User login
Dyshidroticlike Contact Dermatitis and Paronychia Resulting From a Dip Powder Manicure
To the Editor:
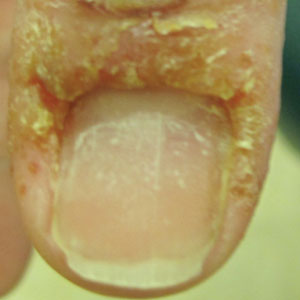
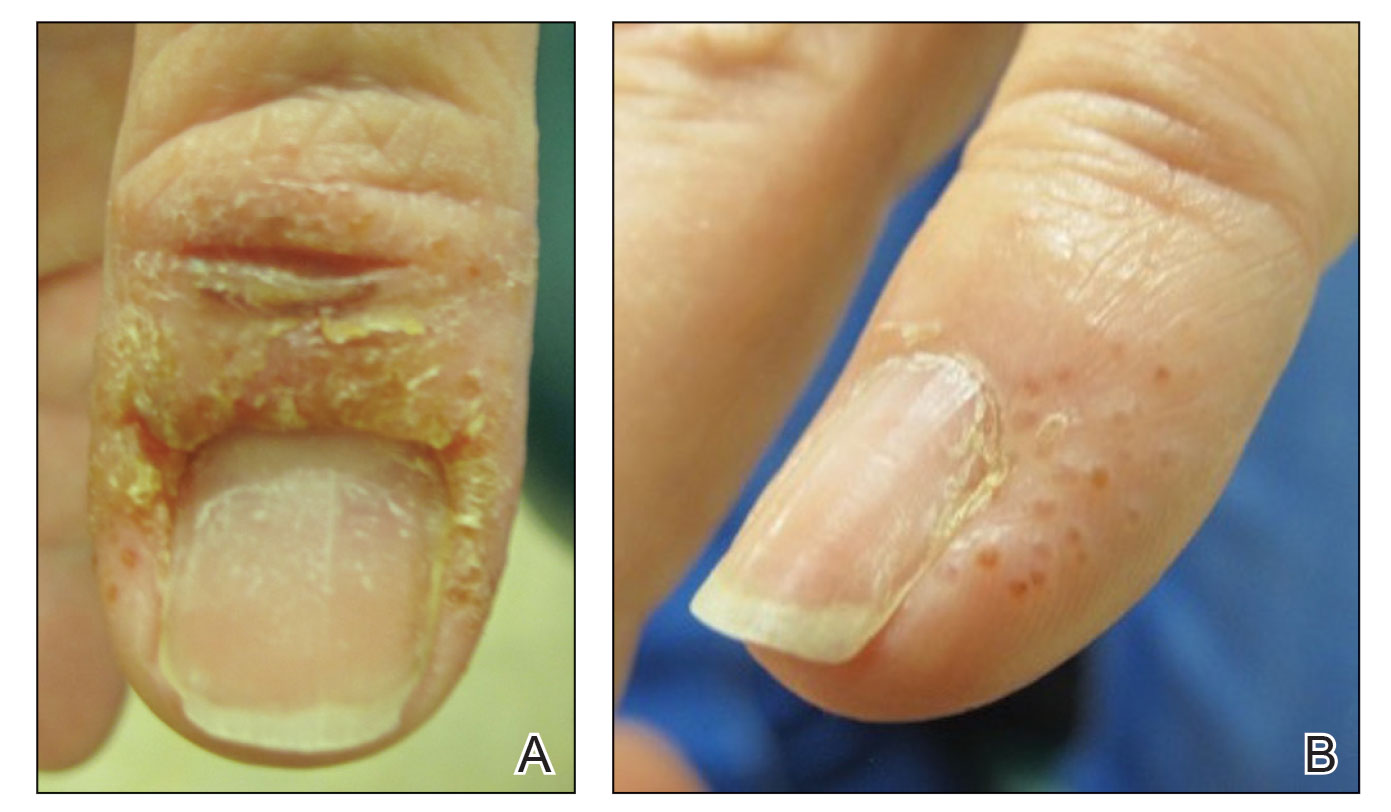
A 58-year-old woman presented to our dermatology clinic with a pruritic weeping eruption circumferentially on the distal digits of both hands of 5 weeks’ duration. The patient disclosed that she had been receiving dip powder manicures at a local nail salon approximately every 2 weeks over the last 3 to 6 months. She had received frequent acrylic nail extensions over the last 8 years prior to starting the dip powder manicures. Physical examination revealed well-demarcated eczematous plaques involving the lateral and proximal nail folds of the right thumb with an overlying serous crust and loss of the cuticle (Figure 1A). Erythematous plaques with firm deep-seated microvesicles also were present on the other digits, distributed distal to the distal interphalangeal joints (Figure 1B). She was diagnosed with dyshidroticlike contact dermatitis and paronychia. Treatment included phenol 1.5% colorless solution and clobetasol ointment 0.05% for twice-daily application to the affected areas. The patient also was advised to stop receiving manicures. At 1-month follow-up, the paronychia had resolved and the dermatitis had nearly resolved.
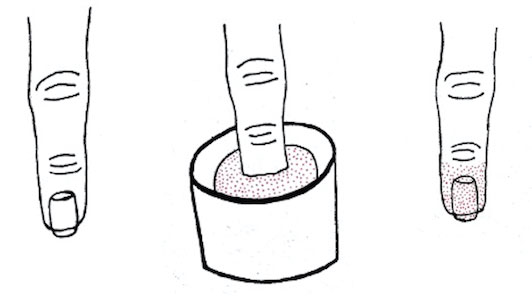
Dip powder manicures use a wet adhesive base coat with acrylic powder and an activator topcoat to initiate a chemical reaction that hardens and sets the nail polish. The colored powder typically is applied by dipping the digit up to the distal interphalangeal joint into a small container of loose powder and then brushing away the excess (Figure 2). Acrylate, a chemical present in dip powders, is a known allergen and has been associated with the development of allergic contact dermatitis and onychodystrophy in patients after receiving acrylic and UV-cured gel polish manicures.1,2 Inadequate sanitation practices at nail salons also have been associated with infection transmission.3,4 Additionally, the news media has covered the potential risk of infection due to contamination from reused dip manicure powder and the use of communal powder containers.5
To increase clinical awareness of the dip manicure technique, we describe the presentation and successful treatment of dyshidroticlike contact dermatitis and paronychia that occurred in a patient after she received a dip powder manicure. Dermatoses and infection limited to the distal phalanges will present in patients more frequently as dip powder manicures continue to increase in popularity and frequency.
- Baran R. Nail cosmetics: allergies and irritations. Am J Clin Dermatol. 2002;3:547-555.
- Chen AF, Chimento SM, Hu S, et al. Nail damage from gel polish manicure. J Cosmet Dermatol. 2012;11:27-29.
- Schmidt AN, Zic JA, Boyd AS. Pedicure-associated Mycobacterium chelonae infection in a hospitalized patient. J Am Acad Dermatol. 2014;71:E248-E250.
- Sniezek PJ, Graham BS, Busch HB, et al. Rapidly growing mycobacterial infections after pedicures. Arch Dermatol. 2003;139:629-634.
- Joseph T. You could be risking an infection with nail dipping. NBC Universal Media, LLC. Updated July 11, 2019. Accessed June 7, 2023. https://www.nbcmiami.com/news/local/You-Could-Be-Risking-an-Infection-with-Nail-Dipping-512550372.html
To the Editor:
A 58-year-old woman presented to our dermatology clinic with a pruritic weeping eruption circumferentially on the distal digits of both hands of 5 weeks’ duration. The patient disclosed that she had been receiving dip powder manicures at a local nail salon approximately every 2 weeks over the last 3 to 6 months. She had received frequent acrylic nail extensions over the last 8 years prior to starting the dip powder manicures. Physical examination revealed well-demarcated eczematous plaques involving the lateral and proximal nail folds of the right thumb with an overlying serous crust and loss of the cuticle (Figure 1A). Erythematous plaques with firm deep-seated microvesicles also were present on the other digits, distributed distal to the distal interphalangeal joints (Figure 1B). She was diagnosed with dyshidroticlike contact dermatitis and paronychia. Treatment included phenol 1.5% colorless solution and clobetasol ointment 0.05% for twice-daily application to the affected areas. The patient also was advised to stop receiving manicures. At 1-month follow-up, the paronychia had resolved and the dermatitis had nearly resolved.

Dip powder manicures use a wet adhesive base coat with acrylic powder and an activator topcoat to initiate a chemical reaction that hardens and sets the nail polish. The colored powder typically is applied by dipping the digit up to the distal interphalangeal joint into a small container of loose powder and then brushing away the excess (Figure 2). Acrylate, a chemical present in dip powders, is a known allergen and has been associated with the development of allergic contact dermatitis and onychodystrophy in patients after receiving acrylic and UV-cured gel polish manicures.1,2 Inadequate sanitation practices at nail salons also have been associated with infection transmission.3,4 Additionally, the news media has covered the potential risk of infection due to contamination from reused dip manicure powder and the use of communal powder containers.5

To increase clinical awareness of the dip manicure technique, we describe the presentation and successful treatment of dyshidroticlike contact dermatitis and paronychia that occurred in a patient after she received a dip powder manicure. Dermatoses and infection limited to the distal phalanges will present in patients more frequently as dip powder manicures continue to increase in popularity and frequency.
To the Editor:
A 58-year-old woman presented to our dermatology clinic with a pruritic weeping eruption circumferentially on the distal digits of both hands of 5 weeks’ duration. The patient disclosed that she had been receiving dip powder manicures at a local nail salon approximately every 2 weeks over the last 3 to 6 months. She had received frequent acrylic nail extensions over the last 8 years prior to starting the dip powder manicures. Physical examination revealed well-demarcated eczematous plaques involving the lateral and proximal nail folds of the right thumb with an overlying serous crust and loss of the cuticle (Figure 1A). Erythematous plaques with firm deep-seated microvesicles also were present on the other digits, distributed distal to the distal interphalangeal joints (Figure 1B). She was diagnosed with dyshidroticlike contact dermatitis and paronychia. Treatment included phenol 1.5% colorless solution and clobetasol ointment 0.05% for twice-daily application to the affected areas. The patient also was advised to stop receiving manicures. At 1-month follow-up, the paronychia had resolved and the dermatitis had nearly resolved.

