User login
Etanercept biosimilar proves effective, tolerated in phase III trial
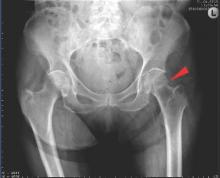
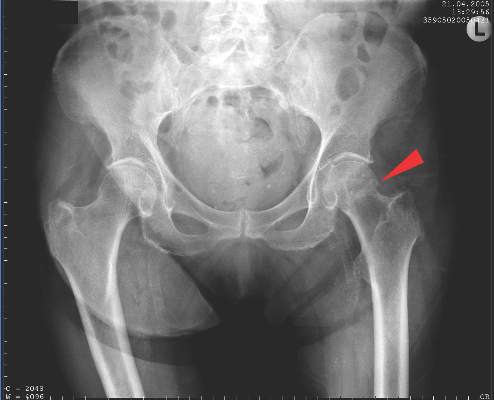
LONDON – Biosimilar etanercept (CHS-0214) is as effective and well tolerated as etanercept (Enbrel) for the treatment of rheumatoid arthritis according to the results of a randomized, double-blind, phase III trial conducted in 13 countries.
The primary endpoint of an American College of Rheumatology (ACR) 20 at 24 weeks was achieved by 91% of patients given CHS-0214 and 90.6% of those given etanercept, giving a treatment difference of just 0.4%. The percentages of patients achieving ACR 50 (67.6% and 63.7%) and ACR 70 (38.3% and 37.9%) were also comparable.
These are the first clinical data to be presented on this biosimilar, which is a fusion protein comprising the soluble human p75 tumor necrosis factor (TNF) receptor and the Fc region of human immunoglobulin G1.
“Like all biosimilars CHD-0214 has undergone extensive analytical characterization, which had demonstrated highly similar structure and function to etanercept,” study investigator James O’Dell, MD, of the University of Nebraska Medical Center, Omaha, said at the European Congress of Rheumatology. “Studies have demonstrated that CHS-0214 is similar to etanercept with regard to in vitro pharmacology, in vivo pharmacokinetics, and toxicology,” he observed.
A total of 644 patients with moderate to severe rheumatoid arthritis who had an inadequate response to methotrexate were enrolled in the phase III trial and randomized to receive CHS-0214 or etanercept at a subcutaneous dose of 50 mg once a week for 24 weeks. After the double-blind period had ended, patients could continue on open-label CHS-0214 for another 24 weeks. Assessment of efficacy was performed on 512 patients as an issue with the production of the biosimilar resulted in a dosing interruption for 132 patients.
Remission, defined as a DAS28-CRP (Disease Activity Score 28–C-reactive protein) score of less than 2.6, was achieved in a similar percentage of subjects in the CHS-0214 and etanercept groups, at 40.6% and 42.4%, respectively.
There was a similar percentage of any adverse event (60.8% vs. 65%) occurring among patients receiving the biosimilar and those getting etanercept. Treatment-related adverse events (16.4% vs. 21.9%), and treatment-related serious adverse events (0.9% vs. 0.3%) were also comparable. Drug-drug antibodies occurred in numerically fewer patients treated with the biosimilar than with etanercept (1.3% vs. 4.7%).
Asked after his presentation about why he thought the ACR responses seen in the study were so high, Dr. O’Dell said: “We were surprised by that.” There are a number of potential explanations, he suggested. “We had a remarkable completion rate in the trial, 95%, so we lost very few patients, and we are in the process of analyzing other trials to see if that is a major factor.” In addition, patients were on relatively lower doses of methotrexate than in some other trials “because a quarter of them came from Japan,” he said. Patients were also all biologic naive and many were recruited from countries where they don’t have the opportunity to be exposed to a lot of therapies.
“The studies on all biosimilars so far suggest that they are indeed biosimilar,” Dr. O’Dell said in an interview. So how might clinicians begin to choose between the various biosimilars? “Well, there is very, very little data to compare any biologic to another biologic,” he answered.
“You haven’t seen studies comparing one TNF inhibitor to another TNF inhibitor. So it’s not surprising that we don’t have data that compare this biosimilar to that biosimilar,” he added. Such studies are probably also unlikely to ever be conducted so the choice of biosimilar, like anti-TNF therapy is probably going to be dictated by national and local guidelines, and medical insurance policies.
“Today, the biosimilars look biosimilar, so I have no problems if somebody tells me I have to start a biosimilar as opposed to the innovator product. I do have a problem if they tell me I have to switch, because that’s interchangeability and that’s a whole different story,” he noted.
Dr. O’Dell observed, however, that data from the DANBIO registry presented during the same session had shown that an enforced national switch from infliximab (Remicade) to biosimilar infliximab had shown that this did not appear to pose a problem in routine care in Denmark. Indeed 3 months’ clinical outcomes in patients with RA, psoriatic arthritis, or axial spondyloarthritis who were switched appeared to be comparable. Whether this is true across all the biosimilars needs to be established.
Coherus Biosciences funded the study. Dr. O’Dell has been a study investigator for, received research grants from, or acted as a speaker or consultant to Coherus Biosciences, Medac, Eli Lilly, Bristol-Myers Squibb, GlaxoSmithKline, Antares, and Crescendo.
LONDON – Biosimilar etanercept (CHS-0214) is as effective and well tolerated as etanercept (Enbrel) for the treatment of rheumatoid arthritis according to the results of a randomized, double-blind, phase III trial conducted in 13 countries.
The primary endpoint of an American College of Rheumatology (ACR) 20 at 24 weeks was achieved by 91% of patients given CHS-0214 and 90.6% of those given etanercept, giving a treatment difference of just 0.4%. The percentages of patients achieving ACR 50 (67.6% and 63.7%) and ACR 70 (38.3% and 37.9%) were also comparable.
These are the first clinical data to be presented on this biosimilar, which is a fusion protein comprising the soluble human p75 tumor necrosis factor (TNF) receptor and the Fc region of human immunoglobulin G1.
“Like all biosimilars CHD-0214 has undergone extensive analytical characterization, which had demonstrated highly similar structure and function to etanercept,” study investigator James O’Dell, MD, of the University of Nebraska Medical Center, Omaha, said at the European Congress of Rheumatology. “Studies have demonstrated that CHS-0214 is similar to etanercept with regard to in vitro pharmacology, in vivo pharmacokinetics, and toxicology,” he observed.
A total of 644 patients with moderate to severe rheumatoid arthritis who had an inadequate response to methotrexate were enrolled in the phase III trial and randomized to receive CHS-0214 or etanercept at a subcutaneous dose of 50 mg once a week for 24 weeks. After the double-blind period had ended, patients could continue on open-label CHS-0214 for another 24 weeks. Assessment of efficacy was performed on 512 patients as an issue with the production of the biosimilar resulted in a dosing interruption for 132 patients.
Remission, defined as a DAS28-CRP (Disease Activity Score 28–C-reactive protein) score of less than 2.6, was achieved in a similar percentage of subjects in the CHS-0214 and etanercept groups, at 40.6% and 42.4%, respectively.
There was a similar percentage of any adverse event (60.8% vs. 65%) occurring among patients receiving the biosimilar and those getting etanercept. Treatment-related adverse events (16.4% vs. 21.9%), and treatment-related serious adverse events (0.9% vs. 0.3%) were also comparable. Drug-drug antibodies occurred in numerically fewer patients treated with the biosimilar than with etanercept (1.3% vs. 4.7%).
Asked after his presentation about why he thought the ACR responses seen in the study were so high, Dr. O’Dell said: “We were surprised by that.” There are a number of potential explanations, he suggested. “We had a remarkable completion rate in the trial, 95%, so we lost very few patients, and we are in the process of analyzing other trials to see if that is a major factor.” In addition, patients were on relatively lower doses of methotrexate than in some other trials “because a quarter of them came from Japan,” he said. Patients were also all biologic naive and many were recruited from countries where they don’t have the opportunity to be exposed to a lot of therapies.
“The studies on all biosimilars so far suggest that they are indeed biosimilar,” Dr. O’Dell said in an interview. So how might clinicians begin to choose between the various biosimilars? “Well, there is very, very little data to compare any biologic to another biologic,” he answered.
“You haven’t seen studies comparing one TNF inhibitor to another TNF inhibitor. So it’s not surprising that we don’t have data that compare this biosimilar to that biosimilar,” he added. Such studies are probably also unlikely to ever be conducted so the choice of biosimilar, like anti-TNF therapy is probably going to be dictated by national and local guidelines, and medical insurance policies.
“Today, the biosimilars look biosimilar, so I have no problems if somebody tells me I have to start a biosimilar as opposed to the innovator product. I do have a problem if they tell me I have to switch, because that’s interchangeability and that’s a whole different story,” he noted.
Dr. O’Dell observed, however, that data from the DANBIO registry presented during the same session had shown that an enforced national switch from infliximab (Remicade) to biosimilar infliximab had shown that this did not appear to pose a problem in routine care in Denmark. Indeed 3 months’ clinical outcomes in patients with RA, psoriatic arthritis, or axial spondyloarthritis who were switched appeared to be comparable. Whether this is true across all the biosimilars needs to be established.
Coherus Biosciences funded the study. Dr. O’Dell has been a study investigator for, received research grants from, or acted as a speaker or consultant to Coherus Biosciences, Medac, Eli Lilly, Bristol-Myers Squibb, GlaxoSmithKline, Antares, and Crescendo.
LONDON – Biosimilar etanercept (CHS-0214) is as effective and well tolerated as etanercept (Enbrel) for the treatment of rheumatoid arthritis according to the results of a randomized, double-blind, phase III trial conducted in 13 countries.
The primary endpoint of an American College of Rheumatology (ACR) 20 at 24 weeks was achieved by 91% of patients given CHS-0214 and 90.6% of those given etanercept, giving a treatment difference of just 0.4%. The percentages of patients achieving ACR 50 (67.6% and 63.7%) and ACR 70 (38.3% and 37.9%) were also comparable.
These are the first clinical data to be presented on this biosimilar, which is a fusion protein comprising the soluble human p75 tumor necrosis factor (TNF) receptor and the Fc region of human immunoglobulin G1.
“Like all biosimilars CHD-0214 has undergone extensive analytical characterization, which had demonstrated highly similar structure and function to etanercept,” study investigator James O’Dell, MD, of the University of Nebraska Medical Center, Omaha, said at the European Congress of Rheumatology. “Studies have demonstrated that CHS-0214 is similar to etanercept with regard to in vitro pharmacology, in vivo pharmacokinetics, and toxicology,” he observed.
A total of 644 patients with moderate to severe rheumatoid arthritis who had an inadequate response to methotrexate were enrolled in the phase III trial and randomized to receive CHS-0214 or etanercept at a subcutaneous dose of 50 mg once a week for 24 weeks. After the double-blind period had ended, patients could continue on open-label CHS-0214 for another 24 weeks. Assessment of efficacy was performed on 512 patients as an issue with the production of the biosimilar resulted in a dosing interruption for 132 patients.
Remission, defined as a DAS28-CRP (Disease Activity Score 28–C-reactive protein) score of less than 2.6, was achieved in a similar percentage of subjects in the CHS-0214 and etanercept groups, at 40.6% and 42.4%, respectively.
There was a similar percentage of any adverse event (60.8% vs. 65%) occurring among patients receiving the biosimilar and those getting etanercept. Treatment-related adverse events (16.4% vs. 21.9%), and treatment-related serious adverse events (0.9% vs. 0.3%) were also comparable. Drug-drug antibodies occurred in numerically fewer patients treated with the biosimilar than with etanercept (1.3% vs. 4.7%).
Asked after his presentation about why he thought the ACR responses seen in the study were so high, Dr. O’Dell said: “We were surprised by that.” There are a number of potential explanations, he suggested. “We had a remarkable completion rate in the trial, 95%, so we lost very few patients, and we are in the process of analyzing other trials to see if that is a major factor.” In addition, patients were on relatively lower doses of methotrexate than in some other trials “because a quarter of them came from Japan,” he said. Patients were also all biologic naive and many were recruited from countries where they don’t have the opportunity to be exposed to a lot of therapies.
“The studies on all biosimilars so far suggest that they are indeed biosimilar,” Dr. O’Dell said in an interview. So how might clinicians begin to choose between the various biosimilars? “Well, there is very, very little data to compare any biologic to another biologic,” he answered.
“You haven’t seen studies comparing one TNF inhibitor to another TNF inhibitor. So it’s not surprising that we don’t have data that compare this biosimilar to that biosimilar,” he added. Such studies are probably also unlikely to ever be conducted so the choice of biosimilar, like anti-TNF therapy is probably going to be dictated by national and local guidelines, and medical insurance policies.
“Today, the biosimilars look biosimilar, so I have no problems if somebody tells me I have to start a biosimilar as opposed to the innovator product. I do have a problem if they tell me I have to switch, because that’s interchangeability and that’s a whole different story,” he noted.
Dr. O’Dell observed, however, that data from the DANBIO registry presented during the same session had shown that an enforced national switch from infliximab (Remicade) to biosimilar infliximab had shown that this did not appear to pose a problem in routine care in Denmark. Indeed 3 months’ clinical outcomes in patients with RA, psoriatic arthritis, or axial spondyloarthritis who were switched appeared to be comparable. Whether this is true across all the biosimilars needs to be established.
Coherus Biosciences funded the study. Dr. O’Dell has been a study investigator for, received research grants from, or acted as a speaker or consultant to Coherus Biosciences, Medac, Eli Lilly, Bristol-Myers Squibb, GlaxoSmithKline, Antares, and Crescendo.
AT THE EULAR 2016 CONGRESS
Key clinical point: Biosimilar etanercept (CHS-0214) was shown to be as effective and well tolerated as etanercept.
Major finding: ACR 20 at 24 weeks (primary endpoint) was achieved by 91% given the biosimilar and 90.6% of those given etanercept.
Data source: Multicenter, randomized, double-blind, phase III trial comparing the etanercept biosimilar CHS-0215 with etanercept in 644 patients with rheumatoid arthritis.
Disclosures: Coherus Biosciences funded the study. Dr. O’Dell has been a study investigator for, received research grants from, or acted as a speaker or consultant to Coherus Biosciences, Medac, Eli Lilly, Bristol-Myers Squibb, GlaxoSmithKline, Antares, and Crescendo.
JAK-1 inhibitors heading for validation in phase III trials
LONDON – ABT-494 and filgotinib – two investigational and highly selective oral Janus kinase-1 inhibitors – are both showing promise in the treatment of patients with rheumatoid arthritis, according to the results of two separate phase II studies presented at the European Congress of Rheumatology.
In the BALANCE-2 study, 62%-80% patients who had an inadequate response to methotrexate alone achieved the primary endpoint of an ACR20 response after 12 weeks of combination treatment with methotrexate and ABT-494, depending on the dose used, versus 46% for placebo plus methotrexate. The secondary endpoint of ACR50 was reached by a respective 38%-50% vs. 18%, and ACR70 response was achieved by 16%-28% vs. 6%.
And in the DARWIN-1 study, 56%-79% of patients treated with different doses of filgotinib plus methotrexate achieved the trial’s primary endpoint, which was again ACR20 at 12 weeks, versus 44% for placebo plus methotrexate. ACR50 and ACR70 responses were also similarly high and maintained up to 24 weeks of follow-up.
Both drugs had safety and tolerability data that supported their further development, the respective study investigators said.
“I think the results are rather straightforward. There was significant improvement in signs and symptoms of RA with fast onset,” said René Westhovens, MD, PhD, of the University of Leuven (Belgium), who presented the data from the DARWIN-1 study. “These robust data support the future development of filgotinib in RA,” he said.
Mark Genovese, MD, of Stanford (Calif.) University, who presented the findings of the BALANCE-2 study, said: “ABT-494 has been shown to have significant improvements in symptoms and signs [of RA] based on our endpoints of ACR [response], DAS[28], and CDAI [clinical disease activity index].” Like ACR50 and ACR70, DAS28 and CDAI were secondary efficacy endpoints and showed significantly greater changes from baseline versus placebo, started from around 2 weeks.
In an interview, Peter Taylor, PhD, who chaired the session at the meeting where the findings were presented, said: “We’ve seen a lot of data about JAK inhibitors at various stages of development at EULAR 2016, with varying selectivity, and the clinical data unequivocally validates Janus kinases as a therapeutic target.”
Dr. Taylor, the Norman Collisson Professor of Musculoskeletal Sciences at the University of Oxford (England), added: “[JAK inhibitors] show very significant promise with favorable safety data overall, but there are subtle differences between the drugs which need further detailed analysis to understand what it means in a clinical context.”
BALANCE-2 was a double-blind, placebo-controlled, dose-ranging phase IIB study designed to look at the safety and efficacy of ABT-494 in adult patients with moderately to severely active rheumatoid arthritis who had an inadequate response to methotrexate. Five doses of ABT-494 were tested: four given twice-daily (3, 6, 12, and 18 mg) and one given once-daily (24 mg). A total of 300 patients were enrolled and 299 were randomized, 50 to placebo, 50 each to the once-daily doses, and 49 to the twice-daily dose group. The mean weekly methotrexate dose at baseline was 14-16 mg across the groups.
“In general, the safety and tolerability of ABT-494 was satisfactory at the doses tested, consistent with what would have been expected,” Dr. Genovese said.
There was a numerically higher rate of any adverse event in the groups treated with ABT-494, at 40%, 46%, 58%, and 50% for the twice-daily regimens of 3, 6, 12, and 18 mg, as well as 35% for the once-daily 24-mg dose. The rate was 26% for placebo plus methotrexate.
Infections occurred in a respective 20%, 14%, 24%, 22% across the twice-daily dosing groups, respectively, compared with 18% for the once-daily 24-mg dose and 14% for placebo plus methotrexate, he noted. While there were some grade 2-3 abnormalities in liver enzymes and dose-related decreases in hemoglobin seen at higher doses, these did not appear to have significant clinical impact. The ratio of high-density lipoprotein cholesterol (HDL-C) to low-density lipoprotein cholesterol (LDL-C) was also affected slightly.
DARWIN-1 involved a total of 599 enrolled and 594 randomized and exposed patients with RA treated with placebo plus methotrexate or methotrexate plus one of six dosing regimens of filgotinib: 50, 100, or 200 mg once daily, or 25, 50, or 100 mg twice daily, for 24 weeks, with around 85 patients in each group. Each patient previously had an inadequate response to methotrexate alone. At the 12-week halfway point, patients taking placebo and the 50-mg dose could be reassigned to filgotinib 100 mg once daily or 50 mg twice daily if their tender or swollen joint counts had not improved. The mean weekly dose of methotrexate at baseline was 16.4-17.5 mg across the groups.
In addition to the improved ACR responses, significant improvements with filgotinib versus placebo were seen in the secondary endpoints of DAS28 (including DAS28 based on C-reactive protein), CDAI, and the Health Assessment Questionnaire-Disability Index.
