User login
Pan-coronavirus vaccines may be key to fighting future pandemics
As the COVID-19 pandemic winds down – for the time being at least – efforts are ramping up to develop next-generation vaccines that can protect against future novel coronaviruses and variants. Several projects are presenting clever combinations of viral parts to the immune system that evoke a robust and hopefully lasting response.
The coming generation of “pan” vaccines aims to tamp down SARS-CoV-2, its closest relatives, and whatever may come into tamer respiratory viruses like the common cold. Whatever the eventual components of this new generation of vaccines, experts agree on the goal: preventing severe disease and death. And a broader approach is critical.
“All the vaccines have been amazing. But we’re playing a whack-a-mole game with the variants. We need to take a step back and ask if a pan-variant vaccine is possible. That’s important because Omicron isn’t the last variant,” said Jacob Lemieux, MD, PhD, instructor in medicine and infectious disease specialist at Massachusetts General Hospital, Boston.
A broad spectrum vaccine
The drive to create a vaccine that would deter multiple coronaviruses arose early, among many researchers. An article published in Nature in May 2020 by National Institute of Allergy and Infectious Diseases researcher Luca T. Giurgea, MD, and colleagues said it all in the title: “Universal coronavirus vaccines: the time to start is now.”
Their concerns? The diversity of bat coronaviruses poised to jump into humans; the high mutability of the spike gene that the immune response recognizes; and the persistence of mutations in an RNA virus, which can’t repair errors.
Work on broader vaccines began in several labs as SARS-CoV-2 spawned variant after variant.
On Sept. 28, NIAID announced funding for developing ‘pan-coronavirus’ vaccines – the quotation marks theirs to indicate that a magic bullet against any new coronavirus is unrealistic. “These new awards are designed to look ahead and prepare for the next generation of coronaviruses with pandemic potential,” said NIAID director Anthony S. Fauci, MD. An initial three awards went to groups at the University of Wisconsin, Brigham and Women’s Hospital, and Duke University.
President Biden mentioned the NIAID funding in his State of the Union Address. He also talked about how the Biomedical Advanced Research and Development Authority, founded in 2006 to prepare for public health emergencies, is spearheading development of new vaccine platforms and vaccines that target a broader swath of pathogen parts.
Meanwhile, individual researchers from eclectic fields are finding new ways to prevent future pandemics.
Artem Babaian, PhD, a computational biologist at the University of Cambridge (England), had the idea to probe National Institutes of Health genome databases, going back more than a decade, for overlooked novel coronaviruses. He started the project while he was between jobs as the pandemic was unfurling, using a telltale enzyme unique to the RNA viruses to fish out COVID cousins. The work is published in Nature and the data freely available at serratus.io.
Among the nearly 132,000 novel RNA viruses Dr. Babaian’s team found, 9 were from previously unrecognized coronaviruses. The novel nine came from “ecologically diverse sources”: a seahorse, an axolotl, an eel, and several fishes. Deciphering the topographies of these coronaviruses may provide clues to developing vaccines that stay ahead of future pandemics.
But optics are important in keeping expectations reasonable. “‘Universal vaccine’ is a misnomer. I think about it as ‘broad spectrum vaccines.’ It’s critical to be up front that these vaccines can never guarantee immunity against all coronaviruses. There are no absolutes in biology, but they hopefully will work against the dangers that we do know exist. A vaccine that mimics exposure to many coronaviruses could protect against a currently unknown coronavirus, especially if slower-evolving antigens are included,” Dr. Babaian said in an interview.
Nikolai Petrovsky, MD, PhD, of Flinders University, Adelaide, and the biotechnology company Vaccine Pty, agrees, calling a literal pan-coronavirus vaccine a “pipe dream. What I do think is achievable is a broadly protective, pan–CoV-19 vaccine – I can say that because we have already developed and tested it, combining antigens rather than trying just one that can do everything.”
Immunity lures
The broader vaccines in development display viral antigens, such as spike proteins, to the immune system on diverse frameworks. Here are a few approaches.
Ferritin nanoparticles: A candidate vaccine from the emerging infectious diseases branch of Water Reed National Military Medical Center began phase 1 human trials in April 2021. Called SpFN, the vaccine consists of arrays of ferritin nanoparticles linked to spike proteins from various variants and species. Ferritin is a protein that binds and stores iron in the body.
“The repetitive and ordered display of the coronavirus spike protein on a multifaced nanoparticle may stimulate immunity in such a way as to translate into significantly broader protection,” said Walter Reed’s branch director and vaccine coinventor Kayvon Modjarrad, MD, PhD.
A second vaccine targets only the “bullseye” part of the spike that the virus uses to attach and gain access to human cells, called the receptor-binding domain (RBD), of SARS-CoV-2 variants and of the virus behind the original SARS. The preclinical data appeared in Science Translational Medicine.
Barton Haynes, MD and colleagues at the Duke Human Vaccine Institute are also using ferritin to design and develop a “pan-betacoronavirus vaccine,” referring to the genus to which SARS-CoV-2 belongs. They say their results in macaques, published in Nature, “demonstrate that current mRNA-based vaccines may provide some protection from future outbreaks of zoonotic betacoronaviruses.”
Mosaic nanoparticles: Graduate student Alexander Cohen is leading an effort at CalTech, in the lab of Pamela Bjorkman, PhD, that uses nanoparticles consisting of proteins from a bacterium (Strep pyogenes) to which RBDs from spike proteins of four or eight different betacoronaviruses are attached. The strategy demonstrates that the whole is greater than the sum of the parts.
“Alex’s results show that it is possible to raise diverse neutralizing antibody responses, even against coronavirus strains that were not represented on the injected nanoparticle. We are hopeful that this technology could be used to protect against future animal coronaviruses that cross into humans,” said Dr. Björkman. The work appeared in Science.
Candidate vaccines from Inovio Pharmaceuticals also use a mosaic spike strategy, but with DNA rings (plasmids) rather than nanoparticles. One version works against pre-Omicron variants and is being tested against Omicron, and another with “pan–COVID-19” coverage has tested well in animal models. Inovio’s vaccines are delivered into the skin using a special device that applies an electric pulse that increases the cells’ permeability.
Chimeric spikes: Yet another approach is to fashion vaccines from various parts of the betacoronaviruses that are most closely related to SARS-CoV-2 – the pathogens behind Middle East respiratory syndrome and severe acute respiratory syndrome as well as several bat viruses and a few pangolin ones. The abundance and ubiquity of these viruses provide a toolbox of sorts, with instructions written in the language of RNA, from which to select, dissect, recombine, and customize vaccines.
“SARS-like viruses can recombine and exhibit great genetic diversity in several parts of the genome. We designed chimeric spikes to improve coverage of a multiplexed vaccine,” said David Martinez, PhD.
His team at the University of North Carolina at Chapel Hill has developed mRNA vaccines that deliver “scrambled coronavirus spikes” representing various parts, not just the RBD, as described in Science.
In mice, the chimeric vaccines elicit robust T- and B-cell immune responses, which stimulate antibody production and control other facets of building immunity.
Beyond the spike bullseye
The challenge of developing pan-coronavirus vaccines is dual. “The very best vaccines are highly specific to each strain, and the universal vaccines have to sacrifice effectiveness to get broad coverage. Life is a trade-off.” Dr. Petrovsky told this news organization.
Efforts to broaden vaccine efficacy venture beyond targeting the RBD bullseyes of the spike triplets that festoon the virus. Some projects are focusing on less changeable spike parts that are more alike among less closely related coronaviruses than is the mutation-prone RBD. For example, the peptides that twist into the “stem-helix” portion of the part of the spike that adheres to host cells are the basis of some candidate vaccines now in preclinical studies.
Still other vaccines aren’t spike based at all. French company Osivax, for example, is working on a vaccine that targets the nucleocapsid protein that shields the viral RNA. The hope is that presenting various faces of the pathogen may spark immunity beyond an initial antibody rush and evoke more diverse and lasting T-cell responses.
With the myriad efforts to back up the first generation of COVID-19 vaccines with new ones offering broader protection, it appears that science may have finally learned from history.
“After the SARS outbreak, we lost interest and failed to complete development of a vaccine for use in case of a recurrent outbreak. We must not make the same mistake again,” Dr. Giurgea and colleagues wrote in their Nature article about universal coronavirus vaccines.
A version of this article first appeared on Medscape.com.
As the COVID-19 pandemic winds down – for the time being at least – efforts are ramping up to develop next-generation vaccines that can protect against future novel coronaviruses and variants. Several projects are presenting clever combinations of viral parts to the immune system that evoke a robust and hopefully lasting response.
The coming generation of “pan” vaccines aims to tamp down SARS-CoV-2, its closest relatives, and whatever may come into tamer respiratory viruses like the common cold. Whatever the eventual components of this new generation of vaccines, experts agree on the goal: preventing severe disease and death. And a broader approach is critical.
“All the vaccines have been amazing. But we’re playing a whack-a-mole game with the variants. We need to take a step back and ask if a pan-variant vaccine is possible. That’s important because Omicron isn’t the last variant,” said Jacob Lemieux, MD, PhD, instructor in medicine and infectious disease specialist at Massachusetts General Hospital, Boston.
A broad spectrum vaccine
The drive to create a vaccine that would deter multiple coronaviruses arose early, among many researchers. An article published in Nature in May 2020 by National Institute of Allergy and Infectious Diseases researcher Luca T. Giurgea, MD, and colleagues said it all in the title: “Universal coronavirus vaccines: the time to start is now.”
Their concerns? The diversity of bat coronaviruses poised to jump into humans; the high mutability of the spike gene that the immune response recognizes; and the persistence of mutations in an RNA virus, which can’t repair errors.
Work on broader vaccines began in several labs as SARS-CoV-2 spawned variant after variant.
On Sept. 28, NIAID announced funding for developing ‘pan-coronavirus’ vaccines – the quotation marks theirs to indicate that a magic bullet against any new coronavirus is unrealistic. “These new awards are designed to look ahead and prepare for the next generation of coronaviruses with pandemic potential,” said NIAID director Anthony S. Fauci, MD. An initial three awards went to groups at the University of Wisconsin, Brigham and Women’s Hospital, and Duke University.
President Biden mentioned the NIAID funding in his State of the Union Address. He also talked about how the Biomedical Advanced Research and Development Authority, founded in 2006 to prepare for public health emergencies, is spearheading development of new vaccine platforms and vaccines that target a broader swath of pathogen parts.
Meanwhile, individual researchers from eclectic fields are finding new ways to prevent future pandemics.
Artem Babaian, PhD, a computational biologist at the University of Cambridge (England), had the idea to probe National Institutes of Health genome databases, going back more than a decade, for overlooked novel coronaviruses. He started the project while he was between jobs as the pandemic was unfurling, using a telltale enzyme unique to the RNA viruses to fish out COVID cousins. The work is published in Nature and the data freely available at serratus.io.
Among the nearly 132,000 novel RNA viruses Dr. Babaian’s team found, 9 were from previously unrecognized coronaviruses. The novel nine came from “ecologically diverse sources”: a seahorse, an axolotl, an eel, and several fishes. Deciphering the topographies of these coronaviruses may provide clues to developing vaccines that stay ahead of future pandemics.
But optics are important in keeping expectations reasonable. “‘Universal vaccine’ is a misnomer. I think about it as ‘broad spectrum vaccines.’ It’s critical to be up front that these vaccines can never guarantee immunity against all coronaviruses. There are no absolutes in biology, but they hopefully will work against the dangers that we do know exist. A vaccine that mimics exposure to many coronaviruses could protect against a currently unknown coronavirus, especially if slower-evolving antigens are included,” Dr. Babaian said in an interview.
Nikolai Petrovsky, MD, PhD, of Flinders University, Adelaide, and the biotechnology company Vaccine Pty, agrees, calling a literal pan-coronavirus vaccine a “pipe dream. What I do think is achievable is a broadly protective, pan–CoV-19 vaccine – I can say that because we have already developed and tested it, combining antigens rather than trying just one that can do everything.”
Immunity lures
The broader vaccines in development display viral antigens, such as spike proteins, to the immune system on diverse frameworks. Here are a few approaches.
Ferritin nanoparticles: A candidate vaccine from the emerging infectious diseases branch of Water Reed National Military Medical Center began phase 1 human trials in April 2021. Called SpFN, the vaccine consists of arrays of ferritin nanoparticles linked to spike proteins from various variants and species. Ferritin is a protein that binds and stores iron in the body.
“The repetitive and ordered display of the coronavirus spike protein on a multifaced nanoparticle may stimulate immunity in such a way as to translate into significantly broader protection,” said Walter Reed’s branch director and vaccine coinventor Kayvon Modjarrad, MD, PhD.
A second vaccine targets only the “bullseye” part of the spike that the virus uses to attach and gain access to human cells, called the receptor-binding domain (RBD), of SARS-CoV-2 variants and of the virus behind the original SARS. The preclinical data appeared in Science Translational Medicine.
Barton Haynes, MD and colleagues at the Duke Human Vaccine Institute are also using ferritin to design and develop a “pan-betacoronavirus vaccine,” referring to the genus to which SARS-CoV-2 belongs. They say their results in macaques, published in Nature, “demonstrate that current mRNA-based vaccines may provide some protection from future outbreaks of zoonotic betacoronaviruses.”
Mosaic nanoparticles: Graduate student Alexander Cohen is leading an effort at CalTech, in the lab of Pamela Bjorkman, PhD, that uses nanoparticles consisting of proteins from a bacterium (Strep pyogenes) to which RBDs from spike proteins of four or eight different betacoronaviruses are attached. The strategy demonstrates that the whole is greater than the sum of the parts.
“Alex’s results show that it is possible to raise diverse neutralizing antibody responses, even against coronavirus strains that were not represented on the injected nanoparticle. We are hopeful that this technology could be used to protect against future animal coronaviruses that cross into humans,” said Dr. Björkman. The work appeared in Science.
Candidate vaccines from Inovio Pharmaceuticals also use a mosaic spike strategy, but with DNA rings (plasmids) rather than nanoparticles. One version works against pre-Omicron variants and is being tested against Omicron, and another with “pan–COVID-19” coverage has tested well in animal models. Inovio’s vaccines are delivered into the skin using a special device that applies an electric pulse that increases the cells’ permeability.
Chimeric spikes: Yet another approach is to fashion vaccines from various parts of the betacoronaviruses that are most closely related to SARS-CoV-2 – the pathogens behind Middle East respiratory syndrome and severe acute respiratory syndrome as well as several bat viruses and a few pangolin ones. The abundance and ubiquity of these viruses provide a toolbox of sorts, with instructions written in the language of RNA, from which to select, dissect, recombine, and customize vaccines.
“SARS-like viruses can recombine and exhibit great genetic diversity in several parts of the genome. We designed chimeric spikes to improve coverage of a multiplexed vaccine,” said David Martinez, PhD.
His team at the University of North Carolina at Chapel Hill has developed mRNA vaccines that deliver “scrambled coronavirus spikes” representing various parts, not just the RBD, as described in Science.
In mice, the chimeric vaccines elicit robust T- and B-cell immune responses, which stimulate antibody production and control other facets of building immunity.
Beyond the spike bullseye
The challenge of developing pan-coronavirus vaccines is dual. “The very best vaccines are highly specific to each strain, and the universal vaccines have to sacrifice effectiveness to get broad coverage. Life is a trade-off.” Dr. Petrovsky told this news organization.
Efforts to broaden vaccine efficacy venture beyond targeting the RBD bullseyes of the spike triplets that festoon the virus. Some projects are focusing on less changeable spike parts that are more alike among less closely related coronaviruses than is the mutation-prone RBD. For example, the peptides that twist into the “stem-helix” portion of the part of the spike that adheres to host cells are the basis of some candidate vaccines now in preclinical studies.
Still other vaccines aren’t spike based at all. French company Osivax, for example, is working on a vaccine that targets the nucleocapsid protein that shields the viral RNA. The hope is that presenting various faces of the pathogen may spark immunity beyond an initial antibody rush and evoke more diverse and lasting T-cell responses.
With the myriad efforts to back up the first generation of COVID-19 vaccines with new ones offering broader protection, it appears that science may have finally learned from history.
“After the SARS outbreak, we lost interest and failed to complete development of a vaccine for use in case of a recurrent outbreak. We must not make the same mistake again,” Dr. Giurgea and colleagues wrote in their Nature article about universal coronavirus vaccines.
A version of this article first appeared on Medscape.com.
As the COVID-19 pandemic winds down – for the time being at least – efforts are ramping up to develop next-generation vaccines that can protect against future novel coronaviruses and variants. Several projects are presenting clever combinations of viral parts to the immune system that evoke a robust and hopefully lasting response.
The coming generation of “pan” vaccines aims to tamp down SARS-CoV-2, its closest relatives, and whatever may come into tamer respiratory viruses like the common cold. Whatever the eventual components of this new generation of vaccines, experts agree on the goal: preventing severe disease and death. And a broader approach is critical.
“All the vaccines have been amazing. But we’re playing a whack-a-mole game with the variants. We need to take a step back and ask if a pan-variant vaccine is possible. That’s important because Omicron isn’t the last variant,” said Jacob Lemieux, MD, PhD, instructor in medicine and infectious disease specialist at Massachusetts General Hospital, Boston.
A broad spectrum vaccine
The drive to create a vaccine that would deter multiple coronaviruses arose early, among many researchers. An article published in Nature in May 2020 by National Institute of Allergy and Infectious Diseases researcher Luca T. Giurgea, MD, and colleagues said it all in the title: “Universal coronavirus vaccines: the time to start is now.”
Their concerns? The diversity of bat coronaviruses poised to jump into humans; the high mutability of the spike gene that the immune response recognizes; and the persistence of mutations in an RNA virus, which can’t repair errors.
Work on broader vaccines began in several labs as SARS-CoV-2 spawned variant after variant.
On Sept. 28, NIAID announced funding for developing ‘pan-coronavirus’ vaccines – the quotation marks theirs to indicate that a magic bullet against any new coronavirus is unrealistic. “These new awards are designed to look ahead and prepare for the next generation of coronaviruses with pandemic potential,” said NIAID director Anthony S. Fauci, MD. An initial three awards went to groups at the University of Wisconsin, Brigham and Women’s Hospital, and Duke University.
President Biden mentioned the NIAID funding in his State of the Union Address. He also talked about how the Biomedical Advanced Research and Development Authority, founded in 2006 to prepare for public health emergencies, is spearheading development of new vaccine platforms and vaccines that target a broader swath of pathogen parts.
Meanwhile, individual researchers from eclectic fields are finding new ways to prevent future pandemics.
Artem Babaian, PhD, a computational biologist at the University of Cambridge (England), had the idea to probe National Institutes of Health genome databases, going back more than a decade, for overlooked novel coronaviruses. He started the project while he was between jobs as the pandemic was unfurling, using a telltale enzyme unique to the RNA viruses to fish out COVID cousins. The work is published in Nature and the data freely available at serratus.io.
Among the nearly 132,000 novel RNA viruses Dr. Babaian’s team found, 9 were from previously unrecognized coronaviruses. The novel nine came from “ecologically diverse sources”: a seahorse, an axolotl, an eel, and several fishes. Deciphering the topographies of these coronaviruses may provide clues to developing vaccines that stay ahead of future pandemics.
