User login
The clinical utility of newly approved angiogenic markers for identifying patients at risk for adverse outcomes due to preeclampsia
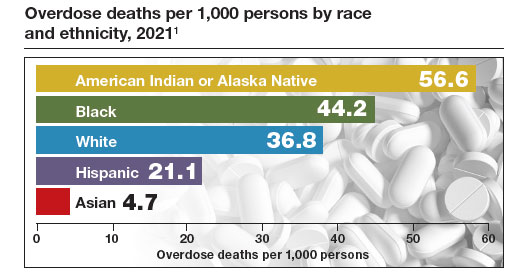
In the United States there is an epidemic of hypertensive disorders in pregnancy, with 16% of pregnant people being diagnosed with preeclampsia, gestational hypertension, chronic hypertension, preeclampsia superimposed on chronic hypertension, HELLP, or eclampsia.1 Preeclampsia with severe features increases the maternal risk for stroke, pulmonary edema, kidney injury, abruption, and fetal and maternal death. Preeclampsia also increases the fetal risk for growth restriction, oligohydramnios, and preterm birth.
Angiogenic factors and the pathophysiology of preeclampsia—From bench to bedside
The pathophysiology of preeclampsia is not fully characterized, but a leading theory is that placental ischemia causes increased placental production of anti-angiogenesis factors and a decrease in placental production of pro-angiogenesis factors.2-4 Clinical studies support the theory that preeclampsia is associated with an increase in placental production of anti-angiogenesis factors, including soluble fms-like tyrosine kinase 1 (sFlt-1) and soluble endoglin, and a decrease in the placental production of pro-angiogenesis factors, including placental growth factor (PlGF).5-15
The US Food and Drug Administration (FDA) has recently approved an assay for the measurement of sFlt-1 (Brahms sFlt-1 Kryptor) and PlGF (Brahms sFlt-1 Kryptor) (Thermo Fisher Scientific; Waltham, Massachusetts).16 This editorial focuses on the current and evolving indications for the measurement of sFlt-1 and PlGF in obstetric practice.
FDA approval of a preeclampsia blood test
The FDA approval of the tests to measure sFlt-1 and PlGF is narrowly tailored and focused on using the sFlt-1/PlGF ratio to assess the risk of progression to preeclampsia with severe features within 2 weeks among hospitalized patients with a hypertensive disorder in pregnancy with a singleton pregnancy between 23 weeks 0 days (23w0d) and 34w6d gestation.16 The test is meant to be used in conjunction with other laboratory tests and clinical assessment. The FDA advises that the test results should not be used to diagnose preeclampsia, nor should they be used to determine the timing of delivery or timing of patient discharge.16 The sFlt-1 and PIGF measurements are both reported as pg/mL, and the sFlt-1/PlGF ratio has no units.
The FDA approval is based on clinical studies that demonstrate the effectiveness of the test in predicting the progression of a hypertensive disorder in pregnancy to preeclampsia with severe features within 2 weeks of testing. In one study, the sFlt-1/PlGF ratio was measured in 556 pregnant patients with a singleton pregnancy who were between 23w0d and 34w6d gestation and hospitalized with a hypertensivedisorder in pregnancy without severe features at study enrollment.15 Those patients receiving intravenous heparin were excluded because of the effect of heparin on sFlt-1 levels. Participants’ mean age was 31.7 years, and their mean gestational age was 30w3d. The patients’ mean body mass index (BMI) was 34.2 kg/m2, with mean maximal blood pressure (BP) at enrollment of 159 mm Hg systolic and 95 mm Hg diastolic.
In this cohort, 31% of enrolled patients progressed to preeclampsia with severe features within 2 weeks. At enrollment, the median sFlt-1/PlGF ratio was greater among the patients who progressed to preeclampsia with severe features than among those who did not have progression to preeclampsia with severe features (291 vs 7). An elevated sFlt-1/PlGF ratio (determined to be a ratio ≥ 40) predicted that patients would progress to severe preeclampsiawith severe features—with positive and negative predictive values of 65% and 96%, respectively. Among the subgroup of patients with a history of chronic hypertension, an sFlt-1/PlGF ratio ≥ 40 had positive and negative predictive values of 59% and 94%, respectively. Focusing the analysis on patients who self-reported their race as Black, representing 30% of the cohort, the positive and negative predictive values for a sFlt-1/PlGF ratio ≥ 40 were 66% and 99%, respectively.15
Receiver-operating curve analyses were used to compare the predictive performance of sFlt-1/PlGF measurement versus standard clinical factors and standard laboratory results, including systolic and diastolic BP; levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and creatinine; and platelet count.15 The area under the curve for predicting progression to preeclampsia with severe features was much greater for the sFlt-1/PlGF test (0.92) than for systolic (0.67) and diastolic BP (0.70), AST level (0.66), ALT level (0.61), creatinine level (0.65), and platelet count (0.57).15 These results demonstrate that measuring sFlt-1/PlGF ratios is a much better way to predict the progression of preeclampsia to severe disease than measuring standard clinical and laboratory results.
Patients with a sFlt-1/PlGF ratio ≥ 40 had higher rates of adverse maternal outcomes including severe hypertension, abruption, stroke, eclampsia, pulmonary edema, thrombocytopenia, low platelets, and/or coagulation disorder, than those patients with a ratio < 40, (16.1% vs 2.8%, respectively; relative risk [RR], 5.8; 95% confidence interval [CI], 2.8 to 12.2).15 Adverse fetal and neonatal outcomes (including fetal death, small for gestational age and early delivery due to progression of disease) were more common among patients with a sFlt-1/PlGF ratio of ≥ 40 (80% vs 26%; RR, 3.1; 95% CI, 2.5–3.8).15 Many other studies support the hypothesis that the sFlt-1/PlGF ratio is predictive of adverse outcomes among patients with hypertensive disorders in pregnancy.6-15
Applying the bottom-line study findings. Patients with a hypertensive disorder in pregnancy and a sFlt-1/PlGF ratio < 40 have a low risk of progressing to preeclampsia with severe features over the following 2 weeks, with a negative predictive value of 96%. The remarkably high negative predictive value of a sFlt-1/PlGF ratio < 40 will help obstetricians generate a care plan that optimizes the use of limited health care resources. Conversely, about two-thirds of patients with a hypertensive disorder in pregnancy and a sFlt-1/PlGF test ≥ 40 will progress to preeclampsia with severe features and may need to prepare for a preterm delivery.
Continue to: Clinical utility of the sFlt-1/PlGF ratio in obstetric triage...
Clinical utility of the sFlt-1/PlGF ratio in obstetric triage
Measurement of the sFlt-1/PlGF ratio may help guide clinical care among patients referred to obstetric triage or admitted to the hospital for the evaluation of suspected preeclampsia. In one study, 402 patients with a singleton pregnancy referred to the hospital for evaluation of suspected preeclampsia, had a standard evaluation plus measurement of an sFlt-1/PlGF ratio.13 The clinicians caring for the patients did not have access to the sFlt-1/PlGF test results. In this cohort, 16% of the patients developed preeclampsia with severe features in the 2 weeks following the initial assessment in triage. In this cohort, a normal sFlt-1/PlGF ratio reliably predicted which patients were not going to develop preeclampsia with severe features over the following 2 weeks, with a negative predictive value of 98%. Among the patients with an elevated sFlt-1/PlGF ratio, however, the positive predictive value of the test was 47% for developing preeclampsia with severe features within the 2 weeks following initial evaluation. Among patients < 34 weeks’ gestation, an elevated sFlt-1/PlGF ratio had a positive predictive value of 65%, and a negative predictive value of 98%. Other studies also have reported that the sFlt-1/PlGF ratio is of value for assessing the risk for progression to preeclampsia with severe features in patients being evaluated for suspected preeclampsia.6,17,18
In obstetric triage, it is difficult to predict the clinical course of patients referred for the evaluation of suspected preeclampsia based on BP measurements or standard laboratory tests. The sFlt-1/PlGF test will help clinicians identify patients at low and high risk of progressing to preeclampsia with severe features.19 Patients with a normal sFlt-1/PlGF test are at low risk of developing preeclampsia with severe features over the following 2 weeks. Patients with an elevated sFlt-1/PlGF test are at higher risk of progressing to preeclampsia with severe features and may warrant more intensive obstetric care. An enhanced care program might include:
- patient education
- remote monitoring of BP or hospitalization
- more frequent assessment of fetal well-being and growth
- administration of glucocorticoids to advance fetal maturity, if indicated by the gestational age.
Twin pregnancy complicated by preeclampsia
Twin pregnancy is associated with a high risk of developing preeclampsia and fetal growth restriction. For patients with a twin pregnancy and a hypertensive disorder in pregnancy, an elevated sFlt-1/PlGF ratio is associated with the need for delivery within 2 weeks and an increased rate of adverse maternal and neonatal outcomes. In a retrospective study involving 164 patients with twin pregnancy first evaluated for suspected preeclampsia at a median gestational age of 33w4d, the sFlt-1/PlGF ratio was positively correlated with progression of preeclampsia without severe features to severe features within 2 weeks.20 In this cohort, at the initial evaluation for suspected preeclampsia, the sFlt-1/PlGF ratio was lower among patients who did not need delivery within 2 weeks compared with those who were delivered within 2 weeks, 24 versus 84 (P<.001). The mean sFlt-1/PlGF ratio was 99 among patients who needed delivery within 1 week following the initial evaluation for suspected preeclampsia. Among patients who delivered within 1 week of presentation, the reasons for delivery were the development of severe hypertension, severe dyspnea, placental abruption, rising levels of serum liver function enzymes, and/or onset of the HELLP syndrome.
An important finding in this study is that a normal
The sFlt-1/PlGF test is a welcome addition to OB care
FDA approval of laboratory tests to measure circulating levels of sFlt-1 and PlGF will advance obstetric practice by identifying patients with a hypertensive disorder in pregnancy who are at low and high risk of developing preeclampsia with severe features within 2 weeks of the test. No laboratory test can replace the clinical judgment of obstetricians who are responsible for balancing the maternal and fetal risks that can occur in the management of a patient with a hypertensive disorder in pregnancy. The
- Ford ND, Cox S, Ko JY, et al. Hypertensive disorders in pregnancy and mortality at delivery hospitalization-United States 2017-2019. Morb Mortal Week Report. 2022;71:585-591.
- Nagamatsu T, Fujii T, Kusumi M, et al. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: an implication for placental vascular development and the pathophysiology of preeclampsia. Endocrinology. 2004;145:4838-4445.
- Rana S, Lemoine E, Granger JP, et al. Preeclampsia: pathophysiology, challenges and perspectives. Circ Res. 2019;124:1094-1112.
- Rana S, Burke SD, Karumanchi SA. Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders. Am J Obstet Gynecol. 2022(2S):S1019-S1034.
- Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672-683.
- Chaiworapongsa T, Romero R, Savasan ZA, et al. Maternal plasma concentrations of angiogenic/ anti-angiogenic factors are of prognostic value in patients presenting to the obstetrical triage area with the suspicion of preeclampsia. J Matern Fetal Neonatal Med. 2011;24:1187-1207.
- Rana S, Powe CE, Salahuddin S, et al. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation. 2012;125:911-919.
- Moore AG, Young H, Keller JM, et al. Angiogenic biomarkers for prediction of maternal and neonatal complications in suspected preeclampsia. J Matern Fetal Neonatal Med. 2012;25:2651-2657.
- Verlohren S, Herraiz I, Lapaire O, et al. The sFlt-1/ PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol. 2012;206:58.e1-e8.
- Verlohren S, Herraiz I, Lapaire O, et al. New gestational phase-specific cutoff values for the use of soluble fms-like tyrosine kinase-1/placental growth factor ratio as a diagnostic test for preeclampsia. Hypertension. 2014;63:346-352.
- Zeisler H, Llurba E, Chantraine F, et al. Predictive value of the sFlt-1/PlGF ratio in women with suspected preeclampsia. N Engl J Med. 2016;374:1322.
- Duckworth S, Griffin M, Seed PT, et al. Diagnostic biomarkers in women with suspected preeclampsia in a prospective multicenter study. Obstet Gynecol. 2016;128:245-252.
- Rana S, Salahuddin S, Mueller A, et al. Angiogenic biomarkers in triage and risk for preeclampsia with severe features. Pregnancy Hyertens. 2018;13:100-106.
- Bian X, Biswas A, Huang X, et al. Short-term prediction of adverse outcomes using the sFlt-1/PlGF ratio in Asian women with suspected preeclampsia. Hypertension. 2019;74:164-172.
- Thadhani R, Lemoine E, Rana S, et al. Circulating angiogenic factor levels in hypertensive disorders of pregnancy. N Engl J Med Evidence. 2022. doi 10.1056/EVIDoa2200161.
- US Food and Drug Administration. FDA approval letter for an assay to measure sFlt-1 and PlGF. May 18, 2023. https://www.accessdata.fda.gov/cdrh _docs/pdf22/DEN220027.pdf
- Chaiworapongsa T, Romero R, Korzeniewski SJ, et al. Plasma concentrations of angiogenic/ anti-angiogenic factors have prognostic value in women presenting with suspected preeclampsia to the obstetrical triage area: a prospective study. J Matern Fetal Neonatal Med. 2014;27:132-144.
- Palomaki GE, Haddow JE, Haddow HR, et al. Modeling risk for severe adverse outcomes using angiogenic factor measurements in women with suspected preterm preeclampsia. Prenat Diagn. 2015;35:386-393.
- Verlohren S, Brennecke SP, Galindo A, et al. Clinical interpretation and implementation of the sFlt-1/PlGF ratio in the prediction, diagnosis and management of preeclampsia. Pregnancy Hyper. 2022;27:42-50.
- Binder J, Palmrich P, Pateisky P, et al. The prognostic value of angiogenic markers in twin pregnancies to predict delivery due to maternal complications of preeclampsia. Hypertension. 2020;76:176-183.
- Sapantzoglou I, Rouvali A, Koutras A, et al. sFlt-1, PlGF, the sFlt-1/PlGF ratio and their association with pre-eclampsia in twin pregnancies- a review of the literature. Medicina. 2023;59:1232.
- Satorres E, Martinez-Varea A, Diago-Almela V. sFlt-1/PlGF ratio as a predictor of pregnancy outcomes in twin pregnancies: a systematic review. J Matern Fetal Neonatal Med. 2023;36:2230514.
- Rana S, Hacker MR, Modest AM, et al. Circulating angiogenic factors and risk of adverse maternal and perinatal outcomes in twin pregnancies with suspected preeclampsia. Hypertension. 2012;60:451-458.

In the United States there is an epidemic of hypertensive disorders in pregnancy, with 16% of pregnant people being diagnosed with preeclampsia, gestational hypertension, chronic hypertension, preeclampsia superimposed on chronic hypertension, HELLP, or eclampsia.1 Preeclampsia with severe features increases the maternal risk for stroke, pulmonary edema, kidney injury, abruption, and fetal and maternal death. Preeclampsia also increases the fetal risk for growth restriction, oligohydramnios, and preterm birth.
Angiogenic factors and the pathophysiology of preeclampsia—From bench to bedside
The pathophysiology of preeclampsia is not fully characterized, but a leading theory is that placental ischemia causes increased placental production of anti-angiogenesis factors and a decrease in placental production of pro-angiogenesis factors.2-4 Clinical studies support the theory that preeclampsia is associated with an increase in placental production of anti-angiogenesis factors, including soluble fms-like tyrosine kinase 1 (sFlt-1) and soluble endoglin, and a decrease in the placental production of pro-angiogenesis factors, including placental growth factor (PlGF).5-15
The US Food and Drug Administration (FDA) has recently approved an assay for the measurement of sFlt-1 (Brahms sFlt-1 Kryptor) and PlGF (Brahms sFlt-1 Kryptor) (Thermo Fisher Scientific; Waltham, Massachusetts).16 This editorial focuses on the current and evolving indications for the measurement of sFlt-1 and PlGF in obstetric practice.
FDA approval of a preeclampsia blood test
The FDA approval of the tests to measure sFlt-1 and PlGF is narrowly tailored and focused on using the sFlt-1/PlGF ratio to assess the risk of progression to preeclampsia with severe features within 2 weeks among hospitalized patients with a hypertensive disorder in pregnancy with a singleton pregnancy between 23 weeks 0 days (23w0d) and 34w6d gestation.16 The test is meant to be used in conjunction with other laboratory tests and clinical assessment. The FDA advises that the test results should not be used to diagnose preeclampsia, nor should they be used to determine the timing of delivery or timing of patient discharge.16 The sFlt-1 and PIGF measurements are both reported as pg/mL, and the sFlt-1/PlGF ratio has no units.
The FDA approval is based on clinical studies that demonstrate the effectiveness of the test in predicting the progression of a hypertensive disorder in pregnancy to preeclampsia with severe features within 2 weeks of testing. In one study, the sFlt-1/PlGF ratio was measured in 556 pregnant patients with a singleton pregnancy who were between 23w0d and 34w6d gestation and hospitalized with a hypertensivedisorder in pregnancy without severe features at study enrollment.15 Those patients receiving intravenous heparin were excluded because of the effect of heparin on sFlt-1 levels. Participants’ mean age was 31.7 years, and their mean gestational age was 30w3d. The patients’ mean body mass index (BMI) was 34.2 kg/m2, with mean maximal blood pressure (BP) at enrollment of 159 mm Hg systolic and 95 mm Hg diastolic.
In this cohort, 31% of enrolled patients progressed to preeclampsia with severe features within 2 weeks. At enrollment, the median sFlt-1/PlGF ratio was greater among the patients who progressed to preeclampsia with severe features than among those who did not have progression to preeclampsia with severe features (291 vs 7). An elevated sFlt-1/PlGF ratio (determined to be a ratio ≥ 40) predicted that patients would progress to severe preeclampsiawith severe features—with positive and negative predictive values of 65% and 96%, respectively. Among the subgroup of patients with a history of chronic hypertension, an sFlt-1/PlGF ratio ≥ 40 had positive and negative predictive values of 59% and 94%, respectively. Focusing the analysis on patients who self-reported their race as Black, representing 30% of the cohort, the positive and negative predictive values for a sFlt-1/PlGF ratio ≥ 40 were 66% and 99%, respectively.15
Receiver-operating curve analyses were used to compare the predictive performance of sFlt-1/PlGF measurement versus standard clinical factors and standard laboratory results, including systolic and diastolic BP; levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and creatinine; and platelet count.15 The area under the curve for predicting progression to preeclampsia with severe features was much greater for the sFlt-1/PlGF test (0.92) than for systolic (0.67) and diastolic BP (0.70), AST level (0.66), ALT level (0.61), creatinine level (0.65), and platelet count (0.57).15 These results demonstrate that measuring sFlt-1/PlGF ratios is a much better way to predict the progression of preeclampsia to severe disease than measuring standard clinical and laboratory results.
Patients with a sFlt-1/PlGF ratio ≥ 40 had higher rates of adverse maternal outcomes including severe hypertension, abruption, stroke, eclampsia, pulmonary edema, thrombocytopenia, low platelets, and/or coagulation disorder, than those patients with a ratio < 40, (16.1% vs 2.8%, respectively; relative risk [RR], 5.8; 95% confidence interval [CI], 2.8 to 12.2).15 Adverse fetal and neonatal outcomes (including fetal death, small for gestational age and early delivery due to progression of disease) were more common among patients with a sFlt-1/PlGF ratio of ≥ 40 (80% vs 26%; RR, 3.1; 95% CI, 2.5–3.8).15 Many other studies support the hypothesis that the sFlt-1/PlGF ratio is predictive of adverse outcomes among patients with hypertensive disorders in pregnancy.6-15
Applying the bottom-line study findings. Patients with a hypertensive disorder in pregnancy and a sFlt-1/PlGF ratio < 40 have a low risk of progressing to preeclampsia with severe features over the following 2 weeks, with a negative predictive value of 96%. The remarkably high negative predictive value of a sFlt-1/PlGF ratio < 40 will help obstetricians generate a care plan that optimizes the use of limited health care resources. Conversely, about two-thirds of patients with a hypertensive disorder in pregnancy and a sFlt-1/PlGF test ≥ 40 will progress to preeclampsia with severe features and may need to prepare for a preterm delivery.
Continue to: Clinical utility of the sFlt-1/PlGF ratio in obstetric triage...
Clinical utility of the sFlt-1/PlGF ratio in obstetric triage
Measurement of the sFlt-1/PlGF ratio may help guide clinical care among patients referred to obstetric triage or admitted to the hospital for the evaluation of suspected preeclampsia. In one study, 402 patients with a singleton pregnancy referred to the hospital for evaluation of suspected preeclampsia, had a standard evaluation plus measurement of an sFlt-1/PlGF ratio.13 The clinicians caring for the patients did not have access to the sFlt-1/PlGF test results. In this cohort, 16% of the patients developed preeclampsia with severe features in the 2 weeks following the initial assessment in triage. In this cohort, a normal sFlt-1/PlGF ratio reliably predicted which patients were not going to develop preeclampsia with severe features over the following 2 weeks, with a negative predictive value of 98%. Among the patients with an elevated sFlt-1/PlGF ratio, however, the positive predictive value of the test was 47% for developing preeclampsia with severe features within the 2 weeks following initial evaluation. Among patients < 34 weeks’ gestation, an elevated sFlt-1/PlGF ratio had a positive predictive value of 65%, and a negative predictive value of 98%. Other studies also have reported that the sFlt-1/PlGF ratio is of value for assessing the risk for progression to preeclampsia with severe features in patients being evaluated for suspected preeclampsia.6,17,18
In obstetric triage, it is difficult to predict the clinical course of patients referred for the evaluation of suspected preeclampsia based on BP measurements or standard laboratory tests. The sFlt-1/PlGF test will help clinicians identify patients at low and high risk of progressing to preeclampsia with severe features.19 Patients with a normal sFlt-1/PlGF test are at low risk of developing preeclampsia with severe features over the following 2 weeks. Patients with an elevated sFlt-1/PlGF test are at higher risk of progressing to preeclampsia with severe features and may warrant more intensive obstetric care. An enhanced care program might include:
- patient education
- remote monitoring of BP or hospitalization
- more frequent assessment of fetal well-being and growth
- administration of glucocorticoids to advance fetal maturity, if indicated by the gestational age.
Twin pregnancy complicated by preeclampsia
Twin pregnancy is associated with a high risk of developing preeclampsia and fetal growth restriction. For patients with a twin pregnancy and a hypertensive disorder in pregnancy, an elevated sFlt-1/PlGF ratio is associated with the need for delivery within 2 weeks and an increased rate of adverse maternal and neonatal outcomes. In a retrospective study involving 164 patients with twin pregnancy first evaluated for suspected preeclampsia at a median gestational age of 33w4d, the sFlt-1/PlGF ratio was positively correlated with progression of preeclampsia without severe features to severe features within 2 weeks.20 In this cohort, at the initial evaluation for suspected preeclampsia, the sFlt-1/PlGF ratio was lower among patients who did not need delivery within 2 weeks compared with those who were delivered within 2 weeks, 24 versus 84 (P<.001). The mean sFlt-1/PlGF ratio was 99 among patients who needed delivery within 1 week following the initial evaluation for suspected preeclampsia. Among patients who delivered within 1 week of presentation, the reasons for delivery were the development of severe hypertension, severe dyspnea, placental abruption, rising levels of serum liver function enzymes, and/or onset of the HELLP syndrome.
An important finding in this study is that a normal
The sFlt-1/PlGF test is a welcome addition to OB care
FDA approval of laboratory tests to measure circulating levels of sFlt-1 and PlGF will advance obstetric practice by identifying patients with a hypertensive disorder in pregnancy who are at low and high risk of developing preeclampsia with severe features within 2 weeks of the test. No laboratory test can replace the clinical judgment of obstetricians who are responsible for balancing the maternal and fetal risks that can occur in the management of a patient with a hypertensive disorder in pregnancy. The

In the United States there is an epidemic of hypertensive disorders in pregnancy, with 16% of pregnant people being diagnosed with preeclampsia, gestational hypertension, chronic hypertension, preeclampsia superimposed on chronic hypertension, HELLP, or eclampsia.1 Preeclampsia with severe features increases the maternal risk for stroke, pulmonary edema, kidney injury, abruption, and fetal and maternal death. Preeclampsia also increases the fetal risk for growth restriction, oligohydramnios, and preterm birth.
Angiogenic factors and the pathophysiology of preeclampsia—From bench to bedside
The pathophysiology of preeclampsia is not fully characterized, but a leading theory is that placental ischemia causes increased placental production of anti-angiogenesis factors and a decrease in placental production of pro-angiogenesis factors.2-4 Clinical studies support the theory that preeclampsia is associated with an increase in placental production of anti-angiogenesis factors, including soluble fms-like tyrosine kinase 1 (sFlt-1) and soluble endoglin, and a decrease in the placental production of pro-angiogenesis factors, including placental growth factor (PlGF).5-15
The US Food and Drug Administration (FDA) has recently approved an assay for the measurement of sFlt-1 (Brahms sFlt-1 Kryptor) and PlGF (Brahms sFlt-1 Kryptor) (Thermo Fisher Scientific; Waltham, Massachusetts).16 This editorial focuses on the current and evolving indications for the measurement of sFlt-1 and PlGF in obstetric practice.
FDA approval of a preeclampsia blood test
The FDA approval of the tests to measure sFlt-1 and PlGF is narrowly tailored and focused on using the sFlt-1/PlGF ratio to assess the risk of progression to preeclampsia with severe features within 2 weeks among hospitalized patients with a hypertensive disorder in pregnancy with a singleton pregnancy between 23 weeks 0 days (23w0d) and 34w6d gestation.16 The test is meant to be used in conjunction with other laboratory tests and clinical assessment. The FDA advises that the test results should not be used to diagnose preeclampsia, nor should they be used to determine the timing of delivery or timing of patient discharge.16 The sFlt-1 and PIGF measurements are both reported as pg/mL, and the sFlt-1/PlGF ratio has no units.
The FDA approval is based on clinical studies that demonstrate the effectiveness of the test in predicting the progression of a hypertensive disorder in pregnancy to preeclampsia with severe features within 2 weeks of testing. In one study, the sFlt-1/PlGF ratio was measured in 556 pregnant patients with a singleton pregnancy who were between 23w0d and 34w6d gestation and hospitalized with a hypertensivedisorder in pregnancy without severe features at study enrollment.15 Those patients receiving intravenous heparin were excluded because of the effect of heparin on sFlt-1 levels. Participants’ mean age was 31.7 years, and their mean gestational age was 30w3d. The patients’ mean body mass index (BMI) was 34.2 kg/m2, with mean maximal blood pressure (BP) at enrollment of 159 mm Hg systolic and 95 mm Hg diastolic.
In this cohort, 31% of enrolled patients progressed to preeclampsia with severe features within 2 weeks. At enrollment, the median sFlt-1/PlGF ratio was greater among the patients who progressed to preeclampsia with severe features than among those who did not have progression to preeclampsia with severe features (291 vs 7). An elevated sFlt-1/PlGF ratio (determined to be a ratio ≥ 40) predicted that patients would progress to severe preeclampsiawith severe features—with positive and negative predictive values of 65% and 96%, respectively. Among the subgroup of patients with a history of chronic hypertension, an sFlt-1/PlGF ratio ≥ 40 had positive and negative predictive values of 59% and 94%, respectively. Focusing the analysis on patients who self-reported their race as Black, representing 30% of the cohort, the positive and negative predictive values for a sFlt-1/PlGF ratio ≥ 40 were 66% and 99%, respectively.15
Receiver-operating curve analyses were used to compare the predictive performance of sFlt-1/PlGF measurement versus standard clinical factors and standard laboratory results, including systolic and diastolic BP; levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and creatinine; and platelet count.15 The area under the curve for predicting progression to preeclampsia with severe features was much greater for the sFlt-1/PlGF test (0.92) than for systolic (0.67) and diastolic BP (0.70), AST level (0.66), ALT level (0.61), creatinine level (0.65), and platelet count (0.57).15 These results demonstrate that measuring sFlt-1/PlGF ratios is a much better way to predict the progression of preeclampsia to severe disease than measuring standard clinical and laboratory results.
Patients with a sFlt-1/PlGF ratio ≥ 40 had higher rates of adverse maternal outcomes including severe hypertension, abruption, stroke, eclampsia, pulmonary edema, thrombocytopenia, low platelets, and/or coagulation disorder, than those patients with a ratio < 40, (16.1% vs 2.8%, respectively; relative risk [RR], 5.8; 95% confidence interval [CI], 2.8 to 12.2).15 Adverse fetal and neonatal outcomes (including fetal death, small for gestational age and early delivery due to progression of disease) were more common among patients with a sFlt-1/PlGF ratio of ≥ 40 (80% vs 26%; RR, 3.1; 95% CI, 2.5–3.8).15 Many other studies support the hypothesis that the sFlt-1/PlGF ratio is predictive of adverse outcomes among patients with hypertensive disorders in pregnancy.6-15
Applying the bottom-line study findings. Patients with a hypertensive disorder in pregnancy and a sFlt-1/PlGF ratio < 40 have a low risk of progressing to preeclampsia with severe features over the following 2 weeks, with a negative predictive value of 96%. The remarkably high negative predictive value of a sFlt-1/PlGF ratio < 40 will help obstetricians generate a care plan that optimizes the use of limited health care resources. Conversely, about two-thirds of patients with a hypertensive disorder in pregnancy and a sFlt-1/PlGF test ≥ 40 will progress to preeclampsia with severe features and may need to prepare for a preterm delivery.
Continue to: Clinical utility of the sFlt-1/PlGF ratio in obstetric triage...
