User login
Isotretinoin Meets COVID-19: Revisiting a Fragmented Paradigm
We cannot solve our problems with the same thinking we used when we created them.
Albert Einstein
Amidst the myriad of disruptions and corollary solutions budding from the ongoing global COVID-19 pandemic, management of acne with isotretinoin underwent a makeover. Firstly, as with any pharmaceutical prescribed in the last 1 to 2 years, patients asked the compelling question, “Will this prescription put me at higher risk for COVID-19?”, resulting in a complex set of answers from both clinical and basic science perspectives. Further, the practical use of telemedicine for clinical visits and pregnancy test reporting altered the shape of isotretinoin physician-patient communication and follow-up. Finally, the combination of these circumstances spurred us to revisit common quandaries in prescribing this drug: Can we trust what patients tell us when they are taking isotretinoin? Do we need to monitor laboratory values and follow patients on isotretinoin as closely and as frequently as we have in the past? Does the Risk Evaluation and Mitigation Strategy (REMS) program of iPLEDGE hold true utility?
Impact of COVID-19 on Isotretinoin Use
Isotretinoin may have varying influence on the ease of host entry and virulence of COVID-19. Because the majority of patients experience some degree of mucous membrane desiccation on isotretinoin, it originally was postulated that disruption of the nasal mucosa, thereby uncovering the basal epithelial layer where angiotensin-converting enzyme 2 (ACE2) receptors are expressed, could increase the risk for viral invasion, as ACE2 is the host receptor for COVID-19 entry.1,2 On the other hand, a study of 672 medications and their effect on regulation of ACE2 levels stratified isotretinoin in the highest category of ACE2 downregulators, therefore theoretically preventing cellular entry and replication of the virus.3 In conferring with many of my colleagues and reviewing available literature, I found that these data did not summarily deter providers from initiating or continuing isotretinoin during the pandemic, and research is ongoing to particularly earmark isotretinoin as a possible COVID-19 therapy option.4,5 Despite this, and despite the lower risk for COVID-19 in the customary isotretinoin adolescent and young adult age range, an Italian study reported that 14.7% of patients (5/34) prematurely interrupted isotretinoin therapy during lockdown because of fear of COVID-19 infection.6 Data also suggest that college towns (akin to where I practice, rife with isotretinoin-eligible patients) reflected higher COVID-19 infection and death rates, likely due to dense cohabitation and intermittent migration of students and staff to and from campuses and within their communities.7 Approximately 30% of my patients on isotretinoin in the last 18 months reported having COVID-19 at some point during the pandemic, though no data exist to guide us on whether isotretinoin should be discontinued in this scenario; my patients typically continued the drug unless their primary health care team discouraged it, and in those cases, all of them resumed it after COVID-19 symptomatology resolved.
Last spring, the US Department of Health and Human Services and the US Food and Drug Administration announced that health care professionals who prescribe and/or dispense drugs subject to REMS with laboratory testing or imaging requirements should consider whether there are compelling reasons not to complete the required testing/imaging during the current public health emergency and use their best medical judgment in weighing the benefits and risks of continuing treatment in the absence of these tests. It also was stressed that prescribers should effectively communicate with their patients regarding these benefits, risks, and altered protocols.8 Further, the iPLEDGE program concurred that telemedicine was an acceptable visit type for both initiating and maintaining isotretinoin, and home pregnancy tests were valid for females of childbearing potential if an accurate testing date and results were communicated by patients to the prescriber in the required reporting windows.9 This allowed dermatologists to foster what was one of our most important roles as outpatient clinicians during the pandemic: to maintain normalcy, continuity, and support for as many patients as possible.
Isotretinoin and Telemedicine
During the pandemic, continuation of isotretinoin therapy proved easier than initiating it, given that patients could access and maintain a clear connection to the online visit platform, display understanding of the REMS mandates (along with a guardian present for a minor), perform a home pregnancy test and report the result followed by the quiz (for females), and collect the prescription in the allotted window. For new patients, the absence of a detailed in-person examination and rapport with the patient (and guardians when applicable) as well as misalignment of the date of iPLEDGE registration with the timing of the pregnancy test results and prescribing window were more onerous using digital or mailed versions of consent forms and photodocumentation of urine pregnancy test results. This tangle of requirements perpetuated missed prescribing windows, increased patient portal and phone messages, resulted in more time on the phone with the iPLEDGE help desk, and intensified angst for clinical staff.
These telemedicine visits also required validation of the patient’s geographic location to verify the billability of the visit and whether the patient was in a secure location to have a US Health Insurance Portability and Accountability Act–compliant conversation as well as the abstract notion that the timing and result of the pregnancy tests for females reflected a true nonpregnant state.10,11 Verification of the pregnancy tests in these situations was approached by either the patient reporting the outcome verbally or displaying the pregnancy test kit result in a video or photograph form for the medical record, all of which leave room for error, doubt, and lower sensitivity than laboratory-based collection. That being said, the increased implementation of telemedicine visits during the pandemic sustained patient access, decreased cost with less laboratory testing and reduced time away from work or school, and resulted in high patient satisfaction with their care.12 Additionally, it allowed providers to continue to more comfortably inch away from frequent in-person serologic cholesterol and hepatic testing during therapy based on mounting data that it is not indicated.13
Accordingly, the complicated concepts of trust, practicality, and sustainability for the safe and effective management of isotretinoin patients re-emerged. For example, prior to COVID-19, we trusted patients who said they were regularly taking their oral contraceptives or were truly practicing abstinence as a form of contraception. During the pandemic, we then added a layer of trust with home pregnancy test reporting. If the patient or guardian signed the isotretinoin consent form and understood the risks of the medication, ideally the physician-patient relationship fostered the optimal goals of honest conversation, adherence to the drug, safety, and clear skin. However, there is yet another trust assay: iPLEDGE, in turn, trusts that we are reporting patient data accurately, provoking us to reiterate questions we asked ourselves before the pandemic. Is the extra provider and staff clerical work and validation necessary, compounded by prior data that iPLEDGE’s capacity to limit pregnancy-related morbidity with isotretinoin has been called into question in the last decade?14 Do males need to be followed every month? Is laboratory monitoring still necessary for all isotretinoin candidates? Will post–COVID-19 data show that during various versions of the lockdown, an increased number of isotretinoin patients developed unmonitored morbidity, including transaminitis, hypertriglyceridemia, and an increase in pregnancies? How long will telemedicine visits for isotretinoin be reimbursable beyond the pandemic? Are there other models to enhance and improve isotretinoin teledermatology and compliance?15
Final Thoughts
Dermatologists’ experience managing high volumes of isotretinoin patients paired with the creativity to maintain meaningful (and truthful) patient connections and decrease administrative burden lie front and center in 2021. Because the COVID-19 pandemic remains ambient with a dearth of data to guide us, I pose the questions above as points for commiseration and catapults for future study, discussion, collaboration, and innovation. Perhaps the neo–COVID-19 world provided us with the spark we needed to metaphorically clean up the dusty isotretinoin tenets that have frayed our time and patience so we can maintain excellent care for this worthy population.
- Hamming I, Timens W, Bulthuis MLC, et al. Tissue disruption of ACE2 protein, the functional receptor for SARS coronavirus. a first step in understanding SARS pathogenesis. J Pathol. 2004;203:631-637.
- British Association of Dermatologists. COVID-19—isotretinoin guidance. Published March 26, 2020. Accessed June 21, 2021. https://www.bad.org.uk/shared/get-file.ashx?itemtype=document&id=6661
- Sinha S, Cheng K, Schäffer AA, et al. In vitro and in vivo identification of clinically approved drugs that modify ACE2 expression. Mol Syst Biol. 2020;16:E9628.
- Öǧüt ND, Kutlu Ö, Erbaǧcı E. Oral isotretinoin treatment in patients with acne vulgaris during the COVID-19 pandemic: a retrospective cohort study in a tertiary care hospital [published online April 22, 2021]. J Cosmet Dermatol. doi:10.1111/jocd.14168
- Isotretinoin in treatment of COVID-19. National Library of Medicine website. ClinicalTrials.gov identifier: NCT04361422. Updated September 23, 2020. Accessed June 21, 2021. https://clinicaltrials.gov/ct2/show/NCT04361422
- Donnarumma M, Nocerino M, Lauro W, et al. Isotretinoin in acne treatment during the coronavirus disease 2019 (COVID-19): a retrospective analysis of adherence to therapy and side effects [published online December 22, 2020]. Dermatol Ther. 2021;34:E14677.
- Ivory D, Gebeloff R, Mervosh S. Young people have less COVID-19 risk, but in college towns, deaths rose fast. The New York Times. December 12, 2020. Accessed June 21, 2021. https://www.nytimes.com/2020/12/12/us/covid-colleges-nursing-homes.html
- US Food and Drug Administration. Coronavirus (COVID-19) update: FDA provides update on patient access to certain REMS drugs during COVID-19 public health emergency. Published March 22, 2020. Accessed June 21, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-provides-update-patient-access-certain-rems-drugs-during-covid-19
- Haelle T. iPledge allows at-home pregnancy tests during pandemic. Dermatology News. Published April 3, 2020. Accessed June 28, 2021. https://www.mdedge.com/dermatology/article/220186/acne/ipledge-allows-home-pregnancy-tests-during-pandemic
- Bressler MY, Siegel DM, Markowitz O. Virtual dermatology: a COVID-19 update. Cutis. 2020;105:163-164; E2.
- Telemedicine key issues and policy. Federation of State Medical Boards website. Accessed June 28, 2021. https://www.fsmb.org/advocacy/telemedicine
- Ruggiero A, Megna M, Annunziata MC, et al. Teledermatology for acne during COVID-19: high patients’ satisfaction in spite of the emergency. J Eur Acad Dermatol Venereol. 2020;34:E662-E663.
- Barbieri JS, Shin DB, Wang S, et al. The clinical utility of laboratory monitoring during isotretinoin therapy for acne and changes to monitoring practices over time. J Am Acad Dermatol. 2020;82:72-79.
- Tkachenko E, Singer S, Sharma P, et al. US Food and Drug Administration reports of pregnancy and pregnancy-related adverse events associated with isotretinoin. JAMA Dermatol. 2019;155:1175-1179.
- Das S, et al. Asynchronous telemedicine for isotretinoin management: a direct care pilot [published online January 21, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.01.039
We cannot solve our problems with the same thinking we used when we created them.
Albert Einstein
Amidst the myriad of disruptions and corollary solutions budding from the ongoing global COVID-19 pandemic, management of acne with isotretinoin underwent a makeover. Firstly, as with any pharmaceutical prescribed in the last 1 to 2 years, patients asked the compelling question, “Will this prescription put me at higher risk for COVID-19?”, resulting in a complex set of answers from both clinical and basic science perspectives. Further, the practical use of telemedicine for clinical visits and pregnancy test reporting altered the shape of isotretinoin physician-patient communication and follow-up. Finally, the combination of these circumstances spurred us to revisit common quandaries in prescribing this drug: Can we trust what patients tell us when they are taking isotretinoin? Do we need to monitor laboratory values and follow patients on isotretinoin as closely and as frequently as we have in the past? Does the Risk Evaluation and Mitigation Strategy (REMS) program of iPLEDGE hold true utility?
Impact of COVID-19 on Isotretinoin Use
Isotretinoin may have varying influence on the ease of host entry and virulence of COVID-19. Because the majority of patients experience some degree of mucous membrane desiccation on isotretinoin, it originally was postulated that disruption of the nasal mucosa, thereby uncovering the basal epithelial layer where angiotensin-converting enzyme 2 (ACE2) receptors are expressed, could increase the risk for viral invasion, as ACE2 is the host receptor for COVID-19 entry.1,2 On the other hand, a study of 672 medications and their effect on regulation of ACE2 levels stratified isotretinoin in the highest category of ACE2 downregulators, therefore theoretically preventing cellular entry and replication of the virus.3 In conferring with many of my colleagues and reviewing available literature, I found that these data did not summarily deter providers from initiating or continuing isotretinoin during the pandemic, and research is ongoing to particularly earmark isotretinoin as a possible COVID-19 therapy option.4,5 Despite this, and despite the lower risk for COVID-19 in the customary isotretinoin adolescent and young adult age range, an Italian study reported that 14.7% of patients (5/34) prematurely interrupted isotretinoin therapy during lockdown because of fear of COVID-19 infection.6 Data also suggest that college towns (akin to where I practice, rife with isotretinoin-eligible patients) reflected higher COVID-19 infection and death rates, likely due to dense cohabitation and intermittent migration of students and staff to and from campuses and within their communities.7 Approximately 30% of my patients on isotretinoin in the last 18 months reported having COVID-19 at some point during the pandemic, though no data exist to guide us on whether isotretinoin should be discontinued in this scenario; my patients typically continued the drug unless their primary health care team discouraged it, and in those cases, all of them resumed it after COVID-19 symptomatology resolved.
Last spring, the US Department of Health and Human Services and the US Food and Drug Administration announced that health care professionals who prescribe and/or dispense drugs subject to REMS with laboratory testing or imaging requirements should consider whether there are compelling reasons not to complete the required testing/imaging during the current public health emergency and use their best medical judgment in weighing the benefits and risks of continuing treatment in the absence of these tests. It also was stressed that prescribers should effectively communicate with their patients regarding these benefits, risks, and altered protocols.8 Further, the iPLEDGE program concurred that telemedicine was an acceptable visit type for both initiating and maintaining isotretinoin, and home pregnancy tests were valid for females of childbearing potential if an accurate testing date and results were communicated by patients to the prescriber in the required reporting windows.9 This allowed dermatologists to foster what was one of our most important roles as outpatient clinicians during the pandemic: to maintain normalcy, continuity, and support for as many patients as possible.
Isotretinoin and Telemedicine
During the pandemic, continuation of isotretinoin therapy proved easier than initiating it, given that patients could access and maintain a clear connection to the online visit platform, display understanding of the REMS mandates (along with a guardian present for a minor), perform a home pregnancy test and report the result followed by the quiz (for females), and collect the prescription in the allotted window. For new patients, the absence of a detailed in-person examination and rapport with the patient (and guardians when applicable) as well as misalignment of the date of iPLEDGE registration with the timing of the pregnancy test results and prescribing window were more onerous using digital or mailed versions of consent forms and photodocumentation of urine pregnancy test results. This tangle of requirements perpetuated missed prescribing windows, increased patient portal and phone messages, resulted in more time on the phone with the iPLEDGE help desk, and intensified angst for clinical staff.
These telemedicine visits also required validation of the patient’s geographic location to verify the billability of the visit and whether the patient was in a secure location to have a US Health Insurance Portability and Accountability Act–compliant conversation as well as the abstract notion that the timing and result of the pregnancy tests for females reflected a true nonpregnant state.10,11 Verification of the pregnancy tests in these situations was approached by either the patient reporting the outcome verbally or displaying the pregnancy test kit result in a video or photograph form for the medical record, all of which leave room for error, doubt, and lower sensitivity than laboratory-based collection. That being said, the increased implementation of telemedicine visits during the pandemic sustained patient access, decreased cost with less laboratory testing and reduced time away from work or school, and resulted in high patient satisfaction with their care.12 Additionally, it allowed providers to continue to more comfortably inch away from frequent in-person serologic cholesterol and hepatic testing during therapy based on mounting data that it is not indicated.13
Accordingly, the complicated concepts of trust, practicality, and sustainability for the safe and effective management of isotretinoin patients re-emerged. For example, prior to COVID-19, we trusted patients who said they were regularly taking their oral contraceptives or were truly practicing abstinence as a form of contraception. During the pandemic, we then added a layer of trust with home pregnancy test reporting. If the patient or guardian signed the isotretinoin consent form and understood the risks of the medication, ideally the physician-patient relationship fostered the optimal goals of honest conversation, adherence to the drug, safety, and clear skin. However, there is yet another trust assay: iPLEDGE, in turn, trusts that we are reporting patient data accurately, provoking us to reiterate questions we asked ourselves before the pandemic. Is the extra provider and staff clerical work and validation necessary, compounded by prior data that iPLEDGE’s capacity to limit pregnancy-related morbidity with isotretinoin has been called into question in the last decade?14 Do males need to be followed every month? Is laboratory monitoring still necessary for all isotretinoin candidates? Will post–COVID-19 data show that during various versions of the lockdown, an increased number of isotretinoin patients developed unmonitored morbidity, including transaminitis, hypertriglyceridemia, and an increase in pregnancies? How long will telemedicine visits for isotretinoin be reimbursable beyond the pandemic? Are there other models to enhance and improve isotretinoin teledermatology and compliance?15
Final Thoughts
Dermatologists’ experience managing high volumes of isotretinoin patients paired with the creativity to maintain meaningful (and truthful) patient connections and decrease administrative burden lie front and center in 2021. Because the COVID-19 pandemic remains ambient with a dearth of data to guide us, I pose the questions above as points for commiseration and catapults for future study, discussion, collaboration, and innovation. Perhaps the neo–COVID-19 world provided us with the spark we needed to metaphorically clean up the dusty isotretinoin tenets that have frayed our time and patience so we can maintain excellent care for this worthy population.
We cannot solve our problems with the same thinking we used when we created them.
Albert Einstein
Amidst the myriad of disruptions and corollary solutions budding from the ongoing global COVID-19 pandemic, management of acne with isotretinoin underwent a makeover. Firstly, as with any pharmaceutical prescribed in the last 1 to 2 years, patients asked the compelling question, “Will this prescription put me at higher risk for COVID-19?”, resulting in a complex set of answers from both clinical and basic science perspectives. Further, the practical use of telemedicine for clinical visits and pregnancy test reporting altered the shape of isotretinoin physician-patient communication and follow-up. Finally, the combination of these circumstances spurred us to revisit common quandaries in prescribing this drug: Can we trust what patients tell us when they are taking isotretinoin? Do we need to monitor laboratory values and follow patients on isotretinoin as closely and as frequently as we have in the past? Does the Risk Evaluation and Mitigation Strategy (REMS) program of iPLEDGE hold true utility?
Impact of COVID-19 on Isotretinoin Use
Isotretinoin may have varying influence on the ease of host entry and virulence of COVID-19. Because the majority of patients experience some degree of mucous membrane desiccation on isotretinoin, it originally was postulated that disruption of the nasal mucosa, thereby uncovering the basal epithelial layer where angiotensin-converting enzyme 2 (ACE2) receptors are expressed, could increase the risk for viral invasion, as ACE2 is the host receptor for COVID-19 entry.1,2 On the other hand, a study of 672 medications and their effect on regulation of ACE2 levels stratified isotretinoin in the highest category of ACE2 downregulators, therefore theoretically preventing cellular entry and replication of the virus.3 In conferring with many of my colleagues and reviewing available literature, I found that these data did not summarily deter providers from initiating or continuing isotretinoin during the pandemic, and research is ongoing to particularly earmark isotretinoin as a possible COVID-19 therapy option.4,5 Despite this, and despite the lower risk for COVID-19 in the customary isotretinoin adolescent and young adult age range, an Italian study reported that 14.7% of patients (5/34) prematurely interrupted isotretinoin therapy during lockdown because of fear of COVID-19 infection.6 Data also suggest that college towns (akin to where I practice, rife with isotretinoin-eligible patients) reflected higher COVID-19 infection and death rates, likely due to dense cohabitation and intermittent migration of students and staff to and from campuses and within their communities.7 Approximately 30% of my patients on isotretinoin in the last 18 months reported having COVID-19 at some point during the pandemic, though no data exist to guide us on whether isotretinoin should be discontinued in this scenario; my patients typically continued the drug unless their primary health care team discouraged it, and in those cases, all of them resumed it after COVID-19 symptomatology resolved.
Last spring, the US Department of Health and Human Services and the US Food and Drug Administration announced that health care professionals who prescribe and/or dispense drugs subject to REMS with laboratory testing or imaging requirements should consider whether there are compelling reasons not to complete the required testing/imaging during the current public health emergency and use their best medical judgment in weighing the benefits and risks of continuing treatment in the absence of these tests. It also was stressed that prescribers should effectively communicate with their patients regarding these benefits, risks, and altered protocols.8 Further, the iPLEDGE program concurred that telemedicine was an acceptable visit type for both initiating and maintaining isotretinoin, and home pregnancy tests were valid for females of childbearing potential if an accurate testing date and results were communicated by patients to the prescriber in the required reporting windows.9 This allowed dermatologists to foster what was one of our most important roles as outpatient clinicians during the pandemic: to maintain normalcy, continuity, and support for as many patients as possible.
Isotretinoin and Telemedicine
During the pandemic, continuation of isotretinoin therapy proved easier than initiating it, given that patients could access and maintain a clear connection to the online visit platform, display understanding of the REMS mandates (along with a guardian present for a minor), perform a home pregnancy test and report the result followed by the quiz (for females), and collect the prescription in the allotted window. For new patients, the absence of a detailed in-person examination and rapport with the patient (and guardians when applicable) as well as misalignment of the date of iPLEDGE registration with the timing of the pregnancy test results and prescribing window were more onerous using digital or mailed versions of consent forms and photodocumentation of urine pregnancy test results. This tangle of requirements perpetuated missed prescribing windows, increased patient portal and phone messages, resulted in more time on the phone with the iPLEDGE help desk, and intensified angst for clinical staff.
