User login
Identifying Melanoma With Dermoscopy
Latex Hypersensitivity to Injection Devices for Biologic Therapies in Psoriasis Patients
An allergic reaction is an exaggerated immune response that is known as a type I or immediate hypersensitivity reaction when provoked by reexposure to an allergen or antigen. Upon initial exposure to the antigen, dendritic cells bind it for presentation to helper T (TH2) lymphocytes. The TH2 cells then interact with B cells, stimulating them to become plasma cells and produce IgE antibodies to the antigen. When exposed to the same allergen a second time, IgE antibodies bind the allergen and cross-link on mast cells and basophils in the blood. Cross-linking stimulates degranulation of the cells, releasing histamine, leukotrienes, prostaglandins, and other cytokines. The major effects of the release of these mediators include vasodilation, increased vascular permeability, and bronchoconstriction. Leukotrienes also are responsible for chemotaxis of white blood cells, further propagating the immune response.1
Effects of a type I hypersensitivity reaction can be either local or systemic, resulting in symptoms ranging from mild irritation to anaphylactic shock and death. Latex allergy is a common example of a type I hypersensitivity reaction. Latex is found in many medical products, including gloves, rubber, elastics, blood pressure cuffs, bandages, dressings, and syringes. Reactions can include runny nose, tearing eyes, itching, hives, wheals, wheezing, and in rare cases anaphylaxis.2 Diagnosis can be suspected based on history and physical examination. Screening is performed with radioallergosorbent testing, which identifies specific IgE antibodies to latex; however, the reported sensitivity and specificity for the latex-specific IgE antibody varies widely in the literature, and the test cannot reliably rule in or rule out a true latex allergy.3
Allergic responses to latex in psoriasis patients receiving frequent injections with biologic agents are not commonly reported in the literature. We report the case of a patient with a long history of psoriasis who developed an allergic response after exposure to injection devices that contained latex components while undergoing treatment with biologic agents.
Case Report
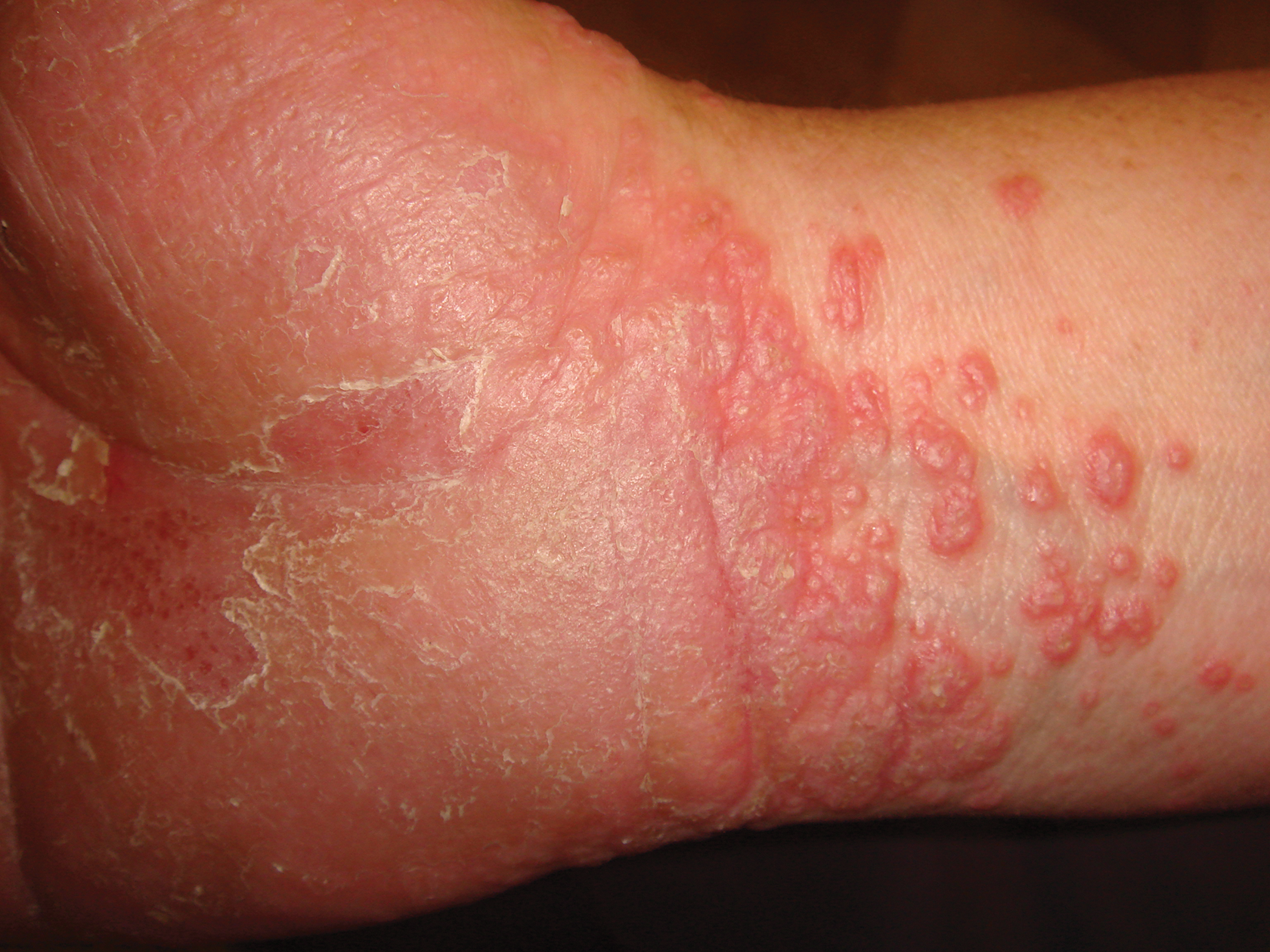
A 72-year-old man presented with an extensive history of severe psoriasis with frequent flares. Treatment with topical agents and etanercept 6 months prior at an outside facility failed. At the time of presentation, the patient had more than 10% body surface area (BSA) involvement, which included the scalp, legs, chest, and back. He subsequently was started on ustekinumab injections. He initially responded well to therapy, but after 8 months of treatment, he began to have recurrent episodes of acute eruptive rashes over the trunk with associated severe pruritus that reproducibly recurred within 24 hours after each ustekinumab injection. It was decided to discontinue ustekinumab due to concern for intolerance, and the patient was switched to secukinumab.
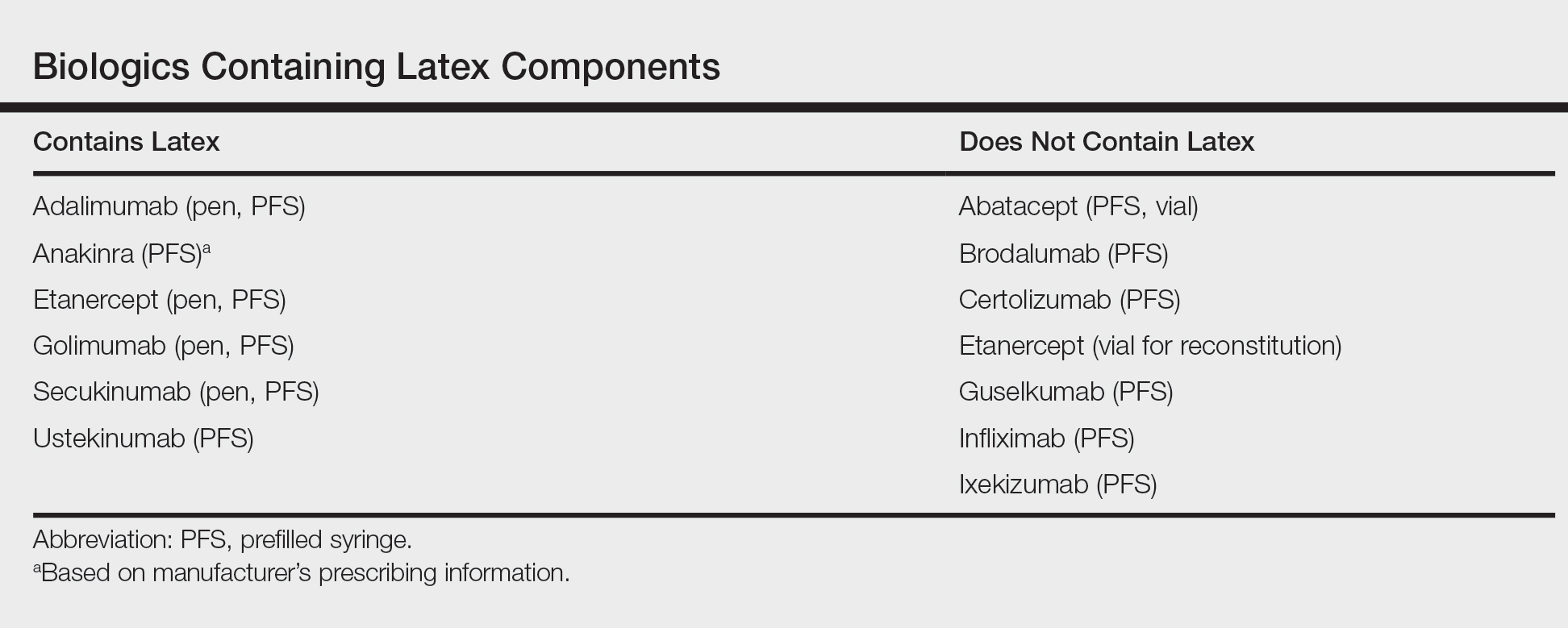
After starting secukinumab, the patient's BSA involvement was reduced to 2% after 1 month; however, he began to develop an eruptive rash with severe pruritus again that reproducibly recurred after each secukinumab injection. On physical examination the patient had ill-defined, confluent, erythematous patches over much of the trunk and extremities. Punch biopsies of the eruptive dermatitis showed spongiform psoriasis and eosinophils with dermal hypersensitivity, consistent with a drug eruption. Upon further questioning, the patient noted that he had a long history of a strong latex allergy and he would develop a blistering dermatitis when coming into contact with latex, which caused a high suspicion for a latex allergy as the cause of the patient's acute dermatitis flares from his prior ustekinumab and secukinumab injections. Although it was confirmed with the manufacturers that both the ustekinumab syringe and secukinumab pen did not contain latex, the caps of these medications (and many other biologic injections) do have latex (Table). Other differential diagnoses included an atypical paradoxical psoriasis flare and a drug eruption to secukinumab, which previously has been reported.4
Based on the suspected cause of the eruption, the patient was instructed not to touch the cap of the secukinumab pen. Despite this recommendation, the rash was still present at the next appointment 1 month later. Repeat punch biopsy showed similar findings to the one prior with likely dermal hypersensitivity. The rash improved with steroid injections and continued to improve after holding the secukinumab for 1 month.
After resolution of the hypersensitivity reaction, the patient was started on ixekizumab, which does not contain latex in any component according to the manufacturer. After 2 months of treatment, the patient had 2% BSA involvement of psoriasis and has had no further reports of itching, rash, or other symptoms of a hypersensitivity reaction. On follow-up, the patient's psoriasis symptoms continue to be controlled without further reactions after injections of ixekizumab. Radioallergosorbent testing was not performed due to the lack of specificity and sensitivity of the test3 as well as the patient's known history of latex allergy and characteristic dermatitis that developed after exposure to latex and resolution with removal of the agent. These clinical manifestations are highly indicative of a type I hypersensitivity to injection devices that contain latex components during biologic therapy.
Comment
Allergic responses to latex are most commonly seen in those exposed to gloves or rubber, but little has been reported on reactions to injections with pens or syringes that contain latex components. Some case reports have demonstrated allergic responses in diabetic patients receiving insulin injections.5,6 MacCracken et al5 reported the case of a young boy who had an allergic response to an insulin injection with a syringe containing latex. The patient had a history of bladder exstrophy with a recent diagnosis of diabetes mellitus. It is well known that patients with spina bifida and other conditions who undergo frequent urological procedures more commonly develop latex allergies. This patient reported a history of swollen lips after a dentist visit, presumably due to contact with latex gloves. Because of the suspected allergy, his first insulin injection was given using a glass syringe and insulin was withdrawn with the top removed due to the top containing latex. He did not experience any complications. After being injected later with insulin drawn through the top using a syringe that contained latex, he developed a flare-up of a 0.5-cm erythematous wheal within minutes with associated pruritus.5
Towse et al6 described another patient with diabetes who developed a local allergic reaction at the site of insulin injections. Workup by the physician ruled out insulin allergy but showed elevated latex-specific IgE antibodies. Future insulin draws through a latex-containing top produced a wheal at the injection site. After switching to latex-free syringes, the allergic reaction resolved.6
Latex allergies are common in medical practice, as latex is found in a wide variety of medical supplies, including syringes used for injections and their caps. Physicians need to be aware of latex allergies in their patients and exercise extreme caution in the use of latex-containing products. In the treatment of psoriasis, care must be given when injecting biologic agents. Although many injection devices contain latex limited to the cap, it may be enough to invoke an allergic response. If such a response is elicited, therapy with injection devices that do not contain latex in either the cap or syringe should be considered.
