User login
Gut microbiota and symptoms of psychosis: Is there a link?
The human microbiota refers to the collection of bacteria, archaea, eukarya, and viruses that reside within the human body. The term gut microbiome indicates the composition of these microbes and genetic codes in the intestine.1 Harkening back to the ancient Greek physician Galen, who treated gastrointestinal (GI) symptoms to relieve mental disturbances such as psychosis, the gut has been a therapeutic target in schizophrenia long before antipsychotics and the DSM.2 In recent years, research into the gut microbiome has drastically increased, with genetic sequencing affording a more precise look into the specific bacteria that call the human intestines their home. This has led to the recognition that the gut microbiome may be severely disrupted in schizophrenia, a condition known as dysbiosis. Preliminary research suggests that gut bacteria are more helpful than many human genes in distinguishing individuals with schizophrenia from their healthy counterparts.3,4 In this article, we discuss the potential role of the gut microbiome in schizophrenia, including new research correlating clinical symptoms of psychosis with dysbiosis. We also provide recommendations for promoting a healthy gut microbiome.
The enteric brain across life
The composition of our bodies is far more microbiota than human. Strikingly, microbiota cells in the gut outnumber human cells, and the distal gut alone hosts bacteria with 100 times the genetic content of the entire genome.5 The intricate meshwork of nerves in the gut is often called the enteric brain because the gut consists of 100 million neurons and synthesizes many neuroactive chemicals implicated in mood disorders and psychosis, including serotonin, dopamine, gamma-aminobutyric acid (GABA), and acetylcholine.6 The variety of neuroimmunologic, hormonal, and metabolic paths by which the gutmicrobiome and the brain interact are collectively known as the gut-microbiota-brainaxis.7
How do we acquire our gut microbiome, and how does it come to influence ourbrain and behavior? On the first day of life, as babies pass through the birth canal, they are bathed in their mother’s vaginal microbiota. In the following weeks, the microbiome expands and colonizes the gut as bacteria are introduced from environmental sources such as skin-to-skin contact and breastmilk.8 The microbiome continues to evolve throughout early life. As children expand their diets and navigate new aspects of the physical world, additional bacteria join the unseen ecosystem growing inside.9 The development of the microbiome coincides with the development of the brain. From preclinical studies, we know the gut microbiome mediates important aspects of neurodevelopment such as the formation of the blood-brain barrier (BBB), synaptic pruning, glial activation, and myelination.10 Interestingly, many of the risk factors for schizophrenia are associated with gut dysbiosis, including obstetric complications, infections treated with antibiotics, and urbanization.11-15
Throughout human life, the gut and brain remain in close communication. The gut microbiota continue to produce monoamines, along with other metabolites that are able to cross the BBB.6 The HPA axis, stimulation of the immune system, and the vagus nerve all provide highways of communication between the gut and the brain.7 The relationship between the enteric brain and cephalic brain continues through life, even up to a person’s final hour. One autopsy study that is often cited (but soberingly, cannot be found online) allegedly revealed that 92% of schizophrenia patients had developed colitis by the time of death.16,17
First-episode psychosis and antipsychotic treatment
For patients with schizophrenia, first-episode psychosis (FEP) represents a cocktail of mounting genetic and environmental factors. Typically, by the time a patient receives psychiatric care, they present with characteristic psychotic symptoms—hallucinations, delusions, bizarre behavior, and unusual thought process—along with a unique gut microbiome profile.
This disrupted microbiome coincides with a marked state of inflammation in the intestines. Inflammation triggers increased endothelial barrier permeability, similar to the way immune signals increase capillary permeability to allow immune cells into the periphery of the blood. Specific gut bacteria play specific roles in maintaining the gut barrier.18,19 Disruptions in the bacteria that maintain the gut barrier, combined with inflammation, contribute to a leaky gut. A leaky gut barrier allows bacterial and immune products to more easily enter the bloodstream and then the brain, which is a potential source of neuroinflammation in schizophrenia.20 This increase in gut permeability (leaky gut syndrome) is likely one of several reasons low-grade inflammation is common in schizophrenia—numerous studies show higher serum levels of proinflammatory cytokines along with antibacterial immunoglobulins in patients with FEP.21,22
Fortunately, antipsychotics, especially the second-generation agents, help restore a healthy gut microbiome and have substantial anti-inflammatory properties.23,24 These medications interact heavily with the gut microbiome: they have been found to have antibiotic properties, even in doses lower than would normally reach the gut microbiome.25 In humans, a randomized controlled trial of probiotic supplementation for schizophrenia patients taking antipsychotics showed a reduction in GI symptoms but no significant improvement in psychotic symptoms.26
Dysbiosis in schizophrenia: cause or effect?
There is no consensus on what constitutes a healthy gut microbiome because the gut microbiome is highly variable, even among healthy individuals, and can change quickly. Those who adopt new diets, for example, see drastic shifts in the gut microbiome within a few days.27 Despite this variation, the main separation between a healthy and dysbiotic gut comes from the diversity of bacteria present in the gut—a healthy gut microbiome is associated with increased diversity. Numerous disease states have been associated with decreased bacterial diversity, including Clostridium difficile infection, Parkinson disease, depression, Crohn disease, and schizophrenia spectrum disorders.28,29
Although there are ethical limitations to studying causality in humans directly, animal models have provided a great deal of insight into the gut microbiome’s role in the development of schizophrenia. A recent study used fecal transplant to provide the gut microbiome from patients with schizophrenia to a group of germ-free mice and compared these animals to a group of mice that received a fecal transplant from individuals with a healthy gut microbiome. The mice receiving the schizophrenia microbiome showed an increased startle response and hyperactivity.3 This was consistent with mouse models of schizophrenia, although with obvious limitations.30 In addition, the brains of these animals showed changes in glutamate, glutamine, and GABA in the hippocampus; these chemicals play a role in the neurophysiology of schizophrenia.3,31 This study has not yet been replicated, and considerable variation remains within the schizophrenia biosignature.
Continue to: Clinical symptoms of psychosis and the gut microbiome
Clinical symptoms of psychosis and the gut microbiome
Previous literature has grouped patients with schizophrenia spectrum disorders as 1 unified study group. But as is the case with many psychiatric conditions, there is a great deal of heterogeneity in neurobiology, genetics, and microbiome composition among individuals with schizophrenia.32
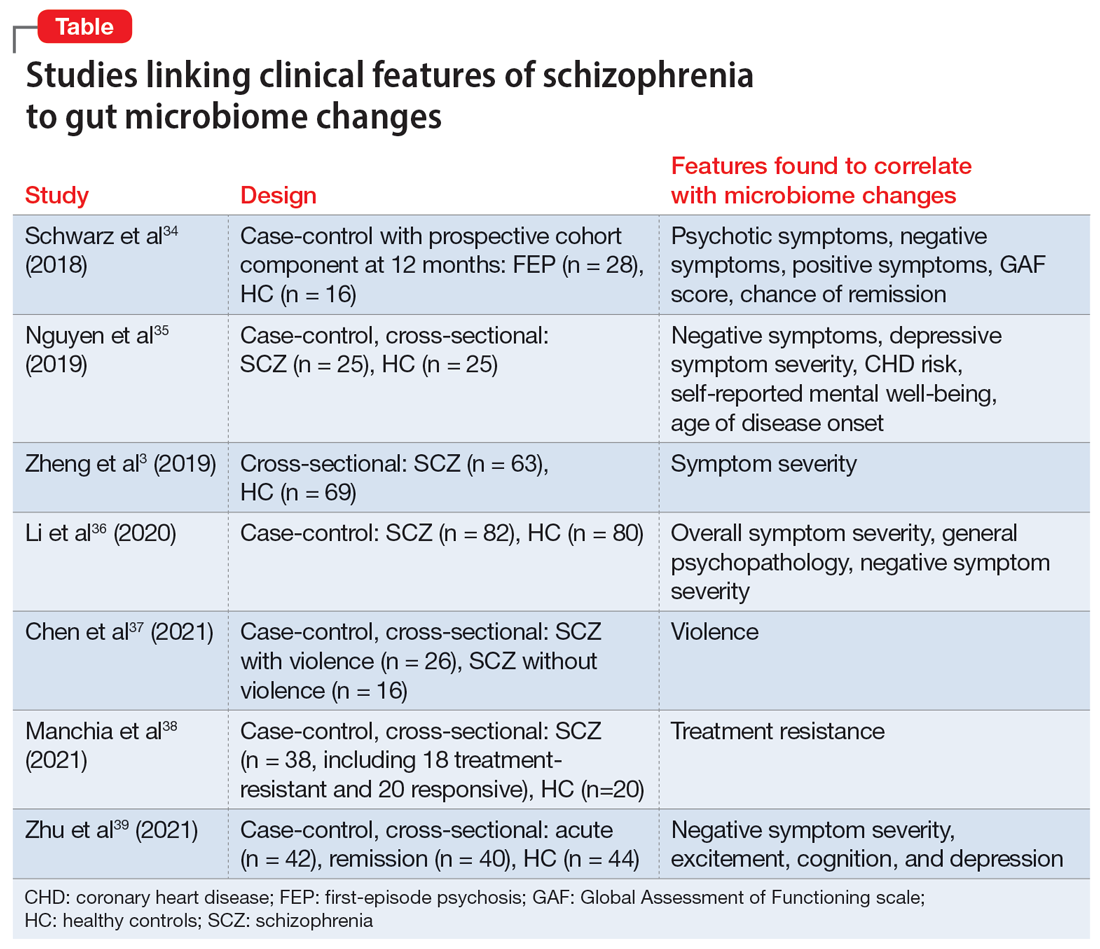
Researchers have begun to investigate ways in which the gut microbiome varies regarding the clinical symptoms of psychosis.33 The Table3,34-39 provides an overview of 7 human studies of gut microbiome changes relating to clinical features of schizophrenia. In these studies, researchers have found correlations between the gut microbiome and a tendency toward violence,37 cognitive deficits,34-36,39 depressive symptoms,35,39 and numerous other clinical features of psychosis. Most of these correlations have not yet been replicated by further studies. But among studies with similar clinical questions, 3 reported changes in gut microbiome correlated with overall symptom severity, and 4 studies correlated changes with negative symptom severity. In 2 studies,3,34Lachnospiraceae was correlated with worsened symptom severity. However, this may have been the result of poor control for antipsychotic use, as 1 study in bipolar patients found that Lachnospiraceae was increased in those taking antipsychotics compared to those who were not treated with antipsychotics.40 The specific shifts in bacteria seen for overall symptom and negative symptom severity were not consistent across studies. This is not surprising because the gut microbiome varies with diet and geographic region,41 and patients in these studies were from a variety of regions. Multiple studies demonstrated gut microbiome alterations for patients with more severe negative symptoms. This is particularly interesting because negative symptoms are often difficult to treat and do not respond to antipsychotics.42 This research suggests the gut microbiome may be helpful in developing future treatments for patients with negative symptoms that do not respond to existing treatments.
Research of probiotic supplementation for ameliorating symptoms of schizophrenia has yielded mixed results.43 It is possible that studies of probiotic supplementation have failed to consider the variations in the gut microbiome among individuals with schizophrenia. A better understanding of the variations in gut microbiome may allow for the development of more personalized interventions.
Recommendations for a healthy gut microbiome
In addition to antipsychotics, many other evidence-based interventions can be used to help restore a healthy gut microbiome in patients with schizophrenia. To improve the gut microbiome, we suggest discussing the following changes with patients:
- Quitting smoking. Smoking is common among patients with schizophrenia but decreases gut microbiome diversity.44
- Avoiding excessive alcohol use. Excessive alcohol use contributes to dysbiosis and increased intestinal permeability.45 Moderate alcohol consumption does not appear to have the same harmful effects on the microbiome.46
- Avoiding the use of recreational drugs, including marijuana, which impact the gut microbiome.47
- Consuming a diet rich in fiber.48 Presently, there is not enough evidence to recommend probiotic supplementation to reduce symptoms of schizophrenia.41 Similar to probiotics, fermented foods contain Lactobacillus, a bacterial species that produces lactic acid.49Lactobacillus is enriched in the gut microbiome in some neurodegenerative diseases, and lactic acid can be neurotoxic at high levels.50-52 Therefore, clinicians should not explicitly recommend fermented foods under the assumption of improved brain health. A diet rich in soluble fiber has been consistently shown to promote anti-inflammatory bacteria and is much more likely to be beneficial.53,54 Soluble fiber is found in foods such as fruits, vegetables, beans, and oats.
- Exercising can increase microbiome diversity and provide anti-inflammatory effects in the gut.55,56 A recent review found that steady-state aerobic and high-intensity exercise interventions have positive effects on mood, cognition, and other negative symptoms in patients with schizophrenia.55
- Minimizing stress. Psychological stress and physiological stress from untreated medical conditions are toxic to healthy gut bacteria and weaken the gut barrier.57
- Mitigating exposure to pollution. Environmental pollution, including exposures to air pollution, heavy metals, and pesticides, disrupts the gut microbiome.58
The American Heart Association publishes lifestyle recommendations for individuals with heart disease and the National Institutes of Health publishes lifestyle recommendations for patients with chronic kidney disease. This leads us to question why the American Psychiatric Association has not published lifestyle recommendations for those with severe mental illness. The effects of lifestyle on both the gut microbiome and symptom mitigation is critical. With increasingly shortened appointments, standardized guidelines would benefit psychiatrists and patients alike.
Bottom Line
The gut microbiome is connected to the clinical symptoms of psychosis via a variety of hormonal, neuroimmune, and metabolic mechanisms active across the lifespan. Despite advances in research, there is still much to be understood regarding this relationship. Clinicians should discuss with patients ways to promote a healthy gut microbiome, including consuming a diet rich in fiber, avoiding use of recreational drugs, and exercising regularly.
Related Resources
- Nocera A, Nasrallah HA. The association of the gut microbiota with clinical features in schizophrenia. Behav Sci. 2022;12(4):89.
- Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
1. Bäckhed F, Ley RE, Sonnenburg JL, et al. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915-1920. doi:10.1126/science.1104816
2. Jackson SW. Galen—on mental disorders. J Hist Behav Sci. 1969;5(4):365-384. doi:10.1002/1520-6696(196910)5:4<365::AID-JHBS2300050408>3.0.CO;2-9
3. Zheng P, Zeng B, Liu M, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv. 2019;5(2):eaau8317. doi:10.1126/sciadv.aau8317
4. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421-427. doi:10.1038/nature13595
5. Gill SR, Pop M, DeBoy RT, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312(5778):1355-1359. doi:10.1126/science.1124234
6. Alam R, Abdolmaleky HM, Zhou JR. Microbiome, inflammation, epigenetic alterations, and mental diseases. Am J Med Genet B Neuropsychiatr Genet. 2017;174(6):651-660. doi:10.1002/ajmg.b.32567
7. Cryan JF, O’Riordan KJ, Cowan CSM, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877-2013. doi:10.1152/physrev.00018.2018
8. Mueller NT, Bakacs E, Combellick J, et al. The infant microbiome development: mom matters. Trends Mol Med. 2015;21(2):109-117. doi:10.1016/j.molmed.2014.12.002
9. Fouhy F, Watkins C, Hill CJ, et al. Perinatal factors affect the gut microbiota up to four years after birth. Nat Commun. 2019;10(1):1517. doi:10.1038/s41467-019-09252-4
10. Sharon G, Sampson TR, Geschwind DH, et al. The central nervous system and the gut microbiome. Cell. 2016;167(4):915-932. doi:10.1016/j.cell.2016.10.027
11. Hill CJ, Lynch DB, Murphy K, et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome. 2017;5:4. doi:10.1186/s40168-016-0213-y
12. Gareau MG, Wine E, Rodrigues DM, et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60(3):307-317. doi:10.1136/gut.2009.202515
13. Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016;8(343):343ra82. doi:10.1126/scitranslmed.aad7121
14. Mancabelli L, Milani C, Lugli GA, et al. Meta-analysis of the human gut microbiome from urbanized and pre-agricultural populations. Environ Microbiol. 2017;19(4):1379-1390. doi:10.1111/1462-2920.13692
15. Stilo SA, Murray RM. Non-genetic factors in schizophrenia. Curr Psychiatry Rep. 2019;21(10):100. doi:10.1007/s11920-019-1091-3
16. Buscaino VM. Patologia extraneurale della schizofrenia: fegato, tubo digerente, sistema reticolo-endoteliale. Acta Neurologica. 1953;VIII:1-60.
17. Hemmings G. Schizophrenia. Lancet. 2004;364(9442):1312-1313. doi:10.1016/S0140- 6736(04)17181-X
18. Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292(5519):1115-1118. doi:10.1126/science.1058709
19. Ewaschuk JB, Diaz H, Meddings L, et al. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol-Gastrointest Liver Physiol. 2008;295(5):G1025-G1034. doi:10.1152/ajpgi.90227.2008
20. Alhasson F, Das S, Seth R, et al. Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS One. 2017;12(3):e0172914. doi:10.1371/journal.pone.0172914
21. Fillman SG, Cloonan N, Catts VS, et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18(2):206-214. doi:10.1038/mp.2012.110
22. Miller BJ, Buckley P, Seabolt W, et al. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663-671. doi:10.1016/j.biopsych.2011.04.013
23. Al-Amin M, Uddin MMN, Reza HM. Effects of antipsychotics on the inflammatory response system of patients with schizophrenia in peripheral blood mononuclear cell cultures. Clin Psychopharmacol Neurosci. 2013;11(3):144-151. doi:10.9758/cpn.2013.11.3.144
24. Yuan X, Zhang P, Wang Y, et al. Changes in metabolism and microbiota after 24-week risperidone treatment in drug naïve, normal weight patients with first episode schizophrenia. Schizophr Res. 2018;201:299-306. doi:10.1016/j.schres.2018.05.017
25. Maier L, Pruteanu M, Kuhn M, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555(7698):623-628. doi:10.1038/nature25979
26. Dickerson FB, Stallings C, Origoni A, et al. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: a randomized, placebo-controlled trial. Prim Care Companion CNS Disord. 2014;15(1):PCC.13m01579. doi:10.4088/PCC.13m01579
27. David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559-563. doi:10.1038/nature12820
28. Bien J, Palagani V, Bozko P. The intestinal microbiota dysbiosis and Clostridium difficile infection: is there a relationship with inflammatory bowel disease? Ther Adv Gastroenterol. 2013;6(1):53-68. doi:10.1177/1756283X12454590
29. Cryan JF, O’Riordan KJ, Sandhu K, et al. The gut microbiome in neurological disorders. Lancet Neurol. 2020;19(2):179-194. doi:10.1016/S1474-4422(19)30356-4
30. Jones CA, Watson DJG, Fone KCF. Animal models of schizophrenia. Br J Pharmacol. 2011;164(4):1162-1194. doi:10.1111/j.1476-5381.2011.01386.x
31. Schmidt MJ, Mirnics K. Neurodevelopment, GABA system dysfunction, and schizophrenia. Neuropsychopharmacology. 2015;40(1):190-206. doi:10.1038/npp.2014.95
32. Nasrallah, HA. The daunting challenge of schizophrenia: hundreds of biotypes and dozens of theories. Curr. Psychiatry 2018;17(12):4-6,50.
33. Nocera A, Nasrallah HA. The association of the gut microbiota with clinical features in schizophrenia. Behav Sci (Basel). 2022;12(4):89. doi:10.3390/bs12040089
34. Schwarz E, Maukonen J, Hyytiäinen T, et al. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr Res. 2018;192:398-403. doi:10.1016/j.schres.2017.04.017
35. Nguyen TT, Kosciolek T, Maldonado Y, et al. Differences in gut microbiome composition between persons with chronic schizophrenia and healthy comparison subjects. Schizophr Res. 2019;204:23-29. doi:10.1016/j.schres.2018.09.014
36. Li S, Zhuo M, Huang X, et al. Altered gut microbiota associated with symptom severity in schizophrenia. PeerJ. 2020;8:e9574. doi:10.7717/peerj.9574
37. Chen X, Xu J, Wang H, et al. Profiling the differences of gut microbial structure between schizophrenia patients with and without violent behaviors based on 16S rRNA gene sequencing. Int J Legal Med. 2021;135(1):131-141. doi:10.1007/s00414-020-02439-1
38. Manchia M, Fontana A, Panebianco C, et al. Involvement of gut microbiota in schizophrenia and treatment resistance to antipsychotics. Biomedicines. 2021;9(8):875. doi:10.3390/biomedicines9080875
39. Zhu C, Zheng M, Ali U, et al. Association between abundance of haemophilus in the gut microbiota and negative symptoms of schizophrenia. Front Psychiatry. 2021;12:685910. doi:10.3389/fpsyt.2021.685910
40. Flowers SA, Evans SJ, Ward KM, et al. Interaction between atypical antipsychotics and the gut microbiome in a bipolar disease cohort. Pharmacotherapy. 2017;37(3):261-267. doi:10.1002/phar.1890
41. Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222-227. doi:10.1038/nature11053
42. Buchanan RW. Persistent negative symptoms in schizophrenia: an overview. Schizophr Bull. 2007;33(4):1013-1022. doi:10.1093/schbul/sb1057
43. Liu JCW, Gorbovskaya I, Hahn MK, et al. The gut microbiome in schizophrenia and the potential benefits of prebiotic and probiotic treatment. Nutrients. 2021;13(4):1152. doi:10.3390/nu13041152
44. Biedermann L, Zeitz J, Mwinyi J, et al. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PloS One. 2013;8(3):e59260. doi:10.1371/journal.pone.0059260
45. Leclercq S, Matamoros S, Cani PD, et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci. 2014;111(42):e4485-e4493. doi:10.1073/pnas.1415174111
46. Hernández-Quiroz F, Nirmalkar K, Villalobos-Flores LE, et al. Influence of moderate beer consumption on human gut microbiota and its impact on fasting glucose and ß-cell function. Alcohol. 2020;85:77-94. doi:10.1016/j.alcohol.2019.05.006
47. Panee J, Gerschenson M, Chang L. Associations between microbiota, mitochondrial function, and cognition in chronic marijuana users. J Neuroimmune Pharmacol. 2018;13(1):113-122. doi:10.1007/s11481-017-9767-0
48. Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105-108. doi:10.1126/science.1208344
49. Rezac S, Kok CR, Heermann M, et al. Fermented foods as a dietary source of live organisms. Front Microbiol. 2018;9:1785. doi:10.3389/fmicb.2018.01785
50. Chen X, Zhang Y, Wang H, et al. The regulatory effects of lactic acid on neuropsychiatric disorders. Discover Ment Health. 2022;2(1). doi:10.1007/s44192-022-00011-4
51. Karbownik MS, Mokros Ł, Dobielska M, et al. Association between consumption of fermented food and food-derived prebiotics with cognitive performance, depressive, and anxiety symptoms in psychiatrically healthy medical students under psychological stress: a prospective cohort study. Front Nutr. 2022;9:850249. doi:10.3389/fnut.2022.850249
52. Romano S, Savva GM, Bedarf JR, et al. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Parkinsons Dis. 2021;7(1):27. doi:10.1038/s41531-021-00156-z
53. Bourassa MW, Alim I, Bultman SJ, et al. Butyrate, neuroepigenetics and the gut microbiome: can a high fiber diet improve brain health? Neurosci Lett. 2016;625:56-63. doi:10.1016/j.neulet.2016.02.009
54. Matt SM, Allen JM, Lawson MA, et al. Butyrate and dietary soluble fiber improve neuroinflammation associated with aging in mice. Front Immunol. 2018;9:1832. doi:10.3389/fimmu.2018.01832
55. Mittal VA, Vargas T, Osborne KJ, et al. Exercise treatments for psychosis: a review. Curr Treat Options Psychiatry. 2017;4(2):152-166. doi:10.1007/s40501-017-0112-2
56. Estaki M, Pither J, Baumeister P, et al. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome. 2016;4(1):42. doi:10.1186/s40168-016-0189-7
57. Karl JP, Margolis LM, Madslien EH, et al. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am J Physiol-Gastrointest Liver Physiol. 2017;312(6):G559-G571. doi:10.1152/ajpgi.00066.2017
58. Claus SP, Guillou H, Ellero-Simatos S. The gut microbiota: a major player in the toxicity of environmental pollutants? NPJ Biofilms Microbiomes. 2016;2:16003. doi:10.1038/npjbiofilms.2016.3
The human microbiota refers to the collection of bacteria, archaea, eukarya, and viruses that reside within the human body. The term gut microbiome indicates the composition of these microbes and genetic codes in the intestine.1 Harkening back to the ancient Greek physician Galen, who treated gastrointestinal (GI) symptoms to relieve mental disturbances such as psychosis, the gut has been a therapeutic target in schizophrenia long before antipsychotics and the DSM.2 In recent years, research into the gut microbiome has drastically increased, with genetic sequencing affording a more precise look into the specific bacteria that call the human intestines their home. This has led to the recognition that the gut microbiome may be severely disrupted in schizophrenia, a condition known as dysbiosis. Preliminary research suggests that gut bacteria are more helpful than many human genes in distinguishing individuals with schizophrenia from their healthy counterparts.3,4 In this article, we discuss the potential role of the gut microbiome in schizophrenia, including new research correlating clinical symptoms of psychosis with dysbiosis. We also provide recommendations for promoting a healthy gut microbiome.
The enteric brain across life
The composition of our bodies is far more microbiota than human. Strikingly, microbiota cells in the gut outnumber human cells, and the distal gut alone hosts bacteria with 100 times the genetic content of the entire genome.5 The intricate meshwork of nerves in the gut is often called the enteric brain because the gut consists of 100 million neurons and synthesizes many neuroactive chemicals implicated in mood disorders and psychosis, including serotonin, dopamine, gamma-aminobutyric acid (GABA), and acetylcholine.6 The variety of neuroimmunologic, hormonal, and metabolic paths by which the gutmicrobiome and the brain interact are collectively known as the gut-microbiota-brainaxis.7
How do we acquire our gut microbiome, and how does it come to influence ourbrain and behavior? On the first day of life, as babies pass through the birth canal, they are bathed in their mother’s vaginal microbiota. In the following weeks, the microbiome expands and colonizes the gut as bacteria are introduced from environmental sources such as skin-to-skin contact and breastmilk.8 The microbiome continues to evolve throughout early life. As children expand their diets and navigate new aspects of the physical world, additional bacteria join the unseen ecosystem growing inside.9 The development of the microbiome coincides with the development of the brain. From preclinical studies, we know the gut microbiome mediates important aspects of neurodevelopment such as the formation of the blood-brain barrier (BBB), synaptic pruning, glial activation, and myelination.10 Interestingly, many of the risk factors for schizophrenia are associated with gut dysbiosis, including obstetric complications, infections treated with antibiotics, and urbanization.11-15
Throughout human life, the gut and brain remain in close communication. The gut microbiota continue to produce monoamines, along with other metabolites that are able to cross the BBB.6 The HPA axis, stimulation of the immune system, and the vagus nerve all provide highways of communication between the gut and the brain.7 The relationship between the enteric brain and cephalic brain continues through life, even up to a person’s final hour. One autopsy study that is often cited (but soberingly, cannot be found online) allegedly revealed that 92% of schizophrenia patients had developed colitis by the time of death.16,17
First-episode psychosis and antipsychotic treatment
For patients with schizophrenia, first-episode psychosis (FEP) represents a cocktail of mounting genetic and environmental factors. Typically, by the time a patient receives psychiatric care, they present with characteristic psychotic symptoms—hallucinations, delusions, bizarre behavior, and unusual thought process—along with a unique gut microbiome profile.
This disrupted microbiome coincides with a marked state of inflammation in the intestines. Inflammation triggers increased endothelial barrier permeability, similar to the way immune signals increase capillary permeability to allow immune cells into the periphery of the blood. Specific gut bacteria play specific roles in maintaining the gut barrier.18,19 Disruptions in the bacteria that maintain the gut barrier, combined with inflammation, contribute to a leaky gut. A leaky gut barrier allows bacterial and immune products to more easily enter the bloodstream and then the brain, which is a potential source of neuroinflammation in schizophrenia.20 This increase in gut permeability (leaky gut syndrome) is likely one of several reasons low-grade inflammation is common in schizophrenia—numerous studies show higher serum levels of proinflammatory cytokines along with antibacterial immunoglobulins in patients with FEP.21,22
Fortunately, antipsychotics, especially the second-generation agents, help restore a healthy gut microbiome and have substantial anti-inflammatory properties.23,24 These medications interact heavily with the gut microbiome: they have been found to have antibiotic properties, even in doses lower than would normally reach the gut microbiome.25 In humans, a randomized controlled trial of probiotic supplementation for schizophrenia patients taking antipsychotics showed a reduction in GI symptoms but no significant improvement in psychotic symptoms.26
Dysbiosis in schizophrenia: cause or effect?
There is no consensus on what constitutes a healthy gut microbiome because the gut microbiome is highly variable, even among healthy individuals, and can change quickly. Those who adopt new diets, for example, see drastic shifts in the gut microbiome within a few days.27 Despite this variation, the main separation between a healthy and dysbiotic gut comes from the diversity of bacteria present in the gut—a healthy gut microbiome is associated with increased diversity. Numerous disease states have been associated with decreased bacterial diversity, including Clostridium difficile infection, Parkinson disease, depression, Crohn disease, and schizophrenia spectrum disorders.28,29
Although there are ethical limitations to studying causality in humans directly, animal models have provided a great deal of insight into the gut microbiome’s role in the development of schizophrenia. A recent study used fecal transplant to provide the gut microbiome from patients with schizophrenia to a group of germ-free mice and compared these animals to a group of mice that received a fecal transplant from individuals with a healthy gut microbiome. The mice receiving the schizophrenia microbiome showed an increased startle response and hyperactivity.3 This was consistent with mouse models of schizophrenia, although with obvious limitations.30 In addition, the brains of these animals showed changes in glutamate, glutamine, and GABA in the hippocampus; these chemicals play a role in the neurophysiology of schizophrenia.3,31 This study has not yet been replicated, and considerable variation remains within the schizophrenia biosignature.
Continue to: Clinical symptoms of psychosis and the gut microbiome
Clinical symptoms of psychosis and the gut microbiome
Previous literature has grouped patients with schizophrenia spectrum disorders as 1 unified study group. But as is the case with many psychiatric conditions, there is a great deal of heterogeneity in neurobiology, genetics, and microbiome composition among individuals with schizophrenia.32
Researchers have begun to investigate ways in which the gut microbiome varies regarding the clinical symptoms of psychosis.33 The Table3,34-39 provides an overview of 7 human studies of gut microbiome changes relating to clinical features of schizophrenia. In these studies, researchers have found correlations between the gut microbiome and a tendency toward violence,37 cognitive deficits,34-36,39 depressive symptoms,35,39 and numerous other clinical features of psychosis. Most of these correlations have not yet been replicated by further studies. But among studies with similar clinical questions, 3 reported changes in gut microbiome correlated with overall symptom severity, and 4 studies correlated changes with negative symptom severity. In 2 studies,3,34Lachnospiraceae was correlated with worsened symptom severity. However, this may have been the result of poor control for antipsychotic use, as 1 study in bipolar patients found that Lachnospiraceae was increased in those taking antipsychotics compared to those who were not treated with antipsychotics.40 The specific shifts in bacteria seen for overall symptom and negative symptom severity were not consistent across studies. This is not surprising because the gut microbiome varies with diet and geographic region,41 and patients in these studies were from a variety of regions. Multiple studies demonstrated gut microbiome alterations for patients with more severe negative symptoms. This is particularly interesting because negative symptoms are often difficult to treat and do not respond to antipsychotics.42 This research suggests the gut microbiome may be helpful in developing future treatments for patients with negative symptoms that do not respond to existing treatments.
Research of probiotic supplementation for ameliorating symptoms of schizophrenia has yielded mixed results.43 It is possible that studies of probiotic supplementation have failed to consider the variations in the gut microbiome among individuals with schizophrenia. A better understanding of the variations in gut microbiome may allow for the development of more personalized interventions.

Recommendations for a healthy gut microbiome
In addition to antipsychotics, many other evidence-based interventions can be used to help restore a healthy gut microbiome in patients with schizophrenia. To improve the gut microbiome, we suggest discussing the following changes with patients:
- Quitting smoking. Smoking is common among patients with schizophrenia but decreases gut microbiome diversity.44
- Avoiding excessive alcohol use. Excessive alcohol use contributes to dysbiosis and increased intestinal permeability.45 Moderate alcohol consumption does not appear to have the same harmful effects on the microbiome.46
- Avoiding the use of recreational drugs, including marijuana, which impact the gut microbiome.47
- Consuming a diet rich in fiber.48 Presently, there is not enough evidence to recommend probiotic supplementation to reduce symptoms of schizophrenia.41 Similar to probiotics, fermented foods contain Lactobacillus, a bacterial species that produces lactic acid.49Lactobacillus is enriched in the gut microbiome in some neurodegenerative diseases, and lactic acid can be neurotoxic at high levels.50-52 Therefore, clinicians should not explicitly recommend fermented foods under the assumption of improved brain health. A diet rich in soluble fiber has been consistently shown to promote anti-inflammatory bacteria and is much more likely to be beneficial.53,54 Soluble fiber is found in foods such as fruits, vegetables, beans, and oats.
- Exercising can increase microbiome diversity and provide anti-inflammatory effects in the gut.55,56 A recent review found that steady-state aerobic and high-intensity exercise interventions have positive effects on mood, cognition, and other negative symptoms in patients with schizophrenia.55
- Minimizing stress. Psychological stress and physiological stress from untreated medical conditions are toxic to healthy gut bacteria and weaken the gut barrier.57
- Mitigating exposure to pollution. Environmental pollution, including exposures to air pollution, heavy metals, and pesticides, disrupts the gut microbiome.58
The American Heart Association publishes lifestyle recommendations for individuals with heart disease and the National Institutes of Health publishes lifestyle recommendations for patients with chronic kidney disease. This leads us to question why the American Psychiatric Association has not published lifestyle recommendations for those with severe mental illness. The effects of lifestyle on both the gut microbiome and symptom mitigation is critical. With increasingly shortened appointments, standardized guidelines would benefit psychiatrists and patients alike.
Bottom Line
The gut microbiome is connected to the clinical symptoms of psychosis via a variety of hormonal, neuroimmune, and metabolic mechanisms active across the lifespan. Despite advances in research, there is still much to be understood regarding this relationship. Clinicians should discuss with patients ways to promote a healthy gut microbiome, including consuming a diet rich in fiber, avoiding use of recreational drugs, and exercising regularly.
