User login
Centrifugally Spreading Lymphocutaneous Sporotrichosis: A Rare Cutaneous Manifestation
To the Editor:
Sporotrichosis refers to a subacute to chronic fungal infection that usually involves the cutaneous and subcutaneous tissues and is caused by the introduction of Sporothrix, a dimorphic fungus, through the skin. We present a case of chronic atypical lymphocutaneous sporotrichosis.
A 46-year-old man presented to the outpatient dermatology clinic for follow-up for a rash on the right leg that spread to the thigh and became painful and pruritic. It initially developed 8 years prior to the current presentation after he sustained trauma to the leg from an electroshock weapon. One year prior to the current presentation, he had presented to the emergency department and was prescribed doxycycline 100 mg twice daily for 7 days as well as bacitracin ointment. He also was instructed to follow up with dermatology, but a lack of health insurance and other socioeconomic barriers prevented him from seeking dermatologic care. Nine months later, he again presented to the emergency department due to a motor vehicle accident. Computed tomography (CT) of the right leg revealed exophytic dermal masses, inflammatory stranding of the subcutaneous tissue, and right inguinal lymph nodes measuring up to 1.4 cm; there was no osteoarticular involvement. At that time, the patient was applying gentian violet to the skin lesions and taking hydroxyzine 50 mg 3 times daily as needed for pruritus with minimal relief. Financial support was provided for follow-up with dermatology, which occurred almost 5 months later.
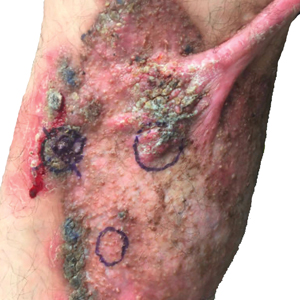
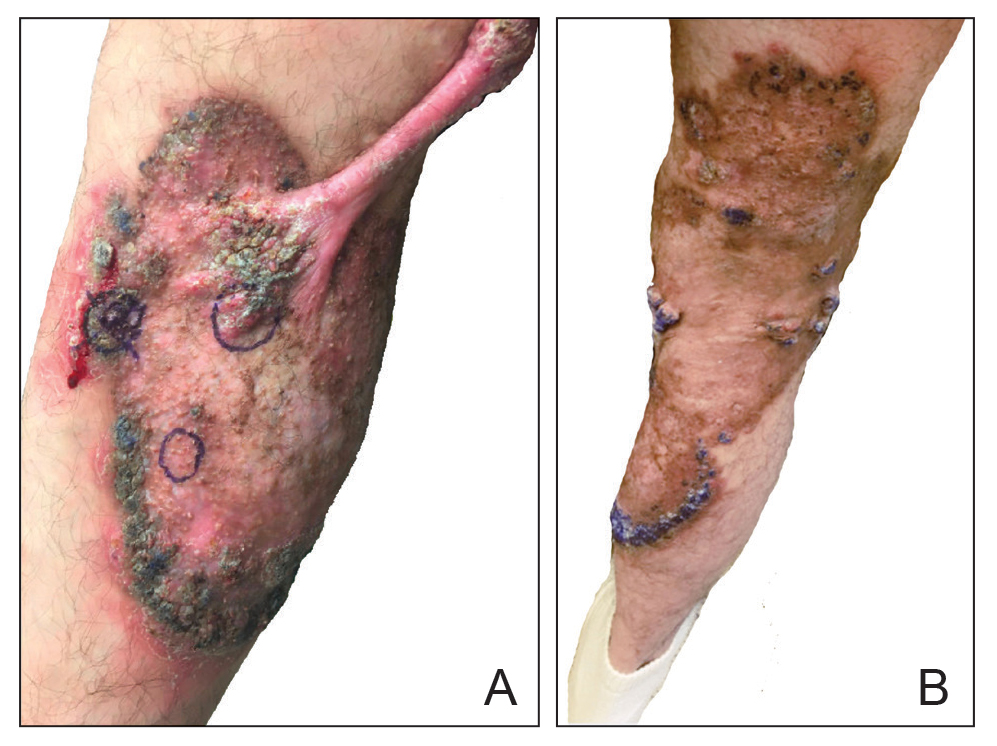
At the current presentation, physical examination revealed a large annular plaque with verrucous, scaly, erythematous borders and a hypopigmented atrophic center extending from the medial aspect of the right leg to the posterior thigh. Numerous pink, scaly, crusted nodules were scattered primarily along the periphery, with some evidence of draining sinus tracts. In addition, a fibrotic pink linear plaque extended from the medial right leg to the popliteal fossa, consistent with a keloid. Violet staining along the periphery of the lesion also was appreciated secondary to the application of topical gentian violet (Figure 1).
Based on the chronic history and morphology, a diagnosis of a chronic fungal or atypical mycobacterial infection was favored. In particular, chromoblastomycosis, cutaneous tuberculosis (eg, scrofuloderma, lupus vulgaris, tuberculosis verrucosa cutis), and atypical mycobacterial infection were highest on the differential, as these conditions often exhibit annular, nodular, verrucous, and/or atrophic lesions. The nodularity, crusting, and draining sinus tracts also raised the possibility of mycetoma. Given the extension of the lesion from the lower to upper leg, a sporotrichoid infection also was considered but was thought to be less likely based on the annular configuration.
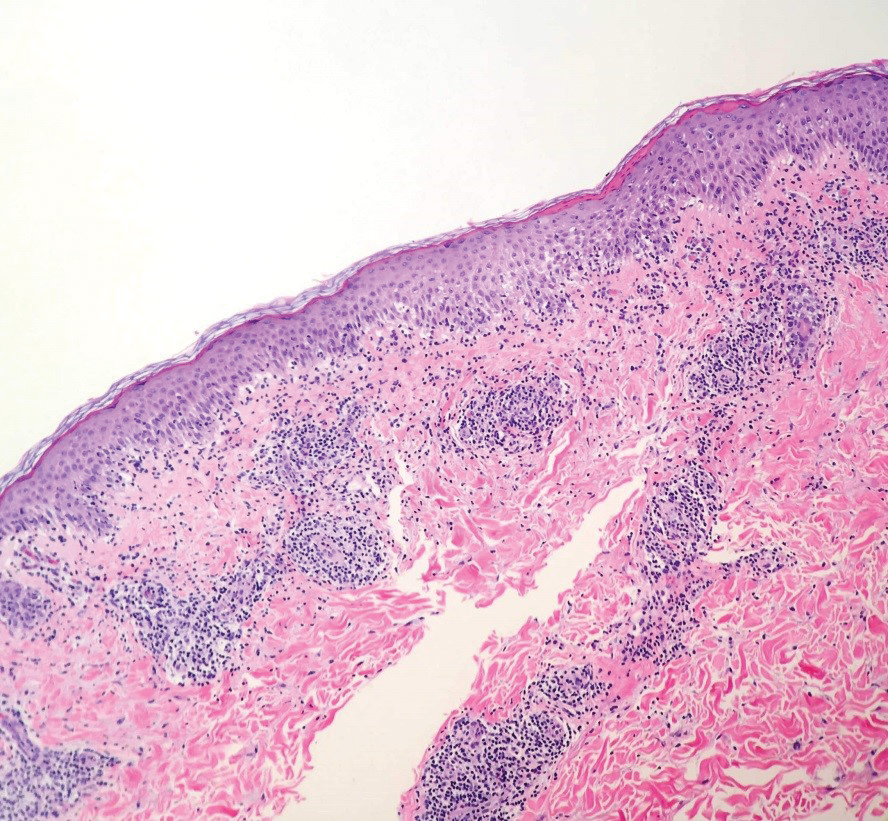
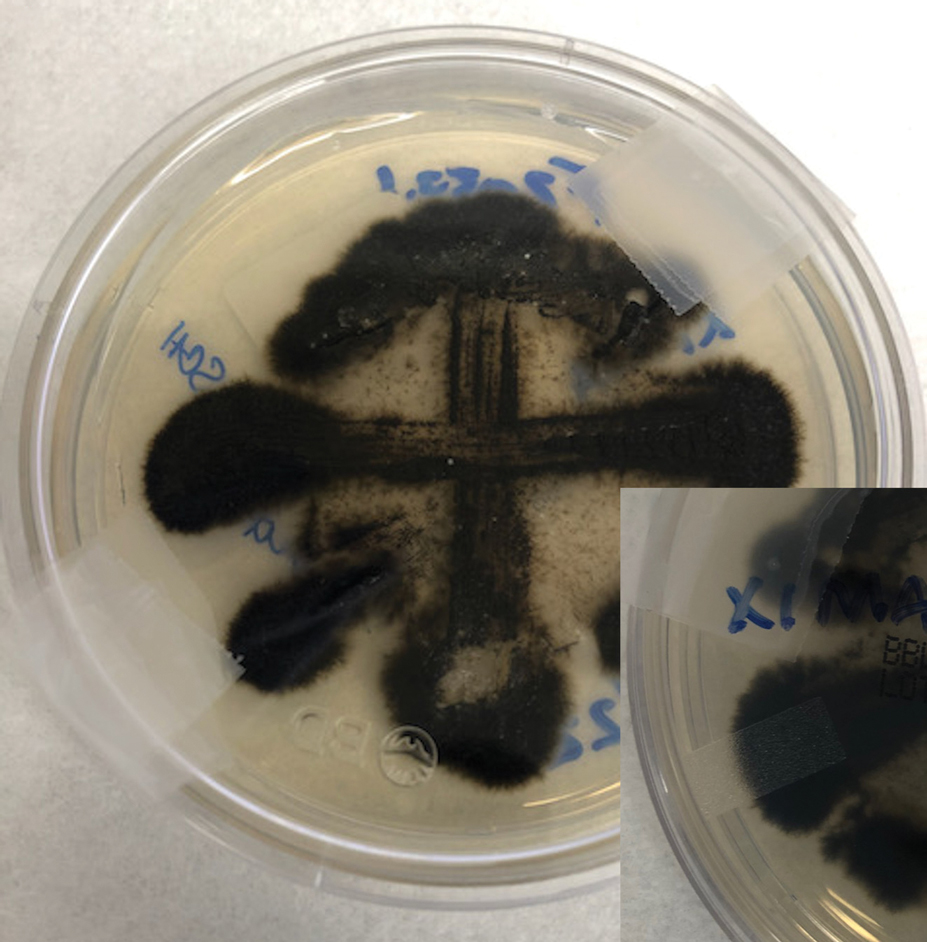
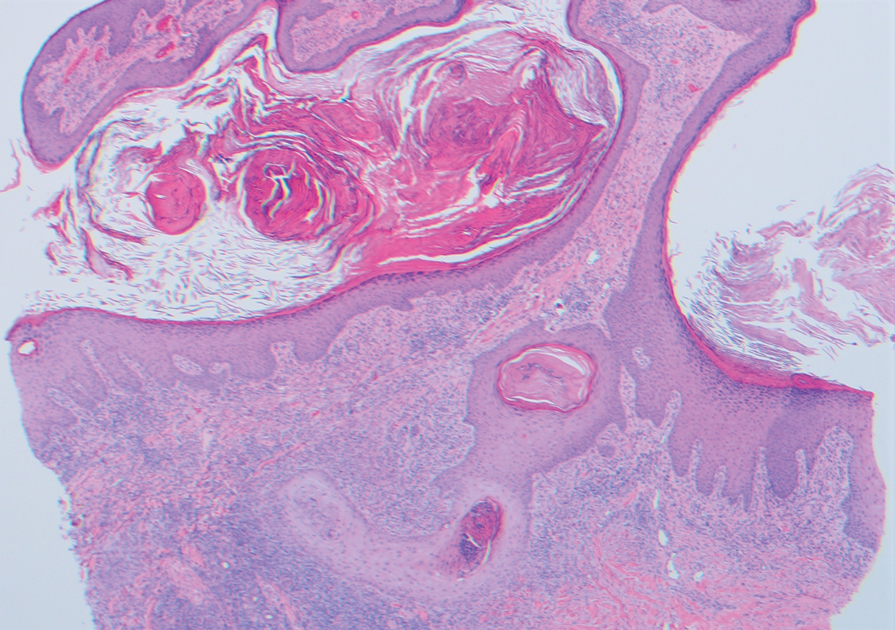
Two 4-mm punch biopsies were taken from a peripheral nodule—one for routine histology and another for bacterial, fungal, and mycobacterial cultures. An interferon-gamma release assay also was ordered to evaluate for immune responses indicative of prior Mycobacterium tuberculosis infection, but the patient did not obtain this for unknown reasons. Histology demonstrated pseudoepitheliomatous hyperplasia and necrotizing granulomas, which suggested an infectious etiology, but no organisms were identified on tissue staining and all cultures were negative for growth at 6 weeks. The patient was asked to return at that point, and 4 additional scouting biopsies were performed and sent for routine histology, M tuberculosis nucleic acid amplification testing, and microbiologic cultures (ie, bacterial, mycobacterial, fungal, nocardia, actinomycetes). Within 1 week, a filamentous organism with pigmentation visible on the front and back of a Sabouraud dextrose agar plate was identified on fungal culture (Figure 2). Microscopic evaluation of this mold with lactophenol blue stain revealed thin septate hyphae with conidiophores arising at right angles that bore clusters of microconidia (Figure 3). Sequencing analysis ultimately identified this organism as Sporothrix schenckii. Routine histology demonstrated pseudoepitheliomatous hyperplasia with scattered intraepidermal collections of neutrophils (Figure 4). The dermis showed a dense, superficial, and deep infiltrate composed of lymphocytes, histiocytes, and plasma cells with occasional neutrophils and eosinophils. A Grocott-Gomori methenamine-silver stain revealed a cluster of ovoid yeast forms within the stratum corneum (Figure 5). The patient was referred to infectious disease for follow-up and treatment.
