User login
How does gender-affirming hormone therapy affect QOL in transgender patients?
Evidence summary
GAHT may improve depression and quality of life, but not anxiety
A well-done systematic review of transgender men and transgender women demonstrated that GAHT of more than a year’s duration was associated with modestly improved standardized scores for QOL, depression, and possibly anxiety.1 It was also associated with improved scores for depression in transgender adolescents.
The authors identified 15 prospective cohort studies (n = 626 transgender adults [mean age, 25-34 years]; 198 transgender adolescent girls and boys [mean age, 15-16 years]), 2 retrospective cohort studies (n = 1756 adults; mean age, 25-32 years), and 4 cross-sectional studies (n = 336 adults; mean age, 30-37 years).
Researchers recruited participants using strict eligibility criteria (psychiatric evaluation and formal diagnosis of gender dysphoria), with no prior history of GAHT, largely from gender-affirming specialty clinics at university hospitals. Most studies were conducted after the year 2000, predominantly in Europe (8 studies in Italy; 2 each in Belgium, the Netherlands, the United States, and Spain).
GAHT comprised testosterone for transgender men (14 studies used injectable testosterone cypionate, enanthate, undecanoate, or transdermal gels), estrogens (usually with an anti-androgen such as cyproterone acetate or spironolactone) for transgender women (10 studies used transdermal, oral, or injectable estradiol valerate or conjugated estrogens), and gonadotropin-releasing hormone (GnRH) therapy for transgender adolescents (3 studies).
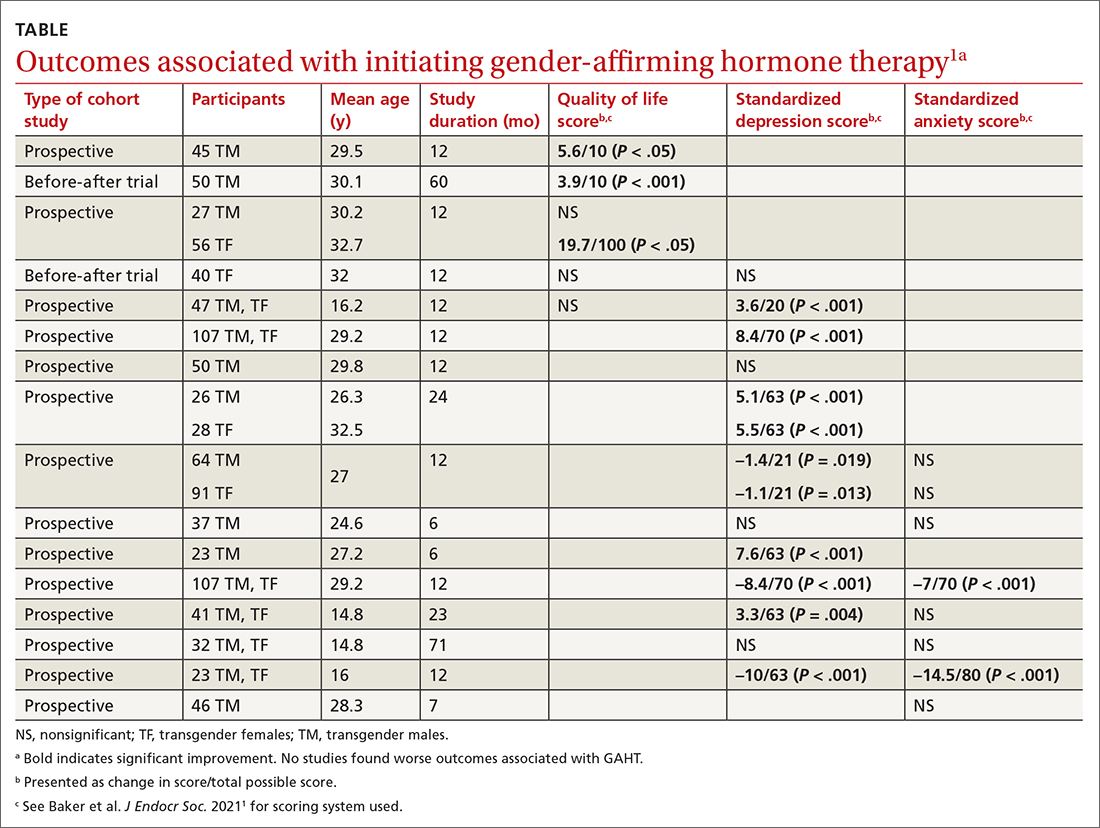
Researchers evaluated the outcomes of QOL, depression, and anxiety with standardized scores on validated screening tools and suicide (2 studies) by medical records. GAHT in adult transgender men and transgender women was associated with modest improvements in QOL (3 of 5 studies) and depression (8 of 12 studies), and some improvement in anxiety scores (2 of 8 studies; see TABLE1). There was insufficient evidence to determine whether GAHT had any effect on suicide. In adolescent transgender girls and boys, GAHT was associated with modest improvements in depression but not QOL or anxiety scores.
The authors rated the strength of evidence from the included studies as low, based on study quality (small study sizes, uncontrolled confounding factors, and risk of bias in study designs).
Additional research supports GAHT’s association with improved outcomes
Three studies, published after the systematic review, evaluated outcomes before and after GAHT and found similar results. All studies recruited treatment-seeking participants from specialty clinics.
Continue to: An Australian propsective longitudinal..
An Australian prospective longitudinal controlled study (n = 77 transgender adults; 103 cisgender controls) evaluated GAHT outcomes after 6 months and found a significant reduction in gender dysphoria scores in both transgender males (adjusted mean difference [aMD] = –6.8; 95% CI, –8.7 to –4.9; P < .001) and transgender females (aMD = –4.2; 95% CI, –6.2 to –2.2; P < .001) vs controls. QOL scores (emotional well-being, social functioning) improved only for transgender males (well-being: aMD = +7.5; 95% CI, 1.3 to 13.6; P < .018; social functioning: aMD = +12.5; 95% CI, 2.8 to 22.2; P = .011).2
A US prospective cohort study (n = 104 adolescents; mean age, 16 years) examined the effect of GnRH and/or GAHT over a 12-month period and found significant decreases in standardized scores for depression (adjusted odds ratio [aOR] = 0.4; 95% CI, 0.17-0.95) and suicidality (aOR = 0.27; 95% CI, 0.11-0.65) but not for anxiety. Participants who did not receive hormonal interventions had increased scores for depression and suicidality at 3 and 6 months’ follow-up.3
A prospective cohort study from the UK (n = 178 transgender adults) examined outcomes before and after GAHT treatment over 18 months and found significant decreases in standardized scores for depression (transgender males: –2.1; 95% CI, –3.2 to –1.2; P < .001; transgender females: –1.9; 95% CI, –2.8 to –1.0; P < .001) but not for anxiety.4
A large US study shows GAHT may reduce depression scores
A recent large cross-sectional study from the United States (n = 11,914 transgender or nonbinary youth, ages 13-24 years) found that receiving GAHT was associated with significantly lower odds of recent depression (aOR = 0.73; P < .001) and suicidality (aOR = 0.74; P < .001) compared to those who wanted GAHT but did not receive it. The authors were unable to differentiate the effects of receiving GAHT from the effects of parental support for their child’s gender identity, which may be a confounding factor.5
Recommendations from others
The World Professional Association for Transgender Health Standards of Care state that “gender incongruence that causes clinically significant distress and impairment often requires medically necessary clinical interventions” and recommends “health care professionals initiate and continue gender-affirming hormone therapy … due to demonstrated improvement in psychosocial functioning and quality of life.”6 The Endocrine Society Position Statement on Transgender Health states that “medical intervention for transgender youth and adults (including … hormone therapy) is effective, relatively safe (when appropriately monitored), and has been established as the standard of care.”7 The American Academy of Family Physicians “supports gender-affirming care as an evidence-informed intervention that can promote health equity for gender-diverse individuals.”8
Editor’s takeaway
Family physicians commonly address many factors that can impact the QOL for our patients with gender dysphoria: lack of fixed residence, underemployment, food insecurity, and trauma. GAHT, especially in male-to-female transgender patients, may further improve QOL without evidence of harm.
1. Baker KE, Wilson LM, Sharma R, et al. Hormone therapy, mental health, and quality of life among transgender people: a systematic review. J Endocr Soc. 2021;5:bvab011. doi: 10.1210/jendso/bvab011
2. Foster Skewis L, Bretherton I, Leemaqz, SY, et al. Short-term effects of gender-affirming hormone therapy on dysphoria and quality of life in transgender individuals: a prospective controlled study. Front Endocrinol (Lausanne). 2021;12:717766.
3. Tordoff DM, Wanta JW, Collin A, et al. Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Netw Open. 2022;5:e220978. doi: 10.1001/jamanetworkopen.2022.0978
4. Aldridge Z, Patel S, Guo B, et al. Long-term effect of gender-affirming hormone treatment on depression and anxiety symptoms in transgender people: a prospective cohort study. Andrology. 2021;9:1808-1816. doi: 10.1111/andr.12884
5. Green AE, DeChants JP, Price MN, et al. Association of gender-affirming hormone therapy with depression, thoughts of suicide, and attempted suicide among transgender and nonbinary youth. J Adolesc Health. 2022;70:643-649. doi: 10.1016/j.jadohealth.2021.10.036
6. World Professional Association for Transgender Health. Standards of Care for the Health of Transsexual, Transgender, and Gender Nonconforming People. 8th version. Published 2022. Accessed November 17, 2022. www.wpath.org/publications/soc
7. Endocrine Society. Transgender health: an Endocrine Society position statement. Updated December 16, 2020. Accessed November 17, 2022. www.endocrine.org/advocacy/position-statements/transgender-health
8. American Academy of Family Physicians. Care for the transgender and gender nonbinary patient. Updated September 2022. Accessed November 17, 2022. www.aafp.org/about/policies/all/transgender-nonbinary.html
Evidence summary
GAHT may improve depression and quality of life, but not anxiety
A well-done systematic review of transgender men and transgender women demonstrated that GAHT of more than a year’s duration was associated with modestly improved standardized scores for QOL, depression, and possibly anxiety.1 It was also associated with improved scores for depression in transgender adolescents.
The authors identified 15 prospective cohort studies (n = 626 transgender adults [mean age, 25-34 years]; 198 transgender adolescent girls and boys [mean age, 15-16 years]), 2 retrospective cohort studies (n = 1756 adults; mean age, 25-32 years), and 4 cross-sectional studies (n = 336 adults; mean age, 30-37 years).
Researchers recruited participants using strict eligibility criteria (psychiatric evaluation and formal diagnosis of gender dysphoria), with no prior history of GAHT, largely from gender-affirming specialty clinics at university hospitals. Most studies were conducted after the year 2000, predominantly in Europe (8 studies in Italy; 2 each in Belgium, the Netherlands, the United States, and Spain).
GAHT comprised testosterone for transgender men (14 studies used injectable testosterone cypionate, enanthate, undecanoate, or transdermal gels), estrogens (usually with an anti-androgen such as cyproterone acetate or spironolactone) for transgender women (10 studies used transdermal, oral, or injectable estradiol valerate or conjugated estrogens), and gonadotropin-releasing hormone (GnRH) therapy for transgender adolescents (3 studies).
Researchers evaluated the outcomes of QOL, depression, and anxiety with standardized scores on validated screening tools and suicide (2 studies) by medical records. GAHT in adult transgender men and transgender women was associated with modest improvements in QOL (3 of 5 studies) and depression (8 of 12 studies), and some improvement in anxiety scores (2 of 8 studies; see TABLE1). There was insufficient evidence to determine whether GAHT had any effect on suicide. In adolescent transgender girls and boys, GAHT was associated with modest improvements in depression but not QOL or anxiety scores.

The authors rated the strength of evidence from the included studies as low, based on study quality (small study sizes, uncontrolled confounding factors, and risk of bias in study designs).
Additional research supports GAHT’s association with improved outcomes
Three studies, published after the systematic review, evaluated outcomes before and after GAHT and found similar results. All studies recruited treatment-seeking participants from specialty clinics.
Continue to: An Australian propsective longitudinal..
An Australian prospective longitudinal controlled study (n = 77 transgender adults; 103 cisgender controls) evaluated GAHT outcomes after 6 months and found a significant reduction in gender dysphoria scores in both transgender males (adjusted mean difference [aMD] = –6.8; 95% CI, –8.7 to –4.9; P < .001) and transgender females (aMD = –4.2; 95% CI, –6.2 to –2.2; P < .001) vs controls. QOL scores (emotional well-being, social functioning) improved only for transgender males (well-being: aMD = +7.5; 95% CI, 1.3 to 13.6; P < .018; social functioning: aMD = +12.5; 95% CI, 2.8 to 22.2; P = .011).2
A US prospective cohort study (n = 104 adolescents; mean age, 16 years) examined the effect of GnRH and/or GAHT over a 12-month period and found significant decreases in standardized scores for depression (adjusted odds ratio [aOR] = 0.4; 95% CI, 0.17-0.95) and suicidality (aOR = 0.27; 95% CI, 0.11-0.65) but not for anxiety. Participants who did not receive hormonal interventions had increased scores for depression and suicidality at 3 and 6 months’ follow-up.3
A prospective cohort study from the UK (n = 178 transgender adults) examined outcomes before and after GAHT treatment over 18 months and found significant decreases in standardized scores for depression (transgender males: –2.1; 95% CI, –3.2 to –1.2; P < .001; transgender females: –1.9; 95% CI, –2.8 to –1.0; P < .001) but not for anxiety.4
A large US study shows GAHT may reduce depression scores
A recent large cross-sectional study from the United States (n = 11,914 transgender or nonbinary youth, ages 13-24 years) found that receiving GAHT was associated with significantly lower odds of recent depression (aOR = 0.73; P < .001) and suicidality (aOR = 0.74; P < .001) compared to those who wanted GAHT but did not receive it. The authors were unable to differentiate the effects of receiving GAHT from the effects of parental support for their child’s gender identity, which may be a confounding factor.5
Recommendations from others
The World Professional Association for Transgender Health Standards of Care state that “gender incongruence that causes clinically significant distress and impairment often requires medically necessary clinical interventions” and recommends “health care professionals initiate and continue gender-affirming hormone therapy … due to demonstrated improvement in psychosocial functioning and quality of life.”6 The Endocrine Society Position Statement on Transgender Health states that “medical intervention for transgender youth and adults (including … hormone therapy) is effective, relatively safe (when appropriately monitored), and has been established as the standard of care.”7 The American Academy of Family Physicians “supports gender-affirming care as an evidence-informed intervention that can promote health equity for gender-diverse individuals.”8
Editor’s takeaway
Family physicians commonly address many factors that can impact the QOL for our patients with gender dysphoria: lack of fixed residence, underemployment, food insecurity, and trauma. GAHT, especially in male-to-female transgender patients, may further improve QOL without evidence of harm.
Evidence summary
GAHT may improve depression and quality of life, but not anxiety
A well-done systematic review of transgender men and transgender women demonstrated that GAHT of more than a year’s duration was associated with modestly improved standardized scores for QOL, depression, and possibly anxiety.1 It was also associated with improved scores for depression in transgender adolescents.
The authors identified 15 prospective cohort studies (n = 626 transgender adults [mean age, 25-34 years]; 198 transgender adolescent girls and boys [mean age, 15-16 years]), 2 retrospective cohort studies (n = 1756 adults; mean age, 25-32 years), and 4 cross-sectional studies (n = 336 adults; mean age, 30-37 years).
Researchers recruited participants using strict eligibility criteria (psychiatric evaluation and formal diagnosis of gender dysphoria), with no prior history of GAHT, largely from gender-affirming specialty clinics at university hospitals. Most studies were conducted after the year 2000, predominantly in Europe (8 studies in Italy; 2 each in Belgium, the Netherlands, the United States, and Spain).
GAHT comprised testosterone for transgender men (14 studies used injectable testosterone cypionate, enanthate, undecanoate, or transdermal gels), estrogens (usually with an anti-androgen such as cyproterone acetate or spironolactone) for transgender women (10 studies used transdermal, oral, or injectable estradiol valerate or conjugated estrogens), and gonadotropin-releasing hormone (GnRH) therapy for transgender adolescents (3 studies).
Researchers evaluated the outcomes of QOL, depression, and anxiety with standardized scores on validated screening tools and suicide (2 studies) by medical records. GAHT in adult transgender men and transgender women was associated with modest improvements in QOL (3 of 5 studies) and depression (8 of 12 studies), and some improvement in anxiety scores (2 of 8 studies; see TABLE1). There was insufficient evidence to determine whether GAHT had any effect on suicide. In adolescent transgender girls and boys, GAHT was associated with modest improvements in depression but not QOL or anxiety scores.

The authors rated the strength of evidence from the included studies as low, based on study quality (small study sizes, uncontrolled confounding factors, and risk of bias in study designs).
Additional research supports GAHT’s association with improved outcomes
Three studies, published after the systematic review, evaluated outcomes before and after GAHT and found similar results. All studies recruited treatment-seeking participants from specialty clinics.
Continue to: An Australian propsective longitudinal..
An Australian prospective longitudinal controlled study (n = 77 transgender adults; 103 cisgender controls) evaluated GAHT outcomes after 6 months and found a significant reduction in gender dysphoria scores in both transgender males (adjusted mean difference [aMD] = –6.8; 95% CI, –8.7 to –4.9; P < .001) and transgender females (aMD = –4.2; 95% CI, –6.2 to –2.2; P < .001) vs controls. QOL scores (emotional well-being, social functioning) improved only for transgender males (well-being: aMD = +7.5; 95% CI, 1.3 to 13.6; P < .018; social functioning: aMD = +12.5; 95% CI, 2.8 to 22.2; P = .011).2
A US prospective cohort study (n = 104 adolescents; mean age, 16 years) examined the effect of GnRH and/or GAHT over a 12-month period and found significant decreases in standardized scores for depression (adjusted odds ratio [aOR] = 0.4; 95% CI, 0.17-0.95) and suicidality (aOR = 0.27; 95% CI, 0.11-0.65) but not for anxiety. Participants who did not receive hormonal interventions had increased scores for depression and suicidality at 3 and 6 months’ follow-up.3
A prospective cohort study from the UK (n = 178 transgender adults) examined outcomes before and after GAHT treatment over 18 months and found significant decreases in standardized scores for depression (transgender males: –2.1; 95% CI, –3.2 to –1.2; P < .001; transgender females: –1.9; 95% CI, –2.8 to –1.0; P < .001) but not for anxiety.4
A large US study shows GAHT may reduce depression scores
A recent large cross-sectional study from the United States (n = 11,914 transgender or nonbinary youth, ages 13-24 years) found that receiving GAHT was associated with significantly lower odds of recent depression (aOR = 0.73; P < .001) and suicidality (aOR = 0.74; P < .001) compared to those who wanted GAHT but did not receive it. The authors were unable to differentiate the effects of receiving GAHT from the effects of parental support for their child’s gender identity, which may be a confounding factor.5
Recommendations from others
The World Professional Association for Transgender Health Standards of Care state that “gender incongruence that causes clinically significant distress and impairment often requires medically necessary clinical interventions” and recommends “health care professionals initiate and continue gender-affirming hormone therapy … due to demonstrated improvement in psychosocial functioning and quality of life.”6 The Endocrine Society Position Statement on Transgender Health states that “medical intervention for transgender youth and adults (including … hormone therapy) is effective, relatively safe (when appropriately monitored), and has been established as the standard of care.”7 The American Academy of Family Physicians “supports gender-affirming care as an evidence-informed intervention that can promote health equity for gender-diverse individuals.”8
Editor’s takeaway
Family physicians commonly address many factors that can impact the QOL for our patients with gender dysphoria: lack of fixed residence, underemployment, food insecurity, and trauma. GAHT, especially in male-to-female transgender patients, may further improve QOL without evidence of harm.
1. Baker KE, Wilson LM, Sharma R, et al. Hormone therapy, mental health, and quality of life among transgender people: a systematic review. J Endocr Soc. 2021;5:bvab011. doi: 10.1210/jendso/bvab011
2. Foster Skewis L, Bretherton I, Leemaqz, SY, et al. Short-term effects of gender-affirming hormone therapy on dysphoria and quality of life in transgender individuals: a prospective controlled study. Front Endocrinol (Lausanne). 2021;12:717766.
3. Tordoff DM, Wanta JW, Collin A, et al. Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Netw Open. 2022;5:e220978. doi: 10.1001/jamanetworkopen.2022.0978
4. Aldridge Z, Patel S, Guo B, et al. Long-term effect of gender-affirming hormone treatment on depression and anxiety symptoms in transgender people: a prospective cohort study. Andrology. 2021;9:1808-1816. doi: 10.1111/andr.12884
5. Green AE, DeChants JP, Price MN, et al. Association of gender-affirming hormone therapy with depression, thoughts of suicide, and attempted suicide among transgender and nonbinary youth. J Adolesc Health. 2022;70:643-649. doi: 10.1016/j.jadohealth.2021.10.036
6. World Professional Association for Transgender Health. Standards of Care for the Health of Transsexual, Transgender, and Gender Nonconforming People. 8th version. Published 2022. Accessed November 17, 2022. www.wpath.org/publications/soc
7. Endocrine Society. Transgender health: an Endocrine Society position statement. Updated December 16, 2020. Accessed November 17, 2022. www.endocrine.org/advocacy/position-statements/transgender-health
8. American Academy of Family Physicians. Care for the transgender and gender nonbinary patient. Updated September 2022. Accessed November 17, 2022. www.aafp.org/about/policies/all/transgender-nonbinary.html
1. Baker KE, Wilson LM, Sharma R, et al. Hormone therapy, mental health, and quality of life among transgender people: a systematic review. J Endocr Soc. 2021;5:bvab011. doi: 10.1210/jendso/bvab011
2. Foster Skewis L, Bretherton I, Leemaqz, SY, et al. Short-term effects of gender-affirming hormone therapy on dysphoria and quality of life in transgender individuals: a prospective controlled study. Front Endocrinol (Lausanne). 2021;12:717766.
3. Tordoff DM, Wanta JW, Collin A, et al. Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Netw Open. 2022;5:e220978. doi: 10.1001/jamanetworkopen.2022.0978
4. Aldridge Z, Patel S, Guo B, et al. Long-term effect of gender-affirming hormone treatment on depression and anxiety symptoms in transgender people: a prospective cohort study. Andrology. 2021;9:1808-1816. doi: 10.1111/andr.12884
5. Green AE, DeChants JP, Price MN, et al. Association of gender-affirming hormone therapy with depression, thoughts of suicide, and attempted suicide among transgender and nonbinary youth. J Adolesc Health. 2022;70:643-649. doi: 10.1016/j.jadohealth.2021.10.036
6. World Professional Association for Transgender Health. Standards of Care for the Health of Transsexual, Transgender, and Gender Nonconforming People. 8th version. Published 2022. Accessed November 17, 2022. www.wpath.org/publications/soc
7. Endocrine Society. Transgender health: an Endocrine Society position statement. Updated December 16, 2020. Accessed November 17, 2022. www.endocrine.org/advocacy/position-statements/transgender-health
8. American Academy of Family Physicians. Care for the transgender and gender nonbinary patient. Updated September 2022. Accessed November 17, 2022. www.aafp.org/about/policies/all/transgender-nonbinary.html
EVIDENCE-BASED ANSWER:
There are modest effects on depression but not anxiety. Gender-affirming hormone therapy (GAHT) is associated with modest improvements in standardized scores for quality of life (QOL) and depression in adult male-to-female and female-to-male transgender people and modest improvements in depression scores in transgender adolescents, but the effect on anxiety is uncertain (strength of recommendation [SOR]: B, based on a preponderance of low-quality prospective cohort studies with inconsistent results).
GAHT is associated with reduced gender dysphoria and decreased suicidality (SOR: B, based on a prospective cohort study). However, there is insufficient evidence to determine any effect on suicide completion. No studies associated GAHT with worsened QOL, depression, or anxiety scores.
How accurate is transcutaneous bilirubin testing in newborns with darker skin tones?