There were infrequent serious adverse events, which included serious infections, and adverse events leading to discontinuations, Dr. Westhovens observed, and nothing that would not have been expected or different from placebo. There was a small decrease in neutrophil counts and increase in creatinine, but neither had any clinical consequences. Interestingly, there was a dose-dependent increase in hemoglobin but no reduction in lymphocyte counts, he said. HDL-C increased more than LDL-C.
Five phase III trials with ABT-494 are currently underway in patients with RA:
• SELECT-COMPARE will enroll an estimated 1,500 RA patients who have had an inadequate response to a stable dose of methotrexate and will compare additional treatment with ABT-494 against additional treatment with adalimumab (Humira) or placebo.
• SELECT-NEXT will enroll an estimated 600 RA patients who have had an inadequate response to stable doses of conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) and are then given ABT-494 or placebo on top.
• SELECT-BEYOND will enroll around 450 RA patients on stable csDMARDs who have an inadequate response or intolerance to biologic DMARDs and compare adding ABT-494 or placebo.
• SELECT-MONOTHERAPY will enroll 600 RA patients who have had an inadequate methotrexate response and compare ABT-494 monotherapy to methotrexate monotherapy.
• SELECT-EARLY will enroll 975 methotrexate-naive, moderately-to-severely active RA patients and compare giving ABT-494 monotherapy to methotrexate monotherapy.
Most of these trials should have primary endpoint data available for analysis by mid to late 2017 or 2018 and be finished by 2020 or 2021.
Filgotinib, formerly known as GLPG0634, is also about to enter phase III trials, but the details of these trials have not yet been revealed other than that they will begin mid-2016.
The BALANCE-2 study was funded by AbbVie. Dr. Genovese is a consultant for, and has received grants from AbbVie, Eli Lilly, Astellas, Vertex, Pfizer, Galapagos, and Gilead.
The DARWIN-1 study was funded by Galapagos. Dr. Westhovens is the principal investigator for the study. He also disclosed receiving research funding from Roche and speaker’s honoraria from Bristol-Myers Squibb.
Dr. Taylor was not involved in either study but has consulted for Eli Lilly, Pfizer, and Galapagos.
LONDON – ABT-494 and filgotinib – two investigational and highly selective oral Janus kinase-1 inhibitors – are both showing promise in the treatment of patients with rheumatoid arthritis, according to the results of two separate phase II studies presented at the European Congress of Rheumatology.
In the BALANCE-2 study, 62%-80% patients who had an inadequate response to methotrexate alone achieved the primary endpoint of an ACR20 response after 12 weeks of combination treatment with methotrexate and ABT-494, depending on the dose used, versus 46% for placebo plus methotrexate. The secondary endpoint of ACR50 was reached by a respective 38%-50% vs. 18%, and ACR70 response was achieved by 16%-28% vs. 6%.
And in the DARWIN-1 study, 56%-79% of patients treated with different doses of filgotinib plus methotrexate achieved the trial’s primary endpoint, which was again ACR20 at 12 weeks, versus 44% for placebo plus methotrexate. ACR50 and ACR70 responses were also similarly high and maintained up to 24 weeks of follow-up.
Both drugs had safety and tolerability data that supported their further development, the respective study investigators said.
“I think the results are rather straightforward. There was significant improvement in signs and symptoms of RA with fast onset,” said René Westhovens, MD, PhD, of the University of Leuven (Belgium), who presented the data from the DARWIN-1 study. “These robust data support the future development of filgotinib in RA,” he said.
Mark Genovese, MD, of Stanford (Calif.) University, who presented the findings of the BALANCE-2 study, said: “ABT-494 has been shown to have significant improvements in symptoms and signs [of RA] based on our endpoints of ACR [response], DAS[28], and CDAI [clinical disease activity index].” Like ACR50 and ACR70, DAS28 and CDAI were secondary efficacy endpoints and showed significantly greater changes from baseline versus placebo, started from around 2 weeks.
In an interview, Peter Taylor, PhD, who chaired the session at the meeting where the findings were presented, said: “We’ve seen a lot of data about JAK inhibitors at various stages of development at EULAR 2016, with varying selectivity, and the clinical data unequivocally validates Janus kinases as a therapeutic target.”
Dr. Taylor, the Norman Collisson Professor of Musculoskeletal Sciences at the University of Oxford (England), added: “[JAK inhibitors] show very significant promise with favorable safety data overall, but there are subtle differences between the drugs which need further detailed analysis to understand what it means in a clinical context.”
BALANCE-2 was a double-blind, placebo-controlled, dose-ranging phase IIB study designed to look at the safety and efficacy of ABT-494 in adult patients with moderately to severely active rheumatoid arthritis who had an inadequate response to methotrexate. Five doses of ABT-494 were tested: four given twice-daily (3, 6, 12, and 18 mg) and one given once-daily (24 mg). A total of 300 patients were enrolled and 299 were randomized, 50 to placebo, 50 each to the once-daily doses, and 49 to the twice-daily dose group. The mean weekly methotrexate dose at baseline was 14-16 mg across the groups.
“In general, the safety and tolerability of ABT-494 was satisfactory at the doses tested, consistent with what would have been expected,” Dr. Genovese said.
There was a numerically higher rate of any adverse event in the groups treated with ABT-494, at 40%, 46%, 58%, and 50% for the twice-daily regimens of 3, 6, 12, and 18 mg, as well as 35% for the once-daily 24-mg dose. The rate was 26% for placebo plus methotrexate.
Infections occurred in a respective 20%, 14%, 24%, 22% across the twice-daily dosing groups, respectively, compared with 18% for the once-daily 24-mg dose and 14% for placebo plus methotrexate, he noted. While there were some grade 2-3 abnormalities in liver enzymes and dose-related decreases in hemoglobin seen at higher doses, these did not appear to have significant clinical impact. The ratio of high-density lipoprotein cholesterol (HDL-C) to low-density lipoprotein cholesterol (LDL-C) was also affected slightly.
DARWIN-1 involved a total of 599 enrolled and 594 randomized and exposed patients with RA treated with placebo plus methotrexate or methotrexate plus one of six dosing regimens of filgotinib: 50, 100, or 200 mg once daily, or 25, 50, or 100 mg twice daily, for 24 weeks, with around 85 patients in each group. Each patient previously had an inadequate response to methotrexate alone. At the 12-week halfway point, patients taking placebo and the 50-mg dose could be reassigned to filgotinib 100 mg once daily or 50 mg twice daily if their tender or swollen joint counts had not improved. The mean weekly dose of methotrexate at baseline was 16.4-17.5 mg across the groups.
In addition to the improved ACR responses, significant improvements with filgotinib versus placebo were seen in the secondary endpoints of DAS28 (including DAS28 based on C-reactive protein), CDAI, and the Health Assessment Questionnaire-Disability Index.
There were infrequent serious adverse events, which included serious infections, and adverse events leading to discontinuations, Dr. Westhovens observed, and nothing that would not have been expected or different from placebo. There was a small decrease in neutrophil counts and increase in creatinine, but neither had any clinical consequences. Interestingly, there was a dose-dependent increase in hemoglobin but no reduction in lymphocyte counts, he said. HDL-C increased more than LDL-C.
Five phase III trials with ABT-494 are currently underway in patients with RA:
• SELECT-COMPARE will enroll an estimated 1,500 RA patients who have had an inadequate response to a stable dose of methotrexate and will compare additional treatment with ABT-494 against additional treatment with adalimumab (Humira) or placebo.
• SELECT-NEXT will enroll an estimated 600 RA patients who have had an inadequate response to stable doses of conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) and are then given ABT-494 or placebo on top.
• SELECT-BEYOND will enroll around 450 RA patients on stable csDMARDs who have an inadequate response or intolerance to biologic DMARDs and compare adding ABT-494 or placebo.
• SELECT-MONOTHERAPY will enroll 600 RA patients who have had an inadequate methotrexate response and compare ABT-494 monotherapy to methotrexate monotherapy.
• SELECT-EARLY will enroll 975 methotrexate-naive, moderately-to-severely active RA patients and compare giving ABT-494 monotherapy to methotrexate monotherapy.
Most of these trials should have primary endpoint data available for analysis by mid to late 2017 or 2018 and be finished by 2020 or 2021.
Filgotinib, formerly known as GLPG0634, is also about to enter phase III trials, but the details of these trials have not yet been revealed other than that they will begin mid-2016.
The BALANCE-2 study was funded by AbbVie. Dr. Genovese is a consultant for, and has received grants from AbbVie, Eli Lilly, Astellas, Vertex, Pfizer, Galapagos, and Gilead.
The DARWIN-1 study was funded by Galapagos. Dr. Westhovens is the principal investigator for the study. He also disclosed receiving research funding from Roche and speaker’s honoraria from Bristol-Myers Squibb.
Dr. Taylor was not involved in either study but has consulted for Eli Lilly, Pfizer, and Galapagos.
LONDON – ABT-494 and filgotinib – two investigational and highly selective oral Janus kinase-1 inhibitors – are both showing promise in the treatment of patients with rheumatoid arthritis, according to the results of two separate phase II studies presented at the European Congress of Rheumatology.
In the BALANCE-2 study, 62%-80% patients who had an inadequate response to methotrexate alone achieved the primary endpoint of an ACR20 response after 12 weeks of combination treatment with methotrexate and ABT-494, depending on the dose used, versus 46% for placebo plus methotrexate. The secondary endpoint of ACR50 was reached by a respective 38%-50% vs. 18%, and ACR70 response was achieved by 16%-28% vs. 6%.
And in the DARWIN-1 study, 56%-79% of patients treated with different doses of filgotinib plus methotrexate achieved the trial’s primary endpoint, which was again ACR20 at 12 weeks, versus 44% for placebo plus methotrexate. ACR50 and ACR70 responses were also similarly high and maintained up to 24 weeks of follow-up.
Both drugs had safety and tolerability data that supported their further development, the respective study investigators said.
“I think the results are rather straightforward. There was significant improvement in signs and symptoms of RA with fast onset,” said René Westhovens, MD, PhD, of the University of Leuven (Belgium), who presented the data from the DARWIN-1 study. “These robust data support the future development of filgotinib in RA,” he said.
Mark Genovese, MD, of Stanford (Calif.) University, who presented the findings of the BALANCE-2 study, said: “ABT-494 has been shown to have significant improvements in symptoms and signs [of RA] based on our endpoints of ACR [response], DAS[28], and CDAI [clinical disease activity index].” Like ACR50 and ACR70, DAS28 and CDAI were secondary efficacy endpoints and showed significantly greater changes from baseline versus placebo, started from around 2 weeks.
In an interview, Peter Taylor, PhD, who chaired the session at the meeting where the findings were presented, said: “We’ve seen a lot of data about JAK inhibitors at various stages of development at EULAR 2016, with varying selectivity, and the clinical data unequivocally validates Janus kinases as a therapeutic target.”
Dr. Taylor, the Norman Collisson Professor of Musculoskeletal Sciences at the University of Oxford (England), added: “[JAK inhibitors] show very significant promise with favorable safety data overall, but there are subtle differences between the drugs which need further detailed analysis to understand what it means in a clinical context.”
BALANCE-2 was a double-blind, placebo-controlled, dose-ranging phase IIB study designed to look at the safety and efficacy of ABT-494 in adult patients with moderately to severely active rheumatoid arthritis who had an inadequate response to methotrexate. Five doses of ABT-494 were tested: four given twice-daily (3, 6, 12, and 18 mg) and one given once-daily (24 mg). A total of 300 patients were enrolled and 299 were randomized, 50 to placebo, 50 each to the once-daily doses, and 49 to the twice-daily dose group. The mean weekly methotrexate dose at baseline was 14-16 mg across the groups.
“In general, the safety and tolerability of ABT-494 was satisfactory at the doses tested, consistent with what would have been expected,” Dr. Genovese said.
There was a numerically higher rate of any adverse event in the groups treated with ABT-494, at 40%, 46%, 58%, and 50% for the twice-daily regimens of 3, 6, 12, and 18 mg, as well as 35% for the once-daily 24-mg dose. The rate was 26% for placebo plus methotrexate.
Infections occurred in a respective 20%, 14%, 24%, 22% across the twice-daily dosing groups, respectively, compared with 18% for the once-daily 24-mg dose and 14% for placebo plus methotrexate, he noted. While there were some grade 2-3 abnormalities in liver enzymes and dose-related decreases in hemoglobin seen at higher doses, these did not appear to have significant clinical impact. The ratio of high-density lipoprotein cholesterol (HDL-C) to low-density lipoprotein cholesterol (LDL-C) was also affected slightly.
DARWIN-1 involved a total of 599 enrolled and 594 randomized and exposed patients with RA treated with placebo plus methotrexate or methotrexate plus one of six dosing regimens of filgotinib: 50, 100, or 200 mg once daily, or 25, 50, or 100 mg twice daily, for 24 weeks, with around 85 patients in each group. Each patient previously had an inadequate response to methotrexate alone. At the 12-week halfway point, patients taking placebo and the 50-mg dose could be reassigned to filgotinib 100 mg once daily or 50 mg twice daily if their tender or swollen joint counts had not improved. The mean weekly dose of methotrexate at baseline was 16.4-17.5 mg across the groups.
In addition to the improved ACR responses, significant improvements with filgotinib versus placebo were seen in the secondary endpoints of DAS28 (including DAS28 based on C-reactive protein), CDAI, and the Health Assessment Questionnaire-Disability Index.
There were infrequent serious adverse events, which included serious infections, and adverse events leading to discontinuations, Dr. Westhovens observed, and nothing that would not have been expected or different from placebo. There was a small decrease in neutrophil counts and increase in creatinine, but neither had any clinical consequences. Interestingly, there was a dose-dependent increase in hemoglobin but no reduction in lymphocyte counts, he said. HDL-C increased more than LDL-C.
Five phase III trials with ABT-494 are currently underway in patients with RA:
• SELECT-COMPARE will enroll an estimated 1,500 RA patients who have had an inadequate response to a stable dose of methotrexate and will compare additional treatment with ABT-494 against additional treatment with adalimumab (Humira) or placebo.
• SELECT-NEXT will enroll an estimated 600 RA patients who have had an inadequate response to stable doses of conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) and are then given ABT-494 or placebo on top.
• SELECT-BEYOND will enroll around 450 RA patients on stable csDMARDs who have an inadequate response or intolerance to biologic DMARDs and compare adding ABT-494 or placebo.
• SELECT-MONOTHERAPY will enroll 600 RA patients who have had an inadequate methotrexate response and compare ABT-494 monotherapy to methotrexate monotherapy.
• SELECT-EARLY will enroll 975 methotrexate-naive, moderately-to-severely active RA patients and compare giving ABT-494 monotherapy to methotrexate monotherapy.
Most of these trials should have primary endpoint data available for analysis by mid to late 2017 or 2018 and be finished by 2020 or 2021.
Filgotinib, formerly known as GLPG0634, is also about to enter phase III trials, but the details of these trials have not yet been revealed other than that they will begin mid-2016.
The BALANCE-2 study was funded by AbbVie. Dr. Genovese is a consultant for, and has received grants from AbbVie, Eli Lilly, Astellas, Vertex, Pfizer, Galapagos, and Gilead.
The DARWIN-1 study was funded by Galapagos. Dr. Westhovens is the principal investigator for the study. He also disclosed receiving research funding from Roche and speaker’s honoraria from Bristol-Myers Squibb.
Dr. Taylor was not involved in either study but has consulted for Eli Lilly, Pfizer, and Galapagos.
Key clinical point: Two new oral Janus kinase inhibitors selective for JAK-1 have shown promising efficacy and safety in separate phase II trials.
Major finding: ACR20 responses were achieved by 62%-80% of patients treated with different dosing regimens of ABT-494 plus methotrexate (vs. 46% with placebo plus methotrexate) in one trial and by 56%-79% of patients treated with different dosing regimens of filgotinib plus methotrexate (vs. 44% with placebo and methotrexate) in the other.
Data source: Two phase II studies addressing the efficacy and safety of the selective JAK-1 inhibitors ABT-494 and filgotinib in patients with rheumatoid arthritis and an inadequate response to methotrexate.
Disclosures: The BALANCE-2 study was funded by AbbVie. Dr. Genovese is a consultant for, and has received grants from AbbVie, Eli Lilly, Astellas, Vertex, Pfizer, Galapagos, and Gilead. The DARWIN-1 study was funded by Galapagos. Dr. Westhovens is the principal investigator for the study. He also disclosed receiving research funding from Roche and speaker’s honoraria from Bristol-Myers Squibb. Dr. Taylor has consulted for Eli Lilly, Pfizer, and Galapagos.
New fragility fracture recommendations emphasize coordination of care
LONDON – The European League Against Rheumatism and the European Federation of National Associations of Orthopaedics and Traumatology have joined forces to develop recommendations for the prevention and management of fragility fractures.
Such fractures are common in men and women over the age of 50 years and can lead to repeat fracture in some patients. The recommendations are unique as they are the first to consider both acute orthopedic and postfracture rheumatologic care, said Willem F. Lems, MD, PhD, of the Amsterdam Rheumatology and Immunology Centre.
At the European Congress of Rheumatology, Dr. Lems provided an overview of the draft recommendations, noting that there would be several overarching principles, one of which recognized the multidisciplinary nature of caring for someone with a fragility fracture. An important point is not who is taking care of the patient, but that the patient is given the best possible care within the multidisciplinary framework.
What constitutes optimal care of course depends on the clinical situation, notably the type of fracture and the age of the patient, and optimal care in all phases of presentation (pre-, peri- and postoperative) can have an important effect on a patient’s outcome. The prevention of subsequent fractures is a key focus, with the recommendation that all patients should be investigated systematically and those deemed at high risk for another fracture should be prescribed both pharmacologic and nonpharmacologic interventions as appropriate. Patient education is also considered important.
As for all EULAR-developed recommendations, standard procedures were followed that involved convening an expert scientific advisory committee and using the Delphi technique to come up with the most important research questions that would be used to formulate the final 10 recommendations. Four of the recommendations cover the acute care setting and six provide advice on postfracture care.
The first of the acute care recommendations looks at pre- and perioperative management of a fragility fracture and highlights that, within 24-48 hours of admission, patients should receive adequate pain and fluid management and treatment, including early surgery if appropriate. This is based on evidence that better outcomes can be achieved in terms of both morbidity and mortality if patients can be seen and managed quickly.
Another of the acute care recommendations focuses on orthogeriatric care, noting that the orthopedic surgeon and a dedicated orthogeriatric team should work together, particularly for elderly patients who have suffered a hip fracture. Key elements here are the management of and prevention of delirium, deep vein thrombosis, pressure sores, and malnutrition.
