User login
Centrifugally Spreading Lymphocutaneous Sporotrichosis: A Rare Cutaneous Manifestation
To the Editor:
Sporotrichosis refers to a subacute to chronic fungal infection that usually involves the cutaneous and subcutaneous tissues and is caused by the introduction of Sporothrix, a dimorphic fungus, through the skin. We present a case of chronic atypical lymphocutaneous sporotrichosis.
A 46-year-old man presented to the outpatient dermatology clinic for follow-up for a rash on the right leg that spread to the thigh and became painful and pruritic. It initially developed 8 years prior to the current presentation after he sustained trauma to the leg from an electroshock weapon. One year prior to the current presentation, he had presented to the emergency department and was prescribed doxycycline 100 mg twice daily for 7 days as well as bacitracin ointment. He also was instructed to follow up with dermatology, but a lack of health insurance and other socioeconomic barriers prevented him from seeking dermatologic care. Nine months later, he again presented to the emergency department due to a motor vehicle accident. Computed tomography (CT) of the right leg revealed exophytic dermal masses, inflammatory stranding of the subcutaneous tissue, and right inguinal lymph nodes measuring up to 1.4 cm; there was no osteoarticular involvement. At that time, the patient was applying gentian violet to the skin lesions and taking hydroxyzine 50 mg 3 times daily as needed for pruritus with minimal relief. Financial support was provided for follow-up with dermatology, which occurred almost 5 months later.
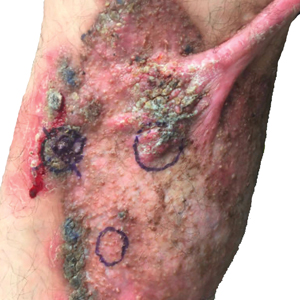
At the current presentation, physical examination revealed a large annular plaque with verrucous, scaly, erythematous borders and a hypopigmented atrophic center extending from the medial aspect of the right leg to the posterior thigh. Numerous pink, scaly, crusted nodules were scattered primarily along the periphery, with some evidence of draining sinus tracts. In addition, a fibrotic pink linear plaque extended from the medial right leg to the popliteal fossa, consistent with a keloid. Violet staining along the periphery of the lesion also was appreciated secondary to the application of topical gentian violet (Figure 1).
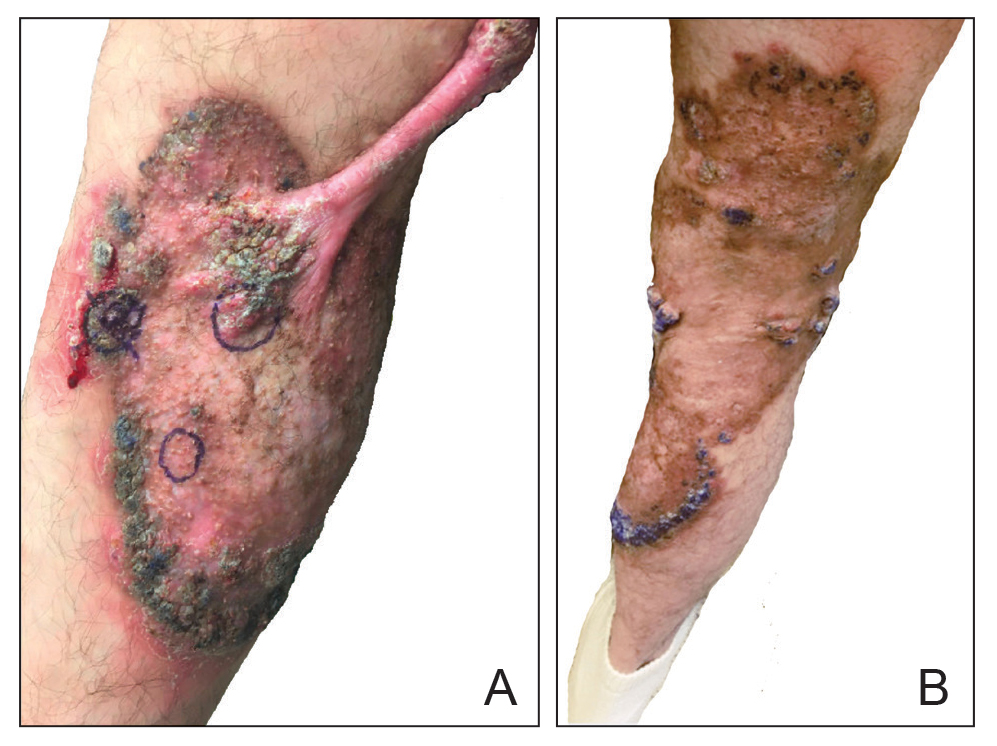
Based on the chronic history and morphology, a diagnosis of a chronic fungal or atypical mycobacterial infection was favored. In particular, chromoblastomycosis, cutaneous tuberculosis (eg, scrofuloderma, lupus vulgaris, tuberculosis verrucosa cutis), and atypical mycobacterial infection were highest on the differential, as these conditions often exhibit annular, nodular, verrucous, and/or atrophic lesions. The nodularity, crusting, and draining sinus tracts also raised the possibility of mycetoma. Given the extension of the lesion from the lower to upper leg, a sporotrichoid infection also was considered but was thought to be less likely based on the annular configuration.
