User login
ISTANBUL, TURKEY – The case for omalizumab as a novel, safe, and highly effective therapy for H1-antihistamine-refractory chronic idiopathic/spontaneous urticaria received a boost in the form of two new phase III clinical trials presented at the annual congress of the European Academy of Dermatology and Venereology.
The two new multicenter, randomized, double-blind, placebo-controlled studies join another trial published earlier this year (N. Engl. J. Med. 2013;368:924-35). Together, the three trials total nearly 1,000 participants aged 12-75 years who remained severely affected by chronic idiopathic/spontaneous urticaria despite treatment with nonsedating H1-antihistamines, the sole approved form of treatment.
The GLACIAL trial, one of the new studies, set the bar even higher: Participants not only had to be refractory to H1-antihistamines at up to four times the approved dose, but also to off-label H2-antihistamines and/or leukotriene receptor antagonists.
The results were highly consistent across all three clinical trials – 80%-90% of patients demonstrated a dramatic clinical response to omalizumab in dose-dependent fashion. The top dose studied, 300 mg per subcutaneous injection once every 4 weeks, brought the greatest reductions in itching, wheals, and days with angioedema, Dr. Marcus Maurer at the meeting, where he presented the phase III data from the GLACIAL and ASTERIA I trials.
"Omalizumab is the new hope in urticaria treatment. The response is super-fast. We saw a massive and rapid decline in urticaria activity as early as in the first days after the first injection," he said. "In fact, the vast majority of responders respond within the first week of treatment. The disease activity then stays down as long as treatment continues," noted Dr. Maurer, professor of dermatology and allergy at Charité University Hospital, Berlin.
"The other important thing is, as soon as you stop the drug, you stop the benefit. Patients go back to baseline," he added. "So omalizumab is a very effective way to control disease activity, but it’s no cure. It doesn’t make the disease go away, it makes the symptoms go away. There is spontaneous remission in chronic idiopathic/spontaneous urticaria, but until that happens, patients need treatment that makes them free of symptoms. I’ve had patients on omalizumab for 5-6 years now."
As for the safety profile, Dr. Maurer called it "boring."
"This is a safe drug. This is a drug that doesn’t have any differences in its safety profile compared to placebo. The number and types of adverse events are virtually identical," he said.
Less than a week after Dr. Maurer’s presentation of the GLACIAL and ASTERIA I phase III trial results in Istanbul, Genentech filed an application with the Food and Drug Administration requesting approval of a supplemental indication for omalizumab in the treatment of chronic idiopathic/spontaneous urticaria in patients who remain symptomatic despite H1-antihistamine therapy at approved doses. A decision by the agency is expected in the spring of 2014.
Omalizumab is a humanized anti-IgE monoclonal antibody already approved as Xolair for treatment of moderate to severe allergic asthma. Unlike in allergic asthma, however, the response of chronic urticaria to omalizumab is independent of serum IgE level. Indeed, IgE levels are only modestly elevated in patients with chronic idiopathic/spontaneous urticaria – nothing like the high levels that can occur in allergic asthma. It’s not useful to measure IgE as a guide to the likelihood that chronic idiopathic/spontaneous urticaria will respond to omalizumab, Dr. Maurer advised.
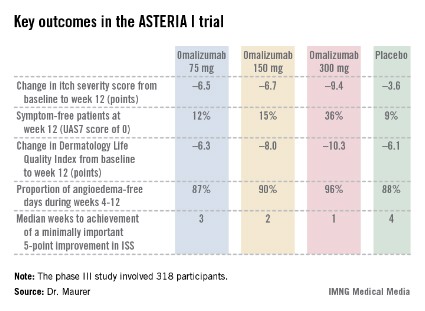
The ASTERIA I trial was arguably the most clinically relevant of the three phase III studies because it employed omalizumab as add-on therapy to H1-antihistamines at approved doses. The 318 participants had a baseline mean weekly Itch Severity Scale (ISS) score of 14.3 out of a possible 21 despite being on H1-antihistamines. Their baseline urticaria activity score over 7 days (UAS7) was 31.1 on a scale of 0-42.
