User login
Hepatitis C virus: Prevention, screening, and interpretation of assays
Screening for hepatitis C virus (HCV) infection in high-risk populations can identify, early on, people at risk of progressive liver disease who may benefit from antiviral therapy and counseling. The US Centers for Disease Control and Prevention (CDC) recommends that all people be assessed for HCV risk factors and that those with risk factors be screened for HCV antibodies (anti-HCV),1 and members of the national societies of gastroenterology and hepatology have endorsed this recommendation.2
Unfortunately, rates at which primary care patients are assessed for risk factors and the rates at which patients at higher risk are screened remain below the goals set by the CDC.3–6 All health care practitioners need to understand how to establish or exclude a diagnosis of HCV infection and to interpret the tests correctly.
WHY SCREEN FOR HCV?
HCV infection is a major public health problem and a leading cause of chronic liver disease. In the United States, an estimated 3.2 million persons (1.3% of the population) have been infected.7 However, in the inner-city primary care setting the rate of HCV infection is as high as 8%, and in Veterans Administration populations it is 17%.8,9 The worldwide prevalence of HCV infection is 2.0%, corresponding to 140 million persons.
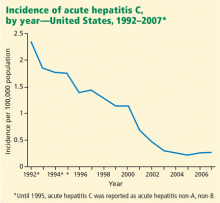
Screening of blood products has led to a decline in the incidence of acute hepatitis C since the late 1980s, although rates have reached a plateau in recent years (Figure 1).10
Approximately 20% of patients infected with HCV develop a serious sequela, such as severe fibrosis, cirrhosis, end-stage liver disease, or hepatocellular carcinoma. Currently, HCV infection causes an estimated 8,000 to 10,000 deaths annually in the United States, and that number is predicted to triple in the next 10 to 20 years. Furthermore, HCV-related disease is the leading indication for liver transplantation in the United States, and it is estimated to cost $600 million to $1 billion annually in medical expenses and loss of work.8
Screening can reduce adverse outcomes
HCV screening has several potential benefits. By detecting HCV infection early, screening facilitates virologic suppression, as treatment earlier in the course of the disease is more effective than later.11,12 Further, early diagnosis together with patient education and subsequent lifestyle modifications may reduce the risk of transmission of HCV infection to other people.13,14
Antiviral therapy with pegylated interferons and ribavirin can cure hepatitis C in up to 90% of cases, depending on the viral genotype15–17 (see discussion of HCV genotypes below). In addition, treatment slows the progression of fibrosis.18 The incidence of hepatocellular carcinoma is lower in patients who achieve a sustained virologic response to antiviral therapy.19 Finally, antiviral therapy prolongs survival.20
New drug therapies are being developed and may, we hope, be even more effective than current drugs. Inhibitors of HCV-specific enzymes such as NS3/4 protease, combined with pegylated interferons and ribavirin, are in phase III clinical trials. These drugs are expected to be available for clinical practice within the next 2 years.21–23 Additionally, nitazoxanide (Alinia), an inducer of eIF2a and PKR phosphorylation, has been shown to increase the treatment response to HCV genotype 4. Studies24 are currently under way in patients infected with HCV genotype 1.
Screening is cost-effective
The National Hepatitis Surveillance Program25 calculated the cost of screening for HCV to be $1,246 per case detected. However, a more vigorous analysis of the same data using several different models to incorporate risk factors based on history revealed costs between $357 and $1,047 per case detected. This compares favorably with the cost of screening for other diseases that physicians routinely screen for.
Antiviral combination therapy for chronic hepatitis C has been shown to be effective in terms of quality-adjusted life-years gained and cost-effectiveness in several studies.26–28
HOW TO SCREEN
To test everyone in the general population would be neither cost-effective nor practical, which is why the CDC recommends that serologic screening for HCV infection be done only in people who have well-established risk factors for it.1,5
Therefore, screening should begin by obtaining a relevant medical history as part of a routine health evaluation. But how should this be done?
McGinn et al29 asked 1,000 patients attending an inner-city clinic to fill out a 27-item questionnaire assessing five “domains” of risk factors for HCV: work, medical, exposure, personal care, and social history. Afterward, they tested all 1,000 patients. They found that the risk factors that best predicted positive results on testing were in three domains: medical (eg, blood transfusions, dialysis, other medical procedures, and elevated liver enzymes), exposure (past contact with another person’s blood), and social history (eg, illicit drug use, incarceration, and sexual activity).
The National Hepatitis Surveillance Program25 explored the cost and yield of several screening strategies for hepatitis C, ie, testing only in patients who had a greater than 7% likelihood of infection based on an empirically derived mathematical model; testing only if significant risk factors were revealed in a simple questionnaire; or testing only if the alanine aminotransferase (ALT) level was elevated. The predictive mathematical model was the most effective and efficient means of deciding who should be tested.
Unfortunately, such a model is too cumbersome to be clinically applicable, and clinical prediction tools for HCV screening have been underused.
GROUPS AT HIGH RISK OF HCV
Groups at risk of HCV infection can be classified as being at high, intermediate, or low risk. The American Association for the Study of Liver Diseases2 rates the level of evidence for screening in all of the following risk groups as class I (ie, there is evidence or general agreement that it is beneficial, useful, and effective) and level B (ie, the data are derived from non-randomized studies).
Intravenous drug abusers
Intravenous drug abuse is the strongest independent risk factor for HCV infection.30–33 It has been the main route of HCV infection over the past decades and currently accounts for 60% of HCV transmission in the United States.7,10,34–37
Hemophilia patients treated with clotting factor concentrates produced before 1987
HCV seroprevalence is very high in patients with hemophilia who received infusions of plasma-derived clotting factor concentrates before 1987.38 In these patients, the HCV genotypes are predominantly 1 and 3, and to a lesser extent genotype 2.39,40 These genotypes likely reflect the prior exposures of the plasma donors.41 (See discussion of HCV genotypes below.) Individuals receiving clotting factor concentrates prepared from plasma pools were at high risk of HCV infection until effective procedures to inactivate viruses were introduced in 1985 (factor VIII) and 1987 (factor IX).42
People infected with HIV
About 25% of people infected with human immunodeficiency virus (HIV) in the Western world also have chronic HCV infection.43 Progression of liver disease is accelerated in HIV-HCV coinfection, and the risk of cirrhosis is twice as high.44
However, about 6% of HIV-positive patients fail to develop HCV antibodies when infected. Thus, HCV RNA should be assessed in HIV patients with unexplained liver disease who are negative for anti-HCV.45
The distribution of HCV genotypes in HIV-infected patients reflects the route of transmission. Genotype 1b accounts for 66% of posttransfusion HCV infections, while genotypes 1a and 3a are more common in intravenous drug users.
GROUPS AT INTERMEDIATE RISK OF HCV
Recipients of blood transfusions before 1992
Before 1992, blood transfusions carried a risk of HCV infection of up to 7% with each unit transfused. Prospective studies of transfusion recipients in the United States found that rates of posttransfusion hepatitis in the 1960s exceeded 20%,36 since most patients received multiple units of blood.
In the mid-1970s, before HCV had been identified, available diagnostic tests indicated that 90% of cases of posttransfusion hepatitis were not caused by hepatitis A or hepatitis B viruses. By this time, the move to all-volunteer blood donors instead of paid donors had reduced the risk of posttransfusion hepatitis to 10%.22,37,46
Although non-A, non-B hepatitis was first recognized because of its association with blood transfusion, population-based sentinel surveillance showed that it accounted for 15% to 20% of cases of community-acquired viral hepatitis in the United States.35 The advent of molecular cloning in 1988 indicated that non-A, non-B hepatitis was primarily caused by HCV.47–52
Screening of blood has reduced the rate of posttransfusion hepatitis C by a factor of about 10,000, to a current rate of 1 per million transfusions.53 The few cases that still occur are due to newly infected people donating blood before they have developed antibodies to the virus, which can take up to 8 weeks.54
Recipients of solid-organ transplants before 1992
Before organ donors were screened for HCV, recipients of solid-organ transplants from infected donors had a high risk of acquiring HCV infection. Transmission rates in different cohorts ranged from 30% to 80%.55 In an attempt to improve the safety of organ transplantation, many transplant centers now screen donors for anti-HCV and test for HCV RNA for verification.
A related problem is pre-existing HCV infection in transplant recipients. Izopet et al56 reported that, in renal transplant recipients with preexisting HCV infection, the HCV RNA titer rose about 10 times (1 log) higher after transplantation, owing to the immunosuppressive drugs that transplant recipients must take. Although this higher viral load does not affect the progression of fibrosis in all patients, the effect of immunosuppressive therapy on liver disease results in a worse outcome for some, and it reduces survival beginning in the second decade after kidney transplantation.56
Additionally, treatment of HCV infection in transplant recipients may pose a challenge, as those receiving immunosuppressive therapy with tacrolimus (Prograf) or cyclosporine (Sandimmune) may develop some degree of renal insufficiency, complicating the use of ribavirin (Rebetol) and subjecting patients to a higher risk of severe anemia. Furthermore, interferon therapy increases the risk of renal allograft rejection and, accordingly, is not often used in renal transplant recipients.
Patients with unexplained elevated aminotransferase levels
HCV infection affects an estimated 1.8% of the general population, but the rate is much higher in people with ALT levels over 40 U/L. Most patients with chronic hepatitis C have no symptoms or only mild symptoms and minimally elevated levels of ALT and aspartate aminotransferase (AST)—ie, two to five times higher than the upper limit of normal.
The first step in the workup of aminotransferase elevations is to confirm the abnormality by repeating the blood test. If an elevation is confirmed, further investigation is warranted. A directed history and physical examination is important and may disclose risk factors, raising clinical suspicion of a particular disease.
Some caveats: The proportion of patients with HCV viremia who have abnormally high aminotransferase levels ranges between only 54% and 66%.57–59 In patients with risk factors for HCV infection and abnormal liver enzyme levels, HCV infection is probable but not certain. Also, liver enzyme tests do not reveal the extent of hepatic injury or reflect the true status of hepatic function.60
Infants born to infected mothers
Children born to HCV-positive women should be tested for anti-HCV no sooner than age 12 months, when passively transferred maternal anti-HCV declines below detectable levels. If earlier diagnosis of HCV infection is desired, a real-time polymerase chain reaction (PCR) test for HCV RNA can be done at or after the infant's first “well-child” visit at age 1 to 2 months.
If positive for either anti-HCV or HCV RNA, children should be evaluated for liver disease, and those with persistently elevated ALT levels should be referred to a specialist for medical management.2,5
GROUPS AT LOW RISK OF HCV
People who have had sexual relations with multiple or infected partners
Sexual activity is associated with a low but measurable risk of transmission of HCV. Large population-based studies, including the National Hepatitis Surveillance Program,25 found an independent association between HCV infection and having sexual relations with multiple partners or with a partner who is infected with HCV.
The CDC reported that 15% to 20% of patients with acute hepatitis C had a history of sexual exposure but no other risk factors. Two-thirds of them had an anti-HCV-positive sexual partner, and one-third reported having had more than two partners in the 6 months before illness.5
More data are needed to determine the risk of and the factors related to transmission of HCV between long-term steady partners as well as in persons with high-risk sexual practices, including whether other sexually transmitted diseases promote transmission of HCV by influencing viral load or modifying mucosal barriers.
Health care workers exposed to HCV, eg, by needlestick
The prevalence of HCV infection in health care workers is no greater than that in the general population, averaging 1% to 2%, and is actually 10 times lower than that of hepatitis B virus infection.47,48,61,62
However, within the disciplines, some groups have a higher prevalence of HCV infection, suggesting that some occupations carry a higher risk. In two US studies, the prevalence of HCV infection was higher in oral surgeons (2.0% and 9.3%) than in other dentists (0.7% and 0.97%).63,64
In a single study that evaluated risk factors for infection, a history of needlestick injury was the only occupational risk factor that was independently associated with HCV infection.65 The average incidence of anti-HCV seroconversion after a needlestick or after an injury with a sharp object contaminated by an HCV-positive source is 1.8% (range 0%–7%).66–69
Although no studies of incidence have documented transmission via mucous membrane or nonintact skin exposures, transmission of HCV from blood splashes to the conjunctiva have been described.70,71
It is worth noting that exposure to blood from unclean needles used in tattooing or body piercing also confers a risk of HCV infection.
SEROLOGIC SCREENING TESTS FOR HCV
- Serologic assays that detect specific antibody to HCV (anti-HCV)
- Molecular assays that detect viral RNA.
Initial serologic screening tests for anti-HCV
Two EIAs are approved for clinical use:
- Abbott HCV EIA 2.0 (Abbott Laboratories, Abbott Park, IL)
- Ortho HCV Version 3.0 enzyme-linked immunosorbent assay (ELISA) (Ortho-Clinical Diagnostics, Rochester, NY).
One enhanced chemiluminescence immunoassay is also approved:
- Vitros Anti-HCV assay (Ortho-Clinical Diagnostics). In practical terms, this test is equivalent to the two EIAs, and the discussion below about EIAs applies to this test as well.
These third-generation tests are highly sensitive (> 99%) and specific (99%) in immunocompetent patients, and eliminate the need for a confirmatory immunoblot assay in patients with clinical liver disease, particularly those with risk factors for HCV infection.
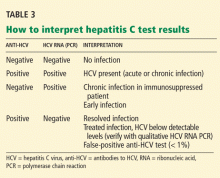
False-positive results are rare now, but they were common with earlier generations of these assays. Most false-positive results occur in patients with autoimmune liver disease or hypergammaglobulinemia who have normal liver enzyme levels and no risk factors for HCV infection. In fact, all positive anti-HCV results should be followed up with an HCV RNA test.
False-negative results are also uncommon, usually occurring only in immunosuppressed patients (eg, organ transplant recipients and HIV-positive patients) and in patients on long-term hemodialysis. Therefore, patients with a history of hemodialysis should be considered for an HCV RNA assay rather than an EIA. Measurement of ALT will not be useful because ALT levels are lower in patients with end-stage renal disease. In most other clinical situations, the HCV EIA is an outstanding screening test for HCV infection because of its high sensitivity and relatively low cost (< $50).
Although the specificity of these tests is good, the predictive value of a positive result varies substantially by the pretest probability of HCV infection. For example, in a group of injection-drug users who are very likely to have ongoing or remote infection, all positive HCV EIA results are likely truly positive.74 On the other hand, in healthy blood donors, up to half of all positive third-generation EIA tests are falsely positive.75
Important points
- A positive anti-HCV antibody test does not distinguish acute from chronic disease or active from past infection, nor is it a sign of immunity or protection.
- A positive anti-HCV EIA requires HCV RNA measurement to discriminate between current infection on the one hand, and either resolved HCV infection or a false-positive result on the other.
- A positive EIA anti-HCV test is a marker that hepatitis C may be present, and it must be followed by confirmatory HCV RNA testing.
- Physicians should be mindful of the potential tribulations associated with false-positive tests. A false-positive test may result in harm to patients that is difficult to measure, such as anxiety, labeling in the medical record, and detrimental effects on close relationships.
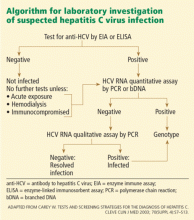
CONFIRMATORY TESTING WITH ASSAYS FOR HCV RNA
As stated above, a positive result on an anti-HCV EIA needs to be confirmed with an assay for HCV RNA, of which there are two types, ie, qualitative and quantitative.
Each involves trade-offs. Qualitative assays are more sensitive and detect more cases, but they provide no information about the amount of virus (viral load). Quantitative assays are less sensitive, so a negative result does not completely exclude hepatitis C, although they can still can detect 95% of cases. They do, however, measure the viral load.
Therefore, the type of test to use depends on the patient’s risk profile, the goals of testing, and the setting in which future care will be provided. The primary objective when a patient has a positive EIA test is to determine whether he or she has ongoing infection, a goal most expeditiously achieved using a qualitative assay. However, since a quantitative assay can detect the vast majority of cases of active HCV infection, many clinicians select this as the test of first choice when the probability of HCV is high (eg, in a patient with risk factors and abnormal liver tests). If the pretest probability is low, a qualitative assay is the better choice.
Many commercial assays are available for detecting (qualitative assays) or measuring (quantitative assays) HCV RNA.
Qualitative HCV RNA assays
The approved qualitative assays are:
- Amplicor HCV Test, version 2.0 (Roche Molecular Diagnostics, Pleasanton, CA)
- Cobas Amplicor HCV Test, version 2.0 (Roche Molecular Diagnostics)
- Ampliscreen (Roche Molecular Diagnostics)
- Versant HCV RNA Qualitative Assay (Siemens Healthcare Diagnostics, Deerfield, IL)
- Procleix HIV-1/HCV Assay (Chiron, Emeryville, CA).
Quantitative HCV RNA assays
The approved quantitative assays are:
- Amplicor HCV Monitor (Roche Molecular Diagnostics)
- Cobas Amplicor HCV Monitor, version 2.0 (Roche Molecular Diagnostics)
- Versant HCV RNA 3.0 Assay (bDNA) (Siemens Healthcare Diagnostics)
- Cobas Taqman HCV Test (Roche Molecular Diagnostics).
Quantitative tests use target amplification with PCR, transcription-mediated amplification (TMA), or a signal amplification technique such as a branched DNA (bDNA) assay. The sensitivity varies for different types of amplification. TMA assays appear to be the most sensitive for detecting HCV RNA.
The latest innovation is real-time PCR, which shortens the typical time for PCR processing from 1.5 hours to 35 minutes. It may also detect relapsed HCV infection earlier than regular PCR. With the recent availability of real-time PCR assays, which have sensitivities of 10 to 50 IU/mL, many experts feel there is no longer a need for qualitative assays.74 In fact, many laboratories no longer offer qualitative testing. The Cleveland Clinic laboratory has recently stopped offering this test.
Because RNA testing is widely available, the recombinant immunoblot assay (RIBA) has become obsolete in diagnosing HCV infection, except in special circumstances. Currently, the primary purpose of RIBA testing is to distinguish between resolved HCV infection (EIA-positive, HCV RNA-negative, RIBA-positive) and a false-positive EIA (EIA-positive, HCV RNA-negative, RIBA-negative).
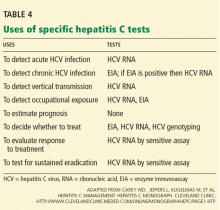
In summary, patients suspected of having acute or chronic HCV infection should first be tested for anti-HCV. Subsequently, HCV RNA testing should be performed in:
- Patients with a positive anti-HCV test
- Patients for whom antiviral treatment is being considered (using a sensitive quantitative assay)
- Patients with unexplained liver disease whose anti-HCV test is negative and who are immunocompromised or suspected of having acute HCV infection.
Significance of the HCV viral load
The significance of the HCV viral load is widely misunderstood. The amount of virus in the blood does not correlate with symptoms, histologic liver injury, or the stage or aggressiveness of disease. Its sole importance is in relation to therapy.
The HCV viral load, measured before treatment, helps predict the likelihood of a treatment response: the lower the pretreatment viral load, the more likely that the patient will respond to current HCV therapies.
Additionally, the pretreatment viral load serves as a baseline for comparison with subsequent measurements during treatment. Patients with HCV genotype 1 who do not achieve more than a 2-log (99%) reduction in viral load by the 12th week of treatment (an early virologic response) have a low response rate, and treatment should generally be stopped, given its cost and side effects.76 However, measuring the viral load to detect an early virologic response is less helpful in patients with HCV genotype 2 or 3 infection, since these patients require only 24 weeks of therapy and most of them clear the virus by week 12 and respond to therapy.
Additionally, patients with genotype 2 or 3 and those with a viral load of less than 600,000 IU/mL have been found to achieve higher rates of sustained virologic response.15 A sustained virologic response is defined as the absence of HCV RNA 24 weeks after stopping treatment and is now considered to be the best predictor of long-term treatment response. A sustained virologic response is generally regarded as a “virologic cure.”
HCV GENOTYPE AFFECTS SUCCESS AND DURATION OF TREATMENT
HCV has at least six major genotypes.1,3–6 Several genotypes are subclassified as “a” or “b” (ie, genotype 1a or 1b); however, these distinctions are of little clinical use.
In the laboratory, HCV genotypes are identified by restriction fragment length polymorphism, by direct sequence analysis, or by reverse hybridization. Once the HCV genotype has been identified, there is no need to repeat the test.
Different genotypes are more common in some areas of the world than in others. Genotype 1 is the one most common in the United States (accounting for 70% to 75% of cases), followed by genotypes 2 and 3 (25%–30%). Genotype 4 is most common in Egypt and the Arabian peninsula.
HCV genotyping is important because it can help predict the likelihood of a response to treatment and in planning the dose and duration of therapy.77 For example, treatment with pegylated interferon plus ribavirin is predicted to work approximately 50% of the time for people with genotype 1, but 80% to 90% of the time for people with genotypes 2 or 3.15–17,78 Additionally, patients with genotype 1 need 12 months of therapy to achieve maximum benefit, whereas those with genotypes 2 and 3 require treatment for only 6 months to achieve maximum benefit.
- Alter MJ, Seeff LB, Bacon BR, Thomas DL, Rigsby MO, Di Bisceglie AM. Testing for hepatitis C virus infection should be routine for persons at increased risk for infection. Ann Intern Med 2004; 141:715–717.
- Ghany MG, Strader DB, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology 2009; 49:1335–1374.
- Shehab TM, Orrego M, Chunduri R, Lok AS. Identification and management of hepatitis C patients in primary care clinics. Am J Gastroenterol 2003; 98:639–644.
- Shehab TM, Sonnad SS, Lok AS. Management of hepatitis C patients by primary care physicians in the USA: results of a national survey. J Viral Hepat 2001; 8:377–383.
- US Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep 1998; 47:1–39.
- US Centers for Disease Control and Prevention. National prevention strategy: a comprehensive strategy for the prevention and control of hepatitis C virus infection and its consequences; summer 2001. http://www.cdc.gov/hepatitis/HCV/Strategy/NatHep-CPrevStrategy.htm. Accessed August 8, 2010.
- Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med 2006; 144:705–714.
- Kim WR. The burden of hepatitis C in the United States. Hepatology 2002; 36(suppl 1):S30–S34.
- Lau DT, Kleiner DE, Ghany MG, Park Y, Schmid P, Hoofnagle JH. 10-Year follow-up after interferon-alpha therapy for chronic hepatitis C. Hepatology 1998; 28:1121–1127.
- Daniels D, Grytdal S, Wasley A; US Centers for Disease Control and Prevention. Surveillance for acute viral hepatitis—United States, 2007. MMWR Surveill Summ 2009; 58:1–27.
- Thomson BJ, Kwong G, Ratib S, et al; Trent HCV Study Group. Response rates to combination therapy for chronic HCV infection in a clinical setting and derivation of probability tables for individual patient management. J Viral Hepat 2008; 15:271–278.
- Hayashi N, Takehara T. Antiviral therapy for chronic hepatitis C: past, present, and future. J Gastroenterol 2006; 41:17–27.
- Gordon FD. Cost-effectiveness of screening patients for hepatitis C. Am J Med 1999; 107:36S–40S.
- Hill L, Henry B, Schweikert S; Prevention Practice Committee, American College of Preventive Medicine. Screening for chronic hepatitis C: American College of Preventive Medicine practice policy statement. Am J Prev Med 2005; 28:327–330.
- Hadziyannis SJ, Sette H, Morgan TR, et al; PEGASYS International Study Group. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med 2004; 140:346–355.
- Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001; 358:958–965.
- Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002; 347:975–982.
- Cammà C, Di Bona D, Schepis F, et al. Effect of peginterferon alfa-2a on liver histology in chronic hepatitis C: a meta-analysis of individual patient data. Hepatology 2004; 39:333–342.
- Yoshida H, Tateishi R, Arakawa Y, et al. Benefit of interferon therapy in hepatocellular carcinoma prevention for individual patients with chronic hepatitis C. Gut 2004; 53:425–430.
- Yoshida H, Arakawa Y, Sata M, et al. Interferon therapy prolonged life expectancy among chronic hepatitis C patients. Gastroenterology 2002; 123:483–491.
- Hézode C, Forestier N, Dusheiko G, et al; PROVE2 Study Team. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med 2009; 360:1839–1850.
- McHutchison JG, Everson GT, Gordon SC, et al; PROVE1 Study Team. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med 2009; 360:1827–1838.
- Berman K, Kwo PY. Boceprevir, an NS3 protease inhibitor of HCV. Clin Liver Dis 2009; 13:429–439.
- Rossignol JF, Elfert A, Keeffe EB. Treatment of chronic hepatitis C using a 4-week lead-in with nitazoxanide before peginterferon plus nitazoxanide. J Clin Gastroenterol 2009 Dec 31; epub ahead of print.
- Lapane KL, Jakiche AF, Sugano D, Weng CS, Carey WD. Hepatitis C infection risk analysis: who should be screened? Comparison of multiple screening strategies based on the National Hepatitis Surveillance Program. Am J Gastroenterol 1998; 93:591–596.
- Wong JB, Davis GL, McHutchison JG, Manns MP, Albrecht JK; International Hepatitis Interventional Therapy Group. Economic and clinical effects of evaluating rapid viral response to peginterferon alfa-2b plus ribavirin for the initial treatment of chronic hepatitis C. Am J Gastroenterol 2003; 98:2354–2362.
- Salomon JA, Weinstein MC, Hammitt JK, Goldie SJ. Cost-effectiveness of treatment for chronic hepatitis C infection in an evolving patient population. JAMA 2003; 290:228–237.
- Sullivan SD, Jensen DM, Bernstein DE, et al. Cost-effectiveness of combination peginterferon alpha-2a and ribavirin compared with interferon alpha-2b and ribavirin in patients with chronic hepatitis C. Am J Gastroenterol 2004; 99:1490–1496.
- McGinn T, O’Connor-Moore N, Alfandre D, Gardenier D, Wisnivesky J. Validation of a hepatitis C screening tool in primary care. Arch Intern Med 2008; 168:2009–2013.
- Kaur S, Rybicki L, Bacon BR, Gollan JL, Rustgi VK, Carey WD. Performance characteristics and results of a large-scale screening program for viral hepatitis and risk factors associated with exposure to viral hepatitis B and C: results of the National Hepatitis Screening Survey. National Hepatitis Surveillance Group. Hepatology 1996; 24:979–986.
- Cheung RC. Epidemiology of hepatitis C virus infection in American veterans. Am J Gastroenterol 2000; 95:740–747.
- Austin GE, Jensen B, Leete J, et al. Prevalence of hepatitis C virus seropositivity among hospitalized US veterans. Am J Med Sci 2000; 319:353–359.
- Yawn BP, Wollan P, Gazzuola L, Kim WR. Diagnosis and 10-year follow-up of a community-based hepatitis C cohort. J Fam Pract 2002; 51:135–140.
- Garfein RS, Doherty MC, Monterroso ER, Thomas DL, Nelson KE, Vlahov D. Prevalence and incidence of hepatitis C virus infection among young adult injection drug users. J Acquir Immune Defic Syndr Hum Retrovirol 1998; 18(suppl 1):S11–S19.
- Alter MJ. The epidemiology of acute and chronic hepatitis C. Clin Liver Dis 1997; 1:559–568,
- Alter MJ, Hadler SC, Judson FN, et al. Risk factors for acute non-A, non-B hepatitis in the United States and association with hepatitis C virus infection. JAMA 1990; 264:2231–2235.
- Wasley A, Miller JT, Finelli L; Centers for Disease Control and Prevention (CDC). Surveillance for acute viral hepatitis—United States, 2005. MMWR Surveill Summ 2007; 56:1–24.
- Goedert JJ, Chen BE, Preiss L, Aledort LM, Rosenberg PS. Reconstruction of the hepatitis C virus epidemic in the US hemophilia population, 1940–1990. Am J Epidemiol 2007; 165:1443–1453.
- Eyster ME, Sherman KE, Goedert JJ, Katsoulidou A, Hatzakis A. Prevalence and changes in hepatitis C virus genotypes among multitransfused persons with hemophilia. The Multicenter Hemophilia Cohort Study. J Infect Dis 1999; 179:1062–1069.
- Yee TT, Griffioen A, Sabin CA, Dusheiko G, Lee CA. The natural history of HCV in a cohort of haemophilic patients infected between 1961 and 1985. Gut 2000; 47:845–851.