The patient later visited a community clinic providing dermatologic care for patients without insurance. He was started on itraconazole 200 mg daily for a total of 6 months until dermatologic clearance of the cutaneous lesions was observed. He was followed by the clinic with laboratory tests including a liver function test. At follow-up 8 months later, a repeat biopsy was performed to ensure histologic clearance of the sporotrichosis, which revealed a dermal scar and no evidence of residual infection.
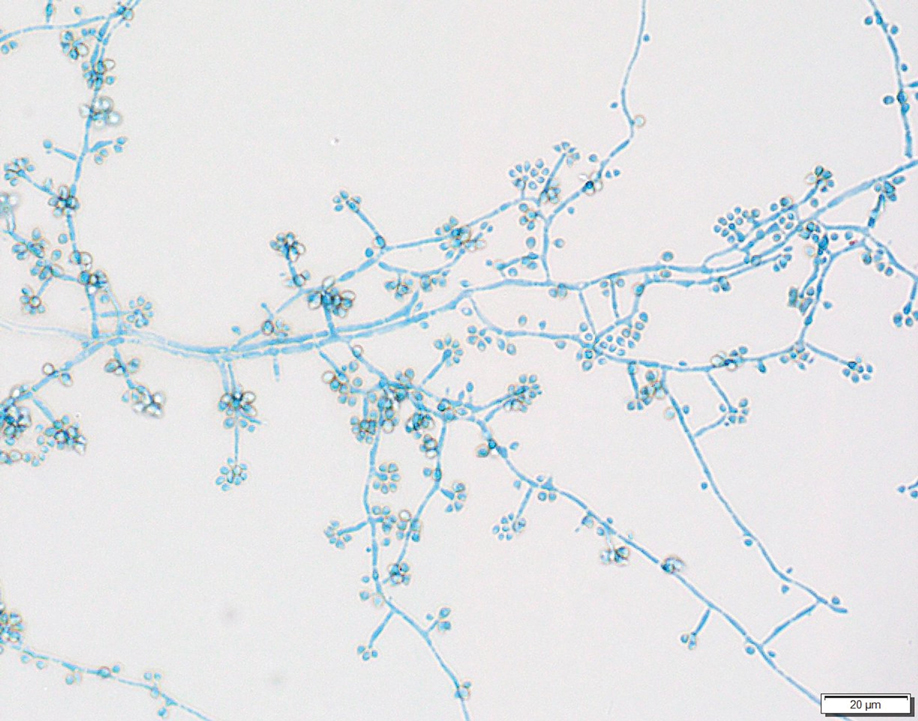
Sporothrix schenckii was first isolated in 1898 by Benjamin Schenck, a student at Johns Hopkins Medicine (Baltimore, Maryland), and identified by a mycologist as sporotricha.1 Species within the genus Sporothrix are unique in that the fungi are both dimorphic (growing as a mold at 25 °C but as a yeast at 37 °C) and dematiaceous (dark pigmentation from melanin is visible on inspection of the anterior and reverse sides of culture plates). Infection usually occurs when cutaneous or subcutaneous tissues are exposed to the fungus via microabrasions; activities thought to contribute to exposure include gardening, agricultural work, animal husbandry, and feline scratches.2 Although skin trauma frequently is considered the primary route of infection, patient recall is variable, with one study noting that only 37.7% of patients recalled trauma and another study similarly demonstrating a patient recall rate of 25%.3,4
Lymphocutaneous sporotrichosis is the most common presentation of the fungal infection,5 and clinical cases may be classified into 1 of 4 categories: (1) lymphangitic lesions—papules at the site of inoculation with spread along the lymphatic channels; (2) localized (fixed) cutaneous lesions—1 or 2 lesions at the inoculation site; (3) disseminated (multifocal) cutaneous lesions; and (4) extracutaneous lesions.6 Extracutaneous manifestations of this infection most notably have been reported as pulmonary disease through inhalation of conidia or through dissemination in immunocompromised hosts.7 Our patient’s infection was categorized as lymphangitic lesions due to spread from the lower to upper leg, albeit in a highly atypical, annular fashion. A review of systems was otherwise negative, and CT ruled out osteoarticular involvement.
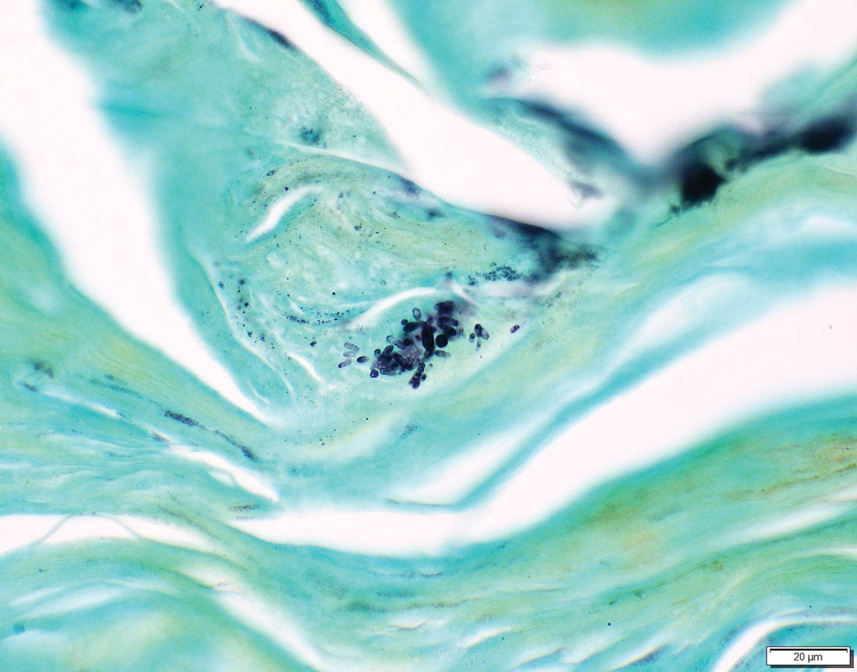
In addition to socioeconomic barriers, several factors contributed to a delayed diagnosis in this patient including the annular presentation with central hypopigmentation and atrophy, negative initial microbiological cultures and lack of visualization of organisms on histopathology, and the consequent need for repeat biopsies. For lymphocutaneous sporotrichosis, the typical presentation consists of a papule or ulcerated nodule at the site of inoculation with subsequent linear spread along lymphatic channels. This classic sporotrichoid pattern is a key diagnostic clue for identifying sporotrichosis but was absent at the time our patient presented for medical care. Rather, the sporotrichoid spread seemed to have occurred in a centrifugal fashion up the leg. Few case reports have documented an annular presentation of lymphocutaneous sporotrichosis,8-13 and one report described central atrophy and hypopigmentation.10 Pain and pruritus, which were present in our patient, rarely are documented.9 Finally, the diagnosis of cutaneous fungal infections may require multiple biopsies due to the variable abundance of viable organisms in tissue specimens as well as the fastidious growth characteristics of these organisms. Furthermore, sensitivity often is low for both fungal and mycobacterial cultures, and cultures may take days to weeks to yield growth.14,15 For these reasons, empiric therapy and repeat biopsies often are pursued if clinical suspicion is high enough.16 Our patient returned for multiple scouting biopsies after the initial tissue culture was negative and was even considered for empiric treatment against Mycobacterium prior to positive fungal cultures.
Another unique aspect of our case was the presence of a keloid. It is difficult to know if this keloid was secondary to the trauma the patient sustained in the inciting incident or formed from the fungal infection. Interestingly, it has been hypothesized that fungal infections may contribute to keloid and hypertrophic scar formation.17 In a case series of 3 patients with either keloids or hypertrophic scars and concomitant tinea infection, there was notable improvement in the appearance of the scars 2 weeks after beginning itraconazole therapy.17 However, it is not yet known if a fungal infection can contribute to the pathogenesis of keloid formation.
As with other aspects of this case, the length of time the patient went without diagnosis and treatment was unusual and may help explain the atypical presentation. Although the incubation period for S schenckii can vary, most reports identify patients as seeking medical attention within 1 year of rash onset.18-20 In our case, the patient was not diagnosed until 8 years after his symptoms began, requiring multiple referrals, multiple health system touchpoints, and an institution-specific financial aid program. As such, this case also highlights the potential need for a multidisciplinary team approach when caring for patients with poor access to health care.
In conclusion, this case illustrates a unique presentation of lymphocutaneous sporotrichosis that may mimic other chronic infections and result in delayed diagnosis. Although lymphangitic sporotrichosis generally is recognized as having a linear distribution, mounting evidence from this report and others suggests an annular presentation also is possible. Pruritus or pain is rare but should not preclude a diagnosis of sporotrichosis if present. For patients with limited access to health care resources, it is especially important to involve multiple members of the health care team, including social workers and specialists, to prevent a protracted and severe course of disease.
- Schenck BR. On refractory subcutaneous abscesses caused by a fungus possibly related to the sporotricha. Bulletin of the Johns Hopkins Hospital. 1898;93:286-290.
- de Lima Barros MB, de Almeida Paes R, Schubach AO. Sporothrix schenckii and sporotrichosis. Clin Microbiol Rev. 2011;24:633-654. doi:10.1128/CMR.00007-11
- Crevasse L, Ellner PD. An outbreak of sporotrichosis in florida. J Am Med Assoc. 1960;173:29-33. doi:10.1001/jama.1960.03020190031006
- Mayorga R, Cáceres A, Toriello C, et al. An endemic area of sporotrichosis in Guatemala [in French]. Sabouraudia. 1978;16:185-198.
- Morris-Jones R. Sporotrichosis. Clin Exp Dermatol. 2002;27:427-431. doi:10.1046/j.1365-2230.2002.01087.x
- Sampaio SA, Da Lacaz CS. Clinical and statistical studies on sporotrichosis in Sao Paulo (Brazil). Article in German. Hautarzt. 1959;10:490-493.
- Ramos-e-Silva M, Vasconcelos C, Carneiro S, et al. Sporotrichosis. Clin Dermatol. 2007;25:181-187. doi:10.1016/j.clindermatol.2006.05.006
- Williams BA, Jennings TA, Rushing EC, et al. Sporotrichosis on the face of a 7-year-old boy following a bicycle accident. Pediatr Dermatol. 2013;30:E246-E247. doi:10.1111/j.1525-1470.2011.01696.x
- Vaishampayan SS, Borde P. An unusual presentation of sporotrichosis. Indian J Dermatol. 2013;58:409. doi:10.4103/0019-5154.117350
- Qin J, Zhang J. Sporotrichosis. N Engl J Med. 2019;380:771. doi:10.1056/NEJMicm1809179
- Patel A, Mudenda V, Lakhi S, et al. A 27-year-old severely immunosuppressed female with misleading clinical features of disseminated cutaneous sporotrichosis. Case Rep Dermatol Med. 2016;2016:1-4. doi:10.1155/2016/9403690
- de Oliveira-Esteves ICMR, Almeida Rosa da Silva G, Eyer-Silva WA, et al. Rapidly progressive disseminated sporotrichosis as the first presentation of HIV infection in a patient with a very low CD4 cell count. Case Rep Infect Dis. 2017;2017:4713140. doi:10.1155/2017/4713140
- Singh S, Bachaspatimayum R, Meetei U, et al. Terbinafine in fixed cutaneous sporotrichosis: a case series. J Clin Diagnostic Res. 2018;12:FR01-FR03. doi:10.7860/JCDR/2018/25315.12223
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280. doi:10.1128/CMR.00053-10
- Peters F, Batinica M, Plum G, et al. Bug or no bug: challenges in diagnosing cutaneous mycobacterial infections. J Ger Soc Dermatol. 2016;14:1227-1236. doi:10.1111/ddg.13001
- Khadka P, Koirala S, Thapaliya J. Cutaneous tuberculosis: clinicopathologic arrays and diagnostic challenges. Dermatol Res Pract. 2018;2018:7201973. doi:10.1155/2018/7201973
- Okada E, Maruyama Y. Are keloids and hypertrophic scars caused by fungal infection? . Plast Reconstr Surg. 2007;120:814-815. doi:10.1097/01.prs.0000278813.23244.3f
- Pappas PG, Tellez I, Deep AE, et al. Sporotrichosis in Peru: description of an area of hyperendemicity. Clin Infect Dis. 2000;30:65-70. doi:10.1086/313607
- McGuinness SL, Boyd R, Kidd S, et al. Epidemiological investigation of an outbreak of cutaneous sporotrichosis, Northern Territory, Australia. BMC Infect Dis. 2016;16:1-7. doi:10.1186/s12879-016-1338-0
- Rojas FD, Fernández MS, Lucchelli JM, et al. Cavitary pulmonary sporotrichosis: case report and literature review. Mycopathologia. 2017;182:1119-1123. doi:10.1007/s11046-017-0197-6
To the Editor:
Sporotrichosis refers to a subacute to chronic fungal infection that usually involves the cutaneous and subcutaneous tissues and is caused by the introduction of Sporothrix, a dimorphic fungus, through the skin. We present a case of chronic atypical lymphocutaneous sporotrichosis.
A 46-year-old man presented to the outpatient dermatology clinic for follow-up for a rash on the right leg that spread to the thigh and became painful and pruritic. It initially developed 8 years prior to the current presentation after he sustained trauma to the leg from an electroshock weapon. One year prior to the current presentation, he had presented to the emergency department and was prescribed doxycycline 100 mg twice daily for 7 days as well as bacitracin ointment. He also was instructed to follow up with dermatology, but a lack of health insurance and other socioeconomic barriers prevented him from seeking dermatologic care. Nine months later, he again presented to the emergency department due to a motor vehicle accident. Computed tomography (CT) of the right leg revealed exophytic dermal masses, inflammatory stranding of the subcutaneous tissue, and right inguinal lymph nodes measuring up to 1.4 cm; there was no osteoarticular involvement. At that time, the patient was applying gentian violet to the skin lesions and taking hydroxyzine 50 mg 3 times daily as needed for pruritus with minimal relief. Financial support was provided for follow-up with dermatology, which occurred almost 5 months later.
