatol. 2014;13:1380-1386.
26. Taieb A, Ortonne JP, Ruzicka T, et al. Superiority of ivermectin 1% cream over metronidazole 0.75% cream in treating inflammatory lesions of rosacea: a randomized, investigator-blinded trial. Br J Dermatol. 2015;172:1103-1110.
27. Koçak M, Ya˘gli S, Vahapo˘glu G, et al. Permethrin 5% cream versus metronidazole 0.75% gel for the treatment of papulopustular rosacea. a randomized double-blind placebo-controlled study. Dermatology (Basel). 2002;205:265-270.
28. Bikowski JB, Del Rosso JQ. Demodex dermatitis: a retrospective analysis of clinical diagnosis and successful treatment with topical crotamiton. J Clin Aesthet Dermatol. 2009;2:20-25.
29. Layton A, Thiboutot D. Emerging therapies in rosacea. J Am Acad Dermatol. 2013;69(6 suppl 1):S57-S65.
30. Pelle MT, Crawford GH, James WD. Rosacea: II. therapy. J Am Acad Dermatol. 2004;51:499-512, quiz 513-514.
31. Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006;54:258-265.
32. Korting HC, Schöllmann C. Tetracycline actions relevant to rosacea treatment. Skin Pharmacol Physiol. 2009;22:287-294.
33. Thomas J, Walker C, Bradshaw M. Long-term use of subantimicrobial dose doxycycline does not lead to changes in antimicrobial susceptibility. J Periodontol. 2000;71:1472-1483.
34. Del Rosso JQ, Webster GF, Jackson M, et al. Two randomized phase III clinical trials evaluating anti-inflammatory dose doxycycline (40-mg doxycycline, USP capsules) administered once daily for treatment of rosacea. J Am Acad Dermatol. 2007;56:791-802.
35. Webster GF. An open-label, community-based, 12-week assessment of the effectiveness and safety of monotherapy with doxycycline 40 mg (30-mg immediate-release and 10-mg delayed-release beads). Cutis. 2010;86(suppl 5):7-15.
36. Del Rosso JQ, Schlessinger J, Werschler P. Comparison of anti-inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. J Drugs Dermatol. 2008;7:573-576.
37. Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 3: a status report on systemic therapies. Cutis. 2014;93:18-28.
38. Torresani C. Clarithromycin: a new perspective in rosacea treatment. Int J Dermatol. 1998;37:347-349.
39. Bakar O, Demircay Z, Gürbüz O. Therapeutic potential of azithromycin in rosacea. Int J Dermatol. 2004;43:151-154.
40. Saihan EM, Burton JL. A double-blind trial of metronidazole versus oxytetracycline therapy for rosacea. Br J Dermatol. 1980;102:443-445.
41. Park H, Del Rosso JQ. Use of oral isotretinoin in the management of rosacea. J Clin Aesthet Dermatol. 2011;4:54-61.
42. Gollnick H, Blume-Peytavi U, Szabo EL, et al. Systemic isotretinoin in the treatment of rosacea—doxycycline-and placebo-controlled, randomized clinical study. J Dtsch Dermatol Ges. 2010;8:505-515.
43. Hofer T. Continuous “microdose” isotretinoin in adult recalcitrant rosacea. Clin Exp Dermatol. 2004;29:204-205.
44. Tanghetti E, Del Rosso JQ, Thiboutot D, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 4: a status report on physical modalities and devices. Cutis. 2014;93:71-76.
45. Alam M, Dover JS, Arndt KA. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol Surg. 2003;29:681-684.
46. Uebelhoer NS, Bogle MA, Stewart B, et al. A split-face comparison study of pulsed 532-nm KTP laser and 595-nm pulsed dye laser in the treatment of facial telangiectasias and diffuse telangiectatic facial erythema. Dermatol Surg. 2007;33:441-448.
47. Alam M, Voravutinon N, Warycha M, et al. Comparative effectiveness of nonpurpuragenic 595-nm pulsed dye laser and microsecond 1064-nm neodymium:yttrium-aluminum-garnet laser for treatment of diffuse facial erythema: a double-blind randomized controlled trial. J Am Acad Dermatol. 2013;69:438-443.
48. Neuhaus IM, Zane LT, Tope WD. Comparative efficacy of nonpurpuragenic pulsed dye laser and intense pulsed light for erythematotelangiectatic rosacea. Dermatol Surg. 2009;35:920-928.
49. Angermeier MC. Treatment of facial vascular lesions with intense pulsed light. J Cutan Laser Ther. 1999;1:95-100.
50. Bryld LE, Jemec GB. Photodynamic therapy in a series of rosacea patients. J Eur Acad Dermatol Venereol. 2007;21:1199-1202.
51. Maden V, Ferguson JE, August PJ. Carbon dioxide laser treatment of rhinophyma: a review of 124 patients. Br J Dermatol. 2009;161:814-818.
52. Fincher EF, Gladstone HB. Use of a dual-mode erbium:YAG laser for the surgical correction of rhinophyma. Arch Facial Plast Surg. 2004;6:267-271.
53. Lloyd KM. Surgical correction of rhinophyma. Arch Dermatol. 1990;126:721-723.
54. Dayan SH, Pritzker RN, Arkins JP. A new treatment regimen for rosacea: onabotulinumtoxinA. J Drugs Dermatol. 2012;11:e76-e79.
55. Park KY, Hyun MY, Jeong SY, et al. Botulinum toxin for the treatment of refractory erythema and flushing of rosacea. Dermatology. 2015;230:299-301.
Practice Points
- As no definitive cure for rosacea exists, effective treatment is aimed at improving the cosmetic symptoms.
- Choice of therapy should be determined on a case-by-case basis as guided by the clinical features most bothersome to the patient.
- A combination of modalities and/or sequential therapy often is required to achieve optimal cosmetic outcomes.
Acne Scarring: A Review of Cosmetic Therapies
Acne vulgaris is one of the most common inflammatory dermatoses affecting nearly all adolescents and a large proportion of adults.1 Incidence rates trend downward with age, but prevalence has been reported to be as high as 51% in individuals aged 20 to 29 years.2 Notably, recent evidence suggests there is an increasing incidence rate of acne among postadolescent women, with the severity associated with the menstrual cycle.3,4 Scarring is a common result of acne and may even occur in the setting of appropriate medical therapy. In particular, some form of facial scarring has been reported to occur in up to 95% of acne patients, with severe scarring in 30% of these patients.5 The detrimental effects of acne scarring are not only limited to impaired cosmetic appearance, as it also has been associated with depression symptoms, suicidal ideation, mental health problems, and general social impairment.6 Given the negative impact of acne scarring on overall health and well-being as well as its permanent nature, early and effective treatment is essential to maximize cosmetic outcomes and minimize long-term deleterious effects.
