User login
European Academy of Dermatology & Venereology (EADV): Annual Congress
Relief for Brachioradial Pruritus
ISTANBUL, TURKEY – The capsaicin 8% patch known as Qutenza appears to be a novel and effective treatment for patients with brachioradial pruritus, according to a preliminary observational study.
"The big advantage of this new treatment strategy is the patient only needs one application of the patch instead of taking drugs daily. Most patients experience relief for 5-6 weeks afterward," Dr. Claudia Zeidler said at the annual congress of the European Academy of Dermatology and Venereology.
Brachioradial pruritus (BRP) is a neuropathic itch often accompanied by pain, burning, and stinging. The condition is localized to the dorsolateral forearms along the distribution of the C-5 or C-6 dermatomes. The condition is more common in women. Imaging studies of patients typically show spinal stenosis or other cervical spinal pathology entailing cervical nerve root compression, according to Dr. Zeidler of the University of Müenster, Germany.
She and her colleagues found that patients with BRP have significantly reduced intraepidermal nerve fiber density in lesional skin, compared with nonlesional skin on the ventral forearm. Patients also have reduced expression of transient receptor potential cation channel subfamily V, member 1 (TRPV1) in lesional skin, compared with nonlesional skin. TRPV1 affects the balance between keratinocyte differentiation and proliferation, and thus can influence epidermal barrier function.
Dr. Zeidler’s study enrolled 16 patients with an average BRP duration of 91 months who had undergone unsuccessful treatment attempts with various combinations of anticonvulsants, antidepressants, and antihistamines. Thirteen of the 16 patients were women, and ranged in age from 47 to 69 years. Affected skin areas were normal in appearance, aside from hyperpigmented scratch lesions that looked like prurigo nodularis in 12 patients. Magnetic resonance imaging showed 15 of the 16 patients had cervical nerve compression or degenerative changes.
Itching relief typically began the day after the in-office Qutenza treatment session. Three weeks post treatment, mean pruritus intensity scores on a 0-10 visual analog scale had dropped from 5.8 pretreatment to 1.1. Mean pain intensity scores fell from 4.2 to 0.8. Significant improvement in quality of life was documented via a reduction in Dermatology Life Quality Index scores from 8.9 pretreatment to 4.3. Levels of TRPV1 in lesional skin were significantly increased 3 weeks post treatment compared with baseline; however, intraepithelial nerve fiber density in lesional skin was not significantly different.
Side effects of the capsaicin 8% patch consisted of mild localized pain and burning lasting for 20 minutes to 2 days in 14 patients.
Additional patient follow-up at 2, 3, and 4 months indicated that the pruritus and pain relief typically lasted 5-6 weeks. Since the conclusion of the study, when symptoms return, patients come in for retreatment.
The 14-by-20-cm capsaicin 8% patch is approved in Europe for the treatment of nondiabetic neuropathic pain and in the United States for management of neuropathic pain associated with postherpetic neuralgia. The patch contains 179 mg of capsaicin. Dr. Zeidler and coworkers were careful to follow the detailed labeling instructions for the patch’s use.
The patch has to be administered by a physician or another health care professional. Self-treatment is not permitted. Removal of hair to promote patch adherence should be accomplished with the use of scissors, not by shaving. To reduce discomfort, the researchers applied topical lidocaine 2.5% for 1 hour prior to putting the patch directly on the itchy area. Upon removing the patch after 1 hour, a cleansing gel that comes with the patch was applied for 1 minute to remove any residual capsaicin.
Dr. Zeidler plans to conduct a randomized controlled trial in patients with BRP to confirm the observational study findings, and also to look at the long-term effects of repeated treatments. Her observational study was supported by Astellas Pharma. She reported having no financial conflicts.
ISTANBUL, TURKEY – The capsaicin 8% patch known as Qutenza appears to be a novel and effective treatment for patients with brachioradial pruritus, according to a preliminary observational study.
"The big advantage of this new treatment strategy is the patient only needs one application of the patch instead of taking drugs daily. Most patients experience relief for 5-6 weeks afterward," Dr. Claudia Zeidler said at the annual congress of the European Academy of Dermatology and Venereology.
Brachioradial pruritus (BRP) is a neuropathic itch often accompanied by pain, burning, and stinging. The condition is localized to the dorsolateral forearms along the distribution of the C-5 or C-6 dermatomes. The condition is more common in women. Imaging studies of patients typically show spinal stenosis or other cervical spinal pathology entailing cervical nerve root compression, according to Dr. Zeidler of the University of Müenster, Germany.
She and her colleagues found that patients with BRP have significantly reduced intraepidermal nerve fiber density in lesional skin, compared with nonlesional skin on the ventral forearm. Patients also have reduced expression of transient receptor potential cation channel subfamily V, member 1 (TRPV1) in lesional skin, compared with nonlesional skin. TRPV1 affects the balance between keratinocyte differentiation and proliferation, and thus can influence epidermal barrier function.
Dr. Zeidler’s study enrolled 16 patients with an average BRP duration of 91 months who had undergone unsuccessful treatment attempts with various combinations of anticonvulsants, antidepressants, and antihistamines. Thirteen of the 16 patients were women, and ranged in age from 47 to 69 years. Affected skin areas were normal in appearance, aside from hyperpigmented scratch lesions that looked like prurigo nodularis in 12 patients. Magnetic resonance imaging showed 15 of the 16 patients had cervical nerve compression or degenerative changes.
Itching relief typically began the day after the in-office Qutenza treatment session. Three weeks post treatment, mean pruritus intensity scores on a 0-10 visual analog scale had dropped from 5.8 pretreatment to 1.1. Mean pain intensity scores fell from 4.2 to 0.8. Significant improvement in quality of life was documented via a reduction in Dermatology Life Quality Index scores from 8.9 pretreatment to 4.3. Levels of TRPV1 in lesional skin were significantly increased 3 weeks post treatment compared with baseline; however, intraepithelial nerve fiber density in lesional skin was not significantly different.
Side effects of the capsaicin 8% patch consisted of mild localized pain and burning lasting for 20 minutes to 2 days in 14 patients.
Additional patient follow-up at 2, 3, and 4 months indicated that the pruritus and pain relief typically lasted 5-6 weeks. Since the conclusion of the study, when symptoms return, patients come in for retreatment.
The 14-by-20-cm capsaicin 8% patch is approved in Europe for the treatment of nondiabetic neuropathic pain and in the United States for management of neuropathic pain associated with postherpetic neuralgia. The patch contains 179 mg of capsaicin. Dr. Zeidler and coworkers were careful to follow the detailed labeling instructions for the patch’s use.
The patch has to be administered by a physician or another health care professional. Self-treatment is not permitted. Removal of hair to promote patch adherence should be accomplished with the use of scissors, not by shaving. To reduce discomfort, the researchers applied topical lidocaine 2.5% for 1 hour prior to putting the patch directly on the itchy area. Upon removing the patch after 1 hour, a cleansing gel that comes with the patch was applied for 1 minute to remove any residual capsaicin.
Dr. Zeidler plans to conduct a randomized controlled trial in patients with BRP to confirm the observational study findings, and also to look at the long-term effects of repeated treatments. Her observational study was supported by Astellas Pharma. She reported having no financial conflicts.
ISTANBUL, TURKEY – The capsaicin 8% patch known as Qutenza appears to be a novel and effective treatment for patients with brachioradial pruritus, according to a preliminary observational study.
"The big advantage of this new treatment strategy is the patient only needs one application of the patch instead of taking drugs daily. Most patients experience relief for 5-6 weeks afterward," Dr. Claudia Zeidler said at the annual congress of the European Academy of Dermatology and Venereology.
Brachioradial pruritus (BRP) is a neuropathic itch often accompanied by pain, burning, and stinging. The condition is localized to the dorsolateral forearms along the distribution of the C-5 or C-6 dermatomes. The condition is more common in women. Imaging studies of patients typically show spinal stenosis or other cervical spinal pathology entailing cervical nerve root compression, according to Dr. Zeidler of the University of Müenster, Germany.
She and her colleagues found that patients with BRP have significantly reduced intraepidermal nerve fiber density in lesional skin, compared with nonlesional skin on the ventral forearm. Patients also have reduced expression of transient receptor potential cation channel subfamily V, member 1 (TRPV1) in lesional skin, compared with nonlesional skin. TRPV1 affects the balance between keratinocyte differentiation and proliferation, and thus can influence epidermal barrier function.
Dr. Zeidler’s study enrolled 16 patients with an average BRP duration of 91 months who had undergone unsuccessful treatment attempts with various combinations of anticonvulsants, antidepressants, and antihistamines. Thirteen of the 16 patients were women, and ranged in age from 47 to 69 years. Affected skin areas were normal in appearance, aside from hyperpigmented scratch lesions that looked like prurigo nodularis in 12 patients. Magnetic resonance imaging showed 15 of the 16 patients had cervical nerve compression or degenerative changes.
Itching relief typically began the day after the in-office Qutenza treatment session. Three weeks post treatment, mean pruritus intensity scores on a 0-10 visual analog scale had dropped from 5.8 pretreatment to 1.1. Mean pain intensity scores fell from 4.2 to 0.8. Significant improvement in quality of life was documented via a reduction in Dermatology Life Quality Index scores from 8.9 pretreatment to 4.3. Levels of TRPV1 in lesional skin were significantly increased 3 weeks post treatment compared with baseline; however, intraepithelial nerve fiber density in lesional skin was not significantly different.
Side effects of the capsaicin 8% patch consisted of mild localized pain and burning lasting for 20 minutes to 2 days in 14 patients.
Additional patient follow-up at 2, 3, and 4 months indicated that the pruritus and pain relief typically lasted 5-6 weeks. Since the conclusion of the study, when symptoms return, patients come in for retreatment.
The 14-by-20-cm capsaicin 8% patch is approved in Europe for the treatment of nondiabetic neuropathic pain and in the United States for management of neuropathic pain associated with postherpetic neuralgia. The patch contains 179 mg of capsaicin. Dr. Zeidler and coworkers were careful to follow the detailed labeling instructions for the patch’s use.
The patch has to be administered by a physician or another health care professional. Self-treatment is not permitted. Removal of hair to promote patch adherence should be accomplished with the use of scissors, not by shaving. To reduce discomfort, the researchers applied topical lidocaine 2.5% for 1 hour prior to putting the patch directly on the itchy area. Upon removing the patch after 1 hour, a cleansing gel that comes with the patch was applied for 1 minute to remove any residual capsaicin.
Dr. Zeidler plans to conduct a randomized controlled trial in patients with BRP to confirm the observational study findings, and also to look at the long-term effects of repeated treatments. Her observational study was supported by Astellas Pharma. She reported having no financial conflicts.
AT THE EADV CONGRESS
Capsaicin 8% patch offers relief for brachioradial pruritus
ISTANBUL, TURKEY – The capsaicin 8% patch known as Qutenza appears to be a novel and effective treatment for patients with brachioradial pruritus, according to a preliminary observational study.
"The big advantage of this new treatment strategy is the patient only needs one application of the patch instead of taking drugs daily. Most patients experience relief for 5-6 weeks afterward," Dr. Claudia Zeidler said at the annual congress of the European Academy of Dermatology and Venereology.
Brachioradial pruritus (BRP) is a neuropathic itch often accompanied by pain, burning, and stinging. The condition is localized to the dorsolateral forearms along the distribution of the C-5 or C-6 dermatomes. The condition is more common in women. Imaging studies of patients typically show spinal stenosis or other cervical spinal pathology entailing cervical nerve root compression, according to Dr. Zeidler of the University of Müenster, Germany.
She and her colleagues found that patients with BRP have significantly reduced intraepidermal nerve fiber density in lesional skin, compared with nonlesional skin on the ventral forearm. Patients also have reduced expression of transient receptor potential cation channel subfamily V, member 1 (TRPV1) in lesional skin, compared with nonlesional skin. TRPV1 affects the balance between keratinocyte differentiation and proliferation, and thus can influence epidermal barrier function.