EVIDENCE SUMMARY
Some evidence suggests overestimation in all skin tones
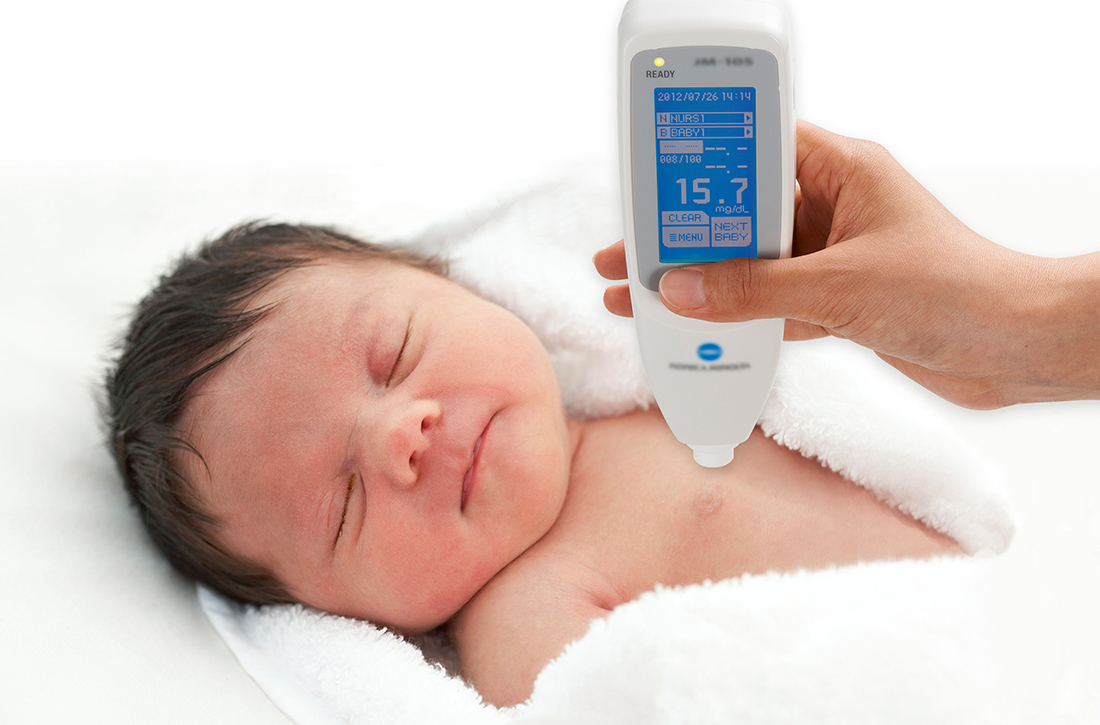
In a prospective diagnostic cohort study of 1553 infants in Nigeria, the accuracy of TcB measurement with 2 transcutaneous bilirubinometers (Konica Minolta/Air Shields JM- 103 and Respironics BiliChek) was analyzed. 1 The study population was derived from neonates delivered in a single maternity hospital in Lagos who were ≥ 35 weeks gestational age or ≥ 2.2 kg.
Using a color scale generated for this population, researchers stratified neonates into 1 of 3 skin tone groups: light brown, medium brown, or dark brown. TcB and TSB paired samples were collected in the first 120 hours of life in all patients. JM-103 recordings comprised 71.9% of TcB readings.
Overall, TcB testing overestimated the TSB by ≥ 2 mg/dL in 64.5% of infants, ≥ 3 mg/dL in 42.7%, and > 4 mg/dL in 25.7%. TcB testing underestimated the TSB by ≥ 2 mg/dL in 1.1% of infants, ≥ 3 mg/dL in 0.5%, and > 4 mg/dL in 0.3%.1
Local variation in skin tone was not associated with changes in overestimation, although the researchers noted that a key limitation of the study was a lack of lighttoned infants for comparison.1
A prospective diagnostic cohort study of 1359 infants in Spain compared TcB measurements to TSB levels using the Dräger Jaundice Meter JM-105.2 Patients included all neonates (gestational age, 36.6 to 41.1 weeks) born at a single hospital in Barcelona.
Using a validated skin tone scale, researchers stratified neonates at 24 hours of life to 1 of 4 skin tones: light (n = 337), medium light (n = 750), medium dark (n = 249), and dark (n = 23). They then obtained TSB samples at 48 to 72 hours of life, along with other routine screening labs and midsternal TcB measurements.
TcB testing tended to overestimate TSB (when < 15 mg/dL) for all skin tones, although to a larger degree for neonates with dark skin tones (mean overestimation, 0.7 mg/dL for light; 1.08 mg/dL for medium light; 1.89 mg/dL for medium dark; and 1.86 mg/dL for dark; P < .001 for light vs medium dark or dark).2
Continue to: Stated limitations...
Stated limitations of the study included relatively low numbers of neonates with dark skin tone, no test of interobserver reliability in skin tone assignment, and enrollment of exclusively healthy neonates with low bilirubin levels.2
Other studies report overestimation in infants with darker skin tone
Two Canadian diagnostic cohort studies also found evidence that TcB testing overestimated TSB in infants with darker skin tones, although TcB test characteristics proved stable over a wide range of bilirubin levels.
The first study enrolled 451 neonates ≥ 35 weeks gestational age at a hospital in Ottawa and assessed TcB using the JM-103 meter.3 The neonates were stratified into light (n = 51), medium (n = 326), and dark (n = 74) skin tones using cosmetic reference color swatches. All had a TcB and TSB obtained within 30 minutes of each other.
TcB testing underestimated TSB in infants with light and medium skin tones and overestimated TSB in infants with darker skin tone (mean difference, –0.88 mg/dL for light; –1.1 mg/dL for medium; and 0.68 mg/dL for dark; P not given). The mean area under the curve (AUC) was ≥ 0.94 for all receiver–operator characteristic (ROC) curves across all skin tones and bilirubin thresholds (AUC range, 0-1, with > 0.8 indicating strong modeling).3
Limitations of the study included failure to check interrater reliability for skin tone assessment, low numbers of infants with elevated bilirubin (≥ 13.5 mg/dL), and very few infants in either the dark or light skin tone groups.3
Continue to: The second Canadian study...
The second Canadian study enrolled 774 infants born at ≥ 37 weeks gestational age in Calgary and assessed TcB with the JM-103.4 Infants were categorized as having light (n = 347), medium (n = 412), and dark (n = 15) skin tones by study nurses, based on reference cosmetic colors. All infants had paired TcB and TSB measurements within 60 minutes of each other and before 120 hours of life.
Multivariate linear regression analysis using medium skin tone as the reference group found a tendency toward low TcB levels in infants with light skin tone and a tendency toward high TcB levels in infants with dark skin tone (adjusted R2 = 0.86). The AUC was ≥ 0.95 for all ROC curves for lightand medium-toned infants at key TSB cutoff points; the study included too few infants with dark skin tone to generate ROC curves for that group.4
Recommendations from others
In 2009, the American Academy of Pediatrics (AAP) recommended universal predischarge screening for hyperbilirubinemia in newborns using either TcB testing or TSB. The AAP statement did not address the effect of skin tone on TcB levels, but did advise regular calibration of TcB and TSB results at the hospital level.5
In 2016, the National Institute for Health and Care Excellence (NICE) updated their guideline on jaundice in newborns younger than 28 days old. NICE recommended visual inspection of all babies for jaundice by examining them in bright natural light and looking for jaundice on blanched skin; it specifically advised checking sclera and gums in infants with darker skin tones.6
The Nigerian researchers noted earlier have published an updated TcB nomogram for their patient population.7
Editor’s takeaway
Even with the small variation of 2 mg/dL or less between transcutaneous and serum bilirubin, and the SOR of C due to lab values being labeled disease-oriented evidence, TcB proves to be useful. In practice, concerning TcB values should lead to serum bilirubin confirmation. This evidence indicates we might be ordering TSB measurements more or less often depending on skin tone, reinforcing the need for review and adjustment of TcB cut-off levels based on the local population.
1. Olusanya BO, Imosemi DO, Emokpae AA. Differences between transcutaneous and serum bilirubin measurements in Black African neonates. Pediatrics. 2016;138:e20160907. doi: 10.1542/ peds.2016-0907
2. Maya-Enero S, Candel-Pau J, Garcia-Garcia J, et al. Reliability of transcutaneous bilirubin determination based on skin color determined by a neonatal skin color scale of our own. Eur J Pediatr. 2021;180:607-616. doi: 10.1007/s00431-020-03885-0
3. Samiee-Zafarghandy S, Feberova J, Williams K, et al. Influence of skin colour on diagnostic accuracy of the jaundice meter JM 103 in newborns. Arch Dis Child Fetal Neonatal Ed. 2014;99: F480-F484. doi: 10.1136/archdischild-2013-305699
4. Wainer S, Rabi Y, Parmar SM, et al. Impact of skin tone on the performance of a transcutaneous jaundice meter. Acta Paediatr. 2009;98:1909-1915. doi: 10.1111/j.1651-2227.2009.01497.x
5. Maisels MJ, Bhutani VK, Bogen D, et al. Hyperbilirubinemia in the newborn infant > or = 35 weeks’ gestation: an update with clarifications. Pediatrics. 2009;124:1193-1198. doi: 10.1542/peds. 2009-0329
6. Amos RC, Jacob H, Leith W. Jaundice in newborn babies under 28 days: NICE guideline 2016 (CG98). Arch Dis Child Educ Pract Ed. 2017;102:207-209. doi: 10.1136/archdischild-2016-311556
7. Olusanya BO, Mabogunje CA, Imosemi DO, et al. Transcutaneous bilirubin nomograms in African neonates. PloS ONE. 2017; 12:e0172058. doi: 10.1371/journal.pone.0172058
EVIDENCE SUMMARY
Some evidence suggests overestimation in all skin tones
In a prospective diagnostic cohort study of 1553 infants in Nigeria, the accuracy of TcB measurement with 2 transcutaneous bilirubinometers (Konica Minolta/Air Shields JM- 103 and Respironics BiliChek) was analyzed. 1 The study population was derived from neonates delivered in a single maternity hospital in Lagos who were ≥ 35 weeks gestational age or ≥ 2.2 kg.
Using a color scale generated for this population, researchers stratified neonates into 1 of 3 skin tone groups: light brown, medium brown, or dark brown. TcB and TSB paired samples were collected in the first 120 hours of life in all patients. JM-103 recordings comprised 71.9% of TcB readings.
Overall, TcB testing overestimated the TSB by ≥ 2 mg/dL in 64.5% of infants, ≥ 3 mg/dL in 42.7%, and > 4 mg/dL in 25.7%. TcB testing underestimated the TSB by ≥ 2 mg/dL in 1.1% of infants, ≥ 3 mg/dL in 0.5%, and > 4 mg/dL in 0.3%.1
Local variation in skin tone was not associated with changes in overestimation, although the researchers noted that a key limitation of the study was a lack of lighttoned infants for comparison.1
A prospective diagnostic cohort study of 1359 infants in Spain compared TcB measurements to TSB levels using the Dräger Jaundice Meter JM-105.2 Patients included all neonates (gestational age, 36.6 to 41.1 weeks) born at a single hospital in Barcelona.
Using a validated skin tone scale, researchers stratified neonates at 24 hours of life to 1 of 4 skin tones: light (n = 337), medium light (n = 750), medium dark (n = 249), and dark (n = 23). They then obtained TSB samples at 48 to 72 hours of life, along with other routine screening labs and midsternal TcB measurements.
TcB testing tended to overestimate TSB (when < 15 mg/dL) for all skin tones, although to a larger degree for neonates with dark skin tones (mean overestimation, 0.7 mg/dL for light; 1.08 mg/dL for medium light; 1.89 mg/dL for medium dark; and 1.86 mg/dL for dark; P < .001 for light vs medium dark or dark).2
Continue to: Stated limitations...
Stated limitations of the study included relatively low numbers of neonates with dark skin tone, no test of interobserver reliability in skin tone assignment, and enrollment of exclusively healthy neonates with low bilirubin levels.2
Other studies report overestimation in infants with darker skin tone
Two Canadian diagnostic cohort studies also found evidence that TcB testing overestimated TSB in infants with darker skin tones, although TcB test characteristics proved stable over a wide range of bilirubin levels.
The first study enrolled 451 neonates ≥ 35 weeks gestational age at a hospital in Ottawa and assessed TcB using the JM-103 meter.3 The neonates were stratified into light (n = 51), medium (n = 326), and dark (n = 74) skin tones using cosmetic reference color swatches. All had a TcB and TSB obtained within 30 minutes of each other.
TcB testing underestimated TSB in infants with light and medium skin tones and overestimated TSB in infants with darker skin tone (mean difference, –0.88 mg/dL for light; –1.1 mg/dL for medium; and 0.68 mg/dL for dark; P not given). The mean area under the curve (AUC) was ≥ 0.94 for all receiver–operator characteristic (ROC) curves across all skin tones and bilirubin thresholds (AUC range, 0-1, with > 0.8 indicating strong modeling).3
Limitations of the study included failure to check interrater reliability for skin tone assessment, low numbers of infants with elevated bilirubin (≥ 13.5 mg/dL), and very few infants in either the dark or light skin tone groups.3
Continue to: The second Canadian study...
The second Canadian study enrolled 774 infants born at ≥ 37 weeks gestational age in Calgary and assessed TcB with the JM-103.4 Infants were categorized as having light (n = 347), medium (n = 412), and dark (n = 15) skin tones by study nurses, based on reference cosmetic colors. All infants had paired TcB and TSB measurements within 60 minutes of each other and before 120 hours of life.
Multivariate linear regression analysis using medium skin tone as the reference group found a tendency toward low TcB levels in infants with light skin tone and a tendency toward high TcB levels in infants with dark skin tone (adjusted R2 = 0.86). The AUC was ≥ 0.95 for all ROC curves for lightand medium-toned infants at key TSB cutoff points; the study included too few infants with dark skin tone to generate ROC curves for that group.4
Recommendations from others
In 2009, the American Academy of Pediatrics (AAP) recommended universal predischarge screening for hyperbilirubinemia in newborns using either TcB testing or TSB. The AAP statement did not address the effect of skin tone on TcB levels, but did advise regular calibration of TcB and TSB results at the hospital level.5
In 2016, the National Institute for Health and Care Excellence (NICE) updated their guideline on jaundice in newborns younger than 28 days old. NICE recommended visual inspection of all babies for jaundice by examining them in bright natural light and looking for jaundice on blanched skin; it specifically advised checking sclera and gums in infants with darker skin tones.6
The Nigerian researchers noted earlier have published an updated TcB nomogram for their patient population.7
Editor’s takeaway
Even with the small variation of 2 mg/dL or less between transcutaneous and serum bilirubin, and the SOR of C due to lab values being labeled disease-oriented evidence, TcB proves to be useful. In practice, concerning TcB values should lead to serum bilirubin confirmation. This evidence indicates we might be ordering TSB measurements more or less often depending on skin tone, reinforcing the need for review and adjustment of TcB cut-off levels based on the local population.
EVIDENCE SUMMARY
Some evidence suggests overestimation in all skin tones
In a prospective diagnostic cohort study of 1553 infants in Nigeria, the accuracy of TcB measurement with 2 transcutaneous bilirubinometers (Konica Minolta/Air Shields JM- 103 and Respironics BiliChek) was analyzed. 1 The study population was derived from neonates delivered in a single maternity hospital in Lagos who were ≥ 35 weeks gestational age or ≥ 2.2 kg.
Using a color scale generated for this population, researchers stratified neonates into 1 of 3 skin tone groups: light brown, medium brown, or dark brown. TcB and TSB paired samples were collected in the first 120 hours of life in all patients. JM-103 recordings comprised 71.9% of TcB readings.
Overall, TcB testing overestimated the TSB by ≥ 2 mg/dL in 64.5% of infants, ≥ 3 mg/dL in 42.7%, and > 4 mg/dL in 25.7%. TcB testing underestimated the TSB by ≥ 2 mg/dL in 1.1% of infants, ≥ 3 mg/dL in 0.5%, and > 4 mg/dL in 0.3%.1
Local variation in skin tone was not associated with changes in overestimation, although the researchers noted that a key limitation of the study was a lack of lighttoned infants for comparison.1
A prospective diagnostic cohort study of 1359 infants in Spain compared TcB measurements to TSB levels using the Dräger Jaundice Meter JM-105.2 Patients included all neonates (gestational age, 36.6 to 41.1 weeks) born at a single hospital in Barcelona.
Using a validated skin tone scale, researchers stratified neonates at 24 hours of life to 1 of 4 skin tones: light (n = 337), medium light (n = 750), medium dark (n = 249), and dark (n = 23). They then obtained TSB samples at 48 to 72 hours of life, along with other routine screening labs and midsternal TcB measurements.
TcB testing tended to overestimate TSB (when < 15 mg/dL) for all skin tones, although to a larger degree for neonates with dark skin tones (mean overestimation, 0.7 mg/dL for light; 1.08 mg/dL for medium light; 1.89 mg/dL for medium dark; and 1.86 mg/dL for dark; P < .001 for light vs medium dark or dark).2
Continue to: Stated limitations...
Stated limitations of the study included relatively low numbers of neonates with dark skin tone, no test of interobserver reliability in skin tone assignment, and enrollment of exclusively healthy neonates with low bilirubin levels.2
Other studies report overestimation in infants with darker skin tone
Two Canadian diagnostic cohort studies also found evidence that TcB testing overestimated TSB in infants with darker skin tones, although TcB test characteristics proved stable over a wide range of bilirubin levels.
The first study enrolled 451 neonates ≥ 35 weeks gestational age at a hospital in Ottawa and assessed TcB using the JM-103 meter.3 The neonates were stratified into light (n = 51), medium (n = 326), and dark (n = 74) skin tones using cosmetic reference color swatches. All had a TcB and TSB obtained within 30 minutes of each other.
TcB testing underestimated TSB in infants with light and medium skin tones and overestimated TSB in infants with darker skin tone (mean difference, –0.88 mg/dL for light; –1.1 mg/dL for medium; and 0.68 mg/dL for dark; P not given). The mean area under the curve (AUC) was ≥ 0.94 for all receiver–operator characteristic (ROC) curves across all skin tones and bilirubin thresholds (AUC range, 0-1, with > 0.8 indicating strong modeling).3
Limitations of the study included failure to check interrater reliability for skin tone assessment, low numbers of infants with elevated bilirubin (≥ 13.5 mg/dL), and very few infants in either the dark or light skin tone groups.3
Continue to: The second Canadian study...
The second Canadian study enrolled 774 infants born at ≥ 37 weeks gestational age in Calgary and assessed TcB with the JM-103.4 Infants were categorized as having light (n = 347), medium (n = 412), and dark (n = 15) skin tones by study nurses, based on reference cosmetic colors. All infants had paired TcB and TSB measurements within 60 minutes of each other and before 120 hours of life.
Multivariate linear regression analysis using medium skin tone as the reference group found a tendency toward low TcB levels in infants with light skin tone and a tendency toward high TcB levels in infants with dark skin tone (adjusted R2 = 0.86). The AUC was ≥ 0.95 for all ROC curves for lightand medium-toned infants at key TSB cutoff points; the study included too few infants with dark skin tone to generate ROC curves for that group.4
Recommendations from others
In 2009, the American Academy of Pediatrics (AAP) recommended universal predischarge screening for hyperbilirubinemia in newborns using either TcB testing or TSB. The AAP statement did not address the effect of skin tone on TcB levels, but did advise regular calibration of TcB and TSB results at the hospital level.5
In 2016, the National Institute for Health and Care Excellence (NICE) updated their guideline on jaundice in newborns younger than 28 days old. NICE recommended visual inspection of all babies for jaundice by examining them in bright natural light and looking for jaundice on blanched skin; it specifically advised checking sclera and gums in infants with darker skin tones.6
The Nigerian researchers noted earlier have published an updated TcB nomogram for their patient population.7
Editor’s takeaway
Even with the small variation of 2 mg/dL or less between transcutaneous and serum bilirubin, and the SOR of C due to lab values being labeled disease-oriented evidence, TcB proves to be useful. In practice, concerning TcB values should lead to serum bilirubin confirmation. This evidence indicates we might be ordering TSB measurements more or less often depending on skin tone, reinforcing the need for review and adjustment of TcB cut-off levels based on the local population.
1. Olusanya BO, Imosemi DO, Emokpae AA. Differences between transcutaneous and serum bilirubin measurements in Black African neonates. Pediatrics. 2016;138:e20160907. doi: 10.1542/ peds.2016-0907
2. Maya-Enero S, Candel-Pau J, Garcia-Garcia J, et al. Reliability of transcutaneous bilirubin determination based on skin color determined by a neonatal skin color scale of our own. Eur J Pediatr. 2021;180:607-616. doi: 10.1007/s00431-020-03885-0
3. Samiee-Zafarghandy S, Feberova J, Williams K, et al. Influence of skin colour on diagnostic accuracy of the jaundice meter JM 103 in newborns. Arch Dis Child Fetal Neonatal Ed. 2014;99: F480-F484. doi: 10.1136/archdischild-2013-305699
4. Wainer S, Rabi Y, Parmar SM, et al. Impact of skin tone on the performance of a transcutaneous jaundice meter. Acta Paediatr. 2009;98:1909-1915. doi: 10.1111/j.1651-2227.2009.01497.x
5. Maisels MJ, Bhutani VK, Bogen D, et al. Hyperbilirubinemia in the newborn infant > or = 35 weeks’ gestation: an update with clarifications. Pediatrics. 2009;124:1193-1198. doi: 10.1542/peds. 2009-0329
6. Amos RC, Jacob H, Leith W. Jaundice in newborn babies under 28 days: NICE guideline 2016 (CG98). Arch Dis Child Educ Pract Ed. 2017;102:207-209. doi: 10.1136/archdischild-2016-311556
7. Olusanya BO, Mabogunje CA, Imosemi DO, et al. Transcutaneous bilirubin nomograms in African neonates. PloS ONE. 2017; 12:e0172058. doi: 10.1371/journal.pone.0172058
1. Olusanya BO, Imosemi DO, Emokpae AA. Differences between transcutaneous and serum bilirubin measurements in Black African neonates. Pediatrics. 2016;138:e20160907. doi: 10.1542/ peds.2016-0907
2. Maya-Enero S, Candel-Pau J, Garcia-Garcia J, et al. Reliability of transcutaneous bilirubin determination based on skin color determined by a neonatal skin color scale of our own. Eur J Pediatr. 2021;180:607-616. doi: 10.1007/s00431-020-03885-0
3. Samiee-Zafarghandy S, Feberova J, Williams K, et al. Influence of skin colour on diagnostic accuracy of the jaundice meter JM 103 in newborns. Arch Dis Child Fetal Neonatal Ed. 2014;99: F480-F484. doi: 10.1136/archdischild-2013-305699
4. Wainer S, Rabi Y, Parmar SM, et al. Impact of skin tone on the performance of a transcutaneous jaundice meter. Acta Paediatr. 2009;98:1909-1915. doi: 10.1111/j.1651-2227.2009.01497.x
5. Maisels MJ, Bhutani VK, Bogen D, et al. Hyperbilirubinemia in the newborn infant > or = 35 weeks’ gestation: an update with clarifications. Pediatrics. 2009;124:1193-1198. doi: 10.1542/peds. 2009-0329
6. Amos RC, Jacob H, Leith W. Jaundice in newborn babies under 28 days: NICE guideline 2016 (CG98). Arch Dis Child Educ Pract Ed. 2017;102:207-209. doi: 10.1136/archdischild-2016-311556
7. Olusanya BO, Mabogunje CA, Imosemi DO, et al. Transcutaneous bilirubin nomograms in African neonates. PloS ONE. 2017; 12:e0172058. doi: 10.1371/journal.pone.0172058
EVIDENCE-BASED ANSWER:
Fairly accurate. Photometric transcutaneous bilirubin (TcB) testing may overestimate total serum bilirubin (TSB) in neonates with darker skin tones by a mean of 0.68 to > 2 mg/dL (strength of recommendation [SOR]: C, diagnostic cohort studies with differing reference standards).
