User login
A minimally invasive treatment for early GI cancers
The treatment of early esophageal, gastric, and colorectal cancer is changing.1 For many years, surgery was the mainstay of treatment for early-stage gastrointestinal cancer. Unfortunately, surgery leads to significant loss of function of the organ, resulting in increased morbidity and decreased quality of life.2
Endoscopic techniques, particularly endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), have been developed and are widely used in Japan, where gastrointestinal cancer is more common than in the West. This article reviews the indications, complications, and outcomes of ESD for early gastrointestinal neoplasms, so that readers will recognize the subset of patients who would benefit from ESD in a Western setting.
ENDOSCOPIC MUCOSAL RESECTION AND SUBMUCOSAL DISSECTION
Since the first therapeutic polypectomy was performed in Japan in 1974, several endoscopic techniques for tumor resection have been developed.3
EMR, one of the most successful and widely used techniques, involves elevating the lesion either with submucosal injection of a solution or with cap suction, and then removing it with a snare.4 Most lesions smaller than 20 mm can be removed in one piece (en bloc).5 Larger lesions are removed in multiple pieces (ie, piecemeal). Unfortunately, some fibrotic lesions, which are usually difficult to lift, cannot be completely removed by EMR.
ESD was first performed in the late 1990s with the aim of overcoming the limitations of EMR in resecting large or fibrotic tumors en bloc.6,7 Since then, ESD technique has been standardized and training centers have been created, especially in Asia, where it is widely used for treatment of early gastric cancer.3,8–10 Since 2012 it has been covered by the Japanese National Health Insurance for treatment of early gastric cancer, and since 2014 for treatment of colorectal malignant tumors measuring 2 to 5 cm.11
Adoption of ESD has been slow in Western countries, where many patients are still referred for surgery or undergo EMR for removal of superficial neoplasms. Reasons for this slow adoption are that gastric cancer is much less common in Western countries, and also that ESD demands a high level of technical skill, is difficult to learn, and is expensive.3,12,13 However, small groups of Western endoscopists have become interested and are advocating it, first studying it on their own and then training in a Japanese center and learning from experts performing the procedure.
Therefore, in a Western setting, ESD should be performed in specialized endoscopy centers and offered to selected patients.1
CANDIDATES SHOULD HAVE EARLY-STAGE, SUPERFICIAL TUMORS
Ideal candidates for endoscopic resection are patients who have early cancer with a negligible risk of lymph node metastasis, such as cancer limited to the mucosa (stage T1a).7 Therefore, to determine the best treatment for a patient with a newly diagnosed gastrointestinal neoplasm, it is mandatory to estimate the depth of invasion.
The depth of invasion is directly correlated with lymph node involvement, which is ultimately the main predictive factor for long-term adverse outcomes of gastrointestinal tumors.4,14–17 Accurate multidisciplinary preprocedure estimations are mandatory, as incorrect evaluations may result in inappropriate therapy and residual cancer.18
Other factors that have been used to predict lymph node involvement include tumor size, macroscopic appearance, histologic differentiation, and lymphatic and vascular involvement.19 Some of these factors can be assessed by special endoscopic techniques (chromoendoscopy and narrow-band imaging with magnifying endoscopy) that allow accurate real-time estimation of the depth of invasion of the lesion.5,17,20–27 Evaluation of microsurface and microvascular arrangements is especially useful for determining the feasibility of ESD in gastric tumors, evaluation of intracapillary loops is useful in esophageal lesions, and assessment of mucosal pit patterns is useful for colorectal lesions.21–29
Endoscopic ultrasonography is another tool that has been used to estimate the depth of the tumor. Although it can differentiate between definite intramucosal and definite submucosal invasive cancers, its ability to confirm minute submucosal invasion is limited. Its use as the sole tumor staging modality is not encouraged, and it should always be used in conjunction with endoscopic evaluation.18
Though the aforementioned factors help stratify patients, pathologic staging is the best predictor of lymph node metastasis. ESD provides adequate specimens for accurate pathologic evaluation, as it removes lesions en bloc.30
All patients found to have risk factors for lymph node metastasis on endoscopic, ultrasonographic, or pathologic analysis should be referred for surgical evaluation.9,19,31,32
ENDOSCOPIC SUBMUCOSAL DISSECTION
Before the procedure, the patient’s physicians need to do the following:
Determine the best type of intervention (EMR, ESD, ablation, surgery) for the specific lesion.3 A multidisciplinary approach is encouraged, with involvement of the internist, gastroenterologist, and surgeon.
Plan for anesthesia, additional consultations, pre- and postprocedural hospital admission, and need for special equipment.33
During the procedure
Define the lateral extent of the lesion using magnification chromoendoscopy or narrow-band imaging. In the stomach, a biopsy sample should be taken from the worst-looking segment and from normal-looking mucosa. Multiple biopsies should be avoided to prevent subsequent fibrosis.33 In the colon, biopsy should be avoided.34
Identify and circumferentially mark the target lesion. Cautery or argon plasma coagulation can be used for making markings at a distance of 5 to 10 mm from the edges.33 This is done to recognize the borders of the lesion, because they can become distorted after submucosal injection.14 This step is unnecessary in colorectal cases, as tumor margins can be adequately visualized after chromoendoscopy.16,35
Lift the lesion by injecting saline, 0.5% hyaluronate, or glycerin to create a submucosal fluid cushion.19,33
Perform a circumferential incision lateral to the mucosal margins to allow for a normal tissue margin.33 Partial incision is performed for esophageal and colorectal ESD to avoid fluid leakage from the submucosal layer, achieving a sustained submucosal lift and safer dissection.16
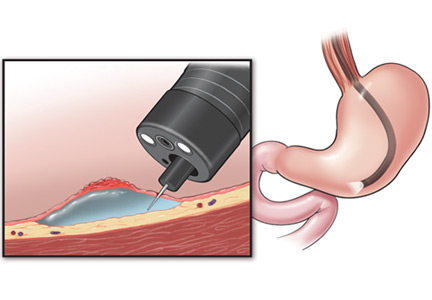
Submucosal dissection. The submucosal layer is dissected with an electrocautery knife until the lesion is completely removed. Dissection should be done carefully to keep the submucosal plane.33 Hemoclips or hemostat forceps can be used to control visible bleeding. The resected specimen is then stretched and fixed to a board using small pins for further histopathologic evaluation.35
Postprocedural monitoring. All patients should be admitted for overnight observation. Those who undergo gastric ESD should receive high-dose acid suppression, and the next day they can be started on a liquid diet.19
STOMACH CANCER
Indications for ESD for stomach cancer in the East
The incidence of gastric cancer is higher in Japan and Korea, where widespread screening programs have led to early identification and early treatment of this disease.36
Pathology studies37 of samples from patients with gastric cancer identified the following as risk factors for lymph node metastasis, which would make ESD unsuitable:
- Undifferentiated type
- Tumors larger than 2 cm
- Lymphatic or venous involvement
- Submucosal invasion
- Ulcerative change.
Based on these findings, the situations in which there was no risk of lymph node involvement (ie, when none of the above factors are present) were accepted as absolute indications for endoscopic resection of early gastric cancer.38 Further histologic studies identified a subset of patients with lesions with very low risk of lymph node metastasis, which outweighed the risk of surgery. Based on these findings, expanded criteria for gastric ESD were proposed,39,40 and the Japanese gastric cancer treatment guidelines now include these expanded preoperative indications9,17 (Table 1).
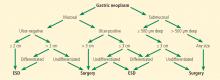
The Japanese Gastric Cancer Association has proposed a treatment algorithm based on the histopathologic evaluation after resection (Figure 2).9
Outcomes
In the largest series of patients who underwent curative ESD for early gastric cancer, the 5-year survival rate was 92.6%, the 5-year disease-specific survival rate was 99.9%, and the 5-year relative survival rate was 105%.41
Similarly, in a Japanese population-based survival analysis, the relative 5-year survival rate for localized gastric cancer was 94.4%.42 Rates of en bloc resection and complete resection with ESD are higher than those with EMR, resulting in a lower risk of local recurrence in selected patients who undergo ESD.8,43,44
Although rare, local recurrence after curative gastric ESD has been reported.45 The annual incidence of local recurrence has been estimated to be 0.84%.46
ESD entails a shorter hospital stay and requires fewer resources than surgery, resulting in lower medical costs (Table 2).44 Additionally, as endoscopic resection is associated with less morbidity, fewer procedure-related adverse events, and fewer complications, ESD could be used as the standard treatment for early gastric cancer.47,48
The Western perspective on endoscopic submucosal dissection for gastric cancer
Since the prevalence of gastric cancer in Western countries is significantly lower than in Japan and Korea, local data and experience are scarce. However, experts performing ESD in the West have adopted the indications of the Japan Gastroenterological Endoscopy Society. The European Society of Gastrointestinal Endoscopy recommends ESD for excision of most superficial gastric neoplasms, with EMR being preferred only in lesions smaller than 15 mm, Paris classification 0 or IIA.5,32
Patients with gastric lesions measuring 15 mm or larger should undergo high-quality endoscopy, preferably chromoendoscopy, to evaluate the mucosal patterns and determine the depth of invasion. If superficial involvement is confirmed, other imaging techniques are not routinely recommended.5 A surgery consult is also recommended.
ESOPHAGEAL CANCER
Indications for ESD for esophageal cancer in the East
Due to the success of ESD for early gastric cancer, this technique is now also used for superficial esophageal neoplasms.19,49 It should be done in a specialized center, as it is more technically difficult than gastric ESD: the esophageal lumen is narrow, the wall is thin, and the esophagus moves with respiration and heartbeat.50 A multidisciplinary approach including an endoscopist, a surgeon, and a pathologist is highly recommended for evaluation and treatment.
EMR is preferred for removal of mucosal cancer, in view of its safety profile and success rates. ESD can be considered in cases of lesions larger than 15 mm, poorly lifting tumors, and those with the possibility of submucosal invasion (Table 3).5,45,49,51
Circumference involvement is critical when determining eligible candidates, as a defect involving more than three-fourths of the esophageal circumference can lead to esophageal strictures.52 Controlled prospective studies have shown promising results from giving intralesional and oral steroids to prevent stricture after ESD, which could potentially overcome this size limitation.53,54
Outcomes for esophageal cancer
ESD has been shown to be safe and effective, achieving en bloc resection in 85% to 100% of patients.19,51 Its advantages over EMR include en bloc resection, complete resection, and high curative rates, resulting in higher recurrence-free survival.2,55,56 Although the incidence of complications such as bleeding, perforation, and stricture formation are higher with ESD, patients usually recover uneventfully.2,19,20
ESD in the esophagus: The Western perspective
As data on the efficacy of EMR vs ESD for the treatment of Barrett esophagus with adenocarcinoma are limited, EMR is the gold standard endoscopic technique for removal of visible esophageal dysplastic lesions.5,51,57 ESD can be considered for tumors larger than 15 mm, for poorly lifting lesions, and if there is suspicion of submucosal invasion.5
Patients should be evaluated by an experienced endoscopist, using an advanced imaging technique such as narrow-band imaging or chromoendoscopy. If suspicious features are found, endoscopic ultrasonography should be considered to confirm submucosal invasion or lymph node involvement.5
COLORECTAL CANCER
Indications for ESD for colorectal cancer in the East
Colon cancer is one of the leading causes of cancer-related deaths worldwide.58 Since ESD has been found to be effective and safe in treating gastric cancer, it has also been used to remove large colorectal tumors.59 However, ESD is not universally accepted in the treatment of colorectal neoplasms due to its greater technical difficulty, longer procedural time, and higher risk of perforating the thinner colonic wall compared with EMR.21,60
Outcomes for colorectal cancer
Tumor size of 50 mm or larger is a risk factor for complications, while a high procedure volume at the center is a protective factor.60
Endoscopic treatment of colorectal cancer: The Western perspective
EMR is the gold standard for removal of superficial colorectal lesions. However, ESD can be considered if there is suspicion of superficial submucosal invasion, especially for lesions larger than 20 mm that cannot be resected en bloc by EMR.32 ESD can also be used for fibrotic lesions not amenable to complete EMR removal, or as a salvage procedure after recurrence after EMR.67 Proper selection of cases is critical.1
Patients who have a superficial colonic lesion should be evaluated by means of high-definition endoscopy and chromoendoscopy to assess the mucosal pattern and establish feasibility of endoscopic resection. If submucosal invasion is suspected, staging with endoscopic ultrasonography or magnetic resonance imaging should be considered.5
FOLLOW-UP AFTER ESD
Endoscopic surveillance after the procedure is recommended, given the persistent risk of metachronous cancer after curative ESD due to its organ-sparing quality.68 Surveillance endoscopy aims to achieve early detection and subsequent endoscopic resection of metachronous lesions.
Histopathologic evaluation assessing the presence of malignant cells in the margins of a resected sample is mandatory for determining the next step in treatment. If margins are negative, follow-up endoscopy can be done every 6 to 12 months. If margins are positive, the approach includes surgery, reattempting ESD or endoscopic surveillance in 3 or 6 months.3,32 Although the surveillance strategy varies according to individual risk of metachronous cancer, it should be continued indefinitely.68
COMPLICATIONS OF ESD
The most common procedure-related complications of ESD are bleeding, perforation, and stricture. Most intraprocedural adverse events can be managed endoscopically.69
Bleeding
Most bleeding occurs during the procedure or early after it and can be controlled with electrocautery.49,69 No episodes of massive bleeding, defined as causing clinical symptoms and requiring transfusion or surgery, have been reported.20,43,55
In gastric ESD, delayed bleeding rates have ranged from 0 to 15.6%.69 Bleeding may be prevented with endoscopic coagulation of visible vessels after dissection has been completed and by proton pump inhibitor therapy.70,71 Excessive coagulation should be avoided to lower the risk of perforation.33
In colorectal ESD the bleeding rate has been reported to be 2.2%; applying coagulation to an area where a blood vessel is suspected before cutting (precoagulation) may prevent subsequent bleeding.21
Perforation
For gastric ESD, perforation rates range from 1.2% to 5.2%.69 Esophageal perforation rates can be up to 4%.49 In colorectal ESD, perforation rates have been reported to be 1.6% to 6.6%.60,72
Although most of the cases were successfully managed with conservative treatment, some required emergency surgery.60,73
Strictures
In a case series of 532 patients undergoing gastric ESD, stricture was reported in 5 patients, all of whom presented with obstructive symptoms.74 Risk factors for post-ESD gastric stenosis are a mucosal defect with a circumferential extent of more than three-fourths or a longitudinal extent of more than 5 cm.75
Strictures are common after esophageal ESD, with rates ranging from 2% to 26%. The risk is higher when longer segments are removed or circumferential resection is performed. As previously mentioned, this complication may be reduced with ingestion or injection of steroids after the procedure.53,54
Surprisingly, ESD of large colorectal lesions involving more than three-fourths of the circumference of the rectum is rarely complicated by stenosis.76
LIMITATIONS OF ESD
ESD requires a high level of technical skill, is time-consuming, and has a higher rate of complications than conventional endoscopic resection. A standardized ESD training system is needed, as the procedure is more difficult than EMR. Training in porcine models has been shown to confer competency in ESD in a Western setting.13,16,33
Colorectal ESD is an even more challenging procedure, given the potential for complications related to its anatomy. Training centers in Japan usually have their trainees first master gastric ESD, then assist in more than 20 colorectal ESDs conducted by experienced endoscopists, and accomplish 30 cases before performing the procedure safely and independently.
As the incidence of gastric cancer is low in Western countries, trainees may also begin with lower rectal lesions, which are easier to remove.77 Incorporation of ESD in the West would require a clear treatment algorithm. It is a complex procedure, with higher rates of complications, a prolonged learning curve, and prolonged procedure time. Therefore, it should be performed in specialized centers and under the special situations discussed here to ensure that the benefits for the patients outweigh the risks.
VALUE OF ENDOSCOPIC SUBMUCOSAL DISSECTION
The optimal method for resecting gastrointestinal neoplasms should be safe, cost-effective, and quick and should also completely remove the lesion. The best treatment strategy takes into account the characteristics of the lesion and the comorbidities and wishes of the patient. Internists should be aware of the multiple options available to achieve the best outcome for the patient.1
Endoscopic resection of superficial gastrointestinal neoplasms, including EMR and ESD, has been a subject of increasing interest due to its minimally invasive and potentially curative character. However, cancer can recur after endoscopic resection because the procedure is organ-sparing.
ESD allows resection of early gastrointestinal tumors with a minimally invasive technique. It can achieve higher curative resection rates and lower recurrence rates compared with EMR. Compared with surgery, ESD leads to less morbidity, fewer procedure-related complications, and lower medical costs. Indications should be rigorously followed to achieve successful treatments in selected patients.
Multiple variables have to be taken into account when deciding which treatment is best, such as tumor characteristics, the patient’s baseline condition, physician expertise, and hospital resources.48 Less-invasive treatments may improve the prognosis of patients. No matter the approach, patients should be treated in specialized treatment centers.
Internal medicine physicians should be aware of the advances in treatments for early gastrointestinal cancer so appropriate options can be considered.
- Burgess NG, Bourke MJ. Endoscopic resection of colorectal lesions: the narrowing divide between East and West. Dig Endosc 2016; 28:296–305.
- Kim DH, Jung HY, Gong EJ, et al. Endoscopic and oncologic outcomes of endoscopic resection for superficial esophageal neoplasm. Gut Liver 2015; 9:470–477.
- Draganov PV, Gotoda T, Chavalitdhamrong D, Wallace MB. Techniques of endoscopic submucosal dissection: application for the Western endoscopist? Gastrointest Endosc 2013; 78:677–688.
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011; 14:101–112.
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2015; 47:829–854.
- Farhat S, Chaussade S, Ponchon T, et al; SFED ESD Study Group. Endoscopic submucosal dissection in a European setting. A multi-institutional report of a technique in development. Endoscopy 2011; 43:664–670.
- Gotoda T, Jung H. Endoscopic resection (endoscopic mucosal resection/endoscopic submucosal dissection) for early gastric cancer. Dig Endosc 2013; 25(suppl 1):55–63.
- Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc 2009; 69:1228–1235.
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011; 14:113–123.
- Ono H. Endoscopic submucosal dissection for early gastric cancer. Chin J Dig Dis 2005; 6:119–121.
- Watanabe T, Itabashi M, Shimada Y, et al; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol 2015; 20:207–239.
- Oyama T, Yahagi N, Ponchon T, Kiesslich T, Berr F. How to establish endoscopic submucosal dissection in Western countries. World J Gastroenterol 2015; 21:11209–11220.
- Bhatt A, Abe S, Kumaravel A, et al. SU1575 Western skill training in endoscopic submucosal dissection (ESD)—an international remote video based study—the WEST ESD Study. Gastrointest Endosc 2015; 81(suppl):AB335–AB336.
- Sano T, Sasako M, Kinoshita T, Maruyama K. Recurrence of early gastric cancer follow-up of 1475 patients and review of the Japanese literature. Cancer 1993; 72:3174–3178.
- Japan Esophageal Society. Japanese classification of esophageal cancer, tenth edition: part I. Esophagus 2009; 6:1–25.
- Bhatt A, Abe S, Kumaravel A, Vargo J, Saito Y. Indications and techniques for endoscopic submucosal dissection. Am J Gastroenterol 2015; 110:784–791.
- Eleftheriadis N, Inoue H, Ikeda H, et al. Definition and staging of early esophageal, gastric and colorectal cancer. J Tumor 2014; 2:161–178.
- Yoshinaga S, Oda I, Nonaka S, Kushima R, Saito Y. Endoscopic ultrasound using ultrasound probes for the diagnosis of early esophageal and gastric cancers. World J Gastrointest Endosc 2012; 4:218–226.
- Stahl M, Mariette C, Haustermans K, Cervantes A, Arnold D; ESMO Guidelines Working Group. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(suppl 6):vi51–vi56.
- Higuchi K, Tanabe S, Azuma M, et al. A phase II study of endoscopic submucosal dissection for superficial esophageal neoplasms (KDOG 0901). Gastrointest Endosc 2013; 78:704–710.
- Sakamoto T, Mori G, Yamada M, et al. Endoscopic submucosal dissection for colorectal neoplasms: a review. World J Gastroenterol 2014; 20:16153–16158.
- Ohta A, Tominaga K, Sakai Y. Efficacy of magnifying colonoscopy for the diagnosis of colorectal neoplasia: comparison with histopathological findings. Dig Endosc 2004; 16:308–314.
- Katagiri A, Fu K, Sano Y, et al. Narrow band imaging with magnifying colonoscopy as diagnostic tool for predicting histology of early colorectal neoplasia. Aliment Pharmacol Ther 2008; 27:1269–1274.
- Fu KI, Kato S, Sano Y, et al. Staging of early colorectal cancers: magnifying colonoscopy versus endoscopic ultrasonography for estimation of depth of invasion. Dig Dis Sci 2008; 53:1886–1892.
- Uraoka T, Saito Y, Ikematsu H, Yamamoto K, Sano Y. Sano’s capillary pattern classification for narrow-band imaging of early colorectal lesions. Dig Endosc 2011; 23(suppl 1):112–115.
- Ikematsu H, Matsuda T, Emura F, et al. Efficacy of capillary pattern type IIIA/IIIB by magnifying narrow band imaging for estimating depth of invasion of early colorectal neoplasms. BMC Gastroenterol 2010;10:33.
- Matsuda T, Fujii T, Saito Y, et al. Efficacy of the invasive/non-invasive pattern by magnifying chromoendoscopy to estimate the depth of invasion of early colorectal neoplasms. Am J Gastroenterol 2008; 103:2700–2706.
- Sato H, Inoue H, Ikeda H, et al. Utility of intrapapillary capillary loops seen on magnifying narrow-band imaging in estimating invasive depth of esophageal squamous cell carcinoma. Endoscopy 2015; 8:122–128.
- Muto M, Yao K, Kaise M, et al. Magnifying endoscopy simple diagnostic algorithm for early gastric cancer (MESDA-G). Dig Endosc 2016; 28:379–393.
- Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D; European Society for Medical Oncology (ESMO); European Society of Surgical Oncology (ESSO); European Society of Radiotherapy and Oncology (ESTRO). Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(suppl 6):vi57–vi63.
- Kuwano H, Nishimura Y, Ohtsu A, et al. Guidelines for diagnosis and treatment of carcinoma of the esophagus. April 2007 edition: part I - Edited by the Japan Esophageal Society. Esophagus 2008; 5:61–73.
- Tanaka S, Kashida H, Saito Y, et al. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc 2015; 27:417–434.
- Gotoda T, Ho KY, Soetikno R, Kaltenbach T, Draganov P. Gastric ESD: current status and future directions of devices and training. Gastrointest Endosc Clin North Am 2014; 24:213–233.
- Saito Y, Sakamoto T, Nakajima T, Matsuda T. Colorectal ESD: current indications and latest technical advances. Gastrointest Endosc Clin N Am 2014; 24:245–255.
- Saito Y, Otake Y, Sakamoto T, et al. Indications for and technical aspects of colorectal endoscopic submucosal dissection. Gut Liver 2013; 7:263–269.
- Saragoni L. Upgrading the definition of early gastric cancer: better staging means more appropriate treatment. Cancer Biol Med 2015; 12:355–361.
- Tsujitani S, Oka S, Saito H, et al. Less invasive surgery for early gastric cancer based on the low probability of lymph node metastasis. Surgery 1999; 125:148–154.
- Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc 2003; 57:567–579.
- Hirasawa T, Gotoda T, Miyata S, et al. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer 2009; 12:148–152.
- Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000; 3:219–225.
- Suzuki H, Oda I, Abe S, et al. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer 2016; 19:198–205.
- Matsuda T, Ajiki W, Marugame T, Ioka A, Tsukuma H, Sobue T; Research Group of Population-Based Cancer Registries of Japan. Population-based survival of cancer patients diagnosed between 1993 and 1999 in Japan: a chronological and international comparative study. Jpn J Clin Oncol 2011; 41:40–51.
- Ahn JY, Jung HY, Choi KD, et al. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc 2011; 74:485–493.
- Kim Y, Kim YW, Choi IJ, et al. Cost comparison between surgical treatments and endoscopic submucosal dissection in patients with early gastric cancer in Korea. Gut Liver 2015; 9:174–180.
- Abe S, Oda I, Nakajima T, et al. A case of local recurrence and distant metastasis following curative endoscopic submucosal dissection of early gastric cancer. Gastric Cancer 2015; 18:188–192.
- Hahn KY, Park JC, Kim EH, et al. Incidence and impact of scheduled endoscopic surveillance on recurrence after curative endoscopic resection for early gastric cancer. Gastrointest Endosc 2016; 84:628–638.e1.
- Wang S, Zhang Z, Liu M, Li S, Jiang C. Endoscopic resection compared with gastrectomy to treat early gastric cancer: a systematic review and meta-analysis. PLoS One 2015; 10:e0144774.
- Kondo A, de Moura EG, Bernardo WM, et al. Endoscopy vs surgery in the treatment of early gastric cancer: systematic review. World J Gastroenterol 2015; 21:13177–13187.
- Kothari S, Kaul V. Endoscopic mucosal resection and endoscopic submucosal dissection for endoscopic therapy of Barrett’s esophagus-related neoplasia. Gastroenterol Clin North Am 2015; 44:317–335.
- Yamashita T, Zeniya A, Ishii H, et al. Endoscopic mucosal resection using a cap-fitted panendoscope and endoscopic submucosal dissection as optimal endoscopic procedures for superficial esophageal carcinoma. Surg Endosc 2011; 25:2541–2546.
- Kagemoto K, Oka S, Tanaka S, et al. Clinical outcomes of endoscopic submucosal dissection for superficial Barrett’s adenocarcinoma. Gastrointest Endosc 2014; 80:239–245.
- Katada C, Muto M, Manabe T, Boku N, Ohtsu A, Yoshida S. Esophageal stenosis after endoscopic mucosal resection of superficial esophageal lesions. Gastrointest Endosc 2003; 57:165–169.
- Hanaoka N, Ishihara R, Takeuchi Y, et al. 1139: A single session of intralesional steroid injection to prevent esophageal stricture after endoscopic submucosal dissection for esophageal squamous cell carcinoma. Gastrointest Endosc 2012; 75(suppl):AB175.
- Yamaguchi N, Isomoto H, Nakayama T, et al. Usefulness of oral prednisolone in the treatment of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Gastrointest Endosc 2011; 73:1115–1121.
- Ono S, Fujishiro M, Niimi K, et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc 2009; 70:860–866.
- Katada C, Muto M, Manabe T, Ohtsu A, Yoshida S. Local recurrence of squamous-cell carcinoma of the esophagus after EMR. Gastrointest Endosc 2005; 61:219–225.
- Hirasawa K, Kokawa A, Oka H, et al. Superficial adenocarcinoma of the esophagogastric junction: long-term results of endoscopic submucosal dissection. Gastrointest Endosc 2010; 72:960–966.
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
- Nakajima T, Saito Y, Tanaka S, et al. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg Endosc 2013; 27:3262–3770.
- Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc 2010; 72:1217–1225.
- Tanaka S, Saitoh Y, Matsuda T, et al; Japanese Society of Gastroenterology. Evidence-based clinical practice guidelines for management of colorectal polyps. J Gastroenterol 2015; 50:252–260.
- Oka S, Tanaka S, Saito Y, et al; Colorectal Endoscopic Resection Standardization Implementation Working Group of the Japanese Society for Cancer of the Colon and Rectum, Tokyo, Japan. Local recurrence after endoscopic resection for large colorectal neoplasia: a multicenter prospective study in Japan. Am J Gastroenterol 2015; 110:697–707.
- Saito Y, Fukuzawa M, Matsuda T, et al. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc 2010; 24:343–352.
- Makazu M, Sakamoto T, So E, et al. Relationship between indeterminate or positive lateral margin and local recurrence after endoscopic resection of colorectal polyps. Endosc Int Open 2015; 3:E252–E257.
- Belderbos TD, Leenders M, Moons LM, Siersema PD. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy 2014; 46:388–402.
- Fujiya M, Tanaka K, Dokoshi T, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc 2015; 81:583–595.
- Rahmi G, Tanaka S, Ohara Y, et al. Efficacy of endoscopic submucosal dissection for residual or recurrent superficial colorectal tumors after endoscopic mucosal resection. J Dig Dis 2015; 16:14–21.
- Abe S, Oda I, Suzuki H, et al. Long-term surveillance and treatment outcomes of metachronous gastric cancer occurring after curative endoscopic submucosal dissection. Endoscopy 2015; 47:1113–1118.
- Oda I, Suzuki H, Nonaka S, Yoshinaga S. Complications of gastric endoscopic submucosal dissection. Dig Endosc 2013; 25(suppl 1):71–78.
- Takizawa K, Oda I, Gotoda T, et al. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection—an analysis of risk factors. Endoscopy 2008; 40:179–183.
- Uedo N, Takeuchi Y, Yamada T, et al. Effect of a proton pump inhibitor or an H2-receptor antagonist on prevention of bleeding from ulcer after endoscopic submucosal dissection of early gastric cancer: a prospective randomized controlled trial. Am J Gastroenterol 2007; 102:1610–1616.
- Hayashi N, Tanaka S, Nishiyama S, et al. Predictors of incomplete resection and perforation associated with endoscopic submucosal dissection for colorectal tumors. Gastrointest Endosc 2014; 79:427–435.
- Suzuki H, Oda I, Sekiguchi M, et al. Management and associated factors of delayed perforation after gastric endoscopic submucosal dissection. World J Gastroenterol 2015; 21:12635–12643.
- Tsunada S, Ogata S, Mannen K, et al. Case series of endoscopic balloon dilation to treat a stricture caused by circumferential resection of the gastric antrum by endoscopic submucosal dissection. Gastrointest Endosc 2008; 67:979–983.
- Coda S, Oda I, Gotoda T, Yokoi C, Kikuchi T, Ono H. Risk factors for cardiac and pyloric stenosis after endoscopic submucosal dissection, and efficacy of endoscopic balloon dilation treatment. Endoscopy 2009; 41:421–426.
- Abe S, Sakamoto T, Takamaru H, et al. Stenosis rates after endoscopic submucosal dissection of large rectal tumors involving greater than three quarters of the luminal circumference. Surg Endosc 2016; 30:5459–5464.
- Sakamoto T, Saito Y, Fukunaga S, Nakajima T, Matsuda T. Learning curve associated with colorectal endoscopic submucosal dissection for endoscopists experienced in gastric endoscopic submucosal dissection. Dis Colon Rectum 2011; 54:1307–1312.
The treatment of early esophageal, gastric, and colorectal cancer is changing.1 For many years, surgery was the mainstay of treatment for early-stage gastrointestinal cancer. Unfortunately, surgery leads to significant loss of function of the organ, resulting in increased morbidity and decreased quality of life.2
Endoscopic techniques, particularly endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), have been developed and are widely used in Japan, where gastrointestinal cancer is more common than in the West. This article reviews the indications, complications, and outcomes of ESD for early gastrointestinal neoplasms, so that readers will recognize the subset of patients who would benefit from ESD in a Western setting.
ENDOSCOPIC MUCOSAL RESECTION AND SUBMUCOSAL DISSECTION
Since the first therapeutic polypectomy was performed in Japan in 1974, several endoscopic techniques for tumor resection have been developed.3
EMR, one of the most successful and widely used techniques, involves elevating the lesion either with submucosal injection of a solution or with cap suction, and then removing it with a snare.4 Most lesions smaller than 20 mm can be removed in one piece (en bloc).5 Larger lesions are removed in multiple pieces (ie, piecemeal). Unfortunately, some fibrotic lesions, which are usually difficult to lift, cannot be completely removed by EMR.
ESD was first performed in the late 1990s with the aim of overcoming the limitations of EMR in resecting large or fibrotic tumors en bloc.6,7 Since then, ESD technique has been standardized and training centers have been created, especially in Asia, where it is widely used for treatment of early gastric cancer.3,8–10 Since 2012 it has been covered by the Japanese National Health Insurance for treatment of early gastric cancer, and since 2014 for treatment of colorectal malignant tumors measuring 2 to 5 cm.11
Adoption of ESD has been slow in Western countries, where many patients are still referred for surgery or undergo EMR for removal of superficial neoplasms. Reasons for this slow adoption are that gastric cancer is much less common in Western countries, and also that ESD demands a high level of technical skill, is difficult to learn, and is expensive.3,12,13 However, small groups of Western endoscopists have become interested and are advocating it, first studying it on their own and then training in a Japanese center and learning from experts performing the procedure.
Therefore, in a Western setting, ESD should be performed in specialized endoscopy centers and offered to selected patients.1
CANDIDATES SHOULD HAVE EARLY-STAGE, SUPERFICIAL TUMORS
Ideal candidates for endoscopic resection are patients who have early cancer with a negligible risk of lymph node metastasis, such as cancer limited to the mucosa (stage T1a).7 Therefore, to determine the best treatment for a patient with a newly diagnosed gastrointestinal neoplasm, it is mandatory to estimate the depth of invasion.
The depth of invasion is directly correlated with lymph node involvement, which is ultimately the main predictive factor for long-term adverse outcomes of gastrointestinal tumors.4,14–17 Accurate multidisciplinary preprocedure estimations are mandatory, as incorrect evaluations may result in inappropriate therapy and residual cancer.18
Other factors that have been used to predict lymph node involvement include tumor size, macroscopic appearance, histologic differentiation, and lymphatic and vascular involvement.19 Some of these factors can be assessed by special endoscopic techniques (chromoendoscopy and narrow-band imaging with magnifying endoscopy) that allow accurate real-time estimation of the depth of invasion of the lesion.5,17,20–27 Evaluation of microsurface and microvascular arrangements is especially useful for determining the feasibility of ESD in gastric tumors, evaluation of intracapillary loops is useful in esophageal lesions, and assessment of mucosal pit patterns is useful for colorectal lesions.21–29
Endoscopic ultrasonography is another tool that has been used to estimate the depth of the tumor. Although it can differentiate between definite intramucosal and definite submucosal invasive cancers, its ability to confirm minute submucosal invasion is limited. Its use as the sole tumor staging modality is not encouraged, and it should always be used in conjunction with endoscopic evaluation.18
Though the aforementioned factors help stratify patients, pathologic staging is the best predictor of lymph node metastasis. ESD provides adequate specimens for accurate pathologic evaluation, as it removes lesions en bloc.30
All patients found to have risk factors for lymph node metastasis on endoscopic, ultrasonographic, or pathologic analysis should be referred for surgical evaluation.9,19,31,32
ENDOSCOPIC SUBMUCOSAL DISSECTION
Before the procedure, the patient’s physicians need to do the following:
Determine the best type of intervention (EMR, ESD, ablation, surgery) for the specific lesion.3 A multidisciplinary approach is encouraged, with involvement of the internist, gastroenterologist, and surgeon.