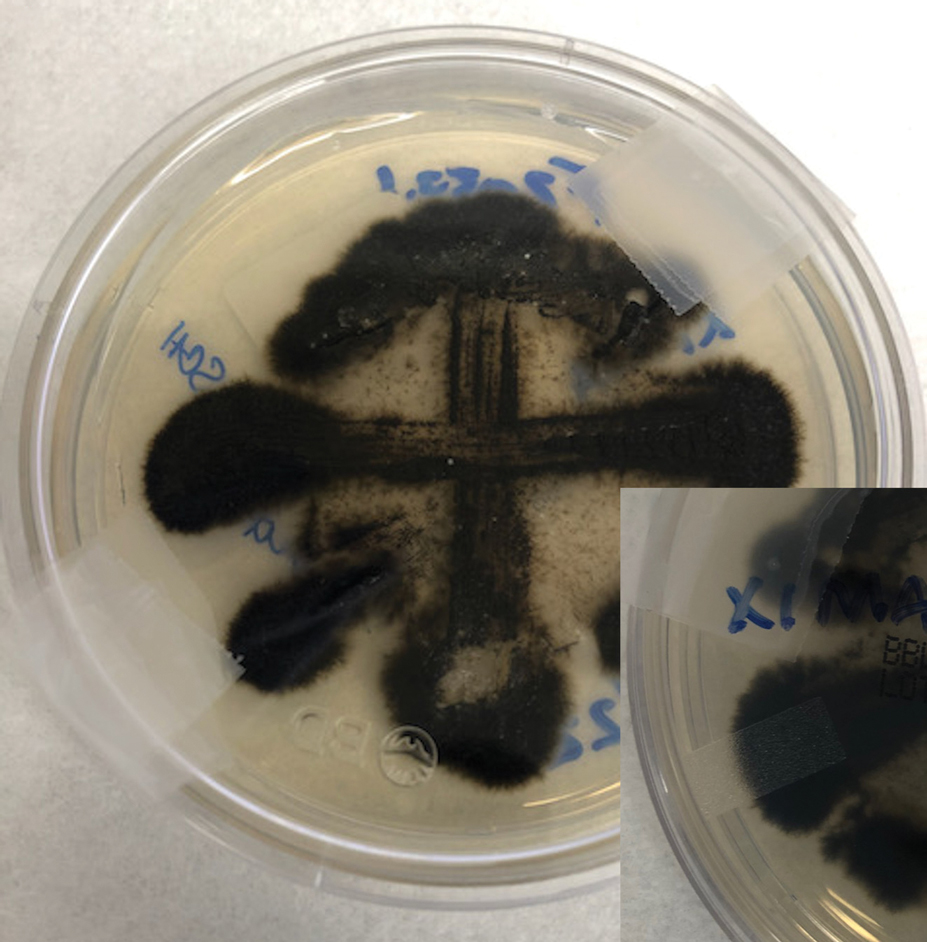
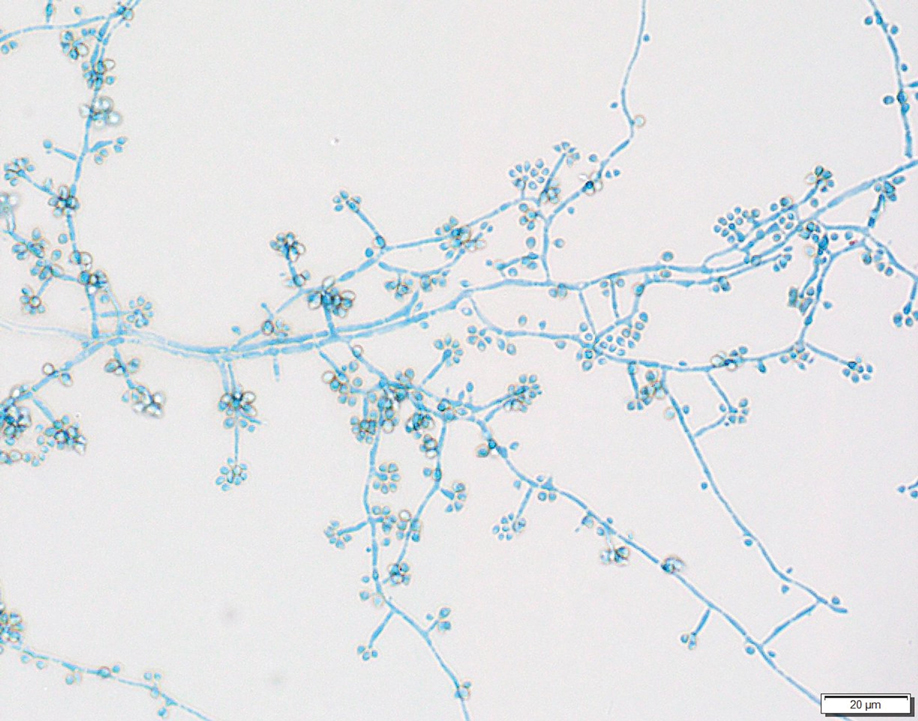
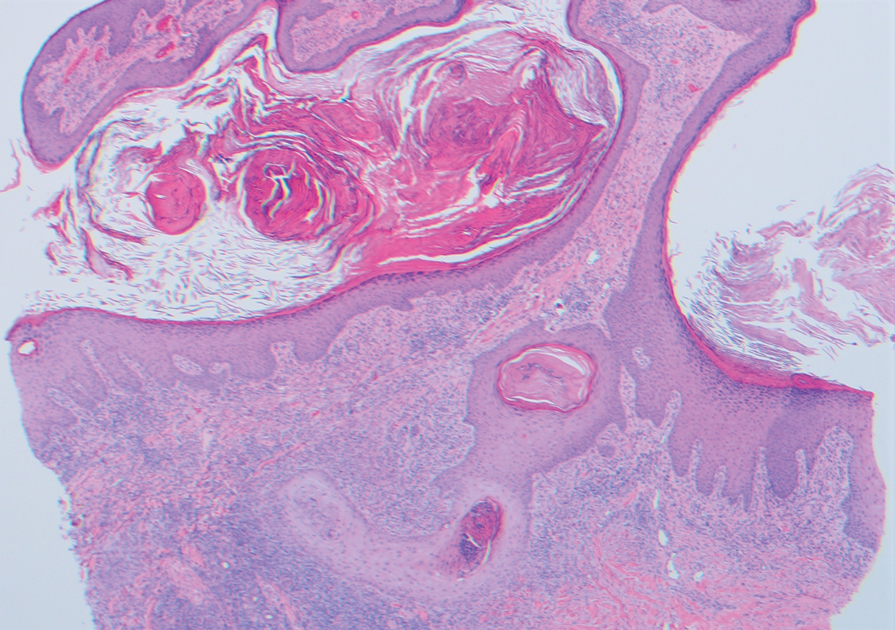
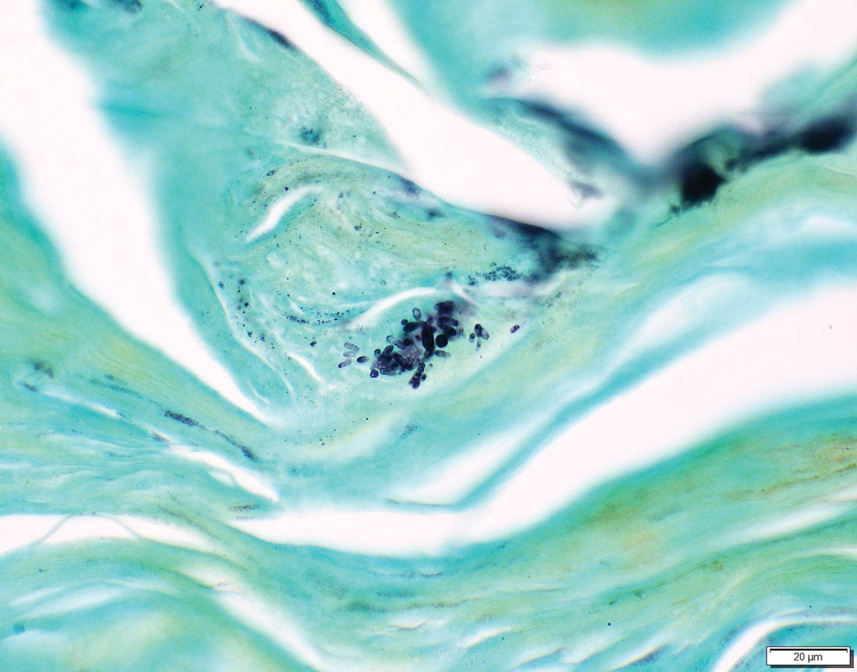
Two 4-mm punch biopsies were taken from a peripheral nodule—one for routine histology and another for bacterial, fungal, and mycobacterial cultures. An interferon-gamma release assay also was ordered to evaluate for immune responses indicative of prior Mycobacterium tuberculosis infection, but the patient did not obtain this for unknown reasons. Histology demonstrated pseudoepitheliomatous hyperplasia and necrotizing granulomas, which suggested an infectious etiology, but no organisms were identified on tissue staining and all cultures were negative for growth at 6 weeks. The patient was asked to return at that point, and 4 additional scouting biopsies were performed and sent for routine histology, M tuberculosis nucleic acid amplification testing, and microbiologic cultures (ie, bacterial, mycobacterial, fungal, nocardia, actinomycetes). Within 1 week, a filamentous organism with pigmentation visible on the front and back of a Sabouraud dextrose agar plate was identified on fungal culture (Figure 2). Microscopic evaluation of this mold with lactophenol blue stain revealed thin septate hyphae with conidiophores arising at right angles that bore clusters of microconidia (Figure 3). Sequencing analysis ultimately identified this organism as Sporothrix schenckii. Routine histology demonstrated pseudoepitheliomatous hyperplasia with scattered intraepidermal collections of neutrophils (Figure 4). The dermis showed a dense, superficial, and deep infiltrate composed of lymphocytes, histiocytes, and plasma cells with occasional neutrophils and eosinophils. A Grocott-Gomori methenamine-silver stain revealed a cluster of ovoid yeast forms within the stratum corneum (Figure 5). The patient was referred to infectious disease for follow-up and treatment.
The patient later visited a community clinic providing dermatologic care for patients without insurance. He was started on itraconazole 200 mg daily for a total of 6 months until dermatologic clearance of the cutaneous lesions was observed. He was followed by the clinic with laboratory tests including a liver function test. At follow-up 8 months later, a repeat biopsy was performed to ensure histologic clearance of the sporotrichosis, which revealed a dermal scar and no evidence of residual infection.
Sporothrix schenckii was first isolated in 1898 by Benjamin Schenck, a student at Johns Hopkins Medicine (Baltimore, Maryland), and identified by a mycologist as sporotricha.1 Species within the genus Sporothrix are unique in that the fungi are both dimorphic (growing as a mold at 25 °C but as a yeast at 37 °C) and dematiaceous (dark pigmentation from melanin is visible on inspection of the anterior and reverse sides of culture plates). Infection usually occurs when cutaneous or subcutaneous tissues are exposed to the fungus via microabrasions; activities thought to contribute to exposure include gardening, agricultural work, animal husbandry, and feline scratches.2 Although skin trauma frequently is considered the primary route of infection, patient recall is variable, with one study noting that only 37.7% of patients recalled trauma and another study similarly demonstrating a patient recall rate of 25%.3,4