Overall, TcB meters retain acceptable accuracy in infants of all skin tones across a range of bilirubin levels, despite being more likely to underestimate lighter skin tones and overestimate darker ones (SOR: C, diagnostic cohort studies with differing reference standards). It is unclear if the higher readings prompt an increase in blood draws or otherwise alter care.
Does an early COPD diagnosis improve long-term outcomes?
EVIDENCE SUMMARY
Early Dx didn’t improve smoking cessation rates or treatment outcomes
A 2016 evidence report and systematic review for the US Preventive Services Task Force (USPSTF) identified no studies directly comparing the effectiveness of COPD screening on patient outcomes, so the authors looked first at studies on the outcomes of screening, followed by studies exploring the effects of early treatment.1
The authors identified 5 fair-quality RCTs (N = 1694) addressing the effect of screening asymptomatic patients for COPD with spirometry on the outcome of smoking cessation. One trial (n = 561) found better 12-month smoking cessation rates in patients who underwent spirometry screening and were given their “lung age” (13.6% vs 6.4% not given a lung age; P < .005; number needed to treat [NNT] = 14). However, a similar study (n = 542) published a year later found no significant difference in quit rates with or without “lung age” discussions (10.9% vs 13%, respectively; P not significant). In the other 3 studies, screening produced no significant effect on smoking cessation rates.1
As for possible early treatment benefits, the review authors identified only 1 RCT (n = 1175) that included any patients with mild COPD (defined as COPD with a forced expiratory volume in 1 second [FEV1] ≥ 80% of predicted normal value). It assessed treatment with inhaled corticosteroids (ICS) in patients with mild COPD who continued to smoke. The trial did not record symptoms (if any) at intake. ICS therapy reduced the frequency of COPD exacerbations (relative risk = 0.63; 95% CI, 0.47-0.85), although patients with milder COPD benefitted little in absolute terms (by 0.02 exacerbations/year).1 The review authors further noted that data were insufficient to make definitive statements about the effect of ICS on dyspnea or health-related quality of life.
But later diagnosis is associated with poorer outcomes
Two recent, large retrospective observational cohort studies, however, have examined the impact of an early vs late COPD diagnosis in patients with dyspnea or other symptoms of COPD.2,3 A later diagnosis was associated with worse outcomes.
In the first study, researchers in Sweden identified patients older than 40 years who had received a new diagnosis of COPD between 2000 and 2014.2 They examined electronic health record data for 6 different “indicators” of COPD during the 5 years prior to date of diagnosis: pneumonia, other respiratory disease, oral steroids, antibiotics for respiratory infection, prescribed drugs for respiratory symptoms, and lung function measurement. Researchers categorized patients as early diagnosis (if they had ≤ 2 indicators prior to diagnosis) or late diagnosis (≥ 3 indicators prior to diagnosis). Compared with early diagnosis (n = 3870), late diagnosis (n = 8827) was associated with
- a higher annual rate of exacerbations within the first 2 years after diagnosis (2.67 vs 1.41; hazard ratio [HR] = 1.89; 95% CI, 1.83-1.96; P < .0001; number of early diagnoses needed to prevent 1 exacerbation in 1 year = 79),
- shorter time to first exacerbation (HR = 1.61; 95% CI, 1.54-1.69; P < .0001), and
- higher direct health care costs (by €1500 per year; no P value given).
Mortality was not different between the groups (HR = 1.04; 95% CI, 0.98-1.11; P = .18).
The second investigation was a similarly designed retrospective observational cohort study using a large UK database.3 Researchers enrolled patients who were at least 40 years old and received a new diagnosis of COPD between 2011 and 2014.
Continue to: Researchers examined electronic...
Researchers examined electronic health record data in the 5 years prior to diagnosis for 7 possible indicators of early COPD: pneumonia, respiratory disease other than pneumonia, chest radiograph, prescription of oral steroids, prescription of antibiotics for lung infection, prescription to manage respiratory disease symptoms, and lung function measurement. Researchers categorized patients as early diagnosis (≥ 2 indicators prior to diagnosis) or late diagnosis (≥ 3 indicators prior to diagnosis). Compared with early diagnosis (n = 3375), late diagnosis (n = 6783) was associated with a higher annual rate of exacerbations over 3-year follow-up (1.09 vs 0.57; adjusted HR = 1.68; 95% CI, 1.59-1.79; P < .0001; or 1 additional exacerbation in 192 patients in 1 year), shorter mean time to first exacerbation (HR = 1.46; 95% CI: 1.38-1.55; P < .0001), and a higher risk of hospitalization within 3 years (rate ratio = 1.18; 95% CI, 1.08-1.28; P = .0001). The researchers did not evaluate for mortality.
Importantly, patients in the late COPD diagnosis group in both trials had higher rates of other severe illnesses that cause dyspnea, including cardiovascular disease and other pulmonary diseases. As a result, dyspnea of other etiologies may have contributed to both the later diagnoses and the poorer clinical outcomes of the late-diagnosis group. Both studies had a high risk of lead-time bias.
Recommendations from others
In 2016, the USPSTF gave a “D” rating (moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits) to screening asymptomatic adults without respiratory symptoms for COPD.4 Likewise, the 2017 Global Initiative for Chronic Obstructive Lung Disease (GOLD) report did not recommend routine screening with spirometry but did advocate trying to make an accurate diagnosis using spirometry in patients with risk factors for COPD and chronic, progressive symptoms.5
Editor’s takeaway
Reasonably good evidence failed to find a benefit from an early COPD diagnosis. Even smoking cessation rates were not improved. Without better disease-modifying treatments, spirometry—the gold standard for confirming a COPD diagnosis—should not be used for screening asymptomatic patients.
1. Guirguis-Blake JM, Senger CA, Webber EM, et al. Screening for chronic obstructive pulmonary disease: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315:1378-1393. doi:10.1001/jama.2016.2654
2. Larsson K, Janson C, Ställberg B, et al. Impact of COPD diagnosis timing on clinical and economic outcomes: the ARCTIC observational cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:995-1008. doi: 10.2147/COPD.S195382
3. Kostikas K, Price D, Gutzwiller FS, et al. Clinical impact and healthcare resource utilization associated with early versus late COPD diagnosis in patients from UK CPRD database. Int J Chron Obstruct Pulmon Dis. 2020;15:1729-1738. doi: 10.2147/COPD.S255414
4. US Preventive Services Task Force; Siu AL, Bibbins-Domingo K, Grossman DC, et al. Screening for chronic obstructive pulmonary disease: US Preventive Services Task Force recommendation statement. JAMA. 2016;315:1372-1377. doi: 10.1001/jama.2016.2638
5. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195:557-582. doi: 10.1164/rccm.201701-0218PP
EVIDENCE SUMMARY
Early Dx didn’t improve smoking cessation rates or treatment outcomes
A 2016 evidence report and systematic review for the US Preventive Services Task Force (USPSTF) identified no studies directly comparing the effectiveness of COPD screening on patient outcomes, so the authors looked first at studies on the outcomes of screening, followed by studies exploring the effects of early treatment.1
The authors identified 5 fair-quality RCTs (N = 1694) addressing the effect of screening asymptomatic patients for COPD with spirometry on the outcome of smoking cessation. One trial (n = 561) found better 12-month smoking cessation rates in patients who underwent spirometry screening and were given their “lung age” (13.6% vs 6.4% not given a lung age; P < .005; number needed to treat [NNT] = 14). However, a similar study (n = 542) published a year later found no significant difference in quit rates with or without “lung age” discussions (10.9% vs 13%, respectively; P not significant). In the other 3 studies, screening produced no significant effect on smoking cessation rates.1
As for possible early treatment benefits, the review authors identified only 1 RCT (n = 1175) that included any patients with mild COPD (defined as COPD with a forced expiratory volume in 1 second [FEV1] ≥ 80% of predicted normal value). It assessed treatment with inhaled corticosteroids (ICS) in patients with mild COPD who continued to smoke. The trial did not record symptoms (if any) at intake. ICS therapy reduced the frequency of COPD exacerbations (relative risk = 0.63; 95% CI, 0.47-0.85), although patients with milder COPD benefitted little in absolute terms (by 0.02 exacerbations/year).1 The review authors further noted that data were insufficient to make definitive statements about the effect of ICS on dyspnea or health-related quality of life.
But later diagnosis is associated with poorer outcomes
Two recent, large retrospective observational cohort studies, however, have examined the impact of an early vs late COPD diagnosis in patients with dyspnea or other symptoms of COPD.2,3 A later diagnosis was associated with worse outcomes.
In the first study, researchers in Sweden identified patients older than 40 years who had received a new diagnosis of COPD between 2000 and 2014.2 They examined electronic health record data for 6 different “indicators” of COPD during the 5 years prior to date of diagnosis: pneumonia, other respiratory disease, oral steroids, antibiotics for respiratory infection, prescribed drugs for respiratory symptoms, and lung function measurement. Researchers categorized patients as early diagnosis (if they had ≤ 2 indicators prior to diagnosis) or late diagnosis (≥ 3 indicators prior to diagnosis). Compared with early diagnosis (n = 3870), late diagnosis (n = 8827) was associated with
- a higher annual rate of exacerbations within the first 2 years after diagnosis (2.67 vs 1.41; hazard ratio [HR] = 1.89; 95% CI, 1.83-1.96; P < .0001; number of early diagnoses needed to prevent 1 exacerbation in 1 year = 79),
- shorter time to first exacerbation (HR = 1.61; 95% CI, 1.54-1.69; P < .0001), and
- higher direct health care costs (by €1500 per year; no P value given).
Mortality was not different between the groups (HR = 1.04; 95% CI, 0.98-1.11; P = .18).
The second investigation was a similarly designed retrospective observational cohort study using a large UK database.3 Researchers enrolled patients who were at least 40 years old and received a new diagnosis of COPD between 2011 and 2014.
Continue to: Researchers examined electronic...
Researchers examined electronic health record data in the 5 years prior to diagnosis for 7 possible indicators of early COPD: pneumonia, respiratory disease other than pneumonia, chest radiograph, prescription of oral steroids, prescription of antibiotics for lung infection, prescription to manage respiratory disease symptoms, and lung function measurement. Researchers categorized patients as early diagnosis (≥ 2 indicators prior to diagnosis) or late diagnosis (≥ 3 indicators prior to diagnosis). Compared with early diagnosis (n = 3375), late diagnosis (n = 6783) was associated with a higher annual rate of exacerbations over 3-year follow-up (1.09 vs 0.57; adjusted HR = 1.68; 95% CI, 1.59-1.79; P < .0001; or 1 additional exacerbation in 192 patients in 1 year), shorter mean time to first exacerbation (HR = 1.46; 95% CI: 1.38-1.55; P < .0001), and a higher risk of hospitalization within 3 years (rate ratio = 1.18; 95% CI, 1.08-1.28; P = .0001). The researchers did not evaluate for mortality.
Importantly, patients in the late COPD diagnosis group in both trials had higher rates of other severe illnesses that cause dyspnea, including cardiovascular disease and other pulmonary diseases. As a result, dyspnea of other etiologies may have contributed to both the later diagnoses and the poorer clinical outcomes of the late-diagnosis group. Both studies had a high risk of lead-time bias.
Recommendations from others
In 2016, the USPSTF gave a “D” rating (moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits) to screening asymptomatic adults without respiratory symptoms for COPD.4 Likewise, the 2017 Global Initiative for Chronic Obstructive Lung Disease (GOLD) report did not recommend routine screening with spirometry but did advocate trying to make an accurate diagnosis using spirometry in patients with risk factors for COPD and chronic, progressive symptoms.5
Editor’s takeaway
Reasonably good evidence failed to find a benefit from an early COPD diagnosis. Even smoking cessation rates were not improved. Without better disease-modifying treatments, spirometry—the gold standard for confirming a COPD diagnosis—should not be used for screening asymptomatic patients.
EVIDENCE SUMMARY
Early Dx didn’t improve smoking cessation rates or treatment outcomes
A 2016 evidence report and systematic review for the US Preventive Services Task Force (USPSTF) identified no studies directly comparing the effectiveness of COPD screening on patient outcomes, so the authors looked first at studies on the outcomes of screening, followed by studies exploring the effects of early treatment.1
The authors identified 5 fair-quality RCTs (N = 1694) addressing the effect of screening asymptomatic patients for COPD with spirometry on the outcome of smoking cessation. One trial (n = 561) found better 12-month smoking cessation rates in patients who underwent spirometry screening and were given their “lung age” (13.6% vs 6.4% not given a lung age; P < .005; number needed to treat [NNT] = 14). However, a similar study (n = 542) published a year later found no significant difference in quit rates with or without “lung age” discussions (10.9% vs 13%, respectively; P not significant). In the other 3 studies, screening produced no significant effect on smoking cessation rates.1
As for possible early treatment benefits, the review authors identified only 1 RCT (n = 1175) that included any patients with mild COPD (defined as COPD with a forced expiratory volume in 1 second [FEV1] ≥ 80% of predicted normal value). It assessed treatment with inhaled corticosteroids (ICS) in patients with mild COPD who continued to smoke. The trial did not record symptoms (if any) at intake. ICS therapy reduced the frequency of COPD exacerbations (relative risk = 0.63; 95% CI, 0.47-0.85), although patients with milder COPD benefitted little in absolute terms (by 0.02 exacerbations/year).1 The review authors further noted that data were insufficient to make definitive statements about the effect of ICS on dyspnea or health-related quality of life.
But later diagnosis is associated with poorer outcomes
Two recent, large retrospective observational cohort studies, however, have examined the impact of an early vs late COPD diagnosis in patients with dyspnea or other symptoms of COPD.2,3 A later diagnosis was associated with worse outcomes.
In the first study, researchers in Sweden identified patients older than 40 years who had received a new diagnosis of COPD between 2000 and 2014.2 They examined electronic health record data for 6 different “indicators” of COPD during the 5 years prior to date of diagnosis: pneumonia, other respiratory disease, oral steroids, antibiotics for respiratory infection, prescribed drugs for respiratory symptoms, and lung function measurement. Researchers categorized patients as early diagnosis (if they had ≤ 2 indicators prior to diagnosis) or late diagnosis (≥ 3 indicators prior to diagnosis). Compared with early diagnosis (n = 3870), late diagnosis (n = 8827) was associated with
- a higher annual rate of exacerbations within the first 2 years after diagnosis (2.67 vs 1.41; hazard ratio [HR] = 1.89; 95% CI, 1.83-1.96; P < .0001; number of early diagnoses needed to prevent 1 exacerbation in 1 year = 79),
- shorter time to first exacerbation (HR = 1.61; 95% CI, 1.54-1.69; P < .0001), and
- higher direct health care costs (by €1500 per year; no P value given).
Mortality was not different between the groups (HR = 1.04; 95% CI, 0.98-1.11; P = .18).
The second investigation was a similarly designed retrospective observational cohort study using a large UK database.3 Researchers enrolled patients who were at least 40 years old and received a new diagnosis of COPD between 2011 and 2014.
Continue to: Researchers examined electronic...
Researchers examined electronic health record data in the 5 years prior to diagnosis for 7 possible indicators of early COPD: pneumonia, respiratory disease other than pneumonia, chest radiograph, prescription of oral steroids, prescription of antibiotics for lung infection, prescription to manage respiratory disease symptoms, and lung function measurement. Researchers categorized patients as early diagnosis (≥ 2 indicators prior to diagnosis) or late diagnosis (≥ 3 indicators prior to diagnosis). Compared with early diagnosis (n = 3375), late diagnosis (n = 6783) was associated with a higher annual rate of exacerbations over 3-year follow-up (1.09 vs 0.57; adjusted HR = 1.68; 95% CI, 1.59-1.79; P < .0001; or 1 additional exacerbation in 192 patients in 1 year), shorter mean time to first exacerbation (HR = 1.46; 95% CI: 1.38-1.55; P < .0001), and a higher risk of hospitalization within 3 years (rate ratio = 1.18; 95% CI, 1.08-1.28; P = .0001). The researchers did not evaluate for mortality.
Importantly, patients in the late COPD diagnosis group in both trials had higher rates of other severe illnesses that cause dyspnea, including cardiovascular disease and other pulmonary diseases. As a result, dyspnea of other etiologies may have contributed to both the later diagnoses and the poorer clinical outcomes of the late-diagnosis group. Both studies had a high risk of lead-time bias.
Recommendations from others
In 2016, the USPSTF gave a “D” rating (moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits) to screening asymptomatic adults without respiratory symptoms for COPD.4 Likewise, the 2017 Global Initiative for Chronic Obstructive Lung Disease (GOLD) report did not recommend routine screening with spirometry but did advocate trying to make an accurate diagnosis using spirometry in patients with risk factors for COPD and chronic, progressive symptoms.5
Editor’s takeaway
Reasonably good evidence failed to find a benefit from an early COPD diagnosis. Even smoking cessation rates were not improved. Without better disease-modifying treatments, spirometry—the gold standard for confirming a COPD diagnosis—should not be used for screening asymptomatic patients.
1. Guirguis-Blake JM, Senger CA, Webber EM, et al. Screening for chronic obstructive pulmonary disease: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315:1378-1393. doi:10.1001/jama.2016.2654
2. Larsson K, Janson C, Ställberg B, et al. Impact of COPD diagnosis timing on clinical and economic outcomes: the ARCTIC observational cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:995-1008. doi: 10.2147/COPD.S195382
3. Kostikas K, Price D, Gutzwiller FS, et al. Clinical impact and healthcare resource utilization associated with early versus late COPD diagnosis in patients from UK CPRD database. Int J Chron Obstruct Pulmon Dis. 2020;15:1729-1738. doi: 10.2147/COPD.S255414
4. US Preventive Services Task Force; Siu AL, Bibbins-Domingo K, Grossman DC, et al. Screening for chronic obstructive pulmonary disease: US Preventive Services Task Force recommendation statement. JAMA. 2016;315:1372-1377. doi: 10.1001/jama.2016.2638
5. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195:557-582. doi: 10.1164/rccm.201701-0218PP
1. Guirguis-Blake JM, Senger CA, Webber EM, et al. Screening for chronic obstructive pulmonary disease: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315:1378-1393. doi:10.1001/jama.2016.2654
2. Larsson K, Janson C, Ställberg B, et al. Impact of COPD diagnosis timing on clinical and economic outcomes: the ARCTIC observational cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:995-1008. doi: 10.2147/COPD.S195382
3. Kostikas K, Price D, Gutzwiller FS, et al. Clinical impact and healthcare resource utilization associated with early versus late COPD diagnosis in patients from UK CPRD database. Int J Chron Obstruct Pulmon Dis. 2020;15:1729-1738. doi: 10.2147/COPD.S255414
4. US Preventive Services Task Force; Siu AL, Bibbins-Domingo K, Grossman DC, et al. Screening for chronic obstructive pulmonary disease: US Preventive Services Task Force recommendation statement. JAMA. 2016;315:1372-1377. doi: 10.1001/jama.2016.2638
5. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195:557-582. doi: 10.1164/rccm.201701-0218PP
EVIDENCE-BASED ANSWER:
It depends. A diagnosis of chronic obstructive pulmonary disease (COPD) made using screening spirometry in patients without symptoms does not change the course of the disease or alter smoking rates (strength of recommendation [SOR]: A, preponderance of evidence from multiple randomized controlled trials [RCTs]). However, once a patient develops symptoms of lung disease, a delayed diagnosis is associated with poorer outcomes (SOR: B, cohort studies). Active case finding (including the use of spirometry) is recommended for patients with risk factors for COPD who present with consistent symptoms (SOR: C, expert opinion).
Does adjunctive oxytocin infusion during balloon cervical ripening improve labor induction?
Evidence summary
Time to delivery is shortened with combined therapy
Two recent high-quality meta-analyses investigated the effect of adding oxytocin to transcervical Foley balloon placement for cervical dilation. A network meta-analysis, including 30 RCTs (with 6465 pregnant patients), examined the efficacy of multiple combinations of cervical ripening methods.1 A subset of 7 trials (n = 1313) compared oxytocin infusion with transcervical Foley (inflated to 30-60 mL) to Foley alone. Patients were at > 24 weeks’ gestation with a live fetus and undergoing elective or medical induction of labor; exclusion criteria were standard contraindications to vaginal delivery.
Compared to Foley alone, Foley plus oxytocin reduced both the time to the primary outcome of vaginal delivery (mean duration [MD] = –4.2 h; 95% CI, –1.9 to –6.5) and the time to overall (vaginal and cesarean) delivery (MD = –3.1 h; 95% CI, –1.5 to –4.6). There were no differences in rates of cesarean section, chorioamnionitis, epidural use, or neonatal intensive care unit admission. This analysis did not stratify by parity.1
In a standard meta-analysis, researchers identified 6 RCTs (N = 1133) comparing transcervical Foley balloon and oxytocin to Foley balloon alone for cervical ripening in pregnant patients at > 23 weeks’ gestation (1 trial was limited to patients at > 37 weeks’ gestation).2 Foley balloons were inflated with 30 to 60 mL saline, and oxytocin infusions started at 1 to 2 mU/min and were titrated up to 10 to 40 mU/min. Balloon time was usually 12 hours, but not always stated.
The authors found no statistically significant difference in cesarean rates (the primary outcome) between Foley plus oxytocin vs Foley alone (relative risk [RR] = 0.91; 95% CI, 0.76-1.1). Overall delivery within 12 hours was more likely with combined therapy (RR of remaining pregnant = 0.46; 95% CI, 0.34-0.63), but delivery at 24 hours was not (RR = 0.94; 95% CI, 0.92-1.05). However, in a sub-analysis by parity, nulliparous women who received combined therapy had higher overall delivery rates in 24 hours than did multiparous women (RR = 0.77; 95% CI, 0.62-0.97).2
Adding oxytocin may allow shorter transcervical balloon times
One recent RCT (N = 177) compared labor induction with oxytocin and a single trans-cervical balloon (Cook catheter with only the intrauterine balloon inflated) removed at either 6 or 12 hours.3 Patients were pregnant women (mean age, 31 years) with a term singleton vertex pregnancy, a Bishop score ≤ 6, and no contraindications to vaginal delivery. All patients received a balloon inflated to 60 mL with an oxytocin infusion (2-30 mU/min). The intervention group had the balloon removed at 6 hours, while the control group had it removed at 12 hours.
The mean Bishop score changed by 6 points in each group. Time to overall delivery (the primary outcome) was significantly shorter with 6 hours of balloon time than with 12 hours (19.2 vs 24.3 h; P < .04). Overall delivery within 24 hours was also significantly more likely in the 6-hour group (67.4% vs 47.4%; P < .01), although vaginal delivery in 24 hours did not change (74% vs 59%; P = .07). No differences were seen in cesarean delivery rates or maternal or neonatal morbidity rates.
A look at fixed-dose vs titrated oxytocin
Another RCT (N = 116) examined the effectiveness of cervical ripening using a Foley balloon plus either fixed-dose or titrated low-dose oxytocin.4 Patients (mean age, 26 years) had singleton pregnancies at ≥ 37 weeks’ gestation with a Bishop score < 6 and presented for induction of labor. Foley balloons were inflated to 30 mL, and patients received either a fixed oxytocin infusion of 2 mU/min or a titrated infusion starting at 1 mU/min, increasing by 2 mU/min every 30 minutes to a maximum of 20 mU/min.
Continue to: Thre was no statistically...
There was no statistically significant difference in median time from Foley placement to overall delivery (the primary outcome) between the fixed low-dose and incremental low-dose groups in either nulliparous women (24 vs 19 h; P = .18) or multiparous women (16 vs 12 h; P = .68). The authors acknowledged the study may have been underpowered to detect a true difference.
Recommendations from others
A 2009 Practice Bulletin from the American College of Obstetricians and Gynecologists (ACOG) recommended the Foley catheter as a reasonable and effective alternative to prostaglandins for cervical ripening and the induction of labor (based on good-quality evidence).5 The guideline stated that Foley catheter placement before oxytocin induction reduced both the duration of labor and risk of cesarean delivery, but that the use of oxytocin along with a Foley catheter did not appear to shorten the time to delivery.