But optics are important in keeping expectations reasonable. “‘Universal vaccine’ is a misnomer. I think about it as ‘broad spectrum vaccines.’ It’s critical to be up front that these vaccines can never guarantee immunity against all coronaviruses. There are no absolutes in biology, but they hopefully will work against the dangers that we do know exist. A vaccine that mimics exposure to many coronaviruses could protect against a currently unknown coronavirus, especially if slower-evolving antigens are included,” Dr. Babaian said in an interview.
Nikolai Petrovsky, MD, PhD, of Flinders University, Adelaide, and the biotechnology company Vaccine Pty, agrees, calling a literal pan-coronavirus vaccine a “pipe dream. What I do think is achievable is a broadly protective, pan–CoV-19 vaccine – I can say that because we have already developed and tested it, combining antigens rather than trying just one that can do everything.”
Immunity lures
The broader vaccines in development display viral antigens, such as spike proteins, to the immune system on diverse frameworks. Here are a few approaches.
Ferritin nanoparticles: A candidate vaccine from the emerging infectious diseases branch of Water Reed National Military Medical Center began phase 1 human trials in April 2021. Called SpFN, the vaccine consists of arrays of ferritin nanoparticles linked to spike proteins from various variants and species. Ferritin is a protein that binds and stores iron in the body.
“The repetitive and ordered display of the coronavirus spike protein on a multifaced nanoparticle may stimulate immunity in such a way as to translate into significantly broader protection,” said Walter Reed’s branch director and vaccine coinventor Kayvon Modjarrad, MD, PhD.
A second vaccine targets only the “bullseye” part of the spike that the virus uses to attach and gain access to human cells, called the receptor-binding domain (RBD), of SARS-CoV-2 variants and of the virus behind the original SARS. The preclinical data appeared in Science Translational Medicine.
Barton Haynes, MD and colleagues at the Duke Human Vaccine Institute are also using ferritin to design and develop a “pan-betacoronavirus vaccine,” referring to the genus to which SARS-CoV-2 belongs. They say their results in macaques, published in Nature, “demonstrate that current mRNA-based vaccines may provide some protection from future outbreaks of zoonotic betacoronaviruses.”
Mosaic nanoparticles: Graduate student Alexander Cohen is leading an effort at CalTech, in the lab of Pamela Bjorkman, PhD, that uses nanoparticles consisting of proteins from a bacterium (Strep pyogenes) to which RBDs from spike proteins of four or eight different betacoronaviruses are attached. The strategy demonstrates that the whole is greater than the sum of the parts.
“Alex’s results show that it is possible to raise diverse neutralizing antibody responses, even against coronavirus strains that were not represented on the injected nanoparticle. We are hopeful that this technology could be used to protect against future animal coronaviruses that cross into humans,” said Dr. Björkman. The work appeared in Science.
Candidate vaccines from Inovio Pharmaceuticals also use a mosaic spike strategy, but with DNA rings (plasmids) rather than nanoparticles. One version works against pre-Omicron variants and is being tested against Omicron, and another with “pan–COVID-19” coverage has tested well in animal models. Inovio’s vaccines are delivered into the skin using a special device that applies an electric pulse that increases the cells’ permeability.
Chimeric spikes: Yet another approach is to fashion vaccines from various parts of the betacoronaviruses that are most closely related to SARS-CoV-2 – the pathogens behind Middle East respiratory syndrome and severe acute respiratory syndrome as well as several bat viruses and a few pangolin ones. The abundance and ubiquity of these viruses provide a toolbox of sorts, with instructions written in the language of RNA, from which to select, dissect, recombine, and customize vaccines.
“SARS-like viruses can recombine and exhibit great genetic diversity in several parts of the genome. We designed chimeric spikes to improve coverage of a multiplexed vaccine,” said David Martinez, PhD.
His team at the University of North Carolina at Chapel Hill has developed mRNA vaccines that deliver “scrambled coronavirus spikes” representing various parts, not just the RBD, as described in Science.
In mice, the chimeric vaccines elicit robust T- and B-cell immune responses, which stimulate antibody production and control other facets of building immunity.
Beyond the spike bullseye
The challenge of developing pan-coronavirus vaccines is dual. “The very best vaccines are highly specific to each strain, and the universal vaccines have to sacrifice effectiveness to get broad coverage. Life is a trade-off.” Dr. Petrovsky told this news organization.
Efforts to broaden vaccine efficacy venture beyond targeting the RBD bullseyes of the spike triplets that festoon the virus. Some projects are focusing on less changeable spike parts that are more alike among less closely related coronaviruses than is the mutation-prone RBD. For example, the peptides that twist into the “stem-helix” portion of the part of the spike that adheres to host cells are the basis of some candidate vaccines now in preclinical studies.
Still other vaccines aren’t spike based at all. French company Osivax, for example, is working on a vaccine that targets the nucleocapsid protein that shields the viral RNA. The hope is that presenting various faces of the pathogen may spark immunity beyond an initial antibody rush and evoke more diverse and lasting T-cell responses.
With the myriad efforts to back up the first generation of COVID-19 vaccines with new ones offering broader protection, it appears that science may have finally learned from history.
“After the SARS outbreak, we lost interest and failed to complete development of a vaccine for use in case of a recurrent outbreak. We must not make the same mistake again,” Dr. Giurgea and colleagues wrote in their Nature article about universal coronavirus vaccines.
A version of this article first appeared on Medscape.com.
Vaccination alone won’t counter rise of resistant variants: Study
Relaxation of nonpharmaceutical interventions once vaccination of the population has reached a tipping point short of herd immunity can increase the probability of the emergence of a resistant strain that natural selection then favors, according to new findings of a modeling study published online on July 30, 2021, in Scientific Reports.
Although vaccination is the best strategy for controlling viral spread, changes in our behavior and mindset will be increasingly required to stay ahead of vaccine-resistant strains, according to the authors of the report.
“We have become accustomed to thinking of the pandemic from the point of view of epidemiology, and advised to reduce transmission and the number of people getting sick and the death rate. As the pandemic spreads across years, there will be a new dimension to our thinking, both for policymakers and the public. And that’s the evolutionary perspective,” coauthor Fyodor Kondrashov, PhD, an evolutionary biologist at the Institute of Science and Technology, Klosterneuburg, Austria, said at a press briefing on July 299.
The coming “change of mentality” that Dr. Kondrashov foresees should reassure people that masking and social distancing even after being vaccinated aren’t futile. “It decreases the possibility that a vaccine-resistant strain is running around. We’re not just trying to prevent the spread, but the evolution of novel variants, which are so rare at this point that we haven’t yet identified them,” he said.
The study focused on evolution generically, rather than on specific variants. “We took the classical model used to study epidemiology of pandemics, the SIR [susceptible, infected, recovered] model, and we modified it to study the dynamics of rare mutations associated with emergence of a vaccine-resistant strain,” Simon A. Rella, the lead author of the study and a PhD student at the Institute of Science and Technology, explained at the briefing.
The researchers simulated the probability that a vaccine-resistant strain will emerge in a population of 10,000,000 individuals over 3 years, with vaccinations beginning after the first year. For eight scenarios, rates of infection, recovery, death, vaccination, and mutation and the percentage of individuals with resistant viral strains were factors in the model.
The model also simulated waves of low and high transmission, similar to the effects of large-scale interventions such as lockdowns.
Three factors
The study showed that a trio of factors increases the probability of a vaccine-resistant strain taking hold: slow rates of vaccination, high number of infected individuals, and faster mutation rate
These factors, Mr. Rella said, are obvious to some degree. “Every infected individual is like a mini-bioreactor, increasing the risk that mutations will appear that will endow the virus with the property of avoiding the immune system primed by a vaccine.”
Not as obvious, Mr. Rella added, is that, when most people are vaccinated, a vaccine-resistant strain has an advantage over the original strain and spreads faster.
But we can stop it, he said. “Our model shows that if at the time a vaccine campaign is close to finishing and nonpharmacological interventions are maintained, then there’s a chance to completely remove the vaccine-resistant mutations from the virus population.”
In scenarios in which a resistant strain became established, resistance initially emerged after about 60% of the population had been vaccinated. That makes nonpharmaceutical interventions such as masking and social distancing vitally important. Just under 50% of the U.S. population over the age of 12 has been fully vaccinated, according to the Centers for Disease Control and Prevention.
“Our results suggest that policymakers and individuals should consider maintaining nonpharmaceutical interventions and transmission-reducing behaviors throughout the entire vaccination period,” the investigators concluded.
A ‘powerful force’
“We hope for the best, that vaccine resistance has not developed, but caution that evolution is a very powerful force, and maintaining some precautions during vaccination may help to control that evolution,” said Dr. Kondrashov.
The investigators are relying on epidemiologists to determine which measures are most effective.
“It’s necessary to vaccinate as many people as fast as possible and as globally as possible and to maintain some level of nonpharmaceutical intervention to ensure rare variants have a chance to be suppressed instead of spread,” concluded Dr. Kondrashov.
He’s pessimistic because many countries are still having difficulty accessing vaccines, and vaccine efficacy wanes slightly over time. The authors warned that “the emergence of a partially or fully vaccine-resistant strain and its eventual establishment appears inevitable.”
The worst-case scenario is familiar to population biologists: rounds of “vaccine development playing catch up in the evolutionary arms race against novel strains,” the authors wrote.
Limitations of the study are that some parameters of the rate of evolution for vaccine-resistant strains aren’t known, and in creating the model, consideration was not given to effects of increased testing, rigorous contact tracing, rates of viral genome sequencing, and travel restrictions.
Rather, the model illustrates general principals by which vaccine resistance can evolve, Dr. Kondrashov said.
A version of this article first appeared on Medscape.com.
Relaxation of nonpharmaceutical interventions once vaccination of the population has reached a tipping point short of herd immunity can increase the probability of the emergence of a resistant strain that natural selection then favors, according to new findings of a modeling study published online on July 30, 2021, in Scientific Reports.
Although vaccination is the best strategy for controlling viral spread, changes in our behavior and mindset will be increasingly required to stay ahead of vaccine-resistant strains, according to the authors of the report.
“We have become accustomed to thinking of the pandemic from the point of view of epidemiology, and advised to reduce transmission and the number of people getting sick and the death rate. As the pandemic spreads across years, there will be a new dimension to our thinking, both for policymakers and the public. And that’s the evolutionary perspective,” coauthor Fyodor Kondrashov, PhD, an evolutionary biologist at the Institute of Science and Technology, Klosterneuburg, Austria, said at a press briefing on July 299.
The coming “change of mentality” that Dr. Kondrashov foresees should reassure people that masking and social distancing even after being vaccinated aren’t futile. “It decreases the possibility that a vaccine-resistant strain is running around. We’re not just trying to prevent the spread, but the evolution of novel variants, which are so rare at this point that we haven’t yet identified them,” he said.
The study focused on evolution generically, rather than on specific variants. “We took the classical model used to study epidemiology of pandemics, the SIR [susceptible, infected, recovered] model, and we modified it to study the dynamics of rare mutations associated with emergence of a vaccine-resistant strain,” Simon A. Rella, the lead author of the study and a PhD student at the Institute of Science and Technology, explained at the briefing.
The researchers simulated the probability that a vaccine-resistant strain will emerge in a population of 10,000,000 individuals over 3 years, with vaccinations beginning after the first year. For eight scenarios, rates of infection, recovery, death, vaccination, and mutation and the percentage of individuals with resistant viral strains were factors in the model.
The model also simulated waves of low and high transmission, similar to the effects of large-scale interventions such as lockdowns.
Three factors
The study showed that a trio of factors increases the probability of a vaccine-resistant strain taking hold: slow rates of vaccination, high number of infected individuals, and faster mutation rate
These factors, Mr. Rella said, are obvious to some degree. “Every infected individual is like a mini-bioreactor, increasing the risk that mutations will appear that will endow the virus with the property of avoiding the immune system primed by a vaccine.”
Not as obvious, Mr. Rella added, is that, when most people are vaccinated, a vaccine-resistant strain has an advantage over the original strain and spreads faster.
But we can stop it, he said. “Our model shows that if at the time a vaccine campaign is close to finishing and nonpharmacological interventions are maintained, then there’s a chance to completely remove the vaccine-resistant mutations from the virus population.”
In scenarios in which a resistant strain became established, resistance initially emerged after about 60% of the population had been vaccinated. That makes nonpharmaceutical interventions such as masking and social distancing vitally important. Just under 50% of the U.S. population over the age of 12 has been fully vaccinated, according to the Centers for Disease Control and Prevention.
“Our results suggest that policymakers and individuals should consider maintaining nonpharmaceutical interventions and transmission-reducing behaviors throughout the entire vaccination period,” the investigators concluded.
A ‘powerful force’
“We hope for the best, that vaccine resistance has not developed, but caution that evolution is a very powerful force, and maintaining some precautions during vaccination may help to control that evolution,” said Dr. Kondrashov.
The investigators are relying on epidemiologists to determine which measures are most effective.
“It’s necessary to vaccinate as many people as fast as possible and as globally as possible and to maintain some level of nonpharmaceutical intervention to ensure rare variants have a chance to be suppressed instead of spread,” concluded Dr. Kondrashov.
He’s pessimistic because many countries are still having difficulty accessing vaccines, and vaccine efficacy wanes slightly over time. The authors warned that “the emergence of a partially or fully vaccine-resistant strain and its eventual establishment appears inevitable.”
The worst-case scenario is familiar to population biologists: rounds of “vaccine development playing catch up in the evolutionary arms race against novel strains,” the authors wrote.
Limitations of the study are that some parameters of the rate of evolution for vaccine-resistant strains aren’t known, and in creating the model, consideration was not given to effects of increased testing, rigorous contact tracing, rates of viral genome sequencing, and travel restrictions.
Rather, the model illustrates general principals by which vaccine resistance can evolve, Dr. Kondrashov said.
A version of this article first appeared on Medscape.com.
Relaxation of nonpharmaceutical interventions once vaccination of the population has reached a tipping point short of herd immunity can increase the probability of the emergence of a resistant strain that natural selection then favors, according to new findings of a modeling study published online on July 30, 2021, in Scientific Reports.
Although vaccination is the best strategy for controlling viral spread, changes in our behavior and mindset will be increasingly required to stay ahead of vaccine-resistant strains, according to the authors of the report.
“We have become accustomed to thinking of the pandemic from the point of view of epidemiology, and advised to reduce transmission and the number of people getting sick and the death rate. As the pandemic spreads across years, there will be a new dimension to our thinking, both for policymakers and the public. And that’s the evolutionary perspective,” coauthor Fyodor Kondrashov, PhD, an evolutionary biologist at the Institute of Science and Technology, Klosterneuburg, Austria, said at a press briefing on July 299.
The coming “change of mentality” that Dr. Kondrashov foresees should reassure people that masking and social distancing even after being vaccinated aren’t futile. “It decreases the possibility that a vaccine-resistant strain is running around. We’re not just trying to prevent the spread, but the evolution of novel variants, which are so rare at this point that we haven’t yet identified them,” he said.
The study focused on evolution generically, rather than on specific variants. “We took the classical model used to study epidemiology of pandemics, the SIR [susceptible, infected, recovered] model, and we modified it to study the dynamics of rare mutations associated with emergence of a vaccine-resistant strain,” Simon A. Rella, the lead author of the study and a PhD student at the Institute of Science and Technology, explained at the briefing.
The researchers simulated the probability that a vaccine-resistant strain will emerge in a population of 10,000,000 individuals over 3 years, with vaccinations beginning after the first year. For eight scenarios, rates of infection, recovery, death, vaccination, and mutation and the percentage of individuals with resistant viral strains were factors in the model.
The model also simulated waves of low and high transmission, similar to the effects of large-scale interventions such as lockdowns.
Three factors
The study showed that a trio of factors increases the probability of a vaccine-resistant strain taking hold: slow rates of vaccination, high number of infected individuals, and faster mutation rate
These factors, Mr. Rella said, are obvious to some degree. “Every infected individual is like a mini-bioreactor, increasing the risk that mutations will appear that will endow the virus with the property of avoiding the immune system primed by a vaccine.”
Not as obvious, Mr. Rella added, is that, when most people are vaccinated, a vaccine-resistant strain has an advantage over the original strain and spreads faster.
But we can stop it, he said. “Our model shows that if at the time a vaccine campaign is close to finishing and nonpharmacological interventions are maintained, then there’s a chance to completely remove the vaccine-resistant mutations from the virus population.”
In scenarios in which a resistant strain became established, resistance initially emerged after about 60% of the population had been vaccinated. That makes nonpharmaceutical interventions such as masking and social distancing vitally important. Just under 50% of the U.S. population over the age of 12 has been fully vaccinated, according to the Centers for Disease Control and Prevention.
“Our results suggest that policymakers and individuals should consider maintaining nonpharmaceutical interventions and transmission-reducing behaviors throughout the entire vaccination period,” the investigators concluded.
A ‘powerful force’
“We hope for the best, that vaccine resistance has not developed, but caution that evolution is a very powerful force, and maintaining some precautions during vaccination may help to control that evolution,” said Dr. Kondrashov.
The investigators are relying on epidemiologists to determine which measures are most effective.
“It’s necessary to vaccinate as many people as fast as possible and as globally as possible and to maintain some level of nonpharmaceutical intervention to ensure rare variants have a chance to be suppressed instead of spread,” concluded Dr. Kondrashov.
He’s pessimistic because many countries are still having difficulty accessing vaccines, and vaccine efficacy wanes slightly over time. The authors warned that “the emergence of a partially or fully vaccine-resistant strain and its eventual establishment appears inevitable.”
The worst-case scenario is familiar to population biologists: rounds of “vaccine development playing catch up in the evolutionary arms race against novel strains,” the authors wrote.
Limitations of the study are that some parameters of the rate of evolution for vaccine-resistant strains aren’t known, and in creating the model, consideration was not given to effects of increased testing, rigorous contact tracing, rates of viral genome sequencing, and travel restrictions.
Rather, the model illustrates general principals by which vaccine resistance can evolve, Dr. Kondrashov said.
A version of this article first appeared on Medscape.com.
Ear tubes no better than antibiotics for otitis media in young kids
The debate over tympanostomy tubes versus antibiotics for recurrent acute otitis media (AOM) in young children is long-standing. Now, results of a randomized controlled trial show that tubes do not significantly lower the rate of episodes, compared with antibiotics, and medical management doesn’t increase antibiotic resistance.
“We found no evidence of microbial resistance from treating with antibiotics. If there’s not an impact on resistance, why take unnecessary chances on complications of surgery?” lead author Alejandro Hoberman, MD, from Children’s Hospital of Pittsburgh, said in an interview.
The study by Dr. Hoberman and colleagues was published May 13 in the New England Journal of Medicine.
AOM is the most frequent condition diagnosed in children in the United States after the common cold, affecting five of six children younger than 3 years. It is the leading indication for antimicrobial treatment, and tympanostomy tube insertion is the most frequently performed pediatric operation after the newborn period.
Randomized controlled clinical trials were conducted in the 1980s, but by the 1990s, questions of overuse arose. The American Academy of Otolaryngology–Head and Neck Surgery Foundation published the first clinical practice guidelines in 2013.
Parents must weigh the pros and cons. The use of tubes may avoid or delay the next round of drugs, but tubes cost more and introduce small risks (anesthesia, refractory otorrhea, tube blockage, premature dislocation or extrusion, and mild conductive hearing loss).
“We addressed issues that plagued older studies – a longer-term follow-up of 2 years, validated diagnoses of infection to determine eligibility – and used rating scales to measure quality of life,” Dr. Hoberman said.
