User login
Assessing Attributes of Topical Vehicles for the Treatment of Acne, Atopic Dermatitis, and Plaque Psoriasis
The skin care market includes topical product formulations (eg, foams, lotions, ointments, creams) for a number of dermatologic therapeutic targets; however, there is limited information available regarding patient preference for product vehicles by specific dermatologic disease. There are few studies in the literature examining patient adherence to topical medications.1 In the current study, 6 focus groups comprised of patients with 3 dermatologic conditions of interest—acne, atopic dermatitis (AD), or plaque psoriasis (PP)—were surveyed to gain a better understanding of treatment preferences among patients and attributes of various formulations that are most desirable and important to this patient population.
Patient preference for a vehicle is relevant to treatment adherence in patients with conditions such as acne, AD, and PP because noncompliance is a major factor in the high rates of failure that have been associated with topical dermatologic treatments.2 The aesthetic attributes of a given vehicle formulation depend on the disease state being treated, the site of application, and the length of treatment.3 A limited number of studies have linked patient preferences to the attributes of topical medications. In one study, participants preferred an aqueous gel formulation compared with previously used topical treatments for AD.3 In another study, participants ranked the following properties of a hypothetical topical medication as most important: gel formulation, room temperature storage, product life of up to 18 months once opened, application with fingers, and a once-daily regimen.2 A third study found that foams were preferred among other formulations (ie, creams, gels, and ointments) for a variety of disease states, including AD, PP, and seborrheic dermatitis.4 The current study uses a qualitative analysis to further explore patient preferences of vehicle attributes for the topical treatment of acne, AD, and PP.
Methods
Study Participants
Six focus groups were conducted with patients who were using topical prescription medications for the treatment of acne, AD, or PP from March to April 2012. Participants were recruited from Raleigh, North Carolina, and New York, New York, with 3 focus groups (1 for each condition) from each city.
Following institutional review board approval of the study processes and recruitment materials, participants meeting the following criteria were included in the study: 18 years or older; diagnosed with acne, AD, or PP by a physician (participants who currently reported more than 1 of these conditions were not eligible); diagnosed with the respective skin condition at least 6 months prior to screening; current or prior use of topical prescription medications in at least 2 different vehicle formulations (eg, cream and foam, ointment and gel); use of at least 1 topical prescription treatment 5 or more times per month; and English speaking and able to provide written consent.
Study Design
A semistructured discussion guide was developed to ensure consistency in the topics surveyed among all 6 focus groups, and the same 2 moderators conducted the discussion for all 6 groups. At the beginning of the study, after providing written informed consent, participants were given an overview of the study and were asked general questions intended to get the participants talking about their experiences with their respective conditions. To avoid or minimize bias, participants were only asked open-ended questions designed to ascertain what symptoms they experienced in relation to their respective conditions. Finally, the discussions were focused on the topical prescription treatments that participants had tried and the properties of each treatment they liked and disliked.
The focus groups were recorded (audio) and transcribed; transcriptions were then verified through an iterative process of technical and editorial review. Analysis of the results was conducted by evaluation and review of the field notes as well as the transcripts from the focus groups.
Results
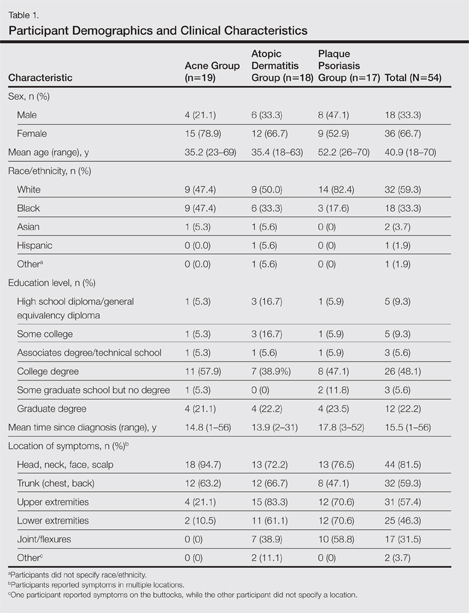
A total of 54 participants were surveyed (average age, 40.9 years). Although the average age of participants in the acne and AD groups was generally the same (35.2 and 35.4 years, respectively), the average age of participants in the PP group was higher (52.2 years). The majority of participants were white females who had at least a college degree. On average, participants had been diagnosed with their respective condition approximately 15.5 years prior to screening. Participant demographics and clinical characteristics are presented in more detail in Table 1.
