User login
Study: TNF inhibitors improve extraintestinal IBD manifestations
SAN DIEGO – Tumor necrosis factor inhibitors improved the extraintestinal manifestations of inflammatory bowel disease (IBD) among more than half of affected patients, according to a national cohort study.
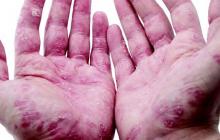
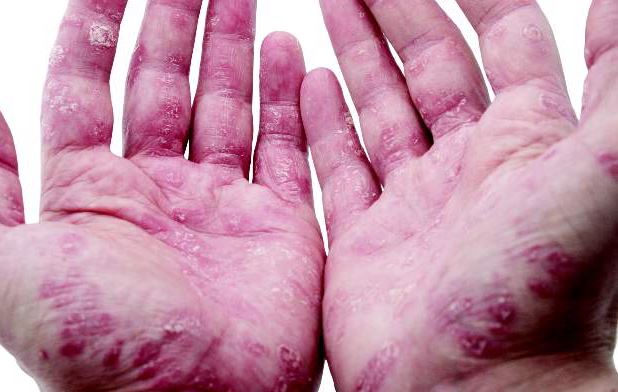
“The best response rates were for psoriasis, aphthous stomatitis, uveitis, and peripheral arthritis,” said Dr. Thomas Greuter of University Hospital in Zürich. Patients responded similarly whether they received oral infliximab or subcutaneous adalimumab or certolizumab, he noted.
IBD often is associated with debilitating disorders of the skin, joints, eyes, and hepatobiliary tract, but “due to the lack of randomized, controlled trials, the therapy of extraintestinal manifestations remains rather empirical,” Dr. Greuter said at the annual Digestive Disease Week.
To study the role of anti-TNF agents in treating these disorders, he and his associates analyzed data for 1,249 patients from the national Swiss IBD Cohort Study between 2006 and 2010. Patients were typically in their mid-30s and had lived with IBD for about 9 years, he said.
A total of 366 patients (29%) had at least one extraintestinal manifestation of IBD – most commonly peripheral arthritis (75%), followed by aphthous stomatitis (24%), and ankylosing spondylitis (22%). In all, 213 (58%) patients received at least one anti-TNF agent, and 40% received the prescription specifically for extraintestinal manifestations. Nearly two-thirds of the patients received infliximab, while 22% received adalimumab and 15% received certolizumab.
About 55% of patients improved on anti-TNF therapy over an average of 7 years of follow-up, Dr. Greuter and his associates reported. Among all three anti-TNF agents, response rates ranged from 100% for psoriasis, to 80% for erythema nodosum and stomatitis, to 73% for arthritis and uveitis, to 50% for pyoderma granulosum. Overall rates of improvement were slightly higher for infliximab than for the other two drugs, but “adalimumab and certolizumab were used mostly as a second or a third-line anti-TNF agent, and the response rate to a second or third-line treatment was lower than for the first one,” Dr. Greuter said. Some patients also received corticosteroids and immunomodulators, but excluding this subgroup had little effect on rates of response to anti-TNF therapy, he added.
Dr. Greuter also reported that 11 patients (about 5% of the cohort) developed 14 new extraintestinal manifestations after starting anti-TNF agents – usually peripheral arthritis, but also pyoderma granulosum, aphthous stomatitis, psoriasis, and uveitis. “We cannot say if this was primary, or a side effect of treatment,” he said. These disorders usually improved if patients stayed on their anti-TNF agent, he added.
About two-thirds of patients in the cohort were female, more than three-quarters had Crohn’s disease, 19% had ulcerative colitis, and 3% had indeterminate colitis, he noted.
A research grant from the Swiss National Science Foundation funded the study. Dr. Greuter had no disclosures.
SAN DIEGO – Tumor necrosis factor inhibitors improved the extraintestinal manifestations of inflammatory bowel disease (IBD) among more than half of affected patients, according to a national cohort study.
“The best response rates were for psoriasis, aphthous stomatitis, uveitis, and peripheral arthritis,” said Dr. Thomas Greuter of University Hospital in Zürich. Patients responded similarly whether they received oral infliximab or subcutaneous adalimumab or certolizumab, he noted.
IBD often is associated with debilitating disorders of the skin, joints, eyes, and hepatobiliary tract, but “due to the lack of randomized, controlled trials, the therapy of extraintestinal manifestations remains rather empirical,” Dr. Greuter said at the annual Digestive Disease Week.
To study the role of anti-TNF agents in treating these disorders, he and his associates analyzed data for 1,249 patients from the national Swiss IBD Cohort Study between 2006 and 2010. Patients were typically in their mid-30s and had lived with IBD for about 9 years, he said.
A total of 366 patients (29%) had at least one extraintestinal manifestation of IBD – most commonly peripheral arthritis (75%), followed by aphthous stomatitis (24%), and ankylosing spondylitis (22%). In all, 213 (58%) patients received at least one anti-TNF agent, and 40% received the prescription specifically for extraintestinal manifestations. Nearly two-thirds of the patients received infliximab, while 22% received adalimumab and 15% received certolizumab.
About 55% of patients improved on anti-TNF therapy over an average of 7 years of follow-up, Dr. Greuter and his associates reported. Among all three anti-TNF agents, response rates ranged from 100% for psoriasis, to 80% for erythema nodosum and stomatitis, to 73% for arthritis and uveitis, to 50% for pyoderma granulosum. Overall rates of improvement were slightly higher for infliximab than for the other two drugs, but “adalimumab and certolizumab were used mostly as a second or a third-line anti-TNF agent, and the response rate to a second or third-line treatment was lower than for the first one,” Dr. Greuter said. Some patients also received corticosteroids and immunomodulators, but excluding this subgroup had little effect on rates of response to anti-TNF therapy, he added.
Dr. Greuter also reported that 11 patients (about 5% of the cohort) developed 14 new extraintestinal manifestations after starting anti-TNF agents – usually peripheral arthritis, but also pyoderma granulosum, aphthous stomatitis, psoriasis, and uveitis. “We cannot say if this was primary, or a side effect of treatment,” he said. These disorders usually improved if patients stayed on their anti-TNF agent, he added.
About two-thirds of patients in the cohort were female, more than three-quarters had Crohn’s disease, 19% had ulcerative colitis, and 3% had indeterminate colitis, he noted.
A research grant from the Swiss National Science Foundation funded the study. Dr. Greuter had no disclosures.
SAN DIEGO – Tumor necrosis factor inhibitors improved the extraintestinal manifestations of inflammatory bowel disease (IBD) among more than half of affected patients, according to a national cohort study.
“The best response rates were for psoriasis, aphthous stomatitis, uveitis, and peripheral arthritis,” said Dr. Thomas Greuter of University Hospital in Zürich. Patients responded similarly whether they received oral infliximab or subcutaneous adalimumab or certolizumab, he noted.
IBD often is associated with debilitating disorders of the skin, joints, eyes, and hepatobiliary tract, but “due to the lack of randomized, controlled trials, the therapy of extraintestinal manifestations remains rather empirical,” Dr. Greuter said at the annual Digestive Disease Week.
To study the role of anti-TNF agents in treating these disorders, he and his associates analyzed data for 1,249 patients from the national Swiss IBD Cohort Study between 2006 and 2010. Patients were typically in their mid-30s and had lived with IBD for about 9 years, he said.
A total of 366 patients (29%) had at least one extraintestinal manifestation of IBD – most commonly peripheral arthritis (75%), followed by aphthous stomatitis (24%), and ankylosing spondylitis (22%). In all, 213 (58%) patients received at least one anti-TNF agent, and 40% received the prescription specifically for extraintestinal manifestations. Nearly two-thirds of the patients received infliximab, while 22% received adalimumab and 15% received certolizumab.
About 55% of patients improved on anti-TNF therapy over an average of 7 years of follow-up, Dr. Greuter and his associates reported. Among all three anti-TNF agents, response rates ranged from 100% for psoriasis, to 80% for erythema nodosum and stomatitis, to 73% for arthritis and uveitis, to 50% for pyoderma granulosum. Overall rates of improvement were slightly higher for infliximab than for the other two drugs, but “adalimumab and certolizumab were used mostly as a second or a third-line anti-TNF agent, and the response rate to a second or third-line treatment was lower than for the first one,” Dr. Greuter said. Some patients also received corticosteroids and immunomodulators, but excluding this subgroup had little effect on rates of response to anti-TNF therapy, he added.
Dr. Greuter also reported that 11 patients (about 5% of the cohort) developed 14 new extraintestinal manifestations after starting anti-TNF agents – usually peripheral arthritis, but also pyoderma granulosum, aphthous stomatitis, psoriasis, and uveitis. “We cannot say if this was primary, or a side effect of treatment,” he said. These disorders usually improved if patients stayed on their anti-TNF agent, he added.
About two-thirds of patients in the cohort were female, more than three-quarters had Crohn’s disease, 19% had ulcerative colitis, and 3% had indeterminate colitis, he noted.
A research grant from the Swiss National Science Foundation funded the study. Dr. Greuter had no disclosures.
AT DDW® 2016
Key clinical point: Tumor necrosis factor inhibitors improved the extraintestinal manifestations of inflammatory bowel disease among more than half of affected patients.
Major finding: About 55% of patients who received infliximab, adalimumab, or certolizumab had a clinical response.
Data source: A study of 1,249 patients with IBD from a national cohort.
Disclosures: A research grant from the Swiss National Science Foundation funded the study. Dr. Greuter had no disclosures.
Women underrepresented as GI division chiefs
SAN DIEGO – Despite an increase in women entering the gastroenterology field, a new study finds that they are severely underrepresented in academia as fellowship program directors and division chiefs.
In addition, most female program directors have lower academic ranks than did their male counterparts.
“A lack of female leadership can detract future women from entering the field,” said study lead author Dr. Sonali Paul, a gastroenterologist and research fellow in medicine at Massachusetts General Hospital in Boston. “Further research is needed to understand these barriers, whether they’re due to personal choice or more systemic issues. Understanding the challenges women face in academic promotions will allow for policy changes.”
According to statistics quoted in the study, women made up more than a third of gastroenterology fellowship positions and 18% of the academic workforce in 2013. At the same time, more than half of medical graduates are women.
“The role of women in GI is important, especially given that studies have shown that women prefer female endoscopists,” Dr. Paul said. And women make up significant portions of the patient load in GI clinical practices, she said.
