User login
Biomarker duo rapidly identifies serious bacterial infections
MALMO, SWEDEN – The combination of serum procalcitonin and C-reactive protein levels upon admission to a pediatric ICU displayed high utility for early diagnosis of serious bacterial infection in critically ill children in a large prospective observational study presented at the annual meeting of the European Society for Paediatric Infectious Diseases.
This winning combination significantly outperformed neutrophil gelatinase-associated lipocalin, activated partial thromboplastin time, and resistin, both individually and in various combinations, for the vital task of making a rapid distinction between infectious and noninfectious causes of pediatric systemic inflammatory response syndrome, reported Enitan D. Carrol, MD, professor of pediatric infection at the University of Liverpool (England).
“One of the clinical dilemmas we face in intensive care is being able to differentiate between infectious and noninfectious causes of systemic inflammatory response syndrome. This is important because we need to identify which children have life-threatening infections so that we can promptly initiate antimicrobial therapy,” she explained.
One in four deaths in pediatric ICUs are infection related, Dr. Carrol noted.
“There is an urgent need for infection markers which, firstly, change early in the course of bacterial infection, secondly, correlate with real-time clinical progression, and thirdly, have a rapid turn-around time to allow effective clinical decision making,” she observed.
The combination of procalcitonin and C-reactive protein (CRP) levels measured at admission fits the bill, Dr. Carrol continued. Of the five biomarkers evaluated in her study – all backed by some supporting evidence of efficacy in earlier studies – the top two individual performers in terms of negative predictive value (NPV) were a CRP less than 4.2 mg/dL with a negative NPV of 99%, and a procalcitonin less than 1.52 ng/mL with an NPV of 96%. The positive predictive value of each of the biomarkers was 37%. The sensitivity and specificity of procalcitonin for diagnosis of serious bacterial infection were 78% and 80%, respectively. For CRP, the figures were 93% and 76%.
The combination of procalcitonin and CRP outperformed a multitude of other two-, three-, and four-biomarker combinations tested, with an area under the curve of 93% for combined sensitivity and specificity.
The study included 657 children admitted to the pediatric ICU at Alder Hey Children’s Hospital in Liverpool with systemic inflammatory response syndrome. All had blood samples measured for the five biomarkers on days 1-7. Clinicians were blinded as to the biomarker results. Ninety-two (14%) patients were ultimately found to have a serious bacterial infection – essentially, bacterial meningitis or septic shock – and 565 (86%) had a nonbacterial etiology.
The 28-day mortality rate was 9% in the group with serious bacterial infection, significantly higher than the 2% rate in the group with other causes of their systemic inflammatory response syndrome.
Longitudinal trends in procalcitonin and CRP as evidenced in the study can be used in clinical decision making, according to Dr. Carrol. Mean values of procalcitonin plummeted by 80% from day 1 to day 5 in response to antimicrobial therapy in the group with serious bacterial infections. In contrast, CRP levels rose sharply from day 1 to a peak on day 2, then fell, although the 50% drop from day 2 to day 5 in response to antimicrobial therapy wasn’t as pronounced as the change in procalcitonin.
“There is an additive benefit for both biomarkers compared with CRP alone. The problem with CRP on admission, as I’ve demonstrated in this study, is it often hasn’t risen yet early after admission. So although it gave the best area under the curve of any of the biomarkers, I think that combined with procalcitonin you get a much better descriminator,” Dr. Carrol said.
The median duration of ICU stay in the patients with serious bacterial infection at admission was 5 days, compared with 3 days when the cause of systemic inflammatory response syndrome lay elsewhere. Their median duration of ventilation was significantly longer, too: 4 days versus 2 in children without a serious bacterial infection.
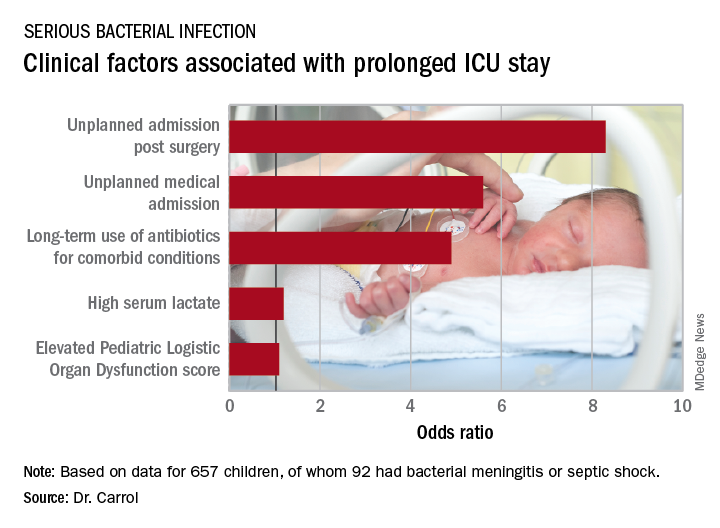
Stepwise logistic regression analysis pinpointed several clinical variables as being associated with prolonged ICU stay.
