User login
Guidelines for the management of hepatitis B virus (HBV) infection can be daunting to clinicians. Further, although established practice guidelines can provide direction, treatment of chronic HBV infection is characterized by uncertainties that can hinder optimal patient care. Reservations about when to initiate and terminate therapy, cost issues, and the development of resistance to therapy are among the factors that impede adequate treatment. This article offers a straightforward roadmap for the management of chronic HBV infection, based on interpretation of recently released guidelines,1–3 and strategies for preventing and managing resistance to antiviral therapy.
DECIDING TO TREAT
Key factors: Viral load and ALT
Two important factors influencing the decision to treat are viral load (HBV DNA) and alanine aminotransferase (ALT) level; although these are relatively straightforward measures, other factors can cause clinicians to avoid or delay treatment.
A simple guideline is to discuss treatment with any patient who is positive for HBV DNA. The most recent guidelines for the treatment of HBV infection, published by the European Association for the Study of the Liver (EASL), recommend an HBV DNA level of 2,000 copies/mL as a threshold for initiating therapy; this recommendation applies to patients who are either positive or negative for hepatitis B e antigen (HBeAg).3
The Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-Hepatitis B Virus (REVEAL-HBV) study investigators used ultrasensitive polymerase chain reaction (PCR) to quantify HBV DNA levels and conducted a time-dependent multiple Cox regression analysis of HBV DNA level and the risk of hepatocellular carcinoma (HCC).4,5 The length of time at a given DNA level was weighted in determining the adjusted hazard ratio. With an HBV DNA level less than 300 copies/mL defined as the reference group, risk of HCC increased commensurate with increasing HBV DNA level; even at levels ranging from 300 to 10,000 copies/mL, longer duration of HBV DNA positivity increased risk. This group also found HBV DNA level to be an independent risk factor for cirrhosis.
Patients who are HBV DNA negative are at much lower risk of cirrhosis and HCC than HBV DNA–positive patients; HBV DNA–negative patients being treated with antiviral drugs are much less likely to develop resistance to treatment, provided that first-line medications such as tenofovir or entecavir are used.
The definition of a “healthy” ALT level is controversial. In my opinion, an abnormal ALT is greater than 19 IU/mL for women and greater than 25 IU/mL for men; in either setting, treatment should be instituted if the patient is HBV DNA positive. This position is supported by a recently published algorithm,6 a recent National Institutes of Health conference on management of HBV,7 and other sources.8–12
Barriers to optimal treatment
Patient reluctance to undergo invasive tests, concerns about resistance, confusion about when to initiate therapy, cost, and other issues can impede timely and effective treatment of HBV infection.
Invasive studies. Liver histology is a key driver for initiating treatment, but many patients resist undergoing a liver biopsy. Ultrasonography has enabled noninvasive determination of spleen size, portal vein size, and liver tissue and surface heterogeneity; noninvasive assessments such as measurement of aspartate aminotransferase, varices, serum markers of fibrosis, and platelet count may provide clues to advanced liver fibrosis. Eventually, ultrasonographic elastography to measure liver stiffness and magnetic resonance scans may be common in clinical practice for noninvasive evaluation of liver damage. Ultimately, however, liver biopsy remains a valuable tool to motivate patients with chronic HBV infection to initiate and continue antiviral therapy.
Rationales for avoiding or delaying treatment. Concern about the development of resistance to treatment, as with antiviral therapy directed against human immunodeficiency virus (HIV), is one reason not to treat. The absence of clear guidelines regarding the appropriate time to terminate therapy has also led to avoidance or delay of treatment. The lack of risk calculators similar to the Framingham risk score, which estimates the risk of coronary heart disease, has limited the treatment of chronic HBV infection.
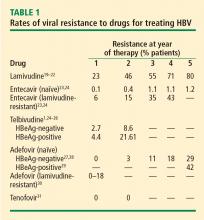
Cost. Cost must be examined in relation to the cost of resistance developing and the cost of treating complications. Lamivudine, considered a third-line treatment for chronic HBV infection, is an inexpensive drug. However, up to 70% of patients will develop resistance to lamivudine over 5 years3,6; most will require combination therapy, with its attendant costs, and may eventually require transplants or experience poor clinical outcomes. Although the initial costs of potent first-line therapies (tenofovir, entecavir, and pegylated interferon) are high, cost modeling shows that they are less expensive over the long term when the overall cost of care is considered.13,14
GOALS OF THERAPY: VIRAL LOAD SUPPRESSION, SEROCONVERSION
Profound suppression of viral load reduces the risk of resistance and is the ultimate goal of therapy for HBV infection. We can infer from recent data15 that achieving HBV DNA negativity has led to improved outcomes in patients with chronic HBV infection; ie, with the increased use of antiviral drugs in the United States over the past 2 decades, the number of liver transplants for end-stage liver disease has fallen dramatically,15 suggesting that profound suppression of viral loads has translated into fewer cases of liver failure and less need for transplants.
Over the same period, the number of patients diagnosed annually with HCC has increased by 146%.15 One interpretation of these data is that patients with chronic HBV infection are living longer, allowing time for HCC to develop. In addition, aggressive surveillance guidelines may account for the increased number of HCC cases since 1990. If detected early, HCC is curable by liver transplant at a rate exceeding 80%.16–18
In discussing treatment duration with patients, I present the ultimate goal of therapy as loss of HB surface antigen (HBsAg), or seroconversion to anti-HBs. At our clinic, we monitor HBsAg at least annually when patients are on long-term therapy.
The cost-effectiveness of treating all patients until they are HBsAg negative needs to be assessed. Incremental cost-effectiveness ratios per quality-adjusted life-year are key to identifying the best course of action.
TREATMENT OPTIONS
Nucleoside analogues
Lamivudine. The incidence of lamivudine resistance increases with increased treatment duration, reaching a peak of 80% after 5 years of treatment19–22; use of this agent eventually requires combination therapy. For this reason, lamivudine is considered a third-line drug and is not recommended as a first-line therapy.
Entecavir. Entecavir induces profound suppression of HBV DNA (to undetectable levels by weeks 24 to 36) in patients who are HBeAg positive or negative, regardless of baseline HBV DNA levels; resistance rates are very low in treatment-naïve patients,23 and entecavir is therefore considered first-line therapy. More than 90% of HBeAg-positive or -negative patients who are adherent to entecavir are HBV DNA negative at 5 years.24 Loss of HBsAg is 5% in entecavir-treated patients at follow-up of approximately 80 weeks, which is roughly double the rate of HBsAg loss with lamivudine.32
Telbivudine. Telbivudine has a secondary role in treatment of HBV infection. In a study by Lai et al,25,26 the cumulative incidence of telbivudine resistance and virologic breakthrough in HBeAg-positive patients rose from nearly 5% after 1 year to 22% after 2 years of treatment. Although the incidence was lower in HBeAg-negative patients, rates of genotypic resistance with virologic breakthrough rose to 9% in this population.
Since these results report genotypic resistance and virologic breakthrough, the rates of genotypic resistance in these patients may actually be higher than reported. Indeed, genotypic resistance was detected in 6.8% of the entire study population after 1 year of treatment. In this study, it must be remembered that patients with HBV DNA levels that were detectable by PCR (≥ 300 copies/mL) but were less than 1,000 copies/mL were not assessed for resistance.
Because of high rates of resistance associated with telbivudine, its role in the treatment of chronic HBV is secondary. I may use it in pregnant patients because most other nucleoside analogues are category C drugs and telbivudine is a category B agent (see “Management of hepatitis B in pregnancy: Weighing the options”). There are risks of myositis and neuropathy with telbivudine; although these risks are low, I mention them to patients when discussing a treatment plan.
Nucleotide analogues
Adefovir. Adefovir is considered second-line or add-on therapy when resistance to lamivudine develops because of its low potency in suppressing viral load. At 48 weeks, only 12% of HBeAg-positive patients are HBV DNA negative when treated with adefovir monotherapy.33,34
In a phase 3 clinical trial, genotypic resistance to adefovir was detected in 29% of HBeAg-negative patients treated for up to 5 years.27 The probability of resistance with virologic breakthrough was 3%, 8%, 14%, and 20% after 2, 3, 4, and 5 years of treatment, respectively.
In patients infected with lamivudine-resistant HBV, the probability of adefovir resistance is reduced by adding adefovir to ongoing lamivudine therapy, according to data from a large retrospective comparative study.35 In patients treated with adefovir monotherapy, the probability of virologic breakthrough (defined as > 1 log10 rebound in HBV DNA compared with on-treatment nadir) reached 30% over 36 months. In patients treated with add-on adefovir, the probability of virologic breakthrough was reduced to 6%. Similarly, the probability of adefovir resistance over 36 months of treatment was greater in the adefovir monotherapy group (16%) than in the add-on adefovir group (0%).
Although adefovir resistance is observed infrequently when adefovir is added to lamivudine, the effectiveness of adding adefovir is still limited by its low potency.
