User login
SGLT2 inhibitors: Real-world data show benefits outweigh risks
Starting therapy with an SGLT2 inhibitor versus a glucagon-like peptide-1 (GLP-1) receptor agonist was associated with more lower limb amputations, nonvertebral fractures, and genital infections, but these risks need to be balanced against cardiovascular and renoprotective benefits, according to the researchers.
The analysis showed that there would be 2.1 more lower limb amputations, 2.5 more nonvertebral fractures, and 41 more genital infections per 1,000 patients per year among those receiving SGLT2 inhibitors versus an equal number of patients receiving GLP-1 agonists, lead author Edouard Fu, PhD, explained to this news organization in an email.
“On the other hand, we know from the evidence from randomized controlled trials that taking an SGLT2 inhibitor compared with placebo lowers the risk of developing kidney failure,” said Dr. Fu, who is a research fellow in the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital, Boston.
“For instance,” he continued, “in the DAPA-CKD clinical trial, dapagliflozin versus placebo led to 29 fewer events per 1,000 patients per year of the composite outcome (50% decline in estimated glomerular filtration rate [eGFR], kidney failure, cardiovascular or kidney death).”
In the CREDENCE trial, canagliflozin versus placebo led to 18 fewer events per 1,000 person-years for the composite outcome of doubling of serum creatinine, kidney failure, and cardiovascular or kidney death.
And in the EMPA-KIDNEY study, empagliflozin versus placebo led to 21 fewer events per 1,000 person-years for the composite outcome of progression of kidney disease or cardiovascular death.
“Thus, benefits would still outweigh the risks,” Dr. Fu emphasized.
‘Quantifies absolute rate of events among routine care patients’
“The importance of our paper,” he summarized, “is that it quantifies the absolute rate of events among routine care patients and may be used to inform shared decision-making.”
The analysis also found that the risks of diabetic ketoacidosis (DKA), hypovolemia, hypoglycemia, and severe urinary tract infection (UTI) were similar with SGLT2 inhibitors versus GLP-1 agonists, but the risk of developing acute kidney injury (AKI) was lower with an SGLT2 inhibitor.
“Our study can help inform patient-physician decision-making regarding risks and benefits before prescribing SGLT2 inhibitors in this population” of patients with CKD and diabetes treated in clinical practice, the researchers conclude, “but needs to be interpreted in light of its limitations, including residual confounding, short follow-up time, and the use of diagnosis codes to identify patients with CKD.”
The study was recently published in the Clinical Journal of the American Society of Nephrology.
Slow uptake, safety concerns
SGLT2 inhibitors are recommended as first-line therapy in patients with type 2 diabetes and CKD who have an eGFR equal to or greater than 20 mL/min per 1.73 m2, and thus are at high risk for cardiovascular disease and kidney disease progression, Dr. Fu and colleagues write.
However, studies report that as few as 6% of patients with CKD and type 2 diabetes are currently prescribed SGLT2 inhibitors in the United States.
This slow uptake of SGLT2 inhibitors among patients with CKD may be partly due to concerns about DKA, fractures, amputations, and urogenital infections observed in clinical trials.
However, such trials are generally underpowered to assess rare adverse events, use monitoring protocols to lower the risk of adverse events, and include a highly selected patient population, and so safety in routine clinical practice is often unclear.
To examine this, the researchers identified health insurance claims data from 96,128 individuals (from Optum, IBM MarketScan, and Medicare databases) who were 18 years or older (65 years or older for Medicare) and had type 2 diabetes and at least one inpatient or two outpatient diagnostic codes for stage 3 or 4 CKD.
Of these patients, 32,192 had a newly filled prescription for an SGLT2 inhibitor (empagliflozin, dapagliflozin, canagliflozin, or ertugliflozin) and 63,936 had a newly filled prescription for a GLP-1 agonist (liraglutide, dulaglutide, semaglutide, exenatide, albiglutide, or lixisenatide) between April 2013, when the first SGLT2 inhibitor was available in the United States, and 2021.
The researchers matched 28,847 individuals who were initiated on an SGLT2 inhibitor with an equal number who were initiated on a GLP-1 agonist, based on propensity scores, adjusting for more than 120 baseline characteristics.
Safety outcomes were based on previously identified potential safety signals.
Patients who were initiated on an SGLT2 inhibitor had 1.30-fold, 2.13-fold, and 3.08-fold higher risks of having a nonvertebral fracture, a lower limb amputation, and a genital infection, respectively, compared with patients who were initiated on a GLP-1 agonist, after a mean on-treatment time of 7.5 months,
Risks of DKA, hypovolemia, hypoglycemia, and severe UTI were similar in both groups.
Patients initiated on an SGLT2 inhibitor versus a GLP-1 agonist had a lower risk of AKI (hazard ratio, 0.93) equivalent to 6.75 fewer cases of AKI per 1,000 patients per year.
Patients had higher risks for lower limb amputation, genital infections, and nonvertebral fractures with SGLT2 inhibitors versus GLP-1 agonists across most of the prespecified subgroups by age, sex, cardiovascular disease, heart failure, and use of metformin, insulin, or sulfonylurea, but with wider confidence intervals.
Dr. Fu was supported by a Rubicon grant from the Dutch Research Council and has reported no relevant financial relationships. Disclosures for the other authors are listed with the article.
A version of this article originally appeared on Medscape.com.
Starting therapy with an SGLT2 inhibitor versus a glucagon-like peptide-1 (GLP-1) receptor agonist was associated with more lower limb amputations, nonvertebral fractures, and genital infections, but these risks need to be balanced against cardiovascular and renoprotective benefits, according to the researchers.
The analysis showed that there would be 2.1 more lower limb amputations, 2.5 more nonvertebral fractures, and 41 more genital infections per 1,000 patients per year among those receiving SGLT2 inhibitors versus an equal number of patients receiving GLP-1 agonists, lead author Edouard Fu, PhD, explained to this news organization in an email.
“On the other hand, we know from the evidence from randomized controlled trials that taking an SGLT2 inhibitor compared with placebo lowers the risk of developing kidney failure,” said Dr. Fu, who is a research fellow in the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital, Boston.
“For instance,” he continued, “in the DAPA-CKD clinical trial, dapagliflozin versus placebo led to 29 fewer events per 1,000 patients per year of the composite outcome (50% decline in estimated glomerular filtration rate [eGFR], kidney failure, cardiovascular or kidney death).”
In the CREDENCE trial, canagliflozin versus placebo led to 18 fewer events per 1,000 person-years for the composite outcome of doubling of serum creatinine, kidney failure, and cardiovascular or kidney death.
And in the EMPA-KIDNEY study, empagliflozin versus placebo led to 21 fewer events per 1,000 person-years for the composite outcome of progression of kidney disease or cardiovascular death.
“Thus, benefits would still outweigh the risks,” Dr. Fu emphasized.
‘Quantifies absolute rate of events among routine care patients’
“The importance of our paper,” he summarized, “is that it quantifies the absolute rate of events among routine care patients and may be used to inform shared decision-making.”
The analysis also found that the risks of diabetic ketoacidosis (DKA), hypovolemia, hypoglycemia, and severe urinary tract infection (UTI) were similar with SGLT2 inhibitors versus GLP-1 agonists, but the risk of developing acute kidney injury (AKI) was lower with an SGLT2 inhibitor.
“Our study can help inform patient-physician decision-making regarding risks and benefits before prescribing SGLT2 inhibitors in this population” of patients with CKD and diabetes treated in clinical practice, the researchers conclude, “but needs to be interpreted in light of its limitations, including residual confounding, short follow-up time, and the use of diagnosis codes to identify patients with CKD.”
The study was recently published in the Clinical Journal of the American Society of Nephrology.
Slow uptake, safety concerns
SGLT2 inhibitors are recommended as first-line therapy in patients with type 2 diabetes and CKD who have an eGFR equal to or greater than 20 mL/min per 1.73 m2, and thus are at high risk for cardiovascular disease and kidney disease progression, Dr. Fu and colleagues write.
However, studies report that as few as 6% of patients with CKD and type 2 diabetes are currently prescribed SGLT2 inhibitors in the United States.
This slow uptake of SGLT2 inhibitors among patients with CKD may be partly due to concerns about DKA, fractures, amputations, and urogenital infections observed in clinical trials.
However, such trials are generally underpowered to assess rare adverse events, use monitoring protocols to lower the risk of adverse events, and include a highly selected patient population, and so safety in routine clinical practice is often unclear.
To examine this, the researchers identified health insurance claims data from 96,128 individuals (from Optum, IBM MarketScan, and Medicare databases) who were 18 years or older (65 years or older for Medicare) and had type 2 diabetes and at least one inpatient or two outpatient diagnostic codes for stage 3 or 4 CKD.
Of these patients, 32,192 had a newly filled prescription for an SGLT2 inhibitor (empagliflozin, dapagliflozin, canagliflozin, or ertugliflozin) and 63,936 had a newly filled prescription for a GLP-1 agonist (liraglutide, dulaglutide, semaglutide, exenatide, albiglutide, or lixisenatide) between April 2013, when the first SGLT2 inhibitor was available in the United States, and 2021.
The researchers matched 28,847 individuals who were initiated on an SGLT2 inhibitor with an equal number who were initiated on a GLP-1 agonist, based on propensity scores, adjusting for more than 120 baseline characteristics.
Safety outcomes were based on previously identified potential safety signals.
Patients who were initiated on an SGLT2 inhibitor had 1.30-fold, 2.13-fold, and 3.08-fold higher risks of having a nonvertebral fracture, a lower limb amputation, and a genital infection, respectively, compared with patients who were initiated on a GLP-1 agonist, after a mean on-treatment time of 7.5 months,
Risks of DKA, hypovolemia, hypoglycemia, and severe UTI were similar in both groups.
Patients initiated on an SGLT2 inhibitor versus a GLP-1 agonist had a lower risk of AKI (hazard ratio, 0.93) equivalent to 6.75 fewer cases of AKI per 1,000 patients per year.
Patients had higher risks for lower limb amputation, genital infections, and nonvertebral fractures with SGLT2 inhibitors versus GLP-1 agonists across most of the prespecified subgroups by age, sex, cardiovascular disease, heart failure, and use of metformin, insulin, or sulfonylurea, but with wider confidence intervals.
Dr. Fu was supported by a Rubicon grant from the Dutch Research Council and has reported no relevant financial relationships. Disclosures for the other authors are listed with the article.
A version of this article originally appeared on Medscape.com.
Starting therapy with an SGLT2 inhibitor versus a glucagon-like peptide-1 (GLP-1) receptor agonist was associated with more lower limb amputations, nonvertebral fractures, and genital infections, but these risks need to be balanced against cardiovascular and renoprotective benefits, according to the researchers.
The analysis showed that there would be 2.1 more lower limb amputations, 2.5 more nonvertebral fractures, and 41 more genital infections per 1,000 patients per year among those receiving SGLT2 inhibitors versus an equal number of patients receiving GLP-1 agonists, lead author Edouard Fu, PhD, explained to this news organization in an email.
“On the other hand, we know from the evidence from randomized controlled trials that taking an SGLT2 inhibitor compared with placebo lowers the risk of developing kidney failure,” said Dr. Fu, who is a research fellow in the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital, Boston.
“For instance,” he continued, “in the DAPA-CKD clinical trial, dapagliflozin versus placebo led to 29 fewer events per 1,000 patients per year of the composite outcome (50% decline in estimated glomerular filtration rate [eGFR], kidney failure, cardiovascular or kidney death).”
In the CREDENCE trial, canagliflozin versus placebo led to 18 fewer events per 1,000 person-years for the composite outcome of doubling of serum creatinine, kidney failure, and cardiovascular or kidney death.
And in the EMPA-KIDNEY study, empagliflozin versus placebo led to 21 fewer events per 1,000 person-years for the composite outcome of progression of kidney disease or cardiovascular death.
“Thus, benefits would still outweigh the risks,” Dr. Fu emphasized.
‘Quantifies absolute rate of events among routine care patients’
“The importance of our paper,” he summarized, “is that it quantifies the absolute rate of events among routine care patients and may be used to inform shared decision-making.”
The analysis also found that the risks of diabetic ketoacidosis (DKA), hypovolemia, hypoglycemia, and severe urinary tract infection (UTI) were similar with SGLT2 inhibitors versus GLP-1 agonists, but the risk of developing acute kidney injury (AKI) was lower with an SGLT2 inhibitor.
“Our study can help inform patient-physician decision-making regarding risks and benefits before prescribing SGLT2 inhibitors in this population” of patients with CKD and diabetes treated in clinical practice, the researchers conclude, “but needs to be interpreted in light of its limitations, including residual confounding, short follow-up time, and the use of diagnosis codes to identify patients with CKD.”
The study was recently published in the Clinical Journal of the American Society of Nephrology.
Slow uptake, safety concerns
SGLT2 inhibitors are recommended as first-line therapy in patients with type 2 diabetes and CKD who have an eGFR equal to or greater than 20 mL/min per 1.73 m2, and thus are at high risk for cardiovascular disease and kidney disease progression, Dr. Fu and colleagues write.
However, studies report that as few as 6% of patients with CKD and type 2 diabetes are currently prescribed SGLT2 inhibitors in the United States.
This slow uptake of SGLT2 inhibitors among patients with CKD may be partly due to concerns about DKA, fractures, amputations, and urogenital infections observed in clinical trials.
However, such trials are generally underpowered to assess rare adverse events, use monitoring protocols to lower the risk of adverse events, and include a highly selected patient population, and so safety in routine clinical practice is often unclear.
To examine this, the researchers identified health insurance claims data from 96,128 individuals (from Optum, IBM MarketScan, and Medicare databases) who were 18 years or older (65 years or older for Medicare) and had type 2 diabetes and at least one inpatient or two outpatient diagnostic codes for stage 3 or 4 CKD.
Of these patients, 32,192 had a newly filled prescription for an SGLT2 inhibitor (empagliflozin, dapagliflozin, canagliflozin, or ertugliflozin) and 63,936 had a newly filled prescription for a GLP-1 agonist (liraglutide, dulaglutide, semaglutide, exenatide, albiglutide, or lixisenatide) between April 2013, when the first SGLT2 inhibitor was available in the United States, and 2021.
The researchers matched 28,847 individuals who were initiated on an SGLT2 inhibitor with an equal number who were initiated on a GLP-1 agonist, based on propensity scores, adjusting for more than 120 baseline characteristics.
Safety outcomes were based on previously identified potential safety signals.
Patients who were initiated on an SGLT2 inhibitor had 1.30-fold, 2.13-fold, and 3.08-fold higher risks of having a nonvertebral fracture, a lower limb amputation, and a genital infection, respectively, compared with patients who were initiated on a GLP-1 agonist, after a mean on-treatment time of 7.5 months,
Risks of DKA, hypovolemia, hypoglycemia, and severe UTI were similar in both groups.
Patients initiated on an SGLT2 inhibitor versus a GLP-1 agonist had a lower risk of AKI (hazard ratio, 0.93) equivalent to 6.75 fewer cases of AKI per 1,000 patients per year.
Patients had higher risks for lower limb amputation, genital infections, and nonvertebral fractures with SGLT2 inhibitors versus GLP-1 agonists across most of the prespecified subgroups by age, sex, cardiovascular disease, heart failure, and use of metformin, insulin, or sulfonylurea, but with wider confidence intervals.
Dr. Fu was supported by a Rubicon grant from the Dutch Research Council and has reported no relevant financial relationships. Disclosures for the other authors are listed with the article.
A version of this article originally appeared on Medscape.com.
Longer life after bariatric surgery, but suicide risk in young
Death from cardiovascular disease, cancer, and diabetes was 29%, 43%, and 72% lower, respectively, in the bariatric surgery patients versus nonsurgery peers, during a mean follow-up of 13 years (all P > .001).
However, the youngest group of bariatric surgery patients – who were 18-34 years old – had a fivefold increased risk of suicide during follow-up compared with their peers who did not undergo surgery (P = .001).
These findings are from a retrospective study in Utah that matched close to 22,000 patients with severe obesity who underwent Roux-en-Y gastric bypass, gastric banding, sleeve gastrectomy, or duodenal switch from 1982 to 2018 with an equal number of nonsurgery individuals.
The study, by Ted D. Adams, PhD, MPH, and colleagues, was published online in Obesity.
‘Impressive’ data, in men too, but psychological screening important
The overall improved survival and decreased deaths from diabetes, heart disease, and cancer over this long follow-up are “impressive,” Dr. Adams, of Intermountain Surgical Specialties/Digestive Health Clinical Program, Salt Lake City, said in an interview.
Previous studies have not shown a survival benefit from bariatric surgery versus no surgery in men, he said. However, “because we had a fair number of male patients and because of the length of follow-up, we did show that the improved mortality was not only evident for the female patients but also for the male patients,” Dr. Adams stressed.
Finding increased suicide rates among bariatric surgical patients who underwent surgery at a younger age (18-34 years) shows that “we need to try and determine who is at risk for suicide,” according to Dr. Adams.
Patients with severe obesity, especially younger ones, “may need more aggressive presurgical psychological screening and postsurgery follow-up,” wrote Dr. Adams and colleagues.
The findings may also “stimulate important research related to the discovery of physiologic and biomolecular mechanisms leading to nonsurgical treatment that results in weight loss and improved mortality similar to that achieved by bariatric surgery,” they suggested.
Close to 1 in 10 Americans has severe obesity
The prevalence of severe obesity (BMI ≥ 40 kg/m2) in the United States has increased from 4.7% during 1999-2000 to 9.2% during 2017-2018, based on National Health and Nutrition Examination Survey (NHANES) data, the researchers noted.
They previously published a study of long-term mortality in 7,925 patients who had gastric bypass surgery from 1984 to 2002 matched with patients with the same BMI who did not have bariatric surgery and were followed out to 2002.
The current study extends the follow-up through 2021, doubles the number of bypass patients, and includes three newer types of bariatric surgery.
The researchers matched 21,873 patients aged 18-80 who had Roux-en-Y gastric bypass, gastric banding, sleeve gastrectomy, or duodenal switch during 1982-2018 in Utah (from the Utah Population Database) with people of the same BMI category, age category (18-34, 35-44, 45-54, and 55-80 years), and sex (from Utah driver license data).
Most patients were women (79%) and most were White (94% and 85%). They had a mean age of 42 years and a mean BMI of 46 kg/m2.
Most patients had Roux-en-Y gastric bypass (69%), and the rest had sleeve gastrectomy (14%), gastric banding (12%), and duodenal switch (4.8%).
During follow-up, 13.5% of patients in the bariatric surgery group and 14.6% of people in the nonsurgery group died.
Overall, all-cause mortality was 16% lower in patients who had bariatric surgery versus matched nonsurgical participants; it was 14% lower in women and 21% lower in men (all P < .001).
All-cause mortality was significantly lower in patients who had bariatric surgery when they were 35-44, 45-54, and 55-80 years old compared with matched peers who did not have surgery.
However, the findings “should not imply patients necessarily postpone surgery until older age,” the researchers cautioned, “as postsurgical complications have been shown to increase with increasing age at surgery and surgical postponement may result in worsened clinical status related to certain conditions such as orthopedic joint health.”
The researchers found significantly improved all-cause mortality following either type of surgery (gastric bypass, gastric banding, and sleeve gastrectomy) compared with no surgery.
Along with fewer deaths from cardiovascular disease, cancer, and diabetes, deaths from lung disease were 39% lower in the surgery group than in the nonsurgery group.
However, in the youngest group (age 18-34), deaths from cirrhosis of the liver were significantly higher in the patients who had bariatric surgery, and rates of suicide were significantly greater for both females and males, compared with similar people who did not undergo surgery.
The study was supported by grants from Ethicon Endo-Surgery (Johnson & Johnson); the National Institute of Diabetes and Digestive and Kidney Diseases, a division of the National Institutes of Health; U.S. Public Health Service; and Intermountain Research and Medical Foundation of Intermountain Healthcare. Dr. Adams disclosed ties to Ethicon Endo-Surgery and Intermountain Healthcare. A coauthor reported ties with Biomedical Research Program at Weill Cornell Medicine in Qatar, a program funded by the Qatar Foundation. The other authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Death from cardiovascular disease, cancer, and diabetes was 29%, 43%, and 72% lower, respectively, in the bariatric surgery patients versus nonsurgery peers, during a mean follow-up of 13 years (all P > .001).
However, the youngest group of bariatric surgery patients – who were 18-34 years old – had a fivefold increased risk of suicide during follow-up compared with their peers who did not undergo surgery (P = .001).
These findings are from a retrospective study in Utah that matched close to 22,000 patients with severe obesity who underwent Roux-en-Y gastric bypass, gastric banding, sleeve gastrectomy, or duodenal switch from 1982 to 2018 with an equal number of nonsurgery individuals.
The study, by Ted D. Adams, PhD, MPH, and colleagues, was published online in Obesity.
‘Impressive’ data, in men too, but psychological screening important
The overall improved survival and decreased deaths from diabetes, heart disease, and cancer over this long follow-up are “impressive,” Dr. Adams, of Intermountain Surgical Specialties/Digestive Health Clinical Program, Salt Lake City, said in an interview.
Previous studies have not shown a survival benefit from bariatric surgery versus no surgery in men, he said. However, “because we had a fair number of male patients and because of the length of follow-up, we did show that the improved mortality was not only evident for the female patients but also for the male patients,” Dr. Adams stressed.
Finding increased suicide rates among bariatric surgical patients who underwent surgery at a younger age (18-34 years) shows that “we need to try and determine who is at risk for suicide,” according to Dr. Adams.
Patients with severe obesity, especially younger ones, “may need more aggressive presurgical psychological screening and postsurgery follow-up,” wrote Dr. Adams and colleagues.
The findings may also “stimulate important research related to the discovery of physiologic and biomolecular mechanisms leading to nonsurgical treatment that results in weight loss and improved mortality similar to that achieved by bariatric surgery,” they suggested.