In addition, initiation of antibiotic therapy prior to admission to the pediatric ICU was associated with a 50% reduction in the likelihood of a prolonged ICU stay. “This reflects the fact that early antibiotics give you a better prognosis if you have sepsis,” according to Dr. Carrol.
She and her coinvestigators now have embarked on a multicenter U.K. study looking at the impact of procalcitonin to guide duration of antimicrobial therapy in critically ill children.
The Alder Hey study was funded by the U.K. National Institute for Health Research. Dr. Carrol reported having no financial conflicts. Although she serves as a consultant to several health care companies, all remuneration goes directly to the University of Liverpool.
MALMO, SWEDEN – The combination of serum procalcitonin and C-reactive protein levels upon admission to a pediatric ICU displayed high utility for early diagnosis of serious bacterial infection in critically ill children in a large prospective observational study presented at the annual meeting of the European Society for Paediatric Infectious Diseases.
This winning combination significantly outperformed neutrophil gelatinase-associated lipocalin, activated partial thromboplastin time, and resistin, both individually and in various combinations, for the vital task of making a rapid distinction between infectious and noninfectious causes of pediatric systemic inflammatory response syndrome, reported Enitan D. Carrol, MD, professor of pediatric infection at the University of Liverpool (England).
“One of the clinical dilemmas we face in intensive care is being able to differentiate between infectious and noninfectious causes of systemic inflammatory response syndrome. This is important because we need to identify which children have life-threatening infections so that we can promptly initiate antimicrobial therapy,” she explained.
One in four deaths in pediatric ICUs are infection related, Dr. Carrol noted.
“There is an urgent need for infection markers which, firstly, change early in the course of bacterial infection, secondly, correlate with real-time clinical progression, and thirdly, have a rapid turn-around time to allow effective clinical decision making,” she observed.
The combination of procalcitonin and C-reactive protein (CRP) levels measured at admission fits the bill, Dr. Carrol continued. Of the five biomarkers evaluated in her study – all backed by some supporting evidence of efficacy in earlier studies – the top two individual performers in terms of negative predictive value (NPV) were a CRP less than 4.2 mg/dL with a negative NPV of 99%, and a procalcitonin less than 1.52 ng/mL with an NPV of 96%. The positive predictive value of each of the biomarkers was 37%. The sensitivity and specificity of procalcitonin for diagnosis of serious bacterial infection were 78% and 80%, respectively. For CRP, the figures were 93% and 76%.
The combination of procalcitonin and CRP outperformed a multitude of other two-, three-, and four-biomarker combinations tested, with an area under the curve of 93% for combined sensitivity and specificity.
The study included 657 children admitted to the pediatric ICU at Alder Hey Children’s Hospital in Liverpool with systemic inflammatory response syndrome. All had blood samples measured for the five biomarkers on days 1-7. Clinicians were blinded as to the biomarker results. Ninety-two (14%) patients were ultimately found to have a serious bacterial infection – essentially, bacterial meningitis or septic shock – and 565 (86%) had a nonbacterial etiology.
The 28-day mortality rate was 9% in the group with serious bacterial infection, significantly higher than the 2% rate in the group with other causes of their systemic inflammatory response syndrome.
Longitudinal trends in procalcitonin and CRP as evidenced in the study can be used in clinical decision making, according to Dr. Carrol. Mean values of procalcitonin plummeted by 80% from day 1 to day 5 in response to antimicrobial therapy in the group with serious bacterial infections. In contrast, CRP levels rose sharply from day 1 to a peak on day 2, then fell, although the 50% drop from day 2 to day 5 in response to antimicrobial therapy wasn’t as pronounced as the change in procalcitonin.
“There is an additive benefit for both biomarkers compared with CRP alone. The problem with CRP on admission, as I’ve demonstrated in this study, is it often hasn’t risen yet early after admission. So although it gave the best area under the curve of any of the biomarkers, I think that combined with procalcitonin you get a much better descriminator,” Dr. Carrol said.
The median duration of ICU stay in the patients with serious bacterial infection at admission was 5 days, compared with 3 days when the cause of systemic inflammatory response syndrome lay elsewhere. Their median duration of ventilation was significantly longer, too: 4 days versus 2 in children without a serious bacterial infection.
Stepwise logistic regression analysis pinpointed several clinical variables as being associated with prolonged ICU stay.

In addition, initiation of antibiotic therapy prior to admission to the pediatric ICU was associated with a 50% reduction in the likelihood of a prolonged ICU stay. “This reflects the fact that early antibiotics give you a better prognosis if you have sepsis,” according to Dr. Carrol.
She and her coinvestigators now have embarked on a multicenter U.K. study looking at the impact of procalcitonin to guide duration of antimicrobial therapy in critically ill children.
The Alder Hey study was funded by the U.K. National Institute for Health Research. Dr. Carrol reported having no financial conflicts. Although she serves as a consultant to several health care companies, all remuneration goes directly to the University of Liverpool.
MALMO, SWEDEN – The combination of serum procalcitonin and C-reactive protein levels upon admission to a pediatric ICU displayed high utility for early diagnosis of serious bacterial infection in critically ill children in a large prospective observational study presented at the annual meeting of the European Society for Paediatric Infectious Diseases.