But it hasn’t been clear if the increase in fellowship positions has corresponded to a higher number of women in positions of academic leadership.
To better understand the situation, Dr. Paul and her associates analyzed the gender and academic rank of GI fellowship program directors and division chiefs in 2015. The researchers analyzed websites and social media to gather the data and also made direct contacts, Dr. Paul said.
At 165 academic GI programs, 85% of these positions were held by men. Of program directors, 81% were male; 89% of division chiefs were male.
Of the men who hold these positions, half were program directors and half were division chiefs. Among women, however, 67% were program directors and 33% were division chiefs.
There was also a disparity in terms of academic experience. Female division chiefs were less likely to be professors (46%), compared with male division chiefs (82%).
Why the disparity? One possible explanation is a “leaky pipeline,” Dr. Paul said. This refers to the fact that women leave academic medicine at higher rates than men do.
Family obligations are one explanation, she said, “but I don’t think that explains everything in the process. A lot of women have felt that promotion was of no benefit to them and that there was a lack of encouragement.”
Dr. Paul notes that the study has limitations. It’s cross sectional, and misclassification errors are possible.
During a discussion period, a questioner noted that it takes time for an academic physician to move up: “Shouldn’t we look at the fraction of graduates in 1996 who were female and now are professors? It’s unusual for someone at less than a professor rank to be division chief, as compared with program director.” Dr. Paul agreed that this would be a good avenue of research.
In addition, she said, “it will be important to examine if these gender disparities are unique to gastroenterology or if they extend into other specialties that are more female dominant, such as rheumatology or geriatrics.”
SAN DIEGO – Despite an increase in women entering the gastroenterology field, a new study finds that they are severely underrepresented in academia as fellowship program directors and division chiefs.
In addition, most female program directors have lower academic ranks than did their male counterparts.
“A lack of female leadership can detract future women from entering the field,” said study lead author Dr. Sonali Paul, a gastroenterologist and research fellow in medicine at Massachusetts General Hospital in Boston. “Further research is needed to understand these barriers, whether they’re due to personal choice or more systemic issues. Understanding the challenges women face in academic promotions will allow for policy changes.”
According to statistics quoted in the study, women made up more than a third of gastroenterology fellowship positions and 18% of the academic workforce in 2013. At the same time, more than half of medical graduates are women.
“The role of women in GI is important, especially given that studies have shown that women prefer female endoscopists,” Dr. Paul said. And women make up significant portions of the patient load in GI clinical practices, she said.
But it hasn’t been clear if the increase in fellowship positions has corresponded to a higher number of women in positions of academic leadership.
To better understand the situation, Dr. Paul and her associates analyzed the gender and academic rank of GI fellowship program directors and division chiefs in 2015. The researchers analyzed websites and social media to gather the data and also made direct contacts, Dr. Paul said.
At 165 academic GI programs, 85% of these positions were held by men. Of program directors, 81% were male; 89% of division chiefs were male.
Of the men who hold these positions, half were program directors and half were division chiefs. Among women, however, 67% were program directors and 33% were division chiefs.
There was also a disparity in terms of academic experience. Female division chiefs were less likely to be professors (46%), compared with male division chiefs (82%).
Why the disparity? One possible explanation is a “leaky pipeline,” Dr. Paul said. This refers to the fact that women leave academic medicine at higher rates than men do.
Family obligations are one explanation, she said, “but I don’t think that explains everything in the process. A lot of women have felt that promotion was of no benefit to them and that there was a lack of encouragement.”
Dr. Paul notes that the study has limitations. It’s cross sectional, and misclassification errors are possible.
During a discussion period, a questioner noted that it takes time for an academic physician to move up: “Shouldn’t we look at the fraction of graduates in 1996 who were female and now are professors? It’s unusual for someone at less than a professor rank to be division chief, as compared with program director.” Dr. Paul agreed that this would be a good avenue of research.
In addition, she said, “it will be important to examine if these gender disparities are unique to gastroenterology or if they extend into other specialties that are more female dominant, such as rheumatology or geriatrics.”
SAN DIEGO – Despite an increase in women entering the gastroenterology field, a new study finds that they are severely underrepresented in academia as fellowship program directors and division chiefs.
In addition, most female program directors have lower academic ranks than did their male counterparts.
“A lack of female leadership can detract future women from entering the field,” said study lead author Dr. Sonali Paul, a gastroenterologist and research fellow in medicine at Massachusetts General Hospital in Boston. “Further research is needed to understand these barriers, whether they’re due to personal choice or more systemic issues. Understanding the challenges women face in academic promotions will allow for policy changes.”
According to statistics quoted in the study, women made up more than a third of gastroenterology fellowship positions and 18% of the academic workforce in 2013. At the same time, more than half of medical graduates are women.
“The role of women in GI is important, especially given that studies have shown that women prefer female endoscopists,” Dr. Paul said. And women make up significant portions of the patient load in GI clinical practices, she said.
But it hasn’t been clear if the increase in fellowship positions has corresponded to a higher number of women in positions of academic leadership.
To better understand the situation, Dr. Paul and her associates analyzed the gender and academic rank of GI fellowship program directors and division chiefs in 2015. The researchers analyzed websites and social media to gather the data and also made direct contacts, Dr. Paul said.
At 165 academic GI programs, 85% of these positions were held by men. Of program directors, 81% were male; 89% of division chiefs were male.
Of the men who hold these positions, half were program directors and half were division chiefs. Among women, however, 67% were program directors and 33% were division chiefs.
There was also a disparity in terms of academic experience. Female division chiefs were less likely to be professors (46%), compared with male division chiefs (82%).
Why the disparity? One possible explanation is a “leaky pipeline,” Dr. Paul said. This refers to the fact that women leave academic medicine at higher rates than men do.
Family obligations are one explanation, she said, “but I don’t think that explains everything in the process. A lot of women have felt that promotion was of no benefit to them and that there was a lack of encouragement.”
Dr. Paul notes that the study has limitations. It’s cross sectional, and misclassification errors are possible.
During a discussion period, a questioner noted that it takes time for an academic physician to move up: “Shouldn’t we look at the fraction of graduates in 1996 who were female and now are professors? It’s unusual for someone at less than a professor rank to be division chief, as compared with program director.” Dr. Paul agreed that this would be a good avenue of research.
In addition, she said, “it will be important to examine if these gender disparities are unique to gastroenterology or if they extend into other specialties that are more female dominant, such as rheumatology or geriatrics.”
AT DDW® 2016
Increase infliximab dosing to heal Crohn’s fistulas
SAN DIEGO – It takes more than the usual 5 mg/kg of infliximab to heal perianal fistulas in some Crohn’s disease patients, according to a multicenter review of 117 cases.
The investigators aimed for trough levels of at least 10-20 mcg/mL, higher than the usual target of perhaps 7 mcg/mL or less. Doing so generally required 10 mg/kg every 8 weeks, but a few patients needed 10 mg/kg or even 15 mg/kg every 4 weeks. Fistulas healed in 63 (54%) patients and closed in 36 (31%).
Troughs of at least 10 mcg/mL and a history of dose escalation were both independent predictors of fistula healing, defined as the absence of drainage. Patients with fistula healing had significantly higher infliximab levels compared to those with active fistulas (18.5 vs. 6.5 mcg/mL; P less than .0001), and there was an incremental gain in healing with higher infliximab levels up to 50 mcg/mL. The association between infliximab levels and fistula healing had an area under the curve of 0.82 (P less than .0001) and the association with fistula closure was 0.69 (P = .014).
“Infliximab levels needed to achieve fistula healing were higher than what [has] been described for mucosal healing. Achieving higher infliximab levels in patients with Crohn’s disease and perianal fistulas may improve outcomes and should be considered in a treat-to-target strategy. At least 10-20 mcg/mL may be needed to achieve fistula healing in some patients,” concluded investigator Dr. Andres J. Yarur, a gastroenterologist at the Medical College of Wisconsin, Milwaukee.
“Obviously, in somebody with active perianal fistulas” at a trough of 30 mcg/mL, “it’s time to try something else, but I don’t give up on infliximab at a level of 7 mcg/mL, like a lot of people do. I’m kind of aggressive with these patients.” There wasn’t a formal assessment of adverse events, but “we have not really seen any side effects,” he said at the annual Digestive Disease Week meeting. One audience member remarked that the findings will likely change how he treats Crohn’s fistulas.
Dr. Yarur said he wasn’t surprised by the findings, “but I have more reassurance now” that aggressive dosing is the way to go for aggressive Crohn’s.
The patients were an average of 39 years old, with a mean disease duration of 12 years; about half were women. They were on infliximab for a median of 19 months. Infliximab antibodies dropped the likelihood of healing (odds ratio, 0.04; 95% confidence interval, 0.005-0.3; P less than .001) and closure (OR 0.038; 95% CI, 0.005-0.3; P less than .0001). About two-thirds of the patients were on concomitant immunomodulators, but they did not increase the odds of healing (OR 1.7; 95% CI, 0.8-3.7; P = .157).
“We usually start at a standard dose of 5 mg/kg, and then start checking troughs at week 14, before the first maintenance dose, and adjust” upward to hit the trough target. However, dosing also depends on “how much I think the patient needs. I don’t think we should standardize trough levels,” Dr. Yarur said. The team checks levels with the homogeneous mobility-shift assay from Prometheus Laboratories. “The type of assay you use is very important,” because interpretation of the results varies from one to the next.
De-escalation is on a case-by-case basis. “I would be very careful de-escalating somebody [who] has very aggressive disease. Many times patients don’t want to go down. They say ‘I feel good doc. I have no side effects. Leave me on my dose,’ ” he said.
There was a lack of cross-sectional imaging in the study; the investigators couldn’t distinguish simple fistulas from complex ones in their review.
Dr. Yarur had no disclosures. There was no industry funding for the work.
SAN DIEGO – It takes more than the usual 5 mg/kg of infliximab to heal perianal fistulas in some Crohn’s disease patients, according to a multicenter review of 117 cases.
The investigators aimed for trough levels of at least 10-20 mcg/mL, higher than the usual target of perhaps 7 mcg/mL or less. Doing so generally required 10 mg/kg every 8 weeks, but a few patients needed 10 mg/kg or even 15 mg/kg every 4 weeks. Fistulas healed in 63 (54%) patients and closed in 36 (31%).