Close to 1 in 10 Americans has severe obesity
The prevalence of severe obesity (BMI ≥ 40 kg/m2) in the United States has increased from 4.7% during 1999-2000 to 9.2% during 2017-2018, based on National Health and Nutrition Examination Survey (NHANES) data, the researchers noted.
They previously published a study of long-term mortality in 7,925 patients who had gastric bypass surgery from 1984 to 2002 matched with patients with the same BMI who did not have bariatric surgery and were followed out to 2002.
The current study extends the follow-up through 2021, doubles the number of bypass patients, and includes three newer types of bariatric surgery.
The researchers matched 21,873 patients aged 18-80 who had Roux-en-Y gastric bypass, gastric banding, sleeve gastrectomy, or duodenal switch during 1982-2018 in Utah (from the Utah Population Database) with people of the same BMI category, age category (18-34, 35-44, 45-54, and 55-80 years), and sex (from Utah driver license data).
Most patients were women (79%) and most were White (94% and 85%). They had a mean age of 42 years and a mean BMI of 46 kg/m2.
Most patients had Roux-en-Y gastric bypass (69%), and the rest had sleeve gastrectomy (14%), gastric banding (12%), and duodenal switch (4.8%).
During follow-up, 13.5% of patients in the bariatric surgery group and 14.6% of people in the nonsurgery group died.
Overall, all-cause mortality was 16% lower in patients who had bariatric surgery versus matched nonsurgical participants; it was 14% lower in women and 21% lower in men (all P < .001).
All-cause mortality was significantly lower in patients who had bariatric surgery when they were 35-44, 45-54, and 55-80 years old compared with matched peers who did not have surgery.
However, the findings “should not imply patients necessarily postpone surgery until older age,” the researchers cautioned, “as postsurgical complications have been shown to increase with increasing age at surgery and surgical postponement may result in worsened clinical status related to certain conditions such as orthopedic joint health.”
The researchers found significantly improved all-cause mortality following either type of surgery (gastric bypass, gastric banding, and sleeve gastrectomy) compared with no surgery.
Along with fewer deaths from cardiovascular disease, cancer, and diabetes, deaths from lung disease were 39% lower in the surgery group than in the nonsurgery group.
However, in the youngest group (age 18-34), deaths from cirrhosis of the liver were significantly higher in the patients who had bariatric surgery, and rates of suicide were significantly greater for both females and males, compared with similar people who did not undergo surgery.
The study was supported by grants from Ethicon Endo-Surgery (Johnson & Johnson); the National Institute of Diabetes and Digestive and Kidney Diseases, a division of the National Institutes of Health; U.S. Public Health Service; and Intermountain Research and Medical Foundation of Intermountain Healthcare. Dr. Adams disclosed ties to Ethicon Endo-Surgery and Intermountain Healthcare. A coauthor reported ties with Biomedical Research Program at Weill Cornell Medicine in Qatar, a program funded by the Qatar Foundation. The other authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Death from cardiovascular disease, cancer, and diabetes was 29%, 43%, and 72% lower, respectively, in the bariatric surgery patients versus nonsurgery peers, during a mean follow-up of 13 years (all P > .001).
However, the youngest group of bariatric surgery patients – who were 18-34 years old – had a fivefold increased risk of suicide during follow-up compared with their peers who did not undergo surgery (P = .001).
These findings are from a retrospective study in Utah that matched close to 22,000 patients with severe obesity who underwent Roux-en-Y gastric bypass, gastric banding, sleeve gastrectomy, or duodenal switch from 1982 to 2018 with an equal number of nonsurgery individuals.
The study, by Ted D. Adams, PhD, MPH, and colleagues, was published online in Obesity.
‘Impressive’ data, in men too, but psychological screening important
The overall improved survival and decreased deaths from diabetes, heart disease, and cancer over this long follow-up are “impressive,” Dr. Adams, of Intermountain Surgical Specialties/Digestive Health Clinical Program, Salt Lake City, said in an interview.
Previous studies have not shown a survival benefit from bariatric surgery versus no surgery in men, he said. However, “because we had a fair number of male patients and because of the length of follow-up, we did show that the improved mortality was not only evident for the female patients but also for the male patients,” Dr. Adams stressed.
Finding increased suicide rates among bariatric surgical patients who underwent surgery at a younger age (18-34 years) shows that “we need to try and determine who is at risk for suicide,” according to Dr. Adams.
Patients with severe obesity, especially younger ones, “may need more aggressive presurgical psychological screening and postsurgery follow-up,” wrote Dr. Adams and colleagues.
The findings may also “stimulate important research related to the discovery of physiologic and biomolecular mechanisms leading to nonsurgical treatment that results in weight loss and improved mortality similar to that achieved by bariatric surgery,” they suggested.
Close to 1 in 10 Americans has severe obesity
The prevalence of severe obesity (BMI ≥ 40 kg/m2) in the United States has increased from 4.7% during 1999-2000 to 9.2% during 2017-2018, based on National Health and Nutrition Examination Survey (NHANES) data, the researchers noted.
They previously published a study of long-term mortality in 7,925 patients who had gastric bypass surgery from 1984 to 2002 matched with patients with the same BMI who did not have bariatric surgery and were followed out to 2002.
The current study extends the follow-up through 2021, doubles the number of bypass patients, and includes three newer types of bariatric surgery.
The researchers matched 21,873 patients aged 18-80 who had Roux-en-Y gastric bypass, gastric banding, sleeve gastrectomy, or duodenal switch during 1982-2018 in Utah (from the Utah Population Database) with people of the same BMI category, age category (18-34, 35-44, 45-54, and 55-80 years), and sex (from Utah driver license data).
Most patients were women (79%) and most were White (94% and 85%). They had a mean age of 42 years and a mean BMI of 46 kg/m2.
Most patients had Roux-en-Y gastric bypass (69%), and the rest had sleeve gastrectomy (14%), gastric banding (12%), and duodenal switch (4.8%).
During follow-up, 13.5% of patients in the bariatric surgery group and 14.6% of people in the nonsurgery group died.
Overall, all-cause mortality was 16% lower in patients who had bariatric surgery versus matched nonsurgical participants; it was 14% lower in women and 21% lower in men (all P < .001).
All-cause mortality was significantly lower in patients who had bariatric surgery when they were 35-44, 45-54, and 55-80 years old compared with matched peers who did not have surgery.
However, the findings “should not imply patients necessarily postpone surgery until older age,” the researchers cautioned, “as postsurgical complications have been shown to increase with increasing age at surgery and surgical postponement may result in worsened clinical status related to certain conditions such as orthopedic joint health.”
The researchers found significantly improved all-cause mortality following either type of surgery (gastric bypass, gastric banding, and sleeve gastrectomy) compared with no surgery.
Along with fewer deaths from cardiovascular disease, cancer, and diabetes, deaths from lung disease were 39% lower in the surgery group than in the nonsurgery group.
However, in the youngest group (age 18-34), deaths from cirrhosis of the liver were significantly higher in the patients who had bariatric surgery, and rates of suicide were significantly greater for both females and males, compared with similar people who did not undergo surgery.
The study was supported by grants from Ethicon Endo-Surgery (Johnson & Johnson); the National Institute of Diabetes and Digestive and Kidney Diseases, a division of the National Institutes of Health; U.S. Public Health Service; and Intermountain Research and Medical Foundation of Intermountain Healthcare. Dr. Adams disclosed ties to Ethicon Endo-Surgery and Intermountain Healthcare. A coauthor reported ties with Biomedical Research Program at Weill Cornell Medicine in Qatar, a program funded by the Qatar Foundation. The other authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM OBESITY
Persistent gaps in drug use by patients with type 2 diabetes
Adults with mainly type 2 diabetes had gaps in the use of medications for managing blood glucose, hypertension, and lipids, in an analysis of nationally representative U.S. survey data.
A mean of 19.5%, 17.1%, and 43.3% of survey participants had inconsistent use of glucose-, BP-, or lipid-lowering medications, respectively, over 2 years in a series of successive 2-year surveys in 2005-2019.
A new group of participants was enrolled for each successive 2-year survey.
“We found persistent and sometimes increasing gaps in continuity of use of these [glycemia, hypertension, and lipid] treatments at the national level,” the researchers wrote.
Moreover, “this outcome was found despite long-lasting guidelines that generally recommend medications as an ongoing part of therapy for adults with type 2 diabetes to reduce macrovascular and microvascular disease risk,” they stressed.
The data did not distinguish between type 1 and type 2 diabetes, but more than 90% of diabetes diagnoses in the United States are type 2 diabetes, the researchers noted.
Therefore, it is “correct, our findings primarily reflect type 2 diabetes,” lead author Puneet Kaur Chehal, PhD, assistant professor, Emory University, Atlanta, clarified in an email.
“The clinical guidelines for treatment of type 1 diabetes are distinct,” she added, so “it is difficult to draw any conclusions from our study for this population.”
“To observe national trends in continuous use decrease at the same time that diabetes complications are increasing and physicians are guided to shift away from treat-to-target and towards individual patient needs certainly caught our attention,” she said.
“Our findings highlight the need for additional research to understand what is going on here,” according to Dr. Chehal.
“We did not observe levels of glucose (or blood pressure and lipids) to explore if the decrease in glucose-lowering drugs was warranted,” she added. “Our evidence of differences in continuity in use across subgroups (by race/ethnicity, payer, and age) does warrant further analysis of whether the decreasing trends we observe are lapses in access or deliberate changes in treatment.”
The study was published online in JAMA Network Open.
Investigating trends in medication adherence
Type 2 diabetes is a chronic condition and medications to control blood glucose, BP, and lipids lower the risk of diabetes-associated complications, Dr. Chehal and colleagues wrote.
After years of improvement, these cardiometabolic parameters plateaued and even decreased in 2013-2021, in parallel with increasing rates of diabetes complications, especially in younger adults, certain ethnic minority groups, and people with increased risks.
Suboptimal medication adherence among people with type 2 diabetes is associated with preventable complications and onset of heart disease, kidney disease, or diabetic neuropathy, which can lead to amputation.
However, previous studies of medication adherence were typically limited to patients covered by Medicare or commercial insurance, or studies only had 1-year follow-up.
Therefore, the researchers performed a cross-sectional analysis of a series of 2-year data from the Medical Expenditure Panel Survey (MEPS), in which participants reply to five interviews in 2 years and new participants are selected each year.
The researchers analyzed data from 15,237 adults aged 18 and older with type 2 diabetes who participated in 1 of 14 2-year MEPS survey panels in 2005-2019.
About half of participants (47.4%) were age 45-64 and about half (54.2%) were women. They were also racially diverse (43% non-Latino White, 25% Latino, and 24% non-Latino Black).
Participants were classified as having “inconsistent use” of glucose-lowering medication, for example, if they did not fill at least one prescription for a glucose-lowering drug in each of the 2 years.
“As long as [the medication] was some type of glucose-, blood pressure–, or lipid-lowering medication and was filled, it counted as continued use for that category,” Dr. Chehal explained.
They are preparing another paper that explores changes in medication regimens.
The current study showed continued use of glucose-lowering medication in both years decreased from 84.5% in 2005-2006 to 77.4% in 2018-2019, no use of glucose-lowering medication in either of the 2 years increased from 8.1% in 2005-2006 to 12.9% in 2018-2019, inconsistent use of glucose-lowering medication increased from 3.3% in 2005-2006 to 7.1% in 2018-2019, and new use of glucose-lowering medications in year 2 fluctuated between 2% and 4% across panels.
It also showed inconsistent use of BP-lowering medication increased from 3.9% in 2005-2006 to 9.0% in 2016-2017 and inconsistent use of lipid-lowering medication increased to a high of 9.9% in 2017-2018.
Younger and Black participants were less likely to consistently use glucose-lowering medication, Latino patients were less likely to consistently use BP-lowering medications, and Black and Latino patients were less likely to continuously use lipid-lowering medications. Uninsured adults were more likely to use no medications or use medications inconsistently.
“Changes and inconsistencies in payer formularies and out-of-pocket cost burden, especially among adults with no or insufficient insurance (i.e., Medicare Part D), remain prominent issues,” according to Dr. Chehal and colleagues.
“Decreases in continuity in use of glucose-lowering medications in recent panels may explain worsening diabetes complications,” they wrote.
This may be partly caused by recommended decreases in sulfonylurea and thiazolidinedione use and increased prescribing of new and more cost-prohibitive medications, they suggested.
Or this may be caused by the shift away from treating aggressively until a target is achieved toward individualizing treatment based on a patient’s age, phenotype, or comorbidities (for example, kidney disease).
The study was supported by a grant from MSD, a subsidiary of Merck, to Emory University. Some of the researchers received grants from Merck for the submitted work or were partially supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health to the Georgia Center for Diabetes Translation Research. Dr. Chehal reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Adults with mainly type 2 diabetes had gaps in the use of medications for managing blood glucose, hypertension, and lipids, in an analysis of nationally representative U.S. survey data.
A mean of 19.5%, 17.1%, and 43.3% of survey participants had inconsistent use of glucose-, BP-, or lipid-lowering medications, respectively, over 2 years in a series of successive 2-year surveys in 2005-2019.
A new group of participants was enrolled for each successive 2-year survey.
“We found persistent and sometimes increasing gaps in continuity of use of these [glycemia, hypertension, and lipid] treatments at the national level,” the researchers wrote.
Moreover, “this outcome was found despite long-lasting guidelines that generally recommend medications as an ongoing part of therapy for adults with type 2 diabetes to reduce macrovascular and microvascular disease risk,” they stressed.
The data did not distinguish between type 1 and type 2 diabetes, but more than 90% of diabetes diagnoses in the United States are type 2 diabetes, the researchers noted.
Therefore, it is “correct, our findings primarily reflect type 2 diabetes,” lead author Puneet Kaur Chehal, PhD, assistant professor, Emory University, Atlanta, clarified in an email.
“The clinical guidelines for treatment of type 1 diabetes are distinct,” she added, so “it is difficult to draw any conclusions from our study for this population.”
“To observe national trends in continuous use decrease at the same time that diabetes complications are increasing and physicians are guided to shift away from treat-to-target and towards individual patient needs certainly caught our attention,” she said.
“Our findings highlight the need for additional research to understand what is going on here,” according to Dr. Chehal.
“We did not observe levels of glucose (or blood pressure and lipids) to explore if the decrease in glucose-lowering drugs was warranted,” she added. “Our evidence of differences in continuity in use across subgroups (by race/ethnicity, payer, and age) does warrant further analysis of whether the decreasing trends we observe are lapses in access or deliberate changes in treatment.”
The study was published online in JAMA Network Open.
Investigating trends in medication adherence
Type 2 diabetes is a chronic condition and medications to control blood glucose, BP, and lipids lower the risk of diabetes-associated complications, Dr. Chehal and colleagues wrote.
After years of improvement, these cardiometabolic parameters plateaued and even decreased in 2013-2021, in parallel with increasing rates of diabetes complications, especially in younger adults, certain ethnic minority groups, and people with increased risks.
Suboptimal medication adherence among people with type 2 diabetes is associated with preventable complications and onset of heart disease, kidney disease, or diabetic neuropathy, which can lead to amputation.
However, previous studies of medication adherence were typically limited to patients covered by Medicare or commercial insurance, or studies only had 1-year follow-up.
Therefore, the researchers performed a cross-sectional analysis of a series of 2-year data from the Medical Expenditure Panel Survey (MEPS), in which participants reply to five interviews in 2 years and new participants are selected each year.
The researchers analyzed data from 15,237 adults aged 18 and older with type 2 diabetes who participated in 1 of 14 2-year MEPS survey panels in 2005-2019.
About half of participants (47.4%) were age 45-64 and about half (54.2%) were women. They were also racially diverse (43% non-Latino White, 25% Latino, and 24% non-Latino Black).
Participants were classified as having “inconsistent use” of glucose-lowering medication, for example, if they did not fill at least one prescription for a glucose-lowering drug in each of the 2 years.
“As long as [the medication] was some type of glucose-, blood pressure–, or lipid-lowering medication and was filled, it counted as continued use for that category,” Dr. Chehal explained.
They are preparing another paper that explores changes in medication regimens.
The current study showed continued use of glucose-lowering medication in both years decreased from 84.5% in 2005-2006 to 77.4% in 2018-2019, no use of glucose-lowering medication in either of the 2 years increased from 8.1% in 2005-2006 to 12.9% in 2018-2019, inconsistent use of glucose-lowering medication increased from 3.3% in 2005-2006 to 7.1% in 2018-2019, and new use of glucose-lowering medications in year 2 fluctuated between 2% and 4% across panels.
It also showed inconsistent use of BP-lowering medication increased from 3.9% in 2005-2006 to 9.0% in 2016-2017 and inconsistent use of lipid-lowering medication increased to a high of 9.9% in 2017-2018.
Younger and Black participants were less likely to consistently use glucose-lowering medication, Latino patients were less likely to consistently use BP-lowering medications, and Black and Latino patients were less likely to continuously use lipid-lowering medications. Uninsured adults were more likely to use no medications or use medications inconsistently.
“Changes and inconsistencies in payer formularies and out-of-pocket cost burden, especially among adults with no or insufficient insurance (i.e., Medicare Part D), remain prominent issues,” according to Dr. Chehal and colleagues.
“Decreases in continuity in use of glucose-lowering medications in recent panels may explain worsening diabetes complications,” they wrote.
This may be partly caused by recommended decreases in sulfonylurea and thiazolidinedione use and increased prescribing of new and more cost-prohibitive medications, they suggested.
Or this may be caused by the shift away from treating aggressively until a target is achieved toward individualizing treatment based on a patient’s age, phenotype, or comorbidities (for example, kidney disease).
The study was supported by a grant from MSD, a subsidiary of Merck, to Emory University. Some of the researchers received grants from Merck for the submitted work or were partially supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health to the Georgia Center for Diabetes Translation Research. Dr. Chehal reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Adults with mainly type 2 diabetes had gaps in the use of medications for managing blood glucose, hypertension, and lipids, in an analysis of nationally representative U.S. survey data.
A mean of 19.5%, 17.1%, and 43.3% of survey participants had inconsistent use of glucose-, BP-, or lipid-lowering medications, respectively, over 2 years in a series of successive 2-year surveys in 2005-2019.
A new group of participants was enrolled for each successive 2-year survey.
“We found persistent and sometimes increasing gaps in continuity of use of these [glycemia, hypertension, and lipid] treatments at the national level,” the researchers wrote.
Moreover, “this outcome was found despite long-lasting guidelines that generally recommend medications as an ongoing part of therapy for adults with type 2 diabetes to reduce macrovascular and microvascular disease risk,” they stressed.
The data did not distinguish between type 1 and type 2 diabetes, but more than 90% of diabetes diagnoses in the United States are type 2 diabetes, the researchers noted.
Therefore, it is “correct, our findings primarily reflect type 2 diabetes,” lead author Puneet Kaur Chehal, PhD, assistant professor, Emory University, Atlanta, clarified in an email.
“The clinical guidelines for treatment of type 1 diabetes are distinct,” she added, so “it is difficult to draw any conclusions from our study for this population.”
“To observe national trends in continuous use decrease at the same time that diabetes complications are increasing and physicians are guided to shift away from treat-to-target and towards individual patient needs certainly caught our attention,” she said.
“Our findings highlight the need for additional research to understand what is going on here,” according to Dr. Chehal.
“We did not observe levels of glucose (or blood pressure and lipids) to explore if the decrease in glucose-lowering drugs was warranted,” she added. “Our evidence of differences in continuity in use across subgroups (by race/ethnicity, payer, and age) does warrant further analysis of whether the decreasing trends we observe are lapses in access or deliberate changes in treatment.”
The study was published online in JAMA Network Open.
Investigating trends in medication adherence
Type 2 diabetes is a chronic condition and medications to control blood glucose, BP, and lipids lower the risk of diabetes-associated complications, Dr. Chehal and colleagues wrote.
After years of improvement, these cardiometabolic parameters plateaued and even decreased in 2013-2021, in parallel with increasing rates of diabetes complications, especially in younger adults, certain ethnic minority groups, and people with increased risks.
Suboptimal medication adherence among people with type 2 diabetes is associated with preventable complications and onset of heart disease, kidney disease, or diabetic neuropathy, which can lead to amputation.
However, previous studies of medication adherence were typically limited to patients covered by Medicare or commercial insurance, or studies only had 1-year follow-up.
Therefore, the researchers performed a cross-sectional analysis of a series of 2-year data from the Medical Expenditure Panel Survey (MEPS), in which participants reply to five interviews in 2 years and new participants are selected each year.
The researchers analyzed data from 15,237 adults aged 18 and older with type 2 diabetes who participated in 1 of 14 2-year MEPS survey panels in 2005-2019.
About half of participants (47.4%) were age 45-64 and about half (54.2%) were women. They were also racially diverse (43% non-Latino White, 25% Latino, and 24% non-Latino Black).
Participants were classified as having “inconsistent use” of glucose-lowering medication, for example, if they did not fill at least one prescription for a glucose-lowering drug in each of the 2 years.
“As long as [the medication] was some type of glucose-, blood pressure–, or lipid-lowering medication and was filled, it counted as continued use for that category,” Dr. Chehal explained.
They are preparing another paper that explores changes in medication regimens.
The current study showed continued use of glucose-lowering medication in both years decreased from 84.5% in 2005-2006 to 77.4% in 2018-2019, no use of glucose-lowering medication in either of the 2 years increased from 8.1% in 2005-2006 to 12.9% in 2018-2019, inconsistent use of glucose-lowering medication increased from 3.3% in 2005-2006 to 7.1% in 2018-2019, and new use of glucose-lowering medications in year 2 fluctuated between 2% and 4% across panels.
