User login
The role of hepatitis B virus (HBV) as a risk factor for the development of hepatocellular carcinoma (HCC) is well established. Not every patient with HBV infection develops HCC; yet, the current guidelines issued by the American Association for the Study of Liver Diseases1 recommend screening all patients who have HBV infection when they reach certain ages associated with increased risk. Improved identification of risk factors specifically associated with the likelihood of developing HCC may spare some patients the burden of unnecessary testing. This article reviews up-to-date information that will help identify patients who are at risk of HCC based on factors with more specificity than age, and considers whether treatment can alter their risk.
ASSESSING RISK
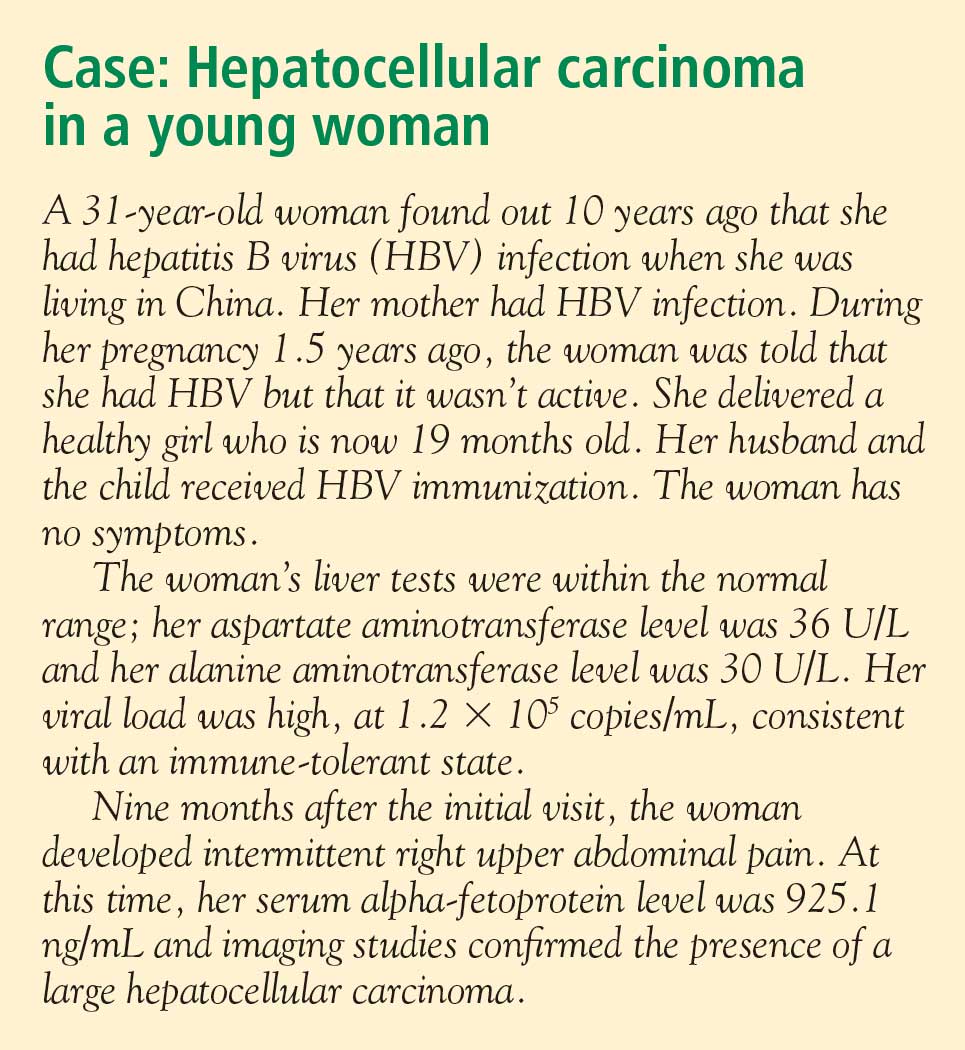
Several factors are associated with increased risk of developing HCC (see “Case: Hepatocellular carcinoma in a young woman”):
- An elevated serum alanine aminotransferase (ALT) level signifies the presence of active disease and increases risk, particularly if the ALT is persistently or intermittently elevated over years.
- Persistently elevated alpha-fetoprotein level is a reflection of enhanced regenerative state in the liver; the increased rate of cell division increases the risk of mutation, leading to increased risk of HCC.
- A low platelet count suggests the presence of cirrhosis, which itself increases the risk of HCC.
- Histologic risk factors revealed at biopsy include dysplasia, geographic morphologic changes that suggest clonal populations of cells, and a positive stain for proliferating cell nuclear antigen.
