User login
Acantholytic Anaplastic Extramammary Paget Disease
To the Editor:
Extramammary Paget disease (EMPD) is a rare intraepidermal neoplasm with glandular differentiation that is classically known as a mimicker of Bowen disease (squamous cell carcinoma in situ of the skin) due to their histologic similarities.1,2 However, acantholytic anaplastic EMPD (AAEMPD) is a rare variant that can pose a particularly difficult diagnostic challenge because of its histologic similarity to benign acantholytic disorders and other malignant neoplasms. Major histologic features suggestive of AAEMPD include full-thickness atypia of the epidermis, loss of nuclear polarity, marked cytologic anaplasia, intraepidermal acantholysis, and Paget cells.3 The differential diagnosis of EMPD typically includes Bowen disease and pagetoid Bowen disease, but the acantholytic anaplastic variant more often is confused with intraepidermal acantholytic lesions such as acantholytic dyskeratosis of the genitocrural area, familial benign pemphigus (Hailey-Hailey disease), pemphigus vulgaris, and acantholytic Bowen disease. Immunohistochemistry (IHC) studies to assist in the definitive diagnosis of AAEMPD are strongly advised because of these difficulties in diagnosis.4 Cases of EMPD with an acantholytic appearance have rarely been reported in the literature.5-7
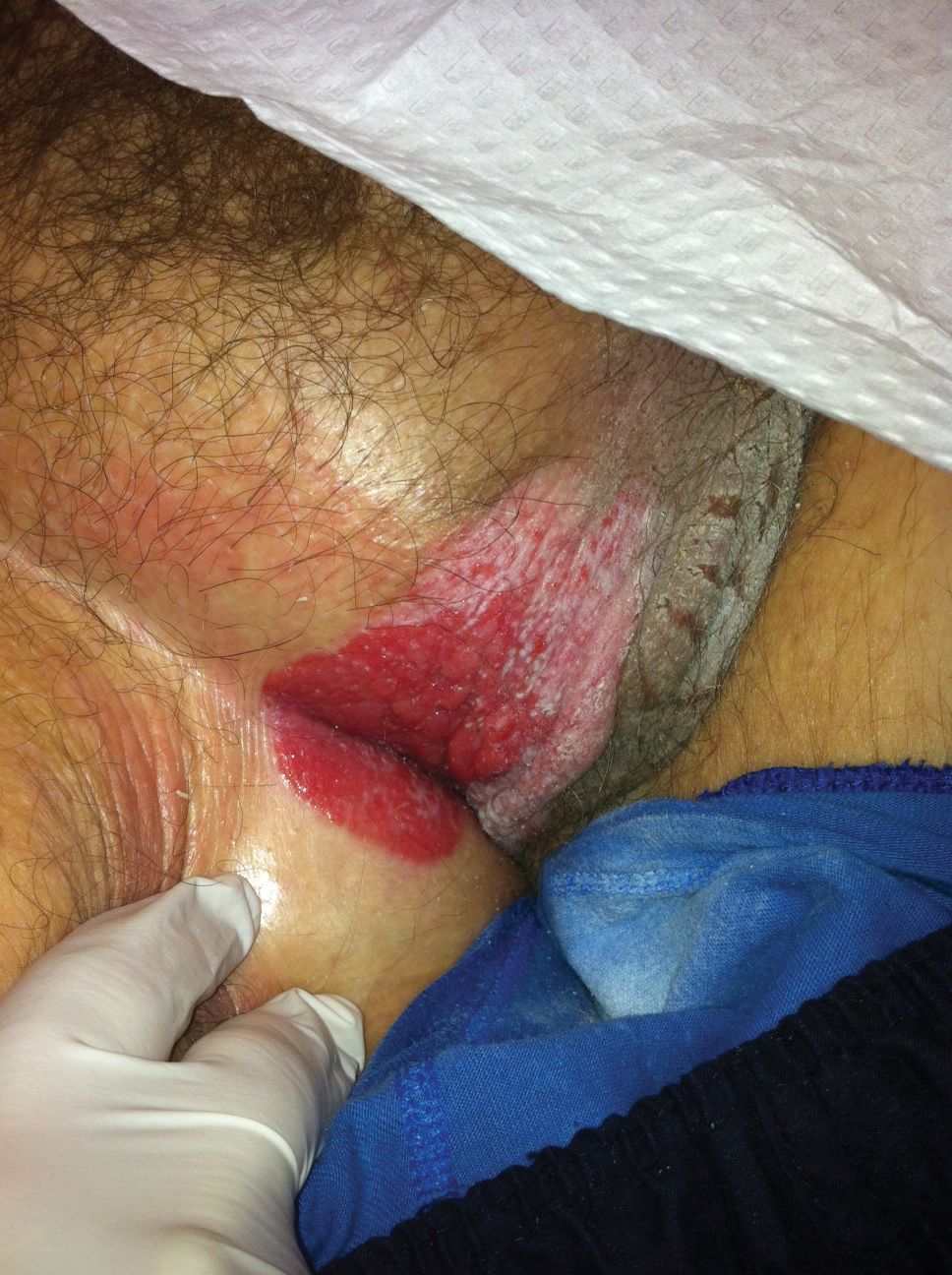
A 78-year-old man with a history of arthritis, heart disease, hypertension, and gastrointestinal disease presented for evaluation of a tender lesion of the right genitocrural crease of 5 years’ duration. He had no history of cutaneous or internal malignancy. Previously the lesion had been treated by dermatology with a variety of topical products including antifungal and antibiotic creams with no improvement. Physical examination revealed a well-defined, 7×5-cm, tender, erythematous, macerated plaque on the right upper inner thigh adjacent to the scrotum with an odor possibly due to secondary infection (Figure 1).
plaque on the right upper inner thigh adjacent to the scrotum.
A biopsy of the lesion was performed, and the specimen was submitted for pathologic examination. Bacterial cultures taken at the time of biopsy revealed polybacterial colonization with Acinetobacter, Morganella, and mixed skin flora. The patient was treated with a 10-day course of oral sulfamethoxazole 800 mg and trimethoprim 160 mg twice daily once culture results returned. The biopsy results were communicated to the patient; however, he subsequently relocated, assumed care at another facility, and has since been lost to follow-up.
The biopsy specimen was examined grossly, serially sectioned, and submitted for routine processing with hematoxylin and eosin, periodic acid–Schiff, and Hale colloidal iron staining. Routine IHC was performed with antibodies to cytokeratin (CK) 7, CK20, carcinoembryonic antigen (CEA), pancytokeratin (CKAE1/AE3), and low- molecular-weight cytokeratin (LMWCK).
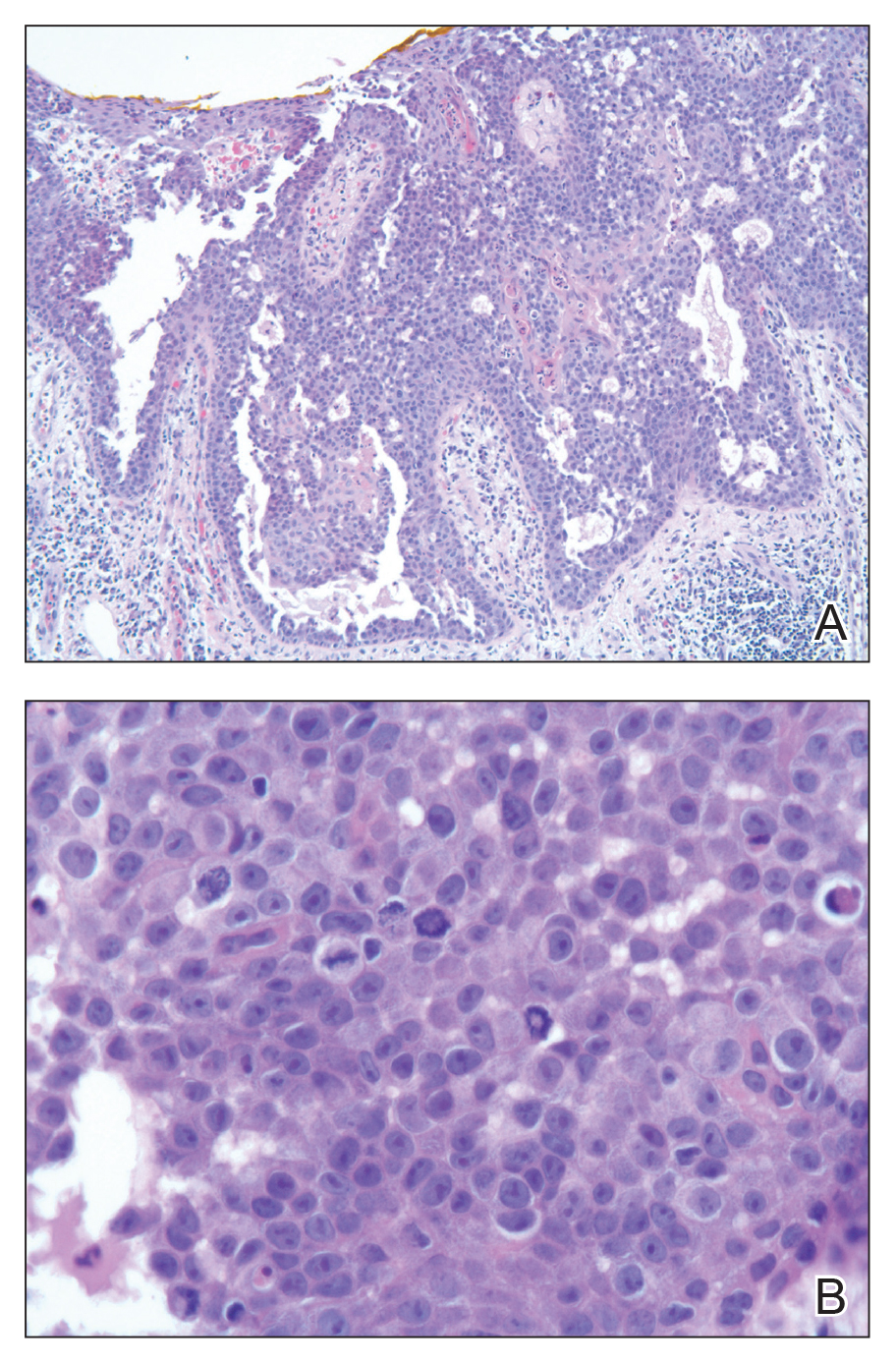
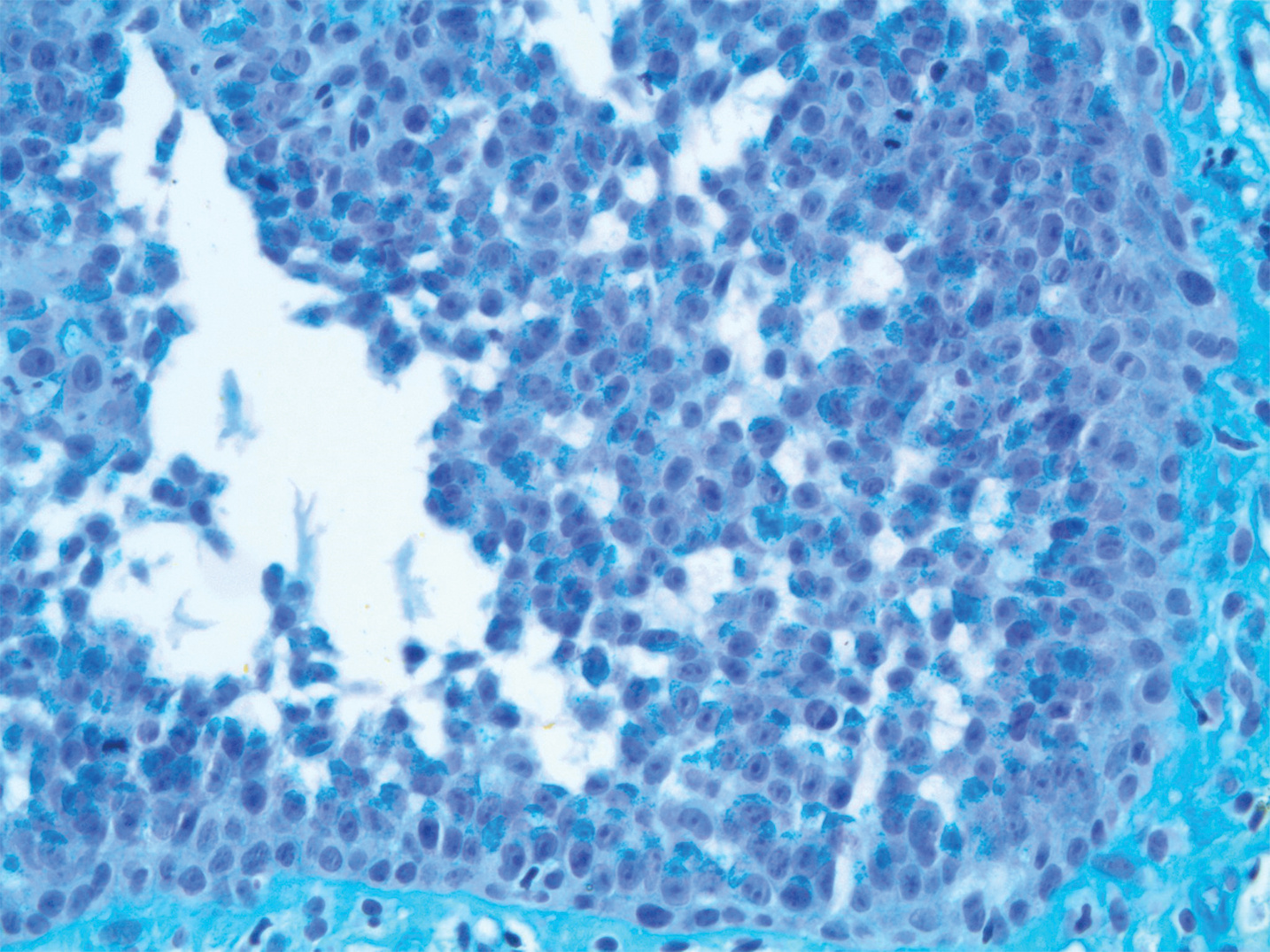
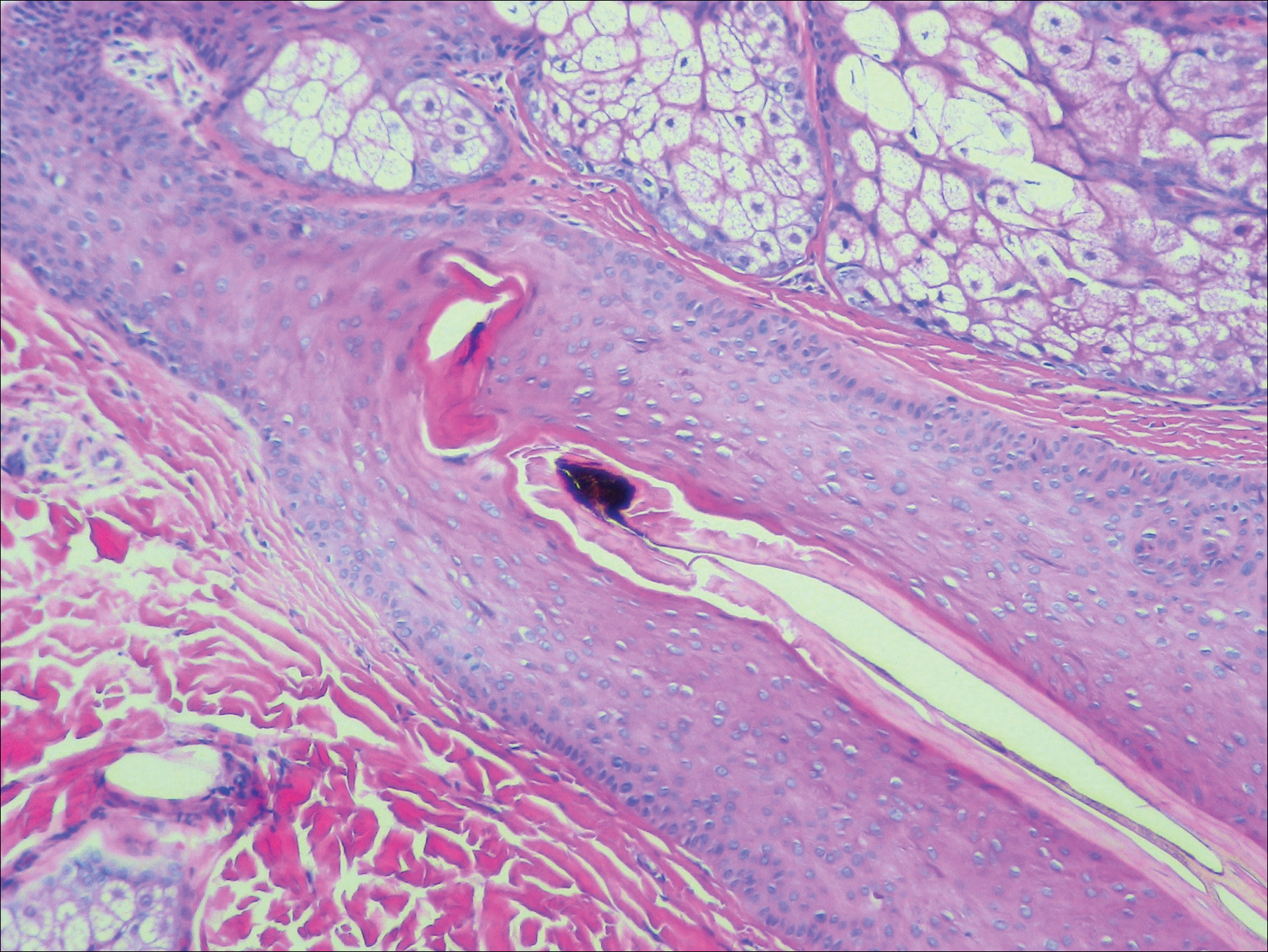
Pathologic examination of the biopsy showed prominent acanthosis of the epidermis composed of a proliferation of epithelial cells with associated full-thickness suprabasal acantholysis (Figure 2A). On inspection at higher magnification, the neoplastic cells demonstrated anaplasia as cytologic atypia with prominent and frequently multiple nucleoli, scant cytoplasm, and a high nuclear to cytoplasmic ratio (Figure 2B). There was a marked increase in mitotic activity with as many as 5 mitotic figures per high-power field. A fairly dense mixed inflammatory infiltrate comprised of lymphocytes, plasma cells, neutrophils, and eosinophils was present in the dermis. No fungal elements were observed on periodic acid–Schiff staining. The vast majority of tumor cells demonstrated moderate to abundant cytoplasmic mucin on Hale colloidal iron staining (Figure 3).
(H&E, original magnification ×400).
Immunohistochemistry staining of tumor cells was positive for CK7, CEA, pancytokeratin (CKAE1/AE3), and LMWCK. The tumor cells were negative for CK20. On the basis of the histopathologic and IHC findings, the patient was diagnosed with AAEMPD.
Extramammary Paget disease is a rare intraepidermal neoplasm with glandular differentiation. The most commonly involved sites are the anogenital areas including the vulvar, perianal, perineal, scrotal, and penile regions, as well as other areas rich in apocrine glands such as the axillae.8 Extramammary Paget disease most commonly originates as a primary intraepidermal neoplasm (type 1 EMPD), but an underlying malignant neoplasm that spreads intraepithelially is seen in a minority of cases (types 2 and 3 EMPD). In the vulva, type 1a refers to cutaneous noninvasive Paget disease, type 1b refers to dermal invasion of Paget disease, type 1c refers to vulvar adenocarcinoma–associated Paget disease, type 2 refers to rectal/anal adenocarcinoma–associated Paget disease, and type 3 refers to urogenital neoplasia–associated Paget disease.9
The acantholytic anaplastic variant of EMPD can be challenging to diagnose because of its similarities to many other lesions, including acantholytic dyskeratosis of the genitocrural area, familial benign pemphigus (Hailey-Hailey disease), pemphigus vulgaris, Bowen disease, pagetoid Bowen disease, and acantholytic Bowen disease. Major histologic features of AAEMPD include full-thickness atypia of the epidermis, loss of nuclear polarity, marked cytologic anaplasia, intraepidermal acantholysis, and Paget cells.3 The acantholytic anaplastic variant of EMPD can be differentiated from other diagnoses using IHC studies, with findings indicative of AAEMPD outlined below.