It also showed inconsistent use of BP-lowering medication increased from 3.9% in 2005-2006 to 9.0% in 2016-2017 and inconsistent use of lipid-lowering medication increased to a high of 9.9% in 2017-2018.
Younger and Black participants were less likely to consistently use glucose-lowering medication, Latino patients were less likely to consistently use BP-lowering medications, and Black and Latino patients were less likely to continuously use lipid-lowering medications. Uninsured adults were more likely to use no medications or use medications inconsistently.
“Changes and inconsistencies in payer formularies and out-of-pocket cost burden, especially among adults with no or insufficient insurance (i.e., Medicare Part D), remain prominent issues,” according to Dr. Chehal and colleagues.
“Decreases in continuity in use of glucose-lowering medications in recent panels may explain worsening diabetes complications,” they wrote.
This may be partly caused by recommended decreases in sulfonylurea and thiazolidinedione use and increased prescribing of new and more cost-prohibitive medications, they suggested.
Or this may be caused by the shift away from treating aggressively until a target is achieved toward individualizing treatment based on a patient’s age, phenotype, or comorbidities (for example, kidney disease).
The study was supported by a grant from MSD, a subsidiary of Merck, to Emory University. Some of the researchers received grants from Merck for the submitted work or were partially supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health to the Georgia Center for Diabetes Translation Research. Dr. Chehal reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM JAMA NETWORK OPEN
More type 2 diabetes deaths from cancer than heart disease
Cancer appears to have overtaken cardiovascular disease (CVD) as a leading cause of death in adults with type 2 diabetes, a 20-year population study in England suggests.
The researchers found that, from 1998 to 2018, in more than 130,000 adults aged 35 and older with type 2 diabetes, all-cause mortality declined for all ages, but cancer mortality increased for those aged 75 and older; people with type 2 diabetes who were smokers had higher and steadily increasing cancer mortality rates; and people with type 2 diabetes had more than twice the rate of colorectal, pancreatic, liver, and endometrial cancer mortality than age- and sex-matched individuals in the general population.
The findings suggest that “cancer prevention strategies therefore deserve at least a similar level of attention as cardiovascular disease prevention, particularly in older people and for some cancers such as liver, colorectal, and pancreatic cancer,” the researchers wrote.
Tailored cancer prevention and early-detection strategies are needed to address persistent inequalities in the older population, the most deprived, and smokers, they added.
Breast cancer rates in younger women with type 2 diabetes rising
According to the researchers, “early cancer detection through changes to existing screening [programs], or more in-depth investigations for suspected/nonspecific symptoms, may reduce the number of avoidable cancer deaths in people with type 2 diabetes.”
Moreover, breast cancer rates in younger women with type 2 diabetes are rising by 4.1% per year, they wrote, which suggests such women are high risk and should be screened at a younger age, but screening age would need to be determined in cost-effectiveness analyses.
The study by Suping Ling, PhD, and colleagues was published online in Diabetologia.
Results challenge belief that preventing CVD is priority in type 2 diabetes
“The prevention of cardiovascular disease has been, and is still considered, a priority in people with diabetes,” the researchers wrote.
“Our results challenge this view by showing that cancer may have overtaken cardiovascular disease as a leading cause of death in people with type 2 diabetes.”
“The proportion of cancer deaths out of all-cause deaths remains high (> 30%) in young ages, and it was steadily increasing in older ages,” Dr. Ling, from the department of noncommunicable disease epidemiology, London School of Hygiene & Tropical Medicine, said in a comment.
“Combined with previous studies reporting decreasing CVD mortality rates,” she said, “we concluded that cancer might have overtaken CVD as the leading cause of death in people with type 2 diabetes.”
Many evidence-based cancer-prevention strategies related to lifestyle (such as being physically active, being a healthy weight, eating a better diet, stopping smoking, as summarized by the World Cancer Research Fund), are helpful for preventing both cancer and CVD, Ling observed.
However, in the medical community, many additional efforts were made for monitoring, early detection, and innovating medications for CVD, she noted. “Therefore, we would like to propose a similar level of attention and effort for cancer in people with type 2 diabetes.”
Deaths from cancer vs. all causes in patients with diabetes
The researchers identified 137,804 patients aged 35 and older who were newly diagnosed with type 2 diabetes from 1998 to 2018 in general practices in the UK that were part of the Clinical Practice Research Datalink.
Patients were a median age of 64 years and 45% were women. Most (83%) were White, followed by South Asian (3.5%), Black (2.0%), and other (3%); 8.4% had missing information for race. Patients had a median body mass index (BMI) of 30.6 kg/m2.
Researchers divided patients into socioeconomic quintiles of most to least deprived based on income, employment, education, and other factors. During a median follow-up of 8.4 years, there were 39,212 deaths (28.5%).
Cancer mortality in subgroups of patients with type 2 diabetes
Researchers analyzed annual deaths from cancer and from all causes over 20 years in subgroups of patients with type 2 diabetes.
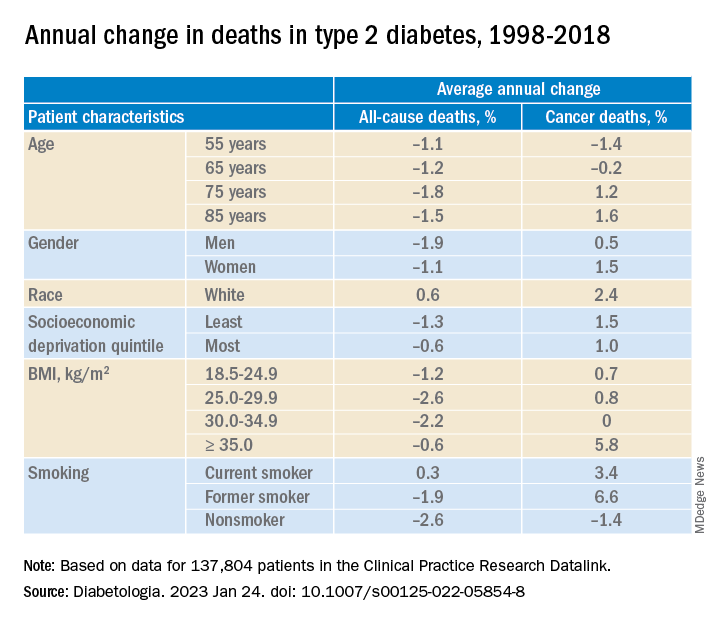
In adults with type 2 diabetes, the average percentage change in cancer mortality per year, from 1998 to 2018 decreased in people aged 55 and 65 (–1.4% and –0.2%, respectively), but increased in people aged 75 and 85 (1.2% and 1.6%, respectively); increased more in women than in men (1.5% vs 1.0%), although women had lower cancer mortality than men; and increased more in the least deprived (wealthiest) individuals than in the most deprived (1.5% vs 1.0%). Cancer mortality rates were consistently higher in the most deprived individuals, Dr. Ling noted.
Cancer mortality also increased more in people with class III obesity (BMI ≥ 35) versus normal weight (5.8% vs 0.7%) and versus other weights. In addition, there was an upward trend in cancer mortality in people who were White or former/current smokers.
Deaths from specific cancers in diabetes vs. general population
Next, researchers determined cancer mortality ratios – the cancer mortality of the patients with diabetes divided by the cancer mortality of the general population.
They determined this for all cancers, the four most common cancers in the United Kingdom (lung, colorectal, breast, and prostate), and cancers caused by type 2 diabetes (pancreatic, liver, gallbladder, and endometrial cancer), standardized by sex and age.
Mortality from all cancer was 18% higher in patients with type 2 diabetes, compared with the general population.
Overall, mortality from colorectal cancer, pancreatic cancer, and liver cancer was 2.4 times, 2.12 times, and 2.13 times higher, respectively, in patients with type 2 diabetes than in the general population.
Mortality from breast cancer was 9% higher and mortality from endometrial cancer was 2.08 times higher in women with type 2 diabetes than in women in the general population.
There was a constant upward trend for mortality rates for pancreatic, liver, and lung cancer at all ages, colorectal cancer at most ages, breast cancer at younger ages, and prostate and endometrial cancer at older ages.
The study was funded by Hope Against Cancer. Dr. Ling reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cancer appears to have overtaken cardiovascular disease (CVD) as a leading cause of death in adults with type 2 diabetes, a 20-year population study in England suggests.
The researchers found that, from 1998 to 2018, in more than 130,000 adults aged 35 and older with type 2 diabetes, all-cause mortality declined for all ages, but cancer mortality increased for those aged 75 and older; people with type 2 diabetes who were smokers had higher and steadily increasing cancer mortality rates; and people with type 2 diabetes had more than twice the rate of colorectal, pancreatic, liver, and endometrial cancer mortality than age- and sex-matched individuals in the general population.
The findings suggest that “cancer prevention strategies therefore deserve at least a similar level of attention as cardiovascular disease prevention, particularly in older people and for some cancers such as liver, colorectal, and pancreatic cancer,” the researchers wrote.
Tailored cancer prevention and early-detection strategies are needed to address persistent inequalities in the older population, the most deprived, and smokers, they added.
Breast cancer rates in younger women with type 2 diabetes rising
According to the researchers, “early cancer detection through changes to existing screening [programs], or more in-depth investigations for suspected/nonspecific symptoms, may reduce the number of avoidable cancer deaths in people with type 2 diabetes.”
Moreover, breast cancer rates in younger women with type 2 diabetes are rising by 4.1% per year, they wrote, which suggests such women are high risk and should be screened at a younger age, but screening age would need to be determined in cost-effectiveness analyses.
The study by Suping Ling, PhD, and colleagues was published online in Diabetologia.
Results challenge belief that preventing CVD is priority in type 2 diabetes
“The prevention of cardiovascular disease has been, and is still considered, a priority in people with diabetes,” the researchers wrote.
“Our results challenge this view by showing that cancer may have overtaken cardiovascular disease as a leading cause of death in people with type 2 diabetes.”
“The proportion of cancer deaths out of all-cause deaths remains high (> 30%) in young ages, and it was steadily increasing in older ages,” Dr. Ling, from the department of noncommunicable disease epidemiology, London School of Hygiene & Tropical Medicine, said in a comment.
“Combined with previous studies reporting decreasing CVD mortality rates,” she said, “we concluded that cancer might have overtaken CVD as the leading cause of death in people with type 2 diabetes.”
Many evidence-based cancer-prevention strategies related to lifestyle (such as being physically active, being a healthy weight, eating a better diet, stopping smoking, as summarized by the World Cancer Research Fund), are helpful for preventing both cancer and CVD, Ling observed.
However, in the medical community, many additional efforts were made for monitoring, early detection, and innovating medications for CVD, she noted. “Therefore, we would like to propose a similar level of attention and effort for cancer in people with type 2 diabetes.”
Deaths from cancer vs. all causes in patients with diabetes
The researchers identified 137,804 patients aged 35 and older who were newly diagnosed with type 2 diabetes from 1998 to 2018 in general practices in the UK that were part of the Clinical Practice Research Datalink.
Patients were a median age of 64 years and 45% were women. Most (83%) were White, followed by South Asian (3.5%), Black (2.0%), and other (3%); 8.4% had missing information for race. Patients had a median body mass index (BMI) of 30.6 kg/m2.
Researchers divided patients into socioeconomic quintiles of most to least deprived based on income, employment, education, and other factors. During a median follow-up of 8.4 years, there were 39,212 deaths (28.5%).
Cancer mortality in subgroups of patients with type 2 diabetes
Researchers analyzed annual deaths from cancer and from all causes over 20 years in subgroups of patients with type 2 diabetes.

In adults with type 2 diabetes, the average percentage change in cancer mortality per year, from 1998 to 2018 decreased in people aged 55 and 65 (–1.4% and –0.2%, respectively), but increased in people aged 75 and 85 (1.2% and 1.6%, respectively); increased more in women than in men (1.5% vs 1.0%), although women had lower cancer mortality than men; and increased more in the least deprived (wealthiest) individuals than in the most deprived (1.5% vs 1.0%). Cancer mortality rates were consistently higher in the most deprived individuals, Dr. Ling noted.
Cancer mortality also increased more in people with class III obesity (BMI ≥ 35) versus normal weight (5.8% vs 0.7%) and versus other weights. In addition, there was an upward trend in cancer mortality in people who were White or former/current smokers.
Deaths from specific cancers in diabetes vs. general population
Next, researchers determined cancer mortality ratios – the cancer mortality of the patients with diabetes divided by the cancer mortality of the general population.
They determined this for all cancers, the four most common cancers in the United Kingdom (lung, colorectal, breast, and prostate), and cancers caused by type 2 diabetes (pancreatic, liver, gallbladder, and endometrial cancer), standardized by sex and age.
Mortality from all cancer was 18% higher in patients with type 2 diabetes, compared with the general population.
Overall, mortality from colorectal cancer, pancreatic cancer, and liver cancer was 2.4 times, 2.12 times, and 2.13 times higher, respectively, in patients with type 2 diabetes than in the general population.
Mortality from breast cancer was 9% higher and mortality from endometrial cancer was 2.08 times higher in women with type 2 diabetes than in women in the general population.
There was a constant upward trend for mortality rates for pancreatic, liver, and lung cancer at all ages, colorectal cancer at most ages, breast cancer at younger ages, and prostate and endometrial cancer at older ages.
The study was funded by Hope Against Cancer. Dr. Ling reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cancer appears to have overtaken cardiovascular disease (CVD) as a leading cause of death in adults with type 2 diabetes, a 20-year population study in England suggests.
The researchers found that, from 1998 to 2018, in more than 130,000 adults aged 35 and older with type 2 diabetes, all-cause mortality declined for all ages, but cancer mortality increased for those aged 75 and older; people with type 2 diabetes who were smokers had higher and steadily increasing cancer mortality rates; and people with type 2 diabetes had more than twice the rate of colorectal, pancreatic, liver, and endometrial cancer mortality than age- and sex-matched individuals in the general population.
The findings suggest that “cancer prevention strategies therefore deserve at least a similar level of attention as cardiovascular disease prevention, particularly in older people and for some cancers such as liver, colorectal, and pancreatic cancer,” the researchers wrote.
Tailored cancer prevention and early-detection strategies are needed to address persistent inequalities in the older population, the most deprived, and smokers, they added.
Breast cancer rates in younger women with type 2 diabetes rising
According to the researchers, “early cancer detection through changes to existing screening [programs], or more in-depth investigations for suspected/nonspecific symptoms, may reduce the number of avoidable cancer deaths in people with type 2 diabetes.”
Moreover, breast cancer rates in younger women with type 2 diabetes are rising by 4.1% per year, they wrote, which suggests such women are high risk and should be screened at a younger age, but screening age would need to be determined in cost-effectiveness analyses.
The study by Suping Ling, PhD, and colleagues was published online in Diabetologia.
Results challenge belief that preventing CVD is priority in type 2 diabetes
“The prevention of cardiovascular disease has been, and is still considered, a priority in people with diabetes,” the researchers wrote.
“Our results challenge this view by showing that cancer may have overtaken cardiovascular disease as a leading cause of death in people with type 2 diabetes.”
“The proportion of cancer deaths out of all-cause deaths remains high (> 30%) in young ages, and it was steadily increasing in older ages,” Dr. Ling, from the department of noncommunicable disease epidemiology, London School of Hygiene & Tropical Medicine, said in a comment.
“Combined with previous studies reporting decreasing CVD mortality rates,” she said, “we concluded that cancer might have overtaken CVD as the leading cause of death in people with type 2 diabetes.”
Many evidence-based cancer-prevention strategies related to lifestyle (such as being physically active, being a healthy weight, eating a better diet, stopping smoking, as summarized by the World Cancer Research Fund), are helpful for preventing both cancer and CVD, Ling observed.
However, in the medical community, many additional efforts were made for monitoring, early detection, and innovating medications for CVD, she noted. “Therefore, we would like to propose a similar level of attention and effort for cancer in people with type 2 diabetes.”
Deaths from cancer vs. all causes in patients with diabetes
The researchers identified 137,804 patients aged 35 and older who were newly diagnosed with type 2 diabetes from 1998 to 2018 in general practices in the UK that were part of the Clinical Practice Research Datalink.
Patients were a median age of 64 years and 45% were women. Most (83%) were White, followed by South Asian (3.5%), Black (2.0%), and other (3%); 8.4% had missing information for race. Patients had a median body mass index (BMI) of 30.6 kg/m2.
Researchers divided patients into socioeconomic quintiles of most to least deprived based on income, employment, education, and other factors. During a median follow-up of 8.4 years, there were 39,212 deaths (28.5%).
Cancer mortality in subgroups of patients with type 2 diabetes
Researchers analyzed annual deaths from cancer and from all causes over 20 years in subgroups of patients with type 2 diabetes.

In adults with type 2 diabetes, the average percentage change in cancer mortality per year, from 1998 to 2018 decreased in people aged 55 and 65 (–1.4% and –0.2%, respectively), but increased in people aged 75 and 85 (1.2% and 1.6%, respectively); increased more in women than in men (1.5% vs 1.0%), although women had lower cancer mortality than men; and increased more in the least deprived (wealthiest) individuals than in the most deprived (1.5% vs 1.0%). Cancer mortality rates were consistently higher in the most deprived individuals, Dr. Ling noted.
Cancer mortality also increased more in people with class III obesity (BMI ≥ 35) versus normal weight (5.8% vs 0.7%) and versus other weights. In addition, there was an upward trend in cancer mortality in people who were White or former/current smokers.
Deaths from specific cancers in diabetes vs. general population
Next, researchers determined cancer mortality ratios – the cancer mortality of the patients with diabetes divided by the cancer mortality of the general population.
They determined this for all cancers, the four most common cancers in the United Kingdom (lung, colorectal, breast, and prostate), and cancers caused by type 2 diabetes (pancreatic, liver, gallbladder, and endometrial cancer), standardized by sex and age.
Mortality from all cancer was 18% higher in patients with type 2 diabetes, compared with the general population.
Overall, mortality from colorectal cancer, pancreatic cancer, and liver cancer was 2.4 times, 2.12 times, and 2.13 times higher, respectively, in patients with type 2 diabetes than in the general population.
Mortality from breast cancer was 9% higher and mortality from endometrial cancer was 2.08 times higher in women with type 2 diabetes than in women in the general population.
There was a constant upward trend for mortality rates for pancreatic, liver, and lung cancer at all ages, colorectal cancer at most ages, breast cancer at younger ages, and prostate and endometrial cancer at older ages.
The study was funded by Hope Against Cancer. Dr. Ling reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM DIABETOLOGIA
New osteoporosis guideline says start with a bisphosphonate
This is the first update for 5 years since the previous guidance was published in 2017.
It strongly recommends initial therapy with bisphosphonates for postmenopausal women with osteoporosis, as well as men with osteoporosis, among other recommendations.
However, the author of an accompanying editorial, Susan M. Ott, MD, says: “The decision to start a bisphosphonate is actually not that easy.”
She also queries some of the other recommendations in the guidance.
Her editorial, along with the guideline by Amir Qaseem, MD, PhD, MPH, and colleagues, and systematic review by Chelsea Ayers, MPH, and colleagues, were published in the Annals of Internal Medicine.
Ryan D. Mire, MD, MACP, president of the ACP, gave a brief overview of the new guidance in a video.
Systematic review
The ACP commissioned a review of the evidence because it says new data have emerged on the efficacy of newer medications for osteoporosis and low bone mass, as well as treatment comparisons, and treatment in men.
The review authors identified 34 randomized controlled trials (in 100 publications) and 36 observational studies, which evaluated the following pharmacologic interventions:
- Antiresorptive drugs: four bisphosphonates (alendronate, ibandronate, risedronate, zoledronate) and a RANK ligand inhibitor (denosumab).
- Anabolic drugs: an analog of human parathyroid hormone (PTH)–related protein (abaloparatide), recombinant human PTH (teriparatide), and a sclerostin inhibitor (romosozumab).
- Estrogen agonists: selective estrogen receptor modulators (bazedoxifene, raloxifene).
The authors focused on effectiveness and harms of active drugs compared with placebo or bisphosphonates.
Major changes from 2017 guidelines, some questions
“Though there are many nuanced changes in this [2023 guideline] version, perhaps the major change is the explicit hierarchy of pharmacologic recommendations: bisphosphonates first, then denosumab,” Thomas G. Cooney, MD, senior author of the clinical guideline, explained in an interview.
“Bisphosphonates had the most favorable balance among benefits, harms, patient values and preferences, and cost among the examined drugs in postmenopausal females with primary osteoporosis,” Dr. Cooney, professor of medicine, Oregon Health & Science University, Portland, noted, as is stated in the guideline.
“Denosumab also had a favorable long-term net benefit, but bisphosphonates are much cheaper than other pharmacologic treatments and available in generic formulations,” the document states.
The new guideline suggests use of denosumab as second-line pharmacotherapy in adults who have contraindications to or experience adverse effects with bisphosphonates.
The choice among bisphosphonates (alendronate, risedronate, zoledronic acid) would be based on a patient-centered discussion between physician and patient, addressing costs (often related to insurance), delivery-mode preferences (oral versus intravenous), and “values,” which includes the patient’s priorities, concerns, and expectations regarding their health care, Dr. Cooney explained.
Another update in the new guideline is, “We also clarify the specific, albeit more limited, role of sclerostin inhibitors and recombinant PTH ‘to reduce the risk of fractures only in females with primary osteoporosis with very high-risk of fracture’,” Dr. Cooney noted.
In addition, the guideline now states, “treatment to reduce the risk of fractures in males rather than limiting it to ‘vertebral fracture’ in men,” as in the 2017 guideline.
It also explicitly includes denosumab as second-line therapy for men, Dr. Cooney noted, but as in 2017, the strength of evidence in men remains low.
“Finally, we also clarified that in females over the age of 65 with low bone mass or osteopenia that an individualized approach be taken to treatment (similar to last guideline), but if treatment is initiated, that a bisphosphonate be used (new content),” he said.
