User login
Experts present first recommendations for treating acne fulminans
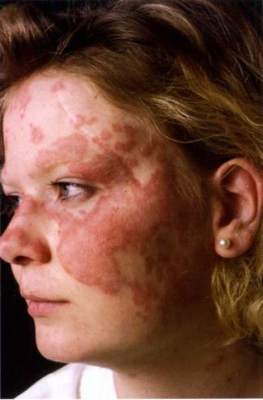
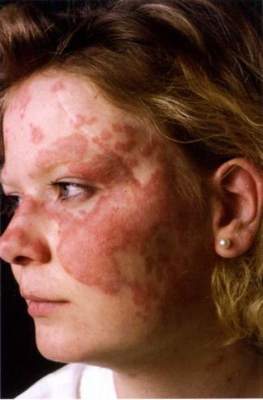
SCOTTSDALE, ARIZ. – Patients with acne fulminans should first begin corticosteroid monotherapy before adding isotretinoin, according to the first evidence-based consensus recommendations on this disease.
Antibiotics should not be used as first line treatment or monotherapy for acne fulminans, according to the experts who drafted the recommendations. Acne fulminans is an uncommon, understudied, and severe disorder that “typically manifests as an explosive worsening and ulceration of skin lesions, and can be associated with fever, bone pain, and other systemic symptoms,” noted panel co-chairs Dr. Andrea Zaenglein, professor of dermatology and pediatric dermatology, Pennsylvania State University, Hershey, and Dr. Sheila Friedlander, professor of medicine and pediatrics, University of California, San Diego, together with their associates.
The recommendations – based on a full literature review, a 5-hour audioconference, and two rounds of surveys to achieve consensus on these topics – were summarized in a poster presented at the annual meeting of the Society for Investigative Dermatology.
Until now, there have been no clear guidelines on the pathogenesis, treatment, and prevention of acne fulminans, according to the panelists.
Consensus definitions
“Acne fulminans is not just an extreme form of acne, but rather, a distinct, likely auto-inflammatory disorder,” the experts stated. Affected patients typically have an “abrupt, dramatic flare of inflammatory acne, with erosions, and with or without crusts, hemorrhagic nodules/plaques, and systemic findings.”
Systemic involvement is uncommon, but when present, includes fever, malaise, bone pain, arthralgias, erythema nodosum, and leukocytosis, they noted. Some patients also have anemia, an elevated erythrocyte sedimentation rate, and an increased C-reactive protein level. Radiography typically reveals osteolytic lesions of the sternum, clavicles, sacroiliac joints, and hips.
Acne fulminans is most often triggered by isotretinoin therapy, but can occur without it, the panel said. Isotretinoin-induced acne fulminans can have systemic involvement, but usually does not.
Corticosteroids
Patients should start corticosteroid monotherapy at a dose of 0.5 to 1.0 mg per kg per day, according to the recommendations. Patients with systemic involvement should receive steroids for at least 4 weeks, and other patients should continue steroids for at least 2 weeks and until all lesions have healed.
Oral corticosteroids should be tapered slowly over about 4 to 8 weeks, first by halving the dose to a physiologic dose each week, and then by dosing every other day for 2 weeks. Topical corticosteroids also can be used for eroded sites with granulation tissue, they noted.
Isotretinoin
Ironically, isotretinoin is both a treatment and a potential trigger of acne fulminans, and the recommendations included detailed guidance on its use.
Patients should wait at least 2 weeks after crusting resolves before starting isotretinoin, and should overlap isotretinoin with corticosteroids for at least 4 weeks, the experts emphasized. They recommended starting isotretinoin at 0.1 mg per kg per day, and waiting at least 2 months to increase this dose. Because patients clear at different rates, there is no universal optimal cumulative dose of isotretinoin, they noted.
If patients on isotretinoin develop flare, crusts, and erosions, they should halt treatment and either start corticosteroids, or increase the steroid dose to 1.0 mg per kg. If crusts and erosions persist, the panelists recommended considering cyclosporine, biologics, or dapsone.
If hemorrhagic crusts or erosions resolve after stopping isotretinoin, it can be restarted at the initial dose of 0.1 mg per kg and overlapped with steroids for 4 weeks.
If flares, crusts, and erosions begin when patients on isotretinoin are tapering corticosteroids, then steroids should be continued without tapering, isotretinoin should be stopped temporarily, and it should be restarted at 0.1 mg per kg after crusts and erosions have healed. This dose should be continued for 4 weeks, and then slowly increased as tolerated.
Antibiotics
Antibiotics are not useful as monotherapy for first-line therapy for acne fulminans, according to the recommendations. But to avoid isotretinoin-induced acne fulminans, the experts often pretreat patients with oral antibiotics before starting isotretinoin, and may continue oral antibiotics during the initial phase of isotretinoin treatment. However, there have been no prospective studies supporting this approach, they noted.
Other treatment considerations
Acne fulminans lesions should not be debrided, the experts emphasized. Acne fulminans associated with SAPHO (synovitis, acne, pustulosis, hyperostosis, and osteitis), PAPA (pyogenic arthritis, pyoderma gangrenosum, and acne), and PAPASH (pyogenic arthritis, pyoderma gangrenosum, acne, and hidradenitis suppurativa) has responded successfully to tumor necrosis factor–alpha inhibitors and interleukin-1 receptor antagonists, they noted. Pulsed dye laser also has been used to successfully treat acne fulminans, particularly when there is associated granulation tissue, they stated.
Reducing the risk of pseudotumor cerebri syndrome
Several drugs used to treat acne fulminans have been linked to pseudotumor cerebri syndrome, the experts cautioned. “Among the tetracyclines, minocycline carries the highest risk,” they noted. Both isotretinoin and corticosteroids have been linked to pseudotumor cerebri syndrome, but clinicians can reduce this risk by avoiding an abrupt steroid taper, they emphasized.
The panel included physicians from the University of California, San Diego; Pennsylvania State University, Hershey; State University of New York, Brooklyn; Cornell University, New York; Touro University California, Vallejo; the University of California, San Francisco; the University of Pennsylvania, Philadelphia; and Jefferson Medical College, Philadelphia. They did not disclose conflicts of interest.
SCOTTSDALE, ARIZ. – Patients with acne fulminans should first begin corticosteroid monotherapy before adding isotretinoin, according to the first evidence-based consensus recommendations on this disease.
Antibiotics should not be used as first line treatment or monotherapy for acne fulminans, according to the experts who drafted the recommendations. Acne fulminans is an uncommon, understudied, and severe disorder that “typically manifests as an explosive worsening and ulceration of skin lesions, and can be associated with fever, bone pain, and other systemic symptoms,” noted panel co-chairs Dr. Andrea Zaenglein, professor of dermatology and pediatric dermatology, Pennsylvania State University, Hershey, and Dr. Sheila Friedlander, professor of medicine and pediatrics, University of California, San Diego, together with their associates.
The recommendations – based on a full literature review, a 5-hour audioconference, and two rounds of surveys to achieve consensus on these topics – were summarized in a poster presented at the annual meeting of the Society for Investigative Dermatology.
Until now, there have been no clear guidelines on the pathogenesis, treatment, and prevention of acne fulminans, according to the panelists.
Consensus definitions
“Acne fulminans is not just an extreme form of acne, but rather, a distinct, likely auto-inflammatory disorder,” the experts stated. Affected patients typically have an “abrupt, dramatic flare of inflammatory acne, with erosions, and with or without crusts, hemorrhagic nodules/plaques, and systemic findings.”
Systemic involvement is uncommon, but when present, includes fever, malaise, bone pain, arthralgias, erythema nodosum, and leukocytosis, they noted. Some patients also have anemia, an elevated erythrocyte sedimentation rate, and an increased C-reactive protein level. Radiography typically reveals osteolytic lesions of the sternum, clavicles, sacroiliac joints, and hips.
Acne fulminans is most often triggered by isotretinoin therapy, but can occur without it, the panel said. Isotretinoin-induced acne fulminans can have systemic involvement, but usually does not.
Corticosteroids
Patients should start corticosteroid monotherapy at a dose of 0.5 to 1.0 mg per kg per day, according to the recommendations. Patients with systemic involvement should receive steroids for at least 4 weeks, and other patients should continue steroids for at least 2 weeks and until all lesions have healed.
Oral corticosteroids should be tapered slowly over about 4 to 8 weeks, first by halving the dose to a physiologic dose each week, and then by dosing every other day for 2 weeks. Topical corticosteroids also can be used for eroded sites with granulation tissue, they noted.
Isotretinoin
Ironically, isotretinoin is both a treatment and a potential trigger of acne fulminans, and the recommendations included detailed guidance on its use.
Patients should wait at least 2 weeks after crusting resolves before starting isotretinoin, and should overlap isotretinoin with corticosteroids for at least 4 weeks, the experts emphasized. They recommended starting isotretinoin at 0.1 mg per kg per day, and waiting at least 2 months to increase this dose. Because patients clear at different rates, there is no universal optimal cumulative dose of isotretinoin, they noted.
If patients on isotretinoin develop flare, crusts, and erosions, they should halt treatment and either start corticosteroids, or increase the steroid dose to 1.0 mg per kg. If crusts and erosions persist, the panelists recommended considering cyclosporine, biologics, or dapsone.
If hemorrhagic crusts or erosions resolve after stopping isotretinoin, it can be restarted at the initial dose of 0.1 mg per kg and overlapped with steroids for 4 weeks.
If flares, crusts, and erosions begin when patients on isotretinoin are tapering corticosteroids, then steroids should be continued without tapering, isotretinoin should be stopped temporarily, and it should be restarted at 0.1 mg per kg after crusts and erosions have healed. This dose should be continued for 4 weeks, and then slowly increased as tolerated.
Antibiotics
Antibiotics are not useful as monotherapy for first-line therapy for acne fulminans, according to the recommendations. But to avoid isotretinoin-induced acne fulminans, the experts often pretreat patients with oral antibiotics before starting isotretinoin, and may continue oral antibiotics during the initial phase of isotretinoin treatment. However, there have been no prospective studies supporting this approach, they noted.
Other treatment considerations
Acne fulminans lesions should not be debrided, the experts emphasized. Acne fulminans associated with SAPHO (synovitis, acne, pustulosis, hyperostosis, and osteitis), PAPA (pyogenic arthritis, pyoderma gangrenosum, and acne), and PAPASH (pyogenic arthritis, pyoderma gangrenosum, acne, and hidradenitis suppurativa) has responded successfully to tumor necrosis factor–alpha inhibitors and interleukin-1 receptor antagonists, they noted. Pulsed dye laser also has been used to successfully treat acne fulminans, particularly when there is associated granulation tissue, they stated.
Reducing the risk of pseudotumor cerebri syndrome
Several drugs used to treat acne fulminans have been linked to pseudotumor cerebri syndrome, the experts cautioned. “Among the tetracyclines, minocycline carries the highest risk,” they noted. Both isotretinoin and corticosteroids have been linked to pseudotumor cerebri syndrome, but clinicians can reduce this risk by avoiding an abrupt steroid taper, they emphasized.
The panel included physicians from the University of California, San Diego; Pennsylvania State University, Hershey; State University of New York, Brooklyn; Cornell University, New York; Touro University California, Vallejo; the University of California, San Francisco; the University of Pennsylvania, Philadelphia; and Jefferson Medical College, Philadelphia. They did not disclose conflicts of interest.
SCOTTSDALE, ARIZ. – Patients with acne fulminans should first begin corticosteroid monotherapy before adding isotretinoin, according to the first evidence-based consensus recommendations on this disease.
Antibiotics should not be used as first line treatment or monotherapy for acne fulminans, according to the experts who drafted the recommendations. Acne fulminans is an uncommon, understudied, and severe disorder that “typically manifests as an explosive worsening and ulceration of skin lesions, and can be associated with fever, bone pain, and other systemic symptoms,” noted panel co-chairs Dr. Andrea Zaenglein, professor of dermatology and pediatric dermatology, Pennsylvania State University, Hershey, and Dr. Sheila Friedlander, professor of medicine and pediatrics, University of California, San Diego, together with their associates.
The recommendations – based on a full literature review, a 5-hour audioconference, and two rounds of surveys to achieve consensus on these topics – were summarized in a poster presented at the annual meeting of the Society for Investigative Dermatology.
Until now, there have been no clear guidelines on the pathogenesis, treatment, and prevention of acne fulminans, according to the panelists.