- Druce HM. Allergic and nonallergic rhinitis. In: Middleton EM Jr, Reed CE, Ellis EF, et al, eds. Allergy: Principles and Practice. 5th ed. Vol 1. St. Louis, MO: Mosby; 1998:1005-1016.
- Rochford C, Milles M. A review of the pathophysiology, diagnosis, and management of allergic reactions in the dental office. Quintessence Int. 2011;42:149-156.
- Hamilton RG, Peterson EL, Ownby DR. Clinical and laboratory-based methods in the diagnosis of natural rubber latex allergy. J Allergy Clin Immunol. 2002;110(2 suppl):S47-S56.
- Shibata M, Sawada Y, Yamaguchi T, et al. Drug eruption caused by secukinumab. Eur J Dermatol. 2017;27:67-68.
- MacCracken J, Stenger P, Jackson T. Latex allergy in diabetic patients: a call for latex-free insulin tops. Diabetes Care. 1996;19:184.
- Towse A, O'Brien M, Twarog FJ, et al. Local reaction secondary to insulin injection: a potential role for latex antigens in insulin vials and syringes. Diabetes Care. 1995;18:1195-1197.
An allergic reaction is an exaggerated immune response that is known as a type I or immediate hypersensitivity reaction when provoked by reexposure to an allergen or antigen. Upon initial exposure to the antigen, dendritic cells bind it for presentation to helper T (TH2) lymphocytes. The TH2 cells then interact with B cells, stimulating them to become plasma cells and produce IgE antibodies to the antigen. When exposed to the same allergen a second time, IgE antibodies bind the allergen and cross-link on mast cells and basophils in the blood. Cross-linking stimulates degranulation of the cells, releasing histamine, leukotrienes, prostaglandins, and other cytokines. The major effects of the release of these mediators include vasodilation, increased vascular permeability, and bronchoconstriction. Leukotrienes also are responsible for chemotaxis of white blood cells, further propagating the immune response.1
Effects of a type I hypersensitivity reaction can be either local or systemic, resulting in symptoms ranging from mild irritation to anaphylactic shock and death. Latex allergy is a common example of a type I hypersensitivity reaction. Latex is found in many medical products, including gloves, rubber, elastics, blood pressure cuffs, bandages, dressings, and syringes. Reactions can include runny nose, tearing eyes, itching, hives, wheals, wheezing, and in rare cases anaphylaxis.2 Diagnosis can be suspected based on history and physical examination. Screening is performed with radioallergosorbent testing, which identifies specific IgE antibodies to latex; however, the reported sensitivity and specificity for the latex-specific IgE antibody varies widely in the literature, and the test cannot reliably rule in or rule out a true latex allergy.3
Allergic responses to latex in psoriasis patients receiving frequent injections with biologic agents are not commonly reported in the literature. We report the case of a patient with a long history of psoriasis who developed an allergic response after exposure to injection devices that contained latex components while undergoing treatment with biologic agents.
Case Report
A 72-year-old man presented with an extensive history of severe psoriasis with frequent flares. Treatment with topical agents and etanercept 6 months prior at an outside facility failed. At the time of presentation, the patient had more than 10% body surface area (BSA) involvement, which included the scalp, legs, chest, and back. He subsequently was started on ustekinumab injections. He initially responded well to therapy, but after 8 months of treatment, he began to have recurrent episodes of acute eruptive rashes over the trunk with associated severe pruritus that reproducibly recurred within 24 hours after each ustekinumab injection. It was decided to discontinue ustekinumab due to concern for intolerance, and the patient was switched to secukinumab.
After starting secukinumab, the patient's BSA involvement was reduced to 2% after 1 month; however, he began to develop an eruptive rash with severe pruritus again that reproducibly recurred after each secukinumab injection. On physical examination the patient had ill-defined, confluent, erythematous patches over much of the trunk and extremities. Punch biopsies of the eruptive dermatitis showed spongiform psoriasis and eosinophils with dermal hypersensitivity, consistent with a drug eruption. Upon further questioning, the patient noted that he had a long history of a strong latex allergy and he would develop a blistering dermatitis when coming into contact with latex, which caused a high suspicion for a latex allergy as the cause of the patient's acute dermatitis flares from his prior ustekinumab and secukinumab injections. Although it was confirmed with the manufacturers that both the ustekinumab syringe and secukinumab pen did not contain latex, the caps of these medications (and many other biologic injections) do have latex (Table). Other differential diagnoses included an atypical paradoxical psoriasis flare and a drug eruption to secukinumab, which previously has been reported.4
Based on the suspected cause of the eruption, the patient was instructed not to touch the cap of the secukinumab pen. Despite this recommendation, the rash was still present at the next appointment 1 month later. Repeat punch biopsy showed similar findings to the one prior with likely dermal hypersensitivity. The rash improved with steroid injections and continued to improve after holding the secukinumab for 1 month.
After resolution of the hypersensitivity reaction, the patient was started on ixekizumab, which does not contain latex in any component according to the manufacturer. After 2 months of treatment, the patient had 2% BSA involvement of psoriasis and has had no further reports of itching, rash, or other symptoms of a hypersensitivity reaction. On follow-up, the patient's psoriasis symptoms continue to be controlled without further reactions after injections of ixekizumab. Radioallergosorbent testing was not performed due to the lack of specificity and sensitivity of the test3 as well as the patient's known history of latex allergy and characteristic dermatitis that developed after exposure to latex and resolution with removal of the agent. These clinical manifestations are highly indicative of a type I hypersensitivity to injection devices that contain latex components during biologic therapy.
Comment
Allergic responses to latex are most commonly seen in those exposed to gloves or rubber, but little has been reported on reactions to injections with pens or syringes that contain latex components. Some case reports have demonstrated allergic responses in diabetic patients receiving insulin injections.5,6 MacCracken et al5 reported the case of a young boy who had an allergic response to an insulin injection with a syringe containing latex. The patient had a history of bladder exstrophy with a recent diagnosis of diabetes mellitus. It is well known that patients with spina bifida and other conditions who undergo frequent urological procedures more commonly develop latex allergies. This patient reported a history of swollen lips after a dentist visit, presumably due to contact with latex gloves. Because of the suspected allergy, his first insulin injection was given using a glass syringe and insulin was withdrawn with the top removed due to the top containing latex. He did not experience any complications. After being injected later with insulin drawn through the top using a syringe that contained latex, he developed a flare-up of a 0.5-cm erythematous wheal within minutes with associated pruritus.5
Towse et al6 described another patient with diabetes who developed a local allergic reaction at the site of insulin injections. Workup by the physician ruled out insulin allergy but showed elevated latex-specific IgE antibodies. Future insulin draws through a latex-containing top produced a wheal at the injection site. After switching to latex-free syringes, the allergic reaction resolved.6
Latex allergies are common in medical practice, as latex is found in a wide variety of medical supplies, including syringes used for injections and their caps. Physicians need to be aware of latex allergies in their patients and exercise extreme caution in the use of latex-containing products. In the treatment of psoriasis, care must be given when injecting biologic agents. Although many injection devices contain latex limited to the cap, it may be enough to invoke an allergic response. If such a response is elicited, therapy with injection devices that do not contain latex in either the cap or syringe should be considered.
An allergic reaction is an exaggerated immune response that is known as a type I or immediate hypersensitivity reaction when provoked by reexposure to an allergen or antigen. Upon initial exposure to the antigen, dendritic cells bind it for presentation to helper T (TH2) lymphocytes. The TH2 cells then interact with B cells, stimulating them to become plasma cells and produce IgE antibodies to the antigen. When exposed to the same allergen a second time, IgE antibodies bind the allergen and cross-link on mast cells and basophils in the blood. Cross-linking stimulates degranulation of the cells, releasing histamine, leukotrienes, prostaglandins, and other cytokines. The major effects of the release of these mediators include vasodilation, increased vascular permeability, and bronchoconstriction. Leukotrienes also are responsible for chemotaxis of white blood cells, further propagating the immune response.1
Effects of a type I hypersensitivity reaction can be either local or systemic, resulting in symptoms ranging from mild irritation to anaphylactic shock and death. Latex allergy is a common example of a type I hypersensitivity reaction. Latex is found in many medical products, including gloves, rubber, elastics, blood pressure cuffs, bandages, dressings, and syringes. Reactions can include runny nose, tearing eyes, itching, hives, wheals, wheezing, and in rare cases anaphylaxis.2 Diagnosis can be suspected based on history and physical examination. Screening is performed with radioallergosorbent testing, which identifies specific IgE antibodies to latex; however, the reported sensitivity and specificity for the latex-specific IgE antibody varies widely in the literature, and the test cannot reliably rule in or rule out a true latex allergy.3
Allergic responses to latex in psoriasis patients receiving frequent injections with biologic agents are not commonly reported in the literature. We report the case of a patient with a long history of psoriasis who developed an allergic response after exposure to injection devices that contained latex components while undergoing treatment with biologic agents.
Case Report
A 72-year-old man presented with an extensive history of severe psoriasis with frequent flares. Treatment with topical agents and etanercept 6 months prior at an outside facility failed. At the time of presentation, the patient had more than 10% body surface area (BSA) involvement, which included the scalp, legs, chest, and back. He subsequently was started on ustekinumab injections. He initially responded well to therapy, but after 8 months of treatment, he began to have recurrent episodes of acute eruptive rashes over the trunk with associated severe pruritus that reproducibly recurred within 24 hours after each ustekinumab injection. It was decided to discontinue ustekinumab due to concern for intolerance, and the patient was switched to secukinumab.
After starting secukinumab, the patient's BSA involvement was reduced to 2% after 1 month; however, he began to develop an eruptive rash with severe pruritus again that reproducibly recurred after each secukinumab injection. On physical examination the patient had ill-defined, confluent, erythematous patches over much of the trunk and extremities. Punch biopsies of the eruptive dermatitis showed spongiform psoriasis and eosinophils with dermal hypersensitivity, consistent with a drug eruption. Upon further questioning, the patient noted that he had a long history of a strong latex allergy and he would develop a blistering dermatitis when coming into contact with latex, which caused a high suspicion for a latex allergy as the cause of the patient's acute dermatitis flares from his prior ustekinumab and secukinumab injections. Although it was confirmed with the manufacturers that both the ustekinumab syringe and secukinumab pen did not contain latex, the caps of these medications (and many other biologic injections) do have latex (Table). Other differential diagnoses included an atypical paradoxical psoriasis flare and a drug eruption to secukinumab, which previously has been reported.4
Based on the suspected cause of the eruption, the patient was instructed not to touch the cap of the secukinumab pen. Despite this recommendation, the rash was still present at the next appointment 1 month later. Repeat punch biopsy showed similar findings to the one prior with likely dermal hypersensitivity. The rash improved with steroid injections and continued to improve after holding the secukinumab for 1 month.
After resolution of the hypersensitivity reaction, the patient was started on ixekizumab, which does not contain latex in any component according to the manufacturer. After 2 months of treatment, the patient had 2% BSA involvement of psoriasis and has had no further reports of itching, rash, or other symptoms of a hypersensitivity reaction. On follow-up, the patient's psoriasis symptoms continue to be controlled without further reactions after injections of ixekizumab. Radioallergosorbent testing was not performed due to the lack of specificity and sensitivity of the test3 as well as the patient's known history of latex allergy and characteristic dermatitis that developed after exposure to latex and resolution with removal of the agent. These clinical manifestations are highly indicative of a type I hypersensitivity to injection devices that contain latex components during biologic therapy.
Comment
Allergic responses to latex are most commonly seen in those exposed to gloves or rubber, but little has been reported on reactions to injections with pens or syringes that contain latex components. Some case reports have demonstrated allergic responses in diabetic patients receiving insulin injections.5,6 MacCracken et al5 reported the case of a young boy who had an allergic response to an insulin injection with a syringe containing latex. The patient had a history of bladder exstrophy with a recent diagnosis of diabetes mellitus. It is well known that patients with spina bifida and other conditions who undergo frequent urological procedures more commonly develop latex allergies. This patient reported a history of swollen lips after a dentist visit, presumably due to contact with latex gloves. Because of the suspected allergy, his first insulin injection was given using a glass syringe and insulin was withdrawn with the top removed due to the top containing latex. He did not experience any complications. After being injected later with insulin drawn through the top using a syringe that contained latex, he developed a flare-up of a 0.5-cm erythematous wheal within minutes with associated pruritus.5
Towse et al6 described another patient with diabetes who developed a local allergic reaction at the site of insulin injections. Workup by the physician ruled out insulin allergy but showed elevated latex-specific IgE antibodies. Future insulin draws through a latex-containing top produced a wheal at the injection site. After switching to latex-free syringes, the allergic reaction resolved.6
Latex allergies are common in medical practice, as latex is found in a wide variety of medical supplies, including syringes used for injections and their caps. Physicians need to be aware of latex allergies in their patients and exercise extreme caution in the use of latex-containing products. In the treatment of psoriasis, care must be given when injecting biologic agents. Although many injection devices contain latex limited to the cap, it may be enough to invoke an allergic response. If such a response is elicited, therapy with injection devices that do not contain latex in either the cap or syringe should be considered.
