User login
Sniffing Out Malignant Melanoma: A Case of Canine Olfactory Detection
To the Editor:
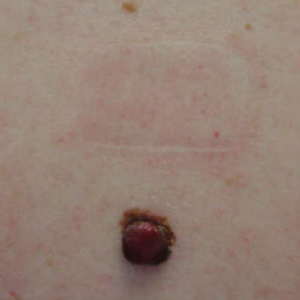
A 43-year-old woman presented with a mole on the central back that had been present since childhood and had changed and grown over the last few years. The patient reported that her 2-year-old rescue dog frequently sniffed the mole and would subsequently get agitated and try to scratch and bite the lesion. This behavior prompted the patient to visit a dermatologist.
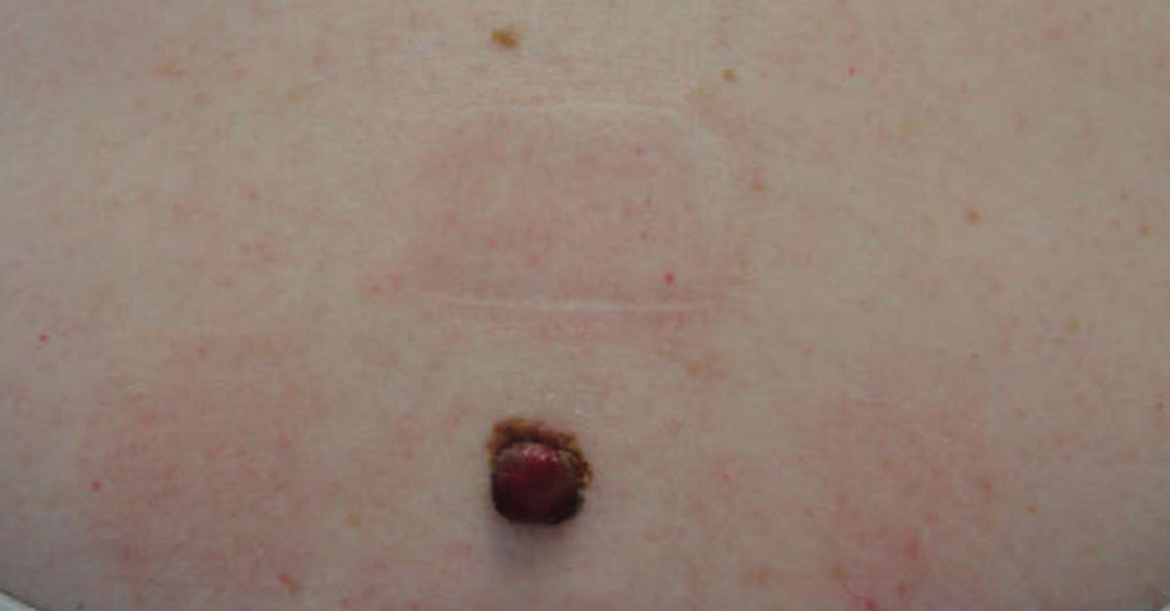
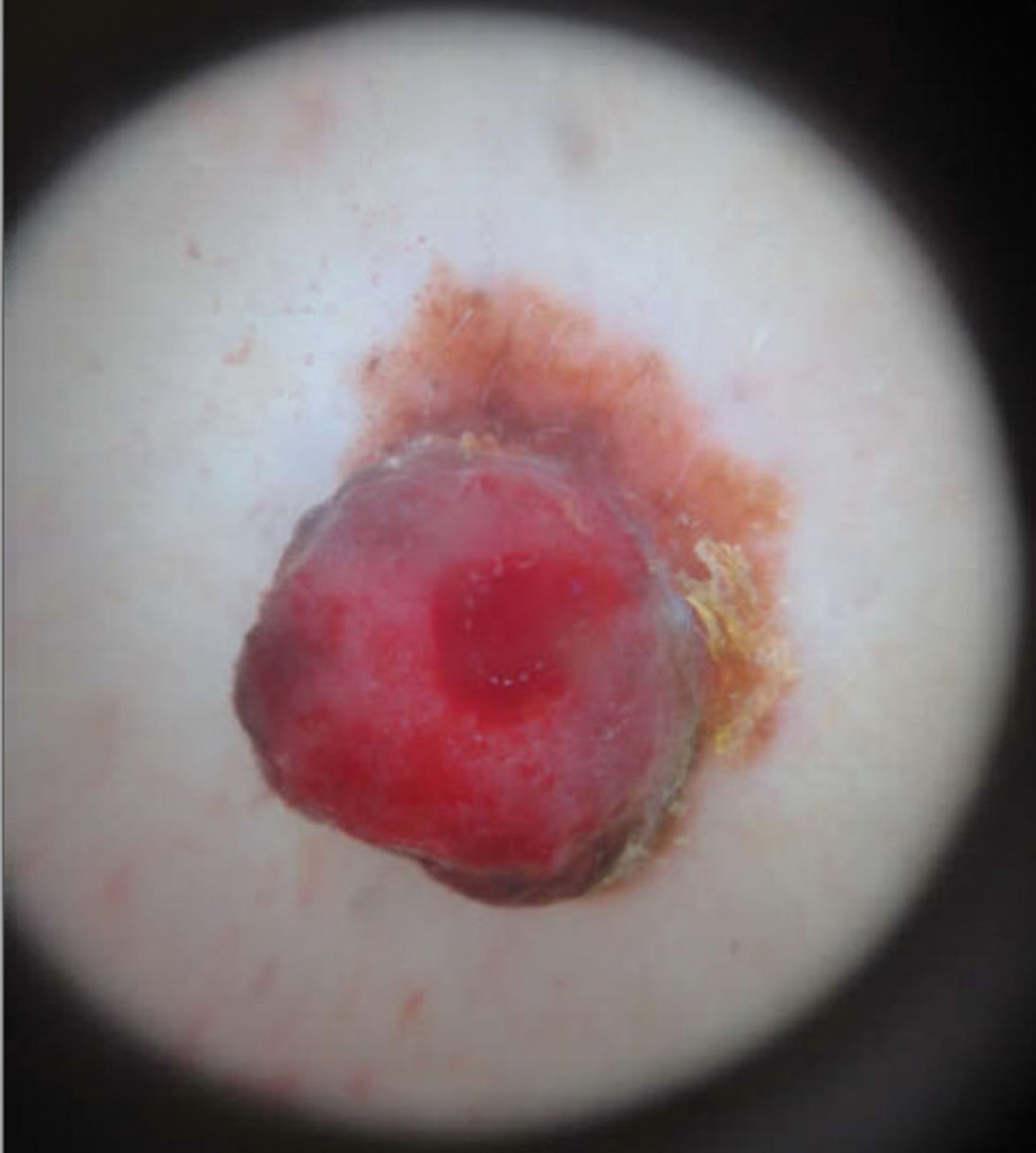
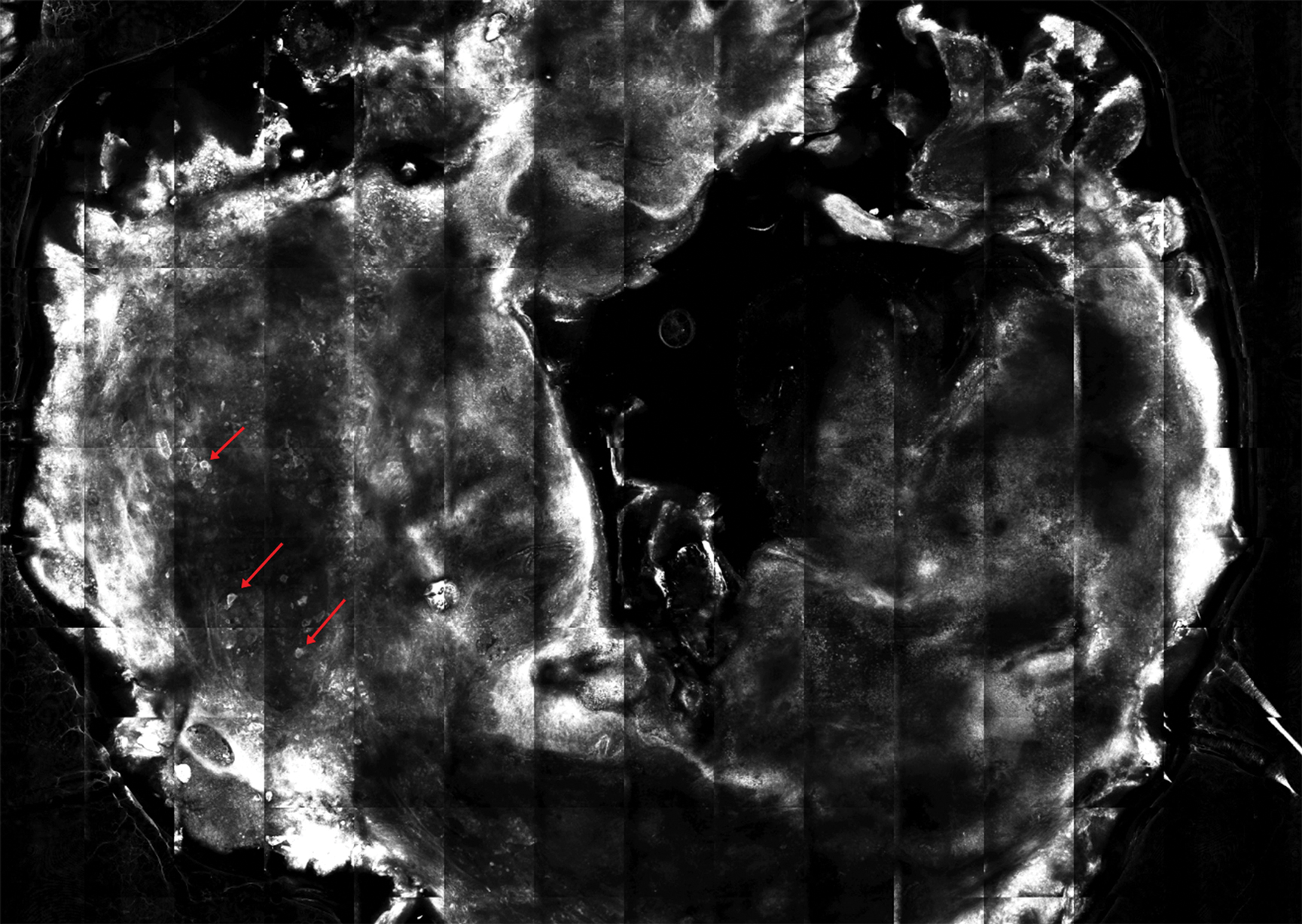
She reported no personal history of melanoma or nonmelanoma skin cancer, tanning booth exposure, blistering sunburns, or use of immunosuppressant medications. Her family history was remarkable for basal cell carcinoma in her father but no family history of melanoma. Physical examination revealed a 1.2×1.5-cm brown patch along with a 1×1-cm ulcerated nodule on the lower aspect of the lesion (Figure 1). Dermoscopy showed a blue-white veil and an irregular vascular pattern (Figure 2). No cervical, axillary, or inguinal lymphadenopathy was appreciated on physical examination. Reflectance confocal microscopy showed pagetoid spread of atypical round melanocytes as well as melanocytes in the stratum corneum (Figure 3).