Dip powder manicures use a wet adhesive base coat with acrylic powder and an activator topcoat to initiate a chemical reaction that hardens and sets the nail polish. The colored powder typically is applied by dipping the digit up to the distal interphalangeal joint into a small container of loose powder and then brushing away the excess (Figure 2). Acrylate, a chemical present in dip powders, is a known allergen and has been associated with the development of allergic contact dermatitis and onychodystrophy in patients after receiving acrylic and UV-cured gel polish manicures.1,2 Inadequate sanitation practices at nail salons also have been associated with infection transmission.3,4 Additionally, the news media has covered the potential risk of infection due to contamination from reused dip manicure powder and the use of communal powder containers.5

To increase clinical awareness of the dip manicure technique, we describe the presentation and successful treatment of dyshidroticlike contact dermatitis and paronychia that occurred in a patient after she received a dip powder manicure. Dermatoses and infection limited to the distal phalanges will present in patients more frequently as dip powder manicures continue to increase in popularity and frequency.
- Baran R. Nail cosmetics: allergies and irritations. Am J Clin Dermatol. 2002;3:547-555.
- Chen AF, Chimento SM, Hu S, et al. Nail damage from gel polish manicure. J Cosmet Dermatol. 2012;11:27-29.
- Schmidt AN, Zic JA, Boyd AS. Pedicure-associated Mycobacterium chelonae infection in a hospitalized patient. J Am Acad Dermatol. 2014;71:E248-E250.
- Sniezek PJ, Graham BS, Busch HB, et al. Rapidly growing mycobacterial infections after pedicures. Arch Dermatol. 2003;139:629-634.
- Joseph T. You could be risking an infection with nail dipping. NBC Universal Media, LLC. Updated July 11, 2019. Accessed June 7, 2023. https://www.nbcmiami.com/news/local/You-Could-Be-Risking-an-Infection-with-Nail-Dipping-512550372.html
- Baran R. Nail cosmetics: allergies and irritations. Am J Clin Dermatol. 2002;3:547-555.
- Chen AF, Chimento SM, Hu S, et al. Nail damage from gel polish manicure. J Cosmet Dermatol. 2012;11:27-29.
- Schmidt AN, Zic JA, Boyd AS. Pedicure-associated Mycobacterium chelonae infection in a hospitalized patient. J Am Acad Dermatol. 2014;71:E248-E250.
- Sniezek PJ, Graham BS, Busch HB, et al. Rapidly growing mycobacterial infections after pedicures. Arch Dermatol. 2003;139:629-634.
- Joseph T. You could be risking an infection with nail dipping. NBC Universal Media, LLC. Updated July 11, 2019. Accessed June 7, 2023. https://www.nbcmiami.com/news/local/You-Could-Be-Risking-an-Infection-with-Nail-Dipping-512550372.html
Practice Points
- Manicures performed at nail salons have been associated with the development of paronychia due to inadequate sanitation practices and contact dermatitis caused by acrylates present in nail polish.
- The dip powder manicure is a relatively new manicure technique. The distribution of dermatoses and infection limited to the distal phalanges will present in patients more frequently as dip powder manicures continue to increase in popularity and are performed more frequently.
Spontaneous ecchymoses
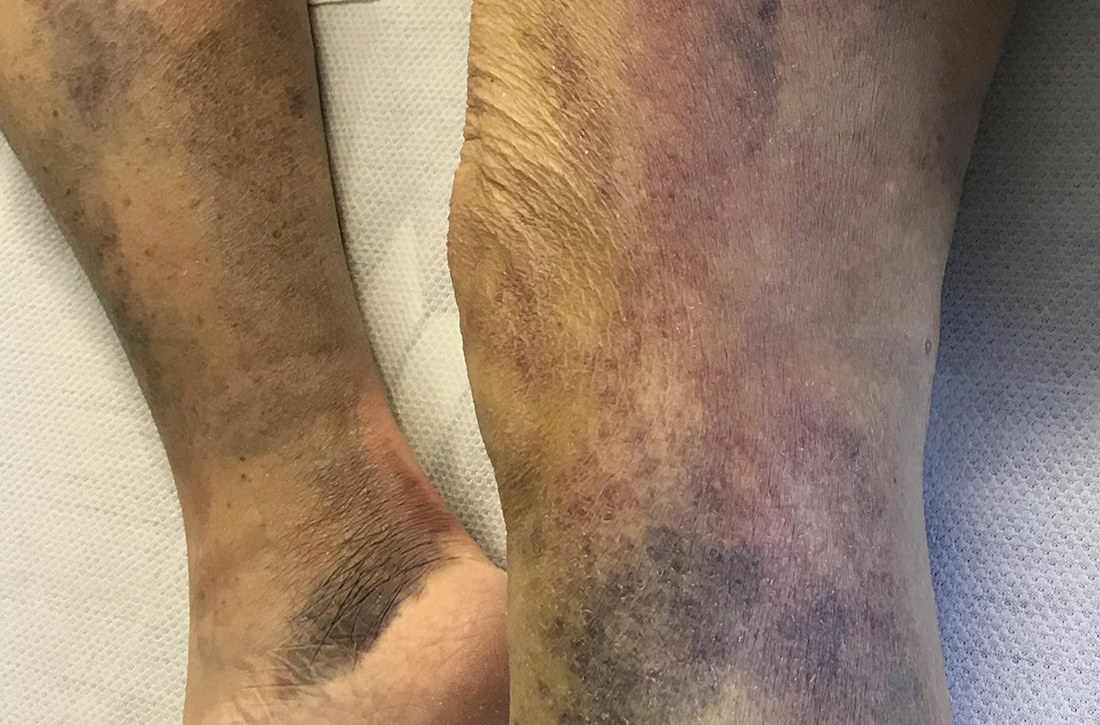
A 65-YEAR-OLD WOMAN was brought into the emergency department by her daughter for spontaneous bruising, fatigue, and weakness of several weeks’ duration. She denied taking any medications or illicit drugs and had not experienced any falls or trauma. On a daily basis, she smoked 5 to 7 cigarettes and drank 6 or 7 beers, as had been her custom for several years. The patient lived alone and was grieving the death of her beloved dog, who had died a month earlier. She reported that since the death of her dog, her diet, which hadn’t been especially good to begin with, had deteriorated; it now consisted of beer and crackers.
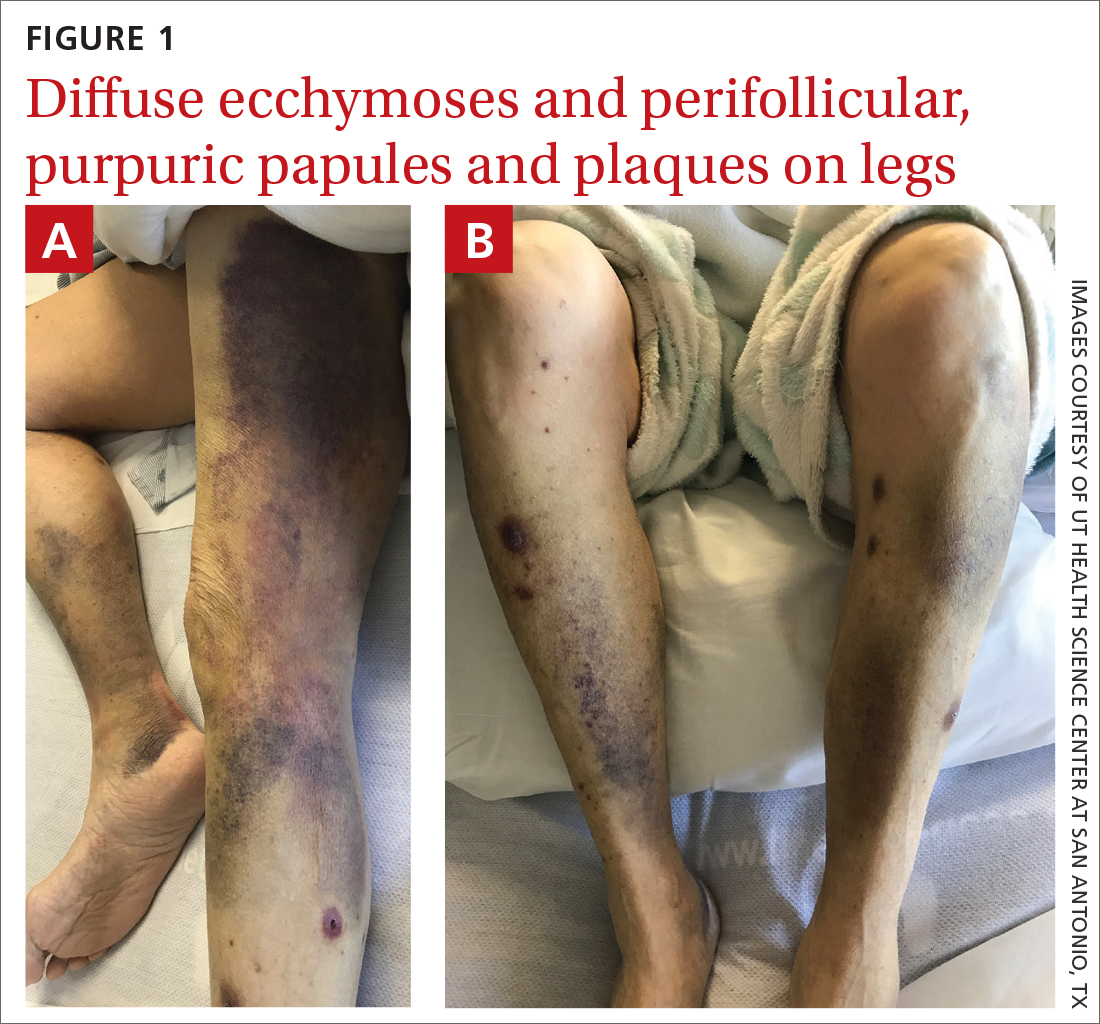
On admission, she was mildly tachycardic (105 beats/min) with a blood pressure of 125/66 mm Hg. Physical examination revealed a frail-appearing woman who was in no acute distress but was unable to stand without assistance. She had diffuse ecchymoses and perifollicular, purpuric, hyperkeratotic papules and plaques on both of her legs (FIGURES 1A and 1B). In addition, she had faint perifollicular purpuric macules on her upper back. An oral examination revealed poor dentition.