- Druce HM. Allergic and nonallergic rhinitis. In: Middleton EM Jr, Reed CE, Ellis EF, et al, eds. Allergy: Principles and Practice. 5th ed. Vol 1. St. Louis, MO: Mosby; 1998:1005-1016.
- Rochford C, Milles M. A review of the pathophysiology, diagnosis, and management of allergic reactions in the dental office. Quintessence Int. 2011;42:149-156.
- Hamilton RG, Peterson EL, Ownby DR. Clinical and laboratory-based methods in the diagnosis of natural rubber latex allergy. J Allergy Clin Immunol. 2002;110(2 suppl):S47-S56.
- Shibata M, Sawada Y, Yamaguchi T, et al. Drug eruption caused by secukinumab. Eur J Dermatol. 2017;27:67-68.
- MacCracken J, Stenger P, Jackson T. Latex allergy in diabetic patients: a call for latex-free insulin tops. Diabetes Care. 1996;19:184.
- Towse A, O'Brien M, Twarog FJ, et al. Local reaction secondary to insulin injection: a potential role for latex antigens in insulin vials and syringes. Diabetes Care. 1995;18:1195-1197.
- Druce HM. Allergic and nonallergic rhinitis. In: Middleton EM Jr, Reed CE, Ellis EF, et al, eds. Allergy: Principles and Practice. 5th ed. Vol 1. St. Louis, MO: Mosby; 1998:1005-1016.
- Rochford C, Milles M. A review of the pathophysiology, diagnosis, and management of allergic reactions in the dental office. Quintessence Int. 2011;42:149-156.
- Hamilton RG, Peterson EL, Ownby DR. Clinical and laboratory-based methods in the diagnosis of natural rubber latex allergy. J Allergy Clin Immunol. 2002;110(2 suppl):S47-S56.
- Shibata M, Sawada Y, Yamaguchi T, et al. Drug eruption caused by secukinumab. Eur J Dermatol. 2017;27:67-68.
- MacCracken J, Stenger P, Jackson T. Latex allergy in diabetic patients: a call for latex-free insulin tops. Diabetes Care. 1996;19:184.
- Towse A, O'Brien M, Twarog FJ, et al. Local reaction secondary to insulin injection: a potential role for latex antigens in insulin vials and syringes. Diabetes Care. 1995;18:1195-1197.
Using Dermoscopy to Identify Melanoma and Improve Diagnostic Discrimination (FULL)
From 1982 to 2011, the melanoma incidence rate doubled in the US.1 In 2018, an estimated 87,290 cases of melanoma in situ and 91,270 cases of invasive melanoma will be diagnosed in the US, and 9,320 deaths will be attributable to melanoma.2 Early detection of melanoma is critically important to reduce melanoma-related mortality, with 5-year survival rates as high as 97% at stage 1A vs a 20% 5-year survival when there is distant metastasis.2,3 Melanoma is particularly relevant for medical providers working with veterans because melanoma disproportionately affects service members with an incidence rate ratio of 1.62 (95% confidence interval [CI], 1.40-1.86) compared with that of the general population.4
Biopsy is the definitive diagnostic tool for melanoma. Histologic analysis differentiates melanoma from seborrheic keratoses, pigmented nevi, dermatofibromas, and other pigmented lesions that can resemble melanoma on clinical examination. However, biopsy must be used judiciously as unnecessary biopsies contribute to health care costs and leave scars, which can have psychosocial implications. With benign nevi outnumbering melanoma about 2 million to 1, biopsy is indicated once a threshold of suspicion is obtained.5
Dermoscopic Tool
Dermoscopy is a microscopy-based tool to improve noninvasive diagnostic discrimination of skin lesions based on color and structure analysis. Coloration provides an indication of the composition of elements present in the skin with keratin appearing yellow, blood appearing red, and collagen appearing white. Coloration also suggests pigment depth as melanin appears black when located in the stratum corneum, brown when located deeper in the epidermis, and blue when located in the dermis.6 Finally, characteristic histopathologic alterations in the dermoepidermal junction, rete ridges, pigment-containing cells, and/or melanocyte granules that occur in melanoma are recognizable with dermoscopy.6
In 2001, Bafounta and colleagues performed the first meta-analysis on the efficacy of dermoscopy compared with that of clinical evaluation, finding that dermoscopy performed specifically by dermatology-trained clinicians improved the accuracy of identifying melanoma from an odds ratio of 16 (95% CI, 9-31) with naked eye examination to 76 (95% CI, 25-223) with dermoscopy.7
More recently, Terushkin and colleagues demonstrated that diagnosis specificity improves when a general dermatologist is trained in dermoscopic pattern recognition. Naked eye examination produced a benign to malignant ratio (BMR) of 18.4:1, indicating that about 18 of 19 biopsies considered suspicious for melanoma ultimately yielded benign melanocytic lesions. Although the BMR for the general dermatologist experienced an increase after dermoscopy training, the ratio eventually decreased to 7.9:1.8
Dermoscopic Analysis
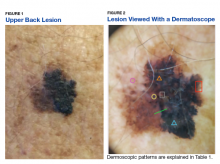
Some of the common patterns recognized in melanoma are demonstrated in Figures 1 and 2. Figure 1 is a close-up of a patient’s upper back showing a solitary asymmetric melanocytic lesion containing multiple red, brown, black, and blue hues.
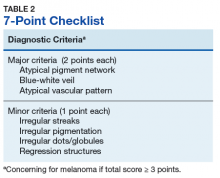
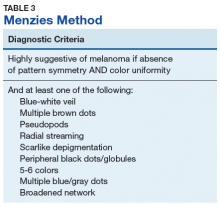
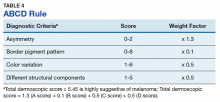
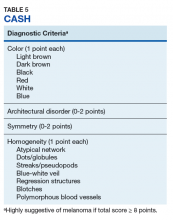
Pattern analysis, the dermoscopic interpretation method preferred by pigmented lesion specialists, requires simultaneously assessing numerous lesion patterns that vary depending on body site.10 Alternative dermoscopic algorithms that focus on the most common features of melanoma have been developed to aid practitioners with the interpretation of dermoscopy findings: the 7-point checklist, the Menzies method, the ABCD rule, and the CASH algorithm (Tables 2, 3, 4, and 5).
Argenziano and colleagues developed the 7-point checklist in 1998. Two points are assigned to the lesion for each of the major criteria and 1 point for each minor criteria.
The Menzies method was developed by Menzies and colleagues in 1996. To be classified as melanoma, the pigmented lesion must show an absence of pattern symmetry and color uniformity while simultaneously exhibiting at least one of the following: blue-white veil, multiple brown dots, pseudopods, radial streaming, scarlike depigmentation, peripheral block dots/globules, 5 to 6 colors, multiple blue/gray dots, and a broadened network.12
The ABCD rule is a more technical dermoscopic evaluation algorithm developed in 1994 by Stolz and colleagues. This method yields a numeric value called the total dermoscopic score (TDS) based on Asymmetry, Border pigment pattern, Color variation, and 5 Different structural components.
Henning and colleagues developed the CASH algorithm in 2006 with the intention of assisting less experienced dermoscopy users with lesion evaluation.14 This algorithm tallies points for Color, Architectural disorder, Symmetry, and Homogeneity. One point is attributed to a lesion for each light brown, dark brown, black, red, white, and/or blue region present. Architectural disorder is assigned a point value between 0 and 2, with 0 indicating the absence of or minimal architectural disorder, 1 indicating moderate disorder, and 2 indicating marked disorder. Symmetry is assigned a point value between 0 and 2, with 0 points assigned to a lesion that exhibits biaxial symmetry, 1 point assigned to a lesion that exhibits monoaxial symmetry, and 2 points assigned to a lesion that exhibits biaxial asymmetry. Finally, 1 point is attributed to a lesion for evidence of each of the following: atypical network, dots/globules, streaks/pseudopods, blue-white veil, regression structures, blotches > 10% of the overall lesion size, and polymorphous blood vessels. The lesion in Figure 2 scores 16 points out of the maximum total CASH score of 17. Any lesion scoring 8 or more is suggestive of malignant melanoma.14
Finally, the TADA method was developed by Rogers and colleagues in 2016.15 This method uses sequential questions to evaluate lesions. First, “Does the lesion exhibit clear dermoscopic evidence of an angioma, dermatofibroma, or seborrheic keratosis?” If “yes,” then no additional dermoscopic evaluation is necessary, and it is recommended to monitor the lesion. If the answer to the first question is “no,” then ask, “Does the lesion exhibit architectural disorder?” The presence of architectural disorder is based on an overall impression of the lesion, which includes symmetry with regard to structures and colors. Any lesion deemed to exhibit architectural disorder should be biopsied. If the lesion has no architectural disorder, the third question is, “Does the lesion contain any starburst patterns, blue-black or gray coloration, shiny white structures, negative networks, ulcers or erosions, and/or vessels?” If “yes,” then the lesion should be biopsied. Since the lesion in Figure 2 exhibits marked architectural disorder in terms of symmetry and color, analysis of the lesion with the TADA method would yield a recommendation for biopsy.15
Dermoscopy in Practice
A. Bernard Ackerman, MD, a key figure in the modern era of dermatopathology, wrote an editorial for the Journal of the American Academy of Dermatology in 1985 titled “No one should die of malignant melanoma.” The editorial highlighted that the visual changes associated with melanoma often manifest years prior to malignant invasion and advocated that all physicians should have competence in melanoma detection, specifically mentioning the importance of training primary care physicians (PCPs), dermatologists, and pathologists in this regard.16 This sentiment is equally as true now as it was in 1985.
Naked eye examination paired with an evaluation of patient risk factors for melanoma, including fair skin types, significant sun exposure history, history of sunburn, geographic location, and personal and family history of melanoma, are the foundation of melanoma detection efforts.17 Studies suggest that the triage skills of PCPs could be improved in regard to the evaluation of pigmented lesions. Primary care residents, for instance, did not accurately diagnose 40% of malignant melanoma cases.18,19 Additionally, a meta-analysis demonstrated that PCP accuracy when diagnosing malignant melanoma ranged between 49% and 80%, significantly less than the 85% to 89% exhibited by practicing dermatologists.19 Dermoscopy could be incorporated as an element of the skin examination to enhance lesion discrimination among PCPs, as it has proven use in dermatologic practice.
Dermoscopy is not readily used by PCPs. A survey study of 705 family practitioners in the US performed by Morris and colleagues demonstrated that only 8.3% of participants currently use a dermatoscope to evaluate pigmented lesions.20 The most common barriers to dermoscopy use cited by PCPs in the US include the cost of the dermatoscope, the time required to acquire proficiency, and the lack of financial reimbursement.16 True utilization of dermoscopy among PCPs is higher than this figure suggests due to the number of PCPs who access dermoscopic evaluations via teledermatology. All 21 Veterans Integrated Services Networks of the Veterans Health Administration (VHA) system, for instance, participate in teledermatology and jointly employ more than 1,150 trained telehealth clinical technicians who created a collective 107,000 teledermatology encounters with and without dermoscopy for evaluation by dermatologists in the most recent fiscal year(Martin Weinstock, written communication, October 2017). Nonetheless, it is necessary to determine the contribution that wider utilization of dermoscopy among PCPs would have on melanoma surveillance.
Studies show that dermoscopic algorithms improve the sensitivity while slightly decreasing the specificity of PCPs to detect melanoma compared with that of the naked eye examination. Dolianitis and colleagues demonstrated that a baseline sensitivity of 60.9% for melanoma detection improved to 85.4% with the 7-point checklist, 85.4% with Menzies method, and 77.5% with the ABCD rule, while the baseline specificity of 85.4% moderated to 73.0%, 77.7%, and 80.4%, respectively, among 61 medical practitioners after studying dermoscopy techniques from 2 CDs.21 Westerhoff and colleagues performed a randomized controlled trial with 74 PCPs to determine the effect of a minimal intervention on melanoma diagnostic accuracy. The intervention consisted of providing participants in the experimental group with an atlas of microscopic features common to melanoma to be read at the participants’ leisure, a 1-hour presentation on microscopy, and a 25-questionpractice quiz. Participants randomized to the intervention group improved their diagnostic accuracy from 57.8% to 75.9% with the use of dermoscopy. This group also experiencedimproved accuracy in its clinical diagnosis of melanoma from 54.6% to 62.7%.22
Argenziano and colleagues demonstrated similar results after PCPs attended a 4-hour workshop on dermoscopy. The 73 PCPs in this study evaluated 2,522 lesions randomized to naked eye examination or dermoscopy. The BMR, calculated from the data provided, improved from 12.6:1 to 10.5:1, respectively, when dermoscopy was incorporated into lesion analysis, while the sensitivity increased from 54.1% to 79.2% and the negative predictive value increased from 95.8% to 98.1%. It is important to note that the BMR and negative predictive value improved in tandem, indicating that PCPs were more discriminatory with their referrals for evaluation by dermatology while capturing a greater percentage of melanomas.23
These studies are not without limitations that preclude broad generalizations. For example, Dolianitis and colleagues and Westerhoff and colleagues provided participants with dermoscopic images of the lesions to be evaluated instead of requiring personal use of a dermatoscope, whereas the study by Argenziano and colleagues incorporated only 6 histopathologically proven malignant melanomas into each of the naked eye examination and the experimental dermoscopy groups.21-23 Yet these studies suggest that broader use of dermoscopy among PCPs could improve the accuracy of melanoma detection given clinically relevant training.