The use of estrogen, treatment duration, drug discontinuation, and serial bone mineral density monitoring were not addressed in this guideline, but will likely be evaluated within 2 to 3 years.
‘Osteoporosis treatment: Not easy’ – editorial
In her editorial, Dr. Ott writes: “The data about bisphosphonates may seem overwhelmingly positive, leading to strong recommendations for their use to treat osteoporosis, but the decision to start a bisphosphonate is actually not that easy.”
“A strong recommendation should be given only when future studies are unlikely to change it,” continues Dr. Ott, professor of medicine, University of Washington, Seattle.
“Yet, data already suggest that, in patients with serious osteoporosis, treatment should start with anabolic medications because previous treatment with either bisphosphonates or denosumab will prevent the anabolic response of newer medications.”
“Starting with bisphosphonate will change the bone so it will not respond to the newer medicines, and then a patient will lose the chance for getting the best improvement,” Dr. Ott clarified in an email to this news organization.
But, in fact, the new guidance does suggest that, to reduce the risk of fractures in females with primary osteoporosis at very high risk of fracture, one should consider use of the sclerostin inhibitor romosozumab (moderate-certainty evidence) or recombinant human parathyroid hormone (teriparatide) (low-certainty evidence) followed by a bisphosphonate (conditional recommendation).
Dr. Ott said: “If the [fracture] risk is high, then we should start with an anabolic medication for 1-2 years. If the risk is medium, then use a bisphosphonate for up to 5 years, and then stop and monitor the patient for signs that the medicine is wearing off,” based on blood and urine tests.
‘We need medicines that will stop bone aging’
Osteopenia is defined by an arbitrary bone density measurement, Dr. Ott explained. “About half of women over 65 will have osteopenia, and by age 85 there are hardly any ‘normal’ women left.”
“We need medicines that will stop bone aging, which might sound impossible, but we should still try,” she continued.
“In the meantime, while waiting on new discoveries,” Dr. Ott said, “I would not use bisphosphonates in patients who did not already have a fracture or whose bone density T-score was better than –2.5 because, in the major study, alendronate did not prevent fractures in this group.”
Many people are worried about bisphosphonates because of problems with the jaw or femur. These are real, but they are very rare during the first 5 years of treatment, Dr. Ott noted. Then the risk starts to rise, up to more than 1 in 1,000 after 8 years. So people can get the benefits of these drugs with very low risk for 5 years.
“An immediate [guideline] update is necessary to address the severity of bone loss and the high risk for vertebral fractures after discontinuation of denosumab,” Dr. Ott urged.
“I don’t agree with using denosumab for osteoporosis as a second-line treatment,” she said. “I would use it only in patients who have cancer or unusually high bone resorption. You have to get a dose strictly every 6 months, and if you need to stop, it is recommended to treat with bisphosphonates. Denosumab is a poor choice for somebody who does not want to take a bisphosphonate. Many patients and even too many doctors do not realize how serious it can be to skip a dose.”
“I also think that men could be treated with anabolic medications,” Dr. Ott said. “Clinical trials show they respond the same as women. Many men have osteoporosis as a consequence of low testosterone, and then they can usually be treated with testosterone. Osteoporosis in men is a serious problem that is too often ignored – almost reverse discrimination.”
It is also unfortunate that the review and recommendations do not address estrogen, one of the most effective medications to prevent osteoporotic fractures, according to Dr. Ott.
Clinical considerations in addition to drug types
The new guideline also advises:
- Clinicians treating adults with osteoporosis should encourage adherence to recommended treatments and healthy lifestyle habits, including exercise, and counseling to evaluate and prevent falls.
- All adults with osteopenia or osteoporosis should have adequate calcium and vitamin D intake, as part of fracture prevention.
- Clinicians should assess baseline fracture risk based on bone density, fracture history, fracture risk factors, and response to prior osteoporosis treatments.
- Current evidence suggests that more than 3-5 years of bisphosphonate therapy reduces risk for new vertebral but not other fractures; however, it also increases risk for long-term harms. Therefore, clinicians should consider stopping bisphosphonate treatment after 5 years unless the patient has a strong indication for treatment continuation.
- The decision for a bisphosphonate holiday (temporary discontinuation) and its duration should be based on baseline fracture risk, medication half-life in bone, and benefits and harms.
- Women treated with an anabolic agent who discontinue it should be offered an antiresorptive agent to preserve gains and because of serious risk for rebound and multiple vertebral fractures.
- Adults older than 65 years with osteoporosis may be at increased risk for falls or other adverse events because of drug interactions.
- Transgender persons have variable risk for low bone mass.
The review and guideline were funded by the ACP. Dr. Ott has reported no relevant disclosures. Relevant financial disclosures for other authors are listed with the guideline and review.
A version of this article first appeared on Medscape.com.
This is the first update for 5 years since the previous guidance was published in 2017.
It strongly recommends initial therapy with bisphosphonates for postmenopausal women with osteoporosis, as well as men with osteoporosis, among other recommendations.
However, the author of an accompanying editorial, Susan M. Ott, MD, says: “The decision to start a bisphosphonate is actually not that easy.”
She also queries some of the other recommendations in the guidance.
Her editorial, along with the guideline by Amir Qaseem, MD, PhD, MPH, and colleagues, and systematic review by Chelsea Ayers, MPH, and colleagues, were published in the Annals of Internal Medicine.
Ryan D. Mire, MD, MACP, president of the ACP, gave a brief overview of the new guidance in a video.
Systematic review
The ACP commissioned a review of the evidence because it says new data have emerged on the efficacy of newer medications for osteoporosis and low bone mass, as well as treatment comparisons, and treatment in men.
The review authors identified 34 randomized controlled trials (in 100 publications) and 36 observational studies, which evaluated the following pharmacologic interventions:
- Antiresorptive drugs: four bisphosphonates (alendronate, ibandronate, risedronate, zoledronate) and a RANK ligand inhibitor (denosumab).
- Anabolic drugs: an analog of human parathyroid hormone (PTH)–related protein (abaloparatide), recombinant human PTH (teriparatide), and a sclerostin inhibitor (romosozumab).
- Estrogen agonists: selective estrogen receptor modulators (bazedoxifene, raloxifene).
The authors focused on effectiveness and harms of active drugs compared with placebo or bisphosphonates.
Major changes from 2017 guidelines, some questions
“Though there are many nuanced changes in this [2023 guideline] version, perhaps the major change is the explicit hierarchy of pharmacologic recommendations: bisphosphonates first, then denosumab,” Thomas G. Cooney, MD, senior author of the clinical guideline, explained in an interview.
“Bisphosphonates had the most favorable balance among benefits, harms, patient values and preferences, and cost among the examined drugs in postmenopausal females with primary osteoporosis,” Dr. Cooney, professor of medicine, Oregon Health & Science University, Portland, noted, as is stated in the guideline.
“Denosumab also had a favorable long-term net benefit, but bisphosphonates are much cheaper than other pharmacologic treatments and available in generic formulations,” the document states.
The new guideline suggests use of denosumab as second-line pharmacotherapy in adults who have contraindications to or experience adverse effects with bisphosphonates.
The choice among bisphosphonates (alendronate, risedronate, zoledronic acid) would be based on a patient-centered discussion between physician and patient, addressing costs (often related to insurance), delivery-mode preferences (oral versus intravenous), and “values,” which includes the patient’s priorities, concerns, and expectations regarding their health care, Dr. Cooney explained.
Another update in the new guideline is, “We also clarify the specific, albeit more limited, role of sclerostin inhibitors and recombinant PTH ‘to reduce the risk of fractures only in females with primary osteoporosis with very high-risk of fracture’,” Dr. Cooney noted.
In addition, the guideline now states, “treatment to reduce the risk of fractures in males rather than limiting it to ‘vertebral fracture’ in men,” as in the 2017 guideline.
It also explicitly includes denosumab as second-line therapy for men, Dr. Cooney noted, but as in 2017, the strength of evidence in men remains low.
“Finally, we also clarified that in females over the age of 65 with low bone mass or osteopenia that an individualized approach be taken to treatment (similar to last guideline), but if treatment is initiated, that a bisphosphonate be used (new content),” he said.
The use of estrogen, treatment duration, drug discontinuation, and serial bone mineral density monitoring were not addressed in this guideline, but will likely be evaluated within 2 to 3 years.
‘Osteoporosis treatment: Not easy’ – editorial
In her editorial, Dr. Ott writes: “The data about bisphosphonates may seem overwhelmingly positive, leading to strong recommendations for their use to treat osteoporosis, but the decision to start a bisphosphonate is actually not that easy.”
“A strong recommendation should be given only when future studies are unlikely to change it,” continues Dr. Ott, professor of medicine, University of Washington, Seattle.
“Yet, data already suggest that, in patients with serious osteoporosis, treatment should start with anabolic medications because previous treatment with either bisphosphonates or denosumab will prevent the anabolic response of newer medications.”
“Starting with bisphosphonate will change the bone so it will not respond to the newer medicines, and then a patient will lose the chance for getting the best improvement,” Dr. Ott clarified in an email to this news organization.
But, in fact, the new guidance does suggest that, to reduce the risk of fractures in females with primary osteoporosis at very high risk of fracture, one should consider use of the sclerostin inhibitor romosozumab (moderate-certainty evidence) or recombinant human parathyroid hormone (teriparatide) (low-certainty evidence) followed by a bisphosphonate (conditional recommendation).
Dr. Ott said: “If the [fracture] risk is high, then we should start with an anabolic medication for 1-2 years. If the risk is medium, then use a bisphosphonate for up to 5 years, and then stop and monitor the patient for signs that the medicine is wearing off,” based on blood and urine tests.
‘We need medicines that will stop bone aging’
Osteopenia is defined by an arbitrary bone density measurement, Dr. Ott explained. “About half of women over 65 will have osteopenia, and by age 85 there are hardly any ‘normal’ women left.”
“We need medicines that will stop bone aging, which might sound impossible, but we should still try,” she continued.
“In the meantime, while waiting on new discoveries,” Dr. Ott said, “I would not use bisphosphonates in patients who did not already have a fracture or whose bone density T-score was better than –2.5 because, in the major study, alendronate did not prevent fractures in this group.”
Many people are worried about bisphosphonates because of problems with the jaw or femur. These are real, but they are very rare during the first 5 years of treatment, Dr. Ott noted. Then the risk starts to rise, up to more than 1 in 1,000 after 8 years. So people can get the benefits of these drugs with very low risk for 5 years.
“An immediate [guideline] update is necessary to address the severity of bone loss and the high risk for vertebral fractures after discontinuation of denosumab,” Dr. Ott urged.
“I don’t agree with using denosumab for osteoporosis as a second-line treatment,” she said. “I would use it only in patients who have cancer or unusually high bone resorption. You have to get a dose strictly every 6 months, and if you need to stop, it is recommended to treat with bisphosphonates. Denosumab is a poor choice for somebody who does not want to take a bisphosphonate. Many patients and even too many doctors do not realize how serious it can be to skip a dose.”
“I also think that men could be treated with anabolic medications,” Dr. Ott said. “Clinical trials show they respond the same as women. Many men have osteoporosis as a consequence of low testosterone, and then they can usually be treated with testosterone. Osteoporosis in men is a serious problem that is too often ignored – almost reverse discrimination.”
It is also unfortunate that the review and recommendations do not address estrogen, one of the most effective medications to prevent osteoporotic fractures, according to Dr. Ott.
Clinical considerations in addition to drug types
The new guideline also advises:
- Clinicians treating adults with osteoporosis should encourage adherence to recommended treatments and healthy lifestyle habits, including exercise, and counseling to evaluate and prevent falls.
- All adults with osteopenia or osteoporosis should have adequate calcium and vitamin D intake, as part of fracture prevention.
- Clinicians should assess baseline fracture risk based on bone density, fracture history, fracture risk factors, and response to prior osteoporosis treatments.
- Current evidence suggests that more than 3-5 years of bisphosphonate therapy reduces risk for new vertebral but not other fractures; however, it also increases risk for long-term harms. Therefore, clinicians should consider stopping bisphosphonate treatment after 5 years unless the patient has a strong indication for treatment continuation.
- The decision for a bisphosphonate holiday (temporary discontinuation) and its duration should be based on baseline fracture risk, medication half-life in bone, and benefits and harms.
- Women treated with an anabolic agent who discontinue it should be offered an antiresorptive agent to preserve gains and because of serious risk for rebound and multiple vertebral fractures.
- Adults older than 65 years with osteoporosis may be at increased risk for falls or other adverse events because of drug interactions.
- Transgender persons have variable risk for low bone mass.
The review and guideline were funded by the ACP. Dr. Ott has reported no relevant disclosures. Relevant financial disclosures for other authors are listed with the guideline and review.
A version of this article first appeared on Medscape.com.
This is the first update for 5 years since the previous guidance was published in 2017.
It strongly recommends initial therapy with bisphosphonates for postmenopausal women with osteoporosis, as well as men with osteoporosis, among other recommendations.
However, the author of an accompanying editorial, Susan M. Ott, MD, says: “The decision to start a bisphosphonate is actually not that easy.”
She also queries some of the other recommendations in the guidance.
Her editorial, along with the guideline by Amir Qaseem, MD, PhD, MPH, and colleagues, and systematic review by Chelsea Ayers, MPH, and colleagues, were published in the Annals of Internal Medicine.
Ryan D. Mire, MD, MACP, president of the ACP, gave a brief overview of the new guidance in a video.
Systematic review
The ACP commissioned a review of the evidence because it says new data have emerged on the efficacy of newer medications for osteoporosis and low bone mass, as well as treatment comparisons, and treatment in men.
The review authors identified 34 randomized controlled trials (in 100 publications) and 36 observational studies, which evaluated the following pharmacologic interventions:
- Antiresorptive drugs: four bisphosphonates (alendronate, ibandronate, risedronate, zoledronate) and a RANK ligand inhibitor (denosumab).
- Anabolic drugs: an analog of human parathyroid hormone (PTH)–related protein (abaloparatide), recombinant human PTH (teriparatide), and a sclerostin inhibitor (romosozumab).
- Estrogen agonists: selective estrogen receptor modulators (bazedoxifene, raloxifene).
The authors focused on effectiveness and harms of active drugs compared with placebo or bisphosphonates.
Major changes from 2017 guidelines, some questions
“Though there are many nuanced changes in this [2023 guideline] version, perhaps the major change is the explicit hierarchy of pharmacologic recommendations: bisphosphonates first, then denosumab,” Thomas G. Cooney, MD, senior author of the clinical guideline, explained in an interview.
“Bisphosphonates had the most favorable balance among benefits, harms, patient values and preferences, and cost among the examined drugs in postmenopausal females with primary osteoporosis,” Dr. Cooney, professor of medicine, Oregon Health & Science University, Portland, noted, as is stated in the guideline.
“Denosumab also had a favorable long-term net benefit, but bisphosphonates are much cheaper than other pharmacologic treatments and available in generic formulations,” the document states.
The new guideline suggests use of denosumab as second-line pharmacotherapy in adults who have contraindications to or experience adverse effects with bisphosphonates.
The choice among bisphosphonates (alendronate, risedronate, zoledronic acid) would be based on a patient-centered discussion between physician and patient, addressing costs (often related to insurance), delivery-mode preferences (oral versus intravenous), and “values,” which includes the patient’s priorities, concerns, and expectations regarding their health care, Dr. Cooney explained.
Another update in the new guideline is, “We also clarify the specific, albeit more limited, role of sclerostin inhibitors and recombinant PTH ‘to reduce the risk of fractures only in females with primary osteoporosis with very high-risk of fracture’,” Dr. Cooney noted.
In addition, the guideline now states, “treatment to reduce the risk of fractures in males rather than limiting it to ‘vertebral fracture’ in men,” as in the 2017 guideline.
It also explicitly includes denosumab as second-line therapy for men, Dr. Cooney noted, but as in 2017, the strength of evidence in men remains low.
“Finally, we also clarified that in females over the age of 65 with low bone mass or osteopenia that an individualized approach be taken to treatment (similar to last guideline), but if treatment is initiated, that a bisphosphonate be used (new content),” he said.
The use of estrogen, treatment duration, drug discontinuation, and serial bone mineral density monitoring were not addressed in this guideline, but will likely be evaluated within 2 to 3 years.
‘Osteoporosis treatment: Not easy’ – editorial
In her editorial, Dr. Ott writes: “The data about bisphosphonates may seem overwhelmingly positive, leading to strong recommendations for their use to treat osteoporosis, but the decision to start a bisphosphonate is actually not that easy.”
“A strong recommendation should be given only when future studies are unlikely to change it,” continues Dr. Ott, professor of medicine, University of Washington, Seattle.
“Yet, data already suggest that, in patients with serious osteoporosis, treatment should start with anabolic medications because previous treatment with either bisphosphonates or denosumab will prevent the anabolic response of newer medications.”
“Starting with bisphosphonate will change the bone so it will not respond to the newer medicines, and then a patient will lose the chance for getting the best improvement,” Dr. Ott clarified in an email to this news organization.
But, in fact, the new guidance does suggest that, to reduce the risk of fractures in females with primary osteoporosis at very high risk of fracture, one should consider use of the sclerostin inhibitor romosozumab (moderate-certainty evidence) or recombinant human parathyroid hormone (teriparatide) (low-certainty evidence) followed by a bisphosphonate (conditional recommendation).
Dr. Ott said: “If the [fracture] risk is high, then we should start with an anabolic medication for 1-2 years. If the risk is medium, then use a bisphosphonate for up to 5 years, and then stop and monitor the patient for signs that the medicine is wearing off,” based on blood and urine tests.
‘We need medicines that will stop bone aging’
Osteopenia is defined by an arbitrary bone density measurement, Dr. Ott explained. “About half of women over 65 will have osteopenia, and by age 85 there are hardly any ‘normal’ women left.”
“We need medicines that will stop bone aging, which might sound impossible, but we should still try,” she continued.
“In the meantime, while waiting on new discoveries,” Dr. Ott said, “I would not use bisphosphonates in patients who did not already have a fracture or whose bone density T-score was better than –2.5 because, in the major study, alendronate did not prevent fractures in this group.”
Many people are worried about bisphosphonates because of problems with the jaw or femur. These are real, but they are very rare during the first 5 years of treatment, Dr. Ott noted. Then the risk starts to rise, up to more than 1 in 1,000 after 8 years. So people can get the benefits of these drugs with very low risk for 5 years.
“An immediate [guideline] update is necessary to address the severity of bone loss and the high risk for vertebral fractures after discontinuation of denosumab,” Dr. Ott urged.
“I don’t agree with using denosumab for osteoporosis as a second-line treatment,” she said. “I would use it only in patients who have cancer or unusually high bone resorption. You have to get a dose strictly every 6 months, and if you need to stop, it is recommended to treat with bisphosphonates. Denosumab is a poor choice for somebody who does not want to take a bisphosphonate. Many patients and even too many doctors do not realize how serious it can be to skip a dose.”
“I also think that men could be treated with anabolic medications,” Dr. Ott said. “Clinical trials show they respond the same as women. Many men have osteoporosis as a consequence of low testosterone, and then they can usually be treated with testosterone. Osteoporosis in men is a serious problem that is too often ignored – almost reverse discrimination.”
It is also unfortunate that the review and recommendations do not address estrogen, one of the most effective medications to prevent osteoporotic fractures, according to Dr. Ott.
Clinical considerations in addition to drug types
The new guideline also advises:
- Clinicians treating adults with osteoporosis should encourage adherence to recommended treatments and healthy lifestyle habits, including exercise, and counseling to evaluate and prevent falls.
- All adults with osteopenia or osteoporosis should have adequate calcium and vitamin D intake, as part of fracture prevention.
- Clinicians should assess baseline fracture risk based on bone density, fracture history, fracture risk factors, and response to prior osteoporosis treatments.
- Current evidence suggests that more than 3-5 years of bisphosphonate therapy reduces risk for new vertebral but not other fractures; however, it also increases risk for long-term harms. Therefore, clinicians should consider stopping bisphosphonate treatment after 5 years unless the patient has a strong indication for treatment continuation.
- The decision for a bisphosphonate holiday (temporary discontinuation) and its duration should be based on baseline fracture risk, medication half-life in bone, and benefits and harms.
- Women treated with an anabolic agent who discontinue it should be offered an antiresorptive agent to preserve gains and because of serious risk for rebound and multiple vertebral fractures.
- Adults older than 65 years with osteoporosis may be at increased risk for falls or other adverse events because of drug interactions.
- Transgender persons have variable risk for low bone mass.
The review and guideline were funded by the ACP. Dr. Ott has reported no relevant disclosures. Relevant financial disclosures for other authors are listed with the guideline and review.
A version of this article first appeared on Medscape.com.
FROM THE ANNALS OF INTERNAL MEDICINE
Rosuvastatin again linked with risks to kidneys
Rosuvastatin for cholesterol lowering was associated with slightly greater risks for kidney harm than atorvastatin, risks that were greater at higher-dose levels, in a large retrospective cohort study.
The most potent statin on the market, rosuvastatin has been linked with excess risk for kidney damage compared with atorvastatin in case reports and small trials, but there has been little surveillance of the issue following its approval in 2003.
The current analysis “is one of the first and largest real-world studies” examining rosuvastatin versus atorvastatin for risk for hematuria, proteinuria, and kidney failure with replacement therapy – dialysis or transplantation – across a range of estimated glomerular filtration rates (eGFR) in a heterogeneous population, the researchers write.
“Our findings suggest the need for greater care in prescribing and monitoring of rosuvastatin, particularly in patients who are receiving high doses” or have severe chronic kidney disease (CKD), they concluded in their report published online in the Journal of the American Society of Nephrology.