Acne scarring can be broadly divided into 2 major categories: atrophic and hypertrophic. Atrophic scarring is more common and is characterized by an overall localized reduction in collagen content. Clinically, atrophic scars present as depressions in the skin secondary to inflammatory fibrous contractions induced by acne. This type of scarring can be further divided into various subtypes based on morphologic criteria (eg, size, depth), such as boxcar, ice pick, and rolling scars.7 Conversely, hypertrophic scarring is characterized by an overall increase in collagen content and presents as firm raised lesions. Hypertrophic scars should be distinguished from keloid scars, as the former will not outgrow the margins of the original wound while the latter will.8 Treatment of acne scarring is based on scar type and can be accomplished through a variety of medical and surgical modalities (Table). In this article, we review some of the most commonly utilized therapies for both atrophic and hypertrophic acne scarring with a focus on cosmetic outcomes. It is important to keep in mind, however, that the best treatment is to prevent the occurrence of acne scarring through early and proactive treatment of acne.9
Dermabrasion
Dermabrasion is a decades-old technique that employs the use of a motorized device equipped with an abrasive material to physically remove the superficial layers of the skin, thus inducing the wound-healing process with subsequent formation of new collagen.10 In the same vein, microdermabrasion utilizes aluminum oxide crystals ejected from a nozzle to induce superficial microlacerations.11 This technique is most successful when used to soften scar edges in superficial atrophic scars of the rolling or boxcar subtypes.12 Dermabrasion has been shown to be equally as effective as laser therapy in the treatment of facial scars but is reported to have a much greater risk for adverse effects (AEs)(eg, erythema, edema) that may last for several weeks posttherapy.13,14 Dermabrasion is a particularly operator-dependent technique for which outcomes may vary depending on operator experience. As such, it is not generally recommended as a first-line therapy given its risks and relatively modest results; however, dermabrasion can be a useful adjunct when performed in the right setting. This technique, in addition to laser resurfacing, should be used with caution in patients who have recently taken or currently are taking isotretinoin, as several case series have reported postprocedural development of hypertrophic or keloid scars,15-17 but these findings subsequently were questioned in the literature.18
Laser Therapy
Laser technology has advanced tremendously over the last few decades and there are now a multitude of available lasers that are capable of variable depth penetration and energy delivery patterns. Common to all, however, is the ability to induce localized thermal damage with eventual collagen remodeling. Lasers can be divided into 2 major categories: ablative and nonablative. Ablative lasers cause epidermal destruction, while nonablative lasers are able to selectively target dermal layers without disrupting the overlying epithelium. Generally speaking, ablative lasers are more effective than nonablative lasers in the treatment of atrophic scars, with reported mean improvements of up to 81%.19 This increased efficacy comes with an increased risk for AEs such as postinflammatory hyperpigmentation, prolonged posttreatment erythema, and formation of additional scarring.20 Both ablative and nonablative lasers can be applied in the more recently developed technology of fractional photothermolysis. With this method, noncontiguous microscopic columns of thermal injury surrounded by zones of viable tissue are created, which is in contrast to the traditional manner of inducing broad thermal injury. Fractional ablative lasers can achieve efficacy rates similar to traditional ablative lasers with a reduced risk for permanent scarring or dispigmentation.21 Notably, recent studies have shown promising results for the use of fractional ablative lasers as a mechanism to enhance drug delivery of topically applied medications such as poly-L-lactic acid and triamcinolone acetonide in the treatment of atrophic and hypertrophic scars, respectively.22,23
Lasers also play a role in the treatment of hypertrophic acne scars with the use of nonablative pulsed dye lasers. These lasers cause selective thermolysis of dermal vasculature, and average clinical improvements in hypertrophic scars of 67.5% after a single treatment have been reported.24 Temporary postoperative purpura and long-term hyperpigmentation are reported outcomes of this therapy.20
Radiofrequency
Nonablative radiofrequency (RF) is a relatively novel technique that creates an electric current in the dermis at preset depths to induce thermal damage and eventual collagen synthesis. There are a variety of modalities for which RF can be applied, but microneedle bipolar RF and fractional bipolar RF treatments offer the best results for atrophic acne scars. Improvements in scar appearance of 25% to 75% have been reported after several treatment sessions.25 Better results have been reported in the treatment of ice pick scars as compared to more superficial scars,26 but additional studies will be necessary to validate this claim. Adverse effects are largely limited to temporary erythema and posttreatment scabbing.27
Subcision
Subcision is a more physically intensive technique useful for treatment of superficial atrophic acne scars. This method involves the use of a small needle that is inserted into the periphery of a scar before being moved in a back-and-forth manner underneath the base of the scar to loosen the fibrotic adhesions that result in the depressed appearance of the scar. Additionally, loosening of the tissue and resultant bleeding creates a potential space for future collagen deposition during the subsequent wound-healing phase. Subcision has a reported success rate of 50% to 60% in the treatment of rolling scars, and prospective, randomized, split-face trials have indicated that the short-term outcomes of subcision are superior to dermal fillers while being equally effective long-term.28,29 Of note, a small percentage of patients may develop a localized nodule at the site of treatment, which can be resolved with intralesional steroids.11
Skin Needling
Skin needling, also referred to as collagen induction therapy, utilizes vertical needle punctures rather than the horizontally directed punctures that are used in subcision and can be used to treat rolling and boxcar scars. Traditionally, a small roller equipped with rows of small needles typically ranging in size from 0.5 to 3.0 mm in length is passed over the skin using gentle pressure, puncturing the superficial layers of the skin to loosen fibrotic adhesions and induce collagen synthesis. This procedure may be repeated several times within a single session or over multiple sessions depending on the depth and quality of the scars. This technique has been reported to reduce scar depth up to 25% after 2 sessions.30
Punch Techniques
Punch techniques are useful for treatment of deeper atrophic acne scarring, for which most other treatment modalities are not particularly effective. A punch excision approximately equal to the scar size is first performed, which may then be followed by either removal of the scar tissue with subsequent suturing, graft replacement of the removed tissue, or elevation of the already established scar tissue to the level of surrounding skin where it is then held in place by sutures or adhesive skin closure material. Success rates with this method are largely limited to case series, but punch techniques are reported to be efficacious, especially for treatment of ice pick scars. Risks for this method include graft failure, graft depression, and formation of sinus tracts.31
Chemical Peels
Chemicals peels traditionally employ the use of acidic compounds to strip away the outer layers of skin to variable depths depending on the concentration of the agent being applied. Chemical peels are not generally recommended for application in a nonspecific manner in the treatment of acne scars given the relatively mild cosmetic improvements seen and the high rate of AEs such as pigmentary alterations and additional scar formation.12 Rather, clinicians should employ the CROSS (chemical reconstruction of skin scars) technique, in which peel agents such as trichloroacetic acid are applied in high concentrations only to areas of atrophic scarring. Use of this method can minimize AEs while simultaneously achieving high success rates, with excellent results in 100% (32/32) of patients after 5 to 6 treatment sessions.32 This method has been successful for hard-to-treat ice pick scars.33
Soft-Tissue Augmentation
Soft-tissue augmentation is another effective treatment of superficial atrophic acne scarring that utilizes injections of collagen fillers such as hyaluronic acid, calcium hydroxylapatite, poly-L-lactic acid, silicone, and even autologous fat to replace lost tissue volume while simultaneously inducing collagen production via stretching of dermal fibroblasts.34 These treatments may require multiple sessions for cosmetic improvement but have shown considerable efficacy in the treatment of atrophic acne scars. Hyaluronic acid has been reported to be particularly effective for rolling scars.12 However, these compounds only provide temporary results, thus requiring repeated treatments to maintain cosmetic outcomes. Permanent options include the recently US Food and Drug Administration–approved polymethylmethacrylate microspheres suspended in bovine collagen as well as the novel technique of autologous fibroblast transfer. These options are relatively new, but initial double-blind, randomized, controlled trials have shown minimal AEs with substantial improvements in 64% to 100% of atrophic scars treated.35,36
Intralesional Therapy
Intralesional corticosteroid injections are a mainstay treatment of hypertrophic acne scarring and are believed to exert their effects by decreasing fibroblast proliferation and promoting collagen degradation.37 Treatment with steroids generally is effective, with reported improvement in 75% (6/8) of patients and complete flattening in 50% (4/8) of lesions according to one study.38 Development of hypopigmentation, dermal atrophy, and telangiectasia are potential sequelae of this treatment.37
5-Fluorouracil, bleomycin, and verapamil also have been used with good results as intralesional treatments of hypertrophic scars, but these agents typically are reserved for cases of corticosteroid failure. Such compounds are thought to mediate their effects through inhibition of dermal fibroblast proliferation.39 Results with these therapies are varied, but greater than 75% improvement is seen in most cases. Adverse effects include injection-site ulceration and hyperpigmentation.39
Cryotherapy
Contact cryotherapy has been studied as treatment of hypertrophic acne scars. The exact mechanism through which scars are reduced is unclear, but it is hypothesized that the physical damage caused by freezing and thrombosis lead to collagen restructuring. According to one study, cryotherapy was reported to achieve good or excellent results in 76% (29/38) of cases.40 Permanent pigmentary alterations are a possible AE.
