User login
Taking cardiac pacing from boring to super cool
For the past 2 decades, catheter ablation stole most of the excitement in electrophysiology. Cardiac pacing was seen as necessary but boring. His-bundle pacing earned only modest attention.
But at the annual scientific sessions of the Heart Rhythm Society, cardiac pacing consolidated its comeback and entered the super-cool category.
Not one but three late-breaking clinical trials considered the role of pacing the heart’s conduction system for both preventive and therapeutic purposes. Conduction system pacing, or CSP as we call it, includes pacing the His bundle or the left bundle branch. Left bundle–branch pacing has now largely replaced His-bundle pacing.
Before I tell you about the studies, let’s review why CSP disrupts the status quo.
The core idea goes back to basic physiology: After the impulse leaves the atrioventricular node, the heart’s specialized conduction system allows rapid and synchronous conduction to both the right and left ventricles.
Standard cardiac pacing means fixing a pacing lead into the muscle of the right ventricle. From that spot, conduction spreads via slower muscle-to-muscle conduction, which leads to a wide QRS complex and the right ventricle contracts before the left ventricle.
While such dyssynchronous contraction is better than no contraction, this approach leads to a pacing-induced cardiomyopathy in a substantial number of cases. (The incidence reported in many studies varies widely.)
The most disruptive effect of conduction system pacing is that it is a form of cardiac resynchronization therapy (CRT). And that is nifty because, until recently, resynchronizing the ventricles required placing two ventricular leads: one in the right ventricle and the other in the coronary sinus to pace the left ventricle.
Left bundle-branch pacing vs. biventricular pacing
The first of the three HRS studies is the LBBP-RESYNC randomized controlled trial led by Jiangang Zou, MD, PhD, and performed in multiple centers in China. It compared the efficacy of left bundle–branch pacing (LBBP) with that of conventional biventricular pacing in 40 patients with heart failure who were eligible for CRT. The primary endpoint was the change in left ventricular ejection fraction (LVEF) from baseline to 6-month follow-up.
The results favored LBBP. Although both pacing techniques improved LVEF from baseline, the between-group difference in LVEF was greater in the LBBP arm than the biventricular pacing arm by a statistically significant 5.6% (95% confidence interval, 0.3%-10.9%). Secondary endpoints, such as reductions in left ventricular end-systolic volume, N-terminal of the prohormone brain natriuretic peptide, and QRS duration, also favored LBBP.
Conduction system pacing vs. biventricular pacing
A second late-breaking study, from the Geisinger group, led by Pugazhendhi Vijayaraman, MD, was simultaneously published in Heart Rhythm.
This nonrandomized observational study compared nearly 500 patients eligible for CRT treated at two health systems. One group favors conduction system pacing and the other does traditional biventricular pacing, which set up a two-armed comparison.
CSP was accomplished by LBBP (65%) and His-bundle pacing (35%).
The primary endpoint of death or first hospitalization for heart failure occurred in 28.3% of patients in the CSP arm versus 38.4% of the biventricular arm (hazard ratio, 1.52; 95% CI, 1.08-2.09). QRS duration and LVEF also improved from baseline in both groups.
LBB area pacing as a bailout for failed CRT
The Geisinger group also presented and published an international multicenter study that assessed the feasibility of LBBP as a bailout when standard biventricular pacing did not work – because of inadequate coronary sinus anatomy or CRT nonresponse, defined as lack of clinical or echocardiographic improvement.
This series included 212 patients in whom CRT failed and who underwent attempted LBBP pacing. The bailout was successful in 200 patients (91%). The primary endpoint was defined as an increase in LVEF above 5% on echocardiography.
During 12-month follow-up, 61% of patients had an improvement in LVEF above 5% and nearly 30% had a “super-response,” defined as a 20% or greater increase or normalization of LVEF. Similar to the previous studies, LBBP resulted in shorter QRS duration and improved echocardiography parameters.
Am I persuaded?
I was an early adopter of His-bundle pacing. When successful, it delivered both aesthetically pleasing QRS complexes and clinical efficacy. But there were many challenges: it is technically difficult, and capture thresholds are often high at implant and get higher over time, which leads to shorter battery life.
Pacing the left bundle branch mitigates these challenges. Here, the operator approaches from the right side and screws the lead a few millimeters into the septum, so the tip of the lead can capture the left bundle or one of its branches. This allows activation of the heart’s specialized conduction system and thus synchronizes right and left ventricle contraction.
Although there is a learning curve, LBBP is technically easier than His-bundle pacing and ultimately results in far better pacing and sensing parameters. What’s more, the preferred lead for LBBP has a stellar efficacy record – over years.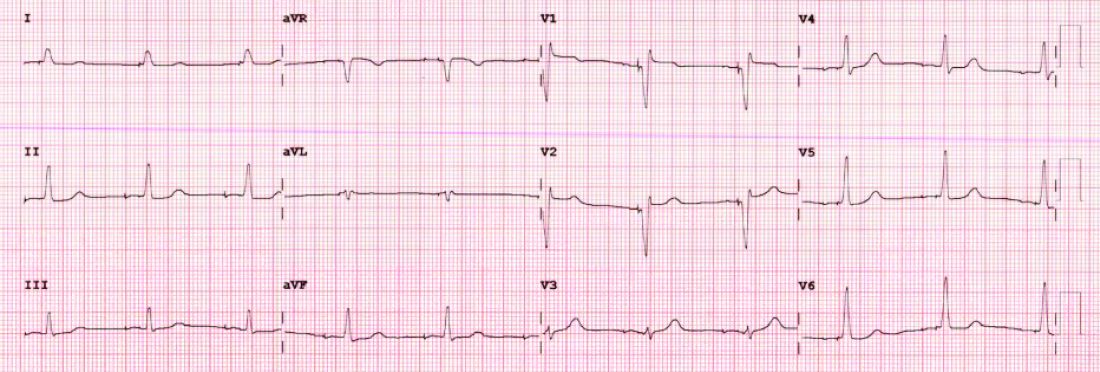
I have become enthralled by the gorgeous QRS complexes from LBBP. The ability to pace the heart without creating dyssynchrony infuses me with joy. I chose cardiology largely because of the beauty of the ECG.
But as a medical conservative who is cautious about unproven therapies, I have questions. How is LBBP defined? Is left septal pacing good enough, or do you need actual left bundle capture? What about long-term performance of a lead in the septum?
Biventricular pacing has set a high bar because it has been proven effective for reducing hard clinical outcomes in large randomized controlled trials.