Clinical utility of the sFlt-1/PlGF ratio in obstetric triage
Measurement of the sFlt-1/PlGF ratio may help guide clinical care among patients referred to obstetric triage or admitted to the hospital for the evaluation of suspected preeclampsia. In one study, 402 patients with a singleton pregnancy referred to the hospital for evaluation of suspected preeclampsia, had a standard evaluation plus measurement of an sFlt-1/PlGF ratio.13 The clinicians caring for the patients did not have access to the sFlt-1/PlGF test results. In this cohort, 16% of the patients developed preeclampsia with severe features in the 2 weeks following the initial assessment in triage. In this cohort, a normal sFlt-1/PlGF ratio reliably predicted which patients were not going to develop preeclampsia with severe features over the following 2 weeks, with a negative predictive value of 98%. Among the patients with an elevated sFlt-1/PlGF ratio, however, the positive predictive value of the test was 47% for developing preeclampsia with severe features within the 2 weeks following initial evaluation. Among patients < 34 weeks’ gestation, an elevated sFlt-1/PlGF ratio had a positive predictive value of 65%, and a negative predictive value of 98%. Other studies also have reported that the sFlt-1/PlGF ratio is of value for assessing the risk for progression to preeclampsia with severe features in patients being evaluated for suspected preeclampsia.6,17,18
In obstetric triage, it is difficult to predict the clinical course of patients referred for the evaluation of suspected preeclampsia based on BP measurements or standard laboratory tests. The sFlt-1/PlGF test will help clinicians identify patients at low and high risk of progressing to preeclampsia with severe features.19 Patients with a normal sFlt-1/PlGF test are at low risk of developing preeclampsia with severe features over the following 2 weeks. Patients with an elevated sFlt-1/PlGF test are at higher risk of progressing to preeclampsia with severe features and may warrant more intensive obstetric care. An enhanced care program might include:
- patient education
- remote monitoring of BP or hospitalization
- more frequent assessment of fetal well-being and growth
- administration of glucocorticoids to advance fetal maturity, if indicated by the gestational age.
Twin pregnancy complicated by preeclampsia
Twin pregnancy is associated with a high risk of developing preeclampsia and fetal growth restriction. For patients with a twin pregnancy and a hypertensive disorder in pregnancy, an elevated sFlt-1/PlGF ratio is associated with the need for delivery within 2 weeks and an increased rate of adverse maternal and neonatal outcomes. In a retrospective study involving 164 patients with twin pregnancy first evaluated for suspected preeclampsia at a median gestational age of 33w4d, the sFlt-1/PlGF ratio was positively correlated with progression of preeclampsia without severe features to severe features within 2 weeks.20 In this cohort, at the initial evaluation for suspected preeclampsia, the sFlt-1/PlGF ratio was lower among patients who did not need delivery within 2 weeks compared with those who were delivered within 2 weeks, 24 versus 84 (P<.001). The mean sFlt-1/PlGF ratio was 99 among patients who needed delivery within 1 week following the initial evaluation for suspected preeclampsia. Among patients who delivered within 1 week of presentation, the reasons for delivery were the development of severe hypertension, severe dyspnea, placental abruption, rising levels of serum liver function enzymes, and/or onset of the HELLP syndrome.
An important finding in this study is that a normal
The sFlt-1/PlGF test is a welcome addition to OB care
FDA approval of laboratory tests to measure circulating levels of sFlt-1 and PlGF will advance obstetric practice by identifying patients with a hypertensive disorder in pregnancy who are at low and high risk of developing preeclampsia with severe features within 2 weeks of the test. No laboratory test can replace the clinical judgment of obstetricians who are responsible for balancing the maternal and fetal risks that can occur in the management of a patient with a hypertensive disorder in pregnancy. The
- Ford ND, Cox S, Ko JY, et al. Hypertensive disorders in pregnancy and mortality at delivery hospitalization-United States 2017-2019. Morb Mortal Week Report. 2022;71:585-591.
- Nagamatsu T, Fujii T, Kusumi M, et al. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: an implication for placental vascular development and the pathophysiology of preeclampsia. Endocrinology. 2004;145:4838-4445.
- Rana S, Lemoine E, Granger JP, et al. Preeclampsia: pathophysiology, challenges and perspectives. Circ Res. 2019;124:1094-1112.
- Rana S, Burke SD, Karumanchi SA. Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders. Am J Obstet Gynecol. 2022(2S):S1019-S1034.
- Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672-683.
- Chaiworapongsa T, Romero R, Savasan ZA, et al. Maternal plasma concentrations of angiogenic/ anti-angiogenic factors are of prognostic value in patients presenting to the obstetrical triage area with the suspicion of preeclampsia. J Matern Fetal Neonatal Med. 2011;24:1187-1207.
- Rana S, Powe CE, Salahuddin S, et al. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation. 2012;125:911-919.
- Moore AG, Young H, Keller JM, et al. Angiogenic biomarkers for prediction of maternal and neonatal complications in suspected preeclampsia. J Matern Fetal Neonatal Med. 2012;25:2651-2657.
- Verlohren S, Herraiz I, Lapaire O, et al. The sFlt-1/ PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol. 2012;206:58.e1-e8.
- Verlohren S, Herraiz I, Lapaire O, et al. New gestational phase-specific cutoff values for the use of soluble fms-like tyrosine kinase-1/placental growth factor ratio as a diagnostic test for preeclampsia. Hypertension. 2014;63:346-352.
- Zeisler H, Llurba E, Chantraine F, et al. Predictive value of the sFlt-1/PlGF ratio in women with suspected preeclampsia. N Engl J Med. 2016;374:1322.
- Duckworth S, Griffin M, Seed PT, et al. Diagnostic biomarkers in women with suspected preeclampsia in a prospective multicenter study. Obstet Gynecol. 2016;128:245-252.
- Rana S, Salahuddin S, Mueller A, et al. Angiogenic biomarkers in triage and risk for preeclampsia with severe features. Pregnancy Hyertens. 2018;13:100-106.
- Bian X, Biswas A, Huang X, et al. Short-term prediction of adverse outcomes using the sFlt-1/PlGF ratio in Asian women with suspected preeclampsia. Hypertension. 2019;74:164-172.
- Thadhani R, Lemoine E, Rana S, et al. Circulating angiogenic factor levels in hypertensive disorders of pregnancy. N Engl J Med Evidence. 2022. doi 10.1056/EVIDoa2200161.
- US Food and Drug Administration. FDA approval letter for an assay to measure sFlt-1 and PlGF. May 18, 2023. https://www.accessdata.fda.gov/cdrh _docs/pdf22/DEN220027.pdf
- Chaiworapongsa T, Romero R, Korzeniewski SJ, et al. Plasma concentrations of angiogenic/ anti-angiogenic factors have prognostic value in women presenting with suspected preeclampsia to the obstetrical triage area: a prospective study. J Matern Fetal Neonatal Med. 2014;27:132-144.
- Palomaki GE, Haddow JE, Haddow HR, et al. Modeling risk for severe adverse outcomes using angiogenic factor measurements in women with suspected preterm preeclampsia. Prenat Diagn. 2015;35:386-393.
- Verlohren S, Brennecke SP, Galindo A, et al. Clinical interpretation and implementation of the sFlt-1/PlGF ratio in the prediction, diagnosis and management of preeclampsia. Pregnancy Hyper. 2022;27:42-50.
- Binder J, Palmrich P, Pateisky P, et al. The prognostic value of angiogenic markers in twin pregnancies to predict delivery due to maternal complications of preeclampsia. Hypertension. 2020;76:176-183.
- Sapantzoglou I, Rouvali A, Koutras A, et al. sFlt-1, PlGF, the sFlt-1/PlGF ratio and their association with pre-eclampsia in twin pregnancies- a review of the literature. Medicina. 2023;59:1232.
- Satorres E, Martinez-Varea A, Diago-Almela V. sFlt-1/PlGF ratio as a predictor of pregnancy outcomes in twin pregnancies: a systematic review. J Matern Fetal Neonatal Med. 2023;36:2230514.
- Rana S, Hacker MR, Modest AM, et al. Circulating angiogenic factors and risk of adverse maternal and perinatal outcomes in twin pregnancies with suspected preeclampsia. Hypertension. 2012;60:451-458.
- Ford ND, Cox S, Ko JY, et al. Hypertensive disorders in pregnancy and mortality at delivery hospitalization-United States 2017-2019. Morb Mortal Week Report. 2022;71:585-591.
- Nagamatsu T, Fujii T, Kusumi M, et al. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: an implication for placental vascular development and the pathophysiology of preeclampsia. Endocrinology. 2004;145:4838-4445.
- Rana S, Lemoine E, Granger JP, et al. Preeclampsia: pathophysiology, challenges and perspectives. Circ Res. 2019;124:1094-1112.
- Rana S, Burke SD, Karumanchi SA. Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders. Am J Obstet Gynecol. 2022(2S):S1019-S1034.
- Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672-683.
- Chaiworapongsa T, Romero R, Savasan ZA, et al. Maternal plasma concentrations of angiogenic/ anti-angiogenic factors are of prognostic value in patients presenting to the obstetrical triage area with the suspicion of preeclampsia. J Matern Fetal Neonatal Med. 2011;24:1187-1207.
- Rana S, Powe CE, Salahuddin S, et al. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation. 2012;125:911-919.
- Moore AG, Young H, Keller JM, et al. Angiogenic biomarkers for prediction of maternal and neonatal complications in suspected preeclampsia. J Matern Fetal Neonatal Med. 2012;25:2651-2657.
- Verlohren S, Herraiz I, Lapaire O, et al. The sFlt-1/ PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol. 2012;206:58.e1-e8.
- Verlohren S, Herraiz I, Lapaire O, et al. New gestational phase-specific cutoff values for the use of soluble fms-like tyrosine kinase-1/placental growth factor ratio as a diagnostic test for preeclampsia. Hypertension. 2014;63:346-352.
- Zeisler H, Llurba E, Chantraine F, et al. Predictive value of the sFlt-1/PlGF ratio in women with suspected preeclampsia. N Engl J Med. 2016;374:1322.
- Duckworth S, Griffin M, Seed PT, et al. Diagnostic biomarkers in women with suspected preeclampsia in a prospective multicenter study. Obstet Gynecol. 2016;128:245-252.
- Rana S, Salahuddin S, Mueller A, et al. Angiogenic biomarkers in triage and risk for preeclampsia with severe features. Pregnancy Hyertens. 2018;13:100-106.
- Bian X, Biswas A, Huang X, et al. Short-term prediction of adverse outcomes using the sFlt-1/PlGF ratio in Asian women with suspected preeclampsia. Hypertension. 2019;74:164-172.
- Thadhani R, Lemoine E, Rana S, et al. Circulating angiogenic factor levels in hypertensive disorders of pregnancy. N Engl J Med Evidence. 2022. doi 10.1056/EVIDoa2200161.
- US Food and Drug Administration. FDA approval letter for an assay to measure sFlt-1 and PlGF. May 18, 2023. https://www.accessdata.fda.gov/cdrh _docs/pdf22/DEN220027.pdf
- Chaiworapongsa T, Romero R, Korzeniewski SJ, et al. Plasma concentrations of angiogenic/ anti-angiogenic factors have prognostic value in women presenting with suspected preeclampsia to the obstetrical triage area: a prospective study. J Matern Fetal Neonatal Med. 2014;27:132-144.
- Palomaki GE, Haddow JE, Haddow HR, et al. Modeling risk for severe adverse outcomes using angiogenic factor measurements in women with suspected preterm preeclampsia. Prenat Diagn. 2015;35:386-393.
- Verlohren S, Brennecke SP, Galindo A, et al. Clinical interpretation and implementation of the sFlt-1/PlGF ratio in the prediction, diagnosis and management of preeclampsia. Pregnancy Hyper. 2022;27:42-50.
- Binder J, Palmrich P, Pateisky P, et al. The prognostic value of angiogenic markers in twin pregnancies to predict delivery due to maternal complications of preeclampsia. Hypertension. 2020;76:176-183.
- Sapantzoglou I, Rouvali A, Koutras A, et al. sFlt-1, PlGF, the sFlt-1/PlGF ratio and their association with pre-eclampsia in twin pregnancies- a review of the literature. Medicina. 2023;59:1232.
- Satorres E, Martinez-Varea A, Diago-Almela V. sFlt-1/PlGF ratio as a predictor of pregnancy outcomes in twin pregnancies: a systematic review. J Matern Fetal Neonatal Med. 2023;36:2230514.
- Rana S, Hacker MR, Modest AM, et al. Circulating angiogenic factors and risk of adverse maternal and perinatal outcomes in twin pregnancies with suspected preeclampsia. Hypertension. 2012;60:451-458.
Valedictory
All that’s bright must fade,
The brightest still the fleetest;
All that’s sweet was made
But to be lost when sweetest.
Thomas Moore
I sometimes hold it half a sin
To put in words the grief I feel;
For words, like Nature, half reveal
And half conceal the Soul within.
Alfred, Lord Tennyson, In Memoriam
Dear Readers,
I have sad news to share with you. This is the last issue of
During my travels around the country over the past 2 decades, countless psychiatrists have told me that
As the saying goes: All good things eventually come to an end. I am so grateful to have had the opportunity to collaborate with a wonderful, highly competent editorial staff, as well as with outstanding colleagues who served on the editorial board all those years. A special shout-out to Jeff Bauer, the publishing staff editor, with whom I worked so closely. I very much appreciated all the authors and peer reviewers who contributed timely clinical articles month after month and made
This has been a unique journey for all of us who strived to transform
All that’s bright must fade,
The brightest still the fleetest;
All that’s sweet was made
But to be lost when sweetest.
Thomas Moore
I sometimes hold it half a sin
To put in words the grief I feel;
For words, like Nature, half reveal
And half conceal the Soul within.
Alfred, Lord Tennyson, In Memoriam
Dear Readers,
I have sad news to share with you. This is the last issue of
During my travels around the country over the past 2 decades, countless psychiatrists have told me that
As the saying goes: All good things eventually come to an end. I am so grateful to have had the opportunity to collaborate with a wonderful, highly competent editorial staff, as well as with outstanding colleagues who served on the editorial board all those years. A special shout-out to Jeff Bauer, the publishing staff editor, with whom I worked so closely. I very much appreciated all the authors and peer reviewers who contributed timely clinical articles month after month and made
This has been a unique journey for all of us who strived to transform
All that’s bright must fade,
The brightest still the fleetest;
All that’s sweet was made
But to be lost when sweetest.
Thomas Moore
I sometimes hold it half a sin
To put in words the grief I feel;
For words, like Nature, half reveal
And half conceal the Soul within.
Alfred, Lord Tennyson, In Memoriam
Dear Readers,
I have sad news to share with you. This is the last issue of
During my travels around the country over the past 2 decades, countless psychiatrists have told me that
As the saying goes: All good things eventually come to an end. I am so grateful to have had the opportunity to collaborate with a wonderful, highly competent editorial staff, as well as with outstanding colleagues who served on the editorial board all those years. A special shout-out to Jeff Bauer, the publishing staff editor, with whom I worked so closely. I very much appreciated all the authors and peer reviewers who contributed timely clinical articles month after month and made
This has been a unique journey for all of us who strived to transform
Breakthroughs in the prevention of RSV disease among infants
Respiratory syncytial virus (RSV) is a negative-sense, single-stranded, ribonucleic acid (RNA) virus that is a member of Pneumoviridae family. Two subtypes, A and B, and multiple genotypes circulate during fall and winter seasonal outbreaks of RSV.1 RSV can cause severe lower respiratory tract disease including bronchiolitis, pneumonia, respiratory failure, and death. Each year, RSV disease causes the hospitalization of 1.5% to 2% of children younger than 6 months of age, resulting in 100 to 300 deaths.2 For infants younger than 1 year, RSV infection is the leading cause of hospitalization.3 In 2023, two new treatments have become available to prevent RSV disease: nirsevimab and RSVPreF vaccine.
Nirsevimab
Nirsevimab is an antibody to an RSV antigen. It has a long half-life and is approved for administration to infants, providing passive immunization. In contrast, administration of the RSVPreF vaccine to pregnant persons elicits active maternal immunity, resulting in the production of anti-RSV antibodies that are transferred to the fetus, resulting in passive immunity in the infant. Seasonal administration of nirsevimab and the RSV vaccine maximizes benefit to the infant and conserves limited health care resources. In temperate regions in the United States, the RSV infection season typically begins in October and peaks in December through mid-February and ends in April or May.4,5 In southern Florida, the RSV season often begins in August to September, peaks in November through December, and ends in March.4,5
This editorial reviews 3 strategies for prevention of RSV infection in infants, including:
- universal treatment of newborns with nirsevimab
- immunization of pregnant persons with an RSVpreF vaccine in the third trimester appropriately timed to occur just before the beginning or during RSV infection season
- prioritizing universal maternal RSV vaccination with reflex administration of nirsevimab to newborns when the pregnant person was not vaccinated.6
Of note, there are no studies that have evaluated the effectiveness of combining RSVpreF vaccine and nirsevimab. The Centers for Disease Control and Prevention (CDC) does not recommend combining both RSV vaccination of pregnant persons plus nirsevimab treatment of the infant, except in limited circumstances, such as for immunocompromised pregnant people with limited antibody production or newborns who have a massive transfusion, which dilutes antibody titres.6
RSV prevention strategy 1
Universal treatment of newborns and infants with nirsevimab
Nirsevimab (Beyfortus, Sanofi and AstraZeneca) is an IgG 1-kappa monoclonal antibody with a long half-life that targets the prefusion conformation of the RSV F-protein, resulting in passive immunity to infection.7 Passive immunization results in rapid protection against infection because it does not require activation of the immune system. Nirsevimab is long acting due to amino acid substitutions in the Fc region, increasing binding to the neonatal Fc receptor, which protects IgG antibodies from degradation, thereby extending the antibody half-life. The terminal halflife of nirsevimab is 71 days, and the duration of protection following a single dose is at least 5 months.
Nirsevimab is approved by the US Food and Drug Administration (FDA) for all neonates and infants born or entering their first RSV infection season and for children up to 24 months of age who are vulnerable to severe RSV during their second RSV infection season. For infants born outside the RSV infection season, nirsevimab should be administered once prior to the start of the next RSV infection season.7 Nirsevimab is administered as a single intramuscular injection at a dose of 50 mg for neonates and infants < 5 kg in weight and a dose of 100 mg for neonates and infants ≥ 5 kg in weight.7 The list average wholesale price for both doses is $594.8 Nirsevimab is contraindicated for patients with a serious hypersensitivity reaction to nirsevimab or its excipients.7 In clinical trials, adverse reactions including rash and injection site reaction were reported in 1.2% of participants.7 Some RSV variants may be resistant to neutralization with nirsevimab.7,9
In a randomized clinical trial, 1,490 infants born ≥ 35 weeks’ gestation, the rates of medically-attended RSV lower respiratory tract disease (MA RSV LRTD) through 150 days of follow-up in the placebo and nirsevimab groups were 5.0% and 1.2%, respectively (P < .001).7,10 Compared with placebo, nirsevimab reduced hospitalizations due to RSV LRTD by 60% through 150 days of follow up. In a randomized clinical trial enrolling 1,453 infants born between 29 weeks’ and < 35 weeks’ gestation, the rates of MA RSV LRTD through 150 days of follow up in the placebo and nirsevimab groups were 9.5% and 2.6%, respectively (P < .001). In this study of infants born preterm, compared with placebo, nirsevimab reduced hospitalization due to RSV LRTD by 70% through 150 days of follow up.7 Nirsevimab is thought to be cost-effective at the current price per dose, but more data are needed to precisely define the magnitude of the health care savings associated with universal nirsevimab administration.11-13 The CDC reports that the incremental cost-effectiveness ratio (ICER) per quality-adjusted life year (QALY) of nirsevimab administration to infants is approximately $250,000, given an estimated cost of $500 for one dose of vaccine.14
Universal passive vaccination of newborns is recommended by many state departments of public health, which can provide the vaccine without cost to clinicians and health care facilities participating in the children’s vaccination program.
Continue to: RSV prevention strategy 2...
RSV prevention strategy 2
Universal RSV vaccination of pregnant persons from September through January
The RSVpreF vaccine (Abryvso, Pfizer) is approved by the FDA for the active immunization of pregnant persons between 32 through 36 weeks’ gestation for the prevention of RSV LRTD in infants from birth through 6 months of age.15 Administration of the RSVpreF vaccine to pregnant people elicits the formation of antiRSV antibodies that are transferred transplacentally to the fetus, resulting in the protection of the infant from RSV during the first 6 months of life. The RSVpreF vaccine also is approved to prevent RSV LRTD in people aged ≥ 60 years.
The RSVpreF vaccine contains the prefusion form of the RSV fusion (F) protein responsible for viral entry into host cells. The vaccine contains 60 µg of both RSV preF A and preF B recombinant proteins. The vaccine is administered as a single intramuscular dose in a volume of 0.5 mL. The vaccine is provided in a vial in a lyophilized form and must be reconstituted prior to administration. The average wholesale price of RSVPreF vaccine is $354.16 The vaccine is contraindicated for people who have had an allergic reaction to any component of the vaccine. The most commonly reported adverse reaction is injection site pain (41%).15 The FDA reports a “numerical imbalance in preterm births in Abrysvo recipients compared to placebo recipients” (5.7% vs 4.7%), and “available data are insufficient to establish or exclude a causal relationship between preterm birth and Abrysvo.”15 In rabbits there is no evidence of developmental toxicity and congenital anomalies associated with the RSVpreF vaccine. In human studies, no differences in the rate of congenital anomalies or fetal deaths were noted between RSVpreF vaccine and placebo.
In a clinical trial, 6,975 pregnant participants 24 through 36 weeks’ gestation were randomly assigned to receive a placebo or the RSVpreF vaccine.15,17 After birth, follow-up of infants at 180 days, showed that the rates of MA RSV LRTD among the infants in the placebo and RSVpreF vaccine groups were 3.4% and 1.6%, respectively. At 180 days, the reported rates of severe RSV LRTD in the placebo and RSVpreF vaccine groups were 1.8% and 0.5%, respectively. In this study, among the subset of pregnant participants who received the RSVpreF vaccine (n = 1,572) or placebo (n = 1,539) at 32 through 36 weeks’ gestation, the rates of MA RSV LRTD among the infants in the placebo and RSVpreF vaccine groups were 3.6% and 1.5%, respectively. In the subset of pregnant participants vaccinated at 32 through 36 weeks’ gestation, at 180 days postvaccination, the reported rates of severe RSV LRTD in the placebo and RSVpreF vaccine groups were 1.6% and 0.4%, respectively.15
The CDC has recommended that the RSVpreF vaccine be administered to pregnant people 32 through 36 weeks’ gestation from September through the end of January in most of the continental United States to reduce the rate of RSV LRTD in infants.6 September was selected because it is 1 to 2 months before the start of the RSV season, and it takes at least 14 days for maternal vaccination to result in transplacental transfer of protective antibodies to the fetus. January was selected because it is 2 to 3 months before the anticipated end of the RSV season.6 The CDC also noted that, for regions with a different pattern of RSV seasonality, clinicians should follow the guidance of local public health officials. This applies to the states of Alaska, southern Florida, Hawaii, and Puerto Rico.6 The CDC recommended that infants born < 34 weeks’ gestation should receive nirsevimab.6
Maternal RSV vaccination is thought to be cost-effective for reducing RSV LRTD in infants. However, the cost-effectiveness analyses are sensitive to the pricing of the two main options: maternal RSV vaccination and nirsevimab.
It is estimated that nirsevimab may provide greater protection than maternal RSV vaccination from RSV LRTD, but the maternal RSVpreF vaccine is priced lower than nirsevimab.18 Focusing administration of RSVpreF vaccine from September through January of the RSV infection season is thought to maximize benefits to infants and reduce total cost of the vaccination program.19 With year-round RSVpreF vaccine dosing, the estimated ICER per quality-adjusted life-year (QALY) is approximately $400,000, whereas seasonal dosing reduces the cost to approximately $170,000.19
RSV prevention strategy 3
Vaccinate pregnant persons; reflex to newborn treatment with nirsevimab if maternal RSV vaccination did not occur
RSVpreF vaccination to all pregnant persons 32 through 36 weeks’ gestation during RSV infection season is not likely to result in 100% adherence. For instance, in a CDC-conducted survey only 47% of pregnant persons received an influenza vaccine.2 Newborns whose mothers did not receive an RSVpreF vaccine will need to be considered for treatment with nirsevimab. Collaboration and communication among obstetricians and pediatricians will be needed to avoid miscommunication and missed opportunities to treat newborns during the birth hospitalization. Enhancements in electronic health records, linking the mother’s vaccination record with the newborn’s medical record plus an added feature of electronic alerts when the mother did not receive an appropriately timed RSVpreF vaccine would improve the communication of important clinical information to the pediatrician.
Next steps for the upcoming peak RSV season
We are currently in the 2023–2024 RSV infection season and can expect a peak in cases of RSV between December 2023 and February 2024. The CDC recommends protecting all infants against RSV-associated LRTD. The options are to administer the maternal RSVpreF vaccine to pregnant persons or treating the infant with nirsevimab. The vaccine is just now becoming available for administration in regional pharmacies, physician practices, and health systems. Obstetrician-gynecologists should follow the recommendation of their state department of public health. As noted above, many state departments of public health are recommending that all newborns receive nirsevimab. For clinicians in those states, RSVPreF vaccination of pregnant persons is not a priority. ●
- Tramuto F, Massimo Maida C, Mazzucco W, et al. Molecular epidemiology and genetic diversity of human respiratory syncytial virus in Sicily during pre- and post-COVID-19 surveillance season. Pathogens. 2023;12:1099.
- Boudreau M, Vadlamudi NK, Bastien N, et al. Pediatric RSV-associated hospitalizations before and during the COVID-19 pandemic. JAMA Netw Open. 2023;6:e2336863.
- Leader S, Kohlhase K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. J Pediatr. 2003;143(5 Suppl):S127-132.
- Hamid S, Winn A, Parikh R, et al. Seasonality of respiratory syncytial virus-United States 2017-2023. MMWR Morb Mortal Wkly Rep. 2023;72:355-361.
- Rose EB, Wheatley A, Langley G, et al. Respiratory syncytial virus seasonality-United States 2014-2017. MMWR Morb Mortal Wkly Rep. 2018;67:71-76.
- Fleming-Dutra KE, Jones JM, Roper LE, et al. Use of Pfizer respiratory syncytial virus vaccine during pregnancy for the prevention of respiratory syncytial virus associated lower respiratory tract disease in infants: recommendations of the Advisory Committee on Immunization Practices- United States 2023. MMWR Morb Mortal Wkly Rep. October 6, 2023. Accessed October 9, 2023. https://www.cdc.gov/mmwr/volumes/72/wr /mm7241e1.htm#print
- FDA package insert for Beyfortus. Accessed October 9, 2023. https://www.accessdata.fda.gov /drugsatfda_docs/label/2023/761328s000lbl.pdf
- Lexicomp. Nirsevimab: Drug information – UpToDate. Accessed October 9, 2023. https://www. wolterskluwer.com/en/solutions/lexicomp
- Ahani B, Tuffy KM, Aksyuk A, et al. Molecular and phenotypic characterization of RSV infections in infants during two nirsevimab randomized clinical trials. Nat Commun. 2023;14:4347.
- Hammitt LL, Dagan R, Yuan Y, et al. Nirsevimab for prevention of RSV in late-preterm and term infants. N Engl J Med. 2022;386:837-846.
- Li X, Bilcke J, Vazquez-Fernandez L, et al. Costeffectiveness of respiratory syncytial virus disease protection strategies: maternal vaccine versus seasonal or year-round monoclonal antibody program in Norwegian children. J Infect Dis. 2022;226(Suppl 1):S95-S101.
- Hodgson D, Koltai M, Krauer F, et al. Optimal respiratory syncytial virus intervention programmes using nirsevimab in England and Wales. Vaccine. 2022;40:7151-7157.
- Yu T, Padula WV, Yieh L, et al. Cost-effectiveness of nirsevimab and palivizumab for respiratory syncytial virus prophylaxis in preterm infants 29-34 6/7 weeks’ gestation in the United States. Pediatr Neonatal. 2023;04:015.
- Jones J. Evidence to recommendations framework: nirsevimab in infants. Accessed October 27, 2023. https://www.cdc.gov/vaccines/acip/meet ings/downloads/slides-2023-02/slides-02-23/rsv -pediatric-04-jones-508.pdf
- Abrysvo [package insert]. Pfizer; New York, New York. August 2023.
- Lexicomp. Recombinant respiratory syncytial virus vaccine (RSVPreF) (Abrysvo): Drug information - UpToDate. Accessed October 9, 2023. https://www.wolterskluwer.com/en/solutions /lexicomp
- Kampmann B, Madhi SA, Munjal I, et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med. 2023;388: 1451-1464.
- Baral R, Higgins D, Regan K, et al. Impact and costeffectiveness of potential interventions against infant respiratory syncytial virus (RSV) in 131 lowincome and middle-income countries using a static cohort model. BMJ Open. 2021;11:e046563.
- Fleming-Dutra KE. Evidence to recommendations framework updates: Pfizer maternal RSVpreF vaccine. June 22, 2023. Accessed October 27, 2023. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.cdc.gov/vaccines/acip /meetings/downloads/slides-2023-06-21-23/03 -RSV-Mat-Ped-Fleming-Dutra-508.pdf
- Razzaghi H, Kahn KE, Calhoun K, et al. Influenza, Tdap and COVID-19 vaccination coverage and hesitancy among pregnant women-United States, April 2023. MMWR Morb Mortal Wkly Rep.
Respiratory syncytial virus (RSV) is a negative-sense, single-stranded, ribonucleic acid (RNA) virus that is a member of Pneumoviridae family. Two subtypes, A and B, and multiple genotypes circulate during fall and winter seasonal outbreaks of RSV.1 RSV can cause severe lower respiratory tract disease including bronchiolitis, pneumonia, respiratory failure, and death. Each year, RSV disease causes the hospitalization of 1.5% to 2% of children younger than 6 months of age, resulting in 100 to 300 deaths.2 For infants younger than 1 year, RSV infection is the leading cause of hospitalization.3 In 2023, two new treatments have become available to prevent RSV disease: nirsevimab and RSVPreF vaccine.