As for actual fracture treatment, a balanced approach is advised when deciding upon a surgical or nonsurgical approach, especially because this is likely to be an older population with other comorbidities. Only one in three vertebral fractures are symptomatic and only about 10% of patients will be hospitalized for pain. Analgesics, modifying activities, and bracing can be options here. Surgical options for distal radial fracture, hip fracture, and trochanteric and femoral neck fractures are included.
The fourth recommendation looks at the organization of postfracture care and the need for a systematic approach to identify those who may be at risk for subsequent fractures, starting with the suggestion that any patient older than 50 years with a recent fracture should be assessed. The fifth recommendation addresses ways to evaluate this risk, such as looking at the clinical risk factors, performing bone scans and imaging, and screening for underlying osteoporosis or metabolic disorders.
Implementation is the next step, and the sixth recommendation suggests ways these recommendations could be integrated into routine practice. Often one of the biggest barriers to effective postfracture care is the lack of patient, and sometimes clinician, awareness of the risk for a subsequent fracture. This recommendation looks at the role of a possible local fracture liaison service or facilitator to coordinate between the various members of the multidisciplinary team from secondary (orthopedic surgeons, rheumatologists, endocrinologists, and geriatricians) to primary care.
The seventh recommendation addresses rehabilitation and the need to initiate physical training and muscle strengthening as early as possible after the initial fracture, with long-term continuation of balance training and fall prevention.
The final three recommendations focus on how to educate patients about their risk factors, need for follow-up, and the duration of any pharmacologic or nonpharmacologic therapy that they may need. Nonpharmacologic options might include stopping smoking, limiting alcohol intake, as well as taking supplements such as calcium or vitamin D. There will be specific guidance on the use of calcium and vitamin D, which have both pros and cons, but the optimal dosage appears to be 1,000–1,200 mg/day for calcium and 800 IU/day for vitamin D.
Pharmacologic options to prevent subsequent fragility fractures include the bisphosphonates alendronate, risedronate, and zoledronic acid (Reclast), and also the monoclonal antibody denosumab (Prolia). These are the only drugs that have been shown to reduced the risk for vertebral, nonvertebral, and hip fractures in primary analyses. Adherence, tolerance, and regular monitoring are key, and a five-step plan is suggested to aid clinical decision making that covers case finding, risk evaluation, differential diagnosis, treatment, and follow-up.
The recommendations are being finalized and should be available for publication later this year. The recommendations task force also plans to propose a research agenda.
Dr. Lems had no relevant disclosures.
LONDON – The European League Against Rheumatism and the European Federation of National Associations of Orthopaedics and Traumatology have joined forces to develop recommendations for the prevention and management of fragility fractures.
Such fractures are common in men and women over the age of 50 years and can lead to repeat fracture in some patients. The recommendations are unique as they are the first to consider both acute orthopedic and postfracture rheumatologic care, said Willem F. Lems, MD, PhD, of the Amsterdam Rheumatology and Immunology Centre.
At the European Congress of Rheumatology, Dr. Lems provided an overview of the draft recommendations, noting that there would be several overarching principles, one of which recognized the multidisciplinary nature of caring for someone with a fragility fracture. An important point is not who is taking care of the patient, but that the patient is given the best possible care within the multidisciplinary framework.
What constitutes optimal care of course depends on the clinical situation, notably the type of fracture and the age of the patient, and optimal care in all phases of presentation (pre-, peri- and postoperative) can have an important effect on a patient’s outcome. The prevention of subsequent fractures is a key focus, with the recommendation that all patients should be investigated systematically and those deemed at high risk for another fracture should be prescribed both pharmacologic and nonpharmacologic interventions as appropriate. Patient education is also considered important.
As for all EULAR-developed recommendations, standard procedures were followed that involved convening an expert scientific advisory committee and using the Delphi technique to come up with the most important research questions that would be used to formulate the final 10 recommendations. Four of the recommendations cover the acute care setting and six provide advice on postfracture care.
The first of the acute care recommendations looks at pre- and perioperative management of a fragility fracture and highlights that, within 24-48 hours of admission, patients should receive adequate pain and fluid management and treatment, including early surgery if appropriate. This is based on evidence that better outcomes can be achieved in terms of both morbidity and mortality if patients can be seen and managed quickly.
Another of the acute care recommendations focuses on orthogeriatric care, noting that the orthopedic surgeon and a dedicated orthogeriatric team should work together, particularly for elderly patients who have suffered a hip fracture. Key elements here are the management of and prevention of delirium, deep vein thrombosis, pressure sores, and malnutrition.
As for actual fracture treatment, a balanced approach is advised when deciding upon a surgical or nonsurgical approach, especially because this is likely to be an older population with other comorbidities. Only one in three vertebral fractures are symptomatic and only about 10% of patients will be hospitalized for pain. Analgesics, modifying activities, and bracing can be options here. Surgical options for distal radial fracture, hip fracture, and trochanteric and femoral neck fractures are included.
The fourth recommendation looks at the organization of postfracture care and the need for a systematic approach to identify those who may be at risk for subsequent fractures, starting with the suggestion that any patient older than 50 years with a recent fracture should be assessed. The fifth recommendation addresses ways to evaluate this risk, such as looking at the clinical risk factors, performing bone scans and imaging, and screening for underlying osteoporosis or metabolic disorders.
Implementation is the next step, and the sixth recommendation suggests ways these recommendations could be integrated into routine practice. Often one of the biggest barriers to effective postfracture care is the lack of patient, and sometimes clinician, awareness of the risk for a subsequent fracture. This recommendation looks at the role of a possible local fracture liaison service or facilitator to coordinate between the various members of the multidisciplinary team from secondary (orthopedic surgeons, rheumatologists, endocrinologists, and geriatricians) to primary care.
The seventh recommendation addresses rehabilitation and the need to initiate physical training and muscle strengthening as early as possible after the initial fracture, with long-term continuation of balance training and fall prevention.
The final three recommendations focus on how to educate patients about their risk factors, need for follow-up, and the duration of any pharmacologic or nonpharmacologic therapy that they may need. Nonpharmacologic options might include stopping smoking, limiting alcohol intake, as well as taking supplements such as calcium or vitamin D. There will be specific guidance on the use of calcium and vitamin D, which have both pros and cons, but the optimal dosage appears to be 1,000–1,200 mg/day for calcium and 800 IU/day for vitamin D.
Pharmacologic options to prevent subsequent fragility fractures include the bisphosphonates alendronate, risedronate, and zoledronic acid (Reclast), and also the monoclonal antibody denosumab (Prolia). These are the only drugs that have been shown to reduced the risk for vertebral, nonvertebral, and hip fractures in primary analyses. Adherence, tolerance, and regular monitoring are key, and a five-step plan is suggested to aid clinical decision making that covers case finding, risk evaluation, differential diagnosis, treatment, and follow-up.
The recommendations are being finalized and should be available for publication later this year. The recommendations task force also plans to propose a research agenda.
Dr. Lems had no relevant disclosures.
LONDON – The European League Against Rheumatism and the European Federation of National Associations of Orthopaedics and Traumatology have joined forces to develop recommendations for the prevention and management of fragility fractures.
Such fractures are common in men and women over the age of 50 years and can lead to repeat fracture in some patients. The recommendations are unique as they are the first to consider both acute orthopedic and postfracture rheumatologic care, said Willem F. Lems, MD, PhD, of the Amsterdam Rheumatology and Immunology Centre.
At the European Congress of Rheumatology, Dr. Lems provided an overview of the draft recommendations, noting that there would be several overarching principles, one of which recognized the multidisciplinary nature of caring for someone with a fragility fracture. An important point is not who is taking care of the patient, but that the patient is given the best possible care within the multidisciplinary framework.
What constitutes optimal care of course depends on the clinical situation, notably the type of fracture and the age of the patient, and optimal care in all phases of presentation (pre-, peri- and postoperative) can have an important effect on a patient’s outcome. The prevention of subsequent fractures is a key focus, with the recommendation that all patients should be investigated systematically and those deemed at high risk for another fracture should be prescribed both pharmacologic and nonpharmacologic interventions as appropriate. Patient education is also considered important.
As for all EULAR-developed recommendations, standard procedures were followed that involved convening an expert scientific advisory committee and using the Delphi technique to come up with the most important research questions that would be used to formulate the final 10 recommendations. Four of the recommendations cover the acute care setting and six provide advice on postfracture care.
The first of the acute care recommendations looks at pre- and perioperative management of a fragility fracture and highlights that, within 24-48 hours of admission, patients should receive adequate pain and fluid management and treatment, including early surgery if appropriate. This is based on evidence that better outcomes can be achieved in terms of both morbidity and mortality if patients can be seen and managed quickly.
Another of the acute care recommendations focuses on orthogeriatric care, noting that the orthopedic surgeon and a dedicated orthogeriatric team should work together, particularly for elderly patients who have suffered a hip fracture. Key elements here are the management of and prevention of delirium, deep vein thrombosis, pressure sores, and malnutrition.
As for actual fracture treatment, a balanced approach is advised when deciding upon a surgical or nonsurgical approach, especially because this is likely to be an older population with other comorbidities. Only one in three vertebral fractures are symptomatic and only about 10% of patients will be hospitalized for pain. Analgesics, modifying activities, and bracing can be options here. Surgical options for distal radial fracture, hip fracture, and trochanteric and femoral neck fractures are included.
The fourth recommendation looks at the organization of postfracture care and the need for a systematic approach to identify those who may be at risk for subsequent fractures, starting with the suggestion that any patient older than 50 years with a recent fracture should be assessed. The fifth recommendation addresses ways to evaluate this risk, such as looking at the clinical risk factors, performing bone scans and imaging, and screening for underlying osteoporosis or metabolic disorders.
Implementation is the next step, and the sixth recommendation suggests ways these recommendations could be integrated into routine practice. Often one of the biggest barriers to effective postfracture care is the lack of patient, and sometimes clinician, awareness of the risk for a subsequent fracture. This recommendation looks at the role of a possible local fracture liaison service or facilitator to coordinate between the various members of the multidisciplinary team from secondary (orthopedic surgeons, rheumatologists, endocrinologists, and geriatricians) to primary care.
The seventh recommendation addresses rehabilitation and the need to initiate physical training and muscle strengthening as early as possible after the initial fracture, with long-term continuation of balance training and fall prevention.
The final three recommendations focus on how to educate patients about their risk factors, need for follow-up, and the duration of any pharmacologic or nonpharmacologic therapy that they may need. Nonpharmacologic options might include stopping smoking, limiting alcohol intake, as well as taking supplements such as calcium or vitamin D. There will be specific guidance on the use of calcium and vitamin D, which have both pros and cons, but the optimal dosage appears to be 1,000–1,200 mg/day for calcium and 800 IU/day for vitamin D.
Pharmacologic options to prevent subsequent fragility fractures include the bisphosphonates alendronate, risedronate, and zoledronic acid (Reclast), and also the monoclonal antibody denosumab (Prolia). These are the only drugs that have been shown to reduced the risk for vertebral, nonvertebral, and hip fractures in primary analyses. Adherence, tolerance, and regular monitoring are key, and a five-step plan is suggested to aid clinical decision making that covers case finding, risk evaluation, differential diagnosis, treatment, and follow-up.
The recommendations are being finalized and should be available for publication later this year. The recommendations task force also plans to propose a research agenda.
Dr. Lems had no relevant disclosures.
AT THE EULAR 2016 CONGRESS
HCQ eye toxicity needs experience to assess
LONDON – Retinopathy in patients taking long-term hydroxychloroquine for rheumatic conditions requires assessment by those experienced with specialized ophthalmic imaging, according to study findings presented at the European Congress of Rheumatology.
Nonspecific abnormalities, which often are unrelated to hydroxychloroquine (HCQ), can be seen with many of the tests recommended by current ophthalmology guidelines. These changes need “careful interpretation by retina specialists,” the study’s investigators wrote in a poster presentation.
HCQ is used widely for the treatment of systemic lupus erythematosus (SLE), rheumatoid arthritis, and many other inflammatory or autoimmune conditions, but it can cause irreversible eye damage and is often associated with prolonged (greater than 5 years) use. Specifically, it can cause a type of end-stage retinopathy called bull’s-eye maculopathy, which is where the fovea becomes hyperpigmented, much like the bull’s-eye on a dartboard. This can lead to substantial vision loss (blind spots) if not caught early.
Although it is reasonably rare to develop end-stage retinopathy, there is currently no treatment for HCQ-induced retinopathy. Stopping the drug may not necessarily stop the retinal damage, and drug withdrawal may not be an option in many patients given the lack of alternative options to treat the symptoms of SLE, study author and ophthalmologist Syed Mahmood Ali Shah, MBBS, MD, said in an interview.
Dr. Shah and his associates at Johns Hopkins University in Baltimore reported on applying the 2011 American Academy of Ophthalmology (AAO) guidelines on screening for HCQ retinopathy (Ophthalmology. 2011;118:415-22) to an academic practice. They also estimated the prevalence of HCQ retinopathy among 135 consecutively treated patients with SLE using recommended tests. The mean duration of HCQ use was 12.5 years.
The 2011 AAO guidelines – which in March 2016 were updated (Ophthalmology 2016 Jun;123:1386-94) – recommended the use of three “ancillary” tests in addition to the usual clinical ophthalmic examination and assessment of visual fields: optical coherence tomography (OCT), fundus autofluorescence (FAF), and multifocal electroretinography (mfERG). Dr. Shah and his colleagues used these three tests together with eye-tracking microperimetry (MP) as a substitute for Humphrey Visual Fields (HVF), which is a common visual field test used in the United States.
One difference between the 2011 guidelines and 2016 revision is that “the baseline exam can now be performed relying [only] on the fundus exam, with additional imaging required only for abnormal patients,” Dr. Shah said. “Overall, the guidelines have not changed on how often and how much you follow up,” he added. “The change is that there is no need to do these tests at baseline unless changes of the fundus are present.” However, OCT has become more widely used in many offices and has been recognized as the most useful objective test and shall be performed if there are any abnormal findings of the fundus.
A total of 266 eyes were examined using these imaging methods and interpreted by experienced retina specialists. Overall, HCQ-related abnormalities were noted in 14 (5%) eyes using OCT, 18 (7%) using FAF, 27 (10%) eyes using mfERG, and 20 (7%) using MP.
MP had the lowest discrepancy between the overall number of eyes with abnormalities (72 [27%] of 266) detected and the number of eyes with abnormalities related to HCQ (20 [28%] of 72), followed by OCT (21% and 25%, respectively), FAF (19% and 35%) and mfERG (37% and 28%). Only four patients (3%) showed changes in all four tests suggestive of HCQ retinopathy.
In the absence of baseline data from the AAO recommended ancillary tests before the use of HCQ, “it may be difficult to interpret changes seen on these tests since most of the screenings are done by regular ophthalmologists who lack the equipment and experience with specialized testing such as mfERG, FAF, and OCT,” Dr. Shah and his coauthors noted. “We found a substantial number of cases with abnormalities unrelated to HCQ.”
Giving some practical advice, Dr. Shah noted that “before a patient starts treatment with HCQ, they should undergo a baseline ophthalmic assessment. Then if the patient complains of any vision changes, even if they have been taking the drug for less than 5 years, they should be reassessed.”
While repeat follow-up is, of course, necessary, he intimated that it is necessary to find a balance of risk and cost in regard to the frequency of screening for drug-related damage. “The American Academy of Ophthalmology currently recommends that a baseline fundus exam be performed shortly after starting HCQ. Ancillary OCT and visual fields shall only be performed if the fundus is abnormal at this baseline exam. However, since most retina specialists get OCT and visual field testing anyway it is wise to look at these as well,” he suggested. After 5 years of using the drug, they must be seen more regularly, and this is the point when ophthalmologists can decide if this should be every 6 months or annually, with the latter recommended by the AAO guidelines for patients with no additional risk factors.
The study was supported by noncommercial grants. Dr. Shah had no conflicts of interest to disclose.
LONDON – Retinopathy in patients taking long-term hydroxychloroquine for rheumatic conditions requires assessment by those experienced with specialized ophthalmic imaging, according to study findings presented at the European Congress of Rheumatology.
Nonspecific abnormalities, which often are unrelated to hydroxychloroquine (HCQ), can be seen with many of the tests recommended by current ophthalmology guidelines. These changes need “careful interpretation by retina specialists,” the study’s investigators wrote in a poster presentation.
HCQ is used widely for the treatment of systemic lupus erythematosus (SLE), rheumatoid arthritis, and many other inflammatory or autoimmune conditions, but it can cause irreversible eye damage and is often associated with prolonged (greater than 5 years) use. Specifically, it can cause a type of end-stage retinopathy called bull’s-eye maculopathy, which is where the fovea becomes hyperpigmented, much like the bull’s-eye on a dartboard. This can lead to substantial vision loss (blind spots) if not caught early.
Although it is reasonably rare to develop end-stage retinopathy, there is currently no treatment for HCQ-induced retinopathy. Stopping the drug may not necessarily stop the retinal damage, and drug withdrawal may not be an option in many patients given the lack of alternative options to treat the symptoms of SLE, study author and ophthalmologist Syed Mahmood Ali Shah, MBBS, MD, said in an interview.
Dr. Shah and his associates at Johns Hopkins University in Baltimore reported on applying the 2011 American Academy of Ophthalmology (AAO) guidelines on screening for HCQ retinopathy (Ophthalmology. 2011;118:415-22) to an academic practice. They also estimated the prevalence of HCQ retinopathy among 135 consecutively treated patients with SLE using recommended tests. The mean duration of HCQ use was 12.5 years.
The 2011 AAO guidelines – which in March 2016 were updated (Ophthalmology 2016 Jun;123:1386-94) – recommended the use of three “ancillary” tests in addition to the usual clinical ophthalmic examination and assessment of visual fields: optical coherence tomography (OCT), fundus autofluorescence (FAF), and multifocal electroretinography (mfERG). Dr. Shah and his colleagues used these three tests together with eye-tracking microperimetry (MP) as a substitute for Humphrey Visual Fields (HVF), which is a common visual field test used in the United States.
One difference between the 2011 guidelines and 2016 revision is that “the baseline exam can now be performed relying [only] on the fundus exam, with additional imaging required only for abnormal patients,” Dr. Shah said. “Overall, the guidelines have not changed on how often and how much you follow up,” he added. “The change is that there is no need to do these tests at baseline unless changes of the fundus are present.” However, OCT has become more widely used in many offices and has been recognized as the most useful objective test and shall be performed if there are any abnormal findings of the fundus.
A total of 266 eyes were examined using these imaging methods and interpreted by experienced retina specialists. Overall, HCQ-related abnormalities were noted in 14 (5%) eyes using OCT, 18 (7%) using FAF, 27 (10%) eyes using mfERG, and 20 (7%) using MP.