Several additional studies identify positive correlations associated with dermoscopy use among PCPs. A recent survey of 425 French general practitioners found that 8% of the study participants acknowledged owning a dermatoscope. Dermatoscope owners spent a statistically significant longer time analyzing each pigmented skin lesions, exhibited greater confidence in their analysis of pigmented lesions, and issued fewer overall referrals to dermatologists.24
Similarly, Rosendahl and colleagues evaluated the number needed to treat (NNT) (equivalent to the BMR) among 193 Australian PCPs and found that the NNT was inversely correlated to the frequency with which the physicians used dermoscopy. However, it was difficult to determine the definitive cause of the reduced NNT in this study because a similar effect was observed when NNT was evaluated based on general practitioner subspecialization.25 Again, despite limitations, these studies suggest that increased dermoscopy use among PCPs could reduce the morbidity of lifelong scarring as well as the short-term anxiety associated with a possible melanoma diagnosis.
Limitations
Even in the hands of a trained dermatologist, dermoscopy has limitations. Featureless melanoma is a term applied to melanoma lesions bereft of classical findings on both naked eye examination and dermoscopy. Menzies, a dermatologic pioneer in dermoscopy, acknowledged this limitation in 1996 while showing that 8% of melanomas evaded dermoscopic detection. He proceeded to discuss the importance of clinical history in melanoma detection because all of the featureless melanomas exhibited recent changes in size, shape, and/or color.26 More recently, sequential dermoscopy (mole mapping) imaging has been implemented to successfully identify these lesions.27 Thus, dermoscopy cannot replace dermatologists trained in the art of visual assessment with honed clinical diagnostic acumen. Rather, dermoscopy is a tool to enhance the assessment of clinically suspicious lesions and aid diagnostic discrimination of uncertain pigmented lesions.
Conclusion
Primary care physicians are on the frontline of medicine and often the first to have the opportunity to detect the presence of melanoma. Notably, 52.2% of the 884.7 million medical office visits performed annually in the US are with PCPs.28 Despite the benefits, dermoscopy is not uniformly used by dermatologists in the US. Of dermatologists practicing for more than 20 years, 76.2% use dermoscopy compared with 97.8% of dermatologists in practice for less than 5 years. This illustrates an increased use in tandem with dermatology residencies integrating dermoscopy training as a component of the curriculum, showing the importance of incorporating dermoscopy into medical school and residency training for PCPs..29-31 Guidelines regarding dermoscopy training and dermoscopic evaluation algorithms should be established, routinely taught in medical education, and actively incorporated into training curriculum for PCPs in order to improve patient care and realize the potential health care savings associated with the early diagnosis and treatment of melanoma. Dermoscopic-teledermatology consultations present a viable opportunity within the VHA to expedite access to care for veterans and simultaneously offer evaluative feedback on lesions to referring PCPs, as skilled, dermoscopy-trained dermatologists render the diagnoses. Given the devastating mortality rate of melanoma, continued multidisciplinary education on identifying melanoma is of the utmost importance for patient care. Widespread implementation of dermoscopy and dermoscopic-teledermatology consultations could save lives and slow the ever-increasing economic burden associated with melanoma treatment, costing $1.467 billion in 2016.32
1. Guy GP Jr, Thomas CC, Thompson T, Watson M, Massetti GM, Richardson LC. Vital signs: melanoma incidence and mortality trends and projections-United States, 1982-2030. MMWR Morb Mortal Wkly Rep. 2015;64(21):591-596.
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
3. American Cancer Society. Cancer facts & figures 2017. Atlanta: American Cancer Society; 2017. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf. Accessed April 19, 2018.
4. Lea CS, Efird JT, Toland AE, Lewis DR, Phillips CJ. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Mil Med. 2014;179(3):247-253.
5. Thomas L, Puig S. Dermoscopy, digital dermoscopy and other diagnostic tools in the early detection of melanoma and follow-up of high-risk skin cancer patients. Acta Derm Venereol. 2017;97(218):14-21.
6. Marghoob AA, Usatine RP, Jaimes N. Dermoscopy for the family physician. Am Fam Physician. 2013;88(7):441-450.
7. Bafounta ML, Beauchet A, Aegerter P, Saiag P. Is dermoscopy (epiluminescence microscopy) useful for the diagnosis of melanoma? Results of a meta-analysis using techniques adapted to the evaluation of diagnostic tests. Arch Dermatol. 2001;137(10):1343-1350.
8. Terushkin V, Warycha M, Levy M, Kopf AW, Cohen DE, Polsky D. Analysis of the benign to malignant ratio of lesions biopsied by a general dermatologist before and after the adoption of dermoscopy. Arch Dermatol. 2010;146(3):343-344.
9. Wolner ZJ, Yélamos O, Liopyris K, Rogers T, Marchetti MA, Marghoob AA. Enhancing skin cancer diagnosis with dermoscopy. Dermatol Clin. 2017;35(4):417-437.
10. Carli P, Quercioli E, Sestini S, et al. Pattern analysis, not simplified algorithms, is the most reliable method for teaching dermoscopy for melanoma diagnosis to residents in dermatology. Br J Dermatol. 2003;148(5):981-984.
11. Argenziano G, Fabbrocini G, Carli P, De Giorgi V, Sammarco E, Delfino M. Epiluminescence microscopy for the diagnosis of doubtful melanocytic skin lesions. Comparison of the ABCD rule of dermatoscopy and a new 7-point checklist based on pattern analysis. Arch Dermatol. 1998;134(12):1563-1570.
12. Menzies SW, Ingvar C, Crotty KA, McCarthy WH. Frequency and morphologic characteristics of invasive melanomas lacking specific surface microscopic features. Arch Dermatol. 1996;132(10):1178-1182.
13. Nachbar F, Stolz W, Merkle T, et al. The ABCD rule of dermatoscopy. High prospective value in the diagnosis of doubtful melanocytic skin lesions. J Am Acad Dermatol. 1994;30(4):551-559.
14. Henning JS, Dusza SW, Wang SQ, et al. The CASH (color, architecture, symmetry, and homogeneity) algorithm for dermoscopy. J Am Acad Dermatol. 2007;56(1):45-52.
15. Rogers T, Marino M, Dusza SW, Bajaj S, Marchetti MA, Marghoob A. Triage amalgamated dermoscopic algorithm (TADA) for skin cancer screening. Dermatol Pract Concept. 2017;7(2):39-46.
16. Ackerman AB. No one should die of malignant melanoma. J Am Acad Dermatol. 1985;12(1):115-116.
17. Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: II: sun exposure. Eur J Cancer. 2005;41(1):45-60.
18. Gerbert B, Maurer T, Berger T, et al. Primary care physicians as gatekeepers in managed care. Primary care physicians’ and dermatologists’ skills at secondary prevention of skin cancer. Arch Dermatol. 1996;132(9):1030-1038.
19. Corbo MD, Wismer J. Agreement between dermatologists and primary care practitioners in the diagnosis of malignant melanoma: review of the literature. J Cutan Med Surg. 2012;16(5):306-310.
20. Morris JB, Alfonso SV, Hernandez N, Fernández MI. Examining the factors associated with past and present dermoscopy use among family physicians. Dermatol Pract Concept. 2017;7(4):63-70.
21. Dolianitis C, Kelly J, Wolfe R, Simpson P. Comparative performance of 4 dermoscopic algorithms by nonexperts for the diagnosis of melanocytic lesions. Arch Dermatol. 2005;141(8):1008-1014.
22. Westerhoff K, Mccarthy WH, Menzies SW. Increase in the sensitivity for melanoma diagnosis by primary care physicians using skin surface microscopy. Br J Dermatol. 2000;143(5):1016-1020.
23. Argenziano G, Puig S, Zalaudek I, et al. Dermoscopy improves accuracy of primary care physicians to triage lesions suggestive of skin cancer. J Clin Oncol. 2006;24(12):1877-1882.
24. Chappuis P, Duru G, Marchal O, Girier P, Dalle S, Thomas L. Dermoscopy, a useful tool for general practitioners in melanoma screening: a nationwide survey. Br J Dermatol. 2016;175(4):744-750.
25. Rosendahl C, Williams G, Eley D, et al. The impact of subspecialization and dermatoscopy use on accuracy of melanoma diagnosis among primary care doctors in Australia. J Am Acad Dermatol. 2012;67(5):846-852.
26. Menzies SW, Ingvar C, Crotty KA, McCarthy WH. Frequency and morphologic characteristics of invasive melanomas lacking specific surface microscopic features. Arch Dermatol. 1996;132(10):1178-1182.
27. Kittler H, Guitera P, Riedl E, et al. Identification of clinically featureless incipient melanoma using sequential dermoscopy imaging. Arch Dermatol. 2006;142(9):1113-1119.
28. Centers for Disease Control and Prevention. Ambulatory care use and physician office visits. https://www.cdc.gov/nchs/fastats/physician-visits.htm. Updated May 3, 2017. Accessed April 10, 2018.
29. Murzaku EC, Hayan S, Rao BK. Methods and rates of dermoscopy usage: a cross-sectional survey of US dermatologists stratified by years in practice. J Am Acad Dermatol. 2014;71(2):393-395.
30. Nehal KS, Oliveria SA, Marghoob AA, et al. Use of and beliefs about dermoscopy in the management of patients with pigmented lesions: a survey of dermatology residency programmes in the United States. Melanoma Res. 2002;12(6):601-605.
31. Wu TP, Newlove T, Smith L, Vuong CH, Stein JA, Polsky D. The importance of dedicated dermoscopy training during residency: a survey of US dermatology chief residents. J Am Acad Dermatol. 2013;68(6):1000-1005.
32. Lim HW, Collins SAB, Resneck JS Jr, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76(5):958-972
From 1982 to 2011, the melanoma incidence rate doubled in the US.1 In 2018, an estimated 87,290 cases of melanoma in situ and 91,270 cases of invasive melanoma will be diagnosed in the US, and 9,320 deaths will be attributable to melanoma.2 Early detection of melanoma is critically important to reduce melanoma-related mortality, with 5-year survival rates as high as 97% at stage 1A vs a 20% 5-year survival when there is distant metastasis.2,3 Melanoma is particularly relevant for medical providers working with veterans because melanoma disproportionately affects service members with an incidence rate ratio of 1.62 (95% confidence interval [CI], 1.40-1.86) compared with that of the general population.4
Biopsy is the definitive diagnostic tool for melanoma. Histologic analysis differentiates melanoma from seborrheic keratoses, pigmented nevi, dermatofibromas, and other pigmented lesions that can resemble melanoma on clinical examination. However, biopsy must be used judiciously as unnecessary biopsies contribute to health care costs and leave scars, which can have psychosocial implications. With benign nevi outnumbering melanoma about 2 million to 1, biopsy is indicated once a threshold of suspicion is obtained.5
Dermoscopic Tool
Dermoscopy is a microscopy-based tool to improve noninvasive diagnostic discrimination of skin lesions based on color and structure analysis. Coloration provides an indication of the composition of elements present in the skin with keratin appearing yellow, blood appearing red, and collagen appearing white. Coloration also suggests pigment depth as melanin appears black when located in the stratum corneum, brown when located deeper in the epidermis, and blue when located in the dermis.6 Finally, characteristic histopathologic alterations in the dermoepidermal junction, rete ridges, pigment-containing cells, and/or melanocyte granules that occur in melanoma are recognizable with dermoscopy.6
In 2001, Bafounta and colleagues performed the first meta-analysis on the efficacy of dermoscopy compared with that of clinical evaluation, finding that dermoscopy performed specifically by dermatology-trained clinicians improved the accuracy of identifying melanoma from an odds ratio of 16 (95% CI, 9-31) with naked eye examination to 76 (95% CI, 25-223) with dermoscopy.7
More recently, Terushkin and colleagues demonstrated that diagnosis specificity improves when a general dermatologist is trained in dermoscopic pattern recognition. Naked eye examination produced a benign to malignant ratio (BMR) of 18.4:1, indicating that about 18 of 19 biopsies considered suspicious for melanoma ultimately yielded benign melanocytic lesions. Although the BMR for the general dermatologist experienced an increase after dermoscopy training, the ratio eventually decreased to 7.9:1.8
Dermoscopic Analysis
Some of the common patterns recognized in melanoma are demonstrated in Figures 1 and 2. Figure 1 is a close-up of a patient’s upper back showing a solitary asymmetric melanocytic lesion containing multiple red, brown, black, and blue hues.
Pattern analysis, the dermoscopic interpretation method preferred by pigmented lesion specialists, requires simultaneously assessing numerous lesion patterns that vary depending on body site.10 Alternative dermoscopic algorithms that focus on the most common features of melanoma have been developed to aid practitioners with the interpretation of dermoscopy findings: the 7-point checklist, the Menzies method, the ABCD rule, and the CASH algorithm (Tables 2, 3, 4, and 5).
