User login
Long COVID mimics other postviral conditions
When Jaime Seltzer first heard about a new virus that was spreading globally early in 2020, she was on full alert. As an advocate for the post-viral condition known as myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), she worried about a new wave of people having long-term disabilities.
“The hair on my arms stood on end,” said Ms. Seltzer, director of scientific and medical outreach at the advocacy group MEAction and a consultant researcher at Stanford University.
Ms. Seltzer, who has had ME/CFS herself, said she wondered.
Sure enough, later in 2020, reports began emerging about people with extreme fatigue, postexertion crashes, brain fog, unrefreshing sleep, and dizziness when standing up months after a bout with the then-new viral illness. Those same symptoms had been designated as “core criteria” of ME/CFS by the National Academy of Medicine in a 2015 report.
Now, advocates like Ms. Seltzer are hoping the research and medical communities will give ME/CFS and other postviral illnesses the same attention they have increasingly focused on long COVID.
The emergence of long COVID was no surprise to researchers who study ME/CFS, because the same set of symptoms has arisen after many other viruses.
“This for all the world looks like ME/CFS. We think they are frighteningly similar, if not identical,” said David M. Systrom, MD, a pulmonary and critical care medicine specialist at Brigham and Women’s Hospital in Boston, who studies people with both diagnoses.
The actual numbers are hard to determine, since many people who meet ME/CFS criteria aren’t formally diagnosed. But a combined analysis of data from several studies published in March found that about one in three people had fatigue and about one in five reported having a hard time with thinking and memory 12 or more weeks after they had COVID-19.
According to some estimates, about half of people with long COVID will meet the criteria for ME/CFS, whether they’re given that specific diagnosis or not.
Other conditions that often exist with ME/CFS are also being seen in people with long COVID, including postural orthostatic tachycardia syndrome, which causes people to feel dizzy when they stand, along with other symptoms; other problems with the autonomic nervous system, which controls body systems such as heart rate, blood pressure, and digestion, known together as dysautonomia; and a condition related to allergies called mast cell activation disorder.
Post–acute infection syndromes have been linked to a long list of viruses, including Ebola, the 2003-2004 SARS virus, and Epstein-Barr – the virus most commonly associated with ME/CFS.
The problem in clinical medicine is that once an infection has cleared, the teaching has been that the person should no longer feel sick, said Nancy G. Klimas, MD, director of the Institute for Neuro-Immune Medicine at Nova Southeastern University in Miami. “I was taught that there has to be an antigen [such as a viral protein] in the system to drive the immune system to make it create sickness, and the immune system should shut off when it’s done,” she said.
Thus, if virus is gone and other routine lab tests come up negative, doctors often deem the person’s reported symptoms to be psychological, which can upset patients, Anthony Komaroff, MD, of Brigham and Women’s Hospital in Boston, wrote in July 2021.
Only recently have doctors started to appreciate the idea that the immune system may be overreacting long term, Dr. Klimas said.
Now, long COVID appears to be speeding up that recognition. Dr. Systrom said he has “absolutely” seen a change in attitude among fellow doctors who had been skeptical of ME/CFS as a “real” illness because there’s no test for it.
“I’m very keenly aware of a large group of health care professionals who really had not bought into the concept of ME/CFS as a real disease who have had an epiphany of sorts with long COVID and now, in a backwards way, have applied that same thinking to their very same patients with ME/CFS,” he said.
Science showing ‘frighteningly similar’ symptoms
Dr. Systrom has spent several years researching how ME/CFS patients cannot tolerate exercise and now is doing similar studies in people with long COVID. “Several months into the pandemic, we began receiving reports of patients who had survived COVID and maybe even had a relatively mild disease ... and as the summer of 2020 moved into the fall, it became apparent that there was a subset of patients who for all the world appeared to meet ME/CFS clinical criteria,” he said.
Using bicycle exercise tests on long COVID patients with catheters placed in their veins, Dr. Systrom and associates have shown a lack of exercise capacity that isn’t caused by heart or lung disease but instead is related to abnormal nerves and blood vessels, just as they’d shown previously in ME/CFS patient.
Avindra Nath, MD, senior investigator and clinical director of intramural research at the National Institute of Neurological Disorders and Stroke, Bethesda, Md., was doing a deep-dive scientific study on ME/CFS when the COVID-19 pandemic hit. Since then, he›s begun another study using the same protocol and sophisticated laboratory measurement to evaluate people with long COVID.
“As terrible as [long COVID] is, it’s kind of a blessing in disguise for ME/CFS because there’s just so much overlap between the two and they could very well be in many ways one in the same thing. The problem with studying ME/CFS is oftentimes you didn’t know what the trigger was. You see patients many years later, then try to backtrack and find out what happened,” said Dr. Nath, a neuroimmunologist.
With long COVID, on the other hand, “we know when they got infected and when their symptoms actually started, so it becomes much more uniform. ... It gives us an opportunity to maybe solve certain things in a much more well-defined population and try to find answers.”
Advocacy groups want to see more.
In February 2021, Solve M.E. launched the Long COVID Alliance, made up of several organizations, companies, and people with a goal to influence policy and speed up research into a range of postviral illnesses.
Solve M.E. has also pushed for inclusion of language regarding ME/CFS and related conditions into congressional bills addressing long COVID, including those that call for funding of research and clinical care.
“On the political front, we’ve really capitalized on a moment in time in which we have the spotlight,” said Emily Taylor, vice president of advocacy and engagement for Solve M.E.
“One of the hardest parts about ME/CFS is how to show that it’s real when it’s invisible. Most people agree that COVID is real and therefore if somebody gets ME/CFS after COVID, it’s real,” she said.
The advocacy groups are now pushing for non-COVID postinfection illnesses to be included in efforts aimed at helping people with long COVID, with mixed results. For example, the RECOVER Initiative, established in February 2021 with $1.5 billion in funding from Congress to the National Institutes of Health, is specifically for studying long COVID and does not fund research into other postinfection illnesses, although representatives from the ME/CFS community are advisers.
Language addressing ME/CFS and other postinfectious chronic illnesses has been included in several long COVID bills now pending in Congress, including the Care for Long COVID Act in the Senate and its companion COVID-19 Long Haulers Act in the House. “Our goal is to push for passage of a long COVID bill by the end of the year,” Ms. Taylor said.
A version of this article first appeared on WebMD.com.
When Jaime Seltzer first heard about a new virus that was spreading globally early in 2020, she was on full alert. As an advocate for the post-viral condition known as myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), she worried about a new wave of people having long-term disabilities.
“The hair on my arms stood on end,” said Ms. Seltzer, director of scientific and medical outreach at the advocacy group MEAction and a consultant researcher at Stanford University.
Ms. Seltzer, who has had ME/CFS herself, said she wondered.
Sure enough, later in 2020, reports began emerging about people with extreme fatigue, postexertion crashes, brain fog, unrefreshing sleep, and dizziness when standing up months after a bout with the then-new viral illness. Those same symptoms had been designated as “core criteria” of ME/CFS by the National Academy of Medicine in a 2015 report.
Now, advocates like Ms. Seltzer are hoping the research and medical communities will give ME/CFS and other postviral illnesses the same attention they have increasingly focused on long COVID.
The emergence of long COVID was no surprise to researchers who study ME/CFS, because the same set of symptoms has arisen after many other viruses.
“This for all the world looks like ME/CFS. We think they are frighteningly similar, if not identical,” said David M. Systrom, MD, a pulmonary and critical care medicine specialist at Brigham and Women’s Hospital in Boston, who studies people with both diagnoses.
The actual numbers are hard to determine, since many people who meet ME/CFS criteria aren’t formally diagnosed. But a combined analysis of data from several studies published in March found that about one in three people had fatigue and about one in five reported having a hard time with thinking and memory 12 or more weeks after they had COVID-19.
According to some estimates, about half of people with long COVID will meet the criteria for ME/CFS, whether they’re given that specific diagnosis or not.
Other conditions that often exist with ME/CFS are also being seen in people with long COVID, including postural orthostatic tachycardia syndrome, which causes people to feel dizzy when they stand, along with other symptoms; other problems with the autonomic nervous system, which controls body systems such as heart rate, blood pressure, and digestion, known together as dysautonomia; and a condition related to allergies called mast cell activation disorder.
Post–acute infection syndromes have been linked to a long list of viruses, including Ebola, the 2003-2004 SARS virus, and Epstein-Barr – the virus most commonly associated with ME/CFS.
The problem in clinical medicine is that once an infection has cleared, the teaching has been that the person should no longer feel sick, said Nancy G. Klimas, MD, director of the Institute for Neuro-Immune Medicine at Nova Southeastern University in Miami. “I was taught that there has to be an antigen [such as a viral protein] in the system to drive the immune system to make it create sickness, and the immune system should shut off when it’s done,” she said.
Thus, if virus is gone and other routine lab tests come up negative, doctors often deem the person’s reported symptoms to be psychological, which can upset patients, Anthony Komaroff, MD, of Brigham and Women’s Hospital in Boston, wrote in July 2021.
Only recently have doctors started to appreciate the idea that the immune system may be overreacting long term, Dr. Klimas said.
Now, long COVID appears to be speeding up that recognition. Dr. Systrom said he has “absolutely” seen a change in attitude among fellow doctors who had been skeptical of ME/CFS as a “real” illness because there’s no test for it.
“I’m very keenly aware of a large group of health care professionals who really had not bought into the concept of ME/CFS as a real disease who have had an epiphany of sorts with long COVID and now, in a backwards way, have applied that same thinking to their very same patients with ME/CFS,” he said.
Science showing ‘frighteningly similar’ symptoms
Dr. Systrom has spent several years researching how ME/CFS patients cannot tolerate exercise and now is doing similar studies in people with long COVID. “Several months into the pandemic, we began receiving reports of patients who had survived COVID and maybe even had a relatively mild disease ... and as the summer of 2020 moved into the fall, it became apparent that there was a subset of patients who for all the world appeared to meet ME/CFS clinical criteria,” he said.
Using bicycle exercise tests on long COVID patients with catheters placed in their veins, Dr. Systrom and associates have shown a lack of exercise capacity that isn’t caused by heart or lung disease but instead is related to abnormal nerves and blood vessels, just as they’d shown previously in ME/CFS patient.
Avindra Nath, MD, senior investigator and clinical director of intramural research at the National Institute of Neurological Disorders and Stroke, Bethesda, Md., was doing a deep-dive scientific study on ME/CFS when the COVID-19 pandemic hit. Since then, he›s begun another study using the same protocol and sophisticated laboratory measurement to evaluate people with long COVID.
“As terrible as [long COVID] is, it’s kind of a blessing in disguise for ME/CFS because there’s just so much overlap between the two and they could very well be in many ways one in the same thing. The problem with studying ME/CFS is oftentimes you didn’t know what the trigger was. You see patients many years later, then try to backtrack and find out what happened,” said Dr. Nath, a neuroimmunologist.
With long COVID, on the other hand, “we know when they got infected and when their symptoms actually started, so it becomes much more uniform. ... It gives us an opportunity to maybe solve certain things in a much more well-defined population and try to find answers.”
Advocacy groups want to see more.
In February 2021, Solve M.E. launched the Long COVID Alliance, made up of several organizations, companies, and people with a goal to influence policy and speed up research into a range of postviral illnesses.
Solve M.E. has also pushed for inclusion of language regarding ME/CFS and related conditions into congressional bills addressing long COVID, including those that call for funding of research and clinical care.
“On the political front, we’ve really capitalized on a moment in time in which we have the spotlight,” said Emily Taylor, vice president of advocacy and engagement for Solve M.E.
“One of the hardest parts about ME/CFS is how to show that it’s real when it’s invisible. Most people agree that COVID is real and therefore if somebody gets ME/CFS after COVID, it’s real,” she said.
The advocacy groups are now pushing for non-COVID postinfection illnesses to be included in efforts aimed at helping people with long COVID, with mixed results. For example, the RECOVER Initiative, established in February 2021 with $1.5 billion in funding from Congress to the National Institutes of Health, is specifically for studying long COVID and does not fund research into other postinfection illnesses, although representatives from the ME/CFS community are advisers.
Language addressing ME/CFS and other postinfectious chronic illnesses has been included in several long COVID bills now pending in Congress, including the Care for Long COVID Act in the Senate and its companion COVID-19 Long Haulers Act in the House. “Our goal is to push for passage of a long COVID bill by the end of the year,” Ms. Taylor said.
A version of this article first appeared on WebMD.com.
When Jaime Seltzer first heard about a new virus that was spreading globally early in 2020, she was on full alert. As an advocate for the post-viral condition known as myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), she worried about a new wave of people having long-term disabilities.
“The hair on my arms stood on end,” said Ms. Seltzer, director of scientific and medical outreach at the advocacy group MEAction and a consultant researcher at Stanford University.
Ms. Seltzer, who has had ME/CFS herself, said she wondered.
Sure enough, later in 2020, reports began emerging about people with extreme fatigue, postexertion crashes, brain fog, unrefreshing sleep, and dizziness when standing up months after a bout with the then-new viral illness. Those same symptoms had been designated as “core criteria” of ME/CFS by the National Academy of Medicine in a 2015 report.
Now, advocates like Ms. Seltzer are hoping the research and medical communities will give ME/CFS and other postviral illnesses the same attention they have increasingly focused on long COVID.
The emergence of long COVID was no surprise to researchers who study ME/CFS, because the same set of symptoms has arisen after many other viruses.
“This for all the world looks like ME/CFS. We think they are frighteningly similar, if not identical,” said David M. Systrom, MD, a pulmonary and critical care medicine specialist at Brigham and Women’s Hospital in Boston, who studies people with both diagnoses.
The actual numbers are hard to determine, since many people who meet ME/CFS criteria aren’t formally diagnosed. But a combined analysis of data from several studies published in March found that about one in three people had fatigue and about one in five reported having a hard time with thinking and memory 12 or more weeks after they had COVID-19.
According to some estimates, about half of people with long COVID will meet the criteria for ME/CFS, whether they’re given that specific diagnosis or not.
