User login
The management of hepatitis B virus (HBV) infection in pregnancy is complex. Because infection with HBV in infancy often leads to chronic disease, prevention of perinatal, or vertical, transmission is a worthy goal; yet, prophylactic therapy during pregnancy is not well studied. This article explores the consequences of HBV infection during pregnancy, the specific risks imposed by high viral load, the evidence to support preemptive antiviral therapy, and the timing of therapy during pregnancy.
PERINATAL TRANSMISSION
Perinatal transmission is the most common mode of HBV transmission worldwide; however, the maternal screening programs and universal vaccination in newborns with active and passive immunoprophylaxis have dramatically reduced HBV transmission rates. According to recent data from the US Centers for Disease Control and Prevention, prenatal screening for hepatitis B surface antigen (HBsAg) in the United States is nearly universal; 97% of pregnant women undergo screening before delivery.1 Further, among infants at risk of acquiring HBV infection, 92% complete the three-dose vaccination series by the time they are 3 years old. There is some nationwide variation, however, in the appropriate administration of immunoprophylaxis to infants exposed perinatally, ranging from 78% in Louisiana to 99.8% in one California health maintenance organization.2
Perinatal transmission of HBV infection has declined steadily in the United States over the past 2 decades, consistent with the successful implementation of universal screening of pregnant women and vaccination policies.3 Outside the United States, however, many high-prevalence countries lack vaccination coverage and perinatal transmission is common. In 87 countries with a prevalence of HBV infection that exceeds 8%, the infant vaccine coverage was only 36%.4
Risk of chronic infection
The risk of progression to chronic HBV infection is inversely proportional to the age at which the infection was acquired. Without immunoprophylaxis, up to 90% of infants born to hepatitis B e antigen (HBeAg)-positive mothers become HBV carriers. In comparison, 20% to 30% of children infected between age 1 year and 5 years, and fewer than 5% of immunocompetent adults, become HBV carriers.5–7
If the mother is positive for both HBsAg and HBeAg and her baby does not receive immunoprophylaxis, the risk of the baby developing chronic HBV infection by age 6 months is 70% to 90%.8–10 Of those exposed in early childhood, 28.8% are HBsAg positive by age 4 years.5 These data underscore the need for early vaccination.
In a study of 402 HBsAg-positive pregnant women in China, Xu et al11 found that 3.7% of their newborn infants were HBsAg positive within 24 hours of birth. Of the women who were HBeAg positive, the intrauterine infection rate was 9.8%. Analysis of placental tissue for HBsAg, hepatitis B core antigen (HBcAg), and viral load (HBV DNA) uncovered an overall placental infection rate of 44.6%.
Transplacental transmission of HBV has been observed in multiple studies, especially when mothers are positive for HBsAg and HBeAg and have high viral loads. Among mothers positive for HBeAg, Burk et al12 found an odds ratio of 147 for a persistently infected infant when the maternal HBV DNA level was at least 1.4 ng/mL compared with less than .005 ng/mL. Among the HBeAg-negative mothers, the odds ratio for a persistently infected infant was 19.2 with high versus low maternal HBV DNA levels.
Importance of maternal viremia
Despite successful screening and vaccination programs, high maternal HBV DNA correlates in some studies with perinatal transmission. Wiseman et al13 studied 298 chronically HBV-infected women and their infants, who were tested for HBV at age 9 months. Interim analysis showed a transmission rate of 8.5% for infants born to mothers with virus levels greater than 8 log10 copies/mL. These data suggest that perinatal transmission may still be occurring despite the use of effective active and passive immunoprophylaxis. Additional studies are needed to assess the potential risk reduction associated with treatment of high maternal viremia during pregnancy.
Maternal HBV DNA positivity was associated with a high rate of intrauterine transmission of HBV in a program in India in which 11,524 woman were screened for HBV infection.14 Babies of the 133 women found to be positive at the time of birth were screened for HBsAg, HBeAg, and HBV DNA in serum and cord blood. Of 127 deliveries in which the mothers were positive for HBV DNA, 66% of infants had HBV DNA in their cord blood and 41% had serum markers that were positive at birth. Maternal HBV DNA greater than 1.5 X 105 copies/mL was significantly associated with intrauterine transmission (P = .025), whereas mode of delivery and maternal HBeAg status were not. This study adds to the concern that in some cases, the vaccine and hepatitis B immune globulin (HBIg) given at the time of birth may not prevent infection in those born already infected and further supports the need to assess the treatment of pregnant women with high viral titers.
TREATMENT DURING PREGNANCY
The use of active and passive immunoprophylaxis to reduce the risk of perinatal transmission of HBV is well accepted in clinical practice. HBIg given at the time of birth in combination with three doses of the recombinant hepatitis B vaccine given over the first 6 months of life has been up to 95% effective in preventing perinatal transmission. As noted above, however, the risk of perinatal transmission of HBV increases as the mother’s viral load increases. In one series of mothers with high viral loads, this risk was as high as 28%.15
It stands to reason that if the mother’s viral load can be reduced at the time of birth, the risk of perinatal transmission could also be reduced (see “Case: Minimizing risk in a 29-year-old woman”). In fact, lamivudine treatment of highly viremic HBsAg-positive women during the final months of pregnancy appears safe and may effectively reduce the risk of perinatal transmission of HBV, even in the setting of HBV vaccination plus HBIg.

Evidence for third-trimester treatment
van Zonneveld et al15 studied eight HBeAg-positive women with HBV DNA levels of 1.2 X 109 copies/mL or greater who were treated with 150 mg/day of lamivudine after the 34th week of pregnancy, and compared the rates of perinatal transmission between them and 24 matched historical controls who did not receive treatment. All children received standard immunoprophylaxis at birth and were followed for 12 months. In five of the eight treated mothers, viral load declined to less than 1.2 X 108 copies/mL. Of the eight infants born to treated mothers, four were HBsAg positive at birth, but only one remained positive at 1 year. This 12.5% rate of perinatal transmission was substantially lower than the 28% rate observed among the controls. No adverse events occurred with lamivudine in this study.