At the current presentation, physical examination revealed a large annular plaque with verrucous, scaly, erythematous borders and a hypopigmented atrophic center extending from the medial aspect of the right leg to the posterior thigh. Numerous pink, scaly, crusted nodules were scattered primarily along the periphery, with some evidence of draining sinus tracts. In addition, a fibrotic pink linear plaque extended from the medial right leg to the popliteal fossa, consistent with a keloid. Violet staining along the periphery of the lesion also was appreciated secondary to the application of topical gentian violet (Figure 1).

Based on the chronic history and morphology, a diagnosis of a chronic fungal or atypical mycobacterial infection was favored. In particular, chromoblastomycosis, cutaneous tuberculosis (eg, scrofuloderma, lupus vulgaris, tuberculosis verrucosa cutis), and atypical mycobacterial infection were highest on the differential, as these conditions often exhibit annular, nodular, verrucous, and/or atrophic lesions. The nodularity, crusting, and draining sinus tracts also raised the possibility of mycetoma. Given the extension of the lesion from the lower to upper leg, a sporotrichoid infection also was considered but was thought to be less likely based on the annular configuration.
Two 4-mm punch biopsies were taken from a peripheral nodule—one for routine histology and another for bacterial, fungal, and mycobacterial cultures. An interferon-gamma release assay also was ordered to evaluate for immune responses indicative of prior Mycobacterium tuberculosis infection, but the patient did not obtain this for unknown reasons. Histology demonstrated pseudoepitheliomatous hyperplasia and necrotizing granulomas, which suggested an infectious etiology, but no organisms were identified on tissue staining and all cultures were negative for growth at 6 weeks. The patient was asked to return at that point, and 4 additional scouting biopsies were performed and sent for routine histology, M tuberculosis nucleic acid amplification testing, and microbiologic cultures (ie, bacterial, mycobacterial, fungal, nocardia, actinomycetes). Within 1 week, a filamentous organism with pigmentation visible on the front and back of a Sabouraud dextrose agar plate was identified on fungal culture (Figure 2). Microscopic evaluation of this mold with lactophenol blue stain revealed thin septate hyphae with conidiophores arising at right angles that bore clusters of microconidia (Figure 3). Sequencing analysis ultimately identified this organism as Sporothrix schenckii. Routine histology demonstrated pseudoepitheliomatous hyperplasia with scattered intraepidermal collections of neutrophils (Figure 4). The dermis showed a dense, superficial, and deep infiltrate composed of lymphocytes, histiocytes, and plasma cells with occasional neutrophils and eosinophils. A Grocott-Gomori methenamine-silver stain revealed a cluster of ovoid yeast forms within the stratum corneum (Figure 5). The patient was referred to infectious disease for follow-up and treatment.

The patient later visited a community clinic providing dermatologic care for patients without insurance. He was started on itraconazole 200 mg daily for a total of 6 months until dermatologic clearance of the cutaneous lesions was observed. He was followed by the clinic with laboratory tests including a liver function test. At follow-up 8 months later, a repeat biopsy was performed to ensure histologic clearance of the sporotrichosis, which revealed a dermal scar and no evidence of residual infection.

Sporothrix schenckii was first isolated in 1898 by Benjamin Schenck, a student at Johns Hopkins Medicine (Baltimore, Maryland), and identified by a mycologist as sporotricha.1 Species within the genus Sporothrix are unique in that the fungi are both dimorphic (growing as a mold at 25 °C but as a yeast at 37 °C) and dematiaceous (dark pigmentation from melanin is visible on inspection of the anterior and reverse sides of culture plates). Infection usually occurs when cutaneous or subcutaneous tissues are exposed to the fungus via microabrasions; activities thought to contribute to exposure include gardening, agricultural work, animal husbandry, and feline scratches.2 Although skin trauma frequently is considered the primary route of infection, patient recall is variable, with one study noting that only 37.7% of patients recalled trauma and another study similarly demonstrating a patient recall rate of 25%.3,4

Lymphocutaneous sporotrichosis is the most common presentation of the fungal infection,5 and clinical cases may be classified into 1 of 4 categories: (1) lymphangitic lesions—papules at the site of inoculation with spread along the lymphatic channels; (2) localized (fixed) cutaneous lesions—1 or 2 lesions at the inoculation site; (3) disseminated (multifocal) cutaneous lesions; and (4) extracutaneous lesions.6 Extracutaneous manifestations of this infection most notably have been reported as pulmonary disease through inhalation of conidia or through dissemination in immunocompromised hosts.7 Our patient’s infection was categorized as lymphangitic lesions due to spread from the lower to upper leg, albeit in a highly atypical, annular fashion. A review of systems was otherwise negative, and CT ruled out osteoarticular involvement.

In addition to socioeconomic barriers, several factors contributed to a delayed diagnosis in this patient including the annular presentation with central hypopigmentation and atrophy, negative initial microbiological cultures and lack of visualization of organisms on histopathology, and the consequent need for repeat biopsies. For lymphocutaneous sporotrichosis, the typical presentation consists of a papule or ulcerated nodule at the site of inoculation with subsequent linear spread along lymphatic channels. This classic sporotrichoid pattern is a key diagnostic clue for identifying sporotrichosis but was absent at the time our patient presented for medical care. Rather, the sporotrichoid spread seemed to have occurred in a centrifugal fashion up the leg. Few case reports have documented an annular presentation of lymphocutaneous sporotrichosis,8-13 and one report described central atrophy and hypopigmentation.10 Pain and pruritus, which were present in our patient, rarely are documented.9 Finally, the diagnosis of cutaneous fungal infections may require multiple biopsies due to the variable abundance of viable organisms in tissue specimens as well as the fastidious growth characteristics of these organisms. Furthermore, sensitivity often is low for both fungal and mycobacterial cultures, and cultures may take days to weeks to yield growth.14,15 For these reasons, empiric therapy and repeat biopsies often are pursued if clinical suspicion is high enough.16 Our patient returned for multiple scouting biopsies after the initial tissue culture was negative and was even considered for empiric treatment against Mycobacterium prior to positive fungal cultures.
Another unique aspect of our case was the presence of a keloid. It is difficult to know if this keloid was secondary to the trauma the patient sustained in the inciting incident or formed from the fungal infection. Interestingly, it has been hypothesized that fungal infections may contribute to keloid and hypertrophic scar formation.17 In a case series of 3 patients with either keloids or hypertrophic scars and concomitant tinea infection, there was notable improvement in the appearance of the scars 2 weeks after beginning itraconazole therapy.17 However, it is not yet known if a fungal infection can contribute to the pathogenesis of keloid formation.
As with other aspects of this case, the length of time the patient went without diagnosis and treatment was unusual and may help explain the atypical presentation. Although the incubation period for S schenckii can vary, most reports identify patients as seeking medical attention within 1 year of rash onset.18-20 In our case, the patient was not diagnosed until 8 years after his symptoms began, requiring multiple referrals, multiple health system touchpoints, and an institution-specific financial aid program. As such, this case also highlights the potential need for a multidisciplinary team approach when caring for patients with poor access to health care.
In conclusion, this case illustrates a unique presentation of lymphocutaneous sporotrichosis that may mimic other chronic infections and result in delayed diagnosis. Although lymphangitic sporotrichosis generally is recognized as having a linear distribution, mounting evidence from this report and others suggests an annular presentation also is possible. Pruritus or pain is rare but should not preclude a diagnosis of sporotrichosis if present. For patients with limited access to health care resources, it is especially important to involve multiple members of the health care team, including social workers and specialists, to prevent a protracted and severe course of disease.
To the Editor:
Sporotrichosis refers to a subacute to chronic fungal infection that usually involves the cutaneous and subcutaneous tissues and is caused by the introduction of Sporothrix, a dimorphic fungus, through the skin. We present a case of chronic atypical lymphocutaneous sporotrichosis.
A 46-year-old man presented to the outpatient dermatology clinic for follow-up for a rash on the right leg that spread to the thigh and became painful and pruritic. It initially developed 8 years prior to the current presentation after he sustained trauma to the leg from an electroshock weapon. One year prior to the current presentation, he had presented to the emergency department and was prescribed doxycycline 100 mg twice daily for 7 days as well as bacitracin ointment. He also was instructed to follow up with dermatology, but a lack of health insurance and other socioeconomic barriers prevented him from seeking dermatologic care. Nine months later, he again presented to the emergency department due to a motor vehicle accident. Computed tomography (CT) of the right leg revealed exophytic dermal masses, inflammatory stranding of the subcutaneous tissue, and right inguinal lymph nodes measuring up to 1.4 cm; there was no osteoarticular involvement. At that time, the patient was applying gentian violet to the skin lesions and taking hydroxyzine 50 mg 3 times daily as needed for pruritus with minimal relief. Financial support was provided for follow-up with dermatology, which occurred almost 5 months later.
At the current presentation, physical examination revealed a large annular plaque with verrucous, scaly, erythematous borders and a hypopigmented atrophic center extending from the medial aspect of the right leg to the posterior thigh. Numerous pink, scaly, crusted nodules were scattered primarily along the periphery, with some evidence of draining sinus tracts. In addition, a fibrotic pink linear plaque extended from the medial right leg to the popliteal fossa, consistent with a keloid. Violet staining along the periphery of the lesion also was appreciated secondary to the application of topical gentian violet (Figure 1).

Based on the chronic history and morphology, a diagnosis of a chronic fungal or atypical mycobacterial infection was favored. In particular, chromoblastomycosis, cutaneous tuberculosis (eg, scrofuloderma, lupus vulgaris, tuberculosis verrucosa cutis), and atypical mycobacterial infection were highest on the differential, as these conditions often exhibit annular, nodular, verrucous, and/or atrophic lesions. The nodularity, crusting, and draining sinus tracts also raised the possibility of mycetoma. Given the extension of the lesion from the lower to upper leg, a sporotrichoid infection also was considered but was thought to be less likely based on the annular configuration.
Two 4-mm punch biopsies were taken from a peripheral nodule—one for routine histology and another for bacterial, fungal, and mycobacterial cultures. An interferon-gamma release assay also was ordered to evaluate for immune responses indicative of prior Mycobacterium tuberculosis infection, but the patient did not obtain this for unknown reasons. Histology demonstrated pseudoepitheliomatous hyperplasia and necrotizing granulomas, which suggested an infectious etiology, but no organisms were identified on tissue staining and all cultures were negative for growth at 6 weeks. The patient was asked to return at that point, and 4 additional scouting biopsies were performed and sent for routine histology, M tuberculosis nucleic acid amplification testing, and microbiologic cultures (ie, bacterial, mycobacterial, fungal, nocardia, actinomycetes). Within 1 week, a filamentous organism with pigmentation visible on the front and back of a Sabouraud dextrose agar plate was identified on fungal culture (Figure 2). Microscopic evaluation of this mold with lactophenol blue stain revealed thin septate hyphae with conidiophores arising at right angles that bore clusters of microconidia (Figure 3). Sequencing analysis ultimately identified this organism as Sporothrix schenckii. Routine histology demonstrated pseudoepitheliomatous hyperplasia with scattered intraepidermal collections of neutrophils (Figure 4). The dermis showed a dense, superficial, and deep infiltrate composed of lymphocytes, histiocytes, and plasma cells with occasional neutrophils and eosinophils. A Grocott-Gomori methenamine-silver stain revealed a cluster of ovoid yeast forms within the stratum corneum (Figure 5). The patient was referred to infectious disease for follow-up and treatment.

The patient later visited a community clinic providing dermatologic care for patients without insurance. He was started on itraconazole 200 mg daily for a total of 6 months until dermatologic clearance of the cutaneous lesions was observed. He was followed by the clinic with laboratory tests including a liver function test. At follow-up 8 months later, a repeat biopsy was performed to ensure histologic clearance of the sporotrichosis, which revealed a dermal scar and no evidence of residual infection.

Sporothrix schenckii was first isolated in 1898 by Benjamin Schenck, a student at Johns Hopkins Medicine (Baltimore, Maryland), and identified by a mycologist as sporotricha.1 Species within the genus Sporothrix are unique in that the fungi are both dimorphic (growing as a mold at 25 °C but as a yeast at 37 °C) and dematiaceous (dark pigmentation from melanin is visible on inspection of the anterior and reverse sides of culture plates). Infection usually occurs when cutaneous or subcutaneous tissues are exposed to the fungus via microabrasions; activities thought to contribute to exposure include gardening, agricultural work, animal husbandry, and feline scratches.2 Although skin trauma frequently is considered the primary route of infection, patient recall is variable, with one study noting that only 37.7% of patients recalled trauma and another study similarly demonstrating a patient recall rate of 25%.3,4

Lymphocutaneous sporotrichosis is the most common presentation of the fungal infection,5 and clinical cases may be classified into 1 of 4 categories: (1) lymphangitic lesions—papules at the site of inoculation with spread along the lymphatic channels; (2) localized (fixed) cutaneous lesions—1 or 2 lesions at the inoculation site; (3) disseminated (multifocal) cutaneous lesions; and (4) extracutaneous lesions.6 Extracutaneous manifestations of this infection most notably have been reported as pulmonary disease through inhalation of conidia or through dissemination in immunocompromised hosts.7 Our patient’s infection was categorized as lymphangitic lesions due to spread from the lower to upper leg, albeit in a highly atypical, annular fashion. A review of systems was otherwise negative, and CT ruled out osteoarticular involvement.