At the time of screening, participants reported prior or present use of topical prescription medications in various formulations for treatment of their respective conditions. The most commonly reported vehicles across all 3 conditions were creams and ointments, followed by lotions, gels, and foams.
Symptoms Across Conditions
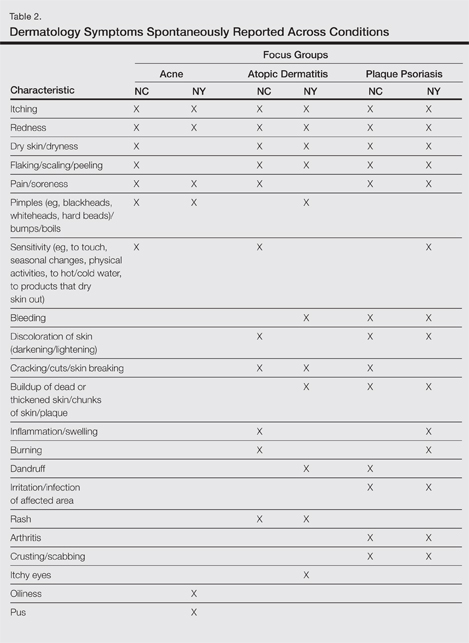
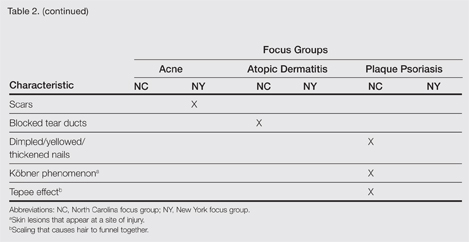
At the beginning of the study, participants reported symptoms they experienced in association with their respective skin conditions. Itching and redness were the only symptoms reported across all 3 conditions in all 6 focus groups. Dry skin/dryness, flaking/scaling/peeling, and pain were reported by at least 1 participant in 5 of 6 focus groups. Other common symptoms reported in at least 3 focus groups included pimples/bumps/boils, sensitivity, bleeding, discoloration of skin, cracking/cuts/skin breaking, and buildup of dead or thickened skin/chunks of skin/plaque. As shown in Table 2, there was more variability in the symptoms reported across the 2 acne groups compared with those reported by the AD and PP groups. Table 2 displays all symptoms spontaneously reported across each of the 6 focus groups. Symptoms are listed according to the terms/descriptions provided by focus group participants.
Results by Condition
Unless otherwise noted, participant responses generally were consistent across the North Carolina and New York focus groups. Results from the study, which included a total of 54 participants, suggested a high degree of similarity among preferences for topical treatment attributes across the 3 conditions. Moisturizing was the single attribute that was mentioned across all 3 conditions in all 6 focus groups as an important characteristic in a topical dermatologic treatment. Other attributes mentioned by at least 1 participant in 5 of 6 focus groups included the following: absorbs/disappears/dries quickly, available in various formulations, does not bleach or stain skin/hair/clothing, is not greasy/oily, is not sticky/tacky, is long lasting/long acting/stays on/lasts through sweating or hand washing, is fragrance or odor free, is easy to apply/simple to use, and can use all the time.
