User login
Pneumonia Readmission Validation
Hospital readmissions are emblematic of the numerous challenges facing the US health care system. Despite high levels of spending, nearly 20% of Medicare beneficiaries are readmitted within 30 days of hospital discharge, many readmissions are considered preventable, and rates vary widely by hospital and region.1 Further, while readmissions have been estimated to cost taxpayers as much as $17 billion annually, the current fee‐for‐service method of paying for the acute care needs of seniors rewards hospitals financially for readmission, not their prevention.2
Pneumonia is the second most common reason for hospitalization among Medicare beneficiaries, accounting for approximately 650,000 admissions annually,3 and has been a focus of national quality‐improvement efforts for more than a decade.4, 5 Despite improvements in key processes of care, rates of readmission within 30 days of discharge following a hospitalization for pneumonia have been reported to vary from 10% to 24%.68 Among several factors, readmissions are believed to be influenced by the quality of both inpatient and outpatient care, and by care‐coordination activities occurring in the transition from inpatient to outpatient status.912
Public reporting of hospital performance is considered a key strategy for improving quality, reducing costs, and increasing the value of hospital care, both in the US and worldwide.13 In 2009, the Centers for Medicare & Medicaid Services (CMS) expanded its reporting initiatives by adding risk‐adjusted hospital readmission rates for acute myocardial infarction, heart failure, and pneumonia to the Hospital Compare website.14, 15 Readmission rates are an attractive focus for public reporting for several reasons. First, in contrast to most process‐based measures of quality (eg, whether a patient with pneumonia received a particular antibiotic), a readmission is an adverse outcome that matters to patients and families.16 Second, unlike process measures whose assessment requires detailed review of medical records, readmissions can be easily determined from standard hospital claims. Finally, readmissions are costly, and their prevention could yield substantial savings to society.
A necessary prerequisite for public reporting of readmission is a validated, risk‐adjusted measure that can be used to track performance over time and can facilitate comparisons across institutions. Toward this end, we describe the development, validation, and results of a National Quality Forum‐approved and CMS‐adopted model to estimate hospital‐specific, risk‐standardized, 30‐day readmission rates for Medicare patients hospitalized with pneumonia.17
METHODS
Data Sources
We used 20052006 claims data from Medicare inpatient, outpatient, and carrier (physician) Standard Analytic Files to develop and validate the administrative model. The Medicare Enrollment Database was used to determine Medicare fee‐for‐service enrollment and mortality statuses. A medical record model, used for additional validation of the administrative model, was developed using information abstracted from the charts of 75,616 pneumonia cases from 19982001 as part of the National Pneumonia Project, a CMS quality improvement initiative.18
Study Cohort
We identified hospitalizations of patients 65 years of age and older with a principal diagnosis of pneumonia (International Classification of Diseases, 9th Revision, Clinical Modification codes 480.XX, 481, 482.XX, 483.X, 485, 486, 487.0) as potential index pneumonia admissions. Because our focus was readmission for patients discharged from acute care settings, we excluded admissions in which patients died or were transferred to another acute care facility. Additionally, we restricted analysis to patients who had been enrolled in fee‐for‐service Medicare Parts A and B, for at least 12 months prior to their pneumonia hospitalization, so that we could use diagnostic codes from all inpatient and outpatient encounters during that period to enhance identification of comorbidities.
Outcome
The outcome was 30‐day readmission, defined as occurrence of at least one hospitalization for any cause within 30 days of discharge after an index admission. Readmissions were identified from hospital claims data, and were attributed to the hospital that had discharged the patient. A 30‐day time frame was selected because it is a clinically meaningful period during which hospitals can be expected to collaborate with other organizations and providers to implement measures to reduce the risk of rehospitalization.
Candidate and Final Model Variables
Candidate variables for the administrative claims model were selected by a clinician team from 189 diagnostic groups included in the Hierarchical Condition Category (HCC) clinical classification system.19 The HCC clinical classification system was developed for CMS in preparation for all‐encounter risk adjustment for Medicare Advantage (managed care). Under the HCC algorithm, the 15,000+ ICD‐9‐CM diagnosis codes are assigned to one of 189 clinically‐coherent condition categories (CCs). We used the April 2008 version of the ICD‐9‐CM to CC assignment map, which is maintained by CMS and posted at
The final risk‐adjustment model included 39 variables selected by the team of clinicians and analysts, primarily based on their clinical relevance but also with knowledge of the strength of their statistical association with readmission outcome (Table 1). For each patient, the presence or absence of these conditions was assessed from multiple sources, including secondary diagnoses during the index admission, principal and secondary diagnoses from hospital admissions in the 12 months prior to the index admission, and diagnoses from hospital outpatient and physician encounters 12 months before the index admission. A small number of CCs were considered to represent potential complications of care (eg, bleeding). Because we did not want to adjust for complications of care occurring during the index admission, a patient was not considered to have one of these conditions unless it was also present in at least one encounter prior to the index admission.
| Variable | Frequencies | Estimate | Standard Error | Odds Ratio | 95% CI | |
|---|---|---|---|---|---|---|
| ||||||
| Intercept | 2.395 | 0.021 | ||||
| Age 65 (years above 65, continuous) | 0.0001 | 0.001 | 1.000 | 0.998 | 1.001 | |
| Male | 45 | 0.071 | 0.012 | 1.073 | 1.048 | 1.099 |
| History of CABG | 5.2 | 0.179 | 0.027 | 0.836 | 0.793 | 0.881 |
| Metastatic cancer and acute leukemia (CC 7) | 4.3 | 0.177 | 0.029 | 1.194 | 1.128 | 1.263 |
| Lung, upper digestive tract, and other severe cancers (CC 8) | 6.0 | 0.256 | 0.024 | 1.292 | 1.232 | 1.354 |
| Diabetes and DM complications (CC 15‐20, 119, 120) | 36 | 0.059 | 0.012 | 1.061 | 1.036 | 1.087 |
| Disorders of fluid/electrolyte/acid‐base (CC 22, 23) | 34 | 0.149 | 0.013 | 1.160 | 1.131 | 1.191 |
| Iron deficiency and other/unspecified anemias and blood disease (CC 47) | 46 | 0.118 | 0.012 | 1.126 | 1.099 | 1.153 |
| Other psychiatric disorders (CC 60) | 12 | 0.108 | 0.017 | 1.114 | 1.077 | 1.151 |
| Cardio‐respiratory failure and shock (CC 79) | 16 | 0.114 | 0.016 | 1.121 | 1.087 | 1.156 |
| Congestive heart failure (CC 80) | 39 | 0.151 | 0.014 | 1.163 | 1.133 | 1.194 |
| Chronic atherosclerosis (CC 83, 84) | 47 | 0.051 | 0.013 | 1.053 | 1.027 | 1.079 |
| Valvular and rheumatic heart disease (CC 86) | 23 | 0.062 | 0.014 | 1.064 | 1.036 | 1.093 |
| Arrhythmias (CC 92, 93) | 38 | 0.126 | 0.013 | 1.134 | 1.107 | 1.163 |
| Vascular or circulatory disease (CC 104‐106) | 38 | 0.088 | 0.012 | 1.092 | 1.066 | 1.119 |
| COPD (CC 108) | 58 | 0.186 | 0.013 | 1.205 | 1.175 | 1.235 |
| Fibrosis of lung and other chronic lung disorders (CC 109) | 17 | 0.086 | 0.015 | 1.090 | 1.059 | 1.122 |
| Renal failure (CC 131) | 17 | 0.147 | 0.016 | 1.158 | 1.122 | 1.196 |
| Protein‐calorie malnutrition (CC 21) | 7.9 | 0.121 | 0.020 | 1.129 | 1.086 | 1.173 |
| History of infection (CC 1, 3‐6) | 35 | 0.068 | 0.012 | 1.071 | 1.045 | 1.097 |
| Severe hematological disorders (CC 44) | 3.6 | 0.117 | 0.028 | 1.125 | 1.064 | 1.188 |
| Decubitus ulcer or chronic skin ulcer (CC 148, 149) | 10 | 0.101 | 0.018 | 1.106 | 1.067 | 1.146 |
| History of pneumonia (CC 111‐113) | 44 | 0.065 | 0.013 | 1.067 | 1.041 | 1.094 |
| Vertebral fractures (CC 157) | 5.1 | 0.113 | 0.024 | 1.120 | 1.068 | 1.174 |
| Other injuries (CC 162) | 32 | 0.061 | 0.012 | 1.063 | 1.038 | 1.089 |
| Urinary tract infection (CC 135) | 26 | 0.064 | 0.014 | 1.066 | 1.038 | 1.095 |
| Lymphatic, head and neck, brain, and other major cancers; breast, prostate, colorectal, and other cancers and tumors (CC 9‐10) | 16 | 0.050 | 0.016 | 1.051 | 1.018 | 1.084 |
| End‐stage renal disease or dialysis (CC 129, 130) | 1.9 | 0.131 | 0.037 | 1.140 | 1.060 | 1.226 |
| Drug/alcohol abuse/dependence/psychosis (CC 51‐53) | 12 | 0.081 | 0.017 | 1.084 | 1.048 | 1.121 |
| Septicemia/shock (CC 2) | 6.3 | 0.094 | 0.022 | 1.098 | 1.052 | 1.146 |
| Other gastrointestinal disorders (CC 36) | 56 | 0.073 | 0.012 | 1.076 | 1.051 | 1.102 |
| Acute coronary syndrome (CC 81, 82) | 8.3 | 0.126 | 0.019 | 1.134 | 1.092 | 1.178 |
| Pleural effusion/pneumothorax (CC 114) | 12 | 0.083 | 0.017 | 1.086 | 1.051 | 1.123 |
| Other urinary tract disorders (CC 136) | 24 | 0.059 | 0.014 | 1.061 | 1.033 | 1.090 |
| Stroke (CC 95, 96) | 10 | 0.047 | 0.019 | 1.049 | 1.011 | 1.088 |
| Dementia and senility (CC 49, 50) | 27 | 0.031 | 0.014 | 1.031 | 1.004 | 1.059 |
| Hemiplegia, paraplegia, paralysis, functional disability (CC 67‐69, 100‐102, 177, 178) | 7.4 | 0.068 | 0.021 | 1.070 | 1.026 | 1.116 |
| Other lung disorders (CC 115) | 45 | 0.005 | 0.012 | 1.005 | 0.982 | 1.030 |
| Major psychiatric disorders (CC 54‐56) | 11 | 0.038 | 0.018 | 1.038 | 1.003 | 1.075 |
| Asthma (CC 110) | 12 | 0.006 | 0.018 | 1.006 | 0.972 | 1.041 |
Model Derivation
For the development of the administrative claims model, we randomly sampled half of 2006 hospitalizations that met inclusion criteria. To assess model performance at the patient level, we calculated the area under the receiver operating curve (AUC), and calculated observed readmission rates in the lowest and highest deciles on the basis of predicted readmission probabilities. We also compared performance with a null model, a model that adjusted for age and sex, and a model that included all candidate variables.20
Risk‐Standardized Readmission Rates
Using hierarchical logistic regression, we modeled the log‐odds of readmission within 30 days of discharge from an index pneumonia admission as a function of patient demographic and clinical characteristics, and a random hospital‐specific intercept. This strategy accounts for within‐hospital correlation, or clustering, of observed outcomes, and models the assumption that underlying differences in quality among hospitals being evaluated lead to systematic differences in outcomes. We then calculated hospital‐specific readmission rates as the ratio of predicted‐to‐expected readmissions (similar to observed/expected ratio), multiplied by the national unadjusted ratea form of indirect standardization. Predicted number of readmissions in each hospital is estimated given the same patient mix and its estimated hospital‐specific intercept. Expected number of readmissions in each hospital is estimated using its patient mix and the average hospital‐specific intercept. To assess hospital performance in any given year, we re‐estimate model coefficients using that year's data.
Model Validation: Administrative Claims
We compared the model performance in the development sample with its performance in the sample from the 2006 data that was not selected for the development set, and separately among pneumonia admissions in 2005. The model was recalibrated in each validation set.
Model Validation: Medical Record Abstraction
We developed a separate medical record‐based model of readmission risk using information from charts that had previously been abstracted as part of CMS's National Pneumonia Project. To select variables for this model, the clinician team: 1) reviewed the list of variables that were included in a medical record model that was previously developed for validating the National Quality Forum‐approved pneumonia mortality measure; 2) reviewed a list of other potential candidate variables available in the National Pneumonia Project dataset; and 3) reviewed variables that emerged as potentially important predictors of readmission, based on a systematic review of the literature that was conducted as part of measure development. This selection process resulted in a final medical record model that included 35 variables.
We linked patients in the National Pneumonia Project cohort to their Medicare claims data, including claims from one year before the index hospitalization, so that we could calculate risk‐standardized readmission rates in this cohort separately using medical record and claims‐based models. This analysis was conducted at the state level, for the 50 states plus the District of Columbia and Puerto Rico, because medical record data were unavailable in sufficient numbers to permit hospital‐level comparisons. To examine the relationship between risk‐standardized rates obtained from medical record and administrative data models, we estimated a linear regression model describing the association between the two rates, weighting each state by number of index hospitalizations, and calculated the correlation coefficient and the intercept and slope of this equation. A slope close to 1 and an intercept close to 0 would provide evidence that risk‐standardized state readmission rates from the medical record and claims models were similar. We also calculated the difference between state risk‐standardized readmission rates from the two models.
Analyses were conducted with the use of SAS version 9.1.3 (SAS Institute Inc, Cary, NC). Models were fitted separately for the National Pneumonia Project and 2006 cohort. We estimated the hierarchical models using the GLIMMIX procedure in SAS. The Human Investigation Committee at the Yale School of Medicine approved an exemption for the authors to use CMS claims and enrollment data for research analyses and publication.
RESULTS
Model Derivation and Performance
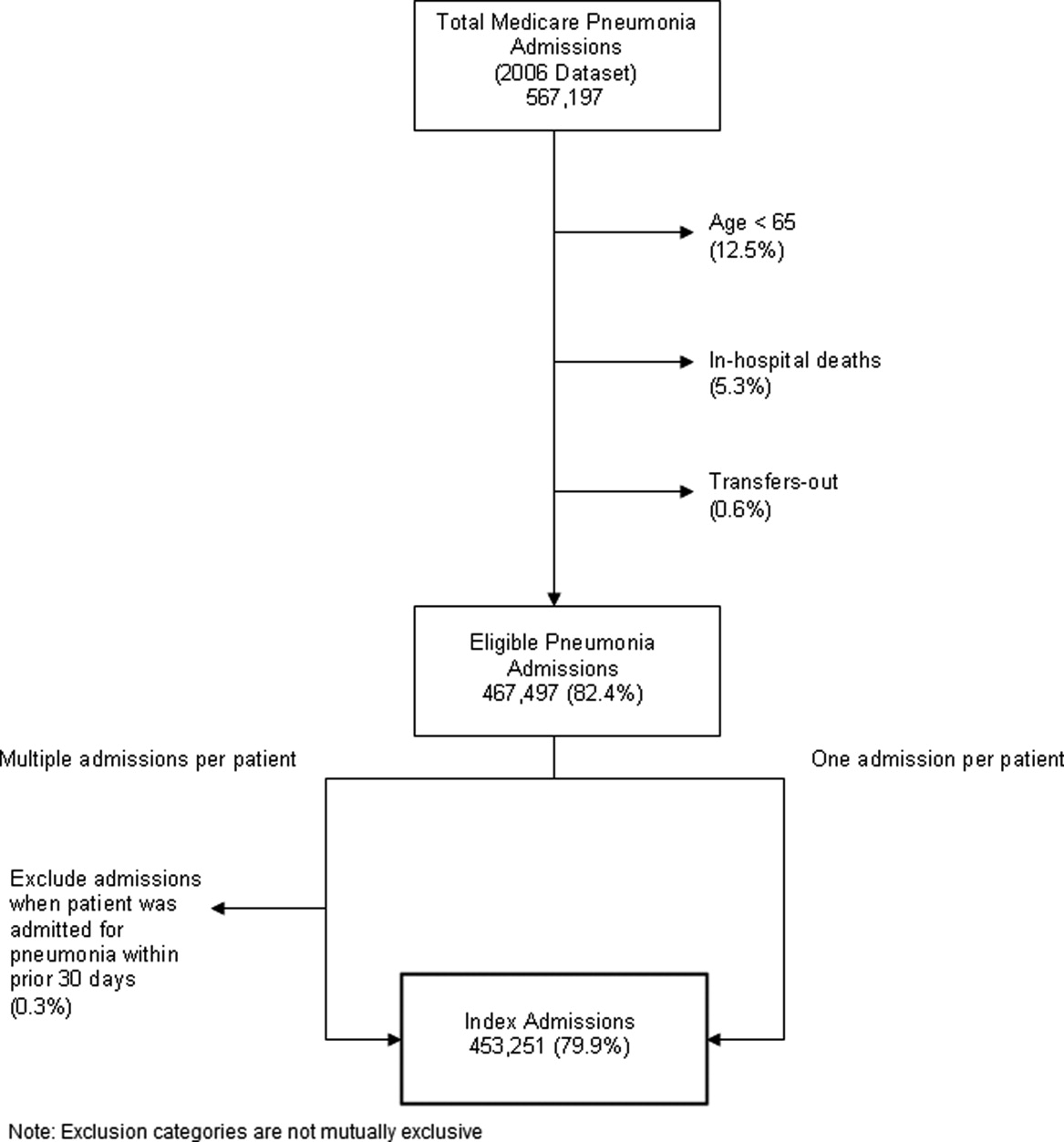
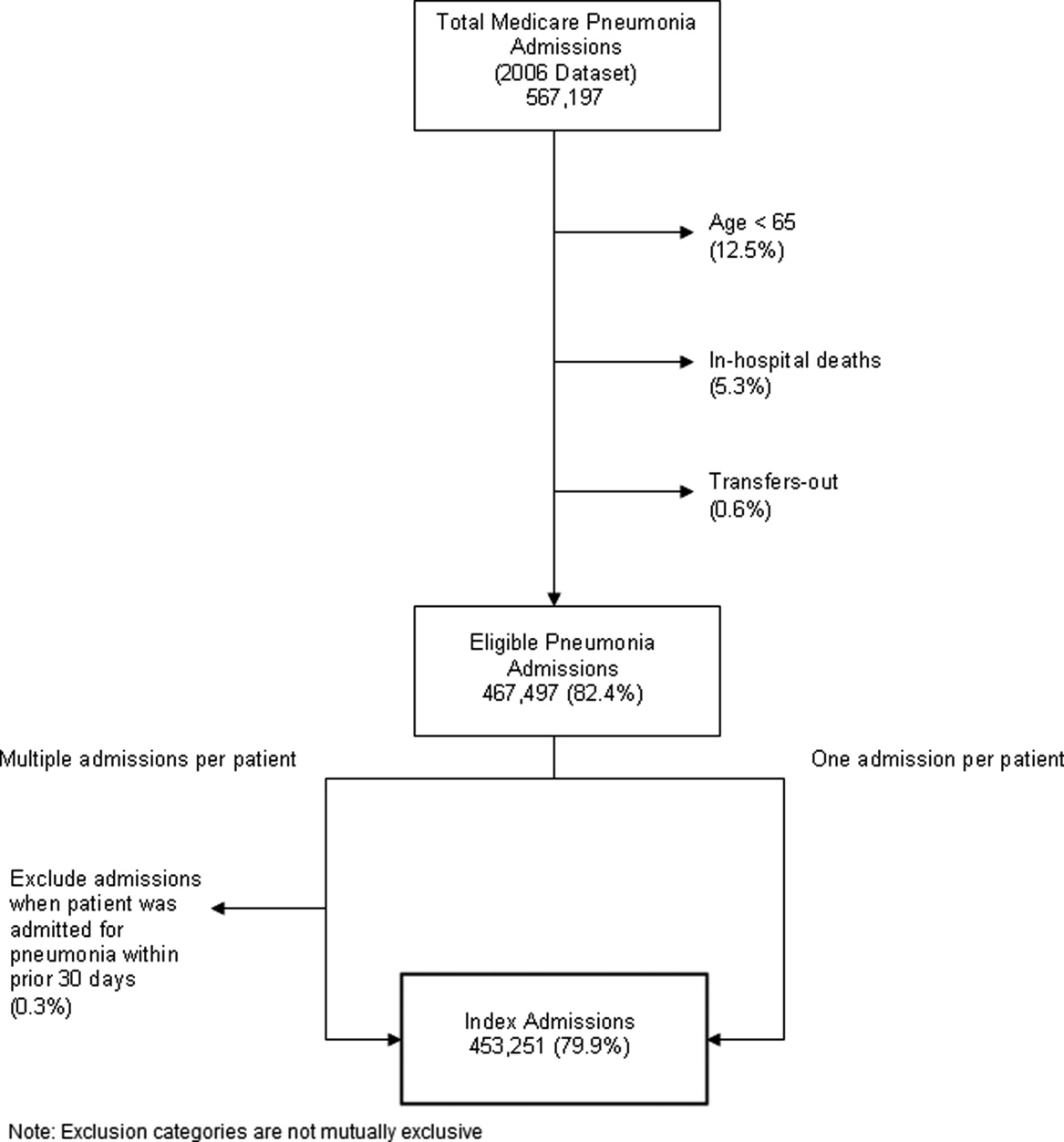
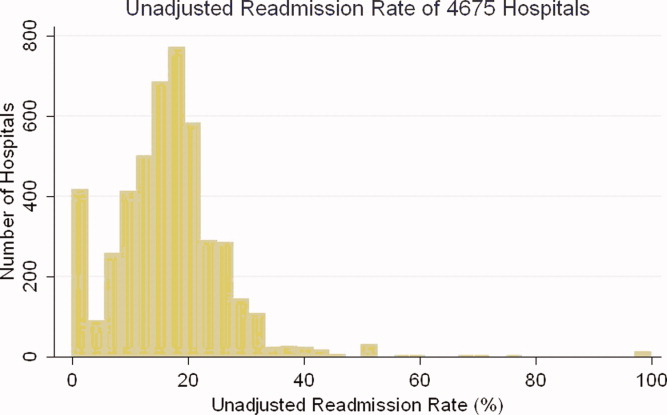
After exclusions were applied, the 2006 sample included 453,251 pneumonia hospitalizations (Figure 1). The development sample consisted of 226,545 hospitalizations at 4675 hospitals, with an overall unadjusted 30‐day readmission rate of 17.4%. In 11,694 index cases (5.2%), the patient died within 30 days without being readmitted. Median readmission rate was 16.3%, 25th and 75th percentile rates were 11.1% and 21.3%, and at the 10th and 90th percentile, hospital readmission rates ranged from 4.6% to 26.7% (Figure 2).
The claims model included 39 variables (age, sex, and 37 clinical variables) (Table 1). The mean age of the cohort was 80.0 years, with 55.5% women and 11.1% nonwhite patients. Mean observed readmission rate in the development sample ranged from 9% in the lowest decile of predicted pneumonia readmission rates to 32% in the highest predicted decile, a range of 23%. The AUC was 0.63. For comparison, a model with only age and sex had an AUC of 0.51, and a model with all candidate variables had an AUC equal to 0.63 (Table 2).
| Calibration (0, 1)* | Discrimination | Residuals Lack of Fit (Pearson Residual Fall %) | Model 2 (No. of Covariates) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictive Ability (Lowest Decile, Highest Decile) | AUC | (<2) | (2, 0) | (0, 2) | (2+) | ||||
| |||||||||
| Development sample | |||||||||
| 2006 | (1st half) N = 226,545 | (0, 1) | (0.09, 0.32) | 0.63 | 0 | 82.62 | 7.39 | 9.99 | 6,843 (40) |
| Validation sample | |||||||||
| 2006 | (2nd half) N = 226,706 | (0.002, 0.997) | (0.09, 0.31) | 0.63 | 0 | 82.55 | 7.45 | 9.99 | 6,870 (40) |
| 2005 | N = 536,015 | (0.035, 1.008) | (0.08, 0.31) | 0.63 | 0 | 82.67 | 7.31 | 10.03 | 16,241 (40) |
Hospital Risk‐Standardized Readmission Rates
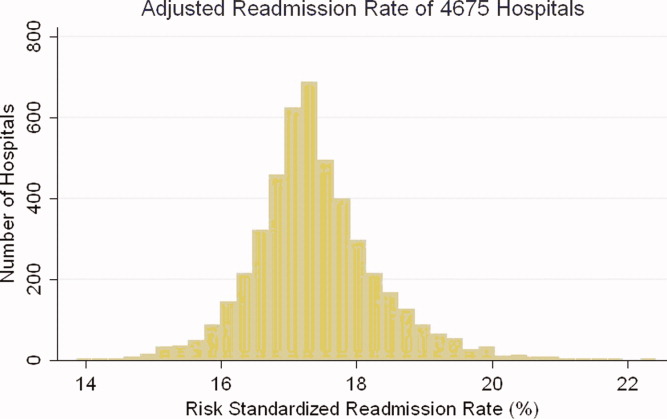
Risk‐standardized readmission rates varied across hospitals (Figure 3). Median risk‐standardized readmission rate was 17.3%, and the 25th and 75th percentiles were 16.9% and 17.9%, respectively. The 5th percentile was 16.0% and the 95th percentile was 19.1%. Odds of readmission for a hospital one standard deviation above average was 1.4 times that of a hospital one standard deviation below average.
Administrative Model Validation
In the remaining 50% of pneumonia index hospitalizations from 2006, and the entire 2005 cohort, regression coefficients and standard errors of model variables were similar to those in the development data set. Model performance using 2005 data was consistent with model performance using the 2006 development and validation half‐samples (Table 2).
Medical Record Validation
After exclusions, the medical record sample taken from the National Pneumonia Project included 47,429 cases, with an unadjusted 30‐day readmission rate of 17.0%. The final medical record risk‐adjustment model included a total of 35 variables, whose prevalence and association with readmission risk varied modestly (Table 3). Performance of the medical record and administrative models was similar (areas under the ROC curve 0.59 and 0.63, respectively) (Table 4). Additionally, in the administrative model, predicted readmission rates ranged from 8% in the lowest predicted decile to 30% in the highest predicted decile, while in the medical record model, the corresponding rates varied from 10% to 26%.
| Variable | Percent | Estimate | Standard Error | Odds Ratio | 95% CI | |
|---|---|---|---|---|---|---|
| ||||||
| Age 65, mean (SD) | 15.24 (7.87) | 0.003 | 0.002 | 0.997 | 0.993 | 1.000 |
| Male | 46.18 | 0.122 | 0.025 | 1.130 | 1.075 | 1.188 |
| Nursing home resident | 17.71 | 0.035 | 0.037 | 1.036 | 0.963 | 1.114 |
| Neoplastic disease | 6.80 | 0.130 | 0.049 | 1.139 | 1.034 | 1.254 |
| Liver disease | 1.04 | 0.089 | 0.123 | 0.915 | 0.719 | 1.164 |
| History of heart failure | 28.98 | 0.234 | 0.029 | 1.264 | 1.194 | 1.339 |
| History of renal disease | 8.51 | 0.188 | 0.047 | 1.206 | 1.100 | 1.323 |
| Altered mental status | 17.95 | 0.009 | 0.034 | 1.009 | 0.944 | 1.080 |
| Pleural effusion | 21.20 | 0.165 | 0.030 | 1.179 | 1.111 | 1.251 |
| BUN 30 mg/dl | 23.28 | 0.160 | 0.033 | 1.174 | 1.100 | 1.252 |
| BUN missing | 14.56 | 0.101 | 0.185 | 0.904 | 0.630 | 1.298 |
| Systolic BP <90 mmHg | 2.95 | 0.068 | 0.070 | 1.070 | 0.932 | 1.228 |
| Systolic BP missing | 11.21 | 0.149 | 0.425 | 1.160 | 0.504 | 2.669 |
| Pulse 125/min | 7.73 | 0.036 | 0.047 | 1.036 | 0.945 | 1.137 |
| Pulse missing | 11.22 | 0.210 | 0.405 | 1.234 | 0.558 | 2.729 |
| Respiratory rate 30/min | 16.38 | 0.079 | 0.034 | 1.082 | 1.012 | 1.157 |
| Respiratory rate missing | 11.39 | 0.204 | 0.240 | 1.226 | 0.765 | 1.964 |
| Sodium <130 mmol/L | 4.82 | 0.136 | 0.057 | 1.145 | 1.025 | 1.280 |
| Sodium missing | 14.39 | 0.049 | 0.143 | 1.050 | 0.793 | 1.391 |
| Glucose 250 mg/dl | 5.19 | 0.005 | 0.057 | 0.995 | 0.889 | 1.114 |
| Glucose missing | 15.44 | 0.156 | 0.105 | 0.855 | 0.696 | 1.051 |
| Hematocrit <30% | 7.77 | 0.270 | 0.044 | 1.310 | 1.202 | 1.428 |
| Hematocrit missing | 13.62 | 0.071 | 0.135 | 0.932 | 0.715 | 1.215 |
| Creatinine 2.5 mg/dL | 4.68 | 0.109 | 0.062 | 1.115 | 0.989 | 1.258 |
| Creatinine missing | 14.63 | 0.200 | 0.167 | 1.221 | 0.880 | 1.695 |
| WBC 6‐12 b/L | 38.04 | 0.021 | 0.049 | 0.979 | 0.889 | 1.079 |
| WBC >12 b/L | 41.45 | 0.068 | 0.049 | 0.934 | 0.848 | 1.029 |
| WBC missing | 12.85 | 0.167 | 0.162 | 1.181 | 0.860 | 1.623 |
| Immunosuppressive therapy | 15.01 | 0.347 | 0.035 | 1.415 | 1.321 | 1.516 |
| Chronic lung disease | 42.16 | 0.137 | 0.028 | 1.147 | 1.086 | 1.211 |
| Coronary artery disease | 39.57 | 0.150 | 0.028 | 1.162 | 1.100 | 1.227 |
| Diabetes mellitus | 20.90 | 0.137 | 0.033 | 1.147 | 1.076 | 1.223 |
| Alcohol/drug abuse | 3.40 | 0.099 | 0.071 | 0.906 | 0.788 | 1.041 |
| Dementia/Alzheimer's disease | 16.38 | 0.125 | 0.038 | 1.133 | 1.052 | 1.222 |
| Splenectomy | 0.44 | 0.016 | 0.186 | 1.016 | 0.706 | 1.463 |
| Model | Calibration (0, 1)* | Discrimination | Residuals Lack of Fit (Pearson Residual Fall %) | Model 2 (No. of Covariates) | ||||
|---|---|---|---|---|---|---|---|---|
| Predictive Ability (Lowest Decile, Highest Decile) | AUC | (<2) | (2, 0) | (0, 2) | (2+) | |||
| ||||||||
| Medical Record Model Development Sample (NP) | ||||||||
| N = 47,429 No. of 30‐day readmissions = 8,042 | (1, 0) | (0.10, 0.26) | 0.59 | 0 | 83.04 | 5.28 | 11.68 | 710 (35) |
| Linked Administrative Model Validation Sample | ||||||||
| N = 47,429 No. of 30‐day readmissions = 8,042 | (1, 0) | (0.08, 0.30) | 0.63 | 0 | 83.04 | 6.94 | 10.01 | 1,414 (40) |
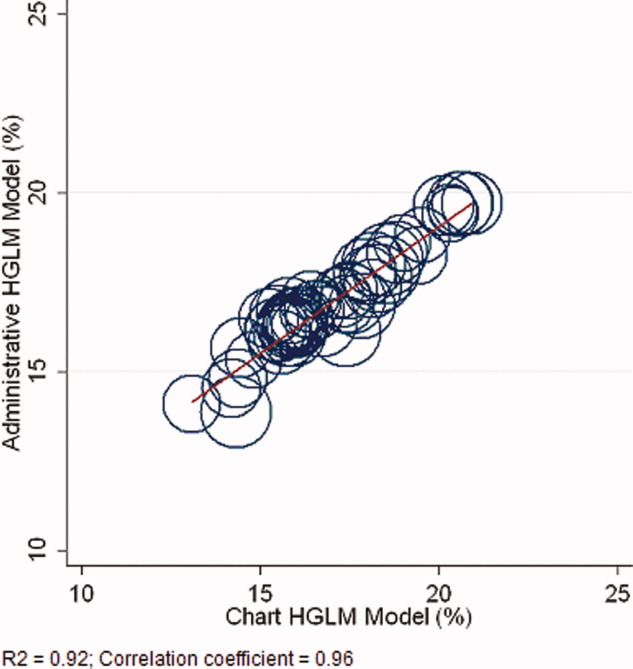
The correlation coefficient of the estimated state‐specific standardized readmission rates from the administrative and medical record models was 0.96, and the proportion of the variance explained by the model was 0.92 (Figure 4).
DISCUSSION
We have described the development, validation, and results of a hospital, 30‐day, risk‐standardized readmission model for pneumonia that was created to support current federal transparency initiatives. The model uses administrative claims data from Medicare fee‐for‐service patients and produces results that are comparable to a model based on information obtained through manual abstraction of medical records. We observed an overall 30‐day readmission rate of 17%, and our analyses revealed substantial variation across US hospitals, suggesting that improvement by lower performing institutions is an achievable goal.
Because more than one in six pneumonia patients are rehospitalized shortly after discharge, and because pneumonia hospitalizations represent an enormous expense to the Medicare program, prevention of readmissions is now widely recognized to offer a substantial opportunity to improve patient outcomes while simultaneously lowering health care costs. Accordingly, promotion of strategies to reduce readmission rates has become a key priority for payers and quality‐improvement organizations. These range from policy‐level attempts to stimulate change, such as publicly reporting hospital readmission rates on government websites, to establishing accreditation standardssuch as the Joint Commission's requirement to accurately reconcile medications, to the creation of quality improvement collaboratives focused on sharing best practices across institutions. Regardless of the approach taken, a valid, risk‐adjusted measure of performance is required to evaluate and track performance over time. The measure we have described meets the National Quality Forum's measure evaluation criteria in that it addresses an important clinical topic for which there appears to be significant opportunities for improvement, the measure is precisely defined and has been subjected to validity and reliability testing, it is risk‐adjusted based on patient clinical factors present at the start of care, is feasible to produce, and is understandable by a broad range of potential users.21 Because hospitalists are the physicians primarily responsible for the care of patients with pneumonia at US hospitals, and because they frequently serve as the physician champions for quality improvement activities related to pneumonia, it is especially important that they maintain a thorough understanding of the measures and methodologies underlying current efforts to measure hospital performance.
Several features of our approach warrant additional comment. First, we deliberately chose to measure all readmission events rather than attempt to discriminate between potentially preventable and nonpreventable readmissions. From the patient perspective, readmission for any reason is a concern, and limiting the measure to pneumonia‐related readmissions could make it susceptible to gaming by hospitals. Moreover, determining whether a readmission is related to a potential quality problem is not straightforward. For example, a patient with pneumonia whose discharge medications were prescribed incorrectly may be readmitted with a hip fracture following an episode of syncope. It would be inappropriate to treat this readmission as unrelated to the care the patient received for pneumonia. Additionally, while our approach does not presume that every readmission is preventable, the goal is to reduce the risk of readmissions generally (not just in narrowly defined subpopulations), and successful interventions to reduce rehospitalization have typically demonstrated reductions in all‐cause readmission.9, 22 Second, deaths that occurred within 30 days of discharge, yet that were not accompanied by a hospital readmission, were not counted as a readmission outcome. While it may seem inappropriate to treat a postdischarge death as a nonevent (rather than censoring or excluding such cases), alternative analytic approaches, such as using a hierarchical survival model, are not currently computationally feasible with large national data sets. Fortunately, only a relatively small proportion of discharges fell into this category (5.2% of index cases in the 2006 development sample died within 30 days of discharge without being readmitted). An alternative approach to handling the competing outcome of death would have been to use a composite outcome of readmission or death. However, we believe that it is important to report the outcomes separately because factors that predict readmission and mortality may differ, and when making comparisons across hospitals it would not be possible to determine whether differences in rate were due to readmission or mortality. Third, while the patient‐level readmission model showed only modest discrimination, we intentionally excluded covariates such as race and socioeconomic status, as well as in‐hospital events and potential complications of care, and whether patients were discharged home or to a skilled nursing facility. While these variables could have improved predictive ability, they may be directly or indirectly related to quality or supply factors that should not be included in a model that seeks to control for patient clinical characteristics. For example, if hospitals with a large share of poor patients have higher readmission rates, then including income in the model will obscure differences that are important to identify. While we believe that the decision to exclude such factors in the model is in the best interest of patients, and supports efforts to reduce health inequality in society more generally, we also recognize that hospitals that care for a disproportionate share of poor patients are likely to require additional resources to overcome these social factors. Fourth, we limited the analysis to patients with a principal diagnosis of pneumonia, and chose not to also include those with a principal diagnosis of sepsis or respiratory failure coupled with a secondary diagnosis of pneumonia. While the broader definition is used by CMS in the National Pneumonia Project, that initiative relied on chart abstraction to differentiate pneumonia present at the time of admission from cases developing as a complication of hospitalization. Additionally, we did not attempt to differentiate between community‐acquired and healthcare‐associated pneumonia, however our approach is consistent with the National Pneumonia Project and Pneumonia Patient Outcomes Research Team.18 Fifth, while our model estimates readmission rates at the hospital level, we recognize that readmissions are influenced by a complex and extensive range of factors. In this context, greater cooperation between hospitals and other care providers will almost certainly be required in order to achieve dramatic improvement in readmission rates, which in turn will depend upon changes to the way serious illness is paid for. Some options that have recently been described include imposing financial penalties for early readmission, extending the boundaries of case‐based payment beyond hospital discharge, and bundling payments between hospitals and physicians.2325
Our measure has several limitations. First, our models were developed and validated using Medicare data, and the results may not apply to pneumonia patients less than 65 years of age. However, most patients hospitalized with pneumonia in the US are 65 or older. In addition, we were unable to test the model with a Medicare managed care population, because data are not currently available on such patients. Finally, the medical record‐based validation was conducted by state‐level analysis because the sample size was insufficient to carry this out at the hospital level.
In conclusion, more than 17% of Medicare beneficiaries are readmitted within 30 days following discharge after a hospitalization for pneumonia, and rates vary substantially across institutions. The development of a valid measure of hospital performance and public reporting are important first steps towards focusing attention on this problem. Actual improvement will now depend on whether hospitals and partner organizations are successful at identifying and implementing effective methods to prevent readmission.
- ,,.Rehospitalizations among patients in the Medicare Fee‐for‐Service Program.N Engl J Med.2009;360(14):1418–1428.
- Medicare Payment Advisory Commission.Report to the Congress: Promoting Greater Efficiency in Medicare.2007.
- ,,,,. HCUP Facts and Figures: Statistics on Hospital‐based Care in the United States, 2007.2009. Available at: http://www.hcup‐us.ahrq.gov/reports.jsp. Accessed November 7, 2009.
- Centers for Medicare 353(3):255–264.
- ,,,.Trends in postdischarge mortality and readmissions: has length of stay declined too far?Arch Intern Med.2004;164(5):538–544.
- ,,,.Short‐term outcomes and their predictors for patients hospitalized with community‐acquired pneumonia.Heart Lung.2004;33(5):301–307.
- ,,, et al.Improved clinical outcomes with utilization of a community‐acquired pneumonia guideline.Chest.2006;130(3):794–799.
- ,,,,.Associations between initial antimicrobial therapy and medical outcomes for hospitalized elderly patients with pneumonia.Arch Intern Med.1999;159(21):2562–2572.
- ,.Hospital readmissions as a measure of quality of health care: advantages and limitations.Arch Intern Med.2000;160(8):1074–1081.
- ,,,.The care transitions intervention: results of a randomized controlled trial.Arch Intern Med.2006;166(17):1822–1828.
- Corrigan JM, Eden J, Smith BM, eds.Leadership by Example: Coordinating Government Roles in Improving Health Care Quality. Committee on Enhancing Federal Healthcare Quality Programs.Washington, DC:National Academies Press,2003.
- Medicare.gov—Hospital Compare. Available at: http://www.hospitalcompare.hhs.gov/Hospital/Search/Welcome.asp?version=default1(1):29–37.
- ,,,,.Measuring performance for treating heart attacks and heart failure: the case for outcomes measurement.Health Aff.2007;26(1):75–85.
- NQF‐Endorsed® Standards. Available at: http://www.qualityforum.org/Measures_List.aspx. Accessed November 6,2009.
- ,,,,.Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community‐acquired pneumonia.Arch Intern Med.2004;164(6):637–644.
- ,,. Diagnostic Cost Group Hierarchical Condition Category Models for Medicare Risk Adjustment. Report prepared for the Health Care Financing Administration. Health Economics Research, Inc;2000. Available at: http://www.cms.hhs.gov/Reports/Reports/ItemDetail.asp?ItemID=CMS023176. Accessed November 7, 2009.
- .Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis.1st ed.New York:Springer;2006.
- National Quality Forum—Measure Evaluation Criteria.2008. Available at: http://www.qualityforum.org/uploadedFiles/Quality_Forum/Measuring_Performance/Consensus_Development_Process%E2%80%99s_Principle/EvalCriteria2008–08‐28Final.pdf?n=4701.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial.JAMA.1999;281(7):613–620.
- .Paying for care episodes and care coordination.N Engl J Med.2007;356(11):1166–1168.
- .Health care reform—toward more freedom, and responsibility, for physicians.N Engl J Med.2009;361(6):623–628.
- .Beyond pay for performance—emerging models of provider‐payment reform.N Engl J Med.2008;359(12):1197–1200.
Hospital readmissions are emblematic of the numerous challenges facing the US health care system. Despite high levels of spending, nearly 20% of Medicare beneficiaries are readmitted within 30 days of hospital discharge, many readmissions are considered preventable, and rates vary widely by hospital and region.1 Further, while readmissions have been estimated to cost taxpayers as much as $17 billion annually, the current fee‐for‐service method of paying for the acute care needs of seniors rewards hospitals financially for readmission, not their prevention.2
Pneumonia is the second most common reason for hospitalization among Medicare beneficiaries, accounting for approximately 650,000 admissions annually,3 and has been a focus of national quality‐improvement efforts for more than a decade.4, 5 Despite improvements in key processes of care, rates of readmission within 30 days of discharge following a hospitalization for pneumonia have been reported to vary from 10% to 24%.68 Among several factors, readmissions are believed to be influenced by the quality of both inpatient and outpatient care, and by care‐coordination activities occurring in the transition from inpatient to outpatient status.912
Public reporting of hospital performance is considered a key strategy for improving quality, reducing costs, and increasing the value of hospital care, both in the US and worldwide.13 In 2009, the Centers for Medicare & Medicaid Services (CMS) expanded its reporting initiatives by adding risk‐adjusted hospital readmission rates for acute myocardial infarction, heart failure, and pneumonia to the Hospital Compare website.14, 15 Readmission rates are an attractive focus for public reporting for several reasons. First, in contrast to most process‐based measures of quality (eg, whether a patient with pneumonia received a particular antibiotic), a readmission is an adverse outcome that matters to patients and families.16 Second, unlike process measures whose assessment requires detailed review of medical records, readmissions can be easily determined from standard hospital claims. Finally, readmissions are costly, and their prevention could yield substantial savings to society.
A necessary prerequisite for public reporting of readmission is a validated, risk‐adjusted measure that can be used to track performance over time and can facilitate comparisons across institutions. Toward this end, we describe the development, validation, and results of a National Quality Forum‐approved and CMS‐adopted model to estimate hospital‐specific, risk‐standardized, 30‐day readmission rates for Medicare patients hospitalized with pneumonia.17
METHODS
Data Sources
We used 20052006 claims data from Medicare inpatient, outpatient, and carrier (physician) Standard Analytic Files to develop and validate the administrative model. The Medicare Enrollment Database was used to determine Medicare fee‐for‐service enrollment and mortality statuses. A medical record model, used for additional validation of the administrative model, was developed using information abstracted from the charts of 75,616 pneumonia cases from 19982001 as part of the National Pneumonia Project, a CMS quality improvement initiative.18
Study Cohort
We identified hospitalizations of patients 65 years of age and older with a principal diagnosis of pneumonia (International Classification of Diseases, 9th Revision, Clinical Modification codes 480.XX, 481, 482.XX, 483.X, 485, 486, 487.0) as potential index pneumonia admissions. Because our focus was readmission for patients discharged from acute care settings, we excluded admissions in which patients died or were transferred to another acute care facility. Additionally, we restricted analysis to patients who had been enrolled in fee‐for‐service Medicare Parts A and B, for at least 12 months prior to their pneumonia hospitalization, so that we could use diagnostic codes from all inpatient and outpatient encounters during that period to enhance identification of comorbidities.
Outcome
The outcome was 30‐day readmission, defined as occurrence of at least one hospitalization for any cause within 30 days of discharge after an index admission. Readmissions were identified from hospital claims data, and were attributed to the hospital that had discharged the patient. A 30‐day time frame was selected because it is a clinically meaningful period during which hospitals can be expected to collaborate with other organizations and providers to implement measures to reduce the risk of rehospitalization.
Candidate and Final Model Variables
Candidate variables for the administrative claims model were selected by a clinician team from 189 diagnostic groups included in the Hierarchical Condition Category (HCC) clinical classification system.19 The HCC clinical classification system was developed for CMS in preparation for all‐encounter risk adjustment for Medicare Advantage (managed care). Under the HCC algorithm, the 15,000+ ICD‐9‐CM diagnosis codes are assigned to one of 189 clinically‐coherent condition categories (CCs). We used the April 2008 version of the ICD‐9‐CM to CC assignment map, which is maintained by CMS and posted at
The final risk‐adjustment model included 39 variables selected by the team of clinicians and analysts, primarily based on their clinical relevance but also with knowledge of the strength of their statistical association with readmission outcome (Table 1). For each patient, the presence or absence of these conditions was assessed from multiple sources, including secondary diagnoses during the index admission, principal and secondary diagnoses from hospital admissions in the 12 months prior to the index admission, and diagnoses from hospital outpatient and physician encounters 12 months before the index admission. A small number of CCs were considered to represent potential complications of care (eg, bleeding). Because we did not want to adjust for complications of care occurring during the index admission, a patient was not considered to have one of these conditions unless it was also present in at least one encounter prior to the index admission.
| Variable | Frequencies | Estimate | Standard Error | Odds Ratio | 95% CI | |
|---|---|---|---|---|---|---|
| ||||||
| Intercept | 2.395 | 0.021 | ||||
| Age 65 (years above 65, continuous) | 0.0001 | 0.001 | 1.000 | 0.998 | 1.001 | |
| Male | 45 | 0.071 | 0.012 | 1.073 | 1.048 | 1.099 |
| History of CABG | 5.2 | 0.179 | 0.027 | 0.836 | 0.793 | 0.881 |
| Metastatic cancer and acute leukemia (CC 7) | 4.3 | 0.177 | 0.029 | 1.194 | 1.128 | 1.263 |
| Lung, upper digestive tract, and other severe cancers (CC 8) | 6.0 | 0.256 | 0.024 | 1.292 | 1.232 | 1.354 |
| Diabetes and DM complications (CC 15‐20, 119, 120) | 36 | 0.059 | 0.012 | 1.061 | 1.036 | 1.087 |
| Disorders of fluid/electrolyte/acid‐base (CC 22, 23) | 34 | 0.149 | 0.013 | 1.160 | 1.131 | 1.191 |
| Iron deficiency and other/unspecified anemias and blood disease (CC 47) | 46 | 0.118 | 0.012 | 1.126 | 1.099 | 1.153 |
| Other psychiatric disorders (CC 60) | 12 | 0.108 | 0.017 | 1.114 | 1.077 | 1.151 |
| Cardio‐respiratory failure and shock (CC 79) | 16 | 0.114 | 0.016 | 1.121 | 1.087 | 1.156 |
| Congestive heart failure (CC 80) | 39 | 0.151 | 0.014 | 1.163 | 1.133 | 1.194 |
| Chronic atherosclerosis (CC 83, 84) | 47 | 0.051 | 0.013 | 1.053 | 1.027 | 1.079 |
| Valvular and rheumatic heart disease (CC 86) | 23 | 0.062 | 0.014 | 1.064 | 1.036 | 1.093 |
| Arrhythmias (CC 92, 93) | 38 | 0.126 | 0.013 | 1.134 | 1.107 | 1.163 |
| Vascular or circulatory disease (CC 104‐106) | 38 | 0.088 | 0.012 | 1.092 | 1.066 | 1.119 |
| COPD (CC 108) | 58 | 0.186 | 0.013 | 1.205 | 1.175 | 1.235 |
| Fibrosis of lung and other chronic lung disorders (CC 109) | 17 | 0.086 | 0.015 | 1.090 | 1.059 | 1.122 |
| Renal failure (CC 131) | 17 | 0.147 | 0.016 | 1.158 | 1.122 | 1.196 |
| Protein‐calorie malnutrition (CC 21) | 7.9 | 0.121 | 0.020 | 1.129 | 1.086 | 1.173 |
| History of infection (CC 1, 3‐6) | 35 | 0.068 | 0.012 | 1.071 | 1.045 | 1.097 |
| Severe hematological disorders (CC 44) | 3.6 | 0.117 | 0.028 | 1.125 | 1.064 | 1.188 |
| Decubitus ulcer or chronic skin ulcer (CC 148, 149) | 10 | 0.101 | 0.018 | 1.106 | 1.067 | 1.146 |
| History of pneumonia (CC 111‐113) | 44 | 0.065 | 0.013 | 1.067 | 1.041 | 1.094 |
| Vertebral fractures (CC 157) | 5.1 | 0.113 | 0.024 | 1.120 | 1.068 | 1.174 |
| Other injuries (CC 162) | 32 | 0.061 | 0.012 | 1.063 | 1.038 | 1.089 |
| Urinary tract infection (CC 135) | 26 | 0.064 | 0.014 | 1.066 | 1.038 | 1.095 |
| Lymphatic, head and neck, brain, and other major cancers; breast, prostate, colorectal, and other cancers and tumors (CC 9‐10) | 16 | 0.050 | 0.016 | 1.051 | 1.018 | 1.084 |
| End‐stage renal disease or dialysis (CC 129, 130) | 1.9 | 0.131 | 0.037 | 1.140 | 1.060 | 1.226 |
| Drug/alcohol abuse/dependence/psychosis (CC 51‐53) | 12 | 0.081 | 0.017 | 1.084 | 1.048 | 1.121 |
| Septicemia/shock (CC 2) | 6.3 | 0.094 | 0.022 | 1.098 | 1.052 | 1.146 |
| Other gastrointestinal disorders (CC 36) | 56 | 0.073 | 0.012 | 1.076 | 1.051 | 1.102 |
| Acute coronary syndrome (CC 81, 82) | 8.3 | 0.126 | 0.019 | 1.134 | 1.092 | 1.178 |
| Pleural effusion/pneumothorax (CC 114) | 12 | 0.083 | 0.017 | 1.086 | 1.051 | 1.123 |
| Other urinary tract disorders (CC 136) | 24 | 0.059 | 0.014 | 1.061 | 1.033 | 1.090 |
| Stroke (CC 95, 96) | 10 | 0.047 | 0.019 | 1.049 | 1.011 | 1.088 |
| Dementia and senility (CC 49, 50) | 27 | 0.031 | 0.014 | 1.031 | 1.004 | 1.059 |
| Hemiplegia, paraplegia, paralysis, functional disability (CC 67‐69, 100‐102, 177, 178) | 7.4 | 0.068 | 0.021 | 1.070 | 1.026 | 1.116 |
| Other lung disorders (CC 115) | 45 | 0.005 | 0.012 | 1.005 | 0.982 | 1.030 |
| Major psychiatric disorders (CC 54‐56) | 11 | 0.038 | 0.018 | 1.038 | 1.003 | 1.075 |
| Asthma (CC 110) | 12 | 0.006 | 0.018 | 1.006 | 0.972 | 1.041 |
Model Derivation
For the development of the administrative claims model, we randomly sampled half of 2006 hospitalizations that met inclusion criteria. To assess model performance at the patient level, we calculated the area under the receiver operating curve (AUC), and calculated observed readmission rates in the lowest and highest deciles on the basis of predicted readmission probabilities. We also compared performance with a null model, a model that adjusted for age and sex, and a model that included all candidate variables.20
Risk‐Standardized Readmission Rates
Using hierarchical logistic regression, we modeled the log‐odds of readmission within 30 days of discharge from an index pneumonia admission as a function of patient demographic and clinical characteristics, and a random hospital‐specific intercept. This strategy accounts for within‐hospital correlation, or clustering, of observed outcomes, and models the assumption that underlying differences in quality among hospitals being evaluated lead to systematic differences in outcomes. We then calculated hospital‐specific readmission rates as the ratio of predicted‐to‐expected readmissions (similar to observed/expected ratio), multiplied by the national unadjusted ratea form of indirect standardization. Predicted number of readmissions in each hospital is estimated given the same patient mix and its estimated hospital‐specific intercept. Expected number of readmissions in each hospital is estimated using its patient mix and the average hospital‐specific intercept. To assess hospital performance in any given year, we re‐estimate model coefficients using that year's data.
Model Validation: Administrative Claims
We compared the model performance in the development sample with its performance in the sample from the 2006 data that was not selected for the development set, and separately among pneumonia admissions in 2005. The model was recalibrated in each validation set.
Model Validation: Medical Record Abstraction
We developed a separate medical record‐based model of readmission risk using information from charts that had previously been abstracted as part of CMS's National Pneumonia Project. To select variables for this model, the clinician team: 1) reviewed the list of variables that were included in a medical record model that was previously developed for validating the National Quality Forum‐approved pneumonia mortality measure; 2) reviewed a list of other potential candidate variables available in the National Pneumonia Project dataset; and 3) reviewed variables that emerged as potentially important predictors of readmission, based on a systematic review of the literature that was conducted as part of measure development. This selection process resulted in a final medical record model that included 35 variables.
We linked patients in the National Pneumonia Project cohort to their Medicare claims data, including claims from one year before the index hospitalization, so that we could calculate risk‐standardized readmission rates in this cohort separately using medical record and claims‐based models. This analysis was conducted at the state level, for the 50 states plus the District of Columbia and Puerto Rico, because medical record data were unavailable in sufficient numbers to permit hospital‐level comparisons. To examine the relationship between risk‐standardized rates obtained from medical record and administrative data models, we estimated a linear regression model describing the association between the two rates, weighting each state by number of index hospitalizations, and calculated the correlation coefficient and the intercept and slope of this equation. A slope close to 1 and an intercept close to 0 would provide evidence that risk‐standardized state readmission rates from the medical record and claims models were similar. We also calculated the difference between state risk‐standardized readmission rates from the two models.
Analyses were conducted with the use of SAS version 9.1.3 (SAS Institute Inc, Cary, NC). Models were fitted separately for the National Pneumonia Project and 2006 cohort. We estimated the hierarchical models using the GLIMMIX procedure in SAS. The Human Investigation Committee at the Yale School of Medicine approved an exemption for the authors to use CMS claims and enrollment data for research analyses and publication.
RESULTS
Model Derivation and Performance
After exclusions were applied, the 2006 sample included 453,251 pneumonia hospitalizations (Figure 1). The development sample consisted of 226,545 hospitalizations at 4675 hospitals, with an overall unadjusted 30‐day readmission rate of 17.4%. In 11,694 index cases (5.2%), the patient died within 30 days without being readmitted. Median readmission rate was 16.3%, 25th and 75th percentile rates were 11.1% and 21.3%, and at the 10th and 90th percentile, hospital readmission rates ranged from 4.6% to 26.7% (Figure 2).


