User login
How does gender-affirming hormone therapy affect QOL in transgender patients?
Evidence summary
GAHT may improve depression and quality of life, but not anxiety
A well-done systematic review of transgender men and transgender women demonstrated that GAHT of more than a year’s duration was associated with modestly improved standardized scores for QOL, depression, and possibly anxiety.1 It was also associated with improved scores for depression in transgender adolescents.
The authors identified 15 prospective cohort studies (n = 626 transgender adults [mean age, 25-34 years]; 198 transgender adolescent girls and boys [mean age, 15-16 years]), 2 retrospective cohort studies (n = 1756 adults; mean age, 25-32 years), and 4 cross-sectional studies (n = 336 adults; mean age, 30-37 years).
Researchers recruited participants using strict eligibility criteria (psychiatric evaluation and formal diagnosis of gender dysphoria), with no prior history of GAHT, largely from gender-affirming specialty clinics at university hospitals. Most studies were conducted after the year 2000, predominantly in Europe (8 studies in Italy; 2 each in Belgium, the Netherlands, the United States, and Spain).
GAHT comprised testosterone for transgender men (14 studies used injectable testosterone cypionate, enanthate, undecanoate, or transdermal gels), estrogens (usually with an anti-androgen such as cyproterone acetate or spironolactone) for transgender women (10 studies used transdermal, oral, or injectable estradiol valerate or conjugated estrogens), and gonadotropin-releasing hormone (GnRH) therapy for transgender adolescents (3 studies).
Researchers evaluated the outcomes of QOL, depression, and anxiety with standardized scores on validated screening tools and suicide (2 studies) by medical records. GAHT in adult transgender men and transgender women was associated with modest improvements in QOL (3 of 5 studies) and depression (8 of 12 studies), and some improvement in anxiety scores (2 of 8 studies; see TABLE1). There was insufficient evidence to determine whether GAHT had any effect on suicide. In adolescent transgender girls and boys, GAHT was associated with modest improvements in depression but not QOL or anxiety scores.
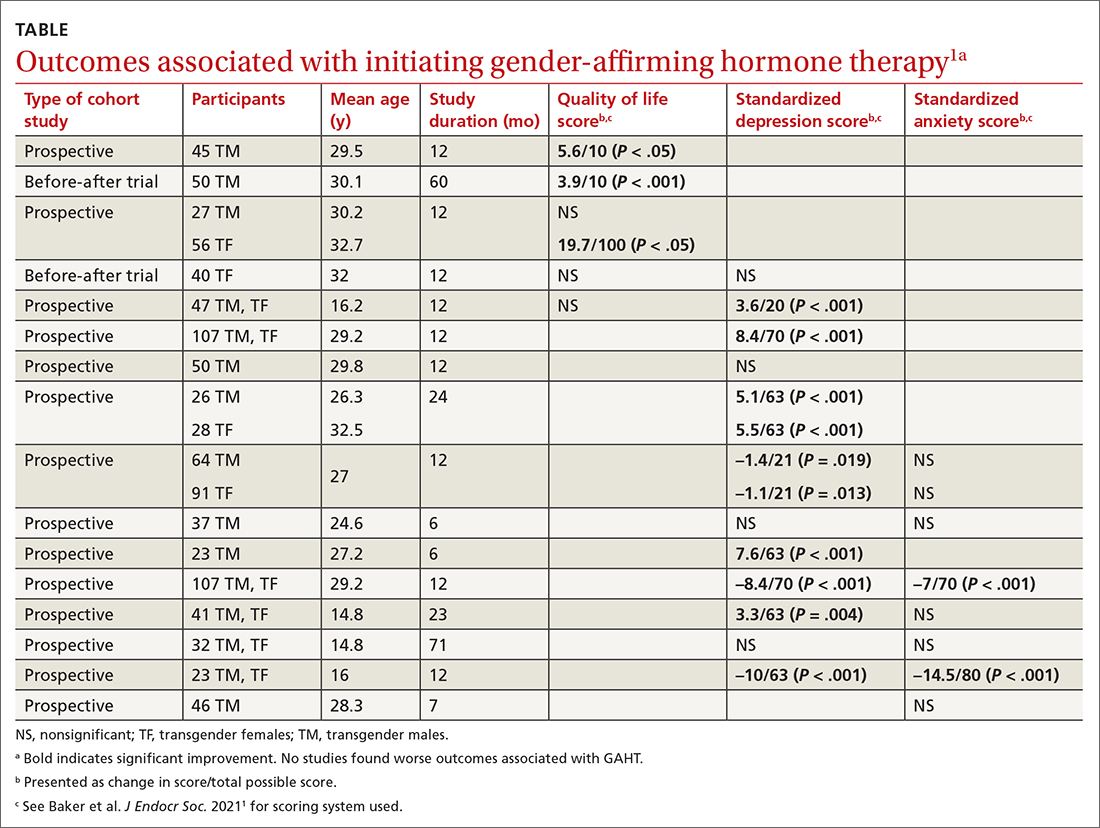
The authors rated the strength of evidence from the included studies as low, based on study quality (small study sizes, uncontrolled confounding factors, and risk of bias in study designs).
Additional research supports GAHT’s association with improved outcomes
Three studies, published after the systematic review, evaluated outcomes before and after GAHT and found similar results. All studies recruited treatment-seeking participants from specialty clinics.
Continue to: An Australian propsective longitudinal..
An Australian prospective longitudinal controlled study (n = 77 transgender adults; 103 cisgender controls) evaluated GAHT outcomes after 6 months and found a significant reduction in gender dysphoria scores in both transgender males (adjusted mean difference [aMD] = –6.8; 95% CI, –8.7 to –4.9; P < .001) and transgender females (aMD = –4.2; 95% CI, –6.2 to –2.2; P < .001) vs controls. QOL scores (emotional well-being, social functioning) improved only for transgender males (well-being: aMD = +7.5; 95% CI, 1.3 to 13.6; P < .018; social functioning: aMD = +12.5; 95% CI, 2.8 to 22.2; P = .011).2
A US prospective cohort study (n = 104 adolescents; mean age, 16 years) examined the effect of GnRH and/or GAHT over a 12-month period and found significant decreases in standardized scores for depression (adjusted odds ratio [aOR] = 0.4; 95% CI, 0.17-0.95) and suicidality (aOR = 0.27; 95% CI, 0.11-0.65) but not for anxiety. Participants who did not receive hormonal interventions had increased scores for depression and suicidality at 3 and 6 months’ follow-up.3
A prospective cohort study from the UK (n = 178 transgender adults) examined outcomes before and after GAHT treatment over 18 months and found significant decreases in standardized scores for depression (transgender males: –2.1; 95% CI, –3.2 to –1.2; P < .001; transgender females: –1.9; 95% CI, –2.8 to –1.0; P < .001) but not for anxiety.4
A large US study shows GAHT may reduce depression scores
A recent large cross-sectional study from the United States (n = 11,914 transgender or nonbinary youth, ages 13-24 years) found that receiving GAHT was associated with significantly lower odds of recent depression (aOR = 0.73; P < .001) and suicidality (aOR = 0.74; P < .001) compared to those who wanted GAHT but did not receive it. The authors were unable to differentiate the effects of receiving GAHT from the effects of parental support for their child’s gender identity, which may be a confounding factor.5
Recommendations from others
The World Professional Association for Transgender Health Standards of Care state that “gender incongruence that causes clinically significant distress and impairment often requires medically necessary clinical interventions” and recommends “health care professionals initiate and continue gender-affirming hormone therapy … due to demonstrated improvement in psychosocial functioning and quality of life.”6 The Endocrine Society Position Statement on Transgender Health states that “medical intervention for transgender youth and adults (including … hormone therapy) is effective, relatively safe (when appropriately monitored), and has been established as the standard of care.”7 The American Academy of Family Physicians “supports gender-affirming care as an evidence-informed intervention that can promote health equity for gender-diverse individuals.”8
Editor’s takeaway
Family physicians commonly address many factors that can impact the QOL for our patients with gender dysphoria: lack of fixed residence, underemployment, food insecurity, and trauma. GAHT, especially in male-to-female transgender patients, may further improve QOL without evidence of harm.
1. Baker KE, Wilson LM, Sharma R, et al. Hormone therapy, mental health, and quality of life among transgender people: a systematic review. J Endocr Soc. 2021;5:bvab011. doi: 10.1210/jendso/bvab011
2. Foster Skewis L, Bretherton I, Leemaqz, SY, et al. Short-term effects of gender-affirming hormone therapy on dysphoria and quality of life in transgender individuals: a prospective controlled study. Front Endocrinol (Lausanne). 2021;12:717766.
3. Tordoff DM, Wanta JW, Collin A, et al. Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Netw Open. 2022;5:e220978. doi: 10.1001/jamanetworkopen.2022.0978
4. Aldridge Z, Patel S, Guo B, et al. Long-term effect of gender-affirming hormone treatment on depression and anxiety symptoms in transgender people: a prospective cohort study. Andrology. 2021;9:1808-1816. doi: 10.1111/andr.12884
5. Green AE, DeChants JP, Price MN, et al. Association of gender-affirming hormone therapy with depression, thoughts of suicide, and attempted suicide among transgender and nonbinary youth. J Adolesc Health. 2022;70:643-649. doi: 10.1016/j.jadohealth.2021.10.036
6. World Professional Association for Transgender Health. Standards of Care for the Health of Transsexual, Transgender, and Gender Nonconforming People. 8th version. Published 2022. Accessed November 17, 2022. www.wpath.org/publications/soc
7. Endocrine Society. Transgender health: an Endocrine Society position statement. Updated December 16, 2020. Accessed November 17, 2022. www.endocrine.org/advocacy/position-statements/transgender-health
8. American Academy of Family Physicians. Care for the transgender and gender nonbinary patient. Updated September 2022. Accessed November 17, 2022. www.aafp.org/about/policies/all/transgender-nonbinary.html
Evidence summary
GAHT may improve depression and quality of life, but not anxiety
A well-done systematic review of transgender men and transgender women demonstrated that GAHT of more than a year’s duration was associated with modestly improved standardized scores for QOL, depression, and possibly anxiety.1 It was also associated with improved scores for depression in transgender adolescents.
The authors identified 15 prospective cohort studies (n = 626 transgender adults [mean age, 25-34 years]; 198 transgender adolescent girls and boys [mean age, 15-16 years]), 2 retrospective cohort studies (n = 1756 adults; mean age, 25-32 years), and 4 cross-sectional studies (n = 336 adults; mean age, 30-37 years).
Researchers recruited participants using strict eligibility criteria (psychiatric evaluation and formal diagnosis of gender dysphoria), with no prior history of GAHT, largely from gender-affirming specialty clinics at university hospitals. Most studies were conducted after the year 2000, predominantly in Europe (8 studies in Italy; 2 each in Belgium, the Netherlands, the United States, and Spain).
GAHT comprised testosterone for transgender men (14 studies used injectable testosterone cypionate, enanthate, undecanoate, or transdermal gels), estrogens (usually with an anti-androgen such as cyproterone acetate or spironolactone) for transgender women (10 studies used transdermal, oral, or injectable estradiol valerate or conjugated estrogens), and gonadotropin-releasing hormone (GnRH) therapy for transgender adolescents (3 studies).
Researchers evaluated the outcomes of QOL, depression, and anxiety with standardized scores on validated screening tools and suicide (2 studies) by medical records. GAHT in adult transgender men and transgender women was associated with modest improvements in QOL (3 of 5 studies) and depression (8 of 12 studies), and some improvement in anxiety scores (2 of 8 studies; see TABLE1). There was insufficient evidence to determine whether GAHT had any effect on suicide. In adolescent transgender girls and boys, GAHT was associated with modest improvements in depression but not QOL or anxiety scores.

The authors rated the strength of evidence from the included studies as low, based on study quality (small study sizes, uncontrolled confounding factors, and risk of bias in study designs).
Additional research supports GAHT’s association with improved outcomes
Three studies, published after the systematic review, evaluated outcomes before and after GAHT and found similar results. All studies recruited treatment-seeking participants from specialty clinics.
Continue to: An Australian propsective longitudinal..
An Australian prospective longitudinal controlled study (n = 77 transgender adults; 103 cisgender controls) evaluated GAHT outcomes after 6 months and found a significant reduction in gender dysphoria scores in both transgender males (adjusted mean difference [aMD] = –6.8; 95% CI, –8.7 to –4.9; P < .001) and transgender females (aMD = –4.2; 95% CI, –6.2 to –2.2; P < .001) vs controls. QOL scores (emotional well-being, social functioning) improved only for transgender males (well-being: aMD = +7.5; 95% CI, 1.3 to 13.6; P < .018; social functioning: aMD = +12.5; 95% CI, 2.8 to 22.2; P = .011).2
A US prospective cohort study (n = 104 adolescents; mean age, 16 years) examined the effect of GnRH and/or GAHT over a 12-month period and found significant decreases in standardized scores for depression (adjusted odds ratio [aOR] = 0.4; 95% CI, 0.17-0.95) and suicidality (aOR = 0.27; 95% CI, 0.11-0.65) but not for anxiety. Participants who did not receive hormonal interventions had increased scores for depression and suicidality at 3 and 6 months’ follow-up.3
A prospective cohort study from the UK (n = 178 transgender adults) examined outcomes before and after GAHT treatment over 18 months and found significant decreases in standardized scores for depression (transgender males: –2.1; 95% CI, –3.2 to –1.2; P < .001; transgender females: –1.9; 95% CI, –2.8 to –1.0; P < .001) but not for anxiety.4
A large US study shows GAHT may reduce depression scores
A recent large cross-sectional study from the United States (n = 11,914 transgender or nonbinary youth, ages 13-24 years) found that receiving GAHT was associated with significantly lower odds of recent depression (aOR = 0.73; P < .001) and suicidality (aOR = 0.74; P < .001) compared to those who wanted GAHT but did not receive it. The authors were unable to differentiate the effects of receiving GAHT from the effects of parental support for their child’s gender identity, which may be a confounding factor.5
Recommendations from others
The World Professional Association for Transgender Health Standards of Care state that “gender incongruence that causes clinically significant distress and impairment often requires medically necessary clinical interventions” and recommends “health care professionals initiate and continue gender-affirming hormone therapy … due to demonstrated improvement in psychosocial functioning and quality of life.”6 The Endocrine Society Position Statement on Transgender Health states that “medical intervention for transgender youth and adults (including … hormone therapy) is effective, relatively safe (when appropriately monitored), and has been established as the standard of care.”7 The American Academy of Family Physicians “supports gender-affirming care as an evidence-informed intervention that can promote health equity for gender-diverse individuals.”8
Editor’s takeaway
Family physicians commonly address many factors that can impact the QOL for our patients with gender dysphoria: lack of fixed residence, underemployment, food insecurity, and trauma. GAHT, especially in male-to-female transgender patients, may further improve QOL without evidence of harm.
Evidence summary
GAHT may improve depression and quality of life, but not anxiety
A well-done systematic review of transgender men and transgender women demonstrated that GAHT of more than a year’s duration was associated with modestly improved standardized scores for QOL, depression, and possibly anxiety.1 It was also associated with improved scores for depression in transgender adolescents.
The authors identified 15 prospective cohort studies (n = 626 transgender adults [mean age, 25-34 years]; 198 transgender adolescent girls and boys [mean age, 15-16 years]), 2 retrospective cohort studies (n = 1756 adults; mean age, 25-32 years), and 4 cross-sectional studies (n = 336 adults; mean age, 30-37 years).
Researchers recruited participants using strict eligibility criteria (psychiatric evaluation and formal diagnosis of gender dysphoria), with no prior history of GAHT, largely from gender-affirming specialty clinics at university hospitals. Most studies were conducted after the year 2000, predominantly in Europe (8 studies in Italy; 2 each in Belgium, the Netherlands, the United States, and Spain).
GAHT comprised testosterone for transgender men (14 studies used injectable testosterone cypionate, enanthate, undecanoate, or transdermal gels), estrogens (usually with an anti-androgen such as cyproterone acetate or spironolactone) for transgender women (10 studies used transdermal, oral, or injectable estradiol valerate or conjugated estrogens), and gonadotropin-releasing hormone (GnRH) therapy for transgender adolescents (3 studies).
Researchers evaluated the outcomes of QOL, depression, and anxiety with standardized scores on validated screening tools and suicide (2 studies) by medical records. GAHT in adult transgender men and transgender women was associated with modest improvements in QOL (3 of 5 studies) and depression (8 of 12 studies), and some improvement in anxiety scores (2 of 8 studies; see TABLE1). There was insufficient evidence to determine whether GAHT had any effect on suicide. In adolescent transgender girls and boys, GAHT was associated with modest improvements in depression but not QOL or anxiety scores.

The authors rated the strength of evidence from the included studies as low, based on study quality (small study sizes, uncontrolled confounding factors, and risk of bias in study designs).
Additional research supports GAHT’s association with improved outcomes
Three studies, published after the systematic review, evaluated outcomes before and after GAHT and found similar results. All studies recruited treatment-seeking participants from specialty clinics.
Continue to: An Australian propsective longitudinal..
An Australian prospective longitudinal controlled study (n = 77 transgender adults; 103 cisgender controls) evaluated GAHT outcomes after 6 months and found a significant reduction in gender dysphoria scores in both transgender males (adjusted mean difference [aMD] = –6.8; 95% CI, –8.7 to –4.9; P < .001) and transgender females (aMD = –4.2; 95% CI, –6.2 to –2.2; P < .001) vs controls. QOL scores (emotional well-being, social functioning) improved only for transgender males (well-being: aMD = +7.5; 95% CI, 1.3 to 13.6; P < .018; social functioning: aMD = +12.5; 95% CI, 2.8 to 22.2; P = .011).2
A US prospective cohort study (n = 104 adolescents; mean age, 16 years) examined the effect of GnRH and/or GAHT over a 12-month period and found significant decreases in standardized scores for depression (adjusted odds ratio [aOR] = 0.4; 95% CI, 0.17-0.95) and suicidality (aOR = 0.27; 95% CI, 0.11-0.65) but not for anxiety. Participants who did not receive hormonal interventions had increased scores for depression and suicidality at 3 and 6 months’ follow-up.3
A prospective cohort study from the UK (n = 178 transgender adults) examined outcomes before and after GAHT treatment over 18 months and found significant decreases in standardized scores for depression (transgender males: –2.1; 95% CI, –3.2 to –1.2; P < .001; transgender females: –1.9; 95% CI, –2.8 to –1.0; P < .001) but not for anxiety.4
A large US study shows GAHT may reduce depression scores
A recent large cross-sectional study from the United States (n = 11,914 transgender or nonbinary youth, ages 13-24 years) found that receiving GAHT was associated with significantly lower odds of recent depression (aOR = 0.73; P < .001) and suicidality (aOR = 0.74; P < .001) compared to those who wanted GAHT but did not receive it. The authors were unable to differentiate the effects of receiving GAHT from the effects of parental support for their child’s gender identity, which may be a confounding factor.5
Recommendations from others
The World Professional Association for Transgender Health Standards of Care state that “gender incongruence that causes clinically significant distress and impairment often requires medically necessary clinical interventions” and recommends “health care professionals initiate and continue gender-affirming hormone therapy … due to demonstrated improvement in psychosocial functioning and quality of life.”6 The Endocrine Society Position Statement on Transgender Health states that “medical intervention for transgender youth and adults (including … hormone therapy) is effective, relatively safe (when appropriately monitored), and has been established as the standard of care.”7 The American Academy of Family Physicians “supports gender-affirming care as an evidence-informed intervention that can promote health equity for gender-diverse individuals.”8
Editor’s takeaway
Family physicians commonly address many factors that can impact the QOL for our patients with gender dysphoria: lack of fixed residence, underemployment, food insecurity, and trauma. GAHT, especially in male-to-female transgender patients, may further improve QOL without evidence of harm.
1. Baker KE, Wilson LM, Sharma R, et al. Hormone therapy, mental health, and quality of life among transgender people: a systematic review. J Endocr Soc. 2021;5:bvab011. doi: 10.1210/jendso/bvab011
2. Foster Skewis L, Bretherton I, Leemaqz, SY, et al. Short-term effects of gender-affirming hormone therapy on dysphoria and quality of life in transgender individuals: a prospective controlled study. Front Endocrinol (Lausanne). 2021;12:717766.
3. Tordoff DM, Wanta JW, Collin A, et al. Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Netw Open. 2022;5:e220978. doi: 10.1001/jamanetworkopen.2022.0978
4. Aldridge Z, Patel S, Guo B, et al. Long-term effect of gender-affirming hormone treatment on depression and anxiety symptoms in transgender people: a prospective cohort study. Andrology. 2021;9:1808-1816. doi: 10.1111/andr.12884
5. Green AE, DeChants JP, Price MN, et al. Association of gender-affirming hormone therapy with depression, thoughts of suicide, and attempted suicide among transgender and nonbinary youth. J Adolesc Health. 2022;70:643-649. doi: 10.1016/j.jadohealth.2021.10.036
6. World Professional Association for Transgender Health. Standards of Care for the Health of Transsexual, Transgender, and Gender Nonconforming People. 8th version. Published 2022. Accessed November 17, 2022. www.wpath.org/publications/soc
7. Endocrine Society. Transgender health: an Endocrine Society position statement. Updated December 16, 2020. Accessed November 17, 2022. www.endocrine.org/advocacy/position-statements/transgender-health
8. American Academy of Family Physicians. Care for the transgender and gender nonbinary patient. Updated September 2022. Accessed November 17, 2022. www.aafp.org/about/policies/all/transgender-nonbinary.html
1. Baker KE, Wilson LM, Sharma R, et al. Hormone therapy, mental health, and quality of life among transgender people: a systematic review. J Endocr Soc. 2021;5:bvab011. doi: 10.1210/jendso/bvab011
2. Foster Skewis L, Bretherton I, Leemaqz, SY, et al. Short-term effects of gender-affirming hormone therapy on dysphoria and quality of life in transgender individuals: a prospective controlled study. Front Endocrinol (Lausanne). 2021;12:717766.