In a multicenter, randomized, double-blind, placebo-controlled study in China and the Philippines, Xu et al16 assessed outcomes among 114 HBsAg-positive pregnant women who had high viral loads (HBV DNA > 1,000 mEq/mL). The women were randomized to placebo or treatment with lamivudine starting at 32 weeks of gestation and continuing until 4 weeks postpartum. All of the infants received standard vaccine plus HBIg.
The mothers treated with lamivudine were more likely (98%) to have a reduction in their viral loads to less than 1,000 mEq/mL than the controls (31%). This reduction in viral load translated to improved outcomes for the infants of mothers receiving lamivudine. At 1 year, 18% of infants born to mothers treated with lamivudine were HBsAg positive compared with 39% of infants born to mothers randomized to placebo (P = .014). The rate of HBV DNA positivity at 1 year was reduced by more than half among the infants born to actively treated mothers compared with those who received placebo (20% vs 46%, respectively; P = .003). There was no difference in the rate of adverse events between the treatment and control groups in either the mothers or the infants.
The Xu study suggests that the use of lamivudine in the third trimester in mothers with high viral loads may effectively reduce the risk of perinatal transmission beyond what can be achieved with active and passive immunoprophylaxis. As this study has been presented in abstract form only, we await the final analysis of these data. This therapy appears to be relatively safe for both mother and infant, although the optimal timing and duration of therapy is still unclear.
Treatment options during pregnancy
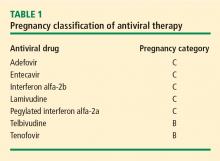
Most human experience with antiviral drug therapy in pregnancy has been with lamivudine. More than 4,600 women have been exposed to the drug during their second or third trimesters.17 Even though lamivudine is classified as FDA pregnancy risk category C, it is associated with a risk of birth defects (2.2% to 2.4%) that is no higher than the baseline birth defect rate.17
Of the two agents classified as FDA pregnancy risk category B, only tenofovir received this classification based on data collected in human exposure. The experience with tenofovir in pregnant women consists of 606 women in their first trimester and 336 in their second trimester.17 The rate of birth defects associated with tenofovir ranges from 1.5% (second-trimester use) to 2.3% (first-trimester use), which is similar to the background rate.17 Telbivudine received its pregnancy risk category B rating based on animal studies; there are few human pregnancy registry data.
Nonpegylated interferon alfa-2b has been shown to have abortifacient effects in rhesus monkeys at 15 and 30 million IU/kg (estimated human equivalent of 5 and 10 million IU/kg, based on body surface area adjustment for a 60-kg adult). Peginterferon alfa-2b should therefore be assumed to also have abortifacient potential, as there are no adequate and well-controlled studies in pregnant women. Peginterferon alfa-2b is to be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. It is recommended for use in fertile women only when they are using effective contraception during the treatment period. Pegylated interferon alfa-2a is approved for treatment of chronic HBV infection, but is not recommended for use during pregnancy.
MANAGEMENT STRATEGY
If the mother is HBsAg positive in the first trimester, history of perinatal transmission and an assessment of viral load at week 28 guide further management decisions. All children of HBsAg-positive mothers receive HBIg in addition to vaccination at birth.
Women with high viral loads can be considered for treatment with antiviral therapy, but a comprehensive discussion of risks and benefits needs to take place before opting for treatment as the data are too limited at this time to advocate therapy. One strategy for therapy is the use of lamivudine, tenofovir, or telbivudine starting at 32 weeks of pregnancy; the HBV DNA level that warrants treatment depends on the presence or absence of a history of perinatal transmission. If a previous child was HBV positive, concerns about the risk of perinatal transmission may be higher, so the threshold for treatment may be lower (HBV DNA > 106 copies/mL) than if the previous child were not positive for HBV. If the previous child was not HBV positive, treatment might be considered with HBV DNA levels greater than 108 copies/mL.
SUMMARY
Although a case can be made for treatment of HBV infection during pregnancy, the risks and benefits must be weighed carefully. The benefits of treatment appear to be most pronounced in cases with high maternal viremia; in such instances, treatment should be considered and discussed with the patient at the start of the third trimester. Viable treatment choices are limited to lamivudine, tenofovir, and telbivudine. Of these, lamivudine and tenofovir appear to be the therapeutic options with reasonable human exposure and safety data in pregnancy.
DISCUSSION
William D. Carey, MD: Referring to your case patient, assume that you treat her with tenofovir and her viral load declines. She delivers her baby and then undergoes a thorough workup, including a liver biopsy, that shows no particular liver damage. What would you do?
Tram T. Tran, MD: There are two separate issues: treating the baby and treating the mother. When you’re treating a mother in her third trimester, your goal is to prevent perinatal transmission of HBV. Once the baby is delivered, treated with HBIg, and vaccinated, then your attention turns to the mother. You can then decide based on treatment guidelines and your clinical judgment whether you want to treat the mom.
The period immediately after birth is a time of treatment uncertainty in mothers who choose to breastfeed, because the nucleoside analogues are likely passed in breast milk to some unknown degree, and it’s probably unwise to expose the child this way. In a mother who chooses to breastfeed, I would stop the medication after the delivery, by which time the baby will have received HBIg and the vaccine. When treatment is stopped, you have to think about the potential for a flare; although clinically significant flares are uncommon, the mother should be monitored after stopping treatment. After she stops breastfeeding, you can decide whether to treat her.
Robert G. Gish, MD: What are the effects of tenofovir on bone? Do you talk to your patients about it, and is it an issue during pregnancy or after the baby is delivered?
Dr. Tran: Some data show a decrease in bone mineral density with tenofovir in the human immunodeficiency virus patient population. I definitely talk to my patients about all the potential risks associated with these medicines as, naturally, pregnant women will be very sensitive to any possible risk to their unborn child. Lamivudine probably has the safest profile in pregnancy, given its large body of human experience; however, it is now classified as an FDA pregnancy risk category C drug, whereas tenofovir is classified as category B. This may make a difference to some clinicians.