Lymphocutaneous sporotrichosis is the most common presentation of the fungal infection,5 and clinical cases may be classified into 1 of 4 categories: (1) lymphangitic lesions—papules at the site of inoculation with spread along the lymphatic channels; (2) localized (fixed) cutaneous lesions—1 or 2 lesions at the inoculation site; (3) disseminated (multifocal) cutaneous lesions; and (4) extracutaneous lesions.6 Extracutaneous manifestations of this infection most notably have been reported as pulmonary disease through inhalation of conidia or through dissemination in immunocompromised hosts.7 Our patient’s infection was categorized as lymphangitic lesions due to spread from the lower to upper leg, albeit in a highly atypical, annular fashion. A review of systems was otherwise negative, and CT ruled out osteoarticular involvement.
In addition to socioeconomic barriers, several factors contributed to a delayed diagnosis in this patient including the annular presentation with central hypopigmentation and atrophy, negative initial microbiological cultures and lack of visualization of organisms on histopathology, and the consequent need for repeat biopsies. For lymphocutaneous sporotrichosis, the typical presentation consists of a papule or ulcerated nodule at the site of inoculation with subsequent linear spread along lymphatic channels. This classic sporotrichoid pattern is a key diagnostic clue for identifying sporotrichosis but was absent at the time our patient presented for medical care. Rather, the sporotrichoid spread seemed to have occurred in a centrifugal fashion up the leg. Few case reports have documented an annular presentation of lymphocutaneous sporotrichosis,8-13 and one report described central atrophy and hypopigmentation.10 Pain and pruritus, which were present in our patient, rarely are documented.9 Finally, the diagnosis of cutaneous fungal infections may require multiple biopsies due to the variable abundance of viable organisms in tissue specimens as well as the fastidious growth characteristics of these organisms. Furthermore, sensitivity often is low for both fungal and mycobacterial cultures, and cultures may take days to weeks to yield growth.14,15 For these reasons, empiric therapy and repeat biopsies often are pursued if clinical suspicion is high enough.16 Our patient returned for multiple scouting biopsies after the initial tissue culture was negative and was even considered for empiric treatment against Mycobacterium prior to positive fungal cultures.