Related Resources
- Nocera A, Nasrallah HA. The association of the gut microbiota with clinical features in schizophrenia. Behav Sci. 2022;12(4):89.
- Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
The human microbiota refers to the collection of bacteria, archaea, eukarya, and viruses that reside within the human body. The term gut microbiome indicates the composition of these microbes and genetic codes in the intestine.1 Harkening back to the ancient Greek physician Galen, who treated gastrointestinal (GI) symptoms to relieve mental disturbances such as psychosis, the gut has been a therapeutic target in schizophrenia long before antipsychotics and the DSM.2 In recent years, research into the gut microbiome has drastically increased, with genetic sequencing affording a more precise look into the specific bacteria that call the human intestines their home. This has led to the recognition that the gut microbiome may be severely disrupted in schizophrenia, a condition known as dysbiosis. Preliminary research suggests that gut bacteria are more helpful than many human genes in distinguishing individuals with schizophrenia from their healthy counterparts.3,4 In this article, we discuss the potential role of the gut microbiome in schizophrenia, including new research correlating clinical symptoms of psychosis with dysbiosis. We also provide recommendations for promoting a healthy gut microbiome.
The enteric brain across life
The composition of our bodies is far more microbiota than human. Strikingly, microbiota cells in the gut outnumber human cells, and the distal gut alone hosts bacteria with 100 times the genetic content of the entire genome.5 The intricate meshwork of nerves in the gut is often called the enteric brain because the gut consists of 100 million neurons and synthesizes many neuroactive chemicals implicated in mood disorders and psychosis, including serotonin, dopamine, gamma-aminobutyric acid (GABA), and acetylcholine.6 The variety of neuroimmunologic, hormonal, and metabolic paths by which the gutmicrobiome and the brain interact are collectively known as the gut-microbiota-brainaxis.7
How do we acquire our gut microbiome, and how does it come to influence ourbrain and behavior? On the first day of life, as babies pass through the birth canal, they are bathed in their mother’s vaginal microbiota. In the following weeks, the microbiome expands and colonizes the gut as bacteria are introduced from environmental sources such as skin-to-skin contact and breastmilk.8 The microbiome continues to evolve throughout early life. As children expand their diets and navigate new aspects of the physical world, additional bacteria join the unseen ecosystem growing inside.9 The development of the microbiome coincides with the development of the brain. From preclinical studies, we know the gut microbiome mediates important aspects of neurodevelopment such as the formation of the blood-brain barrier (BBB), synaptic pruning, glial activation, and myelination.10 Interestingly, many of the risk factors for schizophrenia are associated with gut dysbiosis, including obstetric complications, infections treated with antibiotics, and urbanization.11-15
Throughout human life, the gut and brain remain in close communication. The gut microbiota continue to produce monoamines, along with other metabolites that are able to cross the BBB.6 The HPA axis, stimulation of the immune system, and the vagus nerve all provide highways of communication between the gut and the brain.7 The relationship between the enteric brain and cephalic brain continues through life, even up to a person’s final hour. One autopsy study that is often cited (but soberingly, cannot be found online) allegedly revealed that 92% of schizophrenia patients had developed colitis by the time of death.16,17
First-episode psychosis and antipsychotic treatment
For patients with schizophrenia, first-episode psychosis (FEP) represents a cocktail of mounting genetic and environmental factors. Typically, by the time a patient receives psychiatric care, they present with characteristic psychotic symptoms—hallucinations, delusions, bizarre behavior, and unusual thought process—along with a unique gut microbiome profile.
This disrupted microbiome coincides with a marked state of inflammation in the intestines. Inflammation triggers increased endothelial barrier permeability, similar to the way immune signals increase capillary permeability to allow immune cells into the periphery of the blood. Specific gut bacteria play specific roles in maintaining the gut barrier.18,19 Disruptions in the bacteria that maintain the gut barrier, combined with inflammation, contribute to a leaky gut. A leaky gut barrier allows bacterial and immune products to more easily enter the bloodstream and then the brain, which is a potential source of neuroinflammation in schizophrenia.20 This increase in gut permeability (leaky gut syndrome) is likely one of several reasons low-grade inflammation is common in schizophrenia—numerous studies show higher serum levels of proinflammatory cytokines along with antibacterial immunoglobulins in patients with FEP.21,22
Fortunately, antipsychotics, especially the second-generation agents, help restore a healthy gut microbiome and have substantial anti-inflammatory properties.23,24 These medications interact heavily with the gut microbiome: they have been found to have antibiotic properties, even in doses lower than would normally reach the gut microbiome.25 In humans, a randomized controlled trial of probiotic supplementation for schizophrenia patients taking antipsychotics showed a reduction in GI symptoms but no significant improvement in psychotic symptoms.26
Dysbiosis in schizophrenia: cause or effect?
There is no consensus on what constitutes a healthy gut microbiome because the gut microbiome is highly variable, even among healthy individuals, and can change quickly. Those who adopt new diets, for example, see drastic shifts in the gut microbiome within a few days.27 Despite this variation, the main separation between a healthy and dysbiotic gut comes from the diversity of bacteria present in the gut—a healthy gut microbiome is associated with increased diversity. Numerous disease states have been associated with decreased bacterial diversity, including Clostridium difficile infection, Parkinson disease, depression, Crohn disease, and schizophrenia spectrum disorders.28,29
Although there are ethical limitations to studying causality in humans directly, animal models have provided a great deal of insight into the gut microbiome’s role in the development of schizophrenia. A recent study used fecal transplant to provide the gut microbiome from patients with schizophrenia to a group of germ-free mice and compared these animals to a group of mice that received a fecal transplant from individuals with a healthy gut microbiome. The mice receiving the schizophrenia microbiome showed an increased startle response and hyperactivity.3 This was consistent with mouse models of schizophrenia, although with obvious limitations.30 In addition, the brains of these animals showed changes in glutamate, glutamine, and GABA in the hippocampus; these chemicals play a role in the neurophysiology of schizophrenia.3,31 This study has not yet been replicated, and considerable variation remains within the schizophrenia biosignature.
Continue to: Clinical symptoms of psychosis and the gut microbiome
Clinical symptoms of psychosis and the gut microbiome
Previous literature has grouped patients with schizophrenia spectrum disorders as 1 unified study group. But as is the case with many psychiatric conditions, there is a great deal of heterogeneity in neurobiology, genetics, and microbiome composition among individuals with schizophrenia.32
Researchers have begun to investigate ways in which the gut microbiome varies regarding the clinical symptoms of psychosis.33 The Table3,34-39 provides an overview of 7 human studies of gut microbiome changes relating to clinical features of schizophrenia. In these studies, researchers have found correlations between the gut microbiome and a tendency toward violence,37 cognitive deficits,34-36,39 depressive symptoms,35,39 and numerous other clinical features of psychosis. Most of these correlations have not yet been replicated by further studies. But among studies with similar clinical questions, 3 reported changes in gut microbiome correlated with overall symptom severity, and 4 studies correlated changes with negative symptom severity. In 2 studies,3,34Lachnospiraceae was correlated with worsened symptom severity. However, this may have been the result of poor control for antipsychotic use, as 1 study in bipolar patients found that Lachnospiraceae was increased in those taking antipsychotics compared to those who were not treated with antipsychotics.40 The specific shifts in bacteria seen for overall symptom and negative symptom severity were not consistent across studies. This is not surprising because the gut microbiome varies with diet and geographic region,41 and patients in these studies were from a variety of regions. Multiple studies demonstrated gut microbiome alterations for patients with more severe negative symptoms. This is particularly interesting because negative symptoms are often difficult to treat and do not respond to antipsychotics.42 This research suggests the gut microbiome may be helpful in developing future treatments for patients with negative symptoms that do not respond to existing treatments.
Research of probiotic supplementation for ameliorating symptoms of schizophrenia has yielded mixed results.43 It is possible that studies of probiotic supplementation have failed to consider the variations in the gut microbiome among individuals with schizophrenia. A better understanding of the variations in gut microbiome may allow for the development of more personalized interventions.

Recommendations for a healthy gut microbiome
In addition to antipsychotics, many other evidence-based interventions can be used to help restore a healthy gut microbiome in patients with schizophrenia. To improve the gut microbiome, we suggest discussing the following changes with patients:
- Quitting smoking. Smoking is common among patients with schizophrenia but decreases gut microbiome diversity.44
- Avoiding excessive alcohol use. Excessive alcohol use contributes to dysbiosis and increased intestinal permeability.45 Moderate alcohol consumption does not appear to have the same harmful effects on the microbiome.46
- Avoiding the use of recreational drugs, including marijuana, which impact the gut microbiome.47
- Consuming a diet rich in fiber.48 Presently, there is not enough evidence to recommend probiotic supplementation to reduce symptoms of schizophrenia.41 Similar to probiotics, fermented foods contain Lactobacillus, a bacterial species that produces lactic acid.49Lactobacillus is enriched in the gut microbiome in some neurodegenerative diseases, and lactic acid can be neurotoxic at high levels.50-52 Therefore, clinicians should not explicitly recommend fermented foods under the assumption of improved brain health. A diet rich in soluble fiber has been consistently shown to promote anti-inflammatory bacteria and is much more likely to be beneficial.53,54 Soluble fiber is found in foods such as fruits, vegetables, beans, and oats.
- Exercising can increase microbiome diversity and provide anti-inflammatory effects in the gut.55,56 A recent review found that steady-state aerobic and high-intensity exercise interventions have positive effects on mood, cognition, and other negative symptoms in patients with schizophrenia.55
- Minimizing stress. Psychological stress and physiological stress from untreated medical conditions are toxic to healthy gut bacteria and weaken the gut barrier.57
- Mitigating exposure to pollution. Environmental pollution, including exposures to air pollution, heavy metals, and pesticides, disrupts the gut microbiome.58
The American Heart Association publishes lifestyle recommendations for individuals with heart disease and the National Institutes of Health publishes lifestyle recommendations for patients with chronic kidney disease. This leads us to question why the American Psychiatric Association has not published lifestyle recommendations for those with severe mental illness. The effects of lifestyle on both the gut microbiome and symptom mitigation is critical. With increasingly shortened appointments, standardized guidelines would benefit psychiatrists and patients alike.
Bottom Line
The gut microbiome is connected to the clinical symptoms of psychosis via a variety of hormonal, neuroimmune, and metabolic mechanisms active across the lifespan. Despite advances in research, there is still much to be understood regarding this relationship. Clinicians should discuss with patients ways to promote a healthy gut microbiome, including consuming a diet rich in fiber, avoiding use of recreational drugs, and exercising regularly.
Related Resources
- Nocera A, Nasrallah HA. The association of the gut microbiota with clinical features in schizophrenia. Behav Sci. 2022;12(4):89.
- Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
1. Bäckhed F, Ley RE, Sonnenburg JL, et al. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915-1920. doi:10.1126/science.1104816
2. Jackson SW. Galen—on mental disorders. J Hist Behav Sci. 1969;5(4):365-384. doi:10.1002/1520-6696(196910)5:4<365::AID-JHBS2300050408>3.0.CO;2-9
3. Zheng P, Zeng B, Liu M, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv. 2019;5(2):eaau8317. doi:10.1126/sciadv.aau8317
4. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421-427. doi:10.1038/nature13595
5. Gill SR, Pop M, DeBoy RT, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312(5778):1355-1359. doi:10.1126/science.1124234
6. Alam R, Abdolmaleky HM, Zhou JR. Microbiome, inflammation, epigenetic alterations, and mental diseases. Am J Med Genet B Neuropsychiatr Genet. 2017;174(6):651-660. doi:10.1002/ajmg.b.32567
7. Cryan JF, O’Riordan KJ, Cowan CSM, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877-2013. doi:10.1152/physrev.00018.2018
8. Mueller NT, Bakacs E, Combellick J, et al. The infant microbiome development: mom matters. Trends Mol Med. 2015;21(2):109-117. doi:10.1016/j.molmed.2014.12.002
9. Fouhy F, Watkins C, Hill CJ, et al. Perinatal factors affect the gut microbiota up to four years after birth. Nat Commun. 2019;10(1):1517. doi:10.1038/s41467-019-09252-4
10. Sharon G, Sampson TR, Geschwind DH, et al. The central nervous system and the gut microbiome. Cell. 2016;167(4):915-932. doi:10.1016/j.cell.2016.10.027
11. Hill CJ, Lynch DB, Murphy K, et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome. 2017;5:4. doi:10.1186/s40168-016-0213-y
12. Gareau MG, Wine E, Rodrigues DM, et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60(3):307-317. doi:10.1136/gut.2009.202515
13. Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016;8(343):343ra82. doi:10.1126/scitranslmed.aad7121
14. Mancabelli L, Milani C, Lugli GA, et al. Meta-analysis of the human gut microbiome from urbanized and pre-agricultural populations. Environ Microbiol. 2017;19(4):1379-1390. doi:10.1111/1462-2920.13692
15. Stilo SA, Murray RM. Non-genetic factors in schizophrenia. Curr Psychiatry Rep. 2019;21(10):100. doi:10.1007/s11920-019-1091-3
16. Buscaino VM. Patologia extraneurale della schizofrenia: fegato, tubo digerente, sistema reticolo-endoteliale. Acta Neurologica. 1953;VIII:1-60.
17. Hemmings G. Schizophrenia. Lancet. 2004;364(9442):1312-1313. doi:10.1016/S0140- 6736(04)17181-X
18. Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292(5519):1115-1118. doi:10.1126/science.1058709
19. Ewaschuk JB, Diaz H, Meddings L, et al. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol-Gastrointest Liver Physiol. 2008;295(5):G1025-G1034. doi:10.1152/ajpgi.90227.2008
20. Alhasson F, Das S, Seth R, et al. Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS One. 2017;12(3):e0172914. doi:10.1371/journal.pone.0172914
21. Fillman SG, Cloonan N, Catts VS, et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18(2):206-214. doi:10.1038/mp.2012.110
22. Miller BJ, Buckley P, Seabolt W, et al. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663-671. doi:10.1016/j.biopsych.2011.04.013
23. Al-Amin M, Uddin MMN, Reza HM. Effects of antipsychotics on the inflammatory response system of patients with schizophrenia in peripheral blood mononuclear cell cultures. Clin Psychopharmacol Neurosci. 2013;11(3):144-151. doi:10.9758/cpn.2013.11.3.144
24. Yuan X, Zhang P, Wang Y, et al. Changes in metabolism and microbiota after 24-week risperidone treatment in drug naïve, normal weight patients with first episode schizophrenia. Schizophr Res. 2018;201:299-306. doi:10.1016/j.schres.2018.05.017
25. Maier L, Pruteanu M, Kuhn M, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555(7698):623-628. doi:10.1038/nature25979
26. Dickerson FB, Stallings C, Origoni A, et al. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: a randomized, placebo-controlled trial. Prim Care Companion CNS Disord. 2014;15(1):PCC.13m01579. doi:10.4088/PCC.13m01579
27. David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559-563. doi:10.1038/nature12820
28. Bien J, Palagani V, Bozko P. The intestinal microbiota dysbiosis and Clostridium difficile infection: is there a relationship with inflammatory bowel disease? Ther Adv Gastroenterol. 2013;6(1):53-68. doi:10.1177/1756283X12454590
29. Cryan JF, O’Riordan KJ, Sandhu K, et al. The gut microbiome in neurological disorders. Lancet Neurol. 2020;19(2):179-194. doi:10.1016/S1474-4422(19)30356-4
30. Jones CA, Watson DJG, Fone KCF. Animal models of schizophrenia. Br J Pharmacol. 2011;164(4):1162-1194. doi:10.1111/j.1476-5381.2011.01386.x
31. Schmidt MJ, Mirnics K. Neurodevelopment, GABA system dysfunction, and schizophrenia. Neuropsychopharmacology. 2015;40(1):190-206. doi:10.1038/npp.2014.95
32. Nasrallah, HA. The daunting challenge of schizophrenia: hundreds of biotypes and dozens of theories. Curr. Psychiatry 2018;17(12):4-6,50.
33. Nocera A, Nasrallah HA. The association of the gut microbiota with clinical features in schizophrenia. Behav Sci (Basel). 2022;12(4):89. doi:10.3390/bs12040089
34. Schwarz E, Maukonen J, Hyytiäinen T, et al. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr Res. 2018;192:398-403. doi:10.1016/j.schres.2017.04.017
35. Nguyen TT, Kosciolek T, Maldonado Y, et al. Differences in gut microbiome composition between persons with chronic schizophrenia and healthy comparison subjects. Schizophr Res. 2019;204:23-29. doi:10.1016/j.schres.2018.09.014
36. Li S, Zhuo M, Huang X, et al. Altered gut microbiota associated with symptom severity in schizophrenia. PeerJ. 2020;8:e9574. doi:10.7717/peerj.9574
37. Chen X, Xu J, Wang H, et al. Profiling the differences of gut microbial structure between schizophrenia patients with and without violent behaviors based on 16S rRNA gene sequencing. Int J Legal Med. 2021;135(1):131-141. doi:10.1007/s00414-020-02439-1
38. Manchia M, Fontana A, Panebianco C, et al. Involvement of gut microbiota in schizophrenia and treatment resistance to antipsychotics. Biomedicines. 2021;9(8):875. doi:10.3390/biomedicines9080875
39. Zhu C, Zheng M, Ali U, et al. Association between abundance of haemophilus in the gut microbiota and negative symptoms of schizophrenia. Front Psychiatry. 2021;12:685910. doi:10.3389/fpsyt.2021.685910
40. Flowers SA, Evans SJ, Ward KM, et al. Interaction between atypical antipsychotics and the gut microbiome in a bipolar disease cohort. Pharmacotherapy. 2017;37(3):261-267. doi:10.1002/phar.1890
41. Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222-227. doi:10.1038/nature11053
42. Buchanan RW. Persistent negative symptoms in schizophrenia: an overview. Schizophr Bull. 2007;33(4):1013-1022. doi:10.1093/schbul/sb1057
43. Liu JCW, Gorbovskaya I, Hahn MK, et al. The gut microbiome in schizophrenia and the potential benefits of prebiotic and probiotic treatment. Nutrients. 2021;13(4):1152. doi:10.3390/nu13041152
44. Biedermann L, Zeitz J, Mwinyi J, et al. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PloS One. 2013;8(3):e59260. doi:10.1371/journal.pone.0059260
45. Leclercq S, Matamoros S, Cani PD, et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci. 2014;111(42):e4485-e4493. doi:10.1073/pnas.1415174111
46. Hernández-Quiroz F, Nirmalkar K, Villalobos-Flores LE, et al. Influence of moderate beer consumption on human gut microbiota and its impact on fasting glucose and ß-cell function. Alcohol. 2020;85:77-94. doi:10.1016/j.alcohol.2019.05.006
47. Panee J, Gerschenson M, Chang L. Associations between microbiota, mitochondrial function, and cognition in chronic marijuana users. J Neuroimmune Pharmacol. 2018;13(1):113-122. doi:10.1007/s11481-017-9767-0
48. Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105-108. doi:10.1126/science.1208344
49. Rezac S, Kok CR, Heermann M, et al. Fermented foods as a dietary source of live organisms. Front Microbiol. 2018;9:1785. doi:10.3389/fmicb.2018.01785
50. Chen X, Zhang Y, Wang H, et al. The regulatory effects of lactic acid on neuropsychiatric disorders. Discover Ment Health. 2022;2(1). doi:10.1007/s44192-022-00011-4
51. Karbownik MS, Mokros Ł, Dobielska M, et al. Association between consumption of fermented food and food-derived prebiotics with cognitive performance, depressive, and anxiety symptoms in psychiatrically healthy medical students under psychological stress: a prospective cohort study. Front Nutr. 2022;9:850249. doi:10.3389/fnut.2022.850249
52. Romano S, Savva GM, Bedarf JR, et al. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Parkinsons Dis. 2021;7(1):27. doi:10.1038/s41531-021-00156-z
53. Bourassa MW, Alim I, Bultman SJ, et al. Butyrate, neuroepigenetics and the gut microbiome: can a high fiber diet improve brain health? Neurosci Lett. 2016;625:56-63. doi:10.1016/j.neulet.2016.02.009
54. Matt SM, Allen JM, Lawson MA, et al. Butyrate and dietary soluble fiber improve neuroinflammation associated with aging in mice. Front Immunol. 2018;9:1832. doi:10.3389/fimmu.2018.01832
55. Mittal VA, Vargas T, Osborne KJ, et al. Exercise treatments for psychosis: a review. Curr Treat Options Psychiatry. 2017;4(2):152-166. doi:10.1007/s40501-017-0112-2
56. Estaki M, Pither J, Baumeister P, et al. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome. 2016;4(1):42. doi:10.1186/s40168-016-0189-7
57. Karl JP, Margolis LM, Madslien EH, et al. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am J Physiol-Gastrointest Liver Physiol. 2017;312(6):G559-G571. doi:10.1152/ajpgi.00066.2017
58. Claus SP, Guillou H, Ellero-Simatos S. The gut microbiota: a major player in the toxicity of environmental pollutants? NPJ Biofilms Microbiomes. 2016;2:16003. doi:10.1038/npjbiofilms.2016.3
1. Bäckhed F, Ley RE, Sonnenburg JL, et al. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915-1920. doi:10.1126/science.1104816
2. Jackson SW. Galen—on mental disorders. J Hist Behav Sci. 1969;5(4):365-384. doi:10.1002/1520-6696(196910)5:4<365::AID-JHBS2300050408>3.0.CO;2-9
3. Zheng P, Zeng B, Liu M, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv. 2019;5(2):eaau8317. doi:10.1126/sciadv.aau8317
4. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421-427. doi:10.1038/nature13595
5. Gill SR, Pop M, DeBoy RT, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312(5778):1355-1359. doi:10.1126/science.1124234
6. Alam R, Abdolmaleky HM, Zhou JR. Microbiome, inflammation, epigenetic alterations, and mental diseases. Am J Med Genet B Neuropsychiatr Genet. 2017;174(6):651-660. doi:10.1002/ajmg.b.32567
7. Cryan JF, O’Riordan KJ, Cowan CSM, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877-2013. doi:10.1152/physrev.00018.2018
8. Mueller NT, Bakacs E, Combellick J, et al. The infant microbiome development: mom matters. Trends Mol Med. 2015;21(2):109-117. doi:10.1016/j.molmed.2014.12.002
9. Fouhy F, Watkins C, Hill CJ, et al. Perinatal factors affect the gut microbiota up to four years after birth. Nat Commun. 2019;10(1):1517. doi:10.1038/s41467-019-09252-4
10. Sharon G, Sampson TR, Geschwind DH, et al. The central nervous system and the gut microbiome. Cell. 2016;167(4):915-932. doi:10.1016/j.cell.2016.10.027
11. Hill CJ, Lynch DB, Murphy K, et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome. 2017;5:4. doi:10.1186/s40168-016-0213-y
12. Gareau MG, Wine E, Rodrigues DM, et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60(3):307-317. doi:10.1136/gut.2009.202515
13. Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016;8(343):343ra82. doi:10.1126/scitranslmed.aad7121
14. Mancabelli L, Milani C, Lugli GA, et al. Meta-analysis of the human gut microbiome from urbanized and pre-agricultural populations. Environ Microbiol. 2017;19(4):1379-1390. doi:10.1111/1462-2920.13692
15. Stilo SA, Murray RM. Non-genetic factors in schizophrenia. Curr Psychiatry Rep. 2019;21(10):100. doi:10.1007/s11920-019-1091-3
16. Buscaino VM. Patologia extraneurale della schizofrenia: fegato, tubo digerente, sistema reticolo-endoteliale. Acta Neurologica. 1953;VIII:1-60.
17. Hemmings G. Schizophrenia. Lancet. 2004;364(9442):1312-1313. doi:10.1016/S0140- 6736(04)17181-X
18. Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292(5519):1115-1118. doi:10.1126/science.1058709
19. Ewaschuk JB, Diaz H, Meddings L, et al. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol-Gastrointest Liver Physiol. 2008;295(5):G1025-G1034. doi:10.1152/ajpgi.90227.2008
20. Alhasson F, Das S, Seth R, et al. Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS One. 2017;12(3):e0172914. doi:10.1371/journal.pone.0172914
21. Fillman SG, Cloonan N, Catts VS, et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18(2):206-214. doi:10.1038/mp.2012.110
22. Miller BJ, Buckley P, Seabolt W, et al. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663-671. doi:10.1016/j.biopsych.2011.04.013
23. Al-Amin M, Uddin MMN, Reza HM. Effects of antipsychotics on the inflammatory response system of patients with schizophrenia in peripheral blood mononuclear cell cultures. Clin Psychopharmacol Neurosci. 2013;11(3):144-151. doi:10.9758/cpn.2013.11.3.144
24. Yuan X, Zhang P, Wang Y, et al. Changes in metabolism and microbiota after 24-week risperidone treatment in drug naïve, normal weight patients with first episode schizophrenia. Schizophr Res. 2018;201:299-306. doi:10.1016/j.schres.2018.05.017
25. Maier L, Pruteanu M, Kuhn M, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555(7698):623-628. doi:10.1038/nature25979
26. Dickerson FB, Stallings C, Origoni A, et al. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: a randomized, placebo-controlled trial. Prim Care Companion CNS Disord. 2014;15(1):PCC.13m01579. doi:10.4088/PCC.13m01579
27. David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559-563. doi:10.1038/nature12820
28. Bien J, Palagani V, Bozko P. The intestinal microbiota dysbiosis and Clostridium difficile infection: is there a relationship with inflammatory bowel disease? Ther Adv Gastroenterol. 2013;6(1):53-68. doi:10.1177/1756283X12454590
29. Cryan JF, O’Riordan KJ, Sandhu K, et al. The gut microbiome in neurological disorders. Lancet Neurol. 2020;19(2):179-194. doi:10.1016/S1474-4422(19)30356-4
30. Jones CA, Watson DJG, Fone KCF. Animal models of schizophrenia. Br J Pharmacol. 2011;164(4):1162-1194. doi:10.1111/j.1476-5381.2011.01386.x
31. Schmidt MJ, Mirnics K. Neurodevelopment, GABA system dysfunction, and schizophrenia. Neuropsychopharmacology. 2015;40(1):190-206. doi:10.1038/npp.2014.95
32. Nasrallah, HA. The daunting challenge of schizophrenia: hundreds of biotypes and dozens of theories. Curr. Psychiatry 2018;17(12):4-6,50.
33. Nocera A, Nasrallah HA. The association of the gut microbiota with clinical features in schizophrenia. Behav Sci (Basel). 2022;12(4):89. doi:10.3390/bs12040089
34. Schwarz E, Maukonen J, Hyytiäinen T, et al. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr Res. 2018;192:398-403. doi:10.1016/j.schres.2017.04.017
35. Nguyen TT, Kosciolek T, Maldonado Y, et al. Differences in gut microbiome composition between persons with chronic schizophrenia and healthy comparison subjects. Schizophr Res. 2019;204:23-29. doi:10.1016/j.schres.2018.09.014
36. Li S, Zhuo M, Huang X, et al. Altered gut microbiota associated with symptom severity in schizophrenia. PeerJ. 2020;8:e9574. doi:10.7717/peerj.9574
37. Chen X, Xu J, Wang H, et al. Profiling the differences of gut microbial structure between schizophrenia patients with and without violent behaviors based on 16S rRNA gene sequencing. Int J Legal Med. 2021;135(1):131-141. doi:10.1007/s00414-020-02439-1
38. Manchia M, Fontana A, Panebianco C, et al. Involvement of gut microbiota in schizophrenia and treatment resistance to antipsychotics. Biomedicines. 2021;9(8):875. doi:10.3390/biomedicines9080875
39. Zhu C, Zheng M, Ali U, et al. Association between abundance of haemophilus in the gut microbiota and negative symptoms of schizophrenia. Front Psychiatry. 2021;12:685910. doi:10.3389/fpsyt.2021.685910
40. Flowers SA, Evans SJ, Ward KM, et al. Interaction between atypical antipsychotics and the gut microbiome in a bipolar disease cohort. Pharmacotherapy. 2017;37(3):261-267. doi:10.1002/phar.1890
41. Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222-227. doi:10.1038/nature11053
42. Buchanan RW. Persistent negative symptoms in schizophrenia: an overview. Schizophr Bull. 2007;33(4):1013-1022. doi:10.1093/schbul/sb1057
43. Liu JCW, Gorbovskaya I, Hahn MK, et al. The gut microbiome in schizophrenia and the potential benefits of prebiotic and probiotic treatment. Nutrients. 2021;13(4):1152. doi:10.3390/nu13041152
44. Biedermann L, Zeitz J, Mwinyi J, et al. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PloS One. 2013;8(3):e59260. doi:10.1371/journal.pone.0059260
45. Leclercq S, Matamoros S, Cani PD, et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci. 2014;111(42):e4485-e4493. doi:10.1073/pnas.1415174111
46. Hernández-Quiroz F, Nirmalkar K, Villalobos-Flores LE, et al. Influence of moderate beer consumption on human gut microbiota and its impact on fasting glucose and ß-cell function. Alcohol. 2020;85:77-94. doi:10.1016/j.alcohol.2019.05.006
47. Panee J, Gerschenson M, Chang L. Associations between microbiota, mitochondrial function, and cognition in chronic marijuana users. J Neuroimmune Pharmacol. 2018;13(1):113-122. doi:10.1007/s11481-017-9767-0
48. Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105-108. doi:10.1126/science.1208344
49. Rezac S, Kok CR, Heermann M, et al. Fermented foods as a dietary source of live organisms. Front Microbiol. 2018;9:1785. doi:10.3389/fmicb.2018.01785
50. Chen X, Zhang Y, Wang H, et al. The regulatory effects of lactic acid on neuropsychiatric disorders. Discover Ment Health. 2022;2(1). doi:10.1007/s44192-022-00011-4
51. Karbownik MS, Mokros Ł, Dobielska M, et al. Association between consumption of fermented food and food-derived prebiotics with cognitive performance, depressive, and anxiety symptoms in psychiatrically healthy medical students under psychological stress: a prospective cohort study. Front Nutr. 2022;9:850249. doi:10.3389/fnut.2022.850249
52. Romano S, Savva GM, Bedarf JR, et al. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Parkinsons Dis. 2021;7(1):27. doi:10.1038/s41531-021-00156-z
53. Bourassa MW, Alim I, Bultman SJ, et al. Butyrate, neuroepigenetics and the gut microbiome: can a high fiber diet improve brain health? Neurosci Lett. 2016;625:56-63. doi:10.1016/j.neulet.2016.02.009
54. Matt SM, Allen JM, Lawson MA, et al. Butyrate and dietary soluble fiber improve neuroinflammation associated with aging in mice. Front Immunol. 2018;9:1832. doi:10.3389/fimmu.2018.01832
55. Mittal VA, Vargas T, Osborne KJ, et al. Exercise treatments for psychosis: a review. Curr Treat Options Psychiatry. 2017;4(2):152-166. doi:10.1007/s40501-017-0112-2
56. Estaki M, Pither J, Baumeister P, et al. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome. 2016;4(1):42. doi:10.1186/s40168-016-0189-7
57. Karl JP, Margolis LM, Madslien EH, et al. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am J Physiol-Gastrointest Liver Physiol. 2017;312(6):G559-G571. doi:10.1152/ajpgi.00066.2017
58. Claus SP, Guillou H, Ellero-Simatos S. The gut microbiota: a major player in the toxicity of environmental pollutants? NPJ Biofilms Microbiomes. 2016;2:16003. doi:10.1038/npjbiofilms.2016.3
Depression and schizophrenia: Many biological and clinical similarities
Clinicians generally regard major depressive disorder (MDD) and schizophrenia as 2 separate and distinct psychiatric brain disorders. However, despite some differences, those 2 psychiatric syndromes have numerous similarities across clinical features and neurobiologic parameters.
Biological similarities
Both disorders share the following variables:
- Highly genetic in etiology but with environmental influences and epigenetics
- Associated with childhood maltreatment, abuse, or neglect
- Disrupted neuroplasticity, especially shrinkage in hippocampal volume
- Significant drop in brain-derived neurotrophic factor resulting in decreased neurogenesis
- Extensive white matter pathology across interhemispheric and intrahemispheric bundles
- Increased levels of serum cortisol, a stress hormone and inflammatory biomarker
- Hypofrontal cerebral blood flow during acute episodes of both MDD and schizophrenia
- Reduced dendritic spines (in number and size) and impaired experiential neuroplasticity
- Neuroinflammation (eg, cytokines, tumor necrosis factor-alpha, C-reactive protein) during acute episodes
- Elevated oxidative stress biomarkers, indicating an increase in free radicals
- Overactive default mode network associated with ruminations in MDD and “daydreaming” in schizophrenia
- Decrease in gamma-aminobutyric acid (GABA) and its inhibitory activity, translating into dysregulation of glutamatergic pathways and other neurotransmitters
- Immune dysregulation and comorbid autoimmune disorders
Clinical similarities
- Psychotic symptoms, especially delusional thinking such as paranoia in schizophrenia and severe self-deprecation in MDD
- Significantly elevated lifetime suicide risk
- Cognitive impairment (more severe in schizophrenia across several cognitive functions)
- Similarity of depressive and negative symptoms (especially anhedonia, apathy, restricted facial expression, social withdrawal)
- Antidepressant medications im-prove depressive and negative symptoms (though not completely in the case of negative symptoms of schizophrenia)
- Both have treatment-resistant subtypes that fail to respond to standard therapies
- Both are associated with comorbid generalized anxiety disorder
- Both are associated with comorbid obsessive-compulsive disorder
- Both are associated with serious alcohol and drug use
- Early mortality from general medical conditions, especially cardiovascular risks due to obesity, diabetes, hypertension, dyslipidemia
- Elevated risk of dementia with aging compared to the unaffected general population
- Opioids improve MDD and psychosis (buprenorphine in MDD and morphine in schizophrenia)
- Several second-generation antipsychotic medications are approved for both MDD and schizophrenia
- Electroconvulsive therapy is effective when pharmacotherapy fails in both MDD and schizophrenia
Biological differences
- Glutamate N-methyl-D-aspartate receptor antagonists (eg, ketamine) improve MDD but worsen schizophrenia
- Muscarinic agonists improve psychosis but worsen depression
- High pain threshold in schizophrenia (pain insensitivity) and low threshold in MDD (in which pain is a common comorbidity)
- Cortical thinning more severe in schizophrenia
- Hippocampal atrophy is reversible with successful treatment in MDD but not in schizophrenia
- Hypofrontality is reversible with remission in MDD but not in schizophrenia
Clinical differences
- Auditory and visual hallucinations are more common in schizophrenia than in MDD
- Anosognosia is common in schizophrenia but not in MDD
- Implausible delusions are more common in schizophrenia than in MDD
- Mood-congruent delusions are more common in MDD than in schizophrenia
- Sadness, crying, pessimism, and self-deprecation are common in MDD but not in schizophrenia
- Achieving full remission is more common in MDD than in schizophrenia
- Long-acting injectable medications are available for schizophrenia but not for MDD
- Evidence-based psychotherapy, without pharmacotherapy, is more likely to be effective in MDD than in schizophrenia
A transdiagnostic model of psychopathology
The significant overlap between MDD and schizophrenia should not be surprising. They are both generated by the same organ, the human brain, with disrupted neurochemical and physiological circuits in the brain.