- Schrag SJ, Arnold KE, Mohle-Boetani JC, et al. Prenatal screening for infectious diseases and opportunities for prevention. Obstet Gynecol 2003; 102:753–760.
- Shepard CW, Simard EP, Finelli L, Fiore AE, Bell BP. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol Rev 2006; 28:112–125.
- Mast EE, Weinbaum CM, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep 2006; 55(RR-16):1–33.
- Centers for Disease Control and Prevention (CDC). Implementation of newborn hepatitis B vaccination—worldwide, 2006. MMWR Morb Mortal Wkly Rep 2008; 57:1249–1252.
- McMahon BJ, Alward WL, Hall DB, et al. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. J Infect Dis 1985; 151:599–603.
- Tassopoulos NC, Papaevangelou GJ, Sjogren MH, Roumeliotou-Karayannis A, Gerin JL, Purcell RH. Natural history of acute hepatitis B surface antigen-positive hepatitis in Greek adults. Gastroenterology 1987; 92:1844–1850.
- Chang MH. Natural history of hepatitis B virus infection in children. J Gastroenterol Hepatol 2000; 15(suppl):E16–E19.
- Beasley RP, Trepo C, Stevens CE, Szmuness W. The e antigen and vertical transmission of hepatitis B surface antigen. Am J Epidemiol 1977; 105:94–98.
- Wong VC, Ip HM, Reesink HW, et al. Prevention of the HBsAg carrier state in newborn infants of mothers who are chronic carriers of HBsAg and HBeAg by administration of hepatitis-B vaccine and hepatitis-B immunoglobulin: double-blind randomised placebo-controlled study. Lancet 1984; 1(8383):921–926.
- Okada K, Kamiyama I, Inomata M, Imai M, Miyakawa Y. e antigen and anti-e in the serum of asymptomatic carrier mothers as indicators of positive and negative transmission of hepatitis B virus to their infants. N Engl J Med 1976; 294:746–749.
- Xu DZ, Yan YP, Choi BC, et al. Risk factors and mechanism of transplacental transmission of hepatitis B virus: a case-control study. J Med Virol 2002; 67:20–26.
- Burk RD, Hwang LY, Ho GY, Shafritz DA, Beasley RP. Outcome of perinatal hepatitis B virus exposure is dependent on maternal virus load. J Infect Dis 1994; 170:1418–1423.
- Wiseman E, Fraser MA, Holden S, et al. Perinatal transmission of hepatitis B virus: viral load and HBeAg status are significant risk factors. Presented at: 59th Annual Meeting of the American Association for the Study of Liver Diseases; October 31–November 4, 2008; San Francisco, CA. Abstract 827.
- Pande C, Kumar A, Patra S, Trivedi SS, Dutta AK, Sarin SK. High maternal hepatitis B virus DNA levels but not HBeAg positivity predicts perinatal transmission of hepatitis B to the newborn. Presented at: Digestive Disease Week; May 17–22, 2008; San Diego, CA. Abstract 252.
- van Zonneveld M, van Nunen AB, Niesters HG, de Man RA, Schalm SW, Janssen HL. Lamivudine treatment during pregnancy to prevent perinatal transmission of hepatitis B virus infection. J Viral Hepat 2003; 10:294–297.
- Xu W-M, Cui Y-T, Wang L, et al. Efficacy and safety of lamivudine in late pregnancy for the prevention of mother-child transmission of hepatitis B: a multicentre, randomised, double-blind, placebo-controlled study [AASLD abstract 246]. Hepatology 2004; 40:272A.
- Antiretroviral Pregnancy Registry Steering Committee. Antiretroviral pregnancy registry international interim report for 1 January 1989 through 31 July 2008. Wilmington, NC: Registry Coordinating Center; 2008. Antiretroviral Pregnancy Registry Web site. http://www.apregistry.com/forms/interim_report.pdf. Accessed December 17, 2008.
- Tran TT, Keeffe EB. Management of the pregnant hepatitis B patient. Current Hepatitis Reports 2008; 7:12–17.
The management of hepatitis B virus (HBV) infection in pregnancy is complex. Because infection with HBV in infancy often leads to chronic disease, prevention of perinatal, or vertical, transmission is a worthy goal; yet, prophylactic therapy during pregnancy is not well studied. This article explores the consequences of HBV infection during pregnancy, the specific risks imposed by high viral load, the evidence to support preemptive antiviral therapy, and the timing of therapy during pregnancy.
PERINATAL TRANSMISSION
Perinatal transmission is the most common mode of HBV transmission worldwide; however, the maternal screening programs and universal vaccination in newborns with active and passive immunoprophylaxis have dramatically reduced HBV transmission rates. According to recent data from the US Centers for Disease Control and Prevention, prenatal screening for hepatitis B surface antigen (HBsAg) in the United States is nearly universal; 97% of pregnant women undergo screening before delivery.1 Further, among infants at risk of acquiring HBV infection, 92% complete the three-dose vaccination series by the time they are 3 years old. There is some nationwide variation, however, in the appropriate administration of immunoprophylaxis to infants exposed perinatally, ranging from 78% in Louisiana to 99.8% in one California health maintenance organization.2
Perinatal transmission of HBV infection has declined steadily in the United States over the past 2 decades, consistent with the successful implementation of universal screening of pregnant women and vaccination policies.3 Outside the United States, however, many high-prevalence countries lack vaccination coverage and perinatal transmission is common. In 87 countries with a prevalence of HBV infection that exceeds 8%, the infant vaccine coverage was only 36%.4
Risk of chronic infection
The risk of progression to chronic HBV infection is inversely proportional to the age at which the infection was acquired. Without immunoprophylaxis, up to 90% of infants born to hepatitis B e antigen (HBeAg)-positive mothers become HBV carriers. In comparison, 20% to 30% of children infected between age 1 year and 5 years, and fewer than 5% of immunocompetent adults, become HBV carriers.5–7
If the mother is positive for both HBsAg and HBeAg and her baby does not receive immunoprophylaxis, the risk of the baby developing chronic HBV infection by age 6 months is 70% to 90%.8–10 Of those exposed in early childhood, 28.8% are HBsAg positive by age 4 years.5 These data underscore the need for early vaccination.