Another unique aspect of our case was the presence of a keloid. It is difficult to know if this keloid was secondary to the trauma the patient sustained in the inciting incident or formed from the fungal infection. Interestingly, it has been hypothesized that fungal infections may contribute to keloid and hypertrophic scar formation.17 In a case series of 3 patients with either keloids or hypertrophic scars and concomitant tinea infection, there was notable improvement in the appearance of the scars 2 weeks after beginning itraconazole therapy.17 However, it is not yet known if a fungal infection can contribute to the pathogenesis of keloid formation.
As with other aspects of this case, the length of time the patient went without diagnosis and treatment was unusual and may help explain the atypical presentation. Although the incubation period for S schenckii can vary, most reports identify patients as seeking medical attention within 1 year of rash onset.18-20 In our case, the patient was not diagnosed until 8 years after his symptoms began, requiring multiple referrals, multiple health system touchpoints, and an institution-specific financial aid program. As such, this case also highlights the potential need for a multidisciplinary team approach when caring for patients with poor access to health care.
In conclusion, this case illustrates a unique presentation of lymphocutaneous sporotrichosis that may mimic other chronic infections and result in delayed diagnosis. Although lymphangitic sporotrichosis generally is recognized as having a linear distribution, mounting evidence from this report and others suggests an annular presentation also is possible. Pruritus or pain is rare but should not preclude a diagnosis of sporotrichosis if present. For patients with limited access to health care resources, it is especially important to involve multiple members of the health care team, including social workers and specialists, to prevent a protracted and severe course of disease.
- Schenck BR. On refractory subcutaneous abscesses caused by a fungus possibly related to the sporotricha. Bulletin of the Johns Hopkins Hospital. 1898;93:286-290.
- de Lima Barros MB, de Almeida Paes R, Schubach AO. Sporothrix schenckii and sporotrichosis. Clin Microbiol Rev. 2011;24:633-654. doi:10.1128/CMR.00007-11
- Crevasse L, Ellner PD. An outbreak of sporotrichosis in florida. J Am Med Assoc. 1960;173:29-33. doi:10.1001/jama.1960.03020190031006
- Mayorga R, Cáceres A, Toriello C, et al. An endemic area of sporotrichosis in Guatemala [in French]. Sabouraudia. 1978;16:185-198.
- Morris-Jones R. Sporotrichosis. Clin Exp Dermatol. 2002;27:427-431. doi:10.1046/j.1365-2230.2002.01087.x
- Sampaio SA, Da Lacaz CS. Clinical and statistical studies on sporotrichosis in Sao Paulo (Brazil). Article in German. Hautarzt. 1959;10:490-493.
- Ramos-e-Silva M, Vasconcelos C, Carneiro S, et al. Sporotrichosis. Clin Dermatol. 2007;25:181-187. doi:10.1016/j.clindermatol.2006.05.006
- Williams BA, Jennings TA, Rushing EC, et al. Sporotrichosis on the face of a 7-year-old boy following a bicycle accident. Pediatr Dermatol. 2013;30:E246-E247. doi:10.1111/j.1525-1470.2011.01696.x
- Vaishampayan SS, Borde P. An unusual presentation of sporotrichosis. Indian J Dermatol. 2013;58:409. doi:10.4103/0019-5154.117350
- Qin J, Zhang J. Sporotrichosis. N Engl J Med. 2019;380:771. doi:10.1056/NEJMicm1809179
- Patel A, Mudenda V, Lakhi S, et al. A 27-year-old severely immunosuppressed female with misleading clinical features of disseminated cutaneous sporotrichosis. Case Rep Dermatol Med. 2016;2016:1-4. doi:10.1155/2016/9403690
- de Oliveira-Esteves ICMR, Almeida Rosa da Silva G, Eyer-Silva WA, et al. Rapidly progressive disseminated sporotrichosis as the first presentation of HIV infection in a patient with a very low CD4 cell count. Case Rep Infect Dis. 2017;2017:4713140. doi:10.1155/2017/4713140
- Singh S, Bachaspatimayum R, Meetei U, et al. Terbinafine in fixed cutaneous sporotrichosis: a case series. J Clin Diagnostic Res. 2018;12:FR01-FR03. doi:10.7860/JCDR/2018/25315.12223
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280. doi:10.1128/CMR.00053-10
- Peters F, Batinica M, Plum G, et al. Bug or no bug: challenges in diagnosing cutaneous mycobacterial infections. J Ger Soc Dermatol. 2016;14:1227-1236. doi:10.1111/ddg.13001
- Khadka P, Koirala S, Thapaliya J. Cutaneous tuberculosis: clinicopathologic arrays and diagnostic challenges. Dermatol Res Pract. 2018;2018:7201973. doi:10.1155/2018/7201973
- Okada E, Maruyama Y. Are keloids and hypertrophic scars caused by fungal infection? . Plast Reconstr Surg. 2007;120:814-815. doi:10.1097/01.prs.0000278813.23244.3f
- Pappas PG, Tellez I, Deep AE, et al. Sporotrichosis in Peru: description of an area of hyperendemicity. Clin Infect Dis. 2000;30:65-70. doi:10.1086/313607
- McGuinness SL, Boyd R, Kidd S, et al. Epidemiological investigation of an outbreak of cutaneous sporotrichosis, Northern Territory, Australia. BMC Infect Dis. 2016;16:1-7. doi:10.1186/s12879-016-1338-0
- Rojas FD, Fernández MS, Lucchelli JM, et al. Cavitary pulmonary sporotrichosis: case report and literature review. Mycopathologia. 2017;182:1119-1123. doi:10.1007/s11046-017-0197-6
To the Editor:
Sporotrichosis refers to a subacute to chronic fungal infection that usually involves the cutaneous and subcutaneous tissues and is caused by the introduction of Sporothrix, a dimorphic fungus, through the skin. We present a case of chronic atypical lymphocutaneous sporotrichosis.
A 46-year-old man presented to the outpatient dermatology clinic for follow-up for a rash on the right leg that spread to the thigh and became painful and pruritic. It initially developed 8 years prior to the current presentation after he sustained trauma to the leg from an electroshock weapon. One year prior to the current presentation, he had presented to the emergency department and was prescribed doxycycline 100 mg twice daily for 7 days as well as bacitracin ointment. He also was instructed to follow up with dermatology, but a lack of health insurance and other socioeconomic barriers prevented him from seeking dermatologic care. Nine months later, he again presented to the emergency department due to a motor vehicle accident. Computed tomography (CT) of the right leg revealed exophytic dermal masses, inflammatory stranding of the subcutaneous tissue, and right inguinal lymph nodes measuring up to 1.4 cm; there was no osteoarticular involvement. At that time, the patient was applying gentian violet to the skin lesions and taking hydroxyzine 50 mg 3 times daily as needed for pruritus with minimal relief. Financial support was provided for follow-up with dermatology, which occurred almost 5 months later.