Nirsevimab
Nirsevimab is an antibody to an RSV antigen. It has a long half-life and is approved for administration to infants, providing passive immunization. In contrast, administration of the RSVPreF vaccine to pregnant persons elicits active maternal immunity, resulting in the production of anti-RSV antibodies that are transferred to the fetus, resulting in passive immunity in the infant. Seasonal administration of nirsevimab and the RSV vaccine maximizes benefit to the infant and conserves limited health care resources. In temperate regions in the United States, the RSV infection season typically begins in October and peaks in December through mid-February and ends in April or May.4,5 In southern Florida, the RSV season often begins in August to September, peaks in November through December, and ends in March.4,5
This editorial reviews 3 strategies for prevention of RSV infection in infants, including:
- universal treatment of newborns with nirsevimab
- immunization of pregnant persons with an RSVpreF vaccine in the third trimester appropriately timed to occur just before the beginning or during RSV infection season
- prioritizing universal maternal RSV vaccination with reflex administration of nirsevimab to newborns when the pregnant person was not vaccinated.6
Of note, there are no studies that have evaluated the effectiveness of combining RSVpreF vaccine and nirsevimab. The Centers for Disease Control and Prevention (CDC) does not recommend combining both RSV vaccination of pregnant persons plus nirsevimab treatment of the infant, except in limited circumstances, such as for immunocompromised pregnant people with limited antibody production or newborns who have a massive transfusion, which dilutes antibody titres.6
RSV prevention strategy 1
Universal treatment of newborns and infants with nirsevimab
Nirsevimab (Beyfortus, Sanofi and AstraZeneca) is an IgG 1-kappa monoclonal antibody with a long half-life that targets the prefusion conformation of the RSV F-protein, resulting in passive immunity to infection.7 Passive immunization results in rapid protection against infection because it does not require activation of the immune system. Nirsevimab is long acting due to amino acid substitutions in the Fc region, increasing binding to the neonatal Fc receptor, which protects IgG antibodies from degradation, thereby extending the antibody half-life. The terminal halflife of nirsevimab is 71 days, and the duration of protection following a single dose is at least 5 months.
Nirsevimab is approved by the US Food and Drug Administration (FDA) for all neonates and infants born or entering their first RSV infection season and for children up to 24 months of age who are vulnerable to severe RSV during their second RSV infection season. For infants born outside the RSV infection season, nirsevimab should be administered once prior to the start of the next RSV infection season.7 Nirsevimab is administered as a single intramuscular injection at a dose of 50 mg for neonates and infants < 5 kg in weight and a dose of 100 mg for neonates and infants ≥ 5 kg in weight.7 The list average wholesale price for both doses is $594.8 Nirsevimab is contraindicated for patients with a serious hypersensitivity reaction to nirsevimab or its excipients.7 In clinical trials, adverse reactions including rash and injection site reaction were reported in 1.2% of participants.7 Some RSV variants may be resistant to neutralization with nirsevimab.7,9
In a randomized clinical trial, 1,490 infants born ≥ 35 weeks’ gestation, the rates of medically-attended RSV lower respiratory tract disease (MA RSV LRTD) through 150 days of follow-up in the placebo and nirsevimab groups were 5.0% and 1.2%, respectively (P < .001).7,10 Compared with placebo, nirsevimab reduced hospitalizations due to RSV LRTD by 60% through 150 days of follow up. In a randomized clinical trial enrolling 1,453 infants born between 29 weeks’ and < 35 weeks’ gestation, the rates of MA RSV LRTD through 150 days of follow up in the placebo and nirsevimab groups were 9.5% and 2.6%, respectively (P < .001). In this study of infants born preterm, compared with placebo, nirsevimab reduced hospitalization due to RSV LRTD by 70% through 150 days of follow up.7 Nirsevimab is thought to be cost-effective at the current price per dose, but more data are needed to precisely define the magnitude of the health care savings associated with universal nirsevimab administration.11-13 The CDC reports that the incremental cost-effectiveness ratio (ICER) per quality-adjusted life year (QALY) of nirsevimab administration to infants is approximately $250,000, given an estimated cost of $500 for one dose of vaccine.14
Universal passive vaccination of newborns is recommended by many state departments of public health, which can provide the vaccine without cost to clinicians and health care facilities participating in the children’s vaccination program.
Continue to: RSV prevention strategy 2...
RSV prevention strategy 2
Universal RSV vaccination of pregnant persons from September through January
The RSVpreF vaccine (Abryvso, Pfizer) is approved by the FDA for the active immunization of pregnant persons between 32 through 36 weeks’ gestation for the prevention of RSV LRTD in infants from birth through 6 months of age.15 Administration of the RSVpreF vaccine to pregnant people elicits the formation of antiRSV antibodies that are transferred transplacentally to the fetus, resulting in the protection of the infant from RSV during the first 6 months of life. The RSVpreF vaccine also is approved to prevent RSV LRTD in people aged ≥ 60 years.
The RSVpreF vaccine contains the prefusion form of the RSV fusion (F) protein responsible for viral entry into host cells. The vaccine contains 60 µg of both RSV preF A and preF B recombinant proteins. The vaccine is administered as a single intramuscular dose in a volume of 0.5 mL. The vaccine is provided in a vial in a lyophilized form and must be reconstituted prior to administration. The average wholesale price of RSVPreF vaccine is $354.16 The vaccine is contraindicated for people who have had an allergic reaction to any component of the vaccine. The most commonly reported adverse reaction is injection site pain (41%).15 The FDA reports a “numerical imbalance in preterm births in Abrysvo recipients compared to placebo recipients” (5.7% vs 4.7%), and “available data are insufficient to establish or exclude a causal relationship between preterm birth and Abrysvo.”15 In rabbits there is no evidence of developmental toxicity and congenital anomalies associated with the RSVpreF vaccine. In human studies, no differences in the rate of congenital anomalies or fetal deaths were noted between RSVpreF vaccine and placebo.
In a clinical trial, 6,975 pregnant participants 24 through 36 weeks’ gestation were randomly assigned to receive a placebo or the RSVpreF vaccine.15,17 After birth, follow-up of infants at 180 days, showed that the rates of MA RSV LRTD among the infants in the placebo and RSVpreF vaccine groups were 3.4% and 1.6%, respectively. At 180 days, the reported rates of severe RSV LRTD in the placebo and RSVpreF vaccine groups were 1.8% and 0.5%, respectively. In this study, among the subset of pregnant participants who received the RSVpreF vaccine (n = 1,572) or placebo (n = 1,539) at 32 through 36 weeks’ gestation, the rates of MA RSV LRTD among the infants in the placebo and RSVpreF vaccine groups were 3.6% and 1.5%, respectively. In the subset of pregnant participants vaccinated at 32 through 36 weeks’ gestation, at 180 days postvaccination, the reported rates of severe RSV LRTD in the placebo and RSVpreF vaccine groups were 1.6% and 0.4%, respectively.15
The CDC has recommended that the RSVpreF vaccine be administered to pregnant people 32 through 36 weeks’ gestation from September through the end of January in most of the continental United States to reduce the rate of RSV LRTD in infants.6 September was selected because it is 1 to 2 months before the start of the RSV season, and it takes at least 14 days for maternal vaccination to result in transplacental transfer of protective antibodies to the fetus. January was selected because it is 2 to 3 months before the anticipated end of the RSV season.6 The CDC also noted that, for regions with a different pattern of RSV seasonality, clinicians should follow the guidance of local public health officials. This applies to the states of Alaska, southern Florida, Hawaii, and Puerto Rico.6 The CDC recommended that infants born < 34 weeks’ gestation should receive nirsevimab.6
Maternal RSV vaccination is thought to be cost-effective for reducing RSV LRTD in infants. However, the cost-effectiveness analyses are sensitive to the pricing of the two main options: maternal RSV vaccination and nirsevimab.
It is estimated that nirsevimab may provide greater protection than maternal RSV vaccination from RSV LRTD, but the maternal RSVpreF vaccine is priced lower than nirsevimab.18 Focusing administration of RSVpreF vaccine from September through January of the RSV infection season is thought to maximize benefits to infants and reduce total cost of the vaccination program.19 With year-round RSVpreF vaccine dosing, the estimated ICER per quality-adjusted life-year (QALY) is approximately $400,000, whereas seasonal dosing reduces the cost to approximately $170,000.19
RSV prevention strategy 3
Vaccinate pregnant persons; reflex to newborn treatment with nirsevimab if maternal RSV vaccination did not occur
RSVpreF vaccination to all pregnant persons 32 through 36 weeks’ gestation during RSV infection season is not likely to result in 100% adherence. For instance, in a CDC-conducted survey only 47% of pregnant persons received an influenza vaccine.2 Newborns whose mothers did not receive an RSVpreF vaccine will need to be considered for treatment with nirsevimab. Collaboration and communication among obstetricians and pediatricians will be needed to avoid miscommunication and missed opportunities to treat newborns during the birth hospitalization. Enhancements in electronic health records, linking the mother’s vaccination record with the newborn’s medical record plus an added feature of electronic alerts when the mother did not receive an appropriately timed RSVpreF vaccine would improve the communication of important clinical information to the pediatrician.
Next steps for the upcoming peak RSV season
We are currently in the 2023–2024 RSV infection season and can expect a peak in cases of RSV between December 2023 and February 2024. The CDC recommends protecting all infants against RSV-associated LRTD. The options are to administer the maternal RSVpreF vaccine to pregnant persons or treating the infant with nirsevimab. The vaccine is just now becoming available for administration in regional pharmacies, physician practices, and health systems. Obstetrician-gynecologists should follow the recommendation of their state department of public health. As noted above, many state departments of public health are recommending that all newborns receive nirsevimab. For clinicians in those states, RSVPreF vaccination of pregnant persons is not a priority. ●
Respiratory syncytial virus (RSV) is a negative-sense, single-stranded, ribonucleic acid (RNA) virus that is a member of Pneumoviridae family. Two subtypes, A and B, and multiple genotypes circulate during fall and winter seasonal outbreaks of RSV.1 RSV can cause severe lower respiratory tract disease including bronchiolitis, pneumonia, respiratory failure, and death. Each year, RSV disease causes the hospitalization of 1.5% to 2% of children younger than 6 months of age, resulting in 100 to 300 deaths.2 For infants younger than 1 year, RSV infection is the leading cause of hospitalization.3 In 2023, two new treatments have become available to prevent RSV disease: nirsevimab and RSVPreF vaccine.
Nirsevimab
Nirsevimab is an antibody to an RSV antigen. It has a long half-life and is approved for administration to infants, providing passive immunization. In contrast, administration of the RSVPreF vaccine to pregnant persons elicits active maternal immunity, resulting in the production of anti-RSV antibodies that are transferred to the fetus, resulting in passive immunity in the infant. Seasonal administration of nirsevimab and the RSV vaccine maximizes benefit to the infant and conserves limited health care resources. In temperate regions in the United States, the RSV infection season typically begins in October and peaks in December through mid-February and ends in April or May.4,5 In southern Florida, the RSV season often begins in August to September, peaks in November through December, and ends in March.4,5
This editorial reviews 3 strategies for prevention of RSV infection in infants, including:
- universal treatment of newborns with nirsevimab
- immunization of pregnant persons with an RSVpreF vaccine in the third trimester appropriately timed to occur just before the beginning or during RSV infection season
- prioritizing universal maternal RSV vaccination with reflex administration of nirsevimab to newborns when the pregnant person was not vaccinated.6
Of note, there are no studies that have evaluated the effectiveness of combining RSVpreF vaccine and nirsevimab. The Centers for Disease Control and Prevention (CDC) does not recommend combining both RSV vaccination of pregnant persons plus nirsevimab treatment of the infant, except in limited circumstances, such as for immunocompromised pregnant people with limited antibody production or newborns who have a massive transfusion, which dilutes antibody titres.6
RSV prevention strategy 1
Universal treatment of newborns and infants with nirsevimab
Nirsevimab (Beyfortus, Sanofi and AstraZeneca) is an IgG 1-kappa monoclonal antibody with a long half-life that targets the prefusion conformation of the RSV F-protein, resulting in passive immunity to infection.7 Passive immunization results in rapid protection against infection because it does not require activation of the immune system. Nirsevimab is long acting due to amino acid substitutions in the Fc region, increasing binding to the neonatal Fc receptor, which protects IgG antibodies from degradation, thereby extending the antibody half-life. The terminal halflife of nirsevimab is 71 days, and the duration of protection following a single dose is at least 5 months.
Nirsevimab is approved by the US Food and Drug Administration (FDA) for all neonates and infants born or entering their first RSV infection season and for children up to 24 months of age who are vulnerable to severe RSV during their second RSV infection season. For infants born outside the RSV infection season, nirsevimab should be administered once prior to the start of the next RSV infection season.7 Nirsevimab is administered as a single intramuscular injection at a dose of 50 mg for neonates and infants < 5 kg in weight and a dose of 100 mg for neonates and infants ≥ 5 kg in weight.7 The list average wholesale price for both doses is $594.8 Nirsevimab is contraindicated for patients with a serious hypersensitivity reaction to nirsevimab or its excipients.7 In clinical trials, adverse reactions including rash and injection site reaction were reported in 1.2% of participants.7 Some RSV variants may be resistant to neutralization with nirsevimab.7,9
In a randomized clinical trial, 1,490 infants born ≥ 35 weeks’ gestation, the rates of medically-attended RSV lower respiratory tract disease (MA RSV LRTD) through 150 days of follow-up in the placebo and nirsevimab groups were 5.0% and 1.2%, respectively (P < .001).7,10 Compared with placebo, nirsevimab reduced hospitalizations due to RSV LRTD by 60% through 150 days of follow up. In a randomized clinical trial enrolling 1,453 infants born between 29 weeks’ and < 35 weeks’ gestation, the rates of MA RSV LRTD through 150 days of follow up in the placebo and nirsevimab groups were 9.5% and 2.6%, respectively (P < .001). In this study of infants born preterm, compared with placebo, nirsevimab reduced hospitalization due to RSV LRTD by 70% through 150 days of follow up.7 Nirsevimab is thought to be cost-effective at the current price per dose, but more data are needed to precisely define the magnitude of the health care savings associated with universal nirsevimab administration.11-13 The CDC reports that the incremental cost-effectiveness ratio (ICER) per quality-adjusted life year (QALY) of nirsevimab administration to infants is approximately $250,000, given an estimated cost of $500 for one dose of vaccine.14
Universal passive vaccination of newborns is recommended by many state departments of public health, which can provide the vaccine without cost to clinicians and health care facilities participating in the children’s vaccination program.
Continue to: RSV prevention strategy 2...
RSV prevention strategy 2
Universal RSV vaccination of pregnant persons from September through January
The RSVpreF vaccine (Abryvso, Pfizer) is approved by the FDA for the active immunization of pregnant persons between 32 through 36 weeks’ gestation for the prevention of RSV LRTD in infants from birth through 6 months of age.15 Administration of the RSVpreF vaccine to pregnant people elicits the formation of antiRSV antibodies that are transferred transplacentally to the fetus, resulting in the protection of the infant from RSV during the first 6 months of life. The RSVpreF vaccine also is approved to prevent RSV LRTD in people aged ≥ 60 years.
The RSVpreF vaccine contains the prefusion form of the RSV fusion (F) protein responsible for viral entry into host cells. The vaccine contains 60 µg of both RSV preF A and preF B recombinant proteins. The vaccine is administered as a single intramuscular dose in a volume of 0.5 mL. The vaccine is provided in a vial in a lyophilized form and must be reconstituted prior to administration. The average wholesale price of RSVPreF vaccine is $354.16 The vaccine is contraindicated for people who have had an allergic reaction to any component of the vaccine. The most commonly reported adverse reaction is injection site pain (41%).15 The FDA reports a “numerical imbalance in preterm births in Abrysvo recipients compared to placebo recipients” (5.7% vs 4.7%), and “available data are insufficient to establish or exclude a causal relationship between preterm birth and Abrysvo.”15 In rabbits there is no evidence of developmental toxicity and congenital anomalies associated with the RSVpreF vaccine. In human studies, no differences in the rate of congenital anomalies or fetal deaths were noted between RSVpreF vaccine and placebo.
In a clinical trial, 6,975 pregnant participants 24 through 36 weeks’ gestation were randomly assigned to receive a placebo or the RSVpreF vaccine.15,17 After birth, follow-up of infants at 180 days, showed that the rates of MA RSV LRTD among the infants in the placebo and RSVpreF vaccine groups were 3.4% and 1.6%, respectively. At 180 days, the reported rates of severe RSV LRTD in the placebo and RSVpreF vaccine groups were 1.8% and 0.5%, respectively. In this study, among the subset of pregnant participants who received the RSVpreF vaccine (n = 1,572) or placebo (n = 1,539) at 32 through 36 weeks’ gestation, the rates of MA RSV LRTD among the infants in the placebo and RSVpreF vaccine groups were 3.6% and 1.5%, respectively. In the subset of pregnant participants vaccinated at 32 through 36 weeks’ gestation, at 180 days postvaccination, the reported rates of severe RSV LRTD in the placebo and RSVpreF vaccine groups were 1.6% and 0.4%, respectively.15
The CDC has recommended that the RSVpreF vaccine be administered to pregnant people 32 through 36 weeks’ gestation from September through the end of January in most of the continental United States to reduce the rate of RSV LRTD in infants.6 September was selected because it is 1 to 2 months before the start of the RSV season, and it takes at least 14 days for maternal vaccination to result in transplacental transfer of protective antibodies to the fetus. January was selected because it is 2 to 3 months before the anticipated end of the RSV season.6 The CDC also noted that, for regions with a different pattern of RSV seasonality, clinicians should follow the guidance of local public health officials. This applies to the states of Alaska, southern Florida, Hawaii, and Puerto Rico.6 The CDC recommended that infants born < 34 weeks’ gestation should receive nirsevimab.6
Maternal RSV vaccination is thought to be cost-effective for reducing RSV LRTD in infants. However, the cost-effectiveness analyses are sensitive to the pricing of the two main options: maternal RSV vaccination and nirsevimab.
It is estimated that nirsevimab may provide greater protection than maternal RSV vaccination from RSV LRTD, but the maternal RSVpreF vaccine is priced lower than nirsevimab.18 Focusing administration of RSVpreF vaccine from September through January of the RSV infection season is thought to maximize benefits to infants and reduce total cost of the vaccination program.19 With year-round RSVpreF vaccine dosing, the estimated ICER per quality-adjusted life-year (QALY) is approximately $400,000, whereas seasonal dosing reduces the cost to approximately $170,000.19
RSV prevention strategy 3
Vaccinate pregnant persons; reflex to newborn treatment with nirsevimab if maternal RSV vaccination did not occur
RSVpreF vaccination to all pregnant persons 32 through 36 weeks’ gestation during RSV infection season is not likely to result in 100% adherence. For instance, in a CDC-conducted survey only 47% of pregnant persons received an influenza vaccine.2 Newborns whose mothers did not receive an RSVpreF vaccine will need to be considered for treatment with nirsevimab. Collaboration and communication among obstetricians and pediatricians will be needed to avoid miscommunication and missed opportunities to treat newborns during the birth hospitalization. Enhancements in electronic health records, linking the mother’s vaccination record with the newborn’s medical record plus an added feature of electronic alerts when the mother did not receive an appropriately timed RSVpreF vaccine would improve the communication of important clinical information to the pediatrician.
Next steps for the upcoming peak RSV season
We are currently in the 2023–2024 RSV infection season and can expect a peak in cases of RSV between December 2023 and February 2024. The CDC recommends protecting all infants against RSV-associated LRTD. The options are to administer the maternal RSVpreF vaccine to pregnant persons or treating the infant with nirsevimab. The vaccine is just now becoming available for administration in regional pharmacies, physician practices, and health systems. Obstetrician-gynecologists should follow the recommendation of their state department of public health. As noted above, many state departments of public health are recommending that all newborns receive nirsevimab. For clinicians in those states, RSVPreF vaccination of pregnant persons is not a priority. ●
- Tramuto F, Massimo Maida C, Mazzucco W, et al. Molecular epidemiology and genetic diversity of human respiratory syncytial virus in Sicily during pre- and post-COVID-19 surveillance season. Pathogens. 2023;12:1099.
- Boudreau M, Vadlamudi NK, Bastien N, et al. Pediatric RSV-associated hospitalizations before and during the COVID-19 pandemic. JAMA Netw Open. 2023;6:e2336863.
- Leader S, Kohlhase K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. J Pediatr. 2003;143(5 Suppl):S127-132.
- Hamid S, Winn A, Parikh R, et al. Seasonality of respiratory syncytial virus-United States 2017-2023. MMWR Morb Mortal Wkly Rep. 2023;72:355-361.
- Rose EB, Wheatley A, Langley G, et al. Respiratory syncytial virus seasonality-United States 2014-2017. MMWR Morb Mortal Wkly Rep. 2018;67:71-76.
- Fleming-Dutra KE, Jones JM, Roper LE, et al. Use of Pfizer respiratory syncytial virus vaccine during pregnancy for the prevention of respiratory syncytial virus associated lower respiratory tract disease in infants: recommendations of the Advisory Committee on Immunization Practices- United States 2023. MMWR Morb Mortal Wkly Rep. October 6, 2023. Accessed October 9, 2023. https://www.cdc.gov/mmwr/volumes/72/wr /mm7241e1.htm#print
- FDA package insert for Beyfortus. Accessed October 9, 2023. https://www.accessdata.fda.gov /drugsatfda_docs/label/2023/761328s000lbl.pdf
- Lexicomp. Nirsevimab: Drug information – UpToDate. Accessed October 9, 2023. https://www. wolterskluwer.com/en/solutions/lexicomp
- Ahani B, Tuffy KM, Aksyuk A, et al. Molecular and phenotypic characterization of RSV infections in infants during two nirsevimab randomized clinical trials. Nat Commun. 2023;14:4347.
- Hammitt LL, Dagan R, Yuan Y, et al. Nirsevimab for prevention of RSV in late-preterm and term infants. N Engl J Med. 2022;386:837-846.
- Li X, Bilcke J, Vazquez-Fernandez L, et al. Costeffectiveness of respiratory syncytial virus disease protection strategies: maternal vaccine versus seasonal or year-round monoclonal antibody program in Norwegian children. J Infect Dis. 2022;226(Suppl 1):S95-S101.
- Hodgson D, Koltai M, Krauer F, et al. Optimal respiratory syncytial virus intervention programmes using nirsevimab in England and Wales. Vaccine. 2022;40:7151-7157.
- Yu T, Padula WV, Yieh L, et al. Cost-effectiveness of nirsevimab and palivizumab for respiratory syncytial virus prophylaxis in preterm infants 29-34 6/7 weeks’ gestation in the United States. Pediatr Neonatal. 2023;04:015.
- Jones J. Evidence to recommendations framework: nirsevimab in infants. Accessed October 27, 2023. https://www.cdc.gov/vaccines/acip/meet ings/downloads/slides-2023-02/slides-02-23/rsv -pediatric-04-jones-508.pdf
- Abrysvo [package insert]. Pfizer; New York, New York. August 2023.
- Lexicomp. Recombinant respiratory syncytial virus vaccine (RSVPreF) (Abrysvo): Drug information - UpToDate. Accessed October 9, 2023. https://www.wolterskluwer.com/en/solutions /lexicomp
- Kampmann B, Madhi SA, Munjal I, et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med. 2023;388: 1451-1464.
- Baral R, Higgins D, Regan K, et al. Impact and costeffectiveness of potential interventions against infant respiratory syncytial virus (RSV) in 131 lowincome and middle-income countries using a static cohort model. BMJ Open. 2021;11:e046563.
- Fleming-Dutra KE. Evidence to recommendations framework updates: Pfizer maternal RSVpreF vaccine. June 22, 2023. Accessed October 27, 2023. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.cdc.gov/vaccines/acip /meetings/downloads/slides-2023-06-21-23/03 -RSV-Mat-Ped-Fleming-Dutra-508.pdf
- Razzaghi H, Kahn KE, Calhoun K, et al. Influenza, Tdap and COVID-19 vaccination coverage and hesitancy among pregnant women-United States, April 2023. MMWR Morb Mortal Wkly Rep.
- Tramuto F, Massimo Maida C, Mazzucco W, et al. Molecular epidemiology and genetic diversity of human respiratory syncytial virus in Sicily during pre- and post-COVID-19 surveillance season. Pathogens. 2023;12:1099.
- Boudreau M, Vadlamudi NK, Bastien N, et al. Pediatric RSV-associated hospitalizations before and during the COVID-19 pandemic. JAMA Netw Open. 2023;6:e2336863.
- Leader S, Kohlhase K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. J Pediatr. 2003;143(5 Suppl):S127-132.
- Hamid S, Winn A, Parikh R, et al. Seasonality of respiratory syncytial virus-United States 2017-2023. MMWR Morb Mortal Wkly Rep. 2023;72:355-361.
- Rose EB, Wheatley A, Langley G, et al. Respiratory syncytial virus seasonality-United States 2014-2017. MMWR Morb Mortal Wkly Rep. 2018;67:71-76.
- Fleming-Dutra KE, Jones JM, Roper LE, et al. Use of Pfizer respiratory syncytial virus vaccine during pregnancy for the prevention of respiratory syncytial virus associated lower respiratory tract disease in infants: recommendations of the Advisory Committee on Immunization Practices- United States 2023. MMWR Morb Mortal Wkly Rep. October 6, 2023. Accessed October 9, 2023. https://www.cdc.gov/mmwr/volumes/72/wr /mm7241e1.htm#print
- FDA package insert for Beyfortus. Accessed October 9, 2023. https://www.accessdata.fda.gov /drugsatfda_docs/label/2023/761328s000lbl.pdf
- Lexicomp. Nirsevimab: Drug information – UpToDate. Accessed October 9, 2023. https://www. wolterskluwer.com/en/solutions/lexicomp
- Ahani B, Tuffy KM, Aksyuk A, et al. Molecular and phenotypic characterization of RSV infections in infants during two nirsevimab randomized clinical trials. Nat Commun. 2023;14:4347.
- Hammitt LL, Dagan R, Yuan Y, et al. Nirsevimab for prevention of RSV in late-preterm and term infants. N Engl J Med. 2022;386:837-846.
- Li X, Bilcke J, Vazquez-Fernandez L, et al. Costeffectiveness of respiratory syncytial virus disease protection strategies: maternal vaccine versus seasonal or year-round monoclonal antibody program in Norwegian children. J Infect Dis. 2022;226(Suppl 1):S95-S101.
- Hodgson D, Koltai M, Krauer F, et al. Optimal respiratory syncytial virus intervention programmes using nirsevimab in England and Wales. Vaccine. 2022;40:7151-7157.
- Yu T, Padula WV, Yieh L, et al. Cost-effectiveness of nirsevimab and palivizumab for respiratory syncytial virus prophylaxis in preterm infants 29-34 6/7 weeks’ gestation in the United States. Pediatr Neonatal. 2023;04:015.
- Jones J. Evidence to recommendations framework: nirsevimab in infants. Accessed October 27, 2023. https://www.cdc.gov/vaccines/acip/meet ings/downloads/slides-2023-02/slides-02-23/rsv -pediatric-04-jones-508.pdf
- Abrysvo [package insert]. Pfizer; New York, New York. August 2023.
- Lexicomp. Recombinant respiratory syncytial virus vaccine (RSVPreF) (Abrysvo): Drug information - UpToDate. Accessed October 9, 2023. https://www.wolterskluwer.com/en/solutions /lexicomp
- Kampmann B, Madhi SA, Munjal I, et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med. 2023;388: 1451-1464.
- Baral R, Higgins D, Regan K, et al. Impact and costeffectiveness of potential interventions against infant respiratory syncytial virus (RSV) in 131 lowincome and middle-income countries using a static cohort model. BMJ Open. 2021;11:e046563.
- Fleming-Dutra KE. Evidence to recommendations framework updates: Pfizer maternal RSVpreF vaccine. June 22, 2023. Accessed October 27, 2023. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.cdc.gov/vaccines/acip /meetings/downloads/slides-2023-06-21-23/03 -RSV-Mat-Ped-Fleming-Dutra-508.pdf
- Razzaghi H, Kahn KE, Calhoun K, et al. Influenza, Tdap and COVID-19 vaccination coverage and hesitancy among pregnant women-United States, April 2023. MMWR Morb Mortal Wkly Rep.
Brain structural and cognitive changes during pregnancy
Pregnancy is unquestionably a major milestone in a woman’s life. During gestation, her body shape noticeably changes, but the invisible structural and cognitive changes in her brain are more striking. Some of those neurobiological changes are short-term, while others are long-lasting, well beyond delivery, and even into old age.
Physiological changes during pregnancy are extraordinary. The dramatic increases in estrogen, progesterone, and glucocorticoids help maintain pregnancy, ensure safe delivery of the baby, and trigger maternal behavior. However, other important changes also occur in the mother’s cardiac output, blood volume, renal function, respiratory output, and immune adaptations to accommodate the growth of the fetus. Gene expression also occurs to accomplish those changes, and there are lifelong repercussions from those drastic physiological changes.
During pregnancy, the brain is exposed to escalating levels of hormones released from the placenta, which the woman had never experienced. Those hormones regulate neuroplasticity, neuroinflammation, behavior, and cognition.
Structural brain changes1-6
Brain volume declines during pregnancy, reaching a nadir at the time of parturition. However, recovery occurs within 5 months after delivery. During the postpartum period, gray matter volume increases in the first 3 to 4 weeks, especially in areas involved in maternal behavior, including the amygdala, prefrontal cortex, and hypothalamus. Hippocampal gray matter decreases at 2 months postpartum compared to preconception levels, and reductions can still be observed up to 2 years following delivery. Gray matter reductions occur in multiple brain regions involved in social cognition, including the superior temporal gyrus, medial and inferior frontal cortex, fusiform areas, and hippocampus. Those changes correlate with positive maternal attachment. It is noteworthy that neural activity is highest in areas with reduced gray volume, so a decline in brain volume is associated with enhanced maternal attachment. Interestingly, those changes occur in fathers, too.