Editor’s takeaway
High-quality evidence shows us that the addition of oxytocin to balloon cervical ripening shortens the time to delivery. This newer evidence may prompt an update to the 2009 ACOG statement.
1. Orr L, Reisinger-Kindle K, Roy A, et al. Combination of Foley and prostaglandins versus Foley and oxytocin for cervical ripening: a network meta-analysis. Am J Obstet Gynecol. 2020;223:743.e1-743.e17. doi: 10.1016/j.ajog.2020.05.007
2. Gallagher LT, Gardner B, Rahman M, et al. Cervical ripening using Foley balloon with or without oxytocin: a systematic review and meta-analysis. Am J Perinatol. 2019;36:406-421. doi: 10.1055/s-0038-1668577
3. Lassey SC, Haber HR, Kanbergs A, et al. Six vs twelve hours of single balloon catheter placement with oxytocin administration for labor induction: a randomized controlled trial. Am J Obstet Gynecol. 2021:S0002-9378(21)00185-X. doi: 10.1016/j.ajog.2021.03.021
4. Fitzpatrick CB, Grotegut CA, Bishop TS, et al. Cervical ripening with Foley balloon plus fixed versus incremental low-dose oxytocin: a randomized controlled trial. J Matern Fetal Neonatal Med. 2012;25:1006-1010. doi: 10.3109/14767058.2011.607522
5. ACOG Practice Bulletin No. 107: Induction of labor. Obstet Gynecol. 2009;114(2 pt 1):386-397. doi: 10.1097/AOG.0b013e3181b48ef5
Evidence summary
Time to delivery is shortened with combined therapy
Two recent high-quality meta-analyses investigated the effect of adding oxytocin to transcervical Foley balloon placement for cervical dilation. A network meta-analysis, including 30 RCTs (with 6465 pregnant patients), examined the efficacy of multiple combinations of cervical ripening methods.1 A subset of 7 trials (n = 1313) compared oxytocin infusion with transcervical Foley (inflated to 30-60 mL) to Foley alone. Patients were at > 24 weeks’ gestation with a live fetus and undergoing elective or medical induction of labor; exclusion criteria were standard contraindications to vaginal delivery.
Compared to Foley alone, Foley plus oxytocin reduced both the time to the primary outcome of vaginal delivery (mean duration [MD] = –4.2 h; 95% CI, –1.9 to –6.5) and the time to overall (vaginal and cesarean) delivery (MD = –3.1 h; 95% CI, –1.5 to –4.6). There were no differences in rates of cesarean section, chorioamnionitis, epidural use, or neonatal intensive care unit admission. This analysis did not stratify by parity.1
In a standard meta-analysis, researchers identified 6 RCTs (N = 1133) comparing transcervical Foley balloon and oxytocin to Foley balloon alone for cervical ripening in pregnant patients at > 23 weeks’ gestation (1 trial was limited to patients at > 37 weeks’ gestation).2 Foley balloons were inflated with 30 to 60 mL saline, and oxytocin infusions started at 1 to 2 mU/min and were titrated up to 10 to 40 mU/min. Balloon time was usually 12 hours, but not always stated.
The authors found no statistically significant difference in cesarean rates (the primary outcome) between Foley plus oxytocin vs Foley alone (relative risk [RR] = 0.91; 95% CI, 0.76-1.1). Overall delivery within 12 hours was more likely with combined therapy (RR of remaining pregnant = 0.46; 95% CI, 0.34-0.63), but delivery at 24 hours was not (RR = 0.94; 95% CI, 0.92-1.05). However, in a sub-analysis by parity, nulliparous women who received combined therapy had higher overall delivery rates in 24 hours than did multiparous women (RR = 0.77; 95% CI, 0.62-0.97).2
Adding oxytocin may allow shorter transcervical balloon times
One recent RCT (N = 177) compared labor induction with oxytocin and a single trans-cervical balloon (Cook catheter with only the intrauterine balloon inflated) removed at either 6 or 12 hours.3 Patients were pregnant women (mean age, 31 years) with a term singleton vertex pregnancy, a Bishop score ≤ 6, and no contraindications to vaginal delivery. All patients received a balloon inflated to 60 mL with an oxytocin infusion (2-30 mU/min). The intervention group had the balloon removed at 6 hours, while the control group had it removed at 12 hours.
The mean Bishop score changed by 6 points in each group. Time to overall delivery (the primary outcome) was significantly shorter with 6 hours of balloon time than with 12 hours (19.2 vs 24.3 h; P < .04). Overall delivery within 24 hours was also significantly more likely in the 6-hour group (67.4% vs 47.4%; P < .01), although vaginal delivery in 24 hours did not change (74% vs 59%; P = .07). No differences were seen in cesarean delivery rates or maternal or neonatal morbidity rates.
A look at fixed-dose vs titrated oxytocin
Another RCT (N = 116) examined the effectiveness of cervical ripening using a Foley balloon plus either fixed-dose or titrated low-dose oxytocin.4 Patients (mean age, 26 years) had singleton pregnancies at ≥ 37 weeks’ gestation with a Bishop score < 6 and presented for induction of labor. Foley balloons were inflated to 30 mL, and patients received either a fixed oxytocin infusion of 2 mU/min or a titrated infusion starting at 1 mU/min, increasing by 2 mU/min every 30 minutes to a maximum of 20 mU/min.
Continue to: Thre was no statistically...
There was no statistically significant difference in median time from Foley placement to overall delivery (the primary outcome) between the fixed low-dose and incremental low-dose groups in either nulliparous women (24 vs 19 h; P = .18) or multiparous women (16 vs 12 h; P = .68). The authors acknowledged the study may have been underpowered to detect a true difference.
Recommendations from others
A 2009 Practice Bulletin from the American College of Obstetricians and Gynecologists (ACOG) recommended the Foley catheter as a reasonable and effective alternative to prostaglandins for cervical ripening and the induction of labor (based on good-quality evidence).5 The guideline stated that Foley catheter placement before oxytocin induction reduced both the duration of labor and risk of cesarean delivery, but that the use of oxytocin along with a Foley catheter did not appear to shorten the time to delivery.
Editor’s takeaway
High-quality evidence shows us that the addition of oxytocin to balloon cervical ripening shortens the time to delivery. This newer evidence may prompt an update to the 2009 ACOG statement.
Evidence summary
Time to delivery is shortened with combined therapy
Two recent high-quality meta-analyses investigated the effect of adding oxytocin to transcervical Foley balloon placement for cervical dilation. A network meta-analysis, including 30 RCTs (with 6465 pregnant patients), examined the efficacy of multiple combinations of cervical ripening methods.1 A subset of 7 trials (n = 1313) compared oxytocin infusion with transcervical Foley (inflated to 30-60 mL) to Foley alone. Patients were at > 24 weeks’ gestation with a live fetus and undergoing elective or medical induction of labor; exclusion criteria were standard contraindications to vaginal delivery.
Compared to Foley alone, Foley plus oxytocin reduced both the time to the primary outcome of vaginal delivery (mean duration [MD] = –4.2 h; 95% CI, –1.9 to –6.5) and the time to overall (vaginal and cesarean) delivery (MD = –3.1 h; 95% CI, –1.5 to –4.6). There were no differences in rates of cesarean section, chorioamnionitis, epidural use, or neonatal intensive care unit admission. This analysis did not stratify by parity.1
In a standard meta-analysis, researchers identified 6 RCTs (N = 1133) comparing transcervical Foley balloon and oxytocin to Foley balloon alone for cervical ripening in pregnant patients at > 23 weeks’ gestation (1 trial was limited to patients at > 37 weeks’ gestation).2 Foley balloons were inflated with 30 to 60 mL saline, and oxytocin infusions started at 1 to 2 mU/min and were titrated up to 10 to 40 mU/min. Balloon time was usually 12 hours, but not always stated.
The authors found no statistically significant difference in cesarean rates (the primary outcome) between Foley plus oxytocin vs Foley alone (relative risk [RR] = 0.91; 95% CI, 0.76-1.1). Overall delivery within 12 hours was more likely with combined therapy (RR of remaining pregnant = 0.46; 95% CI, 0.34-0.63), but delivery at 24 hours was not (RR = 0.94; 95% CI, 0.92-1.05). However, in a sub-analysis by parity, nulliparous women who received combined therapy had higher overall delivery rates in 24 hours than did multiparous women (RR = 0.77; 95% CI, 0.62-0.97).2
Adding oxytocin may allow shorter transcervical balloon times
One recent RCT (N = 177) compared labor induction with oxytocin and a single trans-cervical balloon (Cook catheter with only the intrauterine balloon inflated) removed at either 6 or 12 hours.3 Patients were pregnant women (mean age, 31 years) with a term singleton vertex pregnancy, a Bishop score ≤ 6, and no contraindications to vaginal delivery. All patients received a balloon inflated to 60 mL with an oxytocin infusion (2-30 mU/min). The intervention group had the balloon removed at 6 hours, while the control group had it removed at 12 hours.
The mean Bishop score changed by 6 points in each group. Time to overall delivery (the primary outcome) was significantly shorter with 6 hours of balloon time than with 12 hours (19.2 vs 24.3 h; P < .04). Overall delivery within 24 hours was also significantly more likely in the 6-hour group (67.4% vs 47.4%; P < .01), although vaginal delivery in 24 hours did not change (74% vs 59%; P = .07). No differences were seen in cesarean delivery rates or maternal or neonatal morbidity rates.
A look at fixed-dose vs titrated oxytocin
Another RCT (N = 116) examined the effectiveness of cervical ripening using a Foley balloon plus either fixed-dose or titrated low-dose oxytocin.4 Patients (mean age, 26 years) had singleton pregnancies at ≥ 37 weeks’ gestation with a Bishop score < 6 and presented for induction of labor. Foley balloons were inflated to 30 mL, and patients received either a fixed oxytocin infusion of 2 mU/min or a titrated infusion starting at 1 mU/min, increasing by 2 mU/min every 30 minutes to a maximum of 20 mU/min.
Continue to: Thre was no statistically...
There was no statistically significant difference in median time from Foley placement to overall delivery (the primary outcome) between the fixed low-dose and incremental low-dose groups in either nulliparous women (24 vs 19 h; P = .18) or multiparous women (16 vs 12 h; P = .68). The authors acknowledged the study may have been underpowered to detect a true difference.
Recommendations from others
A 2009 Practice Bulletin from the American College of Obstetricians and Gynecologists (ACOG) recommended the Foley catheter as a reasonable and effective alternative to prostaglandins for cervical ripening and the induction of labor (based on good-quality evidence).5 The guideline stated that Foley catheter placement before oxytocin induction reduced both the duration of labor and risk of cesarean delivery, but that the use of oxytocin along with a Foley catheter did not appear to shorten the time to delivery.
Editor’s takeaway
High-quality evidence shows us that the addition of oxytocin to balloon cervical ripening shortens the time to delivery. This newer evidence may prompt an update to the 2009 ACOG statement.
1. Orr L, Reisinger-Kindle K, Roy A, et al. Combination of Foley and prostaglandins versus Foley and oxytocin for cervical ripening: a network meta-analysis. Am J Obstet Gynecol. 2020;223:743.e1-743.e17. doi: 10.1016/j.ajog.2020.05.007
2. Gallagher LT, Gardner B, Rahman M, et al. Cervical ripening using Foley balloon with or without oxytocin: a systematic review and meta-analysis. Am J Perinatol. 2019;36:406-421. doi: 10.1055/s-0038-1668577
3. Lassey SC, Haber HR, Kanbergs A, et al. Six vs twelve hours of single balloon catheter placement with oxytocin administration for labor induction: a randomized controlled trial. Am J Obstet Gynecol. 2021:S0002-9378(21)00185-X. doi: 10.1016/j.ajog.2021.03.021
4. Fitzpatrick CB, Grotegut CA, Bishop TS, et al. Cervical ripening with Foley balloon plus fixed versus incremental low-dose oxytocin: a randomized controlled trial. J Matern Fetal Neonatal Med. 2012;25:1006-1010. doi: 10.3109/14767058.2011.607522
5. ACOG Practice Bulletin No. 107: Induction of labor. Obstet Gynecol. 2009;114(2 pt 1):386-397. doi: 10.1097/AOG.0b013e3181b48ef5
1. Orr L, Reisinger-Kindle K, Roy A, et al. Combination of Foley and prostaglandins versus Foley and oxytocin for cervical ripening: a network meta-analysis. Am J Obstet Gynecol. 2020;223:743.e1-743.e17. doi: 10.1016/j.ajog.2020.05.007
2. Gallagher LT, Gardner B, Rahman M, et al. Cervical ripening using Foley balloon with or without oxytocin: a systematic review and meta-analysis. Am J Perinatol. 2019;36:406-421. doi: 10.1055/s-0038-1668577
3. Lassey SC, Haber HR, Kanbergs A, et al. Six vs twelve hours of single balloon catheter placement with oxytocin administration for labor induction: a randomized controlled trial. Am J Obstet Gynecol. 2021:S0002-9378(21)00185-X. doi: 10.1016/j.ajog.2021.03.021
4. Fitzpatrick CB, Grotegut CA, Bishop TS, et al. Cervical ripening with Foley balloon plus fixed versus incremental low-dose oxytocin: a randomized controlled trial. J Matern Fetal Neonatal Med. 2012;25:1006-1010. doi: 10.3109/14767058.2011.607522
5. ACOG Practice Bulletin No. 107: Induction of labor. Obstet Gynecol. 2009;114(2 pt 1):386-397. doi: 10.1097/AOG.0b013e3181b48ef5
EVIDENCE-BASED ANSWER:
YES. Compared to the use of a transcervical balloon alone, combined cervical ripening with a balloon catheter and oxytocin shortens the time to overall delivery by 3 hours and the time to vaginal delivery by 4 hours, without altering the rate of cesarean section (strength of recommendation [SOR]: A, network meta-analysis). The effect is more pronounced in nulliparous patients (SOR: A, meta-analysis).
When combined therapy is used, 6 hours of balloon time may result in faster delivery than 12 hours (SOR: B, single randomized controlled trial [RCT]). Fixed-dose oxytocin and titrated oxytocin appear to have similar effect when combined with a cervical ripening balloon (SOR: C, underpowered RCT).
Can family physicians accurately screen for AAA with point-of-care ultrasound?
EVIDENCE SUMMARY
Meta-analysis demonstrates accuracy of nonradiologist providers with POCUS
A systematic review and meta-analysis (11 studies; 946 exams) compared nonradiologist-performed AAA screening with POCUS vs radiologist-performed aortic imaging as a gold standard. Eight trials involved emergency medicine physicians (718 exams); 1 trial, surgical residents (104 exams); 1 trial, primary care internal medicine physicians (79 exams); and 1 trial, rural family physicians (45 exams). The majority of studies were conducted in Ireland, the United Kingdom, Australia, and Canada, with 4 trials performed in the United States.1
Researchers compared all POCUS exam findings with radiologist-performed imaging (using ultrasound, computed tomography, magnetic resonance imaging, or angiography) and with operative findings or pathology where available. There were 193 true positives, 8 false-positives, 740 true negatives, and 5 false-negatives. Primary care physicians identified 6 patients with AAA, with no false-positives or false-negatives. Overall, POCUS demonstrated a sensitivity of 0.975 (95% CI, 0.942 to 0.992) and a specificity of 0.989 (95% CI, 0.979 to 0.995).1
Nonradiologist providers received POCUS training as follows: emergency medicine residents, 5 hours to 3 days; emergency medicine physicians, 4 to 24 hours of didactics, 50 AAA scans, or American College of Emergency Medicine certification; and primary care physicians, 2.3 hours or 50 AAA scans. Information on training for surgical residents was not supplied. The authors rated the studies for quality (10-14 points on the 14-point QUADA quality score) and heterogeneity (I2 = 0 for sensitivity and I2 = .38 for specificity).1
European studies support FPs’ ability to diagnose AAA with POCUS
Two subsequent prospective diagnostic accuracy studies both found that POCUS performed by family physicians had 100% concordance with radiologist overread. The first study (in Spain) included 106 men (ages 50 and older; mean, 69 years) with chronic hypertension or a history of tobacco use. One family physician underwent training (duration not reported) by a radiologist, including experience measuring standard cross-sections of the aorta. Radiologists reviewed all POCUS images, which identified 6 patients with AAA (confirmed by CT scan). The concordance between the family physician and the radiologists was absolute (kappa = 1.0; sensitivity and specificity, 100%; positive and negative predictive values, both 1.0).2
The second study (in Denmark) compared 29 POCUS screenings for AAA performed by 5 family physicians vs a gold standard of a radiologist-performed abdominal ultrasound blinded to previous ultrasound findings. Four of the family physicians were board certified and 1 was a final-year resident in training. They all underwent a 3-day ultrasonography course that included initial e-learning followed by 2 days of hands-on training; all passed a final certification exam. The family physicians identified 1 patient with AAA. Radiologists overread all the scans and found 100% agreement with the 1 positive AAA and the 28 negative scans.3
Recommendations from others
In 2019, the US Preventive Services Task Force (USPSTF) offered a Grade “B” (moderate net benefit) recommendation for screening with ultrasonography for AAA in men ages 65 to 75 years who have ever smoked, and a Grade “C” recommendation (small net benefit) for screening men ages 65 to 75 years who have never smoked.4 In 2017, the Canadian Task Force on Preventive Health Care recommended screening all men ages 65 to 80 years with 1 ultrasound exam for AAA (weak recommendation; moderate-quality evidence). The Canadian Task Force also noted that, with adequate training, AAA screening could be performed in a family practice setting.5
Editor’s takeaway
While these studies evaluating POCUS performed by nonradiologists included a small number of family physicians, their finding that all participants (attending physicians and residents) demonstrate high sensitivity and specificity for AAA detection with relatively limited training bodes well for more widespread use of the technology. Offering POCUS to detect AAAs in family physician offices has the potential to dramatically improve access to USPSTF-recommended screening.
1. Concannon E, McHugh S, Healy DA, et al. Diagnostic accuracy of non-radiologist performed ultrasound for abdominal aortic aneurysm: systematic review and meta-analysis. Int J Clin Pract. 2014;9:1122-1129. doi: 10.1111/ijcp.12453
2. Sisó-Almirall A, Gilabert Solé R, Bru Saumell C, et al. Feasibility of hand-held-ultrasonography in the screening of abdominal aortic aneurysms and abdominal aortic atherosclerosis [article in Spanish]. Med Clin (Barc). 2013;141:417-422. doi: 10.1016/j.medcli.2013.02.038
3. Lindgaard K, Riisgaard L. ‘Validation of ultrasound examinations performed by general practitioners’. Scand J Prim Health Care. 2017;3:256-261. doi: 10.1080/02813432.2017.1358437
4. US Preventive Task Force. Screening for abdominal aortic aneurysm: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:2211-2218. doi:10.1001/jama.2019.18928
5. Canadian Task Force on Preventive Health Care. Recommendations on screening for abdominal aortic aneurysm in primary care. CMAJ. 2017;189:E1137-E1145. doi: 10.1503/cmaj.170118
EVIDENCE SUMMARY
Meta-analysis demonstrates accuracy of nonradiologist providers with POCUS
A systematic review and meta-analysis (11 studies; 946 exams) compared nonradiologist-performed AAA screening with POCUS vs radiologist-performed aortic imaging as a gold standard. Eight trials involved emergency medicine physicians (718 exams); 1 trial, surgical residents (104 exams); 1 trial, primary care internal medicine physicians (79 exams); and 1 trial, rural family physicians (45 exams). The majority of studies were conducted in Ireland, the United Kingdom, Australia, and Canada, with 4 trials performed in the United States.1
Researchers compared all POCUS exam findings with radiologist-performed imaging (using ultrasound, computed tomography, magnetic resonance imaging, or angiography) and with operative findings or pathology where available. There were 193 true positives, 8 false-positives, 740 true negatives, and 5 false-negatives. Primary care physicians identified 6 patients with AAA, with no false-positives or false-negatives. Overall, POCUS demonstrated a sensitivity of 0.975 (95% CI, 0.942 to 0.992) and a specificity of 0.989 (95% CI, 0.979 to 0.995).1
Nonradiologist providers received POCUS training as follows: emergency medicine residents, 5 hours to 3 days; emergency medicine physicians, 4 to 24 hours of didactics, 50 AAA scans, or American College of Emergency Medicine certification; and primary care physicians, 2.3 hours or 50 AAA scans. Information on training for surgical residents was not supplied. The authors rated the studies for quality (10-14 points on the 14-point QUADA quality score) and heterogeneity (I2 = 0 for sensitivity and I2 = .38 for specificity).1
European studies support FPs’ ability to diagnose AAA with POCUS
Two subsequent prospective diagnostic accuracy studies both found that POCUS performed by family physicians had 100% concordance with radiologist overread. The first study (in Spain) included 106 men (ages 50 and older; mean, 69 years) with chronic hypertension or a history of tobacco use. One family physician underwent training (duration not reported) by a radiologist, including experience measuring standard cross-sections of the aorta. Radiologists reviewed all POCUS images, which identified 6 patients with AAA (confirmed by CT scan). The concordance between the family physician and the radiologists was absolute (kappa = 1.0; sensitivity and specificity, 100%; positive and negative predictive values, both 1.0).2
The second study (in Denmark) compared 29 POCUS screenings for AAA performed by 5 family physicians vs a gold standard of a radiologist-performed abdominal ultrasound blinded to previous ultrasound findings. Four of the family physicians were board certified and 1 was a final-year resident in training. They all underwent a 3-day ultrasonography course that included initial e-learning followed by 2 days of hands-on training; all passed a final certification exam. The family physicians identified 1 patient with AAA. Radiologists overread all the scans and found 100% agreement with the 1 positive AAA and the 28 negative scans.3
Recommendations from others
In 2019, the US Preventive Services Task Force (USPSTF) offered a Grade “B” (moderate net benefit) recommendation for screening with ultrasonography for AAA in men ages 65 to 75 years who have ever smoked, and a Grade “C” recommendation (small net benefit) for screening men ages 65 to 75 years who have never smoked.4 In 2017, the Canadian Task Force on Preventive Health Care recommended screening all men ages 65 to 80 years with 1 ultrasound exam for AAA (weak recommendation; moderate-quality evidence). The Canadian Task Force also noted that, with adequate training, AAA screening could be performed in a family practice setting.5
Editor’s takeaway
While these studies evaluating POCUS performed by nonradiologists included a small number of family physicians, their finding that all participants (attending physicians and residents) demonstrate high sensitivity and specificity for AAA detection with relatively limited training bodes well for more widespread use of the technology. Offering POCUS to detect AAAs in family physician offices has the potential to dramatically improve access to USPSTF-recommended screening.