The analysis included close to 1 million patients in the United States who were newly prescribed rosuvastatin or atorvastatin from 2011 through 2019; they were followed a median of 3.1 years. Among the findings:
- Users of rosuvastatin had an 8% higher risk for hematuria, a 17% higher risk for proteinuria, and a 15% higher risk for kidney failure with replacement therapy, compared with those on atorvastatin
- The two groups avoided MI and stroke to similar extents
- About 44% of patients with severe CKD G4+ (eGFR < 30 mL/min per 1.73 m2) were prescribed a higher rosuvastatin dosage than the maximum 10 mg/day recommended for such patients by the Food and Drug Administration.
From this study, “we do not know why the adherence of FDA dosing recommendation for rosuvastatin in patients with severe CKD is low,” lead author Jung-Im Shin, MD, PhD, said in an interview.
“It is likely that not many clinicians are aware of rosuvastatin’s dosing recommendations [in severe CKD], or potential risks of hematuria or proteinuria,” speculated Dr. Shin, assistant professor at Johns Hopkins University, Baltimore.
“High-dose rosuvastatin [and its cardiovascular benefits] may not merit the risk, even if small, particularly in low eGFR,” she said. “Our study provides the opportunity to increase awareness of this clinical issue.”
“Future studies are warranted to shed light on the discrepancy between real-world practice and FDA dosing recommendations for high-dose rosuvastatin,” the researchers noted.
‘Greater awareness and education are key’
Invited to comment, Swapnil Hiremath, MD, a nephrologist at the Ottawa Hospital Research Institute, noted that the higher risk for nephrotoxicity with high-dose rosuvastatin versus high-dose atorvastatin was shown in the PLANET 1 trial published in 2015 and in, for example, a case report published in 2016 – which the researchers also mention.
“I was personally surprised” at the high proportion of patients with severe CKD who received higher than recommended doses of rosuvastatin, said Dr. Hiremath, who is also an associate professor at the University of Ottawa and a Freely Filtered podcaster, and not associated with the current study.
“We do see this occasionally,” he continued, “but either because someone is targeting LDL [cholesterol] and hasn’t noted the GFR, or possibly the patient was started on a high dose a long time ago and the kidney function has declined, and no one has noted the high dose.”
“Greater awareness and education are key,” observed Dr. Hiremath. “My personal bias is to have renal pharmacists involved in multidisciplinary clinics when GFR [is] less than 30 or so,” he said. “There are so many other tricky medicine/interaction issues” in patients with kidney disease.
Nevertheless, “I would be careful in drawing too many conclusions from an observational study,” Dr. Hiremath added. “There’s always the threat of residual confounding and selection bias,” which the researchers acknowledge, “and especially competing risks.”
For example, “if there is less cardiovascular death with rosuvastatin, then more people will remain alive to develop kidney failure.”
Dosing in practice unclear
Atorvastatin at 40-mg and 80-mg dosages and rosuvastatin at 20 mg and 40 mg are the only two statins considered high-intensity, the researchers noted.
Development of an 80-mg dosage for rosuvastatin was dropped because of hematuria and proteinuria safety signals highlighted at the time of rosuvastatin’s FDA approval.
However, there has been little postmarketing surveillance to assess real-world risk from high-intensity rosuvastatin, and it remains unclear whether and to what extent clinical practice adheres to the starting dosage recommended by the FDA in severe CKD, 5 mg/day with a maximum of 10 mg/day, the report noted.
The researchers analyzed deidentified electronic health record data from 40 health care organizations in the United States from the OptumLabs Data Warehouse database. They entered 152,101 new rosuvastatin users and 795,799 new atorvastatin users, and excluded patients with a history of rhabdomyolysis.
Patients in the two groups were similar with respect to CKD prevalence, cardiovascular risk factors, and demographics. Their age averaged 60 years, 48% were women, and 82% were White.
Hematuria was defined as dipstick hematuria > + or the presence of more than 3 red blood cells per high-power field in urine microscopy, at least twice. Proteinuria was defined as dipstick proteinuria > ++ or urine albumin-to-creatinine ratio greater than 300 mg/g at least twice.
Overall, 2.9% of patients had hematuria (3.4% of the rosuvastatin group and 2.8% of those taking atorvastatin) and 1% of patients had proteinuria (1.2% and 0.9%, respectively).
After balancing baseline characteristics in both groups using inverse probability of treatment weighting, rosuvastatin treatment, compared with atorvastatin, was associated with significantly greater risks for hematuria (hazard ratio, 1.08), proteinuria (HR, 1.17), and kidney failure requiring replacement therapy (HR, 1.15).
Patients with eGFR less than 30 mL/min per 1.73 m2 had an approximately twofold higher risk for hematuria and ninefold higher risk for proteinuria during the follow-up compared with patients with eGFR of at least 60 mL/min per 1.73 m2.
Patients with eGFR less than 30 mL/min per 1.73 m2 were commonly prescribed high-dose rosuvastatin (29.9% received the 20-mg dose and 14% the 40-mg dose), contrary to the labeling recommendation.
Dr. Shin reported receiving research Funding from the National Institutes of Health and Merck; disclosures for the other authors are in the report. Dr. Hiremath reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rosuvastatin for cholesterol lowering was associated with slightly greater risks for kidney harm than atorvastatin, risks that were greater at higher-dose levels, in a large retrospective cohort study.
The most potent statin on the market, rosuvastatin has been linked with excess risk for kidney damage compared with atorvastatin in case reports and small trials, but there has been little surveillance of the issue following its approval in 2003.
The current analysis “is one of the first and largest real-world studies” examining rosuvastatin versus atorvastatin for risk for hematuria, proteinuria, and kidney failure with replacement therapy – dialysis or transplantation – across a range of estimated glomerular filtration rates (eGFR) in a heterogeneous population, the researchers write.
“Our findings suggest the need for greater care in prescribing and monitoring of rosuvastatin, particularly in patients who are receiving high doses” or have severe chronic kidney disease (CKD), they concluded in their report published online in the Journal of the American Society of Nephrology.
The analysis included close to 1 million patients in the United States who were newly prescribed rosuvastatin or atorvastatin from 2011 through 2019; they were followed a median of 3.1 years. Among the findings:
- Users of rosuvastatin had an 8% higher risk for hematuria, a 17% higher risk for proteinuria, and a 15% higher risk for kidney failure with replacement therapy, compared with those on atorvastatin
- The two groups avoided MI and stroke to similar extents
- About 44% of patients with severe CKD G4+ (eGFR < 30 mL/min per 1.73 m2) were prescribed a higher rosuvastatin dosage than the maximum 10 mg/day recommended for such patients by the Food and Drug Administration.
From this study, “we do not know why the adherence of FDA dosing recommendation for rosuvastatin in patients with severe CKD is low,” lead author Jung-Im Shin, MD, PhD, said in an interview.
“It is likely that not many clinicians are aware of rosuvastatin’s dosing recommendations [in severe CKD], or potential risks of hematuria or proteinuria,” speculated Dr. Shin, assistant professor at Johns Hopkins University, Baltimore.
“High-dose rosuvastatin [and its cardiovascular benefits] may not merit the risk, even if small, particularly in low eGFR,” she said. “Our study provides the opportunity to increase awareness of this clinical issue.”
“Future studies are warranted to shed light on the discrepancy between real-world practice and FDA dosing recommendations for high-dose rosuvastatin,” the researchers noted.
‘Greater awareness and education are key’
Invited to comment, Swapnil Hiremath, MD, a nephrologist at the Ottawa Hospital Research Institute, noted that the higher risk for nephrotoxicity with high-dose rosuvastatin versus high-dose atorvastatin was shown in the PLANET 1 trial published in 2015 and in, for example, a case report published in 2016 – which the researchers also mention.
“I was personally surprised” at the high proportion of patients with severe CKD who received higher than recommended doses of rosuvastatin, said Dr. Hiremath, who is also an associate professor at the University of Ottawa and a Freely Filtered podcaster, and not associated with the current study.
“We do see this occasionally,” he continued, “but either because someone is targeting LDL [cholesterol] and hasn’t noted the GFR, or possibly the patient was started on a high dose a long time ago and the kidney function has declined, and no one has noted the high dose.”
“Greater awareness and education are key,” observed Dr. Hiremath. “My personal bias is to have renal pharmacists involved in multidisciplinary clinics when GFR [is] less than 30 or so,” he said. “There are so many other tricky medicine/interaction issues” in patients with kidney disease.
Nevertheless, “I would be careful in drawing too many conclusions from an observational study,” Dr. Hiremath added. “There’s always the threat of residual confounding and selection bias,” which the researchers acknowledge, “and especially competing risks.”
For example, “if there is less cardiovascular death with rosuvastatin, then more people will remain alive to develop kidney failure.”
Dosing in practice unclear
Atorvastatin at 40-mg and 80-mg dosages and rosuvastatin at 20 mg and 40 mg are the only two statins considered high-intensity, the researchers noted.
Development of an 80-mg dosage for rosuvastatin was dropped because of hematuria and proteinuria safety signals highlighted at the time of rosuvastatin’s FDA approval.
However, there has been little postmarketing surveillance to assess real-world risk from high-intensity rosuvastatin, and it remains unclear whether and to what extent clinical practice adheres to the starting dosage recommended by the FDA in severe CKD, 5 mg/day with a maximum of 10 mg/day, the report noted.
The researchers analyzed deidentified electronic health record data from 40 health care organizations in the United States from the OptumLabs Data Warehouse database. They entered 152,101 new rosuvastatin users and 795,799 new atorvastatin users, and excluded patients with a history of rhabdomyolysis.
Patients in the two groups were similar with respect to CKD prevalence, cardiovascular risk factors, and demographics. Their age averaged 60 years, 48% were women, and 82% were White.
Hematuria was defined as dipstick hematuria > + or the presence of more than 3 red blood cells per high-power field in urine microscopy, at least twice. Proteinuria was defined as dipstick proteinuria > ++ or urine albumin-to-creatinine ratio greater than 300 mg/g at least twice.
Overall, 2.9% of patients had hematuria (3.4% of the rosuvastatin group and 2.8% of those taking atorvastatin) and 1% of patients had proteinuria (1.2% and 0.9%, respectively).
After balancing baseline characteristics in both groups using inverse probability of treatment weighting, rosuvastatin treatment, compared with atorvastatin, was associated with significantly greater risks for hematuria (hazard ratio, 1.08), proteinuria (HR, 1.17), and kidney failure requiring replacement therapy (HR, 1.15).
Patients with eGFR less than 30 mL/min per 1.73 m2 had an approximately twofold higher risk for hematuria and ninefold higher risk for proteinuria during the follow-up compared with patients with eGFR of at least 60 mL/min per 1.73 m2.
Patients with eGFR less than 30 mL/min per 1.73 m2 were commonly prescribed high-dose rosuvastatin (29.9% received the 20-mg dose and 14% the 40-mg dose), contrary to the labeling recommendation.
Dr. Shin reported receiving research Funding from the National Institutes of Health and Merck; disclosures for the other authors are in the report. Dr. Hiremath reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rosuvastatin for cholesterol lowering was associated with slightly greater risks for kidney harm than atorvastatin, risks that were greater at higher-dose levels, in a large retrospective cohort study.
The most potent statin on the market, rosuvastatin has been linked with excess risk for kidney damage compared with atorvastatin in case reports and small trials, but there has been little surveillance of the issue following its approval in 2003.
The current analysis “is one of the first and largest real-world studies” examining rosuvastatin versus atorvastatin for risk for hematuria, proteinuria, and kidney failure with replacement therapy – dialysis or transplantation – across a range of estimated glomerular filtration rates (eGFR) in a heterogeneous population, the researchers write.
“Our findings suggest the need for greater care in prescribing and monitoring of rosuvastatin, particularly in patients who are receiving high doses” or have severe chronic kidney disease (CKD), they concluded in their report published online in the Journal of the American Society of Nephrology.
The analysis included close to 1 million patients in the United States who were newly prescribed rosuvastatin or atorvastatin from 2011 through 2019; they were followed a median of 3.1 years. Among the findings:
- Users of rosuvastatin had an 8% higher risk for hematuria, a 17% higher risk for proteinuria, and a 15% higher risk for kidney failure with replacement therapy, compared with those on atorvastatin
- The two groups avoided MI and stroke to similar extents
- About 44% of patients with severe CKD G4+ (eGFR < 30 mL/min per 1.73 m2) were prescribed a higher rosuvastatin dosage than the maximum 10 mg/day recommended for such patients by the Food and Drug Administration.
From this study, “we do not know why the adherence of FDA dosing recommendation for rosuvastatin in patients with severe CKD is low,” lead author Jung-Im Shin, MD, PhD, said in an interview.
“It is likely that not many clinicians are aware of rosuvastatin’s dosing recommendations [in severe CKD], or potential risks of hematuria or proteinuria,” speculated Dr. Shin, assistant professor at Johns Hopkins University, Baltimore.
“High-dose rosuvastatin [and its cardiovascular benefits] may not merit the risk, even if small, particularly in low eGFR,” she said. “Our study provides the opportunity to increase awareness of this clinical issue.”
“Future studies are warranted to shed light on the discrepancy between real-world practice and FDA dosing recommendations for high-dose rosuvastatin,” the researchers noted.
‘Greater awareness and education are key’
Invited to comment, Swapnil Hiremath, MD, a nephrologist at the Ottawa Hospital Research Institute, noted that the higher risk for nephrotoxicity with high-dose rosuvastatin versus high-dose atorvastatin was shown in the PLANET 1 trial published in 2015 and in, for example, a case report published in 2016 – which the researchers also mention.
“I was personally surprised” at the high proportion of patients with severe CKD who received higher than recommended doses of rosuvastatin, said Dr. Hiremath, who is also an associate professor at the University of Ottawa and a Freely Filtered podcaster, and not associated with the current study.
“We do see this occasionally,” he continued, “but either because someone is targeting LDL [cholesterol] and hasn’t noted the GFR, or possibly the patient was started on a high dose a long time ago and the kidney function has declined, and no one has noted the high dose.”
“Greater awareness and education are key,” observed Dr. Hiremath. “My personal bias is to have renal pharmacists involved in multidisciplinary clinics when GFR [is] less than 30 or so,” he said. “There are so many other tricky medicine/interaction issues” in patients with kidney disease.
Nevertheless, “I would be careful in drawing too many conclusions from an observational study,” Dr. Hiremath added. “There’s always the threat of residual confounding and selection bias,” which the researchers acknowledge, “and especially competing risks.”
For example, “if there is less cardiovascular death with rosuvastatin, then more people will remain alive to develop kidney failure.”
Dosing in practice unclear
Atorvastatin at 40-mg and 80-mg dosages and rosuvastatin at 20 mg and 40 mg are the only two statins considered high-intensity, the researchers noted.
Development of an 80-mg dosage for rosuvastatin was dropped because of hematuria and proteinuria safety signals highlighted at the time of rosuvastatin’s FDA approval.
However, there has been little postmarketing surveillance to assess real-world risk from high-intensity rosuvastatin, and it remains unclear whether and to what extent clinical practice adheres to the starting dosage recommended by the FDA in severe CKD, 5 mg/day with a maximum of 10 mg/day, the report noted.
The researchers analyzed deidentified electronic health record data from 40 health care organizations in the United States from the OptumLabs Data Warehouse database. They entered 152,101 new rosuvastatin users and 795,799 new atorvastatin users, and excluded patients with a history of rhabdomyolysis.
Patients in the two groups were similar with respect to CKD prevalence, cardiovascular risk factors, and demographics. Their age averaged 60 years, 48% were women, and 82% were White.
Hematuria was defined as dipstick hematuria > + or the presence of more than 3 red blood cells per high-power field in urine microscopy, at least twice. Proteinuria was defined as dipstick proteinuria > ++ or urine albumin-to-creatinine ratio greater than 300 mg/g at least twice.
Overall, 2.9% of patients had hematuria (3.4% of the rosuvastatin group and 2.8% of those taking atorvastatin) and 1% of patients had proteinuria (1.2% and 0.9%, respectively).
After balancing baseline characteristics in both groups using inverse probability of treatment weighting, rosuvastatin treatment, compared with atorvastatin, was associated with significantly greater risks for hematuria (hazard ratio, 1.08), proteinuria (HR, 1.17), and kidney failure requiring replacement therapy (HR, 1.15).
Patients with eGFR less than 30 mL/min per 1.73 m2 had an approximately twofold higher risk for hematuria and ninefold higher risk for proteinuria during the follow-up compared with patients with eGFR of at least 60 mL/min per 1.73 m2.
Patients with eGFR less than 30 mL/min per 1.73 m2 were commonly prescribed high-dose rosuvastatin (29.9% received the 20-mg dose and 14% the 40-mg dose), contrary to the labeling recommendation.
Dr. Shin reported receiving research Funding from the National Institutes of Health and Merck; disclosures for the other authors are in the report. Dr. Hiremath reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN SOCIETY OF NEPHROLOGY
Jury still out on cardiovascular safety of testosterone
Despite a new meta-analysis claiming to show that testosterone replacement therapy for men with hypogonadism does not increase the risk of cardiovascular outcomes such as myocardial infarction or stroke, experts say the jury is still out.
A more definitive answer for cardiovascular safety of testosterone therapy will come from the TRAVERSE dedicated cardiovascular outcome trial, sponsored by AbbVie, which will have up to 5 years of follow-up, with results expected later this year.
The current meta-analysis by Jemma Hudson of Aberdeen (Scotland) University and colleagues was published online in The Lancet Healthy Longevity. The work will also be presented June 13 at ENDO 2022, the Endocrine Society’s annual meeting in Atlanta, Georgia, by senior author Channa Y. Jayasena, MD, PhD.
In 2014, the U.S. Food and Drug Administration mandated a label on testosterone products warning of possible increased cardiovascular risks and to reserve the therapy for symptomatic hypogonadism only. In contrast, the European Medicines Agency concluded that when hypogonadism is properly diagnosed and managed, there is currently no clear, consistent evidence that testosterone therapy causes increased cardiovascular risk.
To address this uncertainty, Dr. Hudson and colleagues formed a global collaborative to obtain individual patient data on cardiovascular outcomes from randomized controlled trials of testosterone therapy for men with hypogonadism.
They pooled data from 35 trials published from 1992 to Aug. 27, 2018, including 17 trials (3,431 patients) for which the researchers obtained patient-level data. The individual trials were 3-12 months long, except for one 3-year trial.
During a mean follow-up of 9.5 months, there was no significant increase in cardiovascular outcomes in men randomized to testosterone therapy versus placebo (odds ratio, 1.07; P = .62), nor were there any significantly increased risks of death, stroke, or different types of cardiovascular outcome, although those numbers were small.
This is “the most comprehensive study to date investigating the safety of testosterone treatment of hypogonadism,” according to the researchers. “The current results provide some reassurance about the short-term to medium-term safety of testosterone to treat male hypogonadism,” they conclude.
However, they also acknowledge that “long-term data are needed to fully evaluate the safety of testosterone.”
Erin D. Michos, MD, coauthor of an accompanying editorial, told this news organization, “This study doesn’t say to me that low testosterone necessarily needs to be treated. It’s still not indicated in people just for a low number [for blood testosterone] with less-severe symptoms. It really comes down to each individual person, how symptomatic they are, and their cardiovascular risk.”
‘Trial is not definitive’
Dr. Michos is not the only person to be skeptical. Together with Steven Nissen, MD, an investigator for the TRAVERSE trial, she agrees that this new evidence is not yet decisive, largely because the individual trials in the meta-analysis were short and not designed as cardiovascular outcome trials.
Dr. Nissen, a cardiologist at Cleveland Clinic, added that the individual trials were heterogeneous, with “very few real cardiovascular events,” so the meta-analysis “is not definitive,” he said in an interview.
While this meta-analysis “that pooled together a lot of smaller studies is reassuring that there’s no signal of harm, it’s really inconclusive because the follow-up was really short – a mean of only 9.5 months – and you really need a larger study with longer follow up to be more conclusive,” Dr. Michos noted.
“We should have more data soon” from TRAVERSE, said Dr. Michos, from the division of cardiology, Johns Hopkins University, Baltimore, who is not involved with that study.
Meanwhile, “I don’t think [this analysis] changes the current recommendations,” she said.
“We should continue to use caution as indicated by the FDA label and only use testosterone therapy selectively in people who have true symptoms of hypogonadism,” and be cautious about using it particularly in men at higher cardiovascular risk because of family history or known personal heart disease.
On the other hand, the meta-analysis did not show harm, she noted, “so we don’t necessarily need to pull patients off therapy if they are already taking it. But I wouldn’t right now just start new patients on it unless they had a strong indication.”
“Certainly, great caution is advised regarding the use of testosterone replacement therapy in people with established atherosclerosis due to the findings of plaque progression in the testosterone trials and the excess cardiovascular events observed in the TOM trial, write Dr. Michos and fellow editorialist Matthew J. Budoff, MD, of University of California, Los Angeles, in their editorial.
Earlier data inconclusive
Testosterone concentrations progressively decline in men with advancing age, at about 2% per year, Dr. Michos and Dr. Budoff write. In addition, men with obesity or with diabetes have low levels of testosterone, Dr. Michos noted.
Low testosterone blood levels have been associated with insulin resistance, inflammation, dyslipidemia, and atherosclerosis. Testosterone replacement therapy has been used to increase libido, improve erectile dysfunction, and boost energy levels, mood, and muscle strength.
But it is well known that testosterone increases hematocrit, which has the potential to increase the risk of venous thromboembolism.
Two large observational studies have reported increased risks of myocardial infarction, stroke, and death in men taking testosterone, compared with nonusers, but the study designs have been widely criticized, Dr. Hudson and coauthors say in their article.
A placebo-controlled trial was stopped early by its data- and safety-monitoring board following increased cardiovascular events in men aged 65 and older who received 6 months of testosterone. Other controlled trials have not observed these effects, but none was sufficiently powered.