Troughs of at least 10 mcg/mL and a history of dose escalation were both independent predictors of fistula healing, defined as the absence of drainage. Patients with fistula healing had significantly higher infliximab levels compared to those with active fistulas (18.5 vs. 6.5 mcg/mL; P less than .0001), and there was an incremental gain in healing with higher infliximab levels up to 50 mcg/mL. The association between infliximab levels and fistula healing had an area under the curve of 0.82 (P less than .0001) and the association with fistula closure was 0.69 (P = .014).
“Infliximab levels needed to achieve fistula healing were higher than what [has] been described for mucosal healing. Achieving higher infliximab levels in patients with Crohn’s disease and perianal fistulas may improve outcomes and should be considered in a treat-to-target strategy. At least 10-20 mcg/mL may be needed to achieve fistula healing in some patients,” concluded investigator Dr. Andres J. Yarur, a gastroenterologist at the Medical College of Wisconsin, Milwaukee.
“Obviously, in somebody with active perianal fistulas” at a trough of 30 mcg/mL, “it’s time to try something else, but I don’t give up on infliximab at a level of 7 mcg/mL, like a lot of people do. I’m kind of aggressive with these patients.” There wasn’t a formal assessment of adverse events, but “we have not really seen any side effects,” he said at the annual Digestive Disease Week meeting. One audience member remarked that the findings will likely change how he treats Crohn’s fistulas.
Dr. Yarur said he wasn’t surprised by the findings, “but I have more reassurance now” that aggressive dosing is the way to go for aggressive Crohn’s.
The patients were an average of 39 years old, with a mean disease duration of 12 years; about half were women. They were on infliximab for a median of 19 months. Infliximab antibodies dropped the likelihood of healing (odds ratio, 0.04; 95% confidence interval, 0.005-0.3; P less than .001) and closure (OR 0.038; 95% CI, 0.005-0.3; P less than .0001). About two-thirds of the patients were on concomitant immunomodulators, but they did not increase the odds of healing (OR 1.7; 95% CI, 0.8-3.7; P = .157).
“We usually start at a standard dose of 5 mg/kg, and then start checking troughs at week 14, before the first maintenance dose, and adjust” upward to hit the trough target. However, dosing also depends on “how much I think the patient needs. I don’t think we should standardize trough levels,” Dr. Yarur said. The team checks levels with the homogeneous mobility-shift assay from Prometheus Laboratories. “The type of assay you use is very important,” because interpretation of the results varies from one to the next.
De-escalation is on a case-by-case basis. “I would be very careful de-escalating somebody [who] has very aggressive disease. Many times patients don’t want to go down. They say ‘I feel good doc. I have no side effects. Leave me on my dose,’ ” he said.
There was a lack of cross-sectional imaging in the study; the investigators couldn’t distinguish simple fistulas from complex ones in their review.
Dr. Yarur had no disclosures. There was no industry funding for the work.
SAN DIEGO – It takes more than the usual 5 mg/kg of infliximab to heal perianal fistulas in some Crohn’s disease patients, according to a multicenter review of 117 cases.
The investigators aimed for trough levels of at least 10-20 mcg/mL, higher than the usual target of perhaps 7 mcg/mL or less. Doing so generally required 10 mg/kg every 8 weeks, but a few patients needed 10 mg/kg or even 15 mg/kg every 4 weeks. Fistulas healed in 63 (54%) patients and closed in 36 (31%).
Troughs of at least 10 mcg/mL and a history of dose escalation were both independent predictors of fistula healing, defined as the absence of drainage. Patients with fistula healing had significantly higher infliximab levels compared to those with active fistulas (18.5 vs. 6.5 mcg/mL; P less than .0001), and there was an incremental gain in healing with higher infliximab levels up to 50 mcg/mL. The association between infliximab levels and fistula healing had an area under the curve of 0.82 (P less than .0001) and the association with fistula closure was 0.69 (P = .014).
“Infliximab levels needed to achieve fistula healing were higher than what [has] been described for mucosal healing. Achieving higher infliximab levels in patients with Crohn’s disease and perianal fistulas may improve outcomes and should be considered in a treat-to-target strategy. At least 10-20 mcg/mL may be needed to achieve fistula healing in some patients,” concluded investigator Dr. Andres J. Yarur, a gastroenterologist at the Medical College of Wisconsin, Milwaukee.
“Obviously, in somebody with active perianal fistulas” at a trough of 30 mcg/mL, “it’s time to try something else, but I don’t give up on infliximab at a level of 7 mcg/mL, like a lot of people do. I’m kind of aggressive with these patients.” There wasn’t a formal assessment of adverse events, but “we have not really seen any side effects,” he said at the annual Digestive Disease Week meeting. One audience member remarked that the findings will likely change how he treats Crohn’s fistulas.
Dr. Yarur said he wasn’t surprised by the findings, “but I have more reassurance now” that aggressive dosing is the way to go for aggressive Crohn’s.
The patients were an average of 39 years old, with a mean disease duration of 12 years; about half were women. They were on infliximab for a median of 19 months. Infliximab antibodies dropped the likelihood of healing (odds ratio, 0.04; 95% confidence interval, 0.005-0.3; P less than .001) and closure (OR 0.038; 95% CI, 0.005-0.3; P less than .0001). About two-thirds of the patients were on concomitant immunomodulators, but they did not increase the odds of healing (OR 1.7; 95% CI, 0.8-3.7; P = .157).
“We usually start at a standard dose of 5 mg/kg, and then start checking troughs at week 14, before the first maintenance dose, and adjust” upward to hit the trough target. However, dosing also depends on “how much I think the patient needs. I don’t think we should standardize trough levels,” Dr. Yarur said. The team checks levels with the homogeneous mobility-shift assay from Prometheus Laboratories. “The type of assay you use is very important,” because interpretation of the results varies from one to the next.
De-escalation is on a case-by-case basis. “I would be very careful de-escalating somebody [who] has very aggressive disease. Many times patients don’t want to go down. They say ‘I feel good doc. I have no side effects. Leave me on my dose,’ ” he said.
There was a lack of cross-sectional imaging in the study; the investigators couldn’t distinguish simple fistulas from complex ones in their review.
Dr. Yarur had no disclosures. There was no industry funding for the work.
AT DDW 2016
Key clinical point: Infliximab trough levels of at least 10-20 mcg/mL are needed to heal fistulas in some Crohn’s patients.
Major finding: Patients with fistula healing had significantly higher infliximab trough levels, compared with those with active fistulas (18.5 vs. 6.5 mcg/mL; P less than .0001).
Data source: Multicenter review of 117 patients
Disclosures: There was no industry funding for the work, and the presenter had no disclosures.
VIDEO: Endoscopic pyloromyotomy works for gastroparesis when meds don’t
SAN DIEGO – Gastric peroral endoscopic myotomy, a novel procedure for gastroparesis, restored gastric emptying in 30 refractory patients at Johns Hopkins University, Baltimore, and elsewhere in the largest series to date for the technique.
Drug therapy had failed, and Botox injections and transpyloric stenting weren’t helping much. On gastric emptying scans (GES), patients had around 40% of solid meals in their stomachs at 4 hours. Their gastroparesis was related mostly to diabetes and postoperative complications, but about a quarter of the cases were idiopathic.
Twenty-six patients (87%) responded to gastric peroral endoscopic myotomy (G-POEM) during a median follow-up of 5.5 months. Nausea, vomiting, and abdominal pain resolved or improved in most. On repeat GES in 17 patients, emptying time normalized in about half and improved in a third. Overall, patients had 17% of solid meals in their stomachs at 4 hours. G-POEM took an average of 72 minutes, and patients were in the hospital for about 3 days. One patient in the series developed pneumoperitoneum, and another had a prepyloric ulcer.
“The problem with transpyloric stents is that they migrate,” said investigator Dr. Mouen A. Khashab, director of therapeutic endoscopy at Johns Hopkins University. “G-POEM offers a permanent solution with few side effects. You have to be good at doing POEM in the esophagus first, as a prerequisite.”
In an interview at the annual Digestive Disease Week, Dr. Khashab explained the procedure in detail, as well as how he incorporates it into his practice and the patient population most likely to benefit.
SAN DIEGO – Gastric peroral endoscopic myotomy, a novel procedure for gastroparesis, restored gastric emptying in 30 refractory patients at Johns Hopkins University, Baltimore, and elsewhere in the largest series to date for the technique.
Drug therapy had failed, and Botox injections and transpyloric stenting weren’t helping much. On gastric emptying scans (GES), patients had around 40% of solid meals in their stomachs at 4 hours. Their gastroparesis was related mostly to diabetes and postoperative complications, but about a quarter of the cases were idiopathic.
Twenty-six patients (87%) responded to gastric peroral endoscopic myotomy (G-POEM) during a median follow-up of 5.5 months. Nausea, vomiting, and abdominal pain resolved or improved in most. On repeat GES in 17 patients, emptying time normalized in about half and improved in a third. Overall, patients had 17% of solid meals in their stomachs at 4 hours. G-POEM took an average of 72 minutes, and patients were in the hospital for about 3 days. One patient in the series developed pneumoperitoneum, and another had a prepyloric ulcer.
“The problem with transpyloric stents is that they migrate,” said investigator Dr. Mouen A. Khashab, director of therapeutic endoscopy at Johns Hopkins University. “G-POEM offers a permanent solution with few side effects. You have to be good at doing POEM in the esophagus first, as a prerequisite.”
In an interview at the annual Digestive Disease Week, Dr. Khashab explained the procedure in detail, as well as how he incorporates it into his practice and the patient population most likely to benefit.
SAN DIEGO – Gastric peroral endoscopic myotomy, a novel procedure for gastroparesis, restored gastric emptying in 30 refractory patients at Johns Hopkins University, Baltimore, and elsewhere in the largest series to date for the technique.
Drug therapy had failed, and Botox injections and transpyloric stenting weren’t helping much. On gastric emptying scans (GES), patients had around 40% of solid meals in their stomachs at 4 hours. Their gastroparesis was related mostly to diabetes and postoperative complications, but about a quarter of the cases were idiopathic.
Twenty-six patients (87%) responded to gastric peroral endoscopic myotomy (G-POEM) during a median follow-up of 5.5 months. Nausea, vomiting, and abdominal pain resolved or improved in most. On repeat GES in 17 patients, emptying time normalized in about half and improved in a third. Overall, patients had 17% of solid meals in their stomachs at 4 hours. G-POEM took an average of 72 minutes, and patients were in the hospital for about 3 days. One patient in the series developed pneumoperitoneum, and another had a prepyloric ulcer.
