User login
About 17% of COVID-19 survivors retest positive in follow-up study
For reasons unknown, about one in six people who recovered from COVID-19 subsequently retested positive at least 2 weeks later, researchers reported in a study in Italy.
Sore throat and rhinitis were the only symptoms associated with a positive result. “Patients who continued to have respiratory symptoms, especially, were more likely to have a new positive test result,” lead author Francesco Landi, MD, PhD, said in an interview.
“This suggests the persistence of respiratory symptoms should not be underestimated and should be adequately assessed in all patients considered recovered from COVID-19,” he said.
“The study results are interesting,” Akiko Iwasaki, PhD, an immunobiologist at Yale University and the Howard Hughes Medical Institute, both in New Haven, Conn.,, said in an interview. “There are other reports of RNA detection postdischarge, but this study ... found that only two symptoms out of many – sore throat and rhinitis – were higher in those with PCR [polymerase chain reaction]-positive status.”
The study was published online Sept. 18 in the American Journal of Preventive Medicine.
The findings could carry important implications for people who continue to be symptomatic. “It is reasonable to be cautious and avoid close contact with others, wear a face mask, and possibly undergo an additional nasopharyngeal swab,” said Dr. Landi, associate professor of internal medicine at Catholic University of the Sacred Heart in Rome.
“One of most interesting findings is that persistent symptoms do not correlate with PCR positivity, suggesting that symptoms are in many cases not due to ongoing viral replication,” Jonathan Karn, PhD, professor and chair of the department of molecular biology and microbiology at Case Western Reserve University, Cleveland, said in an interview.
“The key technical problem, which they have discussed, is that a viral RNA signal in the PCR assay does not necessarily mean that infectious virus is present,” Dr. Karn said. He added that new comprehensive viral RNA analyses would be needed to answer this question.
Official COVID-19 recovery
To identify risk factors and COVID-19 survivors more likely to retest positive, Dr. Landi and members of the Gemelli Against COVID-19 Post-Acute Care Study Group evaluated 131 people after hospital discharge.
All participants met World Health Organization criteria for release from isolation, including two negative test results at least 24 hours apart, and were studied between April 21 and May 21. Mean age was 56 and 39% were women. Only a slightly higher mean body mass index of 27.6 kg/m2 in the positive group versus 25.9 in the negative group, was significant.
Although 51% of survivors reported fatigue, 44% had dyspnea, and 17% were coughing, the rates did not differ significantly between groups. In contrast, 18% of positive survivors and 4% of negative survivors had a sore throat (P = .04), and 27% versus 12%, respectively, reported rhinitis (P = .05).
People returned for follow-up visits a mean 17 days after the second negative swab test.
Asymptomatic COVID-19 carriers
“These findings indicate that a noteworthy rate of recovered patients with COVID-19 could still be asymptomatic carriers of the virus,” the researchers noted in the paper. “Even in the absence of specific guidelines, the 22 patients who tested positive for COVID-19 again were suggested to quarantine for a second time.”
No family member or close contact of the positive survivors reported SARS-CoV-2 infection. All patients continued to wear masks and observe social distancing recommendations, which makes it “very difficult to affirm whether these patients were really contagious,” the researchers noted.
Next steps
Evaluating all COVID-19 survivors to identify any who retest positive “will be a crucial contribution to a better understanding of both the natural history of COVID-19 as well as the public health implications of viral shedding,” the authors wrote.
One study limitation is that the reverse transcriptase–PCR test reveals genetic sequences specific to COVID-19. “It is important to underline that this is not a viral culture and cannot determine whether the virus is viable and transmissible,” the researchers noted.
“In this respect, we are trying to better understand if the persistence of long-time positive [reverse transcriptase]–PCR test for COVID-19 is really correlated to a potential contagiousness,” they added.
Dr. Landi and colleagues said their findings should be considered preliminary, and larger data samples are warranted to validate the results.
Dr. Landi and Dr. Karn disclosed no relevant financial relationships. Dr. Iwasaki disclosed a research grant from Condair, a 5% or greater equity interest in RIGImmune, and income of $250 or more from PureTec.
A version of this article originally appeared on Medscape.com.
For reasons unknown, about one in six people who recovered from COVID-19 subsequently retested positive at least 2 weeks later, researchers reported in a study in Italy.
Sore throat and rhinitis were the only symptoms associated with a positive result. “Patients who continued to have respiratory symptoms, especially, were more likely to have a new positive test result,” lead author Francesco Landi, MD, PhD, said in an interview.
“This suggests the persistence of respiratory symptoms should not be underestimated and should be adequately assessed in all patients considered recovered from COVID-19,” he said.
“The study results are interesting,” Akiko Iwasaki, PhD, an immunobiologist at Yale University and the Howard Hughes Medical Institute, both in New Haven, Conn.,, said in an interview. “There are other reports of RNA detection postdischarge, but this study ... found that only two symptoms out of many – sore throat and rhinitis – were higher in those with PCR [polymerase chain reaction]-positive status.”
The study was published online Sept. 18 in the American Journal of Preventive Medicine.
The findings could carry important implications for people who continue to be symptomatic. “It is reasonable to be cautious and avoid close contact with others, wear a face mask, and possibly undergo an additional nasopharyngeal swab,” said Dr. Landi, associate professor of internal medicine at Catholic University of the Sacred Heart in Rome.
“One of most interesting findings is that persistent symptoms do not correlate with PCR positivity, suggesting that symptoms are in many cases not due to ongoing viral replication,” Jonathan Karn, PhD, professor and chair of the department of molecular biology and microbiology at Case Western Reserve University, Cleveland, said in an interview.
“The key technical problem, which they have discussed, is that a viral RNA signal in the PCR assay does not necessarily mean that infectious virus is present,” Dr. Karn said. He added that new comprehensive viral RNA analyses would be needed to answer this question.
Official COVID-19 recovery
To identify risk factors and COVID-19 survivors more likely to retest positive, Dr. Landi and members of the Gemelli Against COVID-19 Post-Acute Care Study Group evaluated 131 people after hospital discharge.
All participants met World Health Organization criteria for release from isolation, including two negative test results at least 24 hours apart, and were studied between April 21 and May 21. Mean age was 56 and 39% were women. Only a slightly higher mean body mass index of 27.6 kg/m2 in the positive group versus 25.9 in the negative group, was significant.
Although 51% of survivors reported fatigue, 44% had dyspnea, and 17% were coughing, the rates did not differ significantly between groups. In contrast, 18% of positive survivors and 4% of negative survivors had a sore throat (P = .04), and 27% versus 12%, respectively, reported rhinitis (P = .05).
People returned for follow-up visits a mean 17 days after the second negative swab test.
Asymptomatic COVID-19 carriers
“These findings indicate that a noteworthy rate of recovered patients with COVID-19 could still be asymptomatic carriers of the virus,” the researchers noted in the paper. “Even in the absence of specific guidelines, the 22 patients who tested positive for COVID-19 again were suggested to quarantine for a second time.”
No family member or close contact of the positive survivors reported SARS-CoV-2 infection. All patients continued to wear masks and observe social distancing recommendations, which makes it “very difficult to affirm whether these patients were really contagious,” the researchers noted.
Next steps
Evaluating all COVID-19 survivors to identify any who retest positive “will be a crucial contribution to a better understanding of both the natural history of COVID-19 as well as the public health implications of viral shedding,” the authors wrote.
One study limitation is that the reverse transcriptase–PCR test reveals genetic sequences specific to COVID-19. “It is important to underline that this is not a viral culture and cannot determine whether the virus is viable and transmissible,” the researchers noted.
“In this respect, we are trying to better understand if the persistence of long-time positive [reverse transcriptase]–PCR test for COVID-19 is really correlated to a potential contagiousness,” they added.
Dr. Landi and colleagues said their findings should be considered preliminary, and larger data samples are warranted to validate the results.
Dr. Landi and Dr. Karn disclosed no relevant financial relationships. Dr. Iwasaki disclosed a research grant from Condair, a 5% or greater equity interest in RIGImmune, and income of $250 or more from PureTec.
A version of this article originally appeared on Medscape.com.
For reasons unknown, about one in six people who recovered from COVID-19 subsequently retested positive at least 2 weeks later, researchers reported in a study in Italy.
Sore throat and rhinitis were the only symptoms associated with a positive result. “Patients who continued to have respiratory symptoms, especially, were more likely to have a new positive test result,” lead author Francesco Landi, MD, PhD, said in an interview.
“This suggests the persistence of respiratory symptoms should not be underestimated and should be adequately assessed in all patients considered recovered from COVID-19,” he said.
“The study results are interesting,” Akiko Iwasaki, PhD, an immunobiologist at Yale University and the Howard Hughes Medical Institute, both in New Haven, Conn.,, said in an interview. “There are other reports of RNA detection postdischarge, but this study ... found that only two symptoms out of many – sore throat and rhinitis – were higher in those with PCR [polymerase chain reaction]-positive status.”
The study was published online Sept. 18 in the American Journal of Preventive Medicine.
The findings could carry important implications for people who continue to be symptomatic. “It is reasonable to be cautious and avoid close contact with others, wear a face mask, and possibly undergo an additional nasopharyngeal swab,” said Dr. Landi, associate professor of internal medicine at Catholic University of the Sacred Heart in Rome.
“One of most interesting findings is that persistent symptoms do not correlate with PCR positivity, suggesting that symptoms are in many cases not due to ongoing viral replication,” Jonathan Karn, PhD, professor and chair of the department of molecular biology and microbiology at Case Western Reserve University, Cleveland, said in an interview.
“The key technical problem, which they have discussed, is that a viral RNA signal in the PCR assay does not necessarily mean that infectious virus is present,” Dr. Karn said. He added that new comprehensive viral RNA analyses would be needed to answer this question.
Official COVID-19 recovery
To identify risk factors and COVID-19 survivors more likely to retest positive, Dr. Landi and members of the Gemelli Against COVID-19 Post-Acute Care Study Group evaluated 131 people after hospital discharge.
All participants met World Health Organization criteria for release from isolation, including two negative test results at least 24 hours apart, and were studied between April 21 and May 21. Mean age was 56 and 39% were women. Only a slightly higher mean body mass index of 27.6 kg/m2 in the positive group versus 25.9 in the negative group, was significant.
Although 51% of survivors reported fatigue, 44% had dyspnea, and 17% were coughing, the rates did not differ significantly between groups. In contrast, 18% of positive survivors and 4% of negative survivors had a sore throat (P = .04), and 27% versus 12%, respectively, reported rhinitis (P = .05).
People returned for follow-up visits a mean 17 days after the second negative swab test.
Asymptomatic COVID-19 carriers
“These findings indicate that a noteworthy rate of recovered patients with COVID-19 could still be asymptomatic carriers of the virus,” the researchers noted in the paper. “Even in the absence of specific guidelines, the 22 patients who tested positive for COVID-19 again were suggested to quarantine for a second time.”
No family member or close contact of the positive survivors reported SARS-CoV-2 infection. All patients continued to wear masks and observe social distancing recommendations, which makes it “very difficult to affirm whether these patients were really contagious,” the researchers noted.
Next steps
Evaluating all COVID-19 survivors to identify any who retest positive “will be a crucial contribution to a better understanding of both the natural history of COVID-19 as well as the public health implications of viral shedding,” the authors wrote.
One study limitation is that the reverse transcriptase–PCR test reveals genetic sequences specific to COVID-19. “It is important to underline that this is not a viral culture and cannot determine whether the virus is viable and transmissible,” the researchers noted.
“In this respect, we are trying to better understand if the persistence of long-time positive [reverse transcriptase]–PCR test for COVID-19 is really correlated to a potential contagiousness,” they added.
Dr. Landi and colleagues said their findings should be considered preliminary, and larger data samples are warranted to validate the results.
Dr. Landi and Dr. Karn disclosed no relevant financial relationships. Dr. Iwasaki disclosed a research grant from Condair, a 5% or greater equity interest in RIGImmune, and income of $250 or more from PureTec.
A version of this article originally appeared on Medscape.com.
CDC panel takes on COVID vaccine rollout, risks, and side effects
Federal advisers who will help determine which Americans get the first COVID vaccines took an in-depth look Oct. 30 at the challenges they face in selecting priority groups.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) will face two key decisions once a COVID vaccine wins clearance from the US Food and Drug Administration (FDA).
ACIP will need to decide whether to recommend its use in adults (the age group in which vaccines are currently being tested). The group will also need to offer direction on which groups should get priority in vaccine allocation, inasmuch as early supplies will not be sufficient to vaccinate everyone.
At the Oct. 30 meeting, CDC’s Kathleen Dooling, MD, MPH, suggested that ACIP plan on tackling these issues as two separate questions when it comes time to weigh in on an approved vaccine. Although there was no formal vote among ACIP members at the meeting, Dooling’s proposal for tackling a future recommendation in a two-part fashion drew positive feedback.
ACIP member Katherine A. Poehling, MD, MPH, suggested that the panel and CDC be ready to reexamine the situation frequently regarding COVID vaccination. “Perhaps we could think about reviewing data on a monthly basis and updating the recommendation, so that we can account for the concerns and balance both the benefits and the [potential] harm,” Poehling said.
Dooling agreed. “Both the vaccine recommendation and allocation will be revisited in what is a very dynamic situation,” Dooling replied to Poehling. “So all new evidence will be brought to ACIP, and certainly the allocation as vaccine distribution proceeds will need to be adjusted accordingly.”
Ethics and limited evidence
During the meeting, ACIP members repeatedly expressed discomfort with the prospect of having to weigh in on widespread use of COVID vaccines on the basis of limited evidence.
Within months, FDA may opt for a special clearance, known as an emergency use authorization (EUA), for one or more of the experimental COVID vaccines now in advanced testing. Many of FDA’s past EUA clearances were granted for test kits. For those EUA approvals, the agency considered risks of false results but not longer-term, direct harm to patients from these products.
With a COVID vaccine, there will be strong pressure to distribute doses as quickly as possible with the hope of curbing the pandemic, which has already led to more than 229,000 deaths in the United States alone and has disrupted lives and economies around the world. But questions will persist about the possibility of serious complications from these vaccines, ACIP members noted.
“My personal struggle is the ethical side and how to balance these two,” said ACIP member Robert L. Atmar, MD, of Baylor College of Medicine, Houston, Texas, who noted that he expects his fellow panelists to share this concern.
Currently, four experimental COVID vaccines likely to be used in the United States have advanced to phase 3 testing. Pfizer Inc and BioNtech have enrolled more than 42,000 participants in a test of their candidate, BNT162b2 vaccine, and rival Moderna has enrolled about 30,000 participants in a test of its mRNA-1273 vaccine, CDC staff said.
The other two advanced COVID vaccine candidates have overcome recent hurdles. AstraZeneca Plc on Oct. 23 announced that FDA had removed a hold on the testing of its AZD1222 vaccine candidate; the trial will enroll approximately 30,000 people. Johnson & Johnson’s Janssen unit also announced that day the lifting of a safety pause for its Ad26.COV2.S vaccine; the phase 3 trial for that vaccine will enroll approximately 60,000 volunteers. Federal agencies, states, and territories have developed plans for future distribution of COVID vaccines, CDC staff said in briefing materials for today’s ACIP meeting.
Several ACIP members raised many of the same concerns that members of an FDA advisory committee raised at a meeting earlier in October. ACIP and FDA advisers honed in on the FDA’s decision to set a median follow-up duration of 2 months in phase 3 trials in connection with expected EUA applications for COVID-19 vaccines.
“I struggle with following people for 2 months after their second vaccination as a time point to start making final decisions about safety,” said ACIP member Sharon E. Frey, MD, a professor at St. Louis University School of Medicine, St. Louis, Missouri. “I just want to put that out there.”
Medical front line, then who?
There is consensus that healthcare workers be in the first stage ― Phase 1 ― of distribution. That recommendation was made in a report from the National Academies of Sciences, Engineering, and Medicine (NASEM). Phase 1A would include first responders; Phase 1B might include people of all ages who have two or more comorbidities that put them at significantly higher risk for COVID-19 or death, as well as older adults living in congregate or overcrowded settings, the NASEM report said.
A presentation from the CDC’s Matthew Biggerstaff, ScD, MPH, underscored challenges in distributing what are expected to be limited initial supplies of COVID vaccines.
Biggerstaff showed several scenarios the CDC’s Data, Analytics, and Modeling Task Force had studied. The initial allocation of vaccines would be for healthcare workers, followed by what the CDC called Phase 1B.
Choices for a rollout may include next giving COVID vaccines to people at high risk, such as persons who have one or more chronic medical conditions, including heart disease, diabetes, kidney disease, or obesity. Other options for the rollout could be to vaccinate people aged 65 years and older or essential workers whose employment puts them in contact with the public, thus raising the risk of contracting the virus.
The CDC’s research found that the greatest impact in preventing death was to initially vaccinate adults aged 65 and older in Phase 1B. The agency staff described this approach as likely to result in an about “1 to 11% increase in averted deaths across the scenarios.”