The study population was typical for patients with moderate to severe chronic idiopathic/spontaneous urticaria refractory to first-line therapy with H1-antihistamines. The average age was 41 years, three-quarters were female, and the mean body mass index was 29 kg/m2. The patients were diagnosed with chronic idiopathic/spontaneous urticaria an average of 4 years earlier and had been on an average of five previous unsuccessful medications for the condition. Half of the patients had angioedema.
Study participants received six subcutaneous doses of omalizumab at either 75-, 150-, or 300-mg doses; or placebo at 4-week intervals. They were then observed for 16 weeks. The primary study endpoint was the change in weekly ISS from baseline to week 12. The mean reduction was 3.6 points in the control group, significantly less than the 6.5-point fall in the low-dose omalizumab arm, the 6.7-point reduction with omalizumab 150 mg, or the 9.4-point decrease with omalizumab at 300 mg.
Omalizumab-treated patients also fared significantly better than did the placebo group in all secondary endpoints.
The anti-IgE biologic was well tolerated, with no new safety signals arising in ASTERIA I or the other two phase III trials beyond the agent’s well-established safety profile in treating allergic asthma.
The diagnosis of chronic idiopathic/spontaneous urticaria is based upon the finding of itchy hives and/or angioedema for 6 weeks or more with no specific external trigger. The estimated prevalence in the general population is 0.5%-1%. More than half of affected patients continue to experience symptoms despite treatment with H1-antihistamines.
The phase III trials were sponsored by Genentech and Novartis, which are jointly developing omalizumab for the treatment of chronic idiopathic/spontaneous urticaria. Dr. Maurer has received research grants from, and served on the advisory boards of, those pharmaceutical companies, as well as more than a dozen others.
ISTANBUL, TURKEY – The case for omalizumab as a novel, safe, and highly effective therapy for H1-antihistamine-refractory chronic idiopathic/spontaneous urticaria received a boost in the form of two new phase III clinical trials presented at the annual congress of the European Academy of Dermatology and Venereology.
The two new multicenter, randomized, double-blind, placebo-controlled studies join another trial published earlier this year (N. Engl. J. Med. 2013;368:924-35). Together, the three trials total nearly 1,000 participants aged 12-75 years who remained severely affected by chronic idiopathic/spontaneous urticaria despite treatment with nonsedating H1-antihistamines, the sole approved form of treatment.

The GLACIAL trial, one of the new studies, set the bar even higher: Participants not only had to be refractory to H1-antihistamines at up to four times the approved dose, but also to off-label H2-antihistamines and/or leukotriene receptor antagonists.
The results were highly consistent across all three clinical trials – 80%-90% of patients demonstrated a dramatic clinical response to omalizumab in dose-dependent fashion. The top dose studied, 300 mg per subcutaneous injection once every 4 weeks, brought the greatest reductions in itching, wheals, and days with angioedema, Dr. Marcus Maurer at the meeting, where he presented the phase III data from the GLACIAL and ASTERIA I trials.
"Omalizumab is the new hope in urticaria treatment. The response is super-fast. We saw a massive and rapid decline in urticaria activity as early as in the first days after the first injection," he said. "In fact, the vast majority of responders respond within the first week of treatment. The disease activity then stays down as long as treatment continues," noted Dr. Maurer, professor of dermatology and allergy at Charité University Hospital, Berlin.
"The other important thing is, as soon as you stop the drug, you stop the benefit. Patients go back to baseline," he added. "So omalizumab is a very effective way to control disease activity, but it’s no cure. It doesn’t make the disease go away, it makes the symptoms go away. There is spontaneous remission in chronic idiopathic/spontaneous urticaria, but until that happens, patients need treatment that makes them free of symptoms. I’ve had patients on omalizumab for 5-6 years now."
As for the safety profile, Dr. Maurer called it "boring."
"This is a safe drug. This is a drug that doesn’t have any differences in its safety profile compared to placebo. The number and types of adverse events are virtually identical," he said.
Less than a week after Dr. Maurer’s presentation of the GLACIAL and ASTERIA I phase III trial results in Istanbul, Genentech filed an application with the Food and Drug Administration requesting approval of a supplemental indication for omalizumab in the treatment of chronic idiopathic/spontaneous urticaria in patients who remain symptomatic despite H1-antihistamine therapy at approved doses. A decision by the agency is expected in the spring of 2014.