- Lee C, Dusheiko G. The natural history and antiviral treatment of hepatitis C in haemophilia. Haemophilia 2002; 8:322–329.
- Makris M, Garson JA, Ring CJ, Tuke PW, Tedder RS, Preston FE. Hepatitis C viral RNA in clotting factor concentrates and the development of hepatitis in recipients. Blood 1993; 81:1898–1902.
- Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis 2002; 34:831–837.
- Sulkowski MS. The HIV-coinfected patient: managing viral hepatitis. J Acquir Immune Defic Syndr 2007; 45(suppl 2):S36–S37.
- Bonacini M, Lin HJ, Hollinger FB. Effect of coexisting HIV-1 infection on the diagnosis and evaluation of hepatitis C virus. J Acquir Immune Defic Syndr 2001; 26:340–344.
- Garfein RS, Vlahov D, Galai N, Doherty MC, Nelson KE. Viral infections in short-term injection drug users: the prevalence of the hepatitis C, hepatitis B, human immunodeficiency, and human T-lymphotropic viruses. Am J Public Health 1996; 86:655–661.
- Bell J, Batey RG, Farrell GC, Crewe EB, Cunningham AL, Byth K. Hepatitis C virus in intravenous drug users. Med J Aust 1990; 153:274–276.
- Villano SA, Vlahov D, Nelson KE, Lyles CM, Cohn S, Thomas DL. Incidence and risk factors for hepatitis C among injection drug users in Baltimore, Maryland. J Clin Microbiol 1997; 35:3274–3277.
- Patrick DM, Tyndall MW, Cornelisse PG, et al. Incidence of hepatitis C virus infection among injection drug users during an outbreak of HIV infection. CMAJ 2001; 165:889–895.
- Seeff LB, Wright EC, Zimmerman HJ, McCollum RW. VA cooperative study of post-transfusion hepatitis, 1969-1974: incidence and characteristics of hepatitis and responsible risk factors. Am J Med Sci 1975; 270:355–362.
- Feinstone SM, Kapikian AZ, Purcell RH, Alter HJ, Holland PV. Transfusion-associated hepatitis not due to viral hepatitis type A or B. N Engl J Med 1975; 292:767–770.
- Alter HJ, Holland PV, Purcell RH, et al. Posttransfusion hepatitis after exclusion of commercial and hepatitis-B antigen-positive donors. Ann Intern Med. 1972; 77:691–699.
- Blajchman MA, Vamvakas EC. The continuing risk of transfusion-transmitted infections. N Engl J Med 2006; 355:1303–1305.
- Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med 2001; 345:41–52.
- Roth D, Zucker K, Cirocco R, et al. The impact of hepatitis C virus infection on renal allograft recipients. Kidney Int 1994; 45:238–244.
- Izopet J, Rostaing L, Sandres K, et al. Longitudinal analysis of hepatitis C virus replication and liver fibrosis progression in renal transplant recipients. J Infect Dis 2000; 181:852–858.
- Dubois F, Desenclos JC, Mariotte N, Goudeau A. Hepatitis C in a French population-based survey, 1994: seroprevalence, frequency of viremia, genotype distribution, and risk factors. The Collaborative Study Group. Hepatology 1997; 25:1490–1496.
- Bellentani S, Pozzato G, Saccoccio G, et al. Clinical course and risk factors of hepatitis C virus related liver disease in the general population: report from the Dionysos study. Gut 1999; 44:874–880.
- Alberti A, Noventa F, Benvegnù L, Boccato S, Gatta A. Prevalence of liver disease in a population of asymptomatic persons with hepatitis C virus infection. Ann Intern Med 2002; 137:961–964.
- Shiffman ML, Diago M, Tran A, et al. Chronic hepatitis C in patients with persistently normal alanine transaminase levels. Clin Gastroenterol Hepatol 2006; 4:645–652.
- Stary A, Kopp W, Hofmann H, Heller-Vitouch C, Kunz C. Seroepidemiologic study of hepatitis C virus in sexually transmitted disease risk groups. Sex Transm Dis 1992; 19:252–258.
- Weinstock HS, Bolan G, Reingold AL, Polish LB. Hepatitis C virus infection among patients attending a clinic for sexually transmitted diseases. JAMA 1993; 269:392–394.
- Thomas DL, Gruninger SE, Siew C, Joy ED, Quinn TC. Occupational risk of hepatitis C infections among general dentists and oral surgeons in North America. Am J Med 1996; 100:41–45.
- Klein RS, Freeman K, Taylor PE, Stevens CE. Occupational risk for hepatitis C virus infection among New York City dentists. Lancet 1991; 338:1539–1542.
- Polish LB, Tong MJ, Co RL, Coleman PJ, Alter MJ. Risk factors for hepatitis C virus infection among health care personnel in a community hospital. Am J Infect Control 1993; 21:196–200.
- Alter MJ. Occupational exposure to hepatitis C virus: a dilemma. Infect Control Hosp Epidemiol 1994; 15:742–744.
- Lanphear BP, Linnemann CC, Cannon CG, DeRonde MM, Pendy L, Kerley LM. Hepatitis C virus infection in healthcare workers: risk of exposure and infection. Infect Control Hosp Epidemiol 1994; 15:745–750.
- Puro V, Petrosillo N, Ippolito G. Risk of hepatitis C seroconversion after occupational exposures in health care workers. Italian Study Group on Occupational Risk of HIV and Other Bloodborne Infections. Am J Infect Control 1995; 23:273–277.
- Mitsui T, Iwano K, Masuko K, et al. Hepatitis C virus infection in medical personnel after needlestick accident. Hepatology 1992; 16:1109–1114.
- Sartori M, La Terra G, Aglietta M, Manzin A, Navino C, Verzetti G. Transmission of hepatitis C via blood splash into conjunctiva. Scand J Infect Dis 1993; 25:270–271.
- Ippolito G, Puro V, Petrosillo N, De Carli G, Micheloni G, Magliano E. Simultaneous infection with HIV and hepatitis C virus following occupational conjunctival blood exposure. JAMA 1998; 280:28.
- Carey W. Tests and screening strategies for the diagnosis of hepatitis C. Cleve Clin J Med 2003; 70(suppl 4):S7–S13.
- Carey WD, Jeffers L, Kugelmas M, et al; Hepatitis C management. Hepatitis C Monograph. Cleveland Clinic; www.clevelandclinicmeded.com/online/monograph/hepc/page1.htm. Accessed 7/30/2010.
- Scott JD, Gretch DR. Molecular diagnostics of hepatitis C virus infection: a systematic review. JAMA 2007; 297:724–732.
- Bowden DS, Berzsenyi MD. Chronic hepatitis C virus infection: genotyping and its clinical role. Future Microbiol 2006; 1:103–112.
- Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002; 347:975–982.
- Poynard T, McHutchison J, Davis GL, et al. Impact of interferon alfa-2b and ribavirin on progression of liver fibrosis in patients with chronic hepatitis C. Hepatology 2000; 32:1131–1137.
- Zeuzem S. Interferon-based therapy for chronic hepatitis C: current and future perspectives. Nat Clin Pract Gastroenterol Hepatol 2008; 5:610–622.
Screening for hepatitis C virus (HCV) infection in high-risk populations can identify, early on, people at risk of progressive liver disease who may benefit from antiviral therapy and counseling. The US Centers for Disease Control and Prevention (CDC) recommends that all people be assessed for HCV risk factors and that those with risk factors be screened for HCV antibodies (anti-HCV),1 and members of the national societies of gastroenterology and hepatology have endorsed this recommendation.2
Unfortunately, rates at which primary care patients are assessed for risk factors and the rates at which patients at higher risk are screened remain below the goals set by the CDC.3–6 All health care practitioners need to understand how to establish or exclude a diagnosis of HCV infection and to interpret the tests correctly.
WHY SCREEN FOR HCV?
HCV infection is a major public health problem and a leading cause of chronic liver disease. In the United States, an estimated 3.2 million persons (1.3% of the population) have been infected.7 However, in the inner-city primary care setting the rate of HCV infection is as high as 8%, and in Veterans Administration populations it is 17%.8,9 The worldwide prevalence of HCV infection is 2.0%, corresponding to 140 million persons.
Screening of blood products has led to a decline in the incidence of acute hepatitis C since the late 1980s, although rates have reached a plateau in recent years (Figure 1).10
Approximately 20% of patients infected with HCV develop a serious sequela, such as severe fibrosis, cirrhosis, end-stage liver disease, or hepatocellular carcinoma. Currently, HCV infection causes an estimated 8,000 to 10,000 deaths annually in the United States, and that number is predicted to triple in the next 10 to 20 years. Furthermore, HCV-related disease is the leading indication for liver transplantation in the United States, and it is estimated to cost $600 million to $1 billion annually in medical expenses and loss of work.8
Screening can reduce adverse outcomes
HCV screening has several potential benefits. By detecting HCV infection early, screening facilitates virologic suppression, as treatment earlier in the course of the disease is more effective than later.11,12 Further, early diagnosis together with patient education and subsequent lifestyle modifications may reduce the risk of transmission of HCV infection to other people.13,14
Antiviral therapy with pegylated interferons and ribavirin can cure hepatitis C in up to 90% of cases, depending on the viral genotype15–17 (see discussion of HCV genotypes below). In addition, treatment slows the progression of fibrosis.18 The incidence of hepatocellular carcinoma is lower in patients who achieve a sustained virologic response to antiviral therapy.19 Finally, antiviral therapy prolongs survival.20
New drug therapies are being developed and may, we hope, be even more effective than current drugs. Inhibitors of HCV-specific enzymes such as NS3/4 protease, combined with pegylated interferons and ribavirin, are in phase III clinical trials. These drugs are expected to be available for clinical practice within the next 2 years.21–23 Additionally, nitazoxanide (Alinia), an inducer of eIF2a and PKR phosphorylation, has been shown to increase the treatment response to HCV genotype 4. Studies24 are currently under way in patients infected with HCV genotype 1.
Screening is cost-effective
The National Hepatitis Surveillance Program25 calculated the cost of screening for HCV to be $1,246 per case detected. However, a more vigorous analysis of the same data using several different models to incorporate risk factors based on history revealed costs between $357 and $1,047 per case detected. This compares favorably with the cost of screening for other diseases that physicians routinely screen for.
Antiviral combination therapy for chronic hepatitis C has been shown to be effective in terms of quality-adjusted life-years gained and cost-effectiveness in several studies.26–28
HOW TO SCREEN
To test everyone in the general population would be neither cost-effective nor practical, which is why the CDC recommends that serologic screening for HCV infection be done only in people who have well-established risk factors for it.1,5
Therefore, screening should begin by obtaining a relevant medical history as part of a routine health evaluation. But how should this be done?
McGinn et al29 asked 1,000 patients attending an inner-city clinic to fill out a 27-item questionnaire assessing five “domains” of risk factors for HCV: work, medical, exposure, personal care, and social history. Afterward, they tested all 1,000 patients. They found that the risk factors that best predicted positive results on testing were in three domains: medical (eg, blood transfusions, dialysis, other medical procedures, and elevated liver enzymes), exposure (past contact with another person’s blood), and social history (eg, illicit drug use, incarceration, and sexual activity).
The National Hepatitis Surveillance Program25 explored the cost and yield of several screening strategies for hepatitis C, ie, testing only in patients who had a greater than 7% likelihood of infection based on an empirically derived mathematical model; testing only if significant risk factors were revealed in a simple questionnaire; or testing only if the alanine aminotransferase (ALT) level was elevated. The predictive mathematical model was the most effective and efficient means of deciding who should be tested.
Unfortunately, such a model is too cumbersome to be clinically applicable, and clinical prediction tools for HCV screening have been underused.
GROUPS AT HIGH RISK OF HCV
Groups at risk of HCV infection can be classified as being at high, intermediate, or low risk. The American Association for the Study of Liver Diseases2 rates the level of evidence for screening in all of the following risk groups as class I (ie, there is evidence or general agreement that it is beneficial, useful, and effective) and level B (ie, the data are derived from non-randomized studies).
Intravenous drug abusers
Intravenous drug abuse is the strongest independent risk factor for HCV infection.30–33 It has been the main route of HCV infection over the past decades and currently accounts for 60% of HCV transmission in the United States.7,10,34–37
Hemophilia patients treated with clotting factor concentrates produced before 1987
HCV seroprevalence is very high in patients with hemophilia who received infusions of plasma-derived clotting factor concentrates before 1987.38 In these patients, the HCV genotypes are predominantly 1 and 3, and to a lesser extent genotype 2.39,40 These genotypes likely reflect the prior exposures of the plasma donors.41 (See discussion of HCV genotypes below.) Individuals receiving clotting factor concentrates prepared from plasma pools were at high risk of HCV infection until effective procedures to inactivate viruses were introduced in 1985 (factor VIII) and 1987 (factor IX).42
People infected with HIV
About 25% of people infected with human immunodeficiency virus (HIV) in the Western world also have chronic HCV infection.43 Progression of liver disease is accelerated in HIV-HCV coinfection, and the risk of cirrhosis is twice as high.44
However, about 6% of HIV-positive patients fail to develop HCV antibodies when infected. Thus, HCV RNA should be assessed in HIV patients with unexplained liver disease who are negative for anti-HCV.45
The distribution of HCV genotypes in HIV-infected patients reflects the route of transmission. Genotype 1b accounts for 66% of posttransfusion HCV infections, while genotypes 1a and 3a are more common in intravenous drug users.
GROUPS AT INTERMEDIATE RISK OF HCV
Recipients of blood transfusions before 1992
Before 1992, blood transfusions carried a risk of HCV infection of up to 7% with each unit transfused. Prospective studies of transfusion recipients in the United States found that rates of posttransfusion hepatitis in the 1960s exceeded 20%,36 since most patients received multiple units of blood.
In the mid-1970s, before HCV had been identified, available diagnostic tests indicated that 90% of cases of posttransfusion hepatitis were not caused by hepatitis A or hepatitis B viruses. By this time, the move to all-volunteer blood donors instead of paid donors had reduced the risk of posttransfusion hepatitis to 10%.22,37,46
Although non-A, non-B hepatitis was first recognized because of its association with blood transfusion, population-based sentinel surveillance showed that it accounted for 15% to 20% of cases of community-acquired viral hepatitis in the United States.35 The advent of molecular cloning in 1988 indicated that non-A, non-B hepatitis was primarily caused by HCV.47–52
Screening of blood has reduced the rate of posttransfusion hepatitis C by a factor of about 10,000, to a current rate of 1 per million transfusions.53 The few cases that still occur are due to newly infected people donating blood before they have developed antibodies to the virus, which can take up to 8 weeks.54
Recipients of solid-organ transplants before 1992
Before organ donors were screened for HCV, recipients of solid-organ transplants from infected donors had a high risk of acquiring HCV infection. Transmission rates in different cohorts ranged from 30% to 80%.55 In an attempt to improve the safety of organ transplantation, many transplant centers now screen donors for anti-HCV and test for HCV RNA for verification.
A related problem is pre-existing HCV infection in transplant recipients. Izopet et al56 reported that, in renal transplant recipients with preexisting HCV infection, the HCV RNA titer rose about 10 times (1 log) higher after transplantation, owing to the immunosuppressive drugs that transplant recipients must take. Although this higher viral load does not affect the progression of fibrosis in all patients, the effect of immunosuppressive therapy on liver disease results in a worse outcome for some, and it reduces survival beginning in the second decade after kidney transplantation.56
Additionally, treatment of HCV infection in transplant recipients may pose a challenge, as those receiving immunosuppressive therapy with tacrolimus (Prograf) or cyclosporine (Sandimmune) may develop some degree of renal insufficiency, complicating the use of ribavirin (Rebetol) and subjecting patients to a higher risk of severe anemia. Furthermore, interferon therapy increases the risk of renal allograft rejection and, accordingly, is not often used in renal transplant recipients.
Patients with unexplained elevated aminotransferase levels
HCV infection affects an estimated 1.8% of the general population, but the rate is much higher in people with ALT levels over 40 U/L. Most patients with chronic hepatitis C have no symptoms or only mild symptoms and minimally elevated levels of ALT and aspartate aminotransferase (AST)—ie, two to five times higher than the upper limit of normal.
The first step in the workup of aminotransferase elevations is to confirm the abnormality by repeating the blood test. If an elevation is confirmed, further investigation is warranted. A directed history and physical examination is important and may disclose risk factors, raising clinical suspicion of a particular disease.
Some caveats: The proportion of patients with HCV viremia who have abnormally high aminotransferase levels ranges between only 54% and 66%.57–59 In patients with risk factors for HCV infection and abnormal liver enzyme levels, HCV infection is probable but not certain. Also, liver enzyme tests do not reveal the extent of hepatic injury or reflect the true status of hepatic function.60
Infants born to infected mothers
Children born to HCV-positive women should be tested for anti-HCV no sooner than age 12 months, when passively transferred maternal anti-HCV declines below detectable levels. If earlier diagnosis of HCV infection is desired, a real-time polymerase chain reaction (PCR) test for HCV RNA can be done at or after the infant's first “well-child” visit at age 1 to 2 months.
If positive for either anti-HCV or HCV RNA, children should be evaluated for liver disease, and those with persistently elevated ALT levels should be referred to a specialist for medical management.2,5
GROUPS AT LOW RISK OF HCV
People who have had sexual relations with multiple or infected partners
Sexual activity is associated with a low but measurable risk of transmission of HCV. Large population-based studies, including the National Hepatitis Surveillance Program,25 found an independent association between HCV infection and having sexual relations with multiple partners or with a partner who is infected with HCV.
The CDC reported that 15% to 20% of patients with acute hepatitis C had a history of sexual exposure but no other risk factors. Two-thirds of them had an anti-HCV-positive sexual partner, and one-third reported having had more than two partners in the 6 months before illness.5
More data are needed to determine the risk of and the factors related to transmission of HCV between long-term steady partners as well as in persons with high-risk sexual practices, including whether other sexually transmitted diseases promote transmission of HCV by influencing viral load or modifying mucosal barriers.
Health care workers exposed to HCV, eg, by needlestick
The prevalence of HCV infection in health care workers is no greater than that in the general population, averaging 1% to 2%, and is actually 10 times lower than that of hepatitis B virus infection.47,48,61,62
However, within the disciplines, some groups have a higher prevalence of HCV infection, suggesting that some occupations carry a higher risk. In two US studies, the prevalence of HCV infection was higher in oral surgeons (2.0% and 9.3%) than in other dentists (0.7% and 0.97%).63,64
In a single study that evaluated risk factors for infection, a history of needlestick injury was the only occupational risk factor that was independently associated with HCV infection.65 The average incidence of anti-HCV seroconversion after a needlestick or after an injury with a sharp object contaminated by an HCV-positive source is 1.8% (range 0%–7%).66–69
Although no studies of incidence have documented transmission via mucous membrane or nonintact skin exposures, transmission of HCV from blood splashes to the conjunctiva have been described.70,71
It is worth noting that exposure to blood from unclean needles used in tattooing or body piercing also confers a risk of HCV infection.
SEROLOGIC SCREENING TESTS FOR HCV
- Serologic assays that detect specific antibody to HCV (anti-HCV)
- Molecular assays that detect viral RNA.
Initial serologic screening tests for anti-HCV
Two EIAs are approved for clinical use:
- Abbott HCV EIA 2.0 (Abbott Laboratories, Abbott Park, IL)
- Ortho HCV Version 3.0 enzyme-linked immunosorbent assay (ELISA) (Ortho-Clinical Diagnostics, Rochester, NY).
One enhanced chemiluminescence immunoassay is also approved:
- Vitros Anti-HCV assay (Ortho-Clinical Diagnostics). In practical terms, this test is equivalent to the two EIAs, and the discussion below about EIAs applies to this test as well.
These third-generation tests are highly sensitive (> 99%) and specific (99%) in immunocompetent patients, and eliminate the need for a confirmatory immunoblot assay in patients with clinical liver disease, particularly those with risk factors for HCV infection.
False-positive results are rare now, but they were common with earlier generations of these assays. Most false-positive results occur in patients with autoimmune liver disease or hypergammaglobulinemia who have normal liver enzyme levels and no risk factors for HCV infection. In fact, all positive anti-HCV results should be followed up with an HCV RNA test.
False-negative results are also uncommon, usually occurring only in immunosuppressed patients (eg, organ transplant recipients and HIV-positive patients) and in patients on long-term hemodialysis. Therefore, patients with a history of hemodialysis should be considered for an HCV RNA assay rather than an EIA. Measurement of ALT will not be useful because ALT levels are lower in patients with end-stage renal disease. In most other clinical situations, the HCV EIA is an outstanding screening test for HCV infection because of its high sensitivity and relatively low cost (< $50).
Although the specificity of these tests is good, the predictive value of a positive result varies substantially by the pretest probability of HCV infection. For example, in a group of injection-drug users who are very likely to have ongoing or remote infection, all positive HCV EIA results are likely truly positive.74 On the other hand, in healthy blood donors, up to half of all positive third-generation EIA tests are falsely positive.75
Important points
- A positive anti-HCV antibody test does not distinguish acute from chronic disease or active from past infection, nor is it a sign of immunity or protection.
- A positive anti-HCV EIA requires HCV RNA measurement to discriminate between current infection on the one hand, and either resolved HCV infection or a false-positive result on the other.
- A positive EIA anti-HCV test is a marker that hepatitis C may be present, and it must be followed by confirmatory HCV RNA testing.
- Physicians should be mindful of the potential tribulations associated with false-positive tests. A false-positive test may result in harm to patients that is difficult to measure, such as anxiety, labeling in the medical record, and detrimental effects on close relationships.
CONFIRMATORY TESTING WITH ASSAYS FOR HCV RNA
As stated above, a positive result on an anti-HCV EIA needs to be confirmed with an assay for HCV RNA, of which there are two types, ie, qualitative and quantitative.
Each involves trade-offs. Qualitative assays are more sensitive and detect more cases, but they provide no information about the amount of virus (viral load). Quantitative assays are less sensitive, so a negative result does not completely exclude hepatitis C, although they can still can detect 95% of cases. They do, however, measure the viral load.
Therefore, the type of test to use depends on the patient’s risk profile, the goals of testing, and the setting in which future care will be provided. The primary objective when a patient has a positive EIA test is to determine whether he or she has ongoing infection, a goal most expeditiously achieved using a qualitative assay. However, since a quantitative assay can detect the vast majority of cases of active HCV infection, many clinicians select this as the test of first choice when the probability of HCV is high (eg, in a patient with risk factors and abnormal liver tests). If the pretest probability is low, a qualitative assay is the better choice.
Many commercial assays are available for detecting (qualitative assays) or measuring (quantitative assays) HCV RNA.
Qualitative HCV RNA assays
The approved qualitative assays are:
- Amplicor HCV Test, version 2.0 (Roche Molecular Diagnostics, Pleasanton, CA)
- Cobas Amplicor HCV Test, version 2.0 (Roche Molecular Diagnostics)
- Ampliscreen (Roche Molecular Diagnostics)
- Versant HCV RNA Qualitative Assay (Siemens Healthcare Diagnostics, Deerfield, IL)
- Procleix HIV-1/HCV Assay (Chiron, Emeryville, CA).
Quantitative HCV RNA assays
The approved quantitative assays are:
- Amplicor HCV Monitor (Roche Molecular Diagnostics)
- Cobas Amplicor HCV Monitor, version 2.0 (Roche Molecular Diagnostics)
- Versant HCV RNA 3.0 Assay (bDNA) (Siemens Healthcare Diagnostics)
- Cobas Taqman HCV Test (Roche Molecular Diagnostics).
Quantitative tests use target amplification with PCR, transcription-mediated amplification (TMA), or a signal amplification technique such as a branched DNA (bDNA) assay. The sensitivity varies for different types of amplification. TMA assays appear to be the most sensitive for detecting HCV RNA.
The latest innovation is real-time PCR, which shortens the typical time for PCR processing from 1.5 hours to 35 minutes. It may also detect relapsed HCV infection earlier than regular PCR. With the recent availability of real-time PCR assays, which have sensitivities of 10 to 50 IU/mL, many experts feel there is no longer a need for qualitative assays.74 In fact, many laboratories no longer offer qualitative testing. The Cleveland Clinic laboratory has recently stopped offering this test.
Because RNA testing is widely available, the recombinant immunoblot assay (RIBA) has become obsolete in diagnosing HCV infection, except in special circumstances. Currently, the primary purpose of RIBA testing is to distinguish between resolved HCV infection (EIA-positive, HCV RNA-negative, RIBA-positive) and a false-positive EIA (EIA-positive, HCV RNA-negative, RIBA-negative).
In summary, patients suspected of having acute or chronic HCV infection should first be tested for anti-HCV. Subsequently, HCV RNA testing should be performed in:
- Patients with a positive anti-HCV test
- Patients for whom antiviral treatment is being considered (using a sensitive quantitative assay)
- Patients with unexplained liver disease whose anti-HCV test is negative and who are immunocompromised or suspected of having acute HCV infection.
Significance of the HCV viral load
The significance of the HCV viral load is widely misunderstood. The amount of virus in the blood does not correlate with symptoms, histologic liver injury, or the stage or aggressiveness of disease. Its sole importance is in relation to therapy.
The HCV viral load, measured before treatment, helps predict the likelihood of a treatment response: the lower the pretreatment viral load, the more likely that the patient will respond to current HCV therapies.
Additionally, the pretreatment viral load serves as a baseline for comparison with subsequent measurements during treatment. Patients with HCV genotype 1 who do not achieve more than a 2-log (99%) reduction in viral load by the 12th week of treatment (an early virologic response) have a low response rate, and treatment should generally be stopped, given its cost and side effects.76 However, measuring the viral load to detect an early virologic response is less helpful in patients with HCV genotype 2 or 3 infection, since these patients require only 24 weeks of therapy and most of them clear the virus by week 12 and respond to therapy.
Additionally, patients with genotype 2 or 3 and those with a viral load of less than 600,000 IU/mL have been found to achieve higher rates of sustained virologic response.15 A sustained virologic response is defined as the absence of HCV RNA 24 weeks after stopping treatment and is now considered to be the best predictor of long-term treatment response. A sustained virologic response is generally regarded as a “virologic cure.”
HCV GENOTYPE AFFECTS SUCCESS AND DURATION OF TREATMENT
HCV has at least six major genotypes.1,3–6 Several genotypes are subclassified as “a” or “b” (ie, genotype 1a or 1b); however, these distinctions are of little clinical use.
In the laboratory, HCV genotypes are identified by restriction fragment length polymorphism, by direct sequence analysis, or by reverse hybridization. Once the HCV genotype has been identified, there is no need to repeat the test.
Different genotypes are more common in some areas of the world than in others. Genotype 1 is the one most common in the United States (accounting for 70% to 75% of cases), followed by genotypes 2 and 3 (25%–30%). Genotype 4 is most common in Egypt and the Arabian peninsula.
HCV genotyping is important because it can help predict the likelihood of a response to treatment and in planning the dose and duration of therapy.77 For example, treatment with pegylated interferon plus ribavirin is predicted to work approximately 50% of the time for people with genotype 1, but 80% to 90% of the time for people with genotypes 2 or 3.15–17,78 Additionally, patients with genotype 1 need 12 months of therapy to achieve maximum benefit, whereas those with genotypes 2 and 3 require treatment for only 6 months to achieve maximum benefit.
Screening for hepatitis C virus (HCV) infection in high-risk populations can identify, early on, people at risk of progressive liver disease who may benefit from antiviral therapy and counseling. The US Centers for Disease Control and Prevention (CDC) recommends that all people be assessed for HCV risk factors and that those with risk factors be screened for HCV antibodies (anti-HCV),1 and members of the national societies of gastroenterology and hepatology have endorsed this recommendation.2
Unfortunately, rates at which primary care patients are assessed for risk factors and the rates at which patients at higher risk are screened remain below the goals set by the CDC.3–6 All health care practitioners need to understand how to establish or exclude a diagnosis of HCV infection and to interpret the tests correctly.
WHY SCREEN FOR HCV?
HCV infection is a major public health problem and a leading cause of chronic liver disease. In the United States, an estimated 3.2 million persons (1.3% of the population) have been infected.7 However, in the inner-city primary care setting the rate of HCV infection is as high as 8%, and in Veterans Administration populations it is 17%.8,9 The worldwide prevalence of HCV infection is 2.0%, corresponding to 140 million persons.