Plan for anesthesia, additional consultations, pre- and postprocedural hospital admission, and need for special equipment.33
During the procedure
Define the lateral extent of the lesion using magnification chromoendoscopy or narrow-band imaging. In the stomach, a biopsy sample should be taken from the worst-looking segment and from normal-looking mucosa. Multiple biopsies should be avoided to prevent subsequent fibrosis.33 In the colon, biopsy should be avoided.34
Identify and circumferentially mark the target lesion. Cautery or argon plasma coagulation can be used for making markings at a distance of 5 to 10 mm from the edges.33 This is done to recognize the borders of the lesion, because they can become distorted after submucosal injection.14 This step is unnecessary in colorectal cases, as tumor margins can be adequately visualized after chromoendoscopy.16,35
Lift the lesion by injecting saline, 0.5% hyaluronate, or glycerin to create a submucosal fluid cushion.19,33
Perform a circumferential incision lateral to the mucosal margins to allow for a normal tissue margin.33 Partial incision is performed for esophageal and colorectal ESD to avoid fluid leakage from the submucosal layer, achieving a sustained submucosal lift and safer dissection.16
Submucosal dissection. The submucosal layer is dissected with an electrocautery knife until the lesion is completely removed. Dissection should be done carefully to keep the submucosal plane.33 Hemoclips or hemostat forceps can be used to control visible bleeding. The resected specimen is then stretched and fixed to a board using small pins for further histopathologic evaluation.35
Postprocedural monitoring. All patients should be admitted for overnight observation. Those who undergo gastric ESD should receive high-dose acid suppression, and the next day they can be started on a liquid diet.19
STOMACH CANCER
Indications for ESD for stomach cancer in the East
The incidence of gastric cancer is higher in Japan and Korea, where widespread screening programs have led to early identification and early treatment of this disease.36
Pathology studies37 of samples from patients with gastric cancer identified the following as risk factors for lymph node metastasis, which would make ESD unsuitable:
- Undifferentiated type
- Tumors larger than 2 cm
- Lymphatic or venous involvement
- Submucosal invasion
- Ulcerative change.
Based on these findings, the situations in which there was no risk of lymph node involvement (ie, when none of the above factors are present) were accepted as absolute indications for endoscopic resection of early gastric cancer.38 Further histologic studies identified a subset of patients with lesions with very low risk of lymph node metastasis, which outweighed the risk of surgery. Based on these findings, expanded criteria for gastric ESD were proposed,39,40 and the Japanese gastric cancer treatment guidelines now include these expanded preoperative indications9,17 (Table 1).
The Japanese Gastric Cancer Association has proposed a treatment algorithm based on the histopathologic evaluation after resection (Figure 2).9
Outcomes
In the largest series of patients who underwent curative ESD for early gastric cancer, the 5-year survival rate was 92.6%, the 5-year disease-specific survival rate was 99.9%, and the 5-year relative survival rate was 105%.41
Similarly, in a Japanese population-based survival analysis, the relative 5-year survival rate for localized gastric cancer was 94.4%.42 Rates of en bloc resection and complete resection with ESD are higher than those with EMR, resulting in a lower risk of local recurrence in selected patients who undergo ESD.8,43,44
Although rare, local recurrence after curative gastric ESD has been reported.45 The annual incidence of local recurrence has been estimated to be 0.84%.46
ESD entails a shorter hospital stay and requires fewer resources than surgery, resulting in lower medical costs (Table 2).44 Additionally, as endoscopic resection is associated with less morbidity, fewer procedure-related adverse events, and fewer complications, ESD could be used as the standard treatment for early gastric cancer.47,48
The Western perspective on endoscopic submucosal dissection for gastric cancer
Since the prevalence of gastric cancer in Western countries is significantly lower than in Japan and Korea, local data and experience are scarce. However, experts performing ESD in the West have adopted the indications of the Japan Gastroenterological Endoscopy Society. The European Society of Gastrointestinal Endoscopy recommends ESD for excision of most superficial gastric neoplasms, with EMR being preferred only in lesions smaller than 15 mm, Paris classification 0 or IIA.5,32
Patients with gastric lesions measuring 15 mm or larger should undergo high-quality endoscopy, preferably chromoendoscopy, to evaluate the mucosal patterns and determine the depth of invasion. If superficial involvement is confirmed, other imaging techniques are not routinely recommended.5 A surgery consult is also recommended.
ESOPHAGEAL CANCER
Indications for ESD for esophageal cancer in the East
Due to the success of ESD for early gastric cancer, this technique is now also used for superficial esophageal neoplasms.19,49 It should be done in a specialized center, as it is more technically difficult than gastric ESD: the esophageal lumen is narrow, the wall is thin, and the esophagus moves with respiration and heartbeat.50 A multidisciplinary approach including an endoscopist, a surgeon, and a pathologist is highly recommended for evaluation and treatment.
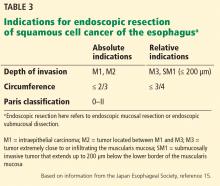
EMR is preferred for removal of mucosal cancer, in view of its safety profile and success rates. ESD can be considered in cases of lesions larger than 15 mm, poorly lifting tumors, and those with the possibility of submucosal invasion (Table 3).5,45,49,51
Circumference involvement is critical when determining eligible candidates, as a defect involving more than three-fourths of the esophageal circumference can lead to esophageal strictures.52 Controlled prospective studies have shown promising results from giving intralesional and oral steroids to prevent stricture after ESD, which could potentially overcome this size limitation.53,54
Outcomes for esophageal cancer
ESD has been shown to be safe and effective, achieving en bloc resection in 85% to 100% of patients.19,51 Its advantages over EMR include en bloc resection, complete resection, and high curative rates, resulting in higher recurrence-free survival.2,55,56 Although the incidence of complications such as bleeding, perforation, and stricture formation are higher with ESD, patients usually recover uneventfully.2,19,20
ESD in the esophagus: The Western perspective
As data on the efficacy of EMR vs ESD for the treatment of Barrett esophagus with adenocarcinoma are limited, EMR is the gold standard endoscopic technique for removal of visible esophageal dysplastic lesions.5,51,57 ESD can be considered for tumors larger than 15 mm, for poorly lifting lesions, and if there is suspicion of submucosal invasion.5
Patients should be evaluated by an experienced endoscopist, using an advanced imaging technique such as narrow-band imaging or chromoendoscopy. If suspicious features are found, endoscopic ultrasonography should be considered to confirm submucosal invasion or lymph node involvement.5
COLORECTAL CANCER
Indications for ESD for colorectal cancer in the East
Colon cancer is one of the leading causes of cancer-related deaths worldwide.58 Since ESD has been found to be effective and safe in treating gastric cancer, it has also been used to remove large colorectal tumors.59 However, ESD is not universally accepted in the treatment of colorectal neoplasms due to its greater technical difficulty, longer procedural time, and higher risk of perforating the thinner colonic wall compared with EMR.21,60
Outcomes for colorectal cancer
Tumor size of 50 mm or larger is a risk factor for complications, while a high procedure volume at the center is a protective factor.60
Endoscopic treatment of colorectal cancer: The Western perspective
EMR is the gold standard for removal of superficial colorectal lesions. However, ESD can be considered if there is suspicion of superficial submucosal invasion, especially for lesions larger than 20 mm that cannot be resected en bloc by EMR.32 ESD can also be used for fibrotic lesions not amenable to complete EMR removal, or as a salvage procedure after recurrence after EMR.67 Proper selection of cases is critical.1
Patients who have a superficial colonic lesion should be evaluated by means of high-definition endoscopy and chromoendoscopy to assess the mucosal pattern and establish feasibility of endoscopic resection. If submucosal invasion is suspected, staging with endoscopic ultrasonography or magnetic resonance imaging should be considered.5
FOLLOW-UP AFTER ESD
Endoscopic surveillance after the procedure is recommended, given the persistent risk of metachronous cancer after curative ESD due to its organ-sparing quality.68 Surveillance endoscopy aims to achieve early detection and subsequent endoscopic resection of metachronous lesions.
Histopathologic evaluation assessing the presence of malignant cells in the margins of a resected sample is mandatory for determining the next step in treatment. If margins are negative, follow-up endoscopy can be done every 6 to 12 months. If margins are positive, the approach includes surgery, reattempting ESD or endoscopic surveillance in 3 or 6 months.3,32 Although the surveillance strategy varies according to individual risk of metachronous cancer, it should be continued indefinitely.68
COMPLICATIONS OF ESD
The most common procedure-related complications of ESD are bleeding, perforation, and stricture. Most intraprocedural adverse events can be managed endoscopically.69
Bleeding
Most bleeding occurs during the procedure or early after it and can be controlled with electrocautery.49,69 No episodes of massive bleeding, defined as causing clinical symptoms and requiring transfusion or surgery, have been reported.20,43,55
In gastric ESD, delayed bleeding rates have ranged from 0 to 15.6%.69 Bleeding may be prevented with endoscopic coagulation of visible vessels after dissection has been completed and by proton pump inhibitor therapy.70,71 Excessive coagulation should be avoided to lower the risk of perforation.33
In colorectal ESD the bleeding rate has been reported to be 2.2%; applying coagulation to an area where a blood vessel is suspected before cutting (precoagulation) may prevent subsequent bleeding.21
Perforation
For gastric ESD, perforation rates range from 1.2% to 5.2%.69 Esophageal perforation rates can be up to 4%.49 In colorectal ESD, perforation rates have been reported to be 1.6% to 6.6%.60,72
Although most of the cases were successfully managed with conservative treatment, some required emergency surgery.60,73
Strictures
In a case series of 532 patients undergoing gastric ESD, stricture was reported in 5 patients, all of whom presented with obstructive symptoms.74 Risk factors for post-ESD gastric stenosis are a mucosal defect with a circumferential extent of more than three-fourths or a longitudinal extent of more than 5 cm.75
Strictures are common after esophageal ESD, with rates ranging from 2% to 26%. The risk is higher when longer segments are removed or circumferential resection is performed. As previously mentioned, this complication may be reduced with ingestion or injection of steroids after the procedure.53,54
Surprisingly, ESD of large colorectal lesions involving more than three-fourths of the circumference of the rectum is rarely complicated by stenosis.76
LIMITATIONS OF ESD
ESD requires a high level of technical skill, is time-consuming, and has a higher rate of complications than conventional endoscopic resection. A standardized ESD training system is needed, as the procedure is more difficult than EMR. Training in porcine models has been shown to confer competency in ESD in a Western setting.13,16,33
Colorectal ESD is an even more challenging procedure, given the potential for complications related to its anatomy. Training centers in Japan usually have their trainees first master gastric ESD, then assist in more than 20 colorectal ESDs conducted by experienced endoscopists, and accomplish 30 cases before performing the procedure safely and independently.
As the incidence of gastric cancer is low in Western countries, trainees may also begin with lower rectal lesions, which are easier to remove.77 Incorporation of ESD in the West would require a clear treatment algorithm. It is a complex procedure, with higher rates of complications, a prolonged learning curve, and prolonged procedure time. Therefore, it should be performed in specialized centers and under the special situations discussed here to ensure that the benefits for the patients outweigh the risks.
VALUE OF ENDOSCOPIC SUBMUCOSAL DISSECTION
The optimal method for resecting gastrointestinal neoplasms should be safe, cost-effective, and quick and should also completely remove the lesion. The best treatment strategy takes into account the characteristics of the lesion and the comorbidities and wishes of the patient. Internists should be aware of the multiple options available to achieve the best outcome for the patient.1
Endoscopic resection of superficial gastrointestinal neoplasms, including EMR and ESD, has been a subject of increasing interest due to its minimally invasive and potentially curative character. However, cancer can recur after endoscopic resection because the procedure is organ-sparing.
ESD allows resection of early gastrointestinal tumors with a minimally invasive technique. It can achieve higher curative resection rates and lower recurrence rates compared with EMR. Compared with surgery, ESD leads to less morbidity, fewer procedure-related complications, and lower medical costs. Indications should be rigorously followed to achieve successful treatments in selected patients.
Multiple variables have to be taken into account when deciding which treatment is best, such as tumor characteristics, the patient’s baseline condition, physician expertise, and hospital resources.48 Less-invasive treatments may improve the prognosis of patients. No matter the approach, patients should be treated in specialized treatment centers.
Internal medicine physicians should be aware of the advances in treatments for early gastrointestinal cancer so appropriate options can be considered.
The treatment of early esophageal, gastric, and colorectal cancer is changing.1 For many years, surgery was the mainstay of treatment for early-stage gastrointestinal cancer. Unfortunately, surgery leads to significant loss of function of the organ, resulting in increased morbidity and decreased quality of life.2
Endoscopic techniques, particularly endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), have been developed and are widely used in Japan, where gastrointestinal cancer is more common than in the West. This article reviews the indications, complications, and outcomes of ESD for early gastrointestinal neoplasms, so that readers will recognize the subset of patients who would benefit from ESD in a Western setting.
ENDOSCOPIC MUCOSAL RESECTION AND SUBMUCOSAL DISSECTION
Since the first therapeutic polypectomy was performed in Japan in 1974, several endoscopic techniques for tumor resection have been developed.3
EMR, one of the most successful and widely used techniques, involves elevating the lesion either with submucosal injection of a solution or with cap suction, and then removing it with a snare.4 Most lesions smaller than 20 mm can be removed in one piece (en bloc).5 Larger lesions are removed in multiple pieces (ie, piecemeal). Unfortunately, some fibrotic lesions, which are usually difficult to lift, cannot be completely removed by EMR.
ESD was first performed in the late 1990s with the aim of overcoming the limitations of EMR in resecting large or fibrotic tumors en bloc.6,7 Since then, ESD technique has been standardized and training centers have been created, especially in Asia, where it is widely used for treatment of early gastric cancer.3,8–10 Since 2012 it has been covered by the Japanese National Health Insurance for treatment of early gastric cancer, and since 2014 for treatment of colorectal malignant tumors measuring 2 to 5 cm.11
Adoption of ESD has been slow in Western countries, where many patients are still referred for surgery or undergo EMR for removal of superficial neoplasms. Reasons for this slow adoption are that gastric cancer is much less common in Western countries, and also that ESD demands a high level of technical skill, is difficult to learn, and is expensive.3,12,13 However, small groups of Western endoscopists have become interested and are advocating it, first studying it on their own and then training in a Japanese center and learning from experts performing the procedure.
Therefore, in a Western setting, ESD should be performed in specialized endoscopy centers and offered to selected patients.1
CANDIDATES SHOULD HAVE EARLY-STAGE, SUPERFICIAL TUMORS
Ideal candidates for endoscopic resection are patients who have early cancer with a negligible risk of lymph node metastasis, such as cancer limited to the mucosa (stage T1a).7 Therefore, to determine the best treatment for a patient with a newly diagnosed gastrointestinal neoplasm, it is mandatory to estimate the depth of invasion.
The depth of invasion is directly correlated with lymph node involvement, which is ultimately the main predictive factor for long-term adverse outcomes of gastrointestinal tumors.4,14–17 Accurate multidisciplinary preprocedure estimations are mandatory, as incorrect evaluations may result in inappropriate therapy and residual cancer.18
Other factors that have been used to predict lymph node involvement include tumor size, macroscopic appearance, histologic differentiation, and lymphatic and vascular involvement.19 Some of these factors can be assessed by special endoscopic techniques (chromoendoscopy and narrow-band imaging with magnifying endoscopy) that allow accurate real-time estimation of the depth of invasion of the lesion.5,17,20–27 Evaluation of microsurface and microvascular arrangements is especially useful for determining the feasibility of ESD in gastric tumors, evaluation of intracapillary loops is useful in esophageal lesions, and assessment of mucosal pit patterns is useful for colorectal lesions.21–29
Endoscopic ultrasonography is another tool that has been used to estimate the depth of the tumor. Although it can differentiate between definite intramucosal and definite submucosal invasive cancers, its ability to confirm minute submucosal invasion is limited. Its use as the sole tumor staging modality is not encouraged, and it should always be used in conjunction with endoscopic evaluation.18
Though the aforementioned factors help stratify patients, pathologic staging is the best predictor of lymph node metastasis. ESD provides adequate specimens for accurate pathologic evaluation, as it removes lesions en bloc.30
All patients found to have risk factors for lymph node metastasis on endoscopic, ultrasonographic, or pathologic analysis should be referred for surgical evaluation.9,19,31,32
ENDOSCOPIC SUBMUCOSAL DISSECTION
Before the procedure, the patient’s physicians need to do the following:
Determine the best type of intervention (EMR, ESD, ablation, surgery) for the specific lesion.3 A multidisciplinary approach is encouraged, with involvement of the internist, gastroenterologist, and surgeon.
Plan for anesthesia, additional consultations, pre- and postprocedural hospital admission, and need for special equipment.33
During the procedure
Define the lateral extent of the lesion using magnification chromoendoscopy or narrow-band imaging. In the stomach, a biopsy sample should be taken from the worst-looking segment and from normal-looking mucosa. Multiple biopsies should be avoided to prevent subsequent fibrosis.33 In the colon, biopsy should be avoided.34
Identify and circumferentially mark the target lesion. Cautery or argon plasma coagulation can be used for making markings at a distance of 5 to 10 mm from the edges.33 This is done to recognize the borders of the lesion, because they can become distorted after submucosal injection.14 This step is unnecessary in colorectal cases, as tumor margins can be adequately visualized after chromoendoscopy.16,35
Lift the lesion by injecting saline, 0.5% hyaluronate, or glycerin to create a submucosal fluid cushion.19,33
Perform a circumferential incision lateral to the mucosal margins to allow for a normal tissue margin.33 Partial incision is performed for esophageal and colorectal ESD to avoid fluid leakage from the submucosal layer, achieving a sustained submucosal lift and safer dissection.16
Submucosal dissection. The submucosal layer is dissected with an electrocautery knife until the lesion is completely removed. Dissection should be done carefully to keep the submucosal plane.33 Hemoclips or hemostat forceps can be used to control visible bleeding. The resected specimen is then stretched and fixed to a board using small pins for further histopathologic evaluation.35
Postprocedural monitoring. All patients should be admitted for overnight observation. Those who undergo gastric ESD should receive high-dose acid suppression, and the next day they can be started on a liquid diet.19
STOMACH CANCER
Indications for ESD for stomach cancer in the East
The incidence of gastric cancer is higher in Japan and Korea, where widespread screening programs have led to early identification and early treatment of this disease.36
Pathology studies37 of samples from patients with gastric cancer identified the following as risk factors for lymph node metastasis, which would make ESD unsuitable:
- Undifferentiated type
- Tumors larger than 2 cm
- Lymphatic or venous involvement
- Submucosal invasion
- Ulcerative change.
Based on these findings, the situations in which there was no risk of lymph node involvement (ie, when none of the above factors are present) were accepted as absolute indications for endoscopic resection of early gastric cancer.38 Further histologic studies identified a subset of patients with lesions with very low risk of lymph node metastasis, which outweighed the risk of surgery. Based on these findings, expanded criteria for gastric ESD were proposed,39,40 and the Japanese gastric cancer treatment guidelines now include these expanded preoperative indications9,17 (Table 1).
The Japanese Gastric Cancer Association has proposed a treatment algorithm based on the histopathologic evaluation after resection (Figure 2).9
Outcomes
In the largest series of patients who underwent curative ESD for early gastric cancer, the 5-year survival rate was 92.6%, the 5-year disease-specific survival rate was 99.9%, and the 5-year relative survival rate was 105%.41
Similarly, in a Japanese population-based survival analysis, the relative 5-year survival rate for localized gastric cancer was 94.4%.42 Rates of en bloc resection and complete resection with ESD are higher than those with EMR, resulting in a lower risk of local recurrence in selected patients who undergo ESD.8,43,44
Although rare, local recurrence after curative gastric ESD has been reported.45 The annual incidence of local recurrence has been estimated to be 0.84%.46
ESD entails a shorter hospital stay and requires fewer resources than surgery, resulting in lower medical costs (Table 2).44 Additionally, as endoscopic resection is associated with less morbidity, fewer procedure-related adverse events, and fewer complications, ESD could be used as the standard treatment for early gastric cancer.47,48
The Western perspective on endoscopic submucosal dissection for gastric cancer
Since the prevalence of gastric cancer in Western countries is significantly lower than in Japan and Korea, local data and experience are scarce. However, experts performing ESD in the West have adopted the indications of the Japan Gastroenterological Endoscopy Society. The European Society of Gastrointestinal Endoscopy recommends ESD for excision of most superficial gastric neoplasms, with EMR being preferred only in lesions smaller than 15 mm, Paris classification 0 or IIA.5,32
Patients with gastric lesions measuring 15 mm or larger should undergo high-quality endoscopy, preferably chromoendoscopy, to evaluate the mucosal patterns and determine the depth of invasion. If superficial involvement is confirmed, other imaging techniques are not routinely recommended.5 A surgery consult is also recommended.
ESOPHAGEAL CANCER
Indications for ESD for esophageal cancer in the East
Due to the success of ESD for early gastric cancer, this technique is now also used for superficial esophageal neoplasms.19,49 It should be done in a specialized center, as it is more technically difficult than gastric ESD: the esophageal lumen is narrow, the wall is thin, and the esophagus moves with respiration and heartbeat.50 A multidisciplinary approach including an endoscopist, a surgeon, and a pathologist is highly recommended for evaluation and treatment.
EMR is preferred for removal of mucosal cancer, in view of its safety profile and success rates. ESD can be considered in cases of lesions larger than 15 mm, poorly lifting tumors, and those with the possibility of submucosal invasion (Table 3).5,45,49,51
Circumference involvement is critical when determining eligible candidates, as a defect involving more than three-fourths of the esophageal circumference can lead to esophageal strictures.52 Controlled prospective studies have shown promising results from giving intralesional and oral steroids to prevent stricture after ESD, which could potentially overcome this size limitation.53,54
Outcomes for esophageal cancer
ESD has been shown to be safe and effective, achieving en bloc resection in 85% to 100% of patients.19,51 Its advantages over EMR include en bloc resection, complete resection, and high curative rates, resulting in higher recurrence-free survival.2,55,56 Although the incidence of complications such as bleeding, perforation, and stricture formation are higher with ESD, patients usually recover uneventfully.2,19,20
ESD in the esophagus: The Western perspective
As data on the efficacy of EMR vs ESD for the treatment of Barrett esophagus with adenocarcinoma are limited, EMR is the gold standard endoscopic technique for removal of visible esophageal dysplastic lesions.5,51,57 ESD can be considered for tumors larger than 15 mm, for poorly lifting lesions, and if there is suspicion of submucosal invasion.5
Patients should be evaluated by an experienced endoscopist, using an advanced imaging technique such as narrow-band imaging or chromoendoscopy. If suspicious features are found, endoscopic ultrasonography should be considered to confirm submucosal invasion or lymph node involvement.5
COLORECTAL CANCER
Indications for ESD for colorectal cancer in the East
Colon cancer is one of the leading causes of cancer-related deaths worldwide.58 Since ESD has been found to be effective and safe in treating gastric cancer, it has also been used to remove large colorectal tumors.59 However, ESD is not universally accepted in the treatment of colorectal neoplasms due to its greater technical difficulty, longer procedural time, and higher risk of perforating the thinner colonic wall compared with EMR.21,60
Outcomes for colorectal cancer
Tumor size of 50 mm or larger is a risk factor for complications, while a high procedure volume at the center is a protective factor.60
Endoscopic treatment of colorectal cancer: The Western perspective
EMR is the gold standard for removal of superficial colorectal lesions. However, ESD can be considered if there is suspicion of superficial submucosal invasion, especially for lesions larger than 20 mm that cannot be resected en bloc by EMR.32 ESD can also be used for fibrotic lesions not amenable to complete EMR removal, or as a salvage procedure after recurrence after EMR.67 Proper selection of cases is critical.1
Patients who have a superficial colonic lesion should be evaluated by means of high-definition endoscopy and chromoendoscopy to assess the mucosal pattern and establish feasibility of endoscopic resection. If submucosal invasion is suspected, staging with endoscopic ultrasonography or magnetic resonance imaging should be considered.5
FOLLOW-UP AFTER ESD
Endoscopic surveillance after the procedure is recommended, given the persistent risk of metachronous cancer after curative ESD due to its organ-sparing quality.68 Surveillance endoscopy aims to achieve early detection and subsequent endoscopic resection of metachronous lesions.
Histopathologic evaluation assessing the presence of malignant cells in the margins of a resected sample is mandatory for determining the next step in treatment. If margins are negative, follow-up endoscopy can be done every 6 to 12 months. If margins are positive, the approach includes surgery, reattempting ESD or endoscopic surveillance in 3 or 6 months.3,32 Although the surveillance strategy varies according to individual risk of metachronous cancer, it should be continued indefinitely.68
COMPLICATIONS OF ESD
The most common procedure-related complications of ESD are bleeding, perforation, and stricture. Most intraprocedural adverse events can be managed endoscopically.69
Bleeding
Most bleeding occurs during the procedure or early after it and can be controlled with electrocautery.49,69 No episodes of massive bleeding, defined as causing clinical symptoms and requiring transfusion or surgery, have been reported.20,43,55
In gastric ESD, delayed bleeding rates have ranged from 0 to 15.6%.69 Bleeding may be prevented with endoscopic coagulation of visible vessels after dissection has been completed and by proton pump inhibitor therapy.70,71 Excessive coagulation should be avoided to lower the risk of perforation.33
In colorectal ESD the bleeding rate has been reported to be 2.2%; applying coagulation to an area where a blood vessel is suspected before cutting (precoagulation) may prevent subsequent bleeding.21
Perforation
For gastric ESD, perforation rates range from 1.2% to 5.2%.69 Esophageal perforation rates can be up to 4%.49 In colorectal ESD, perforation rates have been reported to be 1.6% to 6.6%.60,72
Although most of the cases were successfully managed with conservative treatment, some required emergency surgery.60,73
Strictures
In a case series of 532 patients undergoing gastric ESD, stricture was reported in 5 patients, all of whom presented with obstructive symptoms.74 Risk factors for post-ESD gastric stenosis are a mucosal defect with a circumferential extent of more than three-fourths or a longitudinal extent of more than 5 cm.75
Strictures are common after esophageal ESD, with rates ranging from 2% to 26%. The risk is higher when longer segments are removed or circumferential resection is performed. As previously mentioned, this complication may be reduced with ingestion or injection of steroids after the procedure.53,54
Surprisingly, ESD of large colorectal lesions involving more than three-fourths of the circumference of the rectum is rarely complicated by stenosis.76
LIMITATIONS OF ESD
ESD requires a high level of technical skill, is time-consuming, and has a higher rate of complications than conventional endoscopic resection. A standardized ESD training system is needed, as the procedure is more difficult than EMR. Training in porcine models has been shown to confer competency in ESD in a Western setting.13,16,33
Colorectal ESD is an even more challenging procedure, given the potential for complications related to its anatomy. Training centers in Japan usually have their trainees first master gastric ESD, then assist in more than 20 colorectal ESDs conducted by experienced endoscopists, and accomplish 30 cases before performing the procedure safely and independently.
As the incidence of gastric cancer is low in Western countries, trainees may also begin with lower rectal lesions, which are easier to remove.77 Incorporation of ESD in the West would require a clear treatment algorithm. It is a complex procedure, with higher rates of complications, a prolonged learning curve, and prolonged procedure time. Therefore, it should be performed in specialized centers and under the special situations discussed here to ensure that the benefits for the patients outweigh the risks.
VALUE OF ENDOSCOPIC SUBMUCOSAL DISSECTION
The optimal method for resecting gastrointestinal neoplasms should be safe, cost-effective, and quick and should also completely remove the lesion. The best treatment strategy takes into account the characteristics of the lesion and the comorbidities and wishes of the patient. Internists should be aware of the multiple options available to achieve the best outcome for the patient.1
Endoscopic resection of superficial gastrointestinal neoplasms, including EMR and ESD, has been a subject of increasing interest due to its minimally invasive and potentially curative character. However, cancer can recur after endoscopic resection because the procedure is organ-sparing.
ESD allows resection of early gastrointestinal tumors with a minimally invasive technique. It can achieve higher curative resection rates and lower recurrence rates compared with EMR. Compared with surgery, ESD leads to less morbidity, fewer procedure-related complications, and lower medical costs. Indications should be rigorously followed to achieve successful treatments in selected patients.
Multiple variables have to be taken into account when deciding which treatment is best, such as tumor characteristics, the patient’s baseline condition, physician expertise, and hospital resources.48 Less-invasive treatments may improve the prognosis of patients. No matter the approach, patients should be treated in specialized treatment centers.
Internal medicine physicians should be aware of the advances in treatments for early gastrointestinal cancer so appropriate options can be considered.
- Burgess NG, Bourke MJ. Endoscopic resection of colorectal lesions: the narrowing divide between East and West. Dig Endosc 2016; 28:296–305.
- Kim DH, Jung HY, Gong EJ, et al. Endoscopic and oncologic outcomes of endoscopic resection for superficial esophageal neoplasm. Gut Liver 2015; 9:470–477.
- Draganov PV, Gotoda T, Chavalitdhamrong D, Wallace MB. Techniques of endoscopic submucosal dissection: application for the Western endoscopist? Gastrointest Endosc 2013; 78:677–688.
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011; 14:101–112.
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2015; 47:829–854.
- Farhat S, Chaussade S, Ponchon T, et al; SFED ESD Study Group. Endoscopic submucosal dissection in a European setting. A multi-institutional report of a technique in development. Endoscopy 2011; 43:664–670.
- Gotoda T, Jung H. Endoscopic resection (endoscopic mucosal resection/endoscopic submucosal dissection) for early gastric cancer. Dig Endosc 2013; 25(suppl 1):55–63.
- Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc 2009; 69:1228–1235.
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011; 14:113–123.
- Ono H. Endoscopic submucosal dissection for early gastric cancer. Chin J Dig Dis 2005; 6:119–121.
- Watanabe T, Itabashi M, Shimada Y, et al; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol 2015; 20:207–239.
- Oyama T, Yahagi N, Ponchon T, Kiesslich T, Berr F. How to establish endoscopic submucosal dissection in Western countries. World J Gastroenterol 2015; 21:11209–11220.
- Bhatt A, Abe S, Kumaravel A, et al. SU1575 Western skill training in endoscopic submucosal dissection (ESD)—an international remote video based study—the WEST ESD Study. Gastrointest Endosc 2015; 81(suppl):AB335–AB336.
- Sano T, Sasako M, Kinoshita T, Maruyama K. Recurrence of early gastric cancer follow-up of 1475 patients and review of the Japanese literature. Cancer 1993; 72:3174–3178.
- Japan Esophageal Society. Japanese classification of esophageal cancer, tenth edition: part I. Esophagus 2009; 6:1–25.
- Bhatt A, Abe S, Kumaravel A, Vargo J, Saito Y. Indications and techniques for endoscopic submucosal dissection. Am J Gastroenterol 2015; 110:784–791.
- Eleftheriadis N, Inoue H, Ikeda H, et al. Definition and staging of early esophageal, gastric and colorectal cancer. J Tumor 2014; 2:161–178.
- Yoshinaga S, Oda I, Nonaka S, Kushima R, Saito Y. Endoscopic ultrasound using ultrasound probes for the diagnosis of early esophageal and gastric cancers. World J Gastrointest Endosc 2012; 4:218–226.
- Stahl M, Mariette C, Haustermans K, Cervantes A, Arnold D; ESMO Guidelines Working Group. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(suppl 6):vi51–vi56.
- Higuchi K, Tanabe S, Azuma M, et al. A phase II study of endoscopic submucosal dissection for superficial esophageal neoplasms (KDOG 0901). Gastrointest Endosc 2013; 78:704–710.
- Sakamoto T, Mori G, Yamada M, et al. Endoscopic submucosal dissection for colorectal neoplasms: a review. World J Gastroenterol 2014; 20:16153–16158.
- Ohta A, Tominaga K, Sakai Y. Efficacy of magnifying colonoscopy for the diagnosis of colorectal neoplasia: comparison with histopathological findings. Dig Endosc 2004; 16:308–314.
- Katagiri A, Fu K, Sano Y, et al. Narrow band imaging with magnifying colonoscopy as diagnostic tool for predicting histology of early colorectal neoplasia. Aliment Pharmacol Ther 2008; 27:1269–1274.
- Fu KI, Kato S, Sano Y, et al. Staging of early colorectal cancers: magnifying colonoscopy versus endoscopic ultrasonography for estimation of depth of invasion. Dig Dis Sci 2008; 53:1886–1892.
- Uraoka T, Saito Y, Ikematsu H, Yamamoto K, Sano Y. Sano’s capillary pattern classification for narrow-band imaging of early colorectal lesions. Dig Endosc 2011; 23(suppl 1):112–115.
- Ikematsu H, Matsuda T, Emura F, et al. Efficacy of capillary pattern type IIIA/IIIB by magnifying narrow band imaging for estimating depth of invasion of early colorectal neoplasms. BMC Gastroenterol 2010;10:33.
- Matsuda T, Fujii T, Saito Y, et al. Efficacy of the invasive/non-invasive pattern by magnifying chromoendoscopy to estimate the depth of invasion of early colorectal neoplasms. Am J Gastroenterol 2008; 103:2700–2706.
- Sato H, Inoue H, Ikeda H, et al. Utility of intrapapillary capillary loops seen on magnifying narrow-band imaging in estimating invasive depth of esophageal squamous cell carcinoma. Endoscopy 2015; 8:122–128.
- Muto M, Yao K, Kaise M, et al. Magnifying endoscopy simple diagnostic algorithm for early gastric cancer (MESDA-G). Dig Endosc 2016; 28:379–393.
- Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D; European Society for Medical Oncology (ESMO); European Society of Surgical Oncology (ESSO); European Society of Radiotherapy and Oncology (ESTRO). Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(suppl 6):vi57–vi63.
- Kuwano H, Nishimura Y, Ohtsu A, et al. Guidelines for diagnosis and treatment of carcinoma of the esophagus. April 2007 edition: part I - Edited by the Japan Esophageal Society. Esophagus 2008; 5:61–73.
- Tanaka S, Kashida H, Saito Y, et al. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc 2015; 27:417–434.
- Gotoda T, Ho KY, Soetikno R, Kaltenbach T, Draganov P. Gastric ESD: current status and future directions of devices and training. Gastrointest Endosc Clin North Am 2014; 24:213–233.
- Saito Y, Sakamoto T, Nakajima T, Matsuda T. Colorectal ESD: current indications and latest technical advances. Gastrointest Endosc Clin N Am 2014; 24:245–255.
- Saito Y, Otake Y, Sakamoto T, et al. Indications for and technical aspects of colorectal endoscopic submucosal dissection. Gut Liver 2013; 7:263–269.
- Saragoni L. Upgrading the definition of early gastric cancer: better staging means more appropriate treatment. Cancer Biol Med 2015; 12:355–361.
- Tsujitani S, Oka S, Saito H, et al. Less invasive surgery for early gastric cancer based on the low probability of lymph node metastasis. Surgery 1999; 125:148–154.
- Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc 2003; 57:567–579.
- Hirasawa T, Gotoda T, Miyata S, et al. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer 2009; 12:148–152.
- Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000; 3:219–225.
- Suzuki H, Oda I, Abe S, et al. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer 2016; 19:198–205.
- Matsuda T, Ajiki W, Marugame T, Ioka A, Tsukuma H, Sobue T; Research Group of Population-Based Cancer Registries of Japan. Population-based survival of cancer patients diagnosed between 1993 and 1999 in Japan: a chronological and international comparative study. Jpn J Clin Oncol 2011; 41:40–51.
- Ahn JY, Jung HY, Choi KD, et al. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc 2011; 74:485–493.
- Kim Y, Kim YW, Choi IJ, et al. Cost comparison between surgical treatments and endoscopic submucosal dissection in patients with early gastric cancer in Korea. Gut Liver 2015; 9:174–180.
- Abe S, Oda I, Nakajima T, et al. A case of local recurrence and distant metastasis following curative endoscopic submucosal dissection of early gastric cancer. Gastric Cancer 2015; 18:188–192.