These telemedicine visits also required validation of the patient’s geographic location to verify the billability of the visit and whether the patient was in a secure location to have a US Health Insurance Portability and Accountability Act–compliant conversation as well as the abstract notion that the timing and result of the pregnancy tests for females reflected a true nonpregnant state.10,11 Verification of the pregnancy tests in these situations was approached by either the patient reporting the outcome verbally or displaying the pregnancy test kit result in a video or photograph form for the medical record, all of which leave room for error, doubt, and lower sensitivity than laboratory-based collection. That being said, the increased implementation of telemedicine visits during the pandemic sustained patient access, decreased cost with less laboratory testing and reduced time away from work or school, and resulted in high patient satisfaction with their care.12 Additionally, it allowed providers to continue to more comfortably inch away from frequent in-person serologic cholesterol and hepatic testing during therapy based on mounting data that it is not indicated.13
Accordingly, the complicated concepts of trust, practicality, and sustainability for the safe and effective management of isotretinoin patients re-emerged. For example, prior to COVID-19, we trusted patients who said they were regularly taking their oral contraceptives or were truly practicing abstinence as a form of contraception. During the pandemic, we then added a layer of trust with home pregnancy test reporting. If the patient or guardian signed the isotretinoin consent form and understood the risks of the medication, ideally the physician-patient relationship fostered the optimal goals of honest conversation, adherence to the drug, safety, and clear skin. However, there is yet another trust assay: iPLEDGE, in turn, trusts that we are reporting patient data accurately, provoking us to reiterate questions we asked ourselves before the pandemic. Is the extra provider and staff clerical work and validation necessary, compounded by prior data that iPLEDGE’s capacity to limit pregnancy-related morbidity with isotretinoin has been called into question in the last decade?14 Do males need to be followed every month? Is laboratory monitoring still necessary for all isotretinoin candidates? Will post–COVID-19 data show that during various versions of the lockdown, an increased number of isotretinoin patients developed unmonitored morbidity, including transaminitis, hypertriglyceridemia, and an increase in pregnancies? How long will telemedicine visits for isotretinoin be reimbursable beyond the pandemic? Are there other models to enhance and improve isotretinoin teledermatology and compliance?15
Final Thoughts
Dermatologists’ experience managing high volumes of isotretinoin patients paired with the creativity to maintain meaningful (and truthful) patient connections and decrease administrative burden lie front and center in 2021. Because the COVID-19 pandemic remains ambient with a dearth of data to guide us, I pose the questions above as points for commiseration and catapults for future study, discussion, collaboration, and innovation. Perhaps the neo–COVID-19 world provided us with the spark we needed to metaphorically clean up the dusty isotretinoin tenets that have frayed our time and patience so we can maintain excellent care for this worthy population.
- Hamming I, Timens W, Bulthuis MLC, et al. Tissue disruption of ACE2 protein, the functional receptor for SARS coronavirus. a first step in understanding SARS pathogenesis. J Pathol. 2004;203:631-637.
- British Association of Dermatologists. COVID-19—isotretinoin guidance. Published March 26, 2020. Accessed June 21, 2021. https://www.bad.org.uk/shared/get-file.ashx?itemtype=document&id=6661
- Sinha S, Cheng K, Schäffer AA, et al. In vitro and in vivo identification of clinically approved drugs that modify ACE2 expression. Mol Syst Biol. 2020;16:E9628.
- Öǧüt ND, Kutlu Ö, Erbaǧcı E. Oral isotretinoin treatment in patients with acne vulgaris during the COVID-19 pandemic: a retrospective cohort study in a tertiary care hospital [published online April 22, 2021]. J Cosmet Dermatol. doi:10.1111/jocd.14168
- Isotretinoin in treatment of COVID-19. National Library of Medicine website. ClinicalTrials.gov identifier: NCT04361422. Updated September 23, 2020. Accessed June 21, 2021. https://clinicaltrials.gov/ct2/show/NCT04361422
- Donnarumma M, Nocerino M, Lauro W, et al. Isotretinoin in acne treatment during the coronavirus disease 2019 (COVID-19): a retrospective analysis of adherence to therapy and side effects [published online December 22, 2020]. Dermatol Ther. 2021;34:E14677.
- Ivory D, Gebeloff R, Mervosh S. Young people have less COVID-19 risk, but in college towns, deaths rose fast. The New York Times. December 12, 2020. Accessed June 21, 2021. https://www.nytimes.com/2020/12/12/us/covid-colleges-nursing-homes.html
- US Food and Drug Administration. Coronavirus (COVID-19) update: FDA provides update on patient access to certain REMS drugs during COVID-19 public health emergency. Published March 22, 2020. Accessed June 21, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-provides-update-patient-access-certain-rems-drugs-during-covid-19
- Haelle T. iPledge allows at-home pregnancy tests during pandemic. Dermatology News. Published April 3, 2020. Accessed June 28, 2021. https://www.mdedge.com/dermatology/article/220186/acne/ipledge-allows-home-pregnancy-tests-during-pandemic
- Bressler MY, Siegel DM, Markowitz O. Virtual dermatology: a COVID-19 update. Cutis. 2020;105:163-164; E2.
- Telemedicine key issues and policy. Federation of State Medical Boards website. Accessed June 28, 2021. https://www.fsmb.org/advocacy/telemedicine
- Ruggiero A, Megna M, Annunziata MC, et al. Teledermatology for acne during COVID-19: high patients’ satisfaction in spite of the emergency. J Eur Acad Dermatol Venereol. 2020;34:E662-E663.
- Barbieri JS, Shin DB, Wang S, et al. The clinical utility of laboratory monitoring during isotretinoin therapy for acne and changes to monitoring practices over time. J Am Acad Dermatol. 2020;82:72-79.
- Tkachenko E, Singer S, Sharma P, et al. US Food and Drug Administration reports of pregnancy and pregnancy-related adverse events associated with isotretinoin. JAMA Dermatol. 2019;155:1175-1179.
- Das S, et al. Asynchronous telemedicine for isotretinoin management: a direct care pilot [published online January 21, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.01.039
- Hamming I, Timens W, Bulthuis MLC, et al. Tissue disruption of ACE2 protein, the functional receptor for SARS coronavirus. a first step in understanding SARS pathogenesis. J Pathol. 2004;203:631-637.
- British Association of Dermatologists. COVID-19—isotretinoin guidance. Published March 26, 2020. Accessed June 21, 2021. https://www.bad.org.uk/shared/get-file.ashx?itemtype=document&id=6661
- Sinha S, Cheng K, Schäffer AA, et al. In vitro and in vivo identification of clinically approved drugs that modify ACE2 expression. Mol Syst Biol. 2020;16:E9628.
- Öǧüt ND, Kutlu Ö, Erbaǧcı E. Oral isotretinoin treatment in patients with acne vulgaris during the COVID-19 pandemic: a retrospective cohort study in a tertiary care hospital [published online April 22, 2021]. J Cosmet Dermatol. doi:10.1111/jocd.14168
- Isotretinoin in treatment of COVID-19. National Library of Medicine website. ClinicalTrials.gov identifier: NCT04361422. Updated September 23, 2020. Accessed June 21, 2021. https://clinicaltrials.gov/ct2/show/NCT04361422
- Donnarumma M, Nocerino M, Lauro W, et al. Isotretinoin in acne treatment during the coronavirus disease 2019 (COVID-19): a retrospective analysis of adherence to therapy and side effects [published online December 22, 2020]. Dermatol Ther. 2021;34:E14677.
- Ivory D, Gebeloff R, Mervosh S. Young people have less COVID-19 risk, but in college towns, deaths rose fast. The New York Times. December 12, 2020. Accessed June 21, 2021. https://www.nytimes.com/2020/12/12/us/covid-colleges-nursing-homes.html
- US Food and Drug Administration. Coronavirus (COVID-19) update: FDA provides update on patient access to certain REMS drugs during COVID-19 public health emergency. Published March 22, 2020. Accessed June 21, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-provides-update-patient-access-certain-rems-drugs-during-covid-19
- Haelle T. iPledge allows at-home pregnancy tests during pandemic. Dermatology News. Published April 3, 2020. Accessed June 28, 2021. https://www.mdedge.com/dermatology/article/220186/acne/ipledge-allows-home-pregnancy-tests-during-pandemic
- Bressler MY, Siegel DM, Markowitz O. Virtual dermatology: a COVID-19 update. Cutis. 2020;105:163-164; E2.
- Telemedicine key issues and policy. Federation of State Medical Boards website. Accessed June 28, 2021. https://www.fsmb.org/advocacy/telemedicine
- Ruggiero A, Megna M, Annunziata MC, et al. Teledermatology for acne during COVID-19: high patients’ satisfaction in spite of the emergency. J Eur Acad Dermatol Venereol. 2020;34:E662-E663.
- Barbieri JS, Shin DB, Wang S, et al. The clinical utility of laboratory monitoring during isotretinoin therapy for acne and changes to monitoring practices over time. J Am Acad Dermatol. 2020;82:72-79.
- Tkachenko E, Singer S, Sharma P, et al. US Food and Drug Administration reports of pregnancy and pregnancy-related adverse events associated with isotretinoin. JAMA Dermatol. 2019;155:1175-1179.
- Das S, et al. Asynchronous telemedicine for isotretinoin management: a direct care pilot [published online January 21, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.01.039
Keratotic Papule on the Abdomen
The Diagnosis: Hypergranulotic Dyscornification
Hypergranulotic dyscornification (HD) is a rarely reported reaction pattern present in benign solitary keratoses with only few reports to date. It may be an underrecognized reaction pattern based on the paucity of reported cases as well as the histologic similarities to other entities. It has been hypothesized that this pattern reflects an underlying keratin mutation or disorder of keratinization.1
Clinically, HD most commonly presents as a waxy, tan-colored, solitary keratosis generally found on the lower limbs, trunk, or back in individuals aged 20 to 60 years.1,2 Histopathology shows marked hyperkeratosis, papillomatosis, and clumped basophilic keratohyalin granules within the corneocytes with digitated epidermal hyperplasia. There is abnormal cornification across the entire lesion with papillomatosis and marked hypergranulosis.3 There often are homogeneous orthokeratotic mounds of large, dull, eosinophilic-staining anucleate keratinocytes that are sharply demarcated from the thickened granular layer.1,2 Within the spinous, granular, and corneal layers, there is a pale, gray-staining, basophilic, cytoplasmic substance intercellularly.1
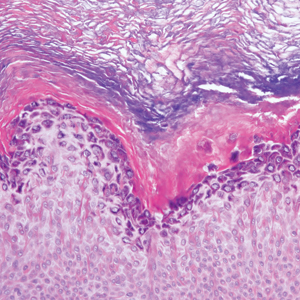
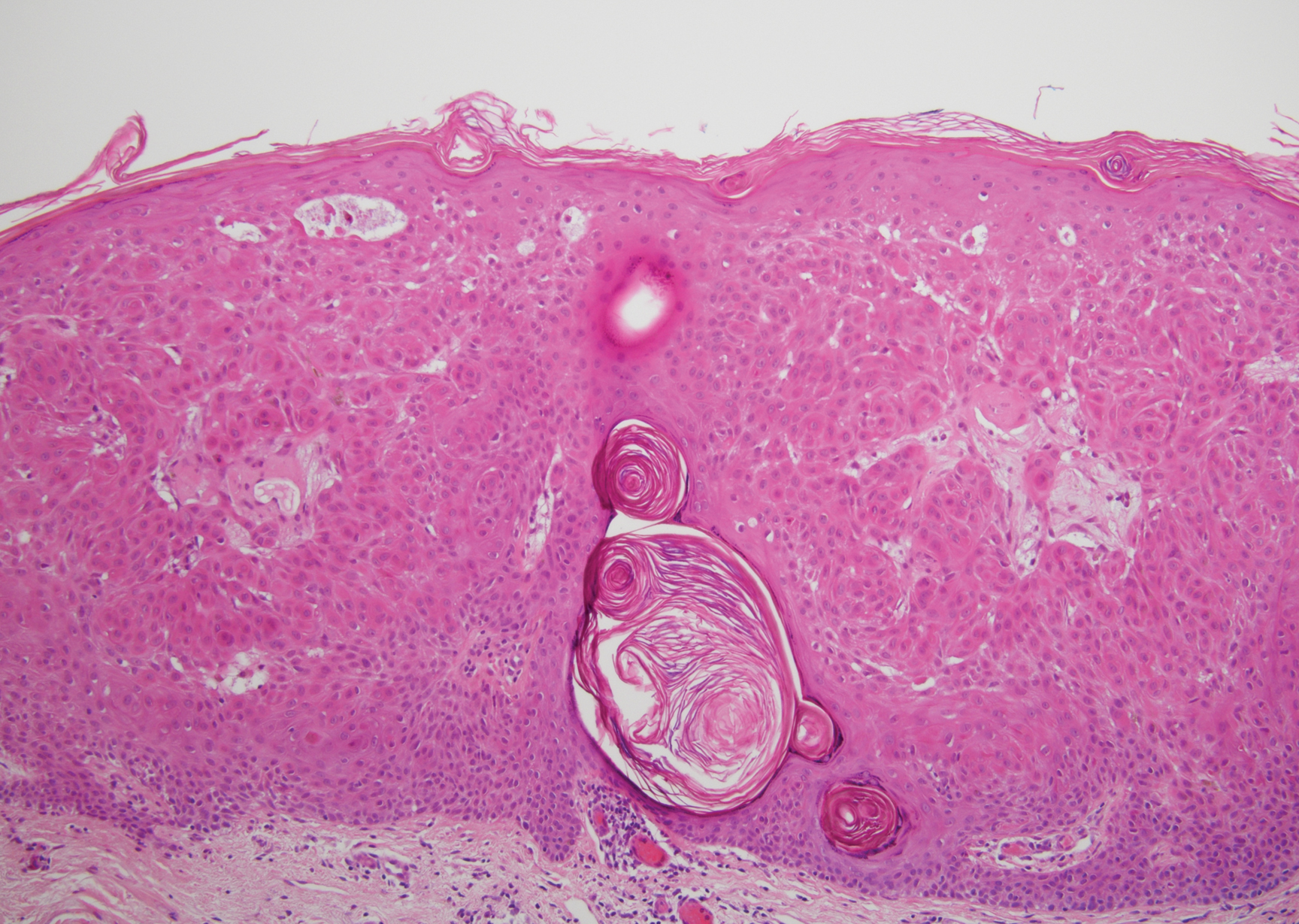
Histopathologically, HD may be mistaken for several other entities both benign and malignant.1 Epidermolytic hyperkeratosis can be a genetic disorder, an incidental finding in a variety of skin conditions, or an isolated lesion.4 The genetic syndrome, caused by mutation in keratins 1 or 10, clinically presents with hyperkeratosis, erosions, blisters, and thickening of the epidermis, often with a corrugated appearance. Epidermal nevi findings often are seen in conjunction with histologic changes of epidermolytic hyperkeratosis caused by mutation. Solitary lesions also can resemble seborrheic keratosis or verruca. In all examples of epidermolytic hyperkeratosis, the histopathologic findings are identical.4 The granular layer is thickened, and coarse keratohyalin granules aggregate in the suprabasal cells.5 There is acantholysis with perinuclear vacuolization in the spinous and granular layers with characteristic pale cytoplasmic areas devoid of keratin filaments (Figure 1). The basal layer may be hyperproliferative.5