Childbearing improves stroke outcomes in middle age, but body weight will increase. The risk of Alzheimer’s disease increases with a higher number of gestations, but longevity is higher if the pregnancy occurs at an older age. Reproduction is also associated with shorter telomeres, which can elevate the risk of cancer, inflammation, diabetes, and dementia.
Cognitive changes7-10
The term “pregnancy brain” refers to cognitive changes during pregnancy and postpartum; these include decreased memory and concentration, absent-mindedness, heightened reactivity to threatening stimuli, and a decrease in motivation and executive functions. After delivery a mother has increased empathy (sometimes referred to as Theory of Mind) and greater activation in brain structures involved in empathy, including the paracingulate cortex, the posterior cingulate, and the insula. Also, the mirror neuron system becomes more activated in response to a woman’s own children compared to unfamiliar children. This incudes the ventral premotor cortex, the inferior frontal gyrus, and the posterior parietal cortex.
Certain forms of memory are impaired during pregnancy and early postpartum, including verbal free recall and working memory, as well as executive functions. Those are believed to correlate with glucocorticoids and estrogen levels.
Continue to: The following cognitive functions...
The following cognitive functions increase between the first and second trimester: verbal memory, attention, executive functions processing speed, verbal, and visuospatial abilities. Interestingly, mothers of a male fetus outperformed mothers of a female fetus on working memory and spatial ability.
Other changes11-16
- Cells from the fetus can traffic to the mother’s body and create microchimeric cells, which have short-term benefits (healing some of the other’s organs as stem cells do) but long-term downsides include future brain disorders such as Parkinson’s disease or Alzheimer’s disease, as well as autoimmune diseases and various types of cancer. The reverse also occurs with cells transferring from the mother to the fetus, persisting into infancy and childhood.
- Postpartum psychosis is associated with reductions in the volumes of the anterior cingulate, left parahippocampal gyrus, and superior temporal gyrus.
- A woman’s white matter increases during pregnancy compared to preconception. This is attributed to the high levels of prolactin, which proliferates oligodendrocytes, the glial cells that continuously manufacture myelin.
- The pituitary gland increases by 200% to 300% during pregnancy and returns to pre-pregnancy levels approximately 8 months following delivery. Prolactin also mediates the production of brain cells in the hippocampus (ie, neurogenesis).
- Sexual activity, even without pregnancy, increases neurogenesis. Plasma levels of prolactin increase significantly following an orgasm in both men and women, which indicates that sexual activity has beneficial brain effects.
- With pregnancy, the immune system shifts from proinflammatory to anti-inflammatory signaling. This protects the fetus from being attacked and rejected as foreign tissue. However, at the end of pregnancy, there is a “burst” of proinflammatory signaling, which serves as a major trigger to induce uterine contractions and initiate labor (to expel the foreign tissue).
- Brain levels of the anti-inflammatory cytokine interleukin-6 increase in the postpartum period, which represents a significant modification in the neuroimmune environment, and the maternal brain assumes an inflammatory-resistant state, which has cognitive and neuroplasticity implications. However, this neuroimmune dysregulation is implicated in postpartum depression and anxiety.
- Older females who were never pregnant or only had 1 pregnancy had better overall cognitive functioning than females who became pregnant at an young age.
- In animal studies, reproduction alleviates the negative effects of aging on several hippocampal functions, especially neurogenesis. Dendritic spine density in the CA1 region of the hippocampus is higher in pregnancy and early postpartum period compared to nulliparous females (based on animal studies).
Pregnancy is indispensable for the perpetuation of the species. Its hormonal, physiologic, neurobiological, and cognitive correlates are extensive. The cognitive changes in the postpartum period are designed by evolution to prepare a woman to care for her newborn and to ensure its survival. But the biological sequelae of pregnancy extend to the rest of a woman’s life and may predispose her to immune and brain disorders as she ages.
1. Barba-Müller E, Craddock S, Carmona S, et al. Brain plasticity in pregnancy and the postpartum period: links to maternal caregiving and mental health. Arch Womens Ment Health. 2019;22(2):289-299.
2. Pawluski JL, Hoekzema E, Leuner B, et al. Less can be more: fine tuning the maternal brain. Neurosci Biobehav Rev. 2022;133:104475. doi:10.1016/j.neubiorev.2021.11.045
3. Hoekzema E, Barba-Müller E, Pozzobon C, et al. Pregnancy leads to long-lasting changes in human brain structure. Nat Neurosci. 2017;20(2):287-296.
4. Cárdenas EF, Kujawa A, Humphreys KL. Neurobiological changes during the peripartum period: implications for health and behavior. Soc Cogn Affect Neurosci. 2020;15(10):1097-1110.
5. Eid RS, Chaiton JA, Lieblich SE, et al. Early and late effects of maternal experience on hippocampal neurogenesis, microglia, and the circulating cytokine milieu. Neurobiol Aging. 2019;78:1-17.
6. Galea LA, Leuner B, Slattery DA. Hippocampal plasticity during the peripartum period: influence of sex steroids, stress and ageing. J Neuroendocrinol. 2014;26(10):641-648.
7. Henry JF, Sherwin BB. Hormones and cognitive functioning during late pregnancy and postpartum: a longitudinal study. Behav Neurosci. 2012;126(1):73-85.
8. Barda G, Mizrachi Y, Borokchovich I, et al. The effect of pregnancy on maternal cognition. Sci Rep. 2011;11(1)12187. doi:10.1038/s41598-021-91504-9
9. Davies SJ, Lum JA, Skouteris H, et al. Cognitive impairment during pregnancy: a meta-analysis. Med J Aust. 2018;208(1):35-40.
10. Pownall M, Hutter RRC, Rockliffe L, et al. Memory and mood changes in pregnancy: a qualitative content analysis of women’s first-hand accounts. J Reprod Infant Psychol. 2023;41(5):516-527.
11. Hoekzema E, Barba-Müller E, Pozzobon C, et al. Pregnancy leads to long-lasting changes in human brain structure. Nat Neurosci. 2017;20(2):287-296.
12. Duarte-Guterman P, Leuner B, Galea LAM. The long and short term effects of motherhood on the brain. Front Neuroendocrinol. 2019;53:100740. doi:10.1016/j.yfrne.2019.02.004
13. Haim A, Julian D, Albin-Brooks C, et al. A survey of neuroimmune changes in pregnant and postpartum female rats. Brain Behav Immun. 2017;59:67-78.
14. Benson JC, Malyuk DF, Madhavan A, et al. Pituitary volume changes in pregnancy and the post-partum period. Neuroradiol J. 2023. doi:10.1177/19714009231196470
15. Schepanski S, Chini M, Sternemann V, et al. Pregnancy-induced maternal microchimerism shapes neurodevelopment and behavior in mice. Nat Commun. 2022;13(1):4571. doi:10.1038/s41467-022-32230-2
16. Larsen CM, Grattan DR. Prolactin, neurogenesis, and maternal behaviors. Brain Behav Immun. 2012;26(2):201-209.
Pregnancy is unquestionably a major milestone in a woman’s life. During gestation, her body shape noticeably changes, but the invisible structural and cognitive changes in her brain are more striking. Some of those neurobiological changes are short-term, while others are long-lasting, well beyond delivery, and even into old age.
Physiological changes during pregnancy are extraordinary. The dramatic increases in estrogen, progesterone, and glucocorticoids help maintain pregnancy, ensure safe delivery of the baby, and trigger maternal behavior. However, other important changes also occur in the mother’s cardiac output, blood volume, renal function, respiratory output, and immune adaptations to accommodate the growth of the fetus. Gene expression also occurs to accomplish those changes, and there are lifelong repercussions from those drastic physiological changes.
During pregnancy, the brain is exposed to escalating levels of hormones released from the placenta, which the woman had never experienced. Those hormones regulate neuroplasticity, neuroinflammation, behavior, and cognition.
Structural brain changes1-6
Brain volume declines during pregnancy, reaching a nadir at the time of parturition. However, recovery occurs within 5 months after delivery. During the postpartum period, gray matter volume increases in the first 3 to 4 weeks, especially in areas involved in maternal behavior, including the amygdala, prefrontal cortex, and hypothalamus. Hippocampal gray matter decreases at 2 months postpartum compared to preconception levels, and reductions can still be observed up to 2 years following delivery. Gray matter reductions occur in multiple brain regions involved in social cognition, including the superior temporal gyrus, medial and inferior frontal cortex, fusiform areas, and hippocampus. Those changes correlate with positive maternal attachment. It is noteworthy that neural activity is highest in areas with reduced gray volume, so a decline in brain volume is associated with enhanced maternal attachment. Interestingly, those changes occur in fathers, too.
Childbearing improves stroke outcomes in middle age, but body weight will increase. The risk of Alzheimer’s disease increases with a higher number of gestations, but longevity is higher if the pregnancy occurs at an older age. Reproduction is also associated with shorter telomeres, which can elevate the risk of cancer, inflammation, diabetes, and dementia.
Cognitive changes7-10
The term “pregnancy brain” refers to cognitive changes during pregnancy and postpartum; these include decreased memory and concentration, absent-mindedness, heightened reactivity to threatening stimuli, and a decrease in motivation and executive functions. After delivery a mother has increased empathy (sometimes referred to as Theory of Mind) and greater activation in brain structures involved in empathy, including the paracingulate cortex, the posterior cingulate, and the insula. Also, the mirror neuron system becomes more activated in response to a woman’s own children compared to unfamiliar children. This incudes the ventral premotor cortex, the inferior frontal gyrus, and the posterior parietal cortex.
Certain forms of memory are impaired during pregnancy and early postpartum, including verbal free recall and working memory, as well as executive functions. Those are believed to correlate with glucocorticoids and estrogen levels.
Continue to: The following cognitive functions...
The following cognitive functions increase between the first and second trimester: verbal memory, attention, executive functions processing speed, verbal, and visuospatial abilities. Interestingly, mothers of a male fetus outperformed mothers of a female fetus on working memory and spatial ability.
Other changes11-16
- Cells from the fetus can traffic to the mother’s body and create microchimeric cells, which have short-term benefits (healing some of the other’s organs as stem cells do) but long-term downsides include future brain disorders such as Parkinson’s disease or Alzheimer’s disease, as well as autoimmune diseases and various types of cancer. The reverse also occurs with cells transferring from the mother to the fetus, persisting into infancy and childhood.
- Postpartum psychosis is associated with reductions in the volumes of the anterior cingulate, left parahippocampal gyrus, and superior temporal gyrus.
- A woman’s white matter increases during pregnancy compared to preconception. This is attributed to the high levels of prolactin, which proliferates oligodendrocytes, the glial cells that continuously manufacture myelin.
- The pituitary gland increases by 200% to 300% during pregnancy and returns to pre-pregnancy levels approximately 8 months following delivery. Prolactin also mediates the production of brain cells in the hippocampus (ie, neurogenesis).
- Sexual activity, even without pregnancy, increases neurogenesis. Plasma levels of prolactin increase significantly following an orgasm in both men and women, which indicates that sexual activity has beneficial brain effects.
- With pregnancy, the immune system shifts from proinflammatory to anti-inflammatory signaling. This protects the fetus from being attacked and rejected as foreign tissue. However, at the end of pregnancy, there is a “burst” of proinflammatory signaling, which serves as a major trigger to induce uterine contractions and initiate labor (to expel the foreign tissue).
- Brain levels of the anti-inflammatory cytokine interleukin-6 increase in the postpartum period, which represents a significant modification in the neuroimmune environment, and the maternal brain assumes an inflammatory-resistant state, which has cognitive and neuroplasticity implications. However, this neuroimmune dysregulation is implicated in postpartum depression and anxiety.
- Older females who were never pregnant or only had 1 pregnancy had better overall cognitive functioning than females who became pregnant at an young age.
- In animal studies, reproduction alleviates the negative effects of aging on several hippocampal functions, especially neurogenesis. Dendritic spine density in the CA1 region of the hippocampus is higher in pregnancy and early postpartum period compared to nulliparous females (based on animal studies).
Pregnancy is indispensable for the perpetuation of the species. Its hormonal, physiologic, neurobiological, and cognitive correlates are extensive. The cognitive changes in the postpartum period are designed by evolution to prepare a woman to care for her newborn and to ensure its survival. But the biological sequelae of pregnancy extend to the rest of a woman’s life and may predispose her to immune and brain disorders as she ages.
Pregnancy is unquestionably a major milestone in a woman’s life. During gestation, her body shape noticeably changes, but the invisible structural and cognitive changes in her brain are more striking. Some of those neurobiological changes are short-term, while others are long-lasting, well beyond delivery, and even into old age.
Physiological changes during pregnancy are extraordinary. The dramatic increases in estrogen, progesterone, and glucocorticoids help maintain pregnancy, ensure safe delivery of the baby, and trigger maternal behavior. However, other important changes also occur in the mother’s cardiac output, blood volume, renal function, respiratory output, and immune adaptations to accommodate the growth of the fetus. Gene expression also occurs to accomplish those changes, and there are lifelong repercussions from those drastic physiological changes.
During pregnancy, the brain is exposed to escalating levels of hormones released from the placenta, which the woman had never experienced. Those hormones regulate neuroplasticity, neuroinflammation, behavior, and cognition.
Structural brain changes1-6
Brain volume declines during pregnancy, reaching a nadir at the time of parturition. However, recovery occurs within 5 months after delivery. During the postpartum period, gray matter volume increases in the first 3 to 4 weeks, especially in areas involved in maternal behavior, including the amygdala, prefrontal cortex, and hypothalamus. Hippocampal gray matter decreases at 2 months postpartum compared to preconception levels, and reductions can still be observed up to 2 years following delivery. Gray matter reductions occur in multiple brain regions involved in social cognition, including the superior temporal gyrus, medial and inferior frontal cortex, fusiform areas, and hippocampus. Those changes correlate with positive maternal attachment. It is noteworthy that neural activity is highest in areas with reduced gray volume, so a decline in brain volume is associated with enhanced maternal attachment. Interestingly, those changes occur in fathers, too.
Childbearing improves stroke outcomes in middle age, but body weight will increase. The risk of Alzheimer’s disease increases with a higher number of gestations, but longevity is higher if the pregnancy occurs at an older age. Reproduction is also associated with shorter telomeres, which can elevate the risk of cancer, inflammation, diabetes, and dementia.
Cognitive changes7-10
The term “pregnancy brain” refers to cognitive changes during pregnancy and postpartum; these include decreased memory and concentration, absent-mindedness, heightened reactivity to threatening stimuli, and a decrease in motivation and executive functions. After delivery a mother has increased empathy (sometimes referred to as Theory of Mind) and greater activation in brain structures involved in empathy, including the paracingulate cortex, the posterior cingulate, and the insula. Also, the mirror neuron system becomes more activated in response to a woman’s own children compared to unfamiliar children. This incudes the ventral premotor cortex, the inferior frontal gyrus, and the posterior parietal cortex.
Certain forms of memory are impaired during pregnancy and early postpartum, including verbal free recall and working memory, as well as executive functions. Those are believed to correlate with glucocorticoids and estrogen levels.
Continue to: The following cognitive functions...
The following cognitive functions increase between the first and second trimester: verbal memory, attention, executive functions processing speed, verbal, and visuospatial abilities. Interestingly, mothers of a male fetus outperformed mothers of a female fetus on working memory and spatial ability.
Other changes11-16
- Cells from the fetus can traffic to the mother’s body and create microchimeric cells, which have short-term benefits (healing some of the other’s organs as stem cells do) but long-term downsides include future brain disorders such as Parkinson’s disease or Alzheimer’s disease, as well as autoimmune diseases and various types of cancer. The reverse also occurs with cells transferring from the mother to the fetus, persisting into infancy and childhood.
- Postpartum psychosis is associated with reductions in the volumes of the anterior cingulate, left parahippocampal gyrus, and superior temporal gyrus.
- A woman’s white matter increases during pregnancy compared to preconception. This is attributed to the high levels of prolactin, which proliferates oligodendrocytes, the glial cells that continuously manufacture myelin.
- The pituitary gland increases by 200% to 300% during pregnancy and returns to pre-pregnancy levels approximately 8 months following delivery. Prolactin also mediates the production of brain cells in the hippocampus (ie, neurogenesis).
- Sexual activity, even without pregnancy, increases neurogenesis. Plasma levels of prolactin increase significantly following an orgasm in both men and women, which indicates that sexual activity has beneficial brain effects.
- With pregnancy, the immune system shifts from proinflammatory to anti-inflammatory signaling. This protects the fetus from being attacked and rejected as foreign tissue. However, at the end of pregnancy, there is a “burst” of proinflammatory signaling, which serves as a major trigger to induce uterine contractions and initiate labor (to expel the foreign tissue).
- Brain levels of the anti-inflammatory cytokine interleukin-6 increase in the postpartum period, which represents a significant modification in the neuroimmune environment, and the maternal brain assumes an inflammatory-resistant state, which has cognitive and neuroplasticity implications. However, this neuroimmune dysregulation is implicated in postpartum depression and anxiety.
- Older females who were never pregnant or only had 1 pregnancy had better overall cognitive functioning than females who became pregnant at an young age.
- In animal studies, reproduction alleviates the negative effects of aging on several hippocampal functions, especially neurogenesis. Dendritic spine density in the CA1 region of the hippocampus is higher in pregnancy and early postpartum period compared to nulliparous females (based on animal studies).
Pregnancy is indispensable for the perpetuation of the species. Its hormonal, physiologic, neurobiological, and cognitive correlates are extensive. The cognitive changes in the postpartum period are designed by evolution to prepare a woman to care for her newborn and to ensure its survival. But the biological sequelae of pregnancy extend to the rest of a woman’s life and may predispose her to immune and brain disorders as she ages.
1. Barba-Müller E, Craddock S, Carmona S, et al. Brain plasticity in pregnancy and the postpartum period: links to maternal caregiving and mental health. Arch Womens Ment Health. 2019;22(2):289-299.
2. Pawluski JL, Hoekzema E, Leuner B, et al. Less can be more: fine tuning the maternal brain. Neurosci Biobehav Rev. 2022;133:104475. doi:10.1016/j.neubiorev.2021.11.045
3. Hoekzema E, Barba-Müller E, Pozzobon C, et al. Pregnancy leads to long-lasting changes in human brain structure. Nat Neurosci. 2017;20(2):287-296.
4. Cárdenas EF, Kujawa A, Humphreys KL. Neurobiological changes during the peripartum period: implications for health and behavior. Soc Cogn Affect Neurosci. 2020;15(10):1097-1110.
5. Eid RS, Chaiton JA, Lieblich SE, et al. Early and late effects of maternal experience on hippocampal neurogenesis, microglia, and the circulating cytokine milieu. Neurobiol Aging. 2019;78:1-17.
6. Galea LA, Leuner B, Slattery DA. Hippocampal plasticity during the peripartum period: influence of sex steroids, stress and ageing. J Neuroendocrinol. 2014;26(10):641-648.
7. Henry JF, Sherwin BB. Hormones and cognitive functioning during late pregnancy and postpartum: a longitudinal study. Behav Neurosci. 2012;126(1):73-85.
8. Barda G, Mizrachi Y, Borokchovich I, et al. The effect of pregnancy on maternal cognition. Sci Rep. 2011;11(1)12187. doi:10.1038/s41598-021-91504-9
9. Davies SJ, Lum JA, Skouteris H, et al. Cognitive impairment during pregnancy: a meta-analysis. Med J Aust. 2018;208(1):35-40.
10. Pownall M, Hutter RRC, Rockliffe L, et al. Memory and mood changes in pregnancy: a qualitative content analysis of women’s first-hand accounts. J Reprod Infant Psychol. 2023;41(5):516-527.
11. Hoekzema E, Barba-Müller E, Pozzobon C, et al. Pregnancy leads to long-lasting changes in human brain structure. Nat Neurosci. 2017;20(2):287-296.
12. Duarte-Guterman P, Leuner B, Galea LAM. The long and short term effects of motherhood on the brain. Front Neuroendocrinol. 2019;53:100740. doi:10.1016/j.yfrne.2019.02.004
13. Haim A, Julian D, Albin-Brooks C, et al. A survey of neuroimmune changes in pregnant and postpartum female rats. Brain Behav Immun. 2017;59:67-78.
14. Benson JC, Malyuk DF, Madhavan A, et al. Pituitary volume changes in pregnancy and the post-partum period. Neuroradiol J. 2023. doi:10.1177/19714009231196470
15. Schepanski S, Chini M, Sternemann V, et al. Pregnancy-induced maternal microchimerism shapes neurodevelopment and behavior in mice. Nat Commun. 2022;13(1):4571. doi:10.1038/s41467-022-32230-2
16. Larsen CM, Grattan DR. Prolactin, neurogenesis, and maternal behaviors. Brain Behav Immun. 2012;26(2):201-209.
1. Barba-Müller E, Craddock S, Carmona S, et al. Brain plasticity in pregnancy and the postpartum period: links to maternal caregiving and mental health. Arch Womens Ment Health. 2019;22(2):289-299.
2. Pawluski JL, Hoekzema E, Leuner B, et al. Less can be more: fine tuning the maternal brain. Neurosci Biobehav Rev. 2022;133:104475. doi:10.1016/j.neubiorev.2021.11.045
3. Hoekzema E, Barba-Müller E, Pozzobon C, et al. Pregnancy leads to long-lasting changes in human brain structure. Nat Neurosci. 2017;20(2):287-296.
4. Cárdenas EF, Kujawa A, Humphreys KL. Neurobiological changes during the peripartum period: implications for health and behavior. Soc Cogn Affect Neurosci. 2020;15(10):1097-1110.
5. Eid RS, Chaiton JA, Lieblich SE, et al. Early and late effects of maternal experience on hippocampal neurogenesis, microglia, and the circulating cytokine milieu. Neurobiol Aging. 2019;78:1-17.
6. Galea LA, Leuner B, Slattery DA. Hippocampal plasticity during the peripartum period: influence of sex steroids, stress and ageing. J Neuroendocrinol. 2014;26(10):641-648.
7. Henry JF, Sherwin BB. Hormones and cognitive functioning during late pregnancy and postpartum: a longitudinal study. Behav Neurosci. 2012;126(1):73-85.
8. Barda G, Mizrachi Y, Borokchovich I, et al. The effect of pregnancy on maternal cognition. Sci Rep. 2011;11(1)12187. doi:10.1038/s41598-021-91504-9
9. Davies SJ, Lum JA, Skouteris H, et al. Cognitive impairment during pregnancy: a meta-analysis. Med J Aust. 2018;208(1):35-40.
10. Pownall M, Hutter RRC, Rockliffe L, et al. Memory and mood changes in pregnancy: a qualitative content analysis of women’s first-hand accounts. J Reprod Infant Psychol. 2023;41(5):516-527.
11. Hoekzema E, Barba-Müller E, Pozzobon C, et al. Pregnancy leads to long-lasting changes in human brain structure. Nat Neurosci. 2017;20(2):287-296.
12. Duarte-Guterman P, Leuner B, Galea LAM. The long and short term effects of motherhood on the brain. Front Neuroendocrinol. 2019;53:100740. doi:10.1016/j.yfrne.2019.02.004
13. Haim A, Julian D, Albin-Brooks C, et al. A survey of neuroimmune changes in pregnant and postpartum female rats. Brain Behav Immun. 2017;59:67-78.
14. Benson JC, Malyuk DF, Madhavan A, et al. Pituitary volume changes in pregnancy and the post-partum period. Neuroradiol J. 2023. doi:10.1177/19714009231196470
15. Schepanski S, Chini M, Sternemann V, et al. Pregnancy-induced maternal microchimerism shapes neurodevelopment and behavior in mice. Nat Commun. 2022;13(1):4571. doi:10.1038/s41467-022-32230-2
16. Larsen CM, Grattan DR. Prolactin, neurogenesis, and maternal behaviors. Brain Behav Immun. 2012;26(2):201-209.
Nonhormonal medication treatment of VMS
VMS, also known as hot flashes, night sweats, or cold sweats, occur for the majority of perimenopausal and menopausal women.1 In one study, the mean duration of clinically significant VMS was 5 years, and one-third of participants continued to have bothersome hot flashes 10 or more years after the onset of menopause.2 VMS may contribute to disrupted sleep patterns and depressed mood.3
All obstetrician-gynecologists know that estradiol and other estrogens are highly effective in the treatment of bothersome VMS. A meta-analysis reported that the frequency of VMS was reduced by 60% to 80% with oral estradiol (1 mg/day), transdermal estradiol(0.05 mg/day), and conjugated estrogen (0.625 mg).4 Breast tenderness and irregular uterine bleeding are common side effects of estrogen treatment of VMS. Estrogen treatment is contraindicated in patients with estrogen-responsive cancers, coronary heart disease, myocardial infarction, stroke, venous thromboembolism, and some cases of inherited thrombophilia. For these patients, an important option is the nonhormonal treatment of VMS, and several nonhormonal medications have been demonstrated to be effective therapy (TABLE 1). In this editorial I will review the medication treatment of VMS with escitalopram, paroxetine, gabapentin, and fezolinetant.
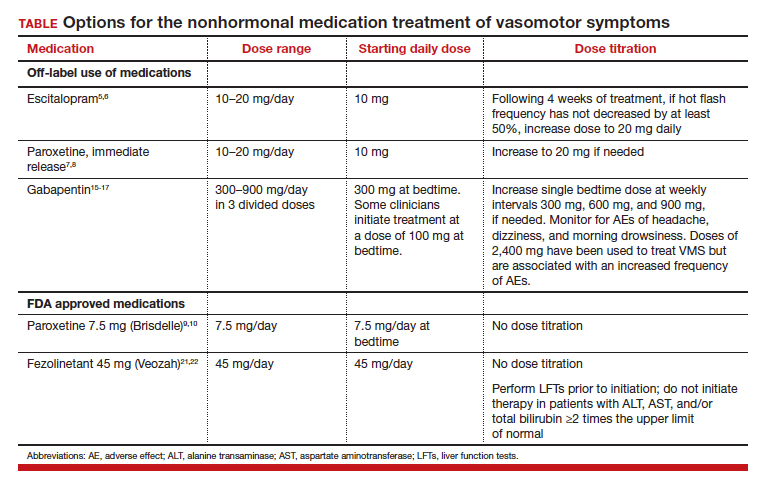
Escitalopram and paroxetine
Escitalopram and paroxetine have been shown to reduce VMS more than placebo in multiple clinical trials.5-10 In addition, escitalopram and paroxetine, at the doses tested, may be more effective for the treatment of VMS than sertraline, citalopram, or fluoxetine.11 In one trial assessing the efficacy of escitalopram to treat VMS, 205 patients with VMS were randomly assigned to 8 weeks of treatment with placebo or escitalopram.5 The initial escitalopram dose was 10 mg daily. At week 4:
- if VMS frequency was reduced by ≥ 50%, the patient remained on the 10-mg dose
- if VMS frequency was reduced by < 50%, the escitalopram dose was increased to 20 mg daily.
Following 8 weeks of treatment, the frequency of VMS decreased for patients in the placebo and escitalopram groups by 33% and 47%, respectively. Similar results have been reported in other studies.6
Paroxetine at a dose of 7.5 mg/day administered at bedtime is approved by the US Food and Drug Administration (FDA) for the treatment of VMS. In a pivotal study, 1,112 patients with VMS were randomly assigned to receive a placebo or paroxetine 7.5 mg at bedtime.9 In the 12-week study the reported decrease in mean weekly frequency of VMS for patients in the placebo and paroxetine groups were -37 and -44, respectively.9 Paroxetine 7.5 mg also reduced awakenings per night attributed to VMS and increased nighttime sleep duration.10
Depressed mood is prevalent among perimenopausal and postmenopausal patients.12 Prescribing escitalopram or paroxetine for VMS also may improve mood. Venlafaxine and desvenlafaxine are effective for the treatment of VMS;13,14 however, I seldom prescribe these medications for VMS because in my experience they are associated with more bothersome side effects, including dry mouth, decreased appetite, nausea, and insomnia than escitalopram or low-dose paroxetine.
Gabapentin
Numerous randomized clinical trials have reported that gabapentin is superior to placebo for the treatment of VMS.15 In one trial, 420 patients with breast cancer and VMS were randomly assigned to 8 weeks of treatment with placebo, gabapentin 300 mg/day (G300), or gabapentin 900 mg/day (G900) in 3 divided doses.16 Following 8 weeks of treatment, reduction in hot-flash severity score among patients receiving placebo, G300, or G900 was 15%, 31%, and 46%, respectively. Fatigue and somnolence were reported more frequently among patients taking gabapentin 900 mg/day. In a small trial, 60 patients with VMS were randomized to receive placebo, conjugated estrogen (0.2625 mg/day),or gabapentin (target dose of 2,400 mg/day in 3 divided doses).17 Following 12 weeks of treatment, the patient-reported decrease in VMS for those taking placebo, estrogen, or gabapentin was 54%, 72%, and 71%, respectively.
High-dose gabapentin treatment was associated with side effects of headache and dizziness more often than placebo or estrogen. Although gabapentin is not a treatment for insomnia, in my practice if a menopausal patient has prominent and bothersome symptoms of sleep disturbance and mild VMS symptoms, I will consider a trial of low-dose gabapentin. Some experts recommend initiating gabapentin at a dose of 100 mgdaily before bedtime to assess the effectiveness of a low dose that seldom causes significant side effects.