Meta-analysis results
Dr. Hudson and colleagues performed a meta-analysis of 35 trials in 5,601 men aged 18 years and older with low baseline testosterone (≤ 350 nmol/dL) who had been randomized to testosterone replacement therapy or placebo for at least 3 months, for which there were data on mortality, stroke, and cardiovascular outcomes.
The men were a mean age of 65, had a mean body mass index of 30 kg/m2, and most (88%) were White. A quarter had angina, 8% had a previous myocardial infarction, and 27% had diabetes.
Cardiovascular and cerebrovascular outcomes were not primary outcomes.
During a mean follow-up of 9.5 months, in the 13 trials that provided this information, the rate of cardiovascular events was similar in the men who received testosterone (120/1,601, 7.5%) compared with those who received placebo (110/1,519, 7.2%).
In the 14 trials that provided this information, fewer deaths were reported during testosterone treatment (6/1,621, 0.4%) than during placebo treatment (12/1,537, 0.8%), but these numbers were too small to establish whether testosterone reduced mortality risk.
The most common cardiovascular events were arrhythmia, followed by coronary heart disease, heart failure, and myocardial infarction.
Patient age, baseline testosterone, smoking status, or diabetes status were not associated with cardiovascular risk.
The only detected adverse effects were edema and a modest lowering of HDL cholesterol.
“Men who develop sexual dysfunction, unexplained anemia, or osteoporosis should be tested for low testosterone,” senior author of the meta-analysis Dr. Jayasena said in an email to this news organization.
However, Dr. Jayasena added, “Mass screening for testosterone has no benefit in asymptomatic men.”
“Older men may still benefit from testosterone, but only if they have the clinical features [of hypogonadism] and low testosterone levels,” he concluded.
The current study is supported by the Health Technology Assessment program of the National Institute for Health Research. The TRAVERSE trial is sponsored by AbbVie. Dr. Jayasena has reported receiving research grants from LogixX Pharma. Dr. Hudson has reported no relevant financial relationships. Disclosures for the other authors are listed in the article. Dr. Michos has reported receiving support from the Amato Fund in Women’s Cardiovascular Health at Johns Hopkins School of Medicine and serving on medical advisory boards for Novartis, Esperion, Amarin, and AstraZeneca outside the submitted work. Dr. Budoff has reported receiving grant support from General Electric.
A version of this article first appeared on Medscape.com.
Despite a new meta-analysis claiming to show that testosterone replacement therapy for men with hypogonadism does not increase the risk of cardiovascular outcomes such as myocardial infarction or stroke, experts say the jury is still out.
A more definitive answer for cardiovascular safety of testosterone therapy will come from the TRAVERSE dedicated cardiovascular outcome trial, sponsored by AbbVie, which will have up to 5 years of follow-up, with results expected later this year.
The current meta-analysis by Jemma Hudson of Aberdeen (Scotland) University and colleagues was published online in The Lancet Healthy Longevity. The work will also be presented June 13 at ENDO 2022, the Endocrine Society’s annual meeting in Atlanta, Georgia, by senior author Channa Y. Jayasena, MD, PhD.
In 2014, the U.S. Food and Drug Administration mandated a label on testosterone products warning of possible increased cardiovascular risks and to reserve the therapy for symptomatic hypogonadism only. In contrast, the European Medicines Agency concluded that when hypogonadism is properly diagnosed and managed, there is currently no clear, consistent evidence that testosterone therapy causes increased cardiovascular risk.
To address this uncertainty, Dr. Hudson and colleagues formed a global collaborative to obtain individual patient data on cardiovascular outcomes from randomized controlled trials of testosterone therapy for men with hypogonadism.
They pooled data from 35 trials published from 1992 to Aug. 27, 2018, including 17 trials (3,431 patients) for which the researchers obtained patient-level data. The individual trials were 3-12 months long, except for one 3-year trial.
During a mean follow-up of 9.5 months, there was no significant increase in cardiovascular outcomes in men randomized to testosterone therapy versus placebo (odds ratio, 1.07; P = .62), nor were there any significantly increased risks of death, stroke, or different types of cardiovascular outcome, although those numbers were small.
This is “the most comprehensive study to date investigating the safety of testosterone treatment of hypogonadism,” according to the researchers. “The current results provide some reassurance about the short-term to medium-term safety of testosterone to treat male hypogonadism,” they conclude.
However, they also acknowledge that “long-term data are needed to fully evaluate the safety of testosterone.”
Erin D. Michos, MD, coauthor of an accompanying editorial, told this news organization, “This study doesn’t say to me that low testosterone necessarily needs to be treated. It’s still not indicated in people just for a low number [for blood testosterone] with less-severe symptoms. It really comes down to each individual person, how symptomatic they are, and their cardiovascular risk.”
‘Trial is not definitive’
Dr. Michos is not the only person to be skeptical. Together with Steven Nissen, MD, an investigator for the TRAVERSE trial, she agrees that this new evidence is not yet decisive, largely because the individual trials in the meta-analysis were short and not designed as cardiovascular outcome trials.
Dr. Nissen, a cardiologist at Cleveland Clinic, added that the individual trials were heterogeneous, with “very few real cardiovascular events,” so the meta-analysis “is not definitive,” he said in an interview.
While this meta-analysis “that pooled together a lot of smaller studies is reassuring that there’s no signal of harm, it’s really inconclusive because the follow-up was really short – a mean of only 9.5 months – and you really need a larger study with longer follow up to be more conclusive,” Dr. Michos noted.
“We should have more data soon” from TRAVERSE, said Dr. Michos, from the division of cardiology, Johns Hopkins University, Baltimore, who is not involved with that study.
Meanwhile, “I don’t think [this analysis] changes the current recommendations,” she said.
“We should continue to use caution as indicated by the FDA label and only use testosterone therapy selectively in people who have true symptoms of hypogonadism,” and be cautious about using it particularly in men at higher cardiovascular risk because of family history or known personal heart disease.
On the other hand, the meta-analysis did not show harm, she noted, “so we don’t necessarily need to pull patients off therapy if they are already taking it. But I wouldn’t right now just start new patients on it unless they had a strong indication.”
“Certainly, great caution is advised regarding the use of testosterone replacement therapy in people with established atherosclerosis due to the findings of plaque progression in the testosterone trials and the excess cardiovascular events observed in the TOM trial, write Dr. Michos and fellow editorialist Matthew J. Budoff, MD, of University of California, Los Angeles, in their editorial.
Earlier data inconclusive
Testosterone concentrations progressively decline in men with advancing age, at about 2% per year, Dr. Michos and Dr. Budoff write. In addition, men with obesity or with diabetes have low levels of testosterone, Dr. Michos noted.
Low testosterone blood levels have been associated with insulin resistance, inflammation, dyslipidemia, and atherosclerosis. Testosterone replacement therapy has been used to increase libido, improve erectile dysfunction, and boost energy levels, mood, and muscle strength.
But it is well known that testosterone increases hematocrit, which has the potential to increase the risk of venous thromboembolism.
Two large observational studies have reported increased risks of myocardial infarction, stroke, and death in men taking testosterone, compared with nonusers, but the study designs have been widely criticized, Dr. Hudson and coauthors say in their article.
A placebo-controlled trial was stopped early by its data- and safety-monitoring board following increased cardiovascular events in men aged 65 and older who received 6 months of testosterone. Other controlled trials have not observed these effects, but none was sufficiently powered.
Meta-analysis results
Dr. Hudson and colleagues performed a meta-analysis of 35 trials in 5,601 men aged 18 years and older with low baseline testosterone (≤ 350 nmol/dL) who had been randomized to testosterone replacement therapy or placebo for at least 3 months, for which there were data on mortality, stroke, and cardiovascular outcomes.
The men were a mean age of 65, had a mean body mass index of 30 kg/m2, and most (88%) were White. A quarter had angina, 8% had a previous myocardial infarction, and 27% had diabetes.
Cardiovascular and cerebrovascular outcomes were not primary outcomes.
During a mean follow-up of 9.5 months, in the 13 trials that provided this information, the rate of cardiovascular events was similar in the men who received testosterone (120/1,601, 7.5%) compared with those who received placebo (110/1,519, 7.2%).
In the 14 trials that provided this information, fewer deaths were reported during testosterone treatment (6/1,621, 0.4%) than during placebo treatment (12/1,537, 0.8%), but these numbers were too small to establish whether testosterone reduced mortality risk.
The most common cardiovascular events were arrhythmia, followed by coronary heart disease, heart failure, and myocardial infarction.
Patient age, baseline testosterone, smoking status, or diabetes status were not associated with cardiovascular risk.
The only detected adverse effects were edema and a modest lowering of HDL cholesterol.
“Men who develop sexual dysfunction, unexplained anemia, or osteoporosis should be tested for low testosterone,” senior author of the meta-analysis Dr. Jayasena said in an email to this news organization.
However, Dr. Jayasena added, “Mass screening for testosterone has no benefit in asymptomatic men.”
“Older men may still benefit from testosterone, but only if they have the clinical features [of hypogonadism] and low testosterone levels,” he concluded.
The current study is supported by the Health Technology Assessment program of the National Institute for Health Research. The TRAVERSE trial is sponsored by AbbVie. Dr. Jayasena has reported receiving research grants from LogixX Pharma. Dr. Hudson has reported no relevant financial relationships. Disclosures for the other authors are listed in the article. Dr. Michos has reported receiving support from the Amato Fund in Women’s Cardiovascular Health at Johns Hopkins School of Medicine and serving on medical advisory boards for Novartis, Esperion, Amarin, and AstraZeneca outside the submitted work. Dr. Budoff has reported receiving grant support from General Electric.
A version of this article first appeared on Medscape.com.
Despite a new meta-analysis claiming to show that testosterone replacement therapy for men with hypogonadism does not increase the risk of cardiovascular outcomes such as myocardial infarction or stroke, experts say the jury is still out.
A more definitive answer for cardiovascular safety of testosterone therapy will come from the TRAVERSE dedicated cardiovascular outcome trial, sponsored by AbbVie, which will have up to 5 years of follow-up, with results expected later this year.
The current meta-analysis by Jemma Hudson of Aberdeen (Scotland) University and colleagues was published online in The Lancet Healthy Longevity. The work will also be presented June 13 at ENDO 2022, the Endocrine Society’s annual meeting in Atlanta, Georgia, by senior author Channa Y. Jayasena, MD, PhD.
In 2014, the U.S. Food and Drug Administration mandated a label on testosterone products warning of possible increased cardiovascular risks and to reserve the therapy for symptomatic hypogonadism only. In contrast, the European Medicines Agency concluded that when hypogonadism is properly diagnosed and managed, there is currently no clear, consistent evidence that testosterone therapy causes increased cardiovascular risk.
To address this uncertainty, Dr. Hudson and colleagues formed a global collaborative to obtain individual patient data on cardiovascular outcomes from randomized controlled trials of testosterone therapy for men with hypogonadism.
They pooled data from 35 trials published from 1992 to Aug. 27, 2018, including 17 trials (3,431 patients) for which the researchers obtained patient-level data. The individual trials were 3-12 months long, except for one 3-year trial.
During a mean follow-up of 9.5 months, there was no significant increase in cardiovascular outcomes in men randomized to testosterone therapy versus placebo (odds ratio, 1.07; P = .62), nor were there any significantly increased risks of death, stroke, or different types of cardiovascular outcome, although those numbers were small.
This is “the most comprehensive study to date investigating the safety of testosterone treatment of hypogonadism,” according to the researchers. “The current results provide some reassurance about the short-term to medium-term safety of testosterone to treat male hypogonadism,” they conclude.
However, they also acknowledge that “long-term data are needed to fully evaluate the safety of testosterone.”
Erin D. Michos, MD, coauthor of an accompanying editorial, told this news organization, “This study doesn’t say to me that low testosterone necessarily needs to be treated. It’s still not indicated in people just for a low number [for blood testosterone] with less-severe symptoms. It really comes down to each individual person, how symptomatic they are, and their cardiovascular risk.”
‘Trial is not definitive’
Dr. Michos is not the only person to be skeptical. Together with Steven Nissen, MD, an investigator for the TRAVERSE trial, she agrees that this new evidence is not yet decisive, largely because the individual trials in the meta-analysis were short and not designed as cardiovascular outcome trials.
Dr. Nissen, a cardiologist at Cleveland Clinic, added that the individual trials were heterogeneous, with “very few real cardiovascular events,” so the meta-analysis “is not definitive,” he said in an interview.
While this meta-analysis “that pooled together a lot of smaller studies is reassuring that there’s no signal of harm, it’s really inconclusive because the follow-up was really short – a mean of only 9.5 months – and you really need a larger study with longer follow up to be more conclusive,” Dr. Michos noted.
“We should have more data soon” from TRAVERSE, said Dr. Michos, from the division of cardiology, Johns Hopkins University, Baltimore, who is not involved with that study.
Meanwhile, “I don’t think [this analysis] changes the current recommendations,” she said.
“We should continue to use caution as indicated by the FDA label and only use testosterone therapy selectively in people who have true symptoms of hypogonadism,” and be cautious about using it particularly in men at higher cardiovascular risk because of family history or known personal heart disease.
On the other hand, the meta-analysis did not show harm, she noted, “so we don’t necessarily need to pull patients off therapy if they are already taking it. But I wouldn’t right now just start new patients on it unless they had a strong indication.”
“Certainly, great caution is advised regarding the use of testosterone replacement therapy in people with established atherosclerosis due to the findings of plaque progression in the testosterone trials and the excess cardiovascular events observed in the TOM trial, write Dr. Michos and fellow editorialist Matthew J. Budoff, MD, of University of California, Los Angeles, in their editorial.
Earlier data inconclusive
Testosterone concentrations progressively decline in men with advancing age, at about 2% per year, Dr. Michos and Dr. Budoff write. In addition, men with obesity or with diabetes have low levels of testosterone, Dr. Michos noted.
Low testosterone blood levels have been associated with insulin resistance, inflammation, dyslipidemia, and atherosclerosis. Testosterone replacement therapy has been used to increase libido, improve erectile dysfunction, and boost energy levels, mood, and muscle strength.
But it is well known that testosterone increases hematocrit, which has the potential to increase the risk of venous thromboembolism.
Two large observational studies have reported increased risks of myocardial infarction, stroke, and death in men taking testosterone, compared with nonusers, but the study designs have been widely criticized, Dr. Hudson and coauthors say in their article.
A placebo-controlled trial was stopped early by its data- and safety-monitoring board following increased cardiovascular events in men aged 65 and older who received 6 months of testosterone. Other controlled trials have not observed these effects, but none was sufficiently powered.
Meta-analysis results
Dr. Hudson and colleagues performed a meta-analysis of 35 trials in 5,601 men aged 18 years and older with low baseline testosterone (≤ 350 nmol/dL) who had been randomized to testosterone replacement therapy or placebo for at least 3 months, for which there were data on mortality, stroke, and cardiovascular outcomes.
The men were a mean age of 65, had a mean body mass index of 30 kg/m2, and most (88%) were White. A quarter had angina, 8% had a previous myocardial infarction, and 27% had diabetes.
Cardiovascular and cerebrovascular outcomes were not primary outcomes.
During a mean follow-up of 9.5 months, in the 13 trials that provided this information, the rate of cardiovascular events was similar in the men who received testosterone (120/1,601, 7.5%) compared with those who received placebo (110/1,519, 7.2%).
In the 14 trials that provided this information, fewer deaths were reported during testosterone treatment (6/1,621, 0.4%) than during placebo treatment (12/1,537, 0.8%), but these numbers were too small to establish whether testosterone reduced mortality risk.
The most common cardiovascular events were arrhythmia, followed by coronary heart disease, heart failure, and myocardial infarction.
Patient age, baseline testosterone, smoking status, or diabetes status were not associated with cardiovascular risk.
The only detected adverse effects were edema and a modest lowering of HDL cholesterol.
“Men who develop sexual dysfunction, unexplained anemia, or osteoporosis should be tested for low testosterone,” senior author of the meta-analysis Dr. Jayasena said in an email to this news organization.
However, Dr. Jayasena added, “Mass screening for testosterone has no benefit in asymptomatic men.”
“Older men may still benefit from testosterone, but only if they have the clinical features [of hypogonadism] and low testosterone levels,” he concluded.
The current study is supported by the Health Technology Assessment program of the National Institute for Health Research. The TRAVERSE trial is sponsored by AbbVie. Dr. Jayasena has reported receiving research grants from LogixX Pharma. Dr. Hudson has reported no relevant financial relationships. Disclosures for the other authors are listed in the article. Dr. Michos has reported receiving support from the Amato Fund in Women’s Cardiovascular Health at Johns Hopkins School of Medicine and serving on medical advisory boards for Novartis, Esperion, Amarin, and AstraZeneca outside the submitted work. Dr. Budoff has reported receiving grant support from General Electric.
A version of this article first appeared on Medscape.com.
FROM THE LANCET HEALTHY LONGEVITY
ADA updates on finerenone, SGLT2 inhibitors, and race-based eGFR
As it gears up for the first in-person scientific sessions for 3 years, the American Diabetes Association has issued an addendum to its most recent annual clinical practice recommendations published in December 2021, the 2022 Standards of Medical Care in Diabetes, based on recent trial evidence and consensus.
The update informs clinicians about:
- The effect of the nonsteroidal mineralocorticoid antagonist (Kerendia) on cardiovascular outcomes in patients with type 2 diabetes and chronic kidney disease.
- The effect of a sodium-glucose cotransporter 2 (SGLT2) inhibitor on heart failure and renal outcomes in patients with type 2 diabetes.
The National Kidney Foundation and the American Society of Nephrology Task Force recommendation to remove race in the formula for calculating estimated glomerular filtration rate (eGFR).
“This is the fifth year that we are able to update the Standards of Care after it has been published through our Living Standards of Care updates, making it possible to give diabetes care providers the most important information and the latest evidence relevant to their practice,” Robert A. Gabbay, MD, PhD, ADA chief scientific and medical officer, said in a press release from the organization.
The addendum, entitled, “Living Standards of Care,” updates Section 10, “Cardiovascular Disease and Risk Management,” and Section 11, “Chronic Kidney Disease and Risk Management” of the 2022 Standards of Medical Care in Diabetes.
The amendments were approved by the ADA Professional Practice Committee, which is responsible for developing the Standards of Care. The American College of Cardiology reviewed and endorsed the section on CVD and risk management.
The Living Standards Update was published online in Diabetes Care.
CVD and risk management
In the Addendum to Section 10, “Cardiovascular Disease and Risk Management,” the committee writes:
- “For patients with type 2 diabetes and chronic kidney disease treated with maximum tolerated doses of ACE inhibitors or angiotensin receptor blockers, addition of finerenone should be considered to improve cardiovascular outcomes and reduce the risk of chronic kidney disease progression. A”
- “Patients with type 2 diabetes and chronic kidney disease should be considered for treatment with finerenone to reduce cardiovascular outcomes and the risk of chronic kidney disease progression.”
- “In patients with type 2 diabetes and established heart failure with either preserved or reduced ejection fraction, an SGLT2 inhibitor [with proven benefit in this patient population] is recommended to reduce risk of worsening heart failure, hospitalizations for heart failure, and cardiovascular death. ”
In the section “Statin Treatment,” the addendum no longer states that “a prospective trial of a newer fibrate ... is ongoing,” because that trial investigating pemafibrate (Kowa), a novel selective peroxisome proliferator-activated receptor alpha modulator (or fibrate), has been discontinued.
Chronic kidney disease and risk management
In the Addendum to Section 11, “Chronic Kidney Disease and Risk Management,” the committee writes:
- “Traditionally, eGFR is calculated from serum creatinine using a validated formula. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation is preferred. ... Historically, a correction factor for muscle mass was included in a modified equation for African Americans; however, due to various issues with inequities, it was decided to the equation such that it applies to all. Hence, a committee was convened, resulting in the recommendation for immediate implementation of the CKD-EPI creatinine equation refit without the race variable in all laboratories in the U.S.” (This is based on an National Kidney Foundation and American Society of Nephrology Task Force recommendation.)
- “Additionally, increased use of cystatin C, especially to confirm estimated GFR in adults who are at risk for or have chronic kidney disease, because combining filtration markers (creatinine and cystatin C) is more accurate and would support better clinical decisions than either marker alone.”
Evidence from clinical trials
The update is based on findings from the following clinical trials:
- Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and Diabetic Kidney Disease (FIDELIO-DKD)
- Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and the Clinical Diagnosis of Diabetic Kidney Disease (FIGARO-DKD)
- FIDELITY, a prespecified pooled analysis of FIDELIO-DKD and FIGARO-DKD
- Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction (EMPEROR-Preserved)
- Effects of Dapagliflozin on Biomarkers, Symptoms and Functional Status in Patients with PRESERVED Ejection Fraction Heart Failure (PRESERVED-HF)
- Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients with Diabetes (PROMINENT).
A version of this article first appeared on Medscape.com.
As it gears up for the first in-person scientific sessions for 3 years, the American Diabetes Association has issued an addendum to its most recent annual clinical practice recommendations published in December 2021, the 2022 Standards of Medical Care in Diabetes, based on recent trial evidence and consensus.
The update informs clinicians about:
- The effect of the nonsteroidal mineralocorticoid antagonist (Kerendia) on cardiovascular outcomes in patients with type 2 diabetes and chronic kidney disease.
- The effect of a sodium-glucose cotransporter 2 (SGLT2) inhibitor on heart failure and renal outcomes in patients with type 2 diabetes.
The National Kidney Foundation and the American Society of Nephrology Task Force recommendation to remove race in the formula for calculating estimated glomerular filtration rate (eGFR).
“This is the fifth year that we are able to update the Standards of Care after it has been published through our Living Standards of Care updates, making it possible to give diabetes care providers the most important information and the latest evidence relevant to their practice,” Robert A. Gabbay, MD, PhD, ADA chief scientific and medical officer, said in a press release from the organization.