Consensus definitions
“Acne fulminans is not just an extreme form of acne, but rather, a distinct, likely auto-inflammatory disorder,” the experts stated. Affected patients typically have an “abrupt, dramatic flare of inflammatory acne, with erosions, and with or without crusts, hemorrhagic nodules/plaques, and systemic findings.”
Systemic involvement is uncommon, but when present, includes fever, malaise, bone pain, arthralgias, erythema nodosum, and leukocytosis, they noted. Some patients also have anemia, an elevated erythrocyte sedimentation rate, and an increased C-reactive protein level. Radiography typically reveals osteolytic lesions of the sternum, clavicles, sacroiliac joints, and hips.
Acne fulminans is most often triggered by isotretinoin therapy, but can occur without it, the panel said. Isotretinoin-induced acne fulminans can have systemic involvement, but usually does not.
Corticosteroids
Patients should start corticosteroid monotherapy at a dose of 0.5 to 1.0 mg per kg per day, according to the recommendations. Patients with systemic involvement should receive steroids for at least 4 weeks, and other patients should continue steroids for at least 2 weeks and until all lesions have healed.
Oral corticosteroids should be tapered slowly over about 4 to 8 weeks, first by halving the dose to a physiologic dose each week, and then by dosing every other day for 2 weeks. Topical corticosteroids also can be used for eroded sites with granulation tissue, they noted.
Isotretinoin
Ironically, isotretinoin is both a treatment and a potential trigger of acne fulminans, and the recommendations included detailed guidance on its use.
Patients should wait at least 2 weeks after crusting resolves before starting isotretinoin, and should overlap isotretinoin with corticosteroids for at least 4 weeks, the experts emphasized. They recommended starting isotretinoin at 0.1 mg per kg per day, and waiting at least 2 months to increase this dose. Because patients clear at different rates, there is no universal optimal cumulative dose of isotretinoin, they noted.
If patients on isotretinoin develop flare, crusts, and erosions, they should halt treatment and either start corticosteroids, or increase the steroid dose to 1.0 mg per kg. If crusts and erosions persist, the panelists recommended considering cyclosporine, biologics, or dapsone.
If hemorrhagic crusts or erosions resolve after stopping isotretinoin, it can be restarted at the initial dose of 0.1 mg per kg and overlapped with steroids for 4 weeks.
If flares, crusts, and erosions begin when patients on isotretinoin are tapering corticosteroids, then steroids should be continued without tapering, isotretinoin should be stopped temporarily, and it should be restarted at 0.1 mg per kg after crusts and erosions have healed. This dose should be continued for 4 weeks, and then slowly increased as tolerated.
Antibiotics
Antibiotics are not useful as monotherapy for first-line therapy for acne fulminans, according to the recommendations. But to avoid isotretinoin-induced acne fulminans, the experts often pretreat patients with oral antibiotics before starting isotretinoin, and may continue oral antibiotics during the initial phase of isotretinoin treatment. However, there have been no prospective studies supporting this approach, they noted.
Other treatment considerations
Acne fulminans lesions should not be debrided, the experts emphasized. Acne fulminans associated with SAPHO (synovitis, acne, pustulosis, hyperostosis, and osteitis), PAPA (pyogenic arthritis, pyoderma gangrenosum, and acne), and PAPASH (pyogenic arthritis, pyoderma gangrenosum, acne, and hidradenitis suppurativa) has responded successfully to tumor necrosis factor–alpha inhibitors and interleukin-1 receptor antagonists, they noted. Pulsed dye laser also has been used to successfully treat acne fulminans, particularly when there is associated granulation tissue, they stated.
Reducing the risk of pseudotumor cerebri syndrome
Several drugs used to treat acne fulminans have been linked to pseudotumor cerebri syndrome, the experts cautioned. “Among the tetracyclines, minocycline carries the highest risk,” they noted. Both isotretinoin and corticosteroids have been linked to pseudotumor cerebri syndrome, but clinicians can reduce this risk by avoiding an abrupt steroid taper, they emphasized.
The panel included physicians from the University of California, San Diego; Pennsylvania State University, Hershey; State University of New York, Brooklyn; Cornell University, New York; Touro University California, Vallejo; the University of California, San Francisco; the University of Pennsylvania, Philadelphia; and Jefferson Medical College, Philadelphia. They did not disclose conflicts of interest.
AT THE 2016 SID ANNUAL MEETING
Fill rates: E-prescriptions beat paper for dermatology inpatients
SCOTTSDALE, ARIZ. – Dermatology patients who received new electronic prescriptions were significantly more likely to fill them than patients given paper prescriptions at a major urban hospital, a study showed.
This is the largest study to directly measure primary nonadherence to dermatologic medications, said Dr. Adewole Adamson of the department of dermatology, University of North Carolina at Chapel Hill. The results belie at least one past study linking e-prescriptions with lower fill rates, he said at the annual meeting of the Society for Investigative Dermatology.
Relatively few large studies have examined adherence to dermatology prescriptions. Furthermore, most looked only at claims data and assessed refill rates, not primary adherence, Dr. Adamson said. “Even fewer studies have focused on e-prescriptions, compared with paper prescriptions, which is astounding, as that is the direction in which we are heading,” he added.
What literature does exist also points to concerns, Dr. Adamson noted, referring to a study in Denmark, which found that half of psoriasis patients never filled their prescriptions (J Am Acad Dermatol. 2008 Jul;59[1]:27-33). Another study found that 27% of patients with acne did not fill all their prescriptions for the disease, he added (JAMA Dermatol. 2015;151[6]:623-6).
For this study, Dr. Adamson and his colleagues retrospectively studied medical charts for 2,579 dermatology outpatients at Parkland Hospital in Dallas between 2011 and 2013. Nearly half the patients were Hispanic, two-thirds were women, and the average age was 48 years. Among these 2,579 patients, 851 received e-prescriptions, and 1,728 received paper prescriptions. Patients received more than 4,600 new dermatology prescriptions – about 1.8 per patient. They could fill these at the hospital or at one of several outlying pharmacies.
The overall rate of complete primary adherence was 59%, while 13% of patients filled only some prescriptions. Thus, almost 28% of patients were completely nonadherent, similar to the findings of previous studies, Dr. Adamson said.
Notably, the rate of primary adherence was 16% higher among patients who received e-prescriptions: Of those who received e-prescriptions, 70% filled all their prescriptions, while another 11% filled some of them. In contrast, only 54% of patients filled all their paper prescriptions (P less than .001), while 14% filled some of them.
For prescriptions overall (written and e-prescriptions), patients aged 60-69 years had the highest fill rate (69%), while patients younger than 30 had the lowest (46%). Sex did not affect fill rates, but native Spanish speakers were somewhat more likely to fill all their prescriptions (62%) than native English speakers (57%).
The more prescriptions patients received, the less likely they were to fill them all. For example, about 60% of patients given one or two prescriptions filled all of them, while only 35% of patients given five prescriptions did so. That finding underscores the importance of simplifying medication regimens, Dr. Adamson said. “In terms of e-prescriptions, the horse is already out of the barn. I don’t think we are going to go back to paper prescriptions. But at least in this population, the data shows that electronic prescribing helps with compliance,” he added.
The study was supported by the University of North Carolina at Chapel Hill and the University of Texas Southwestern Medical Center. Dr. Adamson had no relevant financial disclosures.
SCOTTSDALE, ARIZ. – Dermatology patients who received new electronic prescriptions were significantly more likely to fill them than patients given paper prescriptions at a major urban hospital, a study showed.
This is the largest study to directly measure primary nonadherence to dermatologic medications, said Dr. Adewole Adamson of the department of dermatology, University of North Carolina at Chapel Hill. The results belie at least one past study linking e-prescriptions with lower fill rates, he said at the annual meeting of the Society for Investigative Dermatology.
Relatively few large studies have examined adherence to dermatology prescriptions. Furthermore, most looked only at claims data and assessed refill rates, not primary adherence, Dr. Adamson said. “Even fewer studies have focused on e-prescriptions, compared with paper prescriptions, which is astounding, as that is the direction in which we are heading,” he added.
What literature does exist also points to concerns, Dr. Adamson noted, referring to a study in Denmark, which found that half of psoriasis patients never filled their prescriptions (J Am Acad Dermatol. 2008 Jul;59[1]:27-33). Another study found that 27% of patients with acne did not fill all their prescriptions for the disease, he added (JAMA Dermatol. 2015;151[6]:623-6).
For this study, Dr. Adamson and his colleagues retrospectively studied medical charts for 2,579 dermatology outpatients at Parkland Hospital in Dallas between 2011 and 2013. Nearly half the patients were Hispanic, two-thirds were women, and the average age was 48 years. Among these 2,579 patients, 851 received e-prescriptions, and 1,728 received paper prescriptions. Patients received more than 4,600 new dermatology prescriptions – about 1.8 per patient. They could fill these at the hospital or at one of several outlying pharmacies.
The overall rate of complete primary adherence was 59%, while 13% of patients filled only some prescriptions. Thus, almost 28% of patients were completely nonadherent, similar to the findings of previous studies, Dr. Adamson said.
Notably, the rate of primary adherence was 16% higher among patients who received e-prescriptions: Of those who received e-prescriptions, 70% filled all their prescriptions, while another 11% filled some of them. In contrast, only 54% of patients filled all their paper prescriptions (P less than .001), while 14% filled some of them.
For prescriptions overall (written and e-prescriptions), patients aged 60-69 years had the highest fill rate (69%), while patients younger than 30 had the lowest (46%). Sex did not affect fill rates, but native Spanish speakers were somewhat more likely to fill all their prescriptions (62%) than native English speakers (57%).
The more prescriptions patients received, the less likely they were to fill them all. For example, about 60% of patients given one or two prescriptions filled all of them, while only 35% of patients given five prescriptions did so. That finding underscores the importance of simplifying medication regimens, Dr. Adamson said. “In terms of e-prescriptions, the horse is already out of the barn. I don’t think we are going to go back to paper prescriptions. But at least in this population, the data shows that electronic prescribing helps with compliance,” he added.
The study was supported by the University of North Carolina at Chapel Hill and the University of Texas Southwestern Medical Center. Dr. Adamson had no relevant financial disclosures.
SCOTTSDALE, ARIZ. – Dermatology patients who received new electronic prescriptions were significantly more likely to fill them than patients given paper prescriptions at a major urban hospital, a study showed.
This is the largest study to directly measure primary nonadherence to dermatologic medications, said Dr. Adewole Adamson of the department of dermatology, University of North Carolina at Chapel Hill. The results belie at least one past study linking e-prescriptions with lower fill rates, he said at the annual meeting of the Society for Investigative Dermatology.
Relatively few large studies have examined adherence to dermatology prescriptions. Furthermore, most looked only at claims data and assessed refill rates, not primary adherence, Dr. Adamson said. “Even fewer studies have focused on e-prescriptions, compared with paper prescriptions, which is astounding, as that is the direction in which we are heading,” he added.
What literature does exist also points to concerns, Dr. Adamson noted, referring to a study in Denmark, which found that half of psoriasis patients never filled their prescriptions (J Am Acad Dermatol. 2008 Jul;59[1]:27-33). Another study found that 27% of patients with acne did not fill all their prescriptions for the disease, he added (JAMA Dermatol. 2015;151[6]:623-6).
For this study, Dr. Adamson and his colleagues retrospectively studied medical charts for 2,579 dermatology outpatients at Parkland Hospital in Dallas between 2011 and 2013. Nearly half the patients were Hispanic, two-thirds were women, and the average age was 48 years. Among these 2,579 patients, 851 received e-prescriptions, and 1,728 received paper prescriptions. Patients received more than 4,600 new dermatology prescriptions – about 1.8 per patient. They could fill these at the hospital or at one of several outlying pharmacies.
The overall rate of complete primary adherence was 59%, while 13% of patients filled only some prescriptions. Thus, almost 28% of patients were completely nonadherent, similar to the findings of previous studies, Dr. Adamson said.
Notably, the rate of primary adherence was 16% higher among patients who received e-prescriptions: Of those who received e-prescriptions, 70% filled all their prescriptions, while another 11% filled some of them. In contrast, only 54% of patients filled all their paper prescriptions (P less than .001), while 14% filled some of them.
For prescriptions overall (written and e-prescriptions), patients aged 60-69 years had the highest fill rate (69%), while patients younger than 30 had the lowest (46%). Sex did not affect fill rates, but native Spanish speakers were somewhat more likely to fill all their prescriptions (62%) than native English speakers (57%).