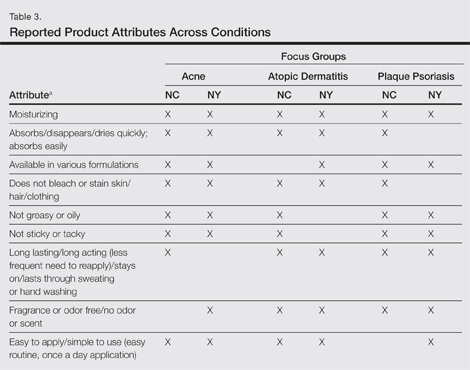
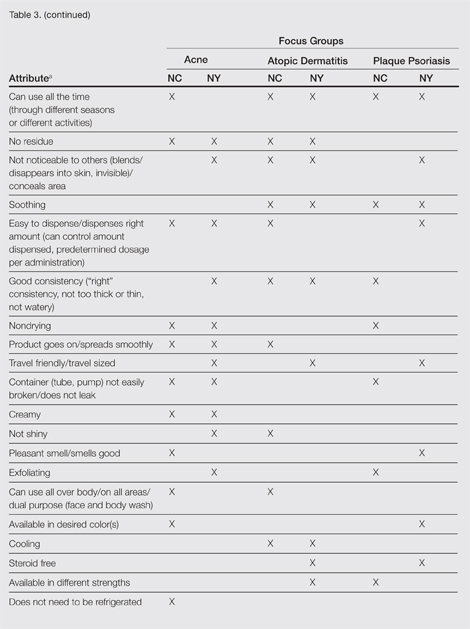
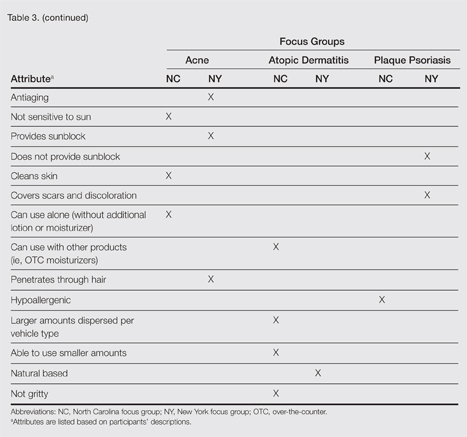
A few condition-specific attributes also were noted. An attribute was considered condition specific if it was mentioned by at least 1 participant in both groups for any condition. Preferred properties for topical medications that were specific to acne patients included the following: easy to dispense/dispenses right amount, nondrying, product goes on/spreads smoothly, container (eg, tube, pump) is not easily broken/does not leak, and creamy. Preferred properties that were specific to AD patients included the following: is not noticeable to others/conceals area, good consistency, and cooling. Preferred properties that were specific to 2 conditions included no residue among acne and AD patients and soothing among AD and PP patients. Preferred properties for topical medications across all focus groups are described in further detail in Table 3.
Regarding vehicle-type preferences, acne patients tended to prefer washes, creams, and lotions; AD patients preferred creams; and psoriasis patients preferred creams, ointments, and foams (particularly for the scalp).
Participants across all 3 conditions reported that during daytime hours (versus at night), they would be less likely to use products that are oily, shiny, thick (eg, ointments, oils); bleach or stain the skin/clothing; interfere with makeup, work, or other activities; or are visible to others. Rather, a majority of participants noted that they were more likely to use thinner, less oily products (eg, creams, lotions) during daytime hours, in social situations, or during certain activities (eg, exercise). Participants across all 3 conditions noted that they only used prescription shampoos when they had enough time to repeatedly rinse their hair to eliminate the smell. Participants across all 6 groups noted that they tended to choose creams or lotions over ointments (when available) during the summer because they considered these products to be lighter and less greasy.
Overall, participants indicated that condition-specific symptoms did not influence their preference for topical formulations; rather, they used whatever prescriptions they currently had. However, in some instances, participants noted that the location of the affected area might influence the type of product selected. For example, some participants tended not to use ointments on the scalp or in locations where their clothes might come into contact with the medicated area to avoid clothes sticking to medicated areas. Other participants used lighter, less stringent products on the face versus other locations. Participants noted that foams were preferred for more discrete localized areas.
Comment
Despite the variability in symptoms and conditions across the study population as well as participants’ varying experience with and access to topical treatments, the attributes participants valued most in topical prescription treatments were relatively consistent across the 3 conditions: moisturizing, absorbs/disappears/dries quickly, available in various formulations, does not bleach or stain skin/hair/clothing, is not greasy or oily, is not sticky or tacky, is long lasting/long acting, is fragrance or odor free, is easy to apply/simple to use, and can use all the time. Preferred vehicle attributes were generally consistent across the 3 conditions, but preferences for vehicle types tended to vary by condition. Although some vehicles were more closely associated with specific attributes (eg, the majority considered lotions to be moisturizing), no one vehicle was associated with the complete list of attributes for the ideal topical medication. Regardless of the symptoms experienced or particular situations/activities, participants noted that they tended to use the topical medications that were available to them, even if intended for different locations of the body, and nearly all reported using more than 1 type of topical treatment.
