User login
The blood-brain barrier (BBB) is an essential barrier of closely spaced cells that regulates entry into the CNS. What passes should be highly regulated to protect the brain from potentially harmful peripheral cells or molecules from the rest of the body. However, research has revealed that the BBB is pathologically permeable in several disease states, including schizophrenia, epilepsy, traumatic brain injury, autism, and DiGeorge syndrome (22q11.2 deletion syndrome, which often presents with symptoms of schizophrenia).1,2 In this article, we discuss potential markers of BBB dysfunction, the consequences of a porous BBB, the effect of BBB permeability on microglial activation, and possible treatment implications.
Detecting a BBB leak
The BBB is composed of microvascular endothelial cell units. Adherens junctions, astrocyte endfeet, and pericytes are all part of these units, but tight junctions have the most significant role in BBB barrier function. Tight junction protein composition varies depending on the location of the endothelium. In the BBB, they are primarily composed of claudin-5, occludin, zonulin, and junction adhesion molecules (JAMs) (Figure). Claudins and occludins are especially important components of the tight junction because they span plasma membranes.3
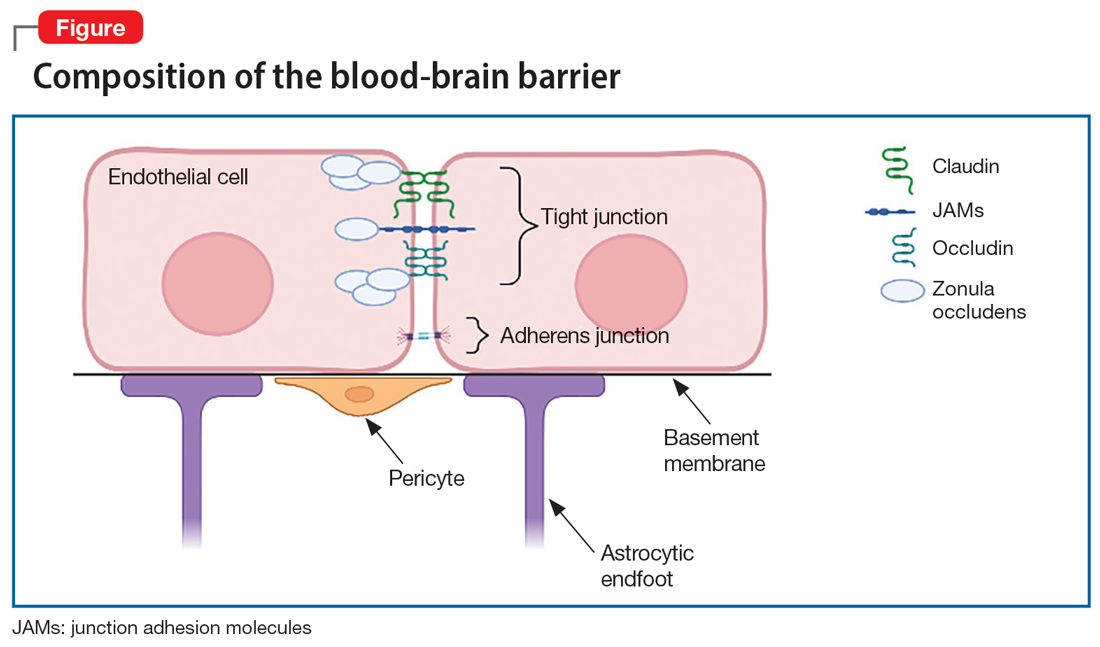
Researchers began to suspect tight junction permeability in schizophrenia while searching for schizophrenia biomarkers. For example, S100B is a marker of astrocytic reactivity to damage. It is increased in schizophrenia, major depressive disorder, and bipolar disorder.4 Studies found elevated S100B specifically in drug-free patients with schizophrenia,5 which prompted research suggesting it could predict the severity of negative symptoms.6 The accuracy of S100B as a biomarker was later complicated by the finding that adipose tissue also secretes S100B. This is problematic due to the high rates of comorbid obesity in psychiatric populations.2
Perhaps a better biomarker is the ratio of albumin in the CSF vs that in peripheral serum. The CSF-to-blood albumin ratio (Q-Alb) is widely considered an acceptable marker of BBB dysfunction because albumin must cross the BBB to alter the ratio. Studies have found a high Q-Alb in neurodegenerative disorders such as multiple sclerosis as well as in schizophrenia, which suggests that some level of BBB dysfunction is occurring. Although the Q-Alb may change slightly when confounded by antipsychotic use or with CSF flow changes,2,4 both S100B and Q-Alb elevation are sufficient for further investigation into tight junction alteration in schizophrenia.
Claudin-5 is a promising factor in detecting BBB permeability. Claudin-5 is deleted in DiGeorge syndrome, which is highly comorbid with schizophrenia and psychosis.1 Mouse knockdown studies show that full suppression of claudin-5 results in psychotic symptoms before fatal seizures,2,7 but a partial absence may enable psychotic symptoms. The same study showed that normally continuous claudin-5 was patchy along blood vessels in the affected sample.7 Follow-up experiments suggest that loss of claudin-5 in schizophrenia is especially prominent in the hippocampus, and there is mixed evidence of a decrease in the prefrontal cortex.8
Outside of claudin-5 alone, JAM-A plays a more regulatory role. It is upstream from an enhancer protein gene that serves as a transcription factor for the claudin-5 promoter, so when JAM-A is deleted, there is less claudin-5.9 However, while this decrease in claudin-5 may be pathological, there could still be various upstream changes that lead to schizophrenia.
What are the consequences of a porous BBB?
Although it is well established that the BBB passes small molecules and solutes, there is significant evidence of inflammatory trafficking in disease states. The BBB moves proinflammatory cytokines, alters transporters, and may even let white blood cells (WBCs) pass through. Immune cell infiltration has different requirements depending on the cell type. T cells rely on integrins, vascular cell adhesion molecule 1 (vCAM1), and intercellular adhesion molecule 1 (iCAM1) for binding, rolling, adhering, crossing, and migration to sites of inflammation.10,11 Both iCAM1 and vCAM1 are elevated in schizophrenia compared to other psychiatric disorders (such as unipolar depression) and correlate with other biomarkers. For example, vCAM1, responsible for recruitment and crossing, is correlated with a high Q-Alb.12 Primarily produced by astrocytes and endothelial cells, iCAM1 plays the largest role in crossing the BBB and migration. Postmortem tissue demonstrates that cytokines upregulate iCAM1 mRNA at the BBB in schizophrenia.13 Increased cytokines are well documented in the inflammatory model of schizophrenia. Interestingly, decreasing claudin-5 also upregulates iCAM1 production.14 Therefore, low baseline claudin-5 may contribute to additional inflammation and symptoms.
Continue to: BBB permeability also results...
BBB permeability also results in a certain pattern of leukocyte and cytokine activity. Interleukin-1 (IL-1), IL-6, and tumor necrosis factor–alpha can all cross the BBB during neuroimmune inflammation,10 but there are abnormal heightened and sustained responses of these molecules in schizophrenia. IL-6 is a proinflammatory cytokine in both acute and chronic inflammation that is expressed by astrocytes, endothelial cells, and microglia.15 IL-6 and its soluble receptor are both elevated in schizophrenia and are associated with white matter degeneration16,17 and an increase in vCAM1.15 This implies that while neuroinflammation in schizophrenia is occurring, additional leukocytes are being recruited and secreting their own cytokines in a chronic destructive positive feedback loop. Meanwhile, atypical IL-10 levels can no longer maintain balanced levels of inflammatory molecules,16 which leads to reduced control of inflammation.