The more prescriptions patients received, the less likely they were to fill them all. For example, about 60% of patients given one or two prescriptions filled all of them, while only 35% of patients given five prescriptions did so. That finding underscores the importance of simplifying medication regimens, Dr. Adamson said. “In terms of e-prescriptions, the horse is already out of the barn. I don’t think we are going to go back to paper prescriptions. But at least in this population, the data shows that electronic prescribing helps with compliance,” he added.
The study was supported by the University of North Carolina at Chapel Hill and the University of Texas Southwestern Medical Center. Dr. Adamson had no relevant financial disclosures.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: The primary adherence rate for electronic dermatology prescriptions was 16% higher than the primary adherence rate for paper prescriptions, dispensed at a large urban hospital.
Major finding: Almost two-thirds (70%) of patients filled all their electronic prescriptions, while only 54% of patients filled all their paper prescriptions (P less than .001).
Data source: A single-center retrospective study evaluating the medical charts of 2,509 dermatology outpatients between 2011 and 2013.
Disclosures: The study was supported by the University of North Carolina at Chapel Hill and the University of Texas Southwestern Medical Center. Dr. Adamson had no relevant financial disclosures.
Stevens-Johnson Syndrome, TEN Not as Rare as Thought
SCOTTSDALE – Stevens-Johnson syndrome and toxic epidermal necrolysis are not quite as rare as previously thought, according to one of the first population-based epidemiologic studies of the disorders.
“The incidence of Stevens-Johnson syndrome appears to be higher than previously reported, though mortality rates are lower,” said Derek Hsu of Northwestern University in Chicago, together with his associates. Both diagnoses were linked to a number of immune system disorders, “suggesting that immune dysregulation contributes to these diseases,” the investigators added.
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are potentially life-threatening mucocutaneous conditions that are usually triggered by medications or infections. In 1991, a population-based study in three U.S. states reported annual incidence rates of 7.1, 2.6, and 6.8 cases per million individuals for SJS, SJS/TEN, and TEN, respectively.
More recent studies have been limited mostly to case series, and the current incidence, mortality, and health care costs of those conditions among adults in the United States were unknown, Mr. Hsu and his coinvestigators noted.
Therefore, they analyzed data for 2009 through 2012 from the Nationwide Inpatient Sample, which covers 20% of hospitalizations in the country. Using validated ICD-9 codes, the researchers extracted information on patients with Stevens-Johnson syndrome and/or toxic epidermal necrolysis. To improve the positive predictive value of the diagnostic codes, they excluded patients with concurrent diagnoses of bullous dermatoses or erythema multiforme major.
The estimated annual incidence of SJS ranged from 8.6 to 9.8 cases per million adults per year, with a mean of 9.3 cases per million population, Mr. Hsu and his coinvestigators reported at the annual meeting of the Society for Investigative Dermatology.
Rates of combined SJS/TEN and TEN were substantially lower, with means of 1.6 and 1.9 cases per million per year, respectively.
Inpatients who were black, Hispanic, Asian, Native American, or of mixed race or ethnicity were more likely to have those diagnoses than were white inpatients, as were Medicare and self-pay patients and patients with relatively more comorbidities.
Patients diagnosed with SJS stayed in the hospital an average of 9.8 days, incurring $21,407 in mean inflation-adjusted treatment costs, while patients with SJS/TEN or TEN stayed an average of more than 16 days, with associated costs of nearly $59,00 and $53,700, respectively. For all three diagnostic categories, length of stay and costs were significantly greater than among inpatients as a whole, Mr. Hsu and his associates noted.
Age- and sex-adjusted case-fatality rates were lower than previously reported, ranging from 3.7% to 7.5% for SJS (average, 4.7%), from 15.7% to 22.3% for SJS/TEN, and from 7.7% to 19% (average, 14.8%) for TEN. The risk of mortality increased very little after the affected body surface exceeded 10%, the researchers noted. Septicemia, other infections, renal failure, cancers, and older age all increased the risk of mortality.
After accounting for race, age, and sex, SJS/TEN were significantly associated with a number of comorbidities, including systemic lupus erythematosus, renal failure, liver disease, epilepsy, and HIV disease, as well as mycoplasma, tuberculosis, and other serious infections, the researchers also found.
In addition, SJS and TEN were significantly more likely among inpatients with non-Hodgkin lymphoma, multiple myeloma, and leukemia. “Further studies are needed to understand the mechanism of these associations,” the investigators said.
The Dermatology Foundation and the Agency for Healthcare Research and Quality funded the study. Mr. Hsu had no disclosures.
SCOTTSDALE – Stevens-Johnson syndrome and toxic epidermal necrolysis are not quite as rare as previously thought, according to one of the first population-based epidemiologic studies of the disorders.
“The incidence of Stevens-Johnson syndrome appears to be higher than previously reported, though mortality rates are lower,” said Derek Hsu of Northwestern University in Chicago, together with his associates. Both diagnoses were linked to a number of immune system disorders, “suggesting that immune dysregulation contributes to these diseases,” the investigators added.
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are potentially life-threatening mucocutaneous conditions that are usually triggered by medications or infections. In 1991, a population-based study in three U.S. states reported annual incidence rates of 7.1, 2.6, and 6.8 cases per million individuals for SJS, SJS/TEN, and TEN, respectively.
More recent studies have been limited mostly to case series, and the current incidence, mortality, and health care costs of those conditions among adults in the United States were unknown, Mr. Hsu and his coinvestigators noted.
Therefore, they analyzed data for 2009 through 2012 from the Nationwide Inpatient Sample, which covers 20% of hospitalizations in the country. Using validated ICD-9 codes, the researchers extracted information on patients with Stevens-Johnson syndrome and/or toxic epidermal necrolysis. To improve the positive predictive value of the diagnostic codes, they excluded patients with concurrent diagnoses of bullous dermatoses or erythema multiforme major.
The estimated annual incidence of SJS ranged from 8.6 to 9.8 cases per million adults per year, with a mean of 9.3 cases per million population, Mr. Hsu and his coinvestigators reported at the annual meeting of the Society for Investigative Dermatology.
Rates of combined SJS/TEN and TEN were substantially lower, with means of 1.6 and 1.9 cases per million per year, respectively.
Inpatients who were black, Hispanic, Asian, Native American, or of mixed race or ethnicity were more likely to have those diagnoses than were white inpatients, as were Medicare and self-pay patients and patients with relatively more comorbidities.
Patients diagnosed with SJS stayed in the hospital an average of 9.8 days, incurring $21,407 in mean inflation-adjusted treatment costs, while patients with SJS/TEN or TEN stayed an average of more than 16 days, with associated costs of nearly $59,00 and $53,700, respectively. For all three diagnostic categories, length of stay and costs were significantly greater than among inpatients as a whole, Mr. Hsu and his associates noted.
Age- and sex-adjusted case-fatality rates were lower than previously reported, ranging from 3.7% to 7.5% for SJS (average, 4.7%), from 15.7% to 22.3% for SJS/TEN, and from 7.7% to 19% (average, 14.8%) for TEN. The risk of mortality increased very little after the affected body surface exceeded 10%, the researchers noted. Septicemia, other infections, renal failure, cancers, and older age all increased the risk of mortality.
After accounting for race, age, and sex, SJS/TEN were significantly associated with a number of comorbidities, including systemic lupus erythematosus, renal failure, liver disease, epilepsy, and HIV disease, as well as mycoplasma, tuberculosis, and other serious infections, the researchers also found.
In addition, SJS and TEN were significantly more likely among inpatients with non-Hodgkin lymphoma, multiple myeloma, and leukemia. “Further studies are needed to understand the mechanism of these associations,” the investigators said.
The Dermatology Foundation and the Agency for Healthcare Research and Quality funded the study. Mr. Hsu had no disclosures.
SCOTTSDALE – Stevens-Johnson syndrome and toxic epidermal necrolysis are not quite as rare as previously thought, according to one of the first population-based epidemiologic studies of the disorders.
“The incidence of Stevens-Johnson syndrome appears to be higher than previously reported, though mortality rates are lower,” said Derek Hsu of Northwestern University in Chicago, together with his associates. Both diagnoses were linked to a number of immune system disorders, “suggesting that immune dysregulation contributes to these diseases,” the investigators added.
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are potentially life-threatening mucocutaneous conditions that are usually triggered by medications or infections. In 1991, a population-based study in three U.S. states reported annual incidence rates of 7.1, 2.6, and 6.8 cases per million individuals for SJS, SJS/TEN, and TEN, respectively.
More recent studies have been limited mostly to case series, and the current incidence, mortality, and health care costs of those conditions among adults in the United States were unknown, Mr. Hsu and his coinvestigators noted.
Therefore, they analyzed data for 2009 through 2012 from the Nationwide Inpatient Sample, which covers 20% of hospitalizations in the country. Using validated ICD-9 codes, the researchers extracted information on patients with Stevens-Johnson syndrome and/or toxic epidermal necrolysis. To improve the positive predictive value of the diagnostic codes, they excluded patients with concurrent diagnoses of bullous dermatoses or erythema multiforme major.
The estimated annual incidence of SJS ranged from 8.6 to 9.8 cases per million adults per year, with a mean of 9.3 cases per million population, Mr. Hsu and his coinvestigators reported at the annual meeting of the Society for Investigative Dermatology.
Rates of combined SJS/TEN and TEN were substantially lower, with means of 1.6 and 1.9 cases per million per year, respectively.
Inpatients who were black, Hispanic, Asian, Native American, or of mixed race or ethnicity were more likely to have those diagnoses than were white inpatients, as were Medicare and self-pay patients and patients with relatively more comorbidities.
Patients diagnosed with SJS stayed in the hospital an average of 9.8 days, incurring $21,407 in mean inflation-adjusted treatment costs, while patients with SJS/TEN or TEN stayed an average of more than 16 days, with associated costs of nearly $59,00 and $53,700, respectively. For all three diagnostic categories, length of stay and costs were significantly greater than among inpatients as a whole, Mr. Hsu and his associates noted.
Age- and sex-adjusted case-fatality rates were lower than previously reported, ranging from 3.7% to 7.5% for SJS (average, 4.7%), from 15.7% to 22.3% for SJS/TEN, and from 7.7% to 19% (average, 14.8%) for TEN. The risk of mortality increased very little after the affected body surface exceeded 10%, the researchers noted. Septicemia, other infections, renal failure, cancers, and older age all increased the risk of mortality.
After accounting for race, age, and sex, SJS/TEN were significantly associated with a number of comorbidities, including systemic lupus erythematosus, renal failure, liver disease, epilepsy, and HIV disease, as well as mycoplasma, tuberculosis, and other serious infections, the researchers also found.
In addition, SJS and TEN were significantly more likely among inpatients with non-Hodgkin lymphoma, multiple myeloma, and leukemia. “Further studies are needed to understand the mechanism of these associations,” the investigators said.
The Dermatology Foundation and the Agency for Healthcare Research and Quality funded the study. Mr. Hsu had no disclosures.
AT THE 2016 SID ANNUAL MEETING
Stevens-Johnson syndrome, TEN not as rare as thought
SCOTTSDALE – Stevens-Johnson syndrome and toxic epidermal necrolysis are not quite as rare as previously thought, according to one of the first population-based epidemiologic studies of the disorders.
“The incidence of Stevens-Johnson syndrome appears to be higher than previously reported, though mortality rates are lower,” said Derek Hsu of Northwestern University in Chicago, together with his associates. Both diagnoses were linked to a number of immune system disorders, “suggesting that immune dysregulation contributes to these diseases,” the investigators added.
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are potentially life-threatening mucocutaneous conditions that are usually triggered by medications or infections. In 1991, a population-based study in three U.S. states reported annual incidence rates of 7.1, 2.6, and 6.8 cases per million individuals for SJS, SJS/TEN, and TEN, respectively.
More recent studies have been limited mostly to case series, and the current incidence, mortality, and health care costs of those conditions among adults in the United States were unknown, Mr. Hsu and his coinvestigators noted.
Therefore, they analyzed data for 2009 through 2012 from the Nationwide Inpatient Sample, which covers 20% of hospitalizations in the country. Using validated ICD-9 codes, the researchers extracted information on patients with Stevens-Johnson syndrome and/or toxic epidermal necrolysis. To improve the positive predictive value of the diagnostic codes, they excluded patients with concurrent diagnoses of bullous dermatoses or erythema multiforme major.