Screening of blood products has led to a decline in the incidence of acute hepatitis C since the late 1980s, although rates have reached a plateau in recent years (Figure 1).10
Approximately 20% of patients infected with HCV develop a serious sequela, such as severe fibrosis, cirrhosis, end-stage liver disease, or hepatocellular carcinoma. Currently, HCV infection causes an estimated 8,000 to 10,000 deaths annually in the United States, and that number is predicted to triple in the next 10 to 20 years. Furthermore, HCV-related disease is the leading indication for liver transplantation in the United States, and it is estimated to cost $600 million to $1 billion annually in medical expenses and loss of work.8
Screening can reduce adverse outcomes
HCV screening has several potential benefits. By detecting HCV infection early, screening facilitates virologic suppression, as treatment earlier in the course of the disease is more effective than later.11,12 Further, early diagnosis together with patient education and subsequent lifestyle modifications may reduce the risk of transmission of HCV infection to other people.13,14
Antiviral therapy with pegylated interferons and ribavirin can cure hepatitis C in up to 90% of cases, depending on the viral genotype15–17 (see discussion of HCV genotypes below). In addition, treatment slows the progression of fibrosis.18 The incidence of hepatocellular carcinoma is lower in patients who achieve a sustained virologic response to antiviral therapy.19 Finally, antiviral therapy prolongs survival.20
New drug therapies are being developed and may, we hope, be even more effective than current drugs. Inhibitors of HCV-specific enzymes such as NS3/4 protease, combined with pegylated interferons and ribavirin, are in phase III clinical trials. These drugs are expected to be available for clinical practice within the next 2 years.21–23 Additionally, nitazoxanide (Alinia), an inducer of eIF2a and PKR phosphorylation, has been shown to increase the treatment response to HCV genotype 4. Studies24 are currently under way in patients infected with HCV genotype 1.
Screening is cost-effective
The National Hepatitis Surveillance Program25 calculated the cost of screening for HCV to be $1,246 per case detected. However, a more vigorous analysis of the same data using several different models to incorporate risk factors based on history revealed costs between $357 and $1,047 per case detected. This compares favorably with the cost of screening for other diseases that physicians routinely screen for.
Antiviral combination therapy for chronic hepatitis C has been shown to be effective in terms of quality-adjusted life-years gained and cost-effectiveness in several studies.26–28
HOW TO SCREEN
To test everyone in the general population would be neither cost-effective nor practical, which is why the CDC recommends that serologic screening for HCV infection be done only in people who have well-established risk factors for it.1,5
Therefore, screening should begin by obtaining a relevant medical history as part of a routine health evaluation. But how should this be done?
McGinn et al29 asked 1,000 patients attending an inner-city clinic to fill out a 27-item questionnaire assessing five “domains” of risk factors for HCV: work, medical, exposure, personal care, and social history. Afterward, they tested all 1,000 patients. They found that the risk factors that best predicted positive results on testing were in three domains: medical (eg, blood transfusions, dialysis, other medical procedures, and elevated liver enzymes), exposure (past contact with another person’s blood), and social history (eg, illicit drug use, incarceration, and sexual activity).
The National Hepatitis Surveillance Program25 explored the cost and yield of several screening strategies for hepatitis C, ie, testing only in patients who had a greater than 7% likelihood of infection based on an empirically derived mathematical model; testing only if significant risk factors were revealed in a simple questionnaire; or testing only if the alanine aminotransferase (ALT) level was elevated. The predictive mathematical model was the most effective and efficient means of deciding who should be tested.
Unfortunately, such a model is too cumbersome to be clinically applicable, and clinical prediction tools for HCV screening have been underused.
GROUPS AT HIGH RISK OF HCV
Groups at risk of HCV infection can be classified as being at high, intermediate, or low risk. The American Association for the Study of Liver Diseases2 rates the level of evidence for screening in all of the following risk groups as class I (ie, there is evidence or general agreement that it is beneficial, useful, and effective) and level B (ie, the data are derived from non-randomized studies).
Intravenous drug abusers
Intravenous drug abuse is the strongest independent risk factor for HCV infection.30–33 It has been the main route of HCV infection over the past decades and currently accounts for 60% of HCV transmission in the United States.7,10,34–37
Hemophilia patients treated with clotting factor concentrates produced before 1987
HCV seroprevalence is very high in patients with hemophilia who received infusions of plasma-derived clotting factor concentrates before 1987.38 In these patients, the HCV genotypes are predominantly 1 and 3, and to a lesser extent genotype 2.39,40 These genotypes likely reflect the prior exposures of the plasma donors.41 (See discussion of HCV genotypes below.) Individuals receiving clotting factor concentrates prepared from plasma pools were at high risk of HCV infection until effective procedures to inactivate viruses were introduced in 1985 (factor VIII) and 1987 (factor IX).42
People infected with HIV
About 25% of people infected with human immunodeficiency virus (HIV) in the Western world also have chronic HCV infection.43 Progression of liver disease is accelerated in HIV-HCV coinfection, and the risk of cirrhosis is twice as high.44
However, about 6% of HIV-positive patients fail to develop HCV antibodies when infected. Thus, HCV RNA should be assessed in HIV patients with unexplained liver disease who are negative for anti-HCV.45
The distribution of HCV genotypes in HIV-infected patients reflects the route of transmission. Genotype 1b accounts for 66% of posttransfusion HCV infections, while genotypes 1a and 3a are more common in intravenous drug users.
GROUPS AT INTERMEDIATE RISK OF HCV
Recipients of blood transfusions before 1992
Before 1992, blood transfusions carried a risk of HCV infection of up to 7% with each unit transfused. Prospective studies of transfusion recipients in the United States found that rates of posttransfusion hepatitis in the 1960s exceeded 20%,36 since most patients received multiple units of blood.
In the mid-1970s, before HCV had been identified, available diagnostic tests indicated that 90% of cases of posttransfusion hepatitis were not caused by hepatitis A or hepatitis B viruses. By this time, the move to all-volunteer blood donors instead of paid donors had reduced the risk of posttransfusion hepatitis to 10%.22,37,46
Although non-A, non-B hepatitis was first recognized because of its association with blood transfusion, population-based sentinel surveillance showed that it accounted for 15% to 20% of cases of community-acquired viral hepatitis in the United States.35 The advent of molecular cloning in 1988 indicated that non-A, non-B hepatitis was primarily caused by HCV.47–52
Screening of blood has reduced the rate of posttransfusion hepatitis C by a factor of about 10,000, to a current rate of 1 per million transfusions.53 The few cases that still occur are due to newly infected people donating blood before they have developed antibodies to the virus, which can take up to 8 weeks.54
Recipients of solid-organ transplants before 1992
Before organ donors were screened for HCV, recipients of solid-organ transplants from infected donors had a high risk of acquiring HCV infection. Transmission rates in different cohorts ranged from 30% to 80%.55 In an attempt to improve the safety of organ transplantation, many transplant centers now screen donors for anti-HCV and test for HCV RNA for verification.
A related problem is pre-existing HCV infection in transplant recipients. Izopet et al56 reported that, in renal transplant recipients with preexisting HCV infection, the HCV RNA titer rose about 10 times (1 log) higher after transplantation, owing to the immunosuppressive drugs that transplant recipients must take. Although this higher viral load does not affect the progression of fibrosis in all patients, the effect of immunosuppressive therapy on liver disease results in a worse outcome for some, and it reduces survival beginning in the second decade after kidney transplantation.56
Additionally, treatment of HCV infection in transplant recipients may pose a challenge, as those receiving immunosuppressive therapy with tacrolimus (Prograf) or cyclosporine (Sandimmune) may develop some degree of renal insufficiency, complicating the use of ribavirin (Rebetol) and subjecting patients to a higher risk of severe anemia. Furthermore, interferon therapy increases the risk of renal allograft rejection and, accordingly, is not often used in renal transplant recipients.
Patients with unexplained elevated aminotransferase levels
HCV infection affects an estimated 1.8% of the general population, but the rate is much higher in people with ALT levels over 40 U/L. Most patients with chronic hepatitis C have no symptoms or only mild symptoms and minimally elevated levels of ALT and aspartate aminotransferase (AST)—ie, two to five times higher than the upper limit of normal.
The first step in the workup of aminotransferase elevations is to confirm the abnormality by repeating the blood test. If an elevation is confirmed, further investigation is warranted. A directed history and physical examination is important and may disclose risk factors, raising clinical suspicion of a particular disease.
Some caveats: The proportion of patients with HCV viremia who have abnormally high aminotransferase levels ranges between only 54% and 66%.57–59 In patients with risk factors for HCV infection and abnormal liver enzyme levels, HCV infection is probable but not certain. Also, liver enzyme tests do not reveal the extent of hepatic injury or reflect the true status of hepatic function.60
Infants born to infected mothers
Children born to HCV-positive women should be tested for anti-HCV no sooner than age 12 months, when passively transferred maternal anti-HCV declines below detectable levels. If earlier diagnosis of HCV infection is desired, a real-time polymerase chain reaction (PCR) test for HCV RNA can be done at or after the infant's first “well-child” visit at age 1 to 2 months.
If positive for either anti-HCV or HCV RNA, children should be evaluated for liver disease, and those with persistently elevated ALT levels should be referred to a specialist for medical management.2,5
GROUPS AT LOW RISK OF HCV
People who have had sexual relations with multiple or infected partners
Sexual activity is associated with a low but measurable risk of transmission of HCV. Large population-based studies, including the National Hepatitis Surveillance Program,25 found an independent association between HCV infection and having sexual relations with multiple partners or with a partner who is infected with HCV.
The CDC reported that 15% to 20% of patients with acute hepatitis C had a history of sexual exposure but no other risk factors. Two-thirds of them had an anti-HCV-positive sexual partner, and one-third reported having had more than two partners in the 6 months before illness.5
More data are needed to determine the risk of and the factors related to transmission of HCV between long-term steady partners as well as in persons with high-risk sexual practices, including whether other sexually transmitted diseases promote transmission of HCV by influencing viral load or modifying mucosal barriers.
Health care workers exposed to HCV, eg, by needlestick
The prevalence of HCV infection in health care workers is no greater than that in the general population, averaging 1% to 2%, and is actually 10 times lower than that of hepatitis B virus infection.47,48,61,62
However, within the disciplines, some groups have a higher prevalence of HCV infection, suggesting that some occupations carry a higher risk. In two US studies, the prevalence of HCV infection was higher in oral surgeons (2.0% and 9.3%) than in other dentists (0.7% and 0.97%).63,64
In a single study that evaluated risk factors for infection, a history of needlestick injury was the only occupational risk factor that was independently associated with HCV infection.65 The average incidence of anti-HCV seroconversion after a needlestick or after an injury with a sharp object contaminated by an HCV-positive source is 1.8% (range 0%–7%).66–69
Although no studies of incidence have documented transmission via mucous membrane or nonintact skin exposures, transmission of HCV from blood splashes to the conjunctiva have been described.70,71
It is worth noting that exposure to blood from unclean needles used in tattooing or body piercing also confers a risk of HCV infection.
SEROLOGIC SCREENING TESTS FOR HCV
- Serologic assays that detect specific antibody to HCV (anti-HCV)
- Molecular assays that detect viral RNA.
Initial serologic screening tests for anti-HCV
Two EIAs are approved for clinical use:
- Abbott HCV EIA 2.0 (Abbott Laboratories, Abbott Park, IL)
- Ortho HCV Version 3.0 enzyme-linked immunosorbent assay (ELISA) (Ortho-Clinical Diagnostics, Rochester, NY).
One enhanced chemiluminescence immunoassay is also approved:
- Vitros Anti-HCV assay (Ortho-Clinical Diagnostics). In practical terms, this test is equivalent to the two EIAs, and the discussion below about EIAs applies to this test as well.
These third-generation tests are highly sensitive (> 99%) and specific (99%) in immunocompetent patients, and eliminate the need for a confirmatory immunoblot assay in patients with clinical liver disease, particularly those with risk factors for HCV infection.
False-positive results are rare now, but they were common with earlier generations of these assays. Most false-positive results occur in patients with autoimmune liver disease or hypergammaglobulinemia who have normal liver enzyme levels and no risk factors for HCV infection. In fact, all positive anti-HCV results should be followed up with an HCV RNA test.
False-negative results are also uncommon, usually occurring only in immunosuppressed patients (eg, organ transplant recipients and HIV-positive patients) and in patients on long-term hemodialysis. Therefore, patients with a history of hemodialysis should be considered for an HCV RNA assay rather than an EIA. Measurement of ALT will not be useful because ALT levels are lower in patients with end-stage renal disease. In most other clinical situations, the HCV EIA is an outstanding screening test for HCV infection because of its high sensitivity and relatively low cost (< $50).
Although the specificity of these tests is good, the predictive value of a positive result varies substantially by the pretest probability of HCV infection. For example, in a group of injection-drug users who are very likely to have ongoing or remote infection, all positive HCV EIA results are likely truly positive.74 On the other hand, in healthy blood donors, up to half of all positive third-generation EIA tests are falsely positive.75
Important points
- A positive anti-HCV antibody test does not distinguish acute from chronic disease or active from past infection, nor is it a sign of immunity or protection.
- A positive anti-HCV EIA requires HCV RNA measurement to discriminate between current infection on the one hand, and either resolved HCV infection or a false-positive result on the other.
- A positive EIA anti-HCV test is a marker that hepatitis C may be present, and it must be followed by confirmatory HCV RNA testing.
- Physicians should be mindful of the potential tribulations associated with false-positive tests. A false-positive test may result in harm to patients that is difficult to measure, such as anxiety, labeling in the medical record, and detrimental effects on close relationships.
CONFIRMATORY TESTING WITH ASSAYS FOR HCV RNA
As stated above, a positive result on an anti-HCV EIA needs to be confirmed with an assay for HCV RNA, of which there are two types, ie, qualitative and quantitative.
Each involves trade-offs. Qualitative assays are more sensitive and detect more cases, but they provide no information about the amount of virus (viral load). Quantitative assays are less sensitive, so a negative result does not completely exclude hepatitis C, although they can still can detect 95% of cases. They do, however, measure the viral load.
Therefore, the type of test to use depends on the patient’s risk profile, the goals of testing, and the setting in which future care will be provided. The primary objective when a patient has a positive EIA test is to determine whether he or she has ongoing infection, a goal most expeditiously achieved using a qualitative assay. However, since a quantitative assay can detect the vast majority of cases of active HCV infection, many clinicians select this as the test of first choice when the probability of HCV is high (eg, in a patient with risk factors and abnormal liver tests). If the pretest probability is low, a qualitative assay is the better choice.
Many commercial assays are available for detecting (qualitative assays) or measuring (quantitative assays) HCV RNA.
Qualitative HCV RNA assays
The approved qualitative assays are:
- Amplicor HCV Test, version 2.0 (Roche Molecular Diagnostics, Pleasanton, CA)
- Cobas Amplicor HCV Test, version 2.0 (Roche Molecular Diagnostics)
- Ampliscreen (Roche Molecular Diagnostics)
- Versant HCV RNA Qualitative Assay (Siemens Healthcare Diagnostics, Deerfield, IL)
- Procleix HIV-1/HCV Assay (Chiron, Emeryville, CA).
Quantitative HCV RNA assays
The approved quantitative assays are:
- Amplicor HCV Monitor (Roche Molecular Diagnostics)
- Cobas Amplicor HCV Monitor, version 2.0 (Roche Molecular Diagnostics)
- Versant HCV RNA 3.0 Assay (bDNA) (Siemens Healthcare Diagnostics)
- Cobas Taqman HCV Test (Roche Molecular Diagnostics).
Quantitative tests use target amplification with PCR, transcription-mediated amplification (TMA), or a signal amplification technique such as a branched DNA (bDNA) assay. The sensitivity varies for different types of amplification. TMA assays appear to be the most sensitive for detecting HCV RNA.
The latest innovation is real-time PCR, which shortens the typical time for PCR processing from 1.5 hours to 35 minutes. It may also detect relapsed HCV infection earlier than regular PCR. With the recent availability of real-time PCR assays, which have sensitivities of 10 to 50 IU/mL, many experts feel there is no longer a need for qualitative assays.74 In fact, many laboratories no longer offer qualitative testing. The Cleveland Clinic laboratory has recently stopped offering this test.
Because RNA testing is widely available, the recombinant immunoblot assay (RIBA) has become obsolete in diagnosing HCV infection, except in special circumstances. Currently, the primary purpose of RIBA testing is to distinguish between resolved HCV infection (EIA-positive, HCV RNA-negative, RIBA-positive) and a false-positive EIA (EIA-positive, HCV RNA-negative, RIBA-negative).
In summary, patients suspected of having acute or chronic HCV infection should first be tested for anti-HCV. Subsequently, HCV RNA testing should be performed in:
- Patients with a positive anti-HCV test
- Patients for whom antiviral treatment is being considered (using a sensitive quantitative assay)
- Patients with unexplained liver disease whose anti-HCV test is negative and who are immunocompromised or suspected of having acute HCV infection.
Significance of the HCV viral load
The significance of the HCV viral load is widely misunderstood. The amount of virus in the blood does not correlate with symptoms, histologic liver injury, or the stage or aggressiveness of disease. Its sole importance is in relation to therapy.
The HCV viral load, measured before treatment, helps predict the likelihood of a treatment response: the lower the pretreatment viral load, the more likely that the patient will respond to current HCV therapies.
Additionally, the pretreatment viral load serves as a baseline for comparison with subsequent measurements during treatment. Patients with HCV genotype 1 who do not achieve more than a 2-log (99%) reduction in viral load by the 12th week of treatment (an early virologic response) have a low response rate, and treatment should generally be stopped, given its cost and side effects.76 However, measuring the viral load to detect an early virologic response is less helpful in patients with HCV genotype 2 or 3 infection, since these patients require only 24 weeks of therapy and most of them clear the virus by week 12 and respond to therapy.
Additionally, patients with genotype 2 or 3 and those with a viral load of less than 600,000 IU/mL have been found to achieve higher rates of sustained virologic response.15 A sustained virologic response is defined as the absence of HCV RNA 24 weeks after stopping treatment and is now considered to be the best predictor of long-term treatment response. A sustained virologic response is generally regarded as a “virologic cure.”
HCV GENOTYPE AFFECTS SUCCESS AND DURATION OF TREATMENT
HCV has at least six major genotypes.1,3–6 Several genotypes are subclassified as “a” or “b” (ie, genotype 1a or 1b); however, these distinctions are of little clinical use.
In the laboratory, HCV genotypes are identified by restriction fragment length polymorphism, by direct sequence analysis, or by reverse hybridization. Once the HCV genotype has been identified, there is no need to repeat the test.
Different genotypes are more common in some areas of the world than in others. Genotype 1 is the one most common in the United States (accounting for 70% to 75% of cases), followed by genotypes 2 and 3 (25%–30%). Genotype 4 is most common in Egypt and the Arabian peninsula.
HCV genotyping is important because it can help predict the likelihood of a response to treatment and in planning the dose and duration of therapy.77 For example, treatment with pegylated interferon plus ribavirin is predicted to work approximately 50% of the time for people with genotype 1, but 80% to 90% of the time for people with genotypes 2 or 3.15–17,78 Additionally, patients with genotype 1 need 12 months of therapy to achieve maximum benefit, whereas those with genotypes 2 and 3 require treatment for only 6 months to achieve maximum benefit.
- Alter MJ, Seeff LB, Bacon BR, Thomas DL, Rigsby MO, Di Bisceglie AM. Testing for hepatitis C virus infection should be routine for persons at increased risk for infection. Ann Intern Med 2004; 141:715–717.
- Ghany MG, Strader DB, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology 2009; 49:1335–1374.
- Shehab TM, Orrego M, Chunduri R, Lok AS. Identification and management of hepatitis C patients in primary care clinics. Am J Gastroenterol 2003; 98:639–644.
- Shehab TM, Sonnad SS, Lok AS. Management of hepatitis C patients by primary care physicians in the USA: results of a national survey. J Viral Hepat 2001; 8:377–383.
- US Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep 1998; 47:1–39.
- US Centers for Disease Control and Prevention. National prevention strategy: a comprehensive strategy for the prevention and control of hepatitis C virus infection and its consequences; summer 2001. http://www.cdc.gov/hepatitis/HCV/Strategy/NatHep-CPrevStrategy.htm. Accessed August 8, 2010.
- Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med 2006; 144:705–714.
- Kim WR. The burden of hepatitis C in the United States. Hepatology 2002; 36(suppl 1):S30–S34.
- Lau DT, Kleiner DE, Ghany MG, Park Y, Schmid P, Hoofnagle JH. 10-Year follow-up after interferon-alpha therapy for chronic hepatitis C. Hepatology 1998; 28:1121–1127.
- Daniels D, Grytdal S, Wasley A; US Centers for Disease Control and Prevention. Surveillance for acute viral hepatitis—United States, 2007. MMWR Surveill Summ 2009; 58:1–27.
- Thomson BJ, Kwong G, Ratib S, et al; Trent HCV Study Group. Response rates to combination therapy for chronic HCV infection in a clinical setting and derivation of probability tables for individual patient management. J Viral Hepat 2008; 15:271–278.
- Hayashi N, Takehara T. Antiviral therapy for chronic hepatitis C: past, present, and future. J Gastroenterol 2006; 41:17–27.
- Gordon FD. Cost-effectiveness of screening patients for hepatitis C. Am J Med 1999; 107:36S–40S.
- Hill L, Henry B, Schweikert S; Prevention Practice Committee, American College of Preventive Medicine. Screening for chronic hepatitis C: American College of Preventive Medicine practice policy statement. Am J Prev Med 2005; 28:327–330.
- Hadziyannis SJ, Sette H, Morgan TR, et al; PEGASYS International Study Group. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med 2004; 140:346–355.
- Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001; 358:958–965.
- Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002; 347:975–982.
- Cammà C, Di Bona D, Schepis F, et al. Effect of peginterferon alfa-2a on liver histology in chronic hepatitis C: a meta-analysis of individual patient data. Hepatology 2004; 39:333–342.
- Yoshida H, Tateishi R, Arakawa Y, et al. Benefit of interferon therapy in hepatocellular carcinoma prevention for individual patients with chronic hepatitis C. Gut 2004; 53:425–430.
- Yoshida H, Arakawa Y, Sata M, et al. Interferon therapy prolonged life expectancy among chronic hepatitis C patients. Gastroenterology 2002; 123:483–491.
- Hézode C, Forestier N, Dusheiko G, et al; PROVE2 Study Team. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med 2009; 360:1839–1850.
- McHutchison JG, Everson GT, Gordon SC, et al; PROVE1 Study Team. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med 2009; 360:1827–1838.
- Berman K, Kwo PY. Boceprevir, an NS3 protease inhibitor of HCV. Clin Liver Dis 2009; 13:429–439.
- Rossignol JF, Elfert A, Keeffe EB. Treatment of chronic hepatitis C using a 4-week lead-in with nitazoxanide before peginterferon plus nitazoxanide. J Clin Gastroenterol 2009 Dec 31; epub ahead of print.
- Lapane KL, Jakiche AF, Sugano D, Weng CS, Carey WD. Hepatitis C infection risk analysis: who should be screened? Comparison of multiple screening strategies based on the National Hepatitis Surveillance Program. Am J Gastroenterol 1998; 93:591–596.
- Wong JB, Davis GL, McHutchison JG, Manns MP, Albrecht JK; International Hepatitis Interventional Therapy Group. Economic and clinical effects of evaluating rapid viral response to peginterferon alfa-2b plus ribavirin for the initial treatment of chronic hepatitis C. Am J Gastroenterol 2003; 98:2354–2362.
- Salomon JA, Weinstein MC, Hammitt JK, Goldie SJ. Cost-effectiveness of treatment for chronic hepatitis C infection in an evolving patient population. JAMA 2003; 290:228–237.
- Sullivan SD, Jensen DM, Bernstein DE, et al. Cost-effectiveness of combination peginterferon alpha-2a and ribavirin compared with interferon alpha-2b and ribavirin in patients with chronic hepatitis C. Am J Gastroenterol 2004; 99:1490–1496.
- McGinn T, O’Connor-Moore N, Alfandre D, Gardenier D, Wisnivesky J. Validation of a hepatitis C screening tool in primary care. Arch Intern Med 2008; 168:2009–2013.
- Kaur S, Rybicki L, Bacon BR, Gollan JL, Rustgi VK, Carey WD. Performance characteristics and results of a large-scale screening program for viral hepatitis and risk factors associated with exposure to viral hepatitis B and C: results of the National Hepatitis Screening Survey. National Hepatitis Surveillance Group. Hepatology 1996; 24:979–986.
- Cheung RC. Epidemiology of hepatitis C virus infection in American veterans. Am J Gastroenterol 2000; 95:740–747.
- Austin GE, Jensen B, Leete J, et al. Prevalence of hepatitis C virus seropositivity among hospitalized US veterans. Am J Med Sci 2000; 319:353–359.
- Yawn BP, Wollan P, Gazzuola L, Kim WR. Diagnosis and 10-year follow-up of a community-based hepatitis C cohort. J Fam Pract 2002; 51:135–140.
- Garfein RS, Doherty MC, Monterroso ER, Thomas DL, Nelson KE, Vlahov D. Prevalence and incidence of hepatitis C virus infection among young adult injection drug users. J Acquir Immune Defic Syndr Hum Retrovirol 1998; 18(suppl 1):S11–S19.
- Alter MJ. The epidemiology of acute and chronic hepatitis C. Clin Liver Dis 1997; 1:559–568,
- Alter MJ, Hadler SC, Judson FN, et al. Risk factors for acute non-A, non-B hepatitis in the United States and association with hepatitis C virus infection. JAMA 1990; 264:2231–2235.
- Wasley A, Miller JT, Finelli L; Centers for Disease Control and Prevention (CDC). Surveillance for acute viral hepatitis—United States, 2005. MMWR Surveill Summ 2007; 56:1–24.
- Goedert JJ, Chen BE, Preiss L, Aledort LM, Rosenberg PS. Reconstruction of the hepatitis C virus epidemic in the US hemophilia population, 1940–1990. Am J Epidemiol 2007; 165:1443–1453.
- Eyster ME, Sherman KE, Goedert JJ, Katsoulidou A, Hatzakis A. Prevalence and changes in hepatitis C virus genotypes among multitransfused persons with hemophilia. The Multicenter Hemophilia Cohort Study. J Infect Dis 1999; 179:1062–1069.
- Yee TT, Griffioen A, Sabin CA, Dusheiko G, Lee CA. The natural history of HCV in a cohort of haemophilic patients infected between 1961 and 1985. Gut 2000; 47:845–851.
- Lee C, Dusheiko G. The natural history and antiviral treatment of hepatitis C in haemophilia. Haemophilia 2002; 8:322–329.
- Makris M, Garson JA, Ring CJ, Tuke PW, Tedder RS, Preston FE. Hepatitis C viral RNA in clotting factor concentrates and the development of hepatitis in recipients. Blood 1993; 81:1898–1902.
- Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis 2002; 34:831–837.
- Sulkowski MS. The HIV-coinfected patient: managing viral hepatitis. J Acquir Immune Defic Syndr 2007; 45(suppl 2):S36–S37.
- Bonacini M, Lin HJ, Hollinger FB. Effect of coexisting HIV-1 infection on the diagnosis and evaluation of hepatitis C virus. J Acquir Immune Defic Syndr 2001; 26:340–344.
- Garfein RS, Vlahov D, Galai N, Doherty MC, Nelson KE. Viral infections in short-term injection drug users: the prevalence of the hepatitis C, hepatitis B, human immunodeficiency, and human T-lymphotropic viruses. Am J Public Health 1996; 86:655–661.
- Bell J, Batey RG, Farrell GC, Crewe EB, Cunningham AL, Byth K. Hepatitis C virus in intravenous drug users. Med J Aust 1990; 153:274–276.
- Villano SA, Vlahov D, Nelson KE, Lyles CM, Cohn S, Thomas DL. Incidence and risk factors for hepatitis C among injection drug users in Baltimore, Maryland. J Clin Microbiol 1997; 35:3274–3277.
- Patrick DM, Tyndall MW, Cornelisse PG, et al. Incidence of hepatitis C virus infection among injection drug users during an outbreak of HIV infection. CMAJ 2001; 165:889–895.