The studies at HRS begin to answer these questions. The randomized controlled trial from China supports the notion that effective LBBP (the investigators rigorously defined left bundle capture) leads to favorable effects on cardiac contraction. The two observational studies reported similarly encouraging findings on cardiac function.
The three studies therefore tentatively support the notion that LBBP actually produces favorable cardiac performance.
Whether LBBP leads to better clinical outcomes remains uncertain. The nonrandomized comparison study, which found better hard outcomes in the CSP arm, cannot be used to infer causality. There is too much risk for selection bias.
But the LBBP bailout study does suggest that this strategy is reasonable when coronary sinus leads fail – especially since the alternative is surgical placement of an epicardial lead on the left ventricle.
At minimum, the HRS studies persuade me that LBBP will likely prevent pacing-induced cardiomyopathy. If I or a family member required a pacemaker, I’d surely want the operator to be skilled at placing a left bundle lead.
While I am confident that conduction system pacing will become a transformative advance in cardiac pacing, aesthetically pleasing ECG patterns are not enough. There remains much to learn with this nascent approach.
The barriers to getting more CSP trials
The challenge going forward will be funding new trials. CSP stands to prevent pacing-induced cardiomyopathy and offer less costly alternatives to standard biventricular pacing for CRT. This is great for patients, but it would mean that fewer higher-cost CRT devices will be sold.
Heart rhythm research is largely industry-funded because in most cases better therapies for patients mean more profits for industry. In the case of CSP, there is no such confluence of interests.
Conduction system pacing has come about because of the efforts of a few tireless champions who not only published extensively but were also skilled at using social media to spread the excitement. Trials have been small and often self-funded.
The data presented at HRS provides enough equipoise to support a large outcomes-based randomized controlled trial. Imagine if our CSP champions were able to find public-funding sources for such future trials.
Now that would be super cool.
Dr. Mandrola practices cardiac electrophysiology in Louisville, Ky., and is a writer and podcaster for Medscape. He participates in clinical research and writes often about the state of medical evidence. He has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
For the past 2 decades, catheter ablation stole most of the excitement in electrophysiology. Cardiac pacing was seen as necessary but boring. His-bundle pacing earned only modest attention.
But at the annual scientific sessions of the Heart Rhythm Society, cardiac pacing consolidated its comeback and entered the super-cool category.
Not one but three late-breaking clinical trials considered the role of pacing the heart’s conduction system for both preventive and therapeutic purposes. Conduction system pacing, or CSP as we call it, includes pacing the His bundle or the left bundle branch. Left bundle–branch pacing has now largely replaced His-bundle pacing.
Before I tell you about the studies, let’s review why CSP disrupts the status quo.
The core idea goes back to basic physiology: After the impulse leaves the atrioventricular node, the heart’s specialized conduction system allows rapid and synchronous conduction to both the right and left ventricles.
Standard cardiac pacing means fixing a pacing lead into the muscle of the right ventricle. From that spot, conduction spreads via slower muscle-to-muscle conduction, which leads to a wide QRS complex and the right ventricle contracts before the left ventricle.
While such dyssynchronous contraction is better than no contraction, this approach leads to a pacing-induced cardiomyopathy in a substantial number of cases. (The incidence reported in many studies varies widely.)
The most disruptive effect of conduction system pacing is that it is a form of cardiac resynchronization therapy (CRT). And that is nifty because, until recently, resynchronizing the ventricles required placing two ventricular leads: one in the right ventricle and the other in the coronary sinus to pace the left ventricle.
Left bundle-branch pacing vs. biventricular pacing
The first of the three HRS studies is the LBBP-RESYNC randomized controlled trial led by Jiangang Zou, MD, PhD, and performed in multiple centers in China. It compared the efficacy of left bundle–branch pacing (LBBP) with that of conventional biventricular pacing in 40 patients with heart failure who were eligible for CRT. The primary endpoint was the change in left ventricular ejection fraction (LVEF) from baseline to 6-month follow-up.
The results favored LBBP. Although both pacing techniques improved LVEF from baseline, the between-group difference in LVEF was greater in the LBBP arm than the biventricular pacing arm by a statistically significant 5.6% (95% confidence interval, 0.3%-10.9%). Secondary endpoints, such as reductions in left ventricular end-systolic volume, N-terminal of the prohormone brain natriuretic peptide, and QRS duration, also favored LBBP.
Conduction system pacing vs. biventricular pacing
A second late-breaking study, from the Geisinger group, led by Pugazhendhi Vijayaraman, MD, was simultaneously published in Heart Rhythm.
This nonrandomized observational study compared nearly 500 patients eligible for CRT treated at two health systems. One group favors conduction system pacing and the other does traditional biventricular pacing, which set up a two-armed comparison.
CSP was accomplished by LBBP (65%) and His-bundle pacing (35%).
The primary endpoint of death or first hospitalization for heart failure occurred in 28.3% of patients in the CSP arm versus 38.4% of the biventricular arm (hazard ratio, 1.52; 95% CI, 1.08-2.09). QRS duration and LVEF also improved from baseline in both groups.
LBB area pacing as a bailout for failed CRT
The Geisinger group also presented and published an international multicenter study that assessed the feasibility of LBBP as a bailout when standard biventricular pacing did not work – because of inadequate coronary sinus anatomy or CRT nonresponse, defined as lack of clinical or echocardiographic improvement.
This series included 212 patients in whom CRT failed and who underwent attempted LBBP pacing. The bailout was successful in 200 patients (91%). The primary endpoint was defined as an increase in LVEF above 5% on echocardiography.
During 12-month follow-up, 61% of patients had an improvement in LVEF above 5% and nearly 30% had a “super-response,” defined as a 20% or greater increase or normalization of LVEF. Similar to the previous studies, LBBP resulted in shorter QRS duration and improved echocardiography parameters.
Am I persuaded?
I was an early adopter of His-bundle pacing. When successful, it delivered both aesthetically pleasing QRS complexes and clinical efficacy. But there were many challenges: it is technically difficult, and capture thresholds are often high at implant and get higher over time, which leads to shorter battery life.
Pacing the left bundle branch mitigates these challenges. Here, the operator approaches from the right side and screws the lead a few millimeters into the septum, so the tip of the lead can capture the left bundle or one of its branches. This allows activation of the heart’s specialized conduction system and thus synchronizes right and left ventricle contraction.