The overlap is also consistent with the emerging transdiagnostic model of psychopathology.1-9 This model proposes that there is a “core” genetic risk for psychopathology with different iterations. The transdiagnostic model is in stark contrast to the prevailing DSM-5, which categorizes psychiatric disorders in “silos,” as if they are completely independent from each other despite many shared features. This is highly debatable according to the substantial evidence that multiple psychiatric disorders share many genes that influence brain development in utero and predispose individuals to a variety of clinical symptoms in adolescence and young adulthood.
The origin of mental illness is being disentangled by emerging research, which is identifying the common links among the various disorders currently listed in DSM-5.10 However, the evolution of psychiatric diagnosis has come full circle from a single entity before DSM, to multiple entities with DSM, and now back to a unified transdiagnostic model that is rapidly emerging.11 This has implications for the FDA’s persistent dogma that clinical trials for new drugs must be targeted for 1 of the DSM-5 categories, a flawed and narrow assumption. Given the accelerating body of evidence for a unified, transdiagnostic model, it makes much more sense for the FDA to approve medications that target a psychiatric symptom that is shared by multiple psychiatric conditions within a transdiagnostic clinical system. When medications are approved for a symptom regardless of a DSM diagnosis, the term “off-label” and its “stigma” will then fade into history, along with the malignant preauthorization racket that was invented by greedy insurance companies that exploit the off-label use of medications (even when an FDA-approved medication for the patient’s condition does not yet exist) simply to deny coverage, lower their expenses, and fatten their profits.
1. Goodkind M, Eickhoff SB, Oathes DJ, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72(4):305-315.
2. Caspi A, Moffitt TE. All for one and one for all: mental disorders in one dimension. Am J Psychiatry. 2018;175(9):831-844.
3. Krueger RF, Easton NR. Transdiagnostic factors in mental disorders. World Psychiatry. 2015;14(1):27-29.
4. Hyman SE. New evidence for shared risk architecture for mental disorders. JAMA Psychiatry. 2019;76(3):235-236.
5. Selzam S, Coleman JRI, Caspi A, et al. A polygenic p factor for major psychiatric disorders. Translational Psychiatry. 2018;8(1):205.
6. Barch DM. What it means to be transdiagnostic and how do we know? Am J Psychiatry. 2020;177(5):370-372.
7. Nasrallah HA. Is there only 1 neurobiologic psychiatric disorder with different clinical expressions? Current Psychiatry. 2015;14(7):10-12.
8. Nasrallah HA. Pleiotropy of psychiatric disorders will reinvent DSM. Current Psychiatry. 2013;12(4):6-7.
9. Nasrallah HA. Beyond DSM-5: clinical and biological features shared by major psychiatric syndromes. Current Psychiatry. 2017;16(10):4-7.
10. Marshall M. Roots of mental illness: researchers are beginning to untangle the common biology that links supposedly distinct psychiatric conditions. Nature. 2020;581:19-21.
11. Kendler KS. From many to one to many--the search for causes of psychiatric illness. JAMA Psychiatry. 2019;76(10):1085-1091.
Clinicians generally regard major depressive disorder (MDD) and schizophrenia as 2 separate and distinct psychiatric brain disorders. However, despite some differences, those 2 psychiatric syndromes have numerous similarities across clinical features and neurobiologic parameters.
Biological similarities
Both disorders share the following variables:
- Highly genetic in etiology but with environmental influences and epigenetics
- Associated with childhood maltreatment, abuse, or neglect
- Disrupted neuroplasticity, especially shrinkage in hippocampal volume
- Significant drop in brain-derived neurotrophic factor resulting in decreased neurogenesis
- Extensive white matter pathology across interhemispheric and intrahemispheric bundles
- Increased levels of serum cortisol, a stress hormone and inflammatory biomarker
- Hypofrontal cerebral blood flow during acute episodes of both MDD and schizophrenia
- Reduced dendritic spines (in number and size) and impaired experiential neuroplasticity
- Neuroinflammation (eg, cytokines, tumor necrosis factor-alpha, C-reactive protein) during acute episodes
- Elevated oxidative stress biomarkers, indicating an increase in free radicals
- Overactive default mode network associated with ruminations in MDD and “daydreaming” in schizophrenia
- Decrease in gamma-aminobutyric acid (GABA) and its inhibitory activity, translating into dysregulation of glutamatergic pathways and other neurotransmitters
- Immune dysregulation and comorbid autoimmune disorders
Clinical similarities
- Psychotic symptoms, especially delusional thinking such as paranoia in schizophrenia and severe self-deprecation in MDD
- Significantly elevated lifetime suicide risk
- Cognitive impairment (more severe in schizophrenia across several cognitive functions)
- Similarity of depressive and negative symptoms (especially anhedonia, apathy, restricted facial expression, social withdrawal)
- Antidepressant medications im-prove depressive and negative symptoms (though not completely in the case of negative symptoms of schizophrenia)
- Both have treatment-resistant subtypes that fail to respond to standard therapies
- Both are associated with comorbid generalized anxiety disorder
- Both are associated with comorbid obsessive-compulsive disorder
- Both are associated with serious alcohol and drug use
- Early mortality from general medical conditions, especially cardiovascular risks due to obesity, diabetes, hypertension, dyslipidemia
- Elevated risk of dementia with aging compared to the unaffected general population
- Opioids improve MDD and psychosis (buprenorphine in MDD and morphine in schizophrenia)
- Several second-generation antipsychotic medications are approved for both MDD and schizophrenia
- Electroconvulsive therapy is effective when pharmacotherapy fails in both MDD and schizophrenia
Biological differences
- Glutamate N-methyl-D-aspartate receptor antagonists (eg, ketamine) improve MDD but worsen schizophrenia
- Muscarinic agonists improve psychosis but worsen depression
- High pain threshold in schizophrenia (pain insensitivity) and low threshold in MDD (in which pain is a common comorbidity)
- Cortical thinning more severe in schizophrenia
- Hippocampal atrophy is reversible with successful treatment in MDD but not in schizophrenia
- Hypofrontality is reversible with remission in MDD but not in schizophrenia
Clinical differences
- Auditory and visual hallucinations are more common in schizophrenia than in MDD
- Anosognosia is common in schizophrenia but not in MDD
- Implausible delusions are more common in schizophrenia than in MDD
- Mood-congruent delusions are more common in MDD than in schizophrenia
- Sadness, crying, pessimism, and self-deprecation are common in MDD but not in schizophrenia
- Achieving full remission is more common in MDD than in schizophrenia
- Long-acting injectable medications are available for schizophrenia but not for MDD
- Evidence-based psychotherapy, without pharmacotherapy, is more likely to be effective in MDD than in schizophrenia
A transdiagnostic model of psychopathology
The significant overlap between MDD and schizophrenia should not be surprising. They are both generated by the same organ, the human brain, with disrupted neurochemical and physiological circuits in the brain.
The overlap is also consistent with the emerging transdiagnostic model of psychopathology.1-9 This model proposes that there is a “core” genetic risk for psychopathology with different iterations. The transdiagnostic model is in stark contrast to the prevailing DSM-5, which categorizes psychiatric disorders in “silos,” as if they are completely independent from each other despite many shared features. This is highly debatable according to the substantial evidence that multiple psychiatric disorders share many genes that influence brain development in utero and predispose individuals to a variety of clinical symptoms in adolescence and young adulthood.
The origin of mental illness is being disentangled by emerging research, which is identifying the common links among the various disorders currently listed in DSM-5.10 However, the evolution of psychiatric diagnosis has come full circle from a single entity before DSM, to multiple entities with DSM, and now back to a unified transdiagnostic model that is rapidly emerging.11 This has implications for the FDA’s persistent dogma that clinical trials for new drugs must be targeted for 1 of the DSM-5 categories, a flawed and narrow assumption. Given the accelerating body of evidence for a unified, transdiagnostic model, it makes much more sense for the FDA to approve medications that target a psychiatric symptom that is shared by multiple psychiatric conditions within a transdiagnostic clinical system. When medications are approved for a symptom regardless of a DSM diagnosis, the term “off-label” and its “stigma” will then fade into history, along with the malignant preauthorization racket that was invented by greedy insurance companies that exploit the off-label use of medications (even when an FDA-approved medication for the patient’s condition does not yet exist) simply to deny coverage, lower their expenses, and fatten their profits.
Clinicians generally regard major depressive disorder (MDD) and schizophrenia as 2 separate and distinct psychiatric brain disorders. However, despite some differences, those 2 psychiatric syndromes have numerous similarities across clinical features and neurobiologic parameters.
Biological similarities
Both disorders share the following variables:
- Highly genetic in etiology but with environmental influences and epigenetics
- Associated with childhood maltreatment, abuse, or neglect
- Disrupted neuroplasticity, especially shrinkage in hippocampal volume
- Significant drop in brain-derived neurotrophic factor resulting in decreased neurogenesis
- Extensive white matter pathology across interhemispheric and intrahemispheric bundles
- Increased levels of serum cortisol, a stress hormone and inflammatory biomarker
- Hypofrontal cerebral blood flow during acute episodes of both MDD and schizophrenia
- Reduced dendritic spines (in number and size) and impaired experiential neuroplasticity
- Neuroinflammation (eg, cytokines, tumor necrosis factor-alpha, C-reactive protein) during acute episodes
- Elevated oxidative stress biomarkers, indicating an increase in free radicals
- Overactive default mode network associated with ruminations in MDD and “daydreaming” in schizophrenia
- Decrease in gamma-aminobutyric acid (GABA) and its inhibitory activity, translating into dysregulation of glutamatergic pathways and other neurotransmitters
- Immune dysregulation and comorbid autoimmune disorders
Clinical similarities
- Psychotic symptoms, especially delusional thinking such as paranoia in schizophrenia and severe self-deprecation in MDD
- Significantly elevated lifetime suicide risk
- Cognitive impairment (more severe in schizophrenia across several cognitive functions)
- Similarity of depressive and negative symptoms (especially anhedonia, apathy, restricted facial expression, social withdrawal)
- Antidepressant medications im-prove depressive and negative symptoms (though not completely in the case of negative symptoms of schizophrenia)
- Both have treatment-resistant subtypes that fail to respond to standard therapies
- Both are associated with comorbid generalized anxiety disorder
- Both are associated with comorbid obsessive-compulsive disorder
- Both are associated with serious alcohol and drug use
- Early mortality from general medical conditions, especially cardiovascular risks due to obesity, diabetes, hypertension, dyslipidemia
- Elevated risk of dementia with aging compared to the unaffected general population
- Opioids improve MDD and psychosis (buprenorphine in MDD and morphine in schizophrenia)
- Several second-generation antipsychotic medications are approved for both MDD and schizophrenia
- Electroconvulsive therapy is effective when pharmacotherapy fails in both MDD and schizophrenia
Biological differences
- Glutamate N-methyl-D-aspartate receptor antagonists (eg, ketamine) improve MDD but worsen schizophrenia
- Muscarinic agonists improve psychosis but worsen depression
- High pain threshold in schizophrenia (pain insensitivity) and low threshold in MDD (in which pain is a common comorbidity)
- Cortical thinning more severe in schizophrenia
- Hippocampal atrophy is reversible with successful treatment in MDD but not in schizophrenia
- Hypofrontality is reversible with remission in MDD but not in schizophrenia
Clinical differences
- Auditory and visual hallucinations are more common in schizophrenia than in MDD
- Anosognosia is common in schizophrenia but not in MDD
- Implausible delusions are more common in schizophrenia than in MDD
- Mood-congruent delusions are more common in MDD than in schizophrenia
- Sadness, crying, pessimism, and self-deprecation are common in MDD but not in schizophrenia
- Achieving full remission is more common in MDD than in schizophrenia
- Long-acting injectable medications are available for schizophrenia but not for MDD
- Evidence-based psychotherapy, without pharmacotherapy, is more likely to be effective in MDD than in schizophrenia
A transdiagnostic model of psychopathology
The significant overlap between MDD and schizophrenia should not be surprising. They are both generated by the same organ, the human brain, with disrupted neurochemical and physiological circuits in the brain.
The overlap is also consistent with the emerging transdiagnostic model of psychopathology.1-9 This model proposes that there is a “core” genetic risk for psychopathology with different iterations. The transdiagnostic model is in stark contrast to the prevailing DSM-5, which categorizes psychiatric disorders in “silos,” as if they are completely independent from each other despite many shared features. This is highly debatable according to the substantial evidence that multiple psychiatric disorders share many genes that influence brain development in utero and predispose individuals to a variety of clinical symptoms in adolescence and young adulthood.
The origin of mental illness is being disentangled by emerging research, which is identifying the common links among the various disorders currently listed in DSM-5.10 However, the evolution of psychiatric diagnosis has come full circle from a single entity before DSM, to multiple entities with DSM, and now back to a unified transdiagnostic model that is rapidly emerging.11 This has implications for the FDA’s persistent dogma that clinical trials for new drugs must be targeted for 1 of the DSM-5 categories, a flawed and narrow assumption. Given the accelerating body of evidence for a unified, transdiagnostic model, it makes much more sense for the FDA to approve medications that target a psychiatric symptom that is shared by multiple psychiatric conditions within a transdiagnostic clinical system. When medications are approved for a symptom regardless of a DSM diagnosis, the term “off-label” and its “stigma” will then fade into history, along with the malignant preauthorization racket that was invented by greedy insurance companies that exploit the off-label use of medications (even when an FDA-approved medication for the patient’s condition does not yet exist) simply to deny coverage, lower their expenses, and fatten their profits.
1. Goodkind M, Eickhoff SB, Oathes DJ, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72(4):305-315.
2. Caspi A, Moffitt TE. All for one and one for all: mental disorders in one dimension. Am J Psychiatry. 2018;175(9):831-844.
3. Krueger RF, Easton NR. Transdiagnostic factors in mental disorders. World Psychiatry. 2015;14(1):27-29.
4. Hyman SE. New evidence for shared risk architecture for mental disorders. JAMA Psychiatry. 2019;76(3):235-236.
5. Selzam S, Coleman JRI, Caspi A, et al. A polygenic p factor for major psychiatric disorders. Translational Psychiatry. 2018;8(1):205.
6. Barch DM. What it means to be transdiagnostic and how do we know? Am J Psychiatry. 2020;177(5):370-372.
7. Nasrallah HA. Is there only 1 neurobiologic psychiatric disorder with different clinical expressions? Current Psychiatry. 2015;14(7):10-12.
8. Nasrallah HA. Pleiotropy of psychiatric disorders will reinvent DSM. Current Psychiatry. 2013;12(4):6-7.
9. Nasrallah HA. Beyond DSM-5: clinical and biological features shared by major psychiatric syndromes. Current Psychiatry. 2017;16(10):4-7.
10. Marshall M. Roots of mental illness: researchers are beginning to untangle the common biology that links supposedly distinct psychiatric conditions. Nature. 2020;581:19-21.
11. Kendler KS. From many to one to many--the search for causes of psychiatric illness. JAMA Psychiatry. 2019;76(10):1085-1091.
1. Goodkind M, Eickhoff SB, Oathes DJ, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72(4):305-315.
2. Caspi A, Moffitt TE. All for one and one for all: mental disorders in one dimension. Am J Psychiatry. 2018;175(9):831-844.
3. Krueger RF, Easton NR. Transdiagnostic factors in mental disorders. World Psychiatry. 2015;14(1):27-29.
4. Hyman SE. New evidence for shared risk architecture for mental disorders. JAMA Psychiatry. 2019;76(3):235-236.
5. Selzam S, Coleman JRI, Caspi A, et al. A polygenic p factor for major psychiatric disorders. Translational Psychiatry. 2018;8(1):205.
6. Barch DM. What it means to be transdiagnostic and how do we know? Am J Psychiatry. 2020;177(5):370-372.
7. Nasrallah HA. Is there only 1 neurobiologic psychiatric disorder with different clinical expressions? Current Psychiatry. 2015;14(7):10-12.
8. Nasrallah HA. Pleiotropy of psychiatric disorders will reinvent DSM. Current Psychiatry. 2013;12(4):6-7.
9. Nasrallah HA. Beyond DSM-5: clinical and biological features shared by major psychiatric syndromes. Current Psychiatry. 2017;16(10):4-7.
10. Marshall M. Roots of mental illness: researchers are beginning to untangle the common biology that links supposedly distinct psychiatric conditions. Nature. 2020;581:19-21.
11. Kendler KS. From many to one to many--the search for causes of psychiatric illness. JAMA Psychiatry. 2019;76(10):1085-1091.
Contemporary psychiatry: A SWOT analysis
Editor’s note: This article was adapted with permission from a version originally published in the Ohio Psychiatric Physician Association’s newsletter, Insight Matters, Fall 2022.
Acknowledging and analyzing strengths, weaknesses, opportunities, and threats (SWOT) is an important tactic many organizations use to develop a strategic plan to grow, move forward, and thrive. A SWOT analysis can provide a “big picture” view of the status and the desired future directions not only for companies but for medical disciplines such as psychiatry. So here are my perspectives on psychiatry’s strengths, weaknesses, opportunities, and threats. It is a work in progress, and I welcome (and encourage) you to send additional items or comments to me at henry.nasrallah@currentpsychiatry.com.
Strengths
- The American Psychiatric Association (APA) is the oldest medical professional organization, established in 1844 (3 years before the American Medical Association)1
- Strong organizational structure and governance, and a “big tent” with several tiers of membership
- Effective, member-driven District Branches
- The medical identity at the core of psychiatry—we are psychiatric physicians2
- Escalating number of senior medical students choosing psychiatry as a career, far more than a decade ago
- High demand for psychiatrists in all settings around the country
- Increased compensation for psychiatrists (market forces of supply and demand)
- Psychiatry is continuously evolving and reinventing itself: seismic shifts in etiopathogenesis, disease conceptualization, terminology, and therapies (4 major shifts over the past century)3
- An abundant body of evidence supporting that all psychiatric disorders are brain disorders and transdiagnostic in nature4
- Many vibrant subspecialty societies
- Substantial number of Tier 1, evidence-based treatments
- Novel mechanisms of action and treatment strategies are being introduced on a regular basis for psychotic and mood disorders5,6
- Advances in neuromodulation techniques to treat a wide spectrum of psychiatric disorders, including electroconvulsive therapy, transcranial magnetic stimulation, vagus nerve stimulation, transcranial direct current stimulation, deep brain stimulation, cranial electric stimulation, epidural cortical stimulation, focused ultrasound, low field magnetic stimulation, magnetic seizure therapy, and near infrared light therapy, with mechanisms that are electric, ultrasound, magnetic, or optical7,8
- Psychiatric physicians develop wisdom by practicing psychiatry (ie, they become more empathic, tolerant of ambiguity, prosocial, introspective, aware of one’s strengths and limitations). Neuroplasticity in the frontal cortex is triggered by conducting psychotherapy9
Weaknesses
- Shrinking workforce due to a static number of residency training slots for 40 years10
- High rate of retirement by aging psychiatrists
- Persistent stigma around mental disorders despite massive scientific and medical advances11
- Still no real parity! We need succinct laws with “teeth”12
- Demedicalization in the public sector, referring to psychiatric physicians as “providers” and labeling patients as “clients”2
- Not enough graduating residents choosing to do subspecialty fellowships (especially geriatric, addiction, psychosomatic psychiatry) to meet escalating societal needs
- Very low presence in rural areas (both psychiatrists and psychiatric hospitals)
- Persistent APA member apathy: only 10% to 15% vote in the APA national elections or volunteer to serve on committees
- Widespread member dissatisfaction with maintenance of certification
- Neuroscience advances are not being translated fast enough for practical clinical applications
- Many in the public at large do not realize psychiatric symptoms are generated from anomalous brain circuits or that psychiatric disorders are highly genetic but also have environmental and epigenetic etiologies
- The DSM diagnostic system needs a paradigm shift: it is still based on a menu of clinical signs and symptoms and is devoid of objective diagnostic measures such as biomarkers4
- Neuroscience literacy among busy psychiatric practitioners is insufficient at a time of explosive growth in basic and clinical neuroscience13
- No effective treatment for alcohol or substance use disorders despite their very high morbidity and mortality
- Major psychiatric disorders are still associated with significant disability (schizophrenia, bipolar disorder, major depressive disorder, anxiety disorders, eating disorders, substance use disorders)
- Suicide rate (other than opioid deaths) has continued to rise in the past 3 decades14
Opportunities
- Potentially momentous clinical applications of the neuroscience breakthroughs
- Collaborative care with primary care physicians and increasing colocalization
- Dramatic increase in public awareness about the importance of mental health due to the COVID-19 pandemic15
- Powerful new data management tools, including machine learning, artificial intelligence, super computers, big data, deep learning, nanotechnology, and metabolomics, all of which are expediting neurobiological discoveries16
- The potential of reclassifying psychiatric disorders as neurological disorders, which will improve reimbursement for patient health care and reduce stigma17
- Emergence of new mechanisms of action of disease etiology, such as microbiota, mitochondrial dysfunction, permeable blood-brain barrier, and neuroimmune dysregulation18,19
- The advent and growth of “precision psychiatry”20
- The tremendous potential of molecular genetics and gene therapy for psychiatric disorders, most of which are genetic in etiology
- Expanding applications of neuroimaging techniques, including morphological, spectroscopic, functional, diffusion tensor imaging, and receptor imaging21
- Epigenetic advances in neuropsychiatric disorders
- Remarkably powerful research methods, such as pluripotent cells (producing neurons from skin cells), optogenetics (activating genes with light), gene-wide association studies, CRISPR (clustered regularly interspaced short palindromic repeats, which serve as genetic scissors to remove and replace abnormal genes), and brain connectomics22
- Psychiatry should develop and promote an “annual mental health checkup” for all age groups, similar to an annual physical exam23
- Focus on the social determinants of health
- Address the unmet mental health needs of individuals who are members of minority groups
- Lobby ferociously for a much larger budget for the National Institute of Mental Health to advance funding for research of serious psychiatric brain disorders
- Remind Congress continuously that the cost of mental illness is $700 billion annually and costs can only be reduced by funding neurobiological research1
- Partner with the pharmaceutical industry instead of demonizing them. They are the only entity that develops medication for psychiatry, where 80% of disorders have no FDA-approved drugs.24 Without the pharmaceutical industry and the help of medications, many psychiatric patients would still be institutionalized and unable to lead a normal life. We must recognize the contributions of pharmaceutical companies to the health of our patients, similar to the warp speed development of vaccines for the deadly coronavirus
- Psychiatric clinicians must refer patients to clinical trials because without patients enrolling in FDA studies, no drug developments can take place
- Many “out-of-the-box” therapies are being developed, such as antiapoptotic therapy, microglia inhibition, mitochondrial repair, white matter fiber remyelination, neuroprotection, and reversing N-methyl-
d -aspartate receptor hypofunction25 - The emerging evidence that psychotherapy is in fact a biological treatment that induces brain changes (neuroplasticity) and can modulate the immune system26
- Druggable genes, providing innovative new medications27
- Reposition psychedelics as revolutionary new treatments28
- Emphasize measurement-based care (rating scales), which can upgrade patient care29
- Because psychosis is associated with brain tissue loss, just like heart attacks are associated with myocardium destruction, psychiatrists must act like cardiologists30 and treat psychotic episodes urgently, like a stroke,31 to reduce the duration of untreated psychosis and improve patient outcomes
Threats
- Antipsychiatry cults continue to disparage and attack psychiatry32
- Health delivery systems are replacing psychiatric physicians with nurse practitioners to lower costs, regardless of quality and experience, and they inappropriately lump them together as “providers”2
- Psychologists continue to seek prescribing privileges with absurdly sketchy, predominantly online training supervised by other psychologists33
- Many legislators and policymakers, as well as the public, still don’t understand the difference between psychiatrists and psychologists, and the extensively disparate medical training in quality and quantity
- A dearth of psychiatric physician-scientists because very few residents are pursuing research fellowships after training34
- Disproportionate emphasis on clinical care and generating clinical revenue (relative value units) in academic institutions, with fewer tenure-track faculty members having protected time to write grants for federal or foundation grants to support their salaries and research operations35
- Meager financial support for teaching in psychiatry departments
- Many seriously psychiatrically ill persons do not have access to psychiatric medical care (and often to primary care as well)
- Many in the public falsely believe psychiatric disorders are hopeless and untreatable, which perpetuates stigma
- Long-acting injectable antipsychotic formulations are not used early enough in patients with psychosis, who are known to have a high nonadherence rate with oral medications following discharge from their first hospitalization. This leads to many recurrences with multiple devastating consequences, including progressive brain tissue loss, treatment resistance, disability, incarceration, and suicide36
- Many clinicians do not have full-text access to all studies indexed in PubMed, which is vital for lifelong learning in a rapidly growing medical discipline such as psychiatry
- Psychiatrists are often unable to prescribe medications shortly after they are approved by the FDA due to the insurance companies’ outrageous preauthorization racket that enforces a fail-first policy with cheaper generics, even if generic medications are associated with safety and tolerability problems37
- The continued use of decades-old first-generation antipsychotic medications despite 32 published studies reporting their neurotoxicity and the death of brain cells38
Using this analysis to benefit our patients
Despite its strengths, psychiatry must overcome its weaknesses, fend off its threats, and exploit its many opportunities. The only way to do that is for psychiatrists to unify and for the APA to provide inspired leadership to achieve the aspirational goals of our field. However, we must adopt “moonshot thinking”39 to magnify the Ss, diminish the Ws, exploit the Os, and stave off the Ts of our SWOT, thereby attaining all our cherished and lofty goals. Ultimately, the greatest beneficiaries will be our patients.
1. Nasrallah HA. 20 reasons to celebrate our APA membership. Current Psychiatry. 2020;19(1):6-9.
2. Nasrallah HA. We are physicians, not providers, and we treat patients, not clients! Current Psychiatry. 2020;19(2):5-8.
3. Nasrallah HA. From bedlam to biomarkers: the transformation of psychiatry’s terminology reflects its 4 conceptual earthquakes. Current Psychiatry. 2015;14(1):5-7.
4. Nasrallah HA. Re-inventing the DSM as a transdiagnostic model: psychiatric disorders are extensively interconnected. Ann Clin Psychiatry. 2021;33(3):148-150.
5. Nasrallah HA. Psychopharmacology 3.0. Current Psychiatry. 2081;17(11):4-7.
6. Nasrallah HA. Reversing depression: a plethora of therapeutic strategies and mechanisms. Current Psychiatry. 2022;21(8):4-6.
7. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Psychopharmacology. 2012;37(1):102-116.
8. Nasrallah HA. Optimal psychiatric treatment: target the brain and avoid the body. Current Psychiatry. 2022;21(12):3-6.
9. Nasrallah HA. Does psychiatry practice make us wise? Current Psychiatry. 2009;8(10):12-14.
10. Buckley PF, Nasrallah HA. The psychiatry workforce pool is shrinking. What are we doing about it? Current Psychiatry. 2016;15(9):23-24,95.
11. Nasrallah HA. A psychiatric manifesto: stigma is hate speech and a hate crime. Current Psychiatry. 2022;21(6):6-8.
12. Nasrallah HA. The travesty of disparity and non-parity. Current Psychiatry. 2014;13(1):8,19.
13. Nasrallah HA. Advancing clinical neuroscience literacy among psychiatric practitioners. Current Psychiatry. 2017;16(9):17-18.
14. Nasrallah HA. The scourge of societal anosognosia about the mentally ill. Current Psychiatry. 2016;15(6):19-24.
15. Nasrallah HA. 10 silver linings of the COVID-19 pandemic. Insight Matters. 2021;45:3-4.
16. Kalenderian H, Nasrallah HA. Artificial intelligence in psychiatry. Current Psychiatry. 2019:18(8):33-38.
17. Nasrallah HA. Let’s tear down the silos and re-unify psychiatry and neurology! Current Psychiatry. 2013;12(8):8-9.
18. Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
19. Schrenk DA, Nasrallah HA. Faulty fences: blood-brain barrier dysfunction in schizophrenia. Current Psychiatry. 2022;21(10):28-32.
20. Nasrallah HA. The dawn of precision psychiatry. Current Psychiatry. 2017;16(12):7-8,11.
21. Nasrallah HA. Today’s psychiatric neuroscience advances were science fiction during my residency. Current Psychiatry 2021;20(4):5-7,12,24.
22. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
23. Nasrallah HA. I have a dream…for psychiatry. Current Psychiatry. 2021;20(11):12-14.
24. Devulapalli KK, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders: the majority of psychiatric diagnoses have no approved drug. Asian J Psychiatry. 2009;2(1):29-36.
25. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
26. Nasrallah HA. Repositioning psychotherapy as a neurobiological intervention. Current Psychiatry. 2013;12(12):18-19.
27. Nasrallah HA. Druggable genes, promiscuous drugs, repurposed medications. Current Psychiatry. 2016;15(5):23,27.
28. Nasrallah HA. Long overdue: measurement-based psychiatric practice. Current Psychiatry. 2009;8(4):14-16.
29. Nasrallah HA. Maddening therapies: how hallucinogens morphed into novel treatments. Current Psychiatry. 2017:16(1):19-21.
30. Nasrallah HA. For first episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
31. Nasrallah HA, Roque A. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
32. Nasrallah HA. The antipsychiatry movement: who and why. Current Psychiatry. 2011;10(12):4,6,53.
33. Nasrallah HA. Prescribing is the culmination of extensive medical training and psychologists do not qualify. Current Psychiatry. 2017;16(6):11-12,14-16.
34. Fenton W, James R, Insel T. Psychiatry residency training, the physician-scientist, and the future of psychiatry. Acad Psychiatry. 2004;28(4):263-266.
35. Balon R, Morreale MK. The precipitous decline of academic medicine in the United States. Ann Clin Psychiatry. 2020;32(4):225-227.
36. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
37. Nasrallah HA. Pre-authorization is illegal, unethical, and adversely disrupts patient care. Current Psychiatry. 2020;19(4):5-11.
38. Nasrallah HA, Chen AT. Multiple neurotoxic effects of haloperidol resulting in neuronal death. Ann Clin Psychiatry. 2017;29(3):195-202.
39. Nasrallah HA. It’s time for moonshot thinking in psychiatry. Current Psychiatry. 2022;21(2):8-10.
Editor’s note: This article was adapted with permission from a version originally published in the Ohio Psychiatric Physician Association’s newsletter, Insight Matters, Fall 2022.
Acknowledging and analyzing strengths, weaknesses, opportunities, and threats (SWOT) is an important tactic many organizations use to develop a strategic plan to grow, move forward, and thrive. A SWOT analysis can provide a “big picture” view of the status and the desired future directions not only for companies but for medical disciplines such as psychiatry. So here are my perspectives on psychiatry’s strengths, weaknesses, opportunities, and threats. It is a work in progress, and I welcome (and encourage) you to send additional items or comments to me at henry.nasrallah@currentpsychiatry.com.