Argenziano and colleagues developed the 7-point checklist in 1998. Two points are assigned to the lesion for each of the major criteria and 1 point for each minor criteria.
The Menzies method was developed by Menzies and colleagues in 1996. To be classified as melanoma, the pigmented lesion must show an absence of pattern symmetry and color uniformity while simultaneously exhibiting at least one of the following: blue-white veil, multiple brown dots, pseudopods, radial streaming, scarlike depigmentation, peripheral block dots/globules, 5 to 6 colors, multiple blue/gray dots, and a broadened network.12
The ABCD rule is a more technical dermoscopic evaluation algorithm developed in 1994 by Stolz and colleagues. This method yields a numeric value called the total dermoscopic score (TDS) based on Asymmetry, Border pigment pattern, Color variation, and 5 Different structural components.
Henning and colleagues developed the CASH algorithm in 2006 with the intention of assisting less experienced dermoscopy users with lesion evaluation.14 This algorithm tallies points for Color, Architectural disorder, Symmetry, and Homogeneity. One point is attributed to a lesion for each light brown, dark brown, black, red, white, and/or blue region present. Architectural disorder is assigned a point value between 0 and 2, with 0 indicating the absence of or minimal architectural disorder, 1 indicating moderate disorder, and 2 indicating marked disorder. Symmetry is assigned a point value between 0 and 2, with 0 points assigned to a lesion that exhibits biaxial symmetry, 1 point assigned to a lesion that exhibits monoaxial symmetry, and 2 points assigned to a lesion that exhibits biaxial asymmetry. Finally, 1 point is attributed to a lesion for evidence of each of the following: atypical network, dots/globules, streaks/pseudopods, blue-white veil, regression structures, blotches > 10% of the overall lesion size, and polymorphous blood vessels. The lesion in Figure 2 scores 16 points out of the maximum total CASH score of 17. Any lesion scoring 8 or more is suggestive of malignant melanoma.14
Finally, the TADA method was developed by Rogers and colleagues in 2016.15 This method uses sequential questions to evaluate lesions. First, “Does the lesion exhibit clear dermoscopic evidence of an angioma, dermatofibroma, or seborrheic keratosis?” If “yes,” then no additional dermoscopic evaluation is necessary, and it is recommended to monitor the lesion. If the answer to the first question is “no,” then ask, “Does the lesion exhibit architectural disorder?” The presence of architectural disorder is based on an overall impression of the lesion, which includes symmetry with regard to structures and colors. Any lesion deemed to exhibit architectural disorder should be biopsied. If the lesion has no architectural disorder, the third question is, “Does the lesion contain any starburst patterns, blue-black or gray coloration, shiny white structures, negative networks, ulcers or erosions, and/or vessels?” If “yes,” then the lesion should be biopsied. Since the lesion in Figure 2 exhibits marked architectural disorder in terms of symmetry and color, analysis of the lesion with the TADA method would yield a recommendation for biopsy.15
Dermoscopy in Practice
A. Bernard Ackerman, MD, a key figure in the modern era of dermatopathology, wrote an editorial for the Journal of the American Academy of Dermatology in 1985 titled “No one should die of malignant melanoma.” The editorial highlighted that the visual changes associated with melanoma often manifest years prior to malignant invasion and advocated that all physicians should have competence in melanoma detection, specifically mentioning the importance of training primary care physicians (PCPs), dermatologists, and pathologists in this regard.16 This sentiment is equally as true now as it was in 1985.
Naked eye examination paired with an evaluation of patient risk factors for melanoma, including fair skin types, significant sun exposure history, history of sunburn, geographic location, and personal and family history of melanoma, are the foundation of melanoma detection efforts.17 Studies suggest that the triage skills of PCPs could be improved in regard to the evaluation of pigmented lesions. Primary care residents, for instance, did not accurately diagnose 40% of malignant melanoma cases.18,19 Additionally, a meta-analysis demonstrated that PCP accuracy when diagnosing malignant melanoma ranged between 49% and 80%, significantly less than the 85% to 89% exhibited by practicing dermatologists.19 Dermoscopy could be incorporated as an element of the skin examination to enhance lesion discrimination among PCPs, as it has proven use in dermatologic practice.
Dermoscopy is not readily used by PCPs. A survey study of 705 family practitioners in the US performed by Morris and colleagues demonstrated that only 8.3% of participants currently use a dermatoscope to evaluate pigmented lesions.20 The most common barriers to dermoscopy use cited by PCPs in the US include the cost of the dermatoscope, the time required to acquire proficiency, and the lack of financial reimbursement.16 True utilization of dermoscopy among PCPs is higher than this figure suggests due to the number of PCPs who access dermoscopic evaluations via teledermatology. All 21 Veterans Integrated Services Networks of the Veterans Health Administration (VHA) system, for instance, participate in teledermatology and jointly employ more than 1,150 trained telehealth clinical technicians who created a collective 107,000 teledermatology encounters with and without dermoscopy for evaluation by dermatologists in the most recent fiscal year(Martin Weinstock, written communication, October 2017). Nonetheless, it is necessary to determine the contribution that wider utilization of dermoscopy among PCPs would have on melanoma surveillance.
Studies show that dermoscopic algorithms improve the sensitivity while slightly decreasing the specificity of PCPs to detect melanoma compared with that of the naked eye examination. Dolianitis and colleagues demonstrated that a baseline sensitivity of 60.9% for melanoma detection improved to 85.4% with the 7-point checklist, 85.4% with Menzies method, and 77.5% with the ABCD rule, while the baseline specificity of 85.4% moderated to 73.0%, 77.7%, and 80.4%, respectively, among 61 medical practitioners after studying dermoscopy techniques from 2 CDs.21 Westerhoff and colleagues performed a randomized controlled trial with 74 PCPs to determine the effect of a minimal intervention on melanoma diagnostic accuracy. The intervention consisted of providing participants in the experimental group with an atlas of microscopic features common to melanoma to be read at the participants’ leisure, a 1-hour presentation on microscopy, and a 25-questionpractice quiz. Participants randomized to the intervention group improved their diagnostic accuracy from 57.8% to 75.9% with the use of dermoscopy. This group also experiencedimproved accuracy in its clinical diagnosis of melanoma from 54.6% to 62.7%.22
Argenziano and colleagues demonstrated similar results after PCPs attended a 4-hour workshop on dermoscopy. The 73 PCPs in this study evaluated 2,522 lesions randomized to naked eye examination or dermoscopy. The BMR, calculated from the data provided, improved from 12.6:1 to 10.5:1, respectively, when dermoscopy was incorporated into lesion analysis, while the sensitivity increased from 54.1% to 79.2% and the negative predictive value increased from 95.8% to 98.1%. It is important to note that the BMR and negative predictive value improved in tandem, indicating that PCPs were more discriminatory with their referrals for evaluation by dermatology while capturing a greater percentage of melanomas.23
These studies are not without limitations that preclude broad generalizations. For example, Dolianitis and colleagues and Westerhoff and colleagues provided participants with dermoscopic images of the lesions to be evaluated instead of requiring personal use of a dermatoscope, whereas the study by Argenziano and colleagues incorporated only 6 histopathologically proven malignant melanomas into each of the naked eye examination and the experimental dermoscopy groups.21-23 Yet these studies suggest that broader use of dermoscopy among PCPs could improve the accuracy of melanoma detection given clinically relevant training.
Several additional studies identify positive correlations associated with dermoscopy use among PCPs. A recent survey of 425 French general practitioners found that 8% of the study participants acknowledged owning a dermatoscope. Dermatoscope owners spent a statistically significant longer time analyzing each pigmented skin lesions, exhibited greater confidence in their analysis of pigmented lesions, and issued fewer overall referrals to dermatologists.24
Similarly, Rosendahl and colleagues evaluated the number needed to treat (NNT) (equivalent to the BMR) among 193 Australian PCPs and found that the NNT was inversely correlated to the frequency with which the physicians used dermoscopy. However, it was difficult to determine the definitive cause of the reduced NNT in this study because a similar effect was observed when NNT was evaluated based on general practitioner subspecialization.25 Again, despite limitations, these studies suggest that increased dermoscopy use among PCPs could reduce the morbidity of lifelong scarring as well as the short-term anxiety associated with a possible melanoma diagnosis.
Limitations
Even in the hands of a trained dermatologist, dermoscopy has limitations. Featureless melanoma is a term applied to melanoma lesions bereft of classical findings on both naked eye examination and dermoscopy. Menzies, a dermatologic pioneer in dermoscopy, acknowledged this limitation in 1996 while showing that 8% of melanomas evaded dermoscopic detection. He proceeded to discuss the importance of clinical history in melanoma detection because all of the featureless melanomas exhibited recent changes in size, shape, and/or color.26 More recently, sequential dermoscopy (mole mapping) imaging has been implemented to successfully identify these lesions.27 Thus, dermoscopy cannot replace dermatologists trained in the art of visual assessment with honed clinical diagnostic acumen. Rather, dermoscopy is a tool to enhance the assessment of clinically suspicious lesions and aid diagnostic discrimination of uncertain pigmented lesions.
Conclusion
Primary care physicians are on the frontline of medicine and often the first to have the opportunity to detect the presence of melanoma. Notably, 52.2% of the 884.7 million medical office visits performed annually in the US are with PCPs.28 Despite the benefits, dermoscopy is not uniformly used by dermatologists in the US. Of dermatologists practicing for more than 20 years, 76.2% use dermoscopy compared with 97.8% of dermatologists in practice for less than 5 years. This illustrates an increased use in tandem with dermatology residencies integrating dermoscopy training as a component of the curriculum, showing the importance of incorporating dermoscopy into medical school and residency training for PCPs..29-31 Guidelines regarding dermoscopy training and dermoscopic evaluation algorithms should be established, routinely taught in medical education, and actively incorporated into training curriculum for PCPs in order to improve patient care and realize the potential health care savings associated with the early diagnosis and treatment of melanoma. Dermoscopic-teledermatology consultations present a viable opportunity within the VHA to expedite access to care for veterans and simultaneously offer evaluative feedback on lesions to referring PCPs, as skilled, dermoscopy-trained dermatologists render the diagnoses. Given the devastating mortality rate of melanoma, continued multidisciplinary education on identifying melanoma is of the utmost importance for patient care. Widespread implementation of dermoscopy and dermoscopic-teledermatology consultations could save lives and slow the ever-increasing economic burden associated with melanoma treatment, costing $1.467 billion in 2016.32
From 1982 to 2011, the melanoma incidence rate doubled in the US.1 In 2018, an estimated 87,290 cases of melanoma in situ and 91,270 cases of invasive melanoma will be diagnosed in the US, and 9,320 deaths will be attributable to melanoma.2 Early detection of melanoma is critically important to reduce melanoma-related mortality, with 5-year survival rates as high as 97% at stage 1A vs a 20% 5-year survival when there is distant metastasis.2,3 Melanoma is particularly relevant for medical providers working with veterans because melanoma disproportionately affects service members with an incidence rate ratio of 1.62 (95% confidence interval [CI], 1.40-1.86) compared with that of the general population.4
Biopsy is the definitive diagnostic tool for melanoma. Histologic analysis differentiates melanoma from seborrheic keratoses, pigmented nevi, dermatofibromas, and other pigmented lesions that can resemble melanoma on clinical examination. However, biopsy must be used judiciously as unnecessary biopsies contribute to health care costs and leave scars, which can have psychosocial implications. With benign nevi outnumbering melanoma about 2 million to 1, biopsy is indicated once a threshold of suspicion is obtained.5
Dermoscopic Tool
Dermoscopy is a microscopy-based tool to improve noninvasive diagnostic discrimination of skin lesions based on color and structure analysis. Coloration provides an indication of the composition of elements present in the skin with keratin appearing yellow, blood appearing red, and collagen appearing white. Coloration also suggests pigment depth as melanin appears black when located in the stratum corneum, brown when located deeper in the epidermis, and blue when located in the dermis.6 Finally, characteristic histopathologic alterations in the dermoepidermal junction, rete ridges, pigment-containing cells, and/or melanocyte granules that occur in melanoma are recognizable with dermoscopy.6
In 2001, Bafounta and colleagues performed the first meta-analysis on the efficacy of dermoscopy compared with that of clinical evaluation, finding that dermoscopy performed specifically by dermatology-trained clinicians improved the accuracy of identifying melanoma from an odds ratio of 16 (95% CI, 9-31) with naked eye examination to 76 (95% CI, 25-223) with dermoscopy.7
More recently, Terushkin and colleagues demonstrated that diagnosis specificity improves when a general dermatologist is trained in dermoscopic pattern recognition. Naked eye examination produced a benign to malignant ratio (BMR) of 18.4:1, indicating that about 18 of 19 biopsies considered suspicious for melanoma ultimately yielded benign melanocytic lesions. Although the BMR for the general dermatologist experienced an increase after dermoscopy training, the ratio eventually decreased to 7.9:1.8
Dermoscopic Analysis
Some of the common patterns recognized in melanoma are demonstrated in Figures 1 and 2. Figure 1 is a close-up of a patient’s upper back showing a solitary asymmetric melanocytic lesion containing multiple red, brown, black, and blue hues.