In addition to socioeconomic barriers, several factors contributed to a delayed diagnosis in this patient including the annular presentation with central hypopigmentation and atrophy, negative initial microbiological cultures and lack of visualization of organisms on histopathology, and the consequent need for repeat biopsies. For lymphocutaneous sporotrichosis, the typical presentation consists of a papule or ulcerated nodule at the site of inoculation with subsequent linear spread along lymphatic channels. This classic sporotrichoid pattern is a key diagnostic clue for identifying sporotrichosis but was absent at the time our patient presented for medical care. Rather, the sporotrichoid spread seemed to have occurred in a centrifugal fashion up the leg. Few case reports have documented an annular presentation of lymphocutaneous sporotrichosis,8-13 and one report described central atrophy and hypopigmentation.10 Pain and pruritus, which were present in our patient, rarely are documented.9 Finally, the diagnosis of cutaneous fungal infections may require multiple biopsies due to the variable abundance of viable organisms in tissue specimens as well as the fastidious growth characteristics of these organisms. Furthermore, sensitivity often is low for both fungal and mycobacterial cultures, and cultures may take days to weeks to yield growth.14,15 For these reasons, empiric therapy and repeat biopsies often are pursued if clinical suspicion is high enough.16 Our patient returned for multiple scouting biopsies after the initial tissue culture was negative and was even considered for empiric treatment against Mycobacterium prior to positive fungal cultures.
Another unique aspect of our case was the presence of a keloid. It is difficult to know if this keloid was secondary to the trauma the patient sustained in the inciting incident or formed from the fungal infection. Interestingly, it has been hypothesized that fungal infections may contribute to keloid and hypertrophic scar formation.17 In a case series of 3 patients with either keloids or hypertrophic scars and concomitant tinea infection, there was notable improvement in the appearance of the scars 2 weeks after beginning itraconazole therapy.17 However, it is not yet known if a fungal infection can contribute to the pathogenesis of keloid formation.
As with other aspects of this case, the length of time the patient went without diagnosis and treatment was unusual and may help explain the atypical presentation. Although the incubation period for S schenckii can vary, most reports identify patients as seeking medical attention within 1 year of rash onset.18-20 In our case, the patient was not diagnosed until 8 years after his symptoms began, requiring multiple referrals, multiple health system touchpoints, and an institution-specific financial aid program. As such, this case also highlights the potential need for a multidisciplinary team approach when caring for patients with poor access to health care.
In conclusion, this case illustrates a unique presentation of lymphocutaneous sporotrichosis that may mimic other chronic infections and result in delayed diagnosis. Although lymphangitic sporotrichosis generally is recognized as having a linear distribution, mounting evidence from this report and others suggests an annular presentation also is possible. Pruritus or pain is rare but should not preclude a diagnosis of sporotrichosis if present. For patients with limited access to health care resources, it is especially important to involve multiple members of the health care team, including social workers and specialists, to prevent a protracted and severe course of disease.
- Schenck BR. On refractory subcutaneous abscesses caused by a fungus possibly related to the sporotricha. Bulletin of the Johns Hopkins Hospital. 1898;93:286-290.
- de Lima Barros MB, de Almeida Paes R, Schubach AO. Sporothrix schenckii and sporotrichosis. Clin Microbiol Rev. 2011;24:633-654. doi:10.1128/CMR.00007-11
- Crevasse L, Ellner PD. An outbreak of sporotrichosis in florida. J Am Med Assoc. 1960;173:29-33. doi:10.1001/jama.1960.03020190031006
- Mayorga R, Cáceres A, Toriello C, et al. An endemic area of sporotrichosis in Guatemala [in French]. Sabouraudia. 1978;16:185-198.
- Morris-Jones R. Sporotrichosis. Clin Exp Dermatol. 2002;27:427-431. doi:10.1046/j.1365-2230.2002.01087.x
- Sampaio SA, Da Lacaz CS. Clinical and statistical studies on sporotrichosis in Sao Paulo (Brazil). Article in German. Hautarzt. 1959;10:490-493.
- Ramos-e-Silva M, Vasconcelos C, Carneiro S, et al. Sporotrichosis. Clin Dermatol. 2007;25:181-187. doi:10.1016/j.clindermatol.2006.05.006
- Williams BA, Jennings TA, Rushing EC, et al. Sporotrichosis on the face of a 7-year-old boy following a bicycle accident. Pediatr Dermatol. 2013;30:E246-E247. doi:10.1111/j.1525-1470.2011.01696.x
- Vaishampayan SS, Borde P. An unusual presentation of sporotrichosis. Indian J Dermatol. 2013;58:409. doi:10.4103/0019-5154.117350
- Qin J, Zhang J. Sporotrichosis. N Engl J Med. 2019;380:771. doi:10.1056/NEJMicm1809179
- Patel A, Mudenda V, Lakhi S, et al. A 27-year-old severely immunosuppressed female with misleading clinical features of disseminated cutaneous sporotrichosis. Case Rep Dermatol Med. 2016;2016:1-4. doi:10.1155/2016/9403690
- de Oliveira-Esteves ICMR, Almeida Rosa da Silva G, Eyer-Silva WA, et al. Rapidly progressive disseminated sporotrichosis as the first presentation of HIV infection in a patient with a very low CD4 cell count. Case Rep Infect Dis. 2017;2017:4713140. doi:10.1155/2017/4713140
- Singh S, Bachaspatimayum R, Meetei U, et al. Terbinafine in fixed cutaneous sporotrichosis: a case series. J Clin Diagnostic Res. 2018;12:FR01-FR03. doi:10.7860/JCDR/2018/25315.12223
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280. doi:10.1128/CMR.00053-10
- Peters F, Batinica M, Plum G, et al. Bug or no bug: challenges in diagnosing cutaneous mycobacterial infections. J Ger Soc Dermatol. 2016;14:1227-1236. doi:10.1111/ddg.13001
- Khadka P, Koirala S, Thapaliya J. Cutaneous tuberculosis: clinicopathologic arrays and diagnostic challenges. Dermatol Res Pract. 2018;2018:7201973. doi:10.1155/2018/7201973
- Okada E, Maruyama Y. Are keloids and hypertrophic scars caused by fungal infection? . Plast Reconstr Surg. 2007;120:814-815. doi:10.1097/01.prs.0000278813.23244.3f
- Pappas PG, Tellez I, Deep AE, et al. Sporotrichosis in Peru: description of an area of hyperendemicity. Clin Infect Dis. 2000;30:65-70. doi:10.1086/313607
- McGuinness SL, Boyd R, Kidd S, et al. Epidemiological investigation of an outbreak of cutaneous sporotrichosis, Northern Territory, Australia. BMC Infect Dis. 2016;16:1-7. doi:10.1186/s12879-016-1338-0
- Rojas FD, Fernández MS, Lucchelli JM, et al. Cavitary pulmonary sporotrichosis: case report and literature review. Mycopathologia. 2017;182:1119-1123. doi:10.1007/s11046-017-0197-6
- Schenck BR. On refractory subcutaneous abscesses caused by a fungus possibly related to the sporotricha. Bulletin of the Johns Hopkins Hospital. 1898;93:286-290.
- de Lima Barros MB, de Almeida Paes R, Schubach AO. Sporothrix schenckii and sporotrichosis. Clin Microbiol Rev. 2011;24:633-654. doi:10.1128/CMR.00007-11
- Crevasse L, Ellner PD. An outbreak of sporotrichosis in florida. J Am Med Assoc. 1960;173:29-33. doi:10.1001/jama.1960.03020190031006
- Mayorga R, Cáceres A, Toriello C, et al. An endemic area of sporotrichosis in Guatemala [in French]. Sabouraudia. 1978;16:185-198.
- Morris-Jones R. Sporotrichosis. Clin Exp Dermatol. 2002;27:427-431. doi:10.1046/j.1365-2230.2002.01087.x
- Sampaio SA, Da Lacaz CS. Clinical and statistical studies on sporotrichosis in Sao Paulo (Brazil). Article in German. Hautarzt. 1959;10:490-493.
- Ramos-e-Silva M, Vasconcelos C, Carneiro S, et al. Sporotrichosis. Clin Dermatol. 2007;25:181-187. doi:10.1016/j.clindermatol.2006.05.006
- Williams BA, Jennings TA, Rushing EC, et al. Sporotrichosis on the face of a 7-year-old boy following a bicycle accident. Pediatr Dermatol. 2013;30:E246-E247. doi:10.1111/j.1525-1470.2011.01696.x
- Vaishampayan SS, Borde P. An unusual presentation of sporotrichosis. Indian J Dermatol. 2013;58:409. doi:10.4103/0019-5154.117350
- Qin J, Zhang J. Sporotrichosis. N Engl J Med. 2019;380:771. doi:10.1056/NEJMicm1809179
- Patel A, Mudenda V, Lakhi S, et al. A 27-year-old severely immunosuppressed female with misleading clinical features of disseminated cutaneous sporotrichosis. Case Rep Dermatol Med. 2016;2016:1-4. doi:10.1155/2016/9403690
- de Oliveira-Esteves ICMR, Almeida Rosa da Silva G, Eyer-Silva WA, et al. Rapidly progressive disseminated sporotrichosis as the first presentation of HIV infection in a patient with a very low CD4 cell count. Case Rep Infect Dis. 2017;2017:4713140. doi:10.1155/2017/4713140
- Singh S, Bachaspatimayum R, Meetei U, et al. Terbinafine in fixed cutaneous sporotrichosis: a case series. J Clin Diagnostic Res. 2018;12:FR01-FR03. doi:10.7860/JCDR/2018/25315.12223
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280. doi:10.1128/CMR.00053-10
- Peters F, Batinica M, Plum G, et al. Bug or no bug: challenges in diagnosing cutaneous mycobacterial infections. J Ger Soc Dermatol. 2016;14:1227-1236. doi:10.1111/ddg.13001
- Khadka P, Koirala S, Thapaliya J. Cutaneous tuberculosis: clinicopathologic arrays and diagnostic challenges. Dermatol Res Pract. 2018;2018:7201973. doi:10.1155/2018/7201973
- Okada E, Maruyama Y. Are keloids and hypertrophic scars caused by fungal infection? . Plast Reconstr Surg. 2007;120:814-815. doi:10.1097/01.prs.0000278813.23244.3f
- Pappas PG, Tellez I, Deep AE, et al. Sporotrichosis in Peru: description of an area of hyperendemicity. Clin Infect Dis. 2000;30:65-70. doi:10.1086/313607
- McGuinness SL, Boyd R, Kidd S, et al. Epidemiological investigation of an outbreak of cutaneous sporotrichosis, Northern Territory, Australia. BMC Infect Dis. 2016;16:1-7. doi:10.1186/s12879-016-1338-0
- Rojas FD, Fernández MS, Lucchelli JM, et al. Cavitary pulmonary sporotrichosis: case report and literature review. Mycopathologia. 2017;182:1119-1123. doi:10.1007/s11046-017-0197-6
Practice Points
- An atypical presentation of lymphocutaneous sporotrichosis may pose challenges to timely diagnosis and treatment.
- Although lymphocutaneous sporotrichosis spreads most commonly in a linear fashion along lymphatic channels, an annular configuration is possible.
- Initial tissue cultures and histopathology of lymphocutaneous sporotrichosis may not yield a diagnosis, necessitating repeat biopsies when clinical suspicion is high.
Retiform Purpura on the Legs
The Diagnosis: Calciphylaxis
Histopathology revealed epidermal and dermal necrosis, a perivascular neutrophilic infiltrate, and scattered microcalcifications within small- and medium-sized subcutaneous vessels, consistent with a diagnosis of calciphylaxis (Figure). Calciphylaxis (also known as calcific uremic arteriolopathy) is a rare, severe, and often fatal vasculopathy that predominately occurs in patients with end-stage renal failure.1 The pathogenesis of calciphylaxis remains poorly understood; however, it generally is thought that an imbalance in calcium homeostasis in susceptible hosts results in the precipitation of calcium phosphate within vessel walls leading to endothelial damage with subsequent thrombotic vasculopathy and ischemic tissue damage. Acquired and congenital hypercoagulable states have been implicated in the pathogenesis of calciphylaxis.2
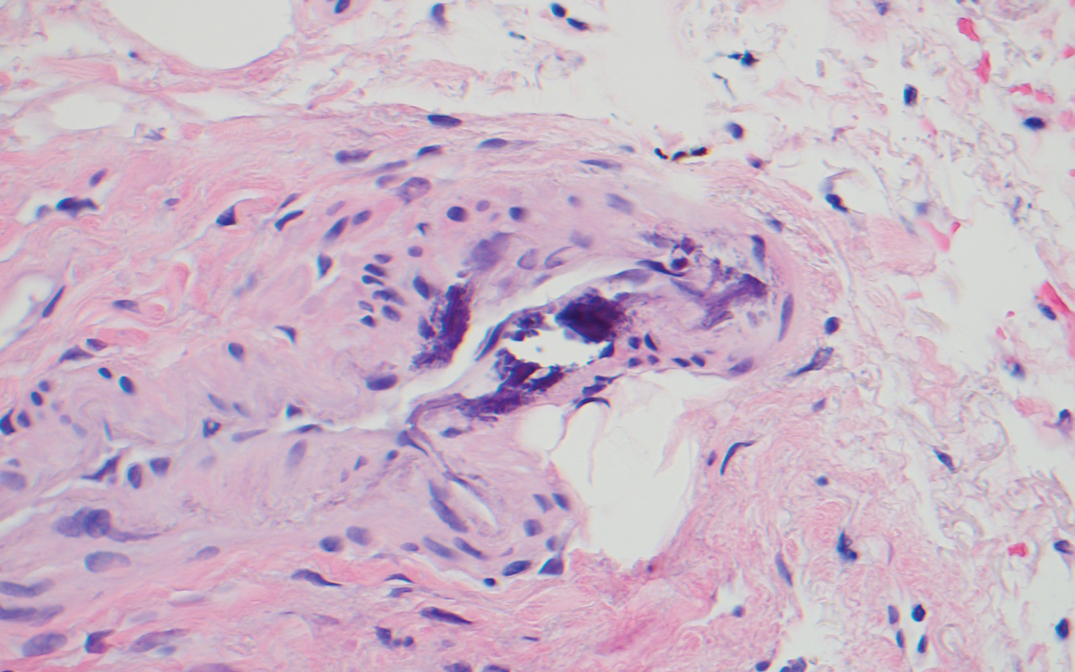
Treatment of calciphylaxis is directed at normalizing abnormal calcium metabolism; removing possible exacerbating agents, such as warfarin, systemic corticosteroids, calcium, and iron; and transitioning patients with end-stage renal disease to hemodialysis, if not already initiated. The treatment approach is multifaceted, and numerous therapies usually are attempted simultaneously. Vitamin K supplementation, low-calcium dialysate, non–calcium carbonate phosphate binders, cinacalcet, becaplermin, bisphosphonates, hyperbaric oxygen, and intravenous sodium thiosulfate all have been utilized with some success. Currently, intravenous sodium thiosulfate is the mainstay therapy for the treatment of calciphylaxis.2 Although the mechanism of sodium thiosulfate is not entirely understood, it is known to have anticalcification, vasodilatory, and antioxidant properties.