The claims model included 39 variables (age, sex, and 37 clinical variables) (Table 1). The mean age of the cohort was 80.0 years, with 55.5% women and 11.1% nonwhite patients. Mean observed readmission rate in the development sample ranged from 9% in the lowest decile of predicted pneumonia readmission rates to 32% in the highest predicted decile, a range of 23%. The AUC was 0.63. For comparison, a model with only age and sex had an AUC of 0.51, and a model with all candidate variables had an AUC equal to 0.63 (Table 2).
| Calibration (0, 1)* | Discrimination | Residuals Lack of Fit (Pearson Residual Fall %) | Model 2 (No. of Covariates) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictive Ability (Lowest Decile, Highest Decile) | AUC | (<2) | (2, 0) | (0, 2) | (2+) | ||||
| |||||||||
| Development sample | |||||||||
| 2006 | (1st half) N = 226,545 | (0, 1) | (0.09, 0.32) | 0.63 | 0 | 82.62 | 7.39 | 9.99 | 6,843 (40) |
| Validation sample | |||||||||
| 2006 | (2nd half) N = 226,706 | (0.002, 0.997) | (0.09, 0.31) | 0.63 | 0 | 82.55 | 7.45 | 9.99 | 6,870 (40) |
| 2005 | N = 536,015 | (0.035, 1.008) | (0.08, 0.31) | 0.63 | 0 | 82.67 | 7.31 | 10.03 | 16,241 (40) |
Hospital Risk‐Standardized Readmission Rates
Risk‐standardized readmission rates varied across hospitals (Figure 3). Median risk‐standardized readmission rate was 17.3%, and the 25th and 75th percentiles were 16.9% and 17.9%, respectively. The 5th percentile was 16.0% and the 95th percentile was 19.1%. Odds of readmission for a hospital one standard deviation above average was 1.4 times that of a hospital one standard deviation below average.

Administrative Model Validation
In the remaining 50% of pneumonia index hospitalizations from 2006, and the entire 2005 cohort, regression coefficients and standard errors of model variables were similar to those in the development data set. Model performance using 2005 data was consistent with model performance using the 2006 development and validation half‐samples (Table 2).
Medical Record Validation
After exclusions, the medical record sample taken from the National Pneumonia Project included 47,429 cases, with an unadjusted 30‐day readmission rate of 17.0%. The final medical record risk‐adjustment model included a total of 35 variables, whose prevalence and association with readmission risk varied modestly (Table 3). Performance of the medical record and administrative models was similar (areas under the ROC curve 0.59 and 0.63, respectively) (Table 4). Additionally, in the administrative model, predicted readmission rates ranged from 8% in the lowest predicted decile to 30% in the highest predicted decile, while in the medical record model, the corresponding rates varied from 10% to 26%.
| Variable | Percent | Estimate | Standard Error | Odds Ratio | 95% CI | |
|---|---|---|---|---|---|---|
| ||||||
| Age 65, mean (SD) | 15.24 (7.87) | 0.003 | 0.002 | 0.997 | 0.993 | 1.000 |
| Male | 46.18 | 0.122 | 0.025 | 1.130 | 1.075 | 1.188 |
| Nursing home resident | 17.71 | 0.035 | 0.037 | 1.036 | 0.963 | 1.114 |
| Neoplastic disease | 6.80 | 0.130 | 0.049 | 1.139 | 1.034 | 1.254 |
| Liver disease | 1.04 | 0.089 | 0.123 | 0.915 | 0.719 | 1.164 |
| History of heart failure | 28.98 | 0.234 | 0.029 | 1.264 | 1.194 | 1.339 |
| History of renal disease | 8.51 | 0.188 | 0.047 | 1.206 | 1.100 | 1.323 |
| Altered mental status | 17.95 | 0.009 | 0.034 | 1.009 | 0.944 | 1.080 |
| Pleural effusion | 21.20 | 0.165 | 0.030 | 1.179 | 1.111 | 1.251 |
| BUN 30 mg/dl | 23.28 | 0.160 | 0.033 | 1.174 | 1.100 | 1.252 |
| BUN missing | 14.56 | 0.101 | 0.185 | 0.904 | 0.630 | 1.298 |
| Systolic BP <90 mmHg | 2.95 | 0.068 | 0.070 | 1.070 | 0.932 | 1.228 |
| Systolic BP missing | 11.21 | 0.149 | 0.425 | 1.160 | 0.504 | 2.669 |
| Pulse 125/min | 7.73 | 0.036 | 0.047 | 1.036 | 0.945 | 1.137 |
| Pulse missing | 11.22 | 0.210 | 0.405 | 1.234 | 0.558 | 2.729 |
| Respiratory rate 30/min | 16.38 | 0.079 | 0.034 | 1.082 | 1.012 | 1.157 |
| Respiratory rate missing | 11.39 | 0.204 | 0.240 | 1.226 | 0.765 | 1.964 |
| Sodium <130 mmol/L | 4.82 | 0.136 | 0.057 | 1.145 | 1.025 | 1.280 |
| Sodium missing | 14.39 | 0.049 | 0.143 | 1.050 | 0.793 | 1.391 |
| Glucose 250 mg/dl | 5.19 | 0.005 | 0.057 | 0.995 | 0.889 | 1.114 |
| Glucose missing | 15.44 | 0.156 | 0.105 | 0.855 | 0.696 | 1.051 |
| Hematocrit <30% | 7.77 | 0.270 | 0.044 | 1.310 | 1.202 | 1.428 |
| Hematocrit missing | 13.62 | 0.071 | 0.135 | 0.932 | 0.715 | 1.215 |
| Creatinine 2.5 mg/dL | 4.68 | 0.109 | 0.062 | 1.115 | 0.989 | 1.258 |
| Creatinine missing | 14.63 | 0.200 | 0.167 | 1.221 | 0.880 | 1.695 |
| WBC 6‐12 b/L | 38.04 | 0.021 | 0.049 | 0.979 | 0.889 | 1.079 |
| WBC >12 b/L | 41.45 | 0.068 | 0.049 | 0.934 | 0.848 | 1.029 |
| WBC missing | 12.85 | 0.167 | 0.162 | 1.181 | 0.860 | 1.623 |
| Immunosuppressive therapy | 15.01 | 0.347 | 0.035 | 1.415 | 1.321 | 1.516 |
| Chronic lung disease | 42.16 | 0.137 | 0.028 | 1.147 | 1.086 | 1.211 |
| Coronary artery disease | 39.57 | 0.150 | 0.028 | 1.162 | 1.100 | 1.227 |
| Diabetes mellitus | 20.90 | 0.137 | 0.033 | 1.147 | 1.076 | 1.223 |
| Alcohol/drug abuse | 3.40 | 0.099 | 0.071 | 0.906 | 0.788 | 1.041 |
| Dementia/Alzheimer's disease | 16.38 | 0.125 | 0.038 | 1.133 | 1.052 | 1.222 |
| Splenectomy | 0.44 | 0.016 | 0.186 | 1.016 | 0.706 | 1.463 |
| Model | Calibration (0, 1)* | Discrimination | Residuals Lack of Fit (Pearson Residual Fall %) | Model 2 (No. of Covariates) | ||||
|---|---|---|---|---|---|---|---|---|
| Predictive Ability (Lowest Decile, Highest Decile) | AUC | (<2) | (2, 0) | (0, 2) | (2+) | |||
| ||||||||
| Medical Record Model Development Sample (NP) | ||||||||
| N = 47,429 No. of 30‐day readmissions = 8,042 | (1, 0) | (0.10, 0.26) | 0.59 | 0 | 83.04 | 5.28 | 11.68 | 710 (35) |
| Linked Administrative Model Validation Sample | ||||||||
| N = 47,429 No. of 30‐day readmissions = 8,042 | (1, 0) | (0.08, 0.30) | 0.63 | 0 | 83.04 | 6.94 | 10.01 | 1,414 (40) |
The correlation coefficient of the estimated state‐specific standardized readmission rates from the administrative and medical record models was 0.96, and the proportion of the variance explained by the model was 0.92 (Figure 4).

DISCUSSION
We have described the development, validation, and results of a hospital, 30‐day, risk‐standardized readmission model for pneumonia that was created to support current federal transparency initiatives. The model uses administrative claims data from Medicare fee‐for‐service patients and produces results that are comparable to a model based on information obtained through manual abstraction of medical records. We observed an overall 30‐day readmission rate of 17%, and our analyses revealed substantial variation across US hospitals, suggesting that improvement by lower performing institutions is an achievable goal.
Because more than one in six pneumonia patients are rehospitalized shortly after discharge, and because pneumonia hospitalizations represent an enormous expense to the Medicare program, prevention of readmissions is now widely recognized to offer a substantial opportunity to improve patient outcomes while simultaneously lowering health care costs. Accordingly, promotion of strategies to reduce readmission rates has become a key priority for payers and quality‐improvement organizations. These range from policy‐level attempts to stimulate change, such as publicly reporting hospital readmission rates on government websites, to establishing accreditation standardssuch as the Joint Commission's requirement to accurately reconcile medications, to the creation of quality improvement collaboratives focused on sharing best practices across institutions. Regardless of the approach taken, a valid, risk‐adjusted measure of performance is required to evaluate and track performance over time. The measure we have described meets the National Quality Forum's measure evaluation criteria in that it addresses an important clinical topic for which there appears to be significant opportunities for improvement, the measure is precisely defined and has been subjected to validity and reliability testing, it is risk‐adjusted based on patient clinical factors present at the start of care, is feasible to produce, and is understandable by a broad range of potential users.21 Because hospitalists are the physicians primarily responsible for the care of patients with pneumonia at US hospitals, and because they frequently serve as the physician champions for quality improvement activities related to pneumonia, it is especially important that they maintain a thorough understanding of the measures and methodologies underlying current efforts to measure hospital performance.
Several features of our approach warrant additional comment. First, we deliberately chose to measure all readmission events rather than attempt to discriminate between potentially preventable and nonpreventable readmissions. From the patient perspective, readmission for any reason is a concern, and limiting the measure to pneumonia‐related readmissions could make it susceptible to gaming by hospitals. Moreover, determining whether a readmission is related to a potential quality problem is not straightforward. For example, a patient with pneumonia whose discharge medications were prescribed incorrectly may be readmitted with a hip fracture following an episode of syncope. It would be inappropriate to treat this readmission as unrelated to the care the patient received for pneumonia. Additionally, while our approach does not presume that every readmission is preventable, the goal is to reduce the risk of readmissions generally (not just in narrowly defined subpopulations), and successful interventions to reduce rehospitalization have typically demonstrated reductions in all‐cause readmission.9, 22 Second, deaths that occurred within 30 days of discharge, yet that were not accompanied by a hospital readmission, were not counted as a readmission outcome. While it may seem inappropriate to treat a postdischarge death as a nonevent (rather than censoring or excluding such cases), alternative analytic approaches, such as using a hierarchical survival model, are not currently computationally feasible with large national data sets. Fortunately, only a relatively small proportion of discharges fell into this category (5.2% of index cases in the 2006 development sample died within 30 days of discharge without being readmitted). An alternative approach to handling the competing outcome of death would have been to use a composite outcome of readmission or death. However, we believe that it is important to report the outcomes separately because factors that predict readmission and mortality may differ, and when making comparisons across hospitals it would not be possible to determine whether differences in rate were due to readmission or mortality. Third, while the patient‐level readmission model showed only modest discrimination, we intentionally excluded covariates such as race and socioeconomic status, as well as in‐hospital events and potential complications of care, and whether patients were discharged home or to a skilled nursing facility. While these variables could have improved predictive ability, they may be directly or indirectly related to quality or supply factors that should not be included in a model that seeks to control for patient clinical characteristics. For example, if hospitals with a large share of poor patients have higher readmission rates, then including income in the model will obscure differences that are important to identify. While we believe that the decision to exclude such factors in the model is in the best interest of patients, and supports efforts to reduce health inequality in society more generally, we also recognize that hospitals that care for a disproportionate share of poor patients are likely to require additional resources to overcome these social factors. Fourth, we limited the analysis to patients with a principal diagnosis of pneumonia, and chose not to also include those with a principal diagnosis of sepsis or respiratory failure coupled with a secondary diagnosis of pneumonia. While the broader definition is used by CMS in the National Pneumonia Project, that initiative relied on chart abstraction to differentiate pneumonia present at the time of admission from cases developing as a complication of hospitalization. Additionally, we did not attempt to differentiate between community‐acquired and healthcare‐associated pneumonia, however our approach is consistent with the National Pneumonia Project and Pneumonia Patient Outcomes Research Team.18 Fifth, while our model estimates readmission rates at the hospital level, we recognize that readmissions are influenced by a complex and extensive range of factors. In this context, greater cooperation between hospitals and other care providers will almost certainly be required in order to achieve dramatic improvement in readmission rates, which in turn will depend upon changes to the way serious illness is paid for. Some options that have recently been described include imposing financial penalties for early readmission, extending the boundaries of case‐based payment beyond hospital discharge, and bundling payments between hospitals and physicians.2325
Our measure has several limitations. First, our models were developed and validated using Medicare data, and the results may not apply to pneumonia patients less than 65 years of age. However, most patients hospitalized with pneumonia in the US are 65 or older. In addition, we were unable to test the model with a Medicare managed care population, because data are not currently available on such patients. Finally, the medical record‐based validation was conducted by state‐level analysis because the sample size was insufficient to carry this out at the hospital level.
In conclusion, more than 17% of Medicare beneficiaries are readmitted within 30 days following discharge after a hospitalization for pneumonia, and rates vary substantially across institutions. The development of a valid measure of hospital performance and public reporting are important first steps towards focusing attention on this problem. Actual improvement will now depend on whether hospitals and partner organizations are successful at identifying and implementing effective methods to prevent readmission.
Hospital readmissions are emblematic of the numerous challenges facing the US health care system. Despite high levels of spending, nearly 20% of Medicare beneficiaries are readmitted within 30 days of hospital discharge, many readmissions are considered preventable, and rates vary widely by hospital and region.1 Further, while readmissions have been estimated to cost taxpayers as much as $17 billion annually, the current fee‐for‐service method of paying for the acute care needs of seniors rewards hospitals financially for readmission, not their prevention.2
Pneumonia is the second most common reason for hospitalization among Medicare beneficiaries, accounting for approximately 650,000 admissions annually,3 and has been a focus of national quality‐improvement efforts for more than a decade.4, 5 Despite improvements in key processes of care, rates of readmission within 30 days of discharge following a hospitalization for pneumonia have been reported to vary from 10% to 24%.68 Among several factors, readmissions are believed to be influenced by the quality of both inpatient and outpatient care, and by care‐coordination activities occurring in the transition from inpatient to outpatient status.912
Public reporting of hospital performance is considered a key strategy for improving quality, reducing costs, and increasing the value of hospital care, both in the US and worldwide.13 In 2009, the Centers for Medicare & Medicaid Services (CMS) expanded its reporting initiatives by adding risk‐adjusted hospital readmission rates for acute myocardial infarction, heart failure, and pneumonia to the Hospital Compare website.14, 15 Readmission rates are an attractive focus for public reporting for several reasons. First, in contrast to most process‐based measures of quality (eg, whether a patient with pneumonia received a particular antibiotic), a readmission is an adverse outcome that matters to patients and families.16 Second, unlike process measures whose assessment requires detailed review of medical records, readmissions can be easily determined from standard hospital claims. Finally, readmissions are costly, and their prevention could yield substantial savings to society.
A necessary prerequisite for public reporting of readmission is a validated, risk‐adjusted measure that can be used to track performance over time and can facilitate comparisons across institutions. Toward this end, we describe the development, validation, and results of a National Quality Forum‐approved and CMS‐adopted model to estimate hospital‐specific, risk‐standardized, 30‐day readmission rates for Medicare patients hospitalized with pneumonia.17
METHODS
Data Sources
We used 20052006 claims data from Medicare inpatient, outpatient, and carrier (physician) Standard Analytic Files to develop and validate the administrative model. The Medicare Enrollment Database was used to determine Medicare fee‐for‐service enrollment and mortality statuses. A medical record model, used for additional validation of the administrative model, was developed using information abstracted from the charts of 75,616 pneumonia cases from 19982001 as part of the National Pneumonia Project, a CMS quality improvement initiative.18
Study Cohort
We identified hospitalizations of patients 65 years of age and older with a principal diagnosis of pneumonia (International Classification of Diseases, 9th Revision, Clinical Modification codes 480.XX, 481, 482.XX, 483.X, 485, 486, 487.0) as potential index pneumonia admissions. Because our focus was readmission for patients discharged from acute care settings, we excluded admissions in which patients died or were transferred to another acute care facility. Additionally, we restricted analysis to patients who had been enrolled in fee‐for‐service Medicare Parts A and B, for at least 12 months prior to their pneumonia hospitalization, so that we could use diagnostic codes from all inpatient and outpatient encounters during that period to enhance identification of comorbidities.
Outcome
The outcome was 30‐day readmission, defined as occurrence of at least one hospitalization for any cause within 30 days of discharge after an index admission. Readmissions were identified from hospital claims data, and were attributed to the hospital that had discharged the patient. A 30‐day time frame was selected because it is a clinically meaningful period during which hospitals can be expected to collaborate with other organizations and providers to implement measures to reduce the risk of rehospitalization.
Candidate and Final Model Variables
Candidate variables for the administrative claims model were selected by a clinician team from 189 diagnostic groups included in the Hierarchical Condition Category (HCC) clinical classification system.19 The HCC clinical classification system was developed for CMS in preparation for all‐encounter risk adjustment for Medicare Advantage (managed care). Under the HCC algorithm, the 15,000+ ICD‐9‐CM diagnosis codes are assigned to one of 189 clinically‐coherent condition categories (CCs). We used the April 2008 version of the ICD‐9‐CM to CC assignment map, which is maintained by CMS and posted at
The final risk‐adjustment model included 39 variables selected by the team of clinicians and analysts, primarily based on their clinical relevance but also with knowledge of the strength of their statistical association with readmission outcome (Table 1). For each patient, the presence or absence of these conditions was assessed from multiple sources, including secondary diagnoses during the index admission, principal and secondary diagnoses from hospital admissions in the 12 months prior to the index admission, and diagnoses from hospital outpatient and physician encounters 12 months before the index admission. A small number of CCs were considered to represent potential complications of care (eg, bleeding). Because we did not want to adjust for complications of care occurring during the index admission, a patient was not considered to have one of these conditions unless it was also present in at least one encounter prior to the index admission.
| Variable | Frequencies | Estimate | Standard Error | Odds Ratio | 95% CI | |
|---|---|---|---|---|---|---|
| ||||||
| Intercept | 2.395 | 0.021 | ||||
| Age 65 (years above 65, continuous) | 0.0001 | 0.001 | 1.000 | 0.998 | 1.001 | |
| Male | 45 | 0.071 | 0.012 | 1.073 | 1.048 | 1.099 |
| History of CABG | 5.2 | 0.179 | 0.027 | 0.836 | 0.793 | 0.881 |
| Metastatic cancer and acute leukemia (CC 7) | 4.3 | 0.177 | 0.029 | 1.194 | 1.128 | 1.263 |
| Lung, upper digestive tract, and other severe cancers (CC 8) | 6.0 | 0.256 | 0.024 | 1.292 | 1.232 | 1.354 |
| Diabetes and DM complications (CC 15‐20, 119, 120) | 36 | 0.059 | 0.012 | 1.061 | 1.036 | 1.087 |
| Disorders of fluid/electrolyte/acid‐base (CC 22, 23) | 34 | 0.149 | 0.013 | 1.160 | 1.131 | 1.191 |
| Iron deficiency and other/unspecified anemias and blood disease (CC 47) | 46 | 0.118 | 0.012 | 1.126 | 1.099 | 1.153 |
| Other psychiatric disorders (CC 60) | 12 | 0.108 | 0.017 | 1.114 | 1.077 | 1.151 |
| Cardio‐respiratory failure and shock (CC 79) | 16 | 0.114 | 0.016 | 1.121 | 1.087 | 1.156 |
| Congestive heart failure (CC 80) | 39 | 0.151 | 0.014 | 1.163 | 1.133 | 1.194 |
| Chronic atherosclerosis (CC 83, 84) | 47 | 0.051 | 0.013 | 1.053 | 1.027 | 1.079 |
| Valvular and rheumatic heart disease (CC 86) | 23 | 0.062 | 0.014 | 1.064 | 1.036 | 1.093 |
| Arrhythmias (CC 92, 93) | 38 | 0.126 | 0.013 | 1.134 | 1.107 | 1.163 |
| Vascular or circulatory disease (CC 104‐106) | 38 | 0.088 | 0.012 | 1.092 | 1.066 | 1.119 |
| COPD (CC 108) | 58 | 0.186 | 0.013 | 1.205 | 1.175 | 1.235 |
| Fibrosis of lung and other chronic lung disorders (CC 109) | 17 | 0.086 | 0.015 | 1.090 | 1.059 | 1.122 |
| Renal failure (CC 131) | 17 | 0.147 | 0.016 | 1.158 | 1.122 | 1.196 |
| Protein‐calorie malnutrition (CC 21) | 7.9 | 0.121 | 0.020 | 1.129 | 1.086 | 1.173 |
| History of infection (CC 1, 3‐6) | 35 | 0.068 | 0.012 | 1.071 | 1.045 | 1.097 |
| Severe hematological disorders (CC 44) | 3.6 | 0.117 | 0.028 | 1.125 | 1.064 | 1.188 |
| Decubitus ulcer or chronic skin ulcer (CC 148, 149) | 10 | 0.101 | 0.018 | 1.106 | 1.067 | 1.146 |
| History of pneumonia (CC 111‐113) | 44 | 0.065 | 0.013 | 1.067 | 1.041 | 1.094 |
| Vertebral fractures (CC 157) | 5.1 | 0.113 | 0.024 | 1.120 | 1.068 | 1.174 |
| Other injuries (CC 162) | 32 | 0.061 | 0.012 | 1.063 | 1.038 | 1.089 |
| Urinary tract infection (CC 135) | 26 | 0.064 | 0.014 | 1.066 | 1.038 | 1.095 |
| Lymphatic, head and neck, brain, and other major cancers; breast, prostate, colorectal, and other cancers and tumors (CC 9‐10) | 16 | 0.050 | 0.016 | 1.051 | 1.018 | 1.084 |
| End‐stage renal disease or dialysis (CC 129, 130) | 1.9 | 0.131 | 0.037 | 1.140 | 1.060 | 1.226 |
| Drug/alcohol abuse/dependence/psychosis (CC 51‐53) | 12 | 0.081 | 0.017 | 1.084 | 1.048 | 1.121 |
| Septicemia/shock (CC 2) | 6.3 | 0.094 | 0.022 | 1.098 | 1.052 | 1.146 |
| Other gastrointestinal disorders (CC 36) | 56 | 0.073 | 0.012 | 1.076 | 1.051 | 1.102 |
| Acute coronary syndrome (CC 81, 82) | 8.3 | 0.126 | 0.019 | 1.134 | 1.092 | 1.178 |
| Pleural effusion/pneumothorax (CC 114) | 12 | 0.083 | 0.017 | 1.086 | 1.051 | 1.123 |
| Other urinary tract disorders (CC 136) | 24 | 0.059 | 0.014 | 1.061 | 1.033 | 1.090 |
| Stroke (CC 95, 96) | 10 | 0.047 | 0.019 | 1.049 | 1.011 | 1.088 |
| Dementia and senility (CC 49, 50) | 27 | 0.031 | 0.014 | 1.031 | 1.004 | 1.059 |
| Hemiplegia, paraplegia, paralysis, functional disability (CC 67‐69, 100‐102, 177, 178) | 7.4 | 0.068 | 0.021 | 1.070 | 1.026 | 1.116 |
| Other lung disorders (CC 115) | 45 | 0.005 | 0.012 | 1.005 | 0.982 | 1.030 |
| Major psychiatric disorders (CC 54‐56) | 11 | 0.038 | 0.018 | 1.038 | 1.003 | 1.075 |
| Asthma (CC 110) | 12 | 0.006 | 0.018 | 1.006 | 0.972 | 1.041 |
Model Derivation
For the development of the administrative claims model, we randomly sampled half of 2006 hospitalizations that met inclusion criteria. To assess model performance at the patient level, we calculated the area under the receiver operating curve (AUC), and calculated observed readmission rates in the lowest and highest deciles on the basis of predicted readmission probabilities. We also compared performance with a null model, a model that adjusted for age and sex, and a model that included all candidate variables.20
Risk‐Standardized Readmission Rates
Using hierarchical logistic regression, we modeled the log‐odds of readmission within 30 days of discharge from an index pneumonia admission as a function of patient demographic and clinical characteristics, and a random hospital‐specific intercept. This strategy accounts for within‐hospital correlation, or clustering, of observed outcomes, and models the assumption that underlying differences in quality among hospitals being evaluated lead to systematic differences in outcomes. We then calculated hospital‐specific readmission rates as the ratio of predicted‐to‐expected readmissions (similar to observed/expected ratio), multiplied by the national unadjusted ratea form of indirect standardization. Predicted number of readmissions in each hospital is estimated given the same patient mix and its estimated hospital‐specific intercept. Expected number of readmissions in each hospital is estimated using its patient mix and the average hospital‐specific intercept. To assess hospital performance in any given year, we re‐estimate model coefficients using that year's data.
Model Validation: Administrative Claims
We compared the model performance in the development sample with its performance in the sample from the 2006 data that was not selected for the development set, and separately among pneumonia admissions in 2005. The model was recalibrated in each validation set.
Model Validation: Medical Record Abstraction
We developed a separate medical record‐based model of readmission risk using information from charts that had previously been abstracted as part of CMS's National Pneumonia Project. To select variables for this model, the clinician team: 1) reviewed the list of variables that were included in a medical record model that was previously developed for validating the National Quality Forum‐approved pneumonia mortality measure; 2) reviewed a list of other potential candidate variables available in the National Pneumonia Project dataset; and 3) reviewed variables that emerged as potentially important predictors of readmission, based on a systematic review of the literature that was conducted as part of measure development. This selection process resulted in a final medical record model that included 35 variables.
We linked patients in the National Pneumonia Project cohort to their Medicare claims data, including claims from one year before the index hospitalization, so that we could calculate risk‐standardized readmission rates in this cohort separately using medical record and claims‐based models. This analysis was conducted at the state level, for the 50 states plus the District of Columbia and Puerto Rico, because medical record data were unavailable in sufficient numbers to permit hospital‐level comparisons. To examine the relationship between risk‐standardized rates obtained from medical record and administrative data models, we estimated a linear regression model describing the association between the two rates, weighting each state by number of index hospitalizations, and calculated the correlation coefficient and the intercept and slope of this equation. A slope close to 1 and an intercept close to 0 would provide evidence that risk‐standardized state readmission rates from the medical record and claims models were similar. We also calculated the difference between state risk‐standardized readmission rates from the two models.
Analyses were conducted with the use of SAS version 9.1.3 (SAS Institute Inc, Cary, NC). Models were fitted separately for the National Pneumonia Project and 2006 cohort. We estimated the hierarchical models using the GLIMMIX procedure in SAS. The Human Investigation Committee at the Yale School of Medicine approved an exemption for the authors to use CMS claims and enrollment data for research analyses and publication.
RESULTS
Model Derivation and Performance
After exclusions were applied, the 2006 sample included 453,251 pneumonia hospitalizations (Figure 1). The development sample consisted of 226,545 hospitalizations at 4675 hospitals, with an overall unadjusted 30‐day readmission rate of 17.4%. In 11,694 index cases (5.2%), the patient died within 30 days without being readmitted. Median readmission rate was 16.3%, 25th and 75th percentile rates were 11.1% and 21.3%, and at the 10th and 90th percentile, hospital readmission rates ranged from 4.6% to 26.7% (Figure 2).


The claims model included 39 variables (age, sex, and 37 clinical variables) (Table 1). The mean age of the cohort was 80.0 years, with 55.5% women and 11.1% nonwhite patients. Mean observed readmission rate in the development sample ranged from 9% in the lowest decile of predicted pneumonia readmission rates to 32% in the highest predicted decile, a range of 23%. The AUC was 0.63. For comparison, a model with only age and sex had an AUC of 0.51, and a model with all candidate variables had an AUC equal to 0.63 (Table 2).
| Calibration (0, 1)* | Discrimination | Residuals Lack of Fit (Pearson Residual Fall %) | Model 2 (No. of Covariates) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictive Ability (Lowest Decile, Highest Decile) | AUC | (<2) | (2, 0) | (0, 2) | (2+) | ||||
| |||||||||
| Development sample | |||||||||
| 2006 | (1st half) N = 226,545 | (0, 1) | (0.09, 0.32) | 0.63 | 0 | 82.62 | 7.39 | 9.99 | 6,843 (40) |
| Validation sample | |||||||||
| 2006 | (2nd half) N = 226,706 | (0.002, 0.997) | (0.09, 0.31) | 0.63 | 0 | 82.55 | 7.45 | 9.99 | 6,870 (40) |
| 2005 | N = 536,015 | (0.035, 1.008) | (0.08, 0.31) | 0.63 | 0 | 82.67 | 7.31 | 10.03 | 16,241 (40) |
Hospital Risk‐Standardized Readmission Rates
Risk‐standardized readmission rates varied across hospitals (Figure 3). Median risk‐standardized readmission rate was 17.3%, and the 25th and 75th percentiles were 16.9% and 17.9%, respectively. The 5th percentile was 16.0% and the 95th percentile was 19.1%. Odds of readmission for a hospital one standard deviation above average was 1.4 times that of a hospital one standard deviation below average.

Administrative Model Validation
In the remaining 50% of pneumonia index hospitalizations from 2006, and the entire 2005 cohort, regression coefficients and standard errors of model variables were similar to those in the development data set. Model performance using 2005 data was consistent with model performance using the 2006 development and validation half‐samples (Table 2).
Medical Record Validation
After exclusions, the medical record sample taken from the National Pneumonia Project included 47,429 cases, with an unadjusted 30‐day readmission rate of 17.0%. The final medical record risk‐adjustment model included a total of 35 variables, whose prevalence and association with readmission risk varied modestly (Table 3). Performance of the medical record and administrative models was similar (areas under the ROC curve 0.59 and 0.63, respectively) (Table 4). Additionally, in the administrative model, predicted readmission rates ranged from 8% in the lowest predicted decile to 30% in the highest predicted decile, while in the medical record model, the corresponding rates varied from 10% to 26%.
| Variable | Percent | Estimate | Standard Error | Odds Ratio | 95% CI | |
|---|---|---|---|---|---|---|
| ||||||
| Age 65, mean (SD) | 15.24 (7.87) | 0.003 | 0.002 | 0.997 | 0.993 | 1.000 |
| Male | 46.18 | 0.122 | 0.025 | 1.130 | 1.075 | 1.188 |
| Nursing home resident | 17.71 | 0.035 | 0.037 | 1.036 | 0.963 | 1.114 |
| Neoplastic disease | 6.80 | 0.130 | 0.049 | 1.139 | 1.034 | 1.254 |
| Liver disease | 1.04 | 0.089 | 0.123 | 0.915 | 0.719 | 1.164 |
| History of heart failure | 28.98 | 0.234 | 0.029 | 1.264 | 1.194 | 1.339 |
| History of renal disease | 8.51 | 0.188 | 0.047 | 1.206 | 1.100 | 1.323 |
| Altered mental status | 17.95 | 0.009 | 0.034 | 1.009 | 0.944 | 1.080 |
| Pleural effusion | 21.20 | 0.165 | 0.030 | 1.179 | 1.111 | 1.251 |
| BUN 30 mg/dl | 23.28 | 0.160 | 0.033 | 1.174 | 1.100 | 1.252 |
| BUN missing | 14.56 | 0.101 | 0.185 | 0.904 | 0.630 | 1.298 |
| Systolic BP <90 mmHg | 2.95 | 0.068 | 0.070 | 1.070 | 0.932 | 1.228 |
| Systolic BP missing | 11.21 | 0.149 | 0.425 | 1.160 | 0.504 | 2.669 |
| Pulse 125/min | 7.73 | 0.036 | 0.047 | 1.036 | 0.945 | 1.137 |
| Pulse missing | 11.22 | 0.210 | 0.405 | 1.234 | 0.558 | 2.729 |
| Respiratory rate 30/min | 16.38 | 0.079 | 0.034 | 1.082 | 1.012 | 1.157 |
| Respiratory rate missing | 11.39 | 0.204 | 0.240 | 1.226 | 0.765 | 1.964 |
| Sodium <130 mmol/L | 4.82 | 0.136 | 0.057 | 1.145 | 1.025 | 1.280 |
| Sodium missing | 14.39 | 0.049 | 0.143 | 1.050 | 0.793 | 1.391 |
| Glucose 250 mg/dl | 5.19 | 0.005 | 0.057 | 0.995 | 0.889 | 1.114 |
| Glucose missing | 15.44 | 0.156 | 0.105 | 0.855 | 0.696 | 1.051 |
| Hematocrit <30% | 7.77 | 0.270 | 0.044 | 1.310 | 1.202 | 1.428 |
| Hematocrit missing | 13.62 | 0.071 | 0.135 | 0.932 | 0.715 | 1.215 |
| Creatinine 2.5 mg/dL | 4.68 | 0.109 | 0.062 | 1.115 | 0.989 | 1.258 |
| Creatinine missing | 14.63 | 0.200 | 0.167 | 1.221 | 0.880 | 1.695 |
| WBC 6‐12 b/L | 38.04 | 0.021 | 0.049 | 0.979 | 0.889 | 1.079 |
| WBC >12 b/L | 41.45 | 0.068 | 0.049 | 0.934 | 0.848 | 1.029 |
| WBC missing | 12.85 | 0.167 | 0.162 | 1.181 | 0.860 | 1.623 |
| Immunosuppressive therapy | 15.01 | 0.347 | 0.035 | 1.415 | 1.321 | 1.516 |
| Chronic lung disease | 42.16 | 0.137 | 0.028 | 1.147 | 1.086 | 1.211 |
| Coronary artery disease | 39.57 | 0.150 | 0.028 | 1.162 | 1.100 | 1.227 |
| Diabetes mellitus | 20.90 | 0.137 | 0.033 | 1.147 | 1.076 | 1.223 |
| Alcohol/drug abuse | 3.40 | 0.099 | 0.071 | 0.906 | 0.788 | 1.041 |
| Dementia/Alzheimer's disease | 16.38 | 0.125 | 0.038 | 1.133 | 1.052 | 1.222 |
| Splenectomy | 0.44 | 0.016 | 0.186 | 1.016 | 0.706 | 1.463 |
| Model | Calibration (0, 1)* | Discrimination | Residuals Lack of Fit (Pearson Residual Fall %) | Model 2 (No. of Covariates) | ||||
|---|---|---|---|---|---|---|---|---|
| Predictive Ability (Lowest Decile, Highest Decile) | AUC | (<2) | (2, 0) | (0, 2) | (2+) | |||
| ||||||||
| Medical Record Model Development Sample (NP) | ||||||||
| N = 47,429 No. of 30‐day readmissions = 8,042 | (1, 0) | (0.10, 0.26) | 0.59 | 0 | 83.04 | 5.28 | 11.68 | 710 (35) |
| Linked Administrative Model Validation Sample | ||||||||
| N = 47,429 No. of 30‐day readmissions = 8,042 | (1, 0) | (0.08, 0.30) | 0.63 | 0 | 83.04 | 6.94 | 10.01 | 1,414 (40) |
The correlation coefficient of the estimated state‐specific standardized readmission rates from the administrative and medical record models was 0.96, and the proportion of the variance explained by the model was 0.92 (Figure 4).