- Hahn KY, Park JC, Kim EH, et al. Incidence and impact of scheduled endoscopic surveillance on recurrence after curative endoscopic resection for early gastric cancer. Gastrointest Endosc 2016; 84:628–638.e1.
- Wang S, Zhang Z, Liu M, Li S, Jiang C. Endoscopic resection compared with gastrectomy to treat early gastric cancer: a systematic review and meta-analysis. PLoS One 2015; 10:e0144774.
- Kondo A, de Moura EG, Bernardo WM, et al. Endoscopy vs surgery in the treatment of early gastric cancer: systematic review. World J Gastroenterol 2015; 21:13177–13187.
- Kothari S, Kaul V. Endoscopic mucosal resection and endoscopic submucosal dissection for endoscopic therapy of Barrett’s esophagus-related neoplasia. Gastroenterol Clin North Am 2015; 44:317–335.
- Yamashita T, Zeniya A, Ishii H, et al. Endoscopic mucosal resection using a cap-fitted panendoscope and endoscopic submucosal dissection as optimal endoscopic procedures for superficial esophageal carcinoma. Surg Endosc 2011; 25:2541–2546.
- Kagemoto K, Oka S, Tanaka S, et al. Clinical outcomes of endoscopic submucosal dissection for superficial Barrett’s adenocarcinoma. Gastrointest Endosc 2014; 80:239–245.
- Katada C, Muto M, Manabe T, Boku N, Ohtsu A, Yoshida S. Esophageal stenosis after endoscopic mucosal resection of superficial esophageal lesions. Gastrointest Endosc 2003; 57:165–169.
- Hanaoka N, Ishihara R, Takeuchi Y, et al. 1139: A single session of intralesional steroid injection to prevent esophageal stricture after endoscopic submucosal dissection for esophageal squamous cell carcinoma. Gastrointest Endosc 2012; 75(suppl):AB175.
- Yamaguchi N, Isomoto H, Nakayama T, et al. Usefulness of oral prednisolone in the treatment of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Gastrointest Endosc 2011; 73:1115–1121.
- Ono S, Fujishiro M, Niimi K, et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc 2009; 70:860–866.
- Katada C, Muto M, Manabe T, Ohtsu A, Yoshida S. Local recurrence of squamous-cell carcinoma of the esophagus after EMR. Gastrointest Endosc 2005; 61:219–225.
- Hirasawa K, Kokawa A, Oka H, et al. Superficial adenocarcinoma of the esophagogastric junction: long-term results of endoscopic submucosal dissection. Gastrointest Endosc 2010; 72:960–966.
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
- Nakajima T, Saito Y, Tanaka S, et al. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg Endosc 2013; 27:3262–3770.
- Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc 2010; 72:1217–1225.
- Tanaka S, Saitoh Y, Matsuda T, et al; Japanese Society of Gastroenterology. Evidence-based clinical practice guidelines for management of colorectal polyps. J Gastroenterol 2015; 50:252–260.
- Oka S, Tanaka S, Saito Y, et al; Colorectal Endoscopic Resection Standardization Implementation Working Group of the Japanese Society for Cancer of the Colon and Rectum, Tokyo, Japan. Local recurrence after endoscopic resection for large colorectal neoplasia: a multicenter prospective study in Japan. Am J Gastroenterol 2015; 110:697–707.
- Saito Y, Fukuzawa M, Matsuda T, et al. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc 2010; 24:343–352.
- Makazu M, Sakamoto T, So E, et al. Relationship between indeterminate or positive lateral margin and local recurrence after endoscopic resection of colorectal polyps. Endosc Int Open 2015; 3:E252–E257.
- Belderbos TD, Leenders M, Moons LM, Siersema PD. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy 2014; 46:388–402.
- Fujiya M, Tanaka K, Dokoshi T, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc 2015; 81:583–595.
- Rahmi G, Tanaka S, Ohara Y, et al. Efficacy of endoscopic submucosal dissection for residual or recurrent superficial colorectal tumors after endoscopic mucosal resection. J Dig Dis 2015; 16:14–21.
- Abe S, Oda I, Suzuki H, et al. Long-term surveillance and treatment outcomes of metachronous gastric cancer occurring after curative endoscopic submucosal dissection. Endoscopy 2015; 47:1113–1118.
- Oda I, Suzuki H, Nonaka S, Yoshinaga S. Complications of gastric endoscopic submucosal dissection. Dig Endosc 2013; 25(suppl 1):71–78.
- Takizawa K, Oda I, Gotoda T, et al. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection—an analysis of risk factors. Endoscopy 2008; 40:179–183.
- Uedo N, Takeuchi Y, Yamada T, et al. Effect of a proton pump inhibitor or an H2-receptor antagonist on prevention of bleeding from ulcer after endoscopic submucosal dissection of early gastric cancer: a prospective randomized controlled trial. Am J Gastroenterol 2007; 102:1610–1616.
- Hayashi N, Tanaka S, Nishiyama S, et al. Predictors of incomplete resection and perforation associated with endoscopic submucosal dissection for colorectal tumors. Gastrointest Endosc 2014; 79:427–435.
- Suzuki H, Oda I, Sekiguchi M, et al. Management and associated factors of delayed perforation after gastric endoscopic submucosal dissection. World J Gastroenterol 2015; 21:12635–12643.
- Tsunada S, Ogata S, Mannen K, et al. Case series of endoscopic balloon dilation to treat a stricture caused by circumferential resection of the gastric antrum by endoscopic submucosal dissection. Gastrointest Endosc 2008; 67:979–983.
- Coda S, Oda I, Gotoda T, Yokoi C, Kikuchi T, Ono H. Risk factors for cardiac and pyloric stenosis after endoscopic submucosal dissection, and efficacy of endoscopic balloon dilation treatment. Endoscopy 2009; 41:421–426.
- Abe S, Sakamoto T, Takamaru H, et al. Stenosis rates after endoscopic submucosal dissection of large rectal tumors involving greater than three quarters of the luminal circumference. Surg Endosc 2016; 30:5459–5464.
- Sakamoto T, Saito Y, Fukunaga S, Nakajima T, Matsuda T. Learning curve associated with colorectal endoscopic submucosal dissection for endoscopists experienced in gastric endoscopic submucosal dissection. Dis Colon Rectum 2011; 54:1307–1312.
- Burgess NG, Bourke MJ. Endoscopic resection of colorectal lesions: the narrowing divide between East and West. Dig Endosc 2016; 28:296–305.
- Kim DH, Jung HY, Gong EJ, et al. Endoscopic and oncologic outcomes of endoscopic resection for superficial esophageal neoplasm. Gut Liver 2015; 9:470–477.
- Draganov PV, Gotoda T, Chavalitdhamrong D, Wallace MB. Techniques of endoscopic submucosal dissection: application for the Western endoscopist? Gastrointest Endosc 2013; 78:677–688.
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011; 14:101–112.
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2015; 47:829–854.
- Farhat S, Chaussade S, Ponchon T, et al; SFED ESD Study Group. Endoscopic submucosal dissection in a European setting. A multi-institutional report of a technique in development. Endoscopy 2011; 43:664–670.
- Gotoda T, Jung H. Endoscopic resection (endoscopic mucosal resection/endoscopic submucosal dissection) for early gastric cancer. Dig Endosc 2013; 25(suppl 1):55–63.
- Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc 2009; 69:1228–1235.
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011; 14:113–123.
- Ono H. Endoscopic submucosal dissection for early gastric cancer. Chin J Dig Dis 2005; 6:119–121.
- Watanabe T, Itabashi M, Shimada Y, et al; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol 2015; 20:207–239.
- Oyama T, Yahagi N, Ponchon T, Kiesslich T, Berr F. How to establish endoscopic submucosal dissection in Western countries. World J Gastroenterol 2015; 21:11209–11220.
- Bhatt A, Abe S, Kumaravel A, et al. SU1575 Western skill training in endoscopic submucosal dissection (ESD)—an international remote video based study—the WEST ESD Study. Gastrointest Endosc 2015; 81(suppl):AB335–AB336.
- Sano T, Sasako M, Kinoshita T, Maruyama K. Recurrence of early gastric cancer follow-up of 1475 patients and review of the Japanese literature. Cancer 1993; 72:3174–3178.
- Japan Esophageal Society. Japanese classification of esophageal cancer, tenth edition: part I. Esophagus 2009; 6:1–25.
- Bhatt A, Abe S, Kumaravel A, Vargo J, Saito Y. Indications and techniques for endoscopic submucosal dissection. Am J Gastroenterol 2015; 110:784–791.
- Eleftheriadis N, Inoue H, Ikeda H, et al. Definition and staging of early esophageal, gastric and colorectal cancer. J Tumor 2014; 2:161–178.
- Yoshinaga S, Oda I, Nonaka S, Kushima R, Saito Y. Endoscopic ultrasound using ultrasound probes for the diagnosis of early esophageal and gastric cancers. World J Gastrointest Endosc 2012; 4:218–226.
- Stahl M, Mariette C, Haustermans K, Cervantes A, Arnold D; ESMO Guidelines Working Group. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(suppl 6):vi51–vi56.
- Higuchi K, Tanabe S, Azuma M, et al. A phase II study of endoscopic submucosal dissection for superficial esophageal neoplasms (KDOG 0901). Gastrointest Endosc 2013; 78:704–710.
- Sakamoto T, Mori G, Yamada M, et al. Endoscopic submucosal dissection for colorectal neoplasms: a review. World J Gastroenterol 2014; 20:16153–16158.
- Ohta A, Tominaga K, Sakai Y. Efficacy of magnifying colonoscopy for the diagnosis of colorectal neoplasia: comparison with histopathological findings. Dig Endosc 2004; 16:308–314.
- Katagiri A, Fu K, Sano Y, et al. Narrow band imaging with magnifying colonoscopy as diagnostic tool for predicting histology of early colorectal neoplasia. Aliment Pharmacol Ther 2008; 27:1269–1274.
- Fu KI, Kato S, Sano Y, et al. Staging of early colorectal cancers: magnifying colonoscopy versus endoscopic ultrasonography for estimation of depth of invasion. Dig Dis Sci 2008; 53:1886–1892.
- Uraoka T, Saito Y, Ikematsu H, Yamamoto K, Sano Y. Sano’s capillary pattern classification for narrow-band imaging of early colorectal lesions. Dig Endosc 2011; 23(suppl 1):112–115.
- Ikematsu H, Matsuda T, Emura F, et al. Efficacy of capillary pattern type IIIA/IIIB by magnifying narrow band imaging for estimating depth of invasion of early colorectal neoplasms. BMC Gastroenterol 2010;10:33.
- Matsuda T, Fujii T, Saito Y, et al. Efficacy of the invasive/non-invasive pattern by magnifying chromoendoscopy to estimate the depth of invasion of early colorectal neoplasms. Am J Gastroenterol 2008; 103:2700–2706.
- Sato H, Inoue H, Ikeda H, et al. Utility of intrapapillary capillary loops seen on magnifying narrow-band imaging in estimating invasive depth of esophageal squamous cell carcinoma. Endoscopy 2015; 8:122–128.
- Muto M, Yao K, Kaise M, et al. Magnifying endoscopy simple diagnostic algorithm for early gastric cancer (MESDA-G). Dig Endosc 2016; 28:379–393.
- Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D; European Society for Medical Oncology (ESMO); European Society of Surgical Oncology (ESSO); European Society of Radiotherapy and Oncology (ESTRO). Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(suppl 6):vi57–vi63.
- Kuwano H, Nishimura Y, Ohtsu A, et al. Guidelines for diagnosis and treatment of carcinoma of the esophagus. April 2007 edition: part I - Edited by the Japan Esophageal Society. Esophagus 2008; 5:61–73.
- Tanaka S, Kashida H, Saito Y, et al. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc 2015; 27:417–434.
- Gotoda T, Ho KY, Soetikno R, Kaltenbach T, Draganov P. Gastric ESD: current status and future directions of devices and training. Gastrointest Endosc Clin North Am 2014; 24:213–233.
- Saito Y, Sakamoto T, Nakajima T, Matsuda T. Colorectal ESD: current indications and latest technical advances. Gastrointest Endosc Clin N Am 2014; 24:245–255.
- Saito Y, Otake Y, Sakamoto T, et al. Indications for and technical aspects of colorectal endoscopic submucosal dissection. Gut Liver 2013; 7:263–269.
- Saragoni L. Upgrading the definition of early gastric cancer: better staging means more appropriate treatment. Cancer Biol Med 2015; 12:355–361.
- Tsujitani S, Oka S, Saito H, et al. Less invasive surgery for early gastric cancer based on the low probability of lymph node metastasis. Surgery 1999; 125:148–154.
- Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc 2003; 57:567–579.
- Hirasawa T, Gotoda T, Miyata S, et al. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer 2009; 12:148–152.
- Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000; 3:219–225.
- Suzuki H, Oda I, Abe S, et al. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer 2016; 19:198–205.
- Matsuda T, Ajiki W, Marugame T, Ioka A, Tsukuma H, Sobue T; Research Group of Population-Based Cancer Registries of Japan. Population-based survival of cancer patients diagnosed between 1993 and 1999 in Japan: a chronological and international comparative study. Jpn J Clin Oncol 2011; 41:40–51.
- Ahn JY, Jung HY, Choi KD, et al. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc 2011; 74:485–493.
- Kim Y, Kim YW, Choi IJ, et al. Cost comparison between surgical treatments and endoscopic submucosal dissection in patients with early gastric cancer in Korea. Gut Liver 2015; 9:174–180.
- Abe S, Oda I, Nakajima T, et al. A case of local recurrence and distant metastasis following curative endoscopic submucosal dissection of early gastric cancer. Gastric Cancer 2015; 18:188–192.
- Hahn KY, Park JC, Kim EH, et al. Incidence and impact of scheduled endoscopic surveillance on recurrence after curative endoscopic resection for early gastric cancer. Gastrointest Endosc 2016; 84:628–638.e1.
- Wang S, Zhang Z, Liu M, Li S, Jiang C. Endoscopic resection compared with gastrectomy to treat early gastric cancer: a systematic review and meta-analysis. PLoS One 2015; 10:e0144774.
- Kondo A, de Moura EG, Bernardo WM, et al. Endoscopy vs surgery in the treatment of early gastric cancer: systematic review. World J Gastroenterol 2015; 21:13177–13187.
- Kothari S, Kaul V. Endoscopic mucosal resection and endoscopic submucosal dissection for endoscopic therapy of Barrett’s esophagus-related neoplasia. Gastroenterol Clin North Am 2015; 44:317–335.
- Yamashita T, Zeniya A, Ishii H, et al. Endoscopic mucosal resection using a cap-fitted panendoscope and endoscopic submucosal dissection as optimal endoscopic procedures for superficial esophageal carcinoma. Surg Endosc 2011; 25:2541–2546.
- Kagemoto K, Oka S, Tanaka S, et al. Clinical outcomes of endoscopic submucosal dissection for superficial Barrett’s adenocarcinoma. Gastrointest Endosc 2014; 80:239–245.
- Katada C, Muto M, Manabe T, Boku N, Ohtsu A, Yoshida S. Esophageal stenosis after endoscopic mucosal resection of superficial esophageal lesions. Gastrointest Endosc 2003; 57:165–169.
- Hanaoka N, Ishihara R, Takeuchi Y, et al. 1139: A single session of intralesional steroid injection to prevent esophageal stricture after endoscopic submucosal dissection for esophageal squamous cell carcinoma. Gastrointest Endosc 2012; 75(suppl):AB175.
- Yamaguchi N, Isomoto H, Nakayama T, et al. Usefulness of oral prednisolone in the treatment of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Gastrointest Endosc 2011; 73:1115–1121.
- Ono S, Fujishiro M, Niimi K, et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc 2009; 70:860–866.
- Katada C, Muto M, Manabe T, Ohtsu A, Yoshida S. Local recurrence of squamous-cell carcinoma of the esophagus after EMR. Gastrointest Endosc 2005; 61:219–225.
- Hirasawa K, Kokawa A, Oka H, et al. Superficial adenocarcinoma of the esophagogastric junction: long-term results of endoscopic submucosal dissection. Gastrointest Endosc 2010; 72:960–966.
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
- Nakajima T, Saito Y, Tanaka S, et al. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg Endosc 2013; 27:3262–3770.
- Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc 2010; 72:1217–1225.
- Tanaka S, Saitoh Y, Matsuda T, et al; Japanese Society of Gastroenterology. Evidence-based clinical practice guidelines for management of colorectal polyps. J Gastroenterol 2015; 50:252–260.
- Oka S, Tanaka S, Saito Y, et al; Colorectal Endoscopic Resection Standardization Implementation Working Group of the Japanese Society for Cancer of the Colon and Rectum, Tokyo, Japan. Local recurrence after endoscopic resection for large colorectal neoplasia: a multicenter prospective study in Japan. Am J Gastroenterol 2015; 110:697–707.
- Saito Y, Fukuzawa M, Matsuda T, et al. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc 2010; 24:343–352.
- Makazu M, Sakamoto T, So E, et al. Relationship between indeterminate or positive lateral margin and local recurrence after endoscopic resection of colorectal polyps. Endosc Int Open 2015; 3:E252–E257.
- Belderbos TD, Leenders M, Moons LM, Siersema PD. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy 2014; 46:388–402.
- Fujiya M, Tanaka K, Dokoshi T, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc 2015; 81:583–595.
- Rahmi G, Tanaka S, Ohara Y, et al. Efficacy of endoscopic submucosal dissection for residual or recurrent superficial colorectal tumors after endoscopic mucosal resection. J Dig Dis 2015; 16:14–21.
- Abe S, Oda I, Suzuki H, et al. Long-term surveillance and treatment outcomes of metachronous gastric cancer occurring after curative endoscopic submucosal dissection. Endoscopy 2015; 47:1113–1118.
- Oda I, Suzuki H, Nonaka S, Yoshinaga S. Complications of gastric endoscopic submucosal dissection. Dig Endosc 2013; 25(suppl 1):71–78.
- Takizawa K, Oda I, Gotoda T, et al. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection—an analysis of risk factors. Endoscopy 2008; 40:179–183.
- Uedo N, Takeuchi Y, Yamada T, et al. Effect of a proton pump inhibitor or an H2-receptor antagonist on prevention of bleeding from ulcer after endoscopic submucosal dissection of early gastric cancer: a prospective randomized controlled trial. Am J Gastroenterol 2007; 102:1610–1616.
- Hayashi N, Tanaka S, Nishiyama S, et al. Predictors of incomplete resection and perforation associated with endoscopic submucosal dissection for colorectal tumors. Gastrointest Endosc 2014; 79:427–435.
- Suzuki H, Oda I, Sekiguchi M, et al. Management and associated factors of delayed perforation after gastric endoscopic submucosal dissection. World J Gastroenterol 2015; 21:12635–12643.
- Tsunada S, Ogata S, Mannen K, et al. Case series of endoscopic balloon dilation to treat a stricture caused by circumferential resection of the gastric antrum by endoscopic submucosal dissection. Gastrointest Endosc 2008; 67:979–983.
- Coda S, Oda I, Gotoda T, Yokoi C, Kikuchi T, Ono H. Risk factors for cardiac and pyloric stenosis after endoscopic submucosal dissection, and efficacy of endoscopic balloon dilation treatment. Endoscopy 2009; 41:421–426.
- Abe S, Sakamoto T, Takamaru H, et al. Stenosis rates after endoscopic submucosal dissection of large rectal tumors involving greater than three quarters of the luminal circumference. Surg Endosc 2016; 30:5459–5464.
- Sakamoto T, Saito Y, Fukunaga S, Nakajima T, Matsuda T. Learning curve associated with colorectal endoscopic submucosal dissection for endoscopists experienced in gastric endoscopic submucosal dissection. Dis Colon Rectum 2011; 54:1307–1312.
KEY POINTS
- ESD is a minimally invasive endoscopic technique with curative potential for patients with superficial GI neoplasia.
- ESD preserves the integrity of the organ while achieving curative resection of large neoplasms.
- ESD is indicated rather than surgery in patients with early GI lesions with a negligible risk of lymph node metastasis.
- Complications of the procedure include bleeding, perforation, and stenosis. Most of these respond to endoscopic treatment.
- Successful ESD requires supportive teamwork among internists, gastroenterologists, pathologists, and surgeons.
Recurrent abdominal pain after laparoscopic cholecystectomy
Four months after undergoing laparoscopic cholecystectomy for symptomatic gallstones, an otherwise healthy 26-year-old woman begins to have episodes of epigastric and back pain similar to what she experienced before the surgery. The surgery was without complications, and her classic biliary colic disappeared afterward. Histologic evaluation of the surgical specimen revealed chronic cholecystitis with multiple small, mixed gallstones.
Now she describes a burning pain in her epigastrium and mid to upper back, starting about 30 minutes after a meal and lasting up to 4 hours. Sometimes it awakens her at night. She avoids eating for fear of inducing the pain. She has occasional chills but no fever, nausea, vomiting, jaundice, or changes in urine or stool color.
Three years ago she was diagnosed with a gastric ulcer induced by taking a nonsteroidal anti-inflammatory drug (NSAID). The ulcer was treated with a proton pump inhibitor for 1 month. She says the ulcer pain was dull and aching, different from her current pain.
Upper endoscopy 4 months ago (ie, before her laparoscopic cholecystectomy) showed no evidence of esophagitis or peptic ulcer disease.
Apart from her gallbladder operation, she has had no other surgery. According to the surgeon’s notes, intraoperative cholangiography was not performed, and no macroscopic changes of acute cholecystitis or difficult biliary anatomy were noted.
The patient does not smoke, does not drink alcohol, is not currently taking any medications, including NSAIDs or over-the-counter medications, and has not taken any recently. Her mother also had symptomatic gallstones requiring cholecystectomy.
On physical examination, only fever
On examination, her temperature is 101.2°F (38.4°C), blood pressure 117/80 mm Hg, heart rate 82 beats per minute, and blood oxygen saturation 99% on room air. Her weight is 138 lb (62.6 kg), height 5 feet 6 inches (168 cm).
There is no jaundice or pallor. Her heart and lung examinations are normal.
No costovertebral angle or spinal tenderness can be elicited.
Her laboratory values are shown in Table 1.
POSTCHOLECYSTECTOMY SYNDROME
1. After cholecystectomy, preoperative symptoms recur in what percentage of patients?
- 10% to 40%
- 50%
- 60%
- 80%
Postcholecystectomy syndrome—the recurrence of symptoms similar to those before the procedure—occurs in 10% to 40% of patients. The time to the onset of symptoms can range from 2 days to up to 25 years.1–4 Women may be at higher risk, with symptoms recurring in 43% vs 28% in men.5
Postcholecystectomy syndrome can have a biliary or a nonbiliary cause. Biliary causes include strictures, retained calculi, dropped calculi, tumors, sphincter of Oddi dysfunction, and calculi in the cystic duct remnant. Nonbiliary causes include functional and organic disorders such as peptic ulcer disease, gastroesophageal reflux, pancreatic disease, hepatocellular disorders, coronary artery disease, irritable bowel syndrome, and intercostal neuritis.
WHAT IS THE NEXT STEP?
2. Which is the most appropriate next step in the workup of this patient?
- Ultrasonography of the right upper quadrant
- Magnetic resonance cholangiopancreatography (MRCP)
- Endoscopic retrograde cholangiopancreatography (ERCP)
- Observation and reassurance
- Review the operative record and consult with the surgeon
Although the patient is presenting with pain and fever, two features of the classic Charcot triad (pain, fever, jaundice) seen in cholangitis (infection of a bile duct), and although cholangitis almost confirms the diagnosis of common bile duct stones in a patient with gallstones (before or after cholecystectomy), other diagnoses to consider are bile duct injury, bile leak, and biloma.
Biloma can be detected with ultrasonography. Bile duct injuries are identified intraoperatively in up to 25% of patients. For those with an unrecognized injury, the clinical presentation is variable and depends on the type of injury. If a bile leak is present, patients present early, at a median of 3 days postoperatively. However, our patient presented with symptoms 4 months after her surgery. Patients with bile duct strictures without bile leak have a longer symptom-free interval and usually present with signs of biliary obstruction. Ultrasonography can then detect biliary dilatation.6
It would be very helpful to review the operative record and to talk to the surgeon to confirm that intraoperative cholangiography had not been done and to determine the level of difficulty of the surgery. (Intraoperative cholangiography involves the introduction of contrast dye into the biliary system by cannulation of the cystic duct or by direct injection into the common bile duct. An intraoperative cholangiogram is considered normal if the entire intrahepatic and extrahepatic biliary tree is seen to be filled with contrast.) A normal cholangiogram has a negative predictive value of 99.8% for the detection of ductal stones. Thus, a normal intraoperative cholangiogram can prevent unnecessary postoperative ECRP, since it almost always indicates a clean bile duct.7
Ultrasonography of the right upper quadrant has a low sensitivity (< 50%) for detecting common bile duct stones. However, it is highly operator-dependent, and it may be twice as sensitive if done by expert radiologists than by less experienced ones. Its limitations include poor visualization of the distal portion of the duct and low sensitivity in patients in whom the common bile duct is minimally dilated and also in patients with small stones. In most studies, however, it had a very high specificity—ie, greater than 95%.8
MRCP has a sensitivity of 82.6% and a specificity of 97.5% in detecting stones in the common bile duct.9 Therefore, normal results on abdominal ultrasonography and MRCP do not completely rule out stones.
Although this patient has a high pretest probability of having common bile duct stones, ERCP should be done only after a thorough review of the previous operative procedure.
Observation and reassurance are not appropriate in a patient with cholangitis, such as this patient, because waiting increases the risk of septicemia.
The patient undergoes ERCP with stone removal
Review of the operative report and discussion with the surgeon confirm that the laparoscopic procedure was uneventful and that intraoperative cholangiography was not done.
Therefore, the patient undergoes ERCP. The major papilla is normal. Cholangiography reveals nondilated common bile and intrahepatic ducts, with faint filling defects in the mid to distal common bile duct. Endoscopic sphincterotomy is performed, and three small stones are extracted from the common bile duct. Repeat balloon-occlusion cholangiography is normal.
The patient tolerates the procedure well and resumes a normal diet and normal activities.
Her pain persists, prompting an emergency room visit
Five days after her ERCP procedure, however, the same burning epigastric pain returns. As before, the pain occurs after eating and does not occur with fasting. At this time, she has no fever or chills.
WHAT IS CAUSING HER PAIN?
3. Which is the most likely cause of her persistent pain?
- Acute pancreatitis after ERCP
- Peptic ulcer disease
- Sphincter of Oddi dysfunction
- Biliary stones
The most likely cause is persistent biliary stones. The common bile duct was recently explored and stones were removed, but she may still have stones in the intrahepatic ducts or in the cystic duct remnant, both of which were unopacified during the ERCP procedure, indicating that either the test was incomplete or a stone is obstructing the passage of contrast. Her persistent symptoms warrant repeating her liver function tests.
Acute pancreatitis is the most common and feared complication of ERCP, and it should be suspected in any patient who develops abdominal pain within 6 hours of the procedure. It is much less likely to develop after 12 hours, however. Risk factors for post-ERCP pancreatitis include patient factors (young age, female sex, history of recurrent pancreatitis), procedural factors (difficult cannulation, minor papilla sphincterotomy), and, less likely, operator-related factors.10–13 In general, the more likely a patient is to have an abnormal and irregular common bile duct or pancreatic duct, the lower the risk of post-ERCP pancreatitis. The importance of operator-dependent factors is not yet clear.10–13
Despite the postprandial pattern of our patient’s pain and her history of gastric ulcer, peptic ulcer disease is unlikely in view of a normal esophagogastroduodenoscopic examination done 4 months earlier, and since she has no recent exposure to NSAIDs.
Sphincter of Oddi dysfunction may explain her symptoms, but she recently underwent endoscopic sphincterotomy, which is regarded as the most definitive treatment.14
WHAT SHOULD BE DONE NEXT?
4. What would be the best next step in her management?
- Repeat ERCP
- MRCP
- Endoscopic ultrasonography
- Observation and reassurance
MRCP is the most appropriate next step, given her recurrent symptoms. Repeat ERCP is not appropriate, since there is no evidence of cholangitis, and since her liver function tests had completely normalized.
A recent systematic review of endoscopic ultrasonography and MRCP for diagnosing choledocholithiasis found both tests to be highly accurate, with no statistically significant differences in sensitivity or specificity between the two.15 However, MRCP has the advantage of being noninvasive and of being able to show intrahepatic stones.
Park et al,16 in a prospective study of 66 patients with primary intrahepatic stones, concluded that MRCP findings were comparable to those of percutaneous transhepatic cholangioscopy, the reference standard for locating intrahepatic stones. The sensitivity, specificity, and accuracy of MRCP for detecting and locating intrahepatic stones were high (97%, 99%, and 98%, respectively).16 However, after sphincterotomy, pneumobilia may create an appearance that can be mistaken for intraductal stones.
She undergoes MRCP
The patient continues to have pain, and she has lost 5 pounds because she is still avoiding eating. At this point, she is beginning to wonder if her symptoms are psychogenic, since all the test results have been normal.
ERCP, MRCP, ULTRASONOGRAPHY?
5. What would be the best next step?
- Reassurance
- Referral to a psychiatrist
- Referral to a pain management clinic
- Endoscopic ultrasonography
- Repeat ERCP
Endoscopic ultrasonography is needed to look for cystic duct stones. Although several tests have shown normal results, the patient’s pain continues as in the previous episodes, making stone disease the most likely cause.
Although no stones were seen on MRCP and ultrasonography, a detailed evaluation for stones in a cystic duct or retained gallbladder remnant was not done satisfactorily.
Reassurance and referral to a psychiatrist or pain management clinic are not appropriate, since an organic cause of her pain has not been completely ruled out.
Findings on endoscopic ultrasonography
Endoscopic ultrasonography is performed and reveals a large (7-mm) stone in the area of the cystic duct remnant or gallbladder remnant (Figure 3). The common bile duct is normal.
CAUSES OF RETAINED GALLBLADDER AND CYSTIC DUCT REMNANT
6. What may have predisposed this patient to a retained gallbladder or cystic duct remnant after her surgery?
- Laparoscopic cholecystectomy
- Not doing intraoperative cholangiography
- Cholecystectomy for acute cholecystitis
- All of the above
All of the above may have contributed.
Postcholecystectomy syndrome can pose a diagnostic and therapeutic challenge, as in our patient. Although it has been reported since the advent of the operation, it is more common after laparoscopic cholecystectomy than after open surgery. One possible cause is stones in a cystic duct remnant, ie, a stub longer than 1 cm.
During open cholecystectomy, the cystic duct is ligated and cut as close to the common bile duct as possible, leaving only a small remnant. In laparoscopic cholecystectomy, it is divided closer to the gallbladder to avoid iatrogenic injury to the common bile duct, leaving a longer remnant. A long cystic duct remnant can be prevented by accurately locating the junction of the gallbladder and the cystic duct during cholecystectomy and by routinely doing intraoperative cholangiography. The presence of stones in a cystic duct or retained gallbladder remnant is a rare cause of postcholecystectomy syndrome, and suspicion is required to make the diagnosis.17–19
We should note that stones may also lurk in the short cystic duct remnant left after open cholecystectomy. In fact, the first case of cystic duct remnant, the so-called reformed gallbladder containing stones, was described in 1912 by Flörcken.20
Intraoperative cholangiography was introduced in 1931 by Mirizzi,21 who recommended its routine use. Since the advent of laparoscopic cholecystectomy in 1988, the routine use of intraoperative cholangiography has been debated. Advocates point to its ability to detect unsuspected calculi and to delineate the biliary anatomy, thus reducing the risk of biliary duct injury.7,22–25 Those who argue against its routine use emphasize the low reported rates of unsuspected stones in the common bile duct (2% to 3%), a longer operative time, the additional cost, and false-positive results that may lead to unnecessary common bile duct exploration. Another argument against its routine use is that most small ductal stones pass spontaneously without significant sequelae.26–28 Surgeons who use intraoperative cholangiography only selectively use it in patients with unclear biliary anatomy and preoperative biochemical or radiologic evidence of choledocholithiasis.
Case continued: She undergoes repeat ERCP
IF STONES ARE DIFFICULT TO EXTRACT
7. If the cystic duct stone were not amenable to endoscopic extraction, what would be the best alternative?
- Extracorporeal shock-wave lithotripsy (ESWL)
- Endoscopic biliary laser lithotripsy
- Repeat laparoscopic cholecystectomy
- All of the above
All of the above are alternatives.
A symptomatic stone in a cystic duct remnant is uncommon and is mentioned in the literature only in case series and case reports.
ESWL is effective for treating bile duct calculi.29 In a cohort of 239 patients with bile duct stones treated by ESWL, Benninger et al30 concluded that endoscopy plus ESWL was a definitive treatment for all patients except one, who subsequently underwent cholecystectomy. Once fragmented, the stones are extracted endoscopically.
Another fragmentation technique that can be offered to patients with stones in the cystic duct that are difficult to extract is contact fragmentation with a holmium laser placed in a transpapillary position under visual guidance.17
Repeat cholecystectomy with removal of stones in the cystic duct remnant (and removal of retained gallbladder remnants and reduction of the cystic duct remnant) has good postoperative results.17,18,31,32
After incomplete cholecystectomy, the cystic duct remnant and the Calot (cystohepatic) triangle are surrounded by inflamed scar tissue, and this was thought to make laparoscopic reoperation difficult.33 However, with advances in surgical technique and increasing experience of surgeons, repeat cholecystectomy can be done laparoscopically. It has now been suggested that laparoscopic exploration to remove the gallbladder remnants is safe and feasible in such patients.34,35
Discharge and follow-up
The patient is discharged home after the procedure. She is still free of symptoms 31 months later.
LESSONS LEARNED
Remnant cystic duct stones are uncommon
The estimated incidence of a retained calculus within the cystic duct remnant after cholecystectomy is less than 2.5%.2,36 In a series of 322 patients who underwent repeat surgery because of postcholecystectomy syndrome, Rogy et al36 found only 8 who had a stone in the cystic duct or gallbladder remnant, and in a series of 371 patients, Zhou el al2 found 4 who had a stone in the cystic duct remnant.
Stones in the cystic duct remnant are difficult to diagnose
Diagnosing stones in surgical remnants of the cystic duct or gallbladder can be difficult. The sensitivity of abdominal ultrasonography in detecting cystic duct stones is low—only 27% in one study, with a specificity of 100% and an accuracy of 75%.37 Ultrasonography may occasionally suggest cystic duct stones by showing an acoustic shadow in the anatomic region of the cystic duct. However, the results should be interpreted with caution.
Determining the accuracy of ERCP and MRCP in detecting cystic duct remnant stones is also difficult, as few cases have been reported and data may be conflicting. In a review of seven patients confirmed to have retained stones in a surgical remnant, Walsh et al17 found that ERCP correctly diagnosed the retained stone in only four out of six patients; MRCP was done in one patient, and it was read as normal.
In three cases of stones in a postsurgical gallbladder remnant, Hassan and Vilmann38 reported that ERCP and MRCP failed to identify the gallbladder remnant in two out of three cases, likely because the remaining structures are small. The diagnosis was finally made by endoscopic ultrasonography, which the authors concluded was a valuable method to visualize a small gallbladder remnant with stones.
Greater suspicion is needed in patients with typical biliary colic after cholecystectomy
Retained gallbladder remnant is described in the literature as a latent complication. The main problem is not the remnant itself but the chance that it harbors retained stones, which can lead to dilatation and inflammation of the remnant.