- Seeff LB, Wright EC, Zimmerman HJ, McCollum RW. VA cooperative study of post-transfusion hepatitis, 1969-1974: incidence and characteristics of hepatitis and responsible risk factors. Am J Med Sci 1975; 270:355–362.
- Feinstone SM, Kapikian AZ, Purcell RH, Alter HJ, Holland PV. Transfusion-associated hepatitis not due to viral hepatitis type A or B. N Engl J Med 1975; 292:767–770.
- Alter HJ, Holland PV, Purcell RH, et al. Posttransfusion hepatitis after exclusion of commercial and hepatitis-B antigen-positive donors. Ann Intern Med. 1972; 77:691–699.
- Blajchman MA, Vamvakas EC. The continuing risk of transfusion-transmitted infections. N Engl J Med 2006; 355:1303–1305.
- Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med 2001; 345:41–52.
- Roth D, Zucker K, Cirocco R, et al. The impact of hepatitis C virus infection on renal allograft recipients. Kidney Int 1994; 45:238–244.
- Izopet J, Rostaing L, Sandres K, et al. Longitudinal analysis of hepatitis C virus replication and liver fibrosis progression in renal transplant recipients. J Infect Dis 2000; 181:852–858.
- Dubois F, Desenclos JC, Mariotte N, Goudeau A. Hepatitis C in a French population-based survey, 1994: seroprevalence, frequency of viremia, genotype distribution, and risk factors. The Collaborative Study Group. Hepatology 1997; 25:1490–1496.
- Bellentani S, Pozzato G, Saccoccio G, et al. Clinical course and risk factors of hepatitis C virus related liver disease in the general population: report from the Dionysos study. Gut 1999; 44:874–880.
- Alberti A, Noventa F, Benvegnù L, Boccato S, Gatta A. Prevalence of liver disease in a population of asymptomatic persons with hepatitis C virus infection. Ann Intern Med 2002; 137:961–964.
- Shiffman ML, Diago M, Tran A, et al. Chronic hepatitis C in patients with persistently normal alanine transaminase levels. Clin Gastroenterol Hepatol 2006; 4:645–652.
- Stary A, Kopp W, Hofmann H, Heller-Vitouch C, Kunz C. Seroepidemiologic study of hepatitis C virus in sexually transmitted disease risk groups. Sex Transm Dis 1992; 19:252–258.
- Weinstock HS, Bolan G, Reingold AL, Polish LB. Hepatitis C virus infection among patients attending a clinic for sexually transmitted diseases. JAMA 1993; 269:392–394.
- Thomas DL, Gruninger SE, Siew C, Joy ED, Quinn TC. Occupational risk of hepatitis C infections among general dentists and oral surgeons in North America. Am J Med 1996; 100:41–45.
- Klein RS, Freeman K, Taylor PE, Stevens CE. Occupational risk for hepatitis C virus infection among New York City dentists. Lancet 1991; 338:1539–1542.
- Polish LB, Tong MJ, Co RL, Coleman PJ, Alter MJ. Risk factors for hepatitis C virus infection among health care personnel in a community hospital. Am J Infect Control 1993; 21:196–200.
- Alter MJ. Occupational exposure to hepatitis C virus: a dilemma. Infect Control Hosp Epidemiol 1994; 15:742–744.
- Lanphear BP, Linnemann CC, Cannon CG, DeRonde MM, Pendy L, Kerley LM. Hepatitis C virus infection in healthcare workers: risk of exposure and infection. Infect Control Hosp Epidemiol 1994; 15:745–750.
- Puro V, Petrosillo N, Ippolito G. Risk of hepatitis C seroconversion after occupational exposures in health care workers. Italian Study Group on Occupational Risk of HIV and Other Bloodborne Infections. Am J Infect Control 1995; 23:273–277.
- Mitsui T, Iwano K, Masuko K, et al. Hepatitis C virus infection in medical personnel after needlestick accident. Hepatology 1992; 16:1109–1114.
- Sartori M, La Terra G, Aglietta M, Manzin A, Navino C, Verzetti G. Transmission of hepatitis C via blood splash into conjunctiva. Scand J Infect Dis 1993; 25:270–271.
- Ippolito G, Puro V, Petrosillo N, De Carli G, Micheloni G, Magliano E. Simultaneous infection with HIV and hepatitis C virus following occupational conjunctival blood exposure. JAMA 1998; 280:28.
- Carey W. Tests and screening strategies for the diagnosis of hepatitis C. Cleve Clin J Med 2003; 70(suppl 4):S7–S13.
- Carey WD, Jeffers L, Kugelmas M, et al; Hepatitis C management. Hepatitis C Monograph. Cleveland Clinic; www.clevelandclinicmeded.com/online/monograph/hepc/page1.htm. Accessed 7/30/2010.
- Scott JD, Gretch DR. Molecular diagnostics of hepatitis C virus infection: a systematic review. JAMA 2007; 297:724–732.
- Bowden DS, Berzsenyi MD. Chronic hepatitis C virus infection: genotyping and its clinical role. Future Microbiol 2006; 1:103–112.
- Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002; 347:975–982.
- Poynard T, McHutchison J, Davis GL, et al. Impact of interferon alfa-2b and ribavirin on progression of liver fibrosis in patients with chronic hepatitis C. Hepatology 2000; 32:1131–1137.
- Zeuzem S. Interferon-based therapy for chronic hepatitis C: current and future perspectives. Nat Clin Pract Gastroenterol Hepatol 2008; 5:610–622.
- Alter MJ, Seeff LB, Bacon BR, Thomas DL, Rigsby MO, Di Bisceglie AM. Testing for hepatitis C virus infection should be routine for persons at increased risk for infection. Ann Intern Med 2004; 141:715–717.
- Ghany MG, Strader DB, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology 2009; 49:1335–1374.
- Shehab TM, Orrego M, Chunduri R, Lok AS. Identification and management of hepatitis C patients in primary care clinics. Am J Gastroenterol 2003; 98:639–644.
- Shehab TM, Sonnad SS, Lok AS. Management of hepatitis C patients by primary care physicians in the USA: results of a national survey. J Viral Hepat 2001; 8:377–383.
- US Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep 1998; 47:1–39.
- US Centers for Disease Control and Prevention. National prevention strategy: a comprehensive strategy for the prevention and control of hepatitis C virus infection and its consequences; summer 2001. http://www.cdc.gov/hepatitis/HCV/Strategy/NatHep-CPrevStrategy.htm. Accessed August 8, 2010.
- Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med 2006; 144:705–714.
- Kim WR. The burden of hepatitis C in the United States. Hepatology 2002; 36(suppl 1):S30–S34.
- Lau DT, Kleiner DE, Ghany MG, Park Y, Schmid P, Hoofnagle JH. 10-Year follow-up after interferon-alpha therapy for chronic hepatitis C. Hepatology 1998; 28:1121–1127.
- Daniels D, Grytdal S, Wasley A; US Centers for Disease Control and Prevention. Surveillance for acute viral hepatitis—United States, 2007. MMWR Surveill Summ 2009; 58:1–27.
- Thomson BJ, Kwong G, Ratib S, et al; Trent HCV Study Group. Response rates to combination therapy for chronic HCV infection in a clinical setting and derivation of probability tables for individual patient management. J Viral Hepat 2008; 15:271–278.
- Hayashi N, Takehara T. Antiviral therapy for chronic hepatitis C: past, present, and future. J Gastroenterol 2006; 41:17–27.
- Gordon FD. Cost-effectiveness of screening patients for hepatitis C. Am J Med 1999; 107:36S–40S.
- Hill L, Henry B, Schweikert S; Prevention Practice Committee, American College of Preventive Medicine. Screening for chronic hepatitis C: American College of Preventive Medicine practice policy statement. Am J Prev Med 2005; 28:327–330.
- Hadziyannis SJ, Sette H, Morgan TR, et al; PEGASYS International Study Group. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med 2004; 140:346–355.
- Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001; 358:958–965.
- Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002; 347:975–982.
- Cammà C, Di Bona D, Schepis F, et al. Effect of peginterferon alfa-2a on liver histology in chronic hepatitis C: a meta-analysis of individual patient data. Hepatology 2004; 39:333–342.
- Yoshida H, Tateishi R, Arakawa Y, et al. Benefit of interferon therapy in hepatocellular carcinoma prevention for individual patients with chronic hepatitis C. Gut 2004; 53:425–430.
- Yoshida H, Arakawa Y, Sata M, et al. Interferon therapy prolonged life expectancy among chronic hepatitis C patients. Gastroenterology 2002; 123:483–491.
- Hézode C, Forestier N, Dusheiko G, et al; PROVE2 Study Team. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med 2009; 360:1839–1850.
- McHutchison JG, Everson GT, Gordon SC, et al; PROVE1 Study Team. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med 2009; 360:1827–1838.
- Berman K, Kwo PY. Boceprevir, an NS3 protease inhibitor of HCV. Clin Liver Dis 2009; 13:429–439.
- Rossignol JF, Elfert A, Keeffe EB. Treatment of chronic hepatitis C using a 4-week lead-in with nitazoxanide before peginterferon plus nitazoxanide. J Clin Gastroenterol 2009 Dec 31; epub ahead of print.
- Lapane KL, Jakiche AF, Sugano D, Weng CS, Carey WD. Hepatitis C infection risk analysis: who should be screened? Comparison of multiple screening strategies based on the National Hepatitis Surveillance Program. Am J Gastroenterol 1998; 93:591–596.
- Wong JB, Davis GL, McHutchison JG, Manns MP, Albrecht JK; International Hepatitis Interventional Therapy Group. Economic and clinical effects of evaluating rapid viral response to peginterferon alfa-2b plus ribavirin for the initial treatment of chronic hepatitis C. Am J Gastroenterol 2003; 98:2354–2362.
- Salomon JA, Weinstein MC, Hammitt JK, Goldie SJ. Cost-effectiveness of treatment for chronic hepatitis C infection in an evolving patient population. JAMA 2003; 290:228–237.
- Sullivan SD, Jensen DM, Bernstein DE, et al. Cost-effectiveness of combination peginterferon alpha-2a and ribavirin compared with interferon alpha-2b and ribavirin in patients with chronic hepatitis C. Am J Gastroenterol 2004; 99:1490–1496.
- McGinn T, O’Connor-Moore N, Alfandre D, Gardenier D, Wisnivesky J. Validation of a hepatitis C screening tool in primary care. Arch Intern Med 2008; 168:2009–2013.
- Kaur S, Rybicki L, Bacon BR, Gollan JL, Rustgi VK, Carey WD. Performance characteristics and results of a large-scale screening program for viral hepatitis and risk factors associated with exposure to viral hepatitis B and C: results of the National Hepatitis Screening Survey. National Hepatitis Surveillance Group. Hepatology 1996; 24:979–986.
- Cheung RC. Epidemiology of hepatitis C virus infection in American veterans. Am J Gastroenterol 2000; 95:740–747.
- Austin GE, Jensen B, Leete J, et al. Prevalence of hepatitis C virus seropositivity among hospitalized US veterans. Am J Med Sci 2000; 319:353–359.
- Yawn BP, Wollan P, Gazzuola L, Kim WR. Diagnosis and 10-year follow-up of a community-based hepatitis C cohort. J Fam Pract 2002; 51:135–140.
- Garfein RS, Doherty MC, Monterroso ER, Thomas DL, Nelson KE, Vlahov D. Prevalence and incidence of hepatitis C virus infection among young adult injection drug users. J Acquir Immune Defic Syndr Hum Retrovirol 1998; 18(suppl 1):S11–S19.
- Alter MJ. The epidemiology of acute and chronic hepatitis C. Clin Liver Dis 1997; 1:559–568,
- Alter MJ, Hadler SC, Judson FN, et al. Risk factors for acute non-A, non-B hepatitis in the United States and association with hepatitis C virus infection. JAMA 1990; 264:2231–2235.
- Wasley A, Miller JT, Finelli L; Centers for Disease Control and Prevention (CDC). Surveillance for acute viral hepatitis—United States, 2005. MMWR Surveill Summ 2007; 56:1–24.
- Goedert JJ, Chen BE, Preiss L, Aledort LM, Rosenberg PS. Reconstruction of the hepatitis C virus epidemic in the US hemophilia population, 1940–1990. Am J Epidemiol 2007; 165:1443–1453.
- Eyster ME, Sherman KE, Goedert JJ, Katsoulidou A, Hatzakis A. Prevalence and changes in hepatitis C virus genotypes among multitransfused persons with hemophilia. The Multicenter Hemophilia Cohort Study. J Infect Dis 1999; 179:1062–1069.
- Yee TT, Griffioen A, Sabin CA, Dusheiko G, Lee CA. The natural history of HCV in a cohort of haemophilic patients infected between 1961 and 1985. Gut 2000; 47:845–851.
- Lee C, Dusheiko G. The natural history and antiviral treatment of hepatitis C in haemophilia. Haemophilia 2002; 8:322–329.
- Makris M, Garson JA, Ring CJ, Tuke PW, Tedder RS, Preston FE. Hepatitis C viral RNA in clotting factor concentrates and the development of hepatitis in recipients. Blood 1993; 81:1898–1902.
- Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis 2002; 34:831–837.
- Sulkowski MS. The HIV-coinfected patient: managing viral hepatitis. J Acquir Immune Defic Syndr 2007; 45(suppl 2):S36–S37.
- Bonacini M, Lin HJ, Hollinger FB. Effect of coexisting HIV-1 infection on the diagnosis and evaluation of hepatitis C virus. J Acquir Immune Defic Syndr 2001; 26:340–344.
- Garfein RS, Vlahov D, Galai N, Doherty MC, Nelson KE. Viral infections in short-term injection drug users: the prevalence of the hepatitis C, hepatitis B, human immunodeficiency, and human T-lymphotropic viruses. Am J Public Health 1996; 86:655–661.
- Bell J, Batey RG, Farrell GC, Crewe EB, Cunningham AL, Byth K. Hepatitis C virus in intravenous drug users. Med J Aust 1990; 153:274–276.
- Villano SA, Vlahov D, Nelson KE, Lyles CM, Cohn S, Thomas DL. Incidence and risk factors for hepatitis C among injection drug users in Baltimore, Maryland. J Clin Microbiol 1997; 35:3274–3277.
- Patrick DM, Tyndall MW, Cornelisse PG, et al. Incidence of hepatitis C virus infection among injection drug users during an outbreak of HIV infection. CMAJ 2001; 165:889–895.
- Seeff LB, Wright EC, Zimmerman HJ, McCollum RW. VA cooperative study of post-transfusion hepatitis, 1969-1974: incidence and characteristics of hepatitis and responsible risk factors. Am J Med Sci 1975; 270:355–362.
- Feinstone SM, Kapikian AZ, Purcell RH, Alter HJ, Holland PV. Transfusion-associated hepatitis not due to viral hepatitis type A or B. N Engl J Med 1975; 292:767–770.
- Alter HJ, Holland PV, Purcell RH, et al. Posttransfusion hepatitis after exclusion of commercial and hepatitis-B antigen-positive donors. Ann Intern Med. 1972; 77:691–699.
- Blajchman MA, Vamvakas EC. The continuing risk of transfusion-transmitted infections. N Engl J Med 2006; 355:1303–1305.
- Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med 2001; 345:41–52.
- Roth D, Zucker K, Cirocco R, et al. The impact of hepatitis C virus infection on renal allograft recipients. Kidney Int 1994; 45:238–244.
- Izopet J, Rostaing L, Sandres K, et al. Longitudinal analysis of hepatitis C virus replication and liver fibrosis progression in renal transplant recipients. J Infect Dis 2000; 181:852–858.
- Dubois F, Desenclos JC, Mariotte N, Goudeau A. Hepatitis C in a French population-based survey, 1994: seroprevalence, frequency of viremia, genotype distribution, and risk factors. The Collaborative Study Group. Hepatology 1997; 25:1490–1496.
- Bellentani S, Pozzato G, Saccoccio G, et al. Clinical course and risk factors of hepatitis C virus related liver disease in the general population: report from the Dionysos study. Gut 1999; 44:874–880.
- Alberti A, Noventa F, Benvegnù L, Boccato S, Gatta A. Prevalence of liver disease in a population of asymptomatic persons with hepatitis C virus infection. Ann Intern Med 2002; 137:961–964.
- Shiffman ML, Diago M, Tran A, et al. Chronic hepatitis C in patients with persistently normal alanine transaminase levels. Clin Gastroenterol Hepatol 2006; 4:645–652.
- Stary A, Kopp W, Hofmann H, Heller-Vitouch C, Kunz C. Seroepidemiologic study of hepatitis C virus in sexually transmitted disease risk groups. Sex Transm Dis 1992; 19:252–258.
- Weinstock HS, Bolan G, Reingold AL, Polish LB. Hepatitis C virus infection among patients attending a clinic for sexually transmitted diseases. JAMA 1993; 269:392–394.
- Thomas DL, Gruninger SE, Siew C, Joy ED, Quinn TC. Occupational risk of hepatitis C infections among general dentists and oral surgeons in North America. Am J Med 1996; 100:41–45.
- Klein RS, Freeman K, Taylor PE, Stevens CE. Occupational risk for hepatitis C virus infection among New York City dentists. Lancet 1991; 338:1539–1542.
- Polish LB, Tong MJ, Co RL, Coleman PJ, Alter MJ. Risk factors for hepatitis C virus infection among health care personnel in a community hospital. Am J Infect Control 1993; 21:196–200.
- Alter MJ. Occupational exposure to hepatitis C virus: a dilemma. Infect Control Hosp Epidemiol 1994; 15:742–744.
- Lanphear BP, Linnemann CC, Cannon CG, DeRonde MM, Pendy L, Kerley LM. Hepatitis C virus infection in healthcare workers: risk of exposure and infection. Infect Control Hosp Epidemiol 1994; 15:745–750.
- Puro V, Petrosillo N, Ippolito G. Risk of hepatitis C seroconversion after occupational exposures in health care workers. Italian Study Group on Occupational Risk of HIV and Other Bloodborne Infections. Am J Infect Control 1995; 23:273–277.
- Mitsui T, Iwano K, Masuko K, et al. Hepatitis C virus infection in medical personnel after needlestick accident. Hepatology 1992; 16:1109–1114.
- Sartori M, La Terra G, Aglietta M, Manzin A, Navino C, Verzetti G. Transmission of hepatitis C via blood splash into conjunctiva. Scand J Infect Dis 1993; 25:270–271.
- Ippolito G, Puro V, Petrosillo N, De Carli G, Micheloni G, Magliano E. Simultaneous infection with HIV and hepatitis C virus following occupational conjunctival blood exposure. JAMA 1998; 280:28.
- Carey W. Tests and screening strategies for the diagnosis of hepatitis C. Cleve Clin J Med 2003; 70(suppl 4):S7–S13.
- Carey WD, Jeffers L, Kugelmas M, et al; Hepatitis C management. Hepatitis C Monograph. Cleveland Clinic; www.clevelandclinicmeded.com/online/monograph/hepc/page1.htm. Accessed 7/30/2010.
- Scott JD, Gretch DR. Molecular diagnostics of hepatitis C virus infection: a systematic review. JAMA 2007; 297:724–732.
- Bowden DS, Berzsenyi MD. Chronic hepatitis C virus infection: genotyping and its clinical role. Future Microbiol 2006; 1:103–112.
- Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002; 347:975–982.
- Poynard T, McHutchison J, Davis GL, et al. Impact of interferon alfa-2b and ribavirin on progression of liver fibrosis in patients with chronic hepatitis C. Hepatology 2000; 32:1131–1137.
- Zeuzem S. Interferon-based therapy for chronic hepatitis C: current and future perspectives. Nat Clin Pract Gastroenterol Hepatol 2008; 5:610–622.
KEY POINTS
- Patients who should be screened include intravenous drug abusers, people infected with human immunodeficiency virus, patients with unexplained elevated alanine aminotransferase levels, infants born to infected mothers, and people with infected sexual partners.
- Patients at risk of HCV infection should be tested for anti-HCV antibody using an enzyme immunoassay (EIA).
- Positive results on anti-HCV EIA testing should be confirmed with an assay for HCV RNA.
- HCV genotyping can help predict the response to therapy. Genotypes 2 or 3 are more likely to respond to therapy than genotype 1.
Managing acute upper GI bleeding, preventing recurrences
Upper gastrointestinal (GI) bleeding is common, costly, and potentially life-threatening. It must be managed promptly and appropriately to prevent adverse outcomes.
More people are admitted to the hospital for upper GI bleeding than for congestive heart failure or deep vein thrombosis. In the United States, the annual rate of hospitalization for upper GI bleeding is estimated to be 165 per 100,000—more than 300,000 hospitalizations per year, at a cost of $2.5 billion.1,2
Furthermore, despite advances in therapy, the case-fatality rate has remained unchanged at 7% to 10%.3 This may be because today’s patients are older and have more comorbidities than those in the past.4
CAUSES OF UPPER GI BLEEDING
Peptic ulcers account for about 60% of severe cases of upper GI bleeding,5 and they are the focus of this paper. Fortunately, up to 80% of bleeding ulcers stop bleeding spontaneously without any intervention.6
Gastroduodenal erosions account for about 12%.3
Varices due to cirrhosis are less common but more dangerous. Variceal bleeding accounts for a relatively small percentage (6%) of upper GI bleeding, but the mortality rate from a single episode of variceal bleeding is 30%, and 60% to 70% of patients die within 1 year, mostly of underlying liver disease.
Less frequent causes include Mallory-Weiss tears, erosive duodenitis, Dieulafoy ulcer (a type of vascular malformation), other vascular lesions, neoplasms, aortoenteric fistula, gastric antral vascular ectasia, and prolapse gastropathy.5
HEMATEMESIS AND MELENA
The most common presenting signs of acute upper GI bleeding are hematemesis (vomiting of blood), “coffee grounds” emesis, and melena (tarry black stools). About 30% of patients with bleeding ulcers present with hematemesis, 20% with melena, and 50% with both.7
Hematochezia (red blood in the stool) usually suggests a lower GI source of bleeding, since blood from an upper source turns black and tarry as it passes through the gut, producing melena. However, up to 5% of patients with bleeding ulcers have hematochezia,7 and it indicates heavy bleeding: bleeding of approximately 1,000 mL into the upper GI tract is needed to cause hematochezia, whereas only 50 to 100 mL is needed to cause melena.8,9 Hematochezia with signs and symptoms of hemodynamic compromise such as syncope, postural hypotension, tachycardia, and shock should therefore direct one’s attention to an upper GI source of bleeding.
Nonspecific features include nausea, vomiting, epigastric pain, vasovagal phenomena, and syncope.
WHAT IS THE PATIENT’S RISK?
An assessment of clinical severity is the first critical task, as it helps in planning treatment. Advanced age, multiple comorbidities, and hemodynamic instability call for aggressive treatment. Apart from this simple clinical rule, scoring systems have been developed.
The Rockall scoring system, the most widely used, gives estimates of the risks of recurrent bleeding and death. It is based on the three clinical factors mentioned above and on two endoscopic ones, awarding points for:
- Age—0 points if less than 60; 1 point if 60 to 79; or 2 points if 80 years or older
- Shock—1 point if the pulse is more than 100; 2 points if the systolic blood pressure is less than 100 mm Hg
- Comorbid illness—2 points for ischemic heart disease, congestive heart failure, or other major comorbidity; 3 points for renal failure, hepatic failure, or metastatic disease
- Endoscopic diagnosis—0 points if no lesion found or a Mallory-Weiss tear; 1 point for peptic ulcer, esophagitis, or erosive disease; 2 points for GI malignancy
- Endoscopic stigmata or recent hemorrhage—0 points for a clean-based ulcer or flat pigmented spot; 2 points for blood in the upper GI tract, active bleeding, a nonbleeding visible vessel, or adherent clot.
The Rockall score can thus range from 0 to 11 points, with an overall score of 0, 1, or 2 associated with an excellent prognosis.10
Other systems that are used less often include the Baylor severity scale and the Acute Physiology and Chronic Health Evaluation (APACHE) II score.
Does the patient have varices?
All variceal bleeding should be considered severe, since the 1-year death rate is so high (up to 70%). Clues pointing to variceal bleeding include previous variceal bleeding, thrombocytopenia, history of liver disease, and signs of liver disease on clinical examination.
All patients suspected of having bleeding varices should be admitted to the intensive care unit for close monitoring and should be given the highest priority, even if they are hemodynamically stable.
Is the patient hemodynamically stable?
Appropriate hemodynamic assessment includes monitoring of heart rate, blood pressure, and mental status. Tachycardia at rest, hypotension, and orthostatic changes in vital signs indicate a considerable loss of blood volume. Low urine output, dry mucous membranes, and sunken neck veins are also useful signs. (Tachycardia may be blunted if the patient is taking a beta-blocker.)
If these signs of hypovolemia are present, the initial management focuses on treating shock and on improving oxygen delivery to the vital organs. This involves repletion of the intravascular volume with intravenous infusions or blood transfusions. Supplemental oxygen also is useful, especially in elderly patients with heart disease.12
Inspection of nasogastric aspirate
In the initial assessment, it is useful to insert a nasogastric tube and inspect the aspirate. If it contains bright red blood, the patient needs an urgent endoscopic evaluation and an intensive level of care13,14; if it contains coffee-grounds material, the patient needs to be admitted to the hospital and to undergo endoscopic evaluation within 24 hours.
However, a normal aspirate does not rule out upper GI bleeding. Aljebreen et al15 found that 15% of patients with upper GI bleeding and normal nasogastric aspirate still had high-risk lesions (ie, visible bleeding or nonbleeding visible vessels) on endoscopy.
ACID-SUPPRESSION HELPS ULCERS HEAL
Acid and pepsin interfere with the healing of ulcers and other nonvariceal upper GI lesions. Further, an acidic environment promotes platelet disaggregation and fibrinolysis and impairs clot formation.16 This suggests that inhibiting gastric acid secretion and raising the gastric pH to 6 or higher may stabilize clots. Moreover, pepsinogen in the stomach is converted to its active form (pepsin) if the pH is less than 4. Therefore, keeping the pH above 4 keeps pepsinogen in an inactive form.
Histamine-2 receptor antagonists
Histamine-2 receptor antagonists were the first drugs to inhibit acid secretion, reversibly blocking histamine-2 receptors on the basolateral membrane of parietal cells. However, these drugs did not prove very useful in managing upper GI bleeding in clinical trials.17,18 In their intravenous form, they often fail to keep the gastric pH at 6 or higher, due to tachyphylaxis.19 The use of this class of drugs has declined in favor of proton pump inhibitors.
Proton pump inhibitors
Proton pump inhibitors reduce both basal and stimulated acid secretion by inhibiting hydrogen-potassium adenosine triphosphatase, the proton pump of the parietal cell.
Multiple studies have shown that proton pump inhibitors raise the gastric pH and keep it high. For example, an infusion of omeprazole (Prilosec) can keep the gastric pH above 6 for 72 hours without inducing tachyphylaxis.20,21
Started after endoscopy. Randomized controlled trials have found proton pump inhibitors to be effective when given in high doses intravenously for 72 hours after successful endoscopic treatment of bleeding ulcers with high-risk endoscopic signs, such as active bleeding or nonbleeding visible vessels.22,23
A meta-analysis indicated that these drugs decrease the incidence of recurrent peptic ulcer bleeding, the need for blood transfusions, the need for surgery, and the duration of hospitalization, but not the mortality rate.24,25 These studies also illustrate the benefit of following up endoscopic treatment to stop the bleeding with an intravenous infusion of a proton pump inhibitor.
The recommended dose of omeprazole for patients with high-risk findings on endoscopy is an 80-mg bolus followed by an 8-mg/hour infusion for 72 hours. After the patient’s condition stabilizes, oral therapy can be substituted for intravenous therapy. In patients with low-risk endoscopic findings (a clean-based ulcer or flat spot), oral proton pump inhibitors in high doses are recommended.
In either case, after the initial bleeding is treated endoscopically and hemostasis is achieved, a proton pump inhibitor is recommended for 6 to 8 weeks, or longer if the patient is also positive for Helicobacter pylori or is on daily treatment with aspirin or a nonsteroidal anti-inflammatory drug (NSAID) that is not selective for cyclo-oxygenase 2 (see below).