Dr. Zeidler’s study enrolled 16 patients with an average BRP duration of 91 months who had undergone unsuccessful treatment attempts with various combinations of anticonvulsants, antidepressants, and antihistamines. Thirteen of the 16 patients were women, and ranged in age from 47 to 69 years. Affected skin areas were normal in appearance, aside from hyperpigmented scratch lesions that looked like prurigo nodularis in 12 patients. Magnetic resonance imaging showed 15 of the 16 patients had cervical nerve compression or degenerative changes.
Itching relief typically began the day after the in-office Qutenza treatment session. Three weeks post treatment, mean pruritus intensity scores on a 0-10 visual analog scale had dropped from 5.8 pretreatment to 1.1. Mean pain intensity scores fell from 4.2 to 0.8. Significant improvement in quality of life was documented via a reduction in Dermatology Life Quality Index scores from 8.9 pretreatment to 4.3. Levels of TRPV1 in lesional skin were significantly increased 3 weeks post treatment compared with baseline; however, intraepithelial nerve fiber density in lesional skin was not significantly different.
Side effects of the capsaicin 8% patch consisted of mild localized pain and burning lasting for 20 minutes to 2 days in 14 patients.
Additional patient follow-up at 2, 3, and 4 months indicated that the pruritus and pain relief typically lasted 5-6 weeks. Since the conclusion of the study, when symptoms return, patients come in for retreatment.
The 14-by-20-cm capsaicin 8% patch is approved in Europe for the treatment of nondiabetic neuropathic pain and in the United States for management of neuropathic pain associated with postherpetic neuralgia. The patch contains 179 mg of capsaicin. Dr. Zeidler and coworkers were careful to follow the detailed labeling instructions for the patch’s use.
The patch has to be administered by a physician or another health care professional. Self-treatment is not permitted. Removal of hair to promote patch adherence should be accomplished with the use of scissors, not by shaving. To reduce discomfort, the researchers applied topical lidocaine 2.5% for 1 hour prior to putting the patch directly on the itchy area. Upon removing the patch after 1 hour, a cleansing gel that comes with the patch was applied for 1 minute to remove any residual capsaicin.
Dr. Zeidler plans to conduct a randomized controlled trial in patients with BRP to confirm the observational study findings, and also to look at the long-term effects of repeated treatments. Her observational study was supported by Astellas Pharma. She reported having no financial conflicts.
ISTANBUL, TURKEY – The capsaicin 8% patch known as Qutenza appears to be a novel and effective treatment for patients with brachioradial pruritus, according to a preliminary observational study.
"The big advantage of this new treatment strategy is the patient only needs one application of the patch instead of taking drugs daily. Most patients experience relief for 5-6 weeks afterward," Dr. Claudia Zeidler said at the annual congress of the European Academy of Dermatology and Venereology.
Brachioradial pruritus (BRP) is a neuropathic itch often accompanied by pain, burning, and stinging. The condition is localized to the dorsolateral forearms along the distribution of the C-5 or C-6 dermatomes. The condition is more common in women. Imaging studies of patients typically show spinal stenosis or other cervical spinal pathology entailing cervical nerve root compression, according to Dr. Zeidler of the University of Müenster, Germany.
She and her colleagues found that patients with BRP have significantly reduced intraepidermal nerve fiber density in lesional skin, compared with nonlesional skin on the ventral forearm. Patients also have reduced expression of transient receptor potential cation channel subfamily V, member 1 (TRPV1) in lesional skin, compared with nonlesional skin. TRPV1 affects the balance between keratinocyte differentiation and proliferation, and thus can influence epidermal barrier function.
Dr. Zeidler’s study enrolled 16 patients with an average BRP duration of 91 months who had undergone unsuccessful treatment attempts with various combinations of anticonvulsants, antidepressants, and antihistamines. Thirteen of the 16 patients were women, and ranged in age from 47 to 69 years. Affected skin areas were normal in appearance, aside from hyperpigmented scratch lesions that looked like prurigo nodularis in 12 patients. Magnetic resonance imaging showed 15 of the 16 patients had cervical nerve compression or degenerative changes.
Itching relief typically began the day after the in-office Qutenza treatment session. Three weeks post treatment, mean pruritus intensity scores on a 0-10 visual analog scale had dropped from 5.8 pretreatment to 1.1. Mean pain intensity scores fell from 4.2 to 0.8. Significant improvement in quality of life was documented via a reduction in Dermatology Life Quality Index scores from 8.9 pretreatment to 4.3. Levels of TRPV1 in lesional skin were significantly increased 3 weeks post treatment compared with baseline; however, intraepithelial nerve fiber density in lesional skin was not significantly different.
Side effects of the capsaicin 8% patch consisted of mild localized pain and burning lasting for 20 minutes to 2 days in 14 patients.
Additional patient follow-up at 2, 3, and 4 months indicated that the pruritus and pain relief typically lasted 5-6 weeks. Since the conclusion of the study, when symptoms return, patients come in for retreatment.
The 14-by-20-cm capsaicin 8% patch is approved in Europe for the treatment of nondiabetic neuropathic pain and in the United States for management of neuropathic pain associated with postherpetic neuralgia. The patch contains 179 mg of capsaicin. Dr. Zeidler and coworkers were careful to follow the detailed labeling instructions for the patch’s use.
The patch has to be administered by a physician or another health care professional. Self-treatment is not permitted. Removal of hair to promote patch adherence should be accomplished with the use of scissors, not by shaving. To reduce discomfort, the researchers applied topical lidocaine 2.5% for 1 hour prior to putting the patch directly on the itchy area. Upon removing the patch after 1 hour, a cleansing gel that comes with the patch was applied for 1 minute to remove any residual capsaicin.
Dr. Zeidler plans to conduct a randomized controlled trial in patients with BRP to confirm the observational study findings, and also to look at the long-term effects of repeated treatments. Her observational study was supported by Astellas Pharma. She reported having no financial conflicts.
ISTANBUL, TURKEY – The capsaicin 8% patch known as Qutenza appears to be a novel and effective treatment for patients with brachioradial pruritus, according to a preliminary observational study.
"The big advantage of this new treatment strategy is the patient only needs one application of the patch instead of taking drugs daily. Most patients experience relief for 5-6 weeks afterward," Dr. Claudia Zeidler said at the annual congress of the European Academy of Dermatology and Venereology.
Brachioradial pruritus (BRP) is a neuropathic itch often accompanied by pain, burning, and stinging. The condition is localized to the dorsolateral forearms along the distribution of the C-5 or C-6 dermatomes. The condition is more common in women. Imaging studies of patients typically show spinal stenosis or other cervical spinal pathology entailing cervical nerve root compression, according to Dr. Zeidler of the University of Müenster, Germany.
She and her colleagues found that patients with BRP have significantly reduced intraepidermal nerve fiber density in lesional skin, compared with nonlesional skin on the ventral forearm. Patients also have reduced expression of transient receptor potential cation channel subfamily V, member 1 (TRPV1) in lesional skin, compared with nonlesional skin. TRPV1 affects the balance between keratinocyte differentiation and proliferation, and thus can influence epidermal barrier function.
Dr. Zeidler’s study enrolled 16 patients with an average BRP duration of 91 months who had undergone unsuccessful treatment attempts with various combinations of anticonvulsants, antidepressants, and antihistamines. Thirteen of the 16 patients were women, and ranged in age from 47 to 69 years. Affected skin areas were normal in appearance, aside from hyperpigmented scratch lesions that looked like prurigo nodularis in 12 patients. Magnetic resonance imaging showed 15 of the 16 patients had cervical nerve compression or degenerative changes.
Itching relief typically began the day after the in-office Qutenza treatment session. Three weeks post treatment, mean pruritus intensity scores on a 0-10 visual analog scale had dropped from 5.8 pretreatment to 1.1. Mean pain intensity scores fell from 4.2 to 0.8. Significant improvement in quality of life was documented via a reduction in Dermatology Life Quality Index scores from 8.9 pretreatment to 4.3. Levels of TRPV1 in lesional skin were significantly increased 3 weeks post treatment compared with baseline; however, intraepithelial nerve fiber density in lesional skin was not significantly different.
Side effects of the capsaicin 8% patch consisted of mild localized pain and burning lasting for 20 minutes to 2 days in 14 patients.
Additional patient follow-up at 2, 3, and 4 months indicated that the pruritus and pain relief typically lasted 5-6 weeks. Since the conclusion of the study, when symptoms return, patients come in for retreatment.
The 14-by-20-cm capsaicin 8% patch is approved in Europe for the treatment of nondiabetic neuropathic pain and in the United States for management of neuropathic pain associated with postherpetic neuralgia. The patch contains 179 mg of capsaicin. Dr. Zeidler and coworkers were careful to follow the detailed labeling instructions for the patch’s use.
The patch has to be administered by a physician or another health care professional. Self-treatment is not permitted. Removal of hair to promote patch adherence should be accomplished with the use of scissors, not by shaving. To reduce discomfort, the researchers applied topical lidocaine 2.5% for 1 hour prior to putting the patch directly on the itchy area. Upon removing the patch after 1 hour, a cleansing gel that comes with the patch was applied for 1 minute to remove any residual capsaicin.
Dr. Zeidler plans to conduct a randomized controlled trial in patients with BRP to confirm the observational study findings, and also to look at the long-term effects of repeated treatments. Her observational study was supported by Astellas Pharma. She reported having no financial conflicts.
AT THE EADV CONGRESS
Major finding: Three weeks after a single hour-long application of the capsaicin 8% patch known as Qutenza, mean itch intensity on a 0-10 visual analog scale in patients with brachioradial pruritus was 1.1, compared with 5.8 pretreatment.
Data source: An uncontrolled prospective observational study involving 16 treated patients with longstanding brachioradial pruritus refractory to various combinations of anticonvulsants, antihistamines, and antidepressants.
Disclosures: The investigator-initiated study was supported by Astellas Pharma. The presenter reported having no financial conflicts.
New Culprit in Allergic Dermatitis
ISTANBUL, TURKEY – Oxidized R-limonene, found in more than 60% of personal care and hygiene products, has been found to be a major cause of fragrance allergy, according to the findings of a large, international, patch test study.
Overall, 5.2% of almost 3,000 patients had a positive patch test result for oxidized limonene, and 37% of those reactions were deemed to be clinically relevant. Shampoos, soaps, perfumes, domestic cleaners, sunscreens, and massage creams are among the household products likely to contain limonene, Dr. Johanna Bråred Christensson said at the annual congress of the European Academy of Dermatology and Venereology. R-limonene is also used in high concentrations in industry as a solvent and degreaser.
R-limonene is structurally a fragrance terpene, with a citrus odor. It is found in nature and produced industrially in mass quantities. In its pure form, R-limonene is nonallergenic or at most a weak allergen. However, when R-limonene is oxidized, it includes limonene hydroperoxides, which Dr. Christensson found to be a contact allergen. This oxidation can occur during storage or handling of R-limonene. The hydroperoxides are often already present in containers of R-limonene when shipped from chemical plants to manufacturers of consumer products, according to Dr. Christensson, a dermatologist at the University of Gothenburg (Sweden).
A stable oxidized R-limonene 3.0% in petrolatum with a controlled 0.33% concentration of limonene hydroperoxides, which is commercially available as a patch test material, was used in the patch test study of 2,900 consecutive dermatitis patients who presented to contact dermatitis clinics in Sweden, Denmark, the United Kingdom, Spain, Australia, and Singapore. Participants completed a questionnaire assessing the relevance of a positive patch test result as indicated by exposure to limonene-containing products on the area of their dermatitis.
The study was funded by grants from national allergy research centers. Dr. Christensson reported having no relevant financial conflicts.
ISTANBUL, TURKEY – Oxidized R-limonene, found in more than 60% of personal care and hygiene products, has been found to be a major cause of fragrance allergy, according to the findings of a large, international, patch test study.
Overall, 5.2% of almost 3,000 patients had a positive patch test result for oxidized limonene, and 37% of those reactions were deemed to be clinically relevant. Shampoos, soaps, perfumes, domestic cleaners, sunscreens, and massage creams are among the household products likely to contain limonene, Dr. Johanna Bråred Christensson said at the annual congress of the European Academy of Dermatology and Venereology. R-limonene is also used in high concentrations in industry as a solvent and degreaser.