The patient can develop symptoms of acute cholecystitis or even acute cholangitis if the stone migrates to the common bile duct. Symptoms can develop as early as 2 weeks or as late as 25 years after laparoscopic cholecystectomy.
Endoscopic ultrasonography may be the best way to look for these remnant stones and to evaluate the bile duct and pancreas. Therefore, it should be part of the diagnostic algorithm in the evaluation of postcholecystectomy pain.
Mixed results with ERCP for extracting cystic duct stones
In case reports of cystic duct calculi after cholecystectomy, ERCP by itself has had mixed results. This traditional means of removing stones may succeed, as in our case. However, the success rate depends largely on anatomic factors such as the position of the stone in the cystic duct, the degree of stone impaction, the diameter of the cystic duct, and the number of valves in the duct.17
Stones in the cystic duct that cannot be extracted with ERCP may benefit from fragmentation techniques in situ via holmium laser followed by endoscopic extraction.
Repeat cholecystectomy is generally advised for any residual gallbladder, and it can be done laparoscopically.
- Lehman GA, Sherman S. Sphincter of Oddi dysfunction (postcholecystectomy syndrome). In:Yamada T, editor. Textbook of Gastroenterology. 2nd ed. Philadelphia: Lippincott; 1995:2251–2262.
- Zhou PH, Liu FL, Yao LQ, Qin XY. Endoscopic diagnosis and treatment of post-cholecystectomy syndrome. Hepatobiliary Pancreat Dis Int 2003; 2:117–120.
- Mergener K, Clavien PA, Branch MS, Baillie J. A stone in a grossly dilated cystic duct stump: a rare cause of postcholecystectomy pain. Am J Gastroenterol 1999; 94:229–231.
- Goenka MK, Kochhar R, Nagi B, Bhasin DK, Chowdhury A, Singh K. Endoscopic retrograde cholangiopancreatography in postcholecystectomy syndrome. J Assoc Physicians India 1996; 44:119–122.
- Bodvall B, Overgaard B. Cystic duct remnant after cholecystectomy: incidence studied by cholegraphy in 500 cases, and significance in 103 reoperations. Ann Surg 1966; 163:382–390.
- Bergman JJ, van den Brink GR, Rauws EA, et al. Treatment of bile duct lesions after laparoscopic cholecystectomy. Gut 1996; 38:141–147.
- Nickkholgh A, Soltaniyekta S, Kalbasi H. Routine versus selective intraoperative cholangiography during laparoscopic cholecystectomy: a survey of 2,130 patients undergoing laparoscopic cholecystectomy. Surg Endosc 2006; 20:868–874.
- Gandolfi L, Torresan F, Solmi L, Puccetti A. The role of ultrasound in biliary and pancreatic diseases. Eur J Ultrasound 2003; 16:141–159.
- Al Samaraee A, Khan U, Almashta Z, Yiannakou Y. Preoperative diagnosis of choledocholithiasis: the role of MRCP. Br J Hosp Med (Lond) 2009; 70:339–343.
- Freeman ML, DiSario JA, Nelson DB, et al. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc 2001; 54:425–434.
- Cheng CL, Sherman S, Watkins JL, et al. Risk factors for post-ERCP pancreatitis: a prospective multicenter study. Am J Gastroenterol 2006; 101:139–147.
- Mehta SN, Pavone E, Barkun JS, Bouchard S, Barkun AN. Predictors of post-ERCP complications in patients with suspected choledocholithiasis. Endoscopy 1998; 30:457–463.
- Badalov N, Tenner S, Baillie J. The prevention, recognition and treatment of post-ERCP pancreatitis. JOP 2009; 10:88–97.
- Geenen JE, Hogan WJ, Dodds WJ, Toouli J, Venu RP. The efficacy of endoscopic sphincterotomy after cholecystectomy in patients with sphincter-of-Oddi dysfunction. N Engl J Med 1989; 320:82–87.
- Verma D, Kapadia A, Eisen GM, Adler DG. EUS vs MRCP for detection of choledocholithiasis. Gastrointest Endosc 2006; 64:248–254.
- Park DH, Kim MH, Lee SS, et al. Accuracy of magnetic resonance cholangiopancreatography for locating hepatolithiasis and detecting accompanying biliary strictures. Endoscopy 2004; 36:987–992.
- Walsh RM, Ponsky JL, Dumot J. Retained gallbladder/cystic duct remnant calculi as a cause of postcholecystectomy pain. Surg Endosc 2002; 16:981–984.
- Tantia O, Jain M, Khanna S, Sen B. Post cholecystectomy syndrome: role of cystic duct stump and re-intervention by laparoscopic surgery. J Minim Access Surg 2008; 4:71–75.
- Palanivelu C, Rangarajan M, Jategaonkar PA, Madankumar MV, Anand NV. Laparoscopic management of remnant cystic duct calculi: a retrospective study. Ann R Coll Surg Engl 2009; 91:25–29.
- Flörcken H. Gallenblasenregeneration mit Steinrecidiv nach Cholecystectomie. Deutsch Z Chir 1912; 113:604.
- Mirizzi PL. La colangiografía durante las operaciones de las vias biliares. Bol Soc Cirug Buenos Aires 1932; 16:1113.
- Soper NJ, Brunt LM. The case for routine operative cholangiography during laparoscopic cholecystectomy. Surg Clin North Am 1994; 74:953–959.
- Cuschieri A, Shimi S, Banting S, Nathanson LK, Pietrabissa A. Intraoperative cholangiography during laparoscopic cholecystectomy. Routine vs selective policy. Surg Endosc 1994; 8:302–305.
- Woods MS, Traverso LW, Kozarek RA, et al. Biliary tract complications of laparoscopic cholecystectomy are detected more frequently with routine intraoperative cholangiography. Surg Endosc 1995; 9:1076–1080.
- Vezakis A, Davides D, Ammori BJ, Martin IG, Larvin M, McMahon MJ. Intraoperative cholangiography during laparoscopic cholecystectomy. Surg Endosc 2000; 14:1118–1122.
- Ladocsi LT, Benitez LD, Filippone DR, Nance FC. Intraoperative cholangiography in laparoscopic cholecystectomy: a review of 734 consecutive cases. Am Surg 1997; 63:150–156.
- Clair DG, Brooks DC. Laparoscopic cholangiography. The case for a selective approach. Surg Clin North Am 1994; 74:961–966.
- Collins C, Maguire D, Ireland A, Fitzgerald E, O’Sullivan GC. A prospective study of common bile duct calculi in patients undergoing laparoscopic cholecystectomy: natural history of choledocholithiasis revisited. Ann Surg 2004; 239:28–33.
- Ponsky LE, Geisinger MA, Ponsky JL, Streem SB. Contemporary ‘urologic’ intervention in the pancreaticobiliary tree. Urology 2001; 57:21–25.
- Benninger J, Rabenstein T, Farnbacher M, Keppler J, Hahn EG, Schneider HT. Extracorporeal shockwave lithotripsy of gallstones in cystic duct remnants and Mirizzi syndrome. Gastrointest Endosc 2004; 60:454–459.
- Demetriades H, Pramateftakis MG, Kanellos I, Angelopoulos S, Mantzoros I, Betsis D. Retained gallbladder remnant after laparoscopic cholecystectomy. J Laparoendosc Adv Surg Tech A 2008; 18:276–279.
- Shaw C, O’Hanlon DM, Fenlon HM, McEntee GP. Cystic duct remnant and the ‘post-cholecystectomy syndrome. ’ Hepatogastroenterology 2004; 51:36–38.
- Rozsos I, Magyaródi Z, Orbán P. Cystic duct syndrome and minimally invasive surgery. [Hungarian] Orv Hetil 1997; 138:2397–2401.
- Chowbey PK, Bandyopadhyay SK, Sharma A, Khullar R, Soni V, Baijal M. Laparoscopic reintervention for residual gallstone disease. Surg Laparosc Endosc Percutan Tech 2003; 13:31–35.
- Clemente G, Giuliante F, Cadeddu F, Nuzzo G. Laparoscopic removal of gallbladder remnant and long cystic stump. Endoscopy 2001; 33:814–815.
- Rogy MA, Függer R, Herbst F, Schulz F. Reoperation after cholecystectomy. The role of the cystic duct stump. HPB Surg 1991; 4:129–134.
- Laing FC, Jeffrey RB. Choledocholithiasis and cystic duct obstruction: difficult ultrasonographic diagnosis. Radiology 1983; 146:475–479.
- Hassan H, Vilmann P. Insufficient cholecystectomy diagnosed by endoscopic ultrasonography. Endoscopy 2004; 36:236–238.
Four months after undergoing laparoscopic cholecystectomy for symptomatic gallstones, an otherwise healthy 26-year-old woman begins to have episodes of epigastric and back pain similar to what she experienced before the surgery. The surgery was without complications, and her classic biliary colic disappeared afterward. Histologic evaluation of the surgical specimen revealed chronic cholecystitis with multiple small, mixed gallstones.
Now she describes a burning pain in her epigastrium and mid to upper back, starting about 30 minutes after a meal and lasting up to 4 hours. Sometimes it awakens her at night. She avoids eating for fear of inducing the pain. She has occasional chills but no fever, nausea, vomiting, jaundice, or changes in urine or stool color.
Three years ago she was diagnosed with a gastric ulcer induced by taking a nonsteroidal anti-inflammatory drug (NSAID). The ulcer was treated with a proton pump inhibitor for 1 month. She says the ulcer pain was dull and aching, different from her current pain.
Upper endoscopy 4 months ago (ie, before her laparoscopic cholecystectomy) showed no evidence of esophagitis or peptic ulcer disease.
Apart from her gallbladder operation, she has had no other surgery. According to the surgeon’s notes, intraoperative cholangiography was not performed, and no macroscopic changes of acute cholecystitis or difficult biliary anatomy were noted.
The patient does not smoke, does not drink alcohol, is not currently taking any medications, including NSAIDs or over-the-counter medications, and has not taken any recently. Her mother also had symptomatic gallstones requiring cholecystectomy.
On physical examination, only fever
On examination, her temperature is 101.2°F (38.4°C), blood pressure 117/80 mm Hg, heart rate 82 beats per minute, and blood oxygen saturation 99% on room air. Her weight is 138 lb (62.6 kg), height 5 feet 6 inches (168 cm).
There is no jaundice or pallor. Her heart and lung examinations are normal.
No costovertebral angle or spinal tenderness can be elicited.
Her laboratory values are shown in Table 1.
POSTCHOLECYSTECTOMY SYNDROME
1. After cholecystectomy, preoperative symptoms recur in what percentage of patients?
- 10% to 40%
- 50%
- 60%
- 80%
Postcholecystectomy syndrome—the recurrence of symptoms similar to those before the procedure—occurs in 10% to 40% of patients. The time to the onset of symptoms can range from 2 days to up to 25 years.1–4 Women may be at higher risk, with symptoms recurring in 43% vs 28% in men.5
Postcholecystectomy syndrome can have a biliary or a nonbiliary cause. Biliary causes include strictures, retained calculi, dropped calculi, tumors, sphincter of Oddi dysfunction, and calculi in the cystic duct remnant. Nonbiliary causes include functional and organic disorders such as peptic ulcer disease, gastroesophageal reflux, pancreatic disease, hepatocellular disorders, coronary artery disease, irritable bowel syndrome, and intercostal neuritis.
WHAT IS THE NEXT STEP?
2. Which is the most appropriate next step in the workup of this patient?
- Ultrasonography of the right upper quadrant
- Magnetic resonance cholangiopancreatography (MRCP)
- Endoscopic retrograde cholangiopancreatography (ERCP)
- Observation and reassurance
- Review the operative record and consult with the surgeon
Although the patient is presenting with pain and fever, two features of the classic Charcot triad (pain, fever, jaundice) seen in cholangitis (infection of a bile duct), and although cholangitis almost confirms the diagnosis of common bile duct stones in a patient with gallstones (before or after cholecystectomy), other diagnoses to consider are bile duct injury, bile leak, and biloma.
Biloma can be detected with ultrasonography. Bile duct injuries are identified intraoperatively in up to 25% of patients. For those with an unrecognized injury, the clinical presentation is variable and depends on the type of injury. If a bile leak is present, patients present early, at a median of 3 days postoperatively. However, our patient presented with symptoms 4 months after her surgery. Patients with bile duct strictures without bile leak have a longer symptom-free interval and usually present with signs of biliary obstruction. Ultrasonography can then detect biliary dilatation.6
It would be very helpful to review the operative record and to talk to the surgeon to confirm that intraoperative cholangiography had not been done and to determine the level of difficulty of the surgery. (Intraoperative cholangiography involves the introduction of contrast dye into the biliary system by cannulation of the cystic duct or by direct injection into the common bile duct. An intraoperative cholangiogram is considered normal if the entire intrahepatic and extrahepatic biliary tree is seen to be filled with contrast.) A normal cholangiogram has a negative predictive value of 99.8% for the detection of ductal stones. Thus, a normal intraoperative cholangiogram can prevent unnecessary postoperative ECRP, since it almost always indicates a clean bile duct.7
Ultrasonography of the right upper quadrant has a low sensitivity (< 50%) for detecting common bile duct stones. However, it is highly operator-dependent, and it may be twice as sensitive if done by expert radiologists than by less experienced ones. Its limitations include poor visualization of the distal portion of the duct and low sensitivity in patients in whom the common bile duct is minimally dilated and also in patients with small stones. In most studies, however, it had a very high specificity—ie, greater than 95%.8
MRCP has a sensitivity of 82.6% and a specificity of 97.5% in detecting stones in the common bile duct.9 Therefore, normal results on abdominal ultrasonography and MRCP do not completely rule out stones.
Although this patient has a high pretest probability of having common bile duct stones, ERCP should be done only after a thorough review of the previous operative procedure.
Observation and reassurance are not appropriate in a patient with cholangitis, such as this patient, because waiting increases the risk of septicemia.
The patient undergoes ERCP with stone removal
Review of the operative report and discussion with the surgeon confirm that the laparoscopic procedure was uneventful and that intraoperative cholangiography was not done.
Therefore, the patient undergoes ERCP. The major papilla is normal. Cholangiography reveals nondilated common bile and intrahepatic ducts, with faint filling defects in the mid to distal common bile duct. Endoscopic sphincterotomy is performed, and three small stones are extracted from the common bile duct. Repeat balloon-occlusion cholangiography is normal.
The patient tolerates the procedure well and resumes a normal diet and normal activities.
Her pain persists, prompting an emergency room visit
Five days after her ERCP procedure, however, the same burning epigastric pain returns. As before, the pain occurs after eating and does not occur with fasting. At this time, she has no fever or chills.
WHAT IS CAUSING HER PAIN?
3. Which is the most likely cause of her persistent pain?
- Acute pancreatitis after ERCP
- Peptic ulcer disease
- Sphincter of Oddi dysfunction
- Biliary stones
The most likely cause is persistent biliary stones. The common bile duct was recently explored and stones were removed, but she may still have stones in the intrahepatic ducts or in the cystic duct remnant, both of which were unopacified during the ERCP procedure, indicating that either the test was incomplete or a stone is obstructing the passage of contrast. Her persistent symptoms warrant repeating her liver function tests.
Acute pancreatitis is the most common and feared complication of ERCP, and it should be suspected in any patient who develops abdominal pain within 6 hours of the procedure. It is much less likely to develop after 12 hours, however. Risk factors for post-ERCP pancreatitis include patient factors (young age, female sex, history of recurrent pancreatitis), procedural factors (difficult cannulation, minor papilla sphincterotomy), and, less likely, operator-related factors.10–13 In general, the more likely a patient is to have an abnormal and irregular common bile duct or pancreatic duct, the lower the risk of post-ERCP pancreatitis. The importance of operator-dependent factors is not yet clear.10–13
Despite the postprandial pattern of our patient’s pain and her history of gastric ulcer, peptic ulcer disease is unlikely in view of a normal esophagogastroduodenoscopic examination done 4 months earlier, and since she has no recent exposure to NSAIDs.
Sphincter of Oddi dysfunction may explain her symptoms, but she recently underwent endoscopic sphincterotomy, which is regarded as the most definitive treatment.14
WHAT SHOULD BE DONE NEXT?
4. What would be the best next step in her management?
- Repeat ERCP
- MRCP
- Endoscopic ultrasonography
- Observation and reassurance
MRCP is the most appropriate next step, given her recurrent symptoms. Repeat ERCP is not appropriate, since there is no evidence of cholangitis, and since her liver function tests had completely normalized.
A recent systematic review of endoscopic ultrasonography and MRCP for diagnosing choledocholithiasis found both tests to be highly accurate, with no statistically significant differences in sensitivity or specificity between the two.15 However, MRCP has the advantage of being noninvasive and of being able to show intrahepatic stones.
Park et al,16 in a prospective study of 66 patients with primary intrahepatic stones, concluded that MRCP findings were comparable to those of percutaneous transhepatic cholangioscopy, the reference standard for locating intrahepatic stones. The sensitivity, specificity, and accuracy of MRCP for detecting and locating intrahepatic stones were high (97%, 99%, and 98%, respectively).16 However, after sphincterotomy, pneumobilia may create an appearance that can be mistaken for intraductal stones.
She undergoes MRCP
The patient continues to have pain, and she has lost 5 pounds because she is still avoiding eating. At this point, she is beginning to wonder if her symptoms are psychogenic, since all the test results have been normal.
ERCP, MRCP, ULTRASONOGRAPHY?
5. What would be the best next step?
- Reassurance
- Referral to a psychiatrist
- Referral to a pain management clinic
- Endoscopic ultrasonography
- Repeat ERCP
Endoscopic ultrasonography is needed to look for cystic duct stones. Although several tests have shown normal results, the patient’s pain continues as in the previous episodes, making stone disease the most likely cause.
Although no stones were seen on MRCP and ultrasonography, a detailed evaluation for stones in a cystic duct or retained gallbladder remnant was not done satisfactorily.
Reassurance and referral to a psychiatrist or pain management clinic are not appropriate, since an organic cause of her pain has not been completely ruled out.
Findings on endoscopic ultrasonography
Endoscopic ultrasonography is performed and reveals a large (7-mm) stone in the area of the cystic duct remnant or gallbladder remnant (Figure 3). The common bile duct is normal.
CAUSES OF RETAINED GALLBLADDER AND CYSTIC DUCT REMNANT
6. What may have predisposed this patient to a retained gallbladder or cystic duct remnant after her surgery?
- Laparoscopic cholecystectomy
- Not doing intraoperative cholangiography
- Cholecystectomy for acute cholecystitis
- All of the above
All of the above may have contributed.
Postcholecystectomy syndrome can pose a diagnostic and therapeutic challenge, as in our patient. Although it has been reported since the advent of the operation, it is more common after laparoscopic cholecystectomy than after open surgery. One possible cause is stones in a cystic duct remnant, ie, a stub longer than 1 cm.
During open cholecystectomy, the cystic duct is ligated and cut as close to the common bile duct as possible, leaving only a small remnant. In laparoscopic cholecystectomy, it is divided closer to the gallbladder to avoid iatrogenic injury to the common bile duct, leaving a longer remnant. A long cystic duct remnant can be prevented by accurately locating the junction of the gallbladder and the cystic duct during cholecystectomy and by routinely doing intraoperative cholangiography. The presence of stones in a cystic duct or retained gallbladder remnant is a rare cause of postcholecystectomy syndrome, and suspicion is required to make the diagnosis.17–19
We should note that stones may also lurk in the short cystic duct remnant left after open cholecystectomy. In fact, the first case of cystic duct remnant, the so-called reformed gallbladder containing stones, was described in 1912 by Flörcken.20
Intraoperative cholangiography was introduced in 1931 by Mirizzi,21 who recommended its routine use. Since the advent of laparoscopic cholecystectomy in 1988, the routine use of intraoperative cholangiography has been debated. Advocates point to its ability to detect unsuspected calculi and to delineate the biliary anatomy, thus reducing the risk of biliary duct injury.7,22–25 Those who argue against its routine use emphasize the low reported rates of unsuspected stones in the common bile duct (2% to 3%), a longer operative time, the additional cost, and false-positive results that may lead to unnecessary common bile duct exploration. Another argument against its routine use is that most small ductal stones pass spontaneously without significant sequelae.26–28 Surgeons who use intraoperative cholangiography only selectively use it in patients with unclear biliary anatomy and preoperative biochemical or radiologic evidence of choledocholithiasis.
Case continued: She undergoes repeat ERCP
IF STONES ARE DIFFICULT TO EXTRACT
7. If the cystic duct stone were not amenable to endoscopic extraction, what would be the best alternative?
- Extracorporeal shock-wave lithotripsy (ESWL)
- Endoscopic biliary laser lithotripsy
- Repeat laparoscopic cholecystectomy
- All of the above
All of the above are alternatives.
A symptomatic stone in a cystic duct remnant is uncommon and is mentioned in the literature only in case series and case reports.
ESWL is effective for treating bile duct calculi.29 In a cohort of 239 patients with bile duct stones treated by ESWL, Benninger et al30 concluded that endoscopy plus ESWL was a definitive treatment for all patients except one, who subsequently underwent cholecystectomy. Once fragmented, the stones are extracted endoscopically.
Another fragmentation technique that can be offered to patients with stones in the cystic duct that are difficult to extract is contact fragmentation with a holmium laser placed in a transpapillary position under visual guidance.17
Repeat cholecystectomy with removal of stones in the cystic duct remnant (and removal of retained gallbladder remnants and reduction of the cystic duct remnant) has good postoperative results.17,18,31,32
After incomplete cholecystectomy, the cystic duct remnant and the Calot (cystohepatic) triangle are surrounded by inflamed scar tissue, and this was thought to make laparoscopic reoperation difficult.33 However, with advances in surgical technique and increasing experience of surgeons, repeat cholecystectomy can be done laparoscopically. It has now been suggested that laparoscopic exploration to remove the gallbladder remnants is safe and feasible in such patients.34,35
Discharge and follow-up
The patient is discharged home after the procedure. She is still free of symptoms 31 months later.
LESSONS LEARNED
Remnant cystic duct stones are uncommon
The estimated incidence of a retained calculus within the cystic duct remnant after cholecystectomy is less than 2.5%.2,36 In a series of 322 patients who underwent repeat surgery because of postcholecystectomy syndrome, Rogy et al36 found only 8 who had a stone in the cystic duct or gallbladder remnant, and in a series of 371 patients, Zhou el al2 found 4 who had a stone in the cystic duct remnant.
Stones in the cystic duct remnant are difficult to diagnose
Diagnosing stones in surgical remnants of the cystic duct or gallbladder can be difficult. The sensitivity of abdominal ultrasonography in detecting cystic duct stones is low—only 27% in one study, with a specificity of 100% and an accuracy of 75%.37 Ultrasonography may occasionally suggest cystic duct stones by showing an acoustic shadow in the anatomic region of the cystic duct. However, the results should be interpreted with caution.
Determining the accuracy of ERCP and MRCP in detecting cystic duct remnant stones is also difficult, as few cases have been reported and data may be conflicting. In a review of seven patients confirmed to have retained stones in a surgical remnant, Walsh et al17 found that ERCP correctly diagnosed the retained stone in only four out of six patients; MRCP was done in one patient, and it was read as normal.
In three cases of stones in a postsurgical gallbladder remnant, Hassan and Vilmann38 reported that ERCP and MRCP failed to identify the gallbladder remnant in two out of three cases, likely because the remaining structures are small. The diagnosis was finally made by endoscopic ultrasonography, which the authors concluded was a valuable method to visualize a small gallbladder remnant with stones.
Greater suspicion is needed in patients with typical biliary colic after cholecystectomy
Retained gallbladder remnant is described in the literature as a latent complication. The main problem is not the remnant itself but the chance that it harbors retained stones, which can lead to dilatation and inflammation of the remnant.
The patient can develop symptoms of acute cholecystitis or even acute cholangitis if the stone migrates to the common bile duct. Symptoms can develop as early as 2 weeks or as late as 25 years after laparoscopic cholecystectomy.
Endoscopic ultrasonography may be the best way to look for these remnant stones and to evaluate the bile duct and pancreas. Therefore, it should be part of the diagnostic algorithm in the evaluation of postcholecystectomy pain.
Mixed results with ERCP for extracting cystic duct stones
In case reports of cystic duct calculi after cholecystectomy, ERCP by itself has had mixed results. This traditional means of removing stones may succeed, as in our case. However, the success rate depends largely on anatomic factors such as the position of the stone in the cystic duct, the degree of stone impaction, the diameter of the cystic duct, and the number of valves in the duct.17
Stones in the cystic duct that cannot be extracted with ERCP may benefit from fragmentation techniques in situ via holmium laser followed by endoscopic extraction.
Repeat cholecystectomy is generally advised for any residual gallbladder, and it can be done laparoscopically.
Four months after undergoing laparoscopic cholecystectomy for symptomatic gallstones, an otherwise healthy 26-year-old woman begins to have episodes of epigastric and back pain similar to what she experienced before the surgery. The surgery was without complications, and her classic biliary colic disappeared afterward. Histologic evaluation of the surgical specimen revealed chronic cholecystitis with multiple small, mixed gallstones.
Now she describes a burning pain in her epigastrium and mid to upper back, starting about 30 minutes after a meal and lasting up to 4 hours. Sometimes it awakens her at night. She avoids eating for fear of inducing the pain. She has occasional chills but no fever, nausea, vomiting, jaundice, or changes in urine or stool color.
Three years ago she was diagnosed with a gastric ulcer induced by taking a nonsteroidal anti-inflammatory drug (NSAID). The ulcer was treated with a proton pump inhibitor for 1 month. She says the ulcer pain was dull and aching, different from her current pain.
Upper endoscopy 4 months ago (ie, before her laparoscopic cholecystectomy) showed no evidence of esophagitis or peptic ulcer disease.
Apart from her gallbladder operation, she has had no other surgery. According to the surgeon’s notes, intraoperative cholangiography was not performed, and no macroscopic changes of acute cholecystitis or difficult biliary anatomy were noted.
The patient does not smoke, does not drink alcohol, is not currently taking any medications, including NSAIDs or over-the-counter medications, and has not taken any recently. Her mother also had symptomatic gallstones requiring cholecystectomy.
On physical examination, only fever
On examination, her temperature is 101.2°F (38.4°C), blood pressure 117/80 mm Hg, heart rate 82 beats per minute, and blood oxygen saturation 99% on room air. Her weight is 138 lb (62.6 kg), height 5 feet 6 inches (168 cm).
There is no jaundice or pallor. Her heart and lung examinations are normal.
No costovertebral angle or spinal tenderness can be elicited.
Her laboratory values are shown in Table 1.
POSTCHOLECYSTECTOMY SYNDROME
1. After cholecystectomy, preoperative symptoms recur in what percentage of patients?
- 10% to 40%
- 50%
- 60%
- 80%
Postcholecystectomy syndrome—the recurrence of symptoms similar to those before the procedure—occurs in 10% to 40% of patients. The time to the onset of symptoms can range from 2 days to up to 25 years.1–4 Women may be at higher risk, with symptoms recurring in 43% vs 28% in men.5
Postcholecystectomy syndrome can have a biliary or a nonbiliary cause. Biliary causes include strictures, retained calculi, dropped calculi, tumors, sphincter of Oddi dysfunction, and calculi in the cystic duct remnant. Nonbiliary causes include functional and organic disorders such as peptic ulcer disease, gastroesophageal reflux, pancreatic disease, hepatocellular disorders, coronary artery disease, irritable bowel syndrome, and intercostal neuritis.
WHAT IS THE NEXT STEP?
2. Which is the most appropriate next step in the workup of this patient?
- Ultrasonography of the right upper quadrant
- Magnetic resonance cholangiopancreatography (MRCP)
- Endoscopic retrograde cholangiopancreatography (ERCP)
- Observation and reassurance
- Review the operative record and consult with the surgeon
Although the patient is presenting with pain and fever, two features of the classic Charcot triad (pain, fever, jaundice) seen in cholangitis (infection of a bile duct), and although cholangitis almost confirms the diagnosis of common bile duct stones in a patient with gallstones (before or after cholecystectomy), other diagnoses to consider are bile duct injury, bile leak, and biloma.
Biloma can be detected with ultrasonography. Bile duct injuries are identified intraoperatively in up to 25% of patients. For those with an unrecognized injury, the clinical presentation is variable and depends on the type of injury. If a bile leak is present, patients present early, at a median of 3 days postoperatively. However, our patient presented with symptoms 4 months after her surgery. Patients with bile duct strictures without bile leak have a longer symptom-free interval and usually present with signs of biliary obstruction. Ultrasonography can then detect biliary dilatation.6
It would be very helpful to review the operative record and to talk to the surgeon to confirm that intraoperative cholangiography had not been done and to determine the level of difficulty of the surgery. (Intraoperative cholangiography involves the introduction of contrast dye into the biliary system by cannulation of the cystic duct or by direct injection into the common bile duct. An intraoperative cholangiogram is considered normal if the entire intrahepatic and extrahepatic biliary tree is seen to be filled with contrast.) A normal cholangiogram has a negative predictive value of 99.8% for the detection of ductal stones. Thus, a normal intraoperative cholangiogram can prevent unnecessary postoperative ECRP, since it almost always indicates a clean bile duct.7
Ultrasonography of the right upper quadrant has a low sensitivity (< 50%) for detecting common bile duct stones. However, it is highly operator-dependent, and it may be twice as sensitive if done by expert radiologists than by less experienced ones. Its limitations include poor visualization of the distal portion of the duct and low sensitivity in patients in whom the common bile duct is minimally dilated and also in patients with small stones. In most studies, however, it had a very high specificity—ie, greater than 95%.8
MRCP has a sensitivity of 82.6% and a specificity of 97.5% in detecting stones in the common bile duct.9 Therefore, normal results on abdominal ultrasonography and MRCP do not completely rule out stones.
Although this patient has a high pretest probability of having common bile duct stones, ERCP should be done only after a thorough review of the previous operative procedure.
Observation and reassurance are not appropriate in a patient with cholangitis, such as this patient, because waiting increases the risk of septicemia.
The patient undergoes ERCP with stone removal
Review of the operative report and discussion with the surgeon confirm that the laparoscopic procedure was uneventful and that intraoperative cholangiography was not done.
Therefore, the patient undergoes ERCP. The major papilla is normal. Cholangiography reveals nondilated common bile and intrahepatic ducts, with faint filling defects in the mid to distal common bile duct. Endoscopic sphincterotomy is performed, and three small stones are extracted from the common bile duct. Repeat balloon-occlusion cholangiography is normal.
The patient tolerates the procedure well and resumes a normal diet and normal activities.
Her pain persists, prompting an emergency room visit
Five days after her ERCP procedure, however, the same burning epigastric pain returns. As before, the pain occurs after eating and does not occur with fasting. At this time, she has no fever or chills.
WHAT IS CAUSING HER PAIN?
3. Which is the most likely cause of her persistent pain?
- Acute pancreatitis after ERCP
- Peptic ulcer disease
- Sphincter of Oddi dysfunction
- Biliary stones
The most likely cause is persistent biliary stones. The common bile duct was recently explored and stones were removed, but she may still have stones in the intrahepatic ducts or in the cystic duct remnant, both of which were unopacified during the ERCP procedure, indicating that either the test was incomplete or a stone is obstructing the passage of contrast. Her persistent symptoms warrant repeating her liver function tests.
Acute pancreatitis is the most common and feared complication of ERCP, and it should be suspected in any patient who develops abdominal pain within 6 hours of the procedure. It is much less likely to develop after 12 hours, however. Risk factors for post-ERCP pancreatitis include patient factors (young age, female sex, history of recurrent pancreatitis), procedural factors (difficult cannulation, minor papilla sphincterotomy), and, less likely, operator-related factors.10–13 In general, the more likely a patient is to have an abnormal and irregular common bile duct or pancreatic duct, the lower the risk of post-ERCP pancreatitis. The importance of operator-dependent factors is not yet clear.10–13
Despite the postprandial pattern of our patient’s pain and her history of gastric ulcer, peptic ulcer disease is unlikely in view of a normal esophagogastroduodenoscopic examination done 4 months earlier, and since she has no recent exposure to NSAIDs.
Sphincter of Oddi dysfunction may explain her symptoms, but she recently underwent endoscopic sphincterotomy, which is regarded as the most definitive treatment.14
WHAT SHOULD BE DONE NEXT?
4. What would be the best next step in her management?
- Repeat ERCP
- MRCP
- Endoscopic ultrasonography
- Observation and reassurance
MRCP is the most appropriate next step, given her recurrent symptoms. Repeat ERCP is not appropriate, since there is no evidence of cholangitis, and since her liver function tests had completely normalized.
A recent systematic review of endoscopic ultrasonography and MRCP for diagnosing choledocholithiasis found both tests to be highly accurate, with no statistically significant differences in sensitivity or specificity between the two.15 However, MRCP has the advantage of being noninvasive and of being able to show intrahepatic stones.
Park et al,16 in a prospective study of 66 patients with primary intrahepatic stones, concluded that MRCP findings were comparable to those of percutaneous transhepatic cholangioscopy, the reference standard for locating intrahepatic stones. The sensitivity, specificity, and accuracy of MRCP for detecting and locating intrahepatic stones were high (97%, 99%, and 98%, respectively).16 However, after sphincterotomy, pneumobilia may create an appearance that can be mistaken for intraductal stones.
She undergoes MRCP
The patient continues to have pain, and she has lost 5 pounds because she is still avoiding eating. At this point, she is beginning to wonder if her symptoms are psychogenic, since all the test results have been normal.
ERCP, MRCP, ULTRASONOGRAPHY?
5. What would be the best next step?
- Reassurance
- Referral to a psychiatrist
- Referral to a pain management clinic
- Endoscopic ultrasonography
- Repeat ERCP
Endoscopic ultrasonography is needed to look for cystic duct stones. Although several tests have shown normal results, the patient’s pain continues as in the previous episodes, making stone disease the most likely cause.
Although no stones were seen on MRCP and ultrasonography, a detailed evaluation for stones in a cystic duct or retained gallbladder remnant was not done satisfactorily.
Reassurance and referral to a psychiatrist or pain management clinic are not appropriate, since an organic cause of her pain has not been completely ruled out.
Findings on endoscopic ultrasonography
Endoscopic ultrasonography is performed and reveals a large (7-mm) stone in the area of the cystic duct remnant or gallbladder remnant (Figure 3). The common bile duct is normal.
CAUSES OF RETAINED GALLBLADDER AND CYSTIC DUCT REMNANT
6. What may have predisposed this patient to a retained gallbladder or cystic duct remnant after her surgery?
- Laparoscopic cholecystectomy
- Not doing intraoperative cholangiography
- Cholecystectomy for acute cholecystitis
- All of the above
All of the above may have contributed.
Postcholecystectomy syndrome can pose a diagnostic and therapeutic challenge, as in our patient. Although it has been reported since the advent of the operation, it is more common after laparoscopic cholecystectomy than after open surgery. One possible cause is stones in a cystic duct remnant, ie, a stub longer than 1 cm.