Strengths
- The American Psychiatric Association (APA) is the oldest medical professional organization, established in 1844 (3 years before the American Medical Association)1
- Strong organizational structure and governance, and a “big tent” with several tiers of membership
- Effective, member-driven District Branches
- The medical identity at the core of psychiatry—we are psychiatric physicians2
- Escalating number of senior medical students choosing psychiatry as a career, far more than a decade ago
- High demand for psychiatrists in all settings around the country
- Increased compensation for psychiatrists (market forces of supply and demand)
- Psychiatry is continuously evolving and reinventing itself: seismic shifts in etiopathogenesis, disease conceptualization, terminology, and therapies (4 major shifts over the past century)3
- An abundant body of evidence supporting that all psychiatric disorders are brain disorders and transdiagnostic in nature4
- Many vibrant subspecialty societies
- Substantial number of Tier 1, evidence-based treatments
- Novel mechanisms of action and treatment strategies are being introduced on a regular basis for psychotic and mood disorders5,6
- Advances in neuromodulation techniques to treat a wide spectrum of psychiatric disorders, including electroconvulsive therapy, transcranial magnetic stimulation, vagus nerve stimulation, transcranial direct current stimulation, deep brain stimulation, cranial electric stimulation, epidural cortical stimulation, focused ultrasound, low field magnetic stimulation, magnetic seizure therapy, and near infrared light therapy, with mechanisms that are electric, ultrasound, magnetic, or optical7,8
- Psychiatric physicians develop wisdom by practicing psychiatry (ie, they become more empathic, tolerant of ambiguity, prosocial, introspective, aware of one’s strengths and limitations). Neuroplasticity in the frontal cortex is triggered by conducting psychotherapy9
Weaknesses
- Shrinking workforce due to a static number of residency training slots for 40 years10
- High rate of retirement by aging psychiatrists
- Persistent stigma around mental disorders despite massive scientific and medical advances11
- Still no real parity! We need succinct laws with “teeth”12
- Demedicalization in the public sector, referring to psychiatric physicians as “providers” and labeling patients as “clients”2
- Not enough graduating residents choosing to do subspecialty fellowships (especially geriatric, addiction, psychosomatic psychiatry) to meet escalating societal needs
- Very low presence in rural areas (both psychiatrists and psychiatric hospitals)
- Persistent APA member apathy: only 10% to 15% vote in the APA national elections or volunteer to serve on committees
- Widespread member dissatisfaction with maintenance of certification
- Neuroscience advances are not being translated fast enough for practical clinical applications
- Many in the public at large do not realize psychiatric symptoms are generated from anomalous brain circuits or that psychiatric disorders are highly genetic but also have environmental and epigenetic etiologies
- The DSM diagnostic system needs a paradigm shift: it is still based on a menu of clinical signs and symptoms and is devoid of objective diagnostic measures such as biomarkers4
- Neuroscience literacy among busy psychiatric practitioners is insufficient at a time of explosive growth in basic and clinical neuroscience13
- No effective treatment for alcohol or substance use disorders despite their very high morbidity and mortality
- Major psychiatric disorders are still associated with significant disability (schizophrenia, bipolar disorder, major depressive disorder, anxiety disorders, eating disorders, substance use disorders)
- Suicide rate (other than opioid deaths) has continued to rise in the past 3 decades14
Opportunities
- Potentially momentous clinical applications of the neuroscience breakthroughs
- Collaborative care with primary care physicians and increasing colocalization
- Dramatic increase in public awareness about the importance of mental health due to the COVID-19 pandemic15
- Powerful new data management tools, including machine learning, artificial intelligence, super computers, big data, deep learning, nanotechnology, and metabolomics, all of which are expediting neurobiological discoveries16
- The potential of reclassifying psychiatric disorders as neurological disorders, which will improve reimbursement for patient health care and reduce stigma17
- Emergence of new mechanisms of action of disease etiology, such as microbiota, mitochondrial dysfunction, permeable blood-brain barrier, and neuroimmune dysregulation18,19
- The advent and growth of “precision psychiatry”20
- The tremendous potential of molecular genetics and gene therapy for psychiatric disorders, most of which are genetic in etiology
- Expanding applications of neuroimaging techniques, including morphological, spectroscopic, functional, diffusion tensor imaging, and receptor imaging21
- Epigenetic advances in neuropsychiatric disorders
- Remarkably powerful research methods, such as pluripotent cells (producing neurons from skin cells), optogenetics (activating genes with light), gene-wide association studies, CRISPR (clustered regularly interspaced short palindromic repeats, which serve as genetic scissors to remove and replace abnormal genes), and brain connectomics22
- Psychiatry should develop and promote an “annual mental health checkup” for all age groups, similar to an annual physical exam23
- Focus on the social determinants of health
- Address the unmet mental health needs of individuals who are members of minority groups
- Lobby ferociously for a much larger budget for the National Institute of Mental Health to advance funding for research of serious psychiatric brain disorders
- Remind Congress continuously that the cost of mental illness is $700 billion annually and costs can only be reduced by funding neurobiological research1
- Partner with the pharmaceutical industry instead of demonizing them. They are the only entity that develops medication for psychiatry, where 80% of disorders have no FDA-approved drugs.24 Without the pharmaceutical industry and the help of medications, many psychiatric patients would still be institutionalized and unable to lead a normal life. We must recognize the contributions of pharmaceutical companies to the health of our patients, similar to the warp speed development of vaccines for the deadly coronavirus
- Psychiatric clinicians must refer patients to clinical trials because without patients enrolling in FDA studies, no drug developments can take place
- Many “out-of-the-box” therapies are being developed, such as antiapoptotic therapy, microglia inhibition, mitochondrial repair, white matter fiber remyelination, neuroprotection, and reversing N-methyl-
d -aspartate receptor hypofunction25 - The emerging evidence that psychotherapy is in fact a biological treatment that induces brain changes (neuroplasticity) and can modulate the immune system26
- Druggable genes, providing innovative new medications27
- Reposition psychedelics as revolutionary new treatments28
- Emphasize measurement-based care (rating scales), which can upgrade patient care29
- Because psychosis is associated with brain tissue loss, just like heart attacks are associated with myocardium destruction, psychiatrists must act like cardiologists30 and treat psychotic episodes urgently, like a stroke,31 to reduce the duration of untreated psychosis and improve patient outcomes
Threats
- Antipsychiatry cults continue to disparage and attack psychiatry32
- Health delivery systems are replacing psychiatric physicians with nurse practitioners to lower costs, regardless of quality and experience, and they inappropriately lump them together as “providers”2
- Psychologists continue to seek prescribing privileges with absurdly sketchy, predominantly online training supervised by other psychologists33
- Many legislators and policymakers, as well as the public, still don’t understand the difference between psychiatrists and psychologists, and the extensively disparate medical training in quality and quantity
- A dearth of psychiatric physician-scientists because very few residents are pursuing research fellowships after training34
- Disproportionate emphasis on clinical care and generating clinical revenue (relative value units) in academic institutions, with fewer tenure-track faculty members having protected time to write grants for federal or foundation grants to support their salaries and research operations35
- Meager financial support for teaching in psychiatry departments
- Many seriously psychiatrically ill persons do not have access to psychiatric medical care (and often to primary care as well)
- Many in the public falsely believe psychiatric disorders are hopeless and untreatable, which perpetuates stigma
- Long-acting injectable antipsychotic formulations are not used early enough in patients with psychosis, who are known to have a high nonadherence rate with oral medications following discharge from their first hospitalization. This leads to many recurrences with multiple devastating consequences, including progressive brain tissue loss, treatment resistance, disability, incarceration, and suicide36
- Many clinicians do not have full-text access to all studies indexed in PubMed, which is vital for lifelong learning in a rapidly growing medical discipline such as psychiatry
- Psychiatrists are often unable to prescribe medications shortly after they are approved by the FDA due to the insurance companies’ outrageous preauthorization racket that enforces a fail-first policy with cheaper generics, even if generic medications are associated with safety and tolerability problems37
- The continued use of decades-old first-generation antipsychotic medications despite 32 published studies reporting their neurotoxicity and the death of brain cells38
Using this analysis to benefit our patients
Despite its strengths, psychiatry must overcome its weaknesses, fend off its threats, and exploit its many opportunities. The only way to do that is for psychiatrists to unify and for the APA to provide inspired leadership to achieve the aspirational goals of our field. However, we must adopt “moonshot thinking”39 to magnify the Ss, diminish the Ws, exploit the Os, and stave off the Ts of our SWOT, thereby attaining all our cherished and lofty goals. Ultimately, the greatest beneficiaries will be our patients.
Editor’s note: This article was adapted with permission from a version originally published in the Ohio Psychiatric Physician Association’s newsletter, Insight Matters, Fall 2022.
Acknowledging and analyzing strengths, weaknesses, opportunities, and threats (SWOT) is an important tactic many organizations use to develop a strategic plan to grow, move forward, and thrive. A SWOT analysis can provide a “big picture” view of the status and the desired future directions not only for companies but for medical disciplines such as psychiatry. So here are my perspectives on psychiatry’s strengths, weaknesses, opportunities, and threats. It is a work in progress, and I welcome (and encourage) you to send additional items or comments to me at henry.nasrallah@currentpsychiatry.com.
Strengths
- The American Psychiatric Association (APA) is the oldest medical professional organization, established in 1844 (3 years before the American Medical Association)1
- Strong organizational structure and governance, and a “big tent” with several tiers of membership
- Effective, member-driven District Branches
- The medical identity at the core of psychiatry—we are psychiatric physicians2
- Escalating number of senior medical students choosing psychiatry as a career, far more than a decade ago
- High demand for psychiatrists in all settings around the country
- Increased compensation for psychiatrists (market forces of supply and demand)
- Psychiatry is continuously evolving and reinventing itself: seismic shifts in etiopathogenesis, disease conceptualization, terminology, and therapies (4 major shifts over the past century)3
- An abundant body of evidence supporting that all psychiatric disorders are brain disorders and transdiagnostic in nature4
- Many vibrant subspecialty societies
- Substantial number of Tier 1, evidence-based treatments
- Novel mechanisms of action and treatment strategies are being introduced on a regular basis for psychotic and mood disorders5,6
- Advances in neuromodulation techniques to treat a wide spectrum of psychiatric disorders, including electroconvulsive therapy, transcranial magnetic stimulation, vagus nerve stimulation, transcranial direct current stimulation, deep brain stimulation, cranial electric stimulation, epidural cortical stimulation, focused ultrasound, low field magnetic stimulation, magnetic seizure therapy, and near infrared light therapy, with mechanisms that are electric, ultrasound, magnetic, or optical7,8
- Psychiatric physicians develop wisdom by practicing psychiatry (ie, they become more empathic, tolerant of ambiguity, prosocial, introspective, aware of one’s strengths and limitations). Neuroplasticity in the frontal cortex is triggered by conducting psychotherapy9
Weaknesses
- Shrinking workforce due to a static number of residency training slots for 40 years10
- High rate of retirement by aging psychiatrists
- Persistent stigma around mental disorders despite massive scientific and medical advances11
- Still no real parity! We need succinct laws with “teeth”12
- Demedicalization in the public sector, referring to psychiatric physicians as “providers” and labeling patients as “clients”2
- Not enough graduating residents choosing to do subspecialty fellowships (especially geriatric, addiction, psychosomatic psychiatry) to meet escalating societal needs
- Very low presence in rural areas (both psychiatrists and psychiatric hospitals)
- Persistent APA member apathy: only 10% to 15% vote in the APA national elections or volunteer to serve on committees
- Widespread member dissatisfaction with maintenance of certification
- Neuroscience advances are not being translated fast enough for practical clinical applications
- Many in the public at large do not realize psychiatric symptoms are generated from anomalous brain circuits or that psychiatric disorders are highly genetic but also have environmental and epigenetic etiologies
- The DSM diagnostic system needs a paradigm shift: it is still based on a menu of clinical signs and symptoms and is devoid of objective diagnostic measures such as biomarkers4
- Neuroscience literacy among busy psychiatric practitioners is insufficient at a time of explosive growth in basic and clinical neuroscience13
- No effective treatment for alcohol or substance use disorders despite their very high morbidity and mortality
- Major psychiatric disorders are still associated with significant disability (schizophrenia, bipolar disorder, major depressive disorder, anxiety disorders, eating disorders, substance use disorders)
- Suicide rate (other than opioid deaths) has continued to rise in the past 3 decades14
Opportunities
- Potentially momentous clinical applications of the neuroscience breakthroughs
- Collaborative care with primary care physicians and increasing colocalization
- Dramatic increase in public awareness about the importance of mental health due to the COVID-19 pandemic15
- Powerful new data management tools, including machine learning, artificial intelligence, super computers, big data, deep learning, nanotechnology, and metabolomics, all of which are expediting neurobiological discoveries16
- The potential of reclassifying psychiatric disorders as neurological disorders, which will improve reimbursement for patient health care and reduce stigma17
- Emergence of new mechanisms of action of disease etiology, such as microbiota, mitochondrial dysfunction, permeable blood-brain barrier, and neuroimmune dysregulation18,19
- The advent and growth of “precision psychiatry”20
- The tremendous potential of molecular genetics and gene therapy for psychiatric disorders, most of which are genetic in etiology
- Expanding applications of neuroimaging techniques, including morphological, spectroscopic, functional, diffusion tensor imaging, and receptor imaging21
- Epigenetic advances in neuropsychiatric disorders
- Remarkably powerful research methods, such as pluripotent cells (producing neurons from skin cells), optogenetics (activating genes with light), gene-wide association studies, CRISPR (clustered regularly interspaced short palindromic repeats, which serve as genetic scissors to remove and replace abnormal genes), and brain connectomics22
- Psychiatry should develop and promote an “annual mental health checkup” for all age groups, similar to an annual physical exam23
- Focus on the social determinants of health
- Address the unmet mental health needs of individuals who are members of minority groups
- Lobby ferociously for a much larger budget for the National Institute of Mental Health to advance funding for research of serious psychiatric brain disorders
- Remind Congress continuously that the cost of mental illness is $700 billion annually and costs can only be reduced by funding neurobiological research1
- Partner with the pharmaceutical industry instead of demonizing them. They are the only entity that develops medication for psychiatry, where 80% of disorders have no FDA-approved drugs.24 Without the pharmaceutical industry and the help of medications, many psychiatric patients would still be institutionalized and unable to lead a normal life. We must recognize the contributions of pharmaceutical companies to the health of our patients, similar to the warp speed development of vaccines for the deadly coronavirus
- Psychiatric clinicians must refer patients to clinical trials because without patients enrolling in FDA studies, no drug developments can take place
- Many “out-of-the-box” therapies are being developed, such as antiapoptotic therapy, microglia inhibition, mitochondrial repair, white matter fiber remyelination, neuroprotection, and reversing N-methyl-
d -aspartate receptor hypofunction25 - The emerging evidence that psychotherapy is in fact a biological treatment that induces brain changes (neuroplasticity) and can modulate the immune system26
- Druggable genes, providing innovative new medications27
- Reposition psychedelics as revolutionary new treatments28
- Emphasize measurement-based care (rating scales), which can upgrade patient care29
- Because psychosis is associated with brain tissue loss, just like heart attacks are associated with myocardium destruction, psychiatrists must act like cardiologists30 and treat psychotic episodes urgently, like a stroke,31 to reduce the duration of untreated psychosis and improve patient outcomes
Threats
- Antipsychiatry cults continue to disparage and attack psychiatry32
- Health delivery systems are replacing psychiatric physicians with nurse practitioners to lower costs, regardless of quality and experience, and they inappropriately lump them together as “providers”2
- Psychologists continue to seek prescribing privileges with absurdly sketchy, predominantly online training supervised by other psychologists33
- Many legislators and policymakers, as well as the public, still don’t understand the difference between psychiatrists and psychologists, and the extensively disparate medical training in quality and quantity
- A dearth of psychiatric physician-scientists because very few residents are pursuing research fellowships after training34
- Disproportionate emphasis on clinical care and generating clinical revenue (relative value units) in academic institutions, with fewer tenure-track faculty members having protected time to write grants for federal or foundation grants to support their salaries and research operations35
- Meager financial support for teaching in psychiatry departments
- Many seriously psychiatrically ill persons do not have access to psychiatric medical care (and often to primary care as well)
- Many in the public falsely believe psychiatric disorders are hopeless and untreatable, which perpetuates stigma
- Long-acting injectable antipsychotic formulations are not used early enough in patients with psychosis, who are known to have a high nonadherence rate with oral medications following discharge from their first hospitalization. This leads to many recurrences with multiple devastating consequences, including progressive brain tissue loss, treatment resistance, disability, incarceration, and suicide36
- Many clinicians do not have full-text access to all studies indexed in PubMed, which is vital for lifelong learning in a rapidly growing medical discipline such as psychiatry
- Psychiatrists are often unable to prescribe medications shortly after they are approved by the FDA due to the insurance companies’ outrageous preauthorization racket that enforces a fail-first policy with cheaper generics, even if generic medications are associated with safety and tolerability problems37
- The continued use of decades-old first-generation antipsychotic medications despite 32 published studies reporting their neurotoxicity and the death of brain cells38
Using this analysis to benefit our patients
Despite its strengths, psychiatry must overcome its weaknesses, fend off its threats, and exploit its many opportunities. The only way to do that is for psychiatrists to unify and for the APA to provide inspired leadership to achieve the aspirational goals of our field. However, we must adopt “moonshot thinking”39 to magnify the Ss, diminish the Ws, exploit the Os, and stave off the Ts of our SWOT, thereby attaining all our cherished and lofty goals. Ultimately, the greatest beneficiaries will be our patients.
1. Nasrallah HA. 20 reasons to celebrate our APA membership. Current Psychiatry. 2020;19(1):6-9.
2. Nasrallah HA. We are physicians, not providers, and we treat patients, not clients! Current Psychiatry. 2020;19(2):5-8.
3. Nasrallah HA. From bedlam to biomarkers: the transformation of psychiatry’s terminology reflects its 4 conceptual earthquakes. Current Psychiatry. 2015;14(1):5-7.
4. Nasrallah HA. Re-inventing the DSM as a transdiagnostic model: psychiatric disorders are extensively interconnected. Ann Clin Psychiatry. 2021;33(3):148-150.
5. Nasrallah HA. Psychopharmacology 3.0. Current Psychiatry. 2081;17(11):4-7.
6. Nasrallah HA. Reversing depression: a plethora of therapeutic strategies and mechanisms. Current Psychiatry. 2022;21(8):4-6.
7. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Psychopharmacology. 2012;37(1):102-116.
8. Nasrallah HA. Optimal psychiatric treatment: target the brain and avoid the body. Current Psychiatry. 2022;21(12):3-6.
9. Nasrallah HA. Does psychiatry practice make us wise? Current Psychiatry. 2009;8(10):12-14.
10. Buckley PF, Nasrallah HA. The psychiatry workforce pool is shrinking. What are we doing about it? Current Psychiatry. 2016;15(9):23-24,95.
11. Nasrallah HA. A psychiatric manifesto: stigma is hate speech and a hate crime. Current Psychiatry. 2022;21(6):6-8.
12. Nasrallah HA. The travesty of disparity and non-parity. Current Psychiatry. 2014;13(1):8,19.
13. Nasrallah HA. Advancing clinical neuroscience literacy among psychiatric practitioners. Current Psychiatry. 2017;16(9):17-18.
14. Nasrallah HA. The scourge of societal anosognosia about the mentally ill. Current Psychiatry. 2016;15(6):19-24.
15. Nasrallah HA. 10 silver linings of the COVID-19 pandemic. Insight Matters. 2021;45:3-4.
16. Kalenderian H, Nasrallah HA. Artificial intelligence in psychiatry. Current Psychiatry. 2019:18(8):33-38.
17. Nasrallah HA. Let’s tear down the silos and re-unify psychiatry and neurology! Current Psychiatry. 2013;12(8):8-9.
18. Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
19. Schrenk DA, Nasrallah HA. Faulty fences: blood-brain barrier dysfunction in schizophrenia. Current Psychiatry. 2022;21(10):28-32.
20. Nasrallah HA. The dawn of precision psychiatry. Current Psychiatry. 2017;16(12):7-8,11.
21. Nasrallah HA. Today’s psychiatric neuroscience advances were science fiction during my residency. Current Psychiatry 2021;20(4):5-7,12,24.
22. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
23. Nasrallah HA. I have a dream…for psychiatry. Current Psychiatry. 2021;20(11):12-14.
24. Devulapalli KK, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders: the majority of psychiatric diagnoses have no approved drug. Asian J Psychiatry. 2009;2(1):29-36.
25. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
26. Nasrallah HA. Repositioning psychotherapy as a neurobiological intervention. Current Psychiatry. 2013;12(12):18-19.
27. Nasrallah HA. Druggable genes, promiscuous drugs, repurposed medications. Current Psychiatry. 2016;15(5):23,27.
28. Nasrallah HA. Long overdue: measurement-based psychiatric practice. Current Psychiatry. 2009;8(4):14-16.
29. Nasrallah HA. Maddening therapies: how hallucinogens morphed into novel treatments. Current Psychiatry. 2017:16(1):19-21.
30. Nasrallah HA. For first episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
31. Nasrallah HA, Roque A. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
32. Nasrallah HA. The antipsychiatry movement: who and why. Current Psychiatry. 2011;10(12):4,6,53.
33. Nasrallah HA. Prescribing is the culmination of extensive medical training and psychologists do not qualify. Current Psychiatry. 2017;16(6):11-12,14-16.
34. Fenton W, James R, Insel T. Psychiatry residency training, the physician-scientist, and the future of psychiatry. Acad Psychiatry. 2004;28(4):263-266.
35. Balon R, Morreale MK. The precipitous decline of academic medicine in the United States. Ann Clin Psychiatry. 2020;32(4):225-227.
36. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
37. Nasrallah HA. Pre-authorization is illegal, unethical, and adversely disrupts patient care. Current Psychiatry. 2020;19(4):5-11.
38. Nasrallah HA, Chen AT. Multiple neurotoxic effects of haloperidol resulting in neuronal death. Ann Clin Psychiatry. 2017;29(3):195-202.
39. Nasrallah HA. It’s time for moonshot thinking in psychiatry. Current Psychiatry. 2022;21(2):8-10.
1. Nasrallah HA. 20 reasons to celebrate our APA membership. Current Psychiatry. 2020;19(1):6-9.
2. Nasrallah HA. We are physicians, not providers, and we treat patients, not clients! Current Psychiatry. 2020;19(2):5-8.
3. Nasrallah HA. From bedlam to biomarkers: the transformation of psychiatry’s terminology reflects its 4 conceptual earthquakes. Current Psychiatry. 2015;14(1):5-7.
4. Nasrallah HA. Re-inventing the DSM as a transdiagnostic model: psychiatric disorders are extensively interconnected. Ann Clin Psychiatry. 2021;33(3):148-150.
5. Nasrallah HA. Psychopharmacology 3.0. Current Psychiatry. 2081;17(11):4-7.
6. Nasrallah HA. Reversing depression: a plethora of therapeutic strategies and mechanisms. Current Psychiatry. 2022;21(8):4-6.
7. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Psychopharmacology. 2012;37(1):102-116.
8. Nasrallah HA. Optimal psychiatric treatment: target the brain and avoid the body. Current Psychiatry. 2022;21(12):3-6.
9. Nasrallah HA. Does psychiatry practice make us wise? Current Psychiatry. 2009;8(10):12-14.
10. Buckley PF, Nasrallah HA. The psychiatry workforce pool is shrinking. What are we doing about it? Current Psychiatry. 2016;15(9):23-24,95.
11. Nasrallah HA. A psychiatric manifesto: stigma is hate speech and a hate crime. Current Psychiatry. 2022;21(6):6-8.
12. Nasrallah HA. The travesty of disparity and non-parity. Current Psychiatry. 2014;13(1):8,19.
13. Nasrallah HA. Advancing clinical neuroscience literacy among psychiatric practitioners. Current Psychiatry. 2017;16(9):17-18.
14. Nasrallah HA. The scourge of societal anosognosia about the mentally ill. Current Psychiatry. 2016;15(6):19-24.
15. Nasrallah HA. 10 silver linings of the COVID-19 pandemic. Insight Matters. 2021;45:3-4.
16. Kalenderian H, Nasrallah HA. Artificial intelligence in psychiatry. Current Psychiatry. 2019:18(8):33-38.
17. Nasrallah HA. Let’s tear down the silos and re-unify psychiatry and neurology! Current Psychiatry. 2013;12(8):8-9.
18. Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
19. Schrenk DA, Nasrallah HA. Faulty fences: blood-brain barrier dysfunction in schizophrenia. Current Psychiatry. 2022;21(10):28-32.
20. Nasrallah HA. The dawn of precision psychiatry. Current Psychiatry. 2017;16(12):7-8,11.
21. Nasrallah HA. Today’s psychiatric neuroscience advances were science fiction during my residency. Current Psychiatry 2021;20(4):5-7,12,24.
22. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
23. Nasrallah HA. I have a dream…for psychiatry. Current Psychiatry. 2021;20(11):12-14.
24. Devulapalli KK, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders: the majority of psychiatric diagnoses have no approved drug. Asian J Psychiatry. 2009;2(1):29-36.
25. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
26. Nasrallah HA. Repositioning psychotherapy as a neurobiological intervention. Current Psychiatry. 2013;12(12):18-19.
27. Nasrallah HA. Druggable genes, promiscuous drugs, repurposed medications. Current Psychiatry. 2016;15(5):23,27.
28. Nasrallah HA. Long overdue: measurement-based psychiatric practice. Current Psychiatry. 2009;8(4):14-16.
29. Nasrallah HA. Maddening therapies: how hallucinogens morphed into novel treatments. Current Psychiatry. 2017:16(1):19-21.
30. Nasrallah HA. For first episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
31. Nasrallah HA, Roque A. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
32. Nasrallah HA. The antipsychiatry movement: who and why. Current Psychiatry. 2011;10(12):4,6,53.
33. Nasrallah HA. Prescribing is the culmination of extensive medical training and psychologists do not qualify. Current Psychiatry. 2017;16(6):11-12,14-16.
34. Fenton W, James R, Insel T. Psychiatry residency training, the physician-scientist, and the future of psychiatry. Acad Psychiatry. 2004;28(4):263-266.
35. Balon R, Morreale MK. The precipitous decline of academic medicine in the United States. Ann Clin Psychiatry. 2020;32(4):225-227.
36. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
37. Nasrallah HA. Pre-authorization is illegal, unethical, and adversely disrupts patient care. Current Psychiatry. 2020;19(4):5-11.
38. Nasrallah HA, Chen AT. Multiple neurotoxic effects of haloperidol resulting in neuronal death. Ann Clin Psychiatry. 2017;29(3):195-202.
39. Nasrallah HA. It’s time for moonshot thinking in psychiatry. Current Psychiatry. 2022;21(2):8-10.
From debate to stalemate and hate: An epidemic of intellectual constipation
Groupthink is hazardous, especially when perfused with religious fervor. It can lead to adopting irrational thinking1 and aversion to new ideas or facts. Tenaciously clinging to 1 ideology as “the absolute truth” precludes an open-minded, constructive debate with any other point of view.
Three historical examples come to mind:
- The discovery of chlorpromazine in 1952 was a scientifically and clinically seismic and transformational event for the treatment of psychosis, which for centuries had been dogmatically deemed irreversible. Jean Delay, MD, the French psychiatrist and co-discoverer of chlorpromazine, was the first physician to witness the magical and dazzling dissolution of delusions and hallucinations in chronically institutionalized patients with psychosis.2 He published his landmark clinical observations and then traveled to the United States to share the great news and present his findings at a large psychiatric conference, hoping to enthrall American psychiatrists with the historic breakthrough in treating psychosis. This was an era in which psychoanalysis dominated American psychiatry (despite its dearth of empirical evidence). Dr. Delay was shocked when the audience of psychoanalysts booed him for saying that psychosis can be treated with a medication instead of with psychoanalysis (which, in the most intense groupthink in the history of psychiatry, they all believed was the only therapy for psychosis). Deeply disheartened, Dr. Delay returned to France and never returned to the United States. This groupthink was a prime example of intellectual constipation. Since then, not surprisingly, psychopharmacology grew meteorically while psychoanalysis declined precipitously.
- The monoamine hypothesis of depression, first propagated 60 years ago, became a groupthink dogma among psychiatric researchers for the next several decades, stultifying broader antidepressant medication development by focusing only on monoamines (eg, serotonin, norepinephrine, and dopamine). More recently, researchers have become more open-minded, and the monoamine hypothesis has taken a backseat to innovative new models of antidepressant therapy based on advances in the pathophysiology of depression, such as glutamatergic, opioid, and sigma pathways as well as neuroplasticity models.3 The consequence of groupthink in antidepressant research was a half-century delay in the development of effective alternative treatments that could have helped millions of patients recover from a life-threatening brain disorder such as major depressive disorder.
- Peptic ulcer and its serious gastritis were long believed to be due to stress and increased stomach acidity. So the groupthink gastroenterologists mocked 2 Australian researchers, Barry Marshall and Robin Warren, when they proposed that peptic ulcer may be due to an infection with a bacterium called Helicobacter pylori, and published their data demonstrating it.4 Marshall and Warren had the last laugh when they were awarded the 2005 Nobel Prize in Medicine and Physiology. It is ironic that even gastroenterologists are not immune to the affliction of intellectual constipation!
Intellectual constipation’s effects on youth
The principle of a civilized debate of contrarian ideas must be inculcated early, especially during college years. Youth should be mentored about not cowering into an ideological cocoon and shun listening to different or opposing points of view.5 Institutions of higher learning are incubators of future leaders. They must provide their young students with a wide diversity of ideas and philosophies and encourage them to critique those ideas, not “shelter” or isolate them from any ideas. Youth need to recognize that the complex societies in which we all live and work are not placid or unidimensional but a hotbed of clashing ideas and perspectives. An open-minded approach to education will inoculate young minds from developing intellectual constipation in adulthood.
Avoiding or insulating oneself from the ideas of others—no matter how disagreeable—leads to cognitive cowardice and behavioral intolerance. Healthy and vibrant debate is necessary as an inoculation against extremism, hate, paranoia, and, ultimately, violence. Psychiatrists help patients to self-reflect, gain insight, and consider changing their view of themselves and the world to help them grow into mature and resilient individuals. But for the millions of people with intellectual constipation, a potent cerebral enema comprised of a salubrious concoction of insight, common sense, and compromise may be the prescription to forestall lethal intellectual ileus.
1. Nasrallah HA. Irrational beliefs: a ubiquitous human trait. Current Psychiatry. 2007;6(2):15-16.
2. Ban TA. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr Dis Treat. 2007;3(4):495-500.
3. Boku S, Nakagawa S, Toda H, et al. Neural basis of major depressive disorder: beyond monoamine hypothesis. Psychiatry Clin Neurosci. 2018;72(1):3-12.
4. Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1(8336):1273-1275.
5. Lukianoff G, Haidt J. The Coddling of the American Mind: How Good Intentions and Bad Ideas Are Setting Up a Generation for Failure. Penguin Books; 2018.
Groupthink is hazardous, especially when perfused with religious fervor. It can lead to adopting irrational thinking1 and aversion to new ideas or facts. Tenaciously clinging to 1 ideology as “the absolute truth” precludes an open-minded, constructive debate with any other point of view.
Three historical examples come to mind:
- The discovery of chlorpromazine in 1952 was a scientifically and clinically seismic and transformational event for the treatment of psychosis, which for centuries had been dogmatically deemed irreversible. Jean Delay, MD, the French psychiatrist and co-discoverer of chlorpromazine, was the first physician to witness the magical and dazzling dissolution of delusions and hallucinations in chronically institutionalized patients with psychosis.2 He published his landmark clinical observations and then traveled to the United States to share the great news and present his findings at a large psychiatric conference, hoping to enthrall American psychiatrists with the historic breakthrough in treating psychosis. This was an era in which psychoanalysis dominated American psychiatry (despite its dearth of empirical evidence). Dr. Delay was shocked when the audience of psychoanalysts booed him for saying that psychosis can be treated with a medication instead of with psychoanalysis (which, in the most intense groupthink in the history of psychiatry, they all believed was the only therapy for psychosis). Deeply disheartened, Dr. Delay returned to France and never returned to the United States. This groupthink was a prime example of intellectual constipation. Since then, not surprisingly, psychopharmacology grew meteorically while psychoanalysis declined precipitously.
- The monoamine hypothesis of depression, first propagated 60 years ago, became a groupthink dogma among psychiatric researchers for the next several decades, stultifying broader antidepressant medication development by focusing only on monoamines (eg, serotonin, norepinephrine, and dopamine). More recently, researchers have become more open-minded, and the monoamine hypothesis has taken a backseat to innovative new models of antidepressant therapy based on advances in the pathophysiology of depression, such as glutamatergic, opioid, and sigma pathways as well as neuroplasticity models.3 The consequence of groupthink in antidepressant research was a half-century delay in the development of effective alternative treatments that could have helped millions of patients recover from a life-threatening brain disorder such as major depressive disorder.
- Peptic ulcer and its serious gastritis were long believed to be due to stress and increased stomach acidity. So the groupthink gastroenterologists mocked 2 Australian researchers, Barry Marshall and Robin Warren, when they proposed that peptic ulcer may be due to an infection with a bacterium called Helicobacter pylori, and published their data demonstrating it.4 Marshall and Warren had the last laugh when they were awarded the 2005 Nobel Prize in Medicine and Physiology. It is ironic that even gastroenterologists are not immune to the affliction of intellectual constipation!
Intellectual constipation’s effects on youth
The principle of a civilized debate of contrarian ideas must be inculcated early, especially during college years. Youth should be mentored about not cowering into an ideological cocoon and shun listening to different or opposing points of view.5 Institutions of higher learning are incubators of future leaders. They must provide their young students with a wide diversity of ideas and philosophies and encourage them to critique those ideas, not “shelter” or isolate them from any ideas. Youth need to recognize that the complex societies in which we all live and work are not placid or unidimensional but a hotbed of clashing ideas and perspectives. An open-minded approach to education will inoculate young minds from developing intellectual constipation in adulthood.
Avoiding or insulating oneself from the ideas of others—no matter how disagreeable—leads to cognitive cowardice and behavioral intolerance. Healthy and vibrant debate is necessary as an inoculation against extremism, hate, paranoia, and, ultimately, violence. Psychiatrists help patients to self-reflect, gain insight, and consider changing their view of themselves and the world to help them grow into mature and resilient individuals. But for the millions of people with intellectual constipation, a potent cerebral enema comprised of a salubrious concoction of insight, common sense, and compromise may be the prescription to forestall lethal intellectual ileus.
Groupthink is hazardous, especially when perfused with religious fervor. It can lead to adopting irrational thinking1 and aversion to new ideas or facts. Tenaciously clinging to 1 ideology as “the absolute truth” precludes an open-minded, constructive debate with any other point of view.
Three historical examples come to mind:
- The discovery of chlorpromazine in 1952 was a scientifically and clinically seismic and transformational event for the treatment of psychosis, which for centuries had been dogmatically deemed irreversible. Jean Delay, MD, the French psychiatrist and co-discoverer of chlorpromazine, was the first physician to witness the magical and dazzling dissolution of delusions and hallucinations in chronically institutionalized patients with psychosis.2 He published his landmark clinical observations and then traveled to the United States to share the great news and present his findings at a large psychiatric conference, hoping to enthrall American psychiatrists with the historic breakthrough in treating psychosis. This was an era in which psychoanalysis dominated American psychiatry (despite its dearth of empirical evidence). Dr. Delay was shocked when the audience of psychoanalysts booed him for saying that psychosis can be treated with a medication instead of with psychoanalysis (which, in the most intense groupthink in the history of psychiatry, they all believed was the only therapy for psychosis). Deeply disheartened, Dr. Delay returned to France and never returned to the United States. This groupthink was a prime example of intellectual constipation. Since then, not surprisingly, psychopharmacology grew meteorically while psychoanalysis declined precipitously.
- The monoamine hypothesis of depression, first propagated 60 years ago, became a groupthink dogma among psychiatric researchers for the next several decades, stultifying broader antidepressant medication development by focusing only on monoamines (eg, serotonin, norepinephrine, and dopamine). More recently, researchers have become more open-minded, and the monoamine hypothesis has taken a backseat to innovative new models of antidepressant therapy based on advances in the pathophysiology of depression, such as glutamatergic, opioid, and sigma pathways as well as neuroplasticity models.3 The consequence of groupthink in antidepressant research was a half-century delay in the development of effective alternative treatments that could have helped millions of patients recover from a life-threatening brain disorder such as major depressive disorder.
- Peptic ulcer and its serious gastritis were long believed to be due to stress and increased stomach acidity. So the groupthink gastroenterologists mocked 2 Australian researchers, Barry Marshall and Robin Warren, when they proposed that peptic ulcer may be due to an infection with a bacterium called Helicobacter pylori, and published their data demonstrating it.4 Marshall and Warren had the last laugh when they were awarded the 2005 Nobel Prize in Medicine and Physiology. It is ironic that even gastroenterologists are not immune to the affliction of intellectual constipation!