Caution should be used in interpreting these findings, as participant preferences are largely dependent on prior experience with different vehicle types. The small number of participants across the 3 conditions in this analysis also limits conclusions that can be drawn from the data sets, as each condition has different though somewhat overlapping treatment algorithms. A focused inquiry into each of these conditions through separate evaluations may provide for a more robust analysis. Future research might employ questionnaire methodology to develop items assessing the attributes of interest (eg, moisturizing ability, rate of absorption, greasiness/oiliness, stickiness/tackiness, fragrance/odor). The final set of desirable attributes may vary depending on the condition (eg, a moisturizing product may be more important to PP and AD patients than acne patients) as well as comparator products.
Acknowledgment—The authors would like to thank Jennifer Gwazdauskas, MBA, Research Triangle Park, North Carolina, of Stiefel, a GSK company, for her assistance with manuscript review.
- Tan X, Feldman SR, Chang J, et al. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9:1263-1271.
- Kellett N, West F, Finlay AY. Conjoint analysis: a novel, rigorous tool for determining patient p for topical antibiotic treatment for acne. a randomised controlled trial. Br J Dermatol. 2006;154:524-532.
- Trookman NS, Rizer RL, Ho ET, et al. The importance of vehicle properties to patients with atopic dermatitis. Cutis. 2011;88:13-17.
- Weiss S, Wyres M, Brundage T. A novel foam vehicle is consistently preferred by patients for dermatologic conditions. J Am Acad Dermatol. 2011;64(suppl 1):AB50.
The skin care market includes topical product formulations (eg, foams, lotions, ointments, creams) for a number of dermatologic therapeutic targets; however, there is limited information available regarding patient preference for product vehicles by specific dermatologic disease. There are few studies in the literature examining patient adherence to topical medications.1 In the current study, 6 focus groups comprised of patients with 3 dermatologic conditions of interest—acne, atopic dermatitis (AD), or plaque psoriasis (PP)—were surveyed to gain a better understanding of treatment preferences among patients and attributes of various formulations that are most desirable and important to this patient population.
Patient preference for a vehicle is relevant to treatment adherence in patients with conditions such as acne, AD, and PP because noncompliance is a major factor in the high rates of failure that have been associated with topical dermatologic treatments.2 The aesthetic attributes of a given vehicle formulation depend on the disease state being treated, the site of application, and the length of treatment.3 A limited number of studies have linked patient preferences to the attributes of topical medications. In one study, participants preferred an aqueous gel formulation compared with previously used topical treatments for AD.3 In another study, participants ranked the following properties of a hypothetical topical medication as most important: gel formulation, room temperature storage, product life of up to 18 months once opened, application with fingers, and a once-daily regimen.2 A third study found that foams were preferred among other formulations (ie, creams, gels, and ointments) for a variety of disease states, including AD, PP, and seborrheic dermatitis.4 The current study uses a qualitative analysis to further explore patient preferences of vehicle attributes for the topical treatment of acne, AD, and PP.
Methods
Study Participants
Six focus groups were conducted with patients who were using topical prescription medications for the treatment of acne, AD, or PP from March to April 2012. Participants were recruited from Raleigh, North Carolina, and New York, New York, with 3 focus groups (1 for each condition) from each city.
Following institutional review board approval of the study processes and recruitment materials, participants meeting the following criteria were included in the study: 18 years or older; diagnosed with acne, AD, or PP by a physician (participants who currently reported more than 1 of these conditions were not eligible); diagnosed with the respective skin condition at least 6 months prior to screening; current or prior use of topical prescription medications in at least 2 different vehicle formulations (eg, cream and foam, ointment and gel); use of at least 1 topical prescription treatment 5 or more times per month; and English speaking and able to provide written consent.
Study Design
A semistructured discussion guide was developed to ensure consistency in the topics surveyed among all 6 focus groups, and the same 2 moderators conducted the discussion for all 6 groups. At the beginning of the study, after providing written informed consent, participants were given an overview of the study and were asked general questions intended to get the participants talking about their experiences with their respective conditions. To avoid or minimize bias, participants were only asked open-ended questions designed to ascertain what symptoms they experienced in relation to their respective conditions. Finally, the discussions were focused on the topical prescription treatments that participants had tried and the properties of each treatment they liked and disliked.