3. Tordoff DM, Wanta JW, Collin A, et al. Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Netw Open. 2022;5:e220978. doi: 10.1001/jamanetworkopen.2022.0978
4. Aldridge Z, Patel S, Guo B, et al. Long-term effect of gender-affirming hormone treatment on depression and anxiety symptoms in transgender people: a prospective cohort study. Andrology. 2021;9:1808-1816. doi: 10.1111/andr.12884
5. Green AE, DeChants JP, Price MN, et al. Association of gender-affirming hormone therapy with depression, thoughts of suicide, and attempted suicide among transgender and nonbinary youth. J Adolesc Health. 2022;70:643-649. doi: 10.1016/j.jadohealth.2021.10.036
6. World Professional Association for Transgender Health. Standards of Care for the Health of Transsexual, Transgender, and Gender Nonconforming People. 8th version. Published 2022. Accessed November 17, 2022. www.wpath.org/publications/soc
7. Endocrine Society. Transgender health: an Endocrine Society position statement. Updated December 16, 2020. Accessed November 17, 2022. www.endocrine.org/advocacy/position-statements/transgender-health
8. American Academy of Family Physicians. Care for the transgender and gender nonbinary patient. Updated September 2022. Accessed November 17, 2022. www.aafp.org/about/policies/all/transgender-nonbinary.html
EVIDENCE-BASED ANSWER:
There are modest effects on depression but not anxiety. Gender-affirming hormone therapy (GAHT) is associated with modest improvements in standardized scores for quality of life (QOL) and depression in adult male-to-female and female-to-male transgender people and modest improvements in depression scores in transgender adolescents, but the effect on anxiety is uncertain (strength of recommendation [SOR]: B, based on a preponderance of low-quality prospective cohort studies with inconsistent results).
GAHT is associated with reduced gender dysphoria and decreased suicidality (SOR: B, based on a prospective cohort study). However, there is insufficient evidence to determine any effect on suicide completion. No studies associated GAHT with worsened QOL, depression, or anxiety scores.
Is event-driven PrEP dosing for HIV as effective as daily dosing?
EVIDENCE SUMMARY
Event-driven PrEP is effective for prevention of HIV transmission
An RCT evaluating the effectiveness of event-driven PrEP in 400 patients at high risk for HIV found that it reduced HIV incidence by 86% compared to placebo. Researchers recruited HIV-negative men or transgender women who had sex with men, who’d had condomless anal sex with at least 2 partners in the previous 6 months, and followed them for a median of 9.3 months for HIV acquisition.1
Patients randomized to event-driven PrEP took tenofovir-emtricitabine (300-200 mg) on the following schedule: 2 pills 2 to 24 hours before intercourse (or 1 pill if they had taken it within the past week), followed by a third pill 24 hours later, and a fourth pill 24 hours after that. When patients had multiple consecutive episodes of intercourse, daily use was continued until 2 days after the last episode. Patients in the control group took placebo pills.1
Event-driven PrEP reduced HIV incidence vs placebo (2 infections vs 14 infections; 0.91 vs 6.6 per 100 person-years; relative risk [RR] = 0.86; P = .002). PrEP produced more gastrointestinal (14% vs 5%; P = .002) and renal (18% vs 10%; P = .03) adverse effects than placebo. Participants took a median of 15 pills per month.1
A post-hoc analysis of the above study, evaluating 270 patients, found that event-driven PrEP reduced HIV incidence by 100% during periods of less frequent sexual encounters. Selected participants had a median of 5 sexual encounters per month (range, 2-10), used a median of 9.5 pills per month (range, 6-13), and represented 134 person-years of follow-up. No HIV infections (0 per 100 person-years; 95% CI, 0-5; P = .013) were diagnosed in the PrEP group and 6 HIV infections (9.2 per 100 person-years; 95% CI, 3.4-20.1) were diagnosed in the placebo group, with a relative reduction of HIV incidence of 100% (95% CI, 39-100).2
For comparison, 2 large open-label trials evaluating daily PrEP found that it reduced HIV incidence by 44%3 and 86%4 vs placebo.
Adherence is better with daily PrEPthan event-driven PrEP
Three prospective cohort trials evaluated PrEP adherence (extent that participants were taking PrEP at the time of sexual encounters) with different dosing regimens and found that event-driven PrEP tended to have lower adherence than daily PrEP. An open-label trial in Bangkok and Harlem (New York City) randomized 357 at-risk patients to 1 of 3 regimens: event-driven (1 tablet before and after sex), time-driven (1 tablet twice weekly with a postsex dose), and daily. Overall, patients with event-driven PrEP had lower adherence than those with daily PrEP (67% event-driven vs 97% daily; P < 0.0001).5
Continue to: In an open-label...
In an open-label prospective cohort trial in Belgium, at-risk patients chose between using event-driven (N = 44) and daily (N = 135) PrEP. Analysis was conducted for both high-risk HIV exposure days (defined as condomless anal receptive intercourse with a new or HIV-positive steady partner with a detectable viral load) and low-risk HIV exposure days (consistent condom use or condomless anal intercourse with a steady partner who is HIV-negative). Over 18 months, lower adherence was demonstrated with event-driven PrEP than with daily PrEP for high-risk days (88% [95% CI, 86%-90%] vs 97.5% [95% CI, 97%-98%]; P < .0001) and also for low-risk days (42% [95% CI, 40%-45%] vs 96% [95% CI, 95%-96%]; P < .0001).6 Researchers diagnosed no new HIV infections in any participant, and the incidence of STIs was the same in both groups.
A third open-label trial evaluated adherence among 178 South African women randomized to event-driven or daily PrEP and found lower sexual event coverage with event-driven PrEP (52% vs 75%; odds ratio = 2.76; 95% CI, 1.68-4.53; P < 0.0006). Four women in each group seroconverted to HIV positive.7
Drug costs, patient preferences, and STI risk are important considerations
Several of the above trials reported use of fewer pills in the event-driven groups, with lower drug costs.2,5,7 A large prospective cohort trial of men who have sex with men (N = 1049) with an average of 10 sexual partners found that most (76%) opted for event-driven PrEP.8 Researchers also reported no difference in STI rates (RR = 1.24 for “at least 1 bacterial STI”; 95% CI, 0.84 to 1.81).8 However, a smaller, open-label prospective cohort trial (N = 200) found that more participants chose daily PrEP than event-driven PrEP (76.5% vs 23.5%), although almost all said they would change their dosing regimen in the next year.9
Recommendations from others
In 2019, the World Health Organization recommended oral PrEP as an additional prevention choice for people at substantial risk for HIV infection and stated that different dosing strategies offer users flexibility, choice, and convenience.10 Also in 2019, the US Preventive Services Task Force published a recommendation that clinicians offer PrEP with effective antiretroviral therapy to patients at high risk for HIV acquisition. They did not specify which regimen to offer.11
Editor’s takeaway
While there are theoretical reasons why event-driven PrEP might not work as well as daily PrEP, we have 1 RCT that suggests the real-world outcomes are similar. Given the apparent effectiveness of either option, the best choice is the one the patient will use. JFP
- Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. NEJM. 2015;373:2237-2246.
- Antoni G, Tremblay C, Delaugerre C, et al. On-demand pre-exposure prophylaxis with tenofovir disoproxil fumarate plus emtricitabine among men who have sex with men with less frequent sexual intercourse: a post-hoc analysis of the ANRS IPERGAY trial. Lancet HIV. 2020;7:e113-e120.
- Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis in men who have sex with men. NEJM. 2010;363:2587-2599.
- McCormack S, Dunn DT, Desai M, et al. Preexposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot of a pragmatic open-label randomized trial. Lancet. 2016;387:53-60.
- Grant RM, Mannheimer S, Hughes JP, et al. Daily and nondaily oral preexposure prophylaxis in men and transgender women who have sex with men: the Human Immunodeficiency Virus Prevention Trials Network 067/ADAPT study. Clin Infect Dis. 2018;66:1712-1721.
 Vuylsteke B, Reyniers T, De Baetselier I, et al. Daily and event-driven pre-exposure prophylaxis for men who have sex with men in Belgium: results of a prospective cohort measuring adherence, sexual behavior and STI incidence. J Intl AIDS Soc. 2019;22:e25407.
Vuylsteke B, Reyniers T, De Baetselier I, et al. Daily and event-driven pre-exposure prophylaxis for men who have sex with men in Belgium: results of a prospective cohort measuring adherence, sexual behavior and STI incidence. J Intl AIDS Soc. 2019;22:e25407.- Bekker LG, Roux S, Sebastien E, et al. Daily and non-daily pre-exposure prophylaxis in African women (HPTN 067/ADAPT Cape Town Trial): a randomized, open-label, phase 2 trial. Lancet HIV. 2018;5:e68-e78.
- Noret M, Balavoine S, Pintado C, et al. Daily or on-demand oral tenofovir disoproxil fumarate/emtricitabine for HIV pre-exposure prophylaxis: experience from a hospital-based clinic in France. AIDS. 2018;32:2161-2169.
- Reyniers T, Nöstlinger C, Laga M, et al. Choosing between daily and event-driven pre-exposure prophylaxis: results of a Belgian PrEP demonstration project. J Acquir Immune Defic Syndr. 2018;79:186-194.
- WHO. What’s the 2+1+1? Event-driven oral pre-exposure prophylaxis to prevent HIV in men who have sex with men: update to WHO’s recommendation on oral PrEP [technical brief]. Published July 2019. Accessed May 14, 2021. https://who.int/hiv/pub/prep/211/en
- US Preventive Services Task Force. Prevention of human immunodeficiency virus (HIV) infection: preexposure prophylaxis [evidence summary]. Published June 11, 2019. Accessed May 14, 2021. www.uspreventiveservicestaskforce.org/uspstf/document/evidence-summary/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
EVIDENCE SUMMARY
Event-driven PrEP is effective for prevention of HIV transmission
An RCT evaluating the effectiveness of event-driven PrEP in 400 patients at high risk for HIV found that it reduced HIV incidence by 86% compared to placebo. Researchers recruited HIV-negative men or transgender women who had sex with men, who’d had condomless anal sex with at least 2 partners in the previous 6 months, and followed them for a median of 9.3 months for HIV acquisition.1
Patients randomized to event-driven PrEP took tenofovir-emtricitabine (300-200 mg) on the following schedule: 2 pills 2 to 24 hours before intercourse (or 1 pill if they had taken it within the past week), followed by a third pill 24 hours later, and a fourth pill 24 hours after that. When patients had multiple consecutive episodes of intercourse, daily use was continued until 2 days after the last episode. Patients in the control group took placebo pills.1
Event-driven PrEP reduced HIV incidence vs placebo (2 infections vs 14 infections; 0.91 vs 6.6 per 100 person-years; relative risk [RR] = 0.86; P = .002). PrEP produced more gastrointestinal (14% vs 5%; P = .002) and renal (18% vs 10%; P = .03) adverse effects than placebo. Participants took a median of 15 pills per month.1
A post-hoc analysis of the above study, evaluating 270 patients, found that event-driven PrEP reduced HIV incidence by 100% during periods of less frequent sexual encounters. Selected participants had a median of 5 sexual encounters per month (range, 2-10), used a median of 9.5 pills per month (range, 6-13), and represented 134 person-years of follow-up. No HIV infections (0 per 100 person-years; 95% CI, 0-5; P = .013) were diagnosed in the PrEP group and 6 HIV infections (9.2 per 100 person-years; 95% CI, 3.4-20.1) were diagnosed in the placebo group, with a relative reduction of HIV incidence of 100% (95% CI, 39-100).2
For comparison, 2 large open-label trials evaluating daily PrEP found that it reduced HIV incidence by 44%3 and 86%4 vs placebo.
Adherence is better with daily PrEPthan event-driven PrEP
Three prospective cohort trials evaluated PrEP adherence (extent that participants were taking PrEP at the time of sexual encounters) with different dosing regimens and found that event-driven PrEP tended to have lower adherence than daily PrEP. An open-label trial in Bangkok and Harlem (New York City) randomized 357 at-risk patients to 1 of 3 regimens: event-driven (1 tablet before and after sex), time-driven (1 tablet twice weekly with a postsex dose), and daily. Overall, patients with event-driven PrEP had lower adherence than those with daily PrEP (67% event-driven vs 97% daily; P < 0.0001).5
Continue to: In an open-label...
In an open-label prospective cohort trial in Belgium, at-risk patients chose between using event-driven (N = 44) and daily (N = 135) PrEP. Analysis was conducted for both high-risk HIV exposure days (defined as condomless anal receptive intercourse with a new or HIV-positive steady partner with a detectable viral load) and low-risk HIV exposure days (consistent condom use or condomless anal intercourse with a steady partner who is HIV-negative). Over 18 months, lower adherence was demonstrated with event-driven PrEP than with daily PrEP for high-risk days (88% [95% CI, 86%-90%] vs 97.5% [95% CI, 97%-98%]; P < .0001) and also for low-risk days (42% [95% CI, 40%-45%] vs 96% [95% CI, 95%-96%]; P < .0001).6 Researchers diagnosed no new HIV infections in any participant, and the incidence of STIs was the same in both groups.
A third open-label trial evaluated adherence among 178 South African women randomized to event-driven or daily PrEP and found lower sexual event coverage with event-driven PrEP (52% vs 75%; odds ratio = 2.76; 95% CI, 1.68-4.53; P < 0.0006). Four women in each group seroconverted to HIV positive.7
Drug costs, patient preferences, and STI risk are important considerations
Several of the above trials reported use of fewer pills in the event-driven groups, with lower drug costs.2,5,7 A large prospective cohort trial of men who have sex with men (N = 1049) with an average of 10 sexual partners found that most (76%) opted for event-driven PrEP.8 Researchers also reported no difference in STI rates (RR = 1.24 for “at least 1 bacterial STI”; 95% CI, 0.84 to 1.81).8 However, a smaller, open-label prospective cohort trial (N = 200) found that more participants chose daily PrEP than event-driven PrEP (76.5% vs 23.5%), although almost all said they would change their dosing regimen in the next year.9
Recommendations from others
In 2019, the World Health Organization recommended oral PrEP as an additional prevention choice for people at substantial risk for HIV infection and stated that different dosing strategies offer users flexibility, choice, and convenience.10 Also in 2019, the US Preventive Services Task Force published a recommendation that clinicians offer PrEP with effective antiretroviral therapy to patients at high risk for HIV acquisition. They did not specify which regimen to offer.11
Editor’s takeaway
While there are theoretical reasons why event-driven PrEP might not work as well as daily PrEP, we have 1 RCT that suggests the real-world outcomes are similar. Given the apparent effectiveness of either option, the best choice is the one the patient will use. JFP
EVIDENCE SUMMARY
Event-driven PrEP is effective for prevention of HIV transmission
An RCT evaluating the effectiveness of event-driven PrEP in 400 patients at high risk for HIV found that it reduced HIV incidence by 86% compared to placebo. Researchers recruited HIV-negative men or transgender women who had sex with men, who’d had condomless anal sex with at least 2 partners in the previous 6 months, and followed them for a median of 9.3 months for HIV acquisition.1
Patients randomized to event-driven PrEP took tenofovir-emtricitabine (300-200 mg) on the following schedule: 2 pills 2 to 24 hours before intercourse (or 1 pill if they had taken it within the past week), followed by a third pill 24 hours later, and a fourth pill 24 hours after that. When patients had multiple consecutive episodes of intercourse, daily use was continued until 2 days after the last episode. Patients in the control group took placebo pills.1
Event-driven PrEP reduced HIV incidence vs placebo (2 infections vs 14 infections; 0.91 vs 6.6 per 100 person-years; relative risk [RR] = 0.86; P = .002). PrEP produced more gastrointestinal (14% vs 5%; P = .002) and renal (18% vs 10%; P = .03) adverse effects than placebo. Participants took a median of 15 pills per month.1
A post-hoc analysis of the above study, evaluating 270 patients, found that event-driven PrEP reduced HIV incidence by 100% during periods of less frequent sexual encounters. Selected participants had a median of 5 sexual encounters per month (range, 2-10), used a median of 9.5 pills per month (range, 6-13), and represented 134 person-years of follow-up. No HIV infections (0 per 100 person-years; 95% CI, 0-5; P = .013) were diagnosed in the PrEP group and 6 HIV infections (9.2 per 100 person-years; 95% CI, 3.4-20.1) were diagnosed in the placebo group, with a relative reduction of HIV incidence of 100% (95% CI, 39-100).2
For comparison, 2 large open-label trials evaluating daily PrEP found that it reduced HIV incidence by 44%3 and 86%4 vs placebo.
Adherence is better with daily PrEPthan event-driven PrEP
Three prospective cohort trials evaluated PrEP adherence (extent that participants were taking PrEP at the time of sexual encounters) with different dosing regimens and found that event-driven PrEP tended to have lower adherence than daily PrEP. An open-label trial in Bangkok and Harlem (New York City) randomized 357 at-risk patients to 1 of 3 regimens: event-driven (1 tablet before and after sex), time-driven (1 tablet twice weekly with a postsex dose), and daily. Overall, patients with event-driven PrEP had lower adherence than those with daily PrEP (67% event-driven vs 97% daily; P < 0.0001).5
Continue to: In an open-label...
In an open-label prospective cohort trial in Belgium, at-risk patients chose between using event-driven (N = 44) and daily (N = 135) PrEP. Analysis was conducted for both high-risk HIV exposure days (defined as condomless anal receptive intercourse with a new or HIV-positive steady partner with a detectable viral load) and low-risk HIV exposure days (consistent condom use or condomless anal intercourse with a steady partner who is HIV-negative). Over 18 months, lower adherence was demonstrated with event-driven PrEP than with daily PrEP for high-risk days (88% [95% CI, 86%-90%] vs 97.5% [95% CI, 97%-98%]; P < .0001) and also for low-risk days (42% [95% CI, 40%-45%] vs 96% [95% CI, 95%-96%]; P < .0001).6 Researchers diagnosed no new HIV infections in any participant, and the incidence of STIs was the same in both groups.
A third open-label trial evaluated adherence among 178 South African women randomized to event-driven or daily PrEP and found lower sexual event coverage with event-driven PrEP (52% vs 75%; odds ratio = 2.76; 95% CI, 1.68-4.53; P < 0.0006). Four women in each group seroconverted to HIV positive.7
Drug costs, patient preferences, and STI risk are important considerations
Several of the above trials reported use of fewer pills in the event-driven groups, with lower drug costs.2,5,7 A large prospective cohort trial of men who have sex with men (N = 1049) with an average of 10 sexual partners found that most (76%) opted for event-driven PrEP.8 Researchers also reported no difference in STI rates (RR = 1.24 for “at least 1 bacterial STI”; 95% CI, 0.84 to 1.81).8 However, a smaller, open-label prospective cohort trial (N = 200) found that more participants chose daily PrEP than event-driven PrEP (76.5% vs 23.5%), although almost all said they would change their dosing regimen in the next year.9
Recommendations from others
In 2019, the World Health Organization recommended oral PrEP as an additional prevention choice for people at substantial risk for HIV infection and stated that different dosing strategies offer users flexibility, choice, and convenience.10 Also in 2019, the US Preventive Services Task Force published a recommendation that clinicians offer PrEP with effective antiretroviral therapy to patients at high risk for HIV acquisition. They did not specify which regimen to offer.11
Editor’s takeaway
While there are theoretical reasons why event-driven PrEP might not work as well as daily PrEP, we have 1 RCT that suggests the real-world outcomes are similar. Given the apparent effectiveness of either option, the best choice is the one the patient will use. JFP
- Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. NEJM. 2015;373:2237-2246.
- Antoni G, Tremblay C, Delaugerre C, et al. On-demand pre-exposure prophylaxis with tenofovir disoproxil fumarate plus emtricitabine among men who have sex with men with less frequent sexual intercourse: a post-hoc analysis of the ANRS IPERGAY trial. Lancet HIV. 2020;7:e113-e120.
- Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis in men who have sex with men. NEJM. 2010;363:2587-2599.
- McCormack S, Dunn DT, Desai M, et al. Preexposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot of a pragmatic open-label randomized trial. Lancet. 2016;387:53-60.
- Grant RM, Mannheimer S, Hughes JP, et al. Daily and nondaily oral preexposure prophylaxis in men and transgender women who have sex with men: the Human Immunodeficiency Virus Prevention Trials Network 067/ADAPT study. Clin Infect Dis. 2018;66:1712-1721.
 Vuylsteke B, Reyniers T, De Baetselier I, et al. Daily and event-driven pre-exposure prophylaxis for men who have sex with men in Belgium: results of a prospective cohort measuring adherence, sexual behavior and STI incidence. J Intl AIDS Soc. 2019;22:e25407.
Vuylsteke B, Reyniers T, De Baetselier I, et al. Daily and event-driven pre-exposure prophylaxis for men who have sex with men in Belgium: results of a prospective cohort measuring adherence, sexual behavior and STI incidence. J Intl AIDS Soc. 2019;22:e25407.- Bekker LG, Roux S, Sebastien E, et al. Daily and non-daily pre-exposure prophylaxis in African women (HPTN 067/ADAPT Cape Town Trial): a randomized, open-label, phase 2 trial. Lancet HIV. 2018;5:e68-e78.
- Noret M, Balavoine S, Pintado C, et al. Daily or on-demand oral tenofovir disoproxil fumarate/emtricitabine for HIV pre-exposure prophylaxis: experience from a hospital-based clinic in France. AIDS. 2018;32:2161-2169.
- Reyniers T, Nöstlinger C, Laga M, et al. Choosing between daily and event-driven pre-exposure prophylaxis: results of a Belgian PrEP demonstration project. J Acquir Immune Defic Syndr. 2018;79:186-194.
- WHO. What’s the 2+1+1? Event-driven oral pre-exposure prophylaxis to prevent HIV in men who have sex with men: update to WHO’s recommendation on oral PrEP [technical brief]. Published July 2019. Accessed May 14, 2021. https://who.int/hiv/pub/prep/211/en
- US Preventive Services Task Force. Prevention of human immunodeficiency virus (HIV) infection: preexposure prophylaxis [evidence summary]. Published June 11, 2019. Accessed May 14, 2021. www.uspreventiveservicestaskforce.org/uspstf/document/evidence-summary/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
- Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. NEJM. 2015;373:2237-2246.