Silicone Dressings
Silicone dressings are a reasonable treatment option for hypertrophic acne scarring given their proven efficacy and minimal risk for AEs. Thin sheets of silicone gels or membranes are applied daily in a topical manner to acne scars and are believed to be therapeutic through a combination of pressure and hydration, which subsequently inhibits fibroblast production of collagen. Notable reductions in scar appearance and size are seen in 60% to 80% of individuals using this method.41 Adverse effects are limited to pruritus and local skin maceration. Patient noncompliance may be an issue, as the silicone dressings may be applied on highly visible areas such as the face. Patients may apply the dressings at night, but efficacy may be reduced.
Conclusion
When determining which treatment options to use in a patient with acne scarring, it is important to first determine the patient’s treatment goals while simultaneously establishing realistic expectations. Important factors to consider are the patient’s preferences regarding treatment risk, duration, and permanence, as well as budget and social or work requirements. As such, treatment plans for each patient should be determined on a case-by-case basis. It also is important to note that a combination of different treatment modalities often is necessary and superior to monotherapy in achieving satisfactory cosmetic outcomes.
1. Ghodsi SZ, Orawa H, Zouboulis CC. Prevalence, severity, and severity risk factors of acne in high school pupils: a community-based study. J Invest Dermatol. 2009;129:2136-2141.
2. Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59.
3. Kim GK, Michaels BB. Post-adolescent acne in women: more common and more clinical considerations. J Drugs Dermatol. 2012;11:708-713.
4. Geller L, Rosen J, Frankel A, et al. Perimenstrual flare of adult acne. J Clin Aesthet Dermatol. 2014;7:30-34.
5. Layton AM, Henderson CA, Cunliffe WJ. A clinical evaluation of acne scarring and its incidence. Clin Exp Dermatol. 1994;19:303-308.
6. Halvorsen JA, Stern RS, Dalgard F, et al. Suicidal ideation, mental health problems, and social impairment are increased in adolescents with acne: a population-based study. J Invest Dermatol. 2011;131:363-370.
7. Jacob CI, Dover JS, Kaminer MS. Acne scarring: a classification system and review of treatment options. J Am Acad Dermatol. 2001;45:109-117.
8. Rivera AE. Acne scarring: a review and current treatment modalities. J Am Acad Dermatol. 2008;59:659-676.
9. Goodman GJ. Acne and acne scarring: why should we treat? Med J Aust. 1999;171:62-63.
10. Frank W. Therapeutic dermabrasion. back to the future. Arch Dermatol. 1994;130:1187-1189.
11. Goodman GJ. Postacne scarring: a review of its pathophysiology and treatment. Dermatol Surg. 2000;26:857-871.
12. Hession MT, Graber EM. Atrophic acne scarring: a review of treatment options. J Clin Aesthet Dermatol. 2015;8:50-58.
13. Levy LL, Zeichner JA. Management of acne scarring, part II: a comparative review of non-laser-based, minimally invasive approaches. Am J Clin Dermatol. 2012;13:331-340.
14. Christophel JJ, Elm C, Endrizzi BT, et al. A randomized controlled trial of fractional laser therapy and dermabrasion for scar resurfacing. Dermatol Surg. 2012;38:595-602.
15. Katz BE, McFarlane DF. Atypical facial scarring after isotretinoin therapy in a patient with previous dermabrasion. J Am Acad Dermatol. 1994;30:852-853.
16. Bernestein LJ, Geronemus RG. Keloid formation with the 585-nm pulsed dye laser during isotretinoin treatment. Arch Dermatol. 1997;133:111-112.
17. Zachariae H. Delayed wound healing and keloid formation following argon laser treatment or dermabrasion during isotretinoin treatment. Br J Dermatol. 1988;118:703-706.
18. Wootton CI, Cartwright RP, Manning P, et al. Should isotretinoin be stopped prior to surgery? a critically appraised topic. Br J Dermatol. 2014;170:239-244.
19. Alster TS, West TB. Resurfacing of atrophic facial acne scars with a high-energy, pulsed carbon dioxide laser. Dermatol Surg. 1996;22:151-155.
20. Sobanko JF, Alster TS. Management of acne scarring, part I: a comparative review of laser surgical approaches. Am J Clin Dermatol. 2012;13:319-330.
21. Cho SB, Lee SJ, Oh SH, et al. Non-ablative 1550nm erbium-glass and ablative 10,600nm carbon dioxide fractional lasers for acne scar: a randomized split-face study with blinded response evaluation. J Eur Acad Dermatol Venereol. 2010;24:921-925.
22. Rkein A, Ozog D, Waibel JS. Treatment of atrophic scars with fractionated CO2 laser facilitating delivery of topically applied poly-L-lactic acid. Dermatol Surg. 2014;40:624-631.
23. Waibel JS, Wulkan AJ, Shumaker PR. Treatment of hypertrophic scars using laser and laser assisted corticosteroid delivery. Lasers Surg Med. 2013;45:135-140.
24. Alster TS, McMeekin TO. Improvement of facial acne scars by the 585-nm flashlamp-pumped pulsed dye laser. J Am Acad Dermatol. 1996;35:79-81.
25. Simmons BJ, Griffith RD, Falto-Aizpurua LA, et al. Use of radiofrequency in cosmetic dermatology: focus on nonablative treatment of acne scars. Clin Cosmet Investig Dermatol. 2014;7:335-339.
26. Ramesh M, Gopal M, Kumar S, et al. Novel technology in the treatment of acne scars: the matrix-tunable radiofrequency technology. J Cutan Aesthet Surg. 2010;3:97-101.
27. Johnson WC. Treatment of pitted scars; punch transplant technique. J Dermatol Surg Oncol. 1986;12:260-265.
28. Alam M, Omura N, Kaminer MS. Subcision for acne scarring: technique and outcomes in 40 patients. Dermatol Surg. 2005;31:310-317.
29. Sage R, Lopiccolo M, Liu A, et al. Subcuticular incision versus naturally sourced porcine collagen filler for acne scars: a randomized split-face comparison. Dermatol Surg. 2011;37:426-431.
30. Fabbrocini G, Annunziata MC, D’arco V, et al. Acne scars: pathogenesis, classification and treatment [published online ahead of print October 14, 2010]. Dermatol Res Pract. 2010;2010:893080.
31. Johnson WC. Treatment of pitted scars: punch transplant technique. J Dermatol Surg Oncol. 1986;12:260-265.
32. Lee JB, Chung WG, Kwahck H, et al. Focal treatment of acne scars with trichloroacetic acid: chemical reconstruction of skin scars method. Dermatol Surg. 2002;28:1017-1021.
33. Bhardwaj D, Khunger N. An assessment of the efficacy and safety of CROSS technique with 100% TCA in the management of ice pick acne scars. J Cutan Aesthet Surg. 2010;3:93-96.
34. Wang F, Garza LA, Kang S, et al. In vivo stimulation of de novo collagen production caused by cross-linked hyaluronic acid dermal filler injections in photodamaged human skin. Arch Dermatol. 2007;143:155-163.
35. Karnik J, Baumann L, Bruce S, et al. A double-blind, randomized, multicenter, controlled trial of suspended polymethylmethacrylate microspheres for the correction of atrophic facial acne scars. J Am Acad Dermatol. 2014;71:77-83.