This winning combination significantly outperformed neutrophil gelatinase-associated lipocalin, activated partial thromboplastin time, and resistin, both individually and in various combinations, for the vital task of making a rapid distinction between infectious and noninfectious causes of pediatric systemic inflammatory response syndrome, reported Enitan D. Carrol, MD, professor of pediatric infection at the University of Liverpool (England).
“One of the clinical dilemmas we face in intensive care is being able to differentiate between infectious and noninfectious causes of systemic inflammatory response syndrome. This is important because we need to identify which children have life-threatening infections so that we can promptly initiate antimicrobial therapy,” she explained.
One in four deaths in pediatric ICUs are infection related, Dr. Carrol noted.
“There is an urgent need for infection markers which, firstly, change early in the course of bacterial infection, secondly, correlate with real-time clinical progression, and thirdly, have a rapid turn-around time to allow effective clinical decision making,” she observed.
The combination of procalcitonin and C-reactive protein (CRP) levels measured at admission fits the bill, Dr. Carrol continued. Of the five biomarkers evaluated in her study – all backed by some supporting evidence of efficacy in earlier studies – the top two individual performers in terms of negative predictive value (NPV) were a CRP less than 4.2 mg/dL with a negative NPV of 99%, and a procalcitonin less than 1.52 ng/mL with an NPV of 96%. The positive predictive value of each of the biomarkers was 37%. The sensitivity and specificity of procalcitonin for diagnosis of serious bacterial infection were 78% and 80%, respectively. For CRP, the figures were 93% and 76%.
The combination of procalcitonin and CRP outperformed a multitude of other two-, three-, and four-biomarker combinations tested, with an area under the curve of 93% for combined sensitivity and specificity.
The study included 657 children admitted to the pediatric ICU at Alder Hey Children’s Hospital in Liverpool with systemic inflammatory response syndrome. All had blood samples measured for the five biomarkers on days 1-7. Clinicians were blinded as to the biomarker results. Ninety-two (14%) patients were ultimately found to have a serious bacterial infection – essentially, bacterial meningitis or septic shock – and 565 (86%) had a nonbacterial etiology.
The 28-day mortality rate was 9% in the group with serious bacterial infection, significantly higher than the 2% rate in the group with other causes of their systemic inflammatory response syndrome.
Longitudinal trends in procalcitonin and CRP as evidenced in the study can be used in clinical decision making, according to Dr. Carrol. Mean values of procalcitonin plummeted by 80% from day 1 to day 5 in response to antimicrobial therapy in the group with serious bacterial infections. In contrast, CRP levels rose sharply from day 1 to a peak on day 2, then fell, although the 50% drop from day 2 to day 5 in response to antimicrobial therapy wasn’t as pronounced as the change in procalcitonin.
“There is an additive benefit for both biomarkers compared with CRP alone. The problem with CRP on admission, as I’ve demonstrated in this study, is it often hasn’t risen yet early after admission. So although it gave the best area under the curve of any of the biomarkers, I think that combined with procalcitonin you get a much better descriminator,” Dr. Carrol said.
The median duration of ICU stay in the patients with serious bacterial infection at admission was 5 days, compared with 3 days when the cause of systemic inflammatory response syndrome lay elsewhere. Their median duration of ventilation was significantly longer, too: 4 days versus 2 in children without a serious bacterial infection.
Stepwise logistic regression analysis pinpointed several clinical variables as being associated with prolonged ICU stay.

In addition, initiation of antibiotic therapy prior to admission to the pediatric ICU was associated with a 50% reduction in the likelihood of a prolonged ICU stay. “This reflects the fact that early antibiotics give you a better prognosis if you have sepsis,” according to Dr. Carrol.
She and her coinvestigators now have embarked on a multicenter U.K. study looking at the impact of procalcitonin to guide duration of antimicrobial therapy in critically ill children.
The Alder Hey study was funded by the U.K. National Institute for Health Research. Dr. Carrol reported having no financial conflicts. Although she serves as a consultant to several health care companies, all remuneration goes directly to the University of Liverpool.
REPORTING FROM ESPID 2018
Key clinical point:
Major finding: The area under the curve combining sensitivity and specificity was 93%.
Study details: This was a prospective, observational, single-center, clinician-blinded study of 657 patients admitted to a pediatric ICU with symptoms of systemic inflammatory response syndrome.
Disclosures: The study was funded by the U.K. National Institute for Health Research. The presenter reported having no relevant financial conflicts. Although she serves as a consultant to several health care companies, all remuneration goes directly to the University of Liverpool.
Impact of varicella vaccination on herpes zoster is not what was expected
MALMO, SWEDEN – The unique 20-year U.S. experience with pediatric universal varicella vaccination hasn’t resulted in the anticipated increase in herpes zoster predicted by the exogenous boosting hypothesis, Lara J. Wolfson, PhD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
In fact, the opposite has occurred. And this finding – based upon hard data – should be of considerable interest to European health officials who have been considering introducing universal varicella vaccination into their national health care systems but have refrained because of theoretical concerns raised by the venerable exogenous boosting hypothesis, noted Dr. Wolfson, director of outcomes research at the Merck Center for Observational and Real-World Evidence, Kenilworth, N.J.
