User login
Hepatitis B virus (HBV) infection is highly prevalent worldwide and is a major cause of morbidity and death. Two billion people globally have been infected with HBV, 350 to 400 million are chronic carriers, and tens of millions of new cases occur annually. Of those infected, 15% to 40% develop HBV complications, namely cirrhosis or hepatocellular carcinoma (HCC).1–3
The high prevalence of HBV infection represents an enormous failure of public health, considering that HBV immunization has been available for an entire generation, and where it has been employed it has been highly effective at reducing the incidence of HBV infection. Immunization, however, has been underused.
This supplement to the Cleveland Clinic Journal of Medicine, derived from a live symposium, aims to enhance awareness of the natural history of HBV infection and clarify its management recommendations with illustrative case histories. The supplement starts with a brief review of HBV terminology, natural history, and epidemiology.
CHRONIC HBV INFECTION TERMINOLOGY
Familiarity with the terms commonly used to describe chronic HBV infection will help clinicians in the management of the disease4:
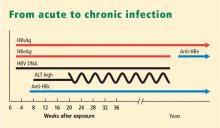
- Chronic HBV infection is defined as presence of hepatitis B surface antigen (HBsAg) for more than 6 months. Those with infection may also express another antigen, HB e antigen (HBeAg), a marker of heightened infectivity. At the same time, those who are HBeAg positive are better responders to antiviral therapy compared with those who are HBeAg negative.
- An inactive HBsAg carrier is an individual who is HBsAg positive with a very low level of circulating virus, liver enzyme levels within normal limits, and a low likelihood of having chronic progressive disease.
- Resolved HBV infection is defined as previous HBV infection with no remaining evidence of active disease. Such individuals test negative for HBsAg and positive for antibody to HBsAg (anti-HBs) and to HB core antigen (anti-HBc). They also have no detectable viral load, or HBV DNA, in their blood. In most instances, they are protected from reinfection.
- Reactivation is the reappearance of HBV infection in someone who is known to be an inactive HBsAg carrier or whose previous HBV infection had resolved (see “Case: Recurrence despite anti-HBs and HBsAg negativity”).
- HBeAg seroconversion is the transition from HBeAg-positive to HBeAg-negative status and development of antibody to HBeAg (anti-HBe), usually accompanied by less active liver disease and lower viral loads.
- HBeAg clearance is disappearance of HBeAg without the development of anti-HBe; reactivation or reversion to HBeAg-positive status can occur.
GEOGRAPHIC DISTRIBUTION OF CHRONIC HBV INFECTION
The global prevalence of HBV varies widely. Regions are divided into areas of low, intermediate, and high prevalence, defined as follows4:
- High prevalence implies that at least 8% of the population is currently infected, with a lifetime likelihood of active or resolved infection greater than 60%. About 45% of the world’s population lives in regions of high prevalence. Among this group, early childhood infections are common, with the virus usually transmitted from mother to infant during the perinatal period.
- Intermediate prevalence is defined as 2% to 7%, with a lifetime risk of infection of 20% to 60%. These regions represent about 43% of the global population. In intermediate-prevalence areas, infections occur in all age groups.
- Low prevalence is defined as less than 2% and represents only 12% of the global population. In these regions, the lifetime risk of infection is less than 20%.
North America is a low-prevalence area except for the northern rim, where Inuit and Yupik Eskimos have a high prevalence, and communities that have a substantial immigrant population from high-prevalence areas, such as sub-Saharan Africa and many parts of Asia.
Chronic HBV infection in the United States
Approximately 1.25 million individuals in the United States are HBsAg carriers.2,4 In Asian Americans and Alaskan natives, the prevalence of HBsAg positivity, or chronic disease, is 5% to 15%.5,6 Similarly, US health statistics sources estimate that among those who are chronically infected, approximately half are Asian American.7 As the Asian American population continues to increase (1.5 million to 7 million from 1970 to 19905,8; 11.9 million in the 2000 US Census8), the total prevalence of chronic HBV infection will increase as well.
