User login
Vedolizumab Scores on Safety, Efficacy for Ulcerative Colitis
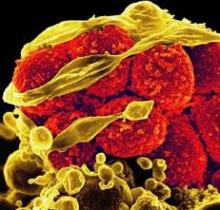
SAN DIEGO – Vedolizumab, a novel drug that selectively blocks lymphocyte trafficking to the gut, was safe and highly effective for inducing and maintaining clinical remission in patients with moderate to severe ulcerative colitis in a pivotal phase III trial.
"If the findings hold up with more detailed analysis, it looks like we’ll have the efficacy of a biologic agent without some of the toxicity issues that we’ve seen with anti-TNF [tumor necrosis factor] drugs," said Dr. William J. Sandborn, professor of medicine and chief of gastroenterology at the University of California, San Diego.
"This potentially could be a first-line drug," for treating moderate to severe ulcerative colitis, said Dr. Sandborn, a coinvestigator in the study.
"Vedolizumab was more effective than placebo for induction and maintenance treatment, including both anti-TNF–exposed and naive patients," Dr. Brian G. Feagan, the study’s lead investigator, said at the annual Digestive Disease Week. In addition, there was little difference between the vedolizumab and placebo groups in terms of adverse events, serious adverse events, and serious infections, said Dr. Feagan, professor of medicine at Western University in London, Ont.
At the end of a year of maintenance treatment, patients kept on the more frequent vedolizumab dosage tested, 300 mg delivered intravenously once every 4 weeks, showed a potent efficacy effect, surpassing the placebo group in corticosteroid-free clinical remissions by 31 percentage points (45% vs. 14%). "Nothing else is that good," Dr. Sandborn said in an interview. "Steroid-free remission with a delta over placebo of 30% is very impressive, especially when you factor in that many of the patients had failed anti-TNF treatment.
"We thought that vedolizumab would be safer than systemic immunosuppression, and I think the data are consistent with that. This will be a first-line treatment," Dr. Feagan said.
Equally important, total worldwide experience with vedolizumab, which now includes about 2,500 patients, has not yet resulted in a single case of progressive multifocal leukoencephalopathy (PML), an adverse effect produced by vedolizumab’s cousin drug, natalizumab (Tysarbi), ’approved for U.S. marketing to treat multiple sclerosis and Crohns disease.
Vedolizumab is a humanized monoclonal antibody that specifically binds to the alpha-4 beta-7 integrin protein that helps move leukocytes into the gut. Natalizumab is a less specific integrin antagonist that affects the protein’s actions and immune-cell trafficking to a variety of body sites, including the brain. Natalizumab "essentially blocks immune surveillance in the brain, and that allows for the JC virus [carried by about 50% of people] to cause PML," explained Dr. Sandborn. In contrast, vedolizumab does not cause "a systemic blockade of lymphocytes trafficking; it only affects a small fraction of lymphocytes, and leaves the brain completely unaffected."
Patients most at risk for PML are those who previously received immunosuppressive treatment with a drug such as azathioprine, methotrexate, or an anti-TNF drug. Patients with that history who received natalizumab for 1-2 years have about a 1 in 500-600 risk for PML, and those who got natalizumab for more than 2 years have a 1% risk.
By comparison, vedolizomab’s clean record based on 2,500 recipients "looks like it might have a very nice safety profile. If people can get comfortable with vedolizumab being different from natalizumab, then it has the potential to be first-line therapy for patients who have the worst prognosis," those who don’t respond to treatment with mesalamine.
The GEMINI I trial enrolled patients at 105 international sites with ulcerative colitis who had a Mayo score of at least 6 and an endoscopic subscore of at least 2 (indicating moderate disease) despite standard treatments. Patients were an average age of 40 years, their average duration of disease was 7 years, and their average Mayo score at entry was 8.5. Roughly 40% had previously received an anti-TNF treatment, about a third had failed on an anti-TNF drug, and just over half of the patients entered the study on a corticosteroid.
The trial included an induction phase that randomized 225 patients to a 6-week regimen with vedolizumab infusions and 149 patients to placebo. The researchers started another 521 ulcerative colitis patients on an open-label induction regimen, and then randomized 373 patients who responded after 6 weeks to maintenance infusion with vedolizumab every 4 weeks, a vedolizumab infusion every 8 weeks, or placebo.
The primary end point of the induction phase was clinical response, defined as a drop in the Mayo score of at least 3 points and at least 30%, plus a drop in the rectal bleeding score of at least 1 point or an absolute rectal bleeding subscore of 1 point or less. Achievement of this end point occurred in 47% of patients on vedolizumab and in 26% of those on placebo, a statistically significant difference. Among patients previously treated with an anti-TNF drug, 39% had a clinical response after 6 weeks on vedolizumab compared with 21% in the placebo arm, a significant difference. Among anti-TNF–naive patients, the rates were 53% and 26%.
The primary end point of the maintenance phase was clinical remission at 52 weeks, defined as a total Mayo score of 2 or less and no subscore greater than 1 point. This end point was met by 45% of patients who received vedolizumab every 4 weeks, by 42% who received the drug every 8 weeks, and by 16% of the placebo patients. Clinical remission without corticosteroid treatment occurred in 45% of patients who got vedolizumab every 4 weeks, 31% who got the drug every 8 weeks, and 14% who took placebo. Among patients with a history of anti-TNF treatment, clinical remission after 1 year occurred in 35% of patients maintained with the drug every 4 weeks, 37% who got the drug every 8 weeks, and 5% who took placebo. A total of 209 patients remained in the study through the 52-week assessment.
On May 11, Millennium Pharmaceuticals, the company developing vedolizumab, announced that the drug significantly surpassed placebo for the treatment of Crohn’s disease in the pivotal, phase III trial for that indication. The company said that it will soon report the full Crohn’s disease results.
The GEMINI I study was funded by Millennium. Dr. Feagan said he has been a consultant to Millennium and to several other drug companies. Dr. Sandborn said he has been a consultant to several drug companies but has no relationship with Millennium.
Vedolizumab is a biological therapy that blocks alpha-4/beta-7 integrins. This mechanism is novel within our armamentarium of available therapies for ulcerative colitis, and vedolizumab is also the first non-systemically acting biologic therapy. The induction and maintenance results of this phase III trial of vedolizumab in moderate to severe ulcerative colitis are very encouraging and represent a major advance in our field. It appears that the impressive safety profile (infection rate was similar to placebo) seen in this trial is likely due to its gut-specific activity.
Furthermore, it is of interest that despite inhibition of lymphocyte trafficking in the gut, there does not appear to be an increased risk of GI infections. If these impressive results pan out in clinical practice, vedolizumab undoubtedly will become a significant option for the management of our ulcerative colitis patients, and will have a prominent place in our treatment algorithms. We eagerly await FDA review and further studies, including the Crohn’s disease trial results.
David T. Rubin, M.D., AGAF, is Professor of Medicine, Associate Section Chief for Education, and Co-Director, Inflammatory Bowel Disease Center, University of Chicago.
Vedolizumab is a biological therapy that blocks alpha-4/beta-7 integrins. This mechanism is novel within our armamentarium of available therapies for ulcerative colitis, and vedolizumab is also the first non-systemically acting biologic therapy. The induction and maintenance results of this phase III trial of vedolizumab in moderate to severe ulcerative colitis are very encouraging and represent a major advance in our field. It appears that the impressive safety profile (infection rate was similar to placebo) seen in this trial is likely due to its gut-specific activity.
Furthermore, it is of interest that despite inhibition of lymphocyte trafficking in the gut, there does not appear to be an increased risk of GI infections. If these impressive results pan out in clinical practice, vedolizumab undoubtedly will become a significant option for the management of our ulcerative colitis patients, and will have a prominent place in our treatment algorithms. We eagerly await FDA review and further studies, including the Crohn’s disease trial results.
David T. Rubin, M.D., AGAF, is Professor of Medicine, Associate Section Chief for Education, and Co-Director, Inflammatory Bowel Disease Center, University of Chicago.
Vedolizumab is a biological therapy that blocks alpha-4/beta-7 integrins. This mechanism is novel within our armamentarium of available therapies for ulcerative colitis, and vedolizumab is also the first non-systemically acting biologic therapy. The induction and maintenance results of this phase III trial of vedolizumab in moderate to severe ulcerative colitis are very encouraging and represent a major advance in our field. It appears that the impressive safety profile (infection rate was similar to placebo) seen in this trial is likely due to its gut-specific activity.
Furthermore, it is of interest that despite inhibition of lymphocyte trafficking in the gut, there does not appear to be an increased risk of GI infections. If these impressive results pan out in clinical practice, vedolizumab undoubtedly will become a significant option for the management of our ulcerative colitis patients, and will have a prominent place in our treatment algorithms. We eagerly await FDA review and further studies, including the Crohn’s disease trial results.
David T. Rubin, M.D., AGAF, is Professor of Medicine, Associate Section Chief for Education, and Co-Director, Inflammatory Bowel Disease Center, University of Chicago.
SAN DIEGO – Vedolizumab, a novel drug that selectively blocks lymphocyte trafficking to the gut, was safe and highly effective for inducing and maintaining clinical remission in patients with moderate to severe ulcerative colitis in a pivotal phase III trial.
"If the findings hold up with more detailed analysis, it looks like we’ll have the efficacy of a biologic agent without some of the toxicity issues that we’ve seen with anti-TNF [tumor necrosis factor] drugs," said Dr. William J. Sandborn, professor of medicine and chief of gastroenterology at the University of California, San Diego.
"This potentially could be a first-line drug," for treating moderate to severe ulcerative colitis, said Dr. Sandborn, a coinvestigator in the study.
"Vedolizumab was more effective than placebo for induction and maintenance treatment, including both anti-TNF–exposed and naive patients," Dr. Brian G. Feagan, the study’s lead investigator, said at the annual Digestive Disease Week. In addition, there was little difference between the vedolizumab and placebo groups in terms of adverse events, serious adverse events, and serious infections, said Dr. Feagan, professor of medicine at Western University in London, Ont.
At the end of a year of maintenance treatment, patients kept on the more frequent vedolizumab dosage tested, 300 mg delivered intravenously once every 4 weeks, showed a potent efficacy effect, surpassing the placebo group in corticosteroid-free clinical remissions by 31 percentage points (45% vs. 14%). "Nothing else is that good," Dr. Sandborn said in an interview. "Steroid-free remission with a delta over placebo of 30% is very impressive, especially when you factor in that many of the patients had failed anti-TNF treatment.
"We thought that vedolizumab would be safer than systemic immunosuppression, and I think the data are consistent with that. This will be a first-line treatment," Dr. Feagan said.
Equally important, total worldwide experience with vedolizumab, which now includes about 2,500 patients, has not yet resulted in a single case of progressive multifocal leukoencephalopathy (PML), an adverse effect produced by vedolizumab’s cousin drug, natalizumab (Tysarbi), ’approved for U.S. marketing to treat multiple sclerosis and Crohns disease.
Vedolizumab is a humanized monoclonal antibody that specifically binds to the alpha-4 beta-7 integrin protein that helps move leukocytes into the gut. Natalizumab is a less specific integrin antagonist that affects the protein’s actions and immune-cell trafficking to a variety of body sites, including the brain. Natalizumab "essentially blocks immune surveillance in the brain, and that allows for the JC virus [carried by about 50% of people] to cause PML," explained Dr. Sandborn. In contrast, vedolizumab does not cause "a systemic blockade of lymphocytes trafficking; it only affects a small fraction of lymphocytes, and leaves the brain completely unaffected."
Patients most at risk for PML are those who previously received immunosuppressive treatment with a drug such as azathioprine, methotrexate, or an anti-TNF drug. Patients with that history who received natalizumab for 1-2 years have about a 1 in 500-600 risk for PML, and those who got natalizumab for more than 2 years have a 1% risk.
By comparison, vedolizomab’s clean record based on 2,500 recipients "looks like it might have a very nice safety profile. If people can get comfortable with vedolizumab being different from natalizumab, then it has the potential to be first-line therapy for patients who have the worst prognosis," those who don’t respond to treatment with mesalamine.
The GEMINI I trial enrolled patients at 105 international sites with ulcerative colitis who had a Mayo score of at least 6 and an endoscopic subscore of at least 2 (indicating moderate disease) despite standard treatments. Patients were an average age of 40 years, their average duration of disease was 7 years, and their average Mayo score at entry was 8.5. Roughly 40% had previously received an anti-TNF treatment, about a third had failed on an anti-TNF drug, and just over half of the patients entered the study on a corticosteroid.
The trial included an induction phase that randomized 225 patients to a 6-week regimen with vedolizumab infusions and 149 patients to placebo. The researchers started another 521 ulcerative colitis patients on an open-label induction regimen, and then randomized 373 patients who responded after 6 weeks to maintenance infusion with vedolizumab every 4 weeks, a vedolizumab infusion every 8 weeks, or placebo.
The primary end point of the induction phase was clinical response, defined as a drop in the Mayo score of at least 3 points and at least 30%, plus a drop in the rectal bleeding score of at least 1 point or an absolute rectal bleeding subscore of 1 point or less. Achievement of this end point occurred in 47% of patients on vedolizumab and in 26% of those on placebo, a statistically significant difference. Among patients previously treated with an anti-TNF drug, 39% had a clinical response after 6 weeks on vedolizumab compared with 21% in the placebo arm, a significant difference. Among anti-TNF–naive patients, the rates were 53% and 26%.