Initially vaccinating essential workers or high-risk adults in Phase 1B would avert the most infections. The agency staff described this approach as yielding about “1 to 5% increase in averted infections across the scenarios,” Biggerstaff said during his presentation.
The following are other findings of the CDC staff:
The earlier the vaccine rollout relative to increasing transmission, the greater the averted percentage and differences between the strategies.
Differences were not substantial in some scenarios.
The need to continue efforts to slow the spread of COVID-19 should be emphasized.
Adverse effects
ACIP members also heard about strategies for tracking potential side effects of future vaccines. A presentation by Tom Shimabukuro, MD, MPH, MBA, from the CDC’s COVID-19 Vaccine Task Force/Vaccine Safety Team, included details about a new smartphone-based active surveillance program for COVID-19 vaccine safety.
Known as v-safe, this system would use Web-based survey monitoring and incorporate text messaging. It would conduct electronic health checks on vaccine recipients, which would occur daily during the first week post vaccination and weekly thereafter for 6 weeks from the time of vaccination.
Clinicians “can play an important role in helping CDC enroll patients in v-safe at the time of vaccination,” Shimabukuro noted in his presentation. This would add another task, though, for clinicians, the CDC staff noted.
Pregnancy and breastfeeding are special concerns
Of special concern with the rollout of a COVID vaccine are recommendations regarding pregnancy and breastfeeding. Women constitute about 75% of the healthcare workforce, CDC staff noted.
At the time the initial ACIP COVID vaccination recommendations are made, there could be approximately 330,000 healthcare personnel who are pregnant or who have recently given birth. Available data indicate potentially increased risks for severe maternal illness and preterm birth associated with SARS-CoV-2 infection, said CDC’s Megan Wallace, DrPH, MPH, in a presentation for the Friday meeting.
In an Oct. 27 letter to ACIP, Chair Jose Romero, the American College of Obstetricians and Gynecologists (ACOG), urged the panel to ensure that pregnant women and new mothers in the healthcare workforce have priority access to a COVID vaccine. Pregnant and lactating women were “noticeably and alarmingly absent from the NASEM vaccine allocation plan for COVID-19,” wrote Christopher M. Zahn, MD, vice president for practice activities at ACOG, in the letter to Romero.
“ACOG urges ACIP to incorporate pregnant and lactating women clearly and explicitly into its COVID-19 vaccine allocation and prioritization framework,” Zahn wrote. “Should an Emergency Use Authorization be executed for one or more COVID-19 vaccines and provide a permissive recommendation for pregnant and lactating women, pregnant health care workers, pregnant first responders, and pregnant individuals with underlying conditions should be prioritized for vaccination alongside their non-pregnant peers.”
This article first appeared on Medscape.com.
Federal advisers who will help determine which Americans get the first COVID vaccines took an in-depth look Oct. 30 at the challenges they face in selecting priority groups.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) will face two key decisions once a COVID vaccine wins clearance from the US Food and Drug Administration (FDA).
ACIP will need to decide whether to recommend its use in adults (the age group in which vaccines are currently being tested). The group will also need to offer direction on which groups should get priority in vaccine allocation, inasmuch as early supplies will not be sufficient to vaccinate everyone.
At the Oct. 30 meeting, CDC’s Kathleen Dooling, MD, MPH, suggested that ACIP plan on tackling these issues as two separate questions when it comes time to weigh in on an approved vaccine. Although there was no formal vote among ACIP members at the meeting, Dooling’s proposal for tackling a future recommendation in a two-part fashion drew positive feedback.
ACIP member Katherine A. Poehling, MD, MPH, suggested that the panel and CDC be ready to reexamine the situation frequently regarding COVID vaccination. “Perhaps we could think about reviewing data on a monthly basis and updating the recommendation, so that we can account for the concerns and balance both the benefits and the [potential] harm,” Poehling said.
Dooling agreed. “Both the vaccine recommendation and allocation will be revisited in what is a very dynamic situation,” Dooling replied to Poehling. “So all new evidence will be brought to ACIP, and certainly the allocation as vaccine distribution proceeds will need to be adjusted accordingly.”
Ethics and limited evidence
During the meeting, ACIP members repeatedly expressed discomfort with the prospect of having to weigh in on widespread use of COVID vaccines on the basis of limited evidence.
Within months, FDA may opt for a special clearance, known as an emergency use authorization (EUA), for one or more of the experimental COVID vaccines now in advanced testing. Many of FDA’s past EUA clearances were granted for test kits. For those EUA approvals, the agency considered risks of false results but not longer-term, direct harm to patients from these products.
With a COVID vaccine, there will be strong pressure to distribute doses as quickly as possible with the hope of curbing the pandemic, which has already led to more than 229,000 deaths in the United States alone and has disrupted lives and economies around the world. But questions will persist about the possibility of serious complications from these vaccines, ACIP members noted.
“My personal struggle is the ethical side and how to balance these two,” said ACIP member Robert L. Atmar, MD, of Baylor College of Medicine, Houston, Texas, who noted that he expects his fellow panelists to share this concern.
Currently, four experimental COVID vaccines likely to be used in the United States have advanced to phase 3 testing. Pfizer Inc and BioNtech have enrolled more than 42,000 participants in a test of their candidate, BNT162b2 vaccine, and rival Moderna has enrolled about 30,000 participants in a test of its mRNA-1273 vaccine, CDC staff said.
The other two advanced COVID vaccine candidates have overcome recent hurdles. AstraZeneca Plc on Oct. 23 announced that FDA had removed a hold on the testing of its AZD1222 vaccine candidate; the trial will enroll approximately 30,000 people. Johnson & Johnson’s Janssen unit also announced that day the lifting of a safety pause for its Ad26.COV2.S vaccine; the phase 3 trial for that vaccine will enroll approximately 60,000 volunteers. Federal agencies, states, and territories have developed plans for future distribution of COVID vaccines, CDC staff said in briefing materials for today’s ACIP meeting.
Several ACIP members raised many of the same concerns that members of an FDA advisory committee raised at a meeting earlier in October. ACIP and FDA advisers honed in on the FDA’s decision to set a median follow-up duration of 2 months in phase 3 trials in connection with expected EUA applications for COVID-19 vaccines.
“I struggle with following people for 2 months after their second vaccination as a time point to start making final decisions about safety,” said ACIP member Sharon E. Frey, MD, a professor at St. Louis University School of Medicine, St. Louis, Missouri. “I just want to put that out there.”
Medical front line, then who?
There is consensus that healthcare workers be in the first stage ― Phase 1 ― of distribution. That recommendation was made in a report from the National Academies of Sciences, Engineering, and Medicine (NASEM). Phase 1A would include first responders; Phase 1B might include people of all ages who have two or more comorbidities that put them at significantly higher risk for COVID-19 or death, as well as older adults living in congregate or overcrowded settings, the NASEM report said.
A presentation from the CDC’s Matthew Biggerstaff, ScD, MPH, underscored challenges in distributing what are expected to be limited initial supplies of COVID vaccines.
Biggerstaff showed several scenarios the CDC’s Data, Analytics, and Modeling Task Force had studied. The initial allocation of vaccines would be for healthcare workers, followed by what the CDC called Phase 1B.
Choices for a rollout may include next giving COVID vaccines to people at high risk, such as persons who have one or more chronic medical conditions, including heart disease, diabetes, kidney disease, or obesity. Other options for the rollout could be to vaccinate people aged 65 years and older or essential workers whose employment puts them in contact with the public, thus raising the risk of contracting the virus.
The CDC’s research found that the greatest impact in preventing death was to initially vaccinate adults aged 65 and older in Phase 1B. The agency staff described this approach as likely to result in an about “1 to 11% increase in averted deaths across the scenarios.”
Initially vaccinating essential workers or high-risk adults in Phase 1B would avert the most infections. The agency staff described this approach as yielding about “1 to 5% increase in averted infections across the scenarios,” Biggerstaff said during his presentation.
The following are other findings of the CDC staff:
The earlier the vaccine rollout relative to increasing transmission, the greater the averted percentage and differences between the strategies.
Differences were not substantial in some scenarios.
The need to continue efforts to slow the spread of COVID-19 should be emphasized.
Adverse effects
ACIP members also heard about strategies for tracking potential side effects of future vaccines. A presentation by Tom Shimabukuro, MD, MPH, MBA, from the CDC’s COVID-19 Vaccine Task Force/Vaccine Safety Team, included details about a new smartphone-based active surveillance program for COVID-19 vaccine safety.
Known as v-safe, this system would use Web-based survey monitoring and incorporate text messaging. It would conduct electronic health checks on vaccine recipients, which would occur daily during the first week post vaccination and weekly thereafter for 6 weeks from the time of vaccination.
Clinicians “can play an important role in helping CDC enroll patients in v-safe at the time of vaccination,” Shimabukuro noted in his presentation. This would add another task, though, for clinicians, the CDC staff noted.
Pregnancy and breastfeeding are special concerns
Of special concern with the rollout of a COVID vaccine are recommendations regarding pregnancy and breastfeeding. Women constitute about 75% of the healthcare workforce, CDC staff noted.
At the time the initial ACIP COVID vaccination recommendations are made, there could be approximately 330,000 healthcare personnel who are pregnant or who have recently given birth. Available data indicate potentially increased risks for severe maternal illness and preterm birth associated with SARS-CoV-2 infection, said CDC’s Megan Wallace, DrPH, MPH, in a presentation for the Friday meeting.
In an Oct. 27 letter to ACIP, Chair Jose Romero, the American College of Obstetricians and Gynecologists (ACOG), urged the panel to ensure that pregnant women and new mothers in the healthcare workforce have priority access to a COVID vaccine. Pregnant and lactating women were “noticeably and alarmingly absent from the NASEM vaccine allocation plan for COVID-19,” wrote Christopher M. Zahn, MD, vice president for practice activities at ACOG, in the letter to Romero.
“ACOG urges ACIP to incorporate pregnant and lactating women clearly and explicitly into its COVID-19 vaccine allocation and prioritization framework,” Zahn wrote. “Should an Emergency Use Authorization be executed for one or more COVID-19 vaccines and provide a permissive recommendation for pregnant and lactating women, pregnant health care workers, pregnant first responders, and pregnant individuals with underlying conditions should be prioritized for vaccination alongside their non-pregnant peers.”
This article first appeared on Medscape.com.
Federal advisers who will help determine which Americans get the first COVID vaccines took an in-depth look Oct. 30 at the challenges they face in selecting priority groups.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) will face two key decisions once a COVID vaccine wins clearance from the US Food and Drug Administration (FDA).
ACIP will need to decide whether to recommend its use in adults (the age group in which vaccines are currently being tested). The group will also need to offer direction on which groups should get priority in vaccine allocation, inasmuch as early supplies will not be sufficient to vaccinate everyone.
At the Oct. 30 meeting, CDC’s Kathleen Dooling, MD, MPH, suggested that ACIP plan on tackling these issues as two separate questions when it comes time to weigh in on an approved vaccine. Although there was no formal vote among ACIP members at the meeting, Dooling’s proposal for tackling a future recommendation in a two-part fashion drew positive feedback.
ACIP member Katherine A. Poehling, MD, MPH, suggested that the panel and CDC be ready to reexamine the situation frequently regarding COVID vaccination. “Perhaps we could think about reviewing data on a monthly basis and updating the recommendation, so that we can account for the concerns and balance both the benefits and the [potential] harm,” Poehling said.
Dooling agreed. “Both the vaccine recommendation and allocation will be revisited in what is a very dynamic situation,” Dooling replied to Poehling. “So all new evidence will be brought to ACIP, and certainly the allocation as vaccine distribution proceeds will need to be adjusted accordingly.”
Ethics and limited evidence
During the meeting, ACIP members repeatedly expressed discomfort with the prospect of having to weigh in on widespread use of COVID vaccines on the basis of limited evidence.
Within months, FDA may opt for a special clearance, known as an emergency use authorization (EUA), for one or more of the experimental COVID vaccines now in advanced testing. Many of FDA’s past EUA clearances were granted for test kits. For those EUA approvals, the agency considered risks of false results but not longer-term, direct harm to patients from these products.
With a COVID vaccine, there will be strong pressure to distribute doses as quickly as possible with the hope of curbing the pandemic, which has already led to more than 229,000 deaths in the United States alone and has disrupted lives and economies around the world. But questions will persist about the possibility of serious complications from these vaccines, ACIP members noted.
“My personal struggle is the ethical side and how to balance these two,” said ACIP member Robert L. Atmar, MD, of Baylor College of Medicine, Houston, Texas, who noted that he expects his fellow panelists to share this concern.
Currently, four experimental COVID vaccines likely to be used in the United States have advanced to phase 3 testing. Pfizer Inc and BioNtech have enrolled more than 42,000 participants in a test of their candidate, BNT162b2 vaccine, and rival Moderna has enrolled about 30,000 participants in a test of its mRNA-1273 vaccine, CDC staff said.
The other two advanced COVID vaccine candidates have overcome recent hurdles. AstraZeneca Plc on Oct. 23 announced that FDA had removed a hold on the testing of its AZD1222 vaccine candidate; the trial will enroll approximately 30,000 people. Johnson & Johnson’s Janssen unit also announced that day the lifting of a safety pause for its Ad26.COV2.S vaccine; the phase 3 trial for that vaccine will enroll approximately 60,000 volunteers. Federal agencies, states, and territories have developed plans for future distribution of COVID vaccines, CDC staff said in briefing materials for today’s ACIP meeting.
Several ACIP members raised many of the same concerns that members of an FDA advisory committee raised at a meeting earlier in October. ACIP and FDA advisers honed in on the FDA’s decision to set a median follow-up duration of 2 months in phase 3 trials in connection with expected EUA applications for COVID-19 vaccines.
“I struggle with following people for 2 months after their second vaccination as a time point to start making final decisions about safety,” said ACIP member Sharon E. Frey, MD, a professor at St. Louis University School of Medicine, St. Louis, Missouri. “I just want to put that out there.”
Medical front line, then who?
There is consensus that healthcare workers be in the first stage ― Phase 1 ― of distribution. That recommendation was made in a report from the National Academies of Sciences, Engineering, and Medicine (NASEM). Phase 1A would include first responders; Phase 1B might include people of all ages who have two or more comorbidities that put them at significantly higher risk for COVID-19 or death, as well as older adults living in congregate or overcrowded settings, the NASEM report said.
A presentation from the CDC’s Matthew Biggerstaff, ScD, MPH, underscored challenges in distributing what are expected to be limited initial supplies of COVID vaccines.
Biggerstaff showed several scenarios the CDC’s Data, Analytics, and Modeling Task Force had studied. The initial allocation of vaccines would be for healthcare workers, followed by what the CDC called Phase 1B.
Choices for a rollout may include next giving COVID vaccines to people at high risk, such as persons who have one or more chronic medical conditions, including heart disease, diabetes, kidney disease, or obesity. Other options for the rollout could be to vaccinate people aged 65 years and older or essential workers whose employment puts them in contact with the public, thus raising the risk of contracting the virus.
The CDC’s research found that the greatest impact in preventing death was to initially vaccinate adults aged 65 and older in Phase 1B. The agency staff described this approach as likely to result in an about “1 to 11% increase in averted deaths across the scenarios.”
Initially vaccinating essential workers or high-risk adults in Phase 1B would avert the most infections. The agency staff described this approach as yielding about “1 to 5% increase in averted infections across the scenarios,” Biggerstaff said during his presentation.
The following are other findings of the CDC staff:
The earlier the vaccine rollout relative to increasing transmission, the greater the averted percentage and differences between the strategies.
Differences were not substantial in some scenarios.
The need to continue efforts to slow the spread of COVID-19 should be emphasized.
Adverse effects
ACIP members also heard about strategies for tracking potential side effects of future vaccines. A presentation by Tom Shimabukuro, MD, MPH, MBA, from the CDC’s COVID-19 Vaccine Task Force/Vaccine Safety Team, included details about a new smartphone-based active surveillance program for COVID-19 vaccine safety.
Known as v-safe, this system would use Web-based survey monitoring and incorporate text messaging. It would conduct electronic health checks on vaccine recipients, which would occur daily during the first week post vaccination and weekly thereafter for 6 weeks from the time of vaccination.
Clinicians “can play an important role in helping CDC enroll patients in v-safe at the time of vaccination,” Shimabukuro noted in his presentation. This would add another task, though, for clinicians, the CDC staff noted.
Pregnancy and breastfeeding are special concerns
Of special concern with the rollout of a COVID vaccine are recommendations regarding pregnancy and breastfeeding. Women constitute about 75% of the healthcare workforce, CDC staff noted.
At the time the initial ACIP COVID vaccination recommendations are made, there could be approximately 330,000 healthcare personnel who are pregnant or who have recently given birth. Available data indicate potentially increased risks for severe maternal illness and preterm birth associated with SARS-CoV-2 infection, said CDC’s Megan Wallace, DrPH, MPH, in a presentation for the Friday meeting.