NATURAL HISTORY OF CHRONIC HBV INFECTION
Chronic HBV usually causes microinflammatory changes that evoke a fibrotic response in the liver, and many infected individuals will eventually develop cirrhosis and are at risk for the development of HCC. Inactive HBsAg carriers often bypass the development of cirrhosis but remain at risk for HCC if their viral load is very high. This is particularly true when infection is acquired in infancy.
The age at acquisition of HBV has a large impact on the likelihood of the disease becoming chronic. The chance of chronic infection is 90% or greater among neonates who become infected with HBV through perinatal transmission. Exposure during adolescence or young adulthood is associated with a 95% or greater likelihood that the disease will be self-limiting.
The typical North American patient with HBV acquires the infection as an adolescent or young adult and is not at risk of HCC unless cirrhosis develops. In most patients who acquire the disease in adolescence or adulthood, the infection resolves after weeks or a few months and they are not at risk of either cirrhosis or HCC. However, an individual such as the one described in the accompanying case, who becomes immunocompromised, is at risk of reactivation of HBV infection (see “Case revisited”).
HBV MODES OF TRANSMISSION
In low-prevalence areas, such as most of North America, most cases of HBV infection are acquired during adolescence to midadulthood, a period during which behaviors that increase the risk of HBV infection (ie, intravenous drug abuse or unprotected sexual activity) are most likely.9,10 Sex workers and homosexuals are at particular risk of sexual transmission of HBV. Intravenous drug abusers and health workers are at risk of parenteral transmission.
In high-prevalence areas, HBV is mostly transmitted during the perinatal period from mother to infant, conferring a high likelihood of chronicity.9,10 Mothers who are HBsAg positive, particularly those who are also HBeAg positive, are much more likely than others to transmit HBV to their offspring.
FACTORS THAT INFLUENCE THE COURSE OF HBV INFECTION
Viral load has emerged as the most significant factor implicated in the development of cirrhosis or HCC. Iloeje et al11 found that viral load predicted progression to cirrhosis among a cohort of nearly 4,000 Taiwanese. Other factors that can influence the course of HBV infection include age at onset, male sex, and comorbidities (ie, alcohol use, human immunodeficiency virus infection, hepatitis C virus infection). Core promoter and precore mutants may affect the likelihood of developing HCC. A genetic signature that predisposes liver cells to proliferate, termed field effects, may also lead to the development of HCC. The influence of smoking and diabetes on the development of HCC in HBV-infected individuals is not well documented.
Reduction or elimination of measurable virus is the current holy grail of treatment; available antiviral therapies are potent tools that lower viral load with the hope of reducing the likelihood of either cirrhosis or HCC.
HBV genotypes may be implicated in the progression of liver disease or the risk of development of HCC. HBV genotypes differ by region and may correlate with ethnicity and disease progression. In a study of 694 US patients with chronic HBV, Chu et al12 found that genotypes A and C were associated with a higher prevalence of HBsAg positivity than other genotypes. Genotypes B and C were the most common among Asian American patients, while genotype A was the most common among Caucasian and African American patients. The authors suggested that HBV genotypes may explain the heterogeneity in the manifestation of the disease.
- Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat 2004; 11:97–107.
- Hepatitis B Foundation. Statistics. Hepatitis B Foundation Web site. http://www.hepb.org/hepb/statistics.htm. Published 2003–2008. Accessed January 9, 2009.
- Hepatitis Foundation International. The ABC’s of hepatitis. Hepatitis Foundation International Web site. http://www.hepfi.org/living/liv_abc.html. Published 2003. Accessed January 9, 2009.
- Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology 2007; 45:507–539.
- Tong MJ, Hwang S-J. Hepatitis B virus infection in Asian Americans. Gastroenterol Clin North Am 1994; 23:523–536.
- McMahon BJ, Schoenberg S, Bulkow L, et al. Seroprevalence of hepatitis B viral markers in 52,000 Alaska natives. Am J Epidemiol 1993; 138:544–549.