Other conditions that often exist with ME/CFS are also being seen in people with long COVID, including postural orthostatic tachycardia syndrome, which causes people to feel dizzy when they stand, along with other symptoms; other problems with the autonomic nervous system, which controls body systems such as heart rate, blood pressure, and digestion, known together as dysautonomia; and a condition related to allergies called mast cell activation disorder.
Post–acute infection syndromes have been linked to a long list of viruses, including Ebola, the 2003-2004 SARS virus, and Epstein-Barr – the virus most commonly associated with ME/CFS.
The problem in clinical medicine is that once an infection has cleared, the teaching has been that the person should no longer feel sick, said Nancy G. Klimas, MD, director of the Institute for Neuro-Immune Medicine at Nova Southeastern University in Miami. “I was taught that there has to be an antigen [such as a viral protein] in the system to drive the immune system to make it create sickness, and the immune system should shut off when it’s done,” she said.
Thus, if virus is gone and other routine lab tests come up negative, doctors often deem the person’s reported symptoms to be psychological, which can upset patients, Anthony Komaroff, MD, of Brigham and Women’s Hospital in Boston, wrote in July 2021.
Only recently have doctors started to appreciate the idea that the immune system may be overreacting long term, Dr. Klimas said.
Now, long COVID appears to be speeding up that recognition. Dr. Systrom said he has “absolutely” seen a change in attitude among fellow doctors who had been skeptical of ME/CFS as a “real” illness because there’s no test for it.
“I’m very keenly aware of a large group of health care professionals who really had not bought into the concept of ME/CFS as a real disease who have had an epiphany of sorts with long COVID and now, in a backwards way, have applied that same thinking to their very same patients with ME/CFS,” he said.
Science showing ‘frighteningly similar’ symptoms
Dr. Systrom has spent several years researching how ME/CFS patients cannot tolerate exercise and now is doing similar studies in people with long COVID. “Several months into the pandemic, we began receiving reports of patients who had survived COVID and maybe even had a relatively mild disease ... and as the summer of 2020 moved into the fall, it became apparent that there was a subset of patients who for all the world appeared to meet ME/CFS clinical criteria,” he said.
Using bicycle exercise tests on long COVID patients with catheters placed in their veins, Dr. Systrom and associates have shown a lack of exercise capacity that isn’t caused by heart or lung disease but instead is related to abnormal nerves and blood vessels, just as they’d shown previously in ME/CFS patient.
Avindra Nath, MD, senior investigator and clinical director of intramural research at the National Institute of Neurological Disorders and Stroke, Bethesda, Md., was doing a deep-dive scientific study on ME/CFS when the COVID-19 pandemic hit. Since then, he›s begun another study using the same protocol and sophisticated laboratory measurement to evaluate people with long COVID.
“As terrible as [long COVID] is, it’s kind of a blessing in disguise for ME/CFS because there’s just so much overlap between the two and they could very well be in many ways one in the same thing. The problem with studying ME/CFS is oftentimes you didn’t know what the trigger was. You see patients many years later, then try to backtrack and find out what happened,” said Dr. Nath, a neuroimmunologist.
With long COVID, on the other hand, “we know when they got infected and when their symptoms actually started, so it becomes much more uniform. ... It gives us an opportunity to maybe solve certain things in a much more well-defined population and try to find answers.”
Advocacy groups want to see more.
In February 2021, Solve M.E. launched the Long COVID Alliance, made up of several organizations, companies, and people with a goal to influence policy and speed up research into a range of postviral illnesses.
Solve M.E. has also pushed for inclusion of language regarding ME/CFS and related conditions into congressional bills addressing long COVID, including those that call for funding of research and clinical care.
“On the political front, we’ve really capitalized on a moment in time in which we have the spotlight,” said Emily Taylor, vice president of advocacy and engagement for Solve M.E.
“One of the hardest parts about ME/CFS is how to show that it’s real when it’s invisible. Most people agree that COVID is real and therefore if somebody gets ME/CFS after COVID, it’s real,” she said.
The advocacy groups are now pushing for non-COVID postinfection illnesses to be included in efforts aimed at helping people with long COVID, with mixed results. For example, the RECOVER Initiative, established in February 2021 with $1.5 billion in funding from Congress to the National Institutes of Health, is specifically for studying long COVID and does not fund research into other postinfection illnesses, although representatives from the ME/CFS community are advisers.
Language addressing ME/CFS and other postinfectious chronic illnesses has been included in several long COVID bills now pending in Congress, including the Care for Long COVID Act in the Senate and its companion COVID-19 Long Haulers Act in the House. “Our goal is to push for passage of a long COVID bill by the end of the year,” Ms. Taylor said.
A version of this article first appeared on WebMD.com.
Teprotumumab eases thyroid eye disease in all, including smokers
Teprotumumab (Tepezza, Horizon Therapeutics), the first-ever medication approved specifically to treat thyroid eye disease, works in patients regardless of age, gender, and smoking status, new research finds.
The data were presented on March 31 by Raymond S. Douglas, MD, director of the thyroid eye disease program at Cedars-Sinai Medical Center, Los Angeles, during a virtual news conference held by the Endocrine Society. The study had been slated for presentation during ENDO 2020, the society’s annual meeting, which was canceled because of the COVID-19 pandemic.
Thyroid eye disease occurs in up to 50% of people with Graves disease, causing a variety of symptoms, such as eye pain, double vision, light sensitivity or difficulty closing the eye, as well as proptosis, or bulging of the eye, and vision-threatening complications. It affects more women than men, and the symptoms can lead to the progressive inability to perform important daily activities, such as driving or working.
Teprotumumab is a fully human monoclonal antibody inhibitor of the insulin-like growth factor-1 (IGF-1) receptor and was approved by the US Food and Drug Administration in January 2020. Prior to that, therapy typically involved steroids or, in severe cases, surgery.
Blocking the IGF-1 receptor leads to reduced inflammation and reversal of retro-orbital tissue expansion and hyaluronan production in the eye orbit. Teprotumumab is given as an infusion once every 3 weeks for a total of eight infusions.
“Exciting to have an agent” that reduces proptosis to this degree
Previously reported pooled phase 2 and phase 3 data from the randomized, placebo-controlled OPTIC trial involving 171 patients showed significantly greater reductions in proptosis, as well as diplopia, and clinical symptoms of inflammation with teprotumumab versus placebo.
“This has really been unheralded in comparison to other medical therapies previously offered,” Dr. Douglas said during the briefing.
Now, the new analysis shows that the drug works across patient subgroups, he added, highlighting in particular the fact that the agent seems to work equally well in smokers and nonsmokers. Smoking leads to a worse prognosis in thyroid eye disease.
Asked to comment, endocrinologist David C. Lieb, MD, of Eastern Virginia Medical School, Norfolk, said in an interview, “It’s reassuring that this drug appears to have benefits in reducing proptosis across multiple age groups, in both genders, and that there are also benefits seen in patients who smoke and who don’t.”
So far Dr. Lieb has two patients who have been prescribed teprotumumab by their ophthalmologists, but it’s too soon to know how they’ll respond.
“I have no first-hand experience yet, but it’s very exciting to have something to offer patients with active Graves eye disease, which causes a lot of disability for people. It makes work difficult and driving difficult. It’s exciting to have an agent that reduces proptosis to the degree that this one does because we haven’t had anything like this before,” he said.
All patient subgroups benefited in combined analysis
A total of 79 patients completed phase 2 and 76 patients completed phase 3 of the OPTIC trial.
Overall, the proportions achieving proptosis reductions of at least 2 mm without deterioration in the fellow eye at week 24 were 77.4% with teprotumumab versus 14.9% with placebo in the intention-to-treat analysis (P < .001), respectively, and 84.8% versus 17.1% in the per-protocol analysis (P < .001). The number needed to treat was 1.6.
Similar results were achieved across all subgroups of patients: those aged 65 and older versus younger than aged 65 years; male versus female; tobacco user versus nonuser; and U.S. versus E.U. study centers (all P < .001).
Overall, the average decrease in proptosis was 3.1 mm, compared to just 0.4 mm with placebo (P < .001). By subgroup, those reductions ranged from 3.55 mm for those aged 65 and older to 2.93 mm for the U.S. group.
The average proptosis reductions with teprotumumab were 2.99 mm among smokers versus 3.20 mm in nonsmokers, but responses in both groups were significant when compared with placebo.
Smoking contributes to the severity of thyroid eye disease and is associated with more optic neuropathy, poorer response to anti-inflammatory treatment, and worse outcomes, Dr. Douglas said. “Smoking appears to preferentially cause fibroblasts in the orbit to increase proinflammatory cytokines. ... It’s reassuring that this medicine does work in smokers since most other medications are much less effective in reducing inflammatory signs in smoking versus nonsmoking patients.”
Most adverse reactions disappeared after infusion stopped
In the pooled studies overall there were no deaths, but there were seven severe adverse events in the teprotumumab group versus one in the placebo group. Two adverse events in the teprotumumab group – diarrhea and infusion-related reaction – were considered treatment-related and led to drug discontinuation. Another adverse event, Hashimoto’s encephalopathy, was deemed possibly related to the drug and also led to discontinuation.
Treatment-emergent adverse events occurred in 79.8% of patients treated with teprotumumab versus 69.8% with placebo. Those occurring in 5% or more of patients included muscle spasms (25% vs. 7%), nausea (17% vs. 9%), alopecia (13% vs. 8%), and diarrhea (12% vs. 8%). Most were well tolerated and tended to resolve after the infusions ended, Dr. Douglas noted, adding muscle spasms tended to occur at night, improved with massage, and were not accompanied by electrolyte abnormalities.
Antidrug antibodies were detected in two teprotumumab-treated patients, one at study day 1 and another at week 3 during the 24-week treatment period. The patient with antibodies at day 1 also tested positive at week 72. “[Antidrug] antibodies appeared to be very uncommon,” Dr. Douglas noted.
The trial was sponsored by Horizon Therapeutics. Dr. Douglas is a consultant for Horizon Therapeutics and Immunovant. Dr. Lieb has reported no relevant financial relationships. The research will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
This article first appeared on Medscape.com.
Teprotumumab (Tepezza, Horizon Therapeutics), the first-ever medication approved specifically to treat thyroid eye disease, works in patients regardless of age, gender, and smoking status, new research finds.
The data were presented on March 31 by Raymond S. Douglas, MD, director of the thyroid eye disease program at Cedars-Sinai Medical Center, Los Angeles, during a virtual news conference held by the Endocrine Society. The study had been slated for presentation during ENDO 2020, the society’s annual meeting, which was canceled because of the COVID-19 pandemic.
Thyroid eye disease occurs in up to 50% of people with Graves disease, causing a variety of symptoms, such as eye pain, double vision, light sensitivity or difficulty closing the eye, as well as proptosis, or bulging of the eye, and vision-threatening complications. It affects more women than men, and the symptoms can lead to the progressive inability to perform important daily activities, such as driving or working.
Teprotumumab is a fully human monoclonal antibody inhibitor of the insulin-like growth factor-1 (IGF-1) receptor and was approved by the US Food and Drug Administration in January 2020. Prior to that, therapy typically involved steroids or, in severe cases, surgery.
Blocking the IGF-1 receptor leads to reduced inflammation and reversal of retro-orbital tissue expansion and hyaluronan production in the eye orbit. Teprotumumab is given as an infusion once every 3 weeks for a total of eight infusions.
“Exciting to have an agent” that reduces proptosis to this degree
Previously reported pooled phase 2 and phase 3 data from the randomized, placebo-controlled OPTIC trial involving 171 patients showed significantly greater reductions in proptosis, as well as diplopia, and clinical symptoms of inflammation with teprotumumab versus placebo.
“This has really been unheralded in comparison to other medical therapies previously offered,” Dr. Douglas said during the briefing.
Now, the new analysis shows that the drug works across patient subgroups, he added, highlighting in particular the fact that the agent seems to work equally well in smokers and nonsmokers. Smoking leads to a worse prognosis in thyroid eye disease.
Asked to comment, endocrinologist David C. Lieb, MD, of Eastern Virginia Medical School, Norfolk, said in an interview, “It’s reassuring that this drug appears to have benefits in reducing proptosis across multiple age groups, in both genders, and that there are also benefits seen in patients who smoke and who don’t.”
So far Dr. Lieb has two patients who have been prescribed teprotumumab by their ophthalmologists, but it’s too soon to know how they’ll respond.
“I have no first-hand experience yet, but it’s very exciting to have something to offer patients with active Graves eye disease, which causes a lot of disability for people. It makes work difficult and driving difficult. It’s exciting to have an agent that reduces proptosis to the degree that this one does because we haven’t had anything like this before,” he said.
All patient subgroups benefited in combined analysis
A total of 79 patients completed phase 2 and 76 patients completed phase 3 of the OPTIC trial.
Overall, the proportions achieving proptosis reductions of at least 2 mm without deterioration in the fellow eye at week 24 were 77.4% with teprotumumab versus 14.9% with placebo in the intention-to-treat analysis (P < .001), respectively, and 84.8% versus 17.1% in the per-protocol analysis (P < .001). The number needed to treat was 1.6.
Similar results were achieved across all subgroups of patients: those aged 65 and older versus younger than aged 65 years; male versus female; tobacco user versus nonuser; and U.S. versus E.U. study centers (all P < .001).
Overall, the average decrease in proptosis was 3.1 mm, compared to just 0.4 mm with placebo (P < .001). By subgroup, those reductions ranged from 3.55 mm for those aged 65 and older to 2.93 mm for the U.S. group.
The average proptosis reductions with teprotumumab were 2.99 mm among smokers versus 3.20 mm in nonsmokers, but responses in both groups were significant when compared with placebo.
Smoking contributes to the severity of thyroid eye disease and is associated with more optic neuropathy, poorer response to anti-inflammatory treatment, and worse outcomes, Dr. Douglas said. “Smoking appears to preferentially cause fibroblasts in the orbit to increase proinflammatory cytokines. ... It’s reassuring that this medicine does work in smokers since most other medications are much less effective in reducing inflammatory signs in smoking versus nonsmoking patients.”
Most adverse reactions disappeared after infusion stopped
In the pooled studies overall there were no deaths, but there were seven severe adverse events in the teprotumumab group versus one in the placebo group. Two adverse events in the teprotumumab group – diarrhea and infusion-related reaction – were considered treatment-related and led to drug discontinuation. Another adverse event, Hashimoto’s encephalopathy, was deemed possibly related to the drug and also led to discontinuation.