In a study of 402 HBsAg-positive pregnant women in China, Xu et al11 found that 3.7% of their newborn infants were HBsAg positive within 24 hours of birth. Of the women who were HBeAg positive, the intrauterine infection rate was 9.8%. Analysis of placental tissue for HBsAg, hepatitis B core antigen (HBcAg), and viral load (HBV DNA) uncovered an overall placental infection rate of 44.6%.
Transplacental transmission of HBV has been observed in multiple studies, especially when mothers are positive for HBsAg and HBeAg and have high viral loads. Among mothers positive for HBeAg, Burk et al12 found an odds ratio of 147 for a persistently infected infant when the maternal HBV DNA level was at least 1.4 ng/mL compared with less than .005 ng/mL. Among the HBeAg-negative mothers, the odds ratio for a persistently infected infant was 19.2 with high versus low maternal HBV DNA levels.
Importance of maternal viremia
Despite successful screening and vaccination programs, high maternal HBV DNA correlates in some studies with perinatal transmission. Wiseman et al13 studied 298 chronically HBV-infected women and their infants, who were tested for HBV at age 9 months. Interim analysis showed a transmission rate of 8.5% for infants born to mothers with virus levels greater than 8 log10 copies/mL. These data suggest that perinatal transmission may still be occurring despite the use of effective active and passive immunoprophylaxis. Additional studies are needed to assess the potential risk reduction associated with treatment of high maternal viremia during pregnancy.
Maternal HBV DNA positivity was associated with a high rate of intrauterine transmission of HBV in a program in India in which 11,524 woman were screened for HBV infection.14 Babies of the 133 women found to be positive at the time of birth were screened for HBsAg, HBeAg, and HBV DNA in serum and cord blood. Of 127 deliveries in which the mothers were positive for HBV DNA, 66% of infants had HBV DNA in their cord blood and 41% had serum markers that were positive at birth. Maternal HBV DNA greater than 1.5 X 105 copies/mL was significantly associated with intrauterine transmission (P = .025), whereas mode of delivery and maternal HBeAg status were not. This study adds to the concern that in some cases, the vaccine and hepatitis B immune globulin (HBIg) given at the time of birth may not prevent infection in those born already infected and further supports the need to assess the treatment of pregnant women with high viral titers.
TREATMENT DURING PREGNANCY
The use of active and passive immunoprophylaxis to reduce the risk of perinatal transmission of HBV is well accepted in clinical practice. HBIg given at the time of birth in combination with three doses of the recombinant hepatitis B vaccine given over the first 6 months of life has been up to 95% effective in preventing perinatal transmission. As noted above, however, the risk of perinatal transmission of HBV increases as the mother’s viral load increases. In one series of mothers with high viral loads, this risk was as high as 28%.15
It stands to reason that if the mother’s viral load can be reduced at the time of birth, the risk of perinatal transmission could also be reduced (see “Case: Minimizing risk in a 29-year-old woman”). In fact, lamivudine treatment of highly viremic HBsAg-positive women during the final months of pregnancy appears safe and may effectively reduce the risk of perinatal transmission of HBV, even in the setting of HBV vaccination plus HBIg.

Evidence for third-trimester treatment
van Zonneveld et al15 studied eight HBeAg-positive women with HBV DNA levels of 1.2 X 109 copies/mL or greater who were treated with 150 mg/day of lamivudine after the 34th week of pregnancy, and compared the rates of perinatal transmission between them and 24 matched historical controls who did not receive treatment. All children received standard immunoprophylaxis at birth and were followed for 12 months. In five of the eight treated mothers, viral load declined to less than 1.2 X 108 copies/mL. Of the eight infants born to treated mothers, four were HBsAg positive at birth, but only one remained positive at 1 year. This 12.5% rate of perinatal transmission was substantially lower than the 28% rate observed among the controls. No adverse events occurred with lamivudine in this study.
In a multicenter, randomized, double-blind, placebo-controlled study in China and the Philippines, Xu et al16 assessed outcomes among 114 HBsAg-positive pregnant women who had high viral loads (HBV DNA > 1,000 mEq/mL). The women were randomized to placebo or treatment with lamivudine starting at 32 weeks of gestation and continuing until 4 weeks postpartum. All of the infants received standard vaccine plus HBIg.
The mothers treated with lamivudine were more likely (98%) to have a reduction in their viral loads to less than 1,000 mEq/mL than the controls (31%). This reduction in viral load translated to improved outcomes for the infants of mothers receiving lamivudine. At 1 year, 18% of infants born to mothers treated with lamivudine were HBsAg positive compared with 39% of infants born to mothers randomized to placebo (P = .014). The rate of HBV DNA positivity at 1 year was reduced by more than half among the infants born to actively treated mothers compared with those who received placebo (20% vs 46%, respectively; P = .003). There was no difference in the rate of adverse events between the treatment and control groups in either the mothers or the infants.
The Xu study suggests that the use of lamivudine in the third trimester in mothers with high viral loads may effectively reduce the risk of perinatal transmission beyond what can be achieved with active and passive immunoprophylaxis. As this study has been presented in abstract form only, we await the final analysis of these data. This therapy appears to be relatively safe for both mother and infant, although the optimal timing and duration of therapy is still unclear.
Treatment options during pregnancy
Most human experience with antiviral drug therapy in pregnancy has been with lamivudine. More than 4,600 women have been exposed to the drug during their second or third trimesters.17 Even though lamivudine is classified as FDA pregnancy risk category C, it is associated with a risk of birth defects (2.2% to 2.4%) that is no higher than the baseline birth defect rate.17
Of the two agents classified as FDA pregnancy risk category B, only tenofovir received this classification based on data collected in human exposure. The experience with tenofovir in pregnant women consists of 606 women in their first trimester and 336 in their second trimester.17 The rate of birth defects associated with tenofovir ranges from 1.5% (second-trimester use) to 2.3% (first-trimester use), which is similar to the background rate.17 Telbivudine received its pregnancy risk category B rating based on animal studies; there are few human pregnancy registry data.