Although there is a learning curve, LBBP is technically easier than His-bundle pacing and ultimately results in far better pacing and sensing parameters. What’s more, the preferred lead for LBBP has a stellar efficacy record – over years.
I have become enthralled by the gorgeous QRS complexes from LBBP. The ability to pace the heart without creating dyssynchrony infuses me with joy. I chose cardiology largely because of the beauty of the ECG.
But as a medical conservative who is cautious about unproven therapies, I have questions. How is LBBP defined? Is left septal pacing good enough, or do you need actual left bundle capture? What about long-term performance of a lead in the septum?
Biventricular pacing has set a high bar because it has been proven effective for reducing hard clinical outcomes in large randomized controlled trials.
The studies at HRS begin to answer these questions. The randomized controlled trial from China supports the notion that effective LBBP (the investigators rigorously defined left bundle capture) leads to favorable effects on cardiac contraction. The two observational studies reported similarly encouraging findings on cardiac function.
The three studies therefore tentatively support the notion that LBBP actually produces favorable cardiac performance.
Whether LBBP leads to better clinical outcomes remains uncertain. The nonrandomized comparison study, which found better hard outcomes in the CSP arm, cannot be used to infer causality. There is too much risk for selection bias.
But the LBBP bailout study does suggest that this strategy is reasonable when coronary sinus leads fail – especially since the alternative is surgical placement of an epicardial lead on the left ventricle.
At minimum, the HRS studies persuade me that LBBP will likely prevent pacing-induced cardiomyopathy. If I or a family member required a pacemaker, I’d surely want the operator to be skilled at placing a left bundle lead.
While I am confident that conduction system pacing will become a transformative advance in cardiac pacing, aesthetically pleasing ECG patterns are not enough. There remains much to learn with this nascent approach.
The barriers to getting more CSP trials
The challenge going forward will be funding new trials. CSP stands to prevent pacing-induced cardiomyopathy and offer less costly alternatives to standard biventricular pacing for CRT. This is great for patients, but it would mean that fewer higher-cost CRT devices will be sold.
Heart rhythm research is largely industry-funded because in most cases better therapies for patients mean more profits for industry. In the case of CSP, there is no such confluence of interests.
Conduction system pacing has come about because of the efforts of a few tireless champions who not only published extensively but were also skilled at using social media to spread the excitement. Trials have been small and often self-funded.
The data presented at HRS provides enough equipoise to support a large outcomes-based randomized controlled trial. Imagine if our CSP champions were able to find public-funding sources for such future trials.
Now that would be super cool.
Dr. Mandrola practices cardiac electrophysiology in Louisville, Ky., and is a writer and podcaster for Medscape. He participates in clinical research and writes often about the state of medical evidence. He has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
For the past 2 decades, catheter ablation stole most of the excitement in electrophysiology. Cardiac pacing was seen as necessary but boring. His-bundle pacing earned only modest attention.
But at the annual scientific sessions of the Heart Rhythm Society, cardiac pacing consolidated its comeback and entered the super-cool category.
Not one but three late-breaking clinical trials considered the role of pacing the heart’s conduction system for both preventive and therapeutic purposes. Conduction system pacing, or CSP as we call it, includes pacing the His bundle or the left bundle branch. Left bundle–branch pacing has now largely replaced His-bundle pacing.
Before I tell you about the studies, let’s review why CSP disrupts the status quo.
The core idea goes back to basic physiology: After the impulse leaves the atrioventricular node, the heart’s specialized conduction system allows rapid and synchronous conduction to both the right and left ventricles.
Standard cardiac pacing means fixing a pacing lead into the muscle of the right ventricle. From that spot, conduction spreads via slower muscle-to-muscle conduction, which leads to a wide QRS complex and the right ventricle contracts before the left ventricle.
While such dyssynchronous contraction is better than no contraction, this approach leads to a pacing-induced cardiomyopathy in a substantial number of cases. (The incidence reported in many studies varies widely.)
The most disruptive effect of conduction system pacing is that it is a form of cardiac resynchronization therapy (CRT). And that is nifty because, until recently, resynchronizing the ventricles required placing two ventricular leads: one in the right ventricle and the other in the coronary sinus to pace the left ventricle.
Left bundle-branch pacing vs. biventricular pacing
The first of the three HRS studies is the LBBP-RESYNC randomized controlled trial led by Jiangang Zou, MD, PhD, and performed in multiple centers in China. It compared the efficacy of left bundle–branch pacing (LBBP) with that of conventional biventricular pacing in 40 patients with heart failure who were eligible for CRT. The primary endpoint was the change in left ventricular ejection fraction (LVEF) from baseline to 6-month follow-up.
The results favored LBBP. Although both pacing techniques improved LVEF from baseline, the between-group difference in LVEF was greater in the LBBP arm than the biventricular pacing arm by a statistically significant 5.6% (95% confidence interval, 0.3%-10.9%). Secondary endpoints, such as reductions in left ventricular end-systolic volume, N-terminal of the prohormone brain natriuretic peptide, and QRS duration, also favored LBBP.
Conduction system pacing vs. biventricular pacing
A second late-breaking study, from the Geisinger group, led by Pugazhendhi Vijayaraman, MD, was simultaneously published in Heart Rhythm.
This nonrandomized observational study compared nearly 500 patients eligible for CRT treated at two health systems. One group favors conduction system pacing and the other does traditional biventricular pacing, which set up a two-armed comparison.
CSP was accomplished by LBBP (65%) and His-bundle pacing (35%).
The primary endpoint of death or first hospitalization for heart failure occurred in 28.3% of patients in the CSP arm versus 38.4% of the biventricular arm (hazard ratio, 1.52; 95% CI, 1.08-2.09). QRS duration and LVEF also improved from baseline in both groups.
LBB area pacing as a bailout for failed CRT
The Geisinger group also presented and published an international multicenter study that assessed the feasibility of LBBP as a bailout when standard biventricular pacing did not work – because of inadequate coronary sinus anatomy or CRT nonresponse, defined as lack of clinical or echocardiographic improvement.
This series included 212 patients in whom CRT failed and who underwent attempted LBBP pacing. The bailout was successful in 200 patients (91%). The primary endpoint was defined as an increase in LVEF above 5% on echocardiography.
During 12-month follow-up, 61% of patients had an improvement in LVEF above 5% and nearly 30% had a “super-response,” defined as a 20% or greater increase or normalization of LVEF. Similar to the previous studies, LBBP resulted in shorter QRS duration and improved echocardiography parameters.
Am I persuaded?