Tenofovir. More than 90% of HBeAg-negative patients and nearly 80% of HBeAg-positive patients treated with tenofovir have persistent virologic responses and HBV DNA levels less than 400 copies/mL by 72 weeks, with minimal side effects.33,34 Marcellin et al reported no development of resistance to tenofovir after 48 weeks of treatment.31 Although the nucleotide analogues have been associated with renal toxicity,36 the risk of renal toxicity associated with tenofovir is 1% or less per year; it can be reduced even further by calculating renal function through the use of the Cockroft-Gault equation or the Modification of Diet in Renal Disease equation prior to therapy and adjusting the dosage accordingly.37
With profound HBV DNA suppression, HBsAg loss occurs in about 5% of tenofovir-treated patients at 64 weeks.33
Treatment with tenofovir in treatment-experienced patients leads to potent suppression of HBV DNA independent of HBV genotype, HBV mutations (YMDD mutations) that signal lamivudine resistance, or HBeAg status at baseline.38 Patients with genotypic resistance to adefovir at baseline had a lower probability of achieving HBV DNA suppression during treatment with tenofovir.
Pegylated interferon
Pegylated interferon has proven useful in subsets of HBV DNA–positive patients. These include patients with genotype A or B who are young, those with high ALT levels (≥ 2 or 3 times the upper limit of normal) and low viral load (< 107 copies/mL), and patients without significant comorbidities.6 Pegylated interferon is also an option for patients who require a defined treatment period (eg, a woman wishing to become pregnant in 1 to 2 years). The patients who would benefit from pegylated interferon as first-line therapy must be better defined, and early markers of virologic response need to be identified.
PREVENTING AND MANAGING RESISTANCE
Antiviral drug resistance has a negative impact on the treatment of patients with chronic HBV infection. The development of resistance can result in virologic breakthrough (a confirmed 1 log10 increase in plasma HBV DNA levels)1; increased ALT levels1,39; and the progression of liver disease,40 including hepatic decompensation, development of HCC, and need for liver transplant. In addition, resistance mutations may re-emerge, with covalently closed circular DNA representing a genetic archive for development of resistance; this can significantly limit future treatment options.41 Early detection and regular monitoring are critical to prevention and management of resistance.
Detection
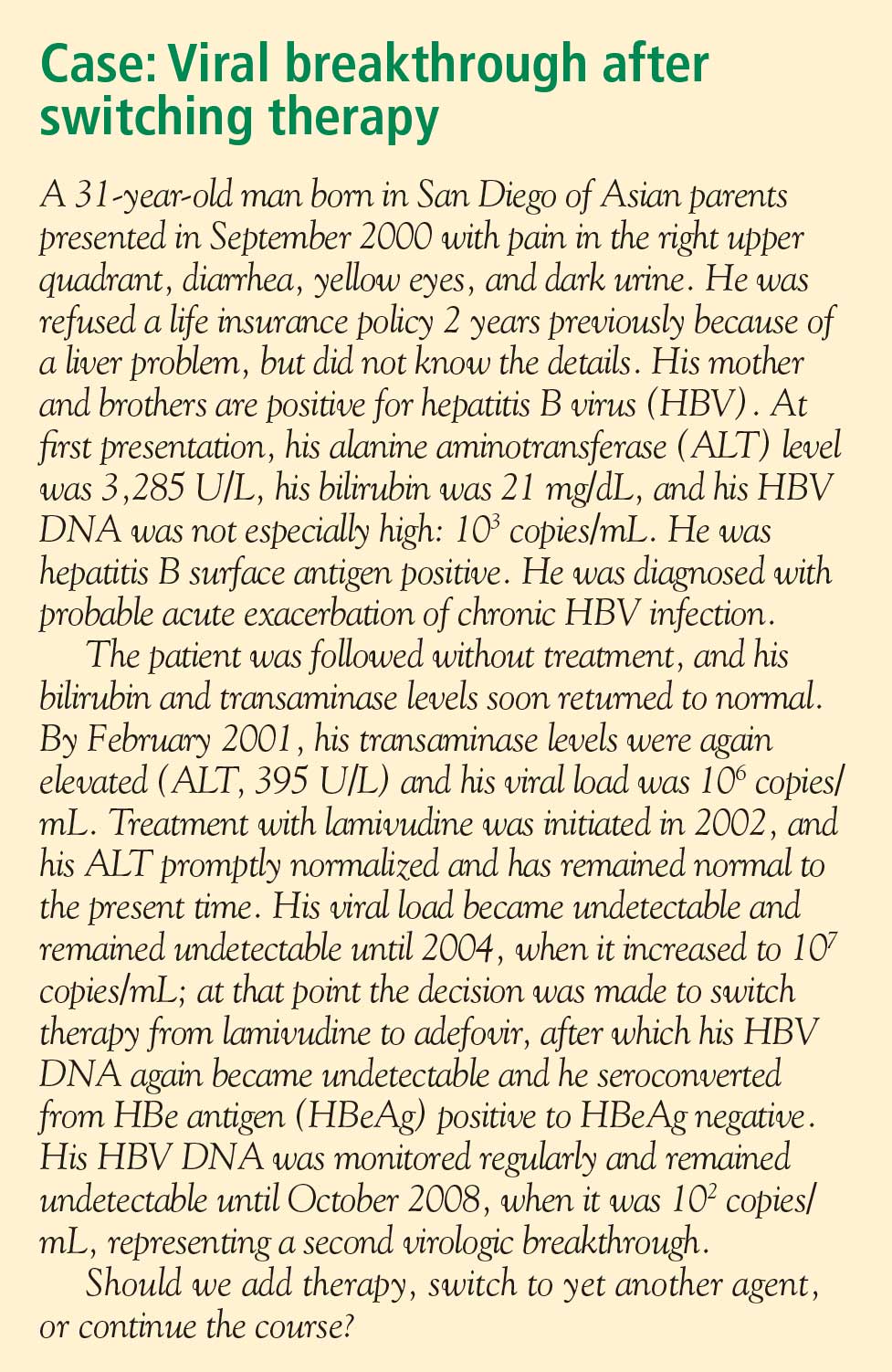
Detecting virologic breakthrough as early as possible increases the likelihood of achieving virologic response. In a study by Rapti and colleagues,42 patients with lamivudine-resistant chronic HBV were treated with a combination of lamivudine and adefovir. The 3-year cumulative probability of virologic response (< 103 copies/mL) was 99% with the addition of adefovir when baseline viral load levels were less than 5 log10 copies/mL, but only 71% when baseline viral loads were greater than 6 log10 copies/mL.
Monitoring
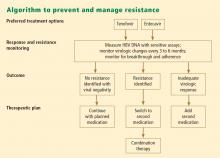
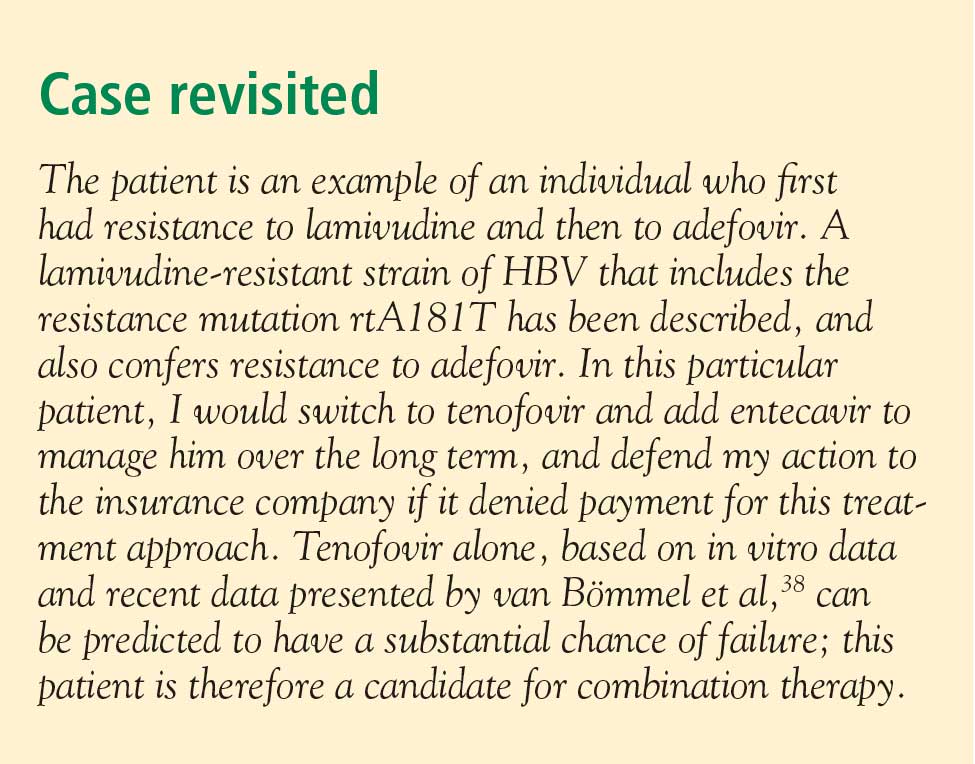
Patient response must be defined correctly. In adherent patients who show an early favorable response to therapy, I advise HBV DNA testing every 3 to 6 months. For those whose response flattens and whose viral load remains high, switching therapy or adding on should be considered. We continue therapy and monitor regularly after HBV DNA reaches an undetectable level. If the response is suboptimal, the treatment regimen is adapted by adding a new agent or switching to an alternative therapy (see “Case revisited”).