The addendum, entitled, “Living Standards of Care,” updates Section 10, “Cardiovascular Disease and Risk Management,” and Section 11, “Chronic Kidney Disease and Risk Management” of the 2022 Standards of Medical Care in Diabetes.
The amendments were approved by the ADA Professional Practice Committee, which is responsible for developing the Standards of Care. The American College of Cardiology reviewed and endorsed the section on CVD and risk management.
The Living Standards Update was published online in Diabetes Care.
CVD and risk management
In the Addendum to Section 10, “Cardiovascular Disease and Risk Management,” the committee writes:
- “For patients with type 2 diabetes and chronic kidney disease treated with maximum tolerated doses of ACE inhibitors or angiotensin receptor blockers, addition of finerenone should be considered to improve cardiovascular outcomes and reduce the risk of chronic kidney disease progression. A”
- “Patients with type 2 diabetes and chronic kidney disease should be considered for treatment with finerenone to reduce cardiovascular outcomes and the risk of chronic kidney disease progression.”
- “In patients with type 2 diabetes and established heart failure with either preserved or reduced ejection fraction, an SGLT2 inhibitor [with proven benefit in this patient population] is recommended to reduce risk of worsening heart failure, hospitalizations for heart failure, and cardiovascular death. ”
In the section “Statin Treatment,” the addendum no longer states that “a prospective trial of a newer fibrate ... is ongoing,” because that trial investigating pemafibrate (Kowa), a novel selective peroxisome proliferator-activated receptor alpha modulator (or fibrate), has been discontinued.
Chronic kidney disease and risk management
In the Addendum to Section 11, “Chronic Kidney Disease and Risk Management,” the committee writes:
- “Traditionally, eGFR is calculated from serum creatinine using a validated formula. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation is preferred. ... Historically, a correction factor for muscle mass was included in a modified equation for African Americans; however, due to various issues with inequities, it was decided to the equation such that it applies to all. Hence, a committee was convened, resulting in the recommendation for immediate implementation of the CKD-EPI creatinine equation refit without the race variable in all laboratories in the U.S.” (This is based on an National Kidney Foundation and American Society of Nephrology Task Force recommendation.)
- “Additionally, increased use of cystatin C, especially to confirm estimated GFR in adults who are at risk for or have chronic kidney disease, because combining filtration markers (creatinine and cystatin C) is more accurate and would support better clinical decisions than either marker alone.”
Evidence from clinical trials
The update is based on findings from the following clinical trials:
- Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and Diabetic Kidney Disease (FIDELIO-DKD)
- Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and the Clinical Diagnosis of Diabetic Kidney Disease (FIGARO-DKD)
- FIDELITY, a prespecified pooled analysis of FIDELIO-DKD and FIGARO-DKD
- Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction (EMPEROR-Preserved)
- Effects of Dapagliflozin on Biomarkers, Symptoms and Functional Status in Patients with PRESERVED Ejection Fraction Heart Failure (PRESERVED-HF)
- Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients with Diabetes (PROMINENT).
A version of this article first appeared on Medscape.com.
As it gears up for the first in-person scientific sessions for 3 years, the American Diabetes Association has issued an addendum to its most recent annual clinical practice recommendations published in December 2021, the 2022 Standards of Medical Care in Diabetes, based on recent trial evidence and consensus.
The update informs clinicians about:
- The effect of the nonsteroidal mineralocorticoid antagonist (Kerendia) on cardiovascular outcomes in patients with type 2 diabetes and chronic kidney disease.
- The effect of a sodium-glucose cotransporter 2 (SGLT2) inhibitor on heart failure and renal outcomes in patients with type 2 diabetes.
The National Kidney Foundation and the American Society of Nephrology Task Force recommendation to remove race in the formula for calculating estimated glomerular filtration rate (eGFR).
“This is the fifth year that we are able to update the Standards of Care after it has been published through our Living Standards of Care updates, making it possible to give diabetes care providers the most important information and the latest evidence relevant to their practice,” Robert A. Gabbay, MD, PhD, ADA chief scientific and medical officer, said in a press release from the organization.
The addendum, entitled, “Living Standards of Care,” updates Section 10, “Cardiovascular Disease and Risk Management,” and Section 11, “Chronic Kidney Disease and Risk Management” of the 2022 Standards of Medical Care in Diabetes.
The amendments were approved by the ADA Professional Practice Committee, which is responsible for developing the Standards of Care. The American College of Cardiology reviewed and endorsed the section on CVD and risk management.
The Living Standards Update was published online in Diabetes Care.
CVD and risk management
In the Addendum to Section 10, “Cardiovascular Disease and Risk Management,” the committee writes:
- “For patients with type 2 diabetes and chronic kidney disease treated with maximum tolerated doses of ACE inhibitors or angiotensin receptor blockers, addition of finerenone should be considered to improve cardiovascular outcomes and reduce the risk of chronic kidney disease progression. A”
- “Patients with type 2 diabetes and chronic kidney disease should be considered for treatment with finerenone to reduce cardiovascular outcomes and the risk of chronic kidney disease progression.”
- “In patients with type 2 diabetes and established heart failure with either preserved or reduced ejection fraction, an SGLT2 inhibitor [with proven benefit in this patient population] is recommended to reduce risk of worsening heart failure, hospitalizations for heart failure, and cardiovascular death. ”
In the section “Statin Treatment,” the addendum no longer states that “a prospective trial of a newer fibrate ... is ongoing,” because that trial investigating pemafibrate (Kowa), a novel selective peroxisome proliferator-activated receptor alpha modulator (or fibrate), has been discontinued.
Chronic kidney disease and risk management
In the Addendum to Section 11, “Chronic Kidney Disease and Risk Management,” the committee writes:
- “Traditionally, eGFR is calculated from serum creatinine using a validated formula. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation is preferred. ... Historically, a correction factor for muscle mass was included in a modified equation for African Americans; however, due to various issues with inequities, it was decided to the equation such that it applies to all. Hence, a committee was convened, resulting in the recommendation for immediate implementation of the CKD-EPI creatinine equation refit without the race variable in all laboratories in the U.S.” (This is based on an National Kidney Foundation and American Society of Nephrology Task Force recommendation.)
- “Additionally, increased use of cystatin C, especially to confirm estimated GFR in adults who are at risk for or have chronic kidney disease, because combining filtration markers (creatinine and cystatin C) is more accurate and would support better clinical decisions than either marker alone.”
Evidence from clinical trials
The update is based on findings from the following clinical trials:
- Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and Diabetic Kidney Disease (FIDELIO-DKD)
- Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and the Clinical Diagnosis of Diabetic Kidney Disease (FIGARO-DKD)
- FIDELITY, a prespecified pooled analysis of FIDELIO-DKD and FIGARO-DKD
- Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction (EMPEROR-Preserved)
- Effects of Dapagliflozin on Biomarkers, Symptoms and Functional Status in Patients with PRESERVED Ejection Fraction Heart Failure (PRESERVED-HF)
- Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients with Diabetes (PROMINENT).
A version of this article first appeared on Medscape.com.
FROM DIABETES CARE
Experts endorse plant-based diet for type 2 diabetes remission
Many adults can achieve remission of type 2 diabetes with a primary intervention consisting of a diet that emphasizes whole, plant-based foods, according to a new publication from the American College of Lifestyle Medicine (ACLM).
The document was developed to assist clinicians treating adults with type 2 diabetes, with the goal of remission using diet as a primary intervention. A panel of 15 experts from seven societies reached consensus on 69 statements.
“A healthy diet is a foundational component of current lifestyle guidelines for treatment of type 2 diabetes, but it is often overlooked because of the lack of physician training and patient awareness,” Felice A. Caldarella, MD, president of the American Association of Clinical Endocrinology (AACE), said in a press release from ACLM.
“The consensus statements produced by this panel of experts are invaluable in bringing awareness to the value of diet for diabetes remission in addition to management,” he summarized.
The initiative was cosponsored by the Endocrine Society, endorsed by AACE, and supported by the Academy of Nutrition and Dietetics. The expert panel also included representatives from the American College of Cardiology, the American Heart Association, and the American Academy of Family Physicians. It was published in the American Journal of Lifestyle Medicine.
“I think many patients would do the challenging work of making lifestyle modifications if it meant remission of [type 2 diabetes] and sparing them the burden and cost of medications or surgery,” said Amy E. Rothberg, MD, PhD, who represented the Endocrine Society on the panel.
“By changing the course of the disease, i.e., if in remission, they are unlikely to get the complications related to [type 2 diabetes],” Dr. Rothberg, professor of nutritional sciences at the University of Michigan, Ann Arbor, told this news organization.
Consensus on 69 statements
The panel members used a modified Delphi process to develop the consensus statement. They identified 49 articles from the literature regarding dietary interventions in adults with type 2 diabetes. They reached consensus on 69 statements that cover seven topics: definitions and basic concepts; diet and remission of type 2 diabetes; dietary specifics and types of diets; adjuvant and alternative interventions; support, monitoring, and adherence to therapy; weight loss; and payment and policy.
Dr. Rothberg identified six key areas:
- Definition of remission: Type 2 diabetes remission is defined as A1c < 6.5% for at least 3 months with no surgery, devices, or active pharmacologic therapy for lowering blood glucose, consistent with the diabetes remission timeline published in 2021 by the American Diabetes Association. Remission does not exclude the possibility of recurrence. Remission is a realistic and achievable goal for some adults with type 2 diabetes.
- High-intensity diet, short duration of diabetes: Patients are more likely to attain remission with a high-intensity diet (e.g., high level of restrictions plus frequent patient contact or counseling) accompanied by physical activity and if the patient has had diabetes for 4 years or less. A high-fiber diet is essential.
- Fewer calories, focus on plant-based foods: Calorie reduction could be achieved by reducing food volume, portion sizes, or energy density, or by using liquid meal replacements, or by a combination of these approaches. It should mainly include whole, plant-based foods (whole grains, vegetables, legumes, fruits, nuts, and seeds) and avoid or minimize meat and other animal products, refined foods, ultra-processed foods, and foods with added fats.
- A very low energy diet as initial intervention is optional: There was consensus that this approach can achieve remission, but there was not agreement that low calorie content was essential for achieving remission, Dr. Rothberg noted.
- Beyond type 2 remission: Diet as a primary intervention can also lower the risk of cardiovascular disease and improve lipoprotein profile.
- Self-management, support, and monitoring: The group recognizes the importance of patient education and support. “This can play a vital role and should be part of any comprehensive lifestyle treatment,” said Dr. Rothberg. The diet and lifestyle strategies should be acceptable to most patients, easy to adhere to, accommodate patient preferences and values, and be culturally sensitive.
Intensive lifestyle change can equate to bariatric surgery
Also invited to comment, Yehuda Handelsman, MD, who coauthored a 2020 type 2 diabetes management algorithm by AACE and the American College of Endocrinology, and was not involved with the current initiative, agrees with the importance of lifestyle in the management of type 2 diabetes but takes issue with a few points.
Most clinicians and experts do not believe that diabetes can be reversed, as such, only controlled, noted Dr. Handelsman, medical director of the Metabolic Institute of America, Tarzana, Calif.
“We always have approached type 2 diabetes treatment with lifestyle – diet, exercise, and (as of late) sleep – as the mainstay of therapy,” he said.
However, most patients do not adhere to diet modifications by 6 months and especially by 1 year, which has led to universal recommendations to add medication to lifestyle from inception, he continued.
Most clinicians have not been trained in lifestyle modalities. And many patients with type 2 diabetes are not adherent to medications, which “led to the relative success of bariatric surgery leading to remission (at least for 3-5 years).”
“Remission, which in broad terms implies the disappearance of signs and symptoms, should be a top priority for individuals with type 2 diabetes,” the consensus statement authors wrote.
“While [bariatric surgery] can induce remission in 25% to 80% of targeted patients, it carries risk and its effectiveness wanes as subjects regain lost weight,” and “more dramatic and intensive [lifestyle] change produces remission rates equivalent to bariatric surgery,” they noted.
Need for more randomized trials
Dr. Handelsman also stressed that remission may be temporary. “Three months or 6 months cannot be a measure of success. We must have at least 1 year,” he added. “In fact, there are data to show that remission requires 3 years.”
Nevertheless, the consensus statement does highlight the importance of lifestyle in remission of diabetes, he agreed.
The expert panel also noted that patients can benefit from a healthy lifestyle, even if they do not attain remission, Dr. Rothberg pointed out.
Moving forward, the statement concludes that “there is ... an ongoing need for additional randomized controlled trials to assess sustainable plant-based dietary interventions with whole or minimally processed foods, as a primary means of treating [type 2 diabetes] with the goal of remission, as well as factors that lead to successful patient adherence and effective dissemination and implementation of such interventions.”
This study was supported by the Lisa Wendel Memorial Foundation. Dr. Rothberg has disclosed being the medical director of Rewind, a virtual platform created for weight control with the goal to “defeat” type 2 diabetes, and a consultant for a study for which Nestle provides product. Dr. Handelsman has disclosed receiving research grants and consultant and speaker honoraria from Amarin, Amgen, Applied Therapeutic, AstraZeneca, Bayer, Boehringer Ingelheim, Corcept, Esperion, Ionis, Mankind, Merck, Merck-Pfizer, Novartis, Novo Nordisk, Regor, Sanofi, and Vertis.
A version of this article first appeared on Medscape.com.
Many adults can achieve remission of type 2 diabetes with a primary intervention consisting of a diet that emphasizes whole, plant-based foods, according to a new publication from the American College of Lifestyle Medicine (ACLM).
The document was developed to assist clinicians treating adults with type 2 diabetes, with the goal of remission using diet as a primary intervention. A panel of 15 experts from seven societies reached consensus on 69 statements.
“A healthy diet is a foundational component of current lifestyle guidelines for treatment of type 2 diabetes, but it is often overlooked because of the lack of physician training and patient awareness,” Felice A. Caldarella, MD, president of the American Association of Clinical Endocrinology (AACE), said in a press release from ACLM.
“The consensus statements produced by this panel of experts are invaluable in bringing awareness to the value of diet for diabetes remission in addition to management,” he summarized.
The initiative was cosponsored by the Endocrine Society, endorsed by AACE, and supported by the Academy of Nutrition and Dietetics. The expert panel also included representatives from the American College of Cardiology, the American Heart Association, and the American Academy of Family Physicians. It was published in the American Journal of Lifestyle Medicine.
“I think many patients would do the challenging work of making lifestyle modifications if it meant remission of [type 2 diabetes] and sparing them the burden and cost of medications or surgery,” said Amy E. Rothberg, MD, PhD, who represented the Endocrine Society on the panel.
“By changing the course of the disease, i.e., if in remission, they are unlikely to get the complications related to [type 2 diabetes],” Dr. Rothberg, professor of nutritional sciences at the University of Michigan, Ann Arbor, told this news organization.
Consensus on 69 statements
The panel members used a modified Delphi process to develop the consensus statement. They identified 49 articles from the literature regarding dietary interventions in adults with type 2 diabetes. They reached consensus on 69 statements that cover seven topics: definitions and basic concepts; diet and remission of type 2 diabetes; dietary specifics and types of diets; adjuvant and alternative interventions; support, monitoring, and adherence to therapy; weight loss; and payment and policy.
Dr. Rothberg identified six key areas:
- Definition of remission: Type 2 diabetes remission is defined as A1c < 6.5% for at least 3 months with no surgery, devices, or active pharmacologic therapy for lowering blood glucose, consistent with the diabetes remission timeline published in 2021 by the American Diabetes Association. Remission does not exclude the possibility of recurrence. Remission is a realistic and achievable goal for some adults with type 2 diabetes.
- High-intensity diet, short duration of diabetes: Patients are more likely to attain remission with a high-intensity diet (e.g., high level of restrictions plus frequent patient contact or counseling) accompanied by physical activity and if the patient has had diabetes for 4 years or less. A high-fiber diet is essential.
- Fewer calories, focus on plant-based foods: Calorie reduction could be achieved by reducing food volume, portion sizes, or energy density, or by using liquid meal replacements, or by a combination of these approaches. It should mainly include whole, plant-based foods (whole grains, vegetables, legumes, fruits, nuts, and seeds) and avoid or minimize meat and other animal products, refined foods, ultra-processed foods, and foods with added fats.
- A very low energy diet as initial intervention is optional: There was consensus that this approach can achieve remission, but there was not agreement that low calorie content was essential for achieving remission, Dr. Rothberg noted.
- Beyond type 2 remission: Diet as a primary intervention can also lower the risk of cardiovascular disease and improve lipoprotein profile.
- Self-management, support, and monitoring: The group recognizes the importance of patient education and support. “This can play a vital role and should be part of any comprehensive lifestyle treatment,” said Dr. Rothberg. The diet and lifestyle strategies should be acceptable to most patients, easy to adhere to, accommodate patient preferences and values, and be culturally sensitive.
Intensive lifestyle change can equate to bariatric surgery
Also invited to comment, Yehuda Handelsman, MD, who coauthored a 2020 type 2 diabetes management algorithm by AACE and the American College of Endocrinology, and was not involved with the current initiative, agrees with the importance of lifestyle in the management of type 2 diabetes but takes issue with a few points.
Most clinicians and experts do not believe that diabetes can be reversed, as such, only controlled, noted Dr. Handelsman, medical director of the Metabolic Institute of America, Tarzana, Calif.
“We always have approached type 2 diabetes treatment with lifestyle – diet, exercise, and (as of late) sleep – as the mainstay of therapy,” he said.
However, most patients do not adhere to diet modifications by 6 months and especially by 1 year, which has led to universal recommendations to add medication to lifestyle from inception, he continued.
Most clinicians have not been trained in lifestyle modalities. And many patients with type 2 diabetes are not adherent to medications, which “led to the relative success of bariatric surgery leading to remission (at least for 3-5 years).”
“Remission, which in broad terms implies the disappearance of signs and symptoms, should be a top priority for individuals with type 2 diabetes,” the consensus statement authors wrote.
“While [bariatric surgery] can induce remission in 25% to 80% of targeted patients, it carries risk and its effectiveness wanes as subjects regain lost weight,” and “more dramatic and intensive [lifestyle] change produces remission rates equivalent to bariatric surgery,” they noted.
Need for more randomized trials
Dr. Handelsman also stressed that remission may be temporary. “Three months or 6 months cannot be a measure of success. We must have at least 1 year,” he added. “In fact, there are data to show that remission requires 3 years.”
Nevertheless, the consensus statement does highlight the importance of lifestyle in remission of diabetes, he agreed.
The expert panel also noted that patients can benefit from a healthy lifestyle, even if they do not attain remission, Dr. Rothberg pointed out.
Moving forward, the statement concludes that “there is ... an ongoing need for additional randomized controlled trials to assess sustainable plant-based dietary interventions with whole or minimally processed foods, as a primary means of treating [type 2 diabetes] with the goal of remission, as well as factors that lead to successful patient adherence and effective dissemination and implementation of such interventions.”
This study was supported by the Lisa Wendel Memorial Foundation. Dr. Rothberg has disclosed being the medical director of Rewind, a virtual platform created for weight control with the goal to “defeat” type 2 diabetes, and a consultant for a study for which Nestle provides product. Dr. Handelsman has disclosed receiving research grants and consultant and speaker honoraria from Amarin, Amgen, Applied Therapeutic, AstraZeneca, Bayer, Boehringer Ingelheim, Corcept, Esperion, Ionis, Mankind, Merck, Merck-Pfizer, Novartis, Novo Nordisk, Regor, Sanofi, and Vertis.
A version of this article first appeared on Medscape.com.
Many adults can achieve remission of type 2 diabetes with a primary intervention consisting of a diet that emphasizes whole, plant-based foods, according to a new publication from the American College of Lifestyle Medicine (ACLM).
The document was developed to assist clinicians treating adults with type 2 diabetes, with the goal of remission using diet as a primary intervention. A panel of 15 experts from seven societies reached consensus on 69 statements.
“A healthy diet is a foundational component of current lifestyle guidelines for treatment of type 2 diabetes, but it is often overlooked because of the lack of physician training and patient awareness,” Felice A. Caldarella, MD, president of the American Association of Clinical Endocrinology (AACE), said in a press release from ACLM.
“The consensus statements produced by this panel of experts are invaluable in bringing awareness to the value of diet for diabetes remission in addition to management,” he summarized.
The initiative was cosponsored by the Endocrine Society, endorsed by AACE, and supported by the Academy of Nutrition and Dietetics. The expert panel also included representatives from the American College of Cardiology, the American Heart Association, and the American Academy of Family Physicians. It was published in the American Journal of Lifestyle Medicine.
“I think many patients would do the challenging work of making lifestyle modifications if it meant remission of [type 2 diabetes] and sparing them the burden and cost of medications or surgery,” said Amy E. Rothberg, MD, PhD, who represented the Endocrine Society on the panel.
“By changing the course of the disease, i.e., if in remission, they are unlikely to get the complications related to [type 2 diabetes],” Dr. Rothberg, professor of nutritional sciences at the University of Michigan, Ann Arbor, told this news organization.
Consensus on 69 statements
The panel members used a modified Delphi process to develop the consensus statement. They identified 49 articles from the literature regarding dietary interventions in adults with type 2 diabetes. They reached consensus on 69 statements that cover seven topics: definitions and basic concepts; diet and remission of type 2 diabetes; dietary specifics and types of diets; adjuvant and alternative interventions; support, monitoring, and adherence to therapy; weight loss; and payment and policy.
Dr. Rothberg identified six key areas:
- Definition of remission: Type 2 diabetes remission is defined as A1c < 6.5% for at least 3 months with no surgery, devices, or active pharmacologic therapy for lowering blood glucose, consistent with the diabetes remission timeline published in 2021 by the American Diabetes Association. Remission does not exclude the possibility of recurrence. Remission is a realistic and achievable goal for some adults with type 2 diabetes.