Retiform purpura clinically is characterized by reticulated, branching, purpuric skin lesions. It occurs following vascular insult by way of vessel lumen occlusion (thrombotic vasculopathy) and less frequently by vessel wall inflammation (vasculitis). The differential diagnosis for retiform purpura includes various causes of microvascular occlusion, including hypercoagulable states and type I cryoglobulinemia, calciphylaxis, infections, autoimmune vasculitic conditions, and embolic causes.3
Cutaneous disease in individuals with antiphospholipid antibodies may present similarly with retiform purpura in the form of necrotizing livedo reticularis, leg ulcers, or widespread cutaneous necrosis. Histopathologic findings include vascular thrombi with partial or complete obstruction of the small- to medium-sized arteries at the dermoepidermal junction, often in the absence of an inflammatory infiltrate.4 True vasculitis is not typical of antiphospholipid syndrome.
Medium vessel vasculitides, such as polyarteritis nodosa, clinically present with livedo reticularis, subcutaneous nodules, and tissue necrosis. Dermatopathologic evaluation of a medium-sized vessel vasculitis would demonstrate a neutrophilic vasculitis involving vessels within the deep dermis and septa of subcutaneous fat.5 Tissue sampling should be deep and wide enough to visualize the pathology, as shallow biopsies may show intraluminal thrombi of the superficial dermal plexus only, while a narrow specimen may result in falsenegative findings due to the focal nature of vessel involvement in conditions such as polyarteritis nodosa.
Type I cryoglobulinemia often is a manifestation of plasma cell dyscrasia and commonly presents with Raynaud phenomenon, livedo reticularis, and acrocyanosis of helices6 ; pathology demonstrates vessel occlusion and erythrocyte extravasation. In contrast, types II and III, also known as mixed cryoglobulinemia, are associated with hepatitis C and autoimmune connective tissue disease. They clinically present as purpuric plaques and nodules that have a propensity to vesiculate and ulcerate.7 Histopathologically, features of leukocytoclastic vasculitis are seen, and direct immunofluorescence demonstrates perivascular granular deposits consisting predominantly of IgM and C3 in the papillary dermis.8
Warfarin therapy, particularly in high initial doses, can induce lesions of cutaneous necrosis, which clinically may resemble the appearance of calciphylaxis. Warfarininduced skin necrosis typically occurs 3 to 5 days after the initiation of therapy and is the result of a temporary prothrombotic state.9 The half-life of antithrombotic protein C is shorter than vitamin K–dependent prothrombotic factors II, X, and IX. Early in warfarin treatment, an acquired state of reduced protein C level exists, which can lead to vessel thrombosis and subsequent cutaneous necrosis. Treatment of warfarin-induced skin necrosis involves cessation of warfarin, supplementation with vitamin K to reverse the effects of warfarin, and the initiation of heparin or low-molecular-weight heparin.9
- Hayashi M. Calciphylaxis: diagnosis and clinical features. Clin Exp Nephrol. 2013;17:498-503.
- Strazzula L, Nigwekar SU, Steele D, et al. Intralesional sodium thiosulfate for the treatment of calciphylaxis. JAMA Dermatol. 2013;149:946-949.
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796.
- Llamas-Velasco M, Alegría V, Santos-Briz Á, et al. Occlusive nonvasculitic vasculopathy. Am J Dermatopathol. 2017;39:637-662.
- Daoud MS, Hutton KP, Gibson LE. Cutaneous periarteritis nodosa: a clinicopathologic study of 79 cases. Br J Dermatol. 1997; 136:706-713.
- Fraser Gibson J, Leventhal JS, King B. Purpuric lesions on acral sites. type I cryoglobulinemia associated with multiple myeloma. JAMA Dermatol. 2015;151:659-660.
- Pakula AS, Garden JM, Roth SI. Mixed cryoglobulinemia and hepatitis C virus infection. J Am Acad Dermatol. 1994;30:143.
- Daoud MS, el-Azhary RA, Gibson LE, et al. Chronic hepatitis C, cryoglobulinemia, and cutaneous necrotizing vasculitis. clinical, pathologic, and immunopathologic study of twelve patients. J Am Acad Dermatol. 1996;34:219-223.
- Nazarian RM, Van Cott EM, Zembowicz A, et al. Warfarin-induced skin necrosis. J Am Acad Dermatol. 2009;61:325-332.
The Diagnosis: Calciphylaxis
Histopathology revealed epidermal and dermal necrosis, a perivascular neutrophilic infiltrate, and scattered microcalcifications within small- and medium-sized subcutaneous vessels, consistent with a diagnosis of calciphylaxis (Figure). Calciphylaxis (also known as calcific uremic arteriolopathy) is a rare, severe, and often fatal vasculopathy that predominately occurs in patients with end-stage renal failure.1 The pathogenesis of calciphylaxis remains poorly understood; however, it generally is thought that an imbalance in calcium homeostasis in susceptible hosts results in the precipitation of calcium phosphate within vessel walls leading to endothelial damage with subsequent thrombotic vasculopathy and ischemic tissue damage. Acquired and congenital hypercoagulable states have been implicated in the pathogenesis of calciphylaxis.2

Treatment of calciphylaxis is directed at normalizing abnormal calcium metabolism; removing possible exacerbating agents, such as warfarin, systemic corticosteroids, calcium, and iron; and transitioning patients with end-stage renal disease to hemodialysis, if not already initiated. The treatment approach is multifaceted, and numerous therapies usually are attempted simultaneously. Vitamin K supplementation, low-calcium dialysate, non–calcium carbonate phosphate binders, cinacalcet, becaplermin, bisphosphonates, hyperbaric oxygen, and intravenous sodium thiosulfate all have been utilized with some success. Currently, intravenous sodium thiosulfate is the mainstay therapy for the treatment of calciphylaxis.2 Although the mechanism of sodium thiosulfate is not entirely understood, it is known to have anticalcification, vasodilatory, and antioxidant properties.
Retiform purpura clinically is characterized by reticulated, branching, purpuric skin lesions. It occurs following vascular insult by way of vessel lumen occlusion (thrombotic vasculopathy) and less frequently by vessel wall inflammation (vasculitis). The differential diagnosis for retiform purpura includes various causes of microvascular occlusion, including hypercoagulable states and type I cryoglobulinemia, calciphylaxis, infections, autoimmune vasculitic conditions, and embolic causes.3
Cutaneous disease in individuals with antiphospholipid antibodies may present similarly with retiform purpura in the form of necrotizing livedo reticularis, leg ulcers, or widespread cutaneous necrosis. Histopathologic findings include vascular thrombi with partial or complete obstruction of the small- to medium-sized arteries at the dermoepidermal junction, often in the absence of an inflammatory infiltrate.4 True vasculitis is not typical of antiphospholipid syndrome.
Medium vessel vasculitides, such as polyarteritis nodosa, clinically present with livedo reticularis, subcutaneous nodules, and tissue necrosis. Dermatopathologic evaluation of a medium-sized vessel vasculitis would demonstrate a neutrophilic vasculitis involving vessels within the deep dermis and septa of subcutaneous fat.5 Tissue sampling should be deep and wide enough to visualize the pathology, as shallow biopsies may show intraluminal thrombi of the superficial dermal plexus only, while a narrow specimen may result in falsenegative findings due to the focal nature of vessel involvement in conditions such as polyarteritis nodosa.
Type I cryoglobulinemia often is a manifestation of plasma cell dyscrasia and commonly presents with Raynaud phenomenon, livedo reticularis, and acrocyanosis of helices6 ; pathology demonstrates vessel occlusion and erythrocyte extravasation. In contrast, types II and III, also known as mixed cryoglobulinemia, are associated with hepatitis C and autoimmune connective tissue disease. They clinically present as purpuric plaques and nodules that have a propensity to vesiculate and ulcerate.7 Histopathologically, features of leukocytoclastic vasculitis are seen, and direct immunofluorescence demonstrates perivascular granular deposits consisting predominantly of IgM and C3 in the papillary dermis.8
Warfarin therapy, particularly in high initial doses, can induce lesions of cutaneous necrosis, which clinically may resemble the appearance of calciphylaxis. Warfarininduced skin necrosis typically occurs 3 to 5 days after the initiation of therapy and is the result of a temporary prothrombotic state.9 The half-life of antithrombotic protein C is shorter than vitamin K–dependent prothrombotic factors II, X, and IX. Early in warfarin treatment, an acquired state of reduced protein C level exists, which can lead to vessel thrombosis and subsequent cutaneous necrosis. Treatment of warfarin-induced skin necrosis involves cessation of warfarin, supplementation with vitamin K to reverse the effects of warfarin, and the initiation of heparin or low-molecular-weight heparin.9
The Diagnosis: Calciphylaxis
Histopathology revealed epidermal and dermal necrosis, a perivascular neutrophilic infiltrate, and scattered microcalcifications within small- and medium-sized subcutaneous vessels, consistent with a diagnosis of calciphylaxis (Figure). Calciphylaxis (also known as calcific uremic arteriolopathy) is a rare, severe, and often fatal vasculopathy that predominately occurs in patients with end-stage renal failure.1 The pathogenesis of calciphylaxis remains poorly understood; however, it generally is thought that an imbalance in calcium homeostasis in susceptible hosts results in the precipitation of calcium phosphate within vessel walls leading to endothelial damage with subsequent thrombotic vasculopathy and ischemic tissue damage. Acquired and congenital hypercoagulable states have been implicated in the pathogenesis of calciphylaxis.2

Treatment of calciphylaxis is directed at normalizing abnormal calcium metabolism; removing possible exacerbating agents, such as warfarin, systemic corticosteroids, calcium, and iron; and transitioning patients with end-stage renal disease to hemodialysis, if not already initiated. The treatment approach is multifaceted, and numerous therapies usually are attempted simultaneously. Vitamin K supplementation, low-calcium dialysate, non–calcium carbonate phosphate binders, cinacalcet, becaplermin, bisphosphonates, hyperbaric oxygen, and intravenous sodium thiosulfate all have been utilized with some success. Currently, intravenous sodium thiosulfate is the mainstay therapy for the treatment of calciphylaxis.2 Although the mechanism of sodium thiosulfate is not entirely understood, it is known to have anticalcification, vasodilatory, and antioxidant properties.
Retiform purpura clinically is characterized by reticulated, branching, purpuric skin lesions. It occurs following vascular insult by way of vessel lumen occlusion (thrombotic vasculopathy) and less frequently by vessel wall inflammation (vasculitis). The differential diagnosis for retiform purpura includes various causes of microvascular occlusion, including hypercoagulable states and type I cryoglobulinemia, calciphylaxis, infections, autoimmune vasculitic conditions, and embolic causes.3
Cutaneous disease in individuals with antiphospholipid antibodies may present similarly with retiform purpura in the form of necrotizing livedo reticularis, leg ulcers, or widespread cutaneous necrosis. Histopathologic findings include vascular thrombi with partial or complete obstruction of the small- to medium-sized arteries at the dermoepidermal junction, often in the absence of an inflammatory infiltrate.4 True vasculitis is not typical of antiphospholipid syndrome.
Medium vessel vasculitides, such as polyarteritis nodosa, clinically present with livedo reticularis, subcutaneous nodules, and tissue necrosis. Dermatopathologic evaluation of a medium-sized vessel vasculitis would demonstrate a neutrophilic vasculitis involving vessels within the deep dermis and septa of subcutaneous fat.5 Tissue sampling should be deep and wide enough to visualize the pathology, as shallow biopsies may show intraluminal thrombi of the superficial dermal plexus only, while a narrow specimen may result in falsenegative findings due to the focal nature of vessel involvement in conditions such as polyarteritis nodosa.