In an Oct. 27 letter to ACIP, Chair Jose Romero, the American College of Obstetricians and Gynecologists (ACOG), urged the panel to ensure that pregnant women and new mothers in the healthcare workforce have priority access to a COVID vaccine. Pregnant and lactating women were “noticeably and alarmingly absent from the NASEM vaccine allocation plan for COVID-19,” wrote Christopher M. Zahn, MD, vice president for practice activities at ACOG, in the letter to Romero.
“ACOG urges ACIP to incorporate pregnant and lactating women clearly and explicitly into its COVID-19 vaccine allocation and prioritization framework,” Zahn wrote. “Should an Emergency Use Authorization be executed for one or more COVID-19 vaccines and provide a permissive recommendation for pregnant and lactating women, pregnant health care workers, pregnant first responders, and pregnant individuals with underlying conditions should be prioritized for vaccination alongside their non-pregnant peers.”
This article first appeared on Medscape.com.
No evidence to guide selection of biologic for severe asthma
Although “biologics have been really revolutionary for the treatment of severe uncontrolled asthma, we still don’t have evidence to know the right drug for the right patient,” said Wendy Moore, MD, of Wake Forest University, Winston-Salem, N.C.
“You start with your best guess and then switch,” she said in an interview.
There are no real-world contemporary measurements of biologic therapy in the United States at this time, Dr. Moore explained during her presentation of findings from the CHRONICLE trial at the annual meeting of the American College of Chest Physicians (CHEST 2020), held virtually this year.
The agents have different targets: omalizumab targets immunoglobulin E, mepolizumab and reslizumab target interleukin (IL)-5, benralizumab targets the IL-5 receptor, and dupilumab targets the common receptor IL-4 receptor A for IL-4 and IL-13.
When the starting biologic doesn’t get the desired results, there is no evidence to show whether another will work better. What we say is, “This one is not working as well as I’d like, let’s try something new?” said Dr. Moore.
However, when looking at data on patients with severe asthma who change from one biologic to another, “I was actually pleased to see that only 10% are switching,” she said in an interview.
But, she added, “if you add that up with the 8% who are stopping, that means that almost 20% don’t get the clinical response they want.”
CHRONICLE trial
In the ongoing observational CHRONICLE trial, Dr. Moore and colleagues assessed biologic initiations, discontinuations, and switches to a different agent.
All 1,884 study participants had a diagnosis of severe asthma and were being treated by an allergist/immunologist or a pulmonologist. All were taking high-dose inhaled corticosteroids and additional controllers, or had received an Food and Drug Administration–approved monoclonal antibody, systemic corticosteroid, or another systemic immunosuppressant for at least half of the previous 12 months.
In the study cohort, 1,219 participants were receiving one biologic and 27 were receiving two.
Before November 2018, “it was almost universally all benralizumab being prescribed.” An earlier preference was omalizumab, which was prescribed to 99% of patients before November 2015 and to 45% from November 2017 to November 2018.
“As new drugs were introduced, patients were switched if the desired outcome was not achieved,” Dr. Moore explained.
Over the 2-year period from February 2018 to February 2020, 134 patients – about 10% of all participants taking a biologic – made 148 switches to another biologic.
“The most common reasons reported for switching were lack of efficacy, worsening of asthma control, or waning efficacy,” Dr. Moore reported.
Of the 101 patients (8%) who discontinued 106 biologics, reasons cited were a worsening of asthma symptoms, a desire to change to a cheaper medication, and a waning of effectiveness.
“It seems that the biologic used depended on when you started and whether you were prescribed by an immunologist or pulmonologist,” said Dr. Moore. “I don’t think we understand the perfect patient for any one of these drugs.”
Large-population studies need to be done on each of the drugs. “You have to look at who’s the super responder, the partial responder, compared with the nonresponders, for each medication, but those comparative studies are unlikely to happen,” she said.
In her own practice, her 175 patients are “pretty evenly split between dupilumab, benralizumab, and mepolizumab.”
I have opinions on what works, said Dr. Moore, but none of it is evidence-based. “Those with upper airway involvement with chronic sinusitis tend to do better with mepolizumab than benralizumab. My opinion,” she emphasized.
“People with nasal problems may do better with dupilumab and mepolizumab,” she added. “Also in my opinion.
“But more likely, the issue is you have a partial responder who’s on a T2 high drug but has a T2 low problem too.”
PATHWAY study
Findings from the phase 2B PATHWAY study showed that tezepelumab reduced exacerbations in patients with uncontrolled asthma better than inhaled corticosteroids, and improved forced expiratory volume in 1 second.
“Adherence was monitored very carefully,” said investigator Jonathan Corren, MD, of the University of California, Los Angeles, who presented the PATHWAY data. This could explain, in part, why some patients in the control group “showed improvement from baseline.”
Before switching to a biologic, “we should always consider some of these issues that might contribute to better asthma control, like patient adherence or the inability to use an inhaler properly,” Dr. Corren said.
Some people have never been “shown how to use their inhalers properly,” said Moore. “Some of them come back fine when we show them.”
Dr. Moore has been on the advisory board for AstraZeneca, Genentech, GlaxoSmithKline (GSK), Regeneron, and Sanofi. Dr. Corren reports receiving honoraria from AstraZeneca.
A version of this article originally appeared on Medscape.com.
Although “biologics have been really revolutionary for the treatment of severe uncontrolled asthma, we still don’t have evidence to know the right drug for the right patient,” said Wendy Moore, MD, of Wake Forest University, Winston-Salem, N.C.
“You start with your best guess and then switch,” she said in an interview.
There are no real-world contemporary measurements of biologic therapy in the United States at this time, Dr. Moore explained during her presentation of findings from the CHRONICLE trial at the annual meeting of the American College of Chest Physicians (CHEST 2020), held virtually this year.
The agents have different targets: omalizumab targets immunoglobulin E, mepolizumab and reslizumab target interleukin (IL)-5, benralizumab targets the IL-5 receptor, and dupilumab targets the common receptor IL-4 receptor A for IL-4 and IL-13.
When the starting biologic doesn’t get the desired results, there is no evidence to show whether another will work better. What we say is, “This one is not working as well as I’d like, let’s try something new?” said Dr. Moore.
However, when looking at data on patients with severe asthma who change from one biologic to another, “I was actually pleased to see that only 10% are switching,” she said in an interview.
But, she added, “if you add that up with the 8% who are stopping, that means that almost 20% don’t get the clinical response they want.”
CHRONICLE trial
In the ongoing observational CHRONICLE trial, Dr. Moore and colleagues assessed biologic initiations, discontinuations, and switches to a different agent.
All 1,884 study participants had a diagnosis of severe asthma and were being treated by an allergist/immunologist or a pulmonologist. All were taking high-dose inhaled corticosteroids and additional controllers, or had received an Food and Drug Administration–approved monoclonal antibody, systemic corticosteroid, or another systemic immunosuppressant for at least half of the previous 12 months.
In the study cohort, 1,219 participants were receiving one biologic and 27 were receiving two.
Before November 2018, “it was almost universally all benralizumab being prescribed.” An earlier preference was omalizumab, which was prescribed to 99% of patients before November 2015 and to 45% from November 2017 to November 2018.
“As new drugs were introduced, patients were switched if the desired outcome was not achieved,” Dr. Moore explained.
Over the 2-year period from February 2018 to February 2020, 134 patients – about 10% of all participants taking a biologic – made 148 switches to another biologic.
“The most common reasons reported for switching were lack of efficacy, worsening of asthma control, or waning efficacy,” Dr. Moore reported.
Of the 101 patients (8%) who discontinued 106 biologics, reasons cited were a worsening of asthma symptoms, a desire to change to a cheaper medication, and a waning of effectiveness.
“It seems that the biologic used depended on when you started and whether you were prescribed by an immunologist or pulmonologist,” said Dr. Moore. “I don’t think we understand the perfect patient for any one of these drugs.”
Large-population studies need to be done on each of the drugs. “You have to look at who’s the super responder, the partial responder, compared with the nonresponders, for each medication, but those comparative studies are unlikely to happen,” she said.
In her own practice, her 175 patients are “pretty evenly split between dupilumab, benralizumab, and mepolizumab.”
I have opinions on what works, said Dr. Moore, but none of it is evidence-based. “Those with upper airway involvement with chronic sinusitis tend to do better with mepolizumab than benralizumab. My opinion,” she emphasized.
“People with nasal problems may do better with dupilumab and mepolizumab,” she added. “Also in my opinion.
“But more likely, the issue is you have a partial responder who’s on a T2 high drug but has a T2 low problem too.”
PATHWAY study
Findings from the phase 2B PATHWAY study showed that tezepelumab reduced exacerbations in patients with uncontrolled asthma better than inhaled corticosteroids, and improved forced expiratory volume in 1 second.
“Adherence was monitored very carefully,” said investigator Jonathan Corren, MD, of the University of California, Los Angeles, who presented the PATHWAY data. This could explain, in part, why some patients in the control group “showed improvement from baseline.”
Before switching to a biologic, “we should always consider some of these issues that might contribute to better asthma control, like patient adherence or the inability to use an inhaler properly,” Dr. Corren said.
Some people have never been “shown how to use their inhalers properly,” said Moore. “Some of them come back fine when we show them.”
Dr. Moore has been on the advisory board for AstraZeneca, Genentech, GlaxoSmithKline (GSK), Regeneron, and Sanofi. Dr. Corren reports receiving honoraria from AstraZeneca.
A version of this article originally appeared on Medscape.com.
Although “biologics have been really revolutionary for the treatment of severe uncontrolled asthma, we still don’t have evidence to know the right drug for the right patient,” said Wendy Moore, MD, of Wake Forest University, Winston-Salem, N.C.
“You start with your best guess and then switch,” she said in an interview.
There are no real-world contemporary measurements of biologic therapy in the United States at this time, Dr. Moore explained during her presentation of findings from the CHRONICLE trial at the annual meeting of the American College of Chest Physicians (CHEST 2020), held virtually this year.
The agents have different targets: omalizumab targets immunoglobulin E, mepolizumab and reslizumab target interleukin (IL)-5, benralizumab targets the IL-5 receptor, and dupilumab targets the common receptor IL-4 receptor A for IL-4 and IL-13.
When the starting biologic doesn’t get the desired results, there is no evidence to show whether another will work better. What we say is, “This one is not working as well as I’d like, let’s try something new?” said Dr. Moore.
However, when looking at data on patients with severe asthma who change from one biologic to another, “I was actually pleased to see that only 10% are switching,” she said in an interview.
But, she added, “if you add that up with the 8% who are stopping, that means that almost 20% don’t get the clinical response they want.”
CHRONICLE trial
In the ongoing observational CHRONICLE trial, Dr. Moore and colleagues assessed biologic initiations, discontinuations, and switches to a different agent.
All 1,884 study participants had a diagnosis of severe asthma and were being treated by an allergist/immunologist or a pulmonologist. All were taking high-dose inhaled corticosteroids and additional controllers, or had received an Food and Drug Administration–approved monoclonal antibody, systemic corticosteroid, or another systemic immunosuppressant for at least half of the previous 12 months.
In the study cohort, 1,219 participants were receiving one biologic and 27 were receiving two.
Before November 2018, “it was almost universally all benralizumab being prescribed.” An earlier preference was omalizumab, which was prescribed to 99% of patients before November 2015 and to 45% from November 2017 to November 2018.
“As new drugs were introduced, patients were switched if the desired outcome was not achieved,” Dr. Moore explained.
Over the 2-year period from February 2018 to February 2020, 134 patients – about 10% of all participants taking a biologic – made 148 switches to another biologic.
“The most common reasons reported for switching were lack of efficacy, worsening of asthma control, or waning efficacy,” Dr. Moore reported.
Of the 101 patients (8%) who discontinued 106 biologics, reasons cited were a worsening of asthma symptoms, a desire to change to a cheaper medication, and a waning of effectiveness.
“It seems that the biologic used depended on when you started and whether you were prescribed by an immunologist or pulmonologist,” said Dr. Moore. “I don’t think we understand the perfect patient for any one of these drugs.”
Large-population studies need to be done on each of the drugs. “You have to look at who’s the super responder, the partial responder, compared with the nonresponders, for each medication, but those comparative studies are unlikely to happen,” she said.
In her own practice, her 175 patients are “pretty evenly split between dupilumab, benralizumab, and mepolizumab.”
I have opinions on what works, said Dr. Moore, but none of it is evidence-based. “Those with upper airway involvement with chronic sinusitis tend to do better with mepolizumab than benralizumab. My opinion,” she emphasized.
“People with nasal problems may do better with dupilumab and mepolizumab,” she added. “Also in my opinion.
“But more likely, the issue is you have a partial responder who’s on a T2 high drug but has a T2 low problem too.”
PATHWAY study
Findings from the phase 2B PATHWAY study showed that tezepelumab reduced exacerbations in patients with uncontrolled asthma better than inhaled corticosteroids, and improved forced expiratory volume in 1 second.
“Adherence was monitored very carefully,” said investigator Jonathan Corren, MD, of the University of California, Los Angeles, who presented the PATHWAY data. This could explain, in part, why some patients in the control group “showed improvement from baseline.”
Before switching to a biologic, “we should always consider some of these issues that might contribute to better asthma control, like patient adherence or the inability to use an inhaler properly,” Dr. Corren said.
Some people have never been “shown how to use their inhalers properly,” said Moore. “Some of them come back fine when we show them.”
Dr. Moore has been on the advisory board for AstraZeneca, Genentech, GlaxoSmithKline (GSK), Regeneron, and Sanofi. Dr. Corren reports receiving honoraria from AstraZeneca.
A version of this article originally appeared on Medscape.com.
Switching to riociguat effective for some patients with PAH not at treatment goal
In patients with intermediate-risk pulmonary arterial hypertension (PAH) who are not at treatment goal on standard therapy, switching to riociguat is a promising strategy across a broad range of patient subgroups, an investigator said at the annual meeting of the American College of Chest Physicians, held virtually this year.
Patients switching to riociguat in the REPLACE study more frequently met the primary efficacy endpoint, compared with patients who remained on a phosphodiesterase-5 (PDE5) inhibitor, said Marius M. Hoeper, MD, of the Clinic for Respiratory Medicine at Hannover (Germany) Medical School.
That clinical benefit of switching to riociguat, a soluble guanylate cyclase (sGC) stimulator, was relatively consistent across patient subgroups including age, sex, PAH subtype, according to Dr. Hoeper.
“At the end of the day, we believe that switching from a PDE5 inhibitor to riociguat can benefit patients with PAH at intermediate risk and may serve as a new strategic option for treatment escalation,” he said in a live virtual presentation of the study results.
About 40% of patients switching to riociguat met the primary endpoint of clinical improvement in absence of clinical worsening versus just 20% of patients who stayed on a PDE5 inhibitor, according to top-line results of the phase 4 REPLACE study, which were reported Sept. 7 at the annual meeting of the European Respiratory Society.
Results of REPLACE presented at the CHEST meeting show a benefit across most patient subgroups, including PAH subtype and whether patients came from monotherapy or combination treatment to riociguat. Some groups did not appear to respond quite as well to switching, including elderly patients, patients with a 6-minute walk distance (6MWD) of less than 320 meters at baseline, and patients switching from tadalafil as opposed to sildenafil. However, these findings were not statistically significant and may have been chance findings, according to Dr. Hoeper.
These results of REPLACE suggest the efficacy of riociguat “across the board” for intermediate-risk PAH patients with inadequate response to standard therapy, said Vijay Balasubramanian, MD, FCCP, clinical professor of medicine at the University of California San Francisco, Fresno.
Based on REPLACE results, switching from a PDE5 inhibitor to riociguat is now a “strong potential option” beyond adding a third drug such as selexipag or an inhaled prostacyclin to usual treatment with a PDE5 inhibitor plus an endothelin receptor antagonist, Dr. Balasubramanian said in an interview.
“We now have an evidence-based option where you can stay on a two-drug regimen and see whether the switch would work just as well,” said Dr. Balasubramanian, vice chair of the Pulmonary Vascular Disease Steering Committee for the American College of Chest Physicians.
REPLACE is a randomized phase 4 study including 226 patients with PAH considered to be at intermediate risk according to World Health Organization functional class III or 6MWD of 165-440 meters. The composite primary endpoint was defined as no clinical worsening (death, disease progression, or hospitalization for worsening PAH) plus clinical improvement on at least two measures including an improvement in 6MWD, achieving WHO functional class I/II, or a decrease in N-terminal pro-brain natriuretic peptide (NT-proBNP).
The primary endpoint of REPLACE was met, showing that 45 patients (41%) who switched to riociguat had clinical improvement without clinical worsening versus 22 patient (20%) who stayed on the PDE5 inhibitor (odds ratio, 2.78; 95% confidence interval, 1.53-5.06; P = .0007), Dr. Hoeper reported.