The researchers randomly assigned children to receive antibiotics or tubes. To be eligible, children had to be 6-35 months of age and have had at least three episodes of AOM within 6 months or at least four episodes within 12 months, including at least one within the preceding 6 months.
The primary outcome was the mean number of episodes of AOM per child-year. Children were assessed at 8-week intervals and within 48 hours of developing symptoms of ear infection. The medically treated children received oral amoxicillin or, if that was ineffective, intramuscular ceftriaxone.
Criteria for determining treatment failure included persistent otorrhea, tympanic membrane perforation, antibiotic-associated diarrhea, reaction to anesthesia, and recurrence of AOM at a frequency equal to the frequency before antibiotic treatment.
In comparing tympanostomy tubes with antibiotics, Dr. Hoberman said, “We were unable to show benefit in the rate of ear infections per child per year over a 2-year period.” As expected, the infection rate fell by about half from the first year to the second in all children.
Overall, the investigators found “no substantial differences between treatment groups” with regard to AOM frequency, percentage of severe episodes, extent of antimicrobial resistance, quality of life for the children, and parental stress.
In an intention-to-treat analysis, the rate of AOM episodes per child-year during the study was 1.48 ± 0.08 for tubes and 1.56 ± 0.08 for antibiotics (P = .66).
However, randomization was not maintained in the intention-to-treat arm. Ten percent (13 of 129) of the children slated to receive tubes didn’t get them because of parental request. Conversely, 16% (54 of 121) of children in the antibiotic group received tubes, 35 (29%) of them in accordance with the trial protocol because of frequent recurrences, and 19 (16%) at parental request.
In a per-protocol analysis, rates of AOM episodes per child-year were 1.47 ± 0.08 for tubes and 1.72 ± 0.11 for antibiotics.
Tubes were associated with longer time until the first ear infection post placement, at a median of 4.34 months, compared with 2.33 months for children who received antibiotics. A smaller percentage of children in the tube group had treatment failure than in the antibiotic group (45% vs. 62%). Children who received tubes also had fewer days per year with symptoms in comparison with the children in the antibiotic group (mean, 2.00 ± 0.29 days vs. 8.33 ± 0.59 days).
The frequency distribution of AOM episodes, the percentage of severe episodes, and antimicrobial resistance detected in respiratory specimens were the same for both groups.
“Hoberman and colleagues add to our knowledge of managing children with recurrent ear infections with a large and rigorous clinical trial showing comparable efficacy of tympanostomy tube insertion, with antibiotic eardrops for new infections versus watchful waiting, with intermittent oral antibiotics, if further ear infections occur,” said Richard M. Rosenfeld, MD, MPH, MBA, distinguished professor and chairman, department of otolaryngology, SUNY Downstate Medical Center, New York.
However, in an accompanying editorial, Ellen R. Wald, MD, from the University of Wisconsin, Madison, pointed out that the sample size was smaller than desired, owing to participants switching groups.
In addition, Dr. Rosenfeld, who was the lead author of the 2013 guidelines, said the study likely underestimates the impact of tubes “because about two-thirds of the children who received them did not have persistent middle-ear fluid at baseline and would not have been candidates for tubes based on the current national guideline on tube indications.”
“Both tubes and intermittent antibiotic therapy are effective for managing recurrent AOM, and parents of children with persistent middle-ear effusion should engage in shared decision-making with their physician to decide on the best management option,” said Dr. Rosenfeld. “When in doubt, watchful waiting is appropriate because many children with recurrent AOM do better over time.”
Dr. Hoberman owns stock in Kaizen Bioscience and holds patents on devices to diagnose and treat AOM. One coauthor consults for Merck. Dr. Wald and Dr. Rosenfeld report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The debate over tympanostomy tubes versus antibiotics for recurrent acute otitis media (AOM) in young children is long-standing. Now, results of a randomized controlled trial show that tubes do not significantly lower the rate of episodes, compared with antibiotics, and medical management doesn’t increase antibiotic resistance.
“We found no evidence of microbial resistance from treating with antibiotics. If there’s not an impact on resistance, why take unnecessary chances on complications of surgery?” lead author Alejandro Hoberman, MD, from Children’s Hospital of Pittsburgh, said in an interview.
The study by Dr. Hoberman and colleagues was published May 13 in the New England Journal of Medicine.
AOM is the most frequent condition diagnosed in children in the United States after the common cold, affecting five of six children younger than 3 years. It is the leading indication for antimicrobial treatment, and tympanostomy tube insertion is the most frequently performed pediatric operation after the newborn period.
Randomized controlled clinical trials were conducted in the 1980s, but by the 1990s, questions of overuse arose. The American Academy of Otolaryngology–Head and Neck Surgery Foundation published the first clinical practice guidelines in 2013.
Parents must weigh the pros and cons. The use of tubes may avoid or delay the next round of drugs, but tubes cost more and introduce small risks (anesthesia, refractory otorrhea, tube blockage, premature dislocation or extrusion, and mild conductive hearing loss).
“We addressed issues that plagued older studies – a longer-term follow-up of 2 years, validated diagnoses of infection to determine eligibility – and used rating scales to measure quality of life,” Dr. Hoberman said.
The researchers randomly assigned children to receive antibiotics or tubes. To be eligible, children had to be 6-35 months of age and have had at least three episodes of AOM within 6 months or at least four episodes within 12 months, including at least one within the preceding 6 months.
The primary outcome was the mean number of episodes of AOM per child-year. Children were assessed at 8-week intervals and within 48 hours of developing symptoms of ear infection. The medically treated children received oral amoxicillin or, if that was ineffective, intramuscular ceftriaxone.
Criteria for determining treatment failure included persistent otorrhea, tympanic membrane perforation, antibiotic-associated diarrhea, reaction to anesthesia, and recurrence of AOM at a frequency equal to the frequency before antibiotic treatment.
In comparing tympanostomy tubes with antibiotics, Dr. Hoberman said, “We were unable to show benefit in the rate of ear infections per child per year over a 2-year period.” As expected, the infection rate fell by about half from the first year to the second in all children.
Overall, the investigators found “no substantial differences between treatment groups” with regard to AOM frequency, percentage of severe episodes, extent of antimicrobial resistance, quality of life for the children, and parental stress.
In an intention-to-treat analysis, the rate of AOM episodes per child-year during the study was 1.48 ± 0.08 for tubes and 1.56 ± 0.08 for antibiotics (P = .66).
However, randomization was not maintained in the intention-to-treat arm. Ten percent (13 of 129) of the children slated to receive tubes didn’t get them because of parental request. Conversely, 16% (54 of 121) of children in the antibiotic group received tubes, 35 (29%) of them in accordance with the trial protocol because of frequent recurrences, and 19 (16%) at parental request.
In a per-protocol analysis, rates of AOM episodes per child-year were 1.47 ± 0.08 for tubes and 1.72 ± 0.11 for antibiotics.
Tubes were associated with longer time until the first ear infection post placement, at a median of 4.34 months, compared with 2.33 months for children who received antibiotics. A smaller percentage of children in the tube group had treatment failure than in the antibiotic group (45% vs. 62%). Children who received tubes also had fewer days per year with symptoms in comparison with the children in the antibiotic group (mean, 2.00 ± 0.29 days vs. 8.33 ± 0.59 days).
The frequency distribution of AOM episodes, the percentage of severe episodes, and antimicrobial resistance detected in respiratory specimens were the same for both groups.
“Hoberman and colleagues add to our knowledge of managing children with recurrent ear infections with a large and rigorous clinical trial showing comparable efficacy of tympanostomy tube insertion, with antibiotic eardrops for new infections versus watchful waiting, with intermittent oral antibiotics, if further ear infections occur,” said Richard M. Rosenfeld, MD, MPH, MBA, distinguished professor and chairman, department of otolaryngology, SUNY Downstate Medical Center, New York.
However, in an accompanying editorial, Ellen R. Wald, MD, from the University of Wisconsin, Madison, pointed out that the sample size was smaller than desired, owing to participants switching groups.
In addition, Dr. Rosenfeld, who was the lead author of the 2013 guidelines, said the study likely underestimates the impact of tubes “because about two-thirds of the children who received them did not have persistent middle-ear fluid at baseline and would not have been candidates for tubes based on the current national guideline on tube indications.”
“Both tubes and intermittent antibiotic therapy are effective for managing recurrent AOM, and parents of children with persistent middle-ear effusion should engage in shared decision-making with their physician to decide on the best management option,” said Dr. Rosenfeld. “When in doubt, watchful waiting is appropriate because many children with recurrent AOM do better over time.”
Dr. Hoberman owns stock in Kaizen Bioscience and holds patents on devices to diagnose and treat AOM. One coauthor consults for Merck. Dr. Wald and Dr. Rosenfeld report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The debate over tympanostomy tubes versus antibiotics for recurrent acute otitis media (AOM) in young children is long-standing. Now, results of a randomized controlled trial show that tubes do not significantly lower the rate of episodes, compared with antibiotics, and medical management doesn’t increase antibiotic resistance.
“We found no evidence of microbial resistance from treating with antibiotics. If there’s not an impact on resistance, why take unnecessary chances on complications of surgery?” lead author Alejandro Hoberman, MD, from Children’s Hospital of Pittsburgh, said in an interview.
The study by Dr. Hoberman and colleagues was published May 13 in the New England Journal of Medicine.
AOM is the most frequent condition diagnosed in children in the United States after the common cold, affecting five of six children younger than 3 years. It is the leading indication for antimicrobial treatment, and tympanostomy tube insertion is the most frequently performed pediatric operation after the newborn period.
Randomized controlled clinical trials were conducted in the 1980s, but by the 1990s, questions of overuse arose. The American Academy of Otolaryngology–Head and Neck Surgery Foundation published the first clinical practice guidelines in 2013.
Parents must weigh the pros and cons. The use of tubes may avoid or delay the next round of drugs, but tubes cost more and introduce small risks (anesthesia, refractory otorrhea, tube blockage, premature dislocation or extrusion, and mild conductive hearing loss).
“We addressed issues that plagued older studies – a longer-term follow-up of 2 years, validated diagnoses of infection to determine eligibility – and used rating scales to measure quality of life,” Dr. Hoberman said.
The researchers randomly assigned children to receive antibiotics or tubes. To be eligible, children had to be 6-35 months of age and have had at least three episodes of AOM within 6 months or at least four episodes within 12 months, including at least one within the preceding 6 months.
The primary outcome was the mean number of episodes of AOM per child-year. Children were assessed at 8-week intervals and within 48 hours of developing symptoms of ear infection. The medically treated children received oral amoxicillin or, if that was ineffective, intramuscular ceftriaxone.
Criteria for determining treatment failure included persistent otorrhea, tympanic membrane perforation, antibiotic-associated diarrhea, reaction to anesthesia, and recurrence of AOM at a frequency equal to the frequency before antibiotic treatment.
In comparing tympanostomy tubes with antibiotics, Dr. Hoberman said, “We were unable to show benefit in the rate of ear infections per child per year over a 2-year period.” As expected, the infection rate fell by about half from the first year to the second in all children.
Overall, the investigators found “no substantial differences between treatment groups” with regard to AOM frequency, percentage of severe episodes, extent of antimicrobial resistance, quality of life for the children, and parental stress.
In an intention-to-treat analysis, the rate of AOM episodes per child-year during the study was 1.48 ± 0.08 for tubes and 1.56 ± 0.08 for antibiotics (P = .66).
However, randomization was not maintained in the intention-to-treat arm. Ten percent (13 of 129) of the children slated to receive tubes didn’t get them because of parental request. Conversely, 16% (54 of 121) of children in the antibiotic group received tubes, 35 (29%) of them in accordance with the trial protocol because of frequent recurrences, and 19 (16%) at parental request.
In a per-protocol analysis, rates of AOM episodes per child-year were 1.47 ± 0.08 for tubes and 1.72 ± 0.11 for antibiotics.
Tubes were associated with longer time until the first ear infection post placement, at a median of 4.34 months, compared with 2.33 months for children who received antibiotics. A smaller percentage of children in the tube group had treatment failure than in the antibiotic group (45% vs. 62%). Children who received tubes also had fewer days per year with symptoms in comparison with the children in the antibiotic group (mean, 2.00 ± 0.29 days vs. 8.33 ± 0.59 days).
The frequency distribution of AOM episodes, the percentage of severe episodes, and antimicrobial resistance detected in respiratory specimens were the same for both groups.
“Hoberman and colleagues add to our knowledge of managing children with recurrent ear infections with a large and rigorous clinical trial showing comparable efficacy of tympanostomy tube insertion, with antibiotic eardrops for new infections versus watchful waiting, with intermittent oral antibiotics, if further ear infections occur,” said Richard M. Rosenfeld, MD, MPH, MBA, distinguished professor and chairman, department of otolaryngology, SUNY Downstate Medical Center, New York.
However, in an accompanying editorial, Ellen R. Wald, MD, from the University of Wisconsin, Madison, pointed out that the sample size was smaller than desired, owing to participants switching groups.
In addition, Dr. Rosenfeld, who was the lead author of the 2013 guidelines, said the study likely underestimates the impact of tubes “because about two-thirds of the children who received them did not have persistent middle-ear fluid at baseline and would not have been candidates for tubes based on the current national guideline on tube indications.”
“Both tubes and intermittent antibiotic therapy are effective for managing recurrent AOM, and parents of children with persistent middle-ear effusion should engage in shared decision-making with their physician to decide on the best management option,” said Dr. Rosenfeld. “When in doubt, watchful waiting is appropriate because many children with recurrent AOM do better over time.”
Dr. Hoberman owns stock in Kaizen Bioscience and holds patents on devices to diagnose and treat AOM. One coauthor consults for Merck. Dr. Wald and Dr. Rosenfeld report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA places clinical hold on sickle cell gene therapy
The Food and Drug Administration placed a clinical hold yesterday on two gene therapy trials for sickle cell disease (SCD) after two recent complications: one participant developed acute myeloid leukemia (AML) and another developed myelodysplastic syndrome (MDS). The sponsoring company, bluebird bio, suspended the trials last week upon learning of the cases.
The company has also put the brakes on a treatment for beta thalassemia already approved in the European Union and the United Kingdom, betibeglogene autotemcel (Zynteglo). The treatment hasn’t been associated with problems but uses the same gene delivery vector, a lentivirus, as that used in the SCD trials.
Overall, the company has enrolled 47 SCD patients and 63 with beta thalassemia in trials.
The gene therapy “space” is one with spectacular successes – for a form of retinal blindness and spinal muscular atrophy – rising against a backdrop of recent setbacks and failures – for Duchenne muscular dystrophy, lipoprotein lipase deficiency, and myotubular myopathy.
A lentiviral vector
The retooled lentivirus used in the SCD trials, LentiGlobin, delivers a beta-globin gene with one amino acid replacement to hematopoietic stem cells outside the patient’s body. The modified cells are then infused back into the patient. The gene therapy reshapes red blood cells, enabling them to circulate through narrow blood vessels without sickling and adhering into painful logjams.
What is worrisome is that in the patient who developed AML, and who received the gene therapy more than 5 years ago, the cancer cells contained the vector. Those test results aren’t yet available for the participant who has MDS.
The finding raises suspicion that the gene therapy had a role in the cancer, but is only correlative.
Lentiviral vectors have a good track record, but the two cases evoke memories of 2 decades ago. In 2001, five children being treated for an inherited immunodeficiency (SCID-X1) with a gamma retroviral vector developed leukemia and one died. Those viruses inserted into an oncogene. Happening 2 years after the death of 18-year-old Jesse Gelsinger in another gene therapy trial, the SCID trial had a chilling effect on the field.
Since then, lentiviral vectors have been reinvented to be “self-inactivating,” minimizing the risk for inserting willy-nilly into a genome. “Lentiviral vectors have been expressly designed to avoid insertional oncogenesis, based on prior experience with the gamma retroviruses. We don’t have evidence that the vector is causative, but our studies will shed some light on whether that’s true in these cases,” said bluebird bio chief scientific officer Philip Gregory, DPhil, on a conference call Feb. 16.
Lentiviral vectors have been successful as the backbone of chimeric antigen receptor T-cell (CAR-T) therapy, which directs modified T cells to certain blood cancers. “Among the hundreds to thousands of patients treated with CAR-T cell therapy, lentivirus vector hasn’t been associated with any malignancies,” said bluebird’s chief medical officer, Dave Davidson, MD.
Jeanne Loring, PhD, director of the Center for Regenerative Medicine at Scripps Research, agreed. “Gene therapy is having some extreme highs and lows these days. Most studies use [adeno-associated viral] vectors, which don’t integrate into the genome. But some people have antibodies to AAV vectors, and AAV is diluted out when cells divide. That’s why lentivirus, which integrates into the genome, is used for blood stem cells and T cells in CAR-T therapy.”
Pinpointing causality
At bluebird bio, investigation into the possible “genetic gymnastics” of the lentivirus vector is focusing on where it integrates into the genome – whether it harpoons an oncogene like the gamma retroviral vectors, or affects genome stability, Dr. Gregory explained. To be causative, the affected gene must be a “driver” of the cancer, and not just a “passenger.”
Another suspect is busulfan, a drug used to “condition” the recipient’s bone marrow, making room for modified stem cells. “It’s possible that busulfan is the main problem, as it is a carcinogen unto itself,” said Paul Knoepfler, PhD, a stem cell researcher at the University of California, Davis.
In addition to the two more recent reports of complications, a third trial participant, who had participated in a phase 1/2 trial, developed MDS in 2018 and died of AML in July 2020. The cancer cells from that patient did not contain viral vectors and the MDS was attributed to busulfan conditioning.
Nick Leschly, chief of bluebird, pointed out the importance of clinical context in implicating the vector. Because SCD itself stresses the bone marrow, patients already face an increased risk of developing blood cancer, he said. “Now layer on other risks of the gene therapy. It’s challenging because we’re dealing with patients who have life expectancy in the mid 40s.” Previous treatments, such the antisickling drug hydroxyurea, may also contribute to patient vulnerability.
A patient’s view
SCD affects more than 100,000 people in the United States, and about 20 million globally. Charles Hough is one of them. He can attest to the severity of the disease as well as the promise of gene therapy
Mr. Hough was diagnosed at age 2, and endured the profound fatigue, pain crises, and even coma characteristic of severe cases. He cited his “rebirth” as Sept. 25, 2018, when he received his first modified stem cells at the National Institutes of Health. Mr. Hough told his story a year ago in a webinar for the National Organization for Rare Disorders. This news organization caught up with him in light of the clinical trial hold.
Although the preparative regimens for the gene therapy were tough, his sickle cell symptoms vanished after gene therapy. Even hearing about the current hold on the clinical trial, Mr. Hough doesn’t regret his participation.
“I had a lot of friends who passed because of the complications from sickle cell. I was always worried that I wouldn’t live to see the next day. Now I don’t have that stress hanging over my head and I feel like I can live a normal life. Becoming sickle cell free was my dream.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration placed a clinical hold yesterday on two gene therapy trials for sickle cell disease (SCD) after two recent complications: one participant developed acute myeloid leukemia (AML) and another developed myelodysplastic syndrome (MDS). The sponsoring company, bluebird bio, suspended the trials last week upon learning of the cases.