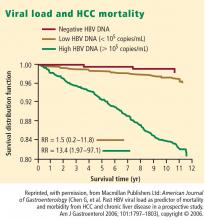
- Viral load (HBV DNA) is a significant predictor of HCC; two recent large, prospective studies—the Haimen City study2,3 and the REVEAL-HBV (Risk Evaluation of Viral Load Evaluation and Associated Liver Disease/Cancer-Hepatitis B Virus) study4—support the importance of this risk factor.
Haimen City study
The REVEAL-HBV study
The REVEAL-HBV study was a multicenter observational cohort study of 23,820 Taiwanese individuals aged 30 to 65 years old who were free of HCC at baseline.4 Of these, 3,653 were seropositive for HBsAg and seronegative for antibodies to hepatitis C virus.
Some 1,619 men and women had serum HBV DNA levels greater than or equal to 104 copies/mL at study entry.4 A direct correlation was observed between baseline HBV DNA levels and the incidence of HCC.During a mean follow-up period of 11.4 years, there were 164 new cases of HCC. In a model that integrated baseline and follow-up HBV DNA levels, the cumulative incidence of HCC ranged from 1.3% of those with undetectable levels of HBV DNA to 14.9% of those with HBV DNA levels of 106 copies/mL or greater. The same association between viral load and incidence of HCC was evident in patients who upon study entry had normal ALT levels and were hepatitis B e antigen (HBeAg) negative, a group previously considered to be inactive carriers of HBV.
The incidence of HCC was higher in the subjects with persistent viremia than in those whose viral load decreased over time, representing a biologic gradient of risk. Compared with the reference group (baseline HBV DNA < 104 copies/mL), the adjusted relative risk was nine times greater in those who maintained HBV DNA levels of 105 copies/mL or greater.
Genotype further defines risk
In addition to viral load, genotype may further define the risk of HCC in HBV carriers aged 30 years or older. In a nested case-control study, genotype C was associated with fivefold increased risk of HCC compared with other genotypes.5 Consistent with other studies, the risk of HCC increased with increasing viral load.
Caveats to the viral load–HCC link
The association between viral load and the development of HCC applies to patients aged 30 years or older, the subjects of the aforementioned studies. Younger patients who present with a high viral load and are HBeAg positive are likely to be in an immune-tolerant phase of HBV infection. Among patients aged 30 years or older, the association between viral load and HCC applies to HBeAg-positive as well as HBeAg-negative status. The longer the HBeAg-positive state is maintained, the greater the risk of developing cirrhosis and HCC, which is a reflection of active disease over a prolonged period. The association applies equally to patients with normal or elevated ALT levels. A risk nomogram is being developed that will help identify patients at highest risk of HCC.6
ALT AS PROGNOSTIC DETERMINANT
The risk of developing liver complications from chronic HBV infection increases with increasing concentrations of ALT. Yuen et al7 followed 3,233 Chinese patients with chronic HBV infection for approximately 4 years. The risk of developing complications from liver disease increased as ALT concentration increased from less than 0.5 times the upper limit of normal (ULN) to two to six times the ULN; ALT levels one to two times the ULN were associated with the highest risk of development of complications.
Interestingly, an ALT level greater than six times the ULN was associated with a significantly lower risk of liver complications. The speculation is that this phenomenon represents inactivation of disease following HBeAg seroconversion.
VIRAL LOAD SUPPRESSION LIMITS DISEASE PROGRESSION
Disease activity may flare during the natural course of chronic HBV infection, and repeated episodes may lead to progressive fibrosis, cirrhosis, and end-stage liver disease, as well as HCC. Patients whose cirrhosis has progressed to end-stage liver disease are candidates for transplant.
Continuous antiviral therapy with lamivudine has been shown to dramatically reduce the risk of complications and disease progression in patients with chronic HBV infection. In a placebo-controlled trial of 651 patients with chronic HBV infection and advanced fibrosis or cirrhosis, those randomized to lamivudine who remained sensitive to the drug had a 7.8% risk of complications over approximately 3 years, compared with a 17.7% risk in the patients randomized to placebo.8 The difference was significant and sizeable enough that the study was terminated after a mean duration of 32.4 months. Patients who developed resistance to lamivudine, caused by a mutation in HBV (YMDD mutation, a sign of lamivudine resistance), lost the protection provided by viral suppression.
The risk of disease progression to cirrhosis or HCC was also significantly lower among HBeAg-positive patients without cirrhosis who were treated with lamivudine for a median of 89.9 months compared with placebo, Yuen et al found.9 As in other studies, patients in whom the YMDD mutation developed lost the protection of viral suppression.
In a retrospective study, Di Marco et al10 also found that a loss of response to lamivudine was associated with higher risk of development of HCC, whereas patients who maintained a response to lamivudine were much less likely to develop progressive disease. The authors found that cirrhosis and loss of antiviral response were independently related to mortality and development of HCC.