The benefit appeared consistent across PAH subgroups, according to Dr. Hoeper. In patients with idiopathic, heritable, or drug- and toxin-induced PAH, the primary endpoint favored riociguat over PDE5 inhibitor, at 45% and 23%, respectively. Similarly, a higher proportion of patients with PAH associated with congenital heart disease or portal hypertension achieved the primary endpoint (46% vs. 8%), as did patients with PAH associated with connective tissue disease (25% vs. 16%).
Adverse events were seen in 71% of riociguat-treated patients and 66% of PDE5 inhibitor–treated patients, according to Dr. Hoeper, who said severe adverse events were more frequent with PDE5-inhibitor treatment, at 17% versus 7% for riociguat. There were three clinical worsening events in the PDE5 inhibitor group leading to death, while a fourth patient died in safety follow-up, according to the reported results, whereas there were no deaths reported with riociguat.
The REPLACE study was cofunded by Bayer AG and Merck Sharpe & Dohme, a subsidiary of Merck & Co. Dr. Hoeper reported receiving fees for consultations or lectures from Acceleron, Actelion, Bayer AG, Janssen, MSD, and Pfizer.
SOURCE: Hoeper MM. CHEST 2020, Abstract A2156-A2159.
In patients with intermediate-risk pulmonary arterial hypertension (PAH) who are not at treatment goal on standard therapy, switching to riociguat is a promising strategy across a broad range of patient subgroups, an investigator said at the annual meeting of the American College of Chest Physicians, held virtually this year.
Patients switching to riociguat in the REPLACE study more frequently met the primary efficacy endpoint, compared with patients who remained on a phosphodiesterase-5 (PDE5) inhibitor, said Marius M. Hoeper, MD, of the Clinic for Respiratory Medicine at Hannover (Germany) Medical School.
That clinical benefit of switching to riociguat, a soluble guanylate cyclase (sGC) stimulator, was relatively consistent across patient subgroups including age, sex, PAH subtype, according to Dr. Hoeper.
“At the end of the day, we believe that switching from a PDE5 inhibitor to riociguat can benefit patients with PAH at intermediate risk and may serve as a new strategic option for treatment escalation,” he said in a live virtual presentation of the study results.
About 40% of patients switching to riociguat met the primary endpoint of clinical improvement in absence of clinical worsening versus just 20% of patients who stayed on a PDE5 inhibitor, according to top-line results of the phase 4 REPLACE study, which were reported Sept. 7 at the annual meeting of the European Respiratory Society.
Results of REPLACE presented at the CHEST meeting show a benefit across most patient subgroups, including PAH subtype and whether patients came from monotherapy or combination treatment to riociguat. Some groups did not appear to respond quite as well to switching, including elderly patients, patients with a 6-minute walk distance (6MWD) of less than 320 meters at baseline, and patients switching from tadalafil as opposed to sildenafil. However, these findings were not statistically significant and may have been chance findings, according to Dr. Hoeper.
These results of REPLACE suggest the efficacy of riociguat “across the board” for intermediate-risk PAH patients with inadequate response to standard therapy, said Vijay Balasubramanian, MD, FCCP, clinical professor of medicine at the University of California San Francisco, Fresno.
Based on REPLACE results, switching from a PDE5 inhibitor to riociguat is now a “strong potential option” beyond adding a third drug such as selexipag or an inhaled prostacyclin to usual treatment with a PDE5 inhibitor plus an endothelin receptor antagonist, Dr. Balasubramanian said in an interview.
“We now have an evidence-based option where you can stay on a two-drug regimen and see whether the switch would work just as well,” said Dr. Balasubramanian, vice chair of the Pulmonary Vascular Disease Steering Committee for the American College of Chest Physicians.
REPLACE is a randomized phase 4 study including 226 patients with PAH considered to be at intermediate risk according to World Health Organization functional class III or 6MWD of 165-440 meters. The composite primary endpoint was defined as no clinical worsening (death, disease progression, or hospitalization for worsening PAH) plus clinical improvement on at least two measures including an improvement in 6MWD, achieving WHO functional class I/II, or a decrease in N-terminal pro-brain natriuretic peptide (NT-proBNP).
The primary endpoint of REPLACE was met, showing that 45 patients (41%) who switched to riociguat had clinical improvement without clinical worsening versus 22 patient (20%) who stayed on the PDE5 inhibitor (odds ratio, 2.78; 95% confidence interval, 1.53-5.06; P = .0007), Dr. Hoeper reported.
The benefit appeared consistent across PAH subgroups, according to Dr. Hoeper. In patients with idiopathic, heritable, or drug- and toxin-induced PAH, the primary endpoint favored riociguat over PDE5 inhibitor, at 45% and 23%, respectively. Similarly, a higher proportion of patients with PAH associated with congenital heart disease or portal hypertension achieved the primary endpoint (46% vs. 8%), as did patients with PAH associated with connective tissue disease (25% vs. 16%).
Adverse events were seen in 71% of riociguat-treated patients and 66% of PDE5 inhibitor–treated patients, according to Dr. Hoeper, who said severe adverse events were more frequent with PDE5-inhibitor treatment, at 17% versus 7% for riociguat. There were three clinical worsening events in the PDE5 inhibitor group leading to death, while a fourth patient died in safety follow-up, according to the reported results, whereas there were no deaths reported with riociguat.
The REPLACE study was cofunded by Bayer AG and Merck Sharpe & Dohme, a subsidiary of Merck & Co. Dr. Hoeper reported receiving fees for consultations or lectures from Acceleron, Actelion, Bayer AG, Janssen, MSD, and Pfizer.
SOURCE: Hoeper MM. CHEST 2020, Abstract A2156-A2159.
In patients with intermediate-risk pulmonary arterial hypertension (PAH) who are not at treatment goal on standard therapy, switching to riociguat is a promising strategy across a broad range of patient subgroups, an investigator said at the annual meeting of the American College of Chest Physicians, held virtually this year.
Patients switching to riociguat in the REPLACE study more frequently met the primary efficacy endpoint, compared with patients who remained on a phosphodiesterase-5 (PDE5) inhibitor, said Marius M. Hoeper, MD, of the Clinic for Respiratory Medicine at Hannover (Germany) Medical School.
That clinical benefit of switching to riociguat, a soluble guanylate cyclase (sGC) stimulator, was relatively consistent across patient subgroups including age, sex, PAH subtype, according to Dr. Hoeper.
“At the end of the day, we believe that switching from a PDE5 inhibitor to riociguat can benefit patients with PAH at intermediate risk and may serve as a new strategic option for treatment escalation,” he said in a live virtual presentation of the study results.
About 40% of patients switching to riociguat met the primary endpoint of clinical improvement in absence of clinical worsening versus just 20% of patients who stayed on a PDE5 inhibitor, according to top-line results of the phase 4 REPLACE study, which were reported Sept. 7 at the annual meeting of the European Respiratory Society.
Results of REPLACE presented at the CHEST meeting show a benefit across most patient subgroups, including PAH subtype and whether patients came from monotherapy or combination treatment to riociguat. Some groups did not appear to respond quite as well to switching, including elderly patients, patients with a 6-minute walk distance (6MWD) of less than 320 meters at baseline, and patients switching from tadalafil as opposed to sildenafil. However, these findings were not statistically significant and may have been chance findings, according to Dr. Hoeper.
These results of REPLACE suggest the efficacy of riociguat “across the board” for intermediate-risk PAH patients with inadequate response to standard therapy, said Vijay Balasubramanian, MD, FCCP, clinical professor of medicine at the University of California San Francisco, Fresno.
Based on REPLACE results, switching from a PDE5 inhibitor to riociguat is now a “strong potential option” beyond adding a third drug such as selexipag or an inhaled prostacyclin to usual treatment with a PDE5 inhibitor plus an endothelin receptor antagonist, Dr. Balasubramanian said in an interview.
“We now have an evidence-based option where you can stay on a two-drug regimen and see whether the switch would work just as well,” said Dr. Balasubramanian, vice chair of the Pulmonary Vascular Disease Steering Committee for the American College of Chest Physicians.
REPLACE is a randomized phase 4 study including 226 patients with PAH considered to be at intermediate risk according to World Health Organization functional class III or 6MWD of 165-440 meters. The composite primary endpoint was defined as no clinical worsening (death, disease progression, or hospitalization for worsening PAH) plus clinical improvement on at least two measures including an improvement in 6MWD, achieving WHO functional class I/II, or a decrease in N-terminal pro-brain natriuretic peptide (NT-proBNP).
The primary endpoint of REPLACE was met, showing that 45 patients (41%) who switched to riociguat had clinical improvement without clinical worsening versus 22 patient (20%) who stayed on the PDE5 inhibitor (odds ratio, 2.78; 95% confidence interval, 1.53-5.06; P = .0007), Dr. Hoeper reported.
The benefit appeared consistent across PAH subgroups, according to Dr. Hoeper. In patients with idiopathic, heritable, or drug- and toxin-induced PAH, the primary endpoint favored riociguat over PDE5 inhibitor, at 45% and 23%, respectively. Similarly, a higher proportion of patients with PAH associated with congenital heart disease or portal hypertension achieved the primary endpoint (46% vs. 8%), as did patients with PAH associated with connective tissue disease (25% vs. 16%).
Adverse events were seen in 71% of riociguat-treated patients and 66% of PDE5 inhibitor–treated patients, according to Dr. Hoeper, who said severe adverse events were more frequent with PDE5-inhibitor treatment, at 17% versus 7% for riociguat. There were three clinical worsening events in the PDE5 inhibitor group leading to death, while a fourth patient died in safety follow-up, according to the reported results, whereas there were no deaths reported with riociguat.
The REPLACE study was cofunded by Bayer AG and Merck Sharpe & Dohme, a subsidiary of Merck & Co. Dr. Hoeper reported receiving fees for consultations or lectures from Acceleron, Actelion, Bayer AG, Janssen, MSD, and Pfizer.
SOURCE: Hoeper MM. CHEST 2020, Abstract A2156-A2159.
FROM CHEST 2020
Score predicts risk for ventilation in COVID-19 patients
A new scoring system can predict whether COVID-19 patients will require invasive mechanical ventilation, researchers report.
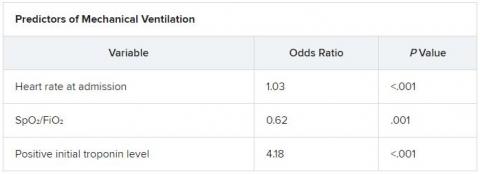
The score uses three variables to predict future risk: heart rate; the ratio of oxygen saturation (SpO2) to fraction of inspired oxygen (FiO2); and a positive troponin I level.
“What excites us is it’s a really benign tool,” said Muhtadi Alnababteh, MD, from the Medstar Washington (D.C.) Hospital Center. “For the first two variables you only need to look at vital signs, no labs or invasive diagnostics.”
“The third part is a simple lab, which is performed universally and can be done in any hospital,” he told this news organization. “We know that even rural hospitals can do this.”
For their retrospective analysis, Dr. Alnababteh and his colleagues assessed 265 adults with confirmed COVID-19 infection who were admitted to a single tertiary care center in March and April. They looked at demographic characteristics, lab results, and clinical and outcome information.
Ultimately, 54 of these patients required invasive mechanical ventilation.
On multiple-regression analysis, the researchers determined that three variables independently predicted the need for invasive mechanical ventilation.
Calibration of the model was good (Hosmer–Lemeshow score, 6.3; P = .39), as was predictive ability (area under the curve, 0.80).
The risk for invasive mechanical ventilation increased as the number of positive variables increased (P < .001), from 15.4% for those with one positive variable, to 29.0% for those with two, to 60.5% for those with three positive variables.
The team established cutoff points for each variable and developed a points-based scoring system to predict risk.
It was an initial surprise that troponin – a cardiac marker – would be a risk factor. “Originally, we thought COVID-19 only affects the lung,” Dr. Alnababteh explained during his presentation at CHEST 2020. Later studies, however, showed it can cause myocarditis symptoms.
The case for looking at cardiac markers was made when a study of young athletes who recovered from COVID-19 after experiencing mild or no symptoms showed that 15% had signs of myocarditis on cardiac MRI.
“If mild COVID disease in young patients caused cardiac injury, you can imagine what it can do to older patients with severe disease,” Alnababteh said.
This tool will help triage patients who are not sick enough for the ICU but are known to be at high risk for ventilation. “It’s one of the biggest decisions you have to make: Where do you send your patient? This score helps determine that,” he said.
The researchers are now working to validate the score and evaluate how it performs, he reported.
Existing scores evaluated for COVID-19 outcome prediction
The MuLBSTA score can also be used to predict outcomes in patients with COVID-19.
A retrospective evaluation of 163 patients was presented at CHEST 2020 by Jurgena Tusha, MD, from Wayne State University in Detroit.
Patients who survived their illness had a mean MuLBSTA score of 8.67, whereas patients who died had a mean score of 13.60.
The score “correlated significantly with mortality, ventilator support, and length of stay, which may be used to provide guidance to screen patients and make further clinical decisions,” Dr. Tusha said in a press release.
“Further studies are required to validate this study in larger patient cohorts,” she added.
The three-variable scoring system is easier to use than the MuLBSTA, and more specific, said Dr. Alnababteh.
“The main difference between our study and the MuLBSTA study is that we came up with a novel score for COVID-19 patients,” he said. “Our study score doesn’t require chest x-rays or blood cultures, and the outcome is need for invasive mechanical ventilation, not mortality.”
A version of this article originally appeared on Medscape.com.
A new scoring system can predict whether COVID-19 patients will require invasive mechanical ventilation, researchers report.
The score uses three variables to predict future risk: heart rate; the ratio of oxygen saturation (SpO2) to fraction of inspired oxygen (FiO2); and a positive troponin I level.
“What excites us is it’s a really benign tool,” said Muhtadi Alnababteh, MD, from the Medstar Washington (D.C.) Hospital Center. “For the first two variables you only need to look at vital signs, no labs or invasive diagnostics.”
“The third part is a simple lab, which is performed universally and can be done in any hospital,” he told this news organization. “We know that even rural hospitals can do this.”
For their retrospective analysis, Dr. Alnababteh and his colleagues assessed 265 adults with confirmed COVID-19 infection who were admitted to a single tertiary care center in March and April. They looked at demographic characteristics, lab results, and clinical and outcome information.
Ultimately, 54 of these patients required invasive mechanical ventilation.
On multiple-regression analysis, the researchers determined that three variables independently predicted the need for invasive mechanical ventilation.
Calibration of the model was good (Hosmer–Lemeshow score, 6.3; P = .39), as was predictive ability (area under the curve, 0.80).
The risk for invasive mechanical ventilation increased as the number of positive variables increased (P < .001), from 15.4% for those with one positive variable, to 29.0% for those with two, to 60.5% for those with three positive variables.
The team established cutoff points for each variable and developed a points-based scoring system to predict risk.
It was an initial surprise that troponin – a cardiac marker – would be a risk factor. “Originally, we thought COVID-19 only affects the lung,” Dr. Alnababteh explained during his presentation at CHEST 2020. Later studies, however, showed it can cause myocarditis symptoms.
The case for looking at cardiac markers was made when a study of young athletes who recovered from COVID-19 after experiencing mild or no symptoms showed that 15% had signs of myocarditis on cardiac MRI.
“If mild COVID disease in young patients caused cardiac injury, you can imagine what it can do to older patients with severe disease,” Alnababteh said.
This tool will help triage patients who are not sick enough for the ICU but are known to be at high risk for ventilation. “It’s one of the biggest decisions you have to make: Where do you send your patient? This score helps determine that,” he said.
The researchers are now working to validate the score and evaluate how it performs, he reported.
Existing scores evaluated for COVID-19 outcome prediction
The MuLBSTA score can also be used to predict outcomes in patients with COVID-19.
A retrospective evaluation of 163 patients was presented at CHEST 2020 by Jurgena Tusha, MD, from Wayne State University in Detroit.
Patients who survived their illness had a mean MuLBSTA score of 8.67, whereas patients who died had a mean score of 13.60.
The score “correlated significantly with mortality, ventilator support, and length of stay, which may be used to provide guidance to screen patients and make further clinical decisions,” Dr. Tusha said in a press release.
“Further studies are required to validate this study in larger patient cohorts,” she added.
The three-variable scoring system is easier to use than the MuLBSTA, and more specific, said Dr. Alnababteh.
“The main difference between our study and the MuLBSTA study is that we came up with a novel score for COVID-19 patients,” he said. “Our study score doesn’t require chest x-rays or blood cultures, and the outcome is need for invasive mechanical ventilation, not mortality.”
A version of this article originally appeared on Medscape.com.
A new scoring system can predict whether COVID-19 patients will require invasive mechanical ventilation, researchers report.
The score uses three variables to predict future risk: heart rate; the ratio of oxygen saturation (SpO2) to fraction of inspired oxygen (FiO2); and a positive troponin I level.
“What excites us is it’s a really benign tool,” said Muhtadi Alnababteh, MD, from the Medstar Washington (D.C.) Hospital Center. “For the first two variables you only need to look at vital signs, no labs or invasive diagnostics.”
“The third part is a simple lab, which is performed universally and can be done in any hospital,” he told this news organization. “We know that even rural hospitals can do this.”