The exogenous boosting hypothesis, which dates back to the mid-1960s, holds that reexposure to wild circulating varicella virus prevents development of herpes zoster later in life. Conversely, by vaccinating children against varicella, opportunities are diminished for reexposure to wild type virus among adults who weren’t vaccinated against varicella, so the hypothesis would predict an increase in the incidence of herpes zoster that should peak 15-35 years after introduction of universal varicella vaccination.
“The same virus that causes varicella in children later reactivates after going dormant in the dorsal root ganglia, and it reactivates as herpes zoster, which is 10 times more severe than chicken pox and leads to 10 times the health care costs. So if in fact implementing a universal varicella vaccine program would lead to an increased incidence of herpes zoster, this would be a bad thing,” the researcher explained.
However, the predictive models based upon the exogenous boosting hypothesis are built upon scanty data. And the models have great difficulty in adjusting for the changes in population dynamics that have occurred in the United States and Western Europe during the past quarter century: namely, declining birth rates coupled with survival to an older age.
Dr. Wolfson presented a retrospective study of deidentified administrative claims data from the MarketScan database covering roughly one-fifth of the U.S. population during 1991-2016. Her analysis broke down the annual incidence of varicella and herpes zoster in three eras: 1991-1995, which was the pre–varicella vaccination period; 1996-2006, when single-dose universal varicella vaccination of children was recommended; and 2007-2016, when two-dose vaccination became standard.
The first key study finding was that herpes zoster rates in the United States already were climbing across all age groups back in 1991-1995; that is, before introduction of universal varicella vaccination. Why? Probably because of those changes in population dynamics, although that’s speculative. The second key finding was that contrary to the exogenous boosting hypothesis prediction that the annual incidence of herpes zoster would accelerate after introduction of universal varicella vaccination, the rate of increase slowed, then plateaued during 2013-2016, most prominently in individuals aged 65 or older.
“In comparing the pre–universal varicella vaccination period to the one- or two-dose period or the total 20 years of vaccination, what we saw consistently across every age group is that herpes zoster is decelerating. There is actually less increase in the rate of herpes zoster than before varicella vaccination,” Dr. Wolfson said.
Uptake of the herpes zoster vaccine, introduced in the United States in 2008, was too low during the study years to account for this trend, she added.
Most dramatically, the incidence of herpes zoster among youths under age 18 years plummeted by 61.4%, from 88 per 100,000 person-years in 1991-1995 to 34 per 100,000 in 2016.
And of course, varicella disease has sharply declined in all age groups following the introduction of universal pediatric varicella vaccination, Dr. Wolfson observed.
Her study was supported by her employer, Merck.
MALMO, SWEDEN – The unique 20-year U.S. experience with pediatric universal varicella vaccination hasn’t resulted in the anticipated increase in herpes zoster predicted by the exogenous boosting hypothesis, Lara J. Wolfson, PhD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
In fact, the opposite has occurred. And this finding – based upon hard data – should be of considerable interest to European health officials who have been considering introducing universal varicella vaccination into their national health care systems but have refrained because of theoretical concerns raised by the venerable exogenous boosting hypothesis, noted Dr. Wolfson, director of outcomes research at the Merck Center for Observational and Real-World Evidence, Kenilworth, N.J.
The exogenous boosting hypothesis, which dates back to the mid-1960s, holds that reexposure to wild circulating varicella virus prevents development of herpes zoster later in life. Conversely, by vaccinating children against varicella, opportunities are diminished for reexposure to wild type virus among adults who weren’t vaccinated against varicella, so the hypothesis would predict an increase in the incidence of herpes zoster that should peak 15-35 years after introduction of universal varicella vaccination.
“The same virus that causes varicella in children later reactivates after going dormant in the dorsal root ganglia, and it reactivates as herpes zoster, which is 10 times more severe than chicken pox and leads to 10 times the health care costs. So if in fact implementing a universal varicella vaccine program would lead to an increased incidence of herpes zoster, this would be a bad thing,” the researcher explained.
However, the predictive models based upon the exogenous boosting hypothesis are built upon scanty data. And the models have great difficulty in adjusting for the changes in population dynamics that have occurred in the United States and Western Europe during the past quarter century: namely, declining birth rates coupled with survival to an older age.
Dr. Wolfson presented a retrospective study of deidentified administrative claims data from the MarketScan database covering roughly one-fifth of the U.S. population during 1991-2016. Her analysis broke down the annual incidence of varicella and herpes zoster in three eras: 1991-1995, which was the pre–varicella vaccination period; 1996-2006, when single-dose universal varicella vaccination of children was recommended; and 2007-2016, when two-dose vaccination became standard.
The first key study finding was that herpes zoster rates in the United States already were climbing across all age groups back in 1991-1995; that is, before introduction of universal varicella vaccination. Why? Probably because of those changes in population dynamics, although that’s speculative. The second key finding was that contrary to the exogenous boosting hypothesis prediction that the annual incidence of herpes zoster would accelerate after introduction of universal varicella vaccination, the rate of increase slowed, then plateaued during 2013-2016, most prominently in individuals aged 65 or older.