Fezolinetant
In a study of genetic variation associated with VMS, investigators discovered that nucleic acid variation in the neurokinin 3 (NK3) receptor was strongly associated with the prevalence of VMS, suggesting that this receptor is in the causal pathway to menopausal VMS.18 Additional research demonstrated that the kisspeptin/neurokinin B/dynorphin (KNDy) neurons, which are involved in the control of hypothalamic thermoregulation, are stimulated by neurokinin B, acting through the NK3 receptor, and suppressed by estradiol. A reduction in hypothalamic estrogen results in unopposed neurokinin B activity, which stimulates KNDy neurons, destabilizing the hypothalamic thermoregulatory center, causing vasodilation, which is perceived as hot flashes and sweating followed by chills.19
Fezolinetant is a high-affinity NK3 receptor antagonist that blocks the activity of neurokinin B, stabilizing the hypothalamic thermoregulatory center, thereby suppressing hot flashes. It is approved by the FDA for the treatment of moderate to severe VMS due to menopause using a fixed dose of 45 mg daily.20 In one clinical trial, 500 menopausal patients with bothersome VMS were randomly assigned to 12 weeks of treatment with placebo, fezolinetant 30 mg/day, or fezolinetant 45 mg/day. Following 12 weeks of treatment, the reported frequency rates of VMS among patients in the placebo, F30, and F45 groups were reduced by 43%, 61%, and 64%, respectively.21 In addition, following 12 weeks of treatment, the severity of VMS rates among patients in the placebo, F30, and F45 groups were reduced by 20%, 26%, and 32%, respectively.
Fezolinetant improved the quality of sleep and was associated with an improvement in patient-reported quality of life. Following 12 weeks of treatment, sleep quality among patients in the placebo, F30, and F45 groups was reported to be “much or moderately better” in 34%, 45%, and 54% of the patients, respectively.21 Similar results were reported in a companion study.22
Fezolinetant is contraindicated for patients with liver cirrhosis or severe renal impairment (estimated glomerular filtration rate of < 30 mL/min/1.73 m2). Before initiating treatment, serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and bilirubin (total and direct). Fezolinetant should not be prescribed if any of these tests are greater than twice the upper limit of normal. These tests should be repeated at 3, 6, and 9 months, and if the patient reports symptoms or signs of liver injury (nausea, vomiting, jaundice). Fezolinetant is metabolized by CYP1A2 and should not be prescribed to patients taking strong CYP1A2 inhibitors. The most common side effects associated with fezolinetant treatment are abdominal pain (4.3%), diarrhea (3.9%), insomnia (3.9%), back pain (3.0%), and hepatic transaminase elevation (2.3%). Fezolinetant has not been thoroughly evaluated in patients older than age 65. Following an oral dose of the medication, the median maximum concentration is reached in 1.5 hours, and the half-life is estimated to be 10 hours.20 Of all the medications discussed in this editorial, fezolinetant is the most expensive.
Effective VMS treatment improves overall health
Estrogen therapy is the gold standard treatment of VMS. However, many menopausal patients with bothersome VMS prefer not to take estrogen, and some have a medical condition that is a contraindication to estrogen treatment. The nonhormonal medication options for the treatment of VMS include escitalopram, paroxetine, gabapentin, and fezolinetant. Patients value the ability to choose the treatment they prefer, among all available hormonal and nonhormonal medication options. For mid-life women, effectively treating bothersome VMS is only one of many interventions that improves health. Optimal health is best achieved with23:
- high-quality diet
- daily physical activity
- appropriate body mass index
- nicotine avoidance
- a healthy sleep schedule
- normal blood pressure, lipid, and glucose levels.
Women who have a high-quality diet; daily physical activity; an appropriate body mass index; and normal blood pressure, cholesterol, and glucose levels are estimated to live 9 disease-free years longer than other women.24 ●
- Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopause transition: study of women’s health across the nation. Am J Pub Health. 2006;1226-1235.
- Freeman EW, Sammel MD, Sanders RJ. Risk of long-term hot flashes after natural menopause: evidence from the Penn Ovarian Aging Study cohort. Menopause. 2014;21:924-932.
- Hatcher KM, Smith RL, Chiang C, et al. Nocturnal hot flashes, but not serum hormone concentrations as a predictor of insomnia in menopausal women: results from the Midlife Women’s Health Study. J Women’s Health. 2023;32:94-101.
- Nelson HD. Commonly used types of postmenopausal estrogen for treatment of hot flashes: scientific review. JAMA. 2004;291:1610.
- Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011;305:267-227.
- Carpenter JS, Guthrie KA, Larson JC, et al. Effect of escitalopram on hot flash interference: a randomized, controlled trial. Fertil Steril. 2012;97:1399-1404.e1.
- Slaton RM, Champion MN, Palmore KB. A review of paroxetine for the treatment of vasomotor symptoms. J Pharm Pract. 2015;28:266-274.
- Stearns V, Slack R, Greep N, et al. Paroxetine is an effective treatment for hot flashes: results from a prospective randomized clinical trial. J Clin Oncol. 2005;23:6919-6930.
- Simon JA, Portman DJ, Kaunitz AM, et al. Lowdose paroxetine 7.5 mg for menopausal vasomotor symptoms: two randomized controlled trials. Menopause. 2013;20:1027-1035.
- Pinkerton JV, Joffe H, Kazempour K, et al. Lowdose paroxetine (7.5 mg) improves sleep in women with vasomotor symptoms associated with menopause. Menopause. 2015;22:50-58.
- Shams T, Firwana B, Habib F, et al. SSRIs for hot flashes: a systematic review and metaanalysis of randomized trials. J Gen Intern Med. 2014;29:204-213.
- Freeman EW. Depression in the menopause transition: risks in the changing hormone milieu as observed in the general population. Womens Midlife Health. 2015;1:2.
- Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet. 2000;356:2059-2063.
- Sun Z, Hao Y, Zhang M. Efficacy and safety of desvenlafaxine treatment for hot flashes associated with menopause: a meta-analysis of randomized controlled trials. Gynecol Obstet Invest. 2013;75:255-262.
- Toulis KA, Tzellos T, Kouvelas D, et al. Gabapentin for the treatment of hot flashes in women with natural or tamoxifen-induced menopause: a systematic review and meta-analysis. Clin Ther. 2009;31:221-235.
- Pandya KJ, Morrow GR, Roscoe JA, et al. Gabapentin for hot flashes in 420 women with breast cancer: a randomized double-blind placebocontrolled trial. Lancet. 2005;366:818-824.
- Reddy SY, Warner H, Guttuso T Jr, et al. Gabapentin, estrogen, and placebo for treating hot flushes: a randomized controlled trial. Obstet Gynecol. 2006;108:41-48.
- Crandall CJ, Manson JE, Hohensee C, et al. Association of genetic variation in the tachykinin receptor 3 locus with hot flashes and night sweats in the Women’s Health Initiative Study. Menopause. 2017;24:252.
- Rance NE, Dacks PA, Mittelman-Smith MA, et al. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neurendocrinol. 2013;34:211-227.
- Veozah (package insert). Astellas Pharma; Northbrook, Illinois. May 2023.
- Johnson KA, Martin N, Nappi RE, et al. Efficacy and safety of fezolinetant in moderate-to-severe vasomotor symptoms associated with menopause: a Phase 3 RCT. J Clin Endocrinol Metab. 2023;108:1981-1997.
- Lederman S, Ottery FD, Cano A, et al. Fezolinetant for treatment of moderate-to-severe vasomotor symptoms associated with menopause (SKYLIGHT 1): a phase 3 randomised controlled study. Lancet. 2023;401:1091-1102.
- Lloyd-Jones DM, Allen NB, Anderson CAM, et al. Life’s essential 8: updating and enhancing the American Heart Association’s construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. 2022;146:e18-43.
- Wang X, Ma H, Li X, et al. Association of cardiovascular health with life expectancy free of cardiovascular disease, diabetes, cancer, and dementia in U.K. adults. JAMA Int Med. 2023;183:340-349.
VMS, also known as hot flashes, night sweats, or cold sweats, occur for the majority of perimenopausal and menopausal women.1 In one study, the mean duration of clinically significant VMS was 5 years, and one-third of participants continued to have bothersome hot flashes 10 or more years after the onset of menopause.2 VMS may contribute to disrupted sleep patterns and depressed mood.3
All obstetrician-gynecologists know that estradiol and other estrogens are highly effective in the treatment of bothersome VMS. A meta-analysis reported that the frequency of VMS was reduced by 60% to 80% with oral estradiol (1 mg/day), transdermal estradiol(0.05 mg/day), and conjugated estrogen (0.625 mg).4 Breast tenderness and irregular uterine bleeding are common side effects of estrogen treatment of VMS. Estrogen treatment is contraindicated in patients with estrogen-responsive cancers, coronary heart disease, myocardial infarction, stroke, venous thromboembolism, and some cases of inherited thrombophilia. For these patients, an important option is the nonhormonal treatment of VMS, and several nonhormonal medications have been demonstrated to be effective therapy (TABLE 1). In this editorial I will review the medication treatment of VMS with escitalopram, paroxetine, gabapentin, and fezolinetant.

Escitalopram and paroxetine
Escitalopram and paroxetine have been shown to reduce VMS more than placebo in multiple clinical trials.5-10 In addition, escitalopram and paroxetine, at the doses tested, may be more effective for the treatment of VMS than sertraline, citalopram, or fluoxetine.11 In one trial assessing the efficacy of escitalopram to treat VMS, 205 patients with VMS were randomly assigned to 8 weeks of treatment with placebo or escitalopram.5 The initial escitalopram dose was 10 mg daily. At week 4:
- if VMS frequency was reduced by ≥ 50%, the patient remained on the 10-mg dose
- if VMS frequency was reduced by < 50%, the escitalopram dose was increased to 20 mg daily.
Following 8 weeks of treatment, the frequency of VMS decreased for patients in the placebo and escitalopram groups by 33% and 47%, respectively. Similar results have been reported in other studies.6
Paroxetine at a dose of 7.5 mg/day administered at bedtime is approved by the US Food and Drug Administration (FDA) for the treatment of VMS. In a pivotal study, 1,112 patients with VMS were randomly assigned to receive a placebo or paroxetine 7.5 mg at bedtime.9 In the 12-week study the reported decrease in mean weekly frequency of VMS for patients in the placebo and paroxetine groups were -37 and -44, respectively.9 Paroxetine 7.5 mg also reduced awakenings per night attributed to VMS and increased nighttime sleep duration.10
Depressed mood is prevalent among perimenopausal and postmenopausal patients.12 Prescribing escitalopram or paroxetine for VMS also may improve mood. Venlafaxine and desvenlafaxine are effective for the treatment of VMS;13,14 however, I seldom prescribe these medications for VMS because in my experience they are associated with more bothersome side effects, including dry mouth, decreased appetite, nausea, and insomnia than escitalopram or low-dose paroxetine.
Gabapentin
Numerous randomized clinical trials have reported that gabapentin is superior to placebo for the treatment of VMS.15 In one trial, 420 patients with breast cancer and VMS were randomly assigned to 8 weeks of treatment with placebo, gabapentin 300 mg/day (G300), or gabapentin 900 mg/day (G900) in 3 divided doses.16 Following 8 weeks of treatment, reduction in hot-flash severity score among patients receiving placebo, G300, or G900 was 15%, 31%, and 46%, respectively. Fatigue and somnolence were reported more frequently among patients taking gabapentin 900 mg/day. In a small trial, 60 patients with VMS were randomized to receive placebo, conjugated estrogen (0.2625 mg/day),or gabapentin (target dose of 2,400 mg/day in 3 divided doses).17 Following 12 weeks of treatment, the patient-reported decrease in VMS for those taking placebo, estrogen, or gabapentin was 54%, 72%, and 71%, respectively.
High-dose gabapentin treatment was associated with side effects of headache and dizziness more often than placebo or estrogen. Although gabapentin is not a treatment for insomnia, in my practice if a menopausal patient has prominent and bothersome symptoms of sleep disturbance and mild VMS symptoms, I will consider a trial of low-dose gabapentin. Some experts recommend initiating gabapentin at a dose of 100 mgdaily before bedtime to assess the effectiveness of a low dose that seldom causes significant side effects.

Fezolinetant
In a study of genetic variation associated with VMS, investigators discovered that nucleic acid variation in the neurokinin 3 (NK3) receptor was strongly associated with the prevalence of VMS, suggesting that this receptor is in the causal pathway to menopausal VMS.18 Additional research demonstrated that the kisspeptin/neurokinin B/dynorphin (KNDy) neurons, which are involved in the control of hypothalamic thermoregulation, are stimulated by neurokinin B, acting through the NK3 receptor, and suppressed by estradiol. A reduction in hypothalamic estrogen results in unopposed neurokinin B activity, which stimulates KNDy neurons, destabilizing the hypothalamic thermoregulatory center, causing vasodilation, which is perceived as hot flashes and sweating followed by chills.19
Fezolinetant is a high-affinity NK3 receptor antagonist that blocks the activity of neurokinin B, stabilizing the hypothalamic thermoregulatory center, thereby suppressing hot flashes. It is approved by the FDA for the treatment of moderate to severe VMS due to menopause using a fixed dose of 45 mg daily.20 In one clinical trial, 500 menopausal patients with bothersome VMS were randomly assigned to 12 weeks of treatment with placebo, fezolinetant 30 mg/day, or fezolinetant 45 mg/day. Following 12 weeks of treatment, the reported frequency rates of VMS among patients in the placebo, F30, and F45 groups were reduced by 43%, 61%, and 64%, respectively.21 In addition, following 12 weeks of treatment, the severity of VMS rates among patients in the placebo, F30, and F45 groups were reduced by 20%, 26%, and 32%, respectively.
Fezolinetant improved the quality of sleep and was associated with an improvement in patient-reported quality of life. Following 12 weeks of treatment, sleep quality among patients in the placebo, F30, and F45 groups was reported to be “much or moderately better” in 34%, 45%, and 54% of the patients, respectively.21 Similar results were reported in a companion study.22
Fezolinetant is contraindicated for patients with liver cirrhosis or severe renal impairment (estimated glomerular filtration rate of < 30 mL/min/1.73 m2). Before initiating treatment, serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and bilirubin (total and direct). Fezolinetant should not be prescribed if any of these tests are greater than twice the upper limit of normal. These tests should be repeated at 3, 6, and 9 months, and if the patient reports symptoms or signs of liver injury (nausea, vomiting, jaundice). Fezolinetant is metabolized by CYP1A2 and should not be prescribed to patients taking strong CYP1A2 inhibitors. The most common side effects associated with fezolinetant treatment are abdominal pain (4.3%), diarrhea (3.9%), insomnia (3.9%), back pain (3.0%), and hepatic transaminase elevation (2.3%). Fezolinetant has not been thoroughly evaluated in patients older than age 65. Following an oral dose of the medication, the median maximum concentration is reached in 1.5 hours, and the half-life is estimated to be 10 hours.20 Of all the medications discussed in this editorial, fezolinetant is the most expensive.
Effective VMS treatment improves overall health
Estrogen therapy is the gold standard treatment of VMS. However, many menopausal patients with bothersome VMS prefer not to take estrogen, and some have a medical condition that is a contraindication to estrogen treatment. The nonhormonal medication options for the treatment of VMS include escitalopram, paroxetine, gabapentin, and fezolinetant. Patients value the ability to choose the treatment they prefer, among all available hormonal and nonhormonal medication options. For mid-life women, effectively treating bothersome VMS is only one of many interventions that improves health. Optimal health is best achieved with23:
- high-quality diet
- daily physical activity
- appropriate body mass index
- nicotine avoidance
- a healthy sleep schedule
- normal blood pressure, lipid, and glucose levels.
Women who have a high-quality diet; daily physical activity; an appropriate body mass index; and normal blood pressure, cholesterol, and glucose levels are estimated to live 9 disease-free years longer than other women.24 ●
VMS, also known as hot flashes, night sweats, or cold sweats, occur for the majority of perimenopausal and menopausal women.1 In one study, the mean duration of clinically significant VMS was 5 years, and one-third of participants continued to have bothersome hot flashes 10 or more years after the onset of menopause.2 VMS may contribute to disrupted sleep patterns and depressed mood.3
All obstetrician-gynecologists know that estradiol and other estrogens are highly effective in the treatment of bothersome VMS. A meta-analysis reported that the frequency of VMS was reduced by 60% to 80% with oral estradiol (1 mg/day), transdermal estradiol(0.05 mg/day), and conjugated estrogen (0.625 mg).4 Breast tenderness and irregular uterine bleeding are common side effects of estrogen treatment of VMS. Estrogen treatment is contraindicated in patients with estrogen-responsive cancers, coronary heart disease, myocardial infarction, stroke, venous thromboembolism, and some cases of inherited thrombophilia. For these patients, an important option is the nonhormonal treatment of VMS, and several nonhormonal medications have been demonstrated to be effective therapy (TABLE 1). In this editorial I will review the medication treatment of VMS with escitalopram, paroxetine, gabapentin, and fezolinetant.

Escitalopram and paroxetine
Escitalopram and paroxetine have been shown to reduce VMS more than placebo in multiple clinical trials.5-10 In addition, escitalopram and paroxetine, at the doses tested, may be more effective for the treatment of VMS than sertraline, citalopram, or fluoxetine.11 In one trial assessing the efficacy of escitalopram to treat VMS, 205 patients with VMS were randomly assigned to 8 weeks of treatment with placebo or escitalopram.5 The initial escitalopram dose was 10 mg daily. At week 4:
- if VMS frequency was reduced by ≥ 50%, the patient remained on the 10-mg dose
- if VMS frequency was reduced by < 50%, the escitalopram dose was increased to 20 mg daily.
Following 8 weeks of treatment, the frequency of VMS decreased for patients in the placebo and escitalopram groups by 33% and 47%, respectively. Similar results have been reported in other studies.6
Paroxetine at a dose of 7.5 mg/day administered at bedtime is approved by the US Food and Drug Administration (FDA) for the treatment of VMS. In a pivotal study, 1,112 patients with VMS were randomly assigned to receive a placebo or paroxetine 7.5 mg at bedtime.9 In the 12-week study the reported decrease in mean weekly frequency of VMS for patients in the placebo and paroxetine groups were -37 and -44, respectively.9 Paroxetine 7.5 mg also reduced awakenings per night attributed to VMS and increased nighttime sleep duration.10
Depressed mood is prevalent among perimenopausal and postmenopausal patients.12 Prescribing escitalopram or paroxetine for VMS also may improve mood. Venlafaxine and desvenlafaxine are effective for the treatment of VMS;13,14 however, I seldom prescribe these medications for VMS because in my experience they are associated with more bothersome side effects, including dry mouth, decreased appetite, nausea, and insomnia than escitalopram or low-dose paroxetine.
Gabapentin
Numerous randomized clinical trials have reported that gabapentin is superior to placebo for the treatment of VMS.15 In one trial, 420 patients with breast cancer and VMS were randomly assigned to 8 weeks of treatment with placebo, gabapentin 300 mg/day (G300), or gabapentin 900 mg/day (G900) in 3 divided doses.16 Following 8 weeks of treatment, reduction in hot-flash severity score among patients receiving placebo, G300, or G900 was 15%, 31%, and 46%, respectively. Fatigue and somnolence were reported more frequently among patients taking gabapentin 900 mg/day. In a small trial, 60 patients with VMS were randomized to receive placebo, conjugated estrogen (0.2625 mg/day),or gabapentin (target dose of 2,400 mg/day in 3 divided doses).17 Following 12 weeks of treatment, the patient-reported decrease in VMS for those taking placebo, estrogen, or gabapentin was 54%, 72%, and 71%, respectively.
High-dose gabapentin treatment was associated with side effects of headache and dizziness more often than placebo or estrogen. Although gabapentin is not a treatment for insomnia, in my practice if a menopausal patient has prominent and bothersome symptoms of sleep disturbance and mild VMS symptoms, I will consider a trial of low-dose gabapentin. Some experts recommend initiating gabapentin at a dose of 100 mgdaily before bedtime to assess the effectiveness of a low dose that seldom causes significant side effects.

Fezolinetant
In a study of genetic variation associated with VMS, investigators discovered that nucleic acid variation in the neurokinin 3 (NK3) receptor was strongly associated with the prevalence of VMS, suggesting that this receptor is in the causal pathway to menopausal VMS.18 Additional research demonstrated that the kisspeptin/neurokinin B/dynorphin (KNDy) neurons, which are involved in the control of hypothalamic thermoregulation, are stimulated by neurokinin B, acting through the NK3 receptor, and suppressed by estradiol. A reduction in hypothalamic estrogen results in unopposed neurokinin B activity, which stimulates KNDy neurons, destabilizing the hypothalamic thermoregulatory center, causing vasodilation, which is perceived as hot flashes and sweating followed by chills.19
Fezolinetant is a high-affinity NK3 receptor antagonist that blocks the activity of neurokinin B, stabilizing the hypothalamic thermoregulatory center, thereby suppressing hot flashes. It is approved by the FDA for the treatment of moderate to severe VMS due to menopause using a fixed dose of 45 mg daily.20 In one clinical trial, 500 menopausal patients with bothersome VMS were randomly assigned to 12 weeks of treatment with placebo, fezolinetant 30 mg/day, or fezolinetant 45 mg/day. Following 12 weeks of treatment, the reported frequency rates of VMS among patients in the placebo, F30, and F45 groups were reduced by 43%, 61%, and 64%, respectively.21 In addition, following 12 weeks of treatment, the severity of VMS rates among patients in the placebo, F30, and F45 groups were reduced by 20%, 26%, and 32%, respectively.
Fezolinetant improved the quality of sleep and was associated with an improvement in patient-reported quality of life. Following 12 weeks of treatment, sleep quality among patients in the placebo, F30, and F45 groups was reported to be “much or moderately better” in 34%, 45%, and 54% of the patients, respectively.21 Similar results were reported in a companion study.22
Fezolinetant is contraindicated for patients with liver cirrhosis or severe renal impairment (estimated glomerular filtration rate of < 30 mL/min/1.73 m2). Before initiating treatment, serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and bilirubin (total and direct). Fezolinetant should not be prescribed if any of these tests are greater than twice the upper limit of normal. These tests should be repeated at 3, 6, and 9 months, and if the patient reports symptoms or signs of liver injury (nausea, vomiting, jaundice). Fezolinetant is metabolized by CYP1A2 and should not be prescribed to patients taking strong CYP1A2 inhibitors. The most common side effects associated with fezolinetant treatment are abdominal pain (4.3%), diarrhea (3.9%), insomnia (3.9%), back pain (3.0%), and hepatic transaminase elevation (2.3%). Fezolinetant has not been thoroughly evaluated in patients older than age 65. Following an oral dose of the medication, the median maximum concentration is reached in 1.5 hours, and the half-life is estimated to be 10 hours.20 Of all the medications discussed in this editorial, fezolinetant is the most expensive.
Effective VMS treatment improves overall health
Estrogen therapy is the gold standard treatment of VMS. However, many menopausal patients with bothersome VMS prefer not to take estrogen, and some have a medical condition that is a contraindication to estrogen treatment. The nonhormonal medication options for the treatment of VMS include escitalopram, paroxetine, gabapentin, and fezolinetant. Patients value the ability to choose the treatment they prefer, among all available hormonal and nonhormonal medication options. For mid-life women, effectively treating bothersome VMS is only one of many interventions that improves health. Optimal health is best achieved with23:
- high-quality diet
- daily physical activity
- appropriate body mass index
- nicotine avoidance
- a healthy sleep schedule
- normal blood pressure, lipid, and glucose levels.
Women who have a high-quality diet; daily physical activity; an appropriate body mass index; and normal blood pressure, cholesterol, and glucose levels are estimated to live 9 disease-free years longer than other women.24 ●
- Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopause transition: study of women’s health across the nation. Am J Pub Health. 2006;1226-1235.
- Freeman EW, Sammel MD, Sanders RJ. Risk of long-term hot flashes after natural menopause: evidence from the Penn Ovarian Aging Study cohort. Menopause. 2014;21:924-932.
- Hatcher KM, Smith RL, Chiang C, et al. Nocturnal hot flashes, but not serum hormone concentrations as a predictor of insomnia in menopausal women: results from the Midlife Women’s Health Study. J Women’s Health. 2023;32:94-101.
- Nelson HD. Commonly used types of postmenopausal estrogen for treatment of hot flashes: scientific review. JAMA. 2004;291:1610.
- Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011;305:267-227.
- Carpenter JS, Guthrie KA, Larson JC, et al. Effect of escitalopram on hot flash interference: a randomized, controlled trial. Fertil Steril. 2012;97:1399-1404.e1.
- Slaton RM, Champion MN, Palmore KB. A review of paroxetine for the treatment of vasomotor symptoms. J Pharm Pract. 2015;28:266-274.
- Stearns V, Slack R, Greep N, et al. Paroxetine is an effective treatment for hot flashes: results from a prospective randomized clinical trial. J Clin Oncol. 2005;23:6919-6930.
- Simon JA, Portman DJ, Kaunitz AM, et al. Lowdose paroxetine 7.5 mg for menopausal vasomotor symptoms: two randomized controlled trials. Menopause. 2013;20:1027-1035.
- Pinkerton JV, Joffe H, Kazempour K, et al. Lowdose paroxetine (7.5 mg) improves sleep in women with vasomotor symptoms associated with menopause. Menopause. 2015;22:50-58.
- Shams T, Firwana B, Habib F, et al. SSRIs for hot flashes: a systematic review and metaanalysis of randomized trials. J Gen Intern Med. 2014;29:204-213.
- Freeman EW. Depression in the menopause transition: risks in the changing hormone milieu as observed in the general population. Womens Midlife Health. 2015;1:2.
- Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet. 2000;356:2059-2063.
- Sun Z, Hao Y, Zhang M. Efficacy and safety of desvenlafaxine treatment for hot flashes associated with menopause: a meta-analysis of randomized controlled trials. Gynecol Obstet Invest. 2013;75:255-262.
- Toulis KA, Tzellos T, Kouvelas D, et al. Gabapentin for the treatment of hot flashes in women with natural or tamoxifen-induced menopause: a systematic review and meta-analysis. Clin Ther. 2009;31:221-235.
- Pandya KJ, Morrow GR, Roscoe JA, et al. Gabapentin for hot flashes in 420 women with breast cancer: a randomized double-blind placebocontrolled trial. Lancet. 2005;366:818-824.
- Reddy SY, Warner H, Guttuso T Jr, et al. Gabapentin, estrogen, and placebo for treating hot flushes: a randomized controlled trial. Obstet Gynecol. 2006;108:41-48.
- Crandall CJ, Manson JE, Hohensee C, et al. Association of genetic variation in the tachykinin receptor 3 locus with hot flashes and night sweats in the Women’s Health Initiative Study. Menopause. 2017;24:252.
- Rance NE, Dacks PA, Mittelman-Smith MA, et al. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neurendocrinol. 2013;34:211-227.
- Veozah (package insert). Astellas Pharma; Northbrook, Illinois. May 2023.
- Johnson KA, Martin N, Nappi RE, et al. Efficacy and safety of fezolinetant in moderate-to-severe vasomotor symptoms associated with menopause: a Phase 3 RCT. J Clin Endocrinol Metab. 2023;108:1981-1997.
- Lederman S, Ottery FD, Cano A, et al. Fezolinetant for treatment of moderate-to-severe vasomotor symptoms associated with menopause (SKYLIGHT 1): a phase 3 randomised controlled study. Lancet. 2023;401:1091-1102.
- Lloyd-Jones DM, Allen NB, Anderson CAM, et al. Life’s essential 8: updating and enhancing the American Heart Association’s construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. 2022;146:e18-43.
- Wang X, Ma H, Li X, et al. Association of cardiovascular health with life expectancy free of cardiovascular disease, diabetes, cancer, and dementia in U.K. adults. JAMA Int Med. 2023;183:340-349.
- Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopause transition: study of women’s health across the nation. Am J Pub Health. 2006;1226-1235.
- Freeman EW, Sammel MD, Sanders RJ. Risk of long-term hot flashes after natural menopause: evidence from the Penn Ovarian Aging Study cohort. Menopause. 2014;21:924-932.
- Hatcher KM, Smith RL, Chiang C, et al. Nocturnal hot flashes, but not serum hormone concentrations as a predictor of insomnia in menopausal women: results from the Midlife Women’s Health Study. J Women’s Health. 2023;32:94-101.
- Nelson HD. Commonly used types of postmenopausal estrogen for treatment of hot flashes: scientific review. JAMA. 2004;291:1610.
- Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011;305:267-227.
- Carpenter JS, Guthrie KA, Larson JC, et al. Effect of escitalopram on hot flash interference: a randomized, controlled trial. Fertil Steril. 2012;97:1399-1404.e1.
- Slaton RM, Champion MN, Palmore KB. A review of paroxetine for the treatment of vasomotor symptoms. J Pharm Pract. 2015;28:266-274.
- Stearns V, Slack R, Greep N, et al. Paroxetine is an effective treatment for hot flashes: results from a prospective randomized clinical trial. J Clin Oncol. 2005;23:6919-6930.
- Simon JA, Portman DJ, Kaunitz AM, et al. Lowdose paroxetine 7.5 mg for menopausal vasomotor symptoms: two randomized controlled trials. Menopause. 2013;20:1027-1035.
- Pinkerton JV, Joffe H, Kazempour K, et al. Lowdose paroxetine (7.5 mg) improves sleep in women with vasomotor symptoms associated with menopause. Menopause. 2015;22:50-58.
- Shams T, Firwana B, Habib F, et al. SSRIs for hot flashes: a systematic review and metaanalysis of randomized trials. J Gen Intern Med. 2014;29:204-213.
- Freeman EW. Depression in the menopause transition: risks in the changing hormone milieu as observed in the general population. Womens Midlife Health. 2015;1:2.
- Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet. 2000;356:2059-2063.
- Sun Z, Hao Y, Zhang M. Efficacy and safety of desvenlafaxine treatment for hot flashes associated with menopause: a meta-analysis of randomized controlled trials. Gynecol Obstet Invest. 2013;75:255-262.