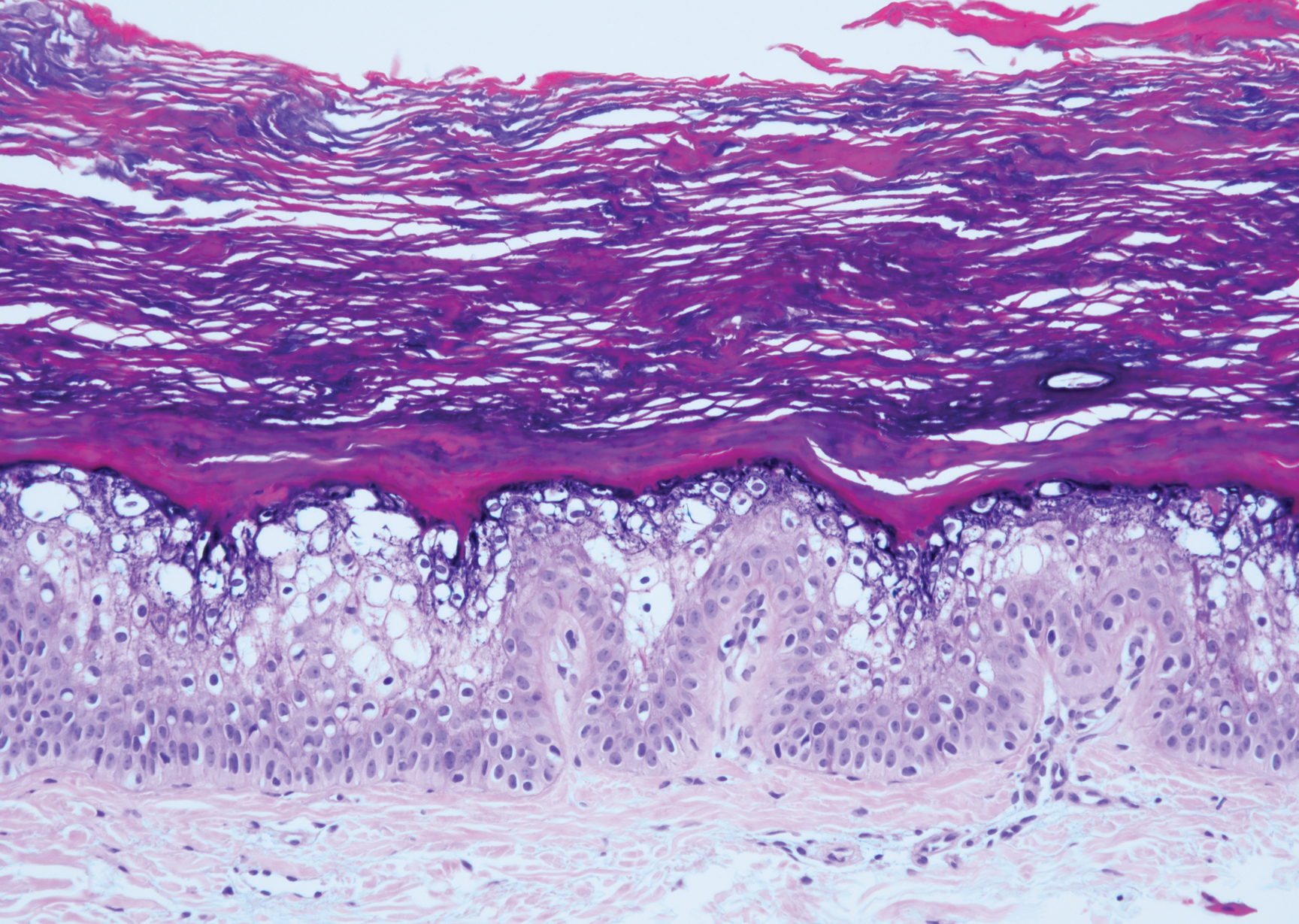
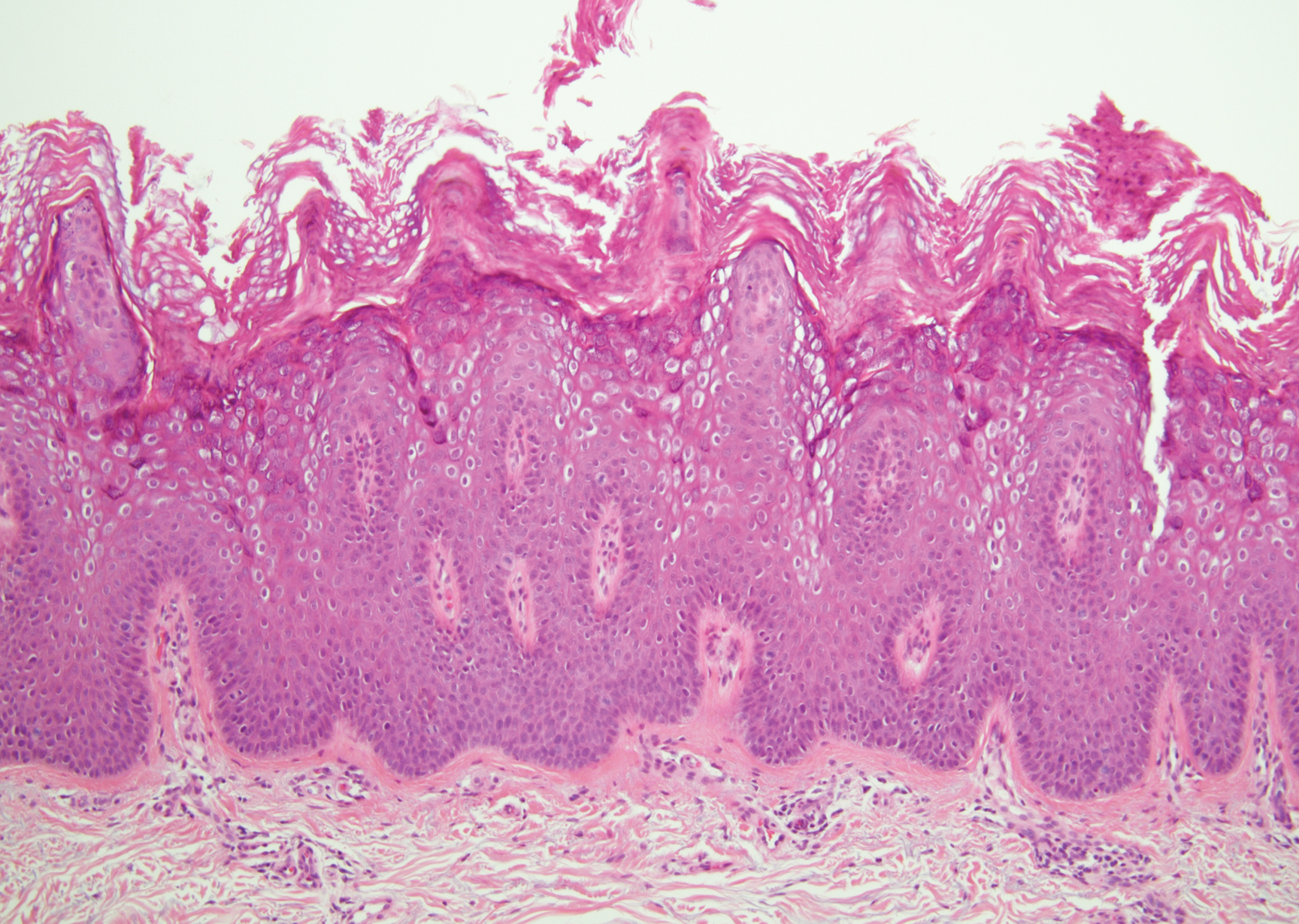
Irritated seborrheic keratosis presents as an exophytic, waxy, dark, sharply demarcated plaque with a stuck-on appearance.6 There is visible keratinization with comedolike openings, fissures and ridges, and scale; it also can contain milialike cysts. Histopathologically there is papillomatosis with prominent rete ridges, often including keratin pseudohorn cysts and squamous eddies. Enlarged capillaries can be seen in the dermal papillae. There is normal cytology with benign sheets of basaloid cells (Figure 2).7 Activating mutation in fibroblast growth factor receptor 3 leads to the growth and thickness of the epidermis that has been identified in these benign lesions.8
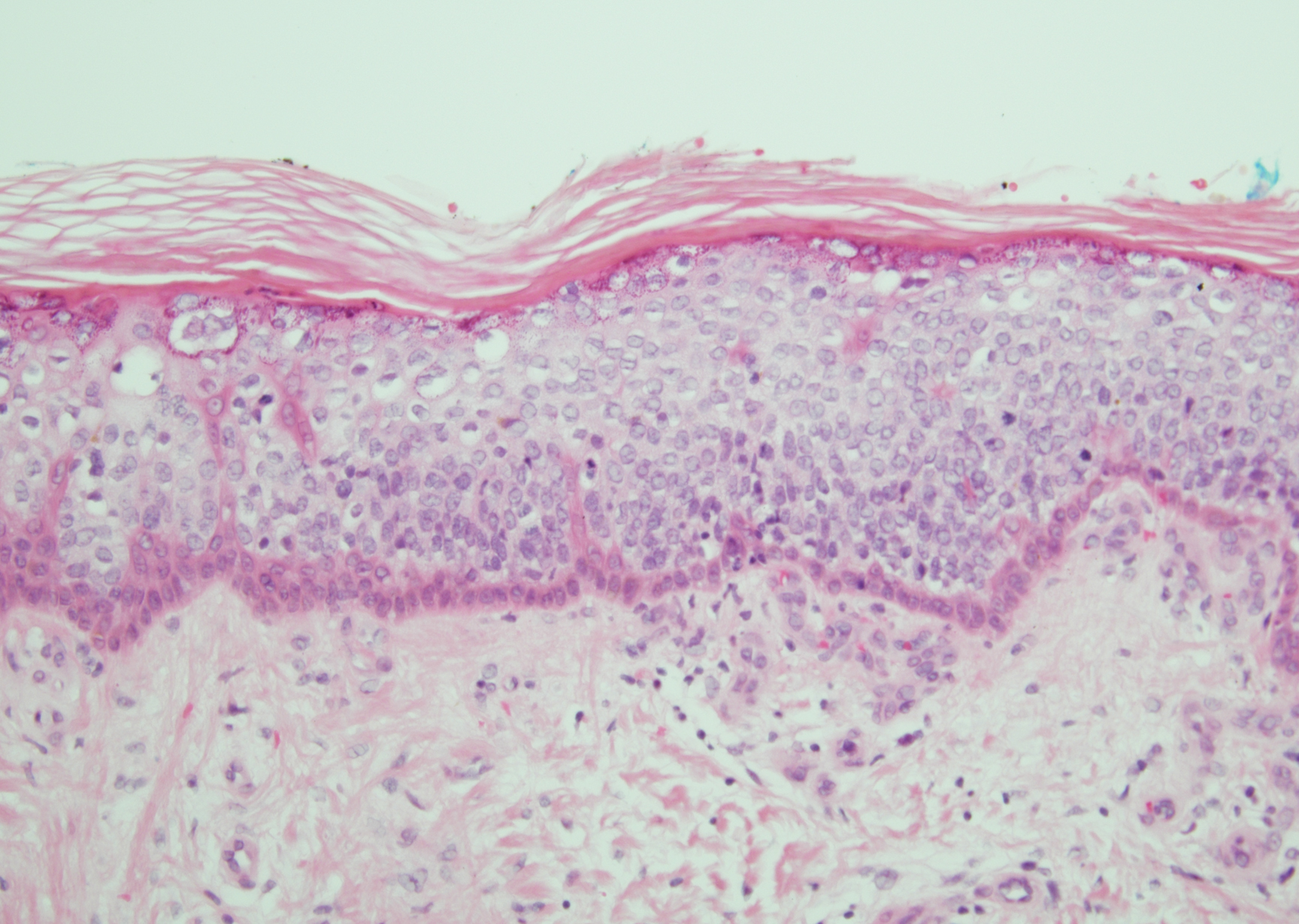
Verruca plana appears as a flesh-colored or reddish, warty, flat-topped papule that often forms clusters. Histopathologically it shows prominent hypergranulosis, thickened stratum spinosum, and vacuolized keratinocytes.9 The nuclei demonstrate a characteristic cytopathic effect of the virion, blurring the nuclear chromatin due to viral particle accumulation, known as koilocytes (Figure 3). The cause is the double-stranded DNA human papillomavirus types 2, 3, and 10.10
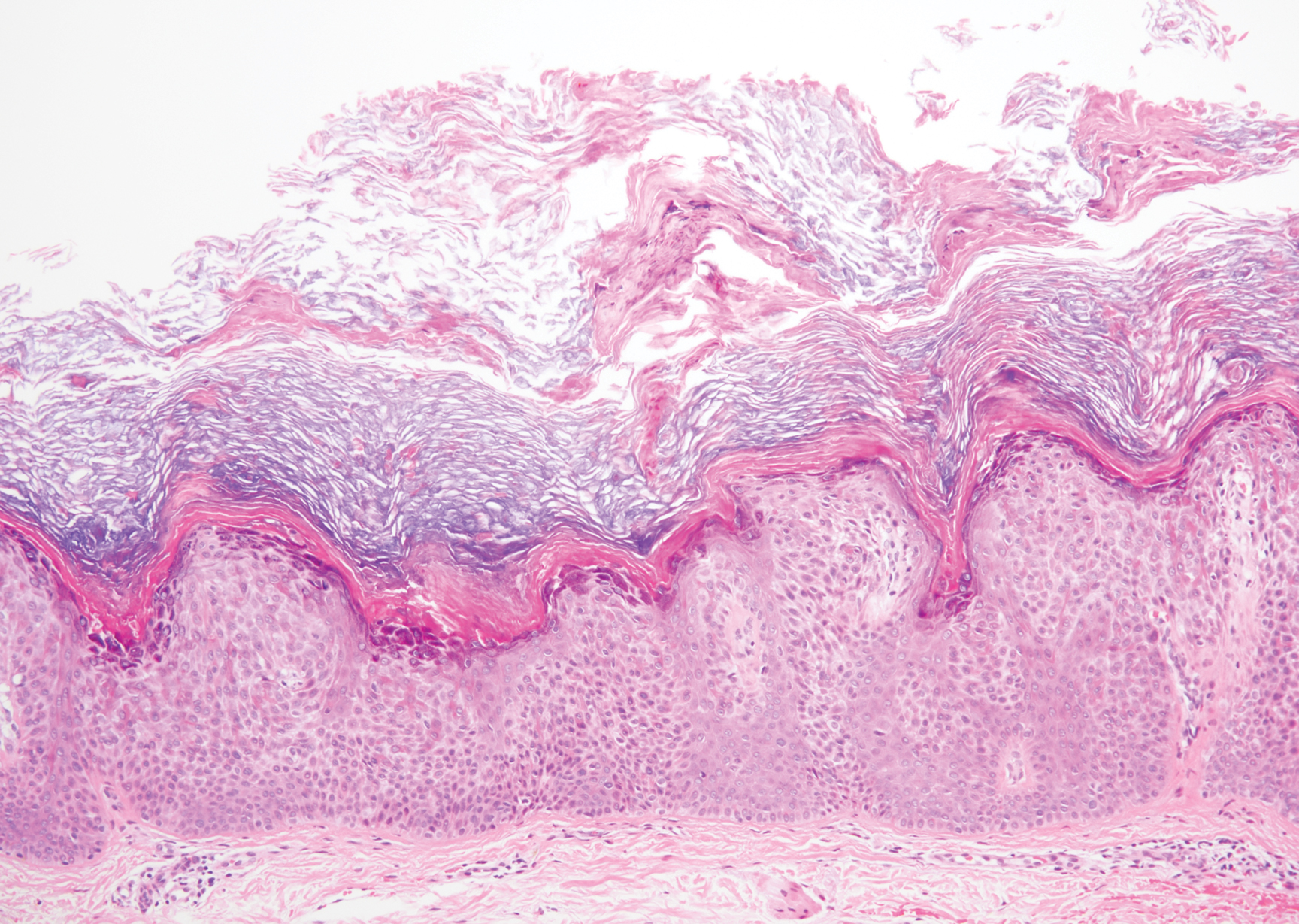
Bowen disease is a form of squamous cell carcinoma in situ characterized by an enlarging, well-demarcated, erythematous plaque with an irregular border and crusting or scaling. Histopathology reveals pleomorphic epidermal keratinization that becomes incorporated in the stratum corneum as parakeratotic nuclei. There is acanthosis, elongation of the rete ridges, and disorganized keratinocytes with atypia.11 The granular and spinous layers show an atypical honeycomb pattern with atypical cellular morphology (Figure 4).12 Bowen disease is a malignant lesion commonly found in older adults on sun-exposed skin that can evolve into invasive squamous cell carcinoma.
- Roy SF, Ko CJ, Moeckel GW, et al. Hypergranulotic dyscornification: 30 cases of a striking epithelial reaction pattern. J Cutan Pathol. 2019;46:742-747.
- Dohse L, Elston D, Lountzis N, et al. Benign hypergranulotic keratosis with dyscornification. J Am Acad Dermatol. 2010;62:AB52.
- Reichel M. Hypergranulotic dyscornification. Am J Dermatopathol. 1999;21:21-24.
- Kumar P, Kumar R, Kumar Mandal RK, et al. Systematized linear epidermolytic hyperkeratosis. Dermatol Online J. 2014;20:21248.
- Peter Rout D, Nair A, Gupta A, et al. Epidermolytic hyperkeratosis: clinical update. Clin Cosmet Investig Dermatol. 2019;12:333-344.
- Ingraffea A. Benign skin neoplasms. Facial Plast Surg Clin North Am. 2013;21:21-32.
- Braun R. Dermoscopy of pigmented seborrheic keratosis. Arch Dermatol. 2002;138:1556.
- Duperret EK, Oh SJ, McNeal A, et al. Activating FGFR3 mutations cause mild hyperplasia in human skin, but are insufficient to drive benign or malignant skin tumors. Cell Cycle. 2014;13:1551-1559.
- Liu H, Chen S, Zhang F, et al. Seborrheic keratosis or verruca plana? a pilot study with confocal laser scanning microscopy. Skin Res Technol. 2010;16:408-412.
- Prieto-Granada CN, Lobo AZC, Mihm MC. Skin infections. In: Kradin RL, ed. Diagnostic Pathology of Infectious Disease. Philadelphia, PA: Saunders Elsevier; 2010:519-616.
- DeCoste R, Moss P, Boutilier R, et al. Bowen disease with invasive mucin-secreting sweat gland differentiation: report of a case and review of the literature. J Cutan Pathol. 2019;46:425-430.
- Ulrich M, Kanitakis J, González S, et al. Evaluation of Bowen disease by in vivo reflectance confocal microscopy. Br J Dermatol. 2011;166:451-453.
The Diagnosis: Hypergranulotic Dyscornification
Hypergranulotic dyscornification (HD) is a rarely reported reaction pattern present in benign solitary keratoses with only few reports to date. It may be an underrecognized reaction pattern based on the paucity of reported cases as well as the histologic similarities to other entities. It has been hypothesized that this pattern reflects an underlying keratin mutation or disorder of keratinization.1
Clinically, HD most commonly presents as a waxy, tan-colored, solitary keratosis generally found on the lower limbs, trunk, or back in individuals aged 20 to 60 years.1,2 Histopathology shows marked hyperkeratosis, papillomatosis, and clumped basophilic keratohyalin granules within the corneocytes with digitated epidermal hyperplasia. There is abnormal cornification across the entire lesion with papillomatosis and marked hypergranulosis.3 There often are homogeneous orthokeratotic mounds of large, dull, eosinophilic-staining anucleate keratinocytes that are sharply demarcated from the thickened granular layer.1,2 Within the spinous, granular, and corneal layers, there is a pale, gray-staining, basophilic, cytoplasmic substance intercellularly.1
Histopathologically, HD may be mistaken for several other entities both benign and malignant.1 Epidermolytic hyperkeratosis can be a genetic disorder, an incidental finding in a variety of skin conditions, or an isolated lesion.4 The genetic syndrome, caused by mutation in keratins 1 or 10, clinically presents with hyperkeratosis, erosions, blisters, and thickening of the epidermis, often with a corrugated appearance. Epidermal nevi findings often are seen in conjunction with histologic changes of epidermolytic hyperkeratosis caused by mutation. Solitary lesions also can resemble seborrheic keratosis or verruca. In all examples of epidermolytic hyperkeratosis, the histopathologic findings are identical.4 The granular layer is thickened, and coarse keratohyalin granules aggregate in the suprabasal cells.5 There is acantholysis with perinuclear vacuolization in the spinous and granular layers with characteristic pale cytoplasmic areas devoid of keratin filaments (Figure 1). The basal layer may be hyperproliferative.5

Irritated seborrheic keratosis presents as an exophytic, waxy, dark, sharply demarcated plaque with a stuck-on appearance.6 There is visible keratinization with comedolike openings, fissures and ridges, and scale; it also can contain milialike cysts. Histopathologically there is papillomatosis with prominent rete ridges, often including keratin pseudohorn cysts and squamous eddies. Enlarged capillaries can be seen in the dermal papillae. There is normal cytology with benign sheets of basaloid cells (Figure 2).7 Activating mutation in fibroblast growth factor receptor 3 leads to the growth and thickness of the epidermis that has been identified in these benign lesions.8

Verruca plana appears as a flesh-colored or reddish, warty, flat-topped papule that often forms clusters. Histopathologically it shows prominent hypergranulosis, thickened stratum spinosum, and vacuolized keratinocytes.9 The nuclei demonstrate a characteristic cytopathic effect of the virion, blurring the nuclear chromatin due to viral particle accumulation, known as koilocytes (Figure 3). The cause is the double-stranded DNA human papillomavirus types 2, 3, and 10.10

Bowen disease is a form of squamous cell carcinoma in situ characterized by an enlarging, well-demarcated, erythematous plaque with an irregular border and crusting or scaling. Histopathology reveals pleomorphic epidermal keratinization that becomes incorporated in the stratum corneum as parakeratotic nuclei. There is acanthosis, elongation of the rete ridges, and disorganized keratinocytes with atypia.11 The granular and spinous layers show an atypical honeycomb pattern with atypical cellular morphology (Figure 4).12 Bowen disease is a malignant lesion commonly found in older adults on sun-exposed skin that can evolve into invasive squamous cell carcinoma.

The Diagnosis: Hypergranulotic Dyscornification
Hypergranulotic dyscornification (HD) is a rarely reported reaction pattern present in benign solitary keratoses with only few reports to date. It may be an underrecognized reaction pattern based on the paucity of reported cases as well as the histologic similarities to other entities. It has been hypothesized that this pattern reflects an underlying keratin mutation or disorder of keratinization.1
Clinically, HD most commonly presents as a waxy, tan-colored, solitary keratosis generally found on the lower limbs, trunk, or back in individuals aged 20 to 60 years.1,2 Histopathology shows marked hyperkeratosis, papillomatosis, and clumped basophilic keratohyalin granules within the corneocytes with digitated epidermal hyperplasia. There is abnormal cornification across the entire lesion with papillomatosis and marked hypergranulosis.3 There often are homogeneous orthokeratotic mounds of large, dull, eosinophilic-staining anucleate keratinocytes that are sharply demarcated from the thickened granular layer.1,2 Within the spinous, granular, and corneal layers, there is a pale, gray-staining, basophilic, cytoplasmic substance intercellularly.1
Histopathologically, HD may be mistaken for several other entities both benign and malignant.1 Epidermolytic hyperkeratosis can be a genetic disorder, an incidental finding in a variety of skin conditions, or an isolated lesion.4 The genetic syndrome, caused by mutation in keratins 1 or 10, clinically presents with hyperkeratosis, erosions, blisters, and thickening of the epidermis, often with a corrugated appearance. Epidermal nevi findings often are seen in conjunction with histologic changes of epidermolytic hyperkeratosis caused by mutation. Solitary lesions also can resemble seborrheic keratosis or verruca. In all examples of epidermolytic hyperkeratosis, the histopathologic findings are identical.4 The granular layer is thickened, and coarse keratohyalin granules aggregate in the suprabasal cells.5 There is acantholysis with perinuclear vacuolization in the spinous and granular layers with characteristic pale cytoplasmic areas devoid of keratin filaments (Figure 1). The basal layer may be hyperproliferative.5

Irritated seborrheic keratosis presents as an exophytic, waxy, dark, sharply demarcated plaque with a stuck-on appearance.6 There is visible keratinization with comedolike openings, fissures and ridges, and scale; it also can contain milialike cysts. Histopathologically there is papillomatosis with prominent rete ridges, often including keratin pseudohorn cysts and squamous eddies. Enlarged capillaries can be seen in the dermal papillae. There is normal cytology with benign sheets of basaloid cells (Figure 2).7 Activating mutation in fibroblast growth factor receptor 3 leads to the growth and thickness of the epidermis that has been identified in these benign lesions.8

Verruca plana appears as a flesh-colored or reddish, warty, flat-topped papule that often forms clusters. Histopathologically it shows prominent hypergranulosis, thickened stratum spinosum, and vacuolized keratinocytes.9 The nuclei demonstrate a characteristic cytopathic effect of the virion, blurring the nuclear chromatin due to viral particle accumulation, known as koilocytes (Figure 3). The cause is the double-stranded DNA human papillomavirus types 2, 3, and 10.10

Bowen disease is a form of squamous cell carcinoma in situ characterized by an enlarging, well-demarcated, erythematous plaque with an irregular border and crusting or scaling. Histopathology reveals pleomorphic epidermal keratinization that becomes incorporated in the stratum corneum as parakeratotic nuclei. There is acanthosis, elongation of the rete ridges, and disorganized keratinocytes with atypia.11 The granular and spinous layers show an atypical honeycomb pattern with atypical cellular morphology (Figure 4).12 Bowen disease is a malignant lesion commonly found in older adults on sun-exposed skin that can evolve into invasive squamous cell carcinoma.