Genetics and immunohistochemistry suggest that the BBB allows the passage of excess B cells and T cells in schizophrenia. Cytokines from WBCs or the BBB during inflammation recruit these additional infiltrating lymphocytes. In gene-wide association studies, there are several genes in schizophrenia important for B cells and T cells in addition to inflammation that interact in a proinflammatory network.16 These cells are also diffusely found in the white matter18 and hippocampal tissue19 of patients with schizophrenia. Taken together, an increase in adhesion molecules, WBCs, and cytokine crosstalk supports a leaky BBB as an important component of the inflammatory model of schizophrenia.
The role of microglia in BBB dysfunction
The effect of BBB permeability on microglial activation is an important caveat in the current research. Although several reports have linked neuroinflammation to confirmed microglial activation in schizophrenia, there is not enough evidence to claim that the BBB alone is the missing link between these theories. Some research suggests that chronic release of cytokines such as IL-6 from macrophages and T cells could increase migration across the BBB for microglial activation.16,20 However, positron emission tomography has shown mixed results at best. Translocator protein (TSPO) is expressed by microglia that are actively secreting cytokines.21 Researchers tracking TSPO changes in relation to BBB alteration have not seen elevated binding in schizophrenia, change due to stage of disease course, or differentiation from low-grade inflammation.21-24 Moreover, TSPO may be confounded by antipsychotic use25 and microglial expression did not correlate with any changes in adhesion molecules.13 TSPO is not an ideal indicator of microglial activation due to BBB breakdown, but that does not bar the possibility of at least a partial contribution to the development of schizophrenia.
Corsi-Zuelli et al26 created a model that attempts to merge BBB permeability and microglial activation through a different medium—T regulatory cells (TRegs). They write that if TRegs mediate interactions between astrocytes and microglia, their hypofunction would impose a prolonged T cell response. The increased access to a high level of IL-6 and its soluble receptors may keep the TRegs hypofunctional in schizophrenia and promote T cell conversion to inflammatory cell types. Experimentally, TReg induction reversed some psychotic symptoms, and greater TReg expression was associated with fewer negative symptoms.26 In an already insufficient BBB, more access to cytokines and leukocytes would sustain inflammation and microglial secretions.
In addition to the issues described regarding the BBB, the blood-CSF barrier at the choroid plexus may also be insufficient in schizophrenia (Box27-31).
Box
The choroid plexus’ primary role is to make CSF, but it also secretes cytokines and to some extent serves as a barrier. Unlike the blood-brain barrier (BBB), the blood-CSF barrier is composed of endothelial cells with fenestrations as well as tight junctions, which make the blood-CSF barrier overall more permeable.27,28 The most unusual finding regarding the choroid plexus in schizophrenia is size. The choroid plexus is physically larger in patients with schizophrenia, and to a lesser extent, in their first-degree relatives.29 A larger choroid plexus is correlated with more severe cognitive symptoms, increased risk for psychosis via biological stress, and significantly higher interleukin-6 (IL-6).27,29 The increased thickness could be an attempt to compensate for hyperactivity and toxic processes in a permeable environment. More circulating cytokines such as IL-6 and tumor necrosis factor–alpha from microglia can trigger an increase in intercellular adhesion molecule 1, resulting in leukocyte attachment and entry.30 Less claudin-5 at the choroid plexus in schizophrenia implicates similar permissive effects as seen at the BBB.31 Although the contribution of blood-CSF barrier dysfunction to schizophrenia requires further study, reduced barrier function outside the BBB is a viable line of inquiry.
Continue to: Caveats about this research
Caveats about this research
There are 3 important points to note about the current research concerning abnormal BBB permeability:
1. BBB dysfunction may exist only in a subset of people diagnosed with schizophrenia. In most human studies, only some patients with schizophrenia demonstrated alterations that suggested pathological BBB permeability. In addition, even when there is BBB dysfunction, it could be a secondary phenomenon, rather than a primary etiologic process.
2. Patient demographics across studies have not always been adequately described. Potential confounds such as obesity, smoking, or antipsychotic use were not consistently recorded or examined as a possible factor.
3. Currently available biomarkers are not perfect. Cytokine elevation, S100B, and Q-Alb are indirect measures of BBB disruption and are found in other disorders. Therefore, they only support the theory of BBB dysfunction in schizophrenia, rather than prove it. They are also not reliable markers for schizophrenia alone. Researchers have pointed out that these markers and proteins work in concert, which necessitates a network analysis approach.16 More research regarding the details of permeability is required to establish more reliable biomarkers and tailored treatment.
Treatment implications
One of the first treatment directions that comes to mind is managing the gaps in the BBB via tight junctions. Presently, there are no FDA-approved medications for altering tight junction proteins, but researchers are exploring potential agents that can induce claudin-5 and reduce inflammation.14 While we wait for such a medication, patients may benefit from existing anti-inflammatory treatments to control immune infiltration and its products. Various anti-inflammatory agents—including cyclooxygenase inhibitors,
Bottom Line
Recent research has revealed that the blood-brain barrier (BBB) is pathologically permeable in several disease states, including schizophrenia. Better characterization of the leaky BBB in schizophrenia has enormous potential in helping us understand how current theories fit together and could serve as a missing puzzle piece in treating schizophrenia.