- Toulis KA, Tzellos T, Kouvelas D, et al. Gabapentin for the treatment of hot flashes in women with natural or tamoxifen-induced menopause: a systematic review and meta-analysis. Clin Ther. 2009;31:221-235.
- Pandya KJ, Morrow GR, Roscoe JA, et al. Gabapentin for hot flashes in 420 women with breast cancer: a randomized double-blind placebocontrolled trial. Lancet. 2005;366:818-824.
- Reddy SY, Warner H, Guttuso T Jr, et al. Gabapentin, estrogen, and placebo for treating hot flushes: a randomized controlled trial. Obstet Gynecol. 2006;108:41-48.
- Crandall CJ, Manson JE, Hohensee C, et al. Association of genetic variation in the tachykinin receptor 3 locus with hot flashes and night sweats in the Women’s Health Initiative Study. Menopause. 2017;24:252.
- Rance NE, Dacks PA, Mittelman-Smith MA, et al. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neurendocrinol. 2013;34:211-227.
- Veozah (package insert). Astellas Pharma; Northbrook, Illinois. May 2023.
- Johnson KA, Martin N, Nappi RE, et al. Efficacy and safety of fezolinetant in moderate-to-severe vasomotor symptoms associated with menopause: a Phase 3 RCT. J Clin Endocrinol Metab. 2023;108:1981-1997.
- Lederman S, Ottery FD, Cano A, et al. Fezolinetant for treatment of moderate-to-severe vasomotor symptoms associated with menopause (SKYLIGHT 1): a phase 3 randomised controlled study. Lancet. 2023;401:1091-1102.
- Lloyd-Jones DM, Allen NB, Anderson CAM, et al. Life’s essential 8: updating and enhancing the American Heart Association’s construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. 2022;146:e18-43.
- Wang X, Ma H, Li X, et al. Association of cardiovascular health with life expectancy free of cardiovascular disease, diabetes, cancer, and dementia in U.K. adults. JAMA Int Med. 2023;183:340-349.
The pandemic has permanently changed us, and its biopsychosocial sequelae linger…
Good riddance COVID-19 pandemic? Alas, that’s wishful thinking.
Many assume the pandemic is in our rearview mirror, but its biological, psychological, and social impacts continue to unfold. Its repercussions are etched into our brain, mind, emotions, behaviors, cognition, and outlook on life. Welcome to Pandemic 2.0.
Think of people who survive a heart attack. They experience multiple changes. Their initial ephemeral thrill of beating death is rapidly tempered with anxiety and worry about a future myocardial infarction and health issues in general. They become more risk-averse and more prone to dysphoria, irritability, and impatience. These individuals adopt a healthy lifestyle (diet and exercise), which they had neglected before. They develop more disciplined personality traits, feel a greater appreciation for being alive, and develop a closer affinity to family and friends. Simple things they had overlooked become more meaningful. They reevaluate their life goals, including career vs personal fulfilment. Some may overindulge in pleasurable activities in case their heart fails again. Some of those changes may be abrupt or transient, while others may become permanent features of their lives. And some may seek psychotherapy, which they may never have considered before.
The pandemic is the equivalent of a “societal cardiac arrest.” Its immediate impact was devastating. Bustling cities suddenly became ghost towns. Schools were closed, and children were locked at home with their parents, who were laid off. Businesses shut down; the economy tanked. Anxiety about being infected and dying skyrocketed, triggering a universal acute stress reaction that worsened the mental health of the population, but especially of the millions with preexisting psychiatric disorders. Routine medical and dental care stopped. Television and social media disseminated alarming updates about massive intensive care unit admissions and morgues overflowing with corpses of COVID-19 victims. Posttraumatic stress disorder (PTSD) was brewing across the nation as everyone faced this life-threatening pandemic.
The warp-speed development of vaccines for COVID-19 was equivalent to a defibrillator for the societal asystole, but the turmoil continued among the frazzled population. Some refused the vaccine due to conspiracy theories about their dangerous adverse effects. Employees in the private sector, state and federal government, and even the military who refused the mandatory vaccination lost their jobs. Controversy about shuttering schools and depriving children of face-to-face learning and socializing prompted some states to keep schools open, in contrast to most other states. Anger escalated about wearing masks, social distancing, and avoiding gatherings such as at restaurants or houses of worship. Cynicism and mistrust sprouted about the competence and reliability of health “experts” due to some conflicting signals, precluding wide adherence to medical advice.
The lingering effects of the COVID-19 pandemic
Those were the immediate repercussions of the pandemic. But what are its lingering effects? The sequelae extend across 1) the health care system; 2) the mental and emotional wellness of the population; 3) education; 4) work culture; 5) the economy; 6) societal operations; 7) technological and digital transformations; 8) mistrust in various societal institutions; 9) lack of confidence in medical information; and 10) preparedness for another pandemic due to a new strain.
As all psychiatrists know, the demand for mental health services continues to surge well after the pandemic has subsided, straining access to outpatient and inpatient care. Multiple lines of evidence confirm a deterioration in the long-term psychological well-being of children and adolescents because of lockdowns, social isolation, and anxiety about their own health and the health of their loved ones, leading to a serious rise in depression and suicidal behavior.1-3
Contunue to: Adults who survived pandemic...
Adults who survived the pandemic experienced grief during 2 very stressful years, with no peace of mind or “normal living.” Many began to contemplate the meaning of life and reevaluate the future, waxing more philosophical and embarking on “personal archeology.” The fragility of life suddenly became a ubiquitous epiphany that changed people’s habits. Working from home, which was necessary during the pandemic, became a preferred option for many, and home became an emotional refuge, not just a physical, brick-and-mortar refuge. Millions decided to quit working altogether (the “great resignation”).
Sexual activity declined precipitously during the pandemic for singles (French kissing became “the kiss of death”) but intercourse increased among couples, eventuating in a significant rise in births after the pandemic (a baby boomlet). Sexual interest among college students declined after the pandemic, which may be either due to fear of getting infected or a sublimation of libido to invest the energy in other, less risky activities.
At the societal level, the pandemic’s sequelae included a major shift to virtual communications, not just in health care (telepsychiatry and telemedicine) but also in business. Technology saved the day during the nadir of the pandemic by enabling psychiatrists and psychotherapists to treat their patients remotely. This was not technologically feasible during the past century’s influenza pandemics (1918, 1957, and 1968).
The intellectual and social development of an entire generation of children was stunted due to the COVID-19 pandemic. Consequences will continue to emerge in the years to come and may have ripple effects on this generation’s functioning. This may have particularly affected children of lower socioeconomic status, whose families cannot afford private schools and who are in dire need of good education to put them on the path of upward mobility.
As for adults who did not get infected by COVID-19, they suffered in 2 ways. First, they experienced a certain degree of brain atrophy, which is known to occur in chronic stress. This is attributed to persistent hypercortisolemia, which is toxic to the hippocampus. PTSD is well known to be associated with hippocampal atrophy.4 Additionally, a significant proportion of adults who contracted the COVID-19 virus and “recovered” were subsequently diagnosed with “long COVID,” with multiple neuropsychiatric symptoms, including psychosis, mania, depression, and panic attacks, as well as memory impairment and loss of the senses of smell and taste. For these individuals, the pandemic has not subsided; they will carry its neuropsychiatric scars for a long time.
Continue to: Economically, the pandemic...
Economically, the pandemic caused a horrific economic setback in its acute phase, which prompted the government to spend trillions to support the unemployed as well as blighted businesses. The economic sequalae of deficit spending of unprecedented proportions due to the pandemic triggered painful inflation that is ongoing. Interestingly, the numerical terms “billion” and “trillion” lost their loftiness as very huge numbers. Few people realize that counting to a billion (at one number per second) would take 31.7 years, while counting to a trillion would take 31,700 years! The inflationary impact of spending $6 trillion (which would take almost 200,000 years to count) becomes mathematically jarring. And despite the heroic measures to support the economy, some business perished, although others were created, changing the human architecture of the economy.
The pandemic drastically suppressed the “hunting and gathering” instinct of humans and demolished the fabled concept of work ethic. The “great resignation,” coupled with a desire to work from home on a mass scale, led to a glut of vacant office space in many large cities, lowering the value of commercial real estate. Following the pandemic, there was an uptick in moving away from urban areas, reflecting a creative destruction and reversal of a decades-long trend to gravitate to cities to work or live.
There was also political fallout from the pandemic. Staying at home is conducive to overdosing on television and social media, leading to an intensification and ossification of political hyperpartisanship and the further displacement of religious beliefs by passionately entrenched political beliefs. This continues to have seismic effects on political stability and harmony in our country. The pandemic may have instigated new models of national voting, which triggered further political friction.
Other examples of the pandemic’s aftereffects include a shortage of lifeguards and truck drivers, replacing the traditional handshake with a first bump, and increased spending on pleasurable activities (reminiscent of the Roaring 20s following the 1918 influenza pandemic), which may reflect an instinct to “live it up” before another deadly pandemic occurs.
Ironically, as I was finishing writing this article in early September 2023, the government announced that COVID-19 cases were again rising and a new vaccine was available for the new viral “strain.”
Here we go again: as the French saying goes: plus ça change, plus c’est la même chose…
1. Chavira DA, Ponting C, Ramos G. The impact of COVID-19 on child and adolescent mental health and treatment considerations. Behav Res Ther. 2022;157:104169. doi:10.1016/j.brat.2022.104169
2. Panchal U, Salazar de Pablo G, Franco M, et al. The impact of COVID-19 lockdown on child and adolescent mental health: systematic review. Eur Child Adolesc Psychiatry. 2023;32:1151-1177.
3. Mazrekaj D, De Witte K. The impact of school closures on learning and mental health of children: lessons from the COVID-19 pandemic. Perspectives on Psychological Science. 2023. https://doi.org/10.1177/17456916231181108
4. Logue MW, van Rooij SJH, Dennis EL, et al. A smaller hippocampal volume in posttraumatic stress disorder: a multisite ENIGMA-PGC study: subcortical volumetry results from posttraumatic stress disorder consortia. Biol Psychiatry. 2018;83(3):244-253.
Good riddance COVID-19 pandemic? Alas, that’s wishful thinking.
Many assume the pandemic is in our rearview mirror, but its biological, psychological, and social impacts continue to unfold. Its repercussions are etched into our brain, mind, emotions, behaviors, cognition, and outlook on life. Welcome to Pandemic 2.0.
Think of people who survive a heart attack. They experience multiple changes. Their initial ephemeral thrill of beating death is rapidly tempered with anxiety and worry about a future myocardial infarction and health issues in general. They become more risk-averse and more prone to dysphoria, irritability, and impatience. These individuals adopt a healthy lifestyle (diet and exercise), which they had neglected before. They develop more disciplined personality traits, feel a greater appreciation for being alive, and develop a closer affinity to family and friends. Simple things they had overlooked become more meaningful. They reevaluate their life goals, including career vs personal fulfilment. Some may overindulge in pleasurable activities in case their heart fails again. Some of those changes may be abrupt or transient, while others may become permanent features of their lives. And some may seek psychotherapy, which they may never have considered before.
The pandemic is the equivalent of a “societal cardiac arrest.” Its immediate impact was devastating. Bustling cities suddenly became ghost towns. Schools were closed, and children were locked at home with their parents, who were laid off. Businesses shut down; the economy tanked. Anxiety about being infected and dying skyrocketed, triggering a universal acute stress reaction that worsened the mental health of the population, but especially of the millions with preexisting psychiatric disorders. Routine medical and dental care stopped. Television and social media disseminated alarming updates about massive intensive care unit admissions and morgues overflowing with corpses of COVID-19 victims. Posttraumatic stress disorder (PTSD) was brewing across the nation as everyone faced this life-threatening pandemic.
The warp-speed development of vaccines for COVID-19 was equivalent to a defibrillator for the societal asystole, but the turmoil continued among the frazzled population. Some refused the vaccine due to conspiracy theories about their dangerous adverse effects. Employees in the private sector, state and federal government, and even the military who refused the mandatory vaccination lost their jobs. Controversy about shuttering schools and depriving children of face-to-face learning and socializing prompted some states to keep schools open, in contrast to most other states. Anger escalated about wearing masks, social distancing, and avoiding gatherings such as at restaurants or houses of worship. Cynicism and mistrust sprouted about the competence and reliability of health “experts” due to some conflicting signals, precluding wide adherence to medical advice.
The lingering effects of the COVID-19 pandemic
Those were the immediate repercussions of the pandemic. But what are its lingering effects? The sequelae extend across 1) the health care system; 2) the mental and emotional wellness of the population; 3) education; 4) work culture; 5) the economy; 6) societal operations; 7) technological and digital transformations; 8) mistrust in various societal institutions; 9) lack of confidence in medical information; and 10) preparedness for another pandemic due to a new strain.
As all psychiatrists know, the demand for mental health services continues to surge well after the pandemic has subsided, straining access to outpatient and inpatient care. Multiple lines of evidence confirm a deterioration in the long-term psychological well-being of children and adolescents because of lockdowns, social isolation, and anxiety about their own health and the health of their loved ones, leading to a serious rise in depression and suicidal behavior.1-3
Contunue to: Adults who survived pandemic...
Adults who survived the pandemic experienced grief during 2 very stressful years, with no peace of mind or “normal living.” Many began to contemplate the meaning of life and reevaluate the future, waxing more philosophical and embarking on “personal archeology.” The fragility of life suddenly became a ubiquitous epiphany that changed people’s habits. Working from home, which was necessary during the pandemic, became a preferred option for many, and home became an emotional refuge, not just a physical, brick-and-mortar refuge. Millions decided to quit working altogether (the “great resignation”).
Sexual activity declined precipitously during the pandemic for singles (French kissing became “the kiss of death”) but intercourse increased among couples, eventuating in a significant rise in births after the pandemic (a baby boomlet). Sexual interest among college students declined after the pandemic, which may be either due to fear of getting infected or a sublimation of libido to invest the energy in other, less risky activities.
At the societal level, the pandemic’s sequelae included a major shift to virtual communications, not just in health care (telepsychiatry and telemedicine) but also in business. Technology saved the day during the nadir of the pandemic by enabling psychiatrists and psychotherapists to treat their patients remotely. This was not technologically feasible during the past century’s influenza pandemics (1918, 1957, and 1968).
The intellectual and social development of an entire generation of children was stunted due to the COVID-19 pandemic. Consequences will continue to emerge in the years to come and may have ripple effects on this generation’s functioning. This may have particularly affected children of lower socioeconomic status, whose families cannot afford private schools and who are in dire need of good education to put them on the path of upward mobility.
As for adults who did not get infected by COVID-19, they suffered in 2 ways. First, they experienced a certain degree of brain atrophy, which is known to occur in chronic stress. This is attributed to persistent hypercortisolemia, which is toxic to the hippocampus. PTSD is well known to be associated with hippocampal atrophy.4 Additionally, a significant proportion of adults who contracted the COVID-19 virus and “recovered” were subsequently diagnosed with “long COVID,” with multiple neuropsychiatric symptoms, including psychosis, mania, depression, and panic attacks, as well as memory impairment and loss of the senses of smell and taste. For these individuals, the pandemic has not subsided; they will carry its neuropsychiatric scars for a long time.
Continue to: Economically, the pandemic...
Economically, the pandemic caused a horrific economic setback in its acute phase, which prompted the government to spend trillions to support the unemployed as well as blighted businesses. The economic sequalae of deficit spending of unprecedented proportions due to the pandemic triggered painful inflation that is ongoing. Interestingly, the numerical terms “billion” and “trillion” lost their loftiness as very huge numbers. Few people realize that counting to a billion (at one number per second) would take 31.7 years, while counting to a trillion would take 31,700 years! The inflationary impact of spending $6 trillion (which would take almost 200,000 years to count) becomes mathematically jarring. And despite the heroic measures to support the economy, some business perished, although others were created, changing the human architecture of the economy.
The pandemic drastically suppressed the “hunting and gathering” instinct of humans and demolished the fabled concept of work ethic. The “great resignation,” coupled with a desire to work from home on a mass scale, led to a glut of vacant office space in many large cities, lowering the value of commercial real estate. Following the pandemic, there was an uptick in moving away from urban areas, reflecting a creative destruction and reversal of a decades-long trend to gravitate to cities to work or live.
There was also political fallout from the pandemic. Staying at home is conducive to overdosing on television and social media, leading to an intensification and ossification of political hyperpartisanship and the further displacement of religious beliefs by passionately entrenched political beliefs. This continues to have seismic effects on political stability and harmony in our country. The pandemic may have instigated new models of national voting, which triggered further political friction.
Other examples of the pandemic’s aftereffects include a shortage of lifeguards and truck drivers, replacing the traditional handshake with a first bump, and increased spending on pleasurable activities (reminiscent of the Roaring 20s following the 1918 influenza pandemic), which may reflect an instinct to “live it up” before another deadly pandemic occurs.
Ironically, as I was finishing writing this article in early September 2023, the government announced that COVID-19 cases were again rising and a new vaccine was available for the new viral “strain.”
Here we go again: as the French saying goes: plus ça change, plus c’est la même chose…
Good riddance COVID-19 pandemic? Alas, that’s wishful thinking.
Many assume the pandemic is in our rearview mirror, but its biological, psychological, and social impacts continue to unfold. Its repercussions are etched into our brain, mind, emotions, behaviors, cognition, and outlook on life. Welcome to Pandemic 2.0.
Think of people who survive a heart attack. They experience multiple changes. Their initial ephemeral thrill of beating death is rapidly tempered with anxiety and worry about a future myocardial infarction and health issues in general. They become more risk-averse and more prone to dysphoria, irritability, and impatience. These individuals adopt a healthy lifestyle (diet and exercise), which they had neglected before. They develop more disciplined personality traits, feel a greater appreciation for being alive, and develop a closer affinity to family and friends. Simple things they had overlooked become more meaningful. They reevaluate their life goals, including career vs personal fulfilment. Some may overindulge in pleasurable activities in case their heart fails again. Some of those changes may be abrupt or transient, while others may become permanent features of their lives. And some may seek psychotherapy, which they may never have considered before.
The pandemic is the equivalent of a “societal cardiac arrest.” Its immediate impact was devastating. Bustling cities suddenly became ghost towns. Schools were closed, and children were locked at home with their parents, who were laid off. Businesses shut down; the economy tanked. Anxiety about being infected and dying skyrocketed, triggering a universal acute stress reaction that worsened the mental health of the population, but especially of the millions with preexisting psychiatric disorders. Routine medical and dental care stopped. Television and social media disseminated alarming updates about massive intensive care unit admissions and morgues overflowing with corpses of COVID-19 victims. Posttraumatic stress disorder (PTSD) was brewing across the nation as everyone faced this life-threatening pandemic.
The warp-speed development of vaccines for COVID-19 was equivalent to a defibrillator for the societal asystole, but the turmoil continued among the frazzled population. Some refused the vaccine due to conspiracy theories about their dangerous adverse effects. Employees in the private sector, state and federal government, and even the military who refused the mandatory vaccination lost their jobs. Controversy about shuttering schools and depriving children of face-to-face learning and socializing prompted some states to keep schools open, in contrast to most other states. Anger escalated about wearing masks, social distancing, and avoiding gatherings such as at restaurants or houses of worship. Cynicism and mistrust sprouted about the competence and reliability of health “experts” due to some conflicting signals, precluding wide adherence to medical advice.
The lingering effects of the COVID-19 pandemic
Those were the immediate repercussions of the pandemic. But what are its lingering effects? The sequelae extend across 1) the health care system; 2) the mental and emotional wellness of the population; 3) education; 4) work culture; 5) the economy; 6) societal operations; 7) technological and digital transformations; 8) mistrust in various societal institutions; 9) lack of confidence in medical information; and 10) preparedness for another pandemic due to a new strain.
As all psychiatrists know, the demand for mental health services continues to surge well after the pandemic has subsided, straining access to outpatient and inpatient care. Multiple lines of evidence confirm a deterioration in the long-term psychological well-being of children and adolescents because of lockdowns, social isolation, and anxiety about their own health and the health of their loved ones, leading to a serious rise in depression and suicidal behavior.1-3
Contunue to: Adults who survived pandemic...
Adults who survived the pandemic experienced grief during 2 very stressful years, with no peace of mind or “normal living.” Many began to contemplate the meaning of life and reevaluate the future, waxing more philosophical and embarking on “personal archeology.” The fragility of life suddenly became a ubiquitous epiphany that changed people’s habits. Working from home, which was necessary during the pandemic, became a preferred option for many, and home became an emotional refuge, not just a physical, brick-and-mortar refuge. Millions decided to quit working altogether (the “great resignation”).
Sexual activity declined precipitously during the pandemic for singles (French kissing became “the kiss of death”) but intercourse increased among couples, eventuating in a significant rise in births after the pandemic (a baby boomlet). Sexual interest among college students declined after the pandemic, which may be either due to fear of getting infected or a sublimation of libido to invest the energy in other, less risky activities.
At the societal level, the pandemic’s sequelae included a major shift to virtual communications, not just in health care (telepsychiatry and telemedicine) but also in business. Technology saved the day during the nadir of the pandemic by enabling psychiatrists and psychotherapists to treat their patients remotely. This was not technologically feasible during the past century’s influenza pandemics (1918, 1957, and 1968).
The intellectual and social development of an entire generation of children was stunted due to the COVID-19 pandemic. Consequences will continue to emerge in the years to come and may have ripple effects on this generation’s functioning. This may have particularly affected children of lower socioeconomic status, whose families cannot afford private schools and who are in dire need of good education to put them on the path of upward mobility.
As for adults who did not get infected by COVID-19, they suffered in 2 ways. First, they experienced a certain degree of brain atrophy, which is known to occur in chronic stress. This is attributed to persistent hypercortisolemia, which is toxic to the hippocampus. PTSD is well known to be associated with hippocampal atrophy.4 Additionally, a significant proportion of adults who contracted the COVID-19 virus and “recovered” were subsequently diagnosed with “long COVID,” with multiple neuropsychiatric symptoms, including psychosis, mania, depression, and panic attacks, as well as memory impairment and loss of the senses of smell and taste. For these individuals, the pandemic has not subsided; they will carry its neuropsychiatric scars for a long time.
Continue to: Economically, the pandemic...
Economically, the pandemic caused a horrific economic setback in its acute phase, which prompted the government to spend trillions to support the unemployed as well as blighted businesses. The economic sequalae of deficit spending of unprecedented proportions due to the pandemic triggered painful inflation that is ongoing. Interestingly, the numerical terms “billion” and “trillion” lost their loftiness as very huge numbers. Few people realize that counting to a billion (at one number per second) would take 31.7 years, while counting to a trillion would take 31,700 years! The inflationary impact of spending $6 trillion (which would take almost 200,000 years to count) becomes mathematically jarring. And despite the heroic measures to support the economy, some business perished, although others were created, changing the human architecture of the economy.
The pandemic drastically suppressed the “hunting and gathering” instinct of humans and demolished the fabled concept of work ethic. The “great resignation,” coupled with a desire to work from home on a mass scale, led to a glut of vacant office space in many large cities, lowering the value of commercial real estate. Following the pandemic, there was an uptick in moving away from urban areas, reflecting a creative destruction and reversal of a decades-long trend to gravitate to cities to work or live.
There was also political fallout from the pandemic. Staying at home is conducive to overdosing on television and social media, leading to an intensification and ossification of political hyperpartisanship and the further displacement of religious beliefs by passionately entrenched political beliefs. This continues to have seismic effects on political stability and harmony in our country. The pandemic may have instigated new models of national voting, which triggered further political friction.
Other examples of the pandemic’s aftereffects include a shortage of lifeguards and truck drivers, replacing the traditional handshake with a first bump, and increased spending on pleasurable activities (reminiscent of the Roaring 20s following the 1918 influenza pandemic), which may reflect an instinct to “live it up” before another deadly pandemic occurs.
Ironically, as I was finishing writing this article in early September 2023, the government announced that COVID-19 cases were again rising and a new vaccine was available for the new viral “strain.”
Here we go again: as the French saying goes: plus ça change, plus c’est la même chose…
1. Chavira DA, Ponting C, Ramos G. The impact of COVID-19 on child and adolescent mental health and treatment considerations. Behav Res Ther. 2022;157:104169. doi:10.1016/j.brat.2022.104169
2. Panchal U, Salazar de Pablo G, Franco M, et al. The impact of COVID-19 lockdown on child and adolescent mental health: systematic review. Eur Child Adolesc Psychiatry. 2023;32:1151-1177.
3. Mazrekaj D, De Witte K. The impact of school closures on learning and mental health of children: lessons from the COVID-19 pandemic. Perspectives on Psychological Science. 2023. https://doi.org/10.1177/17456916231181108
4. Logue MW, van Rooij SJH, Dennis EL, et al. A smaller hippocampal volume in posttraumatic stress disorder: a multisite ENIGMA-PGC study: subcortical volumetry results from posttraumatic stress disorder consortia. Biol Psychiatry. 2018;83(3):244-253.
1. Chavira DA, Ponting C, Ramos G. The impact of COVID-19 on child and adolescent mental health and treatment considerations. Behav Res Ther. 2022;157:104169. doi:10.1016/j.brat.2022.104169
2. Panchal U, Salazar de Pablo G, Franco M, et al. The impact of COVID-19 lockdown on child and adolescent mental health: systematic review. Eur Child Adolesc Psychiatry. 2023;32:1151-1177.
3. Mazrekaj D, De Witte K. The impact of school closures on learning and mental health of children: lessons from the COVID-19 pandemic. Perspectives on Psychological Science. 2023. https://doi.org/10.1177/17456916231181108
4. Logue MW, van Rooij SJH, Dennis EL, et al. A smaller hippocampal volume in posttraumatic stress disorder: a multisite ENIGMA-PGC study: subcortical volumetry results from posttraumatic stress disorder consortia. Biol Psychiatry. 2018;83(3):244-253.
Medication treatment of opioid use disorder in primary care practice: Opportunities and limitations
The Centers for Disease Control and Prevention (CDC) reported 106,699 deaths in 2021 from drug overdose, with the majority being linked to synthetic opioids, including fentanyl and tramadol.1 This number compares with 42,795 deaths due to motor vehicle accidents and 48,183 deaths due to suicide in 2021.2,3 Most of the opioid overdose deaths occurred among people aged 25 to 64 years, the peak age of patients cared for by obstetrician-gynecologists. Among pregnant and postpartum persons, mortality due to drug overdose has increased by 81% between 2017 and 2020.4
Among pregnant and postpartum patients, drug overdose death is more common than suicide, and the risk for drug overdose death appears to be greatest in the year following delivery.5,6 In many cases, postpartum patients with OUD have had multiple contacts with the health care system prior to their death, showing that there is an opportunity for therapeutic intervention before the death occurred.7 Medication-assisted recovery for OUD involves a comprehensive array of interventions including medication, counseling, and social support. Medication treatment of OUD with BUP or methadone reduces the risk for death but is underutilized among patients with OUD.6,8 Recent federal legislation has removed restrictions on the use of BUP, increasing the opportunity for primary care clinicians to prescribe it for the treatment of OUD.9
Screening and diagnosis of OUD
Screening for OUD is recommended for patients who are at risk for opioid misuse (ie, those who are taking/have taken opioid medications). The OWLS (Overuse, Worrying, Losing interest, and feeling Slowed down, sluggish, or sedated) screening tool is used to detect prescription medication OUD and has 4 questions10:
1. In the past 3 months did you use your opioid medicines for other purposes—for example, to help you sleep or to help with stress or worry?
2. In the past 3 months did opioid medicines cause you to feel slowed down, sluggish, or sedated?
3. In the past 3 months did opioid medicines cause you to lose interest in your usual activities?
4. In the past 3 months did you worry about your use of opioid medicines?
Patient agreement with 3 or 4 questions indicates a positive screening test.
If the patient has a positive screening test, a formal diagnosis of OUD can be made using the 11 symptoms outlined in the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.11 The diagnosis of mild (2 to 3 symptoms), moderate (4 to 5 symptoms), or severe OUD (6 or more symptoms) is made based on the number of symptoms the patient reports.
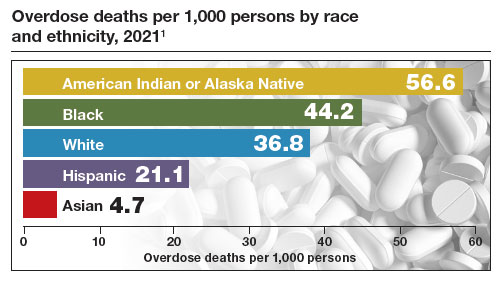
Buprenorphine treatment of OUD in primary care
The role of primary care clinicians in the medication treatment of OUD is increasing. Using a nationwide system that tracks prescription medications, investigators reported that, in 2004, psychiatrists wrote 32.2% of all BUP prescriptions; in 2021, however, only 10% of such prescriptions were provided by psychiatrists, with most prescriptions written by non-psychiatrist physicians, nurse practitioners, and physician assistants that year.12 Innovative telehealth approaches to consultation and medication treatment of OUD are now available—one example is QuickMD.13 Such sites are designed to remove barriers to initiating medication treatment of OUD.
The role of primary care clinicians in the management of OUD using BUP and buprenorphine-naloxone (BUP-NAL) has increased due to many factors, including:
- the removal of US Food and Drug Administration (FDA) barriers to prescribing BUP
- the epidemic of OUD and the small size of the addiction specialist workforce, necessitating that primary care clinicians become engaged in the treatment of OUD
- an increase in unobserved initiation of BUP among ambulatory patients, and a parallel decrease in cases of observed initiation in addiction center settings
- the reframing of OUD as a chronic medical problem, with many similarities to diabetes, obesity, dyslipidemia, and hypertension.