A punch biopsy was performed on her leg, and it revealed noninflammatory dermal hemorrhage without evidence of vasculitis or vasculopathy.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Scurvy
Based on the patient’s appearance and her dietary history, we suspected scurvy, so a serum vitamin C level was ordered. The results took several days to return. In the meantime, additional lab work revealed hyponatremia (sodium, 129 mmol/L; normal range, 135-145 mmol/L), hypokalemia (potassium, 3 mmol/L; normal range, 3.5-5.2 mmol/L), hypophosphatemia (phosphorus, 2.3 mg/dL; normal range, 2.8-4.5 mg/dL); low serum vitamin D (6 ng/mL; normal range, 20-40 ng/mL); and macrocytic anemia (hemoglobin, 7.4 g/dL; normal range, 11-18 g/dL) with a mean corpuscular volume of 101.1 fL (normal range, 80-100 fL). Her iron panel showed normal serum iron and total iron binding capacity with a normal ferritin level (294 ng/mL; normal range, 30-300 ng/mL). A peripheral blood smear test uncovered mild anisocytosis and polychromasia, with no schistocytes. A fecal immunochemical test was negative.
Several days after admission, the results of the patient’s vitamin C test came back. Her levels were undetectable (< 5 µmol/L; normal range, 11-23 µmol/L), confirming that the patient had scurvy.
A health hazard to marinersthat is still around today
Scurvy is a condition that arises from a deficiency of vitamin C, or ascorbic acid. The first known case of scurvy was in 1550 BC.1 Hippocrates termed the condition “ileos ematitis” and stated that “the mouth feels bad; the gums are detached from the teeth; blood runs from the nostrils … ulcerations on the legs … skin is thin.”1 Scurvy was a major health hazard of mariners between the 15thand 18th centuries.2 Today, the deficiency is uncommon in industrialized countries because there are many sources of vitamin C available through diet and vitamin supplements.3 In the United States, the prevalence of vitamin C deficiency is approximately 7%.4
An essential nutrient in humans, vitamin C is required as a cofactor in the synthesis of mature collagen.3 Collagen is found in skin, bone, and endothelium. Inadequate collagen levels can result in poor dermal support of vessels and tissue fragility, leading to hemorrhage, which can occur in nearly any organ system.
Vitamin C deficiency occurs when serum concentration falls below 11.4
Continue to: Scurvy manifests after 8 to 12 weeks
Scurvy manifests after 8 to 12 weeks of inadequate vitamin C intake.1 Patients may initially experience malaise and irritability. Anemia is common. Dermatologic findings include hyperkeratotic lesions, ecchymoses, poor wound healing, gingival swelling with loss of teeth, petechiae, and corkscrew hairs. Perifollicular hemorrhage is a characteristic finding of scurvy, generally seen on the lower extremities, where the capillaries are under higher hydrostatic pressure.3 Patients may also have musculoskeletal involvement with osteopenia or hemarthroses, which may be seen on imaging.3,5 Cardiorespiratory, gastrointestinal, ophthalmologic, and neurologic findings have also been reported.3
Differential is broad; zero in on patient’s history
The differential diagnosis for hemorrhagic skin lesions is extensive and includes scurvy, coagulopathies, trauma, vasculitis, and vasculopathies.
The presence of perifollicular hemorrhage with corkscrew hairs and a dietary history of inadequate vitamin C intake can differentiate scurvy from other conditions. Serum testing revealing low plasma vitamin C will support the diagnosis, but this is an insensitive test, as values increase with recent intake. Leukocyte ascorbic acid concentrations are more representative of total body stores, but impractical for routine use.6 Skin biopsy is not necessary but may help to rule out other conditions.
Ascorbic acid will facilitate a speedy recovery
Treatment of scurvy includes vitamin C replacement. Response is rapid, with improvement to lethargy within several days and disappearance of other manifestations within several weeks.3 Recommendations on supplementation doses and forms vary, but adults require 300 to 1000 mg/d of ascorbic acid for at least 1 week or until clinical symptoms resolve and stores are repleted.3,5,7
During our patient’s hospital stay, she remained stable and improved clinically with vitamin supplementation (ascorbic acid 1 g/d for 3 days, 500 mg/d after that) and physical therapy. She was counseled on a healthy diet, which would include citrus fruits, tomatoes, and leafy vegetables. The patient was also advised to refrain from drinking alcohol and was given information on an alcohol abstinence program.
At her 1-month follow-up, her condition had improved with near resolution of the skin lesions. She reported that she had given up cigarettes and alcohol. She said she’d also begun eating more citrus fruits and leafy vegetables.
1. Maxfield L, Crane JS. Vitamin C deficiency (scurvy). In: StatPearls. StatPearls Publishing; 2020. Accessed on September 13, 2022. www.ncbi.nlm.nih.gov/books/NBK493187/
2. Worral S. A nightmare disease haunted ships during age of discovery. National Geographic. January 15, 2017. Accessed September 21, 2022. www.nationalgeographic.com/science/article/scurvy-disease-discovery-jonathan-lamb
3. Hirschmann JV, Raugi GJ. Adult Scurvy. J Am Acad Dermatol. 1999;41:895-906. doi: 10.1016/s0190-9622(99)70244-6
4. Schleicher RL, Carroll MD, Ford ES, et al. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009;90:1252-1263. doi: 10.3945/ajcn.2008.27016
5. Agarwal A, Shaharyar A, Kumar A, et al. Scurvy in pediatric age group – A disease often forgotten? J Clin Orthop Trauma. 2015;6:101-107. doi: 10.1016/j.jcot.2014.12.003
6. Scurvy and its prevention and control in major emergencies. World Health Organization. February 23, 1999. Accessed September 13, 2022. www.who.int/publications/i/item/WHO-NHD-99.11
7. Weinstein M, Babyn P, Zlotkin S. An orange a day keeps the doctor away: scurvy in the year 2000. Pediatrics. 2001;108:E55. doi: 10.1542/peds.108.3.e55
A 65-YEAR-OLD WOMAN was brought into the emergency department by her daughter for spontaneous bruising, fatigue, and weakness of several weeks’ duration. She denied taking any medications or illicit drugs and had not experienced any falls or trauma. On a daily basis, she smoked 5 to 7 cigarettes and drank 6 or 7 beers, as had been her custom for several years. The patient lived alone and was grieving the death of her beloved dog, who had died a month earlier. She reported that since the death of her dog, her diet, which hadn’t been especially good to begin with, had deteriorated; it now consisted of beer and crackers.
On admission, she was mildly tachycardic (105 beats/min) with a blood pressure of 125/66 mm Hg. Physical examination revealed a frail-appearing woman who was in no acute distress but was unable to stand without assistance. She had diffuse ecchymoses and perifollicular, purpuric, hyperkeratotic papules and plaques on both of her legs (FIGURES 1A and 1B). In addition, she had faint perifollicular purpuric macules on her upper back. An oral examination revealed poor dentition.

A punch biopsy was performed on her leg, and it revealed noninflammatory dermal hemorrhage without evidence of vasculitis or vasculopathy.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Scurvy
Based on the patient’s appearance and her dietary history, we suspected scurvy, so a serum vitamin C level was ordered. The results took several days to return. In the meantime, additional lab work revealed hyponatremia (sodium, 129 mmol/L; normal range, 135-145 mmol/L), hypokalemia (potassium, 3 mmol/L; normal range, 3.5-5.2 mmol/L), hypophosphatemia (phosphorus, 2.3 mg/dL; normal range, 2.8-4.5 mg/dL); low serum vitamin D (6 ng/mL; normal range, 20-40 ng/mL); and macrocytic anemia (hemoglobin, 7.4 g/dL; normal range, 11-18 g/dL) with a mean corpuscular volume of 101.1 fL (normal range, 80-100 fL). Her iron panel showed normal serum iron and total iron binding capacity with a normal ferritin level (294 ng/mL; normal range, 30-300 ng/mL). A peripheral blood smear test uncovered mild anisocytosis and polychromasia, with no schistocytes. A fecal immunochemical test was negative.
Several days after admission, the results of the patient’s vitamin C test came back. Her levels were undetectable (< 5 µmol/L; normal range, 11-23 µmol/L), confirming that the patient had scurvy.
A health hazard to marinersthat is still around today
Scurvy is a condition that arises from a deficiency of vitamin C, or ascorbic acid. The first known case of scurvy was in 1550 BC.1 Hippocrates termed the condition “ileos ematitis” and stated that “the mouth feels bad; the gums are detached from the teeth; blood runs from the nostrils … ulcerations on the legs … skin is thin.”1 Scurvy was a major health hazard of mariners between the 15thand 18th centuries.2 Today, the deficiency is uncommon in industrialized countries because there are many sources of vitamin C available through diet and vitamin supplements.3 In the United States, the prevalence of vitamin C deficiency is approximately 7%.4
An essential nutrient in humans, vitamin C is required as a cofactor in the synthesis of mature collagen.3 Collagen is found in skin, bone, and endothelium. Inadequate collagen levels can result in poor dermal support of vessels and tissue fragility, leading to hemorrhage, which can occur in nearly any organ system.
Vitamin C deficiency occurs when serum concentration falls below 11.4
Continue to: Scurvy manifests after 8 to 12 weeks
Scurvy manifests after 8 to 12 weeks of inadequate vitamin C intake.1 Patients may initially experience malaise and irritability. Anemia is common. Dermatologic findings include hyperkeratotic lesions, ecchymoses, poor wound healing, gingival swelling with loss of teeth, petechiae, and corkscrew hairs. Perifollicular hemorrhage is a characteristic finding of scurvy, generally seen on the lower extremities, where the capillaries are under higher hydrostatic pressure.3 Patients may also have musculoskeletal involvement with osteopenia or hemarthroses, which may be seen on imaging.3,5 Cardiorespiratory, gastrointestinal, ophthalmologic, and neurologic findings have also been reported.3
Differential is broad; zero in on patient’s history
The differential diagnosis for hemorrhagic skin lesions is extensive and includes scurvy, coagulopathies, trauma, vasculitis, and vasculopathies.
The presence of perifollicular hemorrhage with corkscrew hairs and a dietary history of inadequate vitamin C intake can differentiate scurvy from other conditions. Serum testing revealing low plasma vitamin C will support the diagnosis, but this is an insensitive test, as values increase with recent intake. Leukocyte ascorbic acid concentrations are more representative of total body stores, but impractical for routine use.6 Skin biopsy is not necessary but may help to rule out other conditions.