EVIDENCE SUMMARY
Meta-analysis demonstrates accuracy of nonradiologist providers with POCUS
A systematic review and meta-analysis (11 studies; 946 exams) compared nonradiologist-performed AAA screening with POCUS vs radiologist-performed aortic imaging as a gold standard. Eight trials involved emergency medicine physicians (718 exams); 1 trial, surgical residents (104 exams); 1 trial, primary care internal medicine physicians (79 exams); and 1 trial, rural family physicians (45 exams). The majority of studies were conducted in Ireland, the United Kingdom, Australia, and Canada, with 4 trials performed in the United States.1
Researchers compared all POCUS exam findings with radiologist-performed imaging (using ultrasound, computed tomography, magnetic resonance imaging, or angiography) and with operative findings or pathology where available. There were 193 true positives, 8 false-positives, 740 true negatives, and 5 false-negatives. Primary care physicians identified 6 patients with AAA, with no false-positives or false-negatives. Overall, POCUS demonstrated a sensitivity of 0.975 (95% CI, 0.942 to 0.992) and a specificity of 0.989 (95% CI, 0.979 to 0.995).1
Nonradiologist providers received POCUS training as follows: emergency medicine residents, 5 hours to 3 days; emergency medicine physicians, 4 to 24 hours of didactics, 50 AAA scans, or American College of Emergency Medicine certification; and primary care physicians, 2.3 hours or 50 AAA scans. Information on training for surgical residents was not supplied. The authors rated the studies for quality (10-14 points on the 14-point QUADA quality score) and heterogeneity (I2 = 0 for sensitivity and I2 = .38 for specificity).1
European studies support FPs’ ability to diagnose AAA with POCUS
Two subsequent prospective diagnostic accuracy studies both found that POCUS performed by family physicians had 100% concordance with radiologist overread. The first study (in Spain) included 106 men (ages 50 and older; mean, 69 years) with chronic hypertension or a history of tobacco use. One family physician underwent training (duration not reported) by a radiologist, including experience measuring standard cross-sections of the aorta. Radiologists reviewed all POCUS images, which identified 6 patients with AAA (confirmed by CT scan). The concordance between the family physician and the radiologists was absolute (kappa = 1.0; sensitivity and specificity, 100%; positive and negative predictive values, both 1.0).2
The second study (in Denmark) compared 29 POCUS screenings for AAA performed by 5 family physicians vs a gold standard of a radiologist-performed abdominal ultrasound blinded to previous ultrasound findings. Four of the family physicians were board certified and 1 was a final-year resident in training. They all underwent a 3-day ultrasonography course that included initial e-learning followed by 2 days of hands-on training; all passed a final certification exam. The family physicians identified 1 patient with AAA. Radiologists overread all the scans and found 100% agreement with the 1 positive AAA and the 28 negative scans.3
Recommendations from others
In 2019, the US Preventive Services Task Force (USPSTF) offered a Grade “B” (moderate net benefit) recommendation for screening with ultrasonography for AAA in men ages 65 to 75 years who have ever smoked, and a Grade “C” recommendation (small net benefit) for screening men ages 65 to 75 years who have never smoked.4 In 2017, the Canadian Task Force on Preventive Health Care recommended screening all men ages 65 to 80 years with 1 ultrasound exam for AAA (weak recommendation; moderate-quality evidence). The Canadian Task Force also noted that, with adequate training, AAA screening could be performed in a family practice setting.5
Editor’s takeaway
While these studies evaluating POCUS performed by nonradiologists included a small number of family physicians, their finding that all participants (attending physicians and residents) demonstrate high sensitivity and specificity for AAA detection with relatively limited training bodes well for more widespread use of the technology. Offering POCUS to detect AAAs in family physician offices has the potential to dramatically improve access to USPSTF-recommended screening.
1. Concannon E, McHugh S, Healy DA, et al. Diagnostic accuracy of non-radiologist performed ultrasound for abdominal aortic aneurysm: systematic review and meta-analysis. Int J Clin Pract. 2014;9:1122-1129. doi: 10.1111/ijcp.12453
2. Sisó-Almirall A, Gilabert Solé R, Bru Saumell C, et al. Feasibility of hand-held-ultrasonography in the screening of abdominal aortic aneurysms and abdominal aortic atherosclerosis [article in Spanish]. Med Clin (Barc). 2013;141:417-422. doi: 10.1016/j.medcli.2013.02.038
3. Lindgaard K, Riisgaard L. ‘Validation of ultrasound examinations performed by general practitioners’. Scand J Prim Health Care. 2017;3:256-261. doi: 10.1080/02813432.2017.1358437
4. US Preventive Task Force. Screening for abdominal aortic aneurysm: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:2211-2218. doi:10.1001/jama.2019.18928
5. Canadian Task Force on Preventive Health Care. Recommendations on screening for abdominal aortic aneurysm in primary care. CMAJ. 2017;189:E1137-E1145. doi: 10.1503/cmaj.170118
1. Concannon E, McHugh S, Healy DA, et al. Diagnostic accuracy of non-radiologist performed ultrasound for abdominal aortic aneurysm: systematic review and meta-analysis. Int J Clin Pract. 2014;9:1122-1129. doi: 10.1111/ijcp.12453
2. Sisó-Almirall A, Gilabert Solé R, Bru Saumell C, et al. Feasibility of hand-held-ultrasonography in the screening of abdominal aortic aneurysms and abdominal aortic atherosclerosis [article in Spanish]. Med Clin (Barc). 2013;141:417-422. doi: 10.1016/j.medcli.2013.02.038
3. Lindgaard K, Riisgaard L. ‘Validation of ultrasound examinations performed by general practitioners’. Scand J Prim Health Care. 2017;3:256-261. doi: 10.1080/02813432.2017.1358437
4. US Preventive Task Force. Screening for abdominal aortic aneurysm: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:2211-2218. doi:10.1001/jama.2019.18928
5. Canadian Task Force on Preventive Health Care. Recommendations on screening for abdominal aortic aneurysm in primary care. CMAJ. 2017;189:E1137-E1145. doi: 10.1503/cmaj.170118
EVIDENCE-BASED ANSWER:
Likely yes. Point-of-care ultrasound (POCUS) screening for abdominal aortic aneurysm (AAA) by nonradiologist physicians is 98% sensitive and 99% specific, compared with imaging performed by radiologists (strength of recommendation [SOR]: B, meta-analysis of diagnostic accuracy studies mostly involving emergency medicine physicians). European family physicians demonstrated 100% concordance with radiologist readings (SOR: C, very small subsequent diagnostic accuracy studies).
Is event-driven PrEP dosing for HIV as effective as daily dosing?
EVIDENCE SUMMARY
Event-driven PrEP is effective for prevention of HIV transmission
An RCT evaluating the effectiveness of event-driven PrEP in 400 patients at high risk for HIV found that it reduced HIV incidence by 86% compared to placebo. Researchers recruited HIV-negative men or transgender women who had sex with men, who’d had condomless anal sex with at least 2 partners in the previous 6 months, and followed them for a median of 9.3 months for HIV acquisition.1
Patients randomized to event-driven PrEP took tenofovir-emtricitabine (300-200 mg) on the following schedule: 2 pills 2 to 24 hours before intercourse (or 1 pill if they had taken it within the past week), followed by a third pill 24 hours later, and a fourth pill 24 hours after that. When patients had multiple consecutive episodes of intercourse, daily use was continued until 2 days after the last episode. Patients in the control group took placebo pills.1
Event-driven PrEP reduced HIV incidence vs placebo (2 infections vs 14 infections; 0.91 vs 6.6 per 100 person-years; relative risk [RR] = 0.86; P = .002). PrEP produced more gastrointestinal (14% vs 5%; P = .002) and renal (18% vs 10%; P = .03) adverse effects than placebo. Participants took a median of 15 pills per month.1
A post-hoc analysis of the above study, evaluating 270 patients, found that event-driven PrEP reduced HIV incidence by 100% during periods of less frequent sexual encounters. Selected participants had a median of 5 sexual encounters per month (range, 2-10), used a median of 9.5 pills per month (range, 6-13), and represented 134 person-years of follow-up. No HIV infections (0 per 100 person-years; 95% CI, 0-5; P = .013) were diagnosed in the PrEP group and 6 HIV infections (9.2 per 100 person-years; 95% CI, 3.4-20.1) were diagnosed in the placebo group, with a relative reduction of HIV incidence of 100% (95% CI, 39-100).2
For comparison, 2 large open-label trials evaluating daily PrEP found that it reduced HIV incidence by 44%3 and 86%4 vs placebo.
Adherence is better with daily PrEPthan event-driven PrEP
Three prospective cohort trials evaluated PrEP adherence (extent that participants were taking PrEP at the time of sexual encounters) with different dosing regimens and found that event-driven PrEP tended to have lower adherence than daily PrEP. An open-label trial in Bangkok and Harlem (New York City) randomized 357 at-risk patients to 1 of 3 regimens: event-driven (1 tablet before and after sex), time-driven (1 tablet twice weekly with a postsex dose), and daily. Overall, patients with event-driven PrEP had lower adherence than those with daily PrEP (67% event-driven vs 97% daily; P < 0.0001).5
Continue to: In an open-label...
In an open-label prospective cohort trial in Belgium, at-risk patients chose between using event-driven (N = 44) and daily (N = 135) PrEP. Analysis was conducted for both high-risk HIV exposure days (defined as condomless anal receptive intercourse with a new or HIV-positive steady partner with a detectable viral load) and low-risk HIV exposure days (consistent condom use or condomless anal intercourse with a steady partner who is HIV-negative). Over 18 months, lower adherence was demonstrated with event-driven PrEP than with daily PrEP for high-risk days (88% [95% CI, 86%-90%] vs 97.5% [95% CI, 97%-98%]; P < .0001) and also for low-risk days (42% [95% CI, 40%-45%] vs 96% [95% CI, 95%-96%]; P < .0001).6 Researchers diagnosed no new HIV infections in any participant, and the incidence of STIs was the same in both groups.
A third open-label trial evaluated adherence among 178 South African women randomized to event-driven or daily PrEP and found lower sexual event coverage with event-driven PrEP (52% vs 75%; odds ratio = 2.76; 95% CI, 1.68-4.53; P < 0.0006). Four women in each group seroconverted to HIV positive.7
Drug costs, patient preferences, and STI risk are important considerations
Several of the above trials reported use of fewer pills in the event-driven groups, with lower drug costs.2,5,7 A large prospective cohort trial of men who have sex with men (N = 1049) with an average of 10 sexual partners found that most (76%) opted for event-driven PrEP.8 Researchers also reported no difference in STI rates (RR = 1.24 for “at least 1 bacterial STI”; 95% CI, 0.84 to 1.81).8 However, a smaller, open-label prospective cohort trial (N = 200) found that more participants chose daily PrEP than event-driven PrEP (76.5% vs 23.5%), although almost all said they would change their dosing regimen in the next year.9
Recommendations from others
In 2019, the World Health Organization recommended oral PrEP as an additional prevention choice for people at substantial risk for HIV infection and stated that different dosing strategies offer users flexibility, choice, and convenience.10 Also in 2019, the US Preventive Services Task Force published a recommendation that clinicians offer PrEP with effective antiretroviral therapy to patients at high risk for HIV acquisition. They did not specify which regimen to offer.11
Editor’s takeaway
While there are theoretical reasons why event-driven PrEP might not work as well as daily PrEP, we have 1 RCT that suggests the real-world outcomes are similar. Given the apparent effectiveness of either option, the best choice is the one the patient will use. JFP
- Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. NEJM. 2015;373:2237-2246.
- Antoni G, Tremblay C, Delaugerre C, et al. On-demand pre-exposure prophylaxis with tenofovir disoproxil fumarate plus emtricitabine among men who have sex with men with less frequent sexual intercourse: a post-hoc analysis of the ANRS IPERGAY trial. Lancet HIV. 2020;7:e113-e120.
- Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis in men who have sex with men. NEJM. 2010;363:2587-2599.
- McCormack S, Dunn DT, Desai M, et al. Preexposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot of a pragmatic open-label randomized trial. Lancet. 2016;387:53-60.
- Grant RM, Mannheimer S, Hughes JP, et al. Daily and nondaily oral preexposure prophylaxis in men and transgender women who have sex with men: the Human Immunodeficiency Virus Prevention Trials Network 067/ADAPT study. Clin Infect Dis. 2018;66:1712-1721.
 Vuylsteke B, Reyniers T, De Baetselier I, et al. Daily and event-driven pre-exposure prophylaxis for men who have sex with men in Belgium: results of a prospective cohort measuring adherence, sexual behavior and STI incidence. J Intl AIDS Soc. 2019;22:e25407.
Vuylsteke B, Reyniers T, De Baetselier I, et al. Daily and event-driven pre-exposure prophylaxis for men who have sex with men in Belgium: results of a prospective cohort measuring adherence, sexual behavior and STI incidence. J Intl AIDS Soc. 2019;22:e25407.- Bekker LG, Roux S, Sebastien E, et al. Daily and non-daily pre-exposure prophylaxis in African women (HPTN 067/ADAPT Cape Town Trial): a randomized, open-label, phase 2 trial. Lancet HIV. 2018;5:e68-e78.
- Noret M, Balavoine S, Pintado C, et al. Daily or on-demand oral tenofovir disoproxil fumarate/emtricitabine for HIV pre-exposure prophylaxis: experience from a hospital-based clinic in France. AIDS. 2018;32:2161-2169.
- Reyniers T, Nöstlinger C, Laga M, et al. Choosing between daily and event-driven pre-exposure prophylaxis: results of a Belgian PrEP demonstration project. J Acquir Immune Defic Syndr. 2018;79:186-194.
- WHO. What’s the 2+1+1? Event-driven oral pre-exposure prophylaxis to prevent HIV in men who have sex with men: update to WHO’s recommendation on oral PrEP [technical brief]. Published July 2019. Accessed May 14, 2021. https://who.int/hiv/pub/prep/211/en
- US Preventive Services Task Force. Prevention of human immunodeficiency virus (HIV) infection: preexposure prophylaxis [evidence summary]. Published June 11, 2019. Accessed May 14, 2021. www.uspreventiveservicestaskforce.org/uspstf/document/evidence-summary/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
EVIDENCE SUMMARY
Event-driven PrEP is effective for prevention of HIV transmission
An RCT evaluating the effectiveness of event-driven PrEP in 400 patients at high risk for HIV found that it reduced HIV incidence by 86% compared to placebo. Researchers recruited HIV-negative men or transgender women who had sex with men, who’d had condomless anal sex with at least 2 partners in the previous 6 months, and followed them for a median of 9.3 months for HIV acquisition.1
Patients randomized to event-driven PrEP took tenofovir-emtricitabine (300-200 mg) on the following schedule: 2 pills 2 to 24 hours before intercourse (or 1 pill if they had taken it within the past week), followed by a third pill 24 hours later, and a fourth pill 24 hours after that. When patients had multiple consecutive episodes of intercourse, daily use was continued until 2 days after the last episode. Patients in the control group took placebo pills.1
Event-driven PrEP reduced HIV incidence vs placebo (2 infections vs 14 infections; 0.91 vs 6.6 per 100 person-years; relative risk [RR] = 0.86; P = .002). PrEP produced more gastrointestinal (14% vs 5%; P = .002) and renal (18% vs 10%; P = .03) adverse effects than placebo. Participants took a median of 15 pills per month.1
A post-hoc analysis of the above study, evaluating 270 patients, found that event-driven PrEP reduced HIV incidence by 100% during periods of less frequent sexual encounters. Selected participants had a median of 5 sexual encounters per month (range, 2-10), used a median of 9.5 pills per month (range, 6-13), and represented 134 person-years of follow-up. No HIV infections (0 per 100 person-years; 95% CI, 0-5; P = .013) were diagnosed in the PrEP group and 6 HIV infections (9.2 per 100 person-years; 95% CI, 3.4-20.1) were diagnosed in the placebo group, with a relative reduction of HIV incidence of 100% (95% CI, 39-100).2
For comparison, 2 large open-label trials evaluating daily PrEP found that it reduced HIV incidence by 44%3 and 86%4 vs placebo.
Adherence is better with daily PrEPthan event-driven PrEP
Three prospective cohort trials evaluated PrEP adherence (extent that participants were taking PrEP at the time of sexual encounters) with different dosing regimens and found that event-driven PrEP tended to have lower adherence than daily PrEP. An open-label trial in Bangkok and Harlem (New York City) randomized 357 at-risk patients to 1 of 3 regimens: event-driven (1 tablet before and after sex), time-driven (1 tablet twice weekly with a postsex dose), and daily. Overall, patients with event-driven PrEP had lower adherence than those with daily PrEP (67% event-driven vs 97% daily; P < 0.0001).5
Continue to: In an open-label...
In an open-label prospective cohort trial in Belgium, at-risk patients chose between using event-driven (N = 44) and daily (N = 135) PrEP. Analysis was conducted for both high-risk HIV exposure days (defined as condomless anal receptive intercourse with a new or HIV-positive steady partner with a detectable viral load) and low-risk HIV exposure days (consistent condom use or condomless anal intercourse with a steady partner who is HIV-negative). Over 18 months, lower adherence was demonstrated with event-driven PrEP than with daily PrEP for high-risk days (88% [95% CI, 86%-90%] vs 97.5% [95% CI, 97%-98%]; P < .0001) and also for low-risk days (42% [95% CI, 40%-45%] vs 96% [95% CI, 95%-96%]; P < .0001).6 Researchers diagnosed no new HIV infections in any participant, and the incidence of STIs was the same in both groups.
A third open-label trial evaluated adherence among 178 South African women randomized to event-driven or daily PrEP and found lower sexual event coverage with event-driven PrEP (52% vs 75%; odds ratio = 2.76; 95% CI, 1.68-4.53; P < 0.0006). Four women in each group seroconverted to HIV positive.7
Drug costs, patient preferences, and STI risk are important considerations
Several of the above trials reported use of fewer pills in the event-driven groups, with lower drug costs.2,5,7 A large prospective cohort trial of men who have sex with men (N = 1049) with an average of 10 sexual partners found that most (76%) opted for event-driven PrEP.8 Researchers also reported no difference in STI rates (RR = 1.24 for “at least 1 bacterial STI”; 95% CI, 0.84 to 1.81).8 However, a smaller, open-label prospective cohort trial (N = 200) found that more participants chose daily PrEP than event-driven PrEP (76.5% vs 23.5%), although almost all said they would change their dosing regimen in the next year.9
Recommendations from others
In 2019, the World Health Organization recommended oral PrEP as an additional prevention choice for people at substantial risk for HIV infection and stated that different dosing strategies offer users flexibility, choice, and convenience.10 Also in 2019, the US Preventive Services Task Force published a recommendation that clinicians offer PrEP with effective antiretroviral therapy to patients at high risk for HIV acquisition. They did not specify which regimen to offer.11
Editor’s takeaway
While there are theoretical reasons why event-driven PrEP might not work as well as daily PrEP, we have 1 RCT that suggests the real-world outcomes are similar. Given the apparent effectiveness of either option, the best choice is the one the patient will use. JFP
EVIDENCE SUMMARY
Event-driven PrEP is effective for prevention of HIV transmission
An RCT evaluating the effectiveness of event-driven PrEP in 400 patients at high risk for HIV found that it reduced HIV incidence by 86% compared to placebo. Researchers recruited HIV-negative men or transgender women who had sex with men, who’d had condomless anal sex with at least 2 partners in the previous 6 months, and followed them for a median of 9.3 months for HIV acquisition.1
Patients randomized to event-driven PrEP took tenofovir-emtricitabine (300-200 mg) on the following schedule: 2 pills 2 to 24 hours before intercourse (or 1 pill if they had taken it within the past week), followed by a third pill 24 hours later, and a fourth pill 24 hours after that. When patients had multiple consecutive episodes of intercourse, daily use was continued until 2 days after the last episode. Patients in the control group took placebo pills.1
Event-driven PrEP reduced HIV incidence vs placebo (2 infections vs 14 infections; 0.91 vs 6.6 per 100 person-years; relative risk [RR] = 0.86; P = .002). PrEP produced more gastrointestinal (14% vs 5%; P = .002) and renal (18% vs 10%; P = .03) adverse effects than placebo. Participants took a median of 15 pills per month.1
A post-hoc analysis of the above study, evaluating 270 patients, found that event-driven PrEP reduced HIV incidence by 100% during periods of less frequent sexual encounters. Selected participants had a median of 5 sexual encounters per month (range, 2-10), used a median of 9.5 pills per month (range, 6-13), and represented 134 person-years of follow-up. No HIV infections (0 per 100 person-years; 95% CI, 0-5; P = .013) were diagnosed in the PrEP group and 6 HIV infections (9.2 per 100 person-years; 95% CI, 3.4-20.1) were diagnosed in the placebo group, with a relative reduction of HIV incidence of 100% (95% CI, 39-100).2
For comparison, 2 large open-label trials evaluating daily PrEP found that it reduced HIV incidence by 44%3 and 86%4 vs placebo.
Adherence is better with daily PrEPthan event-driven PrEP
Three prospective cohort trials evaluated PrEP adherence (extent that participants were taking PrEP at the time of sexual encounters) with different dosing regimens and found that event-driven PrEP tended to have lower adherence than daily PrEP. An open-label trial in Bangkok and Harlem (New York City) randomized 357 at-risk patients to 1 of 3 regimens: event-driven (1 tablet before and after sex), time-driven (1 tablet twice weekly with a postsex dose), and daily. Overall, patients with event-driven PrEP had lower adherence than those with daily PrEP (67% event-driven vs 97% daily; P < 0.0001).5
Continue to: In an open-label...
In an open-label prospective cohort trial in Belgium, at-risk patients chose between using event-driven (N = 44) and daily (N = 135) PrEP. Analysis was conducted for both high-risk HIV exposure days (defined as condomless anal receptive intercourse with a new or HIV-positive steady partner with a detectable viral load) and low-risk HIV exposure days (consistent condom use or condomless anal intercourse with a steady partner who is HIV-negative). Over 18 months, lower adherence was demonstrated with event-driven PrEP than with daily PrEP for high-risk days (88% [95% CI, 86%-90%] vs 97.5% [95% CI, 97%-98%]; P < .0001) and also for low-risk days (42% [95% CI, 40%-45%] vs 96% [95% CI, 95%-96%]; P < .0001).6 Researchers diagnosed no new HIV infections in any participant, and the incidence of STIs was the same in both groups.
A third open-label trial evaluated adherence among 178 South African women randomized to event-driven or daily PrEP and found lower sexual event coverage with event-driven PrEP (52% vs 75%; odds ratio = 2.76; 95% CI, 1.68-4.53; P < 0.0006). Four women in each group seroconverted to HIV positive.7
Drug costs, patient preferences, and STI risk are important considerations
Several of the above trials reported use of fewer pills in the event-driven groups, with lower drug costs.2,5,7 A large prospective cohort trial of men who have sex with men (N = 1049) with an average of 10 sexual partners found that most (76%) opted for event-driven PrEP.8 Researchers also reported no difference in STI rates (RR = 1.24 for “at least 1 bacterial STI”; 95% CI, 0.84 to 1.81).8 However, a smaller, open-label prospective cohort trial (N = 200) found that more participants chose daily PrEP than event-driven PrEP (76.5% vs 23.5%), although almost all said they would change their dosing regimen in the next year.9
Recommendations from others
In 2019, the World Health Organization recommended oral PrEP as an additional prevention choice for people at substantial risk for HIV infection and stated that different dosing strategies offer users flexibility, choice, and convenience.10 Also in 2019, the US Preventive Services Task Force published a recommendation that clinicians offer PrEP with effective antiretroviral therapy to patients at high risk for HIV acquisition. They did not specify which regimen to offer.11
Editor’s takeaway
While there are theoretical reasons why event-driven PrEP might not work as well as daily PrEP, we have 1 RCT that suggests the real-world outcomes are similar. Given the apparent effectiveness of either option, the best choice is the one the patient will use. JFP
- Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. NEJM. 2015;373:2237-2246.
- Antoni G, Tremblay C, Delaugerre C, et al. On-demand pre-exposure prophylaxis with tenofovir disoproxil fumarate plus emtricitabine among men who have sex with men with less frequent sexual intercourse: a post-hoc analysis of the ANRS IPERGAY trial. Lancet HIV. 2020;7:e113-e120.
- Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis in men who have sex with men. NEJM. 2010;363:2587-2599.
- McCormack S, Dunn DT, Desai M, et al. Preexposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot of a pragmatic open-label randomized trial. Lancet. 2016;387:53-60.
- Grant RM, Mannheimer S, Hughes JP, et al. Daily and nondaily oral preexposure prophylaxis in men and transgender women who have sex with men: the Human Immunodeficiency Virus Prevention Trials Network 067/ADAPT study. Clin Infect Dis. 2018;66:1712-1721.
 Vuylsteke B, Reyniers T, De Baetselier I, et al. Daily and event-driven pre-exposure prophylaxis for men who have sex with men in Belgium: results of a prospective cohort measuring adherence, sexual behavior and STI incidence. J Intl AIDS Soc. 2019;22:e25407.