Started before endoscopy, these drugs reduced the frequency of actively bleeding ulcers, the duration of hospitalization, and the need for endoscopic therapy in a randomized controlled trial.26 A meta-analysis found that significantly fewer patients had signs of recent bleeding on endoscopy if they received a proton pump inhibitor 24 to 48 hours before the procedure, but it did not find any significant difference in important clinical outcomes such as death, recurrent bleeding, or surgery.27 Nevertheless, we believe that intravenous proton pump inhibitor therapy should be started before endoscopy in patients with upper GI bleeding.
Somatostatin analogues
Octreotide (Sandostatin), an analogue of the hormone somatostatin, decreases splanchnic blood flow, decreases secretion of gastric acid and pepsin, and stimulates mucus production. Although it is beneficial in treating upper GI bleeding due to varices, its benefit has not been confirmed in patients with nonvariceal upper GI bleeding.
A meta-analysis revealed that outcomes were better with high-dose intravenous proton pump inhibitor therapy than with octreotide when these drugs were started after endoscopic treatment of acute peptic ulcer bleeding.28 Nevertheless, octreotide may be useful in patients with uncontrolled nonvariceal bleeding who are awaiting endoscopy, since it is relatively safe to use.
ALL PATIENTS NEED ENDOSCOPY
All patients with upper GI bleeding need an upper endoscopic examination to diagnose and assess the risk posed by the bleeding lesion and to treat the lesion, reducing the risk of recurrent bleeding.
How urgently does endoscopy need to be done?
Endoscopy within the first 24 hours of upper GI bleeding is considered the standard of care. Patients with uncontrolled or recurrent bleeding should undergo endoscopy on an urgent basis to control the bleeding and reduce the risk of death.
However, how urgently endoscopy needs to be done is often debated. A multicenter randomized controlled trial compared outcomes in patients who underwent endoscopy within 6 hours of coming to the emergency department vs within 24 hours after the initial evaluation. The study found no significant difference in outcomes between the two groups; however, the group that underwent endoscopy sooner needed fewer transfusions.29
For a better view of the stomach
Gastric lavage improves the view of the gastric fundus but has not been proven to improve outcome.30
Promotility agents such as erythromycin and metoclopramide (Reglan) are also used to empty the stomach for better visualization.31–35 Erythromycin has been shown to improve visualization, shorten the procedure time, and prevent the need for additional endoscopy attempts in two randomized controlled studies.33,34 Furthermore, a cost-effectiveness study confirmed that giving intravenous erythromycin before endoscopy for acute upper GI bleeding saved money and resulted in an increase in quality-adjusted life-years.35
Endoscopy to diagnose bleeding and assess risk
Upper endoscopy is 90% to 95% diagnostic for acute upper GI bleeding.36
Patients at high risk (ie, older than 60 years, with severe comorbidity, or hemodynamically compromised) who have active bleeding (ie, witnessed hematemesis, red blood per nasogastric tube, or fresh blood per rectum) or a nonbleeding visible vessel should be admitted to a monitored bed or intensive care unit. Observation in a regular medical ward is appropriate for high-risk patients found to have an adherent clot. Patients with low-risk findings (eg, a clean ulcer base) are at low risk of recurrent bleeding and may be considered for early hospital discharge with appropriate outpatient follow-up.
Endoscopy to treat bleeding
About 25% of endoscopic procedures performed for upper GI bleeding include some type of treatment,39 such as injections of epinephrine, normal saline, or sclerosants; thermal cautery; argon plasma coagulation; electrocautery; or application of clips or bands. They are all equally effective, and combinations of these therapies are more effective than when they are used individually. A recent meta-analysis found dual therapy to be superior to epinephrine monotherapy in preventing recurrent bleeding, need for surgery, and death.40
How to manage adherent clots is controversial, but recent studies have revealed a significant benefit from removing them and treating the underlying lesions compared with drug therapy alone.43,45
Flat, pigmented spots and nonbleeding ulcers with a clean base do not require endoscopic treatment because the risk of recurrent bleeding is low.
Endoscopic therapy stops the bleeding in more than 90% of patients, but bleeding recurs after endoscopic therapy in 10% to 25%.46 Reversal of any severe coagulopathy with transfusions of platelets or fresh frozen plasma is essential for endoscopic hemostasis. However, coagulopathy at the time of initial bleeding and endoscopy does not appear to be associated with higher rates of recurrent bleeding following endoscopic therapy for nonvariceal upper GI bleeding.47
Patients with refractory bleeding are candidates for angiography or surgery. However, even when endoscopic hemostasis fails, endoscopy is important before angiography or surgery to pinpoint the site of bleeding and diagnose the cause.
A second endoscopic procedure is generally not recommended within 24 hours after the initial procedure.48 However, it is appropriate in cases in which clinical signs indicate recurrent bleeding or if hemostasis during the initial procedure is questionable. A meta-analysis found that routinely repeating endoscopy reduces the rate of recurrent bleeding but not the need for surgery or the risk of death.49
ALL PATIENTS SHOULD BE ADMITTED
VARICEAL BLEEDING
Variceal bleeding, a severe outcome of portal hypertension secondary to cirrhosis, carries a 6-week mortality rate of 10% to 20%.50 In view of the risk, primary prevention is indicated in patients with high-risk varices.
The mainstays of primary and secondary prevention are the nonselective beta-blockers such as nadolol (Corgard) and propranolol (Inderal). Several randomized controlled trials have shown lower rates of recurrent bleeding and death with propranolol or nadolol than with placebo.51 In doses that decrease the heart rate by 25%, beta-blockers have been shown to delay and decrease variceal hemorrhage. However, most patients require prophylactic endoscopic variceal ligation because they cannot tolerate beta-blocker therapy.
In suspected acute variceal bleeding, a somatostatin analogue should be started to decrease the portal pressure, and antibiotics should be started to reduce the risks of infection and death. Vasoactive drugs, ie, somatostatin analogues, should be started before endoscopy and continued for 5 days to reduce the chances of recurrent bleeding.52,53
Terlipressin is the only drug proven to improve the odds of survival in acute variceal bleeding. Although widely used in Europe, it has not been approved for use in the United States.
Octreotide, another option, improves hemostasis to the same extent, although it does not increase the survival rate.54,55 The recommended dose of octreotide for patients with variceal bleeding is a 50-μg intravenous bolus, followed by a 50-μg/hour infusion for 5 days.
Combining endoscopic and drug therapy improves the chances of stopping the bleeding and reduces the risk of recurrent bleeding compared with endoscopic therapy alone.56
Transjugular intrahepatic portosystemic shunting is indicated in recurrent variceal hemorrhage or in those with initial bleeding that is refractory to standard medical and endoscopic therapy. It is not the primary therapy because it doubles the risk of encephalopathy and has a high stent occlusion rate (up to 60%, lower with covered stents).
GI BLEEDING CAN CAUSE ACUTE MYOCARDIAL INFARCTION
The simultaneous presentation of acute myocardial infarction (MI) and GI hemorrhage is very serious and unfortunately common.
An acute MI occurring simultaneously with or after GI bleeding is usually precipitated by massive bleeding causing hypovolemia, hemodynamic compromise, and hypoperfusion. Conversely, the anticoagulant, antiplatelet, or thrombolytic drugs given to treat MI can precipitate GI bleeding (see below).
This distinction is important because the two scenarios have different clinical courses and prognoses. GI bleeding that precipitates an acute MI tends to be massive, whereas GI bleeding after treatment of acute MI tends to be self-limited and often resolves with reversal of underlying coagulopathy.57
Endoscopy carries a higher than average risk in patients with recent acute MI, with all-cause mortality rates as high as 1%.58 (The usual rate is 0.0004%.59) Nevertheless, endoscopy can be safely performed early on in patients with acute MI if it is done under strict monitoring in a coronary care unit.
Several studies have shown that MI patients who present with upper GI bleeding as the inciting event or patients with acute MI who are vomiting blood or who are hemodynamically unstable due to GI bleeding are significantly more likely to have a high-risk lesion and so have the greatest need for endoscopic therapy. Therefore, endoscopic intervention may be offered to MI patients at high risk who have been started on antiplatelet agents.
WARFARIN CAN PRECIPITATE BLEEDING
Acute upper GI bleeding can be a severe complication of long-term oral anticoagulation, not because the drugs cause ulcers, but rather because they exacerbate ulcers that are already present.60 Therefore, when starting warfarin (Coumadin), patients should be evaluated to determine if they have other risk factors for GI bleeding, such as ulcers.
The number of people presenting with upper GI bleeding while on warfarin therapy is increasing because of the expanding indications for long-term anticoagulation therapy, such as atrial fibrillation and deep venous thrombosis.
The risk of GI bleeding in patients who use oral anticoagulants is estimated to be 2.3 to 4.9 times higher than in nonusers.61
The goal international normalized ratio (INR) for patients on warfarin therapy is usually 2.0 to 3.0. Recent studies found that endoscopy can be safely performed in patients with acute GI bleeding whose INR is between 2.0 and 3.0.62,63 Some suggest that both the length of warfarin therapy and the INR affect the risk of bleeding.64,65
Managing patients with an INR higher than 3.0 who have an episode of GI bleeding is always a challenge. It is not uncommon to find pathologic lesions causing GI bleeding in patients who are on warfarin with a supratherapeutic INR, and thus, endoscopy is indicated. However, before endoscopy, reversal of anticoagulation should be considered.
BLEEDING IN PATIENTS ON ANTIPLATELET DRUGS
Aspirin
Aspirin decreases production of prostaglandins in the GI tract, thereby decreasing the protective and restorative properties of the gastric and duodenal mucosa and predisposing to ulcers and bleeding.
The higher the aspirin dose, the higher the risk. Aspirin doubles the risk of upper GI bleeding at daily doses of 75 mg and quadruples it at doses of 300 mg.66 Even doses as low as 10 mg can decrease gastric mucosal prostaglandin production.67 Thus, it appears that there is no risk-free dose of aspirin, and enteric-coated or buffered formulations do not appear to reduce the risk.68–70
The most important risk factor for upper GI bleeding in patients taking aspirin is a history of peptic ulcer bleeding. Approximately 15% of aspirin users who have bleeding from ulcers have recurrent bleeding within 1 year.71
As aspirin-induced GI bleeding becomes more common, health care providers often feel caught between the GI risk and the cardiovascular benefit. When considering whether to discontinue antiplatelet therapy, a cardiologist should be consulted along with a gastroenterologist to weigh the risks of GI bleeding vs thrombosis. To date, there have been no clinical trials published to suggest when antiplatelet therapy should be stopped to optimize GI and cardiovascular outcomes. An alternative is to replace aspirin with another antiplatelet drug that does not induce ulcers.
Clopidogrel
Clopidogrel (Plavix) is recommended for hospitalized patients with acute coronary syndrome who cannot tolerate the GI side effects of aspirin, according to the joint guidelines of the American College of Cardiology and the American Heart Association, with the highest level of evidence.72 This recommendation was largely based on the safety data from the CAPRIE (Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events) trial, in which the incidence of major GI bleeding was lower in the clopidogrel group (0.52%) than in the aspirin group (0.72%; P < .05).73
Aspirin plus a proton pump inhibitor
Patients who have had an episode of upper GI bleeding and who need long-term aspirin therapy should also receive a proton pump inhibitor indefinitely to prevent ulcer recurrence.
In a recent double-blind randomized controlled trial in patients with a history of aspirin-induced bleeding, the combination of low-dose aspirin plus esomeprazole (Nexium) twice a day was superior to clopidogrel by itself in terms of the rate of recurrent bleeding (0.7% vs 8.6%; P < .05).74 A similar trial showed nearly identical results: 0% upper GI bleeding in the group receiving aspirin plus esomeprazole 20 mg daily, vs 13.6% in the clopidogrel group (P = .0019).75 These studies suggest that a once-daily proton pump inhibitor combined with aspirin is a safer alternative than clopidogrel alone.
Clopidogrel plus a proton pump inhibitor
Interestingly, recent studies have shown that omeprazole decreases the antiplatelet effect of clopidogrel, possibly by inhibiting the CYP2C19 enzyme.76 However, concomitant use of pantoprazole (Protonix), lansoprazole (Prevacid), and esomeprazole did not have this effect, suggesting that although all proton pump inhibitors are metabolized to a varying degree by CYP2C19, the interaction between proton pump inhibitors and clopidogrel is not a class effect.77–79 Therefore, pantoprazole, lansoprazole, and esomeprazole may be the appropriate proton pump inhibitors to use with clopidogrel in patients who have a clear indication for the medication, consistent with current guideline recommendations.
Helicobacter pylori infection in antiplatelet drug users
TREATMENT AND PREVENTION OF NSAID-RELATED GI INJURY
About 1 in 20 users of NSAIDs develop GI complications and ulcers of varying degrees of severity, as do one in seven NSAID users over the age of 65. In fact, NSAID use accounts for 30% of hospitalizations for upper GI bleeding and deaths from this cause.82–85 In addition, approximately 15% to 30% of NSAID users have clinically silent but endoscopically evident peptic ulcers.86
NSAIDs contribute to ulcer development by depleting prostaglandins. Thus, misoprostol (Cytotec), a synthetic prostaglandin, has been used to reduce this side effect.
In a clinical trial, misoprostol reduced the incidence of NSAID-associated GI complications by 40%.87 Furthermore, it has been shown to be better than placebo in preventing recurrent gastric ulcers in patients with a history of gastric ulcer who were receiving low-dose aspirin.88
However, misoprostol is rarely used because it can cause diarrhea and abdominal cramping. Rather, the preferred drugs for preventing and treating NSAID- and aspirin-related GI lesions are proton pump inhibitors.
Numerous clinical trials using endoscopic end points showed that proton pump inhibitors in standard doses significantly reduce the incidence of ulcers associated with the use of NSAIDs.89 Proton pump inhibitor therapy has achieved a significant reduction in relative risk of upper GI bleeding in patients who received low-dose aspirin therapy, as confirmed by epidemiologic studies.90,91 The number of NSAID-related ulcers found on endoscopy could be reduced by an estimated 90% simply by using proton pump inhibitors.92
- Lewis JD, Bilker WB, Brensinger C, Farrar JT, Strom BL. Hospitalization and mortality rates from peptic ulcer disease and GI bleeding in the 1990s: relationship to sales of non-steroidal anti-inflammatory drugs and acid suppression medications. Am J Gastroenterol 2002; 97:2540–2549.
- Viviane A, Alan BN. Estimates of costs of hospital stays for variceal and nonvariceal upper gastrointestinal bleeding in the United States. Value Health 2008; 11:1–3.
- Yavorski RT, Wong RK, Maydonovitch C, Battin LS, Furnia A, Amundson DE. Analysis of 3,294 cases of upper gastrointestinal bleeding in military medical facilities. Am J Gastroenterol 1995; 90:568–573.
- Kaplan RC, Heckbert SR, Koepsell TD, et al. Risk factors for hospitalized gastrointestinal bleeding among older persons. Cardiovascular Health Study Investigators. J Am Geriatr Soc 2001; 49:126–133.
- Longstreth GF. Epidemiology of hospitalization for acute upper gastrointestinal hemorrhage: a population-based study. Am J Gastroenterol 1995; 90:206–210.
- Laine L, Peterson WL. Bleeding peptic ulcer. N Engl J Med 1994; 331:717–727.
- Wara P, Stodkilde H. Bleeding pattern before admission as guideline for emergency endoscopy. Scand J Gastroenterol 1985; 20:72–78.
- Jensen DM, Machicado GA. Diagnosis and treatment of severe hematochezia. The role of urgent colonoscopy after purge. Gastroenterology 1988; 95:1569–1574.
- Daniel WA, Egan S. The quantity of blood required to produce a tarry stool. J Am Med Assoc 1939; 113:2232.
- Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal hemorrhage. Gut 1996; 38:316–321.
- Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper-gastrointestinal hemorrhage. Lancet 2000; 356:1318–1321.
- Gralnek IM, Barkun AN, Bardou M. Management of acute bleeding from a peptic ulcer. N Engl J Med 2008; 359:928–937.
- Silverstein FE, Gilbert DA, Tedesco FJ, Buenger NK, Persing J. The national ASGE survey on upper gastrointestinal bleeding II. Clinical prognostic factors. Gastrointest Endosc 1981; 27:80–93.
- Corley DA, Stefan AM, Wolf M, Cook EF, Lee TH. Early indicators of prognosis in upper gastrointestinal hemorrhage. Am J Gastroenterol 1998; 93:336–340.
- Aljebreen AM, Fallone CA, Barkun AN. Nasogastric aspirate predicts high-risk endoscopic lesions in patients with acute upper-GI bleeding. Gastrointest Endosc 2004; 59:172–178.
- Barkun AN, Cockeram AW, Plourde V, Fedorak RN. Review article: acid suppression in non-variceal acute upper gastrointestinal bleeding. Aliment Pharmacol Ther 1999; 13:1565–1584.
- Levine JE, Leontiadis JI, Sharma VK, Howden CW. Meta-analysis: the efficacy of intravenous H2-receptor antagonists in bleeding peptic ulcer. Aliment Pharmacol Ther 2002; 16:1137–1142.
- Walt RP, Cottrell J, Mann SG, Freemantle NP, Langman MJ. Continuous intravenous famotidine for hemorrhage from peptic ulcer. Lancet 1992; 340:1058–1062.
- Labenz J, Peitz U, Leusing C, Tillenburg B, Blum AL, Börsch G. Efficacy of primed infusion with high dose ranitidine and omeprazole to maintain high intragastric pH in patients with peptic ulcer bleeding: a prospective randomized controlled study. Gut 1997; 40:36–41.
- Merki HS, Wilder-Smith CH. Do continuous infusions of omeprazole and ranitidine retain their effect with prolonged dosing? Gastroenterology 1994; 106:60–64.
- Netzer P, Gaia C, Sandoz M, et al. Effect of repeated injection and continuous infusion of omeprazole and ranitidine on intragastric pH over 72 hours. Am J Gastroenterol 1999; 94:351–357.
- Lin HJ, Lo WC, Cheng YC, Perng CL. Role of intravenous omeprazole in patients with high-risk peptic ulcer bleeding after successful endoscopic epinephrine injection: a prospective randomized comparative trial. Am J Gastroenterol 2006; 101:500–505.
- Lau JY, Sung JJ, Lee KK, et al. Effects of intravenous omeprazole on recurrent bleeding after endoscopic treatment of bleeding peptic ulcers. N Engl J Med 2000; 343:310–316.
- Leontiadis GI, Sharma VK, Howden CW. Proton pump inhibitor treatment for acute peptic ulcer bleeding. Cochrane Database Syst Rev 2006;CD002094.
- Andriulli A, Annese V, Caruso N, et al. Proton-pump inhibitors and outcome of endoscopic hemostasis in bleeding peptic ulcers: a series of meta-analyses. Am J Gastroenterol 2005; 100:207–219.
- Lau JY, Leung WK, Wu JC, et al. Omeprazole before endoscopy in patients with gastrointestinal bleeding. N Engl J Med 2007; 356:1631–1640.
- Dorward S, Sreedharan A, Leontiadis GI, Howden CW, Moayyedi P, Forman D. Proton pump inhibitor treatment initiated prior to endoscopic diagnosis in upper gastrointestinal bleeding. Cochrane Database Syst Rev 2006;CD005415.
- Bardou M, Toubouti Y, Benhaberou-Brun D, Rahme E, Barkun AN. Meta-analysis: proton-pump inhibition in high-risk patients with acute peptic ulcer bleeding. Aliment Pharmacol Ther 2005; 21:677–686.
- Bjorkman DJ, Zaman A, Fennerty MB, Lieberman D, Disario JA, Guest-Warnick G. Urgent vs elective endoscopy for acute non-variceal upper-GI bleeding: an effectiveness study. Gastointest Endosc 2004; 60:1–8.
- Lee SD, Kearney DJ. A randomized controlled trial of gastric lavage prior to endoscopy for acute upper gastrointestinal bleeding. J Clin Gastroenterol 2004; 38:861–865.
- Tack J, Janssens J, Vantrappen G, et al. Effect of erythromycin on gastric motility in controls and in diabetic gastroparesis. Gastroenterology 1992; 103:72–79.
- Xynos E, Mantides A, Papageorgiou A, Fountos A, Pechlivanides G, Vassilakis JS. Erythromycin accelerates delayed gastric emptying of solids in patients after truncal vagotomy and pyloroplasty. Eur J Surg 1992; 158:407–411.
- Coffin B, Pocard M, Panis Y, et al; Groupe des endoscopistes de garde á l’AP-HP. Erythromycin improves the quality of EGD in patients with acute upper GI bleeding: a randomized controlled study. Gastrointest Endosc 2002; 56:174–179.
- Frossard JL, Spahr L, Queneau PE, et al. Erythromycin intravenous bolus infusion in acute upper gastrointestinal bleeding: a randomized, controlled, double-blind trial. Gastroenterology 2002; 123:17–23.
- Winstead NS, Wilcox CM. Erythromycin prior to endoscopy for acute upper gastrointestinal hemorrhage: a cost-effectiveness analysis. Aliment Pharmacol Ther 2007; 26:1371–1377.
- Chak A, Cooper GS, Lloyd LE, Kolz CS, Barnhart BA, Wong RC. Effectiveness of endoscopy in patients admitted to the intensive care unit with upper GI hemorrhage. Gastrointest Endosc 2001; 53:6–13.
- Lau JY, Chung SC, Leung JW, Lo KK, Yung MY, Li AK. The evolution of stigmata of hemorrhage in bleeding peptic ulcers: a sequential endoscopic study. Endoscopy 1998; 30:513–518.
- Chung IK, Kim EJ, Lee MS, et al. Endoscopic factors predisposing to rebleeding following endoscopic hemostasis in bleeding peptic ulcers. Endoscopy 2001; 33:969–975.
- Elta GH. Acute nonvariceal upper gastrointestinal hemorrhage. Curr Treat Options Gastroenterol 2002; 5:147–152.
- Marmo R, Rotondano G, Piscopo R, Bianco MA, D’Angella R, Cipolletta L. Dual therapy versus monotherapy in the endoscopic treatment of high-risk bleeding ulcers: a meta-analysis of controlled trials. Am J Gastroenterol 2007; 102:279–289.
- Kovacs TO, Jensen DM. Recent advances in the endoscopic diagnosis and therapy of upper gastrointestinal, small intestinal, and colonic bleeding. Med Clin North Am 2002; 86:1319–1356.
- Kovacs TO, Jensen DM. Endoscopic treatment of ulcer bleeding. Curr Treat Options Gastroenterol 2007; 10:143–148.
- Jensen DM, Kovacs TO, Jutabha R, et al. Randomized trial of medical or endoscopic therapy to prevent recurrent ulcer hemorrhage in patients with adherent clots. Gastroenterology 2002; 123:407–413.
- Jensen DM, Machicado GA. Endoscopic hemostasis of ulcer hemorrhage with injection, thermal, and combination methods. Techniques Gastrointest Endosc 2005; 7:124–131.
- Bleau BL, Gostout CJ, Sherman KE, et al. Recurrent bleeding from peptic ulcer associated with adherent clot: a randomized study comparing endoscopic treatment with medical therapy. Gastrointest Endosc 2002; 56:1–6.
- Lau JY, Sung JJ, Lam YH, et al. Endoscopic retreatment compared with surgery in patients with recurrent bleeding after initial endoscopic control of bleeding ulcers. N Engl J Med 1999; 340:751–756.
- Wolf AT, Wasan SK, Saltzman JR. Impact of anticoagulation on rebleeding following endoscopic therapy for nonvariceal upper gastrointestinal hemorrhage. Am J Gastroenterol 2007; 102:290–296.
- Barkun A, Bardou M, Marshall JK; Nonvariceal Upper GI Bleeding Consensus Conference Group. Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med 2003; 139:843–857.
- Marmo R, Rotondano G, Bianco MA, Piscopo R, Prisco A, Cipolletta L. Outcome of endoscopic treatment for peptic ulcer bleeding: is a second look necessary? A meta-analysis. Gastrointest Endosc 2003; 57:62–67.
- Dell’Era A, deFrancis R, Iannuzzi F. Acute variceal bleeding: pharmacological treatment and primary/secondary prophylaxis. Best Pract Res Clin Gastroenterol 2008; 22:279–294.
- Jalan R, Hayes PC. UK guidelines on the management of variceal hemorrhage in cirrhotic patients. British Society of Gastroenterology. Gut 2000; 46( suppl 3–4):III1–III15.
- Bernard B, Lebrec D, Mathurin P, Opolon P, Poynard T. Beta-adrenergic antagonists in the prevention of gastrointestinal rebleeding in patients with cirrhosis: a meta-analysis. Hepatology 1997; 25:63–70.
- De Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2005; 43:167–176.
- Levacher S, Letoumelin P, Pateron D, Blaise M, Lapandry C, Pourriat JL. Early administration of terlipressin plus glyceryl trinitrate to control active upper gastrointestinal bleeding in cirrhotic patients. Lancet 1995; 346:865–868.
- Abraldes JG, Bosch J. Somatostatin and analogues in portal hypertension. Hepatology 2002; 35:1305–1312.
- Banares R, Albillos A, Rincon D, et al. Endoscopic treatment versus endoscopic plus pharmacological treatment for acute variceal bleeding: a meta analysis. Hepatology 2002; 35:609–615.
- Cappell M. Gastrointenstinal bleeding associated with myocardial infarction. Gastroenterol Clin North Am 2000; 29:423–444.
- Lin S, Konstance R, Jollis J, Fisher DA. The utility of upper endoscopy in patients with concomitant upper gastrointestinal bleeding and acute myocardial infarction. Dig Dis Sci 2006; 51:2377–2383.
- Silvis SE, Nebel O, Rogers G, Sugawa C, Mandelstam P. Endoscopic complications. Results of the 1974 American Society for Gastrointestinal Endoscopy Survey. JAMA 1976; 235:928–930.
- Lanas A, Garcia-Rodriguez LA, Arroyo MT, et al; Investigators of the Asociación Española de Gastroenterología (AEG). Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol 2007; 102:507–515.
- Shorr RI, Ray WA, Daugherty JR, Griffin MR. Concurrent use of nonsteroidal anti-inflammatory drugs and oral anticoagulants places elderly persons at high risk for hemorrhagic peptic ulcer disease. Arch Intern Med 1993; 153:1665–1670.
- Tabibian N. Acute gastrointestinal bleeding in anticoagulated patients: a prospective evaluation. Am J Gastroenterol 1989; 84:10–12.
- Choudari CP, Rajgopal C, Palmer KR. Acute gastrointestinal hemorrhage in anticoagulated patients: diagnoses and response to endoscopic treatment. Gut 1994; 35:464–466.
- Petty GW, Brown RD, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Frequency of major complications of aspirin, warfarin, and intravenous heparin for secondary stroke prevention: a population-based study. Ann Intern Med 1999; 130:14–22.
- Landefeld CS, Rosenblatt MW, Goldman L. Bleeding in outpatients treated with warfarin: relation to the prothrombin time and important remediable lesions. Am J Med 1989; 87:153–159.
- Weil J, Colin-Jones D, Langman M, et al. Prophylactic aspirin and risk of peptic ulcer bleeding. BMJ 1995; 310:827–830.
- Cryer B, Feldman M. Effects of very low dose daily, long-term aspirin therapy on gastric, duodenal, and rectal prostaglandin levels and on mucosal injury in healthy humans. Gastroenterology 1999; 117:17–25.
- De Abajo FJ, Garcia Rodriguez LA. Risk of upper gastrointestinal bleeding and perforation associated with low-dose aspirin as plain and enteric-coated formulations. BMC Clin Pharmacol 2001; 1:1.
- Kelly JP, Kaufman DW, Jurgelon JM, Sheehan J, Koff RS, Shapiro S. Risk of aspirin-associated major upper gastrointestinal bleeding with enteric coated or buffered product. Lancet 1996; 348:1413–1416.
- Garcia Rodriguez LA, Hernandez-Diaz S, De Abajo FJ. Association between aspirin and upper gastrointestinal complications: systematic review of epidemiological studies. Br J Clin Pharmacol 2001; 52:563–571.
- Wilcox CM, Ladabaum U. A patient with high risk of gastrointestinal bleeding requiring nonsteroidal anti-inflammatory drugs. Clin Gastroenterol Hepatol 2006; 4:1090–1093.
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction. J Am Coll Cardiol 2007; 50:e1–e157.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996; 348:1329–1339.