36. Munavalli GS, Smith S, Maslowski JM, et al. Successful treatment of depressed, distensible acne scars using autologous fibroblasts: a multi-site, prospective, double blind, placebo-controlled clinical trial. Dermatol Surg. 2013;39:1226-1236.
37. Leventhal D, Furr M, Reiter D. Treatment of keloids and hypertrophic scars: a meta-analysis and review of the literature. Arch Facial Plast Surg. 2006;8:362-368.
38. Darzi MA, Chowdri NA, Kaul SK, et al. Evaluation of various methods of treating keloids and hypertrophic scars: a 10-year follow-up study. Br J Plast Surg. 1992;45:374-379.
39. Ledon JA, Savas J, Franca K, et al. Intralesional treatment for keloids and hypertrophic scars: a review. Dermatol Surg. 2013;39:1745-1757.
40. Zouboulis CC, Blume U, Büttner P, et al. Outcomes of cryosurgery in keloids and hypertrophic scars. a prospective consecutive trial of case series. Arch Dermatol. 1993;129:1146-1151.
41. Puri N, Talwar A. The efficacy of silicone gel for the treatment of hypertrophic scars and keloids. J Cutan Aesthet Surg. 2009;2:104-106.
Acne vulgaris is one of the most common inflammatory dermatoses affecting nearly all adolescents and a large proportion of adults.1 Incidence rates trend downward with age, but prevalence has been reported to be as high as 51% in individuals aged 20 to 29 years.2 Notably, recent evidence suggests there is an increasing incidence rate of acne among postadolescent women, with the severity associated with the menstrual cycle.3,4 Scarring is a common result of acne and may even occur in the setting of appropriate medical therapy. In particular, some form of facial scarring has been reported to occur in up to 95% of acne patients, with severe scarring in 30% of these patients.5 The detrimental effects of acne scarring are not only limited to impaired cosmetic appearance, as it also has been associated with depression symptoms, suicidal ideation, mental health problems, and general social impairment.6 Given the negative impact of acne scarring on overall health and well-being as well as its permanent nature, early and effective treatment is essential to maximize cosmetic outcomes and minimize long-term deleterious effects.
Acne scarring can be broadly divided into 2 major categories: atrophic and hypertrophic. Atrophic scarring is more common and is characterized by an overall localized reduction in collagen content. Clinically, atrophic scars present as depressions in the skin secondary to inflammatory fibrous contractions induced by acne. This type of scarring can be further divided into various subtypes based on morphologic criteria (eg, size, depth), such as boxcar, ice pick, and rolling scars.7 Conversely, hypertrophic scarring is characterized by an overall increase in collagen content and presents as firm raised lesions. Hypertrophic scars should be distinguished from keloid scars, as the former will not outgrow the margins of the original wound while the latter will.8 Treatment of acne scarring is based on scar type and can be accomplished through a variety of medical and surgical modalities (Table). In this article, we review some of the most commonly utilized therapies for both atrophic and hypertrophic acne scarring with a focus on cosmetic outcomes. It is important to keep in mind, however, that the best treatment is to prevent the occurrence of acne scarring through early and proactive treatment of acne.9
Dermabrasion
Dermabrasion is a decades-old technique that employs the use of a motorized device equipped with an abrasive material to physically remove the superficial layers of the skin, thus inducing the wound-healing process with subsequent formation of new collagen.10 In the same vein, microdermabrasion utilizes aluminum oxide crystals ejected from a nozzle to induce superficial microlacerations.11 This technique is most successful when used to soften scar edges in superficial atrophic scars of the rolling or boxcar subtypes.12 Dermabrasion has been shown to be equally as effective as laser therapy in the treatment of facial scars but is reported to have a much greater risk for adverse effects (AEs)(eg, erythema, edema) that may last for several weeks posttherapy.13,14 Dermabrasion is a particularly operator-dependent technique for which outcomes may vary depending on operator experience. As such, it is not generally recommended as a first-line therapy given its risks and relatively modest results; however, dermabrasion can be a useful adjunct when performed in the right setting. This technique, in addition to laser resurfacing, should be used with caution in patients who have recently taken or currently are taking isotretinoin, as several case series have reported postprocedural development of hypertrophic or keloid scars,15-17 but these findings subsequently were questioned in the literature.18
Laser Therapy
Laser technology has advanced tremendously over the last few decades and there are now a multitude of available lasers that are capable of variable depth penetration and energy delivery patterns. Common to all, however, is the ability to induce localized thermal damage with eventual collagen remodeling. Lasers can be divided into 2 major categories: ablative and nonablative. Ablative lasers cause epidermal destruction, while nonablative lasers are able to selectively target dermal layers without disrupting the overlying epithelium. Generally speaking, ablative lasers are more effective than nonablative lasers in the treatment of atrophic scars, with reported mean improvements of up to 81%.19 This increased efficacy comes with an increased risk for AEs such as postinflammatory hyperpigmentation, prolonged posttreatment erythema, and formation of additional scarring.20 Both ablative and nonablative lasers can be applied in the more recently developed technology of fractional photothermolysis. With this method, noncontiguous microscopic columns of thermal injury surrounded by zones of viable tissue are created, which is in contrast to the traditional manner of inducing broad thermal injury. Fractional ablative lasers can achieve efficacy rates similar to traditional ablative lasers with a reduced risk for permanent scarring or dispigmentation.21 Notably, recent studies have shown promising results for the use of fractional ablative lasers as a mechanism to enhance drug delivery of topically applied medications such as poly-L-lactic acid and triamcinolone acetonide in the treatment of atrophic and hypertrophic scars, respectively.22,23
Lasers also play a role in the treatment of hypertrophic acne scars with the use of nonablative pulsed dye lasers. These lasers cause selective thermolysis of dermal vasculature, and average clinical improvements in hypertrophic scars of 67.5% after a single treatment have been reported.24 Temporary postoperative purpura and long-term hyperpigmentation are reported outcomes of this therapy.20
Radiofrequency
Nonablative radiofrequency (RF) is a relatively novel technique that creates an electric current in the dermis at preset depths to induce thermal damage and eventual collagen synthesis. There are a variety of modalities for which RF can be applied, but microneedle bipolar RF and fractional bipolar RF treatments offer the best results for atrophic acne scars. Improvements in scar appearance of 25% to 75% have been reported after several treatment sessions.25 Better results have been reported in the treatment of ice pick scars as compared to more superficial scars,26 but additional studies will be necessary to validate this claim. Adverse effects are largely limited to temporary erythema and posttreatment scabbing.27
Subcision