- Antoni G, Tremblay C, Delaugerre C, et al. On-demand pre-exposure prophylaxis with tenofovir disoproxil fumarate plus emtricitabine among men who have sex with men with less frequent sexual intercourse: a post-hoc analysis of the ANRS IPERGAY trial. Lancet HIV. 2020;7:e113-e120.
- Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis in men who have sex with men. NEJM. 2010;363:2587-2599.
- McCormack S, Dunn DT, Desai M, et al. Preexposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot of a pragmatic open-label randomized trial. Lancet. 2016;387:53-60.
- Grant RM, Mannheimer S, Hughes JP, et al. Daily and nondaily oral preexposure prophylaxis in men and transgender women who have sex with men: the Human Immunodeficiency Virus Prevention Trials Network 067/ADAPT study. Clin Infect Dis. 2018;66:1712-1721.
 Vuylsteke B, Reyniers T, De Baetselier I, et al. Daily and event-driven pre-exposure prophylaxis for men who have sex with men in Belgium: results of a prospective cohort measuring adherence, sexual behavior and STI incidence. J Intl AIDS Soc. 2019;22:e25407.
Vuylsteke B, Reyniers T, De Baetselier I, et al. Daily and event-driven pre-exposure prophylaxis for men who have sex with men in Belgium: results of a prospective cohort measuring adherence, sexual behavior and STI incidence. J Intl AIDS Soc. 2019;22:e25407.- Bekker LG, Roux S, Sebastien E, et al. Daily and non-daily pre-exposure prophylaxis in African women (HPTN 067/ADAPT Cape Town Trial): a randomized, open-label, phase 2 trial. Lancet HIV. 2018;5:e68-e78.
- Noret M, Balavoine S, Pintado C, et al. Daily or on-demand oral tenofovir disoproxil fumarate/emtricitabine for HIV pre-exposure prophylaxis: experience from a hospital-based clinic in France. AIDS. 2018;32:2161-2169.
- Reyniers T, Nöstlinger C, Laga M, et al. Choosing between daily and event-driven pre-exposure prophylaxis: results of a Belgian PrEP demonstration project. J Acquir Immune Defic Syndr. 2018;79:186-194.
- WHO. What’s the 2+1+1? Event-driven oral pre-exposure prophylaxis to prevent HIV in men who have sex with men: update to WHO’s recommendation on oral PrEP [technical brief]. Published July 2019. Accessed May 14, 2021. https://who.int/hiv/pub/prep/211/en
- US Preventive Services Task Force. Prevention of human immunodeficiency virus (HIV) infection: preexposure prophylaxis [evidence summary]. Published June 11, 2019. Accessed May 14, 2021. www.uspreventiveservicestaskforce.org/uspstf/document/evidence-summary/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
EVIDENCE-BASED ANSWER:
Probably, although there are no head-to-head trials comparing the 2 dosing regimens. Event-driven pre-exposure prophylaxis (PrEP) dosing reduces HIV conversion by 86% compared to placebo (strength of recommendation [SOR]: B, large randomized controlled trial [RCT]). Daily PrEP reduces HIV conversion by 44% to 86% (SOR: B, based on open-label RCTs).
Event-driven PrEP regimens may be associated with lower adherence when compared with daily PrEP regimens (average of 70% for event-driven PrEP vs average of 92% for daily PrEP) (SOR: B, based on open-label and cohort trials). Event-driven PrEP regimens have lower medication costs, and they are associated with no difference in the rate of sexually transmitted infections (STIs) (SOR: B, based on prospective cohort studies). Patients may prefer them to daily regimens (75% choose event-driven PrEP vs 25% choose daily PrEP) (SOR: B, based on the preponderance of prospective cohort studies with conflicting results).
Is metformin effective for reducing weight in obese or overweight adolescents?
EVIDENCE SUMMARY
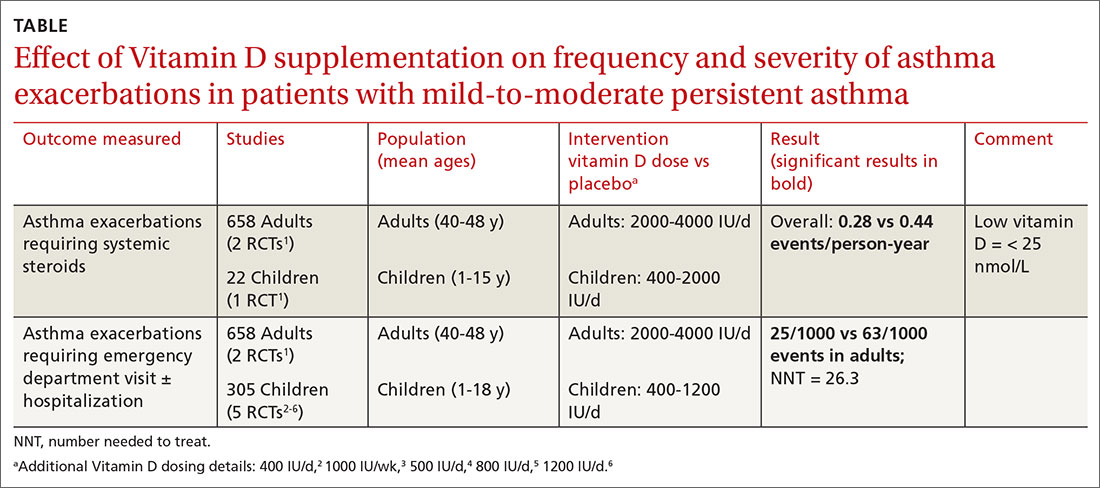
Metformin has modest effects on body weight
A large systematic review and meta-analysis (38 RCTs; n = 2199) published in 2020 evaluated metformin therapy in children and adolescents (including those with metabolic disease, growth problems, and psychological disorders in addition to obesity and overweight).1 Over an average of 6 months, metformin use modestly reduced BMI (weighted mean difference [WMD] = –1.07 kg/m2; 95% CI, –1.43 to –0.72 kg/m2) and body weight (WMD = –2.51 kg; 95% CI, –3.14 to –1.89 kg) for all participants.1
However, the authors also performed a meta-analysis of trials involving obese or overweight youth without other comorbidities. Participants in these trials ranged in age from 7 to 17 years (mean not supplied; most trials, 12-15 years), had a BMI greater than the 95th percentile for age, and took doses of metformin ranging from 1500 to 3000 mg (most trials, 1500-2000 mg/d for 24 weeks).1 In this analysis, metformin reduced body weight (8 trials; n = 616; WMD = –2.06 kg; 95% CI, –3.47 to –0.65 kg) and body fat mass (–1.9%; 95% CI, –3.25% to –0.56%). But it did not reduce BMI (12 trials; n = 826; WMD = –0.76 kg/m2; 95 % CI, –1.61 to 0.08 kg/m2) or improve lean body mass (2 trials; N = 98; WMD = –0.74 kg; 95% CI, –2.4 to 0.91 kg).1
The authors of this meta-analysis did not include an evaluation of the quality of the individual RCTs.
Metformin has benefits but also adverse effects
A 2016 Cochrane systematic review and meta-analysis assessed 8 trials (total n = 543) evaluating metformin vs placebo in adolescents prescribed exercise and lifestyle support.2 This meta-analysis included 4 trials (n = 294) with obese or overweight adolescents that were also included in the newer meta-analysis,1 as well as 4 trials involving obese adolescents with insulin resistance. The authors did not assess the effects of metformin on obese or overweight adolescents separately.
Over 6 months, metformin use reduced BMI (WMD = –1.35 kg/m2; 95% CI –2 to –0.69 kg/m2).2 Metformin commonly produced gastrointestinal symptoms: diarrhea, flatulence (rates not given), and nausea in 15% to 42% compared with 3% to 21% with placebo (no comparison statistic supplied), however rarely to the point of discontinuation (< 5%).2 Nine participants withdrew due to adverse effects: 5 in the metformin group and 4 in the placebo group. The authors rated the quality of the included trials as low to moderate.
An evidence report and systematic review (42 RCTs; total n = 6956) compared the efficacy of several approaches for weight loss in adolescents, including metformin (6 of the 8 RCTs included in the 2020 meta-analysis1) and lifestyle interventions.3 Interventions comprising exercise and diet counseling for > 26 hours over 6 to 12 months produced decreases in BMI (–0.86 kg/m2; 95% CI –1.44 to –0.29 kg/m2) but not weight (–2 kg; 95% CI –3.2 to 1.2 kg).3
Recommendations from others
The US Preventive Services Task Force states that metformin treatment in adolescents who are overweight or obese produces a small reduction in BMI when compared to placebo, but the clinical significance of this reduction is unclear.3
Editor’s takeaway
The idea of using medications for weight loss remains seductive, given how hard it can be for patients to achieve significant, lasting weight loss through lifestyle modification. Evidence suggests that metformin can help in this regard but not enough to recommend it. In addition, metformin therapy is associated with gastrointestinal adverse effects.
1. Sadeghi A, Mousavi SM, Mokhtari T, et al. Metformin therapy reduces obesity indices in children and adolescents: a systematic review and meta-analysis of randomized clinical trials. Child Obes. 2020;16:174-191.
2. Mead E, Atkinson G, Richter B, et al. Drug interventions for the treatment of obesity in children and adolescents. Cochrane Database Syst Rev. 2016;11:CD012436.
3. O’Connor EA, Evans CV, Burda BU, et al. Screening for obesity and intervention for weight management in children and adolescents: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317:2427-2444.
EVIDENCE SUMMARY
Metformin has modest effects on body weight
A large systematic review and meta-analysis (38 RCTs; n = 2199) published in 2020 evaluated metformin therapy in children and adolescents (including those with metabolic disease, growth problems, and psychological disorders in addition to obesity and overweight).1 Over an average of 6 months, metformin use modestly reduced BMI (weighted mean difference [WMD] = –1.07 kg/m2; 95% CI, –1.43 to –0.72 kg/m2) and body weight (WMD = –2.51 kg; 95% CI, –3.14 to –1.89 kg) for all participants.1
However, the authors also performed a meta-analysis of trials involving obese or overweight youth without other comorbidities. Participants in these trials ranged in age from 7 to 17 years (mean not supplied; most trials, 12-15 years), had a BMI greater than the 95th percentile for age, and took doses of metformin ranging from 1500 to 3000 mg (most trials, 1500-2000 mg/d for 24 weeks).1 In this analysis, metformin reduced body weight (8 trials; n = 616; WMD = –2.06 kg; 95% CI, –3.47 to –0.65 kg) and body fat mass (–1.9%; 95% CI, –3.25% to –0.56%). But it did not reduce BMI (12 trials; n = 826; WMD = –0.76 kg/m2; 95 % CI, –1.61 to 0.08 kg/m2) or improve lean body mass (2 trials; N = 98; WMD = –0.74 kg; 95% CI, –2.4 to 0.91 kg).1
The authors of this meta-analysis did not include an evaluation of the quality of the individual RCTs.
Metformin has benefits but also adverse effects
A 2016 Cochrane systematic review and meta-analysis assessed 8 trials (total n = 543) evaluating metformin vs placebo in adolescents prescribed exercise and lifestyle support.2 This meta-analysis included 4 trials (n = 294) with obese or overweight adolescents that were also included in the newer meta-analysis,1 as well as 4 trials involving obese adolescents with insulin resistance. The authors did not assess the effects of metformin on obese or overweight adolescents separately.
Over 6 months, metformin use reduced BMI (WMD = –1.35 kg/m2; 95% CI –2 to –0.69 kg/m2).2 Metformin commonly produced gastrointestinal symptoms: diarrhea, flatulence (rates not given), and nausea in 15% to 42% compared with 3% to 21% with placebo (no comparison statistic supplied), however rarely to the point of discontinuation (< 5%).2 Nine participants withdrew due to adverse effects: 5 in the metformin group and 4 in the placebo group. The authors rated the quality of the included trials as low to moderate.
An evidence report and systematic review (42 RCTs; total n = 6956) compared the efficacy of several approaches for weight loss in adolescents, including metformin (6 of the 8 RCTs included in the 2020 meta-analysis1) and lifestyle interventions.3 Interventions comprising exercise and diet counseling for > 26 hours over 6 to 12 months produced decreases in BMI (–0.86 kg/m2; 95% CI –1.44 to –0.29 kg/m2) but not weight (–2 kg; 95% CI –3.2 to 1.2 kg).3
Recommendations from others
The US Preventive Services Task Force states that metformin treatment in adolescents who are overweight or obese produces a small reduction in BMI when compared to placebo, but the clinical significance of this reduction is unclear.3
Editor’s takeaway
The idea of using medications for weight loss remains seductive, given how hard it can be for patients to achieve significant, lasting weight loss through lifestyle modification. Evidence suggests that metformin can help in this regard but not enough to recommend it. In addition, metformin therapy is associated with gastrointestinal adverse effects.
EVIDENCE SUMMARY
Metformin has modest effects on body weight
A large systematic review and meta-analysis (38 RCTs; n = 2199) published in 2020 evaluated metformin therapy in children and adolescents (including those with metabolic disease, growth problems, and psychological disorders in addition to obesity and overweight).1 Over an average of 6 months, metformin use modestly reduced BMI (weighted mean difference [WMD] = –1.07 kg/m2; 95% CI, –1.43 to –0.72 kg/m2) and body weight (WMD = –2.51 kg; 95% CI, –3.14 to –1.89 kg) for all participants.1
However, the authors also performed a meta-analysis of trials involving obese or overweight youth without other comorbidities. Participants in these trials ranged in age from 7 to 17 years (mean not supplied; most trials, 12-15 years), had a BMI greater than the 95th percentile for age, and took doses of metformin ranging from 1500 to 3000 mg (most trials, 1500-2000 mg/d for 24 weeks).1 In this analysis, metformin reduced body weight (8 trials; n = 616; WMD = –2.06 kg; 95% CI, –3.47 to –0.65 kg) and body fat mass (–1.9%; 95% CI, –3.25% to –0.56%). But it did not reduce BMI (12 trials; n = 826; WMD = –0.76 kg/m2; 95 % CI, –1.61 to 0.08 kg/m2) or improve lean body mass (2 trials; N = 98; WMD = –0.74 kg; 95% CI, –2.4 to 0.91 kg).1
The authors of this meta-analysis did not include an evaluation of the quality of the individual RCTs.
Metformin has benefits but also adverse effects
A 2016 Cochrane systematic review and meta-analysis assessed 8 trials (total n = 543) evaluating metformin vs placebo in adolescents prescribed exercise and lifestyle support.2 This meta-analysis included 4 trials (n = 294) with obese or overweight adolescents that were also included in the newer meta-analysis,1 as well as 4 trials involving obese adolescents with insulin resistance. The authors did not assess the effects of metformin on obese or overweight adolescents separately.
Over 6 months, metformin use reduced BMI (WMD = –1.35 kg/m2; 95% CI –2 to –0.69 kg/m2).2 Metformin commonly produced gastrointestinal symptoms: diarrhea, flatulence (rates not given), and nausea in 15% to 42% compared with 3% to 21% with placebo (no comparison statistic supplied), however rarely to the point of discontinuation (< 5%).2 Nine participants withdrew due to adverse effects: 5 in the metformin group and 4 in the placebo group. The authors rated the quality of the included trials as low to moderate.
An evidence report and systematic review (42 RCTs; total n = 6956) compared the efficacy of several approaches for weight loss in adolescents, including metformin (6 of the 8 RCTs included in the 2020 meta-analysis1) and lifestyle interventions.3 Interventions comprising exercise and diet counseling for > 26 hours over 6 to 12 months produced decreases in BMI (–0.86 kg/m2; 95% CI –1.44 to –0.29 kg/m2) but not weight (–2 kg; 95% CI –3.2 to 1.2 kg).3
Recommendations from others
The US Preventive Services Task Force states that metformin treatment in adolescents who are overweight or obese produces a small reduction in BMI when compared to placebo, but the clinical significance of this reduction is unclear.3
Editor’s takeaway
The idea of using medications for weight loss remains seductive, given how hard it can be for patients to achieve significant, lasting weight loss through lifestyle modification. Evidence suggests that metformin can help in this regard but not enough to recommend it. In addition, metformin therapy is associated with gastrointestinal adverse effects.
1. Sadeghi A, Mousavi SM, Mokhtari T, et al. Metformin therapy reduces obesity indices in children and adolescents: a systematic review and meta-analysis of randomized clinical trials. Child Obes. 2020;16:174-191.
2. Mead E, Atkinson G, Richter B, et al. Drug interventions for the treatment of obesity in children and adolescents. Cochrane Database Syst Rev. 2016;11:CD012436.
3. O’Connor EA, Evans CV, Burda BU, et al. Screening for obesity and intervention for weight management in children and adolescents: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317:2427-2444.
1. Sadeghi A, Mousavi SM, Mokhtari T, et al. Metformin therapy reduces obesity indices in children and adolescents: a systematic review and meta-analysis of randomized clinical trials. Child Obes. 2020;16:174-191.
2. Mead E, Atkinson G, Richter B, et al. Drug interventions for the treatment of obesity in children and adolescents. Cochrane Database Syst Rev. 2016;11:CD012436.
3. O’Connor EA, Evans CV, Burda BU, et al. Screening for obesity and intervention for weight management in children and adolescents: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317:2427-2444.
EVIDENCE-BASED ANSWER:
Yes, to some degree—but it is of uncertain clinical significance. Over a period of 6 months, metformin modestly reduced weight (–2.1 kg) and body fat mass (–1.9%), but not body mass index (BMI) or lean body mass, in adolescents who were overweight or obese. This is comparable to lifestyle interventions (diet and exercise) supported with > 26 hours of counseling, which modestly improved BMI but not weight. (Strength of recommendation [SOR]: A, based on a large meta-analysis of randomized controlled trials [RCTs] of variable quality).
Does concurrent use of clopidogrel and PPIs increase CV risk in patients with ACS?
EVIDENCE SUMMARY
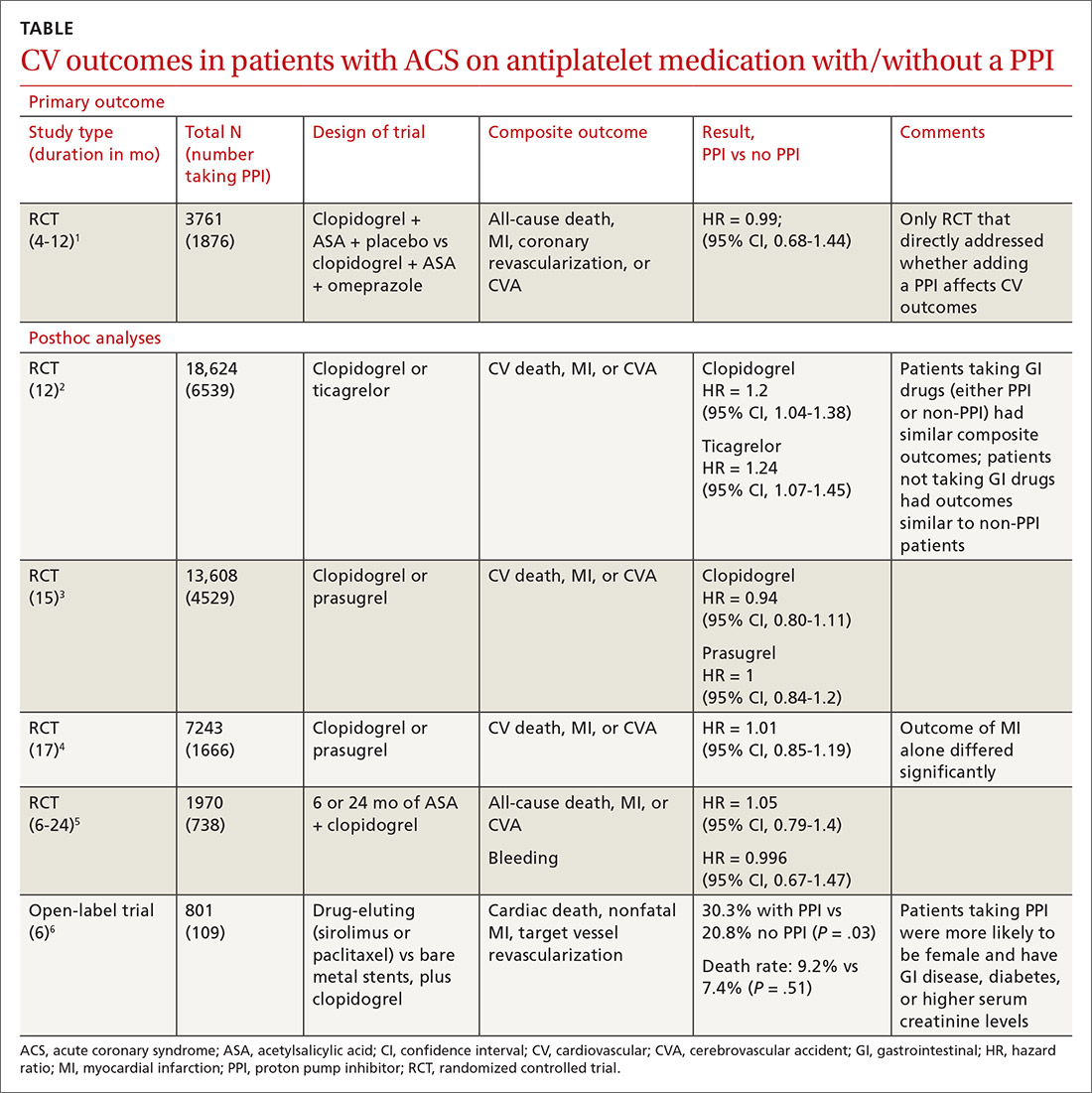
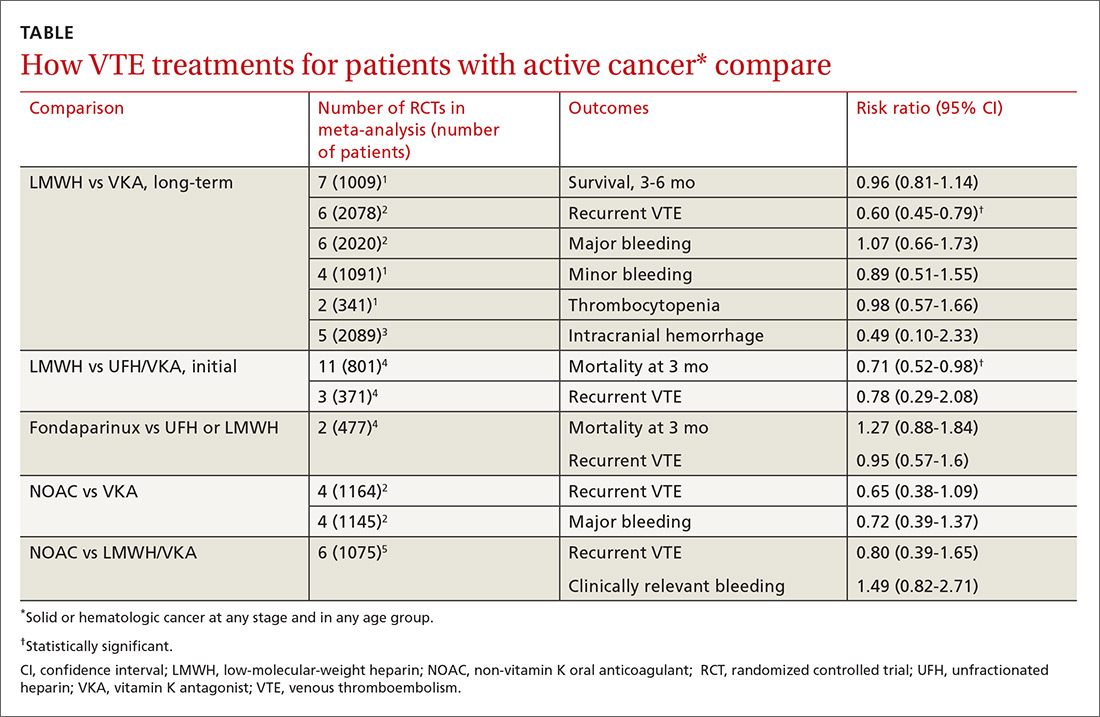
A double-blind, double-dummy, placebo-controlled RCT comparing a combination of clopidogrel, aspirin, and omeprazole with clopidogrel, aspirin, and placebo found no increase in composite CV outcomes with the PPI (TABLE).1 Using a PPI did, however, significantly reduce gastrointestinal (GI) bleeding (hazard ratio [HR] = 0.13; 95% confidence interval [CI], 0.03-0.56).Although several meta-analyses have been conducted, they all rely on this single RCT that directly addresses the question, plus post-hoc analyses of other RCTs.