The estimated annual incidence of SJS ranged from 8.6 to 9.8 cases per million adults per year, with a mean of 9.3 cases per million population, Mr. Hsu and his coinvestigators reported at the annual meeting of the Society for Investigative Dermatology.
Rates of combined SJS/TEN and TEN were substantially lower, with means of 1.6 and 1.9 cases per million per year, respectively.
Inpatients who were black, Hispanic, Asian, Native American, or of mixed race or ethnicity were more likely to have those diagnoses than were white inpatients, as were Medicare and self-pay patients and patients with relatively more comorbidities.
Patients diagnosed with SJS stayed in the hospital an average of 9.8 days, incurring $21,407 in mean inflation-adjusted treatment costs, while patients with SJS/TEN or TEN stayed an average of more than 16 days, with associated costs of nearly $59,00 and $53,700, respectively. For all three diagnostic categories, length of stay and costs were significantly greater than among inpatients as a whole, Mr. Hsu and his associates noted.
Age- and sex-adjusted case-fatality rates were lower than previously reported, ranging from 3.7% to 7.5% for SJS (average, 4.7%), from 15.7% to 22.3% for SJS/TEN, and from 7.7% to 19% (average, 14.8%) for TEN. The risk of mortality increased very little after the affected body surface exceeded 10%, the researchers noted. Septicemia, other infections, renal failure, cancers, and older age all increased the risk of mortality.
After accounting for race, age, and sex, SJS/TEN were significantly associated with a number of comorbidities, including systemic lupus erythematosus, renal failure, liver disease, epilepsy, and HIV disease, as well as mycoplasma, tuberculosis, and other serious infections, the researchers also found.
In addition, SJS and TEN were significantly more likely among inpatients with non-Hodgkin lymphoma, multiple myeloma, and leukemia. “Further studies are needed to understand the mechanism of these associations,” the investigators said.
The Dermatology Foundation and the Agency for Healthcare Research and Quality funded the study. Mr. Hsu had no disclosures.
SCOTTSDALE – Stevens-Johnson syndrome and toxic epidermal necrolysis are not quite as rare as previously thought, according to one of the first population-based epidemiologic studies of the disorders.
“The incidence of Stevens-Johnson syndrome appears to be higher than previously reported, though mortality rates are lower,” said Derek Hsu of Northwestern University in Chicago, together with his associates. Both diagnoses were linked to a number of immune system disorders, “suggesting that immune dysregulation contributes to these diseases,” the investigators added.
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are potentially life-threatening mucocutaneous conditions that are usually triggered by medications or infections. In 1991, a population-based study in three U.S. states reported annual incidence rates of 7.1, 2.6, and 6.8 cases per million individuals for SJS, SJS/TEN, and TEN, respectively.
More recent studies have been limited mostly to case series, and the current incidence, mortality, and health care costs of those conditions among adults in the United States were unknown, Mr. Hsu and his coinvestigators noted.
Therefore, they analyzed data for 2009 through 2012 from the Nationwide Inpatient Sample, which covers 20% of hospitalizations in the country. Using validated ICD-9 codes, the researchers extracted information on patients with Stevens-Johnson syndrome and/or toxic epidermal necrolysis. To improve the positive predictive value of the diagnostic codes, they excluded patients with concurrent diagnoses of bullous dermatoses or erythema multiforme major.
The estimated annual incidence of SJS ranged from 8.6 to 9.8 cases per million adults per year, with a mean of 9.3 cases per million population, Mr. Hsu and his coinvestigators reported at the annual meeting of the Society for Investigative Dermatology.
Rates of combined SJS/TEN and TEN were substantially lower, with means of 1.6 and 1.9 cases per million per year, respectively.
Inpatients who were black, Hispanic, Asian, Native American, or of mixed race or ethnicity were more likely to have those diagnoses than were white inpatients, as were Medicare and self-pay patients and patients with relatively more comorbidities.
Patients diagnosed with SJS stayed in the hospital an average of 9.8 days, incurring $21,407 in mean inflation-adjusted treatment costs, while patients with SJS/TEN or TEN stayed an average of more than 16 days, with associated costs of nearly $59,00 and $53,700, respectively. For all three diagnostic categories, length of stay and costs were significantly greater than among inpatients as a whole, Mr. Hsu and his associates noted.
Age- and sex-adjusted case-fatality rates were lower than previously reported, ranging from 3.7% to 7.5% for SJS (average, 4.7%), from 15.7% to 22.3% for SJS/TEN, and from 7.7% to 19% (average, 14.8%) for TEN. The risk of mortality increased very little after the affected body surface exceeded 10%, the researchers noted. Septicemia, other infections, renal failure, cancers, and older age all increased the risk of mortality.
After accounting for race, age, and sex, SJS/TEN were significantly associated with a number of comorbidities, including systemic lupus erythematosus, renal failure, liver disease, epilepsy, and HIV disease, as well as mycoplasma, tuberculosis, and other serious infections, the researchers also found.
In addition, SJS and TEN were significantly more likely among inpatients with non-Hodgkin lymphoma, multiple myeloma, and leukemia. “Further studies are needed to understand the mechanism of these associations,” the investigators said.
The Dermatology Foundation and the Agency for Healthcare Research and Quality funded the study. Mr. Hsu had no disclosures.
SCOTTSDALE – Stevens-Johnson syndrome and toxic epidermal necrolysis are not quite as rare as previously thought, according to one of the first population-based epidemiologic studies of the disorders.
“The incidence of Stevens-Johnson syndrome appears to be higher than previously reported, though mortality rates are lower,” said Derek Hsu of Northwestern University in Chicago, together with his associates. Both diagnoses were linked to a number of immune system disorders, “suggesting that immune dysregulation contributes to these diseases,” the investigators added.
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are potentially life-threatening mucocutaneous conditions that are usually triggered by medications or infections. In 1991, a population-based study in three U.S. states reported annual incidence rates of 7.1, 2.6, and 6.8 cases per million individuals for SJS, SJS/TEN, and TEN, respectively.
More recent studies have been limited mostly to case series, and the current incidence, mortality, and health care costs of those conditions among adults in the United States were unknown, Mr. Hsu and his coinvestigators noted.
Therefore, they analyzed data for 2009 through 2012 from the Nationwide Inpatient Sample, which covers 20% of hospitalizations in the country. Using validated ICD-9 codes, the researchers extracted information on patients with Stevens-Johnson syndrome and/or toxic epidermal necrolysis. To improve the positive predictive value of the diagnostic codes, they excluded patients with concurrent diagnoses of bullous dermatoses or erythema multiforme major.
The estimated annual incidence of SJS ranged from 8.6 to 9.8 cases per million adults per year, with a mean of 9.3 cases per million population, Mr. Hsu and his coinvestigators reported at the annual meeting of the Society for Investigative Dermatology.
Rates of combined SJS/TEN and TEN were substantially lower, with means of 1.6 and 1.9 cases per million per year, respectively.
Inpatients who were black, Hispanic, Asian, Native American, or of mixed race or ethnicity were more likely to have those diagnoses than were white inpatients, as were Medicare and self-pay patients and patients with relatively more comorbidities.
Patients diagnosed with SJS stayed in the hospital an average of 9.8 days, incurring $21,407 in mean inflation-adjusted treatment costs, while patients with SJS/TEN or TEN stayed an average of more than 16 days, with associated costs of nearly $59,00 and $53,700, respectively. For all three diagnostic categories, length of stay and costs were significantly greater than among inpatients as a whole, Mr. Hsu and his associates noted.
Age- and sex-adjusted case-fatality rates were lower than previously reported, ranging from 3.7% to 7.5% for SJS (average, 4.7%), from 15.7% to 22.3% for SJS/TEN, and from 7.7% to 19% (average, 14.8%) for TEN. The risk of mortality increased very little after the affected body surface exceeded 10%, the researchers noted. Septicemia, other infections, renal failure, cancers, and older age all increased the risk of mortality.
After accounting for race, age, and sex, SJS/TEN were significantly associated with a number of comorbidities, including systemic lupus erythematosus, renal failure, liver disease, epilepsy, and HIV disease, as well as mycoplasma, tuberculosis, and other serious infections, the researchers also found.
In addition, SJS and TEN were significantly more likely among inpatients with non-Hodgkin lymphoma, multiple myeloma, and leukemia. “Further studies are needed to understand the mechanism of these associations,” the investigators said.
The Dermatology Foundation and the Agency for Healthcare Research and Quality funded the study. Mr. Hsu had no disclosures.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: The annual incidence of SJS was 8.6-9.8 cases per million adults per year, with a mean of 9.3 cases.
Major finding: The annual incidence of SJS was 8.6-9.8 cases per million adults per year, with a mean of 9.3. Annual rates of combined SJS/TEN and TEN were substantially lower, averaging 1.6 and 1.9 cases per million individuals per year, respectively.
Data source: An analysis of Nationwide Inpatient Sample data from 2009 through 2012.
Disclosures: The Dermatology Foundation and the Agency for Healthcare Research and Quality funded the study. Mr. Hsu had no disclosures.
Serious Infections Are Increasing Among Psoriasis Inpatients
SCOTTSDALE, ARIZ. – From enterocolitis to MRSA, serious infections are on the rise among inpatients with psoriasis, and psoriasis is an independent risk factor for serious infections, according to findings from large retrospective studies from the United States and the United Kingdom.
Inpatients with psoriasis in the United States also were at greater risk of serious infections, compared with nonpsoriatic inpatients at every time point studied, and serious infections were associated with increased hospital costs, length of stay, and risk of mortality, reported Derek Hsu, a medical student at Northwestern University, Chicago, and his associates. “Research is needed to determine how to reduce the risk of serious infections in patients with psoriasis,” the investigators emphasized.
Psoriasis affects some 7 million adults in the United States. Biologics, which are transforming the treatment landscape for moderate-to-severe psoriasis, “should reduce inherent infectious risk by controlling the inflammatory process and reducing disease severity, [but] these effects may be immunosuppressing and increase the risk of infection in other ways,” according to Mr. Hsu and his associates. For their study, they analyzed data for 2002-2012 from the Nationwide Inpatient Sample, which covers 20% of hospitalizations in the United States. They extracted validated ICD-9 codes for psoriasis and serious infections, and calculated costs of care after adjusting for 2014 inflation, based on the United States Consumer Price Index.
Overall rates of serious infection and rates of pneumonia, MRSA, septicemia, diverticulitis, enterocolitis, encephalitis, and any viral or fungal infection rose significantly among inpatients with psoriasis between 2002 and 2012 (all P-values less than .05). Predictors of serious infections among inpatients with psoriasis included diabetes mellitus, obesity, and being of non-Caucasian race or ethnicity, female, older than 60 years, and on Medicare or Medicaid, the researchers reported at the annual meeting of the Society for Investigative Dermatology.
Furthermore, after controlling for age, sex, and race, psoriasis was a significant risk factor for many different types of serious infections. Among these were cellulitis, herpes simplex virus, infectious arthritis, osteomyelitis, meningitis, influenza, encephalitis, septicemia, enterocolitis, MRSA, methicillin-sensitive Staphylococcus aureus infections, and Clostridium difficile. Further, inpatients with psoriasis were more prone to urinary tract infection, peritonitis or intestinal abscess, appendicitis, tuberculosis, and viral and fungal infections (all P-values less than .05). The average cost of hospital stay for inpatients with psoriasis was more than $2,200 greater when they were diagnosed with one or more serious infections than otherwise, and their average length of hospital stay was 2 days longer.
The study in the United Kingdom included nearly 200,000 patients with psoriasis and almost 1 million patients without psoriasis from The Health Improvement Network electronic medical record database. Between 2002 and 2013, patients without psoriasis developed an estimated 78.5 serious infections per 100,000 person-years, compared with 88.9, 85.7, and 145.7 serious infections per 100,000 person-years, respectively, for all psoriasis patients, patients with mild disease, and patients with severe disease requiring systemic or phototherapy, said Dr. Junko Takeshita and her colleagues at the University of Pennsylvania in Philadelphia. After controlling for many potential demographic and clinical confounders, psoriasis increased the risk of serious infection by about 21% (hazard ratio, 1.21; 95% confidence interval, 1.18-1.23). Patients with severe psoriasis had a 63% greater risk of infection than patients without psoriasis, compared with an 18% increase for patients with mild psoriasis.