Intellectual constipation’s effects on youth
The principle of a civilized debate of contrarian ideas must be inculcated early, especially during college years. Youth should be mentored about not cowering into an ideological cocoon and shun listening to different or opposing points of view.5 Institutions of higher learning are incubators of future leaders. They must provide their young students with a wide diversity of ideas and philosophies and encourage them to critique those ideas, not “shelter” or isolate them from any ideas. Youth need to recognize that the complex societies in which we all live and work are not placid or unidimensional but a hotbed of clashing ideas and perspectives. An open-minded approach to education will inoculate young minds from developing intellectual constipation in adulthood.
Avoiding or insulating oneself from the ideas of others—no matter how disagreeable—leads to cognitive cowardice and behavioral intolerance. Healthy and vibrant debate is necessary as an inoculation against extremism, hate, paranoia, and, ultimately, violence. Psychiatrists help patients to self-reflect, gain insight, and consider changing their view of themselves and the world to help them grow into mature and resilient individuals. But for the millions of people with intellectual constipation, a potent cerebral enema comprised of a salubrious concoction of insight, common sense, and compromise may be the prescription to forestall lethal intellectual ileus.
1. Nasrallah HA. Irrational beliefs: a ubiquitous human trait. Current Psychiatry. 2007;6(2):15-16.
2. Ban TA. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr Dis Treat. 2007;3(4):495-500.
3. Boku S, Nakagawa S, Toda H, et al. Neural basis of major depressive disorder: beyond monoamine hypothesis. Psychiatry Clin Neurosci. 2018;72(1):3-12.
4. Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1(8336):1273-1275.
5. Lukianoff G, Haidt J. The Coddling of the American Mind: How Good Intentions and Bad Ideas Are Setting Up a Generation for Failure. Penguin Books; 2018.
1. Nasrallah HA. Irrational beliefs: a ubiquitous human trait. Current Psychiatry. 2007;6(2):15-16.
2. Ban TA. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr Dis Treat. 2007;3(4):495-500.
3. Boku S, Nakagawa S, Toda H, et al. Neural basis of major depressive disorder: beyond monoamine hypothesis. Psychiatry Clin Neurosci. 2018;72(1):3-12.
4. Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1(8336):1273-1275.
5. Lukianoff G, Haidt J. The Coddling of the American Mind: How Good Intentions and Bad Ideas Are Setting Up a Generation for Failure. Penguin Books; 2018.
Optimal psychiatric treatment: Target the brain and avoid the body
Pharmacotherapy for psychiatric disorders is a mixed blessing. The advent of psychotropic medications since the 1950s (antipsychotics, antidepressants, anxiolytics, mood stabilizers) has revolutionized the treatment of serious psychiatric brain disorders, allowing certain patients to be discharged to the community after a lifetime of institutionalization.
However, like all medications, psychotropic agents are often associated with various potentially intolerable symptoms (Table 1) or safety complications (Table 2) because they interact with every organ in the body besides their intended target, the brain, and its neurochemical circuitry.
Imagine if we could treat our psychiatric patients while bypassing the body and achieve response, remission, and ultimately recovery without any systemic adverse effects. Adherence would dramatically improve, our patients’ quality of life would be enhanced, and the overall effectiveness (defined as the complex package of efficacy, safety, and tolerability) would be superior to current pharmacotherapies. This is important because most psychiatric medications must be taken daily for years, even a lifetime, to avoid a relapse of the illness. Psychiatrists frequently must manage adverse effects or switch the patient to a different medication if a tolerability or safety issue emerges, which is very common in psychiatric practice. A significant part of psychopharmacologic management includes ordering various laboratory tests to monitor adverse reactions in major organs, especially the liver, kidney, and heart. Additionally, psychiatric physicians must be constantly cognizant of medications prescribed by other clinicians for comorbid medical conditions to successfully navigate the turbulent seas of pharmacokinetic interactions.
I am sure you have noticed that whenever you watch a direct-to-consumer commercial for any medication, 90% of the advertisement is a background voice listing the various tolerability and safety complications of the medication as required by the FDA. Interestingly, these ads frequently contain colorful scenery and joyful clips, which I suspect are cleverly designed to distract the audience from focusing on the list of adverse effects.
Benefits of nonpharmacologic treatments
No wonder I am a fan of psychotherapy, a well-established psychiatric treatment modality that completely avoids body tissues. It directly targets the brain without needlessly interacting with any other organ. Psychotherapy’s many benefits (improving insight, enhancing adherence, improving self-esteem, reducing risky behaviors, guiding stress management and coping skills, modifying unhealthy beliefs, and ultimately relieving symptoms such as anxiety and depression) are achieved without any somatic adverse effects! Psychotherapy has also been shown to induce neuroplasticity and reduce inflammatory biomarkers.1 Unlike FDA-approved medications, psychotherapy does not include a “package insert,” 10 to 20 pages (in small print) that mostly focus on warnings, precautions, and sundry physical adverse effects. Even the dosing of psychotherapy is left entirely up to the treating clinician!
Although I have had many gratifying results with pharmacotherapy in my practice, especially in combination with psychotherapy,2 I also have observed excellent outcomes with nonpharmacologic approaches, especially neuromodulation therapies. The best antidepressant I have ever used since my residency training days is electroconvulsive therapy (ECT). My experience is consistent with a large meta-analysis3showing a huge effect size (Cohen d = .91) in contrast to the usual effect size of .3 to .5 for standard antidepressants (except IV ketamine). A recent study showed ECT is even better than the vaunted rapid-acting ketamine,4 which is further evidence of its remarkable efficacy in depression. Neuroimaging studies report that ECT rapidly increases the volume of the hippocampus,5,6 which shrinks in size in patients with unipolar or bipolar depression.
Neuromodulation may very well be the future of psychiatric therapeutics. It targets the brain and avoids the body, thus achieving efficacy with minimal systemic tolerability (ie, patient complaints) (Table 1) or safety (abnormal laboratory test results) issues (Table 2). This sounds ideal, and it is arguably an optimal approach to repairing the brain and healing the mind.
Continue to: ECT is the oldest...
ECT is the oldest neuromodulation technique (developed almost 100 years ago and significantly refined since then). Newer FDA-approved neuromodulation therapies include repetitive transcranial magnetic stimulation (rTMS), which was approved for depression in 2013, obsessive-compulsive disorder (OCD) in 2018, smoking cessation in 2020, and anxious depression in 2021.7 Vagus nerve stimulation (VNS) is used for drug-resistant epilepsy and was later approved for treatment-resistant depression,8,9 but some studies report it can be helpful for fear and anxiety in autism spectrum disorder10 and primary insomnia.11
There are many other neuromodulation therapies in development12 that have not yet been FDA approved (Table 3). The most prominent of these is deep brain stimulation (DBS), which is approved for Parkinson disease and has been reported in many studies to improve treatment-resistant depression13,14 and OCD.15 Another promising neuromodulation therapy is transcranial direct current stimulation (tDCS), which has promising results in schizophrenia16 similar to ECT’s effects in treatment-resistant schizophrenia.17
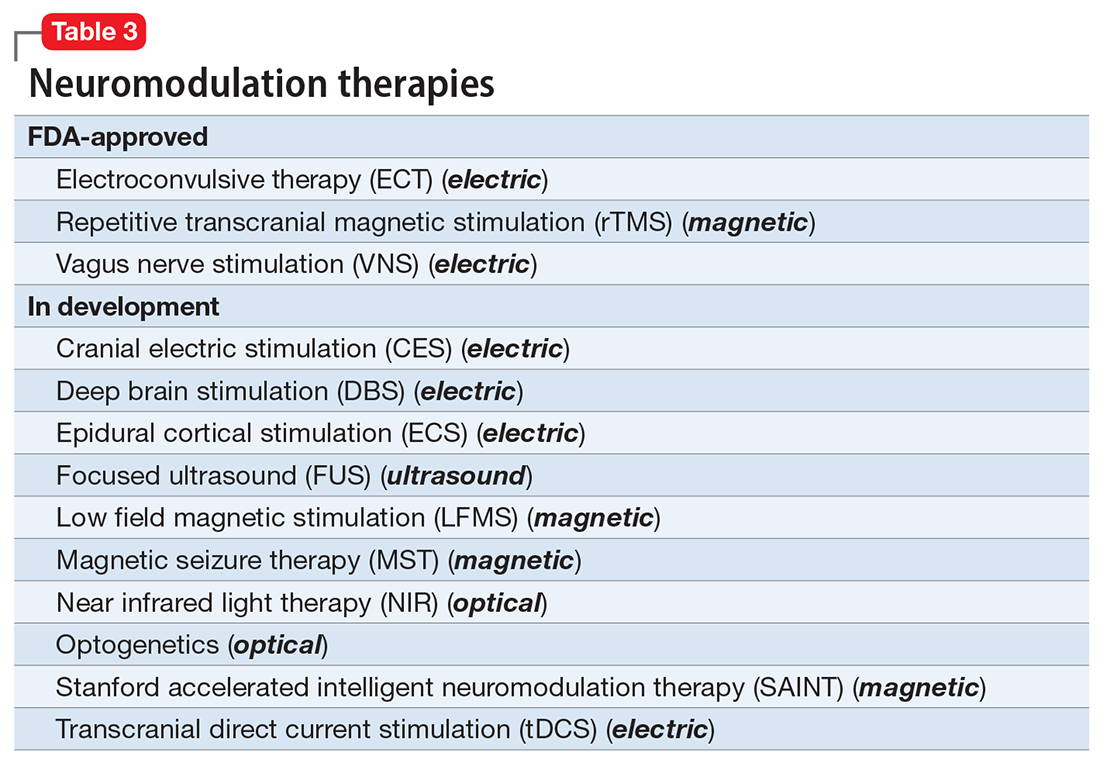
A particularly exciting neuromodulation approach published by Stanford University researchers is Stanford accelerated intelligent neuromodulation therapy (SAINT),18 which uses intermittent theta-burst stimulation (iTBS) daily for 5 days, targeted at the subgenual anterior cingulate gyrus (Brodman area 25). Remarkably, efficacy was rapid, with a very high remission rate (absence of symptoms) in approximately 90% of patients with severe depression.18
The future is bright for neuromodulation therapies, and for a good reason. Why send a chemical agent to every cell and organ in the body when the brain can be targeted directly? As psychiatric neuroscience advances to a point where we can localize the abnormal neurologic circuit in a specific brain region for each psychiatric disorder, it will be possible to treat almost all psychiatric disorders without burdening patients with the intolerable symptoms or safety adverse effects of medications. Psychiatrists should modulate their perspective about the future of psychiatric treatments. And finally, I propose that psychotherapy should be reclassified as a “verbal neuromodulation” technique.
1. Nasrallah HA. Repositioning psychotherapy as a neurobiological intervention. Current Psychiatry. 2013;12(12):18-19.
2. Nasrallah HA. Bipolar disorder: clinical questions beg for answers. Current Psychiatry. 2006;5(12):11-12.
3. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361(9360):799-808.
4. Rhee TG, Shim SR, Forester BP, et al. Efficacy and safety of ketamine vs electroconvulsive therapy among patients with major depressive episode: a systematic review and meta-analysis. JAMA Psychiatry. 2022:e223352. doi:10.1001/jamapsychiatry.2022.3352
5. Nuninga JO, Mandl RCW, Boks MP, et al. Volume increase in the dentate gyrus after electroconvulsive therapy in depressed patients as measured with 7T. Mol Psychiatry. 2020;25(7):1559-1568.
6. Joshi SH, Espinoza RT, Pirnia T, et al. Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry. 2016;79(4):282-292.
7. Rhee TG, Olfson M, Nierenberg AA, et al. 20-year trends in the pharmacologic treatment of bipolar disorder by psychiatrists in outpatient care settings. Am J Psychiatry. 2020;177(8):706-715.
8. Hilz MJ. Transcutaneous vagus nerve stimulation - a brief introduction and overview. Auton Neurosci. 2022;243:103038. doi:10.1016/j.autneu.2022.103038
9. Pigato G, Rosson S, Bresolin N, et al. Vagus nerve stimulation in treatment-resistant depression: a case series of long-term follow-up. J ECT. 2022. doi:10.1097/YCT.0000000000000869
10. Shivaswamy T, Souza RR, Engineer CT, et al. Vagus nerve stimulation as a treatment for fear and anxiety in individuals with autism spectrum disorder. J Psychiatr Brain Sci. 2022;7(4):e220007. doi:10.20900/jpbs.20220007
11. Wu Y, Song L, Wang X, et al. Transcutaneous vagus nerve stimulation could improve the effective rate on the quality of sleep in the treatment of primary insomnia: a randomized control trial. Brain Sci. 2022;12(10):1296. doi:10.3390/brainsci12101296
12. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Neuropsychopharmacology. 2012;37(1):102-116.
13. Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651-660.
14. Choi KS, Mayberg H. Connectomic DBS in major depression. In: Horn A, ed. Connectomic Deep Brain Stimulation. Academic Press; 2022:433-447.
15. Cruz S, Gutiérrez-Rojas L, González-Domenech P, et al. Deep brain stimulation in obsessive-compulsive disorder: results from meta-analysis. Psychiatry Res. 2022;317:114869. doi:10.1016/j.psychres.2022.114869
16. Lisoni J, Baldacci G, Nibbio G, et al. Effects of bilateral, bipolar-nonbalanced, frontal transcranial direct current stimulation (tDCS) on negative symptoms and neurocognition in a sample of patients living with schizophrenia: results of a randomized double-blind sham-controlled trial. J Psychiatr Res. 2022;155:430-442.
17. Sinclair DJ, Zhao S, Qi F, et al. Electroconvulsive therapy for treatment-resistant schizophrenia. Cochrane Database Syst Rev. 2019;3(3):CD011847. doi:10.1002/14651858.CD011847.pub2
18. Cole EJ, Stimpson KH, Bentzley BS, et al. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. Am J Psychiatry. 2020;177(8):716-726.
Pharmacotherapy for psychiatric disorders is a mixed blessing. The advent of psychotropic medications since the 1950s (antipsychotics, antidepressants, anxiolytics, mood stabilizers) has revolutionized the treatment of serious psychiatric brain disorders, allowing certain patients to be discharged to the community after a lifetime of institutionalization.

However, like all medications, psychotropic agents are often associated with various potentially intolerable symptoms (Table 1) or safety complications (Table 2) because they interact with every organ in the body besides their intended target, the brain, and its neurochemical circuitry.

Imagine if we could treat our psychiatric patients while bypassing the body and achieve response, remission, and ultimately recovery without any systemic adverse effects. Adherence would dramatically improve, our patients’ quality of life would be enhanced, and the overall effectiveness (defined as the complex package of efficacy, safety, and tolerability) would be superior to current pharmacotherapies. This is important because most psychiatric medications must be taken daily for years, even a lifetime, to avoid a relapse of the illness. Psychiatrists frequently must manage adverse effects or switch the patient to a different medication if a tolerability or safety issue emerges, which is very common in psychiatric practice. A significant part of psychopharmacologic management includes ordering various laboratory tests to monitor adverse reactions in major organs, especially the liver, kidney, and heart. Additionally, psychiatric physicians must be constantly cognizant of medications prescribed by other clinicians for comorbid medical conditions to successfully navigate the turbulent seas of pharmacokinetic interactions.
I am sure you have noticed that whenever you watch a direct-to-consumer commercial for any medication, 90% of the advertisement is a background voice listing the various tolerability and safety complications of the medication as required by the FDA. Interestingly, these ads frequently contain colorful scenery and joyful clips, which I suspect are cleverly designed to distract the audience from focusing on the list of adverse effects.
Benefits of nonpharmacologic treatments
No wonder I am a fan of psychotherapy, a well-established psychiatric treatment modality that completely avoids body tissues. It directly targets the brain without needlessly interacting with any other organ. Psychotherapy’s many benefits (improving insight, enhancing adherence, improving self-esteem, reducing risky behaviors, guiding stress management and coping skills, modifying unhealthy beliefs, and ultimately relieving symptoms such as anxiety and depression) are achieved without any somatic adverse effects! Psychotherapy has also been shown to induce neuroplasticity and reduce inflammatory biomarkers.1 Unlike FDA-approved medications, psychotherapy does not include a “package insert,” 10 to 20 pages (in small print) that mostly focus on warnings, precautions, and sundry physical adverse effects. Even the dosing of psychotherapy is left entirely up to the treating clinician!
Although I have had many gratifying results with pharmacotherapy in my practice, especially in combination with psychotherapy,2 I also have observed excellent outcomes with nonpharmacologic approaches, especially neuromodulation therapies. The best antidepressant I have ever used since my residency training days is electroconvulsive therapy (ECT). My experience is consistent with a large meta-analysis3showing a huge effect size (Cohen d = .91) in contrast to the usual effect size of .3 to .5 for standard antidepressants (except IV ketamine). A recent study showed ECT is even better than the vaunted rapid-acting ketamine,4 which is further evidence of its remarkable efficacy in depression. Neuroimaging studies report that ECT rapidly increases the volume of the hippocampus,5,6 which shrinks in size in patients with unipolar or bipolar depression.
Neuromodulation may very well be the future of psychiatric therapeutics. It targets the brain and avoids the body, thus achieving efficacy with minimal systemic tolerability (ie, patient complaints) (Table 1) or safety (abnormal laboratory test results) issues (Table 2). This sounds ideal, and it is arguably an optimal approach to repairing the brain and healing the mind.
Continue to: ECT is the oldest...
ECT is the oldest neuromodulation technique (developed almost 100 years ago and significantly refined since then). Newer FDA-approved neuromodulation therapies include repetitive transcranial magnetic stimulation (rTMS), which was approved for depression in 2013, obsessive-compulsive disorder (OCD) in 2018, smoking cessation in 2020, and anxious depression in 2021.7 Vagus nerve stimulation (VNS) is used for drug-resistant epilepsy and was later approved for treatment-resistant depression,8,9 but some studies report it can be helpful for fear and anxiety in autism spectrum disorder10 and primary insomnia.11
There are many other neuromodulation therapies in development12 that have not yet been FDA approved (Table 3). The most prominent of these is deep brain stimulation (DBS), which is approved for Parkinson disease and has been reported in many studies to improve treatment-resistant depression13,14 and OCD.15 Another promising neuromodulation therapy is transcranial direct current stimulation (tDCS), which has promising results in schizophrenia16 similar to ECT’s effects in treatment-resistant schizophrenia.17

A particularly exciting neuromodulation approach published by Stanford University researchers is Stanford accelerated intelligent neuromodulation therapy (SAINT),18 which uses intermittent theta-burst stimulation (iTBS) daily for 5 days, targeted at the subgenual anterior cingulate gyrus (Brodman area 25). Remarkably, efficacy was rapid, with a very high remission rate (absence of symptoms) in approximately 90% of patients with severe depression.18
The future is bright for neuromodulation therapies, and for a good reason. Why send a chemical agent to every cell and organ in the body when the brain can be targeted directly? As psychiatric neuroscience advances to a point where we can localize the abnormal neurologic circuit in a specific brain region for each psychiatric disorder, it will be possible to treat almost all psychiatric disorders without burdening patients with the intolerable symptoms or safety adverse effects of medications. Psychiatrists should modulate their perspective about the future of psychiatric treatments. And finally, I propose that psychotherapy should be reclassified as a “verbal neuromodulation” technique.
Pharmacotherapy for psychiatric disorders is a mixed blessing. The advent of psychotropic medications since the 1950s (antipsychotics, antidepressants, anxiolytics, mood stabilizers) has revolutionized the treatment of serious psychiatric brain disorders, allowing certain patients to be discharged to the community after a lifetime of institutionalization.

However, like all medications, psychotropic agents are often associated with various potentially intolerable symptoms (Table 1) or safety complications (Table 2) because they interact with every organ in the body besides their intended target, the brain, and its neurochemical circuitry.

Imagine if we could treat our psychiatric patients while bypassing the body and achieve response, remission, and ultimately recovery without any systemic adverse effects. Adherence would dramatically improve, our patients’ quality of life would be enhanced, and the overall effectiveness (defined as the complex package of efficacy, safety, and tolerability) would be superior to current pharmacotherapies. This is important because most psychiatric medications must be taken daily for years, even a lifetime, to avoid a relapse of the illness. Psychiatrists frequently must manage adverse effects or switch the patient to a different medication if a tolerability or safety issue emerges, which is very common in psychiatric practice. A significant part of psychopharmacologic management includes ordering various laboratory tests to monitor adverse reactions in major organs, especially the liver, kidney, and heart. Additionally, psychiatric physicians must be constantly cognizant of medications prescribed by other clinicians for comorbid medical conditions to successfully navigate the turbulent seas of pharmacokinetic interactions.
I am sure you have noticed that whenever you watch a direct-to-consumer commercial for any medication, 90% of the advertisement is a background voice listing the various tolerability and safety complications of the medication as required by the FDA. Interestingly, these ads frequently contain colorful scenery and joyful clips, which I suspect are cleverly designed to distract the audience from focusing on the list of adverse effects.
Benefits of nonpharmacologic treatments
No wonder I am a fan of psychotherapy, a well-established psychiatric treatment modality that completely avoids body tissues. It directly targets the brain without needlessly interacting with any other organ. Psychotherapy’s many benefits (improving insight, enhancing adherence, improving self-esteem, reducing risky behaviors, guiding stress management and coping skills, modifying unhealthy beliefs, and ultimately relieving symptoms such as anxiety and depression) are achieved without any somatic adverse effects! Psychotherapy has also been shown to induce neuroplasticity and reduce inflammatory biomarkers.1 Unlike FDA-approved medications, psychotherapy does not include a “package insert,” 10 to 20 pages (in small print) that mostly focus on warnings, precautions, and sundry physical adverse effects. Even the dosing of psychotherapy is left entirely up to the treating clinician!
Although I have had many gratifying results with pharmacotherapy in my practice, especially in combination with psychotherapy,2 I also have observed excellent outcomes with nonpharmacologic approaches, especially neuromodulation therapies. The best antidepressant I have ever used since my residency training days is electroconvulsive therapy (ECT). My experience is consistent with a large meta-analysis3showing a huge effect size (Cohen d = .91) in contrast to the usual effect size of .3 to .5 for standard antidepressants (except IV ketamine). A recent study showed ECT is even better than the vaunted rapid-acting ketamine,4 which is further evidence of its remarkable efficacy in depression. Neuroimaging studies report that ECT rapidly increases the volume of the hippocampus,5,6 which shrinks in size in patients with unipolar or bipolar depression.
Neuromodulation may very well be the future of psychiatric therapeutics. It targets the brain and avoids the body, thus achieving efficacy with minimal systemic tolerability (ie, patient complaints) (Table 1) or safety (abnormal laboratory test results) issues (Table 2). This sounds ideal, and it is arguably an optimal approach to repairing the brain and healing the mind.
Continue to: ECT is the oldest...
ECT is the oldest neuromodulation technique (developed almost 100 years ago and significantly refined since then). Newer FDA-approved neuromodulation therapies include repetitive transcranial magnetic stimulation (rTMS), which was approved for depression in 2013, obsessive-compulsive disorder (OCD) in 2018, smoking cessation in 2020, and anxious depression in 2021.7 Vagus nerve stimulation (VNS) is used for drug-resistant epilepsy and was later approved for treatment-resistant depression,8,9 but some studies report it can be helpful for fear and anxiety in autism spectrum disorder10 and primary insomnia.11
There are many other neuromodulation therapies in development12 that have not yet been FDA approved (Table 3). The most prominent of these is deep brain stimulation (DBS), which is approved for Parkinson disease and has been reported in many studies to improve treatment-resistant depression13,14 and OCD.15 Another promising neuromodulation therapy is transcranial direct current stimulation (tDCS), which has promising results in schizophrenia16 similar to ECT’s effects in treatment-resistant schizophrenia.17

A particularly exciting neuromodulation approach published by Stanford University researchers is Stanford accelerated intelligent neuromodulation therapy (SAINT),18 which uses intermittent theta-burst stimulation (iTBS) daily for 5 days, targeted at the subgenual anterior cingulate gyrus (Brodman area 25). Remarkably, efficacy was rapid, with a very high remission rate (absence of symptoms) in approximately 90% of patients with severe depression.18
The future is bright for neuromodulation therapies, and for a good reason. Why send a chemical agent to every cell and organ in the body when the brain can be targeted directly? As psychiatric neuroscience advances to a point where we can localize the abnormal neurologic circuit in a specific brain region for each psychiatric disorder, it will be possible to treat almost all psychiatric disorders without burdening patients with the intolerable symptoms or safety adverse effects of medications. Psychiatrists should modulate their perspective about the future of psychiatric treatments. And finally, I propose that psychotherapy should be reclassified as a “verbal neuromodulation” technique.
1. Nasrallah HA. Repositioning psychotherapy as a neurobiological intervention. Current Psychiatry. 2013;12(12):18-19.
2. Nasrallah HA. Bipolar disorder: clinical questions beg for answers. Current Psychiatry. 2006;5(12):11-12.
3. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361(9360):799-808.
4. Rhee TG, Shim SR, Forester BP, et al. Efficacy and safety of ketamine vs electroconvulsive therapy among patients with major depressive episode: a systematic review and meta-analysis. JAMA Psychiatry. 2022:e223352. doi:10.1001/jamapsychiatry.2022.3352
5. Nuninga JO, Mandl RCW, Boks MP, et al. Volume increase in the dentate gyrus after electroconvulsive therapy in depressed patients as measured with 7T. Mol Psychiatry. 2020;25(7):1559-1568.
6. Joshi SH, Espinoza RT, Pirnia T, et al. Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry. 2016;79(4):282-292.
7. Rhee TG, Olfson M, Nierenberg AA, et al. 20-year trends in the pharmacologic treatment of bipolar disorder by psychiatrists in outpatient care settings. Am J Psychiatry. 2020;177(8):706-715.
8. Hilz MJ. Transcutaneous vagus nerve stimulation - a brief introduction and overview. Auton Neurosci. 2022;243:103038. doi:10.1016/j.autneu.2022.103038
9. Pigato G, Rosson S, Bresolin N, et al. Vagus nerve stimulation in treatment-resistant depression: a case series of long-term follow-up. J ECT. 2022. doi:10.1097/YCT.0000000000000869
10. Shivaswamy T, Souza RR, Engineer CT, et al. Vagus nerve stimulation as a treatment for fear and anxiety in individuals with autism spectrum disorder. J Psychiatr Brain Sci. 2022;7(4):e220007. doi:10.20900/jpbs.20220007
11. Wu Y, Song L, Wang X, et al. Transcutaneous vagus nerve stimulation could improve the effective rate on the quality of sleep in the treatment of primary insomnia: a randomized control trial. Brain Sci. 2022;12(10):1296. doi:10.3390/brainsci12101296
12. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Neuropsychopharmacology. 2012;37(1):102-116.
13. Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651-660.
14. Choi KS, Mayberg H. Connectomic DBS in major depression. In: Horn A, ed. Connectomic Deep Brain Stimulation. Academic Press; 2022:433-447.
15. Cruz S, Gutiérrez-Rojas L, González-Domenech P, et al. Deep brain stimulation in obsessive-compulsive disorder: results from meta-analysis. Psychiatry Res. 2022;317:114869. doi:10.1016/j.psychres.2022.114869
16. Lisoni J, Baldacci G, Nibbio G, et al. Effects of bilateral, bipolar-nonbalanced, frontal transcranial direct current stimulation (tDCS) on negative symptoms and neurocognition in a sample of patients living with schizophrenia: results of a randomized double-blind sham-controlled trial. J Psychiatr Res. 2022;155:430-442.
17. Sinclair DJ, Zhao S, Qi F, et al. Electroconvulsive therapy for treatment-resistant schizophrenia. Cochrane Database Syst Rev. 2019;3(3):CD011847. doi:10.1002/14651858.CD011847.pub2
18. Cole EJ, Stimpson KH, Bentzley BS, et al. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. Am J Psychiatry. 2020;177(8):716-726.
1. Nasrallah HA. Repositioning psychotherapy as a neurobiological intervention. Current Psychiatry. 2013;12(12):18-19.
2. Nasrallah HA. Bipolar disorder: clinical questions beg for answers. Current Psychiatry. 2006;5(12):11-12.
3. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361(9360):799-808.
4. Rhee TG, Shim SR, Forester BP, et al. Efficacy and safety of ketamine vs electroconvulsive therapy among patients with major depressive episode: a systematic review and meta-analysis. JAMA Psychiatry. 2022:e223352. doi:10.1001/jamapsychiatry.2022.3352
5. Nuninga JO, Mandl RCW, Boks MP, et al. Volume increase in the dentate gyrus after electroconvulsive therapy in depressed patients as measured with 7T. Mol Psychiatry. 2020;25(7):1559-1568.
6. Joshi SH, Espinoza RT, Pirnia T, et al. Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry. 2016;79(4):282-292.
7. Rhee TG, Olfson M, Nierenberg AA, et al. 20-year trends in the pharmacologic treatment of bipolar disorder by psychiatrists in outpatient care settings. Am J Psychiatry. 2020;177(8):706-715.
8. Hilz MJ. Transcutaneous vagus nerve stimulation - a brief introduction and overview. Auton Neurosci. 2022;243:103038. doi:10.1016/j.autneu.2022.103038
9. Pigato G, Rosson S, Bresolin N, et al. Vagus nerve stimulation in treatment-resistant depression: a case series of long-term follow-up. J ECT. 2022. doi:10.1097/YCT.0000000000000869
10. Shivaswamy T, Souza RR, Engineer CT, et al. Vagus nerve stimulation as a treatment for fear and anxiety in individuals with autism spectrum disorder. J Psychiatr Brain Sci. 2022;7(4):e220007. doi:10.20900/jpbs.20220007
11. Wu Y, Song L, Wang X, et al. Transcutaneous vagus nerve stimulation could improve the effective rate on the quality of sleep in the treatment of primary insomnia: a randomized control trial. Brain Sci. 2022;12(10):1296. doi:10.3390/brainsci12101296
12. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Neuropsychopharmacology. 2012;37(1):102-116.
13. Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651-660.
14. Choi KS, Mayberg H. Connectomic DBS in major depression. In: Horn A, ed. Connectomic Deep Brain Stimulation. Academic Press; 2022:433-447.
15. Cruz S, Gutiérrez-Rojas L, González-Domenech P, et al. Deep brain stimulation in obsessive-compulsive disorder: results from meta-analysis. Psychiatry Res. 2022;317:114869. doi:10.1016/j.psychres.2022.114869
16. Lisoni J, Baldacci G, Nibbio G, et al. Effects of bilateral, bipolar-nonbalanced, frontal transcranial direct current stimulation (tDCS) on negative symptoms and neurocognition in a sample of patients living with schizophrenia: results of a randomized double-blind sham-controlled trial. J Psychiatr Res. 2022;155:430-442.
17. Sinclair DJ, Zhao S, Qi F, et al. Electroconvulsive therapy for treatment-resistant schizophrenia. Cochrane Database Syst Rev. 2019;3(3):CD011847. doi:10.1002/14651858.CD011847.pub2
18. Cole EJ, Stimpson KH, Bentzley BS, et al. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. Am J Psychiatry. 2020;177(8):716-726.
More on social entropy
As leaders of the American Psychiatric Association, we received dozens of communications from members who were shocked by the discriminatory and transphobic commentary in the recent editorial “The accelerating societal entropy undermines mental health” (
Specifically, citing “lack of certainty about gender identity in children and adults” as an indicator of societal turmoil that undermines mental health is contrary to the scientific understanding of gender identity. Physicians have professional obligations to advance patients’ well-being and do no harm.
The medical profession, including psychiatry, is at a critical juncture in coming to terms with and dismantling its longstanding history of systemic racism and discrimination. Authors and editors must be aware that harmful and divisive language negatively affects mental health, especially for people who have been subject to discrimination individually and/or as members of historically excluded and/or minoritized groups.
In publishing this editorial,
Rebecca W. Brendel, MD, JD, DFAPA
President
American Psychiatric Association
Saul Levin, MD, MPA, FRCP-E, FRCPsych
CEO and Medical Director
American Psychiatric Association
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
Dr. Nasrallah responds
I regret that the sentence about gender identity in my October editorial was regarded as transphobic and harmful. While the phrasing reflected my patients’ comments to me, I realize my unfortunate choice of words deeply offended individuals who are transgender, who have been subjected to ongoing discrimination and prejudice.
I apologize to our readers; to my American Psychiatric Association LGBTQAI+ friends, colleagues, and relatives; and to the LGBTQAI+ community at large. The sentence has been deleted from the online version of my editorial. This has been a teachable moment for me.
Henry A. Nasrallah, MD
Editor-In-Chief
Continue to: More on psychiatric documentation
More on psychiatric documentation
Dr. Joshi’s helpful discussion of clinical documentation strategies (“Medical record documentation: What to do, and what to avoid,”
The mental health record may not always be as confidential as psychiatrists think (or hope) it is. The Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule, for example, generally does not distinguish between medical and mental health information, nor does it provide special rules for the latter (although certain state laws may do so). HIPAA provides added protections for “psychotherapy notes,” but this category explicitly excludes progress notes that discuss treatment modalities, diagnosis, and clinical milestones. To retain their protected status, psychotherapists’ private, “desk-drawer memory joggers” must never be comingled with the patient chart.1 For mental health professionals, this distinction underscores the importance of keeping personal details broad in the progress note; scandalous or embarrassing narratives recounted in the medical record itself are routinely accessible to the patient and may be lawfully disclosed to others under specified circumstances.
In addition to avoiding speculation and including patient quotes when appropriate, documenting objectively and nonjudgmentally means annotating facts and observations that helped the clinician arrive at their conclusion. For example, “patient appears intoxicated” is less helpful than noting the patient’s slurred speech, impaired gait and/or coordination, and alcohol odor.
Clinical care and its associated documentation are so intertwined that they can become virtually indistinguishable. In a medical malpractice case, the burden is on the plaintiff to prove their injury resulted from substandard care. Some courts, however, have held that missing or incomplete records can effectively shift the burden from the recipient to the provider of care to show that the treatment at issue was rendered non-negligently.2 Statutes of limitations restricting the amount of time in which a patient can sue after an adverse event are sometimes triggered by the date on which they knew or should have known of the alleged malpractice.3 One of the best ways of ascertaining this date, and starting the statute of limitations clock, can be a clear annotation in the medical record that the patient was apprised of an unanticipated outcome or iatrogenic harm. In this way, a timely and thorough note can be critical not just to defending the physician’s quality of care, but potentially to precluding a cognizable lawsuit altogether.