- US Department of Health and Human Services. Hepatitis and Asian Americans. The Office of Minority Health Web site. http://www.omhrc.gov/templates/content.aspx?lvl=3&lvlid=541&ID=6495. Updated May 5, 2008. Accessed January 12, 2009.
- Barnes JS, Bennett CE. The Asian population: 2000. Census 2000 brief. United States Census 2000 Web site. http://www.census.gov/prod/2002pubs/c2kbr01-16.pdf. Published February 2002. Accessed January 12, 2009.
- Lok AS, McMahon BJ; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Chronic hepatitis B. Hepatology 2001; 34:1225–1241.
- Lok AS, Heathcote EJ, Hoofnagle JH. Management of hepatitis B: 2000—summary of a workshop. Gastroenterology 2001; 120:1828–1853.
- Iloeje UH, Yang H-I, Su J, Jen C-L, You S-L, Chen C-J, and The Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-in HBV (the REVEAL-HBV) Study Group. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology 2006; 130:678–686.
- Chu CJ, Keeffe EB, Han SH, et al. Hepatitis B virus genotypes in the United States: results of a nationwide study. Gastroenterology 2003; 125:444–451.
Hepatitis B virus (HBV) infection is highly prevalent worldwide and is a major cause of morbidity and death. Two billion people globally have been infected with HBV, 350 to 400 million are chronic carriers, and tens of millions of new cases occur annually. Of those infected, 15% to 40% develop HBV complications, namely cirrhosis or hepatocellular carcinoma (HCC).1–3
The high prevalence of HBV infection represents an enormous failure of public health, considering that HBV immunization has been available for an entire generation, and where it has been employed it has been highly effective at reducing the incidence of HBV infection. Immunization, however, has been underused.
This supplement to the Cleveland Clinic Journal of Medicine, derived from a live symposium, aims to enhance awareness of the natural history of HBV infection and clarify its management recommendations with illustrative case histories. The supplement starts with a brief review of HBV terminology, natural history, and epidemiology.
CHRONIC HBV INFECTION TERMINOLOGY
Familiarity with the terms commonly used to describe chronic HBV infection will help clinicians in the management of the disease4:
- Chronic HBV infection is defined as presence of hepatitis B surface antigen (HBsAg) for more than 6 months. Those with infection may also express another antigen, HB e antigen (HBeAg), a marker of heightened infectivity. At the same time, those who are HBeAg positive are better responders to antiviral therapy compared with those who are HBeAg negative.
- An inactive HBsAg carrier is an individual who is HBsAg positive with a very low level of circulating virus, liver enzyme levels within normal limits, and a low likelihood of having chronic progressive disease.
- Resolved HBV infection is defined as previous HBV infection with no remaining evidence of active disease. Such individuals test negative for HBsAg and positive for antibody to HBsAg (anti-HBs) and to HB core antigen (anti-HBc). They also have no detectable viral load, or HBV DNA, in their blood. In most instances, they are protected from reinfection.
- Reactivation is the reappearance of HBV infection in someone who is known to be an inactive HBsAg carrier or whose previous HBV infection had resolved (see “Case: Recurrence despite anti-HBs and HBsAg negativity”).
- HBeAg seroconversion is the transition from HBeAg-positive to HBeAg-negative status and development of antibody to HBeAg (anti-HBe), usually accompanied by less active liver disease and lower viral loads.
- HBeAg clearance is disappearance of HBeAg without the development of anti-HBe; reactivation or reversion to HBeAg-positive status can occur.

GEOGRAPHIC DISTRIBUTION OF CHRONIC HBV INFECTION
The global prevalence of HBV varies widely. Regions are divided into areas of low, intermediate, and high prevalence, defined as follows4:
- High prevalence implies that at least 8% of the population is currently infected, with a lifetime likelihood of active or resolved infection greater than 60%. About 45% of the world’s population lives in regions of high prevalence. Among this group, early childhood infections are common, with the virus usually transmitted from mother to infant during the perinatal period.