The primary end point of the maintenance phase was clinical remission at 52 weeks, defined as a total Mayo score of 2 or less and no subscore greater than 1 point. This end point was met by 45% of patients who received vedolizumab every 4 weeks, by 42% who received the drug every 8 weeks, and by 16% of the placebo patients. Clinical remission without corticosteroid treatment occurred in 45% of patients who got vedolizumab every 4 weeks, 31% who got the drug every 8 weeks, and 14% who took placebo. Among patients with a history of anti-TNF treatment, clinical remission after 1 year occurred in 35% of patients maintained with the drug every 4 weeks, 37% who got the drug every 8 weeks, and 5% who took placebo. A total of 209 patients remained in the study through the 52-week assessment.
On May 11, Millennium Pharmaceuticals, the company developing vedolizumab, announced that the drug significantly surpassed placebo for the treatment of Crohn’s disease in the pivotal, phase III trial for that indication. The company said that it will soon report the full Crohn’s disease results.
The GEMINI I study was funded by Millennium. Dr. Feagan said he has been a consultant to Millennium and to several other drug companies. Dr. Sandborn said he has been a consultant to several drug companies but has no relationship with Millennium.
SAN DIEGO – Vedolizumab, a novel drug that selectively blocks lymphocyte trafficking to the gut, was safe and highly effective for inducing and maintaining clinical remission in patients with moderate to severe ulcerative colitis in a pivotal phase III trial.
"If the findings hold up with more detailed analysis, it looks like we’ll have the efficacy of a biologic agent without some of the toxicity issues that we’ve seen with anti-TNF [tumor necrosis factor] drugs," said Dr. William J. Sandborn, professor of medicine and chief of gastroenterology at the University of California, San Diego.
"This potentially could be a first-line drug," for treating moderate to severe ulcerative colitis, said Dr. Sandborn, a coinvestigator in the study.
"Vedolizumab was more effective than placebo for induction and maintenance treatment, including both anti-TNF–exposed and naive patients," Dr. Brian G. Feagan, the study’s lead investigator, said at the annual Digestive Disease Week. In addition, there was little difference between the vedolizumab and placebo groups in terms of adverse events, serious adverse events, and serious infections, said Dr. Feagan, professor of medicine at Western University in London, Ont.
At the end of a year of maintenance treatment, patients kept on the more frequent vedolizumab dosage tested, 300 mg delivered intravenously once every 4 weeks, showed a potent efficacy effect, surpassing the placebo group in corticosteroid-free clinical remissions by 31 percentage points (45% vs. 14%). "Nothing else is that good," Dr. Sandborn said in an interview. "Steroid-free remission with a delta over placebo of 30% is very impressive, especially when you factor in that many of the patients had failed anti-TNF treatment.
"We thought that vedolizumab would be safer than systemic immunosuppression, and I think the data are consistent with that. This will be a first-line treatment," Dr. Feagan said.
Equally important, total worldwide experience with vedolizumab, which now includes about 2,500 patients, has not yet resulted in a single case of progressive multifocal leukoencephalopathy (PML), an adverse effect produced by vedolizumab’s cousin drug, natalizumab (Tysarbi), ’approved for U.S. marketing to treat multiple sclerosis and Crohns disease.
Vedolizumab is a humanized monoclonal antibody that specifically binds to the alpha-4 beta-7 integrin protein that helps move leukocytes into the gut. Natalizumab is a less specific integrin antagonist that affects the protein’s actions and immune-cell trafficking to a variety of body sites, including the brain. Natalizumab "essentially blocks immune surveillance in the brain, and that allows for the JC virus [carried by about 50% of people] to cause PML," explained Dr. Sandborn. In contrast, vedolizumab does not cause "a systemic blockade of lymphocytes trafficking; it only affects a small fraction of lymphocytes, and leaves the brain completely unaffected."
Patients most at risk for PML are those who previously received immunosuppressive treatment with a drug such as azathioprine, methotrexate, or an anti-TNF drug. Patients with that history who received natalizumab for 1-2 years have about a 1 in 500-600 risk for PML, and those who got natalizumab for more than 2 years have a 1% risk.
By comparison, vedolizomab’s clean record based on 2,500 recipients "looks like it might have a very nice safety profile. If people can get comfortable with vedolizumab being different from natalizumab, then it has the potential to be first-line therapy for patients who have the worst prognosis," those who don’t respond to treatment with mesalamine.
The GEMINI I trial enrolled patients at 105 international sites with ulcerative colitis who had a Mayo score of at least 6 and an endoscopic subscore of at least 2 (indicating moderate disease) despite standard treatments. Patients were an average age of 40 years, their average duration of disease was 7 years, and their average Mayo score at entry was 8.5. Roughly 40% had previously received an anti-TNF treatment, about a third had failed on an anti-TNF drug, and just over half of the patients entered the study on a corticosteroid.
The trial included an induction phase that randomized 225 patients to a 6-week regimen with vedolizumab infusions and 149 patients to placebo. The researchers started another 521 ulcerative colitis patients on an open-label induction regimen, and then randomized 373 patients who responded after 6 weeks to maintenance infusion with vedolizumab every 4 weeks, a vedolizumab infusion every 8 weeks, or placebo.
The primary end point of the induction phase was clinical response, defined as a drop in the Mayo score of at least 3 points and at least 30%, plus a drop in the rectal bleeding score of at least 1 point or an absolute rectal bleeding subscore of 1 point or less. Achievement of this end point occurred in 47% of patients on vedolizumab and in 26% of those on placebo, a statistically significant difference. Among patients previously treated with an anti-TNF drug, 39% had a clinical response after 6 weeks on vedolizumab compared with 21% in the placebo arm, a significant difference. Among anti-TNF–naive patients, the rates were 53% and 26%.
The primary end point of the maintenance phase was clinical remission at 52 weeks, defined as a total Mayo score of 2 or less and no subscore greater than 1 point. This end point was met by 45% of patients who received vedolizumab every 4 weeks, by 42% who received the drug every 8 weeks, and by 16% of the placebo patients. Clinical remission without corticosteroid treatment occurred in 45% of patients who got vedolizumab every 4 weeks, 31% who got the drug every 8 weeks, and 14% who took placebo. Among patients with a history of anti-TNF treatment, clinical remission after 1 year occurred in 35% of patients maintained with the drug every 4 weeks, 37% who got the drug every 8 weeks, and 5% who took placebo. A total of 209 patients remained in the study through the 52-week assessment.
On May 11, Millennium Pharmaceuticals, the company developing vedolizumab, announced that the drug significantly surpassed placebo for the treatment of Crohn’s disease in the pivotal, phase III trial for that indication. The company said that it will soon report the full Crohn’s disease results.
The GEMINI I study was funded by Millennium. Dr. Feagan said he has been a consultant to Millennium and to several other drug companies. Dr. Sandborn said he has been a consultant to several drug companies but has no relationship with Millennium.
FROM THE ANNUAL DIGESTIVE DISEASE WEEK
Major Finding: Maintenance therapy with vedolizumab produced a 45% steroid-free clinical remission rate compared with a 14% rate for patients taking placebo.
Data Source: The data came from GEMINI I, an international randomized, controlled phase III trial with 209 patients who remained in the study for 1 year.
Disclosures: The GEMINI I study was funded by Millennium Pharmaceuticals. Dr. Feagan said he has been a consultant to Millennium and to several other drug companies. Dr. Sandborn said he has been a consultant to several drug companies, but has no relationship with Millennium.
Diverticulosis Progression to Diverticulitis Found Surprisingly Rare
SAN DIEGO – A new study has found that people with diverticulosis actually have a low risk of progression to diverticulitis – far lower than the rates of 10%-25% commonly cited in the medical literature.
The actual rate seems to be at most six cases of progression from diverticulosis to diverticulitis for each 1,000 person years of follow-up. And if a stricter definition of diverticulitis is used, the rate for progression is even lower, 1.5 episodes for every 1,000 person-years, Dr. Kamyar Shahedi said at the meeting.
The newly derived rate came from a careful review of more than 2,000 people who were identified with diverticulosis in the VA Greater Los Angeles Healthcare System and followed for as long as 16 years.
The analysis also showed that the risk for developing diverticulitis subsequent to diagnosis of diverticulosis fell markedly with age, dropping by an average of 24% for each added decade of life from the time diverticulosis was first identified, said Dr. Shahedi, a gastroenterologist at the University of California, Los Angeles.
The highest cumulative hazard that people in the study faced for developing diverticulitis was if their diverticulosis was identified when they were in their 40s. The next highest rate of progression occurred among people first identified with diverticulosis in their 50s, and so on, with the lowest risk faced by people first found to have diverticulosis in their 70s.
Dr. Shahedi and his associates suspected that the age-related dimension to the risk for progression may have stemmed from a bias linked to the indication for the colonoscopy that found the diverticulosis, but after adjustment for the colonoscopy indication, younger age remained a significant, independent risk factor for more rapid progression. "Future research should try to explain how and why age affects risk," he said.
Diverticulosis is the most common finding during colonoscopy. Results from a recent review of U.S. adults who underwent colonoscopy during 2001-2005 showed that the procedures identified about 45% with diverticulosis (Gastroenterology 2009;136:741-54).
Citations for a 10%-25% rate of progression of diverticulosis to diverticulitis appear widely in the literature, such as in 1999 diverticulitis guidelines published by the American College of Gastroenterology (Am. J. Gastroenterol. 1999;94:3110-21).
But in the 1999 ACG guidelines, the citation for the 10%-25% rate is a 1975 textbook, and when Dr. Shahedi checked the book he found that the original data behind this rate came out in the 1930s, 1940s, and 1950s. "Few recent studies" have calculated a more contemporary progression rate, and the studies that have been done were small, Dr. Shahedi said.
His study involved a manual chart review of all people in the VA Greater Los Angeles Health Care system identified with diverticulosis during 1996-2011. This system includes 14 community clinics and 1 inpatient medical center and serves about 3 million people.
The manual review excluded people with a prior diagnosis of diverticulosis, and identified 2,222 newly diagnosed cases. The records for these incident diverticulosis cases then underwent further careful review to flag the people who subsequently developed diverticulitis.
The researchers used four different criteria for identifying progression to diverticulitis: a chart diagnosis that included no objective evidence of progression, a diagnosis supported by objective data but without radiographic documentation, a diagnosis supported by imaging, and a diagnosis supported by a surgical specimen.
The review identified 95 of the 2,222 people (4.3%) who progressed to diverticulitis by any of these four criteria, and 23 people (1% of the 2,222) among these 95 whose progression to diverticulitis included documentation by at least one of the two strictest criteria, either radiographic or surgical evidence. The median time to progression to diverticulitis documented by any of the four criteria was 7.1 years.
The 2,222 people with diverticulosis were all veterans, their average age was 67 years, about 98% were men, and their average body mass index was 28.5 kg/m2. About 40% of the group was white, 10% African American, 8% Hispanic, and the rest were of other ethnic groups. Dr. Shahedi noted that the study was limited by examining a VA population that largely included men, it was a retrospective study, and all the people included came from a single health care system.
Dr. Shahedi said that he had no disclosures.
When we diagnose patients with diverticulosis by colonoscopy, they want to know the risk of its progress to the clinically important disease, diverticulitis. Prior data suggested that the risk was as high as 25%, which can be pretty scary, especially given how many people are identified with diverticulosis.
 |
|
The new data reported by Dr. Shahedi show that the rate of progression is much lower. In fact, the rate is so low that if patients are first identified with diverticulosis in their 50s or 60s, it appears that the risk of progression to diverticulitis during the rest of their lives is very small. Those first identified with diverticulosis in their 30s faces a much greater likelihood of eventually developing diverticulitis.
These new data are very helpful because they suggest that the risk is much lower than that usually quoted in the past. There are certain limitations to these new data, which Dr. Shahedi acknowledged, but I believe these numbers more truly reflect what really happens. The methodology they used was much better than what was previously available.
Dr. Philip S. Schoenfeld is a gastroenterologist at the University of Michigan, and chief of gastroenterology at the VA Medical Center, both in Ann Arbor. He said that he has been a consultant to Salix and Ironwood. He made these comments in an interview.
When we diagnose patients with diverticulosis by colonoscopy, they want to know the risk of its progress to the clinically important disease, diverticulitis. Prior data suggested that the risk was as high as 25%, which can be pretty scary, especially given how many people are identified with diverticulosis.
 |
|
The new data reported by Dr. Shahedi show that the rate of progression is much lower. In fact, the rate is so low that if patients are first identified with diverticulosis in their 50s or 60s, it appears that the risk of progression to diverticulitis during the rest of their lives is very small. Those first identified with diverticulosis in their 30s faces a much greater likelihood of eventually developing diverticulitis.
These new data are very helpful because they suggest that the risk is much lower than that usually quoted in the past. There are certain limitations to these new data, which Dr. Shahedi acknowledged, but I believe these numbers more truly reflect what really happens. The methodology they used was much better than what was previously available.
Dr. Philip S. Schoenfeld is a gastroenterologist at the University of Michigan, and chief of gastroenterology at the VA Medical Center, both in Ann Arbor. He said that he has been a consultant to Salix and Ironwood. He made these comments in an interview.
When we diagnose patients with diverticulosis by colonoscopy, they want to know the risk of its progress to the clinically important disease, diverticulitis. Prior data suggested that the risk was as high as 25%, which can be pretty scary, especially given how many people are identified with diverticulosis.
 |
|
The new data reported by Dr. Shahedi show that the rate of progression is much lower. In fact, the rate is so low that if patients are first identified with diverticulosis in their 50s or 60s, it appears that the risk of progression to diverticulitis during the rest of their lives is very small. Those first identified with diverticulosis in their 30s faces a much greater likelihood of eventually developing diverticulitis.
These new data are very helpful because they suggest that the risk is much lower than that usually quoted in the past. There are certain limitations to these new data, which Dr. Shahedi acknowledged, but I believe these numbers more truly reflect what really happens. The methodology they used was much better than what was previously available.