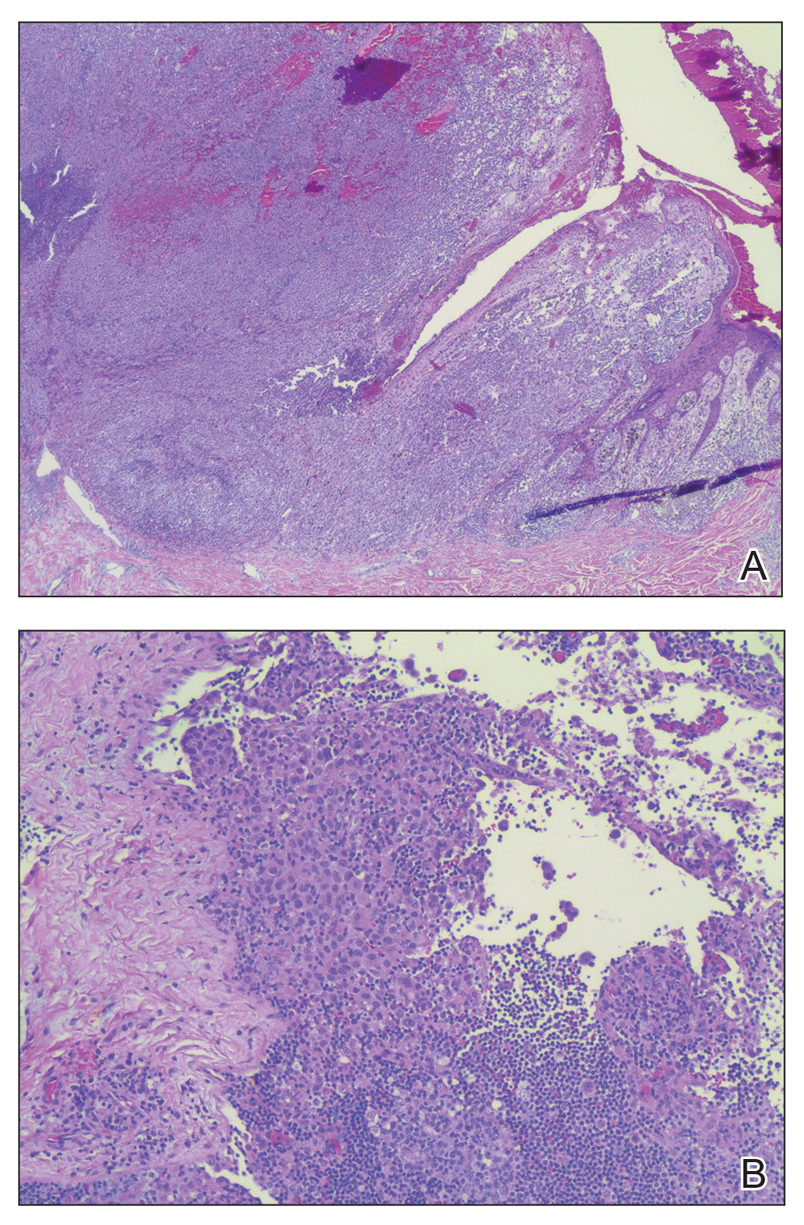
The patient was referred to a surgical oncologist for wide local excision and sentinel lymph node biopsy. Pathology showed a 4-mm-thick melanoma with numerous positive lymph nodes (Figure 4). The patient subsequently underwent a right axillary lymphadenectomy and was diagnosed with stage IIIB malignant melanoma. After surgery, the patient reported that her dog would now sniff her back and calmly rest his head in her lap.
She was treated with ipilimumab but subsequently developed panhypopituitarism, so she was taken off the ipilimumab. Currently, the patient is doing well. She follows up annually for full-body skin examinations and has not had any recurrence in the last 7 years. The patient credits her dog for prompting her to see a dermatologist and saving her life.
Both anecdotal and systematic evidence have emerged on the role of canine olfaction in the detection of lung, breast, colorectal, ovarian, prostate, and skin cancers, including malignant melanoma.1-6 A 1989 case report described a woman who was prompted to seek dermatologic evaluation of a pigmented lesion because her dog consistently targeted the lesion. Excision and subsequent histopathologic examination of the lesion revealed that it was malignant melanoma.5 Another case report described a patient whose dog, which was not trained to detect cancers in humans, persistently licked a lesion behind the patient’s ear that eventually was found to be malignant melanoma.6 These reports have inspired considerable research interest regarding canine olfaction as a potential method to noninvasively screen for and even diagnose malignant melanomas in humans.
Both physiologic and pathologic metabolic processes result in the production of volatile organic compounds (VOCs), or small odorant molecules that evaporate at normal temperatures and pressures.1 Individual cells release VOCs in extremely low concentrations into the blood, urine, feces, and breath, as well as onto the skin’s surface, but there are methods for detecting these VOCs, including gas chromatography–mass spectrometry and canine olfaction.7,8 Pathologic processes, such as infection and malignancy, result in irregular protein synthesis and metabolism, producing new VOCs or differing concentrations of VOCs as compared to normal processes.1
Dimethyl disulfide and dimethyl trisulfide compounds have been identified in malignant melanoma, and these compounds are not produced by normal melanocytes.7 Furthermore, malignant melanoma produces differing quantities of these compounds as compared to normal melanocytes, including isovaleric acid, 2-methylbutyric acid, isoamyl alcohol (3-methyl-1-butanol), and 2-methyl-1-butanol, resulting in a distinct odorant profile that previously has been detected via canine olfaction.7 Canine olfaction can identify odorant molecules at up to 1 part per trillion (a magnitude more sensitive than the currently available gas chromatography–mass spectrometry technologies) and can detect the production of new VOCs or altered VOC ratios due to pathologic processes.1 Systematic studies with dogs that are trained to detect cancers in humans have shown that canine olfaction correctly identified malignant melanomas against healthy skin, benign nevi, and even basal cell carcinomas at higher rates than what would have been expected by chance alone.2,3
Canine olfaction can identify new or altered ratios of odorant VOCs associated with pathologic metabolic processes, and canines can be trained to target odor profiles associated with specific diseases.1 Canine olfaction for melanoma screening and diagnosis may seem appealing, as it provides an easily transportable, real-time, low-cost method compared to other techniques such as gas chromatography–mass spectrometry.1 Although preliminary results have shown that canine olfaction detects melanoma at higher rates than would be expected by chance alone, these findings have not approached clinical utility for the widespread use of canine olfaction as a screening method for melanoma.2,3,9 Further studies are needed to understand the role of canine olfaction in melanoma screening and diagnosis as well as to explore methods to optimize sensitivity and specificity. Until then, patients and dermatologists should not ignore the behavior of dogs toward skin lesions. Dogs may be beneficial in the detection of melanoma and help save lives, as was seen in our case.
- Angle C, Waggoner LP, Ferrando A, et al. Canine detection of the volatilome: a review of implications for pathogen and disease detection. Front Vet Sci. 2016;3:47.
- Pickel D, Mauncy GP, Walker DB, et al. Evidence for canine olfactory detection of melanoma. Applied Animal Behaviour Science. 2004;89:107-116.
- Willis CM, Britton LE, Swindells MA, et al. Invasive melanoma in vivo can be distinguished from basal cell carcinoma, benign naevi and healthy skin by canine olfaction: a proof‐of‐principle study of differential volatile organic compound emission. Br J Dermatol. 2016;175:1020-1029.
- Jezierski T, Walczak M, Ligor T, et al. Study of the art: canine olfaction used for cancer detection on the basis of breath odour. perspectives and limitations. J Breath Res. 2015;9:027001.
- Williams H, Pembroke A. Sniffer dogs in the melanoma clinic? Lancet. 1989;1:734.
- Campbell LF, Farmery L, George SM, et al. Canine olfactory detection of malignant melanoma. BMJ Case Rep. 2013. doi:10.1136/bcr-2013-008566.
- Kwak J, Gallagher M, Ozdener MH, et al. Volatile biomarkers from human melanoma cells. J Chromotogr B Analyt Technol Biomed Life Sci. 2013;931:90-96.