Ascorbic acid will facilitate a speedy recovery
Treatment of scurvy includes vitamin C replacement. Response is rapid, with improvement to lethargy within several days and disappearance of other manifestations within several weeks.3 Recommendations on supplementation doses and forms vary, but adults require 300 to 1000 mg/d of ascorbic acid for at least 1 week or until clinical symptoms resolve and stores are repleted.3,5,7
During our patient’s hospital stay, she remained stable and improved clinically with vitamin supplementation (ascorbic acid 1 g/d for 3 days, 500 mg/d after that) and physical therapy. She was counseled on a healthy diet, which would include citrus fruits, tomatoes, and leafy vegetables. The patient was also advised to refrain from drinking alcohol and was given information on an alcohol abstinence program.
At her 1-month follow-up, her condition had improved with near resolution of the skin lesions. She reported that she had given up cigarettes and alcohol. She said she’d also begun eating more citrus fruits and leafy vegetables.
A 65-YEAR-OLD WOMAN was brought into the emergency department by her daughter for spontaneous bruising, fatigue, and weakness of several weeks’ duration. She denied taking any medications or illicit drugs and had not experienced any falls or trauma. On a daily basis, she smoked 5 to 7 cigarettes and drank 6 or 7 beers, as had been her custom for several years. The patient lived alone and was grieving the death of her beloved dog, who had died a month earlier. She reported that since the death of her dog, her diet, which hadn’t been especially good to begin with, had deteriorated; it now consisted of beer and crackers.
On admission, she was mildly tachycardic (105 beats/min) with a blood pressure of 125/66 mm Hg. Physical examination revealed a frail-appearing woman who was in no acute distress but was unable to stand without assistance. She had diffuse ecchymoses and perifollicular, purpuric, hyperkeratotic papules and plaques on both of her legs (FIGURES 1A and 1B). In addition, she had faint perifollicular purpuric macules on her upper back. An oral examination revealed poor dentition.

A punch biopsy was performed on her leg, and it revealed noninflammatory dermal hemorrhage without evidence of vasculitis or vasculopathy.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Scurvy
Based on the patient’s appearance and her dietary history, we suspected scurvy, so a serum vitamin C level was ordered. The results took several days to return. In the meantime, additional lab work revealed hyponatremia (sodium, 129 mmol/L; normal range, 135-145 mmol/L), hypokalemia (potassium, 3 mmol/L; normal range, 3.5-5.2 mmol/L), hypophosphatemia (phosphorus, 2.3 mg/dL; normal range, 2.8-4.5 mg/dL); low serum vitamin D (6 ng/mL; normal range, 20-40 ng/mL); and macrocytic anemia (hemoglobin, 7.4 g/dL; normal range, 11-18 g/dL) with a mean corpuscular volume of 101.1 fL (normal range, 80-100 fL). Her iron panel showed normal serum iron and total iron binding capacity with a normal ferritin level (294 ng/mL; normal range, 30-300 ng/mL). A peripheral blood smear test uncovered mild anisocytosis and polychromasia, with no schistocytes. A fecal immunochemical test was negative.
Several days after admission, the results of the patient’s vitamin C test came back. Her levels were undetectable (< 5 µmol/L; normal range, 11-23 µmol/L), confirming that the patient had scurvy.
A health hazard to marinersthat is still around today
Scurvy is a condition that arises from a deficiency of vitamin C, or ascorbic acid. The first known case of scurvy was in 1550 BC.1 Hippocrates termed the condition “ileos ematitis” and stated that “the mouth feels bad; the gums are detached from the teeth; blood runs from the nostrils … ulcerations on the legs … skin is thin.”1 Scurvy was a major health hazard of mariners between the 15thand 18th centuries.2 Today, the deficiency is uncommon in industrialized countries because there are many sources of vitamin C available through diet and vitamin supplements.3 In the United States, the prevalence of vitamin C deficiency is approximately 7%.4
An essential nutrient in humans, vitamin C is required as a cofactor in the synthesis of mature collagen.3 Collagen is found in skin, bone, and endothelium. Inadequate collagen levels can result in poor dermal support of vessels and tissue fragility, leading to hemorrhage, which can occur in nearly any organ system.
Vitamin C deficiency occurs when serum concentration falls below 11.4
Continue to: Scurvy manifests after 8 to 12 weeks
Scurvy manifests after 8 to 12 weeks of inadequate vitamin C intake.1 Patients may initially experience malaise and irritability. Anemia is common. Dermatologic findings include hyperkeratotic lesions, ecchymoses, poor wound healing, gingival swelling with loss of teeth, petechiae, and corkscrew hairs. Perifollicular hemorrhage is a characteristic finding of scurvy, generally seen on the lower extremities, where the capillaries are under higher hydrostatic pressure.3 Patients may also have musculoskeletal involvement with osteopenia or hemarthroses, which may be seen on imaging.3,5 Cardiorespiratory, gastrointestinal, ophthalmologic, and neurologic findings have also been reported.3
Differential is broad; zero in on patient’s history
The differential diagnosis for hemorrhagic skin lesions is extensive and includes scurvy, coagulopathies, trauma, vasculitis, and vasculopathies.
The presence of perifollicular hemorrhage with corkscrew hairs and a dietary history of inadequate vitamin C intake can differentiate scurvy from other conditions. Serum testing revealing low plasma vitamin C will support the diagnosis, but this is an insensitive test, as values increase with recent intake. Leukocyte ascorbic acid concentrations are more representative of total body stores, but impractical for routine use.6 Skin biopsy is not necessary but may help to rule out other conditions.
Ascorbic acid will facilitate a speedy recovery
Treatment of scurvy includes vitamin C replacement. Response is rapid, with improvement to lethargy within several days and disappearance of other manifestations within several weeks.3 Recommendations on supplementation doses and forms vary, but adults require 300 to 1000 mg/d of ascorbic acid for at least 1 week or until clinical symptoms resolve and stores are repleted.3,5,7
During our patient’s hospital stay, she remained stable and improved clinically with vitamin supplementation (ascorbic acid 1 g/d for 3 days, 500 mg/d after that) and physical therapy. She was counseled on a healthy diet, which would include citrus fruits, tomatoes, and leafy vegetables. The patient was also advised to refrain from drinking alcohol and was given information on an alcohol abstinence program.
At her 1-month follow-up, her condition had improved with near resolution of the skin lesions. She reported that she had given up cigarettes and alcohol. She said she’d also begun eating more citrus fruits and leafy vegetables.
1. Maxfield L, Crane JS. Vitamin C deficiency (scurvy). In: StatPearls. StatPearls Publishing; 2020. Accessed on September 13, 2022. www.ncbi.nlm.nih.gov/books/NBK493187/
2. Worral S. A nightmare disease haunted ships during age of discovery. National Geographic. January 15, 2017. Accessed September 21, 2022. www.nationalgeographic.com/science/article/scurvy-disease-discovery-jonathan-lamb
3. Hirschmann JV, Raugi GJ. Adult Scurvy. J Am Acad Dermatol. 1999;41:895-906. doi: 10.1016/s0190-9622(99)70244-6
4. Schleicher RL, Carroll MD, Ford ES, et al. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009;90:1252-1263. doi: 10.3945/ajcn.2008.27016
5. Agarwal A, Shaharyar A, Kumar A, et al. Scurvy in pediatric age group – A disease often forgotten? J Clin Orthop Trauma. 2015;6:101-107. doi: 10.1016/j.jcot.2014.12.003
6. Scurvy and its prevention and control in major emergencies. World Health Organization. February 23, 1999. Accessed September 13, 2022. www.who.int/publications/i/item/WHO-NHD-99.11
7. Weinstein M, Babyn P, Zlotkin S. An orange a day keeps the doctor away: scurvy in the year 2000. Pediatrics. 2001;108:E55. doi: 10.1542/peds.108.3.e55
1. Maxfield L, Crane JS. Vitamin C deficiency (scurvy). In: StatPearls. StatPearls Publishing; 2020. Accessed on September 13, 2022. www.ncbi.nlm.nih.gov/books/NBK493187/
2. Worral S. A nightmare disease haunted ships during age of discovery. National Geographic. January 15, 2017. Accessed September 21, 2022. www.nationalgeographic.com/science/article/scurvy-disease-discovery-jonathan-lamb
3. Hirschmann JV, Raugi GJ. Adult Scurvy. J Am Acad Dermatol. 1999;41:895-906. doi: 10.1016/s0190-9622(99)70244-6
4. Schleicher RL, Carroll MD, Ford ES, et al. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009;90:1252-1263. doi: 10.3945/ajcn.2008.27016
5. Agarwal A, Shaharyar A, Kumar A, et al. Scurvy in pediatric age group – A disease often forgotten? J Clin Orthop Trauma. 2015;6:101-107. doi: 10.1016/j.jcot.2014.12.003
6. Scurvy and its prevention and control in major emergencies. World Health Organization. February 23, 1999. Accessed September 13, 2022. www.who.int/publications/i/item/WHO-NHD-99.11
7. Weinstein M, Babyn P, Zlotkin S. An orange a day keeps the doctor away: scurvy in the year 2000. Pediatrics. 2001;108:E55. doi: 10.1542/peds.108.3.e55
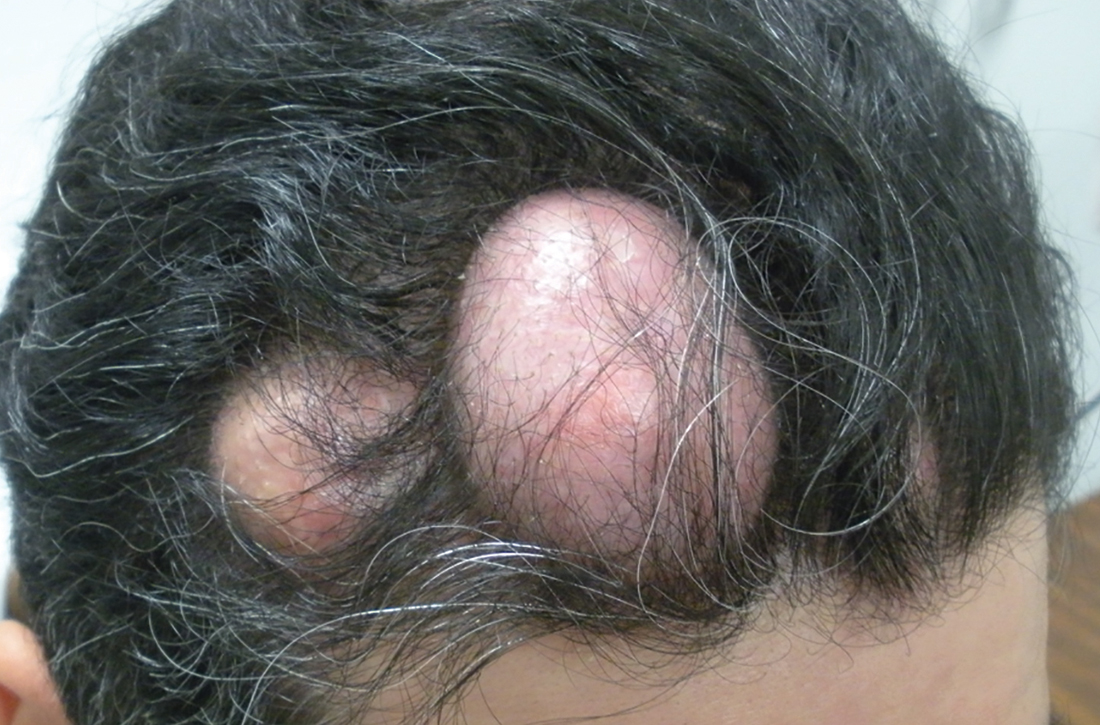
Numerous large nodules on scalp
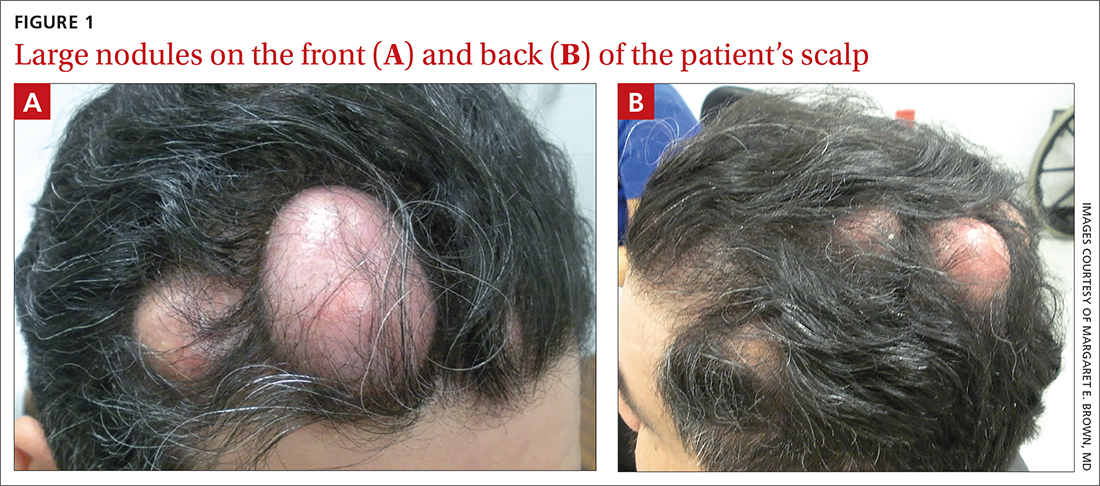
A 31-year-old Hispanic man presented for evaluation of numerous disfiguring growths on his scalp. They first appeared when he was 19 years old. A review of his family history revealed that his father had 2 “cysts” on his body.