- Roy SF, Ko CJ, Moeckel GW, et al. Hypergranulotic dyscornification: 30 cases of a striking epithelial reaction pattern. J Cutan Pathol. 2019;46:742-747.
- Dohse L, Elston D, Lountzis N, et al. Benign hypergranulotic keratosis with dyscornification. J Am Acad Dermatol. 2010;62:AB52.
- Reichel M. Hypergranulotic dyscornification. Am J Dermatopathol. 1999;21:21-24.
- Kumar P, Kumar R, Kumar Mandal RK, et al. Systematized linear epidermolytic hyperkeratosis. Dermatol Online J. 2014;20:21248.
- Peter Rout D, Nair A, Gupta A, et al. Epidermolytic hyperkeratosis: clinical update. Clin Cosmet Investig Dermatol. 2019;12:333-344.
- Ingraffea A. Benign skin neoplasms. Facial Plast Surg Clin North Am. 2013;21:21-32.
- Braun R. Dermoscopy of pigmented seborrheic keratosis. Arch Dermatol. 2002;138:1556.
- Duperret EK, Oh SJ, McNeal A, et al. Activating FGFR3 mutations cause mild hyperplasia in human skin, but are insufficient to drive benign or malignant skin tumors. Cell Cycle. 2014;13:1551-1559.
- Liu H, Chen S, Zhang F, et al. Seborrheic keratosis or verruca plana? a pilot study with confocal laser scanning microscopy. Skin Res Technol. 2010;16:408-412.
- Prieto-Granada CN, Lobo AZC, Mihm MC. Skin infections. In: Kradin RL, ed. Diagnostic Pathology of Infectious Disease. Philadelphia, PA: Saunders Elsevier; 2010:519-616.
- DeCoste R, Moss P, Boutilier R, et al. Bowen disease with invasive mucin-secreting sweat gland differentiation: report of a case and review of the literature. J Cutan Pathol. 2019;46:425-430.
- Ulrich M, Kanitakis J, González S, et al. Evaluation of Bowen disease by in vivo reflectance confocal microscopy. Br J Dermatol. 2011;166:451-453.
- Roy SF, Ko CJ, Moeckel GW, et al. Hypergranulotic dyscornification: 30 cases of a striking epithelial reaction pattern. J Cutan Pathol. 2019;46:742-747.
- Dohse L, Elston D, Lountzis N, et al. Benign hypergranulotic keratosis with dyscornification. J Am Acad Dermatol. 2010;62:AB52.
- Reichel M. Hypergranulotic dyscornification. Am J Dermatopathol. 1999;21:21-24.
- Kumar P, Kumar R, Kumar Mandal RK, et al. Systematized linear epidermolytic hyperkeratosis. Dermatol Online J. 2014;20:21248.
- Peter Rout D, Nair A, Gupta A, et al. Epidermolytic hyperkeratosis: clinical update. Clin Cosmet Investig Dermatol. 2019;12:333-344.
- Ingraffea A. Benign skin neoplasms. Facial Plast Surg Clin North Am. 2013;21:21-32.
- Braun R. Dermoscopy of pigmented seborrheic keratosis. Arch Dermatol. 2002;138:1556.
- Duperret EK, Oh SJ, McNeal A, et al. Activating FGFR3 mutations cause mild hyperplasia in human skin, but are insufficient to drive benign or malignant skin tumors. Cell Cycle. 2014;13:1551-1559.
- Liu H, Chen S, Zhang F, et al. Seborrheic keratosis or verruca plana? a pilot study with confocal laser scanning microscopy. Skin Res Technol. 2010;16:408-412.
- Prieto-Granada CN, Lobo AZC, Mihm MC. Skin infections. In: Kradin RL, ed. Diagnostic Pathology of Infectious Disease. Philadelphia, PA: Saunders Elsevier; 2010:519-616.
- DeCoste R, Moss P, Boutilier R, et al. Bowen disease with invasive mucin-secreting sweat gland differentiation: report of a case and review of the literature. J Cutan Pathol. 2019;46:425-430.
- Ulrich M, Kanitakis J, González S, et al. Evaluation of Bowen disease by in vivo reflectance confocal microscopy. Br J Dermatol. 2011;166:451-453.
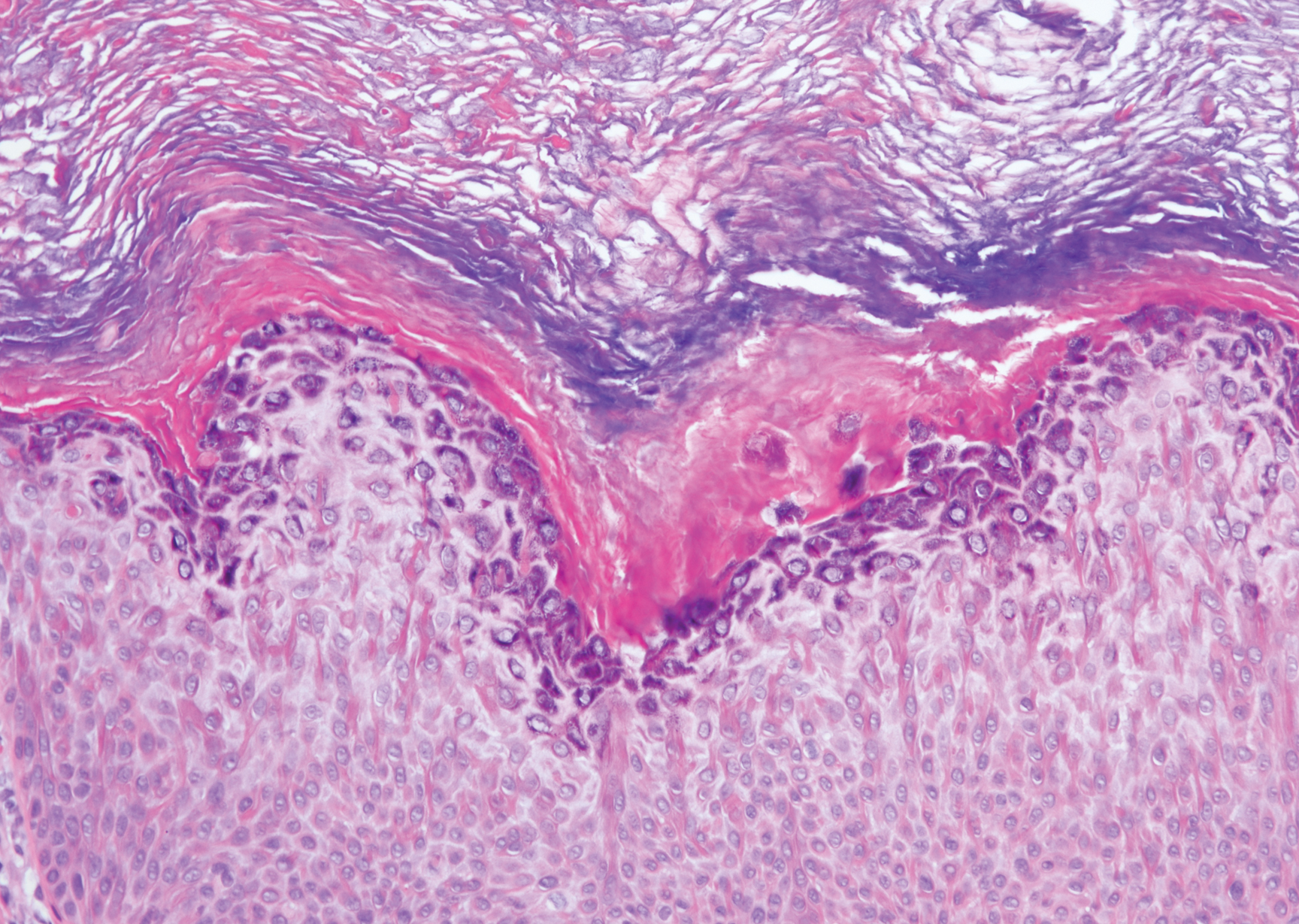
A 59-year-old woman with a history of basal cell carcinoma, uterine and ovarian cancer, and verrucae presented with an asymptomatic 3-mm lesion on the left side of the lower abdomen. Physical examination revealed a waxy, tan-colored, solitary keratosis. A shave biopsy was performed. Histopathology showed hyperkeratosis, focal parakeratosis, papillomatosis, and marked hypergranulosis with pale gray cytoplasm of the spinous-layer keratinocytes.
"Doctor, Do I Need a Skin Check?"
What does your patient need to know at the first visit?
A patient may be scheduled for a total-body skin examination (TBSE) through several routes: primary care referral, continued cancer screening for an at-risk patient or patient transfer, or patient-directed scheduling for general screening regardless of risk factors. At the patient's first visit, it is imperative that the course of the appointment is smooth and predictable for patient comfort and for a thorough and effective examination. The nurse initially solicits salient medical history, particularly personal and family history of skin cancer, current medications, and any acute concerns. The nurse then prepares the patient for the logistics of the TBSE, namely to undress, don a gown that ties and opens in the back, and be seated on the examination table. When I enter the room, the conversation commences with me seated across from the patient, reviewing specifics about his/her history and risk factors. Then the TBSE is executed from head to toe.
Do you broadly recommend TBSE?
Firstly, TBSE is a safe clinical tool, supported by data outlining a lack of notable patient morbidity during the examination, including psychosocial factors, and it is generally well-received by patients (Risica et al). In 2016, the US Preventative Services Task Force (USPSTF) outlined its recommendations regarding screening for skin cancer, concluding that there is insufficient evidence to broadly recommend TBSE. Unfortunately, USPSTF findings amassed data from all types of screenings, including those by nondermatologists, and did not extract specialty-specific benefits and risks to patients. The recommendation also did not outline the influence of TBSE on morbidity and mortality for at-risk groups. The guidelines target primary care practice trends; therefore, specialty societies such as the American Academy of Dermatology issued statements following the USPSTF recommendation outlining these salient clarifications, namely that TBSE detects melanoma and keratinocyte carcinomas earlier than in patients who are not screened. Randomized controlled trials to prove this observation are lacking, particularly because of the ethics of withholding screening from a prospective study group. However, in 2017, Johnson et al outlined the best available survival data in concert with the USPSTF statement to arrive at the most beneficial screening recommendations for patients, specifically targeting risk groups--those with a history of skin cancer, immunosuppression, indoor tanning and/or many blistering sunburns, and several other genetic parameters--for at least annual TBSE.
The technique and reproducibility of TBSE also are not standardized, though they seem to have been endearingly apprenticed but variably implemented through generations of dermatology residents going forward into practice. As it is, depending on patient body surface area, mobility, willingness to disrobe, and adornments (eg, tattoos, hair appliances), multiple factors can restrict full view of a patient's skin. Recently, Helm et al proposed standardizing the TBSE sequence to minimize omitted areas of the body, which may become an imperative tool for streamlined resident teaching and optimal screening encounters.
How do you keep patients compliant with TBSE?
During and following TBSE, I typically outline any lesions of concern and plan for further testing, screening, and behavioral prevention strategies. Frequency of TBSE and importance of compliance are discussed during the visit and reinforced at checkout where the appointment templates are established a year in advance for those with skin cancer. Further, for those with melanoma, their appointment slots are given priority status so that any cancellations or delays are rescheduled preferentially. Particularly during the discussion about TBSE frequency, I emphasize the comparison and importance of this visit akin to other recommended screenings, such as mammograms and colonoscopies, and that we, as dermatologists, are part of their cancer surveillance team.
What do you do if patients refuse your recommendations?
Some patients refuse a gown or removal of certain clothing items (eg, undergarments, socks, wigs). Some patients defer a yearly TBSE upon checkout and schedule an appointment only when a lesion of concern arises. My advice is not to shame patients and to take advantage of as much as the patient is able and comfortable to show us and be present for, welcoming that we have the opportunity to take care of them and screen for cancer in any capacity. In underserved or limited budget practice regions, lesion-directed examination vs TBSE may be the only screening method utilized and may even attract more patients to a screening facility (Hoorens et al).
In the opposite corner are those patients who deem the recommended TBSE interval as too infrequent, which poses a delicate dilemma. In my opinion, these situations present another cohort of risks. Namely, the patient may become (or continue to be) overly fixated on the small details of every skin lesion, and in my experience, they tend to develop the habit of expecting at least 1 biopsy at each visit, typically of a lesion of their choosing. Depending on the validity of this expectation vs my clinical examination, it can lead to a difficult discussion with the patient about oversampling lesions and the potential for many scars, copious reexcisions for ambiguous lesion pathology, and a trend away from prudent clinical care. In addition, multiple visits incur more patient co-pays and time away from school, work, or home. To ease the patient's mind, I advise to call our office for a more acute visit if there is a lesion of concern; I additionally recommend taking a smartphone photograph of a concerning lesion and monitoring it for changes or sending the photograph to our patient portal messaging system so we can evaluate its acuity.
What take-home advice do you give to patients?
As the visit ends, I further explain that home self-examination or examination by a partner between visits is intuitively a valuable screening adjunct for skin cancer. In 2018, the USPSTF recommended behavioral skin cancer prevention counseling and self-examination only for younger-age cohorts with fair skin (6 months to 24 years), but its utility in specialty practice must be qualified. The American Academy of Dermatology Association subsequently issued a statement to support safe sun-protective practices and diligent self-screening for changing lesions, as earlier detection and management of skin cancer can lead to decreased morbidity and mortality from these neoplasms.
Resources for Patients
American Academy of Dermatology's SPOT Skin Cancer
Centers for Disease Control and Prevention: What Screening Tests Are There?
Suggested Readings
AAD statement on USPSTF recommendation on skin cancer screening. Schaumburg, IL: American Academy of Dermatology; July 26, 2016. https://www.aad.org/media/news-releases/aad-statement-on-uspstf. Accessed April 26, 2019.
AADA responds to USPSTF recommendation on skin cancer prevention counseling. Rosemont, IL: American Academy of Dermatology Association; March 20, 2018. https://www.aad.org/media/news-releases/skin-cancer-prevention-counseling. Accessed April 26, 2019.
Helm MF, Hallock KK, Bisbee E, et al. Optimizing the total body skin exam: an observational cohort study [published online February 15, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.02.028.
Hoorens I, Vossaert K, Pil L, et al. Total-body examination vs lesion-directed skin cancer screening. JAMA Dermatol. 2016;152:27-34.
Johnson MM, Leachman SA, Aspinwall LG, et al. Skin cancer screening: recommendations for data-driven screening guidelines and a review of the US Preventive Services Task Force controversy. Melanoma Manag. 2017;4:13-37.
Risica PM, Matthews NH, Dionne L, et al. Psychosocial consequences of skin cancer screening. Prev Med Rep. 2018;10:310-316.
US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:429-435.
US Preventive Services Task Force, Grossman DC, Curry SJ, et al. Behavioral counseling to prevent skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1134-1142.
What does your patient need to know at the first visit?
A patient may be scheduled for a total-body skin examination (TBSE) through several routes: primary care referral, continued cancer screening for an at-risk patient or patient transfer, or patient-directed scheduling for general screening regardless of risk factors. At the patient's first visit, it is imperative that the course of the appointment is smooth and predictable for patient comfort and for a thorough and effective examination. The nurse initially solicits salient medical history, particularly personal and family history of skin cancer, current medications, and any acute concerns. The nurse then prepares the patient for the logistics of the TBSE, namely to undress, don a gown that ties and opens in the back, and be seated on the examination table. When I enter the room, the conversation commences with me seated across from the patient, reviewing specifics about his/her history and risk factors. Then the TBSE is executed from head to toe.
Do you broadly recommend TBSE?
Firstly, TBSE is a safe clinical tool, supported by data outlining a lack of notable patient morbidity during the examination, including psychosocial factors, and it is generally well-received by patients (Risica et al). In 2016, the US Preventative Services Task Force (USPSTF) outlined its recommendations regarding screening for skin cancer, concluding that there is insufficient evidence to broadly recommend TBSE. Unfortunately, USPSTF findings amassed data from all types of screenings, including those by nondermatologists, and did not extract specialty-specific benefits and risks to patients. The recommendation also did not outline the influence of TBSE on morbidity and mortality for at-risk groups. The guidelines target primary care practice trends; therefore, specialty societies such as the American Academy of Dermatology issued statements following the USPSTF recommendation outlining these salient clarifications, namely that TBSE detects melanoma and keratinocyte carcinomas earlier than in patients who are not screened. Randomized controlled trials to prove this observation are lacking, particularly because of the ethics of withholding screening from a prospective study group. However, in 2017, Johnson et al outlined the best available survival data in concert with the USPSTF statement to arrive at the most beneficial screening recommendations for patients, specifically targeting risk groups--those with a history of skin cancer, immunosuppression, indoor tanning and/or many blistering sunburns, and several other genetic parameters--for at least annual TBSE.
The technique and reproducibility of TBSE also are not standardized, though they seem to have been endearingly apprenticed but variably implemented through generations of dermatology residents going forward into practice. As it is, depending on patient body surface area, mobility, willingness to disrobe, and adornments (eg, tattoos, hair appliances), multiple factors can restrict full view of a patient's skin. Recently, Helm et al proposed standardizing the TBSE sequence to minimize omitted areas of the body, which may become an imperative tool for streamlined resident teaching and optimal screening encounters.
How do you keep patients compliant with TBSE?
During and following TBSE, I typically outline any lesions of concern and plan for further testing, screening, and behavioral prevention strategies. Frequency of TBSE and importance of compliance are discussed during the visit and reinforced at checkout where the appointment templates are established a year in advance for those with skin cancer. Further, for those with melanoma, their appointment slots are given priority status so that any cancellations or delays are rescheduled preferentially. Particularly during the discussion about TBSE frequency, I emphasize the comparison and importance of this visit akin to other recommended screenings, such as mammograms and colonoscopies, and that we, as dermatologists, are part of their cancer surveillance team.
What do you do if patients refuse your recommendations?
Some patients refuse a gown or removal of certain clothing items (eg, undergarments, socks, wigs). Some patients defer a yearly TBSE upon checkout and schedule an appointment only when a lesion of concern arises. My advice is not to shame patients and to take advantage of as much as the patient is able and comfortable to show us and be present for, welcoming that we have the opportunity to take care of them and screen for cancer in any capacity. In underserved or limited budget practice regions, lesion-directed examination vs TBSE may be the only screening method utilized and may even attract more patients to a screening facility (Hoorens et al).
In the opposite corner are those patients who deem the recommended TBSE interval as too infrequent, which poses a delicate dilemma. In my opinion, these situations present another cohort of risks. Namely, the patient may become (or continue to be) overly fixated on the small details of every skin lesion, and in my experience, they tend to develop the habit of expecting at least 1 biopsy at each visit, typically of a lesion of their choosing. Depending on the validity of this expectation vs my clinical examination, it can lead to a difficult discussion with the patient about oversampling lesions and the potential for many scars, copious reexcisions for ambiguous lesion pathology, and a trend away from prudent clinical care. In addition, multiple visits incur more patient co-pays and time away from school, work, or home. To ease the patient's mind, I advise to call our office for a more acute visit if there is a lesion of concern; I additionally recommend taking a smartphone photograph of a concerning lesion and monitoring it for changes or sending the photograph to our patient portal messaging system so we can evaluate its acuity.
What take-home advice do you give to patients?
As the visit ends, I further explain that home self-examination or examination by a partner between visits is intuitively a valuable screening adjunct for skin cancer. In 2018, the USPSTF recommended behavioral skin cancer prevention counseling and self-examination only for younger-age cohorts with fair skin (6 months to 24 years), but its utility in specialty practice must be qualified. The American Academy of Dermatology Association subsequently issued a statement to support safe sun-protective practices and diligent self-screening for changing lesions, as earlier detection and management of skin cancer can lead to decreased morbidity and mortality from these neoplasms.
Resources for Patients
American Academy of Dermatology's SPOT Skin Cancer
Centers for Disease Control and Prevention: What Screening Tests Are There?
What does your patient need to know at the first visit?
A patient may be scheduled for a total-body skin examination (TBSE) through several routes: primary care referral, continued cancer screening for an at-risk patient or patient transfer, or patient-directed scheduling for general screening regardless of risk factors. At the patient's first visit, it is imperative that the course of the appointment is smooth and predictable for patient comfort and for a thorough and effective examination. The nurse initially solicits salient medical history, particularly personal and family history of skin cancer, current medications, and any acute concerns. The nurse then prepares the patient for the logistics of the TBSE, namely to undress, don a gown that ties and opens in the back, and be seated on the examination table. When I enter the room, the conversation commences with me seated across from the patient, reviewing specifics about his/her history and risk factors. Then the TBSE is executed from head to toe.
Do you broadly recommend TBSE?