Type I cryoglobulinemia often is a manifestation of plasma cell dyscrasia and commonly presents with Raynaud phenomenon, livedo reticularis, and acrocyanosis of helices6 ; pathology demonstrates vessel occlusion and erythrocyte extravasation. In contrast, types II and III, also known as mixed cryoglobulinemia, are associated with hepatitis C and autoimmune connective tissue disease. They clinically present as purpuric plaques and nodules that have a propensity to vesiculate and ulcerate.7 Histopathologically, features of leukocytoclastic vasculitis are seen, and direct immunofluorescence demonstrates perivascular granular deposits consisting predominantly of IgM and C3 in the papillary dermis.8
Warfarin therapy, particularly in high initial doses, can induce lesions of cutaneous necrosis, which clinically may resemble the appearance of calciphylaxis. Warfarininduced skin necrosis typically occurs 3 to 5 days after the initiation of therapy and is the result of a temporary prothrombotic state.9 The half-life of antithrombotic protein C is shorter than vitamin K–dependent prothrombotic factors II, X, and IX. Early in warfarin treatment, an acquired state of reduced protein C level exists, which can lead to vessel thrombosis and subsequent cutaneous necrosis. Treatment of warfarin-induced skin necrosis involves cessation of warfarin, supplementation with vitamin K to reverse the effects of warfarin, and the initiation of heparin or low-molecular-weight heparin.9
- Hayashi M. Calciphylaxis: diagnosis and clinical features. Clin Exp Nephrol. 2013;17:498-503.
- Strazzula L, Nigwekar SU, Steele D, et al. Intralesional sodium thiosulfate for the treatment of calciphylaxis. JAMA Dermatol. 2013;149:946-949.
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796.
- Llamas-Velasco M, Alegría V, Santos-Briz Á, et al. Occlusive nonvasculitic vasculopathy. Am J Dermatopathol. 2017;39:637-662.
- Daoud MS, Hutton KP, Gibson LE. Cutaneous periarteritis nodosa: a clinicopathologic study of 79 cases. Br J Dermatol. 1997; 136:706-713.
- Fraser Gibson J, Leventhal JS, King B. Purpuric lesions on acral sites. type I cryoglobulinemia associated with multiple myeloma. JAMA Dermatol. 2015;151:659-660.
- Pakula AS, Garden JM, Roth SI. Mixed cryoglobulinemia and hepatitis C virus infection. J Am Acad Dermatol. 1994;30:143.
- Daoud MS, el-Azhary RA, Gibson LE, et al. Chronic hepatitis C, cryoglobulinemia, and cutaneous necrotizing vasculitis. clinical, pathologic, and immunopathologic study of twelve patients. J Am Acad Dermatol. 1996;34:219-223.
- Nazarian RM, Van Cott EM, Zembowicz A, et al. Warfarin-induced skin necrosis. J Am Acad Dermatol. 2009;61:325-332.
- Hayashi M. Calciphylaxis: diagnosis and clinical features. Clin Exp Nephrol. 2013;17:498-503.
- Strazzula L, Nigwekar SU, Steele D, et al. Intralesional sodium thiosulfate for the treatment of calciphylaxis. JAMA Dermatol. 2013;149:946-949.
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796.
- Llamas-Velasco M, Alegría V, Santos-Briz Á, et al. Occlusive nonvasculitic vasculopathy. Am J Dermatopathol. 2017;39:637-662.
- Daoud MS, Hutton KP, Gibson LE. Cutaneous periarteritis nodosa: a clinicopathologic study of 79 cases. Br J Dermatol. 1997; 136:706-713.
- Fraser Gibson J, Leventhal JS, King B. Purpuric lesions on acral sites. type I cryoglobulinemia associated with multiple myeloma. JAMA Dermatol. 2015;151:659-660.
- Pakula AS, Garden JM, Roth SI. Mixed cryoglobulinemia and hepatitis C virus infection. J Am Acad Dermatol. 1994;30:143.
- Daoud MS, el-Azhary RA, Gibson LE, et al. Chronic hepatitis C, cryoglobulinemia, and cutaneous necrotizing vasculitis. clinical, pathologic, and immunopathologic study of twelve patients. J Am Acad Dermatol. 1996;34:219-223.
- Nazarian RM, Van Cott EM, Zembowicz A, et al. Warfarin-induced skin necrosis. J Am Acad Dermatol. 2009;61:325-332.
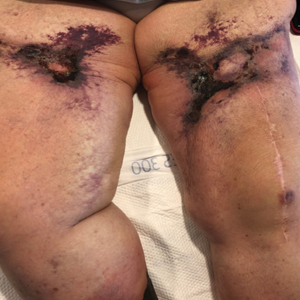
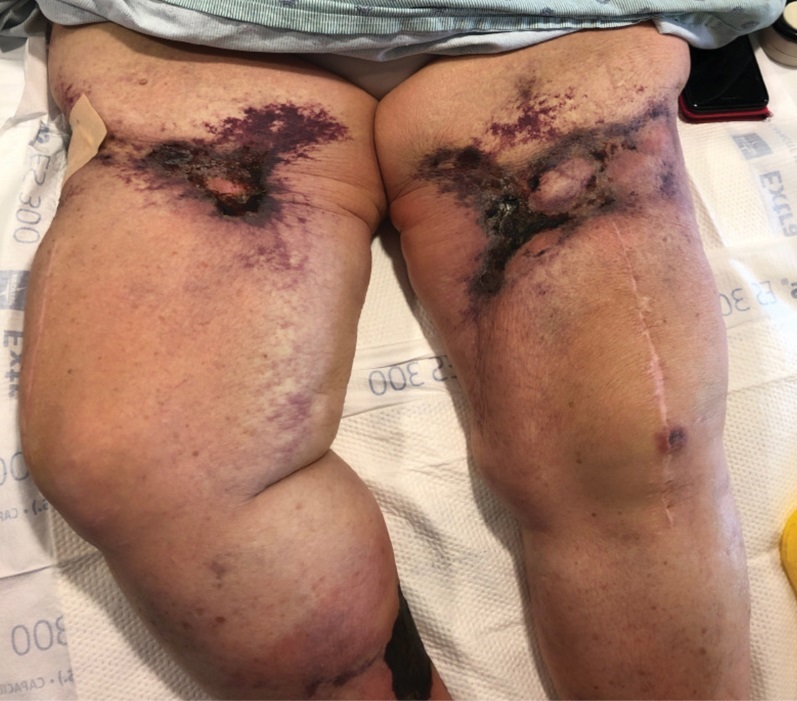
A 70-year-old woman with a medical history of Takayasu arteritis, end-stage renal disease on peritoneal dialysis, coronary artery disease, hypertension, hypothyroidism, and anemia of chronic disease presented to the emergency department with enlarging painful stellate eschars of the legs with associated edema of 3 weeks’ duration. She denied a history of similar-appearing skin lesions. She initially thought the lesions were burns secondary to frequent hot showers for relief of uremic pruritus. For the treatment of these suspected burns prior to hospitalization, she had been applying over-the-counter antibiotic ointments to the affected areas and had completed a 2-week course of oral cephalexin without notable improvement. Physical examination revealed retiform purpura of the legs with large stellate eschars overlying the anteromedial thighs and right medial calf. Computed tomography angiogram of the abdomen and pelvis demonstrated diffuse calcifications of the aortic wall and its associated branches that were most pronounced in the legs without evidence of vessel wall thickening. Punch biopsies were performed, and nephrology, rheumatology, and wound care services were consulted.
Acral Papulovesicular Eruption in a Soldier Following Smallpox Vaccination
Following the attacks of September 11, 2001, heightened concerns over bioterrorism and the potential use of smallpox as a biological weapon made smallpox vaccination a critical component of military readiness. Therefore, the US Military resumed its smallpox vaccination program in 2002 using the first-generation smallpox vaccine (Dryvax, Wyeth Pharmaceuticals), a live vaccinia virus vaccine created in the late 19th century. This vaccine was developed by pooling vaccinia strains from the skin of infected cows1 and had previously been used during the worldwide vaccination campaign in the 1970s. Dryvax was associated with various cardiac and cutaneous complications, from benign hypersensitivity reactions to life-threatening eczema vaccinatum and progressive vaccinia.
Due to concerns that the remaining supply of Dryvax was insufficient to vaccinate the US population in the case of a bioterrorism attack, investigators developed the second-generation smallpox vaccine (ACAM2000, Sanofi Pasteur Biologics Co) using advances in vaccine technology.2 ACAM2000 is a plaque-purified isolate of vaccinia virus propagated in cell culture, thereby reducing contaminants and lot-to-lot variation.1 Clinical trials demonstrated comparable immunogenicity and frequency of adverse events compared with Dryvax,2 and ACAM2000 replaced Dryvax in 2008. However, these trials focused on serious adverse events, such as cardiac complications and postvaccinal encephalitis, with less specific characterization and description of cutaneous eruptions.3
Since 2008, there have been few reports of cutaneous adverse reactions following vaccination with ACAM2000. Beachkofsky et al4 described 7 cases of papulovesicular eruptions and 1 case of generalized vaccinia. Freeman and Lenz5 described 4 cases of papulovesicular eruptions, and there has been 1 case of progressive vaccinia reported in a soldier with newly diagnosed acute myelogenous leukemia.6 Kramer7 described a patient with multiple vesiculopustular lesions secondary to autoinoculation. The distinct pruritic acral papulovesicular eruptions following ACAM2000 vaccination have occurred in healthy military service members at different locations since the introduction of ACAM2000. We describe an additional case of this unique cutaneous eruption, followed by a review of previously described cutaneous adverse events associated with smallpox vaccination.
Case Report
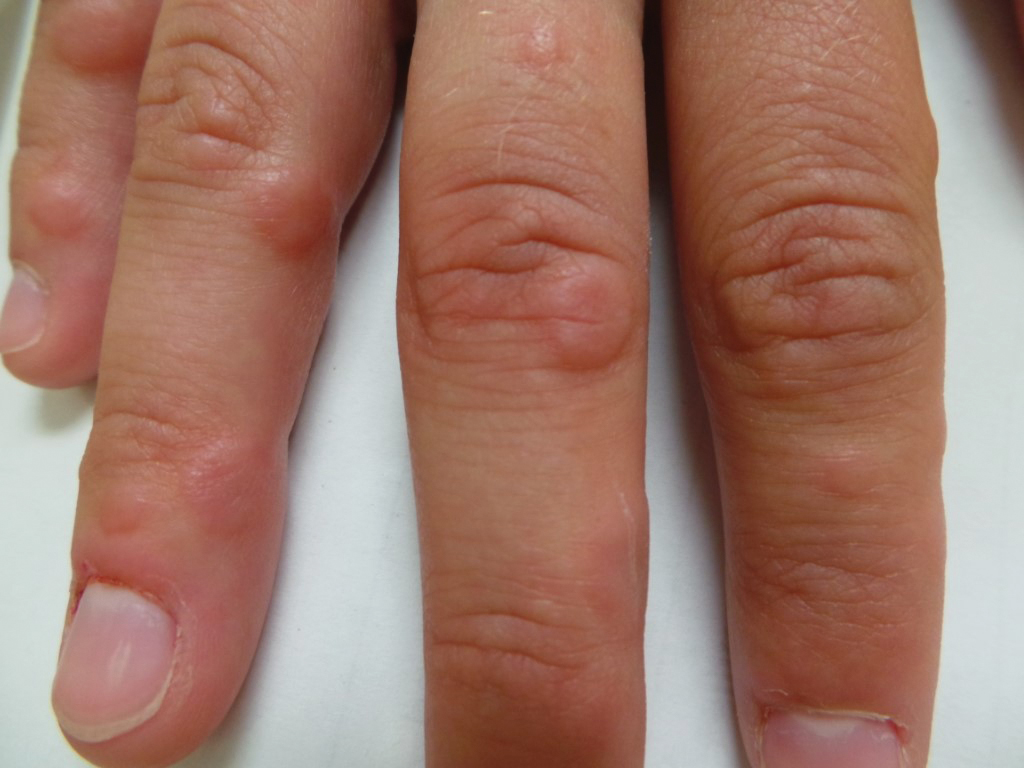
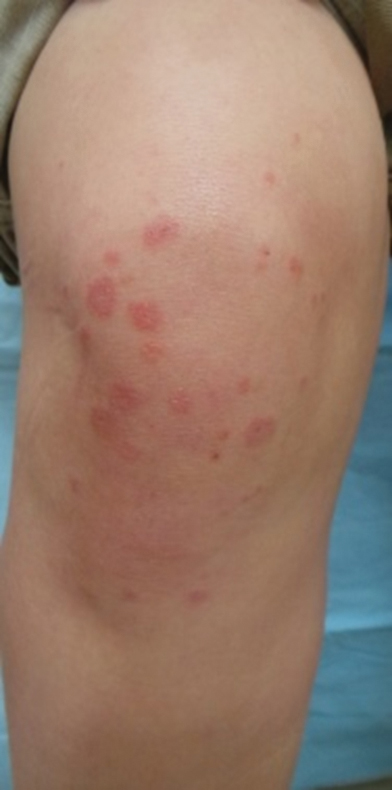
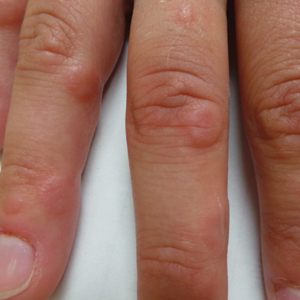
A 21-year-old female soldier who was otherwise healthy presented to the dermatology clinic with a pruritic papular eruption involving the upper and lower extremities of 1 week’s duration. The lesions first appeared 8 days after she received the ACAM2000 vaccine. She received no other concurrent vaccines, had no history of atopic dermatitis, and had no systemic symptoms. Physical examination revealed numerous erythematous indurated papules involving the dorsolateral hands and fingers, as well as the extensor surfaces of the elbows, knees, and thighs (Figures 1 and 2). Based on the clinical presentation, the differential diagnosis included lichen planus, verruca plana, dyshidrotic eczema, and smallpox vaccine reaction. Erythema multiforme was considered; however, the absence of palmoplantar involvement and typical targetoid lesions made this diagnosis less likely.