SUMMARY
Patients with HBV are at risk for cancer, and the risk factors can be identified. Although not yet fully evaluated, awareness of these factors will make the screening process more efficient and less burdensome than current guidelines recommend. The publication and eventual validation of a risk nomogram will facilitate the determination of risk. An especially strong predictor of adverse outcomes, including HCC, is HBV DNA concentration higher than 104 copies/mL, as shown by two recent large studies; further, investigators observed a correlation between HBV DNA level and incidence of HCC.
Antiviral therapy has dramatically reduced the risk of complications and progression of HBV infection. Those who develop resistance to therapy lose the protection provided by viral suppression.
DISCUSSION
William D. Carey, MD: Does biopsy of nontumorous portions of the liver have value, either by showing dysplasia or perhaps through a staining technique, in predicting the development of liver cancer?
Morris Sherman, MD, PhD: I believe that you’re referring to a recent study in which microarray technology was used to identify patients at risk for the development of a de novo tumor after a resection of the first tumor.11 Liver tissue surrounding the tumor was analyzed by microarray technology, and gene expression profiling accurately predicted the development of a new tumor in another part of the liver more than 2 years later. This discovery suggests the presence of a field defect, or a propensity for the development of new tumors in a damaged organ. Patients who have a field defect identified by the microarray technique are at much higher risk of developing a subsequent cancer. These patients might be candidates for liver transplant despite apparent surgical cure of their HCC. However, because the subsequent liver malignancy occurs some time later and is a new primary tumor, the need for transplant is less urgent than it is for a patient with a progressive hepatoma, for example.
Pierre M. Gholam, MD: Do you consider ethnicity in addition to age, viral load, and other factors in your decision to screen patients for HCC?
Dr. Sherman: We traditionally think of ethnicity as a major factor because HBV is concentrated in Asian and African populations. I’m not entirely sure whether ethnicity or the viral genotypes are more important, because the viral genotypes are distributed along ethnic lines. We know that genotypes B and C, which are common in the Far East, are associated with a high rate of progressive liver disease. Genotype D, observed mainly in Middle Eastern and Greek populations, is associated with a much higher rate of progressive liver disease than genotypes common in Western Europe and most of North America. I believe that genotype should be a factor in decisions to screen.
Robert G. Gish, MD: In your case presentation you described the aspartate aminotransferase (AST) and ALT as being normal. New criteria define an AST/ALT of 20 as being “healthy” for a woman. I like the word “healthy” better than “normal.” How would you have described those test results to the patient?
Dr. Sherman: I would have told her that although her AST and ALT levels were within the laboratory reference range, ideally for a young woman the ALT should be closer to 20 U/L. Her actual levels were at least twice the upper range of ideal, and therefore, I believe a biopsy to determine the extent of injury in the liver would be important.
Tram T. Tran, MD: Are there any new serum markers for liver cancer that have promise?
Dr. Sherman: The problem with serum markers, or biomarkers in general, is the confusion over their intended use, such as for screening, risk stratification, or diagnosis.
I assume that your question refers to their potential use in screening, and so far none of the existing biomarkers is adequate to find small tumors. For screening purposes, you ideally want to find tumors that are 2 cm or smaller, and none of the biomarkers is efficient with those small tumors. A biomarker is not needed to identify tumors that are 5 or 6 cm.
- Bruix J, Sherman M. Management of hepatocellular carcinoma. AASLD Practice Guideline. Hepatology 2005; 42:1208–1235.
- Chen G, Lin W, Shen F, Iloeje UH, London WT, Evans AA. Past HBV viral load as predictor of mortality and morbidity from HCC and chronic liver disease in a prospective study. Am J Gastroenterol 2006; 101:1797–1803.
- Chen G, Lin W, Shen F, Iloeje UH, London WT, Evans AA. Chronic hepatitis B virus infection and mortality from nonliver causes: results from the Haimen City cohort study [published online ahead of print January 19, 2005]. Int J Epidemiol 2005; 34:132–137.
- Chen C-J, Yang H-I, Su J, et al; for the REVEAL-HBV Study Group. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006; 295:65–73.
- Yu M-W, Yeh S-H, Chen P-J, et al. Hepatitis B virus genotype and DNA level and hepatocellular carcinoma: a prospective study in men. J Natl Cancer Inst 2005; 97:265–272.
- Chen C-J, Yang H-I, Iloeje UH, et al. A risk function nomogram for predicting HCC in patients with chronic hepatitis B: the REVEAL-HBV study [AASLD abstract S1766]. Gastroenterology 2007; 132(suppl 2):A761.
- Yuen M-F, Yuan H-J, Wong DK-H, et al. Prognostic determinants for chronic hepatitis B in Asians: therapeutic implications. Gut 2005; 54:1610–1614.
- Liaw Y-F, Sung JJY, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004; 351:1521–1531.