- Intermediate prevalence is defined as 2% to 7%, with a lifetime risk of infection of 20% to 60%. These regions represent about 43% of the global population. In intermediate-prevalence areas, infections occur in all age groups.
- Low prevalence is defined as less than 2% and represents only 12% of the global population. In these regions, the lifetime risk of infection is less than 20%.
North America is a low-prevalence area except for the northern rim, where Inuit and Yupik Eskimos have a high prevalence, and communities that have a substantial immigrant population from high-prevalence areas, such as sub-Saharan Africa and many parts of Asia.
Chronic HBV infection in the United States
Approximately 1.25 million individuals in the United States are HBsAg carriers.2,4 In Asian Americans and Alaskan natives, the prevalence of HBsAg positivity, or chronic disease, is 5% to 15%.5,6 Similarly, US health statistics sources estimate that among those who are chronically infected, approximately half are Asian American.7 As the Asian American population continues to increase (1.5 million to 7 million from 1970 to 19905,8; 11.9 million in the 2000 US Census8), the total prevalence of chronic HBV infection will increase as well.
NATURAL HISTORY OF CHRONIC HBV INFECTION
Chronic HBV usually causes microinflammatory changes that evoke a fibrotic response in the liver, and many infected individuals will eventually develop cirrhosis and are at risk for the development of HCC. Inactive HBsAg carriers often bypass the development of cirrhosis but remain at risk for HCC if their viral load is very high. This is particularly true when infection is acquired in infancy.
The age at acquisition of HBV has a large impact on the likelihood of the disease becoming chronic. The chance of chronic infection is 90% or greater among neonates who become infected with HBV through perinatal transmission. Exposure during adolescence or young adulthood is associated with a 95% or greater likelihood that the disease will be self-limiting.
The typical North American patient with HBV acquires the infection as an adolescent or young adult and is not at risk of HCC unless cirrhosis develops. In most patients who acquire the disease in adolescence or adulthood, the infection resolves after weeks or a few months and they are not at risk of either cirrhosis or HCC. However, an individual such as the one described in the accompanying case, who becomes immunocompromised, is at risk of reactivation of HBV infection (see “Case revisited”).

HBV MODES OF TRANSMISSION
In low-prevalence areas, such as most of North America, most cases of HBV infection are acquired during adolescence to midadulthood, a period during which behaviors that increase the risk of HBV infection (ie, intravenous drug abuse or unprotected sexual activity) are most likely.9,10 Sex workers and homosexuals are at particular risk of sexual transmission of HBV. Intravenous drug abusers and health workers are at risk of parenteral transmission.
In high-prevalence areas, HBV is mostly transmitted during the perinatal period from mother to infant, conferring a high likelihood of chronicity.9,10 Mothers who are HBsAg positive, particularly those who are also HBeAg positive, are much more likely than others to transmit HBV to their offspring.
FACTORS THAT INFLUENCE THE COURSE OF HBV INFECTION
Viral load has emerged as the most significant factor implicated in the development of cirrhosis or HCC. Iloeje et al11 found that viral load predicted progression to cirrhosis among a cohort of nearly 4,000 Taiwanese. Other factors that can influence the course of HBV infection include age at onset, male sex, and comorbidities (ie, alcohol use, human immunodeficiency virus infection, hepatitis C virus infection). Core promoter and precore mutants may affect the likelihood of developing HCC. A genetic signature that predisposes liver cells to proliferate, termed field effects, may also lead to the development of HCC. The influence of smoking and diabetes on the development of HCC in HBV-infected individuals is not well documented.
Reduction or elimination of measurable virus is the current holy grail of treatment; available antiviral therapies are potent tools that lower viral load with the hope of reducing the likelihood of either cirrhosis or HCC.