The focus groups were recorded (audio) and transcribed; transcriptions were then verified through an iterative process of technical and editorial review. Analysis of the results was conducted by evaluation and review of the field notes as well as the transcripts from the focus groups.
Results
A total of 54 participants were surveyed (average age, 40.9 years). Although the average age of participants in the acne and AD groups was generally the same (35.2 and 35.4 years, respectively), the average age of participants in the PP group was higher (52.2 years). The majority of participants were white females who had at least a college degree. On average, participants had been diagnosed with their respective condition approximately 15.5 years prior to screening. Participant demographics and clinical characteristics are presented in more detail in Table 1.

At the time of screening, participants reported prior or present use of topical prescription medications in various formulations for treatment of their respective conditions. The most commonly reported vehicles across all 3 conditions were creams and ointments, followed by lotions, gels, and foams.
Symptoms Across Conditions
At the beginning of the study, participants reported symptoms they experienced in association with their respective skin conditions. Itching and redness were the only symptoms reported across all 3 conditions in all 6 focus groups. Dry skin/dryness, flaking/scaling/peeling, and pain were reported by at least 1 participant in 5 of 6 focus groups. Other common symptoms reported in at least 3 focus groups included pimples/bumps/boils, sensitivity, bleeding, discoloration of skin, cracking/cuts/skin breaking, and buildup of dead or thickened skin/chunks of skin/plaque. As shown in Table 2, there was more variability in the symptoms reported across the 2 acne groups compared with those reported by the AD and PP groups. Table 2 displays all symptoms spontaneously reported across each of the 6 focus groups. Symptoms are listed according to the terms/descriptions provided by focus group participants.


Results by Condition
Unless otherwise noted, participant responses generally were consistent across the North Carolina and New York focus groups. Results from the study, which included a total of 54 participants, suggested a high degree of similarity among preferences for topical treatment attributes across the 3 conditions. Moisturizing was the single attribute that was mentioned across all 3 conditions in all 6 focus groups as an important characteristic in a topical dermatologic treatment. Other attributes mentioned by at least 1 participant in 5 of 6 focus groups included the following: absorbs/disappears/dries quickly, available in various formulations, does not bleach or stain skin/hair/clothing, is not greasy/oily, is not sticky/tacky, is long lasting/long acting/stays on/lasts through sweating or hand washing, is fragrance or odor free, is easy to apply/simple to use, and can use all the time.
A few condition-specific attributes also were noted. An attribute was considered condition specific if it was mentioned by at least 1 participant in both groups for any condition. Preferred properties for topical medications that were specific to acne patients included the following: easy to dispense/dispenses right amount, nondrying, product goes on/spreads smoothly, container (eg, tube, pump) is not easily broken/does not leak, and creamy. Preferred properties that were specific to AD patients included the following: is not noticeable to others/conceals area, good consistency, and cooling. Preferred properties that were specific to 2 conditions included no residue among acne and AD patients and soothing among AD and PP patients. Preferred properties for topical medications across all focus groups are described in further detail in Table 3.



Regarding vehicle-type preferences, acne patients tended to prefer washes, creams, and lotions; AD patients preferred creams; and psoriasis patients preferred creams, ointments, and foams (particularly for the scalp).
Participants across all 3 conditions reported that during daytime hours (versus at night), they would be less likely to use products that are oily, shiny, thick (eg, ointments, oils); bleach or stain the skin/clothing; interfere with makeup, work, or other activities; or are visible to others. Rather, a majority of participants noted that they were more likely to use thinner, less oily products (eg, creams, lotions) during daytime hours, in social situations, or during certain activities (eg, exercise). Participants across all 3 conditions noted that they only used prescription shampoos when they had enough time to repeatedly rinse their hair to eliminate the smell. Participants across all 6 groups noted that they tended to choose creams or lotions over ointments (when available) during the summer because they considered these products to be lighter and less greasy.