DISCUSSION
We have described the development, validation, and results of a hospital, 30‐day, risk‐standardized readmission model for pneumonia that was created to support current federal transparency initiatives. The model uses administrative claims data from Medicare fee‐for‐service patients and produces results that are comparable to a model based on information obtained through manual abstraction of medical records. We observed an overall 30‐day readmission rate of 17%, and our analyses revealed substantial variation across US hospitals, suggesting that improvement by lower performing institutions is an achievable goal.
Because more than one in six pneumonia patients are rehospitalized shortly after discharge, and because pneumonia hospitalizations represent an enormous expense to the Medicare program, prevention of readmissions is now widely recognized to offer a substantial opportunity to improve patient outcomes while simultaneously lowering health care costs. Accordingly, promotion of strategies to reduce readmission rates has become a key priority for payers and quality‐improvement organizations. These range from policy‐level attempts to stimulate change, such as publicly reporting hospital readmission rates on government websites, to establishing accreditation standardssuch as the Joint Commission's requirement to accurately reconcile medications, to the creation of quality improvement collaboratives focused on sharing best practices across institutions. Regardless of the approach taken, a valid, risk‐adjusted measure of performance is required to evaluate and track performance over time. The measure we have described meets the National Quality Forum's measure evaluation criteria in that it addresses an important clinical topic for which there appears to be significant opportunities for improvement, the measure is precisely defined and has been subjected to validity and reliability testing, it is risk‐adjusted based on patient clinical factors present at the start of care, is feasible to produce, and is understandable by a broad range of potential users.21 Because hospitalists are the physicians primarily responsible for the care of patients with pneumonia at US hospitals, and because they frequently serve as the physician champions for quality improvement activities related to pneumonia, it is especially important that they maintain a thorough understanding of the measures and methodologies underlying current efforts to measure hospital performance.
Several features of our approach warrant additional comment. First, we deliberately chose to measure all readmission events rather than attempt to discriminate between potentially preventable and nonpreventable readmissions. From the patient perspective, readmission for any reason is a concern, and limiting the measure to pneumonia‐related readmissions could make it susceptible to gaming by hospitals. Moreover, determining whether a readmission is related to a potential quality problem is not straightforward. For example, a patient with pneumonia whose discharge medications were prescribed incorrectly may be readmitted with a hip fracture following an episode of syncope. It would be inappropriate to treat this readmission as unrelated to the care the patient received for pneumonia. Additionally, while our approach does not presume that every readmission is preventable, the goal is to reduce the risk of readmissions generally (not just in narrowly defined subpopulations), and successful interventions to reduce rehospitalization have typically demonstrated reductions in all‐cause readmission.9, 22 Second, deaths that occurred within 30 days of discharge, yet that were not accompanied by a hospital readmission, were not counted as a readmission outcome. While it may seem inappropriate to treat a postdischarge death as a nonevent (rather than censoring or excluding such cases), alternative analytic approaches, such as using a hierarchical survival model, are not currently computationally feasible with large national data sets. Fortunately, only a relatively small proportion of discharges fell into this category (5.2% of index cases in the 2006 development sample died within 30 days of discharge without being readmitted). An alternative approach to handling the competing outcome of death would have been to use a composite outcome of readmission or death. However, we believe that it is important to report the outcomes separately because factors that predict readmission and mortality may differ, and when making comparisons across hospitals it would not be possible to determine whether differences in rate were due to readmission or mortality. Third, while the patient‐level readmission model showed only modest discrimination, we intentionally excluded covariates such as race and socioeconomic status, as well as in‐hospital events and potential complications of care, and whether patients were discharged home or to a skilled nursing facility. While these variables could have improved predictive ability, they may be directly or indirectly related to quality or supply factors that should not be included in a model that seeks to control for patient clinical characteristics. For example, if hospitals with a large share of poor patients have higher readmission rates, then including income in the model will obscure differences that are important to identify. While we believe that the decision to exclude such factors in the model is in the best interest of patients, and supports efforts to reduce health inequality in society more generally, we also recognize that hospitals that care for a disproportionate share of poor patients are likely to require additional resources to overcome these social factors. Fourth, we limited the analysis to patients with a principal diagnosis of pneumonia, and chose not to also include those with a principal diagnosis of sepsis or respiratory failure coupled with a secondary diagnosis of pneumonia. While the broader definition is used by CMS in the National Pneumonia Project, that initiative relied on chart abstraction to differentiate pneumonia present at the time of admission from cases developing as a complication of hospitalization. Additionally, we did not attempt to differentiate between community‐acquired and healthcare‐associated pneumonia, however our approach is consistent with the National Pneumonia Project and Pneumonia Patient Outcomes Research Team.18 Fifth, while our model estimates readmission rates at the hospital level, we recognize that readmissions are influenced by a complex and extensive range of factors. In this context, greater cooperation between hospitals and other care providers will almost certainly be required in order to achieve dramatic improvement in readmission rates, which in turn will depend upon changes to the way serious illness is paid for. Some options that have recently been described include imposing financial penalties for early readmission, extending the boundaries of case‐based payment beyond hospital discharge, and bundling payments between hospitals and physicians.2325
Our measure has several limitations. First, our models were developed and validated using Medicare data, and the results may not apply to pneumonia patients less than 65 years of age. However, most patients hospitalized with pneumonia in the US are 65 or older. In addition, we were unable to test the model with a Medicare managed care population, because data are not currently available on such patients. Finally, the medical record‐based validation was conducted by state‐level analysis because the sample size was insufficient to carry this out at the hospital level.
In conclusion, more than 17% of Medicare beneficiaries are readmitted within 30 days following discharge after a hospitalization for pneumonia, and rates vary substantially across institutions. The development of a valid measure of hospital performance and public reporting are important first steps towards focusing attention on this problem. Actual improvement will now depend on whether hospitals and partner organizations are successful at identifying and implementing effective methods to prevent readmission.
- ,,.Rehospitalizations among patients in the Medicare Fee‐for‐Service Program.N Engl J Med.2009;360(14):1418–1428.
- Medicare Payment Advisory Commission.Report to the Congress: Promoting Greater Efficiency in Medicare.2007.
- ,,,,. HCUP Facts and Figures: Statistics on Hospital‐based Care in the United States, 2007.2009. Available at: http://www.hcup‐us.ahrq.gov/reports.jsp. Accessed November 7, 2009.
- Centers for Medicare 353(3):255–264.
- ,,,.Trends in postdischarge mortality and readmissions: has length of stay declined too far?Arch Intern Med.2004;164(5):538–544.
- ,,,.Short‐term outcomes and their predictors for patients hospitalized with community‐acquired pneumonia.Heart Lung.2004;33(5):301–307.
- ,,, et al.Improved clinical outcomes with utilization of a community‐acquired pneumonia guideline.Chest.2006;130(3):794–799.
- ,,,,.Associations between initial antimicrobial therapy and medical outcomes for hospitalized elderly patients with pneumonia.Arch Intern Med.1999;159(21):2562–2572.
- ,.Hospital readmissions as a measure of quality of health care: advantages and limitations.Arch Intern Med.2000;160(8):1074–1081.
- ,,,.The care transitions intervention: results of a randomized controlled trial.Arch Intern Med.2006;166(17):1822–1828.
- Corrigan JM, Eden J, Smith BM, eds.Leadership by Example: Coordinating Government Roles in Improving Health Care Quality. Committee on Enhancing Federal Healthcare Quality Programs.Washington, DC:National Academies Press,2003.
- Medicare.gov—Hospital Compare. Available at: http://www.hospitalcompare.hhs.gov/Hospital/Search/Welcome.asp?version=default1(1):29–37.
- ,,,,.Measuring performance for treating heart attacks and heart failure: the case for outcomes measurement.Health Aff.2007;26(1):75–85.
- NQF‐Endorsed® Standards. Available at: http://www.qualityforum.org/Measures_List.aspx. Accessed November 6,2009.
- ,,,,.Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community‐acquired pneumonia.Arch Intern Med.2004;164(6):637–644.
- ,,. Diagnostic Cost Group Hierarchical Condition Category Models for Medicare Risk Adjustment. Report prepared for the Health Care Financing Administration. Health Economics Research, Inc;2000. Available at: http://www.cms.hhs.gov/Reports/Reports/ItemDetail.asp?ItemID=CMS023176. Accessed November 7, 2009.
- .Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis.1st ed.New York:Springer;2006.
- National Quality Forum—Measure Evaluation Criteria.2008. Available at: http://www.qualityforum.org/uploadedFiles/Quality_Forum/Measuring_Performance/Consensus_Development_Process%E2%80%99s_Principle/EvalCriteria2008–08‐28Final.pdf?n=4701.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial.JAMA.1999;281(7):613–620.
- .Paying for care episodes and care coordination.N Engl J Med.2007;356(11):1166–1168.
- .Health care reform—toward more freedom, and responsibility, for physicians.N Engl J Med.2009;361(6):623–628.
- .Beyond pay for performance—emerging models of provider‐payment reform.N Engl J Med.2008;359(12):1197–1200.
- ,,.Rehospitalizations among patients in the Medicare Fee‐for‐Service Program.N Engl J Med.2009;360(14):1418–1428.
- Medicare Payment Advisory Commission.Report to the Congress: Promoting Greater Efficiency in Medicare.2007.
- ,,,,. HCUP Facts and Figures: Statistics on Hospital‐based Care in the United States, 2007.2009. Available at: http://www.hcup‐us.ahrq.gov/reports.jsp. Accessed November 7, 2009.
- Centers for Medicare 353(3):255–264.
- ,,,.Trends in postdischarge mortality and readmissions: has length of stay declined too far?Arch Intern Med.2004;164(5):538–544.
- ,,,.Short‐term outcomes and their predictors for patients hospitalized with community‐acquired pneumonia.Heart Lung.2004;33(5):301–307.
- ,,, et al.Improved clinical outcomes with utilization of a community‐acquired pneumonia guideline.Chest.2006;130(3):794–799.
- ,,,,.Associations between initial antimicrobial therapy and medical outcomes for hospitalized elderly patients with pneumonia.Arch Intern Med.1999;159(21):2562–2572.
- ,.Hospital readmissions as a measure of quality of health care: advantages and limitations.Arch Intern Med.2000;160(8):1074–1081.
- ,,,.The care transitions intervention: results of a randomized controlled trial.Arch Intern Med.2006;166(17):1822–1828.
- Corrigan JM, Eden J, Smith BM, eds.Leadership by Example: Coordinating Government Roles in Improving Health Care Quality. Committee on Enhancing Federal Healthcare Quality Programs.Washington, DC:National Academies Press,2003.
- Medicare.gov—Hospital Compare. Available at: http://www.hospitalcompare.hhs.gov/Hospital/Search/Welcome.asp?version=default1(1):29–37.
- ,,,,.Measuring performance for treating heart attacks and heart failure: the case for outcomes measurement.Health Aff.2007;26(1):75–85.
- NQF‐Endorsed® Standards. Available at: http://www.qualityforum.org/Measures_List.aspx. Accessed November 6,2009.
- ,,,,.Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community‐acquired pneumonia.Arch Intern Med.2004;164(6):637–644.
- ,,. Diagnostic Cost Group Hierarchical Condition Category Models for Medicare Risk Adjustment. Report prepared for the Health Care Financing Administration. Health Economics Research, Inc;2000. Available at: http://www.cms.hhs.gov/Reports/Reports/ItemDetail.asp?ItemID=CMS023176. Accessed November 7, 2009.
- .Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis.1st ed.New York:Springer;2006.
- National Quality Forum—Measure Evaluation Criteria.2008. Available at: http://www.qualityforum.org/uploadedFiles/Quality_Forum/Measuring_Performance/Consensus_Development_Process%E2%80%99s_Principle/EvalCriteria2008–08‐28Final.pdf?n=4701.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial.JAMA.1999;281(7):613–620.
- .Paying for care episodes and care coordination.N Engl J Med.2007;356(11):1166–1168.
- .Health care reform—toward more freedom, and responsibility, for physicians.N Engl J Med.2009;361(6):623–628.
- .Beyond pay for performance—emerging models of provider‐payment reform.N Engl J Med.2008;359(12):1197–1200.
Copyright © 2010 Society of Hospital Medicine
Macrolides and Quinolones for AECOPD
Acute exacerbations of chronic obstructive pulmonary disease (AECOPD) are responsible for more than 600,000 hospitalizations annually, resulting in direct costs of over $20 billion.1 Bacterial infections appear responsible for 50% of such exacerbations,25 and current COPD guidelines recommend treatment with antibiotics for patients with severe exacerbations or a change in sputum.1,69 These recommendations are based on a number of small randomized trials, most of which were conducted more than 20 years ago using narrow spectrum antibiotics that are no longer commonly used.10 Only 4 studies, totaling 321 subjects, included hospitalized patients, and most studies excluded patients who required steroids. Because no clinical trials have compared different antibiotic regimens for AECOPD, existing guidelines offer a range of treatment options, including amoxicillin‐clavulonate, macrolides, quinolones, cephalosporins, aminopenicillins, and tetracyclines.
Among hospitalized patients, macrolides and quinolones appear to be the most frequently prescribed antibiotics.11 Both are available in oral formulations, have excellent bioavailability, and are administered once daily. In addition to their antimicrobial activity, macrolides are believed to have antiinflammatory effects, which could be especially advantageous in AECOPD.1214 In trials of chronic bronchitis, however, fluoroquinolones have been shown to reduce the risk of recurrent exacerbation when compared to macrolides.15 The wide variation that has been observed in antibiotic selection for patients hospitalized for AECOPD suggests a high degree of uncertainty among clinicians about the benefits of different treatment options.11 Given the limited evidence from randomized trials, we sought to evaluate the comparative effectiveness of macrolides and quinolones among a large, representative sample of patients hospitalized with AECOPD.
Subjects and Methods
Setting and Subjects
We conducted a retrospective cohort study of all patients hospitalized between January 1 and December 31, 2001 for AECOPD at any 1 of 375 acute care facilities in the United States that participated in Premier's Perspective, a voluntary, fee‐supported database developed for measuring quality and healthcare utilization. Participating hospitals represent all geographical regions, and are primarily small‐sized to medium‐sized nonteaching hospitals located mostly in urban areas. In addition to the information contained in the standard hospital discharge file (Uniform Billing 92) such as patient age, International Classification of Disease, 9th Edition, Clinical Modification (ICD‐9‐CM) codes, the Perspective database includes a date‐stamped log of all billed items, including diagnostic tests, medications, and other treatments, as well as costs, for individual patients. The study was approved by the Institutional Review Board of Baystate Medical Center.
Patients were included if they had a primary diagnosis consistent with AECOPD (ICD‐9 codes 491.21 and 493.22) or a primary diagnosis of respiratory failure (ICD‐9 codes 518.81 and 518.84) paired with secondary diagnosis of AECOPD; they also had to receive at least 2 consecutive days of either a macrolide or a quinolone, started within 48 hours of hospitalization. Patients receiving both antibiotics were excluded, but patients who received additional antibiotics were included. To enhance the specificity of our diagnosis codes, we limited our study to patients age 40 years.16 Because mechanical ventilation initiated after hospital day 2 was an outcome measure, we excluded patients admitted directly to the intensive care unit. We also excluded: those with other bacterial infections, such as pneumonia or cellulitis, who might have another indication for antibiotics; those with a length of stay <2 days, because we could not ascertain whether they received a full course of antibiotics; patients with a secondary diagnosis of pulmonary embolism or pneumothorax; and those whose attending physicians were not internists, family practitioners, hospitalists, pulmonologists, or intensivists. For patients with more than 1 admission during the study period, we included only the first admission.
Data Elements
For each patient, we assessed age, gender, race, marital and insurance status, principal diagnosis, comorbidities, and specialty of the attending physician. Comorbidities were identified from ICD‐9 secondary diagnosis codes and Diagnosis Related Groups using Healthcare Cost and Utilization Project Comorbidity Software (version 3.1), based on the work of Elixhauser et al.17 In addition, to assess disease severity we recorded the presence of chronic pulmonary heart disease, the number of admissions for COPD during the 12 months prior to the index admission, and arterial blood gas testing.18,19 We also identified pharmacy or diagnostic charges for interventions that were recommended in current guidelines (beta‐adrenergic and anticholinergic bronchodilators, steroids, and noninvasive positive‐pressure ventilation); those that were not recommended or were of uncertain benefit (methylxanthine bronchodilators, spirometry/pulmonary function testing, mucolytic medications, chest physiotherapy, and sputum testing); and drugs that might be associated with severe exacerbations or end‐stage COPD (loop diuretics, morphine, and nutritional supplements).1,69 Hospitals were categorized by region (Northeast, South, Midwest, or West), bed size, setting (urban vs. rural), ownership, and teaching status.
Antibiotic Class and Outcome Variables
Our primary predictor variable was the antibiotic initiated during the first 2 hospital days and continued for at least 2 days, regardless of other antibiotics the patient may have received during the course of hospitalization. Because we anticipated low in‐hospital mortality, our primary outcome was a composite measure of treatment failure, defined as initiation of mechanical ventilation after hospital day 2, in‐hospital mortality, or readmission for COPD within 30 days of discharge.20 Secondary outcomes included hospital costs and length of stay, as well as allergic reactions identified by ICD‐9 code, and antibiotic‐associated diarrhea, defined as treatment with either metronidazole or oral vancomycin begun after hospital day 3.
Statistical Analysis
Summary statistics were computed using frequencies and percents for categorical variables; and means, medians, standard deviations, and interquartile ranges for continuous variables. Associations between antibiotic selection and patient and hospital characteristics were assessed using chi‐square tests for categorical variables and z‐tests for continuous variables.
We developed a series of multivariable models to evaluate the impact of initial antibiotic selection on the risk of treatment failure, length of stay, and total cost. In order to account for the effects of within‐hospital correlation, generalized estimating equation (GEE) models with a logit link were used to assess the effect of antibiotic selection on the risk of treatment failure, and identity link models were used for analyses of length of stay and cost. Unadjusted and covariate‐adjusted models for treatment failure were evaluated with and without adjustments for propensity score. A propensity score is the probability that a given patient would receive treatment with a macrolide, derived from a nonparsimonious model in which treatment with a macrolide was considered the outcome. The propensity model included all patient characteristics, other early treatments and tests, comorbidities, hospital and physician characteristics, and selected interaction terms.21 Length of stay and cost were trimmed at 3 standard deviations above the mean, and natural log‐transformed values were modeled due to extreme positive skew. In addition, we carried out matched analyses in which we compared the outcomes of patients who were treated with a macrolide to those with similar propensity scores (ie, with similar likelihood of receiving a macrolide) who received a quinolone.22
Finally, to reduce the threat of residual confounding by indication, which can occur if sicker patients are more likely to receive a particular antibiotic, we developed a grouped treatment model, in which all patients treated at the same hospital were assigned a probability of treatment with a macrolide equal to the overall treatment rate at that hospital.23 This is an adaptation of instrumental variable analysis, a well‐accepted technique in econometrics with growing use in health care.24,25 It attempts to assess whether patients treated at a hospital at which quinolones are used more frequently have better outcomes than patients treated at hospitals at which macrolides are used more frequently, while adjusting for other patient, physician, and hospital variables. It ignores the actual treatment the patient received, and instead substitutes the hospital's rate of macrolide use. By grouping treatment at the hospital level, this method greatly reduces the possibility of residual selection bias, unless hospitals that use a lot of macrolides have patients who differ in a consistent way from hospitals which use mostly quinolones.
All analyses were performed using SAS version 9.1 (SAS Institute, Inc., Cary, NC).
Results
Of 26,248 AECOPD patients treated with antibiotics, 19,608 patients met the inclusion criteria; of these, 6139 (31%) were treated initially with a macrolide; the median age was 70 years; 60% were female; and 78% were white. A total of 86% of patients had a primary diagnosis of obstructive chronic bronchitis with acute exacerbation, and 6% had respiratory failure. The most common comorbidities were hypertension, diabetes, and congestive heart failure. Twenty‐two percent had been admitted at least once in the preceding 12 months. Treatment failure occurred in 7.7% of patients, and 1.3% died in the hospital. Mean length of stay was 4.8 days. Hospital prescribing rates for macrolides varied from 0% to 100%, with a mean of 33% and an interquartile range of 14% to 46% (Supporting Appendix Figure 1).
Compared to patients receiving macrolides, those receiving quinolones were older, more likely to have respiratory failure, to be cared for by a pulmonologist, and to have an admission in the previous year (Table 1). They were also more likely to be treated with bronchodilators, methylxanthines, steroids, diuretics, and noninvasive positive pressure ventilation, and to have an arterial blood gas, but less likely to receive concomitant treatment with a cephalosporin (11% vs. 57%). With the exception of cephalosporin treatment, these differences were small, but due to the large sample were statistically significant. Comorbidities were similar in both groups. Patients in the quinolone group were also more likely to experience treatment failure (8.1% vs. 6.8%), death (1.5% vs. 1.0%), and antibiotic‐associated diarrhea (1.1% vs. 0.5%).
| Complete Cohort | Propensity‐matched Subsample | |||||
|---|---|---|---|---|---|---|
| Characteristic | Quinolone (n = 13469) | Macrolide (n = 6139) | P Value | Quinolone (n = 5610) | Macrolide (n = 5610) | P Value |
| ||||||
| Antibiotics received during hospitalization* [n (%)] | ||||||
| Macrolide | 264 (2) | 6139 (100) | 119 (2) | 5610 (100) | ||
| Quinolone | 13469 (100) | 459 (8) | 5610 (100) | 424 (8) | ||
| Cephalosporin | 1696 (13) | 3579 (59) | <0.001 | 726 (13) | 3305 (59) | <0.001 |
| Tetracycline | 231 (2) | 75 (2) | 0.01 | 101 (2) | 73 (2) | 0.06 |
| Other antibiotics | 397 (3) | 220 (4) | 0.02 | 166 (3) | 193 (3) | 0.03 |
| Age (years) (mean [SD]) | 69.1 (11.4) | 68.2 (11.8) | <0.001 | 68.6 (11.7) | 68.5 (11.7) | 0.58 |
| Male sex (n [%]) | 5447 (40) | 2440 (40) | 0.36 | 2207 (39) | 2196 (39) | 0.85 |
| Race/ethnic group (n [%]) | <0.001 | 0.44 | ||||
| White | 10454 (78) | 4758 (78) | 4359 (78) | 4368 (78) | ||
| Black | 1060 (8) | 540 (9) | 470 (8) | 455 (8) | ||
| Hispanic | 463 (3) | 144 (2) | 157 (3) | 134 (2) | ||
| Other | 1492 (11) | 697 (11) | 624 (11) | 653 (12) | ||
| Primary diagnosis (n [%]) | <0.001 | 0.78 | ||||
| Obstructive chronic bronchitis with acute exacerbation | 11650 (87) | 5298 (86) | 4884 (87) | 4860 (87) | ||
| Chronic obstructive asthma/asthma with COPD | 908 (7) | 569 (9) | 466 (8) | 486 (9) | ||
| Respiratory failure | 911 (7) | 272 (4) | 260 (5) | 264 (5) | ||
| Admissions in the prior year (n [%]) | <0.001 | 0.84 | ||||
| 0 | 9846 (73) | 4654 (76) | 4249 (76) | 4231 (75) | ||
| 1 | 1918 (14) | 816 (13) | 747 (13) | 750 (13) | ||
| 2+ | 1085 (8) | 445 (7) | 397 (7) | 420 (8) | ||
| Missing | 620 (5) | 224 (4) | 217 (4) | 209 (4) | ||
| Physician specialty (n [%]) | <0.001 | 0.84 | ||||
| Internal medicine/hospitalist | 7069 (53) | 3321 (54) | 3032 (54) | 3072 (55) | ||
| Family/general medicine | 3569 (27) | 2074 (34) | 1824 (33) | 1812 (32) | ||
| Pulmonologist | 2776 (21) | 727 (12) | 738 (13) | 711 (13) | ||
| Critical care/emntensivist | 55 (0) | 17 (0) | 16 (0) | 15 (0) | ||
| Tests on hospital day 1 or 2 (n [%]) | ||||||
| Arterial blood gas | 8084 (60) | 3377 (55) | <0.001 | 3195 (57) | 3129 (56) | 0.22 |
| Sputum test | 1741 (13) | 766 (13) | 0.39 | 20 (0) | 16 (0) | 0.62 |
| Medications/therapies on hospital day 1 or 2 (n [%]) | ||||||
| Short‐acting bronchodilators | 7555 (56) | 3242 (53) | <0.001 | 2969 (53) | 2820 (50) | 0.005 |
| Long‐acting beta‐2 agonists | 2068 (15) | 748 (12) | <0.001 | 704 (13) | 719 (13) | 0.69 |
| Methylxanthine bronchodilators | 3051 (23) | 1149 (19) | <0.001 | 1102 (20) | 1093 (20) | 0.85 |
| Steroids | 0.04 | 0.68 | ||||
| Intravenous | 11148 (83) | 4989 (81) | 4547 (81) | 4581 (82) | ||
| Oral | 772 (6) | 376 (6) | 334 (6) | 330 (6) | ||
| Severity indicators (n [%]) | ||||||
| Chronic pulmonary heart disease | 890 (7) | 401 (7) | 0.85 | 337 (6) | 368 (7) | 0.24 |
| Sleep apnea | 586 (4) | 234 (4) | 0.08 | 211 (4) | 218 (4) | 0.77 |
| Noninvasive positive pressure ventilation | 391 (3) | 128 (2) | <0.001 | 128 (2) | 114 (2) | 0.40 |
| Loop diuretics | 4838 (36) | 1971 (32) | <0.001 | 1884 (34) | 1862 (33) | 0.67 |
| Hospital characteristics (n [%]) | ||||||
| Staffed beds | <0.001 | 0.71 | ||||
| 6200 | 3483 (26) | 1688 (28) | 1610 (29) | 1586 (28) | ||
| 201300 | 3132 (23) | 1198 (20) | 1174 (21) | 1154 (21) | ||
| 301500 | 4265 (32) | 2047 (33) | 1809 (32) | 1867 (33) | ||
| 500+ | 2589 (19) | 1206 (20) | 1017 (18) | 1003 (18) | ||
| Hospital region (n [%]) | <0.001 | 0.65 | ||||
| South | 8562 (64) | 3270 (53) | 3212 (57) | 3160 (56) | ||
| Midwest | 2602 (19) | 1444 (24) | 1170 (21) | 1216 (22) | ||
| Northeast | 1163 (9) | 871 (14) | 687 (12) | 704 (13) | ||
| West | 1142 (9) | 554 (9) | 541 (10) | 530 (9) | ||
| Teaching hospital | <0.001 | 0.63 | ||||
| No | 12090 (90) | 5037 (82) | 4896 (87) | 4878 (87) | ||
| Yes | 1379 (10) | 1102 (18) | 714 (13) | 732 (13) | ||
| Comorbidities (n [%]) | ||||||
| Congestive heart failure | 2673 (20) | 1147 (19) | 0.06 | 1081 (19) | 1060 (19) | 0.63 |
| Metastatic cancer | 134 (1) | 27 (0) | <0.001 | 34 (1) | 38 (1) | 0.72 |
| Depression | 1419 (11) | 669 (11) | 0.45 | 598 (11) | 603 (11) | 0.90 |
| Deficiency anemias | 1155 (9) | 476 (8) | 0.05 | 426 (8) | 432 (8) | 0.86 |
| Solid tumor without metastasis | 1487 (11) | 586 (10) | 0.002 | 550 (10) | 552 (10) | 0.97 |
| Hypothyroidism | 1267 (9) | 527 (9) | 0.07 | 481 (9) | 482 (9) | 1.00 |
| Peripheral vascular disease | 821 (6) | 312 (5) | 0.005 | 287 (5) | 288 (5) | 1.00 |
| Paralysis | 165 (1) | 46 (1) | 0.003 | 49 (1) | 51 (1) | 0.92 |
| Obesity | 957 (7) | 435 (7) | 0.98 | 386 (7) | 398 (7) | 0.68 |
| Hypertension | 5793 (43) | 2688 (44) | 0.31 | 2474 (44) | 2468 (44) | 0.92 |
| Diabetes | 0.04 | 0.45 | ||||
| Without chronic complications | 2630 (20) | 1127 (18) | 1057 (19) | 1066 (19) | ||
| With chronic complications | 298 (2) | 116 (2) | 115 (2) | 97 (2) | ||
In the unadjusted analysis, compared to patients receiving quinolones, those treated with macrolides were less likely to experience treatment failure (OR, 0.83; 95% CI, 0.740.94) (Table 2). Adjusting for all patient, hospital, and physician covariates, including the propensity for treatment with macrolides, increased the OR to 0.89 and the results were no longer significant (95% CI, 0.781.01). Propensity matching successfully balanced all measured covariates except for the use of short‐acting bronchodilators and additional antibiotics (Table 1). In the propensity‐matched sample (Figure 1), quinolone‐treated patients were more likely to experience antibiotic‐associated diarrhea (1.2% vs. 0.6%; P = 0.0003) and late mechanical ventilation (1.3% vs. 0.8%; P = 0.02). There were no differences in adjusted cost or length of stay between the 2 groups. The results of the grouped treatment analysis, substituting the hospital's specific rate of macrolide use in place of the actual treatment that each patient received suggested that the 2 antibiotics were associated with similar rates of treatment failure. The OR for a 100% hospital rate of macrolide treatment vs. a 0% rate was 1.01 (95% CI, 0.751.35).
| Treatment Failure | Cost | LOS | ||||
|---|---|---|---|---|---|---|
| Models | OR | 95% CI | Ratio | 95% CI | Ratio | 95% CI |
| ||||||
| Unadjusted | 0.83 | 0.730.93 | 0.98 | 0.971.00 | 0.96 | 0.950.98 |
| Adjusted for propensity score only* | 0.89 | 0.791.01 | 1.00 | 0.981.01 | 0.98 | 0.971.00 |
| Adjusted for covariates | 0.87 | 0.770.99 | 1.00 | 0.991.02 | 0.99 | 0.971.00 |
| Adjusted for covariates and propensity score | 0.89 | 0.781.01 | 1.00 | 0.991.02 | 0.98 | 0.971.00 |
| Matched sample, unadjusted | 0.87 | 0.751.00 | 0.99 | 0.981.01 | 0.99 | 0.971.01 |
| Matched sample, adjusted for unbalanced variables | 0.87 | 0.751.01 | 1.00 | 0.981.02 | 0.99 | 0.971.01 |
| Grouped treatment model, unadjusted | 0.90 | 0.681.19 | 0.97 | 0.891.06 | 0.92 | 0.870.96 |
| Group treatment model, adjusted for covariates∥ | 1.01 | 0.751.35 | 0.96 | 0.881.05 | 0.96 | 0.911.00 |
Discussion
In this large observational study conducted at 375 hospitals, we took advantage of a natural experiment in which antibiotic prescribing patterns varied widely across hospitals to compare the effectiveness of 2 common antibiotic regimens for AECOPD. Treatment with macrolides and quinolones were associated with a similar risk of treatment failure, costs, and length of stay; however, patients treated with macrolides were less likely to experience late mechanical ventilation or treatment for antibiotic‐associated diarrhea.
Despite broad consensus in COPD guidelines that patients with severe acute exacerbations should receive antibiotics, there is little agreement about the preferred empiric agent. Controversy exists regarding antibiotics' comparative effectiveness, and even over which pathogens cause COPD exacerbations. Given the frequency of hospitalization for AECOPD, understanding the comparative effectiveness of treatments in this setting could have important implications for health outcomes and costs. Unfortunately, most antibiotic studies in AECOPD were conducted >20 years ago, using antibiotics that rarely appeared in our sample.26 Consequently, clinical practice guidelines offer conflicting recommendations. For example, the National Institute for Clinical Excellence recommends empirical treatment with an aminopenicillin, a macrolide, or a tetracycline,8 while the American Thoracic Society recommends amoxicillin‐clavulonate or a fluoroquinolone.9
As might be expected in light of so much uncertainty, we found wide variation in prescribing patterns across hospitals. Overall, approximately one‐third of patients received a macrolide and two‐thirds a quinolone. Both regimens provide adequate coverage of H. influenza, S. pneumoniae, and M. catarrhalis, and conform to at least 1 COPD guideline. Nevertheless, patients receiving macrolides often received a cephalosporin as well; this pattern of treatment suggests that antibiotic selection is likely to have been influenced more by guidelines for the treatment of community‐acquired pneumonia than COPD.
Previous studies comparing antibiotic effectiveness suffer from shortcomings that limit their application to patients hospitalized with AECOPD. First, they enrolled patients with chronic bronchitis, and included patients without obstructive lung disease, and most studies included patients as young as 18 years old. Second, many either did not include treatment with steroids or excluded patients receiving more than 10 mg of prednisone daily. Third, almost all enrolled only ambulatory patients.
While there are no studies comparing quinolones and macrolides in patients hospitalized for AECOPD, a meta‐analysis comparing quinolones, macrolides, and amoxicillin‐clavulonate identified 19 trials of ambulatory patients with chronic bronchitis. That study found that all 3 drugs had similar efficacy initially, but that quinolones resulted in the fewest relapses over a 26‐week period.27 Macrolides and quinolones had similar rates of adverse effects. In contrast, we did not find a difference in treatment failure, cost, or length of stay, but did find a higher rate of diarrhea associated with quinolones. Others have also documented an association between fluoroquinolones and C. difficile diarrhea.2830 This trend, first noted in 2001, is of particular concern because the fluoroquinolone‐resistant strains appear to be hypervirulent and have been associated with nosocomial epidemics.3134
Our study has several limitations. First, its observational design leaves open the possibility of selection bias. For this reason we analyzed our data in several ways, including using a grouped treatment approach, an adaptation of the instrumental variable technique, and accepted only those differences which were consistent across all models. Second, our study used claims data, and therefore we could not directly adjust for physiological measures of severity. However, the highly detailed nature of the data allowed us to adjust for numerous tests and treatments that reflected the clinician's assessment of the patient's severity, as well as the number of prior COPD admissions. Third, we cannot exclude the possibility that some patients may have had concurrent pneumonia without an ICD‐9 code. We think that the number would be small because reimbursement for pneumonia is generally higher than for COPD, so hospitals have an incentive to code pneumonia as the principal diagnosis when present. Finally, we compared initial antibiotics only. More than one‐quarter of our patients received an additional antibiotic before discharge. In particular, patients receiving macrolides were often prescribed a concomitant cephalosporin. We do not know to what extent these additional antibiotics may have affected the outcomes.
Despite the large number of patients hospitalized annually for AECOPD, there are no randomized trials comparing different antibiotics in this population. Studies comparing antibiotics in chronic bronchitis can offer little guidance, since they have primarily focused on proving equivalence between existing antibiotics and newer, more expensive formulations.35 Because many of the patients enrolled in such trials do not benefit from antibiotics at all, either because they do not have COPD or because their exacerbation is not caused by bacteria, it is relatively easy to prove equivalence. Given that AECOPD is one of the leading causes of hospitalization in the United States, large, randomized trials comparing the effectiveness of different antibiotics should be a high priority. In the meantime, macrolides (often given together with cephalosporins) and quinolones appear to be equally effective initial antibiotic choices; considering antibiotic‐associated diarrhea, macrolides appear to be the safer of the 2.
- ,,.Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease.Ann Intern Med.2001;134:595–599.
- ,,, et al.Infectious etiologies in acute exacerbation of COPD.Diagn Microbiol Infect Dis.2001;40:95–102.
- ,.Bacterial infections in patients requiring admission for an acute exacerbation of COPD; a 1‐year prospective study.Respir Med.2003;97:770–777.
- ,,, et al.Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease.Arch Intern Med.2005;165:891–897.
- ,.Infection in the pathogenesis and course of chronic obstructive pulmonary disease.N Engl J Med.2008;359:2355–2365.
- ,,, et al.Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary.Am J Respir Crit Care Med.2007;176:532–555.
- ,,, et al.Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2008 update—highlights for primary care.Can Respir J.2008;15(suppl A):1A–8A.
- Chronic obstructive pulmonary disease.National clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care.Thorax.2004;59(suppl 1):1–232.
- ,,, et al.Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper.Eur Respir J.2004;23:932–946.
- ,,,,.Antibiotics for exacerbations of chronic obstructive pulmonary disease.Cochrane Database Syst Rev.2006:CD004403.
- ,,,,,.Quality of care for patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease.Ann Intern Med.2006;144:894–903.
- ,,, et al.Modulation of neutrophil and inflammation markers in chronic obstructive pulmonary disease by short‐term azithromycin treatment.Eur J Pharmacol.2005;517:132–143.
- ,,, et al.Azithromycin modulates neutrophil function and circulating inflammatory mediators in healthy human subjects.Eur J Pharmacol.2002;450:277–289.
- ,,,,,.The effect of clarithromycin on inflammatory markers in chronic obstructive pulmonary disease: preliminary data.Ann Pharmacother.2004;38:1400–1405.
- ,,,.A comparison of gemifloxacin and clarithromycin in acute exacerbations of chronic bronchitis and long‐term clinical outcomes.Clin Ther.2002;24:639–652.
- ,,,.In‐hospital mortality following acute exacerbations of chronic obstructive pulmonary disease.Arch Intern Med.2003;163:1180–1186.
- ,,,.Comorbidity measures for use with administrative data.Med Care.1998;36:8–27.
- ,,, et al.Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments).Am J Respir Crit Care Med.1996;154:959–967.
- ,,.Mortality and mortality‐related factors after hospitalization for acute exacerbation of COPD.Chest.2003;124:459–467.
- ,,, et al.Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease.N Engl J Med.1999;340:1941–1947.
- .Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group.Stat Med.1998;17:2265–2281.
- .Reducing bias in a propensity score matched‐pair sample using greedy matching techniques. Proceedings of the Twenty‐Sixth Annual SAS Users Group International Conference. Cary, NC: SAS Institute;2001,214–216.
- ,,,.Modeling treatment effects on binary outcomes with grouped‐treatment variables and individual covariates.Am J Epidemiol.2002;156:753–760.
- ,,.Does more intensive treatment of acute myocardial infarction in the elderly reduce mortality? Analysis using instrumental variables. [see Comment].JAMA.1994;272:859–866.
- ,,,,,.Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods.JAMA.2007;297:278–285.
- ,,,.Antibiotics in chronic obstructive pulmonary disease exacerbations. A meta‐analysis.JAMA.1995;273:957–960.
- ,,,,.Macrolides, quinolones and amoxicillin/clavulanate for chronic bronchitis: a meta‐analysis.Eur Respir J.2007;29:1127–1137.
- ,,,,.Antimicrobial‐associated risk factors for Clostridium difficile infection.Clin Infect Dis.2008;46(suppl 1):S19–S31.
- ,,, et al.Short‐term and long‐term outcomes of moxifloxacin compared to standard antibiotic treatment in acute exacerbations of chronic bronchitis.Chest.2004;125:953–964.
- ,,,,.Oral gemifloxacin once daily for 5 days compared with sequential therapy with i.v. ceftriaxone/oral cefuroxime (maximum of 10 days) in the treatment of hospitalized patients with acute exacerbations of chronic bronchitis.Respir Med.2003;97:242–249.
- ,,, et al.A large outbreak of Clostridium difficile‐associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use.Infect Control Hosp Epidemiol.2005;26:273–280.
- ,,, et al.An epidemic, toxin gene‐variant strain of Clostridium difficile.N Engl J Med.2005;353:2433–2441.
- ,,, et al.Outbreak of Clostridium difficile infection in a long‐term care facility: association with gatifloxacin use.Clin Infect Dis.2004;38:640–645.
- ,,, et al.A predominantly clonal multi‐institutional outbreak of Clostridium difficile‐associated diarrhea with high morbidity and mortality.N Engl J Med.2005;353:2442–2449.
- ,.No more equivalence trials for antibiotics in exacerbations of COPD, please.Chest.2004;125:811–813.
Acute exacerbations of chronic obstructive pulmonary disease (AECOPD) are responsible for more than 600,000 hospitalizations annually, resulting in direct costs of over $20 billion.1 Bacterial infections appear responsible for 50% of such exacerbations,25 and current COPD guidelines recommend treatment with antibiotics for patients with severe exacerbations or a change in sputum.1,69 These recommendations are based on a number of small randomized trials, most of which were conducted more than 20 years ago using narrow spectrum antibiotics that are no longer commonly used.10 Only 4 studies, totaling 321 subjects, included hospitalized patients, and most studies excluded patients who required steroids. Because no clinical trials have compared different antibiotic regimens for AECOPD, existing guidelines offer a range of treatment options, including amoxicillin‐clavulonate, macrolides, quinolones, cephalosporins, aminopenicillins, and tetracyclines.
Among hospitalized patients, macrolides and quinolones appear to be the most frequently prescribed antibiotics.11 Both are available in oral formulations, have excellent bioavailability, and are administered once daily. In addition to their antimicrobial activity, macrolides are believed to have antiinflammatory effects, which could be especially advantageous in AECOPD.1214 In trials of chronic bronchitis, however, fluoroquinolones have been shown to reduce the risk of recurrent exacerbation when compared to macrolides.15 The wide variation that has been observed in antibiotic selection for patients hospitalized for AECOPD suggests a high degree of uncertainty among clinicians about the benefits of different treatment options.11 Given the limited evidence from randomized trials, we sought to evaluate the comparative effectiveness of macrolides and quinolones among a large, representative sample of patients hospitalized with AECOPD.
Subjects and Methods
Setting and Subjects
We conducted a retrospective cohort study of all patients hospitalized between January 1 and December 31, 2001 for AECOPD at any 1 of 375 acute care facilities in the United States that participated in Premier's Perspective, a voluntary, fee‐supported database developed for measuring quality and healthcare utilization. Participating hospitals represent all geographical regions, and are primarily small‐sized to medium‐sized nonteaching hospitals located mostly in urban areas. In addition to the information contained in the standard hospital discharge file (Uniform Billing 92) such as patient age, International Classification of Disease, 9th Edition, Clinical Modification (ICD‐9‐CM) codes, the Perspective database includes a date‐stamped log of all billed items, including diagnostic tests, medications, and other treatments, as well as costs, for individual patients. The study was approved by the Institutional Review Board of Baystate Medical Center.
Patients were included if they had a primary diagnosis consistent with AECOPD (ICD‐9 codes 491.21 and 493.22) or a primary diagnosis of respiratory failure (ICD‐9 codes 518.81 and 518.84) paired with secondary diagnosis of AECOPD; they also had to receive at least 2 consecutive days of either a macrolide or a quinolone, started within 48 hours of hospitalization. Patients receiving both antibiotics were excluded, but patients who received additional antibiotics were included. To enhance the specificity of our diagnosis codes, we limited our study to patients age 40 years.16 Because mechanical ventilation initiated after hospital day 2 was an outcome measure, we excluded patients admitted directly to the intensive care unit. We also excluded: those with other bacterial infections, such as pneumonia or cellulitis, who might have another indication for antibiotics; those with a length of stay <2 days, because we could not ascertain whether they received a full course of antibiotics; patients with a secondary diagnosis of pulmonary embolism or pneumothorax; and those whose attending physicians were not internists, family practitioners, hospitalists, pulmonologists, or intensivists. For patients with more than 1 admission during the study period, we included only the first admission.
Data Elements
For each patient, we assessed age, gender, race, marital and insurance status, principal diagnosis, comorbidities, and specialty of the attending physician. Comorbidities were identified from ICD‐9 secondary diagnosis codes and Diagnosis Related Groups using Healthcare Cost and Utilization Project Comorbidity Software (version 3.1), based on the work of Elixhauser et al.17 In addition, to assess disease severity we recorded the presence of chronic pulmonary heart disease, the number of admissions for COPD during the 12 months prior to the index admission, and arterial blood gas testing.18,19 We also identified pharmacy or diagnostic charges for interventions that were recommended in current guidelines (beta‐adrenergic and anticholinergic bronchodilators, steroids, and noninvasive positive‐pressure ventilation); those that were not recommended or were of uncertain benefit (methylxanthine bronchodilators, spirometry/pulmonary function testing, mucolytic medications, chest physiotherapy, and sputum testing); and drugs that might be associated with severe exacerbations or end‐stage COPD (loop diuretics, morphine, and nutritional supplements).1,69 Hospitals were categorized by region (Northeast, South, Midwest, or West), bed size, setting (urban vs. rural), ownership, and teaching status.
Antibiotic Class and Outcome Variables
Our primary predictor variable was the antibiotic initiated during the first 2 hospital days and continued for at least 2 days, regardless of other antibiotics the patient may have received during the course of hospitalization. Because we anticipated low in‐hospital mortality, our primary outcome was a composite measure of treatment failure, defined as initiation of mechanical ventilation after hospital day 2, in‐hospital mortality, or readmission for COPD within 30 days of discharge.20 Secondary outcomes included hospital costs and length of stay, as well as allergic reactions identified by ICD‐9 code, and antibiotic‐associated diarrhea, defined as treatment with either metronidazole or oral vancomycin begun after hospital day 3.
Statistical Analysis
Summary statistics were computed using frequencies and percents for categorical variables; and means, medians, standard deviations, and interquartile ranges for continuous variables. Associations between antibiotic selection and patient and hospital characteristics were assessed using chi‐square tests for categorical variables and z‐tests for continuous variables.
We developed a series of multivariable models to evaluate the impact of initial antibiotic selection on the risk of treatment failure, length of stay, and total cost. In order to account for the effects of within‐hospital correlation, generalized estimating equation (GEE) models with a logit link were used to assess the effect of antibiotic selection on the risk of treatment failure, and identity link models were used for analyses of length of stay and cost. Unadjusted and covariate‐adjusted models for treatment failure were evaluated with and without adjustments for propensity score. A propensity score is the probability that a given patient would receive treatment with a macrolide, derived from a nonparsimonious model in which treatment with a macrolide was considered the outcome. The propensity model included all patient characteristics, other early treatments and tests, comorbidities, hospital and physician characteristics, and selected interaction terms.21 Length of stay and cost were trimmed at 3 standard deviations above the mean, and natural log‐transformed values were modeled due to extreme positive skew. In addition, we carried out matched analyses in which we compared the outcomes of patients who were treated with a macrolide to those with similar propensity scores (ie, with similar likelihood of receiving a macrolide) who received a quinolone.22
Finally, to reduce the threat of residual confounding by indication, which can occur if sicker patients are more likely to receive a particular antibiotic, we developed a grouped treatment model, in which all patients treated at the same hospital were assigned a probability of treatment with a macrolide equal to the overall treatment rate at that hospital.23 This is an adaptation of instrumental variable analysis, a well‐accepted technique in econometrics with growing use in health care.24,25 It attempts to assess whether patients treated at a hospital at which quinolones are used more frequently have better outcomes than patients treated at hospitals at which macrolides are used more frequently, while adjusting for other patient, physician, and hospital variables. It ignores the actual treatment the patient received, and instead substitutes the hospital's rate of macrolide use. By grouping treatment at the hospital level, this method greatly reduces the possibility of residual selection bias, unless hospitals that use a lot of macrolides have patients who differ in a consistent way from hospitals which use mostly quinolones.
All analyses were performed using SAS version 9.1 (SAS Institute, Inc., Cary, NC).
Results
Of 26,248 AECOPD patients treated with antibiotics, 19,608 patients met the inclusion criteria; of these, 6139 (31%) were treated initially with a macrolide; the median age was 70 years; 60% were female; and 78% were white. A total of 86% of patients had a primary diagnosis of obstructive chronic bronchitis with acute exacerbation, and 6% had respiratory failure. The most common comorbidities were hypertension, diabetes, and congestive heart failure. Twenty‐two percent had been admitted at least once in the preceding 12 months. Treatment failure occurred in 7.7% of patients, and 1.3% died in the hospital. Mean length of stay was 4.8 days. Hospital prescribing rates for macrolides varied from 0% to 100%, with a mean of 33% and an interquartile range of 14% to 46% (Supporting Appendix Figure 1).
Compared to patients receiving macrolides, those receiving quinolones were older, more likely to have respiratory failure, to be cared for by a pulmonologist, and to have an admission in the previous year (Table 1). They were also more likely to be treated with bronchodilators, methylxanthines, steroids, diuretics, and noninvasive positive pressure ventilation, and to have an arterial blood gas, but less likely to receive concomitant treatment with a cephalosporin (11% vs. 57%). With the exception of cephalosporin treatment, these differences were small, but due to the large sample were statistically significant. Comorbidities were similar in both groups. Patients in the quinolone group were also more likely to experience treatment failure (8.1% vs. 6.8%), death (1.5% vs. 1.0%), and antibiotic‐associated diarrhea (1.1% vs. 0.5%).
| Complete Cohort | Propensity‐matched Subsample | |||||
|---|---|---|---|---|---|---|
| Characteristic | Quinolone (n = 13469) | Macrolide (n = 6139) | P Value | Quinolone (n = 5610) | Macrolide (n = 5610) | P Value |
| ||||||
| Antibiotics received during hospitalization* [n (%)] | ||||||
| Macrolide | 264 (2) | 6139 (100) | 119 (2) | 5610 (100) | ||
| Quinolone | 13469 (100) | 459 (8) | 5610 (100) | 424 (8) | ||
| Cephalosporin | 1696 (13) | 3579 (59) | <0.001 | 726 (13) | 3305 (59) | <0.001 |
| Tetracycline | 231 (2) | 75 (2) | 0.01 | 101 (2) | 73 (2) | 0.06 |
| Other antibiotics | 397 (3) | 220 (4) | 0.02 | 166 (3) | 193 (3) | 0.03 |
| Age (years) (mean [SD]) | 69.1 (11.4) | 68.2 (11.8) | <0.001 | 68.6 (11.7) | 68.5 (11.7) | 0.58 |
| Male sex (n [%]) | 5447 (40) | 2440 (40) | 0.36 | 2207 (39) | 2196 (39) | 0.85 |
| Race/ethnic group (n [%]) | <0.001 | 0.44 | ||||
| White | 10454 (78) | 4758 (78) | 4359 (78) | 4368 (78) | ||
| Black | 1060 (8) | 540 (9) | 470 (8) | 455 (8) | ||
| Hispanic | 463 (3) | 144 (2) | 157 (3) | 134 (2) | ||
| Other | 1492 (11) | 697 (11) | 624 (11) | 653 (12) | ||
| Primary diagnosis (n [%]) | <0.001 | 0.78 | ||||
| Obstructive chronic bronchitis with acute exacerbation | 11650 (87) | 5298 (86) | 4884 (87) | 4860 (87) | ||
| Chronic obstructive asthma/asthma with COPD | 908 (7) | 569 (9) | 466 (8) | 486 (9) | ||
| Respiratory failure | 911 (7) | 272 (4) | 260 (5) | 264 (5) | ||
| Admissions in the prior year (n [%]) | <0.001 | 0.84 | ||||
| 0 | 9846 (73) | 4654 (76) | 4249 (76) | 4231 (75) | ||
| 1 | 1918 (14) | 816 (13) | 747 (13) | 750 (13) | ||
| 2+ | 1085 (8) | 445 (7) | 397 (7) | 420 (8) | ||
| Missing | 620 (5) | 224 (4) | 217 (4) | 209 (4) | ||
| Physician specialty (n [%]) | <0.001 | 0.84 | ||||
| Internal medicine/hospitalist | 7069 (53) | 3321 (54) | 3032 (54) | 3072 (55) | ||
| Family/general medicine | 3569 (27) | 2074 (34) | 1824 (33) | 1812 (32) | ||
| Pulmonologist | 2776 (21) | 727 (12) | 738 (13) | 711 (13) | ||
| Critical care/emntensivist | 55 (0) | 17 (0) | 16 (0) | 15 (0) | ||
| Tests on hospital day 1 or 2 (n [%]) | ||||||
| Arterial blood gas | 8084 (60) | 3377 (55) | <0.001 | 3195 (57) | 3129 (56) | 0.22 |
| Sputum test | 1741 (13) | 766 (13) | 0.39 | 20 (0) | 16 (0) | 0.62 |
| Medications/therapies on hospital day 1 or 2 (n [%]) | ||||||
| Short‐acting bronchodilators | 7555 (56) | 3242 (53) | <0.001 | 2969 (53) | 2820 (50) | 0.005 |
| Long‐acting beta‐2 agonists | 2068 (15) | 748 (12) | <0.001 | 704 (13) | 719 (13) | 0.69 |
| Methylxanthine bronchodilators | 3051 (23) | 1149 (19) | <0.001 | 1102 (20) | 1093 (20) | 0.85 |
| Steroids | 0.04 | 0.68 | ||||
| Intravenous | 11148 (83) | 4989 (81) | 4547 (81) | 4581 (82) | ||
| Oral | 772 (6) | 376 (6) | 334 (6) | 330 (6) | ||
| Severity indicators (n [%]) | ||||||
| Chronic pulmonary heart disease | 890 (7) | 401 (7) | 0.85 | 337 (6) | 368 (7) | 0.24 |
| Sleep apnea | 586 (4) | 234 (4) | 0.08 | 211 (4) | 218 (4) | 0.77 |
| Noninvasive positive pressure ventilation | 391 (3) | 128 (2) | <0.001 | 128 (2) | 114 (2) | 0.40 |
| Loop diuretics | 4838 (36) | 1971 (32) | <0.001 | 1884 (34) | 1862 (33) | 0.67 |
| Hospital characteristics (n [%]) | ||||||
| Staffed beds | <0.001 | 0.71 | ||||
| 6200 | 3483 (26) | 1688 (28) | 1610 (29) | 1586 (28) | ||
| 201300 | 3132 (23) | 1198 (20) | 1174 (21) | 1154 (21) | ||
| 301500 | 4265 (32) | 2047 (33) | 1809 (32) | 1867 (33) | ||
| 500+ | 2589 (19) | 1206 (20) | 1017 (18) | 1003 (18) | ||
| Hospital region (n [%]) | <0.001 | 0.65 | ||||
| South | 8562 (64) | 3270 (53) | 3212 (57) | 3160 (56) | ||
| Midwest | 2602 (19) | 1444 (24) | 1170 (21) | 1216 (22) | ||
| Northeast | 1163 (9) | 871 (14) | 687 (12) | 704 (13) | ||
| West | 1142 (9) | 554 (9) | 541 (10) | 530 (9) | ||
| Teaching hospital | <0.001 | 0.63 | ||||
| No | 12090 (90) | 5037 (82) | 4896 (87) | 4878 (87) | ||
| Yes | 1379 (10) | 1102 (18) | 714 (13) | 732 (13) | ||
| Comorbidities (n [%]) | ||||||
| Congestive heart failure | 2673 (20) | 1147 (19) | 0.06 | 1081 (19) | 1060 (19) | 0.63 |
| Metastatic cancer | 134 (1) | 27 (0) | <0.001 | 34 (1) | 38 (1) | 0.72 |
| Depression | 1419 (11) | 669 (11) | 0.45 | 598 (11) | 603 (11) | 0.90 |
| Deficiency anemias | 1155 (9) | 476 (8) | 0.05 | 426 (8) | 432 (8) | 0.86 |
| Solid tumor without metastasis | 1487 (11) | 586 (10) | 0.002 | 550 (10) | 552 (10) | 0.97 |
| Hypothyroidism | 1267 (9) | 527 (9) | 0.07 | 481 (9) | 482 (9) | 1.00 |
| Peripheral vascular disease | 821 (6) | 312 (5) | 0.005 | 287 (5) | 288 (5) | 1.00 |
| Paralysis | 165 (1) | 46 (1) | 0.003 | 49 (1) | 51 (1) | 0.92 |
| Obesity | 957 (7) | 435 (7) | 0.98 | 386 (7) | 398 (7) | 0.68 |
| Hypertension | 5793 (43) | 2688 (44) | 0.31 | 2474 (44) | 2468 (44) | 0.92 |
| Diabetes | 0.04 | 0.45 | ||||
| Without chronic complications | 2630 (20) | 1127 (18) | 1057 (19) | 1066 (19) | ||
| With chronic complications | 298 (2) | 116 (2) | 115 (2) | 97 (2) | ||
In the unadjusted analysis, compared to patients receiving quinolones, those treated with macrolides were less likely to experience treatment failure (OR, 0.83; 95% CI, 0.740.94) (Table 2). Adjusting for all patient, hospital, and physician covariates, including the propensity for treatment with macrolides, increased the OR to 0.89 and the results were no longer significant (95% CI, 0.781.01). Propensity matching successfully balanced all measured covariates except for the use of short‐acting bronchodilators and additional antibiotics (Table 1). In the propensity‐matched sample (Figure 1), quinolone‐treated patients were more likely to experience antibiotic‐associated diarrhea (1.2% vs. 0.6%; P = 0.0003) and late mechanical ventilation (1.3% vs. 0.8%; P = 0.02). There were no differences in adjusted cost or length of stay between the 2 groups. The results of the grouped treatment analysis, substituting the hospital's specific rate of macrolide use in place of the actual treatment that each patient received suggested that the 2 antibiotics were associated with similar rates of treatment failure. The OR for a 100% hospital rate of macrolide treatment vs. a 0% rate was 1.01 (95% CI, 0.751.35).

| Treatment Failure | Cost | LOS | ||||
|---|---|---|---|---|---|---|
| Models | OR | 95% CI | Ratio | 95% CI | Ratio | 95% CI |
| ||||||
| Unadjusted | 0.83 | 0.730.93 | 0.98 | 0.971.00 | 0.96 | 0.950.98 |
| Adjusted for propensity score only* | 0.89 | 0.791.01 | 1.00 | 0.981.01 | 0.98 | 0.971.00 |
| Adjusted for covariates | 0.87 | 0.770.99 | 1.00 | 0.991.02 | 0.99 | 0.971.00 |
| Adjusted for covariates and propensity score | 0.89 | 0.781.01 | 1.00 | 0.991.02 | 0.98 | 0.971.00 |
| Matched sample, unadjusted | 0.87 | 0.751.00 | 0.99 | 0.981.01 | 0.99 | 0.971.01 |
| Matched sample, adjusted for unbalanced variables | 0.87 | 0.751.01 | 1.00 | 0.981.02 | 0.99 | 0.971.01 |
| Grouped treatment model, unadjusted | 0.90 | 0.681.19 | 0.97 | 0.891.06 | 0.92 | 0.870.96 |
| Group treatment model, adjusted for covariates∥ | 1.01 | 0.751.35 | 0.96 | 0.881.05 | 0.96 | 0.911.00 |
Discussion
In this large observational study conducted at 375 hospitals, we took advantage of a natural experiment in which antibiotic prescribing patterns varied widely across hospitals to compare the effectiveness of 2 common antibiotic regimens for AECOPD. Treatment with macrolides and quinolones were associated with a similar risk of treatment failure, costs, and length of stay; however, patients treated with macrolides were less likely to experience late mechanical ventilation or treatment for antibiotic‐associated diarrhea.
Despite broad consensus in COPD guidelines that patients with severe acute exacerbations should receive antibiotics, there is little agreement about the preferred empiric agent. Controversy exists regarding antibiotics' comparative effectiveness, and even over which pathogens cause COPD exacerbations. Given the frequency of hospitalization for AECOPD, understanding the comparative effectiveness of treatments in this setting could have important implications for health outcomes and costs. Unfortunately, most antibiotic studies in AECOPD were conducted >20 years ago, using antibiotics that rarely appeared in our sample.26 Consequently, clinical practice guidelines offer conflicting recommendations. For example, the National Institute for Clinical Excellence recommends empirical treatment with an aminopenicillin, a macrolide, or a tetracycline,8 while the American Thoracic Society recommends amoxicillin‐clavulonate or a fluoroquinolone.9
As might be expected in light of so much uncertainty, we found wide variation in prescribing patterns across hospitals. Overall, approximately one‐third of patients received a macrolide and two‐thirds a quinolone. Both regimens provide adequate coverage of H. influenza, S. pneumoniae, and M. catarrhalis, and conform to at least 1 COPD guideline. Nevertheless, patients receiving macrolides often received a cephalosporin as well; this pattern of treatment suggests that antibiotic selection is likely to have been influenced more by guidelines for the treatment of community‐acquired pneumonia than COPD.
Previous studies comparing antibiotic effectiveness suffer from shortcomings that limit their application to patients hospitalized with AECOPD. First, they enrolled patients with chronic bronchitis, and included patients without obstructive lung disease, and most studies included patients as young as 18 years old. Second, many either did not include treatment with steroids or excluded patients receiving more than 10 mg of prednisone daily. Third, almost all enrolled only ambulatory patients.
While there are no studies comparing quinolones and macrolides in patients hospitalized for AECOPD, a meta‐analysis comparing quinolones, macrolides, and amoxicillin‐clavulonate identified 19 trials of ambulatory patients with chronic bronchitis. That study found that all 3 drugs had similar efficacy initially, but that quinolones resulted in the fewest relapses over a 26‐week period.27 Macrolides and quinolones had similar rates of adverse effects. In contrast, we did not find a difference in treatment failure, cost, or length of stay, but did find a higher rate of diarrhea associated with quinolones. Others have also documented an association between fluoroquinolones and C. difficile diarrhea.2830 This trend, first noted in 2001, is of particular concern because the fluoroquinolone‐resistant strains appear to be hypervirulent and have been associated with nosocomial epidemics.3134
Our study has several limitations. First, its observational design leaves open the possibility of selection bias. For this reason we analyzed our data in several ways, including using a grouped treatment approach, an adaptation of the instrumental variable technique, and accepted only those differences which were consistent across all models. Second, our study used claims data, and therefore we could not directly adjust for physiological measures of severity. However, the highly detailed nature of the data allowed us to adjust for numerous tests and treatments that reflected the clinician's assessment of the patient's severity, as well as the number of prior COPD admissions. Third, we cannot exclude the possibility that some patients may have had concurrent pneumonia without an ICD‐9 code. We think that the number would be small because reimbursement for pneumonia is generally higher than for COPD, so hospitals have an incentive to code pneumonia as the principal diagnosis when present. Finally, we compared initial antibiotics only. More than one‐quarter of our patients received an additional antibiotic before discharge. In particular, patients receiving macrolides were often prescribed a concomitant cephalosporin. We do not know to what extent these additional antibiotics may have affected the outcomes.
Despite the large number of patients hospitalized annually for AECOPD, there are no randomized trials comparing different antibiotics in this population. Studies comparing antibiotics in chronic bronchitis can offer little guidance, since they have primarily focused on proving equivalence between existing antibiotics and newer, more expensive formulations.35 Because many of the patients enrolled in such trials do not benefit from antibiotics at all, either because they do not have COPD or because their exacerbation is not caused by bacteria, it is relatively easy to prove equivalence. Given that AECOPD is one of the leading causes of hospitalization in the United States, large, randomized trials comparing the effectiveness of different antibiotics should be a high priority. In the meantime, macrolides (often given together with cephalosporins) and quinolones appear to be equally effective initial antibiotic choices; considering antibiotic‐associated diarrhea, macrolides appear to be the safer of the 2.
Acute exacerbations of chronic obstructive pulmonary disease (AECOPD) are responsible for more than 600,000 hospitalizations annually, resulting in direct costs of over $20 billion.1 Bacterial infections appear responsible for 50% of such exacerbations,25 and current COPD guidelines recommend treatment with antibiotics for patients with severe exacerbations or a change in sputum.1,69 These recommendations are based on a number of small randomized trials, most of which were conducted more than 20 years ago using narrow spectrum antibiotics that are no longer commonly used.10 Only 4 studies, totaling 321 subjects, included hospitalized patients, and most studies excluded patients who required steroids. Because no clinical trials have compared different antibiotic regimens for AECOPD, existing guidelines offer a range of treatment options, including amoxicillin‐clavulonate, macrolides, quinolones, cephalosporins, aminopenicillins, and tetracyclines.
Among hospitalized patients, macrolides and quinolones appear to be the most frequently prescribed antibiotics.11 Both are available in oral formulations, have excellent bioavailability, and are administered once daily. In addition to their antimicrobial activity, macrolides are believed to have antiinflammatory effects, which could be especially advantageous in AECOPD.1214 In trials of chronic bronchitis, however, fluoroquinolones have been shown to reduce the risk of recurrent exacerbation when compared to macrolides.15 The wide variation that has been observed in antibiotic selection for patients hospitalized for AECOPD suggests a high degree of uncertainty among clinicians about the benefits of different treatment options.11 Given the limited evidence from randomized trials, we sought to evaluate the comparative effectiveness of macrolides and quinolones among a large, representative sample of patients hospitalized with AECOPD.
Subjects and Methods
Setting and Subjects
We conducted a retrospective cohort study of all patients hospitalized between January 1 and December 31, 2001 for AECOPD at any 1 of 375 acute care facilities in the United States that participated in Premier's Perspective, a voluntary, fee‐supported database developed for measuring quality and healthcare utilization. Participating hospitals represent all geographical regions, and are primarily small‐sized to medium‐sized nonteaching hospitals located mostly in urban areas. In addition to the information contained in the standard hospital discharge file (Uniform Billing 92) such as patient age, International Classification of Disease, 9th Edition, Clinical Modification (ICD‐9‐CM) codes, the Perspective database includes a date‐stamped log of all billed items, including diagnostic tests, medications, and other treatments, as well as costs, for individual patients. The study was approved by the Institutional Review Board of Baystate Medical Center.
Patients were included if they had a primary diagnosis consistent with AECOPD (ICD‐9 codes 491.21 and 493.22) or a primary diagnosis of respiratory failure (ICD‐9 codes 518.81 and 518.84) paired with secondary diagnosis of AECOPD; they also had to receive at least 2 consecutive days of either a macrolide or a quinolone, started within 48 hours of hospitalization. Patients receiving both antibiotics were excluded, but patients who received additional antibiotics were included. To enhance the specificity of our diagnosis codes, we limited our study to patients age 40 years.16 Because mechanical ventilation initiated after hospital day 2 was an outcome measure, we excluded patients admitted directly to the intensive care unit. We also excluded: those with other bacterial infections, such as pneumonia or cellulitis, who might have another indication for antibiotics; those with a length of stay <2 days, because we could not ascertain whether they received a full course of antibiotics; patients with a secondary diagnosis of pulmonary embolism or pneumothorax; and those whose attending physicians were not internists, family practitioners, hospitalists, pulmonologists, or intensivists. For patients with more than 1 admission during the study period, we included only the first admission.
Data Elements
For each patient, we assessed age, gender, race, marital and insurance status, principal diagnosis, comorbidities, and specialty of the attending physician. Comorbidities were identified from ICD‐9 secondary diagnosis codes and Diagnosis Related Groups using Healthcare Cost and Utilization Project Comorbidity Software (version 3.1), based on the work of Elixhauser et al.17 In addition, to assess disease severity we recorded the presence of chronic pulmonary heart disease, the number of admissions for COPD during the 12 months prior to the index admission, and arterial blood gas testing.18,19 We also identified pharmacy or diagnostic charges for interventions that were recommended in current guidelines (beta‐adrenergic and anticholinergic bronchodilators, steroids, and noninvasive positive‐pressure ventilation); those that were not recommended or were of uncertain benefit (methylxanthine bronchodilators, spirometry/pulmonary function testing, mucolytic medications, chest physiotherapy, and sputum testing); and drugs that might be associated with severe exacerbations or end‐stage COPD (loop diuretics, morphine, and nutritional supplements).1,69 Hospitals were categorized by region (Northeast, South, Midwest, or West), bed size, setting (urban vs. rural), ownership, and teaching status.
Antibiotic Class and Outcome Variables
Our primary predictor variable was the antibiotic initiated during the first 2 hospital days and continued for at least 2 days, regardless of other antibiotics the patient may have received during the course of hospitalization. Because we anticipated low in‐hospital mortality, our primary outcome was a composite measure of treatment failure, defined as initiation of mechanical ventilation after hospital day 2, in‐hospital mortality, or readmission for COPD within 30 days of discharge.20 Secondary outcomes included hospital costs and length of stay, as well as allergic reactions identified by ICD‐9 code, and antibiotic‐associated diarrhea, defined as treatment with either metronidazole or oral vancomycin begun after hospital day 3.
Statistical Analysis
Summary statistics were computed using frequencies and percents for categorical variables; and means, medians, standard deviations, and interquartile ranges for continuous variables. Associations between antibiotic selection and patient and hospital characteristics were assessed using chi‐square tests for categorical variables and z‐tests for continuous variables.
We developed a series of multivariable models to evaluate the impact of initial antibiotic selection on the risk of treatment failure, length of stay, and total cost. In order to account for the effects of within‐hospital correlation, generalized estimating equation (GEE) models with a logit link were used to assess the effect of antibiotic selection on the risk of treatment failure, and identity link models were used for analyses of length of stay and cost. Unadjusted and covariate‐adjusted models for treatment failure were evaluated with and without adjustments for propensity score. A propensity score is the probability that a given patient would receive treatment with a macrolide, derived from a nonparsimonious model in which treatment with a macrolide was considered the outcome. The propensity model included all patient characteristics, other early treatments and tests, comorbidities, hospital and physician characteristics, and selected interaction terms.21 Length of stay and cost were trimmed at 3 standard deviations above the mean, and natural log‐transformed values were modeled due to extreme positive skew. In addition, we carried out matched analyses in which we compared the outcomes of patients who were treated with a macrolide to those with similar propensity scores (ie, with similar likelihood of receiving a macrolide) who received a quinolone.22
Finally, to reduce the threat of residual confounding by indication, which can occur if sicker patients are more likely to receive a particular antibiotic, we developed a grouped treatment model, in which all patients treated at the same hospital were assigned a probability of treatment with a macrolide equal to the overall treatment rate at that hospital.23 This is an adaptation of instrumental variable analysis, a well‐accepted technique in econometrics with growing use in health care.24,25 It attempts to assess whether patients treated at a hospital at which quinolones are used more frequently have better outcomes than patients treated at hospitals at which macrolides are used more frequently, while adjusting for other patient, physician, and hospital variables. It ignores the actual treatment the patient received, and instead substitutes the hospital's rate of macrolide use. By grouping treatment at the hospital level, this method greatly reduces the possibility of residual selection bias, unless hospitals that use a lot of macrolides have patients who differ in a consistent way from hospitals which use mostly quinolones.
All analyses were performed using SAS version 9.1 (SAS Institute, Inc., Cary, NC).
Results
Of 26,248 AECOPD patients treated with antibiotics, 19,608 patients met the inclusion criteria; of these, 6139 (31%) were treated initially with a macrolide; the median age was 70 years; 60% were female; and 78% were white. A total of 86% of patients had a primary diagnosis of obstructive chronic bronchitis with acute exacerbation, and 6% had respiratory failure. The most common comorbidities were hypertension, diabetes, and congestive heart failure. Twenty‐two percent had been admitted at least once in the preceding 12 months. Treatment failure occurred in 7.7% of patients, and 1.3% died in the hospital. Mean length of stay was 4.8 days. Hospital prescribing rates for macrolides varied from 0% to 100%, with a mean of 33% and an interquartile range of 14% to 46% (Supporting Appendix Figure 1).
Compared to patients receiving macrolides, those receiving quinolones were older, more likely to have respiratory failure, to be cared for by a pulmonologist, and to have an admission in the previous year (Table 1). They were also more likely to be treated with bronchodilators, methylxanthines, steroids, diuretics, and noninvasive positive pressure ventilation, and to have an arterial blood gas, but less likely to receive concomitant treatment with a cephalosporin (11% vs. 57%). With the exception of cephalosporin treatment, these differences were small, but due to the large sample were statistically significant. Comorbidities were similar in both groups. Patients in the quinolone group were also more likely to experience treatment failure (8.1% vs. 6.8%), death (1.5% vs. 1.0%), and antibiotic‐associated diarrhea (1.1% vs. 0.5%).
| Complete Cohort | Propensity‐matched Subsample | |||||
|---|---|---|---|---|---|---|
| Characteristic | Quinolone (n = 13469) | Macrolide (n = 6139) | P Value | Quinolone (n = 5610) | Macrolide (n = 5610) | P Value |
| ||||||
| Antibiotics received during hospitalization* [n (%)] | ||||||
| Macrolide | 264 (2) | 6139 (100) | 119 (2) | 5610 (100) | ||
| Quinolone | 13469 (100) | 459 (8) | 5610 (100) | 424 (8) | ||
| Cephalosporin | 1696 (13) | 3579 (59) | <0.001 | 726 (13) | 3305 (59) | <0.001 |
| Tetracycline | 231 (2) | 75 (2) | 0.01 | 101 (2) | 73 (2) | 0.06 |
| Other antibiotics | 397 (3) | 220 (4) | 0.02 | 166 (3) | 193 (3) | 0.03 |
| Age (years) (mean [SD]) | 69.1 (11.4) | 68.2 (11.8) | <0.001 | 68.6 (11.7) | 68.5 (11.7) | 0.58 |
| Male sex (n [%]) | 5447 (40) | 2440 (40) | 0.36 | 2207 (39) | 2196 (39) | 0.85 |
| Race/ethnic group (n [%]) | <0.001 | 0.44 | ||||
| White | 10454 (78) | 4758 (78) | 4359 (78) | 4368 (78) | ||
| Black | 1060 (8) | 540 (9) | 470 (8) | 455 (8) | ||
| Hispanic | 463 (3) | 144 (2) | 157 (3) | 134 (2) | ||
| Other | 1492 (11) | 697 (11) | 624 (11) | 653 (12) | ||
| Primary diagnosis (n [%]) | <0.001 | 0.78 | ||||
| Obstructive chronic bronchitis with acute exacerbation | 11650 (87) | 5298 (86) | 4884 (87) | 4860 (87) | ||
| Chronic obstructive asthma/asthma with COPD | 908 (7) | 569 (9) | 466 (8) | 486 (9) | ||
| Respiratory failure | 911 (7) | 272 (4) | 260 (5) | 264 (5) | ||
| Admissions in the prior year (n [%]) | <0.001 | 0.84 | ||||
| 0 | 9846 (73) | 4654 (76) | 4249 (76) | 4231 (75) | ||
| 1 | 1918 (14) | 816 (13) | 747 (13) | 750 (13) | ||
| 2+ | 1085 (8) | 445 (7) | 397 (7) | 420 (8) | ||
| Missing | 620 (5) | 224 (4) | 217 (4) | 209 (4) | ||
| Physician specialty (n [%]) | <0.001 | 0.84 | ||||
| Internal medicine/hospitalist | 7069 (53) | 3321 (54) | 3032 (54) | 3072 (55) | ||
| Family/general medicine | 3569 (27) | 2074 (34) | 1824 (33) | 1812 (32) | ||
| Pulmonologist | 2776 (21) | 727 (12) | 738 (13) | 711 (13) | ||
| Critical care/emntensivist | 55 (0) | 17 (0) | 16 (0) | 15 (0) | ||
| Tests on hospital day 1 or 2 (n [%]) | ||||||
| Arterial blood gas | 8084 (60) | 3377 (55) | <0.001 | 3195 (57) | 3129 (56) | 0.22 |
| Sputum test | 1741 (13) | 766 (13) | 0.39 | 20 (0) | 16 (0) | 0.62 |
| Medications/therapies on hospital day 1 or 2 (n [%]) | ||||||
| Short‐acting bronchodilators | 7555 (56) | 3242 (53) | <0.001 | 2969 (53) | 2820 (50) | 0.005 |
| Long‐acting beta‐2 agonists | 2068 (15) | 748 (12) | <0.001 | 704 (13) | 719 (13) | 0.69 |
| Methylxanthine bronchodilators | 3051 (23) | 1149 (19) | <0.001 | 1102 (20) | 1093 (20) | 0.85 |
| Steroids | 0.04 | 0.68 | ||||
| Intravenous | 11148 (83) | 4989 (81) | 4547 (81) | 4581 (82) | ||
| Oral | 772 (6) | 376 (6) | 334 (6) | 330 (6) | ||
| Severity indicators (n [%]) | ||||||
| Chronic pulmonary heart disease | 890 (7) | 401 (7) | 0.85 | 337 (6) | 368 (7) | 0.24 |
| Sleep apnea | 586 (4) | 234 (4) | 0.08 | 211 (4) | 218 (4) | 0.77 |
| Noninvasive positive pressure ventilation | 391 (3) | 128 (2) | <0.001 | 128 (2) | 114 (2) | 0.40 |
| Loop diuretics | 4838 (36) | 1971 (32) | <0.001 | 1884 (34) | 1862 (33) | 0.67 |
| Hospital characteristics (n [%]) | ||||||
| Staffed beds | <0.001 | 0.71 | ||||
| 6200 | 3483 (26) | 1688 (28) | 1610 (29) | 1586 (28) | ||
| 201300 | 3132 (23) | 1198 (20) | 1174 (21) | 1154 (21) | ||
| 301500 | 4265 (32) | 2047 (33) | 1809 (32) | 1867 (33) | ||
| 500+ | 2589 (19) | 1206 (20) | 1017 (18) | 1003 (18) | ||
| Hospital region (n [%]) | <0.001 | 0.65 | ||||
| South | 8562 (64) | 3270 (53) | 3212 (57) | 3160 (56) | ||
| Midwest | 2602 (19) | 1444 (24) | 1170 (21) | 1216 (22) | ||
| Northeast | 1163 (9) | 871 (14) | 687 (12) | 704 (13) | ||
| West | 1142 (9) | 554 (9) | 541 (10) | 530 (9) | ||
| Teaching hospital | <0.001 | 0.63 | ||||
| No | 12090 (90) | 5037 (82) | 4896 (87) | 4878 (87) | ||
| Yes | 1379 (10) | 1102 (18) | 714 (13) | 732 (13) | ||
| Comorbidities (n [%]) | ||||||
| Congestive heart failure | 2673 (20) | 1147 (19) | 0.06 | 1081 (19) | 1060 (19) | 0.63 |
| Metastatic cancer | 134 (1) | 27 (0) | <0.001 | 34 (1) | 38 (1) | 0.72 |
| Depression | 1419 (11) | 669 (11) | 0.45 | 598 (11) | 603 (11) | 0.90 |
| Deficiency anemias | 1155 (9) | 476 (8) | 0.05 | 426 (8) | 432 (8) | 0.86 |
| Solid tumor without metastasis | 1487 (11) | 586 (10) | 0.002 | 550 (10) | 552 (10) | 0.97 |
| Hypothyroidism | 1267 (9) | 527 (9) | 0.07 | 481 (9) | 482 (9) | 1.00 |
| Peripheral vascular disease | 821 (6) | 312 (5) | 0.005 | 287 (5) | 288 (5) | 1.00 |
| Paralysis | 165 (1) | 46 (1) | 0.003 | 49 (1) | 51 (1) | 0.92 |
| Obesity | 957 (7) | 435 (7) | 0.98 | 386 (7) | 398 (7) | 0.68 |
| Hypertension | 5793 (43) | 2688 (44) | 0.31 | 2474 (44) | 2468 (44) | 0.92 |
| Diabetes | 0.04 | 0.45 | ||||
| Without chronic complications | 2630 (20) | 1127 (18) | 1057 (19) | 1066 (19) | ||
| With chronic complications | 298 (2) | 116 (2) | 115 (2) | 97 (2) | ||
In the unadjusted analysis, compared to patients receiving quinolones, those treated with macrolides were less likely to experience treatment failure (OR, 0.83; 95% CI, 0.740.94) (Table 2). Adjusting for all patient, hospital, and physician covariates, including the propensity for treatment with macrolides, increased the OR to 0.89 and the results were no longer significant (95% CI, 0.781.01). Propensity matching successfully balanced all measured covariates except for the use of short‐acting bronchodilators and additional antibiotics (Table 1). In the propensity‐matched sample (Figure 1), quinolone‐treated patients were more likely to experience antibiotic‐associated diarrhea (1.2% vs. 0.6%; P = 0.0003) and late mechanical ventilation (1.3% vs. 0.8%; P = 0.02). There were no differences in adjusted cost or length of stay between the 2 groups. The results of the grouped treatment analysis, substituting the hospital's specific rate of macrolide use in place of the actual treatment that each patient received suggested that the 2 antibiotics were associated with similar rates of treatment failure. The OR for a 100% hospital rate of macrolide treatment vs. a 0% rate was 1.01 (95% CI, 0.751.35).