Similar to other diseases managed by primary care clinicians, OUD requires long-term chronic treatment with a medicine that, if taken as directed, provides excellent outcomes. Primary care clinicians who prescribe BUP also can optimize longitudinal care for comorbid disorders such as hypertension and diabetes, which are prevalent in people with OUD.
In 2019, New Jersey implemented new guidelines for the treatment of OUD, removing prior authorization barriers, increasing reimbursement for office-based OUD treatment, and establishing regional centers of excellence. The implementation of the new guidelines was followed by a marked increase in BUP prescribers among primary care clinicians, emergency medicine physicians, and advanced practice clinicians.14
To estimate the public health impact of BUP prescribing by primary care clinicians, investigators simulated patient outcomes in 3 scenarios15:
1. primary care clinicians refer patients to addiction specialists for OUD treatment
2. primary care clinicians provide BUP services in their practice
3. primary care clinicians provide BUP and harm reduction kits containing syringes and wound care supplies in their practice.
Strategies 2 and 3 resulted in 14% fewer deaths due to opioid overdose, an increased life expectancy of approximately 2.7 years, and reduced hospital costs. For strategy 3, the incremental cost per life-year saved was $34,400. The investigators noted that prescribing BUP in primary care practice increases practice costs.15
Treatment with BUP reduces death from opioid overdose, improves patient health, decreases use of illicit opioids, and reduces patient cravings for opioids. BUP is a safe medication and is associated with fewer adverse effects than insulin or warfarin.16
Continue to: Methadone treatment of OUD...
Methadone treatment of OUD
Methadone is a full opioid agonist approved by the FDA for the treatment of severe pain or OUD. Methadone treatment of OUD is strictly regulated and typically is ordered and administered at an opioid treatment program that is federally licensed. Methadone for OUD treatment cannot be prescribed by a physician to a pharmacy, limiting its use in primary care practice. Methadone used to treat OUD is ordered and dispensed at opioid-treatment programs. Take-home doses of methadone may be available to patients after adherence to the regimen has been established. When used long-term, higher doses of methadone are associated with better adherence, but these higher doses can cause respiratory depression. In a study of 189 pregnant patients taking methadone to treat OUD, daily doses of 60 mg or greater were associated with better treatment retention at delivery and 60 days postpartum, as well as less use of nonprescription opioids.17 Under limited circumstances methadone can be ordered and dispensed for hospitalized patients with OUD.
Methadone is a pure opioid receptor agonist. Naloxone (NAL) is an opioid receptor antagonist. Buprenorphine (BUP) is a partial opioid receptor agonist-antagonist, which limits overdose risk. BUP often is combined with NAL as a combination formulation, which is thought to reduce the repurposing of BUP for non-prescribed uses. At appropriate treatment dosages, both methadone (≥60 mg) and BUP (≥ 16 mg) are highly effective for the treatment of OUD.1 For patients with health insurance, pharmacy benefits often provide some coverage for preferred products but no coverage for other products. Not all pharmacies carry BUP products. In a study of more than 5,000 pharmacies, approximately 60% reported that they carry and can dispense BUP medications.2
BUP monotherapy is available as generic sublingual tablets, buccal films (Belbuca), formulations for injection (Sublocade), and subcutaneous implants (Probuphine). BUPNAL is available as buccal films (Bunavail), sublingual films (Suboxone), and sublingual tablets (Zubsolv). For BUP-NAL combination productions, the following dose combinations have been reported to have similar effects: BUP-NAL 8 mg/2 mg sublingual film, BUP-NAL 5.7 mg/1.4 mg sublingual tablet, and BUP-NAL 4.2 mg/0.7 mg buccal film.3
When initiating BUP-monotherapy or BUP-NAL treatment for OUD, one approach for unobserved initiation is to instruct the patient to discontinue using opioid agonist drugs and wait for the onset of mild to moderate withdrawal symptoms. The purpose of this step is to avoid precipitating severe withdrawal symptoms caused by giving BUP or BUP-NAL to a patient who has recently used opioid drugs.
If BUP-NAL sublingual films (Suboxone) are prescribed following the onset of mild to moderate withdrawal symptoms, the patient can initiate therapy with a dose of 2 mg BUP/0.5 mg NAL or 4 mg BUP/1 mg NAL. At 60 to 120 minutes following the initial dose, if withdrawal symptoms persist, an additional dose of 4 mg BUP/1 mg NAL can be given. Thereafter, symptoms can be assessed every 60 to 120 minutes and additional doses administered to control symptoms. On the second day of therapy, a maximum of 16 mg of BUP is administered. Over the following days and weeks, if symptoms and cravings persist at a BUP dose of 16 mg, the total daily dose of BUP can be titrated up to 24 mg. For long-term treatment, a commonly prescribed daily dose is 16 mg BUP/4 mg NAL or 24 mg BUP/6 mg NAL. An absolute contraindication to BUP or BUP/NAL treatment is an allergy to the medication, and a relative contraindication is liver failure.
One potential complication of transmucosal BUP or BUP-NAL treatment is a dry mouth (xerostomia), which may contribute to dental disease.4 However, some experts question the quality of the data that contributed to the warning.5,6 Potential dental complications might be prevented by regular oral health examinations, daily flossing and teeth brushing, and stimulation of saliva by sugar-free gum or lozenges.
Primary care clinicians who initiate BUP or BUPNAL treatment for OUD often have a weekly visit with the patient during the initial phase of treatment and then every 3 to 4 weeks during maintenance therapy. Most patients need long-term treatment to achieve the goals of therapy, which include prevention of opioid overdose, reduction of cravings for nonprescription narcotics, and improvement in overall health. BUP and BUP-NAL treatment are effective without formal counseling, but counseling and social work support improve long-term adherence with treatment. Primary care clinicians who have experience with medication treatment of OUD report that their experience convinces them that medication treatment of OUD has similarities to the long-term treatment of diabetes, with antihyperglycemia medicines or the treatment of HIV infection with antiviral medications.
References
1. Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;CD002207.
2. Weiner SG, Qato DM, Faust JS, et al. Pharmacy availability of buprenorphine for opioid use disorder treatment in the U.S. JAMA Netw Open. 2023;6:E2316089.
3. Substance Abuse and Mental Health Services Administration (SAMHSA). Medications for opioid use disorder. SAMHSA website. Accessed August 21, 2023. https ://store.samhsa.gov/sites/default/files/SAMHSA_Digital_Download/PEP 21-02-01-002.pdf
4. FDA warns about dental problems with buprenorphine medicines dissolved in the mouth. FDA website. Accessed August 21, 2023. https ://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-dental-problems-buprenorphine-medicines-dissolved-mouth-treat-opioiduse-disorder#:~:text=What%20did%20FDA%20find%3F,medicines%20 dissolved%20in%20the%20mouth
5. Watson DP, Etmian S, Gastala N. Sublingual buprenorphine-naloxone exposure and dental disease. JAMA. 2023;329:1223-1224.
6. Brothers TD, Lewer D, Bonn M. Sublingual buprenorphine-naloxone exposure and dental disease. JAMA. 2023;329:1224.
Medication treatment of OUD in obstetrics
In the United States, the prevalence of OUD among pregnant patients hospitalized for delivery more than quadrupled from 1999 through 2014.18 BUP and methadone commonly are used to treat OUD during pregnancy.19 Among pregnant patients about 5% of buprenorphine prescriptions are written by obstetricians.20 An innovative approach to initiating BUP for pregnant patients with OUD is to use unobserved initiation, which involves outpatient discontinuation of nonprescription opioids to induce mild to moderate withdrawal symptoms followed by initiation of BUP treatment. In one cohort study, 55 pregnant patients used an unobserved outpatient protocol to initiate BUP treatment; 80% of the patients previously had used methadone or BUP. No patient experienced a precipitated withdrawal and 96% of patients returned for their office visit 1 week after initiation of treatment. Eighty-six percent of patients remained in treatment 3 months following initiation of BUP.21
Compared with methadone, BUP treatment during pregnancy may result in lower rates of neonatal abstinence syndrome. In one study of pregnant patients who were using methadone (n = 5,056) or BUP (n = 11,272) in late pregnancy, neonatal abstinence syndrome was diagnosed in 69.2% and 52.0% of newborns, respectively (adjusted relative risk, 0.73; 95% confidence interval, 0.71–0.75).22 In addition, compared with methadone, the use of BUP was associated with a reduced risk for low birth weight (14.9% vs 8.3%) and a lower risk for preterm birth (24.9% vs 14.4%). In this study, there were no differences in maternal obstetric outcomes when comparing BUP versus methadone treatment. Similar results have been reported in a meta-analysis analyzing the use of methadone and BUP during pregnancy.23 Studies performed to date have not shown an increased risk of congenital anomalies with the use of BUP-NAL during pregnancy.24,25
Although there may be differences in newborn outcomes with BUP and methadone, the American College of Obstetricians and Gynecologists does not recommend switching from methadone to BUP during pregnancy because precipitated withdrawal may occur.26 Based on recent studies, the American Society of Addiction Medicine has advised that it is safe to prescribe pregnant patients either BUP or BUP-NAL.27,28
Medication treatment of OUD with or without intensive counseling
The FDA recently reviewed literature related to the advantages and challenges of combining intensive counseling with medication treatment of OUD.29 The FDA noted that treatment saves lives and encouraged clinicians to initiate medication treatment of OUD or refer the patient to an appropriate clinician or treatment center. Combining medication treatment of OUD with intensive counseling is associated with greater treatment adherence and reduced health care costs. For example, in one study of 4,987 patients with OUD, initiation of counseling within 8 weeks of the start of medication treatment and a BUP dose of 16 mg or greater daily were associated with increased adherence to treatment.30 For patients receiving a BUP dose of less than 16 mg daily, treatment adherence with and without counseling was approximately 325 and 230 days, respectively. When the dose of BUP was 16 mg or greater, treatment adherence with and without counseling was approximately 405 and 320 days, respectively.30
Counseling should always be offered to patients initiating medication treatment of OUD. It should be noted that counseling alone is not a highly effective treatment for OUD.31 The FDA recently advised that the lack of availability of intensive counseling should not prevent clinicians from initiating BUP for the treatment of OUD.29 OUD is associated with a high mortalityrate and if counseling is not possible, medication treatment should be initiated. Substantial evidence demonstrates that medication treatment of OUD is associated with many benefits.16 The FDA advisory committee concluded that OUD treatment decisions should use shared decision making and be supportive and patient centered.29
The opportunities for medication treatment of OUD in primary care practice have expanded due to the recent FDA removal of restrictions on the use of BUP and heightened awareness of the positive public health impact of medication treatment. Challenges to the medication treatment of OUD remain, including stigmatization of OUD, barriers to insurance coverage for BUP, practice costs of treating OUD, and gaps in clinical education. For many pregnant patients, their main point of contact with health care is their obstetrician. By incorporating OUD treatment in pregnancy care, obstetricians will improve the health of the mother and newborn, contributing to the well-being of current and future generations. ●
Experts have recommended several interventions that may help reduce opioid overdose death.1 A consensus recommendation is that people who use drugs should be provided naloxone rescue medication and educated on the proper use of naloxone. Naloxone rescue medication is available in formulations for nasal or parenteral administration. The US Food and Drug Administration (FDA) recently has approved naloxone for over-the-counter status. The American Medical Association has provided a short web video on how to administer nasal naloxone.2 In a small pilot study, obstetricians offered every postpartum patient with naloxone administration education and a 2-dose nasal naloxone pack, with 76% of patients accepting the nasal naloxone pack.3
Many experts recommend that people who use drugs should be advised to never use them alone and to test a small amount of the drug to assess its potency. Many patients who use opioid drugs also take benzodiazepines, which can contribute to respiratory depression.4 Patients should avoid mixing drugs (eg, opioids and benzodiazepines). Some experts recommend that patients who use drugs should be provided take-home fentanyl test strips so they can evaluate their drugs for the presence of fentanyl, a medication that suppresses respiration and contributes to many overdose deaths. In addition, people who use drugs and are interested in reducing their use of drugs or managing overdose risk can be offered initiation of medication treatment of OUD.1
References
1. Wood E, Solomon ED, Hadland SE. Universal precautions for people at risk of opioid overdose in North America. JAMA Int Med. 2023;183:401-402.
2. How to administer Naloxone. AMA website. Accessed August 28, 2023. https://www.ama-assn.org /delivering-care/overdose-epidemic/how-administer-naloxone
3. Naliboff JA, Tharpe N. Universal postpartum naloxone provision: a harm reduction quality improvement project. J Addict Med. 2022;17:360-362.
4. Kelly JC, Raghuraman N, Stout MJ, et al. Home induction of buprenorphine for treatment of opioid use disorder in pregnancy. Obstet Gynecol. 2021;138:655-659.
- Spencer MR, Miniño AM, Warner M. Drug overdose deaths in the United States, 20012021. NCHS Data Brief no 457. Hyattsville, MD, National Center for Health Statistics. 2022. NCHS Data Brief No. 457. Published December 2022. Accessed August 21, 2023. https://www.cdc.gov /nchs/products/databriefs/db457.htm
- US traffic deaths drop slightly in 2022 but still a ‘crisis.’ AP News website. Published April 20, 2023. Accessed August 21, 2023. https://apnews.com /article/traffic-deaths-distracted-driving-crisis -6db6471e273b275920b6c4f9eb7e493b
- Suicide statistics. American Foundation for Suicide Prevention website. Accessed August 21, 2023. https://afsp.org/suicide-statistics/
- Bruzelius E, Martins SS. US Trends in drug overdose mortality among pregnant and postpartum persons, 2017-2020. JAMA. 2022;328:2159-2161.
- Metz TD, Rovner P, Hoffman MC, et al. Maternal deaths from suicide and overdose in Colorado, 2004-2012. Obstet Gynecol. 2016;128:1233-1240.
- Schiff DM, Nielsen T, Terplan M, et al. Fatal and nonfatal overdose among pregnant and postpartum women in Massachusetts. Obstet Gynecol. 2018;132:466-474.
- Goldman-Mellor S, Margerison CE. Maternal drug-related death and suicide are leading causes of postpartum death in California. Am J Obstet Gynecol. 2019;221:489.e1-489.e9.
- Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550.
- Waiver elimination (MAT Act). SAMHSA website. Accessed August 21, 2023. https://www .samhsa.gov/medications-substance-use- disorders/removal-data-waiver-requirement
- Picco L, Middleton M, Bruno R, et al. Validation of the OWLS, a Screening Tool for Measuring Prescription Opioid Use Disorder in Primary Care. Pain Med. 2020;21:2757-2764.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
- Creedon TB, Ali MM, Schuman-Olivier Z. Trends in buprenorphine prescribing for opioid use disorder by psychiatrists in the US from 2003 to 2021. JAMA Health Forum. 2023;4:E230221.
- Quick MD website. Accessed August 21, 2023. https://quick.md/
- Treitler P, Nowels M, Samples H, et al. BUP utilization and prescribing among New Jersey Medicaid beneficiaries after adoption of initiatives designed to improve treatment access. JAMA Netw Open. 2023;6:E2312030.
- Jawa R, Tin Y, Nall S, et al. Estimated clinical outcomes and cost-effectiveness associated with provision of addiction treatment in US primary care clinics. JAMA Netw Open. 2023;6:E237888.
- Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways of opioid use disorder. JAMA Netw Open. 2020;3:E1920622.
- Wilder CM, Hosta D, Winhusen T. Association of methadone dose with substance use and treatment retention in pregnant and postpartum women with opioid use disorder. J Subst Abuse Treat. 2017;80:33-36.
- Haight SC, Ko JY, Tong VT, et al. Opioid use disorder documented at delivery hospitalization - United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2018;67:845-849.
- Xu KY, Jones HE, Schiff DM, et al. Initiation and treatment discontinuation of medications for opioid use disorder in pregnant people compared with nonpregnant people. Obstet Gynecol. 2023;141:845-853.
- Kelly D, Krans EE. Medical specialty of buprenorphine prescribers for pregnant women with opioid use disorder. Am J Obstet Gynecol. 2019;220:502-503.
- Kelly JC, Raghuraman N, Stout MJ, et al. Home induction of buprenorphine for treatment of opioid use disorder in pregnancy. Obstet Gynecol. 2021;138:655-659.
- Suarez EA, Huybrechts KF, Straub L, et al. Buprenorphine versus methadone for opioid use disorder in pregnancy. N Engl J Med. 2022;387:2033-2044.
- Kinsella M, Halliday LO, Shaw M, et al. Buprenorphine compared with methadone in pregnancy: a systematic review and meta-analysis. Subst Use Misuse. 2022;57:1400-1416.
- Jumah NA, Edwards C, Balfour-Boehm J, et al. Observational study of the safety of buprenorphine-naloxone in pregnancy in a rural and remote population. BMJ Open. 2016;6:E011774.
- Mullins N, Galvin SL, Ramage M, et al. Buprenorphine and naloxone versus buprenorphine for opioid use disorder in pregnancy: a cohort study. J Addict Med. 2020;14:185-192.
- Opioid use and opioid use disorder in pregnancy. Committee Opinion No. 711. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2017;130:E81-E94.
- The ASAM National Practice Guideline for the Treatment of Opioid Use Disorder: 2020 Focused Update. J Addict Med. 2020;14(2S suppl 1):1-91.
- Link HM, Jones H, Miller L, et al. Buprenorphinenaloxone use in pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020;2:100179.
- Delphin-Rittmon ME, Cavazzoni P. US Food and Drug Administration website. https://www.fda .gov/media/168027/download
- Eren K, Schuster J, Herschell A, et al. Association of Counseling and Psychotherapy on retention in medication for addiction treatment within a large Medicaid population. J Addict Med. 2022;16:346353.
- Kakko J, Dybrandt Svanborg K, Kreek MJ, et al. 1-year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in Sweden: a randomized, placebo-controlled trial. Lancet. 2003;361:662-668.
The Centers for Disease Control and Prevention (CDC) reported 106,699 deaths in 2021 from drug overdose, with the majority being linked to synthetic opioids, including fentanyl and tramadol.1 This number compares with 42,795 deaths due to motor vehicle accidents and 48,183 deaths due to suicide in 2021.2,3 Most of the opioid overdose deaths occurred among people aged 25 to 64 years, the peak age of patients cared for by obstetrician-gynecologists. Among pregnant and postpartum persons, mortality due to drug overdose has increased by 81% between 2017 and 2020.4
Among pregnant and postpartum patients, drug overdose death is more common than suicide, and the risk for drug overdose death appears to be greatest in the year following delivery.5,6 In many cases, postpartum patients with OUD have had multiple contacts with the health care system prior to their death, showing that there is an opportunity for therapeutic intervention before the death occurred.7 Medication-assisted recovery for OUD involves a comprehensive array of interventions including medication, counseling, and social support. Medication treatment of OUD with BUP or methadone reduces the risk for death but is underutilized among patients with OUD.6,8 Recent federal legislation has removed restrictions on the use of BUP, increasing the opportunity for primary care clinicians to prescribe it for the treatment of OUD.9
Screening and diagnosis of OUD
Screening for OUD is recommended for patients who are at risk for opioid misuse (ie, those who are taking/have taken opioid medications). The OWLS (Overuse, Worrying, Losing interest, and feeling Slowed down, sluggish, or sedated) screening tool is used to detect prescription medication OUD and has 4 questions10:
1. In the past 3 months did you use your opioid medicines for other purposes—for example, to help you sleep or to help with stress or worry?
2. In the past 3 months did opioid medicines cause you to feel slowed down, sluggish, or sedated?
3. In the past 3 months did opioid medicines cause you to lose interest in your usual activities?
4. In the past 3 months did you worry about your use of opioid medicines?
Patient agreement with 3 or 4 questions indicates a positive screening test.
If the patient has a positive screening test, a formal diagnosis of OUD can be made using the 11 symptoms outlined in the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.11 The diagnosis of mild (2 to 3 symptoms), moderate (4 to 5 symptoms), or severe OUD (6 or more symptoms) is made based on the number of symptoms the patient reports.

Buprenorphine treatment of OUD in primary care
The role of primary care clinicians in the medication treatment of OUD is increasing. Using a nationwide system that tracks prescription medications, investigators reported that, in 2004, psychiatrists wrote 32.2% of all BUP prescriptions; in 2021, however, only 10% of such prescriptions were provided by psychiatrists, with most prescriptions written by non-psychiatrist physicians, nurse practitioners, and physician assistants that year.12 Innovative telehealth approaches to consultation and medication treatment of OUD are now available—one example is QuickMD.13 Such sites are designed to remove barriers to initiating medication treatment of OUD.
The role of primary care clinicians in the management of OUD using BUP and buprenorphine-naloxone (BUP-NAL) has increased due to many factors, including:
- the removal of US Food and Drug Administration (FDA) barriers to prescribing BUP
- the epidemic of OUD and the small size of the addiction specialist workforce, necessitating that primary care clinicians become engaged in the treatment of OUD
- an increase in unobserved initiation of BUP among ambulatory patients, and a parallel decrease in cases of observed initiation in addiction center settings
- the reframing of OUD as a chronic medical problem, with many similarities to diabetes, obesity, dyslipidemia, and hypertension.
Similar to other diseases managed by primary care clinicians, OUD requires long-term chronic treatment with a medicine that, if taken as directed, provides excellent outcomes. Primary care clinicians who prescribe BUP also can optimize longitudinal care for comorbid disorders such as hypertension and diabetes, which are prevalent in people with OUD.
In 2019, New Jersey implemented new guidelines for the treatment of OUD, removing prior authorization barriers, increasing reimbursement for office-based OUD treatment, and establishing regional centers of excellence. The implementation of the new guidelines was followed by a marked increase in BUP prescribers among primary care clinicians, emergency medicine physicians, and advanced practice clinicians.14
To estimate the public health impact of BUP prescribing by primary care clinicians, investigators simulated patient outcomes in 3 scenarios15:
1. primary care clinicians refer patients to addiction specialists for OUD treatment
2. primary care clinicians provide BUP services in their practice
3. primary care clinicians provide BUP and harm reduction kits containing syringes and wound care supplies in their practice.
Strategies 2 and 3 resulted in 14% fewer deaths due to opioid overdose, an increased life expectancy of approximately 2.7 years, and reduced hospital costs. For strategy 3, the incremental cost per life-year saved was $34,400. The investigators noted that prescribing BUP in primary care practice increases practice costs.15
Treatment with BUP reduces death from opioid overdose, improves patient health, decreases use of illicit opioids, and reduces patient cravings for opioids. BUP is a safe medication and is associated with fewer adverse effects than insulin or warfarin.16
Continue to: Methadone treatment of OUD...
Methadone treatment of OUD
Methadone is a full opioid agonist approved by the FDA for the treatment of severe pain or OUD. Methadone treatment of OUD is strictly regulated and typically is ordered and administered at an opioid treatment program that is federally licensed. Methadone for OUD treatment cannot be prescribed by a physician to a pharmacy, limiting its use in primary care practice. Methadone used to treat OUD is ordered and dispensed at opioid-treatment programs. Take-home doses of methadone may be available to patients after adherence to the regimen has been established. When used long-term, higher doses of methadone are associated with better adherence, but these higher doses can cause respiratory depression. In a study of 189 pregnant patients taking methadone to treat OUD, daily doses of 60 mg or greater were associated with better treatment retention at delivery and 60 days postpartum, as well as less use of nonprescription opioids.17 Under limited circumstances methadone can be ordered and dispensed for hospitalized patients with OUD.
Methadone is a pure opioid receptor agonist. Naloxone (NAL) is an opioid receptor antagonist. Buprenorphine (BUP) is a partial opioid receptor agonist-antagonist, which limits overdose risk. BUP often is combined with NAL as a combination formulation, which is thought to reduce the repurposing of BUP for non-prescribed uses. At appropriate treatment dosages, both methadone (≥60 mg) and BUP (≥ 16 mg) are highly effective for the treatment of OUD.1 For patients with health insurance, pharmacy benefits often provide some coverage for preferred products but no coverage for other products. Not all pharmacies carry BUP products. In a study of more than 5,000 pharmacies, approximately 60% reported that they carry and can dispense BUP medications.2
BUP monotherapy is available as generic sublingual tablets, buccal films (Belbuca), formulations for injection (Sublocade), and subcutaneous implants (Probuphine). BUPNAL is available as buccal films (Bunavail), sublingual films (Suboxone), and sublingual tablets (Zubsolv). For BUP-NAL combination productions, the following dose combinations have been reported to have similar effects: BUP-NAL 8 mg/2 mg sublingual film, BUP-NAL 5.7 mg/1.4 mg sublingual tablet, and BUP-NAL 4.2 mg/0.7 mg buccal film.3
When initiating BUP-monotherapy or BUP-NAL treatment for OUD, one approach for unobserved initiation is to instruct the patient to discontinue using opioid agonist drugs and wait for the onset of mild to moderate withdrawal symptoms. The purpose of this step is to avoid precipitating severe withdrawal symptoms caused by giving BUP or BUP-NAL to a patient who has recently used opioid drugs.
If BUP-NAL sublingual films (Suboxone) are prescribed following the onset of mild to moderate withdrawal symptoms, the patient can initiate therapy with a dose of 2 mg BUP/0.5 mg NAL or 4 mg BUP/1 mg NAL. At 60 to 120 minutes following the initial dose, if withdrawal symptoms persist, an additional dose of 4 mg BUP/1 mg NAL can be given. Thereafter, symptoms can be assessed every 60 to 120 minutes and additional doses administered to control symptoms. On the second day of therapy, a maximum of 16 mg of BUP is administered. Over the following days and weeks, if symptoms and cravings persist at a BUP dose of 16 mg, the total daily dose of BUP can be titrated up to 24 mg. For long-term treatment, a commonly prescribed daily dose is 16 mg BUP/4 mg NAL or 24 mg BUP/6 mg NAL. An absolute contraindication to BUP or BUP/NAL treatment is an allergy to the medication, and a relative contraindication is liver failure.
One potential complication of transmucosal BUP or BUP-NAL treatment is a dry mouth (xerostomia), which may contribute to dental disease.4 However, some experts question the quality of the data that contributed to the warning.5,6 Potential dental complications might be prevented by regular oral health examinations, daily flossing and teeth brushing, and stimulation of saliva by sugar-free gum or lozenges.
Primary care clinicians who initiate BUP or BUPNAL treatment for OUD often have a weekly visit with the patient during the initial phase of treatment and then every 3 to 4 weeks during maintenance therapy. Most patients need long-term treatment to achieve the goals of therapy, which include prevention of opioid overdose, reduction of cravings for nonprescription narcotics, and improvement in overall health. BUP and BUP-NAL treatment are effective without formal counseling, but counseling and social work support improve long-term adherence with treatment. Primary care clinicians who have experience with medication treatment of OUD report that their experience convinces them that medication treatment of OUD has similarities to the long-term treatment of diabetes, with antihyperglycemia medicines or the treatment of HIV infection with antiviral medications.
References
1. Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;CD002207.
2. Weiner SG, Qato DM, Faust JS, et al. Pharmacy availability of buprenorphine for opioid use disorder treatment in the U.S. JAMA Netw Open. 2023;6:E2316089.
3. Substance Abuse and Mental Health Services Administration (SAMHSA). Medications for opioid use disorder. SAMHSA website. Accessed August 21, 2023. https ://store.samhsa.gov/sites/default/files/SAMHSA_Digital_Download/PEP 21-02-01-002.pdf
4. FDA warns about dental problems with buprenorphine medicines dissolved in the mouth. FDA website. Accessed August 21, 2023. https ://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-dental-problems-buprenorphine-medicines-dissolved-mouth-treat-opioiduse-disorder#:~:text=What%20did%20FDA%20find%3F,medicines%20 dissolved%20in%20the%20mouth
5. Watson DP, Etmian S, Gastala N. Sublingual buprenorphine-naloxone exposure and dental disease. JAMA. 2023;329:1223-1224.