Firstly, TBSE is a safe clinical tool, supported by data outlining a lack of notable patient morbidity during the examination, including psychosocial factors, and it is generally well-received by patients (Risica et al). In 2016, the US Preventative Services Task Force (USPSTF) outlined its recommendations regarding screening for skin cancer, concluding that there is insufficient evidence to broadly recommend TBSE. Unfortunately, USPSTF findings amassed data from all types of screenings, including those by nondermatologists, and did not extract specialty-specific benefits and risks to patients. The recommendation also did not outline the influence of TBSE on morbidity and mortality for at-risk groups. The guidelines target primary care practice trends; therefore, specialty societies such as the American Academy of Dermatology issued statements following the USPSTF recommendation outlining these salient clarifications, namely that TBSE detects melanoma and keratinocyte carcinomas earlier than in patients who are not screened. Randomized controlled trials to prove this observation are lacking, particularly because of the ethics of withholding screening from a prospective study group. However, in 2017, Johnson et al outlined the best available survival data in concert with the USPSTF statement to arrive at the most beneficial screening recommendations for patients, specifically targeting risk groups--those with a history of skin cancer, immunosuppression, indoor tanning and/or many blistering sunburns, and several other genetic parameters--for at least annual TBSE.
The technique and reproducibility of TBSE also are not standardized, though they seem to have been endearingly apprenticed but variably implemented through generations of dermatology residents going forward into practice. As it is, depending on patient body surface area, mobility, willingness to disrobe, and adornments (eg, tattoos, hair appliances), multiple factors can restrict full view of a patient's skin. Recently, Helm et al proposed standardizing the TBSE sequence to minimize omitted areas of the body, which may become an imperative tool for streamlined resident teaching and optimal screening encounters.
How do you keep patients compliant with TBSE?
During and following TBSE, I typically outline any lesions of concern and plan for further testing, screening, and behavioral prevention strategies. Frequency of TBSE and importance of compliance are discussed during the visit and reinforced at checkout where the appointment templates are established a year in advance for those with skin cancer. Further, for those with melanoma, their appointment slots are given priority status so that any cancellations or delays are rescheduled preferentially. Particularly during the discussion about TBSE frequency, I emphasize the comparison and importance of this visit akin to other recommended screenings, such as mammograms and colonoscopies, and that we, as dermatologists, are part of their cancer surveillance team.
What do you do if patients refuse your recommendations?
Some patients refuse a gown or removal of certain clothing items (eg, undergarments, socks, wigs). Some patients defer a yearly TBSE upon checkout and schedule an appointment only when a lesion of concern arises. My advice is not to shame patients and to take advantage of as much as the patient is able and comfortable to show us and be present for, welcoming that we have the opportunity to take care of them and screen for cancer in any capacity. In underserved or limited budget practice regions, lesion-directed examination vs TBSE may be the only screening method utilized and may even attract more patients to a screening facility (Hoorens et al).
In the opposite corner are those patients who deem the recommended TBSE interval as too infrequent, which poses a delicate dilemma. In my opinion, these situations present another cohort of risks. Namely, the patient may become (or continue to be) overly fixated on the small details of every skin lesion, and in my experience, they tend to develop the habit of expecting at least 1 biopsy at each visit, typically of a lesion of their choosing. Depending on the validity of this expectation vs my clinical examination, it can lead to a difficult discussion with the patient about oversampling lesions and the potential for many scars, copious reexcisions for ambiguous lesion pathology, and a trend away from prudent clinical care. In addition, multiple visits incur more patient co-pays and time away from school, work, or home. To ease the patient's mind, I advise to call our office for a more acute visit if there is a lesion of concern; I additionally recommend taking a smartphone photograph of a concerning lesion and monitoring it for changes or sending the photograph to our patient portal messaging system so we can evaluate its acuity.
What take-home advice do you give to patients?
As the visit ends, I further explain that home self-examination or examination by a partner between visits is intuitively a valuable screening adjunct for skin cancer. In 2018, the USPSTF recommended behavioral skin cancer prevention counseling and self-examination only for younger-age cohorts with fair skin (6 months to 24 years), but its utility in specialty practice must be qualified. The American Academy of Dermatology Association subsequently issued a statement to support safe sun-protective practices and diligent self-screening for changing lesions, as earlier detection and management of skin cancer can lead to decreased morbidity and mortality from these neoplasms.
Resources for Patients
American Academy of Dermatology's SPOT Skin Cancer
Centers for Disease Control and Prevention: What Screening Tests Are There?
Suggested Readings
AAD statement on USPSTF recommendation on skin cancer screening. Schaumburg, IL: American Academy of Dermatology; July 26, 2016. https://www.aad.org/media/news-releases/aad-statement-on-uspstf. Accessed April 26, 2019.
AADA responds to USPSTF recommendation on skin cancer prevention counseling. Rosemont, IL: American Academy of Dermatology Association; March 20, 2018. https://www.aad.org/media/news-releases/skin-cancer-prevention-counseling. Accessed April 26, 2019.
Helm MF, Hallock KK, Bisbee E, et al. Optimizing the total body skin exam: an observational cohort study [published online February 15, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.02.028.
Hoorens I, Vossaert K, Pil L, et al. Total-body examination vs lesion-directed skin cancer screening. JAMA Dermatol. 2016;152:27-34.
Johnson MM, Leachman SA, Aspinwall LG, et al. Skin cancer screening: recommendations for data-driven screening guidelines and a review of the US Preventive Services Task Force controversy. Melanoma Manag. 2017;4:13-37.
Risica PM, Matthews NH, Dionne L, et al. Psychosocial consequences of skin cancer screening. Prev Med Rep. 2018;10:310-316.
US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:429-435.
US Preventive Services Task Force, Grossman DC, Curry SJ, et al. Behavioral counseling to prevent skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1134-1142.
Suggested Readings
AAD statement on USPSTF recommendation on skin cancer screening. Schaumburg, IL: American Academy of Dermatology; July 26, 2016. https://www.aad.org/media/news-releases/aad-statement-on-uspstf. Accessed April 26, 2019.
AADA responds to USPSTF recommendation on skin cancer prevention counseling. Rosemont, IL: American Academy of Dermatology Association; March 20, 2018. https://www.aad.org/media/news-releases/skin-cancer-prevention-counseling. Accessed April 26, 2019.
Helm MF, Hallock KK, Bisbee E, et al. Optimizing the total body skin exam: an observational cohort study [published online February 15, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.02.028.
Hoorens I, Vossaert K, Pil L, et al. Total-body examination vs lesion-directed skin cancer screening. JAMA Dermatol. 2016;152:27-34.
Johnson MM, Leachman SA, Aspinwall LG, et al. Skin cancer screening: recommendations for data-driven screening guidelines and a review of the US Preventive Services Task Force controversy. Melanoma Manag. 2017;4:13-37.
Risica PM, Matthews NH, Dionne L, et al. Psychosocial consequences of skin cancer screening. Prev Med Rep. 2018;10:310-316.
US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:429-435.
US Preventive Services Task Force, Grossman DC, Curry SJ, et al. Behavioral counseling to prevent skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1134-1142.
Acne Medications: What Factors Drive Variable Costs?
Economic Stewardship in Acne Management
We are fortunate to have plentiful acne treatment options available that cater to each patient’s clinical examination, predispositions, and triggers, but the choices are daunting amidst the vast prescription and over-the-counter (OTC) topicals available along with disparate insurance and cost nuances. In addition, when prescribing generic oral therapies, it is complicated to parse out regional differences in price, supply, and insurance coverage to advocate best for each patient and land upon the delicate balance between efficacy, safety, and financial stewardship, both at an individual and community level. I will outline some challenges and solutions to the management of acne amidst these complicated factors.
Oral Therapies
For isotretinoin, generic choices, cost, and tiering within insurance plans are perpetual moving targets despite the drug being the only member of its class.1 Prescriber resources include tandem searches of electronic medical record price estimates within each insurance formulary, individual pharmacy search engines, and compilation mobile applications such as GoodRx to select the most affordable version for each patient. As an example of a regional trend, isotretinoin generic coverage by one provider in Pennsylvania shifted earlier this year so that every patient, whether new to isotretinoin or midcourse, required a new prior authorization with more stringent coverage requirements including failure of 2 oral antibiotics. Swiftly thereafter, efforts across the state driven by the Pennsylvania Academy of Dermatology and Dermatologic Surgery and its members and fueled by poignant patient vignettes about fragmented and substandard patient care helped to reverse this policy and remove the prior authorization mandate.2
Tetracyclines have experienced broad cost swings, mostly based on disruption of manufacturing at the limited number of distribution sites in the United States. In 2011, a tetracycline shortage arose due to a major manufacturer’s recall3 and persisted with subsequent material shortages, as doxycycline became the preferred and cheaper member of the class. Doxycycline price tag hikes then occurred following Hurricane Sandy when an East Coast manufacturing site was damaged.3 Spironolactone backorder also has been frequent due to recent raw material shortages and delays in production,3 forcing pharmacies to refill small amounts of medication in various dosage forms despite patients owing the same copayment per prescription.
Topical Therapies
Topical retinoid prescription affordability has always been fraught with difficulty owing to age cutoffs because it is often restricted to patients younger than 25 or 40 years, depending on the plan,4,5 but the availability of adapalene gel 0.1% as an OTC preparation in 2016 has broadened retinoid access and use.6 Prescription benzoyl peroxide (BPO) products alone or in combination with retinoids or topical antibiotics (or other combination topical therapies) comprise a large number of branded prescriptions often not covered by insurance or are only affordable using proprietary and intermittently available coupon cards (eg, BPO-clindamycin, clindamycin-tretinoin, BPO-adapalene); therefore, prescribers tend to dispense the individual ingredients and instruct the patient to compound them at home. Furthermore, BPO products can be purchased in effective concentrations as OTC products, and patients looking to procure more affordable, albeit less effective, topical retinoids that also have less irritation potential reach for OTC nightly retinol creams nestled in the antiaging section of the pharmacy.7
Opportunities to be involved in the larger scaffold of patient advocacy also are plentiful at the state and national levels. For example, with the support of dermatology state societies and the American Academy of Dermatology Association, Pennsylvania House Bill 22118 and similar bills in other states call for reversal of the gag clause that prevents pharmacists from disclosing the best medication price to patients. Also guided by the American Academy of Dermatology Association, prior authorization model legislation to promote transparency across insurers in this haphazard process is emerging across the country.9,10
Final Thoughts
These examples of acne medication access and cost quandaries serve to embody the daily problem-solving that dermatologists execute as part of their growing administrative and economic duties. They also represent worthy efforts to consider on behalf of patients, their pocketbooks, and the prudent use of their dermatologists’ time.
- Borgonjen RJ, de Lange JA, van de Kerkhof PCM. Guideline-based clinical decision support in acne patients receiving isotretinoin: improving adherence and cost-effectiveness. J Eur Acad Dermatol Venereol. 2017;31:e440-e442.
- Oral isotretinoin therapy update. Highmark website. https://content.highmarkprc.com/Files/NewsletterNotices/HotTopics/ht-all-isotretinoin-therapy-update-051718.pdf. Published May 17, 2018. Accessed June 29, 2018.
- Drug shortages. US Food & Drug Administration website. https://www.fda.gov/drugs/drugsafety/drugshortages/default.htm. Updated June 19, 2018. Accessed June 19, 2018.
- Retinoids prior authorization criteria. Blue Cross and Blue Shield of Illinois website. https://www.bcbsil.com/provider/pdf/retinoids.pdf. Published March 2008. Accessed June 18, 2018.
- Davis SA, Huang KE, Feldman SR, et al. Trends in ambulatory health care usage for adult acne. J Cutan Med Surg. 2015;19:377-379.
- FDA approves Differin gel 0.1% for over-the-counter use to treat acne [press release]. Silver Spring, MD: US Food & Drug Administration; July 8, 2016.
- Rosamilia LL. Over-the-counter treatments for acne and rosacea. Semin Cutan Med Surg. 2016;35:87-95.
- An Act Providing for Consumer Prescription Drug Pricing Disclosure, HB 2211, Regular Sess (Pa 2018).
- An Act Providing for Preauthorizations Conducted by Utilization Review Entities Relating to Health Care Services, HB 1293, Regular Sess (Pa 2017).
- Step therapy legislation. American Academy of Dermatology website. https://www.aad.org/advocacy/state-policy/step-therapy-legislation. Accessed June 19, 2018.
We are fortunate to have plentiful acne treatment options available that cater to each patient’s clinical examination, predispositions, and triggers, but the choices are daunting amidst the vast prescription and over-the-counter (OTC) topicals available along with disparate insurance and cost nuances. In addition, when prescribing generic oral therapies, it is complicated to parse out regional differences in price, supply, and insurance coverage to advocate best for each patient and land upon the delicate balance between efficacy, safety, and financial stewardship, both at an individual and community level. I will outline some challenges and solutions to the management of acne amidst these complicated factors.
Oral Therapies
For isotretinoin, generic choices, cost, and tiering within insurance plans are perpetual moving targets despite the drug being the only member of its class.1 Prescriber resources include tandem searches of electronic medical record price estimates within each insurance formulary, individual pharmacy search engines, and compilation mobile applications such as GoodRx to select the most affordable version for each patient. As an example of a regional trend, isotretinoin generic coverage by one provider in Pennsylvania shifted earlier this year so that every patient, whether new to isotretinoin or midcourse, required a new prior authorization with more stringent coverage requirements including failure of 2 oral antibiotics. Swiftly thereafter, efforts across the state driven by the Pennsylvania Academy of Dermatology and Dermatologic Surgery and its members and fueled by poignant patient vignettes about fragmented and substandard patient care helped to reverse this policy and remove the prior authorization mandate.2
Tetracyclines have experienced broad cost swings, mostly based on disruption of manufacturing at the limited number of distribution sites in the United States. In 2011, a tetracycline shortage arose due to a major manufacturer’s recall3 and persisted with subsequent material shortages, as doxycycline became the preferred and cheaper member of the class. Doxycycline price tag hikes then occurred following Hurricane Sandy when an East Coast manufacturing site was damaged.3 Spironolactone backorder also has been frequent due to recent raw material shortages and delays in production,3 forcing pharmacies to refill small amounts of medication in various dosage forms despite patients owing the same copayment per prescription.
Topical Therapies
Topical retinoid prescription affordability has always been fraught with difficulty owing to age cutoffs because it is often restricted to patients younger than 25 or 40 years, depending on the plan,4,5 but the availability of adapalene gel 0.1% as an OTC preparation in 2016 has broadened retinoid access and use.6 Prescription benzoyl peroxide (BPO) products alone or in combination with retinoids or topical antibiotics (or other combination topical therapies) comprise a large number of branded prescriptions often not covered by insurance or are only affordable using proprietary and intermittently available coupon cards (eg, BPO-clindamycin, clindamycin-tretinoin, BPO-adapalene); therefore, prescribers tend to dispense the individual ingredients and instruct the patient to compound them at home. Furthermore, BPO products can be purchased in effective concentrations as OTC products, and patients looking to procure more affordable, albeit less effective, topical retinoids that also have less irritation potential reach for OTC nightly retinol creams nestled in the antiaging section of the pharmacy.7
Opportunities to be involved in the larger scaffold of patient advocacy also are plentiful at the state and national levels. For example, with the support of dermatology state societies and the American Academy of Dermatology Association, Pennsylvania House Bill 22118 and similar bills in other states call for reversal of the gag clause that prevents pharmacists from disclosing the best medication price to patients. Also guided by the American Academy of Dermatology Association, prior authorization model legislation to promote transparency across insurers in this haphazard process is emerging across the country.9,10
Final Thoughts
These examples of acne medication access and cost quandaries serve to embody the daily problem-solving that dermatologists execute as part of their growing administrative and economic duties. They also represent worthy efforts to consider on behalf of patients, their pocketbooks, and the prudent use of their dermatologists’ time.
We are fortunate to have plentiful acne treatment options available that cater to each patient’s clinical examination, predispositions, and triggers, but the choices are daunting amidst the vast prescription and over-the-counter (OTC) topicals available along with disparate insurance and cost nuances. In addition, when prescribing generic oral therapies, it is complicated to parse out regional differences in price, supply, and insurance coverage to advocate best for each patient and land upon the delicate balance between efficacy, safety, and financial stewardship, both at an individual and community level. I will outline some challenges and solutions to the management of acne amidst these complicated factors.
Oral Therapies
For isotretinoin, generic choices, cost, and tiering within insurance plans are perpetual moving targets despite the drug being the only member of its class.1 Prescriber resources include tandem searches of electronic medical record price estimates within each insurance formulary, individual pharmacy search engines, and compilation mobile applications such as GoodRx to select the most affordable version for each patient. As an example of a regional trend, isotretinoin generic coverage by one provider in Pennsylvania shifted earlier this year so that every patient, whether new to isotretinoin or midcourse, required a new prior authorization with more stringent coverage requirements including failure of 2 oral antibiotics. Swiftly thereafter, efforts across the state driven by the Pennsylvania Academy of Dermatology and Dermatologic Surgery and its members and fueled by poignant patient vignettes about fragmented and substandard patient care helped to reverse this policy and remove the prior authorization mandate.2
Tetracyclines have experienced broad cost swings, mostly based on disruption of manufacturing at the limited number of distribution sites in the United States. In 2011, a tetracycline shortage arose due to a major manufacturer’s recall3 and persisted with subsequent material shortages, as doxycycline became the preferred and cheaper member of the class. Doxycycline price tag hikes then occurred following Hurricane Sandy when an East Coast manufacturing site was damaged.3 Spironolactone backorder also has been frequent due to recent raw material shortages and delays in production,3 forcing pharmacies to refill small amounts of medication in various dosage forms despite patients owing the same copayment per prescription.
Topical Therapies
Topical retinoid prescription affordability has always been fraught with difficulty owing to age cutoffs because it is often restricted to patients younger than 25 or 40 years, depending on the plan,4,5 but the availability of adapalene gel 0.1% as an OTC preparation in 2016 has broadened retinoid access and use.6 Prescription benzoyl peroxide (BPO) products alone or in combination with retinoids or topical antibiotics (or other combination topical therapies) comprise a large number of branded prescriptions often not covered by insurance or are only affordable using proprietary and intermittently available coupon cards (eg, BPO-clindamycin, clindamycin-tretinoin, BPO-adapalene); therefore, prescribers tend to dispense the individual ingredients and instruct the patient to compound them at home. Furthermore, BPO products can be purchased in effective concentrations as OTC products, and patients looking to procure more affordable, albeit less effective, topical retinoids that also have less irritation potential reach for OTC nightly retinol creams nestled in the antiaging section of the pharmacy.7
Opportunities to be involved in the larger scaffold of patient advocacy also are plentiful at the state and national levels. For example, with the support of dermatology state societies and the American Academy of Dermatology Association, Pennsylvania House Bill 22118 and similar bills in other states call for reversal of the gag clause that prevents pharmacists from disclosing the best medication price to patients. Also guided by the American Academy of Dermatology Association, prior authorization model legislation to promote transparency across insurers in this haphazard process is emerging across the country.9,10
Final Thoughts
These examples of acne medication access and cost quandaries serve to embody the daily problem-solving that dermatologists execute as part of their growing administrative and economic duties. They also represent worthy efforts to consider on behalf of patients, their pocketbooks, and the prudent use of their dermatologists’ time.
- Borgonjen RJ, de Lange JA, van de Kerkhof PCM. Guideline-based clinical decision support in acne patients receiving isotretinoin: improving adherence and cost-effectiveness. J Eur Acad Dermatol Venereol. 2017;31:e440-e442.
- Oral isotretinoin therapy update. Highmark website. https://content.highmarkprc.com/Files/NewsletterNotices/HotTopics/ht-all-isotretinoin-therapy-update-051718.pdf. Published May 17, 2018. Accessed June 29, 2018.
- Drug shortages. US Food & Drug Administration website. https://www.fda.gov/drugs/drugsafety/drugshortages/default.htm. Updated June 19, 2018. Accessed June 19, 2018.
- Retinoids prior authorization criteria. Blue Cross and Blue Shield of Illinois website. https://www.bcbsil.com/provider/pdf/retinoids.pdf. Published March 2008. Accessed June 18, 2018.
- Davis SA, Huang KE, Feldman SR, et al. Trends in ambulatory health care usage for adult acne. J Cutan Med Surg. 2015;19:377-379.
- FDA approves Differin gel 0.1% for over-the-counter use to treat acne [press release]. Silver Spring, MD: US Food & Drug Administration; July 8, 2016.
- Rosamilia LL. Over-the-counter treatments for acne and rosacea. Semin Cutan Med Surg. 2016;35:87-95.
- An Act Providing for Consumer Prescription Drug Pricing Disclosure, HB 2211, Regular Sess (Pa 2018).
- An Act Providing for Preauthorizations Conducted by Utilization Review Entities Relating to Health Care Services, HB 1293, Regular Sess (Pa 2017).
- Step therapy legislation. American Academy of Dermatology website. https://www.aad.org/advocacy/state-policy/step-therapy-legislation. Accessed June 19, 2018.