Biopsies of lesions on the arm and thigh were performed. Histologic findings revealed interface and spongiotic dermatitis with scattered necrotic keratinocytes and extravasated erythrocytes (Figure 3). There was no evidence of viral cytopathic effects. Similar clinical and histologic findings have been reported in the literature as acral papulovesicular eruptions following smallpox vaccination or papular spongiotic dermatitis of smallpox vaccination.8 The presence of eosinophils was not conspicuous in the current case and was only a notable finding in 1 of 2 cases previously described by Gaertner et al.8 This may simply be due to an idiosyncratic drug reaction. Furthermore, in the cases described by Beachkofsky et al,4 there were essentially 2 histologic groups. The first group demonstrated a dermal hypersensitivity-type reaction, and the second group demonstrated a lymphocytic capillaritis.
Based on these findings, the patient was diagnosed with an acral papulovesicular eruption following smallpox vaccination. Of note, the patient’s presentation was not consistent with other described smallpox vaccine reactions, which included eczema vaccinatum, autoinoculation, generalized vaccinia, and progressive vaccinia. The patient was treated supportively with triamcinolone acetonide cream 0.1%, cool compresses, and oral diphenhydramine as needed for pruritus. The lesions notably improved within the first week of treatment.
Comment
Reported cases of acral papulovesicular eruption4-6 demonstrated an onset of cutaneous symptoms an average of 14 days following vaccination (range, 8–18 days postvaccination). Lesions were benign and self-limited in all cases, with resolution within an average of 25 days (range, 7–71 days). All patients were active-duty military adults with a mean age of 24 years. Supportive treatment varied from topical steroids and oral antihistamines to tapering oral prednisone doses. Of note, all previously reported cases of this reaction occurred in patients who also had received other concurrent or near-concurrent vaccines, including anthrax, hepatitis B, influenza, and typhoid. Our patient represents a unique case of a papulovesicular eruption following smallpox vaccination with no history of concurrent vaccines.
Since the 1970s, smallpox vaccination has been associated with numerous cutaneous reactions, most of which have been reported with the first-generation Dryvax. Minor local reactions occurred in approximately 2% to 6% of vaccinees in clinical trials.9 These reactions included local edema involving the upper arm, satellite lesions within 2.5 cm of the vaccination site, local lymphadenopathy, intense inflammation or viral cellulitis surrounding the inoculation site, and viral lymphangitis tracking to axillary lymph nodes. In clinical trials, these reactions were self-limited and required only symptomatic treatment.9
Autoinoculation is another cutaneous reaction that can occur because Dryvax and ACAM2000 both contain live-attenuated replicating vaccinia virus. Accidental implantation may occur when the high titers of virus present at the vaccine site are subsequently transferred to other sites, especially abnormal mucosa or skin, resulting in an additional primary inoculation site.10
Eczema vaccinatum is a potentially life-threatening reaction that may occur in patients with disruptive skin disorders, such as atopic dermatitis. These patients are at risk for massive confluent vaccinia infection of the skin.10 In patients with atopic dermatitis, the virus rapidly disseminates due to both skin barrier dysfunction and impaired immunomodulation, resulting in large confluent skin lesions and the potential for viremia, septic shock, and death.10,11 Mortality from eczema vaccinatum may be reduced by administration of vaccinia immune globulin.10
The vaccinia virus also may spread hematogenously in healthy individuals,10 resulting in a benign reaction called generalized vaccinia. These patients develop pustules on areas of the skin other than the vaccination site. Although typically benign and self-limited, Beachkofsky et al4 described a case of generalized vaccinia in a healthy 34-year-old man resulting in a rapidly progressive vesiculopustular eruption with associated fever and pancytopenia. The patient made a complete recovery over the course of the following month.4
Alternatively, progressive vaccinia is a severe complication of smallpox vaccination seen in patients with impaired cell-mediated immunity. It also is known as vaccinia gangrenosum or vaccinia necrosum. These patients develop expanding ulcers due to exaggerated viral replication and cell-to-cell spread of the vaccinia virus.10,11 Hematogenous spread may result in viral implantation at distant sites of the body. This disease slowly progresses over weeks to months, and it often is resistant to treatment and fatal in patients with severe T-cell deficiency.10
Acral papulovesicular eruption is a distinct cutaneous adverse event following smallpox vaccination. Although further research is needed to discern the pathogenesis of this reaction, it is benign and self-limited, and patients have fully recovered with supportive care. In addition, a modified vaccinia Ankara vaccine (Bavarian Nordic) was approved by the US Food and Drug Administration in 2019.12,13 It is a nonreplicating attenuated viral vaccine that had fewer adverse events compared to ACAM2000 in clinical trials.13 To date, papulovesicular eruptions have not been reported following vaccination with the modified vaccinia Ankara vaccine; however, continued monitoring will help to further characterize any cutaneous reactions to this newer vaccine.
- Nalca A, Zumbrun EE. ACAM2000: the new smallpox vaccine for United States Strategic National Stockpile. Drug Des Devel Ther. 2010;4:71-79.
- Monath TP, Caldwell JR, Mundt W, et al. ACAM2000 clonal Vero cell culture vaccinia virus (New York City Board of Health strain)—a second-generation smallpox vaccine for biological defense. Int J Infect Dis. 2004;8:S31-S44.
- Thomas TN, Reef S, Neff L, et al. A review of the smallpox vaccine adverse events active surveillance system. Clin Infect Dis. 2008;46:S212-S220.
- Beachkofsky TM, Carrizales SC, Bidinger JJ, et al. Adverse events following smallpox vaccination with ACAM2000 in a military population. Arch Dermatol. 2010;146:656-661.
- Freeman R, Lenz B. Cutaneous reactions associated with ACAM2000 smallpox vaccination in a deploying U.S. Army unit. Mil Med. 2015;180:E152-E156.
- Centers for Disease Control and Prevention. Progressive vaccinia in a military smallpox vaccinee—United States, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:532-536.
- Kramer TR. Post–smallpox vaccination skin eruption in a marine. Mil Med. 2018;183:E649-E653.
- Gaertner EM, Groo S, Kim J. Papular spongiotic dermatitis of smallpox vaccination: report of 2 cases with review of the literature. Arch Pathol Lab Med. 2004;128:1173-1175.
- Fulginiti VA, Papier A, Lane JM, et al. Smallpox vaccination: a review, part I. background, vaccination technique, normal vaccination and revaccination, and expected normal reactions. Clin Infect Dis. 2003;37:241-250.
- Fulginiti VA, Papier A, Lane JM, et al. Smallpox vaccination: a review, part II. adverse events. Clin Infect Dis. 2003;37:251-271.
- Bray M. Understanding smallpox vaccination. J Infect Dis. 2011;203:1037-1039.
- Greenberg RN, Hay CM, Stapleton JT, et al. A randomized, double-blind, placebo-controlled phase II trial investigating the safety and immunogenicity of modified vaccinia ankara smallpox vaccine (MVA-BN®) in 56-80-year-old subjects. PLoS One. 2016;11:E0157335.
- Pittman PR, Hahn M, Lee HS, et al. Phase 3 efficacy trial of modified vaccinia Ankara as a vaccine against smallpox. N Engl J Med. 2019;381:1897-1908.
Following the attacks of September 11, 2001, heightened concerns over bioterrorism and the potential use of smallpox as a biological weapon made smallpox vaccination a critical component of military readiness. Therefore, the US Military resumed its smallpox vaccination program in 2002 using the first-generation smallpox vaccine (Dryvax, Wyeth Pharmaceuticals), a live vaccinia virus vaccine created in the late 19th century. This vaccine was developed by pooling vaccinia strains from the skin of infected cows1 and had previously been used during the worldwide vaccination campaign in the 1970s. Dryvax was associated with various cardiac and cutaneous complications, from benign hypersensitivity reactions to life-threatening eczema vaccinatum and progressive vaccinia.
Due to concerns that the remaining supply of Dryvax was insufficient to vaccinate the US population in the case of a bioterrorism attack, investigators developed the second-generation smallpox vaccine (ACAM2000, Sanofi Pasteur Biologics Co) using advances in vaccine technology.2 ACAM2000 is a plaque-purified isolate of vaccinia virus propagated in cell culture, thereby reducing contaminants and lot-to-lot variation.1 Clinical trials demonstrated comparable immunogenicity and frequency of adverse events compared with Dryvax,2 and ACAM2000 replaced Dryvax in 2008. However, these trials focused on serious adverse events, such as cardiac complications and postvaccinal encephalitis, with less specific characterization and description of cutaneous eruptions.3
Since 2008, there have been few reports of cutaneous adverse reactions following vaccination with ACAM2000. Beachkofsky et al4 described 7 cases of papulovesicular eruptions and 1 case of generalized vaccinia. Freeman and Lenz5 described 4 cases of papulovesicular eruptions, and there has been 1 case of progressive vaccinia reported in a soldier with newly diagnosed acute myelogenous leukemia.6 Kramer7 described a patient with multiple vesiculopustular lesions secondary to autoinoculation. The distinct pruritic acral papulovesicular eruptions following ACAM2000 vaccination have occurred in healthy military service members at different locations since the introduction of ACAM2000. We describe an additional case of this unique cutaneous eruption, followed by a review of previously described cutaneous adverse events associated with smallpox vaccination.
Case Report
A 21-year-old female soldier who was otherwise healthy presented to the dermatology clinic with a pruritic papular eruption involving the upper and lower extremities of 1 week’s duration. The lesions first appeared 8 days after she received the ACAM2000 vaccine. She received no other concurrent vaccines, had no history of atopic dermatitis, and had no systemic symptoms. Physical examination revealed numerous erythematous indurated papules involving the dorsolateral hands and fingers, as well as the extensor surfaces of the elbows, knees, and thighs (Figures 1 and 2). Based on the clinical presentation, the differential diagnosis included lichen planus, verruca plana, dyshidrotic eczema, and smallpox vaccine reaction. Erythema multiforme was considered; however, the absence of palmoplantar involvement and typical targetoid lesions made this diagnosis less likely.
Biopsies of lesions on the arm and thigh were performed. Histologic findings revealed interface and spongiotic dermatitis with scattered necrotic keratinocytes and extravasated erythrocytes (Figure 3). There was no evidence of viral cytopathic effects. Similar clinical and histologic findings have been reported in the literature as acral papulovesicular eruptions following smallpox vaccination or papular spongiotic dermatitis of smallpox vaccination.8 The presence of eosinophils was not conspicuous in the current case and was only a notable finding in 1 of 2 cases previously described by Gaertner et al.8 This may simply be due to an idiosyncratic drug reaction. Furthermore, in the cases described by Beachkofsky et al,4 there were essentially 2 histologic groups. The first group demonstrated a dermal hypersensitivity-type reaction, and the second group demonstrated a lymphocytic capillaritis.
Based on these findings, the patient was diagnosed with an acral papulovesicular eruption following smallpox vaccination. Of note, the patient’s presentation was not consistent with other described smallpox vaccine reactions, which included eczema vaccinatum, autoinoculation, generalized vaccinia, and progressive vaccinia. The patient was treated supportively with triamcinolone acetonide cream 0.1%, cool compresses, and oral diphenhydramine as needed for pruritus. The lesions notably improved within the first week of treatment.
Comment
Reported cases of acral papulovesicular eruption4-6 demonstrated an onset of cutaneous symptoms an average of 14 days following vaccination (range, 8–18 days postvaccination). Lesions were benign and self-limited in all cases, with resolution within an average of 25 days (range, 7–71 days). All patients were active-duty military adults with a mean age of 24 years. Supportive treatment varied from topical steroids and oral antihistamines to tapering oral prednisone doses. Of note, all previously reported cases of this reaction occurred in patients who also had received other concurrent or near-concurrent vaccines, including anthrax, hepatitis B, influenza, and typhoid. Our patient represents a unique case of a papulovesicular eruption following smallpox vaccination with no history of concurrent vaccines.
Since the 1970s, smallpox vaccination has been associated with numerous cutaneous reactions, most of which have been reported with the first-generation Dryvax. Minor local reactions occurred in approximately 2% to 6% of vaccinees in clinical trials.9 These reactions included local edema involving the upper arm, satellite lesions within 2.5 cm of the vaccination site, local lymphadenopathy, intense inflammation or viral cellulitis surrounding the inoculation site, and viral lymphangitis tracking to axillary lymph nodes. In clinical trials, these reactions were self-limited and required only symptomatic treatment.9
Autoinoculation is another cutaneous reaction that can occur because Dryvax and ACAM2000 both contain live-attenuated replicating vaccinia virus. Accidental implantation may occur when the high titers of virus present at the vaccine site are subsequently transferred to other sites, especially abnormal mucosa or skin, resulting in an additional primary inoculation site.10
Eczema vaccinatum is a potentially life-threatening reaction that may occur in patients with disruptive skin disorders, such as atopic dermatitis. These patients are at risk for massive confluent vaccinia infection of the skin.10 In patients with atopic dermatitis, the virus rapidly disseminates due to both skin barrier dysfunction and impaired immunomodulation, resulting in large confluent skin lesions and the potential for viremia, septic shock, and death.10,11 Mortality from eczema vaccinatum may be reduced by administration of vaccinia immune globulin.10
The vaccinia virus also may spread hematogenously in healthy individuals,10 resulting in a benign reaction called generalized vaccinia. These patients develop pustules on areas of the skin other than the vaccination site. Although typically benign and self-limited, Beachkofsky et al4 described a case of generalized vaccinia in a healthy 34-year-old man resulting in a rapidly progressive vesiculopustular eruption with associated fever and pancytopenia. The patient made a complete recovery over the course of the following month.4
Alternatively, progressive vaccinia is a severe complication of smallpox vaccination seen in patients with impaired cell-mediated immunity. It also is known as vaccinia gangrenosum or vaccinia necrosum. These patients develop expanding ulcers due to exaggerated viral replication and cell-to-cell spread of the vaccinia virus.10,11 Hematogenous spread may result in viral implantation at distant sites of the body. This disease slowly progresses over weeks to months, and it often is resistant to treatment and fatal in patients with severe T-cell deficiency.10
Acral papulovesicular eruption is a distinct cutaneous adverse event following smallpox vaccination. Although further research is needed to discern the pathogenesis of this reaction, it is benign and self-limited, and patients have fully recovered with supportive care. In addition, a modified vaccinia Ankara vaccine (Bavarian Nordic) was approved by the US Food and Drug Administration in 2019.12,13 It is a nonreplicating attenuated viral vaccine that had fewer adverse events compared to ACAM2000 in clinical trials.13 To date, papulovesicular eruptions have not been reported following vaccination with the modified vaccinia Ankara vaccine; however, continued monitoring will help to further characterize any cutaneous reactions to this newer vaccine.