MP had the lowest discrepancy between the overall number of eyes with abnormalities (72 [27%] of 266) detected and the number of eyes with abnormalities related to HCQ (20 [28%] of 72), followed by OCT (21% and 25%, respectively), FAF (19% and 35%) and mfERG (37% and 28%). Only four patients (3%) showed changes in all four tests suggestive of HCQ retinopathy.
In the absence of baseline data from the AAO recommended ancillary tests before the use of HCQ, “it may be difficult to interpret changes seen on these tests since most of the screenings are done by regular ophthalmologists who lack the equipment and experience with specialized testing such as mfERG, FAF, and OCT,” Dr. Shah and his coauthors noted. “We found a substantial number of cases with abnormalities unrelated to HCQ.”
Giving some practical advice, Dr. Shah noted that “before a patient starts treatment with HCQ, they should undergo a baseline ophthalmic assessment. Then if the patient complains of any vision changes, even if they have been taking the drug for less than 5 years, they should be reassessed.”
While repeat follow-up is, of course, necessary, he intimated that it is necessary to find a balance of risk and cost in regard to the frequency of screening for drug-related damage. “The American Academy of Ophthalmology currently recommends that a baseline fundus exam be performed shortly after starting HCQ. Ancillary OCT and visual fields shall only be performed if the fundus is abnormal at this baseline exam. However, since most retina specialists get OCT and visual field testing anyway it is wise to look at these as well,” he suggested. After 5 years of using the drug, they must be seen more regularly, and this is the point when ophthalmologists can decide if this should be every 6 months or annually, with the latter recommended by the AAO guidelines for patients with no additional risk factors.
The study was supported by noncommercial grants. Dr. Shah had no conflicts of interest to disclose.
LONDON – Retinopathy in patients taking long-term hydroxychloroquine for rheumatic conditions requires assessment by those experienced with specialized ophthalmic imaging, according to study findings presented at the European Congress of Rheumatology.
Nonspecific abnormalities, which often are unrelated to hydroxychloroquine (HCQ), can be seen with many of the tests recommended by current ophthalmology guidelines. These changes need “careful interpretation by retina specialists,” the study’s investigators wrote in a poster presentation.
HCQ is used widely for the treatment of systemic lupus erythematosus (SLE), rheumatoid arthritis, and many other inflammatory or autoimmune conditions, but it can cause irreversible eye damage and is often associated with prolonged (greater than 5 years) use. Specifically, it can cause a type of end-stage retinopathy called bull’s-eye maculopathy, which is where the fovea becomes hyperpigmented, much like the bull’s-eye on a dartboard. This can lead to substantial vision loss (blind spots) if not caught early.
Although it is reasonably rare to develop end-stage retinopathy, there is currently no treatment for HCQ-induced retinopathy. Stopping the drug may not necessarily stop the retinal damage, and drug withdrawal may not be an option in many patients given the lack of alternative options to treat the symptoms of SLE, study author and ophthalmologist Syed Mahmood Ali Shah, MBBS, MD, said in an interview.
Dr. Shah and his associates at Johns Hopkins University in Baltimore reported on applying the 2011 American Academy of Ophthalmology (AAO) guidelines on screening for HCQ retinopathy (Ophthalmology. 2011;118:415-22) to an academic practice. They also estimated the prevalence of HCQ retinopathy among 135 consecutively treated patients with SLE using recommended tests. The mean duration of HCQ use was 12.5 years.
The 2011 AAO guidelines – which in March 2016 were updated (Ophthalmology 2016 Jun;123:1386-94) – recommended the use of three “ancillary” tests in addition to the usual clinical ophthalmic examination and assessment of visual fields: optical coherence tomography (OCT), fundus autofluorescence (FAF), and multifocal electroretinography (mfERG). Dr. Shah and his colleagues used these three tests together with eye-tracking microperimetry (MP) as a substitute for Humphrey Visual Fields (HVF), which is a common visual field test used in the United States.
One difference between the 2011 guidelines and 2016 revision is that “the baseline exam can now be performed relying [only] on the fundus exam, with additional imaging required only for abnormal patients,” Dr. Shah said. “Overall, the guidelines have not changed on how often and how much you follow up,” he added. “The change is that there is no need to do these tests at baseline unless changes of the fundus are present.” However, OCT has become more widely used in many offices and has been recognized as the most useful objective test and shall be performed if there are any abnormal findings of the fundus.
A total of 266 eyes were examined using these imaging methods and interpreted by experienced retina specialists. Overall, HCQ-related abnormalities were noted in 14 (5%) eyes using OCT, 18 (7%) using FAF, 27 (10%) eyes using mfERG, and 20 (7%) using MP.
MP had the lowest discrepancy between the overall number of eyes with abnormalities (72 [27%] of 266) detected and the number of eyes with abnormalities related to HCQ (20 [28%] of 72), followed by OCT (21% and 25%, respectively), FAF (19% and 35%) and mfERG (37% and 28%). Only four patients (3%) showed changes in all four tests suggestive of HCQ retinopathy.
In the absence of baseline data from the AAO recommended ancillary tests before the use of HCQ, “it may be difficult to interpret changes seen on these tests since most of the screenings are done by regular ophthalmologists who lack the equipment and experience with specialized testing such as mfERG, FAF, and OCT,” Dr. Shah and his coauthors noted. “We found a substantial number of cases with abnormalities unrelated to HCQ.”
Giving some practical advice, Dr. Shah noted that “before a patient starts treatment with HCQ, they should undergo a baseline ophthalmic assessment. Then if the patient complains of any vision changes, even if they have been taking the drug for less than 5 years, they should be reassessed.”
While repeat follow-up is, of course, necessary, he intimated that it is necessary to find a balance of risk and cost in regard to the frequency of screening for drug-related damage. “The American Academy of Ophthalmology currently recommends that a baseline fundus exam be performed shortly after starting HCQ. Ancillary OCT and visual fields shall only be performed if the fundus is abnormal at this baseline exam. However, since most retina specialists get OCT and visual field testing anyway it is wise to look at these as well,” he suggested. After 5 years of using the drug, they must be seen more regularly, and this is the point when ophthalmologists can decide if this should be every 6 months or annually, with the latter recommended by the AAO guidelines for patients with no additional risk factors.
The study was supported by noncommercial grants. Dr. Shah had no conflicts of interest to disclose.
AT THE EULAR 2016 CONGRESS
Key clinical point: Several eye abnormalities can be mistaken for hydroxychloroquine-related eye toxicity, making specialist ophthalmic assessment paramount.
Major finding: Only four patients (3%) showed changes in all four tests suggestive of HCQ retinopathy.
Data source: Observational study of 135 patients with SLE being seen for suspected hydroxychloroquine-related retinopathy at an academic practice
Disclosures: The study was supported by noncommercial grants. Dr. Shah had no conflicts of interest to disclose.
Pharma jousts statistically for an ankylosing spondylitis edge
Now that the interleukin-17 inhibitor secukinumab and tumor necrosis factor inhibitors are competing options for treatment of patients with ankylosing spondylitis, the companies that make those drugs must feel pressure to find some sort of advantage for their agents.
How else to explain the remarkable pair of similar post hoc analyses presented in June at the European Congress of Rheumatology in London? One of the analyses was funded by Novartis – the company that markets secukinumab (Cosentyx) – and included several Novartis employees as coauthors. The second study, presented immediately afterward in the main session at the meeting devoted to ankylosing spondylitis (AS) treatments, had backing from AbbVie, which markets adalimumab (Humira), the largest-selling tumor necrosis factor inhibitor worldwide, and had several AbbVie employees as coauthors.
Both analyses used a “matching adjusted indirect comparison,” a fairly new way to compare the performance of interventions studied in two totally independent trials by propensity matching patients from each of the two trials. It’s purportedly a way to make a legitimate comparison in the absence of head-to-head data.
Making the two reports even more surreal was their use of essentially the same data.
The first report came from Walter P. Maksymowych, MD, an AS clinician and researcher from the University of Alberta, who with his coauthors used data collected on secukinumab in the MEASURE 1 pivotal trial and on adalimumab in the ATLAS pivotal trial. He spent much of his presentation describing the methods behind the indirect comparison, and I don’t think I can be blamed for calling the results of this Novartis-sponsored analysis predictable: overall better performance by secukinumab, compared “indirectly” with adalimumab for clinical responses and patient quality of life.
The second report, the one sponsored by AbbVie, came from Keith A. Betts, PhD, a biostatistician who works for the Analysis Group, an international consulting firm. He also used the ATLAS database as the source for adalimumab outcomes, and differed marginally from Dr. Maksymowych by taking data on secukinumab patients from both the MEASURE 1 and MEASURE 2 pivotal trials. Although Dr. Betts also used the matching adjusted indirect comparison approach and broadened his data source modestly, his results showed a distinctly different outcome: similar efficacy for the two drugs. Dr. Betts also included a cost efficacy analysis, and in this part adalimumab showed superior performance after he factored in the cost per responding AS patient.
During the combined discussion period following the two talks, both presenters defended the legitimacy of their approaches, although Dr. Maksymowych conceded that these indirect comparisons are “hypothesis generating rather than producing a definitive answer.” But a couple of active European AS researchers rose to comment from the floor and discredit the whole process.
“These two presentations show why I am not a proponent of indirect comparisons. The statistical models squeeze the data until they confess,” said Robert Landewé, MD, an AS specialist at the University of Amsterdam. “This is now a commercial rather than a scientific clash between two important drugs. I challenge these companies to perform a head-to-head trial. Indirect comparisons are not good,” he concluded, to a round of audience applause.
“There are so many methodological issues,” said Désirée van der Heijde, MD, another Dutch AS clinician and researcher who rose to critique both studies. “The only thing you can rely on is head-to-head trials.”
I later spoke with Dr. Maksymowych, and he expressed some pessimism about the prospects for a fully-powered, head-to-head trial of an interleukin-17 inhibitor and tumor necrosis factor inhibitor because it would need to enroll so many patients. “Randomized studies of active comparators need to be huge because it’s hard to show improvements when the response rates are high,” he said. Plus, he added, it isn’t entirely about a drug’s efficacy against AS spinal symptoms anyway.
“We also have to think about the impact of treatment on other aspects of this disease, such as psoriasis and colitis, as well as radiographic disease progression,” he said. These aspects of the activity of both classes of drugs have not received much study in AS patients until now.
In other words, the battle between treatment options for AS has just begun, and seems likely to be fought on many fronts.
On Twitter @mitchelzoler
Now that the interleukin-17 inhibitor secukinumab and tumor necrosis factor inhibitors are competing options for treatment of patients with ankylosing spondylitis, the companies that make those drugs must feel pressure to find some sort of advantage for their agents.
How else to explain the remarkable pair of similar post hoc analyses presented in June at the European Congress of Rheumatology in London? One of the analyses was funded by Novartis – the company that markets secukinumab (Cosentyx) – and included several Novartis employees as coauthors. The second study, presented immediately afterward in the main session at the meeting devoted to ankylosing spondylitis (AS) treatments, had backing from AbbVie, which markets adalimumab (Humira), the largest-selling tumor necrosis factor inhibitor worldwide, and had several AbbVie employees as coauthors.
Both analyses used a “matching adjusted indirect comparison,” a fairly new way to compare the performance of interventions studied in two totally independent trials by propensity matching patients from each of the two trials. It’s purportedly a way to make a legitimate comparison in the absence of head-to-head data.
Making the two reports even more surreal was their use of essentially the same data.
The first report came from Walter P. Maksymowych, MD, an AS clinician and researcher from the University of Alberta, who with his coauthors used data collected on secukinumab in the MEASURE 1 pivotal trial and on adalimumab in the ATLAS pivotal trial. He spent much of his presentation describing the methods behind the indirect comparison, and I don’t think I can be blamed for calling the results of this Novartis-sponsored analysis predictable: overall better performance by secukinumab, compared “indirectly” with adalimumab for clinical responses and patient quality of life.
The second report, the one sponsored by AbbVie, came from Keith A. Betts, PhD, a biostatistician who works for the Analysis Group, an international consulting firm. He also used the ATLAS database as the source for adalimumab outcomes, and differed marginally from Dr. Maksymowych by taking data on secukinumab patients from both the MEASURE 1 and MEASURE 2 pivotal trials. Although Dr. Betts also used the matching adjusted indirect comparison approach and broadened his data source modestly, his results showed a distinctly different outcome: similar efficacy for the two drugs. Dr. Betts also included a cost efficacy analysis, and in this part adalimumab showed superior performance after he factored in the cost per responding AS patient.
During the combined discussion period following the two talks, both presenters defended the legitimacy of their approaches, although Dr. Maksymowych conceded that these indirect comparisons are “hypothesis generating rather than producing a definitive answer.” But a couple of active European AS researchers rose to comment from the floor and discredit the whole process.
“These two presentations show why I am not a proponent of indirect comparisons. The statistical models squeeze the data until they confess,” said Robert Landewé, MD, an AS specialist at the University of Amsterdam. “This is now a commercial rather than a scientific clash between two important drugs. I challenge these companies to perform a head-to-head trial. Indirect comparisons are not good,” he concluded, to a round of audience applause.
“There are so many methodological issues,” said Désirée van der Heijde, MD, another Dutch AS clinician and researcher who rose to critique both studies. “The only thing you can rely on is head-to-head trials.”
I later spoke with Dr. Maksymowych, and he expressed some pessimism about the prospects for a fully-powered, head-to-head trial of an interleukin-17 inhibitor and tumor necrosis factor inhibitor because it would need to enroll so many patients. “Randomized studies of active comparators need to be huge because it’s hard to show improvements when the response rates are high,” he said. Plus, he added, it isn’t entirely about a drug’s efficacy against AS spinal symptoms anyway.
“We also have to think about the impact of treatment on other aspects of this disease, such as psoriasis and colitis, as well as radiographic disease progression,” he said. These aspects of the activity of both classes of drugs have not received much study in AS patients until now.
In other words, the battle between treatment options for AS has just begun, and seems likely to be fought on many fronts.
On Twitter @mitchelzoler
Now that the interleukin-17 inhibitor secukinumab and tumor necrosis factor inhibitors are competing options for treatment of patients with ankylosing spondylitis, the companies that make those drugs must feel pressure to find some sort of advantage for their agents.
How else to explain the remarkable pair of similar post hoc analyses presented in June at the European Congress of Rheumatology in London? One of the analyses was funded by Novartis – the company that markets secukinumab (Cosentyx) – and included several Novartis employees as coauthors. The second study, presented immediately afterward in the main session at the meeting devoted to ankylosing spondylitis (AS) treatments, had backing from AbbVie, which markets adalimumab (Humira), the largest-selling tumor necrosis factor inhibitor worldwide, and had several AbbVie employees as coauthors.
Both analyses used a “matching adjusted indirect comparison,” a fairly new way to compare the performance of interventions studied in two totally independent trials by propensity matching patients from each of the two trials. It’s purportedly a way to make a legitimate comparison in the absence of head-to-head data.
Making the two reports even more surreal was their use of essentially the same data.
The first report came from Walter P. Maksymowych, MD, an AS clinician and researcher from the University of Alberta, who with his coauthors used data collected on secukinumab in the MEASURE 1 pivotal trial and on adalimumab in the ATLAS pivotal trial. He spent much of his presentation describing the methods behind the indirect comparison, and I don’t think I can be blamed for calling the results of this Novartis-sponsored analysis predictable: overall better performance by secukinumab, compared “indirectly” with adalimumab for clinical responses and patient quality of life.
The second report, the one sponsored by AbbVie, came from Keith A. Betts, PhD, a biostatistician who works for the Analysis Group, an international consulting firm. He also used the ATLAS database as the source for adalimumab outcomes, and differed marginally from Dr. Maksymowych by taking data on secukinumab patients from both the MEASURE 1 and MEASURE 2 pivotal trials. Although Dr. Betts also used the matching adjusted indirect comparison approach and broadened his data source modestly, his results showed a distinctly different outcome: similar efficacy for the two drugs. Dr. Betts also included a cost efficacy analysis, and in this part adalimumab showed superior performance after he factored in the cost per responding AS patient.
During the combined discussion period following the two talks, both presenters defended the legitimacy of their approaches, although Dr. Maksymowych conceded that these indirect comparisons are “hypothesis generating rather than producing a definitive answer.” But a couple of active European AS researchers rose to comment from the floor and discredit the whole process.
“These two presentations show why I am not a proponent of indirect comparisons. The statistical models squeeze the data until they confess,” said Robert Landewé, MD, an AS specialist at the University of Amsterdam. “This is now a commercial rather than a scientific clash between two important drugs. I challenge these companies to perform a head-to-head trial. Indirect comparisons are not good,” he concluded, to a round of audience applause.
“There are so many methodological issues,” said Désirée van der Heijde, MD, another Dutch AS clinician and researcher who rose to critique both studies. “The only thing you can rely on is head-to-head trials.”
I later spoke with Dr. Maksymowych, and he expressed some pessimism about the prospects for a fully-powered, head-to-head trial of an interleukin-17 inhibitor and tumor necrosis factor inhibitor because it would need to enroll so many patients. “Randomized studies of active comparators need to be huge because it’s hard to show improvements when the response rates are high,” he said. Plus, he added, it isn’t entirely about a drug’s efficacy against AS spinal symptoms anyway.
“We also have to think about the impact of treatment on other aspects of this disease, such as psoriasis and colitis, as well as radiographic disease progression,” he said. These aspects of the activity of both classes of drugs have not received much study in AS patients until now.
In other words, the battle between treatment options for AS has just begun, and seems likely to be fought on many fronts.
On Twitter @mitchelzoler
NSAID plus TNFi linked to less ankylosing spondylitis progression
LONDON – Patients with ankylosing spondylitis who remained on long-term treatment with a nonsteroidal anti-inflammatory drug and a tumor necrosis factor inhibitor had significantly less new spinal-bone formation in a cross-sectional analysis of a multicenter cohort of 527 U.S. patients.
A related analysis of the same cohort also showed significantly less ankylosing spondylitis (AS) progression as measured by radiographic progression among patients who received treatment with a tumor necrosis factor–alpha (TNF) inhibitor for 2.1-3.5 years regardless of their treatment with a nonsteroidal anti-inflammatory drug (NSAID), although this link trended to a stronger effect among the patients taking both, Lianne S. Gensler, MD, reported in a pair of posters at the European Congress of Rheumatology.
These finding suggest “there may be synergy between NSAIDs and TNF inhibitors [for slowing or preventing progression] in a select group of AS patients at high risk for progression,” said Dr. Gensler, a rheumatologist and director of the Ankylosing Spondylitis Clinic at the University of California, San Francisco.
But Dr. Gensler also cautioned that these findings are merely “hypothesis generating” and should not be used as a rationale to place or maintain AS patients on long-term treatment with an NSAID, a TNF inhibitor, or both drugs.