- Borgonjen RJ, de Lange JA, van de Kerkhof PCM. Guideline-based clinical decision support in acne patients receiving isotretinoin: improving adherence and cost-effectiveness. J Eur Acad Dermatol Venereol. 2017;31:e440-e442.
- Oral isotretinoin therapy update. Highmark website. https://content.highmarkprc.com/Files/NewsletterNotices/HotTopics/ht-all-isotretinoin-therapy-update-051718.pdf. Published May 17, 2018. Accessed June 29, 2018.
- Drug shortages. US Food & Drug Administration website. https://www.fda.gov/drugs/drugsafety/drugshortages/default.htm. Updated June 19, 2018. Accessed June 19, 2018.
- Retinoids prior authorization criteria. Blue Cross and Blue Shield of Illinois website. https://www.bcbsil.com/provider/pdf/retinoids.pdf. Published March 2008. Accessed June 18, 2018.
- Davis SA, Huang KE, Feldman SR, et al. Trends in ambulatory health care usage for adult acne. J Cutan Med Surg. 2015;19:377-379.
- FDA approves Differin gel 0.1% for over-the-counter use to treat acne [press release]. Silver Spring, MD: US Food & Drug Administration; July 8, 2016.
- Rosamilia LL. Over-the-counter treatments for acne and rosacea. Semin Cutan Med Surg. 2016;35:87-95.
- An Act Providing for Consumer Prescription Drug Pricing Disclosure, HB 2211, Regular Sess (Pa 2018).
- An Act Providing for Preauthorizations Conducted by Utilization Review Entities Relating to Health Care Services, HB 1293, Regular Sess (Pa 2017).
- Step therapy legislation. American Academy of Dermatology website. https://www.aad.org/advocacy/state-policy/step-therapy-legislation. Accessed June 19, 2018.
Topical 5-Fluorouracil Made Easy?
What is the recent research behind 5-fluorouracil cream 5% combined with calcipotriol ointment 0.005% for actinic keratoses?
Cunningham et al published a randomized double-blind study in which 131 patients with actinic keratoses (AKs) were assigned to either 5-fluorouracil (5-FU) cream 5% combined with calcipotriol (calcipotriene) ointment 0.005% twice daily to the face, scalp, and arms for 4 days, or 5-FU 5% combined with petrolatum applied in the same fashion. There was an 87.8% versus 26.3% mean reduction in the number of AKs and less severe pain, crusting, and ulceration in the study cohort compared to the 5-FU plus petrolatum group.
The same study also investigated immune parameters in these patients and found that the study group preferentially displayed activated thymic stromal lymphopoietin and a CD4 T cell-mediated reaction, among other effects. In prior studies, thymic stromal lymphopoietin has been shown to be upregulated in barrier-defective skin, displays antitumor activity, and is enhanced by topical calcipotriol application based on its original indication for psoriasis.
How do these study results impact patient care?
In a perfect world, every patient could tolerate and afford chemopreventative measures such as 5-FU cream, apply it diffusely to sun-exposed skin, and experience no severe irritant reactions and/or social pariah status. We all know that this product is effective, and we all overprepare patients to use it, knowing that they will call our offices panicked and fearful that they are allergic to or are becoming infected by this cream.
Although further study clearly is needed to determine the optimal application amount, duration of use, and vehicle mix, this new compound utilizing 2 topicals that are familiar to us--5-FU cream approved for AKs and early squamous cell skin cancers and calcipotriol ointment (though available only in cream in the United States currently) for psoriasis--is an encouraging step. Home therapy for AKs and possibly early nonmelanoma skin cancers that is more tolerable, of shorter duration, and in turn more effective than the current options would lessen the burden of treating these lesions surgically or rescheduling 5-FU patients often for irritation reaction education.
How do patients respond to this regimen?
In my own anecdotal experience, this regimen has been well received by patients and often is covered by most insurances when written as 2 separate prescriptions (both in cream vehicle). They still report some irritation, but I prefer to utilize it segmentally instead of treating all sun-exposed areas at once (ie, treat one side of the face/scalp twice daily for 4 days, then the other, or even divide it into smaller segments once the prior segment has healed). This combination, in addition to, for example, adding nicotinamide 500 mg twice daily to a patient's skin cancer chemopreventative sequence, is in my opinion a novel but safe, effective, and well-tolerated field therapy recommendation.
American Academy of Dermatology Actinic Keratosis Overview
American Academy of Family Physicians Actinic Keratoses Information
Suggested Readings
- Cunningham TJ, Tabacchi M, Eliane JP, et al. Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J Clin Invest. 2017;127:106-116.
- Demehri S, Turkoz A, Manivasagam S, et al. Elevated epidermal thymic stromal lymphopoietin levels establish an antitumor environment in the skin. Cancer Cell. 2012;22:494-505.
- Rosamilia LL. Three Cheers for B3? Cutis. July 7, 2015. http://www.mdedge.com/cutis/article/101102/nonmelanoma-skin-cancer/three-cheers-b3. Accessed November 20, 2017.
- Sato-Deguchi E, Imafuku S, Chou B, et al. Topical vitamin D(3) analogues induce thymic stromal lymphopoietin and cathelicidin in psoriatic skin lesions. Br J Dermatol. 2012;167:77-84.
What is the recent research behind 5-fluorouracil cream 5% combined with calcipotriol ointment 0.005% for actinic keratoses?
Cunningham et al published a randomized double-blind study in which 131 patients with actinic keratoses (AKs) were assigned to either 5-fluorouracil (5-FU) cream 5% combined with calcipotriol (calcipotriene) ointment 0.005% twice daily to the face, scalp, and arms for 4 days, or 5-FU 5% combined with petrolatum applied in the same fashion. There was an 87.8% versus 26.3% mean reduction in the number of AKs and less severe pain, crusting, and ulceration in the study cohort compared to the 5-FU plus petrolatum group.
The same study also investigated immune parameters in these patients and found that the study group preferentially displayed activated thymic stromal lymphopoietin and a CD4 T cell-mediated reaction, among other effects. In prior studies, thymic stromal lymphopoietin has been shown to be upregulated in barrier-defective skin, displays antitumor activity, and is enhanced by topical calcipotriol application based on its original indication for psoriasis.
How do these study results impact patient care?
In a perfect world, every patient could tolerate and afford chemopreventative measures such as 5-FU cream, apply it diffusely to sun-exposed skin, and experience no severe irritant reactions and/or social pariah status. We all know that this product is effective, and we all overprepare patients to use it, knowing that they will call our offices panicked and fearful that they are allergic to or are becoming infected by this cream.
Although further study clearly is needed to determine the optimal application amount, duration of use, and vehicle mix, this new compound utilizing 2 topicals that are familiar to us--5-FU cream approved for AKs and early squamous cell skin cancers and calcipotriol ointment (though available only in cream in the United States currently) for psoriasis--is an encouraging step. Home therapy for AKs and possibly early nonmelanoma skin cancers that is more tolerable, of shorter duration, and in turn more effective than the current options would lessen the burden of treating these lesions surgically or rescheduling 5-FU patients often for irritation reaction education.
How do patients respond to this regimen?
In my own anecdotal experience, this regimen has been well received by patients and often is covered by most insurances when written as 2 separate prescriptions (both in cream vehicle). They still report some irritation, but I prefer to utilize it segmentally instead of treating all sun-exposed areas at once (ie, treat one side of the face/scalp twice daily for 4 days, then the other, or even divide it into smaller segments once the prior segment has healed). This combination, in addition to, for example, adding nicotinamide 500 mg twice daily to a patient's skin cancer chemopreventative sequence, is in my opinion a novel but safe, effective, and well-tolerated field therapy recommendation.
American Academy of Dermatology Actinic Keratosis Overview
American Academy of Family Physicians Actinic Keratoses Information
Suggested Readings
- Cunningham TJ, Tabacchi M, Eliane JP, et al. Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J Clin Invest. 2017;127:106-116.
- Demehri S, Turkoz A, Manivasagam S, et al. Elevated epidermal thymic stromal lymphopoietin levels establish an antitumor environment in the skin. Cancer Cell. 2012;22:494-505.
- Rosamilia LL. Three Cheers for B3? Cutis. July 7, 2015. http://www.mdedge.com/cutis/article/101102/nonmelanoma-skin-cancer/three-cheers-b3. Accessed November 20, 2017.
- Sato-Deguchi E, Imafuku S, Chou B, et al. Topical vitamin D(3) analogues induce thymic stromal lymphopoietin and cathelicidin in psoriatic skin lesions. Br J Dermatol. 2012;167:77-84.
What is the recent research behind 5-fluorouracil cream 5% combined with calcipotriol ointment 0.005% for actinic keratoses?
Cunningham et al published a randomized double-blind study in which 131 patients with actinic keratoses (AKs) were assigned to either 5-fluorouracil (5-FU) cream 5% combined with calcipotriol (calcipotriene) ointment 0.005% twice daily to the face, scalp, and arms for 4 days, or 5-FU 5% combined with petrolatum applied in the same fashion. There was an 87.8% versus 26.3% mean reduction in the number of AKs and less severe pain, crusting, and ulceration in the study cohort compared to the 5-FU plus petrolatum group.
The same study also investigated immune parameters in these patients and found that the study group preferentially displayed activated thymic stromal lymphopoietin and a CD4 T cell-mediated reaction, among other effects. In prior studies, thymic stromal lymphopoietin has been shown to be upregulated in barrier-defective skin, displays antitumor activity, and is enhanced by topical calcipotriol application based on its original indication for psoriasis.
How do these study results impact patient care?
In a perfect world, every patient could tolerate and afford chemopreventative measures such as 5-FU cream, apply it diffusely to sun-exposed skin, and experience no severe irritant reactions and/or social pariah status. We all know that this product is effective, and we all overprepare patients to use it, knowing that they will call our offices panicked and fearful that they are allergic to or are becoming infected by this cream.
Although further study clearly is needed to determine the optimal application amount, duration of use, and vehicle mix, this new compound utilizing 2 topicals that are familiar to us--5-FU cream approved for AKs and early squamous cell skin cancers and calcipotriol ointment (though available only in cream in the United States currently) for psoriasis--is an encouraging step. Home therapy for AKs and possibly early nonmelanoma skin cancers that is more tolerable, of shorter duration, and in turn more effective than the current options would lessen the burden of treating these lesions surgically or rescheduling 5-FU patients often for irritation reaction education.
How do patients respond to this regimen?
In my own anecdotal experience, this regimen has been well received by patients and often is covered by most insurances when written as 2 separate prescriptions (both in cream vehicle). They still report some irritation, but I prefer to utilize it segmentally instead of treating all sun-exposed areas at once (ie, treat one side of the face/scalp twice daily for 4 days, then the other, or even divide it into smaller segments once the prior segment has healed). This combination, in addition to, for example, adding nicotinamide 500 mg twice daily to a patient's skin cancer chemopreventative sequence, is in my opinion a novel but safe, effective, and well-tolerated field therapy recommendation.
American Academy of Dermatology Actinic Keratosis Overview
American Academy of Family Physicians Actinic Keratoses Information
Suggested Readings
- Cunningham TJ, Tabacchi M, Eliane JP, et al. Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J Clin Invest. 2017;127:106-116.
- Demehri S, Turkoz A, Manivasagam S, et al. Elevated epidermal thymic stromal lymphopoietin levels establish an antitumor environment in the skin. Cancer Cell. 2012;22:494-505.
- Rosamilia LL. Three Cheers for B3? Cutis. July 7, 2015. http://www.mdedge.com/cutis/article/101102/nonmelanoma-skin-cancer/three-cheers-b3. Accessed November 20, 2017.
- Sato-Deguchi E, Imafuku S, Chou B, et al. Topical vitamin D(3) analogues induce thymic stromal lymphopoietin and cathelicidin in psoriatic skin lesions. Br J Dermatol. 2012;167:77-84.
Rosacea Treatment Schema: An Update
When tasked with outlining updated therapy regimens for rosacea, specific patient vignettes come to mind.
A 53-year-old male golfer presents with years of central facial flushing, prominent telangiectases, erythema, and scattered pink papules. He attempted various over-the-counter topical products indicated for acne, such as salicylic acid scrub and benzoyl peroxide cream, with no improvement and much irritation. Recently, his wife has been helping him apply redness-concealing makeup in the morning and over-the-counter hydrocortisone cream in the evening, which has been slightly helpful.
This patient’s rosacea could conceivably be labeled under the papulopustular rosacea subtype; however, the conventional categories are fluid with subtype overlap and imprecise diagnostic criteria. He also seemed to display features of the erythematotelangiectatic subtype, perhaps with underlying photodamage as well as steroid rebound erythema and/or atrophy.1 Nevertheless, it is a common presentation, and certain baseline tenets should be applied. First, all steroid products and irritants (eg, benzoyl peroxide and salicylic acid ingredients, any scrub vehicle) should be discontinued. Education about avoidance of triggers (ie, sun, heat, spicy food, alcohol, stress is paramount. Because barrier inadequacy is a recent insight into rosacea pathogenesis, mild syndet- or lipid-free cleansers, daily sunscreen, and evening emollients dictate baseline skin care, as does meticulous situation-specific sun protection.2,3 The papular component and immediate erythema in and around the papules can be managed topically (prior to sunscreen or emollient application) with metronidazole gel or cream up to twice daily, ivermectin cream once daily, or azelaic acid gel or foam up to twice daily. Oral doxycycline 40 mg (delayed release) on an empty stomach or 50 mg (immediate release) with food to avert antimicrobial dosing and antibiotic resistance also could be considered if topical therapy is inadequate or irritating, though gastrointestinal comorbidities with rosacea also should be delineated before initiating oral antibiotics.4-6 (Management of this patient’s nonlesional fixed erythema, telangiectases, and flushing is discussed after the next vignette.)
What if a woman presented in a similar fashion as above, only without papules? Her family physician prescribed metronidazole gel twice daily for years with no improvement in flushing, redness, or telangiectases.
Background erythema in rosacea often is persistent with trigger-specific intensification, with or without episodic facial flushing; undoubtedly, these symptoms can be difficult to compartmentalize depending on the clarity of the patient’s history and frequency of clinic visits. The aforementioned baseline skin care and sun-protection regimen applies, and newer topical agents such as α-adrenergics (daily oxymetazoline cream or brimonidine gel) may be considered for persistent erythema; however, irritant potential and rebound erythema are common.7-9 Topical therapies such as metronidazole gel, as in this case, are inadequate for persistent background erythema or flushing. Persistent erythema and telangiectases can be reduced with pulsed dye laser or intense pulsed light modalities, particularly following conservative management of acute inflammation.5 Episodic flushing is poorly controlled with the above tactics, but anecdotally, topical or oral α-adrenergics or oral nonselective beta-blockers could be considered; the latter is also applicable to migraine therapy, which is perhaps comorbid with rosacea.5,10
A 35-year-old Hispanic woman states that the scalp, forehead, and cheeks have been flaky, pink, and pruritic for years. She saw several aestheticians for it and the admixed “acne” on the face, receiving salicylic acid chemical peels with no improvement and much dyspigmentation.
Although underreported, the commingling of rosacea with seborrheic dermatitis is common, perhaps with mutual Demodex mite overpopulation, assigning topical therapies to its management such as daily ivermectin cream or steroid-sparing pimecrolimus cream for inflammatory papules and scaly regions of the face and scalp.11-13 Further, this case exemplifies the increasing incidence and awareness of rosacea in darker skin types, along with its postinflammatory pigmentary perturbations, which necessitate repeated education about barrier control and sun protection.14
A 72-year-old male farmer presents with his wife whoinsists that his nose has been increasing in size for years; she procures a prior driver’s license photograph as proof. She also notes that he has been snoring at night and having more trouble breathing while working outdoors. The patient had not noticed.
Phymatous rosacea may exist as an additional feature of any rosacea subtype or as a singular finding, presenting as actively inflamed, fibrotic/noninflamed, or both. Management, particularly if inflamed, involves baseline gentle skin care and sun protection, avoidance of rosacea triggers, and implementation of oral therapy such as doxycycline or isotretinoin. Many cases, particularly those with a fibrotic component, warrant surgical methods such as fractionated CO2 laser or Shaw scalpel surgical sculpting. These cases frequently demonstrate varying degrees of airway compromise, validating surgery as a legitimate medical, not merely cosmetic, presentation.5,15
Final Thoughts
The Table, constructed as a concise therapy compendium by the ROSacea COnsensus (ROSCO) international panel of dermatologists and ophthalmologists, outlines data-driven and expert experience-based therapies for rosacea.5 This panel asserts that phenotypical features, not rigid subtypes, oblige patient-specific treatment schema. Also, as these cases outline, an evolving understanding of rosacea’s multifaceted pathogenesis, assorted presentations, and frequent pitfalls in daily skin care and initial management require individualized care.
- Tan J, Steinhoff M, Berg M, et al; Rosacea International Study Group. Shortcomings in rosacea diagnosis and classification. Br J Dermatol. 2017;176:197-199.
- Levin J, Miller R. A guide to the ingredients and potential benefits of over-the-counter cleansers and moisturizers for rosacea patients. J Clin Aesthet Dermatol. 2011;4:31-49.
- Del Rosso JQ. Adjunctive skin care in the management of rosacea: cleansers, moisturizers, and photoprotectants. Cutis. 2005;75(suppl 3):17-21;discussion 33-36.
- van Zuuren EJ, Fedorowicz Z. Interventions for rosacea: abridged updated Cochrane systematic review including GRADE assessments [published online August 30, 2015]. Br J Dermatol. 2015;173:651-662.
- Schaller M, Almeida LM, Bewley A, et al. Rosacea treatment update: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:465-471.
- Egeberg A, Weinstock LB, Thvssen EP, et al. Rosacea and gastrointestinal disorders: a population-based cohort study. Br J Dermatol. 2017;176:100-106.
- Layton AM, Schaller M, Homey B, et al. Brimonidine gel 0.33% rapidly improves patient-reported outcomes by controlling facial erythema of rosacea: a randomized, double-blind, vehicle-controlled study. J Eur Acad Dermatol Venereol. 2015;29:2405-2410.
- Docherty JR, Steinhoff M, Lorton D, et al. Multidisciplinary consideration of potential pathophysiologic mechanisms of paradoxical erythema with topical brimonidine therapy [published online August 25, 2016]. Adv Ther. 2016;33:1885-1895.
- Shanler SD, Ondo AL. Successful treatment of the erythema and flushing of rosacea using a topically applied selective alpha1-adrenergic receptor agonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
- Egeberg A, Ashina M, Gaist D, et al. Prevalence and risk of migraine in patients with rosacea: a population-based cohort study. J Am Acad Dermatol. 2017;76:454-458.
- Zhao YE, Peng Y, Wang XL, et al. Facial dermatosis associated with Demodex: a case-control study. J Zhejiang Univ Sci B. 2011;12:1008-1015.
- Siddiqui K, Stein Gold L, Gill J. The efficacy, safety, and tolerability of ivermectin compared with current topical treatments for the inflammatory lesions of rosacea: a network meta-analysis. Springerplus. 2016;5:1151. doi: 10.1186/s40064-016-2819-8.
- Kim MB, Kim GW, Park HJ, et al. Pimecrolimus 1% cream for the treatment of rosacea. J Dermatol. 2011;38:1135-1139.
- Al-Dabagh A, Davis SA, McMichael AJ, et al. Rosacea in skin of color: not a rare diagnosis. Dermatol Online J. 2014;20. pii:13030/qt1mv9r0ss.
- Little SC, Stucker FJ, Compton A, et al. Nuances in the management of rhinophyma. Facial Plast Surg. 2012;28:231-237.
When tasked with outlining updated therapy regimens for rosacea, specific patient vignettes come to mind.
A 53-year-old male golfer presents with years of central facial flushing, prominent telangiectases, erythema, and scattered pink papules. He attempted various over-the-counter topical products indicated for acne, such as salicylic acid scrub and benzoyl peroxide cream, with no improvement and much irritation. Recently, his wife has been helping him apply redness-concealing makeup in the morning and over-the-counter hydrocortisone cream in the evening, which has been slightly helpful.
This patient’s rosacea could conceivably be labeled under the papulopustular rosacea subtype; however, the conventional categories are fluid with subtype overlap and imprecise diagnostic criteria. He also seemed to display features of the erythematotelangiectatic subtype, perhaps with underlying photodamage as well as steroid rebound erythema and/or atrophy.1 Nevertheless, it is a common presentation, and certain baseline tenets should be applied. First, all steroid products and irritants (eg, benzoyl peroxide and salicylic acid ingredients, any scrub vehicle) should be discontinued. Education about avoidance of triggers (ie, sun, heat, spicy food, alcohol, stress is paramount. Because barrier inadequacy is a recent insight into rosacea pathogenesis, mild syndet- or lipid-free cleansers, daily sunscreen, and evening emollients dictate baseline skin care, as does meticulous situation-specific sun protection.2,3 The papular component and immediate erythema in and around the papules can be managed topically (prior to sunscreen or emollient application) with metronidazole gel or cream up to twice daily, ivermectin cream once daily, or azelaic acid gel or foam up to twice daily. Oral doxycycline 40 mg (delayed release) on an empty stomach or 50 mg (immediate release) with food to avert antimicrobial dosing and antibiotic resistance also could be considered if topical therapy is inadequate or irritating, though gastrointestinal comorbidities with rosacea also should be delineated before initiating oral antibiotics.4-6 (Management of this patient’s nonlesional fixed erythema, telangiectases, and flushing is discussed after the next vignette.)