Dr. Philip S. Schoenfeld is a gastroenterologist at the University of Michigan, and chief of gastroenterology at the VA Medical Center, both in Ann Arbor. He said that he has been a consultant to Salix and Ironwood. He made these comments in an interview.
SAN DIEGO – A new study has found that people with diverticulosis actually have a low risk of progression to diverticulitis – far lower than the rates of 10%-25% commonly cited in the medical literature.
The actual rate seems to be at most six cases of progression from diverticulosis to diverticulitis for each 1,000 person years of follow-up. And if a stricter definition of diverticulitis is used, the rate for progression is even lower, 1.5 episodes for every 1,000 person-years, Dr. Kamyar Shahedi said at the meeting.
The newly derived rate came from a careful review of more than 2,000 people who were identified with diverticulosis in the VA Greater Los Angeles Healthcare System and followed for as long as 16 years.
The analysis also showed that the risk for developing diverticulitis subsequent to diagnosis of diverticulosis fell markedly with age, dropping by an average of 24% for each added decade of life from the time diverticulosis was first identified, said Dr. Shahedi, a gastroenterologist at the University of California, Los Angeles.
The highest cumulative hazard that people in the study faced for developing diverticulitis was if their diverticulosis was identified when they were in their 40s. The next highest rate of progression occurred among people first identified with diverticulosis in their 50s, and so on, with the lowest risk faced by people first found to have diverticulosis in their 70s.
Dr. Shahedi and his associates suspected that the age-related dimension to the risk for progression may have stemmed from a bias linked to the indication for the colonoscopy that found the diverticulosis, but after adjustment for the colonoscopy indication, younger age remained a significant, independent risk factor for more rapid progression. "Future research should try to explain how and why age affects risk," he said.
Diverticulosis is the most common finding during colonoscopy. Results from a recent review of U.S. adults who underwent colonoscopy during 2001-2005 showed that the procedures identified about 45% with diverticulosis (Gastroenterology 2009;136:741-54).
Citations for a 10%-25% rate of progression of diverticulosis to diverticulitis appear widely in the literature, such as in 1999 diverticulitis guidelines published by the American College of Gastroenterology (Am. J. Gastroenterol. 1999;94:3110-21).
But in the 1999 ACG guidelines, the citation for the 10%-25% rate is a 1975 textbook, and when Dr. Shahedi checked the book he found that the original data behind this rate came out in the 1930s, 1940s, and 1950s. "Few recent studies" have calculated a more contemporary progression rate, and the studies that have been done were small, Dr. Shahedi said.
His study involved a manual chart review of all people in the VA Greater Los Angeles Health Care system identified with diverticulosis during 1996-2011. This system includes 14 community clinics and 1 inpatient medical center and serves about 3 million people.
The manual review excluded people with a prior diagnosis of diverticulosis, and identified 2,222 newly diagnosed cases. The records for these incident diverticulosis cases then underwent further careful review to flag the people who subsequently developed diverticulitis.
The researchers used four different criteria for identifying progression to diverticulitis: a chart diagnosis that included no objective evidence of progression, a diagnosis supported by objective data but without radiographic documentation, a diagnosis supported by imaging, and a diagnosis supported by a surgical specimen.
The review identified 95 of the 2,222 people (4.3%) who progressed to diverticulitis by any of these four criteria, and 23 people (1% of the 2,222) among these 95 whose progression to diverticulitis included documentation by at least one of the two strictest criteria, either radiographic or surgical evidence. The median time to progression to diverticulitis documented by any of the four criteria was 7.1 years.
The 2,222 people with diverticulosis were all veterans, their average age was 67 years, about 98% were men, and their average body mass index was 28.5 kg/m2. About 40% of the group was white, 10% African American, 8% Hispanic, and the rest were of other ethnic groups. Dr. Shahedi noted that the study was limited by examining a VA population that largely included men, it was a retrospective study, and all the people included came from a single health care system.
Dr. Shahedi said that he had no disclosures.
SAN DIEGO – A new study has found that people with diverticulosis actually have a low risk of progression to diverticulitis – far lower than the rates of 10%-25% commonly cited in the medical literature.
The actual rate seems to be at most six cases of progression from diverticulosis to diverticulitis for each 1,000 person years of follow-up. And if a stricter definition of diverticulitis is used, the rate for progression is even lower, 1.5 episodes for every 1,000 person-years, Dr. Kamyar Shahedi said at the meeting.
The newly derived rate came from a careful review of more than 2,000 people who were identified with diverticulosis in the VA Greater Los Angeles Healthcare System and followed for as long as 16 years.
The analysis also showed that the risk for developing diverticulitis subsequent to diagnosis of diverticulosis fell markedly with age, dropping by an average of 24% for each added decade of life from the time diverticulosis was first identified, said Dr. Shahedi, a gastroenterologist at the University of California, Los Angeles.
The highest cumulative hazard that people in the study faced for developing diverticulitis was if their diverticulosis was identified when they were in their 40s. The next highest rate of progression occurred among people first identified with diverticulosis in their 50s, and so on, with the lowest risk faced by people first found to have diverticulosis in their 70s.
Dr. Shahedi and his associates suspected that the age-related dimension to the risk for progression may have stemmed from a bias linked to the indication for the colonoscopy that found the diverticulosis, but after adjustment for the colonoscopy indication, younger age remained a significant, independent risk factor for more rapid progression. "Future research should try to explain how and why age affects risk," he said.
Diverticulosis is the most common finding during colonoscopy. Results from a recent review of U.S. adults who underwent colonoscopy during 2001-2005 showed that the procedures identified about 45% with diverticulosis (Gastroenterology 2009;136:741-54).
Citations for a 10%-25% rate of progression of diverticulosis to diverticulitis appear widely in the literature, such as in 1999 diverticulitis guidelines published by the American College of Gastroenterology (Am. J. Gastroenterol. 1999;94:3110-21).
But in the 1999 ACG guidelines, the citation for the 10%-25% rate is a 1975 textbook, and when Dr. Shahedi checked the book he found that the original data behind this rate came out in the 1930s, 1940s, and 1950s. "Few recent studies" have calculated a more contemporary progression rate, and the studies that have been done were small, Dr. Shahedi said.
His study involved a manual chart review of all people in the VA Greater Los Angeles Health Care system identified with diverticulosis during 1996-2011. This system includes 14 community clinics and 1 inpatient medical center and serves about 3 million people.
The manual review excluded people with a prior diagnosis of diverticulosis, and identified 2,222 newly diagnosed cases. The records for these incident diverticulosis cases then underwent further careful review to flag the people who subsequently developed diverticulitis.
The researchers used four different criteria for identifying progression to diverticulitis: a chart diagnosis that included no objective evidence of progression, a diagnosis supported by objective data but without radiographic documentation, a diagnosis supported by imaging, and a diagnosis supported by a surgical specimen.
The review identified 95 of the 2,222 people (4.3%) who progressed to diverticulitis by any of these four criteria, and 23 people (1% of the 2,222) among these 95 whose progression to diverticulitis included documentation by at least one of the two strictest criteria, either radiographic or surgical evidence. The median time to progression to diverticulitis documented by any of the four criteria was 7.1 years.
The 2,222 people with diverticulosis were all veterans, their average age was 67 years, about 98% were men, and their average body mass index was 28.5 kg/m2. About 40% of the group was white, 10% African American, 8% Hispanic, and the rest were of other ethnic groups. Dr. Shahedi noted that the study was limited by examining a VA population that largely included men, it was a retrospective study, and all the people included came from a single health care system.
Dr. Shahedi said that he had no disclosures.
FROM THE ANNUAL DIGESTIVE DISEASE WEEK
Major Finding: Among U.S. adults with diverticulosis, progression to strictly-defined diverticulitis occurred 1.5 times per 1,000 person-years.
Data Source: The data came from a review of 2,222 people identified with diverticulosis in the VA Greater Los Angeles Healthcare System during 1996-2011.
Disclosures: Dr. Shahedi said that he had no disclosures.
Routine Sigmoidoscopy Cut Colorectal Cancer Incidence, Mortality
SAN DIEGO – The incidence of colorectal cancer among men and women who underwent flexible sigmoidoscopy, with a repeat screening every 3 or 5 years, was reduced by 21%, compared with those who received usual care, according to the findings of a large, randomized controlled trial.
In addition, results from the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) showed that study participants in the flexible sigmoidoscopy group had a 26% reduction in overall colorectal cancer mortality and a 50% reduction in mortality related to distal colorectal cancer, compared with the usual-care group. In about 22% of cases, colonoscopy was performed as a direct effect of screening with flexible sigmoidoscopy.
Dr. Robert E. Schoen of the division of gastroenterology, hepatology, and nutrition at the University of Pittsburgh Medical Center and his associates randomly assigned 154,900 men and women aged 55-74 years to either screening with a baseline flexible sigmoidoscopy, with a repeat screening every 3 or 5 years, or to usual care, which was defined as "whatever might have been recommended by the patient’s physician," he said.
"The rate of routine colonoscopy after the screening phase was 47.7% in the intervention group and 48.0% in the usual-care group, according to the study.
The investigators followed the study participants for a median of 11.9 years and collected medical records related to follow-up, a diagnosis of cancer, and cancer complications. The primary outcome was death from colorectal cancer, while incidence of the disease was listed as a secondary outcome (N. Engl. J. Med. 2012 May 21 [doi:10.1056/NEJMoa1114635]).
Of the 154,900 study participants, 77,445 were assigned to the flexible sigmoidoscopy group and 77,455 to the usual-care group. The groups were evenly split by gender, more than half (64%) ranged in age from 55-64 years, 86% were white (non-Hispanic), and 34% were college graduates.
During follow-up, the incidence of colorectal cancer was 11.9 cases/10,000 person-years in the intervention group, compared with 15.2 cases/10,000 person-years in the usual care group, which translated into a statistically significant reduction of 21% (P less than .001).
The researchers also reported that there were 2.9 deaths from colorectal cancer/10,000 person-years in the intervention group, compared with 3.9/10,000 person-years in the usual care group, which translated into a statistically significant reduction of 26% (P less than .001). Mortality from distal disease was reduced by 50% in the intervention group, compared with the usual care group (P less than .001), but mortality from proximal disease remained unaffected (P = .81).
"As compared with the distal colon, the proximal colon poses a more difficult challenge for colorectal cancer control because of limitations in bowel preparation, a greater prevalence of advanced serrated adenomas, which are harder to detect than conventional adenomas, and biologic differences, including a greater incidence of BRAF mutation, microsatellite instability, and CpG island methylator phenotype (CIMP)," the researchers wrote.
"Although our protocol was associated with a reduction in the incidence of proximal colorectal cancer, presumably because of the detection and removal of precursor adenomas that would otherwise have progressed to cancer, it apparently did not succeed in identifying and successfully removing a proportionally greater number of precursor lesions destined to develop into fatal colorectal cancers."
At the meeting, Dr. Schoen told this news organization that clinicians "have to become better at detecting subtle polyps in the right [proximal] colon. The hope is that if we get better with colonoscopy we will be able to find not just the polyps that lead to cancer, but also the polyps that lead to fatal cancer."
Dr. Schoen disclosed that he has received stock options from Onconome. The study was funded by the National Cancer Institute.
National guidelines include flexible sigmoidoscopy as a recommended screening strategy; however, use of this test has significantly decreased in the United States. What rationale could one propose to invest in expansion of this strategy?
First, the quality of evidence supporting the effectiveness of screening colonoscopy is inferior to that supporting flexible sigmoidoscopy. Although case-control and prospective cohort studies have been performed, no randomized clinical trial proving that colonoscopy can reduce cancer mortality has yet been published. Moreover, studies have reported that screening colonoscopy lacks benefit in reducing mortality from proximal colorectal cancer.
Second, not all people agree to undergo a screening colonoscopy. We have reported our observations that significantly fewer persons with a recommendation for colonoscopy chose to undergo screening than did persons with a recommendation for alternative screening strategies (Arch. Intern. Med. 2012; 172:575-82).
Finally, health care reform in the United States is likely to alter the manner in which options are presented to patients. As reimbursement moves from fee for service to bundled payments for episodes of care, there will be a renewed focus on delivering evidence-based interventions in a manner that optimizes resource use. Although these efforts must not limit access to beneficial interventions, it is likely that effective strategies that are less resource-intensive will be viewed more favorably by payers and accountable care organizations.
Dr. John M. Inadomi is with the division of gastroenterology in the department of medicine at the University of Washington, Seattle. These comments were extracted from a guest editorial that appeared online May 21, 2012, in the New England Journal of Medicine. (2012 May 21 [doi:10.1056/e1204099]). Dr. Inadomi disclosed that he is a paid consultant to Takeda Pharmaceuticals and Roche Diagnostics.
National guidelines include flexible sigmoidoscopy as a recommended screening strategy; however, use of this test has significantly decreased in the United States. What rationale could one propose to invest in expansion of this strategy?
First, the quality of evidence supporting the effectiveness of screening colonoscopy is inferior to that supporting flexible sigmoidoscopy. Although case-control and prospective cohort studies have been performed, no randomized clinical trial proving that colonoscopy can reduce cancer mortality has yet been published. Moreover, studies have reported that screening colonoscopy lacks benefit in reducing mortality from proximal colorectal cancer.
Second, not all people agree to undergo a screening colonoscopy. We have reported our observations that significantly fewer persons with a recommendation for colonoscopy chose to undergo screening than did persons with a recommendation for alternative screening strategies (Arch. Intern. Med. 2012; 172:575-82).
Finally, health care reform in the United States is likely to alter the manner in which options are presented to patients. As reimbursement moves from fee for service to bundled payments for episodes of care, there will be a renewed focus on delivering evidence-based interventions in a manner that optimizes resource use. Although these efforts must not limit access to beneficial interventions, it is likely that effective strategies that are less resource-intensive will be viewed more favorably by payers and accountable care organizations.