“In comparing the pre–universal varicella vaccination period to the one- or two-dose period or the total 20 years of vaccination, what we saw consistently across every age group is that herpes zoster is decelerating. There is actually less increase in the rate of herpes zoster than before varicella vaccination,” Dr. Wolfson said.
Uptake of the herpes zoster vaccine, introduced in the United States in 2008, was too low during the study years to account for this trend, she added.
Most dramatically, the incidence of herpes zoster among youths under age 18 years plummeted by 61.4%, from 88 per 100,000 person-years in 1991-1995 to 34 per 100,000 in 2016.
And of course, varicella disease has sharply declined in all age groups following the introduction of universal pediatric varicella vaccination, Dr. Wolfson observed.
Her study was supported by her employer, Merck.
MALMO, SWEDEN – The unique 20-year U.S. experience with pediatric universal varicella vaccination hasn’t resulted in the anticipated increase in herpes zoster predicted by the exogenous boosting hypothesis, Lara J. Wolfson, PhD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
In fact, the opposite has occurred. And this finding – based upon hard data – should be of considerable interest to European health officials who have been considering introducing universal varicella vaccination into their national health care systems but have refrained because of theoretical concerns raised by the venerable exogenous boosting hypothesis, noted Dr. Wolfson, director of outcomes research at the Merck Center for Observational and Real-World Evidence, Kenilworth, N.J.
The exogenous boosting hypothesis, which dates back to the mid-1960s, holds that reexposure to wild circulating varicella virus prevents development of herpes zoster later in life. Conversely, by vaccinating children against varicella, opportunities are diminished for reexposure to wild type virus among adults who weren’t vaccinated against varicella, so the hypothesis would predict an increase in the incidence of herpes zoster that should peak 15-35 years after introduction of universal varicella vaccination.
“The same virus that causes varicella in children later reactivates after going dormant in the dorsal root ganglia, and it reactivates as herpes zoster, which is 10 times more severe than chicken pox and leads to 10 times the health care costs. So if in fact implementing a universal varicella vaccine program would lead to an increased incidence of herpes zoster, this would be a bad thing,” the researcher explained.
However, the predictive models based upon the exogenous boosting hypothesis are built upon scanty data. And the models have great difficulty in adjusting for the changes in population dynamics that have occurred in the United States and Western Europe during the past quarter century: namely, declining birth rates coupled with survival to an older age.
Dr. Wolfson presented a retrospective study of deidentified administrative claims data from the MarketScan database covering roughly one-fifth of the U.S. population during 1991-2016. Her analysis broke down the annual incidence of varicella and herpes zoster in three eras: 1991-1995, which was the pre–varicella vaccination period; 1996-2006, when single-dose universal varicella vaccination of children was recommended; and 2007-2016, when two-dose vaccination became standard.
The first key study finding was that herpes zoster rates in the United States already were climbing across all age groups back in 1991-1995; that is, before introduction of universal varicella vaccination. Why? Probably because of those changes in population dynamics, although that’s speculative. The second key finding was that contrary to the exogenous boosting hypothesis prediction that the annual incidence of herpes zoster would accelerate after introduction of universal varicella vaccination, the rate of increase slowed, then plateaued during 2013-2016, most prominently in individuals aged 65 or older.
“In comparing the pre–universal varicella vaccination period to the one- or two-dose period or the total 20 years of vaccination, what we saw consistently across every age group is that herpes zoster is decelerating. There is actually less increase in the rate of herpes zoster than before varicella vaccination,” Dr. Wolfson said.
Uptake of the herpes zoster vaccine, introduced in the United States in 2008, was too low during the study years to account for this trend, she added.
Most dramatically, the incidence of herpes zoster among youths under age 18 years plummeted by 61.4%, from 88 per 100,000 person-years in 1991-1995 to 34 per 100,000 in 2016.
And of course, varicella disease has sharply declined in all age groups following the introduction of universal pediatric varicella vaccination, Dr. Wolfson observed.
Her study was supported by her employer, Merck.
REPORTING FROM ESPID 2018
Key clinical point: The exogenous boosting hypothesis that universal pediatric varicella vaccination would result in an increase in herpes zoster hasn’t been borne out by the U.S. experience.
Major finding: rather than accelerating as some had forecast.
Study details: This was a retrospective study of the annual incidence of varicella and herpes zoster during 1991-2016 in roughly one-fifth of the U.S. population.
Disclosures: The study was sponsored by Merck and presented by a company employee.
Vaccine-related febrile seizures have zero developmental impact
MALMO, SWEDEN – Children who experience a febrile seizure in conjunction with a vaccination have developmental outcomes comparable with those of children who have non–vaccine-related febrile seizures and healthy controls who’ve never had a febrile seizure, according to the first prospective case-control cohort study to examine the issue.
This finding has important implications for clinical practice, Lucy Deng, MD, observed at the annual meeting of the European Society for Paediatric Infectious Diseases.
“Febrile seizures associated with a vaccine can decrease parent and provider confidence in vaccine safety,” the pediatrician noted. Based upon her study results, however, physicians now can offer a truly evidence-based message of reassurance.