| Treatment Failure | Cost | LOS | ||||
|---|---|---|---|---|---|---|
| Models | OR | 95% CI | Ratio | 95% CI | Ratio | 95% CI |
| ||||||
| Unadjusted | 0.83 | 0.730.93 | 0.98 | 0.971.00 | 0.96 | 0.950.98 |
| Adjusted for propensity score only* | 0.89 | 0.791.01 | 1.00 | 0.981.01 | 0.98 | 0.971.00 |
| Adjusted for covariates | 0.87 | 0.770.99 | 1.00 | 0.991.02 | 0.99 | 0.971.00 |
| Adjusted for covariates and propensity score | 0.89 | 0.781.01 | 1.00 | 0.991.02 | 0.98 | 0.971.00 |
| Matched sample, unadjusted | 0.87 | 0.751.00 | 0.99 | 0.981.01 | 0.99 | 0.971.01 |
| Matched sample, adjusted for unbalanced variables | 0.87 | 0.751.01 | 1.00 | 0.981.02 | 0.99 | 0.971.01 |
| Grouped treatment model, unadjusted | 0.90 | 0.681.19 | 0.97 | 0.891.06 | 0.92 | 0.870.96 |
| Group treatment model, adjusted for covariates∥ | 1.01 | 0.751.35 | 0.96 | 0.881.05 | 0.96 | 0.911.00 |
Discussion
In this large observational study conducted at 375 hospitals, we took advantage of a natural experiment in which antibiotic prescribing patterns varied widely across hospitals to compare the effectiveness of 2 common antibiotic regimens for AECOPD. Treatment with macrolides and quinolones were associated with a similar risk of treatment failure, costs, and length of stay; however, patients treated with macrolides were less likely to experience late mechanical ventilation or treatment for antibiotic‐associated diarrhea.
Despite broad consensus in COPD guidelines that patients with severe acute exacerbations should receive antibiotics, there is little agreement about the preferred empiric agent. Controversy exists regarding antibiotics' comparative effectiveness, and even over which pathogens cause COPD exacerbations. Given the frequency of hospitalization for AECOPD, understanding the comparative effectiveness of treatments in this setting could have important implications for health outcomes and costs. Unfortunately, most antibiotic studies in AECOPD were conducted >20 years ago, using antibiotics that rarely appeared in our sample.26 Consequently, clinical practice guidelines offer conflicting recommendations. For example, the National Institute for Clinical Excellence recommends empirical treatment with an aminopenicillin, a macrolide, or a tetracycline,8 while the American Thoracic Society recommends amoxicillin‐clavulonate or a fluoroquinolone.9
As might be expected in light of so much uncertainty, we found wide variation in prescribing patterns across hospitals. Overall, approximately one‐third of patients received a macrolide and two‐thirds a quinolone. Both regimens provide adequate coverage of H. influenza, S. pneumoniae, and M. catarrhalis, and conform to at least 1 COPD guideline. Nevertheless, patients receiving macrolides often received a cephalosporin as well; this pattern of treatment suggests that antibiotic selection is likely to have been influenced more by guidelines for the treatment of community‐acquired pneumonia than COPD.
Previous studies comparing antibiotic effectiveness suffer from shortcomings that limit their application to patients hospitalized with AECOPD. First, they enrolled patients with chronic bronchitis, and included patients without obstructive lung disease, and most studies included patients as young as 18 years old. Second, many either did not include treatment with steroids or excluded patients receiving more than 10 mg of prednisone daily. Third, almost all enrolled only ambulatory patients.
While there are no studies comparing quinolones and macrolides in patients hospitalized for AECOPD, a meta‐analysis comparing quinolones, macrolides, and amoxicillin‐clavulonate identified 19 trials of ambulatory patients with chronic bronchitis. That study found that all 3 drugs had similar efficacy initially, but that quinolones resulted in the fewest relapses over a 26‐week period.27 Macrolides and quinolones had similar rates of adverse effects. In contrast, we did not find a difference in treatment failure, cost, or length of stay, but did find a higher rate of diarrhea associated with quinolones. Others have also documented an association between fluoroquinolones and C. difficile diarrhea.2830 This trend, first noted in 2001, is of particular concern because the fluoroquinolone‐resistant strains appear to be hypervirulent and have been associated with nosocomial epidemics.3134
Our study has several limitations. First, its observational design leaves open the possibility of selection bias. For this reason we analyzed our data in several ways, including using a grouped treatment approach, an adaptation of the instrumental variable technique, and accepted only those differences which were consistent across all models. Second, our study used claims data, and therefore we could not directly adjust for physiological measures of severity. However, the highly detailed nature of the data allowed us to adjust for numerous tests and treatments that reflected the clinician's assessment of the patient's severity, as well as the number of prior COPD admissions. Third, we cannot exclude the possibility that some patients may have had concurrent pneumonia without an ICD‐9 code. We think that the number would be small because reimbursement for pneumonia is generally higher than for COPD, so hospitals have an incentive to code pneumonia as the principal diagnosis when present. Finally, we compared initial antibiotics only. More than one‐quarter of our patients received an additional antibiotic before discharge. In particular, patients receiving macrolides were often prescribed a concomitant cephalosporin. We do not know to what extent these additional antibiotics may have affected the outcomes.
Despite the large number of patients hospitalized annually for AECOPD, there are no randomized trials comparing different antibiotics in this population. Studies comparing antibiotics in chronic bronchitis can offer little guidance, since they have primarily focused on proving equivalence between existing antibiotics and newer, more expensive formulations.35 Because many of the patients enrolled in such trials do not benefit from antibiotics at all, either because they do not have COPD or because their exacerbation is not caused by bacteria, it is relatively easy to prove equivalence. Given that AECOPD is one of the leading causes of hospitalization in the United States, large, randomized trials comparing the effectiveness of different antibiotics should be a high priority. In the meantime, macrolides (often given together with cephalosporins) and quinolones appear to be equally effective initial antibiotic choices; considering antibiotic‐associated diarrhea, macrolides appear to be the safer of the 2.
- ,,.Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease.Ann Intern Med.2001;134:595–599.
- ,,, et al.Infectious etiologies in acute exacerbation of COPD.Diagn Microbiol Infect Dis.2001;40:95–102.
- ,.Bacterial infections in patients requiring admission for an acute exacerbation of COPD; a 1‐year prospective study.Respir Med.2003;97:770–777.
- ,,, et al.Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease.Arch Intern Med.2005;165:891–897.
- ,.Infection in the pathogenesis and course of chronic obstructive pulmonary disease.N Engl J Med.2008;359:2355–2365.
- ,,, et al.Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary.Am J Respir Crit Care Med.2007;176:532–555.
- ,,, et al.Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2008 update—highlights for primary care.Can Respir J.2008;15(suppl A):1A–8A.
- Chronic obstructive pulmonary disease.National clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care.Thorax.2004;59(suppl 1):1–232.
- ,,, et al.Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper.Eur Respir J.2004;23:932–946.
- ,,,,.Antibiotics for exacerbations of chronic obstructive pulmonary disease.Cochrane Database Syst Rev.2006:CD004403.
- ,,,,,.Quality of care for patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease.Ann Intern Med.2006;144:894–903.
- ,,, et al.Modulation of neutrophil and inflammation markers in chronic obstructive pulmonary disease by short‐term azithromycin treatment.Eur J Pharmacol.2005;517:132–143.
- ,,, et al.Azithromycin modulates neutrophil function and circulating inflammatory mediators in healthy human subjects.Eur J Pharmacol.2002;450:277–289.
- ,,,,,.The effect of clarithromycin on inflammatory markers in chronic obstructive pulmonary disease: preliminary data.Ann Pharmacother.2004;38:1400–1405.
- ,,,.A comparison of gemifloxacin and clarithromycin in acute exacerbations of chronic bronchitis and long‐term clinical outcomes.Clin Ther.2002;24:639–652.
- ,,,.In‐hospital mortality following acute exacerbations of chronic obstructive pulmonary disease.Arch Intern Med.2003;163:1180–1186.
- ,,,.Comorbidity measures for use with administrative data.Med Care.1998;36:8–27.
- ,,, et al.Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments).Am J Respir Crit Care Med.1996;154:959–967.
- ,,.Mortality and mortality‐related factors after hospitalization for acute exacerbation of COPD.Chest.2003;124:459–467.
- ,,, et al.Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease.N Engl J Med.1999;340:1941–1947.
- .Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group.Stat Med.1998;17:2265–2281.
- .Reducing bias in a propensity score matched‐pair sample using greedy matching techniques. Proceedings of the Twenty‐Sixth Annual SAS Users Group International Conference. Cary, NC: SAS Institute;2001,214–216.
- ,,,.Modeling treatment effects on binary outcomes with grouped‐treatment variables and individual covariates.Am J Epidemiol.2002;156:753–760.
- ,,.Does more intensive treatment of acute myocardial infarction in the elderly reduce mortality? Analysis using instrumental variables. [see Comment].JAMA.1994;272:859–866.
- ,,,,,.Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods.JAMA.2007;297:278–285.
- ,,,.Antibiotics in chronic obstructive pulmonary disease exacerbations. A meta‐analysis.JAMA.1995;273:957–960.
- ,,,,.Macrolides, quinolones and amoxicillin/clavulanate for chronic bronchitis: a meta‐analysis.Eur Respir J.2007;29:1127–1137.
- ,,,,.Antimicrobial‐associated risk factors for Clostridium difficile infection.Clin Infect Dis.2008;46(suppl 1):S19–S31.
- ,,, et al.Short‐term and long‐term outcomes of moxifloxacin compared to standard antibiotic treatment in acute exacerbations of chronic bronchitis.Chest.2004;125:953–964.
- ,,,,.Oral gemifloxacin once daily for 5 days compared with sequential therapy with i.v. ceftriaxone/oral cefuroxime (maximum of 10 days) in the treatment of hospitalized patients with acute exacerbations of chronic bronchitis.Respir Med.2003;97:242–249.
- ,,, et al.A large outbreak of Clostridium difficile‐associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use.Infect Control Hosp Epidemiol.2005;26:273–280.
- ,,, et al.An epidemic, toxin gene‐variant strain of Clostridium difficile.N Engl J Med.2005;353:2433–2441.
- ,,, et al.Outbreak of Clostridium difficile infection in a long‐term care facility: association with gatifloxacin use.Clin Infect Dis.2004;38:640–645.
- ,,, et al.A predominantly clonal multi‐institutional outbreak of Clostridium difficile‐associated diarrhea with high morbidity and mortality.N Engl J Med.2005;353:2442–2449.
- ,.No more equivalence trials for antibiotics in exacerbations of COPD, please.Chest.2004;125:811–813.
- ,,.Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease.Ann Intern Med.2001;134:595–599.
- ,,, et al.Infectious etiologies in acute exacerbation of COPD.Diagn Microbiol Infect Dis.2001;40:95–102.
- ,.Bacterial infections in patients requiring admission for an acute exacerbation of COPD; a 1‐year prospective study.Respir Med.2003;97:770–777.
- ,,, et al.Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease.Arch Intern Med.2005;165:891–897.
- ,.Infection in the pathogenesis and course of chronic obstructive pulmonary disease.N Engl J Med.2008;359:2355–2365.
- ,,, et al.Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary.Am J Respir Crit Care Med.2007;176:532–555.
- ,,, et al.Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2008 update—highlights for primary care.Can Respir J.2008;15(suppl A):1A–8A.
- Chronic obstructive pulmonary disease.National clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care.Thorax.2004;59(suppl 1):1–232.
- ,,, et al.Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper.Eur Respir J.2004;23:932–946.
- ,,,,.Antibiotics for exacerbations of chronic obstructive pulmonary disease.Cochrane Database Syst Rev.2006:CD004403.
- ,,,,,.Quality of care for patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease.Ann Intern Med.2006;144:894–903.
- ,,, et al.Modulation of neutrophil and inflammation markers in chronic obstructive pulmonary disease by short‐term azithromycin treatment.Eur J Pharmacol.2005;517:132–143.
- ,,, et al.Azithromycin modulates neutrophil function and circulating inflammatory mediators in healthy human subjects.Eur J Pharmacol.2002;450:277–289.
- ,,,,,.The effect of clarithromycin on inflammatory markers in chronic obstructive pulmonary disease: preliminary data.Ann Pharmacother.2004;38:1400–1405.
- ,,,.A comparison of gemifloxacin and clarithromycin in acute exacerbations of chronic bronchitis and long‐term clinical outcomes.Clin Ther.2002;24:639–652.
- ,,,.In‐hospital mortality following acute exacerbations of chronic obstructive pulmonary disease.Arch Intern Med.2003;163:1180–1186.
- ,,,.Comorbidity measures for use with administrative data.Med Care.1998;36:8–27.
- ,,, et al.Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments).Am J Respir Crit Care Med.1996;154:959–967.
- ,,.Mortality and mortality‐related factors after hospitalization for acute exacerbation of COPD.Chest.2003;124:459–467.
- ,,, et al.Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease.N Engl J Med.1999;340:1941–1947.
- .Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group.Stat Med.1998;17:2265–2281.
- .Reducing bias in a propensity score matched‐pair sample using greedy matching techniques. Proceedings of the Twenty‐Sixth Annual SAS Users Group International Conference. Cary, NC: SAS Institute;2001,214–216.
- ,,,.Modeling treatment effects on binary outcomes with grouped‐treatment variables and individual covariates.Am J Epidemiol.2002;156:753–760.
- ,,.Does more intensive treatment of acute myocardial infarction in the elderly reduce mortality? Analysis using instrumental variables. [see Comment].JAMA.1994;272:859–866.
- ,,,,,.Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods.JAMA.2007;297:278–285.
- ,,,.Antibiotics in chronic obstructive pulmonary disease exacerbations. A meta‐analysis.JAMA.1995;273:957–960.
- ,,,,.Macrolides, quinolones and amoxicillin/clavulanate for chronic bronchitis: a meta‐analysis.Eur Respir J.2007;29:1127–1137.
- ,,,,.Antimicrobial‐associated risk factors for Clostridium difficile infection.Clin Infect Dis.2008;46(suppl 1):S19–S31.
- ,,, et al.Short‐term and long‐term outcomes of moxifloxacin compared to standard antibiotic treatment in acute exacerbations of chronic bronchitis.Chest.2004;125:953–964.
- ,,,,.Oral gemifloxacin once daily for 5 days compared with sequential therapy with i.v. ceftriaxone/oral cefuroxime (maximum of 10 days) in the treatment of hospitalized patients with acute exacerbations of chronic bronchitis.Respir Med.2003;97:242–249.
- ,,, et al.A large outbreak of Clostridium difficile‐associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use.Infect Control Hosp Epidemiol.2005;26:273–280.
- ,,, et al.An epidemic, toxin gene‐variant strain of Clostridium difficile.N Engl J Med.2005;353:2433–2441.
- ,,, et al.Outbreak of Clostridium difficile infection in a long‐term care facility: association with gatifloxacin use.Clin Infect Dis.2004;38:640–645.
- ,,, et al.A predominantly clonal multi‐institutional outbreak of Clostridium difficile‐associated diarrhea with high morbidity and mortality.N Engl J Med.2005;353:2442–2449.
- ,.No more equivalence trials for antibiotics in exacerbations of COPD, please.Chest.2004;125:811–813.
Copyright © 2010 Society of Hospital Medicine
Reporting Hospital Quality
Acknowledging striking deficiencies in the quality and safety of healthcare, the Institute of Medicine, policy makers, and payors have called for transformation of the US healthcare system.1 Public reporting of hospital performance is one key strategy for accelerating improvement2 and may improve quality in several ways. First, feedback about performance relative to peers may stimulate quality improvement activities by appealing to professionalism. Second, the desire to preserve one's reputation by not appearing on a list of poor performers may be a powerful incentive. Finally, patients and referring providers could use reports to select high‐quality hospitals, thereby shifting care from low‐quality to high‐quality hospitals and stimulating quality improvement efforts to maintain or enhance market share.
Almost 20 years after New York and Pennsylvania began reporting cardiac surgery outcomes,3 the evidence that public reporting improves healthcare quality is equivocal.4 Moreover, stakeholders have embraced public reporting to differing degrees. Public reporting does lead to greater engagement in quality improvement activities,58 and additional financial incentives provide modest incremental benefits.9 Purchasers, too, are starting to pay attention.10 In New York State, payors appear to contract more with high‐quality surgeons and avoid poorly performing outliers.11 Some payors are creating tiered systems, assigning higher patient copayments for hospitals with poor quality metrics. These new systems have not been rigorously studied and should raise concern among hospitals.12
In contrast to hospitals and payors, patients have been slow to embrace public reporting. In a survey of coronary artery bypass graft (CABG) patients in Pennsylvania, only 2% said that public reporting of mortality rates affected their decision making.13 Eight years later, only 11% of patients sought information about hospitals before deciding on elective major surgery,14 although a majority of patients in both studies expressed interest in the information. It is not clear whether recent proliferation of information on the internet will change patient behavior, but to date public reporting appears not to effect market share.5, 15, 16
Barriers to patients' use of public reporting include difficulty accessing the information, lack of trust, information that is not salient, and data that are difficult to interpret.17 In the absence of consensus on what or how to report, a growing number of organizations, including state and federal government, accrediting bodies, private foundations, and for‐profit companies report a variety of measures relating to structure, processes, and outcomes. Although these sites purport to target consumers, they sometimes offer conflicting information18 and are not easily interpreted by lay readers.19
To realize the benefits of public reporting, and minimize the unintended consequences, rating systems must report salient information in a way that is comprehensible to patients and trusted by the doctors who advise them. At the same time, they should be fair to hospitals and offer useful data for quality improvement. We offer 10 recommendations for improving the public reporting of healthcare quality information: 5 describing what to report and 5 detailing how it should be reported (Figure 1). We also examine 3 leading performance reporting programs to see how well they implement these recommendations.

Recommendations to Make Data Salient for Patients
1. Prioritize Elective Procedures
Hospital quality is not uniform across conditions.2 For data to be salient, then, it should be disease‐specific and focus on common elective procedures, for which consumer choice is possible. Table 1 compares 3 popular reporting services. Hospital Compare, produced by the Center for Medicare Services (CMS, US Department of Health and Human Services, Washington, DC), provides process of care measures for 4 conditions, 3 of which are not elective. The fourth, surgical infection prevention, contains 5 measures3 related to perioperative antibiotics and 2 related to thromboembolism prophylaxisfor all surgical cases. Recently, more conditions have been added, but reports are limited to the number of cases and mean Medicare charge. By year 2011, however, Hospital Compare will offer many new measures, including rates of central line infection, ventilator‐associated pneumonia, and surgical site infection. HealthGrades, a private company, offers comparative mortality rates on over 30 diagnoses, of which 15 can be considered elective, at least some of the time. Only the Leapfrog group, an industry consortium, focuses exclusively on elective procedures, offering volume measures on 7 and outcome measures on 2.
| Rule | Hospital Compare | HealthGrades | Leapfrog | |||
|---|---|---|---|---|---|---|
| ||||||
| 1. Prioritize elective procedures | Yes | 22/28 at least partially elective | Yes | 15/31 at least partially elective | Yes | 7/8 elective |
| 2. Include quality of life and outcome data, if possible | Yes | Mortality for AMI and CHF | Yes | Mortality or complications* | Yes | Outcomes for CABG, PCI, and AVR |
| 3. Include standardized patient satisfaction and service measures | Yes | HCAHPS | No | No | ||
| 4. Offer composite measures that are weighted and evidence‐based | No | No | Specialty excellence award, not evidence‐based | No | ||
| 5. Costs comparisons should include patient prices | Yes | Average Medicare payment | Yes | Charges, health plan and Medicare costs available for a fee | No | |
| 6. Adjust outcomes for severity and risk | Yes | Methodology published on website | Yes | Methodology not public | Yes | Various methodologies published or referenced on website |
| 7. Identify differences not due to chance | Yes | Compares mortality to national mean | Yes | Compares mortality or complications to mean | Yes | Compares mortality to national mean |
| 8. Standardize reporting periods | October 2005 to September 2006 | 2004‐2006 | 12‐24 months, ending 12/31/07 or 6/30/08 | |||
| 9. Avoid use of nonvalidated administrative data | Yes | None used | No | Uses PSIs for safety rating | Yes | None used |
| 10. Utilization rates should be evidence‐based | No | Surgical case volume of Medicare patients | No | Includes Caesarian‐section rates | Yes | Some case volume rates are evidence‐based |
2. Include Quality of Life and Outcome Data
Outcomes are more valuable to patients than process measures, but the risk adjustment needed to compare outcomes requires considerable effort. So far, public reporting of risk‐adjusted outcomes has been limited almost exclusively to mortality. Yet a patient contemplating knee replacement surgery would find no meaningful difference in mortalitythere were only 510 deaths nationally in year 200620but might be interested in whether patients return to full mobility after surgery, and all patients should compare rates of nosocomial infections. For some low‐risk procedures, HealthGrades Inc. (Golden, CO) includes a composite measure of major complications, including complication of an orthopedic implant, stroke, cardiac arrest, excessive bleeding, and some types of infection; CMS will soon add rates of infection and readmission.
3. Include Measures of Patient Experience, Such as Satisfaction and Service Measures
Beyond outcomes, patients want to know about the experience of others.21 Satisfaction surveys should be standardized and made disease‐specific, since patients' experiences may differ between the cardiology suite and the delivery unit. Questions could address the attentiveness of the nursing staff, how well privacy was respected, how easy it was to deal with insurance issues, whether patients were promptly informed of test results, and whether the care team answered questions fully. Medicare has begun reporting patient satisfaction using the Hospital Consumer Assessment of Healthcare Providers (HCAHPS) survey on Hospital Compare, but the data are not disease‐specific and audit a very small number of patients from each institution. Other services are unlikely to perform their own surveys, as multiple surveys would prove burdensome. Social networking sites that allow patients to post their own personal reviews of hospitals and doctors offer an additional if less reliable dimension to traditional public reporting. Such sites are already transforming the market for other industries, such as travel.22
4. Offer Composite Measures That Are Weighted and Evidence‐Based
Interpreting multiple measures, some of which are more important than others, and some of which have better evidence than others, is difficult for health care providers and may be impossible for patients. Is it more important to get aspirin on arrival or at discharge? Also, how does a patient weigh a 1% difference in the number of heart attack patients who get aspirin on arrival against a 14% difference in those who are offered smoking cessation? Because patients may be overwhelmed by data,23 public reports should include evidence‐based, weighted measures of overall care for a given condition, with higher weights attached to those process measures most likely to have clinical benefit, and careful attention to visual representations that convey relative differences.19, 23 More sophisticated measures should be developed to guard against overuse. For example, while hospitals should be rewarded for providing vaccination, they should be penalized for vaccinating the same patient twice.
None of the services we examined provides weighted outcomes. Leapfrog (The Leapfrog Group, Washington, DC) offers a composite snapshot containing 9 pie charts, divided into 4 leaps. The 6 pies representing high‐risk procedures are of equal size, even though 2 of these, esophagectomy and pancreatic resection represent very rare surgeries, even at major medical centers. From a visual perspective, however, these are equivalent to having computerized physician order entry and full‐time intensive care unit staffing, which affect thousands more patients. Similarly, in determining pay‐for‐performance measures, CMS created a composite based on the total number of opportunities of all interventions, weighting all measures equally. Because no validated weighting measures exist, future research will be necessary to achieve this goal. Also, none of the evidence‐based measures contained safeguards against overtreatment.
5. Cost Comparisons Should Include Patient Prices
In an era of patient copayments and deductibles, consumers are increasingly aware of costs. For patients with very high deductible plans or no health insurance, hospital fees are a common cause of bankruptcy.24 Several public reporting agencies, including Hospital Compare and HealthGrades have incorporated Medicare costs into their reported measures, but these have little connection to what patients actually pay. Health sites aimed at consumers should publish the average patient copayment.
Recommendations to Ensure That Data Reflects Hospital Quality
6. Adjust Outcomes for Severity and Risk
Not all bypass operations are the same and not all patients are at equal risk. More difficult operations (eg, CABG for a patient with a previous bypass) will have more complications; similarly, patients with serious comorbidities will experience worse outcomes. Since hospitals which specialize in a procedure will attract complicated cases and higher risk patients, it is important to adjust outcomes to account for these differences. Otherwise, hospitals and surgeons may be discouraged from taking difficult cases. Outside of cardiac surgery, most risk adjustment systems use administrative claims data but vary dramatically in the numbers of variables considered and the underlying proprietary models, which are often criticized as being black boxes that yield discordant results.25 Thus, a hospital's mortality may appear below expected by 1 system and above expected by another. Instead, risk adjustment systems should include clinical data abstracted from patient records using standardized data definitions. Although costly to collect, clinical data offer more predictive information than do administrative data. For example, for heart failure patients undergoing CABG, the ejection fraction predicts mortality better than many stable comorbid diagnoses. A single transparent risk‐adjustment system should be recognized as the industry standard. The American College of Surgeons' standardized risk‐adjusted outcome reporting for the National Surgical Quality Improvement Program (NSQIP) is a good example of such an effort.
7. Identify Differences Not Due to Chance
As a result of random variation, during any period, some hospitals will appear better than average and others worse. Statistical tests should be employed to identify hospitals that differ from the mean, and to allow consumers to compare 2 hospitals directly, with appropriate caveats when the hospitals serve very different patient populations. Medicare's mortality rating system for myocardial infarction identifies only 17 hospitals in the nation as better than average and 7 as worse, out of 4,500 institutions. HealthGrades compares hospitals' actual mortality or complication rates to their predicted rates based on disease‐specific logistic regression models and reports whether the hospital is statistically better or worse than predicted. Hospitals are not compared directly to one another. Given the rarity of mortality in most procedures, other outcome measures will be necessary to distinguish among hospitals.26
8. Standardize Reporting Periods
In a world of continuous quality improvement, public reporting should represent a hospital's recent performance, but reporting periods also need to be long enough to provide a stable estimate of infrequent events, especially at low‐volume institutions. In contrast, the lag time between the end of the reporting period and public availability should be kept to a minimum. We found that reporting periods varied from 1 to 3 years, and did not always cover the same years for all conditions, even on the same website. Some data were 3 years old. Patients will have a hard time making decisions on data that is 1 year old, and hospitals will have little incentive to make improvements that will not be acknowledged for years.
9. Avoid Use of Nonvalidated Administrative Data
Administrative data collected for billing purposes, unlike most clinical data, are already in electronic format, and can inexpensively produce quality rankings using validated models.27 In contrast, screening tools, such as the Agency for Healthcare Research and Quality's patient safety indicators (PSIs), were designed to identify potential quality problems, such as postoperative deep vein thrombosis, for internal quality improvement. Cases identified by the PSI software require additional chart review,28, 29 and should not be used as quality indicators. Even so, HealthGrades reports PSIs and some insurers use them in pay‐for‐performance initiatives. Improvements in PSIs, including present‐on‐admission coding, may increase accuracy,30 but these measures need to be validated before they can be adopted for public reporting.
10. Utilization Rates Should Be Evidence‐Based
Although utilization rates for most procedures vary as much as 2‐fold by state or institution, there is little evidence for a best rate. Nevertheless, HealthGrades reports utilization rates for several obstetrical procedures. At present, there are no standards for these, and it is possible that utilization could be too low in some places. Further research is needed; until then, utilization should not purport to measure quality.
Discussion
The growing commitment to making hospital performance data public could transform the quality and safety of care in the US, introducing competition on quality and price and fostering informed consumer choice. To date, the promise of public reporting remains only partially fulfilled. Few hospitals have done more than comply with regulatory mandates and payer incentives, and consumers have failed to respond. To capture the full benefits of public reporting, we have made 10 recommendations to benefit patients and better engage hospitals. We suggest that reporting be patient‐centered, with an emphasis on making the data useful, meaningful, important, interpretable, and relevant. At the same time, hospitals, which are being judged on their performance, should have a level playing field, with measures that are timely, consistent, severity‐adjusted, evidence‐based, and which foster good clinical care. Of the 3 services we examined, Hospital Compare came closest to meeting these recommendations.
Although this blueprint for public reporting is easy to draft, it is challenging to implement. In particular, some of our suggestions, such as the one regarding risk adjustment, may not currently be feasible, because the complexity and cost of collecting clinical data, even in the era of electronic medical records, may be prohibitive. Until such data are readily available, it may be preferable to report nothing at all, rather than report data that are misleading. In the rush to make hospitals accountable, enthusiasm has often outstripped science,31 and several measures have had to be revised for unintended consequences.32
Any initiative to improve public reporting should have the buy‐in of all stakeholders, but particularly hospitals, which stand to benefit in several ways. By receiving regular feedback, they can focus on improving care, becoming better organizations. These improvements may be rewarded through direct compensation (pay‐for‐performance), decreased costs from complications, or increased market share. Hospitals will be more engaged if the data reflect actual quality, are adequately adjusted for severity, and acknowledge the role of chance. Otherwise, they will merely comply, or worse, look for opportunities to game the system. To succeed, public reporting needs to involve hospitals in establishing standards for reporting and validation, as well as auditing procedures to prevent fraud.33 The Hospital Quality Alliance (HQA, Washington, DC), a first step in this direction, at present has few measures. NSQIP (American College of Surgeons, Chicago, IL) is perhaps a better example of hospitals cooperating to set measurement standards to promote best‐practices. Public release of NSQIP data might accelerate progress. Alternatively, the National Quality Forum (NQF, Washington, DC) could expand its role from endorsing quality measures to include standardizing the way these measures are used in public reporting.
Still, if you build it, will they come? To date, public reporting has not been embraced by the public, despite its stated interest in the information. Several explanations could be offered. First, we may be presenting the wrong data. Process measures and mortality rates are important but represent abstract concepts for most patients. Surveys tell us that patients value most the experiences of other patients.14, 21 They want to know whether their pain will be controlled, whether the doctor will listen to them, whether the nurse will come when they call. The recent advent of the HCAHPS survey (AHRQ, Washington, DC) is another positive step. Stratifying the results by diagnosis and adding a few diagnosis‐specific questions would make HCAHPS even more valuable. Second, the data may not be readily available. Although most public reporting is done on the web, older patients who are deciding about hospitals may not have Internet access. Some reports are still proprietary, and cost could present another obstacle. Finally, even if freely‐available and patient‐centered, the results may not be interpretable by physicians, let alone patients.34
If public reporting is to succeed, it will require measures that better reflect patients' concerns. In order to collect the massive amounts of data required and present them in a timely fashion, better electronic record systems will be necessary. But these are no panacea; others have noted that the Department of Veterans Affairs, a leader in electronic records, still invests considerable time and money to review charts for NSQIP.35 Given the value that Americans place on transparency in other facets of their lives, it is clear that public reporting is here to stay. While much progress has been made over the past 5 years, additional research is needed to better measure quality from the patient's perspective, and to determine how this information can be used to help guide decision‐making, and to reward hospitals for offering the highest‐quality care.
Acknowledgements
The authors thank Kenneth Flax for his help with an earlier version of this manuscript.
- Committee on Quality of Health Care in America IoM.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:National Academy Press;2001.
- ,,,.Care in U.S. hospitals: the Hospital Quality Alliance program.N Engl J Med.2005;353(3):265–274.
- .Achieving and sustaining improved quality: lessons from New York state and cardiac surgery.Health Aff. 20022002;21(4):40–51.
- ,,,,.Systematic review: the evidence that publishing patient care performance data improves quality of care.Ann Intern Med.2008;148(2):111–123.
- ,,.Hospital performance reports: impact on quality, market share, and reputation.Health Aff (Millwood).2005;24(4):1150–1160.
- ,,.Does publicizing hospital performance stimulate quality improvement efforts?Health Aff (Millwood).2003;22(2):84–94.
- ,,,,.Improving the outcomes of coronary artery bypass surgery in New York State.JAMA.1994;271(10):761–766.
- ,,.Declines in hospital mortality associated with a regional initiative to measure hospital performance.Am J Med Qual.1997;12(2):103–112.
- ,,, et al.Public reporting and pay for performance in hospital quality improvement.N Engl J Med.2007;356(5):486–496.
- ,,,,,.Do quality report cards play a role in HMOs' contracting practices? Evidence from New York State.Health Serv Res.2000;35(1 Pt 2):319–332.
- ,,,.Quality of cardiac surgeons and managed care contracting practices.Health Serv Res.2002;37(5):1129–1144.
- ,,,,,.Using performance data to identify preferred hospitals.Health Serv Res.2007;42(6 Pt 1):2109–2119; discussion 2294–2323.
- ,.Use of public performance reports: a survey of patients undergoing cardiac surgery.JAMA.1998;279(20):1638–1642.
- ,,.How do elderly patients decide where to go for major surgery? Telephone interview survey.BMJ.2005;331(7520):821.
- ,,,,,.The effect of publicly reporting hospital performance on market share and risk‐adjusted mortality at high‐mortality hospitals.Med Care.2003;41(6):729–740.
- ,.The predictive accuracy of the New York State coronary artery bypass surgery report‐card system.Health Aff (Millwood).2006;25(3):844–855.
- ,.Publicly disclosed information about the quality of health care: response of the US public.Qual Saf Health Care.2001;10(2):96–103.
- ,,,,.Choosing the best hospital: the limitations of public reporting of hospital quality.Health Aff (Millwood).2008;27(6):1680–1687.
- ,.Will quality report cards help consumers?Health Aff (Millwood).1997;16(3):218–228.
- Agency for Healthcare Research and Quality. HCUPnet, Healthcare Cost and Utilization Project. Available at: http://hcupnet.ahrq.gov. Accessed January 2009.
- ,,.Recovering from cardiac surgery: what patients want you to know.Am J Crit Care.2002;11(4):333–343.
- Trip Advisor. Available at: http://www.tripadvisor.com. Accessed January 2009.
- ,,,,.Less is more in presenting quality information to consumers.Med Care Res Rev.2007;64(2):169–190.
- ,,,.MarketWatch: illness and injury as contributors to bankruptcy.Health Aff (Millwood)2005;(Suppl Web Exclusives):W5‐63–W5‐73.
- .The Lake Wobegon effect: when all the patients are sicker.Am J Med Qual.2006;21(6):365–366.
- ,,.Surgical mortality as an indicator of hospital quality: the problem with small sample size.JAMA.2004;292(7):847–851.
- ,,, et al.An administrative claims model suitable for profiling hospital performance based on 30‐day mortality rates among patients with heart failure.Circulation.2006;113(13):1693–1701.
- ,,,.Can administrative data be used to compare postoperative complication rates across hospitals?Med Care.2002;40(10):856–867.
- ,,,,.Impact of diagnosis‐timing indicators on measures of safety, comorbidity, and case mix groupings from administrative data sources.Med Care.2007;45(8):781–788.
- ,,,,.Do the AHRQ patient safety indicators flag conditions that are present at the time of hospital admission?Med Care.2008;46(5):516–522.
- ,,.The tension between needing to improve care and knowing how to do it.N Engl J Med.2007;357(6):608–613.
- ,,,.Public reporting of antibiotic timing in patients with pneumonia: lessons from a flawed performance measure.Ann Intern Med.2008;149(1):29–32.
- ,,.The GAAP in quality measurement and reporting.JAMA.2007;298(15):1800–1802.
- ,,,.Consumer competencies and the use of comparative quality information: it isn't just about literacy.Med Care Res Rev.2007;64(4):379–394.
- .Performance measurement in search of a path.N Engl J Med.2007;356(9):951–953.
Acknowledging striking deficiencies in the quality and safety of healthcare, the Institute of Medicine, policy makers, and payors have called for transformation of the US healthcare system.1 Public reporting of hospital performance is one key strategy for accelerating improvement2 and may improve quality in several ways. First, feedback about performance relative to peers may stimulate quality improvement activities by appealing to professionalism. Second, the desire to preserve one's reputation by not appearing on a list of poor performers may be a powerful incentive. Finally, patients and referring providers could use reports to select high‐quality hospitals, thereby shifting care from low‐quality to high‐quality hospitals and stimulating quality improvement efforts to maintain or enhance market share.
Almost 20 years after New York and Pennsylvania began reporting cardiac surgery outcomes,3 the evidence that public reporting improves healthcare quality is equivocal.4 Moreover, stakeholders have embraced public reporting to differing degrees. Public reporting does lead to greater engagement in quality improvement activities,58 and additional financial incentives provide modest incremental benefits.9 Purchasers, too, are starting to pay attention.10 In New York State, payors appear to contract more with high‐quality surgeons and avoid poorly performing outliers.11 Some payors are creating tiered systems, assigning higher patient copayments for hospitals with poor quality metrics. These new systems have not been rigorously studied and should raise concern among hospitals.12
In contrast to hospitals and payors, patients have been slow to embrace public reporting. In a survey of coronary artery bypass graft (CABG) patients in Pennsylvania, only 2% said that public reporting of mortality rates affected their decision making.13 Eight years later, only 11% of patients sought information about hospitals before deciding on elective major surgery,14 although a majority of patients in both studies expressed interest in the information. It is not clear whether recent proliferation of information on the internet will change patient behavior, but to date public reporting appears not to effect market share.5, 15, 16
Barriers to patients' use of public reporting include difficulty accessing the information, lack of trust, information that is not salient, and data that are difficult to interpret.17 In the absence of consensus on what or how to report, a growing number of organizations, including state and federal government, accrediting bodies, private foundations, and for‐profit companies report a variety of measures relating to structure, processes, and outcomes. Although these sites purport to target consumers, they sometimes offer conflicting information18 and are not easily interpreted by lay readers.19
To realize the benefits of public reporting, and minimize the unintended consequences, rating systems must report salient information in a way that is comprehensible to patients and trusted by the doctors who advise them. At the same time, they should be fair to hospitals and offer useful data for quality improvement. We offer 10 recommendations for improving the public reporting of healthcare quality information: 5 describing what to report and 5 detailing how it should be reported (Figure 1). We also examine 3 leading performance reporting programs to see how well they implement these recommendations.

Recommendations to Make Data Salient for Patients
1. Prioritize Elective Procedures
Hospital quality is not uniform across conditions.2 For data to be salient, then, it should be disease‐specific and focus on common elective procedures, for which consumer choice is possible. Table 1 compares 3 popular reporting services. Hospital Compare, produced by the Center for Medicare Services (CMS, US Department of Health and Human Services, Washington, DC), provides process of care measures for 4 conditions, 3 of which are not elective. The fourth, surgical infection prevention, contains 5 measures3 related to perioperative antibiotics and 2 related to thromboembolism prophylaxisfor all surgical cases. Recently, more conditions have been added, but reports are limited to the number of cases and mean Medicare charge. By year 2011, however, Hospital Compare will offer many new measures, including rates of central line infection, ventilator‐associated pneumonia, and surgical site infection. HealthGrades, a private company, offers comparative mortality rates on over 30 diagnoses, of which 15 can be considered elective, at least some of the time. Only the Leapfrog group, an industry consortium, focuses exclusively on elective procedures, offering volume measures on 7 and outcome measures on 2.
| Rule | Hospital Compare | HealthGrades | Leapfrog | |||
|---|---|---|---|---|---|---|
| ||||||
| 1. Prioritize elective procedures | Yes | 22/28 at least partially elective | Yes | 15/31 at least partially elective | Yes | 7/8 elective |
| 2. Include quality of life and outcome data, if possible | Yes | Mortality for AMI and CHF | Yes | Mortality or complications* | Yes | Outcomes for CABG, PCI, and AVR |
| 3. Include standardized patient satisfaction and service measures | Yes | HCAHPS | No | No | ||
| 4. Offer composite measures that are weighted and evidence‐based | No | No | Specialty excellence award, not evidence‐based | No | ||
| 5. Costs comparisons should include patient prices | Yes | Average Medicare payment | Yes | Charges, health plan and Medicare costs available for a fee | No | |
| 6. Adjust outcomes for severity and risk | Yes | Methodology published on website | Yes | Methodology not public | Yes | Various methodologies published or referenced on website |
| 7. Identify differences not due to chance | Yes | Compares mortality to national mean | Yes | Compares mortality or complications to mean | Yes | Compares mortality to national mean |
| 8. Standardize reporting periods | October 2005 to September 2006 | 2004‐2006 | 12‐24 months, ending 12/31/07 or 6/30/08 | |||
| 9. Avoid use of nonvalidated administrative data | Yes | None used | No | Uses PSIs for safety rating | Yes | None used |
| 10. Utilization rates should be evidence‐based | No | Surgical case volume of Medicare patients | No | Includes Caesarian‐section rates | Yes | Some case volume rates are evidence‐based |
2. Include Quality of Life and Outcome Data
Outcomes are more valuable to patients than process measures, but the risk adjustment needed to compare outcomes requires considerable effort. So far, public reporting of risk‐adjusted outcomes has been limited almost exclusively to mortality. Yet a patient contemplating knee replacement surgery would find no meaningful difference in mortalitythere were only 510 deaths nationally in year 200620but might be interested in whether patients return to full mobility after surgery, and all patients should compare rates of nosocomial infections. For some low‐risk procedures, HealthGrades Inc. (Golden, CO) includes a composite measure of major complications, including complication of an orthopedic implant, stroke, cardiac arrest, excessive bleeding, and some types of infection; CMS will soon add rates of infection and readmission.
3. Include Measures of Patient Experience, Such as Satisfaction and Service Measures
Beyond outcomes, patients want to know about the experience of others.21 Satisfaction surveys should be standardized and made disease‐specific, since patients' experiences may differ between the cardiology suite and the delivery unit. Questions could address the attentiveness of the nursing staff, how well privacy was respected, how easy it was to deal with insurance issues, whether patients were promptly informed of test results, and whether the care team answered questions fully. Medicare has begun reporting patient satisfaction using the Hospital Consumer Assessment of Healthcare Providers (HCAHPS) survey on Hospital Compare, but the data are not disease‐specific and audit a very small number of patients from each institution. Other services are unlikely to perform their own surveys, as multiple surveys would prove burdensome. Social networking sites that allow patients to post their own personal reviews of hospitals and doctors offer an additional if less reliable dimension to traditional public reporting. Such sites are already transforming the market for other industries, such as travel.22
4. Offer Composite Measures That Are Weighted and Evidence‐Based
Interpreting multiple measures, some of which are more important than others, and some of which have better evidence than others, is difficult for health care providers and may be impossible for patients. Is it more important to get aspirin on arrival or at discharge? Also, how does a patient weigh a 1% difference in the number of heart attack patients who get aspirin on arrival against a 14% difference in those who are offered smoking cessation? Because patients may be overwhelmed by data,23 public reports should include evidence‐based, weighted measures of overall care for a given condition, with higher weights attached to those process measures most likely to have clinical benefit, and careful attention to visual representations that convey relative differences.19, 23 More sophisticated measures should be developed to guard against overuse. For example, while hospitals should be rewarded for providing vaccination, they should be penalized for vaccinating the same patient twice.
None of the services we examined provides weighted outcomes. Leapfrog (The Leapfrog Group, Washington, DC) offers a composite snapshot containing 9 pie charts, divided into 4 leaps. The 6 pies representing high‐risk procedures are of equal size, even though 2 of these, esophagectomy and pancreatic resection represent very rare surgeries, even at major medical centers. From a visual perspective, however, these are equivalent to having computerized physician order entry and full‐time intensive care unit staffing, which affect thousands more patients. Similarly, in determining pay‐for‐performance measures, CMS created a composite based on the total number of opportunities of all interventions, weighting all measures equally. Because no validated weighting measures exist, future research will be necessary to achieve this goal. Also, none of the evidence‐based measures contained safeguards against overtreatment.
5. Cost Comparisons Should Include Patient Prices
In an era of patient copayments and deductibles, consumers are increasingly aware of costs. For patients with very high deductible plans or no health insurance, hospital fees are a common cause of bankruptcy.24 Several public reporting agencies, including Hospital Compare and HealthGrades have incorporated Medicare costs into their reported measures, but these have little connection to what patients actually pay. Health sites aimed at consumers should publish the average patient copayment.
Recommendations to Ensure That Data Reflects Hospital Quality
6. Adjust Outcomes for Severity and Risk
Not all bypass operations are the same and not all patients are at equal risk. More difficult operations (eg, CABG for a patient with a previous bypass) will have more complications; similarly, patients with serious comorbidities will experience worse outcomes. Since hospitals which specialize in a procedure will attract complicated cases and higher risk patients, it is important to adjust outcomes to account for these differences. Otherwise, hospitals and surgeons may be discouraged from taking difficult cases. Outside of cardiac surgery, most risk adjustment systems use administrative claims data but vary dramatically in the numbers of variables considered and the underlying proprietary models, which are often criticized as being black boxes that yield discordant results.25 Thus, a hospital's mortality may appear below expected by 1 system and above expected by another. Instead, risk adjustment systems should include clinical data abstracted from patient records using standardized data definitions. Although costly to collect, clinical data offer more predictive information than do administrative data. For example, for heart failure patients undergoing CABG, the ejection fraction predicts mortality better than many stable comorbid diagnoses. A single transparent risk‐adjustment system should be recognized as the industry standard. The American College of Surgeons' standardized risk‐adjusted outcome reporting for the National Surgical Quality Improvement Program (NSQIP) is a good example of such an effort.
7. Identify Differences Not Due to Chance
As a result of random variation, during any period, some hospitals will appear better than average and others worse. Statistical tests should be employed to identify hospitals that differ from the mean, and to allow consumers to compare 2 hospitals directly, with appropriate caveats when the hospitals serve very different patient populations. Medicare's mortality rating system for myocardial infarction identifies only 17 hospitals in the nation as better than average and 7 as worse, out of 4,500 institutions. HealthGrades compares hospitals' actual mortality or complication rates to their predicted rates based on disease‐specific logistic regression models and reports whether the hospital is statistically better or worse than predicted. Hospitals are not compared directly to one another. Given the rarity of mortality in most procedures, other outcome measures will be necessary to distinguish among hospitals.26
8. Standardize Reporting Periods
In a world of continuous quality improvement, public reporting should represent a hospital's recent performance, but reporting periods also need to be long enough to provide a stable estimate of infrequent events, especially at low‐volume institutions. In contrast, the lag time between the end of the reporting period and public availability should be kept to a minimum. We found that reporting periods varied from 1 to 3 years, and did not always cover the same years for all conditions, even on the same website. Some data were 3 years old. Patients will have a hard time making decisions on data that is 1 year old, and hospitals will have little incentive to make improvements that will not be acknowledged for years.
9. Avoid Use of Nonvalidated Administrative Data
Administrative data collected for billing purposes, unlike most clinical data, are already in electronic format, and can inexpensively produce quality rankings using validated models.27 In contrast, screening tools, such as the Agency for Healthcare Research and Quality's patient safety indicators (PSIs), were designed to identify potential quality problems, such as postoperative deep vein thrombosis, for internal quality improvement. Cases identified by the PSI software require additional chart review,28, 29 and should not be used as quality indicators. Even so, HealthGrades reports PSIs and some insurers use them in pay‐for‐performance initiatives. Improvements in PSIs, including present‐on‐admission coding, may increase accuracy,30 but these measures need to be validated before they can be adopted for public reporting.
10. Utilization Rates Should Be Evidence‐Based
Although utilization rates for most procedures vary as much as 2‐fold by state or institution, there is little evidence for a best rate. Nevertheless, HealthGrades reports utilization rates for several obstetrical procedures. At present, there are no standards for these, and it is possible that utilization could be too low in some places. Further research is needed; until then, utilization should not purport to measure quality.
Discussion
The growing commitment to making hospital performance data public could transform the quality and safety of care in the US, introducing competition on quality and price and fostering informed consumer choice. To date, the promise of public reporting remains only partially fulfilled. Few hospitals have done more than comply with regulatory mandates and payer incentives, and consumers have failed to respond. To capture the full benefits of public reporting, we have made 10 recommendations to benefit patients and better engage hospitals. We suggest that reporting be patient‐centered, with an emphasis on making the data useful, meaningful, important, interpretable, and relevant. At the same time, hospitals, which are being judged on their performance, should have a level playing field, with measures that are timely, consistent, severity‐adjusted, evidence‐based, and which foster good clinical care. Of the 3 services we examined, Hospital Compare came closest to meeting these recommendations.
Although this blueprint for public reporting is easy to draft, it is challenging to implement. In particular, some of our suggestions, such as the one regarding risk adjustment, may not currently be feasible, because the complexity and cost of collecting clinical data, even in the era of electronic medical records, may be prohibitive. Until such data are readily available, it may be preferable to report nothing at all, rather than report data that are misleading. In the rush to make hospitals accountable, enthusiasm has often outstripped science,31 and several measures have had to be revised for unintended consequences.32
Any initiative to improve public reporting should have the buy‐in of all stakeholders, but particularly hospitals, which stand to benefit in several ways. By receiving regular feedback, they can focus on improving care, becoming better organizations. These improvements may be rewarded through direct compensation (pay‐for‐performance), decreased costs from complications, or increased market share. Hospitals will be more engaged if the data reflect actual quality, are adequately adjusted for severity, and acknowledge the role of chance. Otherwise, they will merely comply, or worse, look for opportunities to game the system. To succeed, public reporting needs to involve hospitals in establishing standards for reporting and validation, as well as auditing procedures to prevent fraud.33 The Hospital Quality Alliance (HQA, Washington, DC), a first step in this direction, at present has few measures. NSQIP (American College of Surgeons, Chicago, IL) is perhaps a better example of hospitals cooperating to set measurement standards to promote best‐practices. Public release of NSQIP data might accelerate progress. Alternatively, the National Quality Forum (NQF, Washington, DC) could expand its role from endorsing quality measures to include standardizing the way these measures are used in public reporting.
Still, if you build it, will they come? To date, public reporting has not been embraced by the public, despite its stated interest in the information. Several explanations could be offered. First, we may be presenting the wrong data. Process measures and mortality rates are important but represent abstract concepts for most patients. Surveys tell us that patients value most the experiences of other patients.14, 21 They want to know whether their pain will be controlled, whether the doctor will listen to them, whether the nurse will come when they call. The recent advent of the HCAHPS survey (AHRQ, Washington, DC) is another positive step. Stratifying the results by diagnosis and adding a few diagnosis‐specific questions would make HCAHPS even more valuable. Second, the data may not be readily available. Although most public reporting is done on the web, older patients who are deciding about hospitals may not have Internet access. Some reports are still proprietary, and cost could present another obstacle. Finally, even if freely‐available and patient‐centered, the results may not be interpretable by physicians, let alone patients.34
If public reporting is to succeed, it will require measures that better reflect patients' concerns. In order to collect the massive amounts of data required and present them in a timely fashion, better electronic record systems will be necessary. But these are no panacea; others have noted that the Department of Veterans Affairs, a leader in electronic records, still invests considerable time and money to review charts for NSQIP.35 Given the value that Americans place on transparency in other facets of their lives, it is clear that public reporting is here to stay. While much progress has been made over the past 5 years, additional research is needed to better measure quality from the patient's perspective, and to determine how this information can be used to help guide decision‐making, and to reward hospitals for offering the highest‐quality care.
Acknowledgements
The authors thank Kenneth Flax for his help with an earlier version of this manuscript.
Acknowledging striking deficiencies in the quality and safety of healthcare, the Institute of Medicine, policy makers, and payors have called for transformation of the US healthcare system.1 Public reporting of hospital performance is one key strategy for accelerating improvement2 and may improve quality in several ways. First, feedback about performance relative to peers may stimulate quality improvement activities by appealing to professionalism. Second, the desire to preserve one's reputation by not appearing on a list of poor performers may be a powerful incentive. Finally, patients and referring providers could use reports to select high‐quality hospitals, thereby shifting care from low‐quality to high‐quality hospitals and stimulating quality improvement efforts to maintain or enhance market share.
Almost 20 years after New York and Pennsylvania began reporting cardiac surgery outcomes,3 the evidence that public reporting improves healthcare quality is equivocal.4 Moreover, stakeholders have embraced public reporting to differing degrees. Public reporting does lead to greater engagement in quality improvement activities,58 and additional financial incentives provide modest incremental benefits.9 Purchasers, too, are starting to pay attention.10 In New York State, payors appear to contract more with high‐quality surgeons and avoid poorly performing outliers.11 Some payors are creating tiered systems, assigning higher patient copayments for hospitals with poor quality metrics. These new systems have not been rigorously studied and should raise concern among hospitals.12
In contrast to hospitals and payors, patients have been slow to embrace public reporting. In a survey of coronary artery bypass graft (CABG) patients in Pennsylvania, only 2% said that public reporting of mortality rates affected their decision making.13 Eight years later, only 11% of patients sought information about hospitals before deciding on elective major surgery,14 although a majority of patients in both studies expressed interest in the information. It is not clear whether recent proliferation of information on the internet will change patient behavior, but to date public reporting appears not to effect market share.5, 15, 16
Barriers to patients' use of public reporting include difficulty accessing the information, lack of trust, information that is not salient, and data that are difficult to interpret.17 In the absence of consensus on what or how to report, a growing number of organizations, including state and federal government, accrediting bodies, private foundations, and for‐profit companies report a variety of measures relating to structure, processes, and outcomes. Although these sites purport to target consumers, they sometimes offer conflicting information18 and are not easily interpreted by lay readers.19
To realize the benefits of public reporting, and minimize the unintended consequences, rating systems must report salient information in a way that is comprehensible to patients and trusted by the doctors who advise them. At the same time, they should be fair to hospitals and offer useful data for quality improvement. We offer 10 recommendations for improving the public reporting of healthcare quality information: 5 describing what to report and 5 detailing how it should be reported (Figure 1). We also examine 3 leading performance reporting programs to see how well they implement these recommendations.