- Yuen MF, Seto WK, Chow DHF, et al. Long-term beneficial outcome of Chinese asymptomatic patients with HBeAg-positive chronic hepatitis B on continuous lamivudine therapy: 7-year experience [AASLD abstract 985]. Hepatology 2005; 42(suppl 1):583A.
- Di Marco V, Marzano A, Lampertico P, et al. Clinical outcome of HBeAg-negative chronic hepatitis B in relation to virological response to lamivudine. Hepatology 2004; 40:883–891.
- Hoshida Y, Villaneuva A, Kobayashi M, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med 2008; 359:1995–2004.
The role of hepatitis B virus (HBV) as a risk factor for the development of hepatocellular carcinoma (HCC) is well established. Not every patient with HBV infection develops HCC; yet, the current guidelines issued by the American Association for the Study of Liver Diseases1 recommend screening all patients who have HBV infection when they reach certain ages associated with increased risk. Improved identification of risk factors specifically associated with the likelihood of developing HCC may spare some patients the burden of unnecessary testing. This article reviews up-to-date information that will help identify patients who are at risk of HCC based on factors with more specificity than age, and considers whether treatment can alter their risk.
ASSESSING RISK
Several factors are associated with increased risk of developing HCC (see “Case: Hepatocellular carcinoma in a young woman”):
- An elevated serum alanine aminotransferase (ALT) level signifies the presence of active disease and increases risk, particularly if the ALT is persistently or intermittently elevated over years.
- Persistently elevated alpha-fetoprotein level is a reflection of enhanced regenerative state in the liver; the increased rate of cell division increases the risk of mutation, leading to increased risk of HCC.
- A low platelet count suggests the presence of cirrhosis, which itself increases the risk of HCC.
- Histologic risk factors revealed at biopsy include dysplasia, geographic morphologic changes that suggest clonal populations of cells, and a positive stain for proliferating cell nuclear antigen.
- Viral load (HBV DNA) is a significant predictor of HCC; two recent large, prospective studies—the Haimen City study2,3 and the REVEAL-HBV (Risk Evaluation of Viral Load Evaluation and Associated Liver Disease/Cancer-Hepatitis B Virus) study4—support the importance of this risk factor.

Haimen City study
The REVEAL-HBV study
The REVEAL-HBV study was a multicenter observational cohort study of 23,820 Taiwanese individuals aged 30 to 65 years old who were free of HCC at baseline.4 Of these, 3,653 were seropositive for HBsAg and seronegative for antibodies to hepatitis C virus.
Some 1,619 men and women had serum HBV DNA levels greater than or equal to 104 copies/mL at study entry.4 A direct correlation was observed between baseline HBV DNA levels and the incidence of HCC.During a mean follow-up period of 11.4 years, there were 164 new cases of HCC. In a model that integrated baseline and follow-up HBV DNA levels, the cumulative incidence of HCC ranged from 1.3% of those with undetectable levels of HBV DNA to 14.9% of those with HBV DNA levels of 106 copies/mL or greater. The same association between viral load and incidence of HCC was evident in patients who upon study entry had normal ALT levels and were hepatitis B e antigen (HBeAg) negative, a group previously considered to be inactive carriers of HBV.
The incidence of HCC was higher in the subjects with persistent viremia than in those whose viral load decreased over time, representing a biologic gradient of risk. Compared with the reference group (baseline HBV DNA < 104 copies/mL), the adjusted relative risk was nine times greater in those who maintained HBV DNA levels of 105 copies/mL or greater.
Genotype further defines risk
In addition to viral load, genotype may further define the risk of HCC in HBV carriers aged 30 years or older. In a nested case-control study, genotype C was associated with fivefold increased risk of HCC compared with other genotypes.5 Consistent with other studies, the risk of HCC increased with increasing viral load.
Caveats to the viral load–HCC link
The association between viral load and the development of HCC applies to patients aged 30 years or older, the subjects of the aforementioned studies. Younger patients who present with a high viral load and are HBeAg positive are likely to be in an immune-tolerant phase of HBV infection. Among patients aged 30 years or older, the association between viral load and HCC applies to HBeAg-positive as well as HBeAg-negative status. The longer the HBeAg-positive state is maintained, the greater the risk of developing cirrhosis and HCC, which is a reflection of active disease over a prolonged period. The association applies equally to patients with normal or elevated ALT levels. A risk nomogram is being developed that will help identify patients at highest risk of HCC.6
ALT AS PROGNOSTIC DETERMINANT
The risk of developing liver complications from chronic HBV infection increases with increasing concentrations of ALT. Yuen et al7 followed 3,233 Chinese patients with chronic HBV infection for approximately 4 years. The risk of developing complications from liver disease increased as ALT concentration increased from less than 0.5 times the upper limit of normal (ULN) to two to six times the ULN; ALT levels one to two times the ULN were associated with the highest risk of development of complications.