Vuylsteke B, Reyniers T, De Baetselier I, et al. Daily and event-driven pre-exposure prophylaxis for men who have sex with men in Belgium: results of a prospective cohort measuring adherence, sexual behavior and STI incidence. J Intl AIDS Soc. 2019;22:e25407.- Bekker LG, Roux S, Sebastien E, et al. Daily and non-daily pre-exposure prophylaxis in African women (HPTN 067/ADAPT Cape Town Trial): a randomized, open-label, phase 2 trial. Lancet HIV. 2018;5:e68-e78.
- Noret M, Balavoine S, Pintado C, et al. Daily or on-demand oral tenofovir disoproxil fumarate/emtricitabine for HIV pre-exposure prophylaxis: experience from a hospital-based clinic in France. AIDS. 2018;32:2161-2169.
- Reyniers T, Nöstlinger C, Laga M, et al. Choosing between daily and event-driven pre-exposure prophylaxis: results of a Belgian PrEP demonstration project. J Acquir Immune Defic Syndr. 2018;79:186-194.
- WHO. What’s the 2+1+1? Event-driven oral pre-exposure prophylaxis to prevent HIV in men who have sex with men: update to WHO’s recommendation on oral PrEP [technical brief]. Published July 2019. Accessed May 14, 2021. https://who.int/hiv/pub/prep/211/en
- US Preventive Services Task Force. Prevention of human immunodeficiency virus (HIV) infection: preexposure prophylaxis [evidence summary]. Published June 11, 2019. Accessed May 14, 2021. www.uspreventiveservicestaskforce.org/uspstf/document/evidence-summary/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
- Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. NEJM. 2015;373:2237-2246.
- Antoni G, Tremblay C, Delaugerre C, et al. On-demand pre-exposure prophylaxis with tenofovir disoproxil fumarate plus emtricitabine among men who have sex with men with less frequent sexual intercourse: a post-hoc analysis of the ANRS IPERGAY trial. Lancet HIV. 2020;7:e113-e120.
- Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis in men who have sex with men. NEJM. 2010;363:2587-2599.
- McCormack S, Dunn DT, Desai M, et al. Preexposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot of a pragmatic open-label randomized trial. Lancet. 2016;387:53-60.
- Grant RM, Mannheimer S, Hughes JP, et al. Daily and nondaily oral preexposure prophylaxis in men and transgender women who have sex with men: the Human Immunodeficiency Virus Prevention Trials Network 067/ADAPT study. Clin Infect Dis. 2018;66:1712-1721.
 Vuylsteke B, Reyniers T, De Baetselier I, et al. Daily and event-driven pre-exposure prophylaxis for men who have sex with men in Belgium: results of a prospective cohort measuring adherence, sexual behavior and STI incidence. J Intl AIDS Soc. 2019;22:e25407.
Vuylsteke B, Reyniers T, De Baetselier I, et al. Daily and event-driven pre-exposure prophylaxis for men who have sex with men in Belgium: results of a prospective cohort measuring adherence, sexual behavior and STI incidence. J Intl AIDS Soc. 2019;22:e25407.- Bekker LG, Roux S, Sebastien E, et al. Daily and non-daily pre-exposure prophylaxis in African women (HPTN 067/ADAPT Cape Town Trial): a randomized, open-label, phase 2 trial. Lancet HIV. 2018;5:e68-e78.
- Noret M, Balavoine S, Pintado C, et al. Daily or on-demand oral tenofovir disoproxil fumarate/emtricitabine for HIV pre-exposure prophylaxis: experience from a hospital-based clinic in France. AIDS. 2018;32:2161-2169.
- Reyniers T, Nöstlinger C, Laga M, et al. Choosing between daily and event-driven pre-exposure prophylaxis: results of a Belgian PrEP demonstration project. J Acquir Immune Defic Syndr. 2018;79:186-194.
- WHO. What’s the 2+1+1? Event-driven oral pre-exposure prophylaxis to prevent HIV in men who have sex with men: update to WHO’s recommendation on oral PrEP [technical brief]. Published July 2019. Accessed May 14, 2021. https://who.int/hiv/pub/prep/211/en
- US Preventive Services Task Force. Prevention of human immunodeficiency virus (HIV) infection: preexposure prophylaxis [evidence summary]. Published June 11, 2019. Accessed May 14, 2021. www.uspreventiveservicestaskforce.org/uspstf/document/evidence-summary/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
EVIDENCE-BASED ANSWER:
Probably, although there are no head-to-head trials comparing the 2 dosing regimens. Event-driven pre-exposure prophylaxis (PrEP) dosing reduces HIV conversion by 86% compared to placebo (strength of recommendation [SOR]: B, large randomized controlled trial [RCT]). Daily PrEP reduces HIV conversion by 44% to 86% (SOR: B, based on open-label RCTs).
Event-driven PrEP regimens may be associated with lower adherence when compared with daily PrEP regimens (average of 70% for event-driven PrEP vs average of 92% for daily PrEP) (SOR: B, based on open-label and cohort trials). Event-driven PrEP regimens have lower medication costs, and they are associated with no difference in the rate of sexually transmitted infections (STIs) (SOR: B, based on prospective cohort studies). Patients may prefer them to daily regimens (75% choose event-driven PrEP vs 25% choose daily PrEP) (SOR: B, based on the preponderance of prospective cohort studies with conflicting results).
Is ketamine effective and safe for treatment-resistant depression?
Evidence Summary
Single-dose IV ketamine elicits a short-term response
A meta-analysis of RCTs evaluating a single dose of IV ketamine vs placebo for severe depression found that it increased the chance of a treatment response for up to 1 week afterward. Studies included patients with severe (N = 30), treatment-resistant (N = 40), and psychotic depression (N = 10), based on Diagnostic and Statistical Manual of Mental Disorders–Fifth Edition criteria.1
The primary outcome was treatment response: either an improvement of > 50% on a standardized depression scale or a Clinical Global Impression–Improvement scale score of 1 or 2 (“very much” and “much” improved, respectively, as assessed by a clinician). Ketamine increased the likelihood of short-term response or improvement at 24 hours (3 RCTs; N = 56; odds ratio [OR] = 11; 95% CI, 2-58); at 72 hours (3 RCTs; N = 56; OR = 13; 95% CI, 2-66); and at 7 days (4 RCTs; N = 88; OR = 2.6; 95% CI, 1.1-6.2).1 Response rates equaled placebo at 2 weeks. The authors rated the RCTs as low quality.
Another systematic review of single-dose IV ketamine vs placebo for major depression and bipolar disorder included 3 additional small, low-quality RCTs, 2 of which showed short-term response to ketamine. The authors used Hedge’s g statistic to standardize effect size (a score of magnitude 0.2 indicates a small effect; 0.6, moderate; 1.2, large; and 2, very large). One RCT (n = 26) found a very large 1-day response (effect size: –2; 95% CI, –2.8 to –1.3), and 2 RCTs found conflicting responses at 12 days (RCT with N = 18: effect size: –0.2; 95% CI, –0.4 to 0.02 [no significant response] vs RCT with N = 8: effect size: –1.5; 95% CI, –2.5 to –0.5).2
More frequent dosing of IV ketamine improves symptoms
An RCT (N = 67) evaluating twice- or thrice-weekly IV ketamine vs placebo in patients with recurrent depression (with at least 1 treatment failure) found that ketamine significantly improved standardized depression scores and response rates at 15 days. Patients with clinically significant suicidality were excluded.3
Researchers randomized patients to IV ketamine (0.05 mg/kg) twice or thrice weekly or to saline control and used the 60-point Montgomery-Asberg Depression Rating Scale (MADRS). A response was defined as a reduction of the MADRS score by 50%.
Both ketamine arms produced greater symptom improvement at 15 days, compared to placebo (twice weekly: −18.4 vs −5.7; P < 0.001; thrice weekly: −17.7 vs −3.1; P < 0.001) in addition to higher response rates (twice weekly: 69% vs 15%; P = .005; number needed to treat [NNT] = 2; and thrice-weekly: 54% vs 6%; P = .004; NNT = 2).3 There was no significant difference between twice- or thrice-weekly dosing. The study was flawed by dropouts (N = 57 at 15 days and N = 25 at 28 days), primarily attributed to ketamine adverse effects, that prevented assessment beyond 2 weeks.
Oral ketamine has a moderate effecton depression
A systematic review included 2 low-quality RCTs evaluating oral ketamine vs placebo as adjunctive treatment with sertraline, and oral ketamine vs diclofenac, and found improvement in patients with moderate depression.4 In the first RCT (n = 45), researchers found that oral ketamine (25 mg bid) plus sertraline (25 mg titrated up to 150 mg/d) produced more treatment responses (> 50% reduction on a standardized depression rating scale) than placebo plus sertraline (2 weeks: 85.4% vs 42.5%; P < .001; 6 weeks: 85.4% vs 57.5%; P = .005).4
In the second RCT (n = 23), researchers randomized patients with mild-to-moderate depression and comorbid chronic headaches to take oral ketamine (50 mg tid) or oral diclofenac (50 mg tid) and measured effect size on standardized depression scores at 3 weeks (no difference) and 6 weeks (Cohen d effect size = 0.79 [rated as a moderate effect]; P = .017).4
Nasal esketamine + oral antidepressants boosts treatment response rates
A meta-analysis with 4 RCTs (N = 708) evaluating intranasal esketamine vs placebo as an adjunct to oral antidepressants for patients with predominantly treatment-resistant major depression found that it boosted response rates by about 40%. Researchers randomized patients to intranasal esketamine (mostly 28-84 mg twice weekly for 28 days) or placebo spray as an adjunct to oral antidepressants (duloxetine, escitalopram, sertraline, venlafaxine).
The primary outcomes were treatment response (≥ 50% reduction in depression scores) or remission (a MADRS score < 12). Adjunctive intranasal esketamine produced greater rates of treatment response compared to placebo at 24 hours (21% vs 7%; relative risk [RR] = 8.4; 95% CI, 1.4 to 21.2; P < .02; NNT = 7) and at 28 days (59% vs 43%; RR = 1.4; 95% CI, 1.2 to 1.60; P < .0001; NNT = 6).5 Adjunctive intranasal esketamine also produced greater rates of remission at the end of the study (mostly at 28 days), compared with placebo (41% vs 25%; RR = 1.4; 95% CI, 1.2 to 1.7; P = .0004; NNT = 7).5 The authors rated study quality as moderate to high.
Adverse effects are common, may cause Tx discontinuation
Ketamine-produced adverse effects (AEs) included confusion (2 trials; N = 76; OR = 3.7; 95% CI, 1.1-12) and emotional blunting (1 trial; N = 30; OR = 23; 95% CI, 1.1-489).1
A 2018 systematic review assessed the safety of ketamine in depression after single and repeated dose in 60 studies (N = 899; 20 RCTs, 17 open-label-trials, 20 case series, and 3 retrospective studies). The most common AEs reported were headache (35% of studies), dizziness (33%), dissociation (28%), elevated blood pressure (28%), and blurred vision (23%), with the majority reported to resolve shortly after administration. The most common psychiatric AE was anxiety (15%).6 Included studies varied greatly in design and dosage form, and no meta-analysis could be performed.
Nasal esketamine produced more AEs causing discontinuation than did placebo (5.8% vs 1.5%; RR = 3.5; 95% CI, 1.34-8.9; number needed to harm [NNH] = 23), including blurred vision, dizziness, sedation, nausea, and dysphoria.5A review (5 RCTs and 1 open-label trial; N = 1708) analyzing the cardiac safety profile of intranasal esketamine adjuvant therapy found that it produced transient and asymptomatic blood pressure elevations (OR = 3.2; 95% CI, 1.9-5.8; NNH = 13).7
Recommendations from others
A clinical practice guideline from the US Veterans Administration lists IV ketamine as 1 of the therapeutic options for patients with treatment-resistant depression and suicidal ideation.8 However, a Department of Veterans Affairs Panel restricted its use to a pre-approved case-by-case basis.8
Editor’s takeaway
Physicians with patients facing the all-too-common problem of treatment-resistant major depression will be wondering if ketamine, either by itself or as an augmentation therapy, can help. Unfortunately, the outcomes we report here are too short term to answer that question, and we must await the results of further studies. Augmentation with intranasal esketamine, at a cost of $370/month, might offer some promise.
1. Caddy C, Amit BH, McCloud TL, et al. Ketamine and other glutamate receptor modulators for depression in adults. Cochrane Database Syst Rev. 2015;(9):CD011612.
2. Coyle CM, Laws KR. The use of ketamine as an antidepressant: a systematic review and meta-analysis. Hum Psychopharmacol. 2015;30:152‐163.
3. Singh JB, Fedgchin M, Daly EJ, et al. A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry. 2016;173:816‐826.
4. Rosenblat JD, Carvalho AF, Li M, et al. Oral ketamine for depression: a systematic review. J Clin Psychiatry. 2019;80:18r12475.
5. Zheng W, Cai DB, Xiang YQ, et al. Adjunctive intranasal esketamine for major depressive disorder: a systematic review of randomized double-blind controlled-placebo studies. J Affect Disord. 2020;265:63‐70.
6. Short B, Fong J, Galvez V, et al. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5:65‐78.
7. Doherty T, Wajs E, Melkote R, et al. Cardiac safety of esketamine nasal spray in treatment-resistant depression: results from the Clinical Development Program. CNS Drugs. 2020;34:299‐310.
8. Sall J, Brenner L, Millikan Bell AM, et al. Assessment and management of patients at risk for suicide: synopsis of the 2019 US Department of Veterans Affairs and US Department of Defense Clinical Practice Guidelines. Ann Intern Med. 2019;171:343-353.
Evidence Summary
Single-dose IV ketamine elicits a short-term response
A meta-analysis of RCTs evaluating a single dose of IV ketamine vs placebo for severe depression found that it increased the chance of a treatment response for up to 1 week afterward. Studies included patients with severe (N = 30), treatment-resistant (N = 40), and psychotic depression (N = 10), based on Diagnostic and Statistical Manual of Mental Disorders–Fifth Edition criteria.1
The primary outcome was treatment response: either an improvement of > 50% on a standardized depression scale or a Clinical Global Impression–Improvement scale score of 1 or 2 (“very much” and “much” improved, respectively, as assessed by a clinician). Ketamine increased the likelihood of short-term response or improvement at 24 hours (3 RCTs; N = 56; odds ratio [OR] = 11; 95% CI, 2-58); at 72 hours (3 RCTs; N = 56; OR = 13; 95% CI, 2-66); and at 7 days (4 RCTs; N = 88; OR = 2.6; 95% CI, 1.1-6.2).1 Response rates equaled placebo at 2 weeks. The authors rated the RCTs as low quality.
Another systematic review of single-dose IV ketamine vs placebo for major depression and bipolar disorder included 3 additional small, low-quality RCTs, 2 of which showed short-term response to ketamine. The authors used Hedge’s g statistic to standardize effect size (a score of magnitude 0.2 indicates a small effect; 0.6, moderate; 1.2, large; and 2, very large). One RCT (n = 26) found a very large 1-day response (effect size: –2; 95% CI, –2.8 to –1.3), and 2 RCTs found conflicting responses at 12 days (RCT with N = 18: effect size: –0.2; 95% CI, –0.4 to 0.02 [no significant response] vs RCT with N = 8: effect size: –1.5; 95% CI, –2.5 to –0.5).2
More frequent dosing of IV ketamine improves symptoms
An RCT (N = 67) evaluating twice- or thrice-weekly IV ketamine vs placebo in patients with recurrent depression (with at least 1 treatment failure) found that ketamine significantly improved standardized depression scores and response rates at 15 days. Patients with clinically significant suicidality were excluded.3
Researchers randomized patients to IV ketamine (0.05 mg/kg) twice or thrice weekly or to saline control and used the 60-point Montgomery-Asberg Depression Rating Scale (MADRS). A response was defined as a reduction of the MADRS score by 50%.
Both ketamine arms produced greater symptom improvement at 15 days, compared to placebo (twice weekly: −18.4 vs −5.7; P < 0.001; thrice weekly: −17.7 vs −3.1; P < 0.001) in addition to higher response rates (twice weekly: 69% vs 15%; P = .005; number needed to treat [NNT] = 2; and thrice-weekly: 54% vs 6%; P = .004; NNT = 2).3 There was no significant difference between twice- or thrice-weekly dosing. The study was flawed by dropouts (N = 57 at 15 days and N = 25 at 28 days), primarily attributed to ketamine adverse effects, that prevented assessment beyond 2 weeks.
Oral ketamine has a moderate effecton depression
A systematic review included 2 low-quality RCTs evaluating oral ketamine vs placebo as adjunctive treatment with sertraline, and oral ketamine vs diclofenac, and found improvement in patients with moderate depression.4 In the first RCT (n = 45), researchers found that oral ketamine (25 mg bid) plus sertraline (25 mg titrated up to 150 mg/d) produced more treatment responses (> 50% reduction on a standardized depression rating scale) than placebo plus sertraline (2 weeks: 85.4% vs 42.5%; P < .001; 6 weeks: 85.4% vs 57.5%; P = .005).4
In the second RCT (n = 23), researchers randomized patients with mild-to-moderate depression and comorbid chronic headaches to take oral ketamine (50 mg tid) or oral diclofenac (50 mg tid) and measured effect size on standardized depression scores at 3 weeks (no difference) and 6 weeks (Cohen d effect size = 0.79 [rated as a moderate effect]; P = .017).4
Nasal esketamine + oral antidepressants boosts treatment response rates
A meta-analysis with 4 RCTs (N = 708) evaluating intranasal esketamine vs placebo as an adjunct to oral antidepressants for patients with predominantly treatment-resistant major depression found that it boosted response rates by about 40%. Researchers randomized patients to intranasal esketamine (mostly 28-84 mg twice weekly for 28 days) or placebo spray as an adjunct to oral antidepressants (duloxetine, escitalopram, sertraline, venlafaxine).
The primary outcomes were treatment response (≥ 50% reduction in depression scores) or remission (a MADRS score < 12). Adjunctive intranasal esketamine produced greater rates of treatment response compared to placebo at 24 hours (21% vs 7%; relative risk [RR] = 8.4; 95% CI, 1.4 to 21.2; P < .02; NNT = 7) and at 28 days (59% vs 43%; RR = 1.4; 95% CI, 1.2 to 1.60; P < .0001; NNT = 6).5 Adjunctive intranasal esketamine also produced greater rates of remission at the end of the study (mostly at 28 days), compared with placebo (41% vs 25%; RR = 1.4; 95% CI, 1.2 to 1.7; P = .0004; NNT = 7).5 The authors rated study quality as moderate to high.
Adverse effects are common, may cause Tx discontinuation
Ketamine-produced adverse effects (AEs) included confusion (2 trials; N = 76; OR = 3.7; 95% CI, 1.1-12) and emotional blunting (1 trial; N = 30; OR = 23; 95% CI, 1.1-489).1
A 2018 systematic review assessed the safety of ketamine in depression after single and repeated dose in 60 studies (N = 899; 20 RCTs, 17 open-label-trials, 20 case series, and 3 retrospective studies). The most common AEs reported were headache (35% of studies), dizziness (33%), dissociation (28%), elevated blood pressure (28%), and blurred vision (23%), with the majority reported to resolve shortly after administration. The most common psychiatric AE was anxiety (15%).6 Included studies varied greatly in design and dosage form, and no meta-analysis could be performed.
Nasal esketamine produced more AEs causing discontinuation than did placebo (5.8% vs 1.5%; RR = 3.5; 95% CI, 1.34-8.9; number needed to harm [NNH] = 23), including blurred vision, dizziness, sedation, nausea, and dysphoria.5A review (5 RCTs and 1 open-label trial; N = 1708) analyzing the cardiac safety profile of intranasal esketamine adjuvant therapy found that it produced transient and asymptomatic blood pressure elevations (OR = 3.2; 95% CI, 1.9-5.8; NNH = 13).7
Recommendations from others
A clinical practice guideline from the US Veterans Administration lists IV ketamine as 1 of the therapeutic options for patients with treatment-resistant depression and suicidal ideation.8 However, a Department of Veterans Affairs Panel restricted its use to a pre-approved case-by-case basis.8
Editor’s takeaway
Physicians with patients facing the all-too-common problem of treatment-resistant major depression will be wondering if ketamine, either by itself or as an augmentation therapy, can help. Unfortunately, the outcomes we report here are too short term to answer that question, and we must await the results of further studies. Augmentation with intranasal esketamine, at a cost of $370/month, might offer some promise.
Evidence Summary
Single-dose IV ketamine elicits a short-term response
A meta-analysis of RCTs evaluating a single dose of IV ketamine vs placebo for severe depression found that it increased the chance of a treatment response for up to 1 week afterward. Studies included patients with severe (N = 30), treatment-resistant (N = 40), and psychotic depression (N = 10), based on Diagnostic and Statistical Manual of Mental Disorders–Fifth Edition criteria.1
The primary outcome was treatment response: either an improvement of > 50% on a standardized depression scale or a Clinical Global Impression–Improvement scale score of 1 or 2 (“very much” and “much” improved, respectively, as assessed by a clinician). Ketamine increased the likelihood of short-term response or improvement at 24 hours (3 RCTs; N = 56; odds ratio [OR] = 11; 95% CI, 2-58); at 72 hours (3 RCTs; N = 56; OR = 13; 95% CI, 2-66); and at 7 days (4 RCTs; N = 88; OR = 2.6; 95% CI, 1.1-6.2).1 Response rates equaled placebo at 2 weeks. The authors rated the RCTs as low quality.
Another systematic review of single-dose IV ketamine vs placebo for major depression and bipolar disorder included 3 additional small, low-quality RCTs, 2 of which showed short-term response to ketamine. The authors used Hedge’s g statistic to standardize effect size (a score of magnitude 0.2 indicates a small effect; 0.6, moderate; 1.2, large; and 2, very large). One RCT (n = 26) found a very large 1-day response (effect size: –2; 95% CI, –2.8 to –1.3), and 2 RCTs found conflicting responses at 12 days (RCT with N = 18: effect size: –0.2; 95% CI, –0.4 to 0.02 [no significant response] vs RCT with N = 8: effect size: –1.5; 95% CI, –2.5 to –0.5).2
More frequent dosing of IV ketamine improves symptoms
An RCT (N = 67) evaluating twice- or thrice-weekly IV ketamine vs placebo in patients with recurrent depression (with at least 1 treatment failure) found that ketamine significantly improved standardized depression scores and response rates at 15 days. Patients with clinically significant suicidality were excluded.3
Researchers randomized patients to IV ketamine (0.05 mg/kg) twice or thrice weekly or to saline control and used the 60-point Montgomery-Asberg Depression Rating Scale (MADRS). A response was defined as a reduction of the MADRS score by 50%.
Both ketamine arms produced greater symptom improvement at 15 days, compared to placebo (twice weekly: −18.4 vs −5.7; P < 0.001; thrice weekly: −17.7 vs −3.1; P < 0.001) in addition to higher response rates (twice weekly: 69% vs 15%; P = .005; number needed to treat [NNT] = 2; and thrice-weekly: 54% vs 6%; P = .004; NNT = 2).3 There was no significant difference between twice- or thrice-weekly dosing. The study was flawed by dropouts (N = 57 at 15 days and N = 25 at 28 days), primarily attributed to ketamine adverse effects, that prevented assessment beyond 2 weeks.
Oral ketamine has a moderate effecton depression
A systematic review included 2 low-quality RCTs evaluating oral ketamine vs placebo as adjunctive treatment with sertraline, and oral ketamine vs diclofenac, and found improvement in patients with moderate depression.4 In the first RCT (n = 45), researchers found that oral ketamine (25 mg bid) plus sertraline (25 mg titrated up to 150 mg/d) produced more treatment responses (> 50% reduction on a standardized depression rating scale) than placebo plus sertraline (2 weeks: 85.4% vs 42.5%; P < .001; 6 weeks: 85.4% vs 57.5%; P = .005).4
In the second RCT (n = 23), researchers randomized patients with mild-to-moderate depression and comorbid chronic headaches to take oral ketamine (50 mg tid) or oral diclofenac (50 mg tid) and measured effect size on standardized depression scores at 3 weeks (no difference) and 6 weeks (Cohen d effect size = 0.79 [rated as a moderate effect]; P = .017).4
Nasal esketamine + oral antidepressants boosts treatment response rates
A meta-analysis with 4 RCTs (N = 708) evaluating intranasal esketamine vs placebo as an adjunct to oral antidepressants for patients with predominantly treatment-resistant major depression found that it boosted response rates by about 40%. Researchers randomized patients to intranasal esketamine (mostly 28-84 mg twice weekly for 28 days) or placebo spray as an adjunct to oral antidepressants (duloxetine, escitalopram, sertraline, venlafaxine).