For their retrospective analysis, Dr. Alnababteh and his colleagues assessed 265 adults with confirmed COVID-19 infection who were admitted to a single tertiary care center in March and April. They looked at demographic characteristics, lab results, and clinical and outcome information.
Ultimately, 54 of these patients required invasive mechanical ventilation.
On multiple-regression analysis, the researchers determined that three variables independently predicted the need for invasive mechanical ventilation.
Calibration of the model was good (Hosmer–Lemeshow score, 6.3; P = .39), as was predictive ability (area under the curve, 0.80).
The risk for invasive mechanical ventilation increased as the number of positive variables increased (P < .001), from 15.4% for those with one positive variable, to 29.0% for those with two, to 60.5% for those with three positive variables.
The team established cutoff points for each variable and developed a points-based scoring system to predict risk.
It was an initial surprise that troponin – a cardiac marker – would be a risk factor. “Originally, we thought COVID-19 only affects the lung,” Dr. Alnababteh explained during his presentation at CHEST 2020. Later studies, however, showed it can cause myocarditis symptoms.
The case for looking at cardiac markers was made when a study of young athletes who recovered from COVID-19 after experiencing mild or no symptoms showed that 15% had signs of myocarditis on cardiac MRI.
“If mild COVID disease in young patients caused cardiac injury, you can imagine what it can do to older patients with severe disease,” Alnababteh said.
This tool will help triage patients who are not sick enough for the ICU but are known to be at high risk for ventilation. “It’s one of the biggest decisions you have to make: Where do you send your patient? This score helps determine that,” he said.
The researchers are now working to validate the score and evaluate how it performs, he reported.
Existing scores evaluated for COVID-19 outcome prediction
The MuLBSTA score can also be used to predict outcomes in patients with COVID-19.
A retrospective evaluation of 163 patients was presented at CHEST 2020 by Jurgena Tusha, MD, from Wayne State University in Detroit.
Patients who survived their illness had a mean MuLBSTA score of 8.67, whereas patients who died had a mean score of 13.60.
The score “correlated significantly with mortality, ventilator support, and length of stay, which may be used to provide guidance to screen patients and make further clinical decisions,” Dr. Tusha said in a press release.
“Further studies are required to validate this study in larger patient cohorts,” she added.
The three-variable scoring system is easier to use than the MuLBSTA, and more specific, said Dr. Alnababteh.
“The main difference between our study and the MuLBSTA study is that we came up with a novel score for COVID-19 patients,” he said. “Our study score doesn’t require chest x-rays or blood cultures, and the outcome is need for invasive mechanical ventilation, not mortality.”
A version of this article originally appeared on Medscape.com.
When should students resume sports after a COVID-19 diagnosis?
Many student athletes who test positive for COVID-19 likely can have an uneventful return to their sports after they have rested for 2 weeks in quarantine, doctors suggest.
There are reasons for caution, however, especially when a patient has symptoms that indicate possible cardiac involvement. In these cases, patients should undergo cardiac testing before a physician clears them to return to play, according to guidance from professional associations. Reports of myocarditis in college athletes who tested positive for SARS-CoV-2 but were asymptomatic are among the reasons for concern. Myocarditis may increase the risk of sudden death during exercise.
“The thing that you need to keep in mind is that this is not just a respiratory illness,” David T. Bernhardt, MD, professor of pediatrics, orthopedics, and rehabilitation at the University of Wisconsin in Madison, said in a presentation at the annual meeting of the American Academy of Pediatrics, held virtually this year. High school and college athletes have had cardiac, neurologic, hematologic, and renal problems that “can complicate their recovery and their return to sport.”
Still, children who test positive for COVID-19 tend to have mild illness and often are asymptomatic. “It is more than likely going to be safe for the majority of the student athletes who are in the elementary and middle school age to return to sport,” said Dr. Bernhardt. Given that 18-year-old college freshmen have had cardiac complications, there may be reason for more caution with high school students.
Limited data
The AAP has released interim guidance on returning to sports and recommends that primary care physicians clear all patients with COVID-19 before they resume training. Physicians should screen for cardiac symptoms such as chest pain, shortness of breath, fatigue, palpitations, or syncope.
Those with severe illness should be restricted from exercise and participation for 3-6 months. Primary care physicians, preferably in consultation with pediatric cardiologists, should clear athletes who experience severe illness.
“Most of the recommendations come from the fact that we simply do not know what we do not know with COVID-19,” Susannah Briskin, MD, a coauthor of the interim guidance, said in an interview. “We have to be cautious in returning individuals to play and closely monitor them as we learn more about the disease process and its effect on kids.”
Patients with severe illness could include those who were hospitalized and experienced hypotension or arrhythmias, required intubation or extracorporeal membrane oxygenation (ECMO) support, had kidney or cardiac failure, or developed multisystem inflammatory syndrome in children (MIS-C), said Dr. Briskin, a specialist in pediatric sports medicine at Case Western Reserve University, Cleveland.
“The majority of COVID-19 cases will not present like this in kids. We have no idea how common myocarditis is in kids post infection. We do know that, if anyone has chest pain, shortness of breath, excessive fatigue, syncope [passing out], or arrhythmia [feeling of their heart skipping beats], they should undergo further evaluation for myocarditis,” Dr. Briskin said.
Patients who are asymptomatic or have mild symptoms should rest for 14 days after their positive test. After their infectious period has passed, a doctor should assess for any concerning cardiac symptoms. “Anyone with prolonged fever or moderate symptoms should see their pediatrician and have an EKG performed, at a minimum, prior to return to sports,” Dr. Briskin said. “Anyone with an abnormal EKG or concerning signs or symptoms should be referred on to pediatric cardiology for a further assessment.”
Most patients who Dr. Briskin has seen have been asymptomatic or mildly symptomatic. “They have done well with a gradual return to physical activity,” she said. “We recommend a gradual return so individuals can be monitored for any signs or symptoms concerning for myocarditis. The far majority of individuals likely have an uneventful return to play.”
Mitigating risk
COVID-19 adds elements of uncertainty and complexity to the usual process of mitigating risk in sports, Dr. Bernhardt noted in his lecture. “You are dealing with an infection that we do not know a lot about,” he said. “And we are trying to mitigate risk not only for the individual who may or may not have underlying health problems, but you are also trying to mitigate risk for anybody else involved with the sport, including athletic trainers and team physicians, coaches, spectators, custodial staff, people working at a snack shack, and all the other people that can be involved in a typical sporting type of atmosphere.”
When patients do return to play after an illness, they should gradually increase the training load to avoid injury. In addition, clinicians should screen for depression and anxiety using tools such as the Four-Item Patient Health Questionnaire (PHQ-4) when they see patients. “The pandemic has been quite stressful for everybody, including our high school student athletes,” Dr. Bernhardt said. “Giving everybody a PHQ-4 when they come into clinic right now probably makes sense in terms of the stress levels that all of us are experiencing.”
If a patient screens positive, take additional history and refer for more in-depth mental health evaluation and treatment if warranted. Sharing breathing and relaxation exercises, promoting healthy behaviors, and paying attention to unhealthy strategies also may help, Dr. Bernhardt suggested.
Ultimately, determining when an athlete with COVID-19 can be medically cleared to return to play may be a challenge. There are limited data on epidemiology and clinical presentations that could help identify cardiac injury related to the disease, Dr. Bernhardt said. Guidance from the American College of Cardiology provides a framework for evaluating athletes for return to play, and pediatric cardiologists have discussed how the guidance relates to a pediatric population. Cardiac assessments may include measures of biomarkers such as troponin, B-type natriuretic peptide, and sedimentation rate, along with electrocardiograms, echocardiograms, and cardiac MRI.
Beyond return-to-play decisions, encourage the use of cloth face coverings on the sidelines and away from the playing field, and stress proper quarantining, Dr. Briskin added. Too often, she hears about children not quarantining properly. “Individuals with a known exposure should be quarantined in their house – ideally in a separate room from everyone else. ... When they come out of their room, they should wash their hands well and wear a cloth face covering. They should not be eating with other people.”
Dr. Bernhardt had no relevant disclosures.
Many student athletes who test positive for COVID-19 likely can have an uneventful return to their sports after they have rested for 2 weeks in quarantine, doctors suggest.
There are reasons for caution, however, especially when a patient has symptoms that indicate possible cardiac involvement. In these cases, patients should undergo cardiac testing before a physician clears them to return to play, according to guidance from professional associations. Reports of myocarditis in college athletes who tested positive for SARS-CoV-2 but were asymptomatic are among the reasons for concern. Myocarditis may increase the risk of sudden death during exercise.
“The thing that you need to keep in mind is that this is not just a respiratory illness,” David T. Bernhardt, MD, professor of pediatrics, orthopedics, and rehabilitation at the University of Wisconsin in Madison, said in a presentation at the annual meeting of the American Academy of Pediatrics, held virtually this year. High school and college athletes have had cardiac, neurologic, hematologic, and renal problems that “can complicate their recovery and their return to sport.”
Still, children who test positive for COVID-19 tend to have mild illness and often are asymptomatic. “It is more than likely going to be safe for the majority of the student athletes who are in the elementary and middle school age to return to sport,” said Dr. Bernhardt. Given that 18-year-old college freshmen have had cardiac complications, there may be reason for more caution with high school students.
Limited data
The AAP has released interim guidance on returning to sports and recommends that primary care physicians clear all patients with COVID-19 before they resume training. Physicians should screen for cardiac symptoms such as chest pain, shortness of breath, fatigue, palpitations, or syncope.
Those with severe illness should be restricted from exercise and participation for 3-6 months. Primary care physicians, preferably in consultation with pediatric cardiologists, should clear athletes who experience severe illness.
“Most of the recommendations come from the fact that we simply do not know what we do not know with COVID-19,” Susannah Briskin, MD, a coauthor of the interim guidance, said in an interview. “We have to be cautious in returning individuals to play and closely monitor them as we learn more about the disease process and its effect on kids.”
Patients with severe illness could include those who were hospitalized and experienced hypotension or arrhythmias, required intubation or extracorporeal membrane oxygenation (ECMO) support, had kidney or cardiac failure, or developed multisystem inflammatory syndrome in children (MIS-C), said Dr. Briskin, a specialist in pediatric sports medicine at Case Western Reserve University, Cleveland.
“The majority of COVID-19 cases will not present like this in kids. We have no idea how common myocarditis is in kids post infection. We do know that, if anyone has chest pain, shortness of breath, excessive fatigue, syncope [passing out], or arrhythmia [feeling of their heart skipping beats], they should undergo further evaluation for myocarditis,” Dr. Briskin said.
Patients who are asymptomatic or have mild symptoms should rest for 14 days after their positive test. After their infectious period has passed, a doctor should assess for any concerning cardiac symptoms. “Anyone with prolonged fever or moderate symptoms should see their pediatrician and have an EKG performed, at a minimum, prior to return to sports,” Dr. Briskin said. “Anyone with an abnormal EKG or concerning signs or symptoms should be referred on to pediatric cardiology for a further assessment.”
Most patients who Dr. Briskin has seen have been asymptomatic or mildly symptomatic. “They have done well with a gradual return to physical activity,” she said. “We recommend a gradual return so individuals can be monitored for any signs or symptoms concerning for myocarditis. The far majority of individuals likely have an uneventful return to play.”
Mitigating risk
COVID-19 adds elements of uncertainty and complexity to the usual process of mitigating risk in sports, Dr. Bernhardt noted in his lecture. “You are dealing with an infection that we do not know a lot about,” he said. “And we are trying to mitigate risk not only for the individual who may or may not have underlying health problems, but you are also trying to mitigate risk for anybody else involved with the sport, including athletic trainers and team physicians, coaches, spectators, custodial staff, people working at a snack shack, and all the other people that can be involved in a typical sporting type of atmosphere.”
When patients do return to play after an illness, they should gradually increase the training load to avoid injury. In addition, clinicians should screen for depression and anxiety using tools such as the Four-Item Patient Health Questionnaire (PHQ-4) when they see patients. “The pandemic has been quite stressful for everybody, including our high school student athletes,” Dr. Bernhardt said. “Giving everybody a PHQ-4 when they come into clinic right now probably makes sense in terms of the stress levels that all of us are experiencing.”
If a patient screens positive, take additional history and refer for more in-depth mental health evaluation and treatment if warranted. Sharing breathing and relaxation exercises, promoting healthy behaviors, and paying attention to unhealthy strategies also may help, Dr. Bernhardt suggested.
Ultimately, determining when an athlete with COVID-19 can be medically cleared to return to play may be a challenge. There are limited data on epidemiology and clinical presentations that could help identify cardiac injury related to the disease, Dr. Bernhardt said. Guidance from the American College of Cardiology provides a framework for evaluating athletes for return to play, and pediatric cardiologists have discussed how the guidance relates to a pediatric population. Cardiac assessments may include measures of biomarkers such as troponin, B-type natriuretic peptide, and sedimentation rate, along with electrocardiograms, echocardiograms, and cardiac MRI.
Beyond return-to-play decisions, encourage the use of cloth face coverings on the sidelines and away from the playing field, and stress proper quarantining, Dr. Briskin added. Too often, she hears about children not quarantining properly. “Individuals with a known exposure should be quarantined in their house – ideally in a separate room from everyone else. ... When they come out of their room, they should wash their hands well and wear a cloth face covering. They should not be eating with other people.”
Dr. Bernhardt had no relevant disclosures.
Many student athletes who test positive for COVID-19 likely can have an uneventful return to their sports after they have rested for 2 weeks in quarantine, doctors suggest.
There are reasons for caution, however, especially when a patient has symptoms that indicate possible cardiac involvement. In these cases, patients should undergo cardiac testing before a physician clears them to return to play, according to guidance from professional associations. Reports of myocarditis in college athletes who tested positive for SARS-CoV-2 but were asymptomatic are among the reasons for concern. Myocarditis may increase the risk of sudden death during exercise.
“The thing that you need to keep in mind is that this is not just a respiratory illness,” David T. Bernhardt, MD, professor of pediatrics, orthopedics, and rehabilitation at the University of Wisconsin in Madison, said in a presentation at the annual meeting of the American Academy of Pediatrics, held virtually this year. High school and college athletes have had cardiac, neurologic, hematologic, and renal problems that “can complicate their recovery and their return to sport.”
Still, children who test positive for COVID-19 tend to have mild illness and often are asymptomatic. “It is more than likely going to be safe for the majority of the student athletes who are in the elementary and middle school age to return to sport,” said Dr. Bernhardt. Given that 18-year-old college freshmen have had cardiac complications, there may be reason for more caution with high school students.
Limited data
The AAP has released interim guidance on returning to sports and recommends that primary care physicians clear all patients with COVID-19 before they resume training. Physicians should screen for cardiac symptoms such as chest pain, shortness of breath, fatigue, palpitations, or syncope.
Those with severe illness should be restricted from exercise and participation for 3-6 months. Primary care physicians, preferably in consultation with pediatric cardiologists, should clear athletes who experience severe illness.
“Most of the recommendations come from the fact that we simply do not know what we do not know with COVID-19,” Susannah Briskin, MD, a coauthor of the interim guidance, said in an interview. “We have to be cautious in returning individuals to play and closely monitor them as we learn more about the disease process and its effect on kids.”
Patients with severe illness could include those who were hospitalized and experienced hypotension or arrhythmias, required intubation or extracorporeal membrane oxygenation (ECMO) support, had kidney or cardiac failure, or developed multisystem inflammatory syndrome in children (MIS-C), said Dr. Briskin, a specialist in pediatric sports medicine at Case Western Reserve University, Cleveland.
“The majority of COVID-19 cases will not present like this in kids. We have no idea how common myocarditis is in kids post infection. We do know that, if anyone has chest pain, shortness of breath, excessive fatigue, syncope [passing out], or arrhythmia [feeling of their heart skipping beats], they should undergo further evaluation for myocarditis,” Dr. Briskin said.
Patients who are asymptomatic or have mild symptoms should rest for 14 days after their positive test. After their infectious period has passed, a doctor should assess for any concerning cardiac symptoms. “Anyone with prolonged fever or moderate symptoms should see their pediatrician and have an EKG performed, at a minimum, prior to return to sports,” Dr. Briskin said. “Anyone with an abnormal EKG or concerning signs or symptoms should be referred on to pediatric cardiology for a further assessment.”
Most patients who Dr. Briskin has seen have been asymptomatic or mildly symptomatic. “They have done well with a gradual return to physical activity,” she said. “We recommend a gradual return so individuals can be monitored for any signs or symptoms concerning for myocarditis. The far majority of individuals likely have an uneventful return to play.”
Mitigating risk
COVID-19 adds elements of uncertainty and complexity to the usual process of mitigating risk in sports, Dr. Bernhardt noted in his lecture. “You are dealing with an infection that we do not know a lot about,” he said. “And we are trying to mitigate risk not only for the individual who may or may not have underlying health problems, but you are also trying to mitigate risk for anybody else involved with the sport, including athletic trainers and team physicians, coaches, spectators, custodial staff, people working at a snack shack, and all the other people that can be involved in a typical sporting type of atmosphere.”