Four of 5 analyses find little or no difference in CV outcomes with a PPI
Four of 5 posthoc analyses (which weren’t themselves randomized) of RCTs found unclear or no differences in composite CV outcomes with concurrent use of a PPI and antiplatelet therapy, after multivariate adjustment for differences in populations taking or not taking a PPI.
Posthoc analysis of the largest study found worse CV outcomes for both clopidogrel and ticagrelor with concomitant PPI use.2 However, patients on any GI drugs (PPI or non-PPI) had composite outcomes similar to patients on a PPI (PPI vs non-PPI GI treatment: HR = 0.98; 95% CI, 0.79-1.23), and patients not taking GI drugs had fewer composite outcomes compared with patients on a PPI (clopidogrel vs no GI therapy: HR = 1.29; 95% CI, 1.12-1.49; ticagrelor vs no GI therapy: HR = 1.30; 95% CI, 1.14-1.49). Researchers postulated that because the rate of composite outcomes increased equally for patients on any GI drug, the higher rate of CV adverse events with a PPI might have been related to GI disease rather than PPI use.
A similar posthoc analysis found no differences with or without PPI use among patients with ACS undergoing planned percutaneous coronary intervention (PCI) and assigned to clopidogrel or prasugrel.3 Researchers performed multivariate adjustment for differences in age, gender, ethnicity, and initial presence of unstable angina/non-ST-elevation MI.
A smaller study also found no significant differences in composite CV outcomes in patients using PPIs.4 Patients did have higher rates of MI (HR = 0.62; 95% CI, 0.42-0.91), but they were more likely to be older and have a previous diagnosis of non-ST-elevation MI, higher incidence of previous coronary artery bypass graft surgery, and history of peptic ulcer disease.
The fourth posthoc analysis of an RCT found that concomitant PPI use (91% of patients on lansoprazole) didn’t alter outcomes among patients undergoing PCI and receiving dual antiplatelet therapy with clopidogrel and aspirin.5 Researchers used a multivariate adjustment for differences in age, gender, and renal function and found no difference in outcomes during the 6-month or 24-month period. PPI prescription was at physician discretion. Researchers didn’t assess for dose-dependent effects of PPI.
A fifth, flawed study finds more adverse events with PPIs
A posthoc analysis of a smaller, open-label trial found increased major adverse cardiac events with PPI use among patients taking clopidogrel after PCI.6 Researchers didn’t adjust for differences in populations at baseline, however, and patients taking PPIs were more likely to be female or older and have diabetes, GI disease, or higher serum creatinine levels.
Continue to: Editor's takeaway
Editor’s takeaway
The best evidence (a large RCT) found that adding a PPI to antiplatelet therapy didn’t alter CV outcomes in patients with ACS, but it did reduce GI bleeds. Hopefully this will give providers the confidence to use PPIs, if clinically indicated, in patients taking antiplatelet therapy with clopidogrel or prasugrel.
1. Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363:1909-1917.
2. Goodman SG, Clare R, Pieper KS, et al. Association of proton pump inhibitor use on cardiovascular outcomes with clopidogrel and ticagrelor: insights from the platelet inhibition and patient outcomes trial. Circulation. 2012;125:978-986.
3. O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet. 2009;374:989-997.
4. Nicolau JC, Bhatt DL, Roe MT, et al. Concomitant proton-pump inhibitor use, platelet activity, and clinical outcomes in patients with acute coronary syndromes treated with prasugrel versus clopidogrel and managed without revascularization: insights from the Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes trial. Am Heart J. 2015;170:683-694.e3.
5. Gargiulo G, Costa F, Ariotti S, et al. Impact of proton pump inhibitors on clinical outcomes in patients treated with a 6- or 24-month dual-antiplatelet therapy duration: insights from the PROlonging Dual-antiplatelet treatment after Grading stent-induced Intimal hyperplasia studY trial. Am Heart J. 2016;174:95-102.
6. Burkard T, Kaiser CA, Brunner-La Rocca H, et al. Combined clopidogrel and proton pump inhibitor therapy is associated with higher cardiovascular event rates after percutaneous coronary intervention: a report from the BASKET trial. J Intern Med. 2012;271:257-263.
EVIDENCE SUMMARY
A double-blind, double-dummy, placebo-controlled RCT comparing a combination of clopidogrel, aspirin, and omeprazole with clopidogrel, aspirin, and placebo found no increase in composite CV outcomes with the PPI (TABLE).1 Using a PPI did, however, significantly reduce gastrointestinal (GI) bleeding (hazard ratio [HR] = 0.13; 95% confidence interval [CI], 0.03-0.56).Although several meta-analyses have been conducted, they all rely on this single RCT that directly addresses the question, plus post-hoc analyses of other RCTs.
Four of 5 analyses find little or no difference in CV outcomes with a PPI
Four of 5 posthoc analyses (which weren’t themselves randomized) of RCTs found unclear or no differences in composite CV outcomes with concurrent use of a PPI and antiplatelet therapy, after multivariate adjustment for differences in populations taking or not taking a PPI.
Posthoc analysis of the largest study found worse CV outcomes for both clopidogrel and ticagrelor with concomitant PPI use.2 However, patients on any GI drugs (PPI or non-PPI) had composite outcomes similar to patients on a PPI (PPI vs non-PPI GI treatment: HR = 0.98; 95% CI, 0.79-1.23), and patients not taking GI drugs had fewer composite outcomes compared with patients on a PPI (clopidogrel vs no GI therapy: HR = 1.29; 95% CI, 1.12-1.49; ticagrelor vs no GI therapy: HR = 1.30; 95% CI, 1.14-1.49). Researchers postulated that because the rate of composite outcomes increased equally for patients on any GI drug, the higher rate of CV adverse events with a PPI might have been related to GI disease rather than PPI use.
A similar posthoc analysis found no differences with or without PPI use among patients with ACS undergoing planned percutaneous coronary intervention (PCI) and assigned to clopidogrel or prasugrel.3 Researchers performed multivariate adjustment for differences in age, gender, ethnicity, and initial presence of unstable angina/non-ST-elevation MI.
A smaller study also found no significant differences in composite CV outcomes in patients using PPIs.4 Patients did have higher rates of MI (HR = 0.62; 95% CI, 0.42-0.91), but they were more likely to be older and have a previous diagnosis of non-ST-elevation MI, higher incidence of previous coronary artery bypass graft surgery, and history of peptic ulcer disease.
The fourth posthoc analysis of an RCT found that concomitant PPI use (91% of patients on lansoprazole) didn’t alter outcomes among patients undergoing PCI and receiving dual antiplatelet therapy with clopidogrel and aspirin.5 Researchers used a multivariate adjustment for differences in age, gender, and renal function and found no difference in outcomes during the 6-month or 24-month period. PPI prescription was at physician discretion. Researchers didn’t assess for dose-dependent effects of PPI.
A fifth, flawed study finds more adverse events with PPIs
A posthoc analysis of a smaller, open-label trial found increased major adverse cardiac events with PPI use among patients taking clopidogrel after PCI.6 Researchers didn’t adjust for differences in populations at baseline, however, and patients taking PPIs were more likely to be female or older and have diabetes, GI disease, or higher serum creatinine levels.
Continue to: Editor's takeaway
Editor’s takeaway
The best evidence (a large RCT) found that adding a PPI to antiplatelet therapy didn’t alter CV outcomes in patients with ACS, but it did reduce GI bleeds. Hopefully this will give providers the confidence to use PPIs, if clinically indicated, in patients taking antiplatelet therapy with clopidogrel or prasugrel.
EVIDENCE SUMMARY
A double-blind, double-dummy, placebo-controlled RCT comparing a combination of clopidogrel, aspirin, and omeprazole with clopidogrel, aspirin, and placebo found no increase in composite CV outcomes with the PPI (TABLE).1 Using a PPI did, however, significantly reduce gastrointestinal (GI) bleeding (hazard ratio [HR] = 0.13; 95% confidence interval [CI], 0.03-0.56).Although several meta-analyses have been conducted, they all rely on this single RCT that directly addresses the question, plus post-hoc analyses of other RCTs.
Four of 5 analyses find little or no difference in CV outcomes with a PPI
Four of 5 posthoc analyses (which weren’t themselves randomized) of RCTs found unclear or no differences in composite CV outcomes with concurrent use of a PPI and antiplatelet therapy, after multivariate adjustment for differences in populations taking or not taking a PPI.
Posthoc analysis of the largest study found worse CV outcomes for both clopidogrel and ticagrelor with concomitant PPI use.2 However, patients on any GI drugs (PPI or non-PPI) had composite outcomes similar to patients on a PPI (PPI vs non-PPI GI treatment: HR = 0.98; 95% CI, 0.79-1.23), and patients not taking GI drugs had fewer composite outcomes compared with patients on a PPI (clopidogrel vs no GI therapy: HR = 1.29; 95% CI, 1.12-1.49; ticagrelor vs no GI therapy: HR = 1.30; 95% CI, 1.14-1.49). Researchers postulated that because the rate of composite outcomes increased equally for patients on any GI drug, the higher rate of CV adverse events with a PPI might have been related to GI disease rather than PPI use.
A similar posthoc analysis found no differences with or without PPI use among patients with ACS undergoing planned percutaneous coronary intervention (PCI) and assigned to clopidogrel or prasugrel.3 Researchers performed multivariate adjustment for differences in age, gender, ethnicity, and initial presence of unstable angina/non-ST-elevation MI.
A smaller study also found no significant differences in composite CV outcomes in patients using PPIs.4 Patients did have higher rates of MI (HR = 0.62; 95% CI, 0.42-0.91), but they were more likely to be older and have a previous diagnosis of non-ST-elevation MI, higher incidence of previous coronary artery bypass graft surgery, and history of peptic ulcer disease.
The fourth posthoc analysis of an RCT found that concomitant PPI use (91% of patients on lansoprazole) didn’t alter outcomes among patients undergoing PCI and receiving dual antiplatelet therapy with clopidogrel and aspirin.5 Researchers used a multivariate adjustment for differences in age, gender, and renal function and found no difference in outcomes during the 6-month or 24-month period. PPI prescription was at physician discretion. Researchers didn’t assess for dose-dependent effects of PPI.
A fifth, flawed study finds more adverse events with PPIs
A posthoc analysis of a smaller, open-label trial found increased major adverse cardiac events with PPI use among patients taking clopidogrel after PCI.6 Researchers didn’t adjust for differences in populations at baseline, however, and patients taking PPIs were more likely to be female or older and have diabetes, GI disease, or higher serum creatinine levels.
Continue to: Editor's takeaway
Editor’s takeaway
The best evidence (a large RCT) found that adding a PPI to antiplatelet therapy didn’t alter CV outcomes in patients with ACS, but it did reduce GI bleeds. Hopefully this will give providers the confidence to use PPIs, if clinically indicated, in patients taking antiplatelet therapy with clopidogrel or prasugrel.
1. Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363:1909-1917.
2. Goodman SG, Clare R, Pieper KS, et al. Association of proton pump inhibitor use on cardiovascular outcomes with clopidogrel and ticagrelor: insights from the platelet inhibition and patient outcomes trial. Circulation. 2012;125:978-986.
3. O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet. 2009;374:989-997.
4. Nicolau JC, Bhatt DL, Roe MT, et al. Concomitant proton-pump inhibitor use, platelet activity, and clinical outcomes in patients with acute coronary syndromes treated with prasugrel versus clopidogrel and managed without revascularization: insights from the Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes trial. Am Heart J. 2015;170:683-694.e3.
5. Gargiulo G, Costa F, Ariotti S, et al. Impact of proton pump inhibitors on clinical outcomes in patients treated with a 6- or 24-month dual-antiplatelet therapy duration: insights from the PROlonging Dual-antiplatelet treatment after Grading stent-induced Intimal hyperplasia studY trial. Am Heart J. 2016;174:95-102.
6. Burkard T, Kaiser CA, Brunner-La Rocca H, et al. Combined clopidogrel and proton pump inhibitor therapy is associated with higher cardiovascular event rates after percutaneous coronary intervention: a report from the BASKET trial. J Intern Med. 2012;271:257-263.
1. Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363:1909-1917.
2. Goodman SG, Clare R, Pieper KS, et al. Association of proton pump inhibitor use on cardiovascular outcomes with clopidogrel and ticagrelor: insights from the platelet inhibition and patient outcomes trial. Circulation. 2012;125:978-986.
3. O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet. 2009;374:989-997.
4. Nicolau JC, Bhatt DL, Roe MT, et al. Concomitant proton-pump inhibitor use, platelet activity, and clinical outcomes in patients with acute coronary syndromes treated with prasugrel versus clopidogrel and managed without revascularization: insights from the Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes trial. Am Heart J. 2015;170:683-694.e3.
5. Gargiulo G, Costa F, Ariotti S, et al. Impact of proton pump inhibitors on clinical outcomes in patients treated with a 6- or 24-month dual-antiplatelet therapy duration: insights from the PROlonging Dual-antiplatelet treatment after Grading stent-induced Intimal hyperplasia studY trial. Am Heart J. 2016;174:95-102.
6. Burkard T, Kaiser CA, Brunner-La Rocca H, et al. Combined clopidogrel and proton pump inhibitor therapy is associated with higher cardiovascular event rates after percutaneous coronary intervention: a report from the BASKET trial. J Intern Med. 2012;271:257-263.
EVIDENCE-BASED ANSWER:
No. Adding a proton pump inhibitor (PPI) in patients taking antiplatelet medications such as clopidogrel for acute coronary syndrome (ACS) doesn’t increase the composite risk of cardiovascular (CV) events: CV death, myocardial infarction (MI), and cerebrovascular accident (CVA) (strength of recommendation: B, randomized, controlled trial [RCT] and prepon-derance of posthoc analyses of large RCTs).
Does vitamin D supplementation reduce asthma exacerbations?
EVIDENCE SUMMARY
A Cochrane systematic review of vitamin D for managing asthma performed meta-analyses on RCTs that evaluated several outcomes.1 The review found improvement in the primary outcome of asthma exacerbations requiring systemic steroids, mainly in adult patients, and in the secondary outcomes of emergency department visits or hospitalization, in a mix of adults and children (TABLE1-6).
Most participants had mild-to-moderate asthma; trials lasted 4 to 12 months. Vitamin D dosage regimens varied, with a median daily dose of 900 IU/d (range, 400-4000 IU/d). Six RCTs were rated high-quality, and 1 had unclear risk of bias.
Supplementation reduced exacerbations in patients with low vitamin D levels
A subsequent (2017) systematic review and meta-analysis evaluating the primary outcome of exacerbations requiring steroids7 included another study8 (in addition to the 6 RCTs in the Cochrane review).
When researchers reanalyzed individual participant data from the trials in the Cochrane review, plus the additional RCT, to include baseline vitamin D levels, they found that vitamin D supplementation reduced exacerbations overall (NNT = 7.7) and in patients with low baseline vitamin D levels (25[OH] vitamin D < 25 nmol/L; 92 participants in 3 RCTs; NNT = 4.3) but not in patients with higher baseline levels (764 participants in 6 RCTs). Vitamin D supplementation reduced the asthma exacerbation rate in patients with low baseline vitamin D levels (0.19 vs 0.42 events per participant-year; P = .046).
Smaller benefit found on ED visits and hospitalizations
The Cochrane review, with 2 RCTs with adults (n = 658)1 and 5 RCTs with children (n = 305),2-6 evaluated whether Vitamin D reduced the need for emergency department visits and hospitalization with asthma exacerbations; they found a smaller benefit (NNT = 26.3).
Effects on FEV1, daily asthma symptoms, and serious adverse effects
Several RCTs included in the 2017 meta-analysis found no effect of vitamin D supplementation on FEV1, daily asthma symptoms (evaluated with the standardized Asthma Control Test Score), or reported serious adverse events.2-6,9,10 No deaths occurred in any trial.
Additional findings in children from lower-quality studies
A 2015 systematic review and meta-analysis of RCTs evaluating vitamin D supplementation for children with asthma found11:
- moderate-quality evidence for decreased emergency department visits (1 RCT from India, 100 children ages 3 to 14 years, decrease not specified; P = .015);
- low-quality evidence for reduced exacerbations (6 RCTs [3 RCTs also in Cochrane review], 507 children ages 3 to 17 years; risk ratio = 0.41; 95% confidence interval, 0.27-0.63); and
- low-quality evidence for reduced standardized asthma symptom scores (6 RCTs [2 RCTs also in Cochrane review], 231 children ages 3 to 17 years; amount of reduction not listed; P = .01).
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
No published guidelines discuss using vitamin D in managing asthma. An American Academy of Family Physicians (AAFP) summary of the Cochrane systematic review recommends that family physicians await further studies and updated guidelines before recommending vitamin D for patients with asthma.12 The AAFP also points out that the Endocrine Society has recommended vitamin D supplementation for adults (1500-2000 IU/d) and children (at least 1000 IU/d) at risk for deficiency.
Editor's takeaway
In the meta-analyses highlighted here, researchers evaluated asthma patients with a wide range of ages, baseline vitamin D levels, and vitamin D supplementation protocols. Although vitamin D reduced asthma exacerbations requiring steroids overall, the effect was driven by 3 studies of patients with low baseline vitamin D levels. As a result, disentangling who might benefit the most remains a challenge. The conservative course for now is to manage asthma according to current guidelines and supplement vitamin D in patients at risk for, or with known, deficiency.
, , , . Vitamin D for the management of asthma. Cochrane Database Syst Rev. 2016;9:CD011511.
2. Jensen M, Mailhot G, Alos N, et al. Vitamin D intervention in preschoolers with viral-induced asthma (DIVA): a pilot randomised controlled trial. Trials. 2016;26:17:353.
, , , et al. Correlation of vitamin D with Foxp3 induction and steroid-sparing effect of immunotherapy in asthmatic children. Ann Allergy Asthma Immunol. 2012;109:329-335.
, , , et al. Vitamin D supplementation in children may prevent asthma exacerbation triggered by acute respiratory infection. J Allergy Clin Immunol. 2011;127:1294-1296.
, , , et al. Improved control of childhood asthma with low-dose, short-term vitamin D supplementation: a randomized, double-blind, placebo-controlled trial. Allergy. 2016;71:1001-1009.
, , , et al. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in school children. Am J Clin Nutr. 2010;91:1255-1260.
7. Joliffe DA, Greenberg L, Hooper RL, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet 2017;5:881-890.
8. Kerley CP, Hutchinson K, Cormical L, et al. Vitamin D3 for uncontrolled childhood asthma: a pilot study. Pediatr Allergy Immunol. 2016;27:404-412.
, , , et al. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: the VIDA randomized clinical trial. JAMA. 2014;311:2083-2091.
, , , et al. Double-blind multi-centre randomised controlled trial of vitamin D3 supplementation in adults with inhaled corticosteroid-treated asthma (ViDiAs). Thorax. 2015:70:451-457.
11. Riverin B, Maguire J, Li P. Vitamin D supplementation for childhood asthma: a systematic review and meta-analysis. PLOS One. 2015;10:e0136841.
EVIDENCE SUMMARY
A Cochrane systematic review of vitamin D for managing asthma performed meta-analyses on RCTs that evaluated several outcomes.1 The review found improvement in the primary outcome of asthma exacerbations requiring systemic steroids, mainly in adult patients, and in the secondary outcomes of emergency department visits or hospitalization, in a mix of adults and children (TABLE1-6).
Most participants had mild-to-moderate asthma; trials lasted 4 to 12 months. Vitamin D dosage regimens varied, with a median daily dose of 900 IU/d (range, 400-4000 IU/d). Six RCTs were rated high-quality, and 1 had unclear risk of bias.