Treatment-emergent adverse events occurred in 79.8% of patients treated with teprotumumab versus 69.8% with placebo. Those occurring in 5% or more of patients included muscle spasms (25% vs. 7%), nausea (17% vs. 9%), alopecia (13% vs. 8%), and diarrhea (12% vs. 8%). Most were well tolerated and tended to resolve after the infusions ended, Dr. Douglas noted, adding muscle spasms tended to occur at night, improved with massage, and were not accompanied by electrolyte abnormalities.
Antidrug antibodies were detected in two teprotumumab-treated patients, one at study day 1 and another at week 3 during the 24-week treatment period. The patient with antibodies at day 1 also tested positive at week 72. “[Antidrug] antibodies appeared to be very uncommon,” Dr. Douglas noted.
The trial was sponsored by Horizon Therapeutics. Dr. Douglas is a consultant for Horizon Therapeutics and Immunovant. Dr. Lieb has reported no relevant financial relationships. The research will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
This article first appeared on Medscape.com.
Teprotumumab (Tepezza, Horizon Therapeutics), the first-ever medication approved specifically to treat thyroid eye disease, works in patients regardless of age, gender, and smoking status, new research finds.
The data were presented on March 31 by Raymond S. Douglas, MD, director of the thyroid eye disease program at Cedars-Sinai Medical Center, Los Angeles, during a virtual news conference held by the Endocrine Society. The study had been slated for presentation during ENDO 2020, the society’s annual meeting, which was canceled because of the COVID-19 pandemic.
Thyroid eye disease occurs in up to 50% of people with Graves disease, causing a variety of symptoms, such as eye pain, double vision, light sensitivity or difficulty closing the eye, as well as proptosis, or bulging of the eye, and vision-threatening complications. It affects more women than men, and the symptoms can lead to the progressive inability to perform important daily activities, such as driving or working.
Teprotumumab is a fully human monoclonal antibody inhibitor of the insulin-like growth factor-1 (IGF-1) receptor and was approved by the US Food and Drug Administration in January 2020. Prior to that, therapy typically involved steroids or, in severe cases, surgery.
Blocking the IGF-1 receptor leads to reduced inflammation and reversal of retro-orbital tissue expansion and hyaluronan production in the eye orbit. Teprotumumab is given as an infusion once every 3 weeks for a total of eight infusions.
“Exciting to have an agent” that reduces proptosis to this degree
Previously reported pooled phase 2 and phase 3 data from the randomized, placebo-controlled OPTIC trial involving 171 patients showed significantly greater reductions in proptosis, as well as diplopia, and clinical symptoms of inflammation with teprotumumab versus placebo.
“This has really been unheralded in comparison to other medical therapies previously offered,” Dr. Douglas said during the briefing.
Now, the new analysis shows that the drug works across patient subgroups, he added, highlighting in particular the fact that the agent seems to work equally well in smokers and nonsmokers. Smoking leads to a worse prognosis in thyroid eye disease.
Asked to comment, endocrinologist David C. Lieb, MD, of Eastern Virginia Medical School, Norfolk, said in an interview, “It’s reassuring that this drug appears to have benefits in reducing proptosis across multiple age groups, in both genders, and that there are also benefits seen in patients who smoke and who don’t.”
So far Dr. Lieb has two patients who have been prescribed teprotumumab by their ophthalmologists, but it’s too soon to know how they’ll respond.
“I have no first-hand experience yet, but it’s very exciting to have something to offer patients with active Graves eye disease, which causes a lot of disability for people. It makes work difficult and driving difficult. It’s exciting to have an agent that reduces proptosis to the degree that this one does because we haven’t had anything like this before,” he said.
All patient subgroups benefited in combined analysis
A total of 79 patients completed phase 2 and 76 patients completed phase 3 of the OPTIC trial.
Overall, the proportions achieving proptosis reductions of at least 2 mm without deterioration in the fellow eye at week 24 were 77.4% with teprotumumab versus 14.9% with placebo in the intention-to-treat analysis (P < .001), respectively, and 84.8% versus 17.1% in the per-protocol analysis (P < .001). The number needed to treat was 1.6.
Similar results were achieved across all subgroups of patients: those aged 65 and older versus younger than aged 65 years; male versus female; tobacco user versus nonuser; and U.S. versus E.U. study centers (all P < .001).
Overall, the average decrease in proptosis was 3.1 mm, compared to just 0.4 mm with placebo (P < .001). By subgroup, those reductions ranged from 3.55 mm for those aged 65 and older to 2.93 mm for the U.S. group.
The average proptosis reductions with teprotumumab were 2.99 mm among smokers versus 3.20 mm in nonsmokers, but responses in both groups were significant when compared with placebo.
Smoking contributes to the severity of thyroid eye disease and is associated with more optic neuropathy, poorer response to anti-inflammatory treatment, and worse outcomes, Dr. Douglas said. “Smoking appears to preferentially cause fibroblasts in the orbit to increase proinflammatory cytokines. ... It’s reassuring that this medicine does work in smokers since most other medications are much less effective in reducing inflammatory signs in smoking versus nonsmoking patients.”
Most adverse reactions disappeared after infusion stopped
In the pooled studies overall there were no deaths, but there were seven severe adverse events in the teprotumumab group versus one in the placebo group. Two adverse events in the teprotumumab group – diarrhea and infusion-related reaction – were considered treatment-related and led to drug discontinuation. Another adverse event, Hashimoto’s encephalopathy, was deemed possibly related to the drug and also led to discontinuation.
Treatment-emergent adverse events occurred in 79.8% of patients treated with teprotumumab versus 69.8% with placebo. Those occurring in 5% or more of patients included muscle spasms (25% vs. 7%), nausea (17% vs. 9%), alopecia (13% vs. 8%), and diarrhea (12% vs. 8%). Most were well tolerated and tended to resolve after the infusions ended, Dr. Douglas noted, adding muscle spasms tended to occur at night, improved with massage, and were not accompanied by electrolyte abnormalities.
Antidrug antibodies were detected in two teprotumumab-treated patients, one at study day 1 and another at week 3 during the 24-week treatment period. The patient with antibodies at day 1 also tested positive at week 72. “[Antidrug] antibodies appeared to be very uncommon,” Dr. Douglas noted.
The trial was sponsored by Horizon Therapeutics. Dr. Douglas is a consultant for Horizon Therapeutics and Immunovant. Dr. Lieb has reported no relevant financial relationships. The research will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
This article first appeared on Medscape.com.
Hepatitis B Vaccine Recommended for Adults With Diabetes
ATLANTA – Hepatitis B vaccination should be routinely given to previously unvaccinated adults with diabetes who are younger than 60 years of age, according to recommendations by the Centers for Disease Control’s Advisory Committee on Immunization Practices.
The recommendation is based on the finding that U.S. adults with diabetes patients face a roughly twofold increased risk for hepatitis B infection. Vaccination is advised based on the individual patient’s risk of acquiring hepatitis B infection.
An additional recommendation, which must be published by the CDC before it becomes official, is likely to say that people aged 60 years and older with diabetes who live in nursing homes and assisted living facilities are at increased risk for hepatitis B and should be considered for vaccine prioritization.
Results from CDC investigations identified inadequately cleaned blood glucose monitors as a major route of hepatitis B virus transmission among patients with diabetes, Meredith L. Reilly, a CDC epidemiologist, noted at the earlier annual meeting of the Infectious Diseases Society of America.
"Over the past several years, we’ve observed outbreaks of hepatitis B among patients with diabetes in places where they undergo assisted blood glucose monitoring, with more than one person using the monitor," such as assisted-living facilities, physician offices, and at pharmacies, said Dr. Trudy V. Murphy, head of the vaccine unit in the Division of Viral Hepatitis at the National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention in Atlanta. A total of 24 of 28 outbreaks at long-term care facilities identified different aspects of blood glucose monitoring as the source of the infection."
The advised age cutoff was based on estimates of disease risk and cost effectiveness. An analysis of data from more than 91,000 individuals participating in the CDC’s Emerging Infection Program (EIP) found that among people without traditional risk factors for hepatitis B (use of injectable drugs, unprotected sex with multiple partners), the adjusted odds ratio for acute hepatitis B among those with diabetes was 1.89, compared to those without diabetes, suggesting that diabetes is an independent marker for the infection, according to the CDC’s Dr. Sarah Schillie.
Among people with hepatitis B risk factors, those with diabetes had a 1.1 odds ratio of infection, compared with non-diabetics in that group.
After controlling for sex, race/ethnicity, and age, the overall odds ratio for acute hepatitis B among those with diabetes compared with those without was 2.09 in people aged 23-59, which was statistically significant. Among adults aged 60 and older, the odds ratio was 1.45, which was not significant.
The costs per quality-adjusted life year (QALY) saved of vaccinating adults with diabetes (assuming the private vaccine price), was calculated to be $75,094 for adults aged 20-59, compared with $2,8 million among those aged 60 and older, Dr. Schillie reported.
Many more patients with diabetes are sharing glucose monitors in a wide variety of settings, including households in which more than one family member has diabetes, worksite health clinics, schools, and diabetes camps. "We’re seeing more and more use of one glucose meter for multiple people," noted Dr. Pam Allweiss of the CDC’s Division of Diabetes Translation.
Because a large proportion of people with diabetes who are in institutions are older than 60, the ACIP debated whether to simply recommend the hepatitis vaccine for everyone with diabetes, rather than using cost-effectiveness as a guide for the age cutoff.
Prior to the vote, American College of Physicians liaison Dr. Sandra Fryhofer said that the ACP’s Adult Immunization Technical Advisory Committee supported a universal recommendation: "I urge you to recommend routine hepatitis B vaccination for diabetics of all ages," she told the ACIP.
In an interview after the vote, Dr. Fryhofer said of the College’s vaccine advisory committee, "We’re disappointed that we didn’t have a routine hepatitis B immunization recommendation for all diabetics of all ages. We know that diabetes is the 7th leading cause of death in this country, and the data show that if you have diabetes, it increases your risk of getting hepatitis B by 1.5 to 2 times, and it doubles your risk of death." However, she said, it’s unlikely that ACP will issue a different recommendation from ACIP’s.
Internist William Golden, however, disagreed with the idea of a universal approach. "Vaccinating everyone probably should not be a knee jerk reaction. ... One should first check to see if the patient already has antibodies conferring immunity. Secondly, one should inquire or warn about risks of sharing equipment. Make sure the patient has insurance coverage for the injections to avoid unpleasant surprises. Finally, review the need for frequent home glucose checks," he said. "Stable type 2 diabetics on oral medication may not need home checks more than one or two times a week, if at all. So, in short, assess the risks for the patient before offering a one size fits all strategy," said Dr. Golden, professor of medicine and public health at the University of Arkansas for Medical Sciences, Little Rock.
As CDC employees, Dr. Schillie, Dr. Murphy, and Dr. Allweiss have no financial conflicts. Dr. Fryhofer stated that she had no disclosures.
Mitchel Zoler contributed to this report.
ATLANTA – Hepatitis B vaccination should be routinely given to previously unvaccinated adults with diabetes who are younger than 60 years of age, according to recommendations by the Centers for Disease Control’s Advisory Committee on Immunization Practices.
The recommendation is based on the finding that U.S. adults with diabetes patients face a roughly twofold increased risk for hepatitis B infection. Vaccination is advised based on the individual patient’s risk of acquiring hepatitis B infection.
An additional recommendation, which must be published by the CDC before it becomes official, is likely to say that people aged 60 years and older with diabetes who live in nursing homes and assisted living facilities are at increased risk for hepatitis B and should be considered for vaccine prioritization.
Results from CDC investigations identified inadequately cleaned blood glucose monitors as a major route of hepatitis B virus transmission among patients with diabetes, Meredith L. Reilly, a CDC epidemiologist, noted at the earlier annual meeting of the Infectious Diseases Society of America.
"Over the past several years, we’ve observed outbreaks of hepatitis B among patients with diabetes in places where they undergo assisted blood glucose monitoring, with more than one person using the monitor," such as assisted-living facilities, physician offices, and at pharmacies, said Dr. Trudy V. Murphy, head of the vaccine unit in the Division of Viral Hepatitis at the National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention in Atlanta. A total of 24 of 28 outbreaks at long-term care facilities identified different aspects of blood glucose monitoring as the source of the infection."
The advised age cutoff was based on estimates of disease risk and cost effectiveness. An analysis of data from more than 91,000 individuals participating in the CDC’s Emerging Infection Program (EIP) found that among people without traditional risk factors for hepatitis B (use of injectable drugs, unprotected sex with multiple partners), the adjusted odds ratio for acute hepatitis B among those with diabetes was 1.89, compared to those without diabetes, suggesting that diabetes is an independent marker for the infection, according to the CDC’s Dr. Sarah Schillie.
Among people with hepatitis B risk factors, those with diabetes had a 1.1 odds ratio of infection, compared with non-diabetics in that group.
After controlling for sex, race/ethnicity, and age, the overall odds ratio for acute hepatitis B among those with diabetes compared with those without was 2.09 in people aged 23-59, which was statistically significant. Among adults aged 60 and older, the odds ratio was 1.45, which was not significant.
The costs per quality-adjusted life year (QALY) saved of vaccinating adults with diabetes (assuming the private vaccine price), was calculated to be $75,094 for adults aged 20-59, compared with $2,8 million among those aged 60 and older, Dr. Schillie reported.
Many more patients with diabetes are sharing glucose monitors in a wide variety of settings, including households in which more than one family member has diabetes, worksite health clinics, schools, and diabetes camps. "We’re seeing more and more use of one glucose meter for multiple people," noted Dr. Pam Allweiss of the CDC’s Division of Diabetes Translation.
Because a large proportion of people with diabetes who are in institutions are older than 60, the ACIP debated whether to simply recommend the hepatitis vaccine for everyone with diabetes, rather than using cost-effectiveness as a guide for the age cutoff.
Prior to the vote, American College of Physicians liaison Dr. Sandra Fryhofer said that the ACP’s Adult Immunization Technical Advisory Committee supported a universal recommendation: "I urge you to recommend routine hepatitis B vaccination for diabetics of all ages," she told the ACIP.