For patients who are being treated with tenofovir or entecavir, I typically extend the interval of measuring DNA levels to every 6 months because rates of resistance with these agents are low. If response is suboptimal but resistance is absent, I consider switching to the opposite drug. In those patients with a resistance mutation, I add the other agent.
Managing resistance
Combination therapy has a role in individuals in whom medication has failed to suppress viral load, in the setting of drug resistance, after liver transplant, and in individuals coinfected with HIV (see “Strategies for managing coinfection with hepatitis B virus and HIV”). If patients demonstrate resistance to their current therapy, we examine viral factors, adherence to therapy, and medication availability (eg, cost and insurance coverage). Switching to entecavir in adefovir-resistant patients produces profound suppression of HBV DNA. Patients in whom entecavir or lamivudine have failed may respond to tenofovir, depending on the resistance mutations.
A POTENTIAL FUTURE OPTION
Clevudine is a nucleoside analogue in phase 3 clinical studies in the United States. Its potential role in therapy is not yet clear. To be determined is whether it will induce a long-term, off-treatment viral response, in which case treatment may be able to be terminated earlier, and whether it will show clinically important cross-resistance with other nucleoside analogues. The availability of more sensitive assays to demonstrate the emergence of early viral resistance would enable earlier changes in treatment for more successful outcomes.
SUMMARY
Preventing resistance is crucial to the success of antiviral drug therapy for treatment of chronic HBV; a persistently high viral load increases the risk of cirrhosis and HCC, and resistance is associated with increased HBV DNA levels. The best chance for long-term success depends on initiating therapy before cirrhosis develops, when viral load is still low; profound suppression of viral load using the most potent agents as first-line therapy; and long-term monitoring of HBV DNA. The development of resistance can result in virologic breakthrough and liver complications. Entecavir and tenofovir represent the most effective first-line options to suppress HBV DNA. Because cross-resistance can occur, adding another agent is preferred to switching agents if resistance to initial therapy develops.
- Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology 2007; 45:507–539.
- Liaw Y-F, Leung N, Kao J-H, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int 2008; 2:263–283.
- European Association for the Study of the Liver. EASL clinical practice guidelines: management of chronic hepatitis B. J Hepatol 2009; 50:227–242.
- Chen CJ, Yang HI, Su J, et al. Serial monitoring of viral load and serum alanine aminotransferase level and the risk of hepatocellular carcinoma (HCC): R.E.V.E.A.L.-HBV study update [abstract 141]. J Hepatol 2008; 48(suppl 2):S61.
- Chen JD, Yang HI, Iloeje UH, et al. Liver disease progression in chronic hepatitis B infected persons with normal serum alanine amino transferase level: update from the R.E.V.E.A.L.-HBV study [abstract 644]. J Hepatol 2008; 48(suppl 2):S240.
- Keeffe EG, Dieterich DT, Han S-H B, et al. Special report. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol 2008; 6:1315–1341.
- Sorrell MF, Belongia EA, Costa J, et al. National Institutes of Health Consensus Development Conference statement: management of hepatitis B. Ann Intern Med 2009; 150:104–112.
- Piton A, Poynard T, Imbert-Bismut F, et al. Factors associated with serum alanine aminotransaminase activity in healthy subjects: consequences for the definition of normal values, for selection of blood donors, and for patients with chronic hepatitis C. MULTIVIRC group. Hepatology 1998; 27:1213–1219.
- Kim CH, Nam CM, Jee SH, Khan KH, Oh DK, Suh I. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ 2004; 328:983–986.
- Ioannou GN, Weiss NS, Boyko EJ, Mozaffarian D, Lee SP. Elevated serum alanine aminotransferase activity and calculated risk of coronary heart disease in the United States. Hepatology 2006; 43:1145–1151.
- Puoti C, Magrini A, Stati TN, et al. Clinical, histological, and virological features of hepatitis C virus carriers with persistently normal or abnormal alanine transaminase levels. Hepatology 1997; 26:1393–1398.
- Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med 2002; 137:1–9.
- Deniz B, Buti M, Brosa M, et al. Cost-effectiveness simulation analysis of tenofovir disoproxil fumarate, lamivudine, adefovir dipivoxil, and entecavir of HbeAg negative patients with chronic hepatitis B in Spain [EASL abstract 558]. J Hepatol 2008; 48(suppl 2):S209.
- Deniz B, Everhard R. Cost-effectiveness simulation analysis of tenofovir disoproxil fumarate in HBeAg negative patients with chronic hepatitis B in Italy and France [EASL abstract 559]. J Hepatol 2008; 48(suppl 2):S210.
- Kim W, Benson JT, Hindman A, Brosgart C, Fortner-Burton C. Decline in the need for liver transplantation for end stage liver disease secondary to hepatitis B in the US. Paper presented at: 58th Annual Meeting of the American Association for the Study of Liver Diseases; November 2–6, 2007; Boston, MA. Abstract 12.
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996; 334:693–700.
- Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 1999; 30:1434–1440.
- Yao FY, Bass NM, Nikolai B, et al. A follow-up analysis of the pattern and predictors of dropout from the waiting list for liver transplantation in patients with hepatocellular carcinoma: implications for the current organ allocation policy. Liver Transpl 2003; 9:684–692.
- Lai CL, Ratziu V, Yuen M-F, Poynard T. Viral hepatitis B. Lancet 2003; 362:2089–2094.
- Leung NW, Lai C-L, Chang T-T, et al. Extended lamivudine treatment in patients with chronic hepatitis B enhances hepatitis B e antigen seroconversion rates: results after 3 years of therapy. Hepatology 2001; 33:1527–1532.
- Benhamou Y, Bochet M, Thibault V, et al. Long-term incidence of hepatitis B virus resistance to lamivudine in human immunodeficiency virus-infected patients. Hepatology 1999; 30:1302–1306.
- Lok AS, Lai CL, Leung N, et al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology 2003; 125:1714–1722.
- Colonno RJ, Rose R, Baldick CJ, et al. Entecavir resistance is rare in nucleoside naive patients with hepatitis B. Hepatology 2006; 44:1656–1665.
- Perrillo RP. Current treatment of chronic hepatitis B: benefits and limitations. Semin Liver Dis 2005; 25(suppl 1):20–28.
- Lai C-L, Gane E, Liaw Y-F, et al. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med 2007; 357:2576–2588.
- Lai C-L, Gane E, Hsu C-W, et al. Two-year results from the GLOBE trial in patients with hepatitis B: greater clinical and antiviral efficacy for telbivudine (LDT) vs. lamivudine [AASLD abstract 91]. Hepatology 2006; 44(suppl 1):222A.
- Locarnini S, Qi X, Arterburn S, et al. Incidence and predictors of emergence of adefovir resistant HBV during four years of adefovir dipivoxil therapy for patients with chronic hepatitis B [EASL abstract 36]. J Hepatol 2005; 42(suppl 2):17.
- Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al; Adefovir Dipivoxil 438 Study Group. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B. N Engl J Med 2005; 352:2673–2681.
- Hepsera [package insert]. Foster City, CA: Gilead Sciences, Inc; 2008.
- Lee YS, Suh DJ, Lim YS, et al. Increased risk of adefovir resistance in patients with lamivudine-resistant chronic hepatitis B after 48 weeks of adefovir dipivoxil monotherapy. Hepatology 2006; 43:1385–1391.
- Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med 2008; 359:2442–2455.
- Gish R, Chang T-T, Lai C-L, et al. Hepatitis B surface antigen loss in antiviral-treated patients with HBeAg(+) chronic hepatitis B infection: observations from antiviral-naïve patients treated with entecavir or lamivudine. Paper presented at: 58th Annual Meeting of the American Association for the Study of Liver Diseases; November 2–6, 2007; Boston, MA. Abstract 992.
- Heathcote J, George J, Gordon S, et al. Tenofovir disoproxil fumarate (TDF) for the treatment of HBeAg-positive chronic hepatitis B: week 72 TDF data and week 24 adefovir dipivoxil switch data (study 103) [EASL abstract 71]. J Hepatol 2008; 48(suppl 2):S32.
- Marcellin P, Jacobson I, Habersetzer F, et al. Tenofovir disoproxil fumarate (TDF) for the treatment of HBeAg-negative chronic hepatitis B: week 72 TDF data and week 24 adefovir dipivoxil switch data (study 102) [EASL abstract 57]. J Hepatol 2008; 48(suppl 2):S26.
- Lampertico P, Marzano A, Levrero M, et al. Adefovir and lamivudine combination therapy is superior to adefovir monotherapy for lamivudine-resistant patients with HBeAg-negative chronic hepatitis B [EASL abstract 502]. J Hepatol 2007; 46(suppl 1):S191.
- Ha NB, Ha NB, Trinh HN. Changes in creatinine clearance (CRCL) in chronic hepatitis B (CHB) patients treated with adefovir dipivoxil (ADV) [AASLD abstract 901]. Hepatology 2008; 48:709A–710A.
- Gallant J, Staszewski S, Pozniak AL, et al; for the 903 Study Team. Similar renal safety profile between tenofovir DF (TDF) and stavudine (d4T) using modification of diet in renal disease (MDRD) and Cockcroft-Gault (CG) estimations of glomerular filtration rate (GFR) in antiretroviral-naïve patients through 144 weeks. In: Program and Abstracts of the 45th Interscience Conference on Antimicrobial Agents and Chemotherapy; December 16–19, 2005; Washington, DC. Abstract H-350.