- D’Amico A, Bono R, Pennazza G, et al. Identification of melanoma with a gas sensor array. Skin Res Technol. 2008;14:226-236.
- Elliker KR, Williams HC. Detection of skin cancer odours using dogs: a step forward in melanoma detection training and research methodologies. Br J Dermatol. 2016;175:851-852.
To the Editor:
A 43-year-old woman presented with a mole on the central back that had been present since childhood and had changed and grown over the last few years. The patient reported that her 2-year-old rescue dog frequently sniffed the mole and would subsequently get agitated and try to scratch and bite the lesion. This behavior prompted the patient to visit a dermatologist.
She reported no personal history of melanoma or nonmelanoma skin cancer, tanning booth exposure, blistering sunburns, or use of immunosuppressant medications. Her family history was remarkable for basal cell carcinoma in her father but no family history of melanoma. Physical examination revealed a 1.2×1.5-cm brown patch along with a 1×1-cm ulcerated nodule on the lower aspect of the lesion (Figure 1). Dermoscopy showed a blue-white veil and an irregular vascular pattern (Figure 2). No cervical, axillary, or inguinal lymphadenopathy was appreciated on physical examination. Reflectance confocal microscopy showed pagetoid spread of atypical round melanocytes as well as melanocytes in the stratum corneum (Figure 3).
The patient was referred to a surgical oncologist for wide local excision and sentinel lymph node biopsy. Pathology showed a 4-mm-thick melanoma with numerous positive lymph nodes (Figure 4). The patient subsequently underwent a right axillary lymphadenectomy and was diagnosed with stage IIIB malignant melanoma. After surgery, the patient reported that her dog would now sniff her back and calmly rest his head in her lap.
She was treated with ipilimumab but subsequently developed panhypopituitarism, so she was taken off the ipilimumab. Currently, the patient is doing well. She follows up annually for full-body skin examinations and has not had any recurrence in the last 7 years. The patient credits her dog for prompting her to see a dermatologist and saving her life.
Both anecdotal and systematic evidence have emerged on the role of canine olfaction in the detection of lung, breast, colorectal, ovarian, prostate, and skin cancers, including malignant melanoma.1-6 A 1989 case report described a woman who was prompted to seek dermatologic evaluation of a pigmented lesion because her dog consistently targeted the lesion. Excision and subsequent histopathologic examination of the lesion revealed that it was malignant melanoma.5 Another case report described a patient whose dog, which was not trained to detect cancers in humans, persistently licked a lesion behind the patient’s ear that eventually was found to be malignant melanoma.6 These reports have inspired considerable research interest regarding canine olfaction as a potential method to noninvasively screen for and even diagnose malignant melanomas in humans.
Both physiologic and pathologic metabolic processes result in the production of volatile organic compounds (VOCs), or small odorant molecules that evaporate at normal temperatures and pressures.1 Individual cells release VOCs in extremely low concentrations into the blood, urine, feces, and breath, as well as onto the skin’s surface, but there are methods for detecting these VOCs, including gas chromatography–mass spectrometry and canine olfaction.7,8 Pathologic processes, such as infection and malignancy, result in irregular protein synthesis and metabolism, producing new VOCs or differing concentrations of VOCs as compared to normal processes.1
Dimethyl disulfide and dimethyl trisulfide compounds have been identified in malignant melanoma, and these compounds are not produced by normal melanocytes.7 Furthermore, malignant melanoma produces differing quantities of these compounds as compared to normal melanocytes, including isovaleric acid, 2-methylbutyric acid, isoamyl alcohol (3-methyl-1-butanol), and 2-methyl-1-butanol, resulting in a distinct odorant profile that previously has been detected via canine olfaction.7 Canine olfaction can identify odorant molecules at up to 1 part per trillion (a magnitude more sensitive than the currently available gas chromatography–mass spectrometry technologies) and can detect the production of new VOCs or altered VOC ratios due to pathologic processes.1 Systematic studies with dogs that are trained to detect cancers in humans have shown that canine olfaction correctly identified malignant melanomas against healthy skin, benign nevi, and even basal cell carcinomas at higher rates than what would have been expected by chance alone.2,3
Canine olfaction can identify new or altered ratios of odorant VOCs associated with pathologic metabolic processes, and canines can be trained to target odor profiles associated with specific diseases.1 Canine olfaction for melanoma screening and diagnosis may seem appealing, as it provides an easily transportable, real-time, low-cost method compared to other techniques such as gas chromatography–mass spectrometry.1 Although preliminary results have shown that canine olfaction detects melanoma at higher rates than would be expected by chance alone, these findings have not approached clinical utility for the widespread use of canine olfaction as a screening method for melanoma.2,3,9 Further studies are needed to understand the role of canine olfaction in melanoma screening and diagnosis as well as to explore methods to optimize sensitivity and specificity. Until then, patients and dermatologists should not ignore the behavior of dogs toward skin lesions. Dogs may be beneficial in the detection of melanoma and help save lives, as was seen in our case.
To the Editor:
A 43-year-old woman presented with a mole on the central back that had been present since childhood and had changed and grown over the last few years. The patient reported that her 2-year-old rescue dog frequently sniffed the mole and would subsequently get agitated and try to scratch and bite the lesion. This behavior prompted the patient to visit a dermatologist.
She reported no personal history of melanoma or nonmelanoma skin cancer, tanning booth exposure, blistering sunburns, or use of immunosuppressant medications. Her family history was remarkable for basal cell carcinoma in her father but no family history of melanoma. Physical examination revealed a 1.2×1.5-cm brown patch along with a 1×1-cm ulcerated nodule on the lower aspect of the lesion (Figure 1). Dermoscopy showed a blue-white veil and an irregular vascular pattern (Figure 2). No cervical, axillary, or inguinal lymphadenopathy was appreciated on physical examination. Reflectance confocal microscopy showed pagetoid spread of atypical round melanocytes as well as melanocytes in the stratum corneum (Figure 3).
The patient was referred to a surgical oncologist for wide local excision and sentinel lymph node biopsy. Pathology showed a 4-mm-thick melanoma with numerous positive lymph nodes (Figure 4). The patient subsequently underwent a right axillary lymphadenectomy and was diagnosed with stage IIIB malignant melanoma. After surgery, the patient reported that her dog would now sniff her back and calmly rest his head in her lap.