Omalizumab is a humanized anti-IgE monoclonal antibody already approved as Xolair for treatment of moderate to severe allergic asthma. Unlike in allergic asthma, however, the response of chronic urticaria to omalizumab is independent of serum IgE level. Indeed, IgE levels are only modestly elevated in patients with chronic idiopathic/spontaneous urticaria – nothing like the high levels that can occur in allergic asthma. It’s not useful to measure IgE as a guide to the likelihood that chronic idiopathic/spontaneous urticaria will respond to omalizumab, Dr. Maurer advised.
The ASTERIA I trial was arguably the most clinically relevant of the three phase III studies because it employed omalizumab as add-on therapy to H1-antihistamines at approved doses. The 318 participants had a baseline mean weekly Itch Severity Scale (ISS) score of 14.3 out of a possible 21 despite being on H1-antihistamines. Their baseline urticaria activity score over 7 days (UAS7) was 31.1 on a scale of 0-42.
The study population was typical for patients with moderate to severe chronic idiopathic/spontaneous urticaria refractory to first-line therapy with H1-antihistamines. The average age was 41 years, three-quarters were female, and the mean body mass index was 29 kg/m2. The patients were diagnosed with chronic idiopathic/spontaneous urticaria an average of 4 years earlier and had been on an average of five previous unsuccessful medications for the condition. Half of the patients had angioedema.
Study participants received six subcutaneous doses of omalizumab at either 75-, 150-, or 300-mg doses; or placebo at 4-week intervals. They were then observed for 16 weeks. The primary study endpoint was the change in weekly ISS from baseline to week 12. The mean reduction was 3.6 points in the control group, significantly less than the 6.5-point fall in the low-dose omalizumab arm, the 6.7-point reduction with omalizumab 150 mg, or the 9.4-point decrease with omalizumab at 300 mg.
Omalizumab-treated patients also fared significantly better than did the placebo group in all secondary endpoints.
The anti-IgE biologic was well tolerated, with no new safety signals arising in ASTERIA I or the other two phase III trials beyond the agent’s well-established safety profile in treating allergic asthma.
The diagnosis of chronic idiopathic/spontaneous urticaria is based upon the finding of itchy hives and/or angioedema for 6 weeks or more with no specific external trigger. The estimated prevalence in the general population is 0.5%-1%. More than half of affected patients continue to experience symptoms despite treatment with H1-antihistamines.
The phase III trials were sponsored by Genentech and Novartis, which are jointly developing omalizumab for the treatment of chronic idiopathic/spontaneous urticaria. Dr. Maurer has received research grants from, and served on the advisory boards of, those pharmaceutical companies, as well as more than a dozen others.
ISTANBUL, TURKEY – The case for omalizumab as a novel, safe, and highly effective therapy for H1-antihistamine-refractory chronic idiopathic/spontaneous urticaria received a boost in the form of two new phase III clinical trials presented at the annual congress of the European Academy of Dermatology and Venereology.
The two new multicenter, randomized, double-blind, placebo-controlled studies join another trial published earlier this year (N. Engl. J. Med. 2013;368:924-35). Together, the three trials total nearly 1,000 participants aged 12-75 years who remained severely affected by chronic idiopathic/spontaneous urticaria despite treatment with nonsedating H1-antihistamines, the sole approved form of treatment.

The GLACIAL trial, one of the new studies, set the bar even higher: Participants not only had to be refractory to H1-antihistamines at up to four times the approved dose, but also to off-label H2-antihistamines and/or leukotriene receptor antagonists.
The results were highly consistent across all three clinical trials – 80%-90% of patients demonstrated a dramatic clinical response to omalizumab in dose-dependent fashion. The top dose studied, 300 mg per subcutaneous injection once every 4 weeks, brought the greatest reductions in itching, wheals, and days with angioedema, Dr. Marcus Maurer at the meeting, where he presented the phase III data from the GLACIAL and ASTERIA I trials.
"Omalizumab is the new hope in urticaria treatment. The response is super-fast. We saw a massive and rapid decline in urticaria activity as early as in the first days after the first injection," he said. "In fact, the vast majority of responders respond within the first week of treatment. The disease activity then stays down as long as treatment continues," noted Dr. Maurer, professor of dermatology and allergy at Charité University Hospital, Berlin.