The patient had 10 nodules on his scalp and upper back (Figures 1A and 1B). The ones on his scalp lacked puncta and appeared in a “turban tumor” configuration. The lesions were pink, smooth, and semisoft, and ranged in size from 1 to 6 cm.
Six years earlier, the patient had been seen for evaluation of 20 protuberant nodules. At the time, he had been referred to plastic surgery, where 15 lesions were excised. No other treatment was reported by the patient during the 6-year gap between exams.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Pilar cysts
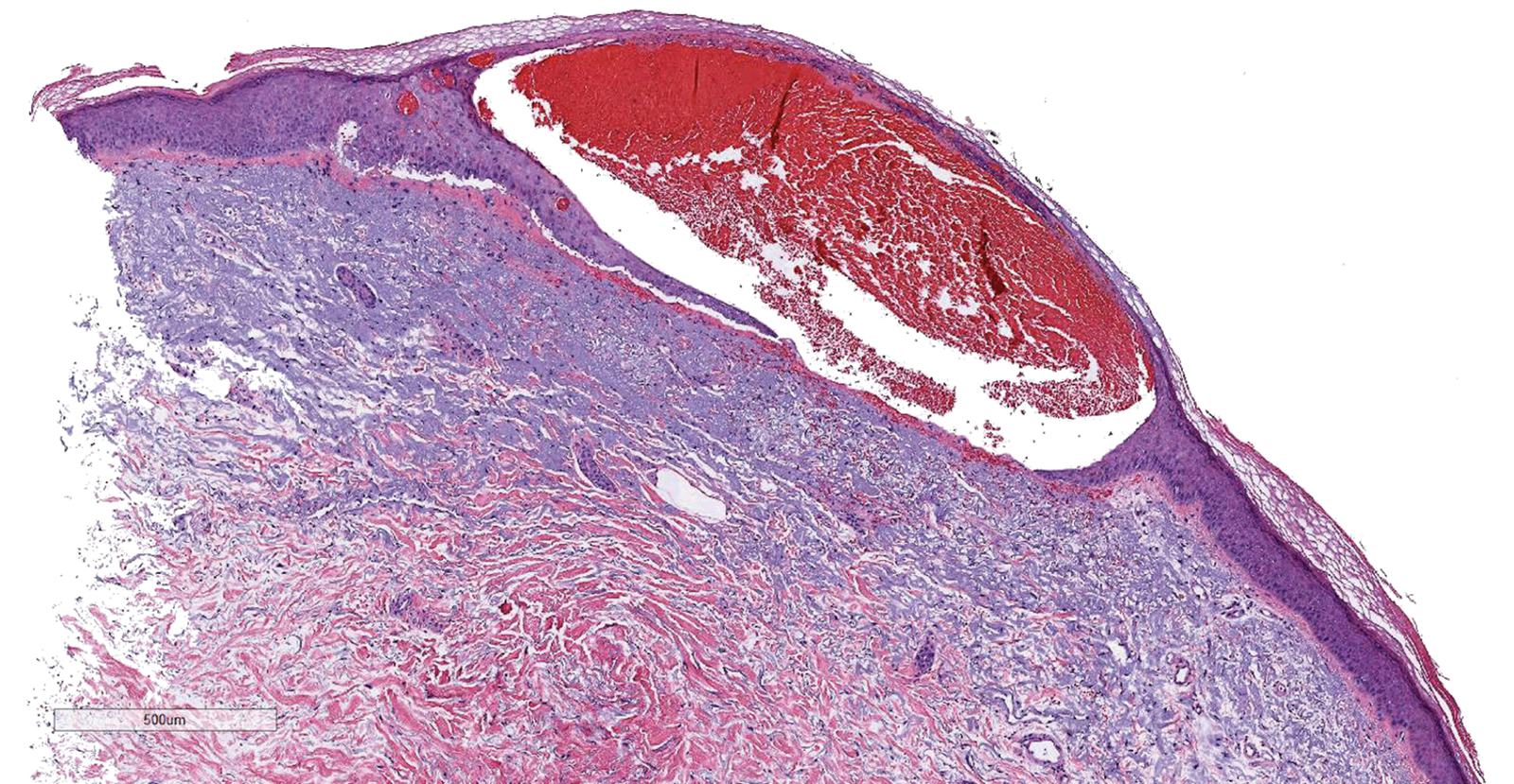
Pilar cysts (PC), also known as trichilemma cysts, wen, or isthmus-catagen cysts, are benign cysts that manifest as smooth, firm, well-circumscribed, pink nodules. PCs originate from the follicular isthmus of the hair’s external root sheath1 and are found in 5% to 10% of the US population.2 Possible sites of appearance include the face, neck, trunk, and extremities, although 90% of PCs develop on the scalp.1 They tend to have an autosomal dominant pattern of inheritance with linkages to the short arm of chromosome 3.3 PCs can occasionally become inflamed following infection or trauma.
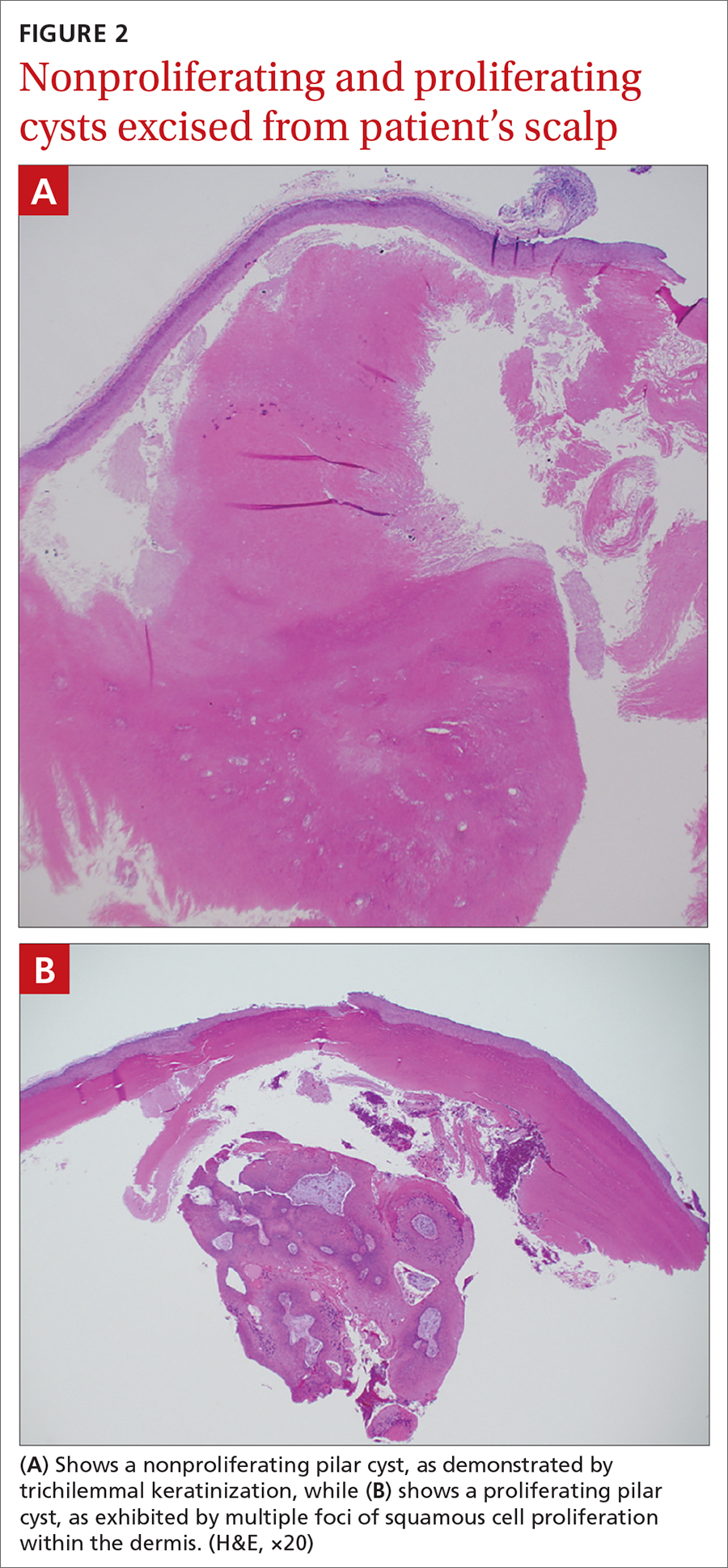
Characteristic histology of PCs demonstrates semisolid, keratin-filled, subepidermal cysts lined by stratified epithelium without a granular layer (trichilemmal keratinization). Lesions excised from this patient’s scalp showed 2 subtypes of PCs: nonproliferating (FIGURE 2A) and proliferating (FIGURE 2B). Subtypes appear similar on exam but can be differentiated on histology.