R-limonene is structurally a fragrance terpene, with a citrus odor. It is found in nature and produced industrially in mass quantities. In its pure form, R-limonene is nonallergenic or at most a weak allergen. However, when R-limonene is oxidized, it includes limonene hydroperoxides, which Dr. Christensson found to be a contact allergen. This oxidation can occur during storage or handling of R-limonene. The hydroperoxides are often already present in containers of R-limonene when shipped from chemical plants to manufacturers of consumer products, according to Dr. Christensson, a dermatologist at the University of Gothenburg (Sweden).
A stable oxidized R-limonene 3.0% in petrolatum with a controlled 0.33% concentration of limonene hydroperoxides, which is commercially available as a patch test material, was used in the patch test study of 2,900 consecutive dermatitis patients who presented to contact dermatitis clinics in Sweden, Denmark, the United Kingdom, Spain, Australia, and Singapore. Participants completed a questionnaire assessing the relevance of a positive patch test result as indicated by exposure to limonene-containing products on the area of their dermatitis.
The study was funded by grants from national allergy research centers. Dr. Christensson reported having no relevant financial conflicts.
ISTANBUL, TURKEY – Oxidized R-limonene, found in more than 60% of personal care and hygiene products, has been found to be a major cause of fragrance allergy, according to the findings of a large, international, patch test study.
Overall, 5.2% of almost 3,000 patients had a positive patch test result for oxidized limonene, and 37% of those reactions were deemed to be clinically relevant. Shampoos, soaps, perfumes, domestic cleaners, sunscreens, and massage creams are among the household products likely to contain limonene, Dr. Johanna Bråred Christensson said at the annual congress of the European Academy of Dermatology and Venereology. R-limonene is also used in high concentrations in industry as a solvent and degreaser.
R-limonene is structurally a fragrance terpene, with a citrus odor. It is found in nature and produced industrially in mass quantities. In its pure form, R-limonene is nonallergenic or at most a weak allergen. However, when R-limonene is oxidized, it includes limonene hydroperoxides, which Dr. Christensson found to be a contact allergen. This oxidation can occur during storage or handling of R-limonene. The hydroperoxides are often already present in containers of R-limonene when shipped from chemical plants to manufacturers of consumer products, according to Dr. Christensson, a dermatologist at the University of Gothenburg (Sweden).
A stable oxidized R-limonene 3.0% in petrolatum with a controlled 0.33% concentration of limonene hydroperoxides, which is commercially available as a patch test material, was used in the patch test study of 2,900 consecutive dermatitis patients who presented to contact dermatitis clinics in Sweden, Denmark, the United Kingdom, Spain, Australia, and Singapore. Participants completed a questionnaire assessing the relevance of a positive patch test result as indicated by exposure to limonene-containing products on the area of their dermatitis.
The study was funded by grants from national allergy research centers. Dr. Christensson reported having no relevant financial conflicts.
AT THE EADV CONGRESS
Oxidized limonene: New culprit in allergic dermatitis
ISTANBUL, TURKEY – Oxidized R-limonene, found in more than 60% of personal care and hygiene products, has been found to be a major cause of fragrance allergy, according to the findings of a large, international, patch test study.
Overall, 5.2% of almost 3,000 patients had a positive patch test result for oxidized limonene, and 37% of those reactions were deemed to be clinically relevant. Shampoos, soaps, perfumes, domestic cleaners, sunscreens, and massage creams are among the household products likely to contain limonene, Dr. Johanna Bråred Christensson said at the annual congress of the European Academy of Dermatology and Venereology. R-limonene is also used in high concentrations in industry as a solvent and degreaser.
R-limonene is structurally a fragrance terpene, with a citrus odor. It is found in nature and produced industrially in mass quantities. In its pure form, R-limonene is nonallergenic or at most a weak allergen. However, when R-limonene is oxidized, it includes limonene hydroperoxides, which Dr. Christensson found to be a contact allergen. This oxidation can occur during storage or handling of R-limonene. The hydroperoxides are often already present in containers of R-limonene when shipped from chemical plants to manufacturers of consumer products, according to Dr. Christensson, a dermatologist at the University of Gothenburg (Sweden).
A stable oxidized R-limonene 3.0% in petrolatum with a controlled 0.33% concentration of limonene hydroperoxides, which is commercially available as a patch test material, was used in the patch test study of 2,900 consecutive dermatitis patients who presented to contact dermatitis clinics in Sweden, Denmark, the United Kingdom, Spain, Australia, and Singapore. Participants completed a questionnaire assessing the relevance of a positive patch test result as indicated by exposure to limonene-containing products on the area of their dermatitis.
The study was funded by grants from national allergy research centers. Dr. Christensson reported having no relevant financial conflicts.
ISTANBUL, TURKEY – Oxidized R-limonene, found in more than 60% of personal care and hygiene products, has been found to be a major cause of fragrance allergy, according to the findings of a large, international, patch test study.
Overall, 5.2% of almost 3,000 patients had a positive patch test result for oxidized limonene, and 37% of those reactions were deemed to be clinically relevant. Shampoos, soaps, perfumes, domestic cleaners, sunscreens, and massage creams are among the household products likely to contain limonene, Dr. Johanna Bråred Christensson said at the annual congress of the European Academy of Dermatology and Venereology. R-limonene is also used in high concentrations in industry as a solvent and degreaser.
R-limonene is structurally a fragrance terpene, with a citrus odor. It is found in nature and produced industrially in mass quantities. In its pure form, R-limonene is nonallergenic or at most a weak allergen. However, when R-limonene is oxidized, it includes limonene hydroperoxides, which Dr. Christensson found to be a contact allergen. This oxidation can occur during storage or handling of R-limonene. The hydroperoxides are often already present in containers of R-limonene when shipped from chemical plants to manufacturers of consumer products, according to Dr. Christensson, a dermatologist at the University of Gothenburg (Sweden).
A stable oxidized R-limonene 3.0% in petrolatum with a controlled 0.33% concentration of limonene hydroperoxides, which is commercially available as a patch test material, was used in the patch test study of 2,900 consecutive dermatitis patients who presented to contact dermatitis clinics in Sweden, Denmark, the United Kingdom, Spain, Australia, and Singapore. Participants completed a questionnaire assessing the relevance of a positive patch test result as indicated by exposure to limonene-containing products on the area of their dermatitis.
The study was funded by grants from national allergy research centers. Dr. Christensson reported having no relevant financial conflicts.
ISTANBUL, TURKEY – Oxidized R-limonene, found in more than 60% of personal care and hygiene products, has been found to be a major cause of fragrance allergy, according to the findings of a large, international, patch test study.
Overall, 5.2% of almost 3,000 patients had a positive patch test result for oxidized limonene, and 37% of those reactions were deemed to be clinically relevant. Shampoos, soaps, perfumes, domestic cleaners, sunscreens, and massage creams are among the household products likely to contain limonene, Dr. Johanna Bråred Christensson said at the annual congress of the European Academy of Dermatology and Venereology. R-limonene is also used in high concentrations in industry as a solvent and degreaser.
R-limonene is structurally a fragrance terpene, with a citrus odor. It is found in nature and produced industrially in mass quantities. In its pure form, R-limonene is nonallergenic or at most a weak allergen. However, when R-limonene is oxidized, it includes limonene hydroperoxides, which Dr. Christensson found to be a contact allergen. This oxidation can occur during storage or handling of R-limonene. The hydroperoxides are often already present in containers of R-limonene when shipped from chemical plants to manufacturers of consumer products, according to Dr. Christensson, a dermatologist at the University of Gothenburg (Sweden).
A stable oxidized R-limonene 3.0% in petrolatum with a controlled 0.33% concentration of limonene hydroperoxides, which is commercially available as a patch test material, was used in the patch test study of 2,900 consecutive dermatitis patients who presented to contact dermatitis clinics in Sweden, Denmark, the United Kingdom, Spain, Australia, and Singapore. Participants completed a questionnaire assessing the relevance of a positive patch test result as indicated by exposure to limonene-containing products on the area of their dermatitis.
The study was funded by grants from national allergy research centers. Dr. Christensson reported having no relevant financial conflicts.
AT THE EADV CONGRESS
Major finding: More than 5% of 2,900 consecutive patch test patients had a positive result for oxidized limonene. More than one-third of the positive results were deemed clinically relevant.
Data source: A prospective study involving 2,900 consecutive dermatitis patients who underwent patch testing at contact dermatitis clinics in six countries.
Disclosures: The study was funded by grants from national allergy research centers. Dr. Christensson reported having no relevant financial conflicts.
Novel topical agent reduces chronic itch
ISTANBUL, TURKEY – A first-in-class topical tyrosine kinase inhibitor markedly reduced chronic pruritus in psoriasis patients in a phase-IIb study.
Moreover, the investigational agent, known for now as CT327, produced no application site reactions or indeed any other adverse events. Nor was it absorbed systemically, Dr. David Roblin reported at the annual congress of the European Academy of Dermatology and Venereology.
A significant unmet need exists for safe and effective therapies for chronic itching, not only in psoriasis but in other diseases for which chronic pruritus figures prominently and has a debilitating effect on quality of life. At present, there is no medication with an indication for treatment of chronic pruritus, noted Dr. Roblin, chief medical officer at Creabilis in Canterbury, England.
Tyrosine kinase is a high-affinity receptor of nerve growth factor. Thus, CT327 targets the sensory neurons implicated in the pathogenesis of chronic pruritis, he explained.
The multicenter, randomized, double-blind clinical trial included 160 patients with mild-to-moderate psoriasis affecting up to 10% of their body surface area. They were assigned to twice-daily application of CT327 at 0.05%, 0.1%, or 0.5% or to the vehicle for 8 weeks. Participants treated all their plaques except those on the face or scalp.
The safety assessment included all 160 patients; however, efficacy endpoints were assessed only in the 108 with at least moderate baseline pruritus as defined by a score greater than 40 mm on a 0-100 mm visual analog scale (VAS).
From a baseline mean pruritus VAS of 65.2, scores decreased at week 8 by 37.1 mm in the CT327 0.05% group, 31.5 mm in patients using CT327 0.1%, 36.4 mm in the 0.5% group, and 16.1 mm in vehicle-treated controls. A 20-mm reduction on the VAS is considered clinically meaningful. Four weeks after treatment stopped, pruritus scores had returned to baseline.
The 108 patients with at least moderate baseline pruritus had a baseline mean Psoriasis Area and Severity Index score, modified to exclude the untreated face and scalp (mPASI), of 9.3. At 8 weeks, patients in the CT327 0.05%, 0.1%, and 0.5% groups averaged mPASI score reductions of 46%, 36%, and 37%, respectively, compared with a 17% drop in the control group.
Of note, there was no correlation between baseline mPASI and pruritus severity scores, which suggests that trying to treat pruritus with agents directed at the inflammatory aspect of psoriasis may be a suboptimal strategy. It’s better to target the sensory neurons involved in the itch, according to Dr. Roblin.
The study was sponsored by Creabilis, which is developing CT327. Dr. Roblin is the company’s medical director.
ISTANBUL, TURKEY – A first-in-class topical tyrosine kinase inhibitor markedly reduced chronic pruritus in psoriasis patients in a phase-IIb study.
Moreover, the investigational agent, known for now as CT327, produced no application site reactions or indeed any other adverse events. Nor was it absorbed systemically, Dr. David Roblin reported at the annual congress of the European Academy of Dermatology and Venereology.
A significant unmet need exists for safe and effective therapies for chronic itching, not only in psoriasis but in other diseases for which chronic pruritus figures prominently and has a debilitating effect on quality of life. At present, there is no medication with an indication for treatment of chronic pruritus, noted Dr. Roblin, chief medical officer at Creabilis in Canterbury, England.
Tyrosine kinase is a high-affinity receptor of nerve growth factor. Thus, CT327 targets the sensory neurons implicated in the pathogenesis of chronic pruritis, he explained.