During open cholecystectomy, the cystic duct is ligated and cut as close to the common bile duct as possible, leaving only a small remnant. In laparoscopic cholecystectomy, it is divided closer to the gallbladder to avoid iatrogenic injury to the common bile duct, leaving a longer remnant. A long cystic duct remnant can be prevented by accurately locating the junction of the gallbladder and the cystic duct during cholecystectomy and by routinely doing intraoperative cholangiography. The presence of stones in a cystic duct or retained gallbladder remnant is a rare cause of postcholecystectomy syndrome, and suspicion is required to make the diagnosis.17–19
We should note that stones may also lurk in the short cystic duct remnant left after open cholecystectomy. In fact, the first case of cystic duct remnant, the so-called reformed gallbladder containing stones, was described in 1912 by Flörcken.20
Intraoperative cholangiography was introduced in 1931 by Mirizzi,21 who recommended its routine use. Since the advent of laparoscopic cholecystectomy in 1988, the routine use of intraoperative cholangiography has been debated. Advocates point to its ability to detect unsuspected calculi and to delineate the biliary anatomy, thus reducing the risk of biliary duct injury.7,22–25 Those who argue against its routine use emphasize the low reported rates of unsuspected stones in the common bile duct (2% to 3%), a longer operative time, the additional cost, and false-positive results that may lead to unnecessary common bile duct exploration. Another argument against its routine use is that most small ductal stones pass spontaneously without significant sequelae.26–28 Surgeons who use intraoperative cholangiography only selectively use it in patients with unclear biliary anatomy and preoperative biochemical or radiologic evidence of choledocholithiasis.
Case continued: She undergoes repeat ERCP
IF STONES ARE DIFFICULT TO EXTRACT
7. If the cystic duct stone were not amenable to endoscopic extraction, what would be the best alternative?
- Extracorporeal shock-wave lithotripsy (ESWL)
- Endoscopic biliary laser lithotripsy
- Repeat laparoscopic cholecystectomy
- All of the above
All of the above are alternatives.
A symptomatic stone in a cystic duct remnant is uncommon and is mentioned in the literature only in case series and case reports.
ESWL is effective for treating bile duct calculi.29 In a cohort of 239 patients with bile duct stones treated by ESWL, Benninger et al30 concluded that endoscopy plus ESWL was a definitive treatment for all patients except one, who subsequently underwent cholecystectomy. Once fragmented, the stones are extracted endoscopically.
Another fragmentation technique that can be offered to patients with stones in the cystic duct that are difficult to extract is contact fragmentation with a holmium laser placed in a transpapillary position under visual guidance.17
Repeat cholecystectomy with removal of stones in the cystic duct remnant (and removal of retained gallbladder remnants and reduction of the cystic duct remnant) has good postoperative results.17,18,31,32
After incomplete cholecystectomy, the cystic duct remnant and the Calot (cystohepatic) triangle are surrounded by inflamed scar tissue, and this was thought to make laparoscopic reoperation difficult.33 However, with advances in surgical technique and increasing experience of surgeons, repeat cholecystectomy can be done laparoscopically. It has now been suggested that laparoscopic exploration to remove the gallbladder remnants is safe and feasible in such patients.34,35
Discharge and follow-up
The patient is discharged home after the procedure. She is still free of symptoms 31 months later.
LESSONS LEARNED
Remnant cystic duct stones are uncommon
The estimated incidence of a retained calculus within the cystic duct remnant after cholecystectomy is less than 2.5%.2,36 In a series of 322 patients who underwent repeat surgery because of postcholecystectomy syndrome, Rogy et al36 found only 8 who had a stone in the cystic duct or gallbladder remnant, and in a series of 371 patients, Zhou el al2 found 4 who had a stone in the cystic duct remnant.
Stones in the cystic duct remnant are difficult to diagnose
Diagnosing stones in surgical remnants of the cystic duct or gallbladder can be difficult. The sensitivity of abdominal ultrasonography in detecting cystic duct stones is low—only 27% in one study, with a specificity of 100% and an accuracy of 75%.37 Ultrasonography may occasionally suggest cystic duct stones by showing an acoustic shadow in the anatomic region of the cystic duct. However, the results should be interpreted with caution.
Determining the accuracy of ERCP and MRCP in detecting cystic duct remnant stones is also difficult, as few cases have been reported and data may be conflicting. In a review of seven patients confirmed to have retained stones in a surgical remnant, Walsh et al17 found that ERCP correctly diagnosed the retained stone in only four out of six patients; MRCP was done in one patient, and it was read as normal.
In three cases of stones in a postsurgical gallbladder remnant, Hassan and Vilmann38 reported that ERCP and MRCP failed to identify the gallbladder remnant in two out of three cases, likely because the remaining structures are small. The diagnosis was finally made by endoscopic ultrasonography, which the authors concluded was a valuable method to visualize a small gallbladder remnant with stones.
Greater suspicion is needed in patients with typical biliary colic after cholecystectomy
Retained gallbladder remnant is described in the literature as a latent complication. The main problem is not the remnant itself but the chance that it harbors retained stones, which can lead to dilatation and inflammation of the remnant.
The patient can develop symptoms of acute cholecystitis or even acute cholangitis if the stone migrates to the common bile duct. Symptoms can develop as early as 2 weeks or as late as 25 years after laparoscopic cholecystectomy.
Endoscopic ultrasonography may be the best way to look for these remnant stones and to evaluate the bile duct and pancreas. Therefore, it should be part of the diagnostic algorithm in the evaluation of postcholecystectomy pain.
Mixed results with ERCP for extracting cystic duct stones
In case reports of cystic duct calculi after cholecystectomy, ERCP by itself has had mixed results. This traditional means of removing stones may succeed, as in our case. However, the success rate depends largely on anatomic factors such as the position of the stone in the cystic duct, the degree of stone impaction, the diameter of the cystic duct, and the number of valves in the duct.17
Stones in the cystic duct that cannot be extracted with ERCP may benefit from fragmentation techniques in situ via holmium laser followed by endoscopic extraction.
Repeat cholecystectomy is generally advised for any residual gallbladder, and it can be done laparoscopically.
- Lehman GA, Sherman S. Sphincter of Oddi dysfunction (postcholecystectomy syndrome). In:Yamada T, editor. Textbook of Gastroenterology. 2nd ed. Philadelphia: Lippincott; 1995:2251–2262.
- Zhou PH, Liu FL, Yao LQ, Qin XY. Endoscopic diagnosis and treatment of post-cholecystectomy syndrome. Hepatobiliary Pancreat Dis Int 2003; 2:117–120.
- Mergener K, Clavien PA, Branch MS, Baillie J. A stone in a grossly dilated cystic duct stump: a rare cause of postcholecystectomy pain. Am J Gastroenterol 1999; 94:229–231.
- Goenka MK, Kochhar R, Nagi B, Bhasin DK, Chowdhury A, Singh K. Endoscopic retrograde cholangiopancreatography in postcholecystectomy syndrome. J Assoc Physicians India 1996; 44:119–122.
- Bodvall B, Overgaard B. Cystic duct remnant after cholecystectomy: incidence studied by cholegraphy in 500 cases, and significance in 103 reoperations. Ann Surg 1966; 163:382–390.
- Bergman JJ, van den Brink GR, Rauws EA, et al. Treatment of bile duct lesions after laparoscopic cholecystectomy. Gut 1996; 38:141–147.
- Nickkholgh A, Soltaniyekta S, Kalbasi H. Routine versus selective intraoperative cholangiography during laparoscopic cholecystectomy: a survey of 2,130 patients undergoing laparoscopic cholecystectomy. Surg Endosc 2006; 20:868–874.
- Gandolfi L, Torresan F, Solmi L, Puccetti A. The role of ultrasound in biliary and pancreatic diseases. Eur J Ultrasound 2003; 16:141–159.
- Al Samaraee A, Khan U, Almashta Z, Yiannakou Y. Preoperative diagnosis of choledocholithiasis: the role of MRCP. Br J Hosp Med (Lond) 2009; 70:339–343.
- Freeman ML, DiSario JA, Nelson DB, et al. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc 2001; 54:425–434.
- Cheng CL, Sherman S, Watkins JL, et al. Risk factors for post-ERCP pancreatitis: a prospective multicenter study. Am J Gastroenterol 2006; 101:139–147.
- Mehta SN, Pavone E, Barkun JS, Bouchard S, Barkun AN. Predictors of post-ERCP complications in patients with suspected choledocholithiasis. Endoscopy 1998; 30:457–463.
- Badalov N, Tenner S, Baillie J. The prevention, recognition and treatment of post-ERCP pancreatitis. JOP 2009; 10:88–97.
- Geenen JE, Hogan WJ, Dodds WJ, Toouli J, Venu RP. The efficacy of endoscopic sphincterotomy after cholecystectomy in patients with sphincter-of-Oddi dysfunction. N Engl J Med 1989; 320:82–87.
- Verma D, Kapadia A, Eisen GM, Adler DG. EUS vs MRCP for detection of choledocholithiasis. Gastrointest Endosc 2006; 64:248–254.
- Park DH, Kim MH, Lee SS, et al. Accuracy of magnetic resonance cholangiopancreatography for locating hepatolithiasis and detecting accompanying biliary strictures. Endoscopy 2004; 36:987–992.
- Walsh RM, Ponsky JL, Dumot J. Retained gallbladder/cystic duct remnant calculi as a cause of postcholecystectomy pain. Surg Endosc 2002; 16:981–984.
- Tantia O, Jain M, Khanna S, Sen B. Post cholecystectomy syndrome: role of cystic duct stump and re-intervention by laparoscopic surgery. J Minim Access Surg 2008; 4:71–75.
- Palanivelu C, Rangarajan M, Jategaonkar PA, Madankumar MV, Anand NV. Laparoscopic management of remnant cystic duct calculi: a retrospective study. Ann R Coll Surg Engl 2009; 91:25–29.
- Flörcken H. Gallenblasenregeneration mit Steinrecidiv nach Cholecystectomie. Deutsch Z Chir 1912; 113:604.
- Mirizzi PL. La colangiografía durante las operaciones de las vias biliares. Bol Soc Cirug Buenos Aires 1932; 16:1113.
- Soper NJ, Brunt LM. The case for routine operative cholangiography during laparoscopic cholecystectomy. Surg Clin North Am 1994; 74:953–959.
- Cuschieri A, Shimi S, Banting S, Nathanson LK, Pietrabissa A. Intraoperative cholangiography during laparoscopic cholecystectomy. Routine vs selective policy. Surg Endosc 1994; 8:302–305.
- Woods MS, Traverso LW, Kozarek RA, et al. Biliary tract complications of laparoscopic cholecystectomy are detected more frequently with routine intraoperative cholangiography. Surg Endosc 1995; 9:1076–1080.
- Vezakis A, Davides D, Ammori BJ, Martin IG, Larvin M, McMahon MJ. Intraoperative cholangiography during laparoscopic cholecystectomy. Surg Endosc 2000; 14:1118–1122.
- Ladocsi LT, Benitez LD, Filippone DR, Nance FC. Intraoperative cholangiography in laparoscopic cholecystectomy: a review of 734 consecutive cases. Am Surg 1997; 63:150–156.
- Clair DG, Brooks DC. Laparoscopic cholangiography. The case for a selective approach. Surg Clin North Am 1994; 74:961–966.
- Collins C, Maguire D, Ireland A, Fitzgerald E, O’Sullivan GC. A prospective study of common bile duct calculi in patients undergoing laparoscopic cholecystectomy: natural history of choledocholithiasis revisited. Ann Surg 2004; 239:28–33.
- Ponsky LE, Geisinger MA, Ponsky JL, Streem SB. Contemporary ‘urologic’ intervention in the pancreaticobiliary tree. Urology 2001; 57:21–25.
- Benninger J, Rabenstein T, Farnbacher M, Keppler J, Hahn EG, Schneider HT. Extracorporeal shockwave lithotripsy of gallstones in cystic duct remnants and Mirizzi syndrome. Gastrointest Endosc 2004; 60:454–459.
- Demetriades H, Pramateftakis MG, Kanellos I, Angelopoulos S, Mantzoros I, Betsis D. Retained gallbladder remnant after laparoscopic cholecystectomy. J Laparoendosc Adv Surg Tech A 2008; 18:276–279.
- Shaw C, O’Hanlon DM, Fenlon HM, McEntee GP. Cystic duct remnant and the ‘post-cholecystectomy syndrome. ’ Hepatogastroenterology 2004; 51:36–38.
- Rozsos I, Magyaródi Z, Orbán P. Cystic duct syndrome and minimally invasive surgery. [Hungarian] Orv Hetil 1997; 138:2397–2401.
- Chowbey PK, Bandyopadhyay SK, Sharma A, Khullar R, Soni V, Baijal M. Laparoscopic reintervention for residual gallstone disease. Surg Laparosc Endosc Percutan Tech 2003; 13:31–35.
- Clemente G, Giuliante F, Cadeddu F, Nuzzo G. Laparoscopic removal of gallbladder remnant and long cystic stump. Endoscopy 2001; 33:814–815.
- Rogy MA, Függer R, Herbst F, Schulz F. Reoperation after cholecystectomy. The role of the cystic duct stump. HPB Surg 1991; 4:129–134.
- Laing FC, Jeffrey RB. Choledocholithiasis and cystic duct obstruction: difficult ultrasonographic diagnosis. Radiology 1983; 146:475–479.
- Hassan H, Vilmann P. Insufficient cholecystectomy diagnosed by endoscopic ultrasonography. Endoscopy 2004; 36:236–238.
- Lehman GA, Sherman S. Sphincter of Oddi dysfunction (postcholecystectomy syndrome). In:Yamada T, editor. Textbook of Gastroenterology. 2nd ed. Philadelphia: Lippincott; 1995:2251–2262.
- Zhou PH, Liu FL, Yao LQ, Qin XY. Endoscopic diagnosis and treatment of post-cholecystectomy syndrome. Hepatobiliary Pancreat Dis Int 2003; 2:117–120.
- Mergener K, Clavien PA, Branch MS, Baillie J. A stone in a grossly dilated cystic duct stump: a rare cause of postcholecystectomy pain. Am J Gastroenterol 1999; 94:229–231.
- Goenka MK, Kochhar R, Nagi B, Bhasin DK, Chowdhury A, Singh K. Endoscopic retrograde cholangiopancreatography in postcholecystectomy syndrome. J Assoc Physicians India 1996; 44:119–122.
- Bodvall B, Overgaard B. Cystic duct remnant after cholecystectomy: incidence studied by cholegraphy in 500 cases, and significance in 103 reoperations. Ann Surg 1966; 163:382–390.
- Bergman JJ, van den Brink GR, Rauws EA, et al. Treatment of bile duct lesions after laparoscopic cholecystectomy. Gut 1996; 38:141–147.
- Nickkholgh A, Soltaniyekta S, Kalbasi H. Routine versus selective intraoperative cholangiography during laparoscopic cholecystectomy: a survey of 2,130 patients undergoing laparoscopic cholecystectomy. Surg Endosc 2006; 20:868–874.
- Gandolfi L, Torresan F, Solmi L, Puccetti A. The role of ultrasound in biliary and pancreatic diseases. Eur J Ultrasound 2003; 16:141–159.
- Al Samaraee A, Khan U, Almashta Z, Yiannakou Y. Preoperative diagnosis of choledocholithiasis: the role of MRCP. Br J Hosp Med (Lond) 2009; 70:339–343.
- Freeman ML, DiSario JA, Nelson DB, et al. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc 2001; 54:425–434.
- Cheng CL, Sherman S, Watkins JL, et al. Risk factors for post-ERCP pancreatitis: a prospective multicenter study. Am J Gastroenterol 2006; 101:139–147.
- Mehta SN, Pavone E, Barkun JS, Bouchard S, Barkun AN. Predictors of post-ERCP complications in patients with suspected choledocholithiasis. Endoscopy 1998; 30:457–463.
- Badalov N, Tenner S, Baillie J. The prevention, recognition and treatment of post-ERCP pancreatitis. JOP 2009; 10:88–97.
- Geenen JE, Hogan WJ, Dodds WJ, Toouli J, Venu RP. The efficacy of endoscopic sphincterotomy after cholecystectomy in patients with sphincter-of-Oddi dysfunction. N Engl J Med 1989; 320:82–87.
- Verma D, Kapadia A, Eisen GM, Adler DG. EUS vs MRCP for detection of choledocholithiasis. Gastrointest Endosc 2006; 64:248–254.
- Park DH, Kim MH, Lee SS, et al. Accuracy of magnetic resonance cholangiopancreatography for locating hepatolithiasis and detecting accompanying biliary strictures. Endoscopy 2004; 36:987–992.
- Walsh RM, Ponsky JL, Dumot J. Retained gallbladder/cystic duct remnant calculi as a cause of postcholecystectomy pain. Surg Endosc 2002; 16:981–984.
- Tantia O, Jain M, Khanna S, Sen B. Post cholecystectomy syndrome: role of cystic duct stump and re-intervention by laparoscopic surgery. J Minim Access Surg 2008; 4:71–75.
- Palanivelu C, Rangarajan M, Jategaonkar PA, Madankumar MV, Anand NV. Laparoscopic management of remnant cystic duct calculi: a retrospective study. Ann R Coll Surg Engl 2009; 91:25–29.
- Flörcken H. Gallenblasenregeneration mit Steinrecidiv nach Cholecystectomie. Deutsch Z Chir 1912; 113:604.
- Mirizzi PL. La colangiografía durante las operaciones de las vias biliares. Bol Soc Cirug Buenos Aires 1932; 16:1113.
- Soper NJ, Brunt LM. The case for routine operative cholangiography during laparoscopic cholecystectomy. Surg Clin North Am 1994; 74:953–959.
- Cuschieri A, Shimi S, Banting S, Nathanson LK, Pietrabissa A. Intraoperative cholangiography during laparoscopic cholecystectomy. Routine vs selective policy. Surg Endosc 1994; 8:302–305.
- Woods MS, Traverso LW, Kozarek RA, et al. Biliary tract complications of laparoscopic cholecystectomy are detected more frequently with routine intraoperative cholangiography. Surg Endosc 1995; 9:1076–1080.
- Vezakis A, Davides D, Ammori BJ, Martin IG, Larvin M, McMahon MJ. Intraoperative cholangiography during laparoscopic cholecystectomy. Surg Endosc 2000; 14:1118–1122.
- Ladocsi LT, Benitez LD, Filippone DR, Nance FC. Intraoperative cholangiography in laparoscopic cholecystectomy: a review of 734 consecutive cases. Am Surg 1997; 63:150–156.
- Clair DG, Brooks DC. Laparoscopic cholangiography. The case for a selective approach. Surg Clin North Am 1994; 74:961–966.
- Collins C, Maguire D, Ireland A, Fitzgerald E, O’Sullivan GC. A prospective study of common bile duct calculi in patients undergoing laparoscopic cholecystectomy: natural history of choledocholithiasis revisited. Ann Surg 2004; 239:28–33.
- Ponsky LE, Geisinger MA, Ponsky JL, Streem SB. Contemporary ‘urologic’ intervention in the pancreaticobiliary tree. Urology 2001; 57:21–25.
- Benninger J, Rabenstein T, Farnbacher M, Keppler J, Hahn EG, Schneider HT. Extracorporeal shockwave lithotripsy of gallstones in cystic duct remnants and Mirizzi syndrome. Gastrointest Endosc 2004; 60:454–459.
- Demetriades H, Pramateftakis MG, Kanellos I, Angelopoulos S, Mantzoros I, Betsis D. Retained gallbladder remnant after laparoscopic cholecystectomy. J Laparoendosc Adv Surg Tech A 2008; 18:276–279.
- Shaw C, O’Hanlon DM, Fenlon HM, McEntee GP. Cystic duct remnant and the ‘post-cholecystectomy syndrome. ’ Hepatogastroenterology 2004; 51:36–38.
- Rozsos I, Magyaródi Z, Orbán P. Cystic duct syndrome and minimally invasive surgery. [Hungarian] Orv Hetil 1997; 138:2397–2401.
- Chowbey PK, Bandyopadhyay SK, Sharma A, Khullar R, Soni V, Baijal M. Laparoscopic reintervention for residual gallstone disease. Surg Laparosc Endosc Percutan Tech 2003; 13:31–35.
- Clemente G, Giuliante F, Cadeddu F, Nuzzo G. Laparoscopic removal of gallbladder remnant and long cystic stump. Endoscopy 2001; 33:814–815.
- Rogy MA, Függer R, Herbst F, Schulz F. Reoperation after cholecystectomy. The role of the cystic duct stump. HPB Surg 1991; 4:129–134.
- Laing FC, Jeffrey RB. Choledocholithiasis and cystic duct obstruction: difficult ultrasonographic diagnosis. Radiology 1983; 146:475–479.
- Hassan H, Vilmann P. Insufficient cholecystectomy diagnosed by endoscopic ultrasonography. Endoscopy 2004; 36:236–238.
Managing acute upper GI bleeding, preventing recurrences
Upper gastrointestinal (GI) bleeding is common, costly, and potentially life-threatening. It must be managed promptly and appropriately to prevent adverse outcomes.
More people are admitted to the hospital for upper GI bleeding than for congestive heart failure or deep vein thrombosis. In the United States, the annual rate of hospitalization for upper GI bleeding is estimated to be 165 per 100,000—more than 300,000 hospitalizations per year, at a cost of $2.5 billion.1,2
Furthermore, despite advances in therapy, the case-fatality rate has remained unchanged at 7% to 10%.3 This may be because today’s patients are older and have more comorbidities than those in the past.4
CAUSES OF UPPER GI BLEEDING
Peptic ulcers account for about 60% of severe cases of upper GI bleeding,5 and they are the focus of this paper. Fortunately, up to 80% of bleeding ulcers stop bleeding spontaneously without any intervention.6
Gastroduodenal erosions account for about 12%.3
Varices due to cirrhosis are less common but more dangerous. Variceal bleeding accounts for a relatively small percentage (6%) of upper GI bleeding, but the mortality rate from a single episode of variceal bleeding is 30%, and 60% to 70% of patients die within 1 year, mostly of underlying liver disease.
Less frequent causes include Mallory-Weiss tears, erosive duodenitis, Dieulafoy ulcer (a type of vascular malformation), other vascular lesions, neoplasms, aortoenteric fistula, gastric antral vascular ectasia, and prolapse gastropathy.5
HEMATEMESIS AND MELENA
The most common presenting signs of acute upper GI bleeding are hematemesis (vomiting of blood), “coffee grounds” emesis, and melena (tarry black stools). About 30% of patients with bleeding ulcers present with hematemesis, 20% with melena, and 50% with both.7
Hematochezia (red blood in the stool) usually suggests a lower GI source of bleeding, since blood from an upper source turns black and tarry as it passes through the gut, producing melena. However, up to 5% of patients with bleeding ulcers have hematochezia,7 and it indicates heavy bleeding: bleeding of approximately 1,000 mL into the upper GI tract is needed to cause hematochezia, whereas only 50 to 100 mL is needed to cause melena.8,9 Hematochezia with signs and symptoms of hemodynamic compromise such as syncope, postural hypotension, tachycardia, and shock should therefore direct one’s attention to an upper GI source of bleeding.
Nonspecific features include nausea, vomiting, epigastric pain, vasovagal phenomena, and syncope.
WHAT IS THE PATIENT’S RISK?
An assessment of clinical severity is the first critical task, as it helps in planning treatment. Advanced age, multiple comorbidities, and hemodynamic instability call for aggressive treatment. Apart from this simple clinical rule, scoring systems have been developed.
The Rockall scoring system, the most widely used, gives estimates of the risks of recurrent bleeding and death. It is based on the three clinical factors mentioned above and on two endoscopic ones, awarding points for:
- Age—0 points if less than 60; 1 point if 60 to 79; or 2 points if 80 years or older
- Shock—1 point if the pulse is more than 100; 2 points if the systolic blood pressure is less than 100 mm Hg
- Comorbid illness—2 points for ischemic heart disease, congestive heart failure, or other major comorbidity; 3 points for renal failure, hepatic failure, or metastatic disease
- Endoscopic diagnosis—0 points if no lesion found or a Mallory-Weiss tear; 1 point for peptic ulcer, esophagitis, or erosive disease; 2 points for GI malignancy
- Endoscopic stigmata or recent hemorrhage—0 points for a clean-based ulcer or flat pigmented spot; 2 points for blood in the upper GI tract, active bleeding, a nonbleeding visible vessel, or adherent clot.
The Rockall score can thus range from 0 to 11 points, with an overall score of 0, 1, or 2 associated with an excellent prognosis.10
Other systems that are used less often include the Baylor severity scale and the Acute Physiology and Chronic Health Evaluation (APACHE) II score.
Does the patient have varices?
All variceal bleeding should be considered severe, since the 1-year death rate is so high (up to 70%). Clues pointing to variceal bleeding include previous variceal bleeding, thrombocytopenia, history of liver disease, and signs of liver disease on clinical examination.
All patients suspected of having bleeding varices should be admitted to the intensive care unit for close monitoring and should be given the highest priority, even if they are hemodynamically stable.
Is the patient hemodynamically stable?
Appropriate hemodynamic assessment includes monitoring of heart rate, blood pressure, and mental status. Tachycardia at rest, hypotension, and orthostatic changes in vital signs indicate a considerable loss of blood volume. Low urine output, dry mucous membranes, and sunken neck veins are also useful signs. (Tachycardia may be blunted if the patient is taking a beta-blocker.)
If these signs of hypovolemia are present, the initial management focuses on treating shock and on improving oxygen delivery to the vital organs. This involves repletion of the intravascular volume with intravenous infusions or blood transfusions. Supplemental oxygen also is useful, especially in elderly patients with heart disease.12
Inspection of nasogastric aspirate
In the initial assessment, it is useful to insert a nasogastric tube and inspect the aspirate. If it contains bright red blood, the patient needs an urgent endoscopic evaluation and an intensive level of care13,14; if it contains coffee-grounds material, the patient needs to be admitted to the hospital and to undergo endoscopic evaluation within 24 hours.
However, a normal aspirate does not rule out upper GI bleeding. Aljebreen et al15 found that 15% of patients with upper GI bleeding and normal nasogastric aspirate still had high-risk lesions (ie, visible bleeding or nonbleeding visible vessels) on endoscopy.
ACID-SUPPRESSION HELPS ULCERS HEAL
Acid and pepsin interfere with the healing of ulcers and other nonvariceal upper GI lesions. Further, an acidic environment promotes platelet disaggregation and fibrinolysis and impairs clot formation.16 This suggests that inhibiting gastric acid secretion and raising the gastric pH to 6 or higher may stabilize clots. Moreover, pepsinogen in the stomach is converted to its active form (pepsin) if the pH is less than 4. Therefore, keeping the pH above 4 keeps pepsinogen in an inactive form.
Histamine-2 receptor antagonists
Histamine-2 receptor antagonists were the first drugs to inhibit acid secretion, reversibly blocking histamine-2 receptors on the basolateral membrane of parietal cells. However, these drugs did not prove very useful in managing upper GI bleeding in clinical trials.17,18 In their intravenous form, they often fail to keep the gastric pH at 6 or higher, due to tachyphylaxis.19 The use of this class of drugs has declined in favor of proton pump inhibitors.
Proton pump inhibitors
Proton pump inhibitors reduce both basal and stimulated acid secretion by inhibiting hydrogen-potassium adenosine triphosphatase, the proton pump of the parietal cell.
Multiple studies have shown that proton pump inhibitors raise the gastric pH and keep it high. For example, an infusion of omeprazole (Prilosec) can keep the gastric pH above 6 for 72 hours without inducing tachyphylaxis.20,21
Started after endoscopy. Randomized controlled trials have found proton pump inhibitors to be effective when given in high doses intravenously for 72 hours after successful endoscopic treatment of bleeding ulcers with high-risk endoscopic signs, such as active bleeding or nonbleeding visible vessels.22,23
A meta-analysis indicated that these drugs decrease the incidence of recurrent peptic ulcer bleeding, the need for blood transfusions, the need for surgery, and the duration of hospitalization, but not the mortality rate.24,25 These studies also illustrate the benefit of following up endoscopic treatment to stop the bleeding with an intravenous infusion of a proton pump inhibitor.
The recommended dose of omeprazole for patients with high-risk findings on endoscopy is an 80-mg bolus followed by an 8-mg/hour infusion for 72 hours. After the patient’s condition stabilizes, oral therapy can be substituted for intravenous therapy. In patients with low-risk endoscopic findings (a clean-based ulcer or flat spot), oral proton pump inhibitors in high doses are recommended.
In either case, after the initial bleeding is treated endoscopically and hemostasis is achieved, a proton pump inhibitor is recommended for 6 to 8 weeks, or longer if the patient is also positive for Helicobacter pylori or is on daily treatment with aspirin or a nonsteroidal anti-inflammatory drug (NSAID) that is not selective for cyclo-oxygenase 2 (see below).
Started before endoscopy, these drugs reduced the frequency of actively bleeding ulcers, the duration of hospitalization, and the need for endoscopic therapy in a randomized controlled trial.26 A meta-analysis found that significantly fewer patients had signs of recent bleeding on endoscopy if they received a proton pump inhibitor 24 to 48 hours before the procedure, but it did not find any significant difference in important clinical outcomes such as death, recurrent bleeding, or surgery.27 Nevertheless, we believe that intravenous proton pump inhibitor therapy should be started before endoscopy in patients with upper GI bleeding.
Somatostatin analogues
Octreotide (Sandostatin), an analogue of the hormone somatostatin, decreases splanchnic blood flow, decreases secretion of gastric acid and pepsin, and stimulates mucus production. Although it is beneficial in treating upper GI bleeding due to varices, its benefit has not been confirmed in patients with nonvariceal upper GI bleeding.
A meta-analysis revealed that outcomes were better with high-dose intravenous proton pump inhibitor therapy than with octreotide when these drugs were started after endoscopic treatment of acute peptic ulcer bleeding.28 Nevertheless, octreotide may be useful in patients with uncontrolled nonvariceal bleeding who are awaiting endoscopy, since it is relatively safe to use.
ALL PATIENTS NEED ENDOSCOPY
All patients with upper GI bleeding need an upper endoscopic examination to diagnose and assess the risk posed by the bleeding lesion and to treat the lesion, reducing the risk of recurrent bleeding.
How urgently does endoscopy need to be done?
Endoscopy within the first 24 hours of upper GI bleeding is considered the standard of care. Patients with uncontrolled or recurrent bleeding should undergo endoscopy on an urgent basis to control the bleeding and reduce the risk of death.
However, how urgently endoscopy needs to be done is often debated. A multicenter randomized controlled trial compared outcomes in patients who underwent endoscopy within 6 hours of coming to the emergency department vs within 24 hours after the initial evaluation. The study found no significant difference in outcomes between the two groups; however, the group that underwent endoscopy sooner needed fewer transfusions.29
For a better view of the stomach
Gastric lavage improves the view of the gastric fundus but has not been proven to improve outcome.30
Promotility agents such as erythromycin and metoclopramide (Reglan) are also used to empty the stomach for better visualization.31–35 Erythromycin has been shown to improve visualization, shorten the procedure time, and prevent the need for additional endoscopy attempts in two randomized controlled studies.33,34 Furthermore, a cost-effectiveness study confirmed that giving intravenous erythromycin before endoscopy for acute upper GI bleeding saved money and resulted in an increase in quality-adjusted life-years.35
Endoscopy to diagnose bleeding and assess risk
Upper endoscopy is 90% to 95% diagnostic for acute upper GI bleeding.36
Patients at high risk (ie, older than 60 years, with severe comorbidity, or hemodynamically compromised) who have active bleeding (ie, witnessed hematemesis, red blood per nasogastric tube, or fresh blood per rectum) or a nonbleeding visible vessel should be admitted to a monitored bed or intensive care unit. Observation in a regular medical ward is appropriate for high-risk patients found to have an adherent clot. Patients with low-risk findings (eg, a clean ulcer base) are at low risk of recurrent bleeding and may be considered for early hospital discharge with appropriate outpatient follow-up.
Endoscopy to treat bleeding
About 25% of endoscopic procedures performed for upper GI bleeding include some type of treatment,39 such as injections of epinephrine, normal saline, or sclerosants; thermal cautery; argon plasma coagulation; electrocautery; or application of clips or bands. They are all equally effective, and combinations of these therapies are more effective than when they are used individually. A recent meta-analysis found dual therapy to be superior to epinephrine monotherapy in preventing recurrent bleeding, need for surgery, and death.40
How to manage adherent clots is controversial, but recent studies have revealed a significant benefit from removing them and treating the underlying lesions compared with drug therapy alone.43,45
Flat, pigmented spots and nonbleeding ulcers with a clean base do not require endoscopic treatment because the risk of recurrent bleeding is low.
Endoscopic therapy stops the bleeding in more than 90% of patients, but bleeding recurs after endoscopic therapy in 10% to 25%.46 Reversal of any severe coagulopathy with transfusions of platelets or fresh frozen plasma is essential for endoscopic hemostasis. However, coagulopathy at the time of initial bleeding and endoscopy does not appear to be associated with higher rates of recurrent bleeding following endoscopic therapy for nonvariceal upper GI bleeding.47
Patients with refractory bleeding are candidates for angiography or surgery. However, even when endoscopic hemostasis fails, endoscopy is important before angiography or surgery to pinpoint the site of bleeding and diagnose the cause.
A second endoscopic procedure is generally not recommended within 24 hours after the initial procedure.48 However, it is appropriate in cases in which clinical signs indicate recurrent bleeding or if hemostasis during the initial procedure is questionable. A meta-analysis found that routinely repeating endoscopy reduces the rate of recurrent bleeding but not the need for surgery or the risk of death.49
ALL PATIENTS SHOULD BE ADMITTED
VARICEAL BLEEDING
Variceal bleeding, a severe outcome of portal hypertension secondary to cirrhosis, carries a 6-week mortality rate of 10% to 20%.50 In view of the risk, primary prevention is indicated in patients with high-risk varices.
The mainstays of primary and secondary prevention are the nonselective beta-blockers such as nadolol (Corgard) and propranolol (Inderal). Several randomized controlled trials have shown lower rates of recurrent bleeding and death with propranolol or nadolol than with placebo.51 In doses that decrease the heart rate by 25%, beta-blockers have been shown to delay and decrease variceal hemorrhage. However, most patients require prophylactic endoscopic variceal ligation because they cannot tolerate beta-blocker therapy.
In suspected acute variceal bleeding, a somatostatin analogue should be started to decrease the portal pressure, and antibiotics should be started to reduce the risks of infection and death. Vasoactive drugs, ie, somatostatin analogues, should be started before endoscopy and continued for 5 days to reduce the chances of recurrent bleeding.52,53
Terlipressin is the only drug proven to improve the odds of survival in acute variceal bleeding. Although widely used in Europe, it has not been approved for use in the United States.
Octreotide, another option, improves hemostasis to the same extent, although it does not increase the survival rate.54,55 The recommended dose of octreotide for patients with variceal bleeding is a 50-μg intravenous bolus, followed by a 50-μg/hour infusion for 5 days.
Combining endoscopic and drug therapy improves the chances of stopping the bleeding and reduces the risk of recurrent bleeding compared with endoscopic therapy alone.56
Transjugular intrahepatic portosystemic shunting is indicated in recurrent variceal hemorrhage or in those with initial bleeding that is refractory to standard medical and endoscopic therapy. It is not the primary therapy because it doubles the risk of encephalopathy and has a high stent occlusion rate (up to 60%, lower with covered stents).
GI BLEEDING CAN CAUSE ACUTE MYOCARDIAL INFARCTION
The simultaneous presentation of acute myocardial infarction (MI) and GI hemorrhage is very serious and unfortunately common.