The proliferative neoplastic cell in EMPD is the Paget cell, which can be identified as a large round cell located in the epidermis with pale-staining cytoplasm, a large nucleus, and sometimes a prominent nucleolus. Paget cells can be distributed singly or in clusters, nests, or glandular structures within the epidermis and adjacent to adnexal structures.10 Extramammary Paget disease can have many patterns, including glandular, acantholysis-like, upper nest, tall nest, budding, and sheetlike.11
Immunohistochemically, Paget cells in EMPD typically express pancytokeratins (CKAE1/AE3), low-molecular-weight/simple epithelial type keratins (CK7, CAM 5.2), sweat gland antigens (epithelial membrane antigen, CEA, gross cystic disease fluid protein 15 [GCDFP15]), mucin 5AC (MUC5AC), and often androgen receptor.12-18 Paget cells contain cytoplasmic mucin and demonstrate prominent cytoplasmic staining with Hale colloidal iron.17 Paget cells typically do not express high-molecular-weight cytokeratin (eg, CK5/6), melanocytic antigens, estrogen receptor, or progesterone receptor.15,18
Immunohistochemical staining has been shown to differ between primary cutaneous (type 1) and secondary (types 2 and 3) EMPD. Primary cutaneous EMPD typically expresses sweat gland markers (CK7+, CK20−, GCDFP15+). Secondary EMPD typically expresses an endodermal phenotype (CK7+, CK20+, GCDFP15−).12
Acantholytic dyskeratosis of the genitocrural area is a rare lesion included in the spectrum of focal acantholytic dyskeratoses described by Ackerman.19 It also has been referred to as papular acantholytic dyskeratosis of the vulva, though histologically similar lesions also have been reported in men.20-22 Histologically, acantholytic dyskeratosis of the genitocrural area has prominent acantholysis and dyskeratosis with corps ronds and grains.19 Familial benign pemphigus (Hailey-Hailey disease) is caused by mutations of the ATP2C1 gene, which encodes for a secretory pathway Ca2+/Mn2+-ATPase pump type 1 (SPCA1) in the Golgi apparatus in keratinocytes.23 Familial benign pemphigus has a histologic appearance similar to acantholytic dyskeratosis of the genitocrural area, but a positive family history of familial benign pemphigus can be used to differentiate the 2 entities from each other due to the autosomal-dominant inheritance pattern of familial benign pemphigus. Both of these disorders can appear similar to AAEMPD because of their extensive intraepidermal acantholysis, but they differ in the lack of Paget cells, intraepidermal atypia, and increased mitotic activity.
Acantholytic Bowen disease is a histologic variant that can be difficult to distinguish from AAEMPD on hematoxylin and eosin–stained sections because of their similar histologic features but can be differentiated by IHC stains.5 Acantholytic Bowen disease expresses high-molecular-weight cytokeratin (eg, CK5/6) but is negative for CK7, CAM 5.2, and CEA. Extramammary Paget disease generally has the opposite pattern: positive staining for CK7, CAM 5.2, and CEA, but negative for high-molecular-weight cytokeratin.13,14,24
Primary cutaneous adenosquamous carcinoma is a rare malignancy of squamous and glandular differentiation known for being locally aggressive and metastatic.25 Histologically, cutaneous adenosquamous carcinoma shows infiltrating nests of neoplastic cells with both squamous and glandular features. It differs notably from AAEMPD in that cutaneous adenosquamous carcinomas tend to arise in the head and arm regions, and their histologic morphology is different. The IHC profiles are similar, with positive staining for CEA, CK7, and mucin; however, they differ in that AAEMPD is negative for high-molecular-weight keratin while cutaneous adenosquamous carcinoma is positive.25
Verrucous carcinoma is an uncommon variant of squamous cell carcinoma with well-differentiated keratinocytes and a blunt pushing border.24 Similar to AAEMPD, this neoplasm can arise in the genital and perineal areas; however, the 2 entities differ considerably in morphology on histologic examination.
Pemphigus vulgaris is an autoimmune intraepidermal blistering disorder of the skin and mucous membranes of which pemphigus vegetans is a subtype.26,27 Pemphigus vulgaris is another diagnosis that can possibly be mimicked by AAEMPD.28 Histologic features of pemphigus vulgaris include intraepidermal acantholysis of keratinocytes immediately above the basal layer of the epidermis. Pemphigus vegetans is similar with the addition of papillomatosis, hyperkeratosis, and an eosinophilic infiltrate.26,27 Immunofluorescence typically demonstrates intercellular C3 and IgG deposits.26 These diseases mimic AAEMPD histologically but differ in their relative lack of atypia and Paget cells.
In summary, we report a case of AAEMPD in a 78-year-old man in whom routine histologic specimens showed marked intraepidermal acantholysis and atypical tumor cells with increased mitoses. The latter finding prompted IHC studies that revealed positive CK7, CEA, pancytokeratin, and LMWCK staining with negative CK20 staining. Hale colloidal iron staining showed moderate to abundant cytoplasmic mucin. The patient was diagnosed with AAEMPD. It is imperative to maintain clinical suspicion for AAEMPD and to examine acantholytic disorders with scrutiny. When there is evidence of atypia or mitoses, use of IHC stains can assist in fully characterizing the lesion.
- Bowen JT. Precancerous dermatosis: a study of two cases of chronic atypical epithelial proliferation. J Cutan Dis. 1912;30:241-255.