What if a woman presented in a similar fashion as above, only without papules? Her family physician prescribed metronidazole gel twice daily for years with no improvement in flushing, redness, or telangiectases.
Background erythema in rosacea often is persistent with trigger-specific intensification, with or without episodic facial flushing; undoubtedly, these symptoms can be difficult to compartmentalize depending on the clarity of the patient’s history and frequency of clinic visits. The aforementioned baseline skin care and sun-protection regimen applies, and newer topical agents such as α-adrenergics (daily oxymetazoline cream or brimonidine gel) may be considered for persistent erythema; however, irritant potential and rebound erythema are common.7-9 Topical therapies such as metronidazole gel, as in this case, are inadequate for persistent background erythema or flushing. Persistent erythema and telangiectases can be reduced with pulsed dye laser or intense pulsed light modalities, particularly following conservative management of acute inflammation.5 Episodic flushing is poorly controlled with the above tactics, but anecdotally, topical or oral α-adrenergics or oral nonselective beta-blockers could be considered; the latter is also applicable to migraine therapy, which is perhaps comorbid with rosacea.5,10
A 35-year-old Hispanic woman states that the scalp, forehead, and cheeks have been flaky, pink, and pruritic for years. She saw several aestheticians for it and the admixed “acne” on the face, receiving salicylic acid chemical peels with no improvement and much dyspigmentation.
Although underreported, the commingling of rosacea with seborrheic dermatitis is common, perhaps with mutual Demodex mite overpopulation, assigning topical therapies to its management such as daily ivermectin cream or steroid-sparing pimecrolimus cream for inflammatory papules and scaly regions of the face and scalp.11-13 Further, this case exemplifies the increasing incidence and awareness of rosacea in darker skin types, along with its postinflammatory pigmentary perturbations, which necessitate repeated education about barrier control and sun protection.14
A 72-year-old male farmer presents with his wife whoinsists that his nose has been increasing in size for years; she procures a prior driver’s license photograph as proof. She also notes that he has been snoring at night and having more trouble breathing while working outdoors. The patient had not noticed.
Phymatous rosacea may exist as an additional feature of any rosacea subtype or as a singular finding, presenting as actively inflamed, fibrotic/noninflamed, or both. Management, particularly if inflamed, involves baseline gentle skin care and sun protection, avoidance of rosacea triggers, and implementation of oral therapy such as doxycycline or isotretinoin. Many cases, particularly those with a fibrotic component, warrant surgical methods such as fractionated CO2 laser or Shaw scalpel surgical sculpting. These cases frequently demonstrate varying degrees of airway compromise, validating surgery as a legitimate medical, not merely cosmetic, presentation.5,15
Final Thoughts
The Table, constructed as a concise therapy compendium by the ROSacea COnsensus (ROSCO) international panel of dermatologists and ophthalmologists, outlines data-driven and expert experience-based therapies for rosacea.5 This panel asserts that phenotypical features, not rigid subtypes, oblige patient-specific treatment schema. Also, as these cases outline, an evolving understanding of rosacea’s multifaceted pathogenesis, assorted presentations, and frequent pitfalls in daily skin care and initial management require individualized care.
When tasked with outlining updated therapy regimens for rosacea, specific patient vignettes come to mind.
A 53-year-old male golfer presents with years of central facial flushing, prominent telangiectases, erythema, and scattered pink papules. He attempted various over-the-counter topical products indicated for acne, such as salicylic acid scrub and benzoyl peroxide cream, with no improvement and much irritation. Recently, his wife has been helping him apply redness-concealing makeup in the morning and over-the-counter hydrocortisone cream in the evening, which has been slightly helpful.
This patient’s rosacea could conceivably be labeled under the papulopustular rosacea subtype; however, the conventional categories are fluid with subtype overlap and imprecise diagnostic criteria. He also seemed to display features of the erythematotelangiectatic subtype, perhaps with underlying photodamage as well as steroid rebound erythema and/or atrophy.1 Nevertheless, it is a common presentation, and certain baseline tenets should be applied. First, all steroid products and irritants (eg, benzoyl peroxide and salicylic acid ingredients, any scrub vehicle) should be discontinued. Education about avoidance of triggers (ie, sun, heat, spicy food, alcohol, stress is paramount. Because barrier inadequacy is a recent insight into rosacea pathogenesis, mild syndet- or lipid-free cleansers, daily sunscreen, and evening emollients dictate baseline skin care, as does meticulous situation-specific sun protection.2,3 The papular component and immediate erythema in and around the papules can be managed topically (prior to sunscreen or emollient application) with metronidazole gel or cream up to twice daily, ivermectin cream once daily, or azelaic acid gel or foam up to twice daily. Oral doxycycline 40 mg (delayed release) on an empty stomach or 50 mg (immediate release) with food to avert antimicrobial dosing and antibiotic resistance also could be considered if topical therapy is inadequate or irritating, though gastrointestinal comorbidities with rosacea also should be delineated before initiating oral antibiotics.4-6 (Management of this patient’s nonlesional fixed erythema, telangiectases, and flushing is discussed after the next vignette.)
What if a woman presented in a similar fashion as above, only without papules? Her family physician prescribed metronidazole gel twice daily for years with no improvement in flushing, redness, or telangiectases.
Background erythema in rosacea often is persistent with trigger-specific intensification, with or without episodic facial flushing; undoubtedly, these symptoms can be difficult to compartmentalize depending on the clarity of the patient’s history and frequency of clinic visits. The aforementioned baseline skin care and sun-protection regimen applies, and newer topical agents such as α-adrenergics (daily oxymetazoline cream or brimonidine gel) may be considered for persistent erythema; however, irritant potential and rebound erythema are common.7-9 Topical therapies such as metronidazole gel, as in this case, are inadequate for persistent background erythema or flushing. Persistent erythema and telangiectases can be reduced with pulsed dye laser or intense pulsed light modalities, particularly following conservative management of acute inflammation.5 Episodic flushing is poorly controlled with the above tactics, but anecdotally, topical or oral α-adrenergics or oral nonselective beta-blockers could be considered; the latter is also applicable to migraine therapy, which is perhaps comorbid with rosacea.5,10
A 35-year-old Hispanic woman states that the scalp, forehead, and cheeks have been flaky, pink, and pruritic for years. She saw several aestheticians for it and the admixed “acne” on the face, receiving salicylic acid chemical peels with no improvement and much dyspigmentation.
Although underreported, the commingling of rosacea with seborrheic dermatitis is common, perhaps with mutual Demodex mite overpopulation, assigning topical therapies to its management such as daily ivermectin cream or steroid-sparing pimecrolimus cream for inflammatory papules and scaly regions of the face and scalp.11-13 Further, this case exemplifies the increasing incidence and awareness of rosacea in darker skin types, along with its postinflammatory pigmentary perturbations, which necessitate repeated education about barrier control and sun protection.14
A 72-year-old male farmer presents with his wife whoinsists that his nose has been increasing in size for years; she procures a prior driver’s license photograph as proof. She also notes that he has been snoring at night and having more trouble breathing while working outdoors. The patient had not noticed.
Phymatous rosacea may exist as an additional feature of any rosacea subtype or as a singular finding, presenting as actively inflamed, fibrotic/noninflamed, or both. Management, particularly if inflamed, involves baseline gentle skin care and sun protection, avoidance of rosacea triggers, and implementation of oral therapy such as doxycycline or isotretinoin. Many cases, particularly those with a fibrotic component, warrant surgical methods such as fractionated CO2 laser or Shaw scalpel surgical sculpting. These cases frequently demonstrate varying degrees of airway compromise, validating surgery as a legitimate medical, not merely cosmetic, presentation.5,15
Final Thoughts
The Table, constructed as a concise therapy compendium by the ROSacea COnsensus (ROSCO) international panel of dermatologists and ophthalmologists, outlines data-driven and expert experience-based therapies for rosacea.5 This panel asserts that phenotypical features, not rigid subtypes, oblige patient-specific treatment schema. Also, as these cases outline, an evolving understanding of rosacea’s multifaceted pathogenesis, assorted presentations, and frequent pitfalls in daily skin care and initial management require individualized care.
- Tan J, Steinhoff M, Berg M, et al; Rosacea International Study Group. Shortcomings in rosacea diagnosis and classification. Br J Dermatol. 2017;176:197-199.
- Levin J, Miller R. A guide to the ingredients and potential benefits of over-the-counter cleansers and moisturizers for rosacea patients. J Clin Aesthet Dermatol. 2011;4:31-49.
- Del Rosso JQ. Adjunctive skin care in the management of rosacea: cleansers, moisturizers, and photoprotectants. Cutis. 2005;75(suppl 3):17-21;discussion 33-36.
- van Zuuren EJ, Fedorowicz Z. Interventions for rosacea: abridged updated Cochrane systematic review including GRADE assessments [published online August 30, 2015]. Br J Dermatol. 2015;173:651-662.
- Schaller M, Almeida LM, Bewley A, et al. Rosacea treatment update: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:465-471.
- Egeberg A, Weinstock LB, Thvssen EP, et al. Rosacea and gastrointestinal disorders: a population-based cohort study. Br J Dermatol. 2017;176:100-106.
- Layton AM, Schaller M, Homey B, et al. Brimonidine gel 0.33% rapidly improves patient-reported outcomes by controlling facial erythema of rosacea: a randomized, double-blind, vehicle-controlled study. J Eur Acad Dermatol Venereol. 2015;29:2405-2410.
- Docherty JR, Steinhoff M, Lorton D, et al. Multidisciplinary consideration of potential pathophysiologic mechanisms of paradoxical erythema with topical brimonidine therapy [published online August 25, 2016]. Adv Ther. 2016;33:1885-1895.
- Shanler SD, Ondo AL. Successful treatment of the erythema and flushing of rosacea using a topically applied selective alpha1-adrenergic receptor agonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
- Egeberg A, Ashina M, Gaist D, et al. Prevalence and risk of migraine in patients with rosacea: a population-based cohort study. J Am Acad Dermatol. 2017;76:454-458.
- Zhao YE, Peng Y, Wang XL, et al. Facial dermatosis associated with Demodex: a case-control study. J Zhejiang Univ Sci B. 2011;12:1008-1015.
- Siddiqui K, Stein Gold L, Gill J. The efficacy, safety, and tolerability of ivermectin compared with current topical treatments for the inflammatory lesions of rosacea: a network meta-analysis. Springerplus. 2016;5:1151. doi: 10.1186/s40064-016-2819-8.
- Kim MB, Kim GW, Park HJ, et al. Pimecrolimus 1% cream for the treatment of rosacea. J Dermatol. 2011;38:1135-1139.
- Al-Dabagh A, Davis SA, McMichael AJ, et al. Rosacea in skin of color: not a rare diagnosis. Dermatol Online J. 2014;20. pii:13030/qt1mv9r0ss.
- Little SC, Stucker FJ, Compton A, et al. Nuances in the management of rhinophyma. Facial Plast Surg. 2012;28:231-237.
- Tan J, Steinhoff M, Berg M, et al; Rosacea International Study Group. Shortcomings in rosacea diagnosis and classification. Br J Dermatol. 2017;176:197-199.
- Levin J, Miller R. A guide to the ingredients and potential benefits of over-the-counter cleansers and moisturizers for rosacea patients. J Clin Aesthet Dermatol. 2011;4:31-49.
- Del Rosso JQ. Adjunctive skin care in the management of rosacea: cleansers, moisturizers, and photoprotectants. Cutis. 2005;75(suppl 3):17-21;discussion 33-36.
- van Zuuren EJ, Fedorowicz Z. Interventions for rosacea: abridged updated Cochrane systematic review including GRADE assessments [published online August 30, 2015]. Br J Dermatol. 2015;173:651-662.
- Schaller M, Almeida LM, Bewley A, et al. Rosacea treatment update: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:465-471.
- Egeberg A, Weinstock LB, Thvssen EP, et al. Rosacea and gastrointestinal disorders: a population-based cohort study. Br J Dermatol. 2017;176:100-106.
- Layton AM, Schaller M, Homey B, et al. Brimonidine gel 0.33% rapidly improves patient-reported outcomes by controlling facial erythema of rosacea: a randomized, double-blind, vehicle-controlled study. J Eur Acad Dermatol Venereol. 2015;29:2405-2410.
- Docherty JR, Steinhoff M, Lorton D, et al. Multidisciplinary consideration of potential pathophysiologic mechanisms of paradoxical erythema with topical brimonidine therapy [published online August 25, 2016]. Adv Ther. 2016;33:1885-1895.
- Shanler SD, Ondo AL. Successful treatment of the erythema and flushing of rosacea using a topically applied selective alpha1-adrenergic receptor agonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
- Egeberg A, Ashina M, Gaist D, et al. Prevalence and risk of migraine in patients with rosacea: a population-based cohort study. J Am Acad Dermatol. 2017;76:454-458.
- Zhao YE, Peng Y, Wang XL, et al. Facial dermatosis associated with Demodex: a case-control study. J Zhejiang Univ Sci B. 2011;12:1008-1015.
- Siddiqui K, Stein Gold L, Gill J. The efficacy, safety, and tolerability of ivermectin compared with current topical treatments for the inflammatory lesions of rosacea: a network meta-analysis. Springerplus. 2016;5:1151. doi: 10.1186/s40064-016-2819-8.
- Kim MB, Kim GW, Park HJ, et al. Pimecrolimus 1% cream for the treatment of rosacea. J Dermatol. 2011;38:1135-1139.
- Al-Dabagh A, Davis SA, McMichael AJ, et al. Rosacea in skin of color: not a rare diagnosis. Dermatol Online J. 2014;20. pii:13030/qt1mv9r0ss.
- Little SC, Stucker FJ, Compton A, et al. Nuances in the management of rhinophyma. Facial Plast Surg. 2012;28:231-237.
Zika Understanding Unfolds
Inundating our popular and academic media circles is information regarding the Zika virus. A recent article by Farahnik et al in the Journal of the American Academy of Dermatology (2016;74:1286-1287) briefly outlines what is known about Zika infection thus far and its dermatologic manifestations. Pairing this article with Centers for Disease Control and Prevention guidelines on the topic, we are presented with an evolving introduction to this new entity. Here’s what we know:
- It is a single-stranded RNA arbovirus in the Flavivirus family transmitted by the bite of Aedes mosquitoes, with cases reported so far in Africa, Asia, and the Americas (particularly southern coastal and island destinations).
- It also is transmitted via transfusion of blood, sexual contact, and mother to fetus.
- There is theoretical risk for fetal microcephaly, intracranial calcifications, and other brain and eye abnormalities.
- Only 1 in 5 affected patients show any systemic manifestations of infection, including self-limited flulike symptoms and nonspecific exanthema, typically sparing acral sites and occurring within 1 to 2 weeks of virus exposure.
- Testing is recommended for pregnant women with possible Zika exposure (ie, travel to an area with active transmission of Zika virus, unprotected sex with a male with this travel history).
- Diagnosis can be made through state health departments, employing real-time reverse transcriptase–polymerase chain reaction (rRT-PCR) or enzyme-linked immunosorbent assay the week after symptom onset using serum, or rRT-PCR 2 weeks after symptom onset using urine. Further antibody testing can be done if a false-negative is suspected, but false-positives also are possible if a patient was exposed to or vaccinated against other flaviviruses (eg, dengue virus, West Nile virus, yellow fever virus)
- Testing is inaccurate if ordered within 7 days or more than 12 weeks following presumed exposure.
- If positive or inconclusive testing arises, serial fetal ultrasonography should be considered; if testing is negative, then a single fetal ultrasound is recommended to detect Zika abnormalities.
- Test results are automatically reported to respective state health departments.
- There is no treatment of this infection aside from supportive care.
What’s the issue?
As with any new outbreak, the applicability to the general population and true risks remain to be seen. Each of our clinics recalls the stark changes in patient intake and screening questions with infections as ubiquitous as methicillin-resistant Staphylococcus aureus to much rarer exposures such as Ebola virus, each with progressive understanding of risk groups, disease manifestations, and eradication and prevention measures.
By mid-June 2016, 30 hits on PubMed addressing Zika had already been cited just within the month, outlining various aspects of the infection, and many specialties, particularly neurology, obstetrics, primary care, infectious disease, and dermatology, are weighing in. Unfortunately, the majority of cases of primary Zika infection do not manifest with skin or systemic symptoms, and even cases that do are nonspecific, exanthematous, and flulike.
Vague as it may be so far, it is nonetheless imperative that clinicians be familiar with what is concretely known about Zika virus and acquaint ourselves with the travel distribution and restrictions, disease risk factors, known sequelae, testing availability and limitations, and reporting guidelines. From personal experience, as I traveled to Belize earlier this year during my first trimester of pregnancy before the travel restrictions were outlined, even obstetricians are not wholly familiar with the manner in which to order testing and the appropriate window to do so. I have been asymptomatic, my blood was drawn in a period of time that exceeded the interval for accurate results (as outlined above) and was therefore inappropriately recommended/ordered, and now serial fetal ultrasonography is being implemented every few weeks.
With lack of ubiquitous knowledge about the infection, clinicians are not universally certain of the appropriate next steps when a patient presents with Zika risk factors, and therefore anxiety remains high for pregnant patients and their contacts. The Centers for Disease Control and Prevention website is the official home base, and we should review it and await their further evolving specific recommendations as more cases unfortunately accumulate.
Have you encountered any patients this year with exposure to or symptoms of Zika infection, and what, if anything, have you outlined for them?
Inundating our popular and academic media circles is information regarding the Zika virus. A recent article by Farahnik et al in the Journal of the American Academy of Dermatology (2016;74:1286-1287) briefly outlines what is known about Zika infection thus far and its dermatologic manifestations. Pairing this article with Centers for Disease Control and Prevention guidelines on the topic, we are presented with an evolving introduction to this new entity. Here’s what we know:
- It is a single-stranded RNA arbovirus in the Flavivirus family transmitted by the bite of Aedes mosquitoes, with cases reported so far in Africa, Asia, and the Americas (particularly southern coastal and island destinations).
- It also is transmitted via transfusion of blood, sexual contact, and mother to fetus.
- There is theoretical risk for fetal microcephaly, intracranial calcifications, and other brain and eye abnormalities.
- Only 1 in 5 affected patients show any systemic manifestations of infection, including self-limited flulike symptoms and nonspecific exanthema, typically sparing acral sites and occurring within 1 to 2 weeks of virus exposure.
- Testing is recommended for pregnant women with possible Zika exposure (ie, travel to an area with active transmission of Zika virus, unprotected sex with a male with this travel history).
- Diagnosis can be made through state health departments, employing real-time reverse transcriptase–polymerase chain reaction (rRT-PCR) or enzyme-linked immunosorbent assay the week after symptom onset using serum, or rRT-PCR 2 weeks after symptom onset using urine. Further antibody testing can be done if a false-negative is suspected, but false-positives also are possible if a patient was exposed to or vaccinated against other flaviviruses (eg, dengue virus, West Nile virus, yellow fever virus)
- Testing is inaccurate if ordered within 7 days or more than 12 weeks following presumed exposure.
- If positive or inconclusive testing arises, serial fetal ultrasonography should be considered; if testing is negative, then a single fetal ultrasound is recommended to detect Zika abnormalities.
- Test results are automatically reported to respective state health departments.
- There is no treatment of this infection aside from supportive care.
What’s the issue?
As with any new outbreak, the applicability to the general population and true risks remain to be seen. Each of our clinics recalls the stark changes in patient intake and screening questions with infections as ubiquitous as methicillin-resistant Staphylococcus aureus to much rarer exposures such as Ebola virus, each with progressive understanding of risk groups, disease manifestations, and eradication and prevention measures.
By mid-June 2016, 30 hits on PubMed addressing Zika had already been cited just within the month, outlining various aspects of the infection, and many specialties, particularly neurology, obstetrics, primary care, infectious disease, and dermatology, are weighing in. Unfortunately, the majority of cases of primary Zika infection do not manifest with skin or systemic symptoms, and even cases that do are nonspecific, exanthematous, and flulike.