Overall, participants indicated that condition-specific symptoms did not influence their preference for topical formulations; rather, they used whatever prescriptions they currently had. However, in some instances, participants noted that the location of the affected area might influence the type of product selected. For example, some participants tended not to use ointments on the scalp or in locations where their clothes might come into contact with the medicated area to avoid clothes sticking to medicated areas. Other participants used lighter, less stringent products on the face versus other locations. Participants noted that foams were preferred for more discrete localized areas.
Comment
Despite the variability in symptoms and conditions across the study population as well as participants’ varying experience with and access to topical treatments, the attributes participants valued most in topical prescription treatments were relatively consistent across the 3 conditions: moisturizing, absorbs/disappears/dries quickly, available in various formulations, does not bleach or stain skin/hair/clothing, is not greasy or oily, is not sticky or tacky, is long lasting/long acting, is fragrance or odor free, is easy to apply/simple to use, and can use all the time. Preferred vehicle attributes were generally consistent across the 3 conditions, but preferences for vehicle types tended to vary by condition. Although some vehicles were more closely associated with specific attributes (eg, the majority considered lotions to be moisturizing), no one vehicle was associated with the complete list of attributes for the ideal topical medication. Regardless of the symptoms experienced or particular situations/activities, participants noted that they tended to use the topical medications that were available to them, even if intended for different locations of the body, and nearly all reported using more than 1 type of topical treatment.
Caution should be used in interpreting these findings, as participant preferences are largely dependent on prior experience with different vehicle types. The small number of participants across the 3 conditions in this analysis also limits conclusions that can be drawn from the data sets, as each condition has different though somewhat overlapping treatment algorithms. A focused inquiry into each of these conditions through separate evaluations may provide for a more robust analysis. Future research might employ questionnaire methodology to develop items assessing the attributes of interest (eg, moisturizing ability, rate of absorption, greasiness/oiliness, stickiness/tackiness, fragrance/odor). The final set of desirable attributes may vary depending on the condition (eg, a moisturizing product may be more important to PP and AD patients than acne patients) as well as comparator products.
Acknowledgment—The authors would like to thank Jennifer Gwazdauskas, MBA, Research Triangle Park, North Carolina, of Stiefel, a GSK company, for her assistance with manuscript review.
The skin care market includes topical product formulations (eg, foams, lotions, ointments, creams) for a number of dermatologic therapeutic targets; however, there is limited information available regarding patient preference for product vehicles by specific dermatologic disease. There are few studies in the literature examining patient adherence to topical medications.1 In the current study, 6 focus groups comprised of patients with 3 dermatologic conditions of interest—acne, atopic dermatitis (AD), or plaque psoriasis (PP)—were surveyed to gain a better understanding of treatment preferences among patients and attributes of various formulations that are most desirable and important to this patient population.
Patient preference for a vehicle is relevant to treatment adherence in patients with conditions such as acne, AD, and PP because noncompliance is a major factor in the high rates of failure that have been associated with topical dermatologic treatments.2 The aesthetic attributes of a given vehicle formulation depend on the disease state being treated, the site of application, and the length of treatment.3 A limited number of studies have linked patient preferences to the attributes of topical medications. In one study, participants preferred an aqueous gel formulation compared with previously used topical treatments for AD.3 In another study, participants ranked the following properties of a hypothetical topical medication as most important: gel formulation, room temperature storage, product life of up to 18 months once opened, application with fingers, and a once-daily regimen.2 A third study found that foams were preferred among other formulations (ie, creams, gels, and ointments) for a variety of disease states, including AD, PP, and seborrheic dermatitis.4 The current study uses a qualitative analysis to further explore patient preferences of vehicle attributes for the topical treatment of acne, AD, and PP.
Methods
Study Participants
Six focus groups were conducted with patients who were using topical prescription medications for the treatment of acne, AD, or PP from March to April 2012. Participants were recruited from Raleigh, North Carolina, and New York, New York, with 3 focus groups (1 for each condition) from each city.
Following institutional review board approval of the study processes and recruitment materials, participants meeting the following criteria were included in the study: 18 years or older; diagnosed with acne, AD, or PP by a physician (participants who currently reported more than 1 of these conditions were not eligible); diagnosed with the respective skin condition at least 6 months prior to screening; current or prior use of topical prescription medications in at least 2 different vehicle formulations (eg, cream and foam, ointment and gel); use of at least 1 topical prescription treatment 5 or more times per month; and English speaking and able to provide written consent.