Nonpegylated interferon alfa-2b has been shown to have abortifacient effects in rhesus monkeys at 15 and 30 million IU/kg (estimated human equivalent of 5 and 10 million IU/kg, based on body surface area adjustment for a 60-kg adult). Peginterferon alfa-2b should therefore be assumed to also have abortifacient potential, as there are no adequate and well-controlled studies in pregnant women. Peginterferon alfa-2b is to be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. It is recommended for use in fertile women only when they are using effective contraception during the treatment period. Pegylated interferon alfa-2a is approved for treatment of chronic HBV infection, but is not recommended for use during pregnancy.
MANAGEMENT STRATEGY
If the mother is HBsAg positive in the first trimester, history of perinatal transmission and an assessment of viral load at week 28 guide further management decisions. All children of HBsAg-positive mothers receive HBIg in addition to vaccination at birth.
Women with high viral loads can be considered for treatment with antiviral therapy, but a comprehensive discussion of risks and benefits needs to take place before opting for treatment as the data are too limited at this time to advocate therapy. One strategy for therapy is the use of lamivudine, tenofovir, or telbivudine starting at 32 weeks of pregnancy; the HBV DNA level that warrants treatment depends on the presence or absence of a history of perinatal transmission. If a previous child was HBV positive, concerns about the risk of perinatal transmission may be higher, so the threshold for treatment may be lower (HBV DNA > 106 copies/mL) than if the previous child were not positive for HBV. If the previous child was not HBV positive, treatment might be considered with HBV DNA levels greater than 108 copies/mL.
SUMMARY
Although a case can be made for treatment of HBV infection during pregnancy, the risks and benefits must be weighed carefully. The benefits of treatment appear to be most pronounced in cases with high maternal viremia; in such instances, treatment should be considered and discussed with the patient at the start of the third trimester. Viable treatment choices are limited to lamivudine, tenofovir, and telbivudine. Of these, lamivudine and tenofovir appear to be the therapeutic options with reasonable human exposure and safety data in pregnancy.
DISCUSSION
William D. Carey, MD: Referring to your case patient, assume that you treat her with tenofovir and her viral load declines. She delivers her baby and then undergoes a thorough workup, including a liver biopsy, that shows no particular liver damage. What would you do?
Tram T. Tran, MD: There are two separate issues: treating the baby and treating the mother. When you’re treating a mother in her third trimester, your goal is to prevent perinatal transmission of HBV. Once the baby is delivered, treated with HBIg, and vaccinated, then your attention turns to the mother. You can then decide based on treatment guidelines and your clinical judgment whether you want to treat the mom.
The period immediately after birth is a time of treatment uncertainty in mothers who choose to breastfeed, because the nucleoside analogues are likely passed in breast milk to some unknown degree, and it’s probably unwise to expose the child this way. In a mother who chooses to breastfeed, I would stop the medication after the delivery, by which time the baby will have received HBIg and the vaccine. When treatment is stopped, you have to think about the potential for a flare; although clinically significant flares are uncommon, the mother should be monitored after stopping treatment. After she stops breastfeeding, you can decide whether to treat her.
Robert G. Gish, MD: What are the effects of tenofovir on bone? Do you talk to your patients about it, and is it an issue during pregnancy or after the baby is delivered?
Dr. Tran: Some data show a decrease in bone mineral density with tenofovir in the human immunodeficiency virus patient population. I definitely talk to my patients about all the potential risks associated with these medicines as, naturally, pregnant women will be very sensitive to any possible risk to their unborn child. Lamivudine probably has the safest profile in pregnancy, given its large body of human experience; however, it is now classified as an FDA pregnancy risk category C drug, whereas tenofovir is classified as category B. This may make a difference to some clinicians.
The management of hepatitis B virus (HBV) infection in pregnancy is complex. Because infection with HBV in infancy often leads to chronic disease, prevention of perinatal, or vertical, transmission is a worthy goal; yet, prophylactic therapy during pregnancy is not well studied. This article explores the consequences of HBV infection during pregnancy, the specific risks imposed by high viral load, the evidence to support preemptive antiviral therapy, and the timing of therapy during pregnancy.
PERINATAL TRANSMISSION
Perinatal transmission is the most common mode of HBV transmission worldwide; however, the maternal screening programs and universal vaccination in newborns with active and passive immunoprophylaxis have dramatically reduced HBV transmission rates. According to recent data from the US Centers for Disease Control and Prevention, prenatal screening for hepatitis B surface antigen (HBsAg) in the United States is nearly universal; 97% of pregnant women undergo screening before delivery.1 Further, among infants at risk of acquiring HBV infection, 92% complete the three-dose vaccination series by the time they are 3 years old. There is some nationwide variation, however, in the appropriate administration of immunoprophylaxis to infants exposed perinatally, ranging from 78% in Louisiana to 99.8% in one California health maintenance organization.2
Perinatal transmission of HBV infection has declined steadily in the United States over the past 2 decades, consistent with the successful implementation of universal screening of pregnant women and vaccination policies.3 Outside the United States, however, many high-prevalence countries lack vaccination coverage and perinatal transmission is common. In 87 countries with a prevalence of HBV infection that exceeds 8%, the infant vaccine coverage was only 36%.4
Risk of chronic infection
The risk of progression to chronic HBV infection is inversely proportional to the age at which the infection was acquired. Without immunoprophylaxis, up to 90% of infants born to hepatitis B e antigen (HBeAg)-positive mothers become HBV carriers. In comparison, 20% to 30% of children infected between age 1 year and 5 years, and fewer than 5% of immunocompetent adults, become HBV carriers.5–7
If the mother is positive for both HBsAg and HBeAg and her baby does not receive immunoprophylaxis, the risk of the baby developing chronic HBV infection by age 6 months is 70% to 90%.8–10 Of those exposed in early childhood, 28.8% are HBsAg positive by age 4 years.5 These data underscore the need for early vaccination.