The multicenter, randomized, double-blind clinical trial included 160 patients with mild-to-moderate psoriasis affecting up to 10% of their body surface area. They were assigned to twice-daily application of CT327 at 0.05%, 0.1%, or 0.5% or to the vehicle for 8 weeks. Participants treated all their plaques except those on the face or scalp.
The safety assessment included all 160 patients; however, efficacy endpoints were assessed only in the 108 with at least moderate baseline pruritus as defined by a score greater than 40 mm on a 0-100 mm visual analog scale (VAS).
From a baseline mean pruritus VAS of 65.2, scores decreased at week 8 by 37.1 mm in the CT327 0.05% group, 31.5 mm in patients using CT327 0.1%, 36.4 mm in the 0.5% group, and 16.1 mm in vehicle-treated controls. A 20-mm reduction on the VAS is considered clinically meaningful. Four weeks after treatment stopped, pruritus scores had returned to baseline.
The 108 patients with at least moderate baseline pruritus had a baseline mean Psoriasis Area and Severity Index score, modified to exclude the untreated face and scalp (mPASI), of 9.3. At 8 weeks, patients in the CT327 0.05%, 0.1%, and 0.5% groups averaged mPASI score reductions of 46%, 36%, and 37%, respectively, compared with a 17% drop in the control group.
Of note, there was no correlation between baseline mPASI and pruritus severity scores, which suggests that trying to treat pruritus with agents directed at the inflammatory aspect of psoriasis may be a suboptimal strategy. It’s better to target the sensory neurons involved in the itch, according to Dr. Roblin.
The study was sponsored by Creabilis, which is developing CT327. Dr. Roblin is the company’s medical director.
ISTANBUL, TURKEY – A first-in-class topical tyrosine kinase inhibitor markedly reduced chronic pruritus in psoriasis patients in a phase-IIb study.
Moreover, the investigational agent, known for now as CT327, produced no application site reactions or indeed any other adverse events. Nor was it absorbed systemically, Dr. David Roblin reported at the annual congress of the European Academy of Dermatology and Venereology.
A significant unmet need exists for safe and effective therapies for chronic itching, not only in psoriasis but in other diseases for which chronic pruritus figures prominently and has a debilitating effect on quality of life. At present, there is no medication with an indication for treatment of chronic pruritus, noted Dr. Roblin, chief medical officer at Creabilis in Canterbury, England.
Tyrosine kinase is a high-affinity receptor of nerve growth factor. Thus, CT327 targets the sensory neurons implicated in the pathogenesis of chronic pruritis, he explained.
The multicenter, randomized, double-blind clinical trial included 160 patients with mild-to-moderate psoriasis affecting up to 10% of their body surface area. They were assigned to twice-daily application of CT327 at 0.05%, 0.1%, or 0.5% or to the vehicle for 8 weeks. Participants treated all their plaques except those on the face or scalp.
The safety assessment included all 160 patients; however, efficacy endpoints were assessed only in the 108 with at least moderate baseline pruritus as defined by a score greater than 40 mm on a 0-100 mm visual analog scale (VAS).
From a baseline mean pruritus VAS of 65.2, scores decreased at week 8 by 37.1 mm in the CT327 0.05% group, 31.5 mm in patients using CT327 0.1%, 36.4 mm in the 0.5% group, and 16.1 mm in vehicle-treated controls. A 20-mm reduction on the VAS is considered clinically meaningful. Four weeks after treatment stopped, pruritus scores had returned to baseline.
The 108 patients with at least moderate baseline pruritus had a baseline mean Psoriasis Area and Severity Index score, modified to exclude the untreated face and scalp (mPASI), of 9.3. At 8 weeks, patients in the CT327 0.05%, 0.1%, and 0.5% groups averaged mPASI score reductions of 46%, 36%, and 37%, respectively, compared with a 17% drop in the control group.
Of note, there was no correlation between baseline mPASI and pruritus severity scores, which suggests that trying to treat pruritus with agents directed at the inflammatory aspect of psoriasis may be a suboptimal strategy. It’s better to target the sensory neurons involved in the itch, according to Dr. Roblin.
The study was sponsored by Creabilis, which is developing CT327. Dr. Roblin is the company’s medical director.
AT THE EADV CONGRESS
Major finding: Treatment of chronic pruritus using various doses of CT327, a novel topical tyrosine kinase inhibitor, resulted in pruritus score reductions of 32-37 points from a mean baseline of 65 on a 0-100 scale.
Data source: This was a randomized, double-blind, multicenter, phase-IIb study including 160 patients with mild-to-moderate psoriasis who applied the investigational agent or its vehicle to their plaques twice daily for 8 weeks.
Disclosures: The study was sponsored by Creabilis. Dr. Roblin is the company’s chief medical officer.
Psoriasis drug research aimed at severe disease
ISTANBUL, TURKEY – The current intensive period of new drug development in psoriasis is cause for enthusiastic celebration by patients and physicians alike. That is, except for one glaring research gap.
Almost all of the promising drugs now in phase-II or -III clinical trials are systemic agents aimed at the minority of psoriasis patients with more severe disease. There’s not nearly as much in the pipeline for the many patients with mild psoriasis who are most appropriately treated with topical therapy, Dr. Hervé Bachelez observed at the annual congress of the European Academy of Dermatology and Venereology.
Indeed, he identified only two promising topical agents advancing through the developmental pipeline: Pfizer’s tofacitinib, a small-molecule Janus kinase inhibitor under study in both topical and oral formulations, and Anacor’s AN2728, a boron-containing molecule that inhibits phosphodiesterase-4 (PDE-4).
Industry’s lack of enthusiasm for developing new topical agents is difficult to understand, given that 84% of all U.S. prescriptions written for psoriasis in 2009 were for topical agents, according to IMS. Moreover, patients clearly believe there is a major unmet need for new and better topicals: a recent survey of 2,151 European psoriasis patients and their dermatologists found that only 45% of patients on topical therapy were satisfied with their treatment. And a mere 35% of dermatologists rated current topical treatments as satisfactory (J. Dermatolog. Treat. 2013;24:193-8).
The great bulk of the action in psoriasis drug development now is focused on injectable biologics and oral small molecules. Among them are three interleukin-17 inhibitors: Novartis’ secukinumab, Amgen’s brodalumab, and Eli Lilly’s ixekizumab. Celgene is developing apremilast as an oral PDE-4 inhibitor. Merck and Janssen Biotech are developing MK-3222 and CNTO 1959, respectively, as interleukin-23 inhibitors.
Dr. Bachelez, professor of dermatology and head of the inflammatory skin diseases unit at Saint Louis University Hospital, Paris, reported serving on the advisory boards of 10 pharmaceutical companies.
ISTANBUL, TURKEY – The current intensive period of new drug development in psoriasis is cause for enthusiastic celebration by patients and physicians alike. That is, except for one glaring research gap.
Almost all of the promising drugs now in phase-II or -III clinical trials are systemic agents aimed at the minority of psoriasis patients with more severe disease. There’s not nearly as much in the pipeline for the many patients with mild psoriasis who are most appropriately treated with topical therapy, Dr. Hervé Bachelez observed at the annual congress of the European Academy of Dermatology and Venereology.
Indeed, he identified only two promising topical agents advancing through the developmental pipeline: Pfizer’s tofacitinib, a small-molecule Janus kinase inhibitor under study in both topical and oral formulations, and Anacor’s AN2728, a boron-containing molecule that inhibits phosphodiesterase-4 (PDE-4).
Industry’s lack of enthusiasm for developing new topical agents is difficult to understand, given that 84% of all U.S. prescriptions written for psoriasis in 2009 were for topical agents, according to IMS. Moreover, patients clearly believe there is a major unmet need for new and better topicals: a recent survey of 2,151 European psoriasis patients and their dermatologists found that only 45% of patients on topical therapy were satisfied with their treatment. And a mere 35% of dermatologists rated current topical treatments as satisfactory (J. Dermatolog. Treat. 2013;24:193-8).
The great bulk of the action in psoriasis drug development now is focused on injectable biologics and oral small molecules. Among them are three interleukin-17 inhibitors: Novartis’ secukinumab, Amgen’s brodalumab, and Eli Lilly’s ixekizumab. Celgene is developing apremilast as an oral PDE-4 inhibitor. Merck and Janssen Biotech are developing MK-3222 and CNTO 1959, respectively, as interleukin-23 inhibitors.
Dr. Bachelez, professor of dermatology and head of the inflammatory skin diseases unit at Saint Louis University Hospital, Paris, reported serving on the advisory boards of 10 pharmaceutical companies.
ISTANBUL, TURKEY – The current intensive period of new drug development in psoriasis is cause for enthusiastic celebration by patients and physicians alike. That is, except for one glaring research gap.
Almost all of the promising drugs now in phase-II or -III clinical trials are systemic agents aimed at the minority of psoriasis patients with more severe disease. There’s not nearly as much in the pipeline for the many patients with mild psoriasis who are most appropriately treated with topical therapy, Dr. Hervé Bachelez observed at the annual congress of the European Academy of Dermatology and Venereology.
Indeed, he identified only two promising topical agents advancing through the developmental pipeline: Pfizer’s tofacitinib, a small-molecule Janus kinase inhibitor under study in both topical and oral formulations, and Anacor’s AN2728, a boron-containing molecule that inhibits phosphodiesterase-4 (PDE-4).
Industry’s lack of enthusiasm for developing new topical agents is difficult to understand, given that 84% of all U.S. prescriptions written for psoriasis in 2009 were for topical agents, according to IMS. Moreover, patients clearly believe there is a major unmet need for new and better topicals: a recent survey of 2,151 European psoriasis patients and their dermatologists found that only 45% of patients on topical therapy were satisfied with their treatment. And a mere 35% of dermatologists rated current topical treatments as satisfactory (J. Dermatolog. Treat. 2013;24:193-8).
The great bulk of the action in psoriasis drug development now is focused on injectable biologics and oral small molecules. Among them are three interleukin-17 inhibitors: Novartis’ secukinumab, Amgen’s brodalumab, and Eli Lilly’s ixekizumab. Celgene is developing apremilast as an oral PDE-4 inhibitor. Merck and Janssen Biotech are developing MK-3222 and CNTO 1959, respectively, as interleukin-23 inhibitors.
Dr. Bachelez, professor of dermatology and head of the inflammatory skin diseases unit at Saint Louis University Hospital, Paris, reported serving on the advisory boards of 10 pharmaceutical companies.
EXPERT ANALYSIS FROM THE EADV CONGRESS
Two new trials bolster omalizumab for urticaria
ISTANBUL, TURKEY – The case for omalizumab as a novel, safe, and highly effective therapy for H1-antihistamine-refractory chronic idiopathic/spontaneous urticaria received a boost in the form of two new phase III clinical trials presented at the annual congress of the European Academy of Dermatology and Venereology.
The two new multicenter, randomized, double-blind, placebo-controlled studies join another trial published earlier this year (N. Engl. J. Med. 2013;368:924-35). Together, the three trials total nearly 1,000 participants aged 12-75 years who remained severely affected by chronic idiopathic/spontaneous urticaria despite treatment with nonsedating H1-antihistamines, the sole approved form of treatment.
The GLACIAL trial, one of the new studies, set the bar even higher: Participants not only had to be refractory to H1-antihistamines at up to four times the approved dose, but also to off-label H2-antihistamines and/or leukotriene receptor antagonists.
The results were highly consistent across all three clinical trials – 80%-90% of patients demonstrated a dramatic clinical response to omalizumab in dose-dependent fashion. The top dose studied, 300 mg per subcutaneous injection once every 4 weeks, brought the greatest reductions in itching, wheals, and days with angioedema, Dr. Marcus Maurer at the meeting, where he presented the phase III data from the GLACIAL and ASTERIA I trials.
"Omalizumab is the new hope in urticaria treatment. The response is super-fast. We saw a massive and rapid decline in urticaria activity as early as in the first days after the first injection," he said. "In fact, the vast majority of responders respond within the first week of treatment. The disease activity then stays down as long as treatment continues," noted Dr. Maurer, professor of dermatology and allergy at Charité University Hospital, Berlin.