The findings show “serious infection, particularly respiratory and skin or soft tissue infections, to be an important and common cause of morbidity among patients with psoriasis, especially those with more severe disease,” Dr. Takeshita and her associates said. Notably, the link between psoriasis and risk of serious infection persisted after excluding patients on immunosuppressive therapies, suggesting “that the greater infection risk is at least partially attributable to more severe psoriasis, itself,” they added.
The analysis of Nationwide Inpatient Sample data was funded by the Agency for Healthcare Research and Quality and by the Dermatology Foundation. The analysis of Health Improvement Network data was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, which is part of the National Institutes of Health, and by the Dermatology Foundation. None of the investigators reported conflicts of interest.
SCOTTSDALE, ARIZ. – From enterocolitis to MRSA, serious infections are on the rise among inpatients with psoriasis, and psoriasis is an independent risk factor for serious infections, according to findings from large retrospective studies from the United States and the United Kingdom.
Inpatients with psoriasis in the United States also were at greater risk of serious infections, compared with nonpsoriatic inpatients at every time point studied, and serious infections were associated with increased hospital costs, length of stay, and risk of mortality, reported Derek Hsu, a medical student at Northwestern University, Chicago, and his associates. “Research is needed to determine how to reduce the risk of serious infections in patients with psoriasis,” the investigators emphasized.
Psoriasis affects some 7 million adults in the United States. Biologics, which are transforming the treatment landscape for moderate-to-severe psoriasis, “should reduce inherent infectious risk by controlling the inflammatory process and reducing disease severity, [but] these effects may be immunosuppressing and increase the risk of infection in other ways,” according to Mr. Hsu and his associates. For their study, they analyzed data for 2002-2012 from the Nationwide Inpatient Sample, which covers 20% of hospitalizations in the United States. They extracted validated ICD-9 codes for psoriasis and serious infections, and calculated costs of care after adjusting for 2014 inflation, based on the United States Consumer Price Index.
Overall rates of serious infection and rates of pneumonia, MRSA, septicemia, diverticulitis, enterocolitis, encephalitis, and any viral or fungal infection rose significantly among inpatients with psoriasis between 2002 and 2012 (all P-values less than .05). Predictors of serious infections among inpatients with psoriasis included diabetes mellitus, obesity, and being of non-Caucasian race or ethnicity, female, older than 60 years, and on Medicare or Medicaid, the researchers reported at the annual meeting of the Society for Investigative Dermatology.
Furthermore, after controlling for age, sex, and race, psoriasis was a significant risk factor for many different types of serious infections. Among these were cellulitis, herpes simplex virus, infectious arthritis, osteomyelitis, meningitis, influenza, encephalitis, septicemia, enterocolitis, MRSA, methicillin-sensitive Staphylococcus aureus infections, and Clostridium difficile. Further, inpatients with psoriasis were more prone to urinary tract infection, peritonitis or intestinal abscess, appendicitis, tuberculosis, and viral and fungal infections (all P-values less than .05). The average cost of hospital stay for inpatients with psoriasis was more than $2,200 greater when they were diagnosed with one or more serious infections than otherwise, and their average length of hospital stay was 2 days longer.
The study in the United Kingdom included nearly 200,000 patients with psoriasis and almost 1 million patients without psoriasis from The Health Improvement Network electronic medical record database. Between 2002 and 2013, patients without psoriasis developed an estimated 78.5 serious infections per 100,000 person-years, compared with 88.9, 85.7, and 145.7 serious infections per 100,000 person-years, respectively, for all psoriasis patients, patients with mild disease, and patients with severe disease requiring systemic or phototherapy, said Dr. Junko Takeshita and her colleagues at the University of Pennsylvania in Philadelphia. After controlling for many potential demographic and clinical confounders, psoriasis increased the risk of serious infection by about 21% (hazard ratio, 1.21; 95% confidence interval, 1.18-1.23). Patients with severe psoriasis had a 63% greater risk of infection than patients without psoriasis, compared with an 18% increase for patients with mild psoriasis.
The findings show “serious infection, particularly respiratory and skin or soft tissue infections, to be an important and common cause of morbidity among patients with psoriasis, especially those with more severe disease,” Dr. Takeshita and her associates said. Notably, the link between psoriasis and risk of serious infection persisted after excluding patients on immunosuppressive therapies, suggesting “that the greater infection risk is at least partially attributable to more severe psoriasis, itself,” they added.
The analysis of Nationwide Inpatient Sample data was funded by the Agency for Healthcare Research and Quality and by the Dermatology Foundation. The analysis of Health Improvement Network data was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, which is part of the National Institutes of Health, and by the Dermatology Foundation. None of the investigators reported conflicts of interest.
SCOTTSDALE, ARIZ. – From enterocolitis to MRSA, serious infections are on the rise among inpatients with psoriasis, and psoriasis is an independent risk factor for serious infections, according to findings from large retrospective studies from the United States and the United Kingdom.
Inpatients with psoriasis in the United States also were at greater risk of serious infections, compared with nonpsoriatic inpatients at every time point studied, and serious infections were associated with increased hospital costs, length of stay, and risk of mortality, reported Derek Hsu, a medical student at Northwestern University, Chicago, and his associates. “Research is needed to determine how to reduce the risk of serious infections in patients with psoriasis,” the investigators emphasized.
Psoriasis affects some 7 million adults in the United States. Biologics, which are transforming the treatment landscape for moderate-to-severe psoriasis, “should reduce inherent infectious risk by controlling the inflammatory process and reducing disease severity, [but] these effects may be immunosuppressing and increase the risk of infection in other ways,” according to Mr. Hsu and his associates. For their study, they analyzed data for 2002-2012 from the Nationwide Inpatient Sample, which covers 20% of hospitalizations in the United States. They extracted validated ICD-9 codes for psoriasis and serious infections, and calculated costs of care after adjusting for 2014 inflation, based on the United States Consumer Price Index.
Overall rates of serious infection and rates of pneumonia, MRSA, septicemia, diverticulitis, enterocolitis, encephalitis, and any viral or fungal infection rose significantly among inpatients with psoriasis between 2002 and 2012 (all P-values less than .05). Predictors of serious infections among inpatients with psoriasis included diabetes mellitus, obesity, and being of non-Caucasian race or ethnicity, female, older than 60 years, and on Medicare or Medicaid, the researchers reported at the annual meeting of the Society for Investigative Dermatology.
Furthermore, after controlling for age, sex, and race, psoriasis was a significant risk factor for many different types of serious infections. Among these were cellulitis, herpes simplex virus, infectious arthritis, osteomyelitis, meningitis, influenza, encephalitis, septicemia, enterocolitis, MRSA, methicillin-sensitive Staphylococcus aureus infections, and Clostridium difficile. Further, inpatients with psoriasis were more prone to urinary tract infection, peritonitis or intestinal abscess, appendicitis, tuberculosis, and viral and fungal infections (all P-values less than .05). The average cost of hospital stay for inpatients with psoriasis was more than $2,200 greater when they were diagnosed with one or more serious infections than otherwise, and their average length of hospital stay was 2 days longer.
The study in the United Kingdom included nearly 200,000 patients with psoriasis and almost 1 million patients without psoriasis from The Health Improvement Network electronic medical record database. Between 2002 and 2013, patients without psoriasis developed an estimated 78.5 serious infections per 100,000 person-years, compared with 88.9, 85.7, and 145.7 serious infections per 100,000 person-years, respectively, for all psoriasis patients, patients with mild disease, and patients with severe disease requiring systemic or phototherapy, said Dr. Junko Takeshita and her colleagues at the University of Pennsylvania in Philadelphia. After controlling for many potential demographic and clinical confounders, psoriasis increased the risk of serious infection by about 21% (hazard ratio, 1.21; 95% confidence interval, 1.18-1.23). Patients with severe psoriasis had a 63% greater risk of infection than patients without psoriasis, compared with an 18% increase for patients with mild psoriasis.
The findings show “serious infection, particularly respiratory and skin or soft tissue infections, to be an important and common cause of morbidity among patients with psoriasis, especially those with more severe disease,” Dr. Takeshita and her associates said. Notably, the link between psoriasis and risk of serious infection persisted after excluding patients on immunosuppressive therapies, suggesting “that the greater infection risk is at least partially attributable to more severe psoriasis, itself,” they added.
The analysis of Nationwide Inpatient Sample data was funded by the Agency for Healthcare Research and Quality and by the Dermatology Foundation. The analysis of Health Improvement Network data was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, which is part of the National Institutes of Health, and by the Dermatology Foundation. None of the investigators reported conflicts of interest.
AT THE 2016 SID ANNUAL MEETING
Serious infections are increasing among psoriasis inpatients
SCOTTSDALE, ARIZ. – From enterocolitis to MRSA, serious infections are on the rise among inpatients with psoriasis, and psoriasis is an independent risk factor for serious infections, according to findings from large retrospective studies from the United States and the United Kingdom.
Inpatients with psoriasis in the United States also were at greater risk of serious infections, compared with nonpsoriatic inpatients at every time point studied, and serious infections were associated with increased hospital costs, length of stay, and risk of mortality, reported Derek Hsu, a medical student at Northwestern University, Chicago, and his associates. “Research is needed to determine how to reduce the risk of serious infections in patients with psoriasis,” the investigators emphasized.
Psoriasis affects some 7 million adults in the United States. Biologics, which are transforming the treatment landscape for moderate-to-severe psoriasis, “should reduce inherent infectious risk by controlling the inflammatory process and reducing disease severity, [but] these effects may be immunosuppressing and increase the risk of infection in other ways,” according to Mr. Hsu and his associates. For their study, they analyzed data for 2002-2012 from the Nationwide Inpatient Sample, which covers 20% of hospitalizations in the United States. They extracted validated ICD-9 codes for psoriasis and serious infections, and calculated costs of care after adjusting for 2014 inflation, based on the United States Consumer Price Index.
Overall rates of serious infection and rates of pneumonia, MRSA, septicemia, diverticulitis, enterocolitis, encephalitis, and any viral or fungal infection rose significantly among inpatients with psoriasis between 2002 and 2012 (all P-values less than .05). Predictors of serious infections among inpatients with psoriasis included diabetes mellitus, obesity, and being of non-Caucasian race or ethnicity, female, older than 60 years, and on Medicare or Medicaid, the researchers reported at the annual meeting of the Society for Investigative Dermatology.
Furthermore, after controlling for age, sex, and race, psoriasis was a significant risk factor for many different types of serious infections. Among these were cellulitis, herpes simplex virus, infectious arthritis, osteomyelitis, meningitis, influenza, encephalitis, septicemia, enterocolitis, MRSA, methicillin-sensitive Staphylococcus aureus infections, and Clostridium difficile. Further, inpatients with psoriasis were more prone to urinary tract infection, peritonitis or intestinal abscess, appendicitis, tuberculosis, and viral and fungal infections (all P-values less than .05). The average cost of hospital stay for inpatients with psoriasis was more than $2,200 greater when they were diagnosed with one or more serious infections than otherwise, and their average length of hospital stay was 2 days longer.
The study in the United Kingdom included nearly 200,000 patients with psoriasis and almost 1 million patients without psoriasis from The Health Improvement Network electronic medical record database. Between 2002 and 2013, patients without psoriasis developed an estimated 78.5 serious infections per 100,000 person-years, compared with 88.9, 85.7, and 145.7 serious infections per 100,000 person-years, respectively, for all psoriasis patients, patients with mild disease, and patients with severe disease requiring systemic or phototherapy, said Dr. Junko Takeshita and her colleagues at the University of Pennsylvania in Philadelphia. After controlling for many potential demographic and clinical confounders, psoriasis increased the risk of serious infection by about 21% (hazard ratio, 1.21; 95% confidence interval, 1.18-1.23). Patients with severe psoriasis had a 63% greater risk of infection than patients without psoriasis, compared with an 18% increase for patients with mild psoriasis.
The findings show “serious infection, particularly respiratory and skin or soft tissue infections, to be an important and common cause of morbidity among patients with psoriasis, especially those with more severe disease,” Dr. Takeshita and her associates said. Notably, the link between psoriasis and risk of serious infection persisted after excluding patients on immunosuppressive therapies, suggesting “that the greater infection risk is at least partially attributable to more severe psoriasis, itself,” they added.