I was an early adopter of His-bundle pacing. When successful, it delivered both aesthetically pleasing QRS complexes and clinical efficacy. But there were many challenges: it is technically difficult, and capture thresholds are often high at implant and get higher over time, which leads to shorter battery life.
Pacing the left bundle branch mitigates these challenges. Here, the operator approaches from the right side and screws the lead a few millimeters into the septum, so the tip of the lead can capture the left bundle or one of its branches. This allows activation of the heart’s specialized conduction system and thus synchronizes right and left ventricle contraction.
Although there is a learning curve, LBBP is technically easier than His-bundle pacing and ultimately results in far better pacing and sensing parameters. What’s more, the preferred lead for LBBP has a stellar efficacy record – over years.
I have become enthralled by the gorgeous QRS complexes from LBBP. The ability to pace the heart without creating dyssynchrony infuses me with joy. I chose cardiology largely because of the beauty of the ECG.
But as a medical conservative who is cautious about unproven therapies, I have questions. How is LBBP defined? Is left septal pacing good enough, or do you need actual left bundle capture? What about long-term performance of a lead in the septum?
Biventricular pacing has set a high bar because it has been proven effective for reducing hard clinical outcomes in large randomized controlled trials.
The studies at HRS begin to answer these questions. The randomized controlled trial from China supports the notion that effective LBBP (the investigators rigorously defined left bundle capture) leads to favorable effects on cardiac contraction. The two observational studies reported similarly encouraging findings on cardiac function.
The three studies therefore tentatively support the notion that LBBP actually produces favorable cardiac performance.
Whether LBBP leads to better clinical outcomes remains uncertain. The nonrandomized comparison study, which found better hard outcomes in the CSP arm, cannot be used to infer causality. There is too much risk for selection bias.
But the LBBP bailout study does suggest that this strategy is reasonable when coronary sinus leads fail – especially since the alternative is surgical placement of an epicardial lead on the left ventricle.
At minimum, the HRS studies persuade me that LBBP will likely prevent pacing-induced cardiomyopathy. If I or a family member required a pacemaker, I’d surely want the operator to be skilled at placing a left bundle lead.
While I am confident that conduction system pacing will become a transformative advance in cardiac pacing, aesthetically pleasing ECG patterns are not enough. There remains much to learn with this nascent approach.
The barriers to getting more CSP trials
The challenge going forward will be funding new trials. CSP stands to prevent pacing-induced cardiomyopathy and offer less costly alternatives to standard biventricular pacing for CRT. This is great for patients, but it would mean that fewer higher-cost CRT devices will be sold.
Heart rhythm research is largely industry-funded because in most cases better therapies for patients mean more profits for industry. In the case of CSP, there is no such confluence of interests.
Conduction system pacing has come about because of the efforts of a few tireless champions who not only published extensively but were also skilled at using social media to spread the excitement. Trials have been small and often self-funded.
The data presented at HRS provides enough equipoise to support a large outcomes-based randomized controlled trial. Imagine if our CSP champions were able to find public-funding sources for such future trials.
Now that would be super cool.
Dr. Mandrola practices cardiac electrophysiology in Louisville, Ky., and is a writer and podcaster for Medscape. He participates in clinical research and writes often about the state of medical evidence. He has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
Cutting dementia risk in AFib: Does rhythm control strategy matter?
The risk for dementia goes up in patients with atrial fibrillation (AFib), but some evidence suggests that risk can be blunted with therapies that restore sinus rhythm. However, a new cohort study suggests that the treatment effect’s magnitude might depend on the rhythm control strategy. It hinted that AFib catheter ablation might be more effective than pharmacologic rhythm control alone at cutting the risk for dementia.
The case-matched study of more than 38,000 adults with AFib saw a 41% reduction (P < .0001) in risk for dementia among those who underwent catheter ablation after attempted rhythm control with antiarrhythmic drugs (AAD), compared with those managed with pharmacologic rhythm control therapy alone.
The observational study comprising 20 years of data comes with big limitations and can’t say for sure whether catheter ablation is better than AAD-only at cutting the dementia risk in AFib. But it and other evidence support the idea, which has yet to be explored in a randomized fashion.
In a secondary finding, the analysis showed a similar reduction in dementia risk from catheter ablation, compared with AAD, in women and in men by 40% and 45%, respectively (P < .0001 for both). The findings are particularly relevant “given the higher life-long risk of dementia among women and the lower likelihood that women will be offered ablation, which has been demonstrated repeatedly,” Emily P. Zeitler, MD, MHS, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire, told this news organization. “I think this is another reason to try to be more generous in offering ablation to women.”
Management of AFib certainly evolved in important ways from 2000 to 2021, the period covered by the study. But a sensitivity analysis based on data from 2010 to 2021 showed “no meaningful differences” in the results, said Dr. Zeitler, who is slated to present the findings April 30 at the Heart Rhythm Society 2022 Scientific Sessions, conducted virtually and live in San Francisco.
Dr. Zeitler acknowledged that the observational study, even with its propensity-matched ablation and AAD cohorts, can only hint at a preference for ablation over AAD for lowering risk for AFib-associated dementia. “We know there’s unmeasured and unfixable confounding between those two groups, so we see this really as hypothesis-generating.”
It was “a well-done analysis,” and the conclusion that the dementia risk was lower with catheter ablation is “absolutely correct,” but only as far as the study and its limitations allow, agreed David Conen, MD, MPH, McMaster University, Hamilton, Ontario, who is not a coauthor.
“Even with propensity matching, you can get rid of some sorts of confounding, but you can never get rid of all selection bias issues.” That, he said when interviewed, takes randomized trials.
Dr. Conen, who is studying cognitive decline in AFib as a SWISS-AF trial principal investigator, pointed to a secondary finding of the analysis as evidence for such confounding. He said the ablation group’s nearly 50% drop (P < .0001) in competing risk for death, compared with patients managed with AAD, isn’t plausible.
The finding “strongly suggests these people were healthier and that there’s some sort of selection bias. They were at lower risk of death, they were at lower risk of dementia, and they were probably also at lower risk of stroke, myocardial infarction, thrombosis, and cancer because they were just probably a little healthier than the others,” Dr. Conen said. The ablation and AAD groups “were two very different populations from the get-go.”
The analysis was based on U.S. insurance and Medicare claims data from AFib patients who either underwent catheter ablation after at least one AAD trial or filled prescriptions for at least two different antiarrhythmic agents in the year after AFib diagnosis. Patients with history of dementia, catheter or surgical AFib ablation, or a valve procedure were excluded.