- van Bömmel F, de Man RA, Stein K, et al. A multicenter analysis of antiviral response after one year of tenofovir monotherapy in HBV-monoinfected patients with prior nucleos(t)ide analog experience [EASL abstract 73]. J Hepatol 2008; 48(suppl 2):S32.
- Fung SK, Lok AS. Management of hepatitis B patients with antiviral resistance. Antivir Ther 2004; 9:1013–1026.
- Gish RG. Chronic hepatitis B virus: treating patients to prevent and manage resistance. US Gastroenterology Review 2007; March:51–54.
- Zoulim F. Mechanism of viral persistence and resistance to nucleoside and nucleotide analogs in chronic hepatitis B virus infection. Antiviral Res 2004; 64:1–15.
- Rapti I, Dimou E, Mitsoula P, Hadziyannis SJ. Adding-on versus switching-to adefovir therapy in lamivudine-resistant HBeAg-negative chronic hepatitis B. Hepatology 2007; 45:307–313.
Guidelines for the management of hepatitis B virus (HBV) infection can be daunting to clinicians. Further, although established practice guidelines can provide direction, treatment of chronic HBV infection is characterized by uncertainties that can hinder optimal patient care. Reservations about when to initiate and terminate therapy, cost issues, and the development of resistance to therapy are among the factors that impede adequate treatment. This article offers a straightforward roadmap for the management of chronic HBV infection, based on interpretation of recently released guidelines,1–3 and strategies for preventing and managing resistance to antiviral therapy.
DECIDING TO TREAT
Key factors: Viral load and ALT
Two important factors influencing the decision to treat are viral load (HBV DNA) and alanine aminotransferase (ALT) level; although these are relatively straightforward measures, other factors can cause clinicians to avoid or delay treatment.
A simple guideline is to discuss treatment with any patient who is positive for HBV DNA. The most recent guidelines for the treatment of HBV infection, published by the European Association for the Study of the Liver (EASL), recommend an HBV DNA level of 2,000 copies/mL as a threshold for initiating therapy; this recommendation applies to patients who are either positive or negative for hepatitis B e antigen (HBeAg).3
The Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-Hepatitis B Virus (REVEAL-HBV) study investigators used ultrasensitive polymerase chain reaction (PCR) to quantify HBV DNA levels and conducted a time-dependent multiple Cox regression analysis of HBV DNA level and the risk of hepatocellular carcinoma (HCC).4,5 The length of time at a given DNA level was weighted in determining the adjusted hazard ratio. With an HBV DNA level less than 300 copies/mL defined as the reference group, risk of HCC increased commensurate with increasing HBV DNA level; even at levels ranging from 300 to 10,000 copies/mL, longer duration of HBV DNA positivity increased risk. This group also found HBV DNA level to be an independent risk factor for cirrhosis.
Patients who are HBV DNA negative are at much lower risk of cirrhosis and HCC than HBV DNA–positive patients; HBV DNA–negative patients being treated with antiviral drugs are much less likely to develop resistance to treatment, provided that first-line medications such as tenofovir or entecavir are used.
The definition of a “healthy” ALT level is controversial. In my opinion, an abnormal ALT is greater than 19 IU/mL for women and greater than 25 IU/mL for men; in either setting, treatment should be instituted if the patient is HBV DNA positive. This position is supported by a recently published algorithm,6 a recent National Institutes of Health conference on management of HBV,7 and other sources.8–12
Barriers to optimal treatment
Patient reluctance to undergo invasive tests, concerns about resistance, confusion about when to initiate therapy, cost, and other issues can impede timely and effective treatment of HBV infection.
Invasive studies. Liver histology is a key driver for initiating treatment, but many patients resist undergoing a liver biopsy. Ultrasonography has enabled noninvasive determination of spleen size, portal vein size, and liver tissue and surface heterogeneity; noninvasive assessments such as measurement of aspartate aminotransferase, varices, serum markers of fibrosis, and platelet count may provide clues to advanced liver fibrosis. Eventually, ultrasonographic elastography to measure liver stiffness and magnetic resonance scans may be common in clinical practice for noninvasive evaluation of liver damage. Ultimately, however, liver biopsy remains a valuable tool to motivate patients with chronic HBV infection to initiate and continue antiviral therapy.
Rationales for avoiding or delaying treatment. Concern about the development of resistance to treatment, as with antiviral therapy directed against human immunodeficiency virus (HIV), is one reason not to treat. The absence of clear guidelines regarding the appropriate time to terminate therapy has also led to avoidance or delay of treatment. The lack of risk calculators similar to the Framingham risk score, which estimates the risk of coronary heart disease, has limited the treatment of chronic HBV infection.
Cost. Cost must be examined in relation to the cost of resistance developing and the cost of treating complications. Lamivudine, considered a third-line treatment for chronic HBV infection, is an inexpensive drug. However, up to 70% of patients will develop resistance to lamivudine over 5 years3,6; most will require combination therapy, with its attendant costs, and may eventually require transplants or experience poor clinical outcomes. Although the initial costs of potent first-line therapies (tenofovir, entecavir, and pegylated interferon) are high, cost modeling shows that they are less expensive over the long term when the overall cost of care is considered.13,14
GOALS OF THERAPY: VIRAL LOAD SUPPRESSION, SEROCONVERSION
Profound suppression of viral load reduces the risk of resistance and is the ultimate goal of therapy for HBV infection. We can infer from recent data15 that achieving HBV DNA negativity has led to improved outcomes in patients with chronic HBV infection; ie, with the increased use of antiviral drugs in the United States over the past 2 decades, the number of liver transplants for end-stage liver disease has fallen dramatically,15 suggesting that profound suppression of viral loads has translated into fewer cases of liver failure and less need for transplants.
Over the same period, the number of patients diagnosed annually with HCC has increased by 146%.15 One interpretation of these data is that patients with chronic HBV infection are living longer, allowing time for HCC to develop. In addition, aggressive surveillance guidelines may account for the increased number of HCC cases since 1990. If detected early, HCC is curable by liver transplant at a rate exceeding 80%.16–18
In discussing treatment duration with patients, I present the ultimate goal of therapy as loss of HB surface antigen (HBsAg), or seroconversion to anti-HBs. At our clinic, we monitor HBsAg at least annually when patients are on long-term therapy.
The cost-effectiveness of treating all patients until they are HBsAg negative needs to be assessed. Incremental cost-effectiveness ratios per quality-adjusted life-year are key to identifying the best course of action.
TREATMENT OPTIONS

Nucleoside analogues
Lamivudine. The incidence of lamivudine resistance increases with increased treatment duration, reaching a peak of 80% after 5 years of treatment19–22; use of this agent eventually requires combination therapy. For this reason, lamivudine is considered a third-line drug and is not recommended as a first-line therapy.
Entecavir. Entecavir induces profound suppression of HBV DNA (to undetectable levels by weeks 24 to 36) in patients who are HBeAg positive or negative, regardless of baseline HBV DNA levels; resistance rates are very low in treatment-naïve patients,23 and entecavir is therefore considered first-line therapy. More than 90% of HBeAg-positive or -negative patients who are adherent to entecavir are HBV DNA negative at 5 years.24 Loss of HBsAg is 5% in entecavir-treated patients at follow-up of approximately 80 weeks, which is roughly double the rate of HBsAg loss with lamivudine.32
Telbivudine. Telbivudine has a secondary role in treatment of HBV infection. In a study by Lai et al,25,26 the cumulative incidence of telbivudine resistance and virologic breakthrough in HBeAg-positive patients rose from nearly 5% after 1 year to 22% after 2 years of treatment. Although the incidence was lower in HBeAg-negative patients, rates of genotypic resistance with virologic breakthrough rose to 9% in this population.
Since these results report genotypic resistance and virologic breakthrough, the rates of genotypic resistance in these patients may actually be higher than reported. Indeed, genotypic resistance was detected in 6.8% of the entire study population after 1 year of treatment. In this study, it must be remembered that patients with HBV DNA levels that were detectable by PCR (≥ 300 copies/mL) but were less than 1,000 copies/mL were not assessed for resistance.
Because of high rates of resistance associated with telbivudine, its role in the treatment of chronic HBV is secondary. I may use it in pregnant patients because most other nucleoside analogues are category C drugs and telbivudine is a category B agent (see “Management of hepatitis B in pregnancy: Weighing the options”). There are risks of myositis and neuropathy with telbivudine; although these risks are low, I mention them to patients when discussing a treatment plan.
Nucleotide analogues
Adefovir. Adefovir is considered second-line or add-on therapy when resistance to lamivudine develops because of its low potency in suppressing viral load. At 48 weeks, only 12% of HBeAg-positive patients are HBV DNA negative when treated with adefovir monotherapy.33,34
In a phase 3 clinical trial, genotypic resistance to adefovir was detected in 29% of HBeAg-negative patients treated for up to 5 years.27 The probability of resistance with virologic breakthrough was 3%, 8%, 14%, and 20% after 2, 3, 4, and 5 years of treatment, respectively.