HBV genotypes may be implicated in the progression of liver disease or the risk of development of HCC. HBV genotypes differ by region and may correlate with ethnicity and disease progression. In a study of 694 US patients with chronic HBV, Chu et al12 found that genotypes A and C were associated with a higher prevalence of HBsAg positivity than other genotypes. Genotypes B and C were the most common among Asian American patients, while genotype A was the most common among Caucasian and African American patients. The authors suggested that HBV genotypes may explain the heterogeneity in the manifestation of the disease.
Hepatitis B virus (HBV) infection is highly prevalent worldwide and is a major cause of morbidity and death. Two billion people globally have been infected with HBV, 350 to 400 million are chronic carriers, and tens of millions of new cases occur annually. Of those infected, 15% to 40% develop HBV complications, namely cirrhosis or hepatocellular carcinoma (HCC).1–3
The high prevalence of HBV infection represents an enormous failure of public health, considering that HBV immunization has been available for an entire generation, and where it has been employed it has been highly effective at reducing the incidence of HBV infection. Immunization, however, has been underused.
This supplement to the Cleveland Clinic Journal of Medicine, derived from a live symposium, aims to enhance awareness of the natural history of HBV infection and clarify its management recommendations with illustrative case histories. The supplement starts with a brief review of HBV terminology, natural history, and epidemiology.
CHRONIC HBV INFECTION TERMINOLOGY
Familiarity with the terms commonly used to describe chronic HBV infection will help clinicians in the management of the disease4:
- Chronic HBV infection is defined as presence of hepatitis B surface antigen (HBsAg) for more than 6 months. Those with infection may also express another antigen, HB e antigen (HBeAg), a marker of heightened infectivity. At the same time, those who are HBeAg positive are better responders to antiviral therapy compared with those who are HBeAg negative.
- An inactive HBsAg carrier is an individual who is HBsAg positive with a very low level of circulating virus, liver enzyme levels within normal limits, and a low likelihood of having chronic progressive disease.
- Resolved HBV infection is defined as previous HBV infection with no remaining evidence of active disease. Such individuals test negative for HBsAg and positive for antibody to HBsAg (anti-HBs) and to HB core antigen (anti-HBc). They also have no detectable viral load, or HBV DNA, in their blood. In most instances, they are protected from reinfection.
- Reactivation is the reappearance of HBV infection in someone who is known to be an inactive HBsAg carrier or whose previous HBV infection had resolved (see “Case: Recurrence despite anti-HBs and HBsAg negativity”).
- HBeAg seroconversion is the transition from HBeAg-positive to HBeAg-negative status and development of antibody to HBeAg (anti-HBe), usually accompanied by less active liver disease and lower viral loads.
- HBeAg clearance is disappearance of HBeAg without the development of anti-HBe; reactivation or reversion to HBeAg-positive status can occur.

GEOGRAPHIC DISTRIBUTION OF CHRONIC HBV INFECTION
The global prevalence of HBV varies widely. Regions are divided into areas of low, intermediate, and high prevalence, defined as follows4:
- High prevalence implies that at least 8% of the population is currently infected, with a lifetime likelihood of active or resolved infection greater than 60%. About 45% of the world’s population lives in regions of high prevalence. Among this group, early childhood infections are common, with the virus usually transmitted from mother to infant during the perinatal period.
- Intermediate prevalence is defined as 2% to 7%, with a lifetime risk of infection of 20% to 60%. These regions represent about 43% of the global population. In intermediate-prevalence areas, infections occur in all age groups.
- Low prevalence is defined as less than 2% and represents only 12% of the global population. In these regions, the lifetime risk of infection is less than 20%.
North America is a low-prevalence area except for the northern rim, where Inuit and Yupik Eskimos have a high prevalence, and communities that have a substantial immigrant population from high-prevalence areas, such as sub-Saharan Africa and many parts of Asia.