In an interview after the vote, Dr. Fryhofer said of the College’s vaccine advisory committee, "We’re disappointed that we didn’t have a routine hepatitis B immunization recommendation for all diabetics of all ages. We know that diabetes is the 7th leading cause of death in this country, and the data show that if you have diabetes, it increases your risk of getting hepatitis B by 1.5 to 2 times, and it doubles your risk of death." However, she said, it’s unlikely that ACP will issue a different recommendation from ACIP’s.
Internist William Golden, however, disagreed with the idea of a universal approach. "Vaccinating everyone probably should not be a knee jerk reaction. ... One should first check to see if the patient already has antibodies conferring immunity. Secondly, one should inquire or warn about risks of sharing equipment. Make sure the patient has insurance coverage for the injections to avoid unpleasant surprises. Finally, review the need for frequent home glucose checks," he said. "Stable type 2 diabetics on oral medication may not need home checks more than one or two times a week, if at all. So, in short, assess the risks for the patient before offering a one size fits all strategy," said Dr. Golden, professor of medicine and public health at the University of Arkansas for Medical Sciences, Little Rock.
As CDC employees, Dr. Schillie, Dr. Murphy, and Dr. Allweiss have no financial conflicts. Dr. Fryhofer stated that she had no disclosures.
Mitchel Zoler contributed to this report.
ATLANTA – Hepatitis B vaccination should be routinely given to previously unvaccinated adults with diabetes who are younger than 60 years of age, according to recommendations by the Centers for Disease Control’s Advisory Committee on Immunization Practices.
The recommendation is based on the finding that U.S. adults with diabetes patients face a roughly twofold increased risk for hepatitis B infection. Vaccination is advised based on the individual patient’s risk of acquiring hepatitis B infection.
An additional recommendation, which must be published by the CDC before it becomes official, is likely to say that people aged 60 years and older with diabetes who live in nursing homes and assisted living facilities are at increased risk for hepatitis B and should be considered for vaccine prioritization.
Results from CDC investigations identified inadequately cleaned blood glucose monitors as a major route of hepatitis B virus transmission among patients with diabetes, Meredith L. Reilly, a CDC epidemiologist, noted at the earlier annual meeting of the Infectious Diseases Society of America.
"Over the past several years, we’ve observed outbreaks of hepatitis B among patients with diabetes in places where they undergo assisted blood glucose monitoring, with more than one person using the monitor," such as assisted-living facilities, physician offices, and at pharmacies, said Dr. Trudy V. Murphy, head of the vaccine unit in the Division of Viral Hepatitis at the National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention in Atlanta. A total of 24 of 28 outbreaks at long-term care facilities identified different aspects of blood glucose monitoring as the source of the infection."
The advised age cutoff was based on estimates of disease risk and cost effectiveness. An analysis of data from more than 91,000 individuals participating in the CDC’s Emerging Infection Program (EIP) found that among people without traditional risk factors for hepatitis B (use of injectable drugs, unprotected sex with multiple partners), the adjusted odds ratio for acute hepatitis B among those with diabetes was 1.89, compared to those without diabetes, suggesting that diabetes is an independent marker for the infection, according to the CDC’s Dr. Sarah Schillie.
Among people with hepatitis B risk factors, those with diabetes had a 1.1 odds ratio of infection, compared with non-diabetics in that group.
After controlling for sex, race/ethnicity, and age, the overall odds ratio for acute hepatitis B among those with diabetes compared with those without was 2.09 in people aged 23-59, which was statistically significant. Among adults aged 60 and older, the odds ratio was 1.45, which was not significant.
The costs per quality-adjusted life year (QALY) saved of vaccinating adults with diabetes (assuming the private vaccine price), was calculated to be $75,094 for adults aged 20-59, compared with $2,8 million among those aged 60 and older, Dr. Schillie reported.
Many more patients with diabetes are sharing glucose monitors in a wide variety of settings, including households in which more than one family member has diabetes, worksite health clinics, schools, and diabetes camps. "We’re seeing more and more use of one glucose meter for multiple people," noted Dr. Pam Allweiss of the CDC’s Division of Diabetes Translation.
Because a large proportion of people with diabetes who are in institutions are older than 60, the ACIP debated whether to simply recommend the hepatitis vaccine for everyone with diabetes, rather than using cost-effectiveness as a guide for the age cutoff.
Prior to the vote, American College of Physicians liaison Dr. Sandra Fryhofer said that the ACP’s Adult Immunization Technical Advisory Committee supported a universal recommendation: "I urge you to recommend routine hepatitis B vaccination for diabetics of all ages," she told the ACIP.
In an interview after the vote, Dr. Fryhofer said of the College’s vaccine advisory committee, "We’re disappointed that we didn’t have a routine hepatitis B immunization recommendation for all diabetics of all ages. We know that diabetes is the 7th leading cause of death in this country, and the data show that if you have diabetes, it increases your risk of getting hepatitis B by 1.5 to 2 times, and it doubles your risk of death." However, she said, it’s unlikely that ACP will issue a different recommendation from ACIP’s.
Internist William Golden, however, disagreed with the idea of a universal approach. "Vaccinating everyone probably should not be a knee jerk reaction. ... One should first check to see if the patient already has antibodies conferring immunity. Secondly, one should inquire or warn about risks of sharing equipment. Make sure the patient has insurance coverage for the injections to avoid unpleasant surprises. Finally, review the need for frequent home glucose checks," he said. "Stable type 2 diabetics on oral medication may not need home checks more than one or two times a week, if at all. So, in short, assess the risks for the patient before offering a one size fits all strategy," said Dr. Golden, professor of medicine and public health at the University of Arkansas for Medical Sciences, Little Rock.
As CDC employees, Dr. Schillie, Dr. Murphy, and Dr. Allweiss have no financial conflicts. Dr. Fryhofer stated that she had no disclosures.
Mitchel Zoler contributed to this report.
FROM A MEETING OF THE CDC’S ADVISORY COMMITTEE ON IMMUNIZATION PRACTICES
Hepatitis B Vaccine Recommended for Adults With Diabetes
ATLANTA – Hepatitis B vaccination should be routinely given to previously unvaccinated adults with diabetes who are younger than 60 years of age, according to recommendations by the Centers for Disease Control’s Advisory Committee on Immunization Practices.
The recommendation is based on the finding that U.S. adults with diabetes patients face a roughly twofold increased risk for hepatitis B infection. Vaccination is advised based on the individual patient’s risk of acquiring hepatitis B infection.
An additional recommendation, which must be published by the CDC before it becomes official, is likely to say that people aged 60 years and older with diabetes who live in nursing homes and assisted living facilities are at increased risk for hepatitis B and should be considered for vaccine prioritization.
Results from CDC investigations identified inadequately cleaned blood glucose monitors as a major route of hepatitis B virus transmission among patients with diabetes, Meredith L. Reilly, a CDC epidemiologist, noted at the earlier annual meeting of the Infectious Diseases Society of America.
"Over the past several years, we’ve observed outbreaks of hepatitis B among patients with diabetes in places where they undergo assisted blood glucose monitoring, with more than one person using the monitor," such as assisted-living facilities, physician offices, and at pharmacies, said Dr. Trudy V. Murphy, head of the vaccine unit in the Division of Viral Hepatitis at the National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention in Atlanta. A total of 24 of 28 outbreaks at long-term care facilities identified different aspects of blood glucose monitoring as the source of the infection."
The advised age cutoff was based on estimates of disease risk and cost effectiveness. An analysis of data from more than 91,000 individuals participating in the CDC’s Emerging Infection Program (EIP) found that among people without traditional risk factors for hepatitis B (use of injectable drugs, unprotected sex with multiple partners), the adjusted odds ratio for acute hepatitis B among those with diabetes was 1.89, compared to those without diabetes, suggesting that diabetes is an independent marker for the infection, according to the CDC’s Dr. Sarah Schillie.
Among people with hepatitis B risk factors, those with diabetes had a 1.1 odds ratio of infection, compared with non-diabetics in that group.
After controlling for sex, race/ethnicity, and age, the overall odds ratio for acute hepatitis B among those with diabetes compared with those without was 2.09 in people aged 23-59, which was statistically significant. Among adults aged 60 and older, the odds ratio was 1.45, which was not significant.
The costs per quality-adjusted life year (QALY) saved of vaccinating adults with diabetes (assuming the private vaccine price), was calculated to be $75,094 for adults aged 20-59, compared with $2,8 million among those aged 60 and older, Dr. Schillie reported.
Many more patients with diabetes are sharing glucose monitors in a wide variety of settings, including households in which more than one family member has diabetes, worksite health clinics, schools, and diabetes camps. "We’re seeing more and more use of one glucose meter for multiple people," noted Dr. Pam Allweiss of the CDC’s Division of Diabetes Translation.
Because a large proportion of people with diabetes who are in institutions are older than 60, the ACIP debated whether to simply recommend the hepatitis vaccine for everyone with diabetes, rather than using cost-effectiveness as a guide for the age cutoff.
Prior to the vote, American College of Physicians liaison Dr. Sandra Fryhofer said that the ACP’s Adult Immunization Technical Advisory Committee supported a universal recommendation: "I urge you to recommend routine hepatitis B vaccination for diabetics of all ages," she told the ACIP.
In an interview after the vote, Dr. Fryhofer said of the College’s vaccine advisory committee, "We’re disappointed that we didn’t have a routine hepatitis B immunization recommendation for all diabetics of all ages. We know that diabetes is the 7th leading cause of death in this country, and the data show that if you have diabetes, it increases your risk of getting hepatitis B by 1.5 to 2 times, and it doubles your risk of death." However, she said, it’s unlikely that ACP will issue a different recommendation from ACIP’s.
Internist William Golden, however, disagreed with the idea of a universal approach. "Vaccinating everyone probably should not be a knee jerk reaction. ... One should first check to see if the patient already has antibodies conferring immunity. Secondly, one should inquire or warn about risks of sharing equipment. Make sure the patient has insurance coverage for the injections to avoid unpleasant surprises. Finally, review the need for frequent home glucose checks," he said. "Stable type 2 diabetics on oral medication may not need home checks more than one or two times a week, if at all. So, in short, assess the risks for the patient before offering a one size fits all strategy," said Dr. Golden, professor of medicine and public health at the University of Arkansas for Medical Sciences, Little Rock.
As CDC employees, Dr. Schillie, Dr. Murphy, and Dr. Allweiss have no financial conflicts. Dr. Fryhofer stated that she had no disclosures.
Mitchel Zoler contributed to this report.
ATLANTA – Hepatitis B vaccination should be routinely given to previously unvaccinated adults with diabetes who are younger than 60 years of age, according to recommendations by the Centers for Disease Control’s Advisory Committee on Immunization Practices.
The recommendation is based on the finding that U.S. adults with diabetes patients face a roughly twofold increased risk for hepatitis B infection. Vaccination is advised based on the individual patient’s risk of acquiring hepatitis B infection.
An additional recommendation, which must be published by the CDC before it becomes official, is likely to say that people aged 60 years and older with diabetes who live in nursing homes and assisted living facilities are at increased risk for hepatitis B and should be considered for vaccine prioritization.
Results from CDC investigations identified inadequately cleaned blood glucose monitors as a major route of hepatitis B virus transmission among patients with diabetes, Meredith L. Reilly, a CDC epidemiologist, noted at the earlier annual meeting of the Infectious Diseases Society of America.
"Over the past several years, we’ve observed outbreaks of hepatitis B among patients with diabetes in places where they undergo assisted blood glucose monitoring, with more than one person using the monitor," such as assisted-living facilities, physician offices, and at pharmacies, said Dr. Trudy V. Murphy, head of the vaccine unit in the Division of Viral Hepatitis at the National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention in Atlanta. A total of 24 of 28 outbreaks at long-term care facilities identified different aspects of blood glucose monitoring as the source of the infection."
The advised age cutoff was based on estimates of disease risk and cost effectiveness. An analysis of data from more than 91,000 individuals participating in the CDC’s Emerging Infection Program (EIP) found that among people without traditional risk factors for hepatitis B (use of injectable drugs, unprotected sex with multiple partners), the adjusted odds ratio for acute hepatitis B among those with diabetes was 1.89, compared to those without diabetes, suggesting that diabetes is an independent marker for the infection, according to the CDC’s Dr. Sarah Schillie.
Among people with hepatitis B risk factors, those with diabetes had a 1.1 odds ratio of infection, compared with non-diabetics in that group.
After controlling for sex, race/ethnicity, and age, the overall odds ratio for acute hepatitis B among those with diabetes compared with those without was 2.09 in people aged 23-59, which was statistically significant. Among adults aged 60 and older, the odds ratio was 1.45, which was not significant.
The costs per quality-adjusted life year (QALY) saved of vaccinating adults with diabetes (assuming the private vaccine price), was calculated to be $75,094 for adults aged 20-59, compared with $2,8 million among those aged 60 and older, Dr. Schillie reported.
Many more patients with diabetes are sharing glucose monitors in a wide variety of settings, including households in which more than one family member has diabetes, worksite health clinics, schools, and diabetes camps. "We’re seeing more and more use of one glucose meter for multiple people," noted Dr. Pam Allweiss of the CDC’s Division of Diabetes Translation.
Because a large proportion of people with diabetes who are in institutions are older than 60, the ACIP debated whether to simply recommend the hepatitis vaccine for everyone with diabetes, rather than using cost-effectiveness as a guide for the age cutoff.
Prior to the vote, American College of Physicians liaison Dr. Sandra Fryhofer said that the ACP’s Adult Immunization Technical Advisory Committee supported a universal recommendation: "I urge you to recommend routine hepatitis B vaccination for diabetics of all ages," she told the ACIP.
In an interview after the vote, Dr. Fryhofer said of the College’s vaccine advisory committee, "We’re disappointed that we didn’t have a routine hepatitis B immunization recommendation for all diabetics of all ages. We know that diabetes is the 7th leading cause of death in this country, and the data show that if you have diabetes, it increases your risk of getting hepatitis B by 1.5 to 2 times, and it doubles your risk of death." However, she said, it’s unlikely that ACP will issue a different recommendation from ACIP’s.