The ablation and AAD-only groups each consisted of 19,066 patients after propensity matching, and the groups were balanced with respect to age, sex, type of insurance, CHA2DS2-VASc scores, and use of renin-angiotensin-system inhibitors, oral anticoagulants, and antiplatelets.
The overall risk for dementia was 1.9% for the ablation group and 3.3% for AAD-only patients (hazard ratio, 0.59; 95% confidence interval, 0.52-0.67). Corresponding HRs by sex were 0.55 (95% CI, 0.46-0.66) for men and 0.60 (95% CI, 0.50-0.72) for women.
The competing risk for death was also significantly decreased in the ablation group (HR, 0.51; 95% CI, 0.46-0.55).
Dr. Zeitler pointed to a randomized trial now in the early stages called Neurocognition and Greater Maintenance of Sinus Rhythm in Atrial Fibrillation, or NOGGIN-AF, which will explore relationships between rhythm control therapy and dementia in patients with AFib, whether catheter ablation or AAD can mitigate that risk, and whether either strategy works better than the other, among other goals.
“I’m optimistic,” she said, “and I think it’s going to add to the growing motivations to get patients ablated more quickly and more broadly.”
The analysis was funded by Biosense-Webster. Dr. Zeitler discloses consulting for Biosense-Webster and Arena Pharmaceuticals (now Pfizer); fees for speaking from Medtronic; and receiving research support from Boston Scientific, Sanofi, and Biosense-Webster. Dr. Conen has previously reported receiving speaker fees from Servier Canada.
A version of this article first appeared on Medscape.com.
The risk for dementia goes up in patients with atrial fibrillation (AFib), but some evidence suggests that risk can be blunted with therapies that restore sinus rhythm. However, a new cohort study suggests that the treatment effect’s magnitude might depend on the rhythm control strategy. It hinted that AFib catheter ablation might be more effective than pharmacologic rhythm control alone at cutting the risk for dementia.
The case-matched study of more than 38,000 adults with AFib saw a 41% reduction (P < .0001) in risk for dementia among those who underwent catheter ablation after attempted rhythm control with antiarrhythmic drugs (AAD), compared with those managed with pharmacologic rhythm control therapy alone.
The observational study comprising 20 years of data comes with big limitations and can’t say for sure whether catheter ablation is better than AAD-only at cutting the dementia risk in AFib. But it and other evidence support the idea, which has yet to be explored in a randomized fashion.
In a secondary finding, the analysis showed a similar reduction in dementia risk from catheter ablation, compared with AAD, in women and in men by 40% and 45%, respectively (P < .0001 for both). The findings are particularly relevant “given the higher life-long risk of dementia among women and the lower likelihood that women will be offered ablation, which has been demonstrated repeatedly,” Emily P. Zeitler, MD, MHS, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire, told this news organization. “I think this is another reason to try to be more generous in offering ablation to women.”
Management of AFib certainly evolved in important ways from 2000 to 2021, the period covered by the study. But a sensitivity analysis based on data from 2010 to 2021 showed “no meaningful differences” in the results, said Dr. Zeitler, who is slated to present the findings April 30 at the Heart Rhythm Society 2022 Scientific Sessions, conducted virtually and live in San Francisco.
Dr. Zeitler acknowledged that the observational study, even with its propensity-matched ablation and AAD cohorts, can only hint at a preference for ablation over AAD for lowering risk for AFib-associated dementia. “We know there’s unmeasured and unfixable confounding between those two groups, so we see this really as hypothesis-generating.”
It was “a well-done analysis,” and the conclusion that the dementia risk was lower with catheter ablation is “absolutely correct,” but only as far as the study and its limitations allow, agreed David Conen, MD, MPH, McMaster University, Hamilton, Ontario, who is not a coauthor.
“Even with propensity matching, you can get rid of some sorts of confounding, but you can never get rid of all selection bias issues.” That, he said when interviewed, takes randomized trials.
Dr. Conen, who is studying cognitive decline in AFib as a SWISS-AF trial principal investigator, pointed to a secondary finding of the analysis as evidence for such confounding. He said the ablation group’s nearly 50% drop (P < .0001) in competing risk for death, compared with patients managed with AAD, isn’t plausible.
The finding “strongly suggests these people were healthier and that there’s some sort of selection bias. They were at lower risk of death, they were at lower risk of dementia, and they were probably also at lower risk of stroke, myocardial infarction, thrombosis, and cancer because they were just probably a little healthier than the others,” Dr. Conen said. The ablation and AAD groups “were two very different populations from the get-go.”
The analysis was based on U.S. insurance and Medicare claims data from AFib patients who either underwent catheter ablation after at least one AAD trial or filled prescriptions for at least two different antiarrhythmic agents in the year after AFib diagnosis. Patients with history of dementia, catheter or surgical AFib ablation, or a valve procedure were excluded.
The ablation and AAD-only groups each consisted of 19,066 patients after propensity matching, and the groups were balanced with respect to age, sex, type of insurance, CHA2DS2-VASc scores, and use of renin-angiotensin-system inhibitors, oral anticoagulants, and antiplatelets.
The overall risk for dementia was 1.9% for the ablation group and 3.3% for AAD-only patients (hazard ratio, 0.59; 95% confidence interval, 0.52-0.67). Corresponding HRs by sex were 0.55 (95% CI, 0.46-0.66) for men and 0.60 (95% CI, 0.50-0.72) for women.
The competing risk for death was also significantly decreased in the ablation group (HR, 0.51; 95% CI, 0.46-0.55).
Dr. Zeitler pointed to a randomized trial now in the early stages called Neurocognition and Greater Maintenance of Sinus Rhythm in Atrial Fibrillation, or NOGGIN-AF, which will explore relationships between rhythm control therapy and dementia in patients with AFib, whether catheter ablation or AAD can mitigate that risk, and whether either strategy works better than the other, among other goals.
“I’m optimistic,” she said, “and I think it’s going to add to the growing motivations to get patients ablated more quickly and more broadly.”
The analysis was funded by Biosense-Webster. Dr. Zeitler discloses consulting for Biosense-Webster and Arena Pharmaceuticals (now Pfizer); fees for speaking from Medtronic; and receiving research support from Boston Scientific, Sanofi, and Biosense-Webster. Dr. Conen has previously reported receiving speaker fees from Servier Canada.
A version of this article first appeared on Medscape.com.