“If you have a child with a vaccine-related febrile seizure, you can give the same advice to those parents as for anyone else who’s had a febrile seizure, in that there is no difference in the clinical outcomes of vaccine-proximate and non–vaccine-proximate febrile seizures. Vaccine-proximate febrile seizures are usually brief, they don’t require any antiepileptic drugs, their length of stay is usually less than a day, and developmentally at 12-24 months post initial febrile seizure, they’re exactly the same as children who’ve never had a seizure before or who’ve had a non-vaccine-related febrile seizure,” said Dr. Deng of the National Centre for Immunisation Research and Surveillance in Sydney.
The impetus for her study was straightforward: “We all know that most children with a history of febrile seizures have normal behavior, intelligence, and academic achievement and do not later develop epilepsy. What we didn’t know before is if all of these facts apply to vaccine-proximate febrile seizures,” she explained.
The clinical severity analysis portion of this prospective case-control cohort study included 1,085 children with febrile seizures seen at five Australian children’s hospitals. Sixty-eight of them had vaccine-proximate febrile seizures, for a 6.6% rate. The febrile seizures in the other 1,027 children didn’t occur within 2 weeks following a vaccination.
Measles vaccine was implicated in 56 of the 68 children with vaccine-proximate febrile seizures, or 82%. Because Australian children receive their first measles-containing vaccine at age 12 months, the average age of the cohort with vaccine-proximate febrile seizures was 13 months, significantly younger than the 20-month average for children with non–vaccine-related febrile seizures.
In a multivariate analysis adjusted for patient age, gender, and history of prior afebrile seizures, the groups with vaccine-proximate and vaccine-unrelated febrile seizures didn’t differ significantly in terms of the proportion with a hospital length of stay greater than 1 day (20% vs. 15%), ICU admission (1.5% vs. 2.3%), seizure duration of more than 15 minutes (16% vs. 12%), repeat seizures within 24 hours (9% vs. 10%), or discharge on antiepileptic medication (4.4% vs. 4.3%).
In the developmental outcomes analysis, 62 of the children with vaccine-proximate febrile seizures, 70 with vaccine-unrelated febrile seizures, and 85 healthy controls with no seizure history underwent formal assessment using the third edition of the Bayley Scales of Infant and Toddler Development 12-24 months after their initial febrile seizure. Scores adjusted for years of maternal education were closely similar in all three groups across all five test domains: cognitive, language, motor, social-emotional, and general-adaptive.
Dr. Deng reported having no financial conflicts of interest regarding the study, which was partially funded by the Australian National Centre for Immunisation Research and Surveillance.
MALMO, SWEDEN – Children who experience a febrile seizure in conjunction with a vaccination have developmental outcomes comparable with those of children who have non–vaccine-related febrile seizures and healthy controls who’ve never had a febrile seizure, according to the first prospective case-control cohort study to examine the issue.
This finding has important implications for clinical practice, Lucy Deng, MD, observed at the annual meeting of the European Society for Paediatric Infectious Diseases.
“Febrile seizures associated with a vaccine can decrease parent and provider confidence in vaccine safety,” the pediatrician noted. Based upon her study results, however, physicians now can offer a truly evidence-based message of reassurance.
“If you have a child with a vaccine-related febrile seizure, you can give the same advice to those parents as for anyone else who’s had a febrile seizure, in that there is no difference in the clinical outcomes of vaccine-proximate and non–vaccine-proximate febrile seizures. Vaccine-proximate febrile seizures are usually brief, they don’t require any antiepileptic drugs, their length of stay is usually less than a day, and developmentally at 12-24 months post initial febrile seizure, they’re exactly the same as children who’ve never had a seizure before or who’ve had a non-vaccine-related febrile seizure,” said Dr. Deng of the National Centre for Immunisation Research and Surveillance in Sydney.
The impetus for her study was straightforward: “We all know that most children with a history of febrile seizures have normal behavior, intelligence, and academic achievement and do not later develop epilepsy. What we didn’t know before is if all of these facts apply to vaccine-proximate febrile seizures,” she explained.
The clinical severity analysis portion of this prospective case-control cohort study included 1,085 children with febrile seizures seen at five Australian children’s hospitals. Sixty-eight of them had vaccine-proximate febrile seizures, for a 6.6% rate. The febrile seizures in the other 1,027 children didn’t occur within 2 weeks following a vaccination.
Measles vaccine was implicated in 56 of the 68 children with vaccine-proximate febrile seizures, or 82%. Because Australian children receive their first measles-containing vaccine at age 12 months, the average age of the cohort with vaccine-proximate febrile seizures was 13 months, significantly younger than the 20-month average for children with non–vaccine-related febrile seizures.
In a multivariate analysis adjusted for patient age, gender, and history of prior afebrile seizures, the groups with vaccine-proximate and vaccine-unrelated febrile seizures didn’t differ significantly in terms of the proportion with a hospital length of stay greater than 1 day (20% vs. 15%), ICU admission (1.5% vs. 2.3%), seizure duration of more than 15 minutes (16% vs. 12%), repeat seizures within 24 hours (9% vs. 10%), or discharge on antiepileptic medication (4.4% vs. 4.3%).