Recommendations to Make Data Salient for Patients
1. Prioritize Elective Procedures
Hospital quality is not uniform across conditions.2 For data to be salient, then, it should be disease‐specific and focus on common elective procedures, for which consumer choice is possible. Table 1 compares 3 popular reporting services. Hospital Compare, produced by the Center for Medicare Services (CMS, US Department of Health and Human Services, Washington, DC), provides process of care measures for 4 conditions, 3 of which are not elective. The fourth, surgical infection prevention, contains 5 measures3 related to perioperative antibiotics and 2 related to thromboembolism prophylaxisfor all surgical cases. Recently, more conditions have been added, but reports are limited to the number of cases and mean Medicare charge. By year 2011, however, Hospital Compare will offer many new measures, including rates of central line infection, ventilator‐associated pneumonia, and surgical site infection. HealthGrades, a private company, offers comparative mortality rates on over 30 diagnoses, of which 15 can be considered elective, at least some of the time. Only the Leapfrog group, an industry consortium, focuses exclusively on elective procedures, offering volume measures on 7 and outcome measures on 2.
| Rule | Hospital Compare | HealthGrades | Leapfrog | |||
|---|---|---|---|---|---|---|
| ||||||
| 1. Prioritize elective procedures | Yes | 22/28 at least partially elective | Yes | 15/31 at least partially elective | Yes | 7/8 elective |
| 2. Include quality of life and outcome data, if possible | Yes | Mortality for AMI and CHF | Yes | Mortality or complications* | Yes | Outcomes for CABG, PCI, and AVR |
| 3. Include standardized patient satisfaction and service measures | Yes | HCAHPS | No | No | ||
| 4. Offer composite measures that are weighted and evidence‐based | No | No | Specialty excellence award, not evidence‐based | No | ||
| 5. Costs comparisons should include patient prices | Yes | Average Medicare payment | Yes | Charges, health plan and Medicare costs available for a fee | No | |
| 6. Adjust outcomes for severity and risk | Yes | Methodology published on website | Yes | Methodology not public | Yes | Various methodologies published or referenced on website |
| 7. Identify differences not due to chance | Yes | Compares mortality to national mean | Yes | Compares mortality or complications to mean | Yes | Compares mortality to national mean |
| 8. Standardize reporting periods | October 2005 to September 2006 | 2004‐2006 | 12‐24 months, ending 12/31/07 or 6/30/08 | |||
| 9. Avoid use of nonvalidated administrative data | Yes | None used | No | Uses PSIs for safety rating | Yes | None used |
| 10. Utilization rates should be evidence‐based | No | Surgical case volume of Medicare patients | No | Includes Caesarian‐section rates | Yes | Some case volume rates are evidence‐based |
2. Include Quality of Life and Outcome Data
Outcomes are more valuable to patients than process measures, but the risk adjustment needed to compare outcomes requires considerable effort. So far, public reporting of risk‐adjusted outcomes has been limited almost exclusively to mortality. Yet a patient contemplating knee replacement surgery would find no meaningful difference in mortalitythere were only 510 deaths nationally in year 200620but might be interested in whether patients return to full mobility after surgery, and all patients should compare rates of nosocomial infections. For some low‐risk procedures, HealthGrades Inc. (Golden, CO) includes a composite measure of major complications, including complication of an orthopedic implant, stroke, cardiac arrest, excessive bleeding, and some types of infection; CMS will soon add rates of infection and readmission.
3. Include Measures of Patient Experience, Such as Satisfaction and Service Measures
Beyond outcomes, patients want to know about the experience of others.21 Satisfaction surveys should be standardized and made disease‐specific, since patients' experiences may differ between the cardiology suite and the delivery unit. Questions could address the attentiveness of the nursing staff, how well privacy was respected, how easy it was to deal with insurance issues, whether patients were promptly informed of test results, and whether the care team answered questions fully. Medicare has begun reporting patient satisfaction using the Hospital Consumer Assessment of Healthcare Providers (HCAHPS) survey on Hospital Compare, but the data are not disease‐specific and audit a very small number of patients from each institution. Other services are unlikely to perform their own surveys, as multiple surveys would prove burdensome. Social networking sites that allow patients to post their own personal reviews of hospitals and doctors offer an additional if less reliable dimension to traditional public reporting. Such sites are already transforming the market for other industries, such as travel.22
4. Offer Composite Measures That Are Weighted and Evidence‐Based
Interpreting multiple measures, some of which are more important than others, and some of which have better evidence than others, is difficult for health care providers and may be impossible for patients. Is it more important to get aspirin on arrival or at discharge? Also, how does a patient weigh a 1% difference in the number of heart attack patients who get aspirin on arrival against a 14% difference in those who are offered smoking cessation? Because patients may be overwhelmed by data,23 public reports should include evidence‐based, weighted measures of overall care for a given condition, with higher weights attached to those process measures most likely to have clinical benefit, and careful attention to visual representations that convey relative differences.19, 23 More sophisticated measures should be developed to guard against overuse. For example, while hospitals should be rewarded for providing vaccination, they should be penalized for vaccinating the same patient twice.
None of the services we examined provides weighted outcomes. Leapfrog (The Leapfrog Group, Washington, DC) offers a composite snapshot containing 9 pie charts, divided into 4 leaps. The 6 pies representing high‐risk procedures are of equal size, even though 2 of these, esophagectomy and pancreatic resection represent very rare surgeries, even at major medical centers. From a visual perspective, however, these are equivalent to having computerized physician order entry and full‐time intensive care unit staffing, which affect thousands more patients. Similarly, in determining pay‐for‐performance measures, CMS created a composite based on the total number of opportunities of all interventions, weighting all measures equally. Because no validated weighting measures exist, future research will be necessary to achieve this goal. Also, none of the evidence‐based measures contained safeguards against overtreatment.
5. Cost Comparisons Should Include Patient Prices
In an era of patient copayments and deductibles, consumers are increasingly aware of costs. For patients with very high deductible plans or no health insurance, hospital fees are a common cause of bankruptcy.24 Several public reporting agencies, including Hospital Compare and HealthGrades have incorporated Medicare costs into their reported measures, but these have little connection to what patients actually pay. Health sites aimed at consumers should publish the average patient copayment.
Recommendations to Ensure That Data Reflects Hospital Quality
6. Adjust Outcomes for Severity and Risk
Not all bypass operations are the same and not all patients are at equal risk. More difficult operations (eg, CABG for a patient with a previous bypass) will have more complications; similarly, patients with serious comorbidities will experience worse outcomes. Since hospitals which specialize in a procedure will attract complicated cases and higher risk patients, it is important to adjust outcomes to account for these differences. Otherwise, hospitals and surgeons may be discouraged from taking difficult cases. Outside of cardiac surgery, most risk adjustment systems use administrative claims data but vary dramatically in the numbers of variables considered and the underlying proprietary models, which are often criticized as being black boxes that yield discordant results.25 Thus, a hospital's mortality may appear below expected by 1 system and above expected by another. Instead, risk adjustment systems should include clinical data abstracted from patient records using standardized data definitions. Although costly to collect, clinical data offer more predictive information than do administrative data. For example, for heart failure patients undergoing CABG, the ejection fraction predicts mortality better than many stable comorbid diagnoses. A single transparent risk‐adjustment system should be recognized as the industry standard. The American College of Surgeons' standardized risk‐adjusted outcome reporting for the National Surgical Quality Improvement Program (NSQIP) is a good example of such an effort.
7. Identify Differences Not Due to Chance
As a result of random variation, during any period, some hospitals will appear better than average and others worse. Statistical tests should be employed to identify hospitals that differ from the mean, and to allow consumers to compare 2 hospitals directly, with appropriate caveats when the hospitals serve very different patient populations. Medicare's mortality rating system for myocardial infarction identifies only 17 hospitals in the nation as better than average and 7 as worse, out of 4,500 institutions. HealthGrades compares hospitals' actual mortality or complication rates to their predicted rates based on disease‐specific logistic regression models and reports whether the hospital is statistically better or worse than predicted. Hospitals are not compared directly to one another. Given the rarity of mortality in most procedures, other outcome measures will be necessary to distinguish among hospitals.26
8. Standardize Reporting Periods
In a world of continuous quality improvement, public reporting should represent a hospital's recent performance, but reporting periods also need to be long enough to provide a stable estimate of infrequent events, especially at low‐volume institutions. In contrast, the lag time between the end of the reporting period and public availability should be kept to a minimum. We found that reporting periods varied from 1 to 3 years, and did not always cover the same years for all conditions, even on the same website. Some data were 3 years old. Patients will have a hard time making decisions on data that is 1 year old, and hospitals will have little incentive to make improvements that will not be acknowledged for years.
9. Avoid Use of Nonvalidated Administrative Data
Administrative data collected for billing purposes, unlike most clinical data, are already in electronic format, and can inexpensively produce quality rankings using validated models.27 In contrast, screening tools, such as the Agency for Healthcare Research and Quality's patient safety indicators (PSIs), were designed to identify potential quality problems, such as postoperative deep vein thrombosis, for internal quality improvement. Cases identified by the PSI software require additional chart review,28, 29 and should not be used as quality indicators. Even so, HealthGrades reports PSIs and some insurers use them in pay‐for‐performance initiatives. Improvements in PSIs, including present‐on‐admission coding, may increase accuracy,30 but these measures need to be validated before they can be adopted for public reporting.
10. Utilization Rates Should Be Evidence‐Based
Although utilization rates for most procedures vary as much as 2‐fold by state or institution, there is little evidence for a best rate. Nevertheless, HealthGrades reports utilization rates for several obstetrical procedures. At present, there are no standards for these, and it is possible that utilization could be too low in some places. Further research is needed; until then, utilization should not purport to measure quality.
Discussion
The growing commitment to making hospital performance data public could transform the quality and safety of care in the US, introducing competition on quality and price and fostering informed consumer choice. To date, the promise of public reporting remains only partially fulfilled. Few hospitals have done more than comply with regulatory mandates and payer incentives, and consumers have failed to respond. To capture the full benefits of public reporting, we have made 10 recommendations to benefit patients and better engage hospitals. We suggest that reporting be patient‐centered, with an emphasis on making the data useful, meaningful, important, interpretable, and relevant. At the same time, hospitals, which are being judged on their performance, should have a level playing field, with measures that are timely, consistent, severity‐adjusted, evidence‐based, and which foster good clinical care. Of the 3 services we examined, Hospital Compare came closest to meeting these recommendations.
Although this blueprint for public reporting is easy to draft, it is challenging to implement. In particular, some of our suggestions, such as the one regarding risk adjustment, may not currently be feasible, because the complexity and cost of collecting clinical data, even in the era of electronic medical records, may be prohibitive. Until such data are readily available, it may be preferable to report nothing at all, rather than report data that are misleading. In the rush to make hospitals accountable, enthusiasm has often outstripped science,31 and several measures have had to be revised for unintended consequences.32
Any initiative to improve public reporting should have the buy‐in of all stakeholders, but particularly hospitals, which stand to benefit in several ways. By receiving regular feedback, they can focus on improving care, becoming better organizations. These improvements may be rewarded through direct compensation (pay‐for‐performance), decreased costs from complications, or increased market share. Hospitals will be more engaged if the data reflect actual quality, are adequately adjusted for severity, and acknowledge the role of chance. Otherwise, they will merely comply, or worse, look for opportunities to game the system. To succeed, public reporting needs to involve hospitals in establishing standards for reporting and validation, as well as auditing procedures to prevent fraud.33 The Hospital Quality Alliance (HQA, Washington, DC), a first step in this direction, at present has few measures. NSQIP (American College of Surgeons, Chicago, IL) is perhaps a better example of hospitals cooperating to set measurement standards to promote best‐practices. Public release of NSQIP data might accelerate progress. Alternatively, the National Quality Forum (NQF, Washington, DC) could expand its role from endorsing quality measures to include standardizing the way these measures are used in public reporting.
Still, if you build it, will they come? To date, public reporting has not been embraced by the public, despite its stated interest in the information. Several explanations could be offered. First, we may be presenting the wrong data. Process measures and mortality rates are important but represent abstract concepts for most patients. Surveys tell us that patients value most the experiences of other patients.14, 21 They want to know whether their pain will be controlled, whether the doctor will listen to them, whether the nurse will come when they call. The recent advent of the HCAHPS survey (AHRQ, Washington, DC) is another positive step. Stratifying the results by diagnosis and adding a few diagnosis‐specific questions would make HCAHPS even more valuable. Second, the data may not be readily available. Although most public reporting is done on the web, older patients who are deciding about hospitals may not have Internet access. Some reports are still proprietary, and cost could present another obstacle. Finally, even if freely‐available and patient‐centered, the results may not be interpretable by physicians, let alone patients.34
If public reporting is to succeed, it will require measures that better reflect patients' concerns. In order to collect the massive amounts of data required and present them in a timely fashion, better electronic record systems will be necessary. But these are no panacea; others have noted that the Department of Veterans Affairs, a leader in electronic records, still invests considerable time and money to review charts for NSQIP.35 Given the value that Americans place on transparency in other facets of their lives, it is clear that public reporting is here to stay. While much progress has been made over the past 5 years, additional research is needed to better measure quality from the patient's perspective, and to determine how this information can be used to help guide decision‐making, and to reward hospitals for offering the highest‐quality care.
Acknowledgements
The authors thank Kenneth Flax for his help with an earlier version of this manuscript.
- Committee on Quality of Health Care in America IoM.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:National Academy Press;2001.
- ,,,.Care in U.S. hospitals: the Hospital Quality Alliance program.N Engl J Med.2005;353(3):265–274.
- .Achieving and sustaining improved quality: lessons from New York state and cardiac surgery.Health Aff. 20022002;21(4):40–51.
- ,,,,.Systematic review: the evidence that publishing patient care performance data improves quality of care.Ann Intern Med.2008;148(2):111–123.
- ,,.Hospital performance reports: impact on quality, market share, and reputation.Health Aff (Millwood).2005;24(4):1150–1160.
- ,,.Does publicizing hospital performance stimulate quality improvement efforts?Health Aff (Millwood).2003;22(2):84–94.
- ,,,,.Improving the outcomes of coronary artery bypass surgery in New York State.JAMA.1994;271(10):761–766.
- ,,.Declines in hospital mortality associated with a regional initiative to measure hospital performance.Am J Med Qual.1997;12(2):103–112.
- ,,, et al.Public reporting and pay for performance in hospital quality improvement.N Engl J Med.2007;356(5):486–496.
- ,,,,,.Do quality report cards play a role in HMOs' contracting practices? Evidence from New York State.Health Serv Res.2000;35(1 Pt 2):319–332.
- ,,,.Quality of cardiac surgeons and managed care contracting practices.Health Serv Res.2002;37(5):1129–1144.
- ,,,,,.Using performance data to identify preferred hospitals.Health Serv Res.2007;42(6 Pt 1):2109–2119; discussion 2294–2323.
- ,.Use of public performance reports: a survey of patients undergoing cardiac surgery.JAMA.1998;279(20):1638–1642.
- ,,.How do elderly patients decide where to go for major surgery? Telephone interview survey.BMJ.2005;331(7520):821.
- ,,,,,.The effect of publicly reporting hospital performance on market share and risk‐adjusted mortality at high‐mortality hospitals.Med Care.2003;41(6):729–740.
- ,.The predictive accuracy of the New York State coronary artery bypass surgery report‐card system.Health Aff (Millwood).2006;25(3):844–855.
- ,.Publicly disclosed information about the quality of health care: response of the US public.Qual Saf Health Care.2001;10(2):96–103.
- ,,,,.Choosing the best hospital: the limitations of public reporting of hospital quality.Health Aff (Millwood).2008;27(6):1680–1687.
- ,.Will quality report cards help consumers?Health Aff (Millwood).1997;16(3):218–228.
- Agency for Healthcare Research and Quality. HCUPnet, Healthcare Cost and Utilization Project. Available at: http://hcupnet.ahrq.gov. Accessed January 2009.
- ,,.Recovering from cardiac surgery: what patients want you to know.Am J Crit Care.2002;11(4):333–343.
- Trip Advisor. Available at: http://www.tripadvisor.com. Accessed January 2009.
- ,,,,.Less is more in presenting quality information to consumers.Med Care Res Rev.2007;64(2):169–190.
- ,,,.MarketWatch: illness and injury as contributors to bankruptcy.Health Aff (Millwood)2005;(Suppl Web Exclusives):W5‐63–W5‐73.
- .The Lake Wobegon effect: when all the patients are sicker.Am J Med Qual.2006;21(6):365–366.
- ,,.Surgical mortality as an indicator of hospital quality: the problem with small sample size.JAMA.2004;292(7):847–851.
- ,,, et al.An administrative claims model suitable for profiling hospital performance based on 30‐day mortality rates among patients with heart failure.Circulation.2006;113(13):1693–1701.
- ,,,.Can administrative data be used to compare postoperative complication rates across hospitals?Med Care.2002;40(10):856–867.
- ,,,,.Impact of diagnosis‐timing indicators on measures of safety, comorbidity, and case mix groupings from administrative data sources.Med Care.2007;45(8):781–788.
- ,,,,.Do the AHRQ patient safety indicators flag conditions that are present at the time of hospital admission?Med Care.2008;46(5):516–522.
- ,,.The tension between needing to improve care and knowing how to do it.N Engl J Med.2007;357(6):608–613.
- ,,,.Public reporting of antibiotic timing in patients with pneumonia: lessons from a flawed performance measure.Ann Intern Med.2008;149(1):29–32.
- ,,.The GAAP in quality measurement and reporting.JAMA.2007;298(15):1800–1802.
- ,,,.Consumer competencies and the use of comparative quality information: it isn't just about literacy.Med Care Res Rev.2007;64(4):379–394.
- .Performance measurement in search of a path.N Engl J Med.2007;356(9):951–953.
- Committee on Quality of Health Care in America IoM.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:National Academy Press;2001.
- ,,,.Care in U.S. hospitals: the Hospital Quality Alliance program.N Engl J Med.2005;353(3):265–274.
- .Achieving and sustaining improved quality: lessons from New York state and cardiac surgery.Health Aff. 20022002;21(4):40–51.
- ,,,,.Systematic review: the evidence that publishing patient care performance data improves quality of care.Ann Intern Med.2008;148(2):111–123.
- ,,.Hospital performance reports: impact on quality, market share, and reputation.Health Aff (Millwood).2005;24(4):1150–1160.
- ,,.Does publicizing hospital performance stimulate quality improvement efforts?Health Aff (Millwood).2003;22(2):84–94.
- ,,,,.Improving the outcomes of coronary artery bypass surgery in New York State.JAMA.1994;271(10):761–766.
- ,,.Declines in hospital mortality associated with a regional initiative to measure hospital performance.Am J Med Qual.1997;12(2):103–112.
- ,,, et al.Public reporting and pay for performance in hospital quality improvement.N Engl J Med.2007;356(5):486–496.
- ,,,,,.Do quality report cards play a role in HMOs' contracting practices? Evidence from New York State.Health Serv Res.2000;35(1 Pt 2):319–332.
- ,,,.Quality of cardiac surgeons and managed care contracting practices.Health Serv Res.2002;37(5):1129–1144.
- ,,,,,.Using performance data to identify preferred hospitals.Health Serv Res.2007;42(6 Pt 1):2109–2119; discussion 2294–2323.
- ,.Use of public performance reports: a survey of patients undergoing cardiac surgery.JAMA.1998;279(20):1638–1642.
- ,,.How do elderly patients decide where to go for major surgery? Telephone interview survey.BMJ.2005;331(7520):821.
- ,,,,,.The effect of publicly reporting hospital performance on market share and risk‐adjusted mortality at high‐mortality hospitals.Med Care.2003;41(6):729–740.
- ,.The predictive accuracy of the New York State coronary artery bypass surgery report‐card system.Health Aff (Millwood).2006;25(3):844–855.
- ,.Publicly disclosed information about the quality of health care: response of the US public.Qual Saf Health Care.2001;10(2):96–103.
- ,,,,.Choosing the best hospital: the limitations of public reporting of hospital quality.Health Aff (Millwood).2008;27(6):1680–1687.
- ,.Will quality report cards help consumers?Health Aff (Millwood).1997;16(3):218–228.
- Agency for Healthcare Research and Quality. HCUPnet, Healthcare Cost and Utilization Project. Available at: http://hcupnet.ahrq.gov. Accessed January 2009.
- ,,.Recovering from cardiac surgery: what patients want you to know.Am J Crit Care.2002;11(4):333–343.
- Trip Advisor. Available at: http://www.tripadvisor.com. Accessed January 2009.
- ,,,,.Less is more in presenting quality information to consumers.Med Care Res Rev.2007;64(2):169–190.
- ,,,.MarketWatch: illness and injury as contributors to bankruptcy.Health Aff (Millwood)2005;(Suppl Web Exclusives):W5‐63–W5‐73.
- .The Lake Wobegon effect: when all the patients are sicker.Am J Med Qual.2006;21(6):365–366.
- ,,.Surgical mortality as an indicator of hospital quality: the problem with small sample size.JAMA.2004;292(7):847–851.
- ,,, et al.An administrative claims model suitable for profiling hospital performance based on 30‐day mortality rates among patients with heart failure.Circulation.2006;113(13):1693–1701.
- ,,,.Can administrative data be used to compare postoperative complication rates across hospitals?Med Care.2002;40(10):856–867.
- ,,,,.Impact of diagnosis‐timing indicators on measures of safety, comorbidity, and case mix groupings from administrative data sources.Med Care.2007;45(8):781–788.
- ,,,,.Do the AHRQ patient safety indicators flag conditions that are present at the time of hospital admission?Med Care.2008;46(5):516–522.
- ,,.The tension between needing to improve care and knowing how to do it.N Engl J Med.2007;357(6):608–613.
- ,,,.Public reporting of antibiotic timing in patients with pneumonia: lessons from a flawed performance measure.Ann Intern Med.2008;149(1):29–32.
- ,,.The GAAP in quality measurement and reporting.JAMA.2007;298(15):1800–1802.
- ,,,.Consumer competencies and the use of comparative quality information: it isn't just about literacy.Med Care Res Rev.2007;64(4):379–394.
- .Performance measurement in search of a path.N Engl J Med.2007;356(9):951–953.
Copyright © 2009 Society of Hospital Medicine
Inappropriate Hospital Prescribing
Medications can be considered inappropriate when their risk outweighs their benefit. The Beers list1 identifies medications that should be avoided in persons 65 years or older because they are ineffective or pose an unnecessarily high risk or because a safer alternative is available. Initially developed in 1991, the list has gained wide acceptance and has been updated twice.2, 3 In July 1999 it was adopted by the Centers for Medicare & Medicaid Services (CMS) for nursing home regulation, and in 2006 the National Committee on Quality Assurance adopted a modified version as a Health Plan Employer Data and Information Set (HEDIS) measure of quality of care for older Americans.4
A number of studies have demonstrated that inappropriate prescribing is common in the ambulatory setting,57 in nursing homes,8, 9 and in emergency departments10, 11 and that exposure to inappropriate medications is associated with increased risk of adverse drug reactions12 and hospitalization.13, 14 Initial studies of hospitalized patients1517 suggest that potentially inappropriate prescribing is also common among elderly inpatients and that reducing the misuse of psychotropic medications can prevent falls.18 We report on the incidence of and risk factors associated with potentially inappropriate prescribing in a large sample of hospitalized elders.
METHODS
Patients
We conducted a retrospective cohort study using data from 384 hospitals participating in Perspective (Premier, Inc., Charlotte, NC), a database developed for measuring quality and health care utilization. Participating hospitals represent all regions of the United States and are primarily small‐ to medium‐sized nonteaching hospitals most of which are in urban areas. Premier collects data elements from participating hospitals via a custom data extract from hospitals' decision support system. Hospitals aggregate the data elements into their decision support systems from multiple information technology systems including billing, medical records, pharmacy, and laboratory systems. In addition to the information contained in the standard hospital discharge file, Perspective includes a date‐stamped log of all billed items, including medications with dose and quantity, for individual patients.
We included patients at least 65 years old admitted between September 1, 2002, and June 30, 2005, with a principal diagnosis of acute myocardial infarction, chronic obstructive pulmonary disease, chest pain, community‐acquired pneumonia, congestive heart failure, ischemic stroke, or urinary tract infection. International Classification of Diseases, Ninth Revision (ICD‐9‐CM) codes were used to identify diagnoses. Patients cared for by an attending physician with a surgical specialty were excluded. The study protocol was approved by the institutional review board of Baystate Medical Center.
Data Elements
For each patient, Perspective contains fields for age, sex, race, marital status, insurance status, principal diagnosis, comorbidities, and specialty of the attending physician. Comorbidities were identified from ICD‐9‐CM secondary diagnosis codes and APR‐DRGs using Healthcare Cost and Utilization Project Comorbidity Software, version 3.1, based on the work of Elixhauser.19 Because almost all patients had Medicare coverage, plans were classified according to managed care status. Finally, for each patient we identified all medications administered, as well as discharge status, readmission rate, total costs, and length of stay. Hospitals were categorized by region (Northeast, South, Midwest, or West), bed size, setting (urban or rural), teaching status, and whether there were geriatricians.
Potentially Inappropriate Prescribing
Using the 2002 updated Beers criteria3 for potentially inappropriate medication (PIM) use in older adults, we identified the total number of PIMs administered to each patient during his or her hospital stay. We classified each PIM as either high or low severity based on the expert consensus expressed in the 1997 update of the Beers criteria.2 The list contains 48 PIMs and an additional 20 that should be avoided in patients with certain conditions. We did not include the second category of PIMs because we did not necessarily have sufficient patient information to make this determination. In addition, some of the standard PIMs, such as laxatives, although inappropriate for chronic outpatient use, could be appropriate in the hospital setting and were excluded from this analysis. Finally, several medications were considered inappropriate only above a given threshold (eg, lorazepam >3.0 mg/day) or for patients without a specific diagnosis (eg, digoxin >0.125 mg/day for patients without atrial fibrillation). We grouped PIMs that had similar side effects into 4 categories: sedatives, anticholinergics, causing orthostasis, or causing bleeding (Fig. 1).
Statistical Analysis
Summary statistics at the patient, physician, and hospital levels were constructed using frequencies and proportions for categorical data and means, standard deviations, medians, interquartile ranges, and box plots for continuous‐scale variables. Patients were identified as receiving a PIM if the drug was administered (above threshold dose where applicable) on at least 1 hospital day. We examined the association of each patient characteristic with use of any PIM, any high‐severity‐rated PIM, and each side effect category using chi‐square statistics. Kruskal‐Wallis analysis of variance was used to examine variation in hospital use rates by each hospital characteristic, and physician use rates for high‐severity PIMs by attending specialty. To examine whether it was feasible to avoid PIMs altogether, we compared individual hospitals as well as individual prescribers within their specialty, limiting the comparison to hospitals that contributed at least 100 patients and to physicians with at least 50 patients.
We developed a multivariable model for any high‐severity medication (HS‐PIM) use that included all patient, physician, and hospital characteristics except length of stay, mortality, cost, discharge status, and readmission rate. A generalized estimating equation model (SAS PROC GENMOD) with a logit link and a subcluster correlation structure was used to account for clustering at the hospital, physician, and diagnosis levels, adjusting for the clustering of primary diagnosis within physician level, nested within hospital level. Effects with P < .10 were retained in the model, and interaction effects were also evaluated for significance. Model fit was assessed using deviance and Pearson chi‐square statistics. All analyses were performed with SAS statistical software, version 9.1 (SAS Institute, Cary, NC).
RESULTS
We identified 519,853 patients at least 65 years old during the study period; 564 were excluded because of missing data for key variables or unclear principal diagnosis. An additional 25,318 were excluded because they were cared for by an attending with a surgical specialty. A total of 493,971 patients were included in the study (Table 1). Mean age was 78 years, and 24% of patients were 85 years or older. Forty‐three percent were male, 71% were white, and 39% were currently married. The most common principal diagnoses were community‐acquired pneumonia, congestive heart failure, and acute myocardial infarction. The most common comorbidities were hypertension, diabetes, and chronic pulmonary disease. Medicare was the primary payer for 91% of subjects, and 13% were in managed care plans. Most patients were cared for by internists (49%), family practitioners (18%), or cardiologists (17%). Only 1% of patients had a geriatrician as attending.
| Characteristic | n (%) |
|---|---|
| |
| Age group | |
| 6574 years | 168,527 (34%) |
| 7584 years | 206,407 (42%) |
| 85+ years | 119,037 (24%) |
| Sex | |
| Male | 212,358 (43%) |
| Female | 281,613 (57%) |
| Race | |
| White | 351,331 (71%) |
| Black | 52,429 (11%) |
| Hispanic | 18,057 (4%) |
| American Indian | 1876 (0%) |
| Asian/Pacific Islander | 5926 (1%) |
| Other | 64,352 (13%) |
| Marital status | |
| Married/partner | 194,496 (39%) |
| Widowed | 155,273 (31%) |
| Single/separated/divorced | 75,964 (15%) |
| Other | 68,238 (14%) |
| Primary diagnosis | |
| Pneumonia | 122,732 (25%) |
| Heart failure | 109,071 (22%) |
| Acute MI | 70,581 (14%) |
| Ischemic stroke | 57,204 (12%) |
| Chest pain | 50,404 (10%) |
| COPD | 44,582 (9%) |
| Urinary tract infection | 39,397 (8%) |
| Comorbidities | |
| Hypertension | 310,163 (63%) |
| Diabetes | 151,755 (31%) |
| Chronic pulmonary disease | 134,900 (27%) |
| Fluid and electrolyte disorders | 128,703 (26%) |
| Deficiency anemias | 92,668 (19%) |
| Congestive heart failure | 69,201 (14%) |
| Hypothyroidism | 68,711 (14%) |
| Peripheral vascular disease | 47,244 (10%) |
| Depression | 41,507 (8%) |
| Other neurological disorders | 40,200 (8%) |
| Renal failure | 38,134 (8%) |
| Obesity | 25,143 (5%) |
| Payer type | |
| Not Managed care | 431,583 (87%) |
| Managed care | 62,388 (13%) |
| Attending physician specialty | |
| Internal medicine (internist) | 241,982 (49%) |
| Family/general medicine | 90,827 (18%) |
| Cardiology | 83,317 (17%) |
| Pulmonology | 21,163 (4%) |
| Hospitalist | 14,924 (3%) |
| Nephrology | 8257 (2%) |
| Neurology | 5800 (1%) |
| Geriatrics | 3099 (1%) |
| Other* | 24,602 (5%) |
| Mortality | |
| Expired | 28,321 (6%) |
| Alive | 465,650 (94%) |
| Discharge status, n (% of survivors) | |
| Home | 323,629 (66%) |
| Nursing care | 119,468 (24%) |
| Transfer/short‐term hospital | 13,531 (3%) |
| Hospice | 9022 (2%) |
| 14‐Day readmission, n (% of survivors) | |
| Yes | 35,309 (8%) |
| No | 430,334 (92%) |
| Length of stay (days), median (IQR) | 4 (2, 7) |
| Total cost (dollars) | $5513 ($3366, $9902) |
Just under half of all patients (49%) received at least 1 PIM, and 6% received 3 or more (Table 2). Thirty‐eight percent received at least 1 drug with a high severity rating (HS‐PIM). The most common PIMs were promethazine, diphenhydramine, propoxyphene, clonidine, amiodarone, and lorazepam (>3 mg/day).
| Patients, n (%) | |
|---|---|
| Number of PIMs | |
| 0 | 254,200 (51%) |
| 1 | 146,028 (30%) |
| 2 | 61,445 (12%) |
| 3 | 22,128 (4%) |
| 4+ | 10,170 (2%) |
| Number of high‐severity‐rated PIMs | |
| 0 | 304,523 (62%) |
| 1 | 129,588 (26%) |
| 2 | 43,739 (9%) |
| 3 | 12,213 (2%) |
| 4+ | 3908 (1%) |
| Use of any PIM by side effect class | |
| Sedatives | 156,384 (32%) |
| Anticholinergic effects | 109,293 (22%) |
| Causing orthostasis | 43,805 (9%) |
| Causing bleeding | 14,744 (3%) |
| Most commonly prescribed | |
| Promethazine | 49,888 (10%) |
| Diphenhydramine | 45,458 (9%) |
| Propoxyphene | 41,786 (8%) |
| Clonidine | 34,765 (7%) |
| Amiodarone | 34,318 (7%) |
| Lorazepam (>3 mg/day) | 25,147 (5%) |
Patient, Physician, and Hospital Factors Associated with PIMs
Patient, physician, and hospital characteristics were all associated with use of PIMs (Table 3). In univariate analyses, older patients were less likely to receive any class of PIM, and this difference was accentuated for HS‐PIMs. Women, American Indians, married people, and those not in managed care plans were slightly more likely to receive PIMs, whereas patients admitted with acute myocardial infarction or congestive heart failure were even more likely to receive PIMs (P < .0001 for all comparisons).
| Patient characteristic | Any PIM n (row %) | Any high‐severity PIM n (row %) | Sedatives n (row %) | Anticholinergic effects n (row %) | Causing orthostasis n (row %) | Causing bleeding n (row %) |
|---|---|---|---|---|---|---|
| ||||||
| Overall | 239,771 (49%) | 189,448 (38%) | 156,384 (32%) | 109,293 (22%) | 43,805 (9%) | 14,744 (3%) |
| Age group | ||||||
| 6574 years | 89,168 (53%) | 72,573 (43%) | 61,399 (36%) | 44,792 (27%) | 15,799 (9%) | 6655 (4%) |
| 7584 years | 100,787 (49%) | 79,595 (39%) | 65,034 (32%) | 45,121 (22%) | 18,519 (9%) | 5727 (3%) |
| 85+ years | 49,816 (42%) | 37,280 (31%) | 29,951 (25%) | 19,380 (16%) | 9487 (8%) | 2362 (2%) |
| Sex | ||||||
| Male | 100,824 (47%) | 79,535 (37%) | 63,591 (30%) | 42,754 (20%) | 17,885 (8%) | 5771 (3%) |
| Female | 138,947 (49%) | 109,913 (39%) | 92,793 (33%) | 66,539 (24%) | 25,920 (9%) | 8973 (3%) |
| Race | ||||||
| White | 173,481 (49%) | 139,941 (40%) | 112,556 (32%) | 81,097 (23%) | 27,555 (8%) | 10,590 (3%) |
| Black | 26,793 (51%) | 18,655 (36%) | 18,720 (36%) | 11,263 (21%) | 8925 (17%) | 1536 (3%) |
| Hispanic | 8509 (47%) | 6370 (35%) | 5549 (31%) | 3505 (19%) | 2047 (11%) | 648 (4%) |
| American Indian | 1091 (58%) | 849 (45%) | 818 (44%) | 563 (30%) | 190 (10%) | 76 (4%) |
| Asian/Pacific Islander | 2386 (40%) | 1896 (32%) | 1420 (24%) | 1023 (17%) | 519 (9%) | 127 (2%) |
| Other | 27,511 (43%) | 21,737 (34%) | 17,321 (27%) | 11,842 (18%) | 4569 (7%) | 1767 (3%) |
| Marital status | ||||||
| Married/partner | 96,874 (50%) | 77,803 (40%) | 63,303 (33%) | 45,042 (23%) | 16,765 (9%) | 5969 (3%) |
| Widowed | 74,622 (48%) | 58,012 (37%) | 48,367 (31%) | 33,516 (22%) | 13,865 (9%) | 4354 (3%) |
| Single/separated/divorced | 36,583 (48%) | 28,799 (38%) | 24,251 (32%) | 16,115 (21%) | 7229 (10%) | 2399 (3%) |
| Other | 31,692 (46%) | 24,834 (36%) | 20,463 (30%) | 14,620 (21%) | 5946 (9%) | 2022 (3%) |
| Primary diagnosis | ||||||
| Pneumonia | 56,845 (46%) | 46,271 (38%) | 35,353 (29%) | 25,484 (21%) | 9184 (7%) | 4155 (3%) |
| Heart failure | 56,460 (52%) | 42,231 (39%) | 34,340 (31%) | 22,093 (20%) | 10,117 (9%) | 1945 (2%) |
| Acute MI | 43,046 (61%) | 37,849 (54%) | 32,560 (46%) | 25,568 (36%) | 4738 (7%) | 2549 (4%) |
| Ischemic stroke | 25,763 (45%) | 17,613 (31%) | 18,500 (32%) | 8742 (15%) | 9644 (17%) | 1384 (2%) |
| Chest pain | 20,655 (41%) | 16,363 (32%) | 13,536 (27%) | 10,520 (21%) | 3474 (7%) | 2027 (4%) |
| COPD | 18,876 (42%) | 14,626 (33%) | 12,087 (27%) | 8096 (18%) | 3209 (7%) | 1139 (3%) |
| Urinary tract infection | 18,126 (46%) | 14,495 (37%) | 10,008 (25%) | 8790 (22%) | 3439 (9%) | 1545 (4%) |
| Payer type | ||||||
| Nonmanaged care | 212,150 (49%) | 168,013 (39%) | 138,679 (32%) | 97,776 (23%) | 38,341 (9%) | 12,868 (3%) |
| Managed care | 27,621 (44%) | 21,435 (34%) | 17,705 (28%) | 11,517 (18%) | 5464 (9%) | 1876 (3%) |
| Attending physician specialty* | ||||||
| Internal medicine (internist%) | 112,664 (47%) | 86,907 (36%) | 71,382 (30%) | 48,746 (20%) | 23,221 (10%) | 7086 (3%) |
| Family/general medicine | 41,303 (45%) | 32,338 (36%) | 25,653 (28%) | 18,274 (20%) | 7660 (8%) | 2852 (3%) |
| Cardiology | 48,485 (58%) | 40,752 (49%) | 34,859 (42%) | 25,792 (31%) | 5455 (7%) | 2542 (3%) |
| Pulmonology | 10,231 (48%) | 8105 (38%) | 6746 (32%) | 4064 (19%) | 1739 (8%) | 574 (3%) |
| Hospitalist | 7003 (47%) | 5443 (36%) | 4447 (30%) | 3179 (21%) | 1471 (10%) | 463 (3%) |
| Nephrology | 4508 (55%) | 3388 (41%) | 3132 (38%) | 2054 (25%) | 1326 (16%) | 198 (2%) |
| Neurology | 2420 (42%) | 1789 (31%) | 1625 (28%) | 851 (15%) | 699 (12%) | 174 (3%) |
| Geriatrics | 1020 (33%) | 785 (25%) | 596 (19%) | 404 (13%) | 196 (6%) | 41 (1%) |
The HS‐PIM prescribing varied substantially by attending specialty (Fig. 2). Internists, family practitioners, and hospitalists all had similar median rates (33%), cardiologists had a higher median rate (48%), and geriatricians had a lower rate (24%). The most common PIM also differed by specialty: whereas promethazine was the most commonly prescribed drug across most specialties, nephrologists and neurologists used clonidine, pulmonologists used lorazepam, and cardiologists used diphenhydramine most often. Among the 8% of physicians who saw at least 50 patients, there was also great variation in each specialty (Fig. 2). Among internists and cardiologists who saw at least 50 patients, the high‐severity PIM usage rate ranged from 0% to more than 90%.
There was substantial variation in PIM usage among hospitals, most notably by region. The mean proportion of patients receiving PIMs ranged from 34% at hospitals in the Northeast to 55% at hospitals in the South (Table 4). Smaller hospitals and those in urban settings had slightly lower rates, as did those that had geriatricians on staff. The teaching status of the hospital had little effect. Variation at the individual hospital level was extreme (Fig. 3). Although half of all hospitals had rates between 43% and 58%, in 7 hospitals with more than 300 encounters each, PIMs were never prescribed for geriatric patients.
| Hospitals Total = 384 n (%) | Patients N = 49,3971 n (%) | Any PIM Mean = 48.2 Mean (SD) | Any high‐severity PIM Mean = 38.7 Mean (SD) | Sedatives Mean = 30.2 Mean (SD) | Anticholinergic effects Mean = 21.5 Mean (SD) | Causing orthostasis Mean = 8.5 Mean (SD) | Causing bleeding Mean = 3.1 Mean (SD) | |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Hospital region | *** | *** | *** | *** | *** | ** | ||
| Midwest | 76 (20%) | 95,791 (19%) | 38.8 (19.7) | 30.0 (16.4) | 24.3 (13.8) | 15.1 (9.9) | 6.9 (6.3) | 3.1 (2.3) |
| Northeast | 47 (12%) | 79,138 (16%) | 34.1 (12.6) | 26.2 (11.2) | 19.0 (9.2) | 13.5 (8.1) | 4.9 (2.3) | 2.1 (1.6) |
| South | 199 (52%) | 260,870 (53%) | 54.5 (10.1) | 42.7 (9.6) | 36.0 (10.8) | 26.4 (8.6) | 10.4 (4.6) | 3.6 (2.5) |
| West | 62 (16%) | 58,172 (12%) | 45.8 (8.1) | 37.4 (7.1) | 27.3 (7.7) | 19.5 (5.7) | 7.4 (4.8) | 2.7 (1.3) |
| Teaching status | ||||||||
| Nonteaching | 297 (77%) | 324,948 (66%) | 47.3 (14.6) | 36.9 (12.3) | 29.8 (12.0) | 21.3 (9.9) | 8.7 (5.4) | 3.3 (2.4) |
| Teaching | 87 (23%) | 169,023 (34%) | 48.2 (16.0) | 38.8 (14.2) | 31.6 (14.5) | 22.1 (10.2) | 7.8 (4.4) | 2.7 (1.5) |
| Staffed beds | * | ** | ||||||
| 22200 | 143 (37%) | 80,741 (16%) | 45.5 (16.9) | 35.2 (14.6) | 27.5 (14.0) | 20.1 (10.3) | 8.0 (6.2) | 3.5 (3.1) |
| 200400 | 137 (36%) | 177,286 (36%) | 47.7 (14.2) | 37.8 (12.0) | 30.5 (11.6) | 22.0 (10.0) | 8.4 (4.7) | 3.0 (1.6) |
| 400+ | 104 (27%) | 235944 (48%) | 50.1 (12.4) | 39.6 (10.6) | 33.5 (10.9) | 22.7 (9.3) | 9.3 (4.2) | 2.9 (1.4) |
| Population serviced | * | * | ** | |||||
| Rural | 119 (31%) | 102,799 (21%) | 48.4 (13.0) | 38.3 (10.6) | 29.2 (11.0) | 23.2 (9.3) | 7.5 (4.0) | 3.7 (3.0) |
| Urban | 265 (69%) | 391,172 (79%) | 47.1 (15.7) | 36.9 (13.7) | 30.6 (13.2) | 20.7 (10.2) | 9.0 (5.6) | 2.9 (1.8) |
| Geriatrician presence | ||||||||
| No | 340 (89%) | 409,281 (83%) | 47.7 (15.3) | 37.6 (13.0) | 30.3 (12.8) | 21.7 (10.0) | 8.4 (5.3) | 3.2 (2.3) |
| Yes | 44 (11%) | 84,690 (17%) | 45.8 (11.4) | 35.5 (10.6) | 29.4 (10.8) | 19.6 (9.4) | 9.3 (4.3) | 2.9 (1.6) |
Multivariable Model
In a multivariable logit model that included all patient, hospital, and physician characteristics and that accounted for clustering at the hospital, physician, and diagnosis levels, several characteristics were associated with HS‐PIM prescribing (Table 5). By far the most important predictor of use was hospital region. Compared with patients at hospitals in the Midwest, patients in the South (OR 1.63, 95% CI 1.591.67) and West (OR 1.43, 95% CI 1.381.47) were more likely and those in the Northeast were less likely (OR 0.85, 95% CI 0.830.88) to receive HS‐PIMs. Larger hospitals had higher HS‐PIM rates than smaller ones, but teaching status and rural or urban setting were not associated with HS‐PIM prescribing. The presence of geriatricians in a hospital was also associated with lower HS‐PIM prescribing for the entire hospital.
| Effect (reference) | Odds ratio | 95% Confidence limits | |
|---|---|---|---|
| Age | |||
| 6574 years | 1.00 | ||
| 7584 years | 0.83 | 0.82 | 0.84 |
| 85+ years | 0.59 | 0.58 | 0.61 |
| Sex | |||
| Female | 1.00 | ||
| Male | 0.85 | 0.83 | 0.86 |
| Race | |||
| White | 1.00 | ||
| Black | 0.78 | 0.76 | 0.80 |
| Hispanic | 0.84 | 0.81 | 0.87 |
| American Indian | 0.97 | 0.88 | 1.07 |
| Asian/Pacific Islander | 0.74 | 0.70 | 0.79 |
| Other | 0.94 | 0.92 | 0.97 |
| Marital Status | |||
| Married/partner | 1.00 | ||
| Single/separated/divorced | 0.96 | 0.94 | 0.98 |
| Widowed | 0.96 | 0.95 | 0.98 |
| Other | 0.93 | 0.90 | 0.95 |
| Primary diagnosis | |||
| Pneumonia | 1.00 | ||
| COPD | 0.83 | 0.81 | 0.85 |
| Heart failure | 1.14 | 1.12 | 1.16 |
| Ischemic stroke | 0.84 | 0.82 | 0.86 |
| Acute MI | 1.95 | 1.90 | 2.01 |
| Urinary tract infection | 1.06 | 1.03 | 1.09 |
| Chest pain | 0.87 | 0.84 | 0.89 |
| Comorbidities (yes or no) | |||
| Hypertension | 0.98 | 0.97 | 0.99 |
| Diabetes | 0.98 | 0.97 | 1.00 |
| Chronic lung disease | 1.11 | 1.10 | 1.13 |
| Fluid and electrolyte disorders | 1.26 | 1.24 | 1.27 |
| Anemia deficiencies | 1.17 | 1.15 | 1.18 |
| Congestive heart failure | 1.34 | 1.32 | 1.37 |
| Hypothyroidism | 1.13 | 1.11 | 1.15 |
| Peripheral vascular disease | 1.09 | 1.06 | 1.11 |
| Depression | 1.38 | 1.35 | 1.41 |
| Neurological disorders | 0.89 | 0.87 | 0.91 |
| Renal failure | 1.23 | 1.20 | 1.26 |
| Obesity | 1.11 | 1.08 | 1.14 |
| Payer type | |||
| Managed care | 1.00 | ||
| Not managed care | 1.04 | 1.02 | 1.06 |
| Attending physician specialty | |||
| Internal medicine | 1.00 | ||
| Cardiology | 1.32 | 1.28 | 1.36 |
| Family/general medicine | 0.99 | 0.97 | 1.01 |
| Geriatrics | 0.69 | 0.61 | 0.78 |
| Hospitalist | 0.90 | 0.84 | 0.96 |
| Nephrology | 1.02 | 0.96 | 1.08 |
| Neurology | 0.93 | 0.86 | 1.00 |
| Pulmonology | 1.10 | 1.05 | 1.15 |
| Setting | |||
| Rural | 1.00 | ||
| Urban | 1.02 | 1.00 | 1.05 |
| Teaching status | |||
| Nonteaching | 1.00 | ||
| Teaching | 1.01 | 0.98 | 1.03 |
| Number of beds | |||
| 22200 | 1.00 | ||
| 200400 | 1.08 | 1.05 | 1.11 |
| 400+ | 1.12 | 1.09 | 1.16 |
| Region | |||
| Midwest | 1.00 | ||
| Northeast | 0.85 | 0.83 | 0.88 |
| South | 1.63 | 1.59 | 1.67 |
| West | 1.43 | 1.38 | 1.47 |
| Geriatrician presence | |||
| No | 1.00 | ||
| Yes | 0.93 | 0.90 | 0.95 |
Physician specialty was also important. Adjusting for diagnosis attenuated some of this association, but compared with internists, cardiologists (OR 1.32, 95% CI 1.281.36) and pulmonologists (OR 1.10, 95% CI 1.051.15) were still more likely, hospitalists (OR 0.90, 95% CI 0.840.96) were less likely, and geriatricians (0.69, 95% CI 0.610.78) were least likely to prescribe any HS‐PIM.
Patient factors were also associated with HS‐PIM use. Compared with patients age 6574 years, patients older than 85 years were much less likely to receive an HS‐PIM (OR 0.59, CI 0.580.61), as to a lesser extent were nonwhites compared with whites and unmarried people compared with those who were married. Compared with patients with pneumonia, those with COPD, stroke, or chest pain were less likely and those with myocardial infarction and congestive heart failure were more likely to receive HS‐PIMs. Patients with a secondary diagnosis of depression were also at high risk (OR 1.38, CI 1.351.41).
DISCUSSION
Although Americans age 65 years and older make up less than 15% of the U.S. population, they consume about one third of all prescription drugs20 and account for one third of all hospital admissions.21 Using the Beers list, numerous studies have documented high rates of potentially inappropriate prescribing for community‐dwelling elderly and nursing home patients and, in some studies, an attendant risk of falling,2224 hip fracture,25, 26 hospitalization,13 or death.14 Applying these same criteria to a large sample of medical inpatients, we found that almost half received a potentially inappropriate drug, most of high severity. Moreover, the PIM prescribing rate varied substantially by region, hospital, and attending physician specialty. Although the use of PIMs was associated with patient age, comorbidities, and primary diagnosis, these patient factors explained only a small portion of the variation in prescribing practices across groups of physicians and hospitals.
Using consensus criteria, Beers originally found that 40% of the residents in 12 nursing homes received at least 1 PIM,8 and studies of community‐dwelling elderly demonstrated rates of 21% to 37%, with little change over time.6, 27, 28 Several small studies have examined inpatient prescribing.16, 17, 29, 30 The largest17 found that only 15% of elderly Italian inpatients received a PIM. Our finding, that 49% of inpatients had received at least 1 PIM, may partially reflect the high prevalence of use among elderly US patients in nursing homes and the community.
Regional variation has been demonstrated for ambulatory patients in the US6 and Europe.31 Zhan et al. found slightly higher rates of PIM use in the Midwest and the South (23%) than in the Northeast and the West (19%). Variation in Europe was greater, with 41% of patients in the Czech Republic versus 5.8% of patients in Denmark receiving at least 1 PIM. We found that region was the strongest predictor of in‐hospital HS‐PIM use, with patients in the South most likely and patients in the Northeast least likely to receive HS‐PIMs. This variation persisted even after adjusting for differences in other patient and hospital factors, suggesting that local custom played a large role in the decision to prescribe HS‐PIMs. Moreover, because outpatient rates are more uniform, these large differences seem limited to inpatient practice.
Patient factors have also been examined. Advanced age was associated with decreased PIM use in some studies17, 28, 31 but not in others.6, 27 We found increasing age to be strongly associated with decreased PIM use, suggesting that in the hospital, at least, doctors take care to avoid prescribing certain drugs to the frail elderly. Women appear to be consistently at higher risk than men,6, 27, 28, 31 and white patients are more at risk than those of other races.6 Our finding that certain diagnoses were associated with higher or lower rates has not been reported previously. The lower rates associated with stroke and COPD suggest that prescribers were aware that these patients were at increased risk of delirium and respiratory depression. The higher rates associated with myocardial infarction may have to do with the use of standardized order sets (eg, cath lab orders) that do not consider the age of the patient going for the procedure.
Admission to a geriatric service32 and intervention by a clinical pharmacist33 have been shown to decrease PIM prescribing at discharge. We noted that patients cared for by a geriatrician had the lowest rates of PIM prescribing during hospitalization as well and that hospitals with geriatricians had lower rates overall, possibly demonstrating that geriatricians had a ripple effect on their colleagues. Hospitalists also had lower rates than internists, supporting the notion that hospitalists provide higher‐quality inpatient care.
Our study had some important limitations. First, we only had access to inpatient administrative records. Thus, we could not identify which medications were continued from home and which were begun in the hospital, nor could we know the indications for which specific drugs were prescribed or who prescribed them. Based on published outpatient rates, however, we could assume that many of the drugs were started in the hospital and that others could have been discontinued but were not. Second, the Beers list was developed by the modified Delphi method; there was little empirical evidence of the danger of specific drugs, although some classes, such as benzodiazepines, opiates and digoxin, have been associated with inpatient falls.18, 3436 Furthermore, our administrative database did not allow us to balance the risks and benefits for particular patients; hence, the medications were only potentially inappropriate, and our study did not address the consequences of such prescribing. Although some of these drugs may be appropriate under certain circumstances, it is unlikely that these circumstances would vary by 60% across geographic regions or that internists would encounter these circumstances more often than do hospitalists. Thus, although we could not identify specific patients who received inappropriate medications, we did identify certain hospitals and even whole regions of the country in which the rate of inappropriate prescribing was high. Third, the Beers list, which was developed for outpatient use, may be less relevant in the inpatient setting. However, given that inpatients have more organ dysfunction and are at higher risk of delirium and falls, it may actually be more applicable to hospitalized patients. We similarly did not distinguish between single and multiple doses because the Beers list does not make such a distinction, and there is no empirical evidence that a single dose is safe. Indeed, patients are often at highest risk of falls immediately after initiation of therapy.3739 We did, however, exclude drugs such as laxatives, which may be appropriate for brief inpatient use but not for chronic use.
Our study also had a number of strengths. The large sample size, representing approximately 5% of annual inpatient admissions in the US over 2 years, offered an instructive look at the recent prescribing patterns of thousands of US physicians. We were able to identify many patient, physician, and hospital factors associated with PIM prescribing that have not previously been reported. Some of these factors, such as advanced age and comorbid diagnoses, suggest that physicians do tailor their treatment to individual patients. Nevertheless, patient factors accounted for only a small portion of the variation in prescribing. The largest variation, associated with regional, hospital, and physician factors, highlights the opportunity for improvement.
At the same time, our findings are encouraging for 2 reasons. First, most inappropriate prescribing involved only a handful of medications, so small changes in prescribing patterns could have a tremendous impact. Second, observing the practice of individual physicians and hospitals reveals what is possible. We found that in most specialties there were physicians who rarely or never used PIMs. We also found 7 hospitals, each with at least 300 cases, where no PIMs were ever prescribed.
Where should hospitals focus their efforts to prevent inappropriate prescribing? Our data highlight the complexity of the problem, which seems daunting. PIM prescribing is spread across all specialties, including geriatrics, and although cardiologists had the highest rate of prescribing, internists, who were more numerous, accounted for a much higher overall number of potentially inappropriate prescriptions. It would be instructive to study the 7 hospitals where PIMs were never prescribed or to interview those physicians who never prescribed PIMs, but the anonymous nature of our data would not allow for this. However, our data do suggest some directions. First, hospitals should become aware of their own rates of PIM use because measurement is the first step in quality improvement. Next, hospitals should focus efforts on reducing the use of the most common drugs. Eliminating just 3 drugs promethazine, diphenhydramine, and propoxyphenewould reduce the use of PIMs in 24% of elderly patients. Enlisting hospital pharmacists and electronic health records and reviewing standard order sets for elderly patients are potentially effective strategies. Finally, increasing the presence of geriatricians and hospitalists would be expected to have a modest impact.
In a representative sample of elderly inpatients, we found that almost half received a potentially inappropriate medication and that the rate of inappropriate prescribing varied widely among doctors and hospitals. Additional research is needed to distinguish which of the Beers drugs are most harmful and which patients are at highest risk. Research should also focus on understanding differences in prescribing patterns, perhaps by studying the outliers at both ends of the quality spectrum, and on techniques to minimize non‐patient‐centered variation.
- ,,,,,.Explicit criteria for determining inappropriate medication use in nursing home residents. UCLA Division of Geriatric Medicine.Arch Intern Med.1991;151:1825–1832.
- .Explicit criteria for determining potentially inappropriate medication use by the elderly. An update.Arch Intern Med.1997;157:1531–1536.
- ,,,,,.Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts.Arch Intern Med.2003;163:2716–2724.
- National Committee on Quality Assurance. Drugs to be Avoided in the Elderly. Available at: http://www.ncqa.org/Programs/HEDIS/2006/Volume2/NDC/DAE_06.xls. Accessed November 20,2006.
- ,,, et al.Inappropriate prescribing for elderly Americans in a large outpatient population.Arch Intern Med.2004;164:1621–1625.
- ,,, et al.Potentially inappropriate medication use in the community‐dwelling elderly: findings from the 1996 Medical Expenditure Panel Survey.JAMA.2001;286:2823–2829.
- ,.Prescribing potentially inappropriate psychotropic medications to the ambulatory elderly.Arch Intern Med.2000;160:2825–2831.
- ,,, et al.Inappropriate medication prescribing in skilled‐nursing facilities.Ann Intern Med.1992;117:684–689.
- ,,, et al.Adverse outcomes associated with inappropriate drug use in nursing homes.Ann Pharmacother.2005;39:405–411.
- ,,.Inappropriate medication administration to the acutely ill elderly: a nationwide emergency department study, 1992–2000.J Am Geriatr Soc.2004;52:1847–1855.
- ,,, et al.Appropriateness of medication selection for older persons in an urban academic emergency department.Acad Emerg Med.1999;6:1232–1242.
- ,,,,,.Use of the Beers criteria to predict adverse drug reactions among first‐visit elderly outpatients.Pharmacotherapy.2005;25:831–838.
- ,,.The association of inappropriate drug use with hospitalisation and mortality: a population‐based study of the very old.Drugs Aging.2005;22(1):69–82.
- ,,,,.Hospitalization and death associated with potentially inappropriate medication prescriptions among elderly nursing home residents.Arch Intern Med.2005;165(1):68–74.
- ,,.Potentially inappropriate prescribing for geriatric inpatients: an acute care of the elderly unit compared to a general medicine service.Consult Pharm.2003;18(1):37–42, 47–39.
- ,,, et al.Inappropriate medication use among frail elderly inpatients.Ann Pharmacother.2004;38(1):9–14.
- ,,,,,.Inappropriate medication use among hospitalized older adults in Italy: results from the Italian Group of Pharmacoepidemiology in the Elderly.Eur J Clin Pharmacol.2003;59(2):157–162.
- ,,,,,.Guided prescription of psychotropic medications for geriatric inpatients.Arch Intern Med.2005;165:802–807.
- ,,,.Comorbidity measures for use with administrative data.Med Care.1998;36(1):8–27.
- ,.Inadequate prescription‐drug coverage for Medicare enrollees—a call to action.N Engl J Med.1999;340:722–728.
- National and regional estimates on hospital use for all patients from the HCUP Nationwide Inpatient Sample (NIS). Agency for Healthcare Research and Quality (AHRQ). Available at: http://hcupnet.ahrq.gov/HCUPnet.jsp. Accessed October 12,2006.
- ,,,,,.Drugs and falls in community‐dwelling older people: a national veterans study.Clin Ther.2006;28:619–630.
- ,,,,,.Psychotropic medications and risk for falls among community‐dwelling frail older people: an observational study.J Gerontol A Biol Sci Med Sci.2005;60:622–626.
- ,,.Drugs and falls in older people: a systematic review and meta‐analysis: I. Psychotropic drugs.J Am Geriatr Soc.1999;47(1):30–39.
- ,,.Propoxyphene use and risk for hip fractures in older adults.Am J Geriatr Pharmacother.2006;4:219–226.
- ,,, et al.Central nervous system active medications and risk for fractures in older women.Arch Intern Med.2003;163:949–957.
- ,,, et al.Potentially inappropriate medication use by elderly persons in U.S. Health Maintenance Organizations, 2000–2001.J Am Geriatr Soc.2005;53:227–232.
- .Inappropriate medication prescribing for elderly ambulatory care patients.Arch Intern Med.2004;164:305–312.
- ,,.Adverse drug reactions in an elderly hospitalised population: inappropriate prescription is a leading cause.Drugs Aging.2005;22:767–777.
- ,,,,.Use of inappropriate medications and their prognostic significance among in‐hospital and nursing home patients with and without dementia in Finland.Drugs Aging.2006;23:333–343.
- ,,, et al.Potentially inappropriate medication use among elderly home care patients in Europe.JAMA.2005;293:1348–1358.
- ,,,,.Impact of hospitalisation in an acute medical geriatric unit on potentially inappropriate medication use.Drugs Aging.2006;23(1):49–59.
- ,.Pharmacists and their effectiveness in ensuring the appropriateness of the chronic medication regimens of geriatric inpatients.Consult Pharm.2004;19:432–436.
- ,,,,,.Benzodiazepines with different half‐life and falling in a hospitalized population: the GIFA study. Gruppo Italiano di Farmacovigilanza nell'Anziano.J Clin Epidemiol.2000;53:1222–1229.
- ,.Relationship between the administration of selected medications and falls in hospitalized elderly patients.Ann Pharmacother.1995;29:354–358.
- .The use of sedative/hypnotic medication and its correlation with falling down in the hospital.Sleep.1996;19:698–701.
- ,,, et al.Benzodiazepine use and hip fractures in the elderly: who is at greatest risk?Arch Intern Med.2004;164:1567–1572.
- ,,,,.Hazardous benzodiazepine regimens in the elderly: effects of half‐life, dosage, and duration on risk of hip fracture.Am J Psychiatry.2001;158:892–898.
- ,,,,.A 5‐year prospective assessment of the risk associated with individual benzodiazepines and doses in new elderly users.J Am Geriatr Soc.2005;53:233–241.
Medications can be considered inappropriate when their risk outweighs their benefit. The Beers list1 identifies medications that should be avoided in persons 65 years or older because they are ineffective or pose an unnecessarily high risk or because a safer alternative is available. Initially developed in 1991, the list has gained wide acceptance and has been updated twice.2, 3 In July 1999 it was adopted by the Centers for Medicare & Medicaid Services (CMS) for nursing home regulation, and in 2006 the National Committee on Quality Assurance adopted a modified version as a Health Plan Employer Data and Information Set (HEDIS) measure of quality of care for older Americans.4
A number of studies have demonstrated that inappropriate prescribing is common in the ambulatory setting,57 in nursing homes,8, 9 and in emergency departments10, 11 and that exposure to inappropriate medications is associated with increased risk of adverse drug reactions12 and hospitalization.13, 14 Initial studies of hospitalized patients1517 suggest that potentially inappropriate prescribing is also common among elderly inpatients and that reducing the misuse of psychotropic medications can prevent falls.18 We report on the incidence of and risk factors associated with potentially inappropriate prescribing in a large sample of hospitalized elders.
METHODS
Patients
We conducted a retrospective cohort study using data from 384 hospitals participating in Perspective (Premier, Inc., Charlotte, NC), a database developed for measuring quality and health care utilization. Participating hospitals represent all regions of the United States and are primarily small‐ to medium‐sized nonteaching hospitals most of which are in urban areas. Premier collects data elements from participating hospitals via a custom data extract from hospitals' decision support system. Hospitals aggregate the data elements into their decision support systems from multiple information technology systems including billing, medical records, pharmacy, and laboratory systems. In addition to the information contained in the standard hospital discharge file, Perspective includes a date‐stamped log of all billed items, including medications with dose and quantity, for individual patients.
We included patients at least 65 years old admitted between September 1, 2002, and June 30, 2005, with a principal diagnosis of acute myocardial infarction, chronic obstructive pulmonary disease, chest pain, community‐acquired pneumonia, congestive heart failure, ischemic stroke, or urinary tract infection. International Classification of Diseases, Ninth Revision (ICD‐9‐CM) codes were used to identify diagnoses. Patients cared for by an attending physician with a surgical specialty were excluded. The study protocol was approved by the institutional review board of Baystate Medical Center.
Data Elements
For each patient, Perspective contains fields for age, sex, race, marital status, insurance status, principal diagnosis, comorbidities, and specialty of the attending physician. Comorbidities were identified from ICD‐9‐CM secondary diagnosis codes and APR‐DRGs using Healthcare Cost and Utilization Project Comorbidity Software, version 3.1, based on the work of Elixhauser.19 Because almost all patients had Medicare coverage, plans were classified according to managed care status. Finally, for each patient we identified all medications administered, as well as discharge status, readmission rate, total costs, and length of stay. Hospitals were categorized by region (Northeast, South, Midwest, or West), bed size, setting (urban or rural), teaching status, and whether there were geriatricians.
Potentially Inappropriate Prescribing
Using the 2002 updated Beers criteria3 for potentially inappropriate medication (PIM) use in older adults, we identified the total number of PIMs administered to each patient during his or her hospital stay. We classified each PIM as either high or low severity based on the expert consensus expressed in the 1997 update of the Beers criteria.2 The list contains 48 PIMs and an additional 20 that should be avoided in patients with certain conditions. We did not include the second category of PIMs because we did not necessarily have sufficient patient information to make this determination. In addition, some of the standard PIMs, such as laxatives, although inappropriate for chronic outpatient use, could be appropriate in the hospital setting and were excluded from this analysis. Finally, several medications were considered inappropriate only above a given threshold (eg, lorazepam >3.0 mg/day) or for patients without a specific diagnosis (eg, digoxin >0.125 mg/day for patients without atrial fibrillation). We grouped PIMs that had similar side effects into 4 categories: sedatives, anticholinergics, causing orthostasis, or causing bleeding (Fig. 1).