- Chan FK, Ching JY, Hung LC, et al. Clopidogrel versus aspirin and esomeprazole to prevent recurrent ulcer bleeding. N Engl J Med 2005; 352:238–244.
- Lai KC, Chu KM, Hui WM, et al. Esomeprazole with aspirin versus clopidogrel for prevention of recurrent gastrointestinal ulcer complications. Clin Gastroenterol Hepatol 2006; 4:860–865.
- Ho MP, Maddox TM, Wang L, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA 2009; 301:937–944.
- Siller-Matula JM, Spiel AO, Lang IM, Kreiner G, Christ G, Jilma B. Effects of pantoprazole and esomeprazole on platelet inhibition by clopidogrel. Am Heart J 2009; 157:148.e1–e5.
- Small DS, Farid NA, Payne CD, et al. Effects of proton pump inhibitor lansoprazole on the pharmacokinetics and pharmacodynamics of prasugel and clopidogrel. J Clin Pharmacol 2008; 48:475–484.
- Ishizaki T, Horai Y. Review article: cytochrome P450 and the metabolism of proton pump inhibitors—emphasis on rabeprazole. Aliment Pharacol Ther 1999; 13 (suppl 3):27–36.
- Lanas A, Fuentes J, Benito R, Serrano P, Bajador E, Sainz R. Helicobacter pylori increases the risk of upper gastrointestinal bleeding in patients taking low-dose aspirin. Aliment Pharmacol Ther 2002; 16:779–786.
- Chan FK. NSAID-Induced peptic ulcers and Helicobacter pylori infection: implications for patient management. Drug Saf 2005; 28:287–300.
- Bombardier V, Laine L, Reicin A, et al; VIGOR Study Group. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis: VIGOR Study Group. N Eng J Med 2000; 343:1520–1528.
- Griffin MR, Ray WA, Schaffner W. Nonsteroidal anti-inflammatory drug use and death from peptic ulcer in elderly persons. Ann Intern Med 1988; 109:359–363.
- Griffin MR, Piper JM, Daugherty JR, Snowden M, Ray WA. Nonsteroidal anti-inflammatory drug use and increased risk for peptic ulcer disease in elderly persons. Ann Intern Med 1991; 114:257–263.
- Smalley WE, Ray WA, Daugherty JR, Griffin MR. Nonsteroidal anti-inflammatory drugs and the incidence of hospitalizations for peptic ulcer disease in elderly persons. Am J Epidemiol 1995; 141:539–545.
- Laine L. Approaches to nonsteroidal anti-inflammatory drug use in the high-risk patient. Gastroenterology 2001; 120:594–606.
- Silverstein FE, Graham DY, Senior JR, et al. Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal anti-inflammatory drugs. A randomized, double blind, placebo controlled trial. Ann Intern Med 1995; 123:241–249.
- Goldstein JL, Huang B, Amer F, Christopoulos NG. Ulcer recurrence in high-risk patients receiving nonsteroidal anti-inflammatory drugs plus low dose aspirin: results of a post hoc subanalysis. Clin Ther 2004; 26:1637–1643.
- Berger JS, Stebbins A, Granger CB, et al. Initial aspirin dose and outcome among ST-elevation myocardial infarction patients treated with fibrinolytic therapy. Circulation 2008; 117:192–199.
- Lanas A, Garcia-Rodriguez LA, Arroyo MT, et al; Investigators of the Asociación Española de Gastroenterología (AEG). Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol 2007; 102:507–515.
- Chin MW, Yong G, Bulsara MK, Rankin J, Forbes GM. Predictive and protective factors associated with upper gastrointestinal bleeding after percutaneous coronary intervention: a case-control study. Am J Gastroenterol 2007; 102:2411–2416.
- Hunt RH, Bazzoli F. Should NSAID/low dose aspirin takers be tested routinely for H. Pylori infection and treated if positive? Implications for primary risk of ulcer and ulcer relapse after initial healing. Aliment Pharmacol Ther 2004; 19 (suppl 1):9–16.
Upper gastrointestinal (GI) bleeding is common, costly, and potentially life-threatening. It must be managed promptly and appropriately to prevent adverse outcomes.
More people are admitted to the hospital for upper GI bleeding than for congestive heart failure or deep vein thrombosis. In the United States, the annual rate of hospitalization for upper GI bleeding is estimated to be 165 per 100,000—more than 300,000 hospitalizations per year, at a cost of $2.5 billion.1,2
Furthermore, despite advances in therapy, the case-fatality rate has remained unchanged at 7% to 10%.3 This may be because today’s patients are older and have more comorbidities than those in the past.4
CAUSES OF UPPER GI BLEEDING
Peptic ulcers account for about 60% of severe cases of upper GI bleeding,5 and they are the focus of this paper. Fortunately, up to 80% of bleeding ulcers stop bleeding spontaneously without any intervention.6
Gastroduodenal erosions account for about 12%.3
Varices due to cirrhosis are less common but more dangerous. Variceal bleeding accounts for a relatively small percentage (6%) of upper GI bleeding, but the mortality rate from a single episode of variceal bleeding is 30%, and 60% to 70% of patients die within 1 year, mostly of underlying liver disease.
Less frequent causes include Mallory-Weiss tears, erosive duodenitis, Dieulafoy ulcer (a type of vascular malformation), other vascular lesions, neoplasms, aortoenteric fistula, gastric antral vascular ectasia, and prolapse gastropathy.5
HEMATEMESIS AND MELENA
The most common presenting signs of acute upper GI bleeding are hematemesis (vomiting of blood), “coffee grounds” emesis, and melena (tarry black stools). About 30% of patients with bleeding ulcers present with hematemesis, 20% with melena, and 50% with both.7
Hematochezia (red blood in the stool) usually suggests a lower GI source of bleeding, since blood from an upper source turns black and tarry as it passes through the gut, producing melena. However, up to 5% of patients with bleeding ulcers have hematochezia,7 and it indicates heavy bleeding: bleeding of approximately 1,000 mL into the upper GI tract is needed to cause hematochezia, whereas only 50 to 100 mL is needed to cause melena.8,9 Hematochezia with signs and symptoms of hemodynamic compromise such as syncope, postural hypotension, tachycardia, and shock should therefore direct one’s attention to an upper GI source of bleeding.
Nonspecific features include nausea, vomiting, epigastric pain, vasovagal phenomena, and syncope.
WHAT IS THE PATIENT’S RISK?
An assessment of clinical severity is the first critical task, as it helps in planning treatment. Advanced age, multiple comorbidities, and hemodynamic instability call for aggressive treatment. Apart from this simple clinical rule, scoring systems have been developed.
The Rockall scoring system, the most widely used, gives estimates of the risks of recurrent bleeding and death. It is based on the three clinical factors mentioned above and on two endoscopic ones, awarding points for:
- Age—0 points if less than 60; 1 point if 60 to 79; or 2 points if 80 years or older
- Shock—1 point if the pulse is more than 100; 2 points if the systolic blood pressure is less than 100 mm Hg
- Comorbid illness—2 points for ischemic heart disease, congestive heart failure, or other major comorbidity; 3 points for renal failure, hepatic failure, or metastatic disease
- Endoscopic diagnosis—0 points if no lesion found or a Mallory-Weiss tear; 1 point for peptic ulcer, esophagitis, or erosive disease; 2 points for GI malignancy
- Endoscopic stigmata or recent hemorrhage—0 points for a clean-based ulcer or flat pigmented spot; 2 points for blood in the upper GI tract, active bleeding, a nonbleeding visible vessel, or adherent clot.
The Rockall score can thus range from 0 to 11 points, with an overall score of 0, 1, or 2 associated with an excellent prognosis.10
Other systems that are used less often include the Baylor severity scale and the Acute Physiology and Chronic Health Evaluation (APACHE) II score.
Does the patient have varices?
All variceal bleeding should be considered severe, since the 1-year death rate is so high (up to 70%). Clues pointing to variceal bleeding include previous variceal bleeding, thrombocytopenia, history of liver disease, and signs of liver disease on clinical examination.
All patients suspected of having bleeding varices should be admitted to the intensive care unit for close monitoring and should be given the highest priority, even if they are hemodynamically stable.
Is the patient hemodynamically stable?
Appropriate hemodynamic assessment includes monitoring of heart rate, blood pressure, and mental status. Tachycardia at rest, hypotension, and orthostatic changes in vital signs indicate a considerable loss of blood volume. Low urine output, dry mucous membranes, and sunken neck veins are also useful signs. (Tachycardia may be blunted if the patient is taking a beta-blocker.)
If these signs of hypovolemia are present, the initial management focuses on treating shock and on improving oxygen delivery to the vital organs. This involves repletion of the intravascular volume with intravenous infusions or blood transfusions. Supplemental oxygen also is useful, especially in elderly patients with heart disease.12
Inspection of nasogastric aspirate
In the initial assessment, it is useful to insert a nasogastric tube and inspect the aspirate. If it contains bright red blood, the patient needs an urgent endoscopic evaluation and an intensive level of care13,14; if it contains coffee-grounds material, the patient needs to be admitted to the hospital and to undergo endoscopic evaluation within 24 hours.
However, a normal aspirate does not rule out upper GI bleeding. Aljebreen et al15 found that 15% of patients with upper GI bleeding and normal nasogastric aspirate still had high-risk lesions (ie, visible bleeding or nonbleeding visible vessels) on endoscopy.
ACID-SUPPRESSION HELPS ULCERS HEAL
Acid and pepsin interfere with the healing of ulcers and other nonvariceal upper GI lesions. Further, an acidic environment promotes platelet disaggregation and fibrinolysis and impairs clot formation.16 This suggests that inhibiting gastric acid secretion and raising the gastric pH to 6 or higher may stabilize clots. Moreover, pepsinogen in the stomach is converted to its active form (pepsin) if the pH is less than 4. Therefore, keeping the pH above 4 keeps pepsinogen in an inactive form.
Histamine-2 receptor antagonists
Histamine-2 receptor antagonists were the first drugs to inhibit acid secretion, reversibly blocking histamine-2 receptors on the basolateral membrane of parietal cells. However, these drugs did not prove very useful in managing upper GI bleeding in clinical trials.17,18 In their intravenous form, they often fail to keep the gastric pH at 6 or higher, due to tachyphylaxis.19 The use of this class of drugs has declined in favor of proton pump inhibitors.
Proton pump inhibitors
Proton pump inhibitors reduce both basal and stimulated acid secretion by inhibiting hydrogen-potassium adenosine triphosphatase, the proton pump of the parietal cell.
Multiple studies have shown that proton pump inhibitors raise the gastric pH and keep it high. For example, an infusion of omeprazole (Prilosec) can keep the gastric pH above 6 for 72 hours without inducing tachyphylaxis.20,21
Started after endoscopy. Randomized controlled trials have found proton pump inhibitors to be effective when given in high doses intravenously for 72 hours after successful endoscopic treatment of bleeding ulcers with high-risk endoscopic signs, such as active bleeding or nonbleeding visible vessels.22,23
A meta-analysis indicated that these drugs decrease the incidence of recurrent peptic ulcer bleeding, the need for blood transfusions, the need for surgery, and the duration of hospitalization, but not the mortality rate.24,25 These studies also illustrate the benefit of following up endoscopic treatment to stop the bleeding with an intravenous infusion of a proton pump inhibitor.
The recommended dose of omeprazole for patients with high-risk findings on endoscopy is an 80-mg bolus followed by an 8-mg/hour infusion for 72 hours. After the patient’s condition stabilizes, oral therapy can be substituted for intravenous therapy. In patients with low-risk endoscopic findings (a clean-based ulcer or flat spot), oral proton pump inhibitors in high doses are recommended.
In either case, after the initial bleeding is treated endoscopically and hemostasis is achieved, a proton pump inhibitor is recommended for 6 to 8 weeks, or longer if the patient is also positive for Helicobacter pylori or is on daily treatment with aspirin or a nonsteroidal anti-inflammatory drug (NSAID) that is not selective for cyclo-oxygenase 2 (see below).
Started before endoscopy, these drugs reduced the frequency of actively bleeding ulcers, the duration of hospitalization, and the need for endoscopic therapy in a randomized controlled trial.26 A meta-analysis found that significantly fewer patients had signs of recent bleeding on endoscopy if they received a proton pump inhibitor 24 to 48 hours before the procedure, but it did not find any significant difference in important clinical outcomes such as death, recurrent bleeding, or surgery.27 Nevertheless, we believe that intravenous proton pump inhibitor therapy should be started before endoscopy in patients with upper GI bleeding.
Somatostatin analogues
Octreotide (Sandostatin), an analogue of the hormone somatostatin, decreases splanchnic blood flow, decreases secretion of gastric acid and pepsin, and stimulates mucus production. Although it is beneficial in treating upper GI bleeding due to varices, its benefit has not been confirmed in patients with nonvariceal upper GI bleeding.
A meta-analysis revealed that outcomes were better with high-dose intravenous proton pump inhibitor therapy than with octreotide when these drugs were started after endoscopic treatment of acute peptic ulcer bleeding.28 Nevertheless, octreotide may be useful in patients with uncontrolled nonvariceal bleeding who are awaiting endoscopy, since it is relatively safe to use.
ALL PATIENTS NEED ENDOSCOPY
All patients with upper GI bleeding need an upper endoscopic examination to diagnose and assess the risk posed by the bleeding lesion and to treat the lesion, reducing the risk of recurrent bleeding.
How urgently does endoscopy need to be done?
Endoscopy within the first 24 hours of upper GI bleeding is considered the standard of care. Patients with uncontrolled or recurrent bleeding should undergo endoscopy on an urgent basis to control the bleeding and reduce the risk of death.
However, how urgently endoscopy needs to be done is often debated. A multicenter randomized controlled trial compared outcomes in patients who underwent endoscopy within 6 hours of coming to the emergency department vs within 24 hours after the initial evaluation. The study found no significant difference in outcomes between the two groups; however, the group that underwent endoscopy sooner needed fewer transfusions.29
For a better view of the stomach
Gastric lavage improves the view of the gastric fundus but has not been proven to improve outcome.30
Promotility agents such as erythromycin and metoclopramide (Reglan) are also used to empty the stomach for better visualization.31–35 Erythromycin has been shown to improve visualization, shorten the procedure time, and prevent the need for additional endoscopy attempts in two randomized controlled studies.33,34 Furthermore, a cost-effectiveness study confirmed that giving intravenous erythromycin before endoscopy for acute upper GI bleeding saved money and resulted in an increase in quality-adjusted life-years.35
Endoscopy to diagnose bleeding and assess risk
Upper endoscopy is 90% to 95% diagnostic for acute upper GI bleeding.36
Patients at high risk (ie, older than 60 years, with severe comorbidity, or hemodynamically compromised) who have active bleeding (ie, witnessed hematemesis, red blood per nasogastric tube, or fresh blood per rectum) or a nonbleeding visible vessel should be admitted to a monitored bed or intensive care unit. Observation in a regular medical ward is appropriate for high-risk patients found to have an adherent clot. Patients with low-risk findings (eg, a clean ulcer base) are at low risk of recurrent bleeding and may be considered for early hospital discharge with appropriate outpatient follow-up.
Endoscopy to treat bleeding
About 25% of endoscopic procedures performed for upper GI bleeding include some type of treatment,39 such as injections of epinephrine, normal saline, or sclerosants; thermal cautery; argon plasma coagulation; electrocautery; or application of clips or bands. They are all equally effective, and combinations of these therapies are more effective than when they are used individually. A recent meta-analysis found dual therapy to be superior to epinephrine monotherapy in preventing recurrent bleeding, need for surgery, and death.40
How to manage adherent clots is controversial, but recent studies have revealed a significant benefit from removing them and treating the underlying lesions compared with drug therapy alone.43,45
Flat, pigmented spots and nonbleeding ulcers with a clean base do not require endoscopic treatment because the risk of recurrent bleeding is low.
Endoscopic therapy stops the bleeding in more than 90% of patients, but bleeding recurs after endoscopic therapy in 10% to 25%.46 Reversal of any severe coagulopathy with transfusions of platelets or fresh frozen plasma is essential for endoscopic hemostasis. However, coagulopathy at the time of initial bleeding and endoscopy does not appear to be associated with higher rates of recurrent bleeding following endoscopic therapy for nonvariceal upper GI bleeding.47
Patients with refractory bleeding are candidates for angiography or surgery. However, even when endoscopic hemostasis fails, endoscopy is important before angiography or surgery to pinpoint the site of bleeding and diagnose the cause.
A second endoscopic procedure is generally not recommended within 24 hours after the initial procedure.48 However, it is appropriate in cases in which clinical signs indicate recurrent bleeding or if hemostasis during the initial procedure is questionable. A meta-analysis found that routinely repeating endoscopy reduces the rate of recurrent bleeding but not the need for surgery or the risk of death.49
ALL PATIENTS SHOULD BE ADMITTED
VARICEAL BLEEDING
Variceal bleeding, a severe outcome of portal hypertension secondary to cirrhosis, carries a 6-week mortality rate of 10% to 20%.50 In view of the risk, primary prevention is indicated in patients with high-risk varices.
The mainstays of primary and secondary prevention are the nonselective beta-blockers such as nadolol (Corgard) and propranolol (Inderal). Several randomized controlled trials have shown lower rates of recurrent bleeding and death with propranolol or nadolol than with placebo.51 In doses that decrease the heart rate by 25%, beta-blockers have been shown to delay and decrease variceal hemorrhage. However, most patients require prophylactic endoscopic variceal ligation because they cannot tolerate beta-blocker therapy.
In suspected acute variceal bleeding, a somatostatin analogue should be started to decrease the portal pressure, and antibiotics should be started to reduce the risks of infection and death. Vasoactive drugs, ie, somatostatin analogues, should be started before endoscopy and continued for 5 days to reduce the chances of recurrent bleeding.52,53
Terlipressin is the only drug proven to improve the odds of survival in acute variceal bleeding. Although widely used in Europe, it has not been approved for use in the United States.
Octreotide, another option, improves hemostasis to the same extent, although it does not increase the survival rate.54,55 The recommended dose of octreotide for patients with variceal bleeding is a 50-μg intravenous bolus, followed by a 50-μg/hour infusion for 5 days.
Combining endoscopic and drug therapy improves the chances of stopping the bleeding and reduces the risk of recurrent bleeding compared with endoscopic therapy alone.56
Transjugular intrahepatic portosystemic shunting is indicated in recurrent variceal hemorrhage or in those with initial bleeding that is refractory to standard medical and endoscopic therapy. It is not the primary therapy because it doubles the risk of encephalopathy and has a high stent occlusion rate (up to 60%, lower with covered stents).
GI BLEEDING CAN CAUSE ACUTE MYOCARDIAL INFARCTION
The simultaneous presentation of acute myocardial infarction (MI) and GI hemorrhage is very serious and unfortunately common.
An acute MI occurring simultaneously with or after GI bleeding is usually precipitated by massive bleeding causing hypovolemia, hemodynamic compromise, and hypoperfusion. Conversely, the anticoagulant, antiplatelet, or thrombolytic drugs given to treat MI can precipitate GI bleeding (see below).
This distinction is important because the two scenarios have different clinical courses and prognoses. GI bleeding that precipitates an acute MI tends to be massive, whereas GI bleeding after treatment of acute MI tends to be self-limited and often resolves with reversal of underlying coagulopathy.57
Endoscopy carries a higher than average risk in patients with recent acute MI, with all-cause mortality rates as high as 1%.58 (The usual rate is 0.0004%.59) Nevertheless, endoscopy can be safely performed early on in patients with acute MI if it is done under strict monitoring in a coronary care unit.
Several studies have shown that MI patients who present with upper GI bleeding as the inciting event or patients with acute MI who are vomiting blood or who are hemodynamically unstable due to GI bleeding are significantly more likely to have a high-risk lesion and so have the greatest need for endoscopic therapy. Therefore, endoscopic intervention may be offered to MI patients at high risk who have been started on antiplatelet agents.
WARFARIN CAN PRECIPITATE BLEEDING
Acute upper GI bleeding can be a severe complication of long-term oral anticoagulation, not because the drugs cause ulcers, but rather because they exacerbate ulcers that are already present.60 Therefore, when starting warfarin (Coumadin), patients should be evaluated to determine if they have other risk factors for GI bleeding, such as ulcers.
The number of people presenting with upper GI bleeding while on warfarin therapy is increasing because of the expanding indications for long-term anticoagulation therapy, such as atrial fibrillation and deep venous thrombosis.
The risk of GI bleeding in patients who use oral anticoagulants is estimated to be 2.3 to 4.9 times higher than in nonusers.61
The goal international normalized ratio (INR) for patients on warfarin therapy is usually 2.0 to 3.0. Recent studies found that endoscopy can be safely performed in patients with acute GI bleeding whose INR is between 2.0 and 3.0.62,63 Some suggest that both the length of warfarin therapy and the INR affect the risk of bleeding.64,65
Managing patients with an INR higher than 3.0 who have an episode of GI bleeding is always a challenge. It is not uncommon to find pathologic lesions causing GI bleeding in patients who are on warfarin with a supratherapeutic INR, and thus, endoscopy is indicated. However, before endoscopy, reversal of anticoagulation should be considered.
BLEEDING IN PATIENTS ON ANTIPLATELET DRUGS
Aspirin
Aspirin decreases production of prostaglandins in the GI tract, thereby decreasing the protective and restorative properties of the gastric and duodenal mucosa and predisposing to ulcers and bleeding.
The higher the aspirin dose, the higher the risk. Aspirin doubles the risk of upper GI bleeding at daily doses of 75 mg and quadruples it at doses of 300 mg.66 Even doses as low as 10 mg can decrease gastric mucosal prostaglandin production.67 Thus, it appears that there is no risk-free dose of aspirin, and enteric-coated or buffered formulations do not appear to reduce the risk.68–70
The most important risk factor for upper GI bleeding in patients taking aspirin is a history of peptic ulcer bleeding. Approximately 15% of aspirin users who have bleeding from ulcers have recurrent bleeding within 1 year.71
As aspirin-induced GI bleeding becomes more common, health care providers often feel caught between the GI risk and the cardiovascular benefit. When considering whether to discontinue antiplatelet therapy, a cardiologist should be consulted along with a gastroenterologist to weigh the risks of GI bleeding vs thrombosis. To date, there have been no clinical trials published to suggest when antiplatelet therapy should be stopped to optimize GI and cardiovascular outcomes. An alternative is to replace aspirin with another antiplatelet drug that does not induce ulcers.
Clopidogrel
Clopidogrel (Plavix) is recommended for hospitalized patients with acute coronary syndrome who cannot tolerate the GI side effects of aspirin, according to the joint guidelines of the American College of Cardiology and the American Heart Association, with the highest level of evidence.72 This recommendation was largely based on the safety data from the CAPRIE (Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events) trial, in which the incidence of major GI bleeding was lower in the clopidogrel group (0.52%) than in the aspirin group (0.72%; P < .05).73
Aspirin plus a proton pump inhibitor
Patients who have had an episode of upper GI bleeding and who need long-term aspirin therapy should also receive a proton pump inhibitor indefinitely to prevent ulcer recurrence.
In a recent double-blind randomized controlled trial in patients with a history of aspirin-induced bleeding, the combination of low-dose aspirin plus esomeprazole (Nexium) twice a day was superior to clopidogrel by itself in terms of the rate of recurrent bleeding (0.7% vs 8.6%; P < .05).74 A similar trial showed nearly identical results: 0% upper GI bleeding in the group receiving aspirin plus esomeprazole 20 mg daily, vs 13.6% in the clopidogrel group (P = .0019).75 These studies suggest that a once-daily proton pump inhibitor combined with aspirin is a safer alternative than clopidogrel alone.
Clopidogrel plus a proton pump inhibitor
Interestingly, recent studies have shown that omeprazole decreases the antiplatelet effect of clopidogrel, possibly by inhibiting the CYP2C19 enzyme.76 However, concomitant use of pantoprazole (Protonix), lansoprazole (Prevacid), and esomeprazole did not have this effect, suggesting that although all proton pump inhibitors are metabolized to a varying degree by CYP2C19, the interaction between proton pump inhibitors and clopidogrel is not a class effect.77–79 Therefore, pantoprazole, lansoprazole, and esomeprazole may be the appropriate proton pump inhibitors to use with clopidogrel in patients who have a clear indication for the medication, consistent with current guideline recommendations.
Helicobacter pylori infection in antiplatelet drug users
TREATMENT AND PREVENTION OF NSAID-RELATED GI INJURY
About 1 in 20 users of NSAIDs develop GI complications and ulcers of varying degrees of severity, as do one in seven NSAID users over the age of 65. In fact, NSAID use accounts for 30% of hospitalizations for upper GI bleeding and deaths from this cause.82–85 In addition, approximately 15% to 30% of NSAID users have clinically silent but endoscopically evident peptic ulcers.86
NSAIDs contribute to ulcer development by depleting prostaglandins. Thus, misoprostol (Cytotec), a synthetic prostaglandin, has been used to reduce this side effect.
In a clinical trial, misoprostol reduced the incidence of NSAID-associated GI complications by 40%.87 Furthermore, it has been shown to be better than placebo in preventing recurrent gastric ulcers in patients with a history of gastric ulcer who were receiving low-dose aspirin.88
However, misoprostol is rarely used because it can cause diarrhea and abdominal cramping. Rather, the preferred drugs for preventing and treating NSAID- and aspirin-related GI lesions are proton pump inhibitors.
Numerous clinical trials using endoscopic end points showed that proton pump inhibitors in standard doses significantly reduce the incidence of ulcers associated with the use of NSAIDs.89 Proton pump inhibitor therapy has achieved a significant reduction in relative risk of upper GI bleeding in patients who received low-dose aspirin therapy, as confirmed by epidemiologic studies.90,91 The number of NSAID-related ulcers found on endoscopy could be reduced by an estimated 90% simply by using proton pump inhibitors.92
Upper gastrointestinal (GI) bleeding is common, costly, and potentially life-threatening. It must be managed promptly and appropriately to prevent adverse outcomes.
More people are admitted to the hospital for upper GI bleeding than for congestive heart failure or deep vein thrombosis. In the United States, the annual rate of hospitalization for upper GI bleeding is estimated to be 165 per 100,000—more than 300,000 hospitalizations per year, at a cost of $2.5 billion.1,2
Furthermore, despite advances in therapy, the case-fatality rate has remained unchanged at 7% to 10%.3 This may be because today’s patients are older and have more comorbidities than those in the past.4
CAUSES OF UPPER GI BLEEDING
Peptic ulcers account for about 60% of severe cases of upper GI bleeding,5 and they are the focus of this paper. Fortunately, up to 80% of bleeding ulcers stop bleeding spontaneously without any intervention.6
Gastroduodenal erosions account for about 12%.3
Varices due to cirrhosis are less common but more dangerous. Variceal bleeding accounts for a relatively small percentage (6%) of upper GI bleeding, but the mortality rate from a single episode of variceal bleeding is 30%, and 60% to 70% of patients die within 1 year, mostly of underlying liver disease.
Less frequent causes include Mallory-Weiss tears, erosive duodenitis, Dieulafoy ulcer (a type of vascular malformation), other vascular lesions, neoplasms, aortoenteric fistula, gastric antral vascular ectasia, and prolapse gastropathy.5
HEMATEMESIS AND MELENA
The most common presenting signs of acute upper GI bleeding are hematemesis (vomiting of blood), “coffee grounds” emesis, and melena (tarry black stools). About 30% of patients with bleeding ulcers present with hematemesis, 20% with melena, and 50% with both.7
Hematochezia (red blood in the stool) usually suggests a lower GI source of bleeding, since blood from an upper source turns black and tarry as it passes through the gut, producing melena. However, up to 5% of patients with bleeding ulcers have hematochezia,7 and it indicates heavy bleeding: bleeding of approximately 1,000 mL into the upper GI tract is needed to cause hematochezia, whereas only 50 to 100 mL is needed to cause melena.8,9 Hematochezia with signs and symptoms of hemodynamic compromise such as syncope, postural hypotension, tachycardia, and shock should therefore direct one’s attention to an upper GI source of bleeding.
Nonspecific features include nausea, vomiting, epigastric pain, vasovagal phenomena, and syncope.
WHAT IS THE PATIENT’S RISK?