She was treated with ipilimumab but subsequently developed panhypopituitarism, so she was taken off the ipilimumab. Currently, the patient is doing well. She follows up annually for full-body skin examinations and has not had any recurrence in the last 7 years. The patient credits her dog for prompting her to see a dermatologist and saving her life.
Both anecdotal and systematic evidence have emerged on the role of canine olfaction in the detection of lung, breast, colorectal, ovarian, prostate, and skin cancers, including malignant melanoma.1-6 A 1989 case report described a woman who was prompted to seek dermatologic evaluation of a pigmented lesion because her dog consistently targeted the lesion. Excision and subsequent histopathologic examination of the lesion revealed that it was malignant melanoma.5 Another case report described a patient whose dog, which was not trained to detect cancers in humans, persistently licked a lesion behind the patient’s ear that eventually was found to be malignant melanoma.6 These reports have inspired considerable research interest regarding canine olfaction as a potential method to noninvasively screen for and even diagnose malignant melanomas in humans.
Both physiologic and pathologic metabolic processes result in the production of volatile organic compounds (VOCs), or small odorant molecules that evaporate at normal temperatures and pressures.1 Individual cells release VOCs in extremely low concentrations into the blood, urine, feces, and breath, as well as onto the skin’s surface, but there are methods for detecting these VOCs, including gas chromatography–mass spectrometry and canine olfaction.7,8 Pathologic processes, such as infection and malignancy, result in irregular protein synthesis and metabolism, producing new VOCs or differing concentrations of VOCs as compared to normal processes.1
Dimethyl disulfide and dimethyl trisulfide compounds have been identified in malignant melanoma, and these compounds are not produced by normal melanocytes.7 Furthermore, malignant melanoma produces differing quantities of these compounds as compared to normal melanocytes, including isovaleric acid, 2-methylbutyric acid, isoamyl alcohol (3-methyl-1-butanol), and 2-methyl-1-butanol, resulting in a distinct odorant profile that previously has been detected via canine olfaction.7 Canine olfaction can identify odorant molecules at up to 1 part per trillion (a magnitude more sensitive than the currently available gas chromatography–mass spectrometry technologies) and can detect the production of new VOCs or altered VOC ratios due to pathologic processes.1 Systematic studies with dogs that are trained to detect cancers in humans have shown that canine olfaction correctly identified malignant melanomas against healthy skin, benign nevi, and even basal cell carcinomas at higher rates than what would have been expected by chance alone.2,3
Canine olfaction can identify new or altered ratios of odorant VOCs associated with pathologic metabolic processes, and canines can be trained to target odor profiles associated with specific diseases.1 Canine olfaction for melanoma screening and diagnosis may seem appealing, as it provides an easily transportable, real-time, low-cost method compared to other techniques such as gas chromatography–mass spectrometry.1 Although preliminary results have shown that canine olfaction detects melanoma at higher rates than would be expected by chance alone, these findings have not approached clinical utility for the widespread use of canine olfaction as a screening method for melanoma.2,3,9 Further studies are needed to understand the role of canine olfaction in melanoma screening and diagnosis as well as to explore methods to optimize sensitivity and specificity. Until then, patients and dermatologists should not ignore the behavior of dogs toward skin lesions. Dogs may be beneficial in the detection of melanoma and help save lives, as was seen in our case.
- Angle C, Waggoner LP, Ferrando A, et al. Canine detection of the volatilome: a review of implications for pathogen and disease detection. Front Vet Sci. 2016;3:47.
- Pickel D, Mauncy GP, Walker DB, et al. Evidence for canine olfactory detection of melanoma. Applied Animal Behaviour Science. 2004;89:107-116.
- Willis CM, Britton LE, Swindells MA, et al. Invasive melanoma in vivo can be distinguished from basal cell carcinoma, benign naevi and healthy skin by canine olfaction: a proof‐of‐principle study of differential volatile organic compound emission. Br J Dermatol. 2016;175:1020-1029.
- Jezierski T, Walczak M, Ligor T, et al. Study of the art: canine olfaction used for cancer detection on the basis of breath odour. perspectives and limitations. J Breath Res. 2015;9:027001.
- Williams H, Pembroke A. Sniffer dogs in the melanoma clinic? Lancet. 1989;1:734.
- Campbell LF, Farmery L, George SM, et al. Canine olfactory detection of malignant melanoma. BMJ Case Rep. 2013. doi:10.1136/bcr-2013-008566.
- Kwak J, Gallagher M, Ozdener MH, et al. Volatile biomarkers from human melanoma cells. J Chromotogr B Analyt Technol Biomed Life Sci. 2013;931:90-96.
- D’Amico A, Bono R, Pennazza G, et al. Identification of melanoma with a gas sensor array. Skin Res Technol. 2008;14:226-236.
- Elliker KR, Williams HC. Detection of skin cancer odours using dogs: a step forward in melanoma detection training and research methodologies. Br J Dermatol. 2016;175:851-852.
- Angle C, Waggoner LP, Ferrando A, et al. Canine detection of the volatilome: a review of implications for pathogen and disease detection. Front Vet Sci. 2016;3:47.
- Pickel D, Mauncy GP, Walker DB, et al. Evidence for canine olfactory detection of melanoma. Applied Animal Behaviour Science. 2004;89:107-116.
- Willis CM, Britton LE, Swindells MA, et al. Invasive melanoma in vivo can be distinguished from basal cell carcinoma, benign naevi and healthy skin by canine olfaction: a proof‐of‐principle study of differential volatile organic compound emission. Br J Dermatol. 2016;175:1020-1029.
- Jezierski T, Walczak M, Ligor T, et al. Study of the art: canine olfaction used for cancer detection on the basis of breath odour. perspectives and limitations. J Breath Res. 2015;9:027001.
- Williams H, Pembroke A. Sniffer dogs in the melanoma clinic? Lancet. 1989;1:734.
- Campbell LF, Farmery L, George SM, et al. Canine olfactory detection of malignant melanoma. BMJ Case Rep. 2013. doi:10.1136/bcr-2013-008566.
- Kwak J, Gallagher M, Ozdener MH, et al. Volatile biomarkers from human melanoma cells. J Chromotogr B Analyt Technol Biomed Life Sci. 2013;931:90-96.
- D’Amico A, Bono R, Pennazza G, et al. Identification of melanoma with a gas sensor array. Skin Res Technol. 2008;14:226-236.
- Elliker KR, Williams HC. Detection of skin cancer odours using dogs: a step forward in melanoma detection training and research methodologies. Br J Dermatol. 2016;175:851-852.
Practice Points
- Physiologic and pathologic processes produce volatile organic compounds in the skin and other tissues.