Interestingly, an ALT level greater than six times the ULN was associated with a significantly lower risk of liver complications. The speculation is that this phenomenon represents inactivation of disease following HBeAg seroconversion.
VIRAL LOAD SUPPRESSION LIMITS DISEASE PROGRESSION
Disease activity may flare during the natural course of chronic HBV infection, and repeated episodes may lead to progressive fibrosis, cirrhosis, and end-stage liver disease, as well as HCC. Patients whose cirrhosis has progressed to end-stage liver disease are candidates for transplant.
Continuous antiviral therapy with lamivudine has been shown to dramatically reduce the risk of complications and disease progression in patients with chronic HBV infection. In a placebo-controlled trial of 651 patients with chronic HBV infection and advanced fibrosis or cirrhosis, those randomized to lamivudine who remained sensitive to the drug had a 7.8% risk of complications over approximately 3 years, compared with a 17.7% risk in the patients randomized to placebo.8 The difference was significant and sizeable enough that the study was terminated after a mean duration of 32.4 months. Patients who developed resistance to lamivudine, caused by a mutation in HBV (YMDD mutation, a sign of lamivudine resistance), lost the protection provided by viral suppression.
The risk of disease progression to cirrhosis or HCC was also significantly lower among HBeAg-positive patients without cirrhosis who were treated with lamivudine for a median of 89.9 months compared with placebo, Yuen et al found.9 As in other studies, patients in whom the YMDD mutation developed lost the protection of viral suppression.
In a retrospective study, Di Marco et al10 also found that a loss of response to lamivudine was associated with higher risk of development of HCC, whereas patients who maintained a response to lamivudine were much less likely to develop progressive disease. The authors found that cirrhosis and loss of antiviral response were independently related to mortality and development of HCC.
SUMMARY
Patients with HBV are at risk for cancer, and the risk factors can be identified. Although not yet fully evaluated, awareness of these factors will make the screening process more efficient and less burdensome than current guidelines recommend. The publication and eventual validation of a risk nomogram will facilitate the determination of risk. An especially strong predictor of adverse outcomes, including HCC, is HBV DNA concentration higher than 104 copies/mL, as shown by two recent large studies; further, investigators observed a correlation between HBV DNA level and incidence of HCC.
Antiviral therapy has dramatically reduced the risk of complications and progression of HBV infection. Those who develop resistance to therapy lose the protection provided by viral suppression.
DISCUSSION
William D. Carey, MD: Does biopsy of nontumorous portions of the liver have value, either by showing dysplasia or perhaps through a staining technique, in predicting the development of liver cancer?
Morris Sherman, MD, PhD: I believe that you’re referring to a recent study in which microarray technology was used to identify patients at risk for the development of a de novo tumor after a resection of the first tumor.11 Liver tissue surrounding the tumor was analyzed by microarray technology, and gene expression profiling accurately predicted the development of a new tumor in another part of the liver more than 2 years later. This discovery suggests the presence of a field defect, or a propensity for the development of new tumors in a damaged organ. Patients who have a field defect identified by the microarray technique are at much higher risk of developing a subsequent cancer. These patients might be candidates for liver transplant despite apparent surgical cure of their HCC. However, because the subsequent liver malignancy occurs some time later and is a new primary tumor, the need for transplant is less urgent than it is for a patient with a progressive hepatoma, for example.
Pierre M. Gholam, MD: Do you consider ethnicity in addition to age, viral load, and other factors in your decision to screen patients for HCC?
Dr. Sherman: We traditionally think of ethnicity as a major factor because HBV is concentrated in Asian and African populations. I’m not entirely sure whether ethnicity or the viral genotypes are more important, because the viral genotypes are distributed along ethnic lines. We know that genotypes B and C, which are common in the Far East, are associated with a high rate of progressive liver disease. Genotype D, observed mainly in Middle Eastern and Greek populations, is associated with a much higher rate of progressive liver disease than genotypes common in Western Europe and most of North America. I believe that genotype should be a factor in decisions to screen.
Robert G. Gish, MD: In your case presentation you described the aspartate aminotransferase (AST) and ALT as being normal. New criteria define an AST/ALT of 20 as being “healthy” for a woman. I like the word “healthy” better than “normal.” How would you have described those test results to the patient?
Dr. Sherman: I would have told her that although her AST and ALT levels were within the laboratory reference range, ideally for a young woman the ALT should be closer to 20 U/L. Her actual levels were at least twice the upper range of ideal, and therefore, I believe a biopsy to determine the extent of injury in the liver would be important.
Tram T. Tran, MD: Are there any new serum markers for liver cancer that have promise?
Dr. Sherman: The problem with serum markers, or biomarkers in general, is the confusion over their intended use, such as for screening, risk stratification, or diagnosis.