In a study of 402 HBsAg-positive pregnant women in China, Xu et al11 found that 3.7% of their newborn infants were HBsAg positive within 24 hours of birth. Of the women who were HBeAg positive, the intrauterine infection rate was 9.8%. Analysis of placental tissue for HBsAg, hepatitis B core antigen (HBcAg), and viral load (HBV DNA) uncovered an overall placental infection rate of 44.6%.
Transplacental transmission of HBV has been observed in multiple studies, especially when mothers are positive for HBsAg and HBeAg and have high viral loads. Among mothers positive for HBeAg, Burk et al12 found an odds ratio of 147 for a persistently infected infant when the maternal HBV DNA level was at least 1.4 ng/mL compared with less than .005 ng/mL. Among the HBeAg-negative mothers, the odds ratio for a persistently infected infant was 19.2 with high versus low maternal HBV DNA levels.
Importance of maternal viremia
Despite successful screening and vaccination programs, high maternal HBV DNA correlates in some studies with perinatal transmission. Wiseman et al13 studied 298 chronically HBV-infected women and their infants, who were tested for HBV at age 9 months. Interim analysis showed a transmission rate of 8.5% for infants born to mothers with virus levels greater than 8 log10 copies/mL. These data suggest that perinatal transmission may still be occurring despite the use of effective active and passive immunoprophylaxis. Additional studies are needed to assess the potential risk reduction associated with treatment of high maternal viremia during pregnancy.
Maternal HBV DNA positivity was associated with a high rate of intrauterine transmission of HBV in a program in India in which 11,524 woman were screened for HBV infection.14 Babies of the 133 women found to be positive at the time of birth were screened for HBsAg, HBeAg, and HBV DNA in serum and cord blood. Of 127 deliveries in which the mothers were positive for HBV DNA, 66% of infants had HBV DNA in their cord blood and 41% had serum markers that were positive at birth. Maternal HBV DNA greater than 1.5 X 105 copies/mL was significantly associated with intrauterine transmission (P = .025), whereas mode of delivery and maternal HBeAg status were not. This study adds to the concern that in some cases, the vaccine and hepatitis B immune globulin (HBIg) given at the time of birth may not prevent infection in those born already infected and further supports the need to assess the treatment of pregnant women with high viral titers.
TREATMENT DURING PREGNANCY
The use of active and passive immunoprophylaxis to reduce the risk of perinatal transmission of HBV is well accepted in clinical practice. HBIg given at the time of birth in combination with three doses of the recombinant hepatitis B vaccine given over the first 6 months of life has been up to 95% effective in preventing perinatal transmission. As noted above, however, the risk of perinatal transmission of HBV increases as the mother’s viral load increases. In one series of mothers with high viral loads, this risk was as high as 28%.15
It stands to reason that if the mother’s viral load can be reduced at the time of birth, the risk of perinatal transmission could also be reduced (see “Case: Minimizing risk in a 29-year-old woman”). In fact, lamivudine treatment of highly viremic HBsAg-positive women during the final months of pregnancy appears safe and may effectively reduce the risk of perinatal transmission of HBV, even in the setting of HBV vaccination plus HBIg.

Evidence for third-trimester treatment
van Zonneveld et al15 studied eight HBeAg-positive women with HBV DNA levels of 1.2 X 109 copies/mL or greater who were treated with 150 mg/day of lamivudine after the 34th week of pregnancy, and compared the rates of perinatal transmission between them and 24 matched historical controls who did not receive treatment. All children received standard immunoprophylaxis at birth and were followed for 12 months. In five of the eight treated mothers, viral load declined to less than 1.2 X 108 copies/mL. Of the eight infants born to treated mothers, four were HBsAg positive at birth, but only one remained positive at 1 year. This 12.5% rate of perinatal transmission was substantially lower than the 28% rate observed among the controls. No adverse events occurred with lamivudine in this study.
In a multicenter, randomized, double-blind, placebo-controlled study in China and the Philippines, Xu et al16 assessed outcomes among 114 HBsAg-positive pregnant women who had high viral loads (HBV DNA > 1,000 mEq/mL). The women were randomized to placebo or treatment with lamivudine starting at 32 weeks of gestation and continuing until 4 weeks postpartum. All of the infants received standard vaccine plus HBIg.
The mothers treated with lamivudine were more likely (98%) to have a reduction in their viral loads to less than 1,000 mEq/mL than the controls (31%). This reduction in viral load translated to improved outcomes for the infants of mothers receiving lamivudine. At 1 year, 18% of infants born to mothers treated with lamivudine were HBsAg positive compared with 39% of infants born to mothers randomized to placebo (P = .014). The rate of HBV DNA positivity at 1 year was reduced by more than half among the infants born to actively treated mothers compared with those who received placebo (20% vs 46%, respectively; P = .003). There was no difference in the rate of adverse events between the treatment and control groups in either the mothers or the infants.
The Xu study suggests that the use of lamivudine in the third trimester in mothers with high viral loads may effectively reduce the risk of perinatal transmission beyond what can be achieved with active and passive immunoprophylaxis. As this study has been presented in abstract form only, we await the final analysis of these data. This therapy appears to be relatively safe for both mother and infant, although the optimal timing and duration of therapy is still unclear.
Treatment options during pregnancy
Most human experience with antiviral drug therapy in pregnancy has been with lamivudine. More than 4,600 women have been exposed to the drug during their second or third trimesters.17 Even though lamivudine is classified as FDA pregnancy risk category C, it is associated with a risk of birth defects (2.2% to 2.4%) that is no higher than the baseline birth defect rate.17
Of the two agents classified as FDA pregnancy risk category B, only tenofovir received this classification based on data collected in human exposure. The experience with tenofovir in pregnant women consists of 606 women in their first trimester and 336 in their second trimester.17 The rate of birth defects associated with tenofovir ranges from 1.5% (second-trimester use) to 2.3% (first-trimester use), which is similar to the background rate.17 Telbivudine received its pregnancy risk category B rating based on animal studies; there are few human pregnancy registry data.