- Jones RE Jr, Austin C, Ackerman AB. Extramammary Paget’s disease: a critical reexamination. Am J Dermatopathol. 1979;1:101-132.
- Rayne SC, Santa Cruz DJ. Anaplastic Paget’s disease. Am J Surg Pathol. 1992;16:1085-1091.
- Wang EC, Kwah YC, Tan WP, et al. Extramammary Paget disease: immunohistochemistry is critical to distinguish potential mimickers. Dermatol Online J. 2012;18:4.
- Du X, Yin X, Zhou N, et al. Extramammary Paget’s disease mimicking acantholytic squamous cell carcinoma in situ: a case report. J Cutan Pathol. 2010;37:683.
- Mobini N. Acantholytic anaplastic Paget’s disease. J Cutan Pathol. 2009;36:374-380.
- Oh YJ, Lew BL, Sim WY. Acantholytic anaplastic extramammary Paget’s disease: a case report and review of the literature. Ann Dermatol. 2011;23:226-230.
- Zollo JD, Zeitouni NC. The Roswell Park Cancer Institute experience with extramammary Paget’s disease. Br J Dermatol. 2000;142:59-65.
- Wilkinson EJ, Brown HM. Vulvar Paget disease of urothelial origin: a report of three cases and a proposed classification of vulvar Paget disease. Hum Pathol. 2002;33:549-554.
- Lam C, Funaro D. Extramammary Paget’s disease: summary of current knowledge. Dermatol Clin. 2010;28:807-826.
- Shiomi T, Yoshida Y, Shomori K, et al. Extramammary Paget’s disease: evaluation of the histopathological patterns of Paget cell proliferation in the epidermis. J Dermatol. 2011;38:1054-1057.
- Goldblum JR, Hart WR. Vulvar Paget’s disease: a clinicopathologic and immunohistochemical study of 19 cases. Am J Surg Pathol. 1997;21:1178-1187.
- Alhumaidi A. Practical immunohistochemistry of epithelial skin tumor. Indian J Dermatol Venerol Leprol. 2012;78:698-708.
- Battles O, Page D, Johnson J. Cytokeratins, CEA and mucin histochemistry in the diagnosis and characterization of extramammary Paget’s disease. Am J Clin Pathol. 1997;108:6-12.
- Kanitakis J. Mammary and extramammary Paget’s disease. J Eur Acad Dermatol Venereol. 2007;21:581-590.
- Krishna M. Diagnosis of metastatic neoplasms: an immunohistochemical approach. Arch Pathol Lab Med. 2010;134:207-215.
- Helm KF, Goellner JR, Peters MS. Immunohistochemical stain in extramammary Paget’s disease. Am J Dermatopathol. 1992;14:402-407.
- Liegl B, Horn L, Moinfar F. Androgen receptors are frequently expressed in mammary and extramammary Paget’s disease. Mod Pathol. 2005;18:1283-288.
- Ackerman AB. Focal acantholytic dyskeratosis. Arch Derm. 1972;106:702-706.
- Dittmer CJ, Hornemann A, Rose C, et al. Successful laser therapy of a papular acantholytic dyskeratosis of the vulva: case report and review of literature. Arch Gynecol Obstet. 2010;291:723-725.
- Roh MR, Choi YJ, Lee KG. Papular acantholytic dyskeratosis of the vulva. J Dermatol. 2009;36:427-429.
- Wong KT, Mihm MC Jr. Acantholytic dermatosis localized to genitalia and crural areas of male patients: a report of three cases. J Cutan Pathol. 1994;21:27-32.
- Hu Z, Bonifas JM, Beech J, et al. Mutations in ATP2C1, encoding a calcium pump, cause Hailey-Hailey disease. Nat Genet. 2000; 24:61-65.
- Elston DM. Malignant tumors of the epidermis. In: Elston DM, Ferringer T, eds. Requisites in Dermatology: Dermatopathology. Philadelphia, PA: Elsevier Limited; 2012:53-68.
- Fu JM, McCalmont T, Yu SS. Adenosquamous carcinoma of the skin: a case series. Arch Dermatol. 2009;145:1152-1158.
- Becker BA, Gaspari AA. Pemphigus vulgaris and vegetans. Dermatol Clin. 1993;11:429-452.
- Rados J. Autoimmune blistering diseases: histologic meaning. Clin Dermatol. 2011;29:377-388.
- Kohler S, Smoller BR. A case of extramammary Paget’s disease mimicking pemphigus vulgaris on histologic examination. Dermatology. 1997;195:54-56.
To the Editor:
Extramammary Paget disease (EMPD) is a rare intraepidermal neoplasm with glandular differentiation that is classically known as a mimicker of Bowen disease (squamous cell carcinoma in situ of the skin) due to their histologic similarities.1,2 However, acantholytic anaplastic EMPD (AAEMPD) is a rare variant that can pose a particularly difficult diagnostic challenge because of its histologic similarity to benign acantholytic disorders and other malignant neoplasms. Major histologic features suggestive of AAEMPD include full-thickness atypia of the epidermis, loss of nuclear polarity, marked cytologic anaplasia, intraepidermal acantholysis, and Paget cells.3 The differential diagnosis of EMPD typically includes Bowen disease and pagetoid Bowen disease, but the acantholytic anaplastic variant more often is confused with intraepidermal acantholytic lesions such as acantholytic dyskeratosis of the genitocrural area, familial benign pemphigus (Hailey-Hailey disease), pemphigus vulgaris, and acantholytic Bowen disease. Immunohistochemistry (IHC) studies to assist in the definitive diagnosis of AAEMPD are strongly advised because of these difficulties in diagnosis.4 Cases of EMPD with an acantholytic appearance have rarely been reported in the literature.5-7
A 78-year-old man with a history of arthritis, heart disease, hypertension, and gastrointestinal disease presented for evaluation of a tender lesion of the right genitocrural crease of 5 years’ duration. He had no history of cutaneous or internal malignancy. Previously the lesion had been treated by dermatology with a variety of topical products including antifungal and antibiotic creams with no improvement. Physical examination revealed a well-defined, 7×5-cm, tender, erythematous, macerated plaque on the right upper inner thigh adjacent to the scrotum with an odor possibly due to secondary infection (Figure 1).
plaque on the right upper inner thigh adjacent to the scrotum.
A biopsy of the lesion was performed, and the specimen was submitted for pathologic examination. Bacterial cultures taken at the time of biopsy revealed polybacterial colonization with Acinetobacter, Morganella, and mixed skin flora. The patient was treated with a 10-day course of oral sulfamethoxazole 800 mg and trimethoprim 160 mg twice daily once culture results returned. The biopsy results were communicated to the patient; however, he subsequently relocated, assumed care at another facility, and has since been lost to follow-up.
The biopsy specimen was examined grossly, serially sectioned, and submitted for routine processing with hematoxylin and eosin, periodic acid–Schiff, and Hale colloidal iron staining. Routine IHC was performed with antibodies to cytokeratin (CK) 7, CK20, carcinoembryonic antigen (CEA), pancytokeratin (CKAE1/AE3), and low- molecular-weight cytokeratin (LMWCK).
Pathologic examination of the biopsy showed prominent acanthosis of the epidermis composed of a proliferation of epithelial cells with associated full-thickness suprabasal acantholysis (Figure 2A). On inspection at higher magnification, the neoplastic cells demonstrated anaplasia as cytologic atypia with prominent and frequently multiple nucleoli, scant cytoplasm, and a high nuclear to cytoplasmic ratio (Figure 2B). There was a marked increase in mitotic activity with as many as 5 mitotic figures per high-power field. A fairly dense mixed inflammatory infiltrate comprised of lymphocytes, plasma cells, neutrophils, and eosinophils was present in the dermis. No fungal elements were observed on periodic acid–Schiff staining. The vast majority of tumor cells demonstrated moderate to abundant cytoplasmic mucin on Hale colloidal iron staining (Figure 3).
(H&E, original magnification ×400).
Immunohistochemistry staining of tumor cells was positive for CK7, CEA, pancytokeratin (CKAE1/AE3), and LMWCK. The tumor cells were negative for CK20. On the basis of the histopathologic and IHC findings, the patient was diagnosed with AAEMPD.
Extramammary Paget disease is a rare intraepidermal neoplasm with glandular differentiation. The most commonly involved sites are the anogenital areas including the vulvar, perianal, perineal, scrotal, and penile regions, as well as other areas rich in apocrine glands such as the axillae.8 Extramammary Paget disease most commonly originates as a primary intraepidermal neoplasm (type 1 EMPD), but an underlying malignant neoplasm that spreads intraepithelially is seen in a minority of cases (types 2 and 3 EMPD). In the vulva, type 1a refers to cutaneous noninvasive Paget disease, type 1b refers to dermal invasion of Paget disease, type 1c refers to vulvar adenocarcinoma–associated Paget disease, type 2 refers to rectal/anal adenocarcinoma–associated Paget disease, and type 3 refers to urogenital neoplasia–associated Paget disease.9
The acantholytic anaplastic variant of EMPD can be challenging to diagnose because of its similarities to many other lesions, including acantholytic dyskeratosis of the genitocrural area, familial benign pemphigus (Hailey-Hailey disease), pemphigus vulgaris, Bowen disease, pagetoid Bowen disease, and acantholytic Bowen disease. Major histologic features of AAEMPD include full-thickness atypia of the epidermis, loss of nuclear polarity, marked cytologic anaplasia, intraepidermal acantholysis, and Paget cells.3 The acantholytic anaplastic variant of EMPD can be differentiated from other diagnoses using IHC studies, with findings indicative of AAEMPD outlined below.
The proliferative neoplastic cell in EMPD is the Paget cell, which can be identified as a large round cell located in the epidermis with pale-staining cytoplasm, a large nucleus, and sometimes a prominent nucleolus. Paget cells can be distributed singly or in clusters, nests, or glandular structures within the epidermis and adjacent to adnexal structures.10 Extramammary Paget disease can have many patterns, including glandular, acantholysis-like, upper nest, tall nest, budding, and sheetlike.11
Immunohistochemically, Paget cells in EMPD typically express pancytokeratins (CKAE1/AE3), low-molecular-weight/simple epithelial type keratins (CK7, CAM 5.2), sweat gland antigens (epithelial membrane antigen, CEA, gross cystic disease fluid protein 15 [GCDFP15]), mucin 5AC (MUC5AC), and often androgen receptor.12-18 Paget cells contain cytoplasmic mucin and demonstrate prominent cytoplasmic staining with Hale colloidal iron.17 Paget cells typically do not express high-molecular-weight cytokeratin (eg, CK5/6), melanocytic antigens, estrogen receptor, or progesterone receptor.15,18
Immunohistochemical staining has been shown to differ between primary cutaneous (type 1) and secondary (types 2 and 3) EMPD. Primary cutaneous EMPD typically expresses sweat gland markers (CK7+, CK20−, GCDFP15+). Secondary EMPD typically expresses an endodermal phenotype (CK7+, CK20+, GCDFP15−).12
Acantholytic dyskeratosis of the genitocrural area is a rare lesion included in the spectrum of focal acantholytic dyskeratoses described by Ackerman.19 It also has been referred to as papular acantholytic dyskeratosis of the vulva, though histologically similar lesions also have been reported in men.20-22 Histologically, acantholytic dyskeratosis of the genitocrural area has prominent acantholysis and dyskeratosis with corps ronds and grains.19 Familial benign pemphigus (Hailey-Hailey disease) is caused by mutations of the ATP2C1 gene, which encodes for a secretory pathway Ca2+/Mn2+-ATPase pump type 1 (SPCA1) in the Golgi apparatus in keratinocytes.23 Familial benign pemphigus has a histologic appearance similar to acantholytic dyskeratosis of the genitocrural area, but a positive family history of familial benign pemphigus can be used to differentiate the 2 entities from each other due to the autosomal-dominant inheritance pattern of familial benign pemphigus. Both of these disorders can appear similar to AAEMPD because of their extensive intraepidermal acantholysis, but they differ in the lack of Paget cells, intraepidermal atypia, and increased mitotic activity.
Acantholytic Bowen disease is a histologic variant that can be difficult to distinguish from AAEMPD on hematoxylin and eosin–stained sections because of their similar histologic features but can be differentiated by IHC stains.5 Acantholytic Bowen disease expresses high-molecular-weight cytokeratin (eg, CK5/6) but is negative for CK7, CAM 5.2, and CEA. Extramammary Paget disease generally has the opposite pattern: positive staining for CK7, CAM 5.2, and CEA, but negative for high-molecular-weight cytokeratin.13,14,24
Primary cutaneous adenosquamous carcinoma is a rare malignancy of squamous and glandular differentiation known for being locally aggressive and metastatic.25 Histologically, cutaneous adenosquamous carcinoma shows infiltrating nests of neoplastic cells with both squamous and glandular features. It differs notably from AAEMPD in that cutaneous adenosquamous carcinomas tend to arise in the head and arm regions, and their histologic morphology is different. The IHC profiles are similar, with positive staining for CEA, CK7, and mucin; however, they differ in that AAEMPD is negative for high-molecular-weight keratin while cutaneous adenosquamous carcinoma is positive.25
Verrucous carcinoma is an uncommon variant of squamous cell carcinoma with well-differentiated keratinocytes and a blunt pushing border.24 Similar to AAEMPD, this neoplasm can arise in the genital and perineal areas; however, the 2 entities differ considerably in morphology on histologic examination.