“The problem with transpyloric stents is that they migrate,” said investigator Dr. Mouen A. Khashab, director of therapeutic endoscopy at Johns Hopkins University. “G-POEM offers a permanent solution with few side effects. You have to be good at doing POEM in the esophagus first, as a prerequisite.”
In an interview at the annual Digestive Disease Week, Dr. Khashab explained the procedure in detail, as well as how he incorporates it into his practice and the patient population most likely to benefit.
AT DDW® 2016
Bezlotoxumab beats placebo at preventing recurrent C. difficile infections in high-risk patients
SAN DIEGO – Bezlotoxumab prevented recurrent Clostridium difficile infections (CDIs) among high-risk patients even more effectively than in the overall populations of the placebo-controlled MODIFY I and MODIFY II trials, according to a report at the annual Digestive Disease Week.
“In those key subpopulations at high risk for recurrence [of C. difficile infection], bezlotoxumab both reduced recurrence and increased rates of global cure,” said Dr. Ciaran P. Kelly of Harvard Medical School and Beth Israel Deaconess Medical Center in Boston. The biologic was especially effective among older adults and patients with at least one recent episode of CDI, Dr. Kelly and his associates found.
Bezlotoxumab is a monoclonal antibody targeting Clostridium difficile toxin B. The international, randomized, double-blind, 12-week MODIFY I and II trials included 2,656 patients with laboratory-confirmed CDI who were randomly assigned to receive either a single intravenous dose of the biologic (10 mg per kg) or placebo in addition to standard care antibiotics – that is, oral metronidazole and vancomycin; intravenous metronidazole with oral vancomycin; oral fidaxomicin; or oral fidaxomicin with intravenous metronidazole.
In both trials, bezlotoxumab was associated with a 10% decrease in rates of recurrent CDI, compared with placebo (P = .0003). Bezlotoxumab also achieved a 9.7% increase in rates of global cure, defined as clinical cure of the initial episode with no recurrence, Dr. Kelly said.
For the current analysis, he and his associates examined the efficacy of bezlotoxumab among patients at increased risk for recurrent CDI. These patients were older than 65 years, were immunocompromised, had a history of recurrent CDI, had been diagnosed with CDI within 6 months, and/or had severe CDI or were infected with hypervirulent, binary toxin positive strain. Most patients in the trials fell into at least one of these categories, Dr. Kelly said.
For each subgroup, bezlotoxumab was associated with lower rates of CDI recurrence and higher rates of global cure than in the overall study population, he emphasized. Compared with placebo, the most dramatic improvements in recurrence and global cure rates were among older patients (a 16% decrease and a 16% increase, respectively), patients with recent CDI (a 16% increase and a 12% decrease), patients with a history of recurrent CDI (a 13% decrease and a 12% increase) and immunocompromised patients (a 13% decrease and a 15% increase).
Neither trial generated a concerning safety signal, according to Dr. Kelly. There were “slight increases” in infusion reactions in the bezlotoxumab arms, but these were mostly minor and short lived, he added. “Serious adverse events were, in fact, slightly more common in the placebo group, mainly because of adverse events related to recurrence.”
The MODIFY trials were funded by Merck. Dr. Kelly reported consulting and advisory relationships with Merck, Sanofi Pasteur, Seres Therapeutics, Summit, and Alba.
SAN DIEGO – Bezlotoxumab prevented recurrent Clostridium difficile infections (CDIs) among high-risk patients even more effectively than in the overall populations of the placebo-controlled MODIFY I and MODIFY II trials, according to a report at the annual Digestive Disease Week.
“In those key subpopulations at high risk for recurrence [of C. difficile infection], bezlotoxumab both reduced recurrence and increased rates of global cure,” said Dr. Ciaran P. Kelly of Harvard Medical School and Beth Israel Deaconess Medical Center in Boston. The biologic was especially effective among older adults and patients with at least one recent episode of CDI, Dr. Kelly and his associates found.
Bezlotoxumab is a monoclonal antibody targeting Clostridium difficile toxin B. The international, randomized, double-blind, 12-week MODIFY I and II trials included 2,656 patients with laboratory-confirmed CDI who were randomly assigned to receive either a single intravenous dose of the biologic (10 mg per kg) or placebo in addition to standard care antibiotics – that is, oral metronidazole and vancomycin; intravenous metronidazole with oral vancomycin; oral fidaxomicin; or oral fidaxomicin with intravenous metronidazole.
In both trials, bezlotoxumab was associated with a 10% decrease in rates of recurrent CDI, compared with placebo (P = .0003). Bezlotoxumab also achieved a 9.7% increase in rates of global cure, defined as clinical cure of the initial episode with no recurrence, Dr. Kelly said.
For the current analysis, he and his associates examined the efficacy of bezlotoxumab among patients at increased risk for recurrent CDI. These patients were older than 65 years, were immunocompromised, had a history of recurrent CDI, had been diagnosed with CDI within 6 months, and/or had severe CDI or were infected with hypervirulent, binary toxin positive strain. Most patients in the trials fell into at least one of these categories, Dr. Kelly said.
For each subgroup, bezlotoxumab was associated with lower rates of CDI recurrence and higher rates of global cure than in the overall study population, he emphasized. Compared with placebo, the most dramatic improvements in recurrence and global cure rates were among older patients (a 16% decrease and a 16% increase, respectively), patients with recent CDI (a 16% increase and a 12% decrease), patients with a history of recurrent CDI (a 13% decrease and a 12% increase) and immunocompromised patients (a 13% decrease and a 15% increase).
Neither trial generated a concerning safety signal, according to Dr. Kelly. There were “slight increases” in infusion reactions in the bezlotoxumab arms, but these were mostly minor and short lived, he added. “Serious adverse events were, in fact, slightly more common in the placebo group, mainly because of adverse events related to recurrence.”
The MODIFY trials were funded by Merck. Dr. Kelly reported consulting and advisory relationships with Merck, Sanofi Pasteur, Seres Therapeutics, Summit, and Alba.
SAN DIEGO – Bezlotoxumab prevented recurrent Clostridium difficile infections (CDIs) among high-risk patients even more effectively than in the overall populations of the placebo-controlled MODIFY I and MODIFY II trials, according to a report at the annual Digestive Disease Week.
“In those key subpopulations at high risk for recurrence [of C. difficile infection], bezlotoxumab both reduced recurrence and increased rates of global cure,” said Dr. Ciaran P. Kelly of Harvard Medical School and Beth Israel Deaconess Medical Center in Boston. The biologic was especially effective among older adults and patients with at least one recent episode of CDI, Dr. Kelly and his associates found.
Bezlotoxumab is a monoclonal antibody targeting Clostridium difficile toxin B. The international, randomized, double-blind, 12-week MODIFY I and II trials included 2,656 patients with laboratory-confirmed CDI who were randomly assigned to receive either a single intravenous dose of the biologic (10 mg per kg) or placebo in addition to standard care antibiotics – that is, oral metronidazole and vancomycin; intravenous metronidazole with oral vancomycin; oral fidaxomicin; or oral fidaxomicin with intravenous metronidazole.
In both trials, bezlotoxumab was associated with a 10% decrease in rates of recurrent CDI, compared with placebo (P = .0003). Bezlotoxumab also achieved a 9.7% increase in rates of global cure, defined as clinical cure of the initial episode with no recurrence, Dr. Kelly said.
For the current analysis, he and his associates examined the efficacy of bezlotoxumab among patients at increased risk for recurrent CDI. These patients were older than 65 years, were immunocompromised, had a history of recurrent CDI, had been diagnosed with CDI within 6 months, and/or had severe CDI or were infected with hypervirulent, binary toxin positive strain. Most patients in the trials fell into at least one of these categories, Dr. Kelly said.
For each subgroup, bezlotoxumab was associated with lower rates of CDI recurrence and higher rates of global cure than in the overall study population, he emphasized. Compared with placebo, the most dramatic improvements in recurrence and global cure rates were among older patients (a 16% decrease and a 16% increase, respectively), patients with recent CDI (a 16% increase and a 12% decrease), patients with a history of recurrent CDI (a 13% decrease and a 12% increase) and immunocompromised patients (a 13% decrease and a 15% increase).
Neither trial generated a concerning safety signal, according to Dr. Kelly. There were “slight increases” in infusion reactions in the bezlotoxumab arms, but these were mostly minor and short lived, he added. “Serious adverse events were, in fact, slightly more common in the placebo group, mainly because of adverse events related to recurrence.”
The MODIFY trials were funded by Merck. Dr. Kelly reported consulting and advisory relationships with Merck, Sanofi Pasteur, Seres Therapeutics, Summit, and Alba.
AT DDW® 2016
Key clinical point: The monoclonal antibody bezlotoxumab prevented recurrent CDIs among patients at high risk for this outcome.
Major finding: Compared with placebo, bezlotoxumab achieved the most dramatic differences in rates of CDI recurrence and global cure for older patients (a 16% decrease and a 16% increase, respectively).
Data source: An analysis of the international, randomized, double-blind, 12-week MODIFY I and II trials, which included 2,656 patients with laboratory-confirmed CDI.
Disclosures: The MODIFY trials were funded by Merck. Dr. Kelly reported consulting and advisory relationships with Merck, Sanofi Pasteur, Seres Therapeutics, Summit, and Alba.
VIDEO: Accelerated infliximab dosing halved colectomy rate in severe ulcerative colitis
SAN DIEGO – Inpatient infliximab rescue for severe, steroid-refractory ulcerative colitis is more likely to work if patients receive a second infusion 3 days after the first, according to a review of 55 University of Michigan, Ann Arbor, patients.
The traditional approach is one inpatient 5-mg/kg infusion, followed by either colectomy or subsequent outpatient infusions, depending on response. In 2013, physicians at the university began offering a second infusion at 72 hours to patients whose C-reactive protein (CRP) levels did not drop below 0.7 mg/dL after their first infusion, and they also began opting more often for 10-mg/kg dosing.
The review found that 90-day colectomy-free survival was 50% in the 16 accelerated-dosing patients, up from 10.2% in the 36 patients treated with the traditional approach (P less than .001). The finding has led to a new, more aggressive infliximab protocol for inpatient ulcerative colitis.
Among patients who did undergo colectomies, postoperative complications were similar between the two groups. But for reasons that are not clear, 30-day postoperative readmission rates were higher in accelerated patients (58% vs. 25%).