Subcision is a more physically intensive technique useful for treatment of superficial atrophic acne scars. This method involves the use of a small needle that is inserted into the periphery of a scar before being moved in a back-and-forth manner underneath the base of the scar to loosen the fibrotic adhesions that result in the depressed appearance of the scar. Additionally, loosening of the tissue and resultant bleeding creates a potential space for future collagen deposition during the subsequent wound-healing phase. Subcision has a reported success rate of 50% to 60% in the treatment of rolling scars, and prospective, randomized, split-face trials have indicated that the short-term outcomes of subcision are superior to dermal fillers while being equally effective long-term.28,29 Of note, a small percentage of patients may develop a localized nodule at the site of treatment, which can be resolved with intralesional steroids.11
Skin Needling
Skin needling, also referred to as collagen induction therapy, utilizes vertical needle punctures rather than the horizontally directed punctures that are used in subcision and can be used to treat rolling and boxcar scars. Traditionally, a small roller equipped with rows of small needles typically ranging in size from 0.5 to 3.0 mm in length is passed over the skin using gentle pressure, puncturing the superficial layers of the skin to loosen fibrotic adhesions and induce collagen synthesis. This procedure may be repeated several times within a single session or over multiple sessions depending on the depth and quality of the scars. This technique has been reported to reduce scar depth up to 25% after 2 sessions.30
Punch Techniques
Punch techniques are useful for treatment of deeper atrophic acne scarring, for which most other treatment modalities are not particularly effective. A punch excision approximately equal to the scar size is first performed, which may then be followed by either removal of the scar tissue with subsequent suturing, graft replacement of the removed tissue, or elevation of the already established scar tissue to the level of surrounding skin where it is then held in place by sutures or adhesive skin closure material. Success rates with this method are largely limited to case series, but punch techniques are reported to be efficacious, especially for treatment of ice pick scars. Risks for this method include graft failure, graft depression, and formation of sinus tracts.31
Chemical Peels
Chemicals peels traditionally employ the use of acidic compounds to strip away the outer layers of skin to variable depths depending on the concentration of the agent being applied. Chemical peels are not generally recommended for application in a nonspecific manner in the treatment of acne scars given the relatively mild cosmetic improvements seen and the high rate of AEs such as pigmentary alterations and additional scar formation.12 Rather, clinicians should employ the CROSS (chemical reconstruction of skin scars) technique, in which peel agents such as trichloroacetic acid are applied in high concentrations only to areas of atrophic scarring. Use of this method can minimize AEs while simultaneously achieving high success rates, with excellent results in 100% (32/32) of patients after 5 to 6 treatment sessions.32 This method has been successful for hard-to-treat ice pick scars.33
Soft-Tissue Augmentation
Soft-tissue augmentation is another effective treatment of superficial atrophic acne scarring that utilizes injections of collagen fillers such as hyaluronic acid, calcium hydroxylapatite, poly-L-lactic acid, silicone, and even autologous fat to replace lost tissue volume while simultaneously inducing collagen production via stretching of dermal fibroblasts.34 These treatments may require multiple sessions for cosmetic improvement but have shown considerable efficacy in the treatment of atrophic acne scars. Hyaluronic acid has been reported to be particularly effective for rolling scars.12 However, these compounds only provide temporary results, thus requiring repeated treatments to maintain cosmetic outcomes. Permanent options include the recently US Food and Drug Administration–approved polymethylmethacrylate microspheres suspended in bovine collagen as well as the novel technique of autologous fibroblast transfer. These options are relatively new, but initial double-blind, randomized, controlled trials have shown minimal AEs with substantial improvements in 64% to 100% of atrophic scars treated.35,36
Intralesional Therapy
Intralesional corticosteroid injections are a mainstay treatment of hypertrophic acne scarring and are believed to exert their effects by decreasing fibroblast proliferation and promoting collagen degradation.37 Treatment with steroids generally is effective, with reported improvement in 75% (6/8) of patients and complete flattening in 50% (4/8) of lesions according to one study.38 Development of hypopigmentation, dermal atrophy, and telangiectasia are potential sequelae of this treatment.37
5-Fluorouracil, bleomycin, and verapamil also have been used with good results as intralesional treatments of hypertrophic scars, but these agents typically are reserved for cases of corticosteroid failure. Such compounds are thought to mediate their effects through inhibition of dermal fibroblast proliferation.39 Results with these therapies are varied, but greater than 75% improvement is seen in most cases. Adverse effects include injection-site ulceration and hyperpigmentation.39
Cryotherapy
Contact cryotherapy has been studied as treatment of hypertrophic acne scars. The exact mechanism through which scars are reduced is unclear, but it is hypothesized that the physical damage caused by freezing and thrombosis lead to collagen restructuring. According to one study, cryotherapy was reported to achieve good or excellent results in 76% (29/38) of cases.40 Permanent pigmentary alterations are a possible AE.
Silicone Dressings
Silicone dressings are a reasonable treatment option for hypertrophic acne scarring given their proven efficacy and minimal risk for AEs. Thin sheets of silicone gels or membranes are applied daily in a topical manner to acne scars and are believed to be therapeutic through a combination of pressure and hydration, which subsequently inhibits fibroblast production of collagen. Notable reductions in scar appearance and size are seen in 60% to 80% of individuals using this method.41 Adverse effects are limited to pruritus and local skin maceration. Patient noncompliance may be an issue, as the silicone dressings may be applied on highly visible areas such as the face. Patients may apply the dressings at night, but efficacy may be reduced.
Conclusion
When determining which treatment options to use in a patient with acne scarring, it is important to first determine the patient’s treatment goals while simultaneously establishing realistic expectations. Important factors to consider are the patient’s preferences regarding treatment risk, duration, and permanence, as well as budget and social or work requirements. As such, treatment plans for each patient should be determined on a case-by-case basis. It also is important to note that a combination of different treatment modalities often is necessary and superior to monotherapy in achieving satisfactory cosmetic outcomes.
Acne vulgaris is one of the most common inflammatory dermatoses affecting nearly all adolescents and a large proportion of adults.1 Incidence rates trend downward with age, but prevalence has been reported to be as high as 51% in individuals aged 20 to 29 years.2 Notably, recent evidence suggests there is an increasing incidence rate of acne among postadolescent women, with the severity associated with the menstrual cycle.3,4 Scarring is a common result of acne and may even occur in the setting of appropriate medical therapy. In particular, some form of facial scarring has been reported to occur in up to 95% of acne patients, with severe scarring in 30% of these patients.5 The detrimental effects of acne scarring are not only limited to impaired cosmetic appearance, as it also has been associated with depression symptoms, suicidal ideation, mental health problems, and general social impairment.6 Given the negative impact of acne scarring on overall health and well-being as well as its permanent nature, early and effective treatment is essential to maximize cosmetic outcomes and minimize long-term deleterious effects.