At the current presentation, physical examination revealed a large annular plaque with verrucous, scaly, erythematous borders and a hypopigmented atrophic center extending from the medial aspect of the right leg to the posterior thigh. Numerous pink, scaly, crusted nodules were scattered primarily along the periphery, with some evidence of draining sinus tracts. In addition, a fibrotic pink linear plaque extended from the medial right leg to the popliteal fossa, consistent with a keloid. Violet staining along the periphery of the lesion also was appreciated secondary to the application of topical gentian violet (Figure 1).

Based on the chronic history and morphology, a diagnosis of a chronic fungal or atypical mycobacterial infection was favored. In particular, chromoblastomycosis, cutaneous tuberculosis (eg, scrofuloderma, lupus vulgaris, tuberculosis verrucosa cutis), and atypical mycobacterial infection were highest on the differential, as these conditions often exhibit annular, nodular, verrucous, and/or atrophic lesions. The nodularity, crusting, and draining sinus tracts also raised the possibility of mycetoma. Given the extension of the lesion from the lower to upper leg, a sporotrichoid infection also was considered but was thought to be less likely based on the annular configuration.
Two 4-mm punch biopsies were taken from a peripheral nodule—one for routine histology and another for bacterial, fungal, and mycobacterial cultures. An interferon-gamma release assay also was ordered to evaluate for immune responses indicative of prior Mycobacterium tuberculosis infection, but the patient did not obtain this for unknown reasons. Histology demonstrated pseudoepitheliomatous hyperplasia and necrotizing granulomas, which suggested an infectious etiology, but no organisms were identified on tissue staining and all cultures were negative for growth at 6 weeks. The patient was asked to return at that point, and 4 additional scouting biopsies were performed and sent for routine histology, M tuberculosis nucleic acid amplification testing, and microbiologic cultures (ie, bacterial, mycobacterial, fungal, nocardia, actinomycetes). Within 1 week, a filamentous organism with pigmentation visible on the front and back of a Sabouraud dextrose agar plate was identified on fungal culture (Figure 2). Microscopic evaluation of this mold with lactophenol blue stain revealed thin septate hyphae with conidiophores arising at right angles that bore clusters of microconidia (Figure 3). Sequencing analysis ultimately identified this organism as Sporothrix schenckii. Routine histology demonstrated pseudoepitheliomatous hyperplasia with scattered intraepidermal collections of neutrophils (Figure 4). The dermis showed a dense, superficial, and deep infiltrate composed of lymphocytes, histiocytes, and plasma cells with occasional neutrophils and eosinophils. A Grocott-Gomori methenamine-silver stain revealed a cluster of ovoid yeast forms within the stratum corneum (Figure 5). The patient was referred to infectious disease for follow-up and treatment.

The patient later visited a community clinic providing dermatologic care for patients without insurance. He was started on itraconazole 200 mg daily for a total of 6 months until dermatologic clearance of the cutaneous lesions was observed. He was followed by the clinic with laboratory tests including a liver function test. At follow-up 8 months later, a repeat biopsy was performed to ensure histologic clearance of the sporotrichosis, which revealed a dermal scar and no evidence of residual infection.

Sporothrix schenckii was first isolated in 1898 by Benjamin Schenck, a student at Johns Hopkins Medicine (Baltimore, Maryland), and identified by a mycologist as sporotricha.1 Species within the genus Sporothrix are unique in that the fungi are both dimorphic (growing as a mold at 25 °C but as a yeast at 37 °C) and dematiaceous (dark pigmentation from melanin is visible on inspection of the anterior and reverse sides of culture plates). Infection usually occurs when cutaneous or subcutaneous tissues are exposed to the fungus via microabrasions; activities thought to contribute to exposure include gardening, agricultural work, animal husbandry, and feline scratches.2 Although skin trauma frequently is considered the primary route of infection, patient recall is variable, with one study noting that only 37.7% of patients recalled trauma and another study similarly demonstrating a patient recall rate of 25%.3,4

Lymphocutaneous sporotrichosis is the most common presentation of the fungal infection,5 and clinical cases may be classified into 1 of 4 categories: (1) lymphangitic lesions—papules at the site of inoculation with spread along the lymphatic channels; (2) localized (fixed) cutaneous lesions—1 or 2 lesions at the inoculation site; (3) disseminated (multifocal) cutaneous lesions; and (4) extracutaneous lesions.6 Extracutaneous manifestations of this infection most notably have been reported as pulmonary disease through inhalation of conidia or through dissemination in immunocompromised hosts.7 Our patient’s infection was categorized as lymphangitic lesions due to spread from the lower to upper leg, albeit in a highly atypical, annular fashion. A review of systems was otherwise negative, and CT ruled out osteoarticular involvement.

In addition to socioeconomic barriers, several factors contributed to a delayed diagnosis in this patient including the annular presentation with central hypopigmentation and atrophy, negative initial microbiological cultures and lack of visualization of organisms on histopathology, and the consequent need for repeat biopsies. For lymphocutaneous sporotrichosis, the typical presentation consists of a papule or ulcerated nodule at the site of inoculation with subsequent linear spread along lymphatic channels. This classic sporotrichoid pattern is a key diagnostic clue for identifying sporotrichosis but was absent at the time our patient presented for medical care. Rather, the sporotrichoid spread seemed to have occurred in a centrifugal fashion up the leg. Few case reports have documented an annular presentation of lymphocutaneous sporotrichosis,8-13 and one report described central atrophy and hypopigmentation.10 Pain and pruritus, which were present in our patient, rarely are documented.9 Finally, the diagnosis of cutaneous fungal infections may require multiple biopsies due to the variable abundance of viable organisms in tissue specimens as well as the fastidious growth characteristics of these organisms. Furthermore, sensitivity often is low for both fungal and mycobacterial cultures, and cultures may take days to weeks to yield growth.14,15 For these reasons, empiric therapy and repeat biopsies often are pursued if clinical suspicion is high enough.16 Our patient returned for multiple scouting biopsies after the initial tissue culture was negative and was even considered for empiric treatment against Mycobacterium prior to positive fungal cultures.