In an interview at the annual Digestive Disease Week, lead investigator Dr. Shail Govani of the University of Michigan explained the thinking behind the new approach, how CRP/albumin ratios come into play, and how to counsel patients in light of the findings.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – Inpatient infliximab rescue for severe, steroid-refractory ulcerative colitis is more likely to work if patients receive a second infusion 3 days after the first, according to a review of 55 University of Michigan, Ann Arbor, patients.
The traditional approach is one inpatient 5-mg/kg infusion, followed by either colectomy or subsequent outpatient infusions, depending on response. In 2013, physicians at the university began offering a second infusion at 72 hours to patients whose C-reactive protein (CRP) levels did not drop below 0.7 mg/dL after their first infusion, and they also began opting more often for 10-mg/kg dosing.
The review found that 90-day colectomy-free survival was 50% in the 16 accelerated-dosing patients, up from 10.2% in the 36 patients treated with the traditional approach (P less than .001). The finding has led to a new, more aggressive infliximab protocol for inpatient ulcerative colitis.
Among patients who did undergo colectomies, postoperative complications were similar between the two groups. But for reasons that are not clear, 30-day postoperative readmission rates were higher in accelerated patients (58% vs. 25%).
In an interview at the annual Digestive Disease Week, lead investigator Dr. Shail Govani of the University of Michigan explained the thinking behind the new approach, how CRP/albumin ratios come into play, and how to counsel patients in light of the findings.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – Inpatient infliximab rescue for severe, steroid-refractory ulcerative colitis is more likely to work if patients receive a second infusion 3 days after the first, according to a review of 55 University of Michigan, Ann Arbor, patients.
The traditional approach is one inpatient 5-mg/kg infusion, followed by either colectomy or subsequent outpatient infusions, depending on response. In 2013, physicians at the university began offering a second infusion at 72 hours to patients whose C-reactive protein (CRP) levels did not drop below 0.7 mg/dL after their first infusion, and they also began opting more often for 10-mg/kg dosing.
The review found that 90-day colectomy-free survival was 50% in the 16 accelerated-dosing patients, up from 10.2% in the 36 patients treated with the traditional approach (P less than .001). The finding has led to a new, more aggressive infliximab protocol for inpatient ulcerative colitis.
Among patients who did undergo colectomies, postoperative complications were similar between the two groups. But for reasons that are not clear, 30-day postoperative readmission rates were higher in accelerated patients (58% vs. 25%).
In an interview at the annual Digestive Disease Week, lead investigator Dr. Shail Govani of the University of Michigan explained the thinking behind the new approach, how CRP/albumin ratios come into play, and how to counsel patients in light of the findings.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT DDW 2016
Ozanimod linked to histologic healing of moderate, severe ulcerative colitis
SAN DIEGO – A new analysis of phase II research finds that the experimental drug ozanimod prompted histologic healing in ulcerative colitis patients at 8 and 32 weeks.
“Ozanimod both induces and maintains remissions in a proportion of patients with refractory ulcerative colitis,” said study coauthor Dr. Stephen B. Hanauer, professor of medicine and medical director of the Digestive Health Center at Northwestern University, Chicago. “It’s a safe and effective oral drug for ulcerative colitis and possibly Crohn’s disease.”
Ozanimod, an experimental drug developed by Celgene and its subsidiary Receptos, has been undergoing review as a treatment for both multiple sclerosis and ulcerative colitis.
“Ozanimod impacts lymphocyte trafficking by retaining lymphocytes in lymph nodes and prevents recirculation. The mechanism of action is impairing S1P [sphingosine-1-phosphate] that ‘traps’ lymphocytes in lymph nodes.” Dr. Hanauer said.
“Other mechanisms by which lymphocyte trafficking has been inhibited have been effective in treating multiple sclerosis and Crohn’s disease (natalizumab) and both ulcerative colitis and Crohn’s disease (vedolizumab),” he said. However, he said, natalizumab has been linked to a risk of progressive multifocal leukoencephalopathy, a rare and mostly fatal brain disease.
Vedolizumab, meanwhile, “is a biologic that requires IV infusions.” By contrast, he says, ozanimod is an oral drug.
In a recent issue of the New England Journal of Medicine, researchers published findings from a phase II randomized, double-blind, placebo-controlled trial of ozanimod in 197 patients with moderate to severe ulcerative colitis. Patients were assigned to high dose (n = 67), low dose (n = 65), or placebo (n = 65) (N Engl J Med. 2016 May;374:1754-62).
At 8 weeks, clinical remission (Mayo Clinic score less than or equal to 2, with no subscore over 1) occurred in 16% of patients who received 1-mg doses (P = .048) and 14% of those who received the 0.5-mg doses (P = .14); the remission rate was 6% for placebo.
The new analysis examined histologic improvement from baseline to 8 and 32 weeks. (Those who reached clinical response continued through 32 weeks.) Improvement was greater in the high-dose group than in the placebo group at week 8 (Geboes score [–4.37 vs. –2.20; P = .0345]) and week 32 (Geboes score [–5.50 vs. –2.24; P = .0033]); low-dose improvement was greater than placebo but didn’t reach statistical significance.
Histologic remission (Geboes score less than 2) occurred in 15/67 (22.4%) for high dose (P = .0705, compared with placebo), 9/65 (13.8%) for low dose (P = .6294, compared with placebo) and 7/65 (10.8%) for placebo at week 8. At week 32, remission was 21/67 (31.3%) for high dose (P = 0.0006, compared with placebo), 15/65 (23.1%) for low dose (P = .0164, compared with placebo) and 5/65 (7.7%) for placebo.
Adverse events were “minor, without significant cardiotoxicity or risk of infections,” Dr. Hanauer said. The events affected 26/67 (38.8%) patients on the high dose, 26/65 (40.0%) on the low dose, and 26/65 (40.0%) on placebo; worsening of ulcerative colitis and anemia were most common, especially in the placebo group.
The cost of the drug is unclear, Dr. Hanauer said.
The patients are now in open-label follow-up, he said.
The study is industry funded by Receptos. Dr. Hanauer is on the ozanimod steering committee and consults for Celgene and its subsidiary research division, Receptos.
SAN DIEGO – A new analysis of phase II research finds that the experimental drug ozanimod prompted histologic healing in ulcerative colitis patients at 8 and 32 weeks.
“Ozanimod both induces and maintains remissions in a proportion of patients with refractory ulcerative colitis,” said study coauthor Dr. Stephen B. Hanauer, professor of medicine and medical director of the Digestive Health Center at Northwestern University, Chicago. “It’s a safe and effective oral drug for ulcerative colitis and possibly Crohn’s disease.”
Ozanimod, an experimental drug developed by Celgene and its subsidiary Receptos, has been undergoing review as a treatment for both multiple sclerosis and ulcerative colitis.
“Ozanimod impacts lymphocyte trafficking by retaining lymphocytes in lymph nodes and prevents recirculation. The mechanism of action is impairing S1P [sphingosine-1-phosphate] that ‘traps’ lymphocytes in lymph nodes.” Dr. Hanauer said.
“Other mechanisms by which lymphocyte trafficking has been inhibited have been effective in treating multiple sclerosis and Crohn’s disease (natalizumab) and both ulcerative colitis and Crohn’s disease (vedolizumab),” he said. However, he said, natalizumab has been linked to a risk of progressive multifocal leukoencephalopathy, a rare and mostly fatal brain disease.
Vedolizumab, meanwhile, “is a biologic that requires IV infusions.” By contrast, he says, ozanimod is an oral drug.
In a recent issue of the New England Journal of Medicine, researchers published findings from a phase II randomized, double-blind, placebo-controlled trial of ozanimod in 197 patients with moderate to severe ulcerative colitis. Patients were assigned to high dose (n = 67), low dose (n = 65), or placebo (n = 65) (N Engl J Med. 2016 May;374:1754-62).
At 8 weeks, clinical remission (Mayo Clinic score less than or equal to 2, with no subscore over 1) occurred in 16% of patients who received 1-mg doses (P = .048) and 14% of those who received the 0.5-mg doses (P = .14); the remission rate was 6% for placebo.
The new analysis examined histologic improvement from baseline to 8 and 32 weeks. (Those who reached clinical response continued through 32 weeks.) Improvement was greater in the high-dose group than in the placebo group at week 8 (Geboes score [–4.37 vs. –2.20; P = .0345]) and week 32 (Geboes score [–5.50 vs. –2.24; P = .0033]); low-dose improvement was greater than placebo but didn’t reach statistical significance.
Histologic remission (Geboes score less than 2) occurred in 15/67 (22.4%) for high dose (P = .0705, compared with placebo), 9/65 (13.8%) for low dose (P = .6294, compared with placebo) and 7/65 (10.8%) for placebo at week 8. At week 32, remission was 21/67 (31.3%) for high dose (P = 0.0006, compared with placebo), 15/65 (23.1%) for low dose (P = .0164, compared with placebo) and 5/65 (7.7%) for placebo.
Adverse events were “minor, without significant cardiotoxicity or risk of infections,” Dr. Hanauer said. The events affected 26/67 (38.8%) patients on the high dose, 26/65 (40.0%) on the low dose, and 26/65 (40.0%) on placebo; worsening of ulcerative colitis and anemia were most common, especially in the placebo group.
The cost of the drug is unclear, Dr. Hanauer said.
The patients are now in open-label follow-up, he said.
The study is industry funded by Receptos. Dr. Hanauer is on the ozanimod steering committee and consults for Celgene and its subsidiary research division, Receptos.
SAN DIEGO – A new analysis of phase II research finds that the experimental drug ozanimod prompted histologic healing in ulcerative colitis patients at 8 and 32 weeks.
“Ozanimod both induces and maintains remissions in a proportion of patients with refractory ulcerative colitis,” said study coauthor Dr. Stephen B. Hanauer, professor of medicine and medical director of the Digestive Health Center at Northwestern University, Chicago. “It’s a safe and effective oral drug for ulcerative colitis and possibly Crohn’s disease.”
Ozanimod, an experimental drug developed by Celgene and its subsidiary Receptos, has been undergoing review as a treatment for both multiple sclerosis and ulcerative colitis.
“Ozanimod impacts lymphocyte trafficking by retaining lymphocytes in lymph nodes and prevents recirculation. The mechanism of action is impairing S1P [sphingosine-1-phosphate] that ‘traps’ lymphocytes in lymph nodes.” Dr. Hanauer said.