The company has also put the brakes on a treatment for beta thalassemia already approved in the European Union and the United Kingdom, betibeglogene autotemcel (Zynteglo). The treatment hasn’t been associated with problems but uses the same gene delivery vector, a lentivirus, as that used in the SCD trials.
Overall, the company has enrolled 47 SCD patients and 63 with beta thalassemia in trials.
The gene therapy “space” is one with spectacular successes – for a form of retinal blindness and spinal muscular atrophy – rising against a backdrop of recent setbacks and failures – for Duchenne muscular dystrophy, lipoprotein lipase deficiency, and myotubular myopathy.
A lentiviral vector
The retooled lentivirus used in the SCD trials, LentiGlobin, delivers a beta-globin gene with one amino acid replacement to hematopoietic stem cells outside the patient’s body. The modified cells are then infused back into the patient. The gene therapy reshapes red blood cells, enabling them to circulate through narrow blood vessels without sickling and adhering into painful logjams.
What is worrisome is that in the patient who developed AML, and who received the gene therapy more than 5 years ago, the cancer cells contained the vector. Those test results aren’t yet available for the participant who has MDS.
The finding raises suspicion that the gene therapy had a role in the cancer, but is only correlative.
Lentiviral vectors have a good track record, but the two cases evoke memories of 2 decades ago. In 2001, five children being treated for an inherited immunodeficiency (SCID-X1) with a gamma retroviral vector developed leukemia and one died. Those viruses inserted into an oncogene. Happening 2 years after the death of 18-year-old Jesse Gelsinger in another gene therapy trial, the SCID trial had a chilling effect on the field.
Since then, lentiviral vectors have been reinvented to be “self-inactivating,” minimizing the risk for inserting willy-nilly into a genome. “Lentiviral vectors have been expressly designed to avoid insertional oncogenesis, based on prior experience with the gamma retroviruses. We don’t have evidence that the vector is causative, but our studies will shed some light on whether that’s true in these cases,” said bluebird bio chief scientific officer Philip Gregory, DPhil, on a conference call Feb. 16.
Lentiviral vectors have been successful as the backbone of chimeric antigen receptor T-cell (CAR-T) therapy, which directs modified T cells to certain blood cancers. “Among the hundreds to thousands of patients treated with CAR-T cell therapy, lentivirus vector hasn’t been associated with any malignancies,” said bluebird’s chief medical officer, Dave Davidson, MD.
Jeanne Loring, PhD, director of the Center for Regenerative Medicine at Scripps Research, agreed. “Gene therapy is having some extreme highs and lows these days. Most studies use [adeno-associated viral] vectors, which don’t integrate into the genome. But some people have antibodies to AAV vectors, and AAV is diluted out when cells divide. That’s why lentivirus, which integrates into the genome, is used for blood stem cells and T cells in CAR-T therapy.”
Pinpointing causality
At bluebird bio, investigation into the possible “genetic gymnastics” of the lentivirus vector is focusing on where it integrates into the genome – whether it harpoons an oncogene like the gamma retroviral vectors, or affects genome stability, Dr. Gregory explained. To be causative, the affected gene must be a “driver” of the cancer, and not just a “passenger.”
Another suspect is busulfan, a drug used to “condition” the recipient’s bone marrow, making room for modified stem cells. “It’s possible that busulfan is the main problem, as it is a carcinogen unto itself,” said Paul Knoepfler, PhD, a stem cell researcher at the University of California, Davis.
In addition to the two more recent reports of complications, a third trial participant, who had participated in a phase 1/2 trial, developed MDS in 2018 and died of AML in July 2020. The cancer cells from that patient did not contain viral vectors and the MDS was attributed to busulfan conditioning.
Nick Leschly, chief of bluebird, pointed out the importance of clinical context in implicating the vector. Because SCD itself stresses the bone marrow, patients already face an increased risk of developing blood cancer, he said. “Now layer on other risks of the gene therapy. It’s challenging because we’re dealing with patients who have life expectancy in the mid 40s.” Previous treatments, such the antisickling drug hydroxyurea, may also contribute to patient vulnerability.
A patient’s view
SCD affects more than 100,000 people in the United States, and about 20 million globally. Charles Hough is one of them. He can attest to the severity of the disease as well as the promise of gene therapy
Mr. Hough was diagnosed at age 2, and endured the profound fatigue, pain crises, and even coma characteristic of severe cases. He cited his “rebirth” as Sept. 25, 2018, when he received his first modified stem cells at the National Institutes of Health. Mr. Hough told his story a year ago in a webinar for the National Organization for Rare Disorders. This news organization caught up with him in light of the clinical trial hold.
Although the preparative regimens for the gene therapy were tough, his sickle cell symptoms vanished after gene therapy. Even hearing about the current hold on the clinical trial, Mr. Hough doesn’t regret his participation.
“I had a lot of friends who passed because of the complications from sickle cell. I was always worried that I wouldn’t live to see the next day. Now I don’t have that stress hanging over my head and I feel like I can live a normal life. Becoming sickle cell free was my dream.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration placed a clinical hold yesterday on two gene therapy trials for sickle cell disease (SCD) after two recent complications: one participant developed acute myeloid leukemia (AML) and another developed myelodysplastic syndrome (MDS). The sponsoring company, bluebird bio, suspended the trials last week upon learning of the cases.
The company has also put the brakes on a treatment for beta thalassemia already approved in the European Union and the United Kingdom, betibeglogene autotemcel (Zynteglo). The treatment hasn’t been associated with problems but uses the same gene delivery vector, a lentivirus, as that used in the SCD trials.
Overall, the company has enrolled 47 SCD patients and 63 with beta thalassemia in trials.
The gene therapy “space” is one with spectacular successes – for a form of retinal blindness and spinal muscular atrophy – rising against a backdrop of recent setbacks and failures – for Duchenne muscular dystrophy, lipoprotein lipase deficiency, and myotubular myopathy.
A lentiviral vector
The retooled lentivirus used in the SCD trials, LentiGlobin, delivers a beta-globin gene with one amino acid replacement to hematopoietic stem cells outside the patient’s body. The modified cells are then infused back into the patient. The gene therapy reshapes red blood cells, enabling them to circulate through narrow blood vessels without sickling and adhering into painful logjams.
What is worrisome is that in the patient who developed AML, and who received the gene therapy more than 5 years ago, the cancer cells contained the vector. Those test results aren’t yet available for the participant who has MDS.
The finding raises suspicion that the gene therapy had a role in the cancer, but is only correlative.
Lentiviral vectors have a good track record, but the two cases evoke memories of 2 decades ago. In 2001, five children being treated for an inherited immunodeficiency (SCID-X1) with a gamma retroviral vector developed leukemia and one died. Those viruses inserted into an oncogene. Happening 2 years after the death of 18-year-old Jesse Gelsinger in another gene therapy trial, the SCID trial had a chilling effect on the field.
Since then, lentiviral vectors have been reinvented to be “self-inactivating,” minimizing the risk for inserting willy-nilly into a genome. “Lentiviral vectors have been expressly designed to avoid insertional oncogenesis, based on prior experience with the gamma retroviruses. We don’t have evidence that the vector is causative, but our studies will shed some light on whether that’s true in these cases,” said bluebird bio chief scientific officer Philip Gregory, DPhil, on a conference call Feb. 16.
Lentiviral vectors have been successful as the backbone of chimeric antigen receptor T-cell (CAR-T) therapy, which directs modified T cells to certain blood cancers. “Among the hundreds to thousands of patients treated with CAR-T cell therapy, lentivirus vector hasn’t been associated with any malignancies,” said bluebird’s chief medical officer, Dave Davidson, MD.
Jeanne Loring, PhD, director of the Center for Regenerative Medicine at Scripps Research, agreed. “Gene therapy is having some extreme highs and lows these days. Most studies use [adeno-associated viral] vectors, which don’t integrate into the genome. But some people have antibodies to AAV vectors, and AAV is diluted out when cells divide. That’s why lentivirus, which integrates into the genome, is used for blood stem cells and T cells in CAR-T therapy.”
Pinpointing causality
At bluebird bio, investigation into the possible “genetic gymnastics” of the lentivirus vector is focusing on where it integrates into the genome – whether it harpoons an oncogene like the gamma retroviral vectors, or affects genome stability, Dr. Gregory explained. To be causative, the affected gene must be a “driver” of the cancer, and not just a “passenger.”
Another suspect is busulfan, a drug used to “condition” the recipient’s bone marrow, making room for modified stem cells. “It’s possible that busulfan is the main problem, as it is a carcinogen unto itself,” said Paul Knoepfler, PhD, a stem cell researcher at the University of California, Davis.
In addition to the two more recent reports of complications, a third trial participant, who had participated in a phase 1/2 trial, developed MDS in 2018 and died of AML in July 2020. The cancer cells from that patient did not contain viral vectors and the MDS was attributed to busulfan conditioning.
Nick Leschly, chief of bluebird, pointed out the importance of clinical context in implicating the vector. Because SCD itself stresses the bone marrow, patients already face an increased risk of developing blood cancer, he said. “Now layer on other risks of the gene therapy. It’s challenging because we’re dealing with patients who have life expectancy in the mid 40s.” Previous treatments, such the antisickling drug hydroxyurea, may also contribute to patient vulnerability.
A patient’s view
SCD affects more than 100,000 people in the United States, and about 20 million globally. Charles Hough is one of them. He can attest to the severity of the disease as well as the promise of gene therapy
Mr. Hough was diagnosed at age 2, and endured the profound fatigue, pain crises, and even coma characteristic of severe cases. He cited his “rebirth” as Sept. 25, 2018, when he received his first modified stem cells at the National Institutes of Health. Mr. Hough told his story a year ago in a webinar for the National Organization for Rare Disorders. This news organization caught up with him in light of the clinical trial hold.
Although the preparative regimens for the gene therapy were tough, his sickle cell symptoms vanished after gene therapy. Even hearing about the current hold on the clinical trial, Mr. Hough doesn’t regret his participation.
“I had a lot of friends who passed because of the complications from sickle cell. I was always worried that I wouldn’t live to see the next day. Now I don’t have that stress hanging over my head and I feel like I can live a normal life. Becoming sickle cell free was my dream.”
A version of this article first appeared on Medscape.com.
Nocturnal oxygen no help for isolated desaturation in COPD
Nocturnal oxygen therapy for patients with COPD and isolated nocturnal oxygen desaturation does not improve survival or delay disease progression, according to findings published Sept. 17 in The New England Journal of Medicine. The new report adds to evidence that the widely implemented and costly practice may be unnecessary.
Patients with COPD who do not qualify for long-term oxygen therapy (LTOT) are commonly prescribed nocturnal oxygen in the belief that it can delay disease progression, possibly by decreasing alveolar hypoventilation and ventilation-perfusion mismatch.
But investigations so far and the new study from the International Nocturnal Oxygen (INOX) Trial have not borne this out.
“There is no indication that nocturnal oxygen has a positive or negative effect on survival or progression to long-term oxygen therapy in patients with nocturnal hypoxemia in COPD. Consequently, there is no reason for physicians to screen for nocturnal hypoxemia in COPD,” study leader Yves Lacasse, MD, told Medscape Medical News.
Lacasse is from the Institut Universitaire de Cardiologie et de Pneumologie de Québec–Université Laval, Quebec, Canada.
The idea that the therapy helps is firmly entrenched.
In the early 1980s, two trials indicated that patients who had COPD and severe chronic daytime hypoxemia benefit from LTOT (15-18 hours a day or longer).
A decade later, two landmark trials (the Nocturnal Oxygen Therapy Trial and the British Medical Research Council Trial) added to evidence that LTOT may prolong life for patients with COPD and severe daytime hypoxemia.
“The good news from both trials was that oxygen saves lives. From this moment, oxygen therapy became a standard of care, and confirmatory trials would be considered unethical,” Lacasse explained.
“Oxygen therapy gained widespread acceptance by official organizations for treatment of most chronic cardiorespiratory conditions complicated by severe hypoxemia, even if proof of efficacy is lacking. New indications emerged, such as isolated nocturnal oxygen desaturation. Even in COPD, inappropriate prescriptions of home oxygen therapy are not unusual. Oxygen is everywhere,” Lacasse continued.
A meta-analysis from 2005 identified two trials that evaluated home oxygen therapy specifically for isolated nocturnal desaturation. Both found no survival benefit from nocturnal oxygen.
The study by Lacasse and colleagues assessed effects on mortality or worsening of disease (progression to LTOT) with 3-4 years of nocturnal oxygen supplementation.
Participants, whose oxygen saturation was less than 90% for at least 30% of the recording time on nocturnal oximetry, received oxygen or ambient air from a sham device as a placebo for at least 4 hours per session. The goal of treatment was nocturnal oxygen saturation exceeding 90% for at least 90% of the recorded time.
The trial protocol excluded patients with severe obesity, apnea, lung cancer, left heart failure, interstitial lung disease, or bronchiectasis.
The study was initially powered in 2010 to include 600 participants, with half to receive placebo. The study assumed mortality of 20% among control patients over 3 years; 20% of patients progressed to LTOT.
When recruiting lagged, the data safety monitoring board and steering committee extended follow-up to 4 years. In 2014, they requested an interim analysis, and recruitment ceased. Overall, 243 patients participated.
Lacasse cited several reasons for the difficulty with recruitment as well as retention: unwillingness to take the risk of receiving placebo instead of a readily available treatment, fading interest over time, and frailty that affects compliance.
Patients in the study came from 28 community or university-affiliated hospitals in Canada, Portugal, Spain, and France. At the 3-year mark, 39% of patients (48 of 123) who were assigned to nocturnal oxygen therapy and 42% (50 of 119) of those taking placebo had met criteria for LTOT or had died (difference, −3.0 percentage points; P = .64). The groups did not differ appreciably in rates of exacerbation and hospitalization.
The researchers could not analyze subgroups because the patients were very similar with regard to the severity of nocturnal oxygen desaturation, Lacasse said.
Economics enters into the picture – home oxygen therapy is second only to hospitalization as the most expensive healthcare expenditure associated with clinical care for COPD in developed countries. “The math is simple. There is enormous potential for saving money if the results of our clinical trial are applied appropriately,” said Lacasse.
William Bailey, MD, professor emeritus of pulmonary, allergy, and critical care medicine at the University of Alabama at Birmingham, agrees that the practice is overused.
“There is a built-in bias in the medical community. Most believe that anyone with lung disease benefits from oxygen. Even some of our investigators had a hard time believing the results. The study was well designed, carefully carried out, and I feel confident that the results are reliable,” he said.
Shawn P. E. Nishi, MD, director of bronchoscopy and advanced pulmonary procedures, division of pulmonary and critical care medicine, the University of Texas Medical Branch, Galveston, Texas, mentioned the study’s main limitation, which the authors readily acknowledge.
“Unfortunately, the trial had difficulty recruiting subjects, with less than half of expected enrollment achieved, and was underpowered to make any conclusions. Other studies have examined nocturnal oxygen use and have not shown a mortality benefit,” Nishi explained.
She added that the study did not evaluate use of LTOT for improving outcomes other than mortality, including quality of life, cardiovascular morbidity, depression, cognitive function, exercise capacity, and frequency of COPD exacerbations or hospitalization.
Other limitations of the study include suboptimal adherence to the therapy and interpretation of the clinical significance on the basis of a survey of Canadian pulmonologists.
This article first appeared on Medscape.com.
Nocturnal oxygen therapy for patients with COPD and isolated nocturnal oxygen desaturation does not improve survival or delay disease progression, according to findings published Sept. 17 in The New England Journal of Medicine. The new report adds to evidence that the widely implemented and costly practice may be unnecessary.
Patients with COPD who do not qualify for long-term oxygen therapy (LTOT) are commonly prescribed nocturnal oxygen in the belief that it can delay disease progression, possibly by decreasing alveolar hypoventilation and ventilation-perfusion mismatch.
But investigations so far and the new study from the International Nocturnal Oxygen (INOX) Trial have not borne this out.
“There is no indication that nocturnal oxygen has a positive or negative effect on survival or progression to long-term oxygen therapy in patients with nocturnal hypoxemia in COPD. Consequently, there is no reason for physicians to screen for nocturnal hypoxemia in COPD,” study leader Yves Lacasse, MD, told Medscape Medical News.
Lacasse is from the Institut Universitaire de Cardiologie et de Pneumologie de Québec–Université Laval, Quebec, Canada.
The idea that the therapy helps is firmly entrenched.
In the early 1980s, two trials indicated that patients who had COPD and severe chronic daytime hypoxemia benefit from LTOT (15-18 hours a day or longer).
A decade later, two landmark trials (the Nocturnal Oxygen Therapy Trial and the British Medical Research Council Trial) added to evidence that LTOT may prolong life for patients with COPD and severe daytime hypoxemia.
“The good news from both trials was that oxygen saves lives. From this moment, oxygen therapy became a standard of care, and confirmatory trials would be considered unethical,” Lacasse explained.
“Oxygen therapy gained widespread acceptance by official organizations for treatment of most chronic cardiorespiratory conditions complicated by severe hypoxemia, even if proof of efficacy is lacking. New indications emerged, such as isolated nocturnal oxygen desaturation. Even in COPD, inappropriate prescriptions of home oxygen therapy are not unusual. Oxygen is everywhere,” Lacasse continued.
A meta-analysis from 2005 identified two trials that evaluated home oxygen therapy specifically for isolated nocturnal desaturation. Both found no survival benefit from nocturnal oxygen.
The study by Lacasse and colleagues assessed effects on mortality or worsening of disease (progression to LTOT) with 3-4 years of nocturnal oxygen supplementation.
Participants, whose oxygen saturation was less than 90% for at least 30% of the recording time on nocturnal oximetry, received oxygen or ambient air from a sham device as a placebo for at least 4 hours per session. The goal of treatment was nocturnal oxygen saturation exceeding 90% for at least 90% of the recorded time.
The trial protocol excluded patients with severe obesity, apnea, lung cancer, left heart failure, interstitial lung disease, or bronchiectasis.
The study was initially powered in 2010 to include 600 participants, with half to receive placebo. The study assumed mortality of 20% among control patients over 3 years; 20% of patients progressed to LTOT.
When recruiting lagged, the data safety monitoring board and steering committee extended follow-up to 4 years. In 2014, they requested an interim analysis, and recruitment ceased. Overall, 243 patients participated.
Lacasse cited several reasons for the difficulty with recruitment as well as retention: unwillingness to take the risk of receiving placebo instead of a readily available treatment, fading interest over time, and frailty that affects compliance.
Patients in the study came from 28 community or university-affiliated hospitals in Canada, Portugal, Spain, and France. At the 3-year mark, 39% of patients (48 of 123) who were assigned to nocturnal oxygen therapy and 42% (50 of 119) of those taking placebo had met criteria for LTOT or had died (difference, −3.0 percentage points; P = .64). The groups did not differ appreciably in rates of exacerbation and hospitalization.
The researchers could not analyze subgroups because the patients were very similar with regard to the severity of nocturnal oxygen desaturation, Lacasse said.
Economics enters into the picture – home oxygen therapy is second only to hospitalization as the most expensive healthcare expenditure associated with clinical care for COPD in developed countries. “The math is simple. There is enormous potential for saving money if the results of our clinical trial are applied appropriately,” said Lacasse.
William Bailey, MD, professor emeritus of pulmonary, allergy, and critical care medicine at the University of Alabama at Birmingham, agrees that the practice is overused.