An acute MI occurring simultaneously with or after GI bleeding is usually precipitated by massive bleeding causing hypovolemia, hemodynamic compromise, and hypoperfusion. Conversely, the anticoagulant, antiplatelet, or thrombolytic drugs given to treat MI can precipitate GI bleeding (see below).
This distinction is important because the two scenarios have different clinical courses and prognoses. GI bleeding that precipitates an acute MI tends to be massive, whereas GI bleeding after treatment of acute MI tends to be self-limited and often resolves with reversal of underlying coagulopathy.57
Endoscopy carries a higher than average risk in patients with recent acute MI, with all-cause mortality rates as high as 1%.58 (The usual rate is 0.0004%.59) Nevertheless, endoscopy can be safely performed early on in patients with acute MI if it is done under strict monitoring in a coronary care unit.
Several studies have shown that MI patients who present with upper GI bleeding as the inciting event or patients with acute MI who are vomiting blood or who are hemodynamically unstable due to GI bleeding are significantly more likely to have a high-risk lesion and so have the greatest need for endoscopic therapy. Therefore, endoscopic intervention may be offered to MI patients at high risk who have been started on antiplatelet agents.
WARFARIN CAN PRECIPITATE BLEEDING
Acute upper GI bleeding can be a severe complication of long-term oral anticoagulation, not because the drugs cause ulcers, but rather because they exacerbate ulcers that are already present.60 Therefore, when starting warfarin (Coumadin), patients should be evaluated to determine if they have other risk factors for GI bleeding, such as ulcers.
The number of people presenting with upper GI bleeding while on warfarin therapy is increasing because of the expanding indications for long-term anticoagulation therapy, such as atrial fibrillation and deep venous thrombosis.
The risk of GI bleeding in patients who use oral anticoagulants is estimated to be 2.3 to 4.9 times higher than in nonusers.61
The goal international normalized ratio (INR) for patients on warfarin therapy is usually 2.0 to 3.0. Recent studies found that endoscopy can be safely performed in patients with acute GI bleeding whose INR is between 2.0 and 3.0.62,63 Some suggest that both the length of warfarin therapy and the INR affect the risk of bleeding.64,65
Managing patients with an INR higher than 3.0 who have an episode of GI bleeding is always a challenge. It is not uncommon to find pathologic lesions causing GI bleeding in patients who are on warfarin with a supratherapeutic INR, and thus, endoscopy is indicated. However, before endoscopy, reversal of anticoagulation should be considered.
BLEEDING IN PATIENTS ON ANTIPLATELET DRUGS
Aspirin
Aspirin decreases production of prostaglandins in the GI tract, thereby decreasing the protective and restorative properties of the gastric and duodenal mucosa and predisposing to ulcers and bleeding.
The higher the aspirin dose, the higher the risk. Aspirin doubles the risk of upper GI bleeding at daily doses of 75 mg and quadruples it at doses of 300 mg.66 Even doses as low as 10 mg can decrease gastric mucosal prostaglandin production.67 Thus, it appears that there is no risk-free dose of aspirin, and enteric-coated or buffered formulations do not appear to reduce the risk.68–70
The most important risk factor for upper GI bleeding in patients taking aspirin is a history of peptic ulcer bleeding. Approximately 15% of aspirin users who have bleeding from ulcers have recurrent bleeding within 1 year.71
As aspirin-induced GI bleeding becomes more common, health care providers often feel caught between the GI risk and the cardiovascular benefit. When considering whether to discontinue antiplatelet therapy, a cardiologist should be consulted along with a gastroenterologist to weigh the risks of GI bleeding vs thrombosis. To date, there have been no clinical trials published to suggest when antiplatelet therapy should be stopped to optimize GI and cardiovascular outcomes. An alternative is to replace aspirin with another antiplatelet drug that does not induce ulcers.
Clopidogrel
Clopidogrel (Plavix) is recommended for hospitalized patients with acute coronary syndrome who cannot tolerate the GI side effects of aspirin, according to the joint guidelines of the American College of Cardiology and the American Heart Association, with the highest level of evidence.72 This recommendation was largely based on the safety data from the CAPRIE (Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events) trial, in which the incidence of major GI bleeding was lower in the clopidogrel group (0.52%) than in the aspirin group (0.72%; P < .05).73
Aspirin plus a proton pump inhibitor
Patients who have had an episode of upper GI bleeding and who need long-term aspirin therapy should also receive a proton pump inhibitor indefinitely to prevent ulcer recurrence.
In a recent double-blind randomized controlled trial in patients with a history of aspirin-induced bleeding, the combination of low-dose aspirin plus esomeprazole (Nexium) twice a day was superior to clopidogrel by itself in terms of the rate of recurrent bleeding (0.7% vs 8.6%; P < .05).74 A similar trial showed nearly identical results: 0% upper GI bleeding in the group receiving aspirin plus esomeprazole 20 mg daily, vs 13.6% in the clopidogrel group (P = .0019).75 These studies suggest that a once-daily proton pump inhibitor combined with aspirin is a safer alternative than clopidogrel alone.
Clopidogrel plus a proton pump inhibitor
Interestingly, recent studies have shown that omeprazole decreases the antiplatelet effect of clopidogrel, possibly by inhibiting the CYP2C19 enzyme.76 However, concomitant use of pantoprazole (Protonix), lansoprazole (Prevacid), and esomeprazole did not have this effect, suggesting that although all proton pump inhibitors are metabolized to a varying degree by CYP2C19, the interaction between proton pump inhibitors and clopidogrel is not a class effect.77–79 Therefore, pantoprazole, lansoprazole, and esomeprazole may be the appropriate proton pump inhibitors to use with clopidogrel in patients who have a clear indication for the medication, consistent with current guideline recommendations.
Helicobacter pylori infection in antiplatelet drug users
TREATMENT AND PREVENTION OF NSAID-RELATED GI INJURY
About 1 in 20 users of NSAIDs develop GI complications and ulcers of varying degrees of severity, as do one in seven NSAID users over the age of 65. In fact, NSAID use accounts for 30% of hospitalizations for upper GI bleeding and deaths from this cause.82–85 In addition, approximately 15% to 30% of NSAID users have clinically silent but endoscopically evident peptic ulcers.86
NSAIDs contribute to ulcer development by depleting prostaglandins. Thus, misoprostol (Cytotec), a synthetic prostaglandin, has been used to reduce this side effect.
In a clinical trial, misoprostol reduced the incidence of NSAID-associated GI complications by 40%.87 Furthermore, it has been shown to be better than placebo in preventing recurrent gastric ulcers in patients with a history of gastric ulcer who were receiving low-dose aspirin.88
However, misoprostol is rarely used because it can cause diarrhea and abdominal cramping. Rather, the preferred drugs for preventing and treating NSAID- and aspirin-related GI lesions are proton pump inhibitors.
Numerous clinical trials using endoscopic end points showed that proton pump inhibitors in standard doses significantly reduce the incidence of ulcers associated with the use of NSAIDs.89 Proton pump inhibitor therapy has achieved a significant reduction in relative risk of upper GI bleeding in patients who received low-dose aspirin therapy, as confirmed by epidemiologic studies.90,91 The number of NSAID-related ulcers found on endoscopy could be reduced by an estimated 90% simply by using proton pump inhibitors.92
- Lewis JD, Bilker WB, Brensinger C, Farrar JT, Strom BL. Hospitalization and mortality rates from peptic ulcer disease and GI bleeding in the 1990s: relationship to sales of non-steroidal anti-inflammatory drugs and acid suppression medications. Am J Gastroenterol 2002; 97:2540–2549.
- Viviane A, Alan BN. Estimates of costs of hospital stays for variceal and nonvariceal upper gastrointestinal bleeding in the United States. Value Health 2008; 11:1–3.
- Yavorski RT, Wong RK, Maydonovitch C, Battin LS, Furnia A, Amundson DE. Analysis of 3,294 cases of upper gastrointestinal bleeding in military medical facilities. Am J Gastroenterol 1995; 90:568–573.
- Kaplan RC, Heckbert SR, Koepsell TD, et al. Risk factors for hospitalized gastrointestinal bleeding among older persons. Cardiovascular Health Study Investigators. J Am Geriatr Soc 2001; 49:126–133.
- Longstreth GF. Epidemiology of hospitalization for acute upper gastrointestinal hemorrhage: a population-based study. Am J Gastroenterol 1995; 90:206–210.
- Laine L, Peterson WL. Bleeding peptic ulcer. N Engl J Med 1994; 331:717–727.
- Wara P, Stodkilde H. Bleeding pattern before admission as guideline for emergency endoscopy. Scand J Gastroenterol 1985; 20:72–78.
- Jensen DM, Machicado GA. Diagnosis and treatment of severe hematochezia. The role of urgent colonoscopy after purge. Gastroenterology 1988; 95:1569–1574.
- Daniel WA, Egan S. The quantity of blood required to produce a tarry stool. J Am Med Assoc 1939; 113:2232.
- Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal hemorrhage. Gut 1996; 38:316–321.
- Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper-gastrointestinal hemorrhage. Lancet 2000; 356:1318–1321.
- Gralnek IM, Barkun AN, Bardou M. Management of acute bleeding from a peptic ulcer. N Engl J Med 2008; 359:928–937.
- Silverstein FE, Gilbert DA, Tedesco FJ, Buenger NK, Persing J. The national ASGE survey on upper gastrointestinal bleeding II. Clinical prognostic factors. Gastrointest Endosc 1981; 27:80–93.
- Corley DA, Stefan AM, Wolf M, Cook EF, Lee TH. Early indicators of prognosis in upper gastrointestinal hemorrhage. Am J Gastroenterol 1998; 93:336–340.
- Aljebreen AM, Fallone CA, Barkun AN. Nasogastric aspirate predicts high-risk endoscopic lesions in patients with acute upper-GI bleeding. Gastrointest Endosc 2004; 59:172–178.
- Barkun AN, Cockeram AW, Plourde V, Fedorak RN. Review article: acid suppression in non-variceal acute upper gastrointestinal bleeding. Aliment Pharmacol Ther 1999; 13:1565–1584.
- Levine JE, Leontiadis JI, Sharma VK, Howden CW. Meta-analysis: the efficacy of intravenous H2-receptor antagonists in bleeding peptic ulcer. Aliment Pharmacol Ther 2002; 16:1137–1142.
- Walt RP, Cottrell J, Mann SG, Freemantle NP, Langman MJ. Continuous intravenous famotidine for hemorrhage from peptic ulcer. Lancet 1992; 340:1058–1062.
- Labenz J, Peitz U, Leusing C, Tillenburg B, Blum AL, Börsch G. Efficacy of primed infusion with high dose ranitidine and omeprazole to maintain high intragastric pH in patients with peptic ulcer bleeding: a prospective randomized controlled study. Gut 1997; 40:36–41.
- Merki HS, Wilder-Smith CH. Do continuous infusions of omeprazole and ranitidine retain their effect with prolonged dosing? Gastroenterology 1994; 106:60–64.
- Netzer P, Gaia C, Sandoz M, et al. Effect of repeated injection and continuous infusion of omeprazole and ranitidine on intragastric pH over 72 hours. Am J Gastroenterol 1999; 94:351–357.
- Lin HJ, Lo WC, Cheng YC, Perng CL. Role of intravenous omeprazole in patients with high-risk peptic ulcer bleeding after successful endoscopic epinephrine injection: a prospective randomized comparative trial. Am J Gastroenterol 2006; 101:500–505.
- Lau JY, Sung JJ, Lee KK, et al. Effects of intravenous omeprazole on recurrent bleeding after endoscopic treatment of bleeding peptic ulcers. N Engl J Med 2000; 343:310–316.
- Leontiadis GI, Sharma VK, Howden CW. Proton pump inhibitor treatment for acute peptic ulcer bleeding. Cochrane Database Syst Rev 2006;CD002094.
- Andriulli A, Annese V, Caruso N, et al. Proton-pump inhibitors and outcome of endoscopic hemostasis in bleeding peptic ulcers: a series of meta-analyses. Am J Gastroenterol 2005; 100:207–219.
- Lau JY, Leung WK, Wu JC, et al. Omeprazole before endoscopy in patients with gastrointestinal bleeding. N Engl J Med 2007; 356:1631–1640.
- Dorward S, Sreedharan A, Leontiadis GI, Howden CW, Moayyedi P, Forman D. Proton pump inhibitor treatment initiated prior to endoscopic diagnosis in upper gastrointestinal bleeding. Cochrane Database Syst Rev 2006;CD005415.
- Bardou M, Toubouti Y, Benhaberou-Brun D, Rahme E, Barkun AN. Meta-analysis: proton-pump inhibition in high-risk patients with acute peptic ulcer bleeding. Aliment Pharmacol Ther 2005; 21:677–686.
- Bjorkman DJ, Zaman A, Fennerty MB, Lieberman D, Disario JA, Guest-Warnick G. Urgent vs elective endoscopy for acute non-variceal upper-GI bleeding: an effectiveness study. Gastointest Endosc 2004; 60:1–8.
- Lee SD, Kearney DJ. A randomized controlled trial of gastric lavage prior to endoscopy for acute upper gastrointestinal bleeding. J Clin Gastroenterol 2004; 38:861–865.
- Tack J, Janssens J, Vantrappen G, et al. Effect of erythromycin on gastric motility in controls and in diabetic gastroparesis. Gastroenterology 1992; 103:72–79.
- Xynos E, Mantides A, Papageorgiou A, Fountos A, Pechlivanides G, Vassilakis JS. Erythromycin accelerates delayed gastric emptying of solids in patients after truncal vagotomy and pyloroplasty. Eur J Surg 1992; 158:407–411.
- Coffin B, Pocard M, Panis Y, et al; Groupe des endoscopistes de garde á l’AP-HP. Erythromycin improves the quality of EGD in patients with acute upper GI bleeding: a randomized controlled study. Gastrointest Endosc 2002; 56:174–179.
- Frossard JL, Spahr L, Queneau PE, et al. Erythromycin intravenous bolus infusion in acute upper gastrointestinal bleeding: a randomized, controlled, double-blind trial. Gastroenterology 2002; 123:17–23.
- Winstead NS, Wilcox CM. Erythromycin prior to endoscopy for acute upper gastrointestinal hemorrhage: a cost-effectiveness analysis. Aliment Pharmacol Ther 2007; 26:1371–1377.
- Chak A, Cooper GS, Lloyd LE, Kolz CS, Barnhart BA, Wong RC. Effectiveness of endoscopy in patients admitted to the intensive care unit with upper GI hemorrhage. Gastrointest Endosc 2001; 53:6–13.
- Lau JY, Chung SC, Leung JW, Lo KK, Yung MY, Li AK. The evolution of stigmata of hemorrhage in bleeding peptic ulcers: a sequential endoscopic study. Endoscopy 1998; 30:513–518.
- Chung IK, Kim EJ, Lee MS, et al. Endoscopic factors predisposing to rebleeding following endoscopic hemostasis in bleeding peptic ulcers. Endoscopy 2001; 33:969–975.
- Elta GH. Acute nonvariceal upper gastrointestinal hemorrhage. Curr Treat Options Gastroenterol 2002; 5:147–152.
- Marmo R, Rotondano G, Piscopo R, Bianco MA, D’Angella R, Cipolletta L. Dual therapy versus monotherapy in the endoscopic treatment of high-risk bleeding ulcers: a meta-analysis of controlled trials. Am J Gastroenterol 2007; 102:279–289.
- Kovacs TO, Jensen DM. Recent advances in the endoscopic diagnosis and therapy of upper gastrointestinal, small intestinal, and colonic bleeding. Med Clin North Am 2002; 86:1319–1356.
- Kovacs TO, Jensen DM. Endoscopic treatment of ulcer bleeding. Curr Treat Options Gastroenterol 2007; 10:143–148.
- Jensen DM, Kovacs TO, Jutabha R, et al. Randomized trial of medical or endoscopic therapy to prevent recurrent ulcer hemorrhage in patients with adherent clots. Gastroenterology 2002; 123:407–413.
- Jensen DM, Machicado GA. Endoscopic hemostasis of ulcer hemorrhage with injection, thermal, and combination methods. Techniques Gastrointest Endosc 2005; 7:124–131.
- Bleau BL, Gostout CJ, Sherman KE, et al. Recurrent bleeding from peptic ulcer associated with adherent clot: a randomized study comparing endoscopic treatment with medical therapy. Gastrointest Endosc 2002; 56:1–6.
- Lau JY, Sung JJ, Lam YH, et al. Endoscopic retreatment compared with surgery in patients with recurrent bleeding after initial endoscopic control of bleeding ulcers. N Engl J Med 1999; 340:751–756.
- Wolf AT, Wasan SK, Saltzman JR. Impact of anticoagulation on rebleeding following endoscopic therapy for nonvariceal upper gastrointestinal hemorrhage. Am J Gastroenterol 2007; 102:290–296.
- Barkun A, Bardou M, Marshall JK; Nonvariceal Upper GI Bleeding Consensus Conference Group. Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med 2003; 139:843–857.
- Marmo R, Rotondano G, Bianco MA, Piscopo R, Prisco A, Cipolletta L. Outcome of endoscopic treatment for peptic ulcer bleeding: is a second look necessary? A meta-analysis. Gastrointest Endosc 2003; 57:62–67.
- Dell’Era A, deFrancis R, Iannuzzi F. Acute variceal bleeding: pharmacological treatment and primary/secondary prophylaxis. Best Pract Res Clin Gastroenterol 2008; 22:279–294.
- Jalan R, Hayes PC. UK guidelines on the management of variceal hemorrhage in cirrhotic patients. British Society of Gastroenterology. Gut 2000; 46( suppl 3–4):III1–III15.
- Bernard B, Lebrec D, Mathurin P, Opolon P, Poynard T. Beta-adrenergic antagonists in the prevention of gastrointestinal rebleeding in patients with cirrhosis: a meta-analysis. Hepatology 1997; 25:63–70.
- De Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2005; 43:167–176.
- Levacher S, Letoumelin P, Pateron D, Blaise M, Lapandry C, Pourriat JL. Early administration of terlipressin plus glyceryl trinitrate to control active upper gastrointestinal bleeding in cirrhotic patients. Lancet 1995; 346:865–868.
- Abraldes JG, Bosch J. Somatostatin and analogues in portal hypertension. Hepatology 2002; 35:1305–1312.
- Banares R, Albillos A, Rincon D, et al. Endoscopic treatment versus endoscopic plus pharmacological treatment for acute variceal bleeding: a meta analysis. Hepatology 2002; 35:609–615.
- Cappell M. Gastrointenstinal bleeding associated with myocardial infarction. Gastroenterol Clin North Am 2000; 29:423–444.
- Lin S, Konstance R, Jollis J, Fisher DA. The utility of upper endoscopy in patients with concomitant upper gastrointestinal bleeding and acute myocardial infarction. Dig Dis Sci 2006; 51:2377–2383.
- Silvis SE, Nebel O, Rogers G, Sugawa C, Mandelstam P. Endoscopic complications. Results of the 1974 American Society for Gastrointestinal Endoscopy Survey. JAMA 1976; 235:928–930.
- Lanas A, Garcia-Rodriguez LA, Arroyo MT, et al; Investigators of the Asociación Española de Gastroenterología (AEG). Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol 2007; 102:507–515.
- Shorr RI, Ray WA, Daugherty JR, Griffin MR. Concurrent use of nonsteroidal anti-inflammatory drugs and oral anticoagulants places elderly persons at high risk for hemorrhagic peptic ulcer disease. Arch Intern Med 1993; 153:1665–1670.
- Tabibian N. Acute gastrointestinal bleeding in anticoagulated patients: a prospective evaluation. Am J Gastroenterol 1989; 84:10–12.
- Choudari CP, Rajgopal C, Palmer KR. Acute gastrointestinal hemorrhage in anticoagulated patients: diagnoses and response to endoscopic treatment. Gut 1994; 35:464–466.
- Petty GW, Brown RD, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Frequency of major complications of aspirin, warfarin, and intravenous heparin for secondary stroke prevention: a population-based study. Ann Intern Med 1999; 130:14–22.
- Landefeld CS, Rosenblatt MW, Goldman L. Bleeding in outpatients treated with warfarin: relation to the prothrombin time and important remediable lesions. Am J Med 1989; 87:153–159.
- Weil J, Colin-Jones D, Langman M, et al. Prophylactic aspirin and risk of peptic ulcer bleeding. BMJ 1995; 310:827–830.
- Cryer B, Feldman M. Effects of very low dose daily, long-term aspirin therapy on gastric, duodenal, and rectal prostaglandin levels and on mucosal injury in healthy humans. Gastroenterology 1999; 117:17–25.
- De Abajo FJ, Garcia Rodriguez LA. Risk of upper gastrointestinal bleeding and perforation associated with low-dose aspirin as plain and enteric-coated formulations. BMC Clin Pharmacol 2001; 1:1.
- Kelly JP, Kaufman DW, Jurgelon JM, Sheehan J, Koff RS, Shapiro S. Risk of aspirin-associated major upper gastrointestinal bleeding with enteric coated or buffered product. Lancet 1996; 348:1413–1416.
- Garcia Rodriguez LA, Hernandez-Diaz S, De Abajo FJ. Association between aspirin and upper gastrointestinal complications: systematic review of epidemiological studies. Br J Clin Pharmacol 2001; 52:563–571.
- Wilcox CM, Ladabaum U. A patient with high risk of gastrointestinal bleeding requiring nonsteroidal anti-inflammatory drugs. Clin Gastroenterol Hepatol 2006; 4:1090–1093.
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction. J Am Coll Cardiol 2007; 50:e1–e157.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996; 348:1329–1339.
- Chan FK, Ching JY, Hung LC, et al. Clopidogrel versus aspirin and esomeprazole to prevent recurrent ulcer bleeding. N Engl J Med 2005; 352:238–244.
- Lai KC, Chu KM, Hui WM, et al. Esomeprazole with aspirin versus clopidogrel for prevention of recurrent gastrointestinal ulcer complications. Clin Gastroenterol Hepatol 2006; 4:860–865.
- Ho MP, Maddox TM, Wang L, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA 2009; 301:937–944.
- Siller-Matula JM, Spiel AO, Lang IM, Kreiner G, Christ G, Jilma B. Effects of pantoprazole and esomeprazole on platelet inhibition by clopidogrel. Am Heart J 2009; 157:148.e1–e5.
- Small DS, Farid NA, Payne CD, et al. Effects of proton pump inhibitor lansoprazole on the pharmacokinetics and pharmacodynamics of prasugel and clopidogrel. J Clin Pharmacol 2008; 48:475–484.
- Ishizaki T, Horai Y. Review article: cytochrome P450 and the metabolism of proton pump inhibitors—emphasis on rabeprazole. Aliment Pharacol Ther 1999; 13 (suppl 3):27–36.
- Lanas A, Fuentes J, Benito R, Serrano P, Bajador E, Sainz R. Helicobacter pylori increases the risk of upper gastrointestinal bleeding in patients taking low-dose aspirin. Aliment Pharmacol Ther 2002; 16:779–786.
- Chan FK. NSAID-Induced peptic ulcers and Helicobacter pylori infection: implications for patient management. Drug Saf 2005; 28:287–300.
- Bombardier V, Laine L, Reicin A, et al; VIGOR Study Group. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis: VIGOR Study Group. N Eng J Med 2000; 343:1520–1528.
- Griffin MR, Ray WA, Schaffner W. Nonsteroidal anti-inflammatory drug use and death from peptic ulcer in elderly persons. Ann Intern Med 1988; 109:359–363.
- Griffin MR, Piper JM, Daugherty JR, Snowden M, Ray WA. Nonsteroidal anti-inflammatory drug use and increased risk for peptic ulcer disease in elderly persons. Ann Intern Med 1991; 114:257–263.
- Smalley WE, Ray WA, Daugherty JR, Griffin MR. Nonsteroidal anti-inflammatory drugs and the incidence of hospitalizations for peptic ulcer disease in elderly persons. Am J Epidemiol 1995; 141:539–545.
- Laine L. Approaches to nonsteroidal anti-inflammatory drug use in the high-risk patient. Gastroenterology 2001; 120:594–606.
- Silverstein FE, Graham DY, Senior JR, et al. Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal anti-inflammatory drugs. A randomized, double blind, placebo controlled trial. Ann Intern Med 1995; 123:241–249.
- Goldstein JL, Huang B, Amer F, Christopoulos NG. Ulcer recurrence in high-risk patients receiving nonsteroidal anti-inflammatory drugs plus low dose aspirin: results of a post hoc subanalysis. Clin Ther 2004; 26:1637–1643.
- Berger JS, Stebbins A, Granger CB, et al. Initial aspirin dose and outcome among ST-elevation myocardial infarction patients treated with fibrinolytic therapy. Circulation 2008; 117:192–199.
- Lanas A, Garcia-Rodriguez LA, Arroyo MT, et al; Investigators of the Asociación Española de Gastroenterología (AEG). Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol 2007; 102:507–515.
- Chin MW, Yong G, Bulsara MK, Rankin J, Forbes GM. Predictive and protective factors associated with upper gastrointestinal bleeding after percutaneous coronary intervention: a case-control study. Am J Gastroenterol 2007; 102:2411–2416.
- Hunt RH, Bazzoli F. Should NSAID/low dose aspirin takers be tested routinely for H. Pylori infection and treated if positive? Implications for primary risk of ulcer and ulcer relapse after initial healing. Aliment Pharmacol Ther 2004; 19 (suppl 1):9–16.
Upper gastrointestinal (GI) bleeding is common, costly, and potentially life-threatening. It must be managed promptly and appropriately to prevent adverse outcomes.
More people are admitted to the hospital for upper GI bleeding than for congestive heart failure or deep vein thrombosis. In the United States, the annual rate of hospitalization for upper GI bleeding is estimated to be 165 per 100,000—more than 300,000 hospitalizations per year, at a cost of $2.5 billion.1,2
Furthermore, despite advances in therapy, the case-fatality rate has remained unchanged at 7% to 10%.3 This may be because today’s patients are older and have more comorbidities than those in the past.4
CAUSES OF UPPER GI BLEEDING
Peptic ulcers account for about 60% of severe cases of upper GI bleeding,5 and they are the focus of this paper. Fortunately, up to 80% of bleeding ulcers stop bleeding spontaneously without any intervention.6
Gastroduodenal erosions account for about 12%.3
Varices due to cirrhosis are less common but more dangerous. Variceal bleeding accounts for a relatively small percentage (6%) of upper GI bleeding, but the mortality rate from a single episode of variceal bleeding is 30%, and 60% to 70% of patients die within 1 year, mostly of underlying liver disease.
Less frequent causes include Mallory-Weiss tears, erosive duodenitis, Dieulafoy ulcer (a type of vascular malformation), other vascular lesions, neoplasms, aortoenteric fistula, gastric antral vascular ectasia, and prolapse gastropathy.5
HEMATEMESIS AND MELENA
The most common presenting signs of acute upper GI bleeding are hematemesis (vomiting of blood), “coffee grounds” emesis, and melena (tarry black stools). About 30% of patients with bleeding ulcers present with hematemesis, 20% with melena, and 50% with both.7
Hematochezia (red blood in the stool) usually suggests a lower GI source of bleeding, since blood from an upper source turns black and tarry as it passes through the gut, producing melena. However, up to 5% of patients with bleeding ulcers have hematochezia,7 and it indicates heavy bleeding: bleeding of approximately 1,000 mL into the upper GI tract is needed to cause hematochezia, whereas only 50 to 100 mL is needed to cause melena.8,9 Hematochezia with signs and symptoms of hemodynamic compromise such as syncope, postural hypotension, tachycardia, and shock should therefore direct one’s attention to an upper GI source of bleeding.
Nonspecific features include nausea, vomiting, epigastric pain, vasovagal phenomena, and syncope.
WHAT IS THE PATIENT’S RISK?
An assessment of clinical severity is the first critical task, as it helps in planning treatment. Advanced age, multiple comorbidities, and hemodynamic instability call for aggressive treatment. Apart from this simple clinical rule, scoring systems have been developed.
The Rockall scoring system, the most widely used, gives estimates of the risks of recurrent bleeding and death. It is based on the three clinical factors mentioned above and on two endoscopic ones, awarding points for:
- Age—0 points if less than 60; 1 point if 60 to 79; or 2 points if 80 years or older
- Shock—1 point if the pulse is more than 100; 2 points if the systolic blood pressure is less than 100 mm Hg
- Comorbid illness—2 points for ischemic heart disease, congestive heart failure, or other major comorbidity; 3 points for renal failure, hepatic failure, or metastatic disease
- Endoscopic diagnosis—0 points if no lesion found or a Mallory-Weiss tear; 1 point for peptic ulcer, esophagitis, or erosive disease; 2 points for GI malignancy
- Endoscopic stigmata or recent hemorrhage—0 points for a clean-based ulcer or flat pigmented spot; 2 points for blood in the upper GI tract, active bleeding, a nonbleeding visible vessel, or adherent clot.
The Rockall score can thus range from 0 to 11 points, with an overall score of 0, 1, or 2 associated with an excellent prognosis.10
Other systems that are used less often include the Baylor severity scale and the Acute Physiology and Chronic Health Evaluation (APACHE) II score.
Does the patient have varices?
All variceal bleeding should be considered severe, since the 1-year death rate is so high (up to 70%). Clues pointing to variceal bleeding include previous variceal bleeding, thrombocytopenia, history of liver disease, and signs of liver disease on clinical examination.
All patients suspected of having bleeding varices should be admitted to the intensive care unit for close monitoring and should be given the highest priority, even if they are hemodynamically stable.
Is the patient hemodynamically stable?
Appropriate hemodynamic assessment includes monitoring of heart rate, blood pressure, and mental status. Tachycardia at rest, hypotension, and orthostatic changes in vital signs indicate a considerable loss of blood volume. Low urine output, dry mucous membranes, and sunken neck veins are also useful signs. (Tachycardia may be blunted if the patient is taking a beta-blocker.)
If these signs of hypovolemia are present, the initial management focuses on treating shock and on improving oxygen delivery to the vital organs. This involves repletion of the intravascular volume with intravenous infusions or blood transfusions. Supplemental oxygen also is useful, especially in elderly patients with heart disease.12
Inspection of nasogastric aspirate
In the initial assessment, it is useful to insert a nasogastric tube and inspect the aspirate. If it contains bright red blood, the patient needs an urgent endoscopic evaluation and an intensive level of care13,14; if it contains coffee-grounds material, the patient needs to be admitted to the hospital and to undergo endoscopic evaluation within 24 hours.
However, a normal aspirate does not rule out upper GI bleeding. Aljebreen et al15 found that 15% of patients with upper GI bleeding and normal nasogastric aspirate still had high-risk lesions (ie, visible bleeding or nonbleeding visible vessels) on endoscopy.
ACID-SUPPRESSION HELPS ULCERS HEAL
Acid and pepsin interfere with the healing of ulcers and other nonvariceal upper GI lesions. Further, an acidic environment promotes platelet disaggregation and fibrinolysis and impairs clot formation.16 This suggests that inhibiting gastric acid secretion and raising the gastric pH to 6 or higher may stabilize clots. Moreover, pepsinogen in the stomach is converted to its active form (pepsin) if the pH is less than 4. Therefore, keeping the pH above 4 keeps pepsinogen in an inactive form.
Histamine-2 receptor antagonists
Histamine-2 receptor antagonists were the first drugs to inhibit acid secretion, reversibly blocking histamine-2 receptors on the basolateral membrane of parietal cells. However, these drugs did not prove very useful in managing upper GI bleeding in clinical trials.17,18 In their intravenous form, they often fail to keep the gastric pH at 6 or higher, due to tachyphylaxis.19 The use of this class of drugs has declined in favor of proton pump inhibitors.
Proton pump inhibitors
Proton pump inhibitors reduce both basal and stimulated acid secretion by inhibiting hydrogen-potassium adenosine triphosphatase, the proton pump of the parietal cell.
Multiple studies have shown that proton pump inhibitors raise the gastric pH and keep it high. For example, an infusion of omeprazole (Prilosec) can keep the gastric pH above 6 for 72 hours without inducing tachyphylaxis.20,21
Started after endoscopy. Randomized controlled trials have found proton pump inhibitors to be effective when given in high doses intravenously for 72 hours after successful endoscopic treatment of bleeding ulcers with high-risk endoscopic signs, such as active bleeding or nonbleeding visible vessels.22,23
A meta-analysis indicated that these drugs decrease the incidence of recurrent peptic ulcer bleeding, the need for blood transfusions, the need for surgery, and the duration of hospitalization, but not the mortality rate.24,25 These studies also illustrate the benefit of following up endoscopic treatment to stop the bleeding with an intravenous infusion of a proton pump inhibitor.
The recommended dose of omeprazole for patients with high-risk findings on endoscopy is an 80-mg bolus followed by an 8-mg/hour infusion for 72 hours. After the patient’s condition stabilizes, oral therapy can be substituted for intravenous therapy. In patients with low-risk endoscopic findings (a clean-based ulcer or flat spot), oral proton pump inhibitors in high doses are recommended.
In either case, after the initial bleeding is treated endoscopically and hemostasis is achieved, a proton pump inhibitor is recommended for 6 to 8 weeks, or longer if the patient is also positive for Helicobacter pylori or is on daily treatment with aspirin or a nonsteroidal anti-inflammatory drug (NSAID) that is not selective for cyclo-oxygenase 2 (see below).
Started before endoscopy, these drugs reduced the frequency of actively bleeding ulcers, the duration of hospitalization, and the need for endoscopic therapy in a randomized controlled trial.26 A meta-analysis found that significantly fewer patients had signs of recent bleeding on endoscopy if they received a proton pump inhibitor 24 to 48 hours before the procedure, but it did not find any significant difference in important clinical outcomes such as death, recurrent bleeding, or surgery.27 Nevertheless, we believe that intravenous proton pump inhibitor therapy should be started before endoscopy in patients with upper GI bleeding.
Somatostatin analogues
Octreotide (Sandostatin), an analogue of the hormone somatostatin, decreases splanchnic blood flow, decreases secretion of gastric acid and pepsin, and stimulates mucus production. Although it is beneficial in treating upper GI bleeding due to varices, its benefit has not been confirmed in patients with nonvariceal upper GI bleeding.
A meta-analysis revealed that outcomes were better with high-dose intravenous proton pump inhibitor therapy than with octreotide when these drugs were started after endoscopic treatment of acute peptic ulcer bleeding.28 Nevertheless, octreotide may be useful in patients with uncontrolled nonvariceal bleeding who are awaiting endoscopy, since it is relatively safe to use.
ALL PATIENTS NEED ENDOSCOPY
All patients with upper GI bleeding need an upper endoscopic examination to diagnose and assess the risk posed by the bleeding lesion and to treat the lesion, reducing the risk of recurrent bleeding.
How urgently does endoscopy need to be done?
Endoscopy within the first 24 hours of upper GI bleeding is considered the standard of care. Patients with uncontrolled or recurrent bleeding should undergo endoscopy on an urgent basis to control the bleeding and reduce the risk of death.