I assume that your question refers to their potential use in screening, and so far none of the existing biomarkers is adequate to find small tumors. For screening purposes, you ideally want to find tumors that are 2 cm or smaller, and none of the biomarkers is efficient with those small tumors. A biomarker is not needed to identify tumors that are 5 or 6 cm.
The role of hepatitis B virus (HBV) as a risk factor for the development of hepatocellular carcinoma (HCC) is well established. Not every patient with HBV infection develops HCC; yet, the current guidelines issued by the American Association for the Study of Liver Diseases1 recommend screening all patients who have HBV infection when they reach certain ages associated with increased risk. Improved identification of risk factors specifically associated with the likelihood of developing HCC may spare some patients the burden of unnecessary testing. This article reviews up-to-date information that will help identify patients who are at risk of HCC based on factors with more specificity than age, and considers whether treatment can alter their risk.
ASSESSING RISK
Several factors are associated with increased risk of developing HCC (see “Case: Hepatocellular carcinoma in a young woman”):
- An elevated serum alanine aminotransferase (ALT) level signifies the presence of active disease and increases risk, particularly if the ALT is persistently or intermittently elevated over years.
- Persistently elevated alpha-fetoprotein level is a reflection of enhanced regenerative state in the liver; the increased rate of cell division increases the risk of mutation, leading to increased risk of HCC.
- A low platelet count suggests the presence of cirrhosis, which itself increases the risk of HCC.
- Histologic risk factors revealed at biopsy include dysplasia, geographic morphologic changes that suggest clonal populations of cells, and a positive stain for proliferating cell nuclear antigen.
- Viral load (HBV DNA) is a significant predictor of HCC; two recent large, prospective studies—the Haimen City study2,3 and the REVEAL-HBV (Risk Evaluation of Viral Load Evaluation and Associated Liver Disease/Cancer-Hepatitis B Virus) study4—support the importance of this risk factor.

Haimen City study
The REVEAL-HBV study
The REVEAL-HBV study was a multicenter observational cohort study of 23,820 Taiwanese individuals aged 30 to 65 years old who were free of HCC at baseline.4 Of these, 3,653 were seropositive for HBsAg and seronegative for antibodies to hepatitis C virus.
Some 1,619 men and women had serum HBV DNA levels greater than or equal to 104 copies/mL at study entry.4 A direct correlation was observed between baseline HBV DNA levels and the incidence of HCC.During a mean follow-up period of 11.4 years, there were 164 new cases of HCC. In a model that integrated baseline and follow-up HBV DNA levels, the cumulative incidence of HCC ranged from 1.3% of those with undetectable levels of HBV DNA to 14.9% of those with HBV DNA levels of 106 copies/mL or greater. The same association between viral load and incidence of HCC was evident in patients who upon study entry had normal ALT levels and were hepatitis B e antigen (HBeAg) negative, a group previously considered to be inactive carriers of HBV.
The incidence of HCC was higher in the subjects with persistent viremia than in those whose viral load decreased over time, representing a biologic gradient of risk. Compared with the reference group (baseline HBV DNA < 104 copies/mL), the adjusted relative risk was nine times greater in those who maintained HBV DNA levels of 105 copies/mL or greater.
Genotype further defines risk
In addition to viral load, genotype may further define the risk of HCC in HBV carriers aged 30 years or older. In a nested case-control study, genotype C was associated with fivefold increased risk of HCC compared with other genotypes.5 Consistent with other studies, the risk of HCC increased with increasing viral load.
Caveats to the viral load–HCC link
The association between viral load and the development of HCC applies to patients aged 30 years or older, the subjects of the aforementioned studies. Younger patients who present with a high viral load and are HBeAg positive are likely to be in an immune-tolerant phase of HBV infection. Among patients aged 30 years or older, the association between viral load and HCC applies to HBeAg-positive as well as HBeAg-negative status. The longer the HBeAg-positive state is maintained, the greater the risk of developing cirrhosis and HCC, which is a reflection of active disease over a prolonged period. The association applies equally to patients with normal or elevated ALT levels. A risk nomogram is being developed that will help identify patients at highest risk of HCC.6
ALT AS PROGNOSTIC DETERMINANT
The risk of developing liver complications from chronic HBV infection increases with increasing concentrations of ALT. Yuen et al7 followed 3,233 Chinese patients with chronic HBV infection for approximately 4 years. The risk of developing complications from liver disease increased as ALT concentration increased from less than 0.5 times the upper limit of normal (ULN) to two to six times the ULN; ALT levels one to two times the ULN were associated with the highest risk of development of complications.
Interestingly, an ALT level greater than six times the ULN was associated with a significantly lower risk of liver complications. The speculation is that this phenomenon represents inactivation of disease following HBeAg seroconversion.