Dr. John M. Inadomi is with the division of gastroenterology in the department of medicine at the University of Washington, Seattle. These comments were extracted from a guest editorial that appeared online May 21, 2012, in the New England Journal of Medicine. (2012 May 21 [doi:10.1056/e1204099]). Dr. Inadomi disclosed that he is a paid consultant to Takeda Pharmaceuticals and Roche Diagnostics.
National guidelines include flexible sigmoidoscopy as a recommended screening strategy; however, use of this test has significantly decreased in the United States. What rationale could one propose to invest in expansion of this strategy?
First, the quality of evidence supporting the effectiveness of screening colonoscopy is inferior to that supporting flexible sigmoidoscopy. Although case-control and prospective cohort studies have been performed, no randomized clinical trial proving that colonoscopy can reduce cancer mortality has yet been published. Moreover, studies have reported that screening colonoscopy lacks benefit in reducing mortality from proximal colorectal cancer.
Second, not all people agree to undergo a screening colonoscopy. We have reported our observations that significantly fewer persons with a recommendation for colonoscopy chose to undergo screening than did persons with a recommendation for alternative screening strategies (Arch. Intern. Med. 2012; 172:575-82).
Finally, health care reform in the United States is likely to alter the manner in which options are presented to patients. As reimbursement moves from fee for service to bundled payments for episodes of care, there will be a renewed focus on delivering evidence-based interventions in a manner that optimizes resource use. Although these efforts must not limit access to beneficial interventions, it is likely that effective strategies that are less resource-intensive will be viewed more favorably by payers and accountable care organizations.
Dr. John M. Inadomi is with the division of gastroenterology in the department of medicine at the University of Washington, Seattle. These comments were extracted from a guest editorial that appeared online May 21, 2012, in the New England Journal of Medicine. (2012 May 21 [doi:10.1056/e1204099]). Dr. Inadomi disclosed that he is a paid consultant to Takeda Pharmaceuticals and Roche Diagnostics.
SAN DIEGO – The incidence of colorectal cancer among men and women who underwent flexible sigmoidoscopy, with a repeat screening every 3 or 5 years, was reduced by 21%, compared with those who received usual care, according to the findings of a large, randomized controlled trial.
In addition, results from the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) showed that study participants in the flexible sigmoidoscopy group had a 26% reduction in overall colorectal cancer mortality and a 50% reduction in mortality related to distal colorectal cancer, compared with the usual-care group. In about 22% of cases, colonoscopy was performed as a direct effect of screening with flexible sigmoidoscopy.
Dr. Robert E. Schoen of the division of gastroenterology, hepatology, and nutrition at the University of Pittsburgh Medical Center and his associates randomly assigned 154,900 men and women aged 55-74 years to either screening with a baseline flexible sigmoidoscopy, with a repeat screening every 3 or 5 years, or to usual care, which was defined as "whatever might have been recommended by the patient’s physician," he said.
"The rate of routine colonoscopy after the screening phase was 47.7% in the intervention group and 48.0% in the usual-care group, according to the study.
The investigators followed the study participants for a median of 11.9 years and collected medical records related to follow-up, a diagnosis of cancer, and cancer complications. The primary outcome was death from colorectal cancer, while incidence of the disease was listed as a secondary outcome (N. Engl. J. Med. 2012 May 21 [doi:10.1056/NEJMoa1114635]).
Of the 154,900 study participants, 77,445 were assigned to the flexible sigmoidoscopy group and 77,455 to the usual-care group. The groups were evenly split by gender, more than half (64%) ranged in age from 55-64 years, 86% were white (non-Hispanic), and 34% were college graduates.
During follow-up, the incidence of colorectal cancer was 11.9 cases/10,000 person-years in the intervention group, compared with 15.2 cases/10,000 person-years in the usual care group, which translated into a statistically significant reduction of 21% (P less than .001).
The researchers also reported that there were 2.9 deaths from colorectal cancer/10,000 person-years in the intervention group, compared with 3.9/10,000 person-years in the usual care group, which translated into a statistically significant reduction of 26% (P less than .001). Mortality from distal disease was reduced by 50% in the intervention group, compared with the usual care group (P less than .001), but mortality from proximal disease remained unaffected (P = .81).
"As compared with the distal colon, the proximal colon poses a more difficult challenge for colorectal cancer control because of limitations in bowel preparation, a greater prevalence of advanced serrated adenomas, which are harder to detect than conventional adenomas, and biologic differences, including a greater incidence of BRAF mutation, microsatellite instability, and CpG island methylator phenotype (CIMP)," the researchers wrote.
"Although our protocol was associated with a reduction in the incidence of proximal colorectal cancer, presumably because of the detection and removal of precursor adenomas that would otherwise have progressed to cancer, it apparently did not succeed in identifying and successfully removing a proportionally greater number of precursor lesions destined to develop into fatal colorectal cancers."
At the meeting, Dr. Schoen told this news organization that clinicians "have to become better at detecting subtle polyps in the right [proximal] colon. The hope is that if we get better with colonoscopy we will be able to find not just the polyps that lead to cancer, but also the polyps that lead to fatal cancer."
Dr. Schoen disclosed that he has received stock options from Onconome. The study was funded by the National Cancer Institute.
SAN DIEGO – The incidence of colorectal cancer among men and women who underwent flexible sigmoidoscopy, with a repeat screening every 3 or 5 years, was reduced by 21%, compared with those who received usual care, according to the findings of a large, randomized controlled trial.
In addition, results from the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) showed that study participants in the flexible sigmoidoscopy group had a 26% reduction in overall colorectal cancer mortality and a 50% reduction in mortality related to distal colorectal cancer, compared with the usual-care group. In about 22% of cases, colonoscopy was performed as a direct effect of screening with flexible sigmoidoscopy.
Dr. Robert E. Schoen of the division of gastroenterology, hepatology, and nutrition at the University of Pittsburgh Medical Center and his associates randomly assigned 154,900 men and women aged 55-74 years to either screening with a baseline flexible sigmoidoscopy, with a repeat screening every 3 or 5 years, or to usual care, which was defined as "whatever might have been recommended by the patient’s physician," he said.
"The rate of routine colonoscopy after the screening phase was 47.7% in the intervention group and 48.0% in the usual-care group, according to the study.
The investigators followed the study participants for a median of 11.9 years and collected medical records related to follow-up, a diagnosis of cancer, and cancer complications. The primary outcome was death from colorectal cancer, while incidence of the disease was listed as a secondary outcome (N. Engl. J. Med. 2012 May 21 [doi:10.1056/NEJMoa1114635]).
Of the 154,900 study participants, 77,445 were assigned to the flexible sigmoidoscopy group and 77,455 to the usual-care group. The groups were evenly split by gender, more than half (64%) ranged in age from 55-64 years, 86% were white (non-Hispanic), and 34% were college graduates.
During follow-up, the incidence of colorectal cancer was 11.9 cases/10,000 person-years in the intervention group, compared with 15.2 cases/10,000 person-years in the usual care group, which translated into a statistically significant reduction of 21% (P less than .001).
The researchers also reported that there were 2.9 deaths from colorectal cancer/10,000 person-years in the intervention group, compared with 3.9/10,000 person-years in the usual care group, which translated into a statistically significant reduction of 26% (P less than .001). Mortality from distal disease was reduced by 50% in the intervention group, compared with the usual care group (P less than .001), but mortality from proximal disease remained unaffected (P = .81).
"As compared with the distal colon, the proximal colon poses a more difficult challenge for colorectal cancer control because of limitations in bowel preparation, a greater prevalence of advanced serrated adenomas, which are harder to detect than conventional adenomas, and biologic differences, including a greater incidence of BRAF mutation, microsatellite instability, and CpG island methylator phenotype (CIMP)," the researchers wrote.
"Although our protocol was associated with a reduction in the incidence of proximal colorectal cancer, presumably because of the detection and removal of precursor adenomas that would otherwise have progressed to cancer, it apparently did not succeed in identifying and successfully removing a proportionally greater number of precursor lesions destined to develop into fatal colorectal cancers."
At the meeting, Dr. Schoen told this news organization that clinicians "have to become better at detecting subtle polyps in the right [proximal] colon. The hope is that if we get better with colonoscopy we will be able to find not just the polyps that lead to cancer, but also the polyps that lead to fatal cancer."
Dr. Schoen disclosed that he has received stock options from Onconome. The study was funded by the National Cancer Institute.
FROM THE ANNUAL DIGESTIVE DISEASE WEEK
Major Finding: The use of flexible sigmoidoscopy for colorectal cancer screening, as compared with usual care, was associated with a 26% reduction in overall colorectal cancer mortality and a 21% reduction in the incidence of colorectal cancer.
Data Source: Findings are based on a study of 77,445 participants in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial.
Disclosures: Dr. Schoen disclosed that he has received stock options from Onconome. The study was funded by the National Cancer Institute.
MRSA Nasal Colonization Predicts MRSA Site Infection in GI Surgery
SAN DIEGO – Nasal colonization of methicillin-resistant Staphylococcus aureus was linked to an increase in surgical site infections and longer hospital stays in patients undergoing major gastrointestinal surgery in a large retrospective study, a finding that surprised investigators who had hypothesized that nasal colonization of the organism, which is not routinely found or colonized in the GI tract, would have little impact on outcome measures.
"Gastrointestinal operations are different from other surgeries in that infectious pathogens are typically organisms found in the gut, not bacteria that colonize in the skin, which is why we didn’t expect to find a correlation," lead investigator Dr. Harry T. Papaconstantinou said during a teleconference reporting the results, which he presented on Sunday, May 20, at the annual Digestive Disease Week conference.
While it’s unlikely that nasal colonization of MRSA necessarily increases the risk of developing a surgical site infection following GI surgery, "it is possible that it might be an indicator of the type of organism that is involved in the infection." For example, in the current analysis, wound culture data were available for 92 patients. In patients who tested positive for MRSA, 70% of the wound infections stemmed from MRSA, compared with only 8.5% in of those who tested negative, he said.
To evaluate the relationship between MRSA nasal colonization and surgical site infection, wound cultures, hospital length of stay, and mortality, Dr. Papaconstantinou, chief of colorectal surgery at Scott and White Memorial Hospital in Temple, Tex., and his colleagues examined the records of patients who underwent major GI surgery at the hospital between December 2007 and August 2009. The patients, who also had nasal swab tests within 24-48 hours after admission, were divided into one of three categories: MRSA-swab positive, methicillin-sensitive Staphylococcus aureus (MSSA)–swab positive, or negative for both.
Of the 1,137 patients, mean age 59.5 years, 73 (6.4%) were MRSA-swab positive, 167 (14.7%) were MSSA-swab positive, and 897 (78.9%) were negative, Dr. Papaconstantinou reported. Surgical site infection was identified in 101 patients (8.9%), and the rate of infection was highest, at 14%, in the MRSA-swab positive patients, compared with 4% and 9%, respectively, in the MSSA-swab positive and negative patients, he said. "When we controlled for other confounding factors, we didn’t find nasal swab to be an independent predictor of surgical site infection, but what we did find was a strong relationship between MRSA-positive nasal colonization and type of organism involved [in the surgical site infection]."
Regarding mean hospital length of stay, the respective durations for the MRSA-swab positive, MSSA-swab positive, and the negative groups overall were 12.5 days, 7.6 days, and 8.8 days, representing a significant increase, said Dr. Papaconstantinou. "By multiregression analysis, we found a MRSA-positive swab to be an independent risk factor for extended length of stay." However, when looking specifically at patients with surgical site infections, the presence of which increased hospital length of stay significantly from 6.2 days to 15.7 days, "there was no between group differences based on nasal swab," he said. Similarly, the 45 deaths in the study population wee distributed evenly across the nasal swab groups.
Based on the findings, Dr. Papaconstantinou said in an interview, "we can conclude that a positive MRSA nasal swab test for colonization is a strong predictor that MRSA-associated surgical site infections will occur in patients undergoing major GI surgery." As such, "we propose the possibility that it might be beneficial to preoperatively screen and decolonize these patients in an effort to reduce the incidence of these infections and improve patient outcomes following surgery." Toward this end, he and his colleagues are anticipating performing such a study and plan on including a cost-benefit analysis to determine whether screening is economically beneficial, he said.
Dr. Papaconstantinou disclosed a financial relationship with Covidien.
SAN DIEGO – Nasal colonization of methicillin-resistant Staphylococcus aureus was linked to an increase in surgical site infections and longer hospital stays in patients undergoing major gastrointestinal surgery in a large retrospective study, a finding that surprised investigators who had hypothesized that nasal colonization of the organism, which is not routinely found or colonized in the GI tract, would have little impact on outcome measures.
"Gastrointestinal operations are different from other surgeries in that infectious pathogens are typically organisms found in the gut, not bacteria that colonize in the skin, which is why we didn’t expect to find a correlation," lead investigator Dr. Harry T. Papaconstantinou said during a teleconference reporting the results, which he presented on Sunday, May 20, at the annual Digestive Disease Week conference.
While it’s unlikely that nasal colonization of MRSA necessarily increases the risk of developing a surgical site infection following GI surgery, "it is possible that it might be an indicator of the type of organism that is involved in the infection." For example, in the current analysis, wound culture data were available for 92 patients. In patients who tested positive for MRSA, 70% of the wound infections stemmed from MRSA, compared with only 8.5% in of those who tested negative, he said.
To evaluate the relationship between MRSA nasal colonization and surgical site infection, wound cultures, hospital length of stay, and mortality, Dr. Papaconstantinou, chief of colorectal surgery at Scott and White Memorial Hospital in Temple, Tex., and his colleagues examined the records of patients who underwent major GI surgery at the hospital between December 2007 and August 2009. The patients, who also had nasal swab tests within 24-48 hours after admission, were divided into one of three categories: MRSA-swab positive, methicillin-sensitive Staphylococcus aureus (MSSA)–swab positive, or negative for both.