Charles G. Kels, JD
Defense Health Agency
San Antonio, Texas
Disclosures
The views expressed are those of the author and do not necessarily reflect those of any government agency, nor do they constitute individualized legal advice. The author reports no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
References
1. 45 CFR Parts 160 and 164, Subparts A and E.
2. Valcin v Public Health Trust, 473 So. 2d 1297 (1984).
3. US v Kubrick, 444 US 111 (1979).
As leaders of the American Psychiatric Association, we received dozens of communications from members who were shocked by the discriminatory and transphobic commentary in the recent editorial “The accelerating societal entropy undermines mental health” (
Specifically, citing “lack of certainty about gender identity in children and adults” as an indicator of societal turmoil that undermines mental health is contrary to the scientific understanding of gender identity. Physicians have professional obligations to advance patients’ well-being and do no harm.
The medical profession, including psychiatry, is at a critical juncture in coming to terms with and dismantling its longstanding history of systemic racism and discrimination. Authors and editors must be aware that harmful and divisive language negatively affects mental health, especially for people who have been subject to discrimination individually and/or as members of historically excluded and/or minoritized groups.
In publishing this editorial,
Rebecca W. Brendel, MD, JD, DFAPA
President
American Psychiatric Association
Saul Levin, MD, MPA, FRCP-E, FRCPsych
CEO and Medical Director
American Psychiatric Association
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
Dr. Nasrallah responds
I regret that the sentence about gender identity in my October editorial was regarded as transphobic and harmful. While the phrasing reflected my patients’ comments to me, I realize my unfortunate choice of words deeply offended individuals who are transgender, who have been subjected to ongoing discrimination and prejudice.
I apologize to our readers; to my American Psychiatric Association LGBTQAI+ friends, colleagues, and relatives; and to the LGBTQAI+ community at large. The sentence has been deleted from the online version of my editorial. This has been a teachable moment for me.
Henry A. Nasrallah, MD
Editor-In-Chief
Continue to: More on psychiatric documentation
More on psychiatric documentation
Dr. Joshi’s helpful discussion of clinical documentation strategies (“Medical record documentation: What to do, and what to avoid,”
The mental health record may not always be as confidential as psychiatrists think (or hope) it is. The Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule, for example, generally does not distinguish between medical and mental health information, nor does it provide special rules for the latter (although certain state laws may do so). HIPAA provides added protections for “psychotherapy notes,” but this category explicitly excludes progress notes that discuss treatment modalities, diagnosis, and clinical milestones. To retain their protected status, psychotherapists’ private, “desk-drawer memory joggers” must never be comingled with the patient chart.1 For mental health professionals, this distinction underscores the importance of keeping personal details broad in the progress note; scandalous or embarrassing narratives recounted in the medical record itself are routinely accessible to the patient and may be lawfully disclosed to others under specified circumstances.
In addition to avoiding speculation and including patient quotes when appropriate, documenting objectively and nonjudgmentally means annotating facts and observations that helped the clinician arrive at their conclusion. For example, “patient appears intoxicated” is less helpful than noting the patient’s slurred speech, impaired gait and/or coordination, and alcohol odor.
Clinical care and its associated documentation are so intertwined that they can become virtually indistinguishable. In a medical malpractice case, the burden is on the plaintiff to prove their injury resulted from substandard care. Some courts, however, have held that missing or incomplete records can effectively shift the burden from the recipient to the provider of care to show that the treatment at issue was rendered non-negligently.2 Statutes of limitations restricting the amount of time in which a patient can sue after an adverse event are sometimes triggered by the date on which they knew or should have known of the alleged malpractice.3 One of the best ways of ascertaining this date, and starting the statute of limitations clock, can be a clear annotation in the medical record that the patient was apprised of an unanticipated outcome or iatrogenic harm. In this way, a timely and thorough note can be critical not just to defending the physician’s quality of care, but potentially to precluding a cognizable lawsuit altogether.
Charles G. Kels, JD
Defense Health Agency
San Antonio, Texas
Disclosures
The views expressed are those of the author and do not necessarily reflect those of any government agency, nor do they constitute individualized legal advice. The author reports no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
References
1. 45 CFR Parts 160 and 164, Subparts A and E.
2. Valcin v Public Health Trust, 473 So. 2d 1297 (1984).
3. US v Kubrick, 444 US 111 (1979).
As leaders of the American Psychiatric Association, we received dozens of communications from members who were shocked by the discriminatory and transphobic commentary in the recent editorial “The accelerating societal entropy undermines mental health” (
Specifically, citing “lack of certainty about gender identity in children and adults” as an indicator of societal turmoil that undermines mental health is contrary to the scientific understanding of gender identity. Physicians have professional obligations to advance patients’ well-being and do no harm.
The medical profession, including psychiatry, is at a critical juncture in coming to terms with and dismantling its longstanding history of systemic racism and discrimination. Authors and editors must be aware that harmful and divisive language negatively affects mental health, especially for people who have been subject to discrimination individually and/or as members of historically excluded and/or minoritized groups.
In publishing this editorial,
Rebecca W. Brendel, MD, JD, DFAPA
President
American Psychiatric Association
Saul Levin, MD, MPA, FRCP-E, FRCPsych
CEO and Medical Director
American Psychiatric Association
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
Dr. Nasrallah responds
I regret that the sentence about gender identity in my October editorial was regarded as transphobic and harmful. While the phrasing reflected my patients’ comments to me, I realize my unfortunate choice of words deeply offended individuals who are transgender, who have been subjected to ongoing discrimination and prejudice.
I apologize to our readers; to my American Psychiatric Association LGBTQAI+ friends, colleagues, and relatives; and to the LGBTQAI+ community at large. The sentence has been deleted from the online version of my editorial. This has been a teachable moment for me.
Henry A. Nasrallah, MD
Editor-In-Chief
Continue to: More on psychiatric documentation
More on psychiatric documentation
Dr. Joshi’s helpful discussion of clinical documentation strategies (“Medical record documentation: What to do, and what to avoid,”
The mental health record may not always be as confidential as psychiatrists think (or hope) it is. The Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule, for example, generally does not distinguish between medical and mental health information, nor does it provide special rules for the latter (although certain state laws may do so). HIPAA provides added protections for “psychotherapy notes,” but this category explicitly excludes progress notes that discuss treatment modalities, diagnosis, and clinical milestones. To retain their protected status, psychotherapists’ private, “desk-drawer memory joggers” must never be comingled with the patient chart.1 For mental health professionals, this distinction underscores the importance of keeping personal details broad in the progress note; scandalous or embarrassing narratives recounted in the medical record itself are routinely accessible to the patient and may be lawfully disclosed to others under specified circumstances.
In addition to avoiding speculation and including patient quotes when appropriate, documenting objectively and nonjudgmentally means annotating facts and observations that helped the clinician arrive at their conclusion. For example, “patient appears intoxicated” is less helpful than noting the patient’s slurred speech, impaired gait and/or coordination, and alcohol odor.
Clinical care and its associated documentation are so intertwined that they can become virtually indistinguishable. In a medical malpractice case, the burden is on the plaintiff to prove their injury resulted from substandard care. Some courts, however, have held that missing or incomplete records can effectively shift the burden from the recipient to the provider of care to show that the treatment at issue was rendered non-negligently.2 Statutes of limitations restricting the amount of time in which a patient can sue after an adverse event are sometimes triggered by the date on which they knew or should have known of the alleged malpractice.3 One of the best ways of ascertaining this date, and starting the statute of limitations clock, can be a clear annotation in the medical record that the patient was apprised of an unanticipated outcome or iatrogenic harm. In this way, a timely and thorough note can be critical not just to defending the physician’s quality of care, but potentially to precluding a cognizable lawsuit altogether.
Charles G. Kels, JD
Defense Health Agency
San Antonio, Texas
Disclosures
The views expressed are those of the author and do not necessarily reflect those of any government agency, nor do they constitute individualized legal advice. The author reports no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
References
1. 45 CFR Parts 160 and 164, Subparts A and E.
2. Valcin v Public Health Trust, 473 So. 2d 1297 (1984).
3. US v Kubrick, 444 US 111 (1979).
Is evolution’s greatest triumph its worst blunder?
Of all the dazzling achievements of evolution, the most glorious by far is the emergence of the advanced human brain, especially the prefrontal cortex. Homo sapiens (the wise humans) are without doubt the most transformative development in the consequential annals of evolution. It was evolution’s spectacular “moonshot.” Ironically, it may also have been the seed of its destruction.
The unprecedented growth of the human brain over the past 7 million years (tripling in size) was a monumental tipping point in evolution that ultimately disrupted the entire orderly cascade of evolution on Planet Earth. Because of their superior intelligence, Homo sapiens have substantially “tinkered” with the foundations of evolution, such as “natural selection” and “survival of the fittest,” and may eventually change the course of evolution, or even reverse it. It should also be recognized that 20% of the human genome is Neanderthal, and the 2022 Nobel Prize in Physiology or Medicine was awarded to Svante Pääbo, the founder of the field of paleogenetics, who demonstrated genetically that Homo sapiens interbred with Homo neanderthalensis (who disappeared 30,000 years ago).
The majestic evolution of the human brain, in both size and complexity, led to monumental changes in the history of humankind compared to their primitive predecessors. Thanks to a superior cerebral cortex, humans developed traits and abilities that were nonexistent, even unimaginable, in the rest of animal kingdom, including primates and other mammals. These include thoughts; speech (hundreds of languages), spoken and written, to communicate among themselves; composed music and created numerous instruments to play it; invented mathematics, physics, and chemistry; developed agriculture to sustain and feed the masses; built homes, palaces, and pyramids, with water and sewage systems; hatched hundreds of religions and built thousands of houses of worship; built machines to transport themselves (cars, trains, ships, planes, and space shuttles); paved airports and countless miles of roads and railways; established companies, universities, hospitals, and research laboratories; built sports facilities such as stadiums for Olympic games and all its athletics; created hotels, restaurants, coffee shops, newspapers, and magazines; discovered the amazing DNA double helix and its genome with 23,000 coding genes containing instructions to build the brain and 200 other body tissues; developed surgeries and invented medications for diseases that would have killed millions every year; and established paper money to replace gold and silver coins. Humans established governments that included monarchies, dictatorships, democracies, and pseudodemocracies; stipulated constitutions, laws, and regulations to maintain various societies; and created several civilizations around the world that thrived and then faded. Over the past century, the advanced human brain elevated human existence to a higher sophistication with technologies such as electricity, phones, computers, internet, artificial intelligence, and machine learning. Using powerful rockets and space stations, humans have begun to expand their influence to the moon and planets of the solar system. Humans are very likely to continue achieving what evolution could never have done without evolving the human brain to become the most powerful force in nature.
The key ingredient of the brain that has enabled humans to achieve so much is the development of an advanced cognition, with superior functions that far exceed those of other living organisms. These include neurocognitive functions such as memory and attention, and executive functions that include planning, problem-solving, decision-making, abstract thinking, and insight. Those cognitive functions generate lofty prose, splendiferous poetry, and heavenly symphonies that inspire those who create it and others. The human brain also developed social cognition, with empathy, theory of mind, recognition of facial expressions, and courtship rituals that can trigger infatuation and love. Homo sapiens can experience a wide range of emotions in addition to love and attachment (necessary for procreation), including shame, guilt, surprise, embarrassment, disgust, and indifference, and a unique sense of right and wrong.
Perhaps the most distinctive human attribute, generated by an advanced prefrontal cortex, is a belief system that includes philosophy, politics, religion, and faith. Hundreds of different religions sprouted throughout human history (each claiming a monopoly on “the truth”), mandating rituals and behaviors, but also promoting a profound and unshakable belief in a divine “higher being” and an afterlife that mitigates the fear of death. Humans, unlike other animals, are painfully aware of mortality and the inevitability of death. Faith is an antidote for thanatophobia. Unfortunately, religious beliefs often generated severe and protracted schisms and warfare, with fatal consequences for their followers.
The anti-evolution aspect of the advanced brain
Despite remarkable talents and achievements, the unprecedented evolutionary expansion of the human brain also has a detrimental downside. The same intellectual power that led to astonishing positive accomplishments has a wicked side as well. While most animals have a predator, humans have become the “omni-predator” that preys on all living things. The balanced ecosystems of animals and plants has been dominated and disrupted by humans. Thousands of species that evolution had so ingeniously spawned became extinct because of human actions. The rainforests, jewels of nature’s plantation system, were victimized by human indifference to the deleterious effects on nature and climate. The excavation of coal and oil, exploited as necessary sources of energy for societal infrastructure, came back to haunt humans with climate consequences. In many ways, human “progress” corrupted evolution and dismantled its components. Survival of the fittest among various species was whittled down to “survival of humans” (and their domesticated animals) at the expense of all other organisms, animals, or plants.
Among Homo sapiens, momentous scientific, medical, and technological advances completely undermined the principle of survival of the fittest. Very premature infants, who would have certainly died, were kept alive. Children with disabling genetic disorders who would have perished in childhood were kept alive into the age of procreation, perpetuating the genetic mutations. The discovery of antibiotic and antiviral medications, and especially vaccines, ensured the survival of millions of humans who would have succumbed to infections. With evolution’s natural selection, humans who survived severe infections without medications would have passed on their “infection-resistant genes” to their progeny. The triumph of human medical progress can be conceptualized as a setback for the principles of evolution.
Continue to: The most malignant...
The most malignant consequence of the exceptional human brain is the evil of which it is capable. Human ingenuity led to the development of weapons of individual killing (guns), large-scale murder (machine guns), and massive destruction (nuclear weapons). And because aggression and warfare are an inherent part of human nature, the most potent predator for a human is another human. The history of humans is riddled with conflict and death on a large scale. Ironically, many wars were instigated by various religious groups around the world, who developed intense hostility towards one another.
There are other downsides to the advanced human brain. It can channel its talents and skills into unimaginably wicked and depraved behaviors, such as premeditated and well-planned murder, slavery, cults, child abuse, domestic abuse, pornography, fascism, dictatorships, and political corruption. Astonishingly, the same brain that can be loving, kind, friendly, and empathetic can suddenly become hateful, vengeful, cruel, vile, sinister, vicious, diabolical, and capable of unimaginable violence and atrocities. The advanced human brain definitely has a very dark side.
Finally, unlike other members of the animal kingdom, the human brain generates its virtual counterpart: the highly complex human mind, which is prone to various maladies, labeled as “psychiatric disorders.” No other animal species develops delusions, hallucinations, thought disorders, melancholia, mania, obsessive-compulsive disorder, generalized anxiety, panic attacks, posttraumatic stress disorder, psychopathy, narcissistic and borderline personality disorders, alcohol addiction, and drug abuse. Homo sapiens are the only species whose members decide to end their own life in large numbers. About 25% of human minds are afflicted with one or more of those psychiatric ailments.1,2 The redeeming grace of the large human brain is that it led to the development of pharmacologic and somatic treatments for most of them, including psychotherapy, which is a uniquely human treatment strategy that can mend many psychiatric disorders.
Evolution may not realize what it hath wrought when it evolved the dramatically expanded human brain, with its extraordinary cognition. This awe-inspiring “biological computer” can be creative and adaptive, with superlative survival abilities, but it can also degenerate and become nefarious, villainous, murderous, and even demonic. The human brain has essentially brought evolution to a screeching halt and may at some point end up destroying Earth and all of its Homo sapien inhabitants, who may foolishly use their weapons of mass destruction. The historic achievement of evolution has become the ultimate example of “the law of unintended consequences.”
1. Robin LN, Regier DA. Psychiatric Disorders in America: The Epidemiologic Catchment Area Study. Free Press; 1990.
2. Johns Hopkins Medicine. Mental Health Disorder Statistics. Accessed October 12, 2022. https://www.hopkinsmedicine.org/health/wellness-and-prevention/mental-health-disorder-statistics
Of all the dazzling achievements of evolution, the most glorious by far is the emergence of the advanced human brain, especially the prefrontal cortex. Homo sapiens (the wise humans) are without doubt the most transformative development in the consequential annals of evolution. It was evolution’s spectacular “moonshot.” Ironically, it may also have been the seed of its destruction.
The unprecedented growth of the human brain over the past 7 million years (tripling in size) was a monumental tipping point in evolution that ultimately disrupted the entire orderly cascade of evolution on Planet Earth. Because of their superior intelligence, Homo sapiens have substantially “tinkered” with the foundations of evolution, such as “natural selection” and “survival of the fittest,” and may eventually change the course of evolution, or even reverse it. It should also be recognized that 20% of the human genome is Neanderthal, and the 2022 Nobel Prize in Physiology or Medicine was awarded to Svante Pääbo, the founder of the field of paleogenetics, who demonstrated genetically that Homo sapiens interbred with Homo neanderthalensis (who disappeared 30,000 years ago).
The majestic evolution of the human brain, in both size and complexity, led to monumental changes in the history of humankind compared to their primitive predecessors. Thanks to a superior cerebral cortex, humans developed traits and abilities that were nonexistent, even unimaginable, in the rest of animal kingdom, including primates and other mammals. These include thoughts; speech (hundreds of languages), spoken and written, to communicate among themselves; composed music and created numerous instruments to play it; invented mathematics, physics, and chemistry; developed agriculture to sustain and feed the masses; built homes, palaces, and pyramids, with water and sewage systems; hatched hundreds of religions and built thousands of houses of worship; built machines to transport themselves (cars, trains, ships, planes, and space shuttles); paved airports and countless miles of roads and railways; established companies, universities, hospitals, and research laboratories; built sports facilities such as stadiums for Olympic games and all its athletics; created hotels, restaurants, coffee shops, newspapers, and magazines; discovered the amazing DNA double helix and its genome with 23,000 coding genes containing instructions to build the brain and 200 other body tissues; developed surgeries and invented medications for diseases that would have killed millions every year; and established paper money to replace gold and silver coins. Humans established governments that included monarchies, dictatorships, democracies, and pseudodemocracies; stipulated constitutions, laws, and regulations to maintain various societies; and created several civilizations around the world that thrived and then faded. Over the past century, the advanced human brain elevated human existence to a higher sophistication with technologies such as electricity, phones, computers, internet, artificial intelligence, and machine learning. Using powerful rockets and space stations, humans have begun to expand their influence to the moon and planets of the solar system. Humans are very likely to continue achieving what evolution could never have done without evolving the human brain to become the most powerful force in nature.
The key ingredient of the brain that has enabled humans to achieve so much is the development of an advanced cognition, with superior functions that far exceed those of other living organisms. These include neurocognitive functions such as memory and attention, and executive functions that include planning, problem-solving, decision-making, abstract thinking, and insight. Those cognitive functions generate lofty prose, splendiferous poetry, and heavenly symphonies that inspire those who create it and others. The human brain also developed social cognition, with empathy, theory of mind, recognition of facial expressions, and courtship rituals that can trigger infatuation and love. Homo sapiens can experience a wide range of emotions in addition to love and attachment (necessary for procreation), including shame, guilt, surprise, embarrassment, disgust, and indifference, and a unique sense of right and wrong.
Perhaps the most distinctive human attribute, generated by an advanced prefrontal cortex, is a belief system that includes philosophy, politics, religion, and faith. Hundreds of different religions sprouted throughout human history (each claiming a monopoly on “the truth”), mandating rituals and behaviors, but also promoting a profound and unshakable belief in a divine “higher being” and an afterlife that mitigates the fear of death. Humans, unlike other animals, are painfully aware of mortality and the inevitability of death. Faith is an antidote for thanatophobia. Unfortunately, religious beliefs often generated severe and protracted schisms and warfare, with fatal consequences for their followers.
The anti-evolution aspect of the advanced brain
Despite remarkable talents and achievements, the unprecedented evolutionary expansion of the human brain also has a detrimental downside. The same intellectual power that led to astonishing positive accomplishments has a wicked side as well. While most animals have a predator, humans have become the “omni-predator” that preys on all living things. The balanced ecosystems of animals and plants has been dominated and disrupted by humans. Thousands of species that evolution had so ingeniously spawned became extinct because of human actions. The rainforests, jewels of nature’s plantation system, were victimized by human indifference to the deleterious effects on nature and climate. The excavation of coal and oil, exploited as necessary sources of energy for societal infrastructure, came back to haunt humans with climate consequences. In many ways, human “progress” corrupted evolution and dismantled its components. Survival of the fittest among various species was whittled down to “survival of humans” (and their domesticated animals) at the expense of all other organisms, animals, or plants.
Among Homo sapiens, momentous scientific, medical, and technological advances completely undermined the principle of survival of the fittest. Very premature infants, who would have certainly died, were kept alive. Children with disabling genetic disorders who would have perished in childhood were kept alive into the age of procreation, perpetuating the genetic mutations. The discovery of antibiotic and antiviral medications, and especially vaccines, ensured the survival of millions of humans who would have succumbed to infections. With evolution’s natural selection, humans who survived severe infections without medications would have passed on their “infection-resistant genes” to their progeny. The triumph of human medical progress can be conceptualized as a setback for the principles of evolution.
Continue to: The most malignant...
The most malignant consequence of the exceptional human brain is the evil of which it is capable. Human ingenuity led to the development of weapons of individual killing (guns), large-scale murder (machine guns), and massive destruction (nuclear weapons). And because aggression and warfare are an inherent part of human nature, the most potent predator for a human is another human. The history of humans is riddled with conflict and death on a large scale. Ironically, many wars were instigated by various religious groups around the world, who developed intense hostility towards one another.
There are other downsides to the advanced human brain. It can channel its talents and skills into unimaginably wicked and depraved behaviors, such as premeditated and well-planned murder, slavery, cults, child abuse, domestic abuse, pornography, fascism, dictatorships, and political corruption. Astonishingly, the same brain that can be loving, kind, friendly, and empathetic can suddenly become hateful, vengeful, cruel, vile, sinister, vicious, diabolical, and capable of unimaginable violence and atrocities. The advanced human brain definitely has a very dark side.
Finally, unlike other members of the animal kingdom, the human brain generates its virtual counterpart: the highly complex human mind, which is prone to various maladies, labeled as “psychiatric disorders.” No other animal species develops delusions, hallucinations, thought disorders, melancholia, mania, obsessive-compulsive disorder, generalized anxiety, panic attacks, posttraumatic stress disorder, psychopathy, narcissistic and borderline personality disorders, alcohol addiction, and drug abuse. Homo sapiens are the only species whose members decide to end their own life in large numbers. About 25% of human minds are afflicted with one or more of those psychiatric ailments.1,2 The redeeming grace of the large human brain is that it led to the development of pharmacologic and somatic treatments for most of them, including psychotherapy, which is a uniquely human treatment strategy that can mend many psychiatric disorders.
Evolution may not realize what it hath wrought when it evolved the dramatically expanded human brain, with its extraordinary cognition. This awe-inspiring “biological computer” can be creative and adaptive, with superlative survival abilities, but it can also degenerate and become nefarious, villainous, murderous, and even demonic. The human brain has essentially brought evolution to a screeching halt and may at some point end up destroying Earth and all of its Homo sapien inhabitants, who may foolishly use their weapons of mass destruction. The historic achievement of evolution has become the ultimate example of “the law of unintended consequences.”
Of all the dazzling achievements of evolution, the most glorious by far is the emergence of the advanced human brain, especially the prefrontal cortex. Homo sapiens (the wise humans) are without doubt the most transformative development in the consequential annals of evolution. It was evolution’s spectacular “moonshot.” Ironically, it may also have been the seed of its destruction.
The unprecedented growth of the human brain over the past 7 million years (tripling in size) was a monumental tipping point in evolution that ultimately disrupted the entire orderly cascade of evolution on Planet Earth. Because of their superior intelligence, Homo sapiens have substantially “tinkered” with the foundations of evolution, such as “natural selection” and “survival of the fittest,” and may eventually change the course of evolution, or even reverse it. It should also be recognized that 20% of the human genome is Neanderthal, and the 2022 Nobel Prize in Physiology or Medicine was awarded to Svante Pääbo, the founder of the field of paleogenetics, who demonstrated genetically that Homo sapiens interbred with Homo neanderthalensis (who disappeared 30,000 years ago).
The majestic evolution of the human brain, in both size and complexity, led to monumental changes in the history of humankind compared to their primitive predecessors. Thanks to a superior cerebral cortex, humans developed traits and abilities that were nonexistent, even unimaginable, in the rest of animal kingdom, including primates and other mammals. These include thoughts; speech (hundreds of languages), spoken and written, to communicate among themselves; composed music and created numerous instruments to play it; invented mathematics, physics, and chemistry; developed agriculture to sustain and feed the masses; built homes, palaces, and pyramids, with water and sewage systems; hatched hundreds of religions and built thousands of houses of worship; built machines to transport themselves (cars, trains, ships, planes, and space shuttles); paved airports and countless miles of roads and railways; established companies, universities, hospitals, and research laboratories; built sports facilities such as stadiums for Olympic games and all its athletics; created hotels, restaurants, coffee shops, newspapers, and magazines; discovered the amazing DNA double helix and its genome with 23,000 coding genes containing instructions to build the brain and 200 other body tissues; developed surgeries and invented medications for diseases that would have killed millions every year; and established paper money to replace gold and silver coins. Humans established governments that included monarchies, dictatorships, democracies, and pseudodemocracies; stipulated constitutions, laws, and regulations to maintain various societies; and created several civilizations around the world that thrived and then faded. Over the past century, the advanced human brain elevated human existence to a higher sophistication with technologies such as electricity, phones, computers, internet, artificial intelligence, and machine learning. Using powerful rockets and space stations, humans have begun to expand their influence to the moon and planets of the solar system. Humans are very likely to continue achieving what evolution could never have done without evolving the human brain to become the most powerful force in nature.
The key ingredient of the brain that has enabled humans to achieve so much is the development of an advanced cognition, with superior functions that far exceed those of other living organisms. These include neurocognitive functions such as memory and attention, and executive functions that include planning, problem-solving, decision-making, abstract thinking, and insight. Those cognitive functions generate lofty prose, splendiferous poetry, and heavenly symphonies that inspire those who create it and others. The human brain also developed social cognition, with empathy, theory of mind, recognition of facial expressions, and courtship rituals that can trigger infatuation and love. Homo sapiens can experience a wide range of emotions in addition to love and attachment (necessary for procreation), including shame, guilt, surprise, embarrassment, disgust, and indifference, and a unique sense of right and wrong.
Perhaps the most distinctive human attribute, generated by an advanced prefrontal cortex, is a belief system that includes philosophy, politics, religion, and faith. Hundreds of different religions sprouted throughout human history (each claiming a monopoly on “the truth”), mandating rituals and behaviors, but also promoting a profound and unshakable belief in a divine “higher being” and an afterlife that mitigates the fear of death. Humans, unlike other animals, are painfully aware of mortality and the inevitability of death. Faith is an antidote for thanatophobia. Unfortunately, religious beliefs often generated severe and protracted schisms and warfare, with fatal consequences for their followers.
The anti-evolution aspect of the advanced brain
Despite remarkable talents and achievements, the unprecedented evolutionary expansion of the human brain also has a detrimental downside. The same intellectual power that led to astonishing positive accomplishments has a wicked side as well. While most animals have a predator, humans have become the “omni-predator” that preys on all living things. The balanced ecosystems of animals and plants has been dominated and disrupted by humans. Thousands of species that evolution had so ingeniously spawned became extinct because of human actions. The rainforests, jewels of nature’s plantation system, were victimized by human indifference to the deleterious effects on nature and climate. The excavation of coal and oil, exploited as necessary sources of energy for societal infrastructure, came back to haunt humans with climate consequences. In many ways, human “progress” corrupted evolution and dismantled its components. Survival of the fittest among various species was whittled down to “survival of humans” (and their domesticated animals) at the expense of all other organisms, animals, or plants.
Among Homo sapiens, momentous scientific, medical, and technological advances completely undermined the principle of survival of the fittest. Very premature infants, who would have certainly died, were kept alive. Children with disabling genetic disorders who would have perished in childhood were kept alive into the age of procreation, perpetuating the genetic mutations. The discovery of antibiotic and antiviral medications, and especially vaccines, ensured the survival of millions of humans who would have succumbed to infections. With evolution’s natural selection, humans who survived severe infections without medications would have passed on their “infection-resistant genes” to their progeny. The triumph of human medical progress can be conceptualized as a setback for the principles of evolution.
Continue to: The most malignant...
The most malignant consequence of the exceptional human brain is the evil of which it is capable. Human ingenuity led to the development of weapons of individual killing (guns), large-scale murder (machine guns), and massive destruction (nuclear weapons). And because aggression and warfare are an inherent part of human nature, the most potent predator for a human is another human. The history of humans is riddled with conflict and death on a large scale. Ironically, many wars were instigated by various religious groups around the world, who developed intense hostility towards one another.
There are other downsides to the advanced human brain. It can channel its talents and skills into unimaginably wicked and depraved behaviors, such as premeditated and well-planned murder, slavery, cults, child abuse, domestic abuse, pornography, fascism, dictatorships, and political corruption. Astonishingly, the same brain that can be loving, kind, friendly, and empathetic can suddenly become hateful, vengeful, cruel, vile, sinister, vicious, diabolical, and capable of unimaginable violence and atrocities. The advanced human brain definitely has a very dark side.
Finally, unlike other members of the animal kingdom, the human brain generates its virtual counterpart: the highly complex human mind, which is prone to various maladies, labeled as “psychiatric disorders.” No other animal species develops delusions, hallucinations, thought disorders, melancholia, mania, obsessive-compulsive disorder, generalized anxiety, panic attacks, posttraumatic stress disorder, psychopathy, narcissistic and borderline personality disorders, alcohol addiction, and drug abuse. Homo sapiens are the only species whose members decide to end their own life in large numbers. About 25% of human minds are afflicted with one or more of those psychiatric ailments.1,2 The redeeming grace of the large human brain is that it led to the development of pharmacologic and somatic treatments for most of them, including psychotherapy, which is a uniquely human treatment strategy that can mend many psychiatric disorders.
Evolution may not realize what it hath wrought when it evolved the dramatically expanded human brain, with its extraordinary cognition. This awe-inspiring “biological computer” can be creative and adaptive, with superlative survival abilities, but it can also degenerate and become nefarious, villainous, murderous, and even demonic. The human brain has essentially brought evolution to a screeching halt and may at some point end up destroying Earth and all of its Homo sapien inhabitants, who may foolishly use their weapons of mass destruction. The historic achievement of evolution has become the ultimate example of “the law of unintended consequences.”
1. Robin LN, Regier DA. Psychiatric Disorders in America: The Epidemiologic Catchment Area Study. Free Press; 1990.
2. Johns Hopkins Medicine. Mental Health Disorder Statistics. Accessed October 12, 2022. https://www.hopkinsmedicine.org/health/wellness-and-prevention/mental-health-disorder-statistics
1. Robin LN, Regier DA. Psychiatric Disorders in America: The Epidemiologic Catchment Area Study. Free Press; 1990.
2. Johns Hopkins Medicine. Mental Health Disorder Statistics. Accessed October 12, 2022. https://www.hopkinsmedicine.org/health/wellness-and-prevention/mental-health-disorder-statistics
Lamotrigine for bipolar depression?
In reading Dr. Nasrallah's August 2022 editorial (“Reversing depression: A plethora of therapeutic strategies and mechanisms,”
Dr. Nasrallah responds
Thanks for your message. Lamotrigine is not FDA-approved for bipolar or unipolar depression, either as monotherapy or as an adjunctive therapy. It has never been approved for mania, either (no efficacy at all). Its only FDA-approved psychiatric indication is maintenance therapy after a patient with bipolar I disorder emerges from mania with the help of one of the antimanic drugs. Yet many clinicians may perceive lamotrigine as useful for bipolar depression because more than 20 years ago the manufacturer sponsored several small studies (not FDA trials). Two studies that showed efficacy were published, but 4 other studies that failed to show efficacy were not published. As a result, many clinicians got the false impression that lamotrigine is an effective antidepressant. I hope this explains why lamotrigine was not included in the list of antidepressants in my editorial.
In reading Dr. Nasrallah's August 2022 editorial (“Reversing depression: A plethora of therapeutic strategies and mechanisms,”
Dr. Nasrallah responds
Thanks for your message. Lamotrigine is not FDA-approved for bipolar or unipolar depression, either as monotherapy or as an adjunctive therapy. It has never been approved for mania, either (no efficacy at all). Its only FDA-approved psychiatric indication is maintenance therapy after a patient with bipolar I disorder emerges from mania with the help of one of the antimanic drugs. Yet many clinicians may perceive lamotrigine as useful for bipolar depression because more than 20 years ago the manufacturer sponsored several small studies (not FDA trials). Two studies that showed efficacy were published, but 4 other studies that failed to show efficacy were not published. As a result, many clinicians got the false impression that lamotrigine is an effective antidepressant. I hope this explains why lamotrigine was not included in the list of antidepressants in my editorial.
In reading Dr. Nasrallah's August 2022 editorial (“Reversing depression: A plethora of therapeutic strategies and mechanisms,”
Dr. Nasrallah responds
Thanks for your message. Lamotrigine is not FDA-approved for bipolar or unipolar depression, either as monotherapy or as an adjunctive therapy. It has never been approved for mania, either (no efficacy at all). Its only FDA-approved psychiatric indication is maintenance therapy after a patient with bipolar I disorder emerges from mania with the help of one of the antimanic drugs. Yet many clinicians may perceive lamotrigine as useful for bipolar depression because more than 20 years ago the manufacturer sponsored several small studies (not FDA trials). Two studies that showed efficacy were published, but 4 other studies that failed to show efficacy were not published. As a result, many clinicians got the false impression that lamotrigine is an effective antidepressant. I hope this explains why lamotrigine was not included in the list of antidepressants in my editorial.
The accelerating societal entropy undermines mental health
According to the second law of thermodynamics, it is inevitable that entropy will continue to increase over time.1 Entropy is a measure of disorder, which can eventuate in chaos and lead to profound uncertainty, with serious psychological consequences.