When patients do return to play after an illness, they should gradually increase the training load to avoid injury. In addition, clinicians should screen for depression and anxiety using tools such as the Four-Item Patient Health Questionnaire (PHQ-4) when they see patients. “The pandemic has been quite stressful for everybody, including our high school student athletes,” Dr. Bernhardt said. “Giving everybody a PHQ-4 when they come into clinic right now probably makes sense in terms of the stress levels that all of us are experiencing.”
If a patient screens positive, take additional history and refer for more in-depth mental health evaluation and treatment if warranted. Sharing breathing and relaxation exercises, promoting healthy behaviors, and paying attention to unhealthy strategies also may help, Dr. Bernhardt suggested.
Ultimately, determining when an athlete with COVID-19 can be medically cleared to return to play may be a challenge. There are limited data on epidemiology and clinical presentations that could help identify cardiac injury related to the disease, Dr. Bernhardt said. Guidance from the American College of Cardiology provides a framework for evaluating athletes for return to play, and pediatric cardiologists have discussed how the guidance relates to a pediatric population. Cardiac assessments may include measures of biomarkers such as troponin, B-type natriuretic peptide, and sedimentation rate, along with electrocardiograms, echocardiograms, and cardiac MRI.
Beyond return-to-play decisions, encourage the use of cloth face coverings on the sidelines and away from the playing field, and stress proper quarantining, Dr. Briskin added. Too often, she hears about children not quarantining properly. “Individuals with a known exposure should be quarantined in their house – ideally in a separate room from everyone else. ... When they come out of their room, they should wash their hands well and wear a cloth face covering. They should not be eating with other people.”
Dr. Bernhardt had no relevant disclosures.
FROM AAP 2020
COVID-19: Convalescent plasma falls short in phase 2 trial
Convalescent plasma may not prevent progression to severe disease or reduce mortality risk in hospitalized patients with moderate COVID-19, based on a phase 2 trial involving more than 400 patients in India.
The PLACID trial offers real-world data with “high generalizability,” according to lead author Anup Agarwal, MD, of the Indian Council of Medical Research, New Delhi, and colleagues.
“Evidence suggests that convalescent plasma collected from survivors of COVID-19 contains receptor binding domain specific antibodies with potent antiviral activity,” the investigators wrote in the BMJ. “However, effective titers of antiviral neutralizing antibodies, optimal timing for convalescent plasma treatment, optimal timing for plasma donation, and the severity class of patients who are likely to benefit from convalescent plasma remain unclear.”
According to Dr. Agarwal and colleagues, case series and observational studies have suggested that convalescent plasma may reduce viral load, hospital stay, and mortality, but randomized controlled trials to date have ended prematurely because of issues with enrollment and design, making PLACID the first randomized controlled trial of its kind to reach completion.
The open-label, multicenter study involved 464 hospitalized adults who tested positive for SARS-CoV-2 via reverse transcription polymerase chain reaction (RT-PCR). Enrollment also required a respiratory rate of more than 24 breaths/min with an oxygen saturation (SpO2) of 93% or less on room air, or a partial pressure of oxygen in arterial blood/fraction of inspired oxygen (PaO2 /FiO2 ) ratio between 200 and 300 mm Hg.
Patients were randomly assigned in a 1:1 ratio to receive either best standard of care (control), or best standard of care plus convalescent plasma, which was given in two doses of 200 mL, 24 hours apart. Patients were assessed via clinical examination, chest imaging, and serial laboratory testing, the latter of which included neutralizing antibody titers on days 0, 3, and 7.
The primary outcome was a 28-day composite of progression to severe disease (PaO2/FiO2 ratio < 100 mm Hg) and all-cause mortality. An array of secondary outcomes were also reported, including symptom resolution, total duration of respiratory support, change in oxygen requirement, and others.
In the convalescent plasma group, 19% of patients progressed to severe disease or died within 28 days, compared with 18% of those in the control group (risk ratio, 1.04; 95% confidence interval, 0.71-1.54), suggesting no statistically significant benefit from the intervention. This lack of benefit was also found in a subgroup analysis of patients with detectable titers of antibodies to SARS-CoV-2, and when progression to severe disease and all-cause mortality were analyzed independently across all patients.
Still, at day 7, patients treated with convalescent plasma were significantly more likely to have resolution of fatigue (RR, 1.21; 95% CI, 1.02-1.42) and shortness of breath (RR, 1.16; 95% CI, 1.02-1.32). And at the same time point, patients treated with convalescent plasma were 20% more likely to test negative for SARS-CoV-2 RNA (RR, 1.2; 95% CI, 1.04-1.5).
In an accompanying editorial, Elizabeth B. Pathak, PhD, of the Women’s Institute for Independent Social Enquiry, Olney, Md., suggested that the reported symptom improvements need to be viewed with skepticism.
“These results should be interpreted with caution, because the trial was not blinded, so knowledge of treatment status could have influenced the reporting of subjective symptoms by patients who survived to day 7,” Dr. Pathak wrote.
Dr. Pathak noted that convalescent plasma did appear to have an antiviral effect, based on the higher rate of negative RNA test results at day 7. She hypothesized that the lack of major corresponding clinical benefit could be explained by detrimental thrombotic processes.
“The net effect of plasma is prothrombotic,” Dr. Pathak wrote, which should raise safety concerns, since “COVID-19 is a life-threatening thrombotic disorder.”
According to Dr. Pathak, large-scale datasets may be giving a false sense of security. She cited a recent safety analysis of 20,000 U.S. patients who received convalescent plasma, in which the investigators excluded 88.2% of cardiac events and 66.3% of thrombotic events, as these were deemed unrelated to transfusion; but this decision was made by the treating physician, without independent review or a defined protocol.
Michael J. Joyner, MD, of the Mayo Clinic in Rochester, Minn., was the lead author of the above safety study, and is leading the Food and Drug Administration expanded access program for convalescent plasma in patients with COVID-19. He suggested that the study by Dr. Agarwal and colleagues was admirable, but flaws in the treatment protocol cast doubt upon the efficacy findings.
“It is very impressive that these investigators performed a large trial of convalescent plasma in the midst of a pandemic,” Dr. Joyner said. “Unfortunately it is unclear how generalizable the findings are because many of the units of plasma had either very low or no antibody titers and because the plasma was given late in the course of the disease. It has been known since at least the 1930s that antibody therapy works best when enough product is given either prophylactically or early in the course of disease.”
Dr. Joyner had a more positive interpretation of the reported symptom improvements.
“It is also interesting to note that while there was no mortality benefit, that – even with the limitations of the study – there was some evidence of improved patient physiology at 7 days,” he said. “So, at one level, [this is] a negative study, but at least [there are] some hints of efficacy given the suboptimal use case in the patients studied.”
The study was funded by the Indian Council of Medical Research, which employs several of the authors and PLACID Trial Collaborators. Dr. Pathak and Dr. Joyner reported no conflicts of interest.
SOURCE: Agarwal A et al. BMJ. 2020 Oct 23. doi: 10.1136/bmj.m3939 .
Convalescent plasma may not prevent progression to severe disease or reduce mortality risk in hospitalized patients with moderate COVID-19, based on a phase 2 trial involving more than 400 patients in India.
The PLACID trial offers real-world data with “high generalizability,” according to lead author Anup Agarwal, MD, of the Indian Council of Medical Research, New Delhi, and colleagues.
“Evidence suggests that convalescent plasma collected from survivors of COVID-19 contains receptor binding domain specific antibodies with potent antiviral activity,” the investigators wrote in the BMJ. “However, effective titers of antiviral neutralizing antibodies, optimal timing for convalescent plasma treatment, optimal timing for plasma donation, and the severity class of patients who are likely to benefit from convalescent plasma remain unclear.”
According to Dr. Agarwal and colleagues, case series and observational studies have suggested that convalescent plasma may reduce viral load, hospital stay, and mortality, but randomized controlled trials to date have ended prematurely because of issues with enrollment and design, making PLACID the first randomized controlled trial of its kind to reach completion.
The open-label, multicenter study involved 464 hospitalized adults who tested positive for SARS-CoV-2 via reverse transcription polymerase chain reaction (RT-PCR). Enrollment also required a respiratory rate of more than 24 breaths/min with an oxygen saturation (SpO2) of 93% or less on room air, or a partial pressure of oxygen in arterial blood/fraction of inspired oxygen (PaO2 /FiO2 ) ratio between 200 and 300 mm Hg.
Patients were randomly assigned in a 1:1 ratio to receive either best standard of care (control), or best standard of care plus convalescent plasma, which was given in two doses of 200 mL, 24 hours apart. Patients were assessed via clinical examination, chest imaging, and serial laboratory testing, the latter of which included neutralizing antibody titers on days 0, 3, and 7.
The primary outcome was a 28-day composite of progression to severe disease (PaO2/FiO2 ratio < 100 mm Hg) and all-cause mortality. An array of secondary outcomes were also reported, including symptom resolution, total duration of respiratory support, change in oxygen requirement, and others.
In the convalescent plasma group, 19% of patients progressed to severe disease or died within 28 days, compared with 18% of those in the control group (risk ratio, 1.04; 95% confidence interval, 0.71-1.54), suggesting no statistically significant benefit from the intervention. This lack of benefit was also found in a subgroup analysis of patients with detectable titers of antibodies to SARS-CoV-2, and when progression to severe disease and all-cause mortality were analyzed independently across all patients.
Still, at day 7, patients treated with convalescent plasma were significantly more likely to have resolution of fatigue (RR, 1.21; 95% CI, 1.02-1.42) and shortness of breath (RR, 1.16; 95% CI, 1.02-1.32). And at the same time point, patients treated with convalescent plasma were 20% more likely to test negative for SARS-CoV-2 RNA (RR, 1.2; 95% CI, 1.04-1.5).
In an accompanying editorial, Elizabeth B. Pathak, PhD, of the Women’s Institute for Independent Social Enquiry, Olney, Md., suggested that the reported symptom improvements need to be viewed with skepticism.
“These results should be interpreted with caution, because the trial was not blinded, so knowledge of treatment status could have influenced the reporting of subjective symptoms by patients who survived to day 7,” Dr. Pathak wrote.
Dr. Pathak noted that convalescent plasma did appear to have an antiviral effect, based on the higher rate of negative RNA test results at day 7. She hypothesized that the lack of major corresponding clinical benefit could be explained by detrimental thrombotic processes.
“The net effect of plasma is prothrombotic,” Dr. Pathak wrote, which should raise safety concerns, since “COVID-19 is a life-threatening thrombotic disorder.”
According to Dr. Pathak, large-scale datasets may be giving a false sense of security. She cited a recent safety analysis of 20,000 U.S. patients who received convalescent plasma, in which the investigators excluded 88.2% of cardiac events and 66.3% of thrombotic events, as these were deemed unrelated to transfusion; but this decision was made by the treating physician, without independent review or a defined protocol.
Michael J. Joyner, MD, of the Mayo Clinic in Rochester, Minn., was the lead author of the above safety study, and is leading the Food and Drug Administration expanded access program for convalescent plasma in patients with COVID-19. He suggested that the study by Dr. Agarwal and colleagues was admirable, but flaws in the treatment protocol cast doubt upon the efficacy findings.
“It is very impressive that these investigators performed a large trial of convalescent plasma in the midst of a pandemic,” Dr. Joyner said. “Unfortunately it is unclear how generalizable the findings are because many of the units of plasma had either very low or no antibody titers and because the plasma was given late in the course of the disease. It has been known since at least the 1930s that antibody therapy works best when enough product is given either prophylactically or early in the course of disease.”
Dr. Joyner had a more positive interpretation of the reported symptom improvements.
“It is also interesting to note that while there was no mortality benefit, that – even with the limitations of the study – there was some evidence of improved patient physiology at 7 days,” he said. “So, at one level, [this is] a negative study, but at least [there are] some hints of efficacy given the suboptimal use case in the patients studied.”
The study was funded by the Indian Council of Medical Research, which employs several of the authors and PLACID Trial Collaborators. Dr. Pathak and Dr. Joyner reported no conflicts of interest.
SOURCE: Agarwal A et al. BMJ. 2020 Oct 23. doi: 10.1136/bmj.m3939 .
Convalescent plasma may not prevent progression to severe disease or reduce mortality risk in hospitalized patients with moderate COVID-19, based on a phase 2 trial involving more than 400 patients in India.
The PLACID trial offers real-world data with “high generalizability,” according to lead author Anup Agarwal, MD, of the Indian Council of Medical Research, New Delhi, and colleagues.
“Evidence suggests that convalescent plasma collected from survivors of COVID-19 contains receptor binding domain specific antibodies with potent antiviral activity,” the investigators wrote in the BMJ. “However, effective titers of antiviral neutralizing antibodies, optimal timing for convalescent plasma treatment, optimal timing for plasma donation, and the severity class of patients who are likely to benefit from convalescent plasma remain unclear.”
According to Dr. Agarwal and colleagues, case series and observational studies have suggested that convalescent plasma may reduce viral load, hospital stay, and mortality, but randomized controlled trials to date have ended prematurely because of issues with enrollment and design, making PLACID the first randomized controlled trial of its kind to reach completion.
The open-label, multicenter study involved 464 hospitalized adults who tested positive for SARS-CoV-2 via reverse transcription polymerase chain reaction (RT-PCR). Enrollment also required a respiratory rate of more than 24 breaths/min with an oxygen saturation (SpO2) of 93% or less on room air, or a partial pressure of oxygen in arterial blood/fraction of inspired oxygen (PaO2 /FiO2 ) ratio between 200 and 300 mm Hg.
Patients were randomly assigned in a 1:1 ratio to receive either best standard of care (control), or best standard of care plus convalescent plasma, which was given in two doses of 200 mL, 24 hours apart. Patients were assessed via clinical examination, chest imaging, and serial laboratory testing, the latter of which included neutralizing antibody titers on days 0, 3, and 7.
The primary outcome was a 28-day composite of progression to severe disease (PaO2/FiO2 ratio < 100 mm Hg) and all-cause mortality. An array of secondary outcomes were also reported, including symptom resolution, total duration of respiratory support, change in oxygen requirement, and others.
In the convalescent plasma group, 19% of patients progressed to severe disease or died within 28 days, compared with 18% of those in the control group (risk ratio, 1.04; 95% confidence interval, 0.71-1.54), suggesting no statistically significant benefit from the intervention. This lack of benefit was also found in a subgroup analysis of patients with detectable titers of antibodies to SARS-CoV-2, and when progression to severe disease and all-cause mortality were analyzed independently across all patients.
Still, at day 7, patients treated with convalescent plasma were significantly more likely to have resolution of fatigue (RR, 1.21; 95% CI, 1.02-1.42) and shortness of breath (RR, 1.16; 95% CI, 1.02-1.32). And at the same time point, patients treated with convalescent plasma were 20% more likely to test negative for SARS-CoV-2 RNA (RR, 1.2; 95% CI, 1.04-1.5).
In an accompanying editorial, Elizabeth B. Pathak, PhD, of the Women’s Institute for Independent Social Enquiry, Olney, Md., suggested that the reported symptom improvements need to be viewed with skepticism.
“These results should be interpreted with caution, because the trial was not blinded, so knowledge of treatment status could have influenced the reporting of subjective symptoms by patients who survived to day 7,” Dr. Pathak wrote.
Dr. Pathak noted that convalescent plasma did appear to have an antiviral effect, based on the higher rate of negative RNA test results at day 7. She hypothesized that the lack of major corresponding clinical benefit could be explained by detrimental thrombotic processes.
“The net effect of plasma is prothrombotic,” Dr. Pathak wrote, which should raise safety concerns, since “COVID-19 is a life-threatening thrombotic disorder.”
According to Dr. Pathak, large-scale datasets may be giving a false sense of security. She cited a recent safety analysis of 20,000 U.S. patients who received convalescent plasma, in which the investigators excluded 88.2% of cardiac events and 66.3% of thrombotic events, as these were deemed unrelated to transfusion; but this decision was made by the treating physician, without independent review or a defined protocol.
Michael J. Joyner, MD, of the Mayo Clinic in Rochester, Minn., was the lead author of the above safety study, and is leading the Food and Drug Administration expanded access program for convalescent plasma in patients with COVID-19. He suggested that the study by Dr. Agarwal and colleagues was admirable, but flaws in the treatment protocol cast doubt upon the efficacy findings.
“It is very impressive that these investigators performed a large trial of convalescent plasma in the midst of a pandemic,” Dr. Joyner said. “Unfortunately it is unclear how generalizable the findings are because many of the units of plasma had either very low or no antibody titers and because the plasma was given late in the course of the disease. It has been known since at least the 1930s that antibody therapy works best when enough product is given either prophylactically or early in the course of disease.”
Dr. Joyner had a more positive interpretation of the reported symptom improvements.