In patients infected with lamivudine-resistant HBV, the probability of adefovir resistance is reduced by adding adefovir to ongoing lamivudine therapy, according to data from a large retrospective comparative study.35 In patients treated with adefovir monotherapy, the probability of virologic breakthrough (defined as > 1 log10 rebound in HBV DNA compared with on-treatment nadir) reached 30% over 36 months. In patients treated with add-on adefovir, the probability of virologic breakthrough was reduced to 6%. Similarly, the probability of adefovir resistance over 36 months of treatment was greater in the adefovir monotherapy group (16%) than in the add-on adefovir group (0%).
Although adefovir resistance is observed infrequently when adefovir is added to lamivudine, the effectiveness of adding adefovir is still limited by its low potency.
Tenofovir. More than 90% of HBeAg-negative patients and nearly 80% of HBeAg-positive patients treated with tenofovir have persistent virologic responses and HBV DNA levels less than 400 copies/mL by 72 weeks, with minimal side effects.33,34 Marcellin et al reported no development of resistance to tenofovir after 48 weeks of treatment.31 Although the nucleotide analogues have been associated with renal toxicity,36 the risk of renal toxicity associated with tenofovir is 1% or less per year; it can be reduced even further by calculating renal function through the use of the Cockroft-Gault equation or the Modification of Diet in Renal Disease equation prior to therapy and adjusting the dosage accordingly.37
With profound HBV DNA suppression, HBsAg loss occurs in about 5% of tenofovir-treated patients at 64 weeks.33
Treatment with tenofovir in treatment-experienced patients leads to potent suppression of HBV DNA independent of HBV genotype, HBV mutations (YMDD mutations) that signal lamivudine resistance, or HBeAg status at baseline.38 Patients with genotypic resistance to adefovir at baseline had a lower probability of achieving HBV DNA suppression during treatment with tenofovir.
Pegylated interferon
Pegylated interferon has proven useful in subsets of HBV DNA–positive patients. These include patients with genotype A or B who are young, those with high ALT levels (≥ 2 or 3 times the upper limit of normal) and low viral load (< 107 copies/mL), and patients without significant comorbidities.6 Pegylated interferon is also an option for patients who require a defined treatment period (eg, a woman wishing to become pregnant in 1 to 2 years). The patients who would benefit from pegylated interferon as first-line therapy must be better defined, and early markers of virologic response need to be identified.
PREVENTING AND MANAGING RESISTANCE
Antiviral drug resistance has a negative impact on the treatment of patients with chronic HBV infection. The development of resistance can result in virologic breakthrough (a confirmed 1 log10 increase in plasma HBV DNA levels)1; increased ALT levels1,39; and the progression of liver disease,40 including hepatic decompensation, development of HCC, and need for liver transplant. In addition, resistance mutations may re-emerge, with covalently closed circular DNA representing a genetic archive for development of resistance; this can significantly limit future treatment options.41 Early detection and regular monitoring are critical to prevention and management of resistance.
Detection
Detecting virologic breakthrough as early as possible increases the likelihood of achieving virologic response. In a study by Rapti and colleagues,42 patients with lamivudine-resistant chronic HBV were treated with a combination of lamivudine and adefovir. The 3-year cumulative probability of virologic response (< 103 copies/mL) was 99% with the addition of adefovir when baseline viral load levels were less than 5 log10 copies/mL, but only 71% when baseline viral loads were greater than 6 log10 copies/mL.
Monitoring
Patient response must be defined correctly. In adherent patients who show an early favorable response to therapy, I advise HBV DNA testing every 3 to 6 months. For those whose response flattens and whose viral load remains high, switching therapy or adding on should be considered. We continue therapy and monitor regularly after HBV DNA reaches an undetectable level. If the response is suboptimal, the treatment regimen is adapted by adding a new agent or switching to an alternative therapy (see “Case revisited”).
For patients who are being treated with tenofovir or entecavir, I typically extend the interval of measuring DNA levels to every 6 months because rates of resistance with these agents are low. If response is suboptimal but resistance is absent, I consider switching to the opposite drug. In those patients with a resistance mutation, I add the other agent.

Managing resistance
Combination therapy has a role in individuals in whom medication has failed to suppress viral load, in the setting of drug resistance, after liver transplant, and in individuals coinfected with HIV (see “Strategies for managing coinfection with hepatitis B virus and HIV”). If patients demonstrate resistance to their current therapy, we examine viral factors, adherence to therapy, and medication availability (eg, cost and insurance coverage). Switching to entecavir in adefovir-resistant patients produces profound suppression of HBV DNA. Patients in whom entecavir or lamivudine have failed may respond to tenofovir, depending on the resistance mutations.
A POTENTIAL FUTURE OPTION
Clevudine is a nucleoside analogue in phase 3 clinical studies in the United States. Its potential role in therapy is not yet clear. To be determined is whether it will induce a long-term, off-treatment viral response, in which case treatment may be able to be terminated earlier, and whether it will show clinically important cross-resistance with other nucleoside analogues. The availability of more sensitive assays to demonstrate the emergence of early viral resistance would enable earlier changes in treatment for more successful outcomes.
SUMMARY
Preventing resistance is crucial to the success of antiviral drug therapy for treatment of chronic HBV; a persistently high viral load increases the risk of cirrhosis and HCC, and resistance is associated with increased HBV DNA levels. The best chance for long-term success depends on initiating therapy before cirrhosis develops, when viral load is still low; profound suppression of viral load using the most potent agents as first-line therapy; and long-term monitoring of HBV DNA. The development of resistance can result in virologic breakthrough and liver complications. Entecavir and tenofovir represent the most effective first-line options to suppress HBV DNA. Because cross-resistance can occur, adding another agent is preferred to switching agents if resistance to initial therapy develops.
Guidelines for the management of hepatitis B virus (HBV) infection can be daunting to clinicians. Further, although established practice guidelines can provide direction, treatment of chronic HBV infection is characterized by uncertainties that can hinder optimal patient care. Reservations about when to initiate and terminate therapy, cost issues, and the development of resistance to therapy are among the factors that impede adequate treatment. This article offers a straightforward roadmap for the management of chronic HBV infection, based on interpretation of recently released guidelines,1–3 and strategies for preventing and managing resistance to antiviral therapy.
DECIDING TO TREAT
Key factors: Viral load and ALT
Two important factors influencing the decision to treat are viral load (HBV DNA) and alanine aminotransferase (ALT) level; although these are relatively straightforward measures, other factors can cause clinicians to avoid or delay treatment.
A simple guideline is to discuss treatment with any patient who is positive for HBV DNA. The most recent guidelines for the treatment of HBV infection, published by the European Association for the Study of the Liver (EASL), recommend an HBV DNA level of 2,000 copies/mL as a threshold for initiating therapy; this recommendation applies to patients who are either positive or negative for hepatitis B e antigen (HBeAg).3
The Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-Hepatitis B Virus (REVEAL-HBV) study investigators used ultrasensitive polymerase chain reaction (PCR) to quantify HBV DNA levels and conducted a time-dependent multiple Cox regression analysis of HBV DNA level and the risk of hepatocellular carcinoma (HCC).4,5 The length of time at a given DNA level was weighted in determining the adjusted hazard ratio. With an HBV DNA level less than 300 copies/mL defined as the reference group, risk of HCC increased commensurate with increasing HBV DNA level; even at levels ranging from 300 to 10,000 copies/mL, longer duration of HBV DNA positivity increased risk. This group also found HBV DNA level to be an independent risk factor for cirrhosis.
Patients who are HBV DNA negative are at much lower risk of cirrhosis and HCC than HBV DNA–positive patients; HBV DNA–negative patients being treated with antiviral drugs are much less likely to develop resistance to treatment, provided that first-line medications such as tenofovir or entecavir are used.
The definition of a “healthy” ALT level is controversial. In my opinion, an abnormal ALT is greater than 19 IU/mL for women and greater than 25 IU/mL for men; in either setting, treatment should be instituted if the patient is HBV DNA positive. This position is supported by a recently published algorithm,6 a recent National Institutes of Health conference on management of HBV,7 and other sources.8–12
Barriers to optimal treatment
Patient reluctance to undergo invasive tests, concerns about resistance, confusion about when to initiate therapy, cost, and other issues can impede timely and effective treatment of HBV infection.
Invasive studies. Liver histology is a key driver for initiating treatment, but many patients resist undergoing a liver biopsy. Ultrasonography has enabled noninvasive determination of spleen size, portal vein size, and liver tissue and surface heterogeneity; noninvasive assessments such as measurement of aspartate aminotransferase, varices, serum markers of fibrosis, and platelet count may provide clues to advanced liver fibrosis. Eventually, ultrasonographic elastography to measure liver stiffness and magnetic resonance scans may be common in clinical practice for noninvasive evaluation of liver damage. Ultimately, however, liver biopsy remains a valuable tool to motivate patients with chronic HBV infection to initiate and continue antiviral therapy.
Rationales for avoiding or delaying treatment. Concern about the development of resistance to treatment, as with antiviral therapy directed against human immunodeficiency virus (HIV), is one reason not to treat. The absence of clear guidelines regarding the appropriate time to terminate therapy has also led to avoidance or delay of treatment. The lack of risk calculators similar to the Framingham risk score, which estimates the risk of coronary heart disease, has limited the treatment of chronic HBV infection.