“Other mechanisms by which lymphocyte trafficking has been inhibited have been effective in treating multiple sclerosis and Crohn’s disease (natalizumab) and both ulcerative colitis and Crohn’s disease (vedolizumab),” he said. However, he said, natalizumab has been linked to a risk of progressive multifocal leukoencephalopathy, a rare and mostly fatal brain disease.
Vedolizumab, meanwhile, “is a biologic that requires IV infusions.” By contrast, he says, ozanimod is an oral drug.
In a recent issue of the New England Journal of Medicine, researchers published findings from a phase II randomized, double-blind, placebo-controlled trial of ozanimod in 197 patients with moderate to severe ulcerative colitis. Patients were assigned to high dose (n = 67), low dose (n = 65), or placebo (n = 65) (N Engl J Med. 2016 May;374:1754-62).
At 8 weeks, clinical remission (Mayo Clinic score less than or equal to 2, with no subscore over 1) occurred in 16% of patients who received 1-mg doses (P = .048) and 14% of those who received the 0.5-mg doses (P = .14); the remission rate was 6% for placebo.
The new analysis examined histologic improvement from baseline to 8 and 32 weeks. (Those who reached clinical response continued through 32 weeks.) Improvement was greater in the high-dose group than in the placebo group at week 8 (Geboes score [–4.37 vs. –2.20; P = .0345]) and week 32 (Geboes score [–5.50 vs. –2.24; P = .0033]); low-dose improvement was greater than placebo but didn’t reach statistical significance.
Histologic remission (Geboes score less than 2) occurred in 15/67 (22.4%) for high dose (P = .0705, compared with placebo), 9/65 (13.8%) for low dose (P = .6294, compared with placebo) and 7/65 (10.8%) for placebo at week 8. At week 32, remission was 21/67 (31.3%) for high dose (P = 0.0006, compared with placebo), 15/65 (23.1%) for low dose (P = .0164, compared with placebo) and 5/65 (7.7%) for placebo.
Adverse events were “minor, without significant cardiotoxicity or risk of infections,” Dr. Hanauer said. The events affected 26/67 (38.8%) patients on the high dose, 26/65 (40.0%) on the low dose, and 26/65 (40.0%) on placebo; worsening of ulcerative colitis and anemia were most common, especially in the placebo group.
The cost of the drug is unclear, Dr. Hanauer said.
The patients are now in open-label follow-up, he said.
The study is industry funded by Receptos. Dr. Hanauer is on the ozanimod steering committee and consults for Celgene and its subsidiary research division, Receptos.
AT DDW® 2016
Key clinical point: In addition to clinical remission, response, and endoscopic mucosal healing, ozanimod appears to offer benefits on the histologic front.
Major finding: Histologic improvement was greater in patients who took higher dose of ozanimod (1 mg) than placebo at week 8 (Geboes score [–4.37 vs. –2.20; P = .0345]) and 32 (Geboes score [–5.50 vs. –2.24; P = .0033]). The lower dose (0.5 mg) showed improvement, but it was not statistically significant.
Data source: Randomized, double-blind, placebo-controlled phase II trial of 197 patients (high dose, 67; low dose, 65; placebo, 65).
Disclosures: The study was industry funded by Receptos. Dr. Hanauer is on the ozanimod steering committee and consults for Celgene and its subsidiary research division, Receptos.
Low-FODMAP diet eased abdominal symptoms in IBS
SAN DIEGO – For patients with diarrhea-predominant irritable bowel syndrome, avoiding FODMAPs (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) improved abdominal pain and bloating significantly more than following standard advice to eat smaller meals and limit caffeine and alcohol, researchers reported.
“Both diets provided adequate relief to about 40%-50% of patients, but the low-FODMAP diet led to significantly greater improvements in abdominal symptoms,” Dr. Shanti L. Eswaran of the University of Michigan, Ann Arbor, said at the annual Digestive Disease Week. Results from the randomized, controlled trial, the first of its kind in the United States, “support a role for the low-FODMAP diet in the treatment of patients with diarrhea-predominant IBS,” she added.
FODMAPs are poorly absorbed or indigestible fermentable carbohydrates that can cause bloating, flatulence, and diarrhea when eaten in excess. Hence, the low-FODMAP diet involves avoiding or limiting foods high in fructose (such as honey and dried fruit), lactose (dairy), fructans (wheat, garlic, and onions), galactans (legumes), and polyols (apples and stone fruits). Several smaller studies have linked a low-FODMAP diet to improvements in IBS, “but the existing data are limited and inconsistent, and there is no randomized, controlled trial data from adults in the United States,” Dr. Eswaran said.
To fill that gap, she and her associates randomly assigned 92 adults meeting Rome III criteria for diarrhea-predominant IBS to follow either a low-FODMAP diet or a control diet that was based on recommendations from the National Institute for Health Care and Excellence (NICE, in the United Kingdom). The modified NICE diet included eating smaller, more frequent meals, limiting caffeine and alcohol, and avoiding foods that patients knew worsened their symptoms. Both groups of patients worked with a dietitian.
At baseline, all patients reported having regular bouts of at least moderate abdominal pain and stool consistency of 5 or higher (that is, looser) on the Bristol Stool Form Scale. In all, 52% of patients on the low-FODMAP diet and 41% of patients on the control diet reported adequate symptom relief during at least one of the last 2 weeks of the study – a statistically similar level of improvement, Dr. Eswaran said. “We were really underpowered for our primary endpoint,” she added. “We had calculated a 30% difference, and we did not get anywhere near that.” In fact, enrollment in the trial ended early because many patients were already putting themselves on the low-FODMAP diet, she added.
But despite its limited power, the study uncovered significant differences in abdominal symptoms with the two diets. More than half of patients on the low-FODMAP diet reported a clinically meaningful improvement in abdominal pain, compared with only 23% of patients on the control diet (P = .008). Likewise, 52% of patients reported clinically meaningful improvement in bloating, compared with about a quarter of patients on the control diet (P = .013). Low-FODMAP patients also were more likely to report improvements in stool consistency (42%, versus 28% for control patients; P = .18). However, there was no evidence that the low-FODMAP diet improved stool consistency or urgency, Dr. Eswaran said.
“Both diets were safe and well tolerated, although dropouts were more common with the low-FODMAP diet,” the researchers noted. Dietary analyses showed that at 4 weeks, the low-FODMAP group was consuming significantly less total carbohydrates, but similar quantities of total calories, protein, fat, dietary fiber, and alcohol as the control group. “The low-FODMAP diet is not designed to be long term, because it is fairly restrictive,” Dr. Eswaran commented. “I think it would be a good idea for the next set of studies to see how long patients can stay on it, and what factors are necessary for them to do so.”
Dr. Eswaran had no disclosures.
SAN DIEGO – For patients with diarrhea-predominant irritable bowel syndrome, avoiding FODMAPs (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) improved abdominal pain and bloating significantly more than following standard advice to eat smaller meals and limit caffeine and alcohol, researchers reported.
“Both diets provided adequate relief to about 40%-50% of patients, but the low-FODMAP diet led to significantly greater improvements in abdominal symptoms,” Dr. Shanti L. Eswaran of the University of Michigan, Ann Arbor, said at the annual Digestive Disease Week. Results from the randomized, controlled trial, the first of its kind in the United States, “support a role for the low-FODMAP diet in the treatment of patients with diarrhea-predominant IBS,” she added.
FODMAPs are poorly absorbed or indigestible fermentable carbohydrates that can cause bloating, flatulence, and diarrhea when eaten in excess. Hence, the low-FODMAP diet involves avoiding or limiting foods high in fructose (such as honey and dried fruit), lactose (dairy), fructans (wheat, garlic, and onions), galactans (legumes), and polyols (apples and stone fruits). Several smaller studies have linked a low-FODMAP diet to improvements in IBS, “but the existing data are limited and inconsistent, and there is no randomized, controlled trial data from adults in the United States,” Dr. Eswaran said.
To fill that gap, she and her associates randomly assigned 92 adults meeting Rome III criteria for diarrhea-predominant IBS to follow either a low-FODMAP diet or a control diet that was based on recommendations from the National Institute for Health Care and Excellence (NICE, in the United Kingdom). The modified NICE diet included eating smaller, more frequent meals, limiting caffeine and alcohol, and avoiding foods that patients knew worsened their symptoms. Both groups of patients worked with a dietitian.
At baseline, all patients reported having regular bouts of at least moderate abdominal pain and stool consistency of 5 or higher (that is, looser) on the Bristol Stool Form Scale. In all, 52% of patients on the low-FODMAP diet and 41% of patients on the control diet reported adequate symptom relief during at least one of the last 2 weeks of the study – a statistically similar level of improvement, Dr. Eswaran said. “We were really underpowered for our primary endpoint,” she added. “We had calculated a 30% difference, and we did not get anywhere near that.” In fact, enrollment in the trial ended early because many patients were already putting themselves on the low-FODMAP diet, she added.
But despite its limited power, the study uncovered significant differences in abdominal symptoms with the two diets. More than half of patients on the low-FODMAP diet reported a clinically meaningful improvement in abdominal pain, compared with only 23% of patients on the control diet (P = .008). Likewise, 52% of patients reported clinically meaningful improvement in bloating, compared with about a quarter of patients on the control diet (P = .013). Low-FODMAP patients also were more likely to report improvements in stool consistency (42%, versus 28% for control patients; P = .18). However, there was no evidence that the low-FODMAP diet improved stool consistency or urgency, Dr. Eswaran said.
“Both diets were safe and well tolerated, although dropouts were more common with the low-FODMAP diet,” the researchers noted. Dietary analyses showed that at 4 weeks, the low-FODMAP group was consuming significantly less total carbohydrates, but similar quantities of total calories, protein, fat, dietary fiber, and alcohol as the control group. “The low-FODMAP diet is not designed to be long term, because it is fairly restrictive,” Dr. Eswaran commented. “I think it would be a good idea for the next set of studies to see how long patients can stay on it, and what factors are necessary for them to do so.”
Dr. Eswaran had no disclosures.
SAN DIEGO – For patients with diarrhea-predominant irritable bowel syndrome, avoiding FODMAPs (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) improved abdominal pain and bloating significantly more than following standard advice to eat smaller meals and limit caffeine and alcohol, researchers reported.
“Both diets provided adequate relief to about 40%-50% of patients, but the low-FODMAP diet led to significantly greater improvements in abdominal symptoms,” Dr. Shanti L. Eswaran of the University of Michigan, Ann Arbor, said at the annual Digestive Disease Week. Results from the randomized, controlled trial, the first of its kind in the United States, “support a role for the low-FODMAP diet in the treatment of patients with diarrhea-predominant IBS,” she added.