Internist William Golden, however, disagreed with the idea of a universal approach. "Vaccinating everyone probably should not be a knee jerk reaction. ... One should first check to see if the patient already has antibodies conferring immunity. Secondly, one should inquire or warn about risks of sharing equipment. Make sure the patient has insurance coverage for the injections to avoid unpleasant surprises. Finally, review the need for frequent home glucose checks," he said. "Stable type 2 diabetics on oral medication may not need home checks more than one or two times a week, if at all. So, in short, assess the risks for the patient before offering a one size fits all strategy," said Dr. Golden, professor of medicine and public health at the University of Arkansas for Medical Sciences, Little Rock.
As CDC employees, Dr. Schillie, Dr. Murphy, and Dr. Allweiss have no financial conflicts. Dr. Fryhofer stated that she had no disclosures.
Mitchel Zoler contributed to this report.
ATLANTA – Hepatitis B vaccination should be routinely given to previously unvaccinated adults with diabetes who are younger than 60 years of age, according to recommendations by the Centers for Disease Control’s Advisory Committee on Immunization Practices.
The recommendation is based on the finding that U.S. adults with diabetes patients face a roughly twofold increased risk for hepatitis B infection. Vaccination is advised based on the individual patient’s risk of acquiring hepatitis B infection.
An additional recommendation, which must be published by the CDC before it becomes official, is likely to say that people aged 60 years and older with diabetes who live in nursing homes and assisted living facilities are at increased risk for hepatitis B and should be considered for vaccine prioritization.
Results from CDC investigations identified inadequately cleaned blood glucose monitors as a major route of hepatitis B virus transmission among patients with diabetes, Meredith L. Reilly, a CDC epidemiologist, noted at the earlier annual meeting of the Infectious Diseases Society of America.
"Over the past several years, we’ve observed outbreaks of hepatitis B among patients with diabetes in places where they undergo assisted blood glucose monitoring, with more than one person using the monitor," such as assisted-living facilities, physician offices, and at pharmacies, said Dr. Trudy V. Murphy, head of the vaccine unit in the Division of Viral Hepatitis at the National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention in Atlanta. A total of 24 of 28 outbreaks at long-term care facilities identified different aspects of blood glucose monitoring as the source of the infection."
The advised age cutoff was based on estimates of disease risk and cost effectiveness. An analysis of data from more than 91,000 individuals participating in the CDC’s Emerging Infection Program (EIP) found that among people without traditional risk factors for hepatitis B (use of injectable drugs, unprotected sex with multiple partners), the adjusted odds ratio for acute hepatitis B among those with diabetes was 1.89, compared to those without diabetes, suggesting that diabetes is an independent marker for the infection, according to the CDC’s Dr. Sarah Schillie.
Among people with hepatitis B risk factors, those with diabetes had a 1.1 odds ratio of infection, compared with non-diabetics in that group.
After controlling for sex, race/ethnicity, and age, the overall odds ratio for acute hepatitis B among those with diabetes compared with those without was 2.09 in people aged 23-59, which was statistically significant. Among adults aged 60 and older, the odds ratio was 1.45, which was not significant.
The costs per quality-adjusted life year (QALY) saved of vaccinating adults with diabetes (assuming the private vaccine price), was calculated to be $75,094 for adults aged 20-59, compared with $2,8 million among those aged 60 and older, Dr. Schillie reported.
Many more patients with diabetes are sharing glucose monitors in a wide variety of settings, including households in which more than one family member has diabetes, worksite health clinics, schools, and diabetes camps. "We’re seeing more and more use of one glucose meter for multiple people," noted Dr. Pam Allweiss of the CDC’s Division of Diabetes Translation.
Because a large proportion of people with diabetes who are in institutions are older than 60, the ACIP debated whether to simply recommend the hepatitis vaccine for everyone with diabetes, rather than using cost-effectiveness as a guide for the age cutoff.
Prior to the vote, American College of Physicians liaison Dr. Sandra Fryhofer said that the ACP’s Adult Immunization Technical Advisory Committee supported a universal recommendation: "I urge you to recommend routine hepatitis B vaccination for diabetics of all ages," she told the ACIP.
In an interview after the vote, Dr. Fryhofer said of the College’s vaccine advisory committee, "We’re disappointed that we didn’t have a routine hepatitis B immunization recommendation for all diabetics of all ages. We know that diabetes is the 7th leading cause of death in this country, and the data show that if you have diabetes, it increases your risk of getting hepatitis B by 1.5 to 2 times, and it doubles your risk of death." However, she said, it’s unlikely that ACP will issue a different recommendation from ACIP’s.
Internist William Golden, however, disagreed with the idea of a universal approach. "Vaccinating everyone probably should not be a knee jerk reaction. ... One should first check to see if the patient already has antibodies conferring immunity. Secondly, one should inquire or warn about risks of sharing equipment. Make sure the patient has insurance coverage for the injections to avoid unpleasant surprises. Finally, review the need for frequent home glucose checks," he said. "Stable type 2 diabetics on oral medication may not need home checks more than one or two times a week, if at all. So, in short, assess the risks for the patient before offering a one size fits all strategy," said Dr. Golden, professor of medicine and public health at the University of Arkansas for Medical Sciences, Little Rock.
As CDC employees, Dr. Schillie, Dr. Murphy, and Dr. Allweiss have no financial conflicts. Dr. Fryhofer stated that she had no disclosures.
Mitchel Zoler contributed to this report.
FROM A MEETING OF THE CDC'S ADVISORY COMMITTEE ON IMMUNIZATION PRACTICES
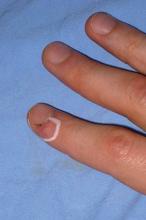
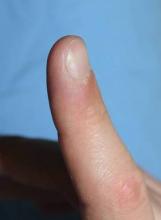
Thermo-Fractional PDT Destroys Persistent Warts
Laser-induced thermotherapy combined with a fractional laser successfully eradicated the majority of persistent warts in a study of 20 patients.
Subclinical infection with human papillomavirus verruca vulgaris infection (due to HPV types 1, 2, and 4) and condyloma acuminata (due to HPV types 6 and 11) often persists after eradication.
Photodynamic therapy (PDT) with topical application of 5-aminolevulinic acid (5-ALA) has previously been shown to be an effective treatment for such warts located in difficult anatomic areas, said Dr. Leonardo Marini, who is in private practice in Trieste, Italy.
However, 5-ALA cannot penetrate deeply into the skin. Therefore, it only has U.S. Food and Drug Administration approval for superficial skin neoplasms, such as multifocal basal cell carcinomas, actinic keratoses, and Bowen's disease.
New strategies for increasing penetration through the stratum corneum include needling and ablative fractional lasers.
Animal data have confirmed the validity of CO2 lasers, showing that transcutaneous microchannels stay open up to 7 hours after a fractional laser procedure. "This opens new horizons for all kinds of topical treatments," Dr. Marini said in an interview.
Thermotherapy performed with an Nd:YAG laser has a damaging effect on viral entities and increases cutaneous reactive circulation, optimizing the conversion of 5-ALA to protoporphyrin IX (PpIX), which is the active light absorber in PDT. "This is why I called the procedure 'thermo-fractional PDT,' " he explained.
His study investigated the sequential combination of 1,064-nm thermotherapy and ablative fractional resurfacing with one of two different types of lasers – CO2 or 2,904-nm Er:YAG – as active trans–stratum corneum penetration enhancers prior to topical application of 5-ALA gel and followed by light-emitting diode (LED) irradiation, he said.
The 10 men and 10 women with resistant plantar and/or digital warts had a mean age of 21 years (range 12-37 years) and Fitzpatrick skin types II-III.
Initially, all the patients' lesions (a total of 57) were treated with full-field, 2,940-nm Er:YAG laser photovaporization to eliminate secondary hyperkeratotic growths.
Then, pre-ALA thermotherapy was performed with three sequential passes of a long-pulse 1064-nm Nd:YAG laser (3-mm spot, 35 ms, 50 J/cm2).
Next, 32 lesions were pretreated with the 2,940-nm Er:YAG laser (0.250-mm spot, 0.175 ms, 12 J/ cm2), while the other 25 warts were treated with a 10.600-nm CO2 ablative fractional laser (0.300-mm spot, 0.900 ms, 14 W).
Ten percent 5-ALA liposome gel was then immediately applied to all lesions and occluded under polyurethane film for 6 hours. Digital blocks and/or infiltration anesthesia with 2% plain mepivacaine were given, and all treated areas received irradiation with a 633-nm LED divided in two sequential exposures of 4 and 16 minutes, separated by a 20-minute interval, Dr. Marini reported.
All of the patients experienced intense post-PDT activation pain immediately after fading of local anesthesia, with a peak level reported at 24 hours postoperatively. Nearly three-quarters of the patients required oral painkillers. The pain progressively faded over the next 48 hours, he noted.
Clinical eradication was achieved at 3 months in 90% of lesions.
No differences were observed between the two different fractional lasers as trans–corneal-intradermal penetration enhancers. No postoperative scarring or infections were observed. Transitory postinflammatory hyperpigmentation occurred on the dorsal hands of two patients in the Er:YAG laser group and two in the CO2 laser group.
No clinical recurrences were observed at 6 months postoperatively.
More studies are needed to determine the optimal treatment timing and sequence, Dr. Marini concluded.
He presented his findings at the annual meeting of the American Society for Laser Medicine and Surgery.
Dr. Marini stated that Deka and Fotona contributed equipment for the study. He had no other conflicts of interest to disclose.
Laser-induced thermotherapy combined with a fractional laser successfully eradicated the majority of persistent warts in a study of 20 patients.
Subclinical infection with human papillomavirus verruca vulgaris infection (due to HPV types 1, 2, and 4) and condyloma acuminata (due to HPV types 6 and 11) often persists after eradication.
Photodynamic therapy (PDT) with topical application of 5-aminolevulinic acid (5-ALA) has previously been shown to be an effective treatment for such warts located in difficult anatomic areas, said Dr. Leonardo Marini, who is in private practice in Trieste, Italy.
However, 5-ALA cannot penetrate deeply into the skin. Therefore, it only has U.S. Food and Drug Administration approval for superficial skin neoplasms, such as multifocal basal cell carcinomas, actinic keratoses, and Bowen's disease.
New strategies for increasing penetration through the stratum corneum include needling and ablative fractional lasers.
Animal data have confirmed the validity of CO2 lasers, showing that transcutaneous microchannels stay open up to 7 hours after a fractional laser procedure. "This opens new horizons for all kinds of topical treatments," Dr. Marini said in an interview.
Thermotherapy performed with an Nd:YAG laser has a damaging effect on viral entities and increases cutaneous reactive circulation, optimizing the conversion of 5-ALA to protoporphyrin IX (PpIX), which is the active light absorber in PDT. "This is why I called the procedure 'thermo-fractional PDT,' " he explained.
His study investigated the sequential combination of 1,064-nm thermotherapy and ablative fractional resurfacing with one of two different types of lasers – CO2 or 2,904-nm Er:YAG – as active trans–stratum corneum penetration enhancers prior to topical application of 5-ALA gel and followed by light-emitting diode (LED) irradiation, he said.
The 10 men and 10 women with resistant plantar and/or digital warts had a mean age of 21 years (range 12-37 years) and Fitzpatrick skin types II-III.
Initially, all the patients' lesions (a total of 57) were treated with full-field, 2,940-nm Er:YAG laser photovaporization to eliminate secondary hyperkeratotic growths.
Then, pre-ALA thermotherapy was performed with three sequential passes of a long-pulse 1064-nm Nd:YAG laser (3-mm spot, 35 ms, 50 J/cm2).
Next, 32 lesions were pretreated with the 2,940-nm Er:YAG laser (0.250-mm spot, 0.175 ms, 12 J/ cm2), while the other 25 warts were treated with a 10.600-nm CO2 ablative fractional laser (0.300-mm spot, 0.900 ms, 14 W).
Ten percent 5-ALA liposome gel was then immediately applied to all lesions and occluded under polyurethane film for 6 hours. Digital blocks and/or infiltration anesthesia with 2% plain mepivacaine were given, and all treated areas received irradiation with a 633-nm LED divided in two sequential exposures of 4 and 16 minutes, separated by a 20-minute interval, Dr. Marini reported.
All of the patients experienced intense post-PDT activation pain immediately after fading of local anesthesia, with a peak level reported at 24 hours postoperatively. Nearly three-quarters of the patients required oral painkillers. The pain progressively faded over the next 48 hours, he noted.
Clinical eradication was achieved at 3 months in 90% of lesions.
No differences were observed between the two different fractional lasers as trans–corneal-intradermal penetration enhancers. No postoperative scarring or infections were observed. Transitory postinflammatory hyperpigmentation occurred on the dorsal hands of two patients in the Er:YAG laser group and two in the CO2 laser group.
No clinical recurrences were observed at 6 months postoperatively.
More studies are needed to determine the optimal treatment timing and sequence, Dr. Marini concluded.
He presented his findings at the annual meeting of the American Society for Laser Medicine and Surgery.
Dr. Marini stated that Deka and Fotona contributed equipment for the study. He had no other conflicts of interest to disclose.
Laser-induced thermotherapy combined with a fractional laser successfully eradicated the majority of persistent warts in a study of 20 patients.
Subclinical infection with human papillomavirus verruca vulgaris infection (due to HPV types 1, 2, and 4) and condyloma acuminata (due to HPV types 6 and 11) often persists after eradication.
Photodynamic therapy (PDT) with topical application of 5-aminolevulinic acid (5-ALA) has previously been shown to be an effective treatment for such warts located in difficult anatomic areas, said Dr. Leonardo Marini, who is in private practice in Trieste, Italy.
However, 5-ALA cannot penetrate deeply into the skin. Therefore, it only has U.S. Food and Drug Administration approval for superficial skin neoplasms, such as multifocal basal cell carcinomas, actinic keratoses, and Bowen's disease.
New strategies for increasing penetration through the stratum corneum include needling and ablative fractional lasers.
Animal data have confirmed the validity of CO2 lasers, showing that transcutaneous microchannels stay open up to 7 hours after a fractional laser procedure. "This opens new horizons for all kinds of topical treatments," Dr. Marini said in an interview.