Related Resources
- Levine A, Strawn JR. The brain’s Twitter system: neuronal extracellular vesicles. Current Psychiatry. 2022;21(6):9-11, 17-19,27. doi:10.12788/cp.0257
Drug Brand Names
Minocycline • Dynacin, Minocin
1. Li Y, Xia Y, Zhu H, et al. Investigation of neurodevelopmental deficits of 22 q11.2 deletion syndrome with a patient-iPSC-derived blood-brain barrier model. Cells. 2021;10(10):2576. doi:10.3390/cells10102576
2. Kealy J, Greene C, Campbell M. Blood-brain barrier regulation in psychiatric disorders. Neurosci Lett. 2020;726:133664. doi:10.1016/j.neulet.2018.06.033
3. Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1-13. doi:10.1016/j.nbd.2003.12.016
4. Futtrup J, Margolinsky R, Benros ME, et al. Blood-brain barrier pathology in patients with severe mental disorders: a systematic review and meta-analysis of biomarkers in case-control studies. Brain Behav Immun Health. 2020;6:100102. doi:10.1016/j.bbih.2020.100102
5. Chen S, Tian L, Chen N, et al. Cognitive dysfunction correlates with elevated serum S100B concentration in drug-free acutely relapsed patients with schizophrenia. Psychiatry Res. 2017;247:6-11. doi:10.1016/j.psychres.2016.09.029
6. Wu YF, Sytwu HK, Lung FW. Human aquaporin 4 gene polymorphisms and haplotypes are associated with serum S100B level and negative symptoms of schizophrenia in a southern Chinese Han population. Front Psychiatry. 2018;9:657. doi:10.3389/fpsyt.2018.00657
7. Greene C, Kealy J, Humphries MM, et al. Dose-dependent expression of claudin-5 is a modifying factor in schizophrenia. Mol Psychiatry. 2018;23(11):2156-2166. doi:10.1038/MP.2017.156
8. Greene C, Hanley N, Campbell M. Blood-brain barrier associated tight junction disruption is a hallmark feature of major psychiatric disorders. Transl Psychiatry. 2020;10(1):373. doi:10.1038/s41398-020-01054-3
9. Kakogiannos N, Ferrari L, Giampietro C, et al. JAM-A acts via C/EBP-α to promote claudin-5 expression and enhance endothelial barrier function. Circ Res. 2020:1056-1073. doi:10.1161/CIRCRESAHA.120.316742
10. Erickson MA, Dohi K, Banks WA. Neuroinflammation: a common pathway in CNS diseases as mediated at the blood-brain barrier. Neuroimmunomodulation. 2012;19(2):121-130. doi:10.1159/000330247
11. Ao LY, Yan YY, Zhou L, et al. Immune cells after ischemic stroke onset: roles, migration, and target intervention. J Mol Neurosci. 2018;66(3):342-355. doi:10.1007/s12031-018-1173-4
12. Meixensberger S, Kuzior H, Fiebich BL, et al. Upregulation of sICAM-1 and sVCAM-1 levels in the cerebrospinal fluid of patients with schizophrenia spectrum disorders. Diagnostics (Basel). 2021;11(7):1134. doi:10.3390diagnostics11071134
13. Cai HQ, Catts VS, Webster MJ, et al. Increased macrophages and changed brain endothelial cell gene expression in the frontal cortex of people with schizophrenia displaying inflammation. Mol Psychiatry. 2020;25(4):761-775. doi:10.1038/s41380-018-0235-x
14. Greene C, Hanley N, Reschke CR, et al. Microvascular stabilization via blood-brain barrier regulation prevents seizure activity. Nat Commun. 2022;13(1):2003. doi:10.1038/s41467-022-29657-y
15. García-Juárez M, Camacho-Morales A. Defining the role of anti- and pro-inflammatory outcomes of interleukin-6 in mental health. Neuroscience. 2022;492:32-46. doi:10.1016/j.neuroscience.2022.03.020
16. Pong S, Karmacharya R, Sofman M, et al. The role of brain microvascular endothelial cell and blood-brain barrier dysfunction in schizophrenia. Complex Psychiatry. 2020;6(1-2):30-46. doi:10.1159/000511552
17. Patel A, Zhu Y, Kuzhikandathil EV, et al. Soluble interleukin-6 receptor induces motor stereotypies and co-localizes with gp130 in regions linked to cortico-striato-thalamo-cortical circuits. PLoS One. 2012;7(7): e41623. doi:10.1371/journal.pone.0041623
18. Schlaaff K, Dobrowolny H, Frodl T, et al. Increased densities of T and B lymphocytes indicate neuroinflammation in subgroups of schizophrenia and mood disorder patients. Brain Behav Immun. 2020;88:497-506. doi:10.1016/j.bbi.2020.04.021
19. Busse S, Busse M, Schiltz K, et al. Different distribution patterns of lymphocytes and microglia in the hippocampus of patients with residual versus paranoid schizophrenia: further evidence for disease course-related immune alterations? Brain Behav Immun. 2012;26(8):1273-1279. doi:10.1016/j.bbi.2012.08.005
20. Na KS, Jung HY, Kim YK. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:277-286. doi:10.1016/j.pnpbp.2012.10.022
21. Conen S, Gregory CJ, Hinz R, et al. Neuroinflammation as measured by positron emission tomography in patients with recent onset and established schizophrenia: implications for immune pathogenesis. Mol Psychiatry. 2021;26(9):5398-5406. doi:10.1038/S41380-020-0829-Y
22. Najjar S, Pahlajani S, De Sanctis V, et al. Neurovascular unit dysfunction and blood-brain barrier hyperpermeability contribute to schizophrenia neurobiology: a theoretical integration of clinical and experimental evidence. Front Psychiatry. 2017;8:83. doi:10.3389/fpsyt.2017.00083
23. Pinjari OF, Dasgupta SK, Okusaga OO. Plasma soluble P-selectin, interleukin-6 and S100B protein in patients with schizophrenia: a pilot study. Psychiatr Q. 2022;93(1):335-345. doi:10.1007/s11126-021-09954-3
24. Di Biase MA, Zalesky A, O’keefe G, et al. PET imaging of putative microglial activation in individuals at ultra-high risk for psychosis, recently diagnosed and chronically ill with schizophrenia. Transl Psychiatry. 2017;7(8):e1225. doi:10.1038/tp.2017.193
25. Holmes SE, Hinz R, Drake RJ, et al. In vivo imaging of brain microglial activity in antipsychotic-free and medicated schizophrenia: a [11C](R)-PK11195 positron emission tomography study. Mol Psychiatry. 2016;21(12):1672-1679. doi:10.1038/mp.2016.180
26. Corsi-Zuelli F, Deakin B, de Lima MHF, et al. T regulatory cells as a potential therapeutic target in psychosis? Current challenges and future perspectives. Brain Behav Immun Health. 2021;17:100330. doi:10.1016/j.bbih.2021.100330
27. Bannai D, Lutz O, Lizano P. Neuroimaging considerations when investigating choroid plexus morphology in idiopathic psychosis. Schizophr Res. 2020;224:19-21. doi:10.1016/j.schres.2020.07.013
28. Hladky SB, Barrand MA. Fluid and ion transfer across the blood-brain and blood-cerebrospinal fluid barriers; a comparative account of mechanisms and roles. Fluids Barriers CNS. 2016;13(1):19. doi:10.1186/s12987-016-0040-3
29. Lizano P, Lutz O, Ling G, et al. Association of choroid plexus enlargement with cognitive, inflammatory, and structural phenotypes across the psychosis spectrum. Am J Psychiatry. 2019;176(7):564-572. doi:10.1176/appi.ajp.2019.18070825
30. Castellani G, Contarini G, Mereu M, et al. Dopamine-mediated immunomodulation affects choroid plexus function. Brain Behav Immun. 2019;81:138-150. doi:10.1016/j.bbi.2019.06.006
31. Bitanihirwe BKY, Lizano P, Woo TW. Deconstructing the functional neuroanatomy of the choroid plexus: an ontogenetic perspective for studying neurodevelopmental and neuropsychiatric disorders. Mol Psychiatry. 2022;1-10. doi:10.1038/s41380-022-01623-6
The blood-brain barrier (BBB) is an essential barrier of closely spaced cells that regulates entry into the CNS. What passes should be highly regulated to protect the brain from potentially harmful peripheral cells or molecules from the rest of the body. However, research has revealed that the BBB is pathologically permeable in several disease states, including schizophrenia, epilepsy, traumatic brain injury, autism, and DiGeorge syndrome (22q11.2 deletion syndrome, which often presents with symptoms of schizophrenia).1,2 In this article, we discuss potential markers of BBB dysfunction, the consequences of a porous BBB, the effect of BBB permeability on microglial activation, and possible treatment implications.