Supplementation reduced exacerbations in patients with low vitamin D levels
A subsequent (2017) systematic review and meta-analysis evaluating the primary outcome of exacerbations requiring steroids7 included another study8 (in addition to the 6 RCTs in the Cochrane review).
When researchers reanalyzed individual participant data from the trials in the Cochrane review, plus the additional RCT, to include baseline vitamin D levels, they found that vitamin D supplementation reduced exacerbations overall (NNT = 7.7) and in patients with low baseline vitamin D levels (25[OH] vitamin D < 25 nmol/L; 92 participants in 3 RCTs; NNT = 4.3) but not in patients with higher baseline levels (764 participants in 6 RCTs). Vitamin D supplementation reduced the asthma exacerbation rate in patients with low baseline vitamin D levels (0.19 vs 0.42 events per participant-year; P = .046).
Smaller benefit found on ED visits and hospitalizations
The Cochrane review, with 2 RCTs with adults (n = 658)1 and 5 RCTs with children (n = 305),2-6 evaluated whether Vitamin D reduced the need for emergency department visits and hospitalization with asthma exacerbations; they found a smaller benefit (NNT = 26.3).
Effects on FEV1, daily asthma symptoms, and serious adverse effects
Several RCTs included in the 2017 meta-analysis found no effect of vitamin D supplementation on FEV1, daily asthma symptoms (evaluated with the standardized Asthma Control Test Score), or reported serious adverse events.2-6,9,10 No deaths occurred in any trial.
Additional findings in children from lower-quality studies
A 2015 systematic review and meta-analysis of RCTs evaluating vitamin D supplementation for children with asthma found11:
- moderate-quality evidence for decreased emergency department visits (1 RCT from India, 100 children ages 3 to 14 years, decrease not specified; P = .015);
- low-quality evidence for reduced exacerbations (6 RCTs [3 RCTs also in Cochrane review], 507 children ages 3 to 17 years; risk ratio = 0.41; 95% confidence interval, 0.27-0.63); and
- low-quality evidence for reduced standardized asthma symptom scores (6 RCTs [2 RCTs also in Cochrane review], 231 children ages 3 to 17 years; amount of reduction not listed; P = .01).
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
No published guidelines discuss using vitamin D in managing asthma. An American Academy of Family Physicians (AAFP) summary of the Cochrane systematic review recommends that family physicians await further studies and updated guidelines before recommending vitamin D for patients with asthma.12 The AAFP also points out that the Endocrine Society has recommended vitamin D supplementation for adults (1500-2000 IU/d) and children (at least 1000 IU/d) at risk for deficiency.
Editor's takeaway
In the meta-analyses highlighted here, researchers evaluated asthma patients with a wide range of ages, baseline vitamin D levels, and vitamin D supplementation protocols. Although vitamin D reduced asthma exacerbations requiring steroids overall, the effect was driven by 3 studies of patients with low baseline vitamin D levels. As a result, disentangling who might benefit the most remains a challenge. The conservative course for now is to manage asthma according to current guidelines and supplement vitamin D in patients at risk for, or with known, deficiency.
EVIDENCE SUMMARY
A Cochrane systematic review of vitamin D for managing asthma performed meta-analyses on RCTs that evaluated several outcomes.1 The review found improvement in the primary outcome of asthma exacerbations requiring systemic steroids, mainly in adult patients, and in the secondary outcomes of emergency department visits or hospitalization, in a mix of adults and children (TABLE1-6).
Most participants had mild-to-moderate asthma; trials lasted 4 to 12 months. Vitamin D dosage regimens varied, with a median daily dose of 900 IU/d (range, 400-4000 IU/d). Six RCTs were rated high-quality, and 1 had unclear risk of bias.
Supplementation reduced exacerbations in patients with low vitamin D levels
A subsequent (2017) systematic review and meta-analysis evaluating the primary outcome of exacerbations requiring steroids7 included another study8 (in addition to the 6 RCTs in the Cochrane review).
When researchers reanalyzed individual participant data from the trials in the Cochrane review, plus the additional RCT, to include baseline vitamin D levels, they found that vitamin D supplementation reduced exacerbations overall (NNT = 7.7) and in patients with low baseline vitamin D levels (25[OH] vitamin D < 25 nmol/L; 92 participants in 3 RCTs; NNT = 4.3) but not in patients with higher baseline levels (764 participants in 6 RCTs). Vitamin D supplementation reduced the asthma exacerbation rate in patients with low baseline vitamin D levels (0.19 vs 0.42 events per participant-year; P = .046).
Smaller benefit found on ED visits and hospitalizations
The Cochrane review, with 2 RCTs with adults (n = 658)1 and 5 RCTs with children (n = 305),2-6 evaluated whether Vitamin D reduced the need for emergency department visits and hospitalization with asthma exacerbations; they found a smaller benefit (NNT = 26.3).
Effects on FEV1, daily asthma symptoms, and serious adverse effects
Several RCTs included in the 2017 meta-analysis found no effect of vitamin D supplementation on FEV1, daily asthma symptoms (evaluated with the standardized Asthma Control Test Score), or reported serious adverse events.2-6,9,10 No deaths occurred in any trial.
Additional findings in children from lower-quality studies
A 2015 systematic review and meta-analysis of RCTs evaluating vitamin D supplementation for children with asthma found11:
- moderate-quality evidence for decreased emergency department visits (1 RCT from India, 100 children ages 3 to 14 years, decrease not specified; P = .015);
- low-quality evidence for reduced exacerbations (6 RCTs [3 RCTs also in Cochrane review], 507 children ages 3 to 17 years; risk ratio = 0.41; 95% confidence interval, 0.27-0.63); and
- low-quality evidence for reduced standardized asthma symptom scores (6 RCTs [2 RCTs also in Cochrane review], 231 children ages 3 to 17 years; amount of reduction not listed; P = .01).
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
No published guidelines discuss using vitamin D in managing asthma. An American Academy of Family Physicians (AAFP) summary of the Cochrane systematic review recommends that family physicians await further studies and updated guidelines before recommending vitamin D for patients with asthma.12 The AAFP also points out that the Endocrine Society has recommended vitamin D supplementation for adults (1500-2000 IU/d) and children (at least 1000 IU/d) at risk for deficiency.
Editor's takeaway
In the meta-analyses highlighted here, researchers evaluated asthma patients with a wide range of ages, baseline vitamin D levels, and vitamin D supplementation protocols. Although vitamin D reduced asthma exacerbations requiring steroids overall, the effect was driven by 3 studies of patients with low baseline vitamin D levels. As a result, disentangling who might benefit the most remains a challenge. The conservative course for now is to manage asthma according to current guidelines and supplement vitamin D in patients at risk for, or with known, deficiency.
, , , . Vitamin D for the management of asthma. Cochrane Database Syst Rev. 2016;9:CD011511.
2. Jensen M, Mailhot G, Alos N, et al. Vitamin D intervention in preschoolers with viral-induced asthma (DIVA): a pilot randomised controlled trial. Trials. 2016;26:17:353.
, , , et al. Correlation of vitamin D with Foxp3 induction and steroid-sparing effect of immunotherapy in asthmatic children. Ann Allergy Asthma Immunol. 2012;109:329-335.
, , , et al. Vitamin D supplementation in children may prevent asthma exacerbation triggered by acute respiratory infection. J Allergy Clin Immunol. 2011;127:1294-1296.
, , , et al. Improved control of childhood asthma with low-dose, short-term vitamin D supplementation: a randomized, double-blind, placebo-controlled trial. Allergy. 2016;71:1001-1009.
, , , et al. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in school children. Am J Clin Nutr. 2010;91:1255-1260.
7. Joliffe DA, Greenberg L, Hooper RL, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet 2017;5:881-890.
8. Kerley CP, Hutchinson K, Cormical L, et al. Vitamin D3 for uncontrolled childhood asthma: a pilot study. Pediatr Allergy Immunol. 2016;27:404-412.
, , , et al. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: the VIDA randomized clinical trial. JAMA. 2014;311:2083-2091.
, , , et al. Double-blind multi-centre randomised controlled trial of vitamin D3 supplementation in adults with inhaled corticosteroid-treated asthma (ViDiAs). Thorax. 2015:70:451-457.
11. Riverin B, Maguire J, Li P. Vitamin D supplementation for childhood asthma: a systematic review and meta-analysis. PLOS One. 2015;10:e0136841.
, , , . Vitamin D for the management of asthma. Cochrane Database Syst Rev. 2016;9:CD011511.
2. Jensen M, Mailhot G, Alos N, et al. Vitamin D intervention in preschoolers with viral-induced asthma (DIVA): a pilot randomised controlled trial. Trials. 2016;26:17:353.
, , , et al. Correlation of vitamin D with Foxp3 induction and steroid-sparing effect of immunotherapy in asthmatic children. Ann Allergy Asthma Immunol. 2012;109:329-335.
, , , et al. Vitamin D supplementation in children may prevent asthma exacerbation triggered by acute respiratory infection. J Allergy Clin Immunol. 2011;127:1294-1296.
, , , et al. Improved control of childhood asthma with low-dose, short-term vitamin D supplementation: a randomized, double-blind, placebo-controlled trial. Allergy. 2016;71:1001-1009.
, , , et al. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in school children. Am J Clin Nutr. 2010;91:1255-1260.
7. Joliffe DA, Greenberg L, Hooper RL, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet 2017;5:881-890.
8. Kerley CP, Hutchinson K, Cormical L, et al. Vitamin D3 for uncontrolled childhood asthma: a pilot study. Pediatr Allergy Immunol. 2016;27:404-412.
, , , et al. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: the VIDA randomized clinical trial. JAMA. 2014;311:2083-2091.
, , , et al. Double-blind multi-centre randomised controlled trial of vitamin D3 supplementation in adults with inhaled corticosteroid-treated asthma (ViDiAs). Thorax. 2015:70:451-457.
11. Riverin B, Maguire J, Li P. Vitamin D supplementation for childhood asthma: a systematic review and meta-analysis. PLOS One. 2015;10:e0136841.
EVIDENCE-BASED ANSWER:
Yes, to some extent it does, and primarily in patients with low vitamin D levels. Supplementation reduces asthma exacerbations requiring systemic steroids by 30% overall in adults and children with mild-to-moderate asthma (number needed to treat [NNT] = 7.7). The outcome is driven by the effect in patients with vitamin D levels < 25 nmol/L (NNT = 4.3), however; supplementation doesn’t decrease exacerbations in patients with higher levels. Supplementation also reduces, by a smaller amount (NNT = 26.3), the odds of exacerbations requiring emergency department care or hospitalization (strength of recommendation [SOR]: A, meta-analysis of randomized controlled trials [RCTs]).
In children, vitamin D supplementation may also reduce exacerbations and improve symptom scores (SOR: C, low-quality RCTs).
Vitamin D doesn’t improve forced expiratory volume in 1 second (FEV1) or standardized asthma control test scores. Also, it isn’t associated with serious adverse effects (SOR: A, meta-analysis of RCTs).
Does screening by primary care providers effectively detect melanoma and other skin cancers?
EVIDENCE SUMMARY
No trials have directly assessed skin cancer morbidity associated with physician visual skin screening. A 2018 ecologic cohort study found no difference in melanoma mortality in a population undergoing a national screening program, although screening was associated with 41% more diagnoses of skin cancer.1 A 2012 cohort study found a reduction in melanoma mortality over 7 years associated with a population-based visual skin cancer screening program compared with similar populations that didn’t undergo specific screening.2 At 12-year follow-up, however, there was no longer a difference in mortality.
Primary care visual screening doesn’t decrease melanoma mortality
German researchers trained 1673 non-dermatologists (64% of general practitioners, obstetrician-gynecologists, and urologists in that region of Germany) and 116 dermatologists (98% in the region) to recognize skin cancer through whole-body visual inspection.1 They recruited and screened 360,000 adults (19% of the population older than 20 years; 74% women) and followed age- and sex-adjusted melanoma mortality over the next 10 years. Non-dermatologists performed most screening exams (77%); 37% of screened positive patients were lost to follow-up.
Melanoma mortality ultimately didn’t change in the screened region, compared with populations in other European countries without national screening programs. Screening detected approximately half of melanoma cases (585/1169) in the region and was associated with 41% greater detection of skin cancers compared with other countries.
Researchers recorded age-adjusted increases in incidence per 100,000 of melanoma from 14.2 (95% confidence interval [CI], 13.3-15.1) to 18 (95% CI, 16.6-19.4), melanoma in situ from 5.8 (95% CI, 5.2-6.4) to 8.5 (95% CI, 7.5-9.5), squamous cell carcinoma from 11.2 (95% CI, 10.6-11.8) to 12.9 (95% CI, 12.0-13.8), and basal cell carcinoma from 60.5 (95% CI, 59.0-62.1) to 78.4 (95% CI, 75.9-80.8).
Visual screening by primary care providers vs screening by dermatologists
A cohort study of 16,383 Australian adults found that visual screening by primary care physicians detected melanoma over 3 years with a sensitivity of 40.2% (95% CIs not supplied) and specificity of 86.1% (95% CI, 85.6-86.6%; positive predictive value = 1.4%).3
A second cohort study, enrolling 7436 adults, that evaluated visual screening by dermatologists and plastic surgeons over 2 years found a sensitivity for melanoma of 49% (95% CI, 34.4-63.7%) and a specificity of 97.6% (95% CI, 97.2-97.9%) with a positive predictive value of 11.9% (95% CI, 7.8-17.2%).4
Visual screening more often detects thinner melanomas
A 3-year case-control study (3762 cases, 3824 controls) that examined the association between visual skin screening by a physician (type of physician not specified) and thickness of melanomas detected found that thin melanomas (≤ 0.75 mm) were more common among screened patients compared with unscreened patients (odds ratio [OR] = 1.38; 95% CI, 1.22-1.56) and thicker melanomas (≥ 0.75 mm) were less common (OR = 0.86; 95% CI, 0.75-0.98).5
Continue to: A systematic review...
A systematic review of 8 observational cohort studies with a total of 200,000 patients found a consistent linear increase in melanoma mortality with increasing tumor thickness.6 The largest study (68,495 patients), which compared melanoma mortality for thinner (< 1 mm) and thicker lesions, reported risk ratios of 2.89 for lesion thicknesses of 1.01 to 2 mm (95% CI, 2.62-3.18); 4.69 for thicknesses of 2.01 to 4 mm (95% CI, 4.24-5.02); and 5.71 for thicknesses > 4 mm (95% CI, 5.10-6.39).
The downside of visual screening: False-positives
The 2012 cohort study, which reported outcomes from 16,000 biopsies performed following visual screening exams, found that 28 biopsies were performed for each diagnosis of melanoma and 9 to 10 biopsies for each basal cell carcinoma.2 Diagnosis rates (number of skin biopsies performed for each case of cancer diagnosed) were equal in men and women for both types of cancer. However, researchers observed more biopsies for each diagnosis of squamous cell carcinoma in women than men (56 vs 28 biopsies per case).
Younger patients underwent more biopsies than older patients for each diagnosis of skin cancer. Women 20 to 34 years of age underwent more biopsies than women 65 years or older for each diagnosis of melanoma (19 additional excisions) and basal cell carcinoma (134 additional excisions). Women 35 to 49 years of age underwent 565 more biopsies for each diagnosis of squamous cell carcinoma than women 65 years or older. Similar patterns applied to men 20 to 34 years of age compared with men 65 years or older (24 additional biopsies per melanoma, 109 per basal cell carcinoma, and 898 per squamous cell carcinoma).
RECOMMENDATIONS
The US Preventive Services Task Force recommendations, based on a systematic review of mostly cohort studies, state that the current evidence is insufficient to assess the balance of benefits and harms of clinician visual skin cancer screening.7,8
The American Academy of Dermatology states that skin cancer screening can save lives and supports research on the benefits and harms of screening in the primary care setting.9
Continue to: Editor's Takeaway
Editor’s Takeaway
Skin cancer screening by primary care physicians is associated with increased detection of skin cancers, including melanomas—even though we have no confirmation that it changes melanoma mortality. It is unclear what the appropriate rate of false-positive screening tests should be, but wider adoption of noninvasive diagnostic techniques such as dermoscopy might reduce unwarranted biopsies.
1. Kaiser M, Schiller J, Schreckenberger C. The effectiveness of a population-based skin cancer screening program: evidence from Germany. Eur J Health Econ. 2018:19:355-367.
2. Waldmann A, Nolte S, Weinstock MA, et al. Skin cancer screening participation and impact on melanoma incidence in Germany—an observational study on incidence trends in regions with and without population-based screening. Br J Cancer. 2012;106:970-974.
3. Aitken JF, Janda M, Elwood M, et al. Clinical outcomes from skin screening clinics within a community-based melanoma screening program. J Am Acad Dermatol. 2006:54:105-114.
4. Fritschi L, Dye SA, Katris P. Validity of melanoma diagnosis in a community-based screening program. Am J Epidemiol. 2006:164:385-390.
5. Aitken JF, Elwood M, Baade PD, et al. Clinical whole-body skin examination reduces the incidence of thick melanomas. Int J Cancer. 2010:126:450-458.
6. Wernli KJ, Henrikson NB, Morrison CC, et al. Screening for Skin Cancer in Adults: An Updated Systematic Evidence Review for the US Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality; 2016. Evidence Synthesis 137.
7. Waldmann A, Nolte S, Geller AC, et al. Frequency of excisions and yields of malignant skin tumors in a population-based screening intervention of 360,288 whole-body examinations. Arch Dermatol. 2012:148:903-910.
8. US Preventive Services Task Force. Screening for Skin Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:429-435.
9. Torres A. AAD statement on USPSTF recommendation on skin cancer screening. July 2016. https://www.aad.org/media/news-releases/aad-statement-on-uspstf 26. Accessed May 2018.
EVIDENCE SUMMARY
No trials have directly assessed skin cancer morbidity associated with physician visual skin screening. A 2018 ecologic cohort study found no difference in melanoma mortality in a population undergoing a national screening program, although screening was associated with 41% more diagnoses of skin cancer.1 A 2012 cohort study found a reduction in melanoma mortality over 7 years associated with a population-based visual skin cancer screening program compared with similar populations that didn’t undergo specific screening.2 At 12-year follow-up, however, there was no longer a difference in mortality.
Primary care visual screening doesn’t decrease melanoma mortality
German researchers trained 1673 non-dermatologists (64% of general practitioners, obstetrician-gynecologists, and urologists in that region of Germany) and 116 dermatologists (98% in the region) to recognize skin cancer through whole-body visual inspection.1 They recruited and screened 360,000 adults (19% of the population older than 20 years; 74% women) and followed age- and sex-adjusted melanoma mortality over the next 10 years. Non-dermatologists performed most screening exams (77%); 37% of screened positive patients were lost to follow-up.
Melanoma mortality ultimately didn’t change in the screened region, compared with populations in other European countries without national screening programs. Screening detected approximately half of melanoma cases (585/1169) in the region and was associated with 41% greater detection of skin cancers compared with other countries.
Researchers recorded age-adjusted increases in incidence per 100,000 of melanoma from 14.2 (95% confidence interval [CI], 13.3-15.1) to 18 (95% CI, 16.6-19.4), melanoma in situ from 5.8 (95% CI, 5.2-6.4) to 8.5 (95% CI, 7.5-9.5), squamous cell carcinoma from 11.2 (95% CI, 10.6-11.8) to 12.9 (95% CI, 12.0-13.8), and basal cell carcinoma from 60.5 (95% CI, 59.0-62.1) to 78.4 (95% CI, 75.9-80.8).
Visual screening by primary care providers vs screening by dermatologists
A cohort study of 16,383 Australian adults found that visual screening by primary care physicians detected melanoma over 3 years with a sensitivity of 40.2% (95% CIs not supplied) and specificity of 86.1% (95% CI, 85.6-86.6%; positive predictive value = 1.4%).3
A second cohort study, enrolling 7436 adults, that evaluated visual screening by dermatologists and plastic surgeons over 2 years found a sensitivity for melanoma of 49% (95% CI, 34.4-63.7%) and a specificity of 97.6% (95% CI, 97.2-97.9%) with a positive predictive value of 11.9% (95% CI, 7.8-17.2%).4
Visual screening more often detects thinner melanomas
A 3-year case-control study (3762 cases, 3824 controls) that examined the association between visual skin screening by a physician (type of physician not specified) and thickness of melanomas detected found that thin melanomas (≤ 0.75 mm) were more common among screened patients compared with unscreened patients (odds ratio [OR] = 1.38; 95% CI, 1.22-1.56) and thicker melanomas (≥ 0.75 mm) were less common (OR = 0.86; 95% CI, 0.75-0.98).5
Continue to: A systematic review...
A systematic review of 8 observational cohort studies with a total of 200,000 patients found a consistent linear increase in melanoma mortality with increasing tumor thickness.6 The largest study (68,495 patients), which compared melanoma mortality for thinner (< 1 mm) and thicker lesions, reported risk ratios of 2.89 for lesion thicknesses of 1.01 to 2 mm (95% CI, 2.62-3.18); 4.69 for thicknesses of 2.01 to 4 mm (95% CI, 4.24-5.02); and 5.71 for thicknesses > 4 mm (95% CI, 5.10-6.39).
The downside of visual screening: False-positives
The 2012 cohort study, which reported outcomes from 16,000 biopsies performed following visual screening exams, found that 28 biopsies were performed for each diagnosis of melanoma and 9 to 10 biopsies for each basal cell carcinoma.2 Diagnosis rates (number of skin biopsies performed for each case of cancer diagnosed) were equal in men and women for both types of cancer. However, researchers observed more biopsies for each diagnosis of squamous cell carcinoma in women than men (56 vs 28 biopsies per case).
Younger patients underwent more biopsies than older patients for each diagnosis of skin cancer. Women 20 to 34 years of age underwent more biopsies than women 65 years or older for each diagnosis of melanoma (19 additional excisions) and basal cell carcinoma (134 additional excisions). Women 35 to 49 years of age underwent 565 more biopsies for each diagnosis of squamous cell carcinoma than women 65 years or older. Similar patterns applied to men 20 to 34 years of age compared with men 65 years or older (24 additional biopsies per melanoma, 109 per basal cell carcinoma, and 898 per squamous cell carcinoma).