Following the attacks of September 11, 2001, heightened concerns over bioterrorism and the potential use of smallpox as a biological weapon made smallpox vaccination a critical component of military readiness. Therefore, the US Military resumed its smallpox vaccination program in 2002 using the first-generation smallpox vaccine (Dryvax, Wyeth Pharmaceuticals), a live vaccinia virus vaccine created in the late 19th century. This vaccine was developed by pooling vaccinia strains from the skin of infected cows1 and had previously been used during the worldwide vaccination campaign in the 1970s. Dryvax was associated with various cardiac and cutaneous complications, from benign hypersensitivity reactions to life-threatening eczema vaccinatum and progressive vaccinia.
Due to concerns that the remaining supply of Dryvax was insufficient to vaccinate the US population in the case of a bioterrorism attack, investigators developed the second-generation smallpox vaccine (ACAM2000, Sanofi Pasteur Biologics Co) using advances in vaccine technology.2 ACAM2000 is a plaque-purified isolate of vaccinia virus propagated in cell culture, thereby reducing contaminants and lot-to-lot variation.1 Clinical trials demonstrated comparable immunogenicity and frequency of adverse events compared with Dryvax,2 and ACAM2000 replaced Dryvax in 2008. However, these trials focused on serious adverse events, such as cardiac complications and postvaccinal encephalitis, with less specific characterization and description of cutaneous eruptions.3
Since 2008, there have been few reports of cutaneous adverse reactions following vaccination with ACAM2000. Beachkofsky et al4 described 7 cases of papulovesicular eruptions and 1 case of generalized vaccinia. Freeman and Lenz5 described 4 cases of papulovesicular eruptions, and there has been 1 case of progressive vaccinia reported in a soldier with newly diagnosed acute myelogenous leukemia.6 Kramer7 described a patient with multiple vesiculopustular lesions secondary to autoinoculation. The distinct pruritic acral papulovesicular eruptions following ACAM2000 vaccination have occurred in healthy military service members at different locations since the introduction of ACAM2000. We describe an additional case of this unique cutaneous eruption, followed by a review of previously described cutaneous adverse events associated with smallpox vaccination.
Case Report
A 21-year-old female soldier who was otherwise healthy presented to the dermatology clinic with a pruritic papular eruption involving the upper and lower extremities of 1 week’s duration. The lesions first appeared 8 days after she received the ACAM2000 vaccine. She received no other concurrent vaccines, had no history of atopic dermatitis, and had no systemic symptoms. Physical examination revealed numerous erythematous indurated papules involving the dorsolateral hands and fingers, as well as the extensor surfaces of the elbows, knees, and thighs (Figures 1 and 2). Based on the clinical presentation, the differential diagnosis included lichen planus, verruca plana, dyshidrotic eczema, and smallpox vaccine reaction. Erythema multiforme was considered; however, the absence of palmoplantar involvement and typical targetoid lesions made this diagnosis less likely.
Biopsies of lesions on the arm and thigh were performed. Histologic findings revealed interface and spongiotic dermatitis with scattered necrotic keratinocytes and extravasated erythrocytes (Figure 3). There was no evidence of viral cytopathic effects. Similar clinical and histologic findings have been reported in the literature as acral papulovesicular eruptions following smallpox vaccination or papular spongiotic dermatitis of smallpox vaccination.8 The presence of eosinophils was not conspicuous in the current case and was only a notable finding in 1 of 2 cases previously described by Gaertner et al.8 This may simply be due to an idiosyncratic drug reaction. Furthermore, in the cases described by Beachkofsky et al,4 there were essentially 2 histologic groups. The first group demonstrated a dermal hypersensitivity-type reaction, and the second group demonstrated a lymphocytic capillaritis.
Based on these findings, the patient was diagnosed with an acral papulovesicular eruption following smallpox vaccination. Of note, the patient’s presentation was not consistent with other described smallpox vaccine reactions, which included eczema vaccinatum, autoinoculation, generalized vaccinia, and progressive vaccinia. The patient was treated supportively with triamcinolone acetonide cream 0.1%, cool compresses, and oral diphenhydramine as needed for pruritus. The lesions notably improved within the first week of treatment.
Comment
Reported cases of acral papulovesicular eruption4-6 demonstrated an onset of cutaneous symptoms an average of 14 days following vaccination (range, 8–18 days postvaccination). Lesions were benign and self-limited in all cases, with resolution within an average of 25 days (range, 7–71 days). All patients were active-duty military adults with a mean age of 24 years. Supportive treatment varied from topical steroids and oral antihistamines to tapering oral prednisone doses. Of note, all previously reported cases of this reaction occurred in patients who also had received other concurrent or near-concurrent vaccines, including anthrax, hepatitis B, influenza, and typhoid. Our patient represents a unique case of a papulovesicular eruption following smallpox vaccination with no history of concurrent vaccines.
Since the 1970s, smallpox vaccination has been associated with numerous cutaneous reactions, most of which have been reported with the first-generation Dryvax. Minor local reactions occurred in approximately 2% to 6% of vaccinees in clinical trials.9 These reactions included local edema involving the upper arm, satellite lesions within 2.5 cm of the vaccination site, local lymphadenopathy, intense inflammation or viral cellulitis surrounding the inoculation site, and viral lymphangitis tracking to axillary lymph nodes. In clinical trials, these reactions were self-limited and required only symptomatic treatment.9
Autoinoculation is another cutaneous reaction that can occur because Dryvax and ACAM2000 both contain live-attenuated replicating vaccinia virus. Accidental implantation may occur when the high titers of virus present at the vaccine site are subsequently transferred to other sites, especially abnormal mucosa or skin, resulting in an additional primary inoculation site.10
Eczema vaccinatum is a potentially life-threatening reaction that may occur in patients with disruptive skin disorders, such as atopic dermatitis. These patients are at risk for massive confluent vaccinia infection of the skin.10 In patients with atopic dermatitis, the virus rapidly disseminates due to both skin barrier dysfunction and impaired immunomodulation, resulting in large confluent skin lesions and the potential for viremia, septic shock, and death.10,11 Mortality from eczema vaccinatum may be reduced by administration of vaccinia immune globulin.10
The vaccinia virus also may spread hematogenously in healthy individuals,10 resulting in a benign reaction called generalized vaccinia. These patients develop pustules on areas of the skin other than the vaccination site. Although typically benign and self-limited, Beachkofsky et al4 described a case of generalized vaccinia in a healthy 34-year-old man resulting in a rapidly progressive vesiculopustular eruption with associated fever and pancytopenia. The patient made a complete recovery over the course of the following month.4
Alternatively, progressive vaccinia is a severe complication of smallpox vaccination seen in patients with impaired cell-mediated immunity. It also is known as vaccinia gangrenosum or vaccinia necrosum. These patients develop expanding ulcers due to exaggerated viral replication and cell-to-cell spread of the vaccinia virus.10,11 Hematogenous spread may result in viral implantation at distant sites of the body. This disease slowly progresses over weeks to months, and it often is resistant to treatment and fatal in patients with severe T-cell deficiency.10
Acral papulovesicular eruption is a distinct cutaneous adverse event following smallpox vaccination. Although further research is needed to discern the pathogenesis of this reaction, it is benign and self-limited, and patients have fully recovered with supportive care. In addition, a modified vaccinia Ankara vaccine (Bavarian Nordic) was approved by the US Food and Drug Administration in 2019.12,13 It is a nonreplicating attenuated viral vaccine that had fewer adverse events compared to ACAM2000 in clinical trials.13 To date, papulovesicular eruptions have not been reported following vaccination with the modified vaccinia Ankara vaccine; however, continued monitoring will help to further characterize any cutaneous reactions to this newer vaccine.
- Nalca A, Zumbrun EE. ACAM2000: the new smallpox vaccine for United States Strategic National Stockpile. Drug Des Devel Ther. 2010;4:71-79.
- Monath TP, Caldwell JR, Mundt W, et al. ACAM2000 clonal Vero cell culture vaccinia virus (New York City Board of Health strain)—a second-generation smallpox vaccine for biological defense. Int J Infect Dis. 2004;8:S31-S44.
- Thomas TN, Reef S, Neff L, et al. A review of the smallpox vaccine adverse events active surveillance system. Clin Infect Dis. 2008;46:S212-S220.
- Beachkofsky TM, Carrizales SC, Bidinger JJ, et al. Adverse events following smallpox vaccination with ACAM2000 in a military population. Arch Dermatol. 2010;146:656-661.
- Freeman R, Lenz B. Cutaneous reactions associated with ACAM2000 smallpox vaccination in a deploying U.S. Army unit. Mil Med. 2015;180:E152-E156.
- Centers for Disease Control and Prevention. Progressive vaccinia in a military smallpox vaccinee—United States, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:532-536.
- Kramer TR. Post–smallpox vaccination skin eruption in a marine. Mil Med. 2018;183:E649-E653.
- Gaertner EM, Groo S, Kim J. Papular spongiotic dermatitis of smallpox vaccination: report of 2 cases with review of the literature. Arch Pathol Lab Med. 2004;128:1173-1175.
- Fulginiti VA, Papier A, Lane JM, et al. Smallpox vaccination: a review, part I. background, vaccination technique, normal vaccination and revaccination, and expected normal reactions. Clin Infect Dis. 2003;37:241-250.
- Fulginiti VA, Papier A, Lane JM, et al. Smallpox vaccination: a review, part II. adverse events. Clin Infect Dis. 2003;37:251-271.
- Bray M. Understanding smallpox vaccination. J Infect Dis. 2011;203:1037-1039.
- Greenberg RN, Hay CM, Stapleton JT, et al. A randomized, double-blind, placebo-controlled phase II trial investigating the safety and immunogenicity of modified vaccinia ankara smallpox vaccine (MVA-BN®) in 56-80-year-old subjects. PLoS One. 2016;11:E0157335.
- Pittman PR, Hahn M, Lee HS, et al. Phase 3 efficacy trial of modified vaccinia Ankara as a vaccine against smallpox. N Engl J Med. 2019;381:1897-1908.
- Nalca A, Zumbrun EE. ACAM2000: the new smallpox vaccine for United States Strategic National Stockpile. Drug Des Devel Ther. 2010;4:71-79.
- Monath TP, Caldwell JR, Mundt W, et al. ACAM2000 clonal Vero cell culture vaccinia virus (New York City Board of Health strain)—a second-generation smallpox vaccine for biological defense. Int J Infect Dis. 2004;8:S31-S44.
- Thomas TN, Reef S, Neff L, et al. A review of the smallpox vaccine adverse events active surveillance system. Clin Infect Dis. 2008;46:S212-S220.
- Beachkofsky TM, Carrizales SC, Bidinger JJ, et al. Adverse events following smallpox vaccination with ACAM2000 in a military population. Arch Dermatol. 2010;146:656-661.
- Freeman R, Lenz B. Cutaneous reactions associated with ACAM2000 smallpox vaccination in a deploying U.S. Army unit. Mil Med. 2015;180:E152-E156.
- Centers for Disease Control and Prevention. Progressive vaccinia in a military smallpox vaccinee—United States, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:532-536.
- Kramer TR. Post–smallpox vaccination skin eruption in a marine. Mil Med. 2018;183:E649-E653.
- Gaertner EM, Groo S, Kim J. Papular spongiotic dermatitis of smallpox vaccination: report of 2 cases with review of the literature. Arch Pathol Lab Med. 2004;128:1173-1175.
- Fulginiti VA, Papier A, Lane JM, et al. Smallpox vaccination: a review, part I. background, vaccination technique, normal vaccination and revaccination, and expected normal reactions. Clin Infect Dis. 2003;37:241-250.
- Fulginiti VA, Papier A, Lane JM, et al. Smallpox vaccination: a review, part II. adverse events. Clin Infect Dis. 2003;37:251-271.
- Bray M. Understanding smallpox vaccination. J Infect Dis. 2011;203:1037-1039.
- Greenberg RN, Hay CM, Stapleton JT, et al. A randomized, double-blind, placebo-controlled phase II trial investigating the safety and immunogenicity of modified vaccinia ankara smallpox vaccine (MVA-BN®) in 56-80-year-old subjects. PLoS One. 2016;11:E0157335.
- Pittman PR, Hahn M, Lee HS, et al. Phase 3 efficacy trial of modified vaccinia Ankara as a vaccine against smallpox. N Engl J Med. 2019;381:1897-1908.
Practice Points
- There are several potential cutaneous adverse reactions associated with smallpox vaccination, ranging from benign self-limited hypersensitivity reactions to life-threatening eczema vaccinatum and progressive vaccinia.
- Acral papulovesicular eruption is a distinct presentation that has been described in the US Military following vaccination with the second-generation live smallpox vaccine (ACAM2000).