Detecting a BBB leak
The BBB is composed of microvascular endothelial cell units. Adherens junctions, astrocyte endfeet, and pericytes are all part of these units, but tight junctions have the most significant role in BBB barrier function. Tight junction protein composition varies depending on the location of the endothelium. In the BBB, they are primarily composed of claudin-5, occludin, zonulin, and junction adhesion molecules (JAMs) (Figure). Claudins and occludins are especially important components of the tight junction because they span plasma membranes.3

Researchers began to suspect tight junction permeability in schizophrenia while searching for schizophrenia biomarkers. For example, S100B is a marker of astrocytic reactivity to damage. It is increased in schizophrenia, major depressive disorder, and bipolar disorder.4 Studies found elevated S100B specifically in drug-free patients with schizophrenia,5 which prompted research suggesting it could predict the severity of negative symptoms.6 The accuracy of S100B as a biomarker was later complicated by the finding that adipose tissue also secretes S100B. This is problematic due to the high rates of comorbid obesity in psychiatric populations.2
Perhaps a better biomarker is the ratio of albumin in the CSF vs that in peripheral serum. The CSF-to-blood albumin ratio (Q-Alb) is widely considered an acceptable marker of BBB dysfunction because albumin must cross the BBB to alter the ratio. Studies have found a high Q-Alb in neurodegenerative disorders such as multiple sclerosis as well as in schizophrenia, which suggests that some level of BBB dysfunction is occurring. Although the Q-Alb may change slightly when confounded by antipsychotic use or with CSF flow changes,2,4 both S100B and Q-Alb elevation are sufficient for further investigation into tight junction alteration in schizophrenia.
Claudin-5 is a promising factor in detecting BBB permeability. Claudin-5 is deleted in DiGeorge syndrome, which is highly comorbid with schizophrenia and psychosis.1 Mouse knockdown studies show that full suppression of claudin-5 results in psychotic symptoms before fatal seizures,2,7 but a partial absence may enable psychotic symptoms. The same study showed that normally continuous claudin-5 was patchy along blood vessels in the affected sample.7 Follow-up experiments suggest that loss of claudin-5 in schizophrenia is especially prominent in the hippocampus, and there is mixed evidence of a decrease in the prefrontal cortex.8
Outside of claudin-5 alone, JAM-A plays a more regulatory role. It is upstream from an enhancer protein gene that serves as a transcription factor for the claudin-5 promoter, so when JAM-A is deleted, there is less claudin-5.9 However, while this decrease in claudin-5 may be pathological, there could still be various upstream changes that lead to schizophrenia.
What are the consequences of a porous BBB?
Although it is well established that the BBB passes small molecules and solutes, there is significant evidence of inflammatory trafficking in disease states. The BBB moves proinflammatory cytokines, alters transporters, and may even let white blood cells (WBCs) pass through. Immune cell infiltration has different requirements depending on the cell type. T cells rely on integrins, vascular cell adhesion molecule 1 (vCAM1), and intercellular adhesion molecule 1 (iCAM1) for binding, rolling, adhering, crossing, and migration to sites of inflammation.10,11 Both iCAM1 and vCAM1 are elevated in schizophrenia compared to other psychiatric disorders (such as unipolar depression) and correlate with other biomarkers. For example, vCAM1, responsible for recruitment and crossing, is correlated with a high Q-Alb.12 Primarily produced by astrocytes and endothelial cells, iCAM1 plays the largest role in crossing the BBB and migration. Postmortem tissue demonstrates that cytokines upregulate iCAM1 mRNA at the BBB in schizophrenia.13 Increased cytokines are well documented in the inflammatory model of schizophrenia. Interestingly, decreasing claudin-5 also upregulates iCAM1 production.14 Therefore, low baseline claudin-5 may contribute to additional inflammation and symptoms.
Continue to: BBB permeability also results...
BBB permeability also results in a certain pattern of leukocyte and cytokine activity. Interleukin-1 (IL-1), IL-6, and tumor necrosis factor–alpha can all cross the BBB during neuroimmune inflammation,10 but there are abnormal heightened and sustained responses of these molecules in schizophrenia. IL-6 is a proinflammatory cytokine in both acute and chronic inflammation that is expressed by astrocytes, endothelial cells, and microglia.15 IL-6 and its soluble receptor are both elevated in schizophrenia and are associated with white matter degeneration16,17 and an increase in vCAM1.15 This implies that while neuroinflammation in schizophrenia is occurring, additional leukocytes are being recruited and secreting their own cytokines in a chronic destructive positive feedback loop. Meanwhile, atypical IL-10 levels can no longer maintain balanced levels of inflammatory molecules,16 which leads to reduced control of inflammation.
Genetics and immunohistochemistry suggest that the BBB allows the passage of excess B cells and T cells in schizophrenia. Cytokines from WBCs or the BBB during inflammation recruit these additional infiltrating lymphocytes. In gene-wide association studies, there are several genes in schizophrenia important for B cells and T cells in addition to inflammation that interact in a proinflammatory network.16 These cells are also diffusely found in the white matter18 and hippocampal tissue19 of patients with schizophrenia. Taken together, an increase in adhesion molecules, WBCs, and cytokine crosstalk supports a leaky BBB as an important component of the inflammatory model of schizophrenia.
The role of microglia in BBB dysfunction
The effect of BBB permeability on microglial activation is an important caveat in the current research. Although several reports have linked neuroinflammation to confirmed microglial activation in schizophrenia, there is not enough evidence to claim that the BBB alone is the missing link between these theories. Some research suggests that chronic release of cytokines such as IL-6 from macrophages and T cells could increase migration across the BBB for microglial activation.16,20 However, positron emission tomography has shown mixed results at best. Translocator protein (TSPO) is expressed by microglia that are actively secreting cytokines.21 Researchers tracking TSPO changes in relation to BBB alteration have not seen elevated binding in schizophrenia, change due to stage of disease course, or differentiation from low-grade inflammation.21-24 Moreover, TSPO may be confounded by antipsychotic use25 and microglial expression did not correlate with any changes in adhesion molecules.13 TSPO is not an ideal indicator of microglial activation due to BBB breakdown, but that does not bar the possibility of at least a partial contribution to the development of schizophrenia.
Corsi-Zuelli et al26 created a model that attempts to merge BBB permeability and microglial activation through a different medium—T regulatory cells (TRegs). They write that if TRegs mediate interactions between astrocytes and microglia, their hypofunction would impose a prolonged T cell response. The increased access to a high level of IL-6 and its soluble receptors may keep the TRegs hypofunctional in schizophrenia and promote T cell conversion to inflammatory cell types. Experimentally, TReg induction reversed some psychotic symptoms, and greater TReg expression was associated with fewer negative symptoms.26 In an already insufficient BBB, more access to cytokines and leukocytes would sustain inflammation and microglial secretions.
In addition to the issues described regarding the BBB, the blood-CSF barrier at the choroid plexus may also be insufficient in schizophrenia (Box27-31).