- Malignant melanocytes release unique volatile organic compounds (VOCs) as well as differing combinations and quantities of VOCs as compared to normal melanocytes.
- Volatile organic compounds released at the skin’s surface can be detected by various methods, including canine olfaction; therefore, unusual canine behavior toward skin lesions should not be ignored.
Analysis of Nail-Related Content in the Basic Dermatology Curriculum
Patients frequently present to dermatologists with nail disorders as their chief concern. Alternatively, nail conditions may be encountered by the examining physician as an incidental finding that may be a clue to underlying systemic disease. Competence in the diagnosis and treatment of nail diseases can drastically improve patient quality of life and can be lifesaving,1 but many dermatologists find management of nail diseases challenging.2 Bridging this educational gap begins with dermatology resident and medical student education. In a collaboration with dermatology educators, the American Academy of Dermatology (AAD) prepared a free online core curriculum for medical students that covers the essential concepts of dermatology. We sought to determine the integration of nail education in the AAD Basic Dermatology Curriculum.
Methods
A cross-sectional study of the AAD Basic Dermatology Curriculum was conducted to determine nail disease content. The curriculum modules were downloaded in June 2018,
Results
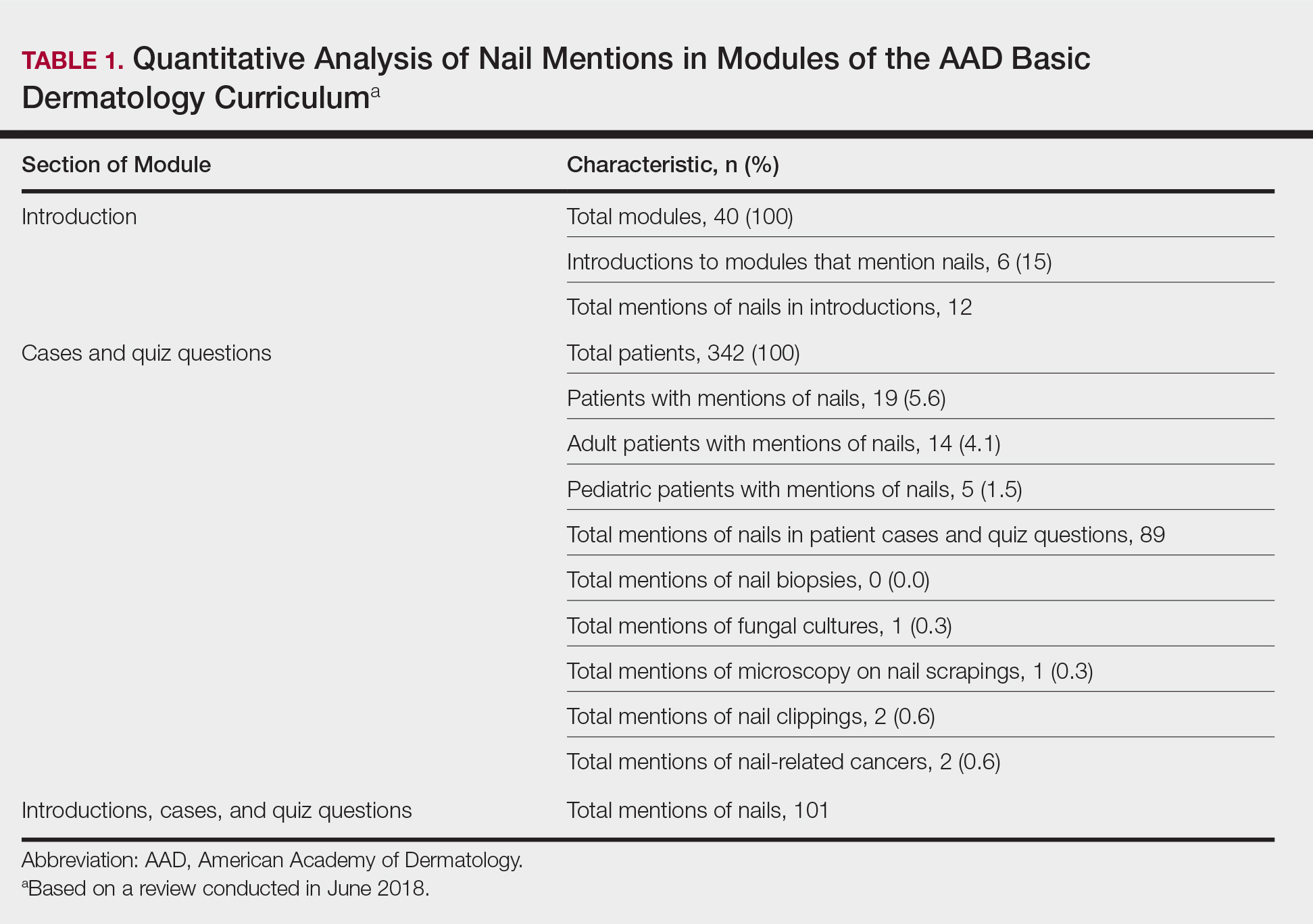
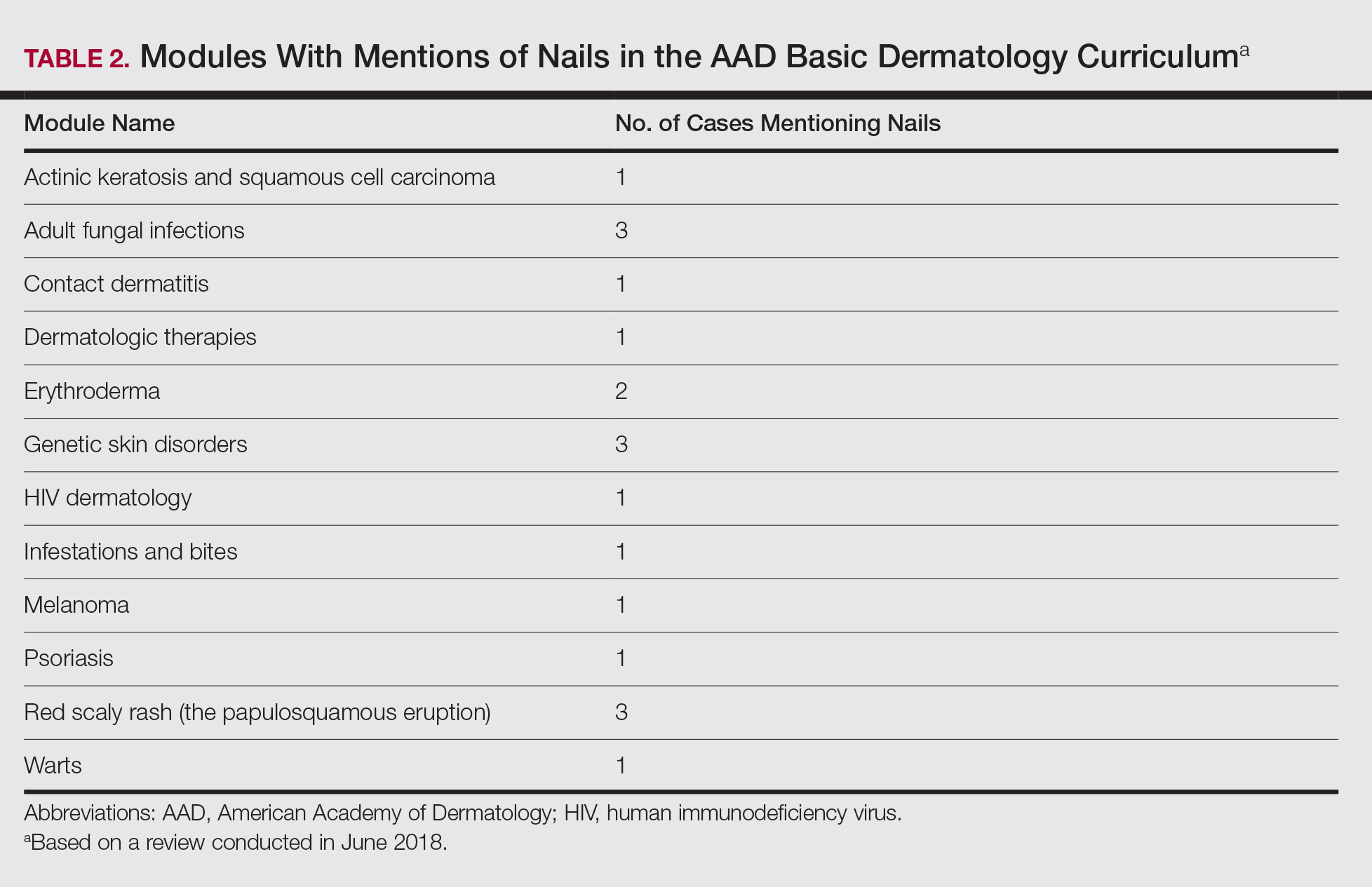
Of 342 patients discussed in cases and quizzes, nails were mentioned for 19 patients (89 times total)(Table 1). Additionally, there were 2 mentions each of nail clippings and nail tumors, 0 mentions of nail biopsies, and 1 mention each of fungal cultures and microscopy on nail scrapings (Table 1). Of the 40 modules, nails were mentioned in 12 modules (Table 2) and 6 introductions to the modules (Table 1). There were no mentions of the terms nails, subungual, or onychomycosis in the learning objectives.3
Comment
Our study demonstrates a paucity of content relevant to nails in the AAD Basic Dermatology Curriculum. Medical students are missing an important opportunity to learn about diagnosis and management of nail conditions and may incorrectly conclude that nail expertise is not essential to becoming a competent board-certified dermatologist.
Particularly concerning is the exclusion of nail examinations in the skin exam module addressing full-body skin examinations (0 mentions in 31 slides). This curriculum may negatively influence medical students and may then follow at the resident level, with a study reporting that 50.3% (69/137) of residents examine nails only when the patient brings it to their attention.4
Most concerning was the inadequate coverage of nail unit melanoma in the melanoma module (1 mention in 53 slides). Furthermore, the ABCDE—asymmetry, border, color, diameter, and evolving—mnemonic for cutaneous melanoma was covered in 6 slides in this module, and the ABCDEF—family history added—mnemonic for nail unit melanoma was completely excluded. Not surprisingly, resident knowledge of melanonychia diagnosis is deficient, with a prior study demonstrating that 62% (88/142) of residents were not confident diagnosing and managing patients with melanonychia, and only 88% (125/142) of residents were aware of the nail melanoma mnemonic.4