“There is a built-in bias in the medical community. Most believe that anyone with lung disease benefits from oxygen. Even some of our investigators had a hard time believing the results. The study was well designed, carefully carried out, and I feel confident that the results are reliable,” he said.
Shawn P. E. Nishi, MD, director of bronchoscopy and advanced pulmonary procedures, division of pulmonary and critical care medicine, the University of Texas Medical Branch, Galveston, Texas, mentioned the study’s main limitation, which the authors readily acknowledge.
“Unfortunately, the trial had difficulty recruiting subjects, with less than half of expected enrollment achieved, and was underpowered to make any conclusions. Other studies have examined nocturnal oxygen use and have not shown a mortality benefit,” Nishi explained.
She added that the study did not evaluate use of LTOT for improving outcomes other than mortality, including quality of life, cardiovascular morbidity, depression, cognitive function, exercise capacity, and frequency of COPD exacerbations or hospitalization.
Other limitations of the study include suboptimal adherence to the therapy and interpretation of the clinical significance on the basis of a survey of Canadian pulmonologists.
This article first appeared on Medscape.com.
Nocturnal oxygen therapy for patients with COPD and isolated nocturnal oxygen desaturation does not improve survival or delay disease progression, according to findings published Sept. 17 in The New England Journal of Medicine. The new report adds to evidence that the widely implemented and costly practice may be unnecessary.
Patients with COPD who do not qualify for long-term oxygen therapy (LTOT) are commonly prescribed nocturnal oxygen in the belief that it can delay disease progression, possibly by decreasing alveolar hypoventilation and ventilation-perfusion mismatch.
But investigations so far and the new study from the International Nocturnal Oxygen (INOX) Trial have not borne this out.
“There is no indication that nocturnal oxygen has a positive or negative effect on survival or progression to long-term oxygen therapy in patients with nocturnal hypoxemia in COPD. Consequently, there is no reason for physicians to screen for nocturnal hypoxemia in COPD,” study leader Yves Lacasse, MD, told Medscape Medical News.
Lacasse is from the Institut Universitaire de Cardiologie et de Pneumologie de Québec–Université Laval, Quebec, Canada.
The idea that the therapy helps is firmly entrenched.
In the early 1980s, two trials indicated that patients who had COPD and severe chronic daytime hypoxemia benefit from LTOT (15-18 hours a day or longer).
A decade later, two landmark trials (the Nocturnal Oxygen Therapy Trial and the British Medical Research Council Trial) added to evidence that LTOT may prolong life for patients with COPD and severe daytime hypoxemia.
“The good news from both trials was that oxygen saves lives. From this moment, oxygen therapy became a standard of care, and confirmatory trials would be considered unethical,” Lacasse explained.
“Oxygen therapy gained widespread acceptance by official organizations for treatment of most chronic cardiorespiratory conditions complicated by severe hypoxemia, even if proof of efficacy is lacking. New indications emerged, such as isolated nocturnal oxygen desaturation. Even in COPD, inappropriate prescriptions of home oxygen therapy are not unusual. Oxygen is everywhere,” Lacasse continued.
A meta-analysis from 2005 identified two trials that evaluated home oxygen therapy specifically for isolated nocturnal desaturation. Both found no survival benefit from nocturnal oxygen.
The study by Lacasse and colleagues assessed effects on mortality or worsening of disease (progression to LTOT) with 3-4 years of nocturnal oxygen supplementation.
Participants, whose oxygen saturation was less than 90% for at least 30% of the recording time on nocturnal oximetry, received oxygen or ambient air from a sham device as a placebo for at least 4 hours per session. The goal of treatment was nocturnal oxygen saturation exceeding 90% for at least 90% of the recorded time.
The trial protocol excluded patients with severe obesity, apnea, lung cancer, left heart failure, interstitial lung disease, or bronchiectasis.
The study was initially powered in 2010 to include 600 participants, with half to receive placebo. The study assumed mortality of 20% among control patients over 3 years; 20% of patients progressed to LTOT.
When recruiting lagged, the data safety monitoring board and steering committee extended follow-up to 4 years. In 2014, they requested an interim analysis, and recruitment ceased. Overall, 243 patients participated.
Lacasse cited several reasons for the difficulty with recruitment as well as retention: unwillingness to take the risk of receiving placebo instead of a readily available treatment, fading interest over time, and frailty that affects compliance.
Patients in the study came from 28 community or university-affiliated hospitals in Canada, Portugal, Spain, and France. At the 3-year mark, 39% of patients (48 of 123) who were assigned to nocturnal oxygen therapy and 42% (50 of 119) of those taking placebo had met criteria for LTOT or had died (difference, −3.0 percentage points; P = .64). The groups did not differ appreciably in rates of exacerbation and hospitalization.
The researchers could not analyze subgroups because the patients were very similar with regard to the severity of nocturnal oxygen desaturation, Lacasse said.
Economics enters into the picture – home oxygen therapy is second only to hospitalization as the most expensive healthcare expenditure associated with clinical care for COPD in developed countries. “The math is simple. There is enormous potential for saving money if the results of our clinical trial are applied appropriately,” said Lacasse.
William Bailey, MD, professor emeritus of pulmonary, allergy, and critical care medicine at the University of Alabama at Birmingham, agrees that the practice is overused.
“There is a built-in bias in the medical community. Most believe that anyone with lung disease benefits from oxygen. Even some of our investigators had a hard time believing the results. The study was well designed, carefully carried out, and I feel confident that the results are reliable,” he said.
Shawn P. E. Nishi, MD, director of bronchoscopy and advanced pulmonary procedures, division of pulmonary and critical care medicine, the University of Texas Medical Branch, Galveston, Texas, mentioned the study’s main limitation, which the authors readily acknowledge.
“Unfortunately, the trial had difficulty recruiting subjects, with less than half of expected enrollment achieved, and was underpowered to make any conclusions. Other studies have examined nocturnal oxygen use and have not shown a mortality benefit,” Nishi explained.
She added that the study did not evaluate use of LTOT for improving outcomes other than mortality, including quality of life, cardiovascular morbidity, depression, cognitive function, exercise capacity, and frequency of COPD exacerbations or hospitalization.
Other limitations of the study include suboptimal adherence to the therapy and interpretation of the clinical significance on the basis of a survey of Canadian pulmonologists.
This article first appeared on Medscape.com.
Distinguishing COVID-19 from flu in kids remains challenging
For children with COVID-19, rates of hospitalization, ICU admission, and ventilator use were similar to those of children with influenza, but rates differed in other respects, according to results of a study published online Sept. 11 in JAMA Network Open.
As winter approaches, distinguishing patients with COVID-19 from those with influenza will become a problem. To assist with that, Xiaoyan Song, PhD, director of the office of infection control and epidemiology at Children’s National Hospital in Washington, D.C., and colleagues investigated commonalities and differences between the clinical symptoms of COVID-19 and influenza in children.
“Distinguishing COVID-19 from flu and other respiratory viral infections remains a challenge to clinicians. Although our study showed that patients with COVID-19 were more likely than patients with flu to report fever, gastrointestinal, and other clinical symptoms at the time of diagnosis, the two groups do have many overlapping clinical symptoms,” Dr. Song said. “Until future data show us otherwise, clinicians need to prepare for managing coinfections of COVID-19 with flu and/or other respiratory viral infections in the upcoming flu season.”
The retrospective cohort study included 315 children diagnosed with laboratory-confirmed COVID-19 between March 25 and May 15, 2020, and 1,402 children diagnosed with laboratory-confirmed seasonal influenza A or influenza B between Oct. 1, 2019, and June 6, 2020, at Children’s National Hospital. The investigation excluded asymptomatic patients who tested positive for COVID-19.
Patients with COVID-19 and patients with influenza were similar with respect to rates of hospitalization (17% vs. 21%; odds ratio, 0.8; 95% confidence interval, 0.6-1.1; P = .15), admission to the ICU (6% vs. 7%; OR, 0.8; 95% CI, 0.5-1.3; P = .42), and use of mechanical ventilation (3% vs. 2%; OR, 1.5; 95% CI, 0.9-2.6; P =.17).
The difference in the duration of ventilation for the two groups was not statistically significant. None of the patients who had COVID-19 or influenza B died, but two patients with influenza A did.
No patients had coinfections, which the researchers attribute to the mid-March shutdown of many schools, which they believe limited the spread of seasonal influenza.
Patients who were hospitalized with COVID-19 were older (median age, 9.7 years; range, 0.06-23.2 years) than those hospitalized with either type of influenza (median age, 4.2 years; range, 0.04-23.1). Patients older than 15 years made up 37% of patients with COVID-19 but only 6% of those with influenza.
Among patients hospitalized with COVID-19, 65% had at least one underlying medical condition, compared with 42% of those hospitalized for either type of influenza (OR, 2.6; 95% CI, 1.4-4.7; P = .002).
The most common underlying condition was neurologic problems from global developmental delay or seizures, identified in 11 patients (20%) hospitalized with COVID-19 and in 24 patients (8%) hospitalized with influenza (OR, 2.8; 95% CI, 1.3-6.2; P = .002). There was no significant difference between the two groups with respect to a history of asthma, cardiac disease, hematologic disease, and cancer.
For both groups, fever and cough were the most frequently reported symptoms at the time of diagnosis. However, more patients hospitalized with COVID-19 reported fever (76% vs. 55%; OR, 2.6; 95% CI, 1.4-5.1; P = 01), diarrhea or vomiting (26% vs. 12%; OR, 2.5; 95% CI, 1.2-5.0; P = .01), headache (11% vs. 3%; OR, 3.9; 95% CI, 1.3-11.5; P = .01), myalgia (22% vs. 7%; OR, 3.9; 95% CI, 1.8-8.5; P = .001), or chest pain (11% vs. 3%; OR, 3.9; 95% CI, 1.3-11.5; P = .01).
The researchers found no statistically significant differences between the two groups in rates of cough, congestion, sore throat, or shortness of breath.
Comparison of the symptom spectrum between COVID-19 and flu differed with respect to influenza type. More patients with COVID-19 reported fever, cough, diarrhea and vomiting, and myalgia than patients hospitalized with influenza A. But rates of fever, cough, diarrhea or vomiting, headache, or chest pain didn’t differ significantly in patients with COVID-19 and those with influenza B.
Larry K. Kociolek, MD, medical director of infection prevention and control at Ann and Robert H. Lurie Children’s Hospital of Chicago, noted the lower age of patients with flu. “Differentiating the two infections, which is difficult if not impossible based on symptoms alone, may have prognostic implications, depending on the age of the child. Because this study was performed outside peak influenza season, when coinfections would be less likely to occur, we must be vigilant about the potential clinical implications of influenza and SARS-CoV-2 coinfection this fall and winter.”
Clinicians will still have to use a combination of symptoms, examinations, and testing to distinguish the two diseases, said Aimee Sznewajs, MD, medical director of the pediatric hospital medicine department at Children’s Minnesota, Minneapolis. “We will continue to test for influenza and COVID-19 prior to hospitalizations and make decisions about whether to hospitalize based on other clinical factors, such as dehydration, oxygen requirement, and vital sign changes.”
Dr. Sznewajs stressed the importance of maintaining public health strategies, including “ensuring all children get the flu vaccine, encouraging mask wearing and hand hygiene, adequate testing to determine which virus is present, and other mitigation measures if the prevalence of COVID-19 is increasing in the community.”
Dr. Song reiterated those points, noting that clinicians need to make the most of the options they have. “Clinicians already have many great tools on hand. It is extremely important to get the flu vaccine now, especially for kids with underlying medical conditions. Diagnostic tests are available for both COVID-19 and flu. Antiviral treatment for flu is available. Judicious use of these tools will protect the health of providers, kids, and well-being at large.”
The authors noted several limitations for the study, including its retrospective design, that the data came from a single center, and that different platforms were used to detect the viruses.
A version of this article originally appeared on Medscape.com.
For children with COVID-19, rates of hospitalization, ICU admission, and ventilator use were similar to those of children with influenza, but rates differed in other respects, according to results of a study published online Sept. 11 in JAMA Network Open.
As winter approaches, distinguishing patients with COVID-19 from those with influenza will become a problem. To assist with that, Xiaoyan Song, PhD, director of the office of infection control and epidemiology at Children’s National Hospital in Washington, D.C., and colleagues investigated commonalities and differences between the clinical symptoms of COVID-19 and influenza in children.
“Distinguishing COVID-19 from flu and other respiratory viral infections remains a challenge to clinicians. Although our study showed that patients with COVID-19 were more likely than patients with flu to report fever, gastrointestinal, and other clinical symptoms at the time of diagnosis, the two groups do have many overlapping clinical symptoms,” Dr. Song said. “Until future data show us otherwise, clinicians need to prepare for managing coinfections of COVID-19 with flu and/or other respiratory viral infections in the upcoming flu season.”
The retrospective cohort study included 315 children diagnosed with laboratory-confirmed COVID-19 between March 25 and May 15, 2020, and 1,402 children diagnosed with laboratory-confirmed seasonal influenza A or influenza B between Oct. 1, 2019, and June 6, 2020, at Children’s National Hospital. The investigation excluded asymptomatic patients who tested positive for COVID-19.
Patients with COVID-19 and patients with influenza were similar with respect to rates of hospitalization (17% vs. 21%; odds ratio, 0.8; 95% confidence interval, 0.6-1.1; P = .15), admission to the ICU (6% vs. 7%; OR, 0.8; 95% CI, 0.5-1.3; P = .42), and use of mechanical ventilation (3% vs. 2%; OR, 1.5; 95% CI, 0.9-2.6; P =.17).
The difference in the duration of ventilation for the two groups was not statistically significant. None of the patients who had COVID-19 or influenza B died, but two patients with influenza A did.
No patients had coinfections, which the researchers attribute to the mid-March shutdown of many schools, which they believe limited the spread of seasonal influenza.
Patients who were hospitalized with COVID-19 were older (median age, 9.7 years; range, 0.06-23.2 years) than those hospitalized with either type of influenza (median age, 4.2 years; range, 0.04-23.1). Patients older than 15 years made up 37% of patients with COVID-19 but only 6% of those with influenza.
Among patients hospitalized with COVID-19, 65% had at least one underlying medical condition, compared with 42% of those hospitalized for either type of influenza (OR, 2.6; 95% CI, 1.4-4.7; P = .002).
The most common underlying condition was neurologic problems from global developmental delay or seizures, identified in 11 patients (20%) hospitalized with COVID-19 and in 24 patients (8%) hospitalized with influenza (OR, 2.8; 95% CI, 1.3-6.2; P = .002). There was no significant difference between the two groups with respect to a history of asthma, cardiac disease, hematologic disease, and cancer.
For both groups, fever and cough were the most frequently reported symptoms at the time of diagnosis. However, more patients hospitalized with COVID-19 reported fever (76% vs. 55%; OR, 2.6; 95% CI, 1.4-5.1; P = 01), diarrhea or vomiting (26% vs. 12%; OR, 2.5; 95% CI, 1.2-5.0; P = .01), headache (11% vs. 3%; OR, 3.9; 95% CI, 1.3-11.5; P = .01), myalgia (22% vs. 7%; OR, 3.9; 95% CI, 1.8-8.5; P = .001), or chest pain (11% vs. 3%; OR, 3.9; 95% CI, 1.3-11.5; P = .01).
The researchers found no statistically significant differences between the two groups in rates of cough, congestion, sore throat, or shortness of breath.
Comparison of the symptom spectrum between COVID-19 and flu differed with respect to influenza type. More patients with COVID-19 reported fever, cough, diarrhea and vomiting, and myalgia than patients hospitalized with influenza A. But rates of fever, cough, diarrhea or vomiting, headache, or chest pain didn’t differ significantly in patients with COVID-19 and those with influenza B.
Larry K. Kociolek, MD, medical director of infection prevention and control at Ann and Robert H. Lurie Children’s Hospital of Chicago, noted the lower age of patients with flu. “Differentiating the two infections, which is difficult if not impossible based on symptoms alone, may have prognostic implications, depending on the age of the child. Because this study was performed outside peak influenza season, when coinfections would be less likely to occur, we must be vigilant about the potential clinical implications of influenza and SARS-CoV-2 coinfection this fall and winter.”
Clinicians will still have to use a combination of symptoms, examinations, and testing to distinguish the two diseases, said Aimee Sznewajs, MD, medical director of the pediatric hospital medicine department at Children’s Minnesota, Minneapolis. “We will continue to test for influenza and COVID-19 prior to hospitalizations and make decisions about whether to hospitalize based on other clinical factors, such as dehydration, oxygen requirement, and vital sign changes.”
Dr. Sznewajs stressed the importance of maintaining public health strategies, including “ensuring all children get the flu vaccine, encouraging mask wearing and hand hygiene, adequate testing to determine which virus is present, and other mitigation measures if the prevalence of COVID-19 is increasing in the community.”
Dr. Song reiterated those points, noting that clinicians need to make the most of the options they have. “Clinicians already have many great tools on hand. It is extremely important to get the flu vaccine now, especially for kids with underlying medical conditions. Diagnostic tests are available for both COVID-19 and flu. Antiviral treatment for flu is available. Judicious use of these tools will protect the health of providers, kids, and well-being at large.”
The authors noted several limitations for the study, including its retrospective design, that the data came from a single center, and that different platforms were used to detect the viruses.
A version of this article originally appeared on Medscape.com.
For children with COVID-19, rates of hospitalization, ICU admission, and ventilator use were similar to those of children with influenza, but rates differed in other respects, according to results of a study published online Sept. 11 in JAMA Network Open.
As winter approaches, distinguishing patients with COVID-19 from those with influenza will become a problem. To assist with that, Xiaoyan Song, PhD, director of the office of infection control and epidemiology at Children’s National Hospital in Washington, D.C., and colleagues investigated commonalities and differences between the clinical symptoms of COVID-19 and influenza in children.
“Distinguishing COVID-19 from flu and other respiratory viral infections remains a challenge to clinicians. Although our study showed that patients with COVID-19 were more likely than patients with flu to report fever, gastrointestinal, and other clinical symptoms at the time of diagnosis, the two groups do have many overlapping clinical symptoms,” Dr. Song said. “Until future data show us otherwise, clinicians need to prepare for managing coinfections of COVID-19 with flu and/or other respiratory viral infections in the upcoming flu season.”
The retrospective cohort study included 315 children diagnosed with laboratory-confirmed COVID-19 between March 25 and May 15, 2020, and 1,402 children diagnosed with laboratory-confirmed seasonal influenza A or influenza B between Oct. 1, 2019, and June 6, 2020, at Children’s National Hospital. The investigation excluded asymptomatic patients who tested positive for COVID-19.
Patients with COVID-19 and patients with influenza were similar with respect to rates of hospitalization (17% vs. 21%; odds ratio, 0.8; 95% confidence interval, 0.6-1.1; P = .15), admission to the ICU (6% vs. 7%; OR, 0.8; 95% CI, 0.5-1.3; P = .42), and use of mechanical ventilation (3% vs. 2%; OR, 1.5; 95% CI, 0.9-2.6; P =.17).
The difference in the duration of ventilation for the two groups was not statistically significant. None of the patients who had COVID-19 or influenza B died, but two patients with influenza A did.
No patients had coinfections, which the researchers attribute to the mid-March shutdown of many schools, which they believe limited the spread of seasonal influenza.