Pattern analysis, the dermoscopic interpretation method preferred by pigmented lesion specialists, requires simultaneously assessing numerous lesion patterns that vary depending on body site.10 Alternative dermoscopic algorithms that focus on the most common features of melanoma have been developed to aid practitioners with the interpretation of dermoscopy findings: the 7-point checklist, the Menzies method, the ABCD rule, and the CASH algorithm (Tables 2, 3, 4, and 5).
Argenziano and colleagues developed the 7-point checklist in 1998. Two points are assigned to the lesion for each of the major criteria and 1 point for each minor criteria.
The Menzies method was developed by Menzies and colleagues in 1996. To be classified as melanoma, the pigmented lesion must show an absence of pattern symmetry and color uniformity while simultaneously exhibiting at least one of the following: blue-white veil, multiple brown dots, pseudopods, radial streaming, scarlike depigmentation, peripheral block dots/globules, 5 to 6 colors, multiple blue/gray dots, and a broadened network.12
The ABCD rule is a more technical dermoscopic evaluation algorithm developed in 1994 by Stolz and colleagues. This method yields a numeric value called the total dermoscopic score (TDS) based on Asymmetry, Border pigment pattern, Color variation, and 5 Different structural components.
Henning and colleagues developed the CASH algorithm in 2006 with the intention of assisting less experienced dermoscopy users with lesion evaluation.14 This algorithm tallies points for Color, Architectural disorder, Symmetry, and Homogeneity. One point is attributed to a lesion for each light brown, dark brown, black, red, white, and/or blue region present. Architectural disorder is assigned a point value between 0 and 2, with 0 indicating the absence of or minimal architectural disorder, 1 indicating moderate disorder, and 2 indicating marked disorder. Symmetry is assigned a point value between 0 and 2, with 0 points assigned to a lesion that exhibits biaxial symmetry, 1 point assigned to a lesion that exhibits monoaxial symmetry, and 2 points assigned to a lesion that exhibits biaxial asymmetry. Finally, 1 point is attributed to a lesion for evidence of each of the following: atypical network, dots/globules, streaks/pseudopods, blue-white veil, regression structures, blotches > 10% of the overall lesion size, and polymorphous blood vessels. The lesion in Figure 2 scores 16 points out of the maximum total CASH score of 17. Any lesion scoring 8 or more is suggestive of malignant melanoma.14
Finally, the TADA method was developed by Rogers and colleagues in 2016.15 This method uses sequential questions to evaluate lesions. First, “Does the lesion exhibit clear dermoscopic evidence of an angioma, dermatofibroma, or seborrheic keratosis?” If “yes,” then no additional dermoscopic evaluation is necessary, and it is recommended to monitor the lesion. If the answer to the first question is “no,” then ask, “Does the lesion exhibit architectural disorder?” The presence of architectural disorder is based on an overall impression of the lesion, which includes symmetry with regard to structures and colors. Any lesion deemed to exhibit architectural disorder should be biopsied. If the lesion has no architectural disorder, the third question is, “Does the lesion contain any starburst patterns, blue-black or gray coloration, shiny white structures, negative networks, ulcers or erosions, and/or vessels?” If “yes,” then the lesion should be biopsied. Since the lesion in Figure 2 exhibits marked architectural disorder in terms of symmetry and color, analysis of the lesion with the TADA method would yield a recommendation for biopsy.15
Dermoscopy in Practice
A. Bernard Ackerman, MD, a key figure in the modern era of dermatopathology, wrote an editorial for the Journal of the American Academy of Dermatology in 1985 titled “No one should die of malignant melanoma.” The editorial highlighted that the visual changes associated with melanoma often manifest years prior to malignant invasion and advocated that all physicians should have competence in melanoma detection, specifically mentioning the importance of training primary care physicians (PCPs), dermatologists, and pathologists in this regard.16 This sentiment is equally as true now as it was in 1985.
Naked eye examination paired with an evaluation of patient risk factors for melanoma, including fair skin types, significant sun exposure history, history of sunburn, geographic location, and personal and family history of melanoma, are the foundation of melanoma detection efforts.17 Studies suggest that the triage skills of PCPs could be improved in regard to the evaluation of pigmented lesions. Primary care residents, for instance, did not accurately diagnose 40% of malignant melanoma cases.18,19 Additionally, a meta-analysis demonstrated that PCP accuracy when diagnosing malignant melanoma ranged between 49% and 80%, significantly less than the 85% to 89% exhibited by practicing dermatologists.19 Dermoscopy could be incorporated as an element of the skin examination to enhance lesion discrimination among PCPs, as it has proven use in dermatologic practice.
Dermoscopy is not readily used by PCPs. A survey study of 705 family practitioners in the US performed by Morris and colleagues demonstrated that only 8.3% of participants currently use a dermatoscope to evaluate pigmented lesions.20 The most common barriers to dermoscopy use cited by PCPs in the US include the cost of the dermatoscope, the time required to acquire proficiency, and the lack of financial reimbursement.16 True utilization of dermoscopy among PCPs is higher than this figure suggests due to the number of PCPs who access dermoscopic evaluations via teledermatology. All 21 Veterans Integrated Services Networks of the Veterans Health Administration (VHA) system, for instance, participate in teledermatology and jointly employ more than 1,150 trained telehealth clinical technicians who created a collective 107,000 teledermatology encounters with and without dermoscopy for evaluation by dermatologists in the most recent fiscal year(Martin Weinstock, written communication, October 2017). Nonetheless, it is necessary to determine the contribution that wider utilization of dermoscopy among PCPs would have on melanoma surveillance.
Studies show that dermoscopic algorithms improve the sensitivity while slightly decreasing the specificity of PCPs to detect melanoma compared with that of the naked eye examination. Dolianitis and colleagues demonstrated that a baseline sensitivity of 60.9% for melanoma detection improved to 85.4% with the 7-point checklist, 85.4% with Menzies method, and 77.5% with the ABCD rule, while the baseline specificity of 85.4% moderated to 73.0%, 77.7%, and 80.4%, respectively, among 61 medical practitioners after studying dermoscopy techniques from 2 CDs.21 Westerhoff and colleagues performed a randomized controlled trial with 74 PCPs to determine the effect of a minimal intervention on melanoma diagnostic accuracy. The intervention consisted of providing participants in the experimental group with an atlas of microscopic features common to melanoma to be read at the participants’ leisure, a 1-hour presentation on microscopy, and a 25-questionpractice quiz. Participants randomized to the intervention group improved their diagnostic accuracy from 57.8% to 75.9% with the use of dermoscopy. This group also experiencedimproved accuracy in its clinical diagnosis of melanoma from 54.6% to 62.7%.22
Argenziano and colleagues demonstrated similar results after PCPs attended a 4-hour workshop on dermoscopy. The 73 PCPs in this study evaluated 2,522 lesions randomized to naked eye examination or dermoscopy. The BMR, calculated from the data provided, improved from 12.6:1 to 10.5:1, respectively, when dermoscopy was incorporated into lesion analysis, while the sensitivity increased from 54.1% to 79.2% and the negative predictive value increased from 95.8% to 98.1%. It is important to note that the BMR and negative predictive value improved in tandem, indicating that PCPs were more discriminatory with their referrals for evaluation by dermatology while capturing a greater percentage of melanomas.23
These studies are not without limitations that preclude broad generalizations. For example, Dolianitis and colleagues and Westerhoff and colleagues provided participants with dermoscopic images of the lesions to be evaluated instead of requiring personal use of a dermatoscope, whereas the study by Argenziano and colleagues incorporated only 6 histopathologically proven malignant melanomas into each of the naked eye examination and the experimental dermoscopy groups.21-23 Yet these studies suggest that broader use of dermoscopy among PCPs could improve the accuracy of melanoma detection given clinically relevant training.
Several additional studies identify positive correlations associated with dermoscopy use among PCPs. A recent survey of 425 French general practitioners found that 8% of the study participants acknowledged owning a dermatoscope. Dermatoscope owners spent a statistically significant longer time analyzing each pigmented skin lesions, exhibited greater confidence in their analysis of pigmented lesions, and issued fewer overall referrals to dermatologists.24
Similarly, Rosendahl and colleagues evaluated the number needed to treat (NNT) (equivalent to the BMR) among 193 Australian PCPs and found that the NNT was inversely correlated to the frequency with which the physicians used dermoscopy. However, it was difficult to determine the definitive cause of the reduced NNT in this study because a similar effect was observed when NNT was evaluated based on general practitioner subspecialization.25 Again, despite limitations, these studies suggest that increased dermoscopy use among PCPs could reduce the morbidity of lifelong scarring as well as the short-term anxiety associated with a possible melanoma diagnosis.
Limitations
Even in the hands of a trained dermatologist, dermoscopy has limitations. Featureless melanoma is a term applied to melanoma lesions bereft of classical findings on both naked eye examination and dermoscopy. Menzies, a dermatologic pioneer in dermoscopy, acknowledged this limitation in 1996 while showing that 8% of melanomas evaded dermoscopic detection. He proceeded to discuss the importance of clinical history in melanoma detection because all of the featureless melanomas exhibited recent changes in size, shape, and/or color.26 More recently, sequential dermoscopy (mole mapping) imaging has been implemented to successfully identify these lesions.27 Thus, dermoscopy cannot replace dermatologists trained in the art of visual assessment with honed clinical diagnostic acumen. Rather, dermoscopy is a tool to enhance the assessment of clinically suspicious lesions and aid diagnostic discrimination of uncertain pigmented lesions.
Conclusion
Primary care physicians are on the frontline of medicine and often the first to have the opportunity to detect the presence of melanoma. Notably, 52.2% of the 884.7 million medical office visits performed annually in the US are with PCPs.28 Despite the benefits, dermoscopy is not uniformly used by dermatologists in the US. Of dermatologists practicing for more than 20 years, 76.2% use dermoscopy compared with 97.8% of dermatologists in practice for less than 5 years. This illustrates an increased use in tandem with dermatology residencies integrating dermoscopy training as a component of the curriculum, showing the importance of incorporating dermoscopy into medical school and residency training for PCPs..29-31 Guidelines regarding dermoscopy training and dermoscopic evaluation algorithms should be established, routinely taught in medical education, and actively incorporated into training curriculum for PCPs in order to improve patient care and realize the potential health care savings associated with the early diagnosis and treatment of melanoma. Dermoscopic-teledermatology consultations present a viable opportunity within the VHA to expedite access to care for veterans and simultaneously offer evaluative feedback on lesions to referring PCPs, as skilled, dermoscopy-trained dermatologists render the diagnoses. Given the devastating mortality rate of melanoma, continued multidisciplinary education on identifying melanoma is of the utmost importance for patient care. Widespread implementation of dermoscopy and dermoscopic-teledermatology consultations could save lives and slow the ever-increasing economic burden associated with melanoma treatment, costing $1.467 billion in 2016.32
1. Guy GP Jr, Thomas CC, Thompson T, Watson M, Massetti GM, Richardson LC. Vital signs: melanoma incidence and mortality trends and projections-United States, 1982-2030. MMWR Morb Mortal Wkly Rep. 2015;64(21):591-596.
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
3. American Cancer Society. Cancer facts & figures 2017. Atlanta: American Cancer Society; 2017. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf. Accessed April 19, 2018.
4. Lea CS, Efird JT, Toland AE, Lewis DR, Phillips CJ. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Mil Med. 2014;179(3):247-253.
5. Thomas L, Puig S. Dermoscopy, digital dermoscopy and other diagnostic tools in the early detection of melanoma and follow-up of high-risk skin cancer patients. Acta Derm Venereol. 2017;97(218):14-21.
6. Marghoob AA, Usatine RP, Jaimes N. Dermoscopy for the family physician. Am Fam Physician. 2013;88(7):441-450.
7. Bafounta ML, Beauchet A, Aegerter P, Saiag P. Is dermoscopy (epiluminescence microscopy) useful for the diagnosis of melanoma? Results of a meta-analysis using techniques adapted to the evaluation of diagnostic tests. Arch Dermatol. 2001;137(10):1343-1350.
8. Terushkin V, Warycha M, Levy M, Kopf AW, Cohen DE, Polsky D. Analysis of the benign to malignant ratio of lesions biopsied by a general dermatologist before and after the adoption of dermoscopy. Arch Dermatol. 2010;146(3):343-344.
9. Wolner ZJ, Yélamos O, Liopyris K, Rogers T, Marchetti MA, Marghoob AA. Enhancing skin cancer diagnosis with dermoscopy. Dermatol Clin. 2017;35(4):417-437.
10. Carli P, Quercioli E, Sestini S, et al. Pattern analysis, not simplified algorithms, is the most reliable method for teaching dermoscopy for melanoma diagnosis to residents in dermatology. Br J Dermatol. 2003;148(5):981-984.
11. Argenziano G, Fabbrocini G, Carli P, De Giorgi V, Sammarco E, Delfino M. Epiluminescence microscopy for the diagnosis of doubtful melanocytic skin lesions. Comparison of the ABCD rule of dermatoscopy and a new 7-point checklist based on pattern analysis. Arch Dermatol. 1998;134(12):1563-1570.