With gradual growth, proliferating PCs can reach up to 25 cm in diameter.1 Rapid growth, size > 5 cm, infiltration, or a non-scalp location may indicate malignancy.4
Differential diagnosis includes lipomas
The differential diagnosis for a lesion such as this includes epidermal inclusion cysts, dermoid cysts, and lipomas. Epidermal inclusion cysts have a punctum, whereas PCs do not. Dermoid cysts are single congenital lesions that manifest much earlier than PCs. Lipomas are easily movable rubbery bulges that appear more frequently in lipid-dense areas of the body.
For this patient, the striking turban tumor–like presentation, with numerous large cysts on the scalp, initially inspired a differential diagnosis including several genetic tumor syndromes. However, unlike the association between Gardner syndrome and numerous epidermoid cysts or Brooke-Spiegler syndrome and spiradenomas, no syndromes have been linked to numerous trichilemmal cysts.
Continue to: Excision is effective
Excision is effective
Excision is the treatment of choice for both proliferating and nonproliferating PCs.5 The local recurrence rate of proliferating PCs is 3.7% with a rare likelihood of transformation to trichilemmal carcinoma.6
Our patient continues to be followed in clinic for monitoring and periodic excision of bothersome cysts.
1. Ramaswamy AS, Manjunatha HK, Sunilkumar B, et al. Morphological spectrum of pilar cysts. N Am J Med Sci. 2013;5:124-128. http://doi.org/10.4103/1947-2714.107532
2. Ibrahim AE, Barikian A, Janom H, et al. Numerous recurrent trichilemmal cysts of the scalp: differential diagnosis and surgical management. J Craniofac Surg. 2012;23:e164-168. http://doi.org/10.1097/SCS.0b013e31824cdbd2
3. Adya KA, Inamadar AC, Palit A. Multiple firm mobile swellings over the scalp. Int J Trichology. 2012;4:98-99. http://doi.org/10.4103/0974-7753.96906
4. Folpe AL, Reisenauer AK, Mentzel T, et al. Proliferating trichilemmal tumors: clinicopathologic evaluation is a guide to biologic behavior. J Cutan Pathol. 2003;30:492-498. http://doi.org/10.1034/j.1600-0560.2003.00041.x
5. Leppard BJ, Sanderson KV. The natural history of trichilemmal cysts. Br J Dermatol. 1976;94:379-390. http://doi.org/10.1111/j.1365-2133.1976.tb06115.x
6. Kim UG, Kook DB, Kim TH, et al. Trichilemmal carcinoma from proliferating trichilemmal cyst on the posterior neck. Arch Craniofac Surg. 2017;18:50-53. http://doi.org/10.7181/acfs.2017.18.1.50
A 31-year-old Hispanic man presented for evaluation of numerous disfiguring growths on his scalp. They first appeared when he was 19 years old. A review of his family history revealed that his father had 2 “cysts” on his body.
The patient had 10 nodules on his scalp and upper back (Figures 1A and 1B). The ones on his scalp lacked puncta and appeared in a “turban tumor” configuration. The lesions were pink, smooth, and semisoft, and ranged in size from 1 to 6 cm.
Six years earlier, the patient had been seen for evaluation of 20 protuberant nodules. At the time, he had been referred to plastic surgery, where 15 lesions were excised. No other treatment was reported by the patient during the 6-year gap between exams.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Pilar cysts
Pilar cysts (PC), also known as trichilemma cysts, wen, or isthmus-catagen cysts, are benign cysts that manifest as smooth, firm, well-circumscribed, pink nodules. PCs originate from the follicular isthmus of the hair’s external root sheath1 and are found in 5% to 10% of the US population.2 Possible sites of appearance include the face, neck, trunk, and extremities, although 90% of PCs develop on the scalp.1 They tend to have an autosomal dominant pattern of inheritance with linkages to the short arm of chromosome 3.3 PCs can occasionally become inflamed following infection or trauma.
Characteristic histology of PCs demonstrates semisolid, keratin-filled, subepidermal cysts lined by stratified epithelium without a granular layer (trichilemmal keratinization). Lesions excised from this patient’s scalp showed 2 subtypes of PCs: nonproliferating (FIGURE 2A) and proliferating (FIGURE 2B). Subtypes appear similar on exam but can be differentiated on histology.
With gradual growth, proliferating PCs can reach up to 25 cm in diameter.1 Rapid growth, size > 5 cm, infiltration, or a non-scalp location may indicate malignancy.4
Differential diagnosis includes lipomas
The differential diagnosis for a lesion such as this includes epidermal inclusion cysts, dermoid cysts, and lipomas. Epidermal inclusion cysts have a punctum, whereas PCs do not. Dermoid cysts are single congenital lesions that manifest much earlier than PCs. Lipomas are easily movable rubbery bulges that appear more frequently in lipid-dense areas of the body.
For this patient, the striking turban tumor–like presentation, with numerous large cysts on the scalp, initially inspired a differential diagnosis including several genetic tumor syndromes. However, unlike the association between Gardner syndrome and numerous epidermoid cysts or Brooke-Spiegler syndrome and spiradenomas, no syndromes have been linked to numerous trichilemmal cysts.
Continue to: Excision is effective
Excision is effective
Excision is the treatment of choice for both proliferating and nonproliferating PCs.5 The local recurrence rate of proliferating PCs is 3.7% with a rare likelihood of transformation to trichilemmal carcinoma.6
Our patient continues to be followed in clinic for monitoring and periodic excision of bothersome cysts.
A 31-year-old Hispanic man presented for evaluation of numerous disfiguring growths on his scalp. They first appeared when he was 19 years old. A review of his family history revealed that his father had 2 “cysts” on his body.
The patient had 10 nodules on his scalp and upper back (Figures 1A and 1B). The ones on his scalp lacked puncta and appeared in a “turban tumor” configuration. The lesions were pink, smooth, and semisoft, and ranged in size from 1 to 6 cm.
Six years earlier, the patient had been seen for evaluation of 20 protuberant nodules. At the time, he had been referred to plastic surgery, where 15 lesions were excised. No other treatment was reported by the patient during the 6-year gap between exams.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Pilar cysts
Pilar cysts (PC), also known as trichilemma cysts, wen, or isthmus-catagen cysts, are benign cysts that manifest as smooth, firm, well-circumscribed, pink nodules. PCs originate from the follicular isthmus of the hair’s external root sheath1 and are found in 5% to 10% of the US population.2 Possible sites of appearance include the face, neck, trunk, and extremities, although 90% of PCs develop on the scalp.1 They tend to have an autosomal dominant pattern of inheritance with linkages to the short arm of chromosome 3.3 PCs can occasionally become inflamed following infection or trauma.
Characteristic histology of PCs demonstrates semisolid, keratin-filled, subepidermal cysts lined by stratified epithelium without a granular layer (trichilemmal keratinization). Lesions excised from this patient’s scalp showed 2 subtypes of PCs: nonproliferating (FIGURE 2A) and proliferating (FIGURE 2B). Subtypes appear similar on exam but can be differentiated on histology.
With gradual growth, proliferating PCs can reach up to 25 cm in diameter.1 Rapid growth, size > 5 cm, infiltration, or a non-scalp location may indicate malignancy.4
Differential diagnosis includes lipomas
The differential diagnosis for a lesion such as this includes epidermal inclusion cysts, dermoid cysts, and lipomas. Epidermal inclusion cysts have a punctum, whereas PCs do not. Dermoid cysts are single congenital lesions that manifest much earlier than PCs. Lipomas are easily movable rubbery bulges that appear more frequently in lipid-dense areas of the body.
For this patient, the striking turban tumor–like presentation, with numerous large cysts on the scalp, initially inspired a differential diagnosis including several genetic tumor syndromes. However, unlike the association between Gardner syndrome and numerous epidermoid cysts or Brooke-Spiegler syndrome and spiradenomas, no syndromes have been linked to numerous trichilemmal cysts.
Continue to: Excision is effective
Excision is effective
Excision is the treatment of choice for both proliferating and nonproliferating PCs.5 The local recurrence rate of proliferating PCs is 3.7% with a rare likelihood of transformation to trichilemmal carcinoma.6
Our patient continues to be followed in clinic for monitoring and periodic excision of bothersome cysts.