“It is also interesting to note that while there was no mortality benefit, that – even with the limitations of the study – there was some evidence of improved patient physiology at 7 days,” he said. “So, at one level, [this is] a negative study, but at least [there are] some hints of efficacy given the suboptimal use case in the patients studied.”
The study was funded by the Indian Council of Medical Research, which employs several of the authors and PLACID Trial Collaborators. Dr. Pathak and Dr. Joyner reported no conflicts of interest.
SOURCE: Agarwal A et al. BMJ. 2020 Oct 23. doi: 10.1136/bmj.m3939 .
FROM BMJ
Selexipag has no effect on daily activity in PAH patients
Selexipag (Uptravi) does not change the level of daily activity of patients with pulmonary arterial hypertension (PAH), results from the phase 4 TRACE trial suggest.
“We had no preconceived idea if this drug would improve exercise capacity,” said Luke Howard, MD, of Imperial College Healthcare NHS Trust in London. It was clear, however, that 6-minute walk tests conducted a few times a year “don’t paint a picture of what daily life is like for patients on selexipag.”
The oral prostacyclin IP receptor agonist is prescribed to slow the progression of PAH and reduce hospital admissions, but there are no studies that show whether it improves quality of life.
Dr. Howard and his team turned to wearable technology to “capture a snapshot of everyday life,” he explained during his presentation at the annual meeting of the American College of Chest Physicians (CHEST 2020), held virtually this year.
The primary concern of the investigators was to get TRACE participants – all with PAH – to wear a wrist device; they did not encourage patients to become more active. “We wanted a true picture of the impact of the drug itself,” he noted.
After 24 months of daily tracking, “there was no benefit to increased daily activity for patients taking this drug,” Dr. Howard said in an interview. “That was a bit deflating.”
The daily activity of TRACE participants was “slightly more elevated” in the selexipag group than in the placebo group. “We saw some numerical drops in activity in the placebo group, and a trend that might make a difference over a longer, bigger study, but not in a statically significant way,” he reported.
In the randomized, blinded trial – the first to track the activity of PAH patients – 53 participants received selexipag and 55 received placebo. All 108 wore a wrist accelerometer (GT9X Link) that counted the number of steps taken each day, providing an indication of daily activity.
Device compliance – the mean number of days in which the device was worn for at least 7 hours during a 14-day predrug period – was similar in the selexipag and placebo groups (13.2 vs 13.0 days).
“We wanted to make sure we had people who were stable and weren’t enrolled in a rehabilitation program; we didn’t want any competing influences,” Dr. Howard explained. All in all, the participants were in pretty good shape. “There was a low risk of a bad outcome.”
The primary endpoint was change in activity from baseline to week 24. The secondary endpoints were PAH-SYMPACT health quality-of-life tests and 6-minute walk distance.
Similar activity levels in both groups
As expected in a population in which the majority of patients meet the criteria for WHO functional class II PAH, all participants had low PAH-SYMPACT domain scores throughout the trial.
All adverse events were “consistent with the known profile” of selexipag, and there were no deaths, Dr. Howard reported.
“We did not show any significant benefit to taking the drug,” he said, but the drug is marketed for the prevention of disease progression, and this finding “doesn’t change that.”
Pulmonary rehabilitation
“Pulmonary rehabilitation is one of the most vital management issues with chronic lung disease,” Riddhi Upadhyay, MD, of Carle Foundation Hospital in Urbana, Ill., said during her CHEST 2020 presentation on improving PAH rehabilitation referral rates.
“We know it improves exercise capacity, lung function, and decreases total hospital stays and recurrent hospital admission,” she explained. And studies have shown that PAH rehabilitation “also reduces frailty and improves quality of life.”
In their study, Dr. Upadhyay and colleagues showed that when pulmonary rehabilitation is added to the discharge order set, referrals increase by 60%.
They attribute their success to “recognizing the benefits of pulmonary rehab and understanding where interventions are required.”
An encouraging takeaway from the TRACE data is that it established that daily activity can be tracked in this patient population. “We think we might need to encourage these patients to get active, maybe combine the drug with a formal rehabilitation program; that might increase motivation,” Dr. Howard said.
“People don’t necessarily do more just because they can,” he noted.
Dr. Howard has received consulting fees from Actelion.
A version of this article originally appeared on Medscape.com.
Selexipag (Uptravi) does not change the level of daily activity of patients with pulmonary arterial hypertension (PAH), results from the phase 4 TRACE trial suggest.
“We had no preconceived idea if this drug would improve exercise capacity,” said Luke Howard, MD, of Imperial College Healthcare NHS Trust in London. It was clear, however, that 6-minute walk tests conducted a few times a year “don’t paint a picture of what daily life is like for patients on selexipag.”
The oral prostacyclin IP receptor agonist is prescribed to slow the progression of PAH and reduce hospital admissions, but there are no studies that show whether it improves quality of life.
Dr. Howard and his team turned to wearable technology to “capture a snapshot of everyday life,” he explained during his presentation at the annual meeting of the American College of Chest Physicians (CHEST 2020), held virtually this year.
The primary concern of the investigators was to get TRACE participants – all with PAH – to wear a wrist device; they did not encourage patients to become more active. “We wanted a true picture of the impact of the drug itself,” he noted.
After 24 months of daily tracking, “there was no benefit to increased daily activity for patients taking this drug,” Dr. Howard said in an interview. “That was a bit deflating.”
The daily activity of TRACE participants was “slightly more elevated” in the selexipag group than in the placebo group. “We saw some numerical drops in activity in the placebo group, and a trend that might make a difference over a longer, bigger study, but not in a statically significant way,” he reported.
In the randomized, blinded trial – the first to track the activity of PAH patients – 53 participants received selexipag and 55 received placebo. All 108 wore a wrist accelerometer (GT9X Link) that counted the number of steps taken each day, providing an indication of daily activity.
Device compliance – the mean number of days in which the device was worn for at least 7 hours during a 14-day predrug period – was similar in the selexipag and placebo groups (13.2 vs 13.0 days).
“We wanted to make sure we had people who were stable and weren’t enrolled in a rehabilitation program; we didn’t want any competing influences,” Dr. Howard explained. All in all, the participants were in pretty good shape. “There was a low risk of a bad outcome.”
The primary endpoint was change in activity from baseline to week 24. The secondary endpoints were PAH-SYMPACT health quality-of-life tests and 6-minute walk distance.
Similar activity levels in both groups
As expected in a population in which the majority of patients meet the criteria for WHO functional class II PAH, all participants had low PAH-SYMPACT domain scores throughout the trial.
All adverse events were “consistent with the known profile” of selexipag, and there were no deaths, Dr. Howard reported.
“We did not show any significant benefit to taking the drug,” he said, but the drug is marketed for the prevention of disease progression, and this finding “doesn’t change that.”
Pulmonary rehabilitation
“Pulmonary rehabilitation is one of the most vital management issues with chronic lung disease,” Riddhi Upadhyay, MD, of Carle Foundation Hospital in Urbana, Ill., said during her CHEST 2020 presentation on improving PAH rehabilitation referral rates.
“We know it improves exercise capacity, lung function, and decreases total hospital stays and recurrent hospital admission,” she explained. And studies have shown that PAH rehabilitation “also reduces frailty and improves quality of life.”
In their study, Dr. Upadhyay and colleagues showed that when pulmonary rehabilitation is added to the discharge order set, referrals increase by 60%.
They attribute their success to “recognizing the benefits of pulmonary rehab and understanding where interventions are required.”
An encouraging takeaway from the TRACE data is that it established that daily activity can be tracked in this patient population. “We think we might need to encourage these patients to get active, maybe combine the drug with a formal rehabilitation program; that might increase motivation,” Dr. Howard said.
“People don’t necessarily do more just because they can,” he noted.
Dr. Howard has received consulting fees from Actelion.
A version of this article originally appeared on Medscape.com.
Selexipag (Uptravi) does not change the level of daily activity of patients with pulmonary arterial hypertension (PAH), results from the phase 4 TRACE trial suggest.
“We had no preconceived idea if this drug would improve exercise capacity,” said Luke Howard, MD, of Imperial College Healthcare NHS Trust in London. It was clear, however, that 6-minute walk tests conducted a few times a year “don’t paint a picture of what daily life is like for patients on selexipag.”
The oral prostacyclin IP receptor agonist is prescribed to slow the progression of PAH and reduce hospital admissions, but there are no studies that show whether it improves quality of life.
Dr. Howard and his team turned to wearable technology to “capture a snapshot of everyday life,” he explained during his presentation at the annual meeting of the American College of Chest Physicians (CHEST 2020), held virtually this year.
The primary concern of the investigators was to get TRACE participants – all with PAH – to wear a wrist device; they did not encourage patients to become more active. “We wanted a true picture of the impact of the drug itself,” he noted.
After 24 months of daily tracking, “there was no benefit to increased daily activity for patients taking this drug,” Dr. Howard said in an interview. “That was a bit deflating.”
The daily activity of TRACE participants was “slightly more elevated” in the selexipag group than in the placebo group. “We saw some numerical drops in activity in the placebo group, and a trend that might make a difference over a longer, bigger study, but not in a statically significant way,” he reported.
In the randomized, blinded trial – the first to track the activity of PAH patients – 53 participants received selexipag and 55 received placebo. All 108 wore a wrist accelerometer (GT9X Link) that counted the number of steps taken each day, providing an indication of daily activity.
Device compliance – the mean number of days in which the device was worn for at least 7 hours during a 14-day predrug period – was similar in the selexipag and placebo groups (13.2 vs 13.0 days).
“We wanted to make sure we had people who were stable and weren’t enrolled in a rehabilitation program; we didn’t want any competing influences,” Dr. Howard explained. All in all, the participants were in pretty good shape. “There was a low risk of a bad outcome.”
The primary endpoint was change in activity from baseline to week 24. The secondary endpoints were PAH-SYMPACT health quality-of-life tests and 6-minute walk distance.
Similar activity levels in both groups
As expected in a population in which the majority of patients meet the criteria for WHO functional class II PAH, all participants had low PAH-SYMPACT domain scores throughout the trial.
All adverse events were “consistent with the known profile” of selexipag, and there were no deaths, Dr. Howard reported.
“We did not show any significant benefit to taking the drug,” he said, but the drug is marketed for the prevention of disease progression, and this finding “doesn’t change that.”
Pulmonary rehabilitation
“Pulmonary rehabilitation is one of the most vital management issues with chronic lung disease,” Riddhi Upadhyay, MD, of Carle Foundation Hospital in Urbana, Ill., said during her CHEST 2020 presentation on improving PAH rehabilitation referral rates.
“We know it improves exercise capacity, lung function, and decreases total hospital stays and recurrent hospital admission,” she explained. And studies have shown that PAH rehabilitation “also reduces frailty and improves quality of life.”
In their study, Dr. Upadhyay and colleagues showed that when pulmonary rehabilitation is added to the discharge order set, referrals increase by 60%.
They attribute their success to “recognizing the benefits of pulmonary rehab and understanding where interventions are required.”
An encouraging takeaway from the TRACE data is that it established that daily activity can be tracked in this patient population. “We think we might need to encourage these patients to get active, maybe combine the drug with a formal rehabilitation program; that might increase motivation,” Dr. Howard said.
“People don’t necessarily do more just because they can,” he noted.
Dr. Howard has received consulting fees from Actelion.
A version of this article originally appeared on Medscape.com.
COVID-19 experience forced residents to quickly improve patient communication skills
While the spring peak of COVID-19 was tough and traumatic for many residents and interns in a New York City health system, the experience may have accelerated their patient communication skills regarding difficult goals-of-care discussions, results of a recent survey suggest.
Breaking bad news was an everyday or every-other-day occurrence at the peak of the pandemic for nearly all of 50 of the trainees surveyed, who had worked at hospitals affiliated with the internal medicine residency program at the at the Icahn School of Medicine at Mount Sinai from March to June 2020.
However, trainees became significantly more comfortable and fluent in goals-of-care discussions during the pandemic, according to Patrick Tobin-Schnittger, MBBS, a third-year internal medicine resident in the Mount Sinai program.
“COVID-19 has obviously made a huge impact on the world, but I think it’s also made a huge impact on a whole generation of junior doctors,” said Dr. Tobin-Schnittger, who presented the findings in a late-breaking abstract session at the CHEST Annual Meeting, held virtually this year.
“It’ll be interesting to see what happens in the future as that generation matures, and I think one of the things is that we’re a lot more comfortable with end-of-life care,” he said in an interview conducted during the conference.
Nevertheless, coping with death may still be a challenge for many residents, according to Dr. Tobin-Schnittger. In the survey, internal medicine residents who had rarely encountered patient deaths suddenly found themselves experiencing deaths weekly, with more than one in five saying they were encountering it every day.
When asked to self-rate themselves according to Bugen’s Coping With Death scale, most participants had scores that suggested their ability to cope was suboptimal, the researcher said.
To help trainees cope with local COVID-19 surges, internal medicine residency programs should be implementing “breaking bad news” workshops and educating house staff on resilience in times of crisis, especially if it can be done virtually, according to Dr. Tobin-Schnittger.
“That could be done pretty quickly, and it could be done remotely so people could practice this from home,” he explained. “They wouldn’t even need to congregate in a big room.”
As a “mini-surge” of COVID-19 cases hits the United States, teaching self-care and coping techniques may also be important, said Mangala Narasimhan, DO, FCCP, director of critical care services at Northwell Health in New York City.
“We’ve had several sessions in our health system of letting people vent, talk about what happened, and tell stories about patients that they are still thinking about and haunted by – there was so much death,” Dr. Narasimhan said in an interview.
“People will be suffering for a long time thinking about what happened in March and April and May, so I think our focus now needs to be how to fix that in any way we can and to support people, as we’re dealing with these increases in numbers,” she said. “I think everyone’s panicking over the increase in numbers, but they’re panicking because of the fear of going through what they went through before.”
Dr. Tobin-Schnittger and colleagues sent their survey to 94 residents and interns in the Mount Sinai program who had worked through the peak of the pandemic. They received 50 responses. Of those individuals, the mean age was 29.5 years, and about 46% had worked for more than 3 years.
Before the pandemic, only 3 of the 50 respondents reported having goals-of-care conversations every day or every other day, while during the pandemic, those conversations were happening at least every other day for 38 of the respondents, survey data show.
Self-reported fluency and comfort with those discussions increased significantly, from a mean of about 50 on a scale of 100 before the pandemic to more than 75 during the pandemic, according to Dr. Tobin-Schnittger.
When asked how they remembered coping with patient death, one respondent described holding up a phone so a dying patient could hear his daughter’s voice. Another reported not being able to sleep at night.
“I constantly would have dreams that my patients were dying and there was nothing I could do about it,” the respondent said in a survey response.
A third respondent described the experience as ”humbling” but said there were rewarding aspects in patient care during the peak of the pandemic, which helped in being able to focus during difficult days.
Three participants (7.7%) said they changed their career plans as a result of the pandemic experience, the researchers reported.
Negative consequences of the peak pandemic experience included anger, anxiety, professional strain, trauma, and emotional distancing, some respondents reported.
However, others called attention to positive outcomes, such as more professional pride, resilience, confidence, and camaraderie.
“While we did encounter a lot of traumatic experiences, overall, there’s a huge sense that there is a lot more camaraderie within our department, but also within other departments,” said Dr. Tobin-Schnittger. “So I think there are some positives that come from this, and I think there’s been a bit of a culture change.”
Dr. Tobin-Schnittger said that he and his coauthors had no conflicts of interest or relationships with commercial interests to report.
SOURCE: Tobin-Schnittger P. CHEST 2020. Late-breaking abstract. doi: 10.1016/j.chest.2020.09.040.
While the spring peak of COVID-19 was tough and traumatic for many residents and interns in a New York City health system, the experience may have accelerated their patient communication skills regarding difficult goals-of-care discussions, results of a recent survey suggest.
Breaking bad news was an everyday or every-other-day occurrence at the peak of the pandemic for nearly all of 50 of the trainees surveyed, who had worked at hospitals affiliated with the internal medicine residency program at the at the Icahn School of Medicine at Mount Sinai from March to June 2020.
However, trainees became significantly more comfortable and fluent in goals-of-care discussions during the pandemic, according to Patrick Tobin-Schnittger, MBBS, a third-year internal medicine resident in the Mount Sinai program.
“COVID-19 has obviously made a huge impact on the world, but I think it’s also made a huge impact on a whole generation of junior doctors,” said Dr. Tobin-Schnittger, who presented the findings in a late-breaking abstract session at the CHEST Annual Meeting, held virtually this year.
“It’ll be interesting to see what happens in the future as that generation matures, and I think one of the things is that we’re a lot more comfortable with end-of-life care,” he said in an interview conducted during the conference.
Nevertheless, coping with death may still be a challenge for many residents, according to Dr. Tobin-Schnittger. In the survey, internal medicine residents who had rarely encountered patient deaths suddenly found themselves experiencing deaths weekly, with more than one in five saying they were encountering it every day.
When asked to self-rate themselves according to Bugen’s Coping With Death scale, most participants had scores that suggested their ability to cope was suboptimal, the researcher said.