The primary outcomes were treatment response (≥ 50% reduction in depression scores) or remission (a MADRS score < 12). Adjunctive intranasal esketamine produced greater rates of treatment response compared to placebo at 24 hours (21% vs 7%; relative risk [RR] = 8.4; 95% CI, 1.4 to 21.2; P < .02; NNT = 7) and at 28 days (59% vs 43%; RR = 1.4; 95% CI, 1.2 to 1.60; P < .0001; NNT = 6).5 Adjunctive intranasal esketamine also produced greater rates of remission at the end of the study (mostly at 28 days), compared with placebo (41% vs 25%; RR = 1.4; 95% CI, 1.2 to 1.7; P = .0004; NNT = 7).5 The authors rated study quality as moderate to high.
Adverse effects are common, may cause Tx discontinuation
Ketamine-produced adverse effects (AEs) included confusion (2 trials; N = 76; OR = 3.7; 95% CI, 1.1-12) and emotional blunting (1 trial; N = 30; OR = 23; 95% CI, 1.1-489).1
A 2018 systematic review assessed the safety of ketamine in depression after single and repeated dose in 60 studies (N = 899; 20 RCTs, 17 open-label-trials, 20 case series, and 3 retrospective studies). The most common AEs reported were headache (35% of studies), dizziness (33%), dissociation (28%), elevated blood pressure (28%), and blurred vision (23%), with the majority reported to resolve shortly after administration. The most common psychiatric AE was anxiety (15%).6 Included studies varied greatly in design and dosage form, and no meta-analysis could be performed.
Nasal esketamine produced more AEs causing discontinuation than did placebo (5.8% vs 1.5%; RR = 3.5; 95% CI, 1.34-8.9; number needed to harm [NNH] = 23), including blurred vision, dizziness, sedation, nausea, and dysphoria.5A review (5 RCTs and 1 open-label trial; N = 1708) analyzing the cardiac safety profile of intranasal esketamine adjuvant therapy found that it produced transient and asymptomatic blood pressure elevations (OR = 3.2; 95% CI, 1.9-5.8; NNH = 13).7
Recommendations from others
A clinical practice guideline from the US Veterans Administration lists IV ketamine as 1 of the therapeutic options for patients with treatment-resistant depression and suicidal ideation.8 However, a Department of Veterans Affairs Panel restricted its use to a pre-approved case-by-case basis.8
Editor’s takeaway
Physicians with patients facing the all-too-common problem of treatment-resistant major depression will be wondering if ketamine, either by itself or as an augmentation therapy, can help. Unfortunately, the outcomes we report here are too short term to answer that question, and we must await the results of further studies. Augmentation with intranasal esketamine, at a cost of $370/month, might offer some promise.
1. Caddy C, Amit BH, McCloud TL, et al. Ketamine and other glutamate receptor modulators for depression in adults. Cochrane Database Syst Rev. 2015;(9):CD011612.
2. Coyle CM, Laws KR. The use of ketamine as an antidepressant: a systematic review and meta-analysis. Hum Psychopharmacol. 2015;30:152‐163.
3. Singh JB, Fedgchin M, Daly EJ, et al. A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry. 2016;173:816‐826.
4. Rosenblat JD, Carvalho AF, Li M, et al. Oral ketamine for depression: a systematic review. J Clin Psychiatry. 2019;80:18r12475.
5. Zheng W, Cai DB, Xiang YQ, et al. Adjunctive intranasal esketamine for major depressive disorder: a systematic review of randomized double-blind controlled-placebo studies. J Affect Disord. 2020;265:63‐70.
6. Short B, Fong J, Galvez V, et al. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5:65‐78.
7. Doherty T, Wajs E, Melkote R, et al. Cardiac safety of esketamine nasal spray in treatment-resistant depression: results from the Clinical Development Program. CNS Drugs. 2020;34:299‐310.
8. Sall J, Brenner L, Millikan Bell AM, et al. Assessment and management of patients at risk for suicide: synopsis of the 2019 US Department of Veterans Affairs and US Department of Defense Clinical Practice Guidelines. Ann Intern Med. 2019;171:343-353.
1. Caddy C, Amit BH, McCloud TL, et al. Ketamine and other glutamate receptor modulators for depression in adults. Cochrane Database Syst Rev. 2015;(9):CD011612.
2. Coyle CM, Laws KR. The use of ketamine as an antidepressant: a systematic review and meta-analysis. Hum Psychopharmacol. 2015;30:152‐163.
3. Singh JB, Fedgchin M, Daly EJ, et al. A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry. 2016;173:816‐826.
4. Rosenblat JD, Carvalho AF, Li M, et al. Oral ketamine for depression: a systematic review. J Clin Psychiatry. 2019;80:18r12475.
5. Zheng W, Cai DB, Xiang YQ, et al. Adjunctive intranasal esketamine for major depressive disorder: a systematic review of randomized double-blind controlled-placebo studies. J Affect Disord. 2020;265:63‐70.
6. Short B, Fong J, Galvez V, et al. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5:65‐78.
7. Doherty T, Wajs E, Melkote R, et al. Cardiac safety of esketamine nasal spray in treatment-resistant depression: results from the Clinical Development Program. CNS Drugs. 2020;34:299‐310.
8. Sall J, Brenner L, Millikan Bell AM, et al. Assessment and management of patients at risk for suicide: synopsis of the 2019 US Department of Veterans Affairs and US Department of Defense Clinical Practice Guidelines. Ann Intern Med. 2019;171:343-353.
EVIDENCE-BASED ANSWER:
MAYBE, but it’s too soon to tell. There is limited evidence that ketamine by itself is effective in the very short term. Single-dose intravenous (IV) ketamine is more likely than placebo (odds ratio = 11-13) to produce improvement (> 50%) in standardized depression scores in 1 to 3 days, lasting up to a week. Twice- or thrice-weekly IV ketamine improves symptom scores by 20%-25% over 2 weeks (strength of recommendation [SOR]: B, meta-analysis of small, low-quality, randomized controlled trials [RCTs] and a single small RCT).
Augmentation of sertraline with daily oral ketamine moderately improves symptom scores for 6 weeks in patients with moderate depression (SOR: B, small, low-quality RCTs).
Augmentation of oral antidepressants (duloxetine, escitalopram, sertraline, venlafaxine) with intranasal esketamine spray improves response and remission rates at 4 weeks (16% for both outcomes) in patients with predominantly treatment-resistant major depression (SOR: A, meta-analysis of RCTs).
Ketamine therapy is associated with confusion, emotional blunting, headache, dizziness, and blurred vision (SOR: A, meta-analyses).
Nasal esketamine spray produces the adverse effects of dizziness, vertigo, and blurred vision severe enough to cause discontinuation in 4% of patients; it also can produce transient elevation of blood pressure (SOR: A, meta-analyses).
Is metformin effective for reducing weight in obese or overweight adolescents?
EVIDENCE SUMMARY
Metformin has modest effects on body weight
A large systematic review and meta-analysis (38 RCTs; n = 2199) published in 2020 evaluated metformin therapy in children and adolescents (including those with metabolic disease, growth problems, and psychological disorders in addition to obesity and overweight).1 Over an average of 6 months, metformin use modestly reduced BMI (weighted mean difference [WMD] = –1.07 kg/m2; 95% CI, –1.43 to –0.72 kg/m2) and body weight (WMD = –2.51 kg; 95% CI, –3.14 to –1.89 kg) for all participants.1
However, the authors also performed a meta-analysis of trials involving obese or overweight youth without other comorbidities. Participants in these trials ranged in age from 7 to 17 years (mean not supplied; most trials, 12-15 years), had a BMI greater than the 95th percentile for age, and took doses of metformin ranging from 1500 to 3000 mg (most trials, 1500-2000 mg/d for 24 weeks).1 In this analysis, metformin reduced body weight (8 trials; n = 616; WMD = –2.06 kg; 95% CI, –3.47 to –0.65 kg) and body fat mass (–1.9%; 95% CI, –3.25% to –0.56%). But it did not reduce BMI (12 trials; n = 826; WMD = –0.76 kg/m2; 95 % CI, –1.61 to 0.08 kg/m2) or improve lean body mass (2 trials; N = 98; WMD = –0.74 kg; 95% CI, –2.4 to 0.91 kg).1
The authors of this meta-analysis did not include an evaluation of the quality of the individual RCTs.
Metformin has benefits but also adverse effects
A 2016 Cochrane systematic review and meta-analysis assessed 8 trials (total n = 543) evaluating metformin vs placebo in adolescents prescribed exercise and lifestyle support.2 This meta-analysis included 4 trials (n = 294) with obese or overweight adolescents that were also included in the newer meta-analysis,1 as well as 4 trials involving obese adolescents with insulin resistance. The authors did not assess the effects of metformin on obese or overweight adolescents separately.
Over 6 months, metformin use reduced BMI (WMD = –1.35 kg/m2; 95% CI –2 to –0.69 kg/m2).2 Metformin commonly produced gastrointestinal symptoms: diarrhea, flatulence (rates not given), and nausea in 15% to 42% compared with 3% to 21% with placebo (no comparison statistic supplied), however rarely to the point of discontinuation (< 5%).2 Nine participants withdrew due to adverse effects: 5 in the metformin group and 4 in the placebo group. The authors rated the quality of the included trials as low to moderate.
An evidence report and systematic review (42 RCTs; total n = 6956) compared the efficacy of several approaches for weight loss in adolescents, including metformin (6 of the 8 RCTs included in the 2020 meta-analysis1) and lifestyle interventions.3 Interventions comprising exercise and diet counseling for > 26 hours over 6 to 12 months produced decreases in BMI (–0.86 kg/m2; 95% CI –1.44 to –0.29 kg/m2) but not weight (–2 kg; 95% CI –3.2 to 1.2 kg).3
Recommendations from others
The US Preventive Services Task Force states that metformin treatment in adolescents who are overweight or obese produces a small reduction in BMI when compared to placebo, but the clinical significance of this reduction is unclear.3
Editor’s takeaway
The idea of using medications for weight loss remains seductive, given how hard it can be for patients to achieve significant, lasting weight loss through lifestyle modification. Evidence suggests that metformin can help in this regard but not enough to recommend it. In addition, metformin therapy is associated with gastrointestinal adverse effects.
1. Sadeghi A, Mousavi SM, Mokhtari T, et al. Metformin therapy reduces obesity indices in children and adolescents: a systematic review and meta-analysis of randomized clinical trials. Child Obes. 2020;16:174-191.
2. Mead E, Atkinson G, Richter B, et al. Drug interventions for the treatment of obesity in children and adolescents. Cochrane Database Syst Rev. 2016;11:CD012436.
3. O’Connor EA, Evans CV, Burda BU, et al. Screening for obesity and intervention for weight management in children and adolescents: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317:2427-2444.
EVIDENCE SUMMARY
Metformin has modest effects on body weight
A large systematic review and meta-analysis (38 RCTs; n = 2199) published in 2020 evaluated metformin therapy in children and adolescents (including those with metabolic disease, growth problems, and psychological disorders in addition to obesity and overweight).1 Over an average of 6 months, metformin use modestly reduced BMI (weighted mean difference [WMD] = –1.07 kg/m2; 95% CI, –1.43 to –0.72 kg/m2) and body weight (WMD = –2.51 kg; 95% CI, –3.14 to –1.89 kg) for all participants.1
However, the authors also performed a meta-analysis of trials involving obese or overweight youth without other comorbidities. Participants in these trials ranged in age from 7 to 17 years (mean not supplied; most trials, 12-15 years), had a BMI greater than the 95th percentile for age, and took doses of metformin ranging from 1500 to 3000 mg (most trials, 1500-2000 mg/d for 24 weeks).1 In this analysis, metformin reduced body weight (8 trials; n = 616; WMD = –2.06 kg; 95% CI, –3.47 to –0.65 kg) and body fat mass (–1.9%; 95% CI, –3.25% to –0.56%). But it did not reduce BMI (12 trials; n = 826; WMD = –0.76 kg/m2; 95 % CI, –1.61 to 0.08 kg/m2) or improve lean body mass (2 trials; N = 98; WMD = –0.74 kg; 95% CI, –2.4 to 0.91 kg).1
The authors of this meta-analysis did not include an evaluation of the quality of the individual RCTs.
Metformin has benefits but also adverse effects
A 2016 Cochrane systematic review and meta-analysis assessed 8 trials (total n = 543) evaluating metformin vs placebo in adolescents prescribed exercise and lifestyle support.2 This meta-analysis included 4 trials (n = 294) with obese or overweight adolescents that were also included in the newer meta-analysis,1 as well as 4 trials involving obese adolescents with insulin resistance. The authors did not assess the effects of metformin on obese or overweight adolescents separately.
Over 6 months, metformin use reduced BMI (WMD = –1.35 kg/m2; 95% CI –2 to –0.69 kg/m2).2 Metformin commonly produced gastrointestinal symptoms: diarrhea, flatulence (rates not given), and nausea in 15% to 42% compared with 3% to 21% with placebo (no comparison statistic supplied), however rarely to the point of discontinuation (< 5%).2 Nine participants withdrew due to adverse effects: 5 in the metformin group and 4 in the placebo group. The authors rated the quality of the included trials as low to moderate.
An evidence report and systematic review (42 RCTs; total n = 6956) compared the efficacy of several approaches for weight loss in adolescents, including metformin (6 of the 8 RCTs included in the 2020 meta-analysis1) and lifestyle interventions.3 Interventions comprising exercise and diet counseling for > 26 hours over 6 to 12 months produced decreases in BMI (–0.86 kg/m2; 95% CI –1.44 to –0.29 kg/m2) but not weight (–2 kg; 95% CI –3.2 to 1.2 kg).3
Recommendations from others
The US Preventive Services Task Force states that metformin treatment in adolescents who are overweight or obese produces a small reduction in BMI when compared to placebo, but the clinical significance of this reduction is unclear.3
Editor’s takeaway
The idea of using medications for weight loss remains seductive, given how hard it can be for patients to achieve significant, lasting weight loss through lifestyle modification. Evidence suggests that metformin can help in this regard but not enough to recommend it. In addition, metformin therapy is associated with gastrointestinal adverse effects.
EVIDENCE SUMMARY
Metformin has modest effects on body weight
A large systematic review and meta-analysis (38 RCTs; n = 2199) published in 2020 evaluated metformin therapy in children and adolescents (including those with metabolic disease, growth problems, and psychological disorders in addition to obesity and overweight).1 Over an average of 6 months, metformin use modestly reduced BMI (weighted mean difference [WMD] = –1.07 kg/m2; 95% CI, –1.43 to –0.72 kg/m2) and body weight (WMD = –2.51 kg; 95% CI, –3.14 to –1.89 kg) for all participants.1
However, the authors also performed a meta-analysis of trials involving obese or overweight youth without other comorbidities. Participants in these trials ranged in age from 7 to 17 years (mean not supplied; most trials, 12-15 years), had a BMI greater than the 95th percentile for age, and took doses of metformin ranging from 1500 to 3000 mg (most trials, 1500-2000 mg/d for 24 weeks).1 In this analysis, metformin reduced body weight (8 trials; n = 616; WMD = –2.06 kg; 95% CI, –3.47 to –0.65 kg) and body fat mass (–1.9%; 95% CI, –3.25% to –0.56%). But it did not reduce BMI (12 trials; n = 826; WMD = –0.76 kg/m2; 95 % CI, –1.61 to 0.08 kg/m2) or improve lean body mass (2 trials; N = 98; WMD = –0.74 kg; 95% CI, –2.4 to 0.91 kg).1
The authors of this meta-analysis did not include an evaluation of the quality of the individual RCTs.
Metformin has benefits but also adverse effects
A 2016 Cochrane systematic review and meta-analysis assessed 8 trials (total n = 543) evaluating metformin vs placebo in adolescents prescribed exercise and lifestyle support.2 This meta-analysis included 4 trials (n = 294) with obese or overweight adolescents that were also included in the newer meta-analysis,1 as well as 4 trials involving obese adolescents with insulin resistance. The authors did not assess the effects of metformin on obese or overweight adolescents separately.
Over 6 months, metformin use reduced BMI (WMD = –1.35 kg/m2; 95% CI –2 to –0.69 kg/m2).2 Metformin commonly produced gastrointestinal symptoms: diarrhea, flatulence (rates not given), and nausea in 15% to 42% compared with 3% to 21% with placebo (no comparison statistic supplied), however rarely to the point of discontinuation (< 5%).2 Nine participants withdrew due to adverse effects: 5 in the metformin group and 4 in the placebo group. The authors rated the quality of the included trials as low to moderate.
An evidence report and systematic review (42 RCTs; total n = 6956) compared the efficacy of several approaches for weight loss in adolescents, including metformin (6 of the 8 RCTs included in the 2020 meta-analysis1) and lifestyle interventions.3 Interventions comprising exercise and diet counseling for > 26 hours over 6 to 12 months produced decreases in BMI (–0.86 kg/m2; 95% CI –1.44 to –0.29 kg/m2) but not weight (–2 kg; 95% CI –3.2 to 1.2 kg).3
Recommendations from others
The US Preventive Services Task Force states that metformin treatment in adolescents who are overweight or obese produces a small reduction in BMI when compared to placebo, but the clinical significance of this reduction is unclear.3
Editor’s takeaway
The idea of using medications for weight loss remains seductive, given how hard it can be for patients to achieve significant, lasting weight loss through lifestyle modification. Evidence suggests that metformin can help in this regard but not enough to recommend it. In addition, metformin therapy is associated with gastrointestinal adverse effects.
1. Sadeghi A, Mousavi SM, Mokhtari T, et al. Metformin therapy reduces obesity indices in children and adolescents: a systematic review and meta-analysis of randomized clinical trials. Child Obes. 2020;16:174-191.
2. Mead E, Atkinson G, Richter B, et al. Drug interventions for the treatment of obesity in children and adolescents. Cochrane Database Syst Rev. 2016;11:CD012436.
3. O’Connor EA, Evans CV, Burda BU, et al. Screening for obesity and intervention for weight management in children and adolescents: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317:2427-2444.
1. Sadeghi A, Mousavi SM, Mokhtari T, et al. Metformin therapy reduces obesity indices in children and adolescents: a systematic review and meta-analysis of randomized clinical trials. Child Obes. 2020;16:174-191.
2. Mead E, Atkinson G, Richter B, et al. Drug interventions for the treatment of obesity in children and adolescents. Cochrane Database Syst Rev. 2016;11:CD012436.
3. O’Connor EA, Evans CV, Burda BU, et al. Screening for obesity and intervention for weight management in children and adolescents: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317:2427-2444.
EVIDENCE-BASED ANSWER:
Yes, to some degree—but it is of uncertain clinical significance. Over a period of 6 months, metformin modestly reduced weight (–2.1 kg) and body fat mass (–1.9%), but not body mass index (BMI) or lean body mass, in adolescents who were overweight or obese. This is comparable to lifestyle interventions (diet and exercise) supported with > 26 hours of counseling, which modestly improved BMI but not weight. (Strength of recommendation [SOR]: A, based on a large meta-analysis of randomized controlled trials [RCTs] of variable quality).
Do electronic reminder systems help patients with T2DM to lose weight?
EVIDENCE SUMMARY
A meta-analysis of 6 RCTs studied the effect of smartphone self-care applications on A1C, weight, blood pressure, and lipids in adult patients with T2DM. All the interventions comprised 4 components: electronic self-management prompts and reminders, personal measuring devices, patient-driven data upload, and remote analysis of the data with feedback. The review excluded studies that used phone calls or lasted fewer than 3 months.
Some improvement in A1C found, but no effect on weight
Telehealth interventions improved A1C more than usual care (6 trials, 884 patients; mean difference = –0.40%; 95% CI, –0.69% to –0.11%).1 A subset of 4 studies with 560 patients evaluated changes in weight. Patients had a mean age of 61 years and average weight of 84 kg (in 3 of 4 studies reporting baseline weight). Aggregate weight loss was insignificant after 3 to 12 months (mean difference = –0.84 kg; 95% CI, –2.04 kg to 0.36 kg, P = .17). Investigators reported no harms. Limitations of the analysis included high heterogeneity in the main outcome of A1C (I2 = 70%) but low heterogeneity within the 4 studies assessing weight (I2 = 30%).
Other, small studies found no change in A1C
Two subsequent small RCTs came to different conclusions than the meta-analysis. One compared the impact of individualized physical activity–based text messages in response to pedometer readings with pedometer use alone.2 It included 126 adult patients (mean age, 50.5 years) with T2DM who had an A1C > 7% and access to an Internet-connected computer. Researchers excluded patients who were unable to perform moderate physical activity or who had cognitive deficits.
At enrollment, researchers supplied all patients with a pedometer and an appointment with a counselor to set goals for physical activity. They sent 2 text messages daily to the intervention group (and none to the control group) based on uploaded pedometer data. One message detailed physical activity progress and the second encouraged increased physical activity. The primary outcome was mean step counts per month; secondary outcomes included A1C and weight measured at 6 months.
The groups showed no significant difference in A1C (mean difference = 0.07%; 95% CI, –0.47% to 0.34%, P = .75) or weight loss (mean difference = 3.1 lb; 95% CI, –24.5 lb to 18.3 lb, P = .77). Many patients (43%) reported difficulty uploading step counts, receiving texts, and responding to texts. The dropout rate was 24%.
A second RCT with 150 patients, using a less elaborate protocol, assessed the effectiveness of tailored text-message reminders compared with nontailored text messages to improve A1C and body mass index (BMI).3 Patients were adult Iranians (mean age, 52.5 years) with T2DM who owned a cell phone and could receive and read text messages.
Patients filled out a diabetic self-care assessment to identify barriers to improving care and were randomized into 3 groups. The first group received tailored text messages (75% addressing the patient’s top 2 barriers to self-care and 25% general messages). The second group received nontailored text messages of encouragement. The control group received no text messages.
Continue to: After 3 months...
After 3 months, BMI was reduced in both messaging groups but not the control group (tailored text = –0.6 kg/m2, nontailored text = –0.5 kg/m2, controls = 0.7 kg/m2; P < .05). A1C levels didn’t change significantly. One limitation of the study was that 30% to 35% of the patients in the intervention group had a university-level education, compared with 12% in the control group.
Recommendations
The Department of Veterans Affairs issued guidelines in 2017 regarding management of patients with T2DM in primary care.4 The guidelines state that all patients should receive individualized self-management education using “modalities tailored to their preferences” (strong recommendation). They further recommend “offering one or more bidirectional telehealth interventions” in coordination with patients’ health care providers (weak recommendation).
The 2017 diabetes self-management recommendations endorsed by the American Diabetes Association state that “strong evidence” shows that incorporating text messaging into diabetes care improves outcomes, enhances feedback loops, and empowers patients.5
Editor’s takeaway
Telehealth offers mechanisms for patients and physicians to enhance communication about health behaviors and health status. But does it alter outcomes? The cited literature suggests that benefits aren’t a forgone conclusion and that acceptability, ease of use, cost, and individualization are critical issues in telehealth design.
1. Cui M, Wu X, Mao J, et al. T2DM self-management via smartphone applications: a systematic review and meta-analysis. PLoS ONE. 2016;11:e0166718.
2. Agboola S, Jethwani K, Lopez L, et al. Text to Move: A randomized controlled trial of a text-messaging program to improve physical activity behaviors in patients with type 2 diabetes mellitus. J Med Internet Res. 2016;18:e307.
3. Peimani M, Rambod C, Omidvar M, et al. Effectiveness of short message service-based intervention (SMS) on self-care in type 2 diabetes: a feasibility study. Prim Care Diabetes. 2016;10:251-258.