Nonpegylated interferon alfa-2b has been shown to have abortifacient effects in rhesus monkeys at 15 and 30 million IU/kg (estimated human equivalent of 5 and 10 million IU/kg, based on body surface area adjustment for a 60-kg adult). Peginterferon alfa-2b should therefore be assumed to also have abortifacient potential, as there are no adequate and well-controlled studies in pregnant women. Peginterferon alfa-2b is to be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. It is recommended for use in fertile women only when they are using effective contraception during the treatment period. Pegylated interferon alfa-2a is approved for treatment of chronic HBV infection, but is not recommended for use during pregnancy.
MANAGEMENT STRATEGY
If the mother is HBsAg positive in the first trimester, history of perinatal transmission and an assessment of viral load at week 28 guide further management decisions. All children of HBsAg-positive mothers receive HBIg in addition to vaccination at birth.
Women with high viral loads can be considered for treatment with antiviral therapy, but a comprehensive discussion of risks and benefits needs to take place before opting for treatment as the data are too limited at this time to advocate therapy. One strategy for therapy is the use of lamivudine, tenofovir, or telbivudine starting at 32 weeks of pregnancy; the HBV DNA level that warrants treatment depends on the presence or absence of a history of perinatal transmission. If a previous child was HBV positive, concerns about the risk of perinatal transmission may be higher, so the threshold for treatment may be lower (HBV DNA > 106 copies/mL) than if the previous child were not positive for HBV. If the previous child was not HBV positive, treatment might be considered with HBV DNA levels greater than 108 copies/mL.
SUMMARY
Although a case can be made for treatment of HBV infection during pregnancy, the risks and benefits must be weighed carefully. The benefits of treatment appear to be most pronounced in cases with high maternal viremia; in such instances, treatment should be considered and discussed with the patient at the start of the third trimester. Viable treatment choices are limited to lamivudine, tenofovir, and telbivudine. Of these, lamivudine and tenofovir appear to be the therapeutic options with reasonable human exposure and safety data in pregnancy.
DISCUSSION
William D. Carey, MD: Referring to your case patient, assume that you treat her with tenofovir and her viral load declines. She delivers her baby and then undergoes a thorough workup, including a liver biopsy, that shows no particular liver damage. What would you do?
Tram T. Tran, MD: There are two separate issues: treating the baby and treating the mother. When you’re treating a mother in her third trimester, your goal is to prevent perinatal transmission of HBV. Once the baby is delivered, treated with HBIg, and vaccinated, then your attention turns to the mother. You can then decide based on treatment guidelines and your clinical judgment whether you want to treat the mom.
The period immediately after birth is a time of treatment uncertainty in mothers who choose to breastfeed, because the nucleoside analogues are likely passed in breast milk to some unknown degree, and it’s probably unwise to expose the child this way. In a mother who chooses to breastfeed, I would stop the medication after the delivery, by which time the baby will have received HBIg and the vaccine. When treatment is stopped, you have to think about the potential for a flare; although clinically significant flares are uncommon, the mother should be monitored after stopping treatment. After she stops breastfeeding, you can decide whether to treat her.
Robert G. Gish, MD: What are the effects of tenofovir on bone? Do you talk to your patients about it, and is it an issue during pregnancy or after the baby is delivered?
Dr. Tran: Some data show a decrease in bone mineral density with tenofovir in the human immunodeficiency virus patient population. I definitely talk to my patients about all the potential risks associated with these medicines as, naturally, pregnant women will be very sensitive to any possible risk to their unborn child. Lamivudine probably has the safest profile in pregnancy, given its large body of human experience; however, it is now classified as an FDA pregnancy risk category C drug, whereas tenofovir is classified as category B. This may make a difference to some clinicians.
- Schrag SJ, Arnold KE, Mohle-Boetani JC, et al. Prenatal screening for infectious diseases and opportunities for prevention. Obstet Gynecol 2003; 102:753–760.
- Shepard CW, Simard EP, Finelli L, Fiore AE, Bell BP. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol Rev 2006; 28:112–125.
- Mast EE, Weinbaum CM, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep 2006; 55(RR-16):1–33.
- Centers for Disease Control and Prevention (CDC). Implementation of newborn hepatitis B vaccination—worldwide, 2006. MMWR Morb Mortal Wkly Rep 2008; 57:1249–1252.
- McMahon BJ, Alward WL, Hall DB, et al. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. J Infect Dis 1985; 151:599–603.
- Tassopoulos NC, Papaevangelou GJ, Sjogren MH, Roumeliotou-Karayannis A, Gerin JL, Purcell RH. Natural history of acute hepatitis B surface antigen-positive hepatitis in Greek adults. Gastroenterology 1987; 92:1844–1850.
- Chang MH. Natural history of hepatitis B virus infection in children. J Gastroenterol Hepatol 2000; 15(suppl):E16–E19.
- Beasley RP, Trepo C, Stevens CE, Szmuness W. The e antigen and vertical transmission of hepatitis B surface antigen. Am J Epidemiol 1977; 105:94–98.
- Wong VC, Ip HM, Reesink HW, et al. Prevention of the HBsAg carrier state in newborn infants of mothers who are chronic carriers of HBsAg and HBeAg by administration of hepatitis-B vaccine and hepatitis-B immunoglobulin: double-blind randomised placebo-controlled study. Lancet 1984; 1(8383):921–926.
- Okada K, Kamiyama I, Inomata M, Imai M, Miyakawa Y. e antigen and anti-e in the serum of asymptomatic carrier mothers as indicators of positive and negative transmission of hepatitis B virus to their infants. N Engl J Med 1976; 294:746–749.
- Xu DZ, Yan YP, Choi BC, et al. Risk factors and mechanism of transplacental transmission of hepatitis B virus: a case-control study. J Med Virol 2002; 67:20–26.
- Burk RD, Hwang LY, Ho GY, Shafritz DA, Beasley RP. Outcome of perinatal hepatitis B virus exposure is dependent on maternal virus load. J Infect Dis 1994; 170:1418–1423.