12. Menzies SW, Ingvar C, Crotty KA, McCarthy WH. Frequency and morphologic characteristics of invasive melanomas lacking specific surface microscopic features. Arch Dermatol. 1996;132(10):1178-1182.
13. Nachbar F, Stolz W, Merkle T, et al. The ABCD rule of dermatoscopy. High prospective value in the diagnosis of doubtful melanocytic skin lesions. J Am Acad Dermatol. 1994;30(4):551-559.
14. Henning JS, Dusza SW, Wang SQ, et al. The CASH (color, architecture, symmetry, and homogeneity) algorithm for dermoscopy. J Am Acad Dermatol. 2007;56(1):45-52.
15. Rogers T, Marino M, Dusza SW, Bajaj S, Marchetti MA, Marghoob A. Triage amalgamated dermoscopic algorithm (TADA) for skin cancer screening. Dermatol Pract Concept. 2017;7(2):39-46.
16. Ackerman AB. No one should die of malignant melanoma. J Am Acad Dermatol. 1985;12(1):115-116.
17. Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: II: sun exposure. Eur J Cancer. 2005;41(1):45-60.
18. Gerbert B, Maurer T, Berger T, et al. Primary care physicians as gatekeepers in managed care. Primary care physicians’ and dermatologists’ skills at secondary prevention of skin cancer. Arch Dermatol. 1996;132(9):1030-1038.
19. Corbo MD, Wismer J. Agreement between dermatologists and primary care practitioners in the diagnosis of malignant melanoma: review of the literature. J Cutan Med Surg. 2012;16(5):306-310.
20. Morris JB, Alfonso SV, Hernandez N, Fernández MI. Examining the factors associated with past and present dermoscopy use among family physicians. Dermatol Pract Concept. 2017;7(4):63-70.
21. Dolianitis C, Kelly J, Wolfe R, Simpson P. Comparative performance of 4 dermoscopic algorithms by nonexperts for the diagnosis of melanocytic lesions. Arch Dermatol. 2005;141(8):1008-1014.
22. Westerhoff K, Mccarthy WH, Menzies SW. Increase in the sensitivity for melanoma diagnosis by primary care physicians using skin surface microscopy. Br J Dermatol. 2000;143(5):1016-1020.
23. Argenziano G, Puig S, Zalaudek I, et al. Dermoscopy improves accuracy of primary care physicians to triage lesions suggestive of skin cancer. J Clin Oncol. 2006;24(12):1877-1882.
24. Chappuis P, Duru G, Marchal O, Girier P, Dalle S, Thomas L. Dermoscopy, a useful tool for general practitioners in melanoma screening: a nationwide survey. Br J Dermatol. 2016;175(4):744-750.
25. Rosendahl C, Williams G, Eley D, et al. The impact of subspecialization and dermatoscopy use on accuracy of melanoma diagnosis among primary care doctors in Australia. J Am Acad Dermatol. 2012;67(5):846-852.
26. Menzies SW, Ingvar C, Crotty KA, McCarthy WH. Frequency and morphologic characteristics of invasive melanomas lacking specific surface microscopic features. Arch Dermatol. 1996;132(10):1178-1182.
27. Kittler H, Guitera P, Riedl E, et al. Identification of clinically featureless incipient melanoma using sequential dermoscopy imaging. Arch Dermatol. 2006;142(9):1113-1119.
28. Centers for Disease Control and Prevention. Ambulatory care use and physician office visits. https://www.cdc.gov/nchs/fastats/physician-visits.htm. Updated May 3, 2017. Accessed April 10, 2018.
29. Murzaku EC, Hayan S, Rao BK. Methods and rates of dermoscopy usage: a cross-sectional survey of US dermatologists stratified by years in practice. J Am Acad Dermatol. 2014;71(2):393-395.
30. Nehal KS, Oliveria SA, Marghoob AA, et al. Use of and beliefs about dermoscopy in the management of patients with pigmented lesions: a survey of dermatology residency programmes in the United States. Melanoma Res. 2002;12(6):601-605.
31. Wu TP, Newlove T, Smith L, Vuong CH, Stein JA, Polsky D. The importance of dedicated dermoscopy training during residency: a survey of US dermatology chief residents. J Am Acad Dermatol. 2013;68(6):1000-1005.
32. Lim HW, Collins SAB, Resneck JS Jr, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76(5):958-972
1. Guy GP Jr, Thomas CC, Thompson T, Watson M, Massetti GM, Richardson LC. Vital signs: melanoma incidence and mortality trends and projections-United States, 1982-2030. MMWR Morb Mortal Wkly Rep. 2015;64(21):591-596.
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
3. American Cancer Society. Cancer facts & figures 2017. Atlanta: American Cancer Society; 2017. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf. Accessed April 19, 2018.
4. Lea CS, Efird JT, Toland AE, Lewis DR, Phillips CJ. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Mil Med. 2014;179(3):247-253.
5. Thomas L, Puig S. Dermoscopy, digital dermoscopy and other diagnostic tools in the early detection of melanoma and follow-up of high-risk skin cancer patients. Acta Derm Venereol. 2017;97(218):14-21.
6. Marghoob AA, Usatine RP, Jaimes N. Dermoscopy for the family physician. Am Fam Physician. 2013;88(7):441-450.
7. Bafounta ML, Beauchet A, Aegerter P, Saiag P. Is dermoscopy (epiluminescence microscopy) useful for the diagnosis of melanoma? Results of a meta-analysis using techniques adapted to the evaluation of diagnostic tests. Arch Dermatol. 2001;137(10):1343-1350.
8. Terushkin V, Warycha M, Levy M, Kopf AW, Cohen DE, Polsky D. Analysis of the benign to malignant ratio of lesions biopsied by a general dermatologist before and after the adoption of dermoscopy. Arch Dermatol. 2010;146(3):343-344.
9. Wolner ZJ, Yélamos O, Liopyris K, Rogers T, Marchetti MA, Marghoob AA. Enhancing skin cancer diagnosis with dermoscopy. Dermatol Clin. 2017;35(4):417-437.
10. Carli P, Quercioli E, Sestini S, et al. Pattern analysis, not simplified algorithms, is the most reliable method for teaching dermoscopy for melanoma diagnosis to residents in dermatology. Br J Dermatol. 2003;148(5):981-984.
11. Argenziano G, Fabbrocini G, Carli P, De Giorgi V, Sammarco E, Delfino M. Epiluminescence microscopy for the diagnosis of doubtful melanocytic skin lesions. Comparison of the ABCD rule of dermatoscopy and a new 7-point checklist based on pattern analysis. Arch Dermatol. 1998;134(12):1563-1570.
12. Menzies SW, Ingvar C, Crotty KA, McCarthy WH. Frequency and morphologic characteristics of invasive melanomas lacking specific surface microscopic features. Arch Dermatol. 1996;132(10):1178-1182.
13. Nachbar F, Stolz W, Merkle T, et al. The ABCD rule of dermatoscopy. High prospective value in the diagnosis of doubtful melanocytic skin lesions. J Am Acad Dermatol. 1994;30(4):551-559.
14. Henning JS, Dusza SW, Wang SQ, et al. The CASH (color, architecture, symmetry, and homogeneity) algorithm for dermoscopy. J Am Acad Dermatol. 2007;56(1):45-52.
15. Rogers T, Marino M, Dusza SW, Bajaj S, Marchetti MA, Marghoob A. Triage amalgamated dermoscopic algorithm (TADA) for skin cancer screening. Dermatol Pract Concept. 2017;7(2):39-46.
16. Ackerman AB. No one should die of malignant melanoma. J Am Acad Dermatol. 1985;12(1):115-116.
17. Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: II: sun exposure. Eur J Cancer. 2005;41(1):45-60.
18. Gerbert B, Maurer T, Berger T, et al. Primary care physicians as gatekeepers in managed care. Primary care physicians’ and dermatologists’ skills at secondary prevention of skin cancer. Arch Dermatol. 1996;132(9):1030-1038.
19. Corbo MD, Wismer J. Agreement between dermatologists and primary care practitioners in the diagnosis of malignant melanoma: review of the literature. J Cutan Med Surg. 2012;16(5):306-310.
20. Morris JB, Alfonso SV, Hernandez N, Fernández MI. Examining the factors associated with past and present dermoscopy use among family physicians. Dermatol Pract Concept. 2017;7(4):63-70.
21. Dolianitis C, Kelly J, Wolfe R, Simpson P. Comparative performance of 4 dermoscopic algorithms by nonexperts for the diagnosis of melanocytic lesions. Arch Dermatol. 2005;141(8):1008-1014.
22. Westerhoff K, Mccarthy WH, Menzies SW. Increase in the sensitivity for melanoma diagnosis by primary care physicians using skin surface microscopy. Br J Dermatol. 2000;143(5):1016-1020.
23. Argenziano G, Puig S, Zalaudek I, et al. Dermoscopy improves accuracy of primary care physicians to triage lesions suggestive of skin cancer. J Clin Oncol. 2006;24(12):1877-1882.
24. Chappuis P, Duru G, Marchal O, Girier P, Dalle S, Thomas L. Dermoscopy, a useful tool for general practitioners in melanoma screening: a nationwide survey. Br J Dermatol. 2016;175(4):744-750.
25. Rosendahl C, Williams G, Eley D, et al. The impact of subspecialization and dermatoscopy use on accuracy of melanoma diagnosis among primary care doctors in Australia. J Am Acad Dermatol. 2012;67(5):846-852.
26. Menzies SW, Ingvar C, Crotty KA, McCarthy WH. Frequency and morphologic characteristics of invasive melanomas lacking specific surface microscopic features. Arch Dermatol. 1996;132(10):1178-1182.
27. Kittler H, Guitera P, Riedl E, et al. Identification of clinically featureless incipient melanoma using sequential dermoscopy imaging. Arch Dermatol. 2006;142(9):1113-1119.
28. Centers for Disease Control and Prevention. Ambulatory care use and physician office visits. https://www.cdc.gov/nchs/fastats/physician-visits.htm. Updated May 3, 2017. Accessed April 10, 2018.
29. Murzaku EC, Hayan S, Rao BK. Methods and rates of dermoscopy usage: a cross-sectional survey of US dermatologists stratified by years in practice. J Am Acad Dermatol. 2014;71(2):393-395.
30. Nehal KS, Oliveria SA, Marghoob AA, et al. Use of and beliefs about dermoscopy in the management of patients with pigmented lesions: a survey of dermatology residency programmes in the United States. Melanoma Res. 2002;12(6):601-605.
31. Wu TP, Newlove T, Smith L, Vuong CH, Stein JA, Polsky D. The importance of dedicated dermoscopy training during residency: a survey of US dermatology chief residents. J Am Acad Dermatol. 2013;68(6):1000-1005.
32. Lim HW, Collins SAB, Resneck JS Jr, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76(5):958-972
Confluent Erythematous Plaques on the Palm
The Diagnosis: Palmoplantar Lichen Planus
A skin biopsy from a lesion on the inner wrist showed an interface pattern with a dense bandlike infiltrate obscuring the dermoepidermal junction coupled with a superficial perivascular infiltrate (Figure). At higher magnification (×10), the histologic features included compact orthokeratosis, wedge-shaped hypergranulosis, vacuolar degeneration of the basal layer, basal dyskeratosis, a dense lymphohistiocytic infiltrate obscuring the basement membrane, and melanophages in the papillary dermis.
Lichen planus (LP) is a common inflammatory disease of the skin presenting with flat-topped, violaceous, polygonal papules with fine white lines (Wickham striae) on the surface. It is recognized and diagnosed clinically by its characteristic appearance. Common areas of LP presentation include the shins, inner thighs, genitalia, trunk, volar aspect of the wrists, and oral mucosa.
Palmoplantar LP can present as erythematous plaques, punctuate keratosis, diffuse keratoderma, or ulcerated lesions. The most common concern among patients with LP is pruritus. One-fourth of patients with LP may present with lesions on the palms and soles, but diffuse palmoplantar hyperkeratosis is rare.1 Lesions typically heal in 1 to 8 months, with an average of 3 months. Palmoplantar LP recurs within 1 year after stopping treatment in one-third of patients.1
The cause of LP is unknown, but the pathophysiology is beginning to be understood. Cytotoxic CD8+ T cells stimulate apoptosis of the keratinocytes. The induction of this mechanism may be due to a self-antigen in a genetically predisposed patient. The evidence for LP being an autoimmune disease is supported by the high female predominance and the association of LP with other autoimmune diseases.2 Patients with LP have an increased chance of coexisting hepatitis C virus. In a cross-sectional study of 303 patients, Lodi et al3 found that approximately 20% of LP patients were hepatitis C virus seropositive.
Treatment options for LP include topical and systemic steroids, tazarotene, acitretin, and immunosuppressive agents.4 Our patient initially was treated with oral cyclosporine 100 mg every morning and oral methotrexate at a dose of 7.5 mg weekly. She also was treated with clobetasol ointment 0.05%. After 3 months, cyclosporine was discontinued. Methotrexate was maintained. At 5 months’ followup there was marked improvement of both clinical and symptomatic concerns with only residual palmoplantar erythema.