In the developmental outcomes analysis, 62 of the children with vaccine-proximate febrile seizures, 70 with vaccine-unrelated febrile seizures, and 85 healthy controls with no seizure history underwent formal assessment using the third edition of the Bayley Scales of Infant and Toddler Development 12-24 months after their initial febrile seizure. Scores adjusted for years of maternal education were closely similar in all three groups across all five test domains: cognitive, language, motor, social-emotional, and general-adaptive.
Dr. Deng reported having no financial conflicts of interest regarding the study, which was partially funded by the Australian National Centre for Immunisation Research and Surveillance.
MALMO, SWEDEN – Children who experience a febrile seizure in conjunction with a vaccination have developmental outcomes comparable with those of children who have non–vaccine-related febrile seizures and healthy controls who’ve never had a febrile seizure, according to the first prospective case-control cohort study to examine the issue.
This finding has important implications for clinical practice, Lucy Deng, MD, observed at the annual meeting of the European Society for Paediatric Infectious Diseases.
“Febrile seizures associated with a vaccine can decrease parent and provider confidence in vaccine safety,” the pediatrician noted. Based upon her study results, however, physicians now can offer a truly evidence-based message of reassurance.
“If you have a child with a vaccine-related febrile seizure, you can give the same advice to those parents as for anyone else who’s had a febrile seizure, in that there is no difference in the clinical outcomes of vaccine-proximate and non–vaccine-proximate febrile seizures. Vaccine-proximate febrile seizures are usually brief, they don’t require any antiepileptic drugs, their length of stay is usually less than a day, and developmentally at 12-24 months post initial febrile seizure, they’re exactly the same as children who’ve never had a seizure before or who’ve had a non-vaccine-related febrile seizure,” said Dr. Deng of the National Centre for Immunisation Research and Surveillance in Sydney.
The impetus for her study was straightforward: “We all know that most children with a history of febrile seizures have normal behavior, intelligence, and academic achievement and do not later develop epilepsy. What we didn’t know before is if all of these facts apply to vaccine-proximate febrile seizures,” she explained.
The clinical severity analysis portion of this prospective case-control cohort study included 1,085 children with febrile seizures seen at five Australian children’s hospitals. Sixty-eight of them had vaccine-proximate febrile seizures, for a 6.6% rate. The febrile seizures in the other 1,027 children didn’t occur within 2 weeks following a vaccination.
Measles vaccine was implicated in 56 of the 68 children with vaccine-proximate febrile seizures, or 82%. Because Australian children receive their first measles-containing vaccine at age 12 months, the average age of the cohort with vaccine-proximate febrile seizures was 13 months, significantly younger than the 20-month average for children with non–vaccine-related febrile seizures.
In a multivariate analysis adjusted for patient age, gender, and history of prior afebrile seizures, the groups with vaccine-proximate and vaccine-unrelated febrile seizures didn’t differ significantly in terms of the proportion with a hospital length of stay greater than 1 day (20% vs. 15%), ICU admission (1.5% vs. 2.3%), seizure duration of more than 15 minutes (16% vs. 12%), repeat seizures within 24 hours (9% vs. 10%), or discharge on antiepileptic medication (4.4% vs. 4.3%).
In the developmental outcomes analysis, 62 of the children with vaccine-proximate febrile seizures, 70 with vaccine-unrelated febrile seizures, and 85 healthy controls with no seizure history underwent formal assessment using the third edition of the Bayley Scales of Infant and Toddler Development 12-24 months after their initial febrile seizure. Scores adjusted for years of maternal education were closely similar in all three groups across all five test domains: cognitive, language, motor, social-emotional, and general-adaptive.
Dr. Deng reported having no financial conflicts of interest regarding the study, which was partially funded by the Australian National Centre for Immunisation Research and Surveillance.
REPORTING FROM ESPID 2018
Key clinical point: Parents now can confidently be reassured that vaccine-proximate febrile seizures have no long-term consequences.
Major finding: as in controls with no seizure history.
Study details: This prospective case-control study comprised 1,180 children at five Australian children’s hospitals.
Disclosures: The study was partially funded by the Australian National Centre for Immunisation Research and Surveillance. The presenter reported having no financial conflicts.
Jump start immunizations in NICU
MALMO, SWEDEN – The neonatal intensive care unit often represents a lost opportunity to bring an infant fully up to date for recommended age-appropriate immunizations – but it needn’t be that way, Raymond C. Stetson, MD, declared at the annual meeting of the European Society for Paediatric Infectious Diseases.
“We were able to find that within our unit a small number of quality improvement measures enabled us to drastically increase our vaccination rate in this population. I think this shows that other units ought to be auditing their immunization rates, and if they find similar root causes of low rates our experience could be generalized to those units as well,” Dr. Stetson said.