Another unique aspect of our case was the presence of a keloid. It is difficult to know if this keloid was secondary to the trauma the patient sustained in the inciting incident or formed from the fungal infection. Interestingly, it has been hypothesized that fungal infections may contribute to keloid and hypertrophic scar formation.17 In a case series of 3 patients with either keloids or hypertrophic scars and concomitant tinea infection, there was notable improvement in the appearance of the scars 2 weeks after beginning itraconazole therapy.17 However, it is not yet known if a fungal infection can contribute to the pathogenesis of keloid formation.
As with other aspects of this case, the length of time the patient went without diagnosis and treatment was unusual and may help explain the atypical presentation. Although the incubation period for S schenckii can vary, most reports identify patients as seeking medical attention within 1 year of rash onset.18-20 In our case, the patient was not diagnosed until 8 years after his symptoms began, requiring multiple referrals, multiple health system touchpoints, and an institution-specific financial aid program. As such, this case also highlights the potential need for a multidisciplinary team approach when caring for patients with poor access to health care.
In conclusion, this case illustrates a unique presentation of lymphocutaneous sporotrichosis that may mimic other chronic infections and result in delayed diagnosis. Although lymphangitic sporotrichosis generally is recognized as having a linear distribution, mounting evidence from this report and others suggests an annular presentation also is possible. Pruritus or pain is rare but should not preclude a diagnosis of sporotrichosis if present. For patients with limited access to health care resources, it is especially important to involve multiple members of the health care team, including social workers and specialists, to prevent a protracted and severe course of disease.
To the Editor:
Sporotrichosis refers to a subacute to chronic fungal infection that usually involves the cutaneous and subcutaneous tissues and is caused by the introduction of Sporothrix, a dimorphic fungus, through the skin. We present a case of chronic atypical lymphocutaneous sporotrichosis.
A 46-year-old man presented to the outpatient dermatology clinic for follow-up for a rash on the right leg that spread to the thigh and became painful and pruritic. It initially developed 8 years prior to the current presentation after he sustained trauma to the leg from an electroshock weapon. One year prior to the current presentation, he had presented to the emergency department and was prescribed doxycycline 100 mg twice daily for 7 days as well as bacitracin ointment. He also was instructed to follow up with dermatology, but a lack of health insurance and other socioeconomic barriers prevented him from seeking dermatologic care. Nine months later, he again presented to the emergency department due to a motor vehicle accident. Computed tomography (CT) of the right leg revealed exophytic dermal masses, inflammatory stranding of the subcutaneous tissue, and right inguinal lymph nodes measuring up to 1.4 cm; there was no osteoarticular involvement. At that time, the patient was applying gentian violet to the skin lesions and taking hydroxyzine 50 mg 3 times daily as needed for pruritus with minimal relief. Financial support was provided for follow-up with dermatology, which occurred almost 5 months later.
At the current presentation, physical examination revealed a large annular plaque with verrucous, scaly, erythematous borders and a hypopigmented atrophic center extending from the medial aspect of the right leg to the posterior thigh. Numerous pink, scaly, crusted nodules were scattered primarily along the periphery, with some evidence of draining sinus tracts. In addition, a fibrotic pink linear plaque extended from the medial right leg to the popliteal fossa, consistent with a keloid. Violet staining along the periphery of the lesion also was appreciated secondary to the application of topical gentian violet (Figure 1).

Based on the chronic history and morphology, a diagnosis of a chronic fungal or atypical mycobacterial infection was favored. In particular, chromoblastomycosis, cutaneous tuberculosis (eg, scrofuloderma, lupus vulgaris, tuberculosis verrucosa cutis), and atypical mycobacterial infection were highest on the differential, as these conditions often exhibit annular, nodular, verrucous, and/or atrophic lesions. The nodularity, crusting, and draining sinus tracts also raised the possibility of mycetoma. Given the extension of the lesion from the lower to upper leg, a sporotrichoid infection also was considered but was thought to be less likely based on the annular configuration.
Two 4-mm punch biopsies were taken from a peripheral nodule—one for routine histology and another for bacterial, fungal, and mycobacterial cultures. An interferon-gamma release assay also was ordered to evaluate for immune responses indicative of prior Mycobacterium tuberculosis infection, but the patient did not obtain this for unknown reasons. Histology demonstrated pseudoepitheliomatous hyperplasia and necrotizing granulomas, which suggested an infectious etiology, but no organisms were identified on tissue staining and all cultures were negative for growth at 6 weeks. The patient was asked to return at that point, and 4 additional scouting biopsies were performed and sent for routine histology, M tuberculosis nucleic acid amplification testing, and microbiologic cultures (ie, bacterial, mycobacterial, fungal, nocardia, actinomycetes). Within 1 week, a filamentous organism with pigmentation visible on the front and back of a Sabouraud dextrose agar plate was identified on fungal culture (Figure 2). Microscopic evaluation of this mold with lactophenol blue stain revealed thin septate hyphae with conidiophores arising at right angles that bore clusters of microconidia (Figure 3). Sequencing analysis ultimately identified this organism as Sporothrix schenckii. Routine histology demonstrated pseudoepitheliomatous hyperplasia with scattered intraepidermal collections of neutrophils (Figure 4). The dermis showed a dense, superficial, and deep infiltrate composed of lymphocytes, histiocytes, and plasma cells with occasional neutrophils and eosinophils. A Grocott-Gomori methenamine-silver stain revealed a cluster of ovoid yeast forms within the stratum corneum (Figure 5). The patient was referred to infectious disease for follow-up and treatment.

The patient later visited a community clinic providing dermatologic care for patients without insurance. He was started on itraconazole 200 mg daily for a total of 6 months until dermatologic clearance of the cutaneous lesions was observed. He was followed by the clinic with laboratory tests including a liver function test. At follow-up 8 months later, a repeat biopsy was performed to ensure histologic clearance of the sporotrichosis, which revealed a dermal scar and no evidence of residual infection.