Cost. Cost must be examined in relation to the cost of resistance developing and the cost of treating complications. Lamivudine, considered a third-line treatment for chronic HBV infection, is an inexpensive drug. However, up to 70% of patients will develop resistance to lamivudine over 5 years3,6; most will require combination therapy, with its attendant costs, and may eventually require transplants or experience poor clinical outcomes. Although the initial costs of potent first-line therapies (tenofovir, entecavir, and pegylated interferon) are high, cost modeling shows that they are less expensive over the long term when the overall cost of care is considered.13,14
GOALS OF THERAPY: VIRAL LOAD SUPPRESSION, SEROCONVERSION
Profound suppression of viral load reduces the risk of resistance and is the ultimate goal of therapy for HBV infection. We can infer from recent data15 that achieving HBV DNA negativity has led to improved outcomes in patients with chronic HBV infection; ie, with the increased use of antiviral drugs in the United States over the past 2 decades, the number of liver transplants for end-stage liver disease has fallen dramatically,15 suggesting that profound suppression of viral loads has translated into fewer cases of liver failure and less need for transplants.
Over the same period, the number of patients diagnosed annually with HCC has increased by 146%.15 One interpretation of these data is that patients with chronic HBV infection are living longer, allowing time for HCC to develop. In addition, aggressive surveillance guidelines may account for the increased number of HCC cases since 1990. If detected early, HCC is curable by liver transplant at a rate exceeding 80%.16–18
In discussing treatment duration with patients, I present the ultimate goal of therapy as loss of HB surface antigen (HBsAg), or seroconversion to anti-HBs. At our clinic, we monitor HBsAg at least annually when patients are on long-term therapy.
The cost-effectiveness of treating all patients until they are HBsAg negative needs to be assessed. Incremental cost-effectiveness ratios per quality-adjusted life-year are key to identifying the best course of action.
TREATMENT OPTIONS

Nucleoside analogues
Lamivudine. The incidence of lamivudine resistance increases with increased treatment duration, reaching a peak of 80% after 5 years of treatment19–22; use of this agent eventually requires combination therapy. For this reason, lamivudine is considered a third-line drug and is not recommended as a first-line therapy.
Entecavir. Entecavir induces profound suppression of HBV DNA (to undetectable levels by weeks 24 to 36) in patients who are HBeAg positive or negative, regardless of baseline HBV DNA levels; resistance rates are very low in treatment-naïve patients,23 and entecavir is therefore considered first-line therapy. More than 90% of HBeAg-positive or -negative patients who are adherent to entecavir are HBV DNA negative at 5 years.24 Loss of HBsAg is 5% in entecavir-treated patients at follow-up of approximately 80 weeks, which is roughly double the rate of HBsAg loss with lamivudine.32
Telbivudine. Telbivudine has a secondary role in treatment of HBV infection. In a study by Lai et al,25,26 the cumulative incidence of telbivudine resistance and virologic breakthrough in HBeAg-positive patients rose from nearly 5% after 1 year to 22% after 2 years of treatment. Although the incidence was lower in HBeAg-negative patients, rates of genotypic resistance with virologic breakthrough rose to 9% in this population.
Since these results report genotypic resistance and virologic breakthrough, the rates of genotypic resistance in these patients may actually be higher than reported. Indeed, genotypic resistance was detected in 6.8% of the entire study population after 1 year of treatment. In this study, it must be remembered that patients with HBV DNA levels that were detectable by PCR (≥ 300 copies/mL) but were less than 1,000 copies/mL were not assessed for resistance.
Because of high rates of resistance associated with telbivudine, its role in the treatment of chronic HBV is secondary. I may use it in pregnant patients because most other nucleoside analogues are category C drugs and telbivudine is a category B agent (see “Management of hepatitis B in pregnancy: Weighing the options”). There are risks of myositis and neuropathy with telbivudine; although these risks are low, I mention them to patients when discussing a treatment plan.
Nucleotide analogues
Adefovir. Adefovir is considered second-line or add-on therapy when resistance to lamivudine develops because of its low potency in suppressing viral load. At 48 weeks, only 12% of HBeAg-positive patients are HBV DNA negative when treated with adefovir monotherapy.33,34
In a phase 3 clinical trial, genotypic resistance to adefovir was detected in 29% of HBeAg-negative patients treated for up to 5 years.27 The probability of resistance with virologic breakthrough was 3%, 8%, 14%, and 20% after 2, 3, 4, and 5 years of treatment, respectively.
In patients infected with lamivudine-resistant HBV, the probability of adefovir resistance is reduced by adding adefovir to ongoing lamivudine therapy, according to data from a large retrospective comparative study.35 In patients treated with adefovir monotherapy, the probability of virologic breakthrough (defined as > 1 log10 rebound in HBV DNA compared with on-treatment nadir) reached 30% over 36 months. In patients treated with add-on adefovir, the probability of virologic breakthrough was reduced to 6%. Similarly, the probability of adefovir resistance over 36 months of treatment was greater in the adefovir monotherapy group (16%) than in the add-on adefovir group (0%).
Although adefovir resistance is observed infrequently when adefovir is added to lamivudine, the effectiveness of adding adefovir is still limited by its low potency.
Tenofovir. More than 90% of HBeAg-negative patients and nearly 80% of HBeAg-positive patients treated with tenofovir have persistent virologic responses and HBV DNA levels less than 400 copies/mL by 72 weeks, with minimal side effects.33,34 Marcellin et al reported no development of resistance to tenofovir after 48 weeks of treatment.31 Although the nucleotide analogues have been associated with renal toxicity,36 the risk of renal toxicity associated with tenofovir is 1% or less per year; it can be reduced even further by calculating renal function through the use of the Cockroft-Gault equation or the Modification of Diet in Renal Disease equation prior to therapy and adjusting the dosage accordingly.37
With profound HBV DNA suppression, HBsAg loss occurs in about 5% of tenofovir-treated patients at 64 weeks.33
Treatment with tenofovir in treatment-experienced patients leads to potent suppression of HBV DNA independent of HBV genotype, HBV mutations (YMDD mutations) that signal lamivudine resistance, or HBeAg status at baseline.38 Patients with genotypic resistance to adefovir at baseline had a lower probability of achieving HBV DNA suppression during treatment with tenofovir.
Pegylated interferon
Pegylated interferon has proven useful in subsets of HBV DNA–positive patients. These include patients with genotype A or B who are young, those with high ALT levels (≥ 2 or 3 times the upper limit of normal) and low viral load (< 107 copies/mL), and patients without significant comorbidities.6 Pegylated interferon is also an option for patients who require a defined treatment period (eg, a woman wishing to become pregnant in 1 to 2 years). The patients who would benefit from pegylated interferon as first-line therapy must be better defined, and early markers of virologic response need to be identified.
PREVENTING AND MANAGING RESISTANCE
Antiviral drug resistance has a negative impact on the treatment of patients with chronic HBV infection. The development of resistance can result in virologic breakthrough (a confirmed 1 log10 increase in plasma HBV DNA levels)1; increased ALT levels1,39; and the progression of liver disease,40 including hepatic decompensation, development of HCC, and need for liver transplant. In addition, resistance mutations may re-emerge, with covalently closed circular DNA representing a genetic archive for development of resistance; this can significantly limit future treatment options.41 Early detection and regular monitoring are critical to prevention and management of resistance.
Detection
Detecting virologic breakthrough as early as possible increases the likelihood of achieving virologic response. In a study by Rapti and colleagues,42 patients with lamivudine-resistant chronic HBV were treated with a combination of lamivudine and adefovir. The 3-year cumulative probability of virologic response (< 103 copies/mL) was 99% with the addition of adefovir when baseline viral load levels were less than 5 log10 copies/mL, but only 71% when baseline viral loads were greater than 6 log10 copies/mL.
Monitoring
Patient response must be defined correctly. In adherent patients who show an early favorable response to therapy, I advise HBV DNA testing every 3 to 6 months. For those whose response flattens and whose viral load remains high, switching therapy or adding on should be considered. We continue therapy and monitor regularly after HBV DNA reaches an undetectable level. If the response is suboptimal, the treatment regimen is adapted by adding a new agent or switching to an alternative therapy (see “Case revisited”).
For patients who are being treated with tenofovir or entecavir, I typically extend the interval of measuring DNA levels to every 6 months because rates of resistance with these agents are low. If response is suboptimal but resistance is absent, I consider switching to the opposite drug. In those patients with a resistance mutation, I add the other agent.

Managing resistance
Combination therapy has a role in individuals in whom medication has failed to suppress viral load, in the setting of drug resistance, after liver transplant, and in individuals coinfected with HIV (see “Strategies for managing coinfection with hepatitis B virus and HIV”). If patients demonstrate resistance to their current therapy, we examine viral factors, adherence to therapy, and medication availability (eg, cost and insurance coverage). Switching to entecavir in adefovir-resistant patients produces profound suppression of HBV DNA. Patients in whom entecavir or lamivudine have failed may respond to tenofovir, depending on the resistance mutations.