It’s well established that premature infants are at increased risk for underimmunization. Dr. Stetson and his coinvestigators deemed the baseline 56% on-time immunization rate in their NICU patients to be unacceptable, because underimmunized infants are more vulnerable to vaccine-preventable illnesses after discharge. So using the quality improvement methodology known as DMAIC – for Define, Measure, Analyze, Improve, Control – the investigators surveyed Mayo NICU physicians and nurses and identified three root causes of the quality gap: lack of staff knowledge of the routine immunization schedule, lack of awareness of when a NICU patient’s vaccines were actually due, and parental vaccine hesitancy.
Session chair Karina Butler, MD, was clearly impressed.
“You make it sound so easy to get such an increment. What were the barriers and obstacles you ran into?” asked Dr. Butler of Temple Street Children’s University Hospital, Dublin.
“Certain providers in our group were a bit more hesitant about giving vaccines,” Dr. Stetson replied. “There had to be a lot of provider education to get them to use the resources we’d created. And parental vaccine hesitancy was a barrier for us. Of that 6% of infants who weren’t fully up to date at discharge, the majority of those were due to parental vaccine hesitancy. I think that’s still a barrier that’s going to need more work.”
Dr. Stetson reported having no relevant financial disclosures.
MALMO, SWEDEN – The neonatal intensive care unit often represents a lost opportunity to bring an infant fully up to date for recommended age-appropriate immunizations – but it needn’t be that way, Raymond C. Stetson, MD, declared at the annual meeting of the European Society for Paediatric Infectious Diseases.
“We were able to find that within our unit a small number of quality improvement measures enabled us to drastically increase our vaccination rate in this population. I think this shows that other units ought to be auditing their immunization rates, and if they find similar root causes of low rates our experience could be generalized to those units as well,” Dr. Stetson said.
It’s well established that premature infants are at increased risk for underimmunization. Dr. Stetson and his coinvestigators deemed the baseline 56% on-time immunization rate in their NICU patients to be unacceptable, because underimmunized infants are more vulnerable to vaccine-preventable illnesses after discharge. So using the quality improvement methodology known as DMAIC – for Define, Measure, Analyze, Improve, Control – the investigators surveyed Mayo NICU physicians and nurses and identified three root causes of the quality gap: lack of staff knowledge of the routine immunization schedule, lack of awareness of when a NICU patient’s vaccines were actually due, and parental vaccine hesitancy.
Session chair Karina Butler, MD, was clearly impressed.
“You make it sound so easy to get such an increment. What were the barriers and obstacles you ran into?” asked Dr. Butler of Temple Street Children’s University Hospital, Dublin.
“Certain providers in our group were a bit more hesitant about giving vaccines,” Dr. Stetson replied. “There had to be a lot of provider education to get them to use the resources we’d created. And parental vaccine hesitancy was a barrier for us. Of that 6% of infants who weren’t fully up to date at discharge, the majority of those were due to parental vaccine hesitancy. I think that’s still a barrier that’s going to need more work.”
Dr. Stetson reported having no relevant financial disclosures.
MALMO, SWEDEN – The neonatal intensive care unit often represents a lost opportunity to bring an infant fully up to date for recommended age-appropriate immunizations – but it needn’t be that way, Raymond C. Stetson, MD, declared at the annual meeting of the European Society for Paediatric Infectious Diseases.
“We were able to find that within our unit a small number of quality improvement measures enabled us to drastically increase our vaccination rate in this population. I think this shows that other units ought to be auditing their immunization rates, and if they find similar root causes of low rates our experience could be generalized to those units as well,” Dr. Stetson said.
It’s well established that premature infants are at increased risk for underimmunization. Dr. Stetson and his coinvestigators deemed the baseline 56% on-time immunization rate in their NICU patients to be unacceptable, because underimmunized infants are more vulnerable to vaccine-preventable illnesses after discharge. So using the quality improvement methodology known as DMAIC – for Define, Measure, Analyze, Improve, Control – the investigators surveyed Mayo NICU physicians and nurses and identified three root causes of the quality gap: lack of staff knowledge of the routine immunization schedule, lack of awareness of when a NICU patient’s vaccines were actually due, and parental vaccine hesitancy.
Session chair Karina Butler, MD, was clearly impressed.
“You make it sound so easy to get such an increment. What were the barriers and obstacles you ran into?” asked Dr. Butler of Temple Street Children’s University Hospital, Dublin.
“Certain providers in our group were a bit more hesitant about giving vaccines,” Dr. Stetson replied. “There had to be a lot of provider education to get them to use the resources we’d created. And parental vaccine hesitancy was a barrier for us. Of that 6% of infants who weren’t fully up to date at discharge, the majority of those were due to parental vaccine hesitancy. I think that’s still a barrier that’s going to need more work.”
Dr. Stetson reported having no relevant financial disclosures.
REPORTING FROM ESPID 2018
Key clinical point:
Major finding: Only 56% of 754 NICU patients from 2015 through mid-2017 were up to date for the ACIP-recommended vaccinations at discharge or transfer. After an intervention, the on-time immunization rate rose to 94% in 155 patients discharged during the first 6 months.
Study details: A study comparing 754 NICU patients prior to intervention and 155 after intervention.
Disclosures: Dr. Stetson reported having no relevant financial disclosures.
Source: Stetson R. E-Poster Discussion Session 04.