"The other important thing is, as soon as you stop the drug, you stop the benefit. Patients go back to baseline," he added. "So omalizumab is a very effective way to control disease activity, but it’s no cure. It doesn’t make the disease go away, it makes the symptoms go away. There is spontaneous remission in chronic idiopathic/spontaneous urticaria, but until that happens, patients need treatment that makes them free of symptoms. I’ve had patients on omalizumab for 5-6 years now."
As for the safety profile, Dr. Maurer called it "boring."
"This is a safe drug. This is a drug that doesn’t have any differences in its safety profile compared to placebo. The number and types of adverse events are virtually identical," he said.
Less than a week after Dr. Maurer’s presentation of the GLACIAL and ASTERIA I phase III trial results in Istanbul, Genentech filed an application with the Food and Drug Administration requesting approval of a supplemental indication for omalizumab in the treatment of chronic idiopathic/spontaneous urticaria in patients who remain symptomatic despite H1-antihistamine therapy at approved doses. A decision by the agency is expected in the spring of 2014.
Omalizumab is a humanized anti-IgE monoclonal antibody already approved as Xolair for treatment of moderate to severe allergic asthma. Unlike in allergic asthma, however, the response of chronic urticaria to omalizumab is independent of serum IgE level. Indeed, IgE levels are only modestly elevated in patients with chronic idiopathic/spontaneous urticaria – nothing like the high levels that can occur in allergic asthma. It’s not useful to measure IgE as a guide to the likelihood that chronic idiopathic/spontaneous urticaria will respond to omalizumab, Dr. Maurer advised.
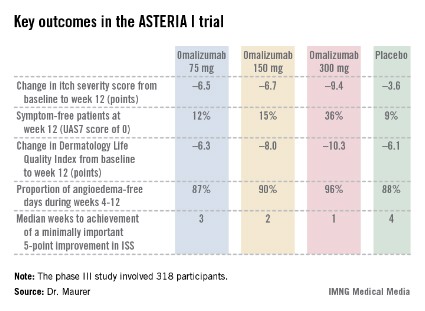
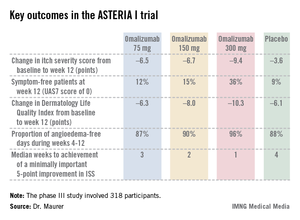
The ASTERIA I trial was arguably the most clinically relevant of the three phase III studies because it employed omalizumab as add-on therapy to H1-antihistamines at approved doses. The 318 participants had a baseline mean weekly Itch Severity Scale (ISS) score of 14.3 out of a possible 21 despite being on H1-antihistamines. Their baseline urticaria activity score over 7 days (UAS7) was 31.1 on a scale of 0-42.
The study population was typical for patients with moderate to severe chronic idiopathic/spontaneous urticaria refractory to first-line therapy with H1-antihistamines. The average age was 41 years, three-quarters were female, and the mean body mass index was 29 kg/m2. The patients were diagnosed with chronic idiopathic/spontaneous urticaria an average of 4 years earlier and had been on an average of five previous unsuccessful medications for the condition. Half of the patients had angioedema.
Study participants received six subcutaneous doses of omalizumab at either 75-, 150-, or 300-mg doses; or placebo at 4-week intervals. They were then observed for 16 weeks. The primary study endpoint was the change in weekly ISS from baseline to week 12. The mean reduction was 3.6 points in the control group, significantly less than the 6.5-point fall in the low-dose omalizumab arm, the 6.7-point reduction with omalizumab 150 mg, or the 9.4-point decrease with omalizumab at 300 mg.
Omalizumab-treated patients also fared significantly better than did the placebo group in all secondary endpoints.
The anti-IgE biologic was well tolerated, with no new safety signals arising in ASTERIA I or the other two phase III trials beyond the agent’s well-established safety profile in treating allergic asthma.
The diagnosis of chronic idiopathic/spontaneous urticaria is based upon the finding of itchy hives and/or angioedema for 6 weeks or more with no specific external trigger. The estimated prevalence in the general population is 0.5%-1%. More than half of affected patients continue to experience symptoms despite treatment with H1-antihistamines.
The phase III trials were sponsored by Genentech and Novartis, which are jointly developing omalizumab for the treatment of chronic idiopathic/spontaneous urticaria. Dr. Maurer has received research grants from, and served on the advisory boards of, those pharmaceutical companies, as well as more than a dozen others.
ISTANBUL, TURKEY – The case for omalizumab as a novel, safe, and highly effective therapy for H1-antihistamine-refractory chronic idiopathic/spontaneous urticaria received a boost in the form of two new phase III clinical trials presented at the annual congress of the European Academy of Dermatology and Venereology.
The two new multicenter, randomized, double-blind, placebo-controlled studies join another trial published earlier this year (N. Engl. J. Med. 2013;368:924-35). Together, the three trials total nearly 1,000 participants aged 12-75 years who remained severely affected by chronic idiopathic/spontaneous urticaria despite treatment with nonsedating H1-antihistamines, the sole approved form of treatment.

The GLACIAL trial, one of the new studies, set the bar even higher: Participants not only had to be refractory to H1-antihistamines at up to four times the approved dose, but also to off-label H2-antihistamines and/or leukotriene receptor antagonists.
The results were highly consistent across all three clinical trials – 80%-90% of patients demonstrated a dramatic clinical response to omalizumab in dose-dependent fashion. The top dose studied, 300 mg per subcutaneous injection once every 4 weeks, brought the greatest reductions in itching, wheals, and days with angioedema, Dr. Marcus Maurer at the meeting, where he presented the phase III data from the GLACIAL and ASTERIA I trials.
"Omalizumab is the new hope in urticaria treatment. The response is super-fast. We saw a massive and rapid decline in urticaria activity as early as in the first days after the first injection," he said. "In fact, the vast majority of responders respond within the first week of treatment. The disease activity then stays down as long as treatment continues," noted Dr. Maurer, professor of dermatology and allergy at Charité University Hospital, Berlin.
"The other important thing is, as soon as you stop the drug, you stop the benefit. Patients go back to baseline," he added. "So omalizumab is a very effective way to control disease activity, but it’s no cure. It doesn’t make the disease go away, it makes the symptoms go away. There is spontaneous remission in chronic idiopathic/spontaneous urticaria, but until that happens, patients need treatment that makes them free of symptoms. I’ve had patients on omalizumab for 5-6 years now."
As for the safety profile, Dr. Maurer called it "boring."
"This is a safe drug. This is a drug that doesn’t have any differences in its safety profile compared to placebo. The number and types of adverse events are virtually identical," he said.
Less than a week after Dr. Maurer’s presentation of the GLACIAL and ASTERIA I phase III trial results in Istanbul, Genentech filed an application with the Food and Drug Administration requesting approval of a supplemental indication for omalizumab in the treatment of chronic idiopathic/spontaneous urticaria in patients who remain symptomatic despite H1-antihistamine therapy at approved doses. A decision by the agency is expected in the spring of 2014.
Omalizumab is a humanized anti-IgE monoclonal antibody already approved as Xolair for treatment of moderate to severe allergic asthma. Unlike in allergic asthma, however, the response of chronic urticaria to omalizumab is independent of serum IgE level. Indeed, IgE levels are only modestly elevated in patients with chronic idiopathic/spontaneous urticaria – nothing like the high levels that can occur in allergic asthma. It’s not useful to measure IgE as a guide to the likelihood that chronic idiopathic/spontaneous urticaria will respond to omalizumab, Dr. Maurer advised.
The ASTERIA I trial was arguably the most clinically relevant of the three phase III studies because it employed omalizumab as add-on therapy to H1-antihistamines at approved doses. The 318 participants had a baseline mean weekly Itch Severity Scale (ISS) score of 14.3 out of a possible 21 despite being on H1-antihistamines. Their baseline urticaria activity score over 7 days (UAS7) was 31.1 on a scale of 0-42.
The study population was typical for patients with moderate to severe chronic idiopathic/spontaneous urticaria refractory to first-line therapy with H1-antihistamines. The average age was 41 years, three-quarters were female, and the mean body mass index was 29 kg/m2. The patients were diagnosed with chronic idiopathic/spontaneous urticaria an average of 4 years earlier and had been on an average of five previous unsuccessful medications for the condition. Half of the patients had angioedema.
Study participants received six subcutaneous doses of omalizumab at either 75-, 150-, or 300-mg doses; or placebo at 4-week intervals. They were then observed for 16 weeks. The primary study endpoint was the change in weekly ISS from baseline to week 12. The mean reduction was 3.6 points in the control group, significantly less than the 6.5-point fall in the low-dose omalizumab arm, the 6.7-point reduction with omalizumab 150 mg, or the 9.4-point decrease with omalizumab at 300 mg.
Omalizumab-treated patients also fared significantly better than did the placebo group in all secondary endpoints.
The anti-IgE biologic was well tolerated, with no new safety signals arising in ASTERIA I or the other two phase III trials beyond the agent’s well-established safety profile in treating allergic asthma.
The diagnosis of chronic idiopathic/spontaneous urticaria is based upon the finding of itchy hives and/or angioedema for 6 weeks or more with no specific external trigger. The estimated prevalence in the general population is 0.5%-1%. More than half of affected patients continue to experience symptoms despite treatment with H1-antihistamines.
The phase III trials were sponsored by Genentech and Novartis, which are jointly developing omalizumab for the treatment of chronic idiopathic/spontaneous urticaria. Dr. Maurer has received research grants from, and served on the advisory boards of, those pharmaceutical companies, as well as more than a dozen others.
ISTANBUL, TURKEY – The case for omalizumab as a novel, safe, and highly effective therapy for H1-antihistamine-refractory chronic idiopathic/spontaneous urticaria received a boost in the form of two new phase III clinical trials presented at the annual congress of the European Academy of Dermatology and Venereology.
The two new multicenter, randomized, double-blind, placebo-controlled studies join another trial published earlier this year (N. Engl. J. Med. 2013;368:924-35). Together, the three trials total nearly 1,000 participants aged 12-75 years who remained severely affected by chronic idiopathic/spontaneous urticaria despite treatment with nonsedating H1-antihistamines, the sole approved form of treatment.

The GLACIAL trial, one of the new studies, set the bar even higher: Participants not only had to be refractory to H1-antihistamines at up to four times the approved dose, but also to off-label H2-antihistamines and/or leukotriene receptor antagonists.
The results were highly consistent across all three clinical trials – 80%-90% of patients demonstrated a dramatic clinical response to omalizumab in dose-dependent fashion. The top dose studied, 300 mg per subcutaneous injection once every 4 weeks, brought the greatest reductions in itching, wheals, and days with angioedema, Dr. Marcus Maurer at the meeting, where he presented the phase III data from the GLACIAL and ASTERIA I trials.
"Omalizumab is the new hope in urticaria treatment. The response is super-fast. We saw a massive and rapid decline in urticaria activity as early as in the first days after the first injection," he said. "In fact, the vast majority of responders respond within the first week of treatment. The disease activity then stays down as long as treatment continues," noted Dr. Maurer, professor of dermatology and allergy at Charité University Hospital, Berlin.
"The other important thing is, as soon as you stop the drug, you stop the benefit. Patients go back to baseline," he added. "So omalizumab is a very effective way to control disease activity, but it’s no cure. It doesn’t make the disease go away, it makes the symptoms go away. There is spontaneous remission in chronic idiopathic/spontaneous urticaria, but until that happens, patients need treatment that makes them free of symptoms. I’ve had patients on omalizumab for 5-6 years now."
As for the safety profile, Dr. Maurer called it "boring."
"This is a safe drug. This is a drug that doesn’t have any differences in its safety profile compared to placebo. The number and types of adverse events are virtually identical," he said.
Less than a week after Dr. Maurer’s presentation of the GLACIAL and ASTERIA I phase III trial results in Istanbul, Genentech filed an application with the Food and Drug Administration requesting approval of a supplemental indication for omalizumab in the treatment of chronic idiopathic/spontaneous urticaria in patients who remain symptomatic despite H1-antihistamine therapy at approved doses. A decision by the agency is expected in the spring of 2014.