“You treat the disease burden. The message is absolutely not to always put AS patients on both types of drugs. When an AS patient is well controlled on a TNF inhibitor alone, I would not tell them to also take a NSAID,” she said in an interview. “This is only relevant for patients who require treatment with both drug classes because of their clinical status.”
As the list of treatment options for patients with AS grows – it now includes NSAIDs, TNF inhibitors, and the interleukin-17 inhibitor secukinumab (Cosentyx) – the impact of these agents on disease progression as assessed by radiography and new bone formation becomes a new dimension to start to consider in addition to the standard criterion of immediate clinical response, Dr. Gensler explained. AS progression “is another factor to think about as we decide on treatment strategies. There is growing evidence that long-term treatment with a tumor necrosis factor inhibitor and with a NSAID have potential roles in disease modification.” But the evidence is indirect, from cohort studies that make cause and effect assessments difficult because of possible unidentified confounding factors, she noted.
“There has never been a randomized, controlled trial examining the disease-modifying effects of a TNF inhibitor because you can’t keep patients on placebo for a long enough time to see this benefit,” Dr. Gensler said. It takes a long time to see progression in AS patients, she noted.
A prior cohort analysis run by Dr. Gensler and her associates found evidence for an effect of long-term treatment with a TNF inhibitor and reduced AS progression measured using the modified Stoke AS Spine Score (mSASSS), compared with AS patients not on a TNF inhibitor in a propensity-score matched analysis of 334 patients. A link between TNF inhibitor use and a discernible difference in mSASSS only occurred when patients were on TNF inhibitor treatment for at least 3.9 years (Arthritis Rheum. 2013 Oct;65[10]:2645-54). In addition, a separate report at the EULAR congress on 168 AS patients maintained on treatment with secukinumab for 2 years showed evidence for slowed progression of mSASSS scores, compared with historical controls as well as with similar patients who were not on secukinumab treatment for as long a period of time.
The new analysis reported by Dr. Gensler looked at 527 AS patients in the multicenter Prospective Study of Outcomes in AS cohort followed for a median of 3.7 years. Clinicians participating in this cohort saw patients every 6 months, and radiographic assessments by mSASSS and for new bone formation occurred every 2 years. At entry into the registry, about 57% of patients received a TNF inhibitor and about 63% received an NSAID, with a third on an NSAID only, 27% on a TNF inhibitor only, 30% on both drugs, and 10% receiving neither drug.
The analysis showed that among patients followed for 2.1-3.5 years, the fraction of patients on a TNF inhibitor who showed progression of their mSASSS was 77% lower than patients not on a TNF inhibitor, a statistically significant difference, Dr. Gensler reported. The researchers saw no statistically significant difference in mSASSS progression rates between the patients on a TNF inhibitor at baseline and those not on a TNF inhibitor at baseline among patients followed for 2 years and among those followed for more than 3.5 years, although the analysis did show nominally higher levels of response among TNF-inhibitor users followed for more than 3.5 years. Dr. Gensler speculated that one reason for the loss of a statistically significant difference during longer follow-up could be that fewer patients reached these levels of prolonged follow-up, making statistically significant differences harder to see. This analysis also showed a strong trend for less progression among the patients treated with an NSAID, a 51% relative reduction in mSASSS progression, compared with patients not taking an NSAID, but this relationship just missed statistical significance.
A second analysis by Dr. Gensler and her associates used data from the same cohort but focused on new bone formation during follow-up. This analysis again showed a statistically significant, 72% reduction in this outcome among patients taking a TNF inhibitor at baseline, compared with those not on a TNF inhibitor, when followed for 2.1-3.5 years, with no statistically significant relationship seen among patients followed for less or more time, Dr. Gensler reported. However, the results from this analysis also showed a statistically significant impact from NSAID treatment: Patients on a TNF inhibitor and an NSAID at baseline had 67% less new bone formation, compared with those who received a TNF inhibitor but were not on an NSAID at baseline.
Dr. Gensler has been a consultant to or investigator funded by AbbVie, Amgen, Janssen, Novartis, and UCB. The Prospective Study of Outcomes in Ankylosing Spondylitis receives no commercial support.
On Twitter @mitchelzoler
LONDON – Patients with ankylosing spondylitis who remained on long-term treatment with a nonsteroidal anti-inflammatory drug and a tumor necrosis factor inhibitor had significantly less new spinal-bone formation in a cross-sectional analysis of a multicenter cohort of 527 U.S. patients.
A related analysis of the same cohort also showed significantly less ankylosing spondylitis (AS) progression as measured by radiographic progression among patients who received treatment with a tumor necrosis factor–alpha (TNF) inhibitor for 2.1-3.5 years regardless of their treatment with a nonsteroidal anti-inflammatory drug (NSAID), although this link trended to a stronger effect among the patients taking both, Lianne S. Gensler, MD, reported in a pair of posters at the European Congress of Rheumatology.
These finding suggest “there may be synergy between NSAIDs and TNF inhibitors [for slowing or preventing progression] in a select group of AS patients at high risk for progression,” said Dr. Gensler, a rheumatologist and director of the Ankylosing Spondylitis Clinic at the University of California, San Francisco.
But Dr. Gensler also cautioned that these findings are merely “hypothesis generating” and should not be used as a rationale to place or maintain AS patients on long-term treatment with an NSAID, a TNF inhibitor, or both drugs.
“You treat the disease burden. The message is absolutely not to always put AS patients on both types of drugs. When an AS patient is well controlled on a TNF inhibitor alone, I would not tell them to also take a NSAID,” she said in an interview. “This is only relevant for patients who require treatment with both drug classes because of their clinical status.”
As the list of treatment options for patients with AS grows – it now includes NSAIDs, TNF inhibitors, and the interleukin-17 inhibitor secukinumab (Cosentyx) – the impact of these agents on disease progression as assessed by radiography and new bone formation becomes a new dimension to start to consider in addition to the standard criterion of immediate clinical response, Dr. Gensler explained. AS progression “is another factor to think about as we decide on treatment strategies. There is growing evidence that long-term treatment with a tumor necrosis factor inhibitor and with a NSAID have potential roles in disease modification.” But the evidence is indirect, from cohort studies that make cause and effect assessments difficult because of possible unidentified confounding factors, she noted.
“There has never been a randomized, controlled trial examining the disease-modifying effects of a TNF inhibitor because you can’t keep patients on placebo for a long enough time to see this benefit,” Dr. Gensler said. It takes a long time to see progression in AS patients, she noted.
A prior cohort analysis run by Dr. Gensler and her associates found evidence for an effect of long-term treatment with a TNF inhibitor and reduced AS progression measured using the modified Stoke AS Spine Score (mSASSS), compared with AS patients not on a TNF inhibitor in a propensity-score matched analysis of 334 patients. A link between TNF inhibitor use and a discernible difference in mSASSS only occurred when patients were on TNF inhibitor treatment for at least 3.9 years (Arthritis Rheum. 2013 Oct;65[10]:2645-54). In addition, a separate report at the EULAR congress on 168 AS patients maintained on treatment with secukinumab for 2 years showed evidence for slowed progression of mSASSS scores, compared with historical controls as well as with similar patients who were not on secukinumab treatment for as long a period of time.
The new analysis reported by Dr. Gensler looked at 527 AS patients in the multicenter Prospective Study of Outcomes in AS cohort followed for a median of 3.7 years. Clinicians participating in this cohort saw patients every 6 months, and radiographic assessments by mSASSS and for new bone formation occurred every 2 years. At entry into the registry, about 57% of patients received a TNF inhibitor and about 63% received an NSAID, with a third on an NSAID only, 27% on a TNF inhibitor only, 30% on both drugs, and 10% receiving neither drug.
The analysis showed that among patients followed for 2.1-3.5 years, the fraction of patients on a TNF inhibitor who showed progression of their mSASSS was 77% lower than patients not on a TNF inhibitor, a statistically significant difference, Dr. Gensler reported. The researchers saw no statistically significant difference in mSASSS progression rates between the patients on a TNF inhibitor at baseline and those not on a TNF inhibitor at baseline among patients followed for 2 years and among those followed for more than 3.5 years, although the analysis did show nominally higher levels of response among TNF-inhibitor users followed for more than 3.5 years. Dr. Gensler speculated that one reason for the loss of a statistically significant difference during longer follow-up could be that fewer patients reached these levels of prolonged follow-up, making statistically significant differences harder to see. This analysis also showed a strong trend for less progression among the patients treated with an NSAID, a 51% relative reduction in mSASSS progression, compared with patients not taking an NSAID, but this relationship just missed statistical significance.
A second analysis by Dr. Gensler and her associates used data from the same cohort but focused on new bone formation during follow-up. This analysis again showed a statistically significant, 72% reduction in this outcome among patients taking a TNF inhibitor at baseline, compared with those not on a TNF inhibitor, when followed for 2.1-3.5 years, with no statistically significant relationship seen among patients followed for less or more time, Dr. Gensler reported. However, the results from this analysis also showed a statistically significant impact from NSAID treatment: Patients on a TNF inhibitor and an NSAID at baseline had 67% less new bone formation, compared with those who received a TNF inhibitor but were not on an NSAID at baseline.
Dr. Gensler has been a consultant to or investigator funded by AbbVie, Amgen, Janssen, Novartis, and UCB. The Prospective Study of Outcomes in Ankylosing Spondylitis receives no commercial support.
On Twitter @mitchelzoler
LONDON – Patients with ankylosing spondylitis who remained on long-term treatment with a nonsteroidal anti-inflammatory drug and a tumor necrosis factor inhibitor had significantly less new spinal-bone formation in a cross-sectional analysis of a multicenter cohort of 527 U.S. patients.
A related analysis of the same cohort also showed significantly less ankylosing spondylitis (AS) progression as measured by radiographic progression among patients who received treatment with a tumor necrosis factor–alpha (TNF) inhibitor for 2.1-3.5 years regardless of their treatment with a nonsteroidal anti-inflammatory drug (NSAID), although this link trended to a stronger effect among the patients taking both, Lianne S. Gensler, MD, reported in a pair of posters at the European Congress of Rheumatology.
These finding suggest “there may be synergy between NSAIDs and TNF inhibitors [for slowing or preventing progression] in a select group of AS patients at high risk for progression,” said Dr. Gensler, a rheumatologist and director of the Ankylosing Spondylitis Clinic at the University of California, San Francisco.
But Dr. Gensler also cautioned that these findings are merely “hypothesis generating” and should not be used as a rationale to place or maintain AS patients on long-term treatment with an NSAID, a TNF inhibitor, or both drugs.
“You treat the disease burden. The message is absolutely not to always put AS patients on both types of drugs. When an AS patient is well controlled on a TNF inhibitor alone, I would not tell them to also take a NSAID,” she said in an interview. “This is only relevant for patients who require treatment with both drug classes because of their clinical status.”
As the list of treatment options for patients with AS grows – it now includes NSAIDs, TNF inhibitors, and the interleukin-17 inhibitor secukinumab (Cosentyx) – the impact of these agents on disease progression as assessed by radiography and new bone formation becomes a new dimension to start to consider in addition to the standard criterion of immediate clinical response, Dr. Gensler explained. AS progression “is another factor to think about as we decide on treatment strategies. There is growing evidence that long-term treatment with a tumor necrosis factor inhibitor and with a NSAID have potential roles in disease modification.” But the evidence is indirect, from cohort studies that make cause and effect assessments difficult because of possible unidentified confounding factors, she noted.
“There has never been a randomized, controlled trial examining the disease-modifying effects of a TNF inhibitor because you can’t keep patients on placebo for a long enough time to see this benefit,” Dr. Gensler said. It takes a long time to see progression in AS patients, she noted.
A prior cohort analysis run by Dr. Gensler and her associates found evidence for an effect of long-term treatment with a TNF inhibitor and reduced AS progression measured using the modified Stoke AS Spine Score (mSASSS), compared with AS patients not on a TNF inhibitor in a propensity-score matched analysis of 334 patients. A link between TNF inhibitor use and a discernible difference in mSASSS only occurred when patients were on TNF inhibitor treatment for at least 3.9 years (Arthritis Rheum. 2013 Oct;65[10]:2645-54). In addition, a separate report at the EULAR congress on 168 AS patients maintained on treatment with secukinumab for 2 years showed evidence for slowed progression of mSASSS scores, compared with historical controls as well as with similar patients who were not on secukinumab treatment for as long a period of time.
The new analysis reported by Dr. Gensler looked at 527 AS patients in the multicenter Prospective Study of Outcomes in AS cohort followed for a median of 3.7 years. Clinicians participating in this cohort saw patients every 6 months, and radiographic assessments by mSASSS and for new bone formation occurred every 2 years. At entry into the registry, about 57% of patients received a TNF inhibitor and about 63% received an NSAID, with a third on an NSAID only, 27% on a TNF inhibitor only, 30% on both drugs, and 10% receiving neither drug.
The analysis showed that among patients followed for 2.1-3.5 years, the fraction of patients on a TNF inhibitor who showed progression of their mSASSS was 77% lower than patients not on a TNF inhibitor, a statistically significant difference, Dr. Gensler reported. The researchers saw no statistically significant difference in mSASSS progression rates between the patients on a TNF inhibitor at baseline and those not on a TNF inhibitor at baseline among patients followed for 2 years and among those followed for more than 3.5 years, although the analysis did show nominally higher levels of response among TNF-inhibitor users followed for more than 3.5 years. Dr. Gensler speculated that one reason for the loss of a statistically significant difference during longer follow-up could be that fewer patients reached these levels of prolonged follow-up, making statistically significant differences harder to see. This analysis also showed a strong trend for less progression among the patients treated with an NSAID, a 51% relative reduction in mSASSS progression, compared with patients not taking an NSAID, but this relationship just missed statistical significance.
A second analysis by Dr. Gensler and her associates used data from the same cohort but focused on new bone formation during follow-up. This analysis again showed a statistically significant, 72% reduction in this outcome among patients taking a TNF inhibitor at baseline, compared with those not on a TNF inhibitor, when followed for 2.1-3.5 years, with no statistically significant relationship seen among patients followed for less or more time, Dr. Gensler reported. However, the results from this analysis also showed a statistically significant impact from NSAID treatment: Patients on a TNF inhibitor and an NSAID at baseline had 67% less new bone formation, compared with those who received a TNF inhibitor but were not on an NSAID at baseline.
Dr. Gensler has been a consultant to or investigator funded by AbbVie, Amgen, Janssen, Novartis, and UCB. The Prospective Study of Outcomes in Ankylosing Spondylitis receives no commercial support.
On Twitter @mitchelzoler
AT THE EULAR 2016 CONGRESS
Key clinical point: Ankylosing spondylitis patients treated with a TNF inhibitor plus an NSAID had the lowest level of new bone formation during treatment for more than 2 years.
Major finding: Patients on a TNF inhibitor and an NSAID had 67% less new bone formation, compared with patients not on an NSAID.
Data source: Cross-sectional cohort study of 527 patients with ankylosing spondylitis enrolled in the Prospective Study of Outcomes in Ankylosing Spondylitis.
Disclosures: Dr. Gensler has been a consultant to or investigator funded by AbbVie, Amgen, Janssen, Novartis, and UCB. The Prospective Study of Outcomes in Ankylosing Spondylitis receives no commercial support.
NSAID plus TNFi linked to less ankylosing spondylitis progression
LONDON – Patients with ankylosing spondylitis who remained on long-term treatment with a nonsteroidal anti-inflammatory drug and a tumor necrosis factor inhibitor had significantly less new spinal-bone formation in a cross-sectional analysis of a multicenter cohort of 527 U.S. patients.
A related analysis of the same cohort also showed significantly less ankylosing spondylitis (AS) progression as measured by radiographic progression among patients who received treatment with a tumor necrosis factor–alpha (TNF) inhibitor for 2.1-3.5 years regardless of their treatment with a nonsteroidal anti-inflammatory drug (NSAID), although this link trended to a stronger effect among the patients taking both, Lianne S. Gensler, MD, reported in a pair of posters at the European Congress of Rheumatology.
These finding suggest “there may be synergy between NSAIDs and TNF inhibitors [for slowing or preventing progression] in a select group of AS patients at high risk for progression,” said Dr. Gensler, a rheumatologist and director of the Ankylosing Spondylitis Clinic at the University of California, San Francisco.
But Dr. Gensler also cautioned that these findings are merely “hypothesis generating” and should not be used as a rationale to place or maintain AS patients on long-term treatment with an NSAID, a TNF inhibitor, or both drugs.
“You treat the disease burden. The message is absolutely not to always put AS patients on both types of drugs. When an AS patient is well controlled on a TNF inhibitor alone, I would not tell them to also take a NSAID,” she said in an interview. “This is only relevant for patients who require treatment with both drug classes because of their clinical status.”
As the list of treatment options for patients with AS grows – it now includes NSAIDs, TNF inhibitors, and the interleukin-17 inhibitor secukinumab (Cosentyx) – the impact of these agents on disease progression as assessed by radiography and new bone formation becomes a new dimension to start to consider in addition to the standard criterion of immediate clinical response, Dr. Gensler explained. AS progression “is another factor to think about as we decide on treatment strategies. There is growing evidence that long-term treatment with a tumor necrosis factor inhibitor and with a NSAID have potential roles in disease modification.” But the evidence is indirect, from cohort studies that make cause and effect assessments difficult because of possible unidentified confounding factors, she noted.
“There has never been a randomized, controlled trial examining the disease-modifying effects of a TNF inhibitor because you can’t keep patients on placebo for a long enough time to see this benefit,” Dr. Gensler said. It takes a long time to see progression in AS patients, she noted.
A prior cohort analysis run by Dr. Gensler and her associates found evidence for an effect of long-term treatment with a TNF inhibitor and reduced AS progression measured using the modified Stoke AS Spine Score (mSASSS), compared with AS patients not on a TNF inhibitor in a propensity-score matched analysis of 334 patients. A link between TNF inhibitor use and a discernible difference in mSASSS only occurred when patients were on TNF inhibitor treatment for at least 3.9 years (Arthritis Rheum. 2013 Oct;65[10]:2645-54). In addition, a separate report at the EULAR congress on 168 AS patients maintained on treatment with secukinumab for 2 years showed evidence for slowed progression of mSASSS scores, compared with historical controls as well as with similar patients who were not on secukinumab treatment for as long a period of time.
The new analysis reported by Dr. Gensler looked at 527 AS patients in the multicenter Prospective Study of Outcomes in AS cohort followed for a median of 3.7 years. Clinicians participating in this cohort saw patients every 6 months, and radiographic assessments by mSASSS and for new bone formation occurred every 2 years. At entry into the registry, about 57% of patients received a TNF inhibitor and about 63% received an NSAID, with a third on an NSAID only, 27% on a TNF inhibitor only, 30% on both drugs, and 10% receiving neither drug.