Of the 1,137 patients, mean age 59.5 years, 73 (6.4%) were MRSA-swab positive, 167 (14.7%) were MSSA-swab positive, and 897 (78.9%) were negative, Dr. Papaconstantinou reported. Surgical site infection was identified in 101 patients (8.9%), and the rate of infection was highest, at 14%, in the MRSA-swab positive patients, compared with 4% and 9%, respectively, in the MSSA-swab positive and negative patients, he said. "When we controlled for other confounding factors, we didn’t find nasal swab to be an independent predictor of surgical site infection, but what we did find was a strong relationship between MRSA-positive nasal colonization and type of organism involved [in the surgical site infection]."
Regarding mean hospital length of stay, the respective durations for the MRSA-swab positive, MSSA-swab positive, and the negative groups overall were 12.5 days, 7.6 days, and 8.8 days, representing a significant increase, said Dr. Papaconstantinou. "By multiregression analysis, we found a MRSA-positive swab to be an independent risk factor for extended length of stay." However, when looking specifically at patients with surgical site infections, the presence of which increased hospital length of stay significantly from 6.2 days to 15.7 days, "there was no between group differences based on nasal swab," he said. Similarly, the 45 deaths in the study population wee distributed evenly across the nasal swab groups.
Based on the findings, Dr. Papaconstantinou said in an interview, "we can conclude that a positive MRSA nasal swab test for colonization is a strong predictor that MRSA-associated surgical site infections will occur in patients undergoing major GI surgery." As such, "we propose the possibility that it might be beneficial to preoperatively screen and decolonize these patients in an effort to reduce the incidence of these infections and improve patient outcomes following surgery." Toward this end, he and his colleagues are anticipating performing such a study and plan on including a cost-benefit analysis to determine whether screening is economically beneficial, he said.
Dr. Papaconstantinou disclosed a financial relationship with Covidien.
SAN DIEGO – Nasal colonization of methicillin-resistant Staphylococcus aureus was linked to an increase in surgical site infections and longer hospital stays in patients undergoing major gastrointestinal surgery in a large retrospective study, a finding that surprised investigators who had hypothesized that nasal colonization of the organism, which is not routinely found or colonized in the GI tract, would have little impact on outcome measures.
"Gastrointestinal operations are different from other surgeries in that infectious pathogens are typically organisms found in the gut, not bacteria that colonize in the skin, which is why we didn’t expect to find a correlation," lead investigator Dr. Harry T. Papaconstantinou said during a teleconference reporting the results, which he presented on Sunday, May 20, at the annual Digestive Disease Week conference.
While it’s unlikely that nasal colonization of MRSA necessarily increases the risk of developing a surgical site infection following GI surgery, "it is possible that it might be an indicator of the type of organism that is involved in the infection." For example, in the current analysis, wound culture data were available for 92 patients. In patients who tested positive for MRSA, 70% of the wound infections stemmed from MRSA, compared with only 8.5% in of those who tested negative, he said.
To evaluate the relationship between MRSA nasal colonization and surgical site infection, wound cultures, hospital length of stay, and mortality, Dr. Papaconstantinou, chief of colorectal surgery at Scott and White Memorial Hospital in Temple, Tex., and his colleagues examined the records of patients who underwent major GI surgery at the hospital between December 2007 and August 2009. The patients, who also had nasal swab tests within 24-48 hours after admission, were divided into one of three categories: MRSA-swab positive, methicillin-sensitive Staphylococcus aureus (MSSA)–swab positive, or negative for both.
Of the 1,137 patients, mean age 59.5 years, 73 (6.4%) were MRSA-swab positive, 167 (14.7%) were MSSA-swab positive, and 897 (78.9%) were negative, Dr. Papaconstantinou reported. Surgical site infection was identified in 101 patients (8.9%), and the rate of infection was highest, at 14%, in the MRSA-swab positive patients, compared with 4% and 9%, respectively, in the MSSA-swab positive and negative patients, he said. "When we controlled for other confounding factors, we didn’t find nasal swab to be an independent predictor of surgical site infection, but what we did find was a strong relationship between MRSA-positive nasal colonization and type of organism involved [in the surgical site infection]."
Regarding mean hospital length of stay, the respective durations for the MRSA-swab positive, MSSA-swab positive, and the negative groups overall were 12.5 days, 7.6 days, and 8.8 days, representing a significant increase, said Dr. Papaconstantinou. "By multiregression analysis, we found a MRSA-positive swab to be an independent risk factor for extended length of stay." However, when looking specifically at patients with surgical site infections, the presence of which increased hospital length of stay significantly from 6.2 days to 15.7 days, "there was no between group differences based on nasal swab," he said. Similarly, the 45 deaths in the study population wee distributed evenly across the nasal swab groups.
Based on the findings, Dr. Papaconstantinou said in an interview, "we can conclude that a positive MRSA nasal swab test for colonization is a strong predictor that MRSA-associated surgical site infections will occur in patients undergoing major GI surgery." As such, "we propose the possibility that it might be beneficial to preoperatively screen and decolonize these patients in an effort to reduce the incidence of these infections and improve patient outcomes following surgery." Toward this end, he and his colleagues are anticipating performing such a study and plan on including a cost-benefit analysis to determine whether screening is economically beneficial, he said.
Dr. Papaconstantinou disclosed a financial relationship with Covidien.
NEWS FROM DIGESTIVE DISEASE WEEK
Major Finding: The surgical site infection rate following major gastrointestinal surgery was 13.7% among patients with positive nasal swab results for MRSA, compared with 4.2% for patients testing positive for MSSA and 9.4% for patients with negative swabs. In patients with for whom wound culture data was available, the rate of MRSA-positive cultures was significantly higher, at 70%, in the MRSA-swab positive group, compared with 8.5% in noncolonized patients.
Data Source: Data were taken from retrospective analysis of medical records for 1,137 patients who underwent major gastrointestinal surgery and had nasal swab tests at Scott and White Memorial Hospital between December 2007 and August 2009. Wound culture data were available for 92 patients.
Disclosures: Dr. Papaconstantinou disclosed a financial relationship with Covidien.
Colonoscopy's Efficacy Confirmed in Average-Risk Adults
SAN DIEGO – Screening colonoscopy showed its efficacy for preventing incident cases of colorectal cancer in prospectively collected data during follow-up of up to 24 years in about 170,000 average-risk Americans.
The finding adds prospectively collected data from a large database of average-risk Americans to the evidence supporting routine colonoscopy screening for colorectal cancer. In contrast, data from the influential National Polyp Study assessed screening colonoscopy in high-risk patients who first underwent polypectomy (N. Engl. J. Med. 2012;366:687-96), Dr. Paul Lochhead said at the annual Digestive Disease Week.
In general, the new findings support current public health recommendations for screening colonoscopy every 10 years in adults aged 50-75 years, but with a few caveats.
The new results showed that screening colonoscopy can significantly cut the risk for new-onset colorectal cancer by 51%, that the benefit from a single colonoscopy screen extended beyond 7 years, and that colonoscopy worked better than screening sigmoidoscopy. The findings also highlighted that colonoscopy was much better at preventing new distal cancers compared with its efficacy for stopping incident proximal tumors, and that when people had two, three, or more screening colonoscopies over time their risk of incident colorectal cancer fell further than after a single screening, said Dr. Lochhead, a gastroenterologist at the University of Aberdeen (Scotland).
"Although a single negative colonoscopy is associated with risk reduction, continued screening may be associated with greater benefit," undercutting the notion that average-risk middle-aged adults should undergo just a single screening colonoscopy with no follow-up screening if the first proves negative, he said.
In addition, "efforts to improve prevention [resulting from] proximal screening are warranted," he added.
"For distal cancers, we saw benefit beyond 10 years, but for proximal cancers we’re less certain about the duration of benefit. It appears there was a pattern of additional risk reduction with multiple screens regardless of whether the cancers were proximal or distal. That is something to bear in mind before we say that once is enough," he said in an interview.
The study used data from two large, prospective, U.S. observational studies: the Nurses’ Health Study, which began in 1976 and initially included 121,700 U.S. women, and the Health Professionals Follow-Up Study, which began in 1986 and included 51,529 men.
In the Nurses’ Health Study, data became available on screening endoscopy starting in 1984, so the data that Dr. Lochhead and his associates used through 2008 included up to 24 years of follow-up. The Health Professionals Follow-Up Study started tracking screening endoscopy in 1988, which gave as many as 20 years of follow-up data through 2008.
For this analysis, the researchers excluded participants in the two studies who had a lower endoscopy prior to the index procedures included in the two databases, those who had prior polyps or any cancer other than nonmelanoma skin cancer before they had their index endoscopy, and participants with inflammatory bowel disease. They calculated multivariate Cox proportional hazard models on the follow-up data that controlled for age, body mass index, smoking, family history of colorectal cancer, regular aspirin use, physical activity, diet, and multivitamin use.
The database included more than 2 million person-years of follow-up from both studies, and during follow-up 2,198 participants developed new-onset colorectal cancer. The majority of participants, constituting nearly 1.4 million person-years of follow-up, underwent no screening endoscopy during the years studied. About 424,000 person years of follow-up came after screening sigmoidoscopy, nearly 300,000 person years of follow-up came following screening colonoscopy, and over 65,000 person-years of follow-up came after polypectomy.
In the multivariate model, compared with no endoscopy, a negative colonoscopy result cut the rate of colorectal cancer by a statistically significant 51%, while negative sigmoidoscopy and polypectomy each cut the subsequent cancer rates by a statistically significant 37%, Dr. Lochhead reported.
When broken down by cancer site – proximal or distal – a negative colonoscopy was the only procedure to cut the risk for incident proximal cancers significantly, reducing the rate by 26% compared with no endoscopy. For distal cancers, colonoscopy cut the rate by 71%, compared with no screening, while sigmoidoscopy and polypectomy each cut the rate by 53%; all these risk reductions for distal cancers were statistically significant.
In the analysis that assessed the durability of protection, screening colonoscopy cut the risk for new colorectal cancers by a statistically significant 34%, compared with no screening, even when incident cancers were tallied more than 7 years following the index colonoscopy procedure. When cancers were divided by location, however, colonoscopy only provided significant protection for the first 3 years, with a risk reduction of 41% compared with no screening. Beyond that, colonoscopy did not produce a statistically significant reduction in incident cancers compared with no screening.
In contrast, for distal cancers the protective benefit of colonoscopy extended beyond 7 years: Screening colonoscopy provided a significant 42% cancer-rate reduction, compared with no screening, more than 7 years out.
The analysis also showed that the statistically significant protective benefit from polypectomy lasted for just 3 years for all cancer locations, with a 52% protection rate compared with no screening. For distal cancers a significant protective effect lasted for 5 years, but for proximal cancers polypectomy did not provide significant protection, even during the first 3 years after the procedure.
An additional multivariate analysis showed cumulative protection from multiple colonoscopies. A single screening colonoscopy cut the rate of incident cancers, both proximal and distal, by a statistically significant 47%, but two colonoscopies cut the risk by 59% and three or more colonoscopies cut the risk by 64%.
Dr. Lochhead said that he had no disclosures.
SAN DIEGO – Screening colonoscopy showed its efficacy for preventing incident cases of colorectal cancer in prospectively collected data during follow-up of up to 24 years in about 170,000 average-risk Americans.
The finding adds prospectively collected data from a large database of average-risk Americans to the evidence supporting routine colonoscopy screening for colorectal cancer. In contrast, data from the influential National Polyp Study assessed screening colonoscopy in high-risk patients who first underwent polypectomy (N. Engl. J. Med. 2012;366:687-96), Dr. Paul Lochhead said at the annual Digestive Disease Week.
In general, the new findings support current public health recommendations for screening colonoscopy every 10 years in adults aged 50-75 years, but with a few caveats.
The new results showed that screening colonoscopy can significantly cut the risk for new-onset colorectal cancer by 51%, that the benefit from a single colonoscopy screen extended beyond 7 years, and that colonoscopy worked better than screening sigmoidoscopy. The findings also highlighted that colonoscopy was much better at preventing new distal cancers compared with its efficacy for stopping incident proximal tumors, and that when people had two, three, or more screening colonoscopies over time their risk of incident colorectal cancer fell further than after a single screening, said Dr. Lochhead, a gastroenterologist at the University of Aberdeen (Scotland).
"Although a single negative colonoscopy is associated with risk reduction, continued screening may be associated with greater benefit," undercutting the notion that average-risk middle-aged adults should undergo just a single screening colonoscopy with no follow-up screening if the first proves negative, he said.
In addition, "efforts to improve prevention [resulting from] proximal screening are warranted," he added.
"For distal cancers, we saw benefit beyond 10 years, but for proximal cancers we’re less certain about the duration of benefit. It appears there was a pattern of additional risk reduction with multiple screens regardless of whether the cancers were proximal or distal. That is something to bear in mind before we say that once is enough," he said in an interview.
The study used data from two large, prospective, U.S. observational studies: the Nurses’ Health Study, which began in 1976 and initially included 121,700 U.S. women, and the Health Professionals Follow-Up Study, which began in 1986 and included 51,529 men.
In the Nurses’ Health Study, data became available on screening endoscopy starting in 1984, so the data that Dr. Lochhead and his associates used through 2008 included up to 24 years of follow-up. The Health Professionals Follow-Up Study started tracking screening endoscopy in 1988, which gave as many as 20 years of follow-up data through 2008.