RECOMMENDATIONS
The US Preventive Services Task Force recommendations, based on a systematic review of mostly cohort studies, state that the current evidence is insufficient to assess the balance of benefits and harms of clinician visual skin cancer screening.7,8
The American Academy of Dermatology states that skin cancer screening can save lives and supports research on the benefits and harms of screening in the primary care setting.9
Continue to: Editor's Takeaway
Editor’s Takeaway
Skin cancer screening by primary care physicians is associated with increased detection of skin cancers, including melanomas—even though we have no confirmation that it changes melanoma mortality. It is unclear what the appropriate rate of false-positive screening tests should be, but wider adoption of noninvasive diagnostic techniques such as dermoscopy might reduce unwarranted biopsies.
EVIDENCE SUMMARY
No trials have directly assessed skin cancer morbidity associated with physician visual skin screening. A 2018 ecologic cohort study found no difference in melanoma mortality in a population undergoing a national screening program, although screening was associated with 41% more diagnoses of skin cancer.1 A 2012 cohort study found a reduction in melanoma mortality over 7 years associated with a population-based visual skin cancer screening program compared with similar populations that didn’t undergo specific screening.2 At 12-year follow-up, however, there was no longer a difference in mortality.
Primary care visual screening doesn’t decrease melanoma mortality
German researchers trained 1673 non-dermatologists (64% of general practitioners, obstetrician-gynecologists, and urologists in that region of Germany) and 116 dermatologists (98% in the region) to recognize skin cancer through whole-body visual inspection.1 They recruited and screened 360,000 adults (19% of the population older than 20 years; 74% women) and followed age- and sex-adjusted melanoma mortality over the next 10 years. Non-dermatologists performed most screening exams (77%); 37% of screened positive patients were lost to follow-up.
Melanoma mortality ultimately didn’t change in the screened region, compared with populations in other European countries without national screening programs. Screening detected approximately half of melanoma cases (585/1169) in the region and was associated with 41% greater detection of skin cancers compared with other countries.
Researchers recorded age-adjusted increases in incidence per 100,000 of melanoma from 14.2 (95% confidence interval [CI], 13.3-15.1) to 18 (95% CI, 16.6-19.4), melanoma in situ from 5.8 (95% CI, 5.2-6.4) to 8.5 (95% CI, 7.5-9.5), squamous cell carcinoma from 11.2 (95% CI, 10.6-11.8) to 12.9 (95% CI, 12.0-13.8), and basal cell carcinoma from 60.5 (95% CI, 59.0-62.1) to 78.4 (95% CI, 75.9-80.8).
Visual screening by primary care providers vs screening by dermatologists
A cohort study of 16,383 Australian adults found that visual screening by primary care physicians detected melanoma over 3 years with a sensitivity of 40.2% (95% CIs not supplied) and specificity of 86.1% (95% CI, 85.6-86.6%; positive predictive value = 1.4%).3
A second cohort study, enrolling 7436 adults, that evaluated visual screening by dermatologists and plastic surgeons over 2 years found a sensitivity for melanoma of 49% (95% CI, 34.4-63.7%) and a specificity of 97.6% (95% CI, 97.2-97.9%) with a positive predictive value of 11.9% (95% CI, 7.8-17.2%).4
Visual screening more often detects thinner melanomas
A 3-year case-control study (3762 cases, 3824 controls) that examined the association between visual skin screening by a physician (type of physician not specified) and thickness of melanomas detected found that thin melanomas (≤ 0.75 mm) were more common among screened patients compared with unscreened patients (odds ratio [OR] = 1.38; 95% CI, 1.22-1.56) and thicker melanomas (≥ 0.75 mm) were less common (OR = 0.86; 95% CI, 0.75-0.98).5
Continue to: A systematic review...
A systematic review of 8 observational cohort studies with a total of 200,000 patients found a consistent linear increase in melanoma mortality with increasing tumor thickness.6 The largest study (68,495 patients), which compared melanoma mortality for thinner (< 1 mm) and thicker lesions, reported risk ratios of 2.89 for lesion thicknesses of 1.01 to 2 mm (95% CI, 2.62-3.18); 4.69 for thicknesses of 2.01 to 4 mm (95% CI, 4.24-5.02); and 5.71 for thicknesses > 4 mm (95% CI, 5.10-6.39).
The downside of visual screening: False-positives
The 2012 cohort study, which reported outcomes from 16,000 biopsies performed following visual screening exams, found that 28 biopsies were performed for each diagnosis of melanoma and 9 to 10 biopsies for each basal cell carcinoma.2 Diagnosis rates (number of skin biopsies performed for each case of cancer diagnosed) were equal in men and women for both types of cancer. However, researchers observed more biopsies for each diagnosis of squamous cell carcinoma in women than men (56 vs 28 biopsies per case).
Younger patients underwent more biopsies than older patients for each diagnosis of skin cancer. Women 20 to 34 years of age underwent more biopsies than women 65 years or older for each diagnosis of melanoma (19 additional excisions) and basal cell carcinoma (134 additional excisions). Women 35 to 49 years of age underwent 565 more biopsies for each diagnosis of squamous cell carcinoma than women 65 years or older. Similar patterns applied to men 20 to 34 years of age compared with men 65 years or older (24 additional biopsies per melanoma, 109 per basal cell carcinoma, and 898 per squamous cell carcinoma).
RECOMMENDATIONS
The US Preventive Services Task Force recommendations, based on a systematic review of mostly cohort studies, state that the current evidence is insufficient to assess the balance of benefits and harms of clinician visual skin cancer screening.7,8
The American Academy of Dermatology states that skin cancer screening can save lives and supports research on the benefits and harms of screening in the primary care setting.9
Continue to: Editor's Takeaway
Editor’s Takeaway
Skin cancer screening by primary care physicians is associated with increased detection of skin cancers, including melanomas—even though we have no confirmation that it changes melanoma mortality. It is unclear what the appropriate rate of false-positive screening tests should be, but wider adoption of noninvasive diagnostic techniques such as dermoscopy might reduce unwarranted biopsies.
1. Kaiser M, Schiller J, Schreckenberger C. The effectiveness of a population-based skin cancer screening program: evidence from Germany. Eur J Health Econ. 2018:19:355-367.
2. Waldmann A, Nolte S, Weinstock MA, et al. Skin cancer screening participation and impact on melanoma incidence in Germany—an observational study on incidence trends in regions with and without population-based screening. Br J Cancer. 2012;106:970-974.
3. Aitken JF, Janda M, Elwood M, et al. Clinical outcomes from skin screening clinics within a community-based melanoma screening program. J Am Acad Dermatol. 2006:54:105-114.
4. Fritschi L, Dye SA, Katris P. Validity of melanoma diagnosis in a community-based screening program. Am J Epidemiol. 2006:164:385-390.
5. Aitken JF, Elwood M, Baade PD, et al. Clinical whole-body skin examination reduces the incidence of thick melanomas. Int J Cancer. 2010:126:450-458.
6. Wernli KJ, Henrikson NB, Morrison CC, et al. Screening for Skin Cancer in Adults: An Updated Systematic Evidence Review for the US Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality; 2016. Evidence Synthesis 137.
7. Waldmann A, Nolte S, Geller AC, et al. Frequency of excisions and yields of malignant skin tumors in a population-based screening intervention of 360,288 whole-body examinations. Arch Dermatol. 2012:148:903-910.
8. US Preventive Services Task Force. Screening for Skin Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:429-435.
9. Torres A. AAD statement on USPSTF recommendation on skin cancer screening. July 2016. https://www.aad.org/media/news-releases/aad-statement-on-uspstf 26. Accessed May 2018.
1. Kaiser M, Schiller J, Schreckenberger C. The effectiveness of a population-based skin cancer screening program: evidence from Germany. Eur J Health Econ. 2018:19:355-367.
2. Waldmann A, Nolte S, Weinstock MA, et al. Skin cancer screening participation and impact on melanoma incidence in Germany—an observational study on incidence trends in regions with and without population-based screening. Br J Cancer. 2012;106:970-974.
3. Aitken JF, Janda M, Elwood M, et al. Clinical outcomes from skin screening clinics within a community-based melanoma screening program. J Am Acad Dermatol. 2006:54:105-114.
4. Fritschi L, Dye SA, Katris P. Validity of melanoma diagnosis in a community-based screening program. Am J Epidemiol. 2006:164:385-390.
5. Aitken JF, Elwood M, Baade PD, et al. Clinical whole-body skin examination reduces the incidence of thick melanomas. Int J Cancer. 2010:126:450-458.
6. Wernli KJ, Henrikson NB, Morrison CC, et al. Screening for Skin Cancer in Adults: An Updated Systematic Evidence Review for the US Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality; 2016. Evidence Synthesis 137.
7. Waldmann A, Nolte S, Geller AC, et al. Frequency of excisions and yields of malignant skin tumors in a population-based screening intervention of 360,288 whole-body examinations. Arch Dermatol. 2012:148:903-910.
8. US Preventive Services Task Force. Screening for Skin Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:429-435.
9. Torres A. AAD statement on USPSTF recommendation on skin cancer screening. July 2016. https://www.aad.org/media/news-releases/aad-statement-on-uspstf 26. Accessed May 2018.
EVIDENCE-BASED ANSWER:
Possibly. No trials have directly assessed detection of melanoma and other skin cancers by primary care providers.
Training a group comprised largely of primary care physicians to perform skin cancer screening was associated with a 41% increase in skin cancer diagnoses but no change in melanoma mortality.
Visual screening for melanoma by primary care physicians is 40% sensitive and 86% specific (compared with 49% and 98%, respectively, for dermatologists and plastic surgeons).
Melanomas found by visual screening are 38% more likely to be thin (≤ 0.75 mm) than melanomas discovered without screening, which correlates with improved outcomes.
Visual skin cancer screening overall is associated with false-positive rates as follows: 28 biopsies for each melanoma detected, 9 to 10 biopsies for each basal cell carcinoma, and 28 to 56 biopsies for squamous cell carcinoma. False-positive rates are higher for women—as much as double the rate for men—and younger patients—as much as 20-fold the rate for older patients (strength of recommendations for all foregoing statements: B, cohort studies).
Does using e-cigarettes increase cigarette smoking in adolescents?
EVIDENCE SUMMARY
A meta-analysis of 9 prospective cohort studies (total 17,389 patients) at least 6 months in duration evaluated the association between e-cigarette exposure and subsequent cigarette smoking in adolescents and young adults.1 It found that smoking was more prevalent in ever-users of e-cigarettes than nonusers at 1 year (23.3% vs 7.2%; odds ratio [OR] = 3.5; 95% confidence interval [CI], 2.38-5.16). The association was even stronger among recent users (within 30 days) of e-cigarettes compared with nonusers (21.5% vs 4.6%; OR = 4.28; 95% CI, 2.52-7.27). The mean age of approximately 80% of participants was 20 years or younger.
Further studies also support a link between e-cigarette and cigarette use
Four subsequent cohort studies also found links between e-cigarette exposure and any level of cigarette smoking (TABLE).2-5 A Canadian study of high school students reported a positive association between recent e-cigarette use (within the previous 30 days) and subsequent daily cigarette usage (OR = 1.79; 95% CI, 1.41-2.28).2 A British study that documented the largest association uniquely validated smoking status with carbon monoxide testing.3 A study of Mexican adolescents found that adolescents who tried e-cigarettes were more likely to smoke cigarettes and also reported an association between e-cigarette use and marijuana use (relative risk [RR] = 1.93; 95% CI, 1.14–3.28).4 A California study that evaluated e-cigarette nicotine level and subsequent cigarette smoking found a dose-dependent response, suggesting an association between nicotine concentration and subsequent uptake of cigarettes.5
RECOMMENDATIONS
A policy statement from The American Academy of Pediatrics Section on Tobacco Control states that youth who use e-cigarettes are more likely to use cigarettes and other tobacco products.6 It recommends that physicians screen patients for use of electronic nicotine delivery systems (ENDS), counsel about immediate and long-term harms and the importance of not using ENDS, and offer current users tobacco cessation counseling (with Food and Drug Administration-approved tobacco dependence treatment).
Editor’s takeaway
While these cohort studies don’t definitively prove causation, they provide the best quality evidence that we are likely to see in support of counseling adolescents against using e-cigarettes, educating them about harms, and offering tobacco cessation measures when appropriate.
1. Soneji S, Barrington-Trimis JL, Willis TA, et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults, a systematic review and meta-analysis. JAMA Pediatr. 2017;171:788-797.
2. Hammond D, Reid JL, Cole AG, et al. Electronic cigarette use and smoking initiation among youth: a longitudinal cohort study. CMAJ. 2017;189:E1328-E1336.
3. Conner M, Grogan S, Simms-Ellis R, et al. Do electronic cigarettes increase cigarette smoking in UK adolescents? Evidence from a 12-month prospective study. Tob Control. 2018;27:365-372.
4. Lozano P, Barrientos-Gutierrez I, Arillo-Santillan E, et al. A longitudinal study of electronic cigarette use and onset of conventional cigarette smoking and marijuana use among Mexican adolescents. Drug Alcohol Depend. 2017;180:427-430.
5. Goldenson NI, Leventhal AM, Stone MD, et al. Associations of electronic cigarette nicotine concentration with subsequent cigarette smoking and vaping levels in adolescents. JAMA Pediatr. 2017;171:1192-1199.
6. Walley SC, Jenssen BP; Section on Tobacco Control. Electronic nicotine delivery systems. Pediatrics. 2015;136:1018-1026.
EVIDENCE SUMMARY
A meta-analysis of 9 prospective cohort studies (total 17,389 patients) at least 6 months in duration evaluated the association between e-cigarette exposure and subsequent cigarette smoking in adolescents and young adults.1 It found that smoking was more prevalent in ever-users of e-cigarettes than nonusers at 1 year (23.3% vs 7.2%; odds ratio [OR] = 3.5; 95% confidence interval [CI], 2.38-5.16). The association was even stronger among recent users (within 30 days) of e-cigarettes compared with nonusers (21.5% vs 4.6%; OR = 4.28; 95% CI, 2.52-7.27). The mean age of approximately 80% of participants was 20 years or younger.
Further studies also support a link between e-cigarette and cigarette use
Four subsequent cohort studies also found links between e-cigarette exposure and any level of cigarette smoking (TABLE).2-5 A Canadian study of high school students reported a positive association between recent e-cigarette use (within the previous 30 days) and subsequent daily cigarette usage (OR = 1.79; 95% CI, 1.41-2.28).2 A British study that documented the largest association uniquely validated smoking status with carbon monoxide testing.3 A study of Mexican adolescents found that adolescents who tried e-cigarettes were more likely to smoke cigarettes and also reported an association between e-cigarette use and marijuana use (relative risk [RR] = 1.93; 95% CI, 1.14–3.28).4 A California study that evaluated e-cigarette nicotine level and subsequent cigarette smoking found a dose-dependent response, suggesting an association between nicotine concentration and subsequent uptake of cigarettes.5
RECOMMENDATIONS
A policy statement from The American Academy of Pediatrics Section on Tobacco Control states that youth who use e-cigarettes are more likely to use cigarettes and other tobacco products.6 It recommends that physicians screen patients for use of electronic nicotine delivery systems (ENDS), counsel about immediate and long-term harms and the importance of not using ENDS, and offer current users tobacco cessation counseling (with Food and Drug Administration-approved tobacco dependence treatment).
Editor’s takeaway
While these cohort studies don’t definitively prove causation, they provide the best quality evidence that we are likely to see in support of counseling adolescents against using e-cigarettes, educating them about harms, and offering tobacco cessation measures when appropriate.
EVIDENCE SUMMARY
A meta-analysis of 9 prospective cohort studies (total 17,389 patients) at least 6 months in duration evaluated the association between e-cigarette exposure and subsequent cigarette smoking in adolescents and young adults.1 It found that smoking was more prevalent in ever-users of e-cigarettes than nonusers at 1 year (23.3% vs 7.2%; odds ratio [OR] = 3.5; 95% confidence interval [CI], 2.38-5.16). The association was even stronger among recent users (within 30 days) of e-cigarettes compared with nonusers (21.5% vs 4.6%; OR = 4.28; 95% CI, 2.52-7.27). The mean age of approximately 80% of participants was 20 years or younger.
Further studies also support a link between e-cigarette and cigarette use
Four subsequent cohort studies also found links between e-cigarette exposure and any level of cigarette smoking (TABLE).2-5 A Canadian study of high school students reported a positive association between recent e-cigarette use (within the previous 30 days) and subsequent daily cigarette usage (OR = 1.79; 95% CI, 1.41-2.28).2 A British study that documented the largest association uniquely validated smoking status with carbon monoxide testing.3 A study of Mexican adolescents found that adolescents who tried e-cigarettes were more likely to smoke cigarettes and also reported an association between e-cigarette use and marijuana use (relative risk [RR] = 1.93; 95% CI, 1.14–3.28).4 A California study that evaluated e-cigarette nicotine level and subsequent cigarette smoking found a dose-dependent response, suggesting an association between nicotine concentration and subsequent uptake of cigarettes.5
RECOMMENDATIONS
A policy statement from The American Academy of Pediatrics Section on Tobacco Control states that youth who use e-cigarettes are more likely to use cigarettes and other tobacco products.6 It recommends that physicians screen patients for use of electronic nicotine delivery systems (ENDS), counsel about immediate and long-term harms and the importance of not using ENDS, and offer current users tobacco cessation counseling (with Food and Drug Administration-approved tobacco dependence treatment).
Editor’s takeaway
While these cohort studies don’t definitively prove causation, they provide the best quality evidence that we are likely to see in support of counseling adolescents against using e-cigarettes, educating them about harms, and offering tobacco cessation measures when appropriate.
1. Soneji S, Barrington-Trimis JL, Willis TA, et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults, a systematic review and meta-analysis. JAMA Pediatr. 2017;171:788-797.
2. Hammond D, Reid JL, Cole AG, et al. Electronic cigarette use and smoking initiation among youth: a longitudinal cohort study. CMAJ. 2017;189:E1328-E1336.
3. Conner M, Grogan S, Simms-Ellis R, et al. Do electronic cigarettes increase cigarette smoking in UK adolescents? Evidence from a 12-month prospective study. Tob Control. 2018;27:365-372.
4. Lozano P, Barrientos-Gutierrez I, Arillo-Santillan E, et al. A longitudinal study of electronic cigarette use and onset of conventional cigarette smoking and marijuana use among Mexican adolescents. Drug Alcohol Depend. 2017;180:427-430.
5. Goldenson NI, Leventhal AM, Stone MD, et al. Associations of electronic cigarette nicotine concentration with subsequent cigarette smoking and vaping levels in adolescents. JAMA Pediatr. 2017;171:1192-1199.
6. Walley SC, Jenssen BP; Section on Tobacco Control. Electronic nicotine delivery systems. Pediatrics. 2015;136:1018-1026.
1. Soneji S, Barrington-Trimis JL, Willis TA, et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults, a systematic review and meta-analysis. JAMA Pediatr. 2017;171:788-797.
2. Hammond D, Reid JL, Cole AG, et al. Electronic cigarette use and smoking initiation among youth: a longitudinal cohort study. CMAJ. 2017;189:E1328-E1336.
3. Conner M, Grogan S, Simms-Ellis R, et al. Do electronic cigarettes increase cigarette smoking in UK adolescents? Evidence from a 12-month prospective study. Tob Control. 2018;27:365-372.
4. Lozano P, Barrientos-Gutierrez I, Arillo-Santillan E, et al. A longitudinal study of electronic cigarette use and onset of conventional cigarette smoking and marijuana use among Mexican adolescents. Drug Alcohol Depend. 2017;180:427-430.
5. Goldenson NI, Leventhal AM, Stone MD, et al. Associations of electronic cigarette nicotine concentration with subsequent cigarette smoking and vaping levels in adolescents. JAMA Pediatr. 2017;171:1192-1199.
6. Walley SC, Jenssen BP; Section on Tobacco Control. Electronic nicotine delivery systems. Pediatrics. 2015;136:1018-1026.
EVIDENCE-BASED ANSWER:
Probably. Electronic cigarette (e-cigarette) use by adolescents is associated with a 2- to 4-fold increase in cigarette smoking over the next year (strength of recommendation: A, meta-analysis and subsequent prospective cohort studies).
Is intra-articular platelet-rich plasma injection an effective treatment for knee OA?
EVIDENCE SUMMARY
PRP vs placebo. Three RCTs compared PRP with saline placebo injections and 2 found that PRP improved the Western Ontario and McMaster Universities Arthritis Index (WOMAC, a standardized scale assessing knee pain, function, and stiffness) by 40% to 70%; the third found 24% to 32% improvements in the EuroQol visual analog scale (EQ-VAS) scores at 6 months1-3 (TABLE1-12).
The first 2 studies enrolled patients (mean age early 60s, approximately 50% women) with clinically and radiographically evaluated knee OA of mostly moderate severity (baseline WOMAC scores about 50).1,2 Investigators in the first RCT injected PRP once in one subgroup and twice in another subgroup, compared with a single injection of saline in a third subgroup.1 They gave 3 weekly injections of PRP or saline in the second RCT.2
The third study enrolled mainly patients with early osteoarthritis (mean age early 50s, slightly more women). Investigators injected PRP 3 times in one subgroup and once (plus 2 saline injections) in another, compared with 3 saline injections, and evaluated patients at baseline and 6 months.3
PRP vs HA. Nine RCTs compared PRP with HA injections. Six studies (673 patients) found no significant difference; 3 studies (376 patients) found that PRP improved standardized knee assessment scores by 35% to 40% at 24-48 weeks.7,8,10 All studies enrolled patients (mean age early 60s, approximately 50% women) with clinically and radiographically evaluated knee OA of mostly moderate severity. In 7 RCTs, 4-6,9-12 investigators injected PRP or HA weekly for 3 weeks, in one RCT8 they gave 4 weekly injections, and in one7they gave 2 PRP injections separated by 4 weeks.