1. Ramaswamy AS, Manjunatha HK, Sunilkumar B, et al. Morphological spectrum of pilar cysts. N Am J Med Sci. 2013;5:124-128. http://doi.org/10.4103/1947-2714.107532
2. Ibrahim AE, Barikian A, Janom H, et al. Numerous recurrent trichilemmal cysts of the scalp: differential diagnosis and surgical management. J Craniofac Surg. 2012;23:e164-168. http://doi.org/10.1097/SCS.0b013e31824cdbd2
3. Adya KA, Inamadar AC, Palit A. Multiple firm mobile swellings over the scalp. Int J Trichology. 2012;4:98-99. http://doi.org/10.4103/0974-7753.96906
4. Folpe AL, Reisenauer AK, Mentzel T, et al. Proliferating trichilemmal tumors: clinicopathologic evaluation is a guide to biologic behavior. J Cutan Pathol. 2003;30:492-498. http://doi.org/10.1034/j.1600-0560.2003.00041.x
5. Leppard BJ, Sanderson KV. The natural history of trichilemmal cysts. Br J Dermatol. 1976;94:379-390. http://doi.org/10.1111/j.1365-2133.1976.tb06115.x
6. Kim UG, Kook DB, Kim TH, et al. Trichilemmal carcinoma from proliferating trichilemmal cyst on the posterior neck. Arch Craniofac Surg. 2017;18:50-53. http://doi.org/10.7181/acfs.2017.18.1.50
1. Ramaswamy AS, Manjunatha HK, Sunilkumar B, et al. Morphological spectrum of pilar cysts. N Am J Med Sci. 2013;5:124-128. http://doi.org/10.4103/1947-2714.107532
2. Ibrahim AE, Barikian A, Janom H, et al. Numerous recurrent trichilemmal cysts of the scalp: differential diagnosis and surgical management. J Craniofac Surg. 2012;23:e164-168. http://doi.org/10.1097/SCS.0b013e31824cdbd2
3. Adya KA, Inamadar AC, Palit A. Multiple firm mobile swellings over the scalp. Int J Trichology. 2012;4:98-99. http://doi.org/10.4103/0974-7753.96906
4. Folpe AL, Reisenauer AK, Mentzel T, et al. Proliferating trichilemmal tumors: clinicopathologic evaluation is a guide to biologic behavior. J Cutan Pathol. 2003;30:492-498. http://doi.org/10.1034/j.1600-0560.2003.00041.x
5. Leppard BJ, Sanderson KV. The natural history of trichilemmal cysts. Br J Dermatol. 1976;94:379-390. http://doi.org/10.1111/j.1365-2133.1976.tb06115.x
6. Kim UG, Kook DB, Kim TH, et al. Trichilemmal carcinoma from proliferating trichilemmal cyst on the posterior neck. Arch Craniofac Surg. 2017;18:50-53. http://doi.org/10.7181/acfs.2017.18.1.50
Hemorrhagic Papular Eruption on the Dorsal Hands
The Diagnosis: Heparin-Induced Bullous Hemorrhagic Dermatosis
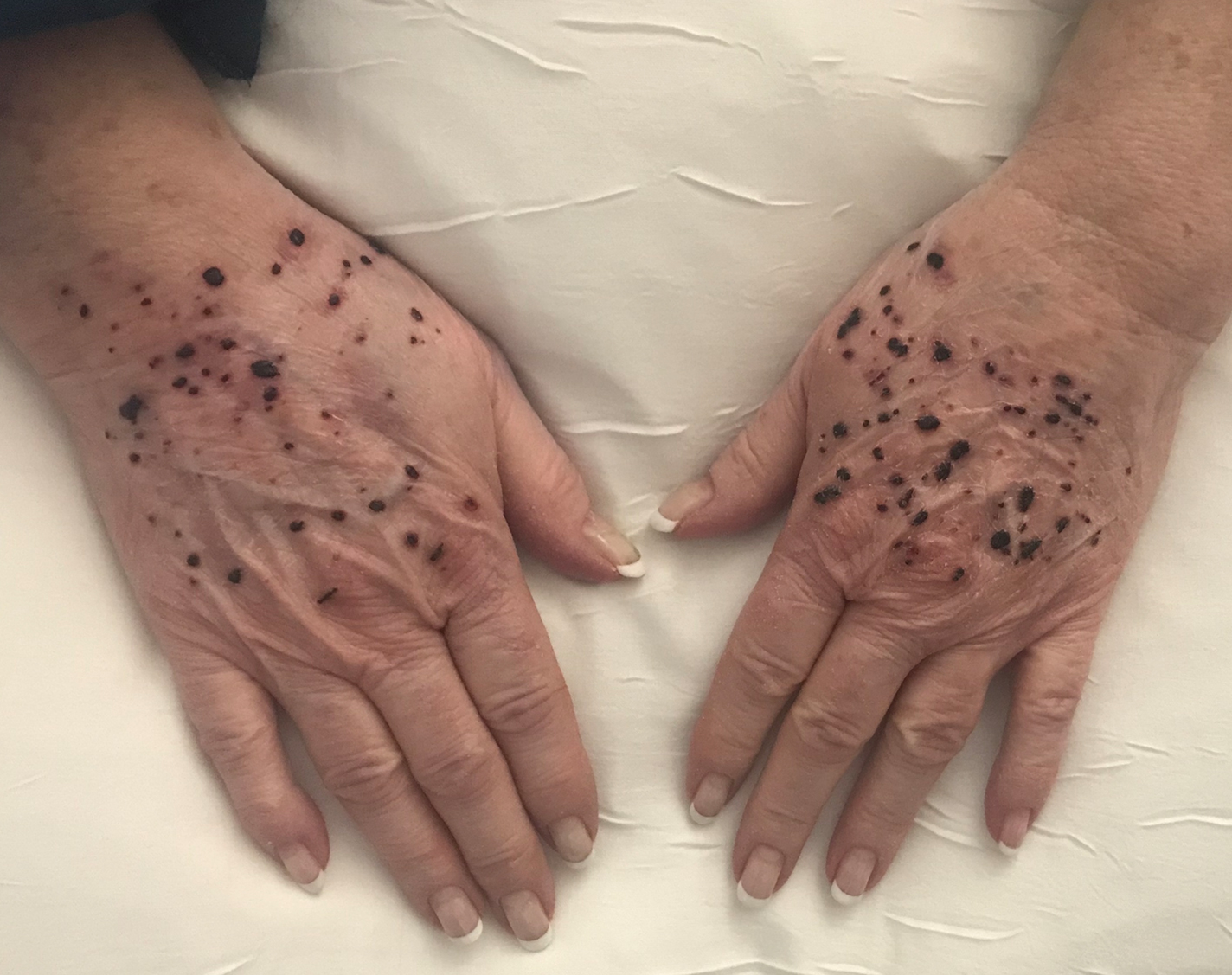
Results of a punch biopsy of one of the hemorrhagic papules revealed a subcorneal hemorrhagic vesicle without underlying vasculitis, vasculopathy, inflammation, or viral changes (Figure). Tissue and blood cultures were sterile. Heparin and platelet factor 4 antibody testing was negative. The patient was diagnosed with heparin induced bullous hemorrhagic dermatosis (BHD). After chest imaging ruled out a pulmonary embolism, anticoagulation therapy was discontinued. Respiratory symptoms improved on antibiotics, and the skin lesions resolved completely within 2 weeks.
Bullous hemorrhagic dermatosis is an uncommon and underrecognized reaction to various anticoagulants. Bullous hemorrhagic dermatosis presents with painless, noninflammatory, hemorrhagic vesicles and bullae occurring at sites distant from anticoagulant administration. The condition was first characterized in 2006 by Perrinaud et al,1 who presented 3 cases in patients treated with heparin and low-molecular-weight heparin. Since then, there have been at least 90 cases reported in the international literature, with elderly men found to be the more affected demographic (male to female ratio, 1.9:1).2 Typically, BHD presents within 1 week of administration of an anticoagulant, but delayed onset has been reported.2 Bullous hemorrhagic dermatosis is most commonly observed with enoxaparin use but also has been described in association with unfractionated heparin, low-molecular-weight heparin products, and warfarin.2
The noninflammatory-appearing hemorrhagic papules and small plaques of BHD generally are seen on the extremities but can occur anywhere on the body including the oral mucosa.3 The differential diagnosis of BHD may include autoimmune vesiculobullous conditions, bullous drug eruptions, herpetic infection, supratherapeutic anticoagulation, porphyria cutanea tarda, amyloidosis, leukocytoclastic vasculitis, angioinvasive infections, and heparin necrosis. Diagnosis of BHD can be made clinically, but a biopsy is useful to exclude other conditions.
Histologically, BHD is characterized by the presence of intraepidermal hemorrhagic bullae without thrombotic, inflammatory, or vasculitic changes. Although heparinrelated skin lesions have been attributed to various mechanisms, including immune-mediated thrombocytopenia, type IV hypersensitivity reactions, type I allergic hypersensitivity reactions, pustulosis, and skin necrosis, the pathogenesis of BHD remains poorly understood.4 The condition has demonstrated koebnerization in some cases.5
In our patient, the absence of histologic inflammation, viral changes, vasculitis, and amyloid deposition helped rule out the other entities in the differential. The absence of heparin and platelet factor 4 antibodies helped exclude heparin necrosis. Direct immunofluorescence testing was not obtained in our patient but may be used to evaluate for an immunobullous etiology.
Management strategies for BHD are variable, and associated evidence is lacking. Treatment of BHD should be considered in the clinical context based on the necessity for anticoagulation and the severity of the eruption. Discontinuation of anticoagulation therapy, if possible, may prevent morbidity in some cases.6 If it is necessary to continue anticoagulation therapy, changing the drug or decreasing the dose are reasonable options. Skin lesions may resolve even if anticoagulation therapy is continued at the same dose.7,8 Concurrent supportive wound care is beneficial.
- Perrinaud A, Jacobi D, Machet MC, et al. Bullous hemorrhagic dermatosis occurring at sites distant from subcutaneous injections of heparin: three cases. J Am Acad Dermatol. 2006;54(suppl):S5-S7.
- Russo A, Curtis S, Balbuena-Merle R, et al. Bullous hemorrhagic dermatosis is an under-recognized side effect of full dose low-molecular weight heparin: a case report and review of the literature [published online July 6, 2018]. Exp Hematol. 2018;7:15.
- Harris HB, Kurth BJ, Lam TK, et al. Heparin-induced bullous hemorrhagic dermatosis confined to the oral mucosa. Cutis. 2019;103:365-366, 370.
- Schindewolf M, Schwaner S, Wolter M, et al. Incidence and causes of heparin-induced skin lesions. CMAJ. 2009;181:477-481.
- Gargallo V, Romero FT, Rodríguez-Peralto JL, et al. Heparin induced bullous hemorrhagic dermatosis at a site distant from the injection. a report of five cases. An Bras Dermatol. 2016;91:857-859.
- Choudhry S, Fishman PM, Hernandez C. Heparin-induced bullous hemorrhagic dermatosis. Cutis. 2013;91:93-98.
- Maldonado Cid P, Moreno Alonso de Celada R, Herranz Pinto P, et al. Bullous hemorrhagic dermatosis at sites distant from subcutaneous injections of heparin: a report of 5 cases. J Am Acad Dermatol. 2012;67:E220-E222.