Pemphigus vulgaris is an autoimmune intraepidermal blistering disorder of the skin and mucous membranes of which pemphigus vegetans is a subtype.26,27 Pemphigus vulgaris is another diagnosis that can possibly be mimicked by AAEMPD.28 Histologic features of pemphigus vulgaris include intraepidermal acantholysis of keratinocytes immediately above the basal layer of the epidermis. Pemphigus vegetans is similar with the addition of papillomatosis, hyperkeratosis, and an eosinophilic infiltrate.26,27 Immunofluorescence typically demonstrates intercellular C3 and IgG deposits.26 These diseases mimic AAEMPD histologically but differ in their relative lack of atypia and Paget cells.
In summary, we report a case of AAEMPD in a 78-year-old man in whom routine histologic specimens showed marked intraepidermal acantholysis and atypical tumor cells with increased mitoses. The latter finding prompted IHC studies that revealed positive CK7, CEA, pancytokeratin, and LMWCK staining with negative CK20 staining. Hale colloidal iron staining showed moderate to abundant cytoplasmic mucin. The patient was diagnosed with AAEMPD. It is imperative to maintain clinical suspicion for AAEMPD and to examine acantholytic disorders with scrutiny. When there is evidence of atypia or mitoses, use of IHC stains can assist in fully characterizing the lesion.
To the Editor:
Extramammary Paget disease (EMPD) is a rare intraepidermal neoplasm with glandular differentiation that is classically known as a mimicker of Bowen disease (squamous cell carcinoma in situ of the skin) due to their histologic similarities.1,2 However, acantholytic anaplastic EMPD (AAEMPD) is a rare variant that can pose a particularly difficult diagnostic challenge because of its histologic similarity to benign acantholytic disorders and other malignant neoplasms. Major histologic features suggestive of AAEMPD include full-thickness atypia of the epidermis, loss of nuclear polarity, marked cytologic anaplasia, intraepidermal acantholysis, and Paget cells.3 The differential diagnosis of EMPD typically includes Bowen disease and pagetoid Bowen disease, but the acantholytic anaplastic variant more often is confused with intraepidermal acantholytic lesions such as acantholytic dyskeratosis of the genitocrural area, familial benign pemphigus (Hailey-Hailey disease), pemphigus vulgaris, and acantholytic Bowen disease. Immunohistochemistry (IHC) studies to assist in the definitive diagnosis of AAEMPD are strongly advised because of these difficulties in diagnosis.4 Cases of EMPD with an acantholytic appearance have rarely been reported in the literature.5-7
A 78-year-old man with a history of arthritis, heart disease, hypertension, and gastrointestinal disease presented for evaluation of a tender lesion of the right genitocrural crease of 5 years’ duration. He had no history of cutaneous or internal malignancy. Previously the lesion had been treated by dermatology with a variety of topical products including antifungal and antibiotic creams with no improvement. Physical examination revealed a well-defined, 7×5-cm, tender, erythematous, macerated plaque on the right upper inner thigh adjacent to the scrotum with an odor possibly due to secondary infection (Figure 1).
plaque on the right upper inner thigh adjacent to the scrotum.
A biopsy of the lesion was performed, and the specimen was submitted for pathologic examination. Bacterial cultures taken at the time of biopsy revealed polybacterial colonization with Acinetobacter, Morganella, and mixed skin flora. The patient was treated with a 10-day course of oral sulfamethoxazole 800 mg and trimethoprim 160 mg twice daily once culture results returned. The biopsy results were communicated to the patient; however, he subsequently relocated, assumed care at another facility, and has since been lost to follow-up.
The biopsy specimen was examined grossly, serially sectioned, and submitted for routine processing with hematoxylin and eosin, periodic acid–Schiff, and Hale colloidal iron staining. Routine IHC was performed with antibodies to cytokeratin (CK) 7, CK20, carcinoembryonic antigen (CEA), pancytokeratin (CKAE1/AE3), and low- molecular-weight cytokeratin (LMWCK).
Pathologic examination of the biopsy showed prominent acanthosis of the epidermis composed of a proliferation of epithelial cells with associated full-thickness suprabasal acantholysis (Figure 2A). On inspection at higher magnification, the neoplastic cells demonstrated anaplasia as cytologic atypia with prominent and frequently multiple nucleoli, scant cytoplasm, and a high nuclear to cytoplasmic ratio (Figure 2B). There was a marked increase in mitotic activity with as many as 5 mitotic figures per high-power field. A fairly dense mixed inflammatory infiltrate comprised of lymphocytes, plasma cells, neutrophils, and eosinophils was present in the dermis. No fungal elements were observed on periodic acid–Schiff staining. The vast majority of tumor cells demonstrated moderate to abundant cytoplasmic mucin on Hale colloidal iron staining (Figure 3).
(H&E, original magnification ×400).
Immunohistochemistry staining of tumor cells was positive for CK7, CEA, pancytokeratin (CKAE1/AE3), and LMWCK. The tumor cells were negative for CK20. On the basis of the histopathologic and IHC findings, the patient was diagnosed with AAEMPD.
Extramammary Paget disease is a rare intraepidermal neoplasm with glandular differentiation. The most commonly involved sites are the anogenital areas including the vulvar, perianal, perineal, scrotal, and penile regions, as well as other areas rich in apocrine glands such as the axillae.8 Extramammary Paget disease most commonly originates as a primary intraepidermal neoplasm (type 1 EMPD), but an underlying malignant neoplasm that spreads intraepithelially is seen in a minority of cases (types 2 and 3 EMPD). In the vulva, type 1a refers to cutaneous noninvasive Paget disease, type 1b refers to dermal invasion of Paget disease, type 1c refers to vulvar adenocarcinoma–associated Paget disease, type 2 refers to rectal/anal adenocarcinoma–associated Paget disease, and type 3 refers to urogenital neoplasia–associated Paget disease.9
The acantholytic anaplastic variant of EMPD can be challenging to diagnose because of its similarities to many other lesions, including acantholytic dyskeratosis of the genitocrural area, familial benign pemphigus (Hailey-Hailey disease), pemphigus vulgaris, Bowen disease, pagetoid Bowen disease, and acantholytic Bowen disease. Major histologic features of AAEMPD include full-thickness atypia of the epidermis, loss of nuclear polarity, marked cytologic anaplasia, intraepidermal acantholysis, and Paget cells.3 The acantholytic anaplastic variant of EMPD can be differentiated from other diagnoses using IHC studies, with findings indicative of AAEMPD outlined below.
The proliferative neoplastic cell in EMPD is the Paget cell, which can be identified as a large round cell located in the epidermis with pale-staining cytoplasm, a large nucleus, and sometimes a prominent nucleolus. Paget cells can be distributed singly or in clusters, nests, or glandular structures within the epidermis and adjacent to adnexal structures.10 Extramammary Paget disease can have many patterns, including glandular, acantholysis-like, upper nest, tall nest, budding, and sheetlike.11
Immunohistochemically, Paget cells in EMPD typically express pancytokeratins (CKAE1/AE3), low-molecular-weight/simple epithelial type keratins (CK7, CAM 5.2), sweat gland antigens (epithelial membrane antigen, CEA, gross cystic disease fluid protein 15 [GCDFP15]), mucin 5AC (MUC5AC), and often androgen receptor.12-18 Paget cells contain cytoplasmic mucin and demonstrate prominent cytoplasmic staining with Hale colloidal iron.17 Paget cells typically do not express high-molecular-weight cytokeratin (eg, CK5/6), melanocytic antigens, estrogen receptor, or progesterone receptor.15,18
Immunohistochemical staining has been shown to differ between primary cutaneous (type 1) and secondary (types 2 and 3) EMPD. Primary cutaneous EMPD typically expresses sweat gland markers (CK7+, CK20−, GCDFP15+). Secondary EMPD typically expresses an endodermal phenotype (CK7+, CK20+, GCDFP15−).12
Acantholytic dyskeratosis of the genitocrural area is a rare lesion included in the spectrum of focal acantholytic dyskeratoses described by Ackerman.19 It also has been referred to as papular acantholytic dyskeratosis of the vulva, though histologically similar lesions also have been reported in men.20-22 Histologically, acantholytic dyskeratosis of the genitocrural area has prominent acantholysis and dyskeratosis with corps ronds and grains.19 Familial benign pemphigus (Hailey-Hailey disease) is caused by mutations of the ATP2C1 gene, which encodes for a secretory pathway Ca2+/Mn2+-ATPase pump type 1 (SPCA1) in the Golgi apparatus in keratinocytes.23 Familial benign pemphigus has a histologic appearance similar to acantholytic dyskeratosis of the genitocrural area, but a positive family history of familial benign pemphigus can be used to differentiate the 2 entities from each other due to the autosomal-dominant inheritance pattern of familial benign pemphigus. Both of these disorders can appear similar to AAEMPD because of their extensive intraepidermal acantholysis, but they differ in the lack of Paget cells, intraepidermal atypia, and increased mitotic activity.
Acantholytic Bowen disease is a histologic variant that can be difficult to distinguish from AAEMPD on hematoxylin and eosin–stained sections because of their similar histologic features but can be differentiated by IHC stains.5 Acantholytic Bowen disease expresses high-molecular-weight cytokeratin (eg, CK5/6) but is negative for CK7, CAM 5.2, and CEA. Extramammary Paget disease generally has the opposite pattern: positive staining for CK7, CAM 5.2, and CEA, but negative for high-molecular-weight cytokeratin.13,14,24
Primary cutaneous adenosquamous carcinoma is a rare malignancy of squamous and glandular differentiation known for being locally aggressive and metastatic.25 Histologically, cutaneous adenosquamous carcinoma shows infiltrating nests of neoplastic cells with both squamous and glandular features. It differs notably from AAEMPD in that cutaneous adenosquamous carcinomas tend to arise in the head and arm regions, and their histologic morphology is different. The IHC profiles are similar, with positive staining for CEA, CK7, and mucin; however, they differ in that AAEMPD is negative for high-molecular-weight keratin while cutaneous adenosquamous carcinoma is positive.25
Verrucous carcinoma is an uncommon variant of squamous cell carcinoma with well-differentiated keratinocytes and a blunt pushing border.24 Similar to AAEMPD, this neoplasm can arise in the genital and perineal areas; however, the 2 entities differ considerably in morphology on histologic examination.
Pemphigus vulgaris is an autoimmune intraepidermal blistering disorder of the skin and mucous membranes of which pemphigus vegetans is a subtype.26,27 Pemphigus vulgaris is another diagnosis that can possibly be mimicked by AAEMPD.28 Histologic features of pemphigus vulgaris include intraepidermal acantholysis of keratinocytes immediately above the basal layer of the epidermis. Pemphigus vegetans is similar with the addition of papillomatosis, hyperkeratosis, and an eosinophilic infiltrate.26,27 Immunofluorescence typically demonstrates intercellular C3 and IgG deposits.26 These diseases mimic AAEMPD histologically but differ in their relative lack of atypia and Paget cells.
In summary, we report a case of AAEMPD in a 78-year-old man in whom routine histologic specimens showed marked intraepidermal acantholysis and atypical tumor cells with increased mitoses. The latter finding prompted IHC studies that revealed positive CK7, CEA, pancytokeratin, and LMWCK staining with negative CK20 staining. Hale colloidal iron staining showed moderate to abundant cytoplasmic mucin. The patient was diagnosed with AAEMPD. It is imperative to maintain clinical suspicion for AAEMPD and to examine acantholytic disorders with scrutiny. When there is evidence of atypia or mitoses, use of IHC stains can assist in fully characterizing the lesion.
- Bowen JT. Precancerous dermatosis: a study of two cases of chronic atypical epithelial proliferation. J Cutan Dis. 1912;30:241-255.
- Jones RE Jr, Austin C, Ackerman AB. Extramammary Paget’s disease: a critical reexamination. Am J Dermatopathol. 1979;1:101-132.
- Rayne SC, Santa Cruz DJ. Anaplastic Paget’s disease. Am J Surg Pathol. 1992;16:1085-1091.
- Wang EC, Kwah YC, Tan WP, et al. Extramammary Paget disease: immunohistochemistry is critical to distinguish potential mimickers. Dermatol Online J. 2012;18:4.
- Du X, Yin X, Zhou N, et al. Extramammary Paget’s disease mimicking acantholytic squamous cell carcinoma in situ: a case report. J Cutan Pathol. 2010;37:683.
- Mobini N. Acantholytic anaplastic Paget’s disease. J Cutan Pathol. 2009;36:374-380.