Three RCTs used the International Knee Documentation Committee (IKDC) score, considered the most reliable standardized scoring system, which quantifies subjective symptoms (pain, stiffness, swelling, giving way), activity (climbing stairs, rising from a chair, squatting, jumping), and function pre- and postintervention.5,9,12 All 3 studies using the IKDC found no difference between PRP and HA injections. Most RCTs used the WOMAC standardized scale, scoring 5 items for pain, 2 for stiffness, and 17 for function.1,2,4,6-8.10
Risk for bias
A systematic review13 that evaluated methodologic quality of the 3 studies comparing PRP with placebo rated 21,3 at high risk of bias and one2 at moderate risk. Another meta-analysis14 performed a quality assessment including 4 of the 9 RCTs,8-10,12 comparing PRP with HA and concluded that 3 had a high risk of bias; the fourth RCT had a moderate risk. No independent quality assessments of the other RCTs were available.4-7,11
RECOMMENDATIONS
The American Academy of Orthopaedic Surgeons doesn’t recommend for or against PRP injections because of insufficient evidence and strongly recommends against HA injections based on multiple RCTs of moderate quality that found no difference between HA and placebo.15
1. Patel S, Dhillon MS, Aggarwal S, et al. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41:356-364.
2. Smith PA. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee arthritis: an FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. Am J Sports Med. 2016;44:884-891.
3. Gorelli G, Gormelli CA, Ataoglu B, et al. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. 2015;25:958-965.
4. Cole BJ, Karas V, Hussey K, et al. Hyaluronic acid versus platelet-rich plasma: a prospective double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med. 2016;45:339-346.
5. Filardo G, Di Matteo B, Di Martino A, et al. Platelet-rich intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med. 2015;43:1575-1582.
6. Sanchez M, Fiz N, Azofra J, et al. A randomized clinical trial evaluating plasma rich in growth factors (PGRF-endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy: J Arth and Related Surg. 2012;28:1070-1078.
7. Raeissadat SA, Rayegani SM, Hassanabadi H, et al. Knee osteoarthritis injection choices: platelet-rich plasma (PRP) versus hyaluronic acid (a one-year randomized clinical trial). Clin Med Insights: Arth Musc Dis. 2015;8:1-8.
8. Cerza F, Carni S, Carcangiu A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40:2822-2827.
9. Filardo G, Kon E, Di Martino B, et al. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskeletal Disorders. 2012;13:229-236.
10. Vaquerizo V, Plasencia MA, Arribas I, et al. Comparison of intra-articular injections of plasma rich in growth factors (PGRF-endoret) versus durolane hyaluronic acid in the treatment of patients with symptomatic osteoarthritis: a randomized controlled trial. Arthroscopy: J Arth and Related Surg. 2013;29:1635-1643.
11. Montanez-Heredia E, Irizar S, Huertas PJ, et al. Intra-articular injections of platelet-rich plasma versus hyaluronic acid in the treatment of osteoarthritis knee pain: a randomized clinical trial in the context of the Spanish national health care system. Intl J Molec Sci. 2016;17:1064-1077.
12. Li M, Zhang C, Ai Z, et al. Therapeutic effectiveness of intra-knee articular injections of platelet-rich plasma on knee articular cartilage degeneration. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011 25:1192-11966. (Article published in Chinese with abstract in English.)
13. Shen L, Yuan T, Chen S, et al. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Ortho Surg Res. 2017;12:16.
14. Laudy ABM, Bakker EWP, Rekers M, et al. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015;49:657-672.
15. American Academy of Orthopaedic Surgeons. Clinical practice guideline on the treatment of osteoarthritis of the knee, 2nd ed. www.aaos.org/cc_files/aaosorg/research/guidelines/treatmentofosteoarthritisofthekneeguideline.pdf. Published May 2013. Accessed February 22, 2019.
EVIDENCE SUMMARY
PRP vs placebo. Three RCTs compared PRP with saline placebo injections and 2 found that PRP improved the Western Ontario and McMaster Universities Arthritis Index (WOMAC, a standardized scale assessing knee pain, function, and stiffness) by 40% to 70%; the third found 24% to 32% improvements in the EuroQol visual analog scale (EQ-VAS) scores at 6 months1-3 (TABLE1-12).
The first 2 studies enrolled patients (mean age early 60s, approximately 50% women) with clinically and radiographically evaluated knee OA of mostly moderate severity (baseline WOMAC scores about 50).1,2 Investigators in the first RCT injected PRP once in one subgroup and twice in another subgroup, compared with a single injection of saline in a third subgroup.1 They gave 3 weekly injections of PRP or saline in the second RCT.2
The third study enrolled mainly patients with early osteoarthritis (mean age early 50s, slightly more women). Investigators injected PRP 3 times in one subgroup and once (plus 2 saline injections) in another, compared with 3 saline injections, and evaluated patients at baseline and 6 months.3
PRP vs HA. Nine RCTs compared PRP with HA injections. Six studies (673 patients) found no significant difference; 3 studies (376 patients) found that PRP improved standardized knee assessment scores by 35% to 40% at 24-48 weeks.7,8,10 All studies enrolled patients (mean age early 60s, approximately 50% women) with clinically and radiographically evaluated knee OA of mostly moderate severity. In 7 RCTs, 4-6,9-12 investigators injected PRP or HA weekly for 3 weeks, in one RCT8 they gave 4 weekly injections, and in one7they gave 2 PRP injections separated by 4 weeks.
Three RCTs used the International Knee Documentation Committee (IKDC) score, considered the most reliable standardized scoring system, which quantifies subjective symptoms (pain, stiffness, swelling, giving way), activity (climbing stairs, rising from a chair, squatting, jumping), and function pre- and postintervention.5,9,12 All 3 studies using the IKDC found no difference between PRP and HA injections. Most RCTs used the WOMAC standardized scale, scoring 5 items for pain, 2 for stiffness, and 17 for function.1,2,4,6-8.10
Risk for bias
A systematic review13 that evaluated methodologic quality of the 3 studies comparing PRP with placebo rated 21,3 at high risk of bias and one2 at moderate risk. Another meta-analysis14 performed a quality assessment including 4 of the 9 RCTs,8-10,12 comparing PRP with HA and concluded that 3 had a high risk of bias; the fourth RCT had a moderate risk. No independent quality assessments of the other RCTs were available.4-7,11
RECOMMENDATIONS
The American Academy of Orthopaedic Surgeons doesn’t recommend for or against PRP injections because of insufficient evidence and strongly recommends against HA injections based on multiple RCTs of moderate quality that found no difference between HA and placebo.15
EVIDENCE SUMMARY
PRP vs placebo. Three RCTs compared PRP with saline placebo injections and 2 found that PRP improved the Western Ontario and McMaster Universities Arthritis Index (WOMAC, a standardized scale assessing knee pain, function, and stiffness) by 40% to 70%; the third found 24% to 32% improvements in the EuroQol visual analog scale (EQ-VAS) scores at 6 months1-3 (TABLE1-12).
The first 2 studies enrolled patients (mean age early 60s, approximately 50% women) with clinically and radiographically evaluated knee OA of mostly moderate severity (baseline WOMAC scores about 50).1,2 Investigators in the first RCT injected PRP once in one subgroup and twice in another subgroup, compared with a single injection of saline in a third subgroup.1 They gave 3 weekly injections of PRP or saline in the second RCT.2
The third study enrolled mainly patients with early osteoarthritis (mean age early 50s, slightly more women). Investigators injected PRP 3 times in one subgroup and once (plus 2 saline injections) in another, compared with 3 saline injections, and evaluated patients at baseline and 6 months.3
PRP vs HA. Nine RCTs compared PRP with HA injections. Six studies (673 patients) found no significant difference; 3 studies (376 patients) found that PRP improved standardized knee assessment scores by 35% to 40% at 24-48 weeks.7,8,10 All studies enrolled patients (mean age early 60s, approximately 50% women) with clinically and radiographically evaluated knee OA of mostly moderate severity. In 7 RCTs, 4-6,9-12 investigators injected PRP or HA weekly for 3 weeks, in one RCT8 they gave 4 weekly injections, and in one7they gave 2 PRP injections separated by 4 weeks.
Three RCTs used the International Knee Documentation Committee (IKDC) score, considered the most reliable standardized scoring system, which quantifies subjective symptoms (pain, stiffness, swelling, giving way), activity (climbing stairs, rising from a chair, squatting, jumping), and function pre- and postintervention.5,9,12 All 3 studies using the IKDC found no difference between PRP and HA injections. Most RCTs used the WOMAC standardized scale, scoring 5 items for pain, 2 for stiffness, and 17 for function.1,2,4,6-8.10
Risk for bias
A systematic review13 that evaluated methodologic quality of the 3 studies comparing PRP with placebo rated 21,3 at high risk of bias and one2 at moderate risk. Another meta-analysis14 performed a quality assessment including 4 of the 9 RCTs,8-10,12 comparing PRP with HA and concluded that 3 had a high risk of bias; the fourth RCT had a moderate risk. No independent quality assessments of the other RCTs were available.4-7,11
RECOMMENDATIONS
The American Academy of Orthopaedic Surgeons doesn’t recommend for or against PRP injections because of insufficient evidence and strongly recommends against HA injections based on multiple RCTs of moderate quality that found no difference between HA and placebo.15
1. Patel S, Dhillon MS, Aggarwal S, et al. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41:356-364.
2. Smith PA. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee arthritis: an FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. Am J Sports Med. 2016;44:884-891.
3. Gorelli G, Gormelli CA, Ataoglu B, et al. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. 2015;25:958-965.
4. Cole BJ, Karas V, Hussey K, et al. Hyaluronic acid versus platelet-rich plasma: a prospective double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med. 2016;45:339-346.
5. Filardo G, Di Matteo B, Di Martino A, et al. Platelet-rich intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med. 2015;43:1575-1582.
6. Sanchez M, Fiz N, Azofra J, et al. A randomized clinical trial evaluating plasma rich in growth factors (PGRF-endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy: J Arth and Related Surg. 2012;28:1070-1078.
7. Raeissadat SA, Rayegani SM, Hassanabadi H, et al. Knee osteoarthritis injection choices: platelet-rich plasma (PRP) versus hyaluronic acid (a one-year randomized clinical trial). Clin Med Insights: Arth Musc Dis. 2015;8:1-8.
8. Cerza F, Carni S, Carcangiu A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40:2822-2827.
9. Filardo G, Kon E, Di Martino B, et al. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskeletal Disorders. 2012;13:229-236.
10. Vaquerizo V, Plasencia MA, Arribas I, et al. Comparison of intra-articular injections of plasma rich in growth factors (PGRF-endoret) versus durolane hyaluronic acid in the treatment of patients with symptomatic osteoarthritis: a randomized controlled trial. Arthroscopy: J Arth and Related Surg. 2013;29:1635-1643.
11. Montanez-Heredia E, Irizar S, Huertas PJ, et al. Intra-articular injections of platelet-rich plasma versus hyaluronic acid in the treatment of osteoarthritis knee pain: a randomized clinical trial in the context of the Spanish national health care system. Intl J Molec Sci. 2016;17:1064-1077.
12. Li M, Zhang C, Ai Z, et al. Therapeutic effectiveness of intra-knee articular injections of platelet-rich plasma on knee articular cartilage degeneration. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011 25:1192-11966. (Article published in Chinese with abstract in English.)
13. Shen L, Yuan T, Chen S, et al. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Ortho Surg Res. 2017;12:16.
14. Laudy ABM, Bakker EWP, Rekers M, et al. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015;49:657-672.
15. American Academy of Orthopaedic Surgeons. Clinical practice guideline on the treatment of osteoarthritis of the knee, 2nd ed. www.aaos.org/cc_files/aaosorg/research/guidelines/treatmentofosteoarthritisofthekneeguideline.pdf. Published May 2013. Accessed February 22, 2019.
1. Patel S, Dhillon MS, Aggarwal S, et al. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41:356-364.
2. Smith PA. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee arthritis: an FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. Am J Sports Med. 2016;44:884-891.
3. Gorelli G, Gormelli CA, Ataoglu B, et al. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. 2015;25:958-965.
4. Cole BJ, Karas V, Hussey K, et al. Hyaluronic acid versus platelet-rich plasma: a prospective double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med. 2016;45:339-346.
5. Filardo G, Di Matteo B, Di Martino A, et al. Platelet-rich intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med. 2015;43:1575-1582.
6. Sanchez M, Fiz N, Azofra J, et al. A randomized clinical trial evaluating plasma rich in growth factors (PGRF-endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy: J Arth and Related Surg. 2012;28:1070-1078.
7. Raeissadat SA, Rayegani SM, Hassanabadi H, et al. Knee osteoarthritis injection choices: platelet-rich plasma (PRP) versus hyaluronic acid (a one-year randomized clinical trial). Clin Med Insights: Arth Musc Dis. 2015;8:1-8.
8. Cerza F, Carni S, Carcangiu A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40:2822-2827.
9. Filardo G, Kon E, Di Martino B, et al. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskeletal Disorders. 2012;13:229-236.
10. Vaquerizo V, Plasencia MA, Arribas I, et al. Comparison of intra-articular injections of plasma rich in growth factors (PGRF-endoret) versus durolane hyaluronic acid in the treatment of patients with symptomatic osteoarthritis: a randomized controlled trial. Arthroscopy: J Arth and Related Surg. 2013;29:1635-1643.
11. Montanez-Heredia E, Irizar S, Huertas PJ, et al. Intra-articular injections of platelet-rich plasma versus hyaluronic acid in the treatment of osteoarthritis knee pain: a randomized clinical trial in the context of the Spanish national health care system. Intl J Molec Sci. 2016;17:1064-1077.
12. Li M, Zhang C, Ai Z, et al. Therapeutic effectiveness of intra-knee articular injections of platelet-rich plasma on knee articular cartilage degeneration. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011 25:1192-11966. (Article published in Chinese with abstract in English.)
13. Shen L, Yuan T, Chen S, et al. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Ortho Surg Res. 2017;12:16.
14. Laudy ABM, Bakker EWP, Rekers M, et al. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015;49:657-672.
15. American Academy of Orthopaedic Surgeons. Clinical practice guideline on the treatment of osteoarthritis of the knee, 2nd ed. www.aaos.org/cc_files/aaosorg/research/guidelines/treatmentofosteoarthritisofthekneeguideline.pdf. Published May 2013. Accessed February 22, 2019.
EVIDENCE-BASED ANSWER:
Probably not, based on the balance of evidence. While low-quality evidence may suggest potential benefit, the balance of evidence suggests it is no better than placebo.
Compared with saline placebo, platelet-rich plasma (PRP) injections may improve standardized scores for knee osteoarthritis (OA) pain, function, and stiffness by 24% to 70% for periods of 6 to 52 weeks in patients with early to moderate OA (strength of recommendation [SOR]: B, small randomized controlled trials [RCTs] with methodologic flaws).
Compared with hyaluronic acid (HA), PRP probably improves scores by a similar amount for periods of 8 to 52 weeks (SOR: B, multiple RCTs with conflicting results favoring no difference). However, since HA alone likely doesn’t improve scores more than placebo (SOR: B, RCTs of moderate quality), if both HA and PRP are about the same, then both are not better than placebo.
Does amniotomy shorten spontaneous labor or improve outcomes?
EVIDENCE SUMMARY
A meta-analysis of 15 RCTs (5583 women) compared intentional artificial rupture of the amniotic membranes during labor (amniotomy) with intention to preserve the membranes (no amniotomy). The study found no differences in any of the measured primary outcomes: length of first stage of labor, cesarean section, maternal satisfaction with childbirth, or Apgar score <7 at 5 minutes.1
Investigators included 9 trials with both nulliparous and multiparous women and 6 trials with only nulliparous women. Thirteen trials compared amniotomy with intention to preserve the membranes, and 2 trials performed amniotomy in the control group if the membranes were intact at full cervical dilation.
Amniotomy doesn’t affect first-stage labor or cesarean risk
Five trials (1127 women) reported no difference in length of the first stage of labor between the amniotomy and no amniotomy groups (mean difference [MD]= −20 minutes; 95% confidence interval [CI], −96 to 55). Subgroups of primiparous and multiparous women showed no difference (MD= −58 minutes; 95% CI, −153 to 37 and MD= +23 minutes; 95% CI, −51 to 97, respectively).
Nine trials (5021 women) reported no significant difference in cesarean section risk overall or when compared by parity, multiparous vs primiparous (risk ratio [RR]= 1.27; 95% CI, 0.99-1.63). One trial (84 women) found no difference in maternal satisfaction scores with childbirth experience. Six trials (3598 women) that reported risk of low Apgar score (<4 at 1 minute or <7 at 5 minutes) found no difference overall (RR=0.53; 95% CI, 0.28-1.00), or when compared by parity (multiparous vs primiparous).
Investigators reported that the included trials varied in quality and described the following limitations: inconsistent or unspecified timing of amniotomy during labor, proportion of women in the control group undergoing amniotomy, and ≥30% of women not getting the allocated treatment in all but one of the trials.
Secondary outcomes: Amniotomy reduces oxytocin use
Eight trials (4264 women) evaluated oxytocin augmentation and found that amniotomy decreased its use in multiparous (RR=0.43; 95% CI, 0.30-0.60), but not primiparous, women.
Eight trials (1927 women) reported length of second stage of labor as a secondary outcome, with no difference overall (MD= −1.33 minutes; 95% CI, −2.92 to 0.26). Amniotomy produced a statistical but not clinically significant shortening in subanalysis of primiparous women (MD= −5.43 minutes; 95% CI, −9.98 to −0.89) but not multiparous women.
Continue to: Three trials...
Three trials (1695 women) evaluated dysfunctional labor, defined as no progress in cervical dilation in 2 hours or ineffective uterine contractions. Amniotomy reduced dysfunctional labor in both primiparous (RR=0.49; 95% CI, 0.33-0.73) and multiparous women (RR=0.44; 95% CI, 0.31-0.62).
No differences found in other maternal and fetal outcomes
Investigators reported no differences in other secondary maternal outcomes: instrumental vaginal birth (10 trials, 5121 women); pain relief (8 trials, 3475 women); postpartum hemorrhage (2 trials, 1822 women); serious maternal morbidity or death (3 trials, 1740 women); umbilical cord prolapse (2 trials, 1615 women); and cesarean section for fetal distress, prolonged labor, or antepartum hemorrhage (1 RCT, 690 women).
Investigators also found no differences in secondary fetal outcomes: serious neonatal morbidity or perinatal death (8 trials, 3397 women); neonatal admission to neonatal intensive care (5 trials, 2686 women); abnormal fetal heart rate tracing in first stage of labor (4 trials, 1284 women); meconium aspiration (2 trials, 1615 women); and fetal acidosis (2 trials, 1014 women). Similarly, 1 RCT (39 women) that compared amniotomy with intent to preserve membranes in spontaneous labors that became prolonged found no difference in cesarean section, maternal satisfaction, or Apgar scores.
A few studies claim shorter labor with amniotomy
However, a later Iranian RCT (300 women) reported that early amniotomy shortened labor (labor duration: 7.5 ± 0.7 hours with amniotomy vs 9.9 ± 1.0 hours without amniotomy; P<.001) and reduced the risk of dystocia (RR=0.81; 95% CI, 0.59-0.90) and cesarean section (RR=0.82; 95% CI, 0.66-0.90).2
A similar Nigerian RCT (214 women) and an Indian RCT (144 women) both claimed that amniotomy also shortened labor (4.7 ± 0.9 hours vs 5.9 ± 1.3, and 3.9 ± 2 hours vs 6.1 ± 2.8 hours, respectively).3,4 In neither trial, however, did investigators explain how the difference was significant when the duration of labor times overlapped within the margin of error.
1. Smyth RMD, Markham C, Dowswell T. Amniotomy for shortening spontaneous labour. Cochrane Database Syst Rev. 2013;(6):CD006167.
2. Ghafarzadeh M, Moeininasab S, Namdari M. Effect of early amniotomy on dystocia risk and cesarean delivery in nulliparous women: a randomized clinical trial. Arch Gynecol Obstet. 2015;292:321-325.
3. Onah LN, Dim CC, Nwagha UI, et al. Effect of early amniotomy on the outcome of spontaneous labour: a randomized controlled trial of pregnant women in Enugu, South-east Nigeria. Afr Health Sci. 2015;15:1097-1103.
4. Vadivelu M, Rathore S, Benjamin SJ, et al. Randomized controlled trial of the effect of amniotomy on the duration of spontaneous labor. Int J Gynaecol Obstet. 2017;138:152-157.
EVIDENCE SUMMARY
A meta-analysis of 15 RCTs (5583 women) compared intentional artificial rupture of the amniotic membranes during labor (amniotomy) with intention to preserve the membranes (no amniotomy). The study found no differences in any of the measured primary outcomes: length of first stage of labor, cesarean section, maternal satisfaction with childbirth, or Apgar score <7 at 5 minutes.1
Investigators included 9 trials with both nulliparous and multiparous women and 6 trials with only nulliparous women. Thirteen trials compared amniotomy with intention to preserve the membranes, and 2 trials performed amniotomy in the control group if the membranes were intact at full cervical dilation.
Amniotomy doesn’t affect first-stage labor or cesarean risk
Five trials (1127 women) reported no difference in length of the first stage of labor between the amniotomy and no amniotomy groups (mean difference [MD]= −20 minutes; 95% confidence interval [CI], −96 to 55). Subgroups of primiparous and multiparous women showed no difference (MD= −58 minutes; 95% CI, −153 to 37 and MD= +23 minutes; 95% CI, −51 to 97, respectively).
Nine trials (5021 women) reported no significant difference in cesarean section risk overall or when compared by parity, multiparous vs primiparous (risk ratio [RR]= 1.27; 95% CI, 0.99-1.63). One trial (84 women) found no difference in maternal satisfaction scores with childbirth experience. Six trials (3598 women) that reported risk of low Apgar score (<4 at 1 minute or <7 at 5 minutes) found no difference overall (RR=0.53; 95% CI, 0.28-1.00), or when compared by parity (multiparous vs primiparous).
Investigators reported that the included trials varied in quality and described the following limitations: inconsistent or unspecified timing of amniotomy during labor, proportion of women in the control group undergoing amniotomy, and ≥30% of women not getting the allocated treatment in all but one of the trials.