Patients who were hospitalized with COVID-19 were older (median age, 9.7 years; range, 0.06-23.2 years) than those hospitalized with either type of influenza (median age, 4.2 years; range, 0.04-23.1). Patients older than 15 years made up 37% of patients with COVID-19 but only 6% of those with influenza.
Among patients hospitalized with COVID-19, 65% had at least one underlying medical condition, compared with 42% of those hospitalized for either type of influenza (OR, 2.6; 95% CI, 1.4-4.7; P = .002).
The most common underlying condition was neurologic problems from global developmental delay or seizures, identified in 11 patients (20%) hospitalized with COVID-19 and in 24 patients (8%) hospitalized with influenza (OR, 2.8; 95% CI, 1.3-6.2; P = .002). There was no significant difference between the two groups with respect to a history of asthma, cardiac disease, hematologic disease, and cancer.
For both groups, fever and cough were the most frequently reported symptoms at the time of diagnosis. However, more patients hospitalized with COVID-19 reported fever (76% vs. 55%; OR, 2.6; 95% CI, 1.4-5.1; P = 01), diarrhea or vomiting (26% vs. 12%; OR, 2.5; 95% CI, 1.2-5.0; P = .01), headache (11% vs. 3%; OR, 3.9; 95% CI, 1.3-11.5; P = .01), myalgia (22% vs. 7%; OR, 3.9; 95% CI, 1.8-8.5; P = .001), or chest pain (11% vs. 3%; OR, 3.9; 95% CI, 1.3-11.5; P = .01).
The researchers found no statistically significant differences between the two groups in rates of cough, congestion, sore throat, or shortness of breath.
Comparison of the symptom spectrum between COVID-19 and flu differed with respect to influenza type. More patients with COVID-19 reported fever, cough, diarrhea and vomiting, and myalgia than patients hospitalized with influenza A. But rates of fever, cough, diarrhea or vomiting, headache, or chest pain didn’t differ significantly in patients with COVID-19 and those with influenza B.
Larry K. Kociolek, MD, medical director of infection prevention and control at Ann and Robert H. Lurie Children’s Hospital of Chicago, noted the lower age of patients with flu. “Differentiating the two infections, which is difficult if not impossible based on symptoms alone, may have prognostic implications, depending on the age of the child. Because this study was performed outside peak influenza season, when coinfections would be less likely to occur, we must be vigilant about the potential clinical implications of influenza and SARS-CoV-2 coinfection this fall and winter.”
Clinicians will still have to use a combination of symptoms, examinations, and testing to distinguish the two diseases, said Aimee Sznewajs, MD, medical director of the pediatric hospital medicine department at Children’s Minnesota, Minneapolis. “We will continue to test for influenza and COVID-19 prior to hospitalizations and make decisions about whether to hospitalize based on other clinical factors, such as dehydration, oxygen requirement, and vital sign changes.”
Dr. Sznewajs stressed the importance of maintaining public health strategies, including “ensuring all children get the flu vaccine, encouraging mask wearing and hand hygiene, adequate testing to determine which virus is present, and other mitigation measures if the prevalence of COVID-19 is increasing in the community.”
Dr. Song reiterated those points, noting that clinicians need to make the most of the options they have. “Clinicians already have many great tools on hand. It is extremely important to get the flu vaccine now, especially for kids with underlying medical conditions. Diagnostic tests are available for both COVID-19 and flu. Antiviral treatment for flu is available. Judicious use of these tools will protect the health of providers, kids, and well-being at large.”
The authors noted several limitations for the study, including its retrospective design, that the data came from a single center, and that different platforms were used to detect the viruses.
A version of this article originally appeared on Medscape.com.
Internists still low for earnings and net worth, report finds
according to the Medscape Internist Debt and Net Worth Report 2020.
The results are from a larger survey that tracked physicians’ efforts to reduce or eliminate debt, save, invest, purchase property, and prepare for retirement. The annual survey was completed just as the COVID-19 pandemic was taking hold, and the findings provide a baseline against which to view the effects of the pandemic, which turned the lives of many physicians upside down.
Conducted from Oct. 4, 2019, until Feb. 10, 2020, the survey represents 17,461 physicians from 29 areas of medicine, including family practice, internal medicine, pediatrics, and obstetrics and gynecology.
Earnings and net worth
Internists earned on average $251,000 annually, sixth from the bottom of the list and approximately half the amount of the most lucrative specialty, orthopedics. The annual salary increased from $243,000 in 2019.
The average annual salary for internists represents a 2.5% increase, compared with the 1.5% increase for specialists, whose annual income rose from $341,000 in 2019 to $346,000 in 2020.
The low earnings for internists are echoed in the findings for net worth: 58% of internists have a net worth of less than $1 million, 37% have between $1 million and $5 million, and 5% have more than $5 million. For comparison, half of all physicians have a net worth of less than $1 million, 42% have between $1 million and $5 million, and 8% have over $5 million.
About 40% of internists indicated that their net worth was less than $500,000, which is the fourth highest of the 29 medical fields surveyed. About 44% of pediatricians, 46% of family practitioners, and 30% of ob.gyns. also reported their net worth at less than $500,000, so the primary care providers share low net worth compared with their colleagues. About 41% of neurologists reported a net worth of less than $500,000.
Only 5% of internists reported a net worth of more than $5 million. The specialties with the most physicians with net worth exceeding $5 million are orthopedists, at 19%, and plastic surgeons and gastroenterologists, each at 16%.
Gender disparity in net worth appears to be lower among internists than in other fields. Among all physicians, 56% of men and 39% of women reported a net worth in excess of $1 million, but among internists, 46% of men and 36% of women did so. About 64% of the internists who took the survey are men, and 34% are women.
Higher net worth tracks clearly with age group, as expected in light of diminishing debt over time and an accumulation of wealth.
Expenses
The top three expenses that internists face are mortgage on primary residence (60%), car loans (36%), and credit card debt (26%); 12% of respondents reported no debt or expenses. Among all physicians, the breakdown of expenses by category is very similar to that for internists.
Paying off school loans affects 24% of internists, which was in the middle of the 29 physician groups. The percentage ranges from physical medicine and rehabilitation at 34% to rheumatology at 15%.
About 42% of internists have a mortgage of less than $300,000, and 30% have no mortgage at all. Figures are similar for all physicians.
Internists are apparently savers and not spenders. Only 8% reported living above their means; 39% indicated that they live below their means. These figures are similar for all physicians who responded to the survey.
About 60% of internists put more than $1,000 a month into tax-deferred accounts. Most internists also contribute to taxable savings accounts, which might reflect the fact that they had contributed the maximum amount to tax-deferred accounts.
Two-fifths of the internists reported having worked with a financial planner. Of the nearly three fourths of responding internists who share finances with a spouse or partner, a few more than half pool resources.
In the world before COVID-19, 31% of internists reported significant financial losses over the previous year, most because of bad investments or problems relating to their practice. Financial losses since that time obviously have another predominant cause – the direct and ripple effects of the pandemic.
As of July 22, primary care providers reported a 55% decrease in revenue and a 20%-30% decrease in patient volume, according to Travis Singleton, senior vice president of Merritt Hawkins, a physician placement and recruiting company. Some practitioners have closed their physical offices because patient demand has plummeted and nonessential office procedures and exams have been postponed or canceled. The use of telemedicine has soared.
Medscape’s Internist Debt and Net Worth Report 2020, and the larger report from which it was derived, may come to serve as a marker between two very different financial worlds for clinical medicine.
A version of this article originally appeared on Medscape.com.
according to the Medscape Internist Debt and Net Worth Report 2020.
The results are from a larger survey that tracked physicians’ efforts to reduce or eliminate debt, save, invest, purchase property, and prepare for retirement. The annual survey was completed just as the COVID-19 pandemic was taking hold, and the findings provide a baseline against which to view the effects of the pandemic, which turned the lives of many physicians upside down.
Conducted from Oct. 4, 2019, until Feb. 10, 2020, the survey represents 17,461 physicians from 29 areas of medicine, including family practice, internal medicine, pediatrics, and obstetrics and gynecology.
Earnings and net worth
Internists earned on average $251,000 annually, sixth from the bottom of the list and approximately half the amount of the most lucrative specialty, orthopedics. The annual salary increased from $243,000 in 2019.
The average annual salary for internists represents a 2.5% increase, compared with the 1.5% increase for specialists, whose annual income rose from $341,000 in 2019 to $346,000 in 2020.
The low earnings for internists are echoed in the findings for net worth: 58% of internists have a net worth of less than $1 million, 37% have between $1 million and $5 million, and 5% have more than $5 million. For comparison, half of all physicians have a net worth of less than $1 million, 42% have between $1 million and $5 million, and 8% have over $5 million.
About 40% of internists indicated that their net worth was less than $500,000, which is the fourth highest of the 29 medical fields surveyed. About 44% of pediatricians, 46% of family practitioners, and 30% of ob.gyns. also reported their net worth at less than $500,000, so the primary care providers share low net worth compared with their colleagues. About 41% of neurologists reported a net worth of less than $500,000.
Only 5% of internists reported a net worth of more than $5 million. The specialties with the most physicians with net worth exceeding $5 million are orthopedists, at 19%, and plastic surgeons and gastroenterologists, each at 16%.
Gender disparity in net worth appears to be lower among internists than in other fields. Among all physicians, 56% of men and 39% of women reported a net worth in excess of $1 million, but among internists, 46% of men and 36% of women did so. About 64% of the internists who took the survey are men, and 34% are women.
Higher net worth tracks clearly with age group, as expected in light of diminishing debt over time and an accumulation of wealth.
Expenses
The top three expenses that internists face are mortgage on primary residence (60%), car loans (36%), and credit card debt (26%); 12% of respondents reported no debt or expenses. Among all physicians, the breakdown of expenses by category is very similar to that for internists.
Paying off school loans affects 24% of internists, which was in the middle of the 29 physician groups. The percentage ranges from physical medicine and rehabilitation at 34% to rheumatology at 15%.
About 42% of internists have a mortgage of less than $300,000, and 30% have no mortgage at all. Figures are similar for all physicians.
Internists are apparently savers and not spenders. Only 8% reported living above their means; 39% indicated that they live below their means. These figures are similar for all physicians who responded to the survey.
About 60% of internists put more than $1,000 a month into tax-deferred accounts. Most internists also contribute to taxable savings accounts, which might reflect the fact that they had contributed the maximum amount to tax-deferred accounts.
Two-fifths of the internists reported having worked with a financial planner. Of the nearly three fourths of responding internists who share finances with a spouse or partner, a few more than half pool resources.
In the world before COVID-19, 31% of internists reported significant financial losses over the previous year, most because of bad investments or problems relating to their practice. Financial losses since that time obviously have another predominant cause – the direct and ripple effects of the pandemic.
As of July 22, primary care providers reported a 55% decrease in revenue and a 20%-30% decrease in patient volume, according to Travis Singleton, senior vice president of Merritt Hawkins, a physician placement and recruiting company. Some practitioners have closed their physical offices because patient demand has plummeted and nonessential office procedures and exams have been postponed or canceled. The use of telemedicine has soared.
Medscape’s Internist Debt and Net Worth Report 2020, and the larger report from which it was derived, may come to serve as a marker between two very different financial worlds for clinical medicine.
A version of this article originally appeared on Medscape.com.
according to the Medscape Internist Debt and Net Worth Report 2020.
The results are from a larger survey that tracked physicians’ efforts to reduce or eliminate debt, save, invest, purchase property, and prepare for retirement. The annual survey was completed just as the COVID-19 pandemic was taking hold, and the findings provide a baseline against which to view the effects of the pandemic, which turned the lives of many physicians upside down.
Conducted from Oct. 4, 2019, until Feb. 10, 2020, the survey represents 17,461 physicians from 29 areas of medicine, including family practice, internal medicine, pediatrics, and obstetrics and gynecology.
Earnings and net worth
Internists earned on average $251,000 annually, sixth from the bottom of the list and approximately half the amount of the most lucrative specialty, orthopedics. The annual salary increased from $243,000 in 2019.
The average annual salary for internists represents a 2.5% increase, compared with the 1.5% increase for specialists, whose annual income rose from $341,000 in 2019 to $346,000 in 2020.
The low earnings for internists are echoed in the findings for net worth: 58% of internists have a net worth of less than $1 million, 37% have between $1 million and $5 million, and 5% have more than $5 million. For comparison, half of all physicians have a net worth of less than $1 million, 42% have between $1 million and $5 million, and 8% have over $5 million.
About 40% of internists indicated that their net worth was less than $500,000, which is the fourth highest of the 29 medical fields surveyed. About 44% of pediatricians, 46% of family practitioners, and 30% of ob.gyns. also reported their net worth at less than $500,000, so the primary care providers share low net worth compared with their colleagues. About 41% of neurologists reported a net worth of less than $500,000.
Only 5% of internists reported a net worth of more than $5 million. The specialties with the most physicians with net worth exceeding $5 million are orthopedists, at 19%, and plastic surgeons and gastroenterologists, each at 16%.
Gender disparity in net worth appears to be lower among internists than in other fields. Among all physicians, 56% of men and 39% of women reported a net worth in excess of $1 million, but among internists, 46% of men and 36% of women did so. About 64% of the internists who took the survey are men, and 34% are women.
Higher net worth tracks clearly with age group, as expected in light of diminishing debt over time and an accumulation of wealth.
Expenses
The top three expenses that internists face are mortgage on primary residence (60%), car loans (36%), and credit card debt (26%); 12% of respondents reported no debt or expenses. Among all physicians, the breakdown of expenses by category is very similar to that for internists.
Paying off school loans affects 24% of internists, which was in the middle of the 29 physician groups. The percentage ranges from physical medicine and rehabilitation at 34% to rheumatology at 15%.
About 42% of internists have a mortgage of less than $300,000, and 30% have no mortgage at all. Figures are similar for all physicians.
Internists are apparently savers and not spenders. Only 8% reported living above their means; 39% indicated that they live below their means. These figures are similar for all physicians who responded to the survey.
About 60% of internists put more than $1,000 a month into tax-deferred accounts. Most internists also contribute to taxable savings accounts, which might reflect the fact that they had contributed the maximum amount to tax-deferred accounts.
Two-fifths of the internists reported having worked with a financial planner. Of the nearly three fourths of responding internists who share finances with a spouse or partner, a few more than half pool resources.
In the world before COVID-19, 31% of internists reported significant financial losses over the previous year, most because of bad investments or problems relating to their practice. Financial losses since that time obviously have another predominant cause – the direct and ripple effects of the pandemic.
As of July 22, primary care providers reported a 55% decrease in revenue and a 20%-30% decrease in patient volume, according to Travis Singleton, senior vice president of Merritt Hawkins, a physician placement and recruiting company. Some practitioners have closed their physical offices because patient demand has plummeted and nonessential office procedures and exams have been postponed or canceled. The use of telemedicine has soared.
Medscape’s Internist Debt and Net Worth Report 2020, and the larger report from which it was derived, may come to serve as a marker between two very different financial worlds for clinical medicine.
A version of this article originally appeared on Medscape.com.
Large study of COVID-19 N.Y.C. hospital cases shows high mortality
according to a report published in JAMA (2020 Apr 22. doi: 10.1001/jama.2020.6775).
The study, which represents the largest cohort of hospitalized patients with COVID-19 in the United States thus far, confirmed that the highest-risk groups are older, male, and those with preexisting hypertension, diabetes, or obesity.
Mortality rates are difficult to compare between studies, emphasizes corresponding author Karina W. Davidson, PhD. Health care systems and resources can affect outcomes as well as patient demographics and the prevalence of comorbidities. In addition, “the speed with which people present with symptoms and where they are in the course of disease” differ between patient series, said Dr. Davidson, professor and senior vice president at the Feinstein Institutes for Medical Research and senior vice president of research, Northwell Health, Manhasset, N.Y.
“But given all of those, we know that our study represents a fairly large sample of consecutive patients. This is what the mortality rate looks like among those requiring hospitalization at the early stage of the pandemic,” Dr. Davidson said.
The large patient sample reflects the diversity of the city and its environs. “It’s a large representative sample of very diverse patients ranging in age from zero (under a year) to 107, from all walks of life and socioeconomic levels,” Dr. Davidson continued. Eight of the 12 participating N.Y.C.–area hospitals are on Long Island, one each in Manhattan and Staten Island, and two in Queens.
For the study, first author Safiya Richardson, MD, MPH, and colleagues in the Northwell COVID-19 Research Consortium analyzed EHRs of 5,700 patients hospitalized with confirmed COVID-19 during March 1, 2020–April 4, 2020.
Overall, 1,151 (20.2%) of the 5,700 patients required mechanical ventilation. As of April 4, 831 (72.2%) of these patients remained in the hospital, 38 (3.3%) were discharged, and 282 (24.5%) had died.
When the authors restricted their analysis to the 2,634 patients whose outcomes (discharge or death) were known at the end of the study, 373 (14.2%) had been treated in the intensive care unit, 320 (12.2%) received invasive mechanical ventilation, 81 (3.2%) received dialysis, and 553 (21%) died.
As seen in other COVID-19 studies, increasing age was associated with a higher risk of death. Of patients receiving mechanical ventilation and whose outcomes (discharge or death) were known, 88.1% died. When stratified by age, the mortality rates for ventilated patients were 76.4% for those aged 18-65 years and 97.2% for those older than 65 years.
Among those who did not require mechanical ventilation and whose outcomes (discharge or death) were known, 19.8% of patients aged 18-65 years died, as did 26.6% of those older than 65 years. No patient under 18 years died during the study period.
“There can be risks with mechanical ventilation, like the development of ventilator-associated pneumonia (VAP), which occurs in 10%-25% of ventilated patients and tends to occur within 5 days. The authors didn’t report data on VAP, but it seems that the mortality for ventilated patients would most likely be attributable to disease severity rather than the ventilation itself,” said Cindy Prins, PhD, director of the Master of Public Health program and clinical associate professor of epidemiology at the University of Florida, Gainesville.
The median follow-up time after discharge was 4.4 days. During the study period, 45 (2.2%) patients were readmitted, with median time to readmission of 3 days.
The most common comorbidities among all 5,700 patients were hypertension (57%), obesity (41%), and diabetes (34%). As has been seen in other patient series, male sex and increasing age were associated with a higher risk for death.
The most surprising finding, Dr. Davidson said, was that fever was uncommon. “Of 5,700 patients requiring admission because of respiratory distress, only a third had fever. So fever should not be a single symptom upon which people make a decision to seek help.”
Dr. Prins was intrigued by the observation that 2% of the patients tested positive for a respiratory virus panel as well as for COVID-19. “Because of a shortage of COVID-19 testing supplies, some hospitals have been running respiratory panels before testing for COVID-19. But this study provides more evidence that a positive result on a respiratory panel does not rule out COVID-19 infection.”
The clinical situation is constantly in flux. “We’ve been seeing since March 8 that the severity of patients has lessened dramatically, and they are coming in later in the disease. Many things are changing, we hope for the better,” Dr. Davidson said.
This article was first published on Medscape.com.
according to a report published in JAMA (2020 Apr 22. doi: 10.1001/jama.2020.6775).
The study, which represents the largest cohort of hospitalized patients with COVID-19 in the United States thus far, confirmed that the highest-risk groups are older, male, and those with preexisting hypertension, diabetes, or obesity.