For this analysis, the researchers excluded participants in the two studies who had a lower endoscopy prior to the index procedures included in the two databases, those who had prior polyps or any cancer other than nonmelanoma skin cancer before they had their index endoscopy, and participants with inflammatory bowel disease. They calculated multivariate Cox proportional hazard models on the follow-up data that controlled for age, body mass index, smoking, family history of colorectal cancer, regular aspirin use, physical activity, diet, and multivitamin use.
The database included more than 2 million person-years of follow-up from both studies, and during follow-up 2,198 participants developed new-onset colorectal cancer. The majority of participants, constituting nearly 1.4 million person-years of follow-up, underwent no screening endoscopy during the years studied. About 424,000 person years of follow-up came after screening sigmoidoscopy, nearly 300,000 person years of follow-up came following screening colonoscopy, and over 65,000 person-years of follow-up came after polypectomy.
In the multivariate model, compared with no endoscopy, a negative colonoscopy result cut the rate of colorectal cancer by a statistically significant 51%, while negative sigmoidoscopy and polypectomy each cut the subsequent cancer rates by a statistically significant 37%, Dr. Lochhead reported.
When broken down by cancer site – proximal or distal – a negative colonoscopy was the only procedure to cut the risk for incident proximal cancers significantly, reducing the rate by 26% compared with no endoscopy. For distal cancers, colonoscopy cut the rate by 71%, compared with no screening, while sigmoidoscopy and polypectomy each cut the rate by 53%; all these risk reductions for distal cancers were statistically significant.
In the analysis that assessed the durability of protection, screening colonoscopy cut the risk for new colorectal cancers by a statistically significant 34%, compared with no screening, even when incident cancers were tallied more than 7 years following the index colonoscopy procedure. When cancers were divided by location, however, colonoscopy only provided significant protection for the first 3 years, with a risk reduction of 41% compared with no screening. Beyond that, colonoscopy did not produce a statistically significant reduction in incident cancers compared with no screening.
In contrast, for distal cancers the protective benefit of colonoscopy extended beyond 7 years: Screening colonoscopy provided a significant 42% cancer-rate reduction, compared with no screening, more than 7 years out.
The analysis also showed that the statistically significant protective benefit from polypectomy lasted for just 3 years for all cancer locations, with a 52% protection rate compared with no screening. For distal cancers a significant protective effect lasted for 5 years, but for proximal cancers polypectomy did not provide significant protection, even during the first 3 years after the procedure.
An additional multivariate analysis showed cumulative protection from multiple colonoscopies. A single screening colonoscopy cut the rate of incident cancers, both proximal and distal, by a statistically significant 47%, but two colonoscopies cut the risk by 59% and three or more colonoscopies cut the risk by 64%.
Dr. Lochhead said that he had no disclosures.
SAN DIEGO – Screening colonoscopy showed its efficacy for preventing incident cases of colorectal cancer in prospectively collected data during follow-up of up to 24 years in about 170,000 average-risk Americans.
The finding adds prospectively collected data from a large database of average-risk Americans to the evidence supporting routine colonoscopy screening for colorectal cancer. In contrast, data from the influential National Polyp Study assessed screening colonoscopy in high-risk patients who first underwent polypectomy (N. Engl. J. Med. 2012;366:687-96), Dr. Paul Lochhead said at the annual Digestive Disease Week.
In general, the new findings support current public health recommendations for screening colonoscopy every 10 years in adults aged 50-75 years, but with a few caveats.
The new results showed that screening colonoscopy can significantly cut the risk for new-onset colorectal cancer by 51%, that the benefit from a single colonoscopy screen extended beyond 7 years, and that colonoscopy worked better than screening sigmoidoscopy. The findings also highlighted that colonoscopy was much better at preventing new distal cancers compared with its efficacy for stopping incident proximal tumors, and that when people had two, three, or more screening colonoscopies over time their risk of incident colorectal cancer fell further than after a single screening, said Dr. Lochhead, a gastroenterologist at the University of Aberdeen (Scotland).
"Although a single negative colonoscopy is associated with risk reduction, continued screening may be associated with greater benefit," undercutting the notion that average-risk middle-aged adults should undergo just a single screening colonoscopy with no follow-up screening if the first proves negative, he said.
In addition, "efforts to improve prevention [resulting from] proximal screening are warranted," he added.
"For distal cancers, we saw benefit beyond 10 years, but for proximal cancers we’re less certain about the duration of benefit. It appears there was a pattern of additional risk reduction with multiple screens regardless of whether the cancers were proximal or distal. That is something to bear in mind before we say that once is enough," he said in an interview.
The study used data from two large, prospective, U.S. observational studies: the Nurses’ Health Study, which began in 1976 and initially included 121,700 U.S. women, and the Health Professionals Follow-Up Study, which began in 1986 and included 51,529 men.
In the Nurses’ Health Study, data became available on screening endoscopy starting in 1984, so the data that Dr. Lochhead and his associates used through 2008 included up to 24 years of follow-up. The Health Professionals Follow-Up Study started tracking screening endoscopy in 1988, which gave as many as 20 years of follow-up data through 2008.
For this analysis, the researchers excluded participants in the two studies who had a lower endoscopy prior to the index procedures included in the two databases, those who had prior polyps or any cancer other than nonmelanoma skin cancer before they had their index endoscopy, and participants with inflammatory bowel disease. They calculated multivariate Cox proportional hazard models on the follow-up data that controlled for age, body mass index, smoking, family history of colorectal cancer, regular aspirin use, physical activity, diet, and multivitamin use.
The database included more than 2 million person-years of follow-up from both studies, and during follow-up 2,198 participants developed new-onset colorectal cancer. The majority of participants, constituting nearly 1.4 million person-years of follow-up, underwent no screening endoscopy during the years studied. About 424,000 person years of follow-up came after screening sigmoidoscopy, nearly 300,000 person years of follow-up came following screening colonoscopy, and over 65,000 person-years of follow-up came after polypectomy.
In the multivariate model, compared with no endoscopy, a negative colonoscopy result cut the rate of colorectal cancer by a statistically significant 51%, while negative sigmoidoscopy and polypectomy each cut the subsequent cancer rates by a statistically significant 37%, Dr. Lochhead reported.
When broken down by cancer site – proximal or distal – a negative colonoscopy was the only procedure to cut the risk for incident proximal cancers significantly, reducing the rate by 26% compared with no endoscopy. For distal cancers, colonoscopy cut the rate by 71%, compared with no screening, while sigmoidoscopy and polypectomy each cut the rate by 53%; all these risk reductions for distal cancers were statistically significant.
In the analysis that assessed the durability of protection, screening colonoscopy cut the risk for new colorectal cancers by a statistically significant 34%, compared with no screening, even when incident cancers were tallied more than 7 years following the index colonoscopy procedure. When cancers were divided by location, however, colonoscopy only provided significant protection for the first 3 years, with a risk reduction of 41% compared with no screening. Beyond that, colonoscopy did not produce a statistically significant reduction in incident cancers compared with no screening.
In contrast, for distal cancers the protective benefit of colonoscopy extended beyond 7 years: Screening colonoscopy provided a significant 42% cancer-rate reduction, compared with no screening, more than 7 years out.
The analysis also showed that the statistically significant protective benefit from polypectomy lasted for just 3 years for all cancer locations, with a 52% protection rate compared with no screening. For distal cancers a significant protective effect lasted for 5 years, but for proximal cancers polypectomy did not provide significant protection, even during the first 3 years after the procedure.
An additional multivariate analysis showed cumulative protection from multiple colonoscopies. A single screening colonoscopy cut the rate of incident cancers, both proximal and distal, by a statistically significant 47%, but two colonoscopies cut the risk by 59% and three or more colonoscopies cut the risk by 64%.
Dr. Lochhead said that he had no disclosures.
FROM THE ANNUAL DIGESTIVE DISEASE WEEK
Major Finding: Screening colonoscopy cut incident colorectal cancers by 51% compared with no endoscopy in a large, average-risk U.S. cohort.
Data Source: The review included prospectively collected data from about 170,000 average-risk U.S. women and men followed for up to 24 years after an index cancer-screening endoscopy.
Disclosures: Dr. Lochhead said that he had no disclosures.
Lymphadenectomy Underused in GI Cancer Surgery
SAN DIEGO – Lymph node removal during gastrointestinal cancer surgery remains underperformed in a large proportion of patients in the United States, although the median number of resected nodes increased from 1998 to 2009.
Those are the key findings of a 10-year analysis of medical records from the National Cancer Institute’s Surveillance Epidemiology and End Results (SEER) database.
Several reports in the literature show a correlation between long-term survival and the removal of possibly metastatic lymph nodes along with the cancerous organ during surgery, Dr. Attila Dubecz explained in an interview at the annual Digestive Disease Week. There are also survival differences based on sex, race or poverty status, and differences in lymph node removal between these groups in certain cancer types, he said. "We wanted to determine if these differences are more related to cancer types therefore the type of operation, for example or to these underprivileged groups."
Using SEER data from 1998 to 2009, Dr. Dubecz of Klinikum Nürnberg (Germany) and his colleagues identified 326,243 patients with a surgically treated GI malignancy. This included 13,165 malignancies in the esophagus, 18,588 in the stomach, 7,666 in the small bowel, 232,345 in the colon, 42,338 in the rectum, and 12,141 in the pancreas.
Adequate lymphadenectomy was defined as removal of at least 15 lymph nodes for cancer of the esophagus and the stomach; at least 12 for cancer of the small bowel, colon, and rectum; and at least 15 for cancer of the pancreas. The researchers evaluated the median number of lymph nodes removed and the prevalence of adequate and/or no lymphadenectomy for each cancer type over the 10-year period. They used multivariate logistic regression analysis to identify factors predicting adequate lymphadenectomy.
Dr. Dubecz, a surgeon, reported that the median number of excised nodes improved over the 10-year period in all types of cancer: from 7 to 13 in esophageal cancer, 8 to 12 in stomach cancer, 2 to 7 in small bowel cancer, 9 to 16 in colon cancer, 8 to 13 in rectal cancer, and 7 to 13 in pancreatic cancer.
In addition, the percentage of patients with an adequate lymphadenectomy (a median of 49% for all types) steadily increased and those with zero nodes removed (a median of 6% for all types) steadily decreased in all types of cancer, "although both remained far from ideal," the researchers wrote.
By 2009, the percentage of patients with adequate lymphadenectomy was 43% for esophageal cancer, 42% for stomach cancer, 35% for small bowel cancer, 77% for colon cancer, 61% for rectal cancer and 42% for pancreatic cancer. Men, patients older than age 65, or those undergoing surgical therapy earlier in the study period and living in areas with high poverty rates were significantly less likely to receive adequate lymphadenectomy (P less than .0001 for all groups).
"The main surprise was that race was an insignificant factor, and gender, age, and socioeconomic differences between the groups with adequate versus inadequate lymph node dissection were also much less [than] between the groups of different cancer types," Dr. Dubecz said at the annual meeting of the Digestive Disease Week.
Dr. Dubecz acknowledged certain limitations of the study, including the potential for misclassification of patient information in the SEER database. "Furthermore, despite being advocated by several practice organizations and consensus panels, the definitions of adequate lymphadenectomy used in this study are not universally accepted," he noted. "Third, our analyses are limited to the available variables in the SEER database with no information regarding patient insurance status, comorbidities, body mass index, or [neo]adjuvant chemotherapy, which could influence lymph node dissection and the disparities."
Dr. Dubecz said he had no relevant financial disclosures.
SAN DIEGO – Lymph node removal during gastrointestinal cancer surgery remains underperformed in a large proportion of patients in the United States, although the median number of resected nodes increased from 1998 to 2009.
Those are the key findings of a 10-year analysis of medical records from the National Cancer Institute’s Surveillance Epidemiology and End Results (SEER) database.
Several reports in the literature show a correlation between long-term survival and the removal of possibly metastatic lymph nodes along with the cancerous organ during surgery, Dr. Attila Dubecz explained in an interview at the annual Digestive Disease Week. There are also survival differences based on sex, race or poverty status, and differences in lymph node removal between these groups in certain cancer types, he said. "We wanted to determine if these differences are more related to cancer types therefore the type of operation, for example or to these underprivileged groups."
Using SEER data from 1998 to 2009, Dr. Dubecz of Klinikum Nürnberg (Germany) and his colleagues identified 326,243 patients with a surgically treated GI malignancy. This included 13,165 malignancies in the esophagus, 18,588 in the stomach, 7,666 in the small bowel, 232,345 in the colon, 42,338 in the rectum, and 12,141 in the pancreas.
Adequate lymphadenectomy was defined as removal of at least 15 lymph nodes for cancer of the esophagus and the stomach; at least 12 for cancer of the small bowel, colon, and rectum; and at least 15 for cancer of the pancreas. The researchers evaluated the median number of lymph nodes removed and the prevalence of adequate and/or no lymphadenectomy for each cancer type over the 10-year period. They used multivariate logistic regression analysis to identify factors predicting adequate lymphadenectomy.
Dr. Dubecz, a surgeon, reported that the median number of excised nodes improved over the 10-year period in all types of cancer: from 7 to 13 in esophageal cancer, 8 to 12 in stomach cancer, 2 to 7 in small bowel cancer, 9 to 16 in colon cancer, 8 to 13 in rectal cancer, and 7 to 13 in pancreatic cancer.
In addition, the percentage of patients with an adequate lymphadenectomy (a median of 49% for all types) steadily increased and those with zero nodes removed (a median of 6% for all types) steadily decreased in all types of cancer, "although both remained far from ideal," the researchers wrote.
By 2009, the percentage of patients with adequate lymphadenectomy was 43% for esophageal cancer, 42% for stomach cancer, 35% for small bowel cancer, 77% for colon cancer, 61% for rectal cancer and 42% for pancreatic cancer. Men, patients older than age 65, or those undergoing surgical therapy earlier in the study period and living in areas with high poverty rates were significantly less likely to receive adequate lymphadenectomy (P less than .0001 for all groups).