The increase in entropy is usually gradual. It took hundreds of years for powerful empires and civilizations to collapse and disappear. Inanimate objects such as a house, a piece of furniture, or a piece of equipment eventually deteriorate and break down over time. Tidy offices will become messy, cluttered, and dirty unless attended to regularly. Living organisms, including humans, inevitably undergo an aging process with cellular senescence, atrophy, and loss of cerebral, muscle, and bone tissue, ending in death. Even human relationships will eventually fracture, wither, and end. The passage of time ruthlessly increases the entropy of everything in life. Even the 13-billion-year-old universe, which currently looks formidable and permanent to us, is inexorably expanding and hurtling towards a calamitous end a few billion years from now.
To slow down, halt, or reverse entropy, work and energy must be invested. A house requires regular maintenance for all its components to avoid deteriorating and becoming uninhabitable (very high entropy). Humans require massive amounts of work during fetal life, infancy, childhood, adolescence, adulthood, and throughout old age. This includes work by parents, teachers, friends, physicians, farmers, and manufacturers of food, clothing, and sundry supplies, all targeted to maintain an individual and slow the rate of entropy. But death is inevitable as the final stage of human entropy.
The brain is an entropic organ.2 Psychiatric disorders can be conceptualized as a neurobiologic consequence of a major rise in brain entropy. The chaos created by high brain entropy will lead to a disruption of basic mental functions such as thought, mood, affect, impulses, behavior, and cognition. Brain entropy increases can be due to genetics or the environment, but most often are due an interaction of both (G x E).
Societal entropy and our patients
Psychiatric patients are deeply influenced by the context in which they live (society). The entropy of contemporary society is rising at an alarming rate, which means that order is rapidly degenerating into disorder at an unprecedented pace. When the COVID-19 pandemic abruptly emerged in early 2020, it was a major public health shock that drastically changed the lives of all citizens and dramatically increased societal entropy. The pandemic led to lockdowns, fear of death, gut-wrenching uncertainty (especially for a whole year before vaccines were developed, but even after), loss of socialization and sexual intimacy, loss of employment, financial straits, and an inability to access routine medical or surgical procedures. Everyone in society developed anxiety and acute stress reaction, but those with pre-existing psychiatric disorders suffered the most with an intensification of their symptoms.
The unforeseen, sudden, and traumatically life-altering pandemic triggered various degrees of posttraumatic stress disorder across all age groups, and painful death in medically compromised individuals and older adults. Both physical and psychological entropy skyrocketed and the “order” of life as we knew it rapidly disintegrated into shambles and disorder. The abrupt traumatic jolt triggered various degrees of deleterious impacts on the brains of all who experienced it in real time. The rise in the psychobiological entropy was unprecedented across the structures of society, especially the population, its vulnerable human component.
But even as the worst of the pandemic is in our rearview mirror and life again has a semblance of normality, the rise of entropy continues to accelerate because we continue to be surrounded and engulfed by countless stressful events in contemporary society. Those nagging stresses continue to transmute order to chaos and metamorphose comforting predictability to entrenched uncertainty:
- Toxic political hyperpartisanship, with intense animus and visceral bidirectional hatred
- Racial tensions, with overt bias across groups
- Economic turmoil, with inflation and threats of recession
- Actual wars and threats of war
- Social media that spreads bad news and distorts facts
- An opioid crisis, with hundreds of thousands of deaths
- Skyrocketing crime, with a decline in policing and quick release of criminals without bail
- A ruthless and arbitrary “cancel culture” that doesn’t even spare the previously revered founders of the republic
- Cognitive dissonance of disparaging Abraham Lincoln despite his major achievement of eliminating slavery by waging a civil war
- The social and medical strife regarding access to abortion.
Continue to: I also would include...
(I also would include some “entropy pet peeves” of mine: Torn clothes as a fashion statement, transforming tattoos from an oddity to a fad, nose rings that disfigure pretty faces, and banishing neckties for men.)
Our role in this scenario
As psychiatrists, we must step up to intensify the work needed to slow down and even reverse the dangerously rising brain entropy in our patients. But that is not an easy task given the implosion of societal norms and traditional values, along with the radicalization of beliefs, with utter intolerance of others’ beliefs. We also face the challenge of maintaining a modicum of resilience and wellness in ourselves, which can be antidotes to entropy.
It’s impossible to stop the inevitability of rising entropy, both physical and psychological, but psychiatrists and other mental health professionals must invest their skills and talents now more than ever to at least slow down the pace of entropy among our patients. Otherwise, psychological chaos and disorder will be quite damaging to their lives, and worsen their outcomes.
1. Ben-Naim A. Entropy Demystified. World Scientific; 2007.
2. Carhart-Harris RL. The entropic brain - revisited. Neuropharmacology. 2018;142:167-178. doi: 10.1016/j.neuropharm.2018.03.010
According to the second law of thermodynamics, it is inevitable that entropy will continue to increase over time.1 Entropy is a measure of disorder, which can eventuate in chaos and lead to profound uncertainty, with serious psychological consequences.
The increase in entropy is usually gradual. It took hundreds of years for powerful empires and civilizations to collapse and disappear. Inanimate objects such as a house, a piece of furniture, or a piece of equipment eventually deteriorate and break down over time. Tidy offices will become messy, cluttered, and dirty unless attended to regularly. Living organisms, including humans, inevitably undergo an aging process with cellular senescence, atrophy, and loss of cerebral, muscle, and bone tissue, ending in death. Even human relationships will eventually fracture, wither, and end. The passage of time ruthlessly increases the entropy of everything in life. Even the 13-billion-year-old universe, which currently looks formidable and permanent to us, is inexorably expanding and hurtling towards a calamitous end a few billion years from now.
To slow down, halt, or reverse entropy, work and energy must be invested. A house requires regular maintenance for all its components to avoid deteriorating and becoming uninhabitable (very high entropy). Humans require massive amounts of work during fetal life, infancy, childhood, adolescence, adulthood, and throughout old age. This includes work by parents, teachers, friends, physicians, farmers, and manufacturers of food, clothing, and sundry supplies, all targeted to maintain an individual and slow the rate of entropy. But death is inevitable as the final stage of human entropy.
The brain is an entropic organ.2 Psychiatric disorders can be conceptualized as a neurobiologic consequence of a major rise in brain entropy. The chaos created by high brain entropy will lead to a disruption of basic mental functions such as thought, mood, affect, impulses, behavior, and cognition. Brain entropy increases can be due to genetics or the environment, but most often are due an interaction of both (G x E).
Societal entropy and our patients
Psychiatric patients are deeply influenced by the context in which they live (society). The entropy of contemporary society is rising at an alarming rate, which means that order is rapidly degenerating into disorder at an unprecedented pace. When the COVID-19 pandemic abruptly emerged in early 2020, it was a major public health shock that drastically changed the lives of all citizens and dramatically increased societal entropy. The pandemic led to lockdowns, fear of death, gut-wrenching uncertainty (especially for a whole year before vaccines were developed, but even after), loss of socialization and sexual intimacy, loss of employment, financial straits, and an inability to access routine medical or surgical procedures. Everyone in society developed anxiety and acute stress reaction, but those with pre-existing psychiatric disorders suffered the most with an intensification of their symptoms.
The unforeseen, sudden, and traumatically life-altering pandemic triggered various degrees of posttraumatic stress disorder across all age groups, and painful death in medically compromised individuals and older adults. Both physical and psychological entropy skyrocketed and the “order” of life as we knew it rapidly disintegrated into shambles and disorder. The abrupt traumatic jolt triggered various degrees of deleterious impacts on the brains of all who experienced it in real time. The rise in the psychobiological entropy was unprecedented across the structures of society, especially the population, its vulnerable human component.
But even as the worst of the pandemic is in our rearview mirror and life again has a semblance of normality, the rise of entropy continues to accelerate because we continue to be surrounded and engulfed by countless stressful events in contemporary society. Those nagging stresses continue to transmute order to chaos and metamorphose comforting predictability to entrenched uncertainty:
- Toxic political hyperpartisanship, with intense animus and visceral bidirectional hatred
- Racial tensions, with overt bias across groups
- Economic turmoil, with inflation and threats of recession
- Actual wars and threats of war
- Social media that spreads bad news and distorts facts
- An opioid crisis, with hundreds of thousands of deaths
- Skyrocketing crime, with a decline in policing and quick release of criminals without bail
- A ruthless and arbitrary “cancel culture” that doesn’t even spare the previously revered founders of the republic
- Cognitive dissonance of disparaging Abraham Lincoln despite his major achievement of eliminating slavery by waging a civil war
- The social and medical strife regarding access to abortion.
Continue to: I also would include...
(I also would include some “entropy pet peeves” of mine: Torn clothes as a fashion statement, transforming tattoos from an oddity to a fad, nose rings that disfigure pretty faces, and banishing neckties for men.)
Our role in this scenario
As psychiatrists, we must step up to intensify the work needed to slow down and even reverse the dangerously rising brain entropy in our patients. But that is not an easy task given the implosion of societal norms and traditional values, along with the radicalization of beliefs, with utter intolerance of others’ beliefs. We also face the challenge of maintaining a modicum of resilience and wellness in ourselves, which can be antidotes to entropy.
It’s impossible to stop the inevitability of rising entropy, both physical and psychological, but psychiatrists and other mental health professionals must invest their skills and talents now more than ever to at least slow down the pace of entropy among our patients. Otherwise, psychological chaos and disorder will be quite damaging to their lives, and worsen their outcomes.
According to the second law of thermodynamics, it is inevitable that entropy will continue to increase over time.1 Entropy is a measure of disorder, which can eventuate in chaos and lead to profound uncertainty, with serious psychological consequences.
The increase in entropy is usually gradual. It took hundreds of years for powerful empires and civilizations to collapse and disappear. Inanimate objects such as a house, a piece of furniture, or a piece of equipment eventually deteriorate and break down over time. Tidy offices will become messy, cluttered, and dirty unless attended to regularly. Living organisms, including humans, inevitably undergo an aging process with cellular senescence, atrophy, and loss of cerebral, muscle, and bone tissue, ending in death. Even human relationships will eventually fracture, wither, and end. The passage of time ruthlessly increases the entropy of everything in life. Even the 13-billion-year-old universe, which currently looks formidable and permanent to us, is inexorably expanding and hurtling towards a calamitous end a few billion years from now.
To slow down, halt, or reverse entropy, work and energy must be invested. A house requires regular maintenance for all its components to avoid deteriorating and becoming uninhabitable (very high entropy). Humans require massive amounts of work during fetal life, infancy, childhood, adolescence, adulthood, and throughout old age. This includes work by parents, teachers, friends, physicians, farmers, and manufacturers of food, clothing, and sundry supplies, all targeted to maintain an individual and slow the rate of entropy. But death is inevitable as the final stage of human entropy.
The brain is an entropic organ.2 Psychiatric disorders can be conceptualized as a neurobiologic consequence of a major rise in brain entropy. The chaos created by high brain entropy will lead to a disruption of basic mental functions such as thought, mood, affect, impulses, behavior, and cognition. Brain entropy increases can be due to genetics or the environment, but most often are due an interaction of both (G x E).
Societal entropy and our patients
Psychiatric patients are deeply influenced by the context in which they live (society). The entropy of contemporary society is rising at an alarming rate, which means that order is rapidly degenerating into disorder at an unprecedented pace. When the COVID-19 pandemic abruptly emerged in early 2020, it was a major public health shock that drastically changed the lives of all citizens and dramatically increased societal entropy. The pandemic led to lockdowns, fear of death, gut-wrenching uncertainty (especially for a whole year before vaccines were developed, but even after), loss of socialization and sexual intimacy, loss of employment, financial straits, and an inability to access routine medical or surgical procedures. Everyone in society developed anxiety and acute stress reaction, but those with pre-existing psychiatric disorders suffered the most with an intensification of their symptoms.
The unforeseen, sudden, and traumatically life-altering pandemic triggered various degrees of posttraumatic stress disorder across all age groups, and painful death in medically compromised individuals and older adults. Both physical and psychological entropy skyrocketed and the “order” of life as we knew it rapidly disintegrated into shambles and disorder. The abrupt traumatic jolt triggered various degrees of deleterious impacts on the brains of all who experienced it in real time. The rise in the psychobiological entropy was unprecedented across the structures of society, especially the population, its vulnerable human component.
But even as the worst of the pandemic is in our rearview mirror and life again has a semblance of normality, the rise of entropy continues to accelerate because we continue to be surrounded and engulfed by countless stressful events in contemporary society. Those nagging stresses continue to transmute order to chaos and metamorphose comforting predictability to entrenched uncertainty:
- Toxic political hyperpartisanship, with intense animus and visceral bidirectional hatred
- Racial tensions, with overt bias across groups
- Economic turmoil, with inflation and threats of recession
- Actual wars and threats of war
- Social media that spreads bad news and distorts facts
- An opioid crisis, with hundreds of thousands of deaths
- Skyrocketing crime, with a decline in policing and quick release of criminals without bail
- A ruthless and arbitrary “cancel culture” that doesn’t even spare the previously revered founders of the republic
- Cognitive dissonance of disparaging Abraham Lincoln despite his major achievement of eliminating slavery by waging a civil war
- The social and medical strife regarding access to abortion.
Continue to: I also would include...
(I also would include some “entropy pet peeves” of mine: Torn clothes as a fashion statement, transforming tattoos from an oddity to a fad, nose rings that disfigure pretty faces, and banishing neckties for men.)
Our role in this scenario
As psychiatrists, we must step up to intensify the work needed to slow down and even reverse the dangerously rising brain entropy in our patients. But that is not an easy task given the implosion of societal norms and traditional values, along with the radicalization of beliefs, with utter intolerance of others’ beliefs. We also face the challenge of maintaining a modicum of resilience and wellness in ourselves, which can be antidotes to entropy.
It’s impossible to stop the inevitability of rising entropy, both physical and psychological, but psychiatrists and other mental health professionals must invest their skills and talents now more than ever to at least slow down the pace of entropy among our patients. Otherwise, psychological chaos and disorder will be quite damaging to their lives, and worsen their outcomes.
1. Ben-Naim A. Entropy Demystified. World Scientific; 2007.
2. Carhart-Harris RL. The entropic brain - revisited. Neuropharmacology. 2018;142:167-178. doi: 10.1016/j.neuropharm.2018.03.010
1. Ben-Naim A. Entropy Demystified. World Scientific; 2007.
2. Carhart-Harris RL. The entropic brain - revisited. Neuropharmacology. 2018;142:167-178. doi: 10.1016/j.neuropharm.2018.03.010
Faulty fences: Blood-brain barrier dysfunction in schizophrenia
The blood-brain barrier (BBB) is an essential barrier of closely spaced cells that regulates entry into the CNS. What passes should be highly regulated to protect the brain from potentially harmful peripheral cells or molecules from the rest of the body. However, research has revealed that the BBB is pathologically permeable in several disease states, including schizophrenia, epilepsy, traumatic brain injury, autism, and DiGeorge syndrome (22q11.2 deletion syndrome, which often presents with symptoms of schizophrenia).1,2 In this article, we discuss potential markers of BBB dysfunction, the consequences of a porous BBB, the effect of BBB permeability on microglial activation, and possible treatment implications.
Detecting a BBB leak
The BBB is composed of microvascular endothelial cell units. Adherens junctions, astrocyte endfeet, and pericytes are all part of these units, but tight junctions have the most significant role in BBB barrier function. Tight junction protein composition varies depending on the location of the endothelium. In the BBB, they are primarily composed of claudin-5, occludin, zonulin, and junction adhesion molecules (JAMs) (Figure). Claudins and occludins are especially important components of the tight junction because they span plasma membranes.3
Researchers began to suspect tight junction permeability in schizophrenia while searching for schizophrenia biomarkers. For example, S100B is a marker of astrocytic reactivity to damage. It is increased in schizophrenia, major depressive disorder, and bipolar disorder.4 Studies found elevated S100B specifically in drug-free patients with schizophrenia,5 which prompted research suggesting it could predict the severity of negative symptoms.6 The accuracy of S100B as a biomarker was later complicated by the finding that adipose tissue also secretes S100B. This is problematic due to the high rates of comorbid obesity in psychiatric populations.2
Perhaps a better biomarker is the ratio of albumin in the CSF vs that in peripheral serum. The CSF-to-blood albumin ratio (Q-Alb) is widely considered an acceptable marker of BBB dysfunction because albumin must cross the BBB to alter the ratio. Studies have found a high Q-Alb in neurodegenerative disorders such as multiple sclerosis as well as in schizophrenia, which suggests that some level of BBB dysfunction is occurring. Although the Q-Alb may change slightly when confounded by antipsychotic use or with CSF flow changes,2,4 both S100B and Q-Alb elevation are sufficient for further investigation into tight junction alteration in schizophrenia.
Claudin-5 is a promising factor in detecting BBB permeability. Claudin-5 is deleted in DiGeorge syndrome, which is highly comorbid with schizophrenia and psychosis.1 Mouse knockdown studies show that full suppression of claudin-5 results in psychotic symptoms before fatal seizures,2,7 but a partial absence may enable psychotic symptoms. The same study showed that normally continuous claudin-5 was patchy along blood vessels in the affected sample.7 Follow-up experiments suggest that loss of claudin-5 in schizophrenia is especially prominent in the hippocampus, and there is mixed evidence of a decrease in the prefrontal cortex.8
Outside of claudin-5 alone, JAM-A plays a more regulatory role. It is upstream from an enhancer protein gene that serves as a transcription factor for the claudin-5 promoter, so when JAM-A is deleted, there is less claudin-5.9 However, while this decrease in claudin-5 may be pathological, there could still be various upstream changes that lead to schizophrenia.
What are the consequences of a porous BBB?
Although it is well established that the BBB passes small molecules and solutes, there is significant evidence of inflammatory trafficking in disease states. The BBB moves proinflammatory cytokines, alters transporters, and may even let white blood cells (WBCs) pass through. Immune cell infiltration has different requirements depending on the cell type. T cells rely on integrins, vascular cell adhesion molecule 1 (vCAM1), and intercellular adhesion molecule 1 (iCAM1) for binding, rolling, adhering, crossing, and migration to sites of inflammation.10,11 Both iCAM1 and vCAM1 are elevated in schizophrenia compared to other psychiatric disorders (such as unipolar depression) and correlate with other biomarkers. For example, vCAM1, responsible for recruitment and crossing, is correlated with a high Q-Alb.12 Primarily produced by astrocytes and endothelial cells, iCAM1 plays the largest role in crossing the BBB and migration. Postmortem tissue demonstrates that cytokines upregulate iCAM1 mRNA at the BBB in schizophrenia.13 Increased cytokines are well documented in the inflammatory model of schizophrenia. Interestingly, decreasing claudin-5 also upregulates iCAM1 production.14 Therefore, low baseline claudin-5 may contribute to additional inflammation and symptoms.
Continue to: BBB permeability also results...
BBB permeability also results in a certain pattern of leukocyte and cytokine activity. Interleukin-1 (IL-1), IL-6, and tumor necrosis factor–alpha can all cross the BBB during neuroimmune inflammation,10 but there are abnormal heightened and sustained responses of these molecules in schizophrenia. IL-6 is a proinflammatory cytokine in both acute and chronic inflammation that is expressed by astrocytes, endothelial cells, and microglia.15 IL-6 and its soluble receptor are both elevated in schizophrenia and are associated with white matter degeneration16,17 and an increase in vCAM1.15 This implies that while neuroinflammation in schizophrenia is occurring, additional leukocytes are being recruited and secreting their own cytokines in a chronic destructive positive feedback loop. Meanwhile, atypical IL-10 levels can no longer maintain balanced levels of inflammatory molecules,16 which leads to reduced control of inflammation.
Genetics and immunohistochemistry suggest that the BBB allows the passage of excess B cells and T cells in schizophrenia. Cytokines from WBCs or the BBB during inflammation recruit these additional infiltrating lymphocytes. In gene-wide association studies, there are several genes in schizophrenia important for B cells and T cells in addition to inflammation that interact in a proinflammatory network.16 These cells are also diffusely found in the white matter18 and hippocampal tissue19 of patients with schizophrenia. Taken together, an increase in adhesion molecules, WBCs, and cytokine crosstalk supports a leaky BBB as an important component of the inflammatory model of schizophrenia.
The role of microglia in BBB dysfunction
The effect of BBB permeability on microglial activation is an important caveat in the current research. Although several reports have linked neuroinflammation to confirmed microglial activation in schizophrenia, there is not enough evidence to claim that the BBB alone is the missing link between these theories. Some research suggests that chronic release of cytokines such as IL-6 from macrophages and T cells could increase migration across the BBB for microglial activation.16,20 However, positron emission tomography has shown mixed results at best. Translocator protein (TSPO) is expressed by microglia that are actively secreting cytokines.21 Researchers tracking TSPO changes in relation to BBB alteration have not seen elevated binding in schizophrenia, change due to stage of disease course, or differentiation from low-grade inflammation.21-24 Moreover, TSPO may be confounded by antipsychotic use25 and microglial expression did not correlate with any changes in adhesion molecules.13 TSPO is not an ideal indicator of microglial activation due to BBB breakdown, but that does not bar the possibility of at least a partial contribution to the development of schizophrenia.
Corsi-Zuelli et al26 created a model that attempts to merge BBB permeability and microglial activation through a different medium—T regulatory cells (TRegs). They write that if TRegs mediate interactions between astrocytes and microglia, their hypofunction would impose a prolonged T cell response. The increased access to a high level of IL-6 and its soluble receptors may keep the TRegs hypofunctional in schizophrenia and promote T cell conversion to inflammatory cell types. Experimentally, TReg induction reversed some psychotic symptoms, and greater TReg expression was associated with fewer negative symptoms.26 In an already insufficient BBB, more access to cytokines and leukocytes would sustain inflammation and microglial secretions.
In addition to the issues described regarding the BBB, the blood-CSF barrier at the choroid plexus may also be insufficient in schizophrenia (Box27-31).
Box
The choroid plexus’ primary role is to make CSF, but it also secretes cytokines and to some extent serves as a barrier. Unlike the blood-brain barrier (BBB), the blood-CSF barrier is composed of endothelial cells with fenestrations as well as tight junctions, which make the blood-CSF barrier overall more permeable.27,28 The most unusual finding regarding the choroid plexus in schizophrenia is size. The choroid plexus is physically larger in patients with schizophrenia, and to a lesser extent, in their first-degree relatives.29 A larger choroid plexus is correlated with more severe cognitive symptoms, increased risk for psychosis via biological stress, and significantly higher interleukin-6 (IL-6).27,29 The increased thickness could be an attempt to compensate for hyperactivity and toxic processes in a permeable environment. More circulating cytokines such as IL-6 and tumor necrosis factor–alpha from microglia can trigger an increase in intercellular adhesion molecule 1, resulting in leukocyte attachment and entry.30 Less claudin-5 at the choroid plexus in schizophrenia implicates similar permissive effects as seen at the BBB.31 Although the contribution of blood-CSF barrier dysfunction to schizophrenia requires further study, reduced barrier function outside the BBB is a viable line of inquiry.
Continue to: Caveats about this research
Caveats about this research
There are 3 important points to note about the current research concerning abnormal BBB permeability:
1. BBB dysfunction may exist only in a subset of people diagnosed with schizophrenia. In most human studies, only some patients with schizophrenia demonstrated alterations that suggested pathological BBB permeability. In addition, even when there is BBB dysfunction, it could be a secondary phenomenon, rather than a primary etiologic process.
2. Patient demographics across studies have not always been adequately described. Potential confounds such as obesity, smoking, or antipsychotic use were not consistently recorded or examined as a possible factor.
3. Currently available biomarkers are not perfect. Cytokine elevation, S100B, and Q-Alb are indirect measures of BBB disruption and are found in other disorders. Therefore, they only support the theory of BBB dysfunction in schizophrenia, rather than prove it. They are also not reliable markers for schizophrenia alone. Researchers have pointed out that these markers and proteins work in concert, which necessitates a network analysis approach.16 More research regarding the details of permeability is required to establish more reliable biomarkers and tailored treatment.
Treatment implications
One of the first treatment directions that comes to mind is managing the gaps in the BBB via tight junctions. Presently, there are no FDA-approved medications for altering tight junction proteins, but researchers are exploring potential agents that can induce claudin-5 and reduce inflammation.14 While we wait for such a medication, patients may benefit from existing anti-inflammatory treatments to control immune infiltration and its products. Various anti-inflammatory agents—including cyclooxygenase inhibitors,
Bottom Line
Recent research has revealed that the blood-brain barrier (BBB) is pathologically permeable in several disease states, including schizophrenia. Better characterization of the leaky BBB in schizophrenia has enormous potential in helping us understand how current theories fit together and could serve as a missing puzzle piece in treating schizophrenia.
Related Resources
- Levine A, Strawn JR. The brain’s Twitter system: neuronal extracellular vesicles. Current Psychiatry. 2022;21(6):9-11, 17-19,27. doi:10.12788/cp.0257
Drug Brand Names
Minocycline • Dynacin, Minocin
1. Li Y, Xia Y, Zhu H, et al. Investigation of neurodevelopmental deficits of 22 q11.2 deletion syndrome with a patient-iPSC-derived blood-brain barrier model. Cells. 2021;10(10):2576. doi:10.3390/cells10102576
2. Kealy J, Greene C, Campbell M. Blood-brain barrier regulation in psychiatric disorders. Neurosci Lett. 2020;726:133664. doi:10.1016/j.neulet.2018.06.033
3. Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1-13. doi:10.1016/j.nbd.2003.12.016
4. Futtrup J, Margolinsky R, Benros ME, et al. Blood-brain barrier pathology in patients with severe mental disorders: a systematic review and meta-analysis of biomarkers in case-control studies. Brain Behav Immun Health. 2020;6:100102. doi:10.1016/j.bbih.2020.100102
5. Chen S, Tian L, Chen N, et al. Cognitive dysfunction correlates with elevated serum S100B concentration in drug-free acutely relapsed patients with schizophrenia. Psychiatry Res. 2017;247:6-11. doi:10.1016/j.psychres.2016.09.029
6. Wu YF, Sytwu HK, Lung FW. Human aquaporin 4 gene polymorphisms and haplotypes are associated with serum S100B level and negative symptoms of schizophrenia in a southern Chinese Han population. Front Psychiatry. 2018;9:657. doi:10.3389/fpsyt.2018.00657
7. Greene C, Kealy J, Humphries MM, et al. Dose-dependent expression of claudin-5 is a modifying factor in schizophrenia. Mol Psychiatry. 2018;23(11):2156-2166. doi:10.1038/MP.2017.156
8. Greene C, Hanley N, Campbell M. Blood-brain barrier associated tight junction disruption is a hallmark feature of major psychiatric disorders. Transl Psychiatry. 2020;10(1):373. doi:10.1038/s41398-020-01054-3
9. Kakogiannos N, Ferrari L, Giampietro C, et al. JAM-A acts via C/EBP-α to promote claudin-5 expression and enhance endothelial barrier function. Circ Res. 2020:1056-1073. doi:10.1161/CIRCRESAHA.120.316742
10. Erickson MA, Dohi K, Banks WA. Neuroinflammation: a common pathway in CNS diseases as mediated at the blood-brain barrier. Neuroimmunomodulation. 2012;19(2):121-130. doi:10.1159/000330247
11. Ao LY, Yan YY, Zhou L, et al. Immune cells after ischemic stroke onset: roles, migration, and target intervention. J Mol Neurosci. 2018;66(3):342-355. doi:10.1007/s12031-018-1173-4
12. Meixensberger S, Kuzior H, Fiebich BL, et al. Upregulation of sICAM-1 and sVCAM-1 levels in the cerebrospinal fluid of patients with schizophrenia spectrum disorders. Diagnostics (Basel). 2021;11(7):1134. doi:10.3390diagnostics11071134
13. Cai HQ, Catts VS, Webster MJ, et al. Increased macrophages and changed brain endothelial cell gene expression in the frontal cortex of people with schizophrenia displaying inflammation. Mol Psychiatry. 2020;25(4):761-775. doi:10.1038/s41380-018-0235-x
14. Greene C, Hanley N, Reschke CR, et al. Microvascular stabilization via blood-brain barrier regulation prevents seizure activity. Nat Commun. 2022;13(1):2003. doi:10.1038/s41467-022-29657-y
15. García-Juárez M, Camacho-Morales A. Defining the role of anti- and pro-inflammatory outcomes of interleukin-6 in mental health. Neuroscience. 2022;492:32-46. doi:10.1016/j.neuroscience.2022.03.020
16. Pong S, Karmacharya R, Sofman M, et al. The role of brain microvascular endothelial cell and blood-brain barrier dysfunction in schizophrenia. Complex Psychiatry. 2020;6(1-2):30-46. doi:10.1159/000511552
17. Patel A, Zhu Y, Kuzhikandathil EV, et al. Soluble interleukin-6 receptor induces motor stereotypies and co-localizes with gp130 in regions linked to cortico-striato-thalamo-cortical circuits. PLoS One. 2012;7(7): e41623. doi:10.1371/journal.pone.0041623
18. Schlaaff K, Dobrowolny H, Frodl T, et al. Increased densities of T and B lymphocytes indicate neuroinflammation in subgroups of schizophrenia and mood disorder patients. Brain Behav Immun. 2020;88:497-506. doi:10.1016/j.bbi.2020.04.021
19. Busse S, Busse M, Schiltz K, et al. Different distribution patterns of lymphocytes and microglia in the hippocampus of patients with residual versus paranoid schizophrenia: further evidence for disease course-related immune alterations? Brain Behav Immun. 2012;26(8):1273-1279. doi:10.1016/j.bbi.2012.08.005
20. Na KS, Jung HY, Kim YK. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:277-286. doi:10.1016/j.pnpbp.2012.10.022
21. Conen S, Gregory CJ, Hinz R, et al. Neuroinflammation as measured by positron emission tomography in patients with recent onset and established schizophrenia: implications for immune pathogenesis. Mol Psychiatry. 2021;26(9):5398-5406. doi:10.1038/S41380-020-0829-Y
22. Najjar S, Pahlajani S, De Sanctis V, et al. Neurovascular unit dysfunction and blood-brain barrier hyperpermeability contribute to schizophrenia neurobiology: a theoretical integration of clinical and experimental evidence. Front Psychiatry. 2017;8:83. doi:10.3389/fpsyt.2017.00083
23. Pinjari OF, Dasgupta SK, Okusaga OO. Plasma soluble P-selectin, interleukin-6 and S100B protein in patients with schizophrenia: a pilot study. Psychiatr Q. 2022;93(1):335-345. doi:10.1007/s11126-021-09954-3
24. Di Biase MA, Zalesky A, O’keefe G, et al. PET imaging of putative microglial activation in individuals at ultra-high risk for psychosis, recently diagnosed and chronically ill with schizophrenia. Transl Psychiatry. 2017;7(8):e1225. doi:10.1038/tp.2017.193
25. Holmes SE, Hinz R, Drake RJ, et al. In vivo imaging of brain microglial activity in antipsychotic-free and medicated schizophrenia: a [11C](R)-PK11195 positron emission tomography study. Mol Psychiatry. 2016;21(12):1672-1679. doi:10.1038/mp.2016.180
26. Corsi-Zuelli F, Deakin B, de Lima MHF, et al. T regulatory cells as a potential therapeutic target in psychosis? Current challenges and future perspectives. Brain Behav Immun Health. 2021;17:100330. doi:10.1016/j.bbih.2021.100330
27. Bannai D, Lutz O, Lizano P. Neuroimaging considerations when investigating choroid plexus morphology in idiopathic psychosis. Schizophr Res. 2020;224:19-21. doi:10.1016/j.schres.2020.07.013
28. Hladky SB, Barrand MA. Fluid and ion transfer across the blood-brain and blood-cerebrospinal fluid barriers; a comparative account of mechanisms and roles. Fluids Barriers CNS. 2016;13(1):19. doi:10.1186/s12987-016-0040-3
29. Lizano P, Lutz O, Ling G, et al. Association of choroid plexus enlargement with cognitive, inflammatory, and structural phenotypes across the psychosis spectrum. Am J Psychiatry. 2019;176(7):564-572. doi:10.1176/appi.ajp.2019.18070825
30. Castellani G, Contarini G, Mereu M, et al. Dopamine-mediated immunomodulation affects choroid plexus function. Brain Behav Immun. 2019;81:138-150. doi:10.1016/j.bbi.2019.06.006
31. Bitanihirwe BKY, Lizano P, Woo TW. Deconstructing the functional neuroanatomy of the choroid plexus: an ontogenetic perspective for studying neurodevelopmental and neuropsychiatric disorders. Mol Psychiatry. 2022;1-10. doi:10.1038/s41380-022-01623-6
The blood-brain barrier (BBB) is an essential barrier of closely spaced cells that regulates entry into the CNS. What passes should be highly regulated to protect the brain from potentially harmful peripheral cells or molecules from the rest of the body. However, research has revealed that the BBB is pathologically permeable in several disease states, including schizophrenia, epilepsy, traumatic brain injury, autism, and DiGeorge syndrome (22q11.2 deletion syndrome, which often presents with symptoms of schizophrenia).1,2 In this article, we discuss potential markers of BBB dysfunction, the consequences of a porous BBB, the effect of BBB permeability on microglial activation, and possible treatment implications.
Detecting a BBB leak
The BBB is composed of microvascular endothelial cell units. Adherens junctions, astrocyte endfeet, and pericytes are all part of these units, but tight junctions have the most significant role in BBB barrier function. Tight junction protein composition varies depending on the location of the endothelium. In the BBB, they are primarily composed of claudin-5, occludin, zonulin, and junction adhesion molecules (JAMs) (Figure). Claudins and occludins are especially important components of the tight junction because they span plasma membranes.3

Researchers began to suspect tight junction permeability in schizophrenia while searching for schizophrenia biomarkers. For example, S100B is a marker of astrocytic reactivity to damage. It is increased in schizophrenia, major depressive disorder, and bipolar disorder.4 Studies found elevated S100B specifically in drug-free patients with schizophrenia,5 which prompted research suggesting it could predict the severity of negative symptoms.6 The accuracy of S100B as a biomarker was later complicated by the finding that adipose tissue also secretes S100B. This is problematic due to the high rates of comorbid obesity in psychiatric populations.2
Perhaps a better biomarker is the ratio of albumin in the CSF vs that in peripheral serum. The CSF-to-blood albumin ratio (Q-Alb) is widely considered an acceptable marker of BBB dysfunction because albumin must cross the BBB to alter the ratio. Studies have found a high Q-Alb in neurodegenerative disorders such as multiple sclerosis as well as in schizophrenia, which suggests that some level of BBB dysfunction is occurring. Although the Q-Alb may change slightly when confounded by antipsychotic use or with CSF flow changes,2,4 both S100B and Q-Alb elevation are sufficient for further investigation into tight junction alteration in schizophrenia.