FODMAPs are poorly absorbed or indigestible fermentable carbohydrates that can cause bloating, flatulence, and diarrhea when eaten in excess. Hence, the low-FODMAP diet involves avoiding or limiting foods high in fructose (such as honey and dried fruit), lactose (dairy), fructans (wheat, garlic, and onions), galactans (legumes), and polyols (apples and stone fruits). Several smaller studies have linked a low-FODMAP diet to improvements in IBS, “but the existing data are limited and inconsistent, and there is no randomized, controlled trial data from adults in the United States,” Dr. Eswaran said.
To fill that gap, she and her associates randomly assigned 92 adults meeting Rome III criteria for diarrhea-predominant IBS to follow either a low-FODMAP diet or a control diet that was based on recommendations from the National Institute for Health Care and Excellence (NICE, in the United Kingdom). The modified NICE diet included eating smaller, more frequent meals, limiting caffeine and alcohol, and avoiding foods that patients knew worsened their symptoms. Both groups of patients worked with a dietitian.
At baseline, all patients reported having regular bouts of at least moderate abdominal pain and stool consistency of 5 or higher (that is, looser) on the Bristol Stool Form Scale. In all, 52% of patients on the low-FODMAP diet and 41% of patients on the control diet reported adequate symptom relief during at least one of the last 2 weeks of the study – a statistically similar level of improvement, Dr. Eswaran said. “We were really underpowered for our primary endpoint,” she added. “We had calculated a 30% difference, and we did not get anywhere near that.” In fact, enrollment in the trial ended early because many patients were already putting themselves on the low-FODMAP diet, she added.
But despite its limited power, the study uncovered significant differences in abdominal symptoms with the two diets. More than half of patients on the low-FODMAP diet reported a clinically meaningful improvement in abdominal pain, compared with only 23% of patients on the control diet (P = .008). Likewise, 52% of patients reported clinically meaningful improvement in bloating, compared with about a quarter of patients on the control diet (P = .013). Low-FODMAP patients also were more likely to report improvements in stool consistency (42%, versus 28% for control patients; P = .18). However, there was no evidence that the low-FODMAP diet improved stool consistency or urgency, Dr. Eswaran said.
“Both diets were safe and well tolerated, although dropouts were more common with the low-FODMAP diet,” the researchers noted. Dietary analyses showed that at 4 weeks, the low-FODMAP group was consuming significantly less total carbohydrates, but similar quantities of total calories, protein, fat, dietary fiber, and alcohol as the control group. “The low-FODMAP diet is not designed to be long term, because it is fairly restrictive,” Dr. Eswaran commented. “I think it would be a good idea for the next set of studies to see how long patients can stay on it, and what factors are necessary for them to do so.”
Dr. Eswaran had no disclosures.
AT DDW® 2016
Key clinical point: A diet low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols was associated with significant and clinically meaningful improvements in the abdominal symptoms of diarrhea-predominant irritable bowel syndrome.
Major finding: About half of patients on the low-FODMAP diet improved, compared with about a quarter of patients on a common-sense control diet.
Data source: A prospective, single-center, single-blind randomized controlled trial of 92 adults with IBS with diarrhea (Rome III).
Disclosures: Dr. Eswaren had no disclosures.
VIDEO: Daily fecal transplants put refractory ulcerative colitis into remission
SAN DIEGO – When fecal transplants don’t work for ulcerative colitis, it’s probably because they aren’t used often enough, according to investigators from the University of New South Wales, Sydney.
The researchers made sure that wasn’t the case in their own double-blind trial, the largest to date of fecal microbiota transplantation (FMT) for ulcerative colitis. Forty-one patients with active, mild to moderate disease (Mayo score 4-10) that was resistant to standard medications were randomized to 150-mL, self-administered fecal enemas 5 days a week for 8 weeks, and 40 others to placebo enemas. Patients were tapered off steroids at study entrance, and each FMT patient received stool from 3-7 unrelated donors.
Steroid-free clinical remission and endoscopic remission or response were achieved in 11 FMT patients (27%), compared with 3 (8%) placebo patients (P = .02). A total of 18 treated patients (44%) and 8 placebo patients (20%) had steroid-free clinical remissions, while 22 treated patients (54%) and 9 patients in the placebo group (23%) had some type of positive clinical response (P less than .01).
Patients were in their mid-30s, on average, with disease durations of about 6 years. More than half were men. About one-quarter were on oral steroids, and more than half were on oral 5-aminosalicylic acid medications, which were allowed during the study. Almost half were on methotrexate or other oral immunomodulators, which were also allowed. Patients were excluded if they had been on a biologic in the previous 12 weeks.
Afterward, 37 patients in the placebo arm opted for open-label FMT. Results were similar, with steroid-free clinical remission and endoscopic remission or response in 10 patients (27%), clinical remission in 17 (46%), and endoscopic remission in 9 (24%).
The anatomical extent of disease did not affect outcome, but patients with more severe endoscopic disease and those on steroids at study entrance didn’t do as well. Three patients flared during the trial, one in the placebo arm and two in the FMT arm, one of whom required colectomy.
The investigators were surprised by the magnitude of the benefit, given the mixed results in previous investigations with less frequent dosing. But they were not surprised that FMT worked.
“In ulcerative colitis, the microbiota appear to be the antigenic driver, so it makes sense that correcting the disturbance” helps, said lead investigator Dr. Sudarshan Paramsothy, a gastroenterologist at the University of New South Wales.
Dr. Paramsothy and his colleagues have a hunch they can do even better. They are looking into the microbiologic factors of donors and patients that influence response, with the ultimate goal of matching the best donor to the best patient. They’re examining maintenance therapy, too; “it’s one thing to induce remission, it’s another thing to maintain remission,” Dr. Paramsothy said.
In an interview at the annual Digestive Disease Week, he explained the technique, the thinking behind it, future directions, and how to counsel patients in light of the findings.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – When fecal transplants don’t work for ulcerative colitis, it’s probably because they aren’t used often enough, according to investigators from the University of New South Wales, Sydney.
The researchers made sure that wasn’t the case in their own double-blind trial, the largest to date of fecal microbiota transplantation (FMT) for ulcerative colitis. Forty-one patients with active, mild to moderate disease (Mayo score 4-10) that was resistant to standard medications were randomized to 150-mL, self-administered fecal enemas 5 days a week for 8 weeks, and 40 others to placebo enemas. Patients were tapered off steroids at study entrance, and each FMT patient received stool from 3-7 unrelated donors.
Steroid-free clinical remission and endoscopic remission or response were achieved in 11 FMT patients (27%), compared with 3 (8%) placebo patients (P = .02). A total of 18 treated patients (44%) and 8 placebo patients (20%) had steroid-free clinical remissions, while 22 treated patients (54%) and 9 patients in the placebo group (23%) had some type of positive clinical response (P less than .01).
Patients were in their mid-30s, on average, with disease durations of about 6 years. More than half were men. About one-quarter were on oral steroids, and more than half were on oral 5-aminosalicylic acid medications, which were allowed during the study. Almost half were on methotrexate or other oral immunomodulators, which were also allowed. Patients were excluded if they had been on a biologic in the previous 12 weeks.
Afterward, 37 patients in the placebo arm opted for open-label FMT. Results were similar, with steroid-free clinical remission and endoscopic remission or response in 10 patients (27%), clinical remission in 17 (46%), and endoscopic remission in 9 (24%).
The anatomical extent of disease did not affect outcome, but patients with more severe endoscopic disease and those on steroids at study entrance didn’t do as well. Three patients flared during the trial, one in the placebo arm and two in the FMT arm, one of whom required colectomy.
The investigators were surprised by the magnitude of the benefit, given the mixed results in previous investigations with less frequent dosing. But they were not surprised that FMT worked.
“In ulcerative colitis, the microbiota appear to be the antigenic driver, so it makes sense that correcting the disturbance” helps, said lead investigator Dr. Sudarshan Paramsothy, a gastroenterologist at the University of New South Wales.
Dr. Paramsothy and his colleagues have a hunch they can do even better. They are looking into the microbiologic factors of donors and patients that influence response, with the ultimate goal of matching the best donor to the best patient. They’re examining maintenance therapy, too; “it’s one thing to induce remission, it’s another thing to maintain remission,” Dr. Paramsothy said.
In an interview at the annual Digestive Disease Week, he explained the technique, the thinking behind it, future directions, and how to counsel patients in light of the findings.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – When fecal transplants don’t work for ulcerative colitis, it’s probably because they aren’t used often enough, according to investigators from the University of New South Wales, Sydney.
The researchers made sure that wasn’t the case in their own double-blind trial, the largest to date of fecal microbiota transplantation (FMT) for ulcerative colitis. Forty-one patients with active, mild to moderate disease (Mayo score 4-10) that was resistant to standard medications were randomized to 150-mL, self-administered fecal enemas 5 days a week for 8 weeks, and 40 others to placebo enemas. Patients were tapered off steroids at study entrance, and each FMT patient received stool from 3-7 unrelated donors.
Steroid-free clinical remission and endoscopic remission or response were achieved in 11 FMT patients (27%), compared with 3 (8%) placebo patients (P = .02). A total of 18 treated patients (44%) and 8 placebo patients (20%) had steroid-free clinical remissions, while 22 treated patients (54%) and 9 patients in the placebo group (23%) had some type of positive clinical response (P less than .01).
Patients were in their mid-30s, on average, with disease durations of about 6 years. More than half were men. About one-quarter were on oral steroids, and more than half were on oral 5-aminosalicylic acid medications, which were allowed during the study. Almost half were on methotrexate or other oral immunomodulators, which were also allowed. Patients were excluded if they had been on a biologic in the previous 12 weeks.
Afterward, 37 patients in the placebo arm opted for open-label FMT. Results were similar, with steroid-free clinical remission and endoscopic remission or response in 10 patients (27%), clinical remission in 17 (46%), and endoscopic remission in 9 (24%).
The anatomical extent of disease did not affect outcome, but patients with more severe endoscopic disease and those on steroids at study entrance didn’t do as well. Three patients flared during the trial, one in the placebo arm and two in the FMT arm, one of whom required colectomy.
The investigators were surprised by the magnitude of the benefit, given the mixed results in previous investigations with less frequent dosing. But they were not surprised that FMT worked.