"The other important thing is, as soon as you stop the drug, you stop the benefit. Patients go back to baseline," he added. "So omalizumab is a very effective way to control disease activity, but it’s no cure. It doesn’t make the disease go away, it makes the symptoms go away. There is spontaneous remission in chronic idiopathic/spontaneous urticaria, but until that happens, patients need treatment that makes them free of symptoms. I’ve had patients on omalizumab for 5-6 years now."
As for the safety profile, Dr. Maurer called it "boring."
"This is a safe drug. This is a drug that doesn’t have any differences in its safety profile compared to placebo. The number and types of adverse events are virtually identical," he said.
Less than a week after Dr. Maurer’s presentation of the GLACIAL and ASTERIA I phase III trial results in Istanbul, Genentech filed an application with the Food and Drug Administration requesting approval of a supplemental indication for omalizumab in the treatment of chronic idiopathic/spontaneous urticaria in patients who remain symptomatic despite H1-antihistamine therapy at approved doses. A decision by the agency is expected in the spring of 2014.
Omalizumab is a humanized anti-IgE monoclonal antibody already approved as Xolair for treatment of moderate to severe allergic asthma. Unlike in allergic asthma, however, the response of chronic urticaria to omalizumab is independent of serum IgE level. Indeed, IgE levels are only modestly elevated in patients with chronic idiopathic/spontaneous urticaria – nothing like the high levels that can occur in allergic asthma. It’s not useful to measure IgE as a guide to the likelihood that chronic idiopathic/spontaneous urticaria will respond to omalizumab, Dr. Maurer advised.
The ASTERIA I trial was arguably the most clinically relevant of the three phase III studies because it employed omalizumab as add-on therapy to H1-antihistamines at approved doses. The 318 participants had a baseline mean weekly Itch Severity Scale (ISS) score of 14.3 out of a possible 21 despite being on H1-antihistamines. Their baseline urticaria activity score over 7 days (UAS7) was 31.1 on a scale of 0-42.
The study population was typical for patients with moderate to severe chronic idiopathic/spontaneous urticaria refractory to first-line therapy with H1-antihistamines. The average age was 41 years, three-quarters were female, and the mean body mass index was 29 kg/m2. The patients were diagnosed with chronic idiopathic/spontaneous urticaria an average of 4 years earlier and had been on an average of five previous unsuccessful medications for the condition. Half of the patients had angioedema.
Study participants received six subcutaneous doses of omalizumab at either 75-, 150-, or 300-mg doses; or placebo at 4-week intervals. They were then observed for 16 weeks. The primary study endpoint was the change in weekly ISS from baseline to week 12. The mean reduction was 3.6 points in the control group, significantly less than the 6.5-point fall in the low-dose omalizumab arm, the 6.7-point reduction with omalizumab 150 mg, or the 9.4-point decrease with omalizumab at 300 mg.
Omalizumab-treated patients also fared significantly better than did the placebo group in all secondary endpoints.
The anti-IgE biologic was well tolerated, with no new safety signals arising in ASTERIA I or the other two phase III trials beyond the agent’s well-established safety profile in treating allergic asthma.
The diagnosis of chronic idiopathic/spontaneous urticaria is based upon the finding of itchy hives and/or angioedema for 6 weeks or more with no specific external trigger. The estimated prevalence in the general population is 0.5%-1%. More than half of affected patients continue to experience symptoms despite treatment with H1-antihistamines.
The phase III trials were sponsored by Genentech and Novartis, which are jointly developing omalizumab for the treatment of chronic idiopathic/spontaneous urticaria. Dr. Maurer has received research grants from, and served on the advisory boards of, those pharmaceutical companies, as well as more than a dozen others.
AT THE EADV CONGRESS
Major finding: Among 318 patients with chronic idiopathic/spontaneous urticaria refractory to H1-antihistamines at standard doses who participated in the phase III ASTERIA I trial, add-on omalizumab at 300 mg resulted in a mean 9.4-point reduction in itch severity score at week 12 compared to a 3.6-point drop with placebo from a baseline score of 14.3.
Data source: ASTERIA I, one of three recent phase III randomized, double-blind, multicenter, placebo-controlled clinical trials of omalizumab for chronic idiopathic/spontaneous urticaria totaling nearly 1,000 participants.
Disclosures: The phase III clinical trials were jointly sponsored by Genentech and Novartis. The presenter reported having received research funds from the two companies as well as serving on their advisory boards.