The risk for dementia goes up in patients with atrial fibrillation (AFib), but some evidence suggests that risk can be blunted with therapies that restore sinus rhythm. However, a new cohort study suggests that the treatment effect’s magnitude might depend on the rhythm control strategy. It hinted that AFib catheter ablation might be more effective than pharmacologic rhythm control alone at cutting the risk for dementia.
The case-matched study of more than 38,000 adults with AFib saw a 41% reduction (P < .0001) in risk for dementia among those who underwent catheter ablation after attempted rhythm control with antiarrhythmic drugs (AAD), compared with those managed with pharmacologic rhythm control therapy alone.
The observational study comprising 20 years of data comes with big limitations and can’t say for sure whether catheter ablation is better than AAD-only at cutting the dementia risk in AFib. But it and other evidence support the idea, which has yet to be explored in a randomized fashion.
In a secondary finding, the analysis showed a similar reduction in dementia risk from catheter ablation, compared with AAD, in women and in men by 40% and 45%, respectively (P < .0001 for both). The findings are particularly relevant “given the higher life-long risk of dementia among women and the lower likelihood that women will be offered ablation, which has been demonstrated repeatedly,” Emily P. Zeitler, MD, MHS, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire, told this news organization. “I think this is another reason to try to be more generous in offering ablation to women.”
Management of AFib certainly evolved in important ways from 2000 to 2021, the period covered by the study. But a sensitivity analysis based on data from 2010 to 2021 showed “no meaningful differences” in the results, said Dr. Zeitler, who is slated to present the findings April 30 at the Heart Rhythm Society 2022 Scientific Sessions, conducted virtually and live in San Francisco.
Dr. Zeitler acknowledged that the observational study, even with its propensity-matched ablation and AAD cohorts, can only hint at a preference for ablation over AAD for lowering risk for AFib-associated dementia. “We know there’s unmeasured and unfixable confounding between those two groups, so we see this really as hypothesis-generating.”
It was “a well-done analysis,” and the conclusion that the dementia risk was lower with catheter ablation is “absolutely correct,” but only as far as the study and its limitations allow, agreed David Conen, MD, MPH, McMaster University, Hamilton, Ontario, who is not a coauthor.
“Even with propensity matching, you can get rid of some sorts of confounding, but you can never get rid of all selection bias issues.” That, he said when interviewed, takes randomized trials.
Dr. Conen, who is studying cognitive decline in AFib as a SWISS-AF trial principal investigator, pointed to a secondary finding of the analysis as evidence for such confounding. He said the ablation group’s nearly 50% drop (P < .0001) in competing risk for death, compared with patients managed with AAD, isn’t plausible.
The finding “strongly suggests these people were healthier and that there’s some sort of selection bias. They were at lower risk of death, they were at lower risk of dementia, and they were probably also at lower risk of stroke, myocardial infarction, thrombosis, and cancer because they were just probably a little healthier than the others,” Dr. Conen said. The ablation and AAD groups “were two very different populations from the get-go.”
The analysis was based on U.S. insurance and Medicare claims data from AFib patients who either underwent catheter ablation after at least one AAD trial or filled prescriptions for at least two different antiarrhythmic agents in the year after AFib diagnosis. Patients with history of dementia, catheter or surgical AFib ablation, or a valve procedure were excluded.
The ablation and AAD-only groups each consisted of 19,066 patients after propensity matching, and the groups were balanced with respect to age, sex, type of insurance, CHA2DS2-VASc scores, and use of renin-angiotensin-system inhibitors, oral anticoagulants, and antiplatelets.
The overall risk for dementia was 1.9% for the ablation group and 3.3% for AAD-only patients (hazard ratio, 0.59; 95% confidence interval, 0.52-0.67). Corresponding HRs by sex were 0.55 (95% CI, 0.46-0.66) for men and 0.60 (95% CI, 0.50-0.72) for women.
The competing risk for death was also significantly decreased in the ablation group (HR, 0.51; 95% CI, 0.46-0.55).
Dr. Zeitler pointed to a randomized trial now in the early stages called Neurocognition and Greater Maintenance of Sinus Rhythm in Atrial Fibrillation, or NOGGIN-AF, which will explore relationships between rhythm control therapy and dementia in patients with AFib, whether catheter ablation or AAD can mitigate that risk, and whether either strategy works better than the other, among other goals.
“I’m optimistic,” she said, “and I think it’s going to add to the growing motivations to get patients ablated more quickly and more broadly.”
The analysis was funded by Biosense-Webster. Dr. Zeitler discloses consulting for Biosense-Webster and Arena Pharmaceuticals (now Pfizer); fees for speaking from Medtronic; and receiving research support from Boston Scientific, Sanofi, and Biosense-Webster. Dr. Conen has previously reported receiving speaker fees from Servier Canada.
A version of this article first appeared on Medscape.com.
Cutting dementia risk in atrial fibrillation: Does rhythm control strategy matter?
The risk for dementia goes up in patients with atrial fibrillation (AFib), but some evidence suggests that risk can be blunted with therapies that restore sinus rhythm. But a new cohort study suggests that the treatment effect’s magnitude might depend on the rhythm control strategy. It hinted that AFib catheter ablation might be more effective than pharmacologic rhythm control alone at cutting the risk for dementia.
The case-matched study of more than 38,000 adults with AFib saw a 41% reduction (P < .0001) in risk for dementia among those who underwent catheter ablation after attempted rhythm control with antiarrhythmic drugs (AAD), compared with those managed with pharmacologic rhythm control therapy alone.
The observational study comprising 20 years of data comes with big limitations and can’t say for sure whether catheter ablation is better than AAD alone at cutting the dementia risk in AFib. But it and other evidence support the idea, which has yet to be explored in a randomized fashion.
In a secondary finding, the analysis showed a similar reduction in dementia risk from catheter ablation, compared with AAD, in women and in men by 40% and 45%, respectively (P < .0001 for both). The findings are particularly relevant “given the higher life-long risk of dementia among women and the lower likelihood that women will be offered ablation, which has been demonstrated repeatedly,” Emily P. Zeitler, MD, MHS, Dartmouth-Hitchcock Medical Center, Lebanon, N.H., said in an interview. “I think this is another reason to try to be more generous in offering ablation to women.”
Management of AFib certainly evolved in important ways from 2000 to 2021, the period covered by the study. But a sensitivity analysis based on data from 2010 to 2021 showed “no meaningful differences” in the results, said Dr. Zeitler, who is slated to present the findings at the annual scientific sessions of the Heart Rhythm Society.