To help trainees cope with local COVID-19 surges, internal medicine residency programs should be implementing “breaking bad news” workshops and educating house staff on resilience in times of crisis, especially if it can be done virtually, according to Dr. Tobin-Schnittger.
“That could be done pretty quickly, and it could be done remotely so people could practice this from home,” he explained. “They wouldn’t even need to congregate in a big room.”
As a “mini-surge” of COVID-19 cases hits the United States, teaching self-care and coping techniques may also be important, said Mangala Narasimhan, DO, FCCP, director of critical care services at Northwell Health in New York City.
“We’ve had several sessions in our health system of letting people vent, talk about what happened, and tell stories about patients that they are still thinking about and haunted by – there was so much death,” Dr. Narasimhan said in an interview.
“People will be suffering for a long time thinking about what happened in March and April and May, so I think our focus now needs to be how to fix that in any way we can and to support people, as we’re dealing with these increases in numbers,” she said. “I think everyone’s panicking over the increase in numbers, but they’re panicking because of the fear of going through what they went through before.”
Dr. Tobin-Schnittger and colleagues sent their survey to 94 residents and interns in the Mount Sinai program who had worked through the peak of the pandemic. They received 50 responses. Of those individuals, the mean age was 29.5 years, and about 46% had worked for more than 3 years.
Before the pandemic, only 3 of the 50 respondents reported having goals-of-care conversations every day or every other day, while during the pandemic, those conversations were happening at least every other day for 38 of the respondents, survey data show.
Self-reported fluency and comfort with those discussions increased significantly, from a mean of about 50 on a scale of 100 before the pandemic to more than 75 during the pandemic, according to Dr. Tobin-Schnittger.
When asked how they remembered coping with patient death, one respondent described holding up a phone so a dying patient could hear his daughter’s voice. Another reported not being able to sleep at night.
“I constantly would have dreams that my patients were dying and there was nothing I could do about it,” the respondent said in a survey response.
A third respondent described the experience as ”humbling” but said there were rewarding aspects in patient care during the peak of the pandemic, which helped in being able to focus during difficult days.
Three participants (7.7%) said they changed their career plans as a result of the pandemic experience, the researchers reported.
Negative consequences of the peak pandemic experience included anger, anxiety, professional strain, trauma, and emotional distancing, some respondents reported.
However, others called attention to positive outcomes, such as more professional pride, resilience, confidence, and camaraderie.
“While we did encounter a lot of traumatic experiences, overall, there’s a huge sense that there is a lot more camaraderie within our department, but also within other departments,” said Dr. Tobin-Schnittger. “So I think there are some positives that come from this, and I think there’s been a bit of a culture change.”
Dr. Tobin-Schnittger said that he and his coauthors had no conflicts of interest or relationships with commercial interests to report.
SOURCE: Tobin-Schnittger P. CHEST 2020. Late-breaking abstract. doi: 10.1016/j.chest.2020.09.040.
While the spring peak of COVID-19 was tough and traumatic for many residents and interns in a New York City health system, the experience may have accelerated their patient communication skills regarding difficult goals-of-care discussions, results of a recent survey suggest.
Breaking bad news was an everyday or every-other-day occurrence at the peak of the pandemic for nearly all of 50 of the trainees surveyed, who had worked at hospitals affiliated with the internal medicine residency program at the at the Icahn School of Medicine at Mount Sinai from March to June 2020.
However, trainees became significantly more comfortable and fluent in goals-of-care discussions during the pandemic, according to Patrick Tobin-Schnittger, MBBS, a third-year internal medicine resident in the Mount Sinai program.
“COVID-19 has obviously made a huge impact on the world, but I think it’s also made a huge impact on a whole generation of junior doctors,” said Dr. Tobin-Schnittger, who presented the findings in a late-breaking abstract session at the CHEST Annual Meeting, held virtually this year.
“It’ll be interesting to see what happens in the future as that generation matures, and I think one of the things is that we’re a lot more comfortable with end-of-life care,” he said in an interview conducted during the conference.
Nevertheless, coping with death may still be a challenge for many residents, according to Dr. Tobin-Schnittger. In the survey, internal medicine residents who had rarely encountered patient deaths suddenly found themselves experiencing deaths weekly, with more than one in five saying they were encountering it every day.
When asked to self-rate themselves according to Bugen’s Coping With Death scale, most participants had scores that suggested their ability to cope was suboptimal, the researcher said.
To help trainees cope with local COVID-19 surges, internal medicine residency programs should be implementing “breaking bad news” workshops and educating house staff on resilience in times of crisis, especially if it can be done virtually, according to Dr. Tobin-Schnittger.
“That could be done pretty quickly, and it could be done remotely so people could practice this from home,” he explained. “They wouldn’t even need to congregate in a big room.”
As a “mini-surge” of COVID-19 cases hits the United States, teaching self-care and coping techniques may also be important, said Mangala Narasimhan, DO, FCCP, director of critical care services at Northwell Health in New York City.
“We’ve had several sessions in our health system of letting people vent, talk about what happened, and tell stories about patients that they are still thinking about and haunted by – there was so much death,” Dr. Narasimhan said in an interview.
“People will be suffering for a long time thinking about what happened in March and April and May, so I think our focus now needs to be how to fix that in any way we can and to support people, as we’re dealing with these increases in numbers,” she said. “I think everyone’s panicking over the increase in numbers, but they’re panicking because of the fear of going through what they went through before.”
Dr. Tobin-Schnittger and colleagues sent their survey to 94 residents and interns in the Mount Sinai program who had worked through the peak of the pandemic. They received 50 responses. Of those individuals, the mean age was 29.5 years, and about 46% had worked for more than 3 years.
Before the pandemic, only 3 of the 50 respondents reported having goals-of-care conversations every day or every other day, while during the pandemic, those conversations were happening at least every other day for 38 of the respondents, survey data show.
Self-reported fluency and comfort with those discussions increased significantly, from a mean of about 50 on a scale of 100 before the pandemic to more than 75 during the pandemic, according to Dr. Tobin-Schnittger.
When asked how they remembered coping with patient death, one respondent described holding up a phone so a dying patient could hear his daughter’s voice. Another reported not being able to sleep at night.
“I constantly would have dreams that my patients were dying and there was nothing I could do about it,” the respondent said in a survey response.
A third respondent described the experience as ”humbling” but said there were rewarding aspects in patient care during the peak of the pandemic, which helped in being able to focus during difficult days.
Three participants (7.7%) said they changed their career plans as a result of the pandemic experience, the researchers reported.
Negative consequences of the peak pandemic experience included anger, anxiety, professional strain, trauma, and emotional distancing, some respondents reported.
However, others called attention to positive outcomes, such as more professional pride, resilience, confidence, and camaraderie.
“While we did encounter a lot of traumatic experiences, overall, there’s a huge sense that there is a lot more camaraderie within our department, but also within other departments,” said Dr. Tobin-Schnittger. “So I think there are some positives that come from this, and I think there’s been a bit of a culture change.”
Dr. Tobin-Schnittger said that he and his coauthors had no conflicts of interest or relationships with commercial interests to report.
SOURCE: Tobin-Schnittger P. CHEST 2020. Late-breaking abstract. doi: 10.1016/j.chest.2020.09.040.
FROM CHEST 2020
Outpatient visits rebound for most specialties to pre-COVID-19 levels
, according to new data.
Overall visits plunged by almost 60% at the low point in late March and did not start recovering until late June, when visits were still off by 10%. Visits began to rise again – by 2% over the March 1 baseline – around Labor Day.
As of Oct. 4, visits had returned to that March 1 baseline, which was slightly higher than in late February, according to data analyzed by Harvard University, the Commonwealth Fund, and the healthcare technology company Phreesia, which helps medical practices with patient registration, insurance verification, and payments, and has data on 50,000 providers in all 50 states.
The study was published online by the Commonwealth Fund.
In-person visits are still down 6% from the March 1 baseline. Telemedicine visits – which surged in mid-April to account for some 13%-14% of visits – have subsided to 6% of visits.
Many states reopened businesses and lifted travel restrictions in early September, benefiting medical practices in some areas. But clinicians in some regions are still facing rising COVID-19 cases, as well as “the challenges of keeping patients and clinicians safe while also maintaining revenue,” wrote the report authors.
Some specialties are still hard hit. For the week starting Oct. 4, visits to pulmonologists were off 20% from March 1. Otolaryngology visits were down 17%, and behavioral health visits were down 14%. Cardiology, allergy/immunology, neurology, gastroenterology, and endocrinology also saw drops of 5%-10% from March.
Patients were flocking to dermatologists, however. Visits were up 17% over baseline. Primary care also was popular, with a 13% increase over March 1.
At the height of the pandemic shutdown in late March, Medicare beneficiaries stayed away from doctors the most. Visits dipped 63%, compared with 56% for the commercially insured, and 52% for those on Medicaid. Now, Medicare visits are up 3% over baseline, while Medicaid visits are down 1% and commercially insured visits have risen 1% from March.
The over-65 age group did not have the steepest drop in visits when analyzed by age. Children aged 3-17 years saw the biggest decline at the height of the shutdown. Infants to 5-year-olds have still not returned to prepandemic visit levels. Those visits are off by 10%-18%. The 65-and-older group is up 4% from March.
Larger practices – with more than six clinicians – have seen the biggest rebound, after having had the largest dip in visits, from a decline of 53% in late March to a 14% rise over that baseline. Practices with fewer than five clinicians are still 6% down from the March baseline.
Wide variation in telemedicine use
The researchers reported a massive gap in the percentage of various specialties that are using telemedicine. At the top end are behavioral health specialists, where 41% of visits are by telemedicine.
The next-closest specialty is endocrinology, which has 14% of visits via telemedicine, on par with rheumatology, neurology, and gastroenterology. At the low end: ophthalmology, with zero virtual visits; otolaryngology (1%), orthopedics (1%), surgery (2%), and dermatology and ob.gyn., both at 3%.
Smaller practices – with fewer than five clinicians – never adopted telemedicine at the rate of the larger practices. During the mid-April peak, about 10% of the smaller practices were using telemedicine in adult primary care practices, compared with 19% of those primary care practices with more than six clinicians.
The gap persists. Currently, 9% of the larger practices are using telemedicine, compared with 4% of small practices.
One-third of all provider organizations analyzed never-adopted telemedicine. And while use continues, it is now mostly minimal. At the April peak, 35% of the practices with telemedicine reported heavy use – that is, in more than 20% of visits. In September, 9% said they had such heavy use.
A version of this article originally appeared on Medscape.com.
, according to new data.
Overall visits plunged by almost 60% at the low point in late March and did not start recovering until late June, when visits were still off by 10%. Visits began to rise again – by 2% over the March 1 baseline – around Labor Day.
As of Oct. 4, visits had returned to that March 1 baseline, which was slightly higher than in late February, according to data analyzed by Harvard University, the Commonwealth Fund, and the healthcare technology company Phreesia, which helps medical practices with patient registration, insurance verification, and payments, and has data on 50,000 providers in all 50 states.
The study was published online by the Commonwealth Fund.
In-person visits are still down 6% from the March 1 baseline. Telemedicine visits – which surged in mid-April to account for some 13%-14% of visits – have subsided to 6% of visits.
Many states reopened businesses and lifted travel restrictions in early September, benefiting medical practices in some areas. But clinicians in some regions are still facing rising COVID-19 cases, as well as “the challenges of keeping patients and clinicians safe while also maintaining revenue,” wrote the report authors.
Some specialties are still hard hit. For the week starting Oct. 4, visits to pulmonologists were off 20% from March 1. Otolaryngology visits were down 17%, and behavioral health visits were down 14%. Cardiology, allergy/immunology, neurology, gastroenterology, and endocrinology also saw drops of 5%-10% from March.
Patients were flocking to dermatologists, however. Visits were up 17% over baseline. Primary care also was popular, with a 13% increase over March 1.
At the height of the pandemic shutdown in late March, Medicare beneficiaries stayed away from doctors the most. Visits dipped 63%, compared with 56% for the commercially insured, and 52% for those on Medicaid. Now, Medicare visits are up 3% over baseline, while Medicaid visits are down 1% and commercially insured visits have risen 1% from March.
The over-65 age group did not have the steepest drop in visits when analyzed by age. Children aged 3-17 years saw the biggest decline at the height of the shutdown. Infants to 5-year-olds have still not returned to prepandemic visit levels. Those visits are off by 10%-18%. The 65-and-older group is up 4% from March.
Larger practices – with more than six clinicians – have seen the biggest rebound, after having had the largest dip in visits, from a decline of 53% in late March to a 14% rise over that baseline. Practices with fewer than five clinicians are still 6% down from the March baseline.
Wide variation in telemedicine use
The researchers reported a massive gap in the percentage of various specialties that are using telemedicine. At the top end are behavioral health specialists, where 41% of visits are by telemedicine.
The next-closest specialty is endocrinology, which has 14% of visits via telemedicine, on par with rheumatology, neurology, and gastroenterology. At the low end: ophthalmology, with zero virtual visits; otolaryngology (1%), orthopedics (1%), surgery (2%), and dermatology and ob.gyn., both at 3%.
Smaller practices – with fewer than five clinicians – never adopted telemedicine at the rate of the larger practices. During the mid-April peak, about 10% of the smaller practices were using telemedicine in adult primary care practices, compared with 19% of those primary care practices with more than six clinicians.
The gap persists. Currently, 9% of the larger practices are using telemedicine, compared with 4% of small practices.
One-third of all provider organizations analyzed never-adopted telemedicine. And while use continues, it is now mostly minimal. At the April peak, 35% of the practices with telemedicine reported heavy use – that is, in more than 20% of visits. In September, 9% said they had such heavy use.
A version of this article originally appeared on Medscape.com.
, according to new data.
Overall visits plunged by almost 60% at the low point in late March and did not start recovering until late June, when visits were still off by 10%. Visits began to rise again – by 2% over the March 1 baseline – around Labor Day.
As of Oct. 4, visits had returned to that March 1 baseline, which was slightly higher than in late February, according to data analyzed by Harvard University, the Commonwealth Fund, and the healthcare technology company Phreesia, which helps medical practices with patient registration, insurance verification, and payments, and has data on 50,000 providers in all 50 states.
The study was published online by the Commonwealth Fund.
In-person visits are still down 6% from the March 1 baseline. Telemedicine visits – which surged in mid-April to account for some 13%-14% of visits – have subsided to 6% of visits.
Many states reopened businesses and lifted travel restrictions in early September, benefiting medical practices in some areas. But clinicians in some regions are still facing rising COVID-19 cases, as well as “the challenges of keeping patients and clinicians safe while also maintaining revenue,” wrote the report authors.
Some specialties are still hard hit. For the week starting Oct. 4, visits to pulmonologists were off 20% from March 1. Otolaryngology visits were down 17%, and behavioral health visits were down 14%. Cardiology, allergy/immunology, neurology, gastroenterology, and endocrinology also saw drops of 5%-10% from March.
Patients were flocking to dermatologists, however. Visits were up 17% over baseline. Primary care also was popular, with a 13% increase over March 1.
At the height of the pandemic shutdown in late March, Medicare beneficiaries stayed away from doctors the most. Visits dipped 63%, compared with 56% for the commercially insured, and 52% for those on Medicaid. Now, Medicare visits are up 3% over baseline, while Medicaid visits are down 1% and commercially insured visits have risen 1% from March.
The over-65 age group did not have the steepest drop in visits when analyzed by age. Children aged 3-17 years saw the biggest decline at the height of the shutdown. Infants to 5-year-olds have still not returned to prepandemic visit levels. Those visits are off by 10%-18%. The 65-and-older group is up 4% from March.
Larger practices – with more than six clinicians – have seen the biggest rebound, after having had the largest dip in visits, from a decline of 53% in late March to a 14% rise over that baseline. Practices with fewer than five clinicians are still 6% down from the March baseline.
Wide variation in telemedicine use
The researchers reported a massive gap in the percentage of various specialties that are using telemedicine. At the top end are behavioral health specialists, where 41% of visits are by telemedicine.
The next-closest specialty is endocrinology, which has 14% of visits via telemedicine, on par with rheumatology, neurology, and gastroenterology. At the low end: ophthalmology, with zero virtual visits; otolaryngology (1%), orthopedics (1%), surgery (2%), and dermatology and ob.gyn., both at 3%.
Smaller practices – with fewer than five clinicians – never adopted telemedicine at the rate of the larger practices. During the mid-April peak, about 10% of the smaller practices were using telemedicine in adult primary care practices, compared with 19% of those primary care practices with more than six clinicians.
The gap persists. Currently, 9% of the larger practices are using telemedicine, compared with 4% of small practices.
One-third of all provider organizations analyzed never-adopted telemedicine. And while use continues, it is now mostly minimal. At the April peak, 35% of the practices with telemedicine reported heavy use – that is, in more than 20% of visits. In September, 9% said they had such heavy use.
A version of this article originally appeared on Medscape.com.