- High-intensity diet, short duration of diabetes: Patients are more likely to attain remission with a high-intensity diet (e.g., high level of restrictions plus frequent patient contact or counseling) accompanied by physical activity and if the patient has had diabetes for 4 years or less. A high-fiber diet is essential.
- Fewer calories, focus on plant-based foods: Calorie reduction could be achieved by reducing food volume, portion sizes, or energy density, or by using liquid meal replacements, or by a combination of these approaches. It should mainly include whole, plant-based foods (whole grains, vegetables, legumes, fruits, nuts, and seeds) and avoid or minimize meat and other animal products, refined foods, ultra-processed foods, and foods with added fats.
- A very low energy diet as initial intervention is optional: There was consensus that this approach can achieve remission, but there was not agreement that low calorie content was essential for achieving remission, Dr. Rothberg noted.
- Beyond type 2 remission: Diet as a primary intervention can also lower the risk of cardiovascular disease and improve lipoprotein profile.
- Self-management, support, and monitoring: The group recognizes the importance of patient education and support. “This can play a vital role and should be part of any comprehensive lifestyle treatment,” said Dr. Rothberg. The diet and lifestyle strategies should be acceptable to most patients, easy to adhere to, accommodate patient preferences and values, and be culturally sensitive.
Intensive lifestyle change can equate to bariatric surgery
Also invited to comment, Yehuda Handelsman, MD, who coauthored a 2020 type 2 diabetes management algorithm by AACE and the American College of Endocrinology, and was not involved with the current initiative, agrees with the importance of lifestyle in the management of type 2 diabetes but takes issue with a few points.
Most clinicians and experts do not believe that diabetes can be reversed, as such, only controlled, noted Dr. Handelsman, medical director of the Metabolic Institute of America, Tarzana, Calif.
“We always have approached type 2 diabetes treatment with lifestyle – diet, exercise, and (as of late) sleep – as the mainstay of therapy,” he said.
However, most patients do not adhere to diet modifications by 6 months and especially by 1 year, which has led to universal recommendations to add medication to lifestyle from inception, he continued.
Most clinicians have not been trained in lifestyle modalities. And many patients with type 2 diabetes are not adherent to medications, which “led to the relative success of bariatric surgery leading to remission (at least for 3-5 years).”
“Remission, which in broad terms implies the disappearance of signs and symptoms, should be a top priority for individuals with type 2 diabetes,” the consensus statement authors wrote.
“While [bariatric surgery] can induce remission in 25% to 80% of targeted patients, it carries risk and its effectiveness wanes as subjects regain lost weight,” and “more dramatic and intensive [lifestyle] change produces remission rates equivalent to bariatric surgery,” they noted.
Need for more randomized trials
Dr. Handelsman also stressed that remission may be temporary. “Three months or 6 months cannot be a measure of success. We must have at least 1 year,” he added. “In fact, there are data to show that remission requires 3 years.”
Nevertheless, the consensus statement does highlight the importance of lifestyle in remission of diabetes, he agreed.
The expert panel also noted that patients can benefit from a healthy lifestyle, even if they do not attain remission, Dr. Rothberg pointed out.
Moving forward, the statement concludes that “there is ... an ongoing need for additional randomized controlled trials to assess sustainable plant-based dietary interventions with whole or minimally processed foods, as a primary means of treating [type 2 diabetes] with the goal of remission, as well as factors that lead to successful patient adherence and effective dissemination and implementation of such interventions.”
This study was supported by the Lisa Wendel Memorial Foundation. Dr. Rothberg has disclosed being the medical director of Rewind, a virtual platform created for weight control with the goal to “defeat” type 2 diabetes, and a consultant for a study for which Nestle provides product. Dr. Handelsman has disclosed receiving research grants and consultant and speaker honoraria from Amarin, Amgen, Applied Therapeutic, AstraZeneca, Bayer, Boehringer Ingelheim, Corcept, Esperion, Ionis, Mankind, Merck, Merck-Pfizer, Novartis, Novo Nordisk, Regor, Sanofi, and Vertis.
A version of this article first appeared on Medscape.com.
Could new therapy for food ‘cues’ improve weight loss?
An intensive 1-year behavior therapy program aimed at changing a person’s response to food “cues” might help people with obesity lose a modest amount of weight, a randomized clinical trial suggests.
“Patients who are food-cue sensitive often feel out of control with their eating; they cannot resist food and/or cannot stop thinking about food,” said lead author Kerri N. Boutelle, PhD.
“Behavioral weight loss skills are not sufficient for these individuals,” so they designed this new approach, Dr. Boutelle, of the University of California, San Diego, explained in a press release.
The regulation of cues (ROC) intervention trains individuals to respond to their hunger and to resist eating highly craved foods (internal management), in contrast to behavioral weight loss programs that focus on counting calories (external management), Dr. Boutelle explained in an email.
The results of the Providing Adult Collaborative Interventions for Ideal Changes (PACIFIC) clinical trial, including follow-up out to 2 years, were published in JAMA Network Open.
Patients in the behavioral weight loss therapy group or the combined ROC and behavioral weight loss therapy group lost more weight at 6 months than patients in the ROC group – but then they slowly regained weight (whereas patients in the ROC group did not).
At 24 months, the three groups had a similar modest weight loss, compared with a control group that did not lose weight.
“We believe these internal management strategies are more durable over time,” said Dr. Boutelle.
However, two obesity experts, who helped develop the Canadian Adult Obesity Clinical Practice Guidelines, cautioned in emails that the intervention is very labor-intensive with less than 5% weight loss.
Four interventions
The trial was conducted at the Center for Healthy Eating and Activity Research at the University of California, San Diego, from December 2015 to December 2019.
Researchers randomized 271 adults with a mean BMI of 35 kg/m2 to one of four interventions:
- Regulation of cues: Patients were not given a prescribed diet but instead were given skills to tolerate cravings and respond better to hunger or satiety cues.
- Behavioral weight loss therapy: Patients were advised to follow a balanced, calorie-deficit diet based on their weight and given related skills.
- Combined regulation of cues plus behavioral weight loss therapy.
- Control: Patients received information about nutrition and stress management plus mindfulness training and were encouraged to find social support.
Therapy was given as 26 group sessions, 90 minutes each, over 12 months, with 16 weekly sessions, four biweekly sessions, and six monthly booster sessions.
Participants were asked to take part in 150 minutes of moderate to high intensity exercise each week and aim for 10,000 steps per day. All patients except those in the control group received a pedometer.
The patients were a mean age of 46 years, 82% were women and 62% were White.
At the end of the 12-month intervention, mean BMI had dropped by –1.18 kg/m2 in the ROC group and by –1.58 kg/m2 and –1.56 kg/m2 in the other two groups, compared with the control group, where BMI was virtually unchanged.
At 24 months follow-up, mean BMI was similar (roughly 33.5 kg/m2) in the ROC, the behavioral weight loss therapy, and the ROC plus behavioral weight loss therapy groups.
There was weight regain from 12 months in the latter two groups but not in the ROC group.
‘Nice study, but not practical’
“This is a nice study, but in no way is it practical,” Sean Wharton, MD, summarized.
“I think it may have difficulty finding its way into everyday practice,” said Dr. Wharton, adjunct professor at McMaster University, Hamilton, Ontario.
Also, “it does not compare ROC to pharmacotherapy,” he added, which is “quickly becoming the gold standard for obesity management. We have learned that adding intensive behavioral therapy – more visits and possibly a liquid diet as part of the weight management and some light group counseling – to pharmacotherapy does not add much.”
However, Dr. Wharton conceded that if an individual did not want, or could not take, pharmacotherapy and had access to ROC sessions, this might be a good option.
“The challenge will be offering this labor-intensive tool to 40% of Americans living with obesity,” he said.
The ROC intervention “is very different than a GP’s office that may see a patient two to three times/year max, with limited supports,” Dr. Wharton pointed out.
“It is labor-intensive, not reproducible in most places, and cannot be sustained forever. There is no evidence that the learning remains past the treatment interval. For example, 2 to 3 years later, are patients still adhering to ROC? Is weight still decreased or do they need to come to classes every month forever?”
‘Modest weight loss, doubtful long-term benefits’
Similarly, Arya M. Sharma, MD, said: “While this [ROC] approach may be helpful for some individuals, given the rather modest weight loss achieved (despite considerable efforts and a cash incentive), the long-term clinical benefits remain doubtful.”
The weight loss of less than 5% over 24 months is “in the ballpark of other behavioral weight-loss interventions,” said Dr. Sharma, of the University of Edmonton, Alberta, and past scientific director of Obesity Canada.
“I’m not convinced” about less weight regain, he added. “The difference between the groups is minimal. While this approach may well help individuals better deal with food cues, it does not change the underlying biology of weight regain.”
“This approach at best may help prevent future weight gain in susceptible individuals,” he speculated. “I would consider this more as a weight-stabilization than a weight-loss strategy.”
Next steps
Insurance doesn’t always cover weight loss with a mental health professional, Dr. Boutelle agreed. “However, there are eating disorder categories that also apply to many of our food-cue-sensitive patients, including binge eating,” she noted.
“We believe that ROC is an alternative model for weight loss that could be offered to patients who are interested or for whom behavioral weight loss has not been successful ... who are highly food-cue-responsive.”
The group is writing a manual about the ROC program to disseminate to other behavior therapists. They are also studying ROC in another clinical trial, Solutions for Hunger and Regulating Eating (SHARE). The ROC program is being offered at the UC San Diego Center for Healthy Eating and Activity Research, of which Dr. Boutelle is director.
The study was supported by grants from the National Institutes of Health. The researchers have reported no relevant financial relationships. Dr. Wharton has reported receiving honoraria and travel expenses and has participated in academic advisory boards for Novo Nordisk, Bausch Health, Eli Lilly, and Janssen. He is the medical director of a medical clinic specializing in weight management and diabetes. Dr. Sharma has reported receiving speakers bureau and consulting fees from Novo Nordisk, Bausch Pharmaceuticals, and AstraZeneca.
A version of this article first appeared on Medscape.com.
An intensive 1-year behavior therapy program aimed at changing a person’s response to food “cues” might help people with obesity lose a modest amount of weight, a randomized clinical trial suggests.
“Patients who are food-cue sensitive often feel out of control with their eating; they cannot resist food and/or cannot stop thinking about food,” said lead author Kerri N. Boutelle, PhD.
“Behavioral weight loss skills are not sufficient for these individuals,” so they designed this new approach, Dr. Boutelle, of the University of California, San Diego, explained in a press release.
The regulation of cues (ROC) intervention trains individuals to respond to their hunger and to resist eating highly craved foods (internal management), in contrast to behavioral weight loss programs that focus on counting calories (external management), Dr. Boutelle explained in an email.
The results of the Providing Adult Collaborative Interventions for Ideal Changes (PACIFIC) clinical trial, including follow-up out to 2 years, were published in JAMA Network Open.
Patients in the behavioral weight loss therapy group or the combined ROC and behavioral weight loss therapy group lost more weight at 6 months than patients in the ROC group – but then they slowly regained weight (whereas patients in the ROC group did not).
At 24 months, the three groups had a similar modest weight loss, compared with a control group that did not lose weight.
“We believe these internal management strategies are more durable over time,” said Dr. Boutelle.
However, two obesity experts, who helped develop the Canadian Adult Obesity Clinical Practice Guidelines, cautioned in emails that the intervention is very labor-intensive with less than 5% weight loss.
Four interventions
The trial was conducted at the Center for Healthy Eating and Activity Research at the University of California, San Diego, from December 2015 to December 2019.
Researchers randomized 271 adults with a mean BMI of 35 kg/m2 to one of four interventions:
- Regulation of cues: Patients were not given a prescribed diet but instead were given skills to tolerate cravings and respond better to hunger or satiety cues.
- Behavioral weight loss therapy: Patients were advised to follow a balanced, calorie-deficit diet based on their weight and given related skills.
- Combined regulation of cues plus behavioral weight loss therapy.
- Control: Patients received information about nutrition and stress management plus mindfulness training and were encouraged to find social support.
Therapy was given as 26 group sessions, 90 minutes each, over 12 months, with 16 weekly sessions, four biweekly sessions, and six monthly booster sessions.
Participants were asked to take part in 150 minutes of moderate to high intensity exercise each week and aim for 10,000 steps per day. All patients except those in the control group received a pedometer.
The patients were a mean age of 46 years, 82% were women and 62% were White.
At the end of the 12-month intervention, mean BMI had dropped by –1.18 kg/m2 in the ROC group and by –1.58 kg/m2 and –1.56 kg/m2 in the other two groups, compared with the control group, where BMI was virtually unchanged.
At 24 months follow-up, mean BMI was similar (roughly 33.5 kg/m2) in the ROC, the behavioral weight loss therapy, and the ROC plus behavioral weight loss therapy groups.
There was weight regain from 12 months in the latter two groups but not in the ROC group.
‘Nice study, but not practical’
“This is a nice study, but in no way is it practical,” Sean Wharton, MD, summarized.
“I think it may have difficulty finding its way into everyday practice,” said Dr. Wharton, adjunct professor at McMaster University, Hamilton, Ontario.
Also, “it does not compare ROC to pharmacotherapy,” he added, which is “quickly becoming the gold standard for obesity management. We have learned that adding intensive behavioral therapy – more visits and possibly a liquid diet as part of the weight management and some light group counseling – to pharmacotherapy does not add much.”
However, Dr. Wharton conceded that if an individual did not want, or could not take, pharmacotherapy and had access to ROC sessions, this might be a good option.
“The challenge will be offering this labor-intensive tool to 40% of Americans living with obesity,” he said.
The ROC intervention “is very different than a GP’s office that may see a patient two to three times/year max, with limited supports,” Dr. Wharton pointed out.
“It is labor-intensive, not reproducible in most places, and cannot be sustained forever. There is no evidence that the learning remains past the treatment interval. For example, 2 to 3 years later, are patients still adhering to ROC? Is weight still decreased or do they need to come to classes every month forever?”
‘Modest weight loss, doubtful long-term benefits’
Similarly, Arya M. Sharma, MD, said: “While this [ROC] approach may be helpful for some individuals, given the rather modest weight loss achieved (despite considerable efforts and a cash incentive), the long-term clinical benefits remain doubtful.”
The weight loss of less than 5% over 24 months is “in the ballpark of other behavioral weight-loss interventions,” said Dr. Sharma, of the University of Edmonton, Alberta, and past scientific director of Obesity Canada.
“I’m not convinced” about less weight regain, he added. “The difference between the groups is minimal. While this approach may well help individuals better deal with food cues, it does not change the underlying biology of weight regain.”
“This approach at best may help prevent future weight gain in susceptible individuals,” he speculated. “I would consider this more as a weight-stabilization than a weight-loss strategy.”
Next steps
Insurance doesn’t always cover weight loss with a mental health professional, Dr. Boutelle agreed. “However, there are eating disorder categories that also apply to many of our food-cue-sensitive patients, including binge eating,” she noted.
“We believe that ROC is an alternative model for weight loss that could be offered to patients who are interested or for whom behavioral weight loss has not been successful ... who are highly food-cue-responsive.”
The group is writing a manual about the ROC program to disseminate to other behavior therapists. They are also studying ROC in another clinical trial, Solutions for Hunger and Regulating Eating (SHARE). The ROC program is being offered at the UC San Diego Center for Healthy Eating and Activity Research, of which Dr. Boutelle is director.
The study was supported by grants from the National Institutes of Health. The researchers have reported no relevant financial relationships. Dr. Wharton has reported receiving honoraria and travel expenses and has participated in academic advisory boards for Novo Nordisk, Bausch Health, Eli Lilly, and Janssen. He is the medical director of a medical clinic specializing in weight management and diabetes. Dr. Sharma has reported receiving speakers bureau and consulting fees from Novo Nordisk, Bausch Pharmaceuticals, and AstraZeneca.
A version of this article first appeared on Medscape.com.
An intensive 1-year behavior therapy program aimed at changing a person’s response to food “cues” might help people with obesity lose a modest amount of weight, a randomized clinical trial suggests.
“Patients who are food-cue sensitive often feel out of control with their eating; they cannot resist food and/or cannot stop thinking about food,” said lead author Kerri N. Boutelle, PhD.
“Behavioral weight loss skills are not sufficient for these individuals,” so they designed this new approach, Dr. Boutelle, of the University of California, San Diego, explained in a press release.
The regulation of cues (ROC) intervention trains individuals to respond to their hunger and to resist eating highly craved foods (internal management), in contrast to behavioral weight loss programs that focus on counting calories (external management), Dr. Boutelle explained in an email.
The results of the Providing Adult Collaborative Interventions for Ideal Changes (PACIFIC) clinical trial, including follow-up out to 2 years, were published in JAMA Network Open.
Patients in the behavioral weight loss therapy group or the combined ROC and behavioral weight loss therapy group lost more weight at 6 months than patients in the ROC group – but then they slowly regained weight (whereas patients in the ROC group did not).
At 24 months, the three groups had a similar modest weight loss, compared with a control group that did not lose weight.
“We believe these internal management strategies are more durable over time,” said Dr. Boutelle.
However, two obesity experts, who helped develop the Canadian Adult Obesity Clinical Practice Guidelines, cautioned in emails that the intervention is very labor-intensive with less than 5% weight loss.
Four interventions
The trial was conducted at the Center for Healthy Eating and Activity Research at the University of California, San Diego, from December 2015 to December 2019.
Researchers randomized 271 adults with a mean BMI of 35 kg/m2 to one of four interventions:
- Regulation of cues: Patients were not given a prescribed diet but instead were given skills to tolerate cravings and respond better to hunger or satiety cues.
- Behavioral weight loss therapy: Patients were advised to follow a balanced, calorie-deficit diet based on their weight and given related skills.
- Combined regulation of cues plus behavioral weight loss therapy.
- Control: Patients received information about nutrition and stress management plus mindfulness training and were encouraged to find social support.
Therapy was given as 26 group sessions, 90 minutes each, over 12 months, with 16 weekly sessions, four biweekly sessions, and six monthly booster sessions.
Participants were asked to take part in 150 minutes of moderate to high intensity exercise each week and aim for 10,000 steps per day. All patients except those in the control group received a pedometer.
The patients were a mean age of 46 years, 82% were women and 62% were White.
At the end of the 12-month intervention, mean BMI had dropped by –1.18 kg/m2 in the ROC group and by –1.58 kg/m2 and –1.56 kg/m2 in the other two groups, compared with the control group, where BMI was virtually unchanged.
At 24 months follow-up, mean BMI was similar (roughly 33.5 kg/m2) in the ROC, the behavioral weight loss therapy, and the ROC plus behavioral weight loss therapy groups.
There was weight regain from 12 months in the latter two groups but not in the ROC group.
‘Nice study, but not practical’
“This is a nice study, but in no way is it practical,” Sean Wharton, MD, summarized.
“I think it may have difficulty finding its way into everyday practice,” said Dr. Wharton, adjunct professor at McMaster University, Hamilton, Ontario.
Also, “it does not compare ROC to pharmacotherapy,” he added, which is “quickly becoming the gold standard for obesity management. We have learned that adding intensive behavioral therapy – more visits and possibly a liquid diet as part of the weight management and some light group counseling – to pharmacotherapy does not add much.”
However, Dr. Wharton conceded that if an individual did not want, or could not take, pharmacotherapy and had access to ROC sessions, this might be a good option.
“The challenge will be offering this labor-intensive tool to 40% of Americans living with obesity,” he said.
The ROC intervention “is very different than a GP’s office that may see a patient two to three times/year max, with limited supports,” Dr. Wharton pointed out.
“It is labor-intensive, not reproducible in most places, and cannot be sustained forever. There is no evidence that the learning remains past the treatment interval. For example, 2 to 3 years later, are patients still adhering to ROC? Is weight still decreased or do they need to come to classes every month forever?”
‘Modest weight loss, doubtful long-term benefits’
Similarly, Arya M. Sharma, MD, said: “While this [ROC] approach may be helpful for some individuals, given the rather modest weight loss achieved (despite considerable efforts and a cash incentive), the long-term clinical benefits remain doubtful.”
The weight loss of less than 5% over 24 months is “in the ballpark of other behavioral weight-loss interventions,” said Dr. Sharma, of the University of Edmonton, Alberta, and past scientific director of Obesity Canada.
“I’m not convinced” about less weight regain, he added. “The difference between the groups is minimal. While this approach may well help individuals better deal with food cues, it does not change the underlying biology of weight regain.”
“This approach at best may help prevent future weight gain in susceptible individuals,” he speculated. “I would consider this more as a weight-stabilization than a weight-loss strategy.”
Next steps
Insurance doesn’t always cover weight loss with a mental health professional, Dr. Boutelle agreed. “However, there are eating disorder categories that also apply to many of our food-cue-sensitive patients, including binge eating,” she noted.
“We believe that ROC is an alternative model for weight loss that could be offered to patients who are interested or for whom behavioral weight loss has not been successful ... who are highly food-cue-responsive.”
The group is writing a manual about the ROC program to disseminate to other behavior therapists. They are also studying ROC in another clinical trial, Solutions for Hunger and Regulating Eating (SHARE). The ROC program is being offered at the UC San Diego Center for Healthy Eating and Activity Research, of which Dr. Boutelle is director.
The study was supported by grants from the National Institutes of Health. The researchers have reported no relevant financial relationships. Dr. Wharton has reported receiving honoraria and travel expenses and has participated in academic advisory boards for Novo Nordisk, Bausch Health, Eli Lilly, and Janssen. He is the medical director of a medical clinic specializing in weight management and diabetes. Dr. Sharma has reported receiving speakers bureau and consulting fees from Novo Nordisk, Bausch Pharmaceuticals, and AstraZeneca.
A version of this article first appeared on Medscape.com.