The analysis of Nationwide Inpatient Sample data was funded by the Agency for Healthcare Research and Quality and by the Dermatology Foundation. The analysis of Health Improvement Network data was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, which is part of the National Institutes of Health, and by the Dermatology Foundation. None of the investigators reported conflicts of interest.
SCOTTSDALE, ARIZ. – From enterocolitis to MRSA, serious infections are on the rise among inpatients with psoriasis, and psoriasis is an independent risk factor for serious infections, according to findings from large retrospective studies from the United States and the United Kingdom.
Inpatients with psoriasis in the United States also were at greater risk of serious infections, compared with nonpsoriatic inpatients at every time point studied, and serious infections were associated with increased hospital costs, length of stay, and risk of mortality, reported Derek Hsu, a medical student at Northwestern University, Chicago, and his associates. “Research is needed to determine how to reduce the risk of serious infections in patients with psoriasis,” the investigators emphasized.
Psoriasis affects some 7 million adults in the United States. Biologics, which are transforming the treatment landscape for moderate-to-severe psoriasis, “should reduce inherent infectious risk by controlling the inflammatory process and reducing disease severity, [but] these effects may be immunosuppressing and increase the risk of infection in other ways,” according to Mr. Hsu and his associates. For their study, they analyzed data for 2002-2012 from the Nationwide Inpatient Sample, which covers 20% of hospitalizations in the United States. They extracted validated ICD-9 codes for psoriasis and serious infections, and calculated costs of care after adjusting for 2014 inflation, based on the United States Consumer Price Index.
Overall rates of serious infection and rates of pneumonia, MRSA, septicemia, diverticulitis, enterocolitis, encephalitis, and any viral or fungal infection rose significantly among inpatients with psoriasis between 2002 and 2012 (all P-values less than .05). Predictors of serious infections among inpatients with psoriasis included diabetes mellitus, obesity, and being of non-Caucasian race or ethnicity, female, older than 60 years, and on Medicare or Medicaid, the researchers reported at the annual meeting of the Society for Investigative Dermatology.
Furthermore, after controlling for age, sex, and race, psoriasis was a significant risk factor for many different types of serious infections. Among these were cellulitis, herpes simplex virus, infectious arthritis, osteomyelitis, meningitis, influenza, encephalitis, septicemia, enterocolitis, MRSA, methicillin-sensitive Staphylococcus aureus infections, and Clostridium difficile. Further, inpatients with psoriasis were more prone to urinary tract infection, peritonitis or intestinal abscess, appendicitis, tuberculosis, and viral and fungal infections (all P-values less than .05). The average cost of hospital stay for inpatients with psoriasis was more than $2,200 greater when they were diagnosed with one or more serious infections than otherwise, and their average length of hospital stay was 2 days longer.
The study in the United Kingdom included nearly 200,000 patients with psoriasis and almost 1 million patients without psoriasis from The Health Improvement Network electronic medical record database. Between 2002 and 2013, patients without psoriasis developed an estimated 78.5 serious infections per 100,000 person-years, compared with 88.9, 85.7, and 145.7 serious infections per 100,000 person-years, respectively, for all psoriasis patients, patients with mild disease, and patients with severe disease requiring systemic or phototherapy, said Dr. Junko Takeshita and her colleagues at the University of Pennsylvania in Philadelphia. After controlling for many potential demographic and clinical confounders, psoriasis increased the risk of serious infection by about 21% (hazard ratio, 1.21; 95% confidence interval, 1.18-1.23). Patients with severe psoriasis had a 63% greater risk of infection than patients without psoriasis, compared with an 18% increase for patients with mild psoriasis.
The findings show “serious infection, particularly respiratory and skin or soft tissue infections, to be an important and common cause of morbidity among patients with psoriasis, especially those with more severe disease,” Dr. Takeshita and her associates said. Notably, the link between psoriasis and risk of serious infection persisted after excluding patients on immunosuppressive therapies, suggesting “that the greater infection risk is at least partially attributable to more severe psoriasis, itself,” they added.
The analysis of Nationwide Inpatient Sample data was funded by the Agency for Healthcare Research and Quality and by the Dermatology Foundation. The analysis of Health Improvement Network data was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, which is part of the National Institutes of Health, and by the Dermatology Foundation. None of the investigators reported conflicts of interest.
SCOTTSDALE, ARIZ. – From enterocolitis to MRSA, serious infections are on the rise among inpatients with psoriasis, and psoriasis is an independent risk factor for serious infections, according to findings from large retrospective studies from the United States and the United Kingdom.
Inpatients with psoriasis in the United States also were at greater risk of serious infections, compared with nonpsoriatic inpatients at every time point studied, and serious infections were associated with increased hospital costs, length of stay, and risk of mortality, reported Derek Hsu, a medical student at Northwestern University, Chicago, and his associates. “Research is needed to determine how to reduce the risk of serious infections in patients with psoriasis,” the investigators emphasized.
Psoriasis affects some 7 million adults in the United States. Biologics, which are transforming the treatment landscape for moderate-to-severe psoriasis, “should reduce inherent infectious risk by controlling the inflammatory process and reducing disease severity, [but] these effects may be immunosuppressing and increase the risk of infection in other ways,” according to Mr. Hsu and his associates. For their study, they analyzed data for 2002-2012 from the Nationwide Inpatient Sample, which covers 20% of hospitalizations in the United States. They extracted validated ICD-9 codes for psoriasis and serious infections, and calculated costs of care after adjusting for 2014 inflation, based on the United States Consumer Price Index.
Overall rates of serious infection and rates of pneumonia, MRSA, septicemia, diverticulitis, enterocolitis, encephalitis, and any viral or fungal infection rose significantly among inpatients with psoriasis between 2002 and 2012 (all P-values less than .05). Predictors of serious infections among inpatients with psoriasis included diabetes mellitus, obesity, and being of non-Caucasian race or ethnicity, female, older than 60 years, and on Medicare or Medicaid, the researchers reported at the annual meeting of the Society for Investigative Dermatology.
Furthermore, after controlling for age, sex, and race, psoriasis was a significant risk factor for many different types of serious infections. Among these were cellulitis, herpes simplex virus, infectious arthritis, osteomyelitis, meningitis, influenza, encephalitis, septicemia, enterocolitis, MRSA, methicillin-sensitive Staphylococcus aureus infections, and Clostridium difficile. Further, inpatients with psoriasis were more prone to urinary tract infection, peritonitis or intestinal abscess, appendicitis, tuberculosis, and viral and fungal infections (all P-values less than .05). The average cost of hospital stay for inpatients with psoriasis was more than $2,200 greater when they were diagnosed with one or more serious infections than otherwise, and their average length of hospital stay was 2 days longer.
The study in the United Kingdom included nearly 200,000 patients with psoriasis and almost 1 million patients without psoriasis from The Health Improvement Network electronic medical record database. Between 2002 and 2013, patients without psoriasis developed an estimated 78.5 serious infections per 100,000 person-years, compared with 88.9, 85.7, and 145.7 serious infections per 100,000 person-years, respectively, for all psoriasis patients, patients with mild disease, and patients with severe disease requiring systemic or phototherapy, said Dr. Junko Takeshita and her colleagues at the University of Pennsylvania in Philadelphia. After controlling for many potential demographic and clinical confounders, psoriasis increased the risk of serious infection by about 21% (hazard ratio, 1.21; 95% confidence interval, 1.18-1.23). Patients with severe psoriasis had a 63% greater risk of infection than patients without psoriasis, compared with an 18% increase for patients with mild psoriasis.
The findings show “serious infection, particularly respiratory and skin or soft tissue infections, to be an important and common cause of morbidity among patients with psoriasis, especially those with more severe disease,” Dr. Takeshita and her associates said. Notably, the link between psoriasis and risk of serious infection persisted after excluding patients on immunosuppressive therapies, suggesting “that the greater infection risk is at least partially attributable to more severe psoriasis, itself,” they added.
The analysis of Nationwide Inpatient Sample data was funded by the Agency for Healthcare Research and Quality and by the Dermatology Foundation. The analysis of Health Improvement Network data was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, which is part of the National Institutes of Health, and by the Dermatology Foundation. None of the investigators reported conflicts of interest.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: Psoriasis is an independent risk factor for serious infections, and serious infections are increasing among inpatients with psoriasis.
Major finding: Overall rates of serious infection and rates of pneumonia, MRSA, septicemia, diverticulitis, enterocolitis, encephalitis, and any viral or fungal infection rose significantly among inpatients in the United States with psoriasis between 2002 and 2012 (all P-values less than .05). In the United Kingdom during the same time period, patients with severe psoriasis had a 63% greater risk of serious infection than patients without psoriasis.
Data source: Analyses of data from the Nationwide Inpatient Sample for 2002 through 2012, and from The Health Improvement Network for 2003 through 2012.
Disclosures: The Nationwide Inpatient Sample analysis was funded by the Agency for Healthcare Research and Quality and the Dermatology Foundation. The analysis of The Health Improvement Network was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, which is part of the National Institutes of Health, and by the Dermatology Foundation. None of the investigators reported conflicts of interest.
Crisaborole’s safety holds up in long-term atopic dermatitis trial
SCOTTSDALE, ARIZ. – The phosphodiesterase-4 inhibitor crisaborole was well tolerated over 6 to 12 months, yielding no major safety signals during a multicenter, open-label extension study of patients with mild-to-moderate atopic dermatitis.
These safety results held up across age groups and over time, said Dr. Lawrence Eichenfield, a dermatologist at Children’s Hospital, San Diego, and at the University of San Diego School of Medicine. “The majority of treatment-emergent adverse events were considered mild to moderate and not related to treatment. There were no reports of long-term cutaneous reactions, such as atrophy or telangiectasia,” he and his associates added.
Atopic dermatitis has lacked widely accepted treatment options. Despite attempts to educate patients and parents about topical steroids, many are afraid to use them, and topical calcineurin inhibitors have a black box warning for cancer risk. Not surprisingly, therefore, a 2% ointment of crisaborole made headlines in 2015 after meeting its efficacy and safety endpoints in two pivotal phase III trials of patients with mild-to-moderate atopic dermatitis. Based on those results, Anacor Pharmaceuticals filed a new drug application for the novel boron-based small molecule in January 2016.
The pivotal trials lasted just 28 days, so to assess long-term safety, Dr. Eichenfield and his associates enrolled a subgroup of 517 patients aged 2 to 72 years into a single-arm, open-label, 48-week extension study of crisaborole. About 31% of participants had received the control vehicle during the pivotal trials, while the rest had received crisaborole and tolerated it well enough to continue using it. Patients applied crisaborole twice daily during treatment cycles of 28 days, and were evaluated on days 1, 8, and 29 for up to 12 treatment cycles. Patients whose skin became clear or almost clear went off treatment, but they were still assessed for adverse effects at the same frequency.
In all, 396 patients used crisaborole for at least 6 months, and 271 completed 12 months of treatment, the researchers reported at the annual meeting of the Society for Investigative Dermatology. Only nine (1.7%) patients stopped treatment during the extension study because of treatment-emergent adverse effects. A total of 65% of patients had at least one treatment-emergent adverse event during the initial phase III trials, the extension study, or both. These were usually mildly or moderately severe and included nasopharyngitis, upper respiratory infections, cough, and/or fever, all of which were considered unrelated to treatment.
Treatment-related adverse events included flares of atopic dermatitis, burning or stinging at the application site, and application site infection, which affected 3.1%, 2.3%, and 1.2%, respectively, of patients in the extension study. None of these events were considered serious. Notably, 11% of patients experienced atopic dermatitis flares in the original phase III trials, the researchers reported. Patients who could not tolerate crisaborole were excluded from the extension study, which might help explain the lower flare rate (3%) with long-term treatment.
“Crisaborole topical ointment, 2%, demonstrated a favorable long-term safety profile for the treatment of patients aged 2 years and older with mild-to-moderate atopic dermatitis,” the researchers concluded. The Food and Drug Administration accepted the new drug application in March.
Anacor Pharmaceuticals makes crisaborole and funded the study. Dr. Eichenfield has served as an investigator and consultant to Anacor. Three coinvestigators also reported affiliations with Anacor.
SCOTTSDALE, ARIZ. – The phosphodiesterase-4 inhibitor crisaborole was well tolerated over 6 to 12 months, yielding no major safety signals during a multicenter, open-label extension study of patients with mild-to-moderate atopic dermatitis.