The analysis showed that among patients followed for 2.1-3.5 years, the fraction of patients on a TNF inhibitor who showed progression of their mSASSS was 77% lower than patients not on a TNF inhibitor, a statistically significant difference, Dr. Gensler reported. The researchers saw no statistically significant difference in mSASSS progression rates between the patients on a TNF inhibitor at baseline and those not on a TNF inhibitor at baseline among patients followed for 2 years and among those followed for more than 3.5 years, although the analysis did show nominally higher levels of response among TNF-inhibitor users followed for more than 3.5 years. Dr. Gensler speculated that one reason for the loss of a statistically significant difference during longer follow-up could be that fewer patients reached these levels of prolonged follow-up, making statistically significant differences harder to see. This analysis also showed a strong trend for less progression among the patients treated with an NSAID, a 51% relative reduction in mSASSS progression, compared with patients not taking an NSAID, but this relationship just missed statistical significance.
A second analysis by Dr. Gensler and her associates used data from the same cohort but focused on new bone formation during follow-up. This analysis again showed a statistically significant, 72% reduction in this outcome among patients taking a TNF inhibitor at baseline, compared with those not on a TNF inhibitor, when followed for 2.1-3.5 years, with no statistically significant relationship seen among patients followed for less or more time, Dr. Gensler reported. However, the results from this analysis also showed a statistically significant impact from NSAID treatment: Patients on a TNF inhibitor and an NSAID at baseline had 67% less new bone formation, compared with those who received a TNF inhibitor but were not on an NSAID at baseline.
Dr. Gensler has been a consultant to or investigator funded by AbbVie, Amgen, Janssen, Novartis, and UCB. The Prospective Study of Outcomes in Ankylosing Spondylitis receives no commercial support.
On Twitter @mitchelzoler
LONDON – Patients with ankylosing spondylitis who remained on long-term treatment with a nonsteroidal anti-inflammatory drug and a tumor necrosis factor inhibitor had significantly less new spinal-bone formation in a cross-sectional analysis of a multicenter cohort of 527 U.S. patients.
A related analysis of the same cohort also showed significantly less ankylosing spondylitis (AS) progression as measured by radiographic progression among patients who received treatment with a tumor necrosis factor–alpha (TNF) inhibitor for 2.1-3.5 years regardless of their treatment with a nonsteroidal anti-inflammatory drug (NSAID), although this link trended to a stronger effect among the patients taking both, Lianne S. Gensler, MD, reported in a pair of posters at the European Congress of Rheumatology.
These finding suggest “there may be synergy between NSAIDs and TNF inhibitors [for slowing or preventing progression] in a select group of AS patients at high risk for progression,” said Dr. Gensler, a rheumatologist and director of the Ankylosing Spondylitis Clinic at the University of California, San Francisco.
But Dr. Gensler also cautioned that these findings are merely “hypothesis generating” and should not be used as a rationale to place or maintain AS patients on long-term treatment with an NSAID, a TNF inhibitor, or both drugs.
“You treat the disease burden. The message is absolutely not to always put AS patients on both types of drugs. When an AS patient is well controlled on a TNF inhibitor alone, I would not tell them to also take a NSAID,” she said in an interview. “This is only relevant for patients who require treatment with both drug classes because of their clinical status.”
As the list of treatment options for patients with AS grows – it now includes NSAIDs, TNF inhibitors, and the interleukin-17 inhibitor secukinumab (Cosentyx) – the impact of these agents on disease progression as assessed by radiography and new bone formation becomes a new dimension to start to consider in addition to the standard criterion of immediate clinical response, Dr. Gensler explained. AS progression “is another factor to think about as we decide on treatment strategies. There is growing evidence that long-term treatment with a tumor necrosis factor inhibitor and with a NSAID have potential roles in disease modification.” But the evidence is indirect, from cohort studies that make cause and effect assessments difficult because of possible unidentified confounding factors, she noted.
“There has never been a randomized, controlled trial examining the disease-modifying effects of a TNF inhibitor because you can’t keep patients on placebo for a long enough time to see this benefit,” Dr. Gensler said. It takes a long time to see progression in AS patients, she noted.
A prior cohort analysis run by Dr. Gensler and her associates found evidence for an effect of long-term treatment with a TNF inhibitor and reduced AS progression measured using the modified Stoke AS Spine Score (mSASSS), compared with AS patients not on a TNF inhibitor in a propensity-score matched analysis of 334 patients. A link between TNF inhibitor use and a discernible difference in mSASSS only occurred when patients were on TNF inhibitor treatment for at least 3.9 years (Arthritis Rheum. 2013 Oct;65[10]:2645-54). In addition, a separate report at the EULAR congress on 168 AS patients maintained on treatment with secukinumab for 2 years showed evidence for slowed progression of mSASSS scores, compared with historical controls as well as with similar patients who were not on secukinumab treatment for as long a period of time.
The new analysis reported by Dr. Gensler looked at 527 AS patients in the multicenter Prospective Study of Outcomes in AS cohort followed for a median of 3.7 years. Clinicians participating in this cohort saw patients every 6 months, and radiographic assessments by mSASSS and for new bone formation occurred every 2 years. At entry into the registry, about 57% of patients received a TNF inhibitor and about 63% received an NSAID, with a third on an NSAID only, 27% on a TNF inhibitor only, 30% on both drugs, and 10% receiving neither drug.
The analysis showed that among patients followed for 2.1-3.5 years, the fraction of patients on a TNF inhibitor who showed progression of their mSASSS was 77% lower than patients not on a TNF inhibitor, a statistically significant difference, Dr. Gensler reported. The researchers saw no statistically significant difference in mSASSS progression rates between the patients on a TNF inhibitor at baseline and those not on a TNF inhibitor at baseline among patients followed for 2 years and among those followed for more than 3.5 years, although the analysis did show nominally higher levels of response among TNF-inhibitor users followed for more than 3.5 years. Dr. Gensler speculated that one reason for the loss of a statistically significant difference during longer follow-up could be that fewer patients reached these levels of prolonged follow-up, making statistically significant differences harder to see. This analysis also showed a strong trend for less progression among the patients treated with an NSAID, a 51% relative reduction in mSASSS progression, compared with patients not taking an NSAID, but this relationship just missed statistical significance.
A second analysis by Dr. Gensler and her associates used data from the same cohort but focused on new bone formation during follow-up. This analysis again showed a statistically significant, 72% reduction in this outcome among patients taking a TNF inhibitor at baseline, compared with those not on a TNF inhibitor, when followed for 2.1-3.5 years, with no statistically significant relationship seen among patients followed for less or more time, Dr. Gensler reported. However, the results from this analysis also showed a statistically significant impact from NSAID treatment: Patients on a TNF inhibitor and an NSAID at baseline had 67% less new bone formation, compared with those who received a TNF inhibitor but were not on an NSAID at baseline.
Dr. Gensler has been a consultant to or investigator funded by AbbVie, Amgen, Janssen, Novartis, and UCB. The Prospective Study of Outcomes in Ankylosing Spondylitis receives no commercial support.
On Twitter @mitchelzoler
LONDON – Patients with ankylosing spondylitis who remained on long-term treatment with a nonsteroidal anti-inflammatory drug and a tumor necrosis factor inhibitor had significantly less new spinal-bone formation in a cross-sectional analysis of a multicenter cohort of 527 U.S. patients.
A related analysis of the same cohort also showed significantly less ankylosing spondylitis (AS) progression as measured by radiographic progression among patients who received treatment with a tumor necrosis factor–alpha (TNF) inhibitor for 2.1-3.5 years regardless of their treatment with a nonsteroidal anti-inflammatory drug (NSAID), although this link trended to a stronger effect among the patients taking both, Lianne S. Gensler, MD, reported in a pair of posters at the European Congress of Rheumatology.
These finding suggest “there may be synergy between NSAIDs and TNF inhibitors [for slowing or preventing progression] in a select group of AS patients at high risk for progression,” said Dr. Gensler, a rheumatologist and director of the Ankylosing Spondylitis Clinic at the University of California, San Francisco.
But Dr. Gensler also cautioned that these findings are merely “hypothesis generating” and should not be used as a rationale to place or maintain AS patients on long-term treatment with an NSAID, a TNF inhibitor, or both drugs.
“You treat the disease burden. The message is absolutely not to always put AS patients on both types of drugs. When an AS patient is well controlled on a TNF inhibitor alone, I would not tell them to also take a NSAID,” she said in an interview. “This is only relevant for patients who require treatment with both drug classes because of their clinical status.”
As the list of treatment options for patients with AS grows – it now includes NSAIDs, TNF inhibitors, and the interleukin-17 inhibitor secukinumab (Cosentyx) – the impact of these agents on disease progression as assessed by radiography and new bone formation becomes a new dimension to start to consider in addition to the standard criterion of immediate clinical response, Dr. Gensler explained. AS progression “is another factor to think about as we decide on treatment strategies. There is growing evidence that long-term treatment with a tumor necrosis factor inhibitor and with a NSAID have potential roles in disease modification.” But the evidence is indirect, from cohort studies that make cause and effect assessments difficult because of possible unidentified confounding factors, she noted.
“There has never been a randomized, controlled trial examining the disease-modifying effects of a TNF inhibitor because you can’t keep patients on placebo for a long enough time to see this benefit,” Dr. Gensler said. It takes a long time to see progression in AS patients, she noted.
A prior cohort analysis run by Dr. Gensler and her associates found evidence for an effect of long-term treatment with a TNF inhibitor and reduced AS progression measured using the modified Stoke AS Spine Score (mSASSS), compared with AS patients not on a TNF inhibitor in a propensity-score matched analysis of 334 patients. A link between TNF inhibitor use and a discernible difference in mSASSS only occurred when patients were on TNF inhibitor treatment for at least 3.9 years (Arthritis Rheum. 2013 Oct;65[10]:2645-54). In addition, a separate report at the EULAR congress on 168 AS patients maintained on treatment with secukinumab for 2 years showed evidence for slowed progression of mSASSS scores, compared with historical controls as well as with similar patients who were not on secukinumab treatment for as long a period of time.
The new analysis reported by Dr. Gensler looked at 527 AS patients in the multicenter Prospective Study of Outcomes in AS cohort followed for a median of 3.7 years. Clinicians participating in this cohort saw patients every 6 months, and radiographic assessments by mSASSS and for new bone formation occurred every 2 years. At entry into the registry, about 57% of patients received a TNF inhibitor and about 63% received an NSAID, with a third on an NSAID only, 27% on a TNF inhibitor only, 30% on both drugs, and 10% receiving neither drug.
The analysis showed that among patients followed for 2.1-3.5 years, the fraction of patients on a TNF inhibitor who showed progression of their mSASSS was 77% lower than patients not on a TNF inhibitor, a statistically significant difference, Dr. Gensler reported. The researchers saw no statistically significant difference in mSASSS progression rates between the patients on a TNF inhibitor at baseline and those not on a TNF inhibitor at baseline among patients followed for 2 years and among those followed for more than 3.5 years, although the analysis did show nominally higher levels of response among TNF-inhibitor users followed for more than 3.5 years. Dr. Gensler speculated that one reason for the loss of a statistically significant difference during longer follow-up could be that fewer patients reached these levels of prolonged follow-up, making statistically significant differences harder to see. This analysis also showed a strong trend for less progression among the patients treated with an NSAID, a 51% relative reduction in mSASSS progression, compared with patients not taking an NSAID, but this relationship just missed statistical significance.
A second analysis by Dr. Gensler and her associates used data from the same cohort but focused on new bone formation during follow-up. This analysis again showed a statistically significant, 72% reduction in this outcome among patients taking a TNF inhibitor at baseline, compared with those not on a TNF inhibitor, when followed for 2.1-3.5 years, with no statistically significant relationship seen among patients followed for less or more time, Dr. Gensler reported. However, the results from this analysis also showed a statistically significant impact from NSAID treatment: Patients on a TNF inhibitor and an NSAID at baseline had 67% less new bone formation, compared with those who received a TNF inhibitor but were not on an NSAID at baseline.
Dr. Gensler has been a consultant to or investigator funded by AbbVie, Amgen, Janssen, Novartis, and UCB. The Prospective Study of Outcomes in Ankylosing Spondylitis receives no commercial support.
On Twitter @mitchelzoler
AT THE EULAR 2016 CONGRESS
Key clinical point: Ankylosing spondylitis patients treated with a TNF inhibitor plus an NSAID had the lowest level of new bone formation during treatment for more than 2 years.
Major finding: Patients on a TNF inhibitor and an NSAID had 67% less new bone formation, compared with patients not on an NSAID.
Data source: Cross-sectional cohort study of 527 patients with ankylosing spondylitis enrolled in the Prospective Study of Outcomes in Ankylosing Spondylitis.
Disclosures: Dr. Gensler has been a consultant to or investigator funded by AbbVie, Amgen, Janssen, Novartis, and UCB. The Prospective Study of Outcomes in Ankylosing Spondylitis receives no commercial support.
VIDEO: TNF inhibitors improved refractory skin disease in juvenile dermatomyositis
LONDON – Tumor necrosis factor–inhibitor treatment improved refractory skin disease in juvenile dermatomyositis patients in the largest observational study of its kind from the United Kingdom and Ireland Juvenile Dermatomyositis Research Group.
Muscle disease in the juvenile dermatomyositis (JDM) patients largely had already improved with conventional therapies prior to treatment with anti–tumor necrosis factor (TNF)-alpha agents, but it did improve further with anti-TNFs.
The effect of TNF inhibitors was most notable for those with skin calcinosis, lead author Dr. Raquel Campanilho-Marques reported at the European Congress of Rheumatology on behalf of her colleagues in the Juvenile Dermatomyositis Research Group.
Some evidence suggests that TNF-alpha might be involved in the pathogenesis of idiopathic inflammatory myopathies, particularly in more prolonged courses of JDM.
But there is limited prior evidence for the efficacy of TNF inhibitors in JDM patients, where small observational studies and case series have shown improved core-set measures of disease activity in patients treated with anti-TNF agents, noted Dr. Campanilho-Marques, a pediatric rheumatologist in the infection, inflammation and rheumatology section at the University College London Institute of Child Health and the Great Ormond Street Hospital for Children NHS Trust in London.
The 67 patients in the study involved those who were enrolled in the JDM Cohort and Biomarker Study, met Bohan and Peter criteria for JDM, and were on anti-TNF therapy at the time of analysis because of nonresponse to conventional therapy, active skin disease, calcinosis, or muscle weakness. They had at least 3 months of anti-TNF therapy and received either infliximab 6 mg/kg every 4 weeks (after a standard initial induction regimen) or adalimumab (Humira) 24 mg/m2 every other week.
A majority of the patients in the study were female (n = 41) and white (n = 54), with a mean age at disease onset of about 5 years. At the time of first use of anti-TNF agents, the patients had a mean age of about 10 years and a mean disease duration of 3.2 years. Treatment with TNF inhibitors lasted for a mean of about 2.5 years.
Of the 67 patients, data were not analyzed for 4 patients; there was insufficient information for 1 patient, while 3 patients had allergic reactions to their anti-TNF therapy on the first or second infusion. The remaining 63 patients included 43 who received infliximab, 4 on adalimumab, and 16 who used both.
Prior to anti-TNF treatment, 52 of 53 patients (98%) were taking methotrexate, azathioprine, hydroxychloroquine, or a combination of those. That declined to 45 of 56 (80%) at the start of anti-TNF therapy and then increased to 44 of 49 (89%) after 12 months of using an anti-TNF agent.
The use of cyclophosphamide declined markedly, from 26 of 65 patients (40%) to 3 of 65 (5%) at the start of TNF inhibition, and then to none after 12 months of anti-TNF therapy. Immunoglobulin therapy also declined, from use in 10%-12% of patients before and at the start of anti-TNF treatment to just 1 of 41 patients (2%) after 12 months of TNF inhibitor therapy.
The median modified Disease Activity Score for skin involvement significantly improved over the course of 12 months of treatment with infliximab, decreasing from 4 to 1. That was also the case for Physician Global Assessment score, as well as muscle outcome measurements on the Childhood Myositis Assessment Scale (CMAS) and the 8-item Manual Muscle Testing (MMT8).
For the 31 patients in the study who had calcinosis, lesions improved (reduced in number and/or size) in 17 patients, including 8 with complete resolution of their lesions. In the other 14 patients, lesions remained stable in 3 (fewer than three lesions) and were widespread or did not improve in 4; the other 7 patients had insufficient data to determine outcomes.
Most patients with muscle involvement already had improved with steroids prior to using anti-TNF drugs. Thus, the improvement in CMAS and MMT8 scores on anti-TNF treatment was not very large, going from about 45 to 53 and from about 74 to 79, respectively.
The investigators did not examine treatment response in relation to muscle-specific antibodies, but Dr. Campanilho-Marques said that it is something they would like to do in the future.
The main indication for anti-TNF agents was active skin disease that had not responded to conventional treatment, noted Dr. Campanilho-Marques, who is also with the departments of rheumatology at the Santa Maria Hospital and the Instituto Português de Reumatologia, both in Lisbon.
For 16 patients who switched from infliximab to adalimumab, the changes in outcome measures were not statistically significant. The switches occurred at a median of 2.35 months after starting infliximab; 10 patients switched because of inefficacy, 4 because of adverse events, and 2 because of patient preference.
After 12 months of anti-TNF therapy, the median prednisolone dose declined from 6 mg to 2.5 mg, but the decline appeared to be driven by five patients who sharply decreased their dose. Seven patients successfully stopped anti-TNF therapy after improvement occurred, Dr. Campanilho-Marques said.
Serious adverse events occurred 12 times during the year-long study period, including nine allergic reactions and three hospitalizations because of infection. Another 19 mild-to-moderate adverse events took place, which involved 15 infections and three local site reactions and skin rash, which led five patients to discontinue the biologic.
Overall, adverse events occurred at a rate of 13.3/100 patient-years, including 5.2 serious events/100 patient-years. One patient died because of a small bowel perforation that was probably secondary to disease-related damage. There were no malignancies or tuberculosis cases.
In a video interview at the meeting, Dr. Campanilho-Marques discussed the study findings and their implications.
The researchers had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LONDON – Tumor necrosis factor–inhibitor treatment improved refractory skin disease in juvenile dermatomyositis patients in the largest observational study of its kind from the United Kingdom and Ireland Juvenile Dermatomyositis Research Group.
Muscle disease in the juvenile dermatomyositis (JDM) patients largely had already improved with conventional therapies prior to treatment with anti–tumor necrosis factor (TNF)-alpha agents, but it did improve further with anti-TNFs.
The effect of TNF inhibitors was most notable for those with skin calcinosis, lead author Dr. Raquel Campanilho-Marques reported at the European Congress of Rheumatology on behalf of her colleagues in the Juvenile Dermatomyositis Research Group.