Secondary outcomes: Amniotomy reduces oxytocin use
Eight trials (4264 women) evaluated oxytocin augmentation and found that amniotomy decreased its use in multiparous (RR=0.43; 95% CI, 0.30-0.60), but not primiparous, women.
Eight trials (1927 women) reported length of second stage of labor as a secondary outcome, with no difference overall (MD= −1.33 minutes; 95% CI, −2.92 to 0.26). Amniotomy produced a statistical but not clinically significant shortening in subanalysis of primiparous women (MD= −5.43 minutes; 95% CI, −9.98 to −0.89) but not multiparous women.
Continue to: Three trials...
Three trials (1695 women) evaluated dysfunctional labor, defined as no progress in cervical dilation in 2 hours or ineffective uterine contractions. Amniotomy reduced dysfunctional labor in both primiparous (RR=0.49; 95% CI, 0.33-0.73) and multiparous women (RR=0.44; 95% CI, 0.31-0.62).
No differences found in other maternal and fetal outcomes
Investigators reported no differences in other secondary maternal outcomes: instrumental vaginal birth (10 trials, 5121 women); pain relief (8 trials, 3475 women); postpartum hemorrhage (2 trials, 1822 women); serious maternal morbidity or death (3 trials, 1740 women); umbilical cord prolapse (2 trials, 1615 women); and cesarean section for fetal distress, prolonged labor, or antepartum hemorrhage (1 RCT, 690 women).
Investigators also found no differences in secondary fetal outcomes: serious neonatal morbidity or perinatal death (8 trials, 3397 women); neonatal admission to neonatal intensive care (5 trials, 2686 women); abnormal fetal heart rate tracing in first stage of labor (4 trials, 1284 women); meconium aspiration (2 trials, 1615 women); and fetal acidosis (2 trials, 1014 women). Similarly, 1 RCT (39 women) that compared amniotomy with intent to preserve membranes in spontaneous labors that became prolonged found no difference in cesarean section, maternal satisfaction, or Apgar scores.
A few studies claim shorter labor with amniotomy
However, a later Iranian RCT (300 women) reported that early amniotomy shortened labor (labor duration: 7.5 ± 0.7 hours with amniotomy vs 9.9 ± 1.0 hours without amniotomy; P<.001) and reduced the risk of dystocia (RR=0.81; 95% CI, 0.59-0.90) and cesarean section (RR=0.82; 95% CI, 0.66-0.90).2
A similar Nigerian RCT (214 women) and an Indian RCT (144 women) both claimed that amniotomy also shortened labor (4.7 ± 0.9 hours vs 5.9 ± 1.3, and 3.9 ± 2 hours vs 6.1 ± 2.8 hours, respectively).3,4 In neither trial, however, did investigators explain how the difference was significant when the duration of labor times overlapped within the margin of error.
EVIDENCE SUMMARY
A meta-analysis of 15 RCTs (5583 women) compared intentional artificial rupture of the amniotic membranes during labor (amniotomy) with intention to preserve the membranes (no amniotomy). The study found no differences in any of the measured primary outcomes: length of first stage of labor, cesarean section, maternal satisfaction with childbirth, or Apgar score <7 at 5 minutes.1
Investigators included 9 trials with both nulliparous and multiparous women and 6 trials with only nulliparous women. Thirteen trials compared amniotomy with intention to preserve the membranes, and 2 trials performed amniotomy in the control group if the membranes were intact at full cervical dilation.
Amniotomy doesn’t affect first-stage labor or cesarean risk
Five trials (1127 women) reported no difference in length of the first stage of labor between the amniotomy and no amniotomy groups (mean difference [MD]= −20 minutes; 95% confidence interval [CI], −96 to 55). Subgroups of primiparous and multiparous women showed no difference (MD= −58 minutes; 95% CI, −153 to 37 and MD= +23 minutes; 95% CI, −51 to 97, respectively).
Nine trials (5021 women) reported no significant difference in cesarean section risk overall or when compared by parity, multiparous vs primiparous (risk ratio [RR]= 1.27; 95% CI, 0.99-1.63). One trial (84 women) found no difference in maternal satisfaction scores with childbirth experience. Six trials (3598 women) that reported risk of low Apgar score (<4 at 1 minute or <7 at 5 minutes) found no difference overall (RR=0.53; 95% CI, 0.28-1.00), or when compared by parity (multiparous vs primiparous).
Investigators reported that the included trials varied in quality and described the following limitations: inconsistent or unspecified timing of amniotomy during labor, proportion of women in the control group undergoing amniotomy, and ≥30% of women not getting the allocated treatment in all but one of the trials.
Secondary outcomes: Amniotomy reduces oxytocin use
Eight trials (4264 women) evaluated oxytocin augmentation and found that amniotomy decreased its use in multiparous (RR=0.43; 95% CI, 0.30-0.60), but not primiparous, women.
Eight trials (1927 women) reported length of second stage of labor as a secondary outcome, with no difference overall (MD= −1.33 minutes; 95% CI, −2.92 to 0.26). Amniotomy produced a statistical but not clinically significant shortening in subanalysis of primiparous women (MD= −5.43 minutes; 95% CI, −9.98 to −0.89) but not multiparous women.
Continue to: Three trials...
Three trials (1695 women) evaluated dysfunctional labor, defined as no progress in cervical dilation in 2 hours or ineffective uterine contractions. Amniotomy reduced dysfunctional labor in both primiparous (RR=0.49; 95% CI, 0.33-0.73) and multiparous women (RR=0.44; 95% CI, 0.31-0.62).
No differences found in other maternal and fetal outcomes
Investigators reported no differences in other secondary maternal outcomes: instrumental vaginal birth (10 trials, 5121 women); pain relief (8 trials, 3475 women); postpartum hemorrhage (2 trials, 1822 women); serious maternal morbidity or death (3 trials, 1740 women); umbilical cord prolapse (2 trials, 1615 women); and cesarean section for fetal distress, prolonged labor, or antepartum hemorrhage (1 RCT, 690 women).
Investigators also found no differences in secondary fetal outcomes: serious neonatal morbidity or perinatal death (8 trials, 3397 women); neonatal admission to neonatal intensive care (5 trials, 2686 women); abnormal fetal heart rate tracing in first stage of labor (4 trials, 1284 women); meconium aspiration (2 trials, 1615 women); and fetal acidosis (2 trials, 1014 women). Similarly, 1 RCT (39 women) that compared amniotomy with intent to preserve membranes in spontaneous labors that became prolonged found no difference in cesarean section, maternal satisfaction, or Apgar scores.
A few studies claim shorter labor with amniotomy
However, a later Iranian RCT (300 women) reported that early amniotomy shortened labor (labor duration: 7.5 ± 0.7 hours with amniotomy vs 9.9 ± 1.0 hours without amniotomy; P<.001) and reduced the risk of dystocia (RR=0.81; 95% CI, 0.59-0.90) and cesarean section (RR=0.82; 95% CI, 0.66-0.90).2
A similar Nigerian RCT (214 women) and an Indian RCT (144 women) both claimed that amniotomy also shortened labor (4.7 ± 0.9 hours vs 5.9 ± 1.3, and 3.9 ± 2 hours vs 6.1 ± 2.8 hours, respectively).3,4 In neither trial, however, did investigators explain how the difference was significant when the duration of labor times overlapped within the margin of error.
1. Smyth RMD, Markham C, Dowswell T. Amniotomy for shortening spontaneous labour. Cochrane Database Syst Rev. 2013;(6):CD006167.
2. Ghafarzadeh M, Moeininasab S, Namdari M. Effect of early amniotomy on dystocia risk and cesarean delivery in nulliparous women: a randomized clinical trial. Arch Gynecol Obstet. 2015;292:321-325.
3. Onah LN, Dim CC, Nwagha UI, et al. Effect of early amniotomy on the outcome of spontaneous labour: a randomized controlled trial of pregnant women in Enugu, South-east Nigeria. Afr Health Sci. 2015;15:1097-1103.
4. Vadivelu M, Rathore S, Benjamin SJ, et al. Randomized controlled trial of the effect of amniotomy on the duration of spontaneous labor. Int J Gynaecol Obstet. 2017;138:152-157.
1. Smyth RMD, Markham C, Dowswell T. Amniotomy for shortening spontaneous labour. Cochrane Database Syst Rev. 2013;(6):CD006167.
2. Ghafarzadeh M, Moeininasab S, Namdari M. Effect of early amniotomy on dystocia risk and cesarean delivery in nulliparous women: a randomized clinical trial. Arch Gynecol Obstet. 2015;292:321-325.
3. Onah LN, Dim CC, Nwagha UI, et al. Effect of early amniotomy on the outcome of spontaneous labour: a randomized controlled trial of pregnant women in Enugu, South-east Nigeria. Afr Health Sci. 2015;15:1097-1103.
4. Vadivelu M, Rathore S, Benjamin SJ, et al. Randomized controlled trial of the effect of amniotomy on the duration of spontaneous labor. Int J Gynaecol Obstet. 2017;138:152-157.
EVIDENCE-BASED ANSWER:
No. Amniotomy neither shortens spontaneous labor nor improves any of the following outcomes: length of first stage of labor, cesarean section rate, maternal satisfaction with childbirth, or Apgar score <7 at 5 minutes (strength of recommendation [SOR]: A, large meta-analyses of randomized controlled trials [RCTs] and a single RCT with conflicting results).
Amniotomy does result in about a 55% reduction of pitocin use in multiparous women, a small (5 minutes) decrease in the duration of second-stage labor in primiparous women, and about a 50% overall reduction in dysfunctional labor—ie, no progress in cervical dilation in 2 hours or ineffective uterine contractions (SOR: A, large meta-analyses of RCTs and a single RCT with conflicting results).
Amniotomy doesn’t improve other maternal outcomes—instrumented vaginal birth; pain relief; postpartum hemorrhage; serious morbidity or death; umbilical cord prolapse; cesarean section for fetal distress, prolonged labor, antepartum hemorrhage—nor fetal outcomes—serious neonatal morbidity or perinatal death; neonatal admission to intensive care; abnormal fetal heart rate tracing in first-stage labor; meconium aspiration; or fetal acidosis (SOR: A, large meta-analyses of RCTs and a single RCT with conflicting results).
What’s the best VTE treatment for patients with cancer?
EVIDENCE SUMMARY
No head-to-head studies or umbrella meta-analyses assess all the main treatments for VTE against each other.
Long-term LMWH decreases VTE recurrence compared with VKA
Two meta-analyses of RCTs evaluating LMWH and VKA for long-term treatment (3-12 months) of confirmed VTE in patients with cancer found that LMWH didn’t change mortality, but reduced the rate of VTE recurrence compared with VKA (40% relative reduction).1,2 The comparison showed no differences in major or minor bleeding or thrombocytopenia between LMWH and VKA (TABLE1-5).
The studies included patients with any solid or hematologic cancer at any stage and from any age group, including children. Overall, the mean age of patients was in the mid 60s; approximately 50% were male when specified. Investigators rated the evidence quality as moderate for VTE, but low for the other outcomes.1
The most recent meta-analysis of the same RCTs comparing LMWH with VKA evaluated intracranial hemorrhage rates and found no difference.3
Initial therapy with LMWH: A look at mortality
A meta-analysis of RCTs that compared LMWH with UFH/VKA for initial treatment of confirmed VTE in adult cancer patients (any type or stage of cancer, mean ages not specified) found that LMWH reduced mortality by 30%, but didn’t affect VTE recurrence or major bleeding.4
The control groups received UFH for 5 to 10 days and then continued with VKA, whereas the experimental groups received different types of LMWH (reviparin, nadroparin, tinzaparin, enoxaparin) initially and for 3 months thereafter. Investigators rated all studies low quality because of imprecision and publication bias favoring LMWH.
Fondaparinux shows no advantage for initial therapy
The same meta-analysis compared initial treatment with fondaparinux and initial therapy with enoxaparin or UFH transitioning to warfarin.4 It found no differences in any outcomes at 3 months. Investigators rated both studies as low quality for recurrent VTE and moderate for mortality and bleeding.
Continue to: Non-vitamin K oral anticoagulants vs LMWH/VKA or VKA
Non-vitamin K oral anticoagulants vs LMWH/VKA or VKA: No differences
A meta-analysis of RCTs comparing NOACs (dabigatran, edoxaban, apixaban, rivaroxaban) with VKA for 6 months found no differences in recurrent VTE or major bleeding.2
A second meta-analysis of RCTs that compared NOACs (rivaroxaban, dabigatran, apixaban) with control (LMWH followed by VKA) in adult cancer patients (mean ages, 54-66 years; 50%-60% men) reported no difference in the composite outcome of recurrent VTE or VTE-related death nor clinically significant bleeding over 1 to 36 months (most RCTs ran 3-12 months).5 Separate comparisons for rivaroxaban and dabigatran found no difference in the composite outcome, and rivaroxaban also produced no difference in clinically-significant bleeding.
RECOMMENDATIONS
The 2016 CHEST guidelines recommend LMWH as first-line treatment for VTE in patients with cancer and indicate no preference between NOACs and VKA for second-line treatment.6
1. Akl EA, Kahale L, Barba M, et al. Anticoagulation for the long-term treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev. 2014;(7):CD006650.
2. Posch F, Königsbrügge O, Zielinski C, et al. Treatment of venous thromboembolism in patients with cancer: A network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res. 2015;136:582-589.
3. Rojas-Hernandez CM, Oo TH, García-Perdomo HA. Risk of intracranial hemorrhage associated with therapeutic anticoagulation for venous thromboembolism in cancer patients: a systematic review and meta-analysis. J Thromb Thrombolysis. 2017;43:233-240.
4. Akl EA, Kahale L, Neumann I, et al. Anticoagulation for the initial treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev. 2014;(6):CD006649.
5. Sardar P, Chatterjee S, Herzog E, et al. New oral anticoagulants in patients with cancer: current state of evidence. Am J Ther. 2015;22:460-468.
6. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315-352.
EVIDENCE SUMMARY
No head-to-head studies or umbrella meta-analyses assess all the main treatments for VTE against each other.
Long-term LMWH decreases VTE recurrence compared with VKA
Two meta-analyses of RCTs evaluating LMWH and VKA for long-term treatment (3-12 months) of confirmed VTE in patients with cancer found that LMWH didn’t change mortality, but reduced the rate of VTE recurrence compared with VKA (40% relative reduction).1,2 The comparison showed no differences in major or minor bleeding or thrombocytopenia between LMWH and VKA (TABLE1-5).
The studies included patients with any solid or hematologic cancer at any stage and from any age group, including children. Overall, the mean age of patients was in the mid 60s; approximately 50% were male when specified. Investigators rated the evidence quality as moderate for VTE, but low for the other outcomes.1
The most recent meta-analysis of the same RCTs comparing LMWH with VKA evaluated intracranial hemorrhage rates and found no difference.3
Initial therapy with LMWH: A look at mortality
A meta-analysis of RCTs that compared LMWH with UFH/VKA for initial treatment of confirmed VTE in adult cancer patients (any type or stage of cancer, mean ages not specified) found that LMWH reduced mortality by 30%, but didn’t affect VTE recurrence or major bleeding.4
The control groups received UFH for 5 to 10 days and then continued with VKA, whereas the experimental groups received different types of LMWH (reviparin, nadroparin, tinzaparin, enoxaparin) initially and for 3 months thereafter. Investigators rated all studies low quality because of imprecision and publication bias favoring LMWH.
Fondaparinux shows no advantage for initial therapy
The same meta-analysis compared initial treatment with fondaparinux and initial therapy with enoxaparin or UFH transitioning to warfarin.4 It found no differences in any outcomes at 3 months. Investigators rated both studies as low quality for recurrent VTE and moderate for mortality and bleeding.
Continue to: Non-vitamin K oral anticoagulants vs LMWH/VKA or VKA
Non-vitamin K oral anticoagulants vs LMWH/VKA or VKA: No differences
A meta-analysis of RCTs comparing NOACs (dabigatran, edoxaban, apixaban, rivaroxaban) with VKA for 6 months found no differences in recurrent VTE or major bleeding.2
A second meta-analysis of RCTs that compared NOACs (rivaroxaban, dabigatran, apixaban) with control (LMWH followed by VKA) in adult cancer patients (mean ages, 54-66 years; 50%-60% men) reported no difference in the composite outcome of recurrent VTE or VTE-related death nor clinically significant bleeding over 1 to 36 months (most RCTs ran 3-12 months).5 Separate comparisons for rivaroxaban and dabigatran found no difference in the composite outcome, and rivaroxaban also produced no difference in clinically-significant bleeding.
RECOMMENDATIONS
The 2016 CHEST guidelines recommend LMWH as first-line treatment for VTE in patients with cancer and indicate no preference between NOACs and VKA for second-line treatment.6
EVIDENCE SUMMARY
No head-to-head studies or umbrella meta-analyses assess all the main treatments for VTE against each other.
Long-term LMWH decreases VTE recurrence compared with VKA
Two meta-analyses of RCTs evaluating LMWH and VKA for long-term treatment (3-12 months) of confirmed VTE in patients with cancer found that LMWH didn’t change mortality, but reduced the rate of VTE recurrence compared with VKA (40% relative reduction).1,2 The comparison showed no differences in major or minor bleeding or thrombocytopenia between LMWH and VKA (TABLE1-5).
The studies included patients with any solid or hematologic cancer at any stage and from any age group, including children. Overall, the mean age of patients was in the mid 60s; approximately 50% were male when specified. Investigators rated the evidence quality as moderate for VTE, but low for the other outcomes.1
The most recent meta-analysis of the same RCTs comparing LMWH with VKA evaluated intracranial hemorrhage rates and found no difference.3
Initial therapy with LMWH: A look at mortality
A meta-analysis of RCTs that compared LMWH with UFH/VKA for initial treatment of confirmed VTE in adult cancer patients (any type or stage of cancer, mean ages not specified) found that LMWH reduced mortality by 30%, but didn’t affect VTE recurrence or major bleeding.4
The control groups received UFH for 5 to 10 days and then continued with VKA, whereas the experimental groups received different types of LMWH (reviparin, nadroparin, tinzaparin, enoxaparin) initially and for 3 months thereafter. Investigators rated all studies low quality because of imprecision and publication bias favoring LMWH.
Fondaparinux shows no advantage for initial therapy
The same meta-analysis compared initial treatment with fondaparinux and initial therapy with enoxaparin or UFH transitioning to warfarin.4 It found no differences in any outcomes at 3 months. Investigators rated both studies as low quality for recurrent VTE and moderate for mortality and bleeding.
Continue to: Non-vitamin K oral anticoagulants vs LMWH/VKA or VKA
Non-vitamin K oral anticoagulants vs LMWH/VKA or VKA: No differences
A meta-analysis of RCTs comparing NOACs (dabigatran, edoxaban, apixaban, rivaroxaban) with VKA for 6 months found no differences in recurrent VTE or major bleeding.2
A second meta-analysis of RCTs that compared NOACs (rivaroxaban, dabigatran, apixaban) with control (LMWH followed by VKA) in adult cancer patients (mean ages, 54-66 years; 50%-60% men) reported no difference in the composite outcome of recurrent VTE or VTE-related death nor clinically significant bleeding over 1 to 36 months (most RCTs ran 3-12 months).5 Separate comparisons for rivaroxaban and dabigatran found no difference in the composite outcome, and rivaroxaban also produced no difference in clinically-significant bleeding.
RECOMMENDATIONS
The 2016 CHEST guidelines recommend LMWH as first-line treatment for VTE in patients with cancer and indicate no preference between NOACs and VKA for second-line treatment.6
1. Akl EA, Kahale L, Barba M, et al. Anticoagulation for the long-term treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev. 2014;(7):CD006650.
2. Posch F, Königsbrügge O, Zielinski C, et al. Treatment of venous thromboembolism in patients with cancer: A network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res. 2015;136:582-589.
3. Rojas-Hernandez CM, Oo TH, García-Perdomo HA. Risk of intracranial hemorrhage associated with therapeutic anticoagulation for venous thromboembolism in cancer patients: a systematic review and meta-analysis. J Thromb Thrombolysis. 2017;43:233-240.
4. Akl EA, Kahale L, Neumann I, et al. Anticoagulation for the initial treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev. 2014;(6):CD006649.
5. Sardar P, Chatterjee S, Herzog E, et al. New oral anticoagulants in patients with cancer: current state of evidence. Am J Ther. 2015;22:460-468.
6. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315-352.
1. Akl EA, Kahale L, Barba M, et al. Anticoagulation for the long-term treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev. 2014;(7):CD006650.
2. Posch F, Königsbrügge O, Zielinski C, et al. Treatment of venous thromboembolism in patients with cancer: A network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res. 2015;136:582-589.
3. Rojas-Hernandez CM, Oo TH, García-Perdomo HA. Risk of intracranial hemorrhage associated with therapeutic anticoagulation for venous thromboembolism in cancer patients: a systematic review and meta-analysis. J Thromb Thrombolysis. 2017;43:233-240.
4. Akl EA, Kahale L, Neumann I, et al. Anticoagulation for the initial treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev. 2014;(6):CD006649.
5. Sardar P, Chatterjee S, Herzog E, et al. New oral anticoagulants in patients with cancer: current state of evidence. Am J Ther. 2015;22:460-468.
6. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315-352.
EVIDENCE-BASED ANSWER:
No head-to-head studies directly compare all the main treatments for venous thromboembolism (VTE) in cancer patients. Long-term treatment (3-12 months) with low-molecular-weight heparin (LMWH) reduces recurrence of VTE by 40% compared with vitamin K antagonists (VKA), but doesn’t change rates of mortality, major or minor bleeding, or intracranial hemorrhage in patients with solid or hematologic cancer at any stage or in any age group. Initial treatment with LMWH reduces mortality by 30% compared with unfractionated heparin (UFH) for 5 to 10 days followed by warfarin, but doesn’t alter recurrent VTE or bleeding. Non-vitamin K oral anticoagulants (NOACs) have risks of recurrent VTE or VTE-related death (composite outcome) and clinically significant bleeding comparable to VKA or LMWH/VKA (strength of recommendation [SOR]: B, meta-analyses of randomized controlled trials [RCTs], mostly of low quality).