Similarly, nail biopsy for melanonychia diagnosis was excluded from the curriculum, whereas skin biopsy was thoroughly discussed in the context of a cutaneous melanoma diagnosis. This deficient teaching may track to the dermatology resident curriculum, as a survey of third-year dermatology residents (N=240) showed that 58% performed 10 or fewer nail procedures, and one-third of residents felt incompetent in nail surgery.5
We acknowledge that the AAD Basic Dermatology Curriculum is simply an introduction to dermatology. However, given that dermatologists are among the major specialists who care for nail patients, we advocate for more content on nail diseases in this curriculum. Nails can easily be incorporated into existing modules, and a new module specifically dedicated to nail disease should be added. Moreover, we envision that our findings will positively reflect on competence in treating nail disease for dermatology residents.
- Lipner SR. Ulcerated nodule of the fingernail. JAMA. 2018;319:713-714.
- Hare AQ, Rich P. Clinical and educational gaps in diagnosis of nail disorders. Dermatol Clin. 2016;34:269-273.
- American Academy of Dermatology. Basic Dermatology Curriculum. https://www.aad.org/education/basic-derm-curriculum. Accessed March 25, 2019.
- Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996.
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483, 483.e1-5.
Patients frequently present to dermatologists with nail disorders as their chief concern. Alternatively, nail conditions may be encountered by the examining physician as an incidental finding that may be a clue to underlying systemic disease. Competence in the diagnosis and treatment of nail diseases can drastically improve patient quality of life and can be lifesaving,1 but many dermatologists find management of nail diseases challenging.2 Bridging this educational gap begins with dermatology resident and medical student education. In a collaboration with dermatology educators, the American Academy of Dermatology (AAD) prepared a free online core curriculum for medical students that covers the essential concepts of dermatology. We sought to determine the integration of nail education in the AAD Basic Dermatology Curriculum.
Methods
A cross-sectional study of the AAD Basic Dermatology Curriculum was conducted to determine nail disease content. The curriculum modules were downloaded in June 2018,
Results
Of 342 patients discussed in cases and quizzes, nails were mentioned for 19 patients (89 times total)(Table 1). Additionally, there were 2 mentions each of nail clippings and nail tumors, 0 mentions of nail biopsies, and 1 mention each of fungal cultures and microscopy on nail scrapings (Table 1). Of the 40 modules, nails were mentioned in 12 modules (Table 2) and 6 introductions to the modules (Table 1). There were no mentions of the terms nails, subungual, or onychomycosis in the learning objectives.3

Comment
Our study demonstrates a paucity of content relevant to nails in the AAD Basic Dermatology Curriculum. Medical students are missing an important opportunity to learn about diagnosis and management of nail conditions and may incorrectly conclude that nail expertise is not essential to becoming a competent board-certified dermatologist.