A POTENTIAL FUTURE OPTION
Clevudine is a nucleoside analogue in phase 3 clinical studies in the United States. Its potential role in therapy is not yet clear. To be determined is whether it will induce a long-term, off-treatment viral response, in which case treatment may be able to be terminated earlier, and whether it will show clinically important cross-resistance with other nucleoside analogues. The availability of more sensitive assays to demonstrate the emergence of early viral resistance would enable earlier changes in treatment for more successful outcomes.
SUMMARY
Preventing resistance is crucial to the success of antiviral drug therapy for treatment of chronic HBV; a persistently high viral load increases the risk of cirrhosis and HCC, and resistance is associated with increased HBV DNA levels. The best chance for long-term success depends on initiating therapy before cirrhosis develops, when viral load is still low; profound suppression of viral load using the most potent agents as first-line therapy; and long-term monitoring of HBV DNA. The development of resistance can result in virologic breakthrough and liver complications. Entecavir and tenofovir represent the most effective first-line options to suppress HBV DNA. Because cross-resistance can occur, adding another agent is preferred to switching agents if resistance to initial therapy develops.
- Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology 2007; 45:507–539.
- Liaw Y-F, Leung N, Kao J-H, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int 2008; 2:263–283.
- European Association for the Study of the Liver. EASL clinical practice guidelines: management of chronic hepatitis B. J Hepatol 2009; 50:227–242.
- Chen CJ, Yang HI, Su J, et al. Serial monitoring of viral load and serum alanine aminotransferase level and the risk of hepatocellular carcinoma (HCC): R.E.V.E.A.L.-HBV study update [abstract 141]. J Hepatol 2008; 48(suppl 2):S61.
- Chen JD, Yang HI, Iloeje UH, et al. Liver disease progression in chronic hepatitis B infected persons with normal serum alanine amino transferase level: update from the R.E.V.E.A.L.-HBV study [abstract 644]. J Hepatol 2008; 48(suppl 2):S240.
- Keeffe EG, Dieterich DT, Han S-H B, et al. Special report. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol 2008; 6:1315–1341.
- Sorrell MF, Belongia EA, Costa J, et al. National Institutes of Health Consensus Development Conference statement: management of hepatitis B. Ann Intern Med 2009; 150:104–112.
- Piton A, Poynard T, Imbert-Bismut F, et al. Factors associated with serum alanine aminotransaminase activity in healthy subjects: consequences for the definition of normal values, for selection of blood donors, and for patients with chronic hepatitis C. MULTIVIRC group. Hepatology 1998; 27:1213–1219.
- Kim CH, Nam CM, Jee SH, Khan KH, Oh DK, Suh I. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ 2004; 328:983–986.
- Ioannou GN, Weiss NS, Boyko EJ, Mozaffarian D, Lee SP. Elevated serum alanine aminotransferase activity and calculated risk of coronary heart disease in the United States. Hepatology 2006; 43:1145–1151.
- Puoti C, Magrini A, Stati TN, et al. Clinical, histological, and virological features of hepatitis C virus carriers with persistently normal or abnormal alanine transaminase levels. Hepatology 1997; 26:1393–1398.
- Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med 2002; 137:1–9.
- Deniz B, Buti M, Brosa M, et al. Cost-effectiveness simulation analysis of tenofovir disoproxil fumarate, lamivudine, adefovir dipivoxil, and entecavir of HbeAg negative patients with chronic hepatitis B in Spain [EASL abstract 558]. J Hepatol 2008; 48(suppl 2):S209.
- Deniz B, Everhard R. Cost-effectiveness simulation analysis of tenofovir disoproxil fumarate in HBeAg negative patients with chronic hepatitis B in Italy and France [EASL abstract 559]. J Hepatol 2008; 48(suppl 2):S210.
- Kim W, Benson JT, Hindman A, Brosgart C, Fortner-Burton C. Decline in the need for liver transplantation for end stage liver disease secondary to hepatitis B in the US. Paper presented at: 58th Annual Meeting of the American Association for the Study of Liver Diseases; November 2–6, 2007; Boston, MA. Abstract 12.
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996; 334:693–700.
- Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 1999; 30:1434–1440.
- Yao FY, Bass NM, Nikolai B, et al. A follow-up analysis of the pattern and predictors of dropout from the waiting list for liver transplantation in patients with hepatocellular carcinoma: implications for the current organ allocation policy. Liver Transpl 2003; 9:684–692.
- Lai CL, Ratziu V, Yuen M-F, Poynard T. Viral hepatitis B. Lancet 2003; 362:2089–2094.
- Leung NW, Lai C-L, Chang T-T, et al. Extended lamivudine treatment in patients with chronic hepatitis B enhances hepatitis B e antigen seroconversion rates: results after 3 years of therapy. Hepatology 2001; 33:1527–1532.
- Benhamou Y, Bochet M, Thibault V, et al. Long-term incidence of hepatitis B virus resistance to lamivudine in human immunodeficiency virus-infected patients. Hepatology 1999; 30:1302–1306.
- Lok AS, Lai CL, Leung N, et al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology 2003; 125:1714–1722.
- Colonno RJ, Rose R, Baldick CJ, et al. Entecavir resistance is rare in nucleoside naive patients with hepatitis B. Hepatology 2006; 44:1656–1665.
- Perrillo RP. Current treatment of chronic hepatitis B: benefits and limitations. Semin Liver Dis 2005; 25(suppl 1):20–28.
- Lai C-L, Gane E, Liaw Y-F, et al. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med 2007; 357:2576–2588.
- Lai C-L, Gane E, Hsu C-W, et al. Two-year results from the GLOBE trial in patients with hepatitis B: greater clinical and antiviral efficacy for telbivudine (LDT) vs. lamivudine [AASLD abstract 91]. Hepatology 2006; 44(suppl 1):222A.
- Locarnini S, Qi X, Arterburn S, et al. Incidence and predictors of emergence of adefovir resistant HBV during four years of adefovir dipivoxil therapy for patients with chronic hepatitis B [EASL abstract 36]. J Hepatol 2005; 42(suppl 2):17.
- Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al; Adefovir Dipivoxil 438 Study Group. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B. N Engl J Med 2005; 352:2673–2681.
- Hepsera [package insert]. Foster City, CA: Gilead Sciences, Inc; 2008.
- Lee YS, Suh DJ, Lim YS, et al. Increased risk of adefovir resistance in patients with lamivudine-resistant chronic hepatitis B after 48 weeks of adefovir dipivoxil monotherapy. Hepatology 2006; 43:1385–1391.
- Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med 2008; 359:2442–2455.
- Gish R, Chang T-T, Lai C-L, et al. Hepatitis B surface antigen loss in antiviral-treated patients with HBeAg(+) chronic hepatitis B infection: observations from antiviral-naïve patients treated with entecavir or lamivudine. Paper presented at: 58th Annual Meeting of the American Association for the Study of Liver Diseases; November 2–6, 2007; Boston, MA. Abstract 992.
- Heathcote J, George J, Gordon S, et al. Tenofovir disoproxil fumarate (TDF) for the treatment of HBeAg-positive chronic hepatitis B: week 72 TDF data and week 24 adefovir dipivoxil switch data (study 103) [EASL abstract 71]. J Hepatol 2008; 48(suppl 2):S32.
- Marcellin P, Jacobson I, Habersetzer F, et al. Tenofovir disoproxil fumarate (TDF) for the treatment of HBeAg-negative chronic hepatitis B: week 72 TDF data and week 24 adefovir dipivoxil switch data (study 102) [EASL abstract 57]. J Hepatol 2008; 48(suppl 2):S26.
- Lampertico P, Marzano A, Levrero M, et al. Adefovir and lamivudine combination therapy is superior to adefovir monotherapy for lamivudine-resistant patients with HBeAg-negative chronic hepatitis B [EASL abstract 502]. J Hepatol 2007; 46(suppl 1):S191.
- Ha NB, Ha NB, Trinh HN. Changes in creatinine clearance (CRCL) in chronic hepatitis B (CHB) patients treated with adefovir dipivoxil (ADV) [AASLD abstract 901]. Hepatology 2008; 48:709A–710A.
- Gallant J, Staszewski S, Pozniak AL, et al; for the 903 Study Team. Similar renal safety profile between tenofovir DF (TDF) and stavudine (d4T) using modification of diet in renal disease (MDRD) and Cockcroft-Gault (CG) estimations of glomerular filtration rate (GFR) in antiretroviral-naïve patients through 144 weeks. In: Program and Abstracts of the 45th Interscience Conference on Antimicrobial Agents and Chemotherapy; December 16–19, 2005; Washington, DC. Abstract H-350.
- van Bömmel F, de Man RA, Stein K, et al. A multicenter analysis of antiviral response after one year of tenofovir monotherapy in HBV-monoinfected patients with prior nucleos(t)ide analog experience [EASL abstract 73]. J Hepatol 2008; 48(suppl 2):S32.
- Fung SK, Lok AS. Management of hepatitis B patients with antiviral resistance. Antivir Ther 2004; 9:1013–1026.
- Gish RG. Chronic hepatitis B virus: treating patients to prevent and manage resistance. US Gastroenterology Review 2007; March:51–54.