Thermotherapy performed with an Nd:YAG laser has a damaging effect on viral entities and increases cutaneous reactive circulation, optimizing the conversion of 5-ALA to protoporphyrin IX (PpIX), which is the active light absorber in PDT. "This is why I called the procedure 'thermo-fractional PDT,' " he explained.
His study investigated the sequential combination of 1,064-nm thermotherapy and ablative fractional resurfacing with one of two different types of lasers – CO2 or 2,904-nm Er:YAG – as active trans–stratum corneum penetration enhancers prior to topical application of 5-ALA gel and followed by light-emitting diode (LED) irradiation, he said.
The 10 men and 10 women with resistant plantar and/or digital warts had a mean age of 21 years (range 12-37 years) and Fitzpatrick skin types II-III.
Initially, all the patients' lesions (a total of 57) were treated with full-field, 2,940-nm Er:YAG laser photovaporization to eliminate secondary hyperkeratotic growths.
Then, pre-ALA thermotherapy was performed with three sequential passes of a long-pulse 1064-nm Nd:YAG laser (3-mm spot, 35 ms, 50 J/cm2).
Next, 32 lesions were pretreated with the 2,940-nm Er:YAG laser (0.250-mm spot, 0.175 ms, 12 J/ cm2), while the other 25 warts were treated with a 10.600-nm CO2 ablative fractional laser (0.300-mm spot, 0.900 ms, 14 W).
Ten percent 5-ALA liposome gel was then immediately applied to all lesions and occluded under polyurethane film for 6 hours. Digital blocks and/or infiltration anesthesia with 2% plain mepivacaine were given, and all treated areas received irradiation with a 633-nm LED divided in two sequential exposures of 4 and 16 minutes, separated by a 20-minute interval, Dr. Marini reported.
All of the patients experienced intense post-PDT activation pain immediately after fading of local anesthesia, with a peak level reported at 24 hours postoperatively. Nearly three-quarters of the patients required oral painkillers. The pain progressively faded over the next 48 hours, he noted.
Clinical eradication was achieved at 3 months in 90% of lesions.
No differences were observed between the two different fractional lasers as trans–corneal-intradermal penetration enhancers. No postoperative scarring or infections were observed. Transitory postinflammatory hyperpigmentation occurred on the dorsal hands of two patients in the Er:YAG laser group and two in the CO2 laser group.
No clinical recurrences were observed at 6 months postoperatively.
More studies are needed to determine the optimal treatment timing and sequence, Dr. Marini concluded.
He presented his findings at the annual meeting of the American Society for Laser Medicine and Surgery.
Dr. Marini stated that Deka and Fotona contributed equipment for the study. He had no other conflicts of interest to disclose.
Management of AD in Children Requires Broad Approach, Expert Says
The management of atopic dermatitis in children requires a comprehensive approach, as with any difficult chronic disease, according to Dr. Moise L. Levy.
"Atopic dermatitis [AD] is no different than any other chronic disease, including the psychosocial issues. It must be approached broadly. Whatever support the child and family need, you provide it," said Dr. Levy, chief of pediatric dermatology at Dell Children's Medical Center, Austin, Texas.
Ongoing education of the family about the importance of maintaining compliance with emollient and topical treatment is essential. Written "action plans" similar to those used with asthma patients can be helpful, Dr. Levy said at the annual Hawaii Dermatology Seminar sponsored by Skin Disease Education Foundation.
Recent evidence has highlighted the interaction between bacterial colonization - not active infection - and worsening of AD symptoms. Interleukins produced during active inflammation of AD appear to impair normal defenses against skin infection, he explained.
One study provides a rationale for the use of bleach baths in children with AD who are colonized with methicillin-resistant Staphylococcus aureus. Thirty-one children aged 6 months to 17 years with moderate to severe AD and clinical signs of bacterial infection received cephalexin for 2 weeks, then were randomized to intranasal mupirocin ointment and twice-weekly sodium hypochlorite baths (½ cup in 40 gallons) or a placebo of intranasal petrolatum plus plain water baths (Pediatrics 2009;123:e808-14).
Eczema Area and Severity Index (EASI) scores were improved at 1 and 3 months in the treatment arm, compared with the placebo group, in all submerged sites (not the head and neck), Dr. Levy reported.
Topical corticosteroids and calcineurin inhibitors can provide excellent control of moderate to severe AD flares, but data also suggest intermittent dosing may help prevent flares in the first place in patients with chronic relapsing disease.
In a two-phase study, 206 children aged 2-15 years with moderate to severe AD were randomized to aclometasone or tacrolimus for 4 days, and then received open-label tacrolimus twice daily for up to 16 weeks. After that, 105 children were randomized to application of either tacrolimus or a vehicle three times per week to clinically normal-appearing skin for up to 40 weeks.
The aclometasone group had better improvement during the acute phase, but there were no differences thereafter. In the long-term phase, the tacrolimus group had more disease-free days compared with the vehicle group, longer time to relapse, and fewer relapse days (Pediatrics 2008;122:e1210-8).
The use of systemic therapies should be reserved for children with the most severe AD, and considered only after aggressive topical therapy has been maximized and compliance with systemics can be ensured, Dr. Levy emphasized.
Currently, cyclosporine is the only one of these agents with sufficient pediatric efficacy and safety data to support its use in children with AD, and still must be used with caution. One pediatric study found that oral cyclosporine use in combination with topical corticosteroids was associated with greater loss of bone mass than was use of corticosteroids alone (Pediatr. Dermatol. 2007;24:613-20).
Although there is a large body of anecdotal evidence supporting the efficacy of other systemic agents such as mycophenolate mofetil, methotrexate, azathioprine, and thiopurine methyltransferase, at this time there are insufficient randomized, prospective clinical data in children with AD to support their use, he said.
The management of AD in children can be extremely challenging. At times, hospitalization and referral to a psychologist may be necessary to address the educational and psychosocial issues that prevent patients and families from maintaining long-term compliance.
One very helpful "treatment" is the American Academy of Dermatology's Camp Discovery (www.campdiscovery.org), where kids with chronic skin disorders can meet, share experiences, and feel "normal." All kids with AD should be offered the chance to go, Dr. Levy said.
Wrist with erythema, lichenification, and crusting (Photo Courtesy: Dr. Moise L. Levy)
He disclosed being on the advisory board of SkinMedica Inc. SDEF and this news organization are owned by Elsevier.
The management of atopic dermatitis in children requires a comprehensive approach, as with any difficult chronic disease, according to Dr. Moise L. Levy.
"Atopic dermatitis [AD] is no different than any other chronic disease, including the psychosocial issues. It must be approached broadly. Whatever support the child and family need, you provide it," said Dr. Levy, chief of pediatric dermatology at Dell Children's Medical Center, Austin, Texas.
Ongoing education of the family about the importance of maintaining compliance with emollient and topical treatment is essential. Written "action plans" similar to those used with asthma patients can be helpful, Dr. Levy said at the annual Hawaii Dermatology Seminar sponsored by Skin Disease Education Foundation.
Recent evidence has highlighted the interaction between bacterial colonization - not active infection - and worsening of AD symptoms. Interleukins produced during active inflammation of AD appear to impair normal defenses against skin infection, he explained.
One study provides a rationale for the use of bleach baths in children with AD who are colonized with methicillin-resistant Staphylococcus aureus. Thirty-one children aged 6 months to 17 years with moderate to severe AD and clinical signs of bacterial infection received cephalexin for 2 weeks, then were randomized to intranasal mupirocin ointment and twice-weekly sodium hypochlorite baths (½ cup in 40 gallons) or a placebo of intranasal petrolatum plus plain water baths (Pediatrics 2009;123:e808-14).
Eczema Area and Severity Index (EASI) scores were improved at 1 and 3 months in the treatment arm, compared with the placebo group, in all submerged sites (not the head and neck), Dr. Levy reported.
Topical corticosteroids and calcineurin inhibitors can provide excellent control of moderate to severe AD flares, but data also suggest intermittent dosing may help prevent flares in the first place in patients with chronic relapsing disease.
In a two-phase study, 206 children aged 2-15 years with moderate to severe AD were randomized to aclometasone or tacrolimus for 4 days, and then received open-label tacrolimus twice daily for up to 16 weeks. After that, 105 children were randomized to application of either tacrolimus or a vehicle three times per week to clinically normal-appearing skin for up to 40 weeks.
The aclometasone group had better improvement during the acute phase, but there were no differences thereafter. In the long-term phase, the tacrolimus group had more disease-free days compared with the vehicle group, longer time to relapse, and fewer relapse days (Pediatrics 2008;122:e1210-8).
The use of systemic therapies should be reserved for children with the most severe AD, and considered only after aggressive topical therapy has been maximized and compliance with systemics can be ensured, Dr. Levy emphasized.
Currently, cyclosporine is the only one of these agents with sufficient pediatric efficacy and safety data to support its use in children with AD, and still must be used with caution. One pediatric study found that oral cyclosporine use in combination with topical corticosteroids was associated with greater loss of bone mass than was use of corticosteroids alone (Pediatr. Dermatol. 2007;24:613-20).
Although there is a large body of anecdotal evidence supporting the efficacy of other systemic agents such as mycophenolate mofetil, methotrexate, azathioprine, and thiopurine methyltransferase, at this time there are insufficient randomized, prospective clinical data in children with AD to support their use, he said.
The management of AD in children can be extremely challenging. At times, hospitalization and referral to a psychologist may be necessary to address the educational and psychosocial issues that prevent patients and families from maintaining long-term compliance.
One very helpful "treatment" is the American Academy of Dermatology's Camp Discovery (www.campdiscovery.org), where kids with chronic skin disorders can meet, share experiences, and feel "normal." All kids with AD should be offered the chance to go, Dr. Levy said.
Wrist with erythema, lichenification, and crusting (Photo Courtesy: Dr. Moise L. Levy)
He disclosed being on the advisory board of SkinMedica Inc. SDEF and this news organization are owned by Elsevier.
The management of atopic dermatitis in children requires a comprehensive approach, as with any difficult chronic disease, according to Dr. Moise L. Levy.
"Atopic dermatitis [AD] is no different than any other chronic disease, including the psychosocial issues. It must be approached broadly. Whatever support the child and family need, you provide it," said Dr. Levy, chief of pediatric dermatology at Dell Children's Medical Center, Austin, Texas.
Ongoing education of the family about the importance of maintaining compliance with emollient and topical treatment is essential. Written "action plans" similar to those used with asthma patients can be helpful, Dr. Levy said at the annual Hawaii Dermatology Seminar sponsored by Skin Disease Education Foundation.
Recent evidence has highlighted the interaction between bacterial colonization - not active infection - and worsening of AD symptoms. Interleukins produced during active inflammation of AD appear to impair normal defenses against skin infection, he explained.
One study provides a rationale for the use of bleach baths in children with AD who are colonized with methicillin-resistant Staphylococcus aureus. Thirty-one children aged 6 months to 17 years with moderate to severe AD and clinical signs of bacterial infection received cephalexin for 2 weeks, then were randomized to intranasal mupirocin ointment and twice-weekly sodium hypochlorite baths (½ cup in 40 gallons) or a placebo of intranasal petrolatum plus plain water baths (Pediatrics 2009;123:e808-14).
Eczema Area and Severity Index (EASI) scores were improved at 1 and 3 months in the treatment arm, compared with the placebo group, in all submerged sites (not the head and neck), Dr. Levy reported.
Topical corticosteroids and calcineurin inhibitors can provide excellent control of moderate to severe AD flares, but data also suggest intermittent dosing may help prevent flares in the first place in patients with chronic relapsing disease.
In a two-phase study, 206 children aged 2-15 years with moderate to severe AD were randomized to aclometasone or tacrolimus for 4 days, and then received open-label tacrolimus twice daily for up to 16 weeks. After that, 105 children were randomized to application of either tacrolimus or a vehicle three times per week to clinically normal-appearing skin for up to 40 weeks.
The aclometasone group had better improvement during the acute phase, but there were no differences thereafter. In the long-term phase, the tacrolimus group had more disease-free days compared with the vehicle group, longer time to relapse, and fewer relapse days (Pediatrics 2008;122:e1210-8).
The use of systemic therapies should be reserved for children with the most severe AD, and considered only after aggressive topical therapy has been maximized and compliance with systemics can be ensured, Dr. Levy emphasized.
Currently, cyclosporine is the only one of these agents with sufficient pediatric efficacy and safety data to support its use in children with AD, and still must be used with caution. One pediatric study found that oral cyclosporine use in combination with topical corticosteroids was associated with greater loss of bone mass than was use of corticosteroids alone (Pediatr. Dermatol. 2007;24:613-20).
Although there is a large body of anecdotal evidence supporting the efficacy of other systemic agents such as mycophenolate mofetil, methotrexate, azathioprine, and thiopurine methyltransferase, at this time there are insufficient randomized, prospective clinical data in children with AD to support their use, he said.
The management of AD in children can be extremely challenging. At times, hospitalization and referral to a psychologist may be necessary to address the educational and psychosocial issues that prevent patients and families from maintaining long-term compliance.
One very helpful "treatment" is the American Academy of Dermatology's Camp Discovery (www.campdiscovery.org), where kids with chronic skin disorders can meet, share experiences, and feel "normal." All kids with AD should be offered the chance to go, Dr. Levy said.
Wrist with erythema, lichenification, and crusting (Photo Courtesy: Dr. Moise L. Levy)
He disclosed being on the advisory board of SkinMedica Inc. SDEF and this news organization are owned by Elsevier.
Screen for CVD Risk in Mentally Ill Patients
VIENNA — A new joint position statement from three European medical organizations is aimed at reducing cardiovascular disease risk and improving diabetes care in people with severe mental illness, as well as improving their overall health.
The statement is from the European Psychiatric Association and supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (www.em-consulte.com/article/223719
People with severe mental illnesses (SMI), including schizophrenia, depression, and bipolar disorder, have worse physical health and reduced life expectancy, compared with the general population. Data suggest that they die years prematurely. It's also much harder for these individuals to access health care services, statement coauthor Dr. Richard Holt said at the briefing.