Chronic HBV infection in the United States
Approximately 1.25 million individuals in the United States are HBsAg carriers.2,4 In Asian Americans and Alaskan natives, the prevalence of HBsAg positivity, or chronic disease, is 5% to 15%.5,6 Similarly, US health statistics sources estimate that among those who are chronically infected, approximately half are Asian American.7 As the Asian American population continues to increase (1.5 million to 7 million from 1970 to 19905,8; 11.9 million in the 2000 US Census8), the total prevalence of chronic HBV infection will increase as well.
NATURAL HISTORY OF CHRONIC HBV INFECTION
Chronic HBV usually causes microinflammatory changes that evoke a fibrotic response in the liver, and many infected individuals will eventually develop cirrhosis and are at risk for the development of HCC. Inactive HBsAg carriers often bypass the development of cirrhosis but remain at risk for HCC if their viral load is very high. This is particularly true when infection is acquired in infancy.
The age at acquisition of HBV has a large impact on the likelihood of the disease becoming chronic. The chance of chronic infection is 90% or greater among neonates who become infected with HBV through perinatal transmission. Exposure during adolescence or young adulthood is associated with a 95% or greater likelihood that the disease will be self-limiting.
The typical North American patient with HBV acquires the infection as an adolescent or young adult and is not at risk of HCC unless cirrhosis develops. In most patients who acquire the disease in adolescence or adulthood, the infection resolves after weeks or a few months and they are not at risk of either cirrhosis or HCC. However, an individual such as the one described in the accompanying case, who becomes immunocompromised, is at risk of reactivation of HBV infection (see “Case revisited”).

HBV MODES OF TRANSMISSION
In low-prevalence areas, such as most of North America, most cases of HBV infection are acquired during adolescence to midadulthood, a period during which behaviors that increase the risk of HBV infection (ie, intravenous drug abuse or unprotected sexual activity) are most likely.9,10 Sex workers and homosexuals are at particular risk of sexual transmission of HBV. Intravenous drug abusers and health workers are at risk of parenteral transmission.
In high-prevalence areas, HBV is mostly transmitted during the perinatal period from mother to infant, conferring a high likelihood of chronicity.9,10 Mothers who are HBsAg positive, particularly those who are also HBeAg positive, are much more likely than others to transmit HBV to their offspring.
FACTORS THAT INFLUENCE THE COURSE OF HBV INFECTION
Viral load has emerged as the most significant factor implicated in the development of cirrhosis or HCC. Iloeje et al11 found that viral load predicted progression to cirrhosis among a cohort of nearly 4,000 Taiwanese. Other factors that can influence the course of HBV infection include age at onset, male sex, and comorbidities (ie, alcohol use, human immunodeficiency virus infection, hepatitis C virus infection). Core promoter and precore mutants may affect the likelihood of developing HCC. A genetic signature that predisposes liver cells to proliferate, termed field effects, may also lead to the development of HCC. The influence of smoking and diabetes on the development of HCC in HBV-infected individuals is not well documented.
Reduction or elimination of measurable virus is the current holy grail of treatment; available antiviral therapies are potent tools that lower viral load with the hope of reducing the likelihood of either cirrhosis or HCC.
HBV genotypes may be implicated in the progression of liver disease or the risk of development of HCC. HBV genotypes differ by region and may correlate with ethnicity and disease progression. In a study of 694 US patients with chronic HBV, Chu et al12 found that genotypes A and C were associated with a higher prevalence of HBsAg positivity than other genotypes. Genotypes B and C were the most common among Asian American patients, while genotype A was the most common among Caucasian and African American patients. The authors suggested that HBV genotypes may explain the heterogeneity in the manifestation of the disease.
- Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat 2004; 11:97–107.
- Hepatitis B Foundation. Statistics. Hepatitis B Foundation Web site. http://www.hepb.org/hepb/statistics.htm. Published 2003–2008. Accessed January 9, 2009.
- Hepatitis Foundation International. The ABC’s of hepatitis. Hepatitis Foundation International Web site. http://www.hepfi.org/living/liv_abc.html. Published 2003. Accessed January 9, 2009.
- Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology 2007; 45:507–539.
- Tong MJ, Hwang S-J. Hepatitis B virus infection in Asian Americans. Gastroenterol Clin North Am 1994; 23:523–536.
- McMahon BJ, Schoenberg S, Bulkow L, et al. Seroprevalence of hepatitis B viral markers in 52,000 Alaska natives. Am J Epidemiol 1993; 138:544–549.
- US Department of Health and Human Services. Hepatitis and Asian Americans. The Office of Minority Health Web site. http://www.omhrc.gov/templates/content.aspx?lvl=3&lvlid=541&ID=6495. Updated May 5, 2008. Accessed January 12, 2009.
- Barnes JS, Bennett CE. The Asian population: 2000. Census 2000 brief. United States Census 2000 Web site. http://www.census.gov/prod/2002pubs/c2kbr01-16.pdf. Published February 2002. Accessed January 12, 2009.
- Lok AS, McMahon BJ; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Chronic hepatitis B. Hepatology 2001; 34:1225–1241.
- Lok AS, Heathcote EJ, Hoofnagle JH. Management of hepatitis B: 2000—summary of a workshop. Gastroenterology 2001; 120:1828–1853.
- Iloeje UH, Yang H-I, Su J, Jen C-L, You S-L, Chen C-J, and The Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-in HBV (the REVEAL-HBV) Study Group. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology 2006; 130:678–686.
- Chu CJ, Keeffe EB, Han SH, et al. Hepatitis B virus genotypes in the United States: results of a nationwide study. Gastroenterology 2003; 125:444–451.
- Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat 2004; 11:97–107.
- Hepatitis B Foundation. Statistics. Hepatitis B Foundation Web site. http://www.hepb.org/hepb/statistics.htm. Published 2003–2008. Accessed January 9, 2009.
- Hepatitis Foundation International. The ABC’s of hepatitis. Hepatitis Foundation International Web site. http://www.hepfi.org/living/liv_abc.html. Published 2003. Accessed January 9, 2009.
- Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology 2007; 45:507–539.
- Tong MJ, Hwang S-J. Hepatitis B virus infection in Asian Americans. Gastroenterol Clin North Am 1994; 23:523–536.
- McMahon BJ, Schoenberg S, Bulkow L, et al. Seroprevalence of hepatitis B viral markers in 52,000 Alaska natives. Am J Epidemiol 1993; 138:544–549.
- US Department of Health and Human Services. Hepatitis and Asian Americans. The Office of Minority Health Web site. http://www.omhrc.gov/templates/content.aspx?lvl=3&lvlid=541&ID=6495. Updated May 5, 2008. Accessed January 12, 2009.
- Barnes JS, Bennett CE. The Asian population: 2000. Census 2000 brief. United States Census 2000 Web site. http://www.census.gov/prod/2002pubs/c2kbr01-16.pdf. Published February 2002. Accessed January 12, 2009.
- Lok AS, McMahon BJ; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Chronic hepatitis B. Hepatology 2001; 34:1225–1241.
- Lok AS, Heathcote EJ, Hoofnagle JH. Management of hepatitis B: 2000—summary of a workshop. Gastroenterology 2001; 120:1828–1853.
- Iloeje UH, Yang H-I, Su J, Jen C-L, You S-L, Chen C-J, and The Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-in HBV (the REVEAL-HBV) Study Group. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology 2006; 130:678–686.
- Chu CJ, Keeffe EB, Han SH, et al. Hepatitis B virus genotypes in the United States: results of a nationwide study. Gastroenterology 2003; 125:444–451.
KEY POINTS
- The prevalence of chronic HBV infection in the United States is expected to increase as Asian immigrants constitute a larger proportion of the US population.
- The chance of chronic infection is 90% or greater with perinatal transmission; conversely, the risk of chronic disease is less than 10% with adult-acquired infection.
- In addition to viral load, predictors of disease progression include age at onset, male sex, and comorbidities.