Box
The choroid plexus’ primary role is to make CSF, but it also secretes cytokines and to some extent serves as a barrier. Unlike the blood-brain barrier (BBB), the blood-CSF barrier is composed of endothelial cells with fenestrations as well as tight junctions, which make the blood-CSF barrier overall more permeable.27,28 The most unusual finding regarding the choroid plexus in schizophrenia is size. The choroid plexus is physically larger in patients with schizophrenia, and to a lesser extent, in their first-degree relatives.29 A larger choroid plexus is correlated with more severe cognitive symptoms, increased risk for psychosis via biological stress, and significantly higher interleukin-6 (IL-6).27,29 The increased thickness could be an attempt to compensate for hyperactivity and toxic processes in a permeable environment. More circulating cytokines such as IL-6 and tumor necrosis factor–alpha from microglia can trigger an increase in intercellular adhesion molecule 1, resulting in leukocyte attachment and entry.30 Less claudin-5 at the choroid plexus in schizophrenia implicates similar permissive effects as seen at the BBB.31 Although the contribution of blood-CSF barrier dysfunction to schizophrenia requires further study, reduced barrier function outside the BBB is a viable line of inquiry.
Continue to: Caveats about this research
Caveats about this research
There are 3 important points to note about the current research concerning abnormal BBB permeability:
1. BBB dysfunction may exist only in a subset of people diagnosed with schizophrenia. In most human studies, only some patients with schizophrenia demonstrated alterations that suggested pathological BBB permeability. In addition, even when there is BBB dysfunction, it could be a secondary phenomenon, rather than a primary etiologic process.
2. Patient demographics across studies have not always been adequately described. Potential confounds such as obesity, smoking, or antipsychotic use were not consistently recorded or examined as a possible factor.
3. Currently available biomarkers are not perfect. Cytokine elevation, S100B, and Q-Alb are indirect measures of BBB disruption and are found in other disorders. Therefore, they only support the theory of BBB dysfunction in schizophrenia, rather than prove it. They are also not reliable markers for schizophrenia alone. Researchers have pointed out that these markers and proteins work in concert, which necessitates a network analysis approach.16 More research regarding the details of permeability is required to establish more reliable biomarkers and tailored treatment.
Treatment implications
One of the first treatment directions that comes to mind is managing the gaps in the BBB via tight junctions. Presently, there are no FDA-approved medications for altering tight junction proteins, but researchers are exploring potential agents that can induce claudin-5 and reduce inflammation.14 While we wait for such a medication, patients may benefit from existing anti-inflammatory treatments to control immune infiltration and its products. Various anti-inflammatory agents—including cyclooxygenase inhibitors,
Bottom Line
Recent research has revealed that the blood-brain barrier (BBB) is pathologically permeable in several disease states, including schizophrenia. Better characterization of the leaky BBB in schizophrenia has enormous potential in helping us understand how current theories fit together and could serve as a missing puzzle piece in treating schizophrenia.
Related Resources
- Levine A, Strawn JR. The brain’s Twitter system: neuronal extracellular vesicles. Current Psychiatry. 2022;21(6):9-11, 17-19,27. doi:10.12788/cp.0257
Drug Brand Names
Minocycline • Dynacin, Minocin
The blood-brain barrier (BBB) is an essential barrier of closely spaced cells that regulates entry into the CNS. What passes should be highly regulated to protect the brain from potentially harmful peripheral cells or molecules from the rest of the body. However, research has revealed that the BBB is pathologically permeable in several disease states, including schizophrenia, epilepsy, traumatic brain injury, autism, and DiGeorge syndrome (22q11.2 deletion syndrome, which often presents with symptoms of schizophrenia).1,2 In this article, we discuss potential markers of BBB dysfunction, the consequences of a porous BBB, the effect of BBB permeability on microglial activation, and possible treatment implications.
Detecting a BBB leak
The BBB is composed of microvascular endothelial cell units. Adherens junctions, astrocyte endfeet, and pericytes are all part of these units, but tight junctions have the most significant role in BBB barrier function. Tight junction protein composition varies depending on the location of the endothelium. In the BBB, they are primarily composed of claudin-5, occludin, zonulin, and junction adhesion molecules (JAMs) (Figure). Claudins and occludins are especially important components of the tight junction because they span plasma membranes.3

Researchers began to suspect tight junction permeability in schizophrenia while searching for schizophrenia biomarkers. For example, S100B is a marker of astrocytic reactivity to damage. It is increased in schizophrenia, major depressive disorder, and bipolar disorder.4 Studies found elevated S100B specifically in drug-free patients with schizophrenia,5 which prompted research suggesting it could predict the severity of negative symptoms.6 The accuracy of S100B as a biomarker was later complicated by the finding that adipose tissue also secretes S100B. This is problematic due to the high rates of comorbid obesity in psychiatric populations.2
Perhaps a better biomarker is the ratio of albumin in the CSF vs that in peripheral serum. The CSF-to-blood albumin ratio (Q-Alb) is widely considered an acceptable marker of BBB dysfunction because albumin must cross the BBB to alter the ratio. Studies have found a high Q-Alb in neurodegenerative disorders such as multiple sclerosis as well as in schizophrenia, which suggests that some level of BBB dysfunction is occurring. Although the Q-Alb may change slightly when confounded by antipsychotic use or with CSF flow changes,2,4 both S100B and Q-Alb elevation are sufficient for further investigation into tight junction alteration in schizophrenia.
Claudin-5 is a promising factor in detecting BBB permeability. Claudin-5 is deleted in DiGeorge syndrome, which is highly comorbid with schizophrenia and psychosis.1 Mouse knockdown studies show that full suppression of claudin-5 results in psychotic symptoms before fatal seizures,2,7 but a partial absence may enable psychotic symptoms. The same study showed that normally continuous claudin-5 was patchy along blood vessels in the affected sample.7 Follow-up experiments suggest that loss of claudin-5 in schizophrenia is especially prominent in the hippocampus, and there is mixed evidence of a decrease in the prefrontal cortex.8
Outside of claudin-5 alone, JAM-A plays a more regulatory role. It is upstream from an enhancer protein gene that serves as a transcription factor for the claudin-5 promoter, so when JAM-A is deleted, there is less claudin-5.9 However, while this decrease in claudin-5 may be pathological, there could still be various upstream changes that lead to schizophrenia.
What are the consequences of a porous BBB?
Although it is well established that the BBB passes small molecules and solutes, there is significant evidence of inflammatory trafficking in disease states. The BBB moves proinflammatory cytokines, alters transporters, and may even let white blood cells (WBCs) pass through. Immune cell infiltration has different requirements depending on the cell type. T cells rely on integrins, vascular cell adhesion molecule 1 (vCAM1), and intercellular adhesion molecule 1 (iCAM1) for binding, rolling, adhering, crossing, and migration to sites of inflammation.10,11 Both iCAM1 and vCAM1 are elevated in schizophrenia compared to other psychiatric disorders (such as unipolar depression) and correlate with other biomarkers. For example, vCAM1, responsible for recruitment and crossing, is correlated with a high Q-Alb.12 Primarily produced by astrocytes and endothelial cells, iCAM1 plays the largest role in crossing the BBB and migration. Postmortem tissue demonstrates that cytokines upregulate iCAM1 mRNA at the BBB in schizophrenia.13 Increased cytokines are well documented in the inflammatory model of schizophrenia. Interestingly, decreasing claudin-5 also upregulates iCAM1 production.14 Therefore, low baseline claudin-5 may contribute to additional inflammation and symptoms.
Continue to: BBB permeability also results...