“In ulcerative colitis, the microbiota appear to be the antigenic driver, so it makes sense that correcting the disturbance” helps, said lead investigator Dr. Sudarshan Paramsothy, a gastroenterologist at the University of New South Wales.
Dr. Paramsothy and his colleagues have a hunch they can do even better. They are looking into the microbiologic factors of donors and patients that influence response, with the ultimate goal of matching the best donor to the best patient. They’re examining maintenance therapy, too; “it’s one thing to induce remission, it’s another thing to maintain remission,” Dr. Paramsothy said.
In an interview at the annual Digestive Disease Week, he explained the technique, the thinking behind it, future directions, and how to counsel patients in light of the findings.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT DDW 2016
Disappointing results for GI bleed prevention in high-risk aspirin users
SAN DIEGO – Neither a proton pump inhibitor nor an H2 antagonist is an optimal choice for users of low-dose aspirin with previously confirmed ulcer bleeding, according to data from a 12-month randomized trial.
“This is one of the largest clinical trials focusing on aspirin users with a history of ulcer bleeding. Previous trials of aspirin users were mostly endoscopic trials. The important message here is that – while PPI may be useful as gastroprotection for patients with a history of ulcer bleed – it seems that neither treatment is sufficiently protective,” Dr. Francis Chan said at the annual Digestive Disease Week.
Findings from earlier research by the same investigators showed that cotreatment with aspirin and a PPI seemed to be effective as secondary prevention for aspirin-induced ulcer bleeding. He noted that PPIs have a warning for high-risk aspirin users, so alternatives are being sought.
In the present randomized trial, even with PPI prophylaxis, 7.9% of these high-risk aspirin users developed recurrent bleed or endoscopic ulcers versus 12.4% of the group treated with an H2 antagonist, a nonsignificant difference.
The prospective, randomized double-blind trial randomized 270 patients in a 1:1 ratio to 1 year of treatment with either 20 mg rabeprazole (a PPI) once daily or 40 mg of the H2 antagonist famotidine once daily. All patients’ ulcers had healed, and all tested negative for Helicobacter pylori prior to randomization. Study participants were taking 80 mg of aspirin daily.
Patients were followed for 12 months. Endoscopy was repeated if there was suspicion of recurrent bleed or they reached 12 months of treatment.
The primary endpoint was a composite of upper gastrointestinal bleed or recurrent ulcer. Secondary endpoints included a composite of recurrent bleed, ulcers visible on endoscopy, and early withdrawal due to severe dyspepsia; lower GI bleeding; and cardiothrombotic events.
Study participants had a mean age of 73 years. At baseline, all patients were negative for H. pylori and hepatitis B virus infection. Both treatment arms were comparable for indication for aspirin. A history of coronary disease was noted in 37% of the PPI group and 40.2% of the H2 antagonist group. A history of cerebrovascular disease was present in 35.5% and 37.1%, respectively.
The source of previous bleeding was comparable in the two groups.
In an intent-to-treat analysis that included all patients who took at least one dose of study medication as well as those who underwent endoscopy at 12 months, 24 cases of suspected bleeding were found: 14 in the PPI group (1 confirmed) and 10 in the H2 antagonist group (4 confirmed). The rate of recurrent bleeding was 5.1% for the PPI and 8.1% for the H2 antagonist.
Lower GI bleeding was reported in 11 patients (8.9%) on the PPI and 6 patients (5%) on the H2 antagonist.
Cardiothrombotic events were reported in five patients: two on the PPI (1.6%) and three on the H2 antagonist (2.5%) .
“We didn’t see any trends for cardiovascular bleeding in either group,” Dr. Chan noted.
An audience member asked what the best way is to treat these patients, given that neither drug provided adequate protection against upper GI bleeding.
“The answer is, I don’t know. We need more study of high-risk patients, and we should study a combination of PPI plus misoprostol. In the absence of larger studies, I currently treat my patients with PPI plus low-dose misoprostol,” he said.
SAN DIEGO – Neither a proton pump inhibitor nor an H2 antagonist is an optimal choice for users of low-dose aspirin with previously confirmed ulcer bleeding, according to data from a 12-month randomized trial.
“This is one of the largest clinical trials focusing on aspirin users with a history of ulcer bleeding. Previous trials of aspirin users were mostly endoscopic trials. The important message here is that – while PPI may be useful as gastroprotection for patients with a history of ulcer bleed – it seems that neither treatment is sufficiently protective,” Dr. Francis Chan said at the annual Digestive Disease Week.
Findings from earlier research by the same investigators showed that cotreatment with aspirin and a PPI seemed to be effective as secondary prevention for aspirin-induced ulcer bleeding. He noted that PPIs have a warning for high-risk aspirin users, so alternatives are being sought.
In the present randomized trial, even with PPI prophylaxis, 7.9% of these high-risk aspirin users developed recurrent bleed or endoscopic ulcers versus 12.4% of the group treated with an H2 antagonist, a nonsignificant difference.
The prospective, randomized double-blind trial randomized 270 patients in a 1:1 ratio to 1 year of treatment with either 20 mg rabeprazole (a PPI) once daily or 40 mg of the H2 antagonist famotidine once daily. All patients’ ulcers had healed, and all tested negative for Helicobacter pylori prior to randomization. Study participants were taking 80 mg of aspirin daily.
Patients were followed for 12 months. Endoscopy was repeated if there was suspicion of recurrent bleed or they reached 12 months of treatment.
The primary endpoint was a composite of upper gastrointestinal bleed or recurrent ulcer. Secondary endpoints included a composite of recurrent bleed, ulcers visible on endoscopy, and early withdrawal due to severe dyspepsia; lower GI bleeding; and cardiothrombotic events.
Study participants had a mean age of 73 years. At baseline, all patients were negative for H. pylori and hepatitis B virus infection. Both treatment arms were comparable for indication for aspirin. A history of coronary disease was noted in 37% of the PPI group and 40.2% of the H2 antagonist group. A history of cerebrovascular disease was present in 35.5% and 37.1%, respectively.
The source of previous bleeding was comparable in the two groups.
In an intent-to-treat analysis that included all patients who took at least one dose of study medication as well as those who underwent endoscopy at 12 months, 24 cases of suspected bleeding were found: 14 in the PPI group (1 confirmed) and 10 in the H2 antagonist group (4 confirmed). The rate of recurrent bleeding was 5.1% for the PPI and 8.1% for the H2 antagonist.
Lower GI bleeding was reported in 11 patients (8.9%) on the PPI and 6 patients (5%) on the H2 antagonist.
Cardiothrombotic events were reported in five patients: two on the PPI (1.6%) and three on the H2 antagonist (2.5%) .
“We didn’t see any trends for cardiovascular bleeding in either group,” Dr. Chan noted.
An audience member asked what the best way is to treat these patients, given that neither drug provided adequate protection against upper GI bleeding.
“The answer is, I don’t know. We need more study of high-risk patients, and we should study a combination of PPI plus misoprostol. In the absence of larger studies, I currently treat my patients with PPI plus low-dose misoprostol,” he said.
SAN DIEGO – Neither a proton pump inhibitor nor an H2 antagonist is an optimal choice for users of low-dose aspirin with previously confirmed ulcer bleeding, according to data from a 12-month randomized trial.
“This is one of the largest clinical trials focusing on aspirin users with a history of ulcer bleeding. Previous trials of aspirin users were mostly endoscopic trials. The important message here is that – while PPI may be useful as gastroprotection for patients with a history of ulcer bleed – it seems that neither treatment is sufficiently protective,” Dr. Francis Chan said at the annual Digestive Disease Week.
Findings from earlier research by the same investigators showed that cotreatment with aspirin and a PPI seemed to be effective as secondary prevention for aspirin-induced ulcer bleeding. He noted that PPIs have a warning for high-risk aspirin users, so alternatives are being sought.
In the present randomized trial, even with PPI prophylaxis, 7.9% of these high-risk aspirin users developed recurrent bleed or endoscopic ulcers versus 12.4% of the group treated with an H2 antagonist, a nonsignificant difference.
The prospective, randomized double-blind trial randomized 270 patients in a 1:1 ratio to 1 year of treatment with either 20 mg rabeprazole (a PPI) once daily or 40 mg of the H2 antagonist famotidine once daily. All patients’ ulcers had healed, and all tested negative for Helicobacter pylori prior to randomization. Study participants were taking 80 mg of aspirin daily.
Patients were followed for 12 months. Endoscopy was repeated if there was suspicion of recurrent bleed or they reached 12 months of treatment.
The primary endpoint was a composite of upper gastrointestinal bleed or recurrent ulcer. Secondary endpoints included a composite of recurrent bleed, ulcers visible on endoscopy, and early withdrawal due to severe dyspepsia; lower GI bleeding; and cardiothrombotic events.
Study participants had a mean age of 73 years. At baseline, all patients were negative for H. pylori and hepatitis B virus infection. Both treatment arms were comparable for indication for aspirin. A history of coronary disease was noted in 37% of the PPI group and 40.2% of the H2 antagonist group. A history of cerebrovascular disease was present in 35.5% and 37.1%, respectively.
The source of previous bleeding was comparable in the two groups.
In an intent-to-treat analysis that included all patients who took at least one dose of study medication as well as those who underwent endoscopy at 12 months, 24 cases of suspected bleeding were found: 14 in the PPI group (1 confirmed) and 10 in the H2 antagonist group (4 confirmed). The rate of recurrent bleeding was 5.1% for the PPI and 8.1% for the H2 antagonist.
Lower GI bleeding was reported in 11 patients (8.9%) on the PPI and 6 patients (5%) on the H2 antagonist.
Cardiothrombotic events were reported in five patients: two on the PPI (1.6%) and three on the H2 antagonist (2.5%) .
“We didn’t see any trends for cardiovascular bleeding in either group,” Dr. Chan noted.
An audience member asked what the best way is to treat these patients, given that neither drug provided adequate protection against upper GI bleeding.
“The answer is, I don’t know. We need more study of high-risk patients, and we should study a combination of PPI plus misoprostol. In the absence of larger studies, I currently treat my patients with PPI plus low-dose misoprostol,” he said.
AT DDW® 2016
Key clinical point: Neither a PPI nor an H2 antagonist provided sufficient gastroprotection in high-risk aspirin users.
Major finding: The rate of recurrent bleeding or endoscopic ulcers was 7.9% with a PPI versus 12.4% with an H2 antagonist.
Data source: A randomized, controlled trial of 270 patients with a previous history of ulcers.
Disclosures: Dr. Chan has received financial support from Pfizer and Eisai.