VIRAL LOAD SUPPRESSION LIMITS DISEASE PROGRESSION
Disease activity may flare during the natural course of chronic HBV infection, and repeated episodes may lead to progressive fibrosis, cirrhosis, and end-stage liver disease, as well as HCC. Patients whose cirrhosis has progressed to end-stage liver disease are candidates for transplant.
Continuous antiviral therapy with lamivudine has been shown to dramatically reduce the risk of complications and disease progression in patients with chronic HBV infection. In a placebo-controlled trial of 651 patients with chronic HBV infection and advanced fibrosis or cirrhosis, those randomized to lamivudine who remained sensitive to the drug had a 7.8% risk of complications over approximately 3 years, compared with a 17.7% risk in the patients randomized to placebo.8 The difference was significant and sizeable enough that the study was terminated after a mean duration of 32.4 months. Patients who developed resistance to lamivudine, caused by a mutation in HBV (YMDD mutation, a sign of lamivudine resistance), lost the protection provided by viral suppression.
The risk of disease progression to cirrhosis or HCC was also significantly lower among HBeAg-positive patients without cirrhosis who were treated with lamivudine for a median of 89.9 months compared with placebo, Yuen et al found.9 As in other studies, patients in whom the YMDD mutation developed lost the protection of viral suppression.
In a retrospective study, Di Marco et al10 also found that a loss of response to lamivudine was associated with higher risk of development of HCC, whereas patients who maintained a response to lamivudine were much less likely to develop progressive disease. The authors found that cirrhosis and loss of antiviral response were independently related to mortality and development of HCC.
SUMMARY
Patients with HBV are at risk for cancer, and the risk factors can be identified. Although not yet fully evaluated, awareness of these factors will make the screening process more efficient and less burdensome than current guidelines recommend. The publication and eventual validation of a risk nomogram will facilitate the determination of risk. An especially strong predictor of adverse outcomes, including HCC, is HBV DNA concentration higher than 104 copies/mL, as shown by two recent large studies; further, investigators observed a correlation between HBV DNA level and incidence of HCC.
Antiviral therapy has dramatically reduced the risk of complications and progression of HBV infection. Those who develop resistance to therapy lose the protection provided by viral suppression.
DISCUSSION
William D. Carey, MD: Does biopsy of nontumorous portions of the liver have value, either by showing dysplasia or perhaps through a staining technique, in predicting the development of liver cancer?
Morris Sherman, MD, PhD: I believe that you’re referring to a recent study in which microarray technology was used to identify patients at risk for the development of a de novo tumor after a resection of the first tumor.11 Liver tissue surrounding the tumor was analyzed by microarray technology, and gene expression profiling accurately predicted the development of a new tumor in another part of the liver more than 2 years later. This discovery suggests the presence of a field defect, or a propensity for the development of new tumors in a damaged organ. Patients who have a field defect identified by the microarray technique are at much higher risk of developing a subsequent cancer. These patients might be candidates for liver transplant despite apparent surgical cure of their HCC. However, because the subsequent liver malignancy occurs some time later and is a new primary tumor, the need for transplant is less urgent than it is for a patient with a progressive hepatoma, for example.
Pierre M. Gholam, MD: Do you consider ethnicity in addition to age, viral load, and other factors in your decision to screen patients for HCC?
Dr. Sherman: We traditionally think of ethnicity as a major factor because HBV is concentrated in Asian and African populations. I’m not entirely sure whether ethnicity or the viral genotypes are more important, because the viral genotypes are distributed along ethnic lines. We know that genotypes B and C, which are common in the Far East, are associated with a high rate of progressive liver disease. Genotype D, observed mainly in Middle Eastern and Greek populations, is associated with a much higher rate of progressive liver disease than genotypes common in Western Europe and most of North America. I believe that genotype should be a factor in decisions to screen.
Robert G. Gish, MD: In your case presentation you described the aspartate aminotransferase (AST) and ALT as being normal. New criteria define an AST/ALT of 20 as being “healthy” for a woman. I like the word “healthy” better than “normal.” How would you have described those test results to the patient?
Dr. Sherman: I would have told her that although her AST and ALT levels were within the laboratory reference range, ideally for a young woman the ALT should be closer to 20 U/L. Her actual levels were at least twice the upper range of ideal, and therefore, I believe a biopsy to determine the extent of injury in the liver would be important.
Tram T. Tran, MD: Are there any new serum markers for liver cancer that have promise?
Dr. Sherman: The problem with serum markers, or biomarkers in general, is the confusion over their intended use, such as for screening, risk stratification, or diagnosis.
I assume that your question refers to their potential use in screening, and so far none of the existing biomarkers is adequate to find small tumors. For screening purposes, you ideally want to find tumors that are 2 cm or smaller, and none of the biomarkers is efficient with those small tumors. A biomarker is not needed to identify tumors that are 5 or 6 cm.