BBB permeability also results in a certain pattern of leukocyte and cytokine activity. Interleukin-1 (IL-1), IL-6, and tumor necrosis factor–alpha can all cross the BBB during neuroimmune inflammation,10 but there are abnormal heightened and sustained responses of these molecules in schizophrenia. IL-6 is a proinflammatory cytokine in both acute and chronic inflammation that is expressed by astrocytes, endothelial cells, and microglia.15 IL-6 and its soluble receptor are both elevated in schizophrenia and are associated with white matter degeneration16,17 and an increase in vCAM1.15 This implies that while neuroinflammation in schizophrenia is occurring, additional leukocytes are being recruited and secreting their own cytokines in a chronic destructive positive feedback loop. Meanwhile, atypical IL-10 levels can no longer maintain balanced levels of inflammatory molecules,16 which leads to reduced control of inflammation.
Genetics and immunohistochemistry suggest that the BBB allows the passage of excess B cells and T cells in schizophrenia. Cytokines from WBCs or the BBB during inflammation recruit these additional infiltrating lymphocytes. In gene-wide association studies, there are several genes in schizophrenia important for B cells and T cells in addition to inflammation that interact in a proinflammatory network.16 These cells are also diffusely found in the white matter18 and hippocampal tissue19 of patients with schizophrenia. Taken together, an increase in adhesion molecules, WBCs, and cytokine crosstalk supports a leaky BBB as an important component of the inflammatory model of schizophrenia.
The role of microglia in BBB dysfunction
The effect of BBB permeability on microglial activation is an important caveat in the current research. Although several reports have linked neuroinflammation to confirmed microglial activation in schizophrenia, there is not enough evidence to claim that the BBB alone is the missing link between these theories. Some research suggests that chronic release of cytokines such as IL-6 from macrophages and T cells could increase migration across the BBB for microglial activation.16,20 However, positron emission tomography has shown mixed results at best. Translocator protein (TSPO) is expressed by microglia that are actively secreting cytokines.21 Researchers tracking TSPO changes in relation to BBB alteration have not seen elevated binding in schizophrenia, change due to stage of disease course, or differentiation from low-grade inflammation.21-24 Moreover, TSPO may be confounded by antipsychotic use25 and microglial expression did not correlate with any changes in adhesion molecules.13 TSPO is not an ideal indicator of microglial activation due to BBB breakdown, but that does not bar the possibility of at least a partial contribution to the development of schizophrenia.
Corsi-Zuelli et al26 created a model that attempts to merge BBB permeability and microglial activation through a different medium—T regulatory cells (TRegs). They write that if TRegs mediate interactions between astrocytes and microglia, their hypofunction would impose a prolonged T cell response. The increased access to a high level of IL-6 and its soluble receptors may keep the TRegs hypofunctional in schizophrenia and promote T cell conversion to inflammatory cell types. Experimentally, TReg induction reversed some psychotic symptoms, and greater TReg expression was associated with fewer negative symptoms.26 In an already insufficient BBB, more access to cytokines and leukocytes would sustain inflammation and microglial secretions.
In addition to the issues described regarding the BBB, the blood-CSF barrier at the choroid plexus may also be insufficient in schizophrenia (Box27-31).
Box
The choroid plexus’ primary role is to make CSF, but it also secretes cytokines and to some extent serves as a barrier. Unlike the blood-brain barrier (BBB), the blood-CSF barrier is composed of endothelial cells with fenestrations as well as tight junctions, which make the blood-CSF barrier overall more permeable.27,28 The most unusual finding regarding the choroid plexus in schizophrenia is size. The choroid plexus is physically larger in patients with schizophrenia, and to a lesser extent, in their first-degree relatives.29 A larger choroid plexus is correlated with more severe cognitive symptoms, increased risk for psychosis via biological stress, and significantly higher interleukin-6 (IL-6).27,29 The increased thickness could be an attempt to compensate for hyperactivity and toxic processes in a permeable environment. More circulating cytokines such as IL-6 and tumor necrosis factor–alpha from microglia can trigger an increase in intercellular adhesion molecule 1, resulting in leukocyte attachment and entry.30 Less claudin-5 at the choroid plexus in schizophrenia implicates similar permissive effects as seen at the BBB.31 Although the contribution of blood-CSF barrier dysfunction to schizophrenia requires further study, reduced barrier function outside the BBB is a viable line of inquiry.
Continue to: Caveats about this research
Caveats about this research
There are 3 important points to note about the current research concerning abnormal BBB permeability:
1. BBB dysfunction may exist only in a subset of people diagnosed with schizophrenia. In most human studies, only some patients with schizophrenia demonstrated alterations that suggested pathological BBB permeability. In addition, even when there is BBB dysfunction, it could be a secondary phenomenon, rather than a primary etiologic process.
2. Patient demographics across studies have not always been adequately described. Potential confounds such as obesity, smoking, or antipsychotic use were not consistently recorded or examined as a possible factor.
3. Currently available biomarkers are not perfect. Cytokine elevation, S100B, and Q-Alb are indirect measures of BBB disruption and are found in other disorders. Therefore, they only support the theory of BBB dysfunction in schizophrenia, rather than prove it. They are also not reliable markers for schizophrenia alone. Researchers have pointed out that these markers and proteins work in concert, which necessitates a network analysis approach.16 More research regarding the details of permeability is required to establish more reliable biomarkers and tailored treatment.
Treatment implications
One of the first treatment directions that comes to mind is managing the gaps in the BBB via tight junctions. Presently, there are no FDA-approved medications for altering tight junction proteins, but researchers are exploring potential agents that can induce claudin-5 and reduce inflammation.14 While we wait for such a medication, patients may benefit from existing anti-inflammatory treatments to control immune infiltration and its products. Various anti-inflammatory agents—including cyclooxygenase inhibitors,
Bottom Line
Recent research has revealed that the blood-brain barrier (BBB) is pathologically permeable in several disease states, including schizophrenia. Better characterization of the leaky BBB in schizophrenia has enormous potential in helping us understand how current theories fit together and could serve as a missing puzzle piece in treating schizophrenia.