- Oh YJ, Lew BL, Sim WY. Acantholytic anaplastic extramammary Paget’s disease: a case report and review of the literature. Ann Dermatol. 2011;23:226-230.
- Zollo JD, Zeitouni NC. The Roswell Park Cancer Institute experience with extramammary Paget’s disease. Br J Dermatol. 2000;142:59-65.
- Wilkinson EJ, Brown HM. Vulvar Paget disease of urothelial origin: a report of three cases and a proposed classification of vulvar Paget disease. Hum Pathol. 2002;33:549-554.
- Lam C, Funaro D. Extramammary Paget’s disease: summary of current knowledge. Dermatol Clin. 2010;28:807-826.
- Shiomi T, Yoshida Y, Shomori K, et al. Extramammary Paget’s disease: evaluation of the histopathological patterns of Paget cell proliferation in the epidermis. J Dermatol. 2011;38:1054-1057.
- Goldblum JR, Hart WR. Vulvar Paget’s disease: a clinicopathologic and immunohistochemical study of 19 cases. Am J Surg Pathol. 1997;21:1178-1187.
- Alhumaidi A. Practical immunohistochemistry of epithelial skin tumor. Indian J Dermatol Venerol Leprol. 2012;78:698-708.
- Battles O, Page D, Johnson J. Cytokeratins, CEA and mucin histochemistry in the diagnosis and characterization of extramammary Paget’s disease. Am J Clin Pathol. 1997;108:6-12.
- Kanitakis J. Mammary and extramammary Paget’s disease. J Eur Acad Dermatol Venereol. 2007;21:581-590.
- Krishna M. Diagnosis of metastatic neoplasms: an immunohistochemical approach. Arch Pathol Lab Med. 2010;134:207-215.
- Helm KF, Goellner JR, Peters MS. Immunohistochemical stain in extramammary Paget’s disease. Am J Dermatopathol. 1992;14:402-407.
- Liegl B, Horn L, Moinfar F. Androgen receptors are frequently expressed in mammary and extramammary Paget’s disease. Mod Pathol. 2005;18:1283-288.
- Ackerman AB. Focal acantholytic dyskeratosis. Arch Derm. 1972;106:702-706.
- Dittmer CJ, Hornemann A, Rose C, et al. Successful laser therapy of a papular acantholytic dyskeratosis of the vulva: case report and review of literature. Arch Gynecol Obstet. 2010;291:723-725.
- Roh MR, Choi YJ, Lee KG. Papular acantholytic dyskeratosis of the vulva. J Dermatol. 2009;36:427-429.
- Wong KT, Mihm MC Jr. Acantholytic dermatosis localized to genitalia and crural areas of male patients: a report of three cases. J Cutan Pathol. 1994;21:27-32.
- Hu Z, Bonifas JM, Beech J, et al. Mutations in ATP2C1, encoding a calcium pump, cause Hailey-Hailey disease. Nat Genet. 2000; 24:61-65.
- Elston DM. Malignant tumors of the epidermis. In: Elston DM, Ferringer T, eds. Requisites in Dermatology: Dermatopathology. Philadelphia, PA: Elsevier Limited; 2012:53-68.
- Fu JM, McCalmont T, Yu SS. Adenosquamous carcinoma of the skin: a case series. Arch Dermatol. 2009;145:1152-1158.
- Becker BA, Gaspari AA. Pemphigus vulgaris and vegetans. Dermatol Clin. 1993;11:429-452.
- Rados J. Autoimmune blistering diseases: histologic meaning. Clin Dermatol. 2011;29:377-388.
- Kohler S, Smoller BR. A case of extramammary Paget’s disease mimicking pemphigus vulgaris on histologic examination. Dermatology. 1997;195:54-56.
- Bowen JT. Precancerous dermatosis: a study of two cases of chronic atypical epithelial proliferation. J Cutan Dis. 1912;30:241-255.
- Jones RE Jr, Austin C, Ackerman AB. Extramammary Paget’s disease: a critical reexamination. Am J Dermatopathol. 1979;1:101-132.
- Rayne SC, Santa Cruz DJ. Anaplastic Paget’s disease. Am J Surg Pathol. 1992;16:1085-1091.
- Wang EC, Kwah YC, Tan WP, et al. Extramammary Paget disease: immunohistochemistry is critical to distinguish potential mimickers. Dermatol Online J. 2012;18:4.
- Du X, Yin X, Zhou N, et al. Extramammary Paget’s disease mimicking acantholytic squamous cell carcinoma in situ: a case report. J Cutan Pathol. 2010;37:683.
- Mobini N. Acantholytic anaplastic Paget’s disease. J Cutan Pathol. 2009;36:374-380.
- Oh YJ, Lew BL, Sim WY. Acantholytic anaplastic extramammary Paget’s disease: a case report and review of the literature. Ann Dermatol. 2011;23:226-230.
- Zollo JD, Zeitouni NC. The Roswell Park Cancer Institute experience with extramammary Paget’s disease. Br J Dermatol. 2000;142:59-65.
- Wilkinson EJ, Brown HM. Vulvar Paget disease of urothelial origin: a report of three cases and a proposed classification of vulvar Paget disease. Hum Pathol. 2002;33:549-554.
- Lam C, Funaro D. Extramammary Paget’s disease: summary of current knowledge. Dermatol Clin. 2010;28:807-826.
- Shiomi T, Yoshida Y, Shomori K, et al. Extramammary Paget’s disease: evaluation of the histopathological patterns of Paget cell proliferation in the epidermis. J Dermatol. 2011;38:1054-1057.
- Goldblum JR, Hart WR. Vulvar Paget’s disease: a clinicopathologic and immunohistochemical study of 19 cases. Am J Surg Pathol. 1997;21:1178-1187.
- Alhumaidi A. Practical immunohistochemistry of epithelial skin tumor. Indian J Dermatol Venerol Leprol. 2012;78:698-708.
- Battles O, Page D, Johnson J. Cytokeratins, CEA and mucin histochemistry in the diagnosis and characterization of extramammary Paget’s disease. Am J Clin Pathol. 1997;108:6-12.
- Kanitakis J. Mammary and extramammary Paget’s disease. J Eur Acad Dermatol Venereol. 2007;21:581-590.
- Krishna M. Diagnosis of metastatic neoplasms: an immunohistochemical approach. Arch Pathol Lab Med. 2010;134:207-215.
- Helm KF, Goellner JR, Peters MS. Immunohistochemical stain in extramammary Paget’s disease. Am J Dermatopathol. 1992;14:402-407.
- Liegl B, Horn L, Moinfar F. Androgen receptors are frequently expressed in mammary and extramammary Paget’s disease. Mod Pathol. 2005;18:1283-288.
- Ackerman AB. Focal acantholytic dyskeratosis. Arch Derm. 1972;106:702-706.
- Dittmer CJ, Hornemann A, Rose C, et al. Successful laser therapy of a papular acantholytic dyskeratosis of the vulva: case report and review of literature. Arch Gynecol Obstet. 2010;291:723-725.
- Roh MR, Choi YJ, Lee KG. Papular acantholytic dyskeratosis of the vulva. J Dermatol. 2009;36:427-429.
- Wong KT, Mihm MC Jr. Acantholytic dermatosis localized to genitalia and crural areas of male patients: a report of three cases. J Cutan Pathol. 1994;21:27-32.
- Hu Z, Bonifas JM, Beech J, et al. Mutations in ATP2C1, encoding a calcium pump, cause Hailey-Hailey disease. Nat Genet. 2000; 24:61-65.
- Elston DM. Malignant tumors of the epidermis. In: Elston DM, Ferringer T, eds. Requisites in Dermatology: Dermatopathology. Philadelphia, PA: Elsevier Limited; 2012:53-68.
- Fu JM, McCalmont T, Yu SS. Adenosquamous carcinoma of the skin: a case series. Arch Dermatol. 2009;145:1152-1158.
- Becker BA, Gaspari AA. Pemphigus vulgaris and vegetans. Dermatol Clin. 1993;11:429-452.
- Rados J. Autoimmune blistering diseases: histologic meaning. Clin Dermatol. 2011;29:377-388.
- Kohler S, Smoller BR. A case of extramammary Paget’s disease mimicking pemphigus vulgaris on histologic examination. Dermatology. 1997;195:54-56.
Practice Points
- The acantholytic anaplastic variant of extramammary Paget disease (EMPD) can be mimicked by many other entities including Bowen disease, acantholytic dyskeratosis of the genitocrural area, and pemphigus vulgaris.
- A good immunohistochemical panel to evaluate for EMPD includes cytokeratin (CK) 7, pancytokeratin (CKAE1/AE3), CK20, and carcinoembryonic antigen.
Diffuse Nonscarring Alopecia
The Diagnosis: Trichotillomania
A scalp punch biopsy revealed pigmented hair casts, an increase in catagen and telogen follicles, and a lack of perifollicular inflammation (Figure). Based on the clinical and histopathological findings, a diagnosis of trichotillomania (TTM) was established.
Trichotillomania is a hairpulling disorder with notable dermatologic and psychiatric overlap. Although previously considered an impulse control disorder, the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition) reclassified it within obsessive-compulsive and related disorders, which also include body dysmorphic disorder and excoriation (skin-picking) disorder. Diagnostic criteria for TTM include the following: the patient must have recurrent pulling out of his/her hair resulting in hair loss despite repeated attempts to stop; underlying medical conditions and other psychiatric diagnoses must be excluded; and the patient must experience distress or impairment in social, occupational, or other areas of functioning from the hairpulling.1 Trichotillomania mainly occurs in children and young adults, with a lifetime prevalence of approximately 1% to 2%.2 The coexistence of a mood or anxiety disorder is common, as seen in our patient.
The diagnosis of TTM requires strong clinical suspicion because patients and their parents/guardians usually deny hairpulling. The main clinical differential diagnosis often is alopecia areata (AA) because both conditions can present as well-defined patches of nonscarring hair loss. Trichoscopy provides an invaluable noninvasive diagnostic tool that can be particularly useful in pediatric patients who may be reluctant to have a scalp biopsy. There are many overlapping trichoscopic findings of TTM and AA, including yellow dots, black dots, broken hairs, coiled hairs, and exclamation mark hairs.3 More specific trichoscopy findings for TTM include flame hairs (wavy proximal hair residue), V-sign (2 shafts within 1 follicle broken at the same length), and tulip hairs (dark, tulip-shaped ends of broken hairs).4 Hair breakage of varying lengths and trichoptilosis (split ends) can be better visualized using trichoscopy and support a diagnosis of TTM over AA.
Androgenetic alopecia (female pattern hair loss) presents with gradual thinning around the part line of the frontal and parietal scalp with trichoscopy showing miniaturization of hairs and decreased follicle density. The moth-eaten-like appearance of alopecia due to secondary syphilis may mimic alopecia areata clinically, but serologic testing can confirm the diagnosis of syphilis. Telogen effluvium does not have the trichoscopic features that are seen in TTM and is clinically distinguished by hair shedding and a positive hair pull test.
Biopsy can provide objective yet nonspecific support for the diagnosis, demonstrating trichomalacia, pigmented hair casts, empty follicles, and an increase in catagen hairs with a lack of inflammation. Normal and damaged hair follicles may be seen in close proximity, and hemorrhage may be seen secondary to trauma. Pigmented hair casts are not specific to TTM and are present in other traumatic hair disorders, such as traction alopecia; therefore, clinical correlation is essential for diagnosis.
Habit reversal training is the most effective treatment of TTM and involves 3 major components: awareness training with self-monitoring, stimulus control, and competing response procedures.5 Although numerous pharmacotherapies have been reported as effective treatments for TTM, a 2013 Cochrane review of 8 randomized controlled trials concluded that no medication has demonstrated reliable efficacy. Reported therapies included selective serotonin reuptake inhibitors, naltrexone, olanzapine, N-acetylcysteine, and clomipramine.6
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
- Schumer MC, Panza KE, Mulqueen JM, et al. Long-term outcome in pediatric trichotillomania. Depress Anxiety. 2015;32:737-743.
- Lencastre A, Tosti A. Role of trichoscopy on children's scalp and hair disorders. Pediatr Dermatol. 2013;30:674-682.
- Rakowska A, Slowinska M, Olszewska M, et al. New trichoscopy findings in trichotillomania: flame hairs, V-sign, hook hairs, hair powder, tulip hairs. Acta Derm Venereol. 2014;94:303-306.
- Morris S, Zickgraf H, Dingfelder H, et al. Habit reversal training in trichotillomania: guide for the clinician. Expert Rev Neurother. 2013;13:1069-1177.
- Rothbart R, Amos T, Siegfried N, et al. Pharmacotherapy for trichotillomania. Cochrane Database Syst Rev. 2013;11:CD007662.
The Diagnosis: Trichotillomania
A scalp punch biopsy revealed pigmented hair casts, an increase in catagen and telogen follicles, and a lack of perifollicular inflammation (Figure). Based on the clinical and histopathological findings, a diagnosis of trichotillomania (TTM) was established.