Acne scarring can be broadly divided into 2 major categories: atrophic and hypertrophic. Atrophic scarring is more common and is characterized by an overall localized reduction in collagen content. Clinically, atrophic scars present as depressions in the skin secondary to inflammatory fibrous contractions induced by acne. This type of scarring can be further divided into various subtypes based on morphologic criteria (eg, size, depth), such as boxcar, ice pick, and rolling scars.7 Conversely, hypertrophic scarring is characterized by an overall increase in collagen content and presents as firm raised lesions. Hypertrophic scars should be distinguished from keloid scars, as the former will not outgrow the margins of the original wound while the latter will.8 Treatment of acne scarring is based on scar type and can be accomplished through a variety of medical and surgical modalities (Table). In this article, we review some of the most commonly utilized therapies for both atrophic and hypertrophic acne scarring with a focus on cosmetic outcomes. It is important to keep in mind, however, that the best treatment is to prevent the occurrence of acne scarring through early and proactive treatment of acne.9
Dermabrasion
Dermabrasion is a decades-old technique that employs the use of a motorized device equipped with an abrasive material to physically remove the superficial layers of the skin, thus inducing the wound-healing process with subsequent formation of new collagen.10 In the same vein, microdermabrasion utilizes aluminum oxide crystals ejected from a nozzle to induce superficial microlacerations.11 This technique is most successful when used to soften scar edges in superficial atrophic scars of the rolling or boxcar subtypes.12 Dermabrasion has been shown to be equally as effective as laser therapy in the treatment of facial scars but is reported to have a much greater risk for adverse effects (AEs)(eg, erythema, edema) that may last for several weeks posttherapy.13,14 Dermabrasion is a particularly operator-dependent technique for which outcomes may vary depending on operator experience. As such, it is not generally recommended as a first-line therapy given its risks and relatively modest results; however, dermabrasion can be a useful adjunct when performed in the right setting. This technique, in addition to laser resurfacing, should be used with caution in patients who have recently taken or currently are taking isotretinoin, as several case series have reported postprocedural development of hypertrophic or keloid scars,15-17 but these findings subsequently were questioned in the literature.18
Laser Therapy
Laser technology has advanced tremendously over the last few decades and there are now a multitude of available lasers that are capable of variable depth penetration and energy delivery patterns. Common to all, however, is the ability to induce localized thermal damage with eventual collagen remodeling. Lasers can be divided into 2 major categories: ablative and nonablative. Ablative lasers cause epidermal destruction, while nonablative lasers are able to selectively target dermal layers without disrupting the overlying epithelium. Generally speaking, ablative lasers are more effective than nonablative lasers in the treatment of atrophic scars, with reported mean improvements of up to 81%.19 This increased efficacy comes with an increased risk for AEs such as postinflammatory hyperpigmentation, prolonged posttreatment erythema, and formation of additional scarring.20 Both ablative and nonablative lasers can be applied in the more recently developed technology of fractional photothermolysis. With this method, noncontiguous microscopic columns of thermal injury surrounded by zones of viable tissue are created, which is in contrast to the traditional manner of inducing broad thermal injury. Fractional ablative lasers can achieve efficacy rates similar to traditional ablative lasers with a reduced risk for permanent scarring or dispigmentation.21 Notably, recent studies have shown promising results for the use of fractional ablative lasers as a mechanism to enhance drug delivery of topically applied medications such as poly-L-lactic acid and triamcinolone acetonide in the treatment of atrophic and hypertrophic scars, respectively.22,23
Lasers also play a role in the treatment of hypertrophic acne scars with the use of nonablative pulsed dye lasers. These lasers cause selective thermolysis of dermal vasculature, and average clinical improvements in hypertrophic scars of 67.5% after a single treatment have been reported.24 Temporary postoperative purpura and long-term hyperpigmentation are reported outcomes of this therapy.20
Radiofrequency
Nonablative radiofrequency (RF) is a relatively novel technique that creates an electric current in the dermis at preset depths to induce thermal damage and eventual collagen synthesis. There are a variety of modalities for which RF can be applied, but microneedle bipolar RF and fractional bipolar RF treatments offer the best results for atrophic acne scars. Improvements in scar appearance of 25% to 75% have been reported after several treatment sessions.25 Better results have been reported in the treatment of ice pick scars as compared to more superficial scars,26 but additional studies will be necessary to validate this claim. Adverse effects are largely limited to temporary erythema and posttreatment scabbing.27
Subcision
Subcision is a more physically intensive technique useful for treatment of superficial atrophic acne scars. This method involves the use of a small needle that is inserted into the periphery of a scar before being moved in a back-and-forth manner underneath the base of the scar to loosen the fibrotic adhesions that result in the depressed appearance of the scar. Additionally, loosening of the tissue and resultant bleeding creates a potential space for future collagen deposition during the subsequent wound-healing phase. Subcision has a reported success rate of 50% to 60% in the treatment of rolling scars, and prospective, randomized, split-face trials have indicated that the short-term outcomes of subcision are superior to dermal fillers while being equally effective long-term.28,29 Of note, a small percentage of patients may develop a localized nodule at the site of treatment, which can be resolved with intralesional steroids.11
Skin Needling
Skin needling, also referred to as collagen induction therapy, utilizes vertical needle punctures rather than the horizontally directed punctures that are used in subcision and can be used to treat rolling and boxcar scars. Traditionally, a small roller equipped with rows of small needles typically ranging in size from 0.5 to 3.0 mm in length is passed over the skin using gentle pressure, puncturing the superficial layers of the skin to loosen fibrotic adhesions and induce collagen synthesis. This procedure may be repeated several times within a single session or over multiple sessions depending on the depth and quality of the scars. This technique has been reported to reduce scar depth up to 25% after 2 sessions.30
Punch Techniques
Punch techniques are useful for treatment of deeper atrophic acne scarring, for which most other treatment modalities are not particularly effective. A punch excision approximately equal to the scar size is first performed, which may then be followed by either removal of the scar tissue with subsequent suturing, graft replacement of the removed tissue, or elevation of the already established scar tissue to the level of surrounding skin where it is then held in place by sutures or adhesive skin closure material. Success rates with this method are largely limited to case series, but punch techniques are reported to be efficacious, especially for treatment of ice pick scars. Risks for this method include graft failure, graft depression, and formation of sinus tracts.31
Chemical Peels
Chemicals peels traditionally employ the use of acidic compounds to strip away the outer layers of skin to variable depths depending on the concentration of the agent being applied. Chemical peels are not generally recommended for application in a nonspecific manner in the treatment of acne scars given the relatively mild cosmetic improvements seen and the high rate of AEs such as pigmentary alterations and additional scar formation.12 Rather, clinicians should employ the CROSS (chemical reconstruction of skin scars) technique, in which peel agents such as trichloroacetic acid are applied in high concentrations only to areas of atrophic scarring. Use of this method can minimize AEs while simultaneously achieving high success rates, with excellent results in 100% (32/32) of patients after 5 to 6 treatment sessions.32 This method has been successful for hard-to-treat ice pick scars.33
Soft-Tissue Augmentation
Soft-tissue augmentation is another effective treatment of superficial atrophic acne scarring that utilizes injections of collagen fillers such as hyaluronic acid, calcium hydroxylapatite, poly-L-lactic acid, silicone, and even autologous fat to replace lost tissue volume while simultaneously inducing collagen production via stretching of dermal fibroblasts.34 These treatments may require multiple sessions for cosmetic improvement but have shown considerable efficacy in the treatment of atrophic acne scars. Hyaluronic acid has been reported to be particularly effective for rolling scars.12 However, these compounds only provide temporary results, thus requiring repeated treatments to maintain cosmetic outcomes. Permanent options include the recently US Food and Drug Administration–approved polymethylmethacrylate microspheres suspended in bovine collagen as well as the novel technique of autologous fibroblast transfer. These options are relatively new, but initial double-blind, randomized, controlled trials have shown minimal AEs with substantial improvements in 64% to 100% of atrophic scars treated.35,36
Intralesional Therapy
Intralesional corticosteroid injections are a mainstay treatment of hypertrophic acne scarring and are believed to exert their effects by decreasing fibroblast proliferation and promoting collagen degradation.37 Treatment with steroids generally is effective, with reported improvement in 75% (6/8) of patients and complete flattening in 50% (4/8) of lesions according to one study.38 Development of hypopigmentation, dermal atrophy, and telangiectasia are potential sequelae of this treatment.37
5-Fluorouracil, bleomycin, and verapamil also have been used with good results as intralesional treatments of hypertrophic scars, but these agents typically are reserved for cases of corticosteroid failure. Such compounds are thought to mediate their effects through inhibition of dermal fibroblast proliferation.39 Results with these therapies are varied, but greater than 75% improvement is seen in most cases. Adverse effects include injection-site ulceration and hyperpigmentation.39
Cryotherapy
Contact cryotherapy has been studied as treatment of hypertrophic acne scars. The exact mechanism through which scars are reduced is unclear, but it is hypothesized that the physical damage caused by freezing and thrombosis lead to collagen restructuring. According to one study, cryotherapy was reported to achieve good or excellent results in 76% (29/38) of cases.40 Permanent pigmentary alterations are a possible AE.
Silicone Dressings
Silicone dressings are a reasonable treatment option for hypertrophic acne scarring given their proven efficacy and minimal risk for AEs. Thin sheets of silicone gels or membranes are applied daily in a topical manner to acne scars and are believed to be therapeutic through a combination of pressure and hydration, which subsequently inhibits fibroblast production of collagen. Notable reductions in scar appearance and size are seen in 60% to 80% of individuals using this method.41 Adverse effects are limited to pruritus and local skin maceration. Patient noncompliance may be an issue, as the silicone dressings may be applied on highly visible areas such as the face. Patients may apply the dressings at night, but efficacy may be reduced.