People with SMI are more likely to be overweight, to smoke, and to have diabetes, hypertension, and dyslipidemia. Antipsychotic medications also can induce weight gain and worsen other metabolic CVD risk factors, he noted.
“The problem is that as well as the devastating effects of SMI, people with bipolar disorder and schizophrenia die on average 10–20 years earlier than the general population,” said Dr. Holt, of the department of endocrinology and metabolism, University of Southampton, England.
Because of the reduced access to physical health care services, the rate of screening for diabetes and CVD is significantly lower than that in the general population. In fact, while about 20% of diabetes in the general population is undiagnosed, that rate is about 70% among people with mental illness. People with mental illness also have high rates of untreated dyslipidemia and hypertension, Dr. Holt noted.
With this new statement, “we have an opportunity here of bringing together psychiatry and physical health services—in both primary care and secondary care—to try to address this problem, to increase the amount of screening for CV risk factors, to identify individuals at high risk, and to then come together with a strategy to treat them,” he said.
Establishing baseline CVD risk at initial presentation is advised, and recommendations are given for assessment of medical history and examination of all CVD risk factors, including lipids, glucose, smoking habit, and blood pressure. Electrocardiography also is recommended. Monitoring should be carried out at regular intervals, depending on the patient's individual risk level. Weight should be closely monitored in patients taking psychotropic medications, the document advises.
Psychiatric centers and diabetes centers should cooperate in the care of patients with SMI and diabetes. A diabetes nurse-educator also should be involved in the care of those on insulin. The document also outlines management of blood lipids and blood pressure, along with smoking cessation counseling.
The choice of psychotropic medications should take into account the potential effects of the agent on CVD risk factors, particularly in patients who are overweight or obese. A dilemma may arise with clozapine, which is recommended by many guidelines as the antipsychotic of choice for patients with refractory schizophrenia, but it is also the antipsychotic associated with the highest risk of weight gain and related CVD risk factors, the document says.
In the United States, a similar set of recommendations from the National Association of State Mental Health Program Directors (NASMHPD) was issued in 2006 and 2008 (www.nasmhpd.org
In an interview, Dr. Joe Parks, the lead author of the NASMHPD papers, said the problem in the United States is that it's often not clear who is responsible for ensuring that evidence-based standards of care are provided.
“Are the recommendations the responsibility of the individual health care provider? Or the local health care organizations such as hospitals and clinics? Or the payers such as private insurers and Medicaid? Because of this lack of accountability, implementation has been fragmented and spotty,” said Dr. Parks, medical director for the Missouri Department of Mental Health, Jefferson City.
Some states have made progress. The New York State Department of Mental Health, for example, has implemented tracking of obesity and blood pressure in its state-operated hospitals and clinics. In Missouri, the Department of Mental Health has just begun to require and fund annual screening and some interventions for obesity, blood pressure, diabetes, high cholesterol, and dyslipidemia in its programs serving people with mental illness.
Several private insurers encourage and attempt to incentivize clinics and individual providers to follow these recommendations, but Dr. Parks said he is unaware of any that actually require compliance.
“If we want to see widespread adoption of these kind of evidence-based standards of care, then the payers—whether private or governmental—need to require it in their contracts.
“Within our current system if you really want something to happen routinely and systematically throughout the health care delivery system, there must be either a legal requirement or financial incentives. Everything else is just wishful thinking,” Dr. Parks said.
'People with bipolar disorder and schizophrenia die on average 10–20 years earlier.'
Source Dr. Holt
'Because of this lack of accountability, implementation has been fragmented and spotty.'
Source Dr. Parks
VIENNA — A new joint position statement from three European medical organizations is aimed at reducing cardiovascular disease risk and improving diabetes care in people with severe mental illness, as well as improving their overall health.
The statement is from the European Psychiatric Association and supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (www.em-consulte.com/article/223719
People with severe mental illnesses (SMI), including schizophrenia, depression, and bipolar disorder, have worse physical health and reduced life expectancy, compared with the general population. Data suggest that they die years prematurely. It's also much harder for these individuals to access health care services, statement coauthor Dr. Richard Holt said at the briefing.
People with SMI are more likely to be overweight, to smoke, and to have diabetes, hypertension, and dyslipidemia. Antipsychotic medications also can induce weight gain and worsen other metabolic CVD risk factors, he noted.
“The problem is that as well as the devastating effects of SMI, people with bipolar disorder and schizophrenia die on average 10–20 years earlier than the general population,” said Dr. Holt, of the department of endocrinology and metabolism, University of Southampton, England.
Because of the reduced access to physical health care services, the rate of screening for diabetes and CVD is significantly lower than that in the general population. In fact, while about 20% of diabetes in the general population is undiagnosed, that rate is about 70% among people with mental illness. People with mental illness also have high rates of untreated dyslipidemia and hypertension, Dr. Holt noted.
With this new statement, “we have an opportunity here of bringing together psychiatry and physical health services—in both primary care and secondary care—to try to address this problem, to increase the amount of screening for CV risk factors, to identify individuals at high risk, and to then come together with a strategy to treat them,” he said.
Establishing baseline CVD risk at initial presentation is advised, and recommendations are given for assessment of medical history and examination of all CVD risk factors, including lipids, glucose, smoking habit, and blood pressure. Electrocardiography also is recommended. Monitoring should be carried out at regular intervals, depending on the patient's individual risk level. Weight should be closely monitored in patients taking psychotropic medications, the document advises.
Psychiatric centers and diabetes centers should cooperate in the care of patients with SMI and diabetes. A diabetes nurse-educator also should be involved in the care of those on insulin. The document also outlines management of blood lipids and blood pressure, along with smoking cessation counseling.
The choice of psychotropic medications should take into account the potential effects of the agent on CVD risk factors, particularly in patients who are overweight or obese. A dilemma may arise with clozapine, which is recommended by many guidelines as the antipsychotic of choice for patients with refractory schizophrenia, but it is also the antipsychotic associated with the highest risk of weight gain and related CVD risk factors, the document says.
In the United States, a similar set of recommendations from the National Association of State Mental Health Program Directors (NASMHPD) was issued in 2006 and 2008 (www.nasmhpd.org
In an interview, Dr. Joe Parks, the lead author of the NASMHPD papers, said the problem in the United States is that it's often not clear who is responsible for ensuring that evidence-based standards of care are provided.
“Are the recommendations the responsibility of the individual health care provider? Or the local health care organizations such as hospitals and clinics? Or the payers such as private insurers and Medicaid? Because of this lack of accountability, implementation has been fragmented and spotty,” said Dr. Parks, medical director for the Missouri Department of Mental Health, Jefferson City.
Some states have made progress. The New York State Department of Mental Health, for example, has implemented tracking of obesity and blood pressure in its state-operated hospitals and clinics. In Missouri, the Department of Mental Health has just begun to require and fund annual screening and some interventions for obesity, blood pressure, diabetes, high cholesterol, and dyslipidemia in its programs serving people with mental illness.
Several private insurers encourage and attempt to incentivize clinics and individual providers to follow these recommendations, but Dr. Parks said he is unaware of any that actually require compliance.
“If we want to see widespread adoption of these kind of evidence-based standards of care, then the payers—whether private or governmental—need to require it in their contracts.
“Within our current system if you really want something to happen routinely and systematically throughout the health care delivery system, there must be either a legal requirement or financial incentives. Everything else is just wishful thinking,” Dr. Parks said.
'People with bipolar disorder and schizophrenia die on average 10–20 years earlier.'
Source Dr. Holt
'Because of this lack of accountability, implementation has been fragmented and spotty.'
Source Dr. Parks
VIENNA — A new joint position statement from three European medical organizations is aimed at reducing cardiovascular disease risk and improving diabetes care in people with severe mental illness, as well as improving their overall health.
The statement is from the European Psychiatric Association and supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (www.em-consulte.com/article/223719
People with severe mental illnesses (SMI), including schizophrenia, depression, and bipolar disorder, have worse physical health and reduced life expectancy, compared with the general population. Data suggest that they die years prematurely. It's also much harder for these individuals to access health care services, statement coauthor Dr. Richard Holt said at the briefing.
People with SMI are more likely to be overweight, to smoke, and to have diabetes, hypertension, and dyslipidemia. Antipsychotic medications also can induce weight gain and worsen other metabolic CVD risk factors, he noted.
“The problem is that as well as the devastating effects of SMI, people with bipolar disorder and schizophrenia die on average 10–20 years earlier than the general population,” said Dr. Holt, of the department of endocrinology and metabolism, University of Southampton, England.
Because of the reduced access to physical health care services, the rate of screening for diabetes and CVD is significantly lower than that in the general population. In fact, while about 20% of diabetes in the general population is undiagnosed, that rate is about 70% among people with mental illness. People with mental illness also have high rates of untreated dyslipidemia and hypertension, Dr. Holt noted.
With this new statement, “we have an opportunity here of bringing together psychiatry and physical health services—in both primary care and secondary care—to try to address this problem, to increase the amount of screening for CV risk factors, to identify individuals at high risk, and to then come together with a strategy to treat them,” he said.
Establishing baseline CVD risk at initial presentation is advised, and recommendations are given for assessment of medical history and examination of all CVD risk factors, including lipids, glucose, smoking habit, and blood pressure. Electrocardiography also is recommended. Monitoring should be carried out at regular intervals, depending on the patient's individual risk level. Weight should be closely monitored in patients taking psychotropic medications, the document advises.
Psychiatric centers and diabetes centers should cooperate in the care of patients with SMI and diabetes. A diabetes nurse-educator also should be involved in the care of those on insulin. The document also outlines management of blood lipids and blood pressure, along with smoking cessation counseling.
The choice of psychotropic medications should take into account the potential effects of the agent on CVD risk factors, particularly in patients who are overweight or obese. A dilemma may arise with clozapine, which is recommended by many guidelines as the antipsychotic of choice for patients with refractory schizophrenia, but it is also the antipsychotic associated with the highest risk of weight gain and related CVD risk factors, the document says.
In the United States, a similar set of recommendations from the National Association of State Mental Health Program Directors (NASMHPD) was issued in 2006 and 2008 (www.nasmhpd.org
In an interview, Dr. Joe Parks, the lead author of the NASMHPD papers, said the problem in the United States is that it's often not clear who is responsible for ensuring that evidence-based standards of care are provided.
“Are the recommendations the responsibility of the individual health care provider? Or the local health care organizations such as hospitals and clinics? Or the payers such as private insurers and Medicaid? Because of this lack of accountability, implementation has been fragmented and spotty,” said Dr. Parks, medical director for the Missouri Department of Mental Health, Jefferson City.
Some states have made progress. The New York State Department of Mental Health, for example, has implemented tracking of obesity and blood pressure in its state-operated hospitals and clinics. In Missouri, the Department of Mental Health has just begun to require and fund annual screening and some interventions for obesity, blood pressure, diabetes, high cholesterol, and dyslipidemia in its programs serving people with mental illness.
Several private insurers encourage and attempt to incentivize clinics and individual providers to follow these recommendations, but Dr. Parks said he is unaware of any that actually require compliance.
“If we want to see widespread adoption of these kind of evidence-based standards of care, then the payers—whether private or governmental—need to require it in their contracts.
“Within our current system if you really want something to happen routinely and systematically throughout the health care delivery system, there must be either a legal requirement or financial incentives. Everything else is just wishful thinking,” Dr. Parks said.
'People with bipolar disorder and schizophrenia die on average 10–20 years earlier.'
Source Dr. Holt
'Because of this lack of accountability, implementation has been fragmented and spotty.'
Source Dr. Parks
Sleep Apnea Associated With Hyperglycemia in Diabetes
COPENHAGEN — Sleep apnea appears to have an immediate elevating effect on nighttime blood glucose levels in people with concomitant type 2 diabetes, Dr. Maria Pallayova said at the annual meeting of the European Association for the Study of Diabetes.
Previous studies have documented the independent association between sleep-disordered breathing (SDB) and abnormal glucose metabolism. However, the findings of this study, which used continuous glucose monitoring, provide a closer look at the immediate glycemic response to apneic episodes.
Medtronic/Minimed's continuous glucose monitoring system (CGMS) was used for several days in 30 patients with type 2 diabetes on diet or oral hypoglycemic therapy. Eight of the patients had severe SDB and a mean hemoglobin A1c level of 7.4%. The other 22, who did not have SDB, had a mean HbA1c level of 6.5%. Those with SDB were referred to a sleep laboratory for overnight polysomnography, and the CGMS data were compared between the two groups, said Dr. Pallayova of the Pavol Jozef Safarik University, Kosice, Slovakia.
In the group without SDB, the CGMS revealed stable normoglycemia throughout the night. In contrast, those with severe untreated SDB had frequent episodes of sleep apnea/hypopnea (mean apnea-hypopnea index 57.64 episodes/hour) with severe oxygen desaturation (oxygen saturation 82.5%, minimal oxygen saturation 49.13%), followed by significant increases in blood glucose of up to 12.3 mmol/L (221 mg/dL).
The nocturnal increment in blood glucose was 1.11 mmol/L (19.98 mg/dL) in the SDB group, significantly greater than the value of 0.2 mmol/L (3.6 mg/dL) seen in the patients without SDB, and was strongly correlated with severe oxygen desaturation. The peak of apnea-induced hyperglycemia tended to occur within 1 hour of severe oxygen desaturation, and the hyperglycemia lasted for a mean of 48 minutes post hypoxia before returning to normal, Dr. Pallayova noted.
The researchers found significant differences in both overall mean nocturnal glucose values—8.24 mmol/L (148.3 mg/dL) in the severe SDB group, compared with 6.15 mmol/L (110 mg/dL) in those without sleep apnea—and morning fasting glucose levels (8.01 vs. 6.6 mmol/L [144.2 vs. 118.8 mg/dL]).
However, there were no significant differences between the groups in daytime CGMS glucose levels, and no associations were seen between arousal frequency and nocturnal hyperglycemia, she reported.
“Obstructive sleep apnea is not only obstruction; it is a cardiovascular and metabolic nightmare,” Dr. Pallayova said.
“We have to be aware of the fact that untreated sleep apnea may adversely influence glucose control and contribute to the development of late diabetes complications,” she added. “Both sleep-disordered breathing and diabetes mellitus are common, serious, treatable, and underdiagnosed, and they require a high index of suspicion.”