Some evidence suggests that TNF-alpha might be involved in the pathogenesis of idiopathic inflammatory myopathies, particularly in more prolonged courses of JDM.
But there is limited prior evidence for the efficacy of TNF inhibitors in JDM patients, where small observational studies and case series have shown improved core-set measures of disease activity in patients treated with anti-TNF agents, noted Dr. Campanilho-Marques, a pediatric rheumatologist in the infection, inflammation and rheumatology section at the University College London Institute of Child Health and the Great Ormond Street Hospital for Children NHS Trust in London.
The 67 patients in the study involved those who were enrolled in the JDM Cohort and Biomarker Study, met Bohan and Peter criteria for JDM, and were on anti-TNF therapy at the time of analysis because of nonresponse to conventional therapy, active skin disease, calcinosis, or muscle weakness. They had at least 3 months of anti-TNF therapy and received either infliximab 6 mg/kg every 4 weeks (after a standard initial induction regimen) or adalimumab (Humira) 24 mg/m2 every other week.
A majority of the patients in the study were female (n = 41) and white (n = 54), with a mean age at disease onset of about 5 years. At the time of first use of anti-TNF agents, the patients had a mean age of about 10 years and a mean disease duration of 3.2 years. Treatment with TNF inhibitors lasted for a mean of about 2.5 years.
Of the 67 patients, data were not analyzed for 4 patients; there was insufficient information for 1 patient, while 3 patients had allergic reactions to their anti-TNF therapy on the first or second infusion. The remaining 63 patients included 43 who received infliximab, 4 on adalimumab, and 16 who used both.
Prior to anti-TNF treatment, 52 of 53 patients (98%) were taking methotrexate, azathioprine, hydroxychloroquine, or a combination of those. That declined to 45 of 56 (80%) at the start of anti-TNF therapy and then increased to 44 of 49 (89%) after 12 months of using an anti-TNF agent.
The use of cyclophosphamide declined markedly, from 26 of 65 patients (40%) to 3 of 65 (5%) at the start of TNF inhibition, and then to none after 12 months of anti-TNF therapy. Immunoglobulin therapy also declined, from use in 10%-12% of patients before and at the start of anti-TNF treatment to just 1 of 41 patients (2%) after 12 months of TNF inhibitor therapy.
The median modified Disease Activity Score for skin involvement significantly improved over the course of 12 months of treatment with infliximab, decreasing from 4 to 1. That was also the case for Physician Global Assessment score, as well as muscle outcome measurements on the Childhood Myositis Assessment Scale (CMAS) and the 8-item Manual Muscle Testing (MMT8).
For the 31 patients in the study who had calcinosis, lesions improved (reduced in number and/or size) in 17 patients, including 8 with complete resolution of their lesions. In the other 14 patients, lesions remained stable in 3 (fewer than three lesions) and were widespread or did not improve in 4; the other 7 patients had insufficient data to determine outcomes.
Most patients with muscle involvement already had improved with steroids prior to using anti-TNF drugs. Thus, the improvement in CMAS and MMT8 scores on anti-TNF treatment was not very large, going from about 45 to 53 and from about 74 to 79, respectively.
The investigators did not examine treatment response in relation to muscle-specific antibodies, but Dr. Campanilho-Marques said that it is something they would like to do in the future.
The main indication for anti-TNF agents was active skin disease that had not responded to conventional treatment, noted Dr. Campanilho-Marques, who is also with the departments of rheumatology at the Santa Maria Hospital and the Instituto Português de Reumatologia, both in Lisbon.
For 16 patients who switched from infliximab to adalimumab, the changes in outcome measures were not statistically significant. The switches occurred at a median of 2.35 months after starting infliximab; 10 patients switched because of inefficacy, 4 because of adverse events, and 2 because of patient preference.
After 12 months of anti-TNF therapy, the median prednisolone dose declined from 6 mg to 2.5 mg, but the decline appeared to be driven by five patients who sharply decreased their dose. Seven patients successfully stopped anti-TNF therapy after improvement occurred, Dr. Campanilho-Marques said.
Serious adverse events occurred 12 times during the year-long study period, including nine allergic reactions and three hospitalizations because of infection. Another 19 mild-to-moderate adverse events took place, which involved 15 infections and three local site reactions and skin rash, which led five patients to discontinue the biologic.
Overall, adverse events occurred at a rate of 13.3/100 patient-years, including 5.2 serious events/100 patient-years. One patient died because of a small bowel perforation that was probably secondary to disease-related damage. There were no malignancies or tuberculosis cases.
In a video interview at the meeting, Dr. Campanilho-Marques discussed the study findings and their implications.
The researchers had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LONDON – Tumor necrosis factor–inhibitor treatment improved refractory skin disease in juvenile dermatomyositis patients in the largest observational study of its kind from the United Kingdom and Ireland Juvenile Dermatomyositis Research Group.
Muscle disease in the juvenile dermatomyositis (JDM) patients largely had already improved with conventional therapies prior to treatment with anti–tumor necrosis factor (TNF)-alpha agents, but it did improve further with anti-TNFs.
The effect of TNF inhibitors was most notable for those with skin calcinosis, lead author Dr. Raquel Campanilho-Marques reported at the European Congress of Rheumatology on behalf of her colleagues in the Juvenile Dermatomyositis Research Group.
Some evidence suggests that TNF-alpha might be involved in the pathogenesis of idiopathic inflammatory myopathies, particularly in more prolonged courses of JDM.
But there is limited prior evidence for the efficacy of TNF inhibitors in JDM patients, where small observational studies and case series have shown improved core-set measures of disease activity in patients treated with anti-TNF agents, noted Dr. Campanilho-Marques, a pediatric rheumatologist in the infection, inflammation and rheumatology section at the University College London Institute of Child Health and the Great Ormond Street Hospital for Children NHS Trust in London.
The 67 patients in the study involved those who were enrolled in the JDM Cohort and Biomarker Study, met Bohan and Peter criteria for JDM, and were on anti-TNF therapy at the time of analysis because of nonresponse to conventional therapy, active skin disease, calcinosis, or muscle weakness. They had at least 3 months of anti-TNF therapy and received either infliximab 6 mg/kg every 4 weeks (after a standard initial induction regimen) or adalimumab (Humira) 24 mg/m2 every other week.
A majority of the patients in the study were female (n = 41) and white (n = 54), with a mean age at disease onset of about 5 years. At the time of first use of anti-TNF agents, the patients had a mean age of about 10 years and a mean disease duration of 3.2 years. Treatment with TNF inhibitors lasted for a mean of about 2.5 years.
Of the 67 patients, data were not analyzed for 4 patients; there was insufficient information for 1 patient, while 3 patients had allergic reactions to their anti-TNF therapy on the first or second infusion. The remaining 63 patients included 43 who received infliximab, 4 on adalimumab, and 16 who used both.
Prior to anti-TNF treatment, 52 of 53 patients (98%) were taking methotrexate, azathioprine, hydroxychloroquine, or a combination of those. That declined to 45 of 56 (80%) at the start of anti-TNF therapy and then increased to 44 of 49 (89%) after 12 months of using an anti-TNF agent.
The use of cyclophosphamide declined markedly, from 26 of 65 patients (40%) to 3 of 65 (5%) at the start of TNF inhibition, and then to none after 12 months of anti-TNF therapy. Immunoglobulin therapy also declined, from use in 10%-12% of patients before and at the start of anti-TNF treatment to just 1 of 41 patients (2%) after 12 months of TNF inhibitor therapy.
The median modified Disease Activity Score for skin involvement significantly improved over the course of 12 months of treatment with infliximab, decreasing from 4 to 1. That was also the case for Physician Global Assessment score, as well as muscle outcome measurements on the Childhood Myositis Assessment Scale (CMAS) and the 8-item Manual Muscle Testing (MMT8).
For the 31 patients in the study who had calcinosis, lesions improved (reduced in number and/or size) in 17 patients, including 8 with complete resolution of their lesions. In the other 14 patients, lesions remained stable in 3 (fewer than three lesions) and were widespread or did not improve in 4; the other 7 patients had insufficient data to determine outcomes.
Most patients with muscle involvement already had improved with steroids prior to using anti-TNF drugs. Thus, the improvement in CMAS and MMT8 scores on anti-TNF treatment was not very large, going from about 45 to 53 and from about 74 to 79, respectively.
The investigators did not examine treatment response in relation to muscle-specific antibodies, but Dr. Campanilho-Marques said that it is something they would like to do in the future.
The main indication for anti-TNF agents was active skin disease that had not responded to conventional treatment, noted Dr. Campanilho-Marques, who is also with the departments of rheumatology at the Santa Maria Hospital and the Instituto Português de Reumatologia, both in Lisbon.
For 16 patients who switched from infliximab to adalimumab, the changes in outcome measures were not statistically significant. The switches occurred at a median of 2.35 months after starting infliximab; 10 patients switched because of inefficacy, 4 because of adverse events, and 2 because of patient preference.
After 12 months of anti-TNF therapy, the median prednisolone dose declined from 6 mg to 2.5 mg, but the decline appeared to be driven by five patients who sharply decreased their dose. Seven patients successfully stopped anti-TNF therapy after improvement occurred, Dr. Campanilho-Marques said.
Serious adverse events occurred 12 times during the year-long study period, including nine allergic reactions and three hospitalizations because of infection. Another 19 mild-to-moderate adverse events took place, which involved 15 infections and three local site reactions and skin rash, which led five patients to discontinue the biologic.
Overall, adverse events occurred at a rate of 13.3/100 patient-years, including 5.2 serious events/100 patient-years. One patient died because of a small bowel perforation that was probably secondary to disease-related damage. There were no malignancies or tuberculosis cases.
In a video interview at the meeting, Dr. Campanilho-Marques discussed the study findings and their implications.
The researchers had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE EULAR 2016 CONGRESS
Key clinical point: TNF inhibitor treatment in patients with juvenile dermatomyositis may be beneficial for skin involvement that is refractory to conventional treatments.
Major finding: The median Modified Disease Activity score for skin involvement significantly improved over 12 months of treatment with infliximab, decreasing from 4 to 1.
Data source: An observational cohort study of 67 JDM patients.
Disclosures: The researchers had no relevant disclosures.
Obesity May Attenuate Anti-TNF Response in Psoriatic Arthritis
LONDON – Patients with psoriatic arthritis appear less likely to achieve a good response to their first anti–tumor necrosis factor (anti-TNF) therapy if they are obese, according to data taken from two Nordic registries.
In a large observational cohort study, obese individuals with psoriatic arthritis (PsA) were significantly less likely than their nonobese counterparts to achieve a European League Against Rheumatism (EULAR) good or moderate response at 6 months (55% vs. 65%, P = .02). The overall odds ratio for achieving a good or moderate response was 0.47 when comparing obese with nonobese individuals.
The findings are potentially important because, with the exception of infliximab, anti-TNF therapy is not currently adjusted according to body weight, said presenting study author Pil Højgaard in an interview at the European Congress of Rheumatology.
Ms. Højgaard, who is an M.D. Ph.D. student at the department of rheumatology, Copenhagen University Hospital Gentofte, Rigshospitalet and the Parker Institute in Copenhagen, noted that obesity was a frequent comorbid condition in patients with PsA and that it is a known proinflammatory condition. As such, obesity could potentially affect immunologic processes, the pharmacokinetics of treatments, and ultimately patient outcomes.
Since TNF-alpha inhibitor (TNFi) treatment fails in around half of all patients with PsA treated in routine care, Ms. Højgaard noted that the aim of the cohort study was to investigate whether obesity could be having any influence on this.
Data on baseline characteristics, EULAR response rates, and drug adherence were obtained for 1,943 patients with PsA prescribed their anti-TNF therapy from two nationwide registries of disease-modifying therapies being used to treat rheumatic conditions in Denmark and Iceland, DANBIO (Rheumatology. 2011;50:69–77) and ICEBIO, respectively.
At baseline, body mass index (BMI) data were available for 1,271 patients and 408 (32%) of these had a BMI of 30 kg/m2 or more and were classed as being obese. The majority (39%) had received a first prescription for adalimumab, with around a quarter each prescribed etanercept (26%) or infliximab (24%), and the remainder prescribed golimumab (7%) and certolizumab (4%).
Compared to the 863 (68%) nonobese individuals, the obese patients were older (47 vs. 49 years, P = .01), less likely to smoke (30% vs. 23%, P = .01), and had higher disease activity measured on the Disease Activity Score 28 (DAS28) (4.4 vs. 4.6, P = .01). Health Assessment Questionnaire scores were also higher in obese than in nonobese individuals (1.1 vs. 0.9, P less than .01), and there were higher tender joint counts (6 vs. 5, P = .01), and higher pain levels assessed on a visual analog scale (VAS). Obese patients also had higher scores on a VAS patient global scale. The median follow-up time was 1.5 years.
Patients who were obese were found to adhere to TNFi treatment for shorter periods of time than nonobese patients, with median durations of 1.76 and 3.08 years, respectively (P less than .001). This discrepancy was most pronounced among men, a finding that may account for the fact that they were less likely to achieve a good EULAR response than their nonobese counterparts (OR = 0.5).
Being obese versus not being obese independently predicted TNFi withdrawal overall (hazard ratio, 1.6), especially in men (HR, 1.8; HR, 1.5 in women). TNFi withdrawal was more likely in obese than in nonobese patients even when individual treatments were considered; adalimumab: HR, 1.6; etanercept: HR, 2.0; infliximab: HR, 1.6.
An association between obesity and reduced response to anti-TNF therapy has also been observed in patients with rheumatoid arthritis, Ms. Højgaard acknowledged. There have also been a few studies of PsA and psoriasis “but to my knowledge, I think in the field of psoriatic arthritis, we are one of the few that have been looking at long-time drug survival,” she said. “We also include quite a lot of patients.”
“Of course this is not a randomized clinical study, so there could be residual confounding factors,” Ms. Højgaard cautioned. “It is always a bit difficult to say something about causality when it is a database study,” she added. “I think what we can see here is that there is an association, but in order to recommend weight loss we need some prospective studies.”
She noted that there was one published clinical study (Ann Rheum Dis. 2014;73:1157–62) that had looked at the benefit of a weight reduction program started at the same time as TNFi initiation in patients with PsA. This found there was a benefit of weight loss on response to TNFis, regardless of the type of diet.
DANBIO is supported by unrestricted grants from Abbott, Pfizer, MSD, Bristol-Myers Squibb, Roche, and UCB-Nordic. The sponsors have had no influence on data collection, analysis, or publication. ICEBIO is part of the electronic medical record system held by the University of Reykjavik and receives no industrial funding. Ms. Højgaard has received speaking fees from Celgene and UCB not related to this work.
LONDON – Patients with psoriatic arthritis appear less likely to achieve a good response to their first anti–tumor necrosis factor (anti-TNF) therapy if they are obese, according to data taken from two Nordic registries.
In a large observational cohort study, obese individuals with psoriatic arthritis (PsA) were significantly less likely than their nonobese counterparts to achieve a European League Against Rheumatism (EULAR) good or moderate response at 6 months (55% vs. 65%, P = .02). The overall odds ratio for achieving a good or moderate response was 0.47 when comparing obese with nonobese individuals.
The findings are potentially important because, with the exception of infliximab, anti-TNF therapy is not currently adjusted according to body weight, said presenting study author Pil Højgaard in an interview at the European Congress of Rheumatology.
Ms. Højgaard, who is an M.D. Ph.D. student at the department of rheumatology, Copenhagen University Hospital Gentofte, Rigshospitalet and the Parker Institute in Copenhagen, noted that obesity was a frequent comorbid condition in patients with PsA and that it is a known proinflammatory condition. As such, obesity could potentially affect immunologic processes, the pharmacokinetics of treatments, and ultimately patient outcomes.
Since TNF-alpha inhibitor (TNFi) treatment fails in around half of all patients with PsA treated in routine care, Ms. Højgaard noted that the aim of the cohort study was to investigate whether obesity could be having any influence on this.
Data on baseline characteristics, EULAR response rates, and drug adherence were obtained for 1,943 patients with PsA prescribed their anti-TNF therapy from two nationwide registries of disease-modifying therapies being used to treat rheumatic conditions in Denmark and Iceland, DANBIO (Rheumatology. 2011;50:69–77) and ICEBIO, respectively.
At baseline, body mass index (BMI) data were available for 1,271 patients and 408 (32%) of these had a BMI of 30 kg/m2 or more and were classed as being obese. The majority (39%) had received a first prescription for adalimumab, with around a quarter each prescribed etanercept (26%) or infliximab (24%), and the remainder prescribed golimumab (7%) and certolizumab (4%).
Compared to the 863 (68%) nonobese individuals, the obese patients were older (47 vs. 49 years, P = .01), less likely to smoke (30% vs. 23%, P = .01), and had higher disease activity measured on the Disease Activity Score 28 (DAS28) (4.4 vs. 4.6, P = .01). Health Assessment Questionnaire scores were also higher in obese than in nonobese individuals (1.1 vs. 0.9, P less than .01), and there were higher tender joint counts (6 vs. 5, P = .01), and higher pain levels assessed on a visual analog scale (VAS). Obese patients also had higher scores on a VAS patient global scale. The median follow-up time was 1.5 years.
Patients who were obese were found to adhere to TNFi treatment for shorter periods of time than nonobese patients, with median durations of 1.76 and 3.08 years, respectively (P less than .001). This discrepancy was most pronounced among men, a finding that may account for the fact that they were less likely to achieve a good EULAR response than their nonobese counterparts (OR = 0.5).
Being obese versus not being obese independently predicted TNFi withdrawal overall (hazard ratio, 1.6), especially in men (HR, 1.8; HR, 1.5 in women). TNFi withdrawal was more likely in obese than in nonobese patients even when individual treatments were considered; adalimumab: HR, 1.6; etanercept: HR, 2.0; infliximab: HR, 1.6.
An association between obesity and reduced response to anti-TNF therapy has also been observed in patients with rheumatoid arthritis, Ms. Højgaard acknowledged. There have also been a few studies of PsA and psoriasis “but to my knowledge, I think in the field of psoriatic arthritis, we are one of the few that have been looking at long-time drug survival,” she said. “We also include quite a lot of patients.”
“Of course this is not a randomized clinical study, so there could be residual confounding factors,” Ms. Højgaard cautioned. “It is always a bit difficult to say something about causality when it is a database study,” she added. “I think what we can see here is that there is an association, but in order to recommend weight loss we need some prospective studies.”
She noted that there was one published clinical study (Ann Rheum Dis. 2014;73:1157–62) that had looked at the benefit of a weight reduction program started at the same time as TNFi initiation in patients with PsA. This found there was a benefit of weight loss on response to TNFis, regardless of the type of diet.