- Snow SC, Pearson DR, Fathi R, et al. Heparin-induced haemorrhagic bullous dermatosis. Clin Exp Dermatol. 2018;43:393-398.
The Diagnosis: Heparin-Induced Bullous Hemorrhagic Dermatosis
Results of a punch biopsy of one of the hemorrhagic papules revealed a subcorneal hemorrhagic vesicle without underlying vasculitis, vasculopathy, inflammation, or viral changes (Figure). Tissue and blood cultures were sterile. Heparin and platelet factor 4 antibody testing was negative. The patient was diagnosed with heparin induced bullous hemorrhagic dermatosis (BHD). After chest imaging ruled out a pulmonary embolism, anticoagulation therapy was discontinued. Respiratory symptoms improved on antibiotics, and the skin lesions resolved completely within 2 weeks.
Bullous hemorrhagic dermatosis is an uncommon and underrecognized reaction to various anticoagulants. Bullous hemorrhagic dermatosis presents with painless, noninflammatory, hemorrhagic vesicles and bullae occurring at sites distant from anticoagulant administration. The condition was first characterized in 2006 by Perrinaud et al,1 who presented 3 cases in patients treated with heparin and low-molecular-weight heparin. Since then, there have been at least 90 cases reported in the international literature, with elderly men found to be the more affected demographic (male to female ratio, 1.9:1).2 Typically, BHD presents within 1 week of administration of an anticoagulant, but delayed onset has been reported.2 Bullous hemorrhagic dermatosis is most commonly observed with enoxaparin use but also has been described in association with unfractionated heparin, low-molecular-weight heparin products, and warfarin.2
The noninflammatory-appearing hemorrhagic papules and small plaques of BHD generally are seen on the extremities but can occur anywhere on the body including the oral mucosa.3 The differential diagnosis of BHD may include autoimmune vesiculobullous conditions, bullous drug eruptions, herpetic infection, supratherapeutic anticoagulation, porphyria cutanea tarda, amyloidosis, leukocytoclastic vasculitis, angioinvasive infections, and heparin necrosis. Diagnosis of BHD can be made clinically, but a biopsy is useful to exclude other conditions.
Histologically, BHD is characterized by the presence of intraepidermal hemorrhagic bullae without thrombotic, inflammatory, or vasculitic changes. Although heparinrelated skin lesions have been attributed to various mechanisms, including immune-mediated thrombocytopenia, type IV hypersensitivity reactions, type I allergic hypersensitivity reactions, pustulosis, and skin necrosis, the pathogenesis of BHD remains poorly understood.4 The condition has demonstrated koebnerization in some cases.5
In our patient, the absence of histologic inflammation, viral changes, vasculitis, and amyloid deposition helped rule out the other entities in the differential. The absence of heparin and platelet factor 4 antibodies helped exclude heparin necrosis. Direct immunofluorescence testing was not obtained in our patient but may be used to evaluate for an immunobullous etiology.
Management strategies for BHD are variable, and associated evidence is lacking. Treatment of BHD should be considered in the clinical context based on the necessity for anticoagulation and the severity of the eruption. Discontinuation of anticoagulation therapy, if possible, may prevent morbidity in some cases.6 If it is necessary to continue anticoagulation therapy, changing the drug or decreasing the dose are reasonable options. Skin lesions may resolve even if anticoagulation therapy is continued at the same dose.7,8 Concurrent supportive wound care is beneficial.
The Diagnosis: Heparin-Induced Bullous Hemorrhagic Dermatosis
Results of a punch biopsy of one of the hemorrhagic papules revealed a subcorneal hemorrhagic vesicle without underlying vasculitis, vasculopathy, inflammation, or viral changes (Figure). Tissue and blood cultures were sterile. Heparin and platelet factor 4 antibody testing was negative. The patient was diagnosed with heparin induced bullous hemorrhagic dermatosis (BHD). After chest imaging ruled out a pulmonary embolism, anticoagulation therapy was discontinued. Respiratory symptoms improved on antibiotics, and the skin lesions resolved completely within 2 weeks.
Bullous hemorrhagic dermatosis is an uncommon and underrecognized reaction to various anticoagulants. Bullous hemorrhagic dermatosis presents with painless, noninflammatory, hemorrhagic vesicles and bullae occurring at sites distant from anticoagulant administration. The condition was first characterized in 2006 by Perrinaud et al,1 who presented 3 cases in patients treated with heparin and low-molecular-weight heparin. Since then, there have been at least 90 cases reported in the international literature, with elderly men found to be the more affected demographic (male to female ratio, 1.9:1).2 Typically, BHD presents within 1 week of administration of an anticoagulant, but delayed onset has been reported.2 Bullous hemorrhagic dermatosis is most commonly observed with enoxaparin use but also has been described in association with unfractionated heparin, low-molecular-weight heparin products, and warfarin.2
The noninflammatory-appearing hemorrhagic papules and small plaques of BHD generally are seen on the extremities but can occur anywhere on the body including the oral mucosa.3 The differential diagnosis of BHD may include autoimmune vesiculobullous conditions, bullous drug eruptions, herpetic infection, supratherapeutic anticoagulation, porphyria cutanea tarda, amyloidosis, leukocytoclastic vasculitis, angioinvasive infections, and heparin necrosis. Diagnosis of BHD can be made clinically, but a biopsy is useful to exclude other conditions.
Histologically, BHD is characterized by the presence of intraepidermal hemorrhagic bullae without thrombotic, inflammatory, or vasculitic changes. Although heparinrelated skin lesions have been attributed to various mechanisms, including immune-mediated thrombocytopenia, type IV hypersensitivity reactions, type I allergic hypersensitivity reactions, pustulosis, and skin necrosis, the pathogenesis of BHD remains poorly understood.4 The condition has demonstrated koebnerization in some cases.5
In our patient, the absence of histologic inflammation, viral changes, vasculitis, and amyloid deposition helped rule out the other entities in the differential. The absence of heparin and platelet factor 4 antibodies helped exclude heparin necrosis. Direct immunofluorescence testing was not obtained in our patient but may be used to evaluate for an immunobullous etiology.
Management strategies for BHD are variable, and associated evidence is lacking. Treatment of BHD should be considered in the clinical context based on the necessity for anticoagulation and the severity of the eruption. Discontinuation of anticoagulation therapy, if possible, may prevent morbidity in some cases.6 If it is necessary to continue anticoagulation therapy, changing the drug or decreasing the dose are reasonable options. Skin lesions may resolve even if anticoagulation therapy is continued at the same dose.7,8 Concurrent supportive wound care is beneficial.
- Perrinaud A, Jacobi D, Machet MC, et al. Bullous hemorrhagic dermatosis occurring at sites distant from subcutaneous injections of heparin: three cases. J Am Acad Dermatol. 2006;54(suppl):S5-S7.
- Russo A, Curtis S, Balbuena-Merle R, et al. Bullous hemorrhagic dermatosis is an under-recognized side effect of full dose low-molecular weight heparin: a case report and review of the literature [published online July 6, 2018]. Exp Hematol. 2018;7:15.
- Harris HB, Kurth BJ, Lam TK, et al. Heparin-induced bullous hemorrhagic dermatosis confined to the oral mucosa. Cutis. 2019;103:365-366, 370.
- Schindewolf M, Schwaner S, Wolter M, et al. Incidence and causes of heparin-induced skin lesions. CMAJ. 2009;181:477-481.
- Gargallo V, Romero FT, Rodríguez-Peralto JL, et al. Heparin induced bullous hemorrhagic dermatosis at a site distant from the injection. a report of five cases. An Bras Dermatol. 2016;91:857-859.
- Choudhry S, Fishman PM, Hernandez C. Heparin-induced bullous hemorrhagic dermatosis. Cutis. 2013;91:93-98.
- Maldonado Cid P, Moreno Alonso de Celada R, Herranz Pinto P, et al. Bullous hemorrhagic dermatosis at sites distant from subcutaneous injections of heparin: a report of 5 cases. J Am Acad Dermatol. 2012;67:E220-E222.
- Snow SC, Pearson DR, Fathi R, et al. Heparin-induced haemorrhagic bullous dermatosis. Clin Exp Dermatol. 2018;43:393-398.
- Perrinaud A, Jacobi D, Machet MC, et al. Bullous hemorrhagic dermatosis occurring at sites distant from subcutaneous injections of heparin: three cases. J Am Acad Dermatol. 2006;54(suppl):S5-S7.
- Russo A, Curtis S, Balbuena-Merle R, et al. Bullous hemorrhagic dermatosis is an under-recognized side effect of full dose low-molecular weight heparin: a case report and review of the literature [published online July 6, 2018]. Exp Hematol. 2018;7:15.
- Harris HB, Kurth BJ, Lam TK, et al. Heparin-induced bullous hemorrhagic dermatosis confined to the oral mucosa. Cutis. 2019;103:365-366, 370.
- Schindewolf M, Schwaner S, Wolter M, et al. Incidence and causes of heparin-induced skin lesions. CMAJ. 2009;181:477-481.
- Gargallo V, Romero FT, Rodríguez-Peralto JL, et al. Heparin induced bullous hemorrhagic dermatosis at a site distant from the injection. a report of five cases. An Bras Dermatol. 2016;91:857-859.
- Choudhry S, Fishman PM, Hernandez C. Heparin-induced bullous hemorrhagic dermatosis. Cutis. 2013;91:93-98.
- Maldonado Cid P, Moreno Alonso de Celada R, Herranz Pinto P, et al. Bullous hemorrhagic dermatosis at sites distant from subcutaneous injections of heparin: a report of 5 cases. J Am Acad Dermatol. 2012;67:E220-E222.
- Snow SC, Pearson DR, Fathi R, et al. Heparin-induced haemorrhagic bullous dermatosis. Clin Exp Dermatol. 2018;43:393-398.
A 66-year-old woman with a history of granulomatous lung disease managed with methotrexate and prednisone, diabetes mellitus, hypertension, and Grave disease was admitted to the hospital for hypoxic respiratory failure. At admission, treatment was empirically initiated for pneumonia with intravenous ceftriaxone and azithromycin. Given the concern of a pulmonary embolism, intravenous heparin also was initiated. Dermatology was consulted for multiple painless blood blisters that erupted on the hands within 24 hours of admission. Physical examination revealed numerous firm hemorrhagic papules on the dorsal hands. Laboratory workup revealed a slightly elevated white blood cell count (11,800/µL [reference range, 4500–11,000/µL]), a normal stable platelet count (231,000/µL [reference range, 150,000– 350,000/µL]), and a normal international normalized ratio.