Related Resources
- Levine A, Strawn JR. The brain’s Twitter system: neuronal extracellular vesicles. Current Psychiatry. 2022;21(6):9-11, 17-19,27. doi:10.12788/cp.0257
Drug Brand Names
Minocycline • Dynacin, Minocin
1. Li Y, Xia Y, Zhu H, et al. Investigation of neurodevelopmental deficits of 22 q11.2 deletion syndrome with a patient-iPSC-derived blood-brain barrier model. Cells. 2021;10(10):2576. doi:10.3390/cells10102576
2. Kealy J, Greene C, Campbell M. Blood-brain barrier regulation in psychiatric disorders. Neurosci Lett. 2020;726:133664. doi:10.1016/j.neulet.2018.06.033
3. Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1-13. doi:10.1016/j.nbd.2003.12.016
4. Futtrup J, Margolinsky R, Benros ME, et al. Blood-brain barrier pathology in patients with severe mental disorders: a systematic review and meta-analysis of biomarkers in case-control studies. Brain Behav Immun Health. 2020;6:100102. doi:10.1016/j.bbih.2020.100102
5. Chen S, Tian L, Chen N, et al. Cognitive dysfunction correlates with elevated serum S100B concentration in drug-free acutely relapsed patients with schizophrenia. Psychiatry Res. 2017;247:6-11. doi:10.1016/j.psychres.2016.09.029
6. Wu YF, Sytwu HK, Lung FW. Human aquaporin 4 gene polymorphisms and haplotypes are associated with serum S100B level and negative symptoms of schizophrenia in a southern Chinese Han population. Front Psychiatry. 2018;9:657. doi:10.3389/fpsyt.2018.00657
7. Greene C, Kealy J, Humphries MM, et al. Dose-dependent expression of claudin-5 is a modifying factor in schizophrenia. Mol Psychiatry. 2018;23(11):2156-2166. doi:10.1038/MP.2017.156
8. Greene C, Hanley N, Campbell M. Blood-brain barrier associated tight junction disruption is a hallmark feature of major psychiatric disorders. Transl Psychiatry. 2020;10(1):373. doi:10.1038/s41398-020-01054-3
9. Kakogiannos N, Ferrari L, Giampietro C, et al. JAM-A acts via C/EBP-α to promote claudin-5 expression and enhance endothelial barrier function. Circ Res. 2020:1056-1073. doi:10.1161/CIRCRESAHA.120.316742
10. Erickson MA, Dohi K, Banks WA. Neuroinflammation: a common pathway in CNS diseases as mediated at the blood-brain barrier. Neuroimmunomodulation. 2012;19(2):121-130. doi:10.1159/000330247
11. Ao LY, Yan YY, Zhou L, et al. Immune cells after ischemic stroke onset: roles, migration, and target intervention. J Mol Neurosci. 2018;66(3):342-355. doi:10.1007/s12031-018-1173-4
12. Meixensberger S, Kuzior H, Fiebich BL, et al. Upregulation of sICAM-1 and sVCAM-1 levels in the cerebrospinal fluid of patients with schizophrenia spectrum disorders. Diagnostics (Basel). 2021;11(7):1134. doi:10.3390diagnostics11071134
13. Cai HQ, Catts VS, Webster MJ, et al. Increased macrophages and changed brain endothelial cell gene expression in the frontal cortex of people with schizophrenia displaying inflammation. Mol Psychiatry. 2020;25(4):761-775. doi:10.1038/s41380-018-0235-x
14. Greene C, Hanley N, Reschke CR, et al. Microvascular stabilization via blood-brain barrier regulation prevents seizure activity. Nat Commun. 2022;13(1):2003. doi:10.1038/s41467-022-29657-y
15. García-Juárez M, Camacho-Morales A. Defining the role of anti- and pro-inflammatory outcomes of interleukin-6 in mental health. Neuroscience. 2022;492:32-46. doi:10.1016/j.neuroscience.2022.03.020
16. Pong S, Karmacharya R, Sofman M, et al. The role of brain microvascular endothelial cell and blood-brain barrier dysfunction in schizophrenia. Complex Psychiatry. 2020;6(1-2):30-46. doi:10.1159/000511552
17. Patel A, Zhu Y, Kuzhikandathil EV, et al. Soluble interleukin-6 receptor induces motor stereotypies and co-localizes with gp130 in regions linked to cortico-striato-thalamo-cortical circuits. PLoS One. 2012;7(7): e41623. doi:10.1371/journal.pone.0041623
18. Schlaaff K, Dobrowolny H, Frodl T, et al. Increased densities of T and B lymphocytes indicate neuroinflammation in subgroups of schizophrenia and mood disorder patients. Brain Behav Immun. 2020;88:497-506. doi:10.1016/j.bbi.2020.04.021
19. Busse S, Busse M, Schiltz K, et al. Different distribution patterns of lymphocytes and microglia in the hippocampus of patients with residual versus paranoid schizophrenia: further evidence for disease course-related immune alterations? Brain Behav Immun. 2012;26(8):1273-1279. doi:10.1016/j.bbi.2012.08.005
20. Na KS, Jung HY, Kim YK. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:277-286. doi:10.1016/j.pnpbp.2012.10.022
21. Conen S, Gregory CJ, Hinz R, et al. Neuroinflammation as measured by positron emission tomography in patients with recent onset and established schizophrenia: implications for immune pathogenesis. Mol Psychiatry. 2021;26(9):5398-5406. doi:10.1038/S41380-020-0829-Y
22. Najjar S, Pahlajani S, De Sanctis V, et al. Neurovascular unit dysfunction and blood-brain barrier hyperpermeability contribute to schizophrenia neurobiology: a theoretical integration of clinical and experimental evidence. Front Psychiatry. 2017;8:83. doi:10.3389/fpsyt.2017.00083
23. Pinjari OF, Dasgupta SK, Okusaga OO. Plasma soluble P-selectin, interleukin-6 and S100B protein in patients with schizophrenia: a pilot study. Psychiatr Q. 2022;93(1):335-345. doi:10.1007/s11126-021-09954-3
24. Di Biase MA, Zalesky A, O’keefe G, et al. PET imaging of putative microglial activation in individuals at ultra-high risk for psychosis, recently diagnosed and chronically ill with schizophrenia. Transl Psychiatry. 2017;7(8):e1225. doi:10.1038/tp.2017.193
25. Holmes SE, Hinz R, Drake RJ, et al. In vivo imaging of brain microglial activity in antipsychotic-free and medicated schizophrenia: a [11C](R)-PK11195 positron emission tomography study. Mol Psychiatry. 2016;21(12):1672-1679. doi:10.1038/mp.2016.180
26. Corsi-Zuelli F, Deakin B, de Lima MHF, et al. T regulatory cells as a potential therapeutic target in psychosis? Current challenges and future perspectives. Brain Behav Immun Health. 2021;17:100330. doi:10.1016/j.bbih.2021.100330
27. Bannai D, Lutz O, Lizano P. Neuroimaging considerations when investigating choroid plexus morphology in idiopathic psychosis. Schizophr Res. 2020;224:19-21. doi:10.1016/j.schres.2020.07.013
28. Hladky SB, Barrand MA. Fluid and ion transfer across the blood-brain and blood-cerebrospinal fluid barriers; a comparative account of mechanisms and roles. Fluids Barriers CNS. 2016;13(1):19. doi:10.1186/s12987-016-0040-3
29. Lizano P, Lutz O, Ling G, et al. Association of choroid plexus enlargement with cognitive, inflammatory, and structural phenotypes across the psychosis spectrum. Am J Psychiatry. 2019;176(7):564-572. doi:10.1176/appi.ajp.2019.18070825
30. Castellani G, Contarini G, Mereu M, et al. Dopamine-mediated immunomodulation affects choroid plexus function. Brain Behav Immun. 2019;81:138-150. doi:10.1016/j.bbi.2019.06.006
31. Bitanihirwe BKY, Lizano P, Woo TW. Deconstructing the functional neuroanatomy of the choroid plexus: an ontogenetic perspective for studying neurodevelopmental and neuropsychiatric disorders. Mol Psychiatry. 2022;1-10. doi:10.1038/s41380-022-01623-6