- Bruix J, Sherman M. Management of hepatocellular carcinoma. AASLD Practice Guideline. Hepatology 2005; 42:1208–1235.
- Chen G, Lin W, Shen F, Iloeje UH, London WT, Evans AA. Past HBV viral load as predictor of mortality and morbidity from HCC and chronic liver disease in a prospective study. Am J Gastroenterol 2006; 101:1797–1803.
- Chen G, Lin W, Shen F, Iloeje UH, London WT, Evans AA. Chronic hepatitis B virus infection and mortality from nonliver causes: results from the Haimen City cohort study [published online ahead of print January 19, 2005]. Int J Epidemiol 2005; 34:132–137.
- Chen C-J, Yang H-I, Su J, et al; for the REVEAL-HBV Study Group. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006; 295:65–73.
- Yu M-W, Yeh S-H, Chen P-J, et al. Hepatitis B virus genotype and DNA level and hepatocellular carcinoma: a prospective study in men. J Natl Cancer Inst 2005; 97:265–272.
- Chen C-J, Yang H-I, Iloeje UH, et al. A risk function nomogram for predicting HCC in patients with chronic hepatitis B: the REVEAL-HBV study [AASLD abstract S1766]. Gastroenterology 2007; 132(suppl 2):A761.
- Yuen M-F, Yuan H-J, Wong DK-H, et al. Prognostic determinants for chronic hepatitis B in Asians: therapeutic implications. Gut 2005; 54:1610–1614.
- Liaw Y-F, Sung JJY, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004; 351:1521–1531.
- Yuen MF, Seto WK, Chow DHF, et al. Long-term beneficial outcome of Chinese asymptomatic patients with HBeAg-positive chronic hepatitis B on continuous lamivudine therapy: 7-year experience [AASLD abstract 985]. Hepatology 2005; 42(suppl 1):583A.
- Di Marco V, Marzano A, Lampertico P, et al. Clinical outcome of HBeAg-negative chronic hepatitis B in relation to virological response to lamivudine. Hepatology 2004; 40:883–891.
- Hoshida Y, Villaneuva A, Kobayashi M, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med 2008; 359:1995–2004.
- Bruix J, Sherman M. Management of hepatocellular carcinoma. AASLD Practice Guideline. Hepatology 2005; 42:1208–1235.
- Chen G, Lin W, Shen F, Iloeje UH, London WT, Evans AA. Past HBV viral load as predictor of mortality and morbidity from HCC and chronic liver disease in a prospective study. Am J Gastroenterol 2006; 101:1797–1803.
- Chen G, Lin W, Shen F, Iloeje UH, London WT, Evans AA. Chronic hepatitis B virus infection and mortality from nonliver causes: results from the Haimen City cohort study [published online ahead of print January 19, 2005]. Int J Epidemiol 2005; 34:132–137.
- Chen C-J, Yang H-I, Su J, et al; for the REVEAL-HBV Study Group. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006; 295:65–73.
- Yu M-W, Yeh S-H, Chen P-J, et al. Hepatitis B virus genotype and DNA level and hepatocellular carcinoma: a prospective study in men. J Natl Cancer Inst 2005; 97:265–272.
- Chen C-J, Yang H-I, Iloeje UH, et al. A risk function nomogram for predicting HCC in patients with chronic hepatitis B: the REVEAL-HBV study [AASLD abstract S1766]. Gastroenterology 2007; 132(suppl 2):A761.
- Yuen M-F, Yuan H-J, Wong DK-H, et al. Prognostic determinants for chronic hepatitis B in Asians: therapeutic implications. Gut 2005; 54:1610–1614.
- Liaw Y-F, Sung JJY, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004; 351:1521–1531.
- Yuen MF, Seto WK, Chow DHF, et al. Long-term beneficial outcome of Chinese asymptomatic patients with HBeAg-positive chronic hepatitis B on continuous lamivudine therapy: 7-year experience [AASLD abstract 985]. Hepatology 2005; 42(suppl 1):583A.
- Di Marco V, Marzano A, Lampertico P, et al. Clinical outcome of HBeAg-negative chronic hepatitis B in relation to virological response to lamivudine. Hepatology 2004; 40:883–891.
- Hoshida Y, Villaneuva A, Kobayashi M, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med 2008; 359:1995–2004.
KEY POINTS
- A high viral load is a significant predictor of the development of hepatocellular carcinoma in patients aged 30 years or older with chronic HBV infection.
- The risk of developing liver complications from chronic HBV infection increases with increasing concentrations of alanine aminotransferase.
- Continuous antiviral therapy to suppress viral load dramatically reduces the risk of complications from HBV infection and reduces the rate of disease progression, as long as patients maintain a therapeutic response.