Statistical Analysis
Summary statistics at the patient, physician, and hospital levels were constructed using frequencies and proportions for categorical data and means, standard deviations, medians, interquartile ranges, and box plots for continuous‐scale variables. Patients were identified as receiving a PIM if the drug was administered (above threshold dose where applicable) on at least 1 hospital day. We examined the association of each patient characteristic with use of any PIM, any high‐severity‐rated PIM, and each side effect category using chi‐square statistics. Kruskal‐Wallis analysis of variance was used to examine variation in hospital use rates by each hospital characteristic, and physician use rates for high‐severity PIMs by attending specialty. To examine whether it was feasible to avoid PIMs altogether, we compared individual hospitals as well as individual prescribers within their specialty, limiting the comparison to hospitals that contributed at least 100 patients and to physicians with at least 50 patients.
We developed a multivariable model for any high‐severity medication (HS‐PIM) use that included all patient, physician, and hospital characteristics except length of stay, mortality, cost, discharge status, and readmission rate. A generalized estimating equation model (SAS PROC GENMOD) with a logit link and a subcluster correlation structure was used to account for clustering at the hospital, physician, and diagnosis levels, adjusting for the clustering of primary diagnosis within physician level, nested within hospital level. Effects with P < .10 were retained in the model, and interaction effects were also evaluated for significance. Model fit was assessed using deviance and Pearson chi‐square statistics. All analyses were performed with SAS statistical software, version 9.1 (SAS Institute, Cary, NC).
RESULTS
We identified 519,853 patients at least 65 years old during the study period; 564 were excluded because of missing data for key variables or unclear principal diagnosis. An additional 25,318 were excluded because they were cared for by an attending with a surgical specialty. A total of 493,971 patients were included in the study (Table 1). Mean age was 78 years, and 24% of patients were 85 years or older. Forty‐three percent were male, 71% were white, and 39% were currently married. The most common principal diagnoses were community‐acquired pneumonia, congestive heart failure, and acute myocardial infarction. The most common comorbidities were hypertension, diabetes, and chronic pulmonary disease. Medicare was the primary payer for 91% of subjects, and 13% were in managed care plans. Most patients were cared for by internists (49%), family practitioners (18%), or cardiologists (17%). Only 1% of patients had a geriatrician as attending.
| Characteristic | n (%) |
|---|---|
| |
| Age group | |
| 6574 years | 168,527 (34%) |
| 7584 years | 206,407 (42%) |
| 85+ years | 119,037 (24%) |
| Sex | |
| Male | 212,358 (43%) |
| Female | 281,613 (57%) |
| Race | |
| White | 351,331 (71%) |
| Black | 52,429 (11%) |
| Hispanic | 18,057 (4%) |
| American Indian | 1876 (0%) |
| Asian/Pacific Islander | 5926 (1%) |
| Other | 64,352 (13%) |
| Marital status | |
| Married/partner | 194,496 (39%) |
| Widowed | 155,273 (31%) |
| Single/separated/divorced | 75,964 (15%) |
| Other | 68,238 (14%) |
| Primary diagnosis | |
| Pneumonia | 122,732 (25%) |
| Heart failure | 109,071 (22%) |
| Acute MI | 70,581 (14%) |
| Ischemic stroke | 57,204 (12%) |
| Chest pain | 50,404 (10%) |
| COPD | 44,582 (9%) |
| Urinary tract infection | 39,397 (8%) |
| Comorbidities | |
| Hypertension | 310,163 (63%) |
| Diabetes | 151,755 (31%) |
| Chronic pulmonary disease | 134,900 (27%) |
| Fluid and electrolyte disorders | 128,703 (26%) |
| Deficiency anemias | 92,668 (19%) |
| Congestive heart failure | 69,201 (14%) |
| Hypothyroidism | 68,711 (14%) |
| Peripheral vascular disease | 47,244 (10%) |
| Depression | 41,507 (8%) |
| Other neurological disorders | 40,200 (8%) |
| Renal failure | 38,134 (8%) |
| Obesity | 25,143 (5%) |
| Payer type | |
| Not Managed care | 431,583 (87%) |
| Managed care | 62,388 (13%) |
| Attending physician specialty | |
| Internal medicine (internist) | 241,982 (49%) |
| Family/general medicine | 90,827 (18%) |
| Cardiology | 83,317 (17%) |
| Pulmonology | 21,163 (4%) |
| Hospitalist | 14,924 (3%) |
| Nephrology | 8257 (2%) |
| Neurology | 5800 (1%) |
| Geriatrics | 3099 (1%) |
| Other* | 24,602 (5%) |
| Mortality | |
| Expired | 28,321 (6%) |
| Alive | 465,650 (94%) |
| Discharge status, n (% of survivors) | |
| Home | 323,629 (66%) |
| Nursing care | 119,468 (24%) |
| Transfer/short‐term hospital | 13,531 (3%) |
| Hospice | 9022 (2%) |
| 14‐Day readmission, n (% of survivors) | |
| Yes | 35,309 (8%) |
| No | 430,334 (92%) |
| Length of stay (days), median (IQR) | 4 (2, 7) |
| Total cost (dollars) | $5513 ($3366, $9902) |
Just under half of all patients (49%) received at least 1 PIM, and 6% received 3 or more (Table 2). Thirty‐eight percent received at least 1 drug with a high severity rating (HS‐PIM). The most common PIMs were promethazine, diphenhydramine, propoxyphene, clonidine, amiodarone, and lorazepam (>3 mg/day).
| Patients, n (%) | |
|---|---|
| Number of PIMs | |
| 0 | 254,200 (51%) |
| 1 | 146,028 (30%) |
| 2 | 61,445 (12%) |
| 3 | 22,128 (4%) |
| 4+ | 10,170 (2%) |
| Number of high‐severity‐rated PIMs | |
| 0 | 304,523 (62%) |
| 1 | 129,588 (26%) |
| 2 | 43,739 (9%) |
| 3 | 12,213 (2%) |
| 4+ | 3908 (1%) |
| Use of any PIM by side effect class | |
| Sedatives | 156,384 (32%) |
| Anticholinergic effects | 109,293 (22%) |
| Causing orthostasis | 43,805 (9%) |
| Causing bleeding | 14,744 (3%) |
| Most commonly prescribed | |
| Promethazine | 49,888 (10%) |
| Diphenhydramine | 45,458 (9%) |
| Propoxyphene | 41,786 (8%) |
| Clonidine | 34,765 (7%) |
| Amiodarone | 34,318 (7%) |
| Lorazepam (>3 mg/day) | 25,147 (5%) |
Patient, Physician, and Hospital Factors Associated with PIMs
Patient, physician, and hospital characteristics were all associated with use of PIMs (Table 3). In univariate analyses, older patients were less likely to receive any class of PIM, and this difference was accentuated for HS‐PIMs. Women, American Indians, married people, and those not in managed care plans were slightly more likely to receive PIMs, whereas patients admitted with acute myocardial infarction or congestive heart failure were even more likely to receive PIMs (P < .0001 for all comparisons).
| Patient characteristic | Any PIM n (row %) | Any high‐severity PIM n (row %) | Sedatives n (row %) | Anticholinergic effects n (row %) | Causing orthostasis n (row %) | Causing bleeding n (row %) |
|---|---|---|---|---|---|---|
| ||||||
| Overall | 239,771 (49%) | 189,448 (38%) | 156,384 (32%) | 109,293 (22%) | 43,805 (9%) | 14,744 (3%) |
| Age group | ||||||
| 6574 years | 89,168 (53%) | 72,573 (43%) | 61,399 (36%) | 44,792 (27%) | 15,799 (9%) | 6655 (4%) |
| 7584 years | 100,787 (49%) | 79,595 (39%) | 65,034 (32%) | 45,121 (22%) | 18,519 (9%) | 5727 (3%) |
| 85+ years | 49,816 (42%) | 37,280 (31%) | 29,951 (25%) | 19,380 (16%) | 9487 (8%) | 2362 (2%) |
| Sex | ||||||
| Male | 100,824 (47%) | 79,535 (37%) | 63,591 (30%) | 42,754 (20%) | 17,885 (8%) | 5771 (3%) |
| Female | 138,947 (49%) | 109,913 (39%) | 92,793 (33%) | 66,539 (24%) | 25,920 (9%) | 8973 (3%) |
| Race | ||||||
| White | 173,481 (49%) | 139,941 (40%) | 112,556 (32%) | 81,097 (23%) | 27,555 (8%) | 10,590 (3%) |
| Black | 26,793 (51%) | 18,655 (36%) | 18,720 (36%) | 11,263 (21%) | 8925 (17%) | 1536 (3%) |
| Hispanic | 8509 (47%) | 6370 (35%) | 5549 (31%) | 3505 (19%) | 2047 (11%) | 648 (4%) |
| American Indian | 1091 (58%) | 849 (45%) | 818 (44%) | 563 (30%) | 190 (10%) | 76 (4%) |
| Asian/Pacific Islander | 2386 (40%) | 1896 (32%) | 1420 (24%) | 1023 (17%) | 519 (9%) | 127 (2%) |
| Other | 27,511 (43%) | 21,737 (34%) | 17,321 (27%) | 11,842 (18%) | 4569 (7%) | 1767 (3%) |
| Marital status | ||||||
| Married/partner | 96,874 (50%) | 77,803 (40%) | 63,303 (33%) | 45,042 (23%) | 16,765 (9%) | 5969 (3%) |
| Widowed | 74,622 (48%) | 58,012 (37%) | 48,367 (31%) | 33,516 (22%) | 13,865 (9%) | 4354 (3%) |
| Single/separated/divorced | 36,583 (48%) | 28,799 (38%) | 24,251 (32%) | 16,115 (21%) | 7229 (10%) | 2399 (3%) |
| Other | 31,692 (46%) | 24,834 (36%) | 20,463 (30%) | 14,620 (21%) | 5946 (9%) | 2022 (3%) |
| Primary diagnosis | ||||||
| Pneumonia | 56,845 (46%) | 46,271 (38%) | 35,353 (29%) | 25,484 (21%) | 9184 (7%) | 4155 (3%) |
| Heart failure | 56,460 (52%) | 42,231 (39%) | 34,340 (31%) | 22,093 (20%) | 10,117 (9%) | 1945 (2%) |
| Acute MI | 43,046 (61%) | 37,849 (54%) | 32,560 (46%) | 25,568 (36%) | 4738 (7%) | 2549 (4%) |
| Ischemic stroke | 25,763 (45%) | 17,613 (31%) | 18,500 (32%) | 8742 (15%) | 9644 (17%) | 1384 (2%) |
| Chest pain | 20,655 (41%) | 16,363 (32%) | 13,536 (27%) | 10,520 (21%) | 3474 (7%) | 2027 (4%) |
| COPD | 18,876 (42%) | 14,626 (33%) | 12,087 (27%) | 8096 (18%) | 3209 (7%) | 1139 (3%) |
| Urinary tract infection | 18,126 (46%) | 14,495 (37%) | 10,008 (25%) | 8790 (22%) | 3439 (9%) | 1545 (4%) |
| Payer type | ||||||
| Nonmanaged care | 212,150 (49%) | 168,013 (39%) | 138,679 (32%) | 97,776 (23%) | 38,341 (9%) | 12,868 (3%) |
| Managed care | 27,621 (44%) | 21,435 (34%) | 17,705 (28%) | 11,517 (18%) | 5464 (9%) | 1876 (3%) |
| Attending physician specialty* | ||||||
| Internal medicine (internist%) | 112,664 (47%) | 86,907 (36%) | 71,382 (30%) | 48,746 (20%) | 23,221 (10%) | 7086 (3%) |
| Family/general medicine | 41,303 (45%) | 32,338 (36%) | 25,653 (28%) | 18,274 (20%) | 7660 (8%) | 2852 (3%) |
| Cardiology | 48,485 (58%) | 40,752 (49%) | 34,859 (42%) | 25,792 (31%) | 5455 (7%) | 2542 (3%) |
| Pulmonology | 10,231 (48%) | 8105 (38%) | 6746 (32%) | 4064 (19%) | 1739 (8%) | 574 (3%) |
| Hospitalist | 7003 (47%) | 5443 (36%) | 4447 (30%) | 3179 (21%) | 1471 (10%) | 463 (3%) |
| Nephrology | 4508 (55%) | 3388 (41%) | 3132 (38%) | 2054 (25%) | 1326 (16%) | 198 (2%) |
| Neurology | 2420 (42%) | 1789 (31%) | 1625 (28%) | 851 (15%) | 699 (12%) | 174 (3%) |
| Geriatrics | 1020 (33%) | 785 (25%) | 596 (19%) | 404 (13%) | 196 (6%) | 41 (1%) |
The HS‐PIM prescribing varied substantially by attending specialty (Fig. 2). Internists, family practitioners, and hospitalists all had similar median rates (33%), cardiologists had a higher median rate (48%), and geriatricians had a lower rate (24%). The most common PIM also differed by specialty: whereas promethazine was the most commonly prescribed drug across most specialties, nephrologists and neurologists used clonidine, pulmonologists used lorazepam, and cardiologists used diphenhydramine most often. Among the 8% of physicians who saw at least 50 patients, there was also great variation in each specialty (Fig. 2). Among internists and cardiologists who saw at least 50 patients, the high‐severity PIM usage rate ranged from 0% to more than 90%.

There was substantial variation in PIM usage among hospitals, most notably by region. The mean proportion of patients receiving PIMs ranged from 34% at hospitals in the Northeast to 55% at hospitals in the South (Table 4). Smaller hospitals and those in urban settings had slightly lower rates, as did those that had geriatricians on staff. The teaching status of the hospital had little effect. Variation at the individual hospital level was extreme (Fig. 3). Although half of all hospitals had rates between 43% and 58%, in 7 hospitals with more than 300 encounters each, PIMs were never prescribed for geriatric patients.

| Hospitals Total = 384 n (%) | Patients N = 49,3971 n (%) | Any PIM Mean = 48.2 Mean (SD) | Any high‐severity PIM Mean = 38.7 Mean (SD) | Sedatives Mean = 30.2 Mean (SD) | Anticholinergic effects Mean = 21.5 Mean (SD) | Causing orthostasis Mean = 8.5 Mean (SD) | Causing bleeding Mean = 3.1 Mean (SD) | |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Hospital region | *** | *** | *** | *** | *** | ** | ||
| Midwest | 76 (20%) | 95,791 (19%) | 38.8 (19.7) | 30.0 (16.4) | 24.3 (13.8) | 15.1 (9.9) | 6.9 (6.3) | 3.1 (2.3) |
| Northeast | 47 (12%) | 79,138 (16%) | 34.1 (12.6) | 26.2 (11.2) | 19.0 (9.2) | 13.5 (8.1) | 4.9 (2.3) | 2.1 (1.6) |
| South | 199 (52%) | 260,870 (53%) | 54.5 (10.1) | 42.7 (9.6) | 36.0 (10.8) | 26.4 (8.6) | 10.4 (4.6) | 3.6 (2.5) |
| West | 62 (16%) | 58,172 (12%) | 45.8 (8.1) | 37.4 (7.1) | 27.3 (7.7) | 19.5 (5.7) | 7.4 (4.8) | 2.7 (1.3) |
| Teaching status | ||||||||
| Nonteaching | 297 (77%) | 324,948 (66%) | 47.3 (14.6) | 36.9 (12.3) | 29.8 (12.0) | 21.3 (9.9) | 8.7 (5.4) | 3.3 (2.4) |
| Teaching | 87 (23%) | 169,023 (34%) | 48.2 (16.0) | 38.8 (14.2) | 31.6 (14.5) | 22.1 (10.2) | 7.8 (4.4) | 2.7 (1.5) |
| Staffed beds | * | ** | ||||||
| 22200 | 143 (37%) | 80,741 (16%) | 45.5 (16.9) | 35.2 (14.6) | 27.5 (14.0) | 20.1 (10.3) | 8.0 (6.2) | 3.5 (3.1) |
| 200400 | 137 (36%) | 177,286 (36%) | 47.7 (14.2) | 37.8 (12.0) | 30.5 (11.6) | 22.0 (10.0) | 8.4 (4.7) | 3.0 (1.6) |
| 400+ | 104 (27%) | 235944 (48%) | 50.1 (12.4) | 39.6 (10.6) | 33.5 (10.9) | 22.7 (9.3) | 9.3 (4.2) | 2.9 (1.4) |
| Population serviced | * | * | ** | |||||
| Rural | 119 (31%) | 102,799 (21%) | 48.4 (13.0) | 38.3 (10.6) | 29.2 (11.0) | 23.2 (9.3) | 7.5 (4.0) | 3.7 (3.0) |
| Urban | 265 (69%) | 391,172 (79%) | 47.1 (15.7) | 36.9 (13.7) | 30.6 (13.2) | 20.7 (10.2) | 9.0 (5.6) | 2.9 (1.8) |
| Geriatrician presence | ||||||||
| No | 340 (89%) | 409,281 (83%) | 47.7 (15.3) | 37.6 (13.0) | 30.3 (12.8) | 21.7 (10.0) | 8.4 (5.3) | 3.2 (2.3) |
| Yes | 44 (11%) | 84,690 (17%) | 45.8 (11.4) | 35.5 (10.6) | 29.4 (10.8) | 19.6 (9.4) | 9.3 (4.3) | 2.9 (1.6) |
Multivariable Model
In a multivariable logit model that included all patient, hospital, and physician characteristics and that accounted for clustering at the hospital, physician, and diagnosis levels, several characteristics were associated with HS‐PIM prescribing (Table 5). By far the most important predictor of use was hospital region. Compared with patients at hospitals in the Midwest, patients in the South (OR 1.63, 95% CI 1.591.67) and West (OR 1.43, 95% CI 1.381.47) were more likely and those in the Northeast were less likely (OR 0.85, 95% CI 0.830.88) to receive HS‐PIMs. Larger hospitals had higher HS‐PIM rates than smaller ones, but teaching status and rural or urban setting were not associated with HS‐PIM prescribing. The presence of geriatricians in a hospital was also associated with lower HS‐PIM prescribing for the entire hospital.
| Effect (reference) | Odds ratio | 95% Confidence limits | |
|---|---|---|---|
| Age | |||
| 6574 years | 1.00 | ||
| 7584 years | 0.83 | 0.82 | 0.84 |
| 85+ years | 0.59 | 0.58 | 0.61 |
| Sex | |||
| Female | 1.00 | ||
| Male | 0.85 | 0.83 | 0.86 |
| Race | |||
| White | 1.00 | ||
| Black | 0.78 | 0.76 | 0.80 |
| Hispanic | 0.84 | 0.81 | 0.87 |
| American Indian | 0.97 | 0.88 | 1.07 |
| Asian/Pacific Islander | 0.74 | 0.70 | 0.79 |
| Other | 0.94 | 0.92 | 0.97 |
| Marital Status | |||
| Married/partner | 1.00 | ||
| Single/separated/divorced | 0.96 | 0.94 | 0.98 |
| Widowed | 0.96 | 0.95 | 0.98 |
| Other | 0.93 | 0.90 | 0.95 |
| Primary diagnosis | |||
| Pneumonia | 1.00 | ||
| COPD | 0.83 | 0.81 | 0.85 |
| Heart failure | 1.14 | 1.12 | 1.16 |
| Ischemic stroke | 0.84 | 0.82 | 0.86 |
| Acute MI | 1.95 | 1.90 | 2.01 |
| Urinary tract infection | 1.06 | 1.03 | 1.09 |
| Chest pain | 0.87 | 0.84 | 0.89 |
| Comorbidities (yes or no) | |||
| Hypertension | 0.98 | 0.97 | 0.99 |
| Diabetes | 0.98 | 0.97 | 1.00 |
| Chronic lung disease | 1.11 | 1.10 | 1.13 |
| Fluid and electrolyte disorders | 1.26 | 1.24 | 1.27 |
| Anemia deficiencies | 1.17 | 1.15 | 1.18 |
| Congestive heart failure | 1.34 | 1.32 | 1.37 |
| Hypothyroidism | 1.13 | 1.11 | 1.15 |
| Peripheral vascular disease | 1.09 | 1.06 | 1.11 |
| Depression | 1.38 | 1.35 | 1.41 |
| Neurological disorders | 0.89 | 0.87 | 0.91 |
| Renal failure | 1.23 | 1.20 | 1.26 |
| Obesity | 1.11 | 1.08 | 1.14 |
| Payer type | |||
| Managed care | 1.00 | ||
| Not managed care | 1.04 | 1.02 | 1.06 |
| Attending physician specialty | |||
| Internal medicine | 1.00 | ||
| Cardiology | 1.32 | 1.28 | 1.36 |
| Family/general medicine | 0.99 | 0.97 | 1.01 |
| Geriatrics | 0.69 | 0.61 | 0.78 |
| Hospitalist | 0.90 | 0.84 | 0.96 |
| Nephrology | 1.02 | 0.96 | 1.08 |
| Neurology | 0.93 | 0.86 | 1.00 |
| Pulmonology | 1.10 | 1.05 | 1.15 |
| Setting | |||
| Rural | 1.00 | ||
| Urban | 1.02 | 1.00 | 1.05 |
| Teaching status | |||
| Nonteaching | 1.00 | ||
| Teaching | 1.01 | 0.98 | 1.03 |
| Number of beds | |||
| 22200 | 1.00 | ||
| 200400 | 1.08 | 1.05 | 1.11 |
| 400+ | 1.12 | 1.09 | 1.16 |
| Region | |||
| Midwest | 1.00 | ||
| Northeast | 0.85 | 0.83 | 0.88 |
| South | 1.63 | 1.59 | 1.67 |
| West | 1.43 | 1.38 | 1.47 |
| Geriatrician presence | |||
| No | 1.00 | ||
| Yes | 0.93 | 0.90 | 0.95 |
Physician specialty was also important. Adjusting for diagnosis attenuated some of this association, but compared with internists, cardiologists (OR 1.32, 95% CI 1.281.36) and pulmonologists (OR 1.10, 95% CI 1.051.15) were still more likely, hospitalists (OR 0.90, 95% CI 0.840.96) were less likely, and geriatricians (0.69, 95% CI 0.610.78) were least likely to prescribe any HS‐PIM.
Patient factors were also associated with HS‐PIM use. Compared with patients age 6574 years, patients older than 85 years were much less likely to receive an HS‐PIM (OR 0.59, CI 0.580.61), as to a lesser extent were nonwhites compared with whites and unmarried people compared with those who were married. Compared with patients with pneumonia, those with COPD, stroke, or chest pain were less likely and those with myocardial infarction and congestive heart failure were more likely to receive HS‐PIMs. Patients with a secondary diagnosis of depression were also at high risk (OR 1.38, CI 1.351.41).
DISCUSSION
Although Americans age 65 years and older make up less than 15% of the U.S. population, they consume about one third of all prescription drugs20 and account for one third of all hospital admissions.21 Using the Beers list, numerous studies have documented high rates of potentially inappropriate prescribing for community‐dwelling elderly and nursing home patients and, in some studies, an attendant risk of falling,2224 hip fracture,25, 26 hospitalization,13 or death.14 Applying these same criteria to a large sample of medical inpatients, we found that almost half received a potentially inappropriate drug, most of high severity. Moreover, the PIM prescribing rate varied substantially by region, hospital, and attending physician specialty. Although the use of PIMs was associated with patient age, comorbidities, and primary diagnosis, these patient factors explained only a small portion of the variation in prescribing practices across groups of physicians and hospitals.
Using consensus criteria, Beers originally found that 40% of the residents in 12 nursing homes received at least 1 PIM,8 and studies of community‐dwelling elderly demonstrated rates of 21% to 37%, with little change over time.6, 27, 28 Several small studies have examined inpatient prescribing.16, 17, 29, 30 The largest17 found that only 15% of elderly Italian inpatients received a PIM. Our finding, that 49% of inpatients had received at least 1 PIM, may partially reflect the high prevalence of use among elderly US patients in nursing homes and the community.
Regional variation has been demonstrated for ambulatory patients in the US6 and Europe.31 Zhan et al. found slightly higher rates of PIM use in the Midwest and the South (23%) than in the Northeast and the West (19%). Variation in Europe was greater, with 41% of patients in the Czech Republic versus 5.8% of patients in Denmark receiving at least 1 PIM. We found that region was the strongest predictor of in‐hospital HS‐PIM use, with patients in the South most likely and patients in the Northeast least likely to receive HS‐PIMs. This variation persisted even after adjusting for differences in other patient and hospital factors, suggesting that local custom played a large role in the decision to prescribe HS‐PIMs. Moreover, because outpatient rates are more uniform, these large differences seem limited to inpatient practice.
Patient factors have also been examined. Advanced age was associated with decreased PIM use in some studies17, 28, 31 but not in others.6, 27 We found increasing age to be strongly associated with decreased PIM use, suggesting that in the hospital, at least, doctors take care to avoid prescribing certain drugs to the frail elderly. Women appear to be consistently at higher risk than men,6, 27, 28, 31 and white patients are more at risk than those of other races.6 Our finding that certain diagnoses were associated with higher or lower rates has not been reported previously. The lower rates associated with stroke and COPD suggest that prescribers were aware that these patients were at increased risk of delirium and respiratory depression. The higher rates associated with myocardial infarction may have to do with the use of standardized order sets (eg, cath lab orders) that do not consider the age of the patient going for the procedure.
Admission to a geriatric service32 and intervention by a clinical pharmacist33 have been shown to decrease PIM prescribing at discharge. We noted that patients cared for by a geriatrician had the lowest rates of PIM prescribing during hospitalization as well and that hospitals with geriatricians had lower rates overall, possibly demonstrating that geriatricians had a ripple effect on their colleagues. Hospitalists also had lower rates than internists, supporting the notion that hospitalists provide higher‐quality inpatient care.
Our study had some important limitations. First, we only had access to inpatient administrative records. Thus, we could not identify which medications were continued from home and which were begun in the hospital, nor could we know the indications for which specific drugs were prescribed or who prescribed them. Based on published outpatient rates, however, we could assume that many of the drugs were started in the hospital and that others could have been discontinued but were not. Second, the Beers list was developed by the modified Delphi method; there was little empirical evidence of the danger of specific drugs, although some classes, such as benzodiazepines, opiates and digoxin, have been associated with inpatient falls.18, 3436 Furthermore, our administrative database did not allow us to balance the risks and benefits for particular patients; hence, the medications were only potentially inappropriate, and our study did not address the consequences of such prescribing. Although some of these drugs may be appropriate under certain circumstances, it is unlikely that these circumstances would vary by 60% across geographic regions or that internists would encounter these circumstances more often than do hospitalists. Thus, although we could not identify specific patients who received inappropriate medications, we did identify certain hospitals and even whole regions of the country in which the rate of inappropriate prescribing was high. Third, the Beers list, which was developed for outpatient use, may be less relevant in the inpatient setting. However, given that inpatients have more organ dysfunction and are at higher risk of delirium and falls, it may actually be more applicable to hospitalized patients. We similarly did not distinguish between single and multiple doses because the Beers list does not make such a distinction, and there is no empirical evidence that a single dose is safe. Indeed, patients are often at highest risk of falls immediately after initiation of therapy.3739 We did, however, exclude drugs such as laxatives, which may be appropriate for brief inpatient use but not for chronic use.
Our study also had a number of strengths. The large sample size, representing approximately 5% of annual inpatient admissions in the US over 2 years, offered an instructive look at the recent prescribing patterns of thousands of US physicians. We were able to identify many patient, physician, and hospital factors associated with PIM prescribing that have not previously been reported. Some of these factors, such as advanced age and comorbid diagnoses, suggest that physicians do tailor their treatment to individual patients. Nevertheless, patient factors accounted for only a small portion of the variation in prescribing. The largest variation, associated with regional, hospital, and physician factors, highlights the opportunity for improvement.
At the same time, our findings are encouraging for 2 reasons. First, most inappropriate prescribing involved only a handful of medications, so small changes in prescribing patterns could have a tremendous impact. Second, observing the practice of individual physicians and hospitals reveals what is possible. We found that in most specialties there were physicians who rarely or never used PIMs. We also found 7 hospitals, each with at least 300 cases, where no PIMs were ever prescribed.
Where should hospitals focus their efforts to prevent inappropriate prescribing? Our data highlight the complexity of the problem, which seems daunting. PIM prescribing is spread across all specialties, including geriatrics, and although cardiologists had the highest rate of prescribing, internists, who were more numerous, accounted for a much higher overall number of potentially inappropriate prescriptions. It would be instructive to study the 7 hospitals where PIMs were never prescribed or to interview those physicians who never prescribed PIMs, but the anonymous nature of our data would not allow for this. However, our data do suggest some directions. First, hospitals should become aware of their own rates of PIM use because measurement is the first step in quality improvement. Next, hospitals should focus efforts on reducing the use of the most common drugs. Eliminating just 3 drugs promethazine, diphenhydramine, and propoxyphenewould reduce the use of PIMs in 24% of elderly patients. Enlisting hospital pharmacists and electronic health records and reviewing standard order sets for elderly patients are potentially effective strategies. Finally, increasing the presence of geriatricians and hospitalists would be expected to have a modest impact.
In a representative sample of elderly inpatients, we found that almost half received a potentially inappropriate medication and that the rate of inappropriate prescribing varied widely among doctors and hospitals. Additional research is needed to distinguish which of the Beers drugs are most harmful and which patients are at highest risk. Research should also focus on understanding differences in prescribing patterns, perhaps by studying the outliers at both ends of the quality spectrum, and on techniques to minimize non‐patient‐centered variation.
Medications can be considered inappropriate when their risk outweighs their benefit. The Beers list1 identifies medications that should be avoided in persons 65 years or older because they are ineffective or pose an unnecessarily high risk or because a safer alternative is available. Initially developed in 1991, the list has gained wide acceptance and has been updated twice.2, 3 In July 1999 it was adopted by the Centers for Medicare & Medicaid Services (CMS) for nursing home regulation, and in 2006 the National Committee on Quality Assurance adopted a modified version as a Health Plan Employer Data and Information Set (HEDIS) measure of quality of care for older Americans.4
A number of studies have demonstrated that inappropriate prescribing is common in the ambulatory setting,57 in nursing homes,8, 9 and in emergency departments10, 11 and that exposure to inappropriate medications is associated with increased risk of adverse drug reactions12 and hospitalization.13, 14 Initial studies of hospitalized patients1517 suggest that potentially inappropriate prescribing is also common among elderly inpatients and that reducing the misuse of psychotropic medications can prevent falls.18 We report on the incidence of and risk factors associated with potentially inappropriate prescribing in a large sample of hospitalized elders.
METHODS
Patients
We conducted a retrospective cohort study using data from 384 hospitals participating in Perspective (Premier, Inc., Charlotte, NC), a database developed for measuring quality and health care utilization. Participating hospitals represent all regions of the United States and are primarily small‐ to medium‐sized nonteaching hospitals most of which are in urban areas. Premier collects data elements from participating hospitals via a custom data extract from hospitals' decision support system. Hospitals aggregate the data elements into their decision support systems from multiple information technology systems including billing, medical records, pharmacy, and laboratory systems. In addition to the information contained in the standard hospital discharge file, Perspective includes a date‐stamped log of all billed items, including medications with dose and quantity, for individual patients.
We included patients at least 65 years old admitted between September 1, 2002, and June 30, 2005, with a principal diagnosis of acute myocardial infarction, chronic obstructive pulmonary disease, chest pain, community‐acquired pneumonia, congestive heart failure, ischemic stroke, or urinary tract infection. International Classification of Diseases, Ninth Revision (ICD‐9‐CM) codes were used to identify diagnoses. Patients cared for by an attending physician with a surgical specialty were excluded. The study protocol was approved by the institutional review board of Baystate Medical Center.
Data Elements
For each patient, Perspective contains fields for age, sex, race, marital status, insurance status, principal diagnosis, comorbidities, and specialty of the attending physician. Comorbidities were identified from ICD‐9‐CM secondary diagnosis codes and APR‐DRGs using Healthcare Cost and Utilization Project Comorbidity Software, version 3.1, based on the work of Elixhauser.19 Because almost all patients had Medicare coverage, plans were classified according to managed care status. Finally, for each patient we identified all medications administered, as well as discharge status, readmission rate, total costs, and length of stay. Hospitals were categorized by region (Northeast, South, Midwest, or West), bed size, setting (urban or rural), teaching status, and whether there were geriatricians.
Potentially Inappropriate Prescribing
Using the 2002 updated Beers criteria3 for potentially inappropriate medication (PIM) use in older adults, we identified the total number of PIMs administered to each patient during his or her hospital stay. We classified each PIM as either high or low severity based on the expert consensus expressed in the 1997 update of the Beers criteria.2 The list contains 48 PIMs and an additional 20 that should be avoided in patients with certain conditions. We did not include the second category of PIMs because we did not necessarily have sufficient patient information to make this determination. In addition, some of the standard PIMs, such as laxatives, although inappropriate for chronic outpatient use, could be appropriate in the hospital setting and were excluded from this analysis. Finally, several medications were considered inappropriate only above a given threshold (eg, lorazepam >3.0 mg/day) or for patients without a specific diagnosis (eg, digoxin >0.125 mg/day for patients without atrial fibrillation). We grouped PIMs that had similar side effects into 4 categories: sedatives, anticholinergics, causing orthostasis, or causing bleeding (Fig. 1).