These safety results held up across age groups and over time, said Dr. Lawrence Eichenfield, a dermatologist at Children’s Hospital, San Diego, and at the University of San Diego School of Medicine. “The majority of treatment-emergent adverse events were considered mild to moderate and not related to treatment. There were no reports of long-term cutaneous reactions, such as atrophy or telangiectasia,” he and his associates added.
Atopic dermatitis has lacked widely accepted treatment options. Despite attempts to educate patients and parents about topical steroids, many are afraid to use them, and topical calcineurin inhibitors have a black box warning for cancer risk. Not surprisingly, therefore, a 2% ointment of crisaborole made headlines in 2015 after meeting its efficacy and safety endpoints in two pivotal phase III trials of patients with mild-to-moderate atopic dermatitis. Based on those results, Anacor Pharmaceuticals filed a new drug application for the novel boron-based small molecule in January 2016.
The pivotal trials lasted just 28 days, so to assess long-term safety, Dr. Eichenfield and his associates enrolled a subgroup of 517 patients aged 2 to 72 years into a single-arm, open-label, 48-week extension study of crisaborole. About 31% of participants had received the control vehicle during the pivotal trials, while the rest had received crisaborole and tolerated it well enough to continue using it. Patients applied crisaborole twice daily during treatment cycles of 28 days, and were evaluated on days 1, 8, and 29 for up to 12 treatment cycles. Patients whose skin became clear or almost clear went off treatment, but they were still assessed for adverse effects at the same frequency.
In all, 396 patients used crisaborole for at least 6 months, and 271 completed 12 months of treatment, the researchers reported at the annual meeting of the Society for Investigative Dermatology. Only nine (1.7%) patients stopped treatment during the extension study because of treatment-emergent adverse effects. A total of 65% of patients had at least one treatment-emergent adverse event during the initial phase III trials, the extension study, or both. These were usually mildly or moderately severe and included nasopharyngitis, upper respiratory infections, cough, and/or fever, all of which were considered unrelated to treatment.
Treatment-related adverse events included flares of atopic dermatitis, burning or stinging at the application site, and application site infection, which affected 3.1%, 2.3%, and 1.2%, respectively, of patients in the extension study. None of these events were considered serious. Notably, 11% of patients experienced atopic dermatitis flares in the original phase III trials, the researchers reported. Patients who could not tolerate crisaborole were excluded from the extension study, which might help explain the lower flare rate (3%) with long-term treatment.
“Crisaborole topical ointment, 2%, demonstrated a favorable long-term safety profile for the treatment of patients aged 2 years and older with mild-to-moderate atopic dermatitis,” the researchers concluded. The Food and Drug Administration accepted the new drug application in March.
Anacor Pharmaceuticals makes crisaborole and funded the study. Dr. Eichenfield has served as an investigator and consultant to Anacor. Three coinvestigators also reported affiliations with Anacor.
SCOTTSDALE, ARIZ. – The phosphodiesterase-4 inhibitor crisaborole was well tolerated over 6 to 12 months, yielding no major safety signals during a multicenter, open-label extension study of patients with mild-to-moderate atopic dermatitis.
These safety results held up across age groups and over time, said Dr. Lawrence Eichenfield, a dermatologist at Children’s Hospital, San Diego, and at the University of San Diego School of Medicine. “The majority of treatment-emergent adverse events were considered mild to moderate and not related to treatment. There were no reports of long-term cutaneous reactions, such as atrophy or telangiectasia,” he and his associates added.
Atopic dermatitis has lacked widely accepted treatment options. Despite attempts to educate patients and parents about topical steroids, many are afraid to use them, and topical calcineurin inhibitors have a black box warning for cancer risk. Not surprisingly, therefore, a 2% ointment of crisaborole made headlines in 2015 after meeting its efficacy and safety endpoints in two pivotal phase III trials of patients with mild-to-moderate atopic dermatitis. Based on those results, Anacor Pharmaceuticals filed a new drug application for the novel boron-based small molecule in January 2016.
The pivotal trials lasted just 28 days, so to assess long-term safety, Dr. Eichenfield and his associates enrolled a subgroup of 517 patients aged 2 to 72 years into a single-arm, open-label, 48-week extension study of crisaborole. About 31% of participants had received the control vehicle during the pivotal trials, while the rest had received crisaborole and tolerated it well enough to continue using it. Patients applied crisaborole twice daily during treatment cycles of 28 days, and were evaluated on days 1, 8, and 29 for up to 12 treatment cycles. Patients whose skin became clear or almost clear went off treatment, but they were still assessed for adverse effects at the same frequency.
In all, 396 patients used crisaborole for at least 6 months, and 271 completed 12 months of treatment, the researchers reported at the annual meeting of the Society for Investigative Dermatology. Only nine (1.7%) patients stopped treatment during the extension study because of treatment-emergent adverse effects. A total of 65% of patients had at least one treatment-emergent adverse event during the initial phase III trials, the extension study, or both. These were usually mildly or moderately severe and included nasopharyngitis, upper respiratory infections, cough, and/or fever, all of which were considered unrelated to treatment.
Treatment-related adverse events included flares of atopic dermatitis, burning or stinging at the application site, and application site infection, which affected 3.1%, 2.3%, and 1.2%, respectively, of patients in the extension study. None of these events were considered serious. Notably, 11% of patients experienced atopic dermatitis flares in the original phase III trials, the researchers reported. Patients who could not tolerate crisaborole were excluded from the extension study, which might help explain the lower flare rate (3%) with long-term treatment.
“Crisaborole topical ointment, 2%, demonstrated a favorable long-term safety profile for the treatment of patients aged 2 years and older with mild-to-moderate atopic dermatitis,” the researchers concluded. The Food and Drug Administration accepted the new drug application in March.
Anacor Pharmaceuticals makes crisaborole and funded the study. Dr. Eichenfield has served as an investigator and consultant to Anacor. Three coinvestigators also reported affiliations with Anacor.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: The topical phosphodiesterase-4 inhibitor crisaborole was safe and well tolerated for up to 48 weeks in patients with mild-to-moderate atopic dermatitis.
Major finding: The most common treatment–related adverse events were atopic dermatitis flare (3%), stinging and burning at the application site (2%), and application site infection (1%). None were serious.
Data source: A single-arm, multicenter, open-label, 48-week extension study of 517 patients with mild-to-moderate atopic dermatitis.
Disclosures: Anacor Pharmaceuticals makes crisaborole and funded the study. Dr. Eichenfield has served as an investigator and consultant to Anacor. Three coinvestigators also reported affiliations with Anacor.
Study Lays Groundwork for Refractory Cutaneous Lupus Treatment Algorithms
SCOTTSDALE, ARIZ. – Thalidomide, methotrexate, dapsone, and belimumab may be the best treatment alternatives for cutaneous lupus erythematosus that is refractory to antimalarials, based on reviews of 15 years of medical records from three large tertiary care centers.
The study is the largest so far to take a comprehensive look at treatments and outcomes in hydroxychloroquine-refractory cutaneous lupus erythematosus (CLE), said Renee Fruchter, a medical student at New York University, who presented the findings at the annual meeting of the Society for Investigative Dermatology.
CLE has no approved treatments in the United States. For affected patients, quality of life is so poor that it resembles that reported by survivors of recent myocardial infarction, according to Ms. Fruchter. Patients with localized CLE can do reasonably well with sun protection and topical and intralesional treatments, but patients with more extensive disease typically need systemic treatment, most often with antimalarials, she noted. However, up to half of CLE patients are refractory to the first-line therapy, hydroxychloroquine (Plaquenil), and treatment options for this CLE subgroup are understudied.
Therefore, Ms. Fruchter and her associates reviewed medical records from patients with CLE treated between 2000 and 2015 at NYU Langone Medical Center, Brigham and Women’s Hospital, and Massachusetts General Hospital. Although the study was retrospective, they used clinical documents and medical photos, when available, to assess treatment response via the validated CLE Disease Area and Severity Index (CLASI).
Among 46 CLE patients who were refractory to hydroxychloroquine, 87% were female and 30% were African American, with an average age of 36 years, Ms. Fruchter said. Nearly three-quarters of patients (73%) had generalized CLE, while the rest had disease localized to the head and neck. As in prior studies, patients exhibited a wide range of CLE subtypes, but most commonly generalized discoid variant, Ms. Fruchter said. Nearly 30% of patients currently smoked, which also resembled prior studies of this risk factor.
Refractory patients received a wide range of systemic agents – most commonly chloroquine (Aralen) and mycophenolate mofetil (Cellcept), followed by quinacrine, dapsone, methotrexate, belimumab (Benlysta), azathioprine, thalidomide (Thalomid), lenalidomide (Revlimid), prednisone, and rituximab (Rituxan), Ms. Fruchter and her associates reported. Although treatment subgroups were small, thalidomide was most effective by far, improving disease by at least 50% in four of five treated patients. In contrast, 5 of 11 patients on methotrexate achieved at least a 50% improvement, as did 4 of 11 patients on dapsone and 3 of 9 patients on belimumab. “Other medications had lower rates of success,” Ms. Fruchter noted. Notably, CLE that failed to respond to antimalarials was also refractory to immunomodulatory therapies, she said. For example, azathioprine failed to induce a substantial clinical response in any of the patients, and only 20% of patients responded to mycophenolate mofetil.
The study also showed that switching to another antimalarial may be effective if patients do not respond to hydroxychloroquine. Fully 53% of hydroxychloroquine nonresponders had a substantial response to quinacrine, while 40% had a substantial response to chloroquine, Ms. Fruchter said.
She had no disclosures.
SCOTTSDALE, ARIZ. – Thalidomide, methotrexate, dapsone, and belimumab may be the best treatment alternatives for cutaneous lupus erythematosus that is refractory to antimalarials, based on reviews of 15 years of medical records from three large tertiary care centers.
The study is the largest so far to take a comprehensive look at treatments and outcomes in hydroxychloroquine-refractory cutaneous lupus erythematosus (CLE), said Renee Fruchter, a medical student at New York University, who presented the findings at the annual meeting of the Society for Investigative Dermatology.
CLE has no approved treatments in the United States. For affected patients, quality of life is so poor that it resembles that reported by survivors of recent myocardial infarction, according to Ms. Fruchter. Patients with localized CLE can do reasonably well with sun protection and topical and intralesional treatments, but patients with more extensive disease typically need systemic treatment, most often with antimalarials, she noted. However, up to half of CLE patients are refractory to the first-line therapy, hydroxychloroquine (Plaquenil), and treatment options for this CLE subgroup are understudied.
Therefore, Ms. Fruchter and her associates reviewed medical records from patients with CLE treated between 2000 and 2015 at NYU Langone Medical Center, Brigham and Women’s Hospital, and Massachusetts General Hospital. Although the study was retrospective, they used clinical documents and medical photos, when available, to assess treatment response via the validated CLE Disease Area and Severity Index (CLASI).
Among 46 CLE patients who were refractory to hydroxychloroquine, 87% were female and 30% were African American, with an average age of 36 years, Ms. Fruchter said. Nearly three-quarters of patients (73%) had generalized CLE, while the rest had disease localized to the head and neck. As in prior studies, patients exhibited a wide range of CLE subtypes, but most commonly generalized discoid variant, Ms. Fruchter said. Nearly 30% of patients currently smoked, which also resembled prior studies of this risk factor.
Refractory patients received a wide range of systemic agents – most commonly chloroquine (Aralen) and mycophenolate mofetil (Cellcept), followed by quinacrine, dapsone, methotrexate, belimumab (Benlysta), azathioprine, thalidomide (Thalomid), lenalidomide (Revlimid), prednisone, and rituximab (Rituxan), Ms. Fruchter and her associates reported. Although treatment subgroups were small, thalidomide was most effective by far, improving disease by at least 50% in four of five treated patients. In contrast, 5 of 11 patients on methotrexate achieved at least a 50% improvement, as did 4 of 11 patients on dapsone and 3 of 9 patients on belimumab. “Other medications had lower rates of success,” Ms. Fruchter noted. Notably, CLE that failed to respond to antimalarials was also refractory to immunomodulatory therapies, she said. For example, azathioprine failed to induce a substantial clinical response in any of the patients, and only 20% of patients responded to mycophenolate mofetil.
The study also showed that switching to another antimalarial may be effective if patients do not respond to hydroxychloroquine. Fully 53% of hydroxychloroquine nonresponders had a substantial response to quinacrine, while 40% had a substantial response to chloroquine, Ms. Fruchter said.