Dr. Zeitler acknowledged that the observational study, even with its propensity-matched ablation and AAD cohorts, can only hint at a preference for ablation over AAD for lowering risk for AFib-associated dementia. “We know there’s unmeasured and unfixable confounding between those two groups, so we see this really as hypothesis-generating.”
It was “a well-done analysis,” and the conclusion that the dementia risk was lower with catheter ablation is “absolutely correct,” but only as far as the study and its limitations allow, agreed David Conen, MD, MPH, McMaster University, Hamilton, Ont., who is not a coauthor.
“Even with propensity matching, you can get rid of some sorts of confounding, but you can never get rid of all selection bias issues.” That, he said when interviewed, takes randomized trials.
Dr. Conen, who is studying cognitive decline in AFib as a SWISS-AF trial principal investigator, pointed to a secondary finding of the analysis as evidence for such confounding. He said the ablation group’s nearly 50% drop (P < .0001) in competing risk for death, compared with patients managed with AAD, isn’t plausible.
The finding “strongly suggests these people were healthier and that there’s some sort of selection bias. They were at lower risk of death, they were at lower risk of dementia, and they were probably also at lower risk of stroke, myocardial infarction, thrombosis, and cancer because they were just probably a little healthier than the others,” Dr. Conen said. The ablation and AAD groups “were two very different populations from the get-go.”
The analysis was based on U.S. insurance and Medicare claims data from AFib patients who either underwent catheter ablation after at least one AAD trial or filled prescriptions for at least two different antiarrhythmic agents in the year after AFib diagnosis. Patients with history of dementia, catheter or surgical AFib ablation, or a valve procedure were excluded.
The ablation and AAD-only groups each consisted of 19,066 patients after propensity matching, and the groups were balanced with respect to age, sex, type of insurance, CHA2DS2-VASc scores, and use of renin-angiotensin system inhibitors, oral anticoagulants, and antiplatelets.
The overall risk for dementia was 1.9% for the ablation group and 3.3% for AAD-only patients (hazard ratio, 0.59; 95% confidence interval, 0.52-0.67). Corresponding HRs by sex were 0.55 (95% CI, 0.46-0.66) for men and 0.60 (95% CI, 0.50-0.72) for women.
The competing risk for death was also significantly decreased in the ablation group (HR, 0.51; 95% CI, 0.46-0.55).
Dr. Zeitler pointed to a randomized trial now in the early stages called Neurocognition and Greater Maintenance of Sinus Rhythm in Atrial Fibrillation, or NOGGIN-AF, which will explore relationships between rhythm control therapy and dementia in patients with AFib, whether catheter ablation or AAD can mitigate that risk, and whether either strategy works better than the other, among other goals.
“I’m optimistic,” she said, “and I think it’s going to add to the growing motivations to get patients ablated more quickly and more broadly.”
The analysis was funded by Biosense-Webster. Dr. Zeitler disclosed consulting for Biosense-Webster and Arena Pharmaceuticals (now Pfizer); fees for speaking from Medtronic; and receiving research support from Boston Scientific, Sanofi, and Biosense-Webster. Dr. Conen has previously reported receiving speaker fees from Servier Canada.
A version of this article first appeared on Medscape.com.
The risk for dementia goes up in patients with atrial fibrillation (AFib), but some evidence suggests that risk can be blunted with therapies that restore sinus rhythm. But a new cohort study suggests that the treatment effect’s magnitude might depend on the rhythm control strategy. It hinted that AFib catheter ablation might be more effective than pharmacologic rhythm control alone at cutting the risk for dementia.
The case-matched study of more than 38,000 adults with AFib saw a 41% reduction (P < .0001) in risk for dementia among those who underwent catheter ablation after attempted rhythm control with antiarrhythmic drugs (AAD), compared with those managed with pharmacologic rhythm control therapy alone.
The observational study comprising 20 years of data comes with big limitations and can’t say for sure whether catheter ablation is better than AAD alone at cutting the dementia risk in AFib. But it and other evidence support the idea, which has yet to be explored in a randomized fashion.
In a secondary finding, the analysis showed a similar reduction in dementia risk from catheter ablation, compared with AAD, in women and in men by 40% and 45%, respectively (P < .0001 for both). The findings are particularly relevant “given the higher life-long risk of dementia among women and the lower likelihood that women will be offered ablation, which has been demonstrated repeatedly,” Emily P. Zeitler, MD, MHS, Dartmouth-Hitchcock Medical Center, Lebanon, N.H., said in an interview. “I think this is another reason to try to be more generous in offering ablation to women.”
Management of AFib certainly evolved in important ways from 2000 to 2021, the period covered by the study. But a sensitivity analysis based on data from 2010 to 2021 showed “no meaningful differences” in the results, said Dr. Zeitler, who is slated to present the findings at the annual scientific sessions of the Heart Rhythm Society.
Dr. Zeitler acknowledged that the observational study, even with its propensity-matched ablation and AAD cohorts, can only hint at a preference for ablation over AAD for lowering risk for AFib-associated dementia. “We know there’s unmeasured and unfixable confounding between those two groups, so we see this really as hypothesis-generating.”
It was “a well-done analysis,” and the conclusion that the dementia risk was lower with catheter ablation is “absolutely correct,” but only as far as the study and its limitations allow, agreed David Conen, MD, MPH, McMaster University, Hamilton, Ont., who is not a coauthor.
“Even with propensity matching, you can get rid of some sorts of confounding, but you can never get rid of all selection bias issues.” That, he said when interviewed, takes randomized trials.
Dr. Conen, who is studying cognitive decline in AFib as a SWISS-AF trial principal investigator, pointed to a secondary finding of the analysis as evidence for such confounding. He said the ablation group’s nearly 50% drop (P < .0001) in competing risk for death, compared with patients managed with AAD, isn’t plausible.
The finding “strongly suggests these people were healthier and that there’s some sort of selection bias. They were at lower risk of death, they were at lower risk of dementia, and they were probably also at lower risk of stroke, myocardial infarction, thrombosis, and cancer because they were just probably a little healthier than the others,” Dr. Conen said. The ablation and AAD groups “were two very different populations from the get-go.”
The analysis was based on U.S. insurance and Medicare claims data from AFib patients who either underwent catheter ablation after at least one AAD trial or filled prescriptions for at least two different antiarrhythmic agents in the year after AFib diagnosis. Patients with history of dementia, catheter or surgical AFib ablation, or a valve procedure were excluded.