Study Design
A semistructured discussion guide was developed to ensure consistency in the topics surveyed among all 6 focus groups, and the same 2 moderators conducted the discussion for all 6 groups. At the beginning of the study, after providing written informed consent, participants were given an overview of the study and were asked general questions intended to get the participants talking about their experiences with their respective conditions. To avoid or minimize bias, participants were only asked open-ended questions designed to ascertain what symptoms they experienced in relation to their respective conditions. Finally, the discussions were focused on the topical prescription treatments that participants had tried and the properties of each treatment they liked and disliked.
The focus groups were recorded (audio) and transcribed; transcriptions were then verified through an iterative process of technical and editorial review. Analysis of the results was conducted by evaluation and review of the field notes as well as the transcripts from the focus groups.
Results
A total of 54 participants were surveyed (average age, 40.9 years). Although the average age of participants in the acne and AD groups was generally the same (35.2 and 35.4 years, respectively), the average age of participants in the PP group was higher (52.2 years). The majority of participants were white females who had at least a college degree. On average, participants had been diagnosed with their respective condition approximately 15.5 years prior to screening. Participant demographics and clinical characteristics are presented in more detail in Table 1.

At the time of screening, participants reported prior or present use of topical prescription medications in various formulations for treatment of their respective conditions. The most commonly reported vehicles across all 3 conditions were creams and ointments, followed by lotions, gels, and foams.
Symptoms Across Conditions
At the beginning of the study, participants reported symptoms they experienced in association with their respective skin conditions. Itching and redness were the only symptoms reported across all 3 conditions in all 6 focus groups. Dry skin/dryness, flaking/scaling/peeling, and pain were reported by at least 1 participant in 5 of 6 focus groups. Other common symptoms reported in at least 3 focus groups included pimples/bumps/boils, sensitivity, bleeding, discoloration of skin, cracking/cuts/skin breaking, and buildup of dead or thickened skin/chunks of skin/plaque. As shown in Table 2, there was more variability in the symptoms reported across the 2 acne groups compared with those reported by the AD and PP groups. Table 2 displays all symptoms spontaneously reported across each of the 6 focus groups. Symptoms are listed according to the terms/descriptions provided by focus group participants.


Results by Condition
Unless otherwise noted, participant responses generally were consistent across the North Carolina and New York focus groups. Results from the study, which included a total of 54 participants, suggested a high degree of similarity among preferences for topical treatment attributes across the 3 conditions. Moisturizing was the single attribute that was mentioned across all 3 conditions in all 6 focus groups as an important characteristic in a topical dermatologic treatment. Other attributes mentioned by at least 1 participant in 5 of 6 focus groups included the following: absorbs/disappears/dries quickly, available in various formulations, does not bleach or stain skin/hair/clothing, is not greasy/oily, is not sticky/tacky, is long lasting/long acting/stays on/lasts through sweating or hand washing, is fragrance or odor free, is easy to apply/simple to use, and can use all the time.
A few condition-specific attributes also were noted. An attribute was considered condition specific if it was mentioned by at least 1 participant in both groups for any condition. Preferred properties for topical medications that were specific to acne patients included the following: easy to dispense/dispenses right amount, nondrying, product goes on/spreads smoothly, container (eg, tube, pump) is not easily broken/does not leak, and creamy. Preferred properties that were specific to AD patients included the following: is not noticeable to others/conceals area, good consistency, and cooling. Preferred properties that were specific to 2 conditions included no residue among acne and AD patients and soothing among AD and PP patients. Preferred properties for topical medications across all focus groups are described in further detail in Table 3.



Regarding vehicle-type preferences, acne patients tended to prefer washes, creams, and lotions; AD patients preferred creams; and psoriasis patients preferred creams, ointments, and foams (particularly for the scalp).
Participants across all 3 conditions reported that during daytime hours (versus at night), they would be less likely to use products that are oily, shiny, thick (eg, ointments, oils); bleach or stain the skin/clothing; interfere with makeup, work, or other activities; or are visible to others. Rather, a majority of participants noted that they were more likely to use thinner, less oily products (eg, creams, lotions) during daytime hours, in social situations, or during certain activities (eg, exercise). Participants across all 3 conditions noted that they only used prescription shampoos when they had enough time to repeatedly rinse their hair to eliminate the smell. Participants across all 6 groups noted that they tended to choose creams or lotions over ointments (when available) during the summer because they considered these products to be lighter and less greasy.