Claudin-5 is a promising factor in detecting BBB permeability. Claudin-5 is deleted in DiGeorge syndrome, which is highly comorbid with schizophrenia and psychosis.1 Mouse knockdown studies show that full suppression of claudin-5 results in psychotic symptoms before fatal seizures,2,7 but a partial absence may enable psychotic symptoms. The same study showed that normally continuous claudin-5 was patchy along blood vessels in the affected sample.7 Follow-up experiments suggest that loss of claudin-5 in schizophrenia is especially prominent in the hippocampus, and there is mixed evidence of a decrease in the prefrontal cortex.8
Outside of claudin-5 alone, JAM-A plays a more regulatory role. It is upstream from an enhancer protein gene that serves as a transcription factor for the claudin-5 promoter, so when JAM-A is deleted, there is less claudin-5.9 However, while this decrease in claudin-5 may be pathological, there could still be various upstream changes that lead to schizophrenia.
What are the consequences of a porous BBB?
Although it is well established that the BBB passes small molecules and solutes, there is significant evidence of inflammatory trafficking in disease states. The BBB moves proinflammatory cytokines, alters transporters, and may even let white blood cells (WBCs) pass through. Immune cell infiltration has different requirements depending on the cell type. T cells rely on integrins, vascular cell adhesion molecule 1 (vCAM1), and intercellular adhesion molecule 1 (iCAM1) for binding, rolling, adhering, crossing, and migration to sites of inflammation.10,11 Both iCAM1 and vCAM1 are elevated in schizophrenia compared to other psychiatric disorders (such as unipolar depression) and correlate with other biomarkers. For example, vCAM1, responsible for recruitment and crossing, is correlated with a high Q-Alb.12 Primarily produced by astrocytes and endothelial cells, iCAM1 plays the largest role in crossing the BBB and migration. Postmortem tissue demonstrates that cytokines upregulate iCAM1 mRNA at the BBB in schizophrenia.13 Increased cytokines are well documented in the inflammatory model of schizophrenia. Interestingly, decreasing claudin-5 also upregulates iCAM1 production.14 Therefore, low baseline claudin-5 may contribute to additional inflammation and symptoms.
Continue to: BBB permeability also results...
BBB permeability also results in a certain pattern of leukocyte and cytokine activity. Interleukin-1 (IL-1), IL-6, and tumor necrosis factor–alpha can all cross the BBB during neuroimmune inflammation,10 but there are abnormal heightened and sustained responses of these molecules in schizophrenia. IL-6 is a proinflammatory cytokine in both acute and chronic inflammation that is expressed by astrocytes, endothelial cells, and microglia.15 IL-6 and its soluble receptor are both elevated in schizophrenia and are associated with white matter degeneration16,17 and an increase in vCAM1.15 This implies that while neuroinflammation in schizophrenia is occurring, additional leukocytes are being recruited and secreting their own cytokines in a chronic destructive positive feedback loop. Meanwhile, atypical IL-10 levels can no longer maintain balanced levels of inflammatory molecules,16 which leads to reduced control of inflammation.
Genetics and immunohistochemistry suggest that the BBB allows the passage of excess B cells and T cells in schizophrenia. Cytokines from WBCs or the BBB during inflammation recruit these additional infiltrating lymphocytes. In gene-wide association studies, there are several genes in schizophrenia important for B cells and T cells in addition to inflammation that interact in a proinflammatory network.16 These cells are also diffusely found in the white matter18 and hippocampal tissue19 of patients with schizophrenia. Taken together, an increase in adhesion molecules, WBCs, and cytokine crosstalk supports a leaky BBB as an important component of the inflammatory model of schizophrenia.
The role of microglia in BBB dysfunction
The effect of BBB permeability on microglial activation is an important caveat in the current research. Although several reports have linked neuroinflammation to confirmed microglial activation in schizophrenia, there is not enough evidence to claim that the BBB alone is the missing link between these theories. Some research suggests that chronic release of cytokines such as IL-6 from macrophages and T cells could increase migration across the BBB for microglial activation.16,20 However, positron emission tomography has shown mixed results at best. Translocator protein (TSPO) is expressed by microglia that are actively secreting cytokines.21 Researchers tracking TSPO changes in relation to BBB alteration have not seen elevated binding in schizophrenia, change due to stage of disease course, or differentiation from low-grade inflammation.21-24 Moreover, TSPO may be confounded by antipsychotic use25 and microglial expression did not correlate with any changes in adhesion molecules.13 TSPO is not an ideal indicator of microglial activation due to BBB breakdown, but that does not bar the possibility of at least a partial contribution to the development of schizophrenia.
Corsi-Zuelli et al26 created a model that attempts to merge BBB permeability and microglial activation through a different medium—T regulatory cells (TRegs). They write that if TRegs mediate interactions between astrocytes and microglia, their hypofunction would impose a prolonged T cell response. The increased access to a high level of IL-6 and its soluble receptors may keep the TRegs hypofunctional in schizophrenia and promote T cell conversion to inflammatory cell types. Experimentally, TReg induction reversed some psychotic symptoms, and greater TReg expression was associated with fewer negative symptoms.26 In an already insufficient BBB, more access to cytokines and leukocytes would sustain inflammation and microglial secretions.
In addition to the issues described regarding the BBB, the blood-CSF barrier at the choroid plexus may also be insufficient in schizophrenia (Box27-31).
Box
The choroid plexus’ primary role is to make CSF, but it also secretes cytokines and to some extent serves as a barrier. Unlike the blood-brain barrier (BBB), the blood-CSF barrier is composed of endothelial cells with fenestrations as well as tight junctions, which make the blood-CSF barrier overall more permeable.27,28 The most unusual finding regarding the choroid plexus in schizophrenia is size. The choroid plexus is physically larger in patients with schizophrenia, and to a lesser extent, in their first-degree relatives.29 A larger choroid plexus is correlated with more severe cognitive symptoms, increased risk for psychosis via biological stress, and significantly higher interleukin-6 (IL-6).27,29 The increased thickness could be an attempt to compensate for hyperactivity and toxic processes in a permeable environment. More circulating cytokines such as IL-6 and tumor necrosis factor–alpha from microglia can trigger an increase in intercellular adhesion molecule 1, resulting in leukocyte attachment and entry.30 Less claudin-5 at the choroid plexus in schizophrenia implicates similar permissive effects as seen at the BBB.31 Although the contribution of blood-CSF barrier dysfunction to schizophrenia requires further study, reduced barrier function outside the BBB is a viable line of inquiry.
Continue to: Caveats about this research
Caveats about this research
There are 3 important points to note about the current research concerning abnormal BBB permeability:
1. BBB dysfunction may exist only in a subset of people diagnosed with schizophrenia. In most human studies, only some patients with schizophrenia demonstrated alterations that suggested pathological BBB permeability. In addition, even when there is BBB dysfunction, it could be a secondary phenomenon, rather than a primary etiologic process.
2. Patient demographics across studies have not always been adequately described. Potential confounds such as obesity, smoking, or antipsychotic use were not consistently recorded or examined as a possible factor.
3. Currently available biomarkers are not perfect. Cytokine elevation, S100B, and Q-Alb are indirect measures of BBB disruption and are found in other disorders. Therefore, they only support the theory of BBB dysfunction in schizophrenia, rather than prove it. They are also not reliable markers for schizophrenia alone. Researchers have pointed out that these markers and proteins work in concert, which necessitates a network analysis approach.16 More research regarding the details of permeability is required to establish more reliable biomarkers and tailored treatment.
Treatment implications
One of the first treatment directions that comes to mind is managing the gaps in the BBB via tight junctions. Presently, there are no FDA-approved medications for altering tight junction proteins, but researchers are exploring potential agents that can induce claudin-5 and reduce inflammation.14 While we wait for such a medication, patients may benefit from existing anti-inflammatory treatments to control immune infiltration and its products. Various anti-inflammatory agents—including cyclooxygenase inhibitors,
Bottom Line
Recent research has revealed that the blood-brain barrier (BBB) is pathologically permeable in several disease states, including schizophrenia. Better characterization of the leaky BBB in schizophrenia has enormous potential in helping us understand how current theories fit together and could serve as a missing puzzle piece in treating schizophrenia.
Related Resources
- Levine A, Strawn JR. The brain’s Twitter system: neuronal extracellular vesicles. Current Psychiatry. 2022;21(6):9-11, 17-19,27. doi:10.12788/cp.0257
Drug Brand Names
Minocycline • Dynacin, Minocin
The blood-brain barrier (BBB) is an essential barrier of closely spaced cells that regulates entry into the CNS. What passes should be highly regulated to protect the brain from potentially harmful peripheral cells or molecules from the rest of the body. However, research has revealed that the BBB is pathologically permeable in several disease states, including schizophrenia, epilepsy, traumatic brain injury, autism, and DiGeorge syndrome (22q11.2 deletion syndrome, which often presents with symptoms of schizophrenia).1,2 In this article, we discuss potential markers of BBB dysfunction, the consequences of a porous BBB, the effect of BBB permeability on microglial activation, and possible treatment implications.
Detecting a BBB leak
The BBB is composed of microvascular endothelial cell units. Adherens junctions, astrocyte endfeet, and pericytes are all part of these units, but tight junctions have the most significant role in BBB barrier function. Tight junction protein composition varies depending on the location of the endothelium. In the BBB, they are primarily composed of claudin-5, occludin, zonulin, and junction adhesion molecules (JAMs) (Figure). Claudins and occludins are especially important components of the tight junction because they span plasma membranes.3

Researchers began to suspect tight junction permeability in schizophrenia while searching for schizophrenia biomarkers. For example, S100B is a marker of astrocytic reactivity to damage. It is increased in schizophrenia, major depressive disorder, and bipolar disorder.4 Studies found elevated S100B specifically in drug-free patients with schizophrenia,5 which prompted research suggesting it could predict the severity of negative symptoms.6 The accuracy of S100B as a biomarker was later complicated by the finding that adipose tissue also secretes S100B. This is problematic due to the high rates of comorbid obesity in psychiatric populations.2
Perhaps a better biomarker is the ratio of albumin in the CSF vs that in peripheral serum. The CSF-to-blood albumin ratio (Q-Alb) is widely considered an acceptable marker of BBB dysfunction because albumin must cross the BBB to alter the ratio. Studies have found a high Q-Alb in neurodegenerative disorders such as multiple sclerosis as well as in schizophrenia, which suggests that some level of BBB dysfunction is occurring. Although the Q-Alb may change slightly when confounded by antipsychotic use or with CSF flow changes,2,4 both S100B and Q-Alb elevation are sufficient for further investigation into tight junction alteration in schizophrenia.
Claudin-5 is a promising factor in detecting BBB permeability. Claudin-5 is deleted in DiGeorge syndrome, which is highly comorbid with schizophrenia and psychosis.1 Mouse knockdown studies show that full suppression of claudin-5 results in psychotic symptoms before fatal seizures,2,7 but a partial absence may enable psychotic symptoms. The same study showed that normally continuous claudin-5 was patchy along blood vessels in the affected sample.7 Follow-up experiments suggest that loss of claudin-5 in schizophrenia is especially prominent in the hippocampus, and there is mixed evidence of a decrease in the prefrontal cortex.8
Outside of claudin-5 alone, JAM-A plays a more regulatory role. It is upstream from an enhancer protein gene that serves as a transcription factor for the claudin-5 promoter, so when JAM-A is deleted, there is less claudin-5.9 However, while this decrease in claudin-5 may be pathological, there could still be various upstream changes that lead to schizophrenia.
What are the consequences of a porous BBB?
Although it is well established that the BBB passes small molecules and solutes, there is significant evidence of inflammatory trafficking in disease states. The BBB moves proinflammatory cytokines, alters transporters, and may even let white blood cells (WBCs) pass through. Immune cell infiltration has different requirements depending on the cell type. T cells rely on integrins, vascular cell adhesion molecule 1 (vCAM1), and intercellular adhesion molecule 1 (iCAM1) for binding, rolling, adhering, crossing, and migration to sites of inflammation.10,11 Both iCAM1 and vCAM1 are elevated in schizophrenia compared to other psychiatric disorders (such as unipolar depression) and correlate with other biomarkers. For example, vCAM1, responsible for recruitment and crossing, is correlated with a high Q-Alb.12 Primarily produced by astrocytes and endothelial cells, iCAM1 plays the largest role in crossing the BBB and migration. Postmortem tissue demonstrates that cytokines upregulate iCAM1 mRNA at the BBB in schizophrenia.13 Increased cytokines are well documented in the inflammatory model of schizophrenia. Interestingly, decreasing claudin-5 also upregulates iCAM1 production.14 Therefore, low baseline claudin-5 may contribute to additional inflammation and symptoms.
Continue to: BBB permeability also results...
BBB permeability also results in a certain pattern of leukocyte and cytokine activity. Interleukin-1 (IL-1), IL-6, and tumor necrosis factor–alpha can all cross the BBB during neuroimmune inflammation,10 but there are abnormal heightened and sustained responses of these molecules in schizophrenia. IL-6 is a proinflammatory cytokine in both acute and chronic inflammation that is expressed by astrocytes, endothelial cells, and microglia.15 IL-6 and its soluble receptor are both elevated in schizophrenia and are associated with white matter degeneration16,17 and an increase in vCAM1.15 This implies that while neuroinflammation in schizophrenia is occurring, additional leukocytes are being recruited and secreting their own cytokines in a chronic destructive positive feedback loop. Meanwhile, atypical IL-10 levels can no longer maintain balanced levels of inflammatory molecules,16 which leads to reduced control of inflammation.
Genetics and immunohistochemistry suggest that the BBB allows the passage of excess B cells and T cells in schizophrenia. Cytokines from WBCs or the BBB during inflammation recruit these additional infiltrating lymphocytes. In gene-wide association studies, there are several genes in schizophrenia important for B cells and T cells in addition to inflammation that interact in a proinflammatory network.16 These cells are also diffusely found in the white matter18 and hippocampal tissue19 of patients with schizophrenia. Taken together, an increase in adhesion molecules, WBCs, and cytokine crosstalk supports a leaky BBB as an important component of the inflammatory model of schizophrenia.
The role of microglia in BBB dysfunction
The effect of BBB permeability on microglial activation is an important caveat in the current research. Although several reports have linked neuroinflammation to confirmed microglial activation in schizophrenia, there is not enough evidence to claim that the BBB alone is the missing link between these theories. Some research suggests that chronic release of cytokines such as IL-6 from macrophages and T cells could increase migration across the BBB for microglial activation.16,20 However, positron emission tomography has shown mixed results at best. Translocator protein (TSPO) is expressed by microglia that are actively secreting cytokines.21 Researchers tracking TSPO changes in relation to BBB alteration have not seen elevated binding in schizophrenia, change due to stage of disease course, or differentiation from low-grade inflammation.21-24 Moreover, TSPO may be confounded by antipsychotic use25 and microglial expression did not correlate with any changes in adhesion molecules.13 TSPO is not an ideal indicator of microglial activation due to BBB breakdown, but that does not bar the possibility of at least a partial contribution to the development of schizophrenia.
Corsi-Zuelli et al26 created a model that attempts to merge BBB permeability and microglial activation through a different medium—T regulatory cells (TRegs). They write that if TRegs mediate interactions between astrocytes and microglia, their hypofunction would impose a prolonged T cell response. The increased access to a high level of IL-6 and its soluble receptors may keep the TRegs hypofunctional in schizophrenia and promote T cell conversion to inflammatory cell types. Experimentally, TReg induction reversed some psychotic symptoms, and greater TReg expression was associated with fewer negative symptoms.26 In an already insufficient BBB, more access to cytokines and leukocytes would sustain inflammation and microglial secretions.
In addition to the issues described regarding the BBB, the blood-CSF barrier at the choroid plexus may also be insufficient in schizophrenia (Box27-31).
Box
The choroid plexus’ primary role is to make CSF, but it also secretes cytokines and to some extent serves as a barrier. Unlike the blood-brain barrier (BBB), the blood-CSF barrier is composed of endothelial cells with fenestrations as well as tight junctions, which make the blood-CSF barrier overall more permeable.27,28 The most unusual finding regarding the choroid plexus in schizophrenia is size. The choroid plexus is physically larger in patients with schizophrenia, and to a lesser extent, in their first-degree relatives.29 A larger choroid plexus is correlated with more severe cognitive symptoms, increased risk for psychosis via biological stress, and significantly higher interleukin-6 (IL-6).27,29 The increased thickness could be an attempt to compensate for hyperactivity and toxic processes in a permeable environment. More circulating cytokines such as IL-6 and tumor necrosis factor–alpha from microglia can trigger an increase in intercellular adhesion molecule 1, resulting in leukocyte attachment and entry.30 Less claudin-5 at the choroid plexus in schizophrenia implicates similar permissive effects as seen at the BBB.31 Although the contribution of blood-CSF barrier dysfunction to schizophrenia requires further study, reduced barrier function outside the BBB is a viable line of inquiry.
Continue to: Caveats about this research
Caveats about this research
There are 3 important points to note about the current research concerning abnormal BBB permeability:
1. BBB dysfunction may exist only in a subset of people diagnosed with schizophrenia. In most human studies, only some patients with schizophrenia demonstrated alterations that suggested pathological BBB permeability. In addition, even when there is BBB dysfunction, it could be a secondary phenomenon, rather than a primary etiologic process.
2. Patient demographics across studies have not always been adequately described. Potential confounds such as obesity, smoking, or antipsychotic use were not consistently recorded or examined as a possible factor.
3. Currently available biomarkers are not perfect. Cytokine elevation, S100B, and Q-Alb are indirect measures of BBB disruption and are found in other disorders. Therefore, they only support the theory of BBB dysfunction in schizophrenia, rather than prove it. They are also not reliable markers for schizophrenia alone. Researchers have pointed out that these markers and proteins work in concert, which necessitates a network analysis approach.16 More research regarding the details of permeability is required to establish more reliable biomarkers and tailored treatment.
Treatment implications
One of the first treatment directions that comes to mind is managing the gaps in the BBB via tight junctions. Presently, there are no FDA-approved medications for altering tight junction proteins, but researchers are exploring potential agents that can induce claudin-5 and reduce inflammation.14 While we wait for such a medication, patients may benefit from existing anti-inflammatory treatments to control immune infiltration and its products. Various anti-inflammatory agents—including cyclooxygenase inhibitors,
Bottom Line
Recent research has revealed that the blood-brain barrier (BBB) is pathologically permeable in several disease states, including schizophrenia. Better characterization of the leaky BBB in schizophrenia has enormous potential in helping us understand how current theories fit together and could serve as a missing puzzle piece in treating schizophrenia.
Related Resources
- Levine A, Strawn JR. The brain’s Twitter system: neuronal extracellular vesicles. Current Psychiatry. 2022;21(6):9-11, 17-19,27. doi:10.12788/cp.0257
Drug Brand Names
Minocycline • Dynacin, Minocin
1. Li Y, Xia Y, Zhu H, et al. Investigation of neurodevelopmental deficits of 22 q11.2 deletion syndrome with a patient-iPSC-derived blood-brain barrier model. Cells. 2021;10(10):2576. doi:10.3390/cells10102576
2. Kealy J, Greene C, Campbell M. Blood-brain barrier regulation in psychiatric disorders. Neurosci Lett. 2020;726:133664. doi:10.1016/j.neulet.2018.06.033
3. Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1-13. doi:10.1016/j.nbd.2003.12.016
4. Futtrup J, Margolinsky R, Benros ME, et al. Blood-brain barrier pathology in patients with severe mental disorders: a systematic review and meta-analysis of biomarkers in case-control studies. Brain Behav Immun Health. 2020;6:100102. doi:10.1016/j.bbih.2020.100102
5. Chen S, Tian L, Chen N, et al. Cognitive dysfunction correlates with elevated serum S100B concentration in drug-free acutely relapsed patients with schizophrenia. Psychiatry Res. 2017;247:6-11. doi:10.1016/j.psychres.2016.09.029
6. Wu YF, Sytwu HK, Lung FW. Human aquaporin 4 gene polymorphisms and haplotypes are associated with serum S100B level and negative symptoms of schizophrenia in a southern Chinese Han population. Front Psychiatry. 2018;9:657. doi:10.3389/fpsyt.2018.00657
7. Greene C, Kealy J, Humphries MM, et al. Dose-dependent expression of claudin-5 is a modifying factor in schizophrenia. Mol Psychiatry. 2018;23(11):2156-2166. doi:10.1038/MP.2017.156
8. Greene C, Hanley N, Campbell M. Blood-brain barrier associated tight junction disruption is a hallmark feature of major psychiatric disorders. Transl Psychiatry. 2020;10(1):373. doi:10.1038/s41398-020-01054-3
9. Kakogiannos N, Ferrari L, Giampietro C, et al. JAM-A acts via C/EBP-α to promote claudin-5 expression and enhance endothelial barrier function. Circ Res. 2020:1056-1073. doi:10.1161/CIRCRESAHA.120.316742
10. Erickson MA, Dohi K, Banks WA. Neuroinflammation: a common pathway in CNS diseases as mediated at the blood-brain barrier. Neuroimmunomodulation. 2012;19(2):121-130. doi:10.1159/000330247
11. Ao LY, Yan YY, Zhou L, et al. Immune cells after ischemic stroke onset: roles, migration, and target intervention. J Mol Neurosci. 2018;66(3):342-355. doi:10.1007/s12031-018-1173-4
12. Meixensberger S, Kuzior H, Fiebich BL, et al. Upregulation of sICAM-1 and sVCAM-1 levels in the cerebrospinal fluid of patients with schizophrenia spectrum disorders. Diagnostics (Basel). 2021;11(7):1134. doi:10.3390diagnostics11071134
13. Cai HQ, Catts VS, Webster MJ, et al. Increased macrophages and changed brain endothelial cell gene expression in the frontal cortex of people with schizophrenia displaying inflammation. Mol Psychiatry. 2020;25(4):761-775. doi:10.1038/s41380-018-0235-x
14. Greene C, Hanley N, Reschke CR, et al. Microvascular stabilization via blood-brain barrier regulation prevents seizure activity. Nat Commun. 2022;13(1):2003. doi:10.1038/s41467-022-29657-y
15. García-Juárez M, Camacho-Morales A. Defining the role of anti- and pro-inflammatory outcomes of interleukin-6 in mental health. Neuroscience. 2022;492:32-46. doi:10.1016/j.neuroscience.2022.03.020
16. Pong S, Karmacharya R, Sofman M, et al. The role of brain microvascular endothelial cell and blood-brain barrier dysfunction in schizophrenia. Complex Psychiatry. 2020;6(1-2):30-46. doi:10.1159/000511552
17. Patel A, Zhu Y, Kuzhikandathil EV, et al. Soluble interleukin-6 receptor induces motor stereotypies and co-localizes with gp130 in regions linked to cortico-striato-thalamo-cortical circuits. PLoS One. 2012;7(7): e41623. doi:10.1371/journal.pone.0041623
18. Schlaaff K, Dobrowolny H, Frodl T, et al. Increased densities of T and B lymphocytes indicate neuroinflammation in subgroups of schizophrenia and mood disorder patients. Brain Behav Immun. 2020;88:497-506. doi:10.1016/j.bbi.2020.04.021
19. Busse S, Busse M, Schiltz K, et al. Different distribution patterns of lymphocytes and microglia in the hippocampus of patients with residual versus paranoid schizophrenia: further evidence for disease course-related immune alterations? Brain Behav Immun. 2012;26(8):1273-1279. doi:10.1016/j.bbi.2012.08.005
20. Na KS, Jung HY, Kim YK. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:277-286. doi:10.1016/j.pnpbp.2012.10.022
21. Conen S, Gregory CJ, Hinz R, et al. Neuroinflammation as measured by positron emission tomography in patients with recent onset and established schizophrenia: implications for immune pathogenesis. Mol Psychiatry. 2021;26(9):5398-5406. doi:10.1038/S41380-020-0829-Y
22. Najjar S, Pahlajani S, De Sanctis V, et al. Neurovascular unit dysfunction and blood-brain barrier hyperpermeability contribute to schizophrenia neurobiology: a theoretical integration of clinical and experimental evidence. Front Psychiatry. 2017;8:83. doi:10.3389/fpsyt.2017.00083
23. Pinjari OF, Dasgupta SK, Okusaga OO. Plasma soluble P-selectin, interleukin-6 and S100B protein in patients with schizophrenia: a pilot study. Psychiatr Q. 2022;93(1):335-345. doi:10.1007/s11126-021-09954-3
24. Di Biase MA, Zalesky A, O’keefe G, et al. PET imaging of putative microglial activation in individuals at ultra-high risk for psychosis, recently diagnosed and chronically ill with schizophrenia. Transl Psychiatry. 2017;7(8):e1225. doi:10.1038/tp.2017.193
25. Holmes SE, Hinz R, Drake RJ, et al. In vivo imaging of brain microglial activity in antipsychotic-free and medicated schizophrenia: a [11C](R)-PK11195 positron emission tomography study. Mol Psychiatry. 2016;21(12):1672-1679. doi:10.1038/mp.2016.180
26. Corsi-Zuelli F, Deakin B, de Lima MHF, et al. T regulatory cells as a potential therapeutic target in psychosis? Current challenges and future perspectives. Brain Behav Immun Health. 2021;17:100330. doi:10.1016/j.bbih.2021.100330
27. Bannai D, Lutz O, Lizano P. Neuroimaging considerations when investigating choroid plexus morphology in idiopathic psychosis. Schizophr Res. 2020;224:19-21. doi:10.1016/j.schres.2020.07.013
28. Hladky SB, Barrand MA. Fluid and ion transfer across the blood-brain and blood-cerebrospinal fluid barriers; a comparative account of mechanisms and roles. Fluids Barriers CNS. 2016;13(1):19. doi:10.1186/s12987-016-0040-3
29. Lizano P, Lutz O, Ling G, et al. Association of choroid plexus enlargement with cognitive, inflammatory, and structural phenotypes across the psychosis spectrum. Am J Psychiatry. 2019;176(7):564-572. doi:10.1176/appi.ajp.2019.18070825
30. Castellani G, Contarini G, Mereu M, et al. Dopamine-mediated immunomodulation affects choroid plexus function. Brain Behav Immun. 2019;81:138-150. doi:10.1016/j.bbi.2019.06.006
31. Bitanihirwe BKY, Lizano P, Woo TW. Deconstructing the functional neuroanatomy of the choroid plexus: an ontogenetic perspective for studying neurodevelopmental and neuropsychiatric disorders. Mol Psychiatry. 2022;1-10. doi:10.1038/s41380-022-01623-6
1. Li Y, Xia Y, Zhu H, et al. Investigation of neurodevelopmental deficits of 22 q11.2 deletion syndrome with a patient-iPSC-derived blood-brain barrier model. Cells. 2021;10(10):2576. doi:10.3390/cells10102576
2. Kealy J, Greene C, Campbell M. Blood-brain barrier regulation in psychiatric disorders. Neurosci Lett. 2020;726:133664. doi:10.1016/j.neulet.2018.06.033
3. Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1-13. doi:10.1016/j.nbd.2003.12.016
4. Futtrup J, Margolinsky R, Benros ME, et al. Blood-brain barrier pathology in patients with severe mental disorders: a systematic review and meta-analysis of biomarkers in case-control studies. Brain Behav Immun Health. 2020;6:100102. doi:10.1016/j.bbih.2020.100102
5. Chen S, Tian L, Chen N, et al. Cognitive dysfunction correlates with elevated serum S100B concentration in drug-free acutely relapsed patients with schizophrenia. Psychiatry Res. 2017;247:6-11. doi:10.1016/j.psychres.2016.09.029
6. Wu YF, Sytwu HK, Lung FW. Human aquaporin 4 gene polymorphisms and haplotypes are associated with serum S100B level and negative symptoms of schizophrenia in a southern Chinese Han population. Front Psychiatry. 2018;9:657. doi:10.3389/fpsyt.2018.00657
7. Greene C, Kealy J, Humphries MM, et al. Dose-dependent expression of claudin-5 is a modifying factor in schizophrenia. Mol Psychiatry. 2018;23(11):2156-2166. doi:10.1038/MP.2017.156
8. Greene C, Hanley N, Campbell M. Blood-brain barrier associated tight junction disruption is a hallmark feature of major psychiatric disorders. Transl Psychiatry. 2020;10(1):373. doi:10.1038/s41398-020-01054-3
9. Kakogiannos N, Ferrari L, Giampietro C, et al. JAM-A acts via C/EBP-α to promote claudin-5 expression and enhance endothelial barrier function. Circ Res. 2020:1056-1073. doi:10.1161/CIRCRESAHA.120.316742
10. Erickson MA, Dohi K, Banks WA. Neuroinflammation: a common pathway in CNS diseases as mediated at the blood-brain barrier. Neuroimmunomodulation. 2012;19(2):121-130. doi:10.1159/000330247
11. Ao LY, Yan YY, Zhou L, et al. Immune cells after ischemic stroke onset: roles, migration, and target intervention. J Mol Neurosci. 2018;66(3):342-355. doi:10.1007/s12031-018-1173-4
12. Meixensberger S, Kuzior H, Fiebich BL, et al. Upregulation of sICAM-1 and sVCAM-1 levels in the cerebrospinal fluid of patients with schizophrenia spectrum disorders. Diagnostics (Basel). 2021;11(7):1134. doi:10.3390diagnostics11071134
13. Cai HQ, Catts VS, Webster MJ, et al. Increased macrophages and changed brain endothelial cell gene expression in the frontal cortex of people with schizophrenia displaying inflammation. Mol Psychiatry. 2020;25(4):761-775. doi:10.1038/s41380-018-0235-x
14. Greene C, Hanley N, Reschke CR, et al. Microvascular stabilization via blood-brain barrier regulation prevents seizure activity. Nat Commun. 2022;13(1):2003. doi:10.1038/s41467-022-29657-y
15. García-Juárez M, Camacho-Morales A. Defining the role of anti- and pro-inflammatory outcomes of interleukin-6 in mental health. Neuroscience. 2022;492:32-46. doi:10.1016/j.neuroscience.2022.03.020
16. Pong S, Karmacharya R, Sofman M, et al. The role of brain microvascular endothelial cell and blood-brain barrier dysfunction in schizophrenia. Complex Psychiatry. 2020;6(1-2):30-46. doi:10.1159/000511552
17. Patel A, Zhu Y, Kuzhikandathil EV, et al. Soluble interleukin-6 receptor induces motor stereotypies and co-localizes with gp130 in regions linked to cortico-striato-thalamo-cortical circuits. PLoS One. 2012;7(7): e41623. doi:10.1371/journal.pone.0041623
18. Schlaaff K, Dobrowolny H, Frodl T, et al. Increased densities of T and B lymphocytes indicate neuroinflammation in subgroups of schizophrenia and mood disorder patients. Brain Behav Immun. 2020;88:497-506. doi:10.1016/j.bbi.2020.04.021
19. Busse S, Busse M, Schiltz K, et al. Different distribution patterns of lymphocytes and microglia in the hippocampus of patients with residual versus paranoid schizophrenia: further evidence for disease course-related immune alterations? Brain Behav Immun. 2012;26(8):1273-1279. doi:10.1016/j.bbi.2012.08.005
20. Na KS, Jung HY, Kim YK. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:277-286. doi:10.1016/j.pnpbp.2012.10.022
21. Conen S, Gregory CJ, Hinz R, et al. Neuroinflammation as measured by positron emission tomography in patients with recent onset and established schizophrenia: implications for immune pathogenesis. Mol Psychiatry. 2021;26(9):5398-5406. doi:10.1038/S41380-020-0829-Y
22. Najjar S, Pahlajani S, De Sanctis V, et al. Neurovascular unit dysfunction and blood-brain barrier hyperpermeability contribute to schizophrenia neurobiology: a theoretical integration of clinical and experimental evidence. Front Psychiatry. 2017;8:83. doi:10.3389/fpsyt.2017.00083
23. Pinjari OF, Dasgupta SK, Okusaga OO. Plasma soluble P-selectin, interleukin-6 and S100B protein in patients with schizophrenia: a pilot study. Psychiatr Q. 2022;93(1):335-345. doi:10.1007/s11126-021-09954-3
24. Di Biase MA, Zalesky A, O’keefe G, et al. PET imaging of putative microglial activation in individuals at ultra-high risk for psychosis, recently diagnosed and chronically ill with schizophrenia. Transl Psychiatry. 2017;7(8):e1225. doi:10.1038/tp.2017.193
25. Holmes SE, Hinz R, Drake RJ, et al. In vivo imaging of brain microglial activity in antipsychotic-free and medicated schizophrenia: a [11C](R)-PK11195 positron emission tomography study. Mol Psychiatry. 2016;21(12):1672-1679. doi:10.1038/mp.2016.180
26. Corsi-Zuelli F, Deakin B, de Lima MHF, et al. T regulatory cells as a potential therapeutic target in psychosis? Current challenges and future perspectives. Brain Behav Immun Health. 2021;17:100330. doi:10.1016/j.bbih.2021.100330
27. Bannai D, Lutz O, Lizano P. Neuroimaging considerations when investigating choroid plexus morphology in idiopathic psychosis. Schizophr Res. 2020;224:19-21. doi:10.1016/j.schres.2020.07.013
28. Hladky SB, Barrand MA. Fluid and ion transfer across the blood-brain and blood-cerebrospinal fluid barriers; a comparative account of mechanisms and roles. Fluids Barriers CNS. 2016;13(1):19. doi:10.1186/s12987-016-0040-3
29. Lizano P, Lutz O, Ling G, et al. Association of choroid plexus enlargement with cognitive, inflammatory, and structural phenotypes across the psychosis spectrum. Am J Psychiatry. 2019;176(7):564-572. doi:10.1176/appi.ajp.2019.18070825
30. Castellani G, Contarini G, Mereu M, et al. Dopamine-mediated immunomodulation affects choroid plexus function. Brain Behav Immun. 2019;81:138-150. doi:10.1016/j.bbi.2019.06.006
31. Bitanihirwe BKY, Lizano P, Woo TW. Deconstructing the functional neuroanatomy of the choroid plexus: an ontogenetic perspective for studying neurodevelopmental and neuropsychiatric disorders. Mol Psychiatry. 2022;1-10. doi:10.1038/s41380-022-01623-6