Sporothrix schenckii was first isolated in 1898 by Benjamin Schenck, a student at Johns Hopkins Medicine (Baltimore, Maryland), and identified by a mycologist as sporotricha.1 Species within the genus Sporothrix are unique in that the fungi are both dimorphic (growing as a mold at 25 °C but as a yeast at 37 °C) and dematiaceous (dark pigmentation from melanin is visible on inspection of the anterior and reverse sides of culture plates). Infection usually occurs when cutaneous or subcutaneous tissues are exposed to the fungus via microabrasions; activities thought to contribute to exposure include gardening, agricultural work, animal husbandry, and feline scratches.2 Although skin trauma frequently is considered the primary route of infection, patient recall is variable, with one study noting that only 37.7% of patients recalled trauma and another study similarly demonstrating a patient recall rate of 25%.3,4

Lymphocutaneous sporotrichosis is the most common presentation of the fungal infection,5 and clinical cases may be classified into 1 of 4 categories: (1) lymphangitic lesions—papules at the site of inoculation with spread along the lymphatic channels; (2) localized (fixed) cutaneous lesions—1 or 2 lesions at the inoculation site; (3) disseminated (multifocal) cutaneous lesions; and (4) extracutaneous lesions.6 Extracutaneous manifestations of this infection most notably have been reported as pulmonary disease through inhalation of conidia or through dissemination in immunocompromised hosts.7 Our patient’s infection was categorized as lymphangitic lesions due to spread from the lower to upper leg, albeit in a highly atypical, annular fashion. A review of systems was otherwise negative, and CT ruled out osteoarticular involvement.

In addition to socioeconomic barriers, several factors contributed to a delayed diagnosis in this patient including the annular presentation with central hypopigmentation and atrophy, negative initial microbiological cultures and lack of visualization of organisms on histopathology, and the consequent need for repeat biopsies. For lymphocutaneous sporotrichosis, the typical presentation consists of a papule or ulcerated nodule at the site of inoculation with subsequent linear spread along lymphatic channels. This classic sporotrichoid pattern is a key diagnostic clue for identifying sporotrichosis but was absent at the time our patient presented for medical care. Rather, the sporotrichoid spread seemed to have occurred in a centrifugal fashion up the leg. Few case reports have documented an annular presentation of lymphocutaneous sporotrichosis,8-13 and one report described central atrophy and hypopigmentation.10 Pain and pruritus, which were present in our patient, rarely are documented.9 Finally, the diagnosis of cutaneous fungal infections may require multiple biopsies due to the variable abundance of viable organisms in tissue specimens as well as the fastidious growth characteristics of these organisms. Furthermore, sensitivity often is low for both fungal and mycobacterial cultures, and cultures may take days to weeks to yield growth.14,15 For these reasons, empiric therapy and repeat biopsies often are pursued if clinical suspicion is high enough.16 Our patient returned for multiple scouting biopsies after the initial tissue culture was negative and was even considered for empiric treatment against Mycobacterium prior to positive fungal cultures.
Another unique aspect of our case was the presence of a keloid. It is difficult to know if this keloid was secondary to the trauma the patient sustained in the inciting incident or formed from the fungal infection. Interestingly, it has been hypothesized that fungal infections may contribute to keloid and hypertrophic scar formation.17 In a case series of 3 patients with either keloids or hypertrophic scars and concomitant tinea infection, there was notable improvement in the appearance of the scars 2 weeks after beginning itraconazole therapy.17 However, it is not yet known if a fungal infection can contribute to the pathogenesis of keloid formation.
As with other aspects of this case, the length of time the patient went without diagnosis and treatment was unusual and may help explain the atypical presentation. Although the incubation period for S schenckii can vary, most reports identify patients as seeking medical attention within 1 year of rash onset.18-20 In our case, the patient was not diagnosed until 8 years after his symptoms began, requiring multiple referrals, multiple health system touchpoints, and an institution-specific financial aid program. As such, this case also highlights the potential need for a multidisciplinary team approach when caring for patients with poor access to health care.
In conclusion, this case illustrates a unique presentation of lymphocutaneous sporotrichosis that may mimic other chronic infections and result in delayed diagnosis. Although lymphangitic sporotrichosis generally is recognized as having a linear distribution, mounting evidence from this report and others suggests an annular presentation also is possible. Pruritus or pain is rare but should not preclude a diagnosis of sporotrichosis if present. For patients with limited access to health care resources, it is especially important to involve multiple members of the health care team, including social workers and specialists, to prevent a protracted and severe course of disease.
- Schenck BR. On refractory subcutaneous abscesses caused by a fungus possibly related to the sporotricha. Bulletin of the Johns Hopkins Hospital. 1898;93:286-290.
- de Lima Barros MB, de Almeida Paes R, Schubach AO. Sporothrix schenckii and sporotrichosis. Clin Microbiol Rev. 2011;24:633-654. doi:10.1128/CMR.00007-11
- Crevasse L, Ellner PD. An outbreak of sporotrichosis in florida. J Am Med Assoc. 1960;173:29-33. doi:10.1001/jama.1960.03020190031006
- Mayorga R, Cáceres A, Toriello C, et al. An endemic area of sporotrichosis in Guatemala [in French]. Sabouraudia. 1978;16:185-198.
- Morris-Jones R. Sporotrichosis. Clin Exp Dermatol. 2002;27:427-431. doi:10.1046/j.1365-2230.2002.01087.x
- Sampaio SA, Da Lacaz CS. Clinical and statistical studies on sporotrichosis in Sao Paulo (Brazil). Article in German. Hautarzt. 1959;10:490-493.