Overall, participants indicated that condition-specific symptoms did not influence their preference for topical formulations; rather, they used whatever prescriptions they currently had. However, in some instances, participants noted that the location of the affected area might influence the type of product selected. For example, some participants tended not to use ointments on the scalp or in locations where their clothes might come into contact with the medicated area to avoid clothes sticking to medicated areas. Other participants used lighter, less stringent products on the face versus other locations. Participants noted that foams were preferred for more discrete localized areas.
Comment
Despite the variability in symptoms and conditions across the study population as well as participants’ varying experience with and access to topical treatments, the attributes participants valued most in topical prescription treatments were relatively consistent across the 3 conditions: moisturizing, absorbs/disappears/dries quickly, available in various formulations, does not bleach or stain skin/hair/clothing, is not greasy or oily, is not sticky or tacky, is long lasting/long acting, is fragrance or odor free, is easy to apply/simple to use, and can use all the time. Preferred vehicle attributes were generally consistent across the 3 conditions, but preferences for vehicle types tended to vary by condition. Although some vehicles were more closely associated with specific attributes (eg, the majority considered lotions to be moisturizing), no one vehicle was associated with the complete list of attributes for the ideal topical medication. Regardless of the symptoms experienced or particular situations/activities, participants noted that they tended to use the topical medications that were available to them, even if intended for different locations of the body, and nearly all reported using more than 1 type of topical treatment.
Caution should be used in interpreting these findings, as participant preferences are largely dependent on prior experience with different vehicle types. The small number of participants across the 3 conditions in this analysis also limits conclusions that can be drawn from the data sets, as each condition has different though somewhat overlapping treatment algorithms. A focused inquiry into each of these conditions through separate evaluations may provide for a more robust analysis. Future research might employ questionnaire methodology to develop items assessing the attributes of interest (eg, moisturizing ability, rate of absorption, greasiness/oiliness, stickiness/tackiness, fragrance/odor). The final set of desirable attributes may vary depending on the condition (eg, a moisturizing product may be more important to PP and AD patients than acne patients) as well as comparator products.
Acknowledgment—The authors would like to thank Jennifer Gwazdauskas, MBA, Research Triangle Park, North Carolina, of Stiefel, a GSK company, for her assistance with manuscript review.
- Tan X, Feldman SR, Chang J, et al. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9:1263-1271.
- Kellett N, West F, Finlay AY. Conjoint analysis: a novel, rigorous tool for determining patient p for topical antibiotic treatment for acne. a randomised controlled trial. Br J Dermatol. 2006;154:524-532.
- Trookman NS, Rizer RL, Ho ET, et al. The importance of vehicle properties to patients with atopic dermatitis. Cutis. 2011;88:13-17.
- Weiss S, Wyres M, Brundage T. A novel foam vehicle is consistently preferred by patients for dermatologic conditions. J Am Acad Dermatol. 2011;64(suppl 1):AB50.
- Tan X, Feldman SR, Chang J, et al. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9:1263-1271.
- Kellett N, West F, Finlay AY. Conjoint analysis: a novel, rigorous tool for determining patient p for topical antibiotic treatment for acne. a randomised controlled trial. Br J Dermatol. 2006;154:524-532.
- Trookman NS, Rizer RL, Ho ET, et al. The importance of vehicle properties to patients with atopic dermatitis. Cutis. 2011;88:13-17.
- Weiss S, Wyres M, Brundage T. A novel foam vehicle is consistently preferred by patients for dermatologic conditions. J Am Acad Dermatol. 2011;64(suppl 1):AB50.
- Patient preference for topical product formulation varies by dermatologic condition being treated.
- Most important vehicle attributes cited across study conditions include moisturizing, absorbs/disappears/dries quickly, available in various formulations, does not bleach or stain skin/hair/clothing, is not greasy or oily, is not sticky or tacky, is long lasting/long acting, is fragrance or odor free, is easy to apply/simple to use, and can use all the time.
- Patient preference for product vehicle is relevant to treatment adherence and may vary depending on the condition being treated.