Omalizumab is a humanized anti-IgE monoclonal antibody already approved as Xolair for treatment of moderate to severe allergic asthma. Unlike in allergic asthma, however, the response of chronic urticaria to omalizumab is independent of serum IgE level. Indeed, IgE levels are only modestly elevated in patients with chronic idiopathic/spontaneous urticaria – nothing like the high levels that can occur in allergic asthma. It’s not useful to measure IgE as a guide to the likelihood that chronic idiopathic/spontaneous urticaria will respond to omalizumab, Dr. Maurer advised.
The ASTERIA I trial was arguably the most clinically relevant of the three phase III studies because it employed omalizumab as add-on therapy to H1-antihistamines at approved doses. The 318 participants had a baseline mean weekly Itch Severity Scale (ISS) score of 14.3 out of a possible 21 despite being on H1-antihistamines. Their baseline urticaria activity score over 7 days (UAS7) was 31.1 on a scale of 0-42.
The study population was typical for patients with moderate to severe chronic idiopathic/spontaneous urticaria refractory to first-line therapy with H1-antihistamines. The average age was 41 years, three-quarters were female, and the mean body mass index was 29 kg/m2. The patients were diagnosed with chronic idiopathic/spontaneous urticaria an average of 4 years earlier and had been on an average of five previous unsuccessful medications for the condition. Half of the patients had angioedema.
Study participants received six subcutaneous doses of omalizumab at either 75-, 150-, or 300-mg doses; or placebo at 4-week intervals. They were then observed for 16 weeks. The primary study endpoint was the change in weekly ISS from baseline to week 12. The mean reduction was 3.6 points in the control group, significantly less than the 6.5-point fall in the low-dose omalizumab arm, the 6.7-point reduction with omalizumab 150 mg, or the 9.4-point decrease with omalizumab at 300 mg.
Omalizumab-treated patients also fared significantly better than did the placebo group in all secondary endpoints.
The anti-IgE biologic was well tolerated, with no new safety signals arising in ASTERIA I or the other two phase III trials beyond the agent’s well-established safety profile in treating allergic asthma.
The diagnosis of chronic idiopathic/spontaneous urticaria is based upon the finding of itchy hives and/or angioedema for 6 weeks or more with no specific external trigger. The estimated prevalence in the general population is 0.5%-1%. More than half of affected patients continue to experience symptoms despite treatment with H1-antihistamines.
The phase III trials were sponsored by Genentech and Novartis, which are jointly developing omalizumab for the treatment of chronic idiopathic/spontaneous urticaria. Dr. Maurer has received research grants from, and served on the advisory boards of, those pharmaceutical companies, as well as more than a dozen others.
AT THE EADV CONGRESS
Major finding: Among 318 patients with chronic idiopathic/spontaneous urticaria refractory to H1-antihistamines at standard doses who participated in the phase III ASTERIA I trial, add-on omalizumab at 300 mg resulted in a mean 9.4-point reduction in itch severity score at week 12 compared to a 3.6-point drop with placebo from a baseline score of 14.3.
Data source: ASTERIA I, one of three recent phase III randomized, double-blind, multicenter, placebo-controlled clinical trials of omalizumab for chronic idiopathic/spontaneous urticaria totaling nearly 1,000 participants.
Disclosures: The phase III clinical trials were jointly sponsored by Genentech and Novartis. The presenter reported having received research funds from the two companies as well as serving on their advisory boards.
ATX-101: ‘You’re gonna like your new chin’
ISTANBUL – Both clinicians and patients gave favorable marks to a novel injectable pharmacologic treatment for removal of unwanted submental fat – the unsightly double chin – in two phase III randomized trials presented at the annual congress of the European Academy of Dermatology and Venereology.
The investigational chin-fat buster, known as ATX-101, is a proprietary purified synthetic form of deoxycholic acid. Upon injection directly into the submental fat, ATX-101 lyses adipocytes by disrupting their cell membranes.
There is an unmet need for a rigorously studied prescription product for nonsurgical treatment of excess submental fat. Not everyone with a double chin is interested in or a good candidate for the established surgical procedures, observed Dr. Berthold Rzany, a dermatologist at Charité University Hospital, Berlin, who presented a 363-patient, randomized, placebo-controlled phase III trial at the meeting. Participants had to have a body mass index no higher than 30 kg/m2, dissatisfaction with the appearance of their submental area, and a physician rating of moderate to severe submental fat. Three-quarters of the subjects were women, with a mean age of 46 years and a mean BMI of 25.7 kg/m2.
One of the two primary efficacy endpoints required at least a 1-point improvement on the 0- to 4-point Clinician-Reported Submental Fat Rating Scale as assessed 12 weeks after the final treatment. This was achieved in 59% of patients randomized to ATX-101 at a dose of 1mg/cm2 and 65% at 2 mg/cm2, both significantly higher rates than the 23% in placebo-treated controls.
The other primary endpoint required a high level of patient satisfaction with the appearance of their face and chin after treatment as expressed in a Subject Self-Rating Scale score of 4 or more on the 0-6 scale. This endpoint was achieved in 53% of patients treated with ATX-101 at 1 mg/cm2, 66% who received the agent at 2 mg/cm2, and 29% of the placebo group.
ATX-101-treated patients were also significantly more likely to report perceived improvement in the visual and psychological impact of their submental fat. For example, 74% of patients who received ATX-101 at 1 mg/cm2 and 80% at 2 mg/cm2 reported improved definition between their chin and neck, compared with baseline and compared with 28% of placebo-treated controls. In addition, 32% of patients who received the lower dose of ATX-101 and 39% who got the higher dose characterized their submental fat as "a great deal better," compared with baseline, as did a mere 7% of controls.
Also, patients who received ATX-101 reported 12 weeks post treatment that they looked less overweight and were less bothered by and self-conscious about their submental fat, compared with controls.
Treatment-emergent induration, redness, bruising, numbness, and/or swelling variously occurred in one-third to two-thirds of ATX-101 recipients. All of these adverse events were more common than in placebo-treated controls. However, the events were transient and mostly mild or moderate in intensity. The exception was injection site pain, which occurred in roughly 80% of ATX-101-treated patients and was mostly moderate to severe, although it lasted a median of only 1 day, according to Dr. Rzany.
The ATX-101 treatment regimen entails up to 50 2-mL fixed-dose subcutaneous injections 1 cm apart per treatment session. Up to four treatment sessions were permitted, each separated by a minimum of 4 weeks.
In a separate presentation, Dr. Benjamin Ascher reported on 360 randomized patients who participated in the other phase III clinical trial. The two studies had the same design and endpoints.
An improvement of at least 1 point on the Clinician-Reported Submental Fat Rating Scale occurred in 58% of patients randomized to ATX-101 at the 1 mg/cm2 dose, 62% of those who received the higher dose, and 35% on placebo. Moreover, 68% of patients who got ATX-101 at 1 mg/cm2 were satisfied with their resultant appearance as reflected in a Subject Self-Rating Scale score of at least 4. So were 65% of those who received the higher dose and 29% of placebo-treated controls. As in the previously mentioned study, indices of self-image and psychological well being were also improved following the aesthetic therapy. Treatment-related adverse events were mostly transient and mild to moderate in intensity, according to Dr. Ascher, who is in the private practice of aesthetic surgery in Paris.
Both phase III studies were supported by Bayer HealthCare and KYTHERA Biopharmaceuticals. Dr. Rzany and Dr. Ascher serve as advisers to the companies.
ISTANBUL – Both clinicians and patients gave favorable marks to a novel injectable pharmacologic treatment for removal of unwanted submental fat – the unsightly double chin – in two phase III randomized trials presented at the annual congress of the European Academy of Dermatology and Venereology.
The investigational chin-fat buster, known as ATX-101, is a proprietary purified synthetic form of deoxycholic acid. Upon injection directly into the submental fat, ATX-101 lyses adipocytes by disrupting their cell membranes.
There is an unmet need for a rigorously studied prescription product for nonsurgical treatment of excess submental fat. Not everyone with a double chin is interested in or a good candidate for the established surgical procedures, observed Dr. Berthold Rzany, a dermatologist at Charité University Hospital, Berlin, who presented a 363-patient, randomized, placebo-controlled phase III trial at the meeting. Participants had to have a body mass index no higher than 30 kg/m2, dissatisfaction with the appearance of their submental area, and a physician rating of moderate to severe submental fat. Three-quarters of the subjects were women, with a mean age of 46 years and a mean BMI of 25.7 kg/m2.
One of the two primary efficacy endpoints required at least a 1-point improvement on the 0- to 4-point Clinician-Reported Submental Fat Rating Scale as assessed 12 weeks after the final treatment. This was achieved in 59% of patients randomized to ATX-101 at a dose of 1mg/cm2 and 65% at 2 mg/cm2, both significantly higher rates than the 23% in placebo-treated controls.
The other primary endpoint required a high level of patient satisfaction with the appearance of their face and chin after treatment as expressed in a Subject Self-Rating Scale score of 4 or more on the 0-6 scale. This endpoint was achieved in 53% of patients treated with ATX-101 at 1 mg/cm2, 66% who received the agent at 2 mg/cm2, and 29% of the placebo group.
ATX-101-treated patients were also significantly more likely to report perceived improvement in the visual and psychological impact of their submental fat. For example, 74% of patients who received ATX-101 at 1 mg/cm2 and 80% at 2 mg/cm2 reported improved definition between their chin and neck, compared with baseline and compared with 28% of placebo-treated controls. In addition, 32% of patients who received the lower dose of ATX-101 and 39% who got the higher dose characterized their submental fat as "a great deal better," compared with baseline, as did a mere 7% of controls.
Also, patients who received ATX-101 reported 12 weeks post treatment that they looked less overweight and were less bothered by and self-conscious about their submental fat, compared with controls.
Treatment-emergent induration, redness, bruising, numbness, and/or swelling variously occurred in one-third to two-thirds of ATX-101 recipients. All of these adverse events were more common than in placebo-treated controls. However, the events were transient and mostly mild or moderate in intensity. The exception was injection site pain, which occurred in roughly 80% of ATX-101-treated patients and was mostly moderate to severe, although it lasted a median of only 1 day, according to Dr. Rzany.
The ATX-101 treatment regimen entails up to 50 2-mL fixed-dose subcutaneous injections 1 cm apart per treatment session. Up to four treatment sessions were permitted, each separated by a minimum of 4 weeks.
In a separate presentation, Dr. Benjamin Ascher reported on 360 randomized patients who participated in the other phase III clinical trial. The two studies had the same design and endpoints.
An improvement of at least 1 point on the Clinician-Reported Submental Fat Rating Scale occurred in 58% of patients randomized to ATX-101 at the 1 mg/cm2 dose, 62% of those who received the higher dose, and 35% on placebo. Moreover, 68% of patients who got ATX-101 at 1 mg/cm2 were satisfied with their resultant appearance as reflected in a Subject Self-Rating Scale score of at least 4. So were 65% of those who received the higher dose and 29% of placebo-treated controls. As in the previously mentioned study, indices of self-image and psychological well being were also improved following the aesthetic therapy. Treatment-related adverse events were mostly transient and mild to moderate in intensity, according to Dr. Ascher, who is in the private practice of aesthetic surgery in Paris.
Both phase III studies were supported by Bayer HealthCare and KYTHERA Biopharmaceuticals. Dr. Rzany and Dr. Ascher serve as advisers to the companies.
ISTANBUL – Both clinicians and patients gave favorable marks to a novel injectable pharmacologic treatment for removal of unwanted submental fat – the unsightly double chin – in two phase III randomized trials presented at the annual congress of the European Academy of Dermatology and Venereology.
The investigational chin-fat buster, known as ATX-101, is a proprietary purified synthetic form of deoxycholic acid. Upon injection directly into the submental fat, ATX-101 lyses adipocytes by disrupting their cell membranes.