"The main surprise was that race was an insignificant factor, and gender, age, and socioeconomic differences between the groups with adequate versus inadequate lymph node dissection were also much less [than] between the groups of different cancer types," Dr. Dubecz said at the annual meeting of the Digestive Disease Week.
Dr. Dubecz acknowledged certain limitations of the study, including the potential for misclassification of patient information in the SEER database. "Furthermore, despite being advocated by several practice organizations and consensus panels, the definitions of adequate lymphadenectomy used in this study are not universally accepted," he noted. "Third, our analyses are limited to the available variables in the SEER database with no information regarding patient insurance status, comorbidities, body mass index, or [neo]adjuvant chemotherapy, which could influence lymph node dissection and the disparities."
Dr. Dubecz said he had no relevant financial disclosures.
SAN DIEGO – Lymph node removal during gastrointestinal cancer surgery remains underperformed in a large proportion of patients in the United States, although the median number of resected nodes increased from 1998 to 2009.
Those are the key findings of a 10-year analysis of medical records from the National Cancer Institute’s Surveillance Epidemiology and End Results (SEER) database.
Several reports in the literature show a correlation between long-term survival and the removal of possibly metastatic lymph nodes along with the cancerous organ during surgery, Dr. Attila Dubecz explained in an interview at the annual Digestive Disease Week. There are also survival differences based on sex, race or poverty status, and differences in lymph node removal between these groups in certain cancer types, he said. "We wanted to determine if these differences are more related to cancer types therefore the type of operation, for example or to these underprivileged groups."
Using SEER data from 1998 to 2009, Dr. Dubecz of Klinikum Nürnberg (Germany) and his colleagues identified 326,243 patients with a surgically treated GI malignancy. This included 13,165 malignancies in the esophagus, 18,588 in the stomach, 7,666 in the small bowel, 232,345 in the colon, 42,338 in the rectum, and 12,141 in the pancreas.
Adequate lymphadenectomy was defined as removal of at least 15 lymph nodes for cancer of the esophagus and the stomach; at least 12 for cancer of the small bowel, colon, and rectum; and at least 15 for cancer of the pancreas. The researchers evaluated the median number of lymph nodes removed and the prevalence of adequate and/or no lymphadenectomy for each cancer type over the 10-year period. They used multivariate logistic regression analysis to identify factors predicting adequate lymphadenectomy.
Dr. Dubecz, a surgeon, reported that the median number of excised nodes improved over the 10-year period in all types of cancer: from 7 to 13 in esophageal cancer, 8 to 12 in stomach cancer, 2 to 7 in small bowel cancer, 9 to 16 in colon cancer, 8 to 13 in rectal cancer, and 7 to 13 in pancreatic cancer.
In addition, the percentage of patients with an adequate lymphadenectomy (a median of 49% for all types) steadily increased and those with zero nodes removed (a median of 6% for all types) steadily decreased in all types of cancer, "although both remained far from ideal," the researchers wrote.
By 2009, the percentage of patients with adequate lymphadenectomy was 43% for esophageal cancer, 42% for stomach cancer, 35% for small bowel cancer, 77% for colon cancer, 61% for rectal cancer and 42% for pancreatic cancer. Men, patients older than age 65, or those undergoing surgical therapy earlier in the study period and living in areas with high poverty rates were significantly less likely to receive adequate lymphadenectomy (P less than .0001 for all groups).
"The main surprise was that race was an insignificant factor, and gender, age, and socioeconomic differences between the groups with adequate versus inadequate lymph node dissection were also much less [than] between the groups of different cancer types," Dr. Dubecz said at the annual meeting of the Digestive Disease Week.
Dr. Dubecz acknowledged certain limitations of the study, including the potential for misclassification of patient information in the SEER database. "Furthermore, despite being advocated by several practice organizations and consensus panels, the definitions of adequate lymphadenectomy used in this study are not universally accepted," he noted. "Third, our analyses are limited to the available variables in the SEER database with no information regarding patient insurance status, comorbidities, body mass index, or [neo]adjuvant chemotherapy, which could influence lymph node dissection and the disparities."
Dr. Dubecz said he had no relevant financial disclosures.
FROM THE ANNUAL DIGESTIVE DISEASE WEEK
Major Finding: By 2009, the percentage of patients with adequate lymphadenectomy during surgery for gastrointestinal cancer was 43% for esophageal cancer, 42% for stomach cancer, 35% for small bowel cancer, 77% for colon cancer, 61% for rectal cancer, and 42% for pancreatic cancer.
Data Source: Findings are based on a 10-year analysis of medical records from 326,243 patients in the National Cancer Institute’s Surveillance Epidemiology and End Results (SEER) database.
Disclosures: Dr. Dubecz said he had no relevant financial disclosures.
Texas Has More Doctors Since Tort Reform
SAN DIEGO – Since the state of Texas implemented medical tort reform in 2003, the number of practicing physicians has increased by 19% per 100,000 population. In addition, hospitals in the state report improved ability to recruit physicians and expand patient services.
Those are key findings from an innovative study presented by Dr. Ronald M. Stewart at the annual Digestive Disease Week.
"Tort reform has been beneficial for all or almost all Texas physicians," Dr. Stewart said in an interview in advance of the meeting. "I have benefited from lower malpractice premiums and a more favorable liability climate in the state. However, the effect of tort reform in a region is probably not the primary driver for physician recruitment and retention. I believe it is permissive – providing the framework for growing physicians relative to the number of patients being served," he explained.
The data show that, in the decade from 2002 to 2012, the Texas population went from 21,779,893 to 26,403,743 – a 21% increase – and the number of Texas physicians rose by 15,611 – a 44% increase (46% in metro areas vs. 9% in nonmetro areas). This absolute change led to an increase of 30 physicians per 100,000 population (19% increase; P less than 0.01).
Nonmetropolitan Texas had a net increase of 115 physicians, but there was no change in the number of physicians per 100,000 in these areas.
Looking at trauma service areas (TSAs), the researchers found that 20 of 22 TSAs had an increase in both number of physicians and physicians per capita. Five had increases of greater than 50%. The increases were generally greater in the central Texas TSAs and TSAs with a larger population size.
"The central part of the state along the I-35 corridor where there are academic medical centers grew significantly," commented Dr. Stewart, professor and chairman of the department of surgery at the University of Texas Health Science Center, San Antonio. "In rural Texas, the absolute number of doctors increased, but per capita it stayed about the same."
For the study, Dr. Stewart and his associates used data from the Texas Medical Board, the U.S. Census Bureau/Texas State Library and Archives Commission, and the Texas Department of State Health Services to compare the rate of physician growth prior to and following tort reform, and the number of licensed physicians per 100,000 population from January 2002 to January 2012.
The information about hospitals was extracted from a 2008 survey by the Texas Hospital Association, which asked hospital and health system administrators from 10 health care systems and 10 independent hospitals to measure the impact of the state medical liability tort reform, which became effective on Sept. 1, 2003. The law includes a $250,000 cap on noneconomic damages in most medical malpractice cases.
The survey of hospital administrators asked how they used the funds saved from their reduced liability coverage costs, and 58% of respondents reported using the funds to expand patient safety programs, 51% used the funds to maintain/expand coverage or services for uninsured/underinsured patients, and 46% used the funds to subsidize various payment shortfalls such as Medicaid.
"Tort reform, as implemented in Texas, was associated with an increase in practicing physicians with resultant improved access to care," Dr. Stewart concluded. "We know that tort reform is beneficial to the physician and hospital, but these data support the notion that it really is better for the patient, too."
When asked to comment on the translatability of the study findings to other states, Dr. Stewart said that comprehensive tort reform "makes an area more attractive to physicians. This is speculation on my part, but I would guess that if you implemented tort reform in other states, it gives those states some degree of an advantage in retaining and recruiting physicians."
He went on to point out that improving access to medical care for patients "is not just about doctors per capita or numbers of doctors; it’s also about what doctors do with their practice. One of the reasons Texas implemented tort reform wasn’t just that we were not keeping pace with our population growth; it was also because physicians were restricting their practice to what they viewed as lower-risk malpractice areas. Our study provides evidence that tort reform has also led to improvements with respect to physicians expanding their care to perceived high-risk areas."
Dr. Stewart said that he had no relevant financial disclosures.
*The headline was updated on May 22, 2012.
SAN DIEGO – Since the state of Texas implemented medical tort reform in 2003, the number of practicing physicians has increased by 19% per 100,000 population. In addition, hospitals in the state report improved ability to recruit physicians and expand patient services.
Those are key findings from an innovative study presented by Dr. Ronald M. Stewart at the annual Digestive Disease Week.
"Tort reform has been beneficial for all or almost all Texas physicians," Dr. Stewart said in an interview in advance of the meeting. "I have benefited from lower malpractice premiums and a more favorable liability climate in the state. However, the effect of tort reform in a region is probably not the primary driver for physician recruitment and retention. I believe it is permissive – providing the framework for growing physicians relative to the number of patients being served," he explained.
The data show that, in the decade from 2002 to 2012, the Texas population went from 21,779,893 to 26,403,743 – a 21% increase – and the number of Texas physicians rose by 15,611 – a 44% increase (46% in metro areas vs. 9% in nonmetro areas). This absolute change led to an increase of 30 physicians per 100,000 population (19% increase; P less than 0.01).
Nonmetropolitan Texas had a net increase of 115 physicians, but there was no change in the number of physicians per 100,000 in these areas.
Looking at trauma service areas (TSAs), the researchers found that 20 of 22 TSAs had an increase in both number of physicians and physicians per capita. Five had increases of greater than 50%. The increases were generally greater in the central Texas TSAs and TSAs with a larger population size.
"The central part of the state along the I-35 corridor where there are academic medical centers grew significantly," commented Dr. Stewart, professor and chairman of the department of surgery at the University of Texas Health Science Center, San Antonio. "In rural Texas, the absolute number of doctors increased, but per capita it stayed about the same."
For the study, Dr. Stewart and his associates used data from the Texas Medical Board, the U.S. Census Bureau/Texas State Library and Archives Commission, and the Texas Department of State Health Services to compare the rate of physician growth prior to and following tort reform, and the number of licensed physicians per 100,000 population from January 2002 to January 2012.
The information about hospitals was extracted from a 2008 survey by the Texas Hospital Association, which asked hospital and health system administrators from 10 health care systems and 10 independent hospitals to measure the impact of the state medical liability tort reform, which became effective on Sept. 1, 2003. The law includes a $250,000 cap on noneconomic damages in most medical malpractice cases.
The survey of hospital administrators asked how they used the funds saved from their reduced liability coverage costs, and 58% of respondents reported using the funds to expand patient safety programs, 51% used the funds to maintain/expand coverage or services for uninsured/underinsured patients, and 46% used the funds to subsidize various payment shortfalls such as Medicaid.
"Tort reform, as implemented in Texas, was associated with an increase in practicing physicians with resultant improved access to care," Dr. Stewart concluded. "We know that tort reform is beneficial to the physician and hospital, but these data support the notion that it really is better for the patient, too."
When asked to comment on the translatability of the study findings to other states, Dr. Stewart said that comprehensive tort reform "makes an area more attractive to physicians. This is speculation on my part, but I would guess that if you implemented tort reform in other states, it gives those states some degree of an advantage in retaining and recruiting physicians."
He went on to point out that improving access to medical care for patients "is not just about doctors per capita or numbers of doctors; it’s also about what doctors do with their practice. One of the reasons Texas implemented tort reform wasn’t just that we were not keeping pace with our population growth; it was also because physicians were restricting their practice to what they viewed as lower-risk malpractice areas. Our study provides evidence that tort reform has also led to improvements with respect to physicians expanding their care to perceived high-risk areas."
Dr. Stewart said that he had no relevant financial disclosures.
*The headline was updated on May 22, 2012.
SAN DIEGO – Since the state of Texas implemented medical tort reform in 2003, the number of practicing physicians has increased by 19% per 100,000 population. In addition, hospitals in the state report improved ability to recruit physicians and expand patient services.
Those are key findings from an innovative study presented by Dr. Ronald M. Stewart at the annual Digestive Disease Week.
"Tort reform has been beneficial for all or almost all Texas physicians," Dr. Stewart said in an interview in advance of the meeting. "I have benefited from lower malpractice premiums and a more favorable liability climate in the state. However, the effect of tort reform in a region is probably not the primary driver for physician recruitment and retention. I believe it is permissive – providing the framework for growing physicians relative to the number of patients being served," he explained.
The data show that, in the decade from 2002 to 2012, the Texas population went from 21,779,893 to 26,403,743 – a 21% increase – and the number of Texas physicians rose by 15,611 – a 44% increase (46% in metro areas vs. 9% in nonmetro areas). This absolute change led to an increase of 30 physicians per 100,000 population (19% increase; P less than 0.01).
Nonmetropolitan Texas had a net increase of 115 physicians, but there was no change in the number of physicians per 100,000 in these areas.
Looking at trauma service areas (TSAs), the researchers found that 20 of 22 TSAs had an increase in both number of physicians and physicians per capita. Five had increases of greater than 50%. The increases were generally greater in the central Texas TSAs and TSAs with a larger population size.
"The central part of the state along the I-35 corridor where there are academic medical centers grew significantly," commented Dr. Stewart, professor and chairman of the department of surgery at the University of Texas Health Science Center, San Antonio. "In rural Texas, the absolute number of doctors increased, but per capita it stayed about the same."
For the study, Dr. Stewart and his associates used data from the Texas Medical Board, the U.S. Census Bureau/Texas State Library and Archives Commission, and the Texas Department of State Health Services to compare the rate of physician growth prior to and following tort reform, and the number of licensed physicians per 100,000 population from January 2002 to January 2012.