Trichotillomania is a hairpulling disorder with notable dermatologic and psychiatric overlap. Although previously considered an impulse control disorder, the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition) reclassified it within obsessive-compulsive and related disorders, which also include body dysmorphic disorder and excoriation (skin-picking) disorder. Diagnostic criteria for TTM include the following: the patient must have recurrent pulling out of his/her hair resulting in hair loss despite repeated attempts to stop; underlying medical conditions and other psychiatric diagnoses must be excluded; and the patient must experience distress or impairment in social, occupational, or other areas of functioning from the hairpulling.1 Trichotillomania mainly occurs in children and young adults, with a lifetime prevalence of approximately 1% to 2%.2 The coexistence of a mood or anxiety disorder is common, as seen in our patient.
The diagnosis of TTM requires strong clinical suspicion because patients and their parents/guardians usually deny hairpulling. The main clinical differential diagnosis often is alopecia areata (AA) because both conditions can present as well-defined patches of nonscarring hair loss. Trichoscopy provides an invaluable noninvasive diagnostic tool that can be particularly useful in pediatric patients who may be reluctant to have a scalp biopsy. There are many overlapping trichoscopic findings of TTM and AA, including yellow dots, black dots, broken hairs, coiled hairs, and exclamation mark hairs.3 More specific trichoscopy findings for TTM include flame hairs (wavy proximal hair residue), V-sign (2 shafts within 1 follicle broken at the same length), and tulip hairs (dark, tulip-shaped ends of broken hairs).4 Hair breakage of varying lengths and trichoptilosis (split ends) can be better visualized using trichoscopy and support a diagnosis of TTM over AA.
Androgenetic alopecia (female pattern hair loss) presents with gradual thinning around the part line of the frontal and parietal scalp with trichoscopy showing miniaturization of hairs and decreased follicle density. The moth-eaten-like appearance of alopecia due to secondary syphilis may mimic alopecia areata clinically, but serologic testing can confirm the diagnosis of syphilis. Telogen effluvium does not have the trichoscopic features that are seen in TTM and is clinically distinguished by hair shedding and a positive hair pull test.
Biopsy can provide objective yet nonspecific support for the diagnosis, demonstrating trichomalacia, pigmented hair casts, empty follicles, and an increase in catagen hairs with a lack of inflammation. Normal and damaged hair follicles may be seen in close proximity, and hemorrhage may be seen secondary to trauma. Pigmented hair casts are not specific to TTM and are present in other traumatic hair disorders, such as traction alopecia; therefore, clinical correlation is essential for diagnosis.
Habit reversal training is the most effective treatment of TTM and involves 3 major components: awareness training with self-monitoring, stimulus control, and competing response procedures.5 Although numerous pharmacotherapies have been reported as effective treatments for TTM, a 2013 Cochrane review of 8 randomized controlled trials concluded that no medication has demonstrated reliable efficacy. Reported therapies included selective serotonin reuptake inhibitors, naltrexone, olanzapine, N-acetylcysteine, and clomipramine.6
The Diagnosis: Trichotillomania
A scalp punch biopsy revealed pigmented hair casts, an increase in catagen and telogen follicles, and a lack of perifollicular inflammation (Figure). Based on the clinical and histopathological findings, a diagnosis of trichotillomania (TTM) was established.

Trichotillomania is a hairpulling disorder with notable dermatologic and psychiatric overlap. Although previously considered an impulse control disorder, the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition) reclassified it within obsessive-compulsive and related disorders, which also include body dysmorphic disorder and excoriation (skin-picking) disorder. Diagnostic criteria for TTM include the following: the patient must have recurrent pulling out of his/her hair resulting in hair loss despite repeated attempts to stop; underlying medical conditions and other psychiatric diagnoses must be excluded; and the patient must experience distress or impairment in social, occupational, or other areas of functioning from the hairpulling.1 Trichotillomania mainly occurs in children and young adults, with a lifetime prevalence of approximately 1% to 2%.2 The coexistence of a mood or anxiety disorder is common, as seen in our patient.
The diagnosis of TTM requires strong clinical suspicion because patients and their parents/guardians usually deny hairpulling. The main clinical differential diagnosis often is alopecia areata (AA) because both conditions can present as well-defined patches of nonscarring hair loss. Trichoscopy provides an invaluable noninvasive diagnostic tool that can be particularly useful in pediatric patients who may be reluctant to have a scalp biopsy. There are many overlapping trichoscopic findings of TTM and AA, including yellow dots, black dots, broken hairs, coiled hairs, and exclamation mark hairs.3 More specific trichoscopy findings for TTM include flame hairs (wavy proximal hair residue), V-sign (2 shafts within 1 follicle broken at the same length), and tulip hairs (dark, tulip-shaped ends of broken hairs).4 Hair breakage of varying lengths and trichoptilosis (split ends) can be better visualized using trichoscopy and support a diagnosis of TTM over AA.
Androgenetic alopecia (female pattern hair loss) presents with gradual thinning around the part line of the frontal and parietal scalp with trichoscopy showing miniaturization of hairs and decreased follicle density. The moth-eaten-like appearance of alopecia due to secondary syphilis may mimic alopecia areata clinically, but serologic testing can confirm the diagnosis of syphilis. Telogen effluvium does not have the trichoscopic features that are seen in TTM and is clinically distinguished by hair shedding and a positive hair pull test.
Biopsy can provide objective yet nonspecific support for the diagnosis, demonstrating trichomalacia, pigmented hair casts, empty follicles, and an increase in catagen hairs with a lack of inflammation. Normal and damaged hair follicles may be seen in close proximity, and hemorrhage may be seen secondary to trauma. Pigmented hair casts are not specific to TTM and are present in other traumatic hair disorders, such as traction alopecia; therefore, clinical correlation is essential for diagnosis.
Habit reversal training is the most effective treatment of TTM and involves 3 major components: awareness training with self-monitoring, stimulus control, and competing response procedures.5 Although numerous pharmacotherapies have been reported as effective treatments for TTM, a 2013 Cochrane review of 8 randomized controlled trials concluded that no medication has demonstrated reliable efficacy. Reported therapies included selective serotonin reuptake inhibitors, naltrexone, olanzapine, N-acetylcysteine, and clomipramine.6
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
- Schumer MC, Panza KE, Mulqueen JM, et al. Long-term outcome in pediatric trichotillomania. Depress Anxiety. 2015;32:737-743.
- Lencastre A, Tosti A. Role of trichoscopy on children's scalp and hair disorders. Pediatr Dermatol. 2013;30:674-682.
- Rakowska A, Slowinska M, Olszewska M, et al. New trichoscopy findings in trichotillomania: flame hairs, V-sign, hook hairs, hair powder, tulip hairs. Acta Derm Venereol. 2014;94:303-306.
- Morris S, Zickgraf H, Dingfelder H, et al. Habit reversal training in trichotillomania: guide for the clinician. Expert Rev Neurother. 2013;13:1069-1177.
- Rothbart R, Amos T, Siegfried N, et al. Pharmacotherapy for trichotillomania. Cochrane Database Syst Rev. 2013;11:CD007662.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
- Schumer MC, Panza KE, Mulqueen JM, et al. Long-term outcome in pediatric trichotillomania. Depress Anxiety. 2015;32:737-743.
- Lencastre A, Tosti A. Role of trichoscopy on children's scalp and hair disorders. Pediatr Dermatol. 2013;30:674-682.
- Rakowska A, Slowinska M, Olszewska M, et al. New trichoscopy findings in trichotillomania: flame hairs, V-sign, hook hairs, hair powder, tulip hairs. Acta Derm Venereol. 2014;94:303-306.
- Morris S, Zickgraf H, Dingfelder H, et al. Habit reversal training in trichotillomania: guide for the clinician. Expert Rev Neurother. 2013;13:1069-1177.
- Rothbart R, Amos T, Siegfried N, et al. Pharmacotherapy for trichotillomania. Cochrane Database Syst Rev. 2013;11:CD007662.
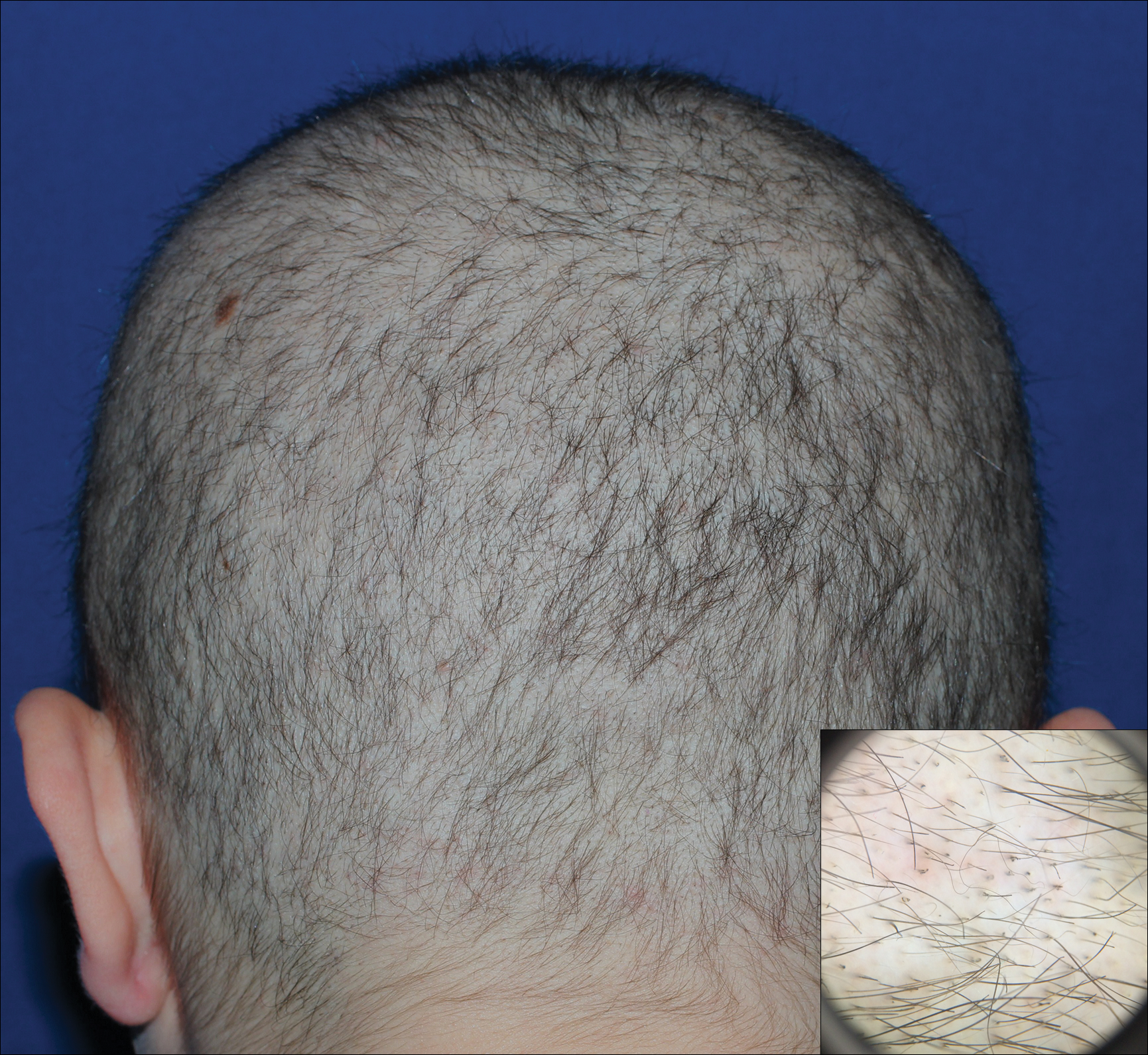
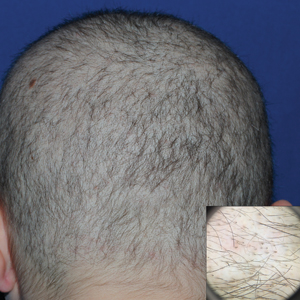
A 19-year-old woman with attention deficit hyperactivity disorder and an anxiety disorder presented with hair loss of 2 years' duration. She initially had small circular bald areas throughout the scalp that had progressed to diffuse hair loss of the entire scalp. She denied recent hairpulling but admitted to a remote prior history of eyelash and eyebrow pulling. She denied any voice changes, acne, or menstrual irregularities. Physical examination revealed short hairs of varying lengths throughout the scalp with no loss of follicles, erythema, scale, or exclamation point hairs. Eyebrows and eyelashes were normal. A hair-pull test was negative. Trichoscopy illuminated variation in hair shaft diameters, as well as short, irregularly broken hairs of different lengths (inset).