Mortality rates are difficult to compare between studies, emphasizes corresponding author Karina W. Davidson, PhD. Health care systems and resources can affect outcomes as well as patient demographics and the prevalence of comorbidities. In addition, “the speed with which people present with symptoms and where they are in the course of disease” differ between patient series, said Dr. Davidson, professor and senior vice president at the Feinstein Institutes for Medical Research and senior vice president of research, Northwell Health, Manhasset, N.Y.
“But given all of those, we know that our study represents a fairly large sample of consecutive patients. This is what the mortality rate looks like among those requiring hospitalization at the early stage of the pandemic,” Dr. Davidson said.
The large patient sample reflects the diversity of the city and its environs. “It’s a large representative sample of very diverse patients ranging in age from zero (under a year) to 107, from all walks of life and socioeconomic levels,” Dr. Davidson continued. Eight of the 12 participating N.Y.C.–area hospitals are on Long Island, one each in Manhattan and Staten Island, and two in Queens.
For the study, first author Safiya Richardson, MD, MPH, and colleagues in the Northwell COVID-19 Research Consortium analyzed EHRs of 5,700 patients hospitalized with confirmed COVID-19 during March 1, 2020–April 4, 2020.
Overall, 1,151 (20.2%) of the 5,700 patients required mechanical ventilation. As of April 4, 831 (72.2%) of these patients remained in the hospital, 38 (3.3%) were discharged, and 282 (24.5%) had died.
When the authors restricted their analysis to the 2,634 patients whose outcomes (discharge or death) were known at the end of the study, 373 (14.2%) had been treated in the intensive care unit, 320 (12.2%) received invasive mechanical ventilation, 81 (3.2%) received dialysis, and 553 (21%) died.
As seen in other COVID-19 studies, increasing age was associated with a higher risk of death. Of patients receiving mechanical ventilation and whose outcomes (discharge or death) were known, 88.1% died. When stratified by age, the mortality rates for ventilated patients were 76.4% for those aged 18-65 years and 97.2% for those older than 65 years.
Among those who did not require mechanical ventilation and whose outcomes (discharge or death) were known, 19.8% of patients aged 18-65 years died, as did 26.6% of those older than 65 years. No patient under 18 years died during the study period.
“There can be risks with mechanical ventilation, like the development of ventilator-associated pneumonia (VAP), which occurs in 10%-25% of ventilated patients and tends to occur within 5 days. The authors didn’t report data on VAP, but it seems that the mortality for ventilated patients would most likely be attributable to disease severity rather than the ventilation itself,” said Cindy Prins, PhD, director of the Master of Public Health program and clinical associate professor of epidemiology at the University of Florida, Gainesville.
The median follow-up time after discharge was 4.4 days. During the study period, 45 (2.2%) patients were readmitted, with median time to readmission of 3 days.
The most common comorbidities among all 5,700 patients were hypertension (57%), obesity (41%), and diabetes (34%). As has been seen in other patient series, male sex and increasing age were associated with a higher risk for death.
The most surprising finding, Dr. Davidson said, was that fever was uncommon. “Of 5,700 patients requiring admission because of respiratory distress, only a third had fever. So fever should not be a single symptom upon which people make a decision to seek help.”
Dr. Prins was intrigued by the observation that 2% of the patients tested positive for a respiratory virus panel as well as for COVID-19. “Because of a shortage of COVID-19 testing supplies, some hospitals have been running respiratory panels before testing for COVID-19. But this study provides more evidence that a positive result on a respiratory panel does not rule out COVID-19 infection.”
The clinical situation is constantly in flux. “We’ve been seeing since March 8 that the severity of patients has lessened dramatically, and they are coming in later in the disease. Many things are changing, we hope for the better,” Dr. Davidson said.
This article was first published on Medscape.com.
according to a report published in JAMA (2020 Apr 22. doi: 10.1001/jama.2020.6775).
The study, which represents the largest cohort of hospitalized patients with COVID-19 in the United States thus far, confirmed that the highest-risk groups are older, male, and those with preexisting hypertension, diabetes, or obesity.
Mortality rates are difficult to compare between studies, emphasizes corresponding author Karina W. Davidson, PhD. Health care systems and resources can affect outcomes as well as patient demographics and the prevalence of comorbidities. In addition, “the speed with which people present with symptoms and where they are in the course of disease” differ between patient series, said Dr. Davidson, professor and senior vice president at the Feinstein Institutes for Medical Research and senior vice president of research, Northwell Health, Manhasset, N.Y.
“But given all of those, we know that our study represents a fairly large sample of consecutive patients. This is what the mortality rate looks like among those requiring hospitalization at the early stage of the pandemic,” Dr. Davidson said.
The large patient sample reflects the diversity of the city and its environs. “It’s a large representative sample of very diverse patients ranging in age from zero (under a year) to 107, from all walks of life and socioeconomic levels,” Dr. Davidson continued. Eight of the 12 participating N.Y.C.–area hospitals are on Long Island, one each in Manhattan and Staten Island, and two in Queens.
For the study, first author Safiya Richardson, MD, MPH, and colleagues in the Northwell COVID-19 Research Consortium analyzed EHRs of 5,700 patients hospitalized with confirmed COVID-19 during March 1, 2020–April 4, 2020.
Overall, 1,151 (20.2%) of the 5,700 patients required mechanical ventilation. As of April 4, 831 (72.2%) of these patients remained in the hospital, 38 (3.3%) were discharged, and 282 (24.5%) had died.
When the authors restricted their analysis to the 2,634 patients whose outcomes (discharge or death) were known at the end of the study, 373 (14.2%) had been treated in the intensive care unit, 320 (12.2%) received invasive mechanical ventilation, 81 (3.2%) received dialysis, and 553 (21%) died.
As seen in other COVID-19 studies, increasing age was associated with a higher risk of death. Of patients receiving mechanical ventilation and whose outcomes (discharge or death) were known, 88.1% died. When stratified by age, the mortality rates for ventilated patients were 76.4% for those aged 18-65 years and 97.2% for those older than 65 years.
Among those who did not require mechanical ventilation and whose outcomes (discharge or death) were known, 19.8% of patients aged 18-65 years died, as did 26.6% of those older than 65 years. No patient under 18 years died during the study period.
“There can be risks with mechanical ventilation, like the development of ventilator-associated pneumonia (VAP), which occurs in 10%-25% of ventilated patients and tends to occur within 5 days. The authors didn’t report data on VAP, but it seems that the mortality for ventilated patients would most likely be attributable to disease severity rather than the ventilation itself,” said Cindy Prins, PhD, director of the Master of Public Health program and clinical associate professor of epidemiology at the University of Florida, Gainesville.
The median follow-up time after discharge was 4.4 days. During the study period, 45 (2.2%) patients were readmitted, with median time to readmission of 3 days.
The most common comorbidities among all 5,700 patients were hypertension (57%), obesity (41%), and diabetes (34%). As has been seen in other patient series, male sex and increasing age were associated with a higher risk for death.
The most surprising finding, Dr. Davidson said, was that fever was uncommon. “Of 5,700 patients requiring admission because of respiratory distress, only a third had fever. So fever should not be a single symptom upon which people make a decision to seek help.”
Dr. Prins was intrigued by the observation that 2% of the patients tested positive for a respiratory virus panel as well as for COVID-19. “Because of a shortage of COVID-19 testing supplies, some hospitals have been running respiratory panels before testing for COVID-19. But this study provides more evidence that a positive result on a respiratory panel does not rule out COVID-19 infection.”
The clinical situation is constantly in flux. “We’ve been seeing since March 8 that the severity of patients has lessened dramatically, and they are coming in later in the disease. Many things are changing, we hope for the better,” Dr. Davidson said.
This article was first published on Medscape.com.
Coronavirus stays in aerosols for hours, on surfaces for days
according to a new study.
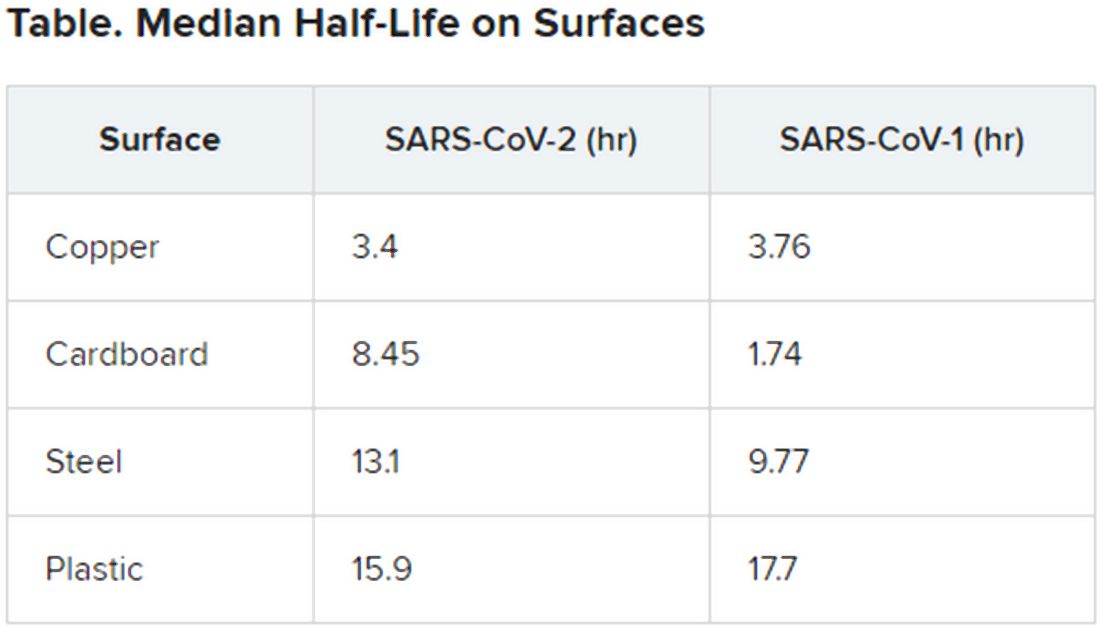
The data indicate that the stability of the new virus is similar to that of SARS-CoV-1, which caused the SARS epidemic, researchers report in an article published on the medRxivpreprint server. (The posted article has been submitted for journal publication but has not been peer reviewed.)
Transmission of SARS-CoV-2, which causes COVID-19, has quickly outstripped the pace of the 2003 SARS epidemic. “Superspread” of the earlier disease arose from infection during medical procedures, in which a single infected individual seeded many secondary cases. In contrast, the novel coronavirus appears to be spread more through human-to-human transmission in a variety of settings.
However, it’s not yet known the extent to which asymptomatic or presymptomatic individuals spread the new virus through daily routine.
To investigate how long SARS-CoV-2 remains infective in the environment, Neeltje van Doremalen, PhD, of the Laboratory of Virology, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, in Hamilton, Montana, and colleagues conducted simulation experiments in which they compared the viability of SARS-CoV-2 with that of SARS-CoV-1 in aerosols and on surfaces.
Among patients infected with SARS-CoV-2, viral loads in the upper respiratory tract are high; as a consequence, respiratory secretion in the form of aerosols (<5 μm) or droplets (>5 mcm) is likely, the authors note.
van Doremalen and colleagues used nebulizers to generate aerosols. Samples of SARS-CoV-1 and SARS-CoV-2 were collecting at 0, 30, 60, 120, and 180 minutes on a gelatin filter. The researchers then tested the infectivity of the viruses on Vero cells grown in culture.
They found that SARS-CoV-2 was largely stable through the full 180-minute test, with only a slight decline at 3 hours. This time course is similar to that of SARS-CoV-1; both viruses have a median half-life in aerosols of 2.7 hours (range, 1.65 hr for SARS-CoV-1, vs 7.24 hr for SARS-CoV-2).
The researchers then tested the viruses on a variety of surfaces for up to 7 days, using humidity values and temperatures designed to mimic “a variety of household and hospital situations.” The volumes of viral exposures that the team used were consistent with amounts found in the human upper and lower respiratory tracts.
For example, they applied 50 mcL of virus-containing solution to a piece of cardboard and then swabbed the surface, at different times, with an additional 1 mcL of medium. Each surface assay was replicated three times.
The novel coronavirus was most stable on plastic and stainless steel, with some virus remaining viable up to 72 hours. However, by that time the viral load had fallen by about three orders of magnitude, indicating exponential decay. This profile was remarkably similar to that of SARS-CoV-1, according to the authors.
However, the two viruses differed in staying power on copper and cardboard. No viable SARS-CoV-2 was detectable on copper after 4 hours or on cardboard after 24 hours. In contrast, SARS-CoV-1 was not viable beyond 8 hours for either copper or cardboard.
“Taken together, our results indicate that aerosol and fomite transmission of HCoV-19 [SARS-CoV-2] are plausible, as the virus can remain viable in aerosols for multiple hours and on surfaces up to days,” the authors conclude.
Andrew Pekosz, PhD, codirector of the Center of Excellence in Influenza Research and Surveillance and director of the Center for Emerging Viruses and Infectious Diseases at the Johns Hopkins Center for Global Health, Baltimore, Maryland, applauds the real-world value of the experiments.
“The PCR [polymerase chain reaction] test used [in other studies] to detect SARS-CoV-2 just detects the virus genome. It doesn’t tell you if the virus was still infectious, or ‘viable.’ That’s why this study is interesting,” Pekosz said. “It focuses on infectious virus, which is the virus that has the potential to transmit and infect another person. What we don’t know yet is how much infectious (viable) virus is needed to initiate infection in another person.”
He suggests that further investigations evaluate other types of environmental surfaces, including lacquered wood that is made into desks and ceramic tiles found in bathrooms and kitchens.
One limitation of the study is that the data for experiments on cardboard were more variable than the data for other surfaces tested.
The investigators and Pekosz have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
according to a new study.

The data indicate that the stability of the new virus is similar to that of SARS-CoV-1, which caused the SARS epidemic, researchers report in an article published on the medRxivpreprint server. (The posted article has been submitted for journal publication but has not been peer reviewed.)
Transmission of SARS-CoV-2, which causes COVID-19, has quickly outstripped the pace of the 2003 SARS epidemic. “Superspread” of the earlier disease arose from infection during medical procedures, in which a single infected individual seeded many secondary cases. In contrast, the novel coronavirus appears to be spread more through human-to-human transmission in a variety of settings.
However, it’s not yet known the extent to which asymptomatic or presymptomatic individuals spread the new virus through daily routine.
To investigate how long SARS-CoV-2 remains infective in the environment, Neeltje van Doremalen, PhD, of the Laboratory of Virology, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, in Hamilton, Montana, and colleagues conducted simulation experiments in which they compared the viability of SARS-CoV-2 with that of SARS-CoV-1 in aerosols and on surfaces.
Among patients infected with SARS-CoV-2, viral loads in the upper respiratory tract are high; as a consequence, respiratory secretion in the form of aerosols (<5 μm) or droplets (>5 mcm) is likely, the authors note.
van Doremalen and colleagues used nebulizers to generate aerosols. Samples of SARS-CoV-1 and SARS-CoV-2 were collecting at 0, 30, 60, 120, and 180 minutes on a gelatin filter. The researchers then tested the infectivity of the viruses on Vero cells grown in culture.
They found that SARS-CoV-2 was largely stable through the full 180-minute test, with only a slight decline at 3 hours. This time course is similar to that of SARS-CoV-1; both viruses have a median half-life in aerosols of 2.7 hours (range, 1.65 hr for SARS-CoV-1, vs 7.24 hr for SARS-CoV-2).
The researchers then tested the viruses on a variety of surfaces for up to 7 days, using humidity values and temperatures designed to mimic “a variety of household and hospital situations.” The volumes of viral exposures that the team used were consistent with amounts found in the human upper and lower respiratory tracts.
For example, they applied 50 mcL of virus-containing solution to a piece of cardboard and then swabbed the surface, at different times, with an additional 1 mcL of medium. Each surface assay was replicated three times.
The novel coronavirus was most stable on plastic and stainless steel, with some virus remaining viable up to 72 hours. However, by that time the viral load had fallen by about three orders of magnitude, indicating exponential decay. This profile was remarkably similar to that of SARS-CoV-1, according to the authors.
However, the two viruses differed in staying power on copper and cardboard. No viable SARS-CoV-2 was detectable on copper after 4 hours or on cardboard after 24 hours. In contrast, SARS-CoV-1 was not viable beyond 8 hours for either copper or cardboard.
“Taken together, our results indicate that aerosol and fomite transmission of HCoV-19 [SARS-CoV-2] are plausible, as the virus can remain viable in aerosols for multiple hours and on surfaces up to days,” the authors conclude.
Andrew Pekosz, PhD, codirector of the Center of Excellence in Influenza Research and Surveillance and director of the Center for Emerging Viruses and Infectious Diseases at the Johns Hopkins Center for Global Health, Baltimore, Maryland, applauds the real-world value of the experiments.
“The PCR [polymerase chain reaction] test used [in other studies] to detect SARS-CoV-2 just detects the virus genome. It doesn’t tell you if the virus was still infectious, or ‘viable.’ That’s why this study is interesting,” Pekosz said. “It focuses on infectious virus, which is the virus that has the potential to transmit and infect another person. What we don’t know yet is how much infectious (viable) virus is needed to initiate infection in another person.”
He suggests that further investigations evaluate other types of environmental surfaces, including lacquered wood that is made into desks and ceramic tiles found in bathrooms and kitchens.
One limitation of the study is that the data for experiments on cardboard were more variable than the data for other surfaces tested.
The investigators and Pekosz have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
according to a new study.

The data indicate that the stability of the new virus is similar to that of SARS-CoV-1, which caused the SARS epidemic, researchers report in an article published on the medRxivpreprint server. (The posted article has been submitted for journal publication but has not been peer reviewed.)
Transmission of SARS-CoV-2, which causes COVID-19, has quickly outstripped the pace of the 2003 SARS epidemic. “Superspread” of the earlier disease arose from infection during medical procedures, in which a single infected individual seeded many secondary cases. In contrast, the novel coronavirus appears to be spread more through human-to-human transmission in a variety of settings.
However, it’s not yet known the extent to which asymptomatic or presymptomatic individuals spread the new virus through daily routine.
To investigate how long SARS-CoV-2 remains infective in the environment, Neeltje van Doremalen, PhD, of the Laboratory of Virology, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, in Hamilton, Montana, and colleagues conducted simulation experiments in which they compared the viability of SARS-CoV-2 with that of SARS-CoV-1 in aerosols and on surfaces.
Among patients infected with SARS-CoV-2, viral loads in the upper respiratory tract are high; as a consequence, respiratory secretion in the form of aerosols (<5 μm) or droplets (>5 mcm) is likely, the authors note.
van Doremalen and colleagues used nebulizers to generate aerosols. Samples of SARS-CoV-1 and SARS-CoV-2 were collecting at 0, 30, 60, 120, and 180 minutes on a gelatin filter. The researchers then tested the infectivity of the viruses on Vero cells grown in culture.
They found that SARS-CoV-2 was largely stable through the full 180-minute test, with only a slight decline at 3 hours. This time course is similar to that of SARS-CoV-1; both viruses have a median half-life in aerosols of 2.7 hours (range, 1.65 hr for SARS-CoV-1, vs 7.24 hr for SARS-CoV-2).
The researchers then tested the viruses on a variety of surfaces for up to 7 days, using humidity values and temperatures designed to mimic “a variety of household and hospital situations.” The volumes of viral exposures that the team used were consistent with amounts found in the human upper and lower respiratory tracts.
For example, they applied 50 mcL of virus-containing solution to a piece of cardboard and then swabbed the surface, at different times, with an additional 1 mcL of medium. Each surface assay was replicated three times.