She had no disclosures.
SCOTTSDALE, ARIZ. – Thalidomide, methotrexate, dapsone, and belimumab may be the best treatment alternatives for cutaneous lupus erythematosus that is refractory to antimalarials, based on reviews of 15 years of medical records from three large tertiary care centers.
The study is the largest so far to take a comprehensive look at treatments and outcomes in hydroxychloroquine-refractory cutaneous lupus erythematosus (CLE), said Renee Fruchter, a medical student at New York University, who presented the findings at the annual meeting of the Society for Investigative Dermatology.
CLE has no approved treatments in the United States. For affected patients, quality of life is so poor that it resembles that reported by survivors of recent myocardial infarction, according to Ms. Fruchter. Patients with localized CLE can do reasonably well with sun protection and topical and intralesional treatments, but patients with more extensive disease typically need systemic treatment, most often with antimalarials, she noted. However, up to half of CLE patients are refractory to the first-line therapy, hydroxychloroquine (Plaquenil), and treatment options for this CLE subgroup are understudied.
Therefore, Ms. Fruchter and her associates reviewed medical records from patients with CLE treated between 2000 and 2015 at NYU Langone Medical Center, Brigham and Women’s Hospital, and Massachusetts General Hospital. Although the study was retrospective, they used clinical documents and medical photos, when available, to assess treatment response via the validated CLE Disease Area and Severity Index (CLASI).
Among 46 CLE patients who were refractory to hydroxychloroquine, 87% were female and 30% were African American, with an average age of 36 years, Ms. Fruchter said. Nearly three-quarters of patients (73%) had generalized CLE, while the rest had disease localized to the head and neck. As in prior studies, patients exhibited a wide range of CLE subtypes, but most commonly generalized discoid variant, Ms. Fruchter said. Nearly 30% of patients currently smoked, which also resembled prior studies of this risk factor.
Refractory patients received a wide range of systemic agents – most commonly chloroquine (Aralen) and mycophenolate mofetil (Cellcept), followed by quinacrine, dapsone, methotrexate, belimumab (Benlysta), azathioprine, thalidomide (Thalomid), lenalidomide (Revlimid), prednisone, and rituximab (Rituxan), Ms. Fruchter and her associates reported. Although treatment subgroups were small, thalidomide was most effective by far, improving disease by at least 50% in four of five treated patients. In contrast, 5 of 11 patients on methotrexate achieved at least a 50% improvement, as did 4 of 11 patients on dapsone and 3 of 9 patients on belimumab. “Other medications had lower rates of success,” Ms. Fruchter noted. Notably, CLE that failed to respond to antimalarials was also refractory to immunomodulatory therapies, she said. For example, azathioprine failed to induce a substantial clinical response in any of the patients, and only 20% of patients responded to mycophenolate mofetil.
The study also showed that switching to another antimalarial may be effective if patients do not respond to hydroxychloroquine. Fully 53% of hydroxychloroquine nonresponders had a substantial response to quinacrine, while 40% had a substantial response to chloroquine, Ms. Fruchter said.
She had no disclosures.
AT THE 2016 SID ANNUAL MEETING
Study lays groundwork for refractory cutaneous lupus treatment algorithms
SCOTTSDALE, ARIZ. – Thalidomide, methotrexate, dapsone, and belimumab may be the best treatment alternatives for cutaneous lupus erythematosus that is refractory to antimalarials, based on reviews of 15 years of medical records from three large tertiary care centers.
The study is the largest so far to take a comprehensive look at treatments and outcomes in hydroxychloroquine-refractory cutaneous lupus erythematosus (CLE), said Renee Fruchter, a medical student at New York University, who presented the findings at the annual meeting of the Society for Investigative Dermatology.
CLE has no approved treatments in the United States. For affected patients, quality of life is so poor that it resembles that reported by survivors of recent myocardial infarction, according to Ms. Fruchter. Patients with localized CLE can do reasonably well with sun protection and topical and intralesional treatments, but patients with more extensive disease typically need systemic treatment, most often with antimalarials, she noted. However, up to half of CLE patients are refractory to the first-line therapy, hydroxychloroquine (Plaquenil), and treatment options for this CLE subgroup are understudied.
Therefore, Ms. Fruchter and her associates reviewed medical records from patients with CLE treated between 2000 and 2015 at NYU Langone Medical Center, Brigham and Women’s Hospital, and Massachusetts General Hospital. Although the study was retrospective, they used clinical documents and medical photos, when available, to assess treatment response via the validated CLE Disease Area and Severity Index (CLASI).
Among 46 CLE patients who were refractory to hydroxychloroquine, 87% were female and 30% were African American, with an average age of 36 years, Ms. Fruchter said. Nearly three-quarters of patients (73%) had generalized CLE, while the rest had disease localized to the head and neck. As in prior studies, patients exhibited a wide range of CLE subtypes, but most commonly generalized discoid variant, Ms. Fruchter said. Nearly 30% of patients currently smoked, which also resembled prior studies of this risk factor.
Refractory patients received a wide range of systemic agents – most commonly chloroquine (Aralen) and mycophenolate mofetil (Cellcept), followed by quinacrine, dapsone, methotrexate, belimumab (Benlysta), azathioprine, thalidomide (Thalomid), lenalidomide (Revlimid), prednisone, and rituximab (Rituxan), Ms. Fruchter and her associates reported. Although treatment subgroups were small, thalidomide was most effective by far, improving disease by at least 50% in four of five treated patients. In contrast, 5 of 11 patients on methotrexate achieved at least a 50% improvement, as did 4 of 11 patients on dapsone and 3 of 9 patients on belimumab. “Other medications had lower rates of success,” Ms. Fruchter noted. Notably, CLE that failed to respond to antimalarials was also refractory to immunomodulatory therapies, she said. For example, azathioprine failed to induce a substantial clinical response in any of the patients, and only 20% of patients responded to mycophenolate mofetil.
The study also showed that switching to another antimalarial may be effective if patients do not respond to hydroxychloroquine. Fully 53% of hydroxychloroquine nonresponders had a substantial response to quinacrine, while 40% had a substantial response to chloroquine, Ms. Fruchter said.
She had no disclosures.
SCOTTSDALE, ARIZ. – Thalidomide, methotrexate, dapsone, and belimumab may be the best treatment alternatives for cutaneous lupus erythematosus that is refractory to antimalarials, based on reviews of 15 years of medical records from three large tertiary care centers.
The study is the largest so far to take a comprehensive look at treatments and outcomes in hydroxychloroquine-refractory cutaneous lupus erythematosus (CLE), said Renee Fruchter, a medical student at New York University, who presented the findings at the annual meeting of the Society for Investigative Dermatology.
CLE has no approved treatments in the United States. For affected patients, quality of life is so poor that it resembles that reported by survivors of recent myocardial infarction, according to Ms. Fruchter. Patients with localized CLE can do reasonably well with sun protection and topical and intralesional treatments, but patients with more extensive disease typically need systemic treatment, most often with antimalarials, she noted. However, up to half of CLE patients are refractory to the first-line therapy, hydroxychloroquine (Plaquenil), and treatment options for this CLE subgroup are understudied.
Therefore, Ms. Fruchter and her associates reviewed medical records from patients with CLE treated between 2000 and 2015 at NYU Langone Medical Center, Brigham and Women’s Hospital, and Massachusetts General Hospital. Although the study was retrospective, they used clinical documents and medical photos, when available, to assess treatment response via the validated CLE Disease Area and Severity Index (CLASI).
Among 46 CLE patients who were refractory to hydroxychloroquine, 87% were female and 30% were African American, with an average age of 36 years, Ms. Fruchter said. Nearly three-quarters of patients (73%) had generalized CLE, while the rest had disease localized to the head and neck. As in prior studies, patients exhibited a wide range of CLE subtypes, but most commonly generalized discoid variant, Ms. Fruchter said. Nearly 30% of patients currently smoked, which also resembled prior studies of this risk factor.
Refractory patients received a wide range of systemic agents – most commonly chloroquine (Aralen) and mycophenolate mofetil (Cellcept), followed by quinacrine, dapsone, methotrexate, belimumab (Benlysta), azathioprine, thalidomide (Thalomid), lenalidomide (Revlimid), prednisone, and rituximab (Rituxan), Ms. Fruchter and her associates reported. Although treatment subgroups were small, thalidomide was most effective by far, improving disease by at least 50% in four of five treated patients. In contrast, 5 of 11 patients on methotrexate achieved at least a 50% improvement, as did 4 of 11 patients on dapsone and 3 of 9 patients on belimumab. “Other medications had lower rates of success,” Ms. Fruchter noted. Notably, CLE that failed to respond to antimalarials was also refractory to immunomodulatory therapies, she said. For example, azathioprine failed to induce a substantial clinical response in any of the patients, and only 20% of patients responded to mycophenolate mofetil.
The study also showed that switching to another antimalarial may be effective if patients do not respond to hydroxychloroquine. Fully 53% of hydroxychloroquine nonresponders had a substantial response to quinacrine, while 40% had a substantial response to chloroquine, Ms. Fruchter said.
She had no disclosures.
SCOTTSDALE, ARIZ. – Thalidomide, methotrexate, dapsone, and belimumab may be the best treatment alternatives for cutaneous lupus erythematosus that is refractory to antimalarials, based on reviews of 15 years of medical records from three large tertiary care centers.
The study is the largest so far to take a comprehensive look at treatments and outcomes in hydroxychloroquine-refractory cutaneous lupus erythematosus (CLE), said Renee Fruchter, a medical student at New York University, who presented the findings at the annual meeting of the Society for Investigative Dermatology.
CLE has no approved treatments in the United States. For affected patients, quality of life is so poor that it resembles that reported by survivors of recent myocardial infarction, according to Ms. Fruchter. Patients with localized CLE can do reasonably well with sun protection and topical and intralesional treatments, but patients with more extensive disease typically need systemic treatment, most often with antimalarials, she noted. However, up to half of CLE patients are refractory to the first-line therapy, hydroxychloroquine (Plaquenil), and treatment options for this CLE subgroup are understudied.
Therefore, Ms. Fruchter and her associates reviewed medical records from patients with CLE treated between 2000 and 2015 at NYU Langone Medical Center, Brigham and Women’s Hospital, and Massachusetts General Hospital. Although the study was retrospective, they used clinical documents and medical photos, when available, to assess treatment response via the validated CLE Disease Area and Severity Index (CLASI).
Among 46 CLE patients who were refractory to hydroxychloroquine, 87% were female and 30% were African American, with an average age of 36 years, Ms. Fruchter said. Nearly three-quarters of patients (73%) had generalized CLE, while the rest had disease localized to the head and neck. As in prior studies, patients exhibited a wide range of CLE subtypes, but most commonly generalized discoid variant, Ms. Fruchter said. Nearly 30% of patients currently smoked, which also resembled prior studies of this risk factor.
Refractory patients received a wide range of systemic agents – most commonly chloroquine (Aralen) and mycophenolate mofetil (Cellcept), followed by quinacrine, dapsone, methotrexate, belimumab (Benlysta), azathioprine, thalidomide (Thalomid), lenalidomide (Revlimid), prednisone, and rituximab (Rituxan), Ms. Fruchter and her associates reported. Although treatment subgroups were small, thalidomide was most effective by far, improving disease by at least 50% in four of five treated patients. In contrast, 5 of 11 patients on methotrexate achieved at least a 50% improvement, as did 4 of 11 patients on dapsone and 3 of 9 patients on belimumab. “Other medications had lower rates of success,” Ms. Fruchter noted. Notably, CLE that failed to respond to antimalarials was also refractory to immunomodulatory therapies, she said. For example, azathioprine failed to induce a substantial clinical response in any of the patients, and only 20% of patients responded to mycophenolate mofetil.
The study also showed that switching to another antimalarial may be effective if patients do not respond to hydroxychloroquine. Fully 53% of hydroxychloroquine nonresponders had a substantial response to quinacrine, while 40% had a substantial response to chloroquine, Ms. Fruchter said.
She had no disclosures.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: Thalidomide, methotrexate, dapsone, and belimumab may be the best alternatives for patients whose cutaneous lupus erythematosus is refractory to antimalarials.
Major finding: Four of five patients treated with thalidomide achieved a response rate of at least 50%.
Data source: A retrospective study of 15 years of medical records from three tertiary care centers.
Disclosures: Ms. Fruchter had no disclosures.