The information about hospitals was extracted from a 2008 survey by the Texas Hospital Association, which asked hospital and health system administrators from 10 health care systems and 10 independent hospitals to measure the impact of the state medical liability tort reform, which became effective on Sept. 1, 2003. The law includes a $250,000 cap on noneconomic damages in most medical malpractice cases.
The survey of hospital administrators asked how they used the funds saved from their reduced liability coverage costs, and 58% of respondents reported using the funds to expand patient safety programs, 51% used the funds to maintain/expand coverage or services for uninsured/underinsured patients, and 46% used the funds to subsidize various payment shortfalls such as Medicaid.
"Tort reform, as implemented in Texas, was associated with an increase in practicing physicians with resultant improved access to care," Dr. Stewart concluded. "We know that tort reform is beneficial to the physician and hospital, but these data support the notion that it really is better for the patient, too."
When asked to comment on the translatability of the study findings to other states, Dr. Stewart said that comprehensive tort reform "makes an area more attractive to physicians. This is speculation on my part, but I would guess that if you implemented tort reform in other states, it gives those states some degree of an advantage in retaining and recruiting physicians."
He went on to point out that improving access to medical care for patients "is not just about doctors per capita or numbers of doctors; it’s also about what doctors do with their practice. One of the reasons Texas implemented tort reform wasn’t just that we were not keeping pace with our population growth; it was also because physicians were restricting their practice to what they viewed as lower-risk malpractice areas. Our study provides evidence that tort reform has also led to improvements with respect to physicians expanding their care to perceived high-risk areas."
Dr. Stewart said that he had no relevant financial disclosures.
*The headline was updated on May 22, 2012.
FROM THE ANNUAL DIGESTIVE DISEASE WEEK
Major Finding: Since medical tort reform became effective in Texas on Sept. 1, 2003, the number of physicians has grown by 30 per 100,000 population, an increase of 19% (P less than 0.01).
Data Source: Analysis of data from the Texas Medical Board, the United States Census Bureau/Texas State Library and Archives Commission, and the Texas Department of State Health Services to compare the rate of physician growth prior to and following tort reform, and the number of licensed physicians per 100,000 population from January 2002 to January 2012.
Disclosures: Dr. Stewart said that he had no relevant financial conflicts to disclose.
Rise in Adolescent NAFLD Outpacing Obesity
Obese children seem to have gotten fatter around the middle over time, and that development may account in part for an observed increase in suspected nonalcoholic fatty liver disease among adolescents.
Nonalcoholic fatty liver disease (NAFLD) in adolescents has nearly tripled from 1998 to 2008, Dr. Miriam Vos said during a teleconference reporting the results of an observational study that she will present on Monday, May 21, at Digestive Disease Week 2012. In a review of nationally representative data from the National Health and Nutrition Examination Survey (NHANES), the tripling of NAFLD cases outpaced a near doubling of adolescent obesity during the same period.
Thus, "our findings suggest that obesity alone does not explain the growing prevalence of the liver disease," she said.
The most common cause of chronic pediatric liver disease, NAFLD has been associated with hypertension, type 2 diabetes, metabolic abnormalities, liver damage, and cancer. Anecdotal data have previously suggested a risk in NAFLD that was linked to obesity in children, but "this finding has not been confirmed in previous studies," said Dr. Vos of Children’s Healthcare of Atlanta. "We wanted to know whether the rates seem high because clinicians are looking more closely [for NAFLD] or because there really are more cases."
The researchers examined the NHANES data sets from 1988 to 2008, which account for 10,359 12- to 18-year-olds after those with incomplete information or known liver disease were excluded.
More conservative cutoff parameters for suspected NAFLD were implemented during the period of the study, so the researchers conducted their analyses using both cutoffs to allow for comparisons with earlier studies, Dr. Vos explained. "Based on the earlier cut-off, [NAFLD] was suspected in adolescents with a BMI in the 85th percentile or higher, and elevated [ALT] levels (defined as greater than 30)," she said. The newly recommended ALT cutoffs are sex-specific; NAFLD is suspected in adolescents in the same BMI range, but at ALT levels greater than 25.8 for boys and 22.1 for girls (Gastroenterology 2010;138:1357-64).
When the sex-specific cutoffs were used, NAFLD rates "increased among all adolescents, from 3.6% to 9.9%," she said.
Dr. Vos said that age, sex, race, and percentage of overweight adolescents did not differ from 1988 to 2008; however, the percentage of overweight adolescents who were obese increased significantly (from 11.2% to 20.6%).
Among overweight adolescents, the prevalence of elevated ALT levels was 13.2% in 2007-2008, which did not represent a significant linear increase over time. Among obese adolescents, however, elevated ALT levels rose from 16.7% to 36.9% from 1988 to 2008. Similar increases were observed in this group when the previous ALT cutoff of 30 was used, as well.
The findings may be limited somewhat by the study’s inclusion criteria, according to Dr. Vos. "It’s tricky to identify NAFLD using population data like this, so we set our definition to look at overweight children who also have elevated serum ALT. By choosing to look only at the overweight children, we might have missed some cases."
Even so, the findings are important from a public health standpoint. "We need to know this kind of information to plan programs that tackle the prevention and treatment of NAFLD, and it also helps us look for clues about why so many children are getting fatty liver disease," Dr. Vos said.
"We need to look beyond just the increase in obesity among children." For example, a further analysis of the cross-sectional data found a parallel increase between NAFLD prevalence and waist circumference. "While the cross-sectional design of our study can’t point to causation, we can hypothesize that the increase in NAFLD may be linked to an increase in visceral adiposity or centrally located fat in kids today," she said, noting that what might be causing such increases is fodder for additional investigation.
Dr. Vos has received financial support in the form of a career award from the National Institute of Diabetes and Digestive and Kidney Diseases, and from the Children’s Digestive Health and Nutrition Foundation. She is the author of "The No-Diet Obesity Solution for Kids" (Bethesda, Md.: AGA Institute Press, 2009), for which she receives royalties.
Obese children seem to have gotten fatter around the middle over time, and that development may account in part for an observed increase in suspected nonalcoholic fatty liver disease among adolescents.
Nonalcoholic fatty liver disease (NAFLD) in adolescents has nearly tripled from 1998 to 2008, Dr. Miriam Vos said during a teleconference reporting the results of an observational study that she will present on Monday, May 21, at Digestive Disease Week 2012. In a review of nationally representative data from the National Health and Nutrition Examination Survey (NHANES), the tripling of NAFLD cases outpaced a near doubling of adolescent obesity during the same period.
Thus, "our findings suggest that obesity alone does not explain the growing prevalence of the liver disease," she said.
The most common cause of chronic pediatric liver disease, NAFLD has been associated with hypertension, type 2 diabetes, metabolic abnormalities, liver damage, and cancer. Anecdotal data have previously suggested a risk in NAFLD that was linked to obesity in children, but "this finding has not been confirmed in previous studies," said Dr. Vos of Children’s Healthcare of Atlanta. "We wanted to know whether the rates seem high because clinicians are looking more closely [for NAFLD] or because there really are more cases."
The researchers examined the NHANES data sets from 1988 to 2008, which account for 10,359 12- to 18-year-olds after those with incomplete information or known liver disease were excluded.
More conservative cutoff parameters for suspected NAFLD were implemented during the period of the study, so the researchers conducted their analyses using both cutoffs to allow for comparisons with earlier studies, Dr. Vos explained. "Based on the earlier cut-off, [NAFLD] was suspected in adolescents with a BMI in the 85th percentile or higher, and elevated [ALT] levels (defined as greater than 30)," she said. The newly recommended ALT cutoffs are sex-specific; NAFLD is suspected in adolescents in the same BMI range, but at ALT levels greater than 25.8 for boys and 22.1 for girls (Gastroenterology 2010;138:1357-64).
When the sex-specific cutoffs were used, NAFLD rates "increased among all adolescents, from 3.6% to 9.9%," she said.
Dr. Vos said that age, sex, race, and percentage of overweight adolescents did not differ from 1988 to 2008; however, the percentage of overweight adolescents who were obese increased significantly (from 11.2% to 20.6%).
Among overweight adolescents, the prevalence of elevated ALT levels was 13.2% in 2007-2008, which did not represent a significant linear increase over time. Among obese adolescents, however, elevated ALT levels rose from 16.7% to 36.9% from 1988 to 2008. Similar increases were observed in this group when the previous ALT cutoff of 30 was used, as well.
The findings may be limited somewhat by the study’s inclusion criteria, according to Dr. Vos. "It’s tricky to identify NAFLD using population data like this, so we set our definition to look at overweight children who also have elevated serum ALT. By choosing to look only at the overweight children, we might have missed some cases."
Even so, the findings are important from a public health standpoint. "We need to know this kind of information to plan programs that tackle the prevention and treatment of NAFLD, and it also helps us look for clues about why so many children are getting fatty liver disease," Dr. Vos said.
"We need to look beyond just the increase in obesity among children." For example, a further analysis of the cross-sectional data found a parallel increase between NAFLD prevalence and waist circumference. "While the cross-sectional design of our study can’t point to causation, we can hypothesize that the increase in NAFLD may be linked to an increase in visceral adiposity or centrally located fat in kids today," she said, noting that what might be causing such increases is fodder for additional investigation.
Dr. Vos has received financial support in the form of a career award from the National Institute of Diabetes and Digestive and Kidney Diseases, and from the Children’s Digestive Health and Nutrition Foundation. She is the author of "The No-Diet Obesity Solution for Kids" (Bethesda, Md.: AGA Institute Press, 2009), for which she receives royalties.
Obese children seem to have gotten fatter around the middle over time, and that development may account in part for an observed increase in suspected nonalcoholic fatty liver disease among adolescents.
Nonalcoholic fatty liver disease (NAFLD) in adolescents has nearly tripled from 1998 to 2008, Dr. Miriam Vos said during a teleconference reporting the results of an observational study that she will present on Monday, May 21, at Digestive Disease Week 2012. In a review of nationally representative data from the National Health and Nutrition Examination Survey (NHANES), the tripling of NAFLD cases outpaced a near doubling of adolescent obesity during the same period.
Thus, "our findings suggest that obesity alone does not explain the growing prevalence of the liver disease," she said.
The most common cause of chronic pediatric liver disease, NAFLD has been associated with hypertension, type 2 diabetes, metabolic abnormalities, liver damage, and cancer. Anecdotal data have previously suggested a risk in NAFLD that was linked to obesity in children, but "this finding has not been confirmed in previous studies," said Dr. Vos of Children’s Healthcare of Atlanta. "We wanted to know whether the rates seem high because clinicians are looking more closely [for NAFLD] or because there really are more cases."
The researchers examined the NHANES data sets from 1988 to 2008, which account for 10,359 12- to 18-year-olds after those with incomplete information or known liver disease were excluded.
More conservative cutoff parameters for suspected NAFLD were implemented during the period of the study, so the researchers conducted their analyses using both cutoffs to allow for comparisons with earlier studies, Dr. Vos explained. "Based on the earlier cut-off, [NAFLD] was suspected in adolescents with a BMI in the 85th percentile or higher, and elevated [ALT] levels (defined as greater than 30)," she said. The newly recommended ALT cutoffs are sex-specific; NAFLD is suspected in adolescents in the same BMI range, but at ALT levels greater than 25.8 for boys and 22.1 for girls (Gastroenterology 2010;138:1357-64).
When the sex-specific cutoffs were used, NAFLD rates "increased among all adolescents, from 3.6% to 9.9%," she said.
Dr. Vos said that age, sex, race, and percentage of overweight adolescents did not differ from 1988 to 2008; however, the percentage of overweight adolescents who were obese increased significantly (from 11.2% to 20.6%).
Among overweight adolescents, the prevalence of elevated ALT levels was 13.2% in 2007-2008, which did not represent a significant linear increase over time. Among obese adolescents, however, elevated ALT levels rose from 16.7% to 36.9% from 1988 to 2008. Similar increases were observed in this group when the previous ALT cutoff of 30 was used, as well.
The findings may be limited somewhat by the study’s inclusion criteria, according to Dr. Vos. "It’s tricky to identify NAFLD using population data like this, so we set our definition to look at overweight children who also have elevated serum ALT. By choosing to look only at the overweight children, we might have missed some cases."
Even so, the findings are important from a public health standpoint. "We need to know this kind of information to plan programs that tackle the prevention and treatment of NAFLD, and it also helps us look for clues about why so many children are getting fatty liver disease," Dr. Vos said.
"We need to look beyond just the increase in obesity among children." For example, a further analysis of the cross-sectional data found a parallel increase between NAFLD prevalence and waist circumference. "While the cross-sectional design of our study can’t point to causation, we can hypothesize that the increase in NAFLD may be linked to an increase in visceral adiposity or centrally located fat in kids today," she said, noting that what might be causing such increases is fodder for additional investigation.
Dr. Vos has received financial support in the form of a career award from the National Institute of Diabetes and Digestive and Kidney Diseases, and from the Children’s Digestive Health and Nutrition Foundation. She is the author of "The No-Diet Obesity Solution for Kids" (Bethesda, Md.: AGA Institute Press, 2009), for which she receives royalties.
FROM THE ANNUAL DIGESTIVE DISEASE WEEK
Major Finding: Among obese adolescents, elevated ALT levels rose from 16.7% in 1988 to 36.9% in 2008.
Data Source: A retrospective analysis of nationally representative data in 12-18 years from the National Health and Examination Survey datasets for 1988-2008.
Disclosures: Dr. Vos has received financial support in the form of a career award from the NIDDK and from the Children’s Digestive Health and Nutrition Foundation. She is the author of "The No-Diet Obesity Solution for Kids" for which she receives royalties.