- Wiseman E, Fraser MA, Holden S, et al. Perinatal transmission of hepatitis B virus: viral load and HBeAg status are significant risk factors. Presented at: 59th Annual Meeting of the American Association for the Study of Liver Diseases; October 31–November 4, 2008; San Francisco, CA. Abstract 827.
- Pande C, Kumar A, Patra S, Trivedi SS, Dutta AK, Sarin SK. High maternal hepatitis B virus DNA levels but not HBeAg positivity predicts perinatal transmission of hepatitis B to the newborn. Presented at: Digestive Disease Week; May 17–22, 2008; San Diego, CA. Abstract 252.
- van Zonneveld M, van Nunen AB, Niesters HG, de Man RA, Schalm SW, Janssen HL. Lamivudine treatment during pregnancy to prevent perinatal transmission of hepatitis B virus infection. J Viral Hepat 2003; 10:294–297.
- Xu W-M, Cui Y-T, Wang L, et al. Efficacy and safety of lamivudine in late pregnancy for the prevention of mother-child transmission of hepatitis B: a multicentre, randomised, double-blind, placebo-controlled study [AASLD abstract 246]. Hepatology 2004; 40:272A.
- Antiretroviral Pregnancy Registry Steering Committee. Antiretroviral pregnancy registry international interim report for 1 January 1989 through 31 July 2008. Wilmington, NC: Registry Coordinating Center; 2008. Antiretroviral Pregnancy Registry Web site. http://www.apregistry.com/forms/interim_report.pdf. Accessed December 17, 2008.
- Tran TT, Keeffe EB. Management of the pregnant hepatitis B patient. Current Hepatitis Reports 2008; 7:12–17.
- Schrag SJ, Arnold KE, Mohle-Boetani JC, et al. Prenatal screening for infectious diseases and opportunities for prevention. Obstet Gynecol 2003; 102:753–760.
- Shepard CW, Simard EP, Finelli L, Fiore AE, Bell BP. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol Rev 2006; 28:112–125.
- Mast EE, Weinbaum CM, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep 2006; 55(RR-16):1–33.
- Centers for Disease Control and Prevention (CDC). Implementation of newborn hepatitis B vaccination—worldwide, 2006. MMWR Morb Mortal Wkly Rep 2008; 57:1249–1252.
- McMahon BJ, Alward WL, Hall DB, et al. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. J Infect Dis 1985; 151:599–603.
- Tassopoulos NC, Papaevangelou GJ, Sjogren MH, Roumeliotou-Karayannis A, Gerin JL, Purcell RH. Natural history of acute hepatitis B surface antigen-positive hepatitis in Greek adults. Gastroenterology 1987; 92:1844–1850.
- Chang MH. Natural history of hepatitis B virus infection in children. J Gastroenterol Hepatol 2000; 15(suppl):E16–E19.
- Beasley RP, Trepo C, Stevens CE, Szmuness W. The e antigen and vertical transmission of hepatitis B surface antigen. Am J Epidemiol 1977; 105:94–98.
- Wong VC, Ip HM, Reesink HW, et al. Prevention of the HBsAg carrier state in newborn infants of mothers who are chronic carriers of HBsAg and HBeAg by administration of hepatitis-B vaccine and hepatitis-B immunoglobulin: double-blind randomised placebo-controlled study. Lancet 1984; 1(8383):921–926.
- Okada K, Kamiyama I, Inomata M, Imai M, Miyakawa Y. e antigen and anti-e in the serum of asymptomatic carrier mothers as indicators of positive and negative transmission of hepatitis B virus to their infants. N Engl J Med 1976; 294:746–749.
- Xu DZ, Yan YP, Choi BC, et al. Risk factors and mechanism of transplacental transmission of hepatitis B virus: a case-control study. J Med Virol 2002; 67:20–26.
- Burk RD, Hwang LY, Ho GY, Shafritz DA, Beasley RP. Outcome of perinatal hepatitis B virus exposure is dependent on maternal virus load. J Infect Dis 1994; 170:1418–1423.
- Wiseman E, Fraser MA, Holden S, et al. Perinatal transmission of hepatitis B virus: viral load and HBeAg status are significant risk factors. Presented at: 59th Annual Meeting of the American Association for the Study of Liver Diseases; October 31–November 4, 2008; San Francisco, CA. Abstract 827.
- Pande C, Kumar A, Patra S, Trivedi SS, Dutta AK, Sarin SK. High maternal hepatitis B virus DNA levels but not HBeAg positivity predicts perinatal transmission of hepatitis B to the newborn. Presented at: Digestive Disease Week; May 17–22, 2008; San Diego, CA. Abstract 252.
- van Zonneveld M, van Nunen AB, Niesters HG, de Man RA, Schalm SW, Janssen HL. Lamivudine treatment during pregnancy to prevent perinatal transmission of hepatitis B virus infection. J Viral Hepat 2003; 10:294–297.
- Xu W-M, Cui Y-T, Wang L, et al. Efficacy and safety of lamivudine in late pregnancy for the prevention of mother-child transmission of hepatitis B: a multicentre, randomised, double-blind, placebo-controlled study [AASLD abstract 246]. Hepatology 2004; 40:272A.
- Antiretroviral Pregnancy Registry Steering Committee. Antiretroviral pregnancy registry international interim report for 1 January 1989 through 31 July 2008. Wilmington, NC: Registry Coordinating Center; 2008. Antiretroviral Pregnancy Registry Web site. http://www.apregistry.com/forms/interim_report.pdf. Accessed December 17, 2008.
- Tran TT, Keeffe EB. Management of the pregnant hepatitis B patient. Current Hepatitis Reports 2008; 7:12–17.
KEY POINTS
- Hepatitis B immune globulin at the time of birth plus three doses of the recombinant hepatitis B vaccine over the first 6 months of life is up to 95% effective in
preventing perinatal transmission. - Despite successful screening and vaccination, perinatal transmission of HBV is still possible if maternal viral load is high.
- Antiviral treatment during the third trimester of pregnancy may reduce perinatal transmission of HBV; the benefit appears most pronounced with high maternal viremia.