Conclusion
When determining which treatment options to use in a patient with acne scarring, it is important to first determine the patient’s treatment goals while simultaneously establishing realistic expectations. Important factors to consider are the patient’s preferences regarding treatment risk, duration, and permanence, as well as budget and social or work requirements. As such, treatment plans for each patient should be determined on a case-by-case basis. It also is important to note that a combination of different treatment modalities often is necessary and superior to monotherapy in achieving satisfactory cosmetic outcomes.
1. Ghodsi SZ, Orawa H, Zouboulis CC. Prevalence, severity, and severity risk factors of acne in high school pupils: a community-based study. J Invest Dermatol. 2009;129:2136-2141.
2. Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59.
3. Kim GK, Michaels BB. Post-adolescent acne in women: more common and more clinical considerations. J Drugs Dermatol. 2012;11:708-713.
4. Geller L, Rosen J, Frankel A, et al. Perimenstrual flare of adult acne. J Clin Aesthet Dermatol. 2014;7:30-34.
5. Layton AM, Henderson CA, Cunliffe WJ. A clinical evaluation of acne scarring and its incidence. Clin Exp Dermatol. 1994;19:303-308.
6. Halvorsen JA, Stern RS, Dalgard F, et al. Suicidal ideation, mental health problems, and social impairment are increased in adolescents with acne: a population-based study. J Invest Dermatol. 2011;131:363-370.
7. Jacob CI, Dover JS, Kaminer MS. Acne scarring: a classification system and review of treatment options. J Am Acad Dermatol. 2001;45:109-117.
8. Rivera AE. Acne scarring: a review and current treatment modalities. J Am Acad Dermatol. 2008;59:659-676.
9. Goodman GJ. Acne and acne scarring: why should we treat? Med J Aust. 1999;171:62-63.
10. Frank W. Therapeutic dermabrasion. back to the future. Arch Dermatol. 1994;130:1187-1189.
11. Goodman GJ. Postacne scarring: a review of its pathophysiology and treatment. Dermatol Surg. 2000;26:857-871.
12. Hession MT, Graber EM. Atrophic acne scarring: a review of treatment options. J Clin Aesthet Dermatol. 2015;8:50-58.
13. Levy LL, Zeichner JA. Management of acne scarring, part II: a comparative review of non-laser-based, minimally invasive approaches. Am J Clin Dermatol. 2012;13:331-340.
14. Christophel JJ, Elm C, Endrizzi BT, et al. A randomized controlled trial of fractional laser therapy and dermabrasion for scar resurfacing. Dermatol Surg. 2012;38:595-602.
15. Katz BE, McFarlane DF. Atypical facial scarring after isotretinoin therapy in a patient with previous dermabrasion. J Am Acad Dermatol. 1994;30:852-853.
16. Bernestein LJ, Geronemus RG. Keloid formation with the 585-nm pulsed dye laser during isotretinoin treatment. Arch Dermatol. 1997;133:111-112.
17. Zachariae H. Delayed wound healing and keloid formation following argon laser treatment or dermabrasion during isotretinoin treatment. Br J Dermatol. 1988;118:703-706.
18. Wootton CI, Cartwright RP, Manning P, et al. Should isotretinoin be stopped prior to surgery? a critically appraised topic. Br J Dermatol. 2014;170:239-244.
19. Alster TS, West TB. Resurfacing of atrophic facial acne scars with a high-energy, pulsed carbon dioxide laser. Dermatol Surg. 1996;22:151-155.
20. Sobanko JF, Alster TS. Management of acne scarring, part I: a comparative review of laser surgical approaches. Am J Clin Dermatol. 2012;13:319-330.
21. Cho SB, Lee SJ, Oh SH, et al. Non-ablative 1550nm erbium-glass and ablative 10,600nm carbon dioxide fractional lasers for acne scar: a randomized split-face study with blinded response evaluation. J Eur Acad Dermatol Venereol. 2010;24:921-925.
22. Rkein A, Ozog D, Waibel JS. Treatment of atrophic scars with fractionated CO2 laser facilitating delivery of topically applied poly-L-lactic acid. Dermatol Surg. 2014;40:624-631.
23. Waibel JS, Wulkan AJ, Shumaker PR. Treatment of hypertrophic scars using laser and laser assisted corticosteroid delivery. Lasers Surg Med. 2013;45:135-140.
24. Alster TS, McMeekin TO. Improvement of facial acne scars by the 585-nm flashlamp-pumped pulsed dye laser. J Am Acad Dermatol. 1996;35:79-81.
25. Simmons BJ, Griffith RD, Falto-Aizpurua LA, et al. Use of radiofrequency in cosmetic dermatology: focus on nonablative treatment of acne scars. Clin Cosmet Investig Dermatol. 2014;7:335-339.
26. Ramesh M, Gopal M, Kumar S, et al. Novel technology in the treatment of acne scars: the matrix-tunable radiofrequency technology. J Cutan Aesthet Surg. 2010;3:97-101.
27. Johnson WC. Treatment of pitted scars; punch transplant technique. J Dermatol Surg Oncol. 1986;12:260-265.
28. Alam M, Omura N, Kaminer MS. Subcision for acne scarring: technique and outcomes in 40 patients. Dermatol Surg. 2005;31:310-317.
29. Sage R, Lopiccolo M, Liu A, et al. Subcuticular incision versus naturally sourced porcine collagen filler for acne scars: a randomized split-face comparison. Dermatol Surg. 2011;37:426-431.
30. Fabbrocini G, Annunziata MC, D’arco V, et al. Acne scars: pathogenesis, classification and treatment [published online ahead of print October 14, 2010]. Dermatol Res Pract. 2010;2010:893080.
31. Johnson WC. Treatment of pitted scars: punch transplant technique. J Dermatol Surg Oncol. 1986;12:260-265.
32. Lee JB, Chung WG, Kwahck H, et al. Focal treatment of acne scars with trichloroacetic acid: chemical reconstruction of skin scars method. Dermatol Surg. 2002;28:1017-1021.
33. Bhardwaj D, Khunger N. An assessment of the efficacy and safety of CROSS technique with 100% TCA in the management of ice pick acne scars. J Cutan Aesthet Surg. 2010;3:93-96.
34. Wang F, Garza LA, Kang S, et al. In vivo stimulation of de novo collagen production caused by cross-linked hyaluronic acid dermal filler injections in photodamaged human skin. Arch Dermatol. 2007;143:155-163.
35. Karnik J, Baumann L, Bruce S, et al. A double-blind, randomized, multicenter, controlled trial of suspended polymethylmethacrylate microspheres for the correction of atrophic facial acne scars. J Am Acad Dermatol. 2014;71:77-83.
36. Munavalli GS, Smith S, Maslowski JM, et al. Successful treatment of depressed, distensible acne scars using autologous fibroblasts: a multi-site, prospective, double blind, placebo-controlled clinical trial. Dermatol Surg. 2013;39:1226-1236.
37. Leventhal D, Furr M, Reiter D. Treatment of keloids and hypertrophic scars: a meta-analysis and review of the literature. Arch Facial Plast Surg. 2006;8:362-368.
38. Darzi MA, Chowdri NA, Kaul SK, et al. Evaluation of various methods of treating keloids and hypertrophic scars: a 10-year follow-up study. Br J Plast Surg. 1992;45:374-379.
39. Ledon JA, Savas J, Franca K, et al. Intralesional treatment for keloids and hypertrophic scars: a review. Dermatol Surg. 2013;39:1745-1757.
40. Zouboulis CC, Blume U, Büttner P, et al. Outcomes of cryosurgery in keloids and hypertrophic scars. a prospective consecutive trial of case series. Arch Dermatol. 1993;129:1146-1151.
41. Puri N, Talwar A. The efficacy of silicone gel for the treatment of hypertrophic scars and keloids. J Cutan Aesthet Surg. 2009;2:104-106.