There is an unmet need for a rigorously studied prescription product for nonsurgical treatment of excess submental fat. Not everyone with a double chin is interested in or a good candidate for the established surgical procedures, observed Dr. Berthold Rzany, a dermatologist at Charité University Hospital, Berlin, who presented a 363-patient, randomized, placebo-controlled phase III trial at the meeting. Participants had to have a body mass index no higher than 30 kg/m2, dissatisfaction with the appearance of their submental area, and a physician rating of moderate to severe submental fat. Three-quarters of the subjects were women, with a mean age of 46 years and a mean BMI of 25.7 kg/m2.
One of the two primary efficacy endpoints required at least a 1-point improvement on the 0- to 4-point Clinician-Reported Submental Fat Rating Scale as assessed 12 weeks after the final treatment. This was achieved in 59% of patients randomized to ATX-101 at a dose of 1mg/cm2 and 65% at 2 mg/cm2, both significantly higher rates than the 23% in placebo-treated controls.
The other primary endpoint required a high level of patient satisfaction with the appearance of their face and chin after treatment as expressed in a Subject Self-Rating Scale score of 4 or more on the 0-6 scale. This endpoint was achieved in 53% of patients treated with ATX-101 at 1 mg/cm2, 66% who received the agent at 2 mg/cm2, and 29% of the placebo group.
ATX-101-treated patients were also significantly more likely to report perceived improvement in the visual and psychological impact of their submental fat. For example, 74% of patients who received ATX-101 at 1 mg/cm2 and 80% at 2 mg/cm2 reported improved definition between their chin and neck, compared with baseline and compared with 28% of placebo-treated controls. In addition, 32% of patients who received the lower dose of ATX-101 and 39% who got the higher dose characterized their submental fat as "a great deal better," compared with baseline, as did a mere 7% of controls.
Also, patients who received ATX-101 reported 12 weeks post treatment that they looked less overweight and were less bothered by and self-conscious about their submental fat, compared with controls.
Treatment-emergent induration, redness, bruising, numbness, and/or swelling variously occurred in one-third to two-thirds of ATX-101 recipients. All of these adverse events were more common than in placebo-treated controls. However, the events were transient and mostly mild or moderate in intensity. The exception was injection site pain, which occurred in roughly 80% of ATX-101-treated patients and was mostly moderate to severe, although it lasted a median of only 1 day, according to Dr. Rzany.
The ATX-101 treatment regimen entails up to 50 2-mL fixed-dose subcutaneous injections 1 cm apart per treatment session. Up to four treatment sessions were permitted, each separated by a minimum of 4 weeks.
In a separate presentation, Dr. Benjamin Ascher reported on 360 randomized patients who participated in the other phase III clinical trial. The two studies had the same design and endpoints.
An improvement of at least 1 point on the Clinician-Reported Submental Fat Rating Scale occurred in 58% of patients randomized to ATX-101 at the 1 mg/cm2 dose, 62% of those who received the higher dose, and 35% on placebo. Moreover, 68% of patients who got ATX-101 at 1 mg/cm2 were satisfied with their resultant appearance as reflected in a Subject Self-Rating Scale score of at least 4. So were 65% of those who received the higher dose and 29% of placebo-treated controls. As in the previously mentioned study, indices of self-image and psychological well being were also improved following the aesthetic therapy. Treatment-related adverse events were mostly transient and mild to moderate in intensity, according to Dr. Ascher, who is in the private practice of aesthetic surgery in Paris.
Both phase III studies were supported by Bayer HealthCare and KYTHERA Biopharmaceuticals. Dr. Rzany and Dr. Ascher serve as advisers to the companies.
AT THE EADV CONGRESS
Major finding: Patients with excess submental fat who received treatment using a novel subcutaneously injectable agent for the nonsurgical reduction of double chins were significantly more likely to be satisfied with their chin’s appearance afterward than were placebo-treated controls.
Data source: The two studies included a total of 723 patients with moderate to severe excess submental fat.
Disclosures: The studies were funded by Bayer HealthCare and KYTHERA Biopharmaceuticals. The presenters have received research grants from and serve as advisers to the companies.
Apremilast promising for Behçet’s syndrome
ISTANBUL, TURKEY – The investigational oral phosphodiesterase-4 inhibitor apremilast showed dramatic efficacy in the treatment of oral and genital ulcers of Behçet’s syndrome in a randomized, double-blind clinical trial.
The multicenter phase II study included 111 Behçet’s syndrome patients with active oral ulcers. They were randomized to 12 weeks of double-blind apremilast at 30 mg twice daily or placebo followed by an additional 12 weeks of open-label apremilast for all, then 4 weeks of post-treatment observation.
Participants had a mean of 2.8 oral ulcers at baseline. The primary study endpoint was the number present at week 12: a mean of 0.5 in the apremilast group, compared with 2.1 in controls, Dr. Gülen Hatemi reported at the annual congress of the European Academy of Dermatology and Venereology.
A total of 71% of patients in the apremilast group were oral ulcer–free at week 12, as were 29% of controls, added Dr. Hatemi, a rheumatologist at Istanbul (Turkey) University.
The benefit of apremilast occurred rapidly. The full response was seen at week 2 and was then maintained throughout the remainder of the 12 weeks of double-blind therapy and the following 12 weeks of open-label apremilast. However, oral ulcers returned in full force quickly after treatment cessation.
Pain from oral ulcers on a 0-100 visual analog scale plummeted in the apremilast group by a mean 44.7 points from a baseline of score of 54. In contrast, pain scores in the control group improved by 16 points, which is less than the 20-point threshold accepted as being clinically meaningful.
Behçet’s syndrome is an immune-mediated small-vessel vasculitis. Its mucocutaneous manifestations can be disabling and resistant to conventional therapies, Dr. Hatemi noted.
Only 16 patients had genital ulcers. All 10 randomized to apremilast were free of the ulcers at week 12, as were three of six affected patients in the control group.
Average scores on the Behçet’s Disease Activity Index dropped from 3.4 at baseline to 2.0 at 12 weeks in the apremilast arm while remaining unchanged in controls. Mean scores on the Behçet’s Disease Quality of Life instrument decreased by 4.5 points from a baseline of 12.6 in apremilast-treated patients, compared with a 1.6-point reduction in controls.
The investigators said no serious adverse events were related to apremilast.
Dr. Hatemi received a research grant from Celgene, the study sponsor, which is also developing the oral drug as a possible treatment for psoriasis, psoriatic arthritis, and ankylosing spondylitis.
ISTANBUL, TURKEY – The investigational oral phosphodiesterase-4 inhibitor apremilast showed dramatic efficacy in the treatment of oral and genital ulcers of Behçet’s syndrome in a randomized, double-blind clinical trial.
The multicenter phase II study included 111 Behçet’s syndrome patients with active oral ulcers. They were randomized to 12 weeks of double-blind apremilast at 30 mg twice daily or placebo followed by an additional 12 weeks of open-label apremilast for all, then 4 weeks of post-treatment observation.
Participants had a mean of 2.8 oral ulcers at baseline. The primary study endpoint was the number present at week 12: a mean of 0.5 in the apremilast group, compared with 2.1 in controls, Dr. Gülen Hatemi reported at the annual congress of the European Academy of Dermatology and Venereology.
A total of 71% of patients in the apremilast group were oral ulcer–free at week 12, as were 29% of controls, added Dr. Hatemi, a rheumatologist at Istanbul (Turkey) University.
The benefit of apremilast occurred rapidly. The full response was seen at week 2 and was then maintained throughout the remainder of the 12 weeks of double-blind therapy and the following 12 weeks of open-label apremilast. However, oral ulcers returned in full force quickly after treatment cessation.
Pain from oral ulcers on a 0-100 visual analog scale plummeted in the apremilast group by a mean 44.7 points from a baseline of score of 54. In contrast, pain scores in the control group improved by 16 points, which is less than the 20-point threshold accepted as being clinically meaningful.
Behçet’s syndrome is an immune-mediated small-vessel vasculitis. Its mucocutaneous manifestations can be disabling and resistant to conventional therapies, Dr. Hatemi noted.
Only 16 patients had genital ulcers. All 10 randomized to apremilast were free of the ulcers at week 12, as were three of six affected patients in the control group.
Average scores on the Behçet’s Disease Activity Index dropped from 3.4 at baseline to 2.0 at 12 weeks in the apremilast arm while remaining unchanged in controls. Mean scores on the Behçet’s Disease Quality of Life instrument decreased by 4.5 points from a baseline of 12.6 in apremilast-treated patients, compared with a 1.6-point reduction in controls.
The investigators said no serious adverse events were related to apremilast.
Dr. Hatemi received a research grant from Celgene, the study sponsor, which is also developing the oral drug as a possible treatment for psoriasis, psoriatic arthritis, and ankylosing spondylitis.
ISTANBUL, TURKEY – The investigational oral phosphodiesterase-4 inhibitor apremilast showed dramatic efficacy in the treatment of oral and genital ulcers of Behçet’s syndrome in a randomized, double-blind clinical trial.
The multicenter phase II study included 111 Behçet’s syndrome patients with active oral ulcers. They were randomized to 12 weeks of double-blind apremilast at 30 mg twice daily or placebo followed by an additional 12 weeks of open-label apremilast for all, then 4 weeks of post-treatment observation.
Participants had a mean of 2.8 oral ulcers at baseline. The primary study endpoint was the number present at week 12: a mean of 0.5 in the apremilast group, compared with 2.1 in controls, Dr. Gülen Hatemi reported at the annual congress of the European Academy of Dermatology and Venereology.
A total of 71% of patients in the apremilast group were oral ulcer–free at week 12, as were 29% of controls, added Dr. Hatemi, a rheumatologist at Istanbul (Turkey) University.
The benefit of apremilast occurred rapidly. The full response was seen at week 2 and was then maintained throughout the remainder of the 12 weeks of double-blind therapy and the following 12 weeks of open-label apremilast. However, oral ulcers returned in full force quickly after treatment cessation.
Pain from oral ulcers on a 0-100 visual analog scale plummeted in the apremilast group by a mean 44.7 points from a baseline of score of 54. In contrast, pain scores in the control group improved by 16 points, which is less than the 20-point threshold accepted as being clinically meaningful.
Behçet’s syndrome is an immune-mediated small-vessel vasculitis. Its mucocutaneous manifestations can be disabling and resistant to conventional therapies, Dr. Hatemi noted.
Only 16 patients had genital ulcers. All 10 randomized to apremilast were free of the ulcers at week 12, as were three of six affected patients in the control group.
Average scores on the Behçet’s Disease Activity Index dropped from 3.4 at baseline to 2.0 at 12 weeks in the apremilast arm while remaining unchanged in controls. Mean scores on the Behçet’s Disease Quality of Life instrument decreased by 4.5 points from a baseline of 12.6 in apremilast-treated patients, compared with a 1.6-point reduction in controls.
The investigators said no serious adverse events were related to apremilast.
Dr. Hatemi received a research grant from Celgene, the study sponsor, which is also developing the oral drug as a possible treatment for psoriasis, psoriatic arthritis, and ankylosing spondylitis.
AT THE EADV CONGRESS
Major finding: The mean number of oral ulcers in Behçet’s syndrome patients treated with apremilast quickly dropped from 2.8 at baseline to 0.5, an improvement maintained during 24 weeks of therapy.
Data source: A randomized, double-blind, multicenter, 28-week phase II study involving 111 Behçet’s syndrome patients with active oral ulcers.
Disclosures: The presenter received a research grant from the study sponsor, Celgene.
Systemic sclerosis overlap syndromes called distinct entity
ISTANBUL – Systemic sclerosis overlap syndromes follow a path of disease progression distinctly different from that of limited or diffuse cutaneous systemic sclerosis, according to a new analysis from the German Network for Systemic Scleroderma.
"This study shows for the first time that patients suffering from systemic sclerosis overlap syndromes should be viewed as a distinct systemic sclerosis subset," Dr. Pia Moinzadeh noted at the annual congress of the European Academy of Dermatology and Venereology.
Those with systemic sclerosis overlap syndromes (SSc-OS) are a heterogeneous subgroup of patients who have the clinical features of SSc according to American College of Rheumatology criteria simultaneously with the characteristic findings of at least one other connective tissue disease, such as dermatomyositis, Sjögren’s syndrome, rheumatoid arthritis, or lupus erythematosus.