Statistical Analysis
Summary statistics at the patient, physician, and hospital levels were constructed using frequencies and proportions for categorical data and means, standard deviations, medians, interquartile ranges, and box plots for continuous‐scale variables. Patients were identified as receiving a PIM if the drug was administered (above threshold dose where applicable) on at least 1 hospital day. We examined the association of each patient characteristic with use of any PIM, any high‐severity‐rated PIM, and each side effect category using chi‐square statistics. Kruskal‐Wallis analysis of variance was used to examine variation in hospital use rates by each hospital characteristic, and physician use rates for high‐severity PIMs by attending specialty. To examine whether it was feasible to avoid PIMs altogether, we compared individual hospitals as well as individual prescribers within their specialty, limiting the comparison to hospitals that contributed at least 100 patients and to physicians with at least 50 patients.
We developed a multivariable model for any high‐severity medication (HS‐PIM) use that included all patient, physician, and hospital characteristics except length of stay, mortality, cost, discharge status, and readmission rate. A generalized estimating equation model (SAS PROC GENMOD) with a logit link and a subcluster correlation structure was used to account for clustering at the hospital, physician, and diagnosis levels, adjusting for the clustering of primary diagnosis within physician level, nested within hospital level. Effects with P < .10 were retained in the model, and interaction effects were also evaluated for significance. Model fit was assessed using deviance and Pearson chi‐square statistics. All analyses were performed with SAS statistical software, version 9.1 (SAS Institute, Cary, NC).
RESULTS
We identified 519,853 patients at least 65 years old during the study period; 564 were excluded because of missing data for key variables or unclear principal diagnosis. An additional 25,318 were excluded because they were cared for by an attending with a surgical specialty. A total of 493,971 patients were included in the study (Table 1). Mean age was 78 years, and 24% of patients were 85 years or older. Forty‐three percent were male, 71% were white, and 39% were currently married. The most common principal diagnoses were community‐acquired pneumonia, congestive heart failure, and acute myocardial infarction. The most common comorbidities were hypertension, diabetes, and chronic pulmonary disease. Medicare was the primary payer for 91% of subjects, and 13% were in managed care plans. Most patients were cared for by internists (49%), family practitioners (18%), or cardiologists (17%). Only 1% of patients had a geriatrician as attending.
| Characteristic | n (%) |
|---|---|
| |
| Age group | |
| 6574 years | 168,527 (34%) |
| 7584 years | 206,407 (42%) |
| 85+ years | 119,037 (24%) |
| Sex | |
| Male | 212,358 (43%) |
| Female | 281,613 (57%) |
| Race | |
| White | 351,331 (71%) |
| Black | 52,429 (11%) |
| Hispanic | 18,057 (4%) |
| American Indian | 1876 (0%) |
| Asian/Pacific Islander | 5926 (1%) |
| Other | 64,352 (13%) |
| Marital status | |
| Married/partner | 194,496 (39%) |
| Widowed | 155,273 (31%) |
| Single/separated/divorced | 75,964 (15%) |
| Other | 68,238 (14%) |
| Primary diagnosis | |
| Pneumonia | 122,732 (25%) |
| Heart failure | 109,071 (22%) |
| Acute MI | 70,581 (14%) |
| Ischemic stroke | 57,204 (12%) |
| Chest pain | 50,404 (10%) |
| COPD | 44,582 (9%) |
| Urinary tract infection | 39,397 (8%) |
| Comorbidities | |
| Hypertension | 310,163 (63%) |
| Diabetes | 151,755 (31%) |
| Chronic pulmonary disease | 134,900 (27%) |
| Fluid and electrolyte disorders | 128,703 (26%) |
| Deficiency anemias | 92,668 (19%) |
| Congestive heart failure | 69,201 (14%) |
| Hypothyroidism | 68,711 (14%) |
| Peripheral vascular disease | 47,244 (10%) |
| Depression | 41,507 (8%) |
| Other neurological disorders | 40,200 (8%) |
| Renal failure | 38,134 (8%) |
| Obesity | 25,143 (5%) |
| Payer type | |
| Not Managed care | 431,583 (87%) |
| Managed care | 62,388 (13%) |
| Attending physician specialty | |
| Internal medicine (internist) | 241,982 (49%) |
| Family/general medicine | 90,827 (18%) |
| Cardiology | 83,317 (17%) |
| Pulmonology | 21,163 (4%) |
| Hospitalist | 14,924 (3%) |
| Nephrology | 8257 (2%) |
| Neurology | 5800 (1%) |
| Geriatrics | 3099 (1%) |
| Other* | 24,602 (5%) |
| Mortality | |
| Expired | 28,321 (6%) |
| Alive | 465,650 (94%) |
| Discharge status, n (% of survivors) | |
| Home | 323,629 (66%) |
| Nursing care | 119,468 (24%) |
| Transfer/short‐term hospital | 13,531 (3%) |
| Hospice | 9022 (2%) |
| 14‐Day readmission, n (% of survivors) | |
| Yes | 35,309 (8%) |
| No | 430,334 (92%) |
| Length of stay (days), median (IQR) | 4 (2, 7) |
| Total cost (dollars) | $5513 ($3366, $9902) |
Just under half of all patients (49%) received at least 1 PIM, and 6% received 3 or more (Table 2). Thirty‐eight percent received at least 1 drug with a high severity rating (HS‐PIM). The most common PIMs were promethazine, diphenhydramine, propoxyphene, clonidine, amiodarone, and lorazepam (>3 mg/day).
| Patients, n (%) | |
|---|---|
| Number of PIMs | |
| 0 | 254,200 (51%) |
| 1 | 146,028 (30%) |
| 2 | 61,445 (12%) |
| 3 | 22,128 (4%) |
| 4+ | 10,170 (2%) |
| Number of high‐severity‐rated PIMs | |
| 0 | 304,523 (62%) |
| 1 | 129,588 (26%) |
| 2 | 43,739 (9%) |
| 3 | 12,213 (2%) |
| 4+ | 3908 (1%) |
| Use of any PIM by side effect class | |
| Sedatives | 156,384 (32%) |
| Anticholinergic effects | 109,293 (22%) |
| Causing orthostasis | 43,805 (9%) |
| Causing bleeding | 14,744 (3%) |
| Most commonly prescribed | |
| Promethazine | 49,888 (10%) |
| Diphenhydramine | 45,458 (9%) |
| Propoxyphene | 41,786 (8%) |
| Clonidine | 34,765 (7%) |
| Amiodarone | 34,318 (7%) |
| Lorazepam (>3 mg/day) | 25,147 (5%) |
Patient, Physician, and Hospital Factors Associated with PIMs
Patient, physician, and hospital characteristics were all associated with use of PIMs (Table 3). In univariate analyses, older patients were less likely to receive any class of PIM, and this difference was accentuated for HS‐PIMs. Women, American Indians, married people, and those not in managed care plans were slightly more likely to receive PIMs, whereas patients admitted with acute myocardial infarction or congestive heart failure were even more likely to receive PIMs (P < .0001 for all comparisons).
| Patient characteristic | Any PIM n (row %) | Any high‐severity PIM n (row %) | Sedatives n (row %) | Anticholinergic effects n (row %) | Causing orthostasis n (row %) | Causing bleeding n (row %) |
|---|---|---|---|---|---|---|
| ||||||
| Overall | 239,771 (49%) | 189,448 (38%) | 156,384 (32%) | 109,293 (22%) | 43,805 (9%) | 14,744 (3%) |
| Age group | ||||||
| 6574 years | 89,168 (53%) | 72,573 (43%) | 61,399 (36%) | 44,792 (27%) | 15,799 (9%) | 6655 (4%) |
| 7584 years | 100,787 (49%) | 79,595 (39%) | 65,034 (32%) | 45,121 (22%) | 18,519 (9%) | 5727 (3%) |
| 85+ years | 49,816 (42%) | 37,280 (31%) | 29,951 (25%) | 19,380 (16%) | 9487 (8%) | 2362 (2%) |
| Sex | ||||||
| Male | 100,824 (47%) | 79,535 (37%) | 63,591 (30%) | 42,754 (20%) | 17,885 (8%) | 5771 (3%) |
| Female | 138,947 (49%) | 109,913 (39%) | 92,793 (33%) | 66,539 (24%) | 25,920 (9%) | 8973 (3%) |
| Race | ||||||
| White | 173,481 (49%) | 139,941 (40%) | 112,556 (32%) | 81,097 (23%) | 27,555 (8%) | 10,590 (3%) |
| Black | 26,793 (51%) | 18,655 (36%) | 18,720 (36%) | 11,263 (21%) | 8925 (17%) | 1536 (3%) |
| Hispanic | 8509 (47%) | 6370 (35%) | 5549 (31%) | 3505 (19%) | 2047 (11%) | 648 (4%) |
| American Indian | 1091 (58%) | 849 (45%) | 818 (44%) | 563 (30%) | 190 (10%) | 76 (4%) |
| Asian/Pacific Islander | 2386 (40%) | 1896 (32%) | 1420 (24%) | 1023 (17%) | 519 (9%) | 127 (2%) |
| Other | 27,511 (43%) | 21,737 (34%) | 17,321 (27%) | 11,842 (18%) | 4569 (7%) | 1767 (3%) |
| Marital status | ||||||
| Married/partner | 96,874 (50%) | 77,803 (40%) | 63,303 (33%) | 45,042 (23%) | 16,765 (9%) | 5969 (3%) |
| Widowed | 74,622 (48%) | 58,012 (37%) | 48,367 (31%) | 33,516 (22%) | 13,865 (9%) | 4354 (3%) |
| Single/separated/divorced | 36,583 (48%) | 28,799 (38%) | 24,251 (32%) | 16,115 (21%) | 7229 (10%) | 2399 (3%) |
| Other | 31,692 (46%) | 24,834 (36%) | 20,463 (30%) | 14,620 (21%) | 5946 (9%) | 2022 (3%) |
| Primary diagnosis | ||||||
| Pneumonia | 56,845 (46%) | 46,271 (38%) | 35,353 (29%) | 25,484 (21%) | 9184 (7%) | 4155 (3%) |
| Heart failure | 56,460 (52%) | 42,231 (39%) | 34,340 (31%) | 22,093 (20%) | 10,117 (9%) | 1945 (2%) |
| Acute MI | 43,046 (61%) | 37,849 (54%) | 32,560 (46%) | 25,568 (36%) | 4738 (7%) | 2549 (4%) |
| Ischemic stroke | 25,763 (45%) | 17,613 (31%) | 18,500 (32%) | 8742 (15%) | 9644 (17%) | 1384 (2%) |
| Chest pain | 20,655 (41%) | 16,363 (32%) | 13,536 (27%) | 10,520 (21%) | 3474 (7%) | 2027 (4%) |
| COPD | 18,876 (42%) | 14,626 (33%) | 12,087 (27%) | 8096 (18%) | 3209 (7%) | 1139 (3%) |
| Urinary tract infection | 18,126 (46%) | 14,495 (37%) | 10,008 (25%) | 8790 (22%) | 3439 (9%) | 1545 (4%) |
| Payer type | ||||||
| Nonmanaged care | 212,150 (49%) | 168,013 (39%) | 138,679 (32%) | 97,776 (23%) | 38,341 (9%) | 12,868 (3%) |
| Managed care | 27,621 (44%) | 21,435 (34%) | 17,705 (28%) | 11,517 (18%) | 5464 (9%) | 1876 (3%) |
| Attending physician specialty* | ||||||
| Internal medicine (internist%) | 112,664 (47%) | 86,907 (36%) | 71,382 (30%) | 48,746 (20%) | 23,221 (10%) | 7086 (3%) |
| Family/general medicine | 41,303 (45%) | 32,338 (36%) | 25,653 (28%) | 18,274 (20%) | 7660 (8%) | 2852 (3%) |
| Cardiology | 48,485 (58%) | 40,752 (49%) | 34,859 (42%) | 25,792 (31%) | 5455 (7%) | 2542 (3%) |
| Pulmonology | 10,231 (48%) | 8105 (38%) | 6746 (32%) | 4064 (19%) | 1739 (8%) | 574 (3%) |
| Hospitalist | 7003 (47%) | 5443 (36%) | 4447 (30%) | 3179 (21%) | 1471 (10%) | 463 (3%) |
| Nephrology | 4508 (55%) | 3388 (41%) | 3132 (38%) | 2054 (25%) | 1326 (16%) | 198 (2%) |
| Neurology | 2420 (42%) | 1789 (31%) | 1625 (28%) | 851 (15%) | 699 (12%) | 174 (3%) |
| Geriatrics | 1020 (33%) | 785 (25%) | 596 (19%) | 404 (13%) | 196 (6%) | 41 (1%) |
The HS‐PIM prescribing varied substantially by attending specialty (Fig. 2). Internists, family practitioners, and hospitalists all had similar median rates (33%), cardiologists had a higher median rate (48%), and geriatricians had a lower rate (24%). The most common PIM also differed by specialty: whereas promethazine was the most commonly prescribed drug across most specialties, nephrologists and neurologists used clonidine, pulmonologists used lorazepam, and cardiologists used diphenhydramine most often. Among the 8% of physicians who saw at least 50 patients, there was also great variation in each specialty (Fig. 2). Among internists and cardiologists who saw at least 50 patients, the high‐severity PIM usage rate ranged from 0% to more than 90%.

There was substantial variation in PIM usage among hospitals, most notably by region. The mean proportion of patients receiving PIMs ranged from 34% at hospitals in the Northeast to 55% at hospitals in the South (Table 4). Smaller hospitals and those in urban settings had slightly lower rates, as did those that had geriatricians on staff. The teaching status of the hospital had little effect. Variation at the individual hospital level was extreme (Fig. 3). Although half of all hospitals had rates between 43% and 58%, in 7 hospitals with more than 300 encounters each, PIMs were never prescribed for geriatric patients.

| Hospitals Total = 384 n (%) | Patients N = 49,3971 n (%) | Any PIM Mean = 48.2 Mean (SD) | Any high‐severity PIM Mean = 38.7 Mean (SD) | Sedatives Mean = 30.2 Mean (SD) | Anticholinergic effects Mean = 21.5 Mean (SD) | Causing orthostasis Mean = 8.5 Mean (SD) | Causing bleeding Mean = 3.1 Mean (SD) | |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Hospital region | *** | *** | *** | *** | *** | ** | ||
| Midwest | 76 (20%) | 95,791 (19%) | 38.8 (19.7) | 30.0 (16.4) | 24.3 (13.8) | 15.1 (9.9) | 6.9 (6.3) | 3.1 (2.3) |
| Northeast | 47 (12%) | 79,138 (16%) | 34.1 (12.6) | 26.2 (11.2) | 19.0 (9.2) | 13.5 (8.1) | 4.9 (2.3) | 2.1 (1.6) |
| South | 199 (52%) | 260,870 (53%) | 54.5 (10.1) | 42.7 (9.6) | 36.0 (10.8) | 26.4 (8.6) | 10.4 (4.6) | 3.6 (2.5) |
| West | 62 (16%) | 58,172 (12%) | 45.8 (8.1) | 37.4 (7.1) | 27.3 (7.7) | 19.5 (5.7) | 7.4 (4.8) | 2.7 (1.3) |
| Teaching status | ||||||||
| Nonteaching | 297 (77%) | 324,948 (66%) | 47.3 (14.6) | 36.9 (12.3) | 29.8 (12.0) | 21.3 (9.9) | 8.7 (5.4) | 3.3 (2.4) |
| Teaching | 87 (23%) | 169,023 (34%) | 48.2 (16.0) | 38.8 (14.2) | 31.6 (14.5) | 22.1 (10.2) | 7.8 (4.4) | 2.7 (1.5) |
| Staffed beds | * | ** | ||||||
| 22200 | 143 (37%) | 80,741 (16%) | 45.5 (16.9) | 35.2 (14.6) | 27.5 (14.0) | 20.1 (10.3) | 8.0 (6.2) | 3.5 (3.1) |
| 200400 | 137 (36%) | 177,286 (36%) | 47.7 (14.2) | 37.8 (12.0) | 30.5 (11.6) | 22.0 (10.0) | 8.4 (4.7) | 3.0 (1.6) |
| 400+ | 104 (27%) | 235944 (48%) | 50.1 (12.4) | 39.6 (10.6) | 33.5 (10.9) | 22.7 (9.3) | 9.3 (4.2) | 2.9 (1.4) |
| Population serviced | * | * | ** | |||||
| Rural | 119 (31%) | 102,799 (21%) | 48.4 (13.0) | 38.3 (10.6) | 29.2 (11.0) | 23.2 (9.3) | 7.5 (4.0) | 3.7 (3.0) |
| Urban | 265 (69%) | 391,172 (79%) | 47.1 (15.7) | 36.9 (13.7) | 30.6 (13.2) | 20.7 (10.2) | 9.0 (5.6) | 2.9 (1.8) |
| Geriatrician presence | ||||||||
| No | 340 (89%) | 409,281 (83%) | 47.7 (15.3) | 37.6 (13.0) | 30.3 (12.8) | 21.7 (10.0) | 8.4 (5.3) | 3.2 (2.3) |
| Yes | 44 (11%) | 84,690 (17%) | 45.8 (11.4) | 35.5 (10.6) | 29.4 (10.8) | 19.6 (9.4) | 9.3 (4.3) | 2.9 (1.6) |
Multivariable Model
In a multivariable logit model that included all patient, hospital, and physician characteristics and that accounted for clustering at the hospital, physician, and diagnosis levels, several characteristics were associated with HS‐PIM prescribing (Table 5). By far the most important predictor of use was hospital region. Compared with patients at hospitals in the Midwest, patients in the South (OR 1.63, 95% CI 1.591.67) and West (OR 1.43, 95% CI 1.381.47) were more likely and those in the Northeast were less likely (OR 0.85, 95% CI 0.830.88) to receive HS‐PIMs. Larger hospitals had higher HS‐PIM rates than smaller ones, but teaching status and rural or urban setting were not associated with HS‐PIM prescribing. The presence of geriatricians in a hospital was also associated with lower HS‐PIM prescribing for the entire hospital.
| Effect (reference) | Odds ratio | 95% Confidence limits | |
|---|---|---|---|
| Age | |||
| 6574 years | 1.00 | ||
| 7584 years | 0.83 | 0.82 | 0.84 |
| 85+ years | 0.59 | 0.58 | 0.61 |
| Sex | |||
| Female | 1.00 | ||
| Male | 0.85 | 0.83 | 0.86 |
| Race | |||
| White | 1.00 | ||
| Black | 0.78 | 0.76 | 0.80 |
| Hispanic | 0.84 | 0.81 | 0.87 |
| American Indian | 0.97 | 0.88 | 1.07 |
| Asian/Pacific Islander | 0.74 | 0.70 | 0.79 |
| Other | 0.94 | 0.92 | 0.97 |
| Marital Status | |||
| Married/partner | 1.00 | ||
| Single/separated/divorced | 0.96 | 0.94 | 0.98 |
| Widowed | 0.96 | 0.95 | 0.98 |
| Other | 0.93 | 0.90 | 0.95 |
| Primary diagnosis | |||
| Pneumonia | 1.00 | ||
| COPD | 0.83 | 0.81 | 0.85 |
| Heart failure | 1.14 | 1.12 | 1.16 |
| Ischemic stroke | 0.84 | 0.82 | 0.86 |
| Acute MI | 1.95 | 1.90 | 2.01 |
| Urinary tract infection | 1.06 | 1.03 | 1.09 |
| Chest pain | 0.87 | 0.84 | 0.89 |
| Comorbidities (yes or no) | |||
| Hypertension | 0.98 | 0.97 | 0.99 |
| Diabetes | 0.98 | 0.97 | 1.00 |
| Chronic lung disease | 1.11 | 1.10 | 1.13 |
| Fluid and electrolyte disorders | 1.26 | 1.24 | 1.27 |
| Anemia deficiencies | 1.17 | 1.15 | 1.18 |
| Congestive heart failure | 1.34 | 1.32 | 1.37 |
| Hypothyroidism | 1.13 | 1.11 | 1.15 |
| Peripheral vascular disease | 1.09 | 1.06 | 1.11 |
| Depression | 1.38 | 1.35 | 1.41 |
| Neurological disorders | 0.89 | 0.87 | 0.91 |
| Renal failure | 1.23 | 1.20 | 1.26 |
| Obesity | 1.11 | 1.08 | 1.14 |
| Payer type | |||
| Managed care | 1.00 | ||
| Not managed care | 1.04 | 1.02 | 1.06 |
| Attending physician specialty | |||
| Internal medicine | 1.00 | ||
| Cardiology | 1.32 | 1.28 | 1.36 |
| Family/general medicine | 0.99 | 0.97 | 1.01 |
| Geriatrics | 0.69 | 0.61 | 0.78 |
| Hospitalist | 0.90 | 0.84 | 0.96 |
| Nephrology | 1.02 | 0.96 | 1.08 |
| Neurology | 0.93 | 0.86 | 1.00 |
| Pulmonology | 1.10 | 1.05 | 1.15 |
| Setting | |||
| Rural | 1.00 | ||
| Urban | 1.02 | 1.00 | 1.05 |
| Teaching status | |||
| Nonteaching | 1.00 | ||
| Teaching | 1.01 | 0.98 | 1.03 |
| Number of beds | |||
| 22200 | 1.00 | ||
| 200400 | 1.08 | 1.05 | 1.11 |
| 400+ | 1.12 | 1.09 | 1.16 |
| Region | |||
| Midwest | 1.00 | ||
| Northeast | 0.85 | 0.83 | 0.88 |
| South | 1.63 | 1.59 | 1.67 |
| West | 1.43 | 1.38 | 1.47 |
| Geriatrician presence | |||
| No | 1.00 | ||
| Yes | 0.93 | 0.90 | 0.95 |
Physician specialty was also important. Adjusting for diagnosis attenuated some of this association, but compared with internists, cardiologists (OR 1.32, 95% CI 1.281.36) and pulmonologists (OR 1.10, 95% CI 1.051.15) were still more likely, hospitalists (OR 0.90, 95% CI 0.840.96) were less likely, and geriatricians (0.69, 95% CI 0.610.78) were least likely to prescribe any HS‐PIM.
Patient factors were also associated with HS‐PIM use. Compared with patients age 6574 years, patients older than 85 years were much less likely to receive an HS‐PIM (OR 0.59, CI 0.580.61), as to a lesser extent were nonwhites compared with whites and unmarried people compared with those who were married. Compared with patients with pneumonia, those with COPD, stroke, or chest pain were less likely and those with myocardial infarction and congestive heart failure were more likely to receive HS‐PIMs. Patients with a secondary diagnosis of depression were also at high risk (OR 1.38, CI 1.351.41).
DISCUSSION
Although Americans age 65 years and older make up less than 15% of the U.S. population, they consume about one third of all prescription drugs20 and account for one third of all hospital admissions.21 Using the Beers list, numerous studies have documented high rates of potentially inappropriate prescribing for community‐dwelling elderly and nursing home patients and, in some studies, an attendant risk of falling,2224 hip fracture,25, 26 hospitalization,13 or death.14 Applying these same criteria to a large sample of medical inpatients, we found that almost half received a potentially inappropriate drug, most of high severity. Moreover, the PIM prescribing rate varied substantially by region, hospital, and attending physician specialty. Although the use of PIMs was associated with patient age, comorbidities, and primary diagnosis, these patient factors explained only a small portion of the variation in prescribing practices across groups of physicians and hospitals.
Using consensus criteria, Beers originally found that 40% of the residents in 12 nursing homes received at least 1 PIM,8 and studies of community‐dwelling elderly demonstrated rates of 21% to 37%, with little change over time.6, 27, 28 Several small studies have examined inpatient prescribing.16, 17, 29, 30 The largest17 found that only 15% of elderly Italian inpatients received a PIM. Our finding, that 49% of inpatients had received at least 1 PIM, may partially reflect the high prevalence of use among elderly US patients in nursing homes and the community.
Regional variation has been demonstrated for ambulatory patients in the US6 and Europe.31 Zhan et al. found slightly higher rates of PIM use in the Midwest and the South (23%) than in the Northeast and the West (19%). Variation in Europe was greater, with 41% of patients in the Czech Republic versus 5.8% of patients in Denmark receiving at least 1 PIM. We found that region was the strongest predictor of in‐hospital HS‐PIM use, with patients in the South most likely and patients in the Northeast least likely to receive HS‐PIMs. This variation persisted even after adjusting for differences in other patient and hospital factors, suggesting that local custom played a large role in the decision to prescribe HS‐PIMs. Moreover, because outpatient rates are more uniform, these large differences seem limited to inpatient practice.
Patient factors have also been examined. Advanced age was associated with decreased PIM use in some studies17, 28, 31 but not in others.6, 27 We found increasing age to be strongly associated with decreased PIM use, suggesting that in the hospital, at least, doctors take care to avoid prescribing certain drugs to the frail elderly. Women appear to be consistently at higher risk than men,6, 27, 28, 31 and white patients are more at risk than those of other races.6 Our finding that certain diagnoses were associated with higher or lower rates has not been reported previously. The lower rates associated with stroke and COPD suggest that prescribers were aware that these patients were at increased risk of delirium and respiratory depression. The higher rates associated with myocardial infarction may have to do with the use of standardized order sets (eg, cath lab orders) that do not consider the age of the patient going for the procedure.
Admission to a geriatric service32 and intervention by a clinical pharmacist33 have been shown to decrease PIM prescribing at discharge. We noted that patients cared for by a geriatrician had the lowest rates of PIM prescribing during hospitalization as well and that hospitals with geriatricians had lower rates overall, possibly demonstrating that geriatricians had a ripple effect on their colleagues. Hospitalists also had lower rates than internists, supporting the notion that hospitalists provide higher‐quality inpatient care.
Our study had some important limitations. First, we only had access to inpatient administrative records. Thus, we could not identify which medications were continued from home and which were begun in the hospital, nor could we know the indications for which specific drugs were prescribed or who prescribed them. Based on published outpatient rates, however, we could assume that many of the drugs were started in the hospital and that others could have been discontinued but were not. Second, the Beers list was developed by the modified Delphi method; there was little empirical evidence of the danger of specific drugs, although some classes, such as benzodiazepines, opiates and digoxin, have been associated with inpatient falls.18, 3436 Furthermore, our administrative database did not allow us to balance the risks and benefits for particular patients; hence, the medications were only potentially inappropriate, and our study did not address the consequences of such prescribing. Although some of these drugs may be appropriate under certain circumstances, it is unlikely that these circumstances would vary by 60% across geographic regions or that internists would encounter these circumstances more often than do hospitalists. Thus, although we could not identify specific patients who received inappropriate medications, we did identify certain hospitals and even whole regions of the country in which the rate of inappropriate prescribing was high. Third, the Beers list, which was developed for outpatient use, may be less relevant in the inpatient setting. However, given that inpatients have more organ dysfunction and are at higher risk of delirium and falls, it may actually be more applicable to hospitalized patients. We similarly did not distinguish between single and multiple doses because the Beers list does not make such a distinction, and there is no empirical evidence that a single dose is safe. Indeed, patients are often at highest risk of falls immediately after initiation of therapy.3739 We did, however, exclude drugs such as laxatives, which may be appropriate for brief inpatient use but not for chronic use.
Our study also had a number of strengths. The large sample size, representing approximately 5% of annual inpatient admissions in the US over 2 years, offered an instructive look at the recent prescribing patterns of thousands of US physicians. We were able to identify many patient, physician, and hospital factors associated with PIM prescribing that have not previously been reported. Some of these factors, such as advanced age and comorbid diagnoses, suggest that physicians do tailor their treatment to individual patients. Nevertheless, patient factors accounted for only a small portion of the variation in prescribing. The largest variation, associated with regional, hospital, and physician factors, highlights the opportunity for improvement.
At the same time, our findings are encouraging for 2 reasons. First, most inappropriate prescribing involved only a handful of medications, so small changes in prescribing patterns could have a tremendous impact. Second, observing the practice of individual physicians and hospitals reveals what is possible. We found that in most specialties there were physicians who rarely or never used PIMs. We also found 7 hospitals, each with at least 300 cases, where no PIMs were ever prescribed.
Where should hospitals focus their efforts to prevent inappropriate prescribing? Our data highlight the complexity of the problem, which seems daunting. PIM prescribing is spread across all specialties, including geriatrics, and although cardiologists had the highest rate of prescribing, internists, who were more numerous, accounted for a much higher overall number of potentially inappropriate prescriptions. It would be instructive to study the 7 hospitals where PIMs were never prescribed or to interview those physicians who never prescribed PIMs, but the anonymous nature of our data would not allow for this. However, our data do suggest some directions. First, hospitals should become aware of their own rates of PIM use because measurement is the first step in quality improvement. Next, hospitals should focus efforts on reducing the use of the most common drugs. Eliminating just 3 drugs promethazine, diphenhydramine, and propoxyphenewould reduce the use of PIMs in 24% of elderly patients. Enlisting hospital pharmacists and electronic health records and reviewing standard order sets for elderly patients are potentially effective strategies. Finally, increasing the presence of geriatricians and hospitalists would be expected to have a modest impact.
In a representative sample of elderly inpatients, we found that almost half received a potentially inappropriate medication and that the rate of inappropriate prescribing varied widely among doctors and hospitals. Additional research is needed to distinguish which of the Beers drugs are most harmful and which patients are at highest risk. Research should also focus on understanding differences in prescribing patterns, perhaps by studying the outliers at both ends of the quality spectrum, and on techniques to minimize non‐patient‐centered variation.
- ,,,,,.Explicit criteria for determining inappropriate medication use in nursing home residents. UCLA Division of Geriatric Medicine.Arch Intern Med.1991;151:1825–1832.
- .Explicit criteria for determining potentially inappropriate medication use by the elderly. An update.Arch Intern Med.1997;157:1531–1536.
- ,,,,,.Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts.Arch Intern Med.2003;163:2716–2724.
- National Committee on Quality Assurance. Drugs to be Avoided in the Elderly. Available at: http://www.ncqa.org/Programs/HEDIS/2006/Volume2/NDC/DAE_06.xls. Accessed November 20,2006.
- ,,, et al.Inappropriate prescribing for elderly Americans in a large outpatient population.Arch Intern Med.2004;164:1621–1625.
- ,,, et al.Potentially inappropriate medication use in the community‐dwelling elderly: findings from the 1996 Medical Expenditure Panel Survey.JAMA.2001;286:2823–2829.
- ,.Prescribing potentially inappropriate psychotropic medications to the ambulatory elderly.Arch Intern Med.2000;160:2825–2831.
- ,,, et al.Inappropriate medication prescribing in skilled‐nursing facilities.Ann Intern Med.1992;117:684–689.
- ,,, et al.Adverse outcomes associated with inappropriate drug use in nursing homes.Ann Pharmacother.2005;39:405–411.
- ,,.Inappropriate medication administration to the acutely ill elderly: a nationwide emergency department study, 1992–2000.J Am Geriatr Soc.2004;52:1847–1855.
- ,,, et al.Appropriateness of medication selection for older persons in an urban academic emergency department.Acad Emerg Med.1999;6:1232–1242.
- ,,,,,.Use of the Beers criteria to predict adverse drug reactions among first‐visit elderly outpatients.Pharmacotherapy.2005;25:831–838.
- ,,.The association of inappropriate drug use with hospitalisation and mortality: a population‐based study of the very old.Drugs Aging.2005;22(1):69–82.
- ,,,,.Hospitalization and death associated with potentially inappropriate medication prescriptions among elderly nursing home residents.Arch Intern Med.2005;165(1):68–74.
- ,,.Potentially inappropriate prescribing for geriatric inpatients: an acute care of the elderly unit compared to a general medicine service.Consult Pharm.2003;18(1):37–42, 47–39.
- ,,, et al.Inappropriate medication use among frail elderly inpatients.Ann Pharmacother.2004;38(1):9–14.
- ,,,,,.Inappropriate medication use among hospitalized older adults in Italy: results from the Italian Group of Pharmacoepidemiology in the Elderly.Eur J Clin Pharmacol.2003;59(2):157–162.
- ,,,,,.Guided prescription of psychotropic medications for geriatric inpatients.Arch Intern Med.2005;165:802–807.
- ,,,.Comorbidity measures for use with administrative data.Med Care.1998;36(1):8–27.
- ,.Inadequate prescription‐drug coverage for Medicare enrollees—a call to action.N Engl J Med.1999;340:722–728.
- National and regional estimates on hospital use for all patients from the HCUP Nationwide Inpatient Sample (NIS). Agency for Healthcare Research and Quality (AHRQ). Available at: http://hcupnet.ahrq.gov/HCUPnet.jsp. Accessed October 12,2006.
- ,,,,,.Drugs and falls in community‐dwelling older people: a national veterans study.Clin Ther.2006;28:619–630.
- ,,,,,.Psychotropic medications and risk for falls among community‐dwelling frail older people: an observational study.J Gerontol A Biol Sci Med Sci.2005;60:622–626.
- ,,.Drugs and falls in older people: a systematic review and meta‐analysis: I. Psychotropic drugs.J Am Geriatr Soc.1999;47(1):30–39.
- ,,.Propoxyphene use and risk for hip fractures in older adults.Am J Geriatr Pharmacother.2006;4:219–226.
- ,,, et al.Central nervous system active medications and risk for fractures in older women.Arch Intern Med.2003;163:949–957.
- ,,, et al.Potentially inappropriate medication use by elderly persons in U.S. Health Maintenance Organizations, 2000–2001.J Am Geriatr Soc.2005;53:227–232.
- .Inappropriate medication prescribing for elderly ambulatory care patients.Arch Intern Med.2004;164:305–312.
- ,,.Adverse drug reactions in an elderly hospitalised population: inappropriate prescription is a leading cause.Drugs Aging.2005;22:767–777.
- ,,,,.Use of inappropriate medications and their prognostic significance among in‐hospital and nursing home patients with and without dementia in Finland.Drugs Aging.2006;23:333–343.
- ,,, et al.Potentially inappropriate medication use among elderly home care patients in Europe.JAMA.2005;293:1348–1358.
- ,,,,.Impact of hospitalisation in an acute medical geriatric unit on potentially inappropriate medication use.Drugs Aging.2006;23(1):49–59.
- ,.Pharmacists and their effectiveness in ensuring the appropriateness of the chronic medication regimens of geriatric inpatients.Consult Pharm.2004;19:432–436.
- ,,,,,.Benzodiazepines with different half‐life and falling in a hospitalized population: the GIFA study. Gruppo Italiano di Farmacovigilanza nell'Anziano.J Clin Epidemiol.2000;53:1222–1229.
- ,.Relationship between the administration of selected medications and falls in hospitalized elderly patients.Ann Pharmacother.1995;29:354–358.
- .The use of sedative/hypnotic medication and its correlation with falling down in the hospital.Sleep.1996;19:698–701.
- ,,, et al.Benzodiazepine use and hip fractures in the elderly: who is at greatest risk?Arch Intern Med.2004;164:1567–1572.
- ,,,,.Hazardous benzodiazepine regimens in the elderly: effects of half‐life, dosage, and duration on risk of hip fracture.Am J Psychiatry.2001;158:892–898.
- ,,,,.A 5‐year prospective assessment of the risk associated with individual benzodiazepines and doses in new elderly users.J Am Geriatr Soc.2005;53:233–241.
- ,,,,,.Explicit criteria for determining inappropriate medication use in nursing home residents. UCLA Division of Geriatric Medicine.Arch Intern Med.1991;151:1825–1832.
- .Explicit criteria for determining potentially inappropriate medication use by the elderly. An update.Arch Intern Med.1997;157:1531–1536.
- ,,,,,.Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts.Arch Intern Med.2003;163:2716–2724.
- National Committee on Quality Assurance. Drugs to be Avoided in the Elderly. Available at: http://www.ncqa.org/Programs/HEDIS/2006/Volume2/NDC/DAE_06.xls. Accessed November 20,2006.
- ,,, et al.Inappropriate prescribing for elderly Americans in a large outpatient population.Arch Intern Med.2004;164:1621–1625.
- ,,, et al.Potentially inappropriate medication use in the community‐dwelling elderly: findings from the 1996 Medical Expenditure Panel Survey.JAMA.2001;286:2823–2829.
- ,.Prescribing potentially inappropriate psychotropic medications to the ambulatory elderly.Arch Intern Med.2000;160:2825–2831.
- ,,, et al.Inappropriate medication prescribing in skilled‐nursing facilities.Ann Intern Med.1992;117:684–689.
- ,,, et al.Adverse outcomes associated with inappropriate drug use in nursing homes.Ann Pharmacother.2005;39:405–411.
- ,,.Inappropriate medication administration to the acutely ill elderly: a nationwide emergency department study, 1992–2000.J Am Geriatr Soc.2004;52:1847–1855.
- ,,, et al.Appropriateness of medication selection for older persons in an urban academic emergency department.Acad Emerg Med.1999;6:1232–1242.
- ,,,,,.Use of the Beers criteria to predict adverse drug reactions among first‐visit elderly outpatients.Pharmacotherapy.2005;25:831–838.
- ,,.The association of inappropriate drug use with hospitalisation and mortality: a population‐based study of the very old.Drugs Aging.2005;22(1):69–82.
- ,,,,.Hospitalization and death associated with potentially inappropriate medication prescriptions among elderly nursing home residents.Arch Intern Med.2005;165(1):68–74.
- ,,.Potentially inappropriate prescribing for geriatric inpatients: an acute care of the elderly unit compared to a general medicine service.Consult Pharm.2003;18(1):37–42, 47–39.
- ,,, et al.Inappropriate medication use among frail elderly inpatients.Ann Pharmacother.2004;38(1):9–14.
- ,,,,,.Inappropriate medication use among hospitalized older adults in Italy: results from the Italian Group of Pharmacoepidemiology in the Elderly.Eur J Clin Pharmacol.2003;59(2):157–162.
- ,,,,,.Guided prescription of psychotropic medications for geriatric inpatients.Arch Intern Med.2005;165:802–807.
- ,,,.Comorbidity measures for use with administrative data.Med Care.1998;36(1):8–27.
- ,.Inadequate prescription‐drug coverage for Medicare enrollees—a call to action.N Engl J Med.1999;340:722–728.
- National and regional estimates on hospital use for all patients from the HCUP Nationwide Inpatient Sample (NIS). Agency for Healthcare Research and Quality (AHRQ). Available at: http://hcupnet.ahrq.gov/HCUPnet.jsp. Accessed October 12,2006.
- ,,,,,.Drugs and falls in community‐dwelling older people: a national veterans study.Clin Ther.2006;28:619–630.
- ,,,,,.Psychotropic medications and risk for falls among community‐dwelling frail older people: an observational study.J Gerontol A Biol Sci Med Sci.2005;60:622–626.
- ,,.Drugs and falls in older people: a systematic review and meta‐analysis: I. Psychotropic drugs.J Am Geriatr Soc.1999;47(1):30–39.
- ,,.Propoxyphene use and risk for hip fractures in older adults.Am J Geriatr Pharmacother.2006;4:219–226.
- ,,, et al.Central nervous system active medications and risk for fractures in older women.Arch Intern Med.2003;163:949–957.
- ,,, et al.Potentially inappropriate medication use by elderly persons in U.S. Health Maintenance Organizations, 2000–2001.J Am Geriatr Soc.2005;53:227–232.
- .Inappropriate medication prescribing for elderly ambulatory care patients.Arch Intern Med.2004;164:305–312.
- ,,.Adverse drug reactions in an elderly hospitalised population: inappropriate prescription is a leading cause.Drugs Aging.2005;22:767–777.
- ,,,,.Use of inappropriate medications and their prognostic significance among in‐hospital and nursing home patients with and without dementia in Finland.Drugs Aging.2006;23:333–343.
- ,,, et al.Potentially inappropriate medication use among elderly home care patients in Europe.JAMA.2005;293:1348–1358.
- ,,,,.Impact of hospitalisation in an acute medical geriatric unit on potentially inappropriate medication use.Drugs Aging.2006;23(1):49–59.
- ,.Pharmacists and their effectiveness in ensuring the appropriateness of the chronic medication regimens of geriatric inpatients.Consult Pharm.2004;19:432–436.
- ,,,,,.Benzodiazepines with different half‐life and falling in a hospitalized population: the GIFA study. Gruppo Italiano di Farmacovigilanza nell'Anziano.J Clin Epidemiol.2000;53:1222–1229.
- ,.Relationship between the administration of selected medications and falls in hospitalized elderly patients.Ann Pharmacother.1995;29:354–358.
- .The use of sedative/hypnotic medication and its correlation with falling down in the hospital.Sleep.1996;19:698–701.
- ,,, et al.Benzodiazepine use and hip fractures in the elderly: who is at greatest risk?Arch Intern Med.2004;164:1567–1572.
- ,,,,.Hazardous benzodiazepine regimens in the elderly: effects of half‐life, dosage, and duration on risk of hip fracture.Am J Psychiatry.2001;158:892–898.
- ,,,,.A 5‐year prospective assessment of the risk associated with individual benzodiazepines and doses in new elderly users.J Am Geriatr Soc.2005;53:233–241.
Copyright © 2008 Society of Hospital Medicine
Physician Attitudes and Use of Computerized Order Entry
It is widely acknowledged that the U.S. health care system is plagued by error and inefficiency and that these factors contribute to as many as 44,000‐98,000 deaths each year in U.S. hospitals. In To Err Is Human: Building a Safer Health System, the Institute of Medicine1 outlined the critical role that information technology can play in improving patient safety and highlighted computerized physician order entry (CPOE) systems for their potential to reduce the frequency of medication errors and to improve the quality of medical care.
Computerized physician order entry systems are specialized software applications that allow physicians to place orders directly into a computer. This process has a number of potential advantages over traditional handwritten ordering, including the ability to structure the ordering process to ensure the completeness of individual orders, to provide clinical decision support through diagnosis‐based order sets, and to automatically check orders for potential drugallergy, drugdrug, and drugfood interactions.2 Finally, entering orders directly into a computer eliminates the problem of transcription‐related errors that stem from the difficulty of interpreting handwriting. In clinical trials, the introduction of CPOE has been shown to reduce the frequency of medication errors, to improve the use of preventive services, and to reduce costs.36 Recognition of the benefits of these systems has not been confined to the medical community. The Leapfrog Organization, a coalition of large businesses in the United States, has chosen CPOE as one of its 3 initial safety leaps and has established a threshold that 70% of medication orders should be entered directly by physicians.7
Although the benefits of CPOE systems are widely recognized, few hospitals have implemented these systems successfully.8, 9 Those that have, have often developed the applications internally, and many have relied on house staff to do most or all of the actual ordering.10 However, most hospitals do not have the expertise for internal development and instead rely on commercially available products. Moreover, most patients hospitalized in the United States are cared for by attending physicians working without the assistance of house staff.11 In light of the importance of successfully implementing CPOE systems in such settings, we assessed the adoption of CPOE by attending physicians at 2 community hospitals where its use was voluntary and examined the characteristics and attitudes associated with use of the system to place orders.
METHODS
Setting and Participants
Baystate Medical Center is a 600‐bed teaching hospital in Springfield, Massachusetts, where approximately 50% of patients are cared for with the assistance of house staff. Franklin Medical Center is a 125‐bed community hospital in rural Greenfield, Massachusetts, and is not a house staff training site. Medical staff membership at the 2 hospitals is largely voluntary. Both institutions share a vendor‐supplied computerized order entry system that was implemented in the early 1990s (E7000, Eclipsys Corporation, Boca Raton, FL). The system provides a structured format for the creation of medication, laboratory, and radiology orders and contains thousands of preconstructed medication order sentences and hundreds of order sets designed to standardize ordering for common diagnoses and procedures. Pharmacists are alerted of potential drugallergy and drugdrug interactions and use clinical judgment about whether to communicate this information to the physician. Although the house staff at Baystate Medical Center is mandated to place orders in the system, attending physicians have no such requirement at either institution. Access to the system is provided though the many fixed workstations located on nursing units, in operating rooms, and in the health sciences library. On a typical medical‐surgical patient care unit most computers are behind the nurses' station, though some are distributed along hallways and in physician charting rooms. No computers are in patient rooms. Although the number varies slightly across units, the average ratio of computers to patient beds is roughly 1 to 1.
Survey
In June 2003 we mailed a 20‐item survey to attending physicians who had been responsible for a minimum of 25 orders during the preceding month at either Baystate or Franklin Medical Center. Orders counted toward this minimum if they had been written, given verbally in person or by phone, or entered directly into the computer by the physician. The survey consisted of 20 questions focused on the topic of computerized order entry. In addition to collecting information about sex and specialty, we asked respondents to describe their use of CPOE during training, their use of computers at home, and, where applicable, their use of computers in their outpatient practices. The survey included questions about how often respondents used the order entry system when caring for hospitalized patients and which features of the system they used. To assess physician attitudes about the order entry process, we asked respondents to consider whether it was faster to place orders directly into the system than it was by handwriting them, whether orders placed in the system were carried out more rapidly, whether placing orders in the system led to fewer medication and other errors, whether order sets were important for the efficient use of the system, whether order sets helped to ensure that important aspects of care did not slip through the cracks, whether the system's user interface supported their work flow, and whether the encouragement of nurses was an important factor in their use of the system. Questions that assessed physician attitudes were presented on a 5‐point Likert scale. Nonrespondents were sent reminder letters along with duplicate surveys twice, approximately 1 and 2 months after the initial mailing. No financial incentive was offered for participation. The study protocol was approved by the Institutional Review Board of Baystate Health System.
Order Entry Rates
Regardless of whether an order is placed directly by a physician into a computer, given verbally, or handwritten, all orders are ultimately entered into the CPOE system. Working with our hospitals' Departments of Information Services, we developed a report that provided physician‐specific information about order entry patterns. For each physician in the study, we determined the total number of orders generated during the month preceding the initial June mailing, as well as the absolute number and percentage of orders of each of the following categories: directly entered, telephone, verbal, and written. Because verbal and telephone orders are required during urgent situations and when physicians give orders from outside the hospital, we calculated and report an adjusted order entry rate as the total number of orders placed directly into the system divided by the sum of the orders entered directly and the number of written orders.
Analysis
Summary statistics for the overall sample were constructed using simple frequencies and proportions for categorical variables and medians and interquartile ranges for continuous variables. We compared characteristics of respondents from the 2 hospitals using chi‐square tests of association for categorical factors and Wilcoxon rank‐sum tests for continuous scale data. We compared the total number of orders placed during the study month and the order entry rates of responders and nonresponders using the Wilcoxon rank‐sum test. We categorized physicians as low (20%), intermediate (21%‐79%), and high (80%) users of the system based on their calculated order entry rate. Responses to each of the attitude questions in the survey were tabulated, and the responses strongly agree and agree were combined for analyses comparing responses. Demographic variables and physician attitudes were tested for associations with order entry rate categories via the Pearson chi‐square for categorical factors, the Mantel‐Haenszel chi‐square for ordered factors, and Kruskal‐Wallis analysis of variance for continuous variables. Initial analyses were stratified by hospital; where no differences in association were found across strata, the data were combined. Statistical tests were performed using SAS version 9.1 (SAS Institute, Cary, NC)
RESULTS
During the study period the target group of physicians placed a total of 135,692 orders, of which 69,654 (51%) were placed directly into the CPOE system, 38,878 (29%) were made using pen and paper, 7,208 (5%) were made verbally, and 19,952 (15%) were placed by telephone. Three hundred and fifty‐six (71%) of the 502 surveys sent out to physicians at the 2 hospitals were returned. Thirteen surveys were excluded from analysis because the respondent was not a physician, and 2 because we were unable to match the survey to system usage data, leaving a total of 341 surveys for analysis. Order entry rates were not computed for an additional 3 physicians who only placed verbal and telephone orders during the study period. Response rates did not differ by clinician specialty (P = .53); compared to those of nonresponders, respondents had a similar median total number of orders (111 vs. 101, P = .67) and a higher median order entry rate (66% vs. 48%, P = .03).
Characteristics of Respondents
Seventy‐two percent of physicians who completed the survey were men; half had graduated from medical school at least 20 years ago, and the median duration of practice at the study institution was 11 years (Table 1). Forty percent practiced internal medicine, 18% were surgeons, and 16% were pediatricians. Thirty‐five percent completed training at an institution that had computerized physician order entry, and 86% cared for patients primarily at Baystate Medical Center. More than half reported they used the system many times each day for patient care, and the features they used most commonly were retrieval of results (95%), placing of orders (78%), and viewing and printing of patient lists (75%). Among those with outpatient practices, 81% used computers in their outpatient practice, and more than half used computers for personal activities at home at least once a day. On average, respondents from Franklin Medical Center had graduated from medical school farther in the past and reported less reliance on the system to carry out all activities other than viewing results.
| Overall n (%) | Baystate n (%) 293 (85.9) | Franklin n (%) 48 (14.1) | Chi square P value | |
|---|---|---|---|---|
| ||||
| Sex | .64 | |||
| Male | 244 (71.6) | 211 (72.0) | 33 (68.8) | |
| Specialty | .24 | |||
| Anesthesia | 23 (6.7) | 23 (7.9) | 0 (0.0) | |
| Internal medicine | 135 (39.6) | 112 (38.2) | 23 (47.9) | |
| Medicine/pediatrics | 13 (3.8) | 6 (2.0) | 7 (14.6) | |
| OB/GYN | 36 (10.6) | 30 (10.2) | 6 (12.5) | |
| Pediatrics | 54 (15.8) | 51 (17.4) | 3 (6.3) | |
| Surgery | 61 (17.9) | 55 (18.8) | 6 (12.5) | |
| Other | 19 (5.6) | 16 (5.5) | 3 (6.3) | |
| Use of CPOE systema | .09 | |||
| Many times a day | 176 (52.2) | 160 (55.0) | 16 (34.8) | |
| At least once a day | 77 (22.9) | 61 (21.0) | 16 (34.8) | |
| A few times a week | 55 (16.3) | 45 (15.5) | 10 (21.7) | |
| Once a week or less | 29 (8.6) | 25 (8.6) | 4 (8.7) | |
| Features useda | ||||
| Viewing and printing patient lists | 254 (75.2) | 212 (72.6) | 42 (91.3) | .01 |
| Looking up results | 320 (94.7) | 277 (94.9) | 43 (93.5) | .70 |
| Viewing current medications | 218 (64.5) | 204 (69.9) | 14 (30.4) | < .01 |
| Placing orders | 263 (77.8) | 244 (83.6) | 19 (41.3) | < .01 |
| Entering discharge summaries | 72 (21.3) | 70 (24.0) | 2 (4.4) | < .01 |
| Use of order setsa | ||||
| Rarely or never | 98 (29.0) | 74 (25.3) | 24 (52.2) | < .01 |
| Minority of patients | 92 (27.2) | 78 (26.7) | 14 (30.4) | |
| Majority of patients | 104 (30.8) | 97 (33.2) | 7 (15.2) | |
| For all or nearly all patients | 44 (13.0) | 43 (14.7) | 1 (2.2) | |
| Percentage of orders placed using order setsa | < .01 | |||
| None | 46 (13.7) | 26 (9.0) | 20 (44.4) | |
| 1%‐25% | 62 (18.5) | 50 (17.2) | 12 (26.7) | |
| 26%‐50% | 29 (8.7) | 23 (7.9) | 6 (13.3) | |
| 51%‐75% | 45 (13.4) | 43 (14.9) | 2 (4.4) | |
| 76%‐99% | 103 (30.8) | 98 (33.8) | 5 (11.1) | |
| All | 50 (14.9) | 50 (17.2) | 0 (0.0) | |
| Use of computer in outpatient practiceab | 243 (81.3) | 206 (80.8) | 37 (84.1) | .60 |
| Personal computer usea | .47 | |||
| At least once a day | 209 (61.7) | 185 (63.4) | 24 (51.1) | |
| Several times a week | 84 (24.8) | 67 (23.0) | 17 (36.2) | |
| A few times a month | 21 (6.2) | 18 (6.2) | 3 (6.4) | |
| Rarely | 25 (7.4) | 22 (7.5) | 3 (6.4) | |
| Training at an institution that had CPOE | 117 (34.7) | 105 (36.1) | 12 (26.1) | 0.19 |
| Use of system to enter orders should be mandatorya | ||||
| Yes | 113 (35.2) | 106 (38.4) | 7 (15.6) | <.01 |
| Median (IQR) | Median (IQR) | Median (IQR) | WilcoxonPvalue | |
| Years since medical school graduationa | 20 (13, 26) | 20 (13, 26) | 24 (17, 28) | .02 |
| Years in practice at study institutiona | 11 (5, 18) | 11 (5, 18) | 13 (7, 19) | .39 |
| Orders directly enteredc | 23 (2, 99) | 27 (5, 108) | 1 (0, 27) | < .01 |
| Orders placed by telephonec | 14 (5, 49) | 12 (3, 38) | 49.5 (16, 123.5) | < .01 |
| Orders placed verballyc | 2 (0, 11) | 3 (0, 13) | 1 (0,3) | < .01 |
| Orders placed in writingc | 21 (4, 73) | 14 (3, 45) | 220 (106.5, 391) | < .01 |
| CPOE ratebc | 66% (3%, 94%) | 76% (19%, 96%) | 0.25% (0%, 17%) | < .01 |
Attitudes Toward Computerized Physician Order Entry
Physicians who completed the survey offered diverse opinions about the impact of computerized order entry on work flow, patient safety, and quality of care. Only 22% believed the system's user interface supported their work flow (Q7), 34% believed it was faster to enter orders directly into the system than to handwrite them (Q1), and 41% believed orders placed into the system were carried out more rapidly (Q2) (Table 2). On the other hand, 63% of respondents believed that placing orders directly into the system led to fewer medication errors (Q3), and 51% stated the system generally reduced medical errors (Q4). Sixty‐nine percent stated order sets were important for efficient use of the system (Q5), and 71% believed order sets served an important decision support role (Q6). Twenty‐six percent stated that the encouragement of nurses was an important factor in their use of the system (Q8). Finally, 35% of attending physicians believed use of the system to place orders should be mandatory.
Characteristics and Attitudes of High, Intermediate, and Low Users
The median order entry rate of respondents was 66%. One hundred and forty‐one (42%) placed at least 80% of their orders directly into the system, whereas 109 (32%) placed no more than 20% of their orders directly in the system (Fig. 1). There was not a significant difference between the low, intermediate, and high use groups in the total number of orders that each physician placed during the study period (Table 3). Sex, years since graduation from medical school, years in practice at the study institution, and use of computers in the outpatient setting were not meaningfully different between the 3 categories of users (Table 3). On the other hand, medical specialty was strongly associated with use of the system, with anesthesiologists, pediatricians, and surgeons the specialties with the largest proportion of high users. Furthermore, physicians who were trained in a CPOE environment and those who reported daily use of computers for personal activities showed the highest levels of adoption. Physicians at Franklin Medical Center showed lower levels of order entry than their counterparts at Baystate.
| Low (20%) n (row %) | Intermediate (20%‐79%) n (row %) | High (80%) n (row %) | P value | |
|---|---|---|---|---|
| ||||
| n = 109 | n = 88 | n = 141 | ||
| Hospital | < .01c | |||
| Baystate | 73 (25) | 79 (27) | 138 (48) | |
| Franklin | 36 (75) | 9 (19) | 3 (6) | |
| Sex | .69c | |||
| Female | 28 (29) | 24 (25) | 43 (45) | |
| Male | 81 (33) | 64 (26) | 98 (40) | |
| Specialty | .0001c | |||
| Anesthesia | 8 (35) | 3 (13) | 12 (52) | |
| Internal medicine | 45 (33) | 37 (27) | 53 (39) | |
| Medicine/pediatrics | 6 (46) | 5 (38) | 2 (15) | |
| OB/GYN | 20 (56) | 12 (33) | 4 (11) | |
| Pediatrics | 13 (24) | 9 (17) | 32 (59) | |
| Surgery | 14 (23) | 21 (34) | 26 (43) | |
| Other | 3 (19) | 1 (6) | 12 (75) | |
| Do you use a computer in your outpatient practice?a | ||||
| Yes | 75 (31) | 61 (25) | 105 (44) | .22c |
| No | 20 (36) | 18 (33) | 17 (31) | |
| Level of personal computer useb | .045d | |||
| Rarely | 11 (44) | 8 (32) | 6 (24) | |
| A few times a month | 7 (33) | 4 (19) | 10 (48) | |
| Several times a week | 28 (35) | 25 (31) | 28 (35) | |
| At least once a day | 62 (30) | 50 (24) | 97 (46) | |
| Training at an institution that had CPOE | .037c | |||
| Yes | 30 (26) | 40 (34) | 46 (40) | |
| No | 76 (35) | 48 (22) | 94 (43) | |
| Median (IQR) | Median (IQR) | Median (IQR) | ||
| Years since graduation from medical school | 21 (16, 28) | 18 (14, 25) | 19 (12, 25) | .06e |
| Years in practice at study institution | 12 (5, 19) | 12 (6, 19) | 12 (6, 17) | .84e |
| Total number of orders placed | 112 (45, 306) | 105 (56, 254) | 113 (44, 382) | .92e |
Use of the system was highly associated with physician attitudes toward CPOE, with the views of intermediate and high users consistently different than those of low users (Fig. 2). The associations found held true regardless of hospital: low, intermediate, and high users from Franklin had similar responses to those from Baystate (P > .05 for all questions), and the data from the 2 hospitals therefore were combined for presentation. Although few physicians believed that the user interface of the system supported their work flow, high and intermediate users were 3 times as likely to share this view than were low users (Q7; Fig. 2). Similarly, 19% of low users, 31% of intermediate users, and 45% of high users believed that entering orders into the system was faster than writing orders (Q1). High and intermediate users of the system were more likely than low users to believe that orders entered into the system were carried out more rapidly (Q2) and led to fewer medication (Q3) and nonmedication (Q4) errors. Regardless of their utilization pattern, most physicians believed that order sets played an important role in promoting efficiency and quality.
DISCUSSION
In this study of the clinical computing practices of physicians at 2 community hospitals, we observed wide variation in the adoption of CPOE by individual attendings. Although roughly one‐third rarely placed orders directly into the system, 42% had an order entry rate of at least 80%. Contrary to our initial expectation, we found little association between a physician's order entry rate with years in practice, duration of exposure to CPOE, or use of computers in the outpatient setting. On the other hand, we observed marked differences in use of the CPOE system across specialty lines and found that physicians who were exposed to CPOE during training and those who were regular users of computers for personal activities were more likely to embrace this technology. Further, we observed important differences between physicians who used the system to place some or most of their orders and those who did so only rarely in their beliefs and attitudes about the impact and benefits of CPOE. Physicians with higher order entry rates were more likely than their colleagues to believe that placing orders electronically was faster than handwriting and that use of the system led to fewer medical errors. These findings should be encouraging to hospitals hoping to implement CPOE because they suggest that successful adoption of CPOE is not limited to physicians who have just completed their residencies or to hospitals with the capability of designing and building their own systems. On the contrary, we documented that women, older physicians, and those with limited CPOE experience were as likely to be frequent users, especially if they perceived CPOE to be safer than handwriting and if they believed the user interface supported the efficient entering of orders.
On the basis of these results we recommend that in addition to purchasing systems that meet physician work‐flow needs and support the efficient entry of orders, hospital leaders should emphasize the quality and safety benefits of CPOE as part of a comprehensive change management strategy. The differences we observed in order entry rates across specialties may have resulted from several factors, including inherent differences in personality type associated with choice of specialty and in the level of customization of a system reflected in which and how many order sets are included. Such findings suggest that when it comes to CPOE, one size does not fit all, and implementation planning should be carried out at the specialty level. Finally, our observation that physicians who had exposure to CPOE during training were more likely to use the system to place orders suggests that the nation's training institutions will play an important role in fostering universal adoption of this technology.
Several earlier studies have reported on physician experiences with CPOE systems. Murff and Kannry12 surveyed 94 internal medicine house staff to compare experiences with 2 CPOE systems: the Department of Veterans Affairs Computerized Patient Record System (CPRS) and a commercially available product. They found striking differences in user satisfaction with numerous aspects of the systems, however they did not address attitudes toward safety or quality, and because house staff were required to place orders electronically they were unable to correlate responses with actual usage patterns. Weiner et al.13 compared the opinions of internal medicine house staff, attendings, fellows, and nurses about the benefits and challenges of using a computerized provider order entry system. In contrast to the findings from our study, Weiner et al. reported that more than half of physicians believed that provider order entry led to a greater number of errors, and only a minority believed the system increased quality of care overall. Finally, Lee et al.14 surveyed medical and surgical house officers and nurses at a large academic medical center about their satisfaction with a locally developed order entry system. They found that attitudes about the impact of the system on productivity and ease of use were more strongly associated with overall satisfaction than having undergone training or experience with personal computers. These findings are congruous with our own observation that beliefs about the speed with which orders are placed are closely associated with actual use of the system. They reported, as have we, that physicians placed a high value on order sets.
Our study had a number of strengths. First, we were able to offer insight into the attitudes and behaviors of a previously neglected, but critically important groupattending physicians who care for patients at community hospitals without the assistance of house staff. Second, whereas previous studies primarily assessed physician satisfaction with CPOE, we explored how physician attitudes about the impact of CPOE on work flow and on safety were associated with actual ordering habits. Information about ordering was obtained directly from the order entry system and not through self‐report. We conducted the study at 2 hospitals, a large urban community teaching hospital and a smaller rural hospital, and focused on a CPOE system that is in use at many institutions throughout the country, thereby increasing the generalizability of our findings. Although adoption of the system by physicians at the 2 hospitals differed, factors that associated with the use of CPOE to place orders were similar. Finally, we surveyed a large number of physicians, had a high response rate, and found only small differences in the utilization patterns of responders and nonresponders, suggesting that our portrayal of the attitudes of physicians was representative of the views of physicians practicing in our community.
The study had a number of weaknesses. First, we cannot be sure whether preexisting beliefs about the benefits of CPOE directly influenced physicians' use of the system or, conversely, if these attitudes developed in response to experience as users. Nevertheless, it seems practical to suggest that hospitals focus on purchasing systems that support the efficient entering of orders while simultaneously adopting a communication and change management strategy that emphasizes the safety and quality benefits of CPOE more broadly. Second, we did not attempt to validate the opinions expressed by physicians about the usability or safety benefits of the system. That said, the purpose of the study was to determine whether physician attitudes toward these issues was associated with the use of the system to place orders. Whether or not this particular CPOE system actually prevented medication errors, most physicians believed it did, a belief strongly associated with the observed order entry rates. Third, we studied a single CPOE system implemented approximately 10 years ago that does not reflect state‐of‐the‐art user interface design or functionality. Nevertheless, our observation about the importance of the user experience is probably no less relevant today. Fourth, we were unable to ascertain every order given by physicians, as some so‐called MD to RN orders may never have made it into the system. Finally, there is a small risk that some written, telephone, and verbal orders may have been randomly or systematically assigned to incorrect physicians, which would have led us to calculate inaccurate utilization rates.
CONCLUSIONS
In a voluntary community hospital environment the adoption of CPOE by attending physicians varies widely. While placing a premium on the purchase of systems that meet the work‐flow needs of physicians and support the efficient entry of orders, hospital leaders can enhance physician adoption of this technology by communicating the role of CPOE in improving quality and safety.
Acknowledgements
The authors thank Gilad Kuperman, MD, PhD, for his thoughtful comments on an earlier version of the manuscript.
- Kohn LT,Corrigan JM,Donaldson MS, eds.To Err Is Human: Building a Safer Health System.Washington, DC:National Academy Press,2000.
- ,.Computer physician order entry: benefits, costs, and issues.Ann Intern Med.2003;139:31–39.
- ,,, et al.Effect of computerized physician order entry and a team intervention on prevention of serious medication errors.JAMA.1998;280:1311–1316.
- ,,,,,.A computerized reminder system to increase the use of preventive care for hospitalized patients.N Engl J Med.2001;345:965–970.
- ,,,.A randomized trial of “corollary orders” to prevent errors of omission.J Am Med Inform Assoc.1997;4:364–375.
- ,,, et al.A computer‐assisted management program for antibiotics and other antiinfective agents.N Engl J Med.1998;338:232–238.
- The Leapfrog Group. Patient Safety Fact Sheet. Available at: http://www.leapfroggroup.org/FactSheets/LF_FactSheet.pdf. Accessed October 6,2004.
- ,,,.Computerized physician order entry in U.S. hospitals: results of a 2002 survey.J Am Med Inform Assoc.2004;11:95–99.
- ,,.U.S. adoption of computerized physician order entry systems.Health Aff.2005;24:1654–1663.
- ,,.Effects of computerized physician order entry and clinical decision support systems on medication safety: a systematic review.Arch Intern Med.2003;163:1409–1416.
- HCUPnet, Healthcare Cost and Utilization Project. Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.ahrq.gov/data/hcup/hcupnet.htm. Accessed October 6,2004.
- ,.Physician satisfaction with two order entry systems.J Am Med Inform Assoc.2001;8:499–509.
- ,,, et al.Contrasting views of physicians and nurses about an inpatient computer‐based provider order‐entry system.J Am Med Inform Assoc.1999;6:234–244.
- ,,,.Implementation of physician order entry: user satisfaction and self‐reported usage patterns.J Am Med Inform Assoc.1996;3:42–55.
It is widely acknowledged that the U.S. health care system is plagued by error and inefficiency and that these factors contribute to as many as 44,000‐98,000 deaths each year in U.S. hospitals. In To Err Is Human: Building a Safer Health System, the Institute of Medicine1 outlined the critical role that information technology can play in improving patient safety and highlighted computerized physician order entry (CPOE) systems for their potential to reduce the frequency of medication errors and to improve the quality of medical care.
Computerized physician order entry systems are specialized software applications that allow physicians to place orders directly into a computer. This process has a number of potential advantages over traditional handwritten ordering, including the ability to structure the ordering process to ensure the completeness of individual orders, to provide clinical decision support through diagnosis‐based order sets, and to automatically check orders for potential drugallergy, drugdrug, and drugfood interactions.2 Finally, entering orders directly into a computer eliminates the problem of transcription‐related errors that stem from the difficulty of interpreting handwriting. In clinical trials, the introduction of CPOE has been shown to reduce the frequency of medication errors, to improve the use of preventive services, and to reduce costs.36 Recognition of the benefits of these systems has not been confined to the medical community. The Leapfrog Organization, a coalition of large businesses in the United States, has chosen CPOE as one of its 3 initial safety leaps and has established a threshold that 70% of medication orders should be entered directly by physicians.7
Although the benefits of CPOE systems are widely recognized, few hospitals have implemented these systems successfully.8, 9 Those that have, have often developed the applications internally, and many have relied on house staff to do most or all of the actual ordering.10 However, most hospitals do not have the expertise for internal development and instead rely on commercially available products. Moreover, most patients hospitalized in the United States are cared for by attending physicians working without the assistance of house staff.11 In light of the importance of successfully implementing CPOE systems in such settings, we assessed the adoption of CPOE by attending physicians at 2 community hospitals where its use was voluntary and examined the characteristics and attitudes associated with use of the system to place orders.
METHODS
Setting and Participants
Baystate Medical Center is a 600‐bed teaching hospital in Springfield, Massachusetts, where approximately 50% of patients are cared for with the assistance of house staff. Franklin Medical Center is a 125‐bed community hospital in rural Greenfield, Massachusetts, and is not a house staff training site. Medical staff membership at the 2 hospitals is largely voluntary. Both institutions share a vendor‐supplied computerized order entry system that was implemented in the early 1990s (E7000, Eclipsys Corporation, Boca Raton, FL). The system provides a structured format for the creation of medication, laboratory, and radiology orders and contains thousands of preconstructed medication order sentences and hundreds of order sets designed to standardize ordering for common diagnoses and procedures. Pharmacists are alerted of potential drugallergy and drugdrug interactions and use clinical judgment about whether to communicate this information to the physician. Although the house staff at Baystate Medical Center is mandated to place orders in the system, attending physicians have no such requirement at either institution. Access to the system is provided though the many fixed workstations located on nursing units, in operating rooms, and in the health sciences library. On a typical medical‐surgical patient care unit most computers are behind the nurses' station, though some are distributed along hallways and in physician charting rooms. No computers are in patient rooms. Although the number varies slightly across units, the average ratio of computers to patient beds is roughly 1 to 1.
Survey
In June 2003 we mailed a 20‐item survey to attending physicians who had been responsible for a minimum of 25 orders during the preceding month at either Baystate or Franklin Medical Center. Orders counted toward this minimum if they had been written, given verbally in person or by phone, or entered directly into the computer by the physician. The survey consisted of 20 questions focused on the topic of computerized order entry. In addition to collecting information about sex and specialty, we asked respondents to describe their use of CPOE during training, their use of computers at home, and, where applicable, their use of computers in their outpatient practices. The survey included questions about how often respondents used the order entry system when caring for hospitalized patients and which features of the system they used. To assess physician attitudes about the order entry process, we asked respondents to consider whether it was faster to place orders directly into the system than it was by handwriting them, whether orders placed in the system were carried out more rapidly, whether placing orders in the system led to fewer medication and other errors, whether order sets were important for the efficient use of the system, whether order sets helped to ensure that important aspects of care did not slip through the cracks, whether the system's user interface supported their work flow, and whether the encouragement of nurses was an important factor in their use of the system. Questions that assessed physician attitudes were presented on a 5‐point Likert scale. Nonrespondents were sent reminder letters along with duplicate surveys twice, approximately 1 and 2 months after the initial mailing. No financial incentive was offered for participation. The study protocol was approved by the Institutional Review Board of Baystate Health System.
Order Entry Rates
Regardless of whether an order is placed directly by a physician into a computer, given verbally, or handwritten, all orders are ultimately entered into the CPOE system. Working with our hospitals' Departments of Information Services, we developed a report that provided physician‐specific information about order entry patterns. For each physician in the study, we determined the total number of orders generated during the month preceding the initial June mailing, as well as the absolute number and percentage of orders of each of the following categories: directly entered, telephone, verbal, and written. Because verbal and telephone orders are required during urgent situations and when physicians give orders from outside the hospital, we calculated and report an adjusted order entry rate as the total number of orders placed directly into the system divided by the sum of the orders entered directly and the number of written orders.
Analysis
Summary statistics for the overall sample were constructed using simple frequencies and proportions for categorical variables and medians and interquartile ranges for continuous variables. We compared characteristics of respondents from the 2 hospitals using chi‐square tests of association for categorical factors and Wilcoxon rank‐sum tests for continuous scale data. We compared the total number of orders placed during the study month and the order entry rates of responders and nonresponders using the Wilcoxon rank‐sum test. We categorized physicians as low (20%), intermediate (21%‐79%), and high (80%) users of the system based on their calculated order entry rate. Responses to each of the attitude questions in the survey were tabulated, and the responses strongly agree and agree were combined for analyses comparing responses. Demographic variables and physician attitudes were tested for associations with order entry rate categories via the Pearson chi‐square for categorical factors, the Mantel‐Haenszel chi‐square for ordered factors, and Kruskal‐Wallis analysis of variance for continuous variables. Initial analyses were stratified by hospital; where no differences in association were found across strata, the data were combined. Statistical tests were performed using SAS version 9.1 (SAS Institute, Cary, NC)
RESULTS
During the study period the target group of physicians placed a total of 135,692 orders, of which 69,654 (51%) were placed directly into the CPOE system, 38,878 (29%) were made using pen and paper, 7,208 (5%) were made verbally, and 19,952 (15%) were placed by telephone. Three hundred and fifty‐six (71%) of the 502 surveys sent out to physicians at the 2 hospitals were returned. Thirteen surveys were excluded from analysis because the respondent was not a physician, and 2 because we were unable to match the survey to system usage data, leaving a total of 341 surveys for analysis. Order entry rates were not computed for an additional 3 physicians who only placed verbal and telephone orders during the study period. Response rates did not differ by clinician specialty (P = .53); compared to those of nonresponders, respondents had a similar median total number of orders (111 vs. 101, P = .67) and a higher median order entry rate (66% vs. 48%, P = .03).
Characteristics of Respondents
Seventy‐two percent of physicians who completed the survey were men; half had graduated from medical school at least 20 years ago, and the median duration of practice at the study institution was 11 years (Table 1). Forty percent practiced internal medicine, 18% were surgeons, and 16% were pediatricians. Thirty‐five percent completed training at an institution that had computerized physician order entry, and 86% cared for patients primarily at Baystate Medical Center. More than half reported they used the system many times each day for patient care, and the features they used most commonly were retrieval of results (95%), placing of orders (78%), and viewing and printing of patient lists (75%). Among those with outpatient practices, 81% used computers in their outpatient practice, and more than half used computers for personal activities at home at least once a day. On average, respondents from Franklin Medical Center had graduated from medical school farther in the past and reported less reliance on the system to carry out all activities other than viewing results.
| Overall n (%) | Baystate n (%) 293 (85.9) | Franklin n (%) 48 (14.1) | Chi square P value | |
|---|---|---|---|---|
| ||||
| Sex | .64 | |||
| Male | 244 (71.6) | 211 (72.0) | 33 (68.8) | |
| Specialty | .24 | |||
| Anesthesia | 23 (6.7) | 23 (7.9) | 0 (0.0) | |
| Internal medicine | 135 (39.6) | 112 (38.2) | 23 (47.9) | |
| Medicine/pediatrics | 13 (3.8) | 6 (2.0) | 7 (14.6) | |
| OB/GYN | 36 (10.6) | 30 (10.2) | 6 (12.5) | |
| Pediatrics | 54 (15.8) | 51 (17.4) | 3 (6.3) | |
| Surgery | 61 (17.9) | 55 (18.8) | 6 (12.5) | |
| Other | 19 (5.6) | 16 (5.5) | 3 (6.3) | |
| Use of CPOE systema | .09 | |||
| Many times a day | 176 (52.2) | 160 (55.0) | 16 (34.8) | |
| At least once a day | 77 (22.9) | 61 (21.0) | 16 (34.8) | |
| A few times a week | 55 (16.3) | 45 (15.5) | 10 (21.7) | |
| Once a week or less | 29 (8.6) | 25 (8.6) | 4 (8.7) | |
| Features useda | ||||
| Viewing and printing patient lists | 254 (75.2) | 212 (72.6) | 42 (91.3) | .01 |
| Looking up results | 320 (94.7) | 277 (94.9) | 43 (93.5) | .70 |
| Viewing current medications | 218 (64.5) | 204 (69.9) | 14 (30.4) | < .01 |
| Placing orders | 263 (77.8) | 244 (83.6) | 19 (41.3) | < .01 |
| Entering discharge summaries | 72 (21.3) | 70 (24.0) | 2 (4.4) | < .01 |
| Use of order setsa | ||||
| Rarely or never | 98 (29.0) | 74 (25.3) | 24 (52.2) | < .01 |
| Minority of patients | 92 (27.2) | 78 (26.7) | 14 (30.4) | |
| Majority of patients | 104 (30.8) | 97 (33.2) | 7 (15.2) | |
| For all or nearly all patients | 44 (13.0) | 43 (14.7) | 1 (2.2) | |
| Percentage of orders placed using order setsa | < .01 | |||
| None | 46 (13.7) | 26 (9.0) | 20 (44.4) | |
| 1%‐25% | 62 (18.5) | 50 (17.2) | 12 (26.7) | |
| 26%‐50% | 29 (8.7) | 23 (7.9) | 6 (13.3) | |
| 51%‐75% | 45 (13.4) | 43 (14.9) | 2 (4.4) | |
| 76%‐99% | 103 (30.8) | 98 (33.8) | 5 (11.1) | |
| All | 50 (14.9) | 50 (17.2) | 0 (0.0) | |
| Use of computer in outpatient practiceab | 243 (81.3) | 206 (80.8) | 37 (84.1) | .60 |
| Personal computer usea | .47 | |||
| At least once a day | 209 (61.7) | 185 (63.4) | 24 (51.1) | |
| Several times a week | 84 (24.8) | 67 (23.0) | 17 (36.2) | |
| A few times a month | 21 (6.2) | 18 (6.2) | 3 (6.4) | |
| Rarely | 25 (7.4) | 22 (7.5) | 3 (6.4) | |
| Training at an institution that had CPOE | 117 (34.7) | 105 (36.1) | 12 (26.1) | 0.19 |
| Use of system to enter orders should be mandatorya | ||||
| Yes | 113 (35.2) | 106 (38.4) | 7 (15.6) | <.01 |
| Median (IQR) | Median (IQR) | Median (IQR) | WilcoxonPvalue | |
| Years since medical school graduationa | 20 (13, 26) | 20 (13, 26) | 24 (17, 28) | .02 |
| Years in practice at study institutiona | 11 (5, 18) | 11 (5, 18) | 13 (7, 19) | .39 |
| Orders directly enteredc | 23 (2, 99) | 27 (5, 108) | 1 (0, 27) | < .01 |
| Orders placed by telephonec | 14 (5, 49) | 12 (3, 38) | 49.5 (16, 123.5) | < .01 |
| Orders placed verballyc | 2 (0, 11) | 3 (0, 13) | 1 (0,3) | < .01 |
| Orders placed in writingc | 21 (4, 73) | 14 (3, 45) | 220 (106.5, 391) | < .01 |
| CPOE ratebc | 66% (3%, 94%) | 76% (19%, 96%) | 0.25% (0%, 17%) | < .01 |
Attitudes Toward Computerized Physician Order Entry
Physicians who completed the survey offered diverse opinions about the impact of computerized order entry on work flow, patient safety, and quality of care. Only 22% believed the system's user interface supported their work flow (Q7), 34% believed it was faster to enter orders directly into the system than to handwrite them (Q1), and 41% believed orders placed into the system were carried out more rapidly (Q2) (Table 2). On the other hand, 63% of respondents believed that placing orders directly into the system led to fewer medication errors (Q3), and 51% stated the system generally reduced medical errors (Q4). Sixty‐nine percent stated order sets were important for efficient use of the system (Q5), and 71% believed order sets served an important decision support role (Q6). Twenty‐six percent stated that the encouragement of nurses was an important factor in their use of the system (Q8). Finally, 35% of attending physicians believed use of the system to place orders should be mandatory.
Characteristics and Attitudes of High, Intermediate, and Low Users
The median order entry rate of respondents was 66%. One hundred and forty‐one (42%) placed at least 80% of their orders directly into the system, whereas 109 (32%) placed no more than 20% of their orders directly in the system (Fig. 1). There was not a significant difference between the low, intermediate, and high use groups in the total number of orders that each physician placed during the study period (Table 3). Sex, years since graduation from medical school, years in practice at the study institution, and use of computers in the outpatient setting were not meaningfully different between the 3 categories of users (Table 3). On the other hand, medical specialty was strongly associated with use of the system, with anesthesiologists, pediatricians, and surgeons the specialties with the largest proportion of high users. Furthermore, physicians who were trained in a CPOE environment and those who reported daily use of computers for personal activities showed the highest levels of adoption. Physicians at Franklin Medical Center showed lower levels of order entry than their counterparts at Baystate.