An assessment of clinical severity is the first critical task, as it helps in planning treatment. Advanced age, multiple comorbidities, and hemodynamic instability call for aggressive treatment. Apart from this simple clinical rule, scoring systems have been developed.
The Rockall scoring system, the most widely used, gives estimates of the risks of recurrent bleeding and death. It is based on the three clinical factors mentioned above and on two endoscopic ones, awarding points for:
- Age—0 points if less than 60; 1 point if 60 to 79; or 2 points if 80 years or older
- Shock—1 point if the pulse is more than 100; 2 points if the systolic blood pressure is less than 100 mm Hg
- Comorbid illness—2 points for ischemic heart disease, congestive heart failure, or other major comorbidity; 3 points for renal failure, hepatic failure, or metastatic disease
- Endoscopic diagnosis—0 points if no lesion found or a Mallory-Weiss tear; 1 point for peptic ulcer, esophagitis, or erosive disease; 2 points for GI malignancy
- Endoscopic stigmata or recent hemorrhage—0 points for a clean-based ulcer or flat pigmented spot; 2 points for blood in the upper GI tract, active bleeding, a nonbleeding visible vessel, or adherent clot.
The Rockall score can thus range from 0 to 11 points, with an overall score of 0, 1, or 2 associated with an excellent prognosis.10
Other systems that are used less often include the Baylor severity scale and the Acute Physiology and Chronic Health Evaluation (APACHE) II score.
Does the patient have varices?
All variceal bleeding should be considered severe, since the 1-year death rate is so high (up to 70%). Clues pointing to variceal bleeding include previous variceal bleeding, thrombocytopenia, history of liver disease, and signs of liver disease on clinical examination.
All patients suspected of having bleeding varices should be admitted to the intensive care unit for close monitoring and should be given the highest priority, even if they are hemodynamically stable.
Is the patient hemodynamically stable?
Appropriate hemodynamic assessment includes monitoring of heart rate, blood pressure, and mental status. Tachycardia at rest, hypotension, and orthostatic changes in vital signs indicate a considerable loss of blood volume. Low urine output, dry mucous membranes, and sunken neck veins are also useful signs. (Tachycardia may be blunted if the patient is taking a beta-blocker.)
If these signs of hypovolemia are present, the initial management focuses on treating shock and on improving oxygen delivery to the vital organs. This involves repletion of the intravascular volume with intravenous infusions or blood transfusions. Supplemental oxygen also is useful, especially in elderly patients with heart disease.12
Inspection of nasogastric aspirate
In the initial assessment, it is useful to insert a nasogastric tube and inspect the aspirate. If it contains bright red blood, the patient needs an urgent endoscopic evaluation and an intensive level of care13,14; if it contains coffee-grounds material, the patient needs to be admitted to the hospital and to undergo endoscopic evaluation within 24 hours.
However, a normal aspirate does not rule out upper GI bleeding. Aljebreen et al15 found that 15% of patients with upper GI bleeding and normal nasogastric aspirate still had high-risk lesions (ie, visible bleeding or nonbleeding visible vessels) on endoscopy.
ACID-SUPPRESSION HELPS ULCERS HEAL
Acid and pepsin interfere with the healing of ulcers and other nonvariceal upper GI lesions. Further, an acidic environment promotes platelet disaggregation and fibrinolysis and impairs clot formation.16 This suggests that inhibiting gastric acid secretion and raising the gastric pH to 6 or higher may stabilize clots. Moreover, pepsinogen in the stomach is converted to its active form (pepsin) if the pH is less than 4. Therefore, keeping the pH above 4 keeps pepsinogen in an inactive form.
Histamine-2 receptor antagonists
Histamine-2 receptor antagonists were the first drugs to inhibit acid secretion, reversibly blocking histamine-2 receptors on the basolateral membrane of parietal cells. However, these drugs did not prove very useful in managing upper GI bleeding in clinical trials.17,18 In their intravenous form, they often fail to keep the gastric pH at 6 or higher, due to tachyphylaxis.19 The use of this class of drugs has declined in favor of proton pump inhibitors.
Proton pump inhibitors
Proton pump inhibitors reduce both basal and stimulated acid secretion by inhibiting hydrogen-potassium adenosine triphosphatase, the proton pump of the parietal cell.
Multiple studies have shown that proton pump inhibitors raise the gastric pH and keep it high. For example, an infusion of omeprazole (Prilosec) can keep the gastric pH above 6 for 72 hours without inducing tachyphylaxis.20,21
Started after endoscopy. Randomized controlled trials have found proton pump inhibitors to be effective when given in high doses intravenously for 72 hours after successful endoscopic treatment of bleeding ulcers with high-risk endoscopic signs, such as active bleeding or nonbleeding visible vessels.22,23
A meta-analysis indicated that these drugs decrease the incidence of recurrent peptic ulcer bleeding, the need for blood transfusions, the need for surgery, and the duration of hospitalization, but not the mortality rate.24,25 These studies also illustrate the benefit of following up endoscopic treatment to stop the bleeding with an intravenous infusion of a proton pump inhibitor.
The recommended dose of omeprazole for patients with high-risk findings on endoscopy is an 80-mg bolus followed by an 8-mg/hour infusion for 72 hours. After the patient’s condition stabilizes, oral therapy can be substituted for intravenous therapy. In patients with low-risk endoscopic findings (a clean-based ulcer or flat spot), oral proton pump inhibitors in high doses are recommended.
In either case, after the initial bleeding is treated endoscopically and hemostasis is achieved, a proton pump inhibitor is recommended for 6 to 8 weeks, or longer if the patient is also positive for Helicobacter pylori or is on daily treatment with aspirin or a nonsteroidal anti-inflammatory drug (NSAID) that is not selective for cyclo-oxygenase 2 (see below).
Started before endoscopy, these drugs reduced the frequency of actively bleeding ulcers, the duration of hospitalization, and the need for endoscopic therapy in a randomized controlled trial.26 A meta-analysis found that significantly fewer patients had signs of recent bleeding on endoscopy if they received a proton pump inhibitor 24 to 48 hours before the procedure, but it did not find any significant difference in important clinical outcomes such as death, recurrent bleeding, or surgery.27 Nevertheless, we believe that intravenous proton pump inhibitor therapy should be started before endoscopy in patients with upper GI bleeding.
Somatostatin analogues
Octreotide (Sandostatin), an analogue of the hormone somatostatin, decreases splanchnic blood flow, decreases secretion of gastric acid and pepsin, and stimulates mucus production. Although it is beneficial in treating upper GI bleeding due to varices, its benefit has not been confirmed in patients with nonvariceal upper GI bleeding.
A meta-analysis revealed that outcomes were better with high-dose intravenous proton pump inhibitor therapy than with octreotide when these drugs were started after endoscopic treatment of acute peptic ulcer bleeding.28 Nevertheless, octreotide may be useful in patients with uncontrolled nonvariceal bleeding who are awaiting endoscopy, since it is relatively safe to use.
ALL PATIENTS NEED ENDOSCOPY
All patients with upper GI bleeding need an upper endoscopic examination to diagnose and assess the risk posed by the bleeding lesion and to treat the lesion, reducing the risk of recurrent bleeding.
How urgently does endoscopy need to be done?
Endoscopy within the first 24 hours of upper GI bleeding is considered the standard of care. Patients with uncontrolled or recurrent bleeding should undergo endoscopy on an urgent basis to control the bleeding and reduce the risk of death.
However, how urgently endoscopy needs to be done is often debated. A multicenter randomized controlled trial compared outcomes in patients who underwent endoscopy within 6 hours of coming to the emergency department vs within 24 hours after the initial evaluation. The study found no significant difference in outcomes between the two groups; however, the group that underwent endoscopy sooner needed fewer transfusions.29
For a better view of the stomach
Gastric lavage improves the view of the gastric fundus but has not been proven to improve outcome.30
Promotility agents such as erythromycin and metoclopramide (Reglan) are also used to empty the stomach for better visualization.31–35 Erythromycin has been shown to improve visualization, shorten the procedure time, and prevent the need for additional endoscopy attempts in two randomized controlled studies.33,34 Furthermore, a cost-effectiveness study confirmed that giving intravenous erythromycin before endoscopy for acute upper GI bleeding saved money and resulted in an increase in quality-adjusted life-years.35
Endoscopy to diagnose bleeding and assess risk
Upper endoscopy is 90% to 95% diagnostic for acute upper GI bleeding.36
Patients at high risk (ie, older than 60 years, with severe comorbidity, or hemodynamically compromised) who have active bleeding (ie, witnessed hematemesis, red blood per nasogastric tube, or fresh blood per rectum) or a nonbleeding visible vessel should be admitted to a monitored bed or intensive care unit. Observation in a regular medical ward is appropriate for high-risk patients found to have an adherent clot. Patients with low-risk findings (eg, a clean ulcer base) are at low risk of recurrent bleeding and may be considered for early hospital discharge with appropriate outpatient follow-up.
Endoscopy to treat bleeding
About 25% of endoscopic procedures performed for upper GI bleeding include some type of treatment,39 such as injections of epinephrine, normal saline, or sclerosants; thermal cautery; argon plasma coagulation; electrocautery; or application of clips or bands. They are all equally effective, and combinations of these therapies are more effective than when they are used individually. A recent meta-analysis found dual therapy to be superior to epinephrine monotherapy in preventing recurrent bleeding, need for surgery, and death.40
How to manage adherent clots is controversial, but recent studies have revealed a significant benefit from removing them and treating the underlying lesions compared with drug therapy alone.43,45
Flat, pigmented spots and nonbleeding ulcers with a clean base do not require endoscopic treatment because the risk of recurrent bleeding is low.
Endoscopic therapy stops the bleeding in more than 90% of patients, but bleeding recurs after endoscopic therapy in 10% to 25%.46 Reversal of any severe coagulopathy with transfusions of platelets or fresh frozen plasma is essential for endoscopic hemostasis. However, coagulopathy at the time of initial bleeding and endoscopy does not appear to be associated with higher rates of recurrent bleeding following endoscopic therapy for nonvariceal upper GI bleeding.47
Patients with refractory bleeding are candidates for angiography or surgery. However, even when endoscopic hemostasis fails, endoscopy is important before angiography or surgery to pinpoint the site of bleeding and diagnose the cause.
A second endoscopic procedure is generally not recommended within 24 hours after the initial procedure.48 However, it is appropriate in cases in which clinical signs indicate recurrent bleeding or if hemostasis during the initial procedure is questionable. A meta-analysis found that routinely repeating endoscopy reduces the rate of recurrent bleeding but not the need for surgery or the risk of death.49
ALL PATIENTS SHOULD BE ADMITTED
VARICEAL BLEEDING
Variceal bleeding, a severe outcome of portal hypertension secondary to cirrhosis, carries a 6-week mortality rate of 10% to 20%.50 In view of the risk, primary prevention is indicated in patients with high-risk varices.
The mainstays of primary and secondary prevention are the nonselective beta-blockers such as nadolol (Corgard) and propranolol (Inderal). Several randomized controlled trials have shown lower rates of recurrent bleeding and death with propranolol or nadolol than with placebo.51 In doses that decrease the heart rate by 25%, beta-blockers have been shown to delay and decrease variceal hemorrhage. However, most patients require prophylactic endoscopic variceal ligation because they cannot tolerate beta-blocker therapy.
In suspected acute variceal bleeding, a somatostatin analogue should be started to decrease the portal pressure, and antibiotics should be started to reduce the risks of infection and death. Vasoactive drugs, ie, somatostatin analogues, should be started before endoscopy and continued for 5 days to reduce the chances of recurrent bleeding.52,53
Terlipressin is the only drug proven to improve the odds of survival in acute variceal bleeding. Although widely used in Europe, it has not been approved for use in the United States.
Octreotide, another option, improves hemostasis to the same extent, although it does not increase the survival rate.54,55 The recommended dose of octreotide for patients with variceal bleeding is a 50-μg intravenous bolus, followed by a 50-μg/hour infusion for 5 days.
Combining endoscopic and drug therapy improves the chances of stopping the bleeding and reduces the risk of recurrent bleeding compared with endoscopic therapy alone.56
Transjugular intrahepatic portosystemic shunting is indicated in recurrent variceal hemorrhage or in those with initial bleeding that is refractory to standard medical and endoscopic therapy. It is not the primary therapy because it doubles the risk of encephalopathy and has a high stent occlusion rate (up to 60%, lower with covered stents).
GI BLEEDING CAN CAUSE ACUTE MYOCARDIAL INFARCTION
The simultaneous presentation of acute myocardial infarction (MI) and GI hemorrhage is very serious and unfortunately common.
An acute MI occurring simultaneously with or after GI bleeding is usually precipitated by massive bleeding causing hypovolemia, hemodynamic compromise, and hypoperfusion. Conversely, the anticoagulant, antiplatelet, or thrombolytic drugs given to treat MI can precipitate GI bleeding (see below).
This distinction is important because the two scenarios have different clinical courses and prognoses. GI bleeding that precipitates an acute MI tends to be massive, whereas GI bleeding after treatment of acute MI tends to be self-limited and often resolves with reversal of underlying coagulopathy.57
Endoscopy carries a higher than average risk in patients with recent acute MI, with all-cause mortality rates as high as 1%.58 (The usual rate is 0.0004%.59) Nevertheless, endoscopy can be safely performed early on in patients with acute MI if it is done under strict monitoring in a coronary care unit.
Several studies have shown that MI patients who present with upper GI bleeding as the inciting event or patients with acute MI who are vomiting blood or who are hemodynamically unstable due to GI bleeding are significantly more likely to have a high-risk lesion and so have the greatest need for endoscopic therapy. Therefore, endoscopic intervention may be offered to MI patients at high risk who have been started on antiplatelet agents.
WARFARIN CAN PRECIPITATE BLEEDING
Acute upper GI bleeding can be a severe complication of long-term oral anticoagulation, not because the drugs cause ulcers, but rather because they exacerbate ulcers that are already present.60 Therefore, when starting warfarin (Coumadin), patients should be evaluated to determine if they have other risk factors for GI bleeding, such as ulcers.
The number of people presenting with upper GI bleeding while on warfarin therapy is increasing because of the expanding indications for long-term anticoagulation therapy, such as atrial fibrillation and deep venous thrombosis.
The risk of GI bleeding in patients who use oral anticoagulants is estimated to be 2.3 to 4.9 times higher than in nonusers.61
The goal international normalized ratio (INR) for patients on warfarin therapy is usually 2.0 to 3.0. Recent studies found that endoscopy can be safely performed in patients with acute GI bleeding whose INR is between 2.0 and 3.0.62,63 Some suggest that both the length of warfarin therapy and the INR affect the risk of bleeding.64,65
Managing patients with an INR higher than 3.0 who have an episode of GI bleeding is always a challenge. It is not uncommon to find pathologic lesions causing GI bleeding in patients who are on warfarin with a supratherapeutic INR, and thus, endoscopy is indicated. However, before endoscopy, reversal of anticoagulation should be considered.
BLEEDING IN PATIENTS ON ANTIPLATELET DRUGS
Aspirin
Aspirin decreases production of prostaglandins in the GI tract, thereby decreasing the protective and restorative properties of the gastric and duodenal mucosa and predisposing to ulcers and bleeding.
The higher the aspirin dose, the higher the risk. Aspirin doubles the risk of upper GI bleeding at daily doses of 75 mg and quadruples it at doses of 300 mg.66 Even doses as low as 10 mg can decrease gastric mucosal prostaglandin production.67 Thus, it appears that there is no risk-free dose of aspirin, and enteric-coated or buffered formulations do not appear to reduce the risk.68–70
The most important risk factor for upper GI bleeding in patients taking aspirin is a history of peptic ulcer bleeding. Approximately 15% of aspirin users who have bleeding from ulcers have recurrent bleeding within 1 year.71
As aspirin-induced GI bleeding becomes more common, health care providers often feel caught between the GI risk and the cardiovascular benefit. When considering whether to discontinue antiplatelet therapy, a cardiologist should be consulted along with a gastroenterologist to weigh the risks of GI bleeding vs thrombosis. To date, there have been no clinical trials published to suggest when antiplatelet therapy should be stopped to optimize GI and cardiovascular outcomes. An alternative is to replace aspirin with another antiplatelet drug that does not induce ulcers.
Clopidogrel
Clopidogrel (Plavix) is recommended for hospitalized patients with acute coronary syndrome who cannot tolerate the GI side effects of aspirin, according to the joint guidelines of the American College of Cardiology and the American Heart Association, with the highest level of evidence.72 This recommendation was largely based on the safety data from the CAPRIE (Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events) trial, in which the incidence of major GI bleeding was lower in the clopidogrel group (0.52%) than in the aspirin group (0.72%; P < .05).73
Aspirin plus a proton pump inhibitor
Patients who have had an episode of upper GI bleeding and who need long-term aspirin therapy should also receive a proton pump inhibitor indefinitely to prevent ulcer recurrence.
In a recent double-blind randomized controlled trial in patients with a history of aspirin-induced bleeding, the combination of low-dose aspirin plus esomeprazole (Nexium) twice a day was superior to clopidogrel by itself in terms of the rate of recurrent bleeding (0.7% vs 8.6%; P < .05).74 A similar trial showed nearly identical results: 0% upper GI bleeding in the group receiving aspirin plus esomeprazole 20 mg daily, vs 13.6% in the clopidogrel group (P = .0019).75 These studies suggest that a once-daily proton pump inhibitor combined with aspirin is a safer alternative than clopidogrel alone.
Clopidogrel plus a proton pump inhibitor
Interestingly, recent studies have shown that omeprazole decreases the antiplatelet effect of clopidogrel, possibly by inhibiting the CYP2C19 enzyme.76 However, concomitant use of pantoprazole (Protonix), lansoprazole (Prevacid), and esomeprazole did not have this effect, suggesting that although all proton pump inhibitors are metabolized to a varying degree by CYP2C19, the interaction between proton pump inhibitors and clopidogrel is not a class effect.77–79 Therefore, pantoprazole, lansoprazole, and esomeprazole may be the appropriate proton pump inhibitors to use with clopidogrel in patients who have a clear indication for the medication, consistent with current guideline recommendations.
Helicobacter pylori infection in antiplatelet drug users
TREATMENT AND PREVENTION OF NSAID-RELATED GI INJURY
About 1 in 20 users of NSAIDs develop GI complications and ulcers of varying degrees of severity, as do one in seven NSAID users over the age of 65. In fact, NSAID use accounts for 30% of hospitalizations for upper GI bleeding and deaths from this cause.82–85 In addition, approximately 15% to 30% of NSAID users have clinically silent but endoscopically evident peptic ulcers.86
NSAIDs contribute to ulcer development by depleting prostaglandins. Thus, misoprostol (Cytotec), a synthetic prostaglandin, has been used to reduce this side effect.
In a clinical trial, misoprostol reduced the incidence of NSAID-associated GI complications by 40%.87 Furthermore, it has been shown to be better than placebo in preventing recurrent gastric ulcers in patients with a history of gastric ulcer who were receiving low-dose aspirin.88
However, misoprostol is rarely used because it can cause diarrhea and abdominal cramping. Rather, the preferred drugs for preventing and treating NSAID- and aspirin-related GI lesions are proton pump inhibitors.
Numerous clinical trials using endoscopic end points showed that proton pump inhibitors in standard doses significantly reduce the incidence of ulcers associated with the use of NSAIDs.89 Proton pump inhibitor therapy has achieved a significant reduction in relative risk of upper GI bleeding in patients who received low-dose aspirin therapy, as confirmed by epidemiologic studies.90,91 The number of NSAID-related ulcers found on endoscopy could be reduced by an estimated 90% simply by using proton pump inhibitors.92
- Lewis JD, Bilker WB, Brensinger C, Farrar JT, Strom BL. Hospitalization and mortality rates from peptic ulcer disease and GI bleeding in the 1990s: relationship to sales of non-steroidal anti-inflammatory drugs and acid suppression medications. Am J Gastroenterol 2002; 97:2540–2549.
- Viviane A, Alan BN. Estimates of costs of hospital stays for variceal and nonvariceal upper gastrointestinal bleeding in the United States. Value Health 2008; 11:1–3.
- Yavorski RT, Wong RK, Maydonovitch C, Battin LS, Furnia A, Amundson DE. Analysis of 3,294 cases of upper gastrointestinal bleeding in military medical facilities. Am J Gastroenterol 1995; 90:568–573.
- Kaplan RC, Heckbert SR, Koepsell TD, et al. Risk factors for hospitalized gastrointestinal bleeding among older persons. Cardiovascular Health Study Investigators. J Am Geriatr Soc 2001; 49:126–133.
- Longstreth GF. Epidemiology of hospitalization for acute upper gastrointestinal hemorrhage: a population-based study. Am J Gastroenterol 1995; 90:206–210.
- Laine L, Peterson WL. Bleeding peptic ulcer. N Engl J Med 1994; 331:717–727.
- Wara P, Stodkilde H. Bleeding pattern before admission as guideline for emergency endoscopy. Scand J Gastroenterol 1985; 20:72–78.
- Jensen DM, Machicado GA. Diagnosis and treatment of severe hematochezia. The role of urgent colonoscopy after purge. Gastroenterology 1988; 95:1569–1574.
- Daniel WA, Egan S. The quantity of blood required to produce a tarry stool. J Am Med Assoc 1939; 113:2232.
- Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal hemorrhage. Gut 1996; 38:316–321.
- Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper-gastrointestinal hemorrhage. Lancet 2000; 356:1318–1321.
- Gralnek IM, Barkun AN, Bardou M. Management of acute bleeding from a peptic ulcer. N Engl J Med 2008; 359:928–937.
- Silverstein FE, Gilbert DA, Tedesco FJ, Buenger NK, Persing J. The national ASGE survey on upper gastrointestinal bleeding II. Clinical prognostic factors. Gastrointest Endosc 1981; 27:80–93.
- Corley DA, Stefan AM, Wolf M, Cook EF, Lee TH. Early indicators of prognosis in upper gastrointestinal hemorrhage. Am J Gastroenterol 1998; 93:336–340.
- Aljebreen AM, Fallone CA, Barkun AN. Nasogastric aspirate predicts high-risk endoscopic lesions in patients with acute upper-GI bleeding. Gastrointest Endosc 2004; 59:172–178.
- Barkun AN, Cockeram AW, Plourde V, Fedorak RN. Review article: acid suppression in non-variceal acute upper gastrointestinal bleeding. Aliment Pharmacol Ther 1999; 13:1565–1584.
- Levine JE, Leontiadis JI, Sharma VK, Howden CW. Meta-analysis: the efficacy of intravenous H2-receptor antagonists in bleeding peptic ulcer. Aliment Pharmacol Ther 2002; 16:1137–1142.
- Walt RP, Cottrell J, Mann SG, Freemantle NP, Langman MJ. Continuous intravenous famotidine for hemorrhage from peptic ulcer. Lancet 1992; 340:1058–1062.
- Labenz J, Peitz U, Leusing C, Tillenburg B, Blum AL, Börsch G. Efficacy of primed infusion with high dose ranitidine and omeprazole to maintain high intragastric pH in patients with peptic ulcer bleeding: a prospective randomized controlled study. Gut 1997; 40:36–41.
- Merki HS, Wilder-Smith CH. Do continuous infusions of omeprazole and ranitidine retain their effect with prolonged dosing? Gastroenterology 1994; 106:60–64.
- Netzer P, Gaia C, Sandoz M, et al. Effect of repeated injection and continuous infusion of omeprazole and ranitidine on intragastric pH over 72 hours. Am J Gastroenterol 1999; 94:351–357.
- Lin HJ, Lo WC, Cheng YC, Perng CL. Role of intravenous omeprazole in patients with high-risk peptic ulcer bleeding after successful endoscopic epinephrine injection: a prospective randomized comparative trial. Am J Gastroenterol 2006; 101:500–505.
- Lau JY, Sung JJ, Lee KK, et al. Effects of intravenous omeprazole on recurrent bleeding after endoscopic treatment of bleeding peptic ulcers. N Engl J Med 2000; 343:310–316.
- Leontiadis GI, Sharma VK, Howden CW. Proton pump inhibitor treatment for acute peptic ulcer bleeding. Cochrane Database Syst Rev 2006;CD002094.
- Andriulli A, Annese V, Caruso N, et al. Proton-pump inhibitors and outcome of endoscopic hemostasis in bleeding peptic ulcers: a series of meta-analyses. Am J Gastroenterol 2005; 100:207–219.
- Lau JY, Leung WK, Wu JC, et al. Omeprazole before endoscopy in patients with gastrointestinal bleeding. N Engl J Med 2007; 356:1631–1640.
- Dorward S, Sreedharan A, Leontiadis GI, Howden CW, Moayyedi P, Forman D. Proton pump inhibitor treatment initiated prior to endoscopic diagnosis in upper gastrointestinal bleeding. Cochrane Database Syst Rev 2006;CD005415.
- Bardou M, Toubouti Y, Benhaberou-Brun D, Rahme E, Barkun AN. Meta-analysis: proton-pump inhibition in high-risk patients with acute peptic ulcer bleeding. Aliment Pharmacol Ther 2005; 21:677–686.
- Bjorkman DJ, Zaman A, Fennerty MB, Lieberman D, Disario JA, Guest-Warnick G. Urgent vs elective endoscopy for acute non-variceal upper-GI bleeding: an effectiveness study. Gastointest Endosc 2004; 60:1–8.
- Lee SD, Kearney DJ. A randomized controlled trial of gastric lavage prior to endoscopy for acute upper gastrointestinal bleeding. J Clin Gastroenterol 2004; 38:861–865.
- Tack J, Janssens J, Vantrappen G, et al. Effect of erythromycin on gastric motility in controls and in diabetic gastroparesis. Gastroenterology 1992; 103:72–79.
- Xynos E, Mantides A, Papageorgiou A, Fountos A, Pechlivanides G, Vassilakis JS. Erythromycin accelerates delayed gastric emptying of solids in patients after truncal vagotomy and pyloroplasty. Eur J Surg 1992; 158:407–411.
- Coffin B, Pocard M, Panis Y, et al; Groupe des endoscopistes de garde á l’AP-HP. Erythromycin improves the quality of EGD in patients with acute upper GI bleeding: a randomized controlled study. Gastrointest Endosc 2002; 56:174–179.
- Frossard JL, Spahr L, Queneau PE, et al. Erythromycin intravenous bolus infusion in acute upper gastrointestinal bleeding: a randomized, controlled, double-blind trial. Gastroenterology 2002; 123:17–23.
- Winstead NS, Wilcox CM. Erythromycin prior to endoscopy for acute upper gastrointestinal hemorrhage: a cost-effectiveness analysis. Aliment Pharmacol Ther 2007; 26:1371–1377.
- Chak A, Cooper GS, Lloyd LE, Kolz CS, Barnhart BA, Wong RC. Effectiveness of endoscopy in patients admitted to the intensive care unit with upper GI hemorrhage. Gastrointest Endosc 2001; 53:6–13.
- Lau JY, Chung SC, Leung JW, Lo KK, Yung MY, Li AK. The evolution of stigmata of hemorrhage in bleeding peptic ulcers: a sequential endoscopic study. Endoscopy 1998; 30:513–518.
- Chung IK, Kim EJ, Lee MS, et al. Endoscopic factors predisposing to rebleeding following endoscopic hemostasis in bleeding peptic ulcers. Endoscopy 2001; 33:969–975.
- Elta GH. Acute nonvariceal upper gastrointestinal hemorrhage. Curr Treat Options Gastroenterol 2002; 5:147–152.
- Marmo R, Rotondano G, Piscopo R, Bianco MA, D’Angella R, Cipolletta L. Dual therapy versus monotherapy in the endoscopic treatment of high-risk bleeding ulcers: a meta-analysis of controlled trials. Am J Gastroenterol 2007; 102:279–289.
- Kovacs TO, Jensen DM. Recent advances in the endoscopic diagnosis and therapy of upper gastrointestinal, small intestinal, and colonic bleeding. Med Clin North Am 2002; 86:1319–1356.
- Kovacs TO, Jensen DM. Endoscopic treatment of ulcer bleeding. Curr Treat Options Gastroenterol 2007; 10:143–148.
- Jensen DM, Kovacs TO, Jutabha R, et al. Randomized trial of medical or endoscopic therapy to prevent recurrent ulcer hemorrhage in patients with adherent clots. Gastroenterology 2002; 123:407–413.