Vague as it may be so far, it is nonetheless imperative that clinicians be familiar with what is concretely known about Zika virus and acquaint ourselves with the travel distribution and restrictions, disease risk factors, known sequelae, testing availability and limitations, and reporting guidelines. From personal experience, as I traveled to Belize earlier this year during my first trimester of pregnancy before the travel restrictions were outlined, even obstetricians are not wholly familiar with the manner in which to order testing and the appropriate window to do so. I have been asymptomatic, my blood was drawn in a period of time that exceeded the interval for accurate results (as outlined above) and was therefore inappropriately recommended/ordered, and now serial fetal ultrasonography is being implemented every few weeks.
With lack of ubiquitous knowledge about the infection, clinicians are not universally certain of the appropriate next steps when a patient presents with Zika risk factors, and therefore anxiety remains high for pregnant patients and their contacts. The Centers for Disease Control and Prevention website is the official home base, and we should review it and await their further evolving specific recommendations as more cases unfortunately accumulate.
Have you encountered any patients this year with exposure to or symptoms of Zika infection, and what, if anything, have you outlined for them?
Inundating our popular and academic media circles is information regarding the Zika virus. A recent article by Farahnik et al in the Journal of the American Academy of Dermatology (2016;74:1286-1287) briefly outlines what is known about Zika infection thus far and its dermatologic manifestations. Pairing this article with Centers for Disease Control and Prevention guidelines on the topic, we are presented with an evolving introduction to this new entity. Here’s what we know:
- It is a single-stranded RNA arbovirus in the Flavivirus family transmitted by the bite of Aedes mosquitoes, with cases reported so far in Africa, Asia, and the Americas (particularly southern coastal and island destinations).
- It also is transmitted via transfusion of blood, sexual contact, and mother to fetus.
- There is theoretical risk for fetal microcephaly, intracranial calcifications, and other brain and eye abnormalities.
- Only 1 in 5 affected patients show any systemic manifestations of infection, including self-limited flulike symptoms and nonspecific exanthema, typically sparing acral sites and occurring within 1 to 2 weeks of virus exposure.
- Testing is recommended for pregnant women with possible Zika exposure (ie, travel to an area with active transmission of Zika virus, unprotected sex with a male with this travel history).
- Diagnosis can be made through state health departments, employing real-time reverse transcriptase–polymerase chain reaction (rRT-PCR) or enzyme-linked immunosorbent assay the week after symptom onset using serum, or rRT-PCR 2 weeks after symptom onset using urine. Further antibody testing can be done if a false-negative is suspected, but false-positives also are possible if a patient was exposed to or vaccinated against other flaviviruses (eg, dengue virus, West Nile virus, yellow fever virus)
- Testing is inaccurate if ordered within 7 days or more than 12 weeks following presumed exposure.
- If positive or inconclusive testing arises, serial fetal ultrasonography should be considered; if testing is negative, then a single fetal ultrasound is recommended to detect Zika abnormalities.
- Test results are automatically reported to respective state health departments.
- There is no treatment of this infection aside from supportive care.
What’s the issue?
As with any new outbreak, the applicability to the general population and true risks remain to be seen. Each of our clinics recalls the stark changes in patient intake and screening questions with infections as ubiquitous as methicillin-resistant Staphylococcus aureus to much rarer exposures such as Ebola virus, each with progressive understanding of risk groups, disease manifestations, and eradication and prevention measures.
By mid-June 2016, 30 hits on PubMed addressing Zika had already been cited just within the month, outlining various aspects of the infection, and many specialties, particularly neurology, obstetrics, primary care, infectious disease, and dermatology, are weighing in. Unfortunately, the majority of cases of primary Zika infection do not manifest with skin or systemic symptoms, and even cases that do are nonspecific, exanthematous, and flulike.
Vague as it may be so far, it is nonetheless imperative that clinicians be familiar with what is concretely known about Zika virus and acquaint ourselves with the travel distribution and restrictions, disease risk factors, known sequelae, testing availability and limitations, and reporting guidelines. From personal experience, as I traveled to Belize earlier this year during my first trimester of pregnancy before the travel restrictions were outlined, even obstetricians are not wholly familiar with the manner in which to order testing and the appropriate window to do so. I have been asymptomatic, my blood was drawn in a period of time that exceeded the interval for accurate results (as outlined above) and was therefore inappropriately recommended/ordered, and now serial fetal ultrasonography is being implemented every few weeks.
With lack of ubiquitous knowledge about the infection, clinicians are not universally certain of the appropriate next steps when a patient presents with Zika risk factors, and therefore anxiety remains high for pregnant patients and their contacts. The Centers for Disease Control and Prevention website is the official home base, and we should review it and await their further evolving specific recommendations as more cases unfortunately accumulate.
Have you encountered any patients this year with exposure to or symptoms of Zika infection, and what, if anything, have you outlined for them?
Nevi, Melanoma, and the Ongoing Argument on Atypia
In a case study published online on March 2 in JAMA Dermatology, Geller et al examined the relationship between total nevi, atypical nevi, and melanoma thickness. The study included 566 patients with melanoma. They were administered written surveys and underwent skin examinations at academic centers in Michigan and California within 3 months of diagnosis, measuring current total nevus count and atypical nevus count in addition to cataloguing melanoma thickness, histologic subtype, patient age, sex, marital status, skin self-examination and physician skin examination tendency, other health care visits, and mode of melanoma discovery.
Many epidemiologic trends were noted, but in summary, most melanoma patients had 0 to 20 total nevi (66.4%) and no atypical nevi (73.3%), a trend most pronounced in older patients (≥60 years). In patients younger than 60 years, higher nevus count (>50) was associated with thinner melanomas (≤2.0 mm), and the presence of more than 5 atypical nevi was associated with thicker melanomas (>2.0 mm).
What’s the issue?Studies clarifying the overall clinical characteristics of patients with aggressive melanomas appear every month in reputable journals, touting that concurrent total nevus count is important; or nevus size is important; or atypia is important; or clinical stigmata, medical history, and family history are important. Who is correct? Is everyone correct? On the pathology arm of the argument, Rosendahl et al (J Am Acad Dermatol. 2015;73:507-512) highlighted the same dilemma in which clinicians do not agree on the histopathologic features of nevi that consistently put patients at risk for individual lesion or de novo melanoma.
For me, each clinic day involves performing many total-body skin examinations, and many of these patients have innumerable nevi and various scars from lesions removed over the years with “atypical mole,” “premelanoma,” “precancer,” and various other self-reported labels. Some lesions may have documented pathology reports, but many do not. Some reports refer to dysplasia as a gradient, some do not. Some reports include molecular testing or clinical markers to grade lesions, and each can vary between institutions and pathologists. On the macroscopic level, clinically atypical nevi do not have a widely agreed upon set of criteria or threshold for biopsy; some clinicians use dermoscopic markers, and others utilize some version of the ABCDE (a=asymmetry; b=border; c=color; d=diameter; e=evolving) features.
The Geller et al study supports that these melanoma patients did not necessarily have more total nevi, and younger patients with aggressive melanoma may have a tendency toward more clinically atypical nevi. Although the study establishes what those institutions and clinicians determined to be atypical, I’m not sure that this is something that most clinicians widely agree upon. Additionally, these features were not paired with histopathologic dysplasia because the lesions were not biopsied.
What I find in conversation with colleagues is that some agree with what Geller et al defined as atypical, but some clinicians do not even refer to nevi as clinically atypical in a medical record unless they have pathology evidence of atypia (or the term their pathologist may use), which may be to avoid controversy regarding legal implications of atypia or “open-note” misunderstanding that the patient may have about this term, likening it to Papanicolaou test premalignancy verbiage.
I am not aware of one dermatologist or dermatopathologist who does not find this quandary to be frustrating. How do any of us really know which patients to follow more often for melanoma surveillance? How does your practice or institution report atypia in the clinical and histopathologic setting, and what do you find are the most important markers for development of melanoma?
In a case study published online on March 2 in JAMA Dermatology, Geller et al examined the relationship between total nevi, atypical nevi, and melanoma thickness. The study included 566 patients with melanoma. They were administered written surveys and underwent skin examinations at academic centers in Michigan and California within 3 months of diagnosis, measuring current total nevus count and atypical nevus count in addition to cataloguing melanoma thickness, histologic subtype, patient age, sex, marital status, skin self-examination and physician skin examination tendency, other health care visits, and mode of melanoma discovery.
Many epidemiologic trends were noted, but in summary, most melanoma patients had 0 to 20 total nevi (66.4%) and no atypical nevi (73.3%), a trend most pronounced in older patients (≥60 years). In patients younger than 60 years, higher nevus count (>50) was associated with thinner melanomas (≤2.0 mm), and the presence of more than 5 atypical nevi was associated with thicker melanomas (>2.0 mm).
What’s the issue?Studies clarifying the overall clinical characteristics of patients with aggressive melanomas appear every month in reputable journals, touting that concurrent total nevus count is important; or nevus size is important; or atypia is important; or clinical stigmata, medical history, and family history are important. Who is correct? Is everyone correct? On the pathology arm of the argument, Rosendahl et al (J Am Acad Dermatol. 2015;73:507-512) highlighted the same dilemma in which clinicians do not agree on the histopathologic features of nevi that consistently put patients at risk for individual lesion or de novo melanoma.
For me, each clinic day involves performing many total-body skin examinations, and many of these patients have innumerable nevi and various scars from lesions removed over the years with “atypical mole,” “premelanoma,” “precancer,” and various other self-reported labels. Some lesions may have documented pathology reports, but many do not. Some reports refer to dysplasia as a gradient, some do not. Some reports include molecular testing or clinical markers to grade lesions, and each can vary between institutions and pathologists. On the macroscopic level, clinically atypical nevi do not have a widely agreed upon set of criteria or threshold for biopsy; some clinicians use dermoscopic markers, and others utilize some version of the ABCDE (a=asymmetry; b=border; c=color; d=diameter; e=evolving) features.
The Geller et al study supports that these melanoma patients did not necessarily have more total nevi, and younger patients with aggressive melanoma may have a tendency toward more clinically atypical nevi. Although the study establishes what those institutions and clinicians determined to be atypical, I’m not sure that this is something that most clinicians widely agree upon. Additionally, these features were not paired with histopathologic dysplasia because the lesions were not biopsied.
What I find in conversation with colleagues is that some agree with what Geller et al defined as atypical, but some clinicians do not even refer to nevi as clinically atypical in a medical record unless they have pathology evidence of atypia (or the term their pathologist may use), which may be to avoid controversy regarding legal implications of atypia or “open-note” misunderstanding that the patient may have about this term, likening it to Papanicolaou test premalignancy verbiage.
I am not aware of one dermatologist or dermatopathologist who does not find this quandary to be frustrating. How do any of us really know which patients to follow more often for melanoma surveillance? How does your practice or institution report atypia in the clinical and histopathologic setting, and what do you find are the most important markers for development of melanoma?
In a case study published online on March 2 in JAMA Dermatology, Geller et al examined the relationship between total nevi, atypical nevi, and melanoma thickness. The study included 566 patients with melanoma. They were administered written surveys and underwent skin examinations at academic centers in Michigan and California within 3 months of diagnosis, measuring current total nevus count and atypical nevus count in addition to cataloguing melanoma thickness, histologic subtype, patient age, sex, marital status, skin self-examination and physician skin examination tendency, other health care visits, and mode of melanoma discovery.
Many epidemiologic trends were noted, but in summary, most melanoma patients had 0 to 20 total nevi (66.4%) and no atypical nevi (73.3%), a trend most pronounced in older patients (≥60 years). In patients younger than 60 years, higher nevus count (>50) was associated with thinner melanomas (≤2.0 mm), and the presence of more than 5 atypical nevi was associated with thicker melanomas (>2.0 mm).
What’s the issue?Studies clarifying the overall clinical characteristics of patients with aggressive melanomas appear every month in reputable journals, touting that concurrent total nevus count is important; or nevus size is important; or atypia is important; or clinical stigmata, medical history, and family history are important. Who is correct? Is everyone correct? On the pathology arm of the argument, Rosendahl et al (J Am Acad Dermatol. 2015;73:507-512) highlighted the same dilemma in which clinicians do not agree on the histopathologic features of nevi that consistently put patients at risk for individual lesion or de novo melanoma.
For me, each clinic day involves performing many total-body skin examinations, and many of these patients have innumerable nevi and various scars from lesions removed over the years with “atypical mole,” “premelanoma,” “precancer,” and various other self-reported labels. Some lesions may have documented pathology reports, but many do not. Some reports refer to dysplasia as a gradient, some do not. Some reports include molecular testing or clinical markers to grade lesions, and each can vary between institutions and pathologists. On the macroscopic level, clinically atypical nevi do not have a widely agreed upon set of criteria or threshold for biopsy; some clinicians use dermoscopic markers, and others utilize some version of the ABCDE (a=asymmetry; b=border; c=color; d=diameter; e=evolving) features.
The Geller et al study supports that these melanoma patients did not necessarily have more total nevi, and younger patients with aggressive melanoma may have a tendency toward more clinically atypical nevi. Although the study establishes what those institutions and clinicians determined to be atypical, I’m not sure that this is something that most clinicians widely agree upon. Additionally, these features were not paired with histopathologic dysplasia because the lesions were not biopsied.
What I find in conversation with colleagues is that some agree with what Geller et al defined as atypical, but some clinicians do not even refer to nevi as clinically atypical in a medical record unless they have pathology evidence of atypia (or the term their pathologist may use), which may be to avoid controversy regarding legal implications of atypia or “open-note” misunderstanding that the patient may have about this term, likening it to Papanicolaou test premalignancy verbiage.
I am not aware of one dermatologist or dermatopathologist who does not find this quandary to be frustrating. How do any of us really know which patients to follow more often for melanoma surveillance? How does your practice or institution report atypia in the clinical and histopathologic setting, and what do you find are the most important markers for development of melanoma?
Investigating Isotretinoin Inconsistencies
In a JAMA Dermatology article published online on December 2, Lee et al challenged the commonly held belief that laboratory studies should be monitored frequently for patients on isotretinoin. In this systematic review and meta-analysis, abnormalities in liver function tests (LFTs), complete blood cell count (CBC), and lipid panel were compared in a set of 22 randomized clinical trials and 4 retrospective studies (1574 patients). Results revealed changes in the mean laboratory values from baseline (99% CI) of the following: aspartate aminotransferase, 22.67 U/L (19.94–25.41 U/L); alanine aminotransferase, 21.77 U/L (18.96–24.59 U/L); alkaline phosphatase, 88.35 U/L (58.94–117.76 U/L); white blood cell count portion of CBC, 6890/µL (5700–8030/µL); lipid panel (triglycerides, 119.98 mg/dL [98.58–141.39 mg/dL]; total cholesterol, 184.74 mg/dL [178.17–191.31 mg/dL]; low-density lipoprotein cholesterol, 109.23 mg/dL [103.68–114.79 mg/dL]; high-density lipoprotein cholesterol, 42.80 mg/dL [39.84–45.76 mg/dL]).
Although these laboratory values were altered as noted above, only 0.5% of patients exhibited test results statistically above or below the mean laboratory values. Additionally, of these laboratory abnormalities, mean changes were not considered to be high risk based on National Institutes of Health clinical center reference ranges.
What’s the issue?
Last year the residents in-training in our department noted variations in what each faculty member was recommending for isotretinoin laboratory monitoring. Practices ranged from initial then monthly full CBC, LFTs, and lipid panel, to those who only checked these laboratory results initially and at 1 month, to those who only performed review of systems-germane parameters. After reviewing the literature, individual preferences, and cost comparisons, a consensus was reached: tests for aspartate aminotransferase, alanine aminotransferase (in lieu of LFT panel), total cholesterol, triglycerides (in lieu of lipid panel), and relevant pregnancy screens would be performed initially, at month 1, and at month 2.
Lee et al also determined that monthly laboratory testing may not be necessary, especially for this low-risk category of patients, but further study is required to determine if there is a standardized way to approach laboratory testing from a safety and economic standpoint, as each dermatologist who prescribes isotretinoin can identify individual cases in which laboratory monitoring was helpful or uncovered individual comorbidities or toxicities in addition to instances where blood work was prohibitively redundant and expensive.
What is your approach to blood work in isotretinoin patients, and can you identify individual patient populations that require more or less stringent laboratory monitoring?
In a JAMA Dermatology article published online on December 2, Lee et al challenged the commonly held belief that laboratory studies should be monitored frequently for patients on isotretinoin. In this systematic review and meta-analysis, abnormalities in liver function tests (LFTs), complete blood cell count (CBC), and lipid panel were compared in a set of 22 randomized clinical trials and 4 retrospective studies (1574 patients). Results revealed changes in the mean laboratory values from baseline (99% CI) of the following: aspartate aminotransferase, 22.67 U/L (19.94–25.41 U/L); alanine aminotransferase, 21.77 U/L (18.96–24.59 U/L); alkaline phosphatase, 88.35 U/L (58.94–117.76 U/L); white blood cell count portion of CBC, 6890/µL (5700–8030/µL); lipid panel (triglycerides, 119.98 mg/dL [98.58–141.39 mg/dL]; total cholesterol, 184.74 mg/dL [178.17–191.31 mg/dL]; low-density lipoprotein cholesterol, 109.23 mg/dL [103.68–114.79 mg/dL]; high-density lipoprotein cholesterol, 42.80 mg/dL [39.84–45.76 mg/dL]).
Although these laboratory values were altered as noted above, only 0.5% of patients exhibited test results statistically above or below the mean laboratory values. Additionally, of these laboratory abnormalities, mean changes were not considered to be high risk based on National Institutes of Health clinical center reference ranges.
What’s the issue?
Last year the residents in-training in our department noted variations in what each faculty member was recommending for isotretinoin laboratory monitoring. Practices ranged from initial then monthly full CBC, LFTs, and lipid panel, to those who only checked these laboratory results initially and at 1 month, to those who only performed review of systems-germane parameters. After reviewing the literature, individual preferences, and cost comparisons, a consensus was reached: tests for aspartate aminotransferase, alanine aminotransferase (in lieu of LFT panel), total cholesterol, triglycerides (in lieu of lipid panel), and relevant pregnancy screens would be performed initially, at month 1, and at month 2.
Lee et al also determined that monthly laboratory testing may not be necessary, especially for this low-risk category of patients, but further study is required to determine if there is a standardized way to approach laboratory testing from a safety and economic standpoint, as each dermatologist who prescribes isotretinoin can identify individual cases in which laboratory monitoring was helpful or uncovered individual comorbidities or toxicities in addition to instances where blood work was prohibitively redundant and expensive.
What is your approach to blood work in isotretinoin patients, and can you identify individual patient populations that require more or less stringent laboratory monitoring?
In a JAMA Dermatology article published online on December 2, Lee et al challenged the commonly held belief that laboratory studies should be monitored frequently for patients on isotretinoin. In this systematic review and meta-analysis, abnormalities in liver function tests (LFTs), complete blood cell count (CBC), and lipid panel were compared in a set of 22 randomized clinical trials and 4 retrospective studies (1574 patients). Results revealed changes in the mean laboratory values from baseline (99% CI) of the following: aspartate aminotransferase, 22.67 U/L (19.94–25.41 U/L); alanine aminotransferase, 21.77 U/L (18.96–24.59 U/L); alkaline phosphatase, 88.35 U/L (58.94–117.76 U/L); white blood cell count portion of CBC, 6890/µL (5700–8030/µL); lipid panel (triglycerides, 119.98 mg/dL [98.58–141.39 mg/dL]; total cholesterol, 184.74 mg/dL [178.17–191.31 mg/dL]; low-density lipoprotein cholesterol, 109.23 mg/dL [103.68–114.79 mg/dL]; high-density lipoprotein cholesterol, 42.80 mg/dL [39.84–45.76 mg/dL]).
Although these laboratory values were altered as noted above, only 0.5% of patients exhibited test results statistically above or below the mean laboratory values. Additionally, of these laboratory abnormalities, mean changes were not considered to be high risk based on National Institutes of Health clinical center reference ranges.
What’s the issue?
Last year the residents in-training in our department noted variations in what each faculty member was recommending for isotretinoin laboratory monitoring. Practices ranged from initial then monthly full CBC, LFTs, and lipid panel, to those who only checked these laboratory results initially and at 1 month, to those who only performed review of systems-germane parameters. After reviewing the literature, individual preferences, and cost comparisons, a consensus was reached: tests for aspartate aminotransferase, alanine aminotransferase (in lieu of LFT panel), total cholesterol, triglycerides (in lieu of lipid panel), and relevant pregnancy screens would be performed initially, at month 1, and at month 2.
Lee et al also determined that monthly laboratory testing may not be necessary, especially for this low-risk category of patients, but further study is required to determine if there is a standardized way to approach laboratory testing from a safety and economic standpoint, as each dermatologist who prescribes isotretinoin can identify individual cases in which laboratory monitoring was helpful or uncovered individual comorbidities or toxicities in addition to instances where blood work was prohibitively redundant and expensive.
What is your approach to blood work in isotretinoin patients, and can you identify individual patient populations that require more or less stringent laboratory monitoring?