COPENHAGEN — Sleep apnea appears to have an immediate elevating effect on nighttime blood glucose levels in people with concomitant type 2 diabetes, Dr. Maria Pallayova said at the annual meeting of the European Association for the Study of Diabetes.
Previous studies have documented the independent association between sleep-disordered breathing (SDB) and abnormal glucose metabolism. However, the findings of this study, which used continuous glucose monitoring, provide a closer look at the immediate glycemic response to apneic episodes.
Medtronic/Minimed's continuous glucose monitoring system (CGMS) was used for several days in 30 patients with type 2 diabetes on diet or oral hypoglycemic therapy. Eight of the patients had severe SDB and a mean hemoglobin A1c level of 7.4%. The other 22, who did not have SDB, had a mean HbA1c level of 6.5%. Those with SDB were referred to a sleep laboratory for overnight polysomnography, and the CGMS data were compared between the two groups, said Dr. Pallayova of the Pavol Jozef Safarik University, Kosice, Slovakia.
In the group without SDB, the CGMS revealed stable normoglycemia throughout the night. In contrast, those with severe untreated SDB had frequent episodes of sleep apnea/hypopnea (mean apnea-hypopnea index 57.64 episodes/hour) with severe oxygen desaturation (oxygen saturation 82.5%, minimal oxygen saturation 49.13%), followed by significant increases in blood glucose of up to 12.3 mmol/L (221 mg/dL).
The nocturnal increment in blood glucose was 1.11 mmol/L (19.98 mg/dL) in the SDB group, significantly greater than the value of 0.2 mmol/L (3.6 mg/dL) seen in the patients without SDB, and was strongly correlated with severe oxygen desaturation. The peak of apnea-induced hyperglycemia tended to occur within 1 hour of severe oxygen desaturation, and the hyperglycemia lasted for a mean of 48 minutes post hypoxia before returning to normal, Dr. Pallayova noted.
The researchers found significant differences in both overall mean nocturnal glucose values—8.24 mmol/L (148.3 mg/dL) in the severe SDB group, compared with 6.15 mmol/L (110 mg/dL) in those without sleep apnea—and morning fasting glucose levels (8.01 vs. 6.6 mmol/L [144.2 vs. 118.8 mg/dL]).
However, there were no significant differences between the groups in daytime CGMS glucose levels, and no associations were seen between arousal frequency and nocturnal hyperglycemia, she reported.
“Obstructive sleep apnea is not only obstruction; it is a cardiovascular and metabolic nightmare,” Dr. Pallayova said.
“We have to be aware of the fact that untreated sleep apnea may adversely influence glucose control and contribute to the development of late diabetes complications,” she added. “Both sleep-disordered breathing and diabetes mellitus are common, serious, treatable, and underdiagnosed, and they require a high index of suspicion.”
COPENHAGEN — Sleep apnea appears to have an immediate elevating effect on nighttime blood glucose levels in people with concomitant type 2 diabetes, Dr. Maria Pallayova said at the annual meeting of the European Association for the Study of Diabetes.
Previous studies have documented the independent association between sleep-disordered breathing (SDB) and abnormal glucose metabolism. However, the findings of this study, which used continuous glucose monitoring, provide a closer look at the immediate glycemic response to apneic episodes.
Medtronic/Minimed's continuous glucose monitoring system (CGMS) was used for several days in 30 patients with type 2 diabetes on diet or oral hypoglycemic therapy. Eight of the patients had severe SDB and a mean hemoglobin A1c level of 7.4%. The other 22, who did not have SDB, had a mean HbA1c level of 6.5%. Those with SDB were referred to a sleep laboratory for overnight polysomnography, and the CGMS data were compared between the two groups, said Dr. Pallayova of the Pavol Jozef Safarik University, Kosice, Slovakia.
In the group without SDB, the CGMS revealed stable normoglycemia throughout the night. In contrast, those with severe untreated SDB had frequent episodes of sleep apnea/hypopnea (mean apnea-hypopnea index 57.64 episodes/hour) with severe oxygen desaturation (oxygen saturation 82.5%, minimal oxygen saturation 49.13%), followed by significant increases in blood glucose of up to 12.3 mmol/L (221 mg/dL).
The nocturnal increment in blood glucose was 1.11 mmol/L (19.98 mg/dL) in the SDB group, significantly greater than the value of 0.2 mmol/L (3.6 mg/dL) seen in the patients without SDB, and was strongly correlated with severe oxygen desaturation. The peak of apnea-induced hyperglycemia tended to occur within 1 hour of severe oxygen desaturation, and the hyperglycemia lasted for a mean of 48 minutes post hypoxia before returning to normal, Dr. Pallayova noted.
The researchers found significant differences in both overall mean nocturnal glucose values—8.24 mmol/L (148.3 mg/dL) in the severe SDB group, compared with 6.15 mmol/L (110 mg/dL) in those without sleep apnea—and morning fasting glucose levels (8.01 vs. 6.6 mmol/L [144.2 vs. 118.8 mg/dL]).
However, there were no significant differences between the groups in daytime CGMS glucose levels, and no associations were seen between arousal frequency and nocturnal hyperglycemia, she reported.
“Obstructive sleep apnea is not only obstruction; it is a cardiovascular and metabolic nightmare,” Dr. Pallayova said.
“We have to be aware of the fact that untreated sleep apnea may adversely influence glucose control and contribute to the development of late diabetes complications,” she added. “Both sleep-disordered breathing and diabetes mellitus are common, serious, treatable, and underdiagnosed, and they require a high index of suspicion.”
Sleep-Disordered Breathing Tied to Hyperglycemia in Type 2
COPENHAGEN — Sleep apnea seems to have an immediate elevating effect on nighttime blood glucose levels in people with concomitant type 2 diabetes, said Dr. Maria Pallayova at the annual meeting of the European Association for the Study of Diabetes.
Previous data have shown the independent association between sleep-disordered breathing (SDB) and abnormal glucose metabolism. These findings provide a look at the immediate glycemic response to apneic episodes.
Medtronic/Minimed's continuous glucose monitoring system (CGMS) was used for several days in 30 patients with type 2 diabetes on diet or oral hypoglycemic therapy. Eight of the patients had severe SDB and a mean hemoglobin A1c level of 7.4%. The 22 who did not have SDB, had a mean HbA1c level of 6.5%. Those with SDB were referred to a sleep laboratory for overnight polysomnography, and the CGMS data were compared between the two groups, said Dr. Pallayova of PJ Safarik University, Kosice, Slovakia.
In the group without SDB, the CGMS revealed stable normoglycemia throughout the night. Those with severe untreated SDB had frequent episodes of sleep apnea/hypopnea (mean apnea-hypopnea index 57.64 episodes/hour) with severe oxygen desaturation (oxygen saturation 83%, minimal oxygen saturation 49%), followed by significant increases in blood glucose of up to 12.3 mmol/L (221 mg/dL).
The nocturnal increment in blood glucose was 1.11 mmol/L (19.98 mg/dL) in the SDB group, significantly greater than the 0.2 mmol/L (3.6 mg/dL) seen in the patients without SDB, and was strongly correlated with severe oxygen desaturation. The researchers found significant differences in both overall mean nocturnal glucose values—8.24 mmol/L (148.3 mg/dL) in the severe SDB group, compared with 6.15 mmol/L (110 mg/dL) in those without sleep apnea—and morning fasting glucose levels (8.01 vs. 6.6 mmol/L [144.2 vs. 118.8 mg/dL]).
COPENHAGEN — Sleep apnea seems to have an immediate elevating effect on nighttime blood glucose levels in people with concomitant type 2 diabetes, said Dr. Maria Pallayova at the annual meeting of the European Association for the Study of Diabetes.
Previous data have shown the independent association between sleep-disordered breathing (SDB) and abnormal glucose metabolism. These findings provide a look at the immediate glycemic response to apneic episodes.
Medtronic/Minimed's continuous glucose monitoring system (CGMS) was used for several days in 30 patients with type 2 diabetes on diet or oral hypoglycemic therapy. Eight of the patients had severe SDB and a mean hemoglobin A1c level of 7.4%. The 22 who did not have SDB, had a mean HbA1c level of 6.5%. Those with SDB were referred to a sleep laboratory for overnight polysomnography, and the CGMS data were compared between the two groups, said Dr. Pallayova of PJ Safarik University, Kosice, Slovakia.
In the group without SDB, the CGMS revealed stable normoglycemia throughout the night. Those with severe untreated SDB had frequent episodes of sleep apnea/hypopnea (mean apnea-hypopnea index 57.64 episodes/hour) with severe oxygen desaturation (oxygen saturation 83%, minimal oxygen saturation 49%), followed by significant increases in blood glucose of up to 12.3 mmol/L (221 mg/dL).
The nocturnal increment in blood glucose was 1.11 mmol/L (19.98 mg/dL) in the SDB group, significantly greater than the 0.2 mmol/L (3.6 mg/dL) seen in the patients without SDB, and was strongly correlated with severe oxygen desaturation. The researchers found significant differences in both overall mean nocturnal glucose values—8.24 mmol/L (148.3 mg/dL) in the severe SDB group, compared with 6.15 mmol/L (110 mg/dL) in those without sleep apnea—and morning fasting glucose levels (8.01 vs. 6.6 mmol/L [144.2 vs. 118.8 mg/dL]).
COPENHAGEN — Sleep apnea seems to have an immediate elevating effect on nighttime blood glucose levels in people with concomitant type 2 diabetes, said Dr. Maria Pallayova at the annual meeting of the European Association for the Study of Diabetes.
Previous data have shown the independent association between sleep-disordered breathing (SDB) and abnormal glucose metabolism. These findings provide a look at the immediate glycemic response to apneic episodes.
Medtronic/Minimed's continuous glucose monitoring system (CGMS) was used for several days in 30 patients with type 2 diabetes on diet or oral hypoglycemic therapy. Eight of the patients had severe SDB and a mean hemoglobin A1c level of 7.4%. The 22 who did not have SDB, had a mean HbA1c level of 6.5%. Those with SDB were referred to a sleep laboratory for overnight polysomnography, and the CGMS data were compared between the two groups, said Dr. Pallayova of PJ Safarik University, Kosice, Slovakia.
In the group without SDB, the CGMS revealed stable normoglycemia throughout the night. Those with severe untreated SDB had frequent episodes of sleep apnea/hypopnea (mean apnea-hypopnea index 57.64 episodes/hour) with severe oxygen desaturation (oxygen saturation 83%, minimal oxygen saturation 49%), followed by significant increases in blood glucose of up to 12.3 mmol/L (221 mg/dL).
The nocturnal increment in blood glucose was 1.11 mmol/L (19.98 mg/dL) in the SDB group, significantly greater than the 0.2 mmol/L (3.6 mg/dL) seen in the patients without SDB, and was strongly correlated with severe oxygen desaturation. The researchers found significant differences in both overall mean nocturnal glucose values—8.24 mmol/L (148.3 mg/dL) in the severe SDB group, compared with 6.15 mmol/L (110 mg/dL) in those without sleep apnea—and morning fasting glucose levels (8.01 vs. 6.6 mmol/L [144.2 vs. 118.8 mg/dL]).
Weight Gain in 5 Years Before Pregnancy May Increase GDM Risk
SAN DIEGO — Weight gain in the 5 years before pregnancy is associated with an increased risk for gestational diabetes, Monique Hedderson reported in a poster at the annual scientific sessions of the American Diabetes Association.
In a nested case-control study including 114 women with gestational diabetes mellitus (GDM) and 95 controls who were members of Kaiser Permanente of Northern California, those who had gained between 1.1 kg and 10.0 kg in the 5 years before their last menstrual period were nearly twice as likely (crude odds ratio 1.98) to have developed GDM during pregnancy than were those whose weight remained within 1 kg of baseline, said Ms. Hedderson, of Kaiser Permanente, Oakland, Calif., and her associates.
The women who developed GDM were older, more likely to be from an ethnic minority group, more likely to be overweight at baseline, and more likely to be primiparous or to have had at least two prior live births.
After adjustment for these factors, the relationship between prepregnancy weight gain and GDM was even stronger, with an odds ratio of 2.58. The relationship with weight loss was again insignificant (OR 0.9).
SAN DIEGO — Weight gain in the 5 years before pregnancy is associated with an increased risk for gestational diabetes, Monique Hedderson reported in a poster at the annual scientific sessions of the American Diabetes Association.
In a nested case-control study including 114 women with gestational diabetes mellitus (GDM) and 95 controls who were members of Kaiser Permanente of Northern California, those who had gained between 1.1 kg and 10.0 kg in the 5 years before their last menstrual period were nearly twice as likely (crude odds ratio 1.98) to have developed GDM during pregnancy than were those whose weight remained within 1 kg of baseline, said Ms. Hedderson, of Kaiser Permanente, Oakland, Calif., and her associates.
The women who developed GDM were older, more likely to be from an ethnic minority group, more likely to be overweight at baseline, and more likely to be primiparous or to have had at least two prior live births.
After adjustment for these factors, the relationship between prepregnancy weight gain and GDM was even stronger, with an odds ratio of 2.58. The relationship with weight loss was again insignificant (OR 0.9).
SAN DIEGO — Weight gain in the 5 years before pregnancy is associated with an increased risk for gestational diabetes, Monique Hedderson reported in a poster at the annual scientific sessions of the American Diabetes Association.
In a nested case-control study including 114 women with gestational diabetes mellitus (GDM) and 95 controls who were members of Kaiser Permanente of Northern California, those who had gained between 1.1 kg and 10.0 kg in the 5 years before their last menstrual period were nearly twice as likely (crude odds ratio 1.98) to have developed GDM during pregnancy than were those whose weight remained within 1 kg of baseline, said Ms. Hedderson, of Kaiser Permanente, Oakland, Calif., and her associates.
The women who developed GDM were older, more likely to be from an ethnic minority group, more likely to be overweight at baseline, and more likely to be primiparous or to have had at least two prior live births.
After adjustment for these factors, the relationship between prepregnancy weight gain and GDM was even stronger, with an odds ratio of 2.58. The relationship with weight loss was again insignificant (OR 0.9).