Counterphobia and Poor Sun Protection Practices in First-Degree Relatives of Melanoma Patients
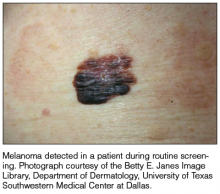
It is widely accepted that there are several factors that may independently elevate an individual’s risk for melanoma, such as a history of childhood sunburns, family history of melanoma, and poor sun protection practices. Several studies have examined risk behaviors in melanoma patients following their diagnosis and have reported findings such as increased UV exposure patterns, persistent tanning bed use, and sun-protective behaviors similar to those of the general population (Figure).1-4
Although first-degree relatives (FDRs) of melanoma patients are at an increased risk for melanoma, they also have been found to exhibit surprisingly poor sun protection practices. In one retrospective analysis, Geller et al5 found that frequent sunburns, high rates of tanning bed use, and low rates of sunscreen use were common among children of health care workers who reported a personal or family history of skin cancer. An independent study reported that merely 37% (37/100) of FDRs of melanoma patients use sunscreen more than half of the time, and considerably fewer wear protective clothing or seek shade while outdoors.6 Given their increased risk for developing melanoma, it is likely to be assumed that FDRs of melanoma patients practice diligent sun protection. The underlying reasons for the failure of this at-risk population to adhere strongly to sun protection practices warrants special attention.
Manne et al7 conducted a survey in a group of FDRs of melanoma patients with self-reported poor sun protection practices to evaluate the demographic, medical, psychological, educational (knowledge of sun protection guidelines), and social influences that correlate with sun protection and sunbathing practices. More effective sun protective behaviors were identified in FDRs with higher education, fewer perceived benefits of sunbathing, more prominent photoaging concerns, and greater sunscreen self-efficacy. The authors concluded that sun-protective behavior in FDRs was not associated with prior knowledge about sunscreen or UV exposure, their relative’s melanoma stage, or physician recommendations for sun protection.7
Factors that have been documented as influencing sun-protective behavior in the general population include knowledge of the benefits of sun protection; attitudes toward tanning and sun protection; subjective norms regarding the beauty and perceived health of a tan; and optimistic bias, which is a cognitive mechanism that causes a person to believe that he/she is at lesser risk for experiencing a negative outcome compared to others. Additionally, sun protection behaviors are influenced by the immediacy of getting the reward (the perceived benefits of tanning) versus the delayed punishment (development of skin cancer).6 Although all of these elements may be important for some individuals, we believe that a subset of FDRs of melanoma patients may be susceptible to the phenomenon known as counterphobia.
Counterphobia is a neurotic response to anxiety in which an individual actively pursues situations that heighten his/her fear rather than fleeing from a feared object or behavior.8 Most insight into counterphobia has come from the experiences of children who have parent(s) with a debilitating or fatal diagnosis. Due to their immature coping mechanisms, some children are at risk for maladaptive behavioral responses. The loss of a parent typically produces severe psychological trauma in all children, but in those who develop counterphobia, it manifests as a heightened fear of death and vulnerability to their parent’s illness. This maladaptive response is dependent on self-identification with the parent, especially among daughters of lost mothers and sons of lost fathers, and this fear remains with the child through adulthood. A survey of 154 motherless daughters found that women aged 19 to 35 years have the highest level of obsessive thoughts of mortality and more than 75% believe they will succumb to their mother’s illness (92% in the case of cancer).9 Despite this fear, children may exhibit health-compromising behaviors related to the diagnoses that led to their parents’ deaths; for example, counterphobia has been identified as a pathologic factor behind sexually promiscuous practices in the children of patients with AIDS, and it also may explain high-risk drinking behavior in a child whose parent died from hepatocellular carcinoma due to a history of alcoholic cirrhosis. Similarly, counterphobia can manifest as the deliberate refusal to undergo a mammogram in a woman whose mother died of breast cancer.9 Psychologists have hypothesized that counterphobic pursuits may result from attempts to master the anxiety associated with fear of injury or death as well as from the notion that attempts at risk-factor reduction are futile, as their death is certain.10
The strong influence of counterphobia on perspectives of health and mortality among individuals affected by early loss of a parent is well documented. An assessment of the subjective life expectancy, death anxiety, and health-related behaviors of college students who lost a parent revealed that these individuals estimated their own life spans to be shorter than college students with 2 living parents.11 Moreover, when students were explicitly instructed to predict their life expectancy based on a purely objective mentality rather than one influenced by personal feelings, the exclusion of emotion yielded a longer projected life span. This finding highlights the magnitude of the psychological forces influencing the ethos of individuals affected by premature parental loss. In the same study, individuals who had experienced early loss of a parent believed they would die of the same condition that caused their parent’s death, a finding accompanied by notably poorer diet and smoking behaviors, which might be expected among those with counterphobic defenses.11
Although Manne et al7 did not find an association between melanoma disease severity and sun-protective behavior in FDRs, the study design did not allow for assessment of potential counterphobic responses, which are most likely to develop in younger individuals who strongly identify with the family member whose disease was disabling or fatal. For example, the study included adult relatives (mean age, 46 years) of melanoma patients diagnosed in the preceding 4 years. Furthermore, fewer than 20% (108/545) of the patients had stage III or IV melanoma, and it was not known if melanoma patients communicated the diagnosis to their family members.7
A practice gap exists in FDRs of melanoma patients who are largely assumed to be practicing adequate, if not heightened, sun protection practices. Given that this group demonstrates poor sun protection practices, it is important to identify reasons for such behavior that may extend beyond what is currently known and may include counterphobia. Based on research performed in other medical conditions, the individuals most at risk for counterphobic responses are young children of patients diagnosed with a disabling or fatal condition, but whether in cases of melanoma counterphobia exists as a maladaptive response and whether such a response may occur in all close relatives, not just offspring, is unknown. Currently, the type of measure(s) that may mitigate poor risk factor modification due to counterphobia, including sun protection practices, is unknown. However, physician knowledge of counterphobic responses as a possibility in close relatives of melanoma patients may improve physician efforts to modify behavior in this unique, high-risk population.
The multimodal pathway of melanoma development suggests that individuals with an underlying genetic predisposition for melanoma who also neglect sun-protective measures are an especially high-risk group.12 As such, targeted education and screening of this patient population may be warranted (Table 1). Although it is incumbent on physicians to incorporate concerted screening, counseling, and focused interventions for newly diagnosed melanoma patients, taking similar measures to counsel and educate immediate relatives who may be at high risk for poor sun protection practices also is encouraged (Table 2).
We believe that recognition of counterphobic behavior is critical in the evaluation of FDRs of melanoma patients. Heightened awareness may bolster primary prevention efforts, especially in our patients with genetic diatheses toward melanoma development.
1. Idorn L, Datta P, Heydenreich J, et al. A 3-year follow-up of sun behavior in patients with cutaneous malignant melanoma [published online ahead of print October 2, 2013]. JAMA Dermatol. doi:10.1001/jamadermatol.2013.5098.
2. Idorn LW, Datta P, Heydenreich J, et al. Sun behaviour after cutaneous malignant melanoma: a study based on ultraviolet radiation measurements and sun diary data [published online ahead of print]. Br J Dermatol. 2013;168:367-373.
3. Mayer D, Layman A, Carlson J. Sun-protection behaviors of melanoma survivors. J Am Acad Dermatol. 2012;66:e9-e10.
4. Lee TK, Brazier AS, Shoveller JA, et al. Sun-related behavior after a diagnosis of cutaneous malignant melanoma. Melanoma Res. 2007;17:51-55.
5. Geller AC, Brooks DR, Colditz GA, et al. Sun protection practices among offspring of women with personal or family history of skin cancer. Pediatrics. 2006;117:e688-e694.
6. Azzarello LM, Dessureault S, Jacobsen PB. Sun-protective behavior among individuals with a family history of melanoma. Cancer Epidemiol Boomarkers Prev. 2006;15:142-145.
7. Manne SL, Coups EJ, Jacobsen PB, et al. Sun protection and sunbathing practices among at-risk family members of patients with melanoma. BMC Public Health. 2011;11:122.
8. Fenichel O. The Psychoanalytic Theory of Neurosis. Oxford, United Kingdom: Taylor & Francis; 1999.
9. Edelman H. Motherless Daughters: The Legacy of Loss. 2nd ed. Cambridge, MA: Da Capo Press; 2006.
10. Poznanski E, Arthur B. The counterphobic defense in children. Child Psychiatry Hum Dev. 1971;1:178-191.
11. Denes-Raj V, Ehrlichman H. Effects of premature parental death on subjective life expectancy, death anxiety, and health behavior. Omega: Journal of Death and Dying. 1991;23:309-321.
12. Hayward NK. Genetics of melanoma predisposition. Oncogene. 2003;22:3053-3062.
13. Arthey S, Clarke VA. Suntanning and sun protection: a review of the psychologial literature. Soc Sci Med. 1995;40:265-274.
It is widely accepted that there are several factors that may independently elevate an individual’s risk for melanoma, such as a history of childhood sunburns, family history of melanoma, and poor sun protection practices. Several studies have examined risk behaviors in melanoma patients following their diagnosis and have reported findings such as increased UV exposure patterns, persistent tanning bed use, and sun-protective behaviors similar to those of the general population (Figure).1-4
Although first-degree relatives (FDRs) of melanoma patients are at an increased risk for melanoma, they also have been found to exhibit surprisingly poor sun protection practices. In one retrospective analysis, Geller et al5 found that frequent sunburns, high rates of tanning bed use, and low rates of sunscreen use were common among children of health care workers who reported a personal or family history of skin cancer. An independent study reported that merely 37% (37/100) of FDRs of melanoma patients use sunscreen more than half of the time, and considerably fewer wear protective clothing or seek shade while outdoors.6 Given their increased risk for developing melanoma, it is likely to be assumed that FDRs of melanoma patients practice diligent sun protection. The underlying reasons for the failure of this at-risk population to adhere strongly to sun protection practices warrants special attention.
Manne et al7 conducted a survey in a group of FDRs of melanoma patients with self-reported poor sun protection practices to evaluate the demographic, medical, psychological, educational (knowledge of sun protection guidelines), and social influences that correlate with sun protection and sunbathing practices. More effective sun protective behaviors were identified in FDRs with higher education, fewer perceived benefits of sunbathing, more prominent photoaging concerns, and greater sunscreen self-efficacy. The authors concluded that sun-protective behavior in FDRs was not associated with prior knowledge about sunscreen or UV exposure, their relative’s melanoma stage, or physician recommendations for sun protection.7
Factors that have been documented as influencing sun-protective behavior in the general population include knowledge of the benefits of sun protection; attitudes toward tanning and sun protection; subjective norms regarding the beauty and perceived health of a tan; and optimistic bias, which is a cognitive mechanism that causes a person to believe that he/she is at lesser risk for experiencing a negative outcome compared to others. Additionally, sun protection behaviors are influenced by the immediacy of getting the reward (the perceived benefits of tanning) versus the delayed punishment (development of skin cancer).6 Although all of these elements may be important for some individuals, we believe that a subset of FDRs of melanoma patients may be susceptible to the phenomenon known as counterphobia.
Counterphobia is a neurotic response to anxiety in which an individual actively pursues situations that heighten his/her fear rather than fleeing from a feared object or behavior.8 Most insight into counterphobia has come from the experiences of children who have parent(s) with a debilitating or fatal diagnosis. Due to their immature coping mechanisms, some children are at risk for maladaptive behavioral responses. The loss of a parent typically produces severe psychological trauma in all children, but in those who develop counterphobia, it manifests as a heightened fear of death and vulnerability to their parent’s illness. This maladaptive response is dependent on self-identification with the parent, especially among daughters of lost mothers and sons of lost fathers, and this fear remains with the child through adulthood. A survey of 154 motherless daughters found that women aged 19 to 35 years have the highest level of obsessive thoughts of mortality and more than 75% believe they will succumb to their mother’s illness (92% in the case of cancer).9 Despite this fear, children may exhibit health-compromising behaviors related to the diagnoses that led to their parents’ deaths; for example, counterphobia has been identified as a pathologic factor behind sexually promiscuous practices in the children of patients with AIDS, and it also may explain high-risk drinking behavior in a child whose parent died from hepatocellular carcinoma due to a history of alcoholic cirrhosis. Similarly, counterphobia can manifest as the deliberate refusal to undergo a mammogram in a woman whose mother died of breast cancer.9 Psychologists have hypothesized that counterphobic pursuits may result from attempts to master the anxiety associated with fear of injury or death as well as from the notion that attempts at risk-factor reduction are futile, as their death is certain.10
The strong influence of counterphobia on perspectives of health and mortality among individuals affected by early loss of a parent is well documented. An assessment of the subjective life expectancy, death anxiety, and health-related behaviors of college students who lost a parent revealed that these individuals estimated their own life spans to be shorter than college students with 2 living parents.11 Moreover, when students were explicitly instructed to predict their life expectancy based on a purely objective mentality rather than one influenced by personal feelings, the exclusion of emotion yielded a longer projected life span. This finding highlights the magnitude of the psychological forces influencing the ethos of individuals affected by premature parental loss. In the same study, individuals who had experienced early loss of a parent believed they would die of the same condition that caused their parent’s death, a finding accompanied by notably poorer diet and smoking behaviors, which might be expected among those with counterphobic defenses.11
Although Manne et al7 did not find an association between melanoma disease severity and sun-protective behavior in FDRs, the study design did not allow for assessment of potential counterphobic responses, which are most likely to develop in younger individuals who strongly identify with the family member whose disease was disabling or fatal. For example, the study included adult relatives (mean age, 46 years) of melanoma patients diagnosed in the preceding 4 years. Furthermore, fewer than 20% (108/545) of the patients had stage III or IV melanoma, and it was not known if melanoma patients communicated the diagnosis to their family members.7
A practice gap exists in FDRs of melanoma patients who are largely assumed to be practicing adequate, if not heightened, sun protection practices. Given that this group demonstrates poor sun protection practices, it is important to identify reasons for such behavior that may extend beyond what is currently known and may include counterphobia. Based on research performed in other medical conditions, the individuals most at risk for counterphobic responses are young children of patients diagnosed with a disabling or fatal condition, but whether in cases of melanoma counterphobia exists as a maladaptive response and whether such a response may occur in all close relatives, not just offspring, is unknown. Currently, the type of measure(s) that may mitigate poor risk factor modification due to counterphobia, including sun protection practices, is unknown. However, physician knowledge of counterphobic responses as a possibility in close relatives of melanoma patients may improve physician efforts to modify behavior in this unique, high-risk population.