4. Guideline summary: VA/DoD clinical practice guideline for the management of type 2 diabetes mellitus in primary care. Rockville, MD: Agency for Healthcare Research and Quality; 2017. www.innovations.ahrq.gov/qualitytools/department-veterans-affairsdepartment-defense-vadod-clinical-practice-guideline-4. Accessed October 26, 2020.
5. Beck J, Greenwood DA, Blanton L, et al. 2017 National Standards for Diabetes Self-Management, Education and Support. Diabetes Care. 2017;40:1409-1419.
EVIDENCE SUMMARY
A meta-analysis of 6 RCTs studied the effect of smartphone self-care applications on A1C, weight, blood pressure, and lipids in adult patients with T2DM. All the interventions comprised 4 components: electronic self-management prompts and reminders, personal measuring devices, patient-driven data upload, and remote analysis of the data with feedback. The review excluded studies that used phone calls or lasted fewer than 3 months.
Some improvement in A1C found, but no effect on weight
Telehealth interventions improved A1C more than usual care (6 trials, 884 patients; mean difference = –0.40%; 95% CI, –0.69% to –0.11%).1 A subset of 4 studies with 560 patients evaluated changes in weight. Patients had a mean age of 61 years and average weight of 84 kg (in 3 of 4 studies reporting baseline weight). Aggregate weight loss was insignificant after 3 to 12 months (mean difference = –0.84 kg; 95% CI, –2.04 kg to 0.36 kg, P = .17). Investigators reported no harms. Limitations of the analysis included high heterogeneity in the main outcome of A1C (I2 = 70%) but low heterogeneity within the 4 studies assessing weight (I2 = 30%).
Other, small studies found no change in A1C
Two subsequent small RCTs came to different conclusions than the meta-analysis. One compared the impact of individualized physical activity–based text messages in response to pedometer readings with pedometer use alone.2 It included 126 adult patients (mean age, 50.5 years) with T2DM who had an A1C > 7% and access to an Internet-connected computer. Researchers excluded patients who were unable to perform moderate physical activity or who had cognitive deficits.
At enrollment, researchers supplied all patients with a pedometer and an appointment with a counselor to set goals for physical activity. They sent 2 text messages daily to the intervention group (and none to the control group) based on uploaded pedometer data. One message detailed physical activity progress and the second encouraged increased physical activity. The primary outcome was mean step counts per month; secondary outcomes included A1C and weight measured at 6 months.
The groups showed no significant difference in A1C (mean difference = 0.07%; 95% CI, –0.47% to 0.34%, P = .75) or weight loss (mean difference = 3.1 lb; 95% CI, –24.5 lb to 18.3 lb, P = .77). Many patients (43%) reported difficulty uploading step counts, receiving texts, and responding to texts. The dropout rate was 24%.
A second RCT with 150 patients, using a less elaborate protocol, assessed the effectiveness of tailored text-message reminders compared with nontailored text messages to improve A1C and body mass index (BMI).3 Patients were adult Iranians (mean age, 52.5 years) with T2DM who owned a cell phone and could receive and read text messages.
Patients filled out a diabetic self-care assessment to identify barriers to improving care and were randomized into 3 groups. The first group received tailored text messages (75% addressing the patient’s top 2 barriers to self-care and 25% general messages). The second group received nontailored text messages of encouragement. The control group received no text messages.
Continue to: After 3 months...
After 3 months, BMI was reduced in both messaging groups but not the control group (tailored text = –0.6 kg/m2, nontailored text = –0.5 kg/m2, controls = 0.7 kg/m2; P < .05). A1C levels didn’t change significantly. One limitation of the study was that 30% to 35% of the patients in the intervention group had a university-level education, compared with 12% in the control group.
Recommendations
The Department of Veterans Affairs issued guidelines in 2017 regarding management of patients with T2DM in primary care.4 The guidelines state that all patients should receive individualized self-management education using “modalities tailored to their preferences” (strong recommendation). They further recommend “offering one or more bidirectional telehealth interventions” in coordination with patients’ health care providers (weak recommendation).
The 2017 diabetes self-management recommendations endorsed by the American Diabetes Association state that “strong evidence” shows that incorporating text messaging into diabetes care improves outcomes, enhances feedback loops, and empowers patients.5
Editor’s takeaway
Telehealth offers mechanisms for patients and physicians to enhance communication about health behaviors and health status. But does it alter outcomes? The cited literature suggests that benefits aren’t a forgone conclusion and that acceptability, ease of use, cost, and individualization are critical issues in telehealth design.
EVIDENCE SUMMARY
A meta-analysis of 6 RCTs studied the effect of smartphone self-care applications on A1C, weight, blood pressure, and lipids in adult patients with T2DM. All the interventions comprised 4 components: electronic self-management prompts and reminders, personal measuring devices, patient-driven data upload, and remote analysis of the data with feedback. The review excluded studies that used phone calls or lasted fewer than 3 months.
Some improvement in A1C found, but no effect on weight
Telehealth interventions improved A1C more than usual care (6 trials, 884 patients; mean difference = –0.40%; 95% CI, –0.69% to –0.11%).1 A subset of 4 studies with 560 patients evaluated changes in weight. Patients had a mean age of 61 years and average weight of 84 kg (in 3 of 4 studies reporting baseline weight). Aggregate weight loss was insignificant after 3 to 12 months (mean difference = –0.84 kg; 95% CI, –2.04 kg to 0.36 kg, P = .17). Investigators reported no harms. Limitations of the analysis included high heterogeneity in the main outcome of A1C (I2 = 70%) but low heterogeneity within the 4 studies assessing weight (I2 = 30%).
Other, small studies found no change in A1C
Two subsequent small RCTs came to different conclusions than the meta-analysis. One compared the impact of individualized physical activity–based text messages in response to pedometer readings with pedometer use alone.2 It included 126 adult patients (mean age, 50.5 years) with T2DM who had an A1C > 7% and access to an Internet-connected computer. Researchers excluded patients who were unable to perform moderate physical activity or who had cognitive deficits.
At enrollment, researchers supplied all patients with a pedometer and an appointment with a counselor to set goals for physical activity. They sent 2 text messages daily to the intervention group (and none to the control group) based on uploaded pedometer data. One message detailed physical activity progress and the second encouraged increased physical activity. The primary outcome was mean step counts per month; secondary outcomes included A1C and weight measured at 6 months.
The groups showed no significant difference in A1C (mean difference = 0.07%; 95% CI, –0.47% to 0.34%, P = .75) or weight loss (mean difference = 3.1 lb; 95% CI, –24.5 lb to 18.3 lb, P = .77). Many patients (43%) reported difficulty uploading step counts, receiving texts, and responding to texts. The dropout rate was 24%.
A second RCT with 150 patients, using a less elaborate protocol, assessed the effectiveness of tailored text-message reminders compared with nontailored text messages to improve A1C and body mass index (BMI).3 Patients were adult Iranians (mean age, 52.5 years) with T2DM who owned a cell phone and could receive and read text messages.
Patients filled out a diabetic self-care assessment to identify barriers to improving care and were randomized into 3 groups. The first group received tailored text messages (75% addressing the patient’s top 2 barriers to self-care and 25% general messages). The second group received nontailored text messages of encouragement. The control group received no text messages.
Continue to: After 3 months...
After 3 months, BMI was reduced in both messaging groups but not the control group (tailored text = –0.6 kg/m2, nontailored text = –0.5 kg/m2, controls = 0.7 kg/m2; P < .05). A1C levels didn’t change significantly. One limitation of the study was that 30% to 35% of the patients in the intervention group had a university-level education, compared with 12% in the control group.
Recommendations
The Department of Veterans Affairs issued guidelines in 2017 regarding management of patients with T2DM in primary care.4 The guidelines state that all patients should receive individualized self-management education using “modalities tailored to their preferences” (strong recommendation). They further recommend “offering one or more bidirectional telehealth interventions” in coordination with patients’ health care providers (weak recommendation).
The 2017 diabetes self-management recommendations endorsed by the American Diabetes Association state that “strong evidence” shows that incorporating text messaging into diabetes care improves outcomes, enhances feedback loops, and empowers patients.5
Editor’s takeaway
Telehealth offers mechanisms for patients and physicians to enhance communication about health behaviors and health status. But does it alter outcomes? The cited literature suggests that benefits aren’t a forgone conclusion and that acceptability, ease of use, cost, and individualization are critical issues in telehealth design.
1. Cui M, Wu X, Mao J, et al. T2DM self-management via smartphone applications: a systematic review and meta-analysis. PLoS ONE. 2016;11:e0166718.
2. Agboola S, Jethwani K, Lopez L, et al. Text to Move: A randomized controlled trial of a text-messaging program to improve physical activity behaviors in patients with type 2 diabetes mellitus. J Med Internet Res. 2016;18:e307.
3. Peimani M, Rambod C, Omidvar M, et al. Effectiveness of short message service-based intervention (SMS) on self-care in type 2 diabetes: a feasibility study. Prim Care Diabetes. 2016;10:251-258.
4. Guideline summary: VA/DoD clinical practice guideline for the management of type 2 diabetes mellitus in primary care. Rockville, MD: Agency for Healthcare Research and Quality; 2017. www.innovations.ahrq.gov/qualitytools/department-veterans-affairsdepartment-defense-vadod-clinical-practice-guideline-4. Accessed October 26, 2020.
5. Beck J, Greenwood DA, Blanton L, et al. 2017 National Standards for Diabetes Self-Management, Education and Support. Diabetes Care. 2017;40:1409-1419.
1. Cui M, Wu X, Mao J, et al. T2DM self-management via smartphone applications: a systematic review and meta-analysis. PLoS ONE. 2016;11:e0166718.
2. Agboola S, Jethwani K, Lopez L, et al. Text to Move: A randomized controlled trial of a text-messaging program to improve physical activity behaviors in patients with type 2 diabetes mellitus. J Med Internet Res. 2016;18:e307.
3. Peimani M, Rambod C, Omidvar M, et al. Effectiveness of short message service-based intervention (SMS) on self-care in type 2 diabetes: a feasibility study. Prim Care Diabetes. 2016;10:251-258.
4. Guideline summary: VA/DoD clinical practice guideline for the management of type 2 diabetes mellitus in primary care. Rockville, MD: Agency for Healthcare Research and Quality; 2017. www.innovations.ahrq.gov/qualitytools/department-veterans-affairsdepartment-defense-vadod-clinical-practice-guideline-4. Accessed October 26, 2020.
5. Beck J, Greenwood DA, Blanton L, et al. 2017 National Standards for Diabetes Self-Management, Education and Support. Diabetes Care. 2017;40:1409-1419.
EVIDENCE-BASED ANSWER:
PROBABLY NOT—but they may augment self-management. Four-component telehealth systems—including electronic reminders, measuring devices, patient-driven data upload, and remote data analysis—likely don’t result in significant weight reductions in adults with type 2 diabetes (T2DM). However, their use may be associated with a decrease in hemoglobin A1C of about 0.4% (strength of recommendation [SOR]: B, meta-analysis of randomized controlled trials [RCTs] and conflicting smaller subsequent RCTs).
Telehealth is considered a reasonable option for augmenting diabetes self-management in patients who are facile with the technology (SOR: C, expert opinion).
Does early introduction of peanuts to an infant’s diet reduce the risk for peanut allergy?
EVIDENCE SUMMARY
A 2016 systematic review identified 2 RCTs that examined whether early introduction of peanuts affects subsequent allergies.1 The first RCT recruited 1303 3-month-old infants from the general population in the United Kingdom.2 All patients had either a negative skin prick test (SPT) to peanuts or a negative oral peanut challenge (if an initial SPT was positive). The control group breastfed exclusively until age 6 months, at which time allergenic foods could be introduced at parental discretion.
Timing doesn’t affect peanut allergy in nonallergic patients
The intervention group received 6 common allergenic foods (peanuts, eggs, cow’s milk, wheat, sesame, and whitefish) twice weekly between ages 3 and 6 months. Researchers then performed double-blinded, placebo-controlled oral food challenges at ages 12 and 36 months.
More patients in the late-introduction group demonstrated peanut allergies by age 36 months than in the early-introduction group, but the difference wasn’t significant (2.5% vs 1.2%; P = 0.11).A key weakness of the study was combining peanuts with other common food allergens.2
Children with eczema, egg allergy benefit from earlier peanut introduction
The second RCT divided 640 infants with severe eczema, egg allergy, or both into 2 groups according to their response to an SPT to peanuts: patients with no wheal and patients with a positive wheal measuring 1 to 4 mm.3 Researchers then randomized patients to either early exposure (peanut products given from ages 4 to 11 months) or avoidance (no peanuts until age 60 months). The primary endpoint was a positive clinical response to oral peanut allergen at age 60 months.
In the negative SPT group (atopic children expected to have a lower risk for allergy), patients introduced to peanuts later had a higher rate of subsequent allergy than children exposed earlier (14% vs 2%; absolute risk reduction [ARR] = 12%; 95% confidence interval [CI], 3%-20%; number needed to treat [NNT] = 9).3
In the positive SPT group (atopic children expected to have a higher risk for allergy), later peanut introduction likewise increased risk compared to earlier introduction (35% vs 11%; ARR = 24%; 95% CI, 5%-43%; NNT = 5). Children in the early-exposure group, however, had more URIs, viral exanthems, gastroenteritis, urticaria, and conjunctivitis (4527 events in the early-exposure group vs 4287 in the avoidance group, P = 0.02; about 1 more event per patient over the course of the study).3
The authors of the systematic review performed a meta-analysis of the 2 RCTs (1793 patients). They concluded that early introduction of peanuts to an infant’s diet (between ages 3 and 11 months) decreased the risk for eventual peanut allergy (relative risk [RR] = 0.29; 95% CI, 0.11-0.74), compared with introduction at or after age 1 year.1 A key weakness, however, was the researchers’ choice to combine trials with very different inclusion criteria (infants with severe eczema and a general population).
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
A 2017 National Institute of Allergy and Infectious Diseases guideline recommends a 3-tiered approach to peanut introduction: 4
- For children with severe eczema or egg allergy who aren’t currently allergic to peanuts (per SPT or immunoglobulin E [IgE] test), the guideline advises adding peanuts to the diet between ages 4 and 6 months. (Patients with positive SPT or IgE should be referred to an allergy specialist.)
- Children with mild or moderate eczema can be introduced to peanuts around age 6 months “in accordance with family preferences and cultural practices.”
- Children with no evidence of allergy or eczema can be “freely introduced” to peanut-containing foods with no specific guidance on age.
Editor’s takeaway
Good-quality evidence supports family physicians encouraging introduction of foods containing peanuts at age 4 to 6 months for children at increased risk because of atopy, allergies, or eczema.
1. Ierodiakonou D, Garcia-Larsen V, Logan A, et al. Timing of allergenic food introduction to the infant diet and risk of allergic or autoimmune disease: a systematic review and meta-analysis. JAMA. 2016;316:1181-1192.
2. Perkin MR, Logan K, Tseng A, et al. Randomized trial of introduction of allergenic foods in breast-fed infants. N Engl J Med. 2016;374:1733-1743.
3. Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
4. Togias A, Cooper SF, Acebal ML, et al. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases–sponsored expert panel. J Allergy Clin Immunol. 2017;139:29-44.
EVIDENCE SUMMARY
A 2016 systematic review identified 2 RCTs that examined whether early introduction of peanuts affects subsequent allergies.1 The first RCT recruited 1303 3-month-old infants from the general population in the United Kingdom.2 All patients had either a negative skin prick test (SPT) to peanuts or a negative oral peanut challenge (if an initial SPT was positive). The control group breastfed exclusively until age 6 months, at which time allergenic foods could be introduced at parental discretion.
Timing doesn’t affect peanut allergy in nonallergic patients
The intervention group received 6 common allergenic foods (peanuts, eggs, cow’s milk, wheat, sesame, and whitefish) twice weekly between ages 3 and 6 months. Researchers then performed double-blinded, placebo-controlled oral food challenges at ages 12 and 36 months.
More patients in the late-introduction group demonstrated peanut allergies by age 36 months than in the early-introduction group, but the difference wasn’t significant (2.5% vs 1.2%; P = 0.11).A key weakness of the study was combining peanuts with other common food allergens.2
Children with eczema, egg allergy benefit from earlier peanut introduction
The second RCT divided 640 infants with severe eczema, egg allergy, or both into 2 groups according to their response to an SPT to peanuts: patients with no wheal and patients with a positive wheal measuring 1 to 4 mm.3 Researchers then randomized patients to either early exposure (peanut products given from ages 4 to 11 months) or avoidance (no peanuts until age 60 months). The primary endpoint was a positive clinical response to oral peanut allergen at age 60 months.
In the negative SPT group (atopic children expected to have a lower risk for allergy), patients introduced to peanuts later had a higher rate of subsequent allergy than children exposed earlier (14% vs 2%; absolute risk reduction [ARR] = 12%; 95% confidence interval [CI], 3%-20%; number needed to treat [NNT] = 9).3
In the positive SPT group (atopic children expected to have a higher risk for allergy), later peanut introduction likewise increased risk compared to earlier introduction (35% vs 11%; ARR = 24%; 95% CI, 5%-43%; NNT = 5). Children in the early-exposure group, however, had more URIs, viral exanthems, gastroenteritis, urticaria, and conjunctivitis (4527 events in the early-exposure group vs 4287 in the avoidance group, P = 0.02; about 1 more event per patient over the course of the study).3
The authors of the systematic review performed a meta-analysis of the 2 RCTs (1793 patients). They concluded that early introduction of peanuts to an infant’s diet (between ages 3 and 11 months) decreased the risk for eventual peanut allergy (relative risk [RR] = 0.29; 95% CI, 0.11-0.74), compared with introduction at or after age 1 year.1 A key weakness, however, was the researchers’ choice to combine trials with very different inclusion criteria (infants with severe eczema and a general population).
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
A 2017 National Institute of Allergy and Infectious Diseases guideline recommends a 3-tiered approach to peanut introduction: 4
- For children with severe eczema or egg allergy who aren’t currently allergic to peanuts (per SPT or immunoglobulin E [IgE] test), the guideline advises adding peanuts to the diet between ages 4 and 6 months. (Patients with positive SPT or IgE should be referred to an allergy specialist.)
- Children with mild or moderate eczema can be introduced to peanuts around age 6 months “in accordance with family preferences and cultural practices.”
- Children with no evidence of allergy or eczema can be “freely introduced” to peanut-containing foods with no specific guidance on age.
Editor’s takeaway
Good-quality evidence supports family physicians encouraging introduction of foods containing peanuts at age 4 to 6 months for children at increased risk because of atopy, allergies, or eczema.
EVIDENCE SUMMARY
A 2016 systematic review identified 2 RCTs that examined whether early introduction of peanuts affects subsequent allergies.1 The first RCT recruited 1303 3-month-old infants from the general population in the United Kingdom.2 All patients had either a negative skin prick test (SPT) to peanuts or a negative oral peanut challenge (if an initial SPT was positive). The control group breastfed exclusively until age 6 months, at which time allergenic foods could be introduced at parental discretion.
Timing doesn’t affect peanut allergy in nonallergic patients
The intervention group received 6 common allergenic foods (peanuts, eggs, cow’s milk, wheat, sesame, and whitefish) twice weekly between ages 3 and 6 months. Researchers then performed double-blinded, placebo-controlled oral food challenges at ages 12 and 36 months.
More patients in the late-introduction group demonstrated peanut allergies by age 36 months than in the early-introduction group, but the difference wasn’t significant (2.5% vs 1.2%; P = 0.11).A key weakness of the study was combining peanuts with other common food allergens.2
Children with eczema, egg allergy benefit from earlier peanut introduction
The second RCT divided 640 infants with severe eczema, egg allergy, or both into 2 groups according to their response to an SPT to peanuts: patients with no wheal and patients with a positive wheal measuring 1 to 4 mm.3 Researchers then randomized patients to either early exposure (peanut products given from ages 4 to 11 months) or avoidance (no peanuts until age 60 months). The primary endpoint was a positive clinical response to oral peanut allergen at age 60 months.
In the negative SPT group (atopic children expected to have a lower risk for allergy), patients introduced to peanuts later had a higher rate of subsequent allergy than children exposed earlier (14% vs 2%; absolute risk reduction [ARR] = 12%; 95% confidence interval [CI], 3%-20%; number needed to treat [NNT] = 9).3
In the positive SPT group (atopic children expected to have a higher risk for allergy), later peanut introduction likewise increased risk compared to earlier introduction (35% vs 11%; ARR = 24%; 95% CI, 5%-43%; NNT = 5). Children in the early-exposure group, however, had more URIs, viral exanthems, gastroenteritis, urticaria, and conjunctivitis (4527 events in the early-exposure group vs 4287 in the avoidance group, P = 0.02; about 1 more event per patient over the course of the study).3
The authors of the systematic review performed a meta-analysis of the 2 RCTs (1793 patients). They concluded that early introduction of peanuts to an infant’s diet (between ages 3 and 11 months) decreased the risk for eventual peanut allergy (relative risk [RR] = 0.29; 95% CI, 0.11-0.74), compared with introduction at or after age 1 year.1 A key weakness, however, was the researchers’ choice to combine trials with very different inclusion criteria (infants with severe eczema and a general population).
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
A 2017 National Institute of Allergy and Infectious Diseases guideline recommends a 3-tiered approach to peanut introduction: 4
- For children with severe eczema or egg allergy who aren’t currently allergic to peanuts (per SPT or immunoglobulin E [IgE] test), the guideline advises adding peanuts to the diet between ages 4 and 6 months. (Patients with positive SPT or IgE should be referred to an allergy specialist.)
- Children with mild or moderate eczema can be introduced to peanuts around age 6 months “in accordance with family preferences and cultural practices.”
- Children with no evidence of allergy or eczema can be “freely introduced” to peanut-containing foods with no specific guidance on age.
Editor’s takeaway
Good-quality evidence supports family physicians encouraging introduction of foods containing peanuts at age 4 to 6 months for children at increased risk because of atopy, allergies, or eczema.
1. Ierodiakonou D, Garcia-Larsen V, Logan A, et al. Timing of allergenic food introduction to the infant diet and risk of allergic or autoimmune disease: a systematic review and meta-analysis. JAMA. 2016;316:1181-1192.
2. Perkin MR, Logan K, Tseng A, et al. Randomized trial of introduction of allergenic foods in breast-fed infants. N Engl J Med. 2016;374:1733-1743.
3. Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
4. Togias A, Cooper SF, Acebal ML, et al. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases–sponsored expert panel. J Allergy Clin Immunol. 2017;139:29-44.
1. Ierodiakonou D, Garcia-Larsen V, Logan A, et al. Timing of allergenic food introduction to the infant diet and risk of allergic or autoimmune disease: a systematic review and meta-analysis. JAMA. 2016;316:1181-1192.
2. Perkin MR, Logan K, Tseng A, et al. Randomized trial of introduction of allergenic foods in breast-fed infants. N Engl J Med. 2016;374:1733-1743.
3. Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
4. Togias A, Cooper SF, Acebal ML, et al. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases–sponsored expert panel. J Allergy Clin Immunol. 2017;139:29-44.
EVIDENCE-BASED ANSWER:
Probably not, unless the child has severe eczema or egg allergy. In a general pediatric population, introducing peanuts early (at age 3 to 6 months) doesn’t appear to alter rates of subsequent peanut allergy compared with introduction after age 6 months (strength of recommendation [SOR]: B, randomized clinical trial [RCT] using multiple potential food allergens).
In children with severe eczema, egg allergy, or both, however, the risk for a peanut allergy is 12% to 24% lower when peanut-containing foods are introduced at age 4 to 11 months than after age 1 year. Early introduction of peanuts is associated with about 1 additional mild virus-associated syndrome (upper respiratory infection [URI], exanthem, conjunctivitis, or gastroenteritis) per patient (SOR: B, RCT).
Introducing peanuts before age 1 year is recommended for atopic children without evidence of pre-existing peanut allergy; an earlier start, at age 4 to 6 months, is advised for infants with severe eczema or egg allergy (SOR: C, expert opinion).