- Ramos-e-Silva M, Vasconcelos C, Carneiro S, et al. Sporotrichosis. Clin Dermatol. 2007;25:181-187. doi:10.1016/j.clindermatol.2006.05.006
- Williams BA, Jennings TA, Rushing EC, et al. Sporotrichosis on the face of a 7-year-old boy following a bicycle accident. Pediatr Dermatol. 2013;30:E246-E247. doi:10.1111/j.1525-1470.2011.01696.x
- Vaishampayan SS, Borde P. An unusual presentation of sporotrichosis. Indian J Dermatol. 2013;58:409. doi:10.4103/0019-5154.117350
- Qin J, Zhang J. Sporotrichosis. N Engl J Med. 2019;380:771. doi:10.1056/NEJMicm1809179
- Patel A, Mudenda V, Lakhi S, et al. A 27-year-old severely immunosuppressed female with misleading clinical features of disseminated cutaneous sporotrichosis. Case Rep Dermatol Med. 2016;2016:1-4. doi:10.1155/2016/9403690
- de Oliveira-Esteves ICMR, Almeida Rosa da Silva G, Eyer-Silva WA, et al. Rapidly progressive disseminated sporotrichosis as the first presentation of HIV infection in a patient with a very low CD4 cell count. Case Rep Infect Dis. 2017;2017:4713140. doi:10.1155/2017/4713140
- Singh S, Bachaspatimayum R, Meetei U, et al. Terbinafine in fixed cutaneous sporotrichosis: a case series. J Clin Diagnostic Res. 2018;12:FR01-FR03. doi:10.7860/JCDR/2018/25315.12223
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280. doi:10.1128/CMR.00053-10
- Peters F, Batinica M, Plum G, et al. Bug or no bug: challenges in diagnosing cutaneous mycobacterial infections. J Ger Soc Dermatol. 2016;14:1227-1236. doi:10.1111/ddg.13001
- Khadka P, Koirala S, Thapaliya J. Cutaneous tuberculosis: clinicopathologic arrays and diagnostic challenges. Dermatol Res Pract. 2018;2018:7201973. doi:10.1155/2018/7201973
- Okada E, Maruyama Y. Are keloids and hypertrophic scars caused by fungal infection? . Plast Reconstr Surg. 2007;120:814-815. doi:10.1097/01.prs.0000278813.23244.3f
- Pappas PG, Tellez I, Deep AE, et al. Sporotrichosis in Peru: description of an area of hyperendemicity. Clin Infect Dis. 2000;30:65-70. doi:10.1086/313607
- McGuinness SL, Boyd R, Kidd S, et al. Epidemiological investigation of an outbreak of cutaneous sporotrichosis, Northern Territory, Australia. BMC Infect Dis. 2016;16:1-7. doi:10.1186/s12879-016-1338-0
- Rojas FD, Fernández MS, Lucchelli JM, et al. Cavitary pulmonary sporotrichosis: case report and literature review. Mycopathologia. 2017;182:1119-1123. doi:10.1007/s11046-017-0197-6
- Schenck BR. On refractory subcutaneous abscesses caused by a fungus possibly related to the sporotricha. Bulletin of the Johns Hopkins Hospital. 1898;93:286-290.
- de Lima Barros MB, de Almeida Paes R, Schubach AO. Sporothrix schenckii and sporotrichosis. Clin Microbiol Rev. 2011;24:633-654. doi:10.1128/CMR.00007-11
- Crevasse L, Ellner PD. An outbreak of sporotrichosis in florida. J Am Med Assoc. 1960;173:29-33. doi:10.1001/jama.1960.03020190031006
- Mayorga R, Cáceres A, Toriello C, et al. An endemic area of sporotrichosis in Guatemala [in French]. Sabouraudia. 1978;16:185-198.
- Morris-Jones R. Sporotrichosis. Clin Exp Dermatol. 2002;27:427-431. doi:10.1046/j.1365-2230.2002.01087.x
- Sampaio SA, Da Lacaz CS. Clinical and statistical studies on sporotrichosis in Sao Paulo (Brazil). Article in German. Hautarzt. 1959;10:490-493.
- Ramos-e-Silva M, Vasconcelos C, Carneiro S, et al. Sporotrichosis. Clin Dermatol. 2007;25:181-187. doi:10.1016/j.clindermatol.2006.05.006
- Williams BA, Jennings TA, Rushing EC, et al. Sporotrichosis on the face of a 7-year-old boy following a bicycle accident. Pediatr Dermatol. 2013;30:E246-E247. doi:10.1111/j.1525-1470.2011.01696.x
- Vaishampayan SS, Borde P. An unusual presentation of sporotrichosis. Indian J Dermatol. 2013;58:409. doi:10.4103/0019-5154.117350
- Qin J, Zhang J. Sporotrichosis. N Engl J Med. 2019;380:771. doi:10.1056/NEJMicm1809179
- Patel A, Mudenda V, Lakhi S, et al. A 27-year-old severely immunosuppressed female with misleading clinical features of disseminated cutaneous sporotrichosis. Case Rep Dermatol Med. 2016;2016:1-4. doi:10.1155/2016/9403690
- de Oliveira-Esteves ICMR, Almeida Rosa da Silva G, Eyer-Silva WA, et al. Rapidly progressive disseminated sporotrichosis as the first presentation of HIV infection in a patient with a very low CD4 cell count. Case Rep Infect Dis. 2017;2017:4713140. doi:10.1155/2017/4713140
- Singh S, Bachaspatimayum R, Meetei U, et al. Terbinafine in fixed cutaneous sporotrichosis: a case series. J Clin Diagnostic Res. 2018;12:FR01-FR03. doi:10.7860/JCDR/2018/25315.12223
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280. doi:10.1128/CMR.00053-10
- Peters F, Batinica M, Plum G, et al. Bug or no bug: challenges in diagnosing cutaneous mycobacterial infections. J Ger Soc Dermatol. 2016;14:1227-1236. doi:10.1111/ddg.13001
- Khadka P, Koirala S, Thapaliya J. Cutaneous tuberculosis: clinicopathologic arrays and diagnostic challenges. Dermatol Res Pract. 2018;2018:7201973. doi:10.1155/2018/7201973
- Okada E, Maruyama Y. Are keloids and hypertrophic scars caused by fungal infection? . Plast Reconstr Surg. 2007;120:814-815. doi:10.1097/01.prs.0000278813.23244.3f
- Pappas PG, Tellez I, Deep AE, et al. Sporotrichosis in Peru: description of an area of hyperendemicity. Clin Infect Dis. 2000;30:65-70. doi:10.1086/313607
- McGuinness SL, Boyd R, Kidd S, et al. Epidemiological investigation of an outbreak of cutaneous sporotrichosis, Northern Territory, Australia. BMC Infect Dis. 2016;16:1-7. doi:10.1186/s12879-016-1338-0
- Rojas FD, Fernández MS, Lucchelli JM, et al. Cavitary pulmonary sporotrichosis: case report and literature review. Mycopathologia. 2017;182:1119-1123. doi:10.1007/s11046-017-0197-6
Practice Points
- An atypical presentation of lymphocutaneous sporotrichosis may pose challenges to timely diagnosis and treatment.
- Although lymphocutaneous sporotrichosis spreads most commonly in a linear fashion along lymphatic channels, an annular configuration is possible.
- Initial tissue cultures and histopathology of lymphocutaneous sporotrichosis may not yield a diagnosis, necessitating repeat biopsies when clinical suspicion is high.