The novel coronavirus was most stable on plastic and stainless steel, with some virus remaining viable up to 72 hours. However, by that time the viral load had fallen by about three orders of magnitude, indicating exponential decay. This profile was remarkably similar to that of SARS-CoV-1, according to the authors.
However, the two viruses differed in staying power on copper and cardboard. No viable SARS-CoV-2 was detectable on copper after 4 hours or on cardboard after 24 hours. In contrast, SARS-CoV-1 was not viable beyond 8 hours for either copper or cardboard.
“Taken together, our results indicate that aerosol and fomite transmission of HCoV-19 [SARS-CoV-2] are plausible, as the virus can remain viable in aerosols for multiple hours and on surfaces up to days,” the authors conclude.
Andrew Pekosz, PhD, codirector of the Center of Excellence in Influenza Research and Surveillance and director of the Center for Emerging Viruses and Infectious Diseases at the Johns Hopkins Center for Global Health, Baltimore, Maryland, applauds the real-world value of the experiments.
“The PCR [polymerase chain reaction] test used [in other studies] to detect SARS-CoV-2 just detects the virus genome. It doesn’t tell you if the virus was still infectious, or ‘viable.’ That’s why this study is interesting,” Pekosz said. “It focuses on infectious virus, which is the virus that has the potential to transmit and infect another person. What we don’t know yet is how much infectious (viable) virus is needed to initiate infection in another person.”
He suggests that further investigations evaluate other types of environmental surfaces, including lacquered wood that is made into desks and ceramic tiles found in bathrooms and kitchens.
One limitation of the study is that the data for experiments on cardboard were more variable than the data for other surfaces tested.
The investigators and Pekosz have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
So you have a COVID-19 patient: How do you treat them?
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
Clinicians are working out how to manage patients with or suspected of having COVID-19.
“Over the past couple of weeks, we’ve been preparing for the oncoming onslaught of patients,” said Lillian Wu, MD, of the HealthPoint network in the Seattle area of greater King County and president elect of the Washington Academy of Family Physicians.
Step One: Triage
The first step, Wu says, is careful triage.
When patients call one of the 17 clinics in the HealthPoint system, nurses gauge how sick they are. High fever? Shortness of breath? Do they have a chronic illness, such as diabetes, cardiovascular disease, or a lung condition, that increases risk for infection and complications?
“If a patient has mild symptoms, we ask them to stay home or to check back in 24 hours, or we’ll reach out to them. For moderate symptoms, we ask them to come in, and [we] clearly mark on the schedule that it is a respiratory patient, who will be sent to a separate area. If the patient is severe, we don’t even see them and send them directly to the hospital to the ER,” Wu told Medscape Medical News.
These categories parallel the World Health Organization’s designations of uncomplicated illness, mild pneumonia, severe pneumonia, acute respiratory distress syndrome, sepsis, and septic shock. The Centers for Disease Control and Prevention (CDC) advises case by case regarding decisions as to outpatient or inpatient assignment.
“Patients who pass the initial phone triage are given masks, separated, and sent to different parts of the clinic or are required to wait in their cars until it’s time to be seen,” Wu said.
Step 2: Hospital Arrival
Once at the hospital, the CDC’s interim guidance kicks in.
“Any patient with fever, cough, and shortness of breath presenting with a history of travel to countries with high ongoing transmission or a credible history of exposure should be promptly evaluated for COVID-19,” said Raghavendra Tirupathi, MD, medical director, Keystone Infectious Diseases/HIV; chair in infection prevention, Summit Health; and clinical assistant professor of medicine, Penn State School of Medicine, Hershey, Pennsylvania.
“We recommend obtaining baseline CBC with differential, basic metabolic panel, liver function tests, and procalcitonin. Clues for COVID-19 include leukopenia, seen in 30% to 45% of patients, and lymphocytopenia, seen in 85% of the patients in the case series from China,” Tirupathi said. He uses a respiratory virus polymerase chain reaction panel to rule out other pathogens.
Wu concurs. “This is the one time we are grateful when someone tests positive for the flu! If flu is negative and other common respiratory infections are negative, then we do a COVID-19 test,” she said.
But test results may be delayed. “At the University of Washington, it takes 8 hours, but commercial labs take up to 4 days,” Wu said. All patients with respiratory symptoms are treated as persons under investigation, for whom isolation precautions are required. In addition, for these patients, use of personal protective equipment by caregivers is required.
For suspected pneumonia, the American College of Radiography recommends chest CT to identify peripheral basal ground-glass opacities characteristic of COVID-19.
However, diagnosis should be based on detection of SARS-CoV-2, because chest images for COVID-19 are nonspecific – associated signs can also be seen in H1N1 influenza, SARS, and MERS.
Step 3: Supportive Care
Once a patient is admitted, supportive care entails “maintaining fluid status and nutrition and supporting physiological functions until we heal. It’s treating complications and organ support, whether that means providing supplementary oxygen all the way to ventilator support, and just waiting it out. If a patient progresses to acute respiratory distress syndrome, it becomes tougher,” said David Liebers, MD, chief medical officer and an infectious disease specialist at Ellis Medicine in Schenectady, New York.
Efforts are ramping up to develop therapeutics. Remdesivir, an investigational antiviral drug developed to treat Ebola and Marburg hemorrhagic fevers, shows activity against SARS-CoV-2 in vitro.
Remdesivir has been used in a few patients on a compassionate-use basis outside of a clinical trial setting. “It’s a nucleotide analogue, and like other drugs of that class, it disrupts nucleic acid production. Some data suggest that it might have some efficacy,” Liebers said.
Antibiotics are reserved for patients suspected of having concomitant bacterial or fungal infections. Liebers said clinicians should be alerted to “the big three” signs of secondary infection – fever, elevated white blood cell count, and lactic acidosis. Immunosuppressed patients are at elevated risk for secondary infection.
Step 4: Managing Complications
Patients do die of COVID-19, mostly through an inability to ventilate, even when supported with oxygen, Liebers told Medscape Medical News. (According to Tirupathi, “The studies from China indicate that from 6%-10% of patients needed ventilators.”)
Liebers continued, “Others may develop sepsis or a syndrome of multisystem organ failure with renal and endothelial collapse, making it difficult to maintain blood pressure. Like with so many pathologies, it is a vicious circle in which everything gets overworked. Off-and-on treatments can sometimes break the cycle: supplementary oxygen, giving red blood cells, dialysis. We support those functions while waiting for healing to occur.”
A facility’s airborne-infection isolation rooms may become filled to capacity, but that isn’t critical, Liebers said. “Airborne precautions are standard to contain measles, tuberculosis, chickenpox, and herpes zoster, in which very small particles spread in the air,” he said.
Consensus is growing that SARS-CoV-2 spreads in large droplets, he added. Private rooms and closed doors may suffice.
Step 5: Discharge
Liebers said that as of now, the million-dollar question regards criteria for discharge.
Patients who clinically improve are sent home with instructions to remain in isolation. They may be tested again for virus before or after discharge.
Liebers and Wu pointed to the experience at EvergreenHealth Medical Center, in Kirkland, Washington, as guidance from the trenches. “They’re the ones who are learning firsthand and passing the experience along to everyone else,” Wu said.
“The situation is unprecedented,” said Liebers, who, like many others, has barely slept these past weeks. “We’re swimming in murky water right now.”
The epidemic in the United States is still months from peaking, Wu emphasized. “There is no vaccine, and many cases are subclinical. COVID-19 has to spread through the country before it infects a critical mass of people who will develop immunity. It’s too late to contain.”
Added Liebers, “It’s a constantly changing situation, and we are still being surprised – not that this wasn’t predicted.”
This article first appeared on Medscape.com.
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
Clinicians are working out how to manage patients with or suspected of having COVID-19.
“Over the past couple of weeks, we’ve been preparing for the oncoming onslaught of patients,” said Lillian Wu, MD, of the HealthPoint network in the Seattle area of greater King County and president elect of the Washington Academy of Family Physicians.
Step One: Triage
The first step, Wu says, is careful triage.
When patients call one of the 17 clinics in the HealthPoint system, nurses gauge how sick they are. High fever? Shortness of breath? Do they have a chronic illness, such as diabetes, cardiovascular disease, or a lung condition, that increases risk for infection and complications?
“If a patient has mild symptoms, we ask them to stay home or to check back in 24 hours, or we’ll reach out to them. For moderate symptoms, we ask them to come in, and [we] clearly mark on the schedule that it is a respiratory patient, who will be sent to a separate area. If the patient is severe, we don’t even see them and send them directly to the hospital to the ER,” Wu told Medscape Medical News.
These categories parallel the World Health Organization’s designations of uncomplicated illness, mild pneumonia, severe pneumonia, acute respiratory distress syndrome, sepsis, and septic shock. The Centers for Disease Control and Prevention (CDC) advises case by case regarding decisions as to outpatient or inpatient assignment.
“Patients who pass the initial phone triage are given masks, separated, and sent to different parts of the clinic or are required to wait in their cars until it’s time to be seen,” Wu said.
Step 2: Hospital Arrival
Once at the hospital, the CDC’s interim guidance kicks in.
“Any patient with fever, cough, and shortness of breath presenting with a history of travel to countries with high ongoing transmission or a credible history of exposure should be promptly evaluated for COVID-19,” said Raghavendra Tirupathi, MD, medical director, Keystone Infectious Diseases/HIV; chair in infection prevention, Summit Health; and clinical assistant professor of medicine, Penn State School of Medicine, Hershey, Pennsylvania.
“We recommend obtaining baseline CBC with differential, basic metabolic panel, liver function tests, and procalcitonin. Clues for COVID-19 include leukopenia, seen in 30% to 45% of patients, and lymphocytopenia, seen in 85% of the patients in the case series from China,” Tirupathi said. He uses a respiratory virus polymerase chain reaction panel to rule out other pathogens.
Wu concurs. “This is the one time we are grateful when someone tests positive for the flu! If flu is negative and other common respiratory infections are negative, then we do a COVID-19 test,” she said.
But test results may be delayed. “At the University of Washington, it takes 8 hours, but commercial labs take up to 4 days,” Wu said. All patients with respiratory symptoms are treated as persons under investigation, for whom isolation precautions are required. In addition, for these patients, use of personal protective equipment by caregivers is required.
For suspected pneumonia, the American College of Radiography recommends chest CT to identify peripheral basal ground-glass opacities characteristic of COVID-19.
However, diagnosis should be based on detection of SARS-CoV-2, because chest images for COVID-19 are nonspecific – associated signs can also be seen in H1N1 influenza, SARS, and MERS.
Step 3: Supportive Care
Once a patient is admitted, supportive care entails “maintaining fluid status and nutrition and supporting physiological functions until we heal. It’s treating complications and organ support, whether that means providing supplementary oxygen all the way to ventilator support, and just waiting it out. If a patient progresses to acute respiratory distress syndrome, it becomes tougher,” said David Liebers, MD, chief medical officer and an infectious disease specialist at Ellis Medicine in Schenectady, New York.
Efforts are ramping up to develop therapeutics. Remdesivir, an investigational antiviral drug developed to treat Ebola and Marburg hemorrhagic fevers, shows activity against SARS-CoV-2 in vitro.
Remdesivir has been used in a few patients on a compassionate-use basis outside of a clinical trial setting. “It’s a nucleotide analogue, and like other drugs of that class, it disrupts nucleic acid production. Some data suggest that it might have some efficacy,” Liebers said.
Antibiotics are reserved for patients suspected of having concomitant bacterial or fungal infections. Liebers said clinicians should be alerted to “the big three” signs of secondary infection – fever, elevated white blood cell count, and lactic acidosis. Immunosuppressed patients are at elevated risk for secondary infection.
Step 4: Managing Complications
Patients do die of COVID-19, mostly through an inability to ventilate, even when supported with oxygen, Liebers told Medscape Medical News. (According to Tirupathi, “The studies from China indicate that from 6%-10% of patients needed ventilators.”)
Liebers continued, “Others may develop sepsis or a syndrome of multisystem organ failure with renal and endothelial collapse, making it difficult to maintain blood pressure. Like with so many pathologies, it is a vicious circle in which everything gets overworked. Off-and-on treatments can sometimes break the cycle: supplementary oxygen, giving red blood cells, dialysis. We support those functions while waiting for healing to occur.”
A facility’s airborne-infection isolation rooms may become filled to capacity, but that isn’t critical, Liebers said. “Airborne precautions are standard to contain measles, tuberculosis, chickenpox, and herpes zoster, in which very small particles spread in the air,” he said.
Consensus is growing that SARS-CoV-2 spreads in large droplets, he added. Private rooms and closed doors may suffice.
Step 5: Discharge
Liebers said that as of now, the million-dollar question regards criteria for discharge.
Patients who clinically improve are sent home with instructions to remain in isolation. They may be tested again for virus before or after discharge.
Liebers and Wu pointed to the experience at EvergreenHealth Medical Center, in Kirkland, Washington, as guidance from the trenches. “They’re the ones who are learning firsthand and passing the experience along to everyone else,” Wu said.
“The situation is unprecedented,” said Liebers, who, like many others, has barely slept these past weeks. “We’re swimming in murky water right now.”
The epidemic in the United States is still months from peaking, Wu emphasized. “There is no vaccine, and many cases are subclinical. COVID-19 has to spread through the country before it infects a critical mass of people who will develop immunity. It’s too late to contain.”
Added Liebers, “It’s a constantly changing situation, and we are still being surprised – not that this wasn’t predicted.”
This article first appeared on Medscape.com.
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
Clinicians are working out how to manage patients with or suspected of having COVID-19.
“Over the past couple of weeks, we’ve been preparing for the oncoming onslaught of patients,” said Lillian Wu, MD, of the HealthPoint network in the Seattle area of greater King County and president elect of the Washington Academy of Family Physicians.
Step One: Triage
The first step, Wu says, is careful triage.
When patients call one of the 17 clinics in the HealthPoint system, nurses gauge how sick they are. High fever? Shortness of breath? Do they have a chronic illness, such as diabetes, cardiovascular disease, or a lung condition, that increases risk for infection and complications?
“If a patient has mild symptoms, we ask them to stay home or to check back in 24 hours, or we’ll reach out to them. For moderate symptoms, we ask them to come in, and [we] clearly mark on the schedule that it is a respiratory patient, who will be sent to a separate area. If the patient is severe, we don’t even see them and send them directly to the hospital to the ER,” Wu told Medscape Medical News.
These categories parallel the World Health Organization’s designations of uncomplicated illness, mild pneumonia, severe pneumonia, acute respiratory distress syndrome, sepsis, and septic shock. The Centers for Disease Control and Prevention (CDC) advises case by case regarding decisions as to outpatient or inpatient assignment.
“Patients who pass the initial phone triage are given masks, separated, and sent to different parts of the clinic or are required to wait in their cars until it’s time to be seen,” Wu said.
Step 2: Hospital Arrival
Once at the hospital, the CDC’s interim guidance kicks in.
“Any patient with fever, cough, and shortness of breath presenting with a history of travel to countries with high ongoing transmission or a credible history of exposure should be promptly evaluated for COVID-19,” said Raghavendra Tirupathi, MD, medical director, Keystone Infectious Diseases/HIV; chair in infection prevention, Summit Health; and clinical assistant professor of medicine, Penn State School of Medicine, Hershey, Pennsylvania.
“We recommend obtaining baseline CBC with differential, basic metabolic panel, liver function tests, and procalcitonin. Clues for COVID-19 include leukopenia, seen in 30% to 45% of patients, and lymphocytopenia, seen in 85% of the patients in the case series from China,” Tirupathi said. He uses a respiratory virus polymerase chain reaction panel to rule out other pathogens.
Wu concurs. “This is the one time we are grateful when someone tests positive for the flu! If flu is negative and other common respiratory infections are negative, then we do a COVID-19 test,” she said.
But test results may be delayed. “At the University of Washington, it takes 8 hours, but commercial labs take up to 4 days,” Wu said. All patients with respiratory symptoms are treated as persons under investigation, for whom isolation precautions are required. In addition, for these patients, use of personal protective equipment by caregivers is required.
For suspected pneumonia, the American College of Radiography recommends chest CT to identify peripheral basal ground-glass opacities characteristic of COVID-19.
However, diagnosis should be based on detection of SARS-CoV-2, because chest images for COVID-19 are nonspecific – associated signs can also be seen in H1N1 influenza, SARS, and MERS.
Step 3: Supportive Care
Once a patient is admitted, supportive care entails “maintaining fluid status and nutrition and supporting physiological functions until we heal. It’s treating complications and organ support, whether that means providing supplementary oxygen all the way to ventilator support, and just waiting it out. If a patient progresses to acute respiratory distress syndrome, it becomes tougher,” said David Liebers, MD, chief medical officer and an infectious disease specialist at Ellis Medicine in Schenectady, New York.
Efforts are ramping up to develop therapeutics. Remdesivir, an investigational antiviral drug developed to treat Ebola and Marburg hemorrhagic fevers, shows activity against SARS-CoV-2 in vitro.
Remdesivir has been used in a few patients on a compassionate-use basis outside of a clinical trial setting. “It’s a nucleotide analogue, and like other drugs of that class, it disrupts nucleic acid production. Some data suggest that it might have some efficacy,” Liebers said.
Antibiotics are reserved for patients suspected of having concomitant bacterial or fungal infections. Liebers said clinicians should be alerted to “the big three” signs of secondary infection – fever, elevated white blood cell count, and lactic acidosis. Immunosuppressed patients are at elevated risk for secondary infection.
Step 4: Managing Complications
Patients do die of COVID-19, mostly through an inability to ventilate, even when supported with oxygen, Liebers told Medscape Medical News. (According to Tirupathi, “The studies from China indicate that from 6%-10% of patients needed ventilators.”)
Liebers continued, “Others may develop sepsis or a syndrome of multisystem organ failure with renal and endothelial collapse, making it difficult to maintain blood pressure. Like with so many pathologies, it is a vicious circle in which everything gets overworked. Off-and-on treatments can sometimes break the cycle: supplementary oxygen, giving red blood cells, dialysis. We support those functions while waiting for healing to occur.”
A facility’s airborne-infection isolation rooms may become filled to capacity, but that isn’t critical, Liebers said. “Airborne precautions are standard to contain measles, tuberculosis, chickenpox, and herpes zoster, in which very small particles spread in the air,” he said.
Consensus is growing that SARS-CoV-2 spreads in large droplets, he added. Private rooms and closed doors may suffice.
Step 5: Discharge
Liebers said that as of now, the million-dollar question regards criteria for discharge.
Patients who clinically improve are sent home with instructions to remain in isolation. They may be tested again for virus before or after discharge.
Liebers and Wu pointed to the experience at EvergreenHealth Medical Center, in Kirkland, Washington, as guidance from the trenches. “They’re the ones who are learning firsthand and passing the experience along to everyone else,” Wu said.
“The situation is unprecedented,” said Liebers, who, like many others, has barely slept these past weeks. “We’re swimming in murky water right now.”
The epidemic in the United States is still months from peaking, Wu emphasized. “There is no vaccine, and many cases are subclinical. COVID-19 has to spread through the country before it infects a critical mass of people who will develop immunity. It’s too late to contain.”
Added Liebers, “It’s a constantly changing situation, and we are still being surprised – not that this wasn’t predicted.”
This article first appeared on Medscape.com.