The differential diagnosis for pruritic palmoplantar hyperkeratosis is large. The most common differential diagnoses include hyperkeratotic eczema, psoriasis, secondary syphilis, and hereditary palmoplantar keratoderma. Lichen planus should be considered in the differential diagnosis of palmoplantar hyperkeratosis. A skin biopsy may be needed, as palmoplantar LP often has an atypical presentation.5
1. Sánchez-Pérez J, Rios Buceta L, Fraga J, et al. Lichen planus with lesions on the palms and/or soles: prevalence and clinicopathological study of 36 patients. Br J Dermatol. 2000;142:310-314.
2. Farhi D, Dupin N. Pathophysiology, etiologic factors, and clinical management of oral lichen planus, part I: facts and controversies. Clin Dermatol. 2010;28:100-108.
3. Lodi G, Giuliani M, Majorana A, et al. Lichen planus and hepatitis C virus: a multicentre study of patients with oral lesions and a systematic review. Br J Dermatol. 2004;151:1172-1181.
4. Karakatsanis G, Patsatsi A, Kastoridou C, et al. Palmoplantar lichen planus with umbilicated papules: an atypical case with rapid therapeutic response to cyclosporin. J Eur Acad Dermatol Venereol. 2007;21:1006-1007.
5. Rotunda AM, Craft N, Haley JC. Hyperkeratotic plaques on the palms and soles. palmoplantar lichen planus, hyperkeratotic variant. Arch Dermatol. 2004;140:1275-1280.
The Diagnosis: Palmoplantar Lichen Planus
A skin biopsy from a lesion on the inner wrist showed an interface pattern with a dense bandlike infiltrate obscuring the dermoepidermal junction coupled with a superficial perivascular infiltrate (Figure). At higher magnification (×10), the histologic features included compact orthokeratosis, wedge-shaped hypergranulosis, vacuolar degeneration of the basal layer, basal dyskeratosis, a dense lymphohistiocytic infiltrate obscuring the basement membrane, and melanophages in the papillary dermis.
Lichen planus (LP) is a common inflammatory disease of the skin presenting with flat-topped, violaceous, polygonal papules with fine white lines (Wickham striae) on the surface. It is recognized and diagnosed clinically by its characteristic appearance. Common areas of LP presentation include the shins, inner thighs, genitalia, trunk, volar aspect of the wrists, and oral mucosa.
Palmoplantar LP can present as erythematous plaques, punctuate keratosis, diffuse keratoderma, or ulcerated lesions. The most common concern among patients with LP is pruritus. One-fourth of patients with LP may present with lesions on the palms and soles, but diffuse palmoplantar hyperkeratosis is rare.1 Lesions typically heal in 1 to 8 months, with an average of 3 months. Palmoplantar LP recurs within 1 year after stopping treatment in one-third of patients.1
The cause of LP is unknown, but the pathophysiology is beginning to be understood. Cytotoxic CD8+ T cells stimulate apoptosis of the keratinocytes. The induction of this mechanism may be due to a self-antigen in a genetically predisposed patient. The evidence for LP being an autoimmune disease is supported by the high female predominance and the association of LP with other autoimmune diseases.2 Patients with LP have an increased chance of coexisting hepatitis C virus. In a cross-sectional study of 303 patients, Lodi et al3 found that approximately 20% of LP patients were hepatitis C virus seropositive.
Treatment options for LP include topical and systemic steroids, tazarotene, acitretin, and immunosuppressive agents.4 Our patient initially was treated with oral cyclosporine 100 mg every morning and oral methotrexate at a dose of 7.5 mg weekly. She also was treated with clobetasol ointment 0.05%. After 3 months, cyclosporine was discontinued. Methotrexate was maintained. At 5 months’ followup there was marked improvement of both clinical and symptomatic concerns with only residual palmoplantar erythema.
The differential diagnosis for pruritic palmoplantar hyperkeratosis is large. The most common differential diagnoses include hyperkeratotic eczema, psoriasis, secondary syphilis, and hereditary palmoplantar keratoderma. Lichen planus should be considered in the differential diagnosis of palmoplantar hyperkeratosis. A skin biopsy may be needed, as palmoplantar LP often has an atypical presentation.5
The Diagnosis: Palmoplantar Lichen Planus
A skin biopsy from a lesion on the inner wrist showed an interface pattern with a dense bandlike infiltrate obscuring the dermoepidermal junction coupled with a superficial perivascular infiltrate (Figure). At higher magnification (×10), the histologic features included compact orthokeratosis, wedge-shaped hypergranulosis, vacuolar degeneration of the basal layer, basal dyskeratosis, a dense lymphohistiocytic infiltrate obscuring the basement membrane, and melanophages in the papillary dermis.
Lichen planus (LP) is a common inflammatory disease of the skin presenting with flat-topped, violaceous, polygonal papules with fine white lines (Wickham striae) on the surface. It is recognized and diagnosed clinically by its characteristic appearance. Common areas of LP presentation include the shins, inner thighs, genitalia, trunk, volar aspect of the wrists, and oral mucosa.
Palmoplantar LP can present as erythematous plaques, punctuate keratosis, diffuse keratoderma, or ulcerated lesions. The most common concern among patients with LP is pruritus. One-fourth of patients with LP may present with lesions on the palms and soles, but diffuse palmoplantar hyperkeratosis is rare.1 Lesions typically heal in 1 to 8 months, with an average of 3 months. Palmoplantar LP recurs within 1 year after stopping treatment in one-third of patients.1
The cause of LP is unknown, but the pathophysiology is beginning to be understood. Cytotoxic CD8+ T cells stimulate apoptosis of the keratinocytes. The induction of this mechanism may be due to a self-antigen in a genetically predisposed patient. The evidence for LP being an autoimmune disease is supported by the high female predominance and the association of LP with other autoimmune diseases.2 Patients with LP have an increased chance of coexisting hepatitis C virus. In a cross-sectional study of 303 patients, Lodi et al3 found that approximately 20% of LP patients were hepatitis C virus seropositive.
Treatment options for LP include topical and systemic steroids, tazarotene, acitretin, and immunosuppressive agents.4 Our patient initially was treated with oral cyclosporine 100 mg every morning and oral methotrexate at a dose of 7.5 mg weekly. She also was treated with clobetasol ointment 0.05%. After 3 months, cyclosporine was discontinued. Methotrexate was maintained. At 5 months’ followup there was marked improvement of both clinical and symptomatic concerns with only residual palmoplantar erythema.
The differential diagnosis for pruritic palmoplantar hyperkeratosis is large. The most common differential diagnoses include hyperkeratotic eczema, psoriasis, secondary syphilis, and hereditary palmoplantar keratoderma. Lichen planus should be considered in the differential diagnosis of palmoplantar hyperkeratosis. A skin biopsy may be needed, as palmoplantar LP often has an atypical presentation.5
1. Sánchez-Pérez J, Rios Buceta L, Fraga J, et al. Lichen planus with lesions on the palms and/or soles: prevalence and clinicopathological study of 36 patients. Br J Dermatol. 2000;142:310-314.
2. Farhi D, Dupin N. Pathophysiology, etiologic factors, and clinical management of oral lichen planus, part I: facts and controversies. Clin Dermatol. 2010;28:100-108.
3. Lodi G, Giuliani M, Majorana A, et al. Lichen planus and hepatitis C virus: a multicentre study of patients with oral lesions and a systematic review. Br J Dermatol. 2004;151:1172-1181.
4. Karakatsanis G, Patsatsi A, Kastoridou C, et al. Palmoplantar lichen planus with umbilicated papules: an atypical case with rapid therapeutic response to cyclosporin. J Eur Acad Dermatol Venereol. 2007;21:1006-1007.
5. Rotunda AM, Craft N, Haley JC. Hyperkeratotic plaques on the palms and soles. palmoplantar lichen planus, hyperkeratotic variant. Arch Dermatol. 2004;140:1275-1280.
1. Sánchez-Pérez J, Rios Buceta L, Fraga J, et al. Lichen planus with lesions on the palms and/or soles: prevalence and clinicopathological study of 36 patients. Br J Dermatol. 2000;142:310-314.
2. Farhi D, Dupin N. Pathophysiology, etiologic factors, and clinical management of oral lichen planus, part I: facts and controversies. Clin Dermatol. 2010;28:100-108.
3. Lodi G, Giuliani M, Majorana A, et al. Lichen planus and hepatitis C virus: a multicentre study of patients with oral lesions and a systematic review. Br J Dermatol. 2004;151:1172-1181.
4. Karakatsanis G, Patsatsi A, Kastoridou C, et al. Palmoplantar lichen planus with umbilicated papules: an atypical case with rapid therapeutic response to cyclosporin. J Eur Acad Dermatol Venereol. 2007;21:1006-1007.
5. Rotunda AM, Craft N, Haley JC. Hyperkeratotic plaques on the palms and soles. palmoplantar lichen planus, hyperkeratotic variant. Arch Dermatol. 2004;140:1275-1280.
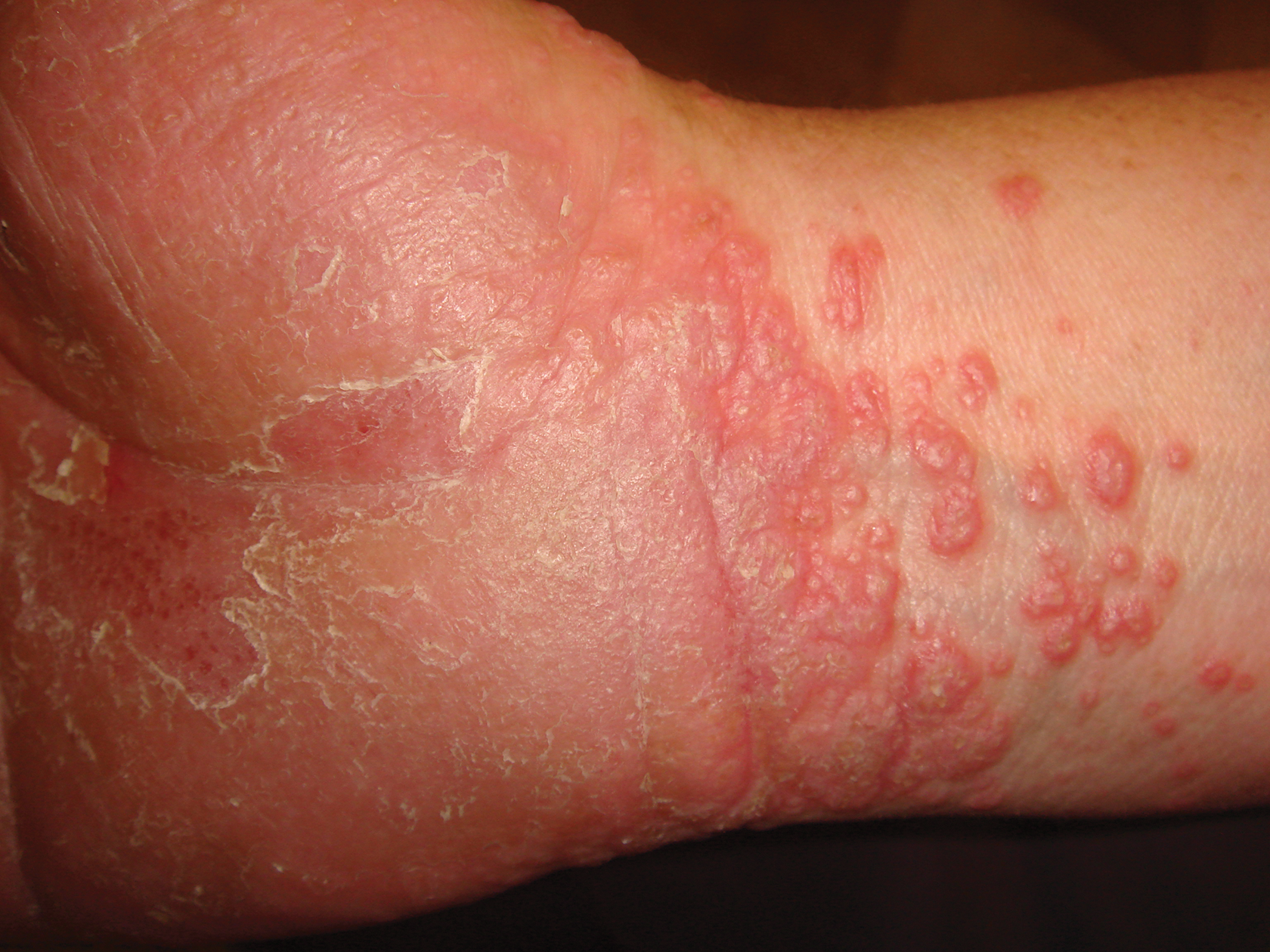
A 45-year-old woman was referred to dermatology by her general internist for the management of a pruritic rash on the hands and feet that was unresponsive to topical steroid creams. The pruritus also was unresponsive to hydroxyzine and aspirin. Erythematous plaques were present on the palms and soles. Physical examination revealed thickened volar skin with a yellowish surface. There were individual papules with atrophic tops at the edge of the plaques on the inner wrists. The patient’s medical history was otherwise unremarkable. Blood tests for glucose and liver function did not reveal any abnormalities.