- Jensen DM, Machicado GA. Endoscopic hemostasis of ulcer hemorrhage with injection, thermal, and combination methods. Techniques Gastrointest Endosc 2005; 7:124–131.
- Bleau BL, Gostout CJ, Sherman KE, et al. Recurrent bleeding from peptic ulcer associated with adherent clot: a randomized study comparing endoscopic treatment with medical therapy. Gastrointest Endosc 2002; 56:1–6.
- Lau JY, Sung JJ, Lam YH, et al. Endoscopic retreatment compared with surgery in patients with recurrent bleeding after initial endoscopic control of bleeding ulcers. N Engl J Med 1999; 340:751–756.
- Wolf AT, Wasan SK, Saltzman JR. Impact of anticoagulation on rebleeding following endoscopic therapy for nonvariceal upper gastrointestinal hemorrhage. Am J Gastroenterol 2007; 102:290–296.
- Barkun A, Bardou M, Marshall JK; Nonvariceal Upper GI Bleeding Consensus Conference Group. Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med 2003; 139:843–857.
- Marmo R, Rotondano G, Bianco MA, Piscopo R, Prisco A, Cipolletta L. Outcome of endoscopic treatment for peptic ulcer bleeding: is a second look necessary? A meta-analysis. Gastrointest Endosc 2003; 57:62–67.
- Dell’Era A, deFrancis R, Iannuzzi F. Acute variceal bleeding: pharmacological treatment and primary/secondary prophylaxis. Best Pract Res Clin Gastroenterol 2008; 22:279–294.
- Jalan R, Hayes PC. UK guidelines on the management of variceal hemorrhage in cirrhotic patients. British Society of Gastroenterology. Gut 2000; 46( suppl 3–4):III1–III15.
- Bernard B, Lebrec D, Mathurin P, Opolon P, Poynard T. Beta-adrenergic antagonists in the prevention of gastrointestinal rebleeding in patients with cirrhosis: a meta-analysis. Hepatology 1997; 25:63–70.
- De Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2005; 43:167–176.
- Levacher S, Letoumelin P, Pateron D, Blaise M, Lapandry C, Pourriat JL. Early administration of terlipressin plus glyceryl trinitrate to control active upper gastrointestinal bleeding in cirrhotic patients. Lancet 1995; 346:865–868.
- Abraldes JG, Bosch J. Somatostatin and analogues in portal hypertension. Hepatology 2002; 35:1305–1312.
- Banares R, Albillos A, Rincon D, et al. Endoscopic treatment versus endoscopic plus pharmacological treatment for acute variceal bleeding: a meta analysis. Hepatology 2002; 35:609–615.
- Cappell M. Gastrointenstinal bleeding associated with myocardial infarction. Gastroenterol Clin North Am 2000; 29:423–444.
- Lin S, Konstance R, Jollis J, Fisher DA. The utility of upper endoscopy in patients with concomitant upper gastrointestinal bleeding and acute myocardial infarction. Dig Dis Sci 2006; 51:2377–2383.
- Silvis SE, Nebel O, Rogers G, Sugawa C, Mandelstam P. Endoscopic complications. Results of the 1974 American Society for Gastrointestinal Endoscopy Survey. JAMA 1976; 235:928–930.
- Lanas A, Garcia-Rodriguez LA, Arroyo MT, et al; Investigators of the Asociación Española de Gastroenterología (AEG). Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol 2007; 102:507–515.
- Shorr RI, Ray WA, Daugherty JR, Griffin MR. Concurrent use of nonsteroidal anti-inflammatory drugs and oral anticoagulants places elderly persons at high risk for hemorrhagic peptic ulcer disease. Arch Intern Med 1993; 153:1665–1670.
- Tabibian N. Acute gastrointestinal bleeding in anticoagulated patients: a prospective evaluation. Am J Gastroenterol 1989; 84:10–12.
- Choudari CP, Rajgopal C, Palmer KR. Acute gastrointestinal hemorrhage in anticoagulated patients: diagnoses and response to endoscopic treatment. Gut 1994; 35:464–466.
- Petty GW, Brown RD, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Frequency of major complications of aspirin, warfarin, and intravenous heparin for secondary stroke prevention: a population-based study. Ann Intern Med 1999; 130:14–22.
- Landefeld CS, Rosenblatt MW, Goldman L. Bleeding in outpatients treated with warfarin: relation to the prothrombin time and important remediable lesions. Am J Med 1989; 87:153–159.
- Weil J, Colin-Jones D, Langman M, et al. Prophylactic aspirin and risk of peptic ulcer bleeding. BMJ 1995; 310:827–830.
- Cryer B, Feldman M. Effects of very low dose daily, long-term aspirin therapy on gastric, duodenal, and rectal prostaglandin levels and on mucosal injury in healthy humans. Gastroenterology 1999; 117:17–25.
- De Abajo FJ, Garcia Rodriguez LA. Risk of upper gastrointestinal bleeding and perforation associated with low-dose aspirin as plain and enteric-coated formulations. BMC Clin Pharmacol 2001; 1:1.
- Kelly JP, Kaufman DW, Jurgelon JM, Sheehan J, Koff RS, Shapiro S. Risk of aspirin-associated major upper gastrointestinal bleeding with enteric coated or buffered product. Lancet 1996; 348:1413–1416.
- Garcia Rodriguez LA, Hernandez-Diaz S, De Abajo FJ. Association between aspirin and upper gastrointestinal complications: systematic review of epidemiological studies. Br J Clin Pharmacol 2001; 52:563–571.
- Wilcox CM, Ladabaum U. A patient with high risk of gastrointestinal bleeding requiring nonsteroidal anti-inflammatory drugs. Clin Gastroenterol Hepatol 2006; 4:1090–1093.
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction. J Am Coll Cardiol 2007; 50:e1–e157.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996; 348:1329–1339.
- Chan FK, Ching JY, Hung LC, et al. Clopidogrel versus aspirin and esomeprazole to prevent recurrent ulcer bleeding. N Engl J Med 2005; 352:238–244.
- Lai KC, Chu KM, Hui WM, et al. Esomeprazole with aspirin versus clopidogrel for prevention of recurrent gastrointestinal ulcer complications. Clin Gastroenterol Hepatol 2006; 4:860–865.
- Ho MP, Maddox TM, Wang L, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA 2009; 301:937–944.
- Siller-Matula JM, Spiel AO, Lang IM, Kreiner G, Christ G, Jilma B. Effects of pantoprazole and esomeprazole on platelet inhibition by clopidogrel. Am Heart J 2009; 157:148.e1–e5.
- Small DS, Farid NA, Payne CD, et al. Effects of proton pump inhibitor lansoprazole on the pharmacokinetics and pharmacodynamics of prasugel and clopidogrel. J Clin Pharmacol 2008; 48:475–484.
- Ishizaki T, Horai Y. Review article: cytochrome P450 and the metabolism of proton pump inhibitors—emphasis on rabeprazole. Aliment Pharacol Ther 1999; 13 (suppl 3):27–36.
- Lanas A, Fuentes J, Benito R, Serrano P, Bajador E, Sainz R. Helicobacter pylori increases the risk of upper gastrointestinal bleeding in patients taking low-dose aspirin. Aliment Pharmacol Ther 2002; 16:779–786.
- Chan FK. NSAID-Induced peptic ulcers and Helicobacter pylori infection: implications for patient management. Drug Saf 2005; 28:287–300.
- Bombardier V, Laine L, Reicin A, et al; VIGOR Study Group. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis: VIGOR Study Group. N Eng J Med 2000; 343:1520–1528.
- Griffin MR, Ray WA, Schaffner W. Nonsteroidal anti-inflammatory drug use and death from peptic ulcer in elderly persons. Ann Intern Med 1988; 109:359–363.
- Griffin MR, Piper JM, Daugherty JR, Snowden M, Ray WA. Nonsteroidal anti-inflammatory drug use and increased risk for peptic ulcer disease in elderly persons. Ann Intern Med 1991; 114:257–263.
- Smalley WE, Ray WA, Daugherty JR, Griffin MR. Nonsteroidal anti-inflammatory drugs and the incidence of hospitalizations for peptic ulcer disease in elderly persons. Am J Epidemiol 1995; 141:539–545.
- Laine L. Approaches to nonsteroidal anti-inflammatory drug use in the high-risk patient. Gastroenterology 2001; 120:594–606.
- Silverstein FE, Graham DY, Senior JR, et al. Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal anti-inflammatory drugs. A randomized, double blind, placebo controlled trial. Ann Intern Med 1995; 123:241–249.
- Goldstein JL, Huang B, Amer F, Christopoulos NG. Ulcer recurrence in high-risk patients receiving nonsteroidal anti-inflammatory drugs plus low dose aspirin: results of a post hoc subanalysis. Clin Ther 2004; 26:1637–1643.
- Berger JS, Stebbins A, Granger CB, et al. Initial aspirin dose and outcome among ST-elevation myocardial infarction patients treated with fibrinolytic therapy. Circulation 2008; 117:192–199.
- Lanas A, Garcia-Rodriguez LA, Arroyo MT, et al; Investigators of the Asociación Española de Gastroenterología (AEG). Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol 2007; 102:507–515.
- Chin MW, Yong G, Bulsara MK, Rankin J, Forbes GM. Predictive and protective factors associated with upper gastrointestinal bleeding after percutaneous coronary intervention: a case-control study. Am J Gastroenterol 2007; 102:2411–2416.
- Hunt RH, Bazzoli F. Should NSAID/low dose aspirin takers be tested routinely for H. Pylori infection and treated if positive? Implications for primary risk of ulcer and ulcer relapse after initial healing. Aliment Pharmacol Ther 2004; 19 (suppl 1):9–16.
- Lewis JD, Bilker WB, Brensinger C, Farrar JT, Strom BL. Hospitalization and mortality rates from peptic ulcer disease and GI bleeding in the 1990s: relationship to sales of non-steroidal anti-inflammatory drugs and acid suppression medications. Am J Gastroenterol 2002; 97:2540–2549.
- Viviane A, Alan BN. Estimates of costs of hospital stays for variceal and nonvariceal upper gastrointestinal bleeding in the United States. Value Health 2008; 11:1–3.
- Yavorski RT, Wong RK, Maydonovitch C, Battin LS, Furnia A, Amundson DE. Analysis of 3,294 cases of upper gastrointestinal bleeding in military medical facilities. Am J Gastroenterol 1995; 90:568–573.
- Kaplan RC, Heckbert SR, Koepsell TD, et al. Risk factors for hospitalized gastrointestinal bleeding among older persons. Cardiovascular Health Study Investigators. J Am Geriatr Soc 2001; 49:126–133.
- Longstreth GF. Epidemiology of hospitalization for acute upper gastrointestinal hemorrhage: a population-based study. Am J Gastroenterol 1995; 90:206–210.
- Laine L, Peterson WL. Bleeding peptic ulcer. N Engl J Med 1994; 331:717–727.
- Wara P, Stodkilde H. Bleeding pattern before admission as guideline for emergency endoscopy. Scand J Gastroenterol 1985; 20:72–78.
- Jensen DM, Machicado GA. Diagnosis and treatment of severe hematochezia. The role of urgent colonoscopy after purge. Gastroenterology 1988; 95:1569–1574.
- Daniel WA, Egan S. The quantity of blood required to produce a tarry stool. J Am Med Assoc 1939; 113:2232.
- Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal hemorrhage. Gut 1996; 38:316–321.
- Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper-gastrointestinal hemorrhage. Lancet 2000; 356:1318–1321.
- Gralnek IM, Barkun AN, Bardou M. Management of acute bleeding from a peptic ulcer. N Engl J Med 2008; 359:928–937.
- Silverstein FE, Gilbert DA, Tedesco FJ, Buenger NK, Persing J. The national ASGE survey on upper gastrointestinal bleeding II. Clinical prognostic factors. Gastrointest Endosc 1981; 27:80–93.
- Corley DA, Stefan AM, Wolf M, Cook EF, Lee TH. Early indicators of prognosis in upper gastrointestinal hemorrhage. Am J Gastroenterol 1998; 93:336–340.
- Aljebreen AM, Fallone CA, Barkun AN. Nasogastric aspirate predicts high-risk endoscopic lesions in patients with acute upper-GI bleeding. Gastrointest Endosc 2004; 59:172–178.
- Barkun AN, Cockeram AW, Plourde V, Fedorak RN. Review article: acid suppression in non-variceal acute upper gastrointestinal bleeding. Aliment Pharmacol Ther 1999; 13:1565–1584.
- Levine JE, Leontiadis JI, Sharma VK, Howden CW. Meta-analysis: the efficacy of intravenous H2-receptor antagonists in bleeding peptic ulcer. Aliment Pharmacol Ther 2002; 16:1137–1142.
- Walt RP, Cottrell J, Mann SG, Freemantle NP, Langman MJ. Continuous intravenous famotidine for hemorrhage from peptic ulcer. Lancet 1992; 340:1058–1062.
- Labenz J, Peitz U, Leusing C, Tillenburg B, Blum AL, Börsch G. Efficacy of primed infusion with high dose ranitidine and omeprazole to maintain high intragastric pH in patients with peptic ulcer bleeding: a prospective randomized controlled study. Gut 1997; 40:36–41.
- Merki HS, Wilder-Smith CH. Do continuous infusions of omeprazole and ranitidine retain their effect with prolonged dosing? Gastroenterology 1994; 106:60–64.
- Netzer P, Gaia C, Sandoz M, et al. Effect of repeated injection and continuous infusion of omeprazole and ranitidine on intragastric pH over 72 hours. Am J Gastroenterol 1999; 94:351–357.
- Lin HJ, Lo WC, Cheng YC, Perng CL. Role of intravenous omeprazole in patients with high-risk peptic ulcer bleeding after successful endoscopic epinephrine injection: a prospective randomized comparative trial. Am J Gastroenterol 2006; 101:500–505.
- Lau JY, Sung JJ, Lee KK, et al. Effects of intravenous omeprazole on recurrent bleeding after endoscopic treatment of bleeding peptic ulcers. N Engl J Med 2000; 343:310–316.
- Leontiadis GI, Sharma VK, Howden CW. Proton pump inhibitor treatment for acute peptic ulcer bleeding. Cochrane Database Syst Rev 2006;CD002094.
- Andriulli A, Annese V, Caruso N, et al. Proton-pump inhibitors and outcome of endoscopic hemostasis in bleeding peptic ulcers: a series of meta-analyses. Am J Gastroenterol 2005; 100:207–219.
- Lau JY, Leung WK, Wu JC, et al. Omeprazole before endoscopy in patients with gastrointestinal bleeding. N Engl J Med 2007; 356:1631–1640.
- Dorward S, Sreedharan A, Leontiadis GI, Howden CW, Moayyedi P, Forman D. Proton pump inhibitor treatment initiated prior to endoscopic diagnosis in upper gastrointestinal bleeding. Cochrane Database Syst Rev 2006;CD005415.
- Bardou M, Toubouti Y, Benhaberou-Brun D, Rahme E, Barkun AN. Meta-analysis: proton-pump inhibition in high-risk patients with acute peptic ulcer bleeding. Aliment Pharmacol Ther 2005; 21:677–686.
- Bjorkman DJ, Zaman A, Fennerty MB, Lieberman D, Disario JA, Guest-Warnick G. Urgent vs elective endoscopy for acute non-variceal upper-GI bleeding: an effectiveness study. Gastointest Endosc 2004; 60:1–8.
- Lee SD, Kearney DJ. A randomized controlled trial of gastric lavage prior to endoscopy for acute upper gastrointestinal bleeding. J Clin Gastroenterol 2004; 38:861–865.
- Tack J, Janssens J, Vantrappen G, et al. Effect of erythromycin on gastric motility in controls and in diabetic gastroparesis. Gastroenterology 1992; 103:72–79.
- Xynos E, Mantides A, Papageorgiou A, Fountos A, Pechlivanides G, Vassilakis JS. Erythromycin accelerates delayed gastric emptying of solids in patients after truncal vagotomy and pyloroplasty. Eur J Surg 1992; 158:407–411.
- Coffin B, Pocard M, Panis Y, et al; Groupe des endoscopistes de garde á l’AP-HP. Erythromycin improves the quality of EGD in patients with acute upper GI bleeding: a randomized controlled study. Gastrointest Endosc 2002; 56:174–179.
- Frossard JL, Spahr L, Queneau PE, et al. Erythromycin intravenous bolus infusion in acute upper gastrointestinal bleeding: a randomized, controlled, double-blind trial. Gastroenterology 2002; 123:17–23.
- Winstead NS, Wilcox CM. Erythromycin prior to endoscopy for acute upper gastrointestinal hemorrhage: a cost-effectiveness analysis. Aliment Pharmacol Ther 2007; 26:1371–1377.
- Chak A, Cooper GS, Lloyd LE, Kolz CS, Barnhart BA, Wong RC. Effectiveness of endoscopy in patients admitted to the intensive care unit with upper GI hemorrhage. Gastrointest Endosc 2001; 53:6–13.
- Lau JY, Chung SC, Leung JW, Lo KK, Yung MY, Li AK. The evolution of stigmata of hemorrhage in bleeding peptic ulcers: a sequential endoscopic study. Endoscopy 1998; 30:513–518.
- Chung IK, Kim EJ, Lee MS, et al. Endoscopic factors predisposing to rebleeding following endoscopic hemostasis in bleeding peptic ulcers. Endoscopy 2001; 33:969–975.
- Elta GH. Acute nonvariceal upper gastrointestinal hemorrhage. Curr Treat Options Gastroenterol 2002; 5:147–152.
- Marmo R, Rotondano G, Piscopo R, Bianco MA, D’Angella R, Cipolletta L. Dual therapy versus monotherapy in the endoscopic treatment of high-risk bleeding ulcers: a meta-analysis of controlled trials. Am J Gastroenterol 2007; 102:279–289.
- Kovacs TO, Jensen DM. Recent advances in the endoscopic diagnosis and therapy of upper gastrointestinal, small intestinal, and colonic bleeding. Med Clin North Am 2002; 86:1319–1356.
- Kovacs TO, Jensen DM. Endoscopic treatment of ulcer bleeding. Curr Treat Options Gastroenterol 2007; 10:143–148.
- Jensen DM, Kovacs TO, Jutabha R, et al. Randomized trial of medical or endoscopic therapy to prevent recurrent ulcer hemorrhage in patients with adherent clots. Gastroenterology 2002; 123:407–413.
- Jensen DM, Machicado GA. Endoscopic hemostasis of ulcer hemorrhage with injection, thermal, and combination methods. Techniques Gastrointest Endosc 2005; 7:124–131.
- Bleau BL, Gostout CJ, Sherman KE, et al. Recurrent bleeding from peptic ulcer associated with adherent clot: a randomized study comparing endoscopic treatment with medical therapy. Gastrointest Endosc 2002; 56:1–6.
- Lau JY, Sung JJ, Lam YH, et al. Endoscopic retreatment compared with surgery in patients with recurrent bleeding after initial endoscopic control of bleeding ulcers. N Engl J Med 1999; 340:751–756.
- Wolf AT, Wasan SK, Saltzman JR. Impact of anticoagulation on rebleeding following endoscopic therapy for nonvariceal upper gastrointestinal hemorrhage. Am J Gastroenterol 2007; 102:290–296.
- Barkun A, Bardou M, Marshall JK; Nonvariceal Upper GI Bleeding Consensus Conference Group. Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med 2003; 139:843–857.
- Marmo R, Rotondano G, Bianco MA, Piscopo R, Prisco A, Cipolletta L. Outcome of endoscopic treatment for peptic ulcer bleeding: is a second look necessary? A meta-analysis. Gastrointest Endosc 2003; 57:62–67.
- Dell’Era A, deFrancis R, Iannuzzi F. Acute variceal bleeding: pharmacological treatment and primary/secondary prophylaxis. Best Pract Res Clin Gastroenterol 2008; 22:279–294.
- Jalan R, Hayes PC. UK guidelines on the management of variceal hemorrhage in cirrhotic patients. British Society of Gastroenterology. Gut 2000; 46( suppl 3–4):III1–III15.
- Bernard B, Lebrec D, Mathurin P, Opolon P, Poynard T. Beta-adrenergic antagonists in the prevention of gastrointestinal rebleeding in patients with cirrhosis: a meta-analysis. Hepatology 1997; 25:63–70.
- De Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2005; 43:167–176.
- Levacher S, Letoumelin P, Pateron D, Blaise M, Lapandry C, Pourriat JL. Early administration of terlipressin plus glyceryl trinitrate to control active upper gastrointestinal bleeding in cirrhotic patients. Lancet 1995; 346:865–868.
- Abraldes JG, Bosch J. Somatostatin and analogues in portal hypertension. Hepatology 2002; 35:1305–1312.
- Banares R, Albillos A, Rincon D, et al. Endoscopic treatment versus endoscopic plus pharmacological treatment for acute variceal bleeding: a meta analysis. Hepatology 2002; 35:609–615.
- Cappell M. Gastrointenstinal bleeding associated with myocardial infarction. Gastroenterol Clin North Am 2000; 29:423–444.
- Lin S, Konstance R, Jollis J, Fisher DA. The utility of upper endoscopy in patients with concomitant upper gastrointestinal bleeding and acute myocardial infarction. Dig Dis Sci 2006; 51:2377–2383.
- Silvis SE, Nebel O, Rogers G, Sugawa C, Mandelstam P. Endoscopic complications. Results of the 1974 American Society for Gastrointestinal Endoscopy Survey. JAMA 1976; 235:928–930.
- Lanas A, Garcia-Rodriguez LA, Arroyo MT, et al; Investigators of the Asociación Española de Gastroenterología (AEG). Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol 2007; 102:507–515.
- Shorr RI, Ray WA, Daugherty JR, Griffin MR. Concurrent use of nonsteroidal anti-inflammatory drugs and oral anticoagulants places elderly persons at high risk for hemorrhagic peptic ulcer disease. Arch Intern Med 1993; 153:1665–1670.
- Tabibian N. Acute gastrointestinal bleeding in anticoagulated patients: a prospective evaluation. Am J Gastroenterol 1989; 84:10–12.
- Choudari CP, Rajgopal C, Palmer KR. Acute gastrointestinal hemorrhage in anticoagulated patients: diagnoses and response to endoscopic treatment. Gut 1994; 35:464–466.
- Petty GW, Brown RD, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Frequency of major complications of aspirin, warfarin, and intravenous heparin for secondary stroke prevention: a population-based study. Ann Intern Med 1999; 130:14–22.
- Landefeld CS, Rosenblatt MW, Goldman L. Bleeding in outpatients treated with warfarin: relation to the prothrombin time and important remediable lesions. Am J Med 1989; 87:153–159.
- Weil J, Colin-Jones D, Langman M, et al. Prophylactic aspirin and risk of peptic ulcer bleeding. BMJ 1995; 310:827–830.
- Cryer B, Feldman M. Effects of very low dose daily, long-term aspirin therapy on gastric, duodenal, and rectal prostaglandin levels and on mucosal injury in healthy humans. Gastroenterology 1999; 117:17–25.
- De Abajo FJ, Garcia Rodriguez LA. Risk of upper gastrointestinal bleeding and perforation associated with low-dose aspirin as plain and enteric-coated formulations. BMC Clin Pharmacol 2001; 1:1.
- Kelly JP, Kaufman DW, Jurgelon JM, Sheehan J, Koff RS, Shapiro S. Risk of aspirin-associated major upper gastrointestinal bleeding with enteric coated or buffered product. Lancet 1996; 348:1413–1416.
- Garcia Rodriguez LA, Hernandez-Diaz S, De Abajo FJ. Association between aspirin and upper gastrointestinal complications: systematic review of epidemiological studies. Br J Clin Pharmacol 2001; 52:563–571.
- Wilcox CM, Ladabaum U. A patient with high risk of gastrointestinal bleeding requiring nonsteroidal anti-inflammatory drugs. Clin Gastroenterol Hepatol 2006; 4:1090–1093.
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction. J Am Coll Cardiol 2007; 50:e1–e157.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996; 348:1329–1339.
- Chan FK, Ching JY, Hung LC, et al. Clopidogrel versus aspirin and esomeprazole to prevent recurrent ulcer bleeding. N Engl J Med 2005; 352:238–244.
- Lai KC, Chu KM, Hui WM, et al. Esomeprazole with aspirin versus clopidogrel for prevention of recurrent gastrointestinal ulcer complications. Clin Gastroenterol Hepatol 2006; 4:860–865.
- Ho MP, Maddox TM, Wang L, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA 2009; 301:937–944.
- Siller-Matula JM, Spiel AO, Lang IM, Kreiner G, Christ G, Jilma B. Effects of pantoprazole and esomeprazole on platelet inhibition by clopidogrel. Am Heart J 2009; 157:148.e1–e5.
- Small DS, Farid NA, Payne CD, et al. Effects of proton pump inhibitor lansoprazole on the pharmacokinetics and pharmacodynamics of prasugel and clopidogrel. J Clin Pharmacol 2008; 48:475–484.
- Ishizaki T, Horai Y. Review article: cytochrome P450 and the metabolism of proton pump inhibitors—emphasis on rabeprazole. Aliment Pharacol Ther 1999; 13 (suppl 3):27–36.
- Lanas A, Fuentes J, Benito R, Serrano P, Bajador E, Sainz R. Helicobacter pylori increases the risk of upper gastrointestinal bleeding in patients taking low-dose aspirin. Aliment Pharmacol Ther 2002; 16:779–786.
- Chan FK. NSAID-Induced peptic ulcers and Helicobacter pylori infection: implications for patient management. Drug Saf 2005; 28:287–300.
- Bombardier V, Laine L, Reicin A, et al; VIGOR Study Group. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis: VIGOR Study Group. N Eng J Med 2000; 343:1520–1528.
- Griffin MR, Ray WA, Schaffner W. Nonsteroidal anti-inflammatory drug use and death from peptic ulcer in elderly persons. Ann Intern Med 1988; 109:359–363.
- Griffin MR, Piper JM, Daugherty JR, Snowden M, Ray WA. Nonsteroidal anti-inflammatory drug use and increased risk for peptic ulcer disease in elderly persons. Ann Intern Med 1991; 114:257–263.
- Smalley WE, Ray WA, Daugherty JR, Griffin MR. Nonsteroidal anti-inflammatory drugs and the incidence of hospitalizations for peptic ulcer disease in elderly persons. Am J Epidemiol 1995; 141:539–545.
- Laine L. Approaches to nonsteroidal anti-inflammatory drug use in the high-risk patient. Gastroenterology 2001; 120:594–606.
- Silverstein FE, Graham DY, Senior JR, et al. Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal anti-inflammatory drugs. A randomized, double blind, placebo controlled trial. Ann Intern Med 1995; 123:241–249.
- Goldstein JL, Huang B, Amer F, Christopoulos NG. Ulcer recurrence in high-risk patients receiving nonsteroidal anti-inflammatory drugs plus low dose aspirin: results of a post hoc subanalysis. Clin Ther 2004; 26:1637–1643.
- Berger JS, Stebbins A, Granger CB, et al. Initial aspirin dose and outcome among ST-elevation myocardial infarction patients treated with fibrinolytic therapy. Circulation 2008; 117:192–199.
- Lanas A, Garcia-Rodriguez LA, Arroyo MT, et al; Investigators of the Asociación Española de Gastroenterología (AEG). Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol 2007; 102:507–515.
- Chin MW, Yong G, Bulsara MK, Rankin J, Forbes GM. Predictive and protective factors associated with upper gastrointestinal bleeding after percutaneous coronary intervention: a case-control study. Am J Gastroenterol 2007; 102:2411–2416.
- Hunt RH, Bazzoli F. Should NSAID/low dose aspirin takers be tested routinely for H. Pylori infection and treated if positive? Implications for primary risk of ulcer and ulcer relapse after initial healing. Aliment Pharmacol Ther 2004; 19 (suppl 1):9–16.
KEY POINTS
- The first priority is to ensure that the patient is hemodynamically stable, which often requires admission to the intensive care unit for monitoring and fluid resuscitation.
- Peptic ulcers account for most cases of upper GI bleeding, but bleeding from varices has a much higher case-fatality rate and always demands aggressive treatment.
- Patients with ulcer disease should be tested and treated for Helicobacter pylori infection.
- Patients with a history of bleeding ulcers who need long-term treatment with aspirin or a nonsteroidal anti-inflammatory drug should also be prescribed a proton pump inhibitor.