The ablation and AAD-only groups each consisted of 19,066 patients after propensity matching, and the groups were balanced with respect to age, sex, type of insurance, CHA2DS2-VASc scores, and use of renin-angiotensin system inhibitors, oral anticoagulants, and antiplatelets.
The overall risk for dementia was 1.9% for the ablation group and 3.3% for AAD-only patients (hazard ratio, 0.59; 95% confidence interval, 0.52-0.67). Corresponding HRs by sex were 0.55 (95% CI, 0.46-0.66) for men and 0.60 (95% CI, 0.50-0.72) for women.
The competing risk for death was also significantly decreased in the ablation group (HR, 0.51; 95% CI, 0.46-0.55).
Dr. Zeitler pointed to a randomized trial now in the early stages called Neurocognition and Greater Maintenance of Sinus Rhythm in Atrial Fibrillation, or NOGGIN-AF, which will explore relationships between rhythm control therapy and dementia in patients with AFib, whether catheter ablation or AAD can mitigate that risk, and whether either strategy works better than the other, among other goals.
“I’m optimistic,” she said, “and I think it’s going to add to the growing motivations to get patients ablated more quickly and more broadly.”
The analysis was funded by Biosense-Webster. Dr. Zeitler disclosed consulting for Biosense-Webster and Arena Pharmaceuticals (now Pfizer); fees for speaking from Medtronic; and receiving research support from Boston Scientific, Sanofi, and Biosense-Webster. Dr. Conen has previously reported receiving speaker fees from Servier Canada.
A version of this article first appeared on Medscape.com.
The risk for dementia goes up in patients with atrial fibrillation (AFib), but some evidence suggests that risk can be blunted with therapies that restore sinus rhythm. But a new cohort study suggests that the treatment effect’s magnitude might depend on the rhythm control strategy. It hinted that AFib catheter ablation might be more effective than pharmacologic rhythm control alone at cutting the risk for dementia.
The case-matched study of more than 38,000 adults with AFib saw a 41% reduction (P < .0001) in risk for dementia among those who underwent catheter ablation after attempted rhythm control with antiarrhythmic drugs (AAD), compared with those managed with pharmacologic rhythm control therapy alone.
The observational study comprising 20 years of data comes with big limitations and can’t say for sure whether catheter ablation is better than AAD alone at cutting the dementia risk in AFib. But it and other evidence support the idea, which has yet to be explored in a randomized fashion.
In a secondary finding, the analysis showed a similar reduction in dementia risk from catheter ablation, compared with AAD, in women and in men by 40% and 45%, respectively (P < .0001 for both). The findings are particularly relevant “given the higher life-long risk of dementia among women and the lower likelihood that women will be offered ablation, which has been demonstrated repeatedly,” Emily P. Zeitler, MD, MHS, Dartmouth-Hitchcock Medical Center, Lebanon, N.H., said in an interview. “I think this is another reason to try to be more generous in offering ablation to women.”
Management of AFib certainly evolved in important ways from 2000 to 2021, the period covered by the study. But a sensitivity analysis based on data from 2010 to 2021 showed “no meaningful differences” in the results, said Dr. Zeitler, who is slated to present the findings at the annual scientific sessions of the Heart Rhythm Society.
Dr. Zeitler acknowledged that the observational study, even with its propensity-matched ablation and AAD cohorts, can only hint at a preference for ablation over AAD for lowering risk for AFib-associated dementia. “We know there’s unmeasured and unfixable confounding between those two groups, so we see this really as hypothesis-generating.”
It was “a well-done analysis,” and the conclusion that the dementia risk was lower with catheter ablation is “absolutely correct,” but only as far as the study and its limitations allow, agreed David Conen, MD, MPH, McMaster University, Hamilton, Ont., who is not a coauthor.
“Even with propensity matching, you can get rid of some sorts of confounding, but you can never get rid of all selection bias issues.” That, he said when interviewed, takes randomized trials.
Dr. Conen, who is studying cognitive decline in AFib as a SWISS-AF trial principal investigator, pointed to a secondary finding of the analysis as evidence for such confounding. He said the ablation group’s nearly 50% drop (P < .0001) in competing risk for death, compared with patients managed with AAD, isn’t plausible.
The finding “strongly suggests these people were healthier and that there’s some sort of selection bias. They were at lower risk of death, they were at lower risk of dementia, and they were probably also at lower risk of stroke, myocardial infarction, thrombosis, and cancer because they were just probably a little healthier than the others,” Dr. Conen said. The ablation and AAD groups “were two very different populations from the get-go.”
The analysis was based on U.S. insurance and Medicare claims data from AFib patients who either underwent catheter ablation after at least one AAD trial or filled prescriptions for at least two different antiarrhythmic agents in the year after AFib diagnosis. Patients with history of dementia, catheter or surgical AFib ablation, or a valve procedure were excluded.
The ablation and AAD-only groups each consisted of 19,066 patients after propensity matching, and the groups were balanced with respect to age, sex, type of insurance, CHA2DS2-VASc scores, and use of renin-angiotensin system inhibitors, oral anticoagulants, and antiplatelets.
The overall risk for dementia was 1.9% for the ablation group and 3.3% for AAD-only patients (hazard ratio, 0.59; 95% confidence interval, 0.52-0.67). Corresponding HRs by sex were 0.55 (95% CI, 0.46-0.66) for men and 0.60 (95% CI, 0.50-0.72) for women.
The competing risk for death was also significantly decreased in the ablation group (HR, 0.51; 95% CI, 0.46-0.55).
Dr. Zeitler pointed to a randomized trial now in the early stages called Neurocognition and Greater Maintenance of Sinus Rhythm in Atrial Fibrillation, or NOGGIN-AF, which will explore relationships between rhythm control therapy and dementia in patients with AFib, whether catheter ablation or AAD can mitigate that risk, and whether either strategy works better than the other, among other goals.
“I’m optimistic,” she said, “and I think it’s going to add to the growing motivations to get patients ablated more quickly and more broadly.”
The analysis was funded by Biosense-Webster. Dr. Zeitler disclosed consulting for Biosense-Webster and Arena Pharmaceuticals (now Pfizer); fees for speaking from Medtronic; and receiving research support from Boston Scientific, Sanofi, and Biosense-Webster. Dr. Conen has previously reported receiving speaker fees from Servier Canada.
A version of this article first appeared on Medscape.com.
HEART RHYTHM 2022