The registry of the German Network for Systemic Scleroderma is a uniquely large and inclusive ongoing prospective data base directed by a joint committee of dermatologists and rheumatologists. More than 6 years ago, when the investigators realized that a sizable fraction of patients enrolled in the national registry didn’t fit within the classic bimodal SSc categorization scheme composed of two subtypes – limited cutaneous and diffuse cutaneous SSc – they proposed three additional SSc subtypes. These are SSc-OS, undifferentiated scleroderma, and sclerosis sine scleroderma.
The focus of Dr. Moinzadeh’s EADV presentation was on SSc-OS. Of 3,240 SSc patients in the German registry, patients with OS comprise 11%, while those with limited cutaneous SSc make up 43%, those with diffuse cutaneous disease account for 31%, and the remainder of patients have undifferentiated scleroderma or sclerosis sine scleroderma.
The validity of the SSc-OS diagnostic construct has been controversial. Some experts have contended that patients with SSc-OS ought to be lumped under the headings of limited cutaneous SSc or diffuse cutaneous SSc, depending on the extent of skin involvement. However, the unparalleled size, inclusiveness, and long-term prospective follow-up provided by the German registry have resulted in evidence that argues to the contrary, according to Dr. Moinzadeh, a rheumatologist at the University of Cologne (Germany).
She reported on 326 patients with SSc-OS, 1,598 with limited cutaneous SSc, and 996 with diffuse cutaneous SSc prospectively followed through the German registry for a mean of 10.1 years. The follow-up data indicate SSc-OS evolves differently from either limited cutaneous SSc or diffuse cutaneous SSc. It also displays a distinctive pattern of organ involvement and autoantibody positivity, she noted.
The group with SSc-OS had a mean modified Rodnan skin score of 6.7, similar to the 7.2 score in the limited cutaneous SSc group and markedly less than the 15.8 in the diffuse cutaneous SSc patients. While this might suggest that patients with SSc-OS have a milder course of disease comparable to limited cutaneous SSc, in fact they progressed more rapidly, with earlier and more widespread significant organ involvement and a higher disease burden than the limited cutaneous SSc group, according to Dr. Moinzadeh.
Patients with SSc-OS developed musculoskeletal involvement far more frequently than others. Sixty-seven percent were affected, compared with 38% of patients with limited cutaneous SSc and 48% with diffuse cutaneous SSc. The patients in the SSc-OS group also developed musculoskeletal involvement earlier.
Lung fibrosis occurred in 38% of patients with SSc-OS, 27% of patients with limited cutaneous SSc, and 63% with diffuse cutaneous SSc. Cardiac involvement occurred in 15% with SSc-OS, 10% with limited cutaneous SSc, and 19% with diffuse cutaneous SSc. The onset of these manifestations of SSc in the SSc-OS subgroup was significantly earlier than in the limited cutaneous SSc patients but later than with diffuse cutaneous SSc.
Progression to esophageal and renal involvement, as well as pulmonary arterial hypertension, in the SSc-OS group occurred significantly earlier than in the limited cutaneous SSC patients, but again was later than with diffuse cutaneous SSC.
The autoantibody status of the three subgroups showed distinct differences (see graphic).
The German Network for Systemic Scleroderma is funded by the German Federal Ministry of Education and Research. Dr. Moinzadeh reported having no financial conflicts.
ISTANBUL – Systemic sclerosis overlap syndromes follow a path of disease progression distinctly different from that of limited or diffuse cutaneous systemic sclerosis, according to a new analysis from the German Network for Systemic Scleroderma.
"This study shows for the first time that patients suffering from systemic sclerosis overlap syndromes should be viewed as a distinct systemic sclerosis subset," Dr. Pia Moinzadeh noted at the annual congress of the European Academy of Dermatology and Venereology.

Those with systemic sclerosis overlap syndromes (SSc-OS) are a heterogeneous subgroup of patients who have the clinical features of SSc according to American College of Rheumatology criteria simultaneously with the characteristic findings of at least one other connective tissue disease, such as dermatomyositis, Sjögren’s syndrome, rheumatoid arthritis, or lupus erythematosus.
The registry of the German Network for Systemic Scleroderma is a uniquely large and inclusive ongoing prospective data base directed by a joint committee of dermatologists and rheumatologists. More than 6 years ago, when the investigators realized that a sizable fraction of patients enrolled in the national registry didn’t fit within the classic bimodal SSc categorization scheme composed of two subtypes – limited cutaneous and diffuse cutaneous SSc – they proposed three additional SSc subtypes. These are SSc-OS, undifferentiated scleroderma, and sclerosis sine scleroderma.
The focus of Dr. Moinzadeh’s EADV presentation was on SSc-OS. Of 3,240 SSc patients in the German registry, patients with OS comprise 11%, while those with limited cutaneous SSc make up 43%, those with diffuse cutaneous disease account for 31%, and the remainder of patients have undifferentiated scleroderma or sclerosis sine scleroderma.
The validity of the SSc-OS diagnostic construct has been controversial. Some experts have contended that patients with SSc-OS ought to be lumped under the headings of limited cutaneous SSc or diffuse cutaneous SSc, depending on the extent of skin involvement. However, the unparalleled size, inclusiveness, and long-term prospective follow-up provided by the German registry have resulted in evidence that argues to the contrary, according to Dr. Moinzadeh, a rheumatologist at the University of Cologne (Germany).
She reported on 326 patients with SSc-OS, 1,598 with limited cutaneous SSc, and 996 with diffuse cutaneous SSc prospectively followed through the German registry for a mean of 10.1 years. The follow-up data indicate SSc-OS evolves differently from either limited cutaneous SSc or diffuse cutaneous SSc. It also displays a distinctive pattern of organ involvement and autoantibody positivity, she noted.
The group with SSc-OS had a mean modified Rodnan skin score of 6.7, similar to the 7.2 score in the limited cutaneous SSc group and markedly less than the 15.8 in the diffuse cutaneous SSc patients. While this might suggest that patients with SSc-OS have a milder course of disease comparable to limited cutaneous SSc, in fact they progressed more rapidly, with earlier and more widespread significant organ involvement and a higher disease burden than the limited cutaneous SSc group, according to Dr. Moinzadeh.
Patients with SSc-OS developed musculoskeletal involvement far more frequently than others. Sixty-seven percent were affected, compared with 38% of patients with limited cutaneous SSc and 48% with diffuse cutaneous SSc. The patients in the SSc-OS group also developed musculoskeletal involvement earlier.
Lung fibrosis occurred in 38% of patients with SSc-OS, 27% of patients with limited cutaneous SSc, and 63% with diffuse cutaneous SSc. Cardiac involvement occurred in 15% with SSc-OS, 10% with limited cutaneous SSc, and 19% with diffuse cutaneous SSc. The onset of these manifestations of SSc in the SSc-OS subgroup was significantly earlier than in the limited cutaneous SSc patients but later than with diffuse cutaneous SSc.
Progression to esophageal and renal involvement, as well as pulmonary arterial hypertension, in the SSc-OS group occurred significantly earlier than in the limited cutaneous SSC patients, but again was later than with diffuse cutaneous SSC.
The autoantibody status of the three subgroups showed distinct differences (see graphic).
The German Network for Systemic Scleroderma is funded by the German Federal Ministry of Education and Research. Dr. Moinzadeh reported having no financial conflicts.
ISTANBUL – Systemic sclerosis overlap syndromes follow a path of disease progression distinctly different from that of limited or diffuse cutaneous systemic sclerosis, according to a new analysis from the German Network for Systemic Scleroderma.
"This study shows for the first time that patients suffering from systemic sclerosis overlap syndromes should be viewed as a distinct systemic sclerosis subset," Dr. Pia Moinzadeh noted at the annual congress of the European Academy of Dermatology and Venereology.

Those with systemic sclerosis overlap syndromes (SSc-OS) are a heterogeneous subgroup of patients who have the clinical features of SSc according to American College of Rheumatology criteria simultaneously with the characteristic findings of at least one other connective tissue disease, such as dermatomyositis, Sjögren’s syndrome, rheumatoid arthritis, or lupus erythematosus.
The registry of the German Network for Systemic Scleroderma is a uniquely large and inclusive ongoing prospective data base directed by a joint committee of dermatologists and rheumatologists. More than 6 years ago, when the investigators realized that a sizable fraction of patients enrolled in the national registry didn’t fit within the classic bimodal SSc categorization scheme composed of two subtypes – limited cutaneous and diffuse cutaneous SSc – they proposed three additional SSc subtypes. These are SSc-OS, undifferentiated scleroderma, and sclerosis sine scleroderma.
The focus of Dr. Moinzadeh’s EADV presentation was on SSc-OS. Of 3,240 SSc patients in the German registry, patients with OS comprise 11%, while those with limited cutaneous SSc make up 43%, those with diffuse cutaneous disease account for 31%, and the remainder of patients have undifferentiated scleroderma or sclerosis sine scleroderma.
The validity of the SSc-OS diagnostic construct has been controversial. Some experts have contended that patients with SSc-OS ought to be lumped under the headings of limited cutaneous SSc or diffuse cutaneous SSc, depending on the extent of skin involvement. However, the unparalleled size, inclusiveness, and long-term prospective follow-up provided by the German registry have resulted in evidence that argues to the contrary, according to Dr. Moinzadeh, a rheumatologist at the University of Cologne (Germany).
She reported on 326 patients with SSc-OS, 1,598 with limited cutaneous SSc, and 996 with diffuse cutaneous SSc prospectively followed through the German registry for a mean of 10.1 years. The follow-up data indicate SSc-OS evolves differently from either limited cutaneous SSc or diffuse cutaneous SSc. It also displays a distinctive pattern of organ involvement and autoantibody positivity, she noted.
The group with SSc-OS had a mean modified Rodnan skin score of 6.7, similar to the 7.2 score in the limited cutaneous SSc group and markedly less than the 15.8 in the diffuse cutaneous SSc patients. While this might suggest that patients with SSc-OS have a milder course of disease comparable to limited cutaneous SSc, in fact they progressed more rapidly, with earlier and more widespread significant organ involvement and a higher disease burden than the limited cutaneous SSc group, according to Dr. Moinzadeh.
Patients with SSc-OS developed musculoskeletal involvement far more frequently than others. Sixty-seven percent were affected, compared with 38% of patients with limited cutaneous SSc and 48% with diffuse cutaneous SSc. The patients in the SSc-OS group also developed musculoskeletal involvement earlier.
Lung fibrosis occurred in 38% of patients with SSc-OS, 27% of patients with limited cutaneous SSc, and 63% with diffuse cutaneous SSc. Cardiac involvement occurred in 15% with SSc-OS, 10% with limited cutaneous SSc, and 19% with diffuse cutaneous SSc. The onset of these manifestations of SSc in the SSc-OS subgroup was significantly earlier than in the limited cutaneous SSc patients but later than with diffuse cutaneous SSc.
Progression to esophageal and renal involvement, as well as pulmonary arterial hypertension, in the SSc-OS group occurred significantly earlier than in the limited cutaneous SSC patients, but again was later than with diffuse cutaneous SSC.
The autoantibody status of the three subgroups showed distinct differences (see graphic).
The German Network for Systemic Scleroderma is funded by the German Federal Ministry of Education and Research. Dr. Moinzadeh reported having no financial conflicts.
AT THE EADV CONGRESS
Major finding: Musculoskeletal involvement occurred in 67% of patients with systemic sclerosis overlap syndromes, compared with 38% of those with limited cutaneous systemic sclerosis and 48% with diffuse cutaneous systemic sclerosis.
Data source: This analysis from the ongoing prospective national registry maintained by the German Network for Systemic Scleroderma included nearly 3,000 patients with systemic sclerosis followed for a mean of 10.1 years.
Disclosures: The German Network for Systemic Scleroderma is funded by the German Federal Ministry of Education and Research. Dr. Moinzadeh reported having no financial conflicts.