1. Li Y, Xia Y, Zhu H, et al. Investigation of neurodevelopmental deficits of 22 q11.2 deletion syndrome with a patient-iPSC-derived blood-brain barrier model. Cells. 2021;10(10):2576. doi:10.3390/cells10102576
2. Kealy J, Greene C, Campbell M. Blood-brain barrier regulation in psychiatric disorders. Neurosci Lett. 2020;726:133664. doi:10.1016/j.neulet.2018.06.033
3. Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1-13. doi:10.1016/j.nbd.2003.12.016
4. Futtrup J, Margolinsky R, Benros ME, et al. Blood-brain barrier pathology in patients with severe mental disorders: a systematic review and meta-analysis of biomarkers in case-control studies. Brain Behav Immun Health. 2020;6:100102. doi:10.1016/j.bbih.2020.100102
5. Chen S, Tian L, Chen N, et al. Cognitive dysfunction correlates with elevated serum S100B concentration in drug-free acutely relapsed patients with schizophrenia. Psychiatry Res. 2017;247:6-11. doi:10.1016/j.psychres.2016.09.029
6. Wu YF, Sytwu HK, Lung FW. Human aquaporin 4 gene polymorphisms and haplotypes are associated with serum S100B level and negative symptoms of schizophrenia in a southern Chinese Han population. Front Psychiatry. 2018;9:657. doi:10.3389/fpsyt.2018.00657
7. Greene C, Kealy J, Humphries MM, et al. Dose-dependent expression of claudin-5 is a modifying factor in schizophrenia. Mol Psychiatry. 2018;23(11):2156-2166. doi:10.1038/MP.2017.156
8. Greene C, Hanley N, Campbell M. Blood-brain barrier associated tight junction disruption is a hallmark feature of major psychiatric disorders. Transl Psychiatry. 2020;10(1):373. doi:10.1038/s41398-020-01054-3
9. Kakogiannos N, Ferrari L, Giampietro C, et al. JAM-A acts via C/EBP-α to promote claudin-5 expression and enhance endothelial barrier function. Circ Res. 2020:1056-1073. doi:10.1161/CIRCRESAHA.120.316742
10. Erickson MA, Dohi K, Banks WA. Neuroinflammation: a common pathway in CNS diseases as mediated at the blood-brain barrier. Neuroimmunomodulation. 2012;19(2):121-130. doi:10.1159/000330247
11. Ao LY, Yan YY, Zhou L, et al. Immune cells after ischemic stroke onset: roles, migration, and target intervention. J Mol Neurosci. 2018;66(3):342-355. doi:10.1007/s12031-018-1173-4
12. Meixensberger S, Kuzior H, Fiebich BL, et al. Upregulation of sICAM-1 and sVCAM-1 levels in the cerebrospinal fluid of patients with schizophrenia spectrum disorders. Diagnostics (Basel). 2021;11(7):1134. doi:10.3390diagnostics11071134
13. Cai HQ, Catts VS, Webster MJ, et al. Increased macrophages and changed brain endothelial cell gene expression in the frontal cortex of people with schizophrenia displaying inflammation. Mol Psychiatry. 2020;25(4):761-775. doi:10.1038/s41380-018-0235-x
14. Greene C, Hanley N, Reschke CR, et al. Microvascular stabilization via blood-brain barrier regulation prevents seizure activity. Nat Commun. 2022;13(1):2003. doi:10.1038/s41467-022-29657-y
15. García-Juárez M, Camacho-Morales A. Defining the role of anti- and pro-inflammatory outcomes of interleukin-6 in mental health. Neuroscience. 2022;492:32-46. doi:10.1016/j.neuroscience.2022.03.020
16. Pong S, Karmacharya R, Sofman M, et al. The role of brain microvascular endothelial cell and blood-brain barrier dysfunction in schizophrenia. Complex Psychiatry. 2020;6(1-2):30-46. doi:10.1159/000511552
17. Patel A, Zhu Y, Kuzhikandathil EV, et al. Soluble interleukin-6 receptor induces motor stereotypies and co-localizes with gp130 in regions linked to cortico-striato-thalamo-cortical circuits. PLoS One. 2012;7(7): e41623. doi:10.1371/journal.pone.0041623
18. Schlaaff K, Dobrowolny H, Frodl T, et al. Increased densities of T and B lymphocytes indicate neuroinflammation in subgroups of schizophrenia and mood disorder patients. Brain Behav Immun. 2020;88:497-506. doi:10.1016/j.bbi.2020.04.021
19. Busse S, Busse M, Schiltz K, et al. Different distribution patterns of lymphocytes and microglia in the hippocampus of patients with residual versus paranoid schizophrenia: further evidence for disease course-related immune alterations? Brain Behav Immun. 2012;26(8):1273-1279. doi:10.1016/j.bbi.2012.08.005
20. Na KS, Jung HY, Kim YK. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:277-286. doi:10.1016/j.pnpbp.2012.10.022
21. Conen S, Gregory CJ, Hinz R, et al. Neuroinflammation as measured by positron emission tomography in patients with recent onset and established schizophrenia: implications for immune pathogenesis. Mol Psychiatry. 2021;26(9):5398-5406. doi:10.1038/S41380-020-0829-Y
22. Najjar S, Pahlajani S, De Sanctis V, et al. Neurovascular unit dysfunction and blood-brain barrier hyperpermeability contribute to schizophrenia neurobiology: a theoretical integration of clinical and experimental evidence. Front Psychiatry. 2017;8:83. doi:10.3389/fpsyt.2017.00083
23. Pinjari OF, Dasgupta SK, Okusaga OO. Plasma soluble P-selectin, interleukin-6 and S100B protein in patients with schizophrenia: a pilot study. Psychiatr Q. 2022;93(1):335-345. doi:10.1007/s11126-021-09954-3
24. Di Biase MA, Zalesky A, O’keefe G, et al. PET imaging of putative microglial activation in individuals at ultra-high risk for psychosis, recently diagnosed and chronically ill with schizophrenia. Transl Psychiatry. 2017;7(8):e1225. doi:10.1038/tp.2017.193
25. Holmes SE, Hinz R, Drake RJ, et al. In vivo imaging of brain microglial activity in antipsychotic-free and medicated schizophrenia: a [11C](R)-PK11195 positron emission tomography study. Mol Psychiatry. 2016;21(12):1672-1679. doi:10.1038/mp.2016.180
26. Corsi-Zuelli F, Deakin B, de Lima MHF, et al. T regulatory cells as a potential therapeutic target in psychosis? Current challenges and future perspectives. Brain Behav Immun Health. 2021;17:100330. doi:10.1016/j.bbih.2021.100330
27. Bannai D, Lutz O, Lizano P. Neuroimaging considerations when investigating choroid plexus morphology in idiopathic psychosis. Schizophr Res. 2020;224:19-21. doi:10.1016/j.schres.2020.07.013
28. Hladky SB, Barrand MA. Fluid and ion transfer across the blood-brain and blood-cerebrospinal fluid barriers; a comparative account of mechanisms and roles. Fluids Barriers CNS. 2016;13(1):19. doi:10.1186/s12987-016-0040-3
29. Lizano P, Lutz O, Ling G, et al. Association of choroid plexus enlargement with cognitive, inflammatory, and structural phenotypes across the psychosis spectrum. Am J Psychiatry. 2019;176(7):564-572. doi:10.1176/appi.ajp.2019.18070825
30. Castellani G, Contarini G, Mereu M, et al. Dopamine-mediated immunomodulation affects choroid plexus function. Brain Behav Immun. 2019;81:138-150. doi:10.1016/j.bbi.2019.06.006
31. Bitanihirwe BKY, Lizano P, Woo TW. Deconstructing the functional neuroanatomy of the choroid plexus: an ontogenetic perspective for studying neurodevelopmental and neuropsychiatric disorders. Mol Psychiatry. 2022;1-10. doi:10.1038/s41380-022-01623-6