Particularly concerning is the exclusion of nail examinations in the skin exam module addressing full-body skin examinations (0 mentions in 31 slides). This curriculum may negatively influence medical students and may then follow at the resident level, with a study reporting that 50.3% (69/137) of residents examine nails only when the patient brings it to their attention.4
Most concerning was the inadequate coverage of nail unit melanoma in the melanoma module (1 mention in 53 slides). Furthermore, the ABCDE—asymmetry, border, color, diameter, and evolving—mnemonic for cutaneous melanoma was covered in 6 slides in this module, and the ABCDEF—family history added—mnemonic for nail unit melanoma was completely excluded. Not surprisingly, resident knowledge of melanonychia diagnosis is deficient, with a prior study demonstrating that 62% (88/142) of residents were not confident diagnosing and managing patients with melanonychia, and only 88% (125/142) of residents were aware of the nail melanoma mnemonic.4
Similarly, nail biopsy for melanonychia diagnosis was excluded from the curriculum, whereas skin biopsy was thoroughly discussed in the context of a cutaneous melanoma diagnosis. This deficient teaching may track to the dermatology resident curriculum, as a survey of third-year dermatology residents (N=240) showed that 58% performed 10 or fewer nail procedures, and one-third of residents felt incompetent in nail surgery.5
We acknowledge that the AAD Basic Dermatology Curriculum is simply an introduction to dermatology. However, given that dermatologists are among the major specialists who care for nail patients, we advocate for more content on nail diseases in this curriculum. Nails can easily be incorporated into existing modules, and a new module specifically dedicated to nail disease should be added. Moreover, we envision that our findings will positively reflect on competence in treating nail disease for dermatology residents.
Patients frequently present to dermatologists with nail disorders as their chief concern. Alternatively, nail conditions may be encountered by the examining physician as an incidental finding that may be a clue to underlying systemic disease. Competence in the diagnosis and treatment of nail diseases can drastically improve patient quality of life and can be lifesaving,1 but many dermatologists find management of nail diseases challenging.2 Bridging this educational gap begins with dermatology resident and medical student education. In a collaboration with dermatology educators, the American Academy of Dermatology (AAD) prepared a free online core curriculum for medical students that covers the essential concepts of dermatology. We sought to determine the integration of nail education in the AAD Basic Dermatology Curriculum.
Methods
A cross-sectional study of the AAD Basic Dermatology Curriculum was conducted to determine nail disease content. The curriculum modules were downloaded in June 2018,
Results
Of 342 patients discussed in cases and quizzes, nails were mentioned for 19 patients (89 times total)(Table 1). Additionally, there were 2 mentions each of nail clippings and nail tumors, 0 mentions of nail biopsies, and 1 mention each of fungal cultures and microscopy on nail scrapings (Table 1). Of the 40 modules, nails were mentioned in 12 modules (Table 2) and 6 introductions to the modules (Table 1). There were no mentions of the terms nails, subungual, or onychomycosis in the learning objectives.3

Comment
Our study demonstrates a paucity of content relevant to nails in the AAD Basic Dermatology Curriculum. Medical students are missing an important opportunity to learn about diagnosis and management of nail conditions and may incorrectly conclude that nail expertise is not essential to becoming a competent board-certified dermatologist.
Particularly concerning is the exclusion of nail examinations in the skin exam module addressing full-body skin examinations (0 mentions in 31 slides). This curriculum may negatively influence medical students and may then follow at the resident level, with a study reporting that 50.3% (69/137) of residents examine nails only when the patient brings it to their attention.4
Most concerning was the inadequate coverage of nail unit melanoma in the melanoma module (1 mention in 53 slides). Furthermore, the ABCDE—asymmetry, border, color, diameter, and evolving—mnemonic for cutaneous melanoma was covered in 6 slides in this module, and the ABCDEF—family history added—mnemonic for nail unit melanoma was completely excluded. Not surprisingly, resident knowledge of melanonychia diagnosis is deficient, with a prior study demonstrating that 62% (88/142) of residents were not confident diagnosing and managing patients with melanonychia, and only 88% (125/142) of residents were aware of the nail melanoma mnemonic.4
Similarly, nail biopsy for melanonychia diagnosis was excluded from the curriculum, whereas skin biopsy was thoroughly discussed in the context of a cutaneous melanoma diagnosis. This deficient teaching may track to the dermatology resident curriculum, as a survey of third-year dermatology residents (N=240) showed that 58% performed 10 or fewer nail procedures, and one-third of residents felt incompetent in nail surgery.5
We acknowledge that the AAD Basic Dermatology Curriculum is simply an introduction to dermatology. However, given that dermatologists are among the major specialists who care for nail patients, we advocate for more content on nail diseases in this curriculum. Nails can easily be incorporated into existing modules, and a new module specifically dedicated to nail disease should be added. Moreover, we envision that our findings will positively reflect on competence in treating nail disease for dermatology residents.
- Lipner SR. Ulcerated nodule of the fingernail. JAMA. 2018;319:713-714.
- Hare AQ, Rich P. Clinical and educational gaps in diagnosis of nail disorders. Dermatol Clin. 2016;34:269-273.
- American Academy of Dermatology. Basic Dermatology Curriculum. https://www.aad.org/education/basic-derm-curriculum. Accessed March 25, 2019.
- Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996.
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483, 483.e1-5.
- Lipner SR. Ulcerated nodule of the fingernail. JAMA. 2018;319:713-714.
- Hare AQ, Rich P. Clinical and educational gaps in diagnosis of nail disorders. Dermatol Clin. 2016;34:269-273.
- American Academy of Dermatology. Basic Dermatology Curriculum. https://www.aad.org/education/basic-derm-curriculum. Accessed March 25, 2019.
- Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996.
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483, 483.e1-5.
Practice Points
- Competence in the diagnosis and treatment of nail diseases can drastically improve patient quality of life and can be lifesaving.
- Education on diagnosis and management of nail conditions is deficient in the American Academy of Dermatology (AAD) Basic Dermatology Curriculum.
- Increased efforts are needed to incorporate relevant nail education materials into the AAD Basic Dermatology Curriculum.