DANBIO is supported by unrestricted grants from Abbott, Pfizer, MSD, Bristol-Myers Squibb, Roche, and UCB-Nordic. The sponsors have had no influence on data collection, analysis, or publication. ICEBIO is part of the electronic medical record system held by the University of Reykjavik and receives no industrial funding. Ms. Højgaard has received speaking fees from Celgene and UCB not related to this work.
LONDON – Patients with psoriatic arthritis appear less likely to achieve a good response to their first anti–tumor necrosis factor (anti-TNF) therapy if they are obese, according to data taken from two Nordic registries.
In a large observational cohort study, obese individuals with psoriatic arthritis (PsA) were significantly less likely than their nonobese counterparts to achieve a European League Against Rheumatism (EULAR) good or moderate response at 6 months (55% vs. 65%, P = .02). The overall odds ratio for achieving a good or moderate response was 0.47 when comparing obese with nonobese individuals.
The findings are potentially important because, with the exception of infliximab, anti-TNF therapy is not currently adjusted according to body weight, said presenting study author Pil Højgaard in an interview at the European Congress of Rheumatology.
Ms. Højgaard, who is an M.D. Ph.D. student at the department of rheumatology, Copenhagen University Hospital Gentofte, Rigshospitalet and the Parker Institute in Copenhagen, noted that obesity was a frequent comorbid condition in patients with PsA and that it is a known proinflammatory condition. As such, obesity could potentially affect immunologic processes, the pharmacokinetics of treatments, and ultimately patient outcomes.
Since TNF-alpha inhibitor (TNFi) treatment fails in around half of all patients with PsA treated in routine care, Ms. Højgaard noted that the aim of the cohort study was to investigate whether obesity could be having any influence on this.
Data on baseline characteristics, EULAR response rates, and drug adherence were obtained for 1,943 patients with PsA prescribed their anti-TNF therapy from two nationwide registries of disease-modifying therapies being used to treat rheumatic conditions in Denmark and Iceland, DANBIO (Rheumatology. 2011;50:69–77) and ICEBIO, respectively.
At baseline, body mass index (BMI) data were available for 1,271 patients and 408 (32%) of these had a BMI of 30 kg/m2 or more and were classed as being obese. The majority (39%) had received a first prescription for adalimumab, with around a quarter each prescribed etanercept (26%) or infliximab (24%), and the remainder prescribed golimumab (7%) and certolizumab (4%).
Compared to the 863 (68%) nonobese individuals, the obese patients were older (47 vs. 49 years, P = .01), less likely to smoke (30% vs. 23%, P = .01), and had higher disease activity measured on the Disease Activity Score 28 (DAS28) (4.4 vs. 4.6, P = .01). Health Assessment Questionnaire scores were also higher in obese than in nonobese individuals (1.1 vs. 0.9, P less than .01), and there were higher tender joint counts (6 vs. 5, P = .01), and higher pain levels assessed on a visual analog scale (VAS). Obese patients also had higher scores on a VAS patient global scale. The median follow-up time was 1.5 years.
Patients who were obese were found to adhere to TNFi treatment for shorter periods of time than nonobese patients, with median durations of 1.76 and 3.08 years, respectively (P less than .001). This discrepancy was most pronounced among men, a finding that may account for the fact that they were less likely to achieve a good EULAR response than their nonobese counterparts (OR = 0.5).
Being obese versus not being obese independently predicted TNFi withdrawal overall (hazard ratio, 1.6), especially in men (HR, 1.8; HR, 1.5 in women). TNFi withdrawal was more likely in obese than in nonobese patients even when individual treatments were considered; adalimumab: HR, 1.6; etanercept: HR, 2.0; infliximab: HR, 1.6.
An association between obesity and reduced response to anti-TNF therapy has also been observed in patients with rheumatoid arthritis, Ms. Højgaard acknowledged. There have also been a few studies of PsA and psoriasis “but to my knowledge, I think in the field of psoriatic arthritis, we are one of the few that have been looking at long-time drug survival,” she said. “We also include quite a lot of patients.”
“Of course this is not a randomized clinical study, so there could be residual confounding factors,” Ms. Højgaard cautioned. “It is always a bit difficult to say something about causality when it is a database study,” she added. “I think what we can see here is that there is an association, but in order to recommend weight loss we need some prospective studies.”
She noted that there was one published clinical study (Ann Rheum Dis. 2014;73:1157–62) that had looked at the benefit of a weight reduction program started at the same time as TNFi initiation in patients with PsA. This found there was a benefit of weight loss on response to TNFis, regardless of the type of diet.
DANBIO is supported by unrestricted grants from Abbott, Pfizer, MSD, Bristol-Myers Squibb, Roche, and UCB-Nordic. The sponsors have had no influence on data collection, analysis, or publication. ICEBIO is part of the electronic medical record system held by the University of Reykjavik and receives no industrial funding. Ms. Højgaard has received speaking fees from Celgene and UCB not related to this work.
Obesity may attenuate anti-TNF response in psoriatic arthritis
LONDON – Patients with psoriatic arthritis appear less likely to achieve a good response to their first anti–tumor necrosis factor (anti-TNF) therapy if they are obese, according to data taken from two Nordic registries.
In a large observational cohort study, obese individuals with psoriatic arthritis (PsA) were significantly less likely than their nonobese counterparts to achieve a European League Against Rheumatism (EULAR) good or moderate response at 6 months (55% vs. 65%, P = .02). The overall odds ratio for achieving a good or moderate response was 0.47 when comparing obese with nonobese individuals.
The findings are potentially important because, with the exception of infliximab, anti-TNF therapy is not currently adjusted according to body weight, said presenting study author Pil Højgaard in an interview at the European Congress of Rheumatology.
Ms. Højgaard, who is an M.D. Ph.D. student at the department of rheumatology, Copenhagen University Hospital Gentofte, Rigshospitalet and the Parker Institute in Copenhagen, noted that obesity was a frequent comorbid condition in patients with PsA and that it is a known proinflammatory condition. As such, obesity could potentially affect immunologic processes, the pharmacokinetics of treatments, and ultimately patient outcomes.
Since TNF-alpha inhibitor (TNFi) treatment fails in around half of all patients with PsA treated in routine care, Ms. Højgaard noted that the aim of the cohort study was to investigate whether obesity could be having any influence on this.
Data on baseline characteristics, EULAR response rates, and drug adherence were obtained for 1,943 patients with PsA prescribed their anti-TNF therapy from two nationwide registries of disease-modifying therapies being used to treat rheumatic conditions in Denmark and Iceland, DANBIO (Rheumatology. 2011;50:69–77) and ICEBIO, respectively.
At baseline, body mass index (BMI) data were available for 1,271 patients and 408 (32%) of these had a BMI of 30 kg/m2 or more and were classed as being obese. The majority (39%) had received a first prescription for adalimumab, with around a quarter each prescribed etanercept (26%) or infliximab (24%), and the remainder prescribed golimumab (7%) and certolizumab (4%).
Compared to the 863 (68%) nonobese individuals, the obese patients were older (47 vs. 49 years, P = .01), less likely to smoke (30% vs. 23%, P = .01), and had higher disease activity measured on the Disease Activity Score 28 (DAS28) (4.4 vs. 4.6, P = .01). Health Assessment Questionnaire scores were also higher in obese than in nonobese individuals (1.1 vs. 0.9, P less than .01), and there were higher tender joint counts (6 vs. 5, P = .01), and higher pain levels assessed on a visual analog scale (VAS). Obese patients also had higher scores on a VAS patient global scale. The median follow-up time was 1.5 years.
Patients who were obese were found to adhere to TNFi treatment for shorter periods of time than nonobese patients, with median durations of 1.76 and 3.08 years, respectively (P less than .001). This discrepancy was most pronounced among men, a finding that may account for the fact that they were less likely to achieve a good EULAR response than their nonobese counterparts (OR = 0.5).
Being obese versus not being obese independently predicted TNFi withdrawal overall (hazard ratio, 1.6), especially in men (HR, 1.8; HR, 1.5 in women). TNFi withdrawal was more likely in obese than in nonobese patients even when individual treatments were considered; adalimumab: HR, 1.6; etanercept: HR, 2.0; infliximab: HR, 1.6.
An association between obesity and reduced response to anti-TNF therapy has also been observed in patients with rheumatoid arthritis, Ms. Højgaard acknowledged. There have also been a few studies of PsA and psoriasis “but to my knowledge, I think in the field of psoriatic arthritis, we are one of the few that have been looking at long-time drug survival,” she said. “We also include quite a lot of patients.”
“Of course this is not a randomized clinical study, so there could be residual confounding factors,” Ms. Højgaard cautioned. “It is always a bit difficult to say something about causality when it is a database study,” she added. “I think what we can see here is that there is an association, but in order to recommend weight loss we need some prospective studies.”
She noted that there was one published clinical study (Ann Rheum Dis. 2014;73:1157–62) that had looked at the benefit of a weight reduction program started at the same time as TNFi initiation in patients with PsA. This found there was a benefit of weight loss on response to TNFis, regardless of the type of diet.
DANBIO is supported by unrestricted grants from Abbott, Pfizer, MSD, Bristol-Myers Squibb, Roche, and UCB-Nordic. The sponsors have had no influence on data collection, analysis, or publication. ICEBIO is part of the electronic medical record system held by the University of Reykjavik and receives no industrial funding. Ms. Højgaard has received speaking fees from Celgene and UCB not related to this work.
LONDON – Patients with psoriatic arthritis appear less likely to achieve a good response to their first anti–tumor necrosis factor (anti-TNF) therapy if they are obese, according to data taken from two Nordic registries.
In a large observational cohort study, obese individuals with psoriatic arthritis (PsA) were significantly less likely than their nonobese counterparts to achieve a European League Against Rheumatism (EULAR) good or moderate response at 6 months (55% vs. 65%, P = .02). The overall odds ratio for achieving a good or moderate response was 0.47 when comparing obese with nonobese individuals.
The findings are potentially important because, with the exception of infliximab, anti-TNF therapy is not currently adjusted according to body weight, said presenting study author Pil Højgaard in an interview at the European Congress of Rheumatology.
Ms. Højgaard, who is an M.D. Ph.D. student at the department of rheumatology, Copenhagen University Hospital Gentofte, Rigshospitalet and the Parker Institute in Copenhagen, noted that obesity was a frequent comorbid condition in patients with PsA and that it is a known proinflammatory condition. As such, obesity could potentially affect immunologic processes, the pharmacokinetics of treatments, and ultimately patient outcomes.
Since TNF-alpha inhibitor (TNFi) treatment fails in around half of all patients with PsA treated in routine care, Ms. Højgaard noted that the aim of the cohort study was to investigate whether obesity could be having any influence on this.
Data on baseline characteristics, EULAR response rates, and drug adherence were obtained for 1,943 patients with PsA prescribed their anti-TNF therapy from two nationwide registries of disease-modifying therapies being used to treat rheumatic conditions in Denmark and Iceland, DANBIO (Rheumatology. 2011;50:69–77) and ICEBIO, respectively.
At baseline, body mass index (BMI) data were available for 1,271 patients and 408 (32%) of these had a BMI of 30 kg/m2 or more and were classed as being obese. The majority (39%) had received a first prescription for adalimumab, with around a quarter each prescribed etanercept (26%) or infliximab (24%), and the remainder prescribed golimumab (7%) and certolizumab (4%).
Compared to the 863 (68%) nonobese individuals, the obese patients were older (47 vs. 49 years, P = .01), less likely to smoke (30% vs. 23%, P = .01), and had higher disease activity measured on the Disease Activity Score 28 (DAS28) (4.4 vs. 4.6, P = .01). Health Assessment Questionnaire scores were also higher in obese than in nonobese individuals (1.1 vs. 0.9, P less than .01), and there were higher tender joint counts (6 vs. 5, P = .01), and higher pain levels assessed on a visual analog scale (VAS). Obese patients also had higher scores on a VAS patient global scale. The median follow-up time was 1.5 years.
Patients who were obese were found to adhere to TNFi treatment for shorter periods of time than nonobese patients, with median durations of 1.76 and 3.08 years, respectively (P less than .001). This discrepancy was most pronounced among men, a finding that may account for the fact that they were less likely to achieve a good EULAR response than their nonobese counterparts (OR = 0.5).
Being obese versus not being obese independently predicted TNFi withdrawal overall (hazard ratio, 1.6), especially in men (HR, 1.8; HR, 1.5 in women). TNFi withdrawal was more likely in obese than in nonobese patients even when individual treatments were considered; adalimumab: HR, 1.6; etanercept: HR, 2.0; infliximab: HR, 1.6.
An association between obesity and reduced response to anti-TNF therapy has also been observed in patients with rheumatoid arthritis, Ms. Højgaard acknowledged. There have also been a few studies of PsA and psoriasis “but to my knowledge, I think in the field of psoriatic arthritis, we are one of the few that have been looking at long-time drug survival,” she said. “We also include quite a lot of patients.”
“Of course this is not a randomized clinical study, so there could be residual confounding factors,” Ms. Højgaard cautioned. “It is always a bit difficult to say something about causality when it is a database study,” she added. “I think what we can see here is that there is an association, but in order to recommend weight loss we need some prospective studies.”
She noted that there was one published clinical study (Ann Rheum Dis. 2014;73:1157–62) that had looked at the benefit of a weight reduction program started at the same time as TNFi initiation in patients with PsA. This found there was a benefit of weight loss on response to TNFis, regardless of the type of diet.
DANBIO is supported by unrestricted grants from Abbott, Pfizer, MSD, Bristol-Myers Squibb, Roche, and UCB-Nordic. The sponsors have had no influence on data collection, analysis, or publication. ICEBIO is part of the electronic medical record system held by the University of Reykjavik and receives no industrial funding. Ms. Højgaard has received speaking fees from Celgene and UCB not related to this work.
LONDON – Patients with psoriatic arthritis appear less likely to achieve a good response to their first anti–tumor necrosis factor (anti-TNF) therapy if they are obese, according to data taken from two Nordic registries.
In a large observational cohort study, obese individuals with psoriatic arthritis (PsA) were significantly less likely than their nonobese counterparts to achieve a European League Against Rheumatism (EULAR) good or moderate response at 6 months (55% vs. 65%, P = .02). The overall odds ratio for achieving a good or moderate response was 0.47 when comparing obese with nonobese individuals.
The findings are potentially important because, with the exception of infliximab, anti-TNF therapy is not currently adjusted according to body weight, said presenting study author Pil Højgaard in an interview at the European Congress of Rheumatology.
Ms. Højgaard, who is an M.D. Ph.D. student at the department of rheumatology, Copenhagen University Hospital Gentofte, Rigshospitalet and the Parker Institute in Copenhagen, noted that obesity was a frequent comorbid condition in patients with PsA and that it is a known proinflammatory condition. As such, obesity could potentially affect immunologic processes, the pharmacokinetics of treatments, and ultimately patient outcomes.
Since TNF-alpha inhibitor (TNFi) treatment fails in around half of all patients with PsA treated in routine care, Ms. Højgaard noted that the aim of the cohort study was to investigate whether obesity could be having any influence on this.
Data on baseline characteristics, EULAR response rates, and drug adherence were obtained for 1,943 patients with PsA prescribed their anti-TNF therapy from two nationwide registries of disease-modifying therapies being used to treat rheumatic conditions in Denmark and Iceland, DANBIO (Rheumatology. 2011;50:69–77) and ICEBIO, respectively.
At baseline, body mass index (BMI) data were available for 1,271 patients and 408 (32%) of these had a BMI of 30 kg/m2 or more and were classed as being obese. The majority (39%) had received a first prescription for adalimumab, with around a quarter each prescribed etanercept (26%) or infliximab (24%), and the remainder prescribed golimumab (7%) and certolizumab (4%).
Compared to the 863 (68%) nonobese individuals, the obese patients were older (47 vs. 49 years, P = .01), less likely to smoke (30% vs. 23%, P = .01), and had higher disease activity measured on the Disease Activity Score 28 (DAS28) (4.4 vs. 4.6, P = .01). Health Assessment Questionnaire scores were also higher in obese than in nonobese individuals (1.1 vs. 0.9, P less than .01), and there were higher tender joint counts (6 vs. 5, P = .01), and higher pain levels assessed on a visual analog scale (VAS). Obese patients also had higher scores on a VAS patient global scale. The median follow-up time was 1.5 years.
Patients who were obese were found to adhere to TNFi treatment for shorter periods of time than nonobese patients, with median durations of 1.76 and 3.08 years, respectively (P less than .001). This discrepancy was most pronounced among men, a finding that may account for the fact that they were less likely to achieve a good EULAR response than their nonobese counterparts (OR = 0.5).
Being obese versus not being obese independently predicted TNFi withdrawal overall (hazard ratio, 1.6), especially in men (HR, 1.8; HR, 1.5 in women). TNFi withdrawal was more likely in obese than in nonobese patients even when individual treatments were considered; adalimumab: HR, 1.6; etanercept: HR, 2.0; infliximab: HR, 1.6.
An association between obesity and reduced response to anti-TNF therapy has also been observed in patients with rheumatoid arthritis, Ms. Højgaard acknowledged. There have also been a few studies of PsA and psoriasis “but to my knowledge, I think in the field of psoriatic arthritis, we are one of the few that have been looking at long-time drug survival,” she said. “We also include quite a lot of patients.”
“Of course this is not a randomized clinical study, so there could be residual confounding factors,” Ms. Højgaard cautioned. “It is always a bit difficult to say something about causality when it is a database study,” she added. “I think what we can see here is that there is an association, but in order to recommend weight loss we need some prospective studies.”
She noted that there was one published clinical study (Ann Rheum Dis. 2014;73:1157–62) that had looked at the benefit of a weight reduction program started at the same time as TNFi initiation in patients with PsA. This found there was a benefit of weight loss on response to TNFis, regardless of the type of diet.
DANBIO is supported by unrestricted grants from Abbott, Pfizer, MSD, Bristol-Myers Squibb, Roche, and UCB-Nordic. The sponsors have had no influence on data collection, analysis, or publication. ICEBIO is part of the electronic medical record system held by the University of Reykjavik and receives no industrial funding. Ms. Højgaard has received speaking fees from Celgene and UCB not related to this work.
Key clinical point: Obesity may reduce response and adherence to tumor necrosis factor–inhibitor therapy in patients with psoriatic arthritis.
Major finding: The EULAR good or moderate response rate at 6 months was 55% in obese vs. 65% in nonobese patients (P = .02).
Data source: Observational cohort study based on two Nordic registries of 1,943 patients with PsA prescribed their first TNFi.
Disclosures: DANBIO is supported by unrestricted grants from Abbott, Pfizer, MSD, Bristol-Myers Squibb, Roche, and UCB-Nordic. The sponsors have had no influence on data collection, analysis, or publication. ICEBIO is part of the electronic medical record system held by the University of Reykjavik and receives no industrial funding. Ms. Højgaard has received speaking fees from Celgene and UCB not related to this work.