| Low (20%) n (row %) | Intermediate (20%‐79%) n (row %) | High (80%) n (row %) | P value | |
|---|---|---|---|---|
| ||||
| n = 109 | n = 88 | n = 141 | ||
| Hospital | < .01c | |||
| Baystate | 73 (25) | 79 (27) | 138 (48) | |
| Franklin | 36 (75) | 9 (19) | 3 (6) | |
| Sex | .69c | |||
| Female | 28 (29) | 24 (25) | 43 (45) | |
| Male | 81 (33) | 64 (26) | 98 (40) | |
| Specialty | .0001c | |||
| Anesthesia | 8 (35) | 3 (13) | 12 (52) | |
| Internal medicine | 45 (33) | 37 (27) | 53 (39) | |
| Medicine/pediatrics | 6 (46) | 5 (38) | 2 (15) | |
| OB/GYN | 20 (56) | 12 (33) | 4 (11) | |
| Pediatrics | 13 (24) | 9 (17) | 32 (59) | |
| Surgery | 14 (23) | 21 (34) | 26 (43) | |
| Other | 3 (19) | 1 (6) | 12 (75) | |
| Do you use a computer in your outpatient practice?a | ||||
| Yes | 75 (31) | 61 (25) | 105 (44) | .22c |
| No | 20 (36) | 18 (33) | 17 (31) | |
| Level of personal computer useb | .045d | |||
| Rarely | 11 (44) | 8 (32) | 6 (24) | |
| A few times a month | 7 (33) | 4 (19) | 10 (48) | |
| Several times a week | 28 (35) | 25 (31) | 28 (35) | |
| At least once a day | 62 (30) | 50 (24) | 97 (46) | |
| Training at an institution that had CPOE | .037c | |||
| Yes | 30 (26) | 40 (34) | 46 (40) | |
| No | 76 (35) | 48 (22) | 94 (43) | |
| Median (IQR) | Median (IQR) | Median (IQR) | ||
| Years since graduation from medical school | 21 (16, 28) | 18 (14, 25) | 19 (12, 25) | .06e |
| Years in practice at study institution | 12 (5, 19) | 12 (6, 19) | 12 (6, 17) | .84e |
| Total number of orders placed | 112 (45, 306) | 105 (56, 254) | 113 (44, 382) | .92e |
Use of the system was highly associated with physician attitudes toward CPOE, with the views of intermediate and high users consistently different than those of low users (Fig. 2). The associations found held true regardless of hospital: low, intermediate, and high users from Franklin had similar responses to those from Baystate (P > .05 for all questions), and the data from the 2 hospitals therefore were combined for presentation. Although few physicians believed that the user interface of the system supported their work flow, high and intermediate users were 3 times as likely to share this view than were low users (Q7; Fig. 2). Similarly, 19% of low users, 31% of intermediate users, and 45% of high users believed that entering orders into the system was faster than writing orders (Q1). High and intermediate users of the system were more likely than low users to believe that orders entered into the system were carried out more rapidly (Q2) and led to fewer medication (Q3) and nonmedication (Q4) errors. Regardless of their utilization pattern, most physicians believed that order sets played an important role in promoting efficiency and quality.

DISCUSSION
In this study of the clinical computing practices of physicians at 2 community hospitals, we observed wide variation in the adoption of CPOE by individual attendings. Although roughly one‐third rarely placed orders directly into the system, 42% had an order entry rate of at least 80%. Contrary to our initial expectation, we found little association between a physician's order entry rate with years in practice, duration of exposure to CPOE, or use of computers in the outpatient setting. On the other hand, we observed marked differences in use of the CPOE system across specialty lines and found that physicians who were exposed to CPOE during training and those who were regular users of computers for personal activities were more likely to embrace this technology. Further, we observed important differences between physicians who used the system to place some or most of their orders and those who did so only rarely in their beliefs and attitudes about the impact and benefits of CPOE. Physicians with higher order entry rates were more likely than their colleagues to believe that placing orders electronically was faster than handwriting and that use of the system led to fewer medical errors. These findings should be encouraging to hospitals hoping to implement CPOE because they suggest that successful adoption of CPOE is not limited to physicians who have just completed their residencies or to hospitals with the capability of designing and building their own systems. On the contrary, we documented that women, older physicians, and those with limited CPOE experience were as likely to be frequent users, especially if they perceived CPOE to be safer than handwriting and if they believed the user interface supported the efficient entering of orders.
On the basis of these results we recommend that in addition to purchasing systems that meet physician work‐flow needs and support the efficient entry of orders, hospital leaders should emphasize the quality and safety benefits of CPOE as part of a comprehensive change management strategy. The differences we observed in order entry rates across specialties may have resulted from several factors, including inherent differences in personality type associated with choice of specialty and in the level of customization of a system reflected in which and how many order sets are included. Such findings suggest that when it comes to CPOE, one size does not fit all, and implementation planning should be carried out at the specialty level. Finally, our observation that physicians who had exposure to CPOE during training were more likely to use the system to place orders suggests that the nation's training institutions will play an important role in fostering universal adoption of this technology.
Several earlier studies have reported on physician experiences with CPOE systems. Murff and Kannry12 surveyed 94 internal medicine house staff to compare experiences with 2 CPOE systems: the Department of Veterans Affairs Computerized Patient Record System (CPRS) and a commercially available product. They found striking differences in user satisfaction with numerous aspects of the systems, however they did not address attitudes toward safety or quality, and because house staff were required to place orders electronically they were unable to correlate responses with actual usage patterns. Weiner et al.13 compared the opinions of internal medicine house staff, attendings, fellows, and nurses about the benefits and challenges of using a computerized provider order entry system. In contrast to the findings from our study, Weiner et al. reported that more than half of physicians believed that provider order entry led to a greater number of errors, and only a minority believed the system increased quality of care overall. Finally, Lee et al.14 surveyed medical and surgical house officers and nurses at a large academic medical center about their satisfaction with a locally developed order entry system. They found that attitudes about the impact of the system on productivity and ease of use were more strongly associated with overall satisfaction than having undergone training or experience with personal computers. These findings are congruous with our own observation that beliefs about the speed with which orders are placed are closely associated with actual use of the system. They reported, as have we, that physicians placed a high value on order sets.
Our study had a number of strengths. First, we were able to offer insight into the attitudes and behaviors of a previously neglected, but critically important groupattending physicians who care for patients at community hospitals without the assistance of house staff. Second, whereas previous studies primarily assessed physician satisfaction with CPOE, we explored how physician attitudes about the impact of CPOE on work flow and on safety were associated with actual ordering habits. Information about ordering was obtained directly from the order entry system and not through self‐report. We conducted the study at 2 hospitals, a large urban community teaching hospital and a smaller rural hospital, and focused on a CPOE system that is in use at many institutions throughout the country, thereby increasing the generalizability of our findings. Although adoption of the system by physicians at the 2 hospitals differed, factors that associated with the use of CPOE to place orders were similar. Finally, we surveyed a large number of physicians, had a high response rate, and found only small differences in the utilization patterns of responders and nonresponders, suggesting that our portrayal of the attitudes of physicians was representative of the views of physicians practicing in our community.
The study had a number of weaknesses. First, we cannot be sure whether preexisting beliefs about the benefits of CPOE directly influenced physicians' use of the system or, conversely, if these attitudes developed in response to experience as users. Nevertheless, it seems practical to suggest that hospitals focus on purchasing systems that support the efficient entering of orders while simultaneously adopting a communication and change management strategy that emphasizes the safety and quality benefits of CPOE more broadly. Second, we did not attempt to validate the opinions expressed by physicians about the usability or safety benefits of the system. That said, the purpose of the study was to determine whether physician attitudes toward these issues was associated with the use of the system to place orders. Whether or not this particular CPOE system actually prevented medication errors, most physicians believed it did, a belief strongly associated with the observed order entry rates. Third, we studied a single CPOE system implemented approximately 10 years ago that does not reflect state‐of‐the‐art user interface design or functionality. Nevertheless, our observation about the importance of the user experience is probably no less relevant today. Fourth, we were unable to ascertain every order given by physicians, as some so‐called MD to RN orders may never have made it into the system. Finally, there is a small risk that some written, telephone, and verbal orders may have been randomly or systematically assigned to incorrect physicians, which would have led us to calculate inaccurate utilization rates.
CONCLUSIONS
In a voluntary community hospital environment the adoption of CPOE by attending physicians varies widely. While placing a premium on the purchase of systems that meet the work‐flow needs of physicians and support the efficient entry of orders, hospital leaders can enhance physician adoption of this technology by communicating the role of CPOE in improving quality and safety.
Acknowledgements
The authors thank Gilad Kuperman, MD, PhD, for his thoughtful comments on an earlier version of the manuscript.
It is widely acknowledged that the U.S. health care system is plagued by error and inefficiency and that these factors contribute to as many as 44,000‐98,000 deaths each year in U.S. hospitals. In To Err Is Human: Building a Safer Health System, the Institute of Medicine1 outlined the critical role that information technology can play in improving patient safety and highlighted computerized physician order entry (CPOE) systems for their potential to reduce the frequency of medication errors and to improve the quality of medical care.
Computerized physician order entry systems are specialized software applications that allow physicians to place orders directly into a computer. This process has a number of potential advantages over traditional handwritten ordering, including the ability to structure the ordering process to ensure the completeness of individual orders, to provide clinical decision support through diagnosis‐based order sets, and to automatically check orders for potential drugallergy, drugdrug, and drugfood interactions.2 Finally, entering orders directly into a computer eliminates the problem of transcription‐related errors that stem from the difficulty of interpreting handwriting. In clinical trials, the introduction of CPOE has been shown to reduce the frequency of medication errors, to improve the use of preventive services, and to reduce costs.36 Recognition of the benefits of these systems has not been confined to the medical community. The Leapfrog Organization, a coalition of large businesses in the United States, has chosen CPOE as one of its 3 initial safety leaps and has established a threshold that 70% of medication orders should be entered directly by physicians.7
Although the benefits of CPOE systems are widely recognized, few hospitals have implemented these systems successfully.8, 9 Those that have, have often developed the applications internally, and many have relied on house staff to do most or all of the actual ordering.10 However, most hospitals do not have the expertise for internal development and instead rely on commercially available products. Moreover, most patients hospitalized in the United States are cared for by attending physicians working without the assistance of house staff.11 In light of the importance of successfully implementing CPOE systems in such settings, we assessed the adoption of CPOE by attending physicians at 2 community hospitals where its use was voluntary and examined the characteristics and attitudes associated with use of the system to place orders.
METHODS
Setting and Participants
Baystate Medical Center is a 600‐bed teaching hospital in Springfield, Massachusetts, where approximately 50% of patients are cared for with the assistance of house staff. Franklin Medical Center is a 125‐bed community hospital in rural Greenfield, Massachusetts, and is not a house staff training site. Medical staff membership at the 2 hospitals is largely voluntary. Both institutions share a vendor‐supplied computerized order entry system that was implemented in the early 1990s (E7000, Eclipsys Corporation, Boca Raton, FL). The system provides a structured format for the creation of medication, laboratory, and radiology orders and contains thousands of preconstructed medication order sentences and hundreds of order sets designed to standardize ordering for common diagnoses and procedures. Pharmacists are alerted of potential drugallergy and drugdrug interactions and use clinical judgment about whether to communicate this information to the physician. Although the house staff at Baystate Medical Center is mandated to place orders in the system, attending physicians have no such requirement at either institution. Access to the system is provided though the many fixed workstations located on nursing units, in operating rooms, and in the health sciences library. On a typical medical‐surgical patient care unit most computers are behind the nurses' station, though some are distributed along hallways and in physician charting rooms. No computers are in patient rooms. Although the number varies slightly across units, the average ratio of computers to patient beds is roughly 1 to 1.
Survey
In June 2003 we mailed a 20‐item survey to attending physicians who had been responsible for a minimum of 25 orders during the preceding month at either Baystate or Franklin Medical Center. Orders counted toward this minimum if they had been written, given verbally in person or by phone, or entered directly into the computer by the physician. The survey consisted of 20 questions focused on the topic of computerized order entry. In addition to collecting information about sex and specialty, we asked respondents to describe their use of CPOE during training, their use of computers at home, and, where applicable, their use of computers in their outpatient practices. The survey included questions about how often respondents used the order entry system when caring for hospitalized patients and which features of the system they used. To assess physician attitudes about the order entry process, we asked respondents to consider whether it was faster to place orders directly into the system than it was by handwriting them, whether orders placed in the system were carried out more rapidly, whether placing orders in the system led to fewer medication and other errors, whether order sets were important for the efficient use of the system, whether order sets helped to ensure that important aspects of care did not slip through the cracks, whether the system's user interface supported their work flow, and whether the encouragement of nurses was an important factor in their use of the system. Questions that assessed physician attitudes were presented on a 5‐point Likert scale. Nonrespondents were sent reminder letters along with duplicate surveys twice, approximately 1 and 2 months after the initial mailing. No financial incentive was offered for participation. The study protocol was approved by the Institutional Review Board of Baystate Health System.
Order Entry Rates
Regardless of whether an order is placed directly by a physician into a computer, given verbally, or handwritten, all orders are ultimately entered into the CPOE system. Working with our hospitals' Departments of Information Services, we developed a report that provided physician‐specific information about order entry patterns. For each physician in the study, we determined the total number of orders generated during the month preceding the initial June mailing, as well as the absolute number and percentage of orders of each of the following categories: directly entered, telephone, verbal, and written. Because verbal and telephone orders are required during urgent situations and when physicians give orders from outside the hospital, we calculated and report an adjusted order entry rate as the total number of orders placed directly into the system divided by the sum of the orders entered directly and the number of written orders.
Analysis
Summary statistics for the overall sample were constructed using simple frequencies and proportions for categorical variables and medians and interquartile ranges for continuous variables. We compared characteristics of respondents from the 2 hospitals using chi‐square tests of association for categorical factors and Wilcoxon rank‐sum tests for continuous scale data. We compared the total number of orders placed during the study month and the order entry rates of responders and nonresponders using the Wilcoxon rank‐sum test. We categorized physicians as low (20%), intermediate (21%‐79%), and high (80%) users of the system based on their calculated order entry rate. Responses to each of the attitude questions in the survey were tabulated, and the responses strongly agree and agree were combined for analyses comparing responses. Demographic variables and physician attitudes were tested for associations with order entry rate categories via the Pearson chi‐square for categorical factors, the Mantel‐Haenszel chi‐square for ordered factors, and Kruskal‐Wallis analysis of variance for continuous variables. Initial analyses were stratified by hospital; where no differences in association were found across strata, the data were combined. Statistical tests were performed using SAS version 9.1 (SAS Institute, Cary, NC)
RESULTS
During the study period the target group of physicians placed a total of 135,692 orders, of which 69,654 (51%) were placed directly into the CPOE system, 38,878 (29%) were made using pen and paper, 7,208 (5%) were made verbally, and 19,952 (15%) were placed by telephone. Three hundred and fifty‐six (71%) of the 502 surveys sent out to physicians at the 2 hospitals were returned. Thirteen surveys were excluded from analysis because the respondent was not a physician, and 2 because we were unable to match the survey to system usage data, leaving a total of 341 surveys for analysis. Order entry rates were not computed for an additional 3 physicians who only placed verbal and telephone orders during the study period. Response rates did not differ by clinician specialty (P = .53); compared to those of nonresponders, respondents had a similar median total number of orders (111 vs. 101, P = .67) and a higher median order entry rate (66% vs. 48%, P = .03).
Characteristics of Respondents
Seventy‐two percent of physicians who completed the survey were men; half had graduated from medical school at least 20 years ago, and the median duration of practice at the study institution was 11 years (Table 1). Forty percent practiced internal medicine, 18% were surgeons, and 16% were pediatricians. Thirty‐five percent completed training at an institution that had computerized physician order entry, and 86% cared for patients primarily at Baystate Medical Center. More than half reported they used the system many times each day for patient care, and the features they used most commonly were retrieval of results (95%), placing of orders (78%), and viewing and printing of patient lists (75%). Among those with outpatient practices, 81% used computers in their outpatient practice, and more than half used computers for personal activities at home at least once a day. On average, respondents from Franklin Medical Center had graduated from medical school farther in the past and reported less reliance on the system to carry out all activities other than viewing results.
| Overall n (%) | Baystate n (%) 293 (85.9) | Franklin n (%) 48 (14.1) | Chi square P value | |
|---|---|---|---|---|
| ||||
| Sex | .64 | |||
| Male | 244 (71.6) | 211 (72.0) | 33 (68.8) | |
| Specialty | .24 | |||
| Anesthesia | 23 (6.7) | 23 (7.9) | 0 (0.0) | |
| Internal medicine | 135 (39.6) | 112 (38.2) | 23 (47.9) | |
| Medicine/pediatrics | 13 (3.8) | 6 (2.0) | 7 (14.6) | |
| OB/GYN | 36 (10.6) | 30 (10.2) | 6 (12.5) | |
| Pediatrics | 54 (15.8) | 51 (17.4) | 3 (6.3) | |
| Surgery | 61 (17.9) | 55 (18.8) | 6 (12.5) | |
| Other | 19 (5.6) | 16 (5.5) | 3 (6.3) | |
| Use of CPOE systema | .09 | |||
| Many times a day | 176 (52.2) | 160 (55.0) | 16 (34.8) | |
| At least once a day | 77 (22.9) | 61 (21.0) | 16 (34.8) | |
| A few times a week | 55 (16.3) | 45 (15.5) | 10 (21.7) | |
| Once a week or less | 29 (8.6) | 25 (8.6) | 4 (8.7) | |
| Features useda | ||||
| Viewing and printing patient lists | 254 (75.2) | 212 (72.6) | 42 (91.3) | .01 |
| Looking up results | 320 (94.7) | 277 (94.9) | 43 (93.5) | .70 |
| Viewing current medications | 218 (64.5) | 204 (69.9) | 14 (30.4) | < .01 |
| Placing orders | 263 (77.8) | 244 (83.6) | 19 (41.3) | < .01 |
| Entering discharge summaries | 72 (21.3) | 70 (24.0) | 2 (4.4) | < .01 |
| Use of order setsa | ||||
| Rarely or never | 98 (29.0) | 74 (25.3) | 24 (52.2) | < .01 |
| Minority of patients | 92 (27.2) | 78 (26.7) | 14 (30.4) | |
| Majority of patients | 104 (30.8) | 97 (33.2) | 7 (15.2) | |
| For all or nearly all patients | 44 (13.0) | 43 (14.7) | 1 (2.2) | |
| Percentage of orders placed using order setsa | < .01 | |||
| None | 46 (13.7) | 26 (9.0) | 20 (44.4) | |
| 1%‐25% | 62 (18.5) | 50 (17.2) | 12 (26.7) | |
| 26%‐50% | 29 (8.7) | 23 (7.9) | 6 (13.3) | |
| 51%‐75% | 45 (13.4) | 43 (14.9) | 2 (4.4) | |
| 76%‐99% | 103 (30.8) | 98 (33.8) | 5 (11.1) | |
| All | 50 (14.9) | 50 (17.2) | 0 (0.0) | |
| Use of computer in outpatient practiceab | 243 (81.3) | 206 (80.8) | 37 (84.1) | .60 |
| Personal computer usea | .47 | |||
| At least once a day | 209 (61.7) | 185 (63.4) | 24 (51.1) | |
| Several times a week | 84 (24.8) | 67 (23.0) | 17 (36.2) | |
| A few times a month | 21 (6.2) | 18 (6.2) | 3 (6.4) | |
| Rarely | 25 (7.4) | 22 (7.5) | 3 (6.4) | |
| Training at an institution that had CPOE | 117 (34.7) | 105 (36.1) | 12 (26.1) | 0.19 |
| Use of system to enter orders should be mandatorya | ||||
| Yes | 113 (35.2) | 106 (38.4) | 7 (15.6) | <.01 |
| Median (IQR) | Median (IQR) | Median (IQR) | WilcoxonPvalue | |
| Years since medical school graduationa | 20 (13, 26) | 20 (13, 26) | 24 (17, 28) | .02 |
| Years in practice at study institutiona | 11 (5, 18) | 11 (5, 18) | 13 (7, 19) | .39 |
| Orders directly enteredc | 23 (2, 99) | 27 (5, 108) | 1 (0, 27) | < .01 |
| Orders placed by telephonec | 14 (5, 49) | 12 (3, 38) | 49.5 (16, 123.5) | < .01 |
| Orders placed verballyc | 2 (0, 11) | 3 (0, 13) | 1 (0,3) | < .01 |
| Orders placed in writingc | 21 (4, 73) | 14 (3, 45) | 220 (106.5, 391) | < .01 |
| CPOE ratebc | 66% (3%, 94%) | 76% (19%, 96%) | 0.25% (0%, 17%) | < .01 |
Attitudes Toward Computerized Physician Order Entry
Physicians who completed the survey offered diverse opinions about the impact of computerized order entry on work flow, patient safety, and quality of care. Only 22% believed the system's user interface supported their work flow (Q7), 34% believed it was faster to enter orders directly into the system than to handwrite them (Q1), and 41% believed orders placed into the system were carried out more rapidly (Q2) (Table 2). On the other hand, 63% of respondents believed that placing orders directly into the system led to fewer medication errors (Q3), and 51% stated the system generally reduced medical errors (Q4). Sixty‐nine percent stated order sets were important for efficient use of the system (Q5), and 71% believed order sets served an important decision support role (Q6). Twenty‐six percent stated that the encouragement of nurses was an important factor in their use of the system (Q8). Finally, 35% of attending physicians believed use of the system to place orders should be mandatory.
Characteristics and Attitudes of High, Intermediate, and Low Users
The median order entry rate of respondents was 66%. One hundred and forty‐one (42%) placed at least 80% of their orders directly into the system, whereas 109 (32%) placed no more than 20% of their orders directly in the system (Fig. 1). There was not a significant difference between the low, intermediate, and high use groups in the total number of orders that each physician placed during the study period (Table 3). Sex, years since graduation from medical school, years in practice at the study institution, and use of computers in the outpatient setting were not meaningfully different between the 3 categories of users (Table 3). On the other hand, medical specialty was strongly associated with use of the system, with anesthesiologists, pediatricians, and surgeons the specialties with the largest proportion of high users. Furthermore, physicians who were trained in a CPOE environment and those who reported daily use of computers for personal activities showed the highest levels of adoption. Physicians at Franklin Medical Center showed lower levels of order entry than their counterparts at Baystate.

| Low (20%) n (row %) | Intermediate (20%‐79%) n (row %) | High (80%) n (row %) | P value | |
|---|---|---|---|---|
| ||||
| n = 109 | n = 88 | n = 141 | ||
| Hospital | < .01c | |||
| Baystate | 73 (25) | 79 (27) | 138 (48) | |
| Franklin | 36 (75) | 9 (19) | 3 (6) | |
| Sex | .69c | |||
| Female | 28 (29) | 24 (25) | 43 (45) | |
| Male | 81 (33) | 64 (26) | 98 (40) | |
| Specialty | .0001c | |||
| Anesthesia | 8 (35) | 3 (13) | 12 (52) | |
| Internal medicine | 45 (33) | 37 (27) | 53 (39) | |
| Medicine/pediatrics | 6 (46) | 5 (38) | 2 (15) | |
| OB/GYN | 20 (56) | 12 (33) | 4 (11) | |
| Pediatrics | 13 (24) | 9 (17) | 32 (59) | |
| Surgery | 14 (23) | 21 (34) | 26 (43) | |
| Other | 3 (19) | 1 (6) | 12 (75) | |
| Do you use a computer in your outpatient practice?a | ||||
| Yes | 75 (31) | 61 (25) | 105 (44) | .22c |
| No | 20 (36) | 18 (33) | 17 (31) | |
| Level of personal computer useb | .045d | |||
| Rarely | 11 (44) | 8 (32) | 6 (24) | |
| A few times a month | 7 (33) | 4 (19) | 10 (48) | |
| Several times a week | 28 (35) | 25 (31) | 28 (35) | |
| At least once a day | 62 (30) | 50 (24) | 97 (46) | |
| Training at an institution that had CPOE | .037c | |||
| Yes | 30 (26) | 40 (34) | 46 (40) | |
| No | 76 (35) | 48 (22) | 94 (43) | |
| Median (IQR) | Median (IQR) | Median (IQR) | ||
| Years since graduation from medical school | 21 (16, 28) | 18 (14, 25) | 19 (12, 25) | .06e |
| Years in practice at study institution | 12 (5, 19) | 12 (6, 19) | 12 (6, 17) | .84e |
| Total number of orders placed | 112 (45, 306) | 105 (56, 254) | 113 (44, 382) | .92e |
Use of the system was highly associated with physician attitudes toward CPOE, with the views of intermediate and high users consistently different than those of low users (Fig. 2). The associations found held true regardless of hospital: low, intermediate, and high users from Franklin had similar responses to those from Baystate (P > .05 for all questions), and the data from the 2 hospitals therefore were combined for presentation. Although few physicians believed that the user interface of the system supported their work flow, high and intermediate users were 3 times as likely to share this view than were low users (Q7; Fig. 2). Similarly, 19% of low users, 31% of intermediate users, and 45% of high users believed that entering orders into the system was faster than writing orders (Q1). High and intermediate users of the system were more likely than low users to believe that orders entered into the system were carried out more rapidly (Q2) and led to fewer medication (Q3) and nonmedication (Q4) errors. Regardless of their utilization pattern, most physicians believed that order sets played an important role in promoting efficiency and quality.

DISCUSSION
In this study of the clinical computing practices of physicians at 2 community hospitals, we observed wide variation in the adoption of CPOE by individual attendings. Although roughly one‐third rarely placed orders directly into the system, 42% had an order entry rate of at least 80%. Contrary to our initial expectation, we found little association between a physician's order entry rate with years in practice, duration of exposure to CPOE, or use of computers in the outpatient setting. On the other hand, we observed marked differences in use of the CPOE system across specialty lines and found that physicians who were exposed to CPOE during training and those who were regular users of computers for personal activities were more likely to embrace this technology. Further, we observed important differences between physicians who used the system to place some or most of their orders and those who did so only rarely in their beliefs and attitudes about the impact and benefits of CPOE. Physicians with higher order entry rates were more likely than their colleagues to believe that placing orders electronically was faster than handwriting and that use of the system led to fewer medical errors. These findings should be encouraging to hospitals hoping to implement CPOE because they suggest that successful adoption of CPOE is not limited to physicians who have just completed their residencies or to hospitals with the capability of designing and building their own systems. On the contrary, we documented that women, older physicians, and those with limited CPOE experience were as likely to be frequent users, especially if they perceived CPOE to be safer than handwriting and if they believed the user interface supported the efficient entering of orders.
On the basis of these results we recommend that in addition to purchasing systems that meet physician work‐flow needs and support the efficient entry of orders, hospital leaders should emphasize the quality and safety benefits of CPOE as part of a comprehensive change management strategy. The differences we observed in order entry rates across specialties may have resulted from several factors, including inherent differences in personality type associated with choice of specialty and in the level of customization of a system reflected in which and how many order sets are included. Such findings suggest that when it comes to CPOE, one size does not fit all, and implementation planning should be carried out at the specialty level. Finally, our observation that physicians who had exposure to CPOE during training were more likely to use the system to place orders suggests that the nation's training institutions will play an important role in fostering universal adoption of this technology.
Several earlier studies have reported on physician experiences with CPOE systems. Murff and Kannry12 surveyed 94 internal medicine house staff to compare experiences with 2 CPOE systems: the Department of Veterans Affairs Computerized Patient Record System (CPRS) and a commercially available product. They found striking differences in user satisfaction with numerous aspects of the systems, however they did not address attitudes toward safety or quality, and because house staff were required to place orders electronically they were unable to correlate responses with actual usage patterns. Weiner et al.13 compared the opinions of internal medicine house staff, attendings, fellows, and nurses about the benefits and challenges of using a computerized provider order entry system. In contrast to the findings from our study, Weiner et al. reported that more than half of physicians believed that provider order entry led to a greater number of errors, and only a minority believed the system increased quality of care overall. Finally, Lee et al.14 surveyed medical and surgical house officers and nurses at a large academic medical center about their satisfaction with a locally developed order entry system. They found that attitudes about the impact of the system on productivity and ease of use were more strongly associated with overall satisfaction than having undergone training or experience with personal computers. These findings are congruous with our own observation that beliefs about the speed with which orders are placed are closely associated with actual use of the system. They reported, as have we, that physicians placed a high value on order sets.
Our study had a number of strengths. First, we were able to offer insight into the attitudes and behaviors of a previously neglected, but critically important groupattending physicians who care for patients at community hospitals without the assistance of house staff. Second, whereas previous studies primarily assessed physician satisfaction with CPOE, we explored how physician attitudes about the impact of CPOE on work flow and on safety were associated with actual ordering habits. Information about ordering was obtained directly from the order entry system and not through self‐report. We conducted the study at 2 hospitals, a large urban community teaching hospital and a smaller rural hospital, and focused on a CPOE system that is in use at many institutions throughout the country, thereby increasing the generalizability of our findings. Although adoption of the system by physicians at the 2 hospitals differed, factors that associated with the use of CPOE to place orders were similar. Finally, we surveyed a large number of physicians, had a high response rate, and found only small differences in the utilization patterns of responders and nonresponders, suggesting that our portrayal of the attitudes of physicians was representative of the views of physicians practicing in our community.
The study had a number of weaknesses. First, we cannot be sure whether preexisting beliefs about the benefits of CPOE directly influenced physicians' use of the system or, conversely, if these attitudes developed in response to experience as users. Nevertheless, it seems practical to suggest that hospitals focus on purchasing systems that support the efficient entering of orders while simultaneously adopting a communication and change management strategy that emphasizes the safety and quality benefits of CPOE more broadly. Second, we did not attempt to validate the opinions expressed by physicians about the usability or safety benefits of the system. That said, the purpose of the study was to determine whether physician attitudes toward these issues was associated with the use of the system to place orders. Whether or not this particular CPOE system actually prevented medication errors, most physicians believed it did, a belief strongly associated with the observed order entry rates. Third, we studied a single CPOE system implemented approximately 10 years ago that does not reflect state‐of‐the‐art user interface design or functionality. Nevertheless, our observation about the importance of the user experience is probably no less relevant today. Fourth, we were unable to ascertain every order given by physicians, as some so‐called MD to RN orders may never have made it into the system. Finally, there is a small risk that some written, telephone, and verbal orders may have been randomly or systematically assigned to incorrect physicians, which would have led us to calculate inaccurate utilization rates.
CONCLUSIONS
In a voluntary community hospital environment the adoption of CPOE by attending physicians varies widely. While placing a premium on the purchase of systems that meet the work‐flow needs of physicians and support the efficient entry of orders, hospital leaders can enhance physician adoption of this technology by communicating the role of CPOE in improving quality and safety.
Acknowledgements
The authors thank Gilad Kuperman, MD, PhD, for his thoughtful comments on an earlier version of the manuscript.
- Kohn LT,Corrigan JM,Donaldson MS, eds.To Err Is Human: Building a Safer Health System.Washington, DC:National Academy Press,2000.
- ,.Computer physician order entry: benefits, costs, and issues.Ann Intern Med.2003;139:31–39.
- ,,, et al.Effect of computerized physician order entry and a team intervention on prevention of serious medication errors.JAMA.1998;280:1311–1316.
- ,,,,,.A computerized reminder system to increase the use of preventive care for hospitalized patients.N Engl J Med.2001;345:965–970.
- ,,,.A randomized trial of “corollary orders” to prevent errors of omission.J Am Med Inform Assoc.1997;4:364–375.
- ,,, et al.A computer‐assisted management program for antibiotics and other antiinfective agents.N Engl J Med.1998;338:232–238.
- The Leapfrog Group. Patient Safety Fact Sheet. Available at: http://www.leapfroggroup.org/FactSheets/LF_FactSheet.pdf. Accessed October 6,2004.
- ,,,.Computerized physician order entry in U.S. hospitals: results of a 2002 survey.J Am Med Inform Assoc.2004;11:95–99.
- ,,.U.S. adoption of computerized physician order entry systems.Health Aff.2005;24:1654–1663.
- ,,.Effects of computerized physician order entry and clinical decision support systems on medication safety: a systematic review.Arch Intern Med.2003;163:1409–1416.
- HCUPnet, Healthcare Cost and Utilization Project. Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.ahrq.gov/data/hcup/hcupnet.htm. Accessed October 6,2004.
- ,.Physician satisfaction with two order entry systems.J Am Med Inform Assoc.2001;8:499–509.
- ,,, et al.Contrasting views of physicians and nurses about an inpatient computer‐based provider order‐entry system.J Am Med Inform Assoc.1999;6:234–244.
- ,,,.Implementation of physician order entry: user satisfaction and self‐reported usage patterns.J Am Med Inform Assoc.1996;3:42–55.
- Kohn LT,Corrigan JM,Donaldson MS, eds.To Err Is Human: Building a Safer Health System.Washington, DC:National Academy Press,2000.
- ,.Computer physician order entry: benefits, costs, and issues.Ann Intern Med.2003;139:31–39.
- ,,, et al.Effect of computerized physician order entry and a team intervention on prevention of serious medication errors.JAMA.1998;280:1311–1316.
- ,,,,,.A computerized reminder system to increase the use of preventive care for hospitalized patients.N Engl J Med.2001;345:965–970.
- ,,,.A randomized trial of “corollary orders” to prevent errors of omission.J Am Med Inform Assoc.1997;4:364–375.
- ,,, et al.A computer‐assisted management program for antibiotics and other antiinfective agents.N Engl J Med.1998;338:232–238.
- The Leapfrog Group. Patient Safety Fact Sheet. Available at: http://www.leapfroggroup.org/FactSheets/LF_FactSheet.pdf. Accessed October 6,2004.
- ,,,.Computerized physician order entry in U.S. hospitals: results of a 2002 survey.J Am Med Inform Assoc.2004;11:95–99.
- ,,.U.S. adoption of computerized physician order entry systems.Health Aff.2005;24:1654–1663.
- ,,.Effects of computerized physician order entry and clinical decision support systems on medication safety: a systematic review.Arch Intern Med.2003;163:1409–1416.
- HCUPnet, Healthcare Cost and Utilization Project. Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.ahrq.gov/data/hcup/hcupnet.htm. Accessed October 6,2004.
- ,.Physician satisfaction with two order entry systems.J Am Med Inform Assoc.2001;8:499–509.
- ,,, et al.Contrasting views of physicians and nurses about an inpatient computer‐based provider order‐entry system.J Am Med Inform Assoc.1999;6:234–244.
- ,,,.Implementation of physician order entry: user satisfaction and self‐reported usage patterns.J Am Med Inform Assoc.1996;3:42–55.
Copyright © 2006 Society of Hospital Medicine