1. Ghodsi SZ, Orawa H, Zouboulis CC. Prevalence, severity, and severity risk factors of acne in high school pupils: a community-based study. J Invest Dermatol. 2009;129:2136-2141.
2. Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59.
3. Kim GK, Michaels BB. Post-adolescent acne in women: more common and more clinical considerations. J Drugs Dermatol. 2012;11:708-713.
4. Geller L, Rosen J, Frankel A, et al. Perimenstrual flare of adult acne. J Clin Aesthet Dermatol. 2014;7:30-34.
5. Layton AM, Henderson CA, Cunliffe WJ. A clinical evaluation of acne scarring and its incidence. Clin Exp Dermatol. 1994;19:303-308.
6. Halvorsen JA, Stern RS, Dalgard F, et al. Suicidal ideation, mental health problems, and social impairment are increased in adolescents with acne: a population-based study. J Invest Dermatol. 2011;131:363-370.
7. Jacob CI, Dover JS, Kaminer MS. Acne scarring: a classification system and review of treatment options. J Am Acad Dermatol. 2001;45:109-117.
8. Rivera AE. Acne scarring: a review and current treatment modalities. J Am Acad Dermatol. 2008;59:659-676.
9. Goodman GJ. Acne and acne scarring: why should we treat? Med J Aust. 1999;171:62-63.
10. Frank W. Therapeutic dermabrasion. back to the future. Arch Dermatol. 1994;130:1187-1189.
11. Goodman GJ. Postacne scarring: a review of its pathophysiology and treatment. Dermatol Surg. 2000;26:857-871.
12. Hession MT, Graber EM. Atrophic acne scarring: a review of treatment options. J Clin Aesthet Dermatol. 2015;8:50-58.
13. Levy LL, Zeichner JA. Management of acne scarring, part II: a comparative review of non-laser-based, minimally invasive approaches. Am J Clin Dermatol. 2012;13:331-340.
14. Christophel JJ, Elm C, Endrizzi BT, et al. A randomized controlled trial of fractional laser therapy and dermabrasion for scar resurfacing. Dermatol Surg. 2012;38:595-602.
15. Katz BE, McFarlane DF. Atypical facial scarring after isotretinoin therapy in a patient with previous dermabrasion. J Am Acad Dermatol. 1994;30:852-853.
16. Bernestein LJ, Geronemus RG. Keloid formation with the 585-nm pulsed dye laser during isotretinoin treatment. Arch Dermatol. 1997;133:111-112.
17. Zachariae H. Delayed wound healing and keloid formation following argon laser treatment or dermabrasion during isotretinoin treatment. Br J Dermatol. 1988;118:703-706.
18. Wootton CI, Cartwright RP, Manning P, et al. Should isotretinoin be stopped prior to surgery? a critically appraised topic. Br J Dermatol. 2014;170:239-244.
19. Alster TS, West TB. Resurfacing of atrophic facial acne scars with a high-energy, pulsed carbon dioxide laser. Dermatol Surg. 1996;22:151-155.
20. Sobanko JF, Alster TS. Management of acne scarring, part I: a comparative review of laser surgical approaches. Am J Clin Dermatol. 2012;13:319-330.
21. Cho SB, Lee SJ, Oh SH, et al. Non-ablative 1550nm erbium-glass and ablative 10,600nm carbon dioxide fractional lasers for acne scar: a randomized split-face study with blinded response evaluation. J Eur Acad Dermatol Venereol. 2010;24:921-925.
22. Rkein A, Ozog D, Waibel JS. Treatment of atrophic scars with fractionated CO2 laser facilitating delivery of topically applied poly-L-lactic acid. Dermatol Surg. 2014;40:624-631.
23. Waibel JS, Wulkan AJ, Shumaker PR. Treatment of hypertrophic scars using laser and laser assisted corticosteroid delivery. Lasers Surg Med. 2013;45:135-140.
24. Alster TS, McMeekin TO. Improvement of facial acne scars by the 585-nm flashlamp-pumped pulsed dye laser. J Am Acad Dermatol. 1996;35:79-81.
25. Simmons BJ, Griffith RD, Falto-Aizpurua LA, et al. Use of radiofrequency in cosmetic dermatology: focus on nonablative treatment of acne scars. Clin Cosmet Investig Dermatol. 2014;7:335-339.
26. Ramesh M, Gopal M, Kumar S, et al. Novel technology in the treatment of acne scars: the matrix-tunable radiofrequency technology. J Cutan Aesthet Surg. 2010;3:97-101.
27. Johnson WC. Treatment of pitted scars; punch transplant technique. J Dermatol Surg Oncol. 1986;12:260-265.
28. Alam M, Omura N, Kaminer MS. Subcision for acne scarring: technique and outcomes in 40 patients. Dermatol Surg. 2005;31:310-317.
29. Sage R, Lopiccolo M, Liu A, et al. Subcuticular incision versus naturally sourced porcine collagen filler for acne scars: a randomized split-face comparison. Dermatol Surg. 2011;37:426-431.
30. Fabbrocini G, Annunziata MC, D’arco V, et al. Acne scars: pathogenesis, classification and treatment [published online ahead of print October 14, 2010]. Dermatol Res Pract. 2010;2010:893080.
31. Johnson WC. Treatment of pitted scars: punch transplant technique. J Dermatol Surg Oncol. 1986;12:260-265.
32. Lee JB, Chung WG, Kwahck H, et al. Focal treatment of acne scars with trichloroacetic acid: chemical reconstruction of skin scars method. Dermatol Surg. 2002;28:1017-1021.
33. Bhardwaj D, Khunger N. An assessment of the efficacy and safety of CROSS technique with 100% TCA in the management of ice pick acne scars. J Cutan Aesthet Surg. 2010;3:93-96.
34. Wang F, Garza LA, Kang S, et al. In vivo stimulation of de novo collagen production caused by cross-linked hyaluronic acid dermal filler injections in photodamaged human skin. Arch Dermatol. 2007;143:155-163.
35. Karnik J, Baumann L, Bruce S, et al. A double-blind, randomized, multicenter, controlled trial of suspended polymethylmethacrylate microspheres for the correction of atrophic facial acne scars. J Am Acad Dermatol. 2014;71:77-83.
36. Munavalli GS, Smith S, Maslowski JM, et al. Successful treatment of depressed, distensible acne scars using autologous fibroblasts: a multi-site, prospective, double blind, placebo-controlled clinical trial. Dermatol Surg. 2013;39:1226-1236.
37. Leventhal D, Furr M, Reiter D. Treatment of keloids and hypertrophic scars: a meta-analysis and review of the literature. Arch Facial Plast Surg. 2006;8:362-368.
38. Darzi MA, Chowdri NA, Kaul SK, et al. Evaluation of various methods of treating keloids and hypertrophic scars: a 10-year follow-up study. Br J Plast Surg. 1992;45:374-379.
39. Ledon JA, Savas J, Franca K, et al. Intralesional treatment for keloids and hypertrophic scars: a review. Dermatol Surg. 2013;39:1745-1757.
40. Zouboulis CC, Blume U, Büttner P, et al. Outcomes of cryosurgery in keloids and hypertrophic scars. a prospective consecutive trial of case series. Arch Dermatol. 1993;129:1146-1151.
41. Puri N, Talwar A. The efficacy of silicone gel for the treatment of hypertrophic scars and keloids. J Cutan Aesthet Surg. 2009;2:104-106.
Practice Points
- Scarring is a common and undesirable outcome of acne vulgaris that can occur even in the setting of appropriate medical management.
- Acne scars can be classified into several different types based on scar quality and appearance. The choice of treatment with medical or surgical measures should be made with respect to the type of scar present.
- A combination of therapeutic modalities often is necessary to achieve optimal cosmetic outcomes in the treatment of both atrophic and hypertrophic acne scars.