However, how urgently endoscopy needs to be done is often debated. A multicenter randomized controlled trial compared outcomes in patients who underwent endoscopy within 6 hours of coming to the emergency department vs within 24 hours after the initial evaluation. The study found no significant difference in outcomes between the two groups; however, the group that underwent endoscopy sooner needed fewer transfusions.29
For a better view of the stomach
Gastric lavage improves the view of the gastric fundus but has not been proven to improve outcome.30
Promotility agents such as erythromycin and metoclopramide (Reglan) are also used to empty the stomach for better visualization.31–35 Erythromycin has been shown to improve visualization, shorten the procedure time, and prevent the need for additional endoscopy attempts in two randomized controlled studies.33,34 Furthermore, a cost-effectiveness study confirmed that giving intravenous erythromycin before endoscopy for acute upper GI bleeding saved money and resulted in an increase in quality-adjusted life-years.35
Endoscopy to diagnose bleeding and assess risk
Upper endoscopy is 90% to 95% diagnostic for acute upper GI bleeding.36
Patients at high risk (ie, older than 60 years, with severe comorbidity, or hemodynamically compromised) who have active bleeding (ie, witnessed hematemesis, red blood per nasogastric tube, or fresh blood per rectum) or a nonbleeding visible vessel should be admitted to a monitored bed or intensive care unit. Observation in a regular medical ward is appropriate for high-risk patients found to have an adherent clot. Patients with low-risk findings (eg, a clean ulcer base) are at low risk of recurrent bleeding and may be considered for early hospital discharge with appropriate outpatient follow-up.
Endoscopy to treat bleeding
About 25% of endoscopic procedures performed for upper GI bleeding include some type of treatment,39 such as injections of epinephrine, normal saline, or sclerosants; thermal cautery; argon plasma coagulation; electrocautery; or application of clips or bands. They are all equally effective, and combinations of these therapies are more effective than when they are used individually. A recent meta-analysis found dual therapy to be superior to epinephrine monotherapy in preventing recurrent bleeding, need for surgery, and death.40
How to manage adherent clots is controversial, but recent studies have revealed a significant benefit from removing them and treating the underlying lesions compared with drug therapy alone.43,45
Flat, pigmented spots and nonbleeding ulcers with a clean base do not require endoscopic treatment because the risk of recurrent bleeding is low.
Endoscopic therapy stops the bleeding in more than 90% of patients, but bleeding recurs after endoscopic therapy in 10% to 25%.46 Reversal of any severe coagulopathy with transfusions of platelets or fresh frozen plasma is essential for endoscopic hemostasis. However, coagulopathy at the time of initial bleeding and endoscopy does not appear to be associated with higher rates of recurrent bleeding following endoscopic therapy for nonvariceal upper GI bleeding.47
Patients with refractory bleeding are candidates for angiography or surgery. However, even when endoscopic hemostasis fails, endoscopy is important before angiography or surgery to pinpoint the site of bleeding and diagnose the cause.
A second endoscopic procedure is generally not recommended within 24 hours after the initial procedure.48 However, it is appropriate in cases in which clinical signs indicate recurrent bleeding or if hemostasis during the initial procedure is questionable. A meta-analysis found that routinely repeating endoscopy reduces the rate of recurrent bleeding but not the need for surgery or the risk of death.49
ALL PATIENTS SHOULD BE ADMITTED
VARICEAL BLEEDING
Variceal bleeding, a severe outcome of portal hypertension secondary to cirrhosis, carries a 6-week mortality rate of 10% to 20%.50 In view of the risk, primary prevention is indicated in patients with high-risk varices.
The mainstays of primary and secondary prevention are the nonselective beta-blockers such as nadolol (Corgard) and propranolol (Inderal). Several randomized controlled trials have shown lower rates of recurrent bleeding and death with propranolol or nadolol than with placebo.51 In doses that decrease the heart rate by 25%, beta-blockers have been shown to delay and decrease variceal hemorrhage. However, most patients require prophylactic endoscopic variceal ligation because they cannot tolerate beta-blocker therapy.
In suspected acute variceal bleeding, a somatostatin analogue should be started to decrease the portal pressure, and antibiotics should be started to reduce the risks of infection and death. Vasoactive drugs, ie, somatostatin analogues, should be started before endoscopy and continued for 5 days to reduce the chances of recurrent bleeding.52,53
Terlipressin is the only drug proven to improve the odds of survival in acute variceal bleeding. Although widely used in Europe, it has not been approved for use in the United States.
Octreotide, another option, improves hemostasis to the same extent, although it does not increase the survival rate.54,55 The recommended dose of octreotide for patients with variceal bleeding is a 50-μg intravenous bolus, followed by a 50-μg/hour infusion for 5 days.
Combining endoscopic and drug therapy improves the chances of stopping the bleeding and reduces the risk of recurrent bleeding compared with endoscopic therapy alone.56
Transjugular intrahepatic portosystemic shunting is indicated in recurrent variceal hemorrhage or in those with initial bleeding that is refractory to standard medical and endoscopic therapy. It is not the primary therapy because it doubles the risk of encephalopathy and has a high stent occlusion rate (up to 60%, lower with covered stents).
GI BLEEDING CAN CAUSE ACUTE MYOCARDIAL INFARCTION
The simultaneous presentation of acute myocardial infarction (MI) and GI hemorrhage is very serious and unfortunately common.
An acute MI occurring simultaneously with or after GI bleeding is usually precipitated by massive bleeding causing hypovolemia, hemodynamic compromise, and hypoperfusion. Conversely, the anticoagulant, antiplatelet, or thrombolytic drugs given to treat MI can precipitate GI bleeding (see below).
This distinction is important because the two scenarios have different clinical courses and prognoses. GI bleeding that precipitates an acute MI tends to be massive, whereas GI bleeding after treatment of acute MI tends to be self-limited and often resolves with reversal of underlying coagulopathy.57
Endoscopy carries a higher than average risk in patients with recent acute MI, with all-cause mortality rates as high as 1%.58 (The usual rate is 0.0004%.59) Nevertheless, endoscopy can be safely performed early on in patients with acute MI if it is done under strict monitoring in a coronary care unit.
Several studies have shown that MI patients who present with upper GI bleeding as the inciting event or patients with acute MI who are vomiting blood or who are hemodynamically unstable due to GI bleeding are significantly more likely to have a high-risk lesion and so have the greatest need for endoscopic therapy. Therefore, endoscopic intervention may be offered to MI patients at high risk who have been started on antiplatelet agents.
WARFARIN CAN PRECIPITATE BLEEDING
Acute upper GI bleeding can be a severe complication of long-term oral anticoagulation, not because the drugs cause ulcers, but rather because they exacerbate ulcers that are already present.60 Therefore, when starting warfarin (Coumadin), patients should be evaluated to determine if they have other risk factors for GI bleeding, such as ulcers.
The number of people presenting with upper GI bleeding while on warfarin therapy is increasing because of the expanding indications for long-term anticoagulation therapy, such as atrial fibrillation and deep venous thrombosis.
The risk of GI bleeding in patients who use oral anticoagulants is estimated to be 2.3 to 4.9 times higher than in nonusers.61
The goal international normalized ratio (INR) for patients on warfarin therapy is usually 2.0 to 3.0. Recent studies found that endoscopy can be safely performed in patients with acute GI bleeding whose INR is between 2.0 and 3.0.62,63 Some suggest that both the length of warfarin therapy and the INR affect the risk of bleeding.64,65
Managing patients with an INR higher than 3.0 who have an episode of GI bleeding is always a challenge. It is not uncommon to find pathologic lesions causing GI bleeding in patients who are on warfarin with a supratherapeutic INR, and thus, endoscopy is indicated. However, before endoscopy, reversal of anticoagulation should be considered.
BLEEDING IN PATIENTS ON ANTIPLATELET DRUGS
Aspirin
Aspirin decreases production of prostaglandins in the GI tract, thereby decreasing the protective and restorative properties of the gastric and duodenal mucosa and predisposing to ulcers and bleeding.
The higher the aspirin dose, the higher the risk. Aspirin doubles the risk of upper GI bleeding at daily doses of 75 mg and quadruples it at doses of 300 mg.66 Even doses as low as 10 mg can decrease gastric mucosal prostaglandin production.67 Thus, it appears that there is no risk-free dose of aspirin, and enteric-coated or buffered formulations do not appear to reduce the risk.68–70
The most important risk factor for upper GI bleeding in patients taking aspirin is a history of peptic ulcer bleeding. Approximately 15% of aspirin users who have bleeding from ulcers have recurrent bleeding within 1 year.71
As aspirin-induced GI bleeding becomes more common, health care providers often feel caught between the GI risk and the cardiovascular benefit. When considering whether to discontinue antiplatelet therapy, a cardiologist should be consulted along with a gastroenterologist to weigh the risks of GI bleeding vs thrombosis. To date, there have been no clinical trials published to suggest when antiplatelet therapy should be stopped to optimize GI and cardiovascular outcomes. An alternative is to replace aspirin with another antiplatelet drug that does not induce ulcers.
Clopidogrel
Clopidogrel (Plavix) is recommended for hospitalized patients with acute coronary syndrome who cannot tolerate the GI side effects of aspirin, according to the joint guidelines of the American College of Cardiology and the American Heart Association, with the highest level of evidence.72 This recommendation was largely based on the safety data from the CAPRIE (Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events) trial, in which the incidence of major GI bleeding was lower in the clopidogrel group (0.52%) than in the aspirin group (0.72%; P < .05).73
Aspirin plus a proton pump inhibitor
Patients who have had an episode of upper GI bleeding and who need long-term aspirin therapy should also receive a proton pump inhibitor indefinitely to prevent ulcer recurrence.
In a recent double-blind randomized controlled trial in patients with a history of aspirin-induced bleeding, the combination of low-dose aspirin plus esomeprazole (Nexium) twice a day was superior to clopidogrel by itself in terms of the rate of recurrent bleeding (0.7% vs 8.6%; P < .05).74 A similar trial showed nearly identical results: 0% upper GI bleeding in the group receiving aspirin plus esomeprazole 20 mg daily, vs 13.6% in the clopidogrel group (P = .0019).75 These studies suggest that a once-daily proton pump inhibitor combined with aspirin is a safer alternative than clopidogrel alone.
Clopidogrel plus a proton pump inhibitor
Interestingly, recent studies have shown that omeprazole decreases the antiplatelet effect of clopidogrel, possibly by inhibiting the CYP2C19 enzyme.76 However, concomitant use of pantoprazole (Protonix), lansoprazole (Prevacid), and esomeprazole did not have this effect, suggesting that although all proton pump inhibitors are metabolized to a varying degree by CYP2C19, the interaction between proton pump inhibitors and clopidogrel is not a class effect.77–79 Therefore, pantoprazole, lansoprazole, and esomeprazole may be the appropriate proton pump inhibitors to use with clopidogrel in patients who have a clear indication for the medication, consistent with current guideline recommendations.
Helicobacter pylori infection in antiplatelet drug users
TREATMENT AND PREVENTION OF NSAID-RELATED GI INJURY
About 1 in 20 users of NSAIDs develop GI complications and ulcers of varying degrees of severity, as do one in seven NSAID users over the age of 65. In fact, NSAID use accounts for 30% of hospitalizations for upper GI bleeding and deaths from this cause.82–85 In addition, approximately 15% to 30% of NSAID users have clinically silent but endoscopically evident peptic ulcers.86
NSAIDs contribute to ulcer development by depleting prostaglandins. Thus, misoprostol (Cytotec), a synthetic prostaglandin, has been used to reduce this side effect.
In a clinical trial, misoprostol reduced the incidence of NSAID-associated GI complications by 40%.87 Furthermore, it has been shown to be better than placebo in preventing recurrent gastric ulcers in patients with a history of gastric ulcer who were receiving low-dose aspirin.88
However, misoprostol is rarely used because it can cause diarrhea and abdominal cramping. Rather, the preferred drugs for preventing and treating NSAID- and aspirin-related GI lesions are proton pump inhibitors.
Numerous clinical trials using endoscopic end points showed that proton pump inhibitors in standard doses significantly reduce the incidence of ulcers associated with the use of NSAIDs.89 Proton pump inhibitor therapy has achieved a significant reduction in relative risk of upper GI bleeding in patients who received low-dose aspirin therapy, as confirmed by epidemiologic studies.90,91 The number of NSAID-related ulcers found on endoscopy could be reduced by an estimated 90% simply by using proton pump inhibitors.92
Upper gastrointestinal (GI) bleeding is common, costly, and potentially life-threatening. It must be managed promptly and appropriately to prevent adverse outcomes.
More people are admitted to the hospital for upper GI bleeding than for congestive heart failure or deep vein thrombosis. In the United States, the annual rate of hospitalization for upper GI bleeding is estimated to be 165 per 100,000—more than 300,000 hospitalizations per year, at a cost of $2.5 billion.1,2
Furthermore, despite advances in therapy, the case-fatality rate has remained unchanged at 7% to 10%.3 This may be because today’s patients are older and have more comorbidities than those in the past.4
CAUSES OF UPPER GI BLEEDING
Peptic ulcers account for about 60% of severe cases of upper GI bleeding,5 and they are the focus of this paper. Fortunately, up to 80% of bleeding ulcers stop bleeding spontaneously without any intervention.6
Gastroduodenal erosions account for about 12%.3
Varices due to cirrhosis are less common but more dangerous. Variceal bleeding accounts for a relatively small percentage (6%) of upper GI bleeding, but the mortality rate from a single episode of variceal bleeding is 30%, and 60% to 70% of patients die within 1 year, mostly of underlying liver disease.
Less frequent causes include Mallory-Weiss tears, erosive duodenitis, Dieulafoy ulcer (a type of vascular malformation), other vascular lesions, neoplasms, aortoenteric fistula, gastric antral vascular ectasia, and prolapse gastropathy.5
HEMATEMESIS AND MELENA
The most common presenting signs of acute upper GI bleeding are hematemesis (vomiting of blood), “coffee grounds” emesis, and melena (tarry black stools). About 30% of patients with bleeding ulcers present with hematemesis, 20% with melena, and 50% with both.7
Hematochezia (red blood in the stool) usually suggests a lower GI source of bleeding, since blood from an upper source turns black and tarry as it passes through the gut, producing melena. However, up to 5% of patients with bleeding ulcers have hematochezia,7 and it indicates heavy bleeding: bleeding of approximately 1,000 mL into the upper GI tract is needed to cause hematochezia, whereas only 50 to 100 mL is needed to cause melena.8,9 Hematochezia with signs and symptoms of hemodynamic compromise such as syncope, postural hypotension, tachycardia, and shock should therefore direct one’s attention to an upper GI source of bleeding.
Nonspecific features include nausea, vomiting, epigastric pain, vasovagal phenomena, and syncope.
WHAT IS THE PATIENT’S RISK?
An assessment of clinical severity is the first critical task, as it helps in planning treatment. Advanced age, multiple comorbidities, and hemodynamic instability call for aggressive treatment. Apart from this simple clinical rule, scoring systems have been developed.
The Rockall scoring system, the most widely used, gives estimates of the risks of recurrent bleeding and death. It is based on the three clinical factors mentioned above and on two endoscopic ones, awarding points for:
- Age—0 points if less than 60; 1 point if 60 to 79; or 2 points if 80 years or older
- Shock—1 point if the pulse is more than 100; 2 points if the systolic blood pressure is less than 100 mm Hg
- Comorbid illness—2 points for ischemic heart disease, congestive heart failure, or other major comorbidity; 3 points for renal failure, hepatic failure, or metastatic disease
- Endoscopic diagnosis—0 points if no lesion found or a Mallory-Weiss tear; 1 point for peptic ulcer, esophagitis, or erosive disease; 2 points for GI malignancy
- Endoscopic stigmata or recent hemorrhage—0 points for a clean-based ulcer or flat pigmented spot; 2 points for blood in the upper GI tract, active bleeding, a nonbleeding visible vessel, or adherent clot.
The Rockall score can thus range from 0 to 11 points, with an overall score of 0, 1, or 2 associated with an excellent prognosis.10
Other systems that are used less often include the Baylor severity scale and the Acute Physiology and Chronic Health Evaluation (APACHE) II score.
Does the patient have varices?
All variceal bleeding should be considered severe, since the 1-year death rate is so high (up to 70%). Clues pointing to variceal bleeding include previous variceal bleeding, thrombocytopenia, history of liver disease, and signs of liver disease on clinical examination.
All patients suspected of having bleeding varices should be admitted to the intensive care unit for close monitoring and should be given the highest priority, even if they are hemodynamically stable.
Is the patient hemodynamically stable?
Appropriate hemodynamic assessment includes monitoring of heart rate, blood pressure, and mental status. Tachycardia at rest, hypotension, and orthostatic changes in vital signs indicate a considerable loss of blood volume. Low urine output, dry mucous membranes, and sunken neck veins are also useful signs. (Tachycardia may be blunted if the patient is taking a beta-blocker.)
If these signs of hypovolemia are present, the initial management focuses on treating shock and on improving oxygen delivery to the vital organs. This involves repletion of the intravascular volume with intravenous infusions or blood transfusions. Supplemental oxygen also is useful, especially in elderly patients with heart disease.12
Inspection of nasogastric aspirate
In the initial assessment, it is useful to insert a nasogastric tube and inspect the aspirate. If it contains bright red blood, the patient needs an urgent endoscopic evaluation and an intensive level of care13,14; if it contains coffee-grounds material, the patient needs to be admitted to the hospital and to undergo endoscopic evaluation within 24 hours.
However, a normal aspirate does not rule out upper GI bleeding. Aljebreen et al15 found that 15% of patients with upper GI bleeding and normal nasogastric aspirate still had high-risk lesions (ie, visible bleeding or nonbleeding visible vessels) on endoscopy.
ACID-SUPPRESSION HELPS ULCERS HEAL
Acid and pepsin interfere with the healing of ulcers and other nonvariceal upper GI lesions. Further, an acidic environment promotes platelet disaggregation and fibrinolysis and impairs clot formation.16 This suggests that inhibiting gastric acid secretion and raising the gastric pH to 6 or higher may stabilize clots. Moreover, pepsinogen in the stomach is converted to its active form (pepsin) if the pH is less than 4. Therefore, keeping the pH above 4 keeps pepsinogen in an inactive form.
Histamine-2 receptor antagonists
Histamine-2 receptor antagonists were the first drugs to inhibit acid secretion, reversibly blocking histamine-2 receptors on the basolateral membrane of parietal cells. However, these drugs did not prove very useful in managing upper GI bleeding in clinical trials.17,18 In their intravenous form, they often fail to keep the gastric pH at 6 or higher, due to tachyphylaxis.19 The use of this class of drugs has declined in favor of proton pump inhibitors.
Proton pump inhibitors
Proton pump inhibitors reduce both basal and stimulated acid secretion by inhibiting hydrogen-potassium adenosine triphosphatase, the proton pump of the parietal cell.
Multiple studies have shown that proton pump inhibitors raise the gastric pH and keep it high. For example, an infusion of omeprazole (Prilosec) can keep the gastric pH above 6 for 72 hours without inducing tachyphylaxis.20,21
Started after endoscopy. Randomized controlled trials have found proton pump inhibitors to be effective when given in high doses intravenously for 72 hours after successful endoscopic treatment of bleeding ulcers with high-risk endoscopic signs, such as active bleeding or nonbleeding visible vessels.22,23
A meta-analysis indicated that these drugs decrease the incidence of recurrent peptic ulcer bleeding, the need for blood transfusions, the need for surgery, and the duration of hospitalization, but not the mortality rate.24,25 These studies also illustrate the benefit of following up endoscopic treatment to stop the bleeding with an intravenous infusion of a proton pump inhibitor.
The recommended dose of omeprazole for patients with high-risk findings on endoscopy is an 80-mg bolus followed by an 8-mg/hour infusion for 72 hours. After the patient’s condition stabilizes, oral therapy can be substituted for intravenous therapy. In patients with low-risk endoscopic findings (a clean-based ulcer or flat spot), oral proton pump inhibitors in high doses are recommended.
In either case, after the initial bleeding is treated endoscopically and hemostasis is achieved, a proton pump inhibitor is recommended for 6 to 8 weeks, or longer if the patient is also positive for Helicobacter pylori or is on daily treatment with aspirin or a nonsteroidal anti-inflammatory drug (NSAID) that is not selective for cyclo-oxygenase 2 (see below).
Started before endoscopy, these drugs reduced the frequency of actively bleeding ulcers, the duration of hospitalization, and the need for endoscopic therapy in a randomized controlled trial.26 A meta-analysis found that significantly fewer patients had signs of recent bleeding on endoscopy if they received a proton pump inhibitor 24 to 48 hours before the procedure, but it did not find any significant difference in important clinical outcomes such as death, recurrent bleeding, or surgery.27 Nevertheless, we believe that intravenous proton pump inhibitor therapy should be started before endoscopy in patients with upper GI bleeding.
Somatostatin analogues
Octreotide (Sandostatin), an analogue of the hormone somatostatin, decreases splanchnic blood flow, decreases secretion of gastric acid and pepsin, and stimulates mucus production. Although it is beneficial in treating upper GI bleeding due to varices, its benefit has not been confirmed in patients with nonvariceal upper GI bleeding.
A meta-analysis revealed that outcomes were better with high-dose intravenous proton pump inhibitor therapy than with octreotide when these drugs were started after endoscopic treatment of acute peptic ulcer bleeding.28 Nevertheless, octreotide may be useful in patients with uncontrolled nonvariceal bleeding who are awaiting endoscopy, since it is relatively safe to use.
ALL PATIENTS NEED ENDOSCOPY
All patients with upper GI bleeding need an upper endoscopic examination to diagnose and assess the risk posed by the bleeding lesion and to treat the lesion, reducing the risk of recurrent bleeding.
How urgently does endoscopy need to be done?
Endoscopy within the first 24 hours of upper GI bleeding is considered the standard of care. Patients with uncontrolled or recurrent bleeding should undergo endoscopy on an urgent basis to control the bleeding and reduce the risk of death.
However, how urgently endoscopy needs to be done is often debated. A multicenter randomized controlled trial compared outcomes in patients who underwent endoscopy within 6 hours of coming to the emergency department vs within 24 hours after the initial evaluation. The study found no significant difference in outcomes between the two groups; however, the group that underwent endoscopy sooner needed fewer transfusions.29
For a better view of the stomach
Gastric lavage improves the view of the gastric fundus but has not been proven to improve outcome.30
Promotility agents such as erythromycin and metoclopramide (Reglan) are also used to empty the stomach for better visualization.31–35 Erythromycin has been shown to improve visualization, shorten the procedure time, and prevent the need for additional endoscopy attempts in two randomized controlled studies.33,34 Furthermore, a cost-effectiveness study confirmed that giving intravenous erythromycin before endoscopy for acute upper GI bleeding saved money and resulted in an increase in quality-adjusted life-years.35
Endoscopy to diagnose bleeding and assess risk
Upper endoscopy is 90% to 95% diagnostic for acute upper GI bleeding.36
Patients at high risk (ie, older than 60 years, with severe comorbidity, or hemodynamically compromised) who have active bleeding (ie, witnessed hematemesis, red blood per nasogastric tube, or fresh blood per rectum) or a nonbleeding visible vessel should be admitted to a monitored bed or intensive care unit. Observation in a regular medical ward is appropriate for high-risk patients found to have an adherent clot. Patients with low-risk findings (eg, a clean ulcer base) are at low risk of recurrent bleeding and may be considered for early hospital discharge with appropriate outpatient follow-up.
Endoscopy to treat bleeding
About 25% of endoscopic procedures performed for upper GI bleeding include some type of treatment,39 such as injections of epinephrine, normal saline, or sclerosants; thermal cautery; argon plasma coagulation; electrocautery; or application of clips or bands. They are all equally effective, and combinations of these therapies are more effective than when they are used individually. A recent meta-analysis found dual therapy to be superior to epinephrine monotherapy in preventing recurrent bleeding, need for surgery, and death.40
How to manage adherent clots is controversial, but recent studies have revealed a significant benefit from removing them and treating the underlying lesions compared with drug therapy alone.43,45
Flat, pigmented spots and nonbleeding ulcers with a clean base do not require endoscopic treatment because the risk of recurrent bleeding is low.
Endoscopic therapy stops the bleeding in more than 90% of patients, but bleeding recurs after endoscopic therapy in 10% to 25%.46 Reversal of any severe coagulopathy with transfusions of platelets or fresh frozen plasma is essential for endoscopic hemostasis. However, coagulopathy at the time of initial bleeding and endoscopy does not appear to be associated with higher rates of recurrent bleeding following endoscopic therapy for nonvariceal upper GI bleeding.47
Patients with refractory bleeding are candidates for angiography or surgery. However, even when endoscopic hemostasis fails, endoscopy is important before angiography or surgery to pinpoint the site of bleeding and diagnose the cause.
A second endoscopic procedure is generally not recommended within 24 hours after the initial procedure.48 However, it is appropriate in cases in which clinical signs indicate recurrent bleeding or if hemostasis during the initial procedure is questionable. A meta-analysis found that routinely repeating endoscopy reduces the rate of recurrent bleeding but not the need for surgery or the risk of death.49
ALL PATIENTS SHOULD BE ADMITTED
VARICEAL BLEEDING
Variceal bleeding, a severe outcome of portal hypertension secondary to cirrhosis, carries a 6-week mortality rate of 10% to 20%.50 In view of the risk, primary prevention is indicated in patients with high-risk varices.
The mainstays of primary and secondary prevention are the nonselective beta-blockers such as nadolol (Corgard) and propranolol (Inderal). Several randomized controlled trials have shown lower rates of recurrent bleeding and death with propranolol or nadolol than with placebo.51 In doses that decrease the heart rate by 25%, beta-blockers have been shown to delay and decrease variceal hemorrhage. However, most patients require prophylactic endoscopic variceal ligation because they cannot tolerate beta-blocker therapy.
In suspected acute variceal bleeding, a somatostatin analogue should be started to decrease the portal pressure, and antibiotics should be started to reduce the risks of infection and death. Vasoactive drugs, ie, somatostatin analogues, should be started before endoscopy and continued for 5 days to reduce the chances of recurrent bleeding.52,53
Terlipressin is the only drug proven to improve the odds of survival in acute variceal bleeding. Although widely used in Europe, it has not been approved for use in the United States.
Octreotide, another option, improves hemostasis to the same extent, although it does not increase the survival rate.54,55 The recommended dose of octreotide for patients with variceal bleeding is a 50-μg intravenous bolus, followed by a 50-μg/hour infusion for 5 days.
Combining endoscopic and drug therapy improves the chances of stopping the bleeding and reduces the risk of recurrent bleeding compared with endoscopic therapy alone.56
Transjugular intrahepatic portosystemic shunting is indicated in recurrent variceal hemorrhage or in those with initial bleeding that is refractory to standard medical and endoscopic therapy. It is not the primary therapy because it doubles the risk of encephalopathy and has a high stent occlusion rate (up to 60%, lower with covered stents).
GI BLEEDING CAN CAUSE ACUTE MYOCARDIAL INFARCTION
The simultaneous presentation of acute myocardial infarction (MI) and GI hemorrhage is very serious and unfortunately common.
An acute MI occurring simultaneously with or after GI bleeding is usually precipitated by massive bleeding causing hypovolemia, hemodynamic compromise, and hypoperfusion. Conversely, the anticoagulant, antiplatelet, or thrombolytic drugs given to treat MI can precipitate GI bleeding (see below).
This distinction is important because the two scenarios have different clinical courses and prognoses. GI bleeding that precipitates an acute MI tends to be massive, whereas GI bleeding after treatment of acute MI tends to be self-limited and often resolves with reversal of underlying coagulopathy.57
Endoscopy carries a higher than average risk in patients with recent acute MI, with all-cause mortality rates as high as 1%.58 (The usual rate is 0.0004%.59) Nevertheless, endoscopy can be safely performed early on in patients with acute MI if it is done under strict monitoring in a coronary care unit.
Several studies have shown that MI patients who present with upper GI bleeding as the inciting event or patients with acute MI who are vomiting blood or who are hemodynamically unstable due to GI bleeding are significantly more likely to have a high-risk lesion and so have the greatest need for endoscopic therapy. Therefore, endoscopic intervention may be offered to MI patients at high risk who have been started on antiplatelet agents.
WARFARIN CAN PRECIPITATE BLEEDING
Acute upper GI bleeding can be a severe complication of long-term oral anticoagulation, not because the drugs cause ulcers, but rather because they exacerbate ulcers that are already present.60 Therefore, when starting warfarin (Coumadin), patients should be evaluated to determine if they have other risk factors for GI bleeding, such as ulcers.
The number of people presenting with upper GI bleeding while on warfarin therapy is increasing because of the expanding indications for long-term anticoagulation therapy, such as atrial fibrillation and deep venous thrombosis.
The risk of GI bleeding in patients who use oral anticoagulants is estimated to be 2.3 to 4.9 times higher than in nonusers.61
The goal international normalized ratio (INR) for patients on warfarin therapy is usually 2.0 to 3.0. Recent studies found that endoscopy can be safely performed in patients with acute GI bleeding whose INR is between 2.0 and 3.0.62,63 Some suggest that both the length of warfarin therapy and the INR affect the risk of bleeding.64,65
Managing patients with an INR higher than 3.0 who have an episode of GI bleeding is always a challenge. It is not uncommon to find pathologic lesions causing GI bleeding in patients who are on warfarin with a supratherapeutic INR, and thus, endoscopy is indicated. However, before endoscopy, reversal of anticoagulation should be considered.
BLEEDING IN PATIENTS ON ANTIPLATELET DRUGS
Aspirin
Aspirin decreases production of prostaglandins in the GI tract, thereby decreasing the protective and restorative properties of the gastric and duodenal mucosa and predisposing to ulcers and bleeding.
The higher the aspirin dose, the higher the risk. Aspirin doubles the risk of upper GI bleeding at daily doses of 75 mg and quadruples it at doses of 300 mg.66 Even doses as low as 10 mg can decrease gastric mucosal prostaglandin production.67 Thus, it appears that there is no risk-free dose of aspirin, and enteric-coated or buffered formulations do not appear to reduce the risk.68–70
The most important risk factor for upper GI bleeding in patients taking aspirin is a history of peptic ulcer bleeding. Approximately 15% of aspirin users who have bleeding from ulcers have recurrent bleeding within 1 year.71
As aspirin-induced GI bleeding becomes more common, health care providers often feel caught between the GI risk and the cardiovascular benefit. When considering whether to discontinue antiplatelet therapy, a cardiologist should be consulted along with a gastroenterologist to weigh the risks of GI bleeding vs thrombosis. To date, there have been no clinical trials published to suggest when antiplatelet therapy should be stopped to optimize GI and cardiovascular outcomes. An alternative is to replace aspirin with another antiplatelet drug that does not induce ulcers.
Clopidogrel
Clopidogrel (Plavix) is recommended for hospitalized patients with acute coronary syndrome who cannot tolerate the GI side effects of aspirin, according to the joint guidelines of the American College of Cardiology and the American Heart Association, with the highest level of evidence.72 This recommendation was largely based on the safety data from the CAPRIE (Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events) trial, in which the incidence of major GI bleeding was lower in the clopidogrel group (0.52%) than in the aspirin group (0.72%; P < .05).73
Aspirin plus a proton pump inhibitor
Patients who have had an episode of upper GI bleeding and who need long-term aspirin therapy should also receive a proton pump inhibitor indefinitely to prevent ulcer recurrence.
In a recent double-blind randomized controlled trial in patients with a history of aspirin-induced bleeding, the combination of low-dose aspirin plus esomeprazole (Nexium) twice a day was superior to clopidogrel by itself in terms of the rate of recurrent bleeding (0.7% vs 8.6%; P < .05).74 A similar trial showed nearly identical results: 0% upper GI bleeding in the group receiving aspirin plus esomeprazole 20 mg daily, vs 13.6% in the clopidogrel group (P = .0019).75 These studies suggest that a once-daily proton pump inhibitor combined with aspirin is a safer alternative than clopidogrel alone.
Clopidogrel plus a proton pump inhibitor
Interestingly, recent studies have shown that omeprazole decreases the antiplatelet effect of clopidogrel, possibly by inhibiting the CYP2C19 enzyme.76 However, concomitant use of pantoprazole (Protonix), lansoprazole (Prevacid), and esomeprazole did not have this effect, suggesting that although all proton pump inhibitors are metabolized to a varying degree by CYP2C19, the interaction between proton pump inhibitors and clopidogrel is not a class effect.77–79 Therefore, pantoprazole, lansoprazole, and esomeprazole may be the appropriate proton pump inhibitors to use with clopidogrel in patients who have a clear indication for the medication, consistent with current guideline recommendations.
Helicobacter pylori infection in antiplatelet drug users
TREATMENT AND PREVENTION OF NSAID-RELATED GI INJURY
About 1 in 20 users of NSAIDs develop GI complications and ulcers of varying degrees of severity, as do one in seven NSAID users over the age of 65. In fact, NSAID use accounts for 30% of hospitalizations for upper GI bleeding and deaths from this cause.82–85 In addition, approximately 15% to 30% of NSAID users have clinically silent but endoscopically evident peptic ulcers.86
NSAIDs contribute to ulcer development by depleting prostaglandins. Thus, misoprostol (Cytotec), a synthetic prostaglandin, has been used to reduce this side effect.
In a clinical trial, misoprostol reduced the incidence of NSAID-associated GI complications by 40%.87 Furthermore, it has been shown to be better than placebo in preventing recurrent gastric ulcers in patients with a history of gastric ulcer who were receiving low-dose aspirin.88
However, misoprostol is rarely used because it can cause diarrhea and abdominal cramping. Rather, the preferred drugs for preventing and treating NSAID- and aspirin-related GI lesions are proton pump inhibitors.
Numerous clinical trials using endoscopic end points showed that proton pump inhibitors in standard doses significantly reduce the incidence of ulcers associated with the use of NSAIDs.89 Proton pump inhibitor therapy has achieved a significant reduction in relative risk of upper GI bleeding in patients who received low-dose aspirin therapy, as confirmed by epidemiologic studies.90,91 The number of NSAID-related ulcers found on endoscopy could be reduced by an estimated 90% simply by using proton pump inhibitors.92
- Lewis JD, Bilker WB, Brensinger C, Farrar JT, Strom BL. Hospitalization and mortality rates from peptic ulcer disease and GI bleeding in the 1990s: relationship to sales of non-steroidal anti-inflammatory drugs and acid suppression medications. Am J Gastroenterol 2002; 97:2540–2549.
- Viviane A, Alan BN. Estimates of costs of hospital stays for variceal and nonvariceal upper gastrointestinal bleeding in the United States. Value Health 2008; 11:1–3.
- Yavorski RT, Wong RK, Maydonovitch C, Battin LS, Furnia A, Amundson DE. Analysis of 3,294 cases of upper gastrointestinal bleeding in military medical facilities. Am J Gastroenterol 1995; 90:568–573.
- Kaplan RC, Heckbert SR, Koepsell TD, et al. Risk factors for hospitalized gastrointestinal bleeding among older persons. Cardiovascular Health Study Investigators. J Am Geriatr Soc 2001; 49:126–133.
- Longstreth GF. Epidemiology of hospitalization for acute upper gastrointestinal hemorrhage: a population-based study. Am J Gastroenterol 1995; 90:206–210.
- Laine L, Peterson WL. Bleeding peptic ulcer. N Engl J Med 1994; 331:717–727.
- Wara P, Stodkilde H. Bleeding pattern before admission as guideline for emergency endoscopy. Scand J Gastroenterol 1985; 20:72–78.
- Jensen DM, Machicado GA. Diagnosis and treatment of severe hematochezia. The role of urgent colonoscopy after purge. Gastroenterology 1988; 95:1569–1574.
- Daniel WA, Egan S. The quantity of blood required to produce a tarry stool. J Am Med Assoc 1939; 113:2232.
- Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal hemorrhage. Gut 1996; 38:316–321.
- Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper-gastrointestinal hemorrhage. Lancet 2000; 356:1318–1321.
- Gralnek IM, Barkun AN, Bardou M. Management of acute bleeding from a peptic ulcer. N Engl J Med 2008; 359:928–937.
- Silverstein FE, Gilbert DA, Tedesco FJ, Buenger NK, Persing J. The national ASGE survey on upper gastrointestinal bleeding II. Clinical prognostic factors. Gastrointest Endosc 1981; 27:80–93.
- Corley DA, Stefan AM, Wolf M, Cook EF, Lee TH. Early indicators of prognosis in upper gastrointestinal hemorrhage. Am J Gastroenterol 1998; 93:336–340.
- Aljebreen AM, Fallone CA, Barkun AN. Nasogastric aspirate predicts high-risk endoscopic lesions in patients with acute upper-GI bleeding. Gastrointest Endosc 2004; 59:172–178.
- Barkun AN, Cockeram AW, Plourde V, Fedorak RN. Review article: acid suppression in non-variceal acute upper gastrointestinal bleeding. Aliment Pharmacol Ther 1999; 13:1565–1584.
- Levine JE, Leontiadis JI, Sharma VK, Howden CW. Meta-analysis: the efficacy of intravenous H2-receptor antagonists in bleeding peptic ulcer. Aliment Pharmacol Ther 2002; 16:1137–1142.
- Walt RP, Cottrell J, Mann SG, Freemantle NP, Langman MJ. Continuous intravenous famotidine for hemorrhage from peptic ulcer. Lancet 1992; 340:1058–1062.
- Labenz J, Peitz U, Leusing C, Tillenburg B, Blum AL, Börsch G. Efficacy of primed infusion with high dose ranitidine and omeprazole to maintain high intragastric pH in patients with peptic ulcer bleeding: a prospective randomized controlled study. Gut 1997; 40:36–41.
- Merki HS, Wilder-Smith CH. Do continuous infusions of omeprazole and ranitidine retain their effect with prolonged dosing? Gastroenterology 1994; 106:60–64.
- Netzer P, Gaia C, Sandoz M, et al. Effect of repeated injection and continuous infusion of omeprazole and ranitidine on intragastric pH over 72 hours. Am J Gastroenterol 1999; 94:351–357.
- Lin HJ, Lo WC, Cheng YC, Perng CL. Role of intravenous omeprazole in patients with high-risk peptic ulcer bleeding after successful endoscopic epinephrine injection: a prospective randomized comparative trial. Am J Gastroenterol 2006; 101:500–505.
- Lau JY, Sung JJ, Lee KK, et al. Effects of intravenous omeprazole on recurrent bleeding after endoscopic treatment of bleeding peptic ulcers. N Engl J Med 2000; 343:310–316.
- Leontiadis GI, Sharma VK, Howden CW. Proton pump inhibitor treatment for acute peptic ulcer bleeding. Cochrane Database Syst Rev 2006;CD002094.
- Andriulli A, Annese V, Caruso N, et al. Proton-pump inhibitors and outcome of endoscopic hemostasis in bleeding peptic ulcers: a series of meta-analyses. Am J Gastroenterol 2005; 100:207–219.
- Lau JY, Leung WK, Wu JC, et al. Omeprazole before endoscopy in patients with gastrointestinal bleeding. N Engl J Med 2007; 356:1631–1640.
- Dorward S, Sreedharan A, Leontiadis GI, Howden CW, Moayyedi P, Forman D. Proton pump inhibitor treatment initiated prior to endoscopic diagnosis in upper gastrointestinal bleeding. Cochrane Database Syst Rev 2006;CD005415.
- Bardou M, Toubouti Y, Benhaberou-Brun D, Rahme E, Barkun AN. Meta-analysis: proton-pump inhibition in high-risk patients with acute peptic ulcer bleeding. Aliment Pharmacol Ther 2005; 21:677–686.
- Bjorkman DJ, Zaman A, Fennerty MB, Lieberman D, Disario JA, Guest-Warnick G. Urgent vs elective endoscopy for acute non-variceal upper-GI bleeding: an effectiveness study. Gastointest Endosc 2004; 60:1–8.
- Lee SD, Kearney DJ. A randomized controlled trial of gastric lavage prior to endoscopy for acute upper gastrointestinal bleeding. J Clin Gastroenterol 2004; 38:861–865.
- Tack J, Janssens J, Vantrappen G, et al. Effect of erythromycin on gastric motility in controls and in diabetic gastroparesis. Gastroenterology 1992; 103:72–79.
- Xynos E, Mantides A, Papageorgiou A, Fountos A, Pechlivanides G, Vassilakis JS. Erythromycin accelerates delayed gastric emptying of solids in patients after truncal vagotomy and pyloroplasty. Eur J Surg 1992; 158:407–411.
- Coffin B, Pocard M, Panis Y, et al; Groupe des endoscopistes de garde á l’AP-HP. Erythromycin improves the quality of EGD in patients with acute upper GI bleeding: a randomized controlled study. Gastrointest Endosc 2002; 56:174–179.
- Frossard JL, Spahr L, Queneau PE, et al. Erythromycin intravenous bolus infusion in acute upper gastrointestinal bleeding: a randomized, controlled, double-blind trial. Gastroenterology 2002; 123:17–23.
- Winstead NS, Wilcox CM. Erythromycin prior to endoscopy for acute upper gastrointestinal hemorrhage: a cost-effectiveness analysis. Aliment Pharmacol Ther 2007; 26:1371–1377.
- Chak A, Cooper GS, Lloyd LE, Kolz CS, Barnhart BA, Wong RC. Effectiveness of endoscopy in patients admitted to the intensive care unit with upper GI hemorrhage. Gastrointest Endosc 2001; 53:6–13.
- Lau JY, Chung SC, Leung JW, Lo KK, Yung MY, Li AK. The evolution of stigmata of hemorrhage in bleeding peptic ulcers: a sequential endoscopic study. Endoscopy 1998; 30:513–518.
- Chung IK, Kim EJ, Lee MS, et al. Endoscopic factors predisposing to rebleeding following endoscopic hemostasis in bleeding peptic ulcers. Endoscopy 2001; 33:969–975.
- Elta GH. Acute nonvariceal upper gastrointestinal hemorrhage. Curr Treat Options Gastroenterol 2002; 5:147–152.
- Marmo R, Rotondano G, Piscopo R, Bianco MA, D’Angella R, Cipolletta L. Dual therapy versus monotherapy in the endoscopic treatment of high-risk bleeding ulcers: a meta-analysis of controlled trials. Am J Gastroenterol 2007; 102:279–289.
- Kovacs TO, Jensen DM. Recent advances in the endoscopic diagnosis and therapy of upper gastrointestinal, small intestinal, and colonic bleeding. Med Clin North Am 2002; 86:1319–1356.
- Kovacs TO, Jensen DM. Endoscopic treatment of ulcer bleeding. Curr Treat Options Gastroenterol 2007; 10:143–148.
- Jensen DM, Kovacs TO, Jutabha R, et al. Randomized trial of medical or endoscopic therapy to prevent recurrent ulcer hemorrhage in patients with adherent clots. Gastroenterology 2002; 123:407–413.
- Jensen DM, Machicado GA. Endoscopic hemostasis of ulcer hemorrhage with injection, thermal, and combination methods. Techniques Gastrointest Endosc 2005; 7:124–131.
- Bleau BL, Gostout CJ, Sherman KE, et al. Recurrent bleeding from peptic ulcer associated with adherent clot: a randomized study comparing endoscopic treatment with medical therapy. Gastrointest Endosc 2002; 56:1–6.
- Lau JY, Sung JJ, Lam YH, et al. Endoscopic retreatment compared with surgery in patients with recurrent bleeding after initial endoscopic control of bleeding ulcers. N Engl J Med 1999; 340:751–756.
- Wolf AT, Wasan SK, Saltzman JR. Impact of anticoagulation on rebleeding following endoscopic therapy for nonvariceal upper gastrointestinal hemorrhage. Am J Gastroenterol 2007; 102:290–296.
- Barkun A, Bardou M, Marshall JK; Nonvariceal Upper GI Bleeding Consensus Conference Group. Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med 2003; 139:843–857.
- Marmo R, Rotondano G, Bianco MA, Piscopo R, Prisco A, Cipolletta L. Outcome of endoscopic treatment for peptic ulcer bleeding: is a second look necessary? A meta-analysis. Gastrointest Endosc 2003; 57:62–67.
- Dell’Era A, deFrancis R, Iannuzzi F. Acute variceal bleeding: pharmacological treatment and primary/secondary prophylaxis. Best Pract Res Clin Gastroenterol 2008; 22:279–294.
- Jalan R, Hayes PC. UK guidelines on the management of variceal hemorrhage in cirrhotic patients. British Society of Gastroenterology. Gut 2000; 46( suppl 3–4):III1–III15.
- Bernard B, Lebrec D, Mathurin P, Opolon P, Poynard T. Beta-adrenergic antagonists in the prevention of gastrointestinal rebleeding in patients with cirrhosis: a meta-analysis. Hepatology 1997; 25:63–70.
- De Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2005; 43:167–176.
- Levacher S, Letoumelin P, Pateron D, Blaise M, Lapandry C, Pourriat JL. Early administration of terlipressin plus glyceryl trinitrate to control active upper gastrointestinal bleeding in cirrhotic patients. Lancet 1995; 346:865–868.
- Abraldes JG, Bosch J. Somatostatin and analogues in portal hypertension. Hepatology 2002; 35:1305–1312.
- Banares R, Albillos A, Rincon D, et al. Endoscopic treatment versus endoscopic plus pharmacological treatment for acute variceal bleeding: a meta analysis. Hepatology 2002; 35:609–615.
- Cappell M. Gastrointenstinal bleeding associated with myocardial infarction. Gastroenterol Clin North Am 2000; 29:423–444.
- Lin S, Konstance R, Jollis J, Fisher DA. The utility of upper endoscopy in patients with concomitant upper gastrointestinal bleeding and acute myocardial infarction. Dig Dis Sci 2006; 51:2377–2383.
- Silvis SE, Nebel O, Rogers G, Sugawa C, Mandelstam P. Endoscopic complications. Results of the 1974 American Society for Gastrointestinal Endoscopy Survey. JAMA 1976; 235:928–930.
- Lanas A, Garcia-Rodriguez LA, Arroyo MT, et al; Investigators of the Asociación Española de Gastroenterología (AEG). Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol 2007; 102:507–515.
- Shorr RI, Ray WA, Daugherty JR, Griffin MR. Concurrent use of nonsteroidal anti-inflammatory drugs and oral anticoagulants places elderly persons at high risk for hemorrhagic peptic ulcer disease. Arch Intern Med 1993; 153:1665–1670.
- Tabibian N. Acute gastrointestinal bleeding in anticoagulated patients: a prospective evaluation. Am J Gastroenterol 1989; 84:10–12.
- Choudari CP, Rajgopal C, Palmer KR. Acute gastrointestinal hemorrhage in anticoagulated patients: diagnoses and response to endoscopic treatment. Gut 1994; 35:464–466.
- Petty GW, Brown RD, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Frequency of major complications of aspirin, warfarin, and intravenous heparin for secondary stroke prevention: a population-based study. Ann Intern Med 1999; 130:14–22.
- Landefeld CS, Rosenblatt MW, Goldman L. Bleeding in outpatients treated with warfarin: relation to the prothrombin time and important remediable lesions. Am J Med 1989; 87:153–159.
- Weil J, Colin-Jones D, Langman M, et al. Prophylactic aspirin and risk of peptic ulcer bleeding. BMJ 1995; 310:827–830.
- Cryer B, Feldman M. Effects of very low dose daily, long-term aspirin therapy on gastric, duodenal, and rectal prostaglandin levels and on mucosal injury in healthy humans. Gastroenterology 1999; 117:17–25.
- De Abajo FJ, Garcia Rodriguez LA. Risk of upper gastrointestinal bleeding and perforation associated with low-dose aspirin as plain and enteric-coated formulations. BMC Clin Pharmacol 2001; 1:1.
- Kelly JP, Kaufman DW, Jurgelon JM, Sheehan J, Koff RS, Shapiro S. Risk of aspirin-associated major upper gastrointestinal bleeding with enteric coated or buffered product. Lancet 1996; 348:1413–1416.
- Garcia Rodriguez LA, Hernandez-Diaz S, De Abajo FJ. Association between aspirin and upper gastrointestinal complications: systematic review of epidemiological studies. Br J Clin Pharmacol 2001; 52:563–571.
- Wilcox CM, Ladabaum U. A patient with high risk of gastrointestinal bleeding requiring nonsteroidal anti-inflammatory drugs. Clin Gastroenterol Hepatol 2006; 4:1090–1093.
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction. J Am Coll Cardiol 2007; 50:e1–e157.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996; 348:1329–1339.
- Chan FK, Ching JY, Hung LC, et al. Clopidogrel versus aspirin and esomeprazole to prevent recurrent ulcer bleeding. N Engl J Med 2005; 352:238–244.
- Lai KC, Chu KM, Hui WM, et al. Esomeprazole with aspirin versus clopidogrel for prevention of recurrent gastrointestinal ulcer complications. Clin Gastroenterol Hepatol 2006; 4:860–865.
- Ho MP, Maddox TM, Wang L, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA 2009; 301:937–944.
- Siller-Matula JM, Spiel AO, Lang IM, Kreiner G, Christ G, Jilma B. Effects of pantoprazole and esomeprazole on platelet inhibition by clopidogrel. Am Heart J 2009; 157:148.e1–e5.
- Small DS, Farid NA, Payne CD, et al. Effects of proton pump inhibitor lansoprazole on the pharmacokinetics and pharmacodynamics of prasugel and clopidogrel. J Clin Pharmacol 2008; 48:475–484.
- Ishizaki T, Horai Y. Review article: cytochrome P450 and the metabolism of proton pump inhibitors—emphasis on rabeprazole. Aliment Pharacol Ther 1999; 13 (suppl 3):27–36.
- Lanas A, Fuentes J, Benito R, Serrano P, Bajador E, Sainz R. Helicobacter pylori increases the risk of upper gastrointestinal bleeding in patients taking low-dose aspirin. Aliment Pharmacol Ther 2002; 16:779–786.
- Chan FK. NSAID-Induced peptic ulcers and Helicobacter pylori infection: implications for patient management. Drug Saf 2005; 28:287–300.
- Bombardier V, Laine L, Reicin A, et al; VIGOR Study Group. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis: VIGOR Study Group. N Eng J Med 2000; 343:1520–1528.
- Griffin MR, Ray WA, Schaffner W. Nonsteroidal anti-inflammatory drug use and death from peptic ulcer in elderly persons. Ann Intern Med 1988; 109:359–363.
- Griffin MR, Piper JM, Daugherty JR, Snowden M, Ray WA. Nonsteroidal anti-inflammatory drug use and increased risk for peptic ulcer disease in elderly persons. Ann Intern Med 1991; 114:257–263.
- Smalley WE, Ray WA, Daugherty JR, Griffin MR. Nonsteroidal anti-inflammatory drugs and the incidence of hospitalizations for peptic ulcer disease in elderly persons. Am J Epidemiol 1995; 141:539–545.
- Laine L. Approaches to nonsteroidal anti-inflammatory drug use in the high-risk patient. Gastroenterology 2001; 120:594–606.
- Silverstein FE, Graham DY, Senior JR, et al. Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal anti-inflammatory drugs. A randomized, double blind, placebo controlled trial. Ann Intern Med 1995; 123:241–249.
- Goldstein JL, Huang B, Amer F, Christopoulos NG. Ulcer recurrence in high-risk patients receiving nonsteroidal anti-inflammatory drugs plus low dose aspirin: results of a post hoc subanalysis. Clin Ther 2004; 26:1637–1643.
- Berger JS, Stebbins A, Granger CB, et al. Initial aspirin dose and outcome among ST-elevation myocardial infarction patients treated with fibrinolytic therapy. Circulation 2008; 117:192–199.
- Lanas A, Garcia-Rodriguez LA, Arroyo MT, et al; Investigators of the Asociación Española de Gastroenterología (AEG). Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol 2007; 102:507–515.
- Chin MW, Yong G, Bulsara MK, Rankin J, Forbes GM. Predictive and protective factors associated with upper gastrointestinal bleeding after percutaneous coronary intervention: a case-control study. Am J Gastroenterol 2007; 102:2411–2416.
- Hunt RH, Bazzoli F. Should NSAID/low dose aspirin takers be tested routinely for H. Pylori infection and treated if positive? Implications for primary risk of ulcer and ulcer relapse after initial healing. Aliment Pharmacol Ther 2004; 19 (suppl 1):9–16.
- Lewis JD, Bilker WB, Brensinger C, Farrar JT, Strom BL. Hospitalization and mortality rates from peptic ulcer disease and GI bleeding in the 1990s: relationship to sales of non-steroidal anti-inflammatory drugs and acid suppression medications. Am J Gastroenterol 2002; 97:2540–2549.
- Viviane A, Alan BN. Estimates of costs of hospital stays for variceal and nonvariceal upper gastrointestinal bleeding in the United States. Value Health 2008; 11:1–3.
- Yavorski RT, Wong RK, Maydonovitch C, Battin LS, Furnia A, Amundson DE. Analysis of 3,294 cases of upper gastrointestinal bleeding in military medical facilities. Am J Gastroenterol 1995; 90:568–573.
- Kaplan RC, Heckbert SR, Koepsell TD, et al. Risk factors for hospitalized gastrointestinal bleeding among older persons. Cardiovascular Health Study Investigators. J Am Geriatr Soc 2001; 49:126–133.
- Longstreth GF. Epidemiology of hospitalization for acute upper gastrointestinal hemorrhage: a population-based study. Am J Gastroenterol 1995; 90:206–210.
- Laine L, Peterson WL. Bleeding peptic ulcer. N Engl J Med 1994; 331:717–727.
- Wara P, Stodkilde H. Bleeding pattern before admission as guideline for emergency endoscopy. Scand J Gastroenterol 1985; 20:72–78.
- Jensen DM, Machicado GA. Diagnosis and treatment of severe hematochezia. The role of urgent colonoscopy after purge. Gastroenterology 1988; 95:1569–1574.
- Daniel WA, Egan S. The quantity of blood required to produce a tarry stool. J Am Med Assoc 1939; 113:2232.
- Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal hemorrhage. Gut 1996; 38:316–321.
- Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper-gastrointestinal hemorrhage. Lancet 2000; 356:1318–1321.
- Gralnek IM, Barkun AN, Bardou M. Management of acute bleeding from a peptic ulcer. N Engl J Med 2008; 359:928–937.
- Silverstein FE, Gilbert DA, Tedesco FJ, Buenger NK, Persing J. The national ASGE survey on upper gastrointestinal bleeding II. Clinical prognostic factors. Gastrointest Endosc 1981; 27:80–93.
- Corley DA, Stefan AM, Wolf M, Cook EF, Lee TH. Early indicators of prognosis in upper gastrointestinal hemorrhage. Am J Gastroenterol 1998; 93:336–340.
- Aljebreen AM, Fallone CA, Barkun AN. Nasogastric aspirate predicts high-risk endoscopic lesions in patients with acute upper-GI bleeding. Gastrointest Endosc 2004; 59:172–178.
- Barkun AN, Cockeram AW, Plourde V, Fedorak RN. Review article: acid suppression in non-variceal acute upper gastrointestinal bleeding. Aliment Pharmacol Ther 1999; 13:1565–1584.
- Levine JE, Leontiadis JI, Sharma VK, Howden CW. Meta-analysis: the efficacy of intravenous H2-receptor antagonists in bleeding peptic ulcer. Aliment Pharmacol Ther 2002; 16:1137–1142.
- Walt RP, Cottrell J, Mann SG, Freemantle NP, Langman MJ. Continuous intravenous famotidine for hemorrhage from peptic ulcer. Lancet 1992; 340:1058–1062.
- Labenz J, Peitz U, Leusing C, Tillenburg B, Blum AL, Börsch G. Efficacy of primed infusion with high dose ranitidine and omeprazole to maintain high intragastric pH in patients with peptic ulcer bleeding: a prospective randomized controlled study. Gut 1997; 40:36–41.
- Merki HS, Wilder-Smith CH. Do continuous infusions of omeprazole and ranitidine retain their effect with prolonged dosing? Gastroenterology 1994; 106:60–64.
- Netzer P, Gaia C, Sandoz M, et al. Effect of repeated injection and continuous infusion of omeprazole and ranitidine on intragastric pH over 72 hours. Am J Gastroenterol 1999; 94:351–357.
- Lin HJ, Lo WC, Cheng YC, Perng CL. Role of intravenous omeprazole in patients with high-risk peptic ulcer bleeding after successful endoscopic epinephrine injection: a prospective randomized comparative trial. Am J Gastroenterol 2006; 101:500–505.
- Lau JY, Sung JJ, Lee KK, et al. Effects of intravenous omeprazole on recurrent bleeding after endoscopic treatment of bleeding peptic ulcers. N Engl J Med 2000; 343:310–316.
- Leontiadis GI, Sharma VK, Howden CW. Proton pump inhibitor treatment for acute peptic ulcer bleeding. Cochrane Database Syst Rev 2006;CD002094.
- Andriulli A, Annese V, Caruso N, et al. Proton-pump inhibitors and outcome of endoscopic hemostasis in bleeding peptic ulcers: a series of meta-analyses. Am J Gastroenterol 2005; 100:207–219.
- Lau JY, Leung WK, Wu JC, et al. Omeprazole before endoscopy in patients with gastrointestinal bleeding. N Engl J Med 2007; 356:1631–1640.
- Dorward S, Sreedharan A, Leontiadis GI, Howden CW, Moayyedi P, Forman D. Proton pump inhibitor treatment initiated prior to endoscopic diagnosis in upper gastrointestinal bleeding. Cochrane Database Syst Rev 2006;CD005415.
- Bardou M, Toubouti Y, Benhaberou-Brun D, Rahme E, Barkun AN. Meta-analysis: proton-pump inhibition in high-risk patients with acute peptic ulcer bleeding. Aliment Pharmacol Ther 2005; 21:677–686.
- Bjorkman DJ, Zaman A, Fennerty MB, Lieberman D, Disario JA, Guest-Warnick G. Urgent vs elective endoscopy for acute non-variceal upper-GI bleeding: an effectiveness study. Gastointest Endosc 2004; 60:1–8.
- Lee SD, Kearney DJ. A randomized controlled trial of gastric lavage prior to endoscopy for acute upper gastrointestinal bleeding. J Clin Gastroenterol 2004; 38:861–865.
- Tack J, Janssens J, Vantrappen G, et al. Effect of erythromycin on gastric motility in controls and in diabetic gastroparesis. Gastroenterology 1992; 103:72–79.
- Xynos E, Mantides A, Papageorgiou A, Fountos A, Pechlivanides G, Vassilakis JS. Erythromycin accelerates delayed gastric emptying of solids in patients after truncal vagotomy and pyloroplasty. Eur J Surg 1992; 158:407–411.
- Coffin B, Pocard M, Panis Y, et al; Groupe des endoscopistes de garde á l’AP-HP. Erythromycin improves the quality of EGD in patients with acute upper GI bleeding: a randomized controlled study. Gastrointest Endosc 2002; 56:174–179.
- Frossard JL, Spahr L, Queneau PE, et al. Erythromycin intravenous bolus infusion in acute upper gastrointestinal bleeding: a randomized, controlled, double-blind trial. Gastroenterology 2002; 123:17–23.
- Winstead NS, Wilcox CM. Erythromycin prior to endoscopy for acute upper gastrointestinal hemorrhage: a cost-effectiveness analysis. Aliment Pharmacol Ther 2007; 26:1371–1377.
- Chak A, Cooper GS, Lloyd LE, Kolz CS, Barnhart BA, Wong RC. Effectiveness of endoscopy in patients admitted to the intensive care unit with upper GI hemorrhage. Gastrointest Endosc 2001; 53:6–13.
- Lau JY, Chung SC, Leung JW, Lo KK, Yung MY, Li AK. The evolution of stigmata of hemorrhage in bleeding peptic ulcers: a sequential endoscopic study. Endoscopy 1998; 30:513–518.
- Chung IK, Kim EJ, Lee MS, et al. Endoscopic factors predisposing to rebleeding following endoscopic hemostasis in bleeding peptic ulcers. Endoscopy 2001; 33:969–975.
- Elta GH. Acute nonvariceal upper gastrointestinal hemorrhage. Curr Treat Options Gastroenterol 2002; 5:147–152.
- Marmo R, Rotondano G, Piscopo R, Bianco MA, D’Angella R, Cipolletta L. Dual therapy versus monotherapy in the endoscopic treatment of high-risk bleeding ulcers: a meta-analysis of controlled trials. Am J Gastroenterol 2007; 102:279–289.
- Kovacs TO, Jensen DM. Recent advances in the endoscopic diagnosis and therapy of upper gastrointestinal, small intestinal, and colonic bleeding. Med Clin North Am 2002; 86:1319–1356.
- Kovacs TO, Jensen DM. Endoscopic treatment of ulcer bleeding. Curr Treat Options Gastroenterol 2007; 10:143–148.
- Jensen DM, Kovacs TO, Jutabha R, et al. Randomized trial of medical or endoscopic therapy to prevent recurrent ulcer hemorrhage in patients with adherent clots. Gastroenterology 2002; 123:407–413.
- Jensen DM, Machicado GA. Endoscopic hemostasis of ulcer hemorrhage with injection, thermal, and combination methods. Techniques Gastrointest Endosc 2005; 7:124–131.
- Bleau BL, Gostout CJ, Sherman KE, et al. Recurrent bleeding from peptic ulcer associated with adherent clot: a randomized study comparing endoscopic treatment with medical therapy. Gastrointest Endosc 2002; 56:1–6.
- Lau JY, Sung JJ, Lam YH, et al. Endoscopic retreatment compared with surgery in patients with recurrent bleeding after initial endoscopic control of bleeding ulcers. N Engl J Med 1999; 340:751–756.
- Wolf AT, Wasan SK, Saltzman JR. Impact of anticoagulation on rebleeding following endoscopic therapy for nonvariceal upper gastrointestinal hemorrhage. Am J Gastroenterol 2007; 102:290–296.
- Barkun A, Bardou M, Marshall JK; Nonvariceal Upper GI Bleeding Consensus Conference Group. Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med 2003; 139:843–857.
- Marmo R, Rotondano G, Bianco MA, Piscopo R, Prisco A, Cipolletta L. Outcome of endoscopic treatment for peptic ulcer bleeding: is a second look necessary? A meta-analysis. Gastrointest Endosc 2003; 57:62–67.
- Dell’Era A, deFrancis R, Iannuzzi F. Acute variceal bleeding: pharmacological treatment and primary/secondary prophylaxis. Best Pract Res Clin Gastroenterol 2008; 22:279–294.
- Jalan R, Hayes PC. UK guidelines on the management of variceal hemorrhage in cirrhotic patients. British Society of Gastroenterology. Gut 2000; 46( suppl 3–4):III1–III15.
- Bernard B, Lebrec D, Mathurin P, Opolon P, Poynard T. Beta-adrenergic antagonists in the prevention of gastrointestinal rebleeding in patients with cirrhosis: a meta-analysis. Hepatology 1997; 25:63–70.
- De Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2005; 43:167–176.
- Levacher S, Letoumelin P, Pateron D, Blaise M, Lapandry C, Pourriat JL. Early administration of terlipressin plus glyceryl trinitrate to control active upper gastrointestinal bleeding in cirrhotic patients. Lancet 1995; 346:865–868.
- Abraldes JG, Bosch J. Somatostatin and analogues in portal hypertension. Hepatology 2002; 35:1305–1312.
- Banares R, Albillos A, Rincon D, et al. Endoscopic treatment versus endoscopic plus pharmacological treatment for acute variceal bleeding: a meta analysis. Hepatology 2002; 35:609–615.
- Cappell M. Gastrointenstinal bleeding associated with myocardial infarction. Gastroenterol Clin North Am 2000; 29:423–444.
- Lin S, Konstance R, Jollis J, Fisher DA. The utility of upper endoscopy in patients with concomitant upper gastrointestinal bleeding and acute myocardial infarction. Dig Dis Sci 2006; 51:2377–2383.
- Silvis SE, Nebel O, Rogers G, Sugawa C, Mandelstam P. Endoscopic complications. Results of the 1974 American Society for Gastrointestinal Endoscopy Survey. JAMA 1976; 235:928–930.
- Lanas A, Garcia-Rodriguez LA, Arroyo MT, et al; Investigators of the Asociación Española de Gastroenterología (AEG). Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol 2007; 102:507–515.
- Shorr RI, Ray WA, Daugherty JR, Griffin MR. Concurrent use of nonsteroidal anti-inflammatory drugs and oral anticoagulants places elderly persons at high risk for hemorrhagic peptic ulcer disease. Arch Intern Med 1993; 153:1665–1670.
- Tabibian N. Acute gastrointestinal bleeding in anticoagulated patients: a prospective evaluation. Am J Gastroenterol 1989; 84:10–12.
- Choudari CP, Rajgopal C, Palmer KR. Acute gastrointestinal hemorrhage in anticoagulated patients: diagnoses and response to endoscopic treatment. Gut 1994; 35:464–466.
- Petty GW, Brown RD, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Frequency of major complications of aspirin, warfarin, and intravenous heparin for secondary stroke prevention: a population-based study. Ann Intern Med 1999; 130:14–22.
- Landefeld CS, Rosenblatt MW, Goldman L. Bleeding in outpatients treated with warfarin: relation to the prothrombin time and important remediable lesions. Am J Med 1989; 87:153–159.
- Weil J, Colin-Jones D, Langman M, et al. Prophylactic aspirin and risk of peptic ulcer bleeding. BMJ 1995; 310:827–830.
- Cryer B, Feldman M. Effects of very low dose daily, long-term aspirin therapy on gastric, duodenal, and rectal prostaglandin levels and on mucosal injury in healthy humans. Gastroenterology 1999; 117:17–25.
- De Abajo FJ, Garcia Rodriguez LA. Risk of upper gastrointestinal bleeding and perforation associated with low-dose aspirin as plain and enteric-coated formulations. BMC Clin Pharmacol 2001; 1:1.
- Kelly JP, Kaufman DW, Jurgelon JM, Sheehan J, Koff RS, Shapiro S. Risk of aspirin-associated major upper gastrointestinal bleeding with enteric coated or buffered product. Lancet 1996; 348:1413–1416.
- Garcia Rodriguez LA, Hernandez-Diaz S, De Abajo FJ. Association between aspirin and upper gastrointestinal complications: systematic review of epidemiological studies. Br J Clin Pharmacol 2001; 52:563–571.
- Wilcox CM, Ladabaum U. A patient with high risk of gastrointestinal bleeding requiring nonsteroidal anti-inflammatory drugs. Clin Gastroenterol Hepatol 2006; 4:1090–1093.
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction. J Am Coll Cardiol 2007; 50:e1–e157.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996; 348:1329–1339.
- Chan FK, Ching JY, Hung LC, et al. Clopidogrel versus aspirin and esomeprazole to prevent recurrent ulcer bleeding. N Engl J Med 2005; 352:238–244.
- Lai KC, Chu KM, Hui WM, et al. Esomeprazole with aspirin versus clopidogrel for prevention of recurrent gastrointestinal ulcer complications. Clin Gastroenterol Hepatol 2006; 4:860–865.
- Ho MP, Maddox TM, Wang L, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA 2009; 301:937–944.
- Siller-Matula JM, Spiel AO, Lang IM, Kreiner G, Christ G, Jilma B. Effects of pantoprazole and esomeprazole on platelet inhibition by clopidogrel. Am Heart J 2009; 157:148.e1–e5.
- Small DS, Farid NA, Payne CD, et al. Effects of proton pump inhibitor lansoprazole on the pharmacokinetics and pharmacodynamics of prasugel and clopidogrel. J Clin Pharmacol 2008; 48:475–484.
- Ishizaki T, Horai Y. Review article: cytochrome P450 and the metabolism of proton pump inhibitors—emphasis on rabeprazole. Aliment Pharacol Ther 1999; 13 (suppl 3):27–36.
- Lanas A, Fuentes J, Benito R, Serrano P, Bajador E, Sainz R. Helicobacter pylori increases the risk of upper gastrointestinal bleeding in patients taking low-dose aspirin. Aliment Pharmacol Ther 2002; 16:779–786.
- Chan FK. NSAID-Induced peptic ulcers and Helicobacter pylori infection: implications for patient management. Drug Saf 2005; 28:287–300.
- Bombardier V, Laine L, Reicin A, et al; VIGOR Study Group. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis: VIGOR Study Group. N Eng J Med 2000; 343:1520–1528.
- Griffin MR, Ray WA, Schaffner W. Nonsteroidal anti-inflammatory drug use and death from peptic ulcer in elderly persons. Ann Intern Med 1988; 109:359–363.
- Griffin MR, Piper JM, Daugherty JR, Snowden M, Ray WA. Nonsteroidal anti-inflammatory drug use and increased risk for peptic ulcer disease in elderly persons. Ann Intern Med 1991; 114:257–263.
- Smalley WE, Ray WA, Daugherty JR, Griffin MR. Nonsteroidal anti-inflammatory drugs and the incidence of hospitalizations for peptic ulcer disease in elderly persons. Am J Epidemiol 1995; 141:539–545.
- Laine L. Approaches to nonsteroidal anti-inflammatory drug use in the high-risk patient. Gastroenterology 2001; 120:594–606.
- Silverstein FE, Graham DY, Senior JR, et al. Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal anti-inflammatory drugs. A randomized, double blind, placebo controlled trial. Ann Intern Med 1995; 123:241–249.
- Goldstein JL, Huang B, Amer F, Christopoulos NG. Ulcer recurrence in high-risk patients receiving nonsteroidal anti-inflammatory drugs plus low dose aspirin: results of a post hoc subanalysis. Clin Ther 2004; 26:1637–1643.
- Berger JS, Stebbins A, Granger CB, et al. Initial aspirin dose and outcome among ST-elevation myocardial infarction patients treated with fibrinolytic therapy. Circulation 2008; 117:192–199.
- Lanas A, Garcia-Rodriguez LA, Arroyo MT, et al; Investigators of the Asociación Española de Gastroenterología (AEG). Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol 2007; 102:507–515.
- Chin MW, Yong G, Bulsara MK, Rankin J, Forbes GM. Predictive and protective factors associated with upper gastrointestinal bleeding after percutaneous coronary intervention: a case-control study. Am J Gastroenterol 2007; 102:2411–2416.
- Hunt RH, Bazzoli F. Should NSAID/low dose aspirin takers be tested routinely for H. Pylori infection and treated if positive? Implications for primary risk of ulcer and ulcer relapse after initial healing. Aliment Pharmacol Ther 2004; 19 (suppl 1):9–16.
KEY POINTS
- The first priority is to ensure that the patient is hemodynamically stable, which often requires admission to the intensive care unit for monitoring and fluid resuscitation.
- Peptic ulcers account for most cases of upper GI bleeding, but bleeding from varices has a much higher case-fatality rate and always demands aggressive treatment.
- Patients with ulcer disease should be tested and treated for Helicobacter pylori infection.
- Patients with a history of bleeding ulcers who need long-term treatment with aspirin or a nonsteroidal anti-inflammatory drug should also be prescribed a proton pump inhibitor.