The multimodal pathway of melanoma development suggests that individuals with an underlying genetic predisposition for melanoma who also neglect sun-protective measures are an especially high-risk group.12 As such, targeted education and screening of this patient population may be warranted (Table 1). Although it is incumbent on physicians to incorporate concerted screening, counseling, and focused interventions for newly diagnosed melanoma patients, taking similar measures to counsel and educate immediate relatives who may be at high risk for poor sun protection practices also is encouraged (Table 2).
We believe that recognition of counterphobic behavior is critical in the evaluation of FDRs of melanoma patients. Heightened awareness may bolster primary prevention efforts, especially in our patients with genetic diatheses toward melanoma development.
It is widely accepted that there are several factors that may independently elevate an individual’s risk for melanoma, such as a history of childhood sunburns, family history of melanoma, and poor sun protection practices. Several studies have examined risk behaviors in melanoma patients following their diagnosis and have reported findings such as increased UV exposure patterns, persistent tanning bed use, and sun-protective behaviors similar to those of the general population (Figure).1-4
Although first-degree relatives (FDRs) of melanoma patients are at an increased risk for melanoma, they also have been found to exhibit surprisingly poor sun protection practices. In one retrospective analysis, Geller et al5 found that frequent sunburns, high rates of tanning bed use, and low rates of sunscreen use were common among children of health care workers who reported a personal or family history of skin cancer. An independent study reported that merely 37% (37/100) of FDRs of melanoma patients use sunscreen more than half of the time, and considerably fewer wear protective clothing or seek shade while outdoors.6 Given their increased risk for developing melanoma, it is likely to be assumed that FDRs of melanoma patients practice diligent sun protection. The underlying reasons for the failure of this at-risk population to adhere strongly to sun protection practices warrants special attention.
Manne et al7 conducted a survey in a group of FDRs of melanoma patients with self-reported poor sun protection practices to evaluate the demographic, medical, psychological, educational (knowledge of sun protection guidelines), and social influences that correlate with sun protection and sunbathing practices. More effective sun protective behaviors were identified in FDRs with higher education, fewer perceived benefits of sunbathing, more prominent photoaging concerns, and greater sunscreen self-efficacy. The authors concluded that sun-protective behavior in FDRs was not associated with prior knowledge about sunscreen or UV exposure, their relative’s melanoma stage, or physician recommendations for sun protection.7
Factors that have been documented as influencing sun-protective behavior in the general population include knowledge of the benefits of sun protection; attitudes toward tanning and sun protection; subjective norms regarding the beauty and perceived health of a tan; and optimistic bias, which is a cognitive mechanism that causes a person to believe that he/she is at lesser risk for experiencing a negative outcome compared to others. Additionally, sun protection behaviors are influenced by the immediacy of getting the reward (the perceived benefits of tanning) versus the delayed punishment (development of skin cancer).6 Although all of these elements may be important for some individuals, we believe that a subset of FDRs of melanoma patients may be susceptible to the phenomenon known as counterphobia.
Counterphobia is a neurotic response to anxiety in which an individual actively pursues situations that heighten his/her fear rather than fleeing from a feared object or behavior.8 Most insight into counterphobia has come from the experiences of children who have parent(s) with a debilitating or fatal diagnosis. Due to their immature coping mechanisms, some children are at risk for maladaptive behavioral responses. The loss of a parent typically produces severe psychological trauma in all children, but in those who develop counterphobia, it manifests as a heightened fear of death and vulnerability to their parent’s illness. This maladaptive response is dependent on self-identification with the parent, especially among daughters of lost mothers and sons of lost fathers, and this fear remains with the child through adulthood. A survey of 154 motherless daughters found that women aged 19 to 35 years have the highest level of obsessive thoughts of mortality and more than 75% believe they will succumb to their mother’s illness (92% in the case of cancer).9 Despite this fear, children may exhibit health-compromising behaviors related to the diagnoses that led to their parents’ deaths; for example, counterphobia has been identified as a pathologic factor behind sexually promiscuous practices in the children of patients with AIDS, and it also may explain high-risk drinking behavior in a child whose parent died from hepatocellular carcinoma due to a history of alcoholic cirrhosis. Similarly, counterphobia can manifest as the deliberate refusal to undergo a mammogram in a woman whose mother died of breast cancer.9 Psychologists have hypothesized that counterphobic pursuits may result from attempts to master the anxiety associated with fear of injury or death as well as from the notion that attempts at risk-factor reduction are futile, as their death is certain.10
The strong influence of counterphobia on perspectives of health and mortality among individuals affected by early loss of a parent is well documented. An assessment of the subjective life expectancy, death anxiety, and health-related behaviors of college students who lost a parent revealed that these individuals estimated their own life spans to be shorter than college students with 2 living parents.11 Moreover, when students were explicitly instructed to predict their life expectancy based on a purely objective mentality rather than one influenced by personal feelings, the exclusion of emotion yielded a longer projected life span. This finding highlights the magnitude of the psychological forces influencing the ethos of individuals affected by premature parental loss. In the same study, individuals who had experienced early loss of a parent believed they would die of the same condition that caused their parent’s death, a finding accompanied by notably poorer diet and smoking behaviors, which might be expected among those with counterphobic defenses.11
Although Manne et al7 did not find an association between melanoma disease severity and sun-protective behavior in FDRs, the study design did not allow for assessment of potential counterphobic responses, which are most likely to develop in younger individuals who strongly identify with the family member whose disease was disabling or fatal. For example, the study included adult relatives (mean age, 46 years) of melanoma patients diagnosed in the preceding 4 years. Furthermore, fewer than 20% (108/545) of the patients had stage III or IV melanoma, and it was not known if melanoma patients communicated the diagnosis to their family members.7
A practice gap exists in FDRs of melanoma patients who are largely assumed to be practicing adequate, if not heightened, sun protection practices. Given that this group demonstrates poor sun protection practices, it is important to identify reasons for such behavior that may extend beyond what is currently known and may include counterphobia. Based on research performed in other medical conditions, the individuals most at risk for counterphobic responses are young children of patients diagnosed with a disabling or fatal condition, but whether in cases of melanoma counterphobia exists as a maladaptive response and whether such a response may occur in all close relatives, not just offspring, is unknown. Currently, the type of measure(s) that may mitigate poor risk factor modification due to counterphobia, including sun protection practices, is unknown. However, physician knowledge of counterphobic responses as a possibility in close relatives of melanoma patients may improve physician efforts to modify behavior in this unique, high-risk population.
The multimodal pathway of melanoma development suggests that individuals with an underlying genetic predisposition for melanoma who also neglect sun-protective measures are an especially high-risk group.12 As such, targeted education and screening of this patient population may be warranted (Table 1). Although it is incumbent on physicians to incorporate concerted screening, counseling, and focused interventions for newly diagnosed melanoma patients, taking similar measures to counsel and educate immediate relatives who may be at high risk for poor sun protection practices also is encouraged (Table 2).
We believe that recognition of counterphobic behavior is critical in the evaluation of FDRs of melanoma patients. Heightened awareness may bolster primary prevention efforts, especially in our patients with genetic diatheses toward melanoma development.
1. Idorn L, Datta P, Heydenreich J, et al. A 3-year follow-up of sun behavior in patients with cutaneous malignant melanoma [published online ahead of print October 2, 2013]. JAMA Dermatol. doi:10.1001/jamadermatol.2013.5098.
2. Idorn LW, Datta P, Heydenreich J, et al. Sun behaviour after cutaneous malignant melanoma: a study based on ultraviolet radiation measurements and sun diary data [published online ahead of print]. Br J Dermatol. 2013;168:367-373.
3. Mayer D, Layman A, Carlson J. Sun-protection behaviors of melanoma survivors. J Am Acad Dermatol. 2012;66:e9-e10.
4. Lee TK, Brazier AS, Shoveller JA, et al. Sun-related behavior after a diagnosis of cutaneous malignant melanoma. Melanoma Res. 2007;17:51-55.
5. Geller AC, Brooks DR, Colditz GA, et al. Sun protection practices among offspring of women with personal or family history of skin cancer. Pediatrics. 2006;117:e688-e694.
6. Azzarello LM, Dessureault S, Jacobsen PB. Sun-protective behavior among individuals with a family history of melanoma. Cancer Epidemiol Boomarkers Prev. 2006;15:142-145.
7. Manne SL, Coups EJ, Jacobsen PB, et al. Sun protection and sunbathing practices among at-risk family members of patients with melanoma. BMC Public Health. 2011;11:122.
8. Fenichel O. The Psychoanalytic Theory of Neurosis. Oxford, United Kingdom: Taylor & Francis; 1999.
9. Edelman H. Motherless Daughters: The Legacy of Loss. 2nd ed. Cambridge, MA: Da Capo Press; 2006.
10. Poznanski E, Arthur B. The counterphobic defense in children. Child Psychiatry Hum Dev. 1971;1:178-191.
11. Denes-Raj V, Ehrlichman H. Effects of premature parental death on subjective life expectancy, death anxiety, and health behavior. Omega: Journal of Death and Dying. 1991;23:309-321.
12. Hayward NK. Genetics of melanoma predisposition. Oncogene. 2003;22:3053-3062.
13. Arthey S, Clarke VA. Suntanning and sun protection: a review of the psychologial literature. Soc Sci Med. 1995;40:265-274.
1. Idorn L, Datta P, Heydenreich J, et al. A 3-year follow-up of sun behavior in patients with cutaneous malignant melanoma [published online ahead of print October 2, 2013]. JAMA Dermatol. doi:10.1001/jamadermatol.2013.5098.
2. Idorn LW, Datta P, Heydenreich J, et al. Sun behaviour after cutaneous malignant melanoma: a study based on ultraviolet radiation measurements and sun diary data [published online ahead of print]. Br J Dermatol. 2013;168:367-373.
3. Mayer D, Layman A, Carlson J. Sun-protection behaviors of melanoma survivors. J Am Acad Dermatol. 2012;66:e9-e10.
4. Lee TK, Brazier AS, Shoveller JA, et al. Sun-related behavior after a diagnosis of cutaneous malignant melanoma. Melanoma Res. 2007;17:51-55.
5. Geller AC, Brooks DR, Colditz GA, et al. Sun protection practices among offspring of women with personal or family history of skin cancer. Pediatrics. 2006;117:e688-e694.
6. Azzarello LM, Dessureault S, Jacobsen PB. Sun-protective behavior among individuals with a family history of melanoma. Cancer Epidemiol Boomarkers Prev. 2006;15:142-145.
7. Manne SL, Coups EJ, Jacobsen PB, et al. Sun protection and sunbathing practices among at-risk family members of patients with melanoma. BMC Public Health. 2011;11:122.
8. Fenichel O. The Psychoanalytic Theory of Neurosis. Oxford, United Kingdom: Taylor & Francis; 1999.
9. Edelman H. Motherless Daughters: The Legacy of Loss. 2nd ed. Cambridge, MA: Da Capo Press; 2006.
10. Poznanski E, Arthur B. The counterphobic defense in children. Child Psychiatry Hum Dev. 1971;1:178-191.
11. Denes-Raj V, Ehrlichman H. Effects of premature parental death on subjective life expectancy, death anxiety, and health behavior. Omega: Journal of Death and Dying. 1991;23:309-321.
12. Hayward NK. Genetics of melanoma predisposition. Oncogene. 2003;22:3053-3062.
13. Arthey S, Clarke VA. Suntanning and sun protection: a review of the psychologial literature. Soc Sci Med. 1995;40:265-274.