- Zoulim F. Mechanism of viral persistence and resistance to nucleoside and nucleotide analogs in chronic hepatitis B virus infection. Antiviral Res 2004; 64:1–15.
- Rapti I, Dimou E, Mitsoula P, Hadziyannis SJ. Adding-on versus switching-to adefovir therapy in lamivudine-resistant HBeAg-negative chronic hepatitis B. Hepatology 2007; 45:307–313.
- Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology 2007; 45:507–539.
- Liaw Y-F, Leung N, Kao J-H, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int 2008; 2:263–283.
- European Association for the Study of the Liver. EASL clinical practice guidelines: management of chronic hepatitis B. J Hepatol 2009; 50:227–242.
- Chen CJ, Yang HI, Su J, et al. Serial monitoring of viral load and serum alanine aminotransferase level and the risk of hepatocellular carcinoma (HCC): R.E.V.E.A.L.-HBV study update [abstract 141]. J Hepatol 2008; 48(suppl 2):S61.
- Chen JD, Yang HI, Iloeje UH, et al. Liver disease progression in chronic hepatitis B infected persons with normal serum alanine amino transferase level: update from the R.E.V.E.A.L.-HBV study [abstract 644]. J Hepatol 2008; 48(suppl 2):S240.
- Keeffe EG, Dieterich DT, Han S-H B, et al. Special report. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol 2008; 6:1315–1341.
- Sorrell MF, Belongia EA, Costa J, et al. National Institutes of Health Consensus Development Conference statement: management of hepatitis B. Ann Intern Med 2009; 150:104–112.
- Piton A, Poynard T, Imbert-Bismut F, et al. Factors associated with serum alanine aminotransaminase activity in healthy subjects: consequences for the definition of normal values, for selection of blood donors, and for patients with chronic hepatitis C. MULTIVIRC group. Hepatology 1998; 27:1213–1219.
- Kim CH, Nam CM, Jee SH, Khan KH, Oh DK, Suh I. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ 2004; 328:983–986.
- Ioannou GN, Weiss NS, Boyko EJ, Mozaffarian D, Lee SP. Elevated serum alanine aminotransferase activity and calculated risk of coronary heart disease in the United States. Hepatology 2006; 43:1145–1151.
- Puoti C, Magrini A, Stati TN, et al. Clinical, histological, and virological features of hepatitis C virus carriers with persistently normal or abnormal alanine transaminase levels. Hepatology 1997; 26:1393–1398.
- Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med 2002; 137:1–9.
- Deniz B, Buti M, Brosa M, et al. Cost-effectiveness simulation analysis of tenofovir disoproxil fumarate, lamivudine, adefovir dipivoxil, and entecavir of HbeAg negative patients with chronic hepatitis B in Spain [EASL abstract 558]. J Hepatol 2008; 48(suppl 2):S209.
- Deniz B, Everhard R. Cost-effectiveness simulation analysis of tenofovir disoproxil fumarate in HBeAg negative patients with chronic hepatitis B in Italy and France [EASL abstract 559]. J Hepatol 2008; 48(suppl 2):S210.
- Kim W, Benson JT, Hindman A, Brosgart C, Fortner-Burton C. Decline in the need for liver transplantation for end stage liver disease secondary to hepatitis B in the US. Paper presented at: 58th Annual Meeting of the American Association for the Study of Liver Diseases; November 2–6, 2007; Boston, MA. Abstract 12.
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996; 334:693–700.
- Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 1999; 30:1434–1440.
- Yao FY, Bass NM, Nikolai B, et al. A follow-up analysis of the pattern and predictors of dropout from the waiting list for liver transplantation in patients with hepatocellular carcinoma: implications for the current organ allocation policy. Liver Transpl 2003; 9:684–692.
- Lai CL, Ratziu V, Yuen M-F, Poynard T. Viral hepatitis B. Lancet 2003; 362:2089–2094.
- Leung NW, Lai C-L, Chang T-T, et al. Extended lamivudine treatment in patients with chronic hepatitis B enhances hepatitis B e antigen seroconversion rates: results after 3 years of therapy. Hepatology 2001; 33:1527–1532.
- Benhamou Y, Bochet M, Thibault V, et al. Long-term incidence of hepatitis B virus resistance to lamivudine in human immunodeficiency virus-infected patients. Hepatology 1999; 30:1302–1306.
- Lok AS, Lai CL, Leung N, et al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology 2003; 125:1714–1722.
- Colonno RJ, Rose R, Baldick CJ, et al. Entecavir resistance is rare in nucleoside naive patients with hepatitis B. Hepatology 2006; 44:1656–1665.
- Perrillo RP. Current treatment of chronic hepatitis B: benefits and limitations. Semin Liver Dis 2005; 25(suppl 1):20–28.
- Lai C-L, Gane E, Liaw Y-F, et al. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med 2007; 357:2576–2588.
- Lai C-L, Gane E, Hsu C-W, et al. Two-year results from the GLOBE trial in patients with hepatitis B: greater clinical and antiviral efficacy for telbivudine (LDT) vs. lamivudine [AASLD abstract 91]. Hepatology 2006; 44(suppl 1):222A.
- Locarnini S, Qi X, Arterburn S, et al. Incidence and predictors of emergence of adefovir resistant HBV during four years of adefovir dipivoxil therapy for patients with chronic hepatitis B [EASL abstract 36]. J Hepatol 2005; 42(suppl 2):17.
- Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al; Adefovir Dipivoxil 438 Study Group. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B. N Engl J Med 2005; 352:2673–2681.
- Hepsera [package insert]. Foster City, CA: Gilead Sciences, Inc; 2008.
- Lee YS, Suh DJ, Lim YS, et al. Increased risk of adefovir resistance in patients with lamivudine-resistant chronic hepatitis B after 48 weeks of adefovir dipivoxil monotherapy. Hepatology 2006; 43:1385–1391.
- Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med 2008; 359:2442–2455.
- Gish R, Chang T-T, Lai C-L, et al. Hepatitis B surface antigen loss in antiviral-treated patients with HBeAg(+) chronic hepatitis B infection: observations from antiviral-naïve patients treated with entecavir or lamivudine. Paper presented at: 58th Annual Meeting of the American Association for the Study of Liver Diseases; November 2–6, 2007; Boston, MA. Abstract 992.
- Heathcote J, George J, Gordon S, et al. Tenofovir disoproxil fumarate (TDF) for the treatment of HBeAg-positive chronic hepatitis B: week 72 TDF data and week 24 adefovir dipivoxil switch data (study 103) [EASL abstract 71]. J Hepatol 2008; 48(suppl 2):S32.
- Marcellin P, Jacobson I, Habersetzer F, et al. Tenofovir disoproxil fumarate (TDF) for the treatment of HBeAg-negative chronic hepatitis B: week 72 TDF data and week 24 adefovir dipivoxil switch data (study 102) [EASL abstract 57]. J Hepatol 2008; 48(suppl 2):S26.
- Lampertico P, Marzano A, Levrero M, et al. Adefovir and lamivudine combination therapy is superior to adefovir monotherapy for lamivudine-resistant patients with HBeAg-negative chronic hepatitis B [EASL abstract 502]. J Hepatol 2007; 46(suppl 1):S191.
- Ha NB, Ha NB, Trinh HN. Changes in creatinine clearance (CRCL) in chronic hepatitis B (CHB) patients treated with adefovir dipivoxil (ADV) [AASLD abstract 901]. Hepatology 2008; 48:709A–710A.
- Gallant J, Staszewski S, Pozniak AL, et al; for the 903 Study Team. Similar renal safety profile between tenofovir DF (TDF) and stavudine (d4T) using modification of diet in renal disease (MDRD) and Cockcroft-Gault (CG) estimations of glomerular filtration rate (GFR) in antiretroviral-naïve patients through 144 weeks. In: Program and Abstracts of the 45th Interscience Conference on Antimicrobial Agents and Chemotherapy; December 16–19, 2005; Washington, DC. Abstract H-350.
- van Bömmel F, de Man RA, Stein K, et al. A multicenter analysis of antiviral response after one year of tenofovir monotherapy in HBV-monoinfected patients with prior nucleos(t)ide analog experience [EASL abstract 73]. J Hepatol 2008; 48(suppl 2):S32.
- Fung SK, Lok AS. Management of hepatitis B patients with antiviral resistance. Antivir Ther 2004; 9:1013–1026.
- Gish RG. Chronic hepatitis B virus: treating patients to prevent and manage resistance. US Gastroenterology Review 2007; March:51–54.
- Zoulim F. Mechanism of viral persistence and resistance to nucleoside and nucleotide analogs in chronic hepatitis B virus infection. Antiviral Res 2004; 64:1–15.
- Rapti I, Dimou E, Mitsoula P, Hadziyannis SJ. Adding-on versus switching-to adefovir therapy in lamivudine-resistant HBeAg-negative chronic hepatitis B. Hepatology 2007; 45:307–313.
KEY POINTS
- Consider treatment for chronic HBV infection for all patients who are positive for HBV DNA, as viral load levels as low as 300 copies/mL confer a risk for hepatocellular carcinoma.
- The goal of therapy is an undetectable level of HBV DNA; initiate therapy with the most potent agent to limit the possibility of resistance.
- Preventing resistance to therapy is crucial for successful treatment of chronic HBV infection.