6. Brothers TD, Lewer D, Bonn M. Sublingual buprenorphine-naloxone exposure and dental disease. JAMA. 2023;329:1224.
Medication treatment of OUD in obstetrics
In the United States, the prevalence of OUD among pregnant patients hospitalized for delivery more than quadrupled from 1999 through 2014.18 BUP and methadone commonly are used to treat OUD during pregnancy.19 Among pregnant patients about 5% of buprenorphine prescriptions are written by obstetricians.20 An innovative approach to initiating BUP for pregnant patients with OUD is to use unobserved initiation, which involves outpatient discontinuation of nonprescription opioids to induce mild to moderate withdrawal symptoms followed by initiation of BUP treatment. In one cohort study, 55 pregnant patients used an unobserved outpatient protocol to initiate BUP treatment; 80% of the patients previously had used methadone or BUP. No patient experienced a precipitated withdrawal and 96% of patients returned for their office visit 1 week after initiation of treatment. Eighty-six percent of patients remained in treatment 3 months following initiation of BUP.21
Compared with methadone, BUP treatment during pregnancy may result in lower rates of neonatal abstinence syndrome. In one study of pregnant patients who were using methadone (n = 5,056) or BUP (n = 11,272) in late pregnancy, neonatal abstinence syndrome was diagnosed in 69.2% and 52.0% of newborns, respectively (adjusted relative risk, 0.73; 95% confidence interval, 0.71–0.75).22 In addition, compared with methadone, the use of BUP was associated with a reduced risk for low birth weight (14.9% vs 8.3%) and a lower risk for preterm birth (24.9% vs 14.4%). In this study, there were no differences in maternal obstetric outcomes when comparing BUP versus methadone treatment. Similar results have been reported in a meta-analysis analyzing the use of methadone and BUP during pregnancy.23 Studies performed to date have not shown an increased risk of congenital anomalies with the use of BUP-NAL during pregnancy.24,25
Although there may be differences in newborn outcomes with BUP and methadone, the American College of Obstetricians and Gynecologists does not recommend switching from methadone to BUP during pregnancy because precipitated withdrawal may occur.26 Based on recent studies, the American Society of Addiction Medicine has advised that it is safe to prescribe pregnant patients either BUP or BUP-NAL.27,28
Medication treatment of OUD with or without intensive counseling
The FDA recently reviewed literature related to the advantages and challenges of combining intensive counseling with medication treatment of OUD.29 The FDA noted that treatment saves lives and encouraged clinicians to initiate medication treatment of OUD or refer the patient to an appropriate clinician or treatment center. Combining medication treatment of OUD with intensive counseling is associated with greater treatment adherence and reduced health care costs. For example, in one study of 4,987 patients with OUD, initiation of counseling within 8 weeks of the start of medication treatment and a BUP dose of 16 mg or greater daily were associated with increased adherence to treatment.30 For patients receiving a BUP dose of less than 16 mg daily, treatment adherence with and without counseling was approximately 325 and 230 days, respectively. When the dose of BUP was 16 mg or greater, treatment adherence with and without counseling was approximately 405 and 320 days, respectively.30
Counseling should always be offered to patients initiating medication treatment of OUD. It should be noted that counseling alone is not a highly effective treatment for OUD.31 The FDA recently advised that the lack of availability of intensive counseling should not prevent clinicians from initiating BUP for the treatment of OUD.29 OUD is associated with a high mortalityrate and if counseling is not possible, medication treatment should be initiated. Substantial evidence demonstrates that medication treatment of OUD is associated with many benefits.16 The FDA advisory committee concluded that OUD treatment decisions should use shared decision making and be supportive and patient centered.29
The opportunities for medication treatment of OUD in primary care practice have expanded due to the recent FDA removal of restrictions on the use of BUP and heightened awareness of the positive public health impact of medication treatment. Challenges to the medication treatment of OUD remain, including stigmatization of OUD, barriers to insurance coverage for BUP, practice costs of treating OUD, and gaps in clinical education. For many pregnant patients, their main point of contact with health care is their obstetrician. By incorporating OUD treatment in pregnancy care, obstetricians will improve the health of the mother and newborn, contributing to the well-being of current and future generations. ●
Experts have recommended several interventions that may help reduce opioid overdose death.1 A consensus recommendation is that people who use drugs should be provided naloxone rescue medication and educated on the proper use of naloxone. Naloxone rescue medication is available in formulations for nasal or parenteral administration. The US Food and Drug Administration (FDA) recently has approved naloxone for over-the-counter status. The American Medical Association has provided a short web video on how to administer nasal naloxone.2 In a small pilot study, obstetricians offered every postpartum patient with naloxone administration education and a 2-dose nasal naloxone pack, with 76% of patients accepting the nasal naloxone pack.3
Many experts recommend that people who use drugs should be advised to never use them alone and to test a small amount of the drug to assess its potency. Many patients who use opioid drugs also take benzodiazepines, which can contribute to respiratory depression.4 Patients should avoid mixing drugs (eg, opioids and benzodiazepines). Some experts recommend that patients who use drugs should be provided take-home fentanyl test strips so they can evaluate their drugs for the presence of fentanyl, a medication that suppresses respiration and contributes to many overdose deaths. In addition, people who use drugs and are interested in reducing their use of drugs or managing overdose risk can be offered initiation of medication treatment of OUD.1
References
1. Wood E, Solomon ED, Hadland SE. Universal precautions for people at risk of opioid overdose in North America. JAMA Int Med. 2023;183:401-402.
2. How to administer Naloxone. AMA website. Accessed August 28, 2023. https://www.ama-assn.org /delivering-care/overdose-epidemic/how-administer-naloxone
3. Naliboff JA, Tharpe N. Universal postpartum naloxone provision: a harm reduction quality improvement project. J Addict Med. 2022;17:360-362.
4. Kelly JC, Raghuraman N, Stout MJ, et al. Home induction of buprenorphine for treatment of opioid use disorder in pregnancy. Obstet Gynecol. 2021;138:655-659.
The Centers for Disease Control and Prevention (CDC) reported 106,699 deaths in 2021 from drug overdose, with the majority being linked to synthetic opioids, including fentanyl and tramadol.1 This number compares with 42,795 deaths due to motor vehicle accidents and 48,183 deaths due to suicide in 2021.2,3 Most of the opioid overdose deaths occurred among people aged 25 to 64 years, the peak age of patients cared for by obstetrician-gynecologists. Among pregnant and postpartum persons, mortality due to drug overdose has increased by 81% between 2017 and 2020.4
Among pregnant and postpartum patients, drug overdose death is more common than suicide, and the risk for drug overdose death appears to be greatest in the year following delivery.5,6 In many cases, postpartum patients with OUD have had multiple contacts with the health care system prior to their death, showing that there is an opportunity for therapeutic intervention before the death occurred.7 Medication-assisted recovery for OUD involves a comprehensive array of interventions including medication, counseling, and social support. Medication treatment of OUD with BUP or methadone reduces the risk for death but is underutilized among patients with OUD.6,8 Recent federal legislation has removed restrictions on the use of BUP, increasing the opportunity for primary care clinicians to prescribe it for the treatment of OUD.9
Screening and diagnosis of OUD
Screening for OUD is recommended for patients who are at risk for opioid misuse (ie, those who are taking/have taken opioid medications). The OWLS (Overuse, Worrying, Losing interest, and feeling Slowed down, sluggish, or sedated) screening tool is used to detect prescription medication OUD and has 4 questions10:
1. In the past 3 months did you use your opioid medicines for other purposes—for example, to help you sleep or to help with stress or worry?
2. In the past 3 months did opioid medicines cause you to feel slowed down, sluggish, or sedated?
3. In the past 3 months did opioid medicines cause you to lose interest in your usual activities?
4. In the past 3 months did you worry about your use of opioid medicines?
Patient agreement with 3 or 4 questions indicates a positive screening test.
If the patient has a positive screening test, a formal diagnosis of OUD can be made using the 11 symptoms outlined in the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.11 The diagnosis of mild (2 to 3 symptoms), moderate (4 to 5 symptoms), or severe OUD (6 or more symptoms) is made based on the number of symptoms the patient reports.

Buprenorphine treatment of OUD in primary care
The role of primary care clinicians in the medication treatment of OUD is increasing. Using a nationwide system that tracks prescription medications, investigators reported that, in 2004, psychiatrists wrote 32.2% of all BUP prescriptions; in 2021, however, only 10% of such prescriptions were provided by psychiatrists, with most prescriptions written by non-psychiatrist physicians, nurse practitioners, and physician assistants that year.12 Innovative telehealth approaches to consultation and medication treatment of OUD are now available—one example is QuickMD.13 Such sites are designed to remove barriers to initiating medication treatment of OUD.
The role of primary care clinicians in the management of OUD using BUP and buprenorphine-naloxone (BUP-NAL) has increased due to many factors, including:
- the removal of US Food and Drug Administration (FDA) barriers to prescribing BUP
- the epidemic of OUD and the small size of the addiction specialist workforce, necessitating that primary care clinicians become engaged in the treatment of OUD
- an increase in unobserved initiation of BUP among ambulatory patients, and a parallel decrease in cases of observed initiation in addiction center settings
- the reframing of OUD as a chronic medical problem, with many similarities to diabetes, obesity, dyslipidemia, and hypertension.
Similar to other diseases managed by primary care clinicians, OUD requires long-term chronic treatment with a medicine that, if taken as directed, provides excellent outcomes. Primary care clinicians who prescribe BUP also can optimize longitudinal care for comorbid disorders such as hypertension and diabetes, which are prevalent in people with OUD.
In 2019, New Jersey implemented new guidelines for the treatment of OUD, removing prior authorization barriers, increasing reimbursement for office-based OUD treatment, and establishing regional centers of excellence. The implementation of the new guidelines was followed by a marked increase in BUP prescribers among primary care clinicians, emergency medicine physicians, and advanced practice clinicians.14
To estimate the public health impact of BUP prescribing by primary care clinicians, investigators simulated patient outcomes in 3 scenarios15:
1. primary care clinicians refer patients to addiction specialists for OUD treatment
2. primary care clinicians provide BUP services in their practice
3. primary care clinicians provide BUP and harm reduction kits containing syringes and wound care supplies in their practice.
Strategies 2 and 3 resulted in 14% fewer deaths due to opioid overdose, an increased life expectancy of approximately 2.7 years, and reduced hospital costs. For strategy 3, the incremental cost per life-year saved was $34,400. The investigators noted that prescribing BUP in primary care practice increases practice costs.15
Treatment with BUP reduces death from opioid overdose, improves patient health, decreases use of illicit opioids, and reduces patient cravings for opioids. BUP is a safe medication and is associated with fewer adverse effects than insulin or warfarin.16
Continue to: Methadone treatment of OUD...
Methadone treatment of OUD
Methadone is a full opioid agonist approved by the FDA for the treatment of severe pain or OUD. Methadone treatment of OUD is strictly regulated and typically is ordered and administered at an opioid treatment program that is federally licensed. Methadone for OUD treatment cannot be prescribed by a physician to a pharmacy, limiting its use in primary care practice. Methadone used to treat OUD is ordered and dispensed at opioid-treatment programs. Take-home doses of methadone may be available to patients after adherence to the regimen has been established. When used long-term, higher doses of methadone are associated with better adherence, but these higher doses can cause respiratory depression. In a study of 189 pregnant patients taking methadone to treat OUD, daily doses of 60 mg or greater were associated with better treatment retention at delivery and 60 days postpartum, as well as less use of nonprescription opioids.17 Under limited circumstances methadone can be ordered and dispensed for hospitalized patients with OUD.
Methadone is a pure opioid receptor agonist. Naloxone (NAL) is an opioid receptor antagonist. Buprenorphine (BUP) is a partial opioid receptor agonist-antagonist, which limits overdose risk. BUP often is combined with NAL as a combination formulation, which is thought to reduce the repurposing of BUP for non-prescribed uses. At appropriate treatment dosages, both methadone (≥60 mg) and BUP (≥ 16 mg) are highly effective for the treatment of OUD.1 For patients with health insurance, pharmacy benefits often provide some coverage for preferred products but no coverage for other products. Not all pharmacies carry BUP products. In a study of more than 5,000 pharmacies, approximately 60% reported that they carry and can dispense BUP medications.2
BUP monotherapy is available as generic sublingual tablets, buccal films (Belbuca), formulations for injection (Sublocade), and subcutaneous implants (Probuphine). BUPNAL is available as buccal films (Bunavail), sublingual films (Suboxone), and sublingual tablets (Zubsolv). For BUP-NAL combination productions, the following dose combinations have been reported to have similar effects: BUP-NAL 8 mg/2 mg sublingual film, BUP-NAL 5.7 mg/1.4 mg sublingual tablet, and BUP-NAL 4.2 mg/0.7 mg buccal film.3
When initiating BUP-monotherapy or BUP-NAL treatment for OUD, one approach for unobserved initiation is to instruct the patient to discontinue using opioid agonist drugs and wait for the onset of mild to moderate withdrawal symptoms. The purpose of this step is to avoid precipitating severe withdrawal symptoms caused by giving BUP or BUP-NAL to a patient who has recently used opioid drugs.
If BUP-NAL sublingual films (Suboxone) are prescribed following the onset of mild to moderate withdrawal symptoms, the patient can initiate therapy with a dose of 2 mg BUP/0.5 mg NAL or 4 mg BUP/1 mg NAL. At 60 to 120 minutes following the initial dose, if withdrawal symptoms persist, an additional dose of 4 mg BUP/1 mg NAL can be given. Thereafter, symptoms can be assessed every 60 to 120 minutes and additional doses administered to control symptoms. On the second day of therapy, a maximum of 16 mg of BUP is administered. Over the following days and weeks, if symptoms and cravings persist at a BUP dose of 16 mg, the total daily dose of BUP can be titrated up to 24 mg. For long-term treatment, a commonly prescribed daily dose is 16 mg BUP/4 mg NAL or 24 mg BUP/6 mg NAL. An absolute contraindication to BUP or BUP/NAL treatment is an allergy to the medication, and a relative contraindication is liver failure.
One potential complication of transmucosal BUP or BUP-NAL treatment is a dry mouth (xerostomia), which may contribute to dental disease.4 However, some experts question the quality of the data that contributed to the warning.5,6 Potential dental complications might be prevented by regular oral health examinations, daily flossing and teeth brushing, and stimulation of saliva by sugar-free gum or lozenges.
Primary care clinicians who initiate BUP or BUPNAL treatment for OUD often have a weekly visit with the patient during the initial phase of treatment and then every 3 to 4 weeks during maintenance therapy. Most patients need long-term treatment to achieve the goals of therapy, which include prevention of opioid overdose, reduction of cravings for nonprescription narcotics, and improvement in overall health. BUP and BUP-NAL treatment are effective without formal counseling, but counseling and social work support improve long-term adherence with treatment. Primary care clinicians who have experience with medication treatment of OUD report that their experience convinces them that medication treatment of OUD has similarities to the long-term treatment of diabetes, with antihyperglycemia medicines or the treatment of HIV infection with antiviral medications.
References
1. Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;CD002207.
2. Weiner SG, Qato DM, Faust JS, et al. Pharmacy availability of buprenorphine for opioid use disorder treatment in the U.S. JAMA Netw Open. 2023;6:E2316089.
3. Substance Abuse and Mental Health Services Administration (SAMHSA). Medications for opioid use disorder. SAMHSA website. Accessed August 21, 2023. https ://store.samhsa.gov/sites/default/files/SAMHSA_Digital_Download/PEP 21-02-01-002.pdf
4. FDA warns about dental problems with buprenorphine medicines dissolved in the mouth. FDA website. Accessed August 21, 2023. https ://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-dental-problems-buprenorphine-medicines-dissolved-mouth-treat-opioiduse-disorder#:~:text=What%20did%20FDA%20find%3F,medicines%20 dissolved%20in%20the%20mouth
5. Watson DP, Etmian S, Gastala N. Sublingual buprenorphine-naloxone exposure and dental disease. JAMA. 2023;329:1223-1224.
6. Brothers TD, Lewer D, Bonn M. Sublingual buprenorphine-naloxone exposure and dental disease. JAMA. 2023;329:1224.
Medication treatment of OUD in obstetrics
In the United States, the prevalence of OUD among pregnant patients hospitalized for delivery more than quadrupled from 1999 through 2014.18 BUP and methadone commonly are used to treat OUD during pregnancy.19 Among pregnant patients about 5% of buprenorphine prescriptions are written by obstetricians.20 An innovative approach to initiating BUP for pregnant patients with OUD is to use unobserved initiation, which involves outpatient discontinuation of nonprescription opioids to induce mild to moderate withdrawal symptoms followed by initiation of BUP treatment. In one cohort study, 55 pregnant patients used an unobserved outpatient protocol to initiate BUP treatment; 80% of the patients previously had used methadone or BUP. No patient experienced a precipitated withdrawal and 96% of patients returned for their office visit 1 week after initiation of treatment. Eighty-six percent of patients remained in treatment 3 months following initiation of BUP.21
Compared with methadone, BUP treatment during pregnancy may result in lower rates of neonatal abstinence syndrome. In one study of pregnant patients who were using methadone (n = 5,056) or BUP (n = 11,272) in late pregnancy, neonatal abstinence syndrome was diagnosed in 69.2% and 52.0% of newborns, respectively (adjusted relative risk, 0.73; 95% confidence interval, 0.71–0.75).22 In addition, compared with methadone, the use of BUP was associated with a reduced risk for low birth weight (14.9% vs 8.3%) and a lower risk for preterm birth (24.9% vs 14.4%). In this study, there were no differences in maternal obstetric outcomes when comparing BUP versus methadone treatment. Similar results have been reported in a meta-analysis analyzing the use of methadone and BUP during pregnancy.23 Studies performed to date have not shown an increased risk of congenital anomalies with the use of BUP-NAL during pregnancy.24,25
Although there may be differences in newborn outcomes with BUP and methadone, the American College of Obstetricians and Gynecologists does not recommend switching from methadone to BUP during pregnancy because precipitated withdrawal may occur.26 Based on recent studies, the American Society of Addiction Medicine has advised that it is safe to prescribe pregnant patients either BUP or BUP-NAL.27,28
Medication treatment of OUD with or without intensive counseling
The FDA recently reviewed literature related to the advantages and challenges of combining intensive counseling with medication treatment of OUD.29 The FDA noted that treatment saves lives and encouraged clinicians to initiate medication treatment of OUD or refer the patient to an appropriate clinician or treatment center. Combining medication treatment of OUD with intensive counseling is associated with greater treatment adherence and reduced health care costs. For example, in one study of 4,987 patients with OUD, initiation of counseling within 8 weeks of the start of medication treatment and a BUP dose of 16 mg or greater daily were associated with increased adherence to treatment.30 For patients receiving a BUP dose of less than 16 mg daily, treatment adherence with and without counseling was approximately 325 and 230 days, respectively. When the dose of BUP was 16 mg or greater, treatment adherence with and without counseling was approximately 405 and 320 days, respectively.30
Counseling should always be offered to patients initiating medication treatment of OUD. It should be noted that counseling alone is not a highly effective treatment for OUD.31 The FDA recently advised that the lack of availability of intensive counseling should not prevent clinicians from initiating BUP for the treatment of OUD.29 OUD is associated with a high mortalityrate and if counseling is not possible, medication treatment should be initiated. Substantial evidence demonstrates that medication treatment of OUD is associated with many benefits.16 The FDA advisory committee concluded that OUD treatment decisions should use shared decision making and be supportive and patient centered.29
The opportunities for medication treatment of OUD in primary care practice have expanded due to the recent FDA removal of restrictions on the use of BUP and heightened awareness of the positive public health impact of medication treatment. Challenges to the medication treatment of OUD remain, including stigmatization of OUD, barriers to insurance coverage for BUP, practice costs of treating OUD, and gaps in clinical education. For many pregnant patients, their main point of contact with health care is their obstetrician. By incorporating OUD treatment in pregnancy care, obstetricians will improve the health of the mother and newborn, contributing to the well-being of current and future generations. ●
Experts have recommended several interventions that may help reduce opioid overdose death.1 A consensus recommendation is that people who use drugs should be provided naloxone rescue medication and educated on the proper use of naloxone. Naloxone rescue medication is available in formulations for nasal or parenteral administration. The US Food and Drug Administration (FDA) recently has approved naloxone for over-the-counter status. The American Medical Association has provided a short web video on how to administer nasal naloxone.2 In a small pilot study, obstetricians offered every postpartum patient with naloxone administration education and a 2-dose nasal naloxone pack, with 76% of patients accepting the nasal naloxone pack.3
Many experts recommend that people who use drugs should be advised to never use them alone and to test a small amount of the drug to assess its potency. Many patients who use opioid drugs also take benzodiazepines, which can contribute to respiratory depression.4 Patients should avoid mixing drugs (eg, opioids and benzodiazepines). Some experts recommend that patients who use drugs should be provided take-home fentanyl test strips so they can evaluate their drugs for the presence of fentanyl, a medication that suppresses respiration and contributes to many overdose deaths. In addition, people who use drugs and are interested in reducing their use of drugs or managing overdose risk can be offered initiation of medication treatment of OUD.1
References
1. Wood E, Solomon ED, Hadland SE. Universal precautions for people at risk of opioid overdose in North America. JAMA Int Med. 2023;183:401-402.
2. How to administer Naloxone. AMA website. Accessed August 28, 2023. https://www.ama-assn.org /delivering-care/overdose-epidemic/how-administer-naloxone
3. Naliboff JA, Tharpe N. Universal postpartum naloxone provision: a harm reduction quality improvement project. J Addict Med. 2022;17:360-362.
4. Kelly JC, Raghuraman N, Stout MJ, et al. Home induction of buprenorphine for treatment of opioid use disorder in pregnancy. Obstet Gynecol. 2021;138:655-659.
- Spencer MR, Miniño AM, Warner M. Drug overdose deaths in the United States, 20012021. NCHS Data Brief no 457. Hyattsville, MD, National Center for Health Statistics. 2022. NCHS Data Brief No. 457. Published December 2022. Accessed August 21, 2023. https://www.cdc.gov /nchs/products/databriefs/db457.htm
- US traffic deaths drop slightly in 2022 but still a ‘crisis.’ AP News website. Published April 20, 2023. Accessed August 21, 2023. https://apnews.com /article/traffic-deaths-distracted-driving-crisis -6db6471e273b275920b6c4f9eb7e493b
- Suicide statistics. American Foundation for Suicide Prevention website. Accessed August 21, 2023. https://afsp.org/suicide-statistics/
- Bruzelius E, Martins SS. US Trends in drug overdose mortality among pregnant and postpartum persons, 2017-2020. JAMA. 2022;328:2159-2161.
- Metz TD, Rovner P, Hoffman MC, et al. Maternal deaths from suicide and overdose in Colorado, 2004-2012. Obstet Gynecol. 2016;128:1233-1240.
- Schiff DM, Nielsen T, Terplan M, et al. Fatal and nonfatal overdose among pregnant and postpartum women in Massachusetts. Obstet Gynecol. 2018;132:466-474.
- Goldman-Mellor S, Margerison CE. Maternal drug-related death and suicide are leading causes of postpartum death in California. Am J Obstet Gynecol. 2019;221:489.e1-489.e9.
- Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550.
- Waiver elimination (MAT Act). SAMHSA website. Accessed August 21, 2023. https://www .samhsa.gov/medications-substance-use- disorders/removal-data-waiver-requirement
- Picco L, Middleton M, Bruno R, et al. Validation of the OWLS, a Screening Tool for Measuring Prescription Opioid Use Disorder in Primary Care. Pain Med. 2020;21:2757-2764.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
- Creedon TB, Ali MM, Schuman-Olivier Z. Trends in buprenorphine prescribing for opioid use disorder by psychiatrists in the US from 2003 to 2021. JAMA Health Forum. 2023;4:E230221.
- Quick MD website. Accessed August 21, 2023. https://quick.md/
- Treitler P, Nowels M, Samples H, et al. BUP utilization and prescribing among New Jersey Medicaid beneficiaries after adoption of initiatives designed to improve treatment access. JAMA Netw Open. 2023;6:E2312030.
- Jawa R, Tin Y, Nall S, et al. Estimated clinical outcomes and cost-effectiveness associated with provision of addiction treatment in US primary care clinics. JAMA Netw Open. 2023;6:E237888.
- Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways of opioid use disorder. JAMA Netw Open. 2020;3:E1920622.
- Wilder CM, Hosta D, Winhusen T. Association of methadone dose with substance use and treatment retention in pregnant and postpartum women with opioid use disorder. J Subst Abuse Treat. 2017;80:33-36.
- Haight SC, Ko JY, Tong VT, et al. Opioid use disorder documented at delivery hospitalization - United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2018;67:845-849.
- Xu KY, Jones HE, Schiff DM, et al. Initiation and treatment discontinuation of medications for opioid use disorder in pregnant people compared with nonpregnant people. Obstet Gynecol. 2023;141:845-853.
- Kelly D, Krans EE. Medical specialty of buprenorphine prescribers for pregnant women with opioid use disorder. Am J Obstet Gynecol. 2019;220:502-503.
- Kelly JC, Raghuraman N, Stout MJ, et al. Home induction of buprenorphine for treatment of opioid use disorder in pregnancy. Obstet Gynecol. 2021;138:655-659.
- Suarez EA, Huybrechts KF, Straub L, et al. Buprenorphine versus methadone for opioid use disorder in pregnancy. N Engl J Med. 2022;387:2033-2044.
- Kinsella M, Halliday LO, Shaw M, et al. Buprenorphine compared with methadone in pregnancy: a systematic review and meta-analysis. Subst Use Misuse. 2022;57:1400-1416.
- Jumah NA, Edwards C, Balfour-Boehm J, et al. Observational study of the safety of buprenorphine-naloxone in pregnancy in a rural and remote population. BMJ Open. 2016;6:E011774.
- Mullins N, Galvin SL, Ramage M, et al. Buprenorphine and naloxone versus buprenorphine for opioid use disorder in pregnancy: a cohort study. J Addict Med. 2020;14:185-192.
- Opioid use and opioid use disorder in pregnancy. Committee Opinion No. 711. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2017;130:E81-E94.
- The ASAM National Practice Guideline for the Treatment of Opioid Use Disorder: 2020 Focused Update. J Addict Med. 2020;14(2S suppl 1):1-91.
- Link HM, Jones H, Miller L, et al. Buprenorphinenaloxone use in pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020;2:100179.
- Delphin-Rittmon ME, Cavazzoni P. US Food and Drug Administration website. https://www.fda .gov/media/168027/download
- Eren K, Schuster J, Herschell A, et al. Association of Counseling and Psychotherapy on retention in medication for addiction treatment within a large Medicaid population. J Addict Med. 2022;16:346353.
- Kakko J, Dybrandt Svanborg K, Kreek MJ, et al. 1-year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in Sweden: a randomized, placebo-controlled trial. Lancet. 2003;361:662-668.
- Spencer MR, Miniño AM, Warner M. Drug overdose deaths in the United States, 20012021. NCHS Data Brief no 457. Hyattsville, MD, National Center for Health Statistics. 2022. NCHS Data Brief No. 457. Published December 2022. Accessed August 21, 2023. https://www.cdc.gov /nchs/products/databriefs/db457.htm
- US traffic deaths drop slightly in 2022 but still a ‘crisis.’ AP News website. Published April 20, 2023. Accessed August 21, 2023. https://apnews.com /article/traffic-deaths-distracted-driving-crisis -6db6471e273b275920b6c4f9eb7e493b
- Suicide statistics. American Foundation for Suicide Prevention website. Accessed August 21, 2023. https://afsp.org/suicide-statistics/
- Bruzelius E, Martins SS. US Trends in drug overdose mortality among pregnant and postpartum persons, 2017-2020. JAMA. 2022;328:2159-2161.
- Metz TD, Rovner P, Hoffman MC, et al. Maternal deaths from suicide and overdose in Colorado, 2004-2012. Obstet Gynecol. 2016;128:1233-1240.
- Schiff DM, Nielsen T, Terplan M, et al. Fatal and nonfatal overdose among pregnant and postpartum women in Massachusetts. Obstet Gynecol. 2018;132:466-474.
- Goldman-Mellor S, Margerison CE. Maternal drug-related death and suicide are leading causes of postpartum death in California. Am J Obstet Gynecol. 2019;221:489.e1-489.e9.
- Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550.
- Waiver elimination (MAT Act). SAMHSA website. Accessed August 21, 2023. https://www .samhsa.gov/medications-substance-use- disorders/removal-data-waiver-requirement
- Picco L, Middleton M, Bruno R, et al. Validation of the OWLS, a Screening Tool for Measuring Prescription Opioid Use Disorder in Primary Care. Pain Med. 2020;21:2757-2764.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
- Creedon TB, Ali MM, Schuman-Olivier Z. Trends in buprenorphine prescribing for opioid use disorder by psychiatrists in the US from 2003 to 2021. JAMA Health Forum. 2023;4:E230221.
- Quick MD website. Accessed August 21, 2023. https://quick.md/
- Treitler P, Nowels M, Samples H, et al. BUP utilization and prescribing among New Jersey Medicaid beneficiaries after adoption of initiatives designed to improve treatment access. JAMA Netw Open. 2023;6:E2312030.
- Jawa R, Tin Y, Nall S, et al. Estimated clinical outcomes and cost-effectiveness associated with provision of addiction treatment in US primary care clinics. JAMA Netw Open. 2023;6:E237888.
- Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways of opioid use disorder. JAMA Netw Open. 2020;3:E1920622.
- Wilder CM, Hosta D, Winhusen T. Association of methadone dose with substance use and treatment retention in pregnant and postpartum women with opioid use disorder. J Subst Abuse Treat. 2017;80:33-36.
- Haight SC, Ko JY, Tong VT, et al. Opioid use disorder documented at delivery hospitalization - United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2018;67:845-849.
- Xu KY, Jones HE, Schiff DM, et al. Initiation and treatment discontinuation of medications for opioid use disorder in pregnant people compared with nonpregnant people. Obstet Gynecol. 2023;141:845-853.
- Kelly D, Krans EE. Medical specialty of buprenorphine prescribers for pregnant women with opioid use disorder. Am J Obstet Gynecol. 2019;220:502-503.
- Kelly JC, Raghuraman N, Stout MJ, et al. Home induction of buprenorphine for treatment of opioid use disorder in pregnancy. Obstet Gynecol. 2021;138:655-659.
- Suarez EA, Huybrechts KF, Straub L, et al. Buprenorphine versus methadone for opioid use disorder in pregnancy. N Engl J Med. 2022;387:2033-2044.
- Kinsella M, Halliday LO, Shaw M, et al. Buprenorphine compared with methadone in pregnancy: a systematic review and meta-analysis. Subst Use Misuse. 2022;57:1400-1416.
- Jumah NA, Edwards C, Balfour-Boehm J, et al. Observational study of the safety of buprenorphine-naloxone in pregnancy in a rural and remote population. BMJ Open. 2016;6:E011774.
- Mullins N, Galvin SL, Ramage M, et al. Buprenorphine and naloxone versus buprenorphine for opioid use disorder in pregnancy: a cohort study. J Addict Med. 2020;14:185-192.
- Opioid use and opioid use disorder in pregnancy. Committee Opinion No. 711. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2017;130:E81-E94.
- The ASAM National Practice Guideline for the Treatment of Opioid Use Disorder: 2020 Focused Update. J Addict Med. 2020;14(2S suppl 1):1-91.
- Link HM, Jones H, Miller L, et al. Buprenorphinenaloxone use in pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020;2:100179.
- Delphin-Rittmon ME, Cavazzoni P. US Food and Drug Administration website. https://www.fda .gov/media/168027/download
- Eren K, Schuster J, Herschell A, et al. Association of Counseling and Psychotherapy on retention in medication for addiction treatment within a large Medicaid population. J Addict Med. 2022;16:346353.
- Kakko J, Dybrandt Svanborg K, Kreek MJ, et al. 1-year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in Sweden: a